User login
CCSs have higher burden of chronic conditions
Adult survivors of childhood cancer have a greater cumulative burden of chronic health conditions than the general public, according to research published in The Lancet.
The study showed that, by age 50, childhood cancer survivors (CCSs) had experienced, on average, 17.1 chronic health conditions, and matched control subjects had experienced 9.2.
“The cumulative burden of chronic disease revealed in this analysis, along with the complexity and severity of chronic conditions some survivors experience, found childhood cancer survivors to be a vulnerable, medically complex population,” said study author Nickhill Bhakta, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
For this study, Dr Bhakta and his colleagues assessed the lifelong impact of 168 chronic health conditions—such as hepatic, thyroid, ocular, and reproductive disorders—on CCSs and control subjects.
The 3010 evaluable CCSs had survived 10 years or longer from their initial cancer diagnosis and were 18 years or older as of June 30, 2015. The 272 controls had no history of pediatric cancer and were matched to CCSs by age and sex.
At age 50, the cumulative incidence of chronic health conditions (of any grade) was 99.9% in CCSs and 96.0% in controls (P<0.0001). The cumulative incidence of grade 3 to 5 chronic health conditions was 96.0% and 84.9%, respectively (P<0.0001).
The cumulative burden for CCSs was 17.1 chronic health conditions, including 4.7 that were grade 3 to 5. For controls, the cumulative burden was 9.2 chronic health conditions, including 2.3 that were grade 3 to 5 (P<0.0001 for both comparisons).
The researchers said second neoplasms, spinal disorders, and pulmonary disease were major contributors to the excess total cumulative burden observed in CCSs. However, there was “notable heterogeneity” in burden according to the patients’ primary cancer diagnosis.
For instance, growth hormone deficiency was in the top 10th percentile of chronic health conditions for survivors of acute lymphoblastic leukemia but not for controls.
And pulmonary function deficits were in the top 10th percentile for survivors of acute myeloid leukemia and Hodgkin lymphoma but not for controls or survivors of acute lymphoblastic leukemia or non-Hodgkin lymphoma.
“This study found that the average childhood cancer survivor has a cumulative burden of chronic disease that requires a significant time investment by healthcare providers to disentangle and manage—time that community providers are unlikely to have,” Dr Bhakta said.
“The results suggest that childhood cancer survivors may benefit from the integrated, specialized healthcare delivery that is being tried for individuals infected with HIV or those with other complex, chronic health problems.” ![]()
Adult survivors of childhood cancer have a greater cumulative burden of chronic health conditions than the general public, according to research published in The Lancet.
The study showed that, by age 50, childhood cancer survivors (CCSs) had experienced, on average, 17.1 chronic health conditions, and matched control subjects had experienced 9.2.
“The cumulative burden of chronic disease revealed in this analysis, along with the complexity and severity of chronic conditions some survivors experience, found childhood cancer survivors to be a vulnerable, medically complex population,” said study author Nickhill Bhakta, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
For this study, Dr Bhakta and his colleagues assessed the lifelong impact of 168 chronic health conditions—such as hepatic, thyroid, ocular, and reproductive disorders—on CCSs and control subjects.
The 3010 evaluable CCSs had survived 10 years or longer from their initial cancer diagnosis and were 18 years or older as of June 30, 2015. The 272 controls had no history of pediatric cancer and were matched to CCSs by age and sex.
At age 50, the cumulative incidence of chronic health conditions (of any grade) was 99.9% in CCSs and 96.0% in controls (P<0.0001). The cumulative incidence of grade 3 to 5 chronic health conditions was 96.0% and 84.9%, respectively (P<0.0001).
The cumulative burden for CCSs was 17.1 chronic health conditions, including 4.7 that were grade 3 to 5. For controls, the cumulative burden was 9.2 chronic health conditions, including 2.3 that were grade 3 to 5 (P<0.0001 for both comparisons).
The researchers said second neoplasms, spinal disorders, and pulmonary disease were major contributors to the excess total cumulative burden observed in CCSs. However, there was “notable heterogeneity” in burden according to the patients’ primary cancer diagnosis.
For instance, growth hormone deficiency was in the top 10th percentile of chronic health conditions for survivors of acute lymphoblastic leukemia but not for controls.
And pulmonary function deficits were in the top 10th percentile for survivors of acute myeloid leukemia and Hodgkin lymphoma but not for controls or survivors of acute lymphoblastic leukemia or non-Hodgkin lymphoma.
“This study found that the average childhood cancer survivor has a cumulative burden of chronic disease that requires a significant time investment by healthcare providers to disentangle and manage—time that community providers are unlikely to have,” Dr Bhakta said.
“The results suggest that childhood cancer survivors may benefit from the integrated, specialized healthcare delivery that is being tried for individuals infected with HIV or those with other complex, chronic health problems.” ![]()
Adult survivors of childhood cancer have a greater cumulative burden of chronic health conditions than the general public, according to research published in The Lancet.
The study showed that, by age 50, childhood cancer survivors (CCSs) had experienced, on average, 17.1 chronic health conditions, and matched control subjects had experienced 9.2.
“The cumulative burden of chronic disease revealed in this analysis, along with the complexity and severity of chronic conditions some survivors experience, found childhood cancer survivors to be a vulnerable, medically complex population,” said study author Nickhill Bhakta, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
For this study, Dr Bhakta and his colleagues assessed the lifelong impact of 168 chronic health conditions—such as hepatic, thyroid, ocular, and reproductive disorders—on CCSs and control subjects.
The 3010 evaluable CCSs had survived 10 years or longer from their initial cancer diagnosis and were 18 years or older as of June 30, 2015. The 272 controls had no history of pediatric cancer and were matched to CCSs by age and sex.
At age 50, the cumulative incidence of chronic health conditions (of any grade) was 99.9% in CCSs and 96.0% in controls (P<0.0001). The cumulative incidence of grade 3 to 5 chronic health conditions was 96.0% and 84.9%, respectively (P<0.0001).
The cumulative burden for CCSs was 17.1 chronic health conditions, including 4.7 that were grade 3 to 5. For controls, the cumulative burden was 9.2 chronic health conditions, including 2.3 that were grade 3 to 5 (P<0.0001 for both comparisons).
The researchers said second neoplasms, spinal disorders, and pulmonary disease were major contributors to the excess total cumulative burden observed in CCSs. However, there was “notable heterogeneity” in burden according to the patients’ primary cancer diagnosis.
For instance, growth hormone deficiency was in the top 10th percentile of chronic health conditions for survivors of acute lymphoblastic leukemia but not for controls.
And pulmonary function deficits were in the top 10th percentile for survivors of acute myeloid leukemia and Hodgkin lymphoma but not for controls or survivors of acute lymphoblastic leukemia or non-Hodgkin lymphoma.
“This study found that the average childhood cancer survivor has a cumulative burden of chronic disease that requires a significant time investment by healthcare providers to disentangle and manage—time that community providers are unlikely to have,” Dr Bhakta said.
“The results suggest that childhood cancer survivors may benefit from the integrated, specialized healthcare delivery that is being tried for individuals infected with HIV or those with other complex, chronic health problems.” ![]()
How to tackle telangiectasias with light
AT MOAS 2017
SAN DIEGO – Multiple light-based devices can be used to treat telangiectasias, often with little or no down time.
During a presentation at the annual Masters of Aesthetics Symposium, Kristen M. Kelly, MD, listed her preferred light-based treatment options for telangiectasias as the pulsed dye laser (PDL), the 532 nm Nd:YAG laser, and the diode laser. The PDL has been around the longest and “is one of the greatest devices for treating vasculature, because it’s very specific,” she said. Purpura can result, but for cosmetic indications, pulse durations of 6 milliseconds or more work well. “Keep in mind, though, that when you’re using longer pulse durations, patients will often require more than one treatment,” she said. “Most people are okay with that, knowing that they’re going to have minimal to no down time with these procedures.”
Dr. Kelly, professor of dermatology and surgery at the University of California, Irvine, noted that erythematotelangiectatic rosacea generally responds well to light-based devices, while papulopustular/inflammatory forms of the condition are best treated with anti-inflammatory agents or antibiotics. Multiple passes or judicious pulse stacking can be used with PDL for treating telangiectases, “but always know what your endpoint is,” Dr. Kelly advised. “You generally don’t want to pulse over purpura when treating telangiectasias; that just increases the risk of adverse effects.”
Settings to consider vary, depending on the patient and the area to be treated. The desired endpoints for telangiectasia are vessel blanching or diminution, or a darker red/purple change to the vessel. “You should not see graying or whitening of the skin; that’s when you’re getting epidermal damage, so you want to watch for that,” she said. For a first pass, Dr. Kelly will often treat with a spot size of 10 mm or 12 mm with a 6-millisecond pulse duration with cryogen spray cooling set at 30 milliseconds with a 30-millisecond delay. If a second pass is required, she will often treat with a spot size of 7 mm and a 6-millisecond pulse duration and slightly higher fluence, again using cryogen spray cooling. “It is important to know your specific device to determine settings,” she said.
The frequency-doubled Nd:YAG laser features a wavelength of 532 nm but requires cautious use in patients with darker skin types or photodamaged skin. The desired endpoint is a darkening of the vessel or vessel disappearance. “Many of these devices have contact cooling, which is an excellent method of cooling, but you have to make sure you keep the contact,” she said. Other cooling options include cryogen spray and the application of cold air. “When you’re doing cold-air cooling, you want to be careful not to point it toward someone’s nose,” she said. “It sounds funny, but this is uncomfortable for the patient.”
Diode lasers with wavelengths of 800-980 nm allow the user to treat larger blood vessels, such as nasal telangiectasias. “Some devices with small spot sizes allow you to trace along the vessel, and you can see it disappear before your eyes, and when the patient leaves, they’re generally very happy,” she said.
Intense pulsed light works well in patients with both pigmentary and vascular changes, especially for poikiloderma with brown and red pigment. “Multiple treatments are required, and there is significant melanin absorption, so you don’t want to treat people with tanned skin,” she said.
Dr. Kelly emphasized the importance of being familiar with the device you use and knowing the desired tissue endpoint. Decisions depend on the patient’s skin type, their tolerance for down time and multiple treatments, and the devices at your disposal. “Patients should be aware of all options, even if you may not have a certain device in your office,” she said. “You can refer them.”
She disclosed having drugs or devices donated by Light Sciences Oncology, Solta Medical, Syneron Candela, ThermiRF, and Novartis. She is also a consultant for MundiPharma, Allergan, and Syneron Candela.
[email protected]
AT MOAS 2017
SAN DIEGO – Multiple light-based devices can be used to treat telangiectasias, often with little or no down time.
During a presentation at the annual Masters of Aesthetics Symposium, Kristen M. Kelly, MD, listed her preferred light-based treatment options for telangiectasias as the pulsed dye laser (PDL), the 532 nm Nd:YAG laser, and the diode laser. The PDL has been around the longest and “is one of the greatest devices for treating vasculature, because it’s very specific,” she said. Purpura can result, but for cosmetic indications, pulse durations of 6 milliseconds or more work well. “Keep in mind, though, that when you’re using longer pulse durations, patients will often require more than one treatment,” she said. “Most people are okay with that, knowing that they’re going to have minimal to no down time with these procedures.”
Dr. Kelly, professor of dermatology and surgery at the University of California, Irvine, noted that erythematotelangiectatic rosacea generally responds well to light-based devices, while papulopustular/inflammatory forms of the condition are best treated with anti-inflammatory agents or antibiotics. Multiple passes or judicious pulse stacking can be used with PDL for treating telangiectases, “but always know what your endpoint is,” Dr. Kelly advised. “You generally don’t want to pulse over purpura when treating telangiectasias; that just increases the risk of adverse effects.”
Settings to consider vary, depending on the patient and the area to be treated. The desired endpoints for telangiectasia are vessel blanching or diminution, or a darker red/purple change to the vessel. “You should not see graying or whitening of the skin; that’s when you’re getting epidermal damage, so you want to watch for that,” she said. For a first pass, Dr. Kelly will often treat with a spot size of 10 mm or 12 mm with a 6-millisecond pulse duration with cryogen spray cooling set at 30 milliseconds with a 30-millisecond delay. If a second pass is required, she will often treat with a spot size of 7 mm and a 6-millisecond pulse duration and slightly higher fluence, again using cryogen spray cooling. “It is important to know your specific device to determine settings,” she said.
The frequency-doubled Nd:YAG laser features a wavelength of 532 nm but requires cautious use in patients with darker skin types or photodamaged skin. The desired endpoint is a darkening of the vessel or vessel disappearance. “Many of these devices have contact cooling, which is an excellent method of cooling, but you have to make sure you keep the contact,” she said. Other cooling options include cryogen spray and the application of cold air. “When you’re doing cold-air cooling, you want to be careful not to point it toward someone’s nose,” she said. “It sounds funny, but this is uncomfortable for the patient.”
Diode lasers with wavelengths of 800-980 nm allow the user to treat larger blood vessels, such as nasal telangiectasias. “Some devices with small spot sizes allow you to trace along the vessel, and you can see it disappear before your eyes, and when the patient leaves, they’re generally very happy,” she said.
Intense pulsed light works well in patients with both pigmentary and vascular changes, especially for poikiloderma with brown and red pigment. “Multiple treatments are required, and there is significant melanin absorption, so you don’t want to treat people with tanned skin,” she said.
Dr. Kelly emphasized the importance of being familiar with the device you use and knowing the desired tissue endpoint. Decisions depend on the patient’s skin type, their tolerance for down time and multiple treatments, and the devices at your disposal. “Patients should be aware of all options, even if you may not have a certain device in your office,” she said. “You can refer them.”
She disclosed having drugs or devices donated by Light Sciences Oncology, Solta Medical, Syneron Candela, ThermiRF, and Novartis. She is also a consultant for MundiPharma, Allergan, and Syneron Candela.
[email protected]
AT MOAS 2017
SAN DIEGO – Multiple light-based devices can be used to treat telangiectasias, often with little or no down time.
During a presentation at the annual Masters of Aesthetics Symposium, Kristen M. Kelly, MD, listed her preferred light-based treatment options for telangiectasias as the pulsed dye laser (PDL), the 532 nm Nd:YAG laser, and the diode laser. The PDL has been around the longest and “is one of the greatest devices for treating vasculature, because it’s very specific,” she said. Purpura can result, but for cosmetic indications, pulse durations of 6 milliseconds or more work well. “Keep in mind, though, that when you’re using longer pulse durations, patients will often require more than one treatment,” she said. “Most people are okay with that, knowing that they’re going to have minimal to no down time with these procedures.”
Dr. Kelly, professor of dermatology and surgery at the University of California, Irvine, noted that erythematotelangiectatic rosacea generally responds well to light-based devices, while papulopustular/inflammatory forms of the condition are best treated with anti-inflammatory agents or antibiotics. Multiple passes or judicious pulse stacking can be used with PDL for treating telangiectases, “but always know what your endpoint is,” Dr. Kelly advised. “You generally don’t want to pulse over purpura when treating telangiectasias; that just increases the risk of adverse effects.”
Settings to consider vary, depending on the patient and the area to be treated. The desired endpoints for telangiectasia are vessel blanching or diminution, or a darker red/purple change to the vessel. “You should not see graying or whitening of the skin; that’s when you’re getting epidermal damage, so you want to watch for that,” she said. For a first pass, Dr. Kelly will often treat with a spot size of 10 mm or 12 mm with a 6-millisecond pulse duration with cryogen spray cooling set at 30 milliseconds with a 30-millisecond delay. If a second pass is required, she will often treat with a spot size of 7 mm and a 6-millisecond pulse duration and slightly higher fluence, again using cryogen spray cooling. “It is important to know your specific device to determine settings,” she said.
The frequency-doubled Nd:YAG laser features a wavelength of 532 nm but requires cautious use in patients with darker skin types or photodamaged skin. The desired endpoint is a darkening of the vessel or vessel disappearance. “Many of these devices have contact cooling, which is an excellent method of cooling, but you have to make sure you keep the contact,” she said. Other cooling options include cryogen spray and the application of cold air. “When you’re doing cold-air cooling, you want to be careful not to point it toward someone’s nose,” she said. “It sounds funny, but this is uncomfortable for the patient.”
Diode lasers with wavelengths of 800-980 nm allow the user to treat larger blood vessels, such as nasal telangiectasias. “Some devices with small spot sizes allow you to trace along the vessel, and you can see it disappear before your eyes, and when the patient leaves, they’re generally very happy,” she said.
Intense pulsed light works well in patients with both pigmentary and vascular changes, especially for poikiloderma with brown and red pigment. “Multiple treatments are required, and there is significant melanin absorption, so you don’t want to treat people with tanned skin,” she said.
Dr. Kelly emphasized the importance of being familiar with the device you use and knowing the desired tissue endpoint. Decisions depend on the patient’s skin type, their tolerance for down time and multiple treatments, and the devices at your disposal. “Patients should be aware of all options, even if you may not have a certain device in your office,” she said. “You can refer them.”
She disclosed having drugs or devices donated by Light Sciences Oncology, Solta Medical, Syneron Candela, ThermiRF, and Novartis. She is also a consultant for MundiPharma, Allergan, and Syneron Candela.
[email protected]
Absorb bioresorbable scaffold folds
The first bioresorbable vascular scaffold to reach clinical practice is no longer available because of poor sales, following nearly a year of bad news for the innovative device.
"We took this decision for commercial reasons, not for safety," Kristina Becker, director of communications at Abbott's Vascular business. Abbott announced that sales of the Absorb scaffold would cease worldwide as of Sept. 14, 2017, adding that the company would continue to follow patients in existing clinical trials according to their protocols.
The device was approved by the Food and Drug Administration in July 2016, but soon after, long-term trial results took an ominous turn. In March 2017, the agency issued a safety alert regarding the Absorb scaffold after release of the 2-year data from the 2,008-patient ABSORB III trial showing a significantly higher rate of target-lesion failure than with the everolimus-eluting metallic Xience stent, also marketed by Abbott.
Meanwhile, 3-year results of the ABSORB II trial, reported last October, revealed a 1% per year rate of late stent thrombosis during both the second and third years following Absorb placement in coronary arteries, the period when the Absorb scaffold was in the process of disappearing. More bad news came in July, when device thrombosis occurred nearly four times more frequently in recipients of the Absorb scaffold than with the Xience stent during 2 years of prospective follow-up in the randomized, investigator-initiated AIDA trial.
Abbott is not giving up on the idea of nonpermanent stents. “We recognize that [bioresorbable scaffold] technologies offer patients the possibility of life without permanent metallic implants, and we will continue to work on a next generation device while monitoring long-term outcomes after stent resorption in current Absorb trials,” the announcement said.
This article was updated 9/8/17.
The first bioresorbable vascular scaffold to reach clinical practice is no longer available because of poor sales, following nearly a year of bad news for the innovative device.
"We took this decision for commercial reasons, not for safety," Kristina Becker, director of communications at Abbott's Vascular business. Abbott announced that sales of the Absorb scaffold would cease worldwide as of Sept. 14, 2017, adding that the company would continue to follow patients in existing clinical trials according to their protocols.
The device was approved by the Food and Drug Administration in July 2016, but soon after, long-term trial results took an ominous turn. In March 2017, the agency issued a safety alert regarding the Absorb scaffold after release of the 2-year data from the 2,008-patient ABSORB III trial showing a significantly higher rate of target-lesion failure than with the everolimus-eluting metallic Xience stent, also marketed by Abbott.
Meanwhile, 3-year results of the ABSORB II trial, reported last October, revealed a 1% per year rate of late stent thrombosis during both the second and third years following Absorb placement in coronary arteries, the period when the Absorb scaffold was in the process of disappearing. More bad news came in July, when device thrombosis occurred nearly four times more frequently in recipients of the Absorb scaffold than with the Xience stent during 2 years of prospective follow-up in the randomized, investigator-initiated AIDA trial.
Abbott is not giving up on the idea of nonpermanent stents. “We recognize that [bioresorbable scaffold] technologies offer patients the possibility of life without permanent metallic implants, and we will continue to work on a next generation device while monitoring long-term outcomes after stent resorption in current Absorb trials,” the announcement said.
This article was updated 9/8/17.
The first bioresorbable vascular scaffold to reach clinical practice is no longer available because of poor sales, following nearly a year of bad news for the innovative device.
"We took this decision for commercial reasons, not for safety," Kristina Becker, director of communications at Abbott's Vascular business. Abbott announced that sales of the Absorb scaffold would cease worldwide as of Sept. 14, 2017, adding that the company would continue to follow patients in existing clinical trials according to their protocols.
The device was approved by the Food and Drug Administration in July 2016, but soon after, long-term trial results took an ominous turn. In March 2017, the agency issued a safety alert regarding the Absorb scaffold after release of the 2-year data from the 2,008-patient ABSORB III trial showing a significantly higher rate of target-lesion failure than with the everolimus-eluting metallic Xience stent, also marketed by Abbott.
Meanwhile, 3-year results of the ABSORB II trial, reported last October, revealed a 1% per year rate of late stent thrombosis during both the second and third years following Absorb placement in coronary arteries, the period when the Absorb scaffold was in the process of disappearing. More bad news came in July, when device thrombosis occurred nearly four times more frequently in recipients of the Absorb scaffold than with the Xience stent during 2 years of prospective follow-up in the randomized, investigator-initiated AIDA trial.
Abbott is not giving up on the idea of nonpermanent stents. “We recognize that [bioresorbable scaffold] technologies offer patients the possibility of life without permanent metallic implants, and we will continue to work on a next generation device while monitoring long-term outcomes after stent resorption in current Absorb trials,” the announcement said.
This article was updated 9/8/17.
Honoring a physician who led by example
In July, mostly unnoticed by Americans, a remarkable physician died in Japan.
Dr. Shigeaki Hinohara was a young 105 years old at the end, still practicing medicine.
When he was born in 1911, the average Japanese lifespan was 40. Due in part to him, it’s now one of the longest on Earth.
No stranger to medical disasters, he cared for those injured in the 1945 firebombing of Tokyo. Fifty years later, still working, he treated 640 victims of the 1995 nerve gas terror attack on the city’s subway. Between them, he survived being taken hostage in a 4-day plane hijacking in 1970.
He didn’t believe in retirement, since keeping busy is good. At the same time he advocated for finding fun in what you were doing.
A staunch opponent of obesity, he advocated a spartan lifestyle. For breakfast he had coffee, milk, and orange juice (the last with a spoonful of olive oil mixed in). For lunch (if he didn’t skip it) hard biscuits and milk. Dinner was vegetables, rice, and a small amount of either beef or fish.
He believed in exercise, even if it was limited to your daily routine. Always take stairs. Carry your own bags and packages. Even in his last months, using a cane, he walked 2,000 steps per day.
At the end, unable to eat, he still led by example. He refused a feeding tube and opted to leave quietly, passing on at home.
Medicine today, including my own field, is full of gadgets. Amazing tests and treatments. I believe in them 100%, and use them, as we all do, to help alleviate suffering and help people live longer and better lives.
But at the same time, we need to keep in mind that prevention is the best treatment. Keeping your mind active is good. Palliative care doesn’t mean you gave up.
In a world of increasing obesity, diabetes, and vascular disease, his simple advice on exercise and eating modestly is a lesson for many, including myself.
Never underestimate the benefits of music and pets.
And always have fun.
Good night, good doctor.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In July, mostly unnoticed by Americans, a remarkable physician died in Japan.
Dr. Shigeaki Hinohara was a young 105 years old at the end, still practicing medicine.
When he was born in 1911, the average Japanese lifespan was 40. Due in part to him, it’s now one of the longest on Earth.
No stranger to medical disasters, he cared for those injured in the 1945 firebombing of Tokyo. Fifty years later, still working, he treated 640 victims of the 1995 nerve gas terror attack on the city’s subway. Between them, he survived being taken hostage in a 4-day plane hijacking in 1970.
He didn’t believe in retirement, since keeping busy is good. At the same time he advocated for finding fun in what you were doing.
A staunch opponent of obesity, he advocated a spartan lifestyle. For breakfast he had coffee, milk, and orange juice (the last with a spoonful of olive oil mixed in). For lunch (if he didn’t skip it) hard biscuits and milk. Dinner was vegetables, rice, and a small amount of either beef or fish.
He believed in exercise, even if it was limited to your daily routine. Always take stairs. Carry your own bags and packages. Even in his last months, using a cane, he walked 2,000 steps per day.
At the end, unable to eat, he still led by example. He refused a feeding tube and opted to leave quietly, passing on at home.
Medicine today, including my own field, is full of gadgets. Amazing tests and treatments. I believe in them 100%, and use them, as we all do, to help alleviate suffering and help people live longer and better lives.
But at the same time, we need to keep in mind that prevention is the best treatment. Keeping your mind active is good. Palliative care doesn’t mean you gave up.
In a world of increasing obesity, diabetes, and vascular disease, his simple advice on exercise and eating modestly is a lesson for many, including myself.
Never underestimate the benefits of music and pets.
And always have fun.
Good night, good doctor.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In July, mostly unnoticed by Americans, a remarkable physician died in Japan.
Dr. Shigeaki Hinohara was a young 105 years old at the end, still practicing medicine.
When he was born in 1911, the average Japanese lifespan was 40. Due in part to him, it’s now one of the longest on Earth.
No stranger to medical disasters, he cared for those injured in the 1945 firebombing of Tokyo. Fifty years later, still working, he treated 640 victims of the 1995 nerve gas terror attack on the city’s subway. Between them, he survived being taken hostage in a 4-day plane hijacking in 1970.
He didn’t believe in retirement, since keeping busy is good. At the same time he advocated for finding fun in what you were doing.
A staunch opponent of obesity, he advocated a spartan lifestyle. For breakfast he had coffee, milk, and orange juice (the last with a spoonful of olive oil mixed in). For lunch (if he didn’t skip it) hard biscuits and milk. Dinner was vegetables, rice, and a small amount of either beef or fish.
He believed in exercise, even if it was limited to your daily routine. Always take stairs. Carry your own bags and packages. Even in his last months, using a cane, he walked 2,000 steps per day.
At the end, unable to eat, he still led by example. He refused a feeding tube and opted to leave quietly, passing on at home.
Medicine today, including my own field, is full of gadgets. Amazing tests and treatments. I believe in them 100%, and use them, as we all do, to help alleviate suffering and help people live longer and better lives.
But at the same time, we need to keep in mind that prevention is the best treatment. Keeping your mind active is good. Palliative care doesn’t mean you gave up.
In a world of increasing obesity, diabetes, and vascular disease, his simple advice on exercise and eating modestly is a lesson for many, including myself.
Never underestimate the benefits of music and pets.
And always have fun.
Good night, good doctor.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
New AML approvals changing the treatment landscape
With a recent flurry of new drug approvals, the treatment landscape for acute myeloid leukemia has expanded, raising new questions about how to incorporate those drugs into patient care.
Until about a decade ago, advances in AML therapy centered mainly around iterations of daunorubicin and cytarabine. Now, novel and targeted agents, many specifically going after mutational byproducts, are yielding some great results and raising hopes for better survival outcomes, Jeffrey Lancet, MD, said in an interview.
“When I go to sleep at night, I often dream about ... 10-year survival rates in the 80% range. And then I wake up ... and I realize this is actually [the survival curve for chronic myeloid leukemia]. This is where we’d like to be [with AML].” Those outcomes are a long way off, but appreciable incremental gains may lie ahead with the recent advances in AML therapy, said Dr. Lancet, chair of the department of malignant hematology at Moffitt Cancer Center in Tampa.
In addition to the new approvals, 16 drugs are in late stage clinical development and will likely contribute to an AML market that is expected to surpass $1.5 billion by 2026, according to projections by the market intelligence company GlobalData.
Vyxeos
The liposome-encapsulated combination of daunorubicin and cytarabine (Vyxeos) was approved in August by the Food and Drug Administration for the treatment of therapy-related AML and AML with myelodysplasia-related changes.
In a phase 3 randomized trial, the fixed-dose combination product was associated with median overall survival of 9.6 months, compared with 5.9 months with a standard combination of cytarabine and daunorubicin (7+3).
“I would envision that Vyxeos will hold and become the primary standard of care for fit chemotherapy-suitable older patients, or any patients for that matter, who are dealing with secondary-like AML or high-risk AML, based on the phase 3 results that we demonstrated,” Dr. Lancet, the principal investigator for the trial, said in an interview.
Asked whether the improved survival with Vyxeos is primarily related to more patients becoming transplant eligible or to significant reductions in disease burden, Dr. Lancet remarked that it’s likely a mixture of both.
The high remission rate with Vyxeos vs. standard 7+3 therapy means Vyxeos has the ability to stand on its own, and “the potential to send more patients to transplant and to get better results.”
“Transplant is part of the continuum of care of AML, including in older patients, and Vyxeos is going to become a standard part of that care,” he remarked. But transplant outcomes were not a predesignated component of the phase 3 trial, and further study will be needed to determine Vyxeos’ role as a bridge to transplant. “At this stage I can reasonably state that it has a role in the upfront therapy of secondary and high-risk AML, regardless of whether the patient is being considered for transplant.”
The early stages of working Vyxeos into the therapeutic mix come with some challenges, however, according to Donna Capozzi, PharmD.
Vyxeos is a fixed-dose combination that comes in vials containing 44 mg daunorubicin and 100 mg cytarabine encapsulated in liposomes. Patient dosing is based on the daunorubicin component and calculated based on body surface area (mg/m2), meaning the cytarabine dose does not need to be calculated. There are both pros and cons to this approach, she explained.
Benefits include a longer half-life with Vyxeos vs. standard 7+3, and the fact that during induction the drug is delivered on days 1, 3, and 5 for 90 minutes rather than continuously for 7 days as with 7+3, Dr. Capozzi said.
The main concern relates to ensuring that the dosing is calculated based on the proper component, she said.
“We had our first patient last week. It was very time consuming, with double and triple checking to make sure everything was correct,” she said. Preparing the drug is also time-consuming, as it involves multiple steps, such as warming, which is not required with standard 7+3; the additional labor factors will have to be built into workflow, she noted.
“The other piece not fully in place right now is building [the use of Vyxeos] into electronic health records,” she said, adding that safeguards put into place through EHRs will also help to streamline the administration process.
For example, cardiac toxicity is a known effect of daunorubicin; the EHR will help track lifetime cumulative dosing of that component, which is otherwise challenging, especially when using a combination product, she said.
The process will get easier over time, as use of Vyxeos becomes more prevalent in practice, she added. “None of these are insurmountable issues.”
Cost is another matter. Based on average wholesale prices, the cost per cycle is approximately $40,000 with Vyxeos vs. about $4,300 for conventional 7+3 therapy, Dr. Capozzi said. Given the differential, there will be a great deal of debate as to which patients will derive the most benefit from Vyxeos, she said.
Also, it will take time to figure out the extent of adverse events. “For liposomal products in general, rash-type side effects can be really significant. Hand-foot syndrome was not reported in the initial trials, but we’ll keep our eyes open to see how that plays out,” she said noting that the one patient treated so far at the University of Pennsylvania is doing very well. “We will learn more with real world experience.”
Oral targeted therapies
Enasidenib (Idhifa) was approved under priority review in August in conjunction with a companion diagnostic IDH2 assay for patients with relapsed or refractory disease and specific mutations in the IDH2 gene. Midostaurin (Rydapt) was approved in April for use in conjunction with standard daunorubicin and cytarabine induction and cytarabine consolidation in adults with FLT3 mutation-positive AML.
In a phase 1 dose escalation study reported at the annual meeting of the European Hematology Association, enasidenib was associated with an overall response rate of 37% in patients with relapsed/refractory AML, including 20.1% complete responses and 7.9% complete responses with incomplete recovery of platelets or incomplete hematologic recovery, 3.7% with partial responses, and 5.1% with a morphologic leukemia-free state. Patients who had a CR had a median overall survival of 22.9 months. For patients with responses other than CR, the median OS was 15.1 months. For patients with no response to the drug, the median OS was 5.6 months, Dr. Eytan M. Stein, of Memorial Sloan Kettering Cancer Center in New York, reported.
Additionally, need for transfusions was reduced in 34% of 157 patients who required transfusions at study entry.
“In a relapsed or refractory group of patients where there’s no true standard of care, this drug definitely represents a major breakthrough and has a lot of utility as a single agent, as a potential bridge to a transplant, and in combination with new or even old drugs – including regular old induction chemotherapy as a way to improve responses and outcomes in the future,” Dr. Lancet said, adding that as an oral agent it has potential for development as a maintenance strategy.
This agent could have a large impact, he said, adding: “I think this sets the paradigm for novel targeted therapies.”
Midostaurin has also emerged as a new standard of care, particularly for younger patients, Dr. Lancet said.
The approval of the multitargeted kinase inhibitor was based on the results of the randomized, placebo-controlled phase 3 RATIFY trial, which demonstrated significantly longer overall and event-free survival vs. placebo and standard chemotherapy in newly diagnosed AML patients with FLT3 gene mutations.
“I think this will be the new comparator for future studies, whatever they may be, for this patient population,” he said.
Dr. Capozzi noted that she has had some difficulty obtaining prior authorization for enasidenib due to its high cost (about $1,000/day).
The drug is taken orally on days 8-21 of a 28-day treatment cycle. In RATIFY, patients who achieved complete remission after induction therapy received four 28-day cycles of consolidation therapy.
Dr. Capozzi noted that the dosing regimen can be confusing, as it changes depending on whether it is used for induction or consolidation. It remains to be seen how these agents will fit into the treatment setting, she said.
Targeted therapies in development
Other targeted therapies in development for AML include an IDH1 inhibitor, the BCL2 inhibitor venetoclax, and several second-generation FLT3 inhibitors such as gilteritinib, Dr. Lancet said.
Venetoclax, which is currently approved for chronic lymphocytic leukemia, has shown single agent activity, but is even more promising in combination with low-dose cytarabine or aza-nucleosides, he noted.
For example, in one recent study reported at the annual congress of the European Hematology Association, response rates in older, newly diagnosed AML patients were as high as 72% for azacitidine plus venetoclax, and 76% for decitabine plus venetoclax.
“So there’s a lot of interest and promise,” Dr. Lancet said, adding that venetoclax may have broad application in AML. “We’ll be seeing a lot more data in the next year or two.”
An unusual aspect of venetoclax, which is used often for CLL, is the need for observation during dose escalation, Dr. Capozzi noted. Patients tend to question the need for admission for observation with the use of an oral agent, thus efforts are underway to develop criteria for outpatient observation.
Otherwise, venetoclax is fairly easy to access and use, and is well tolerated, she said.
“I expect as we learn more about where (venetoclax) fits in, it will be a much more commonplace agent” as part of AML therapy, she said.
Gilteritinib, as well as the second generation FLT3 inhibitors quizartinib and crenolanib, are also of interest in AML. With midostaurin already on the market, however, different strategies are being pursued, Dr. Lancet said.
“I believe gilteritinib is entering the fray in relapsed/refractory disease, and crenolanib is being looked at in the upfront FLT3 AML-positive setting and ultimately will be compared to midostaurin in combination with chemotherapy in that setting,” he added, noting that these drugs have the advantage of being more potent and selective inhibitors of FLT3, and some appear to have the ability to target resistance-conferring mutations.
“It still remains to be determined what the ultimate role will be, especially now that midostaurin is approved as frontline therapy and, in my opinion, will likely be entrenched there for awhile,” he said. “It’s a fairly competitive field right now, but certainly one where there’s a lot of excitement. The encouraging part is the second generation inhibitors, especially crenolanib and gilteritinib, are able to rescue some patients who may have failed primary therapy with an FLT3 inhibitor.”
Future direction and outcomes
So how should one go about selecting therapies, in the absence of data on combining therapies, for patients with multiple mutations?
Ideally, that means teasing out which of the AML patient’s mutations is clonal and the driver of their disease, and which one is subclonal. There are no guarantees, but that seems like a rational way to begin and move the field forward to studies of combination therapies, Dr. Lancet said.
“I think with the right combinations that target leukemias that are mutationally driven, there is potential to treat subsets of patient with very targeted therapies that will lead to prolonged survival. Right now, for the most part, we don’t have drugs for many of the targets that are very important in AML, and we don’t always know which target is driving the disease ... these are considerations that remain to be discovered,” he said. “But I do think that in 10 years we will have the ability with novel drugs and increased understanding of the clinical relevance of these targets to really personalize the approach more so than we are today, and to increase response rates significantly and improve survival as a result.”
Dr. Lancet is a consultant for Jazz Pharmaceuticals, Daiichi Sankyo, and Celgene. Dr. Capozzi reported having no disclosures.
With a recent flurry of new drug approvals, the treatment landscape for acute myeloid leukemia has expanded, raising new questions about how to incorporate those drugs into patient care.
Until about a decade ago, advances in AML therapy centered mainly around iterations of daunorubicin and cytarabine. Now, novel and targeted agents, many specifically going after mutational byproducts, are yielding some great results and raising hopes for better survival outcomes, Jeffrey Lancet, MD, said in an interview.
“When I go to sleep at night, I often dream about ... 10-year survival rates in the 80% range. And then I wake up ... and I realize this is actually [the survival curve for chronic myeloid leukemia]. This is where we’d like to be [with AML].” Those outcomes are a long way off, but appreciable incremental gains may lie ahead with the recent advances in AML therapy, said Dr. Lancet, chair of the department of malignant hematology at Moffitt Cancer Center in Tampa.
In addition to the new approvals, 16 drugs are in late stage clinical development and will likely contribute to an AML market that is expected to surpass $1.5 billion by 2026, according to projections by the market intelligence company GlobalData.
Vyxeos
The liposome-encapsulated combination of daunorubicin and cytarabine (Vyxeos) was approved in August by the Food and Drug Administration for the treatment of therapy-related AML and AML with myelodysplasia-related changes.
In a phase 3 randomized trial, the fixed-dose combination product was associated with median overall survival of 9.6 months, compared with 5.9 months with a standard combination of cytarabine and daunorubicin (7+3).
“I would envision that Vyxeos will hold and become the primary standard of care for fit chemotherapy-suitable older patients, or any patients for that matter, who are dealing with secondary-like AML or high-risk AML, based on the phase 3 results that we demonstrated,” Dr. Lancet, the principal investigator for the trial, said in an interview.
Asked whether the improved survival with Vyxeos is primarily related to more patients becoming transplant eligible or to significant reductions in disease burden, Dr. Lancet remarked that it’s likely a mixture of both.
The high remission rate with Vyxeos vs. standard 7+3 therapy means Vyxeos has the ability to stand on its own, and “the potential to send more patients to transplant and to get better results.”
“Transplant is part of the continuum of care of AML, including in older patients, and Vyxeos is going to become a standard part of that care,” he remarked. But transplant outcomes were not a predesignated component of the phase 3 trial, and further study will be needed to determine Vyxeos’ role as a bridge to transplant. “At this stage I can reasonably state that it has a role in the upfront therapy of secondary and high-risk AML, regardless of whether the patient is being considered for transplant.”
The early stages of working Vyxeos into the therapeutic mix come with some challenges, however, according to Donna Capozzi, PharmD.
Vyxeos is a fixed-dose combination that comes in vials containing 44 mg daunorubicin and 100 mg cytarabine encapsulated in liposomes. Patient dosing is based on the daunorubicin component and calculated based on body surface area (mg/m2), meaning the cytarabine dose does not need to be calculated. There are both pros and cons to this approach, she explained.
Benefits include a longer half-life with Vyxeos vs. standard 7+3, and the fact that during induction the drug is delivered on days 1, 3, and 5 for 90 minutes rather than continuously for 7 days as with 7+3, Dr. Capozzi said.
The main concern relates to ensuring that the dosing is calculated based on the proper component, she said.
“We had our first patient last week. It was very time consuming, with double and triple checking to make sure everything was correct,” she said. Preparing the drug is also time-consuming, as it involves multiple steps, such as warming, which is not required with standard 7+3; the additional labor factors will have to be built into workflow, she noted.
“The other piece not fully in place right now is building [the use of Vyxeos] into electronic health records,” she said, adding that safeguards put into place through EHRs will also help to streamline the administration process.
For example, cardiac toxicity is a known effect of daunorubicin; the EHR will help track lifetime cumulative dosing of that component, which is otherwise challenging, especially when using a combination product, she said.
The process will get easier over time, as use of Vyxeos becomes more prevalent in practice, she added. “None of these are insurmountable issues.”
Cost is another matter. Based on average wholesale prices, the cost per cycle is approximately $40,000 with Vyxeos vs. about $4,300 for conventional 7+3 therapy, Dr. Capozzi said. Given the differential, there will be a great deal of debate as to which patients will derive the most benefit from Vyxeos, she said.
Also, it will take time to figure out the extent of adverse events. “For liposomal products in general, rash-type side effects can be really significant. Hand-foot syndrome was not reported in the initial trials, but we’ll keep our eyes open to see how that plays out,” she said noting that the one patient treated so far at the University of Pennsylvania is doing very well. “We will learn more with real world experience.”
Oral targeted therapies
Enasidenib (Idhifa) was approved under priority review in August in conjunction with a companion diagnostic IDH2 assay for patients with relapsed or refractory disease and specific mutations in the IDH2 gene. Midostaurin (Rydapt) was approved in April for use in conjunction with standard daunorubicin and cytarabine induction and cytarabine consolidation in adults with FLT3 mutation-positive AML.
In a phase 1 dose escalation study reported at the annual meeting of the European Hematology Association, enasidenib was associated with an overall response rate of 37% in patients with relapsed/refractory AML, including 20.1% complete responses and 7.9% complete responses with incomplete recovery of platelets or incomplete hematologic recovery, 3.7% with partial responses, and 5.1% with a morphologic leukemia-free state. Patients who had a CR had a median overall survival of 22.9 months. For patients with responses other than CR, the median OS was 15.1 months. For patients with no response to the drug, the median OS was 5.6 months, Dr. Eytan M. Stein, of Memorial Sloan Kettering Cancer Center in New York, reported.
Additionally, need for transfusions was reduced in 34% of 157 patients who required transfusions at study entry.
“In a relapsed or refractory group of patients where there’s no true standard of care, this drug definitely represents a major breakthrough and has a lot of utility as a single agent, as a potential bridge to a transplant, and in combination with new or even old drugs – including regular old induction chemotherapy as a way to improve responses and outcomes in the future,” Dr. Lancet said, adding that as an oral agent it has potential for development as a maintenance strategy.
This agent could have a large impact, he said, adding: “I think this sets the paradigm for novel targeted therapies.”
Midostaurin has also emerged as a new standard of care, particularly for younger patients, Dr. Lancet said.
The approval of the multitargeted kinase inhibitor was based on the results of the randomized, placebo-controlled phase 3 RATIFY trial, which demonstrated significantly longer overall and event-free survival vs. placebo and standard chemotherapy in newly diagnosed AML patients with FLT3 gene mutations.
“I think this will be the new comparator for future studies, whatever they may be, for this patient population,” he said.
Dr. Capozzi noted that she has had some difficulty obtaining prior authorization for enasidenib due to its high cost (about $1,000/day).
The drug is taken orally on days 8-21 of a 28-day treatment cycle. In RATIFY, patients who achieved complete remission after induction therapy received four 28-day cycles of consolidation therapy.
Dr. Capozzi noted that the dosing regimen can be confusing, as it changes depending on whether it is used for induction or consolidation. It remains to be seen how these agents will fit into the treatment setting, she said.
Targeted therapies in development
Other targeted therapies in development for AML include an IDH1 inhibitor, the BCL2 inhibitor venetoclax, and several second-generation FLT3 inhibitors such as gilteritinib, Dr. Lancet said.
Venetoclax, which is currently approved for chronic lymphocytic leukemia, has shown single agent activity, but is even more promising in combination with low-dose cytarabine or aza-nucleosides, he noted.
For example, in one recent study reported at the annual congress of the European Hematology Association, response rates in older, newly diagnosed AML patients were as high as 72% for azacitidine plus venetoclax, and 76% for decitabine plus venetoclax.
“So there’s a lot of interest and promise,” Dr. Lancet said, adding that venetoclax may have broad application in AML. “We’ll be seeing a lot more data in the next year or two.”
An unusual aspect of venetoclax, which is used often for CLL, is the need for observation during dose escalation, Dr. Capozzi noted. Patients tend to question the need for admission for observation with the use of an oral agent, thus efforts are underway to develop criteria for outpatient observation.
Otherwise, venetoclax is fairly easy to access and use, and is well tolerated, she said.
“I expect as we learn more about where (venetoclax) fits in, it will be a much more commonplace agent” as part of AML therapy, she said.
Gilteritinib, as well as the second generation FLT3 inhibitors quizartinib and crenolanib, are also of interest in AML. With midostaurin already on the market, however, different strategies are being pursued, Dr. Lancet said.
“I believe gilteritinib is entering the fray in relapsed/refractory disease, and crenolanib is being looked at in the upfront FLT3 AML-positive setting and ultimately will be compared to midostaurin in combination with chemotherapy in that setting,” he added, noting that these drugs have the advantage of being more potent and selective inhibitors of FLT3, and some appear to have the ability to target resistance-conferring mutations.
“It still remains to be determined what the ultimate role will be, especially now that midostaurin is approved as frontline therapy and, in my opinion, will likely be entrenched there for awhile,” he said. “It’s a fairly competitive field right now, but certainly one where there’s a lot of excitement. The encouraging part is the second generation inhibitors, especially crenolanib and gilteritinib, are able to rescue some patients who may have failed primary therapy with an FLT3 inhibitor.”
Future direction and outcomes
So how should one go about selecting therapies, in the absence of data on combining therapies, for patients with multiple mutations?
Ideally, that means teasing out which of the AML patient’s mutations is clonal and the driver of their disease, and which one is subclonal. There are no guarantees, but that seems like a rational way to begin and move the field forward to studies of combination therapies, Dr. Lancet said.
“I think with the right combinations that target leukemias that are mutationally driven, there is potential to treat subsets of patient with very targeted therapies that will lead to prolonged survival. Right now, for the most part, we don’t have drugs for many of the targets that are very important in AML, and we don’t always know which target is driving the disease ... these are considerations that remain to be discovered,” he said. “But I do think that in 10 years we will have the ability with novel drugs and increased understanding of the clinical relevance of these targets to really personalize the approach more so than we are today, and to increase response rates significantly and improve survival as a result.”
Dr. Lancet is a consultant for Jazz Pharmaceuticals, Daiichi Sankyo, and Celgene. Dr. Capozzi reported having no disclosures.
With a recent flurry of new drug approvals, the treatment landscape for acute myeloid leukemia has expanded, raising new questions about how to incorporate those drugs into patient care.
Until about a decade ago, advances in AML therapy centered mainly around iterations of daunorubicin and cytarabine. Now, novel and targeted agents, many specifically going after mutational byproducts, are yielding some great results and raising hopes for better survival outcomes, Jeffrey Lancet, MD, said in an interview.
“When I go to sleep at night, I often dream about ... 10-year survival rates in the 80% range. And then I wake up ... and I realize this is actually [the survival curve for chronic myeloid leukemia]. This is where we’d like to be [with AML].” Those outcomes are a long way off, but appreciable incremental gains may lie ahead with the recent advances in AML therapy, said Dr. Lancet, chair of the department of malignant hematology at Moffitt Cancer Center in Tampa.
In addition to the new approvals, 16 drugs are in late stage clinical development and will likely contribute to an AML market that is expected to surpass $1.5 billion by 2026, according to projections by the market intelligence company GlobalData.
Vyxeos
The liposome-encapsulated combination of daunorubicin and cytarabine (Vyxeos) was approved in August by the Food and Drug Administration for the treatment of therapy-related AML and AML with myelodysplasia-related changes.
In a phase 3 randomized trial, the fixed-dose combination product was associated with median overall survival of 9.6 months, compared with 5.9 months with a standard combination of cytarabine and daunorubicin (7+3).
“I would envision that Vyxeos will hold and become the primary standard of care for fit chemotherapy-suitable older patients, or any patients for that matter, who are dealing with secondary-like AML or high-risk AML, based on the phase 3 results that we demonstrated,” Dr. Lancet, the principal investigator for the trial, said in an interview.
Asked whether the improved survival with Vyxeos is primarily related to more patients becoming transplant eligible or to significant reductions in disease burden, Dr. Lancet remarked that it’s likely a mixture of both.
The high remission rate with Vyxeos vs. standard 7+3 therapy means Vyxeos has the ability to stand on its own, and “the potential to send more patients to transplant and to get better results.”
“Transplant is part of the continuum of care of AML, including in older patients, and Vyxeos is going to become a standard part of that care,” he remarked. But transplant outcomes were not a predesignated component of the phase 3 trial, and further study will be needed to determine Vyxeos’ role as a bridge to transplant. “At this stage I can reasonably state that it has a role in the upfront therapy of secondary and high-risk AML, regardless of whether the patient is being considered for transplant.”
The early stages of working Vyxeos into the therapeutic mix come with some challenges, however, according to Donna Capozzi, PharmD.
Vyxeos is a fixed-dose combination that comes in vials containing 44 mg daunorubicin and 100 mg cytarabine encapsulated in liposomes. Patient dosing is based on the daunorubicin component and calculated based on body surface area (mg/m2), meaning the cytarabine dose does not need to be calculated. There are both pros and cons to this approach, she explained.
Benefits include a longer half-life with Vyxeos vs. standard 7+3, and the fact that during induction the drug is delivered on days 1, 3, and 5 for 90 minutes rather than continuously for 7 days as with 7+3, Dr. Capozzi said.
The main concern relates to ensuring that the dosing is calculated based on the proper component, she said.
“We had our first patient last week. It was very time consuming, with double and triple checking to make sure everything was correct,” she said. Preparing the drug is also time-consuming, as it involves multiple steps, such as warming, which is not required with standard 7+3; the additional labor factors will have to be built into workflow, she noted.
“The other piece not fully in place right now is building [the use of Vyxeos] into electronic health records,” she said, adding that safeguards put into place through EHRs will also help to streamline the administration process.
For example, cardiac toxicity is a known effect of daunorubicin; the EHR will help track lifetime cumulative dosing of that component, which is otherwise challenging, especially when using a combination product, she said.
The process will get easier over time, as use of Vyxeos becomes more prevalent in practice, she added. “None of these are insurmountable issues.”
Cost is another matter. Based on average wholesale prices, the cost per cycle is approximately $40,000 with Vyxeos vs. about $4,300 for conventional 7+3 therapy, Dr. Capozzi said. Given the differential, there will be a great deal of debate as to which patients will derive the most benefit from Vyxeos, she said.
Also, it will take time to figure out the extent of adverse events. “For liposomal products in general, rash-type side effects can be really significant. Hand-foot syndrome was not reported in the initial trials, but we’ll keep our eyes open to see how that plays out,” she said noting that the one patient treated so far at the University of Pennsylvania is doing very well. “We will learn more with real world experience.”
Oral targeted therapies
Enasidenib (Idhifa) was approved under priority review in August in conjunction with a companion diagnostic IDH2 assay for patients with relapsed or refractory disease and specific mutations in the IDH2 gene. Midostaurin (Rydapt) was approved in April for use in conjunction with standard daunorubicin and cytarabine induction and cytarabine consolidation in adults with FLT3 mutation-positive AML.
In a phase 1 dose escalation study reported at the annual meeting of the European Hematology Association, enasidenib was associated with an overall response rate of 37% in patients with relapsed/refractory AML, including 20.1% complete responses and 7.9% complete responses with incomplete recovery of platelets or incomplete hematologic recovery, 3.7% with partial responses, and 5.1% with a morphologic leukemia-free state. Patients who had a CR had a median overall survival of 22.9 months. For patients with responses other than CR, the median OS was 15.1 months. For patients with no response to the drug, the median OS was 5.6 months, Dr. Eytan M. Stein, of Memorial Sloan Kettering Cancer Center in New York, reported.
Additionally, need for transfusions was reduced in 34% of 157 patients who required transfusions at study entry.
“In a relapsed or refractory group of patients where there’s no true standard of care, this drug definitely represents a major breakthrough and has a lot of utility as a single agent, as a potential bridge to a transplant, and in combination with new or even old drugs – including regular old induction chemotherapy as a way to improve responses and outcomes in the future,” Dr. Lancet said, adding that as an oral agent it has potential for development as a maintenance strategy.
This agent could have a large impact, he said, adding: “I think this sets the paradigm for novel targeted therapies.”
Midostaurin has also emerged as a new standard of care, particularly for younger patients, Dr. Lancet said.
The approval of the multitargeted kinase inhibitor was based on the results of the randomized, placebo-controlled phase 3 RATIFY trial, which demonstrated significantly longer overall and event-free survival vs. placebo and standard chemotherapy in newly diagnosed AML patients with FLT3 gene mutations.
“I think this will be the new comparator for future studies, whatever they may be, for this patient population,” he said.
Dr. Capozzi noted that she has had some difficulty obtaining prior authorization for enasidenib due to its high cost (about $1,000/day).
The drug is taken orally on days 8-21 of a 28-day treatment cycle. In RATIFY, patients who achieved complete remission after induction therapy received four 28-day cycles of consolidation therapy.
Dr. Capozzi noted that the dosing regimen can be confusing, as it changes depending on whether it is used for induction or consolidation. It remains to be seen how these agents will fit into the treatment setting, she said.
Targeted therapies in development
Other targeted therapies in development for AML include an IDH1 inhibitor, the BCL2 inhibitor venetoclax, and several second-generation FLT3 inhibitors such as gilteritinib, Dr. Lancet said.
Venetoclax, which is currently approved for chronic lymphocytic leukemia, has shown single agent activity, but is even more promising in combination with low-dose cytarabine or aza-nucleosides, he noted.
For example, in one recent study reported at the annual congress of the European Hematology Association, response rates in older, newly diagnosed AML patients were as high as 72% for azacitidine plus venetoclax, and 76% for decitabine plus venetoclax.
“So there’s a lot of interest and promise,” Dr. Lancet said, adding that venetoclax may have broad application in AML. “We’ll be seeing a lot more data in the next year or two.”
An unusual aspect of venetoclax, which is used often for CLL, is the need for observation during dose escalation, Dr. Capozzi noted. Patients tend to question the need for admission for observation with the use of an oral agent, thus efforts are underway to develop criteria for outpatient observation.
Otherwise, venetoclax is fairly easy to access and use, and is well tolerated, she said.
“I expect as we learn more about where (venetoclax) fits in, it will be a much more commonplace agent” as part of AML therapy, she said.
Gilteritinib, as well as the second generation FLT3 inhibitors quizartinib and crenolanib, are also of interest in AML. With midostaurin already on the market, however, different strategies are being pursued, Dr. Lancet said.
“I believe gilteritinib is entering the fray in relapsed/refractory disease, and crenolanib is being looked at in the upfront FLT3 AML-positive setting and ultimately will be compared to midostaurin in combination with chemotherapy in that setting,” he added, noting that these drugs have the advantage of being more potent and selective inhibitors of FLT3, and some appear to have the ability to target resistance-conferring mutations.
“It still remains to be determined what the ultimate role will be, especially now that midostaurin is approved as frontline therapy and, in my opinion, will likely be entrenched there for awhile,” he said. “It’s a fairly competitive field right now, but certainly one where there’s a lot of excitement. The encouraging part is the second generation inhibitors, especially crenolanib and gilteritinib, are able to rescue some patients who may have failed primary therapy with an FLT3 inhibitor.”
Future direction and outcomes
So how should one go about selecting therapies, in the absence of data on combining therapies, for patients with multiple mutations?
Ideally, that means teasing out which of the AML patient’s mutations is clonal and the driver of their disease, and which one is subclonal. There are no guarantees, but that seems like a rational way to begin and move the field forward to studies of combination therapies, Dr. Lancet said.
“I think with the right combinations that target leukemias that are mutationally driven, there is potential to treat subsets of patient with very targeted therapies that will lead to prolonged survival. Right now, for the most part, we don’t have drugs for many of the targets that are very important in AML, and we don’t always know which target is driving the disease ... these are considerations that remain to be discovered,” he said. “But I do think that in 10 years we will have the ability with novel drugs and increased understanding of the clinical relevance of these targets to really personalize the approach more so than we are today, and to increase response rates significantly and improve survival as a result.”
Dr. Lancet is a consultant for Jazz Pharmaceuticals, Daiichi Sankyo, and Celgene. Dr. Capozzi reported having no disclosures.
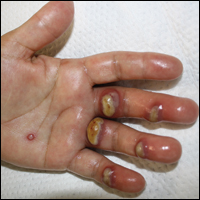
Tender Edematous Nodules on the Hand
The Diagnosis: Ecthyma Contagiosum (Orf)
Orf, or ecthyma contagiosum, is a zoonotic cutaneous infection caused by the orf DNA virus of the genus Parapoxvirus of the family Poxviridae. It is transmitted to humans through direct contact with infected animals, namely sheep and goats, and as such is most commonly seen in patients with occupational exposure to these animals such as butchers, farmers, veterinarians, and shepherds.1,2 Human-to-human transmission is exceedingly rare in immunocompetent patients.2,3 In affected animals, lesions usually are found around the mouth, muzzle, and eyes. In humans, hands are the most commonly affected site, and lesions occur 3 to 10 days after contact. Clinically, the lesions are nonspecific, and our patient presented with tender, erythematous, edematous nodules on the left hand. The differential diagnosis is broad and includes a milker's nodule, pyogenic granuloma, tularemia, anthrax, atypical mycobacterial infection, and sporotrichosis.1,4,5
The diagnosis usually is made with a thorough history and examination, but in cases of uncertainty, routine pathology with hematoxylin and eosin staining, electron microscopy, or real-time polymerase chain reaction may be used.2-4 Histopathologically, lesions demonstrate intraepidermal vesicles, vacuolization of keratinocytes of the upper epidermis with characteristic cytoplasmic inclusion bodies, rete ridge elongation, and dilated vessels in the intervening dermal papillae. Central necrosis may occur in well-developed lesions.2,6 Interestingly, our patient's biopsy exhibited all of these findings (Figure). Immunostains for cytomegalovirus and herpes simplex virus were negative, and Grocott-Gomori methenamine-silver and acid-fast bacillus stains also were negative.

Our patient also developed lymphangitic streaking suggestive of a bacterial superinfection and was treated with a course of intravenous antibiotics. She eventually was discharged with reassurance, wound care instructions, and outpatient antibiotics. She returned to an outside institution's emergency department for further evaluation, and she was admitted for workup. A lesional swab was sent for real-time polymerase chain reaction, which confirmed the diagnosis as orf. When the patient was contacted for follow-up 1 week after biopsy, the hand lesions had notably improved.
Orf is self-limited and typically resolves within 4 to 8 weeks after undergoing evolution through 5 described stages. The maculopapular stage is denoted by enlarging erythematous macule. The targetoid stage is described by a red center within a white halo surrounded by a broader red halo. The nodular stage is self-descriptive. The regenerative and regression stages describe the progressively improving, drier, and crusted nodules.3
Because orf is self-limited, no treatment is required, and patients should be counseled that their lesions should resolve within weeks. Complications include lymphangitis, secondary bacterial infection, and erythema multiforme.1,2,4,5 Immunocompromised patients may develop recalcitrant, giant, or multiple lesions that may be treated with topical imiquimod, topical cidofovir, intralesional interferon alfa, or surgical excision.1,2,4,7
We present a case of orf to remind practitioners of this rare entity. Although the disease is endemic worldwide, it likely is underreported due to its self-limited nature.2,4 A careful history may reveal the diagnosis, and overtreatment with antibiotics, many of which have their own significant side-effect profile, can then be avoided.
Acknowledgment
We thank Eric Behling, MD (Camden, New Jersey), for his contributions in obtaining the histologic images.
- Veraldi S, Nazzaro G, Vaira F, et al. Presentation of orf (ecthyma contagiosum) after sheep slaughtering for religious feasts. Infection. 2014;42:767-769.
- Al-Salam S, Nowotny N, Sohail MR, et al. Ecthyma contagiosum (orf)--report of a human case from the United Arab Emirates and review of the literature. J Cutan Pathol. 2008;35:603-607.
- Thurman RJ, Fitch RW. Images in clinical medicine. contagious ecthyma. N Engl J Med. 2015;372:E12.
- Meier R, Sommacal A, Stahel A, et al. Orf--an orphan disease? JRSM Open. 2015;6:2054270415593718.
- Joseph RH, Haddad FA, Matthews AL, et al. Erythema multiforme after orf virus infection: a report of two cases and literature review. Epidemiol Infect. 2015;143:385-390.
- Xu X, Yun SJ, Erikson L, et al. Diseases caused by viruses. In: Elder DE, Elenitsas R, Rosenbach M, eds. Lever's Histopathology of the Skin. 11th ed. Philadelphia, PA: Wolters Kluwer; 2015:781-815.
- Koufakis T, Katsaitis P, Gabranis I. Orf disease: a report of a case. Braz J Infect Dis. 2014;18:568-569.
The Diagnosis: Ecthyma Contagiosum (Orf)
Orf, or ecthyma contagiosum, is a zoonotic cutaneous infection caused by the orf DNA virus of the genus Parapoxvirus of the family Poxviridae. It is transmitted to humans through direct contact with infected animals, namely sheep and goats, and as such is most commonly seen in patients with occupational exposure to these animals such as butchers, farmers, veterinarians, and shepherds.1,2 Human-to-human transmission is exceedingly rare in immunocompetent patients.2,3 In affected animals, lesions usually are found around the mouth, muzzle, and eyes. In humans, hands are the most commonly affected site, and lesions occur 3 to 10 days after contact. Clinically, the lesions are nonspecific, and our patient presented with tender, erythematous, edematous nodules on the left hand. The differential diagnosis is broad and includes a milker's nodule, pyogenic granuloma, tularemia, anthrax, atypical mycobacterial infection, and sporotrichosis.1,4,5
The diagnosis usually is made with a thorough history and examination, but in cases of uncertainty, routine pathology with hematoxylin and eosin staining, electron microscopy, or real-time polymerase chain reaction may be used.2-4 Histopathologically, lesions demonstrate intraepidermal vesicles, vacuolization of keratinocytes of the upper epidermis with characteristic cytoplasmic inclusion bodies, rete ridge elongation, and dilated vessels in the intervening dermal papillae. Central necrosis may occur in well-developed lesions.2,6 Interestingly, our patient's biopsy exhibited all of these findings (Figure). Immunostains for cytomegalovirus and herpes simplex virus were negative, and Grocott-Gomori methenamine-silver and acid-fast bacillus stains also were negative.

Our patient also developed lymphangitic streaking suggestive of a bacterial superinfection and was treated with a course of intravenous antibiotics. She eventually was discharged with reassurance, wound care instructions, and outpatient antibiotics. She returned to an outside institution's emergency department for further evaluation, and she was admitted for workup. A lesional swab was sent for real-time polymerase chain reaction, which confirmed the diagnosis as orf. When the patient was contacted for follow-up 1 week after biopsy, the hand lesions had notably improved.
Orf is self-limited and typically resolves within 4 to 8 weeks after undergoing evolution through 5 described stages. The maculopapular stage is denoted by enlarging erythematous macule. The targetoid stage is described by a red center within a white halo surrounded by a broader red halo. The nodular stage is self-descriptive. The regenerative and regression stages describe the progressively improving, drier, and crusted nodules.3
Because orf is self-limited, no treatment is required, and patients should be counseled that their lesions should resolve within weeks. Complications include lymphangitis, secondary bacterial infection, and erythema multiforme.1,2,4,5 Immunocompromised patients may develop recalcitrant, giant, or multiple lesions that may be treated with topical imiquimod, topical cidofovir, intralesional interferon alfa, or surgical excision.1,2,4,7
We present a case of orf to remind practitioners of this rare entity. Although the disease is endemic worldwide, it likely is underreported due to its self-limited nature.2,4 A careful history may reveal the diagnosis, and overtreatment with antibiotics, many of which have their own significant side-effect profile, can then be avoided.
Acknowledgment
We thank Eric Behling, MD (Camden, New Jersey), for his contributions in obtaining the histologic images.
The Diagnosis: Ecthyma Contagiosum (Orf)
Orf, or ecthyma contagiosum, is a zoonotic cutaneous infection caused by the orf DNA virus of the genus Parapoxvirus of the family Poxviridae. It is transmitted to humans through direct contact with infected animals, namely sheep and goats, and as such is most commonly seen in patients with occupational exposure to these animals such as butchers, farmers, veterinarians, and shepherds.1,2 Human-to-human transmission is exceedingly rare in immunocompetent patients.2,3 In affected animals, lesions usually are found around the mouth, muzzle, and eyes. In humans, hands are the most commonly affected site, and lesions occur 3 to 10 days after contact. Clinically, the lesions are nonspecific, and our patient presented with tender, erythematous, edematous nodules on the left hand. The differential diagnosis is broad and includes a milker's nodule, pyogenic granuloma, tularemia, anthrax, atypical mycobacterial infection, and sporotrichosis.1,4,5
The diagnosis usually is made with a thorough history and examination, but in cases of uncertainty, routine pathology with hematoxylin and eosin staining, electron microscopy, or real-time polymerase chain reaction may be used.2-4 Histopathologically, lesions demonstrate intraepidermal vesicles, vacuolization of keratinocytes of the upper epidermis with characteristic cytoplasmic inclusion bodies, rete ridge elongation, and dilated vessels in the intervening dermal papillae. Central necrosis may occur in well-developed lesions.2,6 Interestingly, our patient's biopsy exhibited all of these findings (Figure). Immunostains for cytomegalovirus and herpes simplex virus were negative, and Grocott-Gomori methenamine-silver and acid-fast bacillus stains also were negative.

Our patient also developed lymphangitic streaking suggestive of a bacterial superinfection and was treated with a course of intravenous antibiotics. She eventually was discharged with reassurance, wound care instructions, and outpatient antibiotics. She returned to an outside institution's emergency department for further evaluation, and she was admitted for workup. A lesional swab was sent for real-time polymerase chain reaction, which confirmed the diagnosis as orf. When the patient was contacted for follow-up 1 week after biopsy, the hand lesions had notably improved.
Orf is self-limited and typically resolves within 4 to 8 weeks after undergoing evolution through 5 described stages. The maculopapular stage is denoted by enlarging erythematous macule. The targetoid stage is described by a red center within a white halo surrounded by a broader red halo. The nodular stage is self-descriptive. The regenerative and regression stages describe the progressively improving, drier, and crusted nodules.3
Because orf is self-limited, no treatment is required, and patients should be counseled that their lesions should resolve within weeks. Complications include lymphangitis, secondary bacterial infection, and erythema multiforme.1,2,4,5 Immunocompromised patients may develop recalcitrant, giant, or multiple lesions that may be treated with topical imiquimod, topical cidofovir, intralesional interferon alfa, or surgical excision.1,2,4,7
We present a case of orf to remind practitioners of this rare entity. Although the disease is endemic worldwide, it likely is underreported due to its self-limited nature.2,4 A careful history may reveal the diagnosis, and overtreatment with antibiotics, many of which have their own significant side-effect profile, can then be avoided.
Acknowledgment
We thank Eric Behling, MD (Camden, New Jersey), for his contributions in obtaining the histologic images.
- Veraldi S, Nazzaro G, Vaira F, et al. Presentation of orf (ecthyma contagiosum) after sheep slaughtering for religious feasts. Infection. 2014;42:767-769.
- Al-Salam S, Nowotny N, Sohail MR, et al. Ecthyma contagiosum (orf)--report of a human case from the United Arab Emirates and review of the literature. J Cutan Pathol. 2008;35:603-607.
- Thurman RJ, Fitch RW. Images in clinical medicine. contagious ecthyma. N Engl J Med. 2015;372:E12.
- Meier R, Sommacal A, Stahel A, et al. Orf--an orphan disease? JRSM Open. 2015;6:2054270415593718.
- Joseph RH, Haddad FA, Matthews AL, et al. Erythema multiforme after orf virus infection: a report of two cases and literature review. Epidemiol Infect. 2015;143:385-390.
- Xu X, Yun SJ, Erikson L, et al. Diseases caused by viruses. In: Elder DE, Elenitsas R, Rosenbach M, eds. Lever's Histopathology of the Skin. 11th ed. Philadelphia, PA: Wolters Kluwer; 2015:781-815.
- Koufakis T, Katsaitis P, Gabranis I. Orf disease: a report of a case. Braz J Infect Dis. 2014;18:568-569.
- Veraldi S, Nazzaro G, Vaira F, et al. Presentation of orf (ecthyma contagiosum) after sheep slaughtering for religious feasts. Infection. 2014;42:767-769.
- Al-Salam S, Nowotny N, Sohail MR, et al. Ecthyma contagiosum (orf)--report of a human case from the United Arab Emirates and review of the literature. J Cutan Pathol. 2008;35:603-607.
- Thurman RJ, Fitch RW. Images in clinical medicine. contagious ecthyma. N Engl J Med. 2015;372:E12.
- Meier R, Sommacal A, Stahel A, et al. Orf--an orphan disease? JRSM Open. 2015;6:2054270415593718.
- Joseph RH, Haddad FA, Matthews AL, et al. Erythema multiforme after orf virus infection: a report of two cases and literature review. Epidemiol Infect. 2015;143:385-390.
- Xu X, Yun SJ, Erikson L, et al. Diseases caused by viruses. In: Elder DE, Elenitsas R, Rosenbach M, eds. Lever's Histopathology of the Skin. 11th ed. Philadelphia, PA: Wolters Kluwer; 2015:781-815.
- Koufakis T, Katsaitis P, Gabranis I. Orf disease: a report of a case. Braz J Infect Dis. 2014;18:568-569.
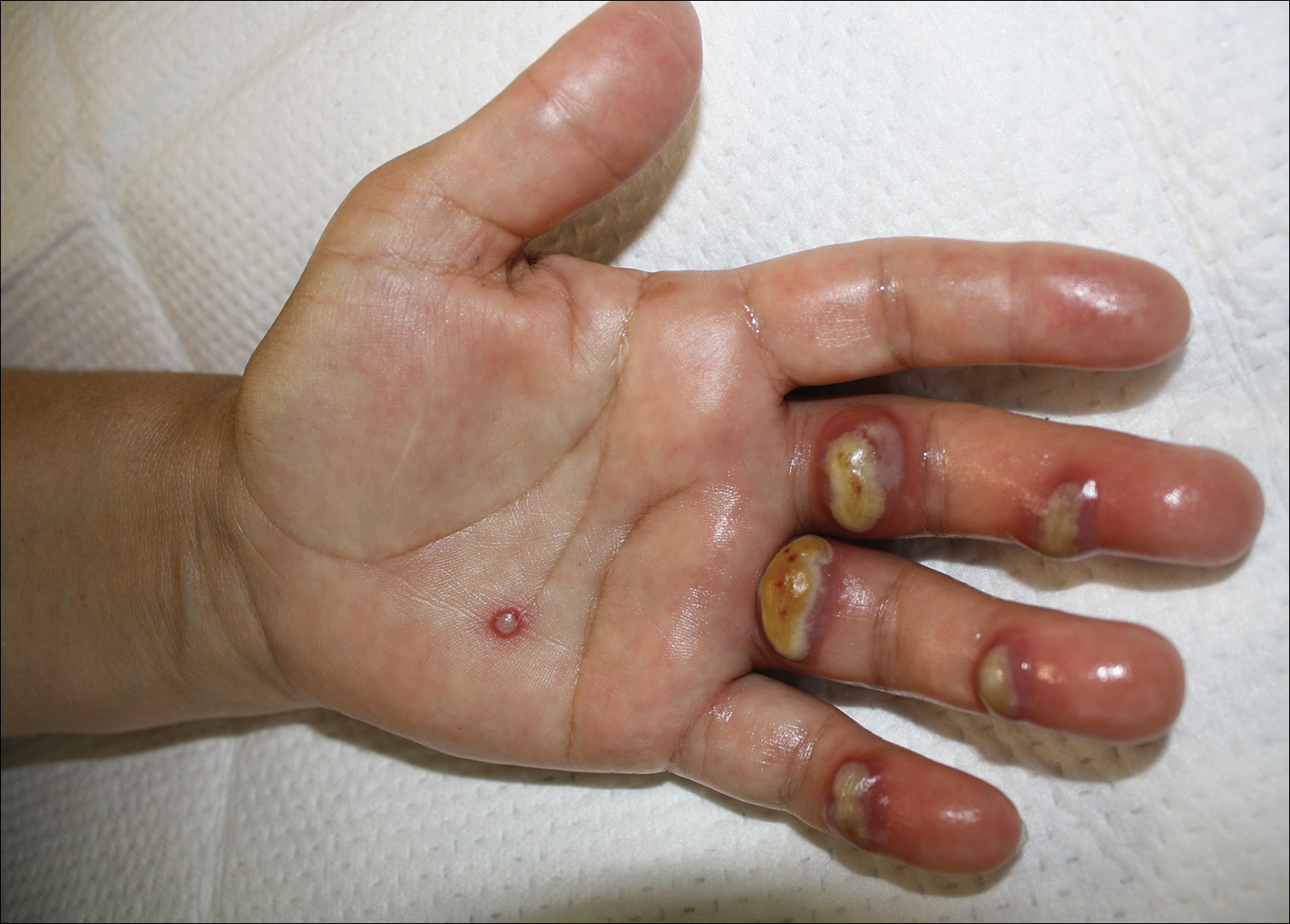
A 57-year-old woman presented to the emergency department (ED) for evaluation of a rash on the left hand of 2 weeks' duration. She described pinpoint red lesions on the left palm, as well as the third, fourth, and fifth fingers, which gradually enlarged and became painful. She denied any specific trauma but recalled cutting her hand on a piece of metal in the ground prior to the onset of the rash. She worked on a farm and bottle-fed sheep and chickens. Physical examination revealed tender edematous nodules with central gray pustules, and the left axillary lymph node was enlarged and tender. Ulceration was not appreciated. Various antibiotics including cephalexin, trimethoprim-sulfamethoxazole, and clindamycin were prescribed during prior ED visits, but she reported no improvement with these medications. She remained afebrile throughout the course of the hand rash, and laboratory workup was consistently unremarkable. Two sets of herpes simplex virus cultures from the ED visits showed no growth, and a hand radiograph also was normal. Medical history included coronary artery disease, myocardial infarction, mitral regurgitation, and hyperlipidemia.
Hospitalist movers and shakers – Sept. 2017
Robert Harrington, MD, recently was tabbed as chief medical officer of SurveyVitals, a health care analytics company specializing in digital patient-experience surveys. Dr. Harrington has 20 years experience, including CMO roles with Reliant Post–Acute Care Solutions and Locum Leaders, a hospitalist staffing firm.
Dr. Harrington is a senior fellow in Hospital Medicine and is past president and member of the board of directors with the Society of Hospital Medicine.
David Northington, DO, has been named the new chief medical officer at Stone County Hospital in Wiggins, Miss. The former hospitalist comes to Stone County after working as chief of staff and chief medical information officer at Memorial Hospital in Gulfport, Miss., where he was also medical director of the hospitalist program.
In addition to his new role, Dr. Northington will serve as medical director of the Woodland Village Nursing Center in Diamondhead, Miss., and the Stone County Nursing and Rehabilitation Center in Wiggins.
Schuyler K. Geller, MD, has been recognized by Continental Who’s Who as a Pinnacle Lifetime Member in the medical field. Dr. Geller works as a full-time hospitalist and a principal consultant for The CopperRidge Group, which provides guidance to patients in health, wellness, and fitness services and products.
In addition to his work at the CopperRidge Group, Dr. Geller is a member of Civil Vision International’s board of directors. He has extensive civilian and military-based experience in the United States, Africa, the Middle East, and South Asia.
A physician leader in the U.S. Air Force, Dr. Geller earned White House Medical Unit commendations for planning and leading the surgical and intensive care unit teams to support President Clinton’s trips to Vietnam and Africa in 2000.
Nikhil Sharma, MD, recently was selected by the International Association of HealthCare Professionals to be part of the Leading Physicians of the World. Dr. Sharma is a hospitalist serving at the Ochsner Health System in New Orleans.
Dr. Sharma, a member of the Southern Hospital Association and the Louisiana Medical Association, began his medical career in 2009 with a residency and fellowship at Ochsner, where he has remained ever since. He specializes in internal medicine and transplants.
I. Carol Nwelue, MD, a longtime hospitalist and the medical director of the Sparrow Medical Group Adult Hospitalist Service, recently received the Sparrow Physician Leadership Award. The award goes to an emerging leader who provides outstanding work in areas such as safety, clinical or service excellence, research, teaching, publishing, teamwork, and innovation.
Dr. Nwelue completed the Sparrow Physician Leadership Academy program, earning recognition for innovation in leadership, as well as practice management.
Laura Jin, MD, recently was promoted to medical director for utilization management at the University of Maryland Shore Regional Health. In her new role, Dr. Jin will identify and facilitate the resolution of utilization issues; in so doing, she will serve as a consultant leader to the health care system, its physicians, its advance practice providers, and the care management team.
Dr. Jin will remain as a hospitalist at Digestive Health Associates while fulfilling the duties in her new position at Shore Regional. She will guide the center on issues such as compliance, level of care, length of stay, resource management, reimbursement, emergency department throughput, and more.
Business Moves
The Mount Sinai Health System and The New Jewish Home, both based in New York City, have extended their relationship to improve care of hospitalized patients who require specialized post-acute or long-term care at a skilled nursing facility. Through the Mount Sinai-New Jewish Home Hospitalist Program, Mount Sinai hospitalists will be charged with providing a seamless transition to The New Jewish Home for patients who need nursing care.
This model will buoy communication and ensure the sharing of vital information between the two venues, reducing the risk of rehospitalization.
Gryphon Investors, based in San Francisco, recently announced it will acquire OB Hospitalist Group, one of the nation’s leading providers of obstetric hospital medicine. The deal with OBHG’s current partner, Ares Management, was finalized in late July.
OBHG, based out of Mauldin, S.C., has a national network of more than 550 OB hospitalists, covering more than 120 hospitals in 28 states. OBHG’s hospitalist program features an obstetric emergency department, providing expectant mothers at partner hospitals with 24/7/365 access to medical care.
Envision Healthcare, based in Nashville, Tenn., and Greenwood Village, Colo., a provider of physician-led services and ambulatory surgery services, has acquired Milwaukee-based Infinity Healthcare. Infinity’s group-physician practice includes more than 340 physicians and providers delivering emergency and hospital medicine, anesthesia, and radiology services.
Robert Harrington, MD, recently was tabbed as chief medical officer of SurveyVitals, a health care analytics company specializing in digital patient-experience surveys. Dr. Harrington has 20 years experience, including CMO roles with Reliant Post–Acute Care Solutions and Locum Leaders, a hospitalist staffing firm.
Dr. Harrington is a senior fellow in Hospital Medicine and is past president and member of the board of directors with the Society of Hospital Medicine.
David Northington, DO, has been named the new chief medical officer at Stone County Hospital in Wiggins, Miss. The former hospitalist comes to Stone County after working as chief of staff and chief medical information officer at Memorial Hospital in Gulfport, Miss., where he was also medical director of the hospitalist program.
In addition to his new role, Dr. Northington will serve as medical director of the Woodland Village Nursing Center in Diamondhead, Miss., and the Stone County Nursing and Rehabilitation Center in Wiggins.
Schuyler K. Geller, MD, has been recognized by Continental Who’s Who as a Pinnacle Lifetime Member in the medical field. Dr. Geller works as a full-time hospitalist and a principal consultant for The CopperRidge Group, which provides guidance to patients in health, wellness, and fitness services and products.
In addition to his work at the CopperRidge Group, Dr. Geller is a member of Civil Vision International’s board of directors. He has extensive civilian and military-based experience in the United States, Africa, the Middle East, and South Asia.
A physician leader in the U.S. Air Force, Dr. Geller earned White House Medical Unit commendations for planning and leading the surgical and intensive care unit teams to support President Clinton’s trips to Vietnam and Africa in 2000.
Nikhil Sharma, MD, recently was selected by the International Association of HealthCare Professionals to be part of the Leading Physicians of the World. Dr. Sharma is a hospitalist serving at the Ochsner Health System in New Orleans.
Dr. Sharma, a member of the Southern Hospital Association and the Louisiana Medical Association, began his medical career in 2009 with a residency and fellowship at Ochsner, where he has remained ever since. He specializes in internal medicine and transplants.
I. Carol Nwelue, MD, a longtime hospitalist and the medical director of the Sparrow Medical Group Adult Hospitalist Service, recently received the Sparrow Physician Leadership Award. The award goes to an emerging leader who provides outstanding work in areas such as safety, clinical or service excellence, research, teaching, publishing, teamwork, and innovation.
Dr. Nwelue completed the Sparrow Physician Leadership Academy program, earning recognition for innovation in leadership, as well as practice management.
Laura Jin, MD, recently was promoted to medical director for utilization management at the University of Maryland Shore Regional Health. In her new role, Dr. Jin will identify and facilitate the resolution of utilization issues; in so doing, she will serve as a consultant leader to the health care system, its physicians, its advance practice providers, and the care management team.
Dr. Jin will remain as a hospitalist at Digestive Health Associates while fulfilling the duties in her new position at Shore Regional. She will guide the center on issues such as compliance, level of care, length of stay, resource management, reimbursement, emergency department throughput, and more.
Business Moves
The Mount Sinai Health System and The New Jewish Home, both based in New York City, have extended their relationship to improve care of hospitalized patients who require specialized post-acute or long-term care at a skilled nursing facility. Through the Mount Sinai-New Jewish Home Hospitalist Program, Mount Sinai hospitalists will be charged with providing a seamless transition to The New Jewish Home for patients who need nursing care.
This model will buoy communication and ensure the sharing of vital information between the two venues, reducing the risk of rehospitalization.
Gryphon Investors, based in San Francisco, recently announced it will acquire OB Hospitalist Group, one of the nation’s leading providers of obstetric hospital medicine. The deal with OBHG’s current partner, Ares Management, was finalized in late July.
OBHG, based out of Mauldin, S.C., has a national network of more than 550 OB hospitalists, covering more than 120 hospitals in 28 states. OBHG’s hospitalist program features an obstetric emergency department, providing expectant mothers at partner hospitals with 24/7/365 access to medical care.
Envision Healthcare, based in Nashville, Tenn., and Greenwood Village, Colo., a provider of physician-led services and ambulatory surgery services, has acquired Milwaukee-based Infinity Healthcare. Infinity’s group-physician practice includes more than 340 physicians and providers delivering emergency and hospital medicine, anesthesia, and radiology services.
Robert Harrington, MD, recently was tabbed as chief medical officer of SurveyVitals, a health care analytics company specializing in digital patient-experience surveys. Dr. Harrington has 20 years experience, including CMO roles with Reliant Post–Acute Care Solutions and Locum Leaders, a hospitalist staffing firm.
Dr. Harrington is a senior fellow in Hospital Medicine and is past president and member of the board of directors with the Society of Hospital Medicine.
David Northington, DO, has been named the new chief medical officer at Stone County Hospital in Wiggins, Miss. The former hospitalist comes to Stone County after working as chief of staff and chief medical information officer at Memorial Hospital in Gulfport, Miss., where he was also medical director of the hospitalist program.
In addition to his new role, Dr. Northington will serve as medical director of the Woodland Village Nursing Center in Diamondhead, Miss., and the Stone County Nursing and Rehabilitation Center in Wiggins.
Schuyler K. Geller, MD, has been recognized by Continental Who’s Who as a Pinnacle Lifetime Member in the medical field. Dr. Geller works as a full-time hospitalist and a principal consultant for The CopperRidge Group, which provides guidance to patients in health, wellness, and fitness services and products.
In addition to his work at the CopperRidge Group, Dr. Geller is a member of Civil Vision International’s board of directors. He has extensive civilian and military-based experience in the United States, Africa, the Middle East, and South Asia.
A physician leader in the U.S. Air Force, Dr. Geller earned White House Medical Unit commendations for planning and leading the surgical and intensive care unit teams to support President Clinton’s trips to Vietnam and Africa in 2000.
Nikhil Sharma, MD, recently was selected by the International Association of HealthCare Professionals to be part of the Leading Physicians of the World. Dr. Sharma is a hospitalist serving at the Ochsner Health System in New Orleans.
Dr. Sharma, a member of the Southern Hospital Association and the Louisiana Medical Association, began his medical career in 2009 with a residency and fellowship at Ochsner, where he has remained ever since. He specializes in internal medicine and transplants.
I. Carol Nwelue, MD, a longtime hospitalist and the medical director of the Sparrow Medical Group Adult Hospitalist Service, recently received the Sparrow Physician Leadership Award. The award goes to an emerging leader who provides outstanding work in areas such as safety, clinical or service excellence, research, teaching, publishing, teamwork, and innovation.
Dr. Nwelue completed the Sparrow Physician Leadership Academy program, earning recognition for innovation in leadership, as well as practice management.
Laura Jin, MD, recently was promoted to medical director for utilization management at the University of Maryland Shore Regional Health. In her new role, Dr. Jin will identify and facilitate the resolution of utilization issues; in so doing, she will serve as a consultant leader to the health care system, its physicians, its advance practice providers, and the care management team.
Dr. Jin will remain as a hospitalist at Digestive Health Associates while fulfilling the duties in her new position at Shore Regional. She will guide the center on issues such as compliance, level of care, length of stay, resource management, reimbursement, emergency department throughput, and more.
Business Moves
The Mount Sinai Health System and The New Jewish Home, both based in New York City, have extended their relationship to improve care of hospitalized patients who require specialized post-acute or long-term care at a skilled nursing facility. Through the Mount Sinai-New Jewish Home Hospitalist Program, Mount Sinai hospitalists will be charged with providing a seamless transition to The New Jewish Home for patients who need nursing care.
This model will buoy communication and ensure the sharing of vital information between the two venues, reducing the risk of rehospitalization.
Gryphon Investors, based in San Francisco, recently announced it will acquire OB Hospitalist Group, one of the nation’s leading providers of obstetric hospital medicine. The deal with OBHG’s current partner, Ares Management, was finalized in late July.
OBHG, based out of Mauldin, S.C., has a national network of more than 550 OB hospitalists, covering more than 120 hospitals in 28 states. OBHG’s hospitalist program features an obstetric emergency department, providing expectant mothers at partner hospitals with 24/7/365 access to medical care.
Envision Healthcare, based in Nashville, Tenn., and Greenwood Village, Colo., a provider of physician-led services and ambulatory surgery services, has acquired Milwaukee-based Infinity Healthcare. Infinity’s group-physician practice includes more than 340 physicians and providers delivering emergency and hospital medicine, anesthesia, and radiology services.
Identifying Pediatric Skull Fracture Using Point-of-Care Ultrasound
Evaluating pediatric patients presenting to the ED with head trauma can be a challenging task for emergency physicians (EPs). Specifically, identifying a nondisplaced skull fracture is not always possible through physical examination alone.1 However, point-of-care (POC) ultrasound permits rapid identification of skull fractures, which in turn assists the EP to determine if advanced imaging studies such as computed tomography (CT) are necessary.
Case
A previously healthy 10-month-old male infant presented to the ED with his mother for evaluation of rhinorrhea, cough, and fever, the onset of which began 24 hours prior to presentation. The patient’s mother reported that the infant continually tugged at his right ear throughout the previous evening and was increasingly irritable, but not inconsolable.
Initial vital signs at presentation were: blood pressure, 95/54 mm Hg; heart rate, 146 beats/min; respiratory rate, 36 beats/min, and temperature, 101.8°F. Oxygen saturation was 96% on room air. The physical examination was notable for an alert well-appearing infant who had a tender nonecchymotic scalp hematoma superior to the right pinna, clear tympanic membranes, crusted mucous bilaterally at the nares, nonlabored respirations, and wheezing throughout the lung fields.
Imaging Technique
To evaluate for skull fractures using POC ultrasound, the area of localized trauma must first be identified.2,3 Evidence of trauma includes an area of focal tenderness, abrasion, soft-tissue swelling, and hematoma.2,3 The presence of any depressed and open cranial injuries are contraindications to ultrasound. In which case, a physician should consult a neurosurgical specialist and obtain a CT scan of the head.
A high-frequency linear probe (5-10 MHz) is used to scan the area of localized trauma; this should be performed in two perpendicular planes using copious gel and light pressure (Figures 2a-2c).
Discussion
Closed head trauma is one of the most common pediatric injuries, accounting for roughly 1.4 million ED visits annually in the United States.5 Four to 12% percent of these minor traumas result in an intracranial injury,2 and the presence of a skull fracture is associated with a 4- to 20-fold increase in risk of underlying intracranial hemorrhage.3
Clinical assessment alone is not always reliable in predicting skull fracture and intracranial injury, especially in children younger than 2 years of age.2,3 Ultrasound is safe, noninvasive, expedient, cost-effective, and well tolerated in the pediatric population for identifying skull fractures,3 and can obviate the need for skull radiographs4 or procedural sedation. Moreover, POC ultrasound can serve as an adjunct to the Pediatric Emergency Care Applied Research Network head injury algorithm for head CT use decision rules if the fracture is not palpable on examination.
Several prospective studies and case reports have demonstrated the usefulness of POC ultrasound in diagnosing pediatric skull fractures in the ED.1-4 Two of the four cases published represented cases in which the EP identified an undisclosed nonaccidental trauma through POC ultrasound. Rabiner et al,3 estimates a combined sensitivity and specificity of 94% and 96%, respectively. It is important to remember that intracranial injury can still occur without an associated skull fracture. As our case demonstrates, POC ultrasound is a useful tool in risk-stratifying minor head trauma in children.
Case Conclusion
The head CT study confirmed a nondisplaced, oblique, and acute-appearing linear fracture of the right parietal bone extending from the squamosal to the lambdoid suture. There was no associated intracranial hemorrhage. The patient was admitted to the hospital for a nonaccidental trauma evaluation. The Department of Children and Family Services was contacted and the patient was discharged in the temporary custody of his maternal grandmother.
Summary
Point-of-care ultrasound is a useful diagnostic tool to rapidly evaluate for, and diagnose skull fractures in pediatric patients. Given its high sensitivity and specificity, ultrasound can help EPs identify occult nondisplaced skull fractures in children.
1. Riera A, Chen L. Ultrasound evaluation of skull fractures in children: a feasibility study. Pediatr Emerg Care. 2012;28(5):420-425. doi:10.1097/PEC.0b013e318252da3b.
2. Parri N, Crosby BJ, Glass C, et al. Ability of emergency ultrasonography to detect pediatric skull fractures: a prospective, observational study. J Emerg Med. 2013;44(1)135-141.
3. Rabiner JE, Friedman LM, Khine H, Avner JR, Tsung JW. Accuracy of point-of-care ultrasound for diagnosis of skull fractures in children. Pediatrics. 2013;131(6):e1757-1764. doi:10.1542/peds.2012-3921.
4. Ramirez-Schrempp D, Vinci RJ, Liteplo AS. Bedside ultrasound in the diagnosis of skull fractures in the pediatric emergency department. Pediatr Emerg Care. 2011;27(4):312-314. doi:10.1097/PEC.0b013e3182131579.
5. Coronado VG, Xu L, Basavaraju SV, et al; Centers for Disease Control and Prevention (CDC). Surveillance for traumatic brain injury-related deaths--United States, 1997-2007. MMWR Surveill Summ. 2011;60(5):1-32.
Evaluating pediatric patients presenting to the ED with head trauma can be a challenging task for emergency physicians (EPs). Specifically, identifying a nondisplaced skull fracture is not always possible through physical examination alone.1 However, point-of-care (POC) ultrasound permits rapid identification of skull fractures, which in turn assists the EP to determine if advanced imaging studies such as computed tomography (CT) are necessary.
Case
A previously healthy 10-month-old male infant presented to the ED with his mother for evaluation of rhinorrhea, cough, and fever, the onset of which began 24 hours prior to presentation. The patient’s mother reported that the infant continually tugged at his right ear throughout the previous evening and was increasingly irritable, but not inconsolable.
Initial vital signs at presentation were: blood pressure, 95/54 mm Hg; heart rate, 146 beats/min; respiratory rate, 36 beats/min, and temperature, 101.8°F. Oxygen saturation was 96% on room air. The physical examination was notable for an alert well-appearing infant who had a tender nonecchymotic scalp hematoma superior to the right pinna, clear tympanic membranes, crusted mucous bilaterally at the nares, nonlabored respirations, and wheezing throughout the lung fields.
Imaging Technique
To evaluate for skull fractures using POC ultrasound, the area of localized trauma must first be identified.2,3 Evidence of trauma includes an area of focal tenderness, abrasion, soft-tissue swelling, and hematoma.2,3 The presence of any depressed and open cranial injuries are contraindications to ultrasound. In which case, a physician should consult a neurosurgical specialist and obtain a CT scan of the head.
A high-frequency linear probe (5-10 MHz) is used to scan the area of localized trauma; this should be performed in two perpendicular planes using copious gel and light pressure (Figures 2a-2c).
Discussion
Closed head trauma is one of the most common pediatric injuries, accounting for roughly 1.4 million ED visits annually in the United States.5 Four to 12% percent of these minor traumas result in an intracranial injury,2 and the presence of a skull fracture is associated with a 4- to 20-fold increase in risk of underlying intracranial hemorrhage.3
Clinical assessment alone is not always reliable in predicting skull fracture and intracranial injury, especially in children younger than 2 years of age.2,3 Ultrasound is safe, noninvasive, expedient, cost-effective, and well tolerated in the pediatric population for identifying skull fractures,3 and can obviate the need for skull radiographs4 or procedural sedation. Moreover, POC ultrasound can serve as an adjunct to the Pediatric Emergency Care Applied Research Network head injury algorithm for head CT use decision rules if the fracture is not palpable on examination.
Several prospective studies and case reports have demonstrated the usefulness of POC ultrasound in diagnosing pediatric skull fractures in the ED.1-4 Two of the four cases published represented cases in which the EP identified an undisclosed nonaccidental trauma through POC ultrasound. Rabiner et al,3 estimates a combined sensitivity and specificity of 94% and 96%, respectively. It is important to remember that intracranial injury can still occur without an associated skull fracture. As our case demonstrates, POC ultrasound is a useful tool in risk-stratifying minor head trauma in children.
Case Conclusion
The head CT study confirmed a nondisplaced, oblique, and acute-appearing linear fracture of the right parietal bone extending from the squamosal to the lambdoid suture. There was no associated intracranial hemorrhage. The patient was admitted to the hospital for a nonaccidental trauma evaluation. The Department of Children and Family Services was contacted and the patient was discharged in the temporary custody of his maternal grandmother.
Summary
Point-of-care ultrasound is a useful diagnostic tool to rapidly evaluate for, and diagnose skull fractures in pediatric patients. Given its high sensitivity and specificity, ultrasound can help EPs identify occult nondisplaced skull fractures in children.
Evaluating pediatric patients presenting to the ED with head trauma can be a challenging task for emergency physicians (EPs). Specifically, identifying a nondisplaced skull fracture is not always possible through physical examination alone.1 However, point-of-care (POC) ultrasound permits rapid identification of skull fractures, which in turn assists the EP to determine if advanced imaging studies such as computed tomography (CT) are necessary.
Case
A previously healthy 10-month-old male infant presented to the ED with his mother for evaluation of rhinorrhea, cough, and fever, the onset of which began 24 hours prior to presentation. The patient’s mother reported that the infant continually tugged at his right ear throughout the previous evening and was increasingly irritable, but not inconsolable.
Initial vital signs at presentation were: blood pressure, 95/54 mm Hg; heart rate, 146 beats/min; respiratory rate, 36 beats/min, and temperature, 101.8°F. Oxygen saturation was 96% on room air. The physical examination was notable for an alert well-appearing infant who had a tender nonecchymotic scalp hematoma superior to the right pinna, clear tympanic membranes, crusted mucous bilaterally at the nares, nonlabored respirations, and wheezing throughout the lung fields.
Imaging Technique
To evaluate for skull fractures using POC ultrasound, the area of localized trauma must first be identified.2,3 Evidence of trauma includes an area of focal tenderness, abrasion, soft-tissue swelling, and hematoma.2,3 The presence of any depressed and open cranial injuries are contraindications to ultrasound. In which case, a physician should consult a neurosurgical specialist and obtain a CT scan of the head.
A high-frequency linear probe (5-10 MHz) is used to scan the area of localized trauma; this should be performed in two perpendicular planes using copious gel and light pressure (Figures 2a-2c).
Discussion
Closed head trauma is one of the most common pediatric injuries, accounting for roughly 1.4 million ED visits annually in the United States.5 Four to 12% percent of these minor traumas result in an intracranial injury,2 and the presence of a skull fracture is associated with a 4- to 20-fold increase in risk of underlying intracranial hemorrhage.3
Clinical assessment alone is not always reliable in predicting skull fracture and intracranial injury, especially in children younger than 2 years of age.2,3 Ultrasound is safe, noninvasive, expedient, cost-effective, and well tolerated in the pediatric population for identifying skull fractures,3 and can obviate the need for skull radiographs4 or procedural sedation. Moreover, POC ultrasound can serve as an adjunct to the Pediatric Emergency Care Applied Research Network head injury algorithm for head CT use decision rules if the fracture is not palpable on examination.
Several prospective studies and case reports have demonstrated the usefulness of POC ultrasound in diagnosing pediatric skull fractures in the ED.1-4 Two of the four cases published represented cases in which the EP identified an undisclosed nonaccidental trauma through POC ultrasound. Rabiner et al,3 estimates a combined sensitivity and specificity of 94% and 96%, respectively. It is important to remember that intracranial injury can still occur without an associated skull fracture. As our case demonstrates, POC ultrasound is a useful tool in risk-stratifying minor head trauma in children.
Case Conclusion
The head CT study confirmed a nondisplaced, oblique, and acute-appearing linear fracture of the right parietal bone extending from the squamosal to the lambdoid suture. There was no associated intracranial hemorrhage. The patient was admitted to the hospital for a nonaccidental trauma evaluation. The Department of Children and Family Services was contacted and the patient was discharged in the temporary custody of his maternal grandmother.
Summary
Point-of-care ultrasound is a useful diagnostic tool to rapidly evaluate for, and diagnose skull fractures in pediatric patients. Given its high sensitivity and specificity, ultrasound can help EPs identify occult nondisplaced skull fractures in children.
1. Riera A, Chen L. Ultrasound evaluation of skull fractures in children: a feasibility study. Pediatr Emerg Care. 2012;28(5):420-425. doi:10.1097/PEC.0b013e318252da3b.
2. Parri N, Crosby BJ, Glass C, et al. Ability of emergency ultrasonography to detect pediatric skull fractures: a prospective, observational study. J Emerg Med. 2013;44(1)135-141.
3. Rabiner JE, Friedman LM, Khine H, Avner JR, Tsung JW. Accuracy of point-of-care ultrasound for diagnosis of skull fractures in children. Pediatrics. 2013;131(6):e1757-1764. doi:10.1542/peds.2012-3921.
4. Ramirez-Schrempp D, Vinci RJ, Liteplo AS. Bedside ultrasound in the diagnosis of skull fractures in the pediatric emergency department. Pediatr Emerg Care. 2011;27(4):312-314. doi:10.1097/PEC.0b013e3182131579.
5. Coronado VG, Xu L, Basavaraju SV, et al; Centers for Disease Control and Prevention (CDC). Surveillance for traumatic brain injury-related deaths--United States, 1997-2007. MMWR Surveill Summ. 2011;60(5):1-32.
1. Riera A, Chen L. Ultrasound evaluation of skull fractures in children: a feasibility study. Pediatr Emerg Care. 2012;28(5):420-425. doi:10.1097/PEC.0b013e318252da3b.
2. Parri N, Crosby BJ, Glass C, et al. Ability of emergency ultrasonography to detect pediatric skull fractures: a prospective, observational study. J Emerg Med. 2013;44(1)135-141.
3. Rabiner JE, Friedman LM, Khine H, Avner JR, Tsung JW. Accuracy of point-of-care ultrasound for diagnosis of skull fractures in children. Pediatrics. 2013;131(6):e1757-1764. doi:10.1542/peds.2012-3921.
4. Ramirez-Schrempp D, Vinci RJ, Liteplo AS. Bedside ultrasound in the diagnosis of skull fractures in the pediatric emergency department. Pediatr Emerg Care. 2011;27(4):312-314. doi:10.1097/PEC.0b013e3182131579.
5. Coronado VG, Xu L, Basavaraju SV, et al; Centers for Disease Control and Prevention (CDC). Surveillance for traumatic brain injury-related deaths--United States, 1997-2007. MMWR Surveill Summ. 2011;60(5):1-32.
Thyroid Cartilage Fracture in Context of Noncompetitive "Horseplay" Wrestling
An isolated thyroid cartilage fracture is very rare.1-5 More interestingly, an isolated thyroid cartilage fracture from a wrestling injury, especially in a non-sports competition context, such as horseplay, has not been previously reported in the literature. Sports-related injuries to the larynx and related structures are uncommon.6,7
Case
A 38-year-old man presented with a complaint of throat pain after wrestling at home, in horseplay, with his 15-year-old son. He reported that when his son placed a choke hold on him, he felt a "crack" in the area of his neck, and soon afterwards felt throat pain with swallowing, along with discomfort with breathing. He also felt a sensation of "fluid building up in his throat." There were no changes noted with his voice and the patient was speaking in full sentences. There was no wheezing or stridor. He denied shortness of breath or any other complaints. He denied pain over the posterior elements of his cervical spine. At the time of the incident, there was no loss of consciousness. Palpation of the neck and chest did not elicit any crepitance to suggest subcutaneous emphysema. The trachea was midline. There was no pain overlying the carotids bilaterally, and the patient had no bruits. The neck examination did not show any surface abnormalities to suggest trauma, such as ecchymosis or swelling. He did have slight tenderness to palpation over the thyroid cartilage.
The patient was sent for a computed tomography (CT) scan of the soft-tissue neck with intravenous (IV) contrast, and a CT scan of the cervical spine. The results showed no cervical spine fracture. However, there was a minimally displaced fracture of the left thyroid cartilage, with soft-tissue swelling that was noted, along with minimal narrowing of the subglottic trachea. There were no abnormal enhancements or fluid collections. No evidence of vocal cord abnormality or asymmetry was seen, and there was no evidence of airway compromise (Figure).
Discussion
Our patient sustained an isolated thyroid cartilage fracture. A thyroid cartilage fracture is a type of laryngeal fracture. Using an anatomic system in which such injuries are classified by location (supraglottic, glottis, or infraglottic), a thyroid cartilage fracture is classified as a supraglottic laryngeal injury.1,2 In our case, the fracture was due to a blunt force mechanism. Most blunt force laryngeal fractures are associated with multiple trauma.8 An isolated thyroid cartilage fracture is very rare.1-5 More interestingly, an isolated thyroid cartilage fracture from a wrestling injury, especially in a non-sports competition context, such as horseplay, has not been previously reported in the literature.
Sports-related injuries to the larynx and related structures are uncommon.6,7 When reported, significant force is usually involved. For example, Tasca et al6 reported a thyroid cartilage fracture from direct blunt trauma (rugby, opponent stamped on patient’s throat) in which the patient presented with pain with swallowing and a lowering of the pitch of his voice. Rejali et al9 reported the case of a midair collision in a soccer match, resulting in an obvious mandibular fracture, but with an arytenoid cartilage fracture that was not initially identified. A football struck a 17-year-old boy with a resulting fracture of the superior cornu of the larynx and a puncture of the laryngeal mucosal wall in a case reported by Saab and Birkinshaw.10 The patient presented with neck pain and dysphagia, as well as subcutaneous air.10 A 21-year-old collegiate basketball player was struck in the neck by a teammate’s head while jumping for a rebound. He sustained a fracture of the thyroid cartilage and a fracture of the anterior cricoid ring.3 Patients with such injuries "may appear deceptively normal when seeking medical attention."8 Kragha2 refers to such injuries as "rare but potentially deadly."
Symptoms can include neck pain, voice changes, pain with swallowing, and shortness of breath. Signs can include tenderness, ecchymosis, and even subcutaneous emphysema. There may be loss of prominence of the thyroid cartilage.3 Tracheal deviation and stridor can occur.10,11 Computed tomography scan and laryngoscopy can be helpful in the diagnostic process; 3-dimensional (3-D) reconstructions may be needed.
Various classification systems have been proposed with related treatment strategies. Percevik et al11 summarized a five-part clinical classification. Group 1 (hematoma, no fracture) and Group 2 (non-displaced fracture) may be treated conservatively. Group 3 (stable, displaced fracture), Group 4 (unstable, displaced fracture), and Group 5 (laryngotracheal disinsertion) are more likely to be treated with surgery.11 Surgical techniques vary and have been refined over time.12
In this case, the patient sustained a thyroid cartilage fracture without the energy and force involved in a motor vehicle collision and without significant sports-related force. It is possible that this injury may have involved neck hyperflexion, rather than direct compressive force. Lin et al,1 described a case of neck hyperflexion in an unrestrained driver, with a resulting isolated thyroid cartilage fracture without direct impact to the neck. Walsh and Trotter5 presented a case of a motorcyclist with a blow to the back of the head, with resulting neck hyperflexion, which resulted in a fracture of the thyroid cartilage. Beato-Martínez et al,13 reported a case of thyroid cartilage fracture following a sneezing episode. The patient presented with odynophagia, dysphonia and neck pain.13 In our review of the literature, we found that only one other similar case has been reported. In that case, a patient experienced a feeling of a neck click, followed by neck pain and hoarseness. He sustained a fracture of the thyroid cartilage.14
In reviewing the hyperflexion mechanism, Lin et al1 noted that isolated thyroid cartilage fractures are rare and that "most of these are caused by direct injury to the neck, except for two patients reported in the literature who sustained isolated thyroid cartilage fractures after sneezing." Lin et al1 proposed an interesting hypothesis—that "the mechanism causing thyroid cartilage fracture during impaction may be the same with sneezing." Sneezing can be associated with sudden and forceful flexion of the neck.
It is certainly possible that this hyperflexion mechanism was involved in our case, given there was no history of significant blunt force to the neck, as in the sports-related injuries discussed. Wrestling holds can produce hyperflexion. The patient described a feeling of a "crack", which is similar to the clicking sound described in one of the sneezing-related cases. An isolated thyroid cartilage fracture is rare in the absence of major trauma. However, as noted by Rejali et al,9 this can create a potential management pitfall. "In the context of non-contact sports, the attendant doctor may not realize the significance of apparently minor head and neck trauma."9
There are no series data to provide us with an exact incidence of airway compromise. However, seemingly minor insults to the anterior neck can cause posterior compression of the larynx and can result in airway compromise.9-11
The CT scan is described as an important imaging modality to rule out cervical spine fracture. Although there was no significant blunt force, the cervical spine was exposed to hyperflexion forces. Another important potential consequence is long-term injury to the vocal cords, with subsequent speech difficulties.11 Computed tomography can visualize the thyroid fracture, but many authors point out that visualization of the vocal cords, with nasopharyngeal laryngoscopy or other modality, is an important adjunct to the CT scan.9-11
Otolaryngologist consultation should be strongly considered. This patient was transferred to a tertiary care center with expertise in thyroid fractures, and planned nasopharyngeal laryngoscopy to be performed at the receiving institution.
Conclusion
Our patient sustained an isolated thyroid cartilage fracture. Most blunt force laryngeal fractures are associated with multiple trauma. An isolated thyroid cartilage fracture is very rare. An isolated thyroid cartilage fracture from a wrestling injury, especially in a non-sports competition context, such as horseplay, has not been previously reported in the literature. Symptoms can include neck pain, voice changes, pain with swallowing, and shortness of breath. Signs can include tenderness, ecchymosis, or even subcutaneous emphysema. There may be loss of the prominence of the thyroid cartilage, tracheal deviation, and stridor. Computed tomography scan imaging with 3-D reconstructions and laryngoscopy can be helpful in the diagnostic process. In our case, the patient sustained a thyroid cartilage fracture without the energy and force involved in a motor vehicle collision and without significant sports-related force. It is possible this injury may have involved neck hyperflexion, rather than direct compressive forces, similar to that described by Lin et al.1 Certainly, there was no history of significant blunt force to the neck on the level of the sports-related injuries discussed.
1. Lin HL, Kuo LC, Chen CW, Cheng YC, Lee WC. Neck hyperflexion causing isolated thyroid cartilage fracture--a case report. Am J Emerg Med. 2008;26(9):1064.e1-e3. doi:10.1016/j.ajem.2008.02.030
2. Kragha KO. Acute traumatic injury of the larynx. Case Reports in Otolaryngology. Volume 2015. Article ID393978. http://dx.doi.org/10.1155/2015/393978
3. Kim JD, Shuler FD, Mo B, Gibbs SR, Belmaggio T, Giangarra CE. Traumatic laryngeal fracture in a collegiate basketball player. Sports Health. 2013;5(3):
273-275.
4. Knopke S, Todt I, Ernst A, Seidl RO. Pseudarthroses of the cornu of the thyroid cartilage. Otolaryngol Head Neck Surg. 2010;143(2):186-189. doi:10.1016/5.otohns.2010.04.011.
5. Walsh PV, Trotter GA. Fracture of the thyroid cartilage associated with full face integral crash helmet. Injury. 1979;11(1):47-48.
6. Tasca RA, Sherman IW, Wood GD. Thyroid cartilage fracture: treatment with biodegradable plates. Br J Oral Maxillofac Surg. 2008;46(2):159-160.
7. Mitrović SM. Blunt external laryngeal trauma. Two case reports. Med Pregl. 2007;60(9-10):489-492.
8. O'Keefe LJ, Maw AR. The dangers of minor blunt laryngeal trauma. J. Laryngol Otol. 1992;106(4):372-373.
9. Rejali SD, Bennett JD, Upile T, Rothera MP. Diagnostic pitfalls in sports related laryngeal injury. Br J Sports Med. 1998;32(2):180-181.
10. Saab M, Birkinshaw R. Blunt laryngeal trauma: an unusual case. Int J Clin Pract. 1997;51(8):527.
11. Pekcevik Y, Ibrahim C, Ülker C. Cricoid and thyroid cartilage fracture, cricothyroid joint dislocation,pseudofracture appearance of the hyoid bone: CT, MRI and laryngoscopic findings. JAEM. 2013;12:170-173.
12. Bent JP 3rd, Porubsky ES. The management of blunt fractures of the thyroid cartilage. Otolaryngol Head Neck Surg. 1994;110(2):195-202. doi: 10:.1177/019459989411000209.
13. Beato Martínez A, Moreno Juara A, López Moya JJ. Fracture of thyroid cartilage after a sneezing episode. Acta Otorrinolaringol Esp. 2007;58(2):73-74.
14. Quinlan PT. Fracture of thyroid cartilage during a sneezing attack. Br Med J. 1950;1(4661):1052.
An isolated thyroid cartilage fracture is very rare.1-5 More interestingly, an isolated thyroid cartilage fracture from a wrestling injury, especially in a non-sports competition context, such as horseplay, has not been previously reported in the literature. Sports-related injuries to the larynx and related structures are uncommon.6,7
Case
A 38-year-old man presented with a complaint of throat pain after wrestling at home, in horseplay, with his 15-year-old son. He reported that when his son placed a choke hold on him, he felt a "crack" in the area of his neck, and soon afterwards felt throat pain with swallowing, along with discomfort with breathing. He also felt a sensation of "fluid building up in his throat." There were no changes noted with his voice and the patient was speaking in full sentences. There was no wheezing or stridor. He denied shortness of breath or any other complaints. He denied pain over the posterior elements of his cervical spine. At the time of the incident, there was no loss of consciousness. Palpation of the neck and chest did not elicit any crepitance to suggest subcutaneous emphysema. The trachea was midline. There was no pain overlying the carotids bilaterally, and the patient had no bruits. The neck examination did not show any surface abnormalities to suggest trauma, such as ecchymosis or swelling. He did have slight tenderness to palpation over the thyroid cartilage.
The patient was sent for a computed tomography (CT) scan of the soft-tissue neck with intravenous (IV) contrast, and a CT scan of the cervical spine. The results showed no cervical spine fracture. However, there was a minimally displaced fracture of the left thyroid cartilage, with soft-tissue swelling that was noted, along with minimal narrowing of the subglottic trachea. There were no abnormal enhancements or fluid collections. No evidence of vocal cord abnormality or asymmetry was seen, and there was no evidence of airway compromise (Figure).
Discussion
Our patient sustained an isolated thyroid cartilage fracture. A thyroid cartilage fracture is a type of laryngeal fracture. Using an anatomic system in which such injuries are classified by location (supraglottic, glottis, or infraglottic), a thyroid cartilage fracture is classified as a supraglottic laryngeal injury.1,2 In our case, the fracture was due to a blunt force mechanism. Most blunt force laryngeal fractures are associated with multiple trauma.8 An isolated thyroid cartilage fracture is very rare.1-5 More interestingly, an isolated thyroid cartilage fracture from a wrestling injury, especially in a non-sports competition context, such as horseplay, has not been previously reported in the literature.
Sports-related injuries to the larynx and related structures are uncommon.6,7 When reported, significant force is usually involved. For example, Tasca et al6 reported a thyroid cartilage fracture from direct blunt trauma (rugby, opponent stamped on patient’s throat) in which the patient presented with pain with swallowing and a lowering of the pitch of his voice. Rejali et al9 reported the case of a midair collision in a soccer match, resulting in an obvious mandibular fracture, but with an arytenoid cartilage fracture that was not initially identified. A football struck a 17-year-old boy with a resulting fracture of the superior cornu of the larynx and a puncture of the laryngeal mucosal wall in a case reported by Saab and Birkinshaw.10 The patient presented with neck pain and dysphagia, as well as subcutaneous air.10 A 21-year-old collegiate basketball player was struck in the neck by a teammate’s head while jumping for a rebound. He sustained a fracture of the thyroid cartilage and a fracture of the anterior cricoid ring.3 Patients with such injuries "may appear deceptively normal when seeking medical attention."8 Kragha2 refers to such injuries as "rare but potentially deadly."
Symptoms can include neck pain, voice changes, pain with swallowing, and shortness of breath. Signs can include tenderness, ecchymosis, and even subcutaneous emphysema. There may be loss of prominence of the thyroid cartilage.3 Tracheal deviation and stridor can occur.10,11 Computed tomography scan and laryngoscopy can be helpful in the diagnostic process; 3-dimensional (3-D) reconstructions may be needed.
Various classification systems have been proposed with related treatment strategies. Percevik et al11 summarized a five-part clinical classification. Group 1 (hematoma, no fracture) and Group 2 (non-displaced fracture) may be treated conservatively. Group 3 (stable, displaced fracture), Group 4 (unstable, displaced fracture), and Group 5 (laryngotracheal disinsertion) are more likely to be treated with surgery.11 Surgical techniques vary and have been refined over time.12
In this case, the patient sustained a thyroid cartilage fracture without the energy and force involved in a motor vehicle collision and without significant sports-related force. It is possible that this injury may have involved neck hyperflexion, rather than direct compressive force. Lin et al,1 described a case of neck hyperflexion in an unrestrained driver, with a resulting isolated thyroid cartilage fracture without direct impact to the neck. Walsh and Trotter5 presented a case of a motorcyclist with a blow to the back of the head, with resulting neck hyperflexion, which resulted in a fracture of the thyroid cartilage. Beato-Martínez et al,13 reported a case of thyroid cartilage fracture following a sneezing episode. The patient presented with odynophagia, dysphonia and neck pain.13 In our review of the literature, we found that only one other similar case has been reported. In that case, a patient experienced a feeling of a neck click, followed by neck pain and hoarseness. He sustained a fracture of the thyroid cartilage.14
In reviewing the hyperflexion mechanism, Lin et al1 noted that isolated thyroid cartilage fractures are rare and that "most of these are caused by direct injury to the neck, except for two patients reported in the literature who sustained isolated thyroid cartilage fractures after sneezing." Lin et al1 proposed an interesting hypothesis—that "the mechanism causing thyroid cartilage fracture during impaction may be the same with sneezing." Sneezing can be associated with sudden and forceful flexion of the neck.
It is certainly possible that this hyperflexion mechanism was involved in our case, given there was no history of significant blunt force to the neck, as in the sports-related injuries discussed. Wrestling holds can produce hyperflexion. The patient described a feeling of a "crack", which is similar to the clicking sound described in one of the sneezing-related cases. An isolated thyroid cartilage fracture is rare in the absence of major trauma. However, as noted by Rejali et al,9 this can create a potential management pitfall. "In the context of non-contact sports, the attendant doctor may not realize the significance of apparently minor head and neck trauma."9
There are no series data to provide us with an exact incidence of airway compromise. However, seemingly minor insults to the anterior neck can cause posterior compression of the larynx and can result in airway compromise.9-11
The CT scan is described as an important imaging modality to rule out cervical spine fracture. Although there was no significant blunt force, the cervical spine was exposed to hyperflexion forces. Another important potential consequence is long-term injury to the vocal cords, with subsequent speech difficulties.11 Computed tomography can visualize the thyroid fracture, but many authors point out that visualization of the vocal cords, with nasopharyngeal laryngoscopy or other modality, is an important adjunct to the CT scan.9-11
Otolaryngologist consultation should be strongly considered. This patient was transferred to a tertiary care center with expertise in thyroid fractures, and planned nasopharyngeal laryngoscopy to be performed at the receiving institution.
Conclusion
Our patient sustained an isolated thyroid cartilage fracture. Most blunt force laryngeal fractures are associated with multiple trauma. An isolated thyroid cartilage fracture is very rare. An isolated thyroid cartilage fracture from a wrestling injury, especially in a non-sports competition context, such as horseplay, has not been previously reported in the literature. Symptoms can include neck pain, voice changes, pain with swallowing, and shortness of breath. Signs can include tenderness, ecchymosis, or even subcutaneous emphysema. There may be loss of the prominence of the thyroid cartilage, tracheal deviation, and stridor. Computed tomography scan imaging with 3-D reconstructions and laryngoscopy can be helpful in the diagnostic process. In our case, the patient sustained a thyroid cartilage fracture without the energy and force involved in a motor vehicle collision and without significant sports-related force. It is possible this injury may have involved neck hyperflexion, rather than direct compressive forces, similar to that described by Lin et al.1 Certainly, there was no history of significant blunt force to the neck on the level of the sports-related injuries discussed.
An isolated thyroid cartilage fracture is very rare.1-5 More interestingly, an isolated thyroid cartilage fracture from a wrestling injury, especially in a non-sports competition context, such as horseplay, has not been previously reported in the literature. Sports-related injuries to the larynx and related structures are uncommon.6,7
Case
A 38-year-old man presented with a complaint of throat pain after wrestling at home, in horseplay, with his 15-year-old son. He reported that when his son placed a choke hold on him, he felt a "crack" in the area of his neck, and soon afterwards felt throat pain with swallowing, along with discomfort with breathing. He also felt a sensation of "fluid building up in his throat." There were no changes noted with his voice and the patient was speaking in full sentences. There was no wheezing or stridor. He denied shortness of breath or any other complaints. He denied pain over the posterior elements of his cervical spine. At the time of the incident, there was no loss of consciousness. Palpation of the neck and chest did not elicit any crepitance to suggest subcutaneous emphysema. The trachea was midline. There was no pain overlying the carotids bilaterally, and the patient had no bruits. The neck examination did not show any surface abnormalities to suggest trauma, such as ecchymosis or swelling. He did have slight tenderness to palpation over the thyroid cartilage.
The patient was sent for a computed tomography (CT) scan of the soft-tissue neck with intravenous (IV) contrast, and a CT scan of the cervical spine. The results showed no cervical spine fracture. However, there was a minimally displaced fracture of the left thyroid cartilage, with soft-tissue swelling that was noted, along with minimal narrowing of the subglottic trachea. There were no abnormal enhancements or fluid collections. No evidence of vocal cord abnormality or asymmetry was seen, and there was no evidence of airway compromise (Figure).
Discussion
Our patient sustained an isolated thyroid cartilage fracture. A thyroid cartilage fracture is a type of laryngeal fracture. Using an anatomic system in which such injuries are classified by location (supraglottic, glottis, or infraglottic), a thyroid cartilage fracture is classified as a supraglottic laryngeal injury.1,2 In our case, the fracture was due to a blunt force mechanism. Most blunt force laryngeal fractures are associated with multiple trauma.8 An isolated thyroid cartilage fracture is very rare.1-5 More interestingly, an isolated thyroid cartilage fracture from a wrestling injury, especially in a non-sports competition context, such as horseplay, has not been previously reported in the literature.
Sports-related injuries to the larynx and related structures are uncommon.6,7 When reported, significant force is usually involved. For example, Tasca et al6 reported a thyroid cartilage fracture from direct blunt trauma (rugby, opponent stamped on patient’s throat) in which the patient presented with pain with swallowing and a lowering of the pitch of his voice. Rejali et al9 reported the case of a midair collision in a soccer match, resulting in an obvious mandibular fracture, but with an arytenoid cartilage fracture that was not initially identified. A football struck a 17-year-old boy with a resulting fracture of the superior cornu of the larynx and a puncture of the laryngeal mucosal wall in a case reported by Saab and Birkinshaw.10 The patient presented with neck pain and dysphagia, as well as subcutaneous air.10 A 21-year-old collegiate basketball player was struck in the neck by a teammate’s head while jumping for a rebound. He sustained a fracture of the thyroid cartilage and a fracture of the anterior cricoid ring.3 Patients with such injuries "may appear deceptively normal when seeking medical attention."8 Kragha2 refers to such injuries as "rare but potentially deadly."
Symptoms can include neck pain, voice changes, pain with swallowing, and shortness of breath. Signs can include tenderness, ecchymosis, and even subcutaneous emphysema. There may be loss of prominence of the thyroid cartilage.3 Tracheal deviation and stridor can occur.10,11 Computed tomography scan and laryngoscopy can be helpful in the diagnostic process; 3-dimensional (3-D) reconstructions may be needed.
Various classification systems have been proposed with related treatment strategies. Percevik et al11 summarized a five-part clinical classification. Group 1 (hematoma, no fracture) and Group 2 (non-displaced fracture) may be treated conservatively. Group 3 (stable, displaced fracture), Group 4 (unstable, displaced fracture), and Group 5 (laryngotracheal disinsertion) are more likely to be treated with surgery.11 Surgical techniques vary and have been refined over time.12
In this case, the patient sustained a thyroid cartilage fracture without the energy and force involved in a motor vehicle collision and without significant sports-related force. It is possible that this injury may have involved neck hyperflexion, rather than direct compressive force. Lin et al,1 described a case of neck hyperflexion in an unrestrained driver, with a resulting isolated thyroid cartilage fracture without direct impact to the neck. Walsh and Trotter5 presented a case of a motorcyclist with a blow to the back of the head, with resulting neck hyperflexion, which resulted in a fracture of the thyroid cartilage. Beato-Martínez et al,13 reported a case of thyroid cartilage fracture following a sneezing episode. The patient presented with odynophagia, dysphonia and neck pain.13 In our review of the literature, we found that only one other similar case has been reported. In that case, a patient experienced a feeling of a neck click, followed by neck pain and hoarseness. He sustained a fracture of the thyroid cartilage.14
In reviewing the hyperflexion mechanism, Lin et al1 noted that isolated thyroid cartilage fractures are rare and that "most of these are caused by direct injury to the neck, except for two patients reported in the literature who sustained isolated thyroid cartilage fractures after sneezing." Lin et al1 proposed an interesting hypothesis—that "the mechanism causing thyroid cartilage fracture during impaction may be the same with sneezing." Sneezing can be associated with sudden and forceful flexion of the neck.
It is certainly possible that this hyperflexion mechanism was involved in our case, given there was no history of significant blunt force to the neck, as in the sports-related injuries discussed. Wrestling holds can produce hyperflexion. The patient described a feeling of a "crack", which is similar to the clicking sound described in one of the sneezing-related cases. An isolated thyroid cartilage fracture is rare in the absence of major trauma. However, as noted by Rejali et al,9 this can create a potential management pitfall. "In the context of non-contact sports, the attendant doctor may not realize the significance of apparently minor head and neck trauma."9
There are no series data to provide us with an exact incidence of airway compromise. However, seemingly minor insults to the anterior neck can cause posterior compression of the larynx and can result in airway compromise.9-11
The CT scan is described as an important imaging modality to rule out cervical spine fracture. Although there was no significant blunt force, the cervical spine was exposed to hyperflexion forces. Another important potential consequence is long-term injury to the vocal cords, with subsequent speech difficulties.11 Computed tomography can visualize the thyroid fracture, but many authors point out that visualization of the vocal cords, with nasopharyngeal laryngoscopy or other modality, is an important adjunct to the CT scan.9-11
Otolaryngologist consultation should be strongly considered. This patient was transferred to a tertiary care center with expertise in thyroid fractures, and planned nasopharyngeal laryngoscopy to be performed at the receiving institution.
Conclusion
Our patient sustained an isolated thyroid cartilage fracture. Most blunt force laryngeal fractures are associated with multiple trauma. An isolated thyroid cartilage fracture is very rare. An isolated thyroid cartilage fracture from a wrestling injury, especially in a non-sports competition context, such as horseplay, has not been previously reported in the literature. Symptoms can include neck pain, voice changes, pain with swallowing, and shortness of breath. Signs can include tenderness, ecchymosis, or even subcutaneous emphysema. There may be loss of the prominence of the thyroid cartilage, tracheal deviation, and stridor. Computed tomography scan imaging with 3-D reconstructions and laryngoscopy can be helpful in the diagnostic process. In our case, the patient sustained a thyroid cartilage fracture without the energy and force involved in a motor vehicle collision and without significant sports-related force. It is possible this injury may have involved neck hyperflexion, rather than direct compressive forces, similar to that described by Lin et al.1 Certainly, there was no history of significant blunt force to the neck on the level of the sports-related injuries discussed.
1. Lin HL, Kuo LC, Chen CW, Cheng YC, Lee WC. Neck hyperflexion causing isolated thyroid cartilage fracture--a case report. Am J Emerg Med. 2008;26(9):1064.e1-e3. doi:10.1016/j.ajem.2008.02.030
2. Kragha KO. Acute traumatic injury of the larynx. Case Reports in Otolaryngology. Volume 2015. Article ID393978. http://dx.doi.org/10.1155/2015/393978
3. Kim JD, Shuler FD, Mo B, Gibbs SR, Belmaggio T, Giangarra CE. Traumatic laryngeal fracture in a collegiate basketball player. Sports Health. 2013;5(3):
273-275.
4. Knopke S, Todt I, Ernst A, Seidl RO. Pseudarthroses of the cornu of the thyroid cartilage. Otolaryngol Head Neck Surg. 2010;143(2):186-189. doi:10.1016/5.otohns.2010.04.011.
5. Walsh PV, Trotter GA. Fracture of the thyroid cartilage associated with full face integral crash helmet. Injury. 1979;11(1):47-48.
6. Tasca RA, Sherman IW, Wood GD. Thyroid cartilage fracture: treatment with biodegradable plates. Br J Oral Maxillofac Surg. 2008;46(2):159-160.
7. Mitrović SM. Blunt external laryngeal trauma. Two case reports. Med Pregl. 2007;60(9-10):489-492.
8. O'Keefe LJ, Maw AR. The dangers of minor blunt laryngeal trauma. J. Laryngol Otol. 1992;106(4):372-373.
9. Rejali SD, Bennett JD, Upile T, Rothera MP. Diagnostic pitfalls in sports related laryngeal injury. Br J Sports Med. 1998;32(2):180-181.
10. Saab M, Birkinshaw R. Blunt laryngeal trauma: an unusual case. Int J Clin Pract. 1997;51(8):527.
11. Pekcevik Y, Ibrahim C, Ülker C. Cricoid and thyroid cartilage fracture, cricothyroid joint dislocation,pseudofracture appearance of the hyoid bone: CT, MRI and laryngoscopic findings. JAEM. 2013;12:170-173.
12. Bent JP 3rd, Porubsky ES. The management of blunt fractures of the thyroid cartilage. Otolaryngol Head Neck Surg. 1994;110(2):195-202. doi: 10:.1177/019459989411000209.
13. Beato Martínez A, Moreno Juara A, López Moya JJ. Fracture of thyroid cartilage after a sneezing episode. Acta Otorrinolaringol Esp. 2007;58(2):73-74.
14. Quinlan PT. Fracture of thyroid cartilage during a sneezing attack. Br Med J. 1950;1(4661):1052.
1. Lin HL, Kuo LC, Chen CW, Cheng YC, Lee WC. Neck hyperflexion causing isolated thyroid cartilage fracture--a case report. Am J Emerg Med. 2008;26(9):1064.e1-e3. doi:10.1016/j.ajem.2008.02.030
2. Kragha KO. Acute traumatic injury of the larynx. Case Reports in Otolaryngology. Volume 2015. Article ID393978. http://dx.doi.org/10.1155/2015/393978
3. Kim JD, Shuler FD, Mo B, Gibbs SR, Belmaggio T, Giangarra CE. Traumatic laryngeal fracture in a collegiate basketball player. Sports Health. 2013;5(3):
273-275.
4. Knopke S, Todt I, Ernst A, Seidl RO. Pseudarthroses of the cornu of the thyroid cartilage. Otolaryngol Head Neck Surg. 2010;143(2):186-189. doi:10.1016/5.otohns.2010.04.011.
5. Walsh PV, Trotter GA. Fracture of the thyroid cartilage associated with full face integral crash helmet. Injury. 1979;11(1):47-48.
6. Tasca RA, Sherman IW, Wood GD. Thyroid cartilage fracture: treatment with biodegradable plates. Br J Oral Maxillofac Surg. 2008;46(2):159-160.
7. Mitrović SM. Blunt external laryngeal trauma. Two case reports. Med Pregl. 2007;60(9-10):489-492.
8. O'Keefe LJ, Maw AR. The dangers of minor blunt laryngeal trauma. J. Laryngol Otol. 1992;106(4):372-373.
9. Rejali SD, Bennett JD, Upile T, Rothera MP. Diagnostic pitfalls in sports related laryngeal injury. Br J Sports Med. 1998;32(2):180-181.
10. Saab M, Birkinshaw R. Blunt laryngeal trauma: an unusual case. Int J Clin Pract. 1997;51(8):527.
11. Pekcevik Y, Ibrahim C, Ülker C. Cricoid and thyroid cartilage fracture, cricothyroid joint dislocation,pseudofracture appearance of the hyoid bone: CT, MRI and laryngoscopic findings. JAEM. 2013;12:170-173.
12. Bent JP 3rd, Porubsky ES. The management of blunt fractures of the thyroid cartilage. Otolaryngol Head Neck Surg. 1994;110(2):195-202. doi: 10:.1177/019459989411000209.
13. Beato Martínez A, Moreno Juara A, López Moya JJ. Fracture of thyroid cartilage after a sneezing episode. Acta Otorrinolaringol Esp. 2007;58(2):73-74.
14. Quinlan PT. Fracture of thyroid cartilage during a sneezing attack. Br Med J. 1950;1(4661):1052.
Retropharyngeal Hematoma in a 90-Year-Old Woman
Case
A 90-year-old woman with chronic obstructive pulmonary disease; hypertension; chronic kidney disease; diastolic dysfunction; severe tricuspid regurgitation; and atrial fibrillation (AF), for which she was taking rivaroxaban, presented to the ED for evaluation of injuries she sustained during a fall. The patient’s family stated that she fell while walking with the assistance of a walker and landed on her face. There was no reported loss of consciousness. Upon arrival at the ED, the patient’s vital signs were: blood pressure, 188/105 mm Hg; heart rate, 91 beats/min; respiratory rate, 20 breaths/min; and temperature, 97.88°F (36.6°C). Oxygen (O2) saturation was 90% on room air, but increased to 98% after the patient received 10 L/min of O2 through a non-rebreather mask.
On physical examination, the patient was awake, alert, and oriented to person, place, and time, with a Glasgow Coma Scale score of 15. She was able to move all four extremities and had 4/5 motor strength in the upper extremities bilaterally, and 3/5 motor strength in the bilateral lower limbs, which her family reported was the same as her baseline. On pulmonary examination, the lungs were clear to auscultation bilaterally and had no stridor. On auscultation she had a regular rate, with no murmurs or rubs.
The patient had nasal bone tenderness with epistaxis that resolved spontaneously and did not require packing; she had no other facial tenderness. The oropharynx was clear. There was mild posterior midline tenderness over C5 and C6, but no skin ecchymosis or neck swelling. Along with the non-rebreather mask, the patient was placed in a neck collar while she awaited transport to radiology for computed tomography (CT) studies.
The CT scan of the cervical spine demonstrated a minimally displaced fracture of the right anterior arch, both sides of the posterior arch of C1, and a comminuted minimally displaced fracture involving the posterior arch and spinous process of C5, with mild retrolisthesis of C5 over C6.
Based on the CT findings, the patient was taken to the operating room (OR) where she underwent awake fiberoptic laryngoscopy. During transfer to the OR, the patient’s O2 dropped to 87%; however, after successful intubation without complication, O2 saturation improved to 95%. After intubation, the patient was admitted to the intensive care unit for observation, and rivaroxaban therapy was discontinued.
A CT scan of the neck postintubation showed a mild interval decrease in the retropharyngeal hematoma, but an increase in the anterior disc space at C5-C6 with mild retrolisthesis, which raised suspicion for an anterior longitudinal ligamentous injury. A repeat CT scan on hospital day 4 revealed a new bleed within the old retropharyngeal hematoma, with no increase in thickness or size of the initial hematoma. The head and neck surgical team kept the patient intubated while awaiting resolution of the hematoma, with no plan of surgery.
On hospital day 6, the patient was transferred to another facility for continued long-term care. She was transitioned to a tracheostomy 4 days later. Follow-up approximately 2 weeks after presentation confirmed complete resolution of the hematoma, and no surgical intervention was required.
Discussion
Overview
Retropharyngeal hematomas are infrequent, but potentially life-threatening complications of cervical fractures, foreign body trauma, infection, violent coughing, and anticoagulation therapy.1 Although retropharyngeal hematomas associated with warfarin have been well described, to our knowledge, there are no reported cases associated with a direct oral anticoagulant (DOAC).2
Though multiple studies have supported the effectiveness and safety of DOACs for prevention of stroke and systemic embolism in patients with AF, the risk of hemorrhage still exists.3 Postmarketing surveillance studies of DOACs report an overall risk of bleeding comparable to warfarin. Gastrointestinal bleeding was found to be slightly higher in patients taking a DOAC compared to those on warfarin, but the risk of intracranial bleeding from DOACs was notably lower.3 With limited effective reversal agents, DOACs present a tremendous challenge in managing acute life-threatening hemorrhage.4
Signs and Symptoms
Patients with retropharyngeal hematomas can present with dyspnea, sore throat, dysphagia, or odynophagia. Neck tenderness and swelling can suggest a retropharyngeal hematoma.5 The diagnosis of a retropharyngeal hemorrhage is of clinical importance because of the possible threat of airway obstruction—which may not be initially detectable clinically, and depends on how quickly the blood fills the retropharyngeal space.1,6
Diagnosis
Computed tomography with intravenous contrast is the imaging study of choice for diagnosing retropharyngeal hematomas in the emergent care setting, and can detect the presence of any associated vertebral facture.5,7,8 Lateral neck X-ray imaging can detect prevertebral swelling, but is not as sensitive as CT and may underestimate the extent of spinal injury; moreover, lesions or early bleeding may be missed.9 In the absence of vertebral fracture on CT imaging, magnetic resonance imaging should be considered to evaluate for possible associated ligamentous injury.9
Treatment and Management
Airway Management. Given the risk of progression to complete airway obstruction, the first step in managing retropharyngeal hematomas is to secure the patient’s airway. Even though the published literature recommends either endotracheal intubation or tracheostomy, the latter should only be considered as a last resort for patients on DOACs because of the increased risk of bleeding.
The fiberoptic approach to endotracheal intubation minimizes the risk of further trauma and rupture of the hematoma.1,10 Once the patient’s airway is secure, the hematoma can be managed conservatively with spinal immobilization and observation for resolution, which may take 2 to 3 weeks.6,11
Surgical Intervention. Some clinicians believe early surgical intervention leads to early recovery and a shorter hospitalization.12 Surgical intervention using a transoral or anterior cervical approach is recommended for large hematomas that fail to regress.6 Surgical intervention may be considered for patients taking warfarin after successful anticoagulation reversal is achieved using fresh frozen plasma (FFP) and vitamin K. However, due to the increased bleeding potential and limited reversal options, there is an increased risk of surgical complications in patients on DOACs.5
Direct Oral Anticoagulation Reversal
The anticoagulation effect of DOACs resolves after five half-lives from the last administered dose, which in the case of rivaroxaban, is between 1 to 2 days.13 Therefore, when emergent surgical intervention is required for a retropharyngeal hematoma, understanding the options and limitations of reversal agents is necessary.
Idarucizumab. Currently the only DOAC anticoagulation reversal agent approved by the US Food and Drug Administration, idarucizumab is only effective for reversing the anticoagulation effects of dabigatran.4,14
Prothrombin Complex Concentrate. Also referred to as factor IX complex, prothrombin complex concentrate (PCC) has been shown to correct prolonged prothrombin time in experimental models of bleeding. Although there is no clinical evidence for its use in DOAC-associated bleeding, PCC should be considered in life-threatening cases, including large or expanding prevertebral hematoma, or other cases in which the potential benefit outweighs the potential risk of thrombosis associated with PCC.4
Fresh Frozen Plasma. In the absence of PCC, FFP may be considered, though there are no data supporting its use as a reversal agent for rivaroxaban.15
Conclusion
Although a rare entity, retropharyngeal hematoma should be suspected in patients with cervical fractures or trauma, especially in the setting of anticoagulation. Early airway management should be considered in a patient with a retropharyngeal hematoma, as symptoms of airway obstruction may be insidious. In patients on DOACs, the potential benefit of earlier resolution with surgical intervention must be strongly weighed against the increased risk of bleeding.
1. Duvillard C, Ballester M, Romanet P. Traumatic retropharyngeal hematoma: a rare and critical pathology needed for early diagnosis. Eur Arch Otorhinolaryngol. 2005;262(9):713-715. doi:10.1007/s00405-004-0767-3.
2. Karmacharya P, Pathak R, Ghimire S, et al. Upper airway hematoma secondary to warfarin therapy: a systematic review of reported cases. N Am J Med Sci. 2015;7(11):494-502. doi:10.4103/1947-2714.170606.
3. Villines TC, Peacock WF. Safety of direct oral anticoagulants: insights from postmarketing studies. Am J Emerg Med. 2016;34(11S):9-13. doi:10.1016/j.ajem.2016.09.047.
4. Levi M. Management of bleeding in patients treated with direct oral anticoagulants. Crit Care. 2016;20:249. doi:10.1186/s13054-016-1413-3.
5. Toker I, Duman Atilla O, Yesilaras M, Ursavas B. Retropharyngeal hematoma due to oral warfarin usage. Turk J Emerg Med. 2014;14(4):182-184. doi:10.5505/1304.7361.2014.25594.
6. Senel AC, Gunduz AK. Retropharyngeal hematoma secondary to minor blunt neck trauma: case report. Rev Bras Anestesiol. 2012;62(5):731-735. doi:10.1016/S0034-7094(12)70171-X.
7. Koulouris G, Pianta M, Stuckey S. The ‘sentinel clot’ sign in spontaneous retropharyngeal hematoma secondary to parathyroid apoplexy. Ear Nose Throat J. 2006;85(9):606-608.
8. Ryan MF, Meurer D, Tyndall JA. Expanding prevertebral soft tissue swelling subsequent to a motor vehicle collision. Case Rep Emerg Med. 2014;2014:870580. doi:10.1155/2014/870580.
9. Parizel PM, van der Zijden T, Gaudino S, et al. Trauma of the spine and spinal cord: imaging strategies. Eur Spine J. 2010;19(suppl 1):S8-S17. doi:10.1007/s00586-009-1123-5.
10. Shaw CB, Bawa R, Snider G, Wax MK. Traumatic retropharyngeal hematoma: a case report. Otolaryngol Head Neck Surg. 1995;113(4):485-488. doi:10.1016/S0194-59989570091-9.
11. Mackenzie JW, Jellicoe JA. Acute upper airway obstruction. Spontaneous retropharyngeal haematoma in a patient with polycythaemia rubra vera. Anaesthesia. 1986;41(1):57-60.
12. Park JH, Jeong EK, Kang DH, Jeon SR. Surgical treatment of a life-threatening large retropharyngeal hematoma after minor trauma: two case reports and a literature review. J Korean Neurosurg Soc. 2015;58(3):304-307. doi:10.3340/jkns.2015.58.3.304.
13. Scaglione F. New oral anticoagulants: comparative pharmacology with vitamin K antagonists. Clin Pharmacokinet. 2013;52(2):69-82. doi:10.1007/s40262-012-0030-9.
14. Christos S, Naples R. Anticoagulation reversal and treatment strategies in major bleeding: update 2016. West J Emerg Med. 2016;17(3):264-270. doi:10.5811/westjem.2016.3.29294. Erratum in: West J Emerg Med. 2016;17(5):669-670.
15. Chai-Adisaksopha C, Hillis C, Lim W, Boonyawat K, Moffat K, Crowther M. Hemodialysis for the treatment of dabigatran-associated bleeding: a case report and systematic review. J Thromb Haemost. 2015;13(10):1790-1798. doi:10.1111/jth.13117.
Case
A 90-year-old woman with chronic obstructive pulmonary disease; hypertension; chronic kidney disease; diastolic dysfunction; severe tricuspid regurgitation; and atrial fibrillation (AF), for which she was taking rivaroxaban, presented to the ED for evaluation of injuries she sustained during a fall. The patient’s family stated that she fell while walking with the assistance of a walker and landed on her face. There was no reported loss of consciousness. Upon arrival at the ED, the patient’s vital signs were: blood pressure, 188/105 mm Hg; heart rate, 91 beats/min; respiratory rate, 20 breaths/min; and temperature, 97.88°F (36.6°C). Oxygen (O2) saturation was 90% on room air, but increased to 98% after the patient received 10 L/min of O2 through a non-rebreather mask.
On physical examination, the patient was awake, alert, and oriented to person, place, and time, with a Glasgow Coma Scale score of 15. She was able to move all four extremities and had 4/5 motor strength in the upper extremities bilaterally, and 3/5 motor strength in the bilateral lower limbs, which her family reported was the same as her baseline. On pulmonary examination, the lungs were clear to auscultation bilaterally and had no stridor. On auscultation she had a regular rate, with no murmurs or rubs.
The patient had nasal bone tenderness with epistaxis that resolved spontaneously and did not require packing; she had no other facial tenderness. The oropharynx was clear. There was mild posterior midline tenderness over C5 and C6, but no skin ecchymosis or neck swelling. Along with the non-rebreather mask, the patient was placed in a neck collar while she awaited transport to radiology for computed tomography (CT) studies.
The CT scan of the cervical spine demonstrated a minimally displaced fracture of the right anterior arch, both sides of the posterior arch of C1, and a comminuted minimally displaced fracture involving the posterior arch and spinous process of C5, with mild retrolisthesis of C5 over C6.
Based on the CT findings, the patient was taken to the operating room (OR) where she underwent awake fiberoptic laryngoscopy. During transfer to the OR, the patient’s O2 dropped to 87%; however, after successful intubation without complication, O2 saturation improved to 95%. After intubation, the patient was admitted to the intensive care unit for observation, and rivaroxaban therapy was discontinued.
A CT scan of the neck postintubation showed a mild interval decrease in the retropharyngeal hematoma, but an increase in the anterior disc space at C5-C6 with mild retrolisthesis, which raised suspicion for an anterior longitudinal ligamentous injury. A repeat CT scan on hospital day 4 revealed a new bleed within the old retropharyngeal hematoma, with no increase in thickness or size of the initial hematoma. The head and neck surgical team kept the patient intubated while awaiting resolution of the hematoma, with no plan of surgery.
On hospital day 6, the patient was transferred to another facility for continued long-term care. She was transitioned to a tracheostomy 4 days later. Follow-up approximately 2 weeks after presentation confirmed complete resolution of the hematoma, and no surgical intervention was required.
Discussion
Overview
Retropharyngeal hematomas are infrequent, but potentially life-threatening complications of cervical fractures, foreign body trauma, infection, violent coughing, and anticoagulation therapy.1 Although retropharyngeal hematomas associated with warfarin have been well described, to our knowledge, there are no reported cases associated with a direct oral anticoagulant (DOAC).2
Though multiple studies have supported the effectiveness and safety of DOACs for prevention of stroke and systemic embolism in patients with AF, the risk of hemorrhage still exists.3 Postmarketing surveillance studies of DOACs report an overall risk of bleeding comparable to warfarin. Gastrointestinal bleeding was found to be slightly higher in patients taking a DOAC compared to those on warfarin, but the risk of intracranial bleeding from DOACs was notably lower.3 With limited effective reversal agents, DOACs present a tremendous challenge in managing acute life-threatening hemorrhage.4
Signs and Symptoms
Patients with retropharyngeal hematomas can present with dyspnea, sore throat, dysphagia, or odynophagia. Neck tenderness and swelling can suggest a retropharyngeal hematoma.5 The diagnosis of a retropharyngeal hemorrhage is of clinical importance because of the possible threat of airway obstruction—which may not be initially detectable clinically, and depends on how quickly the blood fills the retropharyngeal space.1,6
Diagnosis
Computed tomography with intravenous contrast is the imaging study of choice for diagnosing retropharyngeal hematomas in the emergent care setting, and can detect the presence of any associated vertebral facture.5,7,8 Lateral neck X-ray imaging can detect prevertebral swelling, but is not as sensitive as CT and may underestimate the extent of spinal injury; moreover, lesions or early bleeding may be missed.9 In the absence of vertebral fracture on CT imaging, magnetic resonance imaging should be considered to evaluate for possible associated ligamentous injury.9
Treatment and Management
Airway Management. Given the risk of progression to complete airway obstruction, the first step in managing retropharyngeal hematomas is to secure the patient’s airway. Even though the published literature recommends either endotracheal intubation or tracheostomy, the latter should only be considered as a last resort for patients on DOACs because of the increased risk of bleeding.
The fiberoptic approach to endotracheal intubation minimizes the risk of further trauma and rupture of the hematoma.1,10 Once the patient’s airway is secure, the hematoma can be managed conservatively with spinal immobilization and observation for resolution, which may take 2 to 3 weeks.6,11
Surgical Intervention. Some clinicians believe early surgical intervention leads to early recovery and a shorter hospitalization.12 Surgical intervention using a transoral or anterior cervical approach is recommended for large hematomas that fail to regress.6 Surgical intervention may be considered for patients taking warfarin after successful anticoagulation reversal is achieved using fresh frozen plasma (FFP) and vitamin K. However, due to the increased bleeding potential and limited reversal options, there is an increased risk of surgical complications in patients on DOACs.5
Direct Oral Anticoagulation Reversal
The anticoagulation effect of DOACs resolves after five half-lives from the last administered dose, which in the case of rivaroxaban, is between 1 to 2 days.13 Therefore, when emergent surgical intervention is required for a retropharyngeal hematoma, understanding the options and limitations of reversal agents is necessary.
Idarucizumab. Currently the only DOAC anticoagulation reversal agent approved by the US Food and Drug Administration, idarucizumab is only effective for reversing the anticoagulation effects of dabigatran.4,14
Prothrombin Complex Concentrate. Also referred to as factor IX complex, prothrombin complex concentrate (PCC) has been shown to correct prolonged prothrombin time in experimental models of bleeding. Although there is no clinical evidence for its use in DOAC-associated bleeding, PCC should be considered in life-threatening cases, including large or expanding prevertebral hematoma, or other cases in which the potential benefit outweighs the potential risk of thrombosis associated with PCC.4
Fresh Frozen Plasma. In the absence of PCC, FFP may be considered, though there are no data supporting its use as a reversal agent for rivaroxaban.15
Conclusion
Although a rare entity, retropharyngeal hematoma should be suspected in patients with cervical fractures or trauma, especially in the setting of anticoagulation. Early airway management should be considered in a patient with a retropharyngeal hematoma, as symptoms of airway obstruction may be insidious. In patients on DOACs, the potential benefit of earlier resolution with surgical intervention must be strongly weighed against the increased risk of bleeding.
Case
A 90-year-old woman with chronic obstructive pulmonary disease; hypertension; chronic kidney disease; diastolic dysfunction; severe tricuspid regurgitation; and atrial fibrillation (AF), for which she was taking rivaroxaban, presented to the ED for evaluation of injuries she sustained during a fall. The patient’s family stated that she fell while walking with the assistance of a walker and landed on her face. There was no reported loss of consciousness. Upon arrival at the ED, the patient’s vital signs were: blood pressure, 188/105 mm Hg; heart rate, 91 beats/min; respiratory rate, 20 breaths/min; and temperature, 97.88°F (36.6°C). Oxygen (O2) saturation was 90% on room air, but increased to 98% after the patient received 10 L/min of O2 through a non-rebreather mask.
On physical examination, the patient was awake, alert, and oriented to person, place, and time, with a Glasgow Coma Scale score of 15. She was able to move all four extremities and had 4/5 motor strength in the upper extremities bilaterally, and 3/5 motor strength in the bilateral lower limbs, which her family reported was the same as her baseline. On pulmonary examination, the lungs were clear to auscultation bilaterally and had no stridor. On auscultation she had a regular rate, with no murmurs or rubs.
The patient had nasal bone tenderness with epistaxis that resolved spontaneously and did not require packing; she had no other facial tenderness. The oropharynx was clear. There was mild posterior midline tenderness over C5 and C6, but no skin ecchymosis or neck swelling. Along with the non-rebreather mask, the patient was placed in a neck collar while she awaited transport to radiology for computed tomography (CT) studies.
The CT scan of the cervical spine demonstrated a minimally displaced fracture of the right anterior arch, both sides of the posterior arch of C1, and a comminuted minimally displaced fracture involving the posterior arch and spinous process of C5, with mild retrolisthesis of C5 over C6.
Based on the CT findings, the patient was taken to the operating room (OR) where she underwent awake fiberoptic laryngoscopy. During transfer to the OR, the patient’s O2 dropped to 87%; however, after successful intubation without complication, O2 saturation improved to 95%. After intubation, the patient was admitted to the intensive care unit for observation, and rivaroxaban therapy was discontinued.
A CT scan of the neck postintubation showed a mild interval decrease in the retropharyngeal hematoma, but an increase in the anterior disc space at C5-C6 with mild retrolisthesis, which raised suspicion for an anterior longitudinal ligamentous injury. A repeat CT scan on hospital day 4 revealed a new bleed within the old retropharyngeal hematoma, with no increase in thickness or size of the initial hematoma. The head and neck surgical team kept the patient intubated while awaiting resolution of the hematoma, with no plan of surgery.
On hospital day 6, the patient was transferred to another facility for continued long-term care. She was transitioned to a tracheostomy 4 days later. Follow-up approximately 2 weeks after presentation confirmed complete resolution of the hematoma, and no surgical intervention was required.
Discussion
Overview
Retropharyngeal hematomas are infrequent, but potentially life-threatening complications of cervical fractures, foreign body trauma, infection, violent coughing, and anticoagulation therapy.1 Although retropharyngeal hematomas associated with warfarin have been well described, to our knowledge, there are no reported cases associated with a direct oral anticoagulant (DOAC).2
Though multiple studies have supported the effectiveness and safety of DOACs for prevention of stroke and systemic embolism in patients with AF, the risk of hemorrhage still exists.3 Postmarketing surveillance studies of DOACs report an overall risk of bleeding comparable to warfarin. Gastrointestinal bleeding was found to be slightly higher in patients taking a DOAC compared to those on warfarin, but the risk of intracranial bleeding from DOACs was notably lower.3 With limited effective reversal agents, DOACs present a tremendous challenge in managing acute life-threatening hemorrhage.4
Signs and Symptoms
Patients with retropharyngeal hematomas can present with dyspnea, sore throat, dysphagia, or odynophagia. Neck tenderness and swelling can suggest a retropharyngeal hematoma.5 The diagnosis of a retropharyngeal hemorrhage is of clinical importance because of the possible threat of airway obstruction—which may not be initially detectable clinically, and depends on how quickly the blood fills the retropharyngeal space.1,6
Diagnosis
Computed tomography with intravenous contrast is the imaging study of choice for diagnosing retropharyngeal hematomas in the emergent care setting, and can detect the presence of any associated vertebral facture.5,7,8 Lateral neck X-ray imaging can detect prevertebral swelling, but is not as sensitive as CT and may underestimate the extent of spinal injury; moreover, lesions or early bleeding may be missed.9 In the absence of vertebral fracture on CT imaging, magnetic resonance imaging should be considered to evaluate for possible associated ligamentous injury.9
Treatment and Management
Airway Management. Given the risk of progression to complete airway obstruction, the first step in managing retropharyngeal hematomas is to secure the patient’s airway. Even though the published literature recommends either endotracheal intubation or tracheostomy, the latter should only be considered as a last resort for patients on DOACs because of the increased risk of bleeding.
The fiberoptic approach to endotracheal intubation minimizes the risk of further trauma and rupture of the hematoma.1,10 Once the patient’s airway is secure, the hematoma can be managed conservatively with spinal immobilization and observation for resolution, which may take 2 to 3 weeks.6,11
Surgical Intervention. Some clinicians believe early surgical intervention leads to early recovery and a shorter hospitalization.12 Surgical intervention using a transoral or anterior cervical approach is recommended for large hematomas that fail to regress.6 Surgical intervention may be considered for patients taking warfarin after successful anticoagulation reversal is achieved using fresh frozen plasma (FFP) and vitamin K. However, due to the increased bleeding potential and limited reversal options, there is an increased risk of surgical complications in patients on DOACs.5
Direct Oral Anticoagulation Reversal
The anticoagulation effect of DOACs resolves after five half-lives from the last administered dose, which in the case of rivaroxaban, is between 1 to 2 days.13 Therefore, when emergent surgical intervention is required for a retropharyngeal hematoma, understanding the options and limitations of reversal agents is necessary.
Idarucizumab. Currently the only DOAC anticoagulation reversal agent approved by the US Food and Drug Administration, idarucizumab is only effective for reversing the anticoagulation effects of dabigatran.4,14
Prothrombin Complex Concentrate. Also referred to as factor IX complex, prothrombin complex concentrate (PCC) has been shown to correct prolonged prothrombin time in experimental models of bleeding. Although there is no clinical evidence for its use in DOAC-associated bleeding, PCC should be considered in life-threatening cases, including large or expanding prevertebral hematoma, or other cases in which the potential benefit outweighs the potential risk of thrombosis associated with PCC.4
Fresh Frozen Plasma. In the absence of PCC, FFP may be considered, though there are no data supporting its use as a reversal agent for rivaroxaban.15
Conclusion
Although a rare entity, retropharyngeal hematoma should be suspected in patients with cervical fractures or trauma, especially in the setting of anticoagulation. Early airway management should be considered in a patient with a retropharyngeal hematoma, as symptoms of airway obstruction may be insidious. In patients on DOACs, the potential benefit of earlier resolution with surgical intervention must be strongly weighed against the increased risk of bleeding.
1. Duvillard C, Ballester M, Romanet P. Traumatic retropharyngeal hematoma: a rare and critical pathology needed for early diagnosis. Eur Arch Otorhinolaryngol. 2005;262(9):713-715. doi:10.1007/s00405-004-0767-3.
2. Karmacharya P, Pathak R, Ghimire S, et al. Upper airway hematoma secondary to warfarin therapy: a systematic review of reported cases. N Am J Med Sci. 2015;7(11):494-502. doi:10.4103/1947-2714.170606.
3. Villines TC, Peacock WF. Safety of direct oral anticoagulants: insights from postmarketing studies. Am J Emerg Med. 2016;34(11S):9-13. doi:10.1016/j.ajem.2016.09.047.
4. Levi M. Management of bleeding in patients treated with direct oral anticoagulants. Crit Care. 2016;20:249. doi:10.1186/s13054-016-1413-3.
5. Toker I, Duman Atilla O, Yesilaras M, Ursavas B. Retropharyngeal hematoma due to oral warfarin usage. Turk J Emerg Med. 2014;14(4):182-184. doi:10.5505/1304.7361.2014.25594.
6. Senel AC, Gunduz AK. Retropharyngeal hematoma secondary to minor blunt neck trauma: case report. Rev Bras Anestesiol. 2012;62(5):731-735. doi:10.1016/S0034-7094(12)70171-X.
7. Koulouris G, Pianta M, Stuckey S. The ‘sentinel clot’ sign in spontaneous retropharyngeal hematoma secondary to parathyroid apoplexy. Ear Nose Throat J. 2006;85(9):606-608.
8. Ryan MF, Meurer D, Tyndall JA. Expanding prevertebral soft tissue swelling subsequent to a motor vehicle collision. Case Rep Emerg Med. 2014;2014:870580. doi:10.1155/2014/870580.
9. Parizel PM, van der Zijden T, Gaudino S, et al. Trauma of the spine and spinal cord: imaging strategies. Eur Spine J. 2010;19(suppl 1):S8-S17. doi:10.1007/s00586-009-1123-5.
10. Shaw CB, Bawa R, Snider G, Wax MK. Traumatic retropharyngeal hematoma: a case report. Otolaryngol Head Neck Surg. 1995;113(4):485-488. doi:10.1016/S0194-59989570091-9.
11. Mackenzie JW, Jellicoe JA. Acute upper airway obstruction. Spontaneous retropharyngeal haematoma in a patient with polycythaemia rubra vera. Anaesthesia. 1986;41(1):57-60.
12. Park JH, Jeong EK, Kang DH, Jeon SR. Surgical treatment of a life-threatening large retropharyngeal hematoma after minor trauma: two case reports and a literature review. J Korean Neurosurg Soc. 2015;58(3):304-307. doi:10.3340/jkns.2015.58.3.304.
13. Scaglione F. New oral anticoagulants: comparative pharmacology with vitamin K antagonists. Clin Pharmacokinet. 2013;52(2):69-82. doi:10.1007/s40262-012-0030-9.
14. Christos S, Naples R. Anticoagulation reversal and treatment strategies in major bleeding: update 2016. West J Emerg Med. 2016;17(3):264-270. doi:10.5811/westjem.2016.3.29294. Erratum in: West J Emerg Med. 2016;17(5):669-670.
15. Chai-Adisaksopha C, Hillis C, Lim W, Boonyawat K, Moffat K, Crowther M. Hemodialysis for the treatment of dabigatran-associated bleeding: a case report and systematic review. J Thromb Haemost. 2015;13(10):1790-1798. doi:10.1111/jth.13117.
1. Duvillard C, Ballester M, Romanet P. Traumatic retropharyngeal hematoma: a rare and critical pathology needed for early diagnosis. Eur Arch Otorhinolaryngol. 2005;262(9):713-715. doi:10.1007/s00405-004-0767-3.
2. Karmacharya P, Pathak R, Ghimire S, et al. Upper airway hematoma secondary to warfarin therapy: a systematic review of reported cases. N Am J Med Sci. 2015;7(11):494-502. doi:10.4103/1947-2714.170606.
3. Villines TC, Peacock WF. Safety of direct oral anticoagulants: insights from postmarketing studies. Am J Emerg Med. 2016;34(11S):9-13. doi:10.1016/j.ajem.2016.09.047.
4. Levi M. Management of bleeding in patients treated with direct oral anticoagulants. Crit Care. 2016;20:249. doi:10.1186/s13054-016-1413-3.
5. Toker I, Duman Atilla O, Yesilaras M, Ursavas B. Retropharyngeal hematoma due to oral warfarin usage. Turk J Emerg Med. 2014;14(4):182-184. doi:10.5505/1304.7361.2014.25594.
6. Senel AC, Gunduz AK. Retropharyngeal hematoma secondary to minor blunt neck trauma: case report. Rev Bras Anestesiol. 2012;62(5):731-735. doi:10.1016/S0034-7094(12)70171-X.
7. Koulouris G, Pianta M, Stuckey S. The ‘sentinel clot’ sign in spontaneous retropharyngeal hematoma secondary to parathyroid apoplexy. Ear Nose Throat J. 2006;85(9):606-608.
8. Ryan MF, Meurer D, Tyndall JA. Expanding prevertebral soft tissue swelling subsequent to a motor vehicle collision. Case Rep Emerg Med. 2014;2014:870580. doi:10.1155/2014/870580.
9. Parizel PM, van der Zijden T, Gaudino S, et al. Trauma of the spine and spinal cord: imaging strategies. Eur Spine J. 2010;19(suppl 1):S8-S17. doi:10.1007/s00586-009-1123-5.
10. Shaw CB, Bawa R, Snider G, Wax MK. Traumatic retropharyngeal hematoma: a case report. Otolaryngol Head Neck Surg. 1995;113(4):485-488. doi:10.1016/S0194-59989570091-9.
11. Mackenzie JW, Jellicoe JA. Acute upper airway obstruction. Spontaneous retropharyngeal haematoma in a patient with polycythaemia rubra vera. Anaesthesia. 1986;41(1):57-60.
12. Park JH, Jeong EK, Kang DH, Jeon SR. Surgical treatment of a life-threatening large retropharyngeal hematoma after minor trauma: two case reports and a literature review. J Korean Neurosurg Soc. 2015;58(3):304-307. doi:10.3340/jkns.2015.58.3.304.
13. Scaglione F. New oral anticoagulants: comparative pharmacology with vitamin K antagonists. Clin Pharmacokinet. 2013;52(2):69-82. doi:10.1007/s40262-012-0030-9.
14. Christos S, Naples R. Anticoagulation reversal and treatment strategies in major bleeding: update 2016. West J Emerg Med. 2016;17(3):264-270. doi:10.5811/westjem.2016.3.29294. Erratum in: West J Emerg Med. 2016;17(5):669-670.
15. Chai-Adisaksopha C, Hillis C, Lim W, Boonyawat K, Moffat K, Crowther M. Hemodialysis for the treatment of dabigatran-associated bleeding: a case report and systematic review. J Thromb Haemost. 2015;13(10):1790-1798. doi:10.1111/jth.13117.