User login
Sen. Alexander seeks quick, passable steps to stabilize individual markets
WASHINGTON – Sen. Lamar Alexander (R-Tenn.) wants two quick fixes to stabilize the individual health insurance market so that Congress can come together to craft a long-term solution.
There seems to be “general consensus that we should see what can we do, No. 1, on the cost-sharing payments and, No. 2, on amending 1332 [waiver program] to provide flexibility to the states,” Sen. Alexander*, chairman of the Senate Health, Education, Labor, and Pensions Committee said Sept. 6 after the first of four hearings the committee is holding on creating stability for the individual health insurance market.
Chairman Alexander said that he hopes to have a small, passable legislative proposal ready by Sept. 14, in time to allow insurers to make last-minute adjustments to their individual market bids, which are due to state insurance commissioners by Sept. 20.
Sen. Patty Murray (D-Wash.), the committee’s ranking member, stressed the urgent need to pass a quick fix, noting that insurance premiums could rise as much as 20% higher than they would have, if there is no guarantee on the cost-sharing reduction (CSR) payments.
A panel of state insurance commissioners all agreed with the two proposals, though there were suggestions that the CSR payments should be guaranteed for at least a year, if not longer, beyond 2018, which was when Chairman Alexander suggested the guarantee should sunset.
“The CSR funding issue is the single most critical issue you can address to help stabilize insurance markets in 2018,” Julie Mix McPeak, commissioner of the Tennessee Department of Commerce and Insurance, testified at the hearing. She emphasized that it is not an insurance bailout. “CSR funding ensures that some of our most vulnerable consumers receive assistance for copays and deductibles that are required to be paid under federal law and has the effect of reducing proposed premium increases and has a direct impact on the amount of subsidy assistance provided by the federal government.”
“You must permanently fund the cost-sharing reduction payments,” Mike Kreidler, insurance commissioner for the state of Washington, testified during the hearing. “That is something that is going help a great deal in our marketplace.”
The commissioners also advocated for a reinsurance program.
Ms. McPeak called for Congress to “establish a reinsurance mechanism that would stop losses for individual claims at a specified amount to increase market participation by carriers. For the most immediate impact, this backstop mechanism must be federal, as it would be impossible for many states to develop such a program for the 2018 plan year.”
“I urge you to create a federal reinsurance program this year,” Mr. Kreidler said. “Doing this would show your commitment to stabilizing the market. They worked very well in the state of Washington for the first 3 years we had a reinsurance program. We would like to see it continue and go forward.”
In talking about fixing 1332 waivers, which allow states to develop a specific proposal that works within the parameters of the Affordable Care Act but offers flexibility to create programs that are unique to a state’s needs, Lori Wing-Heier, director of the Alaska Division of Insurance talked about her state’s successful efforts to get a wavier for its reinsurance program.
“The waiver process is somewhat onerous in the fact that there is not a defined application to submit,” Ms. Wing-Heier said, leaving it up to states to make sure they are providing all the requested information. “After that, the part that is stifling states right now is the 6-month waiting period before they receive final approval.”
Other issues were raised by both panelists and senators, including the cost of delivering health care, the cost of prescription drugs, and cuts to marketing budgets, but Chairman Alexander said that he did not want to expand a proposal beyond his two points.
“My whole focus right now is: What can we do to take a couple of steps to stabilize the individual market?” he said, although he suggested that if there were clear consensus on other aspects, they could be considered.
The archived hearing can be viewed on the committee’s website.
CORRECTION, 9/8/17: An earlier version of this story misidentified the HELP Committee chairman.
WASHINGTON – Sen. Lamar Alexander (R-Tenn.) wants two quick fixes to stabilize the individual health insurance market so that Congress can come together to craft a long-term solution.
There seems to be “general consensus that we should see what can we do, No. 1, on the cost-sharing payments and, No. 2, on amending 1332 [waiver program] to provide flexibility to the states,” Sen. Alexander*, chairman of the Senate Health, Education, Labor, and Pensions Committee said Sept. 6 after the first of four hearings the committee is holding on creating stability for the individual health insurance market.
Chairman Alexander said that he hopes to have a small, passable legislative proposal ready by Sept. 14, in time to allow insurers to make last-minute adjustments to their individual market bids, which are due to state insurance commissioners by Sept. 20.
Sen. Patty Murray (D-Wash.), the committee’s ranking member, stressed the urgent need to pass a quick fix, noting that insurance premiums could rise as much as 20% higher than they would have, if there is no guarantee on the cost-sharing reduction (CSR) payments.
A panel of state insurance commissioners all agreed with the two proposals, though there were suggestions that the CSR payments should be guaranteed for at least a year, if not longer, beyond 2018, which was when Chairman Alexander suggested the guarantee should sunset.
“The CSR funding issue is the single most critical issue you can address to help stabilize insurance markets in 2018,” Julie Mix McPeak, commissioner of the Tennessee Department of Commerce and Insurance, testified at the hearing. She emphasized that it is not an insurance bailout. “CSR funding ensures that some of our most vulnerable consumers receive assistance for copays and deductibles that are required to be paid under federal law and has the effect of reducing proposed premium increases and has a direct impact on the amount of subsidy assistance provided by the federal government.”
“You must permanently fund the cost-sharing reduction payments,” Mike Kreidler, insurance commissioner for the state of Washington, testified during the hearing. “That is something that is going help a great deal in our marketplace.”
The commissioners also advocated for a reinsurance program.
Ms. McPeak called for Congress to “establish a reinsurance mechanism that would stop losses for individual claims at a specified amount to increase market participation by carriers. For the most immediate impact, this backstop mechanism must be federal, as it would be impossible for many states to develop such a program for the 2018 plan year.”
“I urge you to create a federal reinsurance program this year,” Mr. Kreidler said. “Doing this would show your commitment to stabilizing the market. They worked very well in the state of Washington for the first 3 years we had a reinsurance program. We would like to see it continue and go forward.”
In talking about fixing 1332 waivers, which allow states to develop a specific proposal that works within the parameters of the Affordable Care Act but offers flexibility to create programs that are unique to a state’s needs, Lori Wing-Heier, director of the Alaska Division of Insurance talked about her state’s successful efforts to get a wavier for its reinsurance program.
“The waiver process is somewhat onerous in the fact that there is not a defined application to submit,” Ms. Wing-Heier said, leaving it up to states to make sure they are providing all the requested information. “After that, the part that is stifling states right now is the 6-month waiting period before they receive final approval.”
Other issues were raised by both panelists and senators, including the cost of delivering health care, the cost of prescription drugs, and cuts to marketing budgets, but Chairman Alexander said that he did not want to expand a proposal beyond his two points.
“My whole focus right now is: What can we do to take a couple of steps to stabilize the individual market?” he said, although he suggested that if there were clear consensus on other aspects, they could be considered.
The archived hearing can be viewed on the committee’s website.
CORRECTION, 9/8/17: An earlier version of this story misidentified the HELP Committee chairman.
WASHINGTON – Sen. Lamar Alexander (R-Tenn.) wants two quick fixes to stabilize the individual health insurance market so that Congress can come together to craft a long-term solution.
There seems to be “general consensus that we should see what can we do, No. 1, on the cost-sharing payments and, No. 2, on amending 1332 [waiver program] to provide flexibility to the states,” Sen. Alexander*, chairman of the Senate Health, Education, Labor, and Pensions Committee said Sept. 6 after the first of four hearings the committee is holding on creating stability for the individual health insurance market.
Chairman Alexander said that he hopes to have a small, passable legislative proposal ready by Sept. 14, in time to allow insurers to make last-minute adjustments to their individual market bids, which are due to state insurance commissioners by Sept. 20.
Sen. Patty Murray (D-Wash.), the committee’s ranking member, stressed the urgent need to pass a quick fix, noting that insurance premiums could rise as much as 20% higher than they would have, if there is no guarantee on the cost-sharing reduction (CSR) payments.
A panel of state insurance commissioners all agreed with the two proposals, though there were suggestions that the CSR payments should be guaranteed for at least a year, if not longer, beyond 2018, which was when Chairman Alexander suggested the guarantee should sunset.
“The CSR funding issue is the single most critical issue you can address to help stabilize insurance markets in 2018,” Julie Mix McPeak, commissioner of the Tennessee Department of Commerce and Insurance, testified at the hearing. She emphasized that it is not an insurance bailout. “CSR funding ensures that some of our most vulnerable consumers receive assistance for copays and deductibles that are required to be paid under federal law and has the effect of reducing proposed premium increases and has a direct impact on the amount of subsidy assistance provided by the federal government.”
“You must permanently fund the cost-sharing reduction payments,” Mike Kreidler, insurance commissioner for the state of Washington, testified during the hearing. “That is something that is going help a great deal in our marketplace.”
The commissioners also advocated for a reinsurance program.
Ms. McPeak called for Congress to “establish a reinsurance mechanism that would stop losses for individual claims at a specified amount to increase market participation by carriers. For the most immediate impact, this backstop mechanism must be federal, as it would be impossible for many states to develop such a program for the 2018 plan year.”
“I urge you to create a federal reinsurance program this year,” Mr. Kreidler said. “Doing this would show your commitment to stabilizing the market. They worked very well in the state of Washington for the first 3 years we had a reinsurance program. We would like to see it continue and go forward.”
In talking about fixing 1332 waivers, which allow states to develop a specific proposal that works within the parameters of the Affordable Care Act but offers flexibility to create programs that are unique to a state’s needs, Lori Wing-Heier, director of the Alaska Division of Insurance talked about her state’s successful efforts to get a wavier for its reinsurance program.
“The waiver process is somewhat onerous in the fact that there is not a defined application to submit,” Ms. Wing-Heier said, leaving it up to states to make sure they are providing all the requested information. “After that, the part that is stifling states right now is the 6-month waiting period before they receive final approval.”
Other issues were raised by both panelists and senators, including the cost of delivering health care, the cost of prescription drugs, and cuts to marketing budgets, but Chairman Alexander said that he did not want to expand a proposal beyond his two points.
“My whole focus right now is: What can we do to take a couple of steps to stabilize the individual market?” he said, although he suggested that if there were clear consensus on other aspects, they could be considered.
The archived hearing can be viewed on the committee’s website.
CORRECTION, 9/8/17: An earlier version of this story misidentified the HELP Committee chairman.
AT A SENATE HELP COMMITTEE HEARING
Can High Frequency Oscillations Help Predict Seizures?
A recently published practical guide can help epilepsy specialists record and interpret high-frequency oscillations (HFOs), which have been implicated as promising markers for the disease. Such biomarkers may help better locate areas in which to perform surgery. Researchers offer the following observations:
- To accurately record HFOs, experts emphasize the importance of low noise recording to reduce artifacts.
- Even under ideal conditions, artifacts can still occur, including muscle contractions, movement, and filtering.
- Magnetoencephalography (MEG) and EEG recordings are capable of recording ripples while intracranial EEG can record ripples, fast ripples, and very high frequency oscillations.
- Nonetheless, MEG and intracranial EEG recordings can also experience artifacts.
- Researchers believe that HFOs represent a new type of electromagnetic biomarker that can detect epileptogenic brain tissue, but several technical barriers need to be overcome before clinicians can use them as tools in community practice.
- Software engineers are in the process of developing HFO filters and automatic detection tools to help bring this technology to market.
Zijlmans M, Worrell GA, Dümpelmann M, et al. How to record high-frequency oscillations in epilepsy: A practical guideline. Epilepsia. 2017;58(8):1305-1315. d
A recently published practical guide can help epilepsy specialists record and interpret high-frequency oscillations (HFOs), which have been implicated as promising markers for the disease. Such biomarkers may help better locate areas in which to perform surgery. Researchers offer the following observations:
- To accurately record HFOs, experts emphasize the importance of low noise recording to reduce artifacts.
- Even under ideal conditions, artifacts can still occur, including muscle contractions, movement, and filtering.
- Magnetoencephalography (MEG) and EEG recordings are capable of recording ripples while intracranial EEG can record ripples, fast ripples, and very high frequency oscillations.
- Nonetheless, MEG and intracranial EEG recordings can also experience artifacts.
- Researchers believe that HFOs represent a new type of electromagnetic biomarker that can detect epileptogenic brain tissue, but several technical barriers need to be overcome before clinicians can use them as tools in community practice.
- Software engineers are in the process of developing HFO filters and automatic detection tools to help bring this technology to market.
Zijlmans M, Worrell GA, Dümpelmann M, et al. How to record high-frequency oscillations in epilepsy: A practical guideline. Epilepsia. 2017;58(8):1305-1315. d
A recently published practical guide can help epilepsy specialists record and interpret high-frequency oscillations (HFOs), which have been implicated as promising markers for the disease. Such biomarkers may help better locate areas in which to perform surgery. Researchers offer the following observations:
- To accurately record HFOs, experts emphasize the importance of low noise recording to reduce artifacts.
- Even under ideal conditions, artifacts can still occur, including muscle contractions, movement, and filtering.
- Magnetoencephalography (MEG) and EEG recordings are capable of recording ripples while intracranial EEG can record ripples, fast ripples, and very high frequency oscillations.
- Nonetheless, MEG and intracranial EEG recordings can also experience artifacts.
- Researchers believe that HFOs represent a new type of electromagnetic biomarker that can detect epileptogenic brain tissue, but several technical barriers need to be overcome before clinicians can use them as tools in community practice.
- Software engineers are in the process of developing HFO filters and automatic detection tools to help bring this technology to market.
Zijlmans M, Worrell GA, Dümpelmann M, et al. How to record high-frequency oscillations in epilepsy: A practical guideline. Epilepsia. 2017;58(8):1305-1315. d
New Insights on High Frequency Oscillations and the Association With Epilepsy
High frequency oscillations (HFOs) in certain regions of the brain have been associated with epilepsy. A recent review of the literature on HFOs is shedding new light on their possible role in the pathophysiology of the disease.
- Advances in recording technology and optogenetics have enhanced researchers’ ability to study HFOs.
- Substrates of HFOs linked to epilepsy have been identified with the help of sophisticated computer models.
- In the past, HFOs have been studied in animals and patients with temporal lobe epilepsy, but HFOs have recently been reported in patients with other types of seizures, including neocortical epilepsy, genetically induced epilepsy, and infantile spasms.
- High resolution in vivo imaging, optogenetics, and chemogenetic techniques are poised to help future investigators gain even deeper insights into the role of HFOs in seizures and epilepsy.
- The reviewers postulate that as investigators gain a better understanding of how HFOs are involved in the pathogenesis of epileptic disorders, it will allow them to better classify these diseases and improve diagnosis and treatment.
Jiruska P, Alvarado-Rojas C, Schevon CA, et al. Update on the mechanisms and roles of high-frequency oscillations in seizures and epileptic disorders. Epilepsia. 2017;58(8):1330-1339.
High frequency oscillations (HFOs) in certain regions of the brain have been associated with epilepsy. A recent review of the literature on HFOs is shedding new light on their possible role in the pathophysiology of the disease.
- Advances in recording technology and optogenetics have enhanced researchers’ ability to study HFOs.
- Substrates of HFOs linked to epilepsy have been identified with the help of sophisticated computer models.
- In the past, HFOs have been studied in animals and patients with temporal lobe epilepsy, but HFOs have recently been reported in patients with other types of seizures, including neocortical epilepsy, genetically induced epilepsy, and infantile spasms.
- High resolution in vivo imaging, optogenetics, and chemogenetic techniques are poised to help future investigators gain even deeper insights into the role of HFOs in seizures and epilepsy.
- The reviewers postulate that as investigators gain a better understanding of how HFOs are involved in the pathogenesis of epileptic disorders, it will allow them to better classify these diseases and improve diagnosis and treatment.
Jiruska P, Alvarado-Rojas C, Schevon CA, et al. Update on the mechanisms and roles of high-frequency oscillations in seizures and epileptic disorders. Epilepsia. 2017;58(8):1330-1339.
High frequency oscillations (HFOs) in certain regions of the brain have been associated with epilepsy. A recent review of the literature on HFOs is shedding new light on their possible role in the pathophysiology of the disease.
- Advances in recording technology and optogenetics have enhanced researchers’ ability to study HFOs.
- Substrates of HFOs linked to epilepsy have been identified with the help of sophisticated computer models.
- In the past, HFOs have been studied in animals and patients with temporal lobe epilepsy, but HFOs have recently been reported in patients with other types of seizures, including neocortical epilepsy, genetically induced epilepsy, and infantile spasms.
- High resolution in vivo imaging, optogenetics, and chemogenetic techniques are poised to help future investigators gain even deeper insights into the role of HFOs in seizures and epilepsy.
- The reviewers postulate that as investigators gain a better understanding of how HFOs are involved in the pathogenesis of epileptic disorders, it will allow them to better classify these diseases and improve diagnosis and treatment.
Jiruska P, Alvarado-Rojas C, Schevon CA, et al. Update on the mechanisms and roles of high-frequency oscillations in seizures and epileptic disorders. Epilepsia. 2017;58(8):1330-1339.
A New Statistical Approach for Variability in Seizure Frequency
Researchers have devised a statistical approach that can take into account the variability in seizure frequency associated with a placebo response. In an article published in Epilepsy Research, Goldenholz et al explain their approach in more detail:
- The researchers compared the traditional 50% responder rate to a new measure called the variability-corrected score or Zv.
- The analysis revealed that the new approach can predict the expected frequency of seizures several months in advance.
- When they compared the 50% responder rates to the variability-corrected score, Zv was more useful in telling the difference between patients who had a placebo response and those who had a genuine response to a therapeutic intervention.
- Because Zv scores were able to generate higher statistical power, the authors theorize that they may allow researchers to conduct randomized clinical trials that use fewer patients and cost less than traditional randomized controlled trials.
Goldenholz DM, Goldenholz SR, Moss R, et al. Does accounting for seizure frequency variability increase clinical trial power? [Published online ahead of pint July 25, 2017]. Epilepsy Res. doi.org/10.1016/j.eplepsyres.2017.07.013
Researchers have devised a statistical approach that can take into account the variability in seizure frequency associated with a placebo response. In an article published in Epilepsy Research, Goldenholz et al explain their approach in more detail:
- The researchers compared the traditional 50% responder rate to a new measure called the variability-corrected score or Zv.
- The analysis revealed that the new approach can predict the expected frequency of seizures several months in advance.
- When they compared the 50% responder rates to the variability-corrected score, Zv was more useful in telling the difference between patients who had a placebo response and those who had a genuine response to a therapeutic intervention.
- Because Zv scores were able to generate higher statistical power, the authors theorize that they may allow researchers to conduct randomized clinical trials that use fewer patients and cost less than traditional randomized controlled trials.
Goldenholz DM, Goldenholz SR, Moss R, et al. Does accounting for seizure frequency variability increase clinical trial power? [Published online ahead of pint July 25, 2017]. Epilepsy Res. doi.org/10.1016/j.eplepsyres.2017.07.013
Researchers have devised a statistical approach that can take into account the variability in seizure frequency associated with a placebo response. In an article published in Epilepsy Research, Goldenholz et al explain their approach in more detail:
- The researchers compared the traditional 50% responder rate to a new measure called the variability-corrected score or Zv.
- The analysis revealed that the new approach can predict the expected frequency of seizures several months in advance.
- When they compared the 50% responder rates to the variability-corrected score, Zv was more useful in telling the difference between patients who had a placebo response and those who had a genuine response to a therapeutic intervention.
- Because Zv scores were able to generate higher statistical power, the authors theorize that they may allow researchers to conduct randomized clinical trials that use fewer patients and cost less than traditional randomized controlled trials.
Goldenholz DM, Goldenholz SR, Moss R, et al. Does accounting for seizure frequency variability increase clinical trial power? [Published online ahead of pint July 25, 2017]. Epilepsy Res. doi.org/10.1016/j.eplepsyres.2017.07.013
Half of young men at-risk for HIV maintained protective PrEP levels with monthly visits
Up to 54% of adolescent men who had sex with men maintained tenofovir disphosphate levels consistent with a high degree of anti-HIV protection while attending monthly clinic visits, but this proportion fell as low as 17% after they switched to quarterly visits, according to the results of a first-of-its-kind 48-week, prospective, multicenter trial.
The Adolescent Medicine Trials Network for HIV/AIDS Interventions (Project PrEPare) evaluated the safety, tolerability, and acceptability of tenofovir disoproxil fumarate and emtricitabine among 78 HIV-negative MSM aged 15-17 years who had condomless anal intercourse with a male of positive or unknown HIV status, had multiple male sex partners, or had other high-risk sexual behaviors or sexually transmitted infections within the previous 6 months. Participants averaged 16.5 years old age, 33% were of mixed race or ethnicity, 29% were African American, 21% were white Hispanic, 14% were white, and 3% were Asian or Pacific Islander. They received one cognitive-behavioral risk-reduction session before starting PrEP and were paid $50-$75 to attend follow-up visits every month for 3 months and then quarterly after that.
While attending monthly visits, between 47% and 54% of participants maintained highly protective levels of tenofovir disphosphate (above 700 fmol/punch, based on dried blood spot tests), but this proportion fell as low as 17% after participants switched to quarterly visits. At week 48, the HIV seroconversion rate was 6.4 per 100 person-years (95% confidence interval, 1.3-8.7), underscoring the need to offer PrEP and behavioral support to this population, the researchers emphasized. “The waning adherence, especially with quarterly visits, demonstrates that more time, attention, and resources may need to be allocated to adolescents who are seeking prevention services,” they wrote. “We need to better understand the barriers to adherence and develop more effective ways to enhance adherence for youth who are clinically prescribed PrEP.”
Gilead Sciences provided the study drugs and helped fund the study. The study was supported by grants from the National Institute of Child Health and Human Development, the National Institutes on Drug Abuse and Mental Health, and the Adolescent Medicine Trials Network for HIV/AIDS Interventions. Dr. Hosek had no disclosures. Two coinvestigators disclosed research support and contract work fees from Gilead.
As an adolescent medicine specialist who cares for youth living with and at risk for HIV, I am excited to see data that will help young men at risk for HIV to access and use emtricitabine/tenofovir disoproxil fumarate as pre-exposure prophylaxis (PrEP) in their daily lives.
In the United States, the rates of HIV acquisition are highest among adolescent young men who have sex with men (MSM), and data suggest that 40% of young black MSM will acquire HIV by age 40 years unless prevention efforts improve. Clinician barriers to caring for sexual minority men, combined with young men’s having to feel comfortable disclosing their sexual orientation and sexual history to receive counseling about emtricitabine/tenofovir disoproxil fumarate, will mean we will also need to address the institutional, social, and historical factors in medical settings to effectively increase access to PrEP; otherwise, it may further contribute to the HIV inequity among this population.
Clinicians, public health practitioners, and researchers who aim to improve adolescent access to PrEP will need not only to focus on the developmental and cognitive needs of adolescents but also to address the social contexts of stigma, minority stress, and sexual identity that intersect to affect adherence. This work suggests that adolescents may require more frequent visits than is currently recommended by national guidelines and suggests a need for multiple team members to address structural barriers to accessing PrEP, assist with youths’ interpretation of HIV risk, and support self-efficacy to swallow and adhere to medications.
Renata Arrington-Sanders, MD, MPH, ScM, is at the division of general pediatrics and adolescent medicine, Johns Hopkins University, Baltimore. She reported having no conflicts of interest. These comments are from her editorial (JAMA Ped. 2017 Sep 5. doi: 10.1001/jamapediatrics.2017.2397).
As an adolescent medicine specialist who cares for youth living with and at risk for HIV, I am excited to see data that will help young men at risk for HIV to access and use emtricitabine/tenofovir disoproxil fumarate as pre-exposure prophylaxis (PrEP) in their daily lives.
In the United States, the rates of HIV acquisition are highest among adolescent young men who have sex with men (MSM), and data suggest that 40% of young black MSM will acquire HIV by age 40 years unless prevention efforts improve. Clinician barriers to caring for sexual minority men, combined with young men’s having to feel comfortable disclosing their sexual orientation and sexual history to receive counseling about emtricitabine/tenofovir disoproxil fumarate, will mean we will also need to address the institutional, social, and historical factors in medical settings to effectively increase access to PrEP; otherwise, it may further contribute to the HIV inequity among this population.
Clinicians, public health practitioners, and researchers who aim to improve adolescent access to PrEP will need not only to focus on the developmental and cognitive needs of adolescents but also to address the social contexts of stigma, minority stress, and sexual identity that intersect to affect adherence. This work suggests that adolescents may require more frequent visits than is currently recommended by national guidelines and suggests a need for multiple team members to address structural barriers to accessing PrEP, assist with youths’ interpretation of HIV risk, and support self-efficacy to swallow and adhere to medications.
Renata Arrington-Sanders, MD, MPH, ScM, is at the division of general pediatrics and adolescent medicine, Johns Hopkins University, Baltimore. She reported having no conflicts of interest. These comments are from her editorial (JAMA Ped. 2017 Sep 5. doi: 10.1001/jamapediatrics.2017.2397).
As an adolescent medicine specialist who cares for youth living with and at risk for HIV, I am excited to see data that will help young men at risk for HIV to access and use emtricitabine/tenofovir disoproxil fumarate as pre-exposure prophylaxis (PrEP) in their daily lives.
In the United States, the rates of HIV acquisition are highest among adolescent young men who have sex with men (MSM), and data suggest that 40% of young black MSM will acquire HIV by age 40 years unless prevention efforts improve. Clinician barriers to caring for sexual minority men, combined with young men’s having to feel comfortable disclosing their sexual orientation and sexual history to receive counseling about emtricitabine/tenofovir disoproxil fumarate, will mean we will also need to address the institutional, social, and historical factors in medical settings to effectively increase access to PrEP; otherwise, it may further contribute to the HIV inequity among this population.
Clinicians, public health practitioners, and researchers who aim to improve adolescent access to PrEP will need not only to focus on the developmental and cognitive needs of adolescents but also to address the social contexts of stigma, minority stress, and sexual identity that intersect to affect adherence. This work suggests that adolescents may require more frequent visits than is currently recommended by national guidelines and suggests a need for multiple team members to address structural barriers to accessing PrEP, assist with youths’ interpretation of HIV risk, and support self-efficacy to swallow and adhere to medications.
Renata Arrington-Sanders, MD, MPH, ScM, is at the division of general pediatrics and adolescent medicine, Johns Hopkins University, Baltimore. She reported having no conflicts of interest. These comments are from her editorial (JAMA Ped. 2017 Sep 5. doi: 10.1001/jamapediatrics.2017.2397).
Up to 54% of adolescent men who had sex with men maintained tenofovir disphosphate levels consistent with a high degree of anti-HIV protection while attending monthly clinic visits, but this proportion fell as low as 17% after they switched to quarterly visits, according to the results of a first-of-its-kind 48-week, prospective, multicenter trial.
The Adolescent Medicine Trials Network for HIV/AIDS Interventions (Project PrEPare) evaluated the safety, tolerability, and acceptability of tenofovir disoproxil fumarate and emtricitabine among 78 HIV-negative MSM aged 15-17 years who had condomless anal intercourse with a male of positive or unknown HIV status, had multiple male sex partners, or had other high-risk sexual behaviors or sexually transmitted infections within the previous 6 months. Participants averaged 16.5 years old age, 33% were of mixed race or ethnicity, 29% were African American, 21% were white Hispanic, 14% were white, and 3% were Asian or Pacific Islander. They received one cognitive-behavioral risk-reduction session before starting PrEP and were paid $50-$75 to attend follow-up visits every month for 3 months and then quarterly after that.
While attending monthly visits, between 47% and 54% of participants maintained highly protective levels of tenofovir disphosphate (above 700 fmol/punch, based on dried blood spot tests), but this proportion fell as low as 17% after participants switched to quarterly visits. At week 48, the HIV seroconversion rate was 6.4 per 100 person-years (95% confidence interval, 1.3-8.7), underscoring the need to offer PrEP and behavioral support to this population, the researchers emphasized. “The waning adherence, especially with quarterly visits, demonstrates that more time, attention, and resources may need to be allocated to adolescents who are seeking prevention services,” they wrote. “We need to better understand the barriers to adherence and develop more effective ways to enhance adherence for youth who are clinically prescribed PrEP.”
Gilead Sciences provided the study drugs and helped fund the study. The study was supported by grants from the National Institute of Child Health and Human Development, the National Institutes on Drug Abuse and Mental Health, and the Adolescent Medicine Trials Network for HIV/AIDS Interventions. Dr. Hosek had no disclosures. Two coinvestigators disclosed research support and contract work fees from Gilead.
Up to 54% of adolescent men who had sex with men maintained tenofovir disphosphate levels consistent with a high degree of anti-HIV protection while attending monthly clinic visits, but this proportion fell as low as 17% after they switched to quarterly visits, according to the results of a first-of-its-kind 48-week, prospective, multicenter trial.
The Adolescent Medicine Trials Network for HIV/AIDS Interventions (Project PrEPare) evaluated the safety, tolerability, and acceptability of tenofovir disoproxil fumarate and emtricitabine among 78 HIV-negative MSM aged 15-17 years who had condomless anal intercourse with a male of positive or unknown HIV status, had multiple male sex partners, or had other high-risk sexual behaviors or sexually transmitted infections within the previous 6 months. Participants averaged 16.5 years old age, 33% were of mixed race or ethnicity, 29% were African American, 21% were white Hispanic, 14% were white, and 3% were Asian or Pacific Islander. They received one cognitive-behavioral risk-reduction session before starting PrEP and were paid $50-$75 to attend follow-up visits every month for 3 months and then quarterly after that.
While attending monthly visits, between 47% and 54% of participants maintained highly protective levels of tenofovir disphosphate (above 700 fmol/punch, based on dried blood spot tests), but this proportion fell as low as 17% after participants switched to quarterly visits. At week 48, the HIV seroconversion rate was 6.4 per 100 person-years (95% confidence interval, 1.3-8.7), underscoring the need to offer PrEP and behavioral support to this population, the researchers emphasized. “The waning adherence, especially with quarterly visits, demonstrates that more time, attention, and resources may need to be allocated to adolescents who are seeking prevention services,” they wrote. “We need to better understand the barriers to adherence and develop more effective ways to enhance adherence for youth who are clinically prescribed PrEP.”
Gilead Sciences provided the study drugs and helped fund the study. The study was supported by grants from the National Institute of Child Health and Human Development, the National Institutes on Drug Abuse and Mental Health, and the Adolescent Medicine Trials Network for HIV/AIDS Interventions. Dr. Hosek had no disclosures. Two coinvestigators disclosed research support and contract work fees from Gilead.
FROM JAMA PEDIATRICS
Key clinical point: With monthly clinic visits, about half of adolescent men who had sex with men were able to maintain highly protective blood levels of pre-exposure prophylaxis.
Major finding: Up to 54% of participants maintained tenofovir disphosphate levels above 700 fmol/punch when they attended monthly visits, but as few as 17% did so after switching to quarterly visits.
Data source: A multicenter, open-label clinical trial of 78 high-risk men who had sex with men aged 15-17 years.
Disclosures: Gilead Sciences provided the study drugs and helped fund the study. The study was supported by grants from the National Institute of Child Health and Human Development, the National Institutes on Drug Abuse and Mental Health, and the Adolescent Medicine Trials Network for HIV/AIDS Interventions. Dr. Hosek had no disclosures. Two coinvestigators disclosed research support and contract work fees from Gilead.
Progressing rash on boy’s trunk
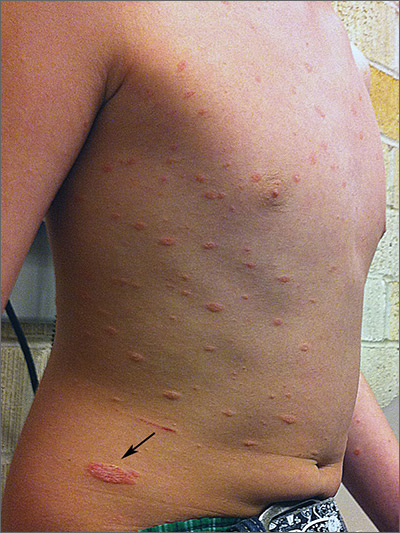
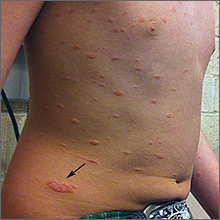
The FP diagnosed pityriasis rosea based on clinical appearance, which included a herald patch (see arrow) that had preceded the eruption. (A herald patch—a solitary, oval, flesh-colored to salmon-colored lesion with scaling at the border—is seen in less than a quarter of pityriasis rosea cases.)
Pityriasis rosea is a papulosquamous eruption of unknown etiology. It occurs at all ages but is most commonly seen between the ages of 10 to 35. Peak incidence occurs between the ages of 20 to 29. Gender distribution is essentially equal.
Pityriasis rosea lesions vary from oval macules to slightly raised plaques (0.5 - 2 cm in size). They are salmon colored (or hyperpigmented in individuals with dark skin), and typically have a collarette of scaling at the border. There are no systemic symptoms, and itching occurs in about 25% of patients. The eruption resolves without treatment in 8 weeks in 80% of patients, but can last up to 3 to 5 months for some. A topical steroid or first-generation oral antihistamine can be prescribed to relieve pruritus.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Henderson D, Usatine R. Pityriasis rosea. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 896-900.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP diagnosed pityriasis rosea based on clinical appearance, which included a herald patch (see arrow) that had preceded the eruption. (A herald patch—a solitary, oval, flesh-colored to salmon-colored lesion with scaling at the border—is seen in less than a quarter of pityriasis rosea cases.)
Pityriasis rosea is a papulosquamous eruption of unknown etiology. It occurs at all ages but is most commonly seen between the ages of 10 to 35. Peak incidence occurs between the ages of 20 to 29. Gender distribution is essentially equal.
Pityriasis rosea lesions vary from oval macules to slightly raised plaques (0.5 - 2 cm in size). They are salmon colored (or hyperpigmented in individuals with dark skin), and typically have a collarette of scaling at the border. There are no systemic symptoms, and itching occurs in about 25% of patients. The eruption resolves without treatment in 8 weeks in 80% of patients, but can last up to 3 to 5 months for some. A topical steroid or first-generation oral antihistamine can be prescribed to relieve pruritus.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Henderson D, Usatine R. Pityriasis rosea. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 896-900.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP diagnosed pityriasis rosea based on clinical appearance, which included a herald patch (see arrow) that had preceded the eruption. (A herald patch—a solitary, oval, flesh-colored to salmon-colored lesion with scaling at the border—is seen in less than a quarter of pityriasis rosea cases.)
Pityriasis rosea is a papulosquamous eruption of unknown etiology. It occurs at all ages but is most commonly seen between the ages of 10 to 35. Peak incidence occurs between the ages of 20 to 29. Gender distribution is essentially equal.
Pityriasis rosea lesions vary from oval macules to slightly raised plaques (0.5 - 2 cm in size). They are salmon colored (or hyperpigmented in individuals with dark skin), and typically have a collarette of scaling at the border. There are no systemic symptoms, and itching occurs in about 25% of patients. The eruption resolves without treatment in 8 weeks in 80% of patients, but can last up to 3 to 5 months for some. A topical steroid or first-generation oral antihistamine can be prescribed to relieve pruritus.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Henderson D, Usatine R. Pityriasis rosea. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 896-900.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Thomas Edwards
Is pain or dependency driving elevated opioid use among long-term cancer survivors?
Rates of opioid prescribing were about 1.2 times higher overall among cancer survivors up to 10 years after diagnosis, compared with matched controls, with more than threefold higher rates of opioid prescriptions for survivors of some cancers, according to a Canadian population-based cohort study.
Searching for the cause of these elevated rates reveals the complexity of the survivorship experience, and may also point the way to areas where there’s work to be done, according to physicians whose practices touch the lives of cancer survivors.
In a retrospective matched cohort study of participants in the Ontario Health Insurance Plan and the Ontario Drug Benefits Program, Rinku Sutradhar, PhD, of the University of Toronto, and her colleagues, identified patients aged 18-64 who had a cancer diagnosis at least 5 years previously. Patients were included only if they had not had a cancer recurrence or another malignancy. When compared 1:1 to age- and sex-matched controls, the 8,601 cancer survivors had a relative rate of opioid prescribing of 1.220 (95% confidence interval [CI], 1.209-1.232), the investigators reported (Cancer 2017 Aug 17. doi: 10.1002/cncr.30839).
Opioid prescribing rates varied according to the type of cancer the survivor had had, with a relative rate of 3.119 for noncolorectal gastrointestinal cancer survivors and a rate of 2.066 for lung cancer survivors. Individuals with nonprostate genitourinary cancers had a prescribing rate of 1.619. All of these differences were statistically significant.
Elevated prescribing rates were not seen in patients with brain, breast, colorectal, head and neck, or prostate cancers. The relative rate of prescribing for hematologic cancers was 1.383, a difference that approached, but did not quite reach, statistical significance (P = .0512).
When multivariable analysis was used to stratify individuals by length of time since cancer diagnosis, a significantly elevated relative rate of opioid prescribing persisted; for those 5-10 years from diagnosis, the relative rate was 1.190 (95% CI, 1.040-1.362, P = .011), while for those diagnosed at least 10 years ago, the relative rate was 1.244 (95% CI, 1.090-1.420; P = .00118).
Multivariable analysis was also used to control for income, rural residence, and comorbidities. However, the study could capture only opioids that were obtained with a prescription, and could not track whether medications were taken by the person for whom they were prescribed, Dr. Sutradhar and her colleagues said.
Cancer survivors may have a higher prevalence of chronic pain than the general population for reasons related both to their initial diagnosis and the sequelae of treatments such as surgery, chemotherapy, and radiation therapy, the investigators noted, adding, “it is also possible that a higher rate of opioid prescribing among survivors is due to a dependency that originated from opioid use earlier in the disease trajectory.”
Because of the potential for opioid use disorder and the many adverse effects that can be associated with long-term opioid use, they said, “primary care providers who treat cancer survivors should be encouraged to critically examine reasons for lingering opioid use among their patients.”
The oncologist’s perspective
Walter M. Stadler, MD, an oncologist who treats genitourinary and hematologic cancers, also wonders whether it’s pain or dependency that’s driving the increased prescribing rates in cancer survivors.
One opportunity to reassess which medications and treatment modalities are appropriate for the cancer survivor, said Dr. Stadler, is at the point of discharge from oncology care, which usually happens at about the 5-year mark for patients with no evidence of disease. Survivorship has received more attention since the 2005 Institute of Medicine report calling for increased attention to cancer survivors’ ongoing care. However, he said, “it’s not clear that we do a very good job in terms of educating either the patient or their primary care physician in regards to the kinds of things that we expect, the kinds of things that need to be done, or even a good summary of the therapy that was provided.”
There are resources that can help, he said. “That’s why organizations like [the American Society for Clinical Oncology] have put together some more formal survivorship plans that should be provided when patients are transitioned.”
The realities of clinical life can get in the way of implementation, though. Oncologists are already stretched thin, and most electronic health record systems don’t integrate well with survivorship documentation. Finding staff who can spend the time to gather and package all the necessary information can also be a problem: “People are expensive, and none of us have extra cash lying around,” said Dr. Stadler.
Still, he said, “like a lot of good papers, this raises some issues and areas for further investigation.” First, he said, physicians must assess whether cancer survivors are having chronic pain, and then sort things out from there. “What are the pain syndromes – and what are we doing about them? – because it’s not something that’s been well addressed.”
What can primary care offer?
Larissa Nekhlyudov, MD, is an internal medicine physician whose clinical practice straddles two domains. She sees patients, including some cancer survivors, as a primary care provider; she also provides care in a survivorship clinic to adult survivors of childhood cancers. There, she is able to focus more on survivorship care, developing a care plan and communicating with primary care providers about care elements her patients need.
It’s reasonable to think that there might be an increased risk for chronic pain syndromes in some of the types of cancer in which elevated opioid prescribing rates were seen, said Dr. Nekhlyudov. “Maybe this is okay.
“Pain in cancer survivors is so multidimensional that it’s quite possible that some of these cancer survivors – gynecologic, lung, other gastrointestinal, genitourinary – might have peripheral neuropathy, adhesions, and so many potential late effects,” said Dr. Nekhlyudov. “However, narcotics are not necessarily the preferred and the only method to treat this pain,” she said, noting that optimal survivorship care might seek to transition these patients to nonopioid therapies or, at least, a multimodal approach.
When she’s wearing her survivorship care hat, said Dr. Nekhlyudov, managing pain medication isn’t always at the top of the to-do list in an office visit. “It’s certainly not uncommon that patients will have a variety of pain issues. But in the survivorship domain, I think that we don’t take the role of managing their pain medications; that piece belongs, really, to their primary care provider,” she said.
“In many ways, it’s difficult to distinguish how much of their pain is related to their cancer, versus not, and figuring out alternatives,” said Dr. Nekhlyudov, applauding the authors’ recognition of the need for a multimodal approach in cancer survivors with pain. However, she said, “that sounds really great on paper, but it’s really not readily available.”
Even in the resource-rich greater Boston area where she practices, said Dr. Nekhlyudov, “it’s very difficult for cancer patients – and noncancer patients – to get hooked into a multidisciplinary, holistic program for pain.”
Although the long-term perspective is helpful, Dr. Nekhlyudov hopes for research that can help identify at what point, and by whom, the opioids were initiated in the cancer survivor population. “What is their trajectory from the time of diagnosis? Are these patients who are started on narcotics during their cancer treatment, and then continue on forever, or are some of these patients being started later, because of late effects?”
In any case, she said, “one of the key pieces is the ownership for this really belongs with both oncologists and the primary care providers.”
Mental health implications of survivorship
Viewing the issue through the lens of mental health offers a slightly different perspective. Thomas B. Strouse, MD, is a psychiatrist who holds the Maddie Katz Chair in palliative care research and education at the University of California, Los Angeles. He said he laments the current “opioidophobia” that calls into question any long-term opioid prescribing.
Acknowledging that there’s certainly a serious nationwide problem with both prescription and nonprescription opioid abuse, Dr. Strouse said he still finds it unfortunate that the current situation has “reactivated for many people a certain set of reflexes that say that any chronic opioid use is always a bad thing. That’s simply not true,” he said.
“Whether opioids are the right treatment for all of those patients, of course, is an entirely fair question. But it’s unfortunate, or wrong, for everybody to approach this article and to say that we know that for all of these patients, chronic opioid therapy is not appropriate,” he added.
Chronic pain that lingers after cancer treatments affects “a very significant minority of cancer survivors,” he said. It’s also true that the meaning of pain can be different for cancer survivors, said Dr. Strouse. For a cancer survivor, “any new pain is cancer pain until proven otherwise,” he said.
Further, pivoting from the attentive, multidisciplinary, wraparound care often received during cancer treatment to the relatively unsupported survivorship experience can be a rough transition for some. Despite the grim reason for the connection, “frequently, it’s the best experience of patients’ lives from a human relations perspective. … We don’t think enough about the loss that the end of cancer treatment may mean for people who may have otherwise unsatisfactory relationships in their lives,” he said.
Dr. Strouse, who works extensively with cancer survivors, said that the elevated rate of opioid prescribing seen in this study “opens the door to a bigger discussion about the challenges in the relatively empty domain of survivorship.” After discharge from cancer care, patients are all too often left without a navigator to help them through the years when, though their treatment is complete, anxiety, financial and social strain, and pain may linger.
The study, he said, should be a call to physicians for “a more meaningful commitment to understanding the burdens of survivorship, and actually offering meaningful clinical services to those people in an integrated and appropriate way.” This might include determining a patient’s absolute minimum opioid requirement, with a goal of getting the patient off opioids, but also making sure the patient has knowledge of and access to alternative pharmacologic and nonpharmacologic treatments for pain. “That seems like a reasonable approach,” said Dr. Strouse.
None of the study’s authors or the physicians interviewed for commentary had relevant conflicts of interest.
[email protected]
On Twitter @karioakes
Rates of opioid prescribing were about 1.2 times higher overall among cancer survivors up to 10 years after diagnosis, compared with matched controls, with more than threefold higher rates of opioid prescriptions for survivors of some cancers, according to a Canadian population-based cohort study.
Searching for the cause of these elevated rates reveals the complexity of the survivorship experience, and may also point the way to areas where there’s work to be done, according to physicians whose practices touch the lives of cancer survivors.
In a retrospective matched cohort study of participants in the Ontario Health Insurance Plan and the Ontario Drug Benefits Program, Rinku Sutradhar, PhD, of the University of Toronto, and her colleagues, identified patients aged 18-64 who had a cancer diagnosis at least 5 years previously. Patients were included only if they had not had a cancer recurrence or another malignancy. When compared 1:1 to age- and sex-matched controls, the 8,601 cancer survivors had a relative rate of opioid prescribing of 1.220 (95% confidence interval [CI], 1.209-1.232), the investigators reported (Cancer 2017 Aug 17. doi: 10.1002/cncr.30839).
Opioid prescribing rates varied according to the type of cancer the survivor had had, with a relative rate of 3.119 for noncolorectal gastrointestinal cancer survivors and a rate of 2.066 for lung cancer survivors. Individuals with nonprostate genitourinary cancers had a prescribing rate of 1.619. All of these differences were statistically significant.
Elevated prescribing rates were not seen in patients with brain, breast, colorectal, head and neck, or prostate cancers. The relative rate of prescribing for hematologic cancers was 1.383, a difference that approached, but did not quite reach, statistical significance (P = .0512).
When multivariable analysis was used to stratify individuals by length of time since cancer diagnosis, a significantly elevated relative rate of opioid prescribing persisted; for those 5-10 years from diagnosis, the relative rate was 1.190 (95% CI, 1.040-1.362, P = .011), while for those diagnosed at least 10 years ago, the relative rate was 1.244 (95% CI, 1.090-1.420; P = .00118).
Multivariable analysis was also used to control for income, rural residence, and comorbidities. However, the study could capture only opioids that were obtained with a prescription, and could not track whether medications were taken by the person for whom they were prescribed, Dr. Sutradhar and her colleagues said.
Cancer survivors may have a higher prevalence of chronic pain than the general population for reasons related both to their initial diagnosis and the sequelae of treatments such as surgery, chemotherapy, and radiation therapy, the investigators noted, adding, “it is also possible that a higher rate of opioid prescribing among survivors is due to a dependency that originated from opioid use earlier in the disease trajectory.”
Because of the potential for opioid use disorder and the many adverse effects that can be associated with long-term opioid use, they said, “primary care providers who treat cancer survivors should be encouraged to critically examine reasons for lingering opioid use among their patients.”
The oncologist’s perspective
Walter M. Stadler, MD, an oncologist who treats genitourinary and hematologic cancers, also wonders whether it’s pain or dependency that’s driving the increased prescribing rates in cancer survivors.
One opportunity to reassess which medications and treatment modalities are appropriate for the cancer survivor, said Dr. Stadler, is at the point of discharge from oncology care, which usually happens at about the 5-year mark for patients with no evidence of disease. Survivorship has received more attention since the 2005 Institute of Medicine report calling for increased attention to cancer survivors’ ongoing care. However, he said, “it’s not clear that we do a very good job in terms of educating either the patient or their primary care physician in regards to the kinds of things that we expect, the kinds of things that need to be done, or even a good summary of the therapy that was provided.”
There are resources that can help, he said. “That’s why organizations like [the American Society for Clinical Oncology] have put together some more formal survivorship plans that should be provided when patients are transitioned.”
The realities of clinical life can get in the way of implementation, though. Oncologists are already stretched thin, and most electronic health record systems don’t integrate well with survivorship documentation. Finding staff who can spend the time to gather and package all the necessary information can also be a problem: “People are expensive, and none of us have extra cash lying around,” said Dr. Stadler.
Still, he said, “like a lot of good papers, this raises some issues and areas for further investigation.” First, he said, physicians must assess whether cancer survivors are having chronic pain, and then sort things out from there. “What are the pain syndromes – and what are we doing about them? – because it’s not something that’s been well addressed.”
What can primary care offer?
Larissa Nekhlyudov, MD, is an internal medicine physician whose clinical practice straddles two domains. She sees patients, including some cancer survivors, as a primary care provider; she also provides care in a survivorship clinic to adult survivors of childhood cancers. There, she is able to focus more on survivorship care, developing a care plan and communicating with primary care providers about care elements her patients need.
It’s reasonable to think that there might be an increased risk for chronic pain syndromes in some of the types of cancer in which elevated opioid prescribing rates were seen, said Dr. Nekhlyudov. “Maybe this is okay.
“Pain in cancer survivors is so multidimensional that it’s quite possible that some of these cancer survivors – gynecologic, lung, other gastrointestinal, genitourinary – might have peripheral neuropathy, adhesions, and so many potential late effects,” said Dr. Nekhlyudov. “However, narcotics are not necessarily the preferred and the only method to treat this pain,” she said, noting that optimal survivorship care might seek to transition these patients to nonopioid therapies or, at least, a multimodal approach.
When she’s wearing her survivorship care hat, said Dr. Nekhlyudov, managing pain medication isn’t always at the top of the to-do list in an office visit. “It’s certainly not uncommon that patients will have a variety of pain issues. But in the survivorship domain, I think that we don’t take the role of managing their pain medications; that piece belongs, really, to their primary care provider,” she said.
“In many ways, it’s difficult to distinguish how much of their pain is related to their cancer, versus not, and figuring out alternatives,” said Dr. Nekhlyudov, applauding the authors’ recognition of the need for a multimodal approach in cancer survivors with pain. However, she said, “that sounds really great on paper, but it’s really not readily available.”
Even in the resource-rich greater Boston area where she practices, said Dr. Nekhlyudov, “it’s very difficult for cancer patients – and noncancer patients – to get hooked into a multidisciplinary, holistic program for pain.”
Although the long-term perspective is helpful, Dr. Nekhlyudov hopes for research that can help identify at what point, and by whom, the opioids were initiated in the cancer survivor population. “What is their trajectory from the time of diagnosis? Are these patients who are started on narcotics during their cancer treatment, and then continue on forever, or are some of these patients being started later, because of late effects?”
In any case, she said, “one of the key pieces is the ownership for this really belongs with both oncologists and the primary care providers.”
Mental health implications of survivorship
Viewing the issue through the lens of mental health offers a slightly different perspective. Thomas B. Strouse, MD, is a psychiatrist who holds the Maddie Katz Chair in palliative care research and education at the University of California, Los Angeles. He said he laments the current “opioidophobia” that calls into question any long-term opioid prescribing.
Acknowledging that there’s certainly a serious nationwide problem with both prescription and nonprescription opioid abuse, Dr. Strouse said he still finds it unfortunate that the current situation has “reactivated for many people a certain set of reflexes that say that any chronic opioid use is always a bad thing. That’s simply not true,” he said.
“Whether opioids are the right treatment for all of those patients, of course, is an entirely fair question. But it’s unfortunate, or wrong, for everybody to approach this article and to say that we know that for all of these patients, chronic opioid therapy is not appropriate,” he added.
Chronic pain that lingers after cancer treatments affects “a very significant minority of cancer survivors,” he said. It’s also true that the meaning of pain can be different for cancer survivors, said Dr. Strouse. For a cancer survivor, “any new pain is cancer pain until proven otherwise,” he said.
Further, pivoting from the attentive, multidisciplinary, wraparound care often received during cancer treatment to the relatively unsupported survivorship experience can be a rough transition for some. Despite the grim reason for the connection, “frequently, it’s the best experience of patients’ lives from a human relations perspective. … We don’t think enough about the loss that the end of cancer treatment may mean for people who may have otherwise unsatisfactory relationships in their lives,” he said.
Dr. Strouse, who works extensively with cancer survivors, said that the elevated rate of opioid prescribing seen in this study “opens the door to a bigger discussion about the challenges in the relatively empty domain of survivorship.” After discharge from cancer care, patients are all too often left without a navigator to help them through the years when, though their treatment is complete, anxiety, financial and social strain, and pain may linger.
The study, he said, should be a call to physicians for “a more meaningful commitment to understanding the burdens of survivorship, and actually offering meaningful clinical services to those people in an integrated and appropriate way.” This might include determining a patient’s absolute minimum opioid requirement, with a goal of getting the patient off opioids, but also making sure the patient has knowledge of and access to alternative pharmacologic and nonpharmacologic treatments for pain. “That seems like a reasonable approach,” said Dr. Strouse.
None of the study’s authors or the physicians interviewed for commentary had relevant conflicts of interest.
[email protected]
On Twitter @karioakes
Rates of opioid prescribing were about 1.2 times higher overall among cancer survivors up to 10 years after diagnosis, compared with matched controls, with more than threefold higher rates of opioid prescriptions for survivors of some cancers, according to a Canadian population-based cohort study.
Searching for the cause of these elevated rates reveals the complexity of the survivorship experience, and may also point the way to areas where there’s work to be done, according to physicians whose practices touch the lives of cancer survivors.
In a retrospective matched cohort study of participants in the Ontario Health Insurance Plan and the Ontario Drug Benefits Program, Rinku Sutradhar, PhD, of the University of Toronto, and her colleagues, identified patients aged 18-64 who had a cancer diagnosis at least 5 years previously. Patients were included only if they had not had a cancer recurrence or another malignancy. When compared 1:1 to age- and sex-matched controls, the 8,601 cancer survivors had a relative rate of opioid prescribing of 1.220 (95% confidence interval [CI], 1.209-1.232), the investigators reported (Cancer 2017 Aug 17. doi: 10.1002/cncr.30839).
Opioid prescribing rates varied according to the type of cancer the survivor had had, with a relative rate of 3.119 for noncolorectal gastrointestinal cancer survivors and a rate of 2.066 for lung cancer survivors. Individuals with nonprostate genitourinary cancers had a prescribing rate of 1.619. All of these differences were statistically significant.
Elevated prescribing rates were not seen in patients with brain, breast, colorectal, head and neck, or prostate cancers. The relative rate of prescribing for hematologic cancers was 1.383, a difference that approached, but did not quite reach, statistical significance (P = .0512).
When multivariable analysis was used to stratify individuals by length of time since cancer diagnosis, a significantly elevated relative rate of opioid prescribing persisted; for those 5-10 years from diagnosis, the relative rate was 1.190 (95% CI, 1.040-1.362, P = .011), while for those diagnosed at least 10 years ago, the relative rate was 1.244 (95% CI, 1.090-1.420; P = .00118).
Multivariable analysis was also used to control for income, rural residence, and comorbidities. However, the study could capture only opioids that were obtained with a prescription, and could not track whether medications were taken by the person for whom they were prescribed, Dr. Sutradhar and her colleagues said.
Cancer survivors may have a higher prevalence of chronic pain than the general population for reasons related both to their initial diagnosis and the sequelae of treatments such as surgery, chemotherapy, and radiation therapy, the investigators noted, adding, “it is also possible that a higher rate of opioid prescribing among survivors is due to a dependency that originated from opioid use earlier in the disease trajectory.”
Because of the potential for opioid use disorder and the many adverse effects that can be associated with long-term opioid use, they said, “primary care providers who treat cancer survivors should be encouraged to critically examine reasons for lingering opioid use among their patients.”
The oncologist’s perspective
Walter M. Stadler, MD, an oncologist who treats genitourinary and hematologic cancers, also wonders whether it’s pain or dependency that’s driving the increased prescribing rates in cancer survivors.
One opportunity to reassess which medications and treatment modalities are appropriate for the cancer survivor, said Dr. Stadler, is at the point of discharge from oncology care, which usually happens at about the 5-year mark for patients with no evidence of disease. Survivorship has received more attention since the 2005 Institute of Medicine report calling for increased attention to cancer survivors’ ongoing care. However, he said, “it’s not clear that we do a very good job in terms of educating either the patient or their primary care physician in regards to the kinds of things that we expect, the kinds of things that need to be done, or even a good summary of the therapy that was provided.”
There are resources that can help, he said. “That’s why organizations like [the American Society for Clinical Oncology] have put together some more formal survivorship plans that should be provided when patients are transitioned.”
The realities of clinical life can get in the way of implementation, though. Oncologists are already stretched thin, and most electronic health record systems don’t integrate well with survivorship documentation. Finding staff who can spend the time to gather and package all the necessary information can also be a problem: “People are expensive, and none of us have extra cash lying around,” said Dr. Stadler.
Still, he said, “like a lot of good papers, this raises some issues and areas for further investigation.” First, he said, physicians must assess whether cancer survivors are having chronic pain, and then sort things out from there. “What are the pain syndromes – and what are we doing about them? – because it’s not something that’s been well addressed.”
What can primary care offer?
Larissa Nekhlyudov, MD, is an internal medicine physician whose clinical practice straddles two domains. She sees patients, including some cancer survivors, as a primary care provider; she also provides care in a survivorship clinic to adult survivors of childhood cancers. There, she is able to focus more on survivorship care, developing a care plan and communicating with primary care providers about care elements her patients need.
It’s reasonable to think that there might be an increased risk for chronic pain syndromes in some of the types of cancer in which elevated opioid prescribing rates were seen, said Dr. Nekhlyudov. “Maybe this is okay.
“Pain in cancer survivors is so multidimensional that it’s quite possible that some of these cancer survivors – gynecologic, lung, other gastrointestinal, genitourinary – might have peripheral neuropathy, adhesions, and so many potential late effects,” said Dr. Nekhlyudov. “However, narcotics are not necessarily the preferred and the only method to treat this pain,” she said, noting that optimal survivorship care might seek to transition these patients to nonopioid therapies or, at least, a multimodal approach.
When she’s wearing her survivorship care hat, said Dr. Nekhlyudov, managing pain medication isn’t always at the top of the to-do list in an office visit. “It’s certainly not uncommon that patients will have a variety of pain issues. But in the survivorship domain, I think that we don’t take the role of managing their pain medications; that piece belongs, really, to their primary care provider,” she said.
“In many ways, it’s difficult to distinguish how much of their pain is related to their cancer, versus not, and figuring out alternatives,” said Dr. Nekhlyudov, applauding the authors’ recognition of the need for a multimodal approach in cancer survivors with pain. However, she said, “that sounds really great on paper, but it’s really not readily available.”
Even in the resource-rich greater Boston area where she practices, said Dr. Nekhlyudov, “it’s very difficult for cancer patients – and noncancer patients – to get hooked into a multidisciplinary, holistic program for pain.”
Although the long-term perspective is helpful, Dr. Nekhlyudov hopes for research that can help identify at what point, and by whom, the opioids were initiated in the cancer survivor population. “What is their trajectory from the time of diagnosis? Are these patients who are started on narcotics during their cancer treatment, and then continue on forever, or are some of these patients being started later, because of late effects?”
In any case, she said, “one of the key pieces is the ownership for this really belongs with both oncologists and the primary care providers.”
Mental health implications of survivorship
Viewing the issue through the lens of mental health offers a slightly different perspective. Thomas B. Strouse, MD, is a psychiatrist who holds the Maddie Katz Chair in palliative care research and education at the University of California, Los Angeles. He said he laments the current “opioidophobia” that calls into question any long-term opioid prescribing.
Acknowledging that there’s certainly a serious nationwide problem with both prescription and nonprescription opioid abuse, Dr. Strouse said he still finds it unfortunate that the current situation has “reactivated for many people a certain set of reflexes that say that any chronic opioid use is always a bad thing. That’s simply not true,” he said.
“Whether opioids are the right treatment for all of those patients, of course, is an entirely fair question. But it’s unfortunate, or wrong, for everybody to approach this article and to say that we know that for all of these patients, chronic opioid therapy is not appropriate,” he added.
Chronic pain that lingers after cancer treatments affects “a very significant minority of cancer survivors,” he said. It’s also true that the meaning of pain can be different for cancer survivors, said Dr. Strouse. For a cancer survivor, “any new pain is cancer pain until proven otherwise,” he said.
Further, pivoting from the attentive, multidisciplinary, wraparound care often received during cancer treatment to the relatively unsupported survivorship experience can be a rough transition for some. Despite the grim reason for the connection, “frequently, it’s the best experience of patients’ lives from a human relations perspective. … We don’t think enough about the loss that the end of cancer treatment may mean for people who may have otherwise unsatisfactory relationships in their lives,” he said.
Dr. Strouse, who works extensively with cancer survivors, said that the elevated rate of opioid prescribing seen in this study “opens the door to a bigger discussion about the challenges in the relatively empty domain of survivorship.” After discharge from cancer care, patients are all too often left without a navigator to help them through the years when, though their treatment is complete, anxiety, financial and social strain, and pain may linger.
The study, he said, should be a call to physicians for “a more meaningful commitment to understanding the burdens of survivorship, and actually offering meaningful clinical services to those people in an integrated and appropriate way.” This might include determining a patient’s absolute minimum opioid requirement, with a goal of getting the patient off opioids, but also making sure the patient has knowledge of and access to alternative pharmacologic and nonpharmacologic treatments for pain. “That seems like a reasonable approach,” said Dr. Strouse.
None of the study’s authors or the physicians interviewed for commentary had relevant conflicts of interest.
[email protected]
On Twitter @karioakes
When the waters recede: Hurricane Harvey and PTSD through indirect trauma
It’s been 5 days since Texas came under siege from Hurricane Harvey and it left up to 51 inches of rain in its wake. Several Southern cities suffered almost complete loss of homes and businesses. The Houston metropolitan area reported 14 deaths, including one of a police officer who was trying to report for duty. Hundreds of thousands of homes have been damaged or lost, and thousands of people are now in makeshift shelters across the city. We have slowly begun the process of repair and rebuilding, and many Houstonians are returning to work. Many others, including well-known local celebrities like J.J. Watt and MattressMack, are volunteering their time and giving money to help those who were not so fortunate. The rescue and recovery efforts have been lauded for the absence of issues tied to politics, religion, or race.
Despite this, we must not forget that this was a natural disaster unlike anything that’s been seen in recent decades. Much like Katrina and Sandy, Hurricane Harvey brought to the people who have lived through the initial trauma the fear, nightmares, emotional distress, and sleep disturbances associated with posttraumatic stress disorder (PTSD). They will require significant support and monitoring to determine whether there is a need for medical intervention, such as cognitive-behavioral therapy, behavioral modification, or pharmacotherapy. However, we are also witnessing something psychiatrists are just becoming more knowledgeable about – PTSD due to indirect trauma.

Taking early warnings in stride
When the anchors and journalists began reporting about a tropical cyclone heading toward the Gulf of Mexico on Aug. 17, most Houstonians – myself included – flipped the channel. Living off the Southern Coast of the United States meant seeing more than our fair share of storm systems, including hurricanes. Each time, no matter the damage or the loss, Texans would pull themselves up by their bootstraps and band together to rebuild their beloved city.
So, it’s no surprise that even as Harvey was upgraded to a hurricane and prepared to breach land, we went about business as usual. However, less than a week later, countless residents of the Lone Star State prepared for what promised to be one of the worst storms in recent history.
Moving to Houston from Dallas for college back in 1998, I fell in love with the city and made it my home. I was here when Tropical Storm Allison made landfall in 2001, leaving up to 37 inches of rain and massive flooding in its wake. The Texas Medical Center, where I was working at the Baylor Human Genome Lab for the summer, suffered about $2 billion worth of damage.
I watched as the images and videos of the city under water splashed across my television screen. I witnessed the floodwaters firsthand as my friends and I carefully drove to an overpass and found a vast body of water where a convergence of three highways used to be visible. I was fortunate not to have been affected by the flood, but the fear of West Nile virus worried me for days because of the mosquito infestation that followed. Eventually, the city recovered, the water receded, and we persevered.
In 2005, in the wake of Katrina, Southern Texans were warned of an impending Category 3 hurricane named Rita. Having been inundated with local and national news coverage of the devastation, and hearing the personal stories of evacuees from New Orleans, Houstonians definitely took more notice this time. More than 3 million people from Houston and the surrounding areas evacuated inland before it arrived, but the chaos resulted in indirect deaths from panicked people trying to leave.
I, along with my two best friends and my boyfriend, were among the many who made the lengthy drive to Dallas, where my parents were anxiously waiting. What should have been a 4-hour drive turned into 10, and that was the result of all the back roads we took to get around the majority of the traffic. There were mass outages around the city, but within a few days, we were all back home. Rita left behind much less damage than predicted, and after the water receded, we persevered.
My third encounter with a hurricane was the Category 2 Ike 3 years later. There were mixed emotions going into this one, with many citizens split between evacuating and staying behind. I was in residency by then, and with only a voluntary evacuation for Houston (compared with a mandatory one in Galveston and the coastal cities), I opted to remain. I had already prepared for the worst by barricading all the glass and stocking up on supplies. In addition, I was living in a two-story townhome in an area considered part of a 200-year flood plain, so I figured I was safe. When Ike struck the city, I was up for several hours listening to the howl of the wind and the insistent smacks of rain against my windows. I left town once the coast was clear, not because of flooding, but because Ike knocked out power and water for much of Houston in the middle of a horridly hot September. I stayed with my parents for about a week until my complex had fixed everything, and seeing that the water had receded, I persevered.
Harvey’s vast destruction
This past week, when Category 4 Hurricane Harvey struck my beloved city, I could not have imagined the losses that were waiting for us. After finishing up a short workday on Friday, Aug. 25, I made my last run for supplies before the weekend. Like many others, I had been keeping an eye on the news as we heard about the destruction Harvey had wreaked on Rockport, South Padre, and Corpus Christi. We all knew that this one was the real deal, that Harvey was going to challenge us in every way possible. For the next 4 days I hunkered down in my house, waiting out the periods of torrential rain while keeping a close eye on the news. At worst, my neighborhood flooded up to the front sidewalk, but water never entered my home, as it did for so many unfortunate individuals. I never lost power, air conditioning, or Internet access. The most distressing thing to happen to me was the inability to leave my home for fear of being caught in the floodwater.
We are #The CityWithNoLimits.
We are #HoustonStrong.
We are #TexasStrong.
When the waters recede, we will persevere.
Jennifer Yen, MD, is a board-certified child, adolescent, and adult private practice psychiatrist in Houston. She also is a clinical assistant professor of psychiatry at Baylor College of Medicine and serves on the Consumers Issues Committee of the American Academy of Child and Adolescent Psychiatry.
It’s been 5 days since Texas came under siege from Hurricane Harvey and it left up to 51 inches of rain in its wake. Several Southern cities suffered almost complete loss of homes and businesses. The Houston metropolitan area reported 14 deaths, including one of a police officer who was trying to report for duty. Hundreds of thousands of homes have been damaged or lost, and thousands of people are now in makeshift shelters across the city. We have slowly begun the process of repair and rebuilding, and many Houstonians are returning to work. Many others, including well-known local celebrities like J.J. Watt and MattressMack, are volunteering their time and giving money to help those who were not so fortunate. The rescue and recovery efforts have been lauded for the absence of issues tied to politics, religion, or race.
Despite this, we must not forget that this was a natural disaster unlike anything that’s been seen in recent decades. Much like Katrina and Sandy, Hurricane Harvey brought to the people who have lived through the initial trauma the fear, nightmares, emotional distress, and sleep disturbances associated with posttraumatic stress disorder (PTSD). They will require significant support and monitoring to determine whether there is a need for medical intervention, such as cognitive-behavioral therapy, behavioral modification, or pharmacotherapy. However, we are also witnessing something psychiatrists are just becoming more knowledgeable about – PTSD due to indirect trauma.

Taking early warnings in stride
When the anchors and journalists began reporting about a tropical cyclone heading toward the Gulf of Mexico on Aug. 17, most Houstonians – myself included – flipped the channel. Living off the Southern Coast of the United States meant seeing more than our fair share of storm systems, including hurricanes. Each time, no matter the damage or the loss, Texans would pull themselves up by their bootstraps and band together to rebuild their beloved city.
So, it’s no surprise that even as Harvey was upgraded to a hurricane and prepared to breach land, we went about business as usual. However, less than a week later, countless residents of the Lone Star State prepared for what promised to be one of the worst storms in recent history.
Moving to Houston from Dallas for college back in 1998, I fell in love with the city and made it my home. I was here when Tropical Storm Allison made landfall in 2001, leaving up to 37 inches of rain and massive flooding in its wake. The Texas Medical Center, where I was working at the Baylor Human Genome Lab for the summer, suffered about $2 billion worth of damage.
I watched as the images and videos of the city under water splashed across my television screen. I witnessed the floodwaters firsthand as my friends and I carefully drove to an overpass and found a vast body of water where a convergence of three highways used to be visible. I was fortunate not to have been affected by the flood, but the fear of West Nile virus worried me for days because of the mosquito infestation that followed. Eventually, the city recovered, the water receded, and we persevered.
In 2005, in the wake of Katrina, Southern Texans were warned of an impending Category 3 hurricane named Rita. Having been inundated with local and national news coverage of the devastation, and hearing the personal stories of evacuees from New Orleans, Houstonians definitely took more notice this time. More than 3 million people from Houston and the surrounding areas evacuated inland before it arrived, but the chaos resulted in indirect deaths from panicked people trying to leave.
I, along with my two best friends and my boyfriend, were among the many who made the lengthy drive to Dallas, where my parents were anxiously waiting. What should have been a 4-hour drive turned into 10, and that was the result of all the back roads we took to get around the majority of the traffic. There were mass outages around the city, but within a few days, we were all back home. Rita left behind much less damage than predicted, and after the water receded, we persevered.
My third encounter with a hurricane was the Category 2 Ike 3 years later. There were mixed emotions going into this one, with many citizens split between evacuating and staying behind. I was in residency by then, and with only a voluntary evacuation for Houston (compared with a mandatory one in Galveston and the coastal cities), I opted to remain. I had already prepared for the worst by barricading all the glass and stocking up on supplies. In addition, I was living in a two-story townhome in an area considered part of a 200-year flood plain, so I figured I was safe. When Ike struck the city, I was up for several hours listening to the howl of the wind and the insistent smacks of rain against my windows. I left town once the coast was clear, not because of flooding, but because Ike knocked out power and water for much of Houston in the middle of a horridly hot September. I stayed with my parents for about a week until my complex had fixed everything, and seeing that the water had receded, I persevered.
Harvey’s vast destruction
This past week, when Category 4 Hurricane Harvey struck my beloved city, I could not have imagined the losses that were waiting for us. After finishing up a short workday on Friday, Aug. 25, I made my last run for supplies before the weekend. Like many others, I had been keeping an eye on the news as we heard about the destruction Harvey had wreaked on Rockport, South Padre, and Corpus Christi. We all knew that this one was the real deal, that Harvey was going to challenge us in every way possible. For the next 4 days I hunkered down in my house, waiting out the periods of torrential rain while keeping a close eye on the news. At worst, my neighborhood flooded up to the front sidewalk, but water never entered my home, as it did for so many unfortunate individuals. I never lost power, air conditioning, or Internet access. The most distressing thing to happen to me was the inability to leave my home for fear of being caught in the floodwater.
We are #The CityWithNoLimits.
We are #HoustonStrong.
We are #TexasStrong.
When the waters recede, we will persevere.
Jennifer Yen, MD, is a board-certified child, adolescent, and adult private practice psychiatrist in Houston. She also is a clinical assistant professor of psychiatry at Baylor College of Medicine and serves on the Consumers Issues Committee of the American Academy of Child and Adolescent Psychiatry.
It’s been 5 days since Texas came under siege from Hurricane Harvey and it left up to 51 inches of rain in its wake. Several Southern cities suffered almost complete loss of homes and businesses. The Houston metropolitan area reported 14 deaths, including one of a police officer who was trying to report for duty. Hundreds of thousands of homes have been damaged or lost, and thousands of people are now in makeshift shelters across the city. We have slowly begun the process of repair and rebuilding, and many Houstonians are returning to work. Many others, including well-known local celebrities like J.J. Watt and MattressMack, are volunteering their time and giving money to help those who were not so fortunate. The rescue and recovery efforts have been lauded for the absence of issues tied to politics, religion, or race.
Despite this, we must not forget that this was a natural disaster unlike anything that’s been seen in recent decades. Much like Katrina and Sandy, Hurricane Harvey brought to the people who have lived through the initial trauma the fear, nightmares, emotional distress, and sleep disturbances associated with posttraumatic stress disorder (PTSD). They will require significant support and monitoring to determine whether there is a need for medical intervention, such as cognitive-behavioral therapy, behavioral modification, or pharmacotherapy. However, we are also witnessing something psychiatrists are just becoming more knowledgeable about – PTSD due to indirect trauma.

Taking early warnings in stride
When the anchors and journalists began reporting about a tropical cyclone heading toward the Gulf of Mexico on Aug. 17, most Houstonians – myself included – flipped the channel. Living off the Southern Coast of the United States meant seeing more than our fair share of storm systems, including hurricanes. Each time, no matter the damage or the loss, Texans would pull themselves up by their bootstraps and band together to rebuild their beloved city.
So, it’s no surprise that even as Harvey was upgraded to a hurricane and prepared to breach land, we went about business as usual. However, less than a week later, countless residents of the Lone Star State prepared for what promised to be one of the worst storms in recent history.
Moving to Houston from Dallas for college back in 1998, I fell in love with the city and made it my home. I was here when Tropical Storm Allison made landfall in 2001, leaving up to 37 inches of rain and massive flooding in its wake. The Texas Medical Center, where I was working at the Baylor Human Genome Lab for the summer, suffered about $2 billion worth of damage.
I watched as the images and videos of the city under water splashed across my television screen. I witnessed the floodwaters firsthand as my friends and I carefully drove to an overpass and found a vast body of water where a convergence of three highways used to be visible. I was fortunate not to have been affected by the flood, but the fear of West Nile virus worried me for days because of the mosquito infestation that followed. Eventually, the city recovered, the water receded, and we persevered.
In 2005, in the wake of Katrina, Southern Texans were warned of an impending Category 3 hurricane named Rita. Having been inundated with local and national news coverage of the devastation, and hearing the personal stories of evacuees from New Orleans, Houstonians definitely took more notice this time. More than 3 million people from Houston and the surrounding areas evacuated inland before it arrived, but the chaos resulted in indirect deaths from panicked people trying to leave.
I, along with my two best friends and my boyfriend, were among the many who made the lengthy drive to Dallas, where my parents were anxiously waiting. What should have been a 4-hour drive turned into 10, and that was the result of all the back roads we took to get around the majority of the traffic. There were mass outages around the city, but within a few days, we were all back home. Rita left behind much less damage than predicted, and after the water receded, we persevered.
My third encounter with a hurricane was the Category 2 Ike 3 years later. There were mixed emotions going into this one, with many citizens split between evacuating and staying behind. I was in residency by then, and with only a voluntary evacuation for Houston (compared with a mandatory one in Galveston and the coastal cities), I opted to remain. I had already prepared for the worst by barricading all the glass and stocking up on supplies. In addition, I was living in a two-story townhome in an area considered part of a 200-year flood plain, so I figured I was safe. When Ike struck the city, I was up for several hours listening to the howl of the wind and the insistent smacks of rain against my windows. I left town once the coast was clear, not because of flooding, but because Ike knocked out power and water for much of Houston in the middle of a horridly hot September. I stayed with my parents for about a week until my complex had fixed everything, and seeing that the water had receded, I persevered.
Harvey’s vast destruction
This past week, when Category 4 Hurricane Harvey struck my beloved city, I could not have imagined the losses that were waiting for us. After finishing up a short workday on Friday, Aug. 25, I made my last run for supplies before the weekend. Like many others, I had been keeping an eye on the news as we heard about the destruction Harvey had wreaked on Rockport, South Padre, and Corpus Christi. We all knew that this one was the real deal, that Harvey was going to challenge us in every way possible. For the next 4 days I hunkered down in my house, waiting out the periods of torrential rain while keeping a close eye on the news. At worst, my neighborhood flooded up to the front sidewalk, but water never entered my home, as it did for so many unfortunate individuals. I never lost power, air conditioning, or Internet access. The most distressing thing to happen to me was the inability to leave my home for fear of being caught in the floodwater.
We are #The CityWithNoLimits.
We are #HoustonStrong.
We are #TexasStrong.
When the waters recede, we will persevere.
Jennifer Yen, MD, is a board-certified child, adolescent, and adult private practice psychiatrist in Houston. She also is a clinical assistant professor of psychiatry at Baylor College of Medicine and serves on the Consumers Issues Committee of the American Academy of Child and Adolescent Psychiatry.
Short Takes
Hospitalized-patient one-year mortality risk (HOMR) score an excellent prognostic tool
The HOMR score, derived from administrative data, accurately predicts mortality. This study derived the score from medical records which providers can access and found it still accurately determines 1-year mortality.
Citation: Casey G, van Walraven C. Prognosticating with the hospitalized-patient one-year mortality risk score using information abstracted from the medical record. J Hosp Med. 2017 April; 12(4):224-30.
New drug for C. difficile recurrence
Bezlotoxumab is now approved to reduce recurrence of Clostridium difficile. This is an injectable human monoclonal antibody to C. difficile toxin and must be used in conjunction with antibiotics.
Citation: U.S. Food and Drug Administration. Drug Label. Available online at https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761046s000lbl.pdf. Accessed 7 May 2017.
Hospitalized-patient one-year mortality risk (HOMR) score an excellent prognostic tool
The HOMR score, derived from administrative data, accurately predicts mortality. This study derived the score from medical records which providers can access and found it still accurately determines 1-year mortality.
Citation: Casey G, van Walraven C. Prognosticating with the hospitalized-patient one-year mortality risk score using information abstracted from the medical record. J Hosp Med. 2017 April; 12(4):224-30.
New drug for C. difficile recurrence
Bezlotoxumab is now approved to reduce recurrence of Clostridium difficile. This is an injectable human monoclonal antibody to C. difficile toxin and must be used in conjunction with antibiotics.
Citation: U.S. Food and Drug Administration. Drug Label. Available online at https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761046s000lbl.pdf. Accessed 7 May 2017.
Hospitalized-patient one-year mortality risk (HOMR) score an excellent prognostic tool
The HOMR score, derived from administrative data, accurately predicts mortality. This study derived the score from medical records which providers can access and found it still accurately determines 1-year mortality.
Citation: Casey G, van Walraven C. Prognosticating with the hospitalized-patient one-year mortality risk score using information abstracted from the medical record. J Hosp Med. 2017 April; 12(4):224-30.
New drug for C. difficile recurrence
Bezlotoxumab is now approved to reduce recurrence of Clostridium difficile. This is an injectable human monoclonal antibody to C. difficile toxin and must be used in conjunction with antibiotics.
Citation: U.S. Food and Drug Administration. Drug Label. Available online at https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761046s000lbl.pdf. Accessed 7 May 2017.