User login
Understanding the human papillomavirus
Human papillomavirus (HPV) is the most prevalent sexually transmitted disease globally. It is causally related to the development of several malignancies, including cervical, anal, and oropharyngeal ones, because of its integration and dysregulation of the genome of infected cells. Fortunately, vaccination is available to prevent development of HPV-related diseases. Understanding this virus, its carcinogenic role, and the importance of prevention through vaccination are critically important for ob.gyns. This column reviews the fundamentals of HPV biology, epidemiology, and carcinogenesis.
Viral anatomy
HPV are members of the A genus of the family Papovaviridae. They contain between 7,800 and 7,900 base pairs. They are nonenveloped, double-stranded DNA viruses with a circular structure. The viral DNA is contained within an icosahedral capsid that measures 45 nm-55 nm. The HPV genome has three critical regions: the long control region, otherwise known as the upstream regulatory region; the early region; and the late region.1
Capsid proteins are similar between groups. Therefore, HPV are categorized into “types” and “subtypes” based on the extent of DNA similarity. There are more than 100 types of HPV in humans.2 The type of HPV is determined by the gene sequences of E6/E7 and L1 and must show more than 10% difference between types. The gene sequences between different subtypes differ by 2%-5%.
Epidemiology of HPV infection
HPV are widely distributed among mammalian species but are species specific. Their tissue affinity varies by type. HPV types 1, 2, and 4 cause common or plantar warts. HPV types 6 and 11 cause condyloma acuminata (genital warts) and low grade dysplasia. HPV types 16 and 18 – in addition to 31 and 52 – are of particular interest to oncologists because they are associated with lower genital tract high grade dysplasia and invasive carcinoma. Infection with HPV 16 is present in about half of invasive cervical cancers, with HPV type 18 present in 20% of cervical cancers. Adenocarcinomas of the cervix are more commonly associated with HPV 18. Anal cancer and oropharyngeal cancer are more commonly associated with HPV 16.3
HPV infections are acquired through cutaneous touching (including hand to genital) and HPV positivity is most commonly present within the first 10 years after sexual debut.4 However, most individuals who acquire HPV do so as a transient infection, which is cleared without sequelae. Those who fail to rapidly clear HPV infection, and in whom it becomes chronic, face an increasing risk of development of dysplasia and invasive carcinoma. The incidence of HPV infection increases again at menopause, but, for these older women, the new finding of HPV detection may be related to reactivation of an earlier infection rather than exclusively new exposure to the virus.5
Diagnosis and testing
HPV infection can be detected through DNA testing, RNA testing, and cellular markers.6
HPV DNA testing was the original form of testing offered. It improved the sensitivity over cytology alone in the detection of precursors to malignancy but had relatively poor specificity, resulting in a high false positive rate and unnecessary referral to colposcopy. The various tests approved by the Food and Drug Administration – Hybrid Capture 2 (HC2), Cervista, and Cobas 4800 – differ in the number and nature of HPV types that they detect.
HPV RNA testing has developed and involves measuring the expression of E6 and E7 RNA. This testing is FDA approved and has the potential to improve upon the specificity of DNA testing procedures by decreasing false positives.
Measurement of cellular markers is currently considered experimental/exploratory and is not yet FDA approved for diagnostic purposes in screening or confirmation of HPV infection or coexisting dysplasia. It involves measuring the downstream cellular targets (such as p16) of E6 or E7 activity.
The mechanism of carcinogenesis
The early region of the HPV genome is downstream from the upstream regulatory region. It codes for proteins involved in viral infection and replication. The two most important genes in the early region are E6 and E7. When integrated into the human genome of the lower genital tract cell, the viral genes E6 and E7 negatively interfere with cell cycle control and mechanisms to halt dysregulation.7
E6 and E7 are considered oncogenes because they cause loss of function of the critical tumor suppressor proteins p53 and the retinoblastoma protein. The p53 protein is typically responsible for controlling cell cycling through the G0/G1 to S phases. It involves stalling cellular mitosis in order to facilitate DNA repair mechanisms in the case of damaged cells, thereby preventing replication of DNA aberrations. The retinoblastoma protein also functions to inhibit cells that have acquired DNA damage from cycling and induces apoptosis in DNA damaged cells. When protein products of E6 and E7 negatively interact with these two tumor suppressor proteins they overcome the cell’s safeguard arrest response.
In the presence of other carcinogens, such as products of tobacco exposure, the increased DNA damage sustained by the genital tract cell is allowed to go relatively unchecked by the HPV coinfection, which has disabled tumor suppressor function. This facilitates immortality of the damaged cell, amplification of additional DNA mutations, and unchecked cellular growth and dysplastic transformation. E6 and E7 are strongly expressed in invasive genital tract lesions to support its important role in carcinogenesis.
HIV coinfection is another factor that promotes carcinogenesis following HPV infection because it inhibits clearance of the virus through T-cell mediated immunosuppression and directly enhances expression of E6 and E7 proteins in the HIV and HPV coinfected cell.8 For these reasons, HIV-positive women are less likely to clear HPV infection and more likely to develop high grade dysplasia or invasive carcinomas.
Prevention and vaccination
HPV vaccinations utilize virus-like particles (VLPs). These VLPs are capsid particles generated from the L1 region of the HPV DNA. The capsid proteins coded for by L1 are highly immunogenic. VLPs are recombinant proteins created in benign biologic systems (such as yeast) and contain no inner DNA core (effectively empty viral capsids) and therefore are not infectious. The L1 gene is incorporated into a plasmid, which is inserted into the nucleus of a eukaryotic cell. Transcription and translation of the L1 gene takes place, creating capsid proteins that self-assemble into VLPs. These VLPs are retrieved and inoculated into candidate patients to illicit an immune response.
Quadrivalent, nine-valent, and bivalent vaccines are available worldwide. However, only the nine-valent vaccine – protective against types 6, 11, 16, 18, 31, 33, 45, 52, and 58 – is available in the United States. This theoretically provides more comprehensive coverage against cervical cancer–causing HPV types, as 70% of cervical cancer is attributable to HPV 16 and 18, but an additional 20% is attributable to HPV 31, 33, 45, 52, and 58. This vaccine also provides protection against the HPV strains that cause genital warts and low-grade dysplastic changes.9
HPV, in most instances, is a transient virus with no sequelae. However, if not cleared from the cells of the lower genital tract, anus, or oropharynx it can result in the breakdown of cellular correction strategies and culminate in invasive carcinoma. Fortunately, highly effective and safe vaccinations are available and should be broadly prescribed.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no relevant financial disclosures.
References
1. Cancer Epidemiol Biomarkers Prev. 1995 Jun;4(4):415-28.
2. Gynecol Oncol. 2011 Apr;121(1):32-42.
3. Cancer Epidemiol Biomarkers Prev. 2008 Jul;17(7):1611-22.
4. JAMA. 2007 Feb 28;297(8):813-9.
5. J Infect Dis. 2013; 207(2): 272-80.
6. J Natl Cancer Inst. 2011 Mar 2;103(5):368-83.
7. J Natl Cancer Inst. 2000 May 3;92(9):690-8.
8. Lancet. 2002 Jan 12;359(9301):108-13.
9. Obstet Gynecol 2017;129:e173–8.
Human papillomavirus (HPV) is the most prevalent sexually transmitted disease globally. It is causally related to the development of several malignancies, including cervical, anal, and oropharyngeal ones, because of its integration and dysregulation of the genome of infected cells. Fortunately, vaccination is available to prevent development of HPV-related diseases. Understanding this virus, its carcinogenic role, and the importance of prevention through vaccination are critically important for ob.gyns. This column reviews the fundamentals of HPV biology, epidemiology, and carcinogenesis.
Viral anatomy
HPV are members of the A genus of the family Papovaviridae. They contain between 7,800 and 7,900 base pairs. They are nonenveloped, double-stranded DNA viruses with a circular structure. The viral DNA is contained within an icosahedral capsid that measures 45 nm-55 nm. The HPV genome has three critical regions: the long control region, otherwise known as the upstream regulatory region; the early region; and the late region.1
Capsid proteins are similar between groups. Therefore, HPV are categorized into “types” and “subtypes” based on the extent of DNA similarity. There are more than 100 types of HPV in humans.2 The type of HPV is determined by the gene sequences of E6/E7 and L1 and must show more than 10% difference between types. The gene sequences between different subtypes differ by 2%-5%.
Epidemiology of HPV infection
HPV are widely distributed among mammalian species but are species specific. Their tissue affinity varies by type. HPV types 1, 2, and 4 cause common or plantar warts. HPV types 6 and 11 cause condyloma acuminata (genital warts) and low grade dysplasia. HPV types 16 and 18 – in addition to 31 and 52 – are of particular interest to oncologists because they are associated with lower genital tract high grade dysplasia and invasive carcinoma. Infection with HPV 16 is present in about half of invasive cervical cancers, with HPV type 18 present in 20% of cervical cancers. Adenocarcinomas of the cervix are more commonly associated with HPV 18. Anal cancer and oropharyngeal cancer are more commonly associated with HPV 16.3
HPV infections are acquired through cutaneous touching (including hand to genital) and HPV positivity is most commonly present within the first 10 years after sexual debut.4 However, most individuals who acquire HPV do so as a transient infection, which is cleared without sequelae. Those who fail to rapidly clear HPV infection, and in whom it becomes chronic, face an increasing risk of development of dysplasia and invasive carcinoma. The incidence of HPV infection increases again at menopause, but, for these older women, the new finding of HPV detection may be related to reactivation of an earlier infection rather than exclusively new exposure to the virus.5
Diagnosis and testing
HPV infection can be detected through DNA testing, RNA testing, and cellular markers.6
HPV DNA testing was the original form of testing offered. It improved the sensitivity over cytology alone in the detection of precursors to malignancy but had relatively poor specificity, resulting in a high false positive rate and unnecessary referral to colposcopy. The various tests approved by the Food and Drug Administration – Hybrid Capture 2 (HC2), Cervista, and Cobas 4800 – differ in the number and nature of HPV types that they detect.
HPV RNA testing has developed and involves measuring the expression of E6 and E7 RNA. This testing is FDA approved and has the potential to improve upon the specificity of DNA testing procedures by decreasing false positives.
Measurement of cellular markers is currently considered experimental/exploratory and is not yet FDA approved for diagnostic purposes in screening or confirmation of HPV infection or coexisting dysplasia. It involves measuring the downstream cellular targets (such as p16) of E6 or E7 activity.
The mechanism of carcinogenesis
The early region of the HPV genome is downstream from the upstream regulatory region. It codes for proteins involved in viral infection and replication. The two most important genes in the early region are E6 and E7. When integrated into the human genome of the lower genital tract cell, the viral genes E6 and E7 negatively interfere with cell cycle control and mechanisms to halt dysregulation.7
E6 and E7 are considered oncogenes because they cause loss of function of the critical tumor suppressor proteins p53 and the retinoblastoma protein. The p53 protein is typically responsible for controlling cell cycling through the G0/G1 to S phases. It involves stalling cellular mitosis in order to facilitate DNA repair mechanisms in the case of damaged cells, thereby preventing replication of DNA aberrations. The retinoblastoma protein also functions to inhibit cells that have acquired DNA damage from cycling and induces apoptosis in DNA damaged cells. When protein products of E6 and E7 negatively interact with these two tumor suppressor proteins they overcome the cell’s safeguard arrest response.
In the presence of other carcinogens, such as products of tobacco exposure, the increased DNA damage sustained by the genital tract cell is allowed to go relatively unchecked by the HPV coinfection, which has disabled tumor suppressor function. This facilitates immortality of the damaged cell, amplification of additional DNA mutations, and unchecked cellular growth and dysplastic transformation. E6 and E7 are strongly expressed in invasive genital tract lesions to support its important role in carcinogenesis.
HIV coinfection is another factor that promotes carcinogenesis following HPV infection because it inhibits clearance of the virus through T-cell mediated immunosuppression and directly enhances expression of E6 and E7 proteins in the HIV and HPV coinfected cell.8 For these reasons, HIV-positive women are less likely to clear HPV infection and more likely to develop high grade dysplasia or invasive carcinomas.
Prevention and vaccination
HPV vaccinations utilize virus-like particles (VLPs). These VLPs are capsid particles generated from the L1 region of the HPV DNA. The capsid proteins coded for by L1 are highly immunogenic. VLPs are recombinant proteins created in benign biologic systems (such as yeast) and contain no inner DNA core (effectively empty viral capsids) and therefore are not infectious. The L1 gene is incorporated into a plasmid, which is inserted into the nucleus of a eukaryotic cell. Transcription and translation of the L1 gene takes place, creating capsid proteins that self-assemble into VLPs. These VLPs are retrieved and inoculated into candidate patients to illicit an immune response.
Quadrivalent, nine-valent, and bivalent vaccines are available worldwide. However, only the nine-valent vaccine – protective against types 6, 11, 16, 18, 31, 33, 45, 52, and 58 – is available in the United States. This theoretically provides more comprehensive coverage against cervical cancer–causing HPV types, as 70% of cervical cancer is attributable to HPV 16 and 18, but an additional 20% is attributable to HPV 31, 33, 45, 52, and 58. This vaccine also provides protection against the HPV strains that cause genital warts and low-grade dysplastic changes.9
HPV, in most instances, is a transient virus with no sequelae. However, if not cleared from the cells of the lower genital tract, anus, or oropharynx it can result in the breakdown of cellular correction strategies and culminate in invasive carcinoma. Fortunately, highly effective and safe vaccinations are available and should be broadly prescribed.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no relevant financial disclosures.
References
1. Cancer Epidemiol Biomarkers Prev. 1995 Jun;4(4):415-28.
2. Gynecol Oncol. 2011 Apr;121(1):32-42.
3. Cancer Epidemiol Biomarkers Prev. 2008 Jul;17(7):1611-22.
4. JAMA. 2007 Feb 28;297(8):813-9.
5. J Infect Dis. 2013; 207(2): 272-80.
6. J Natl Cancer Inst. 2011 Mar 2;103(5):368-83.
7. J Natl Cancer Inst. 2000 May 3;92(9):690-8.
8. Lancet. 2002 Jan 12;359(9301):108-13.
9. Obstet Gynecol 2017;129:e173–8.
Human papillomavirus (HPV) is the most prevalent sexually transmitted disease globally. It is causally related to the development of several malignancies, including cervical, anal, and oropharyngeal ones, because of its integration and dysregulation of the genome of infected cells. Fortunately, vaccination is available to prevent development of HPV-related diseases. Understanding this virus, its carcinogenic role, and the importance of prevention through vaccination are critically important for ob.gyns. This column reviews the fundamentals of HPV biology, epidemiology, and carcinogenesis.
Viral anatomy
HPV are members of the A genus of the family Papovaviridae. They contain between 7,800 and 7,900 base pairs. They are nonenveloped, double-stranded DNA viruses with a circular structure. The viral DNA is contained within an icosahedral capsid that measures 45 nm-55 nm. The HPV genome has three critical regions: the long control region, otherwise known as the upstream regulatory region; the early region; and the late region.1
Capsid proteins are similar between groups. Therefore, HPV are categorized into “types” and “subtypes” based on the extent of DNA similarity. There are more than 100 types of HPV in humans.2 The type of HPV is determined by the gene sequences of E6/E7 and L1 and must show more than 10% difference between types. The gene sequences between different subtypes differ by 2%-5%.
Epidemiology of HPV infection
HPV are widely distributed among mammalian species but are species specific. Their tissue affinity varies by type. HPV types 1, 2, and 4 cause common or plantar warts. HPV types 6 and 11 cause condyloma acuminata (genital warts) and low grade dysplasia. HPV types 16 and 18 – in addition to 31 and 52 – are of particular interest to oncologists because they are associated with lower genital tract high grade dysplasia and invasive carcinoma. Infection with HPV 16 is present in about half of invasive cervical cancers, with HPV type 18 present in 20% of cervical cancers. Adenocarcinomas of the cervix are more commonly associated with HPV 18. Anal cancer and oropharyngeal cancer are more commonly associated with HPV 16.3
HPV infections are acquired through cutaneous touching (including hand to genital) and HPV positivity is most commonly present within the first 10 years after sexual debut.4 However, most individuals who acquire HPV do so as a transient infection, which is cleared without sequelae. Those who fail to rapidly clear HPV infection, and in whom it becomes chronic, face an increasing risk of development of dysplasia and invasive carcinoma. The incidence of HPV infection increases again at menopause, but, for these older women, the new finding of HPV detection may be related to reactivation of an earlier infection rather than exclusively new exposure to the virus.5
Diagnosis and testing
HPV infection can be detected through DNA testing, RNA testing, and cellular markers.6
HPV DNA testing was the original form of testing offered. It improved the sensitivity over cytology alone in the detection of precursors to malignancy but had relatively poor specificity, resulting in a high false positive rate and unnecessary referral to colposcopy. The various tests approved by the Food and Drug Administration – Hybrid Capture 2 (HC2), Cervista, and Cobas 4800 – differ in the number and nature of HPV types that they detect.
HPV RNA testing has developed and involves measuring the expression of E6 and E7 RNA. This testing is FDA approved and has the potential to improve upon the specificity of DNA testing procedures by decreasing false positives.
Measurement of cellular markers is currently considered experimental/exploratory and is not yet FDA approved for diagnostic purposes in screening or confirmation of HPV infection or coexisting dysplasia. It involves measuring the downstream cellular targets (such as p16) of E6 or E7 activity.
The mechanism of carcinogenesis
The early region of the HPV genome is downstream from the upstream regulatory region. It codes for proteins involved in viral infection and replication. The two most important genes in the early region are E6 and E7. When integrated into the human genome of the lower genital tract cell, the viral genes E6 and E7 negatively interfere with cell cycle control and mechanisms to halt dysregulation.7
E6 and E7 are considered oncogenes because they cause loss of function of the critical tumor suppressor proteins p53 and the retinoblastoma protein. The p53 protein is typically responsible for controlling cell cycling through the G0/G1 to S phases. It involves stalling cellular mitosis in order to facilitate DNA repair mechanisms in the case of damaged cells, thereby preventing replication of DNA aberrations. The retinoblastoma protein also functions to inhibit cells that have acquired DNA damage from cycling and induces apoptosis in DNA damaged cells. When protein products of E6 and E7 negatively interact with these two tumor suppressor proteins they overcome the cell’s safeguard arrest response.
In the presence of other carcinogens, such as products of tobacco exposure, the increased DNA damage sustained by the genital tract cell is allowed to go relatively unchecked by the HPV coinfection, which has disabled tumor suppressor function. This facilitates immortality of the damaged cell, amplification of additional DNA mutations, and unchecked cellular growth and dysplastic transformation. E6 and E7 are strongly expressed in invasive genital tract lesions to support its important role in carcinogenesis.
HIV coinfection is another factor that promotes carcinogenesis following HPV infection because it inhibits clearance of the virus through T-cell mediated immunosuppression and directly enhances expression of E6 and E7 proteins in the HIV and HPV coinfected cell.8 For these reasons, HIV-positive women are less likely to clear HPV infection and more likely to develop high grade dysplasia or invasive carcinomas.
Prevention and vaccination
HPV vaccinations utilize virus-like particles (VLPs). These VLPs are capsid particles generated from the L1 region of the HPV DNA. The capsid proteins coded for by L1 are highly immunogenic. VLPs are recombinant proteins created in benign biologic systems (such as yeast) and contain no inner DNA core (effectively empty viral capsids) and therefore are not infectious. The L1 gene is incorporated into a plasmid, which is inserted into the nucleus of a eukaryotic cell. Transcription and translation of the L1 gene takes place, creating capsid proteins that self-assemble into VLPs. These VLPs are retrieved and inoculated into candidate patients to illicit an immune response.
Quadrivalent, nine-valent, and bivalent vaccines are available worldwide. However, only the nine-valent vaccine – protective against types 6, 11, 16, 18, 31, 33, 45, 52, and 58 – is available in the United States. This theoretically provides more comprehensive coverage against cervical cancer–causing HPV types, as 70% of cervical cancer is attributable to HPV 16 and 18, but an additional 20% is attributable to HPV 31, 33, 45, 52, and 58. This vaccine also provides protection against the HPV strains that cause genital warts and low-grade dysplastic changes.9
HPV, in most instances, is a transient virus with no sequelae. However, if not cleared from the cells of the lower genital tract, anus, or oropharynx it can result in the breakdown of cellular correction strategies and culminate in invasive carcinoma. Fortunately, highly effective and safe vaccinations are available and should be broadly prescribed.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no relevant financial disclosures.
References
1. Cancer Epidemiol Biomarkers Prev. 1995 Jun;4(4):415-28.
2. Gynecol Oncol. 2011 Apr;121(1):32-42.
3. Cancer Epidemiol Biomarkers Prev. 2008 Jul;17(7):1611-22.
4. JAMA. 2007 Feb 28;297(8):813-9.
5. J Infect Dis. 2013; 207(2): 272-80.
6. J Natl Cancer Inst. 2011 Mar 2;103(5):368-83.
7. J Natl Cancer Inst. 2000 May 3;92(9):690-8.
8. Lancet. 2002 Jan 12;359(9301):108-13.
9. Obstet Gynecol 2017;129:e173–8.
FDA clears use of reagents to detect hematopoietic neoplasia
The US Food and Drug Administration (FDA) has allowed marketing of the ClearLLab Reagent Panel, a combination of conjugated antibody cocktails designed to aid the detection of hematopoietic neoplasia.
This includes chronic and acute leukemias, non-Hodgkin lymphoma, myeloma, myelodysplastic syndromes, and myeloproliferative neoplasms.
The ClearLLab reagents are intended for in vitro diagnostic use to identify various cell populations by immunophenotyping on an FC 500 flow cytometer.
The reagents are directed against B, T, and myeloid lineage antigens and intended to identify relevant leukocyte surface molecules.
ClearLLab provides 2 T-cell tubes, 2 B-cell tubes, and a myeloid tube, each consisting of pre-mixed 4- to 5-color cocktails. Together, this totals 18 markers as directly conjugated antibodies.
The reagents can be used with peripheral whole blood, bone marrow, and lymph node specimens.
The results obtained via testing with the ClearLLab reagents should be interpreted along with additional clinical and laboratory findings, according to Beckman Coulter, Inc., the company that will be marketing the reagents.
The FDA reviewed data for the ClearLLab reagents through the de novo premarket review pathway, a regulatory pathway for novel, low-to-moderate-risk devices that are not substantially equivalent to an already legally marketed device.
The FDA’s clearance of the ClearLLab reagents was supported by a study designed to demonstrate the reagents’ performance, which was conducted on 279 samples at 4 independent clinical sites.
Results with the ClearLLab reagents were compared to results with alternative detection methods used at the sites.
The ClearLLab results aligned with the study sites’ final diagnosis 93.4% of the time and correctly detected abnormalities 84.2% of the time.
Along with its clearance of the ClearLLab reagents, the FDA is establishing criteria, called special controls, which clarify the agency’s expectations in assuring the reagents’ accuracy, reliability, and clinical relevance.
These special controls, when met along with general controls, provide reasonable assurance of safety and effectiveness for the ClearLLab reagents and similar tools.
The special controls also describe the least burdensome regulatory pathway for future developers of similar diagnostic tests. ![]()
The US Food and Drug Administration (FDA) has allowed marketing of the ClearLLab Reagent Panel, a combination of conjugated antibody cocktails designed to aid the detection of hematopoietic neoplasia.
This includes chronic and acute leukemias, non-Hodgkin lymphoma, myeloma, myelodysplastic syndromes, and myeloproliferative neoplasms.
The ClearLLab reagents are intended for in vitro diagnostic use to identify various cell populations by immunophenotyping on an FC 500 flow cytometer.
The reagents are directed against B, T, and myeloid lineage antigens and intended to identify relevant leukocyte surface molecules.
ClearLLab provides 2 T-cell tubes, 2 B-cell tubes, and a myeloid tube, each consisting of pre-mixed 4- to 5-color cocktails. Together, this totals 18 markers as directly conjugated antibodies.
The reagents can be used with peripheral whole blood, bone marrow, and lymph node specimens.
The results obtained via testing with the ClearLLab reagents should be interpreted along with additional clinical and laboratory findings, according to Beckman Coulter, Inc., the company that will be marketing the reagents.
The FDA reviewed data for the ClearLLab reagents through the de novo premarket review pathway, a regulatory pathway for novel, low-to-moderate-risk devices that are not substantially equivalent to an already legally marketed device.
The FDA’s clearance of the ClearLLab reagents was supported by a study designed to demonstrate the reagents’ performance, which was conducted on 279 samples at 4 independent clinical sites.
Results with the ClearLLab reagents were compared to results with alternative detection methods used at the sites.
The ClearLLab results aligned with the study sites’ final diagnosis 93.4% of the time and correctly detected abnormalities 84.2% of the time.
Along with its clearance of the ClearLLab reagents, the FDA is establishing criteria, called special controls, which clarify the agency’s expectations in assuring the reagents’ accuracy, reliability, and clinical relevance.
These special controls, when met along with general controls, provide reasonable assurance of safety and effectiveness for the ClearLLab reagents and similar tools.
The special controls also describe the least burdensome regulatory pathway for future developers of similar diagnostic tests. ![]()
The US Food and Drug Administration (FDA) has allowed marketing of the ClearLLab Reagent Panel, a combination of conjugated antibody cocktails designed to aid the detection of hematopoietic neoplasia.
This includes chronic and acute leukemias, non-Hodgkin lymphoma, myeloma, myelodysplastic syndromes, and myeloproliferative neoplasms.
The ClearLLab reagents are intended for in vitro diagnostic use to identify various cell populations by immunophenotyping on an FC 500 flow cytometer.
The reagents are directed against B, T, and myeloid lineage antigens and intended to identify relevant leukocyte surface molecules.
ClearLLab provides 2 T-cell tubes, 2 B-cell tubes, and a myeloid tube, each consisting of pre-mixed 4- to 5-color cocktails. Together, this totals 18 markers as directly conjugated antibodies.
The reagents can be used with peripheral whole blood, bone marrow, and lymph node specimens.
The results obtained via testing with the ClearLLab reagents should be interpreted along with additional clinical and laboratory findings, according to Beckman Coulter, Inc., the company that will be marketing the reagents.
The FDA reviewed data for the ClearLLab reagents through the de novo premarket review pathway, a regulatory pathway for novel, low-to-moderate-risk devices that are not substantially equivalent to an already legally marketed device.
The FDA’s clearance of the ClearLLab reagents was supported by a study designed to demonstrate the reagents’ performance, which was conducted on 279 samples at 4 independent clinical sites.
Results with the ClearLLab reagents were compared to results with alternative detection methods used at the sites.
The ClearLLab results aligned with the study sites’ final diagnosis 93.4% of the time and correctly detected abnormalities 84.2% of the time.
Along with its clearance of the ClearLLab reagents, the FDA is establishing criteria, called special controls, which clarify the agency’s expectations in assuring the reagents’ accuracy, reliability, and clinical relevance.
These special controls, when met along with general controls, provide reasonable assurance of safety and effectiveness for the ClearLLab reagents and similar tools.
The special controls also describe the least burdensome regulatory pathway for future developers of similar diagnostic tests. ![]()
Study indicates parent-reported penicillin allergy in question
, according to David Vyles, DO, and his associates at the Medical College of Wisconsin, Milwaukee.
Because there is no process to safely and rapidly diagnose true penicillin allergy in a critical care setting, pediatric providers are reluctant to prescribe penicillin to children with a reported allergy. In this study, a three-tier penicillin testing process was used to evaluate the accuracy of a parent-reported penicillin allergy questionnaire in identifying children likely to be at low risk for penicillin allergy in an ED setting.
“Our results in the current study highlight that the high percentage of patients reporting a penicillin allergy to medical providers are likely inconsistent with true allergy,” concluded Dr. Vyles and his associates.
Read more at Pediatrics (2017. doi: 10.1542/peds.2017-0471).
, according to David Vyles, DO, and his associates at the Medical College of Wisconsin, Milwaukee.
Because there is no process to safely and rapidly diagnose true penicillin allergy in a critical care setting, pediatric providers are reluctant to prescribe penicillin to children with a reported allergy. In this study, a three-tier penicillin testing process was used to evaluate the accuracy of a parent-reported penicillin allergy questionnaire in identifying children likely to be at low risk for penicillin allergy in an ED setting.
“Our results in the current study highlight that the high percentage of patients reporting a penicillin allergy to medical providers are likely inconsistent with true allergy,” concluded Dr. Vyles and his associates.
Read more at Pediatrics (2017. doi: 10.1542/peds.2017-0471).
, according to David Vyles, DO, and his associates at the Medical College of Wisconsin, Milwaukee.
Because there is no process to safely and rapidly diagnose true penicillin allergy in a critical care setting, pediatric providers are reluctant to prescribe penicillin to children with a reported allergy. In this study, a three-tier penicillin testing process was used to evaluate the accuracy of a parent-reported penicillin allergy questionnaire in identifying children likely to be at low risk for penicillin allergy in an ED setting.
“Our results in the current study highlight that the high percentage of patients reporting a penicillin allergy to medical providers are likely inconsistent with true allergy,” concluded Dr. Vyles and his associates.
Read more at Pediatrics (2017. doi: 10.1542/peds.2017-0471).
FROM PEDIATRICS
Daily probiotics have no effect on infections causing child care absences
Results of a double-blind, placebo-controlled study do not support use of probiotics to prevent upper respiratory and gastrointestinal infections in children aged 8-14 months at enrollment, as the probiotics did not reduce infection-related child care absences.
In the study of 290 Danish infants randomly allocated to receive a placebo or a combination of Bifidobacterium animalis subsp lactis (BB-12) and Lactobacillus rhamnosus (LGG) in a dose of 109 colony-forming units of each daily for a 6-month intervention period, there were no differences in absences from child care between the two groups (1.14 days; 95% confidence interval, −0.55 to 2.82), reported Rikke Pilmann Laursen, MSc, and her associates at the University of Copenhagen (Pediatrics. 2017. doi: 10.1542/peds.2017-0735).
Infants were a mean 10 months old at baseline, and 47% were still breastfeeding. The mean age at breastfeeding discontinuation was 12 months.
In terms of the number of children with one or more episodes of an upper respiratory tract infection, the LGG and BB-12 vs. placebo odds ratio was 1.22 (95% CI, 0.74-2.00; P = .43). The number of children with one or more episodes of diarrhea in the LGG and BB-12 vs. placebo odds ratio was 1.42 (95% CI, 0.88-2.32; P = .15).
The effect of probiotics in preventing infections in preschool-aged children has been explored in several studies and recent reviews, suggesting an effect specifically on upper respiratory tract and GI infections in small children. This study diverges in that it examined the effect of a combination of probiotics on absences from child care related to those infections in infants aged 8-14 months at the time of enrollment, whereas previous studies focused on children older than 1 year.
This study was funded by Innovation Fund Denmark, the University of Copenhagen, and Chr Hansen A/S. Dr. Kim Fleischer Michaelsen and Dr. Christian Mølgaard received a grant from Chr Hansen for the current study. None of the other investigators had any relevant financial disclosures.
Any intervention that can prevent child care–associated illnesses can have economic and educational implications, as well as public health ones. However, in the Laursen et al. study, almost 47% of the infants enrolled were breastfed, as opposed to previous studies in which the children participating generally were over 1 year old.
Given the positive effects of breastfeeding in preventing infections and the high percentage of infants who were breastfed in this study, it may be harder to distinguish the effects of the probiotic supplement in the Laursen et al. study. Indirectly, this study suggests additional information on the relative value of a probiotic intervention, compared with breastfeeding and diet, is warranted, and perhaps there is another reason to encourage breastfeeding as long as possible.
Michael D. Cabana, MD, MPH, is a professor of pediatrics, epidemiology and biostatistics and a member of the faculty at the Philip R. Lee Institute for Health Policy Studies at the University of California, San Francisco. Daniel J. Merenstein, MD, is an associate professor of family medicine at Georgetown University, Washington. Pharmavite, Bayer, and Sanofi have provided Georgetown University with consulting funding. Both physicians, who commented in an editorial accompanying the article by Laursen et al. (Pediatrics. 2017. doi: 10.1542/peds.2017-1729), disclosed no financial relationships relevant to this article. Dr. Merenstein has been an expert witness for Proctor & Gamble while Dr. Cabana has served as a consultant for BioGaia, Nestle, Mead Johnson, and Wyeth.
Any intervention that can prevent child care–associated illnesses can have economic and educational implications, as well as public health ones. However, in the Laursen et al. study, almost 47% of the infants enrolled were breastfed, as opposed to previous studies in which the children participating generally were over 1 year old.
Given the positive effects of breastfeeding in preventing infections and the high percentage of infants who were breastfed in this study, it may be harder to distinguish the effects of the probiotic supplement in the Laursen et al. study. Indirectly, this study suggests additional information on the relative value of a probiotic intervention, compared with breastfeeding and diet, is warranted, and perhaps there is another reason to encourage breastfeeding as long as possible.
Michael D. Cabana, MD, MPH, is a professor of pediatrics, epidemiology and biostatistics and a member of the faculty at the Philip R. Lee Institute for Health Policy Studies at the University of California, San Francisco. Daniel J. Merenstein, MD, is an associate professor of family medicine at Georgetown University, Washington. Pharmavite, Bayer, and Sanofi have provided Georgetown University with consulting funding. Both physicians, who commented in an editorial accompanying the article by Laursen et al. (Pediatrics. 2017. doi: 10.1542/peds.2017-1729), disclosed no financial relationships relevant to this article. Dr. Merenstein has been an expert witness for Proctor & Gamble while Dr. Cabana has served as a consultant for BioGaia, Nestle, Mead Johnson, and Wyeth.
Any intervention that can prevent child care–associated illnesses can have economic and educational implications, as well as public health ones. However, in the Laursen et al. study, almost 47% of the infants enrolled were breastfed, as opposed to previous studies in which the children participating generally were over 1 year old.
Given the positive effects of breastfeeding in preventing infections and the high percentage of infants who were breastfed in this study, it may be harder to distinguish the effects of the probiotic supplement in the Laursen et al. study. Indirectly, this study suggests additional information on the relative value of a probiotic intervention, compared with breastfeeding and diet, is warranted, and perhaps there is another reason to encourage breastfeeding as long as possible.
Michael D. Cabana, MD, MPH, is a professor of pediatrics, epidemiology and biostatistics and a member of the faculty at the Philip R. Lee Institute for Health Policy Studies at the University of California, San Francisco. Daniel J. Merenstein, MD, is an associate professor of family medicine at Georgetown University, Washington. Pharmavite, Bayer, and Sanofi have provided Georgetown University with consulting funding. Both physicians, who commented in an editorial accompanying the article by Laursen et al. (Pediatrics. 2017. doi: 10.1542/peds.2017-1729), disclosed no financial relationships relevant to this article. Dr. Merenstein has been an expert witness for Proctor & Gamble while Dr. Cabana has served as a consultant for BioGaia, Nestle, Mead Johnson, and Wyeth.
Results of a double-blind, placebo-controlled study do not support use of probiotics to prevent upper respiratory and gastrointestinal infections in children aged 8-14 months at enrollment, as the probiotics did not reduce infection-related child care absences.
In the study of 290 Danish infants randomly allocated to receive a placebo or a combination of Bifidobacterium animalis subsp lactis (BB-12) and Lactobacillus rhamnosus (LGG) in a dose of 109 colony-forming units of each daily for a 6-month intervention period, there were no differences in absences from child care between the two groups (1.14 days; 95% confidence interval, −0.55 to 2.82), reported Rikke Pilmann Laursen, MSc, and her associates at the University of Copenhagen (Pediatrics. 2017. doi: 10.1542/peds.2017-0735).
Infants were a mean 10 months old at baseline, and 47% were still breastfeeding. The mean age at breastfeeding discontinuation was 12 months.
In terms of the number of children with one or more episodes of an upper respiratory tract infection, the LGG and BB-12 vs. placebo odds ratio was 1.22 (95% CI, 0.74-2.00; P = .43). The number of children with one or more episodes of diarrhea in the LGG and BB-12 vs. placebo odds ratio was 1.42 (95% CI, 0.88-2.32; P = .15).
The effect of probiotics in preventing infections in preschool-aged children has been explored in several studies and recent reviews, suggesting an effect specifically on upper respiratory tract and GI infections in small children. This study diverges in that it examined the effect of a combination of probiotics on absences from child care related to those infections in infants aged 8-14 months at the time of enrollment, whereas previous studies focused on children older than 1 year.
This study was funded by Innovation Fund Denmark, the University of Copenhagen, and Chr Hansen A/S. Dr. Kim Fleischer Michaelsen and Dr. Christian Mølgaard received a grant from Chr Hansen for the current study. None of the other investigators had any relevant financial disclosures.
Results of a double-blind, placebo-controlled study do not support use of probiotics to prevent upper respiratory and gastrointestinal infections in children aged 8-14 months at enrollment, as the probiotics did not reduce infection-related child care absences.
In the study of 290 Danish infants randomly allocated to receive a placebo or a combination of Bifidobacterium animalis subsp lactis (BB-12) and Lactobacillus rhamnosus (LGG) in a dose of 109 colony-forming units of each daily for a 6-month intervention period, there were no differences in absences from child care between the two groups (1.14 days; 95% confidence interval, −0.55 to 2.82), reported Rikke Pilmann Laursen, MSc, and her associates at the University of Copenhagen (Pediatrics. 2017. doi: 10.1542/peds.2017-0735).
Infants were a mean 10 months old at baseline, and 47% were still breastfeeding. The mean age at breastfeeding discontinuation was 12 months.
In terms of the number of children with one or more episodes of an upper respiratory tract infection, the LGG and BB-12 vs. placebo odds ratio was 1.22 (95% CI, 0.74-2.00; P = .43). The number of children with one or more episodes of diarrhea in the LGG and BB-12 vs. placebo odds ratio was 1.42 (95% CI, 0.88-2.32; P = .15).
The effect of probiotics in preventing infections in preschool-aged children has been explored in several studies and recent reviews, suggesting an effect specifically on upper respiratory tract and GI infections in small children. This study diverges in that it examined the effect of a combination of probiotics on absences from child care related to those infections in infants aged 8-14 months at the time of enrollment, whereas previous studies focused on children older than 1 year.
This study was funded by Innovation Fund Denmark, the University of Copenhagen, and Chr Hansen A/S. Dr. Kim Fleischer Michaelsen and Dr. Christian Mølgaard received a grant from Chr Hansen for the current study. None of the other investigators had any relevant financial disclosures.
FROM PEDIATRICS
Key clinical point:
Major finding: Intention-to-treat analysis showed no difference between the probiotics and placebo study groups.
Data source: A randomized, double-blind, placebo-controlled study of 290 infants aged 8-14 months at enrollment.
Disclosures: This study was funded by Innovation Fund Denmark, the University of Copenhagen, and Chr Hansen A/S. Dr. Kim Fleischer Michaelsen and Dr. Christian Mølgaard received a grant from Chr Hansen for the current study. None of the other investigators had any relevant financial disclosures.
How Low Should You Go? Optimizing BP in CKD
Q) I hear providers quote different numbers for target blood pressure in kidney patients. Which are correct?
The answer to this question starts with the word “meta-analysis”—but don’t stop reading! We’ll get down to the basics quickly. Determining the goal blood pressure (BP) for patients with chronic kidney disease (CKD) comes down to three questions.
1. Does the patient have diabetes? The National Kidney Foundation states that the goal BP for a patient with type 2 diabetes, CKD, and urine albumin > 30 mg/dL is < 140/90 mm Hg.1 This is in line with the JNC-8 recommendations for patients with hypertension and CKD, which do not take urine albumin level into consideration.2 It is important to recognize that while many patients with CKD do not have diabetes, those who do have a worse prognosis.3
2. Is the patient African-American? A meta-analysis of nine randomized clinical trials found that lowering BP to < 130/80 mm Hg was linked to a slower decline in glomerular filtration rate (GFR) in non-African-American patients.3 But this BP was not beneficial for African-American patients; in fact, it actually caused a faster decline in GFR.3 Therefore, target BP for African-American patients should be < 140/90 mm Hg.
3. Does the patient have significant albuminuria? An additional subgroup analysis for patients with high levels of proteinuria (defined as > 1 g/d) yielded inconclusive results.3 Patients with proteinuria > 1 g/d tended to have a slower decline in GFR with intensive BP control.3 Proteinuria > 0.5 g/d was correlated with a slowed progression to end-stage renal disease with intensive BP control.3 Again, these were trends and not statistically significant. So, for patients with high levels of proteinuria, it will not hurt to achieve a BP < 130/80 mm Hg, but there is no statistically significant difference between BP < 130/80 mm Hg and BP < 140/90 mm Hg.
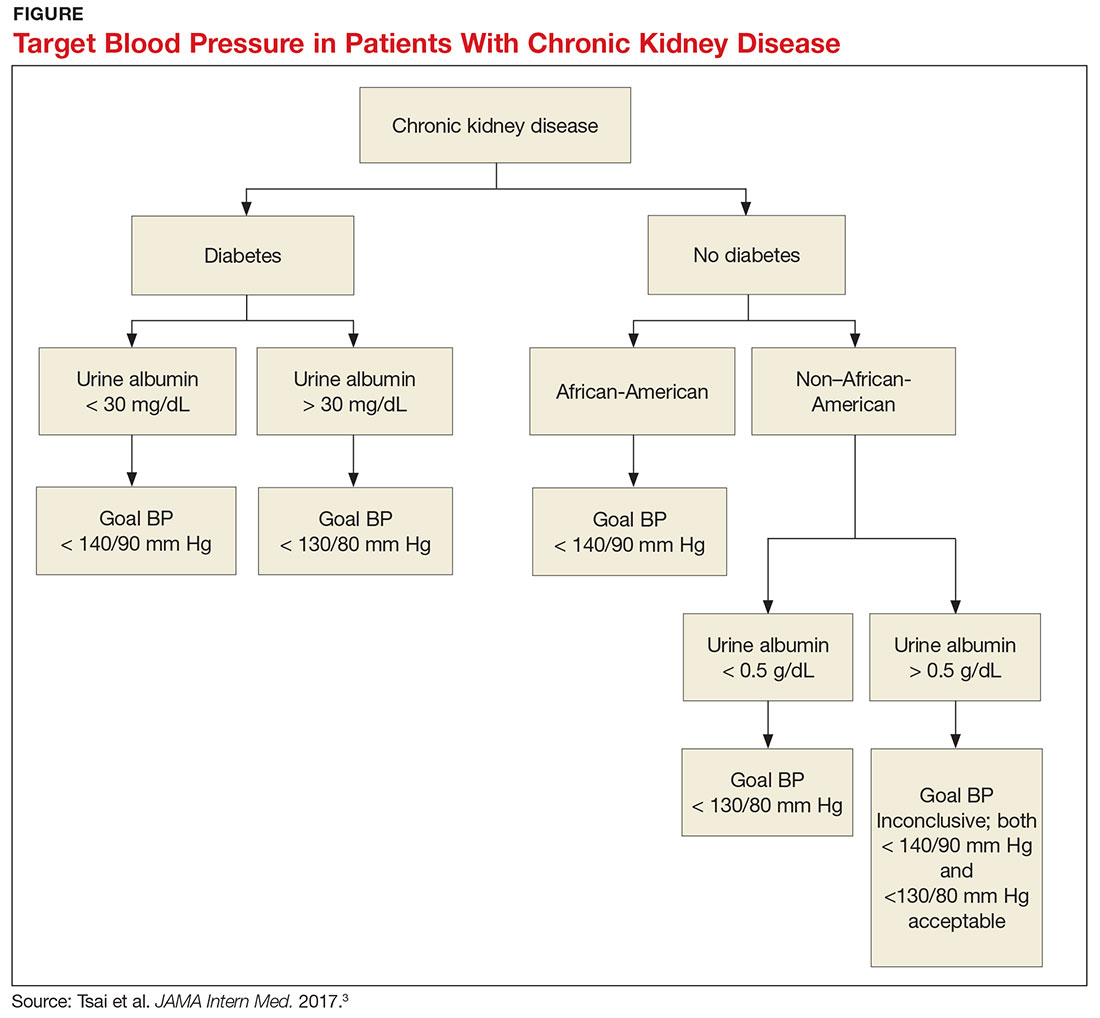
What, then, are the recommendations for an African-American patient with significant proteinuria? While not addressed directly in the analysis, the study results suggest that the goal should still be < 140/90 mm Hg, since the link between race and changes in GFR is statistically significant and the effects of proteinuria are not. Although the recommendations from this review are many, the main points are summarized in the Figure.—RC
Rebecca Clawson, MAT, PA-C
Instructor, PA Program, LSU Health Shreveport, Louisiana
1. Kidney Disease: Improving Global Outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Inter Suppl. 2013;3(1):1-150.
2. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520.
3. Tsai WC, Wu HY, Peng YS, et al. Association of intensive blood pressure control and kidney disease progression in nondiabetic patients with chronic kidney disease: a systematic review and meta-analysis. JAMA Intern Med. 2017;177:792-799.
Q) I hear providers quote different numbers for target blood pressure in kidney patients. Which are correct?
The answer to this question starts with the word “meta-analysis”—but don’t stop reading! We’ll get down to the basics quickly. Determining the goal blood pressure (BP) for patients with chronic kidney disease (CKD) comes down to three questions.
1. Does the patient have diabetes? The National Kidney Foundation states that the goal BP for a patient with type 2 diabetes, CKD, and urine albumin > 30 mg/dL is < 140/90 mm Hg.1 This is in line with the JNC-8 recommendations for patients with hypertension and CKD, which do not take urine albumin level into consideration.2 It is important to recognize that while many patients with CKD do not have diabetes, those who do have a worse prognosis.3
2. Is the patient African-American? A meta-analysis of nine randomized clinical trials found that lowering BP to < 130/80 mm Hg was linked to a slower decline in glomerular filtration rate (GFR) in non-African-American patients.3 But this BP was not beneficial for African-American patients; in fact, it actually caused a faster decline in GFR.3 Therefore, target BP for African-American patients should be < 140/90 mm Hg.
3. Does the patient have significant albuminuria? An additional subgroup analysis for patients with high levels of proteinuria (defined as > 1 g/d) yielded inconclusive results.3 Patients with proteinuria > 1 g/d tended to have a slower decline in GFR with intensive BP control.3 Proteinuria > 0.5 g/d was correlated with a slowed progression to end-stage renal disease with intensive BP control.3 Again, these were trends and not statistically significant. So, for patients with high levels of proteinuria, it will not hurt to achieve a BP < 130/80 mm Hg, but there is no statistically significant difference between BP < 130/80 mm Hg and BP < 140/90 mm Hg.

What, then, are the recommendations for an African-American patient with significant proteinuria? While not addressed directly in the analysis, the study results suggest that the goal should still be < 140/90 mm Hg, since the link between race and changes in GFR is statistically significant and the effects of proteinuria are not. Although the recommendations from this review are many, the main points are summarized in the Figure.—RC
Rebecca Clawson, MAT, PA-C
Instructor, PA Program, LSU Health Shreveport, Louisiana
Q) I hear providers quote different numbers for target blood pressure in kidney patients. Which are correct?
The answer to this question starts with the word “meta-analysis”—but don’t stop reading! We’ll get down to the basics quickly. Determining the goal blood pressure (BP) for patients with chronic kidney disease (CKD) comes down to three questions.
1. Does the patient have diabetes? The National Kidney Foundation states that the goal BP for a patient with type 2 diabetes, CKD, and urine albumin > 30 mg/dL is < 140/90 mm Hg.1 This is in line with the JNC-8 recommendations for patients with hypertension and CKD, which do not take urine albumin level into consideration.2 It is important to recognize that while many patients with CKD do not have diabetes, those who do have a worse prognosis.3
2. Is the patient African-American? A meta-analysis of nine randomized clinical trials found that lowering BP to < 130/80 mm Hg was linked to a slower decline in glomerular filtration rate (GFR) in non-African-American patients.3 But this BP was not beneficial for African-American patients; in fact, it actually caused a faster decline in GFR.3 Therefore, target BP for African-American patients should be < 140/90 mm Hg.
3. Does the patient have significant albuminuria? An additional subgroup analysis for patients with high levels of proteinuria (defined as > 1 g/d) yielded inconclusive results.3 Patients with proteinuria > 1 g/d tended to have a slower decline in GFR with intensive BP control.3 Proteinuria > 0.5 g/d was correlated with a slowed progression to end-stage renal disease with intensive BP control.3 Again, these were trends and not statistically significant. So, for patients with high levels of proteinuria, it will not hurt to achieve a BP < 130/80 mm Hg, but there is no statistically significant difference between BP < 130/80 mm Hg and BP < 140/90 mm Hg.

What, then, are the recommendations for an African-American patient with significant proteinuria? While not addressed directly in the analysis, the study results suggest that the goal should still be < 140/90 mm Hg, since the link between race and changes in GFR is statistically significant and the effects of proteinuria are not. Although the recommendations from this review are many, the main points are summarized in the Figure.—RC
Rebecca Clawson, MAT, PA-C
Instructor, PA Program, LSU Health Shreveport, Louisiana
1. Kidney Disease: Improving Global Outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Inter Suppl. 2013;3(1):1-150.
2. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520.
3. Tsai WC, Wu HY, Peng YS, et al. Association of intensive blood pressure control and kidney disease progression in nondiabetic patients with chronic kidney disease: a systematic review and meta-analysis. JAMA Intern Med. 2017;177:792-799.
1. Kidney Disease: Improving Global Outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Inter Suppl. 2013;3(1):1-150.
2. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520.
3. Tsai WC, Wu HY, Peng YS, et al. Association of intensive blood pressure control and kidney disease progression in nondiabetic patients with chronic kidney disease: a systematic review and meta-analysis. JAMA Intern Med. 2017;177:792-799.
AHRQ identifies interventions, drugs that best target diabetic neuropathy
Contextual influences of trainee characteristics and daily workload on trainee learning preferences
We previously identified key domains of attending attributes for successful rounds1 and adapted them to represent the trainees’ perspective: Teaching Process (eg, sharing decision-making process, physical exam skills), Learning Environment (eg, being approachable, respectful), Role Modeling (eg, teaching by example, bedside manner), and Team Management (eg, efficiency, providing autonomy). Though all domains are necessary, the relative importance may change in response to external pressures. Inpatient service demands and time constraints fluctuate daily due to patient admissions and discharges, educational conference schedules, and concurrent outpatient clinic responsibilities.2–4 Furthermore, the 2011 Accreditation Council for Graduate Medical Education (ACGME) duty hour rules placed greater time pressure on inpatient ward attending rounds.5 It is plausible that these pressures affect trainees’ needs and priorities during rounds.
Therefore, we sought to refine our understanding of the learners’ needs during ward rounds. Because we were interested in the contextual influences of trainee characteristics and workload on their preferences, we used the principles of ecological momentary assessment (EMA) to design a novel system to assess daily changes in trainee priorities and associated workload.
METHODS
Design, Participants, and Setting
In a prospective observational study, we assessed trainee priorities during inpatient rounds. Participants included third- and fourth-year medical students in the University of Alabama at Birmingham (UAB) School of Medicine (Birmingham campus) and residents in the Tinsley Harrison Internal Medicine Residency Training Program assigned to inpatient general medicine ward services from September 2010 to February 2011 (except from December 20, 2010, to January 3, 2011). Three training sites were included in this study: UAB Hospital (>1000-bed, university-based hospital), Birmingham Veterans Affairs Medical Center (300-bed), and Cooper Green Hospital (40-bed county hospital). Each site housed 4 or 5 general medicine ward teams, composed of 1–3 medical students (third or fourth year), 2 first-year residents, 1 upper level resident (postgraduate year 2–4), and 1 attending physician.
Trainees were recruited to participate via e-mail and verbal announcements during program conferences. Participation was voluntary and responses were confidential. As an incentive, the team submitting the highest number of cards per site each month received 1 free lunch. Institutional review boards of all 3 participating hospitals approved this study.
Assessment
Our original domains of attending rounds were derived from groupings and ratings of specific teaching characteristics by attending physicians, residents, and students.1 However, because our goal was to understand the learners’ perspective of successful ward rounds, we revised these domains by limiting the algorithm to data on groupings and ratings by residents and students only. This process resulted in the domains used in this study (Appendix).
We used EMA principles to create a novel system to assess daily variation in trainees’ prioritization of these domains and workload.6,7 EMA-derived assessment tools collect data frequently (several times per week, up to multiple times per day) to identify time-sensitive fluctuations. We designed a pocket-sized daily assessment card for trainees to complete each day after rounds (Figure 1). Trainees were asked to indicate the most important domain for successful ward rounds to them that day and provide individual characteristics (ie, sex and training level) and data on factors we hypothesized were related to perceived work load (ie, patient census, day of call cycle, and number of team members absent on rounds that day). We anticipated the Expectations domain would not be responsive to daily changes in workload because expectations are usually set once on the first day of the rotation, and thus we did not include this domain in our final assessment tool. Assessment cards and locked receptacles were kept in team workrooms for ease of accessibility; cards were collected twice monthly. All data were anonymous.
Analysis
Our unit of analysis was the EMA card. We examined associations between daily domain priority with respondent demographics and workload information using Pearson’s chi-squared analyses, adjusted for clustering effects by team. α was set at 0.05. All analyses were performed by using Stata 13.0 (Stata Statistical Software: Release 13; College Station, TX).
RESULTS
Sex and training level were associated with prioritization of teaching domains. Male trainees were more likely to choose Team Management (P = 0.01) or Teaching Process (P = 0.04) as their preferred domain. Medical students valued Teaching Process 42% of the time, compared with 23% for interns and 21% for upper level residents (P = 0.005). The opposite trend emerged for Team Management: as training level increased, the importance of Team Management increased (P < 0.001). There were no significant trends by training level for the Role Modeling and Learning Environment domains.
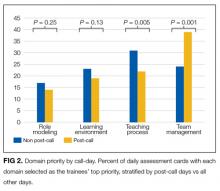
Domain priority was also associated with workload characteristics. On post-call days, Team Management (P < 0.001) was more likely to be selected as the most important domain, but on other days, Teaching Process (P = 0.005) was more often selected (Figure 2). Trainees also selected Team Management as the most important domain with an increasing number of team members absent (P = 0.001), and as the teams’ overall patient census increased (P < 0.001). The Learning Environment and Role Modeling domains’ importance did not vary by call-day or patient census.
DISCUSSION
We used a novel approach to assess contextual factors affecting trainee prioritization of 4 domains that contribute to successful inpatient internal medicine attending rounds. We found training level and workload demands were associated with prioritization of teaching domains. Prioritization of Teaching Process, exemplified by setting aside time to teach, demonstrating physical exam skills, and clear delineation of the attending’s thought process, was inversely associated with training level. On the days with highest workload, Team Management was most likely to be prioritized. Our findings suggest that attending physicians should consider adapting rounding style based on team members’ training levels and workload.
Prior work has described teaching and rounding styles, influences, and priorities in response to workload from the attending physician’s perspective,8–11 and our study extends these reports by providing the complementary perspective of trainees. On days with high workload, trainees prefer the Team Management domain, characterized by organized and efficient rounds, agreement on a clear and consistent plan of care, and being allowed independence and time during rounds to meet other responsibilities.1 These findings support an “empowerment style,” defined by Goldszmidt et al. as using integrated teaching and oversight strategies to support trainees’ progressive independence.9 Though some attending physicians report shifting to a more direct patient care style on days with a high patient census,9 our results suggest that learners instead prefer more independence, being empowered to perform more direct care. While there is an increasing pressure to heighten attending supervision due to concerns about patient safety, restricted work hours, and litigation,12 trainees value being part of the care process and being included as integral members of the care team, which may actually mitigate patient safety risks.8
Our results are consistent with prior studies, reporting that learners at different levels of training have different instructional needs: medical students seek more teaching, and senior residents sought an efficient leader.13,14 Taken together, these studies suggest that attending physicians should tailor rounds to the level of the trainee. For example, it may be beneficial for the attending physician to spend time outside of rounds with students to teach medical knowledge. During rounds, the entire group benefits most from modeling clinical reasoning, discussing new medical evidence, and demonstrating communication skills and leadership.
Our study has limitations. Though our study was performed before the 2011 ACGME duty hour restrictions,5 our results are likely of greater importance and relevance, as our findings ultimately highlight the competing demands of time vs duty. Also, while our study was performed at a single institution, potentially limiting generalizability, we included 3 types of training hospitals, a university, veterans and a county hospital, and found no differences between sites. Additionally, we collected over 1,000 cards over the course of 6 months, assessing rounds of over 50 different attending physicians, suggesting broader applicability. Our overall response rate was low, a typical signal for respondent bias, but because we collected daily assessments, standard interpretation of response rates referring to a one-time survey do not apply.15 We believe we achieved an adequate sample, as the majority of teams participated, the respondent demographics were proportional to the base population eligible to participate, and we received a similar number of cards on all months. Finally, although we were unable to account for clustering effects by individual respondents because response cards were anonymous, we adjusted for clustering effects by team.
Attending physicians may use our findings to adapt teaching techniques to appeal to specific training levels and to external pressures during teaching rounds. Focusing and investing time in teaching medical knowledge and clinical reasoning tailored to each level of learner is paramount on most days. However, days with a high workload may require emphasis on delegating clear, rational treatment plans, when learners are less receptive to traditional didactic methods.
Disclosure
An abstract based on the current analysis was presented at the Society of General Internal Medicine 34th Annual Meeting, April 2011, Phoenix, AZ. Dr. Brita Roy is supported by grant number K12HS023000 from the Agency for Healthcare Research and Quality. The authors have no conflicts to disclose. The opinions expressed in this article are those of the authors alone and do not reflect the views of the Department of Veterans Affairs or the Agency for Healthcare Research and Quality.
1. Roy B, Salanitro AH, Willett L, et al. Using cognitive mapping to identify attributes contributing to successful ward-attending rounds -- a resident and student perspective. J Gen Intern Med. 2010;25(S3):2. PubMed
2. Ben-Menachem T, Estrada C, Young MJ, et al. Balancing service and education: improving internal medicine residencies in the managed care era. Am J Med. 1996;100(2):224–229. PubMed
3. Drolet BC, Bishop KD. Unintended consequences of duty hours regulation. Acad Med. 2012;87(6):680. PubMed
4. Drolet BC, Spalluto LB, Fischer SA. Residents’ perspectives on ACGME regulation of supervision and duty hours--a national survey. N Engl J Med. 2010;363(23):e34. PubMed
5. Nasca TJ, Day SH, Amis ES, Jr. The new recommendations on duty hours from the ACGME Task Force. N Engl J Med. 2010;363(2):e3. PubMed
6. Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. PubMed
7. Moskowitz DS, Young SN. Ecological momentary assessment: what it is and why it is a method of the future in clinical psychopharmacology. J Psychiatry Neurosci. 2006;31(1):13–20. PubMed
8. Wingo MT, Halvorsen AJ, Beckman TJ, Johnson MG, Reed DA. Associations between attending physician workload, teaching effectiveness, and patient safety. J Hosp Med. 2016;11(3):169–173. PubMed
9. Goldszmidt M, Faden L, Dornan T, van Merriënboer J, Bordage G, Lingard L. Attending physician variability: a model of four supervisory styles. Acad Med. 2015;90(11):1541–1546. PubMed
10. Kennedy TJ, Lingard L, Baker GR, Kitchen L, Regehr G. Clinical oversight: conceptualizing the relationship between supervision and safety. J Gen Intern Med. 2007;22(8):1080–1085. PubMed
11. Irby DM. How attending physicians make instructional decisions when conducting teaching rounds. Acad Med. 1992;67(10):630–638. PubMed
12. Kennedy TJ, Regehr G, Baker GR, Lingard LA. Progressive independence in clinical training: a tradition worth defending? Acad Med. 2005;80(10):S106–S111. PubMed
13. Tariq M, Motiwala A, Ali SU, Riaz M, Awan S, Akhter J. The learners’ perspective on internal medicine ward rounds: a cross-sectional study. BMC Med Educ. 2010;10:53. PubMed
14. Certain LK, Guarino AJ, Greenwald JL. Effective multilevel teaching techniques on attending rounds: a pilot survey and systematic review of the literature. Med Teach. 2011;33(12):e644–e650. PubMed
15. Stone AA, Shiffman S. Capturing momentary, self-report data: A proposal for reporting guidelines. Ann Behav Med. 2002;24(3):236–243. PubMed
We previously identified key domains of attending attributes for successful rounds1 and adapted them to represent the trainees’ perspective: Teaching Process (eg, sharing decision-making process, physical exam skills), Learning Environment (eg, being approachable, respectful), Role Modeling (eg, teaching by example, bedside manner), and Team Management (eg, efficiency, providing autonomy). Though all domains are necessary, the relative importance may change in response to external pressures. Inpatient service demands and time constraints fluctuate daily due to patient admissions and discharges, educational conference schedules, and concurrent outpatient clinic responsibilities.2–4 Furthermore, the 2011 Accreditation Council for Graduate Medical Education (ACGME) duty hour rules placed greater time pressure on inpatient ward attending rounds.5 It is plausible that these pressures affect trainees’ needs and priorities during rounds.
Therefore, we sought to refine our understanding of the learners’ needs during ward rounds. Because we were interested in the contextual influences of trainee characteristics and workload on their preferences, we used the principles of ecological momentary assessment (EMA) to design a novel system to assess daily changes in trainee priorities and associated workload.
METHODS
Design, Participants, and Setting
In a prospective observational study, we assessed trainee priorities during inpatient rounds. Participants included third- and fourth-year medical students in the University of Alabama at Birmingham (UAB) School of Medicine (Birmingham campus) and residents in the Tinsley Harrison Internal Medicine Residency Training Program assigned to inpatient general medicine ward services from September 2010 to February 2011 (except from December 20, 2010, to January 3, 2011). Three training sites were included in this study: UAB Hospital (>1000-bed, university-based hospital), Birmingham Veterans Affairs Medical Center (300-bed), and Cooper Green Hospital (40-bed county hospital). Each site housed 4 or 5 general medicine ward teams, composed of 1–3 medical students (third or fourth year), 2 first-year residents, 1 upper level resident (postgraduate year 2–4), and 1 attending physician.
Trainees were recruited to participate via e-mail and verbal announcements during program conferences. Participation was voluntary and responses were confidential. As an incentive, the team submitting the highest number of cards per site each month received 1 free lunch. Institutional review boards of all 3 participating hospitals approved this study.
Assessment
Our original domains of attending rounds were derived from groupings and ratings of specific teaching characteristics by attending physicians, residents, and students.1 However, because our goal was to understand the learners’ perspective of successful ward rounds, we revised these domains by limiting the algorithm to data on groupings and ratings by residents and students only. This process resulted in the domains used in this study (Appendix).
We used EMA principles to create a novel system to assess daily variation in trainees’ prioritization of these domains and workload.6,7 EMA-derived assessment tools collect data frequently (several times per week, up to multiple times per day) to identify time-sensitive fluctuations. We designed a pocket-sized daily assessment card for trainees to complete each day after rounds (Figure 1). Trainees were asked to indicate the most important domain for successful ward rounds to them that day and provide individual characteristics (ie, sex and training level) and data on factors we hypothesized were related to perceived work load (ie, patient census, day of call cycle, and number of team members absent on rounds that day). We anticipated the Expectations domain would not be responsive to daily changes in workload because expectations are usually set once on the first day of the rotation, and thus we did not include this domain in our final assessment tool. Assessment cards and locked receptacles were kept in team workrooms for ease of accessibility; cards were collected twice monthly. All data were anonymous.
Analysis
Our unit of analysis was the EMA card. We examined associations between daily domain priority with respondent demographics and workload information using Pearson’s chi-squared analyses, adjusted for clustering effects by team. α was set at 0.05. All analyses were performed by using Stata 13.0 (Stata Statistical Software: Release 13; College Station, TX).
RESULTS
Sex and training level were associated with prioritization of teaching domains. Male trainees were more likely to choose Team Management (P = 0.01) or Teaching Process (P = 0.04) as their preferred domain. Medical students valued Teaching Process 42% of the time, compared with 23% for interns and 21% for upper level residents (P = 0.005). The opposite trend emerged for Team Management: as training level increased, the importance of Team Management increased (P < 0.001). There were no significant trends by training level for the Role Modeling and Learning Environment domains.
Domain priority was also associated with workload characteristics. On post-call days, Team Management (P < 0.001) was more likely to be selected as the most important domain, but on other days, Teaching Process (P = 0.005) was more often selected (Figure 2). Trainees also selected Team Management as the most important domain with an increasing number of team members absent (P = 0.001), and as the teams’ overall patient census increased (P < 0.001). The Learning Environment and Role Modeling domains’ importance did not vary by call-day or patient census.
DISCUSSION
We used a novel approach to assess contextual factors affecting trainee prioritization of 4 domains that contribute to successful inpatient internal medicine attending rounds. We found training level and workload demands were associated with prioritization of teaching domains. Prioritization of Teaching Process, exemplified by setting aside time to teach, demonstrating physical exam skills, and clear delineation of the attending’s thought process, was inversely associated with training level. On the days with highest workload, Team Management was most likely to be prioritized. Our findings suggest that attending physicians should consider adapting rounding style based on team members’ training levels and workload.
Prior work has described teaching and rounding styles, influences, and priorities in response to workload from the attending physician’s perspective,8–11 and our study extends these reports by providing the complementary perspective of trainees. On days with high workload, trainees prefer the Team Management domain, characterized by organized and efficient rounds, agreement on a clear and consistent plan of care, and being allowed independence and time during rounds to meet other responsibilities.1 These findings support an “empowerment style,” defined by Goldszmidt et al. as using integrated teaching and oversight strategies to support trainees’ progressive independence.9 Though some attending physicians report shifting to a more direct patient care style on days with a high patient census,9 our results suggest that learners instead prefer more independence, being empowered to perform more direct care. While there is an increasing pressure to heighten attending supervision due to concerns about patient safety, restricted work hours, and litigation,12 trainees value being part of the care process and being included as integral members of the care team, which may actually mitigate patient safety risks.8
Our results are consistent with prior studies, reporting that learners at different levels of training have different instructional needs: medical students seek more teaching, and senior residents sought an efficient leader.13,14 Taken together, these studies suggest that attending physicians should tailor rounds to the level of the trainee. For example, it may be beneficial for the attending physician to spend time outside of rounds with students to teach medical knowledge. During rounds, the entire group benefits most from modeling clinical reasoning, discussing new medical evidence, and demonstrating communication skills and leadership.
Our study has limitations. Though our study was performed before the 2011 ACGME duty hour restrictions,5 our results are likely of greater importance and relevance, as our findings ultimately highlight the competing demands of time vs duty. Also, while our study was performed at a single institution, potentially limiting generalizability, we included 3 types of training hospitals, a university, veterans and a county hospital, and found no differences between sites. Additionally, we collected over 1,000 cards over the course of 6 months, assessing rounds of over 50 different attending physicians, suggesting broader applicability. Our overall response rate was low, a typical signal for respondent bias, but because we collected daily assessments, standard interpretation of response rates referring to a one-time survey do not apply.15 We believe we achieved an adequate sample, as the majority of teams participated, the respondent demographics were proportional to the base population eligible to participate, and we received a similar number of cards on all months. Finally, although we were unable to account for clustering effects by individual respondents because response cards were anonymous, we adjusted for clustering effects by team.
Attending physicians may use our findings to adapt teaching techniques to appeal to specific training levels and to external pressures during teaching rounds. Focusing and investing time in teaching medical knowledge and clinical reasoning tailored to each level of learner is paramount on most days. However, days with a high workload may require emphasis on delegating clear, rational treatment plans, when learners are less receptive to traditional didactic methods.
Disclosure
An abstract based on the current analysis was presented at the Society of General Internal Medicine 34th Annual Meeting, April 2011, Phoenix, AZ. Dr. Brita Roy is supported by grant number K12HS023000 from the Agency for Healthcare Research and Quality. The authors have no conflicts to disclose. The opinions expressed in this article are those of the authors alone and do not reflect the views of the Department of Veterans Affairs or the Agency for Healthcare Research and Quality.
We previously identified key domains of attending attributes for successful rounds1 and adapted them to represent the trainees’ perspective: Teaching Process (eg, sharing decision-making process, physical exam skills), Learning Environment (eg, being approachable, respectful), Role Modeling (eg, teaching by example, bedside manner), and Team Management (eg, efficiency, providing autonomy). Though all domains are necessary, the relative importance may change in response to external pressures. Inpatient service demands and time constraints fluctuate daily due to patient admissions and discharges, educational conference schedules, and concurrent outpatient clinic responsibilities.2–4 Furthermore, the 2011 Accreditation Council for Graduate Medical Education (ACGME) duty hour rules placed greater time pressure on inpatient ward attending rounds.5 It is plausible that these pressures affect trainees’ needs and priorities during rounds.
Therefore, we sought to refine our understanding of the learners’ needs during ward rounds. Because we were interested in the contextual influences of trainee characteristics and workload on their preferences, we used the principles of ecological momentary assessment (EMA) to design a novel system to assess daily changes in trainee priorities and associated workload.
METHODS
Design, Participants, and Setting
In a prospective observational study, we assessed trainee priorities during inpatient rounds. Participants included third- and fourth-year medical students in the University of Alabama at Birmingham (UAB) School of Medicine (Birmingham campus) and residents in the Tinsley Harrison Internal Medicine Residency Training Program assigned to inpatient general medicine ward services from September 2010 to February 2011 (except from December 20, 2010, to January 3, 2011). Three training sites were included in this study: UAB Hospital (>1000-bed, university-based hospital), Birmingham Veterans Affairs Medical Center (300-bed), and Cooper Green Hospital (40-bed county hospital). Each site housed 4 or 5 general medicine ward teams, composed of 1–3 medical students (third or fourth year), 2 first-year residents, 1 upper level resident (postgraduate year 2–4), and 1 attending physician.
Trainees were recruited to participate via e-mail and verbal announcements during program conferences. Participation was voluntary and responses were confidential. As an incentive, the team submitting the highest number of cards per site each month received 1 free lunch. Institutional review boards of all 3 participating hospitals approved this study.
Assessment
Our original domains of attending rounds were derived from groupings and ratings of specific teaching characteristics by attending physicians, residents, and students.1 However, because our goal was to understand the learners’ perspective of successful ward rounds, we revised these domains by limiting the algorithm to data on groupings and ratings by residents and students only. This process resulted in the domains used in this study (Appendix).
We used EMA principles to create a novel system to assess daily variation in trainees’ prioritization of these domains and workload.6,7 EMA-derived assessment tools collect data frequently (several times per week, up to multiple times per day) to identify time-sensitive fluctuations. We designed a pocket-sized daily assessment card for trainees to complete each day after rounds (Figure 1). Trainees were asked to indicate the most important domain for successful ward rounds to them that day and provide individual characteristics (ie, sex and training level) and data on factors we hypothesized were related to perceived work load (ie, patient census, day of call cycle, and number of team members absent on rounds that day). We anticipated the Expectations domain would not be responsive to daily changes in workload because expectations are usually set once on the first day of the rotation, and thus we did not include this domain in our final assessment tool. Assessment cards and locked receptacles were kept in team workrooms for ease of accessibility; cards were collected twice monthly. All data were anonymous.
Analysis
Our unit of analysis was the EMA card. We examined associations between daily domain priority with respondent demographics and workload information using Pearson’s chi-squared analyses, adjusted for clustering effects by team. α was set at 0.05. All analyses were performed by using Stata 13.0 (Stata Statistical Software: Release 13; College Station, TX).
RESULTS
Sex and training level were associated with prioritization of teaching domains. Male trainees were more likely to choose Team Management (P = 0.01) or Teaching Process (P = 0.04) as their preferred domain. Medical students valued Teaching Process 42% of the time, compared with 23% for interns and 21% for upper level residents (P = 0.005). The opposite trend emerged for Team Management: as training level increased, the importance of Team Management increased (P < 0.001). There were no significant trends by training level for the Role Modeling and Learning Environment domains.
Domain priority was also associated with workload characteristics. On post-call days, Team Management (P < 0.001) was more likely to be selected as the most important domain, but on other days, Teaching Process (P = 0.005) was more often selected (Figure 2). Trainees also selected Team Management as the most important domain with an increasing number of team members absent (P = 0.001), and as the teams’ overall patient census increased (P < 0.001). The Learning Environment and Role Modeling domains’ importance did not vary by call-day or patient census.
DISCUSSION
We used a novel approach to assess contextual factors affecting trainee prioritization of 4 domains that contribute to successful inpatient internal medicine attending rounds. We found training level and workload demands were associated with prioritization of teaching domains. Prioritization of Teaching Process, exemplified by setting aside time to teach, demonstrating physical exam skills, and clear delineation of the attending’s thought process, was inversely associated with training level. On the days with highest workload, Team Management was most likely to be prioritized. Our findings suggest that attending physicians should consider adapting rounding style based on team members’ training levels and workload.
Prior work has described teaching and rounding styles, influences, and priorities in response to workload from the attending physician’s perspective,8–11 and our study extends these reports by providing the complementary perspective of trainees. On days with high workload, trainees prefer the Team Management domain, characterized by organized and efficient rounds, agreement on a clear and consistent plan of care, and being allowed independence and time during rounds to meet other responsibilities.1 These findings support an “empowerment style,” defined by Goldszmidt et al. as using integrated teaching and oversight strategies to support trainees’ progressive independence.9 Though some attending physicians report shifting to a more direct patient care style on days with a high patient census,9 our results suggest that learners instead prefer more independence, being empowered to perform more direct care. While there is an increasing pressure to heighten attending supervision due to concerns about patient safety, restricted work hours, and litigation,12 trainees value being part of the care process and being included as integral members of the care team, which may actually mitigate patient safety risks.8
Our results are consistent with prior studies, reporting that learners at different levels of training have different instructional needs: medical students seek more teaching, and senior residents sought an efficient leader.13,14 Taken together, these studies suggest that attending physicians should tailor rounds to the level of the trainee. For example, it may be beneficial for the attending physician to spend time outside of rounds with students to teach medical knowledge. During rounds, the entire group benefits most from modeling clinical reasoning, discussing new medical evidence, and demonstrating communication skills and leadership.
Our study has limitations. Though our study was performed before the 2011 ACGME duty hour restrictions,5 our results are likely of greater importance and relevance, as our findings ultimately highlight the competing demands of time vs duty. Also, while our study was performed at a single institution, potentially limiting generalizability, we included 3 types of training hospitals, a university, veterans and a county hospital, and found no differences between sites. Additionally, we collected over 1,000 cards over the course of 6 months, assessing rounds of over 50 different attending physicians, suggesting broader applicability. Our overall response rate was low, a typical signal for respondent bias, but because we collected daily assessments, standard interpretation of response rates referring to a one-time survey do not apply.15 We believe we achieved an adequate sample, as the majority of teams participated, the respondent demographics were proportional to the base population eligible to participate, and we received a similar number of cards on all months. Finally, although we were unable to account for clustering effects by individual respondents because response cards were anonymous, we adjusted for clustering effects by team.
Attending physicians may use our findings to adapt teaching techniques to appeal to specific training levels and to external pressures during teaching rounds. Focusing and investing time in teaching medical knowledge and clinical reasoning tailored to each level of learner is paramount on most days. However, days with a high workload may require emphasis on delegating clear, rational treatment plans, when learners are less receptive to traditional didactic methods.
Disclosure
An abstract based on the current analysis was presented at the Society of General Internal Medicine 34th Annual Meeting, April 2011, Phoenix, AZ. Dr. Brita Roy is supported by grant number K12HS023000 from the Agency for Healthcare Research and Quality. The authors have no conflicts to disclose. The opinions expressed in this article are those of the authors alone and do not reflect the views of the Department of Veterans Affairs or the Agency for Healthcare Research and Quality.
1. Roy B, Salanitro AH, Willett L, et al. Using cognitive mapping to identify attributes contributing to successful ward-attending rounds -- a resident and student perspective. J Gen Intern Med. 2010;25(S3):2. PubMed
2. Ben-Menachem T, Estrada C, Young MJ, et al. Balancing service and education: improving internal medicine residencies in the managed care era. Am J Med. 1996;100(2):224–229. PubMed
3. Drolet BC, Bishop KD. Unintended consequences of duty hours regulation. Acad Med. 2012;87(6):680. PubMed
4. Drolet BC, Spalluto LB, Fischer SA. Residents’ perspectives on ACGME regulation of supervision and duty hours--a national survey. N Engl J Med. 2010;363(23):e34. PubMed
5. Nasca TJ, Day SH, Amis ES, Jr. The new recommendations on duty hours from the ACGME Task Force. N Engl J Med. 2010;363(2):e3. PubMed
6. Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. PubMed
7. Moskowitz DS, Young SN. Ecological momentary assessment: what it is and why it is a method of the future in clinical psychopharmacology. J Psychiatry Neurosci. 2006;31(1):13–20. PubMed
8. Wingo MT, Halvorsen AJ, Beckman TJ, Johnson MG, Reed DA. Associations between attending physician workload, teaching effectiveness, and patient safety. J Hosp Med. 2016;11(3):169–173. PubMed
9. Goldszmidt M, Faden L, Dornan T, van Merriënboer J, Bordage G, Lingard L. Attending physician variability: a model of four supervisory styles. Acad Med. 2015;90(11):1541–1546. PubMed
10. Kennedy TJ, Lingard L, Baker GR, Kitchen L, Regehr G. Clinical oversight: conceptualizing the relationship between supervision and safety. J Gen Intern Med. 2007;22(8):1080–1085. PubMed
11. Irby DM. How attending physicians make instructional decisions when conducting teaching rounds. Acad Med. 1992;67(10):630–638. PubMed
12. Kennedy TJ, Regehr G, Baker GR, Lingard LA. Progressive independence in clinical training: a tradition worth defending? Acad Med. 2005;80(10):S106–S111. PubMed
13. Tariq M, Motiwala A, Ali SU, Riaz M, Awan S, Akhter J. The learners’ perspective on internal medicine ward rounds: a cross-sectional study. BMC Med Educ. 2010;10:53. PubMed
14. Certain LK, Guarino AJ, Greenwald JL. Effective multilevel teaching techniques on attending rounds: a pilot survey and systematic review of the literature. Med Teach. 2011;33(12):e644–e650. PubMed
15. Stone AA, Shiffman S. Capturing momentary, self-report data: A proposal for reporting guidelines. Ann Behav Med. 2002;24(3):236–243. PubMed
1. Roy B, Salanitro AH, Willett L, et al. Using cognitive mapping to identify attributes contributing to successful ward-attending rounds -- a resident and student perspective. J Gen Intern Med. 2010;25(S3):2. PubMed
2. Ben-Menachem T, Estrada C, Young MJ, et al. Balancing service and education: improving internal medicine residencies in the managed care era. Am J Med. 1996;100(2):224–229. PubMed
3. Drolet BC, Bishop KD. Unintended consequences of duty hours regulation. Acad Med. 2012;87(6):680. PubMed
4. Drolet BC, Spalluto LB, Fischer SA. Residents’ perspectives on ACGME regulation of supervision and duty hours--a national survey. N Engl J Med. 2010;363(23):e34. PubMed
5. Nasca TJ, Day SH, Amis ES, Jr. The new recommendations on duty hours from the ACGME Task Force. N Engl J Med. 2010;363(2):e3. PubMed
6. Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. PubMed
7. Moskowitz DS, Young SN. Ecological momentary assessment: what it is and why it is a method of the future in clinical psychopharmacology. J Psychiatry Neurosci. 2006;31(1):13–20. PubMed
8. Wingo MT, Halvorsen AJ, Beckman TJ, Johnson MG, Reed DA. Associations between attending physician workload, teaching effectiveness, and patient safety. J Hosp Med. 2016;11(3):169–173. PubMed
9. Goldszmidt M, Faden L, Dornan T, van Merriënboer J, Bordage G, Lingard L. Attending physician variability: a model of four supervisory styles. Acad Med. 2015;90(11):1541–1546. PubMed
10. Kennedy TJ, Lingard L, Baker GR, Kitchen L, Regehr G. Clinical oversight: conceptualizing the relationship between supervision and safety. J Gen Intern Med. 2007;22(8):1080–1085. PubMed
11. Irby DM. How attending physicians make instructional decisions when conducting teaching rounds. Acad Med. 1992;67(10):630–638. PubMed
12. Kennedy TJ, Regehr G, Baker GR, Lingard LA. Progressive independence in clinical training: a tradition worth defending? Acad Med. 2005;80(10):S106–S111. PubMed
13. Tariq M, Motiwala A, Ali SU, Riaz M, Awan S, Akhter J. The learners’ perspective on internal medicine ward rounds: a cross-sectional study. BMC Med Educ. 2010;10:53. PubMed
14. Certain LK, Guarino AJ, Greenwald JL. Effective multilevel teaching techniques on attending rounds: a pilot survey and systematic review of the literature. Med Teach. 2011;33(12):e644–e650. PubMed
15. Stone AA, Shiffman S. Capturing momentary, self-report data: A proposal for reporting guidelines. Ann Behav Med. 2002;24(3):236–243. PubMed
© 2017 Society of Hospital Medicine
Drug granted PRIME access as treatment for DLBCL
The European Medicines Agency (EMA) has granted polatuzumab vedotin access to the agency’s PRIority MEdicines (PRIME) program.
The access is for polatuzumab vedotin when used in combination with rituximab and bendamustine for the treatment of relapsed or refractory diffuse large B-cell lymphoma (DLBCL).
Polatuzumab vedotin is an anti-CD79b antibody drug conjugate consisting of an anti-CD79b monoclonal antibody that is linked to a microtubule-disrupting agent.
Polatuzumab vedotin is being developed by Roche utilizing Seattle Genetics’ technology.
The goal of the EMA’s PRIME program is to accelerate the development of therapies that may offer a major advantage over existing treatments or benefit patients with no treatment options.
Through PRIME, the EMA offers early and enhanced support to developers in order to optimize development plans and speed regulatory evaluations to potentially bring therapies to patients more quickly.
To be accepted for PRIME, a therapy must demonstrate the potential to benefit patients with unmet medical need through early clinical or nonclinical data.
The acceptance of polatuzumab vedotin in the PRIME program was supported by results from the phase 2 component of the GO29365 study.
Results from this trial were recently presented at the 22nd Congress of the European Hematology Association (EHA) as abstract S468. ![]()
The European Medicines Agency (EMA) has granted polatuzumab vedotin access to the agency’s PRIority MEdicines (PRIME) program.
The access is for polatuzumab vedotin when used in combination with rituximab and bendamustine for the treatment of relapsed or refractory diffuse large B-cell lymphoma (DLBCL).
Polatuzumab vedotin is an anti-CD79b antibody drug conjugate consisting of an anti-CD79b monoclonal antibody that is linked to a microtubule-disrupting agent.
Polatuzumab vedotin is being developed by Roche utilizing Seattle Genetics’ technology.
The goal of the EMA’s PRIME program is to accelerate the development of therapies that may offer a major advantage over existing treatments or benefit patients with no treatment options.
Through PRIME, the EMA offers early and enhanced support to developers in order to optimize development plans and speed regulatory evaluations to potentially bring therapies to patients more quickly.
To be accepted for PRIME, a therapy must demonstrate the potential to benefit patients with unmet medical need through early clinical or nonclinical data.
The acceptance of polatuzumab vedotin in the PRIME program was supported by results from the phase 2 component of the GO29365 study.
Results from this trial were recently presented at the 22nd Congress of the European Hematology Association (EHA) as abstract S468. ![]()
The European Medicines Agency (EMA) has granted polatuzumab vedotin access to the agency’s PRIority MEdicines (PRIME) program.
The access is for polatuzumab vedotin when used in combination with rituximab and bendamustine for the treatment of relapsed or refractory diffuse large B-cell lymphoma (DLBCL).
Polatuzumab vedotin is an anti-CD79b antibody drug conjugate consisting of an anti-CD79b monoclonal antibody that is linked to a microtubule-disrupting agent.
Polatuzumab vedotin is being developed by Roche utilizing Seattle Genetics’ technology.
The goal of the EMA’s PRIME program is to accelerate the development of therapies that may offer a major advantage over existing treatments or benefit patients with no treatment options.
Through PRIME, the EMA offers early and enhanced support to developers in order to optimize development plans and speed regulatory evaluations to potentially bring therapies to patients more quickly.
To be accepted for PRIME, a therapy must demonstrate the potential to benefit patients with unmet medical need through early clinical or nonclinical data.
The acceptance of polatuzumab vedotin in the PRIME program was supported by results from the phase 2 component of the GO29365 study.
Results from this trial were recently presented at the 22nd Congress of the European Hematology Association (EHA) as abstract S468. ![]()
Writing: Do Make a MEAL of It!
Writing for publication fulfills a professional obligation to contribute to the body of knowledge. In the past decade, we’ve seen increases in the number of journals and in the number of NPs and PAs—providing more publishing opportunities and more potential authors. Yet many of my colleagues have little interest in publishing; perhaps they fear the writing process or believe they lack the skills to compose a manuscript.
How, then, do we cajole them into sharing their expertise in written form? Much has been written about why you should write.1,2 What is needed is guidance on the process to help overcome the common barriers to success in publishing. If it’s been a long time since you were in school at all—or at least since you took English Composition 101—allow me to offer a solid starting point for the journey of writing.
A manuscript, in essence, is a collection of paragraphs that follow a traditional flow. But to be sufficiently developed, paragraphs must (1) contain a main idea, (2) be structurally coherent, and (3) maintain a sense of unity around the idea.3 Once authors master the composition of a strong paragraph, the development of the manuscript comes naturally. Here are seven tips—four on content development and three on form—for compiling a manuscript worthy of publication and personal pride.
1. Start strong. Everyone understands the importance of grabbing a reader’s attention right out of the gate, right? A strong topic sentence does more than introduce the subject; it sets the tone for those that follow. A short sentence at the beginning of a paragraph establishes an understanding that a discussion will follow—and offers a preview of what the discussion will entail. But writers need not be limited; a topic sentence can take the form of a question or be placed later in the paragraph. Less experienced authors may prefer to open the paragraph with the topic sentence, however, as this allows for a quick assessment of whether the subsequent sentences follow logically. In other words, start simple—there is room to grow as you gain confidence with writing.
2. Use the MEAL plan. A paragraph should extend from the topic sentence. Collectively, the paragraph’s sentences should follow the steps outlined in the “MEAL plan,” a valuable resource conceptualized by the Duke University Thompson Writing Program.4 The “M” stands for the main topic; “E,” for the evidence that supports or refutes the topic sentence; “A,” for analysis and its importance; and “L,” for the link back to the larger claim (ie, the overall topic of the paper).
Using the MEAL approach is relatively straightforward. Picture yourself as the sender of a message; the reader is your recipient. You need to convey the information to your reader so that he or she understands it.
Limiting the number of words in a sentence and the number of sentences in a paragraph may help. Exceedingly long sentences are cumbersome and threaten to muddle the author’s message. They also pose the risk for improper noun/verb agreement, irregular punctuation, and hindered readability. (But then, what are editors for?)
A paragraph presents a well-formulated argument; one that contains only two sentences is unlikely to support the author’s assertion. Novice writers, however, often go to the other extreme, which dilutes the point. As a rule of thumb, if you can speak the entire paragraph with a single breath, the length is adequate. Reading your work aloud also helps identify hidden hazards.
3. Make connections. The result would be confusing and nonsensical. Similarly, you wouldn’t conclude your paragraph before you’ve started it. Imagine if you tried to clean out a wound after applying a dressing. The key to a good paragraph is cohesion.
If you’re scratching your head at the previous paragraph, you take my next point: All sentences within a paragraph should have a natural flow. In a well-developed paragraph, each sentence directly relates to the one before and the one after; they work together to convey your point. If the topic sentence contains an assertion, the following sentences provide supporting evidence. In medical writing, this evidence comes from citations to published literature.
If you are using the MEAL plan, you have an outline for how to support your topic sentence in a logical manner. You can then link sentences by capitalizing on words or phrases to form bridges that carry the reader through the paragraph.5 These links are created through repetition of key words or through parallel grammatical forms.
4. Pump the brakes. No one likes an abrupt stop. When you’re winding down on a paragraph, you need to signal to the reader that you’re going to shift gears. The last sentence of a paragraph should serve as a natural transition to the next paragraph.6 Equally important is the transition line at the beginning of the next paragraph.
Regardless of where the transition occurs, the goal remains to help the reader follow your logic. A subsequent paragraph can extend or refute the argument that has been presented, or it can introduce an entirely new point. No matter what, you want the reader to be prepared for a change in focus and to stay with you through your transition to a new perspective or subtopic.
On the other hand, if the argument is exhausted—it stops. When your discussion is complete, the transition to the next paragraph is very different (eg, “In summary…”). This is an appropriate time to move to a new paragraph and new section.
Speaking of a new section, let’s switch our focus to form.
5. Take action. There are two types of “voice” in communication: active, in which the subject is taking some form of action (eg, You are reading this article), and passive, in which the action is performed upon the subject (eg, This article is being read by you). Active voice is preferred for several reasons—namely, clarity and space. An active sentence identifies who is doing what. The same conclusion may be reached through a passive sentence, with some effort on the reader’s part—but active sentences tend to be more concise. They also convey a sense of immediacy, hopefully drawing the reader into your article.
6. Punctuate like a pro. Punctuation is important! It provides structure, improving clarity and comprehension. Can you fathom the confusion that would occur if an author misused (or failed to use) appropriate punctuation?
Thankfully, rules regarding punctuation have not changed much over the past century. One notable exception is use of the serial (or “Oxford”) comma, which incites passionate debate among both amateur and professional linguists.7 (For the record, this publication uses the Oxford comma. Just ask the editors: Karen, Ann, and Amy.)
A full review of punctuation is beyond the scope of this article; however, be it known that the most problematic marks—the ones to double-check in your own writing—are the apostrophe (plural or singular possessive use), the semi-colon, the comma (or lack of), and quotation marks.8 To improve your punctuation, obtain a basic grammar handbook or search online.
7. Don’t volley with verbs. Verb tense should remain consistent throughout the paragraph.9 Paragraphs with multiple verb tenses bounce the reader’s mind back and forth. It’s like watching a ping pong match, but less enjoyable.
To check for consistency, print the manuscript and mix the order of the pages. Circle the verbs. Look at the circled words and assess the tense of each. Then, repair the damage.
It is easier to identify these types of problems with a manuscript when you’re reading the pages out of order. Editors will tell you that it is difficult to focus on particulars when you are distracted by the content of the paper. Out-of-order pages are harder to read for “sense,” so you can focus on tense, voice, and punctuation.
In conclusion, I hope I’ve helped you see that writing is a process—some say a craft—but it is one that anyone can tackle with the right mindset and preparation. Most authors have a key message; they may just need help expressing it. Starting with small elements, such as the paragraph, helps the author deliver the message clearly.
1. Danielsen RD. I’d like to write a medical article, but …. Clinician Reviews. 2013;23(12):11-12.
2. Onieal ME. Be the change you wish to see. Clinician Reviews. 2015;25(7):8-9.
3. Monmouth University Tutoring and Writing Services. Paragraphs. www.monmouth.edu/uploadedFiles/Resources_for_Writers/The_Writing_Process/Paragraphs2013.pdf. Accessed June 1, 2017.
4. Duke University Thompson Writing Program. Paragraphing: The MEAL Plan. https://twp.duke.edu/sites/twp.duke.edu/files/file-attachments/meal-plan.original.pdf. Accessed June 1, 2017.
5. Purdue Online Writing Lab. On paragraphs. https://owl.english.purdue.edu/owl/resource/606/01/. Accessed June 1, 2017.
6. The Writing Center at UNC–Chapel Hill. Transitions. http://writingcenter.unc.edu/handouts/transitions/. Accessed June 1, 2017.
7. Edwards A. What is the Oxford comma and why do people care so much about it? www.grammarly.com/blog/what-is-the-oxford-comma-and-why-do-people-care-so-much-about-it/. Accessed June 1, 2017.
8. Endpaper: the Paperblanks blog. Writing Wednesday: 3 most commonly misused punctuation marks. February 1, 2017. http://blog.paperblanks.com/2017/02/writing-wednesday-3-most-commonly-misused-punctuation-marks/. Accessed June 1, 2017.
9. Towson University Online Writing Support. Verb tense consistency. https://webapps.towson.edu/ows/tenseconsistency.htm. Accessed June 1, 2017.
Writing for publication fulfills a professional obligation to contribute to the body of knowledge. In the past decade, we’ve seen increases in the number of journals and in the number of NPs and PAs—providing more publishing opportunities and more potential authors. Yet many of my colleagues have little interest in publishing; perhaps they fear the writing process or believe they lack the skills to compose a manuscript.
How, then, do we cajole them into sharing their expertise in written form? Much has been written about why you should write.1,2 What is needed is guidance on the process to help overcome the common barriers to success in publishing. If it’s been a long time since you were in school at all—or at least since you took English Composition 101—allow me to offer a solid starting point for the journey of writing.
A manuscript, in essence, is a collection of paragraphs that follow a traditional flow. But to be sufficiently developed, paragraphs must (1) contain a main idea, (2) be structurally coherent, and (3) maintain a sense of unity around the idea.3 Once authors master the composition of a strong paragraph, the development of the manuscript comes naturally. Here are seven tips—four on content development and three on form—for compiling a manuscript worthy of publication and personal pride.
1. Start strong. Everyone understands the importance of grabbing a reader’s attention right out of the gate, right? A strong topic sentence does more than introduce the subject; it sets the tone for those that follow. A short sentence at the beginning of a paragraph establishes an understanding that a discussion will follow—and offers a preview of what the discussion will entail. But writers need not be limited; a topic sentence can take the form of a question or be placed later in the paragraph. Less experienced authors may prefer to open the paragraph with the topic sentence, however, as this allows for a quick assessment of whether the subsequent sentences follow logically. In other words, start simple—there is room to grow as you gain confidence with writing.
2. Use the MEAL plan. A paragraph should extend from the topic sentence. Collectively, the paragraph’s sentences should follow the steps outlined in the “MEAL plan,” a valuable resource conceptualized by the Duke University Thompson Writing Program.4 The “M” stands for the main topic; “E,” for the evidence that supports or refutes the topic sentence; “A,” for analysis and its importance; and “L,” for the link back to the larger claim (ie, the overall topic of the paper).
Using the MEAL approach is relatively straightforward. Picture yourself as the sender of a message; the reader is your recipient. You need to convey the information to your reader so that he or she understands it.
Limiting the number of words in a sentence and the number of sentences in a paragraph may help. Exceedingly long sentences are cumbersome and threaten to muddle the author’s message. They also pose the risk for improper noun/verb agreement, irregular punctuation, and hindered readability. (But then, what are editors for?)
A paragraph presents a well-formulated argument; one that contains only two sentences is unlikely to support the author’s assertion. Novice writers, however, often go to the other extreme, which dilutes the point. As a rule of thumb, if you can speak the entire paragraph with a single breath, the length is adequate. Reading your work aloud also helps identify hidden hazards.
3. Make connections. The result would be confusing and nonsensical. Similarly, you wouldn’t conclude your paragraph before you’ve started it. Imagine if you tried to clean out a wound after applying a dressing. The key to a good paragraph is cohesion.
If you’re scratching your head at the previous paragraph, you take my next point: All sentences within a paragraph should have a natural flow. In a well-developed paragraph, each sentence directly relates to the one before and the one after; they work together to convey your point. If the topic sentence contains an assertion, the following sentences provide supporting evidence. In medical writing, this evidence comes from citations to published literature.
If you are using the MEAL plan, you have an outline for how to support your topic sentence in a logical manner. You can then link sentences by capitalizing on words or phrases to form bridges that carry the reader through the paragraph.5 These links are created through repetition of key words or through parallel grammatical forms.
4. Pump the brakes. No one likes an abrupt stop. When you’re winding down on a paragraph, you need to signal to the reader that you’re going to shift gears. The last sentence of a paragraph should serve as a natural transition to the next paragraph.6 Equally important is the transition line at the beginning of the next paragraph.
Regardless of where the transition occurs, the goal remains to help the reader follow your logic. A subsequent paragraph can extend or refute the argument that has been presented, or it can introduce an entirely new point. No matter what, you want the reader to be prepared for a change in focus and to stay with you through your transition to a new perspective or subtopic.
On the other hand, if the argument is exhausted—it stops. When your discussion is complete, the transition to the next paragraph is very different (eg, “In summary…”). This is an appropriate time to move to a new paragraph and new section.
Speaking of a new section, let’s switch our focus to form.
5. Take action. There are two types of “voice” in communication: active, in which the subject is taking some form of action (eg, You are reading this article), and passive, in which the action is performed upon the subject (eg, This article is being read by you). Active voice is preferred for several reasons—namely, clarity and space. An active sentence identifies who is doing what. The same conclusion may be reached through a passive sentence, with some effort on the reader’s part—but active sentences tend to be more concise. They also convey a sense of immediacy, hopefully drawing the reader into your article.
6. Punctuate like a pro. Punctuation is important! It provides structure, improving clarity and comprehension. Can you fathom the confusion that would occur if an author misused (or failed to use) appropriate punctuation?
Thankfully, rules regarding punctuation have not changed much over the past century. One notable exception is use of the serial (or “Oxford”) comma, which incites passionate debate among both amateur and professional linguists.7 (For the record, this publication uses the Oxford comma. Just ask the editors: Karen, Ann, and Amy.)
A full review of punctuation is beyond the scope of this article; however, be it known that the most problematic marks—the ones to double-check in your own writing—are the apostrophe (plural or singular possessive use), the semi-colon, the comma (or lack of), and quotation marks.8 To improve your punctuation, obtain a basic grammar handbook or search online.
7. Don’t volley with verbs. Verb tense should remain consistent throughout the paragraph.9 Paragraphs with multiple verb tenses bounce the reader’s mind back and forth. It’s like watching a ping pong match, but less enjoyable.
To check for consistency, print the manuscript and mix the order of the pages. Circle the verbs. Look at the circled words and assess the tense of each. Then, repair the damage.
It is easier to identify these types of problems with a manuscript when you’re reading the pages out of order. Editors will tell you that it is difficult to focus on particulars when you are distracted by the content of the paper. Out-of-order pages are harder to read for “sense,” so you can focus on tense, voice, and punctuation.
In conclusion, I hope I’ve helped you see that writing is a process—some say a craft—but it is one that anyone can tackle with the right mindset and preparation. Most authors have a key message; they may just need help expressing it. Starting with small elements, such as the paragraph, helps the author deliver the message clearly.
Writing for publication fulfills a professional obligation to contribute to the body of knowledge. In the past decade, we’ve seen increases in the number of journals and in the number of NPs and PAs—providing more publishing opportunities and more potential authors. Yet many of my colleagues have little interest in publishing; perhaps they fear the writing process or believe they lack the skills to compose a manuscript.
How, then, do we cajole them into sharing their expertise in written form? Much has been written about why you should write.1,2 What is needed is guidance on the process to help overcome the common barriers to success in publishing. If it’s been a long time since you were in school at all—or at least since you took English Composition 101—allow me to offer a solid starting point for the journey of writing.
A manuscript, in essence, is a collection of paragraphs that follow a traditional flow. But to be sufficiently developed, paragraphs must (1) contain a main idea, (2) be structurally coherent, and (3) maintain a sense of unity around the idea.3 Once authors master the composition of a strong paragraph, the development of the manuscript comes naturally. Here are seven tips—four on content development and three on form—for compiling a manuscript worthy of publication and personal pride.
1. Start strong. Everyone understands the importance of grabbing a reader’s attention right out of the gate, right? A strong topic sentence does more than introduce the subject; it sets the tone for those that follow. A short sentence at the beginning of a paragraph establishes an understanding that a discussion will follow—and offers a preview of what the discussion will entail. But writers need not be limited; a topic sentence can take the form of a question or be placed later in the paragraph. Less experienced authors may prefer to open the paragraph with the topic sentence, however, as this allows for a quick assessment of whether the subsequent sentences follow logically. In other words, start simple—there is room to grow as you gain confidence with writing.
2. Use the MEAL plan. A paragraph should extend from the topic sentence. Collectively, the paragraph’s sentences should follow the steps outlined in the “MEAL plan,” a valuable resource conceptualized by the Duke University Thompson Writing Program.4 The “M” stands for the main topic; “E,” for the evidence that supports or refutes the topic sentence; “A,” for analysis and its importance; and “L,” for the link back to the larger claim (ie, the overall topic of the paper).
Using the MEAL approach is relatively straightforward. Picture yourself as the sender of a message; the reader is your recipient. You need to convey the information to your reader so that he or she understands it.
Limiting the number of words in a sentence and the number of sentences in a paragraph may help. Exceedingly long sentences are cumbersome and threaten to muddle the author’s message. They also pose the risk for improper noun/verb agreement, irregular punctuation, and hindered readability. (But then, what are editors for?)
A paragraph presents a well-formulated argument; one that contains only two sentences is unlikely to support the author’s assertion. Novice writers, however, often go to the other extreme, which dilutes the point. As a rule of thumb, if you can speak the entire paragraph with a single breath, the length is adequate. Reading your work aloud also helps identify hidden hazards.
3. Make connections. The result would be confusing and nonsensical. Similarly, you wouldn’t conclude your paragraph before you’ve started it. Imagine if you tried to clean out a wound after applying a dressing. The key to a good paragraph is cohesion.
If you’re scratching your head at the previous paragraph, you take my next point: All sentences within a paragraph should have a natural flow. In a well-developed paragraph, each sentence directly relates to the one before and the one after; they work together to convey your point. If the topic sentence contains an assertion, the following sentences provide supporting evidence. In medical writing, this evidence comes from citations to published literature.
If you are using the MEAL plan, you have an outline for how to support your topic sentence in a logical manner. You can then link sentences by capitalizing on words or phrases to form bridges that carry the reader through the paragraph.5 These links are created through repetition of key words or through parallel grammatical forms.
4. Pump the brakes. No one likes an abrupt stop. When you’re winding down on a paragraph, you need to signal to the reader that you’re going to shift gears. The last sentence of a paragraph should serve as a natural transition to the next paragraph.6 Equally important is the transition line at the beginning of the next paragraph.
Regardless of where the transition occurs, the goal remains to help the reader follow your logic. A subsequent paragraph can extend or refute the argument that has been presented, or it can introduce an entirely new point. No matter what, you want the reader to be prepared for a change in focus and to stay with you through your transition to a new perspective or subtopic.
On the other hand, if the argument is exhausted—it stops. When your discussion is complete, the transition to the next paragraph is very different (eg, “In summary…”). This is an appropriate time to move to a new paragraph and new section.
Speaking of a new section, let’s switch our focus to form.
5. Take action. There are two types of “voice” in communication: active, in which the subject is taking some form of action (eg, You are reading this article), and passive, in which the action is performed upon the subject (eg, This article is being read by you). Active voice is preferred for several reasons—namely, clarity and space. An active sentence identifies who is doing what. The same conclusion may be reached through a passive sentence, with some effort on the reader’s part—but active sentences tend to be more concise. They also convey a sense of immediacy, hopefully drawing the reader into your article.
6. Punctuate like a pro. Punctuation is important! It provides structure, improving clarity and comprehension. Can you fathom the confusion that would occur if an author misused (or failed to use) appropriate punctuation?
Thankfully, rules regarding punctuation have not changed much over the past century. One notable exception is use of the serial (or “Oxford”) comma, which incites passionate debate among both amateur and professional linguists.7 (For the record, this publication uses the Oxford comma. Just ask the editors: Karen, Ann, and Amy.)
A full review of punctuation is beyond the scope of this article; however, be it known that the most problematic marks—the ones to double-check in your own writing—are the apostrophe (plural or singular possessive use), the semi-colon, the comma (or lack of), and quotation marks.8 To improve your punctuation, obtain a basic grammar handbook or search online.
7. Don’t volley with verbs. Verb tense should remain consistent throughout the paragraph.9 Paragraphs with multiple verb tenses bounce the reader’s mind back and forth. It’s like watching a ping pong match, but less enjoyable.
To check for consistency, print the manuscript and mix the order of the pages. Circle the verbs. Look at the circled words and assess the tense of each. Then, repair the damage.
It is easier to identify these types of problems with a manuscript when you’re reading the pages out of order. Editors will tell you that it is difficult to focus on particulars when you are distracted by the content of the paper. Out-of-order pages are harder to read for “sense,” so you can focus on tense, voice, and punctuation.
In conclusion, I hope I’ve helped you see that writing is a process—some say a craft—but it is one that anyone can tackle with the right mindset and preparation. Most authors have a key message; they may just need help expressing it. Starting with small elements, such as the paragraph, helps the author deliver the message clearly.
1. Danielsen RD. I’d like to write a medical article, but …. Clinician Reviews. 2013;23(12):11-12.
2. Onieal ME. Be the change you wish to see. Clinician Reviews. 2015;25(7):8-9.
3. Monmouth University Tutoring and Writing Services. Paragraphs. www.monmouth.edu/uploadedFiles/Resources_for_Writers/The_Writing_Process/Paragraphs2013.pdf. Accessed June 1, 2017.
4. Duke University Thompson Writing Program. Paragraphing: The MEAL Plan. https://twp.duke.edu/sites/twp.duke.edu/files/file-attachments/meal-plan.original.pdf. Accessed June 1, 2017.
5. Purdue Online Writing Lab. On paragraphs. https://owl.english.purdue.edu/owl/resource/606/01/. Accessed June 1, 2017.
6. The Writing Center at UNC–Chapel Hill. Transitions. http://writingcenter.unc.edu/handouts/transitions/. Accessed June 1, 2017.
7. Edwards A. What is the Oxford comma and why do people care so much about it? www.grammarly.com/blog/what-is-the-oxford-comma-and-why-do-people-care-so-much-about-it/. Accessed June 1, 2017.
8. Endpaper: the Paperblanks blog. Writing Wednesday: 3 most commonly misused punctuation marks. February 1, 2017. http://blog.paperblanks.com/2017/02/writing-wednesday-3-most-commonly-misused-punctuation-marks/. Accessed June 1, 2017.
9. Towson University Online Writing Support. Verb tense consistency. https://webapps.towson.edu/ows/tenseconsistency.htm. Accessed June 1, 2017.
1. Danielsen RD. I’d like to write a medical article, but …. Clinician Reviews. 2013;23(12):11-12.
2. Onieal ME. Be the change you wish to see. Clinician Reviews. 2015;25(7):8-9.
3. Monmouth University Tutoring and Writing Services. Paragraphs. www.monmouth.edu/uploadedFiles/Resources_for_Writers/The_Writing_Process/Paragraphs2013.pdf. Accessed June 1, 2017.
4. Duke University Thompson Writing Program. Paragraphing: The MEAL Plan. https://twp.duke.edu/sites/twp.duke.edu/files/file-attachments/meal-plan.original.pdf. Accessed June 1, 2017.
5. Purdue Online Writing Lab. On paragraphs. https://owl.english.purdue.edu/owl/resource/606/01/. Accessed June 1, 2017.
6. The Writing Center at UNC–Chapel Hill. Transitions. http://writingcenter.unc.edu/handouts/transitions/. Accessed June 1, 2017.
7. Edwards A. What is the Oxford comma and why do people care so much about it? www.grammarly.com/blog/what-is-the-oxford-comma-and-why-do-people-care-so-much-about-it/. Accessed June 1, 2017.
8. Endpaper: the Paperblanks blog. Writing Wednesday: 3 most commonly misused punctuation marks. February 1, 2017. http://blog.paperblanks.com/2017/02/writing-wednesday-3-most-commonly-misused-punctuation-marks/. Accessed June 1, 2017.
9. Towson University Online Writing Support. Verb tense consistency. https://webapps.towson.edu/ows/tenseconsistency.htm. Accessed June 1, 2017.
‘Chronic Lyme’: Serious bacterial infections reported with unproven treatments
Unproven treatments for so-called chronic Lyme disease can cause serious, even fatal, complications, according to five case reports published in the Morbidity and Mortality Weekly Report.
“Patients, clinicians, and public health practitioners should be aware that treatments for chronic Lyme disease can carry serious risks,” the authors wrote.
Chronic Lyme disease is a nonspecific diagnosis that has no consistent definition. Some clinicians use this label for patients who have a variety of debilitating conditions such as fatigue, generalized pain, and neurologic symptoms, even in the absence of laboratory evidence of Borrelia burgdorferi infection, objective signs of infection, or a history of tick exposure. People who support this diagnosis mistakenly believe that B. burgdorferi can cause longstanding disabling symptoms even when standard testing for the organism is negative, when the truth is that tests for the organism become more sensitive the longer the infection persists, according to Natalie S. Marzec, MD, a resident in preventive medicine at the University of Colorado, Aurora, and her associates.
Patients who cannot obtain symptom relief with conventional clinicians may consult “practitioners who might identify themselves as Lyme disease specialists (‘Lyme literate’ doctors) or from complementary and alternative medicine clinics,” where they are diagnosed with chronic Lyme disease. Such patients have been offered unproven treatments, including extended courses of intravenous antibiotics, infusions of hydrogen peroxide, immunoglobulin therapy, hyperbaric oxygen treatment, electromagnetic frequency therapy, garlic supplements, colloidal silver, and stem-cell transplantation.
Dr. Marzec and her associates presented case reports of five such patients diagnosed with chronic Lyme disease who sustained serious harm from such treatments (MMWR 2017;66[23]:607-9).
A woman in her late 30s with fatigue and joint pain was given a peripherally inserted central catheter (PICC) for IV delivery of ceftriaxone and cefotaxime. After 3 weeks, she developed fever, rash, hypotension, and tachycardia. In the intensive care unit (ICU), she was given broad-spectrum IV antibiotics and vasopressors, and was mechanically ventilated, but died of septic shock related to catheter-associated bacteremia.
An adolescent was told at an alternative medicine clinic that her years of muscle and joint pain, backaches, headaches, and lethargy were due to chronic Lyme disease. A PICC was placed to deliver IV antibiotics for 5 months. She developed pallor, chills, fever, hypotension, and tachycardia consistent with septic shock. Cultures demonstrated Acinetobacter species in her blood and on the PICC, and she required several weeks of ICU care.
A woman in her late 40s was diagnosed as having chronic Lyme disease based on unvalidated tests and was treated for months with intramuscular penicillin, IV ceftriazone, and IV azithromycin administered through a tunneled IV catheter; as well as doxycycline, and the antiparasitic drug tinidazole. She was hospitalized for back pain, shortness of breath, and malaise, and cultures of the catheter and her blood yielded Pseudomonas aeruginosa. She was found to have osteodiscitis caused by the same organism, with destruction of the 9th and 10th vertebrae, and was treated, and her back pain eventually improved.
A woman in her 50s was diagnosed as having amyotrophic lateral sclerosis, but sought a second opinion and was told that she had chronic Lyme disease (along with babesiosis, and Rocky Mountain spotted fever). She was treated with herbs and homeopathic remedies, followed by intensive antimicrobial and antiviral therapies. She developed intractable Clostridium difficile colitis that lasted for 2 years until she died from complications related to amyotrophic lateral sclerosis.
A woman in her 60s who had autoimmune neutropenia, mixed connective tissue disease, and degenerative arthritis was told her neuropathy was due to chronic Lyme disease. She was treated with immunoglobulin administered through an implanted subcutaneous port. After years of treatments, she was hospitalized for fever and back pain, had blood cultures positive for methicillin-sensitive Staphylococcus aureus, was found to have inflammation of the lumbar facet joints, epidural space, and paraspinal muscles – and eventually required surgical drainage of a paraspinal abscess.
“These cases highlight the severity and scope of adverse effects that can be caused by the use of unproven treatments for chronic Lyme disease,” Dr. Marzec and her associates said.
This work was supported by the CDC. Dr. Marzec and her associates reported having no relevant financial disclosures.
Unproven treatments for so-called chronic Lyme disease can cause serious, even fatal, complications, according to five case reports published in the Morbidity and Mortality Weekly Report.
“Patients, clinicians, and public health practitioners should be aware that treatments for chronic Lyme disease can carry serious risks,” the authors wrote.
Chronic Lyme disease is a nonspecific diagnosis that has no consistent definition. Some clinicians use this label for patients who have a variety of debilitating conditions such as fatigue, generalized pain, and neurologic symptoms, even in the absence of laboratory evidence of Borrelia burgdorferi infection, objective signs of infection, or a history of tick exposure. People who support this diagnosis mistakenly believe that B. burgdorferi can cause longstanding disabling symptoms even when standard testing for the organism is negative, when the truth is that tests for the organism become more sensitive the longer the infection persists, according to Natalie S. Marzec, MD, a resident in preventive medicine at the University of Colorado, Aurora, and her associates.
Patients who cannot obtain symptom relief with conventional clinicians may consult “practitioners who might identify themselves as Lyme disease specialists (‘Lyme literate’ doctors) or from complementary and alternative medicine clinics,” where they are diagnosed with chronic Lyme disease. Such patients have been offered unproven treatments, including extended courses of intravenous antibiotics, infusions of hydrogen peroxide, immunoglobulin therapy, hyperbaric oxygen treatment, electromagnetic frequency therapy, garlic supplements, colloidal silver, and stem-cell transplantation.
Dr. Marzec and her associates presented case reports of five such patients diagnosed with chronic Lyme disease who sustained serious harm from such treatments (MMWR 2017;66[23]:607-9).
A woman in her late 30s with fatigue and joint pain was given a peripherally inserted central catheter (PICC) for IV delivery of ceftriaxone and cefotaxime. After 3 weeks, she developed fever, rash, hypotension, and tachycardia. In the intensive care unit (ICU), she was given broad-spectrum IV antibiotics and vasopressors, and was mechanically ventilated, but died of septic shock related to catheter-associated bacteremia.
An adolescent was told at an alternative medicine clinic that her years of muscle and joint pain, backaches, headaches, and lethargy were due to chronic Lyme disease. A PICC was placed to deliver IV antibiotics for 5 months. She developed pallor, chills, fever, hypotension, and tachycardia consistent with septic shock. Cultures demonstrated Acinetobacter species in her blood and on the PICC, and she required several weeks of ICU care.
A woman in her late 40s was diagnosed as having chronic Lyme disease based on unvalidated tests and was treated for months with intramuscular penicillin, IV ceftriazone, and IV azithromycin administered through a tunneled IV catheter; as well as doxycycline, and the antiparasitic drug tinidazole. She was hospitalized for back pain, shortness of breath, and malaise, and cultures of the catheter and her blood yielded Pseudomonas aeruginosa. She was found to have osteodiscitis caused by the same organism, with destruction of the 9th and 10th vertebrae, and was treated, and her back pain eventually improved.
A woman in her 50s was diagnosed as having amyotrophic lateral sclerosis, but sought a second opinion and was told that she had chronic Lyme disease (along with babesiosis, and Rocky Mountain spotted fever). She was treated with herbs and homeopathic remedies, followed by intensive antimicrobial and antiviral therapies. She developed intractable Clostridium difficile colitis that lasted for 2 years until she died from complications related to amyotrophic lateral sclerosis.
A woman in her 60s who had autoimmune neutropenia, mixed connective tissue disease, and degenerative arthritis was told her neuropathy was due to chronic Lyme disease. She was treated with immunoglobulin administered through an implanted subcutaneous port. After years of treatments, she was hospitalized for fever and back pain, had blood cultures positive for methicillin-sensitive Staphylococcus aureus, was found to have inflammation of the lumbar facet joints, epidural space, and paraspinal muscles – and eventually required surgical drainage of a paraspinal abscess.
“These cases highlight the severity and scope of adverse effects that can be caused by the use of unproven treatments for chronic Lyme disease,” Dr. Marzec and her associates said.
This work was supported by the CDC. Dr. Marzec and her associates reported having no relevant financial disclosures.
Unproven treatments for so-called chronic Lyme disease can cause serious, even fatal, complications, according to five case reports published in the Morbidity and Mortality Weekly Report.
“Patients, clinicians, and public health practitioners should be aware that treatments for chronic Lyme disease can carry serious risks,” the authors wrote.
Chronic Lyme disease is a nonspecific diagnosis that has no consistent definition. Some clinicians use this label for patients who have a variety of debilitating conditions such as fatigue, generalized pain, and neurologic symptoms, even in the absence of laboratory evidence of Borrelia burgdorferi infection, objective signs of infection, or a history of tick exposure. People who support this diagnosis mistakenly believe that B. burgdorferi can cause longstanding disabling symptoms even when standard testing for the organism is negative, when the truth is that tests for the organism become more sensitive the longer the infection persists, according to Natalie S. Marzec, MD, a resident in preventive medicine at the University of Colorado, Aurora, and her associates.
Patients who cannot obtain symptom relief with conventional clinicians may consult “practitioners who might identify themselves as Lyme disease specialists (‘Lyme literate’ doctors) or from complementary and alternative medicine clinics,” where they are diagnosed with chronic Lyme disease. Such patients have been offered unproven treatments, including extended courses of intravenous antibiotics, infusions of hydrogen peroxide, immunoglobulin therapy, hyperbaric oxygen treatment, electromagnetic frequency therapy, garlic supplements, colloidal silver, and stem-cell transplantation.
Dr. Marzec and her associates presented case reports of five such patients diagnosed with chronic Lyme disease who sustained serious harm from such treatments (MMWR 2017;66[23]:607-9).
A woman in her late 30s with fatigue and joint pain was given a peripherally inserted central catheter (PICC) for IV delivery of ceftriaxone and cefotaxime. After 3 weeks, she developed fever, rash, hypotension, and tachycardia. In the intensive care unit (ICU), she was given broad-spectrum IV antibiotics and vasopressors, and was mechanically ventilated, but died of septic shock related to catheter-associated bacteremia.
An adolescent was told at an alternative medicine clinic that her years of muscle and joint pain, backaches, headaches, and lethargy were due to chronic Lyme disease. A PICC was placed to deliver IV antibiotics for 5 months. She developed pallor, chills, fever, hypotension, and tachycardia consistent with septic shock. Cultures demonstrated Acinetobacter species in her blood and on the PICC, and she required several weeks of ICU care.
A woman in her late 40s was diagnosed as having chronic Lyme disease based on unvalidated tests and was treated for months with intramuscular penicillin, IV ceftriazone, and IV azithromycin administered through a tunneled IV catheter; as well as doxycycline, and the antiparasitic drug tinidazole. She was hospitalized for back pain, shortness of breath, and malaise, and cultures of the catheter and her blood yielded Pseudomonas aeruginosa. She was found to have osteodiscitis caused by the same organism, with destruction of the 9th and 10th vertebrae, and was treated, and her back pain eventually improved.
A woman in her 50s was diagnosed as having amyotrophic lateral sclerosis, but sought a second opinion and was told that she had chronic Lyme disease (along with babesiosis, and Rocky Mountain spotted fever). She was treated with herbs and homeopathic remedies, followed by intensive antimicrobial and antiviral therapies. She developed intractable Clostridium difficile colitis that lasted for 2 years until she died from complications related to amyotrophic lateral sclerosis.
A woman in her 60s who had autoimmune neutropenia, mixed connective tissue disease, and degenerative arthritis was told her neuropathy was due to chronic Lyme disease. She was treated with immunoglobulin administered through an implanted subcutaneous port. After years of treatments, she was hospitalized for fever and back pain, had blood cultures positive for methicillin-sensitive Staphylococcus aureus, was found to have inflammation of the lumbar facet joints, epidural space, and paraspinal muscles – and eventually required surgical drainage of a paraspinal abscess.
“These cases highlight the severity and scope of adverse effects that can be caused by the use of unproven treatments for chronic Lyme disease,” Dr. Marzec and her associates said.
This work was supported by the CDC. Dr. Marzec and her associates reported having no relevant financial disclosures.
FROM MMWR
Key clinical point: Unproven treatments for so-called chronic Lyme disease cause serious, even fatal, complications.
Major finding:
Data source: Five case reports submitted to the CDC by clinicians, health departments, and individual patients.
Disclosures: This work was supported by the CDC. Dr. Marzec and her associates reported having no relevant financial disclosures.