User login
Reflux Diagnostics: Modern Techniques and Future Directions
Introduction
Chronic esophageal symptoms attributed to gastroesophageal reflux disease (GERD) are common presenting symptoms in gastroenterology, leading to high healthcare costs and adverse quality of life globally.1,2 The clinical diagnosis of GERD hinges on the presence of “troublesome” compatible typical symptoms (heartburn, acid regurgitation) or evidence of mucosal injury on endoscopy (esophagitis, Barrett’s esophagus, peptic stricture).3 With the growing availability of proton pump inhibitors (PPIs), patients and clinicians often utilize an empiric therapeutic trial of PPI as an initial test, with symptom improvement in the absence of alarm symptoms indicating a high likelihood of GERD.4 A meta-analysis of studies that used objective measures of GERD (in this case, 24-hour pH monitoring) showed that the “PPI test” has a sensitivity of 78%, but a specificity of only 54%, as a diagnostic approach to GERD symptoms.5 Apart from noncardiac chest pain, the diagnostic yield is even lower for atypical and extra-esophageal symptoms such as cough or laryngeal symptoms.6
The “nuts and bolts” of reflux testing
Ambulatory reflux testing assesses esophageal reflux burden and symptom-reflux association (SRA). Individual reflux events are identified as either a drop in esophageal pH to less than 4 (acid reflux events), or a sharp decrease in esophageal impedance measurements in a retrograde fashion (impedance-detected reflux events), with subsequent recovery to the baseline in each instance. Ambulatory reflux testing affords insight into three areas: 1) measurement of esophageal acid exposure time (AET); the cumulative time duration when distal esophageal pH is less than 4 at the recording site, reported as a percentage of the recording period; 2) measurement of the number of reflux events both acidic (from pH monitoring) and weakly acidic/alkaline (from impedance monitoring); and 3) quantitative evaluation of the association between reported symptom episodes and reflux events.
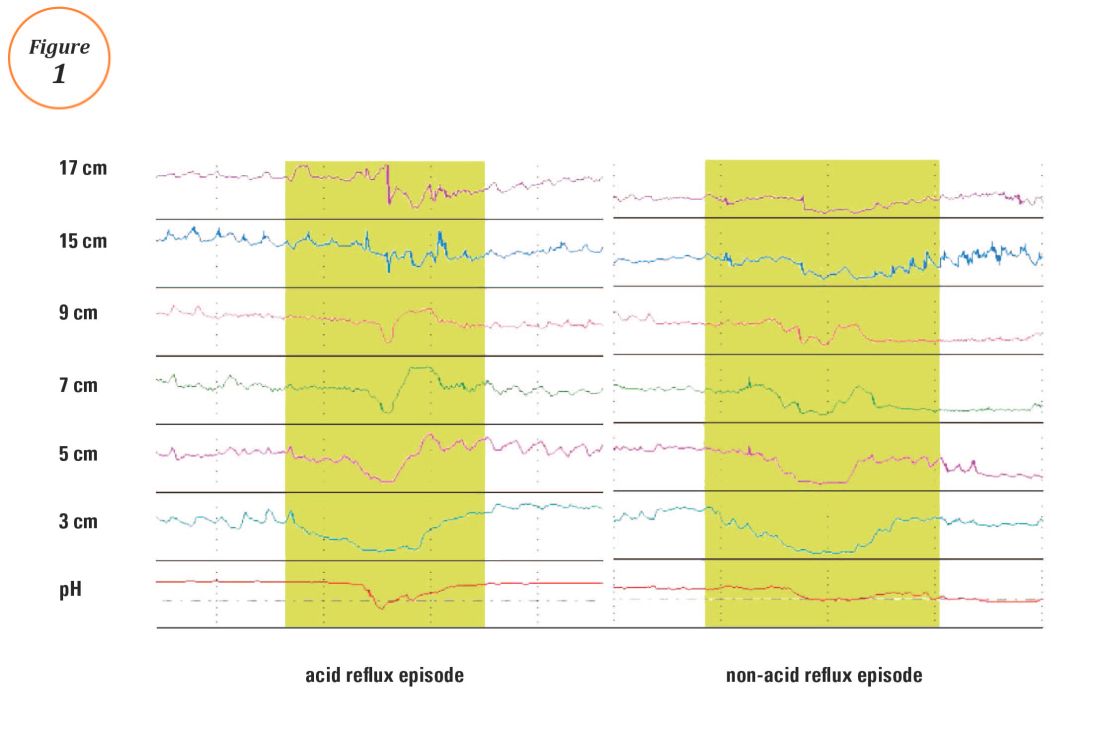
The SI and SAP can be calculated individually for acid-detected reflux events and for impedance-detected reflux events. Since reflux events are better detected with impedance, combined pH-impedance testing increases the yield of detecting positive SRA, especially when performed off PPI therapy.16,17 Because these indices are heavily reliant on patient reporting of symptom episodes, SRA can be overinterpreted;18 positive associations are more clinically useful than negative results in the evaluation of symptoms attributed to GERD.19 Despite these concerns, the two most consistent predictors of symptomatic outcome with antireflux therapy on pH-impedance testing are abnormal AET and positive SAP with impedance-detected reflux events.17
Testing on or off PPI?
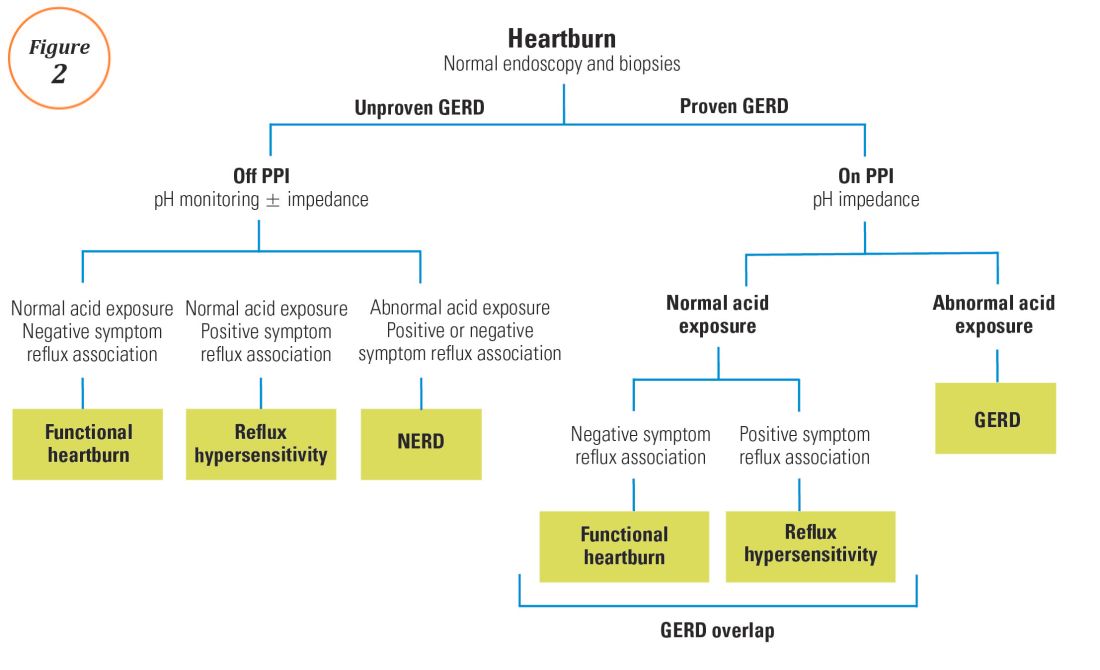
For symptoms attributable to GERD that persist despite properly administered PPI therapy, the 2013 American College of Gastroenterology guidelines suggest upper endoscopy with esophageal biopsies for typical symptoms and appropriate referrals for atypical symptoms.24 However, if these evaluations are unremarkable, reflux monitoring is recommended, with PPI status for testing guided by the pre-test probability of GERD: with a low pre-test probability of GERD, reflux testing is best performed off PPI with either pH or combined pH-impedance testing. In contrast, with a high pre-test probability of GERD, testing is best performed on PPI with combined pH-impedance testing. A similar concept is proposed in the Rome IV approach (Figure 2)23 and on GERD consensus guidelines:7 when heartburn or chest pain persists despite PPI therapy and endoscopy and esophageal biopsies are normal, evidence for GERD (past esophagitis, Barrett’s esophagus, peptic stricture, or prior positive reflux testing) prompts pH-impedance monitoring on PPI therapy (i.e., proven GERD). Those without this evidence for proven GERD (i.e., unproven GERD) are best tested off PPI, and the test utilized can be either pH alone or combined pH-impedance.
GERD phenotypes and management
The presence or absence of the two core metrics on ambulatory reflux monitoring – abnormal AET and positive SRA – can stratify symptomatic GERD patients into phenotypes that predict symptomatic improvement with antireflux therapy and guide management of symptoms (Figure 3).25,26 The presence of both abnormal AET and positive SRA suggests “strong” evidence for GERD, for which symptom improvement is likely with maximization of antireflux therapy, which can include BID PPI, baclofen (to decrease transient LES relaxations), alginates (such as Gaviscon), and consideration of endosopic or surgical antireflux procedures such as fundoplication or magnetic sphincter augmentation. Abnormal AET but negative SRA is regarded as “good” evidence for GERD, for which similar antireflux therapies can be advocated. Normal AET but positive SRA is designated as “reflux hypersensitivity,”23 with increasing proportions of patients meeting this phenotype when tested with combined pH-impedance and off PPI therapy.27 Both normal AET and negative SRA suggest equivocal evidence for GERD and the likely presence of a functional esophageal disorder, such as functional heartburn.23 For reflux hypersensitivity and especially functional esophageal disorders, antireflux therapy is unlikely to be as effective and management can include pharmacologic neuromodulation (such as tricyclic antidepressants administered at bedtime) as well as adjunctive nonpharmacologic approaches (such as stress reduction, relaxation, hypnosis, or cognitive-behavioral therapy).
The future of reflux diagnostics

Conclusions
For esophageal symptoms potentially attributable to GERD that persist despite optimized PPI therapy, esophageal testing should be undertaken, starting with endoscopy and biopsies and proceeding to ambulatory reflux monitoring with HRM. The decisions between pH testing alone versus combined pH-impedance monitoring, and between testing on or off PPI therapy, can be guided either by the pre-test probability of GERD or whether GERD has been proven or unproven in prior evaluations (Figure 2). Elevated AET and positive SRA with impedance-detected reflux events can predict the likelihood of successful management outcomes from antireflux therapy. These two core metrics can be utilized to phenotype GERD and guide management approaches for persisting symptoms (Figure 3). Novel impedance metrics (baseline mucosal impedance, postreflux swallow-induced peristaltic wave index) and markers for esophageal mucosal damage continue to be studied as potential markers for evidence of longitudinal reflux exposure.
Dr. Patel is assistant professor of medicine, division of gastroenterology, Duke University School of Medicine and the Durham Veterans Affairs Medical Center, Durham, N.C. Dr. Gyawali is professor of medicine, division of gastroenterology, Washington University School of Medicine, St. Louis, Mo.
References
1. Shaheen N.J., et al. Am J Gastroenterol. 2006;101:2128-38.
2. Patel A., Gyawali C.P.. Switzerland: Springer International, 2016.
3. Vakil N., et al. Am J Gastroenterol. 2006;101:1900-20; quiz 1943.
4. Fass R., et al. Arch Intern Med. 1999;159:2161-8.
5. Numans M.E., et al. Ann Intern Med. 2004;140:518-27.
6. Shaheen N.J., et al. Aliment Pharmacol Ther. 2011;33:225-34.
7. Roman S., et al. Neurogastroenterol Motil Mar 31. doi: 10.1111/nmo.13067. [Epub ahead of print] 2017.
8. Dellon E.S., et al. Am J Gastroenterol. 2013;108:679-92; quiz 693.
9. Pandolfino JE, Vela MF. Gastrointest Endosc. 2009;69:917-30, 930 e1.
10. Shay S., et al. Am J Gastroenterol. 2004;99:1037-43.
11. Zerbib F., et al. Clin Gastroenterol Hepatol. 2013;11:366-72.
12. Wiener G.J., et al. Am J Gastroenterol 1988;83:358-61.
13. Weusten B.L., et al. Gastroenterology. 1994;107:1741-5.
14. Ghillebert G., et al. Gut 1990;31:738-44.
15. Kushnir V.M., et al. Aliment Pharmacol Ther. 2012;35(9):1080-7.
16. Bredenoord A.J., et al. Am J Gastroenterol. 2006;101:453-9.
17. Patel A., et al. Clin Gastroenterol Hepatol. 2015;13:884-91.
18. Slaughter J.C., et al. Clin Gastroenterol Hepatol. 2011;9:868-74.
19. Kavitt R.T., et al. Am J Gastroenterol. 2012;107:1826-32.
20. Kahrilas P.J., et al. Gastroenterology 2008;135:1383-91, 1391 e1-5.
21. Kessing B.F., et al. Clin Gastroenterol Hepatol. 2011;9:1020-4.
22. Kahrilas P.J., et al. Neurogastroenterol Motil. 2015;27:160-74.
23. Aziz A, et al. Esophageal disorders. Gastroenterology 2016;150:1368-79.
24. Katz P.O., et al. Am J Gastroenterol. 2013;108:308-28; quiz 329.
25. Boeckxstaens G., et al. Gut 2014;63:1185-93.
26. Patel A., et al. Neurogastroenterol Motil. 2016;28:513-21.
27. Patel A., et al. Neurogastroenterol Motil. 2016;28:1382-90.
28. Martinucci I., et al. Neurogastroenterol Motil. 2014;26:546-55.
29. Ates F., et al. Gastroenterology 2015;148:334-43.
30. Kessing B.F., et al. Am J Gastroenterol. 2011;106:2093-7.
31. Patel A., et al. Aliment Pharmacol Ther. 2016;44:890-8.
32. Frazzoni M., et al. Neurogastroenterol Motil. 2016.
33. Frazzoni M., et al. Neurogastroenterol Motil. 2013;25:399-406, e295.
34. Vela M.F., et al. Am J Gastroenterol. 2011;106:844-50.
Introduction
Chronic esophageal symptoms attributed to gastroesophageal reflux disease (GERD) are common presenting symptoms in gastroenterology, leading to high healthcare costs and adverse quality of life globally.1,2 The clinical diagnosis of GERD hinges on the presence of “troublesome” compatible typical symptoms (heartburn, acid regurgitation) or evidence of mucosal injury on endoscopy (esophagitis, Barrett’s esophagus, peptic stricture).3 With the growing availability of proton pump inhibitors (PPIs), patients and clinicians often utilize an empiric therapeutic trial of PPI as an initial test, with symptom improvement in the absence of alarm symptoms indicating a high likelihood of GERD.4 A meta-analysis of studies that used objective measures of GERD (in this case, 24-hour pH monitoring) showed that the “PPI test” has a sensitivity of 78%, but a specificity of only 54%, as a diagnostic approach to GERD symptoms.5 Apart from noncardiac chest pain, the diagnostic yield is even lower for atypical and extra-esophageal symptoms such as cough or laryngeal symptoms.6
The “nuts and bolts” of reflux testing
Ambulatory reflux testing assesses esophageal reflux burden and symptom-reflux association (SRA). Individual reflux events are identified as either a drop in esophageal pH to less than 4 (acid reflux events), or a sharp decrease in esophageal impedance measurements in a retrograde fashion (impedance-detected reflux events), with subsequent recovery to the baseline in each instance. Ambulatory reflux testing affords insight into three areas: 1) measurement of esophageal acid exposure time (AET); the cumulative time duration when distal esophageal pH is less than 4 at the recording site, reported as a percentage of the recording period; 2) measurement of the number of reflux events both acidic (from pH monitoring) and weakly acidic/alkaline (from impedance monitoring); and 3) quantitative evaluation of the association between reported symptom episodes and reflux events.

The SI and SAP can be calculated individually for acid-detected reflux events and for impedance-detected reflux events. Since reflux events are better detected with impedance, combined pH-impedance testing increases the yield of detecting positive SRA, especially when performed off PPI therapy.16,17 Because these indices are heavily reliant on patient reporting of symptom episodes, SRA can be overinterpreted;18 positive associations are more clinically useful than negative results in the evaluation of symptoms attributed to GERD.19 Despite these concerns, the two most consistent predictors of symptomatic outcome with antireflux therapy on pH-impedance testing are abnormal AET and positive SAP with impedance-detected reflux events.17

Testing on or off PPI?
For symptoms attributable to GERD that persist despite properly administered PPI therapy, the 2013 American College of Gastroenterology guidelines suggest upper endoscopy with esophageal biopsies for typical symptoms and appropriate referrals for atypical symptoms.24 However, if these evaluations are unremarkable, reflux monitoring is recommended, with PPI status for testing guided by the pre-test probability of GERD: with a low pre-test probability of GERD, reflux testing is best performed off PPI with either pH or combined pH-impedance testing. In contrast, with a high pre-test probability of GERD, testing is best performed on PPI with combined pH-impedance testing. A similar concept is proposed in the Rome IV approach (Figure 2)23 and on GERD consensus guidelines:7 when heartburn or chest pain persists despite PPI therapy and endoscopy and esophageal biopsies are normal, evidence for GERD (past esophagitis, Barrett’s esophagus, peptic stricture, or prior positive reflux testing) prompts pH-impedance monitoring on PPI therapy (i.e., proven GERD). Those without this evidence for proven GERD (i.e., unproven GERD) are best tested off PPI, and the test utilized can be either pH alone or combined pH-impedance.
GERD phenotypes and management
The presence or absence of the two core metrics on ambulatory reflux monitoring – abnormal AET and positive SRA – can stratify symptomatic GERD patients into phenotypes that predict symptomatic improvement with antireflux therapy and guide management of symptoms (Figure 3).25,26 The presence of both abnormal AET and positive SRA suggests “strong” evidence for GERD, for which symptom improvement is likely with maximization of antireflux therapy, which can include BID PPI, baclofen (to decrease transient LES relaxations), alginates (such as Gaviscon), and consideration of endosopic or surgical antireflux procedures such as fundoplication or magnetic sphincter augmentation. Abnormal AET but negative SRA is regarded as “good” evidence for GERD, for which similar antireflux therapies can be advocated. Normal AET but positive SRA is designated as “reflux hypersensitivity,”23 with increasing proportions of patients meeting this phenotype when tested with combined pH-impedance and off PPI therapy.27 Both normal AET and negative SRA suggest equivocal evidence for GERD and the likely presence of a functional esophageal disorder, such as functional heartburn.23 For reflux hypersensitivity and especially functional esophageal disorders, antireflux therapy is unlikely to be as effective and management can include pharmacologic neuromodulation (such as tricyclic antidepressants administered at bedtime) as well as adjunctive nonpharmacologic approaches (such as stress reduction, relaxation, hypnosis, or cognitive-behavioral therapy).
The future of reflux diagnostics

Conclusions
For esophageal symptoms potentially attributable to GERD that persist despite optimized PPI therapy, esophageal testing should be undertaken, starting with endoscopy and biopsies and proceeding to ambulatory reflux monitoring with HRM. The decisions between pH testing alone versus combined pH-impedance monitoring, and between testing on or off PPI therapy, can be guided either by the pre-test probability of GERD or whether GERD has been proven or unproven in prior evaluations (Figure 2). Elevated AET and positive SRA with impedance-detected reflux events can predict the likelihood of successful management outcomes from antireflux therapy. These two core metrics can be utilized to phenotype GERD and guide management approaches for persisting symptoms (Figure 3). Novel impedance metrics (baseline mucosal impedance, postreflux swallow-induced peristaltic wave index) and markers for esophageal mucosal damage continue to be studied as potential markers for evidence of longitudinal reflux exposure.
Dr. Patel is assistant professor of medicine, division of gastroenterology, Duke University School of Medicine and the Durham Veterans Affairs Medical Center, Durham, N.C. Dr. Gyawali is professor of medicine, division of gastroenterology, Washington University School of Medicine, St. Louis, Mo.
References
1. Shaheen N.J., et al. Am J Gastroenterol. 2006;101:2128-38.
2. Patel A., Gyawali C.P.. Switzerland: Springer International, 2016.
3. Vakil N., et al. Am J Gastroenterol. 2006;101:1900-20; quiz 1943.
4. Fass R., et al. Arch Intern Med. 1999;159:2161-8.
5. Numans M.E., et al. Ann Intern Med. 2004;140:518-27.
6. Shaheen N.J., et al. Aliment Pharmacol Ther. 2011;33:225-34.
7. Roman S., et al. Neurogastroenterol Motil Mar 31. doi: 10.1111/nmo.13067. [Epub ahead of print] 2017.
8. Dellon E.S., et al. Am J Gastroenterol. 2013;108:679-92; quiz 693.
9. Pandolfino JE, Vela MF. Gastrointest Endosc. 2009;69:917-30, 930 e1.
10. Shay S., et al. Am J Gastroenterol. 2004;99:1037-43.
11. Zerbib F., et al. Clin Gastroenterol Hepatol. 2013;11:366-72.
12. Wiener G.J., et al. Am J Gastroenterol 1988;83:358-61.
13. Weusten B.L., et al. Gastroenterology. 1994;107:1741-5.
14. Ghillebert G., et al. Gut 1990;31:738-44.
15. Kushnir V.M., et al. Aliment Pharmacol Ther. 2012;35(9):1080-7.
16. Bredenoord A.J., et al. Am J Gastroenterol. 2006;101:453-9.
17. Patel A., et al. Clin Gastroenterol Hepatol. 2015;13:884-91.
18. Slaughter J.C., et al. Clin Gastroenterol Hepatol. 2011;9:868-74.
19. Kavitt R.T., et al. Am J Gastroenterol. 2012;107:1826-32.
20. Kahrilas P.J., et al. Gastroenterology 2008;135:1383-91, 1391 e1-5.
21. Kessing B.F., et al. Clin Gastroenterol Hepatol. 2011;9:1020-4.
22. Kahrilas P.J., et al. Neurogastroenterol Motil. 2015;27:160-74.
23. Aziz A, et al. Esophageal disorders. Gastroenterology 2016;150:1368-79.
24. Katz P.O., et al. Am J Gastroenterol. 2013;108:308-28; quiz 329.
25. Boeckxstaens G., et al. Gut 2014;63:1185-93.
26. Patel A., et al. Neurogastroenterol Motil. 2016;28:513-21.
27. Patel A., et al. Neurogastroenterol Motil. 2016;28:1382-90.
28. Martinucci I., et al. Neurogastroenterol Motil. 2014;26:546-55.
29. Ates F., et al. Gastroenterology 2015;148:334-43.
30. Kessing B.F., et al. Am J Gastroenterol. 2011;106:2093-7.
31. Patel A., et al. Aliment Pharmacol Ther. 2016;44:890-8.
32. Frazzoni M., et al. Neurogastroenterol Motil. 2016.
33. Frazzoni M., et al. Neurogastroenterol Motil. 2013;25:399-406, e295.
34. Vela M.F., et al. Am J Gastroenterol. 2011;106:844-50.
Introduction
Chronic esophageal symptoms attributed to gastroesophageal reflux disease (GERD) are common presenting symptoms in gastroenterology, leading to high healthcare costs and adverse quality of life globally.1,2 The clinical diagnosis of GERD hinges on the presence of “troublesome” compatible typical symptoms (heartburn, acid regurgitation) or evidence of mucosal injury on endoscopy (esophagitis, Barrett’s esophagus, peptic stricture).3 With the growing availability of proton pump inhibitors (PPIs), patients and clinicians often utilize an empiric therapeutic trial of PPI as an initial test, with symptom improvement in the absence of alarm symptoms indicating a high likelihood of GERD.4 A meta-analysis of studies that used objective measures of GERD (in this case, 24-hour pH monitoring) showed that the “PPI test” has a sensitivity of 78%, but a specificity of only 54%, as a diagnostic approach to GERD symptoms.5 Apart from noncardiac chest pain, the diagnostic yield is even lower for atypical and extra-esophageal symptoms such as cough or laryngeal symptoms.6
The “nuts and bolts” of reflux testing
Ambulatory reflux testing assesses esophageal reflux burden and symptom-reflux association (SRA). Individual reflux events are identified as either a drop in esophageal pH to less than 4 (acid reflux events), or a sharp decrease in esophageal impedance measurements in a retrograde fashion (impedance-detected reflux events), with subsequent recovery to the baseline in each instance. Ambulatory reflux testing affords insight into three areas: 1) measurement of esophageal acid exposure time (AET); the cumulative time duration when distal esophageal pH is less than 4 at the recording site, reported as a percentage of the recording period; 2) measurement of the number of reflux events both acidic (from pH monitoring) and weakly acidic/alkaline (from impedance monitoring); and 3) quantitative evaluation of the association between reported symptom episodes and reflux events.

The SI and SAP can be calculated individually for acid-detected reflux events and for impedance-detected reflux events. Since reflux events are better detected with impedance, combined pH-impedance testing increases the yield of detecting positive SRA, especially when performed off PPI therapy.16,17 Because these indices are heavily reliant on patient reporting of symptom episodes, SRA can be overinterpreted;18 positive associations are more clinically useful than negative results in the evaluation of symptoms attributed to GERD.19 Despite these concerns, the two most consistent predictors of symptomatic outcome with antireflux therapy on pH-impedance testing are abnormal AET and positive SAP with impedance-detected reflux events.17

Testing on or off PPI?
For symptoms attributable to GERD that persist despite properly administered PPI therapy, the 2013 American College of Gastroenterology guidelines suggest upper endoscopy with esophageal biopsies for typical symptoms and appropriate referrals for atypical symptoms.24 However, if these evaluations are unremarkable, reflux monitoring is recommended, with PPI status for testing guided by the pre-test probability of GERD: with a low pre-test probability of GERD, reflux testing is best performed off PPI with either pH or combined pH-impedance testing. In contrast, with a high pre-test probability of GERD, testing is best performed on PPI with combined pH-impedance testing. A similar concept is proposed in the Rome IV approach (Figure 2)23 and on GERD consensus guidelines:7 when heartburn or chest pain persists despite PPI therapy and endoscopy and esophageal biopsies are normal, evidence for GERD (past esophagitis, Barrett’s esophagus, peptic stricture, or prior positive reflux testing) prompts pH-impedance monitoring on PPI therapy (i.e., proven GERD). Those without this evidence for proven GERD (i.e., unproven GERD) are best tested off PPI, and the test utilized can be either pH alone or combined pH-impedance.
GERD phenotypes and management
The presence or absence of the two core metrics on ambulatory reflux monitoring – abnormal AET and positive SRA – can stratify symptomatic GERD patients into phenotypes that predict symptomatic improvement with antireflux therapy and guide management of symptoms (Figure 3).25,26 The presence of both abnormal AET and positive SRA suggests “strong” evidence for GERD, for which symptom improvement is likely with maximization of antireflux therapy, which can include BID PPI, baclofen (to decrease transient LES relaxations), alginates (such as Gaviscon), and consideration of endosopic or surgical antireflux procedures such as fundoplication or magnetic sphincter augmentation. Abnormal AET but negative SRA is regarded as “good” evidence for GERD, for which similar antireflux therapies can be advocated. Normal AET but positive SRA is designated as “reflux hypersensitivity,”23 with increasing proportions of patients meeting this phenotype when tested with combined pH-impedance and off PPI therapy.27 Both normal AET and negative SRA suggest equivocal evidence for GERD and the likely presence of a functional esophageal disorder, such as functional heartburn.23 For reflux hypersensitivity and especially functional esophageal disorders, antireflux therapy is unlikely to be as effective and management can include pharmacologic neuromodulation (such as tricyclic antidepressants administered at bedtime) as well as adjunctive nonpharmacologic approaches (such as stress reduction, relaxation, hypnosis, or cognitive-behavioral therapy).
The future of reflux diagnostics

Conclusions
For esophageal symptoms potentially attributable to GERD that persist despite optimized PPI therapy, esophageal testing should be undertaken, starting with endoscopy and biopsies and proceeding to ambulatory reflux monitoring with HRM. The decisions between pH testing alone versus combined pH-impedance monitoring, and between testing on or off PPI therapy, can be guided either by the pre-test probability of GERD or whether GERD has been proven or unproven in prior evaluations (Figure 2). Elevated AET and positive SRA with impedance-detected reflux events can predict the likelihood of successful management outcomes from antireflux therapy. These two core metrics can be utilized to phenotype GERD and guide management approaches for persisting symptoms (Figure 3). Novel impedance metrics (baseline mucosal impedance, postreflux swallow-induced peristaltic wave index) and markers for esophageal mucosal damage continue to be studied as potential markers for evidence of longitudinal reflux exposure.
Dr. Patel is assistant professor of medicine, division of gastroenterology, Duke University School of Medicine and the Durham Veterans Affairs Medical Center, Durham, N.C. Dr. Gyawali is professor of medicine, division of gastroenterology, Washington University School of Medicine, St. Louis, Mo.
References
1. Shaheen N.J., et al. Am J Gastroenterol. 2006;101:2128-38.
2. Patel A., Gyawali C.P.. Switzerland: Springer International, 2016.
3. Vakil N., et al. Am J Gastroenterol. 2006;101:1900-20; quiz 1943.
4. Fass R., et al. Arch Intern Med. 1999;159:2161-8.
5. Numans M.E., et al. Ann Intern Med. 2004;140:518-27.
6. Shaheen N.J., et al. Aliment Pharmacol Ther. 2011;33:225-34.
7. Roman S., et al. Neurogastroenterol Motil Mar 31. doi: 10.1111/nmo.13067. [Epub ahead of print] 2017.
8. Dellon E.S., et al. Am J Gastroenterol. 2013;108:679-92; quiz 693.
9. Pandolfino JE, Vela MF. Gastrointest Endosc. 2009;69:917-30, 930 e1.
10. Shay S., et al. Am J Gastroenterol. 2004;99:1037-43.
11. Zerbib F., et al. Clin Gastroenterol Hepatol. 2013;11:366-72.
12. Wiener G.J., et al. Am J Gastroenterol 1988;83:358-61.
13. Weusten B.L., et al. Gastroenterology. 1994;107:1741-5.
14. Ghillebert G., et al. Gut 1990;31:738-44.
15. Kushnir V.M., et al. Aliment Pharmacol Ther. 2012;35(9):1080-7.
16. Bredenoord A.J., et al. Am J Gastroenterol. 2006;101:453-9.
17. Patel A., et al. Clin Gastroenterol Hepatol. 2015;13:884-91.
18. Slaughter J.C., et al. Clin Gastroenterol Hepatol. 2011;9:868-74.
19. Kavitt R.T., et al. Am J Gastroenterol. 2012;107:1826-32.
20. Kahrilas P.J., et al. Gastroenterology 2008;135:1383-91, 1391 e1-5.
21. Kessing B.F., et al. Clin Gastroenterol Hepatol. 2011;9:1020-4.
22. Kahrilas P.J., et al. Neurogastroenterol Motil. 2015;27:160-74.
23. Aziz A, et al. Esophageal disorders. Gastroenterology 2016;150:1368-79.
24. Katz P.O., et al. Am J Gastroenterol. 2013;108:308-28; quiz 329.
25. Boeckxstaens G., et al. Gut 2014;63:1185-93.
26. Patel A., et al. Neurogastroenterol Motil. 2016;28:513-21.
27. Patel A., et al. Neurogastroenterol Motil. 2016;28:1382-90.
28. Martinucci I., et al. Neurogastroenterol Motil. 2014;26:546-55.
29. Ates F., et al. Gastroenterology 2015;148:334-43.
30. Kessing B.F., et al. Am J Gastroenterol. 2011;106:2093-7.
31. Patel A., et al. Aliment Pharmacol Ther. 2016;44:890-8.
32. Frazzoni M., et al. Neurogastroenterol Motil. 2016.
33. Frazzoni M., et al. Neurogastroenterol Motil. 2013;25:399-406, e295.
34. Vela M.F., et al. Am J Gastroenterol. 2011;106:844-50.


