User login
Total Eclipse of the Heart
ANSWER
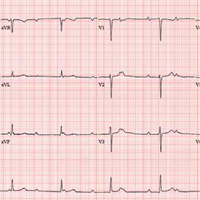
The correct interpretation includes sinus rhythm with complete heart block and a junctional rhythm. Normal sinus rhythm is evidenced by normal-appearing P waves at a rate of about 66 beats/min, although there is some respiratory variation. There is no correlation of the P waves to the QRS complex. A junctional rhythm is diagnosed based on narrow QRS complexes of normal duration (82 ms). The patient underwent implantation of a dual-chamber pacemaker and had a complete recovery.
ANSWER
The correct interpretation includes sinus rhythm with complete heart block and a junctional rhythm. Normal sinus rhythm is evidenced by normal-appearing P waves at a rate of about 66 beats/min, although there is some respiratory variation. There is no correlation of the P waves to the QRS complex. A junctional rhythm is diagnosed based on narrow QRS complexes of normal duration (82 ms). The patient underwent implantation of a dual-chamber pacemaker and had a complete recovery.
ANSWER
The correct interpretation includes sinus rhythm with complete heart block and a junctional rhythm. Normal sinus rhythm is evidenced by normal-appearing P waves at a rate of about 66 beats/min, although there is some respiratory variation. There is no correlation of the P waves to the QRS complex. A junctional rhythm is diagnosed based on narrow QRS complexes of normal duration (82 ms). The patient underwent implantation of a dual-chamber pacemaker and had a complete recovery.
For the past two months, a 72-year-old man has been experiencing dyspnea on exertion “off and on.” In the past three days, this shortness of breath has been more consistent.
When his son drops by to check on him, the father mentions the problem, noting that although he’s usually quite active, he hasn’t “had the stamina” to finish his current woodworking project. The son, a paramedic, checks his father’s pulse; it is regular, but the rate is below 40 beats/min. The son decides to bring him to the emergency department for evaluation.
His medical history is remarkable for hypertension, gout, and cholelithiasis. He underwent a right orchiectomy secondary to trauma in the remote past and had bilateral total knee replacements four years ago. Since the latter procedure, he has been extremely active; prior to his current complaint, he walked three miles each day.
The patient, a retired minister, lost his wife to cancer four years ago and now lives at home alone. He has four sons—one of whom visits twice a week—and two daughters, all of whom are in good health. He has never smoked and only drinks a glass of wine on holidays.
His current medications include metoprolol XL (25 mg/d) for hypertension and acetaminophen as needed for arthritic joint pain. He denies missing or doubling his medication doses. He is allergic to sulfa, which causes an anaphylactic reaction with significant airway narrowing.
The patient wears corrective lenses and hearing aids. He denies recent changes in bowel or bladder function, extreme temperature changes, productive cough, or constitutional symptoms suggestive of indolent infection.
Vital signs include a regular pulse of 50 beats/min; blood pressure, 104/66 mm Hg; respiratory rate, 14 breaths/min-1; and temperature, 98.4°F. His height is 70 in and his weight, 172 lb.
On physical exam, you notice that the patient tires walking 20 feet from the waiting room to the exam room. Otherwise, he is in no apparent distress. He denies chest pain with exertion or dyspnea at rest. His funduscopic exam is normal, his oropharynx is normal, and he is not missing teeth. There are no carotid bruits. The lungs are clear in all fields without evidence of rales, rhonchi, or crackles.
Cardiac exam reveals a grade II/VI early systolic murmur at the left upper sternal border, which does not radiate to the carotid arteries or the back. The abdomen is scaphoid and soft with no palpable masses. Peripheral pulses are strong bilaterally in all extremities. He has evidence of osteoarthritis in his hands and feet, as well as surgical scars consistent with knee replacements. His neurologic exam is intact.
An ECG reveals a ventricular rate of 47 beats/min; no discernable PR interval; QRS duration, 82 ms; QT/QTc interval, 450/398 ms; P axis, 48°; R axis, 14°; and T axis, 26°. What is your interpretation?
Trump administration ends DACA program, stranding medical students
President Trump has ended the Obama administration’s Deferred Action for Childhood Arrivals (DACA) program, a policy that protected immigrants who came to the United States as children from deportation and authorized them to work in the United States.
In a Sept. 5 press conference, Attorney General Jeff Sessions called the DACA program an unconstitutional overreach of executive branch power by the former administration that deliberately sought to undermine the legislative branch. Rollback of the DACA program will begin immediately, Mr. Sessions said, with the program expiring completely on March 5, 2018.
“The effect of this unilateral executive amnesty, among other things, contributed to a surge of minors at the southern border that yielded terrible humanitarian consequences,” Mr. Sessions said during the press conference. “[DACA has] also denied jobs to hundreds of thousands of Americans by allowing those same illegal aliens to take those jobs. To have a lawful system of immigration that serves the national interest, we cannot admit everyone. Therefore the nation must set and enforce a limit on how many immigrants we admit and that means all cannot be accepted.”
In a statement issued shortly after the press conference, President Trump said winding down the DACA program is in the nation’s best interest, and that there can be no principled immigration reform if the executive branch is able to “rewrite or nullify federal laws at will.
“As President, my highest duty is to defend the American people and the Constitution of the United States of America,” President Trump said in the statement. “At the same time, I do not favor punishing children, most of whom are now adults, for the actions of their parents. But we must also recognize that we are nation of opportunity because we are a nation of laws.”
The DACA program was created by the Obama administration in 2012 as a way of protecting young, undocumented immigrants from deportation after Congress repeatedly blocked legislation that would develop such a safe haven. The policy allowed about 800,000 young adults brought to the United States illegally as children to work legally in the U.S. and remain in the country without the fear of deportation.
The program’s end will affect the growing number of medical students with DACA status and likely jeopardize the funding invested in their training. Sixty-two medical schools accept applications from DACA applicants, according to the Association of American Medical Colleges (AAMC). For the 2016 -2017 school year, 113 students with DACA status applied to US medical schools, and there were 65 medical students enrolled who had DACA status. AAMC does not collect data on medical students with DACA status; the National Resident Matching Program, likewise, does not collect data on residents with DACA status.
Loyola University in Chicago is one institute that could be significantly impacted by recension of DACA. The university has accepted more students with DACA status than any other U.S. medical school, and currently has 32 DACA students attending, said Mark Kuczewski, Ph.D., chair of medical education at Loyola University.
“It’s a tragic decision,” he said in an interview. “It once again puts a cloud over these young people who DACA has given the first real opportunity to come out of the shadows, be educated, and serve the community. Now they’re returned back to the situation of uncertainty.”
The decision to end DACA means that current DACA medical students may not be able to finish their training and that those close to completion may not be able to use their degrees in the workforce, Dr. Kuczewski said. Since they are not citizens, DACA students do not qualify for federal student loans, so medical schools must find ways to help DACA students finance their education. A major Catholic health system provides student loan packages for several DACA students at Loyola’s Stritch School of Medicine, Dr. Kuczewski said. However, such loan programs require DACA status. Without DACA or another path to citizenship, medical students in the middle of training will not be able to obtain financial aid to finish their training, he said. The work authorization that DACA provided will also be eliminated.
Dr. Kuczewski said his university plans to advocate strongly for Congress to pass legislation to protect DACA youth, such as the Dream Act of 2017.
“We are going to advocate strongly because we believe this is common sense,” he said. “You don’t just throw away the talents of these young people and the investments they’ve made in their education and the investments we’ve made in them. DACA has given many people the chance to see these young people as students, as employees, as colleagues, and we hope that helps people to mobilize.”
In a statement, Jack Ende, MD, president of the American College of Physicians, said President Trump’s decision to end the DACA program threatens to deny the country the talents of more than half a million individuals making enormous contributions and will also undermine public health and medical education.
“Today’s executive order has the potential to gravely impact public health,” Dr. Ende said in the statement. “We know that noncitizens and undocumented immigrants are more likely to lack health insurance coverage. If the nearly 800,000 people who are currently benefiting from DACA have their protections removed, many will avoid seeking health care in order to reduce the risk of detection and deportation. … Those who seek to serve in the health care professions will be denied that opportunity.”
The Immigration Reform Law Institute praised President Trump’s decision to rescind the DACA program, calling the policy an affront to Congress and a violation of the U.S. Constitution.
“Contrary to former President Obama’s claims, not only is DACA not authorized by federal statute, but prior to the unlawful program, deferred action has only ever been applied to small numbers of illegal aliens on a case-by-case basis,” Dale Wilcox, executive director, said in a statement. “Applying it to approximately 15% of the illegal alien population was never a proper exercise of the president’s discretion under the Constitution and is inconsistent with the president’s duty to take care that the laws be faithfully executed. By rescinding DACA, President Trump has put an end to the previous administration’s flagrant violation of our immigration laws and its abuse of hard-working American taxpayers.”
In a memorandum issued Sept. 5, the Department of Homeland Security said it will begin winding down the DACA program, while providing a limited window in which it will adjudicate certain requests for DACA and associated applications. DHS will adjudicate, on an individual, case-by-case basis, properly filed pending DACA initial requests and associated applications for employment authorization documents that have been accepted by the department as of Sept.5, 2017, according to the memorandum. All DACA initial requests and associated applications filed after this date will be rejected.
[email protected]
On Twitter @legal_med
President Trump has ended the Obama administration’s Deferred Action for Childhood Arrivals (DACA) program, a policy that protected immigrants who came to the United States as children from deportation and authorized them to work in the United States.
In a Sept. 5 press conference, Attorney General Jeff Sessions called the DACA program an unconstitutional overreach of executive branch power by the former administration that deliberately sought to undermine the legislative branch. Rollback of the DACA program will begin immediately, Mr. Sessions said, with the program expiring completely on March 5, 2018.
“The effect of this unilateral executive amnesty, among other things, contributed to a surge of minors at the southern border that yielded terrible humanitarian consequences,” Mr. Sessions said during the press conference. “[DACA has] also denied jobs to hundreds of thousands of Americans by allowing those same illegal aliens to take those jobs. To have a lawful system of immigration that serves the national interest, we cannot admit everyone. Therefore the nation must set and enforce a limit on how many immigrants we admit and that means all cannot be accepted.”
In a statement issued shortly after the press conference, President Trump said winding down the DACA program is in the nation’s best interest, and that there can be no principled immigration reform if the executive branch is able to “rewrite or nullify federal laws at will.
“As President, my highest duty is to defend the American people and the Constitution of the United States of America,” President Trump said in the statement. “At the same time, I do not favor punishing children, most of whom are now adults, for the actions of their parents. But we must also recognize that we are nation of opportunity because we are a nation of laws.”
The DACA program was created by the Obama administration in 2012 as a way of protecting young, undocumented immigrants from deportation after Congress repeatedly blocked legislation that would develop such a safe haven. The policy allowed about 800,000 young adults brought to the United States illegally as children to work legally in the U.S. and remain in the country without the fear of deportation.
The program’s end will affect the growing number of medical students with DACA status and likely jeopardize the funding invested in their training. Sixty-two medical schools accept applications from DACA applicants, according to the Association of American Medical Colleges (AAMC). For the 2016 -2017 school year, 113 students with DACA status applied to US medical schools, and there were 65 medical students enrolled who had DACA status. AAMC does not collect data on medical students with DACA status; the National Resident Matching Program, likewise, does not collect data on residents with DACA status.
Loyola University in Chicago is one institute that could be significantly impacted by recension of DACA. The university has accepted more students with DACA status than any other U.S. medical school, and currently has 32 DACA students attending, said Mark Kuczewski, Ph.D., chair of medical education at Loyola University.
“It’s a tragic decision,” he said in an interview. “It once again puts a cloud over these young people who DACA has given the first real opportunity to come out of the shadows, be educated, and serve the community. Now they’re returned back to the situation of uncertainty.”
The decision to end DACA means that current DACA medical students may not be able to finish their training and that those close to completion may not be able to use their degrees in the workforce, Dr. Kuczewski said. Since they are not citizens, DACA students do not qualify for federal student loans, so medical schools must find ways to help DACA students finance their education. A major Catholic health system provides student loan packages for several DACA students at Loyola’s Stritch School of Medicine, Dr. Kuczewski said. However, such loan programs require DACA status. Without DACA or another path to citizenship, medical students in the middle of training will not be able to obtain financial aid to finish their training, he said. The work authorization that DACA provided will also be eliminated.
Dr. Kuczewski said his university plans to advocate strongly for Congress to pass legislation to protect DACA youth, such as the Dream Act of 2017.
“We are going to advocate strongly because we believe this is common sense,” he said. “You don’t just throw away the talents of these young people and the investments they’ve made in their education and the investments we’ve made in them. DACA has given many people the chance to see these young people as students, as employees, as colleagues, and we hope that helps people to mobilize.”
In a statement, Jack Ende, MD, president of the American College of Physicians, said President Trump’s decision to end the DACA program threatens to deny the country the talents of more than half a million individuals making enormous contributions and will also undermine public health and medical education.
“Today’s executive order has the potential to gravely impact public health,” Dr. Ende said in the statement. “We know that noncitizens and undocumented immigrants are more likely to lack health insurance coverage. If the nearly 800,000 people who are currently benefiting from DACA have their protections removed, many will avoid seeking health care in order to reduce the risk of detection and deportation. … Those who seek to serve in the health care professions will be denied that opportunity.”
The Immigration Reform Law Institute praised President Trump’s decision to rescind the DACA program, calling the policy an affront to Congress and a violation of the U.S. Constitution.
“Contrary to former President Obama’s claims, not only is DACA not authorized by federal statute, but prior to the unlawful program, deferred action has only ever been applied to small numbers of illegal aliens on a case-by-case basis,” Dale Wilcox, executive director, said in a statement. “Applying it to approximately 15% of the illegal alien population was never a proper exercise of the president’s discretion under the Constitution and is inconsistent with the president’s duty to take care that the laws be faithfully executed. By rescinding DACA, President Trump has put an end to the previous administration’s flagrant violation of our immigration laws and its abuse of hard-working American taxpayers.”
In a memorandum issued Sept. 5, the Department of Homeland Security said it will begin winding down the DACA program, while providing a limited window in which it will adjudicate certain requests for DACA and associated applications. DHS will adjudicate, on an individual, case-by-case basis, properly filed pending DACA initial requests and associated applications for employment authorization documents that have been accepted by the department as of Sept.5, 2017, according to the memorandum. All DACA initial requests and associated applications filed after this date will be rejected.
[email protected]
On Twitter @legal_med
President Trump has ended the Obama administration’s Deferred Action for Childhood Arrivals (DACA) program, a policy that protected immigrants who came to the United States as children from deportation and authorized them to work in the United States.
In a Sept. 5 press conference, Attorney General Jeff Sessions called the DACA program an unconstitutional overreach of executive branch power by the former administration that deliberately sought to undermine the legislative branch. Rollback of the DACA program will begin immediately, Mr. Sessions said, with the program expiring completely on March 5, 2018.
“The effect of this unilateral executive amnesty, among other things, contributed to a surge of minors at the southern border that yielded terrible humanitarian consequences,” Mr. Sessions said during the press conference. “[DACA has] also denied jobs to hundreds of thousands of Americans by allowing those same illegal aliens to take those jobs. To have a lawful system of immigration that serves the national interest, we cannot admit everyone. Therefore the nation must set and enforce a limit on how many immigrants we admit and that means all cannot be accepted.”
In a statement issued shortly after the press conference, President Trump said winding down the DACA program is in the nation’s best interest, and that there can be no principled immigration reform if the executive branch is able to “rewrite or nullify federal laws at will.
“As President, my highest duty is to defend the American people and the Constitution of the United States of America,” President Trump said in the statement. “At the same time, I do not favor punishing children, most of whom are now adults, for the actions of their parents. But we must also recognize that we are nation of opportunity because we are a nation of laws.”
The DACA program was created by the Obama administration in 2012 as a way of protecting young, undocumented immigrants from deportation after Congress repeatedly blocked legislation that would develop such a safe haven. The policy allowed about 800,000 young adults brought to the United States illegally as children to work legally in the U.S. and remain in the country without the fear of deportation.
The program’s end will affect the growing number of medical students with DACA status and likely jeopardize the funding invested in their training. Sixty-two medical schools accept applications from DACA applicants, according to the Association of American Medical Colleges (AAMC). For the 2016 -2017 school year, 113 students with DACA status applied to US medical schools, and there were 65 medical students enrolled who had DACA status. AAMC does not collect data on medical students with DACA status; the National Resident Matching Program, likewise, does not collect data on residents with DACA status.
Loyola University in Chicago is one institute that could be significantly impacted by recension of DACA. The university has accepted more students with DACA status than any other U.S. medical school, and currently has 32 DACA students attending, said Mark Kuczewski, Ph.D., chair of medical education at Loyola University.
“It’s a tragic decision,” he said in an interview. “It once again puts a cloud over these young people who DACA has given the first real opportunity to come out of the shadows, be educated, and serve the community. Now they’re returned back to the situation of uncertainty.”
The decision to end DACA means that current DACA medical students may not be able to finish their training and that those close to completion may not be able to use their degrees in the workforce, Dr. Kuczewski said. Since they are not citizens, DACA students do not qualify for federal student loans, so medical schools must find ways to help DACA students finance their education. A major Catholic health system provides student loan packages for several DACA students at Loyola’s Stritch School of Medicine, Dr. Kuczewski said. However, such loan programs require DACA status. Without DACA or another path to citizenship, medical students in the middle of training will not be able to obtain financial aid to finish their training, he said. The work authorization that DACA provided will also be eliminated.
Dr. Kuczewski said his university plans to advocate strongly for Congress to pass legislation to protect DACA youth, such as the Dream Act of 2017.
“We are going to advocate strongly because we believe this is common sense,” he said. “You don’t just throw away the talents of these young people and the investments they’ve made in their education and the investments we’ve made in them. DACA has given many people the chance to see these young people as students, as employees, as colleagues, and we hope that helps people to mobilize.”
In a statement, Jack Ende, MD, president of the American College of Physicians, said President Trump’s decision to end the DACA program threatens to deny the country the talents of more than half a million individuals making enormous contributions and will also undermine public health and medical education.
“Today’s executive order has the potential to gravely impact public health,” Dr. Ende said in the statement. “We know that noncitizens and undocumented immigrants are more likely to lack health insurance coverage. If the nearly 800,000 people who are currently benefiting from DACA have their protections removed, many will avoid seeking health care in order to reduce the risk of detection and deportation. … Those who seek to serve in the health care professions will be denied that opportunity.”
The Immigration Reform Law Institute praised President Trump’s decision to rescind the DACA program, calling the policy an affront to Congress and a violation of the U.S. Constitution.
“Contrary to former President Obama’s claims, not only is DACA not authorized by federal statute, but prior to the unlawful program, deferred action has only ever been applied to small numbers of illegal aliens on a case-by-case basis,” Dale Wilcox, executive director, said in a statement. “Applying it to approximately 15% of the illegal alien population was never a proper exercise of the president’s discretion under the Constitution and is inconsistent with the president’s duty to take care that the laws be faithfully executed. By rescinding DACA, President Trump has put an end to the previous administration’s flagrant violation of our immigration laws and its abuse of hard-working American taxpayers.”
In a memorandum issued Sept. 5, the Department of Homeland Security said it will begin winding down the DACA program, while providing a limited window in which it will adjudicate certain requests for DACA and associated applications. DHS will adjudicate, on an individual, case-by-case basis, properly filed pending DACA initial requests and associated applications for employment authorization documents that have been accepted by the department as of Sept.5, 2017, according to the memorandum. All DACA initial requests and associated applications filed after this date will be rejected.
[email protected]
On Twitter @legal_med
Zika’s 2017 summer less active than 2016
Zika may not have gone away this summer, but it didn’t make a comeback, either.
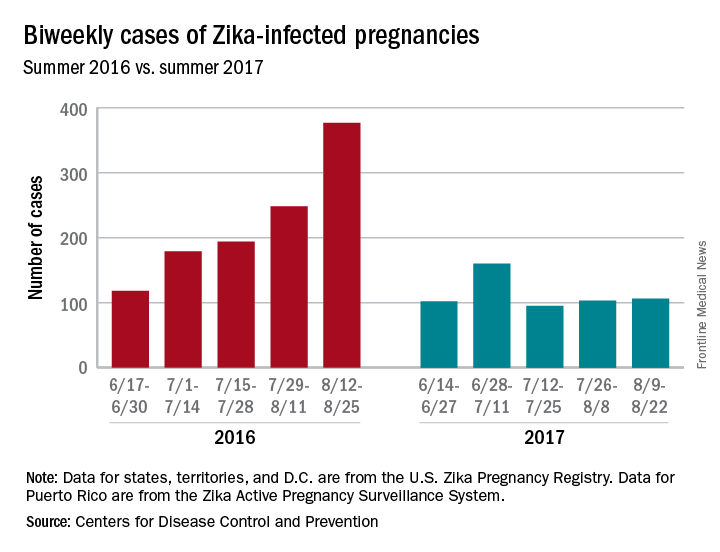
New cases in pregnant women are still being reported, but the numbers are much lower than a year ago, when the infection was kicking into high gear. For the 2 weeks ending Aug. 22, 106 pregnant women with laboratory evidence of Zika virus infection were reported: 43 in the U.S. states and the District of Columbia, and 63 in the U.S. territories, according to the Centers for Disease Control and Prevention.
The total cases reported for the previous 2-week periods, going back to mid-June, look like this: 102 (June 14-27), 160 (June 28–July 11), 95 (July 12-25), and 103 (July 26–Aug. 8). In the summer of 2016, the 2-week period of Aug. 12-25 produced 375 new reports of Zika-infected pregnant women, the CDC data show.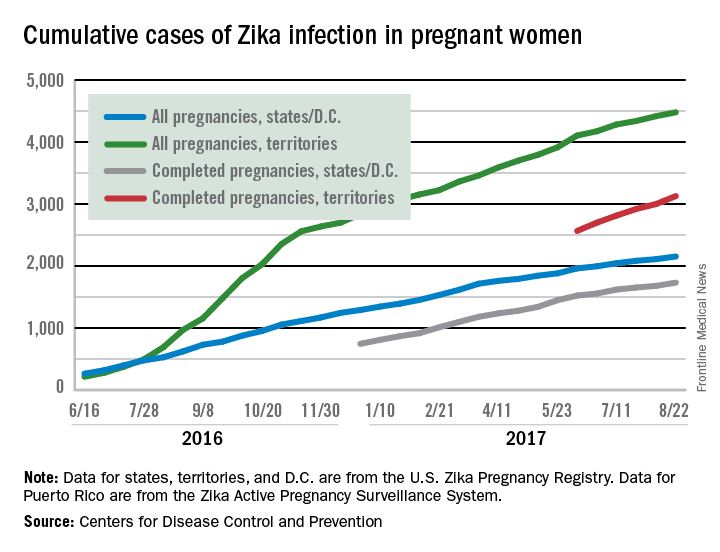
Zika may not have gone away this summer, but it didn’t make a comeback, either.
New cases in pregnant women are still being reported, but the numbers are much lower than a year ago, when the infection was kicking into high gear. For the 2 weeks ending Aug. 22, 106 pregnant women with laboratory evidence of Zika virus infection were reported: 43 in the U.S. states and the District of Columbia, and 63 in the U.S. territories, according to the Centers for Disease Control and Prevention.
The total cases reported for the previous 2-week periods, going back to mid-June, look like this: 102 (June 14-27), 160 (June 28–July 11), 95 (July 12-25), and 103 (July 26–Aug. 8). In the summer of 2016, the 2-week period of Aug. 12-25 produced 375 new reports of Zika-infected pregnant women, the CDC data show.

Zika may not have gone away this summer, but it didn’t make a comeback, either.
New cases in pregnant women are still being reported, but the numbers are much lower than a year ago, when the infection was kicking into high gear. For the 2 weeks ending Aug. 22, 106 pregnant women with laboratory evidence of Zika virus infection were reported: 43 in the U.S. states and the District of Columbia, and 63 in the U.S. territories, according to the Centers for Disease Control and Prevention.
The total cases reported for the previous 2-week periods, going back to mid-June, look like this: 102 (June 14-27), 160 (June 28–July 11), 95 (July 12-25), and 103 (July 26–Aug. 8). In the summer of 2016, the 2-week period of Aug. 12-25 produced 375 new reports of Zika-infected pregnant women, the CDC data show.

No obvious choice for treating pruritus in PBC patients
While multiple options exist for the treatment of pruritus in patients with primary biliary cholangitis (PBC), none have compelling evidence regarding their long-term efficacy and safety, according to a narrative review of literature by Hirsh D. Trivedi, MD, and associates.
There are four treatments commonly used for treating pruritus in PBC patients: bile acid–binding resins, rifampicin, opioid antagonists, and sertraline. In the cases of bile acid–binding resins, rifampicin, and opioid antagonists, significant side effects and a lack of proof of long-term efficacy prevent the treatments from standing out. Sertraline seems to have no significant side effects, but research is lacking, and further investigation is required.
Several experimental treatments for refractory pruritus also exist: These include phototherapy, plasmapheresis, albumin dialysis, nasobiliary drainage, ileal bile acid transporter–inhibitors, methotrexate and colchicine, and fibrates. In extreme cases, liver transplant can also be utilized to reduce pruritus symptoms.
“Our ongoing learning [about] this multifaceted symptom will hopefully lead to the development of more effective therapies and improve the quality of life for patients with primary biliary cholangitis,” the investigators concluded.
Find the full narrative review in the American Journal of Medicine (doi: 10.1016/j.amjmed.2017.01.037).
While multiple options exist for the treatment of pruritus in patients with primary biliary cholangitis (PBC), none have compelling evidence regarding their long-term efficacy and safety, according to a narrative review of literature by Hirsh D. Trivedi, MD, and associates.
There are four treatments commonly used for treating pruritus in PBC patients: bile acid–binding resins, rifampicin, opioid antagonists, and sertraline. In the cases of bile acid–binding resins, rifampicin, and opioid antagonists, significant side effects and a lack of proof of long-term efficacy prevent the treatments from standing out. Sertraline seems to have no significant side effects, but research is lacking, and further investigation is required.
Several experimental treatments for refractory pruritus also exist: These include phototherapy, plasmapheresis, albumin dialysis, nasobiliary drainage, ileal bile acid transporter–inhibitors, methotrexate and colchicine, and fibrates. In extreme cases, liver transplant can also be utilized to reduce pruritus symptoms.
“Our ongoing learning [about] this multifaceted symptom will hopefully lead to the development of more effective therapies and improve the quality of life for patients with primary biliary cholangitis,” the investigators concluded.
Find the full narrative review in the American Journal of Medicine (doi: 10.1016/j.amjmed.2017.01.037).
While multiple options exist for the treatment of pruritus in patients with primary biliary cholangitis (PBC), none have compelling evidence regarding their long-term efficacy and safety, according to a narrative review of literature by Hirsh D. Trivedi, MD, and associates.
There are four treatments commonly used for treating pruritus in PBC patients: bile acid–binding resins, rifampicin, opioid antagonists, and sertraline. In the cases of bile acid–binding resins, rifampicin, and opioid antagonists, significant side effects and a lack of proof of long-term efficacy prevent the treatments from standing out. Sertraline seems to have no significant side effects, but research is lacking, and further investigation is required.
Several experimental treatments for refractory pruritus also exist: These include phototherapy, plasmapheresis, albumin dialysis, nasobiliary drainage, ileal bile acid transporter–inhibitors, methotrexate and colchicine, and fibrates. In extreme cases, liver transplant can also be utilized to reduce pruritus symptoms.
“Our ongoing learning [about] this multifaceted symptom will hopefully lead to the development of more effective therapies and improve the quality of life for patients with primary biliary cholangitis,” the investigators concluded.
Find the full narrative review in the American Journal of Medicine (doi: 10.1016/j.amjmed.2017.01.037).
FROM THE AMERICAN JOURNAL OF MEDICINE
Vaccinate and consider tofacitinib monotherapy to prevent herpes zoster in RA
The results of two studies of tofacitinib treatment for rheumatoid arthritis offer evidence to support the use of the drug without concomitant conventional synthetic disease-modifying antirheumatic drugs in order to reduce the risk of risk of herpes zoster infection and the safety of starting the drug 2-3 weeks after administering live zoster vaccine.
The risk of herpes zoster infection was elevated among rheumatoid arthritis (RA) patients receiving tofacitinib (Xeljanz) with glucocorticoids, compared with those receiving tofacitinib monotherapy, according to an analysis of data from 19 phase 2, phase 3, and long-term extension studies of tofacitinib.
“Further, physicians should continue to consider shingles vaccination prior to starting tofacitinib or biologic therapy,” they wrote.
Dr. Winthrop is the first author of a separate phase 2 study that also appears in Arthritis & Rheumatology, which suggests that live zoster vaccine (LZV) is safe in RA patients who start tofacitinib 2-3 weeks after vaccination. The study also showed that varicella-zoster virus (VZV)-specific humoral and cell-mediated immune responses to LZV are similar in tofacitinib- and placebo-treated patients.
“Our study provides the first data with this vaccine in the RA setting and suggests that these patients, even while using nonbiologic DMARDs at the time of vaccination, are capable of mounting adequate immune responses to this vaccine. Further, our data suggest that the use of tofacitinib following VZV vaccination in the RA setting did not impact negatively the vaccine immunogenicity or the time course of the immune response to the vaccine,” he and his colleagues wrote (Arthritis Rheumatol. 2017 Aug 28. doi: 10.1002/art.40187).
Increased herpes zoster risk in combination therapy
In the first study, herpes zoster (HZ) was reported in 636 of 6,192 patients over a median follow-up of 3 years of tofacitinib exposure, for an incident rate (IR) of 4.0 per 100 patient-years. However, IRs varied by region, ranging from 2.4 in Eastern Europe to 8.0 and 8.4 in Japan and Korea, respectively.
Further, in phase 3 studies, the IRs varied by tofacitinib dose, background use of conventional csDMARDs, and baseline glucocorticoid use; the rates were lowest among patients on tofacitinib monotherapy at a dose of 5 mg twice daily (IR, 0.6), and highest in those on tofacitinib at 10 mg twice daily with csDMARDs and glucocorticoids (IR, 5.4), the investigators found.
Independent risk factors for HZ included age, glucocorticoid use, tofacitinib dose, and enrollment within Asia, they said.
“Shingles, or reactivation of varicella virus, is a common and potentially debilitating illness. Around one-third of the general population will develop HZ in their lifetime, and approximately 10% of these patients develop postherpetic neuralgia which can last months to years and cause significant pain and morbidity,” the investigators wrote, adding that RA patients are at 1.5- to 2-fold greater risk vs. similarly aged individuals in the general population.
RA itself and treatment with glucocorticoids are known to increase HZ risk, but recent data have suggested that Janus kinase inhibitors, such as tofacitinib, and tumor necrosis factor antagonists are also associated with a higher rate of HZ. Additionally, a theoretical risk exists with various csDMARDs, they said.
“Given the increased risk of HZ observed among patients with RA versus the general population and the risk associated with RA therapies, it is possible that risk of HZ may be further increased when such therapies are combined,” they wrote.
Indeed, the findings of the study demonstrate an increased risk of HZ with tofacitinib in combination with glucocorticoids vs. tofacitinib monotherapy.
Further research is necessary to understand why Japanese and Korean patients are at elevated risk, and to understand the mechanism for the effects of combination therapy on VZV reactivation, they concluded.
LZV immunogenicity holds up during tofacitinib treatment
In the second study, 112 patients aged 50 years and older with active RA on background methotrexate received LZV and were then randomized to receive 5 mg tofacitinib twice daily or placebo 2-3 weeks after vaccination.
At 6 weeks after vaccination, VZV-specific IgG geometric mean fold rise (GMFR) was 2.11 and 1.74 in the tofacitinib and placebo patients, respectively; at all postvaccination time points at which VZV-specific IgG levels were evaluated, there was a trend toward numerically higher GMFR in tofacitinib patients, but the differences were not statistically significant. Also, the proportion of patients developing a 1.5-fold or greater postvaccination rise in IgG levels at 6 weeks trended higher for tofacitinib (57.4% vs. 43.4% with placebo).
VZV-specific T-cell GMFR at 6 weeks increased similarly in the groups (1.50 with tofacitinib and 1.29 with placebo).
Serious adverse events occurred in three patients in the tofacitinib group (5.5%) and in none of the placebo patients.
“The three SAEs included one case each of cholangitis and bronchitis, and once case of disseminated primary varicella,” the investigators said, noting that the onset of the latter was 16 days postvaccination, 2 days after starting tofacitinib. The rash resolved with discontinuation of tofacitinib and treatment with valacyclovir for 7 days.
The findings suggest that patients with active RA develop robust immune responses to HZ vaccine, and that starting tofacitinib 2-3 weeks after vaccination has no negative impact on the established immune response.
“Importantly, while our results suggest the vaccine is safe for patients with RA with prior VZV exposure, they also indicate the potential need to either screen for prior exposure before giving this vaccine or waiting longer than 2-3 weeks before starting immunosuppression with tofacitinib,” they said, noting that the current data suggest 4 weeks might be preferable.
Alternatively, testing patients who don’t recollect a history of chickenpox to ensure prior VZV exposure prior to vaccination could also mitigate the risk, they said.
“Further research is necessary to understand the risk of this complication, as well as the long-term effectiveness of this vaccine to prevent HZ in this high-risk population,” they concluded.
Both studies were sponsored by Pfizer. Dr. Winthrop has received research grants from and served as a scientific consultant to Pfizer. Other authors also reported financial relationships with Pfizer.
At a symposium during the 2015 annual meeting of the American College of Rheumatology, William Schaffner, MD, highlighted the connection between the seriousness of an infection, and the respect one has for the solution.
Dr. Schaffner said that “if you don’t fear the infection, you won’t value the solution.” Herpes zoster (HZ) should be feared, and the solution – the live zoster vaccine – valued.
Live zoster vaccine (LZV) was approved in 2006 on the basis of a trial involving more than 38,500 adults over age 60 years, which showed a 51% HZ prevention rate (64% protection in the 60-69 year age group) and a two-thirds reduction in postherpetic neuralgia. Complications and disseminated infection were rare.
HZ vaccination should be offered regardless of a history of varicella infection or prior shingles, as HZ may recur.
There is an imperative need to know who is at risk, when and how they should be vaccinated, and what other risk reduction measures should be considered.
John J. Cush, MD, is director of clinical rheumatology at Baylor Scott & White Research Institute and professor of medicine and rheumatology at Baylor University Medical Center, both in Dallas. His comments are taken from his editorial accompanying the two studies by Winthrop et al. (Arthritis Rheumatol. 2017 Aug 28. doi: 10.1002/art.40188).
At a symposium during the 2015 annual meeting of the American College of Rheumatology, William Schaffner, MD, highlighted the connection between the seriousness of an infection, and the respect one has for the solution.
Dr. Schaffner said that “if you don’t fear the infection, you won’t value the solution.” Herpes zoster (HZ) should be feared, and the solution – the live zoster vaccine – valued.
Live zoster vaccine (LZV) was approved in 2006 on the basis of a trial involving more than 38,500 adults over age 60 years, which showed a 51% HZ prevention rate (64% protection in the 60-69 year age group) and a two-thirds reduction in postherpetic neuralgia. Complications and disseminated infection were rare.
HZ vaccination should be offered regardless of a history of varicella infection or prior shingles, as HZ may recur.
There is an imperative need to know who is at risk, when and how they should be vaccinated, and what other risk reduction measures should be considered.
John J. Cush, MD, is director of clinical rheumatology at Baylor Scott & White Research Institute and professor of medicine and rheumatology at Baylor University Medical Center, both in Dallas. His comments are taken from his editorial accompanying the two studies by Winthrop et al. (Arthritis Rheumatol. 2017 Aug 28. doi: 10.1002/art.40188).
At a symposium during the 2015 annual meeting of the American College of Rheumatology, William Schaffner, MD, highlighted the connection between the seriousness of an infection, and the respect one has for the solution.
Dr. Schaffner said that “if you don’t fear the infection, you won’t value the solution.” Herpes zoster (HZ) should be feared, and the solution – the live zoster vaccine – valued.
Live zoster vaccine (LZV) was approved in 2006 on the basis of a trial involving more than 38,500 adults over age 60 years, which showed a 51% HZ prevention rate (64% protection in the 60-69 year age group) and a two-thirds reduction in postherpetic neuralgia. Complications and disseminated infection were rare.
HZ vaccination should be offered regardless of a history of varicella infection or prior shingles, as HZ may recur.
There is an imperative need to know who is at risk, when and how they should be vaccinated, and what other risk reduction measures should be considered.
John J. Cush, MD, is director of clinical rheumatology at Baylor Scott & White Research Institute and professor of medicine and rheumatology at Baylor University Medical Center, both in Dallas. His comments are taken from his editorial accompanying the two studies by Winthrop et al. (Arthritis Rheumatol. 2017 Aug 28. doi: 10.1002/art.40188).
The results of two studies of tofacitinib treatment for rheumatoid arthritis offer evidence to support the use of the drug without concomitant conventional synthetic disease-modifying antirheumatic drugs in order to reduce the risk of risk of herpes zoster infection and the safety of starting the drug 2-3 weeks after administering live zoster vaccine.
The risk of herpes zoster infection was elevated among rheumatoid arthritis (RA) patients receiving tofacitinib (Xeljanz) with glucocorticoids, compared with those receiving tofacitinib monotherapy, according to an analysis of data from 19 phase 2, phase 3, and long-term extension studies of tofacitinib.
“Further, physicians should continue to consider shingles vaccination prior to starting tofacitinib or biologic therapy,” they wrote.
Dr. Winthrop is the first author of a separate phase 2 study that also appears in Arthritis & Rheumatology, which suggests that live zoster vaccine (LZV) is safe in RA patients who start tofacitinib 2-3 weeks after vaccination. The study also showed that varicella-zoster virus (VZV)-specific humoral and cell-mediated immune responses to LZV are similar in tofacitinib- and placebo-treated patients.
“Our study provides the first data with this vaccine in the RA setting and suggests that these patients, even while using nonbiologic DMARDs at the time of vaccination, are capable of mounting adequate immune responses to this vaccine. Further, our data suggest that the use of tofacitinib following VZV vaccination in the RA setting did not impact negatively the vaccine immunogenicity or the time course of the immune response to the vaccine,” he and his colleagues wrote (Arthritis Rheumatol. 2017 Aug 28. doi: 10.1002/art.40187).
Increased herpes zoster risk in combination therapy
In the first study, herpes zoster (HZ) was reported in 636 of 6,192 patients over a median follow-up of 3 years of tofacitinib exposure, for an incident rate (IR) of 4.0 per 100 patient-years. However, IRs varied by region, ranging from 2.4 in Eastern Europe to 8.0 and 8.4 in Japan and Korea, respectively.
Further, in phase 3 studies, the IRs varied by tofacitinib dose, background use of conventional csDMARDs, and baseline glucocorticoid use; the rates were lowest among patients on tofacitinib monotherapy at a dose of 5 mg twice daily (IR, 0.6), and highest in those on tofacitinib at 10 mg twice daily with csDMARDs and glucocorticoids (IR, 5.4), the investigators found.
Independent risk factors for HZ included age, glucocorticoid use, tofacitinib dose, and enrollment within Asia, they said.
“Shingles, or reactivation of varicella virus, is a common and potentially debilitating illness. Around one-third of the general population will develop HZ in their lifetime, and approximately 10% of these patients develop postherpetic neuralgia which can last months to years and cause significant pain and morbidity,” the investigators wrote, adding that RA patients are at 1.5- to 2-fold greater risk vs. similarly aged individuals in the general population.
RA itself and treatment with glucocorticoids are known to increase HZ risk, but recent data have suggested that Janus kinase inhibitors, such as tofacitinib, and tumor necrosis factor antagonists are also associated with a higher rate of HZ. Additionally, a theoretical risk exists with various csDMARDs, they said.
“Given the increased risk of HZ observed among patients with RA versus the general population and the risk associated with RA therapies, it is possible that risk of HZ may be further increased when such therapies are combined,” they wrote.
Indeed, the findings of the study demonstrate an increased risk of HZ with tofacitinib in combination with glucocorticoids vs. tofacitinib monotherapy.
Further research is necessary to understand why Japanese and Korean patients are at elevated risk, and to understand the mechanism for the effects of combination therapy on VZV reactivation, they concluded.
LZV immunogenicity holds up during tofacitinib treatment
In the second study, 112 patients aged 50 years and older with active RA on background methotrexate received LZV and were then randomized to receive 5 mg tofacitinib twice daily or placebo 2-3 weeks after vaccination.
At 6 weeks after vaccination, VZV-specific IgG geometric mean fold rise (GMFR) was 2.11 and 1.74 in the tofacitinib and placebo patients, respectively; at all postvaccination time points at which VZV-specific IgG levels were evaluated, there was a trend toward numerically higher GMFR in tofacitinib patients, but the differences were not statistically significant. Also, the proportion of patients developing a 1.5-fold or greater postvaccination rise in IgG levels at 6 weeks trended higher for tofacitinib (57.4% vs. 43.4% with placebo).
VZV-specific T-cell GMFR at 6 weeks increased similarly in the groups (1.50 with tofacitinib and 1.29 with placebo).
Serious adverse events occurred in three patients in the tofacitinib group (5.5%) and in none of the placebo patients.
“The three SAEs included one case each of cholangitis and bronchitis, and once case of disseminated primary varicella,” the investigators said, noting that the onset of the latter was 16 days postvaccination, 2 days after starting tofacitinib. The rash resolved with discontinuation of tofacitinib and treatment with valacyclovir for 7 days.
The findings suggest that patients with active RA develop robust immune responses to HZ vaccine, and that starting tofacitinib 2-3 weeks after vaccination has no negative impact on the established immune response.
“Importantly, while our results suggest the vaccine is safe for patients with RA with prior VZV exposure, they also indicate the potential need to either screen for prior exposure before giving this vaccine or waiting longer than 2-3 weeks before starting immunosuppression with tofacitinib,” they said, noting that the current data suggest 4 weeks might be preferable.
Alternatively, testing patients who don’t recollect a history of chickenpox to ensure prior VZV exposure prior to vaccination could also mitigate the risk, they said.
“Further research is necessary to understand the risk of this complication, as well as the long-term effectiveness of this vaccine to prevent HZ in this high-risk population,” they concluded.
Both studies were sponsored by Pfizer. Dr. Winthrop has received research grants from and served as a scientific consultant to Pfizer. Other authors also reported financial relationships with Pfizer.
The results of two studies of tofacitinib treatment for rheumatoid arthritis offer evidence to support the use of the drug without concomitant conventional synthetic disease-modifying antirheumatic drugs in order to reduce the risk of risk of herpes zoster infection and the safety of starting the drug 2-3 weeks after administering live zoster vaccine.
The risk of herpes zoster infection was elevated among rheumatoid arthritis (RA) patients receiving tofacitinib (Xeljanz) with glucocorticoids, compared with those receiving tofacitinib monotherapy, according to an analysis of data from 19 phase 2, phase 3, and long-term extension studies of tofacitinib.
“Further, physicians should continue to consider shingles vaccination prior to starting tofacitinib or biologic therapy,” they wrote.
Dr. Winthrop is the first author of a separate phase 2 study that also appears in Arthritis & Rheumatology, which suggests that live zoster vaccine (LZV) is safe in RA patients who start tofacitinib 2-3 weeks after vaccination. The study also showed that varicella-zoster virus (VZV)-specific humoral and cell-mediated immune responses to LZV are similar in tofacitinib- and placebo-treated patients.
“Our study provides the first data with this vaccine in the RA setting and suggests that these patients, even while using nonbiologic DMARDs at the time of vaccination, are capable of mounting adequate immune responses to this vaccine. Further, our data suggest that the use of tofacitinib following VZV vaccination in the RA setting did not impact negatively the vaccine immunogenicity or the time course of the immune response to the vaccine,” he and his colleagues wrote (Arthritis Rheumatol. 2017 Aug 28. doi: 10.1002/art.40187).
Increased herpes zoster risk in combination therapy
In the first study, herpes zoster (HZ) was reported in 636 of 6,192 patients over a median follow-up of 3 years of tofacitinib exposure, for an incident rate (IR) of 4.0 per 100 patient-years. However, IRs varied by region, ranging from 2.4 in Eastern Europe to 8.0 and 8.4 in Japan and Korea, respectively.
Further, in phase 3 studies, the IRs varied by tofacitinib dose, background use of conventional csDMARDs, and baseline glucocorticoid use; the rates were lowest among patients on tofacitinib monotherapy at a dose of 5 mg twice daily (IR, 0.6), and highest in those on tofacitinib at 10 mg twice daily with csDMARDs and glucocorticoids (IR, 5.4), the investigators found.
Independent risk factors for HZ included age, glucocorticoid use, tofacitinib dose, and enrollment within Asia, they said.
“Shingles, or reactivation of varicella virus, is a common and potentially debilitating illness. Around one-third of the general population will develop HZ in their lifetime, and approximately 10% of these patients develop postherpetic neuralgia which can last months to years and cause significant pain and morbidity,” the investigators wrote, adding that RA patients are at 1.5- to 2-fold greater risk vs. similarly aged individuals in the general population.
RA itself and treatment with glucocorticoids are known to increase HZ risk, but recent data have suggested that Janus kinase inhibitors, such as tofacitinib, and tumor necrosis factor antagonists are also associated with a higher rate of HZ. Additionally, a theoretical risk exists with various csDMARDs, they said.
“Given the increased risk of HZ observed among patients with RA versus the general population and the risk associated with RA therapies, it is possible that risk of HZ may be further increased when such therapies are combined,” they wrote.
Indeed, the findings of the study demonstrate an increased risk of HZ with tofacitinib in combination with glucocorticoids vs. tofacitinib monotherapy.
Further research is necessary to understand why Japanese and Korean patients are at elevated risk, and to understand the mechanism for the effects of combination therapy on VZV reactivation, they concluded.
LZV immunogenicity holds up during tofacitinib treatment
In the second study, 112 patients aged 50 years and older with active RA on background methotrexate received LZV and were then randomized to receive 5 mg tofacitinib twice daily or placebo 2-3 weeks after vaccination.
At 6 weeks after vaccination, VZV-specific IgG geometric mean fold rise (GMFR) was 2.11 and 1.74 in the tofacitinib and placebo patients, respectively; at all postvaccination time points at which VZV-specific IgG levels were evaluated, there was a trend toward numerically higher GMFR in tofacitinib patients, but the differences were not statistically significant. Also, the proportion of patients developing a 1.5-fold or greater postvaccination rise in IgG levels at 6 weeks trended higher for tofacitinib (57.4% vs. 43.4% with placebo).
VZV-specific T-cell GMFR at 6 weeks increased similarly in the groups (1.50 with tofacitinib and 1.29 with placebo).
Serious adverse events occurred in three patients in the tofacitinib group (5.5%) and in none of the placebo patients.
“The three SAEs included one case each of cholangitis and bronchitis, and once case of disseminated primary varicella,” the investigators said, noting that the onset of the latter was 16 days postvaccination, 2 days after starting tofacitinib. The rash resolved with discontinuation of tofacitinib and treatment with valacyclovir for 7 days.
The findings suggest that patients with active RA develop robust immune responses to HZ vaccine, and that starting tofacitinib 2-3 weeks after vaccination has no negative impact on the established immune response.
“Importantly, while our results suggest the vaccine is safe for patients with RA with prior VZV exposure, they also indicate the potential need to either screen for prior exposure before giving this vaccine or waiting longer than 2-3 weeks before starting immunosuppression with tofacitinib,” they said, noting that the current data suggest 4 weeks might be preferable.
Alternatively, testing patients who don’t recollect a history of chickenpox to ensure prior VZV exposure prior to vaccination could also mitigate the risk, they said.
“Further research is necessary to understand the risk of this complication, as well as the long-term effectiveness of this vaccine to prevent HZ in this high-risk population,” they concluded.
Both studies were sponsored by Pfizer. Dr. Winthrop has received research grants from and served as a scientific consultant to Pfizer. Other authors also reported financial relationships with Pfizer.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point:
Major finding: HZ rates were lowest among patients on tofacitinib monotherapy at a dose of 5 mg twice daily (IR, 0.6).
Data source: A phase 2 trial of 112 patients, and a review of 19 studies involving 6,192 patients.
Disclosures: Both studies were sponsored by Pfizer. Dr. Winthrop has received research grants from and served as a scientific consultant to Pfizer. Other authors also reported financial relationships with Pfizer.
Heart failure guidelines updated
Clinical Question: What new evidence is available to guide heart failure (HF) management?
Background: New data has become available since the 2013 HF guidelines.
Study Design: A focused update.
Setting: Ongoing review of HF literature.
Synopsis: Beta-natriuretic peptide (BNP) is recommended to screen at risk patients (IIaB), on admission (IA), and prior to discharge (IIaB). The combination of ARB and neprilysin inhibitor (ARB-NI) is recommended in symptomatic patients with HF with reduced ejection fraction (HFrEF) who are tolerant of ACE inhibition (IB). For these patients, transitioning from ACE-inhibitor to the ARB-NI combination, valsartan-sacubitril significantly reduced hospitalization and mortality. Optimal dose and titration strategies remain unclear. ARB-NIs should not be used in patients with a history of angioedema (IIIC) or within 36 hours of receiving ACE-inhibitors (IIIB). Ivabradine, a selective inhibitor of the If current in the sinoatrial node, is recommended to reduce hospitalizations for patients with HFrEF with stable symptoms with resting sinus heart rate greater than or equal to 70 despite maximally-tolerated beta-blockade (IIaB). Intravenous iron replacement is recommended to improve function and quality of life for patients with symptomatic HF and iron deficiency (IIbB).
Bottom Line: Updates support use of BNP, ARB-NIs, ivabradine, and IV iron for HFrEF.
Citation: Yancy CW, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart failure society of America. Published online, 2017 Apr 28. Circulation. doi: 10.1161/CIR.0000000000000509.
Dr. Sweigart is an assistant professor in the University of Kentucky division of hospital medicine and Lexington VA Medical Center.
Clinical Question: What new evidence is available to guide heart failure (HF) management?
Background: New data has become available since the 2013 HF guidelines.
Study Design: A focused update.
Setting: Ongoing review of HF literature.
Synopsis: Beta-natriuretic peptide (BNP) is recommended to screen at risk patients (IIaB), on admission (IA), and prior to discharge (IIaB). The combination of ARB and neprilysin inhibitor (ARB-NI) is recommended in symptomatic patients with HF with reduced ejection fraction (HFrEF) who are tolerant of ACE inhibition (IB). For these patients, transitioning from ACE-inhibitor to the ARB-NI combination, valsartan-sacubitril significantly reduced hospitalization and mortality. Optimal dose and titration strategies remain unclear. ARB-NIs should not be used in patients with a history of angioedema (IIIC) or within 36 hours of receiving ACE-inhibitors (IIIB). Ivabradine, a selective inhibitor of the If current in the sinoatrial node, is recommended to reduce hospitalizations for patients with HFrEF with stable symptoms with resting sinus heart rate greater than or equal to 70 despite maximally-tolerated beta-blockade (IIaB). Intravenous iron replacement is recommended to improve function and quality of life for patients with symptomatic HF and iron deficiency (IIbB).
Bottom Line: Updates support use of BNP, ARB-NIs, ivabradine, and IV iron for HFrEF.
Citation: Yancy CW, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart failure society of America. Published online, 2017 Apr 28. Circulation. doi: 10.1161/CIR.0000000000000509.
Dr. Sweigart is an assistant professor in the University of Kentucky division of hospital medicine and Lexington VA Medical Center.
Clinical Question: What new evidence is available to guide heart failure (HF) management?
Background: New data has become available since the 2013 HF guidelines.
Study Design: A focused update.
Setting: Ongoing review of HF literature.
Synopsis: Beta-natriuretic peptide (BNP) is recommended to screen at risk patients (IIaB), on admission (IA), and prior to discharge (IIaB). The combination of ARB and neprilysin inhibitor (ARB-NI) is recommended in symptomatic patients with HF with reduced ejection fraction (HFrEF) who are tolerant of ACE inhibition (IB). For these patients, transitioning from ACE-inhibitor to the ARB-NI combination, valsartan-sacubitril significantly reduced hospitalization and mortality. Optimal dose and titration strategies remain unclear. ARB-NIs should not be used in patients with a history of angioedema (IIIC) or within 36 hours of receiving ACE-inhibitors (IIIB). Ivabradine, a selective inhibitor of the If current in the sinoatrial node, is recommended to reduce hospitalizations for patients with HFrEF with stable symptoms with resting sinus heart rate greater than or equal to 70 despite maximally-tolerated beta-blockade (IIaB). Intravenous iron replacement is recommended to improve function and quality of life for patients with symptomatic HF and iron deficiency (IIbB).
Bottom Line: Updates support use of BNP, ARB-NIs, ivabradine, and IV iron for HFrEF.
Citation: Yancy CW, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart failure society of America. Published online, 2017 Apr 28. Circulation. doi: 10.1161/CIR.0000000000000509.
Dr. Sweigart is an assistant professor in the University of Kentucky division of hospital medicine and Lexington VA Medical Center.
Study linked H2 receptor antagonists, but not PPIs, to dementia
A large prospective study of middle-aged and older women found no convincing evidence that using proton pump inhibitors increased their risk of dementia, investigators reported.
However, using H2 receptor antagonists for at least 9 years was associated with a slight decrease in scores of learning and working memory (mean decrease, –0.2; 95% confidence interval, –0.3 to –0.08; P less than .001), Paul Lochhead, MBChB, PhD, and his associates wrote in the October issue of Gastroenterology (doi: 10.1053/j.gastro.2017.06.061). “Since our primary hypothesis related to PPI [proton pump inhibitor] use, our findings for [H2 receptor antagonists] should be interpreted with caution,” they said.
Source: American Gastroenterological Association
In a recent German study of a medical claims database, use of PPIs was associated with a 44% increase in the likelihood of incident dementia (JAMA Neurol. 2016;73:410-6). “The existence of a causal mechanism linking PPI use to dementia is suggested by observations from cellular and animal models of Alzheimer’s disease, where PPI exposure appears to influence amyloid-beta metabolism,” Dr. Lochhead and his associates wrote. “However, other preclinical data on PPIs and Alzheimer’s disease are conflicting.” Noting that cognitive function predicts dementia later in life, they analyzed prospective data on medications and other potential risk factors from 13,864 participants in the Nurses’ Health Study II who had completed Cogstate, a computerized, self-administered neuropsychological battery.
Study participants averaged 61 years old when they underwent cognitive testing, ranging in age from 50 to 70 years. Users of PPIs tended to be older, had more comorbidities, were less physically active, had higher body mass indexes, had less education, and ate a lower-quality diet than women who did not use PPIs. After adjusting for such confounders, using PPIs for 9-14 years was associated with a modest decrease in scores for psychomotor speed and attention (mean score difference, compared with never users, –0.06; 95% CI, –0.11 to 0.00; P = .03). “For comparison, in multivariable models, a 1-year increase in age was associated with mean score decreases of 0.03 for psychomotor speed and attention, 0.02 for learning and working memory, and 0.03 for overall cognition,” the researchers wrote.
Next, they examined links between use of H2 receptor antagonists and cognitive scores among 10,778 study participants who had used PPIs for 2 years or less. Use of H2 receptor antagonists for 9-14 years predicted poorer scores on learning, working memory, and overall cognition, even after controlling for potential confounders (P less than or equal to .002). “The magnitudes of mean score differences were larger than those observed in the analysis of PPI use, particularly for learning and working memory,” the researchers noted. Additionally, PPI use did not predict lower cognitive scores among individuals who had never used H2 receptor antagonists.
On the other hand, using PPIs for 9-14 years was associated with the equivalent of about 2 years of age-related cognitive decline, and controlling for exposure to H2 receptor antagonists weakened even this modest effect, the investigators said. Users and nonusers of PPIs tend to differ on many measures, and analyses of claims data, such as the German study above, are less able to account for these potential confounders, they noted. “Nonjudicious PPI prescribing is especially frequent among the elderly and those with cognitive impairment,” they added. “Therefore, elderly individuals who have frequent contact with health providers are at increased risk of both PPI prescription and dementia diagnosis. This bias may not be completely mitigated by adjustment for comorbidities or polypharmacy.”
The findings regarding H2 receptor antagonists reflect those of three smaller cohort studies, and these medications are known to cause central nervous system effects in the elderly, including delirium, the researchers said. Ranitidine and cimetidine have anticholinergic effects that also could “pose a risk for adverse cognitive effects with long-term use.”
Dr. Lochhead reported having no conflicts. Two coinvestigators disclosed ties to Bayer Healthcare, Pfizer, Aralez Pharmaceuticals, AbbVie, Samsung Bioepis, and Takeda.
Numerous possible PPI-related adverse events have been reported within the past few years; some resultant media attention has caused anxiety for patients. Dementia is a dreaded diagnosis. Therefore, initial reports that PPI treatment might be associated with an increased risk of dementia attracted considerable media attention, much of which was unbalanced and uninformed. There is no obvious biological rationale for such an association, and the risks reported initially were of small magnitude (for example, hazard ratios of approximately 1.4). However, patients cannot reliably assess levels of risk from media coverage that often veers toward sensationalism.
The study by Lochhead et al. is a welcome contribution to the topic of PPI safety. Using the Nurses’ Health Study II database, the investigators measured cognitive function in a large group of female PPI users and nonusers. Unsurprisingly, PPI users were older and sicker than nonusers. There were quantitatively small changes in some measures of cognitive function among PPI users. However, learning and working memory scores, which are more predictive of Alzheimer’s-type cognitive decline, were unaffected by PPI use.
For those prescribers with residual concerns about any association between PPIs and dementia, these prospectively collected data on cognitive function should provide further reassurance. It is appropriate that this study should have been highlighted in GI & Hepatology News, but since it lacks the potential sensationalism of studies that report a putative association, we should not expect it to be discussed on the TV evening news anytime soon!
Colin W. Howden, MD, AGAF, is chief of gastroenterology at University of Tennessee Health Science Center, Memphis. He has been a consultant, investigator, and/or speaker for all PPI manufacturers at some time. He is currently a consultant for Takeda, Aralez, and Pfizer Consumer Health.
Numerous possible PPI-related adverse events have been reported within the past few years; some resultant media attention has caused anxiety for patients. Dementia is a dreaded diagnosis. Therefore, initial reports that PPI treatment might be associated with an increased risk of dementia attracted considerable media attention, much of which was unbalanced and uninformed. There is no obvious biological rationale for such an association, and the risks reported initially were of small magnitude (for example, hazard ratios of approximately 1.4). However, patients cannot reliably assess levels of risk from media coverage that often veers toward sensationalism.
The study by Lochhead et al. is a welcome contribution to the topic of PPI safety. Using the Nurses’ Health Study II database, the investigators measured cognitive function in a large group of female PPI users and nonusers. Unsurprisingly, PPI users were older and sicker than nonusers. There were quantitatively small changes in some measures of cognitive function among PPI users. However, learning and working memory scores, which are more predictive of Alzheimer’s-type cognitive decline, were unaffected by PPI use.
For those prescribers with residual concerns about any association between PPIs and dementia, these prospectively collected data on cognitive function should provide further reassurance. It is appropriate that this study should have been highlighted in GI & Hepatology News, but since it lacks the potential sensationalism of studies that report a putative association, we should not expect it to be discussed on the TV evening news anytime soon!
Colin W. Howden, MD, AGAF, is chief of gastroenterology at University of Tennessee Health Science Center, Memphis. He has been a consultant, investigator, and/or speaker for all PPI manufacturers at some time. He is currently a consultant for Takeda, Aralez, and Pfizer Consumer Health.
Numerous possible PPI-related adverse events have been reported within the past few years; some resultant media attention has caused anxiety for patients. Dementia is a dreaded diagnosis. Therefore, initial reports that PPI treatment might be associated with an increased risk of dementia attracted considerable media attention, much of which was unbalanced and uninformed. There is no obvious biological rationale for such an association, and the risks reported initially were of small magnitude (for example, hazard ratios of approximately 1.4). However, patients cannot reliably assess levels of risk from media coverage that often veers toward sensationalism.
The study by Lochhead et al. is a welcome contribution to the topic of PPI safety. Using the Nurses’ Health Study II database, the investigators measured cognitive function in a large group of female PPI users and nonusers. Unsurprisingly, PPI users were older and sicker than nonusers. There were quantitatively small changes in some measures of cognitive function among PPI users. However, learning and working memory scores, which are more predictive of Alzheimer’s-type cognitive decline, were unaffected by PPI use.
For those prescribers with residual concerns about any association between PPIs and dementia, these prospectively collected data on cognitive function should provide further reassurance. It is appropriate that this study should have been highlighted in GI & Hepatology News, but since it lacks the potential sensationalism of studies that report a putative association, we should not expect it to be discussed on the TV evening news anytime soon!
Colin W. Howden, MD, AGAF, is chief of gastroenterology at University of Tennessee Health Science Center, Memphis. He has been a consultant, investigator, and/or speaker for all PPI manufacturers at some time. He is currently a consultant for Takeda, Aralez, and Pfizer Consumer Health.
A large prospective study of middle-aged and older women found no convincing evidence that using proton pump inhibitors increased their risk of dementia, investigators reported.
However, using H2 receptor antagonists for at least 9 years was associated with a slight decrease in scores of learning and working memory (mean decrease, –0.2; 95% confidence interval, –0.3 to –0.08; P less than .001), Paul Lochhead, MBChB, PhD, and his associates wrote in the October issue of Gastroenterology (doi: 10.1053/j.gastro.2017.06.061). “Since our primary hypothesis related to PPI [proton pump inhibitor] use, our findings for [H2 receptor antagonists] should be interpreted with caution,” they said.
Source: American Gastroenterological Association
In a recent German study of a medical claims database, use of PPIs was associated with a 44% increase in the likelihood of incident dementia (JAMA Neurol. 2016;73:410-6). “The existence of a causal mechanism linking PPI use to dementia is suggested by observations from cellular and animal models of Alzheimer’s disease, where PPI exposure appears to influence amyloid-beta metabolism,” Dr. Lochhead and his associates wrote. “However, other preclinical data on PPIs and Alzheimer’s disease are conflicting.” Noting that cognitive function predicts dementia later in life, they analyzed prospective data on medications and other potential risk factors from 13,864 participants in the Nurses’ Health Study II who had completed Cogstate, a computerized, self-administered neuropsychological battery.
Study participants averaged 61 years old when they underwent cognitive testing, ranging in age from 50 to 70 years. Users of PPIs tended to be older, had more comorbidities, were less physically active, had higher body mass indexes, had less education, and ate a lower-quality diet than women who did not use PPIs. After adjusting for such confounders, using PPIs for 9-14 years was associated with a modest decrease in scores for psychomotor speed and attention (mean score difference, compared with never users, –0.06; 95% CI, –0.11 to 0.00; P = .03). “For comparison, in multivariable models, a 1-year increase in age was associated with mean score decreases of 0.03 for psychomotor speed and attention, 0.02 for learning and working memory, and 0.03 for overall cognition,” the researchers wrote.
Next, they examined links between use of H2 receptor antagonists and cognitive scores among 10,778 study participants who had used PPIs for 2 years or less. Use of H2 receptor antagonists for 9-14 years predicted poorer scores on learning, working memory, and overall cognition, even after controlling for potential confounders (P less than or equal to .002). “The magnitudes of mean score differences were larger than those observed in the analysis of PPI use, particularly for learning and working memory,” the researchers noted. Additionally, PPI use did not predict lower cognitive scores among individuals who had never used H2 receptor antagonists.
On the other hand, using PPIs for 9-14 years was associated with the equivalent of about 2 years of age-related cognitive decline, and controlling for exposure to H2 receptor antagonists weakened even this modest effect, the investigators said. Users and nonusers of PPIs tend to differ on many measures, and analyses of claims data, such as the German study above, are less able to account for these potential confounders, they noted. “Nonjudicious PPI prescribing is especially frequent among the elderly and those with cognitive impairment,” they added. “Therefore, elderly individuals who have frequent contact with health providers are at increased risk of both PPI prescription and dementia diagnosis. This bias may not be completely mitigated by adjustment for comorbidities or polypharmacy.”
The findings regarding H2 receptor antagonists reflect those of three smaller cohort studies, and these medications are known to cause central nervous system effects in the elderly, including delirium, the researchers said. Ranitidine and cimetidine have anticholinergic effects that also could “pose a risk for adverse cognitive effects with long-term use.”
Dr. Lochhead reported having no conflicts. Two coinvestigators disclosed ties to Bayer Healthcare, Pfizer, Aralez Pharmaceuticals, AbbVie, Samsung Bioepis, and Takeda.
A large prospective study of middle-aged and older women found no convincing evidence that using proton pump inhibitors increased their risk of dementia, investigators reported.
However, using H2 receptor antagonists for at least 9 years was associated with a slight decrease in scores of learning and working memory (mean decrease, –0.2; 95% confidence interval, –0.3 to –0.08; P less than .001), Paul Lochhead, MBChB, PhD, and his associates wrote in the October issue of Gastroenterology (doi: 10.1053/j.gastro.2017.06.061). “Since our primary hypothesis related to PPI [proton pump inhibitor] use, our findings for [H2 receptor antagonists] should be interpreted with caution,” they said.
Source: American Gastroenterological Association
In a recent German study of a medical claims database, use of PPIs was associated with a 44% increase in the likelihood of incident dementia (JAMA Neurol. 2016;73:410-6). “The existence of a causal mechanism linking PPI use to dementia is suggested by observations from cellular and animal models of Alzheimer’s disease, where PPI exposure appears to influence amyloid-beta metabolism,” Dr. Lochhead and his associates wrote. “However, other preclinical data on PPIs and Alzheimer’s disease are conflicting.” Noting that cognitive function predicts dementia later in life, they analyzed prospective data on medications and other potential risk factors from 13,864 participants in the Nurses’ Health Study II who had completed Cogstate, a computerized, self-administered neuropsychological battery.
Study participants averaged 61 years old when they underwent cognitive testing, ranging in age from 50 to 70 years. Users of PPIs tended to be older, had more comorbidities, were less physically active, had higher body mass indexes, had less education, and ate a lower-quality diet than women who did not use PPIs. After adjusting for such confounders, using PPIs for 9-14 years was associated with a modest decrease in scores for psychomotor speed and attention (mean score difference, compared with never users, –0.06; 95% CI, –0.11 to 0.00; P = .03). “For comparison, in multivariable models, a 1-year increase in age was associated with mean score decreases of 0.03 for psychomotor speed and attention, 0.02 for learning and working memory, and 0.03 for overall cognition,” the researchers wrote.
Next, they examined links between use of H2 receptor antagonists and cognitive scores among 10,778 study participants who had used PPIs for 2 years or less. Use of H2 receptor antagonists for 9-14 years predicted poorer scores on learning, working memory, and overall cognition, even after controlling for potential confounders (P less than or equal to .002). “The magnitudes of mean score differences were larger than those observed in the analysis of PPI use, particularly for learning and working memory,” the researchers noted. Additionally, PPI use did not predict lower cognitive scores among individuals who had never used H2 receptor antagonists.
On the other hand, using PPIs for 9-14 years was associated with the equivalent of about 2 years of age-related cognitive decline, and controlling for exposure to H2 receptor antagonists weakened even this modest effect, the investigators said. Users and nonusers of PPIs tend to differ on many measures, and analyses of claims data, such as the German study above, are less able to account for these potential confounders, they noted. “Nonjudicious PPI prescribing is especially frequent among the elderly and those with cognitive impairment,” they added. “Therefore, elderly individuals who have frequent contact with health providers are at increased risk of both PPI prescription and dementia diagnosis. This bias may not be completely mitigated by adjustment for comorbidities or polypharmacy.”
The findings regarding H2 receptor antagonists reflect those of three smaller cohort studies, and these medications are known to cause central nervous system effects in the elderly, including delirium, the researchers said. Ranitidine and cimetidine have anticholinergic effects that also could “pose a risk for adverse cognitive effects with long-term use.”
Dr. Lochhead reported having no conflicts. Two coinvestigators disclosed ties to Bayer Healthcare, Pfizer, Aralez Pharmaceuticals, AbbVie, Samsung Bioepis, and Takeda.
FROM GASTROENTEROLOGY
Key clinical point: A large prospective cohort study linked long-term use of H2 receptor antagonists, but not PPIs, to dementia.
Major finding: Use of PPIs did not significantly predict incident dementia in the adjusted analysis. However, using H2 receptor antagonists for at least 9 years was associated with a slight decrease in scores of learning and working memory (mean decrease, –0.2; P less than .001).
Data source: A population-based cohort study of 13,864 middle-aged and older women.
Disclosures: Dr. Lochhead reported having no conflicts. Two coinvestigators disclosed ties to Bayer Healthcare, Pfizer, Aralez Pharmaceuticals, AbbVie, Samsung Bioepis, and Takeda.
Burden of HCV-induced cirrhosis expected to shift from men to women
Prevalence of hepatitis C virus (HCV) complications among women grew at a rate similar to that of men, while mortality among men was nearly double that of women, according to a study conducted through the Veterans Affairs office.
While men still have a higher prevalence of conditions such as cirrhosis, investigators expect to see a shift in the burden of care as women with HCV complications outlive men with similar diagnoses.
“The current and near-term burden in HCV-related cirrhosis was disproportionately attributed to men,” according to Jennifer Kramer, PhD, investigator at the Center for Innovations in Quality, Effectiveness and Safety, Michael E. DeBakey Veterans Affairs Medical Center, Houston. “However, the trends are expected to change after 2020.”
The retrospective cohort study analyzed 264,409 HCV-infected veterans, 7,162 of whom were women, between January 2000 and December 2013.
Investigators found annual average prevalence change (AAPC) among men and women was 13.1% and 15.2%, respectively, for cirrhosis, while overall mortality was 28.7% for men, compared with 15.5% for women (J Viral Hepat. 2017 Aug 16. doi: 10.1111/jvh.12728).
Dr. Kramer and her fellow investigators also found similar rates among decompensated cirrhosis between 15.6% and 16.9% for women and men, respectively, and hepatocellular cancer, 21% and 25.3%, respectively.
Women included in the cohort were, on average, younger (48 years vs. 53 years), were less likely to use alcohol (33% vs. 45%), and were less likely to have contracted diabetes (30% vs. 39%).
While men’s prevalence growth was equal to women’s, male patients are 1.7 times more likely to be infected with HCV (J Hepatol. 2012 Jun 2 doi: 10.1016/j.jhep.2012.05.018), which is reflected in overall incidence rates of complications.
As expected, overall incidence of cirrhosis was higher in men than in women, with incidence rates for men at 28.2% compared with 20.1% of women.
Similar differences were found in rates of decompensated cirrhosis, 18.6% in men compared with 12.4% in women, and hepatocellular cancer, 5.3% in men compared with 1.5% in women.
Shifting trends in burden of care toward women have investigators worried about current HCV treatment practices for female patients.
“The increasing burden of HCV complications in women is concerning,” the researchers wrote. “Studies show that women are less likely to receive antiviral treatment than men.”
Contrary to this claim, antiviral treatment rates among men and women in this study were almost identical: 23.6% of women and 23.3% of men.
While the difference in treatment is not evident, the low rate of treatment for both men and women is another concern for Dr. Kramer and her colleagues.
“In the U.S., HCV infection remains undiagnosed in over 50% of all persons with HCV disease,” the investigators wrote. “Access to highly affective yet expensive direct acting antiviral treatment remains a challenge.”
Findings from this study may not be a true representation of the U.S. HCV-infected population because patients were veterans, with differences such as a higher rate of alcohol use among women.
The researchers reported no relevant financial disclosures.
AGA Resource
Through the HCV Clinical Service Line, AGA offers tools to help you become more efficient, understand quality standards and improve the process of care for patients. Learn more at http://www.gastro.org/patient-care/conditions-diseases/hepatitis-c
[email protected]
On Twitter @eaztweets
Prevalence of hepatitis C virus (HCV) complications among women grew at a rate similar to that of men, while mortality among men was nearly double that of women, according to a study conducted through the Veterans Affairs office.
While men still have a higher prevalence of conditions such as cirrhosis, investigators expect to see a shift in the burden of care as women with HCV complications outlive men with similar diagnoses.
“The current and near-term burden in HCV-related cirrhosis was disproportionately attributed to men,” according to Jennifer Kramer, PhD, investigator at the Center for Innovations in Quality, Effectiveness and Safety, Michael E. DeBakey Veterans Affairs Medical Center, Houston. “However, the trends are expected to change after 2020.”
The retrospective cohort study analyzed 264,409 HCV-infected veterans, 7,162 of whom were women, between January 2000 and December 2013.
Investigators found annual average prevalence change (AAPC) among men and women was 13.1% and 15.2%, respectively, for cirrhosis, while overall mortality was 28.7% for men, compared with 15.5% for women (J Viral Hepat. 2017 Aug 16. doi: 10.1111/jvh.12728).
Dr. Kramer and her fellow investigators also found similar rates among decompensated cirrhosis between 15.6% and 16.9% for women and men, respectively, and hepatocellular cancer, 21% and 25.3%, respectively.
Women included in the cohort were, on average, younger (48 years vs. 53 years), were less likely to use alcohol (33% vs. 45%), and were less likely to have contracted diabetes (30% vs. 39%).
While men’s prevalence growth was equal to women’s, male patients are 1.7 times more likely to be infected with HCV (J Hepatol. 2012 Jun 2 doi: 10.1016/j.jhep.2012.05.018), which is reflected in overall incidence rates of complications.
As expected, overall incidence of cirrhosis was higher in men than in women, with incidence rates for men at 28.2% compared with 20.1% of women.
Similar differences were found in rates of decompensated cirrhosis, 18.6% in men compared with 12.4% in women, and hepatocellular cancer, 5.3% in men compared with 1.5% in women.
Shifting trends in burden of care toward women have investigators worried about current HCV treatment practices for female patients.
“The increasing burden of HCV complications in women is concerning,” the researchers wrote. “Studies show that women are less likely to receive antiviral treatment than men.”
Contrary to this claim, antiviral treatment rates among men and women in this study were almost identical: 23.6% of women and 23.3% of men.
While the difference in treatment is not evident, the low rate of treatment for both men and women is another concern for Dr. Kramer and her colleagues.
“In the U.S., HCV infection remains undiagnosed in over 50% of all persons with HCV disease,” the investigators wrote. “Access to highly affective yet expensive direct acting antiviral treatment remains a challenge.”
Findings from this study may not be a true representation of the U.S. HCV-infected population because patients were veterans, with differences such as a higher rate of alcohol use among women.
The researchers reported no relevant financial disclosures.
AGA Resource
Through the HCV Clinical Service Line, AGA offers tools to help you become more efficient, understand quality standards and improve the process of care for patients. Learn more at http://www.gastro.org/patient-care/conditions-diseases/hepatitis-c
[email protected]
On Twitter @eaztweets
Prevalence of hepatitis C virus (HCV) complications among women grew at a rate similar to that of men, while mortality among men was nearly double that of women, according to a study conducted through the Veterans Affairs office.
While men still have a higher prevalence of conditions such as cirrhosis, investigators expect to see a shift in the burden of care as women with HCV complications outlive men with similar diagnoses.
“The current and near-term burden in HCV-related cirrhosis was disproportionately attributed to men,” according to Jennifer Kramer, PhD, investigator at the Center for Innovations in Quality, Effectiveness and Safety, Michael E. DeBakey Veterans Affairs Medical Center, Houston. “However, the trends are expected to change after 2020.”
The retrospective cohort study analyzed 264,409 HCV-infected veterans, 7,162 of whom were women, between January 2000 and December 2013.
Investigators found annual average prevalence change (AAPC) among men and women was 13.1% and 15.2%, respectively, for cirrhosis, while overall mortality was 28.7% for men, compared with 15.5% for women (J Viral Hepat. 2017 Aug 16. doi: 10.1111/jvh.12728).
Dr. Kramer and her fellow investigators also found similar rates among decompensated cirrhosis between 15.6% and 16.9% for women and men, respectively, and hepatocellular cancer, 21% and 25.3%, respectively.
Women included in the cohort were, on average, younger (48 years vs. 53 years), were less likely to use alcohol (33% vs. 45%), and were less likely to have contracted diabetes (30% vs. 39%).
While men’s prevalence growth was equal to women’s, male patients are 1.7 times more likely to be infected with HCV (J Hepatol. 2012 Jun 2 doi: 10.1016/j.jhep.2012.05.018), which is reflected in overall incidence rates of complications.
As expected, overall incidence of cirrhosis was higher in men than in women, with incidence rates for men at 28.2% compared with 20.1% of women.
Similar differences were found in rates of decompensated cirrhosis, 18.6% in men compared with 12.4% in women, and hepatocellular cancer, 5.3% in men compared with 1.5% in women.
Shifting trends in burden of care toward women have investigators worried about current HCV treatment practices for female patients.
“The increasing burden of HCV complications in women is concerning,” the researchers wrote. “Studies show that women are less likely to receive antiviral treatment than men.”
Contrary to this claim, antiviral treatment rates among men and women in this study were almost identical: 23.6% of women and 23.3% of men.
While the difference in treatment is not evident, the low rate of treatment for both men and women is another concern for Dr. Kramer and her colleagues.
“In the U.S., HCV infection remains undiagnosed in over 50% of all persons with HCV disease,” the investigators wrote. “Access to highly affective yet expensive direct acting antiviral treatment remains a challenge.”
Findings from this study may not be a true representation of the U.S. HCV-infected population because patients were veterans, with differences such as a higher rate of alcohol use among women.
The researchers reported no relevant financial disclosures.
AGA Resource
Through the HCV Clinical Service Line, AGA offers tools to help you become more efficient, understand quality standards and improve the process of care for patients. Learn more at http://www.gastro.org/patient-care/conditions-diseases/hepatitis-c
[email protected]
On Twitter @eaztweets
FROM JOURNAL OF VIRAL HEPATITIS
Key clinical point:
Major finding: Average annual change of cirrhosis prevalence in women was 15.2% for women and 13.1% for men, while total mortality was 15.5% compared with 28.7% for women and men, respectively.
Data source: Retrospective cohort study of 264,409 veterans diagnosed with HCV, from the Veterans Affairs corporate wellness data for January 2000 to December 2013.
Disclosures: The investigators reported no relevant financial disclosures.
Burden of HCV-induced cirrhosis expected to shift from men to women
Prevalence of hepatitis C virus (HCV) complications among women grew at a rate similar to that of men, while mortality among men was nearly double that of women, according to a study conducted through the Veterans Affairs office.
While men still have a higher prevalence of conditions such as cirrhosis, investigators expect to see a shift in the burden of care as women with HCV complications outlive men with similar diagnoses.
“The current and near-term burden in HCV-related cirrhosis was disproportionately attributed to men,” according to Jennifer Kramer, PhD, investigator at the Center for Innovations in Quality, Effectiveness and Safety, Michael E. DeBakey Veterans Affairs Medical Center, Houston. “However, the trends are expected to change after 2020.”
The retrospective cohort study analyzed 264,409 HCV-infected veterans, 7,162 of whom were women, between January 2000 and December 2013.
Investigators found annual average prevalence change (AAPC) among men and women was 13.1% and 15.2%, respectively, for cirrhosis, while overall mortality was 28.7% for men, compared with 15.5% for women (J Viral Hepat. 2017 Aug 16. doi: 10.1111/jvh.12728).
Dr. Kramer and her fellow investigators also found similar rates among decompensated cirrhosis between 15.6% and 16.9% for women and men, respectively, and hepatocellular cancer, 21% and 25.3%, respectively.
Women included in the cohort were, on average, younger (48 years vs. 53 years), were less likely to use alcohol (33% vs. 45%), and were less likely to have contracted diabetes (30% vs. 39%).
While men’s prevalence growth was equal to women’s, male patients are 1.7 times more likely to be infected with HCV (J Hepatol. 2012 Jun 2 doi: 10.1016/j.jhep.2012.05.018), which is reflected in overall incidence rates of complications.
As expected, overall incidence of cirrhosis was higher in men than in women, with incidence rates for men at 28.2% compared with 20.1% of women.
Similar differences were found in rates of decompensated cirrhosis, 18.6% in men compared with 12.4% in women, and hepatocellular cancer, 5.3% in men compared with 1.5% in women.
Shifting trends in burden of care toward women have investigators worried about current HCV treatment practices for female patients.
“The increasing burden of HCV complications in women is concerning,” the researchers wrote. “Studies show that women are less likely to receive antiviral treatment than men.”
Contrary to this claim, antiviral treatment rates among men and women in this study were almost identical: 23.6% of women and 23.3% of men.
While the difference in treatment is not evident, the low rate of treatment for both men and women is another concern for Dr. Kramer and her colleagues.
“In the U.S., HCV infection remains undiagnosed in over 50% of all persons with HCV disease,” the investigators wrote. “Access to highly affective yet expensive direct acting antiviral treatment remains a challenge.”
Findings from this study may not be a true representation of the U.S. HCV-infected population because patients were veterans, with differences such as a higher rate of alcohol use among women.
The researchers reported no relevant financial disclosures.
[email protected]
On Twitter @eaztweets
Prevalence of hepatitis C virus (HCV) complications among women grew at a rate similar to that of men, while mortality among men was nearly double that of women, according to a study conducted through the Veterans Affairs office.
While men still have a higher prevalence of conditions such as cirrhosis, investigators expect to see a shift in the burden of care as women with HCV complications outlive men with similar diagnoses.
“The current and near-term burden in HCV-related cirrhosis was disproportionately attributed to men,” according to Jennifer Kramer, PhD, investigator at the Center for Innovations in Quality, Effectiveness and Safety, Michael E. DeBakey Veterans Affairs Medical Center, Houston. “However, the trends are expected to change after 2020.”
The retrospective cohort study analyzed 264,409 HCV-infected veterans, 7,162 of whom were women, between January 2000 and December 2013.
Investigators found annual average prevalence change (AAPC) among men and women was 13.1% and 15.2%, respectively, for cirrhosis, while overall mortality was 28.7% for men, compared with 15.5% for women (J Viral Hepat. 2017 Aug 16. doi: 10.1111/jvh.12728).
Dr. Kramer and her fellow investigators also found similar rates among decompensated cirrhosis between 15.6% and 16.9% for women and men, respectively, and hepatocellular cancer, 21% and 25.3%, respectively.
Women included in the cohort were, on average, younger (48 years vs. 53 years), were less likely to use alcohol (33% vs. 45%), and were less likely to have contracted diabetes (30% vs. 39%).
While men’s prevalence growth was equal to women’s, male patients are 1.7 times more likely to be infected with HCV (J Hepatol. 2012 Jun 2 doi: 10.1016/j.jhep.2012.05.018), which is reflected in overall incidence rates of complications.
As expected, overall incidence of cirrhosis was higher in men than in women, with incidence rates for men at 28.2% compared with 20.1% of women.
Similar differences were found in rates of decompensated cirrhosis, 18.6% in men compared with 12.4% in women, and hepatocellular cancer, 5.3% in men compared with 1.5% in women.
Shifting trends in burden of care toward women have investigators worried about current HCV treatment practices for female patients.
“The increasing burden of HCV complications in women is concerning,” the researchers wrote. “Studies show that women are less likely to receive antiviral treatment than men.”
Contrary to this claim, antiviral treatment rates among men and women in this study were almost identical: 23.6% of women and 23.3% of men.
While the difference in treatment is not evident, the low rate of treatment for both men and women is another concern for Dr. Kramer and her colleagues.
“In the U.S., HCV infection remains undiagnosed in over 50% of all persons with HCV disease,” the investigators wrote. “Access to highly affective yet expensive direct acting antiviral treatment remains a challenge.”
Findings from this study may not be a true representation of the U.S. HCV-infected population because patients were veterans, with differences such as a higher rate of alcohol use among women.
The researchers reported no relevant financial disclosures.
[email protected]
On Twitter @eaztweets
Prevalence of hepatitis C virus (HCV) complications among women grew at a rate similar to that of men, while mortality among men was nearly double that of women, according to a study conducted through the Veterans Affairs office.
While men still have a higher prevalence of conditions such as cirrhosis, investigators expect to see a shift in the burden of care as women with HCV complications outlive men with similar diagnoses.
“The current and near-term burden in HCV-related cirrhosis was disproportionately attributed to men,” according to Jennifer Kramer, PhD, investigator at the Center for Innovations in Quality, Effectiveness and Safety, Michael E. DeBakey Veterans Affairs Medical Center, Houston. “However, the trends are expected to change after 2020.”
The retrospective cohort study analyzed 264,409 HCV-infected veterans, 7,162 of whom were women, between January 2000 and December 2013.
Investigators found annual average prevalence change (AAPC) among men and women was 13.1% and 15.2%, respectively, for cirrhosis, while overall mortality was 28.7% for men, compared with 15.5% for women (J Viral Hepat. 2017 Aug 16. doi: 10.1111/jvh.12728).
Dr. Kramer and her fellow investigators also found similar rates among decompensated cirrhosis between 15.6% and 16.9% for women and men, respectively, and hepatocellular cancer, 21% and 25.3%, respectively.
Women included in the cohort were, on average, younger (48 years vs. 53 years), were less likely to use alcohol (33% vs. 45%), and were less likely to have contracted diabetes (30% vs. 39%).
While men’s prevalence growth was equal to women’s, male patients are 1.7 times more likely to be infected with HCV (J Hepatol. 2012 Jun 2 doi: 10.1016/j.jhep.2012.05.018), which is reflected in overall incidence rates of complications.
As expected, overall incidence of cirrhosis was higher in men than in women, with incidence rates for men at 28.2% compared with 20.1% of women.
Similar differences were found in rates of decompensated cirrhosis, 18.6% in men compared with 12.4% in women, and hepatocellular cancer, 5.3% in men compared with 1.5% in women.
Shifting trends in burden of care toward women have investigators worried about current HCV treatment practices for female patients.
“The increasing burden of HCV complications in women is concerning,” the researchers wrote. “Studies show that women are less likely to receive antiviral treatment than men.”
Contrary to this claim, antiviral treatment rates among men and women in this study were almost identical: 23.6% of women and 23.3% of men.
While the difference in treatment is not evident, the low rate of treatment for both men and women is another concern for Dr. Kramer and her colleagues.
“In the U.S., HCV infection remains undiagnosed in over 50% of all persons with HCV disease,” the investigators wrote. “Access to highly affective yet expensive direct acting antiviral treatment remains a challenge.”
Findings from this study may not be a true representation of the U.S. HCV-infected population because patients were veterans, with differences such as a higher rate of alcohol use among women.
The researchers reported no relevant financial disclosures.
[email protected]
On Twitter @eaztweets
FROM THE JOURNAL OF VIRAL HEPATITIS
Key clinical point:
Major finding: Average annual change of cirrhosis prevalence in women was 15.2% for women and 13.1% for men, while total mortality was 15.5% compared with 28.7% for women and men, respectively.
Data source: Retrospective cohort study of 264,409 veterans diagnosed with HCV, from the Veterans Affairs corporate wellness data for January 2000 to December 2013.
Disclosures: The investigators reported no relevant financial disclosures.