User login
Management of high-grade pleomorphic sarcoma with colon metastasis
Soft tissue sarcomas (STS) are a heterogeneous group of tumors of mesenchymal origin that represent a rare form of adult malignancy. According to the World Health Organization classification system, there are more than 100 histologic subtypes of sarcoma based on tissue of origin. Staging criteria most commonly use the American Joint Committee on Cancer’s TNM Classification of Malignant Tumours. About 50% of soft tissue sarcomas originate in the lower extremity.1 Advancements in the use of multimodal therapy have reduced the need for amputation and allowed for equally effective treatment strategies that use limb-sparing surgical resections.
Although most sarcoma metastases spread in a hematogenous fashion, nodal spread is underestimated. Certain histologic subtypes carry a higher predilection for nodal involvement: these include rhabdomyosarcoma, synovial sarcoma, epithelioid sarcoma, vascular sarcoma, and clear-cell sarcoma.2-4 Fong and colleagues have reported that 2.6% of sarcomas have lymphatic spread.3 In the current report, we describe a rare observation of locoregional pelvic nodal metastases from a large undifferentiated pleomorphic sarcoma of the right thigh.
Case presentation and summary
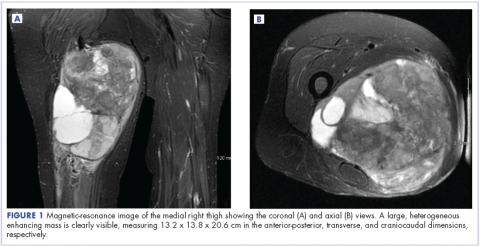
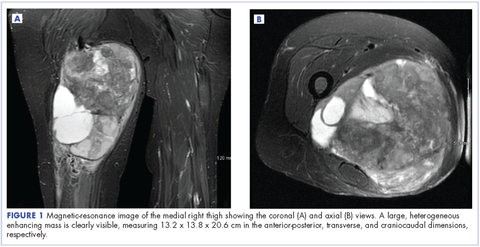
A 63-year-old white woman had a 1-year history of a right thigh mass and an unintentional weight loss of 40 lb. After a year of chiropractic care, she was referred to a physician because of palpable inguinal adenopathy and a 20-cm mass in the medial compartment of the right thigh, with heterogeneous appearance on a magnetic-resonance imaging scan (Figure 1). The patient was referred to the sarcoma transdisciplinary team for evaluation. She was diagnosed by core needle biopsy with a high-grade malignant epithelioid and spindle-cell neoplasm, favoring pleomorphic sarcoma. The metastatic work-up confirmed locoregional right inguinal and retroperitoneal lymph node disease, with 2 lung nodules that were too small to characterize. She also had a paraneoplastic leukocytosis with a white blood cell count of 45,500 cells/ml (normal 10,500 cells/ml).
About 8 months after surgery, she presented to the emergency room with a 3-day history of blood per rectum, anemia, and fatigue. She also reported a weight loss of 10 lb in the previous month. She was admitted for hydration and monitoring. Although computed-tomography (CT) scans of the abdomen and chest done during the previous month and had been interpreted as having no evidence of recurrence or any lymph node disease, the results of an inpatient colonoscopy revealed 2 colonic masses, 1 in the ascending colon and another in the transverse colon. The biopsy findings were consistent with undifferentiated pleomorphic sarcoma, favoring epithelioid histology. The CT scans were re-evaluated in light of these colonoscopic findings. These masses were visible retrospectively on imaging but had been interpreted as stool given the lack of abnormality on imaging 3 months before.
Adequate re-staging was complete, and without other evidence of disease, an extended right hemicolectomy was performed. The postoperative pathology report described geographically 2 distinct masses: a 7-cm mass in the ascending colon, about 3 cm from the ileocecal valve; and a 4-cm mass in the transverse colon, about 7.5 cm from the distal margin of resection. Both masses were identified as high-grade pleomorphic sarcoma. Again, all nodes recovered were negative for malignancy (0/5). Of note is that the background colonic mucosa showed active multifocal colitis with deep inflammatory activity likely consistent with a paraneoplastic syndrome.
Discussion
Surgery remains the primary treatment modality for localized soft tissue sarcoma. Obtaining a margin-free resection, while maintaining optimal function, is the objective with extremity sarcoma. In addition to surgical resection, doxorubicin-based adjuvant chemotherapy remains the standard of care with modest improvement in overall survival and disease-free survival, especially in sarcomas of the extremities.5,6 Gemcitabine also has activity in soft tissue sarcomas7 and might synergize with docetaxel. High response rates of 53% with fixed dose infusion rates of these agents in uterine leiomyosarcoma led the Sarcoma Alliance for Research through Collaboration investigators to consider this regimen for other STS.8 An overall response rate of 16% was noted across all sarcoma subtypes, and undifferentiated pleomorphic sarcoma had a response rate of 32%.
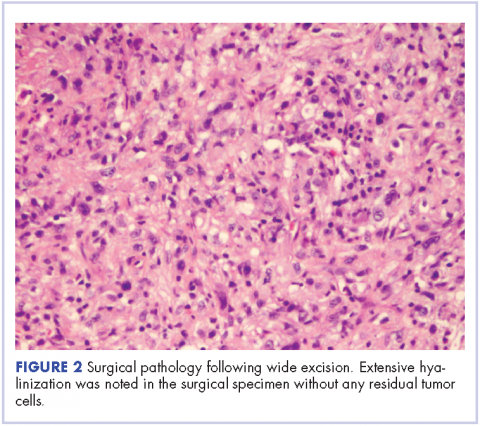
In our experience, a modified schedule of gemcitabine and docetaxel is better tolerated than the standard every 3 weeks regimen or doxorubicin-based chemotherapies. As previously described, gemcitabine and docetaxel were administered to this patient every 2 weeks.9 This regimen, in our experience, is less toxic and preserves the dose intensity of the regimen. A complete pathologic necrosis of the primary tumor and regional nodal basin was observed, as well as pulmonary nodule regression, enabling an R0-wide excision and regional lymphadenectomy. The colonic recurrence was surprising, but easily managed with surgical resection.
Pathologic complete response after neoadjuvant chemotherapy is a rare event, observed in about 10% of patients. However, when observed, complete pathologic necrosis (>95%) provided a distant recurrence-free survival of 100% at 3 years.10-12
Our patient, despite achieving a complete pathologic response and excellent initial local control, ultimately experienced an isolated metastatic recurrence in the colon within 1 year of therapy. Data supports performing metastatectomy for stage IV extremity sarcoma for isolated pulmonary or hepatic burden in selected patients with improved survival rates.13,14 As far as we know, there is no published literature describing isolated colon metastases in the absence of liver burden from lower extremity soft tissue sarcomas, or the outcome of surgical resection and adjuvant therapy in these cases.
In conclusion, high-grade pleomorphic sarcoma often follows an aggressive clinical course with ultimately local and distant recurrence. Use of multimodal therapy may have a role in improving local control. Complete pathologic necrosis is a rare event that is predictive of improved outcome. Our case represents an unusual pattern of recurrence among patients with a complete pathologic response to neoadjuvant therapy with isolated colon metastases. Timely, comprehensive management together with vigilant surveillance remain key priorities in the long-term management of high-risk sarcoma.
1. Lawrence W Jr, Donegan WL, Natarajan N, Mettlin C, Beart R, Winchester D. Adult soft tissue sarcomas. A pattern of care survey of the American College of Surgeons. Ann Surg. 1987;205:349-359.
2. Riad S, Griffin AM, Liberman B, et al. Lymph node metastasis in soft tissue sarcoma in an extremity. Clin Orthop Relat Res. 2004:129-134.
3. Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg. 1993;217:72-77.
4. Mazeron JJ, Suit HD. Lymph nodes as sites of metastases from sarcomas of soft tissue. Cancer. 1987;60:1800-1808.
5. Sarcoma Meta-analysis Collaboration. Adjuvant chemotherapy for localised resectable soft tissue sarcoma of adults: meta-analysis of individual data. Lancet. 1997;350:1647-1654.
6. Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft tissue sarcoma. Cancer. 2008;113:573-581.
7. Patel SR, Gandhi V, Jenkins J, et al. Phase II clinical investigation of gemcitabine in advanced soft tissue sarcomas and window evaluation of dose rate on gemcitabine triphosphate accumulation. J Clin Oncol. 2001;19:3483-3489.
8. Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol. 2007;25:2755-2763.
9. Verschraegen CF, Arias-Pulido H, Lee SJ, et al. Phase IB study of the combination of docetaxel, gemcitabine, and bevacizumab in patients with advanced or recurrent soft tissue sarcoma: the Axtell regimen. Ann Oncol. 2012;23:785-790.
10. MacDermed DM, Miller LL, Peabody TD, et al. Primary tumor necrosis predicts distant control in locally advanced soft tissue sarcomas after preoperative concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1147-1153.
11. Eilber FC, Rosen G, Eckardt J, et al. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19:3203-3209.
12. Shah D, Borys D, Martinez SR, et al. Complete pathologic response to neoadjuvant radiotherapy is predictive of oncological outcome in patients with soft tissue sarcoma. Anticancer Res. 2012;32:3911-3915.
13. Rehders A, Peiper M, Stoecklein NH, et al. Hepatic metastasectomy for soft tissue sarcomas: is it justified? World J Surg. 2009;33:111-117.
14. Pfannschmidt J, Hoffmann H, Schneider T, Dienemann H. Pulmonary metastasectomy for soft tissue sarcomas: is it justified? Recent Results Cancer Res. 2009;179:321-336.
Soft tissue sarcomas (STS) are a heterogeneous group of tumors of mesenchymal origin that represent a rare form of adult malignancy. According to the World Health Organization classification system, there are more than 100 histologic subtypes of sarcoma based on tissue of origin. Staging criteria most commonly use the American Joint Committee on Cancer’s TNM Classification of Malignant Tumours. About 50% of soft tissue sarcomas originate in the lower extremity.1 Advancements in the use of multimodal therapy have reduced the need for amputation and allowed for equally effective treatment strategies that use limb-sparing surgical resections.
Although most sarcoma metastases spread in a hematogenous fashion, nodal spread is underestimated. Certain histologic subtypes carry a higher predilection for nodal involvement: these include rhabdomyosarcoma, synovial sarcoma, epithelioid sarcoma, vascular sarcoma, and clear-cell sarcoma.2-4 Fong and colleagues have reported that 2.6% of sarcomas have lymphatic spread.3 In the current report, we describe a rare observation of locoregional pelvic nodal metastases from a large undifferentiated pleomorphic sarcoma of the right thigh.
Case presentation and summary
A 63-year-old white woman had a 1-year history of a right thigh mass and an unintentional weight loss of 40 lb. After a year of chiropractic care, she was referred to a physician because of palpable inguinal adenopathy and a 20-cm mass in the medial compartment of the right thigh, with heterogeneous appearance on a magnetic-resonance imaging scan (Figure 1). The patient was referred to the sarcoma transdisciplinary team for evaluation. She was diagnosed by core needle biopsy with a high-grade malignant epithelioid and spindle-cell neoplasm, favoring pleomorphic sarcoma. The metastatic work-up confirmed locoregional right inguinal and retroperitoneal lymph node disease, with 2 lung nodules that were too small to characterize. She also had a paraneoplastic leukocytosis with a white blood cell count of 45,500 cells/ml (normal 10,500 cells/ml).
About 8 months after surgery, she presented to the emergency room with a 3-day history of blood per rectum, anemia, and fatigue. She also reported a weight loss of 10 lb in the previous month. She was admitted for hydration and monitoring. Although computed-tomography (CT) scans of the abdomen and chest done during the previous month and had been interpreted as having no evidence of recurrence or any lymph node disease, the results of an inpatient colonoscopy revealed 2 colonic masses, 1 in the ascending colon and another in the transverse colon. The biopsy findings were consistent with undifferentiated pleomorphic sarcoma, favoring epithelioid histology. The CT scans were re-evaluated in light of these colonoscopic findings. These masses were visible retrospectively on imaging but had been interpreted as stool given the lack of abnormality on imaging 3 months before.
Adequate re-staging was complete, and without other evidence of disease, an extended right hemicolectomy was performed. The postoperative pathology report described geographically 2 distinct masses: a 7-cm mass in the ascending colon, about 3 cm from the ileocecal valve; and a 4-cm mass in the transverse colon, about 7.5 cm from the distal margin of resection. Both masses were identified as high-grade pleomorphic sarcoma. Again, all nodes recovered were negative for malignancy (0/5). Of note is that the background colonic mucosa showed active multifocal colitis with deep inflammatory activity likely consistent with a paraneoplastic syndrome.
Discussion
Surgery remains the primary treatment modality for localized soft tissue sarcoma. Obtaining a margin-free resection, while maintaining optimal function, is the objective with extremity sarcoma. In addition to surgical resection, doxorubicin-based adjuvant chemotherapy remains the standard of care with modest improvement in overall survival and disease-free survival, especially in sarcomas of the extremities.5,6 Gemcitabine also has activity in soft tissue sarcomas7 and might synergize with docetaxel. High response rates of 53% with fixed dose infusion rates of these agents in uterine leiomyosarcoma led the Sarcoma Alliance for Research through Collaboration investigators to consider this regimen for other STS.8 An overall response rate of 16% was noted across all sarcoma subtypes, and undifferentiated pleomorphic sarcoma had a response rate of 32%.
In our experience, a modified schedule of gemcitabine and docetaxel is better tolerated than the standard every 3 weeks regimen or doxorubicin-based chemotherapies. As previously described, gemcitabine and docetaxel were administered to this patient every 2 weeks.9 This regimen, in our experience, is less toxic and preserves the dose intensity of the regimen. A complete pathologic necrosis of the primary tumor and regional nodal basin was observed, as well as pulmonary nodule regression, enabling an R0-wide excision and regional lymphadenectomy. The colonic recurrence was surprising, but easily managed with surgical resection.
Pathologic complete response after neoadjuvant chemotherapy is a rare event, observed in about 10% of patients. However, when observed, complete pathologic necrosis (>95%) provided a distant recurrence-free survival of 100% at 3 years.10-12
Our patient, despite achieving a complete pathologic response and excellent initial local control, ultimately experienced an isolated metastatic recurrence in the colon within 1 year of therapy. Data supports performing metastatectomy for stage IV extremity sarcoma for isolated pulmonary or hepatic burden in selected patients with improved survival rates.13,14 As far as we know, there is no published literature describing isolated colon metastases in the absence of liver burden from lower extremity soft tissue sarcomas, or the outcome of surgical resection and adjuvant therapy in these cases.
In conclusion, high-grade pleomorphic sarcoma often follows an aggressive clinical course with ultimately local and distant recurrence. Use of multimodal therapy may have a role in improving local control. Complete pathologic necrosis is a rare event that is predictive of improved outcome. Our case represents an unusual pattern of recurrence among patients with a complete pathologic response to neoadjuvant therapy with isolated colon metastases. Timely, comprehensive management together with vigilant surveillance remain key priorities in the long-term management of high-risk sarcoma.
Soft tissue sarcomas (STS) are a heterogeneous group of tumors of mesenchymal origin that represent a rare form of adult malignancy. According to the World Health Organization classification system, there are more than 100 histologic subtypes of sarcoma based on tissue of origin. Staging criteria most commonly use the American Joint Committee on Cancer’s TNM Classification of Malignant Tumours. About 50% of soft tissue sarcomas originate in the lower extremity.1 Advancements in the use of multimodal therapy have reduced the need for amputation and allowed for equally effective treatment strategies that use limb-sparing surgical resections.
Although most sarcoma metastases spread in a hematogenous fashion, nodal spread is underestimated. Certain histologic subtypes carry a higher predilection for nodal involvement: these include rhabdomyosarcoma, synovial sarcoma, epithelioid sarcoma, vascular sarcoma, and clear-cell sarcoma.2-4 Fong and colleagues have reported that 2.6% of sarcomas have lymphatic spread.3 In the current report, we describe a rare observation of locoregional pelvic nodal metastases from a large undifferentiated pleomorphic sarcoma of the right thigh.
Case presentation and summary
A 63-year-old white woman had a 1-year history of a right thigh mass and an unintentional weight loss of 40 lb. After a year of chiropractic care, she was referred to a physician because of palpable inguinal adenopathy and a 20-cm mass in the medial compartment of the right thigh, with heterogeneous appearance on a magnetic-resonance imaging scan (Figure 1). The patient was referred to the sarcoma transdisciplinary team for evaluation. She was diagnosed by core needle biopsy with a high-grade malignant epithelioid and spindle-cell neoplasm, favoring pleomorphic sarcoma. The metastatic work-up confirmed locoregional right inguinal and retroperitoneal lymph node disease, with 2 lung nodules that were too small to characterize. She also had a paraneoplastic leukocytosis with a white blood cell count of 45,500 cells/ml (normal 10,500 cells/ml).
About 8 months after surgery, she presented to the emergency room with a 3-day history of blood per rectum, anemia, and fatigue. She also reported a weight loss of 10 lb in the previous month. She was admitted for hydration and monitoring. Although computed-tomography (CT) scans of the abdomen and chest done during the previous month and had been interpreted as having no evidence of recurrence or any lymph node disease, the results of an inpatient colonoscopy revealed 2 colonic masses, 1 in the ascending colon and another in the transverse colon. The biopsy findings were consistent with undifferentiated pleomorphic sarcoma, favoring epithelioid histology. The CT scans were re-evaluated in light of these colonoscopic findings. These masses were visible retrospectively on imaging but had been interpreted as stool given the lack of abnormality on imaging 3 months before.
Adequate re-staging was complete, and without other evidence of disease, an extended right hemicolectomy was performed. The postoperative pathology report described geographically 2 distinct masses: a 7-cm mass in the ascending colon, about 3 cm from the ileocecal valve; and a 4-cm mass in the transverse colon, about 7.5 cm from the distal margin of resection. Both masses were identified as high-grade pleomorphic sarcoma. Again, all nodes recovered were negative for malignancy (0/5). Of note is that the background colonic mucosa showed active multifocal colitis with deep inflammatory activity likely consistent with a paraneoplastic syndrome.
Discussion
Surgery remains the primary treatment modality for localized soft tissue sarcoma. Obtaining a margin-free resection, while maintaining optimal function, is the objective with extremity sarcoma. In addition to surgical resection, doxorubicin-based adjuvant chemotherapy remains the standard of care with modest improvement in overall survival and disease-free survival, especially in sarcomas of the extremities.5,6 Gemcitabine also has activity in soft tissue sarcomas7 and might synergize with docetaxel. High response rates of 53% with fixed dose infusion rates of these agents in uterine leiomyosarcoma led the Sarcoma Alliance for Research through Collaboration investigators to consider this regimen for other STS.8 An overall response rate of 16% was noted across all sarcoma subtypes, and undifferentiated pleomorphic sarcoma had a response rate of 32%.
In our experience, a modified schedule of gemcitabine and docetaxel is better tolerated than the standard every 3 weeks regimen or doxorubicin-based chemotherapies. As previously described, gemcitabine and docetaxel were administered to this patient every 2 weeks.9 This regimen, in our experience, is less toxic and preserves the dose intensity of the regimen. A complete pathologic necrosis of the primary tumor and regional nodal basin was observed, as well as pulmonary nodule regression, enabling an R0-wide excision and regional lymphadenectomy. The colonic recurrence was surprising, but easily managed with surgical resection.
Pathologic complete response after neoadjuvant chemotherapy is a rare event, observed in about 10% of patients. However, when observed, complete pathologic necrosis (>95%) provided a distant recurrence-free survival of 100% at 3 years.10-12
Our patient, despite achieving a complete pathologic response and excellent initial local control, ultimately experienced an isolated metastatic recurrence in the colon within 1 year of therapy. Data supports performing metastatectomy for stage IV extremity sarcoma for isolated pulmonary or hepatic burden in selected patients with improved survival rates.13,14 As far as we know, there is no published literature describing isolated colon metastases in the absence of liver burden from lower extremity soft tissue sarcomas, or the outcome of surgical resection and adjuvant therapy in these cases.
In conclusion, high-grade pleomorphic sarcoma often follows an aggressive clinical course with ultimately local and distant recurrence. Use of multimodal therapy may have a role in improving local control. Complete pathologic necrosis is a rare event that is predictive of improved outcome. Our case represents an unusual pattern of recurrence among patients with a complete pathologic response to neoadjuvant therapy with isolated colon metastases. Timely, comprehensive management together with vigilant surveillance remain key priorities in the long-term management of high-risk sarcoma.
1. Lawrence W Jr, Donegan WL, Natarajan N, Mettlin C, Beart R, Winchester D. Adult soft tissue sarcomas. A pattern of care survey of the American College of Surgeons. Ann Surg. 1987;205:349-359.
2. Riad S, Griffin AM, Liberman B, et al. Lymph node metastasis in soft tissue sarcoma in an extremity. Clin Orthop Relat Res. 2004:129-134.
3. Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg. 1993;217:72-77.
4. Mazeron JJ, Suit HD. Lymph nodes as sites of metastases from sarcomas of soft tissue. Cancer. 1987;60:1800-1808.
5. Sarcoma Meta-analysis Collaboration. Adjuvant chemotherapy for localised resectable soft tissue sarcoma of adults: meta-analysis of individual data. Lancet. 1997;350:1647-1654.
6. Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft tissue sarcoma. Cancer. 2008;113:573-581.
7. Patel SR, Gandhi V, Jenkins J, et al. Phase II clinical investigation of gemcitabine in advanced soft tissue sarcomas and window evaluation of dose rate on gemcitabine triphosphate accumulation. J Clin Oncol. 2001;19:3483-3489.
8. Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol. 2007;25:2755-2763.
9. Verschraegen CF, Arias-Pulido H, Lee SJ, et al. Phase IB study of the combination of docetaxel, gemcitabine, and bevacizumab in patients with advanced or recurrent soft tissue sarcoma: the Axtell regimen. Ann Oncol. 2012;23:785-790.
10. MacDermed DM, Miller LL, Peabody TD, et al. Primary tumor necrosis predicts distant control in locally advanced soft tissue sarcomas after preoperative concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1147-1153.
11. Eilber FC, Rosen G, Eckardt J, et al. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19:3203-3209.
12. Shah D, Borys D, Martinez SR, et al. Complete pathologic response to neoadjuvant radiotherapy is predictive of oncological outcome in patients with soft tissue sarcoma. Anticancer Res. 2012;32:3911-3915.
13. Rehders A, Peiper M, Stoecklein NH, et al. Hepatic metastasectomy for soft tissue sarcomas: is it justified? World J Surg. 2009;33:111-117.
14. Pfannschmidt J, Hoffmann H, Schneider T, Dienemann H. Pulmonary metastasectomy for soft tissue sarcomas: is it justified? Recent Results Cancer Res. 2009;179:321-336.
1. Lawrence W Jr, Donegan WL, Natarajan N, Mettlin C, Beart R, Winchester D. Adult soft tissue sarcomas. A pattern of care survey of the American College of Surgeons. Ann Surg. 1987;205:349-359.
2. Riad S, Griffin AM, Liberman B, et al. Lymph node metastasis in soft tissue sarcoma in an extremity. Clin Orthop Relat Res. 2004:129-134.
3. Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg. 1993;217:72-77.
4. Mazeron JJ, Suit HD. Lymph nodes as sites of metastases from sarcomas of soft tissue. Cancer. 1987;60:1800-1808.
5. Sarcoma Meta-analysis Collaboration. Adjuvant chemotherapy for localised resectable soft tissue sarcoma of adults: meta-analysis of individual data. Lancet. 1997;350:1647-1654.
6. Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft tissue sarcoma. Cancer. 2008;113:573-581.
7. Patel SR, Gandhi V, Jenkins J, et al. Phase II clinical investigation of gemcitabine in advanced soft tissue sarcomas and window evaluation of dose rate on gemcitabine triphosphate accumulation. J Clin Oncol. 2001;19:3483-3489.
8. Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol. 2007;25:2755-2763.
9. Verschraegen CF, Arias-Pulido H, Lee SJ, et al. Phase IB study of the combination of docetaxel, gemcitabine, and bevacizumab in patients with advanced or recurrent soft tissue sarcoma: the Axtell regimen. Ann Oncol. 2012;23:785-790.
10. MacDermed DM, Miller LL, Peabody TD, et al. Primary tumor necrosis predicts distant control in locally advanced soft tissue sarcomas after preoperative concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1147-1153.
11. Eilber FC, Rosen G, Eckardt J, et al. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19:3203-3209.
12. Shah D, Borys D, Martinez SR, et al. Complete pathologic response to neoadjuvant radiotherapy is predictive of oncological outcome in patients with soft tissue sarcoma. Anticancer Res. 2012;32:3911-3915.
13. Rehders A, Peiper M, Stoecklein NH, et al. Hepatic metastasectomy for soft tissue sarcomas: is it justified? World J Surg. 2009;33:111-117.
14. Pfannschmidt J, Hoffmann H, Schneider T, Dienemann H. Pulmonary metastasectomy for soft tissue sarcomas: is it justified? Recent Results Cancer Res. 2009;179:321-336.
Recent upturn seen in stroke death rate
A recent increase in the death rate from stroke resulted in more than 32,000 more deaths than would have occurred had the previous long-term decline continued, according to the Centers for Disease Control and Prevention.
The overall age-standardized stroke death rate among adults aged 35 years and older declined from 118.4 per 100,000 in 2000 to 73.3 per 100,000 in 2015, for an average annual percent change of –3.1%. That long-term rate, however, includes a more recent, but statistically nonsignificant, increase of 2.5% a year in 2013-2015, which produced an estimated 32,593 excess deaths based on the previous rate of decline, the CDC investigators said (MMWR 2017 Sep 6;66[early release]:1-7).
“Reasons for the slowing, stalling, and reversing in declines in stroke death rates are not clear. … Recent studies have reported that younger adults have experienced a significant increase in both stroke hospitalizations and in associated stroke risk factors (e.g., hypertension, obesity, diabetes, lipid disorder, and tobacco use). … These changes in modifiable stroke risk factors might present new challenges for stroke prevention and for maintaining a sustained decline in stroke mortality in the United States,” the investigators wrote.
A recent increase in the death rate from stroke resulted in more than 32,000 more deaths than would have occurred had the previous long-term decline continued, according to the Centers for Disease Control and Prevention.
The overall age-standardized stroke death rate among adults aged 35 years and older declined from 118.4 per 100,000 in 2000 to 73.3 per 100,000 in 2015, for an average annual percent change of –3.1%. That long-term rate, however, includes a more recent, but statistically nonsignificant, increase of 2.5% a year in 2013-2015, which produced an estimated 32,593 excess deaths based on the previous rate of decline, the CDC investigators said (MMWR 2017 Sep 6;66[early release]:1-7).
“Reasons for the slowing, stalling, and reversing in declines in stroke death rates are not clear. … Recent studies have reported that younger adults have experienced a significant increase in both stroke hospitalizations and in associated stroke risk factors (e.g., hypertension, obesity, diabetes, lipid disorder, and tobacco use). … These changes in modifiable stroke risk factors might present new challenges for stroke prevention and for maintaining a sustained decline in stroke mortality in the United States,” the investigators wrote.
A recent increase in the death rate from stroke resulted in more than 32,000 more deaths than would have occurred had the previous long-term decline continued, according to the Centers for Disease Control and Prevention.
The overall age-standardized stroke death rate among adults aged 35 years and older declined from 118.4 per 100,000 in 2000 to 73.3 per 100,000 in 2015, for an average annual percent change of –3.1%. That long-term rate, however, includes a more recent, but statistically nonsignificant, increase of 2.5% a year in 2013-2015, which produced an estimated 32,593 excess deaths based on the previous rate of decline, the CDC investigators said (MMWR 2017 Sep 6;66[early release]:1-7).
“Reasons for the slowing, stalling, and reversing in declines in stroke death rates are not clear. … Recent studies have reported that younger adults have experienced a significant increase in both stroke hospitalizations and in associated stroke risk factors (e.g., hypertension, obesity, diabetes, lipid disorder, and tobacco use). … These changes in modifiable stroke risk factors might present new challenges for stroke prevention and for maintaining a sustained decline in stroke mortality in the United States,” the investigators wrote.
FROM MMWR
Intramedullary spinal cord and leptomeningeal metastases presenting as cauda equina syndrome in a patient with melanoma
The incidence of malignant melanoma has been rising in the United States, especially among non-Hispanic white men and women. Death rates have increased for those aged 65 years or older, and incidence rates have increased for all age groups.1 It is a serious public health issue.
Given the unique biology of melanoma, metastatic disease can present in a variety of ways. In most cases, the lymph nodes and lungs are involved.2 The incidence of brain metastases is 10%-40%, however the percentage may be even higher based on reported incidence of autopsy reports.3 The most common forms of metastatic melanoma to the spine are vertebral and intramedullary.4 Specifically, leptomeningeal involvement can be found in 20% of patients in clinical studies and 44%-70% in autopsy series of patients with central nervous system (CNS) metastatic disease.5 Despite its incidence, leptomeningeal disease (LMD) from melanoma is rarely discussed in the literature and the diagnosis may be difficult. Even rarer is the documented presentation of intramedullary spinal cord metastases, or “drop metastases.”6 In our review of the literature, we found no published case reports to date of drop metastases from melanoma causing cauda equina syndrome.
The prognosis of patients with metastatic melanoma with brain metastases is very poor, with a median overall survival of about 4 months reported in several studies.7-9 Prognosis is even worse for patients with leptomeningeal involvement, and median survival without therapy is about 4-6 weeks.10 A combination of intrathecal and systemic chemotherapy can be used to treat LMD.11
Case presentation and summary
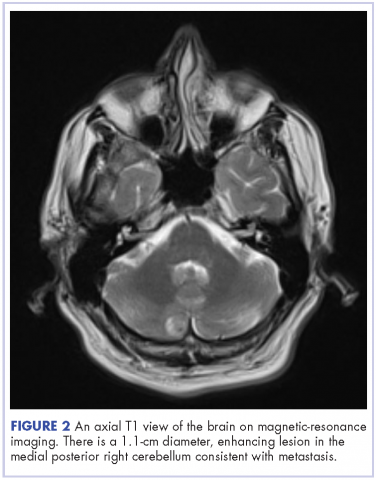
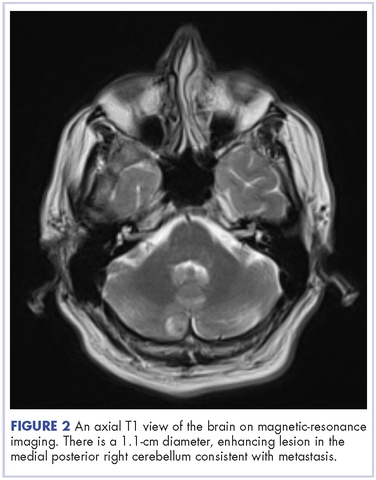
This is the case of a 56-year-old man with history of metastatic melanoma that had been initially diagnosed about 4 years before the current case presentation. Original sites of disease were a supraclavicular lymph node and solitary liver metastasis, both of which were resected. The patient then developed biopsy-proven lung involvement that required left and right wedge resections. Mutation testing for BRAF V600E and BRAF V600K was sent and not detected. Therefore the patient did not receive any BRAF-targeted therapies. Subsequently, recurrent metastatic disease to the brain with 2 dominant lesions in the cerebellum and the occiput as well as numerous small lesions at the gray-white matter junction was identified (Figure 1 and Figure 2).
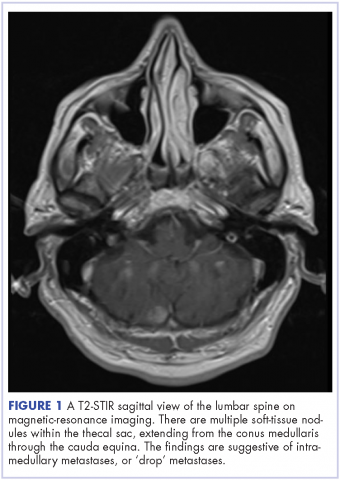
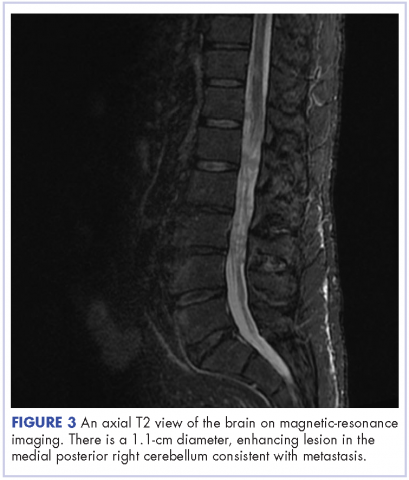
The patient received whole-brain radiation (30 Gy in 10 fractions of 3 Gy each). There was no evidence of disease in his spine at that time. About 2 weeks after completing whole-brain radiation, the patient presented to the hospital with left lower extremity weakness, urinary retention, bowel incontinence, saddle anesthesia, and malaise. The symptoms had begun after he had finished whole-brain radiation and weakness progressed to the point at which he need a cane to be able to walk. A physical examination was significant for hyporreflexia, decreased strength and sensitivity on left lower extremity, saddle anesthesia, and lumbar spinal tenderness to palpation. The results of magnetic-resonance imaging (MRI) of the spine revealed multiple soft-tissue nodules extending from the conus medullaris throughout the cauda equina, consistent with intramedullary metastases, as well as concomitant leptomeningeal involvement (Figure 3).
The patient was started on steroids with minimal improvement in neurologic function. We consulted with our neurosurgery colleagues, but learned that no direct surgical intervention could be performed because of widespread involvement. We then proceeded with radiation, 30 Gy in 10 fractions to the lumbar spine. Intrathecal chemotherapy with methotrexate (12 mg twice a week) was also started, with a plan to complete 4 weeks. Shortly after starting radiation therapy and methotrexate, we observed clinical improvement in the patient, with mildly increased left lower extremity strength and increased ambulation with a physical therapist.
Cerebrospinal fluid studies (CSF) showed clearance of malignant cells after 2 treatments of intrathecal methotrexate as well as improvement in CSF chemistry parameters: the patient’s protein level decreased from 1,095 mg/dL to 42 mg/dL (15-45 mg/dL) and his glucose level increased from 3 mg/dL to 73 mg/dL (40-85 mg/dL) However, after completing 3 weeks of intrathecal chemotherapy, the hospital course was complicated by leukopenia, thrombocytopenia, and spontaneous intracranial hemorrhage. The cytopenias were thought to be secondary to systemic effect of intrathecal methotrexate in conjunction with the radiation treatments to the spine. Intrathecal chemotherapy was held.
The patient was not a candidate for systemic immunotherapy because of his decline in performance status. He continued to deteriorate neurologically, and the family decided to pursue inpatient hospice. He died a week after transfer to hospice and 5 weeks after the initial diagnosis of leptomeningeal and intramedullary metastases.
Conclusions
Although metastatic melanoma to the brain is not uncommon, leptomeningeal and intramedullary drop metastases are an infrequent presentation. Even more rare are intramedullary drop metastasis that are significant enough to cause cauda equina syndrome, as with our patient. The incidence of LMD has increased over the years and may continue to increase, likely because of the improved overall survival and a prolonged control of extracranial disease with newly approved systemic therapeutic drugs, such as molecularly targeted therapy and immunotherapy.12 Intramedullary metastases are extremely rare, but reported incidence has seemed to be increasing due to detection with MRI. Currently there are fewer than 100 case reports of intramedullary spinal cord metastasis.6 In one retrospective study, 40 patients with intramedullary metastatic disease secondary to systemic cancer were identified during 1980-1993.6 About half of those cases were from lung cancer, the second most common was breast cancer.
CNS involvement by melanoma can have debilitating complications and confers a poor prognosis. In another retrospective study, several patient characteristics were found to be associated with significantly shorter survival in patients with known brain metastases, including presence of neurologic symptoms and leptomeningeal involvement.3
Malignant cells can reach the CSF by several routes: direct extension, hematogenous, venous access, venous drainage from bone marrow and cranial and peripheral nerves. Once the tumor has reached the CSF, it can seed any portion of the nervous system that has contact with the CSF and become entangled among the cauda equine.13
Given the rarity of leptomeningeal and intramedullary involvement of melanoma, there are no standard treatment guidelines. Treatment for LMD usually consists of intrathecal and systemic chemotherapy. Commonly used intrathecal agents are methotrexate, liposomal cytarabine, and thiopeta.11 The goals of treatment are to improve or stabilize neurologic status of the patient and ideally prolong survival. The choice of agent for intrathecal chemotherapy is guided by the primary tumor, however, there is no strong evidence to choose one agent over the other.12,14 Methotrexate or cytarabine are generally recommended in the National Comprehensive Cancer Network (NCCN) guidelines. Targeted therapy toward the primary tumor is occasionally used for treatment of LMD, for example rituximab can be given intrathecally for lymphoma,15 and trastuzumab has been given intrathecally for breast cancer.16 No intrathecal targeted agents are currently available for melanoma. Administration of intrathecal chemotherapy is given via lumbar puncture or Ommaya reservoir. Induction intrathecal chemotherapy is recommended by NCCN to be given for 4-6 weeks. The schedule of administration varies based on the agent used. Most systemic chemotherapy has poor CSF penetration, which is the basis behind using chemotherapy intrathecally in these patients.14 However, novel therapies for melanoma, such as ipilimumab, have shown activity in the CNS, and it is not known if intrathecal chemotherapy will continue to play role in the management of LMD.17
Systemic therapy for metastatic melanoma has changed with the development of novel agents, which have shown better efficacy than traditional chemotherapy. The recommendation for first-line systemic therapy of metastatic unresectable melanoma is based on several factors: BRAF mutation status, tempo of disease, and presence or absence of cancer-related symptoms. Immunotherapy for metastatic melanoma that is unresectable includes anti-programmed cell death protein-1 (PD-1) monotherapy (nivolumab or pembrolizumab) or combination therapy with nivolumab plus ipilimumab. Targeted therapy is preferred in cases with an identified BRAF mutation. Combination therapy with dabrafenib plus trametinib or with vemurafenib plus cobimetinib is recommended. Single-agent therapy may also be used with dabrafenib or vemurafenib.18
Ipilimumab is a monoclonal antibody that blocks cytotoxic T-lymphocyte antigen-4 to potentiate an anti-tumor T-cell response that was approved in 2011 by the US Food and Drug Administration for the treatment of melanoma. A randomized, phase 3 clinical trial showed an increase in overall survival in patients with unresectable metastatic disease who had received previous treatment.19 Before that, no therapy had been shown to improve overall survival in patients with metastatic melanoma. Patients with CNS metastases were included in this study.19
The activity of ipilimumab specifically in patients with brain metastasis was further studied in a phase 2 trial that enrolled 72 patients, 1 cohort with symptomatic brain metastases and the other cohort with asymptomatic brain metastases.20 After 12 weeks of therapy, response was assessed by modified World Health Organization criteria for disease control (complete response plus partial response plus stable disease). In all, 18% of patients with asymptomatic brain metastasis achieved disease control, compared with 10% of patients with symptomatic brain metastases. When the brain alone was assessed, 24% of asymptomatic patients and 10% of symptomatic patients achieved disease control. No unexpected toxic effects occurred during the study. Anti-PD1 therapy such as nivolumab, which has shown durable responses in metastatic melanoma, has no published results specifically in patients with active brain metastases.
Of the BRAF-targeted therapy, dabrafenib and vemurafenib have also been studied in patients with brain metastases. For darafenib, 172 patients with BRAF-mutated metastatic melanoma were included in a phase 2 clinical trial that showed an intracranial response of 39% in previously untreated patients and 31% in patients whose brain metastases had progressed after previous local treatment.21 Vemurafenib has also shown intracranial response in a phase 2 clinical trial.22
The role of the aforementioned therapies in patients with metastatic melanoma with CNS disease should not be overlooked because these patients are typically excluded from clinical trials. As already noted, agents such as ipilimumab and the dabrafenib–vemurafenib combination have been studied in patients with brain metastases and have shown disease control, but more studies are needed to truly assess whether there is an improvement in overall survival and whether that will change treatment guidelines. Although patients with parenchymal brain metastases were included in these studies, it is not clear how patients with LMD and intramedullary spinal cord metastases, such as our patient, would be affected. It is also not clear whether intrathecal chemotherapy will continue to play a role in management of metastatic melanoma with LMD, especially if these newer agents have CNS activity in addition to controlling extracranial disease. Although rarely documented, leptomeningeal and intramedullary metastatic disease will likely become increasingly recognized as patients with cancer live longer and diagnostic studies improve. These initial studies showing intracranial disease control show compelling evidence to continue enrolling patients with active CNS disease in clinical trials.
1. Jemal A, Saraiya M, Patel P, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992-2006. J Am Acad Dermatol. 2011;65(5 Suppl 1):S17.e1-S17.e11.
2. Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma: a study of 216 autopsy cases. Am J Surg. 1978;135(6):807-810.
3. Raizer J, Hwu W, Panageas K, et al. Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro Oncol. 2008;10(2):199-207.
4. Sun L, Song Y, Gong Q. Easily misdiagnosed delayed metastatic intraspinal extradural melanoma of the lumbar spine: a case report and review of the literature. Oncol Lett. 2013;5(6):1799-1802.
5. Moseley R, Davies A, Bourne S, et al. Neoplastic meningitis in malignant melanoma: diagnosis with monoclonal antibiodies. J Neurol Neurosurg Psychiatry. 1989;52:991-886.
6. Schiff D, O’Neill B. Intramedullary spinal cord metastases clinical features and treatment outcome. Neurology. 1996;47(4):906-912.
7. Fife KM, Colman MH, Stevens G, et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol. 2004;22(7):1293-1300.
8. Raizer J, Hwu W, Panageas K, et al. Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro Oncol. 2008;10(2):199-207.
9. Sampson JH, Carter JH Jr, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88:11-20.
10. Abernethy AP. Central nervous system tumors. In: Loprinzi C, ed. ASCO-SEP: Medical Oncology Self-evaluation Program. 4th ed. Alexandria, VA: American Society of Clinical Oncology, 2015. Page 396. Print.
11. Pape E, Desmedt E, Zairi , et al. Leptomeningeal metastasis in melanoma: a prospective clinical study of nine patients. In Vivo. 2012;26(6):1079-1086.
12. Pavlidis N. The diagnostic and therapeutic management of leptomeningeal carcinomatosis. Ann Oncol. 2004;15(Suppl 4):iv285-291.
13. DeAngelis L, Posner JB. Neurologic complications of cancer. 2nd ed. New York, NY: Oxford University Press; 2008.
14. Chamberlain, M. Leptomeningeal metastasis. Curr Opin Oncol. 2010;22:627-635.
15. Chamberlain M, Johnston S, Van Horn A, Glantz MJ. Recurrent lymphomatous meningitis treated with intra-CSF rituximab and liposomal ara-C. J Neurooncol. 2009;91(3):271-277.
16. Zagouri F, Sergentanis T, Bartsch R, et al. Intrathecal administration of trastuzumab for the treatment of meningeal carcinomatosis in HER2-positive metastatic breast cancer: a systematic review and pooled analysis. Breast Cancer Res Treat. 2013;139(1):13-22.
17. Silk A, Bassetti M, West BT, Tsien C, Lao CD. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med. 2013;2(6):899-906.
18. [Behind paywall.] National Comprehensive Cancer Network. Melanoma (version 2.2016). http://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf. November 10, 2016. Accessed February 28, 2016
19. Hodi F, O’Day S, McDermott D, et al. Improved survival with ipilimumab in patients with metastatic melanoma. NEJM. 2010;363(8):711-723.
20. Margolin K, Ernstoff M, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459-465.
21. Long G, Trefzer U, Davies M, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087-1095.
22. McArthur GA, Maio M, Arance A, et al. Vemurafenib in metastatic melanoma patients with brain metastases: an open-label, single-arm, phase 2, multicenter study. Ann Oncol. 2017;
The incidence of malignant melanoma has been rising in the United States, especially among non-Hispanic white men and women. Death rates have increased for those aged 65 years or older, and incidence rates have increased for all age groups.1 It is a serious public health issue.
Given the unique biology of melanoma, metastatic disease can present in a variety of ways. In most cases, the lymph nodes and lungs are involved.2 The incidence of brain metastases is 10%-40%, however the percentage may be even higher based on reported incidence of autopsy reports.3 The most common forms of metastatic melanoma to the spine are vertebral and intramedullary.4 Specifically, leptomeningeal involvement can be found in 20% of patients in clinical studies and 44%-70% in autopsy series of patients with central nervous system (CNS) metastatic disease.5 Despite its incidence, leptomeningeal disease (LMD) from melanoma is rarely discussed in the literature and the diagnosis may be difficult. Even rarer is the documented presentation of intramedullary spinal cord metastases, or “drop metastases.”6 In our review of the literature, we found no published case reports to date of drop metastases from melanoma causing cauda equina syndrome.
The prognosis of patients with metastatic melanoma with brain metastases is very poor, with a median overall survival of about 4 months reported in several studies.7-9 Prognosis is even worse for patients with leptomeningeal involvement, and median survival without therapy is about 4-6 weeks.10 A combination of intrathecal and systemic chemotherapy can be used to treat LMD.11
Case presentation and summary
This is the case of a 56-year-old man with history of metastatic melanoma that had been initially diagnosed about 4 years before the current case presentation. Original sites of disease were a supraclavicular lymph node and solitary liver metastasis, both of which were resected. The patient then developed biopsy-proven lung involvement that required left and right wedge resections. Mutation testing for BRAF V600E and BRAF V600K was sent and not detected. Therefore the patient did not receive any BRAF-targeted therapies. Subsequently, recurrent metastatic disease to the brain with 2 dominant lesions in the cerebellum and the occiput as well as numerous small lesions at the gray-white matter junction was identified (Figure 1 and Figure 2).
The patient received whole-brain radiation (30 Gy in 10 fractions of 3 Gy each). There was no evidence of disease in his spine at that time. About 2 weeks after completing whole-brain radiation, the patient presented to the hospital with left lower extremity weakness, urinary retention, bowel incontinence, saddle anesthesia, and malaise. The symptoms had begun after he had finished whole-brain radiation and weakness progressed to the point at which he need a cane to be able to walk. A physical examination was significant for hyporreflexia, decreased strength and sensitivity on left lower extremity, saddle anesthesia, and lumbar spinal tenderness to palpation. The results of magnetic-resonance imaging (MRI) of the spine revealed multiple soft-tissue nodules extending from the conus medullaris throughout the cauda equina, consistent with intramedullary metastases, as well as concomitant leptomeningeal involvement (Figure 3).
The patient was started on steroids with minimal improvement in neurologic function. We consulted with our neurosurgery colleagues, but learned that no direct surgical intervention could be performed because of widespread involvement. We then proceeded with radiation, 30 Gy in 10 fractions to the lumbar spine. Intrathecal chemotherapy with methotrexate (12 mg twice a week) was also started, with a plan to complete 4 weeks. Shortly after starting radiation therapy and methotrexate, we observed clinical improvement in the patient, with mildly increased left lower extremity strength and increased ambulation with a physical therapist.
Cerebrospinal fluid studies (CSF) showed clearance of malignant cells after 2 treatments of intrathecal methotrexate as well as improvement in CSF chemistry parameters: the patient’s protein level decreased from 1,095 mg/dL to 42 mg/dL (15-45 mg/dL) and his glucose level increased from 3 mg/dL to 73 mg/dL (40-85 mg/dL) However, after completing 3 weeks of intrathecal chemotherapy, the hospital course was complicated by leukopenia, thrombocytopenia, and spontaneous intracranial hemorrhage. The cytopenias were thought to be secondary to systemic effect of intrathecal methotrexate in conjunction with the radiation treatments to the spine. Intrathecal chemotherapy was held.
The patient was not a candidate for systemic immunotherapy because of his decline in performance status. He continued to deteriorate neurologically, and the family decided to pursue inpatient hospice. He died a week after transfer to hospice and 5 weeks after the initial diagnosis of leptomeningeal and intramedullary metastases.
Conclusions
Although metastatic melanoma to the brain is not uncommon, leptomeningeal and intramedullary drop metastases are an infrequent presentation. Even more rare are intramedullary drop metastasis that are significant enough to cause cauda equina syndrome, as with our patient. The incidence of LMD has increased over the years and may continue to increase, likely because of the improved overall survival and a prolonged control of extracranial disease with newly approved systemic therapeutic drugs, such as molecularly targeted therapy and immunotherapy.12 Intramedullary metastases are extremely rare, but reported incidence has seemed to be increasing due to detection with MRI. Currently there are fewer than 100 case reports of intramedullary spinal cord metastasis.6 In one retrospective study, 40 patients with intramedullary metastatic disease secondary to systemic cancer were identified during 1980-1993.6 About half of those cases were from lung cancer, the second most common was breast cancer.
CNS involvement by melanoma can have debilitating complications and confers a poor prognosis. In another retrospective study, several patient characteristics were found to be associated with significantly shorter survival in patients with known brain metastases, including presence of neurologic symptoms and leptomeningeal involvement.3
Malignant cells can reach the CSF by several routes: direct extension, hematogenous, venous access, venous drainage from bone marrow and cranial and peripheral nerves. Once the tumor has reached the CSF, it can seed any portion of the nervous system that has contact with the CSF and become entangled among the cauda equine.13
Given the rarity of leptomeningeal and intramedullary involvement of melanoma, there are no standard treatment guidelines. Treatment for LMD usually consists of intrathecal and systemic chemotherapy. Commonly used intrathecal agents are methotrexate, liposomal cytarabine, and thiopeta.11 The goals of treatment are to improve or stabilize neurologic status of the patient and ideally prolong survival. The choice of agent for intrathecal chemotherapy is guided by the primary tumor, however, there is no strong evidence to choose one agent over the other.12,14 Methotrexate or cytarabine are generally recommended in the National Comprehensive Cancer Network (NCCN) guidelines. Targeted therapy toward the primary tumor is occasionally used for treatment of LMD, for example rituximab can be given intrathecally for lymphoma,15 and trastuzumab has been given intrathecally for breast cancer.16 No intrathecal targeted agents are currently available for melanoma. Administration of intrathecal chemotherapy is given via lumbar puncture or Ommaya reservoir. Induction intrathecal chemotherapy is recommended by NCCN to be given for 4-6 weeks. The schedule of administration varies based on the agent used. Most systemic chemotherapy has poor CSF penetration, which is the basis behind using chemotherapy intrathecally in these patients.14 However, novel therapies for melanoma, such as ipilimumab, have shown activity in the CNS, and it is not known if intrathecal chemotherapy will continue to play role in the management of LMD.17
Systemic therapy for metastatic melanoma has changed with the development of novel agents, which have shown better efficacy than traditional chemotherapy. The recommendation for first-line systemic therapy of metastatic unresectable melanoma is based on several factors: BRAF mutation status, tempo of disease, and presence or absence of cancer-related symptoms. Immunotherapy for metastatic melanoma that is unresectable includes anti-programmed cell death protein-1 (PD-1) monotherapy (nivolumab or pembrolizumab) or combination therapy with nivolumab plus ipilimumab. Targeted therapy is preferred in cases with an identified BRAF mutation. Combination therapy with dabrafenib plus trametinib or with vemurafenib plus cobimetinib is recommended. Single-agent therapy may also be used with dabrafenib or vemurafenib.18
Ipilimumab is a monoclonal antibody that blocks cytotoxic T-lymphocyte antigen-4 to potentiate an anti-tumor T-cell response that was approved in 2011 by the US Food and Drug Administration for the treatment of melanoma. A randomized, phase 3 clinical trial showed an increase in overall survival in patients with unresectable metastatic disease who had received previous treatment.19 Before that, no therapy had been shown to improve overall survival in patients with metastatic melanoma. Patients with CNS metastases were included in this study.19
The activity of ipilimumab specifically in patients with brain metastasis was further studied in a phase 2 trial that enrolled 72 patients, 1 cohort with symptomatic brain metastases and the other cohort with asymptomatic brain metastases.20 After 12 weeks of therapy, response was assessed by modified World Health Organization criteria for disease control (complete response plus partial response plus stable disease). In all, 18% of patients with asymptomatic brain metastasis achieved disease control, compared with 10% of patients with symptomatic brain metastases. When the brain alone was assessed, 24% of asymptomatic patients and 10% of symptomatic patients achieved disease control. No unexpected toxic effects occurred during the study. Anti-PD1 therapy such as nivolumab, which has shown durable responses in metastatic melanoma, has no published results specifically in patients with active brain metastases.
Of the BRAF-targeted therapy, dabrafenib and vemurafenib have also been studied in patients with brain metastases. For darafenib, 172 patients with BRAF-mutated metastatic melanoma were included in a phase 2 clinical trial that showed an intracranial response of 39% in previously untreated patients and 31% in patients whose brain metastases had progressed after previous local treatment.21 Vemurafenib has also shown intracranial response in a phase 2 clinical trial.22
The role of the aforementioned therapies in patients with metastatic melanoma with CNS disease should not be overlooked because these patients are typically excluded from clinical trials. As already noted, agents such as ipilimumab and the dabrafenib–vemurafenib combination have been studied in patients with brain metastases and have shown disease control, but more studies are needed to truly assess whether there is an improvement in overall survival and whether that will change treatment guidelines. Although patients with parenchymal brain metastases were included in these studies, it is not clear how patients with LMD and intramedullary spinal cord metastases, such as our patient, would be affected. It is also not clear whether intrathecal chemotherapy will continue to play a role in management of metastatic melanoma with LMD, especially if these newer agents have CNS activity in addition to controlling extracranial disease. Although rarely documented, leptomeningeal and intramedullary metastatic disease will likely become increasingly recognized as patients with cancer live longer and diagnostic studies improve. These initial studies showing intracranial disease control show compelling evidence to continue enrolling patients with active CNS disease in clinical trials.
The incidence of malignant melanoma has been rising in the United States, especially among non-Hispanic white men and women. Death rates have increased for those aged 65 years or older, and incidence rates have increased for all age groups.1 It is a serious public health issue.
Given the unique biology of melanoma, metastatic disease can present in a variety of ways. In most cases, the lymph nodes and lungs are involved.2 The incidence of brain metastases is 10%-40%, however the percentage may be even higher based on reported incidence of autopsy reports.3 The most common forms of metastatic melanoma to the spine are vertebral and intramedullary.4 Specifically, leptomeningeal involvement can be found in 20% of patients in clinical studies and 44%-70% in autopsy series of patients with central nervous system (CNS) metastatic disease.5 Despite its incidence, leptomeningeal disease (LMD) from melanoma is rarely discussed in the literature and the diagnosis may be difficult. Even rarer is the documented presentation of intramedullary spinal cord metastases, or “drop metastases.”6 In our review of the literature, we found no published case reports to date of drop metastases from melanoma causing cauda equina syndrome.
The prognosis of patients with metastatic melanoma with brain metastases is very poor, with a median overall survival of about 4 months reported in several studies.7-9 Prognosis is even worse for patients with leptomeningeal involvement, and median survival without therapy is about 4-6 weeks.10 A combination of intrathecal and systemic chemotherapy can be used to treat LMD.11
Case presentation and summary
This is the case of a 56-year-old man with history of metastatic melanoma that had been initially diagnosed about 4 years before the current case presentation. Original sites of disease were a supraclavicular lymph node and solitary liver metastasis, both of which were resected. The patient then developed biopsy-proven lung involvement that required left and right wedge resections. Mutation testing for BRAF V600E and BRAF V600K was sent and not detected. Therefore the patient did not receive any BRAF-targeted therapies. Subsequently, recurrent metastatic disease to the brain with 2 dominant lesions in the cerebellum and the occiput as well as numerous small lesions at the gray-white matter junction was identified (Figure 1 and Figure 2).
The patient received whole-brain radiation (30 Gy in 10 fractions of 3 Gy each). There was no evidence of disease in his spine at that time. About 2 weeks after completing whole-brain radiation, the patient presented to the hospital with left lower extremity weakness, urinary retention, bowel incontinence, saddle anesthesia, and malaise. The symptoms had begun after he had finished whole-brain radiation and weakness progressed to the point at which he need a cane to be able to walk. A physical examination was significant for hyporreflexia, decreased strength and sensitivity on left lower extremity, saddle anesthesia, and lumbar spinal tenderness to palpation. The results of magnetic-resonance imaging (MRI) of the spine revealed multiple soft-tissue nodules extending from the conus medullaris throughout the cauda equina, consistent with intramedullary metastases, as well as concomitant leptomeningeal involvement (Figure 3).
The patient was started on steroids with minimal improvement in neurologic function. We consulted with our neurosurgery colleagues, but learned that no direct surgical intervention could be performed because of widespread involvement. We then proceeded with radiation, 30 Gy in 10 fractions to the lumbar spine. Intrathecal chemotherapy with methotrexate (12 mg twice a week) was also started, with a plan to complete 4 weeks. Shortly after starting radiation therapy and methotrexate, we observed clinical improvement in the patient, with mildly increased left lower extremity strength and increased ambulation with a physical therapist.
Cerebrospinal fluid studies (CSF) showed clearance of malignant cells after 2 treatments of intrathecal methotrexate as well as improvement in CSF chemistry parameters: the patient’s protein level decreased from 1,095 mg/dL to 42 mg/dL (15-45 mg/dL) and his glucose level increased from 3 mg/dL to 73 mg/dL (40-85 mg/dL) However, after completing 3 weeks of intrathecal chemotherapy, the hospital course was complicated by leukopenia, thrombocytopenia, and spontaneous intracranial hemorrhage. The cytopenias were thought to be secondary to systemic effect of intrathecal methotrexate in conjunction with the radiation treatments to the spine. Intrathecal chemotherapy was held.
The patient was not a candidate for systemic immunotherapy because of his decline in performance status. He continued to deteriorate neurologically, and the family decided to pursue inpatient hospice. He died a week after transfer to hospice and 5 weeks after the initial diagnosis of leptomeningeal and intramedullary metastases.
Conclusions
Although metastatic melanoma to the brain is not uncommon, leptomeningeal and intramedullary drop metastases are an infrequent presentation. Even more rare are intramedullary drop metastasis that are significant enough to cause cauda equina syndrome, as with our patient. The incidence of LMD has increased over the years and may continue to increase, likely because of the improved overall survival and a prolonged control of extracranial disease with newly approved systemic therapeutic drugs, such as molecularly targeted therapy and immunotherapy.12 Intramedullary metastases are extremely rare, but reported incidence has seemed to be increasing due to detection with MRI. Currently there are fewer than 100 case reports of intramedullary spinal cord metastasis.6 In one retrospective study, 40 patients with intramedullary metastatic disease secondary to systemic cancer were identified during 1980-1993.6 About half of those cases were from lung cancer, the second most common was breast cancer.
CNS involvement by melanoma can have debilitating complications and confers a poor prognosis. In another retrospective study, several patient characteristics were found to be associated with significantly shorter survival in patients with known brain metastases, including presence of neurologic symptoms and leptomeningeal involvement.3
Malignant cells can reach the CSF by several routes: direct extension, hematogenous, venous access, venous drainage from bone marrow and cranial and peripheral nerves. Once the tumor has reached the CSF, it can seed any portion of the nervous system that has contact with the CSF and become entangled among the cauda equine.13
Given the rarity of leptomeningeal and intramedullary involvement of melanoma, there are no standard treatment guidelines. Treatment for LMD usually consists of intrathecal and systemic chemotherapy. Commonly used intrathecal agents are methotrexate, liposomal cytarabine, and thiopeta.11 The goals of treatment are to improve or stabilize neurologic status of the patient and ideally prolong survival. The choice of agent for intrathecal chemotherapy is guided by the primary tumor, however, there is no strong evidence to choose one agent over the other.12,14 Methotrexate or cytarabine are generally recommended in the National Comprehensive Cancer Network (NCCN) guidelines. Targeted therapy toward the primary tumor is occasionally used for treatment of LMD, for example rituximab can be given intrathecally for lymphoma,15 and trastuzumab has been given intrathecally for breast cancer.16 No intrathecal targeted agents are currently available for melanoma. Administration of intrathecal chemotherapy is given via lumbar puncture or Ommaya reservoir. Induction intrathecal chemotherapy is recommended by NCCN to be given for 4-6 weeks. The schedule of administration varies based on the agent used. Most systemic chemotherapy has poor CSF penetration, which is the basis behind using chemotherapy intrathecally in these patients.14 However, novel therapies for melanoma, such as ipilimumab, have shown activity in the CNS, and it is not known if intrathecal chemotherapy will continue to play role in the management of LMD.17
Systemic therapy for metastatic melanoma has changed with the development of novel agents, which have shown better efficacy than traditional chemotherapy. The recommendation for first-line systemic therapy of metastatic unresectable melanoma is based on several factors: BRAF mutation status, tempo of disease, and presence or absence of cancer-related symptoms. Immunotherapy for metastatic melanoma that is unresectable includes anti-programmed cell death protein-1 (PD-1) monotherapy (nivolumab or pembrolizumab) or combination therapy with nivolumab plus ipilimumab. Targeted therapy is preferred in cases with an identified BRAF mutation. Combination therapy with dabrafenib plus trametinib or with vemurafenib plus cobimetinib is recommended. Single-agent therapy may also be used with dabrafenib or vemurafenib.18
Ipilimumab is a monoclonal antibody that blocks cytotoxic T-lymphocyte antigen-4 to potentiate an anti-tumor T-cell response that was approved in 2011 by the US Food and Drug Administration for the treatment of melanoma. A randomized, phase 3 clinical trial showed an increase in overall survival in patients with unresectable metastatic disease who had received previous treatment.19 Before that, no therapy had been shown to improve overall survival in patients with metastatic melanoma. Patients with CNS metastases were included in this study.19
The activity of ipilimumab specifically in patients with brain metastasis was further studied in a phase 2 trial that enrolled 72 patients, 1 cohort with symptomatic brain metastases and the other cohort with asymptomatic brain metastases.20 After 12 weeks of therapy, response was assessed by modified World Health Organization criteria for disease control (complete response plus partial response plus stable disease). In all, 18% of patients with asymptomatic brain metastasis achieved disease control, compared with 10% of patients with symptomatic brain metastases. When the brain alone was assessed, 24% of asymptomatic patients and 10% of symptomatic patients achieved disease control. No unexpected toxic effects occurred during the study. Anti-PD1 therapy such as nivolumab, which has shown durable responses in metastatic melanoma, has no published results specifically in patients with active brain metastases.
Of the BRAF-targeted therapy, dabrafenib and vemurafenib have also been studied in patients with brain metastases. For darafenib, 172 patients with BRAF-mutated metastatic melanoma were included in a phase 2 clinical trial that showed an intracranial response of 39% in previously untreated patients and 31% in patients whose brain metastases had progressed after previous local treatment.21 Vemurafenib has also shown intracranial response in a phase 2 clinical trial.22
The role of the aforementioned therapies in patients with metastatic melanoma with CNS disease should not be overlooked because these patients are typically excluded from clinical trials. As already noted, agents such as ipilimumab and the dabrafenib–vemurafenib combination have been studied in patients with brain metastases and have shown disease control, but more studies are needed to truly assess whether there is an improvement in overall survival and whether that will change treatment guidelines. Although patients with parenchymal brain metastases were included in these studies, it is not clear how patients with LMD and intramedullary spinal cord metastases, such as our patient, would be affected. It is also not clear whether intrathecal chemotherapy will continue to play a role in management of metastatic melanoma with LMD, especially if these newer agents have CNS activity in addition to controlling extracranial disease. Although rarely documented, leptomeningeal and intramedullary metastatic disease will likely become increasingly recognized as patients with cancer live longer and diagnostic studies improve. These initial studies showing intracranial disease control show compelling evidence to continue enrolling patients with active CNS disease in clinical trials.
1. Jemal A, Saraiya M, Patel P, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992-2006. J Am Acad Dermatol. 2011;65(5 Suppl 1):S17.e1-S17.e11.
2. Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma: a study of 216 autopsy cases. Am J Surg. 1978;135(6):807-810.
3. Raizer J, Hwu W, Panageas K, et al. Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro Oncol. 2008;10(2):199-207.
4. Sun L, Song Y, Gong Q. Easily misdiagnosed delayed metastatic intraspinal extradural melanoma of the lumbar spine: a case report and review of the literature. Oncol Lett. 2013;5(6):1799-1802.
5. Moseley R, Davies A, Bourne S, et al. Neoplastic meningitis in malignant melanoma: diagnosis with monoclonal antibiodies. J Neurol Neurosurg Psychiatry. 1989;52:991-886.
6. Schiff D, O’Neill B. Intramedullary spinal cord metastases clinical features and treatment outcome. Neurology. 1996;47(4):906-912.
7. Fife KM, Colman MH, Stevens G, et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol. 2004;22(7):1293-1300.
8. Raizer J, Hwu W, Panageas K, et al. Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro Oncol. 2008;10(2):199-207.
9. Sampson JH, Carter JH Jr, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88:11-20.
10. Abernethy AP. Central nervous system tumors. In: Loprinzi C, ed. ASCO-SEP: Medical Oncology Self-evaluation Program. 4th ed. Alexandria, VA: American Society of Clinical Oncology, 2015. Page 396. Print.
11. Pape E, Desmedt E, Zairi , et al. Leptomeningeal metastasis in melanoma: a prospective clinical study of nine patients. In Vivo. 2012;26(6):1079-1086.
12. Pavlidis N. The diagnostic and therapeutic management of leptomeningeal carcinomatosis. Ann Oncol. 2004;15(Suppl 4):iv285-291.
13. DeAngelis L, Posner JB. Neurologic complications of cancer. 2nd ed. New York, NY: Oxford University Press; 2008.
14. Chamberlain, M. Leptomeningeal metastasis. Curr Opin Oncol. 2010;22:627-635.
15. Chamberlain M, Johnston S, Van Horn A, Glantz MJ. Recurrent lymphomatous meningitis treated with intra-CSF rituximab and liposomal ara-C. J Neurooncol. 2009;91(3):271-277.
16. Zagouri F, Sergentanis T, Bartsch R, et al. Intrathecal administration of trastuzumab for the treatment of meningeal carcinomatosis in HER2-positive metastatic breast cancer: a systematic review and pooled analysis. Breast Cancer Res Treat. 2013;139(1):13-22.
17. Silk A, Bassetti M, West BT, Tsien C, Lao CD. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med. 2013;2(6):899-906.
18. [Behind paywall.] National Comprehensive Cancer Network. Melanoma (version 2.2016). http://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf. November 10, 2016. Accessed February 28, 2016
19. Hodi F, O’Day S, McDermott D, et al. Improved survival with ipilimumab in patients with metastatic melanoma. NEJM. 2010;363(8):711-723.
20. Margolin K, Ernstoff M, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459-465.
21. Long G, Trefzer U, Davies M, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087-1095.
22. McArthur GA, Maio M, Arance A, et al. Vemurafenib in metastatic melanoma patients with brain metastases: an open-label, single-arm, phase 2, multicenter study. Ann Oncol. 2017;
1. Jemal A, Saraiya M, Patel P, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992-2006. J Am Acad Dermatol. 2011;65(5 Suppl 1):S17.e1-S17.e11.
2. Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma: a study of 216 autopsy cases. Am J Surg. 1978;135(6):807-810.
3. Raizer J, Hwu W, Panageas K, et al. Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro Oncol. 2008;10(2):199-207.
4. Sun L, Song Y, Gong Q. Easily misdiagnosed delayed metastatic intraspinal extradural melanoma of the lumbar spine: a case report and review of the literature. Oncol Lett. 2013;5(6):1799-1802.
5. Moseley R, Davies A, Bourne S, et al. Neoplastic meningitis in malignant melanoma: diagnosis with monoclonal antibiodies. J Neurol Neurosurg Psychiatry. 1989;52:991-886.
6. Schiff D, O’Neill B. Intramedullary spinal cord metastases clinical features and treatment outcome. Neurology. 1996;47(4):906-912.
7. Fife KM, Colman MH, Stevens G, et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol. 2004;22(7):1293-1300.
8. Raizer J, Hwu W, Panageas K, et al. Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro Oncol. 2008;10(2):199-207.
9. Sampson JH, Carter JH Jr, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88:11-20.
10. Abernethy AP. Central nervous system tumors. In: Loprinzi C, ed. ASCO-SEP: Medical Oncology Self-evaluation Program. 4th ed. Alexandria, VA: American Society of Clinical Oncology, 2015. Page 396. Print.
11. Pape E, Desmedt E, Zairi , et al. Leptomeningeal metastasis in melanoma: a prospective clinical study of nine patients. In Vivo. 2012;26(6):1079-1086.
12. Pavlidis N. The diagnostic and therapeutic management of leptomeningeal carcinomatosis. Ann Oncol. 2004;15(Suppl 4):iv285-291.
13. DeAngelis L, Posner JB. Neurologic complications of cancer. 2nd ed. New York, NY: Oxford University Press; 2008.
14. Chamberlain, M. Leptomeningeal metastasis. Curr Opin Oncol. 2010;22:627-635.
15. Chamberlain M, Johnston S, Van Horn A, Glantz MJ. Recurrent lymphomatous meningitis treated with intra-CSF rituximab and liposomal ara-C. J Neurooncol. 2009;91(3):271-277.
16. Zagouri F, Sergentanis T, Bartsch R, et al. Intrathecal administration of trastuzumab for the treatment of meningeal carcinomatosis in HER2-positive metastatic breast cancer: a systematic review and pooled analysis. Breast Cancer Res Treat. 2013;139(1):13-22.
17. Silk A, Bassetti M, West BT, Tsien C, Lao CD. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med. 2013;2(6):899-906.
18. [Behind paywall.] National Comprehensive Cancer Network. Melanoma (version 2.2016). http://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf. November 10, 2016. Accessed February 28, 2016
19. Hodi F, O’Day S, McDermott D, et al. Improved survival with ipilimumab in patients with metastatic melanoma. NEJM. 2010;363(8):711-723.
20. Margolin K, Ernstoff M, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459-465.
21. Long G, Trefzer U, Davies M, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087-1095.
22. McArthur GA, Maio M, Arance A, et al. Vemurafenib in metastatic melanoma patients with brain metastases: an open-label, single-arm, phase 2, multicenter study. Ann Oncol. 2017;
"It's the Pits"
The several-week duration of this 14-year-old boy’s rash is worrisome to him and his family, despite a lack of other symptoms. Located on his right axilla, the rash was previously diagnosed as “yeast infection” but was unaffected by topical anti-yeast medications (nystatin and clotrimazole creams) and oral fluconazole.
A serious family crisis preceded the appearance of the rash: The parents lost custody of their children, who were then placed under the care of grandparents in another state. The boy lost his family and friends and had to start again in a new school.
EXAMINATION
Additional rashes are present on his face, in and behind his ears, and on focal areas of his genitals. They are all orangish red with faintly scaly surfaces. The scale on his face, ears, and genitals is coarser than that of his axilla, but it is the same salmon-pink. Focal areas of his brows and scalp are also involved.
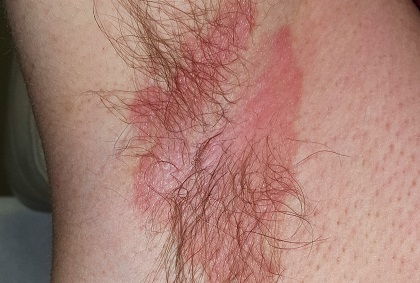
What is the diagnosis?
DISCUSSION
Seborrhea, or seborrheic dermatitis, is most commonly seen on the scalp in the form of dandruff, but it can also flare in other locations. Seborrhea is an adverse inflammatory response to the consumption of sebum by commensal yeast organisms (eg, Pityrosporum) on oil-rich skin. Stress is believed to trigger flares (as exemplified in this case), presumably because it increases the production and outflow of sebum.
Non-dermatology providers often incorrectly diagnose axillary rashes as yeast infections, or seborrhea on the face as fungal infection, simply for lack of a complete differential. The truth is, yeast infections of the skin are unusual. The differential should include psoriasis or eczema, as well as sebopsoriasis (an overlap condition with signs of both seborrhea and psoriasis).
The “trick” to diagnosing seborrhea is to recognize that it is incredibly common (affecting around 30% of the Caucasian population), is often inherited, and can affect multiple sites. When it is seen in one area, corroboration can be sought by locating typical changes elsewhere.
Although there is no cure, control is obtained through use of topical anti-yeast creams (eg, ketoconazole) combined with a mild topical steroid (eg, 2.5% hydrocortisone). Using ketoconazole 2% shampoo for the scalp and other affected areas can be helpful as well. Use of nystatin, however, has long been replaced by more effective alternatives.
With a bit of luck, the patient’s stress will diminish over time, which should be a big help in resolving this problem.
TAKE-HOME LEARNING POINTS
- Seborrhea, or seborrheic dermatitis, is extremely common among those of northern European descent and can affect not only the scalp, but also the face, ears, chest, axillae, and genitals.
- The rash is usually orangish pink and slightly scaly, unless it’s in the axillae, where moisture and friction preclude the formation of significant scale.
- Seborrhea is often misdiagnosed as yeast infection, but the latter is quite unusual on the skin.
- The differential should include psoriasis, eczema, and sebopsoriasis (an overlap condition with signs of both seborrhea and psoriasis).
The several-week duration of this 14-year-old boy’s rash is worrisome to him and his family, despite a lack of other symptoms. Located on his right axilla, the rash was previously diagnosed as “yeast infection” but was unaffected by topical anti-yeast medications (nystatin and clotrimazole creams) and oral fluconazole.
A serious family crisis preceded the appearance of the rash: The parents lost custody of their children, who were then placed under the care of grandparents in another state. The boy lost his family and friends and had to start again in a new school.
EXAMINATION
Additional rashes are present on his face, in and behind his ears, and on focal areas of his genitals. They are all orangish red with faintly scaly surfaces. The scale on his face, ears, and genitals is coarser than that of his axilla, but it is the same salmon-pink. Focal areas of his brows and scalp are also involved.

What is the diagnosis?
DISCUSSION
Seborrhea, or seborrheic dermatitis, is most commonly seen on the scalp in the form of dandruff, but it can also flare in other locations. Seborrhea is an adverse inflammatory response to the consumption of sebum by commensal yeast organisms (eg, Pityrosporum) on oil-rich skin. Stress is believed to trigger flares (as exemplified in this case), presumably because it increases the production and outflow of sebum.
Non-dermatology providers often incorrectly diagnose axillary rashes as yeast infections, or seborrhea on the face as fungal infection, simply for lack of a complete differential. The truth is, yeast infections of the skin are unusual. The differential should include psoriasis or eczema, as well as sebopsoriasis (an overlap condition with signs of both seborrhea and psoriasis).
The “trick” to diagnosing seborrhea is to recognize that it is incredibly common (affecting around 30% of the Caucasian population), is often inherited, and can affect multiple sites. When it is seen in one area, corroboration can be sought by locating typical changes elsewhere.
Although there is no cure, control is obtained through use of topical anti-yeast creams (eg, ketoconazole) combined with a mild topical steroid (eg, 2.5% hydrocortisone). Using ketoconazole 2% shampoo for the scalp and other affected areas can be helpful as well. Use of nystatin, however, has long been replaced by more effective alternatives.
With a bit of luck, the patient’s stress will diminish over time, which should be a big help in resolving this problem.
TAKE-HOME LEARNING POINTS
- Seborrhea, or seborrheic dermatitis, is extremely common among those of northern European descent and can affect not only the scalp, but also the face, ears, chest, axillae, and genitals.
- The rash is usually orangish pink and slightly scaly, unless it’s in the axillae, where moisture and friction preclude the formation of significant scale.
- Seborrhea is often misdiagnosed as yeast infection, but the latter is quite unusual on the skin.
- The differential should include psoriasis, eczema, and sebopsoriasis (an overlap condition with signs of both seborrhea and psoriasis).
The several-week duration of this 14-year-old boy’s rash is worrisome to him and his family, despite a lack of other symptoms. Located on his right axilla, the rash was previously diagnosed as “yeast infection” but was unaffected by topical anti-yeast medications (nystatin and clotrimazole creams) and oral fluconazole.
A serious family crisis preceded the appearance of the rash: The parents lost custody of their children, who were then placed under the care of grandparents in another state. The boy lost his family and friends and had to start again in a new school.
EXAMINATION
Additional rashes are present on his face, in and behind his ears, and on focal areas of his genitals. They are all orangish red with faintly scaly surfaces. The scale on his face, ears, and genitals is coarser than that of his axilla, but it is the same salmon-pink. Focal areas of his brows and scalp are also involved.

What is the diagnosis?
DISCUSSION
Seborrhea, or seborrheic dermatitis, is most commonly seen on the scalp in the form of dandruff, but it can also flare in other locations. Seborrhea is an adverse inflammatory response to the consumption of sebum by commensal yeast organisms (eg, Pityrosporum) on oil-rich skin. Stress is believed to trigger flares (as exemplified in this case), presumably because it increases the production and outflow of sebum.
Non-dermatology providers often incorrectly diagnose axillary rashes as yeast infections, or seborrhea on the face as fungal infection, simply for lack of a complete differential. The truth is, yeast infections of the skin are unusual. The differential should include psoriasis or eczema, as well as sebopsoriasis (an overlap condition with signs of both seborrhea and psoriasis).
The “trick” to diagnosing seborrhea is to recognize that it is incredibly common (affecting around 30% of the Caucasian population), is often inherited, and can affect multiple sites. When it is seen in one area, corroboration can be sought by locating typical changes elsewhere.
Although there is no cure, control is obtained through use of topical anti-yeast creams (eg, ketoconazole) combined with a mild topical steroid (eg, 2.5% hydrocortisone). Using ketoconazole 2% shampoo for the scalp and other affected areas can be helpful as well. Use of nystatin, however, has long been replaced by more effective alternatives.
With a bit of luck, the patient’s stress will diminish over time, which should be a big help in resolving this problem.
TAKE-HOME LEARNING POINTS
- Seborrhea, or seborrheic dermatitis, is extremely common among those of northern European descent and can affect not only the scalp, but also the face, ears, chest, axillae, and genitals.
- The rash is usually orangish pink and slightly scaly, unless it’s in the axillae, where moisture and friction preclude the formation of significant scale.
- Seborrhea is often misdiagnosed as yeast infection, but the latter is quite unusual on the skin.
- The differential should include psoriasis, eczema, and sebopsoriasis (an overlap condition with signs of both seborrhea and psoriasis).
Hyperpigmented Patch on the Leg
The Diagnosis: Lichen Aureus
The clinicopathological findings were diagnostic of lichen aureus (LA). Microscopic examination revealed a relatively sparse, superficial, perivascular and interstitial lymphohistiocytic infiltrate with scattered siderophages in the upper dermis. Extravasation of red blood cells also was noted (Figure 1). An immunohistochemical stain for Melan-A highlighted a normal number and distribution of single melanocytes at the dermoepidermal junction with no evidence of pagetoid scatter. A Perls Prussian blue stain for iron demonstrated abundant hemosiderin in the dermis (Figure 2).
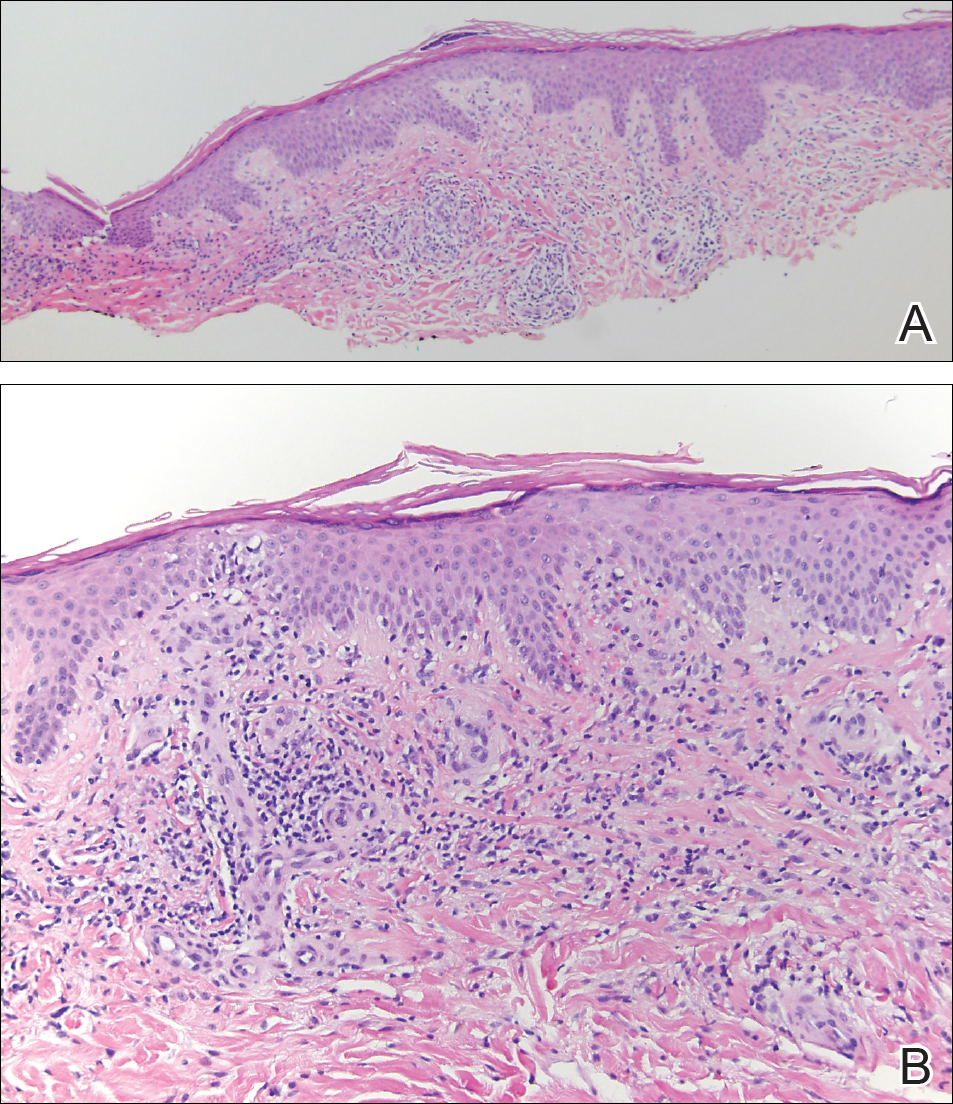
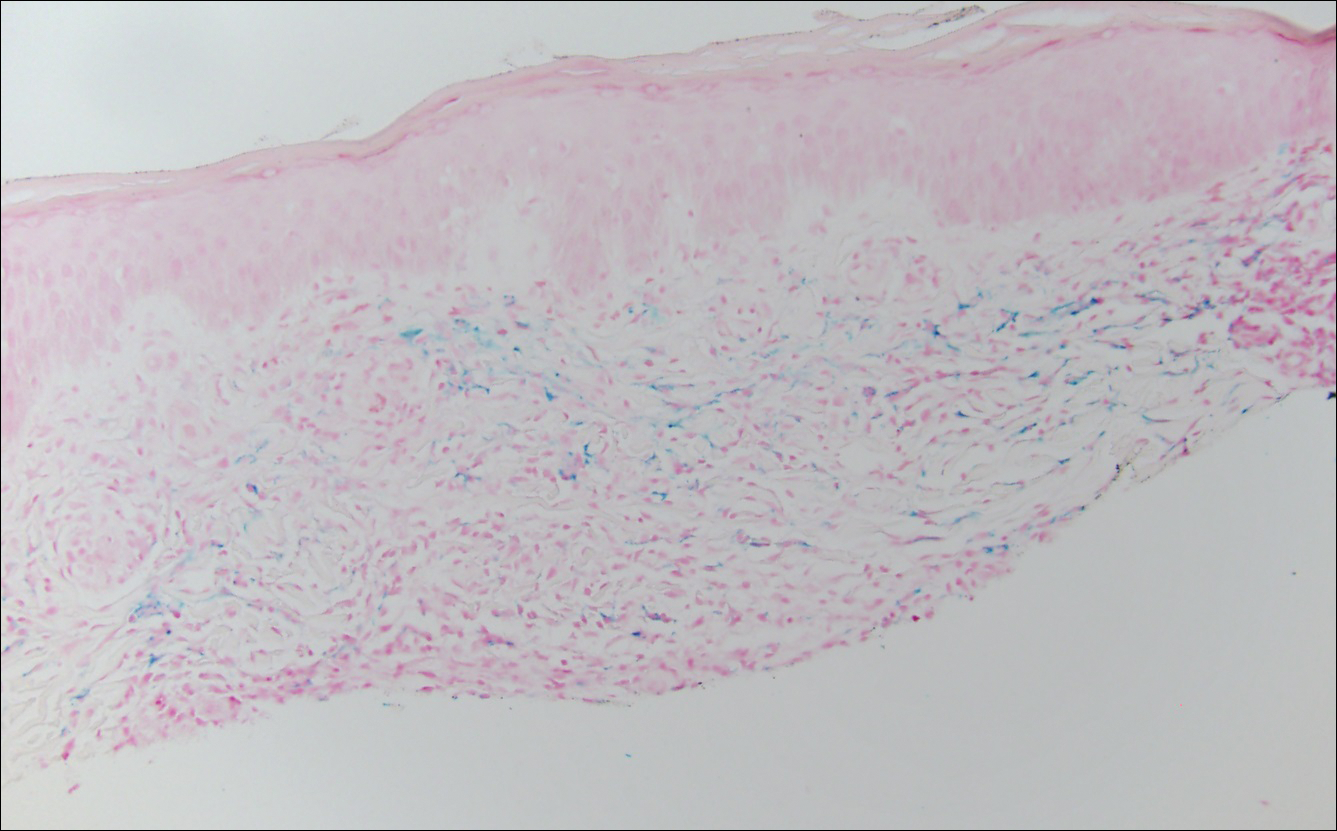
Pigmented purpuric dermatosis (PPD) describes a group of cutaneous lesions that are characterized by petechiae and pigmentary changes. These lesions most commonly present on the lower limbs; however, other sites have been reported.1 This group includes several major clinical forms such as Schamberg disease, LA, purpura annularis telangiectodes of Majocchi, eczematidlike purpura of Doucas and Kapetanakis, and lichenoid PPD of Gougerot and Blum. Lesions typically demonstrate a striking golden brown color clinically and by definition occur in the absence of platelet defects or vasculitis.1
Factors implicated in the pathogenesis of pigmented purpura include gravitational dependency, venous stasis, infection, and drugs.2 It is suggested that cellular immunity may play a role in the development of the disease based on the presence of CD4+ T lymphocytes in the infiltrate and the expression of HLA-DR by these lymphocytes and the keratinocytes.3 Lichen aureus differs in that it relates to increased intravascular pressure from an incompetent valve in an underlying perforating vein.4
Lichen aureus, also referred to as lichen purpuricus, is one major variant of PPD. The name reflects both the characteristic golden brown color and the histopathologic pattern of inflammation.1 Lichen aureus usually presents as a unilateral, asymptomatic, confined single lesion located mainly on the leg,1 though it can develop at other sites or as a localized group of lesions. Extensive lesions have been reported5 and cases with a segmental distribution have been described.6 In contrast, Schamberg disease demonstrates pinhead-sized reddish lesions giving the characteristic cayenne pepper pigmentation. These lesions coalesce to form thumbprint patches that progress proximally.1 Majocchi purpura is annular and telangiectatic, while lichenoid purpura of Gougerot and Blum presents with flat-topped, polygonal, violaceous papules that turn brown over time.
Some authors have championed a role for dermoscopy in diagnosis of LA.7 By dermoscopy, LA demonstrates a diffuse copper background reflecting the lymphohistiocytic dermal infiltrate, red dots and globules representing the extravasated red blood cells and the dilated swollen vessels, and grey dots that reflect the hemosiderin present in the dermis.8
Histologically, LA demonstrates a superficial perivascular infiltrate composed mainly of CD4+ lymphocytes surrounding the superficial capillaries. Over time, red cell extravasation leads to the formation of hemosiderin-laden macrophages, which can be highlighted with Perls Prussian blue stain. A bandlike infiltrate with thin strands of collagen separating it from the epidermis also may be noted.9
An important consideration in the differential diagnosis of PPD is mycosis fungoides (MF). Mycosis fungoides is a cutaneous T-cell lymphoma that clinically presents as a single or multiple hypopigmented or hyperpigmented patches or as erythematous scaly lesions in the patch or plaque stage. These lesions eventually may evolve into tumor stage.10 Mycosis fungoides may mimic PPD clinically and/or histopathologically, and rarely PPD also may precede MF.11 Involvement of the trunk, especially the lower abdomen and buttock region, favors a diagnosis of MF. Typically, histopathologic examination of MF demonstrates an epidermotropic lymphocytic infiltrate composed of atypical cerebriform lymphocytes overlying papillary dermal fibrosis. Although classic MF would be difficult to confuse with PPD, the atrophic lichenoid pattern of MF may show remarkable overlap with PPD.12 Such cases require clinicopathologic correlation, immunophenotyping of the epidermotropic lymphocytes, and occasionally T-cell clonality studies.
Lichen aureus is a chronic persistent disease unless the underlying incompetent perforator vessel is ligated. Various treatments have been used for other forms of pigmented purpura including topical corticosteroids, topical tacrolimus, systemic vasodilators such as prostacyclin and pentoxifylline, and phototherapy.1 Clinical follow-up is recommended for lesions that show some clinical or histopathological overlap with MF. Additional biopsies also may prove useful in establishing a definitive diagnosis in ambiguous cases.
- Sardana K, Sarkar R, Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482-488.
- Newton RC, Raimer SS. Pigmented purpuric eruptions. Dermatol Clin. 1985;3:165-169.
- Aiba S, Tagami H. Immunohistologic studies in Schamberg's disease. evidence for cellular immune reaction in lesional skin. Arch Dermatol. 1988;124:1058-1062.
- English J. Lichen aureus. J Am Acad Dermatol. 1985;12(2, pt 1):377-379.
- Duhra P, Tan CY. Lichen aureus. Br J Dermatol. 1986;114:395.
- Moche J, Glassman S, Modi D, et al. Segmental lichen aureus: a report of two cases treated with methylprednisolone aceponate. Australas J Dermatol. 2011;52:E15-E18.
- Zaballos P, Puig S, Malvehy J. Dermoscopy of pigmented purpuric dermatoses (lichen aureus): a useful tool for clinical diagnosis. Arch Dermatol. 2004;140:1290-1291.
- Portela PS, Melo DF, Ormiga P, et al. Dermoscopy of lichen aureus. An Bras Dermatol. 2013;88:253-255.
- Smoller BR, Kamel OW. Pigmented purpuric eruptions: immunopathologic studies supportive of a common immunophenotype. J Cutan Pathol. 1991;18:423-427.
- Jaffe ES, Harris NL, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. a progress report. Am J Clin Pathol. 1999;111(1 suppl 1):S8-S12.
- Hanna S, Walsh N, D'Intino Y, et al. Mycosis fungoides presenting as pigmented purpuric dermatitis. Pediatr Dermatol. 2006;23:350-354.
- Toro JR, Sander CA, LeBoit PE. Persistent pigmented purpuric dermatitis and mycosis fungoides: simulant, precursor, or both? a study by light microscopy and molecular methods. Am J Dermatopathol. 1997;19:108-118.
The Diagnosis: Lichen Aureus
The clinicopathological findings were diagnostic of lichen aureus (LA). Microscopic examination revealed a relatively sparse, superficial, perivascular and interstitial lymphohistiocytic infiltrate with scattered siderophages in the upper dermis. Extravasation of red blood cells also was noted (Figure 1). An immunohistochemical stain for Melan-A highlighted a normal number and distribution of single melanocytes at the dermoepidermal junction with no evidence of pagetoid scatter. A Perls Prussian blue stain for iron demonstrated abundant hemosiderin in the dermis (Figure 2).


Pigmented purpuric dermatosis (PPD) describes a group of cutaneous lesions that are characterized by petechiae and pigmentary changes. These lesions most commonly present on the lower limbs; however, other sites have been reported.1 This group includes several major clinical forms such as Schamberg disease, LA, purpura annularis telangiectodes of Majocchi, eczematidlike purpura of Doucas and Kapetanakis, and lichenoid PPD of Gougerot and Blum. Lesions typically demonstrate a striking golden brown color clinically and by definition occur in the absence of platelet defects or vasculitis.1
Factors implicated in the pathogenesis of pigmented purpura include gravitational dependency, venous stasis, infection, and drugs.2 It is suggested that cellular immunity may play a role in the development of the disease based on the presence of CD4+ T lymphocytes in the infiltrate and the expression of HLA-DR by these lymphocytes and the keratinocytes.3 Lichen aureus differs in that it relates to increased intravascular pressure from an incompetent valve in an underlying perforating vein.4
Lichen aureus, also referred to as lichen purpuricus, is one major variant of PPD. The name reflects both the characteristic golden brown color and the histopathologic pattern of inflammation.1 Lichen aureus usually presents as a unilateral, asymptomatic, confined single lesion located mainly on the leg,1 though it can develop at other sites or as a localized group of lesions. Extensive lesions have been reported5 and cases with a segmental distribution have been described.6 In contrast, Schamberg disease demonstrates pinhead-sized reddish lesions giving the characteristic cayenne pepper pigmentation. These lesions coalesce to form thumbprint patches that progress proximally.1 Majocchi purpura is annular and telangiectatic, while lichenoid purpura of Gougerot and Blum presents with flat-topped, polygonal, violaceous papules that turn brown over time.
Some authors have championed a role for dermoscopy in diagnosis of LA.7 By dermoscopy, LA demonstrates a diffuse copper background reflecting the lymphohistiocytic dermal infiltrate, red dots and globules representing the extravasated red blood cells and the dilated swollen vessels, and grey dots that reflect the hemosiderin present in the dermis.8
Histologically, LA demonstrates a superficial perivascular infiltrate composed mainly of CD4+ lymphocytes surrounding the superficial capillaries. Over time, red cell extravasation leads to the formation of hemosiderin-laden macrophages, which can be highlighted with Perls Prussian blue stain. A bandlike infiltrate with thin strands of collagen separating it from the epidermis also may be noted.9
An important consideration in the differential diagnosis of PPD is mycosis fungoides (MF). Mycosis fungoides is a cutaneous T-cell lymphoma that clinically presents as a single or multiple hypopigmented or hyperpigmented patches or as erythematous scaly lesions in the patch or plaque stage. These lesions eventually may evolve into tumor stage.10 Mycosis fungoides may mimic PPD clinically and/or histopathologically, and rarely PPD also may precede MF.11 Involvement of the trunk, especially the lower abdomen and buttock region, favors a diagnosis of MF. Typically, histopathologic examination of MF demonstrates an epidermotropic lymphocytic infiltrate composed of atypical cerebriform lymphocytes overlying papillary dermal fibrosis. Although classic MF would be difficult to confuse with PPD, the atrophic lichenoid pattern of MF may show remarkable overlap with PPD.12 Such cases require clinicopathologic correlation, immunophenotyping of the epidermotropic lymphocytes, and occasionally T-cell clonality studies.
Lichen aureus is a chronic persistent disease unless the underlying incompetent perforator vessel is ligated. Various treatments have been used for other forms of pigmented purpura including topical corticosteroids, topical tacrolimus, systemic vasodilators such as prostacyclin and pentoxifylline, and phototherapy.1 Clinical follow-up is recommended for lesions that show some clinical or histopathological overlap with MF. Additional biopsies also may prove useful in establishing a definitive diagnosis in ambiguous cases.
The Diagnosis: Lichen Aureus
The clinicopathological findings were diagnostic of lichen aureus (LA). Microscopic examination revealed a relatively sparse, superficial, perivascular and interstitial lymphohistiocytic infiltrate with scattered siderophages in the upper dermis. Extravasation of red blood cells also was noted (Figure 1). An immunohistochemical stain for Melan-A highlighted a normal number and distribution of single melanocytes at the dermoepidermal junction with no evidence of pagetoid scatter. A Perls Prussian blue stain for iron demonstrated abundant hemosiderin in the dermis (Figure 2).


Pigmented purpuric dermatosis (PPD) describes a group of cutaneous lesions that are characterized by petechiae and pigmentary changes. These lesions most commonly present on the lower limbs; however, other sites have been reported.1 This group includes several major clinical forms such as Schamberg disease, LA, purpura annularis telangiectodes of Majocchi, eczematidlike purpura of Doucas and Kapetanakis, and lichenoid PPD of Gougerot and Blum. Lesions typically demonstrate a striking golden brown color clinically and by definition occur in the absence of platelet defects or vasculitis.1
Factors implicated in the pathogenesis of pigmented purpura include gravitational dependency, venous stasis, infection, and drugs.2 It is suggested that cellular immunity may play a role in the development of the disease based on the presence of CD4+ T lymphocytes in the infiltrate and the expression of HLA-DR by these lymphocytes and the keratinocytes.3 Lichen aureus differs in that it relates to increased intravascular pressure from an incompetent valve in an underlying perforating vein.4
Lichen aureus, also referred to as lichen purpuricus, is one major variant of PPD. The name reflects both the characteristic golden brown color and the histopathologic pattern of inflammation.1 Lichen aureus usually presents as a unilateral, asymptomatic, confined single lesion located mainly on the leg,1 though it can develop at other sites or as a localized group of lesions. Extensive lesions have been reported5 and cases with a segmental distribution have been described.6 In contrast, Schamberg disease demonstrates pinhead-sized reddish lesions giving the characteristic cayenne pepper pigmentation. These lesions coalesce to form thumbprint patches that progress proximally.1 Majocchi purpura is annular and telangiectatic, while lichenoid purpura of Gougerot and Blum presents with flat-topped, polygonal, violaceous papules that turn brown over time.
Some authors have championed a role for dermoscopy in diagnosis of LA.7 By dermoscopy, LA demonstrates a diffuse copper background reflecting the lymphohistiocytic dermal infiltrate, red dots and globules representing the extravasated red blood cells and the dilated swollen vessels, and grey dots that reflect the hemosiderin present in the dermis.8
Histologically, LA demonstrates a superficial perivascular infiltrate composed mainly of CD4+ lymphocytes surrounding the superficial capillaries. Over time, red cell extravasation leads to the formation of hemosiderin-laden macrophages, which can be highlighted with Perls Prussian blue stain. A bandlike infiltrate with thin strands of collagen separating it from the epidermis also may be noted.9
An important consideration in the differential diagnosis of PPD is mycosis fungoides (MF). Mycosis fungoides is a cutaneous T-cell lymphoma that clinically presents as a single or multiple hypopigmented or hyperpigmented patches or as erythematous scaly lesions in the patch or plaque stage. These lesions eventually may evolve into tumor stage.10 Mycosis fungoides may mimic PPD clinically and/or histopathologically, and rarely PPD also may precede MF.11 Involvement of the trunk, especially the lower abdomen and buttock region, favors a diagnosis of MF. Typically, histopathologic examination of MF demonstrates an epidermotropic lymphocytic infiltrate composed of atypical cerebriform lymphocytes overlying papillary dermal fibrosis. Although classic MF would be difficult to confuse with PPD, the atrophic lichenoid pattern of MF may show remarkable overlap with PPD.12 Such cases require clinicopathologic correlation, immunophenotyping of the epidermotropic lymphocytes, and occasionally T-cell clonality studies.
Lichen aureus is a chronic persistent disease unless the underlying incompetent perforator vessel is ligated. Various treatments have been used for other forms of pigmented purpura including topical corticosteroids, topical tacrolimus, systemic vasodilators such as prostacyclin and pentoxifylline, and phototherapy.1 Clinical follow-up is recommended for lesions that show some clinical or histopathological overlap with MF. Additional biopsies also may prove useful in establishing a definitive diagnosis in ambiguous cases.
- Sardana K, Sarkar R, Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482-488.
- Newton RC, Raimer SS. Pigmented purpuric eruptions. Dermatol Clin. 1985;3:165-169.
- Aiba S, Tagami H. Immunohistologic studies in Schamberg's disease. evidence for cellular immune reaction in lesional skin. Arch Dermatol. 1988;124:1058-1062.
- English J. Lichen aureus. J Am Acad Dermatol. 1985;12(2, pt 1):377-379.
- Duhra P, Tan CY. Lichen aureus. Br J Dermatol. 1986;114:395.
- Moche J, Glassman S, Modi D, et al. Segmental lichen aureus: a report of two cases treated with methylprednisolone aceponate. Australas J Dermatol. 2011;52:E15-E18.
- Zaballos P, Puig S, Malvehy J. Dermoscopy of pigmented purpuric dermatoses (lichen aureus): a useful tool for clinical diagnosis. Arch Dermatol. 2004;140:1290-1291.
- Portela PS, Melo DF, Ormiga P, et al. Dermoscopy of lichen aureus. An Bras Dermatol. 2013;88:253-255.
- Smoller BR, Kamel OW. Pigmented purpuric eruptions: immunopathologic studies supportive of a common immunophenotype. J Cutan Pathol. 1991;18:423-427.
- Jaffe ES, Harris NL, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. a progress report. Am J Clin Pathol. 1999;111(1 suppl 1):S8-S12.
- Hanna S, Walsh N, D'Intino Y, et al. Mycosis fungoides presenting as pigmented purpuric dermatitis. Pediatr Dermatol. 2006;23:350-354.
- Toro JR, Sander CA, LeBoit PE. Persistent pigmented purpuric dermatitis and mycosis fungoides: simulant, precursor, or both? a study by light microscopy and molecular methods. Am J Dermatopathol. 1997;19:108-118.
- Sardana K, Sarkar R, Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482-488.
- Newton RC, Raimer SS. Pigmented purpuric eruptions. Dermatol Clin. 1985;3:165-169.
- Aiba S, Tagami H. Immunohistologic studies in Schamberg's disease. evidence for cellular immune reaction in lesional skin. Arch Dermatol. 1988;124:1058-1062.
- English J. Lichen aureus. J Am Acad Dermatol. 1985;12(2, pt 1):377-379.
- Duhra P, Tan CY. Lichen aureus. Br J Dermatol. 1986;114:395.
- Moche J, Glassman S, Modi D, et al. Segmental lichen aureus: a report of two cases treated with methylprednisolone aceponate. Australas J Dermatol. 2011;52:E15-E18.
- Zaballos P, Puig S, Malvehy J. Dermoscopy of pigmented purpuric dermatoses (lichen aureus): a useful tool for clinical diagnosis. Arch Dermatol. 2004;140:1290-1291.
- Portela PS, Melo DF, Ormiga P, et al. Dermoscopy of lichen aureus. An Bras Dermatol. 2013;88:253-255.
- Smoller BR, Kamel OW. Pigmented purpuric eruptions: immunopathologic studies supportive of a common immunophenotype. J Cutan Pathol. 1991;18:423-427.
- Jaffe ES, Harris NL, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. a progress report. Am J Clin Pathol. 1999;111(1 suppl 1):S8-S12.
- Hanna S, Walsh N, D'Intino Y, et al. Mycosis fungoides presenting as pigmented purpuric dermatitis. Pediatr Dermatol. 2006;23:350-354.
- Toro JR, Sander CA, LeBoit PE. Persistent pigmented purpuric dermatitis and mycosis fungoides: simulant, precursor, or both? a study by light microscopy and molecular methods. Am J Dermatopathol. 1997;19:108-118.
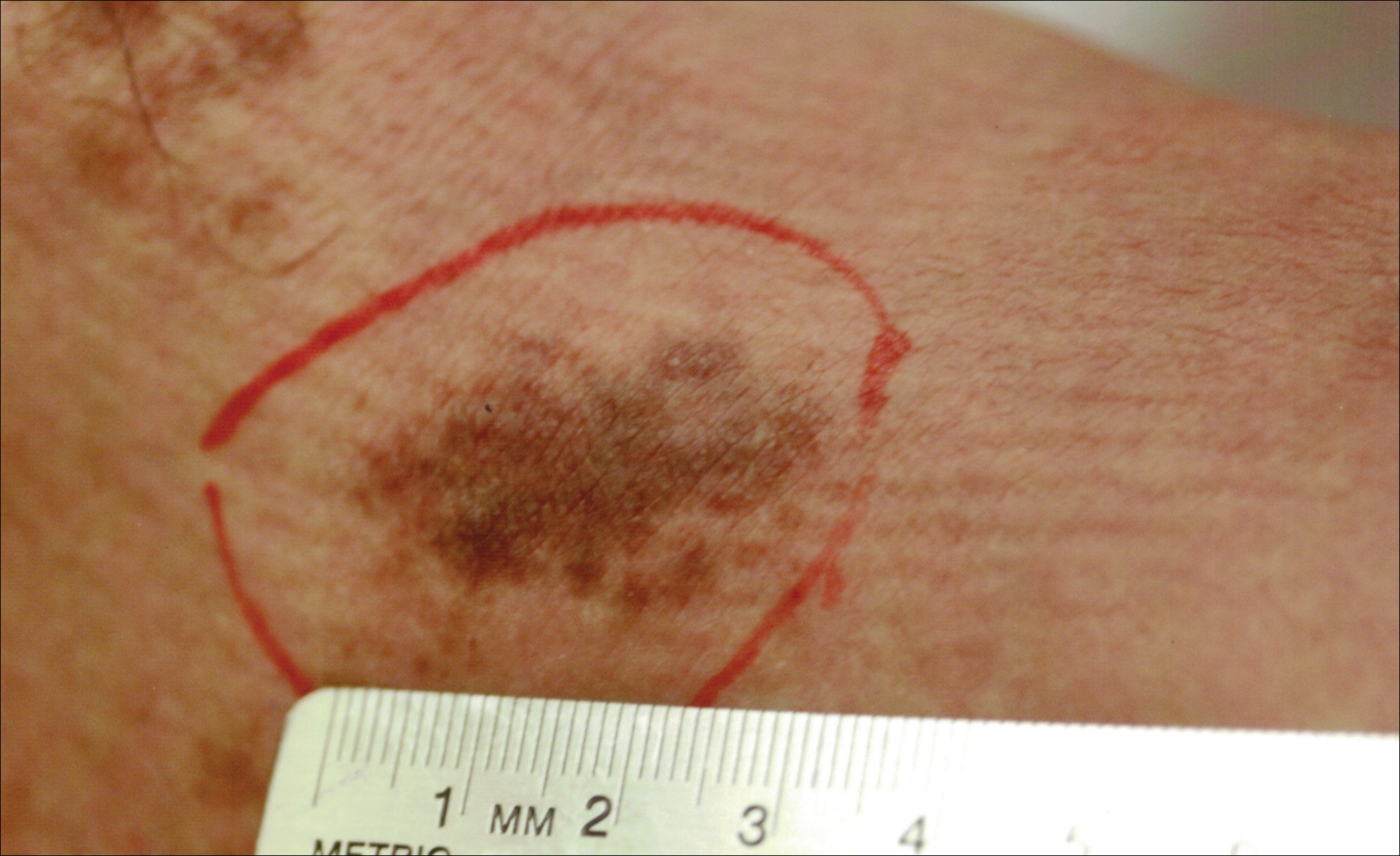
A 32-year-old man presented with an asymptomatic pigmented lesion on the left foot that developed over the course of 4 months. Physical examination revealed a 4-cm asymmetrical, deeply pigmented macule on the left foot. A shave biopsy of the lesion was performed.
How hospitalists can help reduce readmissions
Hospital readmissions are frequent, harmful, and costly. Consider the fact that 18% of Medicare patients can expect to be readmitted within 30 days at a cost of more than $17 billion.1 Recent changes in health care policy aimed at reducing readmission have substantially increased attention to this major health care issue.2
The Affordable Care Act has mandated that the Centers for Medicare & Medicaid Services reduce payment to hospitals with higher-than-expected 30-day readmissions, with its Hospital Readmissions Reduction Program. This has driven rapid growth in the study of patients rehospitalized within 30 days of discharge.3 So what are some strategies that have either been proven to reduce readmissions or show promise in doing so?

An ounce of prevention
In studying inpatient and outpatient physicians’ perspectives regarding factors contributing to readmission,4 Shoshana Herzig, MD, MPH, assistant professor of medicine, Harvard Medical School, and director of Hospital Medicine Research, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, and her colleagues identified some potential preventive strategies.
The most commonly endorsed strategy to prevent readmissions by both primary care physicians and hospitalists surveyed involved improving self-management plans at discharge. “This refers to actions such as providing patient-centered discharge instructions (that is, making sure they are written in language that patients can understand) or asking transition coaches to help facilitate a successful transition,” Dr. Herzig said. “This finding is consistent with the fact that the factor most commonly identified as contributing to readmissions was insufficient patient understanding or ability to self-manage. Combined, these findings suggest that strategies to enhance patient understanding of their illness, care plan, and what to expect after hospital discharge, are likely to be important components of successful readmission reduction programs.”
Provisioning of resources to patients to help them manage their care after discharge is also recommended. For example, engaging nurses or pharmacists who can help with issues that arise after discharge may help keep patients out of the hospital.
“Hospitalists should be aware of what resources are available to help patients manage their care,” Dr. Herzig said. For example, if a patient needs periodic blood pressure monitoring, the hospitalist can tell the patient about free blood pressure checkpoints or suggest a home-automated blood pressure monitor.
The study also showed that improved coordination of care between inpatient and outpatient providers, such as sharing medical records, could reduce readmission rates. “This allows for better inpatient care and increased ability for primary care physicians to react appropriately to issues arising after discharge,” Dr. Herzig said. “In the absence of a shared system, hospitalists should complete discharge summaries in a timely fashion and ensure that they’re promptly transmitted to primary care physicians.”
Dr. Herzig said it’s important to note that hospitalists and primary care physicians had different appraisals of reasons for readmission. Therefore, when designing readmission reduction programs or determining specific services to prevent a readmission for a given patient, it is important for hospitalists to obtain input from primary care physicians to ensure that they address all of the potential contributors to readmission for a given patient.
Interviewing patients regarding readmissions
After involved clinicians and independent physician reviewers performed extensive case reviews of more than 700 readmitted patients,6 Ashley Busuttil, MD, FHM, associate section chief, Hospital Medicine, University of California, Los Angeles Department of Medicine; and executive medical director, Medicine Services, UCLA Department of Medicine, and Erin Dowling, MD, assistant clinical professor, General Internal Medicine, Hospitalist Services, UCLA Medical Center, Santa Monica, Calif., and their colleagues were unable to identify which readmissions could have easily been prevented, and found that readmission causality varied extensively.
Through interviews with patients, the researchers determined that patients were more likely to think that their readmission was preventable if they felt unready for discharge during their initial hospitalization. This was despite the fact that patients met what clinicians would consider “ready” by objective, provider-centric criteria: they were medically stable, they had in-home support services, they had follow-up arranged, and so forth. As such, they wanted to put effort into educating and preparing patients for what home will look and feel like posthospitalization to address their feelings of unreadiness.
To that end, the researchers created an enhanced transition initiative that included showing an educational video near the time of admission and a patient-centered discharge checklist to help patients identify questions they might have after discharge. The discharge checklist asks patients to put themselves in the position of being at home and working through scenarios they may face so they will know how to deal with them. For example, if you have pain, who should you call? What should you do if you run out of medication?
Dr. Dowling believes that the hospitalist will, over time, become essential to assessing patient readiness. “As we learn more about how patients approach discharge, hospitalists’ understanding of patient needs beyond straightforward medical care will be crucial to having smoother transitions of care,” she said.
The researchers also explored pain control. As a health system, UCLA Medical Center has formed a multidisciplinary task force to optimize its approach to pain control. “If we can address comfort – for both patients at high risk of readmission and those that aren’t – we hope we can improve symptom control overall,” Dr. Busuttil said. “It’s not uncommon for patients to feel inadequate symptom control at discharge. While this is likely only one component of all the readmission pieces, a patient who feels that their symptoms are not controlled is likely to feel less ready for discharge. Increasing patient readiness, perhaps by increasing symptom control and improving communication regarding symptom management expectations, is a task that the hospitalist is well positioned to address.”
The researchers also wanted to find out why patients may not use available outpatient resources, and assessed them for decisional conflict – a measure of certainty with decision making – when selecting from multiple options for accessing medical care if they were home postdischarge and began to feel ill again. “Patients with decisional conflict were more likely to state that they would go the emergency room rather than call their primary medical physician or visit an urgent care center,” Dr. Busuttil said.
The health system continues to screen patients for decisional conflict. “When positive, we provide bedside education on when to seek medical care through primary care, urgent care, or the emergency department,” Dr. Busuttil said. “We also provide patients with information on how to access each of these resources.”
While a prior discharge plan may have seemed ideal on paper, time and time again it’s not logistically possible for certain patients. “By having this knowledge gleaned from patient interviews, hospitalists are able to provide feedback to health systems regarding different options of outpatient care that may work for the different patient populations they serve,” Dr. Dowling said.
To understand why one particular patient population is being readmitted requires taking the time to understand that population, Dr. Dowling noted. “While many validated risk stratification tools are available, they may only serve as general guides,” she said. “To impact the population you serve, you must first understand the readmission process as it looks to them.”
Employing the HOSPITAL score
In another effort to reduce hospital readmissions, Jacques Donzé, MD, MSc, associate physician, Bern University Hospital, Switzerland, and research associate, Brigham and Women’s Hospital, Boston, and his colleagues used the HOSPITAL score to identify patients at high risk of 30-day potentially avoidable readmission.
To most efficiently reduce hospital readmissions, hospitals need to target complex and intensive discharge interventions for patients at high risk of potentially avoidable readmission who are more likely to benefit.2 “However, prior research indicates that clinical health care providers are not able to accurately identify which patients are at high risk for readmission,” Dr. Donzé said.
Dr. Donzé believes that several factors may influence the performance of a prediction model, such as the initial selection of the potential predictors, the quality of the derivation method, including readily available predictors commonly available, and including reliable factors that aren’t subject to subjective evaluation. “All of these factors can play a role in the performance and generalizability of the HOSPITAL score,” he said.
When a patient is identified as high risk to be readmitted, hospitalists can take certain actions to prevent readmission. “Interventions are more likely to be effective when they include several components,” Dr. Donzé said. “These include follow-up phone calls and/or home visits, review of the patient’s medication list, patient education, and sending a discharge summary to the patient’s primary care physician in a timely manner. For now, enough evidence for a specific effective multimodal intervention to be generalizable to the majority of patients is lacking.”
Currently, the HOSPITAL score has been validated in approximately 180,000 patients in 14 hospitals across five countries and three continents – always showing good performance and generalizability. The HOSPITAL score includes seven variables readily available before hospital discharge, is easy to use, and is the most widely validated prediction model for readmission, Dr. Donzé said.
Before being implemented into practice, a score should ideally reach the highest level of validation, that is, show its clinical impact. “We expect that the score will not only be able to accurately predict high-risk patients, but using the score will also impact patient care by reducing readmissions when coupled with an appropriate intervention,” Dr. Donzé said.
In summary, research has shown that a variety of methods can be used to reduce hospital readmissions, including studying inpatient and outpatient physicians’ perspectives regarding factors contributing to readmission; interviewing patients regarding readmissions; and identifying patients at high risk of readmission using the HOSPITAL score.
Many researchers are continuing their studies in these areas.
Karen Appold is a medical writer in Pennsylvania.
Using hospitalist reflections as a means to reduce readmissions
Readmission studies and the development of readmission scoring systems and prediction tools rely on data from a large number of patients, typically extracted from administrative databases.
To complement this data, Deanne Kashiwagi, MD, consultant, Hospital Internal Medicine, Mayo Clinic, Rochester, Minn., and her colleagues asked hospitalists to reflect upon the readmissions of patients for whom they cared to add insight into the culture of patient care transitions within the health system.
“We felt there was some value in considering these nuances of the local care environment, which may not be represented in studies drawing from large databases, as potential targets for readmission efforts,” she said.
Dr. Kashiwagi believes that including elements of local practice and culture was the strength of their work. “Groups interested in replicating this reflective process should consider including factors specific to their practices that may contribute to readmission,” she said.
Asking hospitalists to perform reviews has led to implementing changes. Physicians were prompted to schedule earlier follow-up appointments and nurse practitioners and physician assistants have worked to improve the quality of their discharge summaries. The exercise also engaged hospitalists to suggest system changes that might contribute to decreased readmissions, such as a geriatrician-run service (which was recently begun) to provide multidisciplinary acute geriatric care for hospitalized older adults.
“Although large-scale studies are clearly important, readmission review at a more granular level may have merit as well,” Dr. Kashiwagi said, noting that such reviews identify local practice factors that groups may quickly act upon to help decrease readmissions. “Hospitalists readily engaged in this reflective exercise, which yielded actionable information to decrease readmissions.”
In commenting on why a different similar study7 didn’t mimic the results of Mayo Clinic’s study, Dr. Kashiwagi said there were some differences in methodology that may explain the difference in readmission rates. “First, this group excluded patients on dialysis, which in our study was a common comorbidity of our readmitted patients,” she said. “It is also notable that the chart review tool was different. Perhaps there is less representation of local factors, unique to that hospitalist group and their practice culture, than on our review form. These investigators also discussed their readmissions at routine intervals. Additionally, their preintervention readmission rate was lower than Mayo Clinic’s group, and although the readmission rate trended downward postintervention, it did not reach statistical significance.”
References
1. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-28.
2. Donzé JD, Williams MV, Robinson EJ, et al. International validity of the HOSPITAL Score to predict 30-day potentially avoidable hospital readmissions. JAMA Intern Med. 2016 Apr;176(4):496-502.
3. Kashiwagi DT, Burton MC, Hakim FA, et al. Reflective practice: a tool for readmission reduction. Am J Med Qual. 2016 May;31(3):265-71.
4. Herzig SJ, Schnipper JL, Doctoroff L, et al. Physician perspectives on factors contributing to readmissions and potential prevention strategies: a multicenter survey. J Gen Intern Med. 2016 Nov;31(11):1287-93. Epub 2016 Jun 9.
5. Allaudeen N, Schnipper JL, Orav EJ, et al. Inability of providers to predict unplanned readmissions. J Gen Intern Med. 2011 Jul;26(7):771-6.
6. Busuttil A, Howard-Anderson J, Dowling EP, et al. Building a comprehensive patient-centered readmission reduction program [abstract]. J Hosp Med. 2016;11(suppl 1).
7. Rana V, Thapa B, Saini SC, et al. Self-reflection as a tool to increase hospitalist participation in readmission quality improvement. Qual Manag Health Care. 2016 Oct/Dec;25(4):219-24.
Hospital readmissions are frequent, harmful, and costly. Consider the fact that 18% of Medicare patients can expect to be readmitted within 30 days at a cost of more than $17 billion.1 Recent changes in health care policy aimed at reducing readmission have substantially increased attention to this major health care issue.2
The Affordable Care Act has mandated that the Centers for Medicare & Medicaid Services reduce payment to hospitals with higher-than-expected 30-day readmissions, with its Hospital Readmissions Reduction Program. This has driven rapid growth in the study of patients rehospitalized within 30 days of discharge.3 So what are some strategies that have either been proven to reduce readmissions or show promise in doing so?

An ounce of prevention
In studying inpatient and outpatient physicians’ perspectives regarding factors contributing to readmission,4 Shoshana Herzig, MD, MPH, assistant professor of medicine, Harvard Medical School, and director of Hospital Medicine Research, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, and her colleagues identified some potential preventive strategies.
The most commonly endorsed strategy to prevent readmissions by both primary care physicians and hospitalists surveyed involved improving self-management plans at discharge. “This refers to actions such as providing patient-centered discharge instructions (that is, making sure they are written in language that patients can understand) or asking transition coaches to help facilitate a successful transition,” Dr. Herzig said. “This finding is consistent with the fact that the factor most commonly identified as contributing to readmissions was insufficient patient understanding or ability to self-manage. Combined, these findings suggest that strategies to enhance patient understanding of their illness, care plan, and what to expect after hospital discharge, are likely to be important components of successful readmission reduction programs.”
Provisioning of resources to patients to help them manage their care after discharge is also recommended. For example, engaging nurses or pharmacists who can help with issues that arise after discharge may help keep patients out of the hospital.
“Hospitalists should be aware of what resources are available to help patients manage their care,” Dr. Herzig said. For example, if a patient needs periodic blood pressure monitoring, the hospitalist can tell the patient about free blood pressure checkpoints or suggest a home-automated blood pressure monitor.
The study also showed that improved coordination of care between inpatient and outpatient providers, such as sharing medical records, could reduce readmission rates. “This allows for better inpatient care and increased ability for primary care physicians to react appropriately to issues arising after discharge,” Dr. Herzig said. “In the absence of a shared system, hospitalists should complete discharge summaries in a timely fashion and ensure that they’re promptly transmitted to primary care physicians.”
Dr. Herzig said it’s important to note that hospitalists and primary care physicians had different appraisals of reasons for readmission. Therefore, when designing readmission reduction programs or determining specific services to prevent a readmission for a given patient, it is important for hospitalists to obtain input from primary care physicians to ensure that they address all of the potential contributors to readmission for a given patient.
Interviewing patients regarding readmissions
After involved clinicians and independent physician reviewers performed extensive case reviews of more than 700 readmitted patients,6 Ashley Busuttil, MD, FHM, associate section chief, Hospital Medicine, University of California, Los Angeles Department of Medicine; and executive medical director, Medicine Services, UCLA Department of Medicine, and Erin Dowling, MD, assistant clinical professor, General Internal Medicine, Hospitalist Services, UCLA Medical Center, Santa Monica, Calif., and their colleagues were unable to identify which readmissions could have easily been prevented, and found that readmission causality varied extensively.
Through interviews with patients, the researchers determined that patients were more likely to think that their readmission was preventable if they felt unready for discharge during their initial hospitalization. This was despite the fact that patients met what clinicians would consider “ready” by objective, provider-centric criteria: they were medically stable, they had in-home support services, they had follow-up arranged, and so forth. As such, they wanted to put effort into educating and preparing patients for what home will look and feel like posthospitalization to address their feelings of unreadiness.
To that end, the researchers created an enhanced transition initiative that included showing an educational video near the time of admission and a patient-centered discharge checklist to help patients identify questions they might have after discharge. The discharge checklist asks patients to put themselves in the position of being at home and working through scenarios they may face so they will know how to deal with them. For example, if you have pain, who should you call? What should you do if you run out of medication?
Dr. Dowling believes that the hospitalist will, over time, become essential to assessing patient readiness. “As we learn more about how patients approach discharge, hospitalists’ understanding of patient needs beyond straightforward medical care will be crucial to having smoother transitions of care,” she said.
The researchers also explored pain control. As a health system, UCLA Medical Center has formed a multidisciplinary task force to optimize its approach to pain control. “If we can address comfort – for both patients at high risk of readmission and those that aren’t – we hope we can improve symptom control overall,” Dr. Busuttil said. “It’s not uncommon for patients to feel inadequate symptom control at discharge. While this is likely only one component of all the readmission pieces, a patient who feels that their symptoms are not controlled is likely to feel less ready for discharge. Increasing patient readiness, perhaps by increasing symptom control and improving communication regarding symptom management expectations, is a task that the hospitalist is well positioned to address.”
The researchers also wanted to find out why patients may not use available outpatient resources, and assessed them for decisional conflict – a measure of certainty with decision making – when selecting from multiple options for accessing medical care if they were home postdischarge and began to feel ill again. “Patients with decisional conflict were more likely to state that they would go the emergency room rather than call their primary medical physician or visit an urgent care center,” Dr. Busuttil said.
The health system continues to screen patients for decisional conflict. “When positive, we provide bedside education on when to seek medical care through primary care, urgent care, or the emergency department,” Dr. Busuttil said. “We also provide patients with information on how to access each of these resources.”
While a prior discharge plan may have seemed ideal on paper, time and time again it’s not logistically possible for certain patients. “By having this knowledge gleaned from patient interviews, hospitalists are able to provide feedback to health systems regarding different options of outpatient care that may work for the different patient populations they serve,” Dr. Dowling said.
To understand why one particular patient population is being readmitted requires taking the time to understand that population, Dr. Dowling noted. “While many validated risk stratification tools are available, they may only serve as general guides,” she said. “To impact the population you serve, you must first understand the readmission process as it looks to them.”
Employing the HOSPITAL score
In another effort to reduce hospital readmissions, Jacques Donzé, MD, MSc, associate physician, Bern University Hospital, Switzerland, and research associate, Brigham and Women’s Hospital, Boston, and his colleagues used the HOSPITAL score to identify patients at high risk of 30-day potentially avoidable readmission.
To most efficiently reduce hospital readmissions, hospitals need to target complex and intensive discharge interventions for patients at high risk of potentially avoidable readmission who are more likely to benefit.2 “However, prior research indicates that clinical health care providers are not able to accurately identify which patients are at high risk for readmission,” Dr. Donzé said.
Dr. Donzé believes that several factors may influence the performance of a prediction model, such as the initial selection of the potential predictors, the quality of the derivation method, including readily available predictors commonly available, and including reliable factors that aren’t subject to subjective evaluation. “All of these factors can play a role in the performance and generalizability of the HOSPITAL score,” he said.
When a patient is identified as high risk to be readmitted, hospitalists can take certain actions to prevent readmission. “Interventions are more likely to be effective when they include several components,” Dr. Donzé said. “These include follow-up phone calls and/or home visits, review of the patient’s medication list, patient education, and sending a discharge summary to the patient’s primary care physician in a timely manner. For now, enough evidence for a specific effective multimodal intervention to be generalizable to the majority of patients is lacking.”
Currently, the HOSPITAL score has been validated in approximately 180,000 patients in 14 hospitals across five countries and three continents – always showing good performance and generalizability. The HOSPITAL score includes seven variables readily available before hospital discharge, is easy to use, and is the most widely validated prediction model for readmission, Dr. Donzé said.
Before being implemented into practice, a score should ideally reach the highest level of validation, that is, show its clinical impact. “We expect that the score will not only be able to accurately predict high-risk patients, but using the score will also impact patient care by reducing readmissions when coupled with an appropriate intervention,” Dr. Donzé said.
In summary, research has shown that a variety of methods can be used to reduce hospital readmissions, including studying inpatient and outpatient physicians’ perspectives regarding factors contributing to readmission; interviewing patients regarding readmissions; and identifying patients at high risk of readmission using the HOSPITAL score.
Many researchers are continuing their studies in these areas.
Karen Appold is a medical writer in Pennsylvania.
Using hospitalist reflections as a means to reduce readmissions
Readmission studies and the development of readmission scoring systems and prediction tools rely on data from a large number of patients, typically extracted from administrative databases.
To complement this data, Deanne Kashiwagi, MD, consultant, Hospital Internal Medicine, Mayo Clinic, Rochester, Minn., and her colleagues asked hospitalists to reflect upon the readmissions of patients for whom they cared to add insight into the culture of patient care transitions within the health system.
“We felt there was some value in considering these nuances of the local care environment, which may not be represented in studies drawing from large databases, as potential targets for readmission efforts,” she said.
Dr. Kashiwagi believes that including elements of local practice and culture was the strength of their work. “Groups interested in replicating this reflective process should consider including factors specific to their practices that may contribute to readmission,” she said.
Asking hospitalists to perform reviews has led to implementing changes. Physicians were prompted to schedule earlier follow-up appointments and nurse practitioners and physician assistants have worked to improve the quality of their discharge summaries. The exercise also engaged hospitalists to suggest system changes that might contribute to decreased readmissions, such as a geriatrician-run service (which was recently begun) to provide multidisciplinary acute geriatric care for hospitalized older adults.
“Although large-scale studies are clearly important, readmission review at a more granular level may have merit as well,” Dr. Kashiwagi said, noting that such reviews identify local practice factors that groups may quickly act upon to help decrease readmissions. “Hospitalists readily engaged in this reflective exercise, which yielded actionable information to decrease readmissions.”
In commenting on why a different similar study7 didn’t mimic the results of Mayo Clinic’s study, Dr. Kashiwagi said there were some differences in methodology that may explain the difference in readmission rates. “First, this group excluded patients on dialysis, which in our study was a common comorbidity of our readmitted patients,” she said. “It is also notable that the chart review tool was different. Perhaps there is less representation of local factors, unique to that hospitalist group and their practice culture, than on our review form. These investigators also discussed their readmissions at routine intervals. Additionally, their preintervention readmission rate was lower than Mayo Clinic’s group, and although the readmission rate trended downward postintervention, it did not reach statistical significance.”
References
1. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-28.
2. Donzé JD, Williams MV, Robinson EJ, et al. International validity of the HOSPITAL Score to predict 30-day potentially avoidable hospital readmissions. JAMA Intern Med. 2016 Apr;176(4):496-502.
3. Kashiwagi DT, Burton MC, Hakim FA, et al. Reflective practice: a tool for readmission reduction. Am J Med Qual. 2016 May;31(3):265-71.
4. Herzig SJ, Schnipper JL, Doctoroff L, et al. Physician perspectives on factors contributing to readmissions and potential prevention strategies: a multicenter survey. J Gen Intern Med. 2016 Nov;31(11):1287-93. Epub 2016 Jun 9.
5. Allaudeen N, Schnipper JL, Orav EJ, et al. Inability of providers to predict unplanned readmissions. J Gen Intern Med. 2011 Jul;26(7):771-6.
6. Busuttil A, Howard-Anderson J, Dowling EP, et al. Building a comprehensive patient-centered readmission reduction program [abstract]. J Hosp Med. 2016;11(suppl 1).
7. Rana V, Thapa B, Saini SC, et al. Self-reflection as a tool to increase hospitalist participation in readmission quality improvement. Qual Manag Health Care. 2016 Oct/Dec;25(4):219-24.
Hospital readmissions are frequent, harmful, and costly. Consider the fact that 18% of Medicare patients can expect to be readmitted within 30 days at a cost of more than $17 billion.1 Recent changes in health care policy aimed at reducing readmission have substantially increased attention to this major health care issue.2
The Affordable Care Act has mandated that the Centers for Medicare & Medicaid Services reduce payment to hospitals with higher-than-expected 30-day readmissions, with its Hospital Readmissions Reduction Program. This has driven rapid growth in the study of patients rehospitalized within 30 days of discharge.3 So what are some strategies that have either been proven to reduce readmissions or show promise in doing so?

An ounce of prevention
In studying inpatient and outpatient physicians’ perspectives regarding factors contributing to readmission,4 Shoshana Herzig, MD, MPH, assistant professor of medicine, Harvard Medical School, and director of Hospital Medicine Research, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, and her colleagues identified some potential preventive strategies.
The most commonly endorsed strategy to prevent readmissions by both primary care physicians and hospitalists surveyed involved improving self-management plans at discharge. “This refers to actions such as providing patient-centered discharge instructions (that is, making sure they are written in language that patients can understand) or asking transition coaches to help facilitate a successful transition,” Dr. Herzig said. “This finding is consistent with the fact that the factor most commonly identified as contributing to readmissions was insufficient patient understanding or ability to self-manage. Combined, these findings suggest that strategies to enhance patient understanding of their illness, care plan, and what to expect after hospital discharge, are likely to be important components of successful readmission reduction programs.”
Provisioning of resources to patients to help them manage their care after discharge is also recommended. For example, engaging nurses or pharmacists who can help with issues that arise after discharge may help keep patients out of the hospital.
“Hospitalists should be aware of what resources are available to help patients manage their care,” Dr. Herzig said. For example, if a patient needs periodic blood pressure monitoring, the hospitalist can tell the patient about free blood pressure checkpoints or suggest a home-automated blood pressure monitor.
The study also showed that improved coordination of care between inpatient and outpatient providers, such as sharing medical records, could reduce readmission rates. “This allows for better inpatient care and increased ability for primary care physicians to react appropriately to issues arising after discharge,” Dr. Herzig said. “In the absence of a shared system, hospitalists should complete discharge summaries in a timely fashion and ensure that they’re promptly transmitted to primary care physicians.”
Dr. Herzig said it’s important to note that hospitalists and primary care physicians had different appraisals of reasons for readmission. Therefore, when designing readmission reduction programs or determining specific services to prevent a readmission for a given patient, it is important for hospitalists to obtain input from primary care physicians to ensure that they address all of the potential contributors to readmission for a given patient.
Interviewing patients regarding readmissions
After involved clinicians and independent physician reviewers performed extensive case reviews of more than 700 readmitted patients,6 Ashley Busuttil, MD, FHM, associate section chief, Hospital Medicine, University of California, Los Angeles Department of Medicine; and executive medical director, Medicine Services, UCLA Department of Medicine, and Erin Dowling, MD, assistant clinical professor, General Internal Medicine, Hospitalist Services, UCLA Medical Center, Santa Monica, Calif., and their colleagues were unable to identify which readmissions could have easily been prevented, and found that readmission causality varied extensively.
Through interviews with patients, the researchers determined that patients were more likely to think that their readmission was preventable if they felt unready for discharge during their initial hospitalization. This was despite the fact that patients met what clinicians would consider “ready” by objective, provider-centric criteria: they were medically stable, they had in-home support services, they had follow-up arranged, and so forth. As such, they wanted to put effort into educating and preparing patients for what home will look and feel like posthospitalization to address their feelings of unreadiness.
To that end, the researchers created an enhanced transition initiative that included showing an educational video near the time of admission and a patient-centered discharge checklist to help patients identify questions they might have after discharge. The discharge checklist asks patients to put themselves in the position of being at home and working through scenarios they may face so they will know how to deal with them. For example, if you have pain, who should you call? What should you do if you run out of medication?
Dr. Dowling believes that the hospitalist will, over time, become essential to assessing patient readiness. “As we learn more about how patients approach discharge, hospitalists’ understanding of patient needs beyond straightforward medical care will be crucial to having smoother transitions of care,” she said.
The researchers also explored pain control. As a health system, UCLA Medical Center has formed a multidisciplinary task force to optimize its approach to pain control. “If we can address comfort – for both patients at high risk of readmission and those that aren’t – we hope we can improve symptom control overall,” Dr. Busuttil said. “It’s not uncommon for patients to feel inadequate symptom control at discharge. While this is likely only one component of all the readmission pieces, a patient who feels that their symptoms are not controlled is likely to feel less ready for discharge. Increasing patient readiness, perhaps by increasing symptom control and improving communication regarding symptom management expectations, is a task that the hospitalist is well positioned to address.”
The researchers also wanted to find out why patients may not use available outpatient resources, and assessed them for decisional conflict – a measure of certainty with decision making – when selecting from multiple options for accessing medical care if they were home postdischarge and began to feel ill again. “Patients with decisional conflict were more likely to state that they would go the emergency room rather than call their primary medical physician or visit an urgent care center,” Dr. Busuttil said.
The health system continues to screen patients for decisional conflict. “When positive, we provide bedside education on when to seek medical care through primary care, urgent care, or the emergency department,” Dr. Busuttil said. “We also provide patients with information on how to access each of these resources.”
While a prior discharge plan may have seemed ideal on paper, time and time again it’s not logistically possible for certain patients. “By having this knowledge gleaned from patient interviews, hospitalists are able to provide feedback to health systems regarding different options of outpatient care that may work for the different patient populations they serve,” Dr. Dowling said.
To understand why one particular patient population is being readmitted requires taking the time to understand that population, Dr. Dowling noted. “While many validated risk stratification tools are available, they may only serve as general guides,” she said. “To impact the population you serve, you must first understand the readmission process as it looks to them.”
Employing the HOSPITAL score
In another effort to reduce hospital readmissions, Jacques Donzé, MD, MSc, associate physician, Bern University Hospital, Switzerland, and research associate, Brigham and Women’s Hospital, Boston, and his colleagues used the HOSPITAL score to identify patients at high risk of 30-day potentially avoidable readmission.
To most efficiently reduce hospital readmissions, hospitals need to target complex and intensive discharge interventions for patients at high risk of potentially avoidable readmission who are more likely to benefit.2 “However, prior research indicates that clinical health care providers are not able to accurately identify which patients are at high risk for readmission,” Dr. Donzé said.
Dr. Donzé believes that several factors may influence the performance of a prediction model, such as the initial selection of the potential predictors, the quality of the derivation method, including readily available predictors commonly available, and including reliable factors that aren’t subject to subjective evaluation. “All of these factors can play a role in the performance and generalizability of the HOSPITAL score,” he said.
When a patient is identified as high risk to be readmitted, hospitalists can take certain actions to prevent readmission. “Interventions are more likely to be effective when they include several components,” Dr. Donzé said. “These include follow-up phone calls and/or home visits, review of the patient’s medication list, patient education, and sending a discharge summary to the patient’s primary care physician in a timely manner. For now, enough evidence for a specific effective multimodal intervention to be generalizable to the majority of patients is lacking.”
Currently, the HOSPITAL score has been validated in approximately 180,000 patients in 14 hospitals across five countries and three continents – always showing good performance and generalizability. The HOSPITAL score includes seven variables readily available before hospital discharge, is easy to use, and is the most widely validated prediction model for readmission, Dr. Donzé said.
Before being implemented into practice, a score should ideally reach the highest level of validation, that is, show its clinical impact. “We expect that the score will not only be able to accurately predict high-risk patients, but using the score will also impact patient care by reducing readmissions when coupled with an appropriate intervention,” Dr. Donzé said.
In summary, research has shown that a variety of methods can be used to reduce hospital readmissions, including studying inpatient and outpatient physicians’ perspectives regarding factors contributing to readmission; interviewing patients regarding readmissions; and identifying patients at high risk of readmission using the HOSPITAL score.
Many researchers are continuing their studies in these areas.
Karen Appold is a medical writer in Pennsylvania.
Using hospitalist reflections as a means to reduce readmissions
Readmission studies and the development of readmission scoring systems and prediction tools rely on data from a large number of patients, typically extracted from administrative databases.
To complement this data, Deanne Kashiwagi, MD, consultant, Hospital Internal Medicine, Mayo Clinic, Rochester, Minn., and her colleagues asked hospitalists to reflect upon the readmissions of patients for whom they cared to add insight into the culture of patient care transitions within the health system.
“We felt there was some value in considering these nuances of the local care environment, which may not be represented in studies drawing from large databases, as potential targets for readmission efforts,” she said.
Dr. Kashiwagi believes that including elements of local practice and culture was the strength of their work. “Groups interested in replicating this reflective process should consider including factors specific to their practices that may contribute to readmission,” she said.
Asking hospitalists to perform reviews has led to implementing changes. Physicians were prompted to schedule earlier follow-up appointments and nurse practitioners and physician assistants have worked to improve the quality of their discharge summaries. The exercise also engaged hospitalists to suggest system changes that might contribute to decreased readmissions, such as a geriatrician-run service (which was recently begun) to provide multidisciplinary acute geriatric care for hospitalized older adults.
“Although large-scale studies are clearly important, readmission review at a more granular level may have merit as well,” Dr. Kashiwagi said, noting that such reviews identify local practice factors that groups may quickly act upon to help decrease readmissions. “Hospitalists readily engaged in this reflective exercise, which yielded actionable information to decrease readmissions.”
In commenting on why a different similar study7 didn’t mimic the results of Mayo Clinic’s study, Dr. Kashiwagi said there were some differences in methodology that may explain the difference in readmission rates. “First, this group excluded patients on dialysis, which in our study was a common comorbidity of our readmitted patients,” she said. “It is also notable that the chart review tool was different. Perhaps there is less representation of local factors, unique to that hospitalist group and their practice culture, than on our review form. These investigators also discussed their readmissions at routine intervals. Additionally, their preintervention readmission rate was lower than Mayo Clinic’s group, and although the readmission rate trended downward postintervention, it did not reach statistical significance.”
References
1. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-28.
2. Donzé JD, Williams MV, Robinson EJ, et al. International validity of the HOSPITAL Score to predict 30-day potentially avoidable hospital readmissions. JAMA Intern Med. 2016 Apr;176(4):496-502.
3. Kashiwagi DT, Burton MC, Hakim FA, et al. Reflective practice: a tool for readmission reduction. Am J Med Qual. 2016 May;31(3):265-71.
4. Herzig SJ, Schnipper JL, Doctoroff L, et al. Physician perspectives on factors contributing to readmissions and potential prevention strategies: a multicenter survey. J Gen Intern Med. 2016 Nov;31(11):1287-93. Epub 2016 Jun 9.
5. Allaudeen N, Schnipper JL, Orav EJ, et al. Inability of providers to predict unplanned readmissions. J Gen Intern Med. 2011 Jul;26(7):771-6.
6. Busuttil A, Howard-Anderson J, Dowling EP, et al. Building a comprehensive patient-centered readmission reduction program [abstract]. J Hosp Med. 2016;11(suppl 1).
7. Rana V, Thapa B, Saini SC, et al. Self-reflection as a tool to increase hospitalist participation in readmission quality improvement. Qual Manag Health Care. 2016 Oct/Dec;25(4):219-24.
Reduced starting dose of sorafenib for HCC appears safe
Compared with starting patients with hepatocellular carcinoma on a standard dose of sorafenib, reducing the starting dose was associated with reduced treatment costs, yet did not reduce overall survival, a large retrospective analysis showed.
“Our data suggest that the initiation of sorafenib at a reduced dosage may be a safe and reasonable strategy for some patients with HCC,” researchers led by Kim A. Reiss, MD, wrote in the Journal of Oncology.
Dr. Reiss, of the Philadelphia-based Perelman Center for Advanced Medicine, and her associates analyzed data from 4,903 patients with HCC who were prescribed sorafenib at 128 Veterans Health Administration hospitals between January 2006 and April 2015. They used Cox regression analysis to examine the impact of the drug’s starting dose on patient outcomes and cost. The primary endpoint was overall survival of patients who were prescribed a standard starting dose of sorafenib at 800 mg/d, compared with a starting dose of less than 800 mg/d.
The mean age of patients was 62 years, 99% were male, and 61% where white. In an unmatched and unadjusted analysis, the researchers found that compared with patients who received a standard starting dose of sorafenib, those who received a reduced starting dose had more Barcelona Clinic Liver Cancer stage D disease (P less than .001), higher Model for End-Stage Liver Disease Sodium scores (P less than .001), higher Child-Turcotte-Pugh scores (P less than .001), higher Cirrhosis Comorbidity Index scores (P=.01), and a lower overall survival (a median of 200 vs. 233 days, respectively; HR 1.10). However, after propensity score matching and adjustment for potential confounders, the difference in overall survival between the two treatment groups dropped below the noninferiority margin (P less than .001), the investigators reported (J Clin Oncol. 2017 Sept 5 doi.org/10.1200/JCO.2017.73.8245).
In addition, patients who received a reduced starting dose of sorafenib had a significantly lower total cost of the medication ($5,636 vs. $8,661; P less than .001) and were less likely to stop taking sorafenib because of GI effects (8.7% vs. 10.8%; P = .047).
The study was supported by unrestricted research funds from Bayer HealthCare Pharmaceuticals and the VA HIV, Hepatitis, and Related Conditions Programs in the Office of Specialty Care Services. One of the authors, Ronac Mamtani, MD, is supported by National Institutes of Health/National Cancer Institute grant K23CA18718.
Compared with starting patients with hepatocellular carcinoma on a standard dose of sorafenib, reducing the starting dose was associated with reduced treatment costs, yet did not reduce overall survival, a large retrospective analysis showed.
“Our data suggest that the initiation of sorafenib at a reduced dosage may be a safe and reasonable strategy for some patients with HCC,” researchers led by Kim A. Reiss, MD, wrote in the Journal of Oncology.
Dr. Reiss, of the Philadelphia-based Perelman Center for Advanced Medicine, and her associates analyzed data from 4,903 patients with HCC who were prescribed sorafenib at 128 Veterans Health Administration hospitals between January 2006 and April 2015. They used Cox regression analysis to examine the impact of the drug’s starting dose on patient outcomes and cost. The primary endpoint was overall survival of patients who were prescribed a standard starting dose of sorafenib at 800 mg/d, compared with a starting dose of less than 800 mg/d.
The mean age of patients was 62 years, 99% were male, and 61% where white. In an unmatched and unadjusted analysis, the researchers found that compared with patients who received a standard starting dose of sorafenib, those who received a reduced starting dose had more Barcelona Clinic Liver Cancer stage D disease (P less than .001), higher Model for End-Stage Liver Disease Sodium scores (P less than .001), higher Child-Turcotte-Pugh scores (P less than .001), higher Cirrhosis Comorbidity Index scores (P=.01), and a lower overall survival (a median of 200 vs. 233 days, respectively; HR 1.10). However, after propensity score matching and adjustment for potential confounders, the difference in overall survival between the two treatment groups dropped below the noninferiority margin (P less than .001), the investigators reported (J Clin Oncol. 2017 Sept 5 doi.org/10.1200/JCO.2017.73.8245).
In addition, patients who received a reduced starting dose of sorafenib had a significantly lower total cost of the medication ($5,636 vs. $8,661; P less than .001) and were less likely to stop taking sorafenib because of GI effects (8.7% vs. 10.8%; P = .047).
The study was supported by unrestricted research funds from Bayer HealthCare Pharmaceuticals and the VA HIV, Hepatitis, and Related Conditions Programs in the Office of Specialty Care Services. One of the authors, Ronac Mamtani, MD, is supported by National Institutes of Health/National Cancer Institute grant K23CA18718.
Compared with starting patients with hepatocellular carcinoma on a standard dose of sorafenib, reducing the starting dose was associated with reduced treatment costs, yet did not reduce overall survival, a large retrospective analysis showed.
“Our data suggest that the initiation of sorafenib at a reduced dosage may be a safe and reasonable strategy for some patients with HCC,” researchers led by Kim A. Reiss, MD, wrote in the Journal of Oncology.
Dr. Reiss, of the Philadelphia-based Perelman Center for Advanced Medicine, and her associates analyzed data from 4,903 patients with HCC who were prescribed sorafenib at 128 Veterans Health Administration hospitals between January 2006 and April 2015. They used Cox regression analysis to examine the impact of the drug’s starting dose on patient outcomes and cost. The primary endpoint was overall survival of patients who were prescribed a standard starting dose of sorafenib at 800 mg/d, compared with a starting dose of less than 800 mg/d.
The mean age of patients was 62 years, 99% were male, and 61% where white. In an unmatched and unadjusted analysis, the researchers found that compared with patients who received a standard starting dose of sorafenib, those who received a reduced starting dose had more Barcelona Clinic Liver Cancer stage D disease (P less than .001), higher Model for End-Stage Liver Disease Sodium scores (P less than .001), higher Child-Turcotte-Pugh scores (P less than .001), higher Cirrhosis Comorbidity Index scores (P=.01), and a lower overall survival (a median of 200 vs. 233 days, respectively; HR 1.10). However, after propensity score matching and adjustment for potential confounders, the difference in overall survival between the two treatment groups dropped below the noninferiority margin (P less than .001), the investigators reported (J Clin Oncol. 2017 Sept 5 doi.org/10.1200/JCO.2017.73.8245).
In addition, patients who received a reduced starting dose of sorafenib had a significantly lower total cost of the medication ($5,636 vs. $8,661; P less than .001) and were less likely to stop taking sorafenib because of GI effects (8.7% vs. 10.8%; P = .047).
The study was supported by unrestricted research funds from Bayer HealthCare Pharmaceuticals and the VA HIV, Hepatitis, and Related Conditions Programs in the Office of Specialty Care Services. One of the authors, Ronac Mamtani, MD, is supported by National Institutes of Health/National Cancer Institute grant K23CA18718.
FROM THE JOURNAL OF ONCOLOGY
Key clinical point:
Major finding: Patients who received a reduced starting dose of sorafenib had a significantly lower total cost of the medication ($5,636 vs. $8,661; P less than .001) and were less likely to stop taking sorafenib because of GI effects (8.7% vs. 10.8%; P = .047). Overall survival was not significantly different.
Data source: Retrospective data analysis of 4,903 patients from 128 Veterans Health Administration hospitals.
Disclosures: The study was supported by unrestricted research funds from Bayer HealthCare Pharmaceuticals and the VA HIV, Hepatitis, and Related Conditions Programs in the Office of Specialty Care Services. One of the authors, Ronac Mamtani, MD, is supported by National Institutes of Health/National Cancer Institute grant K23CA18718.
Florida health officials prepare for Hurricane Irma
As Irma, the most powerful Atlantic hurricane in recorded history, approaches south Florida, the state’s Department of Health is moving quickly to prepare.
Once Gov. Rick Scott (R) declared a state of emergency in all 67 counties Sept. 4, the department swung into action.
“Florida has a robust emergency operation system and once activated, Florida Department of Health is the lead for State Emergency Function 8 or ESF-8. Hospital evacuations, special needs sheltering, and other tasks are coordinated through that function,” Mara Gambineri, communications director for the department, said in an interview. “We have plans in place and exercise those frequently to prepare for these situations.”
Hospitals near Miami are putting together skeleton crews, making sure there will be adequate personnel for the height of the storm.
“All the hospitals are going into this type of military, alpha team, bravo team approach,” Rodolfo Oviedo, MD, FACS, assistant professor of surgery at Florida State University and surgical fellow at Baptist Hospital of Miami, said in an interview. “We’ll have one specialist per specialty, and we’ll have emergency medical technicians volunteering as well.”
Despite the forecast of Hurricane Irma’s size and strength, Dr. Oviedo said that he is not concerned about local hospitals succumbing to the storm or being caught unprepared.
“This is a city that is used to this, they all have plenty of experience with hurricanes,” Dr. Oviedo said. “These buildings are built to withstand hurricanes, especially the hospitals, and even the older hospitals have been renovated for that.”
Florida adopted new building codes to improve hurricane resilience, including special exterior glazing that can handle high winds, after Hurricane Andrew tore across the state in 1992.
Others already have started to look ahead to when after the storm has passed. The Red Cross is issuing volunteer applications for Irma relief, and has committed to sending enough supplies to shelter 120,000 people by Sept. 8-9, according to a Red Cross update.
FEMA Administrator Brock Long noted that despite the intense recovery efforts ongoing in Texas and Louisiana from Hurricane Harvey, his agency is primed to assist with the impact of Hurricane Irma as well.
“We’re not going to let money get in the way of saving lives,” Mr. Long said in an interview on “CBS This Morning” Sept. 6.
Sen. Marco Rubio (R-Fla.) and Sen. Bill Nelson (D-Fla.) on Sept. 6 requested that additional FEMA funding be added to the Hurricane Harvey disaster relief package currently being considered by Congress.
Floridians “need to know that the federal government is both ready and willing to direct the necessary resources needed to help them in the recovery process,” the senators wrote in a joint letter to Senate leaders. “We strongly urge you to include additional funding in the Hurricane Harvey aid package to account for the additional costs FEMA will likely incur responding to Hurricane Irma.”
At press time, Hurricane Irma was expected to make landfall in south Florida on Sept. 10.
[email protected]
On Twitter @eaztweets
As Irma, the most powerful Atlantic hurricane in recorded history, approaches south Florida, the state’s Department of Health is moving quickly to prepare.
Once Gov. Rick Scott (R) declared a state of emergency in all 67 counties Sept. 4, the department swung into action.
“Florida has a robust emergency operation system and once activated, Florida Department of Health is the lead for State Emergency Function 8 or ESF-8. Hospital evacuations, special needs sheltering, and other tasks are coordinated through that function,” Mara Gambineri, communications director for the department, said in an interview. “We have plans in place and exercise those frequently to prepare for these situations.”
Hospitals near Miami are putting together skeleton crews, making sure there will be adequate personnel for the height of the storm.
“All the hospitals are going into this type of military, alpha team, bravo team approach,” Rodolfo Oviedo, MD, FACS, assistant professor of surgery at Florida State University and surgical fellow at Baptist Hospital of Miami, said in an interview. “We’ll have one specialist per specialty, and we’ll have emergency medical technicians volunteering as well.”
Despite the forecast of Hurricane Irma’s size and strength, Dr. Oviedo said that he is not concerned about local hospitals succumbing to the storm or being caught unprepared.
“This is a city that is used to this, they all have plenty of experience with hurricanes,” Dr. Oviedo said. “These buildings are built to withstand hurricanes, especially the hospitals, and even the older hospitals have been renovated for that.”
Florida adopted new building codes to improve hurricane resilience, including special exterior glazing that can handle high winds, after Hurricane Andrew tore across the state in 1992.
Others already have started to look ahead to when after the storm has passed. The Red Cross is issuing volunteer applications for Irma relief, and has committed to sending enough supplies to shelter 120,000 people by Sept. 8-9, according to a Red Cross update.
FEMA Administrator Brock Long noted that despite the intense recovery efforts ongoing in Texas and Louisiana from Hurricane Harvey, his agency is primed to assist with the impact of Hurricane Irma as well.
“We’re not going to let money get in the way of saving lives,” Mr. Long said in an interview on “CBS This Morning” Sept. 6.
Sen. Marco Rubio (R-Fla.) and Sen. Bill Nelson (D-Fla.) on Sept. 6 requested that additional FEMA funding be added to the Hurricane Harvey disaster relief package currently being considered by Congress.
Floridians “need to know that the federal government is both ready and willing to direct the necessary resources needed to help them in the recovery process,” the senators wrote in a joint letter to Senate leaders. “We strongly urge you to include additional funding in the Hurricane Harvey aid package to account for the additional costs FEMA will likely incur responding to Hurricane Irma.”
At press time, Hurricane Irma was expected to make landfall in south Florida on Sept. 10.
[email protected]
On Twitter @eaztweets
As Irma, the most powerful Atlantic hurricane in recorded history, approaches south Florida, the state’s Department of Health is moving quickly to prepare.
Once Gov. Rick Scott (R) declared a state of emergency in all 67 counties Sept. 4, the department swung into action.
“Florida has a robust emergency operation system and once activated, Florida Department of Health is the lead for State Emergency Function 8 or ESF-8. Hospital evacuations, special needs sheltering, and other tasks are coordinated through that function,” Mara Gambineri, communications director for the department, said in an interview. “We have plans in place and exercise those frequently to prepare for these situations.”
Hospitals near Miami are putting together skeleton crews, making sure there will be adequate personnel for the height of the storm.
“All the hospitals are going into this type of military, alpha team, bravo team approach,” Rodolfo Oviedo, MD, FACS, assistant professor of surgery at Florida State University and surgical fellow at Baptist Hospital of Miami, said in an interview. “We’ll have one specialist per specialty, and we’ll have emergency medical technicians volunteering as well.”
Despite the forecast of Hurricane Irma’s size and strength, Dr. Oviedo said that he is not concerned about local hospitals succumbing to the storm or being caught unprepared.
“This is a city that is used to this, they all have plenty of experience with hurricanes,” Dr. Oviedo said. “These buildings are built to withstand hurricanes, especially the hospitals, and even the older hospitals have been renovated for that.”
Florida adopted new building codes to improve hurricane resilience, including special exterior glazing that can handle high winds, after Hurricane Andrew tore across the state in 1992.
Others already have started to look ahead to when after the storm has passed. The Red Cross is issuing volunteer applications for Irma relief, and has committed to sending enough supplies to shelter 120,000 people by Sept. 8-9, according to a Red Cross update.
FEMA Administrator Brock Long noted that despite the intense recovery efforts ongoing in Texas and Louisiana from Hurricane Harvey, his agency is primed to assist with the impact of Hurricane Irma as well.
“We’re not going to let money get in the way of saving lives,” Mr. Long said in an interview on “CBS This Morning” Sept. 6.
Sen. Marco Rubio (R-Fla.) and Sen. Bill Nelson (D-Fla.) on Sept. 6 requested that additional FEMA funding be added to the Hurricane Harvey disaster relief package currently being considered by Congress.
Floridians “need to know that the federal government is both ready and willing to direct the necessary resources needed to help them in the recovery process,” the senators wrote in a joint letter to Senate leaders. “We strongly urge you to include additional funding in the Hurricane Harvey aid package to account for the additional costs FEMA will likely incur responding to Hurricane Irma.”
At press time, Hurricane Irma was expected to make landfall in south Florida on Sept. 10.
[email protected]
On Twitter @eaztweets
Do staphylococci play a role in acne?
Clues are emerging that Staphylococcus species may be contributors to acne vulgaris, according to a new study that examined changes in the skin microbiota of acne patients undergoing topical treatment.
Comparing topical antibiotic treatment for acne with a novel cosmetic formulation, Brigitte Dreno, MD, PhD, and her colleagues found that normal skin had fewer surface staphylococci than did skin with comedones or papulopustular eruptions (P = .004 for comedones; P = .003 for papules and pustules). Further, the number of staphylococci increased as acne severity increased (P less than .05 for the increase seen between scores of GEA-2 and GEA-3 on the Global Acne Severity Scale).
These results were seen in a split-face study of 26 adults with mild to moderate acne (GEA-2 and GEA-3) that compared a topical 4% erythromycin gel to a “dermocosmetic” containing lipohydroxy, linoleic, and salicylic acids, niacinamide, piroctone olamine, a ceramide, and water from a thermal spring. Each patient used each product on half of his or her face for 28 consecutive days while avoiding use of other skin care products or topical medications during the study period (Exp Dermatol. 2017;26[9]:798-803; doi: 10.1111/exd.13296).
Patients’ acne severity was assessed at days 0, 14, and 28 using the GEA scale, together with a count of inflammatory and noninflammatory lesions. The mean GEA grade for patients at enrollment was 2.4. For each patient, skin microbiota samples were obtained from an area with comedones, an area with papulopustular lesions, and an unaffected area.
Dr. Dreno, professor of dermatology at University Hospital, Nantes, France, and her coauthors noted that the range of bacterial diversity was similar on all the facial areas sampled.
After 28 days of treatment, the antibiotic-treated facial skin had significantly less Actinobacteria species, including corynebacteria and propionibacteria. However, the effect on Staphylococci was limited, “potentially confirming the increased resistance of the bacterium to macrolides,” they wrote.
Facial skin treated with the cosmetic, by contrast, had fewer Actinobacteria and fewer Staphylococcus species by the end of the study period, though staphylococci remained the predominant organisms after treatment. Patients saw a significant reduction in both inflammatory and noninflammatory lesions during the study period (P less than .05), with no significant difference between antibiotic- and cosmetic-treated skin.
The authors said that previous studies have shown that propionibacteria are common commensal organisms, representing up to 30% of the bacteria on healthy skin. For the individuals enrolled in the study, though, propionibacteria made up just 2% of the bacteria on the surface of both healthy skin and skin with acne.
Staphylococci, and S. epidermidis in particular, may use fermentation as a defense against other organisms in the skin microbiota. Since staphylococci are aerobic and facultative anaerobic species, they flourish on the skin surface. Propionibacterium acnes, by contrast, is anaerobic, and primarily inhabits the sebaceous follicle.
The complex interplay of skin microbiota contribute to acne and other dermatologic pathology in ways that are just beginning to be understood. “Even though the association between P. acnes and acne vulgaris is well established, very few studies have investigated the entire facial skin microbiota of patients with acne,” wrote Dr. Dreno and her coauthors.
One of the six study authors are employees of L’Oreal; two are employees of La Roche–Posay Dermatological Laboratory, which funded the study and produced the cosmetic product used by the researchers.
[email protected]
On Twitter @karioakes
Clues are emerging that Staphylococcus species may be contributors to acne vulgaris, according to a new study that examined changes in the skin microbiota of acne patients undergoing topical treatment.
Comparing topical antibiotic treatment for acne with a novel cosmetic formulation, Brigitte Dreno, MD, PhD, and her colleagues found that normal skin had fewer surface staphylococci than did skin with comedones or papulopustular eruptions (P = .004 for comedones; P = .003 for papules and pustules). Further, the number of staphylococci increased as acne severity increased (P less than .05 for the increase seen between scores of GEA-2 and GEA-3 on the Global Acne Severity Scale).
These results were seen in a split-face study of 26 adults with mild to moderate acne (GEA-2 and GEA-3) that compared a topical 4% erythromycin gel to a “dermocosmetic” containing lipohydroxy, linoleic, and salicylic acids, niacinamide, piroctone olamine, a ceramide, and water from a thermal spring. Each patient used each product on half of his or her face for 28 consecutive days while avoiding use of other skin care products or topical medications during the study period (Exp Dermatol. 2017;26[9]:798-803; doi: 10.1111/exd.13296).
Patients’ acne severity was assessed at days 0, 14, and 28 using the GEA scale, together with a count of inflammatory and noninflammatory lesions. The mean GEA grade for patients at enrollment was 2.4. For each patient, skin microbiota samples were obtained from an area with comedones, an area with papulopustular lesions, and an unaffected area.
Dr. Dreno, professor of dermatology at University Hospital, Nantes, France, and her coauthors noted that the range of bacterial diversity was similar on all the facial areas sampled.
After 28 days of treatment, the antibiotic-treated facial skin had significantly less Actinobacteria species, including corynebacteria and propionibacteria. However, the effect on Staphylococci was limited, “potentially confirming the increased resistance of the bacterium to macrolides,” they wrote.
Facial skin treated with the cosmetic, by contrast, had fewer Actinobacteria and fewer Staphylococcus species by the end of the study period, though staphylococci remained the predominant organisms after treatment. Patients saw a significant reduction in both inflammatory and noninflammatory lesions during the study period (P less than .05), with no significant difference between antibiotic- and cosmetic-treated skin.
The authors said that previous studies have shown that propionibacteria are common commensal organisms, representing up to 30% of the bacteria on healthy skin. For the individuals enrolled in the study, though, propionibacteria made up just 2% of the bacteria on the surface of both healthy skin and skin with acne.
Staphylococci, and S. epidermidis in particular, may use fermentation as a defense against other organisms in the skin microbiota. Since staphylococci are aerobic and facultative anaerobic species, they flourish on the skin surface. Propionibacterium acnes, by contrast, is anaerobic, and primarily inhabits the sebaceous follicle.
The complex interplay of skin microbiota contribute to acne and other dermatologic pathology in ways that are just beginning to be understood. “Even though the association between P. acnes and acne vulgaris is well established, very few studies have investigated the entire facial skin microbiota of patients with acne,” wrote Dr. Dreno and her coauthors.
One of the six study authors are employees of L’Oreal; two are employees of La Roche–Posay Dermatological Laboratory, which funded the study and produced the cosmetic product used by the researchers.
[email protected]
On Twitter @karioakes
Clues are emerging that Staphylococcus species may be contributors to acne vulgaris, according to a new study that examined changes in the skin microbiota of acne patients undergoing topical treatment.
Comparing topical antibiotic treatment for acne with a novel cosmetic formulation, Brigitte Dreno, MD, PhD, and her colleagues found that normal skin had fewer surface staphylococci than did skin with comedones or papulopustular eruptions (P = .004 for comedones; P = .003 for papules and pustules). Further, the number of staphylococci increased as acne severity increased (P less than .05 for the increase seen between scores of GEA-2 and GEA-3 on the Global Acne Severity Scale).
These results were seen in a split-face study of 26 adults with mild to moderate acne (GEA-2 and GEA-3) that compared a topical 4% erythromycin gel to a “dermocosmetic” containing lipohydroxy, linoleic, and salicylic acids, niacinamide, piroctone olamine, a ceramide, and water from a thermal spring. Each patient used each product on half of his or her face for 28 consecutive days while avoiding use of other skin care products or topical medications during the study period (Exp Dermatol. 2017;26[9]:798-803; doi: 10.1111/exd.13296).
Patients’ acne severity was assessed at days 0, 14, and 28 using the GEA scale, together with a count of inflammatory and noninflammatory lesions. The mean GEA grade for patients at enrollment was 2.4. For each patient, skin microbiota samples were obtained from an area with comedones, an area with papulopustular lesions, and an unaffected area.
Dr. Dreno, professor of dermatology at University Hospital, Nantes, France, and her coauthors noted that the range of bacterial diversity was similar on all the facial areas sampled.
After 28 days of treatment, the antibiotic-treated facial skin had significantly less Actinobacteria species, including corynebacteria and propionibacteria. However, the effect on Staphylococci was limited, “potentially confirming the increased resistance of the bacterium to macrolides,” they wrote.
Facial skin treated with the cosmetic, by contrast, had fewer Actinobacteria and fewer Staphylococcus species by the end of the study period, though staphylococci remained the predominant organisms after treatment. Patients saw a significant reduction in both inflammatory and noninflammatory lesions during the study period (P less than .05), with no significant difference between antibiotic- and cosmetic-treated skin.
The authors said that previous studies have shown that propionibacteria are common commensal organisms, representing up to 30% of the bacteria on healthy skin. For the individuals enrolled in the study, though, propionibacteria made up just 2% of the bacteria on the surface of both healthy skin and skin with acne.
Staphylococci, and S. epidermidis in particular, may use fermentation as a defense against other organisms in the skin microbiota. Since staphylococci are aerobic and facultative anaerobic species, they flourish on the skin surface. Propionibacterium acnes, by contrast, is anaerobic, and primarily inhabits the sebaceous follicle.
The complex interplay of skin microbiota contribute to acne and other dermatologic pathology in ways that are just beginning to be understood. “Even though the association between P. acnes and acne vulgaris is well established, very few studies have investigated the entire facial skin microbiota of patients with acne,” wrote Dr. Dreno and her coauthors.
One of the six study authors are employees of L’Oreal; two are employees of La Roche–Posay Dermatological Laboratory, which funded the study and produced the cosmetic product used by the researchers.
[email protected]
On Twitter @karioakes
FROM EXPERIMENTAL DERMATOLOGY
Key clinical point:
Major finding: Staphylococci increased as acne severity increased (P less than .05 for increase from GEA-2 to GEA-3 on the Global Acne Severity Scale).
Data source: A split-face study of erythromycin gel and a novel cosmetic product in 26 adult patients with acne.
Disclosures: One of the six study authors are employees of L’Oreal; two are employees of La Roche–Posay Dermatological Laboratory, which funded the study and produced the cosmetic product used by the researchers.
Hospital value-based purchasing is largely ineffective
Over the last 5 years, I’ve periodically devoted this column to providing updates to the Hospital Value-Based Purchasing program. HVBP launched in 2013 as a 5-year mixed upside/downside incentive program with mandatory participation for all U.S. acute care hospitals (critical access, acute inpatient rehabilitation, and long-term acute care hospitals are exempt). The program initially included process and patient experience measures. It later added measures for mortality, efficiency, and patient safety.
For the 2017 version of HVBP, the measures are allocated as follows: eight for patient experience, seven for patient safety (1 of which is a roll up of 11 claims-based measures), three for process, and three for mortality. HVBP uses a budget-neutral funding approach with some winners and some losers but overall net zero spending on the program. It initially put hospitals at risk for 1% of their Medicare inpatient payments (in 2013), with a progressive increase to 2% by this year. HVBP has used a complex approach to determining incentives and penalties, rewarding either improvement or achievement, depending on the baseline performance of the hospital.
Has HVBP improved quality? Two studies looking at the early period of HVBP failed to show improvements in process or patient experience measures and demonstrated no change in mortality for heart failure, pneumonia, or heart attack.1,2 Now that the program is in its 5th and final year, thanks to a recent study by Ryan et al., we have an idea if HVBP is associated with longer-term improvements in quality.3
In the study, Ryan et al. compared hospitals participating in HVBP with critical access hospitals, which are exempt from the program. The study yielded some disappointing, if not surprising, results. Improvements in process and patient experience measures for HVBP hospitals were no greater than those for the control group. HVBP was not associated with a significant reduction in mortality for heart failure or heart attack, but was associated with a mortality reduction for pneumonia. In sum, HVBP was not associated with improvements in process or patient experience, and was not associated with lower mortality, except in pneumonia.
As a program designed to incentivize better quality, where did HVBP go wrong? I believe HVBP simply had too many measures for the cognitive bandwidth of an individual or a team looking to improve quality. The total measure count for 2017 is 21! I submit that a hospitalist working to improve quality can keep top-of-mind one or two measures, possibly three at most. While others have postulated that the amount of dollars at risk are too small, I don’t think that’s the problem. Instead, my sense is that hospitalists and other members of the hospital team have quality improvement in their DNA and, regardless of the size of the financial incentives, will work to improve it as long as they have the right tools. Chief among these are good performance data and the time to focus on a finite number of projects.
What lessons can inform better design in the future? As of January 2017, the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) – representing the biggest change in reimbursement in a generation – progressively exposes doctors and other professionals to upside/downside incentives for quality, resource utilization, use of a certified electronic health record (hospitalists are exempt as they already use the hospital’s EHR), and practice improvement activities.
It would be wise to learn from the shortcomings of HVBP. Namely, if MACRA keeps on its course to incentivize physicians using a complicated formula based on four domains and many more subdomains, it will repeat the mistakes of HVBP and – while creating more administrative burden – likely improve quality very little, if at all. Instead, MACRA should delineate a simple measure set representing improvement activities that physicians and teams can incorporate into their regular work flow without more time taken away from patient care.
The reality is that complicated pay-for-performance programs divert limited available resources away from meaningful improvement activities in order to comply with onerous reporting requirements. As we gain a more nuanced understanding of how these programs work, policy makers should pay attention to the elements of “low-value” and “high-value” incentive systems and apply the “less is more” ethos of high-value care to the next generation of pay-for-performance programs.
Dr. Whitcomb is chief medical officer at Remedy Partners in Darien, Conn., and a cofounder and past president of SHM.
References
1. Ryan AM, Burgess JF, Pesko MF, Borden WB, Dimick JB. “The early effects of Medicare’s mandatory hospital pay-for-performance program” Health Serv Res. 2015;50:81-97.
2. Figueroa JF, Tsugawa Y, Zheng J, Orav EJ, Jha AK. “Association between the Value-Based Purchasing pay for performance program and patient mortality in US hospitals: observational study” BMJ. 2016;353:i2214.
3. Ryan AM, Krinsky S, Maurer KA, Dimick JB. “Changes in Hospital Quality Associated with Hospital Value-Based Purchasing” N Engl J Med. 2017;376:2358-66.
Over the last 5 years, I’ve periodically devoted this column to providing updates to the Hospital Value-Based Purchasing program. HVBP launched in 2013 as a 5-year mixed upside/downside incentive program with mandatory participation for all U.S. acute care hospitals (critical access, acute inpatient rehabilitation, and long-term acute care hospitals are exempt). The program initially included process and patient experience measures. It later added measures for mortality, efficiency, and patient safety.
For the 2017 version of HVBP, the measures are allocated as follows: eight for patient experience, seven for patient safety (1 of which is a roll up of 11 claims-based measures), three for process, and three for mortality. HVBP uses a budget-neutral funding approach with some winners and some losers but overall net zero spending on the program. It initially put hospitals at risk for 1% of their Medicare inpatient payments (in 2013), with a progressive increase to 2% by this year. HVBP has used a complex approach to determining incentives and penalties, rewarding either improvement or achievement, depending on the baseline performance of the hospital.
Has HVBP improved quality? Two studies looking at the early period of HVBP failed to show improvements in process or patient experience measures and demonstrated no change in mortality for heart failure, pneumonia, or heart attack.1,2 Now that the program is in its 5th and final year, thanks to a recent study by Ryan et al., we have an idea if HVBP is associated with longer-term improvements in quality.3
In the study, Ryan et al. compared hospitals participating in HVBP with critical access hospitals, which are exempt from the program. The study yielded some disappointing, if not surprising, results. Improvements in process and patient experience measures for HVBP hospitals were no greater than those for the control group. HVBP was not associated with a significant reduction in mortality for heart failure or heart attack, but was associated with a mortality reduction for pneumonia. In sum, HVBP was not associated with improvements in process or patient experience, and was not associated with lower mortality, except in pneumonia.
As a program designed to incentivize better quality, where did HVBP go wrong? I believe HVBP simply had too many measures for the cognitive bandwidth of an individual or a team looking to improve quality. The total measure count for 2017 is 21! I submit that a hospitalist working to improve quality can keep top-of-mind one or two measures, possibly three at most. While others have postulated that the amount of dollars at risk are too small, I don’t think that’s the problem. Instead, my sense is that hospitalists and other members of the hospital team have quality improvement in their DNA and, regardless of the size of the financial incentives, will work to improve it as long as they have the right tools. Chief among these are good performance data and the time to focus on a finite number of projects.
What lessons can inform better design in the future? As of January 2017, the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) – representing the biggest change in reimbursement in a generation – progressively exposes doctors and other professionals to upside/downside incentives for quality, resource utilization, use of a certified electronic health record (hospitalists are exempt as they already use the hospital’s EHR), and practice improvement activities.
It would be wise to learn from the shortcomings of HVBP. Namely, if MACRA keeps on its course to incentivize physicians using a complicated formula based on four domains and many more subdomains, it will repeat the mistakes of HVBP and – while creating more administrative burden – likely improve quality very little, if at all. Instead, MACRA should delineate a simple measure set representing improvement activities that physicians and teams can incorporate into their regular work flow without more time taken away from patient care.
The reality is that complicated pay-for-performance programs divert limited available resources away from meaningful improvement activities in order to comply with onerous reporting requirements. As we gain a more nuanced understanding of how these programs work, policy makers should pay attention to the elements of “low-value” and “high-value” incentive systems and apply the “less is more” ethos of high-value care to the next generation of pay-for-performance programs.
Dr. Whitcomb is chief medical officer at Remedy Partners in Darien, Conn., and a cofounder and past president of SHM.
References
1. Ryan AM, Burgess JF, Pesko MF, Borden WB, Dimick JB. “The early effects of Medicare’s mandatory hospital pay-for-performance program” Health Serv Res. 2015;50:81-97.
2. Figueroa JF, Tsugawa Y, Zheng J, Orav EJ, Jha AK. “Association between the Value-Based Purchasing pay for performance program and patient mortality in US hospitals: observational study” BMJ. 2016;353:i2214.
3. Ryan AM, Krinsky S, Maurer KA, Dimick JB. “Changes in Hospital Quality Associated with Hospital Value-Based Purchasing” N Engl J Med. 2017;376:2358-66.
Over the last 5 years, I’ve periodically devoted this column to providing updates to the Hospital Value-Based Purchasing program. HVBP launched in 2013 as a 5-year mixed upside/downside incentive program with mandatory participation for all U.S. acute care hospitals (critical access, acute inpatient rehabilitation, and long-term acute care hospitals are exempt). The program initially included process and patient experience measures. It later added measures for mortality, efficiency, and patient safety.
For the 2017 version of HVBP, the measures are allocated as follows: eight for patient experience, seven for patient safety (1 of which is a roll up of 11 claims-based measures), three for process, and three for mortality. HVBP uses a budget-neutral funding approach with some winners and some losers but overall net zero spending on the program. It initially put hospitals at risk for 1% of their Medicare inpatient payments (in 2013), with a progressive increase to 2% by this year. HVBP has used a complex approach to determining incentives and penalties, rewarding either improvement or achievement, depending on the baseline performance of the hospital.
Has HVBP improved quality? Two studies looking at the early period of HVBP failed to show improvements in process or patient experience measures and demonstrated no change in mortality for heart failure, pneumonia, or heart attack.1,2 Now that the program is in its 5th and final year, thanks to a recent study by Ryan et al., we have an idea if HVBP is associated with longer-term improvements in quality.3
In the study, Ryan et al. compared hospitals participating in HVBP with critical access hospitals, which are exempt from the program. The study yielded some disappointing, if not surprising, results. Improvements in process and patient experience measures for HVBP hospitals were no greater than those for the control group. HVBP was not associated with a significant reduction in mortality for heart failure or heart attack, but was associated with a mortality reduction for pneumonia. In sum, HVBP was not associated with improvements in process or patient experience, and was not associated with lower mortality, except in pneumonia.
As a program designed to incentivize better quality, where did HVBP go wrong? I believe HVBP simply had too many measures for the cognitive bandwidth of an individual or a team looking to improve quality. The total measure count for 2017 is 21! I submit that a hospitalist working to improve quality can keep top-of-mind one or two measures, possibly three at most. While others have postulated that the amount of dollars at risk are too small, I don’t think that’s the problem. Instead, my sense is that hospitalists and other members of the hospital team have quality improvement in their DNA and, regardless of the size of the financial incentives, will work to improve it as long as they have the right tools. Chief among these are good performance data and the time to focus on a finite number of projects.
What lessons can inform better design in the future? As of January 2017, the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) – representing the biggest change in reimbursement in a generation – progressively exposes doctors and other professionals to upside/downside incentives for quality, resource utilization, use of a certified electronic health record (hospitalists are exempt as they already use the hospital’s EHR), and practice improvement activities.
It would be wise to learn from the shortcomings of HVBP. Namely, if MACRA keeps on its course to incentivize physicians using a complicated formula based on four domains and many more subdomains, it will repeat the mistakes of HVBP and – while creating more administrative burden – likely improve quality very little, if at all. Instead, MACRA should delineate a simple measure set representing improvement activities that physicians and teams can incorporate into their regular work flow without more time taken away from patient care.
The reality is that complicated pay-for-performance programs divert limited available resources away from meaningful improvement activities in order to comply with onerous reporting requirements. As we gain a more nuanced understanding of how these programs work, policy makers should pay attention to the elements of “low-value” and “high-value” incentive systems and apply the “less is more” ethos of high-value care to the next generation of pay-for-performance programs.
Dr. Whitcomb is chief medical officer at Remedy Partners in Darien, Conn., and a cofounder and past president of SHM.
References
1. Ryan AM, Burgess JF, Pesko MF, Borden WB, Dimick JB. “The early effects of Medicare’s mandatory hospital pay-for-performance program” Health Serv Res. 2015;50:81-97.
2. Figueroa JF, Tsugawa Y, Zheng J, Orav EJ, Jha AK. “Association between the Value-Based Purchasing pay for performance program and patient mortality in US hospitals: observational study” BMJ. 2016;353:i2214.
3. Ryan AM, Krinsky S, Maurer KA, Dimick JB. “Changes in Hospital Quality Associated with Hospital Value-Based Purchasing” N Engl J Med. 2017;376:2358-66.