User login
Insulin degludec decreases rate, severity of hypoglycemic episodes
Insulin degludec decreased both the rate and the severity of hypoglycemic episodes in adults with type 1 and type 2 diabetes, compared with insulin glargine, in two head-to-head trials sponsored by the maker of insulin degludec and reported online July 3 in JAMA.
The two trials had identical randomized double-blind crossover designs. The first involved 501 adults with type 1 diabetes treated at 84 sites in the United States and 6 sites in Poland, and the second involved 721 adults with type 2 diabetes treated at 152 sites in the United States. All the study participants were at risk for hypoglycemia by virtue of experiencing one or more severe hypoglycemic episodes within the preceding year, having moderate chronic renal failure, having a 15-year or longer duration of diabetes, being unaware of their hypoglycemic symptoms, or experiencing severe hypoglycemia within the 3 months preceding baseline.
In both trials, patients were randomized to one type of once-daily insulin for 32 weeks (a 16-week titration period followed by a 16-week maintenance period) and then crossed over to the other type of insulin for 32 weeks. The primary end point in both studies was the rate of overall severe hypoglycemia during the maintenance period. This was defined as either an episode requiring the assistance of another person to administer aid or an episode in which blood glucose measured less than 56 mg/dL.
In the first trial, rates of hypoglycemia were significantly lower with insulin degludec (2,201 episodes per 100 person-years of exposure) than with insulin glargine (2,463 episodes per 100 PYE), demonstrating not just the noninferiority but also the superiority of insulin degludec. In addition, a significantly lower proportion of patients had hypoglycemia with insulin degludec (32.8%) than with insulin glargine (43.1%), said Wendy Lane, MD, of Mountain Diabetes and Endocrine Center, Asheville NC, and her associates.
Regarding the severity of hypoglycemia, the rate of severe episodes was significantly lower with insulin degludec (69 episodes per 100 PYE) than with insulin glargine (92 episodes per 100 PYE). In addition, the proportion of patients who experienced a severe hypoglycemic episode was significantly lower with insulin degludec (10.3%) than with insulin glargine (17.1%).
Both types of insulin reduced hemoglobin A1c levels to 7% or below. Rates of adverse events and serious adverse events were similar between the two study groups, and there were no differences between them in weight change, blood pressure, pulse rate, or laboratory findings, the investigators said (JAMA.2017;318[1]:33-44).
In the second trial, rates of hypoglycemia again were significantly lower with insulin degludec than with insulin glargine (186 vs. 265 episodes per 100 PYE). In addition, the proportion of patients who experienced a severe hypoglycemic episode was 1.6% vs. 2.4%, respectively, said Carol Wysham, MD, of Rockwood Clinic University of Washington, Spokane, and her associates.
As in the first trial, both types of insulin reduced HbA1c levels to the same degree, and rates of adverse events and of serious adverse events were comparable, Dr. Wysham and her associates said (JAMA. 2017;318[1]:45-56).
The dropout rates were similar and higher than expected in both trials, at approximately 20%. Dr. Lane and her associates noted that this may have resulted from “the demanding nature” of the studies, including their 64-week duration; demand for close monitoring of blood glucose; and the requirement of using vials and syringes to maintain treatment blinding, instead of more convenient injector devices.
Both trials were funded by Novo Nordisk, maker of insulin degludec. Dr. Lane reported ties to Novo Nordisk, Insulet Corporation, and Eli Lilly, and her associates reported ties to numerous industry sources. Dr. Wysham reported ties to Novo Nordisk, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, and Sanofi, and her associates reported ties to numerous industry sources.
Given the risks associated with hypoglycemia and the concerns about this adverse effect among patients and their families, any basal insulin that reduces the rate of hypoglycemia represents an advance in the treatment of diabetes.
Both studies were limited in that they had relatively high dropout rates of approximately 20% each. However, it appeared that the patients who completed the study were not substantially different from those who dropped out.
These remarks are from an editorial by Elizabeth R. Seaquist, MD, and Lisa S. Chow, MD, that was published along with the research reports (JAMA 2017;318[1]:31-2).
Dr Seaquist reported a variety of sources of funding from Eli Lilly, Locemia, Medtronic, Sanofi, and Zucera; serving as a member of the International Hypoglycemia Study Group; and serving on the examination committee for the American Board of Internal Medicine. Dr. Chow reported research funding from Eli Lilly.
Given the risks associated with hypoglycemia and the concerns about this adverse effect among patients and their families, any basal insulin that reduces the rate of hypoglycemia represents an advance in the treatment of diabetes.
Both studies were limited in that they had relatively high dropout rates of approximately 20% each. However, it appeared that the patients who completed the study were not substantially different from those who dropped out.
These remarks are from an editorial by Elizabeth R. Seaquist, MD, and Lisa S. Chow, MD, that was published along with the research reports (JAMA 2017;318[1]:31-2).
Dr Seaquist reported a variety of sources of funding from Eli Lilly, Locemia, Medtronic, Sanofi, and Zucera; serving as a member of the International Hypoglycemia Study Group; and serving on the examination committee for the American Board of Internal Medicine. Dr. Chow reported research funding from Eli Lilly.
Given the risks associated with hypoglycemia and the concerns about this adverse effect among patients and their families, any basal insulin that reduces the rate of hypoglycemia represents an advance in the treatment of diabetes.
Both studies were limited in that they had relatively high dropout rates of approximately 20% each. However, it appeared that the patients who completed the study were not substantially different from those who dropped out.
These remarks are from an editorial by Elizabeth R. Seaquist, MD, and Lisa S. Chow, MD, that was published along with the research reports (JAMA 2017;318[1]:31-2).
Dr Seaquist reported a variety of sources of funding from Eli Lilly, Locemia, Medtronic, Sanofi, and Zucera; serving as a member of the International Hypoglycemia Study Group; and serving on the examination committee for the American Board of Internal Medicine. Dr. Chow reported research funding from Eli Lilly.
Insulin degludec decreased both the rate and the severity of hypoglycemic episodes in adults with type 1 and type 2 diabetes, compared with insulin glargine, in two head-to-head trials sponsored by the maker of insulin degludec and reported online July 3 in JAMA.
The two trials had identical randomized double-blind crossover designs. The first involved 501 adults with type 1 diabetes treated at 84 sites in the United States and 6 sites in Poland, and the second involved 721 adults with type 2 diabetes treated at 152 sites in the United States. All the study participants were at risk for hypoglycemia by virtue of experiencing one or more severe hypoglycemic episodes within the preceding year, having moderate chronic renal failure, having a 15-year or longer duration of diabetes, being unaware of their hypoglycemic symptoms, or experiencing severe hypoglycemia within the 3 months preceding baseline.
In both trials, patients were randomized to one type of once-daily insulin for 32 weeks (a 16-week titration period followed by a 16-week maintenance period) and then crossed over to the other type of insulin for 32 weeks. The primary end point in both studies was the rate of overall severe hypoglycemia during the maintenance period. This was defined as either an episode requiring the assistance of another person to administer aid or an episode in which blood glucose measured less than 56 mg/dL.
In the first trial, rates of hypoglycemia were significantly lower with insulin degludec (2,201 episodes per 100 person-years of exposure) than with insulin glargine (2,463 episodes per 100 PYE), demonstrating not just the noninferiority but also the superiority of insulin degludec. In addition, a significantly lower proportion of patients had hypoglycemia with insulin degludec (32.8%) than with insulin glargine (43.1%), said Wendy Lane, MD, of Mountain Diabetes and Endocrine Center, Asheville NC, and her associates.
Regarding the severity of hypoglycemia, the rate of severe episodes was significantly lower with insulin degludec (69 episodes per 100 PYE) than with insulin glargine (92 episodes per 100 PYE). In addition, the proportion of patients who experienced a severe hypoglycemic episode was significantly lower with insulin degludec (10.3%) than with insulin glargine (17.1%).
Both types of insulin reduced hemoglobin A1c levels to 7% or below. Rates of adverse events and serious adverse events were similar between the two study groups, and there were no differences between them in weight change, blood pressure, pulse rate, or laboratory findings, the investigators said (JAMA.2017;318[1]:33-44).
In the second trial, rates of hypoglycemia again were significantly lower with insulin degludec than with insulin glargine (186 vs. 265 episodes per 100 PYE). In addition, the proportion of patients who experienced a severe hypoglycemic episode was 1.6% vs. 2.4%, respectively, said Carol Wysham, MD, of Rockwood Clinic University of Washington, Spokane, and her associates.
As in the first trial, both types of insulin reduced HbA1c levels to the same degree, and rates of adverse events and of serious adverse events were comparable, Dr. Wysham and her associates said (JAMA. 2017;318[1]:45-56).
The dropout rates were similar and higher than expected in both trials, at approximately 20%. Dr. Lane and her associates noted that this may have resulted from “the demanding nature” of the studies, including their 64-week duration; demand for close monitoring of blood glucose; and the requirement of using vials and syringes to maintain treatment blinding, instead of more convenient injector devices.
Both trials were funded by Novo Nordisk, maker of insulin degludec. Dr. Lane reported ties to Novo Nordisk, Insulet Corporation, and Eli Lilly, and her associates reported ties to numerous industry sources. Dr. Wysham reported ties to Novo Nordisk, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, and Sanofi, and her associates reported ties to numerous industry sources.
Insulin degludec decreased both the rate and the severity of hypoglycemic episodes in adults with type 1 and type 2 diabetes, compared with insulin glargine, in two head-to-head trials sponsored by the maker of insulin degludec and reported online July 3 in JAMA.
The two trials had identical randomized double-blind crossover designs. The first involved 501 adults with type 1 diabetes treated at 84 sites in the United States and 6 sites in Poland, and the second involved 721 adults with type 2 diabetes treated at 152 sites in the United States. All the study participants were at risk for hypoglycemia by virtue of experiencing one or more severe hypoglycemic episodes within the preceding year, having moderate chronic renal failure, having a 15-year or longer duration of diabetes, being unaware of their hypoglycemic symptoms, or experiencing severe hypoglycemia within the 3 months preceding baseline.
In both trials, patients were randomized to one type of once-daily insulin for 32 weeks (a 16-week titration period followed by a 16-week maintenance period) and then crossed over to the other type of insulin for 32 weeks. The primary end point in both studies was the rate of overall severe hypoglycemia during the maintenance period. This was defined as either an episode requiring the assistance of another person to administer aid or an episode in which blood glucose measured less than 56 mg/dL.
In the first trial, rates of hypoglycemia were significantly lower with insulin degludec (2,201 episodes per 100 person-years of exposure) than with insulin glargine (2,463 episodes per 100 PYE), demonstrating not just the noninferiority but also the superiority of insulin degludec. In addition, a significantly lower proportion of patients had hypoglycemia with insulin degludec (32.8%) than with insulin glargine (43.1%), said Wendy Lane, MD, of Mountain Diabetes and Endocrine Center, Asheville NC, and her associates.
Regarding the severity of hypoglycemia, the rate of severe episodes was significantly lower with insulin degludec (69 episodes per 100 PYE) than with insulin glargine (92 episodes per 100 PYE). In addition, the proportion of patients who experienced a severe hypoglycemic episode was significantly lower with insulin degludec (10.3%) than with insulin glargine (17.1%).
Both types of insulin reduced hemoglobin A1c levels to 7% or below. Rates of adverse events and serious adverse events were similar between the two study groups, and there were no differences between them in weight change, blood pressure, pulse rate, or laboratory findings, the investigators said (JAMA.2017;318[1]:33-44).
In the second trial, rates of hypoglycemia again were significantly lower with insulin degludec than with insulin glargine (186 vs. 265 episodes per 100 PYE). In addition, the proportion of patients who experienced a severe hypoglycemic episode was 1.6% vs. 2.4%, respectively, said Carol Wysham, MD, of Rockwood Clinic University of Washington, Spokane, and her associates.
As in the first trial, both types of insulin reduced HbA1c levels to the same degree, and rates of adverse events and of serious adverse events were comparable, Dr. Wysham and her associates said (JAMA. 2017;318[1]:45-56).
The dropout rates were similar and higher than expected in both trials, at approximately 20%. Dr. Lane and her associates noted that this may have resulted from “the demanding nature” of the studies, including their 64-week duration; demand for close monitoring of blood glucose; and the requirement of using vials and syringes to maintain treatment blinding, instead of more convenient injector devices.
Both trials were funded by Novo Nordisk, maker of insulin degludec. Dr. Lane reported ties to Novo Nordisk, Insulet Corporation, and Eli Lilly, and her associates reported ties to numerous industry sources. Dr. Wysham reported ties to Novo Nordisk, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, and Sanofi, and her associates reported ties to numerous industry sources.
FROM JAMA
Key clinical point: Insulin degludec decreases the rate and severity of hypoglycemic episodes in adults with type 1 and type 2 diabetes, compared with insulin glargine.
Major finding: Rates of hypoglycemia were significantly lower with insulin degludec (2,201 episodes per 100 person-years of exposure) than with insulin glargine (2,463 episodes per 100 PYE) in type 1 diabetes and in type 2 diabetes (185.6 vs. 265.4 episodes per 100 PYE).
Data source: Two separate multicenter, randomized, double-blind crossover trials involving 501 adults with type 1 and 721 with type 2 diabetes.
Disclosures: Both trials were funded by Novo Nordisk, maker of insulin degludec. Dr. Lane reported ties to Novo Nordisk, Insulet Corporation, and Eli Lilly, and her associates reported ties to numerous industry sources. Dr. Wysham reported ties to Novo Nordisk, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, and Sanofi, and her associates reported ties to numerous industry sources.
Best Practices: Understanding the Role of Delayed Cord Clamping During Cord Blood Collection
Click Here to Read Supplement.
Topics Include:
- The Role of Cord Blood Stem Cells
- Impact of DCC in Cord Blood Collection
- Combining DCC and Cord Blood Collection
- When to Clamp the Umbilical Cord
Faculty/Faculty Disclosure:
Fung Lam, MD
Chair-GYN Quality, Director of Medical Education (OB-GYN), California-Pacific Medical Center
Senior Partner, Golden Gate Obstetrics & Gynecology
Clinical Professor-Department of Obstetrics, Gynecology and Reproductive Sciences
University of California, San Francisco
San Francisco, California
Click Here to Read Supplement.
Topics Include:
- The Role of Cord Blood Stem Cells
- Impact of DCC in Cord Blood Collection
- Combining DCC and Cord Blood Collection
- When to Clamp the Umbilical Cord
Faculty/Faculty Disclosure:
Fung Lam, MD
Chair-GYN Quality, Director of Medical Education (OB-GYN), California-Pacific Medical Center
Senior Partner, Golden Gate Obstetrics & Gynecology
Clinical Professor-Department of Obstetrics, Gynecology and Reproductive Sciences
University of California, San Francisco
San Francisco, California
Click Here to Read Supplement.
Topics Include:
- The Role of Cord Blood Stem Cells
- Impact of DCC in Cord Blood Collection
- Combining DCC and Cord Blood Collection
- When to Clamp the Umbilical Cord
Faculty/Faculty Disclosure:
Fung Lam, MD
Chair-GYN Quality, Director of Medical Education (OB-GYN), California-Pacific Medical Center
Senior Partner, Golden Gate Obstetrics & Gynecology
Clinical Professor-Department of Obstetrics, Gynecology and Reproductive Sciences
University of California, San Francisco
San Francisco, California
Treatment of low bone density or osteoporosis to prevent fractures in men and women
Osteoporosis is defined by a clinically diagnosed fragility fracture or a bone mineral density (BMD) of at least 2.5 SD below the mean for young female adults, usually measured by dual-energy x-ray absorptiometry. Risk factors include age, female sex, post-menopause, hypogonadism or premature ovarian failure, history of cigarette smoking or alcohol consumption (3 or more drinks daily), rheumatoid arthritis, or medications including glucocorticoids, anticoagulants, anticonvulsants, and aromatase inhibitors.
This guideline update focuses on treatment with bisphosphonates (alendronate, risedronate, ibandronate, zoledronic acid) and denosumab. Denosumab, a human monoclonal antibody against RANK-ligand, approved by the Food and Drug Administration for treatment of osteoporosis, has been added to the list of allowed medications since publication of the 2008 guideline. Several therapies have been excluded from the update, including calcitonin, which is no longer widely used for osteoporosis treatment, and etidronate and pamidronate, neither of which are FDA-approved for the prevention of fractures or treatment of osteoporosis. It should be noted that the evidence continues to be insufficient regarding the effectiveness of therapies to prevent fractures or to treat osteoporosis in men.
Bisphosphonates are associated with mild upper GI symptoms, atypical subtrochanteric fracture, and rare osteonecrosis of the jaw. There is no significant association between bisphosphonate use and total cardiovascular adverse events. Evidence is insufficient to associate bisphosphonates with increased cancer risk. Zoledronic acid is associated with atrial fibrillation, arthritis/arthralgias, headaches, hypocalcemia, influenza-like symptoms, and an increased incidence of uveitis/episcleritis. Denosumab is associated with mild upper GI symptoms, rash/eczema, and cellulitis.
While in the past additional medications were recommended for osteoporosis, the current guidelines recommend against using raloxifene, ibandronate, teriparatide, menopausal estrogen therapy, or menopausal estrogen plus progesterone therapy for first-line pharmacologic treatment.
The overall effect of calcium, vitamin D, or exercise alone on fracture risk is uncertain. Calcium and vitamin D may be added to treatment regimens, as a majority of trials with bisphosphonate therapy added this supplementation. Dosages should be considered because excessive dosing has been associated with hypercalcemia. Although previous data suggested an association between calcium supplementation and increased risk for myocardial infarction, moderate-quality evidence shows no association, though there is a risk of kidney stones.
Recommendation: Women who have osteoporosis and receive pharmacologic treatment should be treated for 5 years (weak recommendation; low-quality evidence). The evidence to determine the length of treatment is not strong, so recommendation is an extrapolation from existing evidence. High-risk patients may benefit from more than 5 years of treatment. Data suggests that patients treated with alendronate who had preexisting fractures or those with a BMD of –2.5 or less after 5 years of initial therapy may benefit from continued treatment, because these patients experienced a decreased incidence of new clinical vertebral fractures.
Recommendation: Pharmacologic treatment with bisphosphonates to reduce the risk for vertebral fracture can be offered to men who have clinically recognized osteoporosis (weak recommendation, low-quality evidence). No evidence suggests that outcomes associated with pharmacologic treatment would differ between men and women if based on similar BMDs.
Recommendation: Bone density monitoring is not recommended during the 5-year pharmacologic treatment period for osteoporosis in women (weak recommendation, low-quality evidence). Data showed that most women with normal dual-energy x-ray absorptiometry scores did not progress to osteoporosis within 15 years. Data also does not support monitoring BMD during the initial 5 years of treatment in patients taking pharmacologic agents to treat osteoporosis. Several studies showed that women treated with antiresorptive treatment benefited from reduced fractures with treatment even if BMD did not increase.
Only 10% of women with normal or mild osteopenia develop osteoporosis within 15 years; 10% of women with moderate osteopenia develop osteoporosis within 5 years, and 10% of women with advanced osteopenia develop osteoporosis within 1 year.
Recommendation: The decision about whether to treat osteopenic women older then 65 years of age who are at a high risk for fracture should be based on a discussion of with the patient about their risk of fracture and the risk and benefits of treatment. Clinicians can use their judgment regarding the qualitative risk for fracture, or a validated tool such as the FRAX tool that gives 10-year risk of any major osteoporotic fracture and of hip fracture. The FRAX site recommends consideration of treatment for individuals with low bone mass (T-score between –1.0 and –2.5 at the femoral neck or spine) and a 10-year probability of a hip fracture of at least 3% or a 10-year probability of a major osteoporosis-related fracture greater than 20%.
Bottom line:
Clinicians should offer pharmacologic treatment with alendronate, risedronate, zoledronic acid, or denosumab to reduce the risk of hip and vertebral fractures in women who have known osteoporosis diagnosed as a T score less than –2.5 or those with a fragility fracture. Pharmacologic therapy should be used for 5 years; however, high risk patients may benefit from longer treatment. There is no benefit to bone density monitoring during the 5-year pharmacologic treatment period. In addition, bisphosphonates should be considered in men who have clinically recognized osteoporosis.
Reference:
Qaseem, A, Forciea, MA, McLean RM, Denberg TD. Treatment of Low Bone Density or Osteoporosis to Prevent Fractures in Men and Women: A Clinical Practice Guideline Update From the American College of Physicians. Ann Int Med. 2017;166(11):818-39.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Meizinger is a second year resident in the Family Medicine Residency Program at Abington Jefferson Health.
Osteoporosis is defined by a clinically diagnosed fragility fracture or a bone mineral density (BMD) of at least 2.5 SD below the mean for young female adults, usually measured by dual-energy x-ray absorptiometry. Risk factors include age, female sex, post-menopause, hypogonadism or premature ovarian failure, history of cigarette smoking or alcohol consumption (3 or more drinks daily), rheumatoid arthritis, or medications including glucocorticoids, anticoagulants, anticonvulsants, and aromatase inhibitors.
This guideline update focuses on treatment with bisphosphonates (alendronate, risedronate, ibandronate, zoledronic acid) and denosumab. Denosumab, a human monoclonal antibody against RANK-ligand, approved by the Food and Drug Administration for treatment of osteoporosis, has been added to the list of allowed medications since publication of the 2008 guideline. Several therapies have been excluded from the update, including calcitonin, which is no longer widely used for osteoporosis treatment, and etidronate and pamidronate, neither of which are FDA-approved for the prevention of fractures or treatment of osteoporosis. It should be noted that the evidence continues to be insufficient regarding the effectiveness of therapies to prevent fractures or to treat osteoporosis in men.
Bisphosphonates are associated with mild upper GI symptoms, atypical subtrochanteric fracture, and rare osteonecrosis of the jaw. There is no significant association between bisphosphonate use and total cardiovascular adverse events. Evidence is insufficient to associate bisphosphonates with increased cancer risk. Zoledronic acid is associated with atrial fibrillation, arthritis/arthralgias, headaches, hypocalcemia, influenza-like symptoms, and an increased incidence of uveitis/episcleritis. Denosumab is associated with mild upper GI symptoms, rash/eczema, and cellulitis.
While in the past additional medications were recommended for osteoporosis, the current guidelines recommend against using raloxifene, ibandronate, teriparatide, menopausal estrogen therapy, or menopausal estrogen plus progesterone therapy for first-line pharmacologic treatment.
The overall effect of calcium, vitamin D, or exercise alone on fracture risk is uncertain. Calcium and vitamin D may be added to treatment regimens, as a majority of trials with bisphosphonate therapy added this supplementation. Dosages should be considered because excessive dosing has been associated with hypercalcemia. Although previous data suggested an association between calcium supplementation and increased risk for myocardial infarction, moderate-quality evidence shows no association, though there is a risk of kidney stones.
Recommendation: Women who have osteoporosis and receive pharmacologic treatment should be treated for 5 years (weak recommendation; low-quality evidence). The evidence to determine the length of treatment is not strong, so recommendation is an extrapolation from existing evidence. High-risk patients may benefit from more than 5 years of treatment. Data suggests that patients treated with alendronate who had preexisting fractures or those with a BMD of –2.5 or less after 5 years of initial therapy may benefit from continued treatment, because these patients experienced a decreased incidence of new clinical vertebral fractures.
Recommendation: Pharmacologic treatment with bisphosphonates to reduce the risk for vertebral fracture can be offered to men who have clinically recognized osteoporosis (weak recommendation, low-quality evidence). No evidence suggests that outcomes associated with pharmacologic treatment would differ between men and women if based on similar BMDs.
Recommendation: Bone density monitoring is not recommended during the 5-year pharmacologic treatment period for osteoporosis in women (weak recommendation, low-quality evidence). Data showed that most women with normal dual-energy x-ray absorptiometry scores did not progress to osteoporosis within 15 years. Data also does not support monitoring BMD during the initial 5 years of treatment in patients taking pharmacologic agents to treat osteoporosis. Several studies showed that women treated with antiresorptive treatment benefited from reduced fractures with treatment even if BMD did not increase.
Only 10% of women with normal or mild osteopenia develop osteoporosis within 15 years; 10% of women with moderate osteopenia develop osteoporosis within 5 years, and 10% of women with advanced osteopenia develop osteoporosis within 1 year.
Recommendation: The decision about whether to treat osteopenic women older then 65 years of age who are at a high risk for fracture should be based on a discussion of with the patient about their risk of fracture and the risk and benefits of treatment. Clinicians can use their judgment regarding the qualitative risk for fracture, or a validated tool such as the FRAX tool that gives 10-year risk of any major osteoporotic fracture and of hip fracture. The FRAX site recommends consideration of treatment for individuals with low bone mass (T-score between –1.0 and –2.5 at the femoral neck or spine) and a 10-year probability of a hip fracture of at least 3% or a 10-year probability of a major osteoporosis-related fracture greater than 20%.
Bottom line:
Clinicians should offer pharmacologic treatment with alendronate, risedronate, zoledronic acid, or denosumab to reduce the risk of hip and vertebral fractures in women who have known osteoporosis diagnosed as a T score less than –2.5 or those with a fragility fracture. Pharmacologic therapy should be used for 5 years; however, high risk patients may benefit from longer treatment. There is no benefit to bone density monitoring during the 5-year pharmacologic treatment period. In addition, bisphosphonates should be considered in men who have clinically recognized osteoporosis.
Reference:
Qaseem, A, Forciea, MA, McLean RM, Denberg TD. Treatment of Low Bone Density or Osteoporosis to Prevent Fractures in Men and Women: A Clinical Practice Guideline Update From the American College of Physicians. Ann Int Med. 2017;166(11):818-39.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Meizinger is a second year resident in the Family Medicine Residency Program at Abington Jefferson Health.
Osteoporosis is defined by a clinically diagnosed fragility fracture or a bone mineral density (BMD) of at least 2.5 SD below the mean for young female adults, usually measured by dual-energy x-ray absorptiometry. Risk factors include age, female sex, post-menopause, hypogonadism or premature ovarian failure, history of cigarette smoking or alcohol consumption (3 or more drinks daily), rheumatoid arthritis, or medications including glucocorticoids, anticoagulants, anticonvulsants, and aromatase inhibitors.
This guideline update focuses on treatment with bisphosphonates (alendronate, risedronate, ibandronate, zoledronic acid) and denosumab. Denosumab, a human monoclonal antibody against RANK-ligand, approved by the Food and Drug Administration for treatment of osteoporosis, has been added to the list of allowed medications since publication of the 2008 guideline. Several therapies have been excluded from the update, including calcitonin, which is no longer widely used for osteoporosis treatment, and etidronate and pamidronate, neither of which are FDA-approved for the prevention of fractures or treatment of osteoporosis. It should be noted that the evidence continues to be insufficient regarding the effectiveness of therapies to prevent fractures or to treat osteoporosis in men.
Bisphosphonates are associated with mild upper GI symptoms, atypical subtrochanteric fracture, and rare osteonecrosis of the jaw. There is no significant association between bisphosphonate use and total cardiovascular adverse events. Evidence is insufficient to associate bisphosphonates with increased cancer risk. Zoledronic acid is associated with atrial fibrillation, arthritis/arthralgias, headaches, hypocalcemia, influenza-like symptoms, and an increased incidence of uveitis/episcleritis. Denosumab is associated with mild upper GI symptoms, rash/eczema, and cellulitis.
While in the past additional medications were recommended for osteoporosis, the current guidelines recommend against using raloxifene, ibandronate, teriparatide, menopausal estrogen therapy, or menopausal estrogen plus progesterone therapy for first-line pharmacologic treatment.
The overall effect of calcium, vitamin D, or exercise alone on fracture risk is uncertain. Calcium and vitamin D may be added to treatment regimens, as a majority of trials with bisphosphonate therapy added this supplementation. Dosages should be considered because excessive dosing has been associated with hypercalcemia. Although previous data suggested an association between calcium supplementation and increased risk for myocardial infarction, moderate-quality evidence shows no association, though there is a risk of kidney stones.
Recommendation: Women who have osteoporosis and receive pharmacologic treatment should be treated for 5 years (weak recommendation; low-quality evidence). The evidence to determine the length of treatment is not strong, so recommendation is an extrapolation from existing evidence. High-risk patients may benefit from more than 5 years of treatment. Data suggests that patients treated with alendronate who had preexisting fractures or those with a BMD of –2.5 or less after 5 years of initial therapy may benefit from continued treatment, because these patients experienced a decreased incidence of new clinical vertebral fractures.
Recommendation: Pharmacologic treatment with bisphosphonates to reduce the risk for vertebral fracture can be offered to men who have clinically recognized osteoporosis (weak recommendation, low-quality evidence). No evidence suggests that outcomes associated with pharmacologic treatment would differ between men and women if based on similar BMDs.
Recommendation: Bone density monitoring is not recommended during the 5-year pharmacologic treatment period for osteoporosis in women (weak recommendation, low-quality evidence). Data showed that most women with normal dual-energy x-ray absorptiometry scores did not progress to osteoporosis within 15 years. Data also does not support monitoring BMD during the initial 5 years of treatment in patients taking pharmacologic agents to treat osteoporosis. Several studies showed that women treated with antiresorptive treatment benefited from reduced fractures with treatment even if BMD did not increase.
Only 10% of women with normal or mild osteopenia develop osteoporosis within 15 years; 10% of women with moderate osteopenia develop osteoporosis within 5 years, and 10% of women with advanced osteopenia develop osteoporosis within 1 year.
Recommendation: The decision about whether to treat osteopenic women older then 65 years of age who are at a high risk for fracture should be based on a discussion of with the patient about their risk of fracture and the risk and benefits of treatment. Clinicians can use their judgment regarding the qualitative risk for fracture, or a validated tool such as the FRAX tool that gives 10-year risk of any major osteoporotic fracture and of hip fracture. The FRAX site recommends consideration of treatment for individuals with low bone mass (T-score between –1.0 and –2.5 at the femoral neck or spine) and a 10-year probability of a hip fracture of at least 3% or a 10-year probability of a major osteoporosis-related fracture greater than 20%.
Bottom line:
Clinicians should offer pharmacologic treatment with alendronate, risedronate, zoledronic acid, or denosumab to reduce the risk of hip and vertebral fractures in women who have known osteoporosis diagnosed as a T score less than –2.5 or those with a fragility fracture. Pharmacologic therapy should be used for 5 years; however, high risk patients may benefit from longer treatment. There is no benefit to bone density monitoring during the 5-year pharmacologic treatment period. In addition, bisphosphonates should be considered in men who have clinically recognized osteoporosis.
Reference:
Qaseem, A, Forciea, MA, McLean RM, Denberg TD. Treatment of Low Bone Density or Osteoporosis to Prevent Fractures in Men and Women: A Clinical Practice Guideline Update From the American College of Physicians. Ann Int Med. 2017;166(11):818-39.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Meizinger is a second year resident in the Family Medicine Residency Program at Abington Jefferson Health.
Quality of life preserved with ribociclib + letrozole for advanced breast cancer
CHICAGO – Patients with advanced breast cancer whose aromatase inhibitor therapy was supplemented with a cycline-dependent kinase inhibitor had better progression-free survival with no drop in quality of life. Health-related quality of life for patients on the combination therapy was equivalent to that of patients on monotherapy in most aspects, but patients receiving both therapies had a sustained and clinically meaningful decrease in pain.
Also, the time to definitive deterioration by 10% or more of the global health status/quality of life scale score was similar between treatment arms (hazard ratio [HR] 0.944; 95% confidence interval [CI] 0.720-1.237).
The MONALEESA-2 trial had previously shown that the CDK 4/6 inhibitor ribociclib, when added to the aromatase inhibitor letrozole, significantly improved progression-free survival for postmenopausal patients with hormone receptor-positive, HER2 negative advanced breast cancer, when compared to letrozole in combination with placebo.
Sunil Verma, MD, reported on health-related quality of life and symptoms in the two arms of MONALEESA-2, reporting change from baseline, time to a definitive 10% deterioration, and the mean scores for on-treatment time compared to end of treatment on the global health status/quality of life subscale of the European Organization for Research and Treatment of Cancer 30-item core quality of life questionnaire (EORTC QLQ-30).
“During treatment, overall health-related quality of life was maintained from baseline and was similar in both arms,” said Dr. Verma, the study’s first author. Changes during treatment were not statistically significant, and did not reach the predetermined threshold for a clinically meaningful difference. The effect of such key symptoms as fatigue, nausea, and vomiting on quality of life was similar regardless of whether patients received ribociclib or placebo, he said; though symptom scores were slightly higher for patients in the active arm of the study, the results were not clinically significant.
Reporting validated, cancer-specific patient-reported outcomes from the trial, Dr. Verma, professor and head of the department of oncology at the University of Calgary, (Alta.), sought to “highlight the patient experience with a focus on health-related quality of life and symptoms,” he said during his presentation at the annual meeting of the American Society of Clinical Oncology.
“A clinically meaningful – more than 5-point – improvement from baseline in pain score was maintained up to and including cycle 15 in the ribociclib plus letrozole arm,” said Dr. Verma. The placebo arm had a mild, clinically insignificant improvement at most assessment points. For both treatment arms, pain scores increased a bit above baseline levels at the time of disease progression or end of therapy, he said.
Patients completed the EORTC 30-item core quality of life questionnaire at their screening visit, and then every 8 weeks for the first 18 months. Then, they completed the questionnaires every 12 weeks until they experienced disease progression, died, were lost to follow-up or withdrew from the study, or stopped treatment.
Statistical analysis of the questionnaire results took into account the patients’ baseline scores, treatments received, and how they were stratified. Investigators assessed both statistical significance and the clinical meaningfulness of changes, defined as a change of 5-10 points.
In MONALEESA-2, 334 patients each were allocated to the ribociclib + letrozole arm and the placebo + letrozole arm. Patients in both arms, said Dr. Verma, were very compliant with questionnaire completion. Over 90% of patients who were eligible completed questionnaire through cycle 19 of ribociclib or placebo. He explained that the overall numbers completing the questionnaire declined with time, as more patients had disease progression.
It’s important to include these measures, he said, because patients and their families care about “the quality of the time gained,” so patient-reported outcomes should be a part of risk-benefit assessments for new cancer therapies. “While delaying disease progression may help to maintain patient quality of life, the addition of novel treatments to existing therapies can add toxicities, which may diminish quality of life,” said Dr. Verma.
Dr. Verma reported financial relationships with multiple pharmaceutical companies, including Novartis, which funded the study.
[email protected]
On Twitter @karioakes
CHICAGO – Patients with advanced breast cancer whose aromatase inhibitor therapy was supplemented with a cycline-dependent kinase inhibitor had better progression-free survival with no drop in quality of life. Health-related quality of life for patients on the combination therapy was equivalent to that of patients on monotherapy in most aspects, but patients receiving both therapies had a sustained and clinically meaningful decrease in pain.
Also, the time to definitive deterioration by 10% or more of the global health status/quality of life scale score was similar between treatment arms (hazard ratio [HR] 0.944; 95% confidence interval [CI] 0.720-1.237).
The MONALEESA-2 trial had previously shown that the CDK 4/6 inhibitor ribociclib, when added to the aromatase inhibitor letrozole, significantly improved progression-free survival for postmenopausal patients with hormone receptor-positive, HER2 negative advanced breast cancer, when compared to letrozole in combination with placebo.
Sunil Verma, MD, reported on health-related quality of life and symptoms in the two arms of MONALEESA-2, reporting change from baseline, time to a definitive 10% deterioration, and the mean scores for on-treatment time compared to end of treatment on the global health status/quality of life subscale of the European Organization for Research and Treatment of Cancer 30-item core quality of life questionnaire (EORTC QLQ-30).
“During treatment, overall health-related quality of life was maintained from baseline and was similar in both arms,” said Dr. Verma, the study’s first author. Changes during treatment were not statistically significant, and did not reach the predetermined threshold for a clinically meaningful difference. The effect of such key symptoms as fatigue, nausea, and vomiting on quality of life was similar regardless of whether patients received ribociclib or placebo, he said; though symptom scores were slightly higher for patients in the active arm of the study, the results were not clinically significant.
Reporting validated, cancer-specific patient-reported outcomes from the trial, Dr. Verma, professor and head of the department of oncology at the University of Calgary, (Alta.), sought to “highlight the patient experience with a focus on health-related quality of life and symptoms,” he said during his presentation at the annual meeting of the American Society of Clinical Oncology.
“A clinically meaningful – more than 5-point – improvement from baseline in pain score was maintained up to and including cycle 15 in the ribociclib plus letrozole arm,” said Dr. Verma. The placebo arm had a mild, clinically insignificant improvement at most assessment points. For both treatment arms, pain scores increased a bit above baseline levels at the time of disease progression or end of therapy, he said.
Patients completed the EORTC 30-item core quality of life questionnaire at their screening visit, and then every 8 weeks for the first 18 months. Then, they completed the questionnaires every 12 weeks until they experienced disease progression, died, were lost to follow-up or withdrew from the study, or stopped treatment.
Statistical analysis of the questionnaire results took into account the patients’ baseline scores, treatments received, and how they were stratified. Investigators assessed both statistical significance and the clinical meaningfulness of changes, defined as a change of 5-10 points.
In MONALEESA-2, 334 patients each were allocated to the ribociclib + letrozole arm and the placebo + letrozole arm. Patients in both arms, said Dr. Verma, were very compliant with questionnaire completion. Over 90% of patients who were eligible completed questionnaire through cycle 19 of ribociclib or placebo. He explained that the overall numbers completing the questionnaire declined with time, as more patients had disease progression.
It’s important to include these measures, he said, because patients and their families care about “the quality of the time gained,” so patient-reported outcomes should be a part of risk-benefit assessments for new cancer therapies. “While delaying disease progression may help to maintain patient quality of life, the addition of novel treatments to existing therapies can add toxicities, which may diminish quality of life,” said Dr. Verma.
Dr. Verma reported financial relationships with multiple pharmaceutical companies, including Novartis, which funded the study.
[email protected]
On Twitter @karioakes
CHICAGO – Patients with advanced breast cancer whose aromatase inhibitor therapy was supplemented with a cycline-dependent kinase inhibitor had better progression-free survival with no drop in quality of life. Health-related quality of life for patients on the combination therapy was equivalent to that of patients on monotherapy in most aspects, but patients receiving both therapies had a sustained and clinically meaningful decrease in pain.
Also, the time to definitive deterioration by 10% or more of the global health status/quality of life scale score was similar between treatment arms (hazard ratio [HR] 0.944; 95% confidence interval [CI] 0.720-1.237).
The MONALEESA-2 trial had previously shown that the CDK 4/6 inhibitor ribociclib, when added to the aromatase inhibitor letrozole, significantly improved progression-free survival for postmenopausal patients with hormone receptor-positive, HER2 negative advanced breast cancer, when compared to letrozole in combination with placebo.
Sunil Verma, MD, reported on health-related quality of life and symptoms in the two arms of MONALEESA-2, reporting change from baseline, time to a definitive 10% deterioration, and the mean scores for on-treatment time compared to end of treatment on the global health status/quality of life subscale of the European Organization for Research and Treatment of Cancer 30-item core quality of life questionnaire (EORTC QLQ-30).
“During treatment, overall health-related quality of life was maintained from baseline and was similar in both arms,” said Dr. Verma, the study’s first author. Changes during treatment were not statistically significant, and did not reach the predetermined threshold for a clinically meaningful difference. The effect of such key symptoms as fatigue, nausea, and vomiting on quality of life was similar regardless of whether patients received ribociclib or placebo, he said; though symptom scores were slightly higher for patients in the active arm of the study, the results were not clinically significant.
Reporting validated, cancer-specific patient-reported outcomes from the trial, Dr. Verma, professor and head of the department of oncology at the University of Calgary, (Alta.), sought to “highlight the patient experience with a focus on health-related quality of life and symptoms,” he said during his presentation at the annual meeting of the American Society of Clinical Oncology.
“A clinically meaningful – more than 5-point – improvement from baseline in pain score was maintained up to and including cycle 15 in the ribociclib plus letrozole arm,” said Dr. Verma. The placebo arm had a mild, clinically insignificant improvement at most assessment points. For both treatment arms, pain scores increased a bit above baseline levels at the time of disease progression or end of therapy, he said.
Patients completed the EORTC 30-item core quality of life questionnaire at their screening visit, and then every 8 weeks for the first 18 months. Then, they completed the questionnaires every 12 weeks until they experienced disease progression, died, were lost to follow-up or withdrew from the study, or stopped treatment.
Statistical analysis of the questionnaire results took into account the patients’ baseline scores, treatments received, and how they were stratified. Investigators assessed both statistical significance and the clinical meaningfulness of changes, defined as a change of 5-10 points.
In MONALEESA-2, 334 patients each were allocated to the ribociclib + letrozole arm and the placebo + letrozole arm. Patients in both arms, said Dr. Verma, were very compliant with questionnaire completion. Over 90% of patients who were eligible completed questionnaire through cycle 19 of ribociclib or placebo. He explained that the overall numbers completing the questionnaire declined with time, as more patients had disease progression.
It’s important to include these measures, he said, because patients and their families care about “the quality of the time gained,” so patient-reported outcomes should be a part of risk-benefit assessments for new cancer therapies. “While delaying disease progression may help to maintain patient quality of life, the addition of novel treatments to existing therapies can add toxicities, which may diminish quality of life,” said Dr. Verma.
Dr. Verma reported financial relationships with multiple pharmaceutical companies, including Novartis, which funded the study.
[email protected]
On Twitter @karioakes
AT ASCO 2017
Key clinical point:
Major finding: Quality of life was sustained and pain scores decreased when ribociclib was added to letrozole for patients with advanced breast cancer.
Data source: Double-blind, placebo-controlled phase III trial of letrozole plus ribociclib compared to letrozole plus placebo in 668 patients with advanced hormone receptor-positive, HER-2 negative breast cancer.
Disclosures: Dr. Verma reported financial relationships with multiple companies, including Novartis, which markets ribociclib.
Elevated levels of AST, ALT, and CPK • no family history of liver disease • Dx?
THE CASE
A 26-year-old healthy male veteran with bipolar disorder and post-traumatic stress disorder was referred for a gastroenterology consultation after a routine laboratory evaluation revealed elevated levels of aspartate aminotransferase (AST), 1040 IU/L (normal range, 10-40 IU/L), and alanine aminotransferase (ALT), 334 IU/L (normal range, 7-56 IU/L). He had been taking divalproex and ziprasidone for the previous 2 years, during which time liver test results had been normal.
The patient reported no symptoms in the course of a detailed history. He had no family history of liver disease, drank alcohol infrequently, and didn’t use tobacco. He hadn’t received any blood transfusions and didn’t have tattoos.
The patient indicated that he had recently returned from military deployment and that a week before his laboratory tests, he’d resumed weight training. To boost his workout, he’d begun taking a nutritional supplement supplied by a friend. Further questioning revealed that the supplement was MuscleMeds’ Code Red, which contains 1,3-dimethylamylamine (DMAA). He denied using any other dietary supplements.
The physical examination was unremarkable and additional lab work was unrevealing. Lab results included normal levels of ceruloplasmin, alpha-1 antitrypsin, ferritin, iron, and transferrin. Viral hepatitis serologies revealed immunity to the hepatitis A and B virus. The patient tested negative for Epstein-Barr virus, cytomegalovirus, herpes simplex virus, human immunodeficiency virus, antinuclear antibody, anti-smooth muscle antibody, and antimitochondrial antibody. A toxicology screen was remarkable for cannabinoids. The remainder of the basic metabolic panel and complete blood count were within normal limits.
THE DIAGNOSIS
The patient’s AST and ALT levels prompted measurement of creatine phosphokinase (CPK), which was elevated at 34,270 IU/L (normal range, 22-198 IU/L). We diagnosed rhabdomyolysis in this patient, which can be associated with elevated levels of AST and ALT. When we contacted the patient about the diagnosis, he reported no muscle aches or pains, or other symptoms.
We instructed the patient to increase his fluid intake and refrain from further use of Code Red. Repeat liver tests one month after the initial consultation revealed significant improvement in AST (29 IU/L) and ALT (68 IU/L), as well as a decline in CPK to 743 IU/L.
DISCUSSION
Much debate has surrounded the safety and use of DMAA, also known as methylhexamine or Geranamine, in dietary supplements such as Code Red. Eli Lilly and Company developed and patented DMAA in the 1940s, then trademarked it under the name Forthane as an inhaled nasal decongestant in 1971.1-3 United States Food and Drug Administration (FDA) approval for Forthane was withdrawn in 1983 at Lilly’s request.4 DMAA was reintroduced as a dietary supplement more than a decade ago after the FDA, in 2004, banned supplements containing ephedrine alkaloids, which have effects similar to DMAA.5
DMAA has been used to increase muscle mass, promote weight loss, and improve physical performance; it’s also been used as a recreational drug.6-8 Several case reports have described poor outcomes in patients who consumed DMAA products. In 2012, the deaths of 2 military personnel who used DMAA prompted the FDA to warn manufacturers of DMAA-containing supplements to stop production, but such supplements remain easily available in the United States.6
DMAA’s validity as a dietary supplement is controversial. The claim that DMAA is naturally present in geraniums hasn’t been verified, leading some to question whether an inaccurate description of DMAA as a natural substance was employed to justify its use as a nutritional supplement.9 No published evidence exists to establish DMAA as a dietary ingredient.10,11
A long list of potential adverse effects
DMAA is an indirect sympathomimetic with vasoconstricting and cardiovascular effects.12 Animal studies have shown effects similar to ephedrine and amphetamines.12-15 Marsh and colleagues reported that a single oral dose of 3 mg/kg in a human (210 mg/70 kg) moderately increases heart rate and blood pressure and can lead to confusion and concentration problems.16
Oral intake of DMAA affects the lungs at doses above 4 to 15 mg, the heart after 50 to 75 mg, and blood pressure after 100 mg.17 Because of the drug’s long half-life—24 hours based on urinary excretion rates—Venhuis and Kaste reported that there is a risk from repeated doses within 24 to 36 hours that can lead to steadily stronger pharmacologic effects.17
The use of DMAA has been cited in 5 cases of hemorrhagic stroke, a case of acute heart failure, and the deaths of 2 military personnel who experienced asystole during aerobic exercise.7,8,18-20 These individuals ranged in age from 22 to 41 years.
Initial symptoms included severe headaches, palpitations, dizziness, twitching of extremities, nausea, vomiting, confusion, agitation, and chest pain. The 2 military personnel suffered leg cramps and dyspnea followed by loss of consciousness. Several individuals were hypertensive on presentation to the emergency department with blood pressures as high as 240/120 mm Hg.
THE TAKEAWAY
Our patient presented with transaminitis and was found to have rhabdomyolysis after using DMAA. A few case reports have associated rhabdomyolysis with elevated liver function tests.21,22 We suspect that DMAA use, which has been linked to adverse effects such as hypertension, tachycardia, and muscle aches, may also cause leakage of muscle enzymes and the development of rhabdomyolysis.
Although a single instance can’t prove causation, this case may illustrate additional adverse effects of DMAA beyond the already long list of risks, including hypertension, seizures, cerebral hemorrhage, arrhythmias, myocardial infarction, cardiomyopathy, and death.7,8,18-20,23 It’s important for physicians to recognize that their patients may be using dietary supplements to increase strength, energy, or weight loss and to be aware of the potential adverse effects.
1. Shonle HA, Rohrmann E, inventors; Eli Lilly and Company, assignee. Aminoalkanes. Patent US2350318A. May 30, 1944.
2. Shonle HA, Rohrmann E, inventors; Eli Lilly and Company, assignee. Carbonates of 1-R-1 aminoethanes. Patent US2386273. October 9, 1945.
3. Eli Lilly and Company. Forthane. Registration 0925396, February 1, 1971. United States Patent and Trademark Office.
4. Federal Register. Vol. 48, No. 218/Notices. November 9, 1983.
5. Shipley A. Chemist’s new product contains hidden substance. Washington Post. May 8, 2006:Sports. Available at: http://www.washingtonpost.com/wp-dyn/content/article/2006/05/07/AR2006050700913.html. Accessed June 5, 2017.
6. Gregory PJ. Availability of DMAA supplements despite US Food and Drug Administration action. JAMA Intern Med. 2013;173:164-165.
7. Gee P, Jackson S, Easton J. Another bitter pill: a case of toxicity from DMAA party pills. N Z Med J. 2010;123:124-127.
8. Gee P, Tallon C, Long N, et al. Use of recreational drug 1,3 Dimethylamylamine (DMAA) [corrected] associated with cerebral hemorrhage. Ann Emerg Med. 2012;60:431-434.
9. Ping Z, Jun Q, Qing L. A study on the chemical constituents of geranium oil. Journal of Guizhou Institute of Technology. 1996;25:82-85.
10. Lisi A, Hasick N, Kazlauskas R, et al. Studies of methylhexaneamine in supplements and geranium oil. Drug Test Anal. 2011;3:873-876.
11. Elsohly MA, Gul W, Elsohly KM, et al. Pelargonium oil and methyl hexaneamine (MHA): analytical approaches supporting the absence of MHA in authenticated Pelargonium graveolens plant material and oil. J Anal Toxicol. 2012;36:457-471.
12. Charlier R. [Pharmacology of 2-amino-4-methylhexane]. Arch Int Pharmacodyn Ther. 1950;83:573-584.
13. Ahlquist R. A contribution to the pharmacology of the aliphatic amines. J Pharmacol Exp Ther. 1944;81:235-239.
14. Swanson EE, Chen KK. Comparison of pressor action of aliphatic amines. J Pharmacol Exp Ther. 1946;88:10-13.
15. Swanson EE, Chen KK. Comparison of pressor action of alicyclic derivatives of aliphatic amines. J Pharmacol Exp Ther. 1948;93:423-429.
16. Marsh DF, Howard A, Herring DA. The comparative pharmacology of the isomeric nitrogen methyl substituted heptylamines. J Pharmacol Exp Ther. 1951;103:325-329.
17. Venhuis BJ, Kaste D. Scientific opinion on the regulatory status of 1,3-dimethylamylamine (DMAA). European Journal of Food Research and Review. 2012;2:93-100.
18. Eliason MJ, Eichner A, Cancio A, et al. Case reports: Death of active duty soldiers following ingestion of dietary supplements containing 1,3-dimethylamylamine (DMAA). Mil Med. 2012;177:1455-1459.
19. Young C, Oladipo O, Frasier S, et al. Hemorrhagic stroke in young healthy male following use of sports supplement Jack3d. Mil Med. 2012;177:1450-1454.
20. Salinger L, Daniels B, Sangalli B, et al. Recreational use of a bodybuilding supplement resulting in severe cardiotoxicity. Clin Toxicol (Philadelphia). 2011;49:573-574.
21. Lee GY, Lee H, Kim YJ. Rhabdomyolysis recognized after elevation of liver enzymes following prolonged urologic surgery with lateral decubitus position: a case report. Korean J Anesthesiol. 2011;61:341-343.
22. Karcher C, Dieterich HJ, Schroeder TH. Rhabdomyolysis in an obese patient after total knee arthroplasty. Br J Anaesth. 2006;97:822-824.
23. Karnatovskaia LV, Leoni JC, Freeman ML. Cardiac arrest in a 21-year-old man after ingestion of 1,3-DMAA-containing workout supplement. Clin J Sport Med. 2015;25:e23-e25.
THE CASE
A 26-year-old healthy male veteran with bipolar disorder and post-traumatic stress disorder was referred for a gastroenterology consultation after a routine laboratory evaluation revealed elevated levels of aspartate aminotransferase (AST), 1040 IU/L (normal range, 10-40 IU/L), and alanine aminotransferase (ALT), 334 IU/L (normal range, 7-56 IU/L). He had been taking divalproex and ziprasidone for the previous 2 years, during which time liver test results had been normal.
The patient reported no symptoms in the course of a detailed history. He had no family history of liver disease, drank alcohol infrequently, and didn’t use tobacco. He hadn’t received any blood transfusions and didn’t have tattoos.
The patient indicated that he had recently returned from military deployment and that a week before his laboratory tests, he’d resumed weight training. To boost his workout, he’d begun taking a nutritional supplement supplied by a friend. Further questioning revealed that the supplement was MuscleMeds’ Code Red, which contains 1,3-dimethylamylamine (DMAA). He denied using any other dietary supplements.
The physical examination was unremarkable and additional lab work was unrevealing. Lab results included normal levels of ceruloplasmin, alpha-1 antitrypsin, ferritin, iron, and transferrin. Viral hepatitis serologies revealed immunity to the hepatitis A and B virus. The patient tested negative for Epstein-Barr virus, cytomegalovirus, herpes simplex virus, human immunodeficiency virus, antinuclear antibody, anti-smooth muscle antibody, and antimitochondrial antibody. A toxicology screen was remarkable for cannabinoids. The remainder of the basic metabolic panel and complete blood count were within normal limits.
THE DIAGNOSIS
The patient’s AST and ALT levels prompted measurement of creatine phosphokinase (CPK), which was elevated at 34,270 IU/L (normal range, 22-198 IU/L). We diagnosed rhabdomyolysis in this patient, which can be associated with elevated levels of AST and ALT. When we contacted the patient about the diagnosis, he reported no muscle aches or pains, or other symptoms.
We instructed the patient to increase his fluid intake and refrain from further use of Code Red. Repeat liver tests one month after the initial consultation revealed significant improvement in AST (29 IU/L) and ALT (68 IU/L), as well as a decline in CPK to 743 IU/L.
DISCUSSION
Much debate has surrounded the safety and use of DMAA, also known as methylhexamine or Geranamine, in dietary supplements such as Code Red. Eli Lilly and Company developed and patented DMAA in the 1940s, then trademarked it under the name Forthane as an inhaled nasal decongestant in 1971.1-3 United States Food and Drug Administration (FDA) approval for Forthane was withdrawn in 1983 at Lilly’s request.4 DMAA was reintroduced as a dietary supplement more than a decade ago after the FDA, in 2004, banned supplements containing ephedrine alkaloids, which have effects similar to DMAA.5
DMAA has been used to increase muscle mass, promote weight loss, and improve physical performance; it’s also been used as a recreational drug.6-8 Several case reports have described poor outcomes in patients who consumed DMAA products. In 2012, the deaths of 2 military personnel who used DMAA prompted the FDA to warn manufacturers of DMAA-containing supplements to stop production, but such supplements remain easily available in the United States.6
DMAA’s validity as a dietary supplement is controversial. The claim that DMAA is naturally present in geraniums hasn’t been verified, leading some to question whether an inaccurate description of DMAA as a natural substance was employed to justify its use as a nutritional supplement.9 No published evidence exists to establish DMAA as a dietary ingredient.10,11
A long list of potential adverse effects
DMAA is an indirect sympathomimetic with vasoconstricting and cardiovascular effects.12 Animal studies have shown effects similar to ephedrine and amphetamines.12-15 Marsh and colleagues reported that a single oral dose of 3 mg/kg in a human (210 mg/70 kg) moderately increases heart rate and blood pressure and can lead to confusion and concentration problems.16
Oral intake of DMAA affects the lungs at doses above 4 to 15 mg, the heart after 50 to 75 mg, and blood pressure after 100 mg.17 Because of the drug’s long half-life—24 hours based on urinary excretion rates—Venhuis and Kaste reported that there is a risk from repeated doses within 24 to 36 hours that can lead to steadily stronger pharmacologic effects.17
The use of DMAA has been cited in 5 cases of hemorrhagic stroke, a case of acute heart failure, and the deaths of 2 military personnel who experienced asystole during aerobic exercise.7,8,18-20 These individuals ranged in age from 22 to 41 years.
Initial symptoms included severe headaches, palpitations, dizziness, twitching of extremities, nausea, vomiting, confusion, agitation, and chest pain. The 2 military personnel suffered leg cramps and dyspnea followed by loss of consciousness. Several individuals were hypertensive on presentation to the emergency department with blood pressures as high as 240/120 mm Hg.
THE TAKEAWAY
Our patient presented with transaminitis and was found to have rhabdomyolysis after using DMAA. A few case reports have associated rhabdomyolysis with elevated liver function tests.21,22 We suspect that DMAA use, which has been linked to adverse effects such as hypertension, tachycardia, and muscle aches, may also cause leakage of muscle enzymes and the development of rhabdomyolysis.
Although a single instance can’t prove causation, this case may illustrate additional adverse effects of DMAA beyond the already long list of risks, including hypertension, seizures, cerebral hemorrhage, arrhythmias, myocardial infarction, cardiomyopathy, and death.7,8,18-20,23 It’s important for physicians to recognize that their patients may be using dietary supplements to increase strength, energy, or weight loss and to be aware of the potential adverse effects.
THE CASE
A 26-year-old healthy male veteran with bipolar disorder and post-traumatic stress disorder was referred for a gastroenterology consultation after a routine laboratory evaluation revealed elevated levels of aspartate aminotransferase (AST), 1040 IU/L (normal range, 10-40 IU/L), and alanine aminotransferase (ALT), 334 IU/L (normal range, 7-56 IU/L). He had been taking divalproex and ziprasidone for the previous 2 years, during which time liver test results had been normal.
The patient reported no symptoms in the course of a detailed history. He had no family history of liver disease, drank alcohol infrequently, and didn’t use tobacco. He hadn’t received any blood transfusions and didn’t have tattoos.
The patient indicated that he had recently returned from military deployment and that a week before his laboratory tests, he’d resumed weight training. To boost his workout, he’d begun taking a nutritional supplement supplied by a friend. Further questioning revealed that the supplement was MuscleMeds’ Code Red, which contains 1,3-dimethylamylamine (DMAA). He denied using any other dietary supplements.
The physical examination was unremarkable and additional lab work was unrevealing. Lab results included normal levels of ceruloplasmin, alpha-1 antitrypsin, ferritin, iron, and transferrin. Viral hepatitis serologies revealed immunity to the hepatitis A and B virus. The patient tested negative for Epstein-Barr virus, cytomegalovirus, herpes simplex virus, human immunodeficiency virus, antinuclear antibody, anti-smooth muscle antibody, and antimitochondrial antibody. A toxicology screen was remarkable for cannabinoids. The remainder of the basic metabolic panel and complete blood count were within normal limits.
THE DIAGNOSIS
The patient’s AST and ALT levels prompted measurement of creatine phosphokinase (CPK), which was elevated at 34,270 IU/L (normal range, 22-198 IU/L). We diagnosed rhabdomyolysis in this patient, which can be associated with elevated levels of AST and ALT. When we contacted the patient about the diagnosis, he reported no muscle aches or pains, or other symptoms.
We instructed the patient to increase his fluid intake and refrain from further use of Code Red. Repeat liver tests one month after the initial consultation revealed significant improvement in AST (29 IU/L) and ALT (68 IU/L), as well as a decline in CPK to 743 IU/L.
DISCUSSION
Much debate has surrounded the safety and use of DMAA, also known as methylhexamine or Geranamine, in dietary supplements such as Code Red. Eli Lilly and Company developed and patented DMAA in the 1940s, then trademarked it under the name Forthane as an inhaled nasal decongestant in 1971.1-3 United States Food and Drug Administration (FDA) approval for Forthane was withdrawn in 1983 at Lilly’s request.4 DMAA was reintroduced as a dietary supplement more than a decade ago after the FDA, in 2004, banned supplements containing ephedrine alkaloids, which have effects similar to DMAA.5
DMAA has been used to increase muscle mass, promote weight loss, and improve physical performance; it’s also been used as a recreational drug.6-8 Several case reports have described poor outcomes in patients who consumed DMAA products. In 2012, the deaths of 2 military personnel who used DMAA prompted the FDA to warn manufacturers of DMAA-containing supplements to stop production, but such supplements remain easily available in the United States.6
DMAA’s validity as a dietary supplement is controversial. The claim that DMAA is naturally present in geraniums hasn’t been verified, leading some to question whether an inaccurate description of DMAA as a natural substance was employed to justify its use as a nutritional supplement.9 No published evidence exists to establish DMAA as a dietary ingredient.10,11
A long list of potential adverse effects
DMAA is an indirect sympathomimetic with vasoconstricting and cardiovascular effects.12 Animal studies have shown effects similar to ephedrine and amphetamines.12-15 Marsh and colleagues reported that a single oral dose of 3 mg/kg in a human (210 mg/70 kg) moderately increases heart rate and blood pressure and can lead to confusion and concentration problems.16
Oral intake of DMAA affects the lungs at doses above 4 to 15 mg, the heart after 50 to 75 mg, and blood pressure after 100 mg.17 Because of the drug’s long half-life—24 hours based on urinary excretion rates—Venhuis and Kaste reported that there is a risk from repeated doses within 24 to 36 hours that can lead to steadily stronger pharmacologic effects.17
The use of DMAA has been cited in 5 cases of hemorrhagic stroke, a case of acute heart failure, and the deaths of 2 military personnel who experienced asystole during aerobic exercise.7,8,18-20 These individuals ranged in age from 22 to 41 years.
Initial symptoms included severe headaches, palpitations, dizziness, twitching of extremities, nausea, vomiting, confusion, agitation, and chest pain. The 2 military personnel suffered leg cramps and dyspnea followed by loss of consciousness. Several individuals were hypertensive on presentation to the emergency department with blood pressures as high as 240/120 mm Hg.
THE TAKEAWAY
Our patient presented with transaminitis and was found to have rhabdomyolysis after using DMAA. A few case reports have associated rhabdomyolysis with elevated liver function tests.21,22 We suspect that DMAA use, which has been linked to adverse effects such as hypertension, tachycardia, and muscle aches, may also cause leakage of muscle enzymes and the development of rhabdomyolysis.
Although a single instance can’t prove causation, this case may illustrate additional adverse effects of DMAA beyond the already long list of risks, including hypertension, seizures, cerebral hemorrhage, arrhythmias, myocardial infarction, cardiomyopathy, and death.7,8,18-20,23 It’s important for physicians to recognize that their patients may be using dietary supplements to increase strength, energy, or weight loss and to be aware of the potential adverse effects.
1. Shonle HA, Rohrmann E, inventors; Eli Lilly and Company, assignee. Aminoalkanes. Patent US2350318A. May 30, 1944.
2. Shonle HA, Rohrmann E, inventors; Eli Lilly and Company, assignee. Carbonates of 1-R-1 aminoethanes. Patent US2386273. October 9, 1945.
3. Eli Lilly and Company. Forthane. Registration 0925396, February 1, 1971. United States Patent and Trademark Office.
4. Federal Register. Vol. 48, No. 218/Notices. November 9, 1983.
5. Shipley A. Chemist’s new product contains hidden substance. Washington Post. May 8, 2006:Sports. Available at: http://www.washingtonpost.com/wp-dyn/content/article/2006/05/07/AR2006050700913.html. Accessed June 5, 2017.
6. Gregory PJ. Availability of DMAA supplements despite US Food and Drug Administration action. JAMA Intern Med. 2013;173:164-165.
7. Gee P, Jackson S, Easton J. Another bitter pill: a case of toxicity from DMAA party pills. N Z Med J. 2010;123:124-127.
8. Gee P, Tallon C, Long N, et al. Use of recreational drug 1,3 Dimethylamylamine (DMAA) [corrected] associated with cerebral hemorrhage. Ann Emerg Med. 2012;60:431-434.
9. Ping Z, Jun Q, Qing L. A study on the chemical constituents of geranium oil. Journal of Guizhou Institute of Technology. 1996;25:82-85.
10. Lisi A, Hasick N, Kazlauskas R, et al. Studies of methylhexaneamine in supplements and geranium oil. Drug Test Anal. 2011;3:873-876.
11. Elsohly MA, Gul W, Elsohly KM, et al. Pelargonium oil and methyl hexaneamine (MHA): analytical approaches supporting the absence of MHA in authenticated Pelargonium graveolens plant material and oil. J Anal Toxicol. 2012;36:457-471.
12. Charlier R. [Pharmacology of 2-amino-4-methylhexane]. Arch Int Pharmacodyn Ther. 1950;83:573-584.
13. Ahlquist R. A contribution to the pharmacology of the aliphatic amines. J Pharmacol Exp Ther. 1944;81:235-239.
14. Swanson EE, Chen KK. Comparison of pressor action of aliphatic amines. J Pharmacol Exp Ther. 1946;88:10-13.
15. Swanson EE, Chen KK. Comparison of pressor action of alicyclic derivatives of aliphatic amines. J Pharmacol Exp Ther. 1948;93:423-429.
16. Marsh DF, Howard A, Herring DA. The comparative pharmacology of the isomeric nitrogen methyl substituted heptylamines. J Pharmacol Exp Ther. 1951;103:325-329.
17. Venhuis BJ, Kaste D. Scientific opinion on the regulatory status of 1,3-dimethylamylamine (DMAA). European Journal of Food Research and Review. 2012;2:93-100.
18. Eliason MJ, Eichner A, Cancio A, et al. Case reports: Death of active duty soldiers following ingestion of dietary supplements containing 1,3-dimethylamylamine (DMAA). Mil Med. 2012;177:1455-1459.
19. Young C, Oladipo O, Frasier S, et al. Hemorrhagic stroke in young healthy male following use of sports supplement Jack3d. Mil Med. 2012;177:1450-1454.
20. Salinger L, Daniels B, Sangalli B, et al. Recreational use of a bodybuilding supplement resulting in severe cardiotoxicity. Clin Toxicol (Philadelphia). 2011;49:573-574.
21. Lee GY, Lee H, Kim YJ. Rhabdomyolysis recognized after elevation of liver enzymes following prolonged urologic surgery with lateral decubitus position: a case report. Korean J Anesthesiol. 2011;61:341-343.
22. Karcher C, Dieterich HJ, Schroeder TH. Rhabdomyolysis in an obese patient after total knee arthroplasty. Br J Anaesth. 2006;97:822-824.
23. Karnatovskaia LV, Leoni JC, Freeman ML. Cardiac arrest in a 21-year-old man after ingestion of 1,3-DMAA-containing workout supplement. Clin J Sport Med. 2015;25:e23-e25.
1. Shonle HA, Rohrmann E, inventors; Eli Lilly and Company, assignee. Aminoalkanes. Patent US2350318A. May 30, 1944.
2. Shonle HA, Rohrmann E, inventors; Eli Lilly and Company, assignee. Carbonates of 1-R-1 aminoethanes. Patent US2386273. October 9, 1945.
3. Eli Lilly and Company. Forthane. Registration 0925396, February 1, 1971. United States Patent and Trademark Office.
4. Federal Register. Vol. 48, No. 218/Notices. November 9, 1983.
5. Shipley A. Chemist’s new product contains hidden substance. Washington Post. May 8, 2006:Sports. Available at: http://www.washingtonpost.com/wp-dyn/content/article/2006/05/07/AR2006050700913.html. Accessed June 5, 2017.
6. Gregory PJ. Availability of DMAA supplements despite US Food and Drug Administration action. JAMA Intern Med. 2013;173:164-165.
7. Gee P, Jackson S, Easton J. Another bitter pill: a case of toxicity from DMAA party pills. N Z Med J. 2010;123:124-127.
8. Gee P, Tallon C, Long N, et al. Use of recreational drug 1,3 Dimethylamylamine (DMAA) [corrected] associated with cerebral hemorrhage. Ann Emerg Med. 2012;60:431-434.
9. Ping Z, Jun Q, Qing L. A study on the chemical constituents of geranium oil. Journal of Guizhou Institute of Technology. 1996;25:82-85.
10. Lisi A, Hasick N, Kazlauskas R, et al. Studies of methylhexaneamine in supplements and geranium oil. Drug Test Anal. 2011;3:873-876.
11. Elsohly MA, Gul W, Elsohly KM, et al. Pelargonium oil and methyl hexaneamine (MHA): analytical approaches supporting the absence of MHA in authenticated Pelargonium graveolens plant material and oil. J Anal Toxicol. 2012;36:457-471.
12. Charlier R. [Pharmacology of 2-amino-4-methylhexane]. Arch Int Pharmacodyn Ther. 1950;83:573-584.
13. Ahlquist R. A contribution to the pharmacology of the aliphatic amines. J Pharmacol Exp Ther. 1944;81:235-239.
14. Swanson EE, Chen KK. Comparison of pressor action of aliphatic amines. J Pharmacol Exp Ther. 1946;88:10-13.
15. Swanson EE, Chen KK. Comparison of pressor action of alicyclic derivatives of aliphatic amines. J Pharmacol Exp Ther. 1948;93:423-429.
16. Marsh DF, Howard A, Herring DA. The comparative pharmacology of the isomeric nitrogen methyl substituted heptylamines. J Pharmacol Exp Ther. 1951;103:325-329.
17. Venhuis BJ, Kaste D. Scientific opinion on the regulatory status of 1,3-dimethylamylamine (DMAA). European Journal of Food Research and Review. 2012;2:93-100.
18. Eliason MJ, Eichner A, Cancio A, et al. Case reports: Death of active duty soldiers following ingestion of dietary supplements containing 1,3-dimethylamylamine (DMAA). Mil Med. 2012;177:1455-1459.
19. Young C, Oladipo O, Frasier S, et al. Hemorrhagic stroke in young healthy male following use of sports supplement Jack3d. Mil Med. 2012;177:1450-1454.
20. Salinger L, Daniels B, Sangalli B, et al. Recreational use of a bodybuilding supplement resulting in severe cardiotoxicity. Clin Toxicol (Philadelphia). 2011;49:573-574.
21. Lee GY, Lee H, Kim YJ. Rhabdomyolysis recognized after elevation of liver enzymes following prolonged urologic surgery with lateral decubitus position: a case report. Korean J Anesthesiol. 2011;61:341-343.
22. Karcher C, Dieterich HJ, Schroeder TH. Rhabdomyolysis in an obese patient after total knee arthroplasty. Br J Anaesth. 2006;97:822-824.
23. Karnatovskaia LV, Leoni JC, Freeman ML. Cardiac arrest in a 21-year-old man after ingestion of 1,3-DMAA-containing workout supplement. Clin J Sport Med. 2015;25:e23-e25.
Here’s what’s trending at SHM: July 2017
Updated Clinical Documentation & Coding resources now available
SHM’s Clinical Documentation & Coding for Hospitalists, formerly CODE-H, has been updated for 2017.
“[It’s] an exciting program that offers valuable insight into the coding and billing challenges of hospitalist services. Whether you are a new or seasoned physician, SHM’s Clinical Documentation & Coding for Hospitalists provides you with a solid foundation for documentation, identifies common problems, and offers strategies for success.” – Carol Pohlig, BSN, RN, CPC, ASC Senior Coding and Compliance Specialist
For more information, visit hospitalmedicine.org/codeh.
Registration now open for NP/PA Bootcamp
Whether you’re new to hospital medicine or need a refresher on the latest topics, this course from the AAPA and SHM is perfect for you and offers up to 34.75 AAPA Category 1 CME credits.
At the Adult Hospital Medicine Bootcamp, you will cover commonly encountered diagnoses and diseases of adult hospitalized patients while networking with other hospital-based practitioners. Plus, attend optional pre-courses on reimbursement, hands-on ultrasound or hospital medicine basics.
Join us at the ninth annual Adult Hospital Medicine Boot Camp, September 27 – October 1, 2017, in San Diego. To register and learn more visit: aapa.org/bootcamp.
Learn how your HMG stacks up with the State of Hospital Medicine report
Did you know that hospitalist compensation typically consists of 80% base pay and 20% supplemental income based on production and performance? SHM’s State of Hospital Medicine Report continues to be your best source of information about how hospital medicine groups (HMGs) operate.
Don’t miss the new additions to the report for the 2016 version, including:
• Percentage of the hospital’s total patient volume the HMG was responsible for caring for.
• Presence of medical hospitalists within the HMG focusing their practice in a specific medical subspecialty.
• Value of CME allowances for hospitalists.
• Utilization of prolonged service codes by hospitalists.
• Charge capture methodologies being used by HMGs.
• For academic HMGs, the dollar amount of financial support provided for non-clinical work.
Order your print or digital copy at hospitalmedicine.org/sohm.
Enhance your leadership skills at SHM’s Leadership Academy
SHM’s Leadership Academy is the only leadership program designed specifically for hospitalists. The 2017 meeting will be held October 23 – 26 at the JW Marriott Camelback Inn in Scottsdale, Ariz.
Course highlight: Leadership mastering teamwork
Developed in response to high demand from previous Leadership Academy attendees, this course focuses on strengthening teams and institutions. Participants learn how to critically assess program growth opportunities and develop operational plans; utilize the principles of SWARM intelligence; lead, manage, and motivate teams in complex hospital environments; and develop effective communication strategies.
Upon completion of this course, participants will be able to apply communication strategies that allow others to fully experience their message, lead teams in complex environments to achieve the best results, invest in themselves as leaders to optimize their professional growth and career path, and critically assess program growth opportunities and implement the necessary infrastructure for success.
To view the course schedule, faculty and more visit shmleadershipacademy.org/masteringteamwork.
Improve glycemic control efforts in your hospital with online resources & mentorship
SHM offers a variety of resources to improve glycemic control in your hospital. Glycemic Control Electronic Quality Improvement Programs (eQUIPS) are designed to enhance the efficiency and reliability of your quality improvement efforts to close the gap between best practices and methods for caring for the inpatient with hyperglycemia.
Benefits of SHM’s eQUIPS include:
• Data and performance tracking tools.
• Step-by-step instructions for improving glycemic control, preventing hypoglycemia, and optimizing care of inpatients with hyperglycemia and diabetes.
• An online community and library of tools and documents, including sample order sets and protocols, awareness campaigns, patient educational materials, and supplemental articles.
• Toolkit of clinical tools and interventions, research materials, literature reviews, case studies, teaching slide sets, and more.
SHM’s Glycemic Control Mentored Implementation program sites receive 1 year of individualized mentoring including:
• On-site mentoring and training for the entire care team to help members interpret needs and resource assessments, map system processes, and develop site-specific action and intervention plans.
• Monthly coaching calls with the mentor to develop, modify, and implement interventions, establish evaluation processes, and monitor performance over time.
• SHM-facilitated calls with live webinars with other sites in the collaborative to share success stories and experiences.
• Access to the online community to share ideas, documents, and other resources.
• Data collection and analysis tools to generate on-demand reports and benchmark against other program participants.
Learn more about all of SHM’s Glycemic Control offerings by watching the recorded webinar from June 28, 2017, at hospitalmedicine.org/gc.
Earn CME with SHM’s Learning Portal
SHM’s Learning Portal is the online learning destination for hospitalists, featuring all of SHM’s eLearning initiatives in one place. Members can access over 85 CME credits for free in the Learning Portal.
Featured topics currently include perioperative medicine, anticoagulation, quality improvement, cardiac arrhythmia, and antimicrobial stewardship.
Try out the most popular modules:
• The Role of the Medical Consultant.
• Pulmonary Risk Management in the Perioperative Setting.
• Perioperative Medication Management.
• Venous Thromboembolism Prophylaxis in Surgical Patients.
• Perioperative Cardiac Risk Assessment.
Not a member? Join today or pay a small fee per module. Visit shmlearningportal.org to learn more and earn CME credits today.
Brett Radler is communications specialist at the Society of Hospital Medicine.
Updated Clinical Documentation & Coding resources now available
SHM’s Clinical Documentation & Coding for Hospitalists, formerly CODE-H, has been updated for 2017.
“[It’s] an exciting program that offers valuable insight into the coding and billing challenges of hospitalist services. Whether you are a new or seasoned physician, SHM’s Clinical Documentation & Coding for Hospitalists provides you with a solid foundation for documentation, identifies common problems, and offers strategies for success.” – Carol Pohlig, BSN, RN, CPC, ASC Senior Coding and Compliance Specialist
For more information, visit hospitalmedicine.org/codeh.
Registration now open for NP/PA Bootcamp
Whether you’re new to hospital medicine or need a refresher on the latest topics, this course from the AAPA and SHM is perfect for you and offers up to 34.75 AAPA Category 1 CME credits.
At the Adult Hospital Medicine Bootcamp, you will cover commonly encountered diagnoses and diseases of adult hospitalized patients while networking with other hospital-based practitioners. Plus, attend optional pre-courses on reimbursement, hands-on ultrasound or hospital medicine basics.
Join us at the ninth annual Adult Hospital Medicine Boot Camp, September 27 – October 1, 2017, in San Diego. To register and learn more visit: aapa.org/bootcamp.
Learn how your HMG stacks up with the State of Hospital Medicine report
Did you know that hospitalist compensation typically consists of 80% base pay and 20% supplemental income based on production and performance? SHM’s State of Hospital Medicine Report continues to be your best source of information about how hospital medicine groups (HMGs) operate.
Don’t miss the new additions to the report for the 2016 version, including:
• Percentage of the hospital’s total patient volume the HMG was responsible for caring for.
• Presence of medical hospitalists within the HMG focusing their practice in a specific medical subspecialty.
• Value of CME allowances for hospitalists.
• Utilization of prolonged service codes by hospitalists.
• Charge capture methodologies being used by HMGs.
• For academic HMGs, the dollar amount of financial support provided for non-clinical work.
Order your print or digital copy at hospitalmedicine.org/sohm.
Enhance your leadership skills at SHM’s Leadership Academy
SHM’s Leadership Academy is the only leadership program designed specifically for hospitalists. The 2017 meeting will be held October 23 – 26 at the JW Marriott Camelback Inn in Scottsdale, Ariz.
Course highlight: Leadership mastering teamwork
Developed in response to high demand from previous Leadership Academy attendees, this course focuses on strengthening teams and institutions. Participants learn how to critically assess program growth opportunities and develop operational plans; utilize the principles of SWARM intelligence; lead, manage, and motivate teams in complex hospital environments; and develop effective communication strategies.
Upon completion of this course, participants will be able to apply communication strategies that allow others to fully experience their message, lead teams in complex environments to achieve the best results, invest in themselves as leaders to optimize their professional growth and career path, and critically assess program growth opportunities and implement the necessary infrastructure for success.
To view the course schedule, faculty and more visit shmleadershipacademy.org/masteringteamwork.
Improve glycemic control efforts in your hospital with online resources & mentorship
SHM offers a variety of resources to improve glycemic control in your hospital. Glycemic Control Electronic Quality Improvement Programs (eQUIPS) are designed to enhance the efficiency and reliability of your quality improvement efforts to close the gap between best practices and methods for caring for the inpatient with hyperglycemia.
Benefits of SHM’s eQUIPS include:
• Data and performance tracking tools.
• Step-by-step instructions for improving glycemic control, preventing hypoglycemia, and optimizing care of inpatients with hyperglycemia and diabetes.
• An online community and library of tools and documents, including sample order sets and protocols, awareness campaigns, patient educational materials, and supplemental articles.
• Toolkit of clinical tools and interventions, research materials, literature reviews, case studies, teaching slide sets, and more.
SHM’s Glycemic Control Mentored Implementation program sites receive 1 year of individualized mentoring including:
• On-site mentoring and training for the entire care team to help members interpret needs and resource assessments, map system processes, and develop site-specific action and intervention plans.
• Monthly coaching calls with the mentor to develop, modify, and implement interventions, establish evaluation processes, and monitor performance over time.
• SHM-facilitated calls with live webinars with other sites in the collaborative to share success stories and experiences.
• Access to the online community to share ideas, documents, and other resources.
• Data collection and analysis tools to generate on-demand reports and benchmark against other program participants.
Learn more about all of SHM’s Glycemic Control offerings by watching the recorded webinar from June 28, 2017, at hospitalmedicine.org/gc.
Earn CME with SHM’s Learning Portal
SHM’s Learning Portal is the online learning destination for hospitalists, featuring all of SHM’s eLearning initiatives in one place. Members can access over 85 CME credits for free in the Learning Portal.
Featured topics currently include perioperative medicine, anticoagulation, quality improvement, cardiac arrhythmia, and antimicrobial stewardship.
Try out the most popular modules:
• The Role of the Medical Consultant.
• Pulmonary Risk Management in the Perioperative Setting.
• Perioperative Medication Management.
• Venous Thromboembolism Prophylaxis in Surgical Patients.
• Perioperative Cardiac Risk Assessment.
Not a member? Join today or pay a small fee per module. Visit shmlearningportal.org to learn more and earn CME credits today.
Brett Radler is communications specialist at the Society of Hospital Medicine.
Updated Clinical Documentation & Coding resources now available
SHM’s Clinical Documentation & Coding for Hospitalists, formerly CODE-H, has been updated for 2017.
“[It’s] an exciting program that offers valuable insight into the coding and billing challenges of hospitalist services. Whether you are a new or seasoned physician, SHM’s Clinical Documentation & Coding for Hospitalists provides you with a solid foundation for documentation, identifies common problems, and offers strategies for success.” – Carol Pohlig, BSN, RN, CPC, ASC Senior Coding and Compliance Specialist
For more information, visit hospitalmedicine.org/codeh.
Registration now open for NP/PA Bootcamp
Whether you’re new to hospital medicine or need a refresher on the latest topics, this course from the AAPA and SHM is perfect for you and offers up to 34.75 AAPA Category 1 CME credits.
At the Adult Hospital Medicine Bootcamp, you will cover commonly encountered diagnoses and diseases of adult hospitalized patients while networking with other hospital-based practitioners. Plus, attend optional pre-courses on reimbursement, hands-on ultrasound or hospital medicine basics.
Join us at the ninth annual Adult Hospital Medicine Boot Camp, September 27 – October 1, 2017, in San Diego. To register and learn more visit: aapa.org/bootcamp.
Learn how your HMG stacks up with the State of Hospital Medicine report
Did you know that hospitalist compensation typically consists of 80% base pay and 20% supplemental income based on production and performance? SHM’s State of Hospital Medicine Report continues to be your best source of information about how hospital medicine groups (HMGs) operate.
Don’t miss the new additions to the report for the 2016 version, including:
• Percentage of the hospital’s total patient volume the HMG was responsible for caring for.
• Presence of medical hospitalists within the HMG focusing their practice in a specific medical subspecialty.
• Value of CME allowances for hospitalists.
• Utilization of prolonged service codes by hospitalists.
• Charge capture methodologies being used by HMGs.
• For academic HMGs, the dollar amount of financial support provided for non-clinical work.
Order your print or digital copy at hospitalmedicine.org/sohm.
Enhance your leadership skills at SHM’s Leadership Academy
SHM’s Leadership Academy is the only leadership program designed specifically for hospitalists. The 2017 meeting will be held October 23 – 26 at the JW Marriott Camelback Inn in Scottsdale, Ariz.
Course highlight: Leadership mastering teamwork
Developed in response to high demand from previous Leadership Academy attendees, this course focuses on strengthening teams and institutions. Participants learn how to critically assess program growth opportunities and develop operational plans; utilize the principles of SWARM intelligence; lead, manage, and motivate teams in complex hospital environments; and develop effective communication strategies.
Upon completion of this course, participants will be able to apply communication strategies that allow others to fully experience their message, lead teams in complex environments to achieve the best results, invest in themselves as leaders to optimize their professional growth and career path, and critically assess program growth opportunities and implement the necessary infrastructure for success.
To view the course schedule, faculty and more visit shmleadershipacademy.org/masteringteamwork.
Improve glycemic control efforts in your hospital with online resources & mentorship
SHM offers a variety of resources to improve glycemic control in your hospital. Glycemic Control Electronic Quality Improvement Programs (eQUIPS) are designed to enhance the efficiency and reliability of your quality improvement efforts to close the gap between best practices and methods for caring for the inpatient with hyperglycemia.
Benefits of SHM’s eQUIPS include:
• Data and performance tracking tools.
• Step-by-step instructions for improving glycemic control, preventing hypoglycemia, and optimizing care of inpatients with hyperglycemia and diabetes.
• An online community and library of tools and documents, including sample order sets and protocols, awareness campaigns, patient educational materials, and supplemental articles.
• Toolkit of clinical tools and interventions, research materials, literature reviews, case studies, teaching slide sets, and more.
SHM’s Glycemic Control Mentored Implementation program sites receive 1 year of individualized mentoring including:
• On-site mentoring and training for the entire care team to help members interpret needs and resource assessments, map system processes, and develop site-specific action and intervention plans.
• Monthly coaching calls with the mentor to develop, modify, and implement interventions, establish evaluation processes, and monitor performance over time.
• SHM-facilitated calls with live webinars with other sites in the collaborative to share success stories and experiences.
• Access to the online community to share ideas, documents, and other resources.
• Data collection and analysis tools to generate on-demand reports and benchmark against other program participants.
Learn more about all of SHM’s Glycemic Control offerings by watching the recorded webinar from June 28, 2017, at hospitalmedicine.org/gc.
Earn CME with SHM’s Learning Portal
SHM’s Learning Portal is the online learning destination for hospitalists, featuring all of SHM’s eLearning initiatives in one place. Members can access over 85 CME credits for free in the Learning Portal.
Featured topics currently include perioperative medicine, anticoagulation, quality improvement, cardiac arrhythmia, and antimicrobial stewardship.
Try out the most popular modules:
• The Role of the Medical Consultant.
• Pulmonary Risk Management in the Perioperative Setting.
• Perioperative Medication Management.
• Venous Thromboembolism Prophylaxis in Surgical Patients.
• Perioperative Cardiac Risk Assessment.
Not a member? Join today or pay a small fee per module. Visit shmlearningportal.org to learn more and earn CME credits today.
Brett Radler is communications specialist at the Society of Hospital Medicine.
Stroke: Secondary prevention of ischemic events
Patients who suffer a stroke rarely have just one vascular risk factor. Therefore, the approach to secondary stroke prevention must be multifactorial. In fact, it has been estimated that 80% of recurrent strokes could be prevented through the application of a comprehensive, multifactorial approach that includes lifestyle modification and optimal medical management.1 Such an achievement would save millions of people from disability and functional decline, as well as millions of dollars in related medical costs.
The initial approach to patients with stroke is focused on stabilization and a rapid work-up to identify the most likely etiology. Common causes of stroke include large artery atherosclerosis, cardiac emboli, and small vessel disease; less common causes include dissection, aortic emboli, and non-atherosclerotic vascular disease. If a complete diagnostic work-up is unrevealing, the stroke is said to be cryptogenic. Determining the correct etiology of a stroke is paramount to preventing secondary stroke (FIGURE2-13).
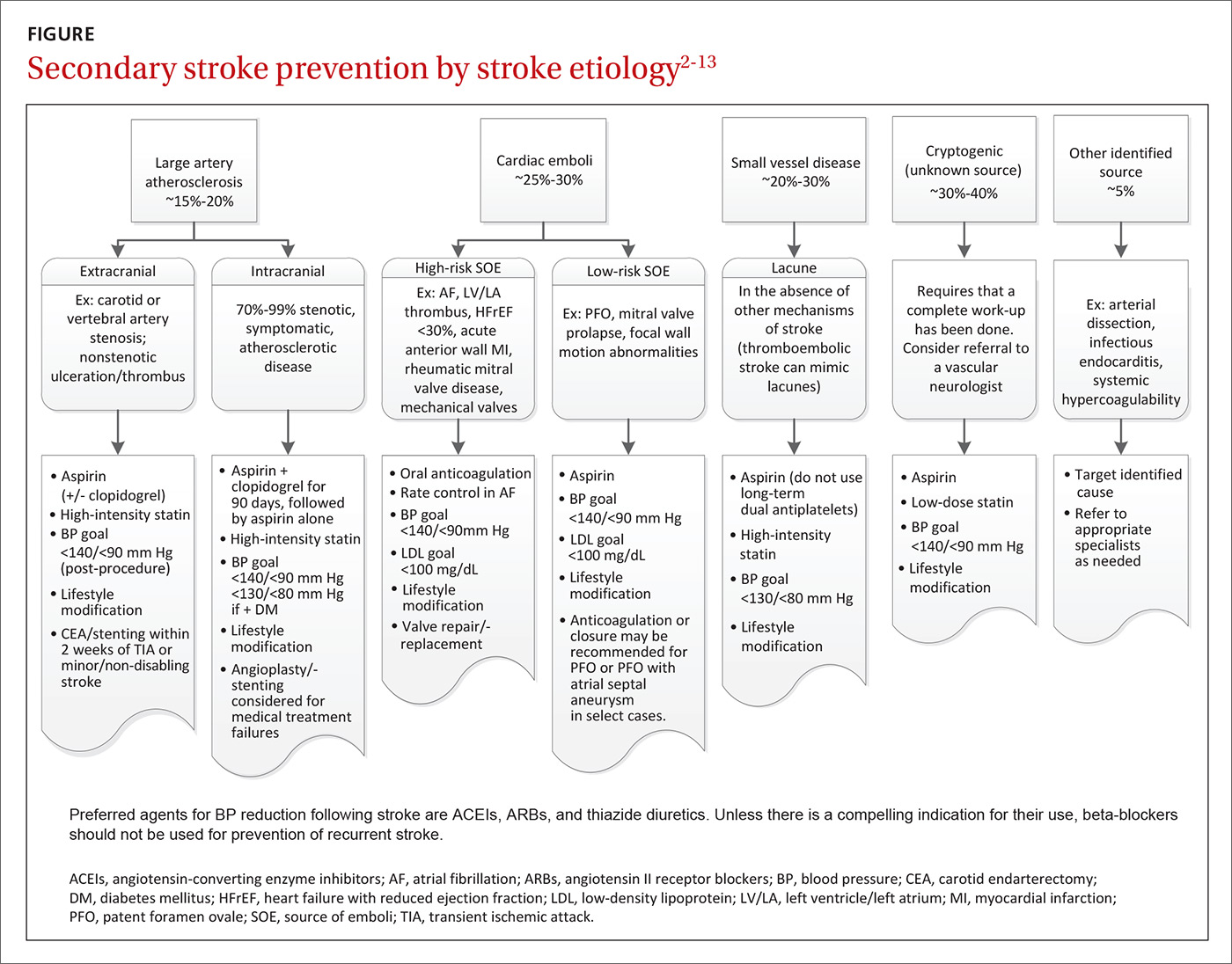
Effective secondary prevention strategies designed to prevent a stroke or transient ischemic attack (TIA) in a patient with a known history of either event include lifestyle modifications, medications, and when appropriate, mechanical interventions. As a primary care physician (PCP), you are uniquely positioned to spearhead the prevention of secondary strokes: Not only are you at the forefront of prevention and the use of techniques such as motivational interviewing, but you also have longstanding relationships with many of your patients. In fact, the success of many interventions is improved by the informed, enduring, and trusting nature of relationships between patients and their PCPs.
In the first part of this 2-part series, we focused on subacute stroke management and outlined the recommended work-up for subacute stroke/TIA (see “Stroke: A road map to subacute management,” 2017;66:366-374). In this part, we focus on secondary prevention. The more common modifiable conditions encountered in primary care are discussed here, while many of the more rare etiologies (hypercoagulable states, sickle cell disease, and vasculitis) are outside the scope of this article.
Lifestyle interventions: Target tobacco use, obesity, alcohol intake
Lifestyle modifications can have a positive impact on many of America’s most prevalent diseases, and stroke is no exception.14 Many of the disease states identified as risk factors for stroke (type 2 diabetes, hypertension, dyslipidemia) are exacerbated by tobacco use, obesity, and excessive alcohol intake.
Does your patient smoke? Up to 25% of all strokes are directly attributable to cigarette smoking.15 Smoking raises an individual’s risk for stroke in a dose-dependent fashion.15,16 One study demonstrated that, compared to never-smokers, women ages 15 to 49 years who smoked a half-pack per day had an odds ratio for ischemic stroke of 2.2; those who smoked 2 packs per day had an odds ratio of 9.1.17 After cessation, stroke risk generally returns to baseline within 5 years.16 Thus, smoking cessation is among the most significant steps a patient can take to reduce the risk of both primary and secondary stroke.
Is your patient overweight? While obesity in and of itself is a risk factor for stroke, a focus on nutrition and physical activity as mechanisms for weight loss is far superior to focusing on either element alone. Physical activity—consisting of at least 40 minutes of moderate intensity aerobic exercise 3 to 4 times per week—and a diet that emphasizes fruits and vegetables, whole grains, and healthy fats, have both independently demonstrated benefits in secondary stroke prevention and are important parts of American College of Cardiology (ACC)/American Heart Association (AHA) guidelines.2,3
The Mediterranean Diet, which emphasizes consumption of fruits and vegetables, legumes, tree nuts, olive oil, and lean protein, has long been associated with cardiovascular benefit.18 One prospective, randomized, single-blinded trial involving approximately 600 patients that looked at secondary prevention of coronary heart disease found that following the diet significantly reduced mortality compared with a usual prudent post-infarct diet (number needed to treat [NNT]=30 over 4 years).19
Is alcohol consumption an issue? Chronic heavy alcohol intake contributes to the development of hemorrhagic and ischemic stroke through multiple mechanisms, including alcohol-induced hypertension, alcoholic cardiomyopathy, and atrial fibrillation (AF). Light or moderate alcohol consumption has a paradoxical mild protective effect on ischemic stroke, thought to possibly be mediated by an increase in high-density lipoprotein (HDL) level and mild antiplatelet effect.3
AHA/American Stroke Association (ASA) guidelines indicate that no more than one standard drink per day for women and 2 drinks per day for men is reasonable.3 Counsel patients who drink in excess of this about the benefits of decreasing alcohol intake or abstaining altogether.
Choosing medications to manage BP, cholesterol, and clotting
Optimize blood pressure control. Blood pressure (BP) plays a critical role in both the management and prevention of stroke and is considered to be the most important modifiable risk factor in both primary and secondary stroke prevention.20 In the first 24 to 48 hours following a cerebral ischemic event that is not eligible for thrombolysis, permissive hypertension (treating BP only if it exceeds 220/120 mm Hg unless there is a concurrent medical illness that requires you do so) is appropriate, as hypotension or rapid fluctuations in BP can be harmful.21
This flexibility does not continue into the subacute phase of management (at a minimum, after the initial 48 hours) or into secondary prevention. Initiation and titration of oral agents to gradually achieve a BP <140/90 mm Hg or a reduction of 10/5 mm Hg for patients already within optimal range are the most widely recognized goals.3,20 Patients with stroke secondary to small vessel disease may benefit from an even lower goal of <130/<80 mm Hg.11 Encourage patients to monitor their BP at home for added accuracy and consistency.22
Pharmacologic BP management is appropriate for patients who are consistently above optimal range despite attempting recommended lifestyle modifications. The data are relatively consistent with respect to the effects of different drug classes after a stroke: beta-blockers have no effect on any outcome; thiazide diuretics significantly reduce stroke and total vascular events; angiotensin-converting enzyme (ACE) inhibitors significantly reduce myocardial infarction (MI); and the combination of an ACE inhibitor and thiazide diuretic reduces stroke, MI, and combined vascular events.4
This has led many stroke specialists to recommend the combination of an ACE inhibitor or angiotensin II receptor blocker (ARB) and a thiazide diuretic as a first-line approach to secondary stroke prevention rather than a beta-blocker (assuming there is no additional indication for a beta-blocker). Similarly, there is ample evidence to show that the magnitude of BP reduction is proportional to the reduction in recurrent vascular events.3
Make use of statin therapy—regardless of LDL. The SPARCL (Stroke Prevention by Aggressive Reduction in Cholesterol Levels) trial5 explored the potential role of statin medication for secondary stroke prevention. Researchers randomly assigned almost 5000 participants who’d had a stroke or TIA one to 6 months before study entry (but had no known history of coronary artery disease) to placebo or a high-intensity statin (80 mg/d atorvastatin). The statin group demonstrated a 4.9-year absolute risk reduction in fatal or nonfatal recurrent stroke of 1.9% (NNT=53).
Given these findings and those from other studies, the AHA and ASA recommend treating patients with stroke or TIA presumed to be of atherosclerotic origin with high-intensity statin therapy, regardless of low-density lipoprotein (LDL) level.3 Of note, statins are not indicated for the secondary prevention of hemorrhagic stroke.
Select antiplatelet therapy based on ischemic stroke subtype. Investigators are still trying to determine the optimal antiplatelet for secondary stroke prevention; it is likely that the ideal choice depends largely on the etiology of the stroke. Trials that did not select patients based on subtype of ischemic stroke have not shown a long-term benefit from dual antiplatelet therapy (clopidogrel and aspirin),23,24 and one double-blind, multicenter trial involving more than 3000 patients with recent stroke secondary to small vessel disease demonstrated harm from such therapy in terms of a significantly increased risk of bleeding and death.6
However, a 2011 study compared aggressive medical management (aspirin 325 mg/d plus clopidogrel 75 mg/d for 90 days) alone to aggressive medical management plus percutaneous transluminal angioplasty and stenting (PTAS). The study involved almost 500 patients who'd had a recent TIA or stroke attributed to intracranial atherosclerotic stenosis. The authors found that the 30-day rate of stroke or death was 14.7% in the PTAS group vs 5.8% in the medical management group.25
Similarly, a randomized double-blind, placebo-controlled trial published in 2013 involving over 5000 patients in China found that short-term use of dual antiplatelets (clopidogrel and aspirin for the first 21 days after an ischemic event, followed by aspirin monotherapy for 90 days) had an absolute risk reduction of 3.5% without increasing the risk of major bleeding in patients with high-risk TIA or minor stroke.26
All stroke patients who do not have an indication for oral anticoagulation should be placed on long-term daily aspirin (75-325 mg); research has shown that lower doses are as effective as higher doses but with a lower risk of adverse gastrointestinal effects, including bleeding.3,20 Aspirin 81 mg/d is a common effective dose.
For patients who cannot tolerate aspirin due to allergy, clopidogrel 75 mg/d is a reasonable alternative. Long-term studies of aspirin vs clopidogrel7 and clopidogrel vs extended-release dipyridamole8 showed no difference in secondary stroke prevention. The International Stroke Trial27 and Chinese Acute Stroke Trial28 both indicate that aspirin should be started as soon as possible after the onset of an acute stroke.
This special population should probably get antiplatelets, too. One recent study explored the use of an antiplatelet vs anticoagulation therapy for stroke patients with carotid artery dissection. The CADISS (Cervical Artery Dissection in Stroke Study) trial29 randomized 250 patients with extracranial carotid and vertebral artery dissection with onset of symptoms within the previous 7 days to either antiplatelet or anticoagulation therapy and found no difference in the primary outcomes of recurrent stroke or death. The study also demonstrated a low risk of recurrent stroke in this population, which was 2% at 3-month follow-up.
Most patients with cervical artery dissection, therefore, are now treated with antiplatelet therapy. That said, situations may still arise in which anticoagulation can be considered, and consultation with a neurologist for guidance on choice of therapy is recommended.
Is an anticoagulant in order? Which agent, when
The most common cause of cardioembolic stroke is AF, which accounts for at least 15% of ischemic strokes, a number that rises in those over the age of 80.20,30,31 A meta-analysis of more than 28,000 patients with non-valvular AF demonstrated that warfarin reduced the risk of stroke by 64%.32
The rate of intracerebral hemorrhages during oral anticoagulation ranges from 0.3% to 0.6% per year.33 The risk of bleeding complications can be mitigated by keeping international normalized ratios ≤3.0, maintaining good BP control, and avoiding concurrent use of antiplatelets in the absence of a clear indication for them.33
Several risk assessment scores, such as the HAS-BLED,34 can help with estimating the risk of hemorrhagic complications, although these scores have their limitations.35,36 Even in an older population (mean age 83 years) with a high risk for falls, warfarin provided a net benefit in a composite endpoint of out-of-hospital death or hospitalization for stroke, MI, or hemorrhage in a retrospective study of over 1200 Medicare beneficiaries.37
AF is not the only cause of cardioembolic stroke to consider. Additional high-risk factors warranting anticoagulation include rheumatic mitral valve disease, the presence of mechanical aortic or mitral valves, known mural thrombus, and acute anterior ST segment elevation myocardial infarctions (STEMIs) with resulting anterior apical dyskinesis/akinesis and concurrent ischemic stroke/TIA.3 (The specific management of each of these situations is beyond the scope of this paper.)
The choice of anticoagulation agent is based on multiple factors, including cost, risk of non-reversible bleeding, drug interactions, renal function, and patient preference. Approved options currently include warfarin/vitamin K antagonist therapy, apixaban, rivaroxaban, dabigatran and edoxaban.3 Choice of therapy will continue to evolve as reversal agents, such as idarucizumab, are developed. Idarucizumab, a reversal agent for dabigatran, received approval from the US Food and Drug Administration in October 2015.38
When to start anticoagulation. There are limited data regarding the optimal timing of initiation of anticoagulation following a stroke; however, a recent multicenter prospective study supported the common practice of initiating anticoagulation therapy within 4 to 14 days of the event.39 Individual patient factors must be taken into consideration, including the size of the stroke (the larger the stroke, the higher the risk for hemorrhagic transformation), BP control, any additional risk factors for bleeding, and the estimated risk of early recurrent stroke.
Bridging patients onto anticoagulation with unfractionated or low-molecular-weight heparin in the setting of acute stroke is not recommended.40 Results from randomized controlled trials involving unfractionated heparin, heparinoids, and low-molecular-weight heparin have not reported any benefit to these agents over aspirin at preventing early stroke recurrence.27,41,42
For immobile or hospitalized patients. Subcutaneous heparin for the prevention of deep vein thrombosis (DVT) during immobility and hospitalization is recommended.43 Patients who cannot tolerate anticoagulation should be maintained on low-dose antiplatelet therapy. Experts do not recommend dual treatment with aspirin and anticoagulation in most cases. However, recent coronary artery stent placement does require temporary dual treatment, with duration dependent on the type of stent placed.
A role for glycemic control? Still to be determined
The specific role of diabetic management in secondary stroke prevention remains unclear. The 2008 ACCORD trial,44 a randomized study involving over 10,000 patients with a median glycated hemoglobin level of 8.1%, investigated intensive hyperglycemic control (targeting a glycated hemoglobin level <6.0% vs <7.9%) as a means of decreasing cardiovascular risk. However, the trial ended 17 months early because of an increase in all-cause mortality in the intensive treatment arm compared with the standard management group. The same trial was also unable to demonstrate a decrease in stroke risk with a decrease in A1c.44
More recently, the IRIS (Insulin Resistance Intervention after Stroke) trial45 (2016) found a 2.8% absolute risk reduction in stroke or MI among participants who had a stroke or TIA in the previous 6 months who were treated with pioglitazone vs placebo over 4.8 years (NNT=36). Participants were required to have insulin resistance, but were excluded if they had diabetes. The authors did, however, report a notable increase in the risk of bone fractures requiring surgery or hospitalization in the pioglitazone arm (5.1% vs 3.2%; number needed to harm [NNH]=53).
The impact this single study should have on standard secondary prevention is not yet clear. The authors concluded, “It seems reasonable to consider individual treatment preference and risk of drug-related adverse events in addition to potential benefits when making patient-specific decisions regarding therapy.”45
Determining whether mechanical interventions are needed
Almost all conditions leading to stroke warrant active medical management, but a few benefit from procedural intervention, as well.
Extracranial carotid atherosclerosis. Carotid endarterectomy or carotid artery stenting is recommended as secondary prevention for patients with a history of stroke or TIA who have ipsilateral high-grade extracranial carotid stenosis of 70% to 99% and, in some cases, 50% to 69%.3,9,20 In patients with mild non-disabling stroke, the optimal timing for these procedures is within 2 weeks of the ischemic event. A delay of 6 weeks is generally preferred for moderate or larger strokes to allow for some healing of the injured brain.
The choice of procedure is based on risk profile, with the most important factor being age. For patients >70 years, endarterectomy is preferred because stenting is associated with an increased risk of stroke.3,9,10 Experts do not recommend either procedure for patients who have had a severe disabling stroke. Generally speaking, these procedures have higher rates of success when they are performed in centers that perform a higher number of these procedures.10
Vertebrobasilar atherosclerosis. Due to generally good compensatory blood flow of the contralateral vertebral artery in the setting of vertebral artery stenosis, and an unacceptably high complication rate of angioplasty and stenting in the basilar artery, medical management is typically the first-line approach. If a patient has recurrent symptoms in the setting of optimal medical management and a focal lesion that is amenable to an endovascular intervention (most commonly a vertebral artery origin high-grade stenosis), angioplasty and stenting may be considered.10
Intracranial atherosclerosis. Similarly, medical management is the preferred strategy for intracranial atherosclerosis. Angioplasty and/or stenting are reserved for complex cases or recurrence despite adherence to secondary stroke prevention measures. Ideally, these patients should be managed with long-term aspirin 81 mg/d, adjunctive clopidogrel 75 mg/d for 90 days post stroke, a high-intensity statin, BP optimization, and any relevant lifestyle interventions.13
Patent foramen ovale. Research to date has not shown that closure of a patent foramen ovale (PFO) is superior to medical therapy for secondary stroke prevention in patients <60 years with cryptogenic stroke.12,46,47 The decision to anticoagulate these patients should be based on the presence or absence of a DVT and not on a PFO alone. In patients with an identified DVT and a contraindication to oral anticoagulation, inferior vena cava filter placement should be considered. For patients with ongoing prothrombotic risk thought to increase the chances of future paradoxical embolism, closure of the PFO may be considered.
CORRESPONDENCE
Stephen A. Martin, MD, EdM, Barre Family Health Center, 151 Worcester Road, Barre, MA 01005; [email protected].
1. Hackam DG, Spence JD. Combining multiple approaches for the secondary prevention of vascular events after stroke: a quantitative modeling study. Stroke. 2007;38:1881-1885.
2. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S76-S99.
3. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236.
4. Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke. 2003;34:2741-2748.
5. Amarenco P, Bogousslavsky J, Callahan A, et al, for the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549-559.
6. Benavente OR, Hart RG, McClure LA, et al, for the SPS3 Investigators. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
7. CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329-1339.
8. Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238-1251.
9. Diethrich EB, N’diaye M, Reid DB. The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST): implications for clinical practice. In: Henry M, Diethrich EB, Polydorou A, eds. The Carotid and Supra-Aortic Trunks: Diagnosis, Angioplasty and Stenting. 2nd ed. Oxford, UK: Wiley-Blackwell; 2011.
10. Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. Circulation. 2011;124:489-532.
11. SPS3 Study Group. Blood pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382:507-515.
12. Carroll JD, Saver JL, Thaler DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092-1100.
13. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993-1003.
14. Romero JR, Morris J, Pikula A. Stroke prevention: modifying risk factors. Ther Adv Cardiovasc Dis. 2008;2:287-303.
15. Hankey GJ. Smoking and risk of stroke. J Cardiovasc Risk. 1999;6:207-211.
16. Shah RS, Cole JW. Smoking and stroke: the more you smoke the more you stroke. Expert Rev Cardiovasc Ther. 2010;8:917-932.
17. Bhat VM, Cole JW, Sorkin JD, et al. Dose-response relationship between cigarette smoking and risk of ischemic stroke in young women. Stroke. 2008;39:2439-2443.
18. Lakkur S, Judd SE. Diet and stroke: recent evidence supporting a Mediterranean-style diet and food in the primary prevention of stroke. Stroke. 2015;46:2007-2011.
19. de Lorgeril M, Salen P, Martin JL, et al. Mediterranean dietary pattern in a randomized trial: prolonged survival and possible reduced cancer rate. Arch Intern Med. 1998;158:1181-1187.
20. Davis SM, Donnan GA. Clinical practice. Secondary prevention after ischemic stroke or transient ischemic attack. N Engl J Med. 2012;366:1914-1922.
21. Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870-947.
22. Magid DJ, Green BB. Home blood pressure monitoring: take it to the bank. JAMA. 2013;310:40-41.
23. Diener H-C, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2004;364:331-337.
24. Bhatt DL, Fox KAA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706-1717.
25. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993-1003.
26. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
27. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet. 1997;349:1569-1581.
28. CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet. 1997;349:1641-1649.
29. CADISS trial investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14:361-367.
30. Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. EAFT (European Atrial Fibrillation Trial) Study Group. Lancet. 1993;342:1255-1262.
31. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983-988.
32. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Int Med. 2007;146:857-867.
33. Hart RG, Tonarelli SB, Pearce LA. Avoiding central nervous system bleeding during antithrombotic therapy. Recent data and ideas. Stroke. 2005;36:1588-1593.
34. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest. 2010;138:1093-1100.
35. Quinn GR, Singer DE, Chang Y, et al. How well do stroke risk scores predict hemorrhage in patients with atrial fibrillation? Am J Cardiol. 2016;118:697-699.
36. Gorman EW, Perkel D, Dennis D, et al. Validation of the HAS-BLED tool in atrial fibrillation patients receiving rivaroxaban. J Atr Fibrillation. 2016;9:1461.
37. Gage BF, Birman-Deych E, Kerzner R, et al. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med. 2005;118:612-617.
38. US Food and Drug Administration. FDA approves Praxbind, the first reversal agent for the anticoagulant Pradaxa. October 16, 2015. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm467300.htm. Accessed May 26, 2017.
39. Paciaroni M, Agnelli G, Falocci N, et al. Early recurrence and cerebral bleeding in patients with acute ischemic stroke and atrial fibrillation: effect of anticoagulation and its timing: the RAF Study. Stroke. 2015;46:2175-2182.
40. Sandercock PA, Counsell C, Kane EJ. Anticoagulants for acute ischaemic stroke. Cochrane Database Syst Rev. 2015;3:CD000024.
41. Bath PM, Lindenstrom E, Boysen G, et al. Tinzaparin in acute ischaemic stroke (TAIST): a randomised aspirin-controlled trial. Lancet. 2001;358:702-710.
42. Berge E, Abdelnoor M, Nakstad PH, et al. Low molecular-weight heparin versus aspirin in patients with acute ischaemic stroke and atrial fibrillation: a double-blind randomised study. HAEST Study Group. Heparin in Acute Embolic Stroke Trial. Lancet. 2000;355:1205-1210.
43. Sherman DG, Albers GW, Bladin C, et al. The efficacy and safety of enoxaparin versus unfractionated heparin for the prevention of venous thromboembolism after acute ischaemic stroke (PREVAIL Study): an open-label randomised comparison. Lancet. 2007;369:1347-1355.
44. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559.
45. Kernan WN, Viscoli CM, Furie KL, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374:1321-1331.
46. Meier B, Kalesan B, Mattle HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083-1091.
47. Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991-999.
Patients who suffer a stroke rarely have just one vascular risk factor. Therefore, the approach to secondary stroke prevention must be multifactorial. In fact, it has been estimated that 80% of recurrent strokes could be prevented through the application of a comprehensive, multifactorial approach that includes lifestyle modification and optimal medical management.1 Such an achievement would save millions of people from disability and functional decline, as well as millions of dollars in related medical costs.
The initial approach to patients with stroke is focused on stabilization and a rapid work-up to identify the most likely etiology. Common causes of stroke include large artery atherosclerosis, cardiac emboli, and small vessel disease; less common causes include dissection, aortic emboli, and non-atherosclerotic vascular disease. If a complete diagnostic work-up is unrevealing, the stroke is said to be cryptogenic. Determining the correct etiology of a stroke is paramount to preventing secondary stroke (FIGURE2-13).

Effective secondary prevention strategies designed to prevent a stroke or transient ischemic attack (TIA) in a patient with a known history of either event include lifestyle modifications, medications, and when appropriate, mechanical interventions. As a primary care physician (PCP), you are uniquely positioned to spearhead the prevention of secondary strokes: Not only are you at the forefront of prevention and the use of techniques such as motivational interviewing, but you also have longstanding relationships with many of your patients. In fact, the success of many interventions is improved by the informed, enduring, and trusting nature of relationships between patients and their PCPs.
In the first part of this 2-part series, we focused on subacute stroke management and outlined the recommended work-up for subacute stroke/TIA (see “Stroke: A road map to subacute management,” 2017;66:366-374). In this part, we focus on secondary prevention. The more common modifiable conditions encountered in primary care are discussed here, while many of the more rare etiologies (hypercoagulable states, sickle cell disease, and vasculitis) are outside the scope of this article.
Lifestyle interventions: Target tobacco use, obesity, alcohol intake
Lifestyle modifications can have a positive impact on many of America’s most prevalent diseases, and stroke is no exception.14 Many of the disease states identified as risk factors for stroke (type 2 diabetes, hypertension, dyslipidemia) are exacerbated by tobacco use, obesity, and excessive alcohol intake.
Does your patient smoke? Up to 25% of all strokes are directly attributable to cigarette smoking.15 Smoking raises an individual’s risk for stroke in a dose-dependent fashion.15,16 One study demonstrated that, compared to never-smokers, women ages 15 to 49 years who smoked a half-pack per day had an odds ratio for ischemic stroke of 2.2; those who smoked 2 packs per day had an odds ratio of 9.1.17 After cessation, stroke risk generally returns to baseline within 5 years.16 Thus, smoking cessation is among the most significant steps a patient can take to reduce the risk of both primary and secondary stroke.
Is your patient overweight? While obesity in and of itself is a risk factor for stroke, a focus on nutrition and physical activity as mechanisms for weight loss is far superior to focusing on either element alone. Physical activity—consisting of at least 40 minutes of moderate intensity aerobic exercise 3 to 4 times per week—and a diet that emphasizes fruits and vegetables, whole grains, and healthy fats, have both independently demonstrated benefits in secondary stroke prevention and are important parts of American College of Cardiology (ACC)/American Heart Association (AHA) guidelines.2,3
The Mediterranean Diet, which emphasizes consumption of fruits and vegetables, legumes, tree nuts, olive oil, and lean protein, has long been associated with cardiovascular benefit.18 One prospective, randomized, single-blinded trial involving approximately 600 patients that looked at secondary prevention of coronary heart disease found that following the diet significantly reduced mortality compared with a usual prudent post-infarct diet (number needed to treat [NNT]=30 over 4 years).19
Is alcohol consumption an issue? Chronic heavy alcohol intake contributes to the development of hemorrhagic and ischemic stroke through multiple mechanisms, including alcohol-induced hypertension, alcoholic cardiomyopathy, and atrial fibrillation (AF). Light or moderate alcohol consumption has a paradoxical mild protective effect on ischemic stroke, thought to possibly be mediated by an increase in high-density lipoprotein (HDL) level and mild antiplatelet effect.3
AHA/American Stroke Association (ASA) guidelines indicate that no more than one standard drink per day for women and 2 drinks per day for men is reasonable.3 Counsel patients who drink in excess of this about the benefits of decreasing alcohol intake or abstaining altogether.
Choosing medications to manage BP, cholesterol, and clotting
Optimize blood pressure control. Blood pressure (BP) plays a critical role in both the management and prevention of stroke and is considered to be the most important modifiable risk factor in both primary and secondary stroke prevention.20 In the first 24 to 48 hours following a cerebral ischemic event that is not eligible for thrombolysis, permissive hypertension (treating BP only if it exceeds 220/120 mm Hg unless there is a concurrent medical illness that requires you do so) is appropriate, as hypotension or rapid fluctuations in BP can be harmful.21
This flexibility does not continue into the subacute phase of management (at a minimum, after the initial 48 hours) or into secondary prevention. Initiation and titration of oral agents to gradually achieve a BP <140/90 mm Hg or a reduction of 10/5 mm Hg for patients already within optimal range are the most widely recognized goals.3,20 Patients with stroke secondary to small vessel disease may benefit from an even lower goal of <130/<80 mm Hg.11 Encourage patients to monitor their BP at home for added accuracy and consistency.22
Pharmacologic BP management is appropriate for patients who are consistently above optimal range despite attempting recommended lifestyle modifications. The data are relatively consistent with respect to the effects of different drug classes after a stroke: beta-blockers have no effect on any outcome; thiazide diuretics significantly reduce stroke and total vascular events; angiotensin-converting enzyme (ACE) inhibitors significantly reduce myocardial infarction (MI); and the combination of an ACE inhibitor and thiazide diuretic reduces stroke, MI, and combined vascular events.4
This has led many stroke specialists to recommend the combination of an ACE inhibitor or angiotensin II receptor blocker (ARB) and a thiazide diuretic as a first-line approach to secondary stroke prevention rather than a beta-blocker (assuming there is no additional indication for a beta-blocker). Similarly, there is ample evidence to show that the magnitude of BP reduction is proportional to the reduction in recurrent vascular events.3
Make use of statin therapy—regardless of LDL. The SPARCL (Stroke Prevention by Aggressive Reduction in Cholesterol Levels) trial5 explored the potential role of statin medication for secondary stroke prevention. Researchers randomly assigned almost 5000 participants who’d had a stroke or TIA one to 6 months before study entry (but had no known history of coronary artery disease) to placebo or a high-intensity statin (80 mg/d atorvastatin). The statin group demonstrated a 4.9-year absolute risk reduction in fatal or nonfatal recurrent stroke of 1.9% (NNT=53).
Given these findings and those from other studies, the AHA and ASA recommend treating patients with stroke or TIA presumed to be of atherosclerotic origin with high-intensity statin therapy, regardless of low-density lipoprotein (LDL) level.3 Of note, statins are not indicated for the secondary prevention of hemorrhagic stroke.
Select antiplatelet therapy based on ischemic stroke subtype. Investigators are still trying to determine the optimal antiplatelet for secondary stroke prevention; it is likely that the ideal choice depends largely on the etiology of the stroke. Trials that did not select patients based on subtype of ischemic stroke have not shown a long-term benefit from dual antiplatelet therapy (clopidogrel and aspirin),23,24 and one double-blind, multicenter trial involving more than 3000 patients with recent stroke secondary to small vessel disease demonstrated harm from such therapy in terms of a significantly increased risk of bleeding and death.6
However, a 2011 study compared aggressive medical management (aspirin 325 mg/d plus clopidogrel 75 mg/d for 90 days) alone to aggressive medical management plus percutaneous transluminal angioplasty and stenting (PTAS). The study involved almost 500 patients who'd had a recent TIA or stroke attributed to intracranial atherosclerotic stenosis. The authors found that the 30-day rate of stroke or death was 14.7% in the PTAS group vs 5.8% in the medical management group.25
Similarly, a randomized double-blind, placebo-controlled trial published in 2013 involving over 5000 patients in China found that short-term use of dual antiplatelets (clopidogrel and aspirin for the first 21 days after an ischemic event, followed by aspirin monotherapy for 90 days) had an absolute risk reduction of 3.5% without increasing the risk of major bleeding in patients with high-risk TIA or minor stroke.26
All stroke patients who do not have an indication for oral anticoagulation should be placed on long-term daily aspirin (75-325 mg); research has shown that lower doses are as effective as higher doses but with a lower risk of adverse gastrointestinal effects, including bleeding.3,20 Aspirin 81 mg/d is a common effective dose.
For patients who cannot tolerate aspirin due to allergy, clopidogrel 75 mg/d is a reasonable alternative. Long-term studies of aspirin vs clopidogrel7 and clopidogrel vs extended-release dipyridamole8 showed no difference in secondary stroke prevention. The International Stroke Trial27 and Chinese Acute Stroke Trial28 both indicate that aspirin should be started as soon as possible after the onset of an acute stroke.
This special population should probably get antiplatelets, too. One recent study explored the use of an antiplatelet vs anticoagulation therapy for stroke patients with carotid artery dissection. The CADISS (Cervical Artery Dissection in Stroke Study) trial29 randomized 250 patients with extracranial carotid and vertebral artery dissection with onset of symptoms within the previous 7 days to either antiplatelet or anticoagulation therapy and found no difference in the primary outcomes of recurrent stroke or death. The study also demonstrated a low risk of recurrent stroke in this population, which was 2% at 3-month follow-up.
Most patients with cervical artery dissection, therefore, are now treated with antiplatelet therapy. That said, situations may still arise in which anticoagulation can be considered, and consultation with a neurologist for guidance on choice of therapy is recommended.
Is an anticoagulant in order? Which agent, when
The most common cause of cardioembolic stroke is AF, which accounts for at least 15% of ischemic strokes, a number that rises in those over the age of 80.20,30,31 A meta-analysis of more than 28,000 patients with non-valvular AF demonstrated that warfarin reduced the risk of stroke by 64%.32
The rate of intracerebral hemorrhages during oral anticoagulation ranges from 0.3% to 0.6% per year.33 The risk of bleeding complications can be mitigated by keeping international normalized ratios ≤3.0, maintaining good BP control, and avoiding concurrent use of antiplatelets in the absence of a clear indication for them.33
Several risk assessment scores, such as the HAS-BLED,34 can help with estimating the risk of hemorrhagic complications, although these scores have their limitations.35,36 Even in an older population (mean age 83 years) with a high risk for falls, warfarin provided a net benefit in a composite endpoint of out-of-hospital death or hospitalization for stroke, MI, or hemorrhage in a retrospective study of over 1200 Medicare beneficiaries.37
AF is not the only cause of cardioembolic stroke to consider. Additional high-risk factors warranting anticoagulation include rheumatic mitral valve disease, the presence of mechanical aortic or mitral valves, known mural thrombus, and acute anterior ST segment elevation myocardial infarctions (STEMIs) with resulting anterior apical dyskinesis/akinesis and concurrent ischemic stroke/TIA.3 (The specific management of each of these situations is beyond the scope of this paper.)
The choice of anticoagulation agent is based on multiple factors, including cost, risk of non-reversible bleeding, drug interactions, renal function, and patient preference. Approved options currently include warfarin/vitamin K antagonist therapy, apixaban, rivaroxaban, dabigatran and edoxaban.3 Choice of therapy will continue to evolve as reversal agents, such as idarucizumab, are developed. Idarucizumab, a reversal agent for dabigatran, received approval from the US Food and Drug Administration in October 2015.38
When to start anticoagulation. There are limited data regarding the optimal timing of initiation of anticoagulation following a stroke; however, a recent multicenter prospective study supported the common practice of initiating anticoagulation therapy within 4 to 14 days of the event.39 Individual patient factors must be taken into consideration, including the size of the stroke (the larger the stroke, the higher the risk for hemorrhagic transformation), BP control, any additional risk factors for bleeding, and the estimated risk of early recurrent stroke.
Bridging patients onto anticoagulation with unfractionated or low-molecular-weight heparin in the setting of acute stroke is not recommended.40 Results from randomized controlled trials involving unfractionated heparin, heparinoids, and low-molecular-weight heparin have not reported any benefit to these agents over aspirin at preventing early stroke recurrence.27,41,42
For immobile or hospitalized patients. Subcutaneous heparin for the prevention of deep vein thrombosis (DVT) during immobility and hospitalization is recommended.43 Patients who cannot tolerate anticoagulation should be maintained on low-dose antiplatelet therapy. Experts do not recommend dual treatment with aspirin and anticoagulation in most cases. However, recent coronary artery stent placement does require temporary dual treatment, with duration dependent on the type of stent placed.
A role for glycemic control? Still to be determined
The specific role of diabetic management in secondary stroke prevention remains unclear. The 2008 ACCORD trial,44 a randomized study involving over 10,000 patients with a median glycated hemoglobin level of 8.1%, investigated intensive hyperglycemic control (targeting a glycated hemoglobin level <6.0% vs <7.9%) as a means of decreasing cardiovascular risk. However, the trial ended 17 months early because of an increase in all-cause mortality in the intensive treatment arm compared with the standard management group. The same trial was also unable to demonstrate a decrease in stroke risk with a decrease in A1c.44
More recently, the IRIS (Insulin Resistance Intervention after Stroke) trial45 (2016) found a 2.8% absolute risk reduction in stroke or MI among participants who had a stroke or TIA in the previous 6 months who were treated with pioglitazone vs placebo over 4.8 years (NNT=36). Participants were required to have insulin resistance, but were excluded if they had diabetes. The authors did, however, report a notable increase in the risk of bone fractures requiring surgery or hospitalization in the pioglitazone arm (5.1% vs 3.2%; number needed to harm [NNH]=53).
The impact this single study should have on standard secondary prevention is not yet clear. The authors concluded, “It seems reasonable to consider individual treatment preference and risk of drug-related adverse events in addition to potential benefits when making patient-specific decisions regarding therapy.”45
Determining whether mechanical interventions are needed
Almost all conditions leading to stroke warrant active medical management, but a few benefit from procedural intervention, as well.
Extracranial carotid atherosclerosis. Carotid endarterectomy or carotid artery stenting is recommended as secondary prevention for patients with a history of stroke or TIA who have ipsilateral high-grade extracranial carotid stenosis of 70% to 99% and, in some cases, 50% to 69%.3,9,20 In patients with mild non-disabling stroke, the optimal timing for these procedures is within 2 weeks of the ischemic event. A delay of 6 weeks is generally preferred for moderate or larger strokes to allow for some healing of the injured brain.
The choice of procedure is based on risk profile, with the most important factor being age. For patients >70 years, endarterectomy is preferred because stenting is associated with an increased risk of stroke.3,9,10 Experts do not recommend either procedure for patients who have had a severe disabling stroke. Generally speaking, these procedures have higher rates of success when they are performed in centers that perform a higher number of these procedures.10
Vertebrobasilar atherosclerosis. Due to generally good compensatory blood flow of the contralateral vertebral artery in the setting of vertebral artery stenosis, and an unacceptably high complication rate of angioplasty and stenting in the basilar artery, medical management is typically the first-line approach. If a patient has recurrent symptoms in the setting of optimal medical management and a focal lesion that is amenable to an endovascular intervention (most commonly a vertebral artery origin high-grade stenosis), angioplasty and stenting may be considered.10
Intracranial atherosclerosis. Similarly, medical management is the preferred strategy for intracranial atherosclerosis. Angioplasty and/or stenting are reserved for complex cases or recurrence despite adherence to secondary stroke prevention measures. Ideally, these patients should be managed with long-term aspirin 81 mg/d, adjunctive clopidogrel 75 mg/d for 90 days post stroke, a high-intensity statin, BP optimization, and any relevant lifestyle interventions.13
Patent foramen ovale. Research to date has not shown that closure of a patent foramen ovale (PFO) is superior to medical therapy for secondary stroke prevention in patients <60 years with cryptogenic stroke.12,46,47 The decision to anticoagulate these patients should be based on the presence or absence of a DVT and not on a PFO alone. In patients with an identified DVT and a contraindication to oral anticoagulation, inferior vena cava filter placement should be considered. For patients with ongoing prothrombotic risk thought to increase the chances of future paradoxical embolism, closure of the PFO may be considered.
CORRESPONDENCE
Stephen A. Martin, MD, EdM, Barre Family Health Center, 151 Worcester Road, Barre, MA 01005; [email protected].
Patients who suffer a stroke rarely have just one vascular risk factor. Therefore, the approach to secondary stroke prevention must be multifactorial. In fact, it has been estimated that 80% of recurrent strokes could be prevented through the application of a comprehensive, multifactorial approach that includes lifestyle modification and optimal medical management.1 Such an achievement would save millions of people from disability and functional decline, as well as millions of dollars in related medical costs.
The initial approach to patients with stroke is focused on stabilization and a rapid work-up to identify the most likely etiology. Common causes of stroke include large artery atherosclerosis, cardiac emboli, and small vessel disease; less common causes include dissection, aortic emboli, and non-atherosclerotic vascular disease. If a complete diagnostic work-up is unrevealing, the stroke is said to be cryptogenic. Determining the correct etiology of a stroke is paramount to preventing secondary stroke (FIGURE2-13).

Effective secondary prevention strategies designed to prevent a stroke or transient ischemic attack (TIA) in a patient with a known history of either event include lifestyle modifications, medications, and when appropriate, mechanical interventions. As a primary care physician (PCP), you are uniquely positioned to spearhead the prevention of secondary strokes: Not only are you at the forefront of prevention and the use of techniques such as motivational interviewing, but you also have longstanding relationships with many of your patients. In fact, the success of many interventions is improved by the informed, enduring, and trusting nature of relationships between patients and their PCPs.
In the first part of this 2-part series, we focused on subacute stroke management and outlined the recommended work-up for subacute stroke/TIA (see “Stroke: A road map to subacute management,” 2017;66:366-374). In this part, we focus on secondary prevention. The more common modifiable conditions encountered in primary care are discussed here, while many of the more rare etiologies (hypercoagulable states, sickle cell disease, and vasculitis) are outside the scope of this article.
Lifestyle interventions: Target tobacco use, obesity, alcohol intake
Lifestyle modifications can have a positive impact on many of America’s most prevalent diseases, and stroke is no exception.14 Many of the disease states identified as risk factors for stroke (type 2 diabetes, hypertension, dyslipidemia) are exacerbated by tobacco use, obesity, and excessive alcohol intake.
Does your patient smoke? Up to 25% of all strokes are directly attributable to cigarette smoking.15 Smoking raises an individual’s risk for stroke in a dose-dependent fashion.15,16 One study demonstrated that, compared to never-smokers, women ages 15 to 49 years who smoked a half-pack per day had an odds ratio for ischemic stroke of 2.2; those who smoked 2 packs per day had an odds ratio of 9.1.17 After cessation, stroke risk generally returns to baseline within 5 years.16 Thus, smoking cessation is among the most significant steps a patient can take to reduce the risk of both primary and secondary stroke.
Is your patient overweight? While obesity in and of itself is a risk factor for stroke, a focus on nutrition and physical activity as mechanisms for weight loss is far superior to focusing on either element alone. Physical activity—consisting of at least 40 minutes of moderate intensity aerobic exercise 3 to 4 times per week—and a diet that emphasizes fruits and vegetables, whole grains, and healthy fats, have both independently demonstrated benefits in secondary stroke prevention and are important parts of American College of Cardiology (ACC)/American Heart Association (AHA) guidelines.2,3
The Mediterranean Diet, which emphasizes consumption of fruits and vegetables, legumes, tree nuts, olive oil, and lean protein, has long been associated with cardiovascular benefit.18 One prospective, randomized, single-blinded trial involving approximately 600 patients that looked at secondary prevention of coronary heart disease found that following the diet significantly reduced mortality compared with a usual prudent post-infarct diet (number needed to treat [NNT]=30 over 4 years).19
Is alcohol consumption an issue? Chronic heavy alcohol intake contributes to the development of hemorrhagic and ischemic stroke through multiple mechanisms, including alcohol-induced hypertension, alcoholic cardiomyopathy, and atrial fibrillation (AF). Light or moderate alcohol consumption has a paradoxical mild protective effect on ischemic stroke, thought to possibly be mediated by an increase in high-density lipoprotein (HDL) level and mild antiplatelet effect.3
AHA/American Stroke Association (ASA) guidelines indicate that no more than one standard drink per day for women and 2 drinks per day for men is reasonable.3 Counsel patients who drink in excess of this about the benefits of decreasing alcohol intake or abstaining altogether.
Choosing medications to manage BP, cholesterol, and clotting
Optimize blood pressure control. Blood pressure (BP) plays a critical role in both the management and prevention of stroke and is considered to be the most important modifiable risk factor in both primary and secondary stroke prevention.20 In the first 24 to 48 hours following a cerebral ischemic event that is not eligible for thrombolysis, permissive hypertension (treating BP only if it exceeds 220/120 mm Hg unless there is a concurrent medical illness that requires you do so) is appropriate, as hypotension or rapid fluctuations in BP can be harmful.21
This flexibility does not continue into the subacute phase of management (at a minimum, after the initial 48 hours) or into secondary prevention. Initiation and titration of oral agents to gradually achieve a BP <140/90 mm Hg or a reduction of 10/5 mm Hg for patients already within optimal range are the most widely recognized goals.3,20 Patients with stroke secondary to small vessel disease may benefit from an even lower goal of <130/<80 mm Hg.11 Encourage patients to monitor their BP at home for added accuracy and consistency.22
Pharmacologic BP management is appropriate for patients who are consistently above optimal range despite attempting recommended lifestyle modifications. The data are relatively consistent with respect to the effects of different drug classes after a stroke: beta-blockers have no effect on any outcome; thiazide diuretics significantly reduce stroke and total vascular events; angiotensin-converting enzyme (ACE) inhibitors significantly reduce myocardial infarction (MI); and the combination of an ACE inhibitor and thiazide diuretic reduces stroke, MI, and combined vascular events.4
This has led many stroke specialists to recommend the combination of an ACE inhibitor or angiotensin II receptor blocker (ARB) and a thiazide diuretic as a first-line approach to secondary stroke prevention rather than a beta-blocker (assuming there is no additional indication for a beta-blocker). Similarly, there is ample evidence to show that the magnitude of BP reduction is proportional to the reduction in recurrent vascular events.3
Make use of statin therapy—regardless of LDL. The SPARCL (Stroke Prevention by Aggressive Reduction in Cholesterol Levels) trial5 explored the potential role of statin medication for secondary stroke prevention. Researchers randomly assigned almost 5000 participants who’d had a stroke or TIA one to 6 months before study entry (but had no known history of coronary artery disease) to placebo or a high-intensity statin (80 mg/d atorvastatin). The statin group demonstrated a 4.9-year absolute risk reduction in fatal or nonfatal recurrent stroke of 1.9% (NNT=53).
Given these findings and those from other studies, the AHA and ASA recommend treating patients with stroke or TIA presumed to be of atherosclerotic origin with high-intensity statin therapy, regardless of low-density lipoprotein (LDL) level.3 Of note, statins are not indicated for the secondary prevention of hemorrhagic stroke.
Select antiplatelet therapy based on ischemic stroke subtype. Investigators are still trying to determine the optimal antiplatelet for secondary stroke prevention; it is likely that the ideal choice depends largely on the etiology of the stroke. Trials that did not select patients based on subtype of ischemic stroke have not shown a long-term benefit from dual antiplatelet therapy (clopidogrel and aspirin),23,24 and one double-blind, multicenter trial involving more than 3000 patients with recent stroke secondary to small vessel disease demonstrated harm from such therapy in terms of a significantly increased risk of bleeding and death.6
However, a 2011 study compared aggressive medical management (aspirin 325 mg/d plus clopidogrel 75 mg/d for 90 days) alone to aggressive medical management plus percutaneous transluminal angioplasty and stenting (PTAS). The study involved almost 500 patients who'd had a recent TIA or stroke attributed to intracranial atherosclerotic stenosis. The authors found that the 30-day rate of stroke or death was 14.7% in the PTAS group vs 5.8% in the medical management group.25
Similarly, a randomized double-blind, placebo-controlled trial published in 2013 involving over 5000 patients in China found that short-term use of dual antiplatelets (clopidogrel and aspirin for the first 21 days after an ischemic event, followed by aspirin monotherapy for 90 days) had an absolute risk reduction of 3.5% without increasing the risk of major bleeding in patients with high-risk TIA or minor stroke.26
All stroke patients who do not have an indication for oral anticoagulation should be placed on long-term daily aspirin (75-325 mg); research has shown that lower doses are as effective as higher doses but with a lower risk of adverse gastrointestinal effects, including bleeding.3,20 Aspirin 81 mg/d is a common effective dose.
For patients who cannot tolerate aspirin due to allergy, clopidogrel 75 mg/d is a reasonable alternative. Long-term studies of aspirin vs clopidogrel7 and clopidogrel vs extended-release dipyridamole8 showed no difference in secondary stroke prevention. The International Stroke Trial27 and Chinese Acute Stroke Trial28 both indicate that aspirin should be started as soon as possible after the onset of an acute stroke.
This special population should probably get antiplatelets, too. One recent study explored the use of an antiplatelet vs anticoagulation therapy for stroke patients with carotid artery dissection. The CADISS (Cervical Artery Dissection in Stroke Study) trial29 randomized 250 patients with extracranial carotid and vertebral artery dissection with onset of symptoms within the previous 7 days to either antiplatelet or anticoagulation therapy and found no difference in the primary outcomes of recurrent stroke or death. The study also demonstrated a low risk of recurrent stroke in this population, which was 2% at 3-month follow-up.
Most patients with cervical artery dissection, therefore, are now treated with antiplatelet therapy. That said, situations may still arise in which anticoagulation can be considered, and consultation with a neurologist for guidance on choice of therapy is recommended.
Is an anticoagulant in order? Which agent, when
The most common cause of cardioembolic stroke is AF, which accounts for at least 15% of ischemic strokes, a number that rises in those over the age of 80.20,30,31 A meta-analysis of more than 28,000 patients with non-valvular AF demonstrated that warfarin reduced the risk of stroke by 64%.32
The rate of intracerebral hemorrhages during oral anticoagulation ranges from 0.3% to 0.6% per year.33 The risk of bleeding complications can be mitigated by keeping international normalized ratios ≤3.0, maintaining good BP control, and avoiding concurrent use of antiplatelets in the absence of a clear indication for them.33
Several risk assessment scores, such as the HAS-BLED,34 can help with estimating the risk of hemorrhagic complications, although these scores have their limitations.35,36 Even in an older population (mean age 83 years) with a high risk for falls, warfarin provided a net benefit in a composite endpoint of out-of-hospital death or hospitalization for stroke, MI, or hemorrhage in a retrospective study of over 1200 Medicare beneficiaries.37
AF is not the only cause of cardioembolic stroke to consider. Additional high-risk factors warranting anticoagulation include rheumatic mitral valve disease, the presence of mechanical aortic or mitral valves, known mural thrombus, and acute anterior ST segment elevation myocardial infarctions (STEMIs) with resulting anterior apical dyskinesis/akinesis and concurrent ischemic stroke/TIA.3 (The specific management of each of these situations is beyond the scope of this paper.)
The choice of anticoagulation agent is based on multiple factors, including cost, risk of non-reversible bleeding, drug interactions, renal function, and patient preference. Approved options currently include warfarin/vitamin K antagonist therapy, apixaban, rivaroxaban, dabigatran and edoxaban.3 Choice of therapy will continue to evolve as reversal agents, such as idarucizumab, are developed. Idarucizumab, a reversal agent for dabigatran, received approval from the US Food and Drug Administration in October 2015.38
When to start anticoagulation. There are limited data regarding the optimal timing of initiation of anticoagulation following a stroke; however, a recent multicenter prospective study supported the common practice of initiating anticoagulation therapy within 4 to 14 days of the event.39 Individual patient factors must be taken into consideration, including the size of the stroke (the larger the stroke, the higher the risk for hemorrhagic transformation), BP control, any additional risk factors for bleeding, and the estimated risk of early recurrent stroke.
Bridging patients onto anticoagulation with unfractionated or low-molecular-weight heparin in the setting of acute stroke is not recommended.40 Results from randomized controlled trials involving unfractionated heparin, heparinoids, and low-molecular-weight heparin have not reported any benefit to these agents over aspirin at preventing early stroke recurrence.27,41,42
For immobile or hospitalized patients. Subcutaneous heparin for the prevention of deep vein thrombosis (DVT) during immobility and hospitalization is recommended.43 Patients who cannot tolerate anticoagulation should be maintained on low-dose antiplatelet therapy. Experts do not recommend dual treatment with aspirin and anticoagulation in most cases. However, recent coronary artery stent placement does require temporary dual treatment, with duration dependent on the type of stent placed.
A role for glycemic control? Still to be determined
The specific role of diabetic management in secondary stroke prevention remains unclear. The 2008 ACCORD trial,44 a randomized study involving over 10,000 patients with a median glycated hemoglobin level of 8.1%, investigated intensive hyperglycemic control (targeting a glycated hemoglobin level <6.0% vs <7.9%) as a means of decreasing cardiovascular risk. However, the trial ended 17 months early because of an increase in all-cause mortality in the intensive treatment arm compared with the standard management group. The same trial was also unable to demonstrate a decrease in stroke risk with a decrease in A1c.44
More recently, the IRIS (Insulin Resistance Intervention after Stroke) trial45 (2016) found a 2.8% absolute risk reduction in stroke or MI among participants who had a stroke or TIA in the previous 6 months who were treated with pioglitazone vs placebo over 4.8 years (NNT=36). Participants were required to have insulin resistance, but were excluded if they had diabetes. The authors did, however, report a notable increase in the risk of bone fractures requiring surgery or hospitalization in the pioglitazone arm (5.1% vs 3.2%; number needed to harm [NNH]=53).
The impact this single study should have on standard secondary prevention is not yet clear. The authors concluded, “It seems reasonable to consider individual treatment preference and risk of drug-related adverse events in addition to potential benefits when making patient-specific decisions regarding therapy.”45
Determining whether mechanical interventions are needed
Almost all conditions leading to stroke warrant active medical management, but a few benefit from procedural intervention, as well.
Extracranial carotid atherosclerosis. Carotid endarterectomy or carotid artery stenting is recommended as secondary prevention for patients with a history of stroke or TIA who have ipsilateral high-grade extracranial carotid stenosis of 70% to 99% and, in some cases, 50% to 69%.3,9,20 In patients with mild non-disabling stroke, the optimal timing for these procedures is within 2 weeks of the ischemic event. A delay of 6 weeks is generally preferred for moderate or larger strokes to allow for some healing of the injured brain.
The choice of procedure is based on risk profile, with the most important factor being age. For patients >70 years, endarterectomy is preferred because stenting is associated with an increased risk of stroke.3,9,10 Experts do not recommend either procedure for patients who have had a severe disabling stroke. Generally speaking, these procedures have higher rates of success when they are performed in centers that perform a higher number of these procedures.10
Vertebrobasilar atherosclerosis. Due to generally good compensatory blood flow of the contralateral vertebral artery in the setting of vertebral artery stenosis, and an unacceptably high complication rate of angioplasty and stenting in the basilar artery, medical management is typically the first-line approach. If a patient has recurrent symptoms in the setting of optimal medical management and a focal lesion that is amenable to an endovascular intervention (most commonly a vertebral artery origin high-grade stenosis), angioplasty and stenting may be considered.10
Intracranial atherosclerosis. Similarly, medical management is the preferred strategy for intracranial atherosclerosis. Angioplasty and/or stenting are reserved for complex cases or recurrence despite adherence to secondary stroke prevention measures. Ideally, these patients should be managed with long-term aspirin 81 mg/d, adjunctive clopidogrel 75 mg/d for 90 days post stroke, a high-intensity statin, BP optimization, and any relevant lifestyle interventions.13
Patent foramen ovale. Research to date has not shown that closure of a patent foramen ovale (PFO) is superior to medical therapy for secondary stroke prevention in patients <60 years with cryptogenic stroke.12,46,47 The decision to anticoagulate these patients should be based on the presence or absence of a DVT and not on a PFO alone. In patients with an identified DVT and a contraindication to oral anticoagulation, inferior vena cava filter placement should be considered. For patients with ongoing prothrombotic risk thought to increase the chances of future paradoxical embolism, closure of the PFO may be considered.
CORRESPONDENCE
Stephen A. Martin, MD, EdM, Barre Family Health Center, 151 Worcester Road, Barre, MA 01005; [email protected].
1. Hackam DG, Spence JD. Combining multiple approaches for the secondary prevention of vascular events after stroke: a quantitative modeling study. Stroke. 2007;38:1881-1885.
2. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S76-S99.
3. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236.
4. Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke. 2003;34:2741-2748.
5. Amarenco P, Bogousslavsky J, Callahan A, et al, for the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549-559.
6. Benavente OR, Hart RG, McClure LA, et al, for the SPS3 Investigators. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
7. CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329-1339.
8. Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238-1251.
9. Diethrich EB, N’diaye M, Reid DB. The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST): implications for clinical practice. In: Henry M, Diethrich EB, Polydorou A, eds. The Carotid and Supra-Aortic Trunks: Diagnosis, Angioplasty and Stenting. 2nd ed. Oxford, UK: Wiley-Blackwell; 2011.
10. Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. Circulation. 2011;124:489-532.
11. SPS3 Study Group. Blood pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382:507-515.
12. Carroll JD, Saver JL, Thaler DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092-1100.
13. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993-1003.
14. Romero JR, Morris J, Pikula A. Stroke prevention: modifying risk factors. Ther Adv Cardiovasc Dis. 2008;2:287-303.
15. Hankey GJ. Smoking and risk of stroke. J Cardiovasc Risk. 1999;6:207-211.
16. Shah RS, Cole JW. Smoking and stroke: the more you smoke the more you stroke. Expert Rev Cardiovasc Ther. 2010;8:917-932.
17. Bhat VM, Cole JW, Sorkin JD, et al. Dose-response relationship between cigarette smoking and risk of ischemic stroke in young women. Stroke. 2008;39:2439-2443.
18. Lakkur S, Judd SE. Diet and stroke: recent evidence supporting a Mediterranean-style diet and food in the primary prevention of stroke. Stroke. 2015;46:2007-2011.
19. de Lorgeril M, Salen P, Martin JL, et al. Mediterranean dietary pattern in a randomized trial: prolonged survival and possible reduced cancer rate. Arch Intern Med. 1998;158:1181-1187.
20. Davis SM, Donnan GA. Clinical practice. Secondary prevention after ischemic stroke or transient ischemic attack. N Engl J Med. 2012;366:1914-1922.
21. Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870-947.
22. Magid DJ, Green BB. Home blood pressure monitoring: take it to the bank. JAMA. 2013;310:40-41.
23. Diener H-C, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2004;364:331-337.
24. Bhatt DL, Fox KAA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706-1717.
25. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993-1003.
26. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
27. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet. 1997;349:1569-1581.
28. CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet. 1997;349:1641-1649.
29. CADISS trial investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14:361-367.
30. Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. EAFT (European Atrial Fibrillation Trial) Study Group. Lancet. 1993;342:1255-1262.
31. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983-988.
32. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Int Med. 2007;146:857-867.
33. Hart RG, Tonarelli SB, Pearce LA. Avoiding central nervous system bleeding during antithrombotic therapy. Recent data and ideas. Stroke. 2005;36:1588-1593.
34. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest. 2010;138:1093-1100.
35. Quinn GR, Singer DE, Chang Y, et al. How well do stroke risk scores predict hemorrhage in patients with atrial fibrillation? Am J Cardiol. 2016;118:697-699.
36. Gorman EW, Perkel D, Dennis D, et al. Validation of the HAS-BLED tool in atrial fibrillation patients receiving rivaroxaban. J Atr Fibrillation. 2016;9:1461.
37. Gage BF, Birman-Deych E, Kerzner R, et al. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med. 2005;118:612-617.
38. US Food and Drug Administration. FDA approves Praxbind, the first reversal agent for the anticoagulant Pradaxa. October 16, 2015. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm467300.htm. Accessed May 26, 2017.
39. Paciaroni M, Agnelli G, Falocci N, et al. Early recurrence and cerebral bleeding in patients with acute ischemic stroke and atrial fibrillation: effect of anticoagulation and its timing: the RAF Study. Stroke. 2015;46:2175-2182.
40. Sandercock PA, Counsell C, Kane EJ. Anticoagulants for acute ischaemic stroke. Cochrane Database Syst Rev. 2015;3:CD000024.
41. Bath PM, Lindenstrom E, Boysen G, et al. Tinzaparin in acute ischaemic stroke (TAIST): a randomised aspirin-controlled trial. Lancet. 2001;358:702-710.
42. Berge E, Abdelnoor M, Nakstad PH, et al. Low molecular-weight heparin versus aspirin in patients with acute ischaemic stroke and atrial fibrillation: a double-blind randomised study. HAEST Study Group. Heparin in Acute Embolic Stroke Trial. Lancet. 2000;355:1205-1210.
43. Sherman DG, Albers GW, Bladin C, et al. The efficacy and safety of enoxaparin versus unfractionated heparin for the prevention of venous thromboembolism after acute ischaemic stroke (PREVAIL Study): an open-label randomised comparison. Lancet. 2007;369:1347-1355.
44. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559.
45. Kernan WN, Viscoli CM, Furie KL, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374:1321-1331.
46. Meier B, Kalesan B, Mattle HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083-1091.
47. Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991-999.
1. Hackam DG, Spence JD. Combining multiple approaches for the secondary prevention of vascular events after stroke: a quantitative modeling study. Stroke. 2007;38:1881-1885.
2. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S76-S99.
3. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236.
4. Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke. 2003;34:2741-2748.
5. Amarenco P, Bogousslavsky J, Callahan A, et al, for the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549-559.
6. Benavente OR, Hart RG, McClure LA, et al, for the SPS3 Investigators. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
7. CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329-1339.
8. Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238-1251.
9. Diethrich EB, N’diaye M, Reid DB. The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST): implications for clinical practice. In: Henry M, Diethrich EB, Polydorou A, eds. The Carotid and Supra-Aortic Trunks: Diagnosis, Angioplasty and Stenting. 2nd ed. Oxford, UK: Wiley-Blackwell; 2011.
10. Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. Circulation. 2011;124:489-532.
11. SPS3 Study Group. Blood pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382:507-515.
12. Carroll JD, Saver JL, Thaler DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092-1100.
13. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993-1003.
14. Romero JR, Morris J, Pikula A. Stroke prevention: modifying risk factors. Ther Adv Cardiovasc Dis. 2008;2:287-303.
15. Hankey GJ. Smoking and risk of stroke. J Cardiovasc Risk. 1999;6:207-211.
16. Shah RS, Cole JW. Smoking and stroke: the more you smoke the more you stroke. Expert Rev Cardiovasc Ther. 2010;8:917-932.
17. Bhat VM, Cole JW, Sorkin JD, et al. Dose-response relationship between cigarette smoking and risk of ischemic stroke in young women. Stroke. 2008;39:2439-2443.
18. Lakkur S, Judd SE. Diet and stroke: recent evidence supporting a Mediterranean-style diet and food in the primary prevention of stroke. Stroke. 2015;46:2007-2011.
19. de Lorgeril M, Salen P, Martin JL, et al. Mediterranean dietary pattern in a randomized trial: prolonged survival and possible reduced cancer rate. Arch Intern Med. 1998;158:1181-1187.
20. Davis SM, Donnan GA. Clinical practice. Secondary prevention after ischemic stroke or transient ischemic attack. N Engl J Med. 2012;366:1914-1922.
21. Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870-947.
22. Magid DJ, Green BB. Home blood pressure monitoring: take it to the bank. JAMA. 2013;310:40-41.
23. Diener H-C, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2004;364:331-337.
24. Bhatt DL, Fox KAA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706-1717.
25. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993-1003.
26. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
27. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet. 1997;349:1569-1581.
28. CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet. 1997;349:1641-1649.
29. CADISS trial investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14:361-367.
30. Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. EAFT (European Atrial Fibrillation Trial) Study Group. Lancet. 1993;342:1255-1262.
31. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983-988.
32. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Int Med. 2007;146:857-867.
33. Hart RG, Tonarelli SB, Pearce LA. Avoiding central nervous system bleeding during antithrombotic therapy. Recent data and ideas. Stroke. 2005;36:1588-1593.
34. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest. 2010;138:1093-1100.
35. Quinn GR, Singer DE, Chang Y, et al. How well do stroke risk scores predict hemorrhage in patients with atrial fibrillation? Am J Cardiol. 2016;118:697-699.
36. Gorman EW, Perkel D, Dennis D, et al. Validation of the HAS-BLED tool in atrial fibrillation patients receiving rivaroxaban. J Atr Fibrillation. 2016;9:1461.
37. Gage BF, Birman-Deych E, Kerzner R, et al. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med. 2005;118:612-617.
38. US Food and Drug Administration. FDA approves Praxbind, the first reversal agent for the anticoagulant Pradaxa. October 16, 2015. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm467300.htm. Accessed May 26, 2017.
39. Paciaroni M, Agnelli G, Falocci N, et al. Early recurrence and cerebral bleeding in patients with acute ischemic stroke and atrial fibrillation: effect of anticoagulation and its timing: the RAF Study. Stroke. 2015;46:2175-2182.
40. Sandercock PA, Counsell C, Kane EJ. Anticoagulants for acute ischaemic stroke. Cochrane Database Syst Rev. 2015;3:CD000024.
41. Bath PM, Lindenstrom E, Boysen G, et al. Tinzaparin in acute ischaemic stroke (TAIST): a randomised aspirin-controlled trial. Lancet. 2001;358:702-710.
42. Berge E, Abdelnoor M, Nakstad PH, et al. Low molecular-weight heparin versus aspirin in patients with acute ischaemic stroke and atrial fibrillation: a double-blind randomised study. HAEST Study Group. Heparin in Acute Embolic Stroke Trial. Lancet. 2000;355:1205-1210.
43. Sherman DG, Albers GW, Bladin C, et al. The efficacy and safety of enoxaparin versus unfractionated heparin for the prevention of venous thromboembolism after acute ischaemic stroke (PREVAIL Study): an open-label randomised comparison. Lancet. 2007;369:1347-1355.
44. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559.
45. Kernan WN, Viscoli CM, Furie KL, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374:1321-1331.
46. Meier B, Kalesan B, Mattle HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083-1091.
47. Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991-999.
From The Journal of Family Practice | 2017;66(7):420-422,424-427.
PRACTICE RECOMMENDATIONS
› Encourage lifestyle modifications, including smoking cessation, alcohol moderation, appropriate diet, and exercise to reduce the risk of recurrent stroke. A
› Optimize blood pressure control using an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker and a thiazide diuretic. A
› Only use beta-blockers if there is another indication for them. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Tips and advice for Essure removal
Essure tubal microinserts were not designed to be removed. However, a small minority of women are requesting removal because of regret, complications, or the development of pelvic pain and other symptoms that may or may not be caused by the device.
Minimally invasive gynecologic surgeons have developed a variety of removal procedures and techniques for these women. There is general agreement that hysteroscopic removal is feasible only in the first 3 months following insertion of the device. After that, laparoscopic removal has become the norm. Small published reports and case series have documented the use of laparoscopic bilateral salpingectomy (BS) with and without hysterectomy, laparoscopic BS with cornuectomy, and laparoscopic salpingostomy, often followed by salpingectomy. There is not yet enough data to demonstrate that one method is superior to another, and we each have our own preferred approaches for preoperative imaging and removal.
Here is some of our advice on working with patients to assess the need for, and possible outcomes of, removal, as well as how to approach the surgery.
Counseling, assessment, and consent
Dr. Cohen: We have to be frank with our patients that symptoms may or may not improve following Essure removal. In a recently published case series of 52 women who underwent Essure removal at our institution, three-quarters of the patients reported near or total improvement in the quality of life. However, a relatively high number – roughly 30% – reported some ongoing symptoms (J Minim Invasive Gynecol. 2017 Jun 6. doi: 10:1016/j.jmig.2017.05.015).
The most common indication for Essure removal in this series was pelvic pain (96%), followed by abnormal uterine bleeding (35%) and patient-reported allergic reaction (21%). The indications were not mutually exclusive.
Importantly, could endometriosis or another underlying condition have developed since placement or worsened over time? Or, could her pelvic pain be worsened because of the cessation of hormonal contraception that coincided with Essure placement, rather than the device itself? For some women, Essure removal alone will not cure their symptoms.
In our cohort of 52 women, interestingly, 44% of those with pelvic pain had one or more concomitant or alternate causes of pain, including endometriosis, adenomyosis, and adhesions.
Dr. Levie: We can’t assume that Essure coils are at fault when patients present with pain and other symptoms, nor can we minimize complaints and concerns. We have to explore them.
It’s important that we inform women that pain may not be related to Essure microinserts. However, if, after thorough evaluation, the patient believes that the coils are the etiology of her pain and I cannot find another reason – or if she has regrets or is concerned about potential problems in the future – I am happy to remove them.
Dr. Yunker: In our case series of 29 women who underwent removal for the primary indication of pelvic pain, 88.5% reported significant relief at their postoperative visit (Contraception. 2016 Aug;94[2]:190-2). This, and other unpublished data, show that patients with gynecologic complaints specifically are the most likely to have resolution of symptoms, compared with those with more systemic or nongynecologic complaints.
Some patients have systemic symptoms that they feel are related and new since the device was placed. My counseling in these cases is that, while I do not have any physiologic evidence that the Essure coil is causing their symptoms, I’m hopeful that symptoms will improve with removal. If they do not, these patients must follow up with their primary care doctor for further work-up.
Device structure and use of imaging
Essure is a 4-cm long device (0.8 mm in diameter) with two parts: an inner coil made of stainless steel and PET fibers, the latter of which induces the fibrosis responsible for tubal occlusion, and an outer coil made of nitinol, a nickel titanium alloy.
Dr. Cohen: While the exact mechanism is unclear, it’s possible that the PET fibers may be drivers of the systemic inflammatory-type symptoms that some women report. Nickel allergies are also possible albeit uncommon. They appear to manifest as rash, urticaria, and other symptoms characteristic of contact allergic reactions.
The brittle nature of the outer coil makes a grasp-and-pull approach disadvantageous, unless you’re removing coils early on hysteroscopically. In general, one must avoid fracturing the outer coil, or parts of the device will be left behind. Pulling too hard may also cause the outer coil to unravel and expand to be quite long, which further increases the risk of fracture.
Hysterosalpingogram (HSG) and ultrasound are typically first-line options for looking at coil position. A diagnostic hysteroscopy may also help identify coils, and intraoperative fluoroscopy may be useful for either the hysteroscopic or laparoscopic approach, if there’s any question about portions of the device not being recovered.
Dr. Levie: Ultrasound is often sufficient for operative planning, but, if it does not detect devices in the cornual region, then further imaging may be warranted.
It’s important to be aware that some devices that appear to have correct placement on ultrasound or HSG may actually be partially tracking subserosally. In these cases, the distal portion of the device may have tracked through the mucosal layer and along the muscularis but below the serosa in the fallopian tube, causing pain. Imaging won’t be helpful in making this diagnosis. It will be identified laparoscopically.
Dr. Yunker: When patients have completed the 3-month HSG (to confirm occlusion of the Fallopian tubes post placement), I will review the images myself rather than relying on the report. Without an HSG – and, in many cases, even when I have it in hand – I will order a plain film x-ray of the abdomen and pelvis to look for coils. In almost all cases, I also order an ultrasound, which is helpful in assessing for ovarian and uterine conditions.
I’ve found plain film imaging to be valuable for identifying additional or misplaced Essure inserts. I have found up to four in one tube. In interpreting x-rays, one must appreciate that the outer coil is not radio-opaque (other than the tiny marker at the end) and will not show up. Occasionally we’ll add hysteroscopy to see how much of a coil is trailing into the uterus, but the ultrasound and x-ray are usually enough.
Some patients ask about postremoval imaging. I do not routinely do this, but I’m not opposed to it.
Surgical techniques
Dr. Cohen: I advise dissecting around each coil without cutting the outer portion and removing the coil intact, resecting all the way down to the interstitial portion of the tube, then proceeding with bilateral salpingectomy to ensure contraception.
If the patient’s symptoms are systemic and possibly reflective of PET fiber reactions, a wedge resection of the cornua may provide more peace of mind that PET fibers will not be left in situ. This procedure can be approached similarly to myomectomy, with the use of hemostatic agents such as misoprostol or vasopressin and suture closure in multiple layers.
If there are multiple coils present in the cavity, one option, to avoid having to pull them all out from the abdominal side, is to transect and remove the intracavity portion of the device hysteroscopically then dissect and remove the tubal/interstitial potion laparoscopically. As a general rule, I send all the removed tissue to pathology.
Dr. Levie: In general, I do a linear salpingostomy after using a uterine manipulator and a grasper to first identify the site of the distal portion of the device. One can usually feel where the tubes bend onto the device.
A bit proximal to where I visually and mentally mark the distal end of the device, I make a 2-3 cm incision over the device. With a fine-tip grasper, I can usually release the distal portion of the inner coil. Using two graspers over the inner and outer coils together and a hand-over-hand motion, I pull without excess traction in the access of the tubes, and the proximal portion will usually follow and deliver fairly simply. If the proximal portion breaks, I advise looking for it hysteroscopically and delivering it through the uterus.
Some surgeons have recommended hysteroscopy at the beginning of the procedure with cutting (using scissors) at the proximal end of the outer coil to avoid its getting caught in the cornua.
Most patients continue to want permanent sterilization, so we proceed with salpingectomy. Sometimes, given underlying pathologies, we’ll decide on laparoscopic or vaginal hysterectomy as well or bilateral salpingectomy without doing the salpingostomy. When hysterectomy is part of the surgery, we don’t need to worry at all about broken devices.
When the device is removed separately from the fallopian tube, one should inspect it afterward to ensure that all four markers of the device – the markers that are recommended by the manufacturer for radiologic confirmation of proper placement – have been delivered.
Dr. Yunker: When everything looks normal on the ultrasound – and when the coils on either HSG and/or plain film x-ray appear to be in the appropriate position in the tubes – then removal of the coils and tubes only is an option.
The closer the coil is to the fimbriae, the easier it is to come straight across the tube as you would in a regular salpingectomy without concern of breaking or cutting the coil. However, the closer the coil is the uterine side, the deeper you’ll need to dissect into the cornual region of the uterus. A cornual wedge resection may be necessary in order to remove the coil intact.
Our procedure has evolved over the years and we have moved away from salpingectomy as a means to dissect out the coils. With the theoretical risk of retained coil fragments and PET fibers, we prefer to remove the coils and tubes en bloc.
Dr. Cohen is director of research and the fellowship program director of minimally invasive gynecologic surgery at Brigham and Women’s Hospital, Boston. She reported that she has no financial disclosures. Dr. Levie is professor and associate chairman of the department of obstetrics and gynecology and women’s health and director of the minimally invasive surgery fellowship at Montefiore Medical Center, New York. Dr. Levie reported that he is an investigator in two studies involving Essure and sat on the Essure medical advisory board for Bayer but did not receive personal renumeration. Dr. Yunker is an assistant professor of obstetrics and gynecology at Vanderbilt University, Nashville. She reported that she is a consultant for Olympus.
Essure tubal microinserts were not designed to be removed. However, a small minority of women are requesting removal because of regret, complications, or the development of pelvic pain and other symptoms that may or may not be caused by the device.
Minimally invasive gynecologic surgeons have developed a variety of removal procedures and techniques for these women. There is general agreement that hysteroscopic removal is feasible only in the first 3 months following insertion of the device. After that, laparoscopic removal has become the norm. Small published reports and case series have documented the use of laparoscopic bilateral salpingectomy (BS) with and without hysterectomy, laparoscopic BS with cornuectomy, and laparoscopic salpingostomy, often followed by salpingectomy. There is not yet enough data to demonstrate that one method is superior to another, and we each have our own preferred approaches for preoperative imaging and removal.
Here is some of our advice on working with patients to assess the need for, and possible outcomes of, removal, as well as how to approach the surgery.
Counseling, assessment, and consent
Dr. Cohen: We have to be frank with our patients that symptoms may or may not improve following Essure removal. In a recently published case series of 52 women who underwent Essure removal at our institution, three-quarters of the patients reported near or total improvement in the quality of life. However, a relatively high number – roughly 30% – reported some ongoing symptoms (J Minim Invasive Gynecol. 2017 Jun 6. doi: 10:1016/j.jmig.2017.05.015).
The most common indication for Essure removal in this series was pelvic pain (96%), followed by abnormal uterine bleeding (35%) and patient-reported allergic reaction (21%). The indications were not mutually exclusive.
Importantly, could endometriosis or another underlying condition have developed since placement or worsened over time? Or, could her pelvic pain be worsened because of the cessation of hormonal contraception that coincided with Essure placement, rather than the device itself? For some women, Essure removal alone will not cure their symptoms.
In our cohort of 52 women, interestingly, 44% of those with pelvic pain had one or more concomitant or alternate causes of pain, including endometriosis, adenomyosis, and adhesions.
Dr. Levie: We can’t assume that Essure coils are at fault when patients present with pain and other symptoms, nor can we minimize complaints and concerns. We have to explore them.
It’s important that we inform women that pain may not be related to Essure microinserts. However, if, after thorough evaluation, the patient believes that the coils are the etiology of her pain and I cannot find another reason – or if she has regrets or is concerned about potential problems in the future – I am happy to remove them.
Dr. Yunker: In our case series of 29 women who underwent removal for the primary indication of pelvic pain, 88.5% reported significant relief at their postoperative visit (Contraception. 2016 Aug;94[2]:190-2). This, and other unpublished data, show that patients with gynecologic complaints specifically are the most likely to have resolution of symptoms, compared with those with more systemic or nongynecologic complaints.
Some patients have systemic symptoms that they feel are related and new since the device was placed. My counseling in these cases is that, while I do not have any physiologic evidence that the Essure coil is causing their symptoms, I’m hopeful that symptoms will improve with removal. If they do not, these patients must follow up with their primary care doctor for further work-up.
Device structure and use of imaging
Essure is a 4-cm long device (0.8 mm in diameter) with two parts: an inner coil made of stainless steel and PET fibers, the latter of which induces the fibrosis responsible for tubal occlusion, and an outer coil made of nitinol, a nickel titanium alloy.
Dr. Cohen: While the exact mechanism is unclear, it’s possible that the PET fibers may be drivers of the systemic inflammatory-type symptoms that some women report. Nickel allergies are also possible albeit uncommon. They appear to manifest as rash, urticaria, and other symptoms characteristic of contact allergic reactions.
The brittle nature of the outer coil makes a grasp-and-pull approach disadvantageous, unless you’re removing coils early on hysteroscopically. In general, one must avoid fracturing the outer coil, or parts of the device will be left behind. Pulling too hard may also cause the outer coil to unravel and expand to be quite long, which further increases the risk of fracture.
Hysterosalpingogram (HSG) and ultrasound are typically first-line options for looking at coil position. A diagnostic hysteroscopy may also help identify coils, and intraoperative fluoroscopy may be useful for either the hysteroscopic or laparoscopic approach, if there’s any question about portions of the device not being recovered.
Dr. Levie: Ultrasound is often sufficient for operative planning, but, if it does not detect devices in the cornual region, then further imaging may be warranted.
It’s important to be aware that some devices that appear to have correct placement on ultrasound or HSG may actually be partially tracking subserosally. In these cases, the distal portion of the device may have tracked through the mucosal layer and along the muscularis but below the serosa in the fallopian tube, causing pain. Imaging won’t be helpful in making this diagnosis. It will be identified laparoscopically.
Dr. Yunker: When patients have completed the 3-month HSG (to confirm occlusion of the Fallopian tubes post placement), I will review the images myself rather than relying on the report. Without an HSG – and, in many cases, even when I have it in hand – I will order a plain film x-ray of the abdomen and pelvis to look for coils. In almost all cases, I also order an ultrasound, which is helpful in assessing for ovarian and uterine conditions.
I’ve found plain film imaging to be valuable for identifying additional or misplaced Essure inserts. I have found up to four in one tube. In interpreting x-rays, one must appreciate that the outer coil is not radio-opaque (other than the tiny marker at the end) and will not show up. Occasionally we’ll add hysteroscopy to see how much of a coil is trailing into the uterus, but the ultrasound and x-ray are usually enough.
Some patients ask about postremoval imaging. I do not routinely do this, but I’m not opposed to it.
Surgical techniques
Dr. Cohen: I advise dissecting around each coil without cutting the outer portion and removing the coil intact, resecting all the way down to the interstitial portion of the tube, then proceeding with bilateral salpingectomy to ensure contraception.
If the patient’s symptoms are systemic and possibly reflective of PET fiber reactions, a wedge resection of the cornua may provide more peace of mind that PET fibers will not be left in situ. This procedure can be approached similarly to myomectomy, with the use of hemostatic agents such as misoprostol or vasopressin and suture closure in multiple layers.
If there are multiple coils present in the cavity, one option, to avoid having to pull them all out from the abdominal side, is to transect and remove the intracavity portion of the device hysteroscopically then dissect and remove the tubal/interstitial potion laparoscopically. As a general rule, I send all the removed tissue to pathology.
Dr. Levie: In general, I do a linear salpingostomy after using a uterine manipulator and a grasper to first identify the site of the distal portion of the device. One can usually feel where the tubes bend onto the device.
A bit proximal to where I visually and mentally mark the distal end of the device, I make a 2-3 cm incision over the device. With a fine-tip grasper, I can usually release the distal portion of the inner coil. Using two graspers over the inner and outer coils together and a hand-over-hand motion, I pull without excess traction in the access of the tubes, and the proximal portion will usually follow and deliver fairly simply. If the proximal portion breaks, I advise looking for it hysteroscopically and delivering it through the uterus.
Some surgeons have recommended hysteroscopy at the beginning of the procedure with cutting (using scissors) at the proximal end of the outer coil to avoid its getting caught in the cornua.
Most patients continue to want permanent sterilization, so we proceed with salpingectomy. Sometimes, given underlying pathologies, we’ll decide on laparoscopic or vaginal hysterectomy as well or bilateral salpingectomy without doing the salpingostomy. When hysterectomy is part of the surgery, we don’t need to worry at all about broken devices.
When the device is removed separately from the fallopian tube, one should inspect it afterward to ensure that all four markers of the device – the markers that are recommended by the manufacturer for radiologic confirmation of proper placement – have been delivered.
Dr. Yunker: When everything looks normal on the ultrasound – and when the coils on either HSG and/or plain film x-ray appear to be in the appropriate position in the tubes – then removal of the coils and tubes only is an option.
The closer the coil is to the fimbriae, the easier it is to come straight across the tube as you would in a regular salpingectomy without concern of breaking or cutting the coil. However, the closer the coil is the uterine side, the deeper you’ll need to dissect into the cornual region of the uterus. A cornual wedge resection may be necessary in order to remove the coil intact.
Our procedure has evolved over the years and we have moved away from salpingectomy as a means to dissect out the coils. With the theoretical risk of retained coil fragments and PET fibers, we prefer to remove the coils and tubes en bloc.
Dr. Cohen is director of research and the fellowship program director of minimally invasive gynecologic surgery at Brigham and Women’s Hospital, Boston. She reported that she has no financial disclosures. Dr. Levie is professor and associate chairman of the department of obstetrics and gynecology and women’s health and director of the minimally invasive surgery fellowship at Montefiore Medical Center, New York. Dr. Levie reported that he is an investigator in two studies involving Essure and sat on the Essure medical advisory board for Bayer but did not receive personal renumeration. Dr. Yunker is an assistant professor of obstetrics and gynecology at Vanderbilt University, Nashville. She reported that she is a consultant for Olympus.
Essure tubal microinserts were not designed to be removed. However, a small minority of women are requesting removal because of regret, complications, or the development of pelvic pain and other symptoms that may or may not be caused by the device.
Minimally invasive gynecologic surgeons have developed a variety of removal procedures and techniques for these women. There is general agreement that hysteroscopic removal is feasible only in the first 3 months following insertion of the device. After that, laparoscopic removal has become the norm. Small published reports and case series have documented the use of laparoscopic bilateral salpingectomy (BS) with and without hysterectomy, laparoscopic BS with cornuectomy, and laparoscopic salpingostomy, often followed by salpingectomy. There is not yet enough data to demonstrate that one method is superior to another, and we each have our own preferred approaches for preoperative imaging and removal.
Here is some of our advice on working with patients to assess the need for, and possible outcomes of, removal, as well as how to approach the surgery.
Counseling, assessment, and consent
Dr. Cohen: We have to be frank with our patients that symptoms may or may not improve following Essure removal. In a recently published case series of 52 women who underwent Essure removal at our institution, three-quarters of the patients reported near or total improvement in the quality of life. However, a relatively high number – roughly 30% – reported some ongoing symptoms (J Minim Invasive Gynecol. 2017 Jun 6. doi: 10:1016/j.jmig.2017.05.015).
The most common indication for Essure removal in this series was pelvic pain (96%), followed by abnormal uterine bleeding (35%) and patient-reported allergic reaction (21%). The indications were not mutually exclusive.
Importantly, could endometriosis or another underlying condition have developed since placement or worsened over time? Or, could her pelvic pain be worsened because of the cessation of hormonal contraception that coincided with Essure placement, rather than the device itself? For some women, Essure removal alone will not cure their symptoms.
In our cohort of 52 women, interestingly, 44% of those with pelvic pain had one or more concomitant or alternate causes of pain, including endometriosis, adenomyosis, and adhesions.
Dr. Levie: We can’t assume that Essure coils are at fault when patients present with pain and other symptoms, nor can we minimize complaints and concerns. We have to explore them.
It’s important that we inform women that pain may not be related to Essure microinserts. However, if, after thorough evaluation, the patient believes that the coils are the etiology of her pain and I cannot find another reason – or if she has regrets or is concerned about potential problems in the future – I am happy to remove them.
Dr. Yunker: In our case series of 29 women who underwent removal for the primary indication of pelvic pain, 88.5% reported significant relief at their postoperative visit (Contraception. 2016 Aug;94[2]:190-2). This, and other unpublished data, show that patients with gynecologic complaints specifically are the most likely to have resolution of symptoms, compared with those with more systemic or nongynecologic complaints.
Some patients have systemic symptoms that they feel are related and new since the device was placed. My counseling in these cases is that, while I do not have any physiologic evidence that the Essure coil is causing their symptoms, I’m hopeful that symptoms will improve with removal. If they do not, these patients must follow up with their primary care doctor for further work-up.
Device structure and use of imaging
Essure is a 4-cm long device (0.8 mm in diameter) with two parts: an inner coil made of stainless steel and PET fibers, the latter of which induces the fibrosis responsible for tubal occlusion, and an outer coil made of nitinol, a nickel titanium alloy.
Dr. Cohen: While the exact mechanism is unclear, it’s possible that the PET fibers may be drivers of the systemic inflammatory-type symptoms that some women report. Nickel allergies are also possible albeit uncommon. They appear to manifest as rash, urticaria, and other symptoms characteristic of contact allergic reactions.
The brittle nature of the outer coil makes a grasp-and-pull approach disadvantageous, unless you’re removing coils early on hysteroscopically. In general, one must avoid fracturing the outer coil, or parts of the device will be left behind. Pulling too hard may also cause the outer coil to unravel and expand to be quite long, which further increases the risk of fracture.
Hysterosalpingogram (HSG) and ultrasound are typically first-line options for looking at coil position. A diagnostic hysteroscopy may also help identify coils, and intraoperative fluoroscopy may be useful for either the hysteroscopic or laparoscopic approach, if there’s any question about portions of the device not being recovered.
Dr. Levie: Ultrasound is often sufficient for operative planning, but, if it does not detect devices in the cornual region, then further imaging may be warranted.
It’s important to be aware that some devices that appear to have correct placement on ultrasound or HSG may actually be partially tracking subserosally. In these cases, the distal portion of the device may have tracked through the mucosal layer and along the muscularis but below the serosa in the fallopian tube, causing pain. Imaging won’t be helpful in making this diagnosis. It will be identified laparoscopically.
Dr. Yunker: When patients have completed the 3-month HSG (to confirm occlusion of the Fallopian tubes post placement), I will review the images myself rather than relying on the report. Without an HSG – and, in many cases, even when I have it in hand – I will order a plain film x-ray of the abdomen and pelvis to look for coils. In almost all cases, I also order an ultrasound, which is helpful in assessing for ovarian and uterine conditions.
I’ve found plain film imaging to be valuable for identifying additional or misplaced Essure inserts. I have found up to four in one tube. In interpreting x-rays, one must appreciate that the outer coil is not radio-opaque (other than the tiny marker at the end) and will not show up. Occasionally we’ll add hysteroscopy to see how much of a coil is trailing into the uterus, but the ultrasound and x-ray are usually enough.
Some patients ask about postremoval imaging. I do not routinely do this, but I’m not opposed to it.
Surgical techniques
Dr. Cohen: I advise dissecting around each coil without cutting the outer portion and removing the coil intact, resecting all the way down to the interstitial portion of the tube, then proceeding with bilateral salpingectomy to ensure contraception.
If the patient’s symptoms are systemic and possibly reflective of PET fiber reactions, a wedge resection of the cornua may provide more peace of mind that PET fibers will not be left in situ. This procedure can be approached similarly to myomectomy, with the use of hemostatic agents such as misoprostol or vasopressin and suture closure in multiple layers.
If there are multiple coils present in the cavity, one option, to avoid having to pull them all out from the abdominal side, is to transect and remove the intracavity portion of the device hysteroscopically then dissect and remove the tubal/interstitial potion laparoscopically. As a general rule, I send all the removed tissue to pathology.
Dr. Levie: In general, I do a linear salpingostomy after using a uterine manipulator and a grasper to first identify the site of the distal portion of the device. One can usually feel where the tubes bend onto the device.
A bit proximal to where I visually and mentally mark the distal end of the device, I make a 2-3 cm incision over the device. With a fine-tip grasper, I can usually release the distal portion of the inner coil. Using two graspers over the inner and outer coils together and a hand-over-hand motion, I pull without excess traction in the access of the tubes, and the proximal portion will usually follow and deliver fairly simply. If the proximal portion breaks, I advise looking for it hysteroscopically and delivering it through the uterus.
Some surgeons have recommended hysteroscopy at the beginning of the procedure with cutting (using scissors) at the proximal end of the outer coil to avoid its getting caught in the cornua.
Most patients continue to want permanent sterilization, so we proceed with salpingectomy. Sometimes, given underlying pathologies, we’ll decide on laparoscopic or vaginal hysterectomy as well or bilateral salpingectomy without doing the salpingostomy. When hysterectomy is part of the surgery, we don’t need to worry at all about broken devices.
When the device is removed separately from the fallopian tube, one should inspect it afterward to ensure that all four markers of the device – the markers that are recommended by the manufacturer for radiologic confirmation of proper placement – have been delivered.
Dr. Yunker: When everything looks normal on the ultrasound – and when the coils on either HSG and/or plain film x-ray appear to be in the appropriate position in the tubes – then removal of the coils and tubes only is an option.
The closer the coil is to the fimbriae, the easier it is to come straight across the tube as you would in a regular salpingectomy without concern of breaking or cutting the coil. However, the closer the coil is the uterine side, the deeper you’ll need to dissect into the cornual region of the uterus. A cornual wedge resection may be necessary in order to remove the coil intact.
Our procedure has evolved over the years and we have moved away from salpingectomy as a means to dissect out the coils. With the theoretical risk of retained coil fragments and PET fibers, we prefer to remove the coils and tubes en bloc.
Dr. Cohen is director of research and the fellowship program director of minimally invasive gynecologic surgery at Brigham and Women’s Hospital, Boston. She reported that she has no financial disclosures. Dr. Levie is professor and associate chairman of the department of obstetrics and gynecology and women’s health and director of the minimally invasive surgery fellowship at Montefiore Medical Center, New York. Dr. Levie reported that he is an investigator in two studies involving Essure and sat on the Essure medical advisory board for Bayer but did not receive personal renumeration. Dr. Yunker is an assistant professor of obstetrics and gynecology at Vanderbilt University, Nashville. She reported that she is a consultant for Olympus.
So you want your Essure device removed ...
Despite recent negative lay press and a boxed safety warning from the Food and Drug Administration, Essure tubal microinserts continue to be a popular method of permanent contraception. It is imperative for patients to understand that this method of contraception cannot be reversed, and thereafter, the only method to achieve pregnancy would be via in vitro fertilization. Furthermore, preoperatively, patients must be counseled that placement of the Essure tubal microinserts may be associated with pelvic pain, abnormal bleeding, and even allergic reaction.
Even with our best effort to properly inform our patients as to the risks and benefits of permanent sterilization via Essure tubal microinserts, secondary to undesired side effects, patients desire their removal. This can be a challenging endeavor for the practitioner, especially if the women is not interested in hysterectomy.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago; director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. He reported having no financial disclosures related to this column.
Despite recent negative lay press and a boxed safety warning from the Food and Drug Administration, Essure tubal microinserts continue to be a popular method of permanent contraception. It is imperative for patients to understand that this method of contraception cannot be reversed, and thereafter, the only method to achieve pregnancy would be via in vitro fertilization. Furthermore, preoperatively, patients must be counseled that placement of the Essure tubal microinserts may be associated with pelvic pain, abnormal bleeding, and even allergic reaction.
Even with our best effort to properly inform our patients as to the risks and benefits of permanent sterilization via Essure tubal microinserts, secondary to undesired side effects, patients desire their removal. This can be a challenging endeavor for the practitioner, especially if the women is not interested in hysterectomy.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago; director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. He reported having no financial disclosures related to this column.
Despite recent negative lay press and a boxed safety warning from the Food and Drug Administration, Essure tubal microinserts continue to be a popular method of permanent contraception. It is imperative for patients to understand that this method of contraception cannot be reversed, and thereafter, the only method to achieve pregnancy would be via in vitro fertilization. Furthermore, preoperatively, patients must be counseled that placement of the Essure tubal microinserts may be associated with pelvic pain, abnormal bleeding, and even allergic reaction.
Even with our best effort to properly inform our patients as to the risks and benefits of permanent sterilization via Essure tubal microinserts, secondary to undesired side effects, patients desire their removal. This can be a challenging endeavor for the practitioner, especially if the women is not interested in hysterectomy.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago; director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. He reported having no financial disclosures related to this column.
Evolving practice in perinatal psychopharmacology: Lessons learned
Over the last 2 decades, there has been a growing interest in establishing a rich evidence base for treatment of psychiatric illness in pregnancy and the postpartum period. It seems as if a week does not go by when we don’t find multiple publications in the scientific literature describing a new finding or confirmation or inconsistency with existing data – whether it is a small prospective cohort study or an elegant analysis of a large administrative database. The goals of these reports center on refining our knowledge of safe treatments for perinatal psychiatric disorders.
Despite these strides, my colleagues and I frequently see a divergence between recommendations in the literature and what is done clinically by those who treat women around reproductive associated psychiatric disturbance – premenstrual dysphoric disorder or psychiatric disorder during pregnancy and the postpartum period. In some cases, scientific evidence has not filtered into day to day practice and some physicians continue to follow practices that, while outdated, make intuitive sense. In other clinical situations, limited evidence is being applied too broadly or data are too sparse to clearly inform practice. Regardless of the reason, we frequently see patients in clinical situations in which we are forced to rethink the clinical rationale for advice they have received or the clinical path taken.
Here is a sample of the clinical scenarios in which we have seen inconsistencies between current practice and the best evidence in perinatal psychiatry or situations in which data are too sparse to inform the clearest clinical path.
1. Discontinuation of antidepressants proximate to conception
Despite multiple studies supporting the high risk for relapse of major depression in women on maintenance antidepressant therapy with a history of recurrent depressive illness, it is still quite common for clinicians to routinely advise women to stop antidepressants while planning a pregnancy or after documentation of a pregnancy, regardless of the severity of the underlying illness. This runs counter to data showing high rates of relapse in women who stop antidepressants proximate to conception, the safety of antidepressants in pregnancy, and the harm to the mother and fetus when depression during pregnancy is untreated.
2. Use of a lower dose of antidepressants during pregnancy
It makes intuitive sense to use the smallest dose of medicine like antidepressant during pregnancy. However, multiple studies show that, at least in nonpregnant patients, the dose that gets patients well is typically the dose that keeps them well. One of the quickest paths to relapse in depression is a reduction in the antidepressant dose after someone has gotten well. This is even more relevant in pregnant patients because pregnancy itself dilutes the plasma level of the antidepressant given the rapidly expanding plasma volume seen in pregnancy. One can debate whether clinicians should empirically increase the dose of antidepressant during pregnancy to sustain plasma level of medication, but lowering the dose of this medication proximate or during pregnancy makes little sense.
3. A switch to sertraline in pregnancy/post partum
Another scenario that my colleagues and I often see is a pregnant patient whose depression was previously well controlled with a particular antidepressant, but whose physician, once she decides to conceive or becomes pregnant, switched her to sertraline.
The idea, which has been around for a long time, is that sertraline is the safest antidepressant for pregnant women because it has robust reproductive safety data and has particularly modest amounts of medication (if detected at all) in the plasma of infants of mothers who breastfeed while using the medicine. While we certainly have more safety data on SSRIs that were manufactured earlier, as compared with antidepressants that became available later, we have now accumulated data that fails to demonstrate a clear signal for teratogenicity across many antidepressants manufactured over the last 2 decades. Identifying an antidepressant for a given patient to which she will respond can be a challenging course for the patient. Achieving euthymia and subsequently switching to sertraline or another medication may only put her at risk for recurrence of depression and its attendant morbidity.
4. A change to a Category B label drug
This is another example of switching a patient to a potentially less effective drug in a somewhat misguided effort at finding a treatment that is safer in pregnancy. While the Food and Drug Administration’s drug category label system was a step forward, or at least a well intentioned effort to give women and their clinicians clearer insight into the reproductive safety of a medication, ultimately, the incomplete nature of the information caused the agency to transition to a new system (see the Pregnancy Labeling and Lactation Rule). Switching a woman to a category B medicine with sparse reproductive safety data instead of a category C medicine, which may not be unsafe but has raised some concerns in animal models, is not a better choice. The new labeling system is a step forward.
5. Discontinuation of lithium during pregnancy
Like discontinuation of antidepressants, the discontinuation of lithium during attempts to conceive in a woman whose illness has been well controlled, is associated with a high risk of relapse. In earlier work, it was sometimes recommended that discontinuation of lithium be considered after a long period of wellness. We have learned over time that this can be a risky move. Even women with a remote history of bipolar disorder appear to be at high risk of relapse when a mood stabilizer is stopped. Exquisite response to medicine does not imply less severe illness. Women who have bipolar disorder who have sustained euthymia on lithium should consider maintaining the safest possible regimen before, during, and after pregnancy despite the known small teratogenic risk associated with fetal exposure to this agent.
6. Try supplements or alternative therapies
Out of a desire to avoid any medication with incomplete reproductive safety data, some women and clinicians make the intuitive leap that “alternative treatments” can mitigate relapse in pregnancy, and they stop pharmacological treatments and switch to supplements or alternative therapies, including acupuncture, massage, or light therapy. Unfortunately, data supporting this clinical maneuver are sparse. Frequently, we see women with past histories of severe depression who have stopped antidepressants and who have started supplements as a substitute and who then relapse. Then, they try to restore euthymia with antidepressants and psychotherapy, and the road to restoration of well-being can be long.
Data on efficacy of alternative therapies continues to evolve and is an exciting and important area of research. However, where these treatments are best employed in the algorithm for treating depression in pregnancy, or at other times, has yet to be adequately defined.
7. Stop breastfeeding or defer antidepressant treatment
Many women continue to be counseled to either stop breastfeeding while using antidepressants or to defer treatment with antidepressants if they wish to breastfeed. Not uncommonly, we see women who are suffering from postpartum depression and who are engaged in psychotherapy but who have deferred treatment with antidepressants despite residual depressive symptoms that impair functioning. Clinicians should keep in mind that data supporting evidence of toxicity in newborns of women using antidepressants while breastfeeding are extremely sparse. Unfortunately, some women with postpartum depression are deferring treatment because they were counseled that it is not compatible with their desire to breastfeed.
8. Use of non-benzodiazepine sedative-hypnotics
Insomnia is a common problem in pregnancy, especially when coupled with comorbid anxiety, and, increasingly, it is being treated with non-benzodiazepine sedative-hypnotics. Clinicians should keep in mind that a known small risk may be better than an unknown risk. If a pregnant woman has severe insomnia, she may benefit from a low dose of a benzodiazepine, such as lorazepam or clonazepam, as opposed to a medication such as zolpidem for which reproductive safety data are particularly limited.
9. Pumping and dumping breast milk
Many women are advised to set an alarm to “pump and dump” their breast milk to minimize their baby’s exposure to antidepressants during breastfeeding. Early literature to pump and dump breast milk at peak antidepressant concentration was of great analytic and theoretical interest but has scant clinical application. As an author of many of those early publications, I can say that we never intended for women to sacrifice precious sleep to dump breast milk with the idea that limiting exposure to trace amounts of antidepressants would have beneficial effects over the long term.
10. Failure to bring up contraception use
We continue to see a 50% unplanned pregnancy rate across sociodemographic groups in the United States. This is a critical statistic because it may affect how treatment is managed. Bringing up the topic of reliable contraception prior to pregnancy allows for planned pregnancy and affords us the time to discuss treatment options and the ability to plot a more thoughtful and safe clinical course. However, often, contraception is not discussed.
One of our goals as clinicians is to, first, do no harm and that continues to be a challenge because the data in perinatal psychiatry is still inconsistent in some areas and there are evidence gaps in others. Nevertheless, our task is to take the best available data along with the patient’s wishes and knowledge of her past clinical history and to then translate that into the best care for the individual.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications.
Over the last 2 decades, there has been a growing interest in establishing a rich evidence base for treatment of psychiatric illness in pregnancy and the postpartum period. It seems as if a week does not go by when we don’t find multiple publications in the scientific literature describing a new finding or confirmation or inconsistency with existing data – whether it is a small prospective cohort study or an elegant analysis of a large administrative database. The goals of these reports center on refining our knowledge of safe treatments for perinatal psychiatric disorders.
Despite these strides, my colleagues and I frequently see a divergence between recommendations in the literature and what is done clinically by those who treat women around reproductive associated psychiatric disturbance – premenstrual dysphoric disorder or psychiatric disorder during pregnancy and the postpartum period. In some cases, scientific evidence has not filtered into day to day practice and some physicians continue to follow practices that, while outdated, make intuitive sense. In other clinical situations, limited evidence is being applied too broadly or data are too sparse to clearly inform practice. Regardless of the reason, we frequently see patients in clinical situations in which we are forced to rethink the clinical rationale for advice they have received or the clinical path taken.
Here is a sample of the clinical scenarios in which we have seen inconsistencies between current practice and the best evidence in perinatal psychiatry or situations in which data are too sparse to inform the clearest clinical path.
1. Discontinuation of antidepressants proximate to conception
Despite multiple studies supporting the high risk for relapse of major depression in women on maintenance antidepressant therapy with a history of recurrent depressive illness, it is still quite common for clinicians to routinely advise women to stop antidepressants while planning a pregnancy or after documentation of a pregnancy, regardless of the severity of the underlying illness. This runs counter to data showing high rates of relapse in women who stop antidepressants proximate to conception, the safety of antidepressants in pregnancy, and the harm to the mother and fetus when depression during pregnancy is untreated.
2. Use of a lower dose of antidepressants during pregnancy
It makes intuitive sense to use the smallest dose of medicine like antidepressant during pregnancy. However, multiple studies show that, at least in nonpregnant patients, the dose that gets patients well is typically the dose that keeps them well. One of the quickest paths to relapse in depression is a reduction in the antidepressant dose after someone has gotten well. This is even more relevant in pregnant patients because pregnancy itself dilutes the plasma level of the antidepressant given the rapidly expanding plasma volume seen in pregnancy. One can debate whether clinicians should empirically increase the dose of antidepressant during pregnancy to sustain plasma level of medication, but lowering the dose of this medication proximate or during pregnancy makes little sense.
3. A switch to sertraline in pregnancy/post partum
Another scenario that my colleagues and I often see is a pregnant patient whose depression was previously well controlled with a particular antidepressant, but whose physician, once she decides to conceive or becomes pregnant, switched her to sertraline.
The idea, which has been around for a long time, is that sertraline is the safest antidepressant for pregnant women because it has robust reproductive safety data and has particularly modest amounts of medication (if detected at all) in the plasma of infants of mothers who breastfeed while using the medicine. While we certainly have more safety data on SSRIs that were manufactured earlier, as compared with antidepressants that became available later, we have now accumulated data that fails to demonstrate a clear signal for teratogenicity across many antidepressants manufactured over the last 2 decades. Identifying an antidepressant for a given patient to which she will respond can be a challenging course for the patient. Achieving euthymia and subsequently switching to sertraline or another medication may only put her at risk for recurrence of depression and its attendant morbidity.
4. A change to a Category B label drug
This is another example of switching a patient to a potentially less effective drug in a somewhat misguided effort at finding a treatment that is safer in pregnancy. While the Food and Drug Administration’s drug category label system was a step forward, or at least a well intentioned effort to give women and their clinicians clearer insight into the reproductive safety of a medication, ultimately, the incomplete nature of the information caused the agency to transition to a new system (see the Pregnancy Labeling and Lactation Rule). Switching a woman to a category B medicine with sparse reproductive safety data instead of a category C medicine, which may not be unsafe but has raised some concerns in animal models, is not a better choice. The new labeling system is a step forward.
5. Discontinuation of lithium during pregnancy
Like discontinuation of antidepressants, the discontinuation of lithium during attempts to conceive in a woman whose illness has been well controlled, is associated with a high risk of relapse. In earlier work, it was sometimes recommended that discontinuation of lithium be considered after a long period of wellness. We have learned over time that this can be a risky move. Even women with a remote history of bipolar disorder appear to be at high risk of relapse when a mood stabilizer is stopped. Exquisite response to medicine does not imply less severe illness. Women who have bipolar disorder who have sustained euthymia on lithium should consider maintaining the safest possible regimen before, during, and after pregnancy despite the known small teratogenic risk associated with fetal exposure to this agent.
6. Try supplements or alternative therapies
Out of a desire to avoid any medication with incomplete reproductive safety data, some women and clinicians make the intuitive leap that “alternative treatments” can mitigate relapse in pregnancy, and they stop pharmacological treatments and switch to supplements or alternative therapies, including acupuncture, massage, or light therapy. Unfortunately, data supporting this clinical maneuver are sparse. Frequently, we see women with past histories of severe depression who have stopped antidepressants and who have started supplements as a substitute and who then relapse. Then, they try to restore euthymia with antidepressants and psychotherapy, and the road to restoration of well-being can be long.
Data on efficacy of alternative therapies continues to evolve and is an exciting and important area of research. However, where these treatments are best employed in the algorithm for treating depression in pregnancy, or at other times, has yet to be adequately defined.
7. Stop breastfeeding or defer antidepressant treatment
Many women continue to be counseled to either stop breastfeeding while using antidepressants or to defer treatment with antidepressants if they wish to breastfeed. Not uncommonly, we see women who are suffering from postpartum depression and who are engaged in psychotherapy but who have deferred treatment with antidepressants despite residual depressive symptoms that impair functioning. Clinicians should keep in mind that data supporting evidence of toxicity in newborns of women using antidepressants while breastfeeding are extremely sparse. Unfortunately, some women with postpartum depression are deferring treatment because they were counseled that it is not compatible with their desire to breastfeed.
8. Use of non-benzodiazepine sedative-hypnotics
Insomnia is a common problem in pregnancy, especially when coupled with comorbid anxiety, and, increasingly, it is being treated with non-benzodiazepine sedative-hypnotics. Clinicians should keep in mind that a known small risk may be better than an unknown risk. If a pregnant woman has severe insomnia, she may benefit from a low dose of a benzodiazepine, such as lorazepam or clonazepam, as opposed to a medication such as zolpidem for which reproductive safety data are particularly limited.
9. Pumping and dumping breast milk
Many women are advised to set an alarm to “pump and dump” their breast milk to minimize their baby’s exposure to antidepressants during breastfeeding. Early literature to pump and dump breast milk at peak antidepressant concentration was of great analytic and theoretical interest but has scant clinical application. As an author of many of those early publications, I can say that we never intended for women to sacrifice precious sleep to dump breast milk with the idea that limiting exposure to trace amounts of antidepressants would have beneficial effects over the long term.
10. Failure to bring up contraception use
We continue to see a 50% unplanned pregnancy rate across sociodemographic groups in the United States. This is a critical statistic because it may affect how treatment is managed. Bringing up the topic of reliable contraception prior to pregnancy allows for planned pregnancy and affords us the time to discuss treatment options and the ability to plot a more thoughtful and safe clinical course. However, often, contraception is not discussed.
One of our goals as clinicians is to, first, do no harm and that continues to be a challenge because the data in perinatal psychiatry is still inconsistent in some areas and there are evidence gaps in others. Nevertheless, our task is to take the best available data along with the patient’s wishes and knowledge of her past clinical history and to then translate that into the best care for the individual.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications.
Over the last 2 decades, there has been a growing interest in establishing a rich evidence base for treatment of psychiatric illness in pregnancy and the postpartum period. It seems as if a week does not go by when we don’t find multiple publications in the scientific literature describing a new finding or confirmation or inconsistency with existing data – whether it is a small prospective cohort study or an elegant analysis of a large administrative database. The goals of these reports center on refining our knowledge of safe treatments for perinatal psychiatric disorders.
Despite these strides, my colleagues and I frequently see a divergence between recommendations in the literature and what is done clinically by those who treat women around reproductive associated psychiatric disturbance – premenstrual dysphoric disorder or psychiatric disorder during pregnancy and the postpartum period. In some cases, scientific evidence has not filtered into day to day practice and some physicians continue to follow practices that, while outdated, make intuitive sense. In other clinical situations, limited evidence is being applied too broadly or data are too sparse to clearly inform practice. Regardless of the reason, we frequently see patients in clinical situations in which we are forced to rethink the clinical rationale for advice they have received or the clinical path taken.
Here is a sample of the clinical scenarios in which we have seen inconsistencies between current practice and the best evidence in perinatal psychiatry or situations in which data are too sparse to inform the clearest clinical path.
1. Discontinuation of antidepressants proximate to conception
Despite multiple studies supporting the high risk for relapse of major depression in women on maintenance antidepressant therapy with a history of recurrent depressive illness, it is still quite common for clinicians to routinely advise women to stop antidepressants while planning a pregnancy or after documentation of a pregnancy, regardless of the severity of the underlying illness. This runs counter to data showing high rates of relapse in women who stop antidepressants proximate to conception, the safety of antidepressants in pregnancy, and the harm to the mother and fetus when depression during pregnancy is untreated.
2. Use of a lower dose of antidepressants during pregnancy
It makes intuitive sense to use the smallest dose of medicine like antidepressant during pregnancy. However, multiple studies show that, at least in nonpregnant patients, the dose that gets patients well is typically the dose that keeps them well. One of the quickest paths to relapse in depression is a reduction in the antidepressant dose after someone has gotten well. This is even more relevant in pregnant patients because pregnancy itself dilutes the plasma level of the antidepressant given the rapidly expanding plasma volume seen in pregnancy. One can debate whether clinicians should empirically increase the dose of antidepressant during pregnancy to sustain plasma level of medication, but lowering the dose of this medication proximate or during pregnancy makes little sense.
3. A switch to sertraline in pregnancy/post partum
Another scenario that my colleagues and I often see is a pregnant patient whose depression was previously well controlled with a particular antidepressant, but whose physician, once she decides to conceive or becomes pregnant, switched her to sertraline.
The idea, which has been around for a long time, is that sertraline is the safest antidepressant for pregnant women because it has robust reproductive safety data and has particularly modest amounts of medication (if detected at all) in the plasma of infants of mothers who breastfeed while using the medicine. While we certainly have more safety data on SSRIs that were manufactured earlier, as compared with antidepressants that became available later, we have now accumulated data that fails to demonstrate a clear signal for teratogenicity across many antidepressants manufactured over the last 2 decades. Identifying an antidepressant for a given patient to which she will respond can be a challenging course for the patient. Achieving euthymia and subsequently switching to sertraline or another medication may only put her at risk for recurrence of depression and its attendant morbidity.
4. A change to a Category B label drug
This is another example of switching a patient to a potentially less effective drug in a somewhat misguided effort at finding a treatment that is safer in pregnancy. While the Food and Drug Administration’s drug category label system was a step forward, or at least a well intentioned effort to give women and their clinicians clearer insight into the reproductive safety of a medication, ultimately, the incomplete nature of the information caused the agency to transition to a new system (see the Pregnancy Labeling and Lactation Rule). Switching a woman to a category B medicine with sparse reproductive safety data instead of a category C medicine, which may not be unsafe but has raised some concerns in animal models, is not a better choice. The new labeling system is a step forward.
5. Discontinuation of lithium during pregnancy
Like discontinuation of antidepressants, the discontinuation of lithium during attempts to conceive in a woman whose illness has been well controlled, is associated with a high risk of relapse. In earlier work, it was sometimes recommended that discontinuation of lithium be considered after a long period of wellness. We have learned over time that this can be a risky move. Even women with a remote history of bipolar disorder appear to be at high risk of relapse when a mood stabilizer is stopped. Exquisite response to medicine does not imply less severe illness. Women who have bipolar disorder who have sustained euthymia on lithium should consider maintaining the safest possible regimen before, during, and after pregnancy despite the known small teratogenic risk associated with fetal exposure to this agent.
6. Try supplements or alternative therapies
Out of a desire to avoid any medication with incomplete reproductive safety data, some women and clinicians make the intuitive leap that “alternative treatments” can mitigate relapse in pregnancy, and they stop pharmacological treatments and switch to supplements or alternative therapies, including acupuncture, massage, or light therapy. Unfortunately, data supporting this clinical maneuver are sparse. Frequently, we see women with past histories of severe depression who have stopped antidepressants and who have started supplements as a substitute and who then relapse. Then, they try to restore euthymia with antidepressants and psychotherapy, and the road to restoration of well-being can be long.
Data on efficacy of alternative therapies continues to evolve and is an exciting and important area of research. However, where these treatments are best employed in the algorithm for treating depression in pregnancy, or at other times, has yet to be adequately defined.
7. Stop breastfeeding or defer antidepressant treatment
Many women continue to be counseled to either stop breastfeeding while using antidepressants or to defer treatment with antidepressants if they wish to breastfeed. Not uncommonly, we see women who are suffering from postpartum depression and who are engaged in psychotherapy but who have deferred treatment with antidepressants despite residual depressive symptoms that impair functioning. Clinicians should keep in mind that data supporting evidence of toxicity in newborns of women using antidepressants while breastfeeding are extremely sparse. Unfortunately, some women with postpartum depression are deferring treatment because they were counseled that it is not compatible with their desire to breastfeed.
8. Use of non-benzodiazepine sedative-hypnotics
Insomnia is a common problem in pregnancy, especially when coupled with comorbid anxiety, and, increasingly, it is being treated with non-benzodiazepine sedative-hypnotics. Clinicians should keep in mind that a known small risk may be better than an unknown risk. If a pregnant woman has severe insomnia, she may benefit from a low dose of a benzodiazepine, such as lorazepam or clonazepam, as opposed to a medication such as zolpidem for which reproductive safety data are particularly limited.
9. Pumping and dumping breast milk
Many women are advised to set an alarm to “pump and dump” their breast milk to minimize their baby’s exposure to antidepressants during breastfeeding. Early literature to pump and dump breast milk at peak antidepressant concentration was of great analytic and theoretical interest but has scant clinical application. As an author of many of those early publications, I can say that we never intended for women to sacrifice precious sleep to dump breast milk with the idea that limiting exposure to trace amounts of antidepressants would have beneficial effects over the long term.
10. Failure to bring up contraception use
We continue to see a 50% unplanned pregnancy rate across sociodemographic groups in the United States. This is a critical statistic because it may affect how treatment is managed. Bringing up the topic of reliable contraception prior to pregnancy allows for planned pregnancy and affords us the time to discuss treatment options and the ability to plot a more thoughtful and safe clinical course. However, often, contraception is not discussed.
One of our goals as clinicians is to, first, do no harm and that continues to be a challenge because the data in perinatal psychiatry is still inconsistent in some areas and there are evidence gaps in others. Nevertheless, our task is to take the best available data along with the patient’s wishes and knowledge of her past clinical history and to then translate that into the best care for the individual.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications.