User login
An ASCO 2017 recap: significant advances continue
As we head into vacation season and the dog days of summer, let’s reflect for a few minutes on some of the very important advances we heard about at this year’s annual meeting of the American Society of Clinical Oncology in Chicago. Nearly 40,000 individuals registered for the conference, an indication of both the interest and the excitement around the new agents and the emerging clinical trial data. Scientific sessions dedicated to the use of combination immunotherapy, the role of antibody drug conjugates, and targeting molecular aberrations with small molecules were among the most popular (p. e236).
In the setting of metastatic breast cancer, several trials produced highly significant results that will positively affect the duration and quality of life for our patients. The use of PARP inhibitors in BRCA-mutated cancers has been shown to be effective in a few areas, particularly advanced ovarian cancer. The OlympiAD study evaluated olaparib monotherapy and a physician’s choice arm (capecitabine, eribulin, or vinorelbine) in BRCA-mutated, HER2-negative metastatic breast cancer. The 2:1 design enrolled 302 patients and demonstrated a 3-month improvement in progression-free survival (PFS) for olaparib compared with the control arm (7.0 vs 4.2 months, respectively; P = .0009). The patient population for this BRCA-mutated trial was relatively young, with a median age of 45 years, and 50% of the women were hormone positive and 30%, platinum resistant.
The CDK4/6 inhibitors continue to be impressive, with the recently reported results from the MONARCH 2 trial showing encouraging PFS and overall response rate results with the addition of the CDK4/6 inhibitor abemaciclib to fulvestrant, a selective estrogen-receptor degrader. In this study, hormone-positive, HER2-negative women who had progressed on previous endocrine therapy were randomized 2:1 to abemaciclib plus fulvestrant or placebo plus fulvestrant. A total of 669 patients were accrued, and after a median follow-up of 19 months, a highly significant PFS difference of 7 months between the abemaciclib–fulvestrant and fulvestrant–only groups was observed (16.4 vs 9.3 months, respectively; P < .0000001) along with an overall response rate of 48.1 months, compared with 21.3 months. Previous findings have demonstrated monotherapy activity for abemaciclib, and the comparisons with palbociclib and ribociclib will be forthcoming, although no comparative trials are underway. These agents will be extensively assessed in a variety of settings, including adjuvantly.
The results of the much anticipated APHINITY study, which evaluated the addition of pertuzumab to trastuzumab in the adjuvant HER2-positive setting, were met with mixed reviews. Patients were included if they had node-positive invasive breast cancer or node-negative tumors of >1.0 cm. A total of 4,804 patients (37% node negative) were enrolled in the study. The intent-to-treat primary endpoint of invasive disease-free survival (DFS) was statistically positive (P = .045), although the 3-year absolute percentages for the pertuzumab–trastuzumab and trastuzumab-only groups were 94.1% and 93.2%, respectively. It should be noted that the planned statistical assumption was for a delta of 2.6% – 91.8% and 89.2%, respectively. Thus, both arms actually did better than had been planned, which was based on historical comparisons, and the node-positive and hormone-negative subgroups trended toward a greater benefit with the addition of pertuzumab. There was, and will continue to be, much debate around the cost–benefit ratio and which patients should be offered the combination. The outstanding results with the addition of pertuzumab in the neoadjuvant setting will continue to be the setting in which the greatest absolute clinical benefit will be seen. It is unusual in this era to see trials this large planned to identify a small difference, and it is likely that resource constraints will make such studies a thing of the past.
The very active hormonal therapies, abiraterone and enzalutimide, for castrate-resistant prostate cancer remain of high interest in the area of clinical trials. The LATITUDE study evaluated a straightforward design that compared abiraterone with placebo in patients who were newly diagnosed with high-risk, metastatic hormone-naïve prostate cancer. Patients in both arms received androgen-deprivation therapy and high risk was defined by having 2 of 3 criteria: a Gleason score of ≥8; 3 or more bone lesions; or visceral disease. Of note is that 1,199 patients were enrolled before publication of the CHAARTED or STAMPEDE results, which established docetaxel as a standard for these patients. The median age in the LATITUDE trial was 68 years, with 17% of patients having visceral disease and 48% having nodal disease, making it a similar patient population to those in the docetaxel studies. The results favoring abiraterone were strikingly positive, with a 38% reduction in the risk of death (P < .0001) and a 53% reduction in the risk of radiographic progression or death (P < .0001). The regimen was well tolerated overall, and it is clear that this option will be widely considered by physicians and their patients.
Two studies addressing the importance of managing symptoms and improving outcomes were also part of the plenary session. The IDEA Collaboration conducted a prospective pooled analysis of 6 phase 3 studies that assessed 3 and 6 months of oxaliplatin-based regimens for stage 3 colon cancer. FOLFOX and CAPOX given to 12,834 patients in 6 studies from the United States, European Union, Canada, Australia, New Zealand, and Japan were evaluated for DFS, treatment compliance, and adverse events. As would be anticipated, fewer side effects, particularly neurotoxicity, and greater compliance were observed in the 3-month group. Although DFS noninferiority for 3 months of therapy was not established statistically, the overall data led the investigators to issue a consensus statement advocating for a risk-based approach in deciding the duration of therapy and recommending 3 months of therapy for patients with stage 3, T1-3N1 disease, and consideration of 6 months therapy for T4 and/ or N2 disease. The investigators also acknowledged the leader and creator of IDEA, the late Daniel Sargent, PhD, of the Mayo Clinic, who passed away far too young after a brief illness last fall (1970-2016).
The second symptom-based study was performed at Memorial Sloan Kettering Cancer Center (MSKCC) in New York and designed by a group of investigators from the Dana-Farber Cancer Institute in Boston; the Mayo Clinic in Rochester, Minnesota; the University of North Carolina in Chapel Hill; and MSKCC (p. e236). The hypothesis was simply that proactive symptom monitoring during chemotherapy would improve symptom management and lead to better outcomes. For the study, 766 patients with advanced solid tumors who were receiving outpatient chemotherapy were randomized to a control arm with standard follow-up or to the intervention arm, on which patients self-reported on 12 common symptoms before and between visits using a web-based tool and received weekly e-mail reminders and nursing alerts. At 6 months, and compared with baseline, the self-reporting patients in the intervention arm experienced an improved quality of life (P < .001). In addition, 7% fewer of the self-reporting patients visited the emergency department (P = .02), and they experienced longer survival by 5 months compared with the standard follow-up group (31.2 vs 26.0 months, respectively; P = .03). Although there are limitations to such a study, the growth in technological advances should create the opportunity to expand on this strategy in further trials and in practice. With such an emphasis in the Medicare Oncology Home Model on decreasing hospital admissions and visits to the emergency department, there should great motivation for all involved to consider incorporating self-reporting into their patterns of care.
A continued emphasis on molecular profiling, personalized and/or precision medicine, and identifying or matching the patient to the best possible therapy or the most appropriate clinical trial remains vital to improving outcomes. Just before the ASCO meeting, the US Food and Drug Administration approved pembrolizumab for the treatment of patients with high-level microsatellite instability (MSI-H) and mismatch-repair deficient (dMMR) cancers, regardless of the site of origin. The approval was based on data from 149 patients with MSI-H or dMMR cancers, which showed a 40% response rate in this group of patients, two-thirds of whom had previously treated colon cancer. This landmark approval of a cancer therapy for a specific molecular profile and not the site of the disease, will certainly shape the future of oncology drug development. One of the highlighted stories at ASCO was the success of the larotrectinib (LOXO 101) tropomyosin receptor kinase inhibitor in patients with the TRK fusion mutations (p. e237). The data, including waterfall charts, swimmer plots, and computed-tomography scans, were impressive in this targeted population with a 76% response rate and a 91% duration of response at 6 months with a mild side effect profile.
In summary, across a variety of cancers, with treatment strategies of an equally diverse nature, we saw practice-changing data from the ASCO meeting that will benefit our patients. Continuing to seek out clinical trial options for patients will be critical in answering the many questions that have emerged and the substantial number of studies that are ongoing with combination immunotherapies, targeted small molecules, and a growing armamentarium of monoclonal antibodies.
As we head into vacation season and the dog days of summer, let’s reflect for a few minutes on some of the very important advances we heard about at this year’s annual meeting of the American Society of Clinical Oncology in Chicago. Nearly 40,000 individuals registered for the conference, an indication of both the interest and the excitement around the new agents and the emerging clinical trial data. Scientific sessions dedicated to the use of combination immunotherapy, the role of antibody drug conjugates, and targeting molecular aberrations with small molecules were among the most popular (p. e236).
In the setting of metastatic breast cancer, several trials produced highly significant results that will positively affect the duration and quality of life for our patients. The use of PARP inhibitors in BRCA-mutated cancers has been shown to be effective in a few areas, particularly advanced ovarian cancer. The OlympiAD study evaluated olaparib monotherapy and a physician’s choice arm (capecitabine, eribulin, or vinorelbine) in BRCA-mutated, HER2-negative metastatic breast cancer. The 2:1 design enrolled 302 patients and demonstrated a 3-month improvement in progression-free survival (PFS) for olaparib compared with the control arm (7.0 vs 4.2 months, respectively; P = .0009). The patient population for this BRCA-mutated trial was relatively young, with a median age of 45 years, and 50% of the women were hormone positive and 30%, platinum resistant.
The CDK4/6 inhibitors continue to be impressive, with the recently reported results from the MONARCH 2 trial showing encouraging PFS and overall response rate results with the addition of the CDK4/6 inhibitor abemaciclib to fulvestrant, a selective estrogen-receptor degrader. In this study, hormone-positive, HER2-negative women who had progressed on previous endocrine therapy were randomized 2:1 to abemaciclib plus fulvestrant or placebo plus fulvestrant. A total of 669 patients were accrued, and after a median follow-up of 19 months, a highly significant PFS difference of 7 months between the abemaciclib–fulvestrant and fulvestrant–only groups was observed (16.4 vs 9.3 months, respectively; P < .0000001) along with an overall response rate of 48.1 months, compared with 21.3 months. Previous findings have demonstrated monotherapy activity for abemaciclib, and the comparisons with palbociclib and ribociclib will be forthcoming, although no comparative trials are underway. These agents will be extensively assessed in a variety of settings, including adjuvantly.
The results of the much anticipated APHINITY study, which evaluated the addition of pertuzumab to trastuzumab in the adjuvant HER2-positive setting, were met with mixed reviews. Patients were included if they had node-positive invasive breast cancer or node-negative tumors of >1.0 cm. A total of 4,804 patients (37% node negative) were enrolled in the study. The intent-to-treat primary endpoint of invasive disease-free survival (DFS) was statistically positive (P = .045), although the 3-year absolute percentages for the pertuzumab–trastuzumab and trastuzumab-only groups were 94.1% and 93.2%, respectively. It should be noted that the planned statistical assumption was for a delta of 2.6% – 91.8% and 89.2%, respectively. Thus, both arms actually did better than had been planned, which was based on historical comparisons, and the node-positive and hormone-negative subgroups trended toward a greater benefit with the addition of pertuzumab. There was, and will continue to be, much debate around the cost–benefit ratio and which patients should be offered the combination. The outstanding results with the addition of pertuzumab in the neoadjuvant setting will continue to be the setting in which the greatest absolute clinical benefit will be seen. It is unusual in this era to see trials this large planned to identify a small difference, and it is likely that resource constraints will make such studies a thing of the past.
The very active hormonal therapies, abiraterone and enzalutimide, for castrate-resistant prostate cancer remain of high interest in the area of clinical trials. The LATITUDE study evaluated a straightforward design that compared abiraterone with placebo in patients who were newly diagnosed with high-risk, metastatic hormone-naïve prostate cancer. Patients in both arms received androgen-deprivation therapy and high risk was defined by having 2 of 3 criteria: a Gleason score of ≥8; 3 or more bone lesions; or visceral disease. Of note is that 1,199 patients were enrolled before publication of the CHAARTED or STAMPEDE results, which established docetaxel as a standard for these patients. The median age in the LATITUDE trial was 68 years, with 17% of patients having visceral disease and 48% having nodal disease, making it a similar patient population to those in the docetaxel studies. The results favoring abiraterone were strikingly positive, with a 38% reduction in the risk of death (P < .0001) and a 53% reduction in the risk of radiographic progression or death (P < .0001). The regimen was well tolerated overall, and it is clear that this option will be widely considered by physicians and their patients.
Two studies addressing the importance of managing symptoms and improving outcomes were also part of the plenary session. The IDEA Collaboration conducted a prospective pooled analysis of 6 phase 3 studies that assessed 3 and 6 months of oxaliplatin-based regimens for stage 3 colon cancer. FOLFOX and CAPOX given to 12,834 patients in 6 studies from the United States, European Union, Canada, Australia, New Zealand, and Japan were evaluated for DFS, treatment compliance, and adverse events. As would be anticipated, fewer side effects, particularly neurotoxicity, and greater compliance were observed in the 3-month group. Although DFS noninferiority for 3 months of therapy was not established statistically, the overall data led the investigators to issue a consensus statement advocating for a risk-based approach in deciding the duration of therapy and recommending 3 months of therapy for patients with stage 3, T1-3N1 disease, and consideration of 6 months therapy for T4 and/ or N2 disease. The investigators also acknowledged the leader and creator of IDEA, the late Daniel Sargent, PhD, of the Mayo Clinic, who passed away far too young after a brief illness last fall (1970-2016).
The second symptom-based study was performed at Memorial Sloan Kettering Cancer Center (MSKCC) in New York and designed by a group of investigators from the Dana-Farber Cancer Institute in Boston; the Mayo Clinic in Rochester, Minnesota; the University of North Carolina in Chapel Hill; and MSKCC (p. e236). The hypothesis was simply that proactive symptom monitoring during chemotherapy would improve symptom management and lead to better outcomes. For the study, 766 patients with advanced solid tumors who were receiving outpatient chemotherapy were randomized to a control arm with standard follow-up or to the intervention arm, on which patients self-reported on 12 common symptoms before and between visits using a web-based tool and received weekly e-mail reminders and nursing alerts. At 6 months, and compared with baseline, the self-reporting patients in the intervention arm experienced an improved quality of life (P < .001). In addition, 7% fewer of the self-reporting patients visited the emergency department (P = .02), and they experienced longer survival by 5 months compared with the standard follow-up group (31.2 vs 26.0 months, respectively; P = .03). Although there are limitations to such a study, the growth in technological advances should create the opportunity to expand on this strategy in further trials and in practice. With such an emphasis in the Medicare Oncology Home Model on decreasing hospital admissions and visits to the emergency department, there should great motivation for all involved to consider incorporating self-reporting into their patterns of care.
A continued emphasis on molecular profiling, personalized and/or precision medicine, and identifying or matching the patient to the best possible therapy or the most appropriate clinical trial remains vital to improving outcomes. Just before the ASCO meeting, the US Food and Drug Administration approved pembrolizumab for the treatment of patients with high-level microsatellite instability (MSI-H) and mismatch-repair deficient (dMMR) cancers, regardless of the site of origin. The approval was based on data from 149 patients with MSI-H or dMMR cancers, which showed a 40% response rate in this group of patients, two-thirds of whom had previously treated colon cancer. This landmark approval of a cancer therapy for a specific molecular profile and not the site of the disease, will certainly shape the future of oncology drug development. One of the highlighted stories at ASCO was the success of the larotrectinib (LOXO 101) tropomyosin receptor kinase inhibitor in patients with the TRK fusion mutations (p. e237). The data, including waterfall charts, swimmer plots, and computed-tomography scans, were impressive in this targeted population with a 76% response rate and a 91% duration of response at 6 months with a mild side effect profile.
In summary, across a variety of cancers, with treatment strategies of an equally diverse nature, we saw practice-changing data from the ASCO meeting that will benefit our patients. Continuing to seek out clinical trial options for patients will be critical in answering the many questions that have emerged and the substantial number of studies that are ongoing with combination immunotherapies, targeted small molecules, and a growing armamentarium of monoclonal antibodies.
As we head into vacation season and the dog days of summer, let’s reflect for a few minutes on some of the very important advances we heard about at this year’s annual meeting of the American Society of Clinical Oncology in Chicago. Nearly 40,000 individuals registered for the conference, an indication of both the interest and the excitement around the new agents and the emerging clinical trial data. Scientific sessions dedicated to the use of combination immunotherapy, the role of antibody drug conjugates, and targeting molecular aberrations with small molecules were among the most popular (p. e236).
In the setting of metastatic breast cancer, several trials produced highly significant results that will positively affect the duration and quality of life for our patients. The use of PARP inhibitors in BRCA-mutated cancers has been shown to be effective in a few areas, particularly advanced ovarian cancer. The OlympiAD study evaluated olaparib monotherapy and a physician’s choice arm (capecitabine, eribulin, or vinorelbine) in BRCA-mutated, HER2-negative metastatic breast cancer. The 2:1 design enrolled 302 patients and demonstrated a 3-month improvement in progression-free survival (PFS) for olaparib compared with the control arm (7.0 vs 4.2 months, respectively; P = .0009). The patient population for this BRCA-mutated trial was relatively young, with a median age of 45 years, and 50% of the women were hormone positive and 30%, platinum resistant.
The CDK4/6 inhibitors continue to be impressive, with the recently reported results from the MONARCH 2 trial showing encouraging PFS and overall response rate results with the addition of the CDK4/6 inhibitor abemaciclib to fulvestrant, a selective estrogen-receptor degrader. In this study, hormone-positive, HER2-negative women who had progressed on previous endocrine therapy were randomized 2:1 to abemaciclib plus fulvestrant or placebo plus fulvestrant. A total of 669 patients were accrued, and after a median follow-up of 19 months, a highly significant PFS difference of 7 months between the abemaciclib–fulvestrant and fulvestrant–only groups was observed (16.4 vs 9.3 months, respectively; P < .0000001) along with an overall response rate of 48.1 months, compared with 21.3 months. Previous findings have demonstrated monotherapy activity for abemaciclib, and the comparisons with palbociclib and ribociclib will be forthcoming, although no comparative trials are underway. These agents will be extensively assessed in a variety of settings, including adjuvantly.
The results of the much anticipated APHINITY study, which evaluated the addition of pertuzumab to trastuzumab in the adjuvant HER2-positive setting, were met with mixed reviews. Patients were included if they had node-positive invasive breast cancer or node-negative tumors of >1.0 cm. A total of 4,804 patients (37% node negative) were enrolled in the study. The intent-to-treat primary endpoint of invasive disease-free survival (DFS) was statistically positive (P = .045), although the 3-year absolute percentages for the pertuzumab–trastuzumab and trastuzumab-only groups were 94.1% and 93.2%, respectively. It should be noted that the planned statistical assumption was for a delta of 2.6% – 91.8% and 89.2%, respectively. Thus, both arms actually did better than had been planned, which was based on historical comparisons, and the node-positive and hormone-negative subgroups trended toward a greater benefit with the addition of pertuzumab. There was, and will continue to be, much debate around the cost–benefit ratio and which patients should be offered the combination. The outstanding results with the addition of pertuzumab in the neoadjuvant setting will continue to be the setting in which the greatest absolute clinical benefit will be seen. It is unusual in this era to see trials this large planned to identify a small difference, and it is likely that resource constraints will make such studies a thing of the past.
The very active hormonal therapies, abiraterone and enzalutimide, for castrate-resistant prostate cancer remain of high interest in the area of clinical trials. The LATITUDE study evaluated a straightforward design that compared abiraterone with placebo in patients who were newly diagnosed with high-risk, metastatic hormone-naïve prostate cancer. Patients in both arms received androgen-deprivation therapy and high risk was defined by having 2 of 3 criteria: a Gleason score of ≥8; 3 or more bone lesions; or visceral disease. Of note is that 1,199 patients were enrolled before publication of the CHAARTED or STAMPEDE results, which established docetaxel as a standard for these patients. The median age in the LATITUDE trial was 68 years, with 17% of patients having visceral disease and 48% having nodal disease, making it a similar patient population to those in the docetaxel studies. The results favoring abiraterone were strikingly positive, with a 38% reduction in the risk of death (P < .0001) and a 53% reduction in the risk of radiographic progression or death (P < .0001). The regimen was well tolerated overall, and it is clear that this option will be widely considered by physicians and their patients.
Two studies addressing the importance of managing symptoms and improving outcomes were also part of the plenary session. The IDEA Collaboration conducted a prospective pooled analysis of 6 phase 3 studies that assessed 3 and 6 months of oxaliplatin-based regimens for stage 3 colon cancer. FOLFOX and CAPOX given to 12,834 patients in 6 studies from the United States, European Union, Canada, Australia, New Zealand, and Japan were evaluated for DFS, treatment compliance, and adverse events. As would be anticipated, fewer side effects, particularly neurotoxicity, and greater compliance were observed in the 3-month group. Although DFS noninferiority for 3 months of therapy was not established statistically, the overall data led the investigators to issue a consensus statement advocating for a risk-based approach in deciding the duration of therapy and recommending 3 months of therapy for patients with stage 3, T1-3N1 disease, and consideration of 6 months therapy for T4 and/ or N2 disease. The investigators also acknowledged the leader and creator of IDEA, the late Daniel Sargent, PhD, of the Mayo Clinic, who passed away far too young after a brief illness last fall (1970-2016).
The second symptom-based study was performed at Memorial Sloan Kettering Cancer Center (MSKCC) in New York and designed by a group of investigators from the Dana-Farber Cancer Institute in Boston; the Mayo Clinic in Rochester, Minnesota; the University of North Carolina in Chapel Hill; and MSKCC (p. e236). The hypothesis was simply that proactive symptom monitoring during chemotherapy would improve symptom management and lead to better outcomes. For the study, 766 patients with advanced solid tumors who were receiving outpatient chemotherapy were randomized to a control arm with standard follow-up or to the intervention arm, on which patients self-reported on 12 common symptoms before and between visits using a web-based tool and received weekly e-mail reminders and nursing alerts. At 6 months, and compared with baseline, the self-reporting patients in the intervention arm experienced an improved quality of life (P < .001). In addition, 7% fewer of the self-reporting patients visited the emergency department (P = .02), and they experienced longer survival by 5 months compared with the standard follow-up group (31.2 vs 26.0 months, respectively; P = .03). Although there are limitations to such a study, the growth in technological advances should create the opportunity to expand on this strategy in further trials and in practice. With such an emphasis in the Medicare Oncology Home Model on decreasing hospital admissions and visits to the emergency department, there should great motivation for all involved to consider incorporating self-reporting into their patterns of care.
A continued emphasis on molecular profiling, personalized and/or precision medicine, and identifying or matching the patient to the best possible therapy or the most appropriate clinical trial remains vital to improving outcomes. Just before the ASCO meeting, the US Food and Drug Administration approved pembrolizumab for the treatment of patients with high-level microsatellite instability (MSI-H) and mismatch-repair deficient (dMMR) cancers, regardless of the site of origin. The approval was based on data from 149 patients with MSI-H or dMMR cancers, which showed a 40% response rate in this group of patients, two-thirds of whom had previously treated colon cancer. This landmark approval of a cancer therapy for a specific molecular profile and not the site of the disease, will certainly shape the future of oncology drug development. One of the highlighted stories at ASCO was the success of the larotrectinib (LOXO 101) tropomyosin receptor kinase inhibitor in patients with the TRK fusion mutations (p. e237). The data, including waterfall charts, swimmer plots, and computed-tomography scans, were impressive in this targeted population with a 76% response rate and a 91% duration of response at 6 months with a mild side effect profile.
In summary, across a variety of cancers, with treatment strategies of an equally diverse nature, we saw practice-changing data from the ASCO meeting that will benefit our patients. Continuing to seek out clinical trial options for patients will be critical in answering the many questions that have emerged and the substantial number of studies that are ongoing with combination immunotherapies, targeted small molecules, and a growing armamentarium of monoclonal antibodies.
FDA places full, partial holds on durvalumab trials
The US Food and Drug Administration (FDA) has placed a partial clinical hold on 5 trials and a full clinical hold on 1 trial of the anti-PD-L1 antibody durvalumab (Imfinzi™).
In these trials, researchers are testing durvalumab in combination with immunomodulatory and chemotherapy agents in patients with multiple myeloma (MM) and lymphomas.
At present, no new patients can be enrolled in any of the 6 trials.
Patients enrolled in the trials on partial clinical hold can remain on treatment if they are receiving clinical benefit.
Patients enrolled in the trial on full clinical hold will discontinue the study treatment.
The FDA’s decision to place these trials on hold is related to risks identified in trials studying another anti-PD-1 agent, pembrolizumab, in MM patients.
Data from the pembrolizumab trials indicate the risks outweigh the benefits when PD-1/PD-L1 treatment is given to MM patients in combination with dexamethasone and pomalidomide or lenalidomide.
In addition, there may be an unfavorable risk-benefit ratio for MM patients receiving PD-1/PD-L1 treatments alone or in other combinations.
With this in mind, the FDA placed the MEDI4736-MM-002 trial on full clinical hold.
MEDI4736-MM-002 is a phase 1b study designed to determine the recommended dose and regimen of durvalumab in combination with lenalidomide, with and without low-dose dexamethasone, in patients with newly diagnosed MM.
The FDA also placed the following trials on partial clinical hold:
- MEDI4736-MM-001: A phase 1b study to determine the recommended dose and regimen of durvalumab either as monotherapy or in combination with pomalidomide, with or without low-dose dexamethasone, in patients with relapsed and refractory MM
- MEDI4736-MM-003: A phase 2 study to determine the safety and efficacy of the combination of durvalumab and daratumumab in patients with relapsed and refractory MM
- MEDI4736-MM-005: A phase 2 study to determine the efficacy of the combination of durvalumab plus daratumumab in patients with relapsed and refractory MM who have progressed while on a current treatment regimen containing daratumumab
- MEDI4736-NHL-001: A phase 1/2 study to assess the safety and tolerability of durvalumab as monotherapy and in combination therapy in patients with lymphomas, including chronic lymphocytic leukemia. The only arm in this trial for which enrollment is suspended is the arm with the durvalumab, lenalidomide, and rituximab combination.
- MEDI4736-DLBCL-001: A phase 2 study to evaluate the safety and clinical activity of durvalumab in combination with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or with lenalidomide plus R-CHOP in patients with previously untreated, high-risk diffuse large B-cell lymphoma.
The trials that will continue to enroll are:
- MEDI4736-MDS-001: A phase 2 study evaluating the efficacy and safety of subcutaneous azacitidine in combination with durvalumab in previously untreated patients with higher-risk myelodysplastic syndromes or in elderly (≥65 years) acute myeloid leukemia patients not eligible for hematopoietic stem cell transplant
- CC-486-MDS-006: A phase 2 study to evaluate the efficacy and safety of CC-486 alone or in combination with durvalumab in patients with myelodysplastic syndromes who fail to achieve an objective response to treatment with azacitidine for injection or decitabine.
Durvalumab is being developed by Celgene Corporation and MedImmune, the global biologics research and development arm of AstraZeneca.
The use of durvalumab in combination with other agents for the treatment of patients with hematologic malignancies is not approved by the FDA, and the safety and efficacy of those combinations has not been established.
Durvalumab has accelerated approval from the FDA to treat patients with locally advanced or metastatic urothelial carcinoma. ![]()
The US Food and Drug Administration (FDA) has placed a partial clinical hold on 5 trials and a full clinical hold on 1 trial of the anti-PD-L1 antibody durvalumab (Imfinzi™).
In these trials, researchers are testing durvalumab in combination with immunomodulatory and chemotherapy agents in patients with multiple myeloma (MM) and lymphomas.
At present, no new patients can be enrolled in any of the 6 trials.
Patients enrolled in the trials on partial clinical hold can remain on treatment if they are receiving clinical benefit.
Patients enrolled in the trial on full clinical hold will discontinue the study treatment.
The FDA’s decision to place these trials on hold is related to risks identified in trials studying another anti-PD-1 agent, pembrolizumab, in MM patients.
Data from the pembrolizumab trials indicate the risks outweigh the benefits when PD-1/PD-L1 treatment is given to MM patients in combination with dexamethasone and pomalidomide or lenalidomide.
In addition, there may be an unfavorable risk-benefit ratio for MM patients receiving PD-1/PD-L1 treatments alone or in other combinations.
With this in mind, the FDA placed the MEDI4736-MM-002 trial on full clinical hold.
MEDI4736-MM-002 is a phase 1b study designed to determine the recommended dose and regimen of durvalumab in combination with lenalidomide, with and without low-dose dexamethasone, in patients with newly diagnosed MM.
The FDA also placed the following trials on partial clinical hold:
- MEDI4736-MM-001: A phase 1b study to determine the recommended dose and regimen of durvalumab either as monotherapy or in combination with pomalidomide, with or without low-dose dexamethasone, in patients with relapsed and refractory MM
- MEDI4736-MM-003: A phase 2 study to determine the safety and efficacy of the combination of durvalumab and daratumumab in patients with relapsed and refractory MM
- MEDI4736-MM-005: A phase 2 study to determine the efficacy of the combination of durvalumab plus daratumumab in patients with relapsed and refractory MM who have progressed while on a current treatment regimen containing daratumumab
- MEDI4736-NHL-001: A phase 1/2 study to assess the safety and tolerability of durvalumab as monotherapy and in combination therapy in patients with lymphomas, including chronic lymphocytic leukemia. The only arm in this trial for which enrollment is suspended is the arm with the durvalumab, lenalidomide, and rituximab combination.
- MEDI4736-DLBCL-001: A phase 2 study to evaluate the safety and clinical activity of durvalumab in combination with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or with lenalidomide plus R-CHOP in patients with previously untreated, high-risk diffuse large B-cell lymphoma.
The trials that will continue to enroll are:
- MEDI4736-MDS-001: A phase 2 study evaluating the efficacy and safety of subcutaneous azacitidine in combination with durvalumab in previously untreated patients with higher-risk myelodysplastic syndromes or in elderly (≥65 years) acute myeloid leukemia patients not eligible for hematopoietic stem cell transplant
- CC-486-MDS-006: A phase 2 study to evaluate the efficacy and safety of CC-486 alone or in combination with durvalumab in patients with myelodysplastic syndromes who fail to achieve an objective response to treatment with azacitidine for injection or decitabine.
Durvalumab is being developed by Celgene Corporation and MedImmune, the global biologics research and development arm of AstraZeneca.
The use of durvalumab in combination with other agents for the treatment of patients with hematologic malignancies is not approved by the FDA, and the safety and efficacy of those combinations has not been established.
Durvalumab has accelerated approval from the FDA to treat patients with locally advanced or metastatic urothelial carcinoma. ![]()
The US Food and Drug Administration (FDA) has placed a partial clinical hold on 5 trials and a full clinical hold on 1 trial of the anti-PD-L1 antibody durvalumab (Imfinzi™).
In these trials, researchers are testing durvalumab in combination with immunomodulatory and chemotherapy agents in patients with multiple myeloma (MM) and lymphomas.
At present, no new patients can be enrolled in any of the 6 trials.
Patients enrolled in the trials on partial clinical hold can remain on treatment if they are receiving clinical benefit.
Patients enrolled in the trial on full clinical hold will discontinue the study treatment.
The FDA’s decision to place these trials on hold is related to risks identified in trials studying another anti-PD-1 agent, pembrolizumab, in MM patients.
Data from the pembrolizumab trials indicate the risks outweigh the benefits when PD-1/PD-L1 treatment is given to MM patients in combination with dexamethasone and pomalidomide or lenalidomide.
In addition, there may be an unfavorable risk-benefit ratio for MM patients receiving PD-1/PD-L1 treatments alone or in other combinations.
With this in mind, the FDA placed the MEDI4736-MM-002 trial on full clinical hold.
MEDI4736-MM-002 is a phase 1b study designed to determine the recommended dose and regimen of durvalumab in combination with lenalidomide, with and without low-dose dexamethasone, in patients with newly diagnosed MM.
The FDA also placed the following trials on partial clinical hold:
- MEDI4736-MM-001: A phase 1b study to determine the recommended dose and regimen of durvalumab either as monotherapy or in combination with pomalidomide, with or without low-dose dexamethasone, in patients with relapsed and refractory MM
- MEDI4736-MM-003: A phase 2 study to determine the safety and efficacy of the combination of durvalumab and daratumumab in patients with relapsed and refractory MM
- MEDI4736-MM-005: A phase 2 study to determine the efficacy of the combination of durvalumab plus daratumumab in patients with relapsed and refractory MM who have progressed while on a current treatment regimen containing daratumumab
- MEDI4736-NHL-001: A phase 1/2 study to assess the safety and tolerability of durvalumab as monotherapy and in combination therapy in patients with lymphomas, including chronic lymphocytic leukemia. The only arm in this trial for which enrollment is suspended is the arm with the durvalumab, lenalidomide, and rituximab combination.
- MEDI4736-DLBCL-001: A phase 2 study to evaluate the safety and clinical activity of durvalumab in combination with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or with lenalidomide plus R-CHOP in patients with previously untreated, high-risk diffuse large B-cell lymphoma.
The trials that will continue to enroll are:
- MEDI4736-MDS-001: A phase 2 study evaluating the efficacy and safety of subcutaneous azacitidine in combination with durvalumab in previously untreated patients with higher-risk myelodysplastic syndromes or in elderly (≥65 years) acute myeloid leukemia patients not eligible for hematopoietic stem cell transplant
- CC-486-MDS-006: A phase 2 study to evaluate the efficacy and safety of CC-486 alone or in combination with durvalumab in patients with myelodysplastic syndromes who fail to achieve an objective response to treatment with azacitidine for injection or decitabine.
Durvalumab is being developed by Celgene Corporation and MedImmune, the global biologics research and development arm of AstraZeneca.
The use of durvalumab in combination with other agents for the treatment of patients with hematologic malignancies is not approved by the FDA, and the safety and efficacy of those combinations has not been established.
Durvalumab has accelerated approval from the FDA to treat patients with locally advanced or metastatic urothelial carcinoma. ![]()
Death prompts dosing suspension in fitusiran trials
Alnylam Pharmaceuticals, Inc. has announced suspension of dosing in all trials of fitusiran, an RNAi therapeutic being developed to treat patients with hemophilia A and B, with and without inhibitors.
Alnylam reported a fatal thrombotic event in a patient with hemophilia A without inhibitors in the phase 2 open-label extension study of fitusiran.
As a result, the company has suspended dosing in all ongoing fitusiran studies pending further review of the event and development of a risk mitigation strategy.
Fitusiran trials include the trial in which the death was reported—a phase 2 open-label extension study of hemophilia A and B patients with and without inhibitors—and the ATLAS phase 3 program, in which patient dosing has not yet begun.
Based on an overall consideration of fitusiran’s benefit-risk profile, Alnylam said it aims to resume or begin dosing in these trials as soon as possible, upon agreement with global regulatory authorities and with appropriate protocol amendments in place for enhanced patient safety monitoring.
“We are deeply saddened to learn of this patient’s death, and we extend our sympathies to his family,” said Akshay Vaishnaw, MD, PhD, executive vice president of research and development at Alnylam.
“We believe that fitusiran holds great promise as a potential treatment option for patients with hemophilia, and we remain fully committed to its ongoing development. Following further investigation of this safety finding, implementation of a risk mitigation strategy, and alignment with global regulatory authorities, we expect to resume fitusiran dosing in our clinical studies as soon as possible, potentially as early as late 2017, with a goal of advancing this innovative investigational medicine to hemophilia patients in need.”
About the patient
Alnylam recently became aware of a fatal serious adverse event in a patient with hemophilia A who was receiving fitusiran in the phase 2 open-label extension study.
Approximately 9 days prior to hospital admission, the patient developed exercise-induced right hip pain that was treated with a total of 3 doses of factor VIII concentrate (31-46 IU/kg) on 3 separate days.
Four days prior to admission, when the patient received his third dose of factor, he developed a severe headache.
While he was initially suspected of having viral meningitis, the patient was diagnosed with subarachnoid hemorrhage on the basis of CT imaging, and he was treated with factor VIII concentrate administered 2 to 3 times daily.
Over a 14-day hospitalization, his medical condition worsened, and the patient died from subsequent cerebral edema.
The initial diagnosis of subarachnoid hemorrhage was reported by the investigator as not related to fitusiran.
For a more complete understanding, Alnylam initiated further investigation of the event, including review of the patient’s CT scans by 3 independent neuro-radiologists.
All 3 neuro-radiologists confirmed that the initiating event was a cerebral venous sinus thrombosis and not a subarachnoid hemorrhage.
As a result of this new information, Alnylam suspended dosing in fitusiran studies in order to further investigate the safety event, now considered to be possibly related to fitusiran, and to develop a risk mitigation plan.
The company also notified study investigators and global regulatory authorities.
About fitusiran
Fitusiran is an investigational, once-monthly, subcutaneously administered RNAi therapeutic targeting antithrombin for the treatment of hemophilia A and B, with and without inhibitors. Fitusiran also has the potential to be used for rare bleeding disorders.
Fitusiran is designed to lower levels of antithrombin with the goal of promoting sufficient thrombin generation to restore hemostasis and prevent bleeding.
The safety and efficacy of fitusiran have not been evaluated by the FDA or any other health authority. ![]()
Alnylam Pharmaceuticals, Inc. has announced suspension of dosing in all trials of fitusiran, an RNAi therapeutic being developed to treat patients with hemophilia A and B, with and without inhibitors.
Alnylam reported a fatal thrombotic event in a patient with hemophilia A without inhibitors in the phase 2 open-label extension study of fitusiran.
As a result, the company has suspended dosing in all ongoing fitusiran studies pending further review of the event and development of a risk mitigation strategy.
Fitusiran trials include the trial in which the death was reported—a phase 2 open-label extension study of hemophilia A and B patients with and without inhibitors—and the ATLAS phase 3 program, in which patient dosing has not yet begun.
Based on an overall consideration of fitusiran’s benefit-risk profile, Alnylam said it aims to resume or begin dosing in these trials as soon as possible, upon agreement with global regulatory authorities and with appropriate protocol amendments in place for enhanced patient safety monitoring.
“We are deeply saddened to learn of this patient’s death, and we extend our sympathies to his family,” said Akshay Vaishnaw, MD, PhD, executive vice president of research and development at Alnylam.
“We believe that fitusiran holds great promise as a potential treatment option for patients with hemophilia, and we remain fully committed to its ongoing development. Following further investigation of this safety finding, implementation of a risk mitigation strategy, and alignment with global regulatory authorities, we expect to resume fitusiran dosing in our clinical studies as soon as possible, potentially as early as late 2017, with a goal of advancing this innovative investigational medicine to hemophilia patients in need.”
About the patient
Alnylam recently became aware of a fatal serious adverse event in a patient with hemophilia A who was receiving fitusiran in the phase 2 open-label extension study.
Approximately 9 days prior to hospital admission, the patient developed exercise-induced right hip pain that was treated with a total of 3 doses of factor VIII concentrate (31-46 IU/kg) on 3 separate days.
Four days prior to admission, when the patient received his third dose of factor, he developed a severe headache.
While he was initially suspected of having viral meningitis, the patient was diagnosed with subarachnoid hemorrhage on the basis of CT imaging, and he was treated with factor VIII concentrate administered 2 to 3 times daily.
Over a 14-day hospitalization, his medical condition worsened, and the patient died from subsequent cerebral edema.
The initial diagnosis of subarachnoid hemorrhage was reported by the investigator as not related to fitusiran.
For a more complete understanding, Alnylam initiated further investigation of the event, including review of the patient’s CT scans by 3 independent neuro-radiologists.
All 3 neuro-radiologists confirmed that the initiating event was a cerebral venous sinus thrombosis and not a subarachnoid hemorrhage.
As a result of this new information, Alnylam suspended dosing in fitusiran studies in order to further investigate the safety event, now considered to be possibly related to fitusiran, and to develop a risk mitigation plan.
The company also notified study investigators and global regulatory authorities.
About fitusiran
Fitusiran is an investigational, once-monthly, subcutaneously administered RNAi therapeutic targeting antithrombin for the treatment of hemophilia A and B, with and without inhibitors. Fitusiran also has the potential to be used for rare bleeding disorders.
Fitusiran is designed to lower levels of antithrombin with the goal of promoting sufficient thrombin generation to restore hemostasis and prevent bleeding.
The safety and efficacy of fitusiran have not been evaluated by the FDA or any other health authority. ![]()
Alnylam Pharmaceuticals, Inc. has announced suspension of dosing in all trials of fitusiran, an RNAi therapeutic being developed to treat patients with hemophilia A and B, with and without inhibitors.
Alnylam reported a fatal thrombotic event in a patient with hemophilia A without inhibitors in the phase 2 open-label extension study of fitusiran.
As a result, the company has suspended dosing in all ongoing fitusiran studies pending further review of the event and development of a risk mitigation strategy.
Fitusiran trials include the trial in which the death was reported—a phase 2 open-label extension study of hemophilia A and B patients with and without inhibitors—and the ATLAS phase 3 program, in which patient dosing has not yet begun.
Based on an overall consideration of fitusiran’s benefit-risk profile, Alnylam said it aims to resume or begin dosing in these trials as soon as possible, upon agreement with global regulatory authorities and with appropriate protocol amendments in place for enhanced patient safety monitoring.
“We are deeply saddened to learn of this patient’s death, and we extend our sympathies to his family,” said Akshay Vaishnaw, MD, PhD, executive vice president of research and development at Alnylam.
“We believe that fitusiran holds great promise as a potential treatment option for patients with hemophilia, and we remain fully committed to its ongoing development. Following further investigation of this safety finding, implementation of a risk mitigation strategy, and alignment with global regulatory authorities, we expect to resume fitusiran dosing in our clinical studies as soon as possible, potentially as early as late 2017, with a goal of advancing this innovative investigational medicine to hemophilia patients in need.”
About the patient
Alnylam recently became aware of a fatal serious adverse event in a patient with hemophilia A who was receiving fitusiran in the phase 2 open-label extension study.
Approximately 9 days prior to hospital admission, the patient developed exercise-induced right hip pain that was treated with a total of 3 doses of factor VIII concentrate (31-46 IU/kg) on 3 separate days.
Four days prior to admission, when the patient received his third dose of factor, he developed a severe headache.
While he was initially suspected of having viral meningitis, the patient was diagnosed with subarachnoid hemorrhage on the basis of CT imaging, and he was treated with factor VIII concentrate administered 2 to 3 times daily.
Over a 14-day hospitalization, his medical condition worsened, and the patient died from subsequent cerebral edema.
The initial diagnosis of subarachnoid hemorrhage was reported by the investigator as not related to fitusiran.
For a more complete understanding, Alnylam initiated further investigation of the event, including review of the patient’s CT scans by 3 independent neuro-radiologists.
All 3 neuro-radiologists confirmed that the initiating event was a cerebral venous sinus thrombosis and not a subarachnoid hemorrhage.
As a result of this new information, Alnylam suspended dosing in fitusiran studies in order to further investigate the safety event, now considered to be possibly related to fitusiran, and to develop a risk mitigation plan.
The company also notified study investigators and global regulatory authorities.
About fitusiran
Fitusiran is an investigational, once-monthly, subcutaneously administered RNAi therapeutic targeting antithrombin for the treatment of hemophilia A and B, with and without inhibitors. Fitusiran also has the potential to be used for rare bleeding disorders.
Fitusiran is designed to lower levels of antithrombin with the goal of promoting sufficient thrombin generation to restore hemostasis and prevent bleeding.
The safety and efficacy of fitusiran have not been evaluated by the FDA or any other health authority. ![]()
FDA places partial hold on trials of nivolumab in MM
The US Food and Drug Administration (FDA) has placed a partial clinical hold on 3 trials of the PD-1 immune checkpoint inhibitor nivolumab (Opdivo).
The trials were designed to investigate nivolumab-based combination regimens in patients with relapsed or refractory multiple myeloma (MM).
The partial clinical hold means patients currently enrolled in these 3 trials can continue treatment if they are experiencing clinical benefit. However, no new patients can be enrolled at this time.
The partial clinical hold is related to risks identified in trials studying another anti-PD-1 agent, pembrolizumab, in MM patients.
Data from the pembrolizumab trials indicate the risks outweigh the benefits when PD-1/PD-L1 treatment is given to MM patients in combination with dexamethasone and pomalidomide or lenalidomide.
In addition, there may be an unfavorable risk-benefit ratio for MM patients receiving PD-1/PD-L1 treatments alone or in other combinations.
With this in mind, the FDA placed the partial hold on the following nivolumab trials:
- CheckMate-602: A randomized, phase 3 trial of combinations of nivolumab, elotuzumab, pomalidomide, and dexamethasone in relapsed and refractory MM
- CheckMate-039: A phase 1 study intended to establish the tolerability of nivolumab and the combination of nivolumab and daratumumab, with or without pomalidomide and dexamethasone, in patients with relapsed or refractory MM
- CA204142: A phase 2 study of elotuzumab in combination with pomalidomide and low-dose dexamethasone, and in combination with nivolumab, in patients with MM who relapsed after or were refractory to prior treatment with lenalidomide.
Other studies of nivolumab will continue as planned.
Bristol-Myers Squibb, the company developing and marketing nivolumab, said it remains steadfast in its commitment to improve outcomes for MM patients and will work closely with the FDA to address concerns.
Nivolumab is currently FDA-approved to treat:
- Adults with classical Hodgkin lymphoma that has relapsed or progressed after autologous hematopoietic stem cell transplant and brentuximab vedotin or after 3 or more lines of therapy, including autologous transplant
- Patients with previously treated metastatic non-small cell lung cancer
- Metastatic melanoma patients
- Advanced renal cell carcinoma patients who received prior anti-angiogenic therapy
- Patients with recurrent or metastatic squamous cell carcinoma of the head and neck on or after platinum-based therapy
- Patients with previously treated locally advanced or metastatic urothelial carcinoma who have disease progression during or following platinum-based chemotherapy
- Patients (≥12 years) with microsatellite instability high or mismatch repair-deficient metastatic colorectal cancer that has progressed following treatment with a fluoropyrimidine, oxaliplatin, and irinotecan.

The US Food and Drug Administration (FDA) has placed a partial clinical hold on 3 trials of the PD-1 immune checkpoint inhibitor nivolumab (Opdivo).
The trials were designed to investigate nivolumab-based combination regimens in patients with relapsed or refractory multiple myeloma (MM).
The partial clinical hold means patients currently enrolled in these 3 trials can continue treatment if they are experiencing clinical benefit. However, no new patients can be enrolled at this time.
The partial clinical hold is related to risks identified in trials studying another anti-PD-1 agent, pembrolizumab, in MM patients.
Data from the pembrolizumab trials indicate the risks outweigh the benefits when PD-1/PD-L1 treatment is given to MM patients in combination with dexamethasone and pomalidomide or lenalidomide.
In addition, there may be an unfavorable risk-benefit ratio for MM patients receiving PD-1/PD-L1 treatments alone or in other combinations.
With this in mind, the FDA placed the partial hold on the following nivolumab trials:
- CheckMate-602: A randomized, phase 3 trial of combinations of nivolumab, elotuzumab, pomalidomide, and dexamethasone in relapsed and refractory MM
- CheckMate-039: A phase 1 study intended to establish the tolerability of nivolumab and the combination of nivolumab and daratumumab, with or without pomalidomide and dexamethasone, in patients with relapsed or refractory MM
- CA204142: A phase 2 study of elotuzumab in combination with pomalidomide and low-dose dexamethasone, and in combination with nivolumab, in patients with MM who relapsed after or were refractory to prior treatment with lenalidomide.
Other studies of nivolumab will continue as planned.
Bristol-Myers Squibb, the company developing and marketing nivolumab, said it remains steadfast in its commitment to improve outcomes for MM patients and will work closely with the FDA to address concerns.
Nivolumab is currently FDA-approved to treat:
- Adults with classical Hodgkin lymphoma that has relapsed or progressed after autologous hematopoietic stem cell transplant and brentuximab vedotin or after 3 or more lines of therapy, including autologous transplant
- Patients with previously treated metastatic non-small cell lung cancer
- Metastatic melanoma patients
- Advanced renal cell carcinoma patients who received prior anti-angiogenic therapy
- Patients with recurrent or metastatic squamous cell carcinoma of the head and neck on or after platinum-based therapy
- Patients with previously treated locally advanced or metastatic urothelial carcinoma who have disease progression during or following platinum-based chemotherapy
- Patients (≥12 years) with microsatellite instability high or mismatch repair-deficient metastatic colorectal cancer that has progressed following treatment with a fluoropyrimidine, oxaliplatin, and irinotecan.

The US Food and Drug Administration (FDA) has placed a partial clinical hold on 3 trials of the PD-1 immune checkpoint inhibitor nivolumab (Opdivo).
The trials were designed to investigate nivolumab-based combination regimens in patients with relapsed or refractory multiple myeloma (MM).
The partial clinical hold means patients currently enrolled in these 3 trials can continue treatment if they are experiencing clinical benefit. However, no new patients can be enrolled at this time.
The partial clinical hold is related to risks identified in trials studying another anti-PD-1 agent, pembrolizumab, in MM patients.
Data from the pembrolizumab trials indicate the risks outweigh the benefits when PD-1/PD-L1 treatment is given to MM patients in combination with dexamethasone and pomalidomide or lenalidomide.
In addition, there may be an unfavorable risk-benefit ratio for MM patients receiving PD-1/PD-L1 treatments alone or in other combinations.
With this in mind, the FDA placed the partial hold on the following nivolumab trials:
- CheckMate-602: A randomized, phase 3 trial of combinations of nivolumab, elotuzumab, pomalidomide, and dexamethasone in relapsed and refractory MM
- CheckMate-039: A phase 1 study intended to establish the tolerability of nivolumab and the combination of nivolumab and daratumumab, with or without pomalidomide and dexamethasone, in patients with relapsed or refractory MM
- CA204142: A phase 2 study of elotuzumab in combination with pomalidomide and low-dose dexamethasone, and in combination with nivolumab, in patients with MM who relapsed after or were refractory to prior treatment with lenalidomide.
Other studies of nivolumab will continue as planned.
Bristol-Myers Squibb, the company developing and marketing nivolumab, said it remains steadfast in its commitment to improve outcomes for MM patients and will work closely with the FDA to address concerns.
Nivolumab is currently FDA-approved to treat:
- Adults with classical Hodgkin lymphoma that has relapsed or progressed after autologous hematopoietic stem cell transplant and brentuximab vedotin or after 3 or more lines of therapy, including autologous transplant
- Patients with previously treated metastatic non-small cell lung cancer
- Metastatic melanoma patients
- Advanced renal cell carcinoma patients who received prior anti-angiogenic therapy
- Patients with recurrent or metastatic squamous cell carcinoma of the head and neck on or after platinum-based therapy
- Patients with previously treated locally advanced or metastatic urothelial carcinoma who have disease progression during or following platinum-based chemotherapy
- Patients (≥12 years) with microsatellite instability high or mismatch repair-deficient metastatic colorectal cancer that has progressed following treatment with a fluoropyrimidine, oxaliplatin, and irinotecan.

Does Diet Matter in Multiple Sclerosis?
Q) What is known about the impact of diet on multiple sclerosis? How can I advise my patients with MS?
Multiple sclerosis (MS) is a chronic inflammatory and degenerative central nervous system disease affecting more than 2.5 million people worldwide. Today, if a Google search is performed for “diet and MS,” more than 67 million results are obtained. Many tout specific protocols as beneficial for MS but have no substantial data to support these claims. This can be confusing for patients as well as providers. How should you advise those who ask for advice on dietary modifications to help control symptoms or disease course?
First, it’s important to remember that individuals with MS have a reduced median lifespan (by about seven years), compared to healthy controls. Furthermore, patients with MS commonly have comorbid conditions—such as diabetes, obesity, and ischemic heart disease—that increase mortality risk.1,2 Diet and nutrition are significant factors that impact the course of these diseases.
We must also bear in mind that patients with MS experience symptoms that may impede their efforts to prepare meals. In a 2008 study of 123 MS patients (more than 50% of whom were overweight or obese), fatigue was cited as a significant factor that limited cooking and food preparation. Cognitive impairment and depression also may affect dietary intake. Interestingly, the average recorded intake for all food groups was less than that recommended in the Dietary Guidelines for Americans.3
A web-based survey conducted by the German MS Society in 2011 revealed that 42% of the 337 respondents had modified their diet due to MS. These modifications included change in intake of fatty acids; decrease or elimination of meat, sugar, and additives; and introduction of a low-carb or Paleo diet.4
Among an international sample of 2,087 MS patients, a significant association was found between a healthy diet and improved quality of life (both physical and mental) and reduced disability. This “healthy consumption” of fruits, vegetables, and dietary fat was also associated with a marginally decreased risk for relapse. Patients who demonstrated increased disease activity were more likely to have poor consumption of fruits, vegetables, and fats and to consume more meat and dairy products.5
There has also been research on specific components of dietary intake. Antioxidant-containing foods, for example, may have an anti-inflammatory effect.6 Vitamin B12 deficiency plays a role in immunomodulatory effect, as well as formation of the myelin sheath, although its role (and the effect of biotin supplementation) in MS disease progression requires further study.7 Also ongoing is research into various calorie-restriction protocols, altering both timing and amount of caloric intake, since some data suggest this strategy reduces leptin, a satiety hormone that increases inflammation and has been shown to promote more aggressive MS in a mouse model.8
In the meantime, what can we conclude about diet and MS? A recent review determined that, although there is insufficient data to support one specific diet, there is sufficient evidence to recommend consumption of fish, foods lower in fat, whole grains, vitamin D, and supplemental omega fatty acids.5
It is important to discuss diet with our MS patients. In the German survey, 82% of patients felt that diet was important, yet only 10% had asked a provider for nutritional advice.4 In another study, patients indicated that food labels were their top source for nutrition information; only 20% sought advice from a nutritionist.3 We need to ask our MS patients if they are following a particular diet and be prepared to discuss potentially beneficial dietary choices with them—and offer referral to a nutritionist to those who require additional direction and support.—SP
Stacey Panasci, MSPAS, PA-C
Springfield Neurology Associates, LLC
Massachusetts
1. Marrie RA, Elliott L, Marriott J, et al. Effect of comorbidity on mortality in multiple sclerosis. Neurology. 2015;85(3):240-247.
2. Langer-Gould A, Brara SM, Beaber BE, Koebnick C. Childhood obesity and risk of pediatric multiple sclerosis and clinically isolated syndrome. Neurology. 2013;80(6):548-552.
3. Goodman S, Gulick EE. Dietary practices of people with multiple sclerosis. Int J MS Care. 2008;10:47-57.
4. Riemann- Lorenz K, Eilers M, von Geldern G, et al. Dietary interventions in multiple sclerosis: development and pilot testing of an evidence based patient education program. PLoS One. 2016;11(10):e0165246.
5. Hadgkiss EJ, Jekinek GA, Weiland TJ, et al. The association of diet with quality of life, disability, and relapse rate in an international sample of people with multiple sclerosis. Nutr Neurosci. 2015;18(3):125-136.
6. Khalili M, Azimi A, Izadi V, et al. Does lipoic acid consumption affect the cytokine profile in multiple sclerosis patients: a double-blind, placebo-controlled, randomized clinical trial. Neuroimmunomodulation. 2014;21(6):291-296.
7. Kocer B, Engur S, Ak F, Yilmaz M. Serum vitamin B12, folate, and homocysteine levels and their association with clinical and electrophysiological parameters in multiple sclerosis.
J Clin Neurosci. 2009;16:399-403.
8. Galgani M, Procaccini C, De Rosa V, et al. Leptin modulates the survival of autoreactive CD4+ T cells through the nutrient/energy-sensing mammalian target of rapamycin signaling pathway. J Immunol. 2010;185(12):7474-7479.
Q) What is known about the impact of diet on multiple sclerosis? How can I advise my patients with MS?
Multiple sclerosis (MS) is a chronic inflammatory and degenerative central nervous system disease affecting more than 2.5 million people worldwide. Today, if a Google search is performed for “diet and MS,” more than 67 million results are obtained. Many tout specific protocols as beneficial for MS but have no substantial data to support these claims. This can be confusing for patients as well as providers. How should you advise those who ask for advice on dietary modifications to help control symptoms or disease course?
First, it’s important to remember that individuals with MS have a reduced median lifespan (by about seven years), compared to healthy controls. Furthermore, patients with MS commonly have comorbid conditions—such as diabetes, obesity, and ischemic heart disease—that increase mortality risk.1,2 Diet and nutrition are significant factors that impact the course of these diseases.
We must also bear in mind that patients with MS experience symptoms that may impede their efforts to prepare meals. In a 2008 study of 123 MS patients (more than 50% of whom were overweight or obese), fatigue was cited as a significant factor that limited cooking and food preparation. Cognitive impairment and depression also may affect dietary intake. Interestingly, the average recorded intake for all food groups was less than that recommended in the Dietary Guidelines for Americans.3
A web-based survey conducted by the German MS Society in 2011 revealed that 42% of the 337 respondents had modified their diet due to MS. These modifications included change in intake of fatty acids; decrease or elimination of meat, sugar, and additives; and introduction of a low-carb or Paleo diet.4
Among an international sample of 2,087 MS patients, a significant association was found between a healthy diet and improved quality of life (both physical and mental) and reduced disability. This “healthy consumption” of fruits, vegetables, and dietary fat was also associated with a marginally decreased risk for relapse. Patients who demonstrated increased disease activity were more likely to have poor consumption of fruits, vegetables, and fats and to consume more meat and dairy products.5
There has also been research on specific components of dietary intake. Antioxidant-containing foods, for example, may have an anti-inflammatory effect.6 Vitamin B12 deficiency plays a role in immunomodulatory effect, as well as formation of the myelin sheath, although its role (and the effect of biotin supplementation) in MS disease progression requires further study.7 Also ongoing is research into various calorie-restriction protocols, altering both timing and amount of caloric intake, since some data suggest this strategy reduces leptin, a satiety hormone that increases inflammation and has been shown to promote more aggressive MS in a mouse model.8
In the meantime, what can we conclude about diet and MS? A recent review determined that, although there is insufficient data to support one specific diet, there is sufficient evidence to recommend consumption of fish, foods lower in fat, whole grains, vitamin D, and supplemental omega fatty acids.5
It is important to discuss diet with our MS patients. In the German survey, 82% of patients felt that diet was important, yet only 10% had asked a provider for nutritional advice.4 In another study, patients indicated that food labels were their top source for nutrition information; only 20% sought advice from a nutritionist.3 We need to ask our MS patients if they are following a particular diet and be prepared to discuss potentially beneficial dietary choices with them—and offer referral to a nutritionist to those who require additional direction and support.—SP
Stacey Panasci, MSPAS, PA-C
Springfield Neurology Associates, LLC
Massachusetts
Q) What is known about the impact of diet on multiple sclerosis? How can I advise my patients with MS?
Multiple sclerosis (MS) is a chronic inflammatory and degenerative central nervous system disease affecting more than 2.5 million people worldwide. Today, if a Google search is performed for “diet and MS,” more than 67 million results are obtained. Many tout specific protocols as beneficial for MS but have no substantial data to support these claims. This can be confusing for patients as well as providers. How should you advise those who ask for advice on dietary modifications to help control symptoms or disease course?
First, it’s important to remember that individuals with MS have a reduced median lifespan (by about seven years), compared to healthy controls. Furthermore, patients with MS commonly have comorbid conditions—such as diabetes, obesity, and ischemic heart disease—that increase mortality risk.1,2 Diet and nutrition are significant factors that impact the course of these diseases.
We must also bear in mind that patients with MS experience symptoms that may impede their efforts to prepare meals. In a 2008 study of 123 MS patients (more than 50% of whom were overweight or obese), fatigue was cited as a significant factor that limited cooking and food preparation. Cognitive impairment and depression also may affect dietary intake. Interestingly, the average recorded intake for all food groups was less than that recommended in the Dietary Guidelines for Americans.3
A web-based survey conducted by the German MS Society in 2011 revealed that 42% of the 337 respondents had modified their diet due to MS. These modifications included change in intake of fatty acids; decrease or elimination of meat, sugar, and additives; and introduction of a low-carb or Paleo diet.4
Among an international sample of 2,087 MS patients, a significant association was found between a healthy diet and improved quality of life (both physical and mental) and reduced disability. This “healthy consumption” of fruits, vegetables, and dietary fat was also associated with a marginally decreased risk for relapse. Patients who demonstrated increased disease activity were more likely to have poor consumption of fruits, vegetables, and fats and to consume more meat and dairy products.5
There has also been research on specific components of dietary intake. Antioxidant-containing foods, for example, may have an anti-inflammatory effect.6 Vitamin B12 deficiency plays a role in immunomodulatory effect, as well as formation of the myelin sheath, although its role (and the effect of biotin supplementation) in MS disease progression requires further study.7 Also ongoing is research into various calorie-restriction protocols, altering both timing and amount of caloric intake, since some data suggest this strategy reduces leptin, a satiety hormone that increases inflammation and has been shown to promote more aggressive MS in a mouse model.8
In the meantime, what can we conclude about diet and MS? A recent review determined that, although there is insufficient data to support one specific diet, there is sufficient evidence to recommend consumption of fish, foods lower in fat, whole grains, vitamin D, and supplemental omega fatty acids.5
It is important to discuss diet with our MS patients. In the German survey, 82% of patients felt that diet was important, yet only 10% had asked a provider for nutritional advice.4 In another study, patients indicated that food labels were their top source for nutrition information; only 20% sought advice from a nutritionist.3 We need to ask our MS patients if they are following a particular diet and be prepared to discuss potentially beneficial dietary choices with them—and offer referral to a nutritionist to those who require additional direction and support.—SP
Stacey Panasci, MSPAS, PA-C
Springfield Neurology Associates, LLC
Massachusetts
1. Marrie RA, Elliott L, Marriott J, et al. Effect of comorbidity on mortality in multiple sclerosis. Neurology. 2015;85(3):240-247.
2. Langer-Gould A, Brara SM, Beaber BE, Koebnick C. Childhood obesity and risk of pediatric multiple sclerosis and clinically isolated syndrome. Neurology. 2013;80(6):548-552.
3. Goodman S, Gulick EE. Dietary practices of people with multiple sclerosis. Int J MS Care. 2008;10:47-57.
4. Riemann- Lorenz K, Eilers M, von Geldern G, et al. Dietary interventions in multiple sclerosis: development and pilot testing of an evidence based patient education program. PLoS One. 2016;11(10):e0165246.
5. Hadgkiss EJ, Jekinek GA, Weiland TJ, et al. The association of diet with quality of life, disability, and relapse rate in an international sample of people with multiple sclerosis. Nutr Neurosci. 2015;18(3):125-136.
6. Khalili M, Azimi A, Izadi V, et al. Does lipoic acid consumption affect the cytokine profile in multiple sclerosis patients: a double-blind, placebo-controlled, randomized clinical trial. Neuroimmunomodulation. 2014;21(6):291-296.
7. Kocer B, Engur S, Ak F, Yilmaz M. Serum vitamin B12, folate, and homocysteine levels and their association with clinical and electrophysiological parameters in multiple sclerosis.
J Clin Neurosci. 2009;16:399-403.
8. Galgani M, Procaccini C, De Rosa V, et al. Leptin modulates the survival of autoreactive CD4+ T cells through the nutrient/energy-sensing mammalian target of rapamycin signaling pathway. J Immunol. 2010;185(12):7474-7479.
1. Marrie RA, Elliott L, Marriott J, et al. Effect of comorbidity on mortality in multiple sclerosis. Neurology. 2015;85(3):240-247.
2. Langer-Gould A, Brara SM, Beaber BE, Koebnick C. Childhood obesity and risk of pediatric multiple sclerosis and clinically isolated syndrome. Neurology. 2013;80(6):548-552.
3. Goodman S, Gulick EE. Dietary practices of people with multiple sclerosis. Int J MS Care. 2008;10:47-57.
4. Riemann- Lorenz K, Eilers M, von Geldern G, et al. Dietary interventions in multiple sclerosis: development and pilot testing of an evidence based patient education program. PLoS One. 2016;11(10):e0165246.
5. Hadgkiss EJ, Jekinek GA, Weiland TJ, et al. The association of diet with quality of life, disability, and relapse rate in an international sample of people with multiple sclerosis. Nutr Neurosci. 2015;18(3):125-136.
6. Khalili M, Azimi A, Izadi V, et al. Does lipoic acid consumption affect the cytokine profile in multiple sclerosis patients: a double-blind, placebo-controlled, randomized clinical trial. Neuroimmunomodulation. 2014;21(6):291-296.
7. Kocer B, Engur S, Ak F, Yilmaz M. Serum vitamin B12, folate, and homocysteine levels and their association with clinical and electrophysiological parameters in multiple sclerosis.
J Clin Neurosci. 2009;16:399-403.
8. Galgani M, Procaccini C, De Rosa V, et al. Leptin modulates the survival of autoreactive CD4+ T cells through the nutrient/energy-sensing mammalian target of rapamycin signaling pathway. J Immunol. 2010;185(12):7474-7479.
Tips for avoiding laser treatment complications
SAN DIEGO – The way Mathew M. Avram, MD, JD, sees it, the best way to avoid complications from using lasers for aesthetic procedures is to trust your own eyes, not the laser device itself.
“Lasers are never perfect,” he said at the annual Masters of Aesthetics Symposium. “The same device made by the same manufacturer may produce highly different outputs at the same setting. Moreover, lasers produce much different energies after they’ve been serviced. So, if you have two devices sitting right next to each other, don’t assume that the energy output on one device is exactly the same as the other device.”
He also warned clinicians against taking a “cookbook approach” to using lasers, such as memorizing settings or using ones recommended by a colleague or a device manufacturer. “Some lasers are not externally calibrated,” said Dr. Avram, who is codirector of the Massachusetts General Hospital/Wellman Laser and Cosmetic Fellowship. “Safe and unsafe laser endpoints and close clinical observation are the best means to avoiding complications. Learn your endpoints. This is true with the selective photothermolysis lasers: the pigment lasers, vascular lasers, and laser hair removal. Unfortunately, when you use nonablative fractional lasers, there really isn’t an endpoint, so it’s going to be more difficult to discern in that case. The key clinical finding is the endpoint, not the energy setting.”
When treating pigmented lesions and tattoos, for example, immediate whitening is the desired endpoint, not tissue splatter. “So, if you see the epidermis fly off with your first pulse, dial it down,” Dr. Avram said. “It sounds obvious, but sometimes, if you’re working quickly, you figure it will be all right. Just stop and make sure you’re seeing what you’re supposed to be seeing.”
The desired endpoints for vascular lasers, meanwhile, include purpura, transient purpura, or vessel clearance. “What you don’t want to see is gray,” he said. Desired endpoints for hair removal include perifollicular edema and erythema. “What you don’t want to see is epidermal change or dermal tightening,” he added. “Observe the skin and the patient. If the skin is reacting strangely, stop and check all of your settings. If the patient is having an inordinate amount of pain, stop and check all of your settings. These are often the clues that can help you avoid harming a patient.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis, Invasix, and Zalea.
SAN DIEGO – The way Mathew M. Avram, MD, JD, sees it, the best way to avoid complications from using lasers for aesthetic procedures is to trust your own eyes, not the laser device itself.
“Lasers are never perfect,” he said at the annual Masters of Aesthetics Symposium. “The same device made by the same manufacturer may produce highly different outputs at the same setting. Moreover, lasers produce much different energies after they’ve been serviced. So, if you have two devices sitting right next to each other, don’t assume that the energy output on one device is exactly the same as the other device.”
He also warned clinicians against taking a “cookbook approach” to using lasers, such as memorizing settings or using ones recommended by a colleague or a device manufacturer. “Some lasers are not externally calibrated,” said Dr. Avram, who is codirector of the Massachusetts General Hospital/Wellman Laser and Cosmetic Fellowship. “Safe and unsafe laser endpoints and close clinical observation are the best means to avoiding complications. Learn your endpoints. This is true with the selective photothermolysis lasers: the pigment lasers, vascular lasers, and laser hair removal. Unfortunately, when you use nonablative fractional lasers, there really isn’t an endpoint, so it’s going to be more difficult to discern in that case. The key clinical finding is the endpoint, not the energy setting.”
When treating pigmented lesions and tattoos, for example, immediate whitening is the desired endpoint, not tissue splatter. “So, if you see the epidermis fly off with your first pulse, dial it down,” Dr. Avram said. “It sounds obvious, but sometimes, if you’re working quickly, you figure it will be all right. Just stop and make sure you’re seeing what you’re supposed to be seeing.”
The desired endpoints for vascular lasers, meanwhile, include purpura, transient purpura, or vessel clearance. “What you don’t want to see is gray,” he said. Desired endpoints for hair removal include perifollicular edema and erythema. “What you don’t want to see is epidermal change or dermal tightening,” he added. “Observe the skin and the patient. If the skin is reacting strangely, stop and check all of your settings. If the patient is having an inordinate amount of pain, stop and check all of your settings. These are often the clues that can help you avoid harming a patient.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis, Invasix, and Zalea.
SAN DIEGO – The way Mathew M. Avram, MD, JD, sees it, the best way to avoid complications from using lasers for aesthetic procedures is to trust your own eyes, not the laser device itself.
“Lasers are never perfect,” he said at the annual Masters of Aesthetics Symposium. “The same device made by the same manufacturer may produce highly different outputs at the same setting. Moreover, lasers produce much different energies after they’ve been serviced. So, if you have two devices sitting right next to each other, don’t assume that the energy output on one device is exactly the same as the other device.”
He also warned clinicians against taking a “cookbook approach” to using lasers, such as memorizing settings or using ones recommended by a colleague or a device manufacturer. “Some lasers are not externally calibrated,” said Dr. Avram, who is codirector of the Massachusetts General Hospital/Wellman Laser and Cosmetic Fellowship. “Safe and unsafe laser endpoints and close clinical observation are the best means to avoiding complications. Learn your endpoints. This is true with the selective photothermolysis lasers: the pigment lasers, vascular lasers, and laser hair removal. Unfortunately, when you use nonablative fractional lasers, there really isn’t an endpoint, so it’s going to be more difficult to discern in that case. The key clinical finding is the endpoint, not the energy setting.”
When treating pigmented lesions and tattoos, for example, immediate whitening is the desired endpoint, not tissue splatter. “So, if you see the epidermis fly off with your first pulse, dial it down,” Dr. Avram said. “It sounds obvious, but sometimes, if you’re working quickly, you figure it will be all right. Just stop and make sure you’re seeing what you’re supposed to be seeing.”
The desired endpoints for vascular lasers, meanwhile, include purpura, transient purpura, or vessel clearance. “What you don’t want to see is gray,” he said. Desired endpoints for hair removal include perifollicular edema and erythema. “What you don’t want to see is epidermal change or dermal tightening,” he added. “Observe the skin and the patient. If the skin is reacting strangely, stop and check all of your settings. If the patient is having an inordinate amount of pain, stop and check all of your settings. These are often the clues that can help you avoid harming a patient.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis, Invasix, and Zalea.
FROM MOAS 2017
New recommendations tout biosimilars for rheumatologic diseases
The available evidence is sufficient to support switching appropriate patients with rheumatologic diseases from a bio-originator agent to an approved biosimilar agent, according to new consensus-based recommendations from an international multidisciplinary task force.
“Treatment with biological agents has dramatically improved the outcome for patients with inflammatory diseases. However, the high cost of these medications has limited access for many patients,” Jonathan Kay, MD, of UMass Memorial Medical Center and the University of Massachusetts, Worcester, and his colleagues wrote on behalf of the Task Force on the Use of Biosimilars to Treat Rheumatological Diseases. Biosimilars of agents no longer protected by patent allow for increased availability at lower costs, they noted. In the European Union, the United States, Japan, and other countries, biosimilars of adalimumab, etanercept, infliximab, and rituximab have been approved, and those for which the bio-originator is no longer protected by patent have been marketed.
The task force, convened in 2016 to address the matter at an international level, included 25 experts from Europe, Japan, and the United States, including 17 rheumatologists, a rheumatologist/regulator, a dermatologist, a gastroenterologist, 2 pharmacologists, 2 patients, and a research fellow. The task force identified five overarching principles and made eight specific recommendations, based on expert opinion and an extensive literature review that yielded 29 relevant full-text papers and 20 relevant abstracts from the 2015 and 2016 American College of Rheumatology and European League Against Rheumatism annual meetings (Ann Rheum Dis. 2017 Sep 2. doi: 10.1136/annrheumdis-2017-211937).
“This statement was intended both to guide clinicians and to serve as a framework for future educational efforts,” they wrote.
The experts based all five overarching principles for the use of biosimilars on level 5, grade D evidence, indicating that they were derived mainly from expert opinion. They determined that:
- Treatment of rheumatic diseases is based on a shared decision-making process between patients and their rheumatologists.
- The contextual aspects of the health care system should be taken into consideration when treatment decisions are made.
- A biosimilar, as approved by authorities in a highly regulated area, is neither better nor worse in efficacy and is not inferior in safety to its bio-originator.
- Patients and health care providers should be informed about the nature of biosimilars, their approval process, and their safety and efficacy.
- Harmonized methods should be established to obtain reliable pharmacovigilance data, including traceability, about both biosimilars and bio-originators.
These principles represent the key issues regarding biosimilars as identified by the task force. As for the specific recommendations, the task force agreed that:
1. The availability of biosimilars must significantly lower the cost of treating an individual patient and increase access to optimal therapy for all patients with rheumatic diseases (level 5, grade D evidence).
2. Approved biosimilars can be used to treat appropriate patients in the same way as their bio-originators (level 1b, grade A evidence, indicating that the recommendation is based on an individual randomized, controlled trial and that the level 1 evidence is consistent).
3. Antidrug antibodies to biosimilars need not be measured in clinical practice as no significant differences have been detected between biosimilars and their bio-originators (level 2b, grade B evidence, indicating that the recommendation is based on an individual cohort study/low-quality randomized, controlled trial and consistent level 2 or 3 evidence).
4. Relevant preclinical and phase 1 data on a biosimilar should be available when phase 3 data are published (level 5, grade D evidence).
5. Confirmation of efficacy and safety in a single indication is sufficient for extrapolation to other diseases for which the bio-originator has been approved because biosimilars are equivalent in physiochemical, functional, and pharmacokinetic properties to the bio-originator (level 5, grade D evidence).
6. Available evidence suggests that a single switch from a bio-originator to one of its biosimilars is safe and effective; there is no reason to expect a different clinical outcome. However, patient perspectives must be considered (level 1b, grade A evidence).
7. Multiple switching between biosimilars and their bio-originators or other biosimilars should be assessed in registries (level 5, grade D evidence).
8. No switch to or among biosimilars should be initiated without the prior awareness of the patient and the treating health care provider (level 5, grade D evidence).
Differing opinions about the use of biosimilars as published by various subspecialty organizations highlight a lack of confidence among many clinicians with respect to appropriate use of the products, but that is changing amid a rapidly growing body of evidence, the task force said. The group achieved a high level of agreement about both the evaluation of biosimilars and their use to treat rheumatologic diseases, reaching 100% consensus for six of the recommendations and 91% and 96% for the other two.
“Data available as of December 2016 support the use of biosimilars by rheumatologists to encourage a fair and competitive market for biologics. Biosimilars now provide an opportunity to expand access to effective but expensive medications, increasing the number of available treatment choices and helping to control rapidly increasing drug expenditures,” they concluded.
The task force’s work was funded by an unrestricted educational grant from Amgen. Dr. Kay and his coauthors reported financial relationships with multiple pharmaceutical companies, many of which are developing biosimilars.
The available evidence is sufficient to support switching appropriate patients with rheumatologic diseases from a bio-originator agent to an approved biosimilar agent, according to new consensus-based recommendations from an international multidisciplinary task force.
“Treatment with biological agents has dramatically improved the outcome for patients with inflammatory diseases. However, the high cost of these medications has limited access for many patients,” Jonathan Kay, MD, of UMass Memorial Medical Center and the University of Massachusetts, Worcester, and his colleagues wrote on behalf of the Task Force on the Use of Biosimilars to Treat Rheumatological Diseases. Biosimilars of agents no longer protected by patent allow for increased availability at lower costs, they noted. In the European Union, the United States, Japan, and other countries, biosimilars of adalimumab, etanercept, infliximab, and rituximab have been approved, and those for which the bio-originator is no longer protected by patent have been marketed.
The task force, convened in 2016 to address the matter at an international level, included 25 experts from Europe, Japan, and the United States, including 17 rheumatologists, a rheumatologist/regulator, a dermatologist, a gastroenterologist, 2 pharmacologists, 2 patients, and a research fellow. The task force identified five overarching principles and made eight specific recommendations, based on expert opinion and an extensive literature review that yielded 29 relevant full-text papers and 20 relevant abstracts from the 2015 and 2016 American College of Rheumatology and European League Against Rheumatism annual meetings (Ann Rheum Dis. 2017 Sep 2. doi: 10.1136/annrheumdis-2017-211937).
“This statement was intended both to guide clinicians and to serve as a framework for future educational efforts,” they wrote.
The experts based all five overarching principles for the use of biosimilars on level 5, grade D evidence, indicating that they were derived mainly from expert opinion. They determined that:
- Treatment of rheumatic diseases is based on a shared decision-making process between patients and their rheumatologists.
- The contextual aspects of the health care system should be taken into consideration when treatment decisions are made.
- A biosimilar, as approved by authorities in a highly regulated area, is neither better nor worse in efficacy and is not inferior in safety to its bio-originator.
- Patients and health care providers should be informed about the nature of biosimilars, their approval process, and their safety and efficacy.
- Harmonized methods should be established to obtain reliable pharmacovigilance data, including traceability, about both biosimilars and bio-originators.
These principles represent the key issues regarding biosimilars as identified by the task force. As for the specific recommendations, the task force agreed that:
1. The availability of biosimilars must significantly lower the cost of treating an individual patient and increase access to optimal therapy for all patients with rheumatic diseases (level 5, grade D evidence).
2. Approved biosimilars can be used to treat appropriate patients in the same way as their bio-originators (level 1b, grade A evidence, indicating that the recommendation is based on an individual randomized, controlled trial and that the level 1 evidence is consistent).
3. Antidrug antibodies to biosimilars need not be measured in clinical practice as no significant differences have been detected between biosimilars and their bio-originators (level 2b, grade B evidence, indicating that the recommendation is based on an individual cohort study/low-quality randomized, controlled trial and consistent level 2 or 3 evidence).
4. Relevant preclinical and phase 1 data on a biosimilar should be available when phase 3 data are published (level 5, grade D evidence).
5. Confirmation of efficacy and safety in a single indication is sufficient for extrapolation to other diseases for which the bio-originator has been approved because biosimilars are equivalent in physiochemical, functional, and pharmacokinetic properties to the bio-originator (level 5, grade D evidence).
6. Available evidence suggests that a single switch from a bio-originator to one of its biosimilars is safe and effective; there is no reason to expect a different clinical outcome. However, patient perspectives must be considered (level 1b, grade A evidence).
7. Multiple switching between biosimilars and their bio-originators or other biosimilars should be assessed in registries (level 5, grade D evidence).
8. No switch to or among biosimilars should be initiated without the prior awareness of the patient and the treating health care provider (level 5, grade D evidence).
Differing opinions about the use of biosimilars as published by various subspecialty organizations highlight a lack of confidence among many clinicians with respect to appropriate use of the products, but that is changing amid a rapidly growing body of evidence, the task force said. The group achieved a high level of agreement about both the evaluation of biosimilars and their use to treat rheumatologic diseases, reaching 100% consensus for six of the recommendations and 91% and 96% for the other two.
“Data available as of December 2016 support the use of biosimilars by rheumatologists to encourage a fair and competitive market for biologics. Biosimilars now provide an opportunity to expand access to effective but expensive medications, increasing the number of available treatment choices and helping to control rapidly increasing drug expenditures,” they concluded.
The task force’s work was funded by an unrestricted educational grant from Amgen. Dr. Kay and his coauthors reported financial relationships with multiple pharmaceutical companies, many of which are developing biosimilars.
The available evidence is sufficient to support switching appropriate patients with rheumatologic diseases from a bio-originator agent to an approved biosimilar agent, according to new consensus-based recommendations from an international multidisciplinary task force.
“Treatment with biological agents has dramatically improved the outcome for patients with inflammatory diseases. However, the high cost of these medications has limited access for many patients,” Jonathan Kay, MD, of UMass Memorial Medical Center and the University of Massachusetts, Worcester, and his colleagues wrote on behalf of the Task Force on the Use of Biosimilars to Treat Rheumatological Diseases. Biosimilars of agents no longer protected by patent allow for increased availability at lower costs, they noted. In the European Union, the United States, Japan, and other countries, biosimilars of adalimumab, etanercept, infliximab, and rituximab have been approved, and those for which the bio-originator is no longer protected by patent have been marketed.
The task force, convened in 2016 to address the matter at an international level, included 25 experts from Europe, Japan, and the United States, including 17 rheumatologists, a rheumatologist/regulator, a dermatologist, a gastroenterologist, 2 pharmacologists, 2 patients, and a research fellow. The task force identified five overarching principles and made eight specific recommendations, based on expert opinion and an extensive literature review that yielded 29 relevant full-text papers and 20 relevant abstracts from the 2015 and 2016 American College of Rheumatology and European League Against Rheumatism annual meetings (Ann Rheum Dis. 2017 Sep 2. doi: 10.1136/annrheumdis-2017-211937).
“This statement was intended both to guide clinicians and to serve as a framework for future educational efforts,” they wrote.
The experts based all five overarching principles for the use of biosimilars on level 5, grade D evidence, indicating that they were derived mainly from expert opinion. They determined that:
- Treatment of rheumatic diseases is based on a shared decision-making process between patients and their rheumatologists.
- The contextual aspects of the health care system should be taken into consideration when treatment decisions are made.
- A biosimilar, as approved by authorities in a highly regulated area, is neither better nor worse in efficacy and is not inferior in safety to its bio-originator.
- Patients and health care providers should be informed about the nature of biosimilars, their approval process, and their safety and efficacy.
- Harmonized methods should be established to obtain reliable pharmacovigilance data, including traceability, about both biosimilars and bio-originators.
These principles represent the key issues regarding biosimilars as identified by the task force. As for the specific recommendations, the task force agreed that:
1. The availability of biosimilars must significantly lower the cost of treating an individual patient and increase access to optimal therapy for all patients with rheumatic diseases (level 5, grade D evidence).
2. Approved biosimilars can be used to treat appropriate patients in the same way as their bio-originators (level 1b, grade A evidence, indicating that the recommendation is based on an individual randomized, controlled trial and that the level 1 evidence is consistent).
3. Antidrug antibodies to biosimilars need not be measured in clinical practice as no significant differences have been detected between biosimilars and their bio-originators (level 2b, grade B evidence, indicating that the recommendation is based on an individual cohort study/low-quality randomized, controlled trial and consistent level 2 or 3 evidence).
4. Relevant preclinical and phase 1 data on a biosimilar should be available when phase 3 data are published (level 5, grade D evidence).
5. Confirmation of efficacy and safety in a single indication is sufficient for extrapolation to other diseases for which the bio-originator has been approved because biosimilars are equivalent in physiochemical, functional, and pharmacokinetic properties to the bio-originator (level 5, grade D evidence).
6. Available evidence suggests that a single switch from a bio-originator to one of its biosimilars is safe and effective; there is no reason to expect a different clinical outcome. However, patient perspectives must be considered (level 1b, grade A evidence).
7. Multiple switching between biosimilars and their bio-originators or other biosimilars should be assessed in registries (level 5, grade D evidence).
8. No switch to or among biosimilars should be initiated without the prior awareness of the patient and the treating health care provider (level 5, grade D evidence).
Differing opinions about the use of biosimilars as published by various subspecialty organizations highlight a lack of confidence among many clinicians with respect to appropriate use of the products, but that is changing amid a rapidly growing body of evidence, the task force said. The group achieved a high level of agreement about both the evaluation of biosimilars and their use to treat rheumatologic diseases, reaching 100% consensus for six of the recommendations and 91% and 96% for the other two.
“Data available as of December 2016 support the use of biosimilars by rheumatologists to encourage a fair and competitive market for biologics. Biosimilars now provide an opportunity to expand access to effective but expensive medications, increasing the number of available treatment choices and helping to control rapidly increasing drug expenditures,” they concluded.
The task force’s work was funded by an unrestricted educational grant from Amgen. Dr. Kay and his coauthors reported financial relationships with multiple pharmaceutical companies, many of which are developing biosimilars.
FROM ANNALS OF THE RHEUMATIC DISEASES
Orange Nodules on the Scalp
The Diagnosis: Rosai-Dorfman Disease
Rosai-Dorfman disease is a rare histiocytic proliferative disorder of unknown etiology. It has 2 forms: limited cutaneous and systemic. The systemic form, also known as sinus histiocytosis with massive lymphadenopathy, affects the lymph nodes and other organs at times. The disease is characterized by a proliferation of histiocytes in the lymph nodes, most commonly in the cervical basin1; however, the inguinal, axillary, mediastinal, or para-aortic nodes also may be affected.1,2 The skin is the most common site of extranodal disease, seen in approximately 10% of cases.1 Cutaneous involvement often is in the facial area but also can be found on the trunk, ears, neck, arms, legs, and genitals. Clinically, skin lesions appear as papules, plaques, and/or nodules.2
Histopathologic examination of Rosai-Dorfman disease generally shows a dense sheetlike dermal infiltrate of large polygonal histiocytes (Figure 1). Histiocytes may display pale pink or clear cytoplasm. The pathognomonic finding is emperipolesis, which consists of histiocytes with engulfed lymphocytes, erythrocytes, plasma cells, and/or granulocytes surrounded by a clear halo. Immunohistochemical staining also is characteristic, with lesional histiocytes showing expression of S-100 protein (Figure 1, inset) and CD68. The associated inflammatory infiltrate is mixed, containing primarily plasma cells but also lymphocytes, neutrophils, and eosinophils.
Blastomycosis (Figure 2) is a systemic infection due to inhalation of Blastomyces dermatitidis conidia. Primary infection occurs in the lungs, and with dissemination the skin is the most common subsequently involved organ.3 Cutaneous blastomycosis shows pseudoepitheliomatous hyperplasia with neutrophilic microabscesses and a dense dermal infiltrate containing suppurative granulomatous inflammation. The nonpigmented yeast phase typically is 8 to 15 µm in length with a refractile cell wall and characteristic single, broad-based budding.3
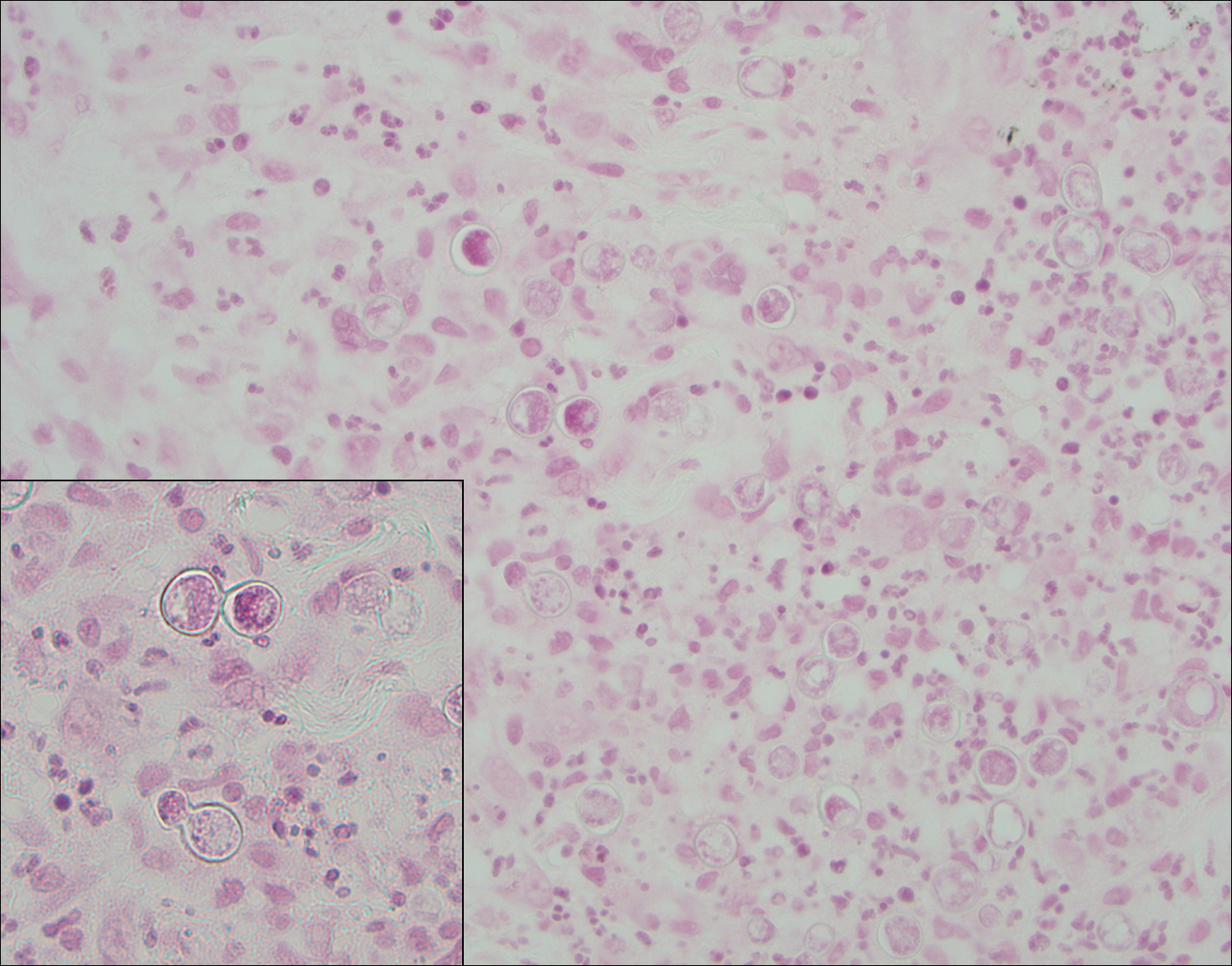
Granuloma faciale (Figure 3) is a rare disease with unknown etiology characterized by reddish brown plaques or nodules most commonly occurring on the face.4,5 Histology shows a dense nodular dermal infiltrate with a grenz zone. The infiltrate is mixed, containing mostly neutrophils with leukocytoclasis and eosinophils. Leukocytoclastic vasculitis is present with associated extravasated erythrocytes. In chronic fibrosing granuloma faciale, lesions can demonstrate fibrosis and hemosiderin deposition, similar to erythema elevatum diutinum.
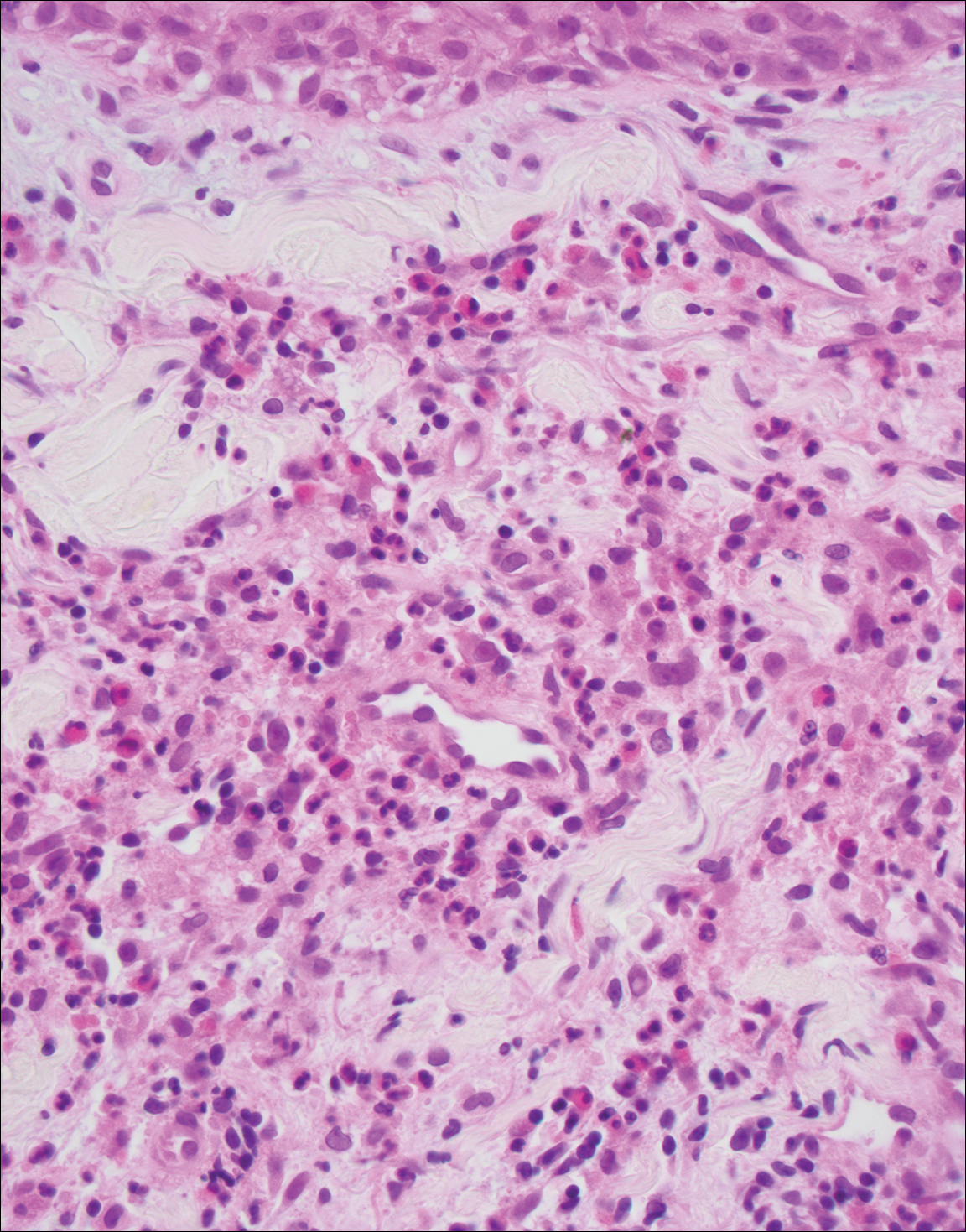
Juvenile xanthogranuloma (Figure 4) is a common histiocytic disease of early childhood, though adult cases have been reported.6 Tumors are found on the head and trunk and are typically firm, reddish yellow papules or nodules.6,7 Histologic examination shows a nodular infiltrate of foamy histiocytes in the superficial dermis. Touton-type multinucleated giant cells with a peripheral rim of xanthomatized foamy cytoplasm and a wreathlike arrangement of nuclei are characteristic. Associated eosinophils are seen. No emperipolesis is present.
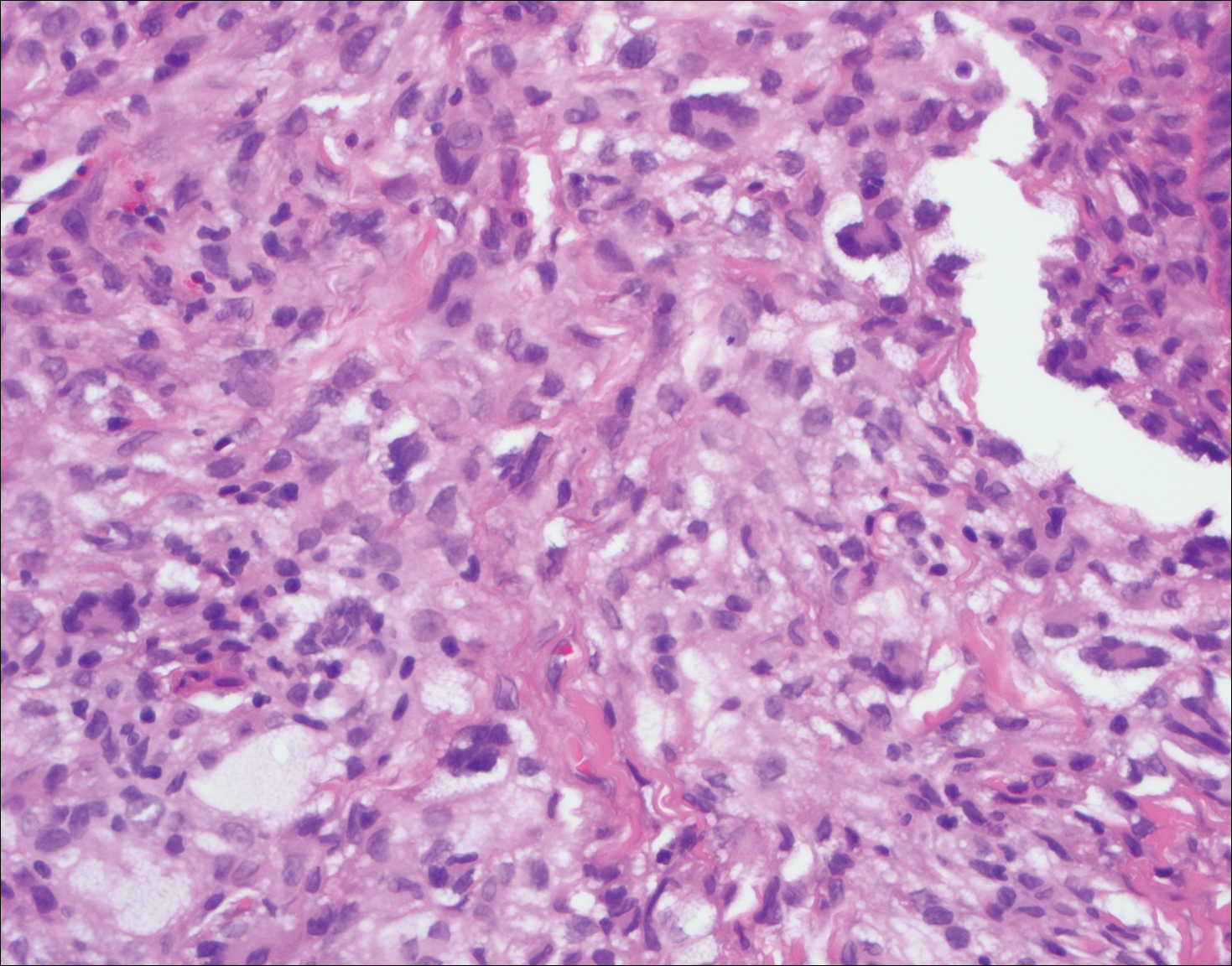
Reticulohistiocytoma (Figure 5) is a benign dermal lesion that presents as solitary or less commonly multiple red-brown papules or nodules.8 Lesions consist of well-delineated nodular aggregates of histiocytes containing a finely granular eosinophilic ground glass cytoplasm. Few, if any, eosinophils are found. The lack of Touton multinucleated giant cells or emperipolesis and lack of expression of S-100 protein helps to distinguish reticulohistiocytoma from other entities in the differential diagnosis.
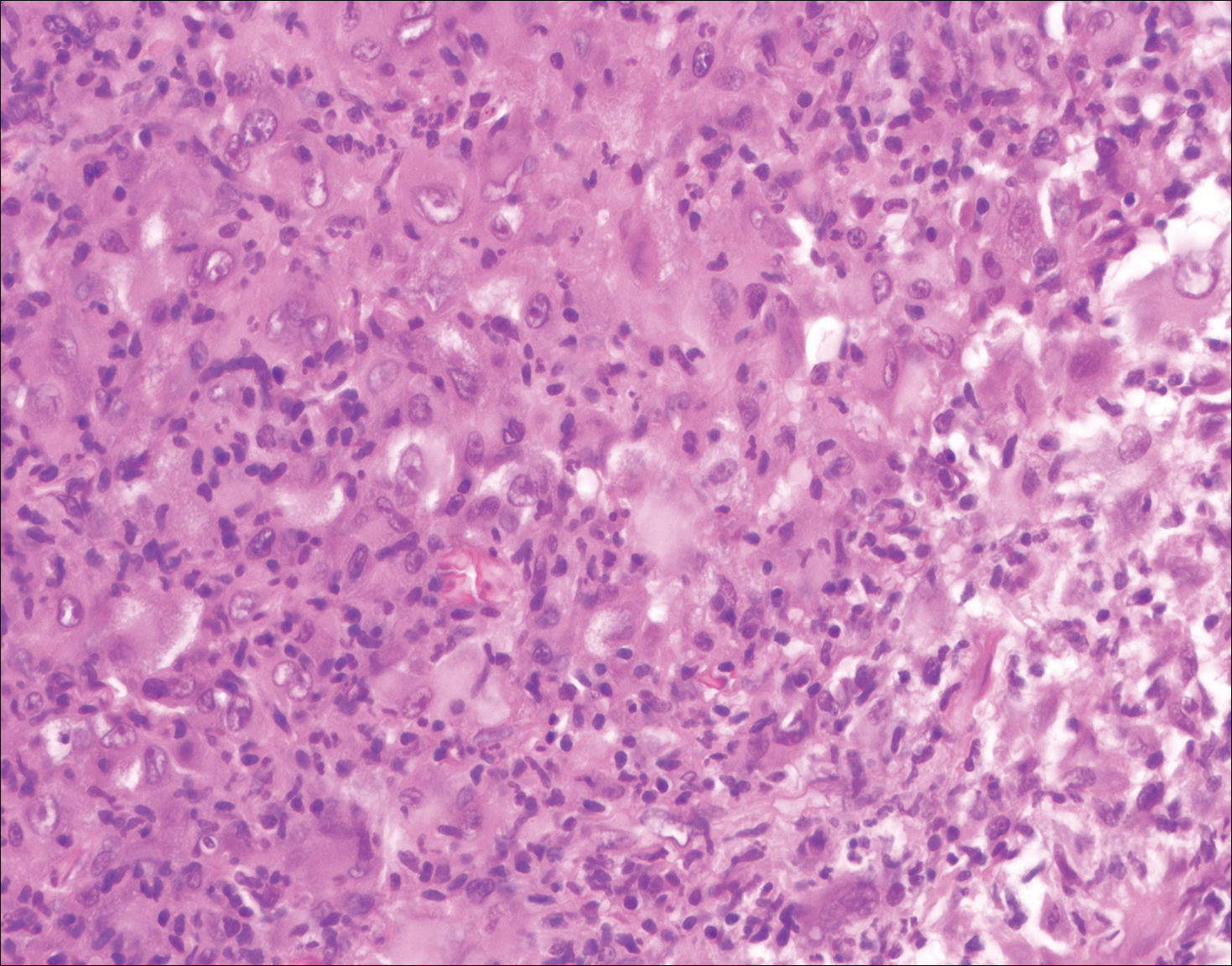
- Foucar E, Rosai J, Dorfman R. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): review of the entity. Semin Diagn Pathol. 1990;7:19-73.
- Kutlubay Z, Bairamov O, Sevim A, et al. Rosai-Dorfman disease: a case report with nodal and cutaneous involvement and review of the literature. Am J Dermatopathol. 2014;36:353-357.
- James WD, Berger TG, Elston DM, eds. Andrews' Diseases of the Skin: Clinical Dermatology. 12th ed. Philadelphia, PA: Elsevier; 2015.
- Wolff K, Johnson R, Saavedra AP. Fitzpatrick's Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill; 2013.
- Marcoval J, Moreno A, Peyrí J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51:269-273.
- Rodriguez J, Ackerman AB. Xanthogranuloma in adults. Arch Dermatol. 1976;112:43-44.
- Tanz WS, Schwartz RA, Janniger CK. Juvenile xanthogranuloma. Cutis. 1994;54:241-245.
- Cohen PR, Lee RA. Adult-onset reticulohistiocytoma presenting as a solitary asymptomatic red knee nodule: report and review of clinical presentations and immunohistochemistry staining features of reticulohistiocytosis. Dermatology Online J. 2014;20. pii:doj_21725.
The Diagnosis: Rosai-Dorfman Disease
Rosai-Dorfman disease is a rare histiocytic proliferative disorder of unknown etiology. It has 2 forms: limited cutaneous and systemic. The systemic form, also known as sinus histiocytosis with massive lymphadenopathy, affects the lymph nodes and other organs at times. The disease is characterized by a proliferation of histiocytes in the lymph nodes, most commonly in the cervical basin1; however, the inguinal, axillary, mediastinal, or para-aortic nodes also may be affected.1,2 The skin is the most common site of extranodal disease, seen in approximately 10% of cases.1 Cutaneous involvement often is in the facial area but also can be found on the trunk, ears, neck, arms, legs, and genitals. Clinically, skin lesions appear as papules, plaques, and/or nodules.2
Histopathologic examination of Rosai-Dorfman disease generally shows a dense sheetlike dermal infiltrate of large polygonal histiocytes (Figure 1). Histiocytes may display pale pink or clear cytoplasm. The pathognomonic finding is emperipolesis, which consists of histiocytes with engulfed lymphocytes, erythrocytes, plasma cells, and/or granulocytes surrounded by a clear halo. Immunohistochemical staining also is characteristic, with lesional histiocytes showing expression of S-100 protein (Figure 1, inset) and CD68. The associated inflammatory infiltrate is mixed, containing primarily plasma cells but also lymphocytes, neutrophils, and eosinophils.

Blastomycosis (Figure 2) is a systemic infection due to inhalation of Blastomyces dermatitidis conidia. Primary infection occurs in the lungs, and with dissemination the skin is the most common subsequently involved organ.3 Cutaneous blastomycosis shows pseudoepitheliomatous hyperplasia with neutrophilic microabscesses and a dense dermal infiltrate containing suppurative granulomatous inflammation. The nonpigmented yeast phase typically is 8 to 15 µm in length with a refractile cell wall and characteristic single, broad-based budding.3

Granuloma faciale (Figure 3) is a rare disease with unknown etiology characterized by reddish brown plaques or nodules most commonly occurring on the face.4,5 Histology shows a dense nodular dermal infiltrate with a grenz zone. The infiltrate is mixed, containing mostly neutrophils with leukocytoclasis and eosinophils. Leukocytoclastic vasculitis is present with associated extravasated erythrocytes. In chronic fibrosing granuloma faciale, lesions can demonstrate fibrosis and hemosiderin deposition, similar to erythema elevatum diutinum.

Juvenile xanthogranuloma (Figure 4) is a common histiocytic disease of early childhood, though adult cases have been reported.6 Tumors are found on the head and trunk and are typically firm, reddish yellow papules or nodules.6,7 Histologic examination shows a nodular infiltrate of foamy histiocytes in the superficial dermis. Touton-type multinucleated giant cells with a peripheral rim of xanthomatized foamy cytoplasm and a wreathlike arrangement of nuclei are characteristic. Associated eosinophils are seen. No emperipolesis is present.

Reticulohistiocytoma (Figure 5) is a benign dermal lesion that presents as solitary or less commonly multiple red-brown papules or nodules.8 Lesions consist of well-delineated nodular aggregates of histiocytes containing a finely granular eosinophilic ground glass cytoplasm. Few, if any, eosinophils are found. The lack of Touton multinucleated giant cells or emperipolesis and lack of expression of S-100 protein helps to distinguish reticulohistiocytoma from other entities in the differential diagnosis.

The Diagnosis: Rosai-Dorfman Disease
Rosai-Dorfman disease is a rare histiocytic proliferative disorder of unknown etiology. It has 2 forms: limited cutaneous and systemic. The systemic form, also known as sinus histiocytosis with massive lymphadenopathy, affects the lymph nodes and other organs at times. The disease is characterized by a proliferation of histiocytes in the lymph nodes, most commonly in the cervical basin1; however, the inguinal, axillary, mediastinal, or para-aortic nodes also may be affected.1,2 The skin is the most common site of extranodal disease, seen in approximately 10% of cases.1 Cutaneous involvement often is in the facial area but also can be found on the trunk, ears, neck, arms, legs, and genitals. Clinically, skin lesions appear as papules, plaques, and/or nodules.2
Histopathologic examination of Rosai-Dorfman disease generally shows a dense sheetlike dermal infiltrate of large polygonal histiocytes (Figure 1). Histiocytes may display pale pink or clear cytoplasm. The pathognomonic finding is emperipolesis, which consists of histiocytes with engulfed lymphocytes, erythrocytes, plasma cells, and/or granulocytes surrounded by a clear halo. Immunohistochemical staining also is characteristic, with lesional histiocytes showing expression of S-100 protein (Figure 1, inset) and CD68. The associated inflammatory infiltrate is mixed, containing primarily plasma cells but also lymphocytes, neutrophils, and eosinophils.

Blastomycosis (Figure 2) is a systemic infection due to inhalation of Blastomyces dermatitidis conidia. Primary infection occurs in the lungs, and with dissemination the skin is the most common subsequently involved organ.3 Cutaneous blastomycosis shows pseudoepitheliomatous hyperplasia with neutrophilic microabscesses and a dense dermal infiltrate containing suppurative granulomatous inflammation. The nonpigmented yeast phase typically is 8 to 15 µm in length with a refractile cell wall and characteristic single, broad-based budding.3

Granuloma faciale (Figure 3) is a rare disease with unknown etiology characterized by reddish brown plaques or nodules most commonly occurring on the face.4,5 Histology shows a dense nodular dermal infiltrate with a grenz zone. The infiltrate is mixed, containing mostly neutrophils with leukocytoclasis and eosinophils. Leukocytoclastic vasculitis is present with associated extravasated erythrocytes. In chronic fibrosing granuloma faciale, lesions can demonstrate fibrosis and hemosiderin deposition, similar to erythema elevatum diutinum.

Juvenile xanthogranuloma (Figure 4) is a common histiocytic disease of early childhood, though adult cases have been reported.6 Tumors are found on the head and trunk and are typically firm, reddish yellow papules or nodules.6,7 Histologic examination shows a nodular infiltrate of foamy histiocytes in the superficial dermis. Touton-type multinucleated giant cells with a peripheral rim of xanthomatized foamy cytoplasm and a wreathlike arrangement of nuclei are characteristic. Associated eosinophils are seen. No emperipolesis is present.

Reticulohistiocytoma (Figure 5) is a benign dermal lesion that presents as solitary or less commonly multiple red-brown papules or nodules.8 Lesions consist of well-delineated nodular aggregates of histiocytes containing a finely granular eosinophilic ground glass cytoplasm. Few, if any, eosinophils are found. The lack of Touton multinucleated giant cells or emperipolesis and lack of expression of S-100 protein helps to distinguish reticulohistiocytoma from other entities in the differential diagnosis.

- Foucar E, Rosai J, Dorfman R. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): review of the entity. Semin Diagn Pathol. 1990;7:19-73.
- Kutlubay Z, Bairamov O, Sevim A, et al. Rosai-Dorfman disease: a case report with nodal and cutaneous involvement and review of the literature. Am J Dermatopathol. 2014;36:353-357.
- James WD, Berger TG, Elston DM, eds. Andrews' Diseases of the Skin: Clinical Dermatology. 12th ed. Philadelphia, PA: Elsevier; 2015.
- Wolff K, Johnson R, Saavedra AP. Fitzpatrick's Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill; 2013.
- Marcoval J, Moreno A, Peyrí J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51:269-273.
- Rodriguez J, Ackerman AB. Xanthogranuloma in adults. Arch Dermatol. 1976;112:43-44.
- Tanz WS, Schwartz RA, Janniger CK. Juvenile xanthogranuloma. Cutis. 1994;54:241-245.
- Cohen PR, Lee RA. Adult-onset reticulohistiocytoma presenting as a solitary asymptomatic red knee nodule: report and review of clinical presentations and immunohistochemistry staining features of reticulohistiocytosis. Dermatology Online J. 2014;20. pii:doj_21725.
- Foucar E, Rosai J, Dorfman R. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): review of the entity. Semin Diagn Pathol. 1990;7:19-73.
- Kutlubay Z, Bairamov O, Sevim A, et al. Rosai-Dorfman disease: a case report with nodal and cutaneous involvement and review of the literature. Am J Dermatopathol. 2014;36:353-357.
- James WD, Berger TG, Elston DM, eds. Andrews' Diseases of the Skin: Clinical Dermatology. 12th ed. Philadelphia, PA: Elsevier; 2015.
- Wolff K, Johnson R, Saavedra AP. Fitzpatrick's Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill; 2013.
- Marcoval J, Moreno A, Peyrí J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51:269-273.
- Rodriguez J, Ackerman AB. Xanthogranuloma in adults. Arch Dermatol. 1976;112:43-44.
- Tanz WS, Schwartz RA, Janniger CK. Juvenile xanthogranuloma. Cutis. 1994;54:241-245.
- Cohen PR, Lee RA. Adult-onset reticulohistiocytoma presenting as a solitary asymptomatic red knee nodule: report and review of clinical presentations and immunohistochemistry staining features of reticulohistiocytosis. Dermatology Online J. 2014;20. pii:doj_21725.
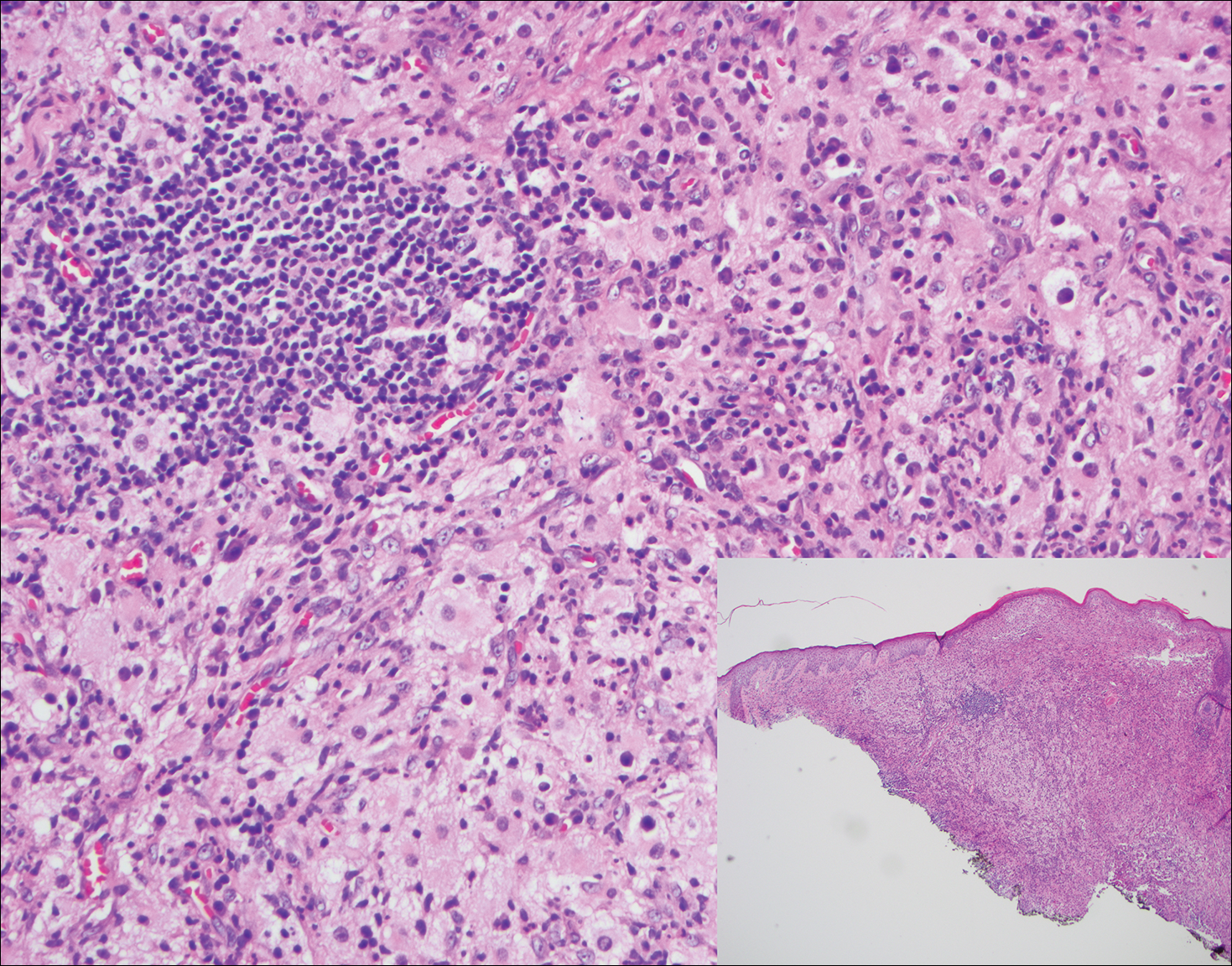
A 59-year-old man presented with itchy and mildly painful nodules on the head and neck of 7 months' duration. The patient denied fever, chills, unintentional weight loss, night sweats, and other systemic symptoms. Physical examination revealed multiple firm pink-orange nodules of varying sizes distributed on the scalp, face, and neck. Right-sided, painless, bulky cervical lymphadenopathy also was noted. An incisional biopsy was performed.
The Atopic Dermatitis Biologic Era Has Begun
Atopic dermatitis (AD) is a vexing multisystem disorder characterized by frequently recurrent, intrusive, and sometimes disabling itch and dermatitis. The itch may be present throughout the day but crescendos at bedtime or 1 to 2 hours after sleep initiation, resulting in disrupted sleep cycles, lack of rest, more hours scratching, daytime somnolence, poor work attendance and performance, and poor school attendance and performance.1
Atopic dermatitis is a lifelong disease that only remits in approximately half of patients.2 There is a need for a disease-specific systemic drug in AD. Phototherapy, cyclosporine, methotrexate, and azathioprine are nonspecific immunosuppressive agents that can be used off label for AD but may or may not be effective.3 Oral or intramuscular corticosteroids are associated with problematic side effects such as weight gain, osteoporosis, fractures, psychological problems, striae, buffalo hump, and steroid withdrawal symptoms and disease aggravation upon withdrawal (ie, flaring to a state worse than prior to steroid initiation).3,4
A biologic medication for AD has been long overdue. Psoriatic biologic medications have been tried in AD with occasional benefit in case reports but no major response in larger trials. Belloni et al5 reviewed early data on off-label usage of biologics approved by the US Food and Drug Administration for psoriasis or other indications applied to AD patients. In their review of cases, they make the point that results are variable and anti-B-cell activity may hold the greatest promise.5 On the other hand, a recent series of 3 patients showed limited response to rituximab in chronic AD,6 while a combination of omalizumab, an anti-IgE medication, and rituximab was helpful in some patients.7 Ultimately, the issue is that nonspecific biologics may or may not address the underlying disease factors in AD. Therefore, there has been a true need for biologic intervention targeted directly at the pathogenic mechanism of AD. Furthermore, the desire for a biologic targeted at AD is paired with the true need to have a medication so targeted that the drug would have little effect on the rest of the immune system, resulting in targeted immunomodulation without secondary risk of infections.
Wait no longer, that era arrived a few months ago with the rapid US Food and Drug Administration approval of dupilumab, an injectable medication used every 2 weeks for the therapy of moderate to severe AD. This fully human monoclonal antibody against the IL-4Rα subunit blocks IL-4 and IL-13, key inflammatory agents in the triggering of production of IgE and eosinophil activation. Even better than the fact that it is targeted are the excellent outcomes in the therapy of moderate to severe AD in adults and the minimal side-effect profile resulting in no requirements for laboratory screening or ongoing monitoring.8
Dupilumab seems to perform well, both clinically and in improving the lives of AD patients. Meta-analysis of trials involving dupilumab has shown improved health-related quality of life outcomes.9,10 Usage of dupilumab alone in clinical trials for 16 weeks (SOLO 1 and SOLO 2) has resulted in stunning reduction in disease severity with a limited side-effect profile, with patients most commonly reporting conjunctivitis.11 In real-world models where dupilumab is added into a regimen of topical corticosteroid usage (LIBERTY AD CHRONOS trial), patients fared even better with the combination, highlighting that this medication may best be used adjunctively to our skin care guidance as dermatologists.12
A new era for AD patients has arrived and we as practitioners are now fortunate to be able to therapeutically reach the worst cases of AD. The new era has only begun with dozens of new agents addressing a variety of interleukin pathways including IL-17 and IL-22 still under development. Ultimately, we hope that ongoing pediatric trials will allow us to glean the role of early disease intervention at the root cause of AD and address our abilities to prevent comorbidities and disease persistence. Will we be able to avert years of disabling disease? The future holds immense hope.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Somanunt S, Chinratanapisit S, Pacharn P, et al. The natural history of atopic dermatitis and its association with Atopic March [published online Dec 12, 2016]. Asian Pac J Allergy Immunol. doi:10.12932/AP0825.
- Sidbury R, Davis DM, Cohen DE, et al; American Academy of Dermatology. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Hajar T, Leshem YA, Hanifin JM, et al; the National Eczema Association Task Force. A systematic review of topical corticosteroid withdrawal ("steroid addiction") in patients with atopic dermatitis and other dermatoses [published online January 13, 2015]. J Am Acad Dermatol. 2015;72:541.e2-549.e2.
- Belloni B, Andres C, Ollert M, et al. Novel immunological approaches in the treatment of atopic eczema. Curr Opin Allergy Clin Immunol. 2008;8:423-427.
- McDonald BS, Jones J, Rustin M. Rituximab as a treatment for severe atopic eczema: failure to improve in three consecutive patients. Clin Exp Dermatol. 2016;41:45-47.
- Sánchez-Ramón S, Eguíluz-Gracia I, Rodríguez-Mazariego ME, et al. Sequential combined therapy with omalizumab and rituximab: a new approach to severe atopic dermatitis. J Investig Allergol Clin Immunol. 2013;23:190-196.
- D'Erme AM, Romanelli M, Chiricozzi A. Spotlight on dupilumab in the treatment of atopic dermatitis: design, development, and potential place in therapy. Drug Des Devel Ther. 2017;11:1473-1480.
- Han Y, Chen Y, Liu X, et al. Efficacy and safety of dupilumab for the treatment of adult atopic dermatitis: a meta-analysis of randomized clinical trials [published online May 4, 2017]. J Allergy Clin Immunol. doi:10.1016/j.jaci.2017.04.015.
- Simpson EL. Dupilumab improves general health-related quality-of-life in patients with moderate-to-severe atopic dermatitis: pooled results from two randomized, controlled phase 3 clinical trials. Dermatol Ther (Heidelb). 2017;7:243-248.
- Simpson EL, Bieber T, Guttman-Yassky E, et al; SOLO 1 and SOLO 2 Investigators. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis [published online Sep 30, 2016]. N Engl J Med. 2016;375:2335-2348.
- Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial [published online May 4, 2017]. Lancet. 2017;389:2287-2303.
Atopic dermatitis (AD) is a vexing multisystem disorder characterized by frequently recurrent, intrusive, and sometimes disabling itch and dermatitis. The itch may be present throughout the day but crescendos at bedtime or 1 to 2 hours after sleep initiation, resulting in disrupted sleep cycles, lack of rest, more hours scratching, daytime somnolence, poor work attendance and performance, and poor school attendance and performance.1
Atopic dermatitis is a lifelong disease that only remits in approximately half of patients.2 There is a need for a disease-specific systemic drug in AD. Phototherapy, cyclosporine, methotrexate, and azathioprine are nonspecific immunosuppressive agents that can be used off label for AD but may or may not be effective.3 Oral or intramuscular corticosteroids are associated with problematic side effects such as weight gain, osteoporosis, fractures, psychological problems, striae, buffalo hump, and steroid withdrawal symptoms and disease aggravation upon withdrawal (ie, flaring to a state worse than prior to steroid initiation).3,4
A biologic medication for AD has been long overdue. Psoriatic biologic medications have been tried in AD with occasional benefit in case reports but no major response in larger trials. Belloni et al5 reviewed early data on off-label usage of biologics approved by the US Food and Drug Administration for psoriasis or other indications applied to AD patients. In their review of cases, they make the point that results are variable and anti-B-cell activity may hold the greatest promise.5 On the other hand, a recent series of 3 patients showed limited response to rituximab in chronic AD,6 while a combination of omalizumab, an anti-IgE medication, and rituximab was helpful in some patients.7 Ultimately, the issue is that nonspecific biologics may or may not address the underlying disease factors in AD. Therefore, there has been a true need for biologic intervention targeted directly at the pathogenic mechanism of AD. Furthermore, the desire for a biologic targeted at AD is paired with the true need to have a medication so targeted that the drug would have little effect on the rest of the immune system, resulting in targeted immunomodulation without secondary risk of infections.
Wait no longer, that era arrived a few months ago with the rapid US Food and Drug Administration approval of dupilumab, an injectable medication used every 2 weeks for the therapy of moderate to severe AD. This fully human monoclonal antibody against the IL-4Rα subunit blocks IL-4 and IL-13, key inflammatory agents in the triggering of production of IgE and eosinophil activation. Even better than the fact that it is targeted are the excellent outcomes in the therapy of moderate to severe AD in adults and the minimal side-effect profile resulting in no requirements for laboratory screening or ongoing monitoring.8
Dupilumab seems to perform well, both clinically and in improving the lives of AD patients. Meta-analysis of trials involving dupilumab has shown improved health-related quality of life outcomes.9,10 Usage of dupilumab alone in clinical trials for 16 weeks (SOLO 1 and SOLO 2) has resulted in stunning reduction in disease severity with a limited side-effect profile, with patients most commonly reporting conjunctivitis.11 In real-world models where dupilumab is added into a regimen of topical corticosteroid usage (LIBERTY AD CHRONOS trial), patients fared even better with the combination, highlighting that this medication may best be used adjunctively to our skin care guidance as dermatologists.12
A new era for AD patients has arrived and we as practitioners are now fortunate to be able to therapeutically reach the worst cases of AD. The new era has only begun with dozens of new agents addressing a variety of interleukin pathways including IL-17 and IL-22 still under development. Ultimately, we hope that ongoing pediatric trials will allow us to glean the role of early disease intervention at the root cause of AD and address our abilities to prevent comorbidities and disease persistence. Will we be able to avert years of disabling disease? The future holds immense hope.
Atopic dermatitis (AD) is a vexing multisystem disorder characterized by frequently recurrent, intrusive, and sometimes disabling itch and dermatitis. The itch may be present throughout the day but crescendos at bedtime or 1 to 2 hours after sleep initiation, resulting in disrupted sleep cycles, lack of rest, more hours scratching, daytime somnolence, poor work attendance and performance, and poor school attendance and performance.1
Atopic dermatitis is a lifelong disease that only remits in approximately half of patients.2 There is a need for a disease-specific systemic drug in AD. Phototherapy, cyclosporine, methotrexate, and azathioprine are nonspecific immunosuppressive agents that can be used off label for AD but may or may not be effective.3 Oral or intramuscular corticosteroids are associated with problematic side effects such as weight gain, osteoporosis, fractures, psychological problems, striae, buffalo hump, and steroid withdrawal symptoms and disease aggravation upon withdrawal (ie, flaring to a state worse than prior to steroid initiation).3,4
A biologic medication for AD has been long overdue. Psoriatic biologic medications have been tried in AD with occasional benefit in case reports but no major response in larger trials. Belloni et al5 reviewed early data on off-label usage of biologics approved by the US Food and Drug Administration for psoriasis or other indications applied to AD patients. In their review of cases, they make the point that results are variable and anti-B-cell activity may hold the greatest promise.5 On the other hand, a recent series of 3 patients showed limited response to rituximab in chronic AD,6 while a combination of omalizumab, an anti-IgE medication, and rituximab was helpful in some patients.7 Ultimately, the issue is that nonspecific biologics may or may not address the underlying disease factors in AD. Therefore, there has been a true need for biologic intervention targeted directly at the pathogenic mechanism of AD. Furthermore, the desire for a biologic targeted at AD is paired with the true need to have a medication so targeted that the drug would have little effect on the rest of the immune system, resulting in targeted immunomodulation without secondary risk of infections.
Wait no longer, that era arrived a few months ago with the rapid US Food and Drug Administration approval of dupilumab, an injectable medication used every 2 weeks for the therapy of moderate to severe AD. This fully human monoclonal antibody against the IL-4Rα subunit blocks IL-4 and IL-13, key inflammatory agents in the triggering of production of IgE and eosinophil activation. Even better than the fact that it is targeted are the excellent outcomes in the therapy of moderate to severe AD in adults and the minimal side-effect profile resulting in no requirements for laboratory screening or ongoing monitoring.8
Dupilumab seems to perform well, both clinically and in improving the lives of AD patients. Meta-analysis of trials involving dupilumab has shown improved health-related quality of life outcomes.9,10 Usage of dupilumab alone in clinical trials for 16 weeks (SOLO 1 and SOLO 2) has resulted in stunning reduction in disease severity with a limited side-effect profile, with patients most commonly reporting conjunctivitis.11 In real-world models where dupilumab is added into a regimen of topical corticosteroid usage (LIBERTY AD CHRONOS trial), patients fared even better with the combination, highlighting that this medication may best be used adjunctively to our skin care guidance as dermatologists.12
A new era for AD patients has arrived and we as practitioners are now fortunate to be able to therapeutically reach the worst cases of AD. The new era has only begun with dozens of new agents addressing a variety of interleukin pathways including IL-17 and IL-22 still under development. Ultimately, we hope that ongoing pediatric trials will allow us to glean the role of early disease intervention at the root cause of AD and address our abilities to prevent comorbidities and disease persistence. Will we be able to avert years of disabling disease? The future holds immense hope.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Somanunt S, Chinratanapisit S, Pacharn P, et al. The natural history of atopic dermatitis and its association with Atopic March [published online Dec 12, 2016]. Asian Pac J Allergy Immunol. doi:10.12932/AP0825.
- Sidbury R, Davis DM, Cohen DE, et al; American Academy of Dermatology. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Hajar T, Leshem YA, Hanifin JM, et al; the National Eczema Association Task Force. A systematic review of topical corticosteroid withdrawal ("steroid addiction") in patients with atopic dermatitis and other dermatoses [published online January 13, 2015]. J Am Acad Dermatol. 2015;72:541.e2-549.e2.
- Belloni B, Andres C, Ollert M, et al. Novel immunological approaches in the treatment of atopic eczema. Curr Opin Allergy Clin Immunol. 2008;8:423-427.
- McDonald BS, Jones J, Rustin M. Rituximab as a treatment for severe atopic eczema: failure to improve in three consecutive patients. Clin Exp Dermatol. 2016;41:45-47.
- Sánchez-Ramón S, Eguíluz-Gracia I, Rodríguez-Mazariego ME, et al. Sequential combined therapy with omalizumab and rituximab: a new approach to severe atopic dermatitis. J Investig Allergol Clin Immunol. 2013;23:190-196.
- D'Erme AM, Romanelli M, Chiricozzi A. Spotlight on dupilumab in the treatment of atopic dermatitis: design, development, and potential place in therapy. Drug Des Devel Ther. 2017;11:1473-1480.
- Han Y, Chen Y, Liu X, et al. Efficacy and safety of dupilumab for the treatment of adult atopic dermatitis: a meta-analysis of randomized clinical trials [published online May 4, 2017]. J Allergy Clin Immunol. doi:10.1016/j.jaci.2017.04.015.
- Simpson EL. Dupilumab improves general health-related quality-of-life in patients with moderate-to-severe atopic dermatitis: pooled results from two randomized, controlled phase 3 clinical trials. Dermatol Ther (Heidelb). 2017;7:243-248.
- Simpson EL, Bieber T, Guttman-Yassky E, et al; SOLO 1 and SOLO 2 Investigators. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis [published online Sep 30, 2016]. N Engl J Med. 2016;375:2335-2348.
- Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial [published online May 4, 2017]. Lancet. 2017;389:2287-2303.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Somanunt S, Chinratanapisit S, Pacharn P, et al. The natural history of atopic dermatitis and its association with Atopic March [published online Dec 12, 2016]. Asian Pac J Allergy Immunol. doi:10.12932/AP0825.
- Sidbury R, Davis DM, Cohen DE, et al; American Academy of Dermatology. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Hajar T, Leshem YA, Hanifin JM, et al; the National Eczema Association Task Force. A systematic review of topical corticosteroid withdrawal ("steroid addiction") in patients with atopic dermatitis and other dermatoses [published online January 13, 2015]. J Am Acad Dermatol. 2015;72:541.e2-549.e2.
- Belloni B, Andres C, Ollert M, et al. Novel immunological approaches in the treatment of atopic eczema. Curr Opin Allergy Clin Immunol. 2008;8:423-427.
- McDonald BS, Jones J, Rustin M. Rituximab as a treatment for severe atopic eczema: failure to improve in three consecutive patients. Clin Exp Dermatol. 2016;41:45-47.
- Sánchez-Ramón S, Eguíluz-Gracia I, Rodríguez-Mazariego ME, et al. Sequential combined therapy with omalizumab and rituximab: a new approach to severe atopic dermatitis. J Investig Allergol Clin Immunol. 2013;23:190-196.
- D'Erme AM, Romanelli M, Chiricozzi A. Spotlight on dupilumab in the treatment of atopic dermatitis: design, development, and potential place in therapy. Drug Des Devel Ther. 2017;11:1473-1480.
- Han Y, Chen Y, Liu X, et al. Efficacy and safety of dupilumab for the treatment of adult atopic dermatitis: a meta-analysis of randomized clinical trials [published online May 4, 2017]. J Allergy Clin Immunol. doi:10.1016/j.jaci.2017.04.015.
- Simpson EL. Dupilumab improves general health-related quality-of-life in patients with moderate-to-severe atopic dermatitis: pooled results from two randomized, controlled phase 3 clinical trials. Dermatol Ther (Heidelb). 2017;7:243-248.
- Simpson EL, Bieber T, Guttman-Yassky E, et al; SOLO 1 and SOLO 2 Investigators. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis [published online Sep 30, 2016]. N Engl J Med. 2016;375:2335-2348.
- Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial [published online May 4, 2017]. Lancet. 2017;389:2287-2303.
‘Making a difference in cancer care’
Survival improves when patients with cancer self-report symptoms
Key clinical point Patients with metastatic cancer who self-reported symptoms experienced significant improvement in overall survival. Major finding Median overall survival with self-reporting of symptoms compared with usual care was 31.2 and 26 months, respectively. Data source A randomized controlled clinical trial of 766 patients. Funding and disclosures This study was supported by the National Institutes of Health and the Conquer Cancer Foundation of the American Society of Clinical Oncology. Dr Basch and Dr Burstein each reported having no disclosures.
Patients with metastatic cancer who self-reported symptoms during routine cancer treatment experienced a number of benefits, including a statistically significant improvement in overall survival, according to findings from a randomized, controlled clinical trial (see p. e184). The median overall survival in 441 patients receiving treatment for metastatic breast, lung, genitourinary, or gynecologic cancer who were randomized to the self-reporting intervention arm was more than 5 months longer (a nearly 20% increase) than in 325 patients receiving standard care (31.2 vs. 26 months), Ethan Basch, MD, of the Lineberger Comprehensive Cancer Center at the University of North Carolina, Chapel Hill, said at the meeting. “Another way to think of this is [in terms of] 5-year survival. At 5 years, 8% more patients were alive in the self-reporting group,” he said.
In addition, 31% of patients in the intervention arm had better quality of life/physical functioning, compared with those in the control arm, and 7% fewer patients in the intervention arm visited an emergency room during the study. The duration of potentially life-prolonging chemotherapy was increased by an average of 2 months in the intervention arm, he said (JAMA. 2017 Jun 4. doi: 10.1001/jama.2017.7156).
Symptoms such as nausea, pain, and fatigue are common among patients with metastatic cancer and can often go undetected by doctors and nurses until they become severe and physically debilitating, Dr Basch added, noting that patients are often hesitant to call the office between visits to report symptoms.
Dr Basch and his colleagues hypothesized that self-reporting of patient symptoms between visits or before a visit while the patient was in the clinic waiting area would prompt earlier intervention and improve symptom control and outcomes.
Study participants were patients at Memorial Sloan Kettering Cancer Center who had advanced solid genitourinary, gynecologic, breast, or lung tumors and who were receiving outpatient chemotherapy. Those assigned to the intervention group used tablet computers and an online web survey system to report on 12 symptoms commonly experienced during chemotherapy. The system triggers an alert to a nurse when a severe or worsening symptom is reported. Patients in the usual care group discussed symptoms during office visits and were encouraged to call the office between visits if they were concerned about symptoms.
Patients remained on the study until discontinuation of all cancer treatment, hospice, or death.
One possible explanation for the findings is that this self-reporting approach prompts clinicians to manage symptoms before they cause serious downstream complications, Dr Basch said.
The approach may also keep patients more physically functional, which is known from previous studies to have a strong association with better survival, and it may also improve management of chemotherapy side effects, enabling longer duration of beneficial cancer treatment. “In oncology, we often are limited in our ability to give life-prolonging treatment because people don’t tolerate it well,” Dr Basch explained.
“This approach should be considered for inclusion in standard symptoms management as a component of high-quality cancer care,” he concluded, noting that efforts are underway to test the next generation of systems to improve communication between patients and care teams and to figure out how best to integrate these tools into oncology practice.
The system used in the this study was designed for research, but a number of companies have tools currently available for patient-reported outcomes, and others are being developed, Dr Basch said. A National Cancer Institute questionnaire, the PRO-CTCAE, is publicly available and can be loaded into patients’ electronic health records for this purpose as well.
Harold J Burstein, MD, of Dana-Farber Cancer Institute, Boston, said the study findings validate the feeling among many clinicians that patient-focused, team-based care can improve outcomes in a meaningful way for patients. If this were a drug … it would be worth tens, if not hundreds of thousands, of dollars per year … We don’t have those same kinds of dollars to help implement these into our electronic health records or our systems. We need to find ways to support that and make it happen,” he said.
— Sharon Worcester
TRK inhibitor shows ‘striking’ activity and durability across diverse cancers
Key clinical point Larotrectinib has good, durable efficacy when used to treat advanced cancers harboring TRK fusions. Major finding The overall response rate was 76%, and 79% of responses were still ongoing at 12 months. Data source An integrated analysis of phase 1 and 2 trials among 55 children and adults having 17 discrete types of advanced cancer with TRK fusions. Funding and disclosures Loxo Oncology funded the study. Dr Hyman disclosed that he has a consulting or advisory role with Atara Biotherapeutics, Chugai Pharma, and CytomX Therapeutics, and that he receives research funding from AstraZeneca and Puma Biotechnology.
Larotrectinib, an oral inhibitor of tropomyosin receptor kinase (TRK), has durable efficacy across diverse adult and pediatric cancers that harbor a genetic aberration known as TRK fusion, according to findings from an analysis of 3 trials reported at the meeting (see p. e184). Fusion of a TRK gene with an unrelated gene leads to uncontrolled signaling in the TRK pathway, potentially causing tumor growth and addiction to this input, David Hyman, MD, chief of early drug development at Memorial Sloan Kettering Cancer Center in New York, explained in a press briefing.
“One of the defining features of TRK fusions is that they are not just found in one cancer type, but in dozens of different cancer types, and not just in adults, but children as well, spanning the entire lifetime of the person,” he noted. They are rare in common cancers and nearly universal in certain uncommon cancers; collectively, they are present in possibly 5,000 cancers diagnosed each year in the United States.
Dr Hyman and his colleagues analyzed data from 55 patients having 17 discrete types of advanced cancer harboring TRK fusions who were treated with larotrectinib in phase 1 and 2 trials. Results showed an overall response rate of 76%, and the majority of responses were still ongoing at 12 months. “I believe these data support larotrectinib as a potential new standard of care for these patients,” he said. “However, I want to emphasize that really recognizing this benefit in the community will require that we test patients more universally for the presence of TRK fusions or other tumor-agnostic biomarkers, such as microsatellite instability.”
Study details
The investigators analyzed data from 3 trials in which patients with advanced TRK fusion-positive solid cancers received larotrectinib (LOXO-101): a phase 1 trial among 8 adult patients, a phase 1/2 trial among 12 pediatric patients (SCOUT), and a phase 2 “basket” trial among 35 adult and adolescent patients (NAVIGATE).
“These patients were identified by local testing,” Dr Hyman noted. “We did not perform central screening to find the TRK fusions, and in fact, 50 different laboratories identified the 55 patients. So in a sense, this really represents the real-world identification of these patients.”
In an integrated analysis, the overall rate of confirmed response as assessed by investigators was 76%, with complete response in 12% of patients and partial response in 64%. Two patients had such deep tumor regression that they experienced downstaging enabling them to undergo potentially curative surgery. Efficacy was consistent regardless of tumor type, which TRK gene was affected, and the fusion partner gene.
Median time to response was 1.8 months. “This is just a reflection of when the first scan was obtained. But in the clinic, patients reported dramatic improvement of their symptoms within days of starting therapy,” Dr Hyman said.
With a median follow-up of 5.8 months, the median duration of response was not yet reached. In all, 79% of responses were still ongoing at 12 months. Median progression-free survival (PFS) was likewise not reached; the 12-month rate was 63%.
The leading treatment-emergent adverse events were fatigue (38%), dizziness (27%), nausea (26%), and anemia (26%). “This is an extremely well tolerated therapy with only 13% of patients requiring any form of dose modification and not a single patient discontinuing due to adverse events,” he said.
It is not clear why some patients had apparent primary resistance to larotrectinib, but their TRK fusion test results may have been incorrect, Dr Hyman speculated. In all, 6 patients developed acquired resistance to larotrectinib; 5 of them were found to have an identical resistance mutation, and 2 went on to receive and have a response to LOXO-195, a next-generation TRK inhibitor that seems to retain activity in the presence of this mutation (Cancer Discov. 2017 June 3. doi: 10.1158/2159-8290.CD-17-0507).
TRK testing
Several next-generation sequencing-based tests already available clinically can pick up TRK fusions, Dr Hyman pointed out. “But it is important for the ordering physician to understand whether the tests they are ordering include fusion detection and, if it’s an option, to select it. Otherwise, they will not find TRK fusions. “The list price for these tests is in the low thousands of dollars,” he noted. In cancers in which sequential single-gene testing is already being done as standard of care, there is “minimal” incremental cost of instead using comprehensive testing that would detect TRK fusions.
Oncologists should be aware that obtaining test results can take weeks, Dr Hyman stressed. “This [testing] should be more broadly adopted and should be adopted at a point in the patient’s treatment [so that they] don’t become too sick, then don’t have an opportunity to be treated even when the test results come back positive.”
— Susan London
QoL preserved with ribociclib-letrozole for advanced breast cancer
Key clinical point Patients who took ribociclib plus letrozole had less pain and no drop in QoL compared with letrozole alone. Major finding QoL was sustained and pain scores decreased when ribociclib was added to letrozole for patients with advanced breast cancer. Data source Double-blind, placebo-controlled phase 3 trial of letrozole plus ribociclib compared with letrozole plus placebo in 668 patients with advanced hormone receptor–positive, HER2-negative breast cancer. Disclosures Dr Verma reported financial relationships with Novartis, which markets ribociclib, and other firms.
Patients with advanced breast cancer whose aromatase inhibitor therapy was supplemented with a cycline-dependent kinase inhibitor had better PFS with no drop in quality of life (QoL). Health-related QoL for patients on the combination therapy was equivalent to that of patients on monotherapy in most aspects, but patients receiving both therapies had a sustained and clinically meaningful decrease in pain.
In addition, the time to definitive deterioration by 10% or more of the global health status/QoL scale score was similar between treatment arms (hazard ratio [HR], 0.944; 95% confidence interval, 0.720-1.237).
The MONALEESA-2 trial had previously shown that the CDK4/6 inhibitor ribociclib, when added to the aromatase inhibitor letrozole, significantly improved PFS for postmenopausal patients with hormone receptor–positive, HER2-negative advanced breast cancer, when compared with letrozole in combination with placebo.
Sunil Verma, MD, reported on health-related QoL and symptoms in the 2 arms of MONALEESA-2, showing change from baseline, time to a definitive 10% deterioration, and the mean scores for on-treatment time compared with end of treatment on the global health-status QoL subscale of the European Organization for Research and Treatment of Cancer's (EORTC's) 30-item core QoL questionnaire.
“During treatment, overall health-related quality of life was maintained from baseline and was similar in both arms,” said Dr Verma, the study’s first author. Changes during treatment were not statistically significant, and did not reach the predetermined threshold for a clinically meaningful difference. The effect of key symptoms such as fatigue, nausea, and vomiting on QoL was similar for patients receiving ribociclib or placebo, he said. Although symptom scores were slightly higher for patients in the active arm of the study, the results were not clinically significant.
Reporting validated, cancer-specific patient-reported outcomes from the trial, Dr Verma, professor and head of the department of oncology at the University of Calgary in Alberta, Canada, sought to “highlight the patient experience with a focus on health-related quality of life and symptoms,” he said during his presentation at the meeting. “A clinically meaningful – more than 5 points – improvement from baseline in pain score was maintained up to and including cycle 15 in the ribociclib plus letrozole arm.” The placebo arm had a mild, clinically insignificant improvement at most assessment points. For both treatment arms, pain scores increased a bit above baseline levels at the time of disease progression or end of therapy, he said.
Patients completed the EORTC 30-item core QoL questionnaire at their screening visit, and then every 8 weeks for the first 18 months. Then, they completed the questionnaires every 12 weeks until they experienced disease progression, died, were lost to follow-up or withdrew from the study, or stopped treatment.
Statistical analysis of the questionnaire results took into account the patients’ baseline scores, treatments received, and how they were stratified. Investigators assessed both statistical significance and the clinical meaningfulness of changes, defined as a change of 5-10 points.
In MONALEESA-2, 334 patients each were allocated to the ribociclib-letrozole arm and the placebo-letrozole arm. Patients in both arms, said Dr Verma, were very compliant with questionnaire completion. More than 90% of patients who were eligible completed questionnaire through cycle 19 of ribociclib or placebo. He explained that the overall numbers completing the questionnaire declined with time, as more patients had disease progression. Dr Verma said it is important to include those measures, because patients and their families care about “the quality of the time gained,” so patient-reported outcomes should be a part of risk-benefit assessments for new cancer therapies. “While delaying disease progression may help maintain patient quality of life, the addition of novel treatments to existing therapies can add toxicities, which may diminish quality of life,” he said.
— Kari Oakes
Ibrutinib and beyond: optimizing therapy in relapsed CLL
Key clinical point Ibrutinib had durable efficacy in relapsed CLL, and combinations with other targeted agents or with CAR-T cells are promising. Major finding Long-term PFS was better with ibrutinib than with ofatumumab (HR, 0.133). The overall response rate with the triplet of ibrutinib, ublituximab, and umbralisib was 100%. In all, 89% of patients achieved no evidence of disease in marrow when anti-CD19 CAR-T cells were added to ibrutinib. Data source An update of a phase 3 randomized trial among 391 patients with previously treated CLL or SLL (RESONATE). A phase 1/1b trial including 19 patients with mainly relapsed or refractory CLL or SLL. A pilot trial among 10 patients with previously treated, mainly higher-risk, CLL or SLL. Disclosures See article text.
Ibrutinib monotherapy
In the phase 3 randomized RESONATE trial, investigators compared ibrutinib with ofatumumab, an anti-CD20 antibody, among 391 patients with CLL or small lymphocytic lymphoma (SLL), with crossover allowed. Initial results favored ibrutinib.
Investigators led by John C Byrd, MD, director of the division of hematology at the Ohio State University Comprehensive Cancer Center in Columbus, reported updated data in a poster session at the meeting, now with a median 44 months of follow-up in the ibrutinib arm.
Median PFS was not reached with ibrutinib, compared with 8.1 months with ofatumumab (HR, 0.133). The 3-year rate of PFS for ibrutinib and ofatumumab was 59% and 3%, respectively.
The pattern was generally similar across patients stratified by cytogenetics (deletion of 17p, deletion of 11q, or neither), IGHV and TP53 mutation status, and previous lines of therapy, reported Dr Byrd. The 3-year overall survival rate for ibrutinib was 74%. In analyses adjusted for crossover, patients given the inhibitor had a markedly lower risk of death than did peers given the antibody (HR, 0.37). The overall response rate with ibrutinib was 91%. Although the rate of complete response increased with follow-up, it was still just 9%.
The most common grade 3 or worse adverse events were neutropenia (23%), pneumonia (17%), and anemia, thrombocytopenia, and hypertension (8% each). Major hemorrhage was reported in 2 patients (1%) in the ibrutinib group, and 3 (2%) in the ofatumumab group.
The investigators emphasized that these long-term results “show that extended treatment with ibrutinib is tolerable and continues to show sustained PFS in previously treated patients with CLL regardless of high-risk cytogenetics, adding that traditional poor prognostic factors for survival with chemoimmunotherapy, including del(17p) and del(11q), were not significant factors predictive of PFS outcomes with ibrutinib.
Funding and disclosures: Pharmacyclics funded the trial. Dr Byrd disclosed that he receives research funding from Pharmacyclics and other companies.
Ibrutinib plus ublituximab and umbralisib
Investigators led by Loretta J Nastoupil, MD, tested a triplet consisting of ibrutinib with ublituximab (another anti-CD20 antibody) and umbralisib (TGR-1202), an oral PI3 kinase-delta inhibitor, in a phase 1/1b trial of 38 patients with generally heavily pretreated leukemias and lymphomas, including 20 with CLL or SLL. Notably, 8 patients with CLL (50%) had a 17p or 11q deletion.
The median time on study was 11.1 months, reported Dr Nastoupil, of the department of lymphoma/myeloma at the University of Texas MD Anderson Cancer Center, Houston. The overall response rate for the 19 evaluable patients with CLL or SLL was 100% (complete response in 32%, partial response in 68%). The main grade 3 or 4 adverse events in the entire trial population were neutropenia (18%) and pyrexia (8%). Only two patients discontinued treatment because of adverse events.
“An expansion cohort is ongoing at the highest dose, and we will clearly need more patients treated and longer follow-up to really know the efficacy,” commented invited discussant Jennifer R Brown, MD, PhD, director of the Chronic Lymphocytic Leukemia Center at the Dana-Farber Cancer Institute in Boston.
Funding and disclosures: TG Therapeutics funded the trial. Dr Nastoupil has received honoraria and research funding from TG Therapeutics, and honoraria from Pharmacyclics.
Ibrutinib plus CAR-T cells
Investigators in a pilot trial led by Saar Gill, MD, PhD, of the hospital of the University of Pennsylvania in Philadelphia, tested the combination of ibrutinib with chimeric antigen receptor-T cells (CAR-T cells) against CD19, on the basis of preclinical evidence of synergy. The trial participants were 10 patients with CLL or SLL who had not achieved complete response with ibrutinib. All had a 17p deletion or a p53 mutation, or a complex karyotype, and some had a known ibrutinib resistance mutation.
At 3 months, 8 of 9 evaluable patients (89%) had no evidence of disease in bone marrow, reported Dr Gill. Seven patients (78%) achieved a complete or partial radiographic response in the spleen and lymph nodes. Overall, the treatment was well tolerated. One patient developed grade 4 tumor lysis syndrome, and two developed grade 3 cytokine release syndrome. But, none required anticytokine therapy.
Most patients remain on ibrutinib and are being monitored. In addition, the researchers plan to treat 25 more patients with the same combination.
Funding and disclosures: Novartis funded the trial. Dr Gill disclosed that he receives research funding from Novartis (institutional) and has patents for CAR-T cells for AML.
— Susan London
Pazopanib falls short as adjuvant therapy for high-risk RCC
Key clinical point Pazopanib is not efficacious for treating resected high-risk locally advanced RCC. Major finding Compared with placebo, pazopanib started at 600 mg daily did not significantly reduce the risk of DFS events (HR, 0.86; P = .16). Data source A phase 3 randomized controlled trial among 1,538 patients who had undergone nephrectomy for high-risk locally advanced RCC (PROTECT trial). Disclosures Novartis Oncology funded the trial. Dr Motzer disclosed that he is a consultant to Novartis and receives research funding from Novartis (institutional).
The antiangiogenic agent pazopanib is not efficacious when used as adjuvant therapy for resected renal cell carcinoma (RCC) with features that confer a high risk of recurrence, investigators in the PROTECT investigators reported at the meeting. “The trial did not meet its primary endpoint,” concluded lead investigator Robert J Motzer, MD, of Memorial Sloan-Kettering Cancer Center in New York. “Pazopanib is not recommended for adjuvant therapy following resection of locally advanced RCC.”
Adjuvant use of other agents in this class has yielded mixed results, Dr Motzer noted, citing the earlier ASSURE trial found that neither sunitinib nor sorafenib improved disease-free survival (DFS) or overall survival (Lancet 2016;387:2008-2016), and the S-TRAC trial that found that sunitinib improved DFS (N Engl J Med. 2016;375:2246-2254).
Pazopanib is an oral multitargeted tyrosine kinase inhibitor active against the vascular endothelial growth factor receptor (VEGFR). The PROTECT trial included 1,538 patients with resected pT2 (high grade), pT3, or greater nonmetastatic clear cell RCC, a highly vascular tumor typically reliant on aberrant signaling in the VEGF pathway. The patients were assigned evenly to receive pazopanib or placebo for 1 year. The starting dose was lowered from 800 mg daily to 600 mg daily (with escalation permitted) after 403 patients had been treated.
In intention-to-treat analysis among patients started on the 600-mg dose and having a median follow-up of about 31 months, DFS was better with pazopanib but not significantly so (HR, 0.86; P = .16). In secondary analyses, pazopanib had a significant DFS benefit in patients started on the 800-mg dose (HR, 0.69; P = .02) and among the entire trial population started on either dose (HR, 0.80; P = .01).
One possible explanation for the differing results seen with the 2 doses was the difference in follow-up, because the 800-mg group was treated earlier in the trial, said Dr Motzer. But with an additional year of blinded follow-up, the benefit in the 600-mg group diminished, but not in the 800-mg group.
Another possibility was the somewhat better performance of the placebo group used for comparison with the lower dose: the 3-year DFS rate with placebo was 64% for the 600-mg comparison but 56% for the 800-mg comparison. “One factor that could explain differences in the outcomes of the placebo groups includes unidentified patient demographic characteristics,” Dr Motzer added.
Overall survival was statistically indistinguishable between the pazopanib and placebo groups, regardless of dose. However, “the results are inconclusive as the data are not yet mature,” he said, with a definitive analysis planned for 2019.
Patients started on the 600-mg dose of pazopanib had a higher rate of grade 3 or 4 adverse events overall compared with those given placebo (60% vs 21%, respectively), driven in part by higher rates of hypertension and increased alanine aminotransferase levels. “Although the intent of modifying the protocol dose of pazopanib from 800 mg to 600 mg was to reduce the rate of discontinuation and improve the safety profile ... both cohorts had similar discontinuation rates and safety profiles,” Dr Motzer noted.
A QoL analysis for the 600-mg group using the 19-item Functional Assessment of Cancer Therapy Kidney Symptom Index showed values were consistently lower with the drug than with placebo during treatment, with a crossing of the threshold for a minimally important difference at week 8. Pharmacokinetic analyses from the trial, reported in a poster at the meeting, showed that in the group starting pazopanib at 600 mg, DFS was longer in patients who achieved higher drug trough concentrations at week 3 or 5.
— Susan London
Survival improves when patients with cancer self-report symptoms
Key clinical point Patients with metastatic cancer who self-reported symptoms experienced significant improvement in overall survival. Major finding Median overall survival with self-reporting of symptoms compared with usual care was 31.2 and 26 months, respectively. Data source A randomized controlled clinical trial of 766 patients. Funding and disclosures This study was supported by the National Institutes of Health and the Conquer Cancer Foundation of the American Society of Clinical Oncology. Dr Basch and Dr Burstein each reported having no disclosures.
Patients with metastatic cancer who self-reported symptoms during routine cancer treatment experienced a number of benefits, including a statistically significant improvement in overall survival, according to findings from a randomized, controlled clinical trial (see p. e184). The median overall survival in 441 patients receiving treatment for metastatic breast, lung, genitourinary, or gynecologic cancer who were randomized to the self-reporting intervention arm was more than 5 months longer (a nearly 20% increase) than in 325 patients receiving standard care (31.2 vs. 26 months), Ethan Basch, MD, of the Lineberger Comprehensive Cancer Center at the University of North Carolina, Chapel Hill, said at the meeting. “Another way to think of this is [in terms of] 5-year survival. At 5 years, 8% more patients were alive in the self-reporting group,” he said.
In addition, 31% of patients in the intervention arm had better quality of life/physical functioning, compared with those in the control arm, and 7% fewer patients in the intervention arm visited an emergency room during the study. The duration of potentially life-prolonging chemotherapy was increased by an average of 2 months in the intervention arm, he said (JAMA. 2017 Jun 4. doi: 10.1001/jama.2017.7156).
Symptoms such as nausea, pain, and fatigue are common among patients with metastatic cancer and can often go undetected by doctors and nurses until they become severe and physically debilitating, Dr Basch added, noting that patients are often hesitant to call the office between visits to report symptoms.
Dr Basch and his colleagues hypothesized that self-reporting of patient symptoms between visits or before a visit while the patient was in the clinic waiting area would prompt earlier intervention and improve symptom control and outcomes.
Study participants were patients at Memorial Sloan Kettering Cancer Center who had advanced solid genitourinary, gynecologic, breast, or lung tumors and who were receiving outpatient chemotherapy. Those assigned to the intervention group used tablet computers and an online web survey system to report on 12 symptoms commonly experienced during chemotherapy. The system triggers an alert to a nurse when a severe or worsening symptom is reported. Patients in the usual care group discussed symptoms during office visits and were encouraged to call the office between visits if they were concerned about symptoms.
Patients remained on the study until discontinuation of all cancer treatment, hospice, or death.
One possible explanation for the findings is that this self-reporting approach prompts clinicians to manage symptoms before they cause serious downstream complications, Dr Basch said.
The approach may also keep patients more physically functional, which is known from previous studies to have a strong association with better survival, and it may also improve management of chemotherapy side effects, enabling longer duration of beneficial cancer treatment. “In oncology, we often are limited in our ability to give life-prolonging treatment because people don’t tolerate it well,” Dr Basch explained.
“This approach should be considered for inclusion in standard symptoms management as a component of high-quality cancer care,” he concluded, noting that efforts are underway to test the next generation of systems to improve communication between patients and care teams and to figure out how best to integrate these tools into oncology practice.
The system used in the this study was designed for research, but a number of companies have tools currently available for patient-reported outcomes, and others are being developed, Dr Basch said. A National Cancer Institute questionnaire, the PRO-CTCAE, is publicly available and can be loaded into patients’ electronic health records for this purpose as well.
Harold J Burstein, MD, of Dana-Farber Cancer Institute, Boston, said the study findings validate the feeling among many clinicians that patient-focused, team-based care can improve outcomes in a meaningful way for patients. If this were a drug … it would be worth tens, if not hundreds of thousands, of dollars per year … We don’t have those same kinds of dollars to help implement these into our electronic health records or our systems. We need to find ways to support that and make it happen,” he said.
— Sharon Worcester
TRK inhibitor shows ‘striking’ activity and durability across diverse cancers
Key clinical point Larotrectinib has good, durable efficacy when used to treat advanced cancers harboring TRK fusions. Major finding The overall response rate was 76%, and 79% of responses were still ongoing at 12 months. Data source An integrated analysis of phase 1 and 2 trials among 55 children and adults having 17 discrete types of advanced cancer with TRK fusions. Funding and disclosures Loxo Oncology funded the study. Dr Hyman disclosed that he has a consulting or advisory role with Atara Biotherapeutics, Chugai Pharma, and CytomX Therapeutics, and that he receives research funding from AstraZeneca and Puma Biotechnology.
Larotrectinib, an oral inhibitor of tropomyosin receptor kinase (TRK), has durable efficacy across diverse adult and pediatric cancers that harbor a genetic aberration known as TRK fusion, according to findings from an analysis of 3 trials reported at the meeting (see p. e184). Fusion of a TRK gene with an unrelated gene leads to uncontrolled signaling in the TRK pathway, potentially causing tumor growth and addiction to this input, David Hyman, MD, chief of early drug development at Memorial Sloan Kettering Cancer Center in New York, explained in a press briefing.
“One of the defining features of TRK fusions is that they are not just found in one cancer type, but in dozens of different cancer types, and not just in adults, but children as well, spanning the entire lifetime of the person,” he noted. They are rare in common cancers and nearly universal in certain uncommon cancers; collectively, they are present in possibly 5,000 cancers diagnosed each year in the United States.
Dr Hyman and his colleagues analyzed data from 55 patients having 17 discrete types of advanced cancer harboring TRK fusions who were treated with larotrectinib in phase 1 and 2 trials. Results showed an overall response rate of 76%, and the majority of responses were still ongoing at 12 months. “I believe these data support larotrectinib as a potential new standard of care for these patients,” he said. “However, I want to emphasize that really recognizing this benefit in the community will require that we test patients more universally for the presence of TRK fusions or other tumor-agnostic biomarkers, such as microsatellite instability.”
Study details
The investigators analyzed data from 3 trials in which patients with advanced TRK fusion-positive solid cancers received larotrectinib (LOXO-101): a phase 1 trial among 8 adult patients, a phase 1/2 trial among 12 pediatric patients (SCOUT), and a phase 2 “basket” trial among 35 adult and adolescent patients (NAVIGATE).
“These patients were identified by local testing,” Dr Hyman noted. “We did not perform central screening to find the TRK fusions, and in fact, 50 different laboratories identified the 55 patients. So in a sense, this really represents the real-world identification of these patients.”
In an integrated analysis, the overall rate of confirmed response as assessed by investigators was 76%, with complete response in 12% of patients and partial response in 64%. Two patients had such deep tumor regression that they experienced downstaging enabling them to undergo potentially curative surgery. Efficacy was consistent regardless of tumor type, which TRK gene was affected, and the fusion partner gene.
Median time to response was 1.8 months. “This is just a reflection of when the first scan was obtained. But in the clinic, patients reported dramatic improvement of their symptoms within days of starting therapy,” Dr Hyman said.
With a median follow-up of 5.8 months, the median duration of response was not yet reached. In all, 79% of responses were still ongoing at 12 months. Median progression-free survival (PFS) was likewise not reached; the 12-month rate was 63%.
The leading treatment-emergent adverse events were fatigue (38%), dizziness (27%), nausea (26%), and anemia (26%). “This is an extremely well tolerated therapy with only 13% of patients requiring any form of dose modification and not a single patient discontinuing due to adverse events,” he said.
It is not clear why some patients had apparent primary resistance to larotrectinib, but their TRK fusion test results may have been incorrect, Dr Hyman speculated. In all, 6 patients developed acquired resistance to larotrectinib; 5 of them were found to have an identical resistance mutation, and 2 went on to receive and have a response to LOXO-195, a next-generation TRK inhibitor that seems to retain activity in the presence of this mutation (Cancer Discov. 2017 June 3. doi: 10.1158/2159-8290.CD-17-0507).
TRK testing
Several next-generation sequencing-based tests already available clinically can pick up TRK fusions, Dr Hyman pointed out. “But it is important for the ordering physician to understand whether the tests they are ordering include fusion detection and, if it’s an option, to select it. Otherwise, they will not find TRK fusions. “The list price for these tests is in the low thousands of dollars,” he noted. In cancers in which sequential single-gene testing is already being done as standard of care, there is “minimal” incremental cost of instead using comprehensive testing that would detect TRK fusions.
Oncologists should be aware that obtaining test results can take weeks, Dr Hyman stressed. “This [testing] should be more broadly adopted and should be adopted at a point in the patient’s treatment [so that they] don’t become too sick, then don’t have an opportunity to be treated even when the test results come back positive.”
— Susan London
QoL preserved with ribociclib-letrozole for advanced breast cancer
Key clinical point Patients who took ribociclib plus letrozole had less pain and no drop in QoL compared with letrozole alone. Major finding QoL was sustained and pain scores decreased when ribociclib was added to letrozole for patients with advanced breast cancer. Data source Double-blind, placebo-controlled phase 3 trial of letrozole plus ribociclib compared with letrozole plus placebo in 668 patients with advanced hormone receptor–positive, HER2-negative breast cancer. Disclosures Dr Verma reported financial relationships with Novartis, which markets ribociclib, and other firms.
Patients with advanced breast cancer whose aromatase inhibitor therapy was supplemented with a cycline-dependent kinase inhibitor had better PFS with no drop in quality of life (QoL). Health-related QoL for patients on the combination therapy was equivalent to that of patients on monotherapy in most aspects, but patients receiving both therapies had a sustained and clinically meaningful decrease in pain.
In addition, the time to definitive deterioration by 10% or more of the global health status/QoL scale score was similar between treatment arms (hazard ratio [HR], 0.944; 95% confidence interval, 0.720-1.237).
The MONALEESA-2 trial had previously shown that the CDK4/6 inhibitor ribociclib, when added to the aromatase inhibitor letrozole, significantly improved PFS for postmenopausal patients with hormone receptor–positive, HER2-negative advanced breast cancer, when compared with letrozole in combination with placebo.
Sunil Verma, MD, reported on health-related QoL and symptoms in the 2 arms of MONALEESA-2, showing change from baseline, time to a definitive 10% deterioration, and the mean scores for on-treatment time compared with end of treatment on the global health-status QoL subscale of the European Organization for Research and Treatment of Cancer's (EORTC's) 30-item core QoL questionnaire.
“During treatment, overall health-related quality of life was maintained from baseline and was similar in both arms,” said Dr Verma, the study’s first author. Changes during treatment were not statistically significant, and did not reach the predetermined threshold for a clinically meaningful difference. The effect of key symptoms such as fatigue, nausea, and vomiting on QoL was similar for patients receiving ribociclib or placebo, he said. Although symptom scores were slightly higher for patients in the active arm of the study, the results were not clinically significant.
Reporting validated, cancer-specific patient-reported outcomes from the trial, Dr Verma, professor and head of the department of oncology at the University of Calgary in Alberta, Canada, sought to “highlight the patient experience with a focus on health-related quality of life and symptoms,” he said during his presentation at the meeting. “A clinically meaningful – more than 5 points – improvement from baseline in pain score was maintained up to and including cycle 15 in the ribociclib plus letrozole arm.” The placebo arm had a mild, clinically insignificant improvement at most assessment points. For both treatment arms, pain scores increased a bit above baseline levels at the time of disease progression or end of therapy, he said.
Patients completed the EORTC 30-item core QoL questionnaire at their screening visit, and then every 8 weeks for the first 18 months. Then, they completed the questionnaires every 12 weeks until they experienced disease progression, died, were lost to follow-up or withdrew from the study, or stopped treatment.
Statistical analysis of the questionnaire results took into account the patients’ baseline scores, treatments received, and how they were stratified. Investigators assessed both statistical significance and the clinical meaningfulness of changes, defined as a change of 5-10 points.
In MONALEESA-2, 334 patients each were allocated to the ribociclib-letrozole arm and the placebo-letrozole arm. Patients in both arms, said Dr Verma, were very compliant with questionnaire completion. More than 90% of patients who were eligible completed questionnaire through cycle 19 of ribociclib or placebo. He explained that the overall numbers completing the questionnaire declined with time, as more patients had disease progression. Dr Verma said it is important to include those measures, because patients and their families care about “the quality of the time gained,” so patient-reported outcomes should be a part of risk-benefit assessments for new cancer therapies. “While delaying disease progression may help maintain patient quality of life, the addition of novel treatments to existing therapies can add toxicities, which may diminish quality of life,” he said.
— Kari Oakes
Ibrutinib and beyond: optimizing therapy in relapsed CLL
Key clinical point Ibrutinib had durable efficacy in relapsed CLL, and combinations with other targeted agents or with CAR-T cells are promising. Major finding Long-term PFS was better with ibrutinib than with ofatumumab (HR, 0.133). The overall response rate with the triplet of ibrutinib, ublituximab, and umbralisib was 100%. In all, 89% of patients achieved no evidence of disease in marrow when anti-CD19 CAR-T cells were added to ibrutinib. Data source An update of a phase 3 randomized trial among 391 patients with previously treated CLL or SLL (RESONATE). A phase 1/1b trial including 19 patients with mainly relapsed or refractory CLL or SLL. A pilot trial among 10 patients with previously treated, mainly higher-risk, CLL or SLL. Disclosures See article text.
Ibrutinib monotherapy
In the phase 3 randomized RESONATE trial, investigators compared ibrutinib with ofatumumab, an anti-CD20 antibody, among 391 patients with CLL or small lymphocytic lymphoma (SLL), with crossover allowed. Initial results favored ibrutinib.
Investigators led by John C Byrd, MD, director of the division of hematology at the Ohio State University Comprehensive Cancer Center in Columbus, reported updated data in a poster session at the meeting, now with a median 44 months of follow-up in the ibrutinib arm.
Median PFS was not reached with ibrutinib, compared with 8.1 months with ofatumumab (HR, 0.133). The 3-year rate of PFS for ibrutinib and ofatumumab was 59% and 3%, respectively.
The pattern was generally similar across patients stratified by cytogenetics (deletion of 17p, deletion of 11q, or neither), IGHV and TP53 mutation status, and previous lines of therapy, reported Dr Byrd. The 3-year overall survival rate for ibrutinib was 74%. In analyses adjusted for crossover, patients given the inhibitor had a markedly lower risk of death than did peers given the antibody (HR, 0.37). The overall response rate with ibrutinib was 91%. Although the rate of complete response increased with follow-up, it was still just 9%.
The most common grade 3 or worse adverse events were neutropenia (23%), pneumonia (17%), and anemia, thrombocytopenia, and hypertension (8% each). Major hemorrhage was reported in 2 patients (1%) in the ibrutinib group, and 3 (2%) in the ofatumumab group.
The investigators emphasized that these long-term results “show that extended treatment with ibrutinib is tolerable and continues to show sustained PFS in previously treated patients with CLL regardless of high-risk cytogenetics, adding that traditional poor prognostic factors for survival with chemoimmunotherapy, including del(17p) and del(11q), were not significant factors predictive of PFS outcomes with ibrutinib.
Funding and disclosures: Pharmacyclics funded the trial. Dr Byrd disclosed that he receives research funding from Pharmacyclics and other companies.
Ibrutinib plus ublituximab and umbralisib
Investigators led by Loretta J Nastoupil, MD, tested a triplet consisting of ibrutinib with ublituximab (another anti-CD20 antibody) and umbralisib (TGR-1202), an oral PI3 kinase-delta inhibitor, in a phase 1/1b trial of 38 patients with generally heavily pretreated leukemias and lymphomas, including 20 with CLL or SLL. Notably, 8 patients with CLL (50%) had a 17p or 11q deletion.
The median time on study was 11.1 months, reported Dr Nastoupil, of the department of lymphoma/myeloma at the University of Texas MD Anderson Cancer Center, Houston. The overall response rate for the 19 evaluable patients with CLL or SLL was 100% (complete response in 32%, partial response in 68%). The main grade 3 or 4 adverse events in the entire trial population were neutropenia (18%) and pyrexia (8%). Only two patients discontinued treatment because of adverse events.
“An expansion cohort is ongoing at the highest dose, and we will clearly need more patients treated and longer follow-up to really know the efficacy,” commented invited discussant Jennifer R Brown, MD, PhD, director of the Chronic Lymphocytic Leukemia Center at the Dana-Farber Cancer Institute in Boston.
Funding and disclosures: TG Therapeutics funded the trial. Dr Nastoupil has received honoraria and research funding from TG Therapeutics, and honoraria from Pharmacyclics.
Ibrutinib plus CAR-T cells
Investigators in a pilot trial led by Saar Gill, MD, PhD, of the hospital of the University of Pennsylvania in Philadelphia, tested the combination of ibrutinib with chimeric antigen receptor-T cells (CAR-T cells) against CD19, on the basis of preclinical evidence of synergy. The trial participants were 10 patients with CLL or SLL who had not achieved complete response with ibrutinib. All had a 17p deletion or a p53 mutation, or a complex karyotype, and some had a known ibrutinib resistance mutation.
At 3 months, 8 of 9 evaluable patients (89%) had no evidence of disease in bone marrow, reported Dr Gill. Seven patients (78%) achieved a complete or partial radiographic response in the spleen and lymph nodes. Overall, the treatment was well tolerated. One patient developed grade 4 tumor lysis syndrome, and two developed grade 3 cytokine release syndrome. But, none required anticytokine therapy.
Most patients remain on ibrutinib and are being monitored. In addition, the researchers plan to treat 25 more patients with the same combination.
Funding and disclosures: Novartis funded the trial. Dr Gill disclosed that he receives research funding from Novartis (institutional) and has patents for CAR-T cells for AML.
— Susan London
Pazopanib falls short as adjuvant therapy for high-risk RCC
Key clinical point Pazopanib is not efficacious for treating resected high-risk locally advanced RCC. Major finding Compared with placebo, pazopanib started at 600 mg daily did not significantly reduce the risk of DFS events (HR, 0.86; P = .16). Data source A phase 3 randomized controlled trial among 1,538 patients who had undergone nephrectomy for high-risk locally advanced RCC (PROTECT trial). Disclosures Novartis Oncology funded the trial. Dr Motzer disclosed that he is a consultant to Novartis and receives research funding from Novartis (institutional).
The antiangiogenic agent pazopanib is not efficacious when used as adjuvant therapy for resected renal cell carcinoma (RCC) with features that confer a high risk of recurrence, investigators in the PROTECT investigators reported at the meeting. “The trial did not meet its primary endpoint,” concluded lead investigator Robert J Motzer, MD, of Memorial Sloan-Kettering Cancer Center in New York. “Pazopanib is not recommended for adjuvant therapy following resection of locally advanced RCC.”
Adjuvant use of other agents in this class has yielded mixed results, Dr Motzer noted, citing the earlier ASSURE trial found that neither sunitinib nor sorafenib improved disease-free survival (DFS) or overall survival (Lancet 2016;387:2008-2016), and the S-TRAC trial that found that sunitinib improved DFS (N Engl J Med. 2016;375:2246-2254).
Pazopanib is an oral multitargeted tyrosine kinase inhibitor active against the vascular endothelial growth factor receptor (VEGFR). The PROTECT trial included 1,538 patients with resected pT2 (high grade), pT3, or greater nonmetastatic clear cell RCC, a highly vascular tumor typically reliant on aberrant signaling in the VEGF pathway. The patients were assigned evenly to receive pazopanib or placebo for 1 year. The starting dose was lowered from 800 mg daily to 600 mg daily (with escalation permitted) after 403 patients had been treated.
In intention-to-treat analysis among patients started on the 600-mg dose and having a median follow-up of about 31 months, DFS was better with pazopanib but not significantly so (HR, 0.86; P = .16). In secondary analyses, pazopanib had a significant DFS benefit in patients started on the 800-mg dose (HR, 0.69; P = .02) and among the entire trial population started on either dose (HR, 0.80; P = .01).
One possible explanation for the differing results seen with the 2 doses was the difference in follow-up, because the 800-mg group was treated earlier in the trial, said Dr Motzer. But with an additional year of blinded follow-up, the benefit in the 600-mg group diminished, but not in the 800-mg group.
Another possibility was the somewhat better performance of the placebo group used for comparison with the lower dose: the 3-year DFS rate with placebo was 64% for the 600-mg comparison but 56% for the 800-mg comparison. “One factor that could explain differences in the outcomes of the placebo groups includes unidentified patient demographic characteristics,” Dr Motzer added.
Overall survival was statistically indistinguishable between the pazopanib and placebo groups, regardless of dose. However, “the results are inconclusive as the data are not yet mature,” he said, with a definitive analysis planned for 2019.
Patients started on the 600-mg dose of pazopanib had a higher rate of grade 3 or 4 adverse events overall compared with those given placebo (60% vs 21%, respectively), driven in part by higher rates of hypertension and increased alanine aminotransferase levels. “Although the intent of modifying the protocol dose of pazopanib from 800 mg to 600 mg was to reduce the rate of discontinuation and improve the safety profile ... both cohorts had similar discontinuation rates and safety profiles,” Dr Motzer noted.
A QoL analysis for the 600-mg group using the 19-item Functional Assessment of Cancer Therapy Kidney Symptom Index showed values were consistently lower with the drug than with placebo during treatment, with a crossing of the threshold for a minimally important difference at week 8. Pharmacokinetic analyses from the trial, reported in a poster at the meeting, showed that in the group starting pazopanib at 600 mg, DFS was longer in patients who achieved higher drug trough concentrations at week 3 or 5.
— Susan London
Survival improves when patients with cancer self-report symptoms
Key clinical point Patients with metastatic cancer who self-reported symptoms experienced significant improvement in overall survival. Major finding Median overall survival with self-reporting of symptoms compared with usual care was 31.2 and 26 months, respectively. Data source A randomized controlled clinical trial of 766 patients. Funding and disclosures This study was supported by the National Institutes of Health and the Conquer Cancer Foundation of the American Society of Clinical Oncology. Dr Basch and Dr Burstein each reported having no disclosures.
Patients with metastatic cancer who self-reported symptoms during routine cancer treatment experienced a number of benefits, including a statistically significant improvement in overall survival, according to findings from a randomized, controlled clinical trial (see p. e184). The median overall survival in 441 patients receiving treatment for metastatic breast, lung, genitourinary, or gynecologic cancer who were randomized to the self-reporting intervention arm was more than 5 months longer (a nearly 20% increase) than in 325 patients receiving standard care (31.2 vs. 26 months), Ethan Basch, MD, of the Lineberger Comprehensive Cancer Center at the University of North Carolina, Chapel Hill, said at the meeting. “Another way to think of this is [in terms of] 5-year survival. At 5 years, 8% more patients were alive in the self-reporting group,” he said.
In addition, 31% of patients in the intervention arm had better quality of life/physical functioning, compared with those in the control arm, and 7% fewer patients in the intervention arm visited an emergency room during the study. The duration of potentially life-prolonging chemotherapy was increased by an average of 2 months in the intervention arm, he said (JAMA. 2017 Jun 4. doi: 10.1001/jama.2017.7156).
Symptoms such as nausea, pain, and fatigue are common among patients with metastatic cancer and can often go undetected by doctors and nurses until they become severe and physically debilitating, Dr Basch added, noting that patients are often hesitant to call the office between visits to report symptoms.
Dr Basch and his colleagues hypothesized that self-reporting of patient symptoms between visits or before a visit while the patient was in the clinic waiting area would prompt earlier intervention and improve symptom control and outcomes.
Study participants were patients at Memorial Sloan Kettering Cancer Center who had advanced solid genitourinary, gynecologic, breast, or lung tumors and who were receiving outpatient chemotherapy. Those assigned to the intervention group used tablet computers and an online web survey system to report on 12 symptoms commonly experienced during chemotherapy. The system triggers an alert to a nurse when a severe or worsening symptom is reported. Patients in the usual care group discussed symptoms during office visits and were encouraged to call the office between visits if they were concerned about symptoms.
Patients remained on the study until discontinuation of all cancer treatment, hospice, or death.
One possible explanation for the findings is that this self-reporting approach prompts clinicians to manage symptoms before they cause serious downstream complications, Dr Basch said.
The approach may also keep patients more physically functional, which is known from previous studies to have a strong association with better survival, and it may also improve management of chemotherapy side effects, enabling longer duration of beneficial cancer treatment. “In oncology, we often are limited in our ability to give life-prolonging treatment because people don’t tolerate it well,” Dr Basch explained.
“This approach should be considered for inclusion in standard symptoms management as a component of high-quality cancer care,” he concluded, noting that efforts are underway to test the next generation of systems to improve communication between patients and care teams and to figure out how best to integrate these tools into oncology practice.
The system used in the this study was designed for research, but a number of companies have tools currently available for patient-reported outcomes, and others are being developed, Dr Basch said. A National Cancer Institute questionnaire, the PRO-CTCAE, is publicly available and can be loaded into patients’ electronic health records for this purpose as well.
Harold J Burstein, MD, of Dana-Farber Cancer Institute, Boston, said the study findings validate the feeling among many clinicians that patient-focused, team-based care can improve outcomes in a meaningful way for patients. If this were a drug … it would be worth tens, if not hundreds of thousands, of dollars per year … We don’t have those same kinds of dollars to help implement these into our electronic health records or our systems. We need to find ways to support that and make it happen,” he said.
— Sharon Worcester
TRK inhibitor shows ‘striking’ activity and durability across diverse cancers
Key clinical point Larotrectinib has good, durable efficacy when used to treat advanced cancers harboring TRK fusions. Major finding The overall response rate was 76%, and 79% of responses were still ongoing at 12 months. Data source An integrated analysis of phase 1 and 2 trials among 55 children and adults having 17 discrete types of advanced cancer with TRK fusions. Funding and disclosures Loxo Oncology funded the study. Dr Hyman disclosed that he has a consulting or advisory role with Atara Biotherapeutics, Chugai Pharma, and CytomX Therapeutics, and that he receives research funding from AstraZeneca and Puma Biotechnology.
Larotrectinib, an oral inhibitor of tropomyosin receptor kinase (TRK), has durable efficacy across diverse adult and pediatric cancers that harbor a genetic aberration known as TRK fusion, according to findings from an analysis of 3 trials reported at the meeting (see p. e184). Fusion of a TRK gene with an unrelated gene leads to uncontrolled signaling in the TRK pathway, potentially causing tumor growth and addiction to this input, David Hyman, MD, chief of early drug development at Memorial Sloan Kettering Cancer Center in New York, explained in a press briefing.
“One of the defining features of TRK fusions is that they are not just found in one cancer type, but in dozens of different cancer types, and not just in adults, but children as well, spanning the entire lifetime of the person,” he noted. They are rare in common cancers and nearly universal in certain uncommon cancers; collectively, they are present in possibly 5,000 cancers diagnosed each year in the United States.
Dr Hyman and his colleagues analyzed data from 55 patients having 17 discrete types of advanced cancer harboring TRK fusions who were treated with larotrectinib in phase 1 and 2 trials. Results showed an overall response rate of 76%, and the majority of responses were still ongoing at 12 months. “I believe these data support larotrectinib as a potential new standard of care for these patients,” he said. “However, I want to emphasize that really recognizing this benefit in the community will require that we test patients more universally for the presence of TRK fusions or other tumor-agnostic biomarkers, such as microsatellite instability.”
Study details
The investigators analyzed data from 3 trials in which patients with advanced TRK fusion-positive solid cancers received larotrectinib (LOXO-101): a phase 1 trial among 8 adult patients, a phase 1/2 trial among 12 pediatric patients (SCOUT), and a phase 2 “basket” trial among 35 adult and adolescent patients (NAVIGATE).
“These patients were identified by local testing,” Dr Hyman noted. “We did not perform central screening to find the TRK fusions, and in fact, 50 different laboratories identified the 55 patients. So in a sense, this really represents the real-world identification of these patients.”
In an integrated analysis, the overall rate of confirmed response as assessed by investigators was 76%, with complete response in 12% of patients and partial response in 64%. Two patients had such deep tumor regression that they experienced downstaging enabling them to undergo potentially curative surgery. Efficacy was consistent regardless of tumor type, which TRK gene was affected, and the fusion partner gene.
Median time to response was 1.8 months. “This is just a reflection of when the first scan was obtained. But in the clinic, patients reported dramatic improvement of their symptoms within days of starting therapy,” Dr Hyman said.
With a median follow-up of 5.8 months, the median duration of response was not yet reached. In all, 79% of responses were still ongoing at 12 months. Median progression-free survival (PFS) was likewise not reached; the 12-month rate was 63%.
The leading treatment-emergent adverse events were fatigue (38%), dizziness (27%), nausea (26%), and anemia (26%). “This is an extremely well tolerated therapy with only 13% of patients requiring any form of dose modification and not a single patient discontinuing due to adverse events,” he said.
It is not clear why some patients had apparent primary resistance to larotrectinib, but their TRK fusion test results may have been incorrect, Dr Hyman speculated. In all, 6 patients developed acquired resistance to larotrectinib; 5 of them were found to have an identical resistance mutation, and 2 went on to receive and have a response to LOXO-195, a next-generation TRK inhibitor that seems to retain activity in the presence of this mutation (Cancer Discov. 2017 June 3. doi: 10.1158/2159-8290.CD-17-0507).
TRK testing
Several next-generation sequencing-based tests already available clinically can pick up TRK fusions, Dr Hyman pointed out. “But it is important for the ordering physician to understand whether the tests they are ordering include fusion detection and, if it’s an option, to select it. Otherwise, they will not find TRK fusions. “The list price for these tests is in the low thousands of dollars,” he noted. In cancers in which sequential single-gene testing is already being done as standard of care, there is “minimal” incremental cost of instead using comprehensive testing that would detect TRK fusions.
Oncologists should be aware that obtaining test results can take weeks, Dr Hyman stressed. “This [testing] should be more broadly adopted and should be adopted at a point in the patient’s treatment [so that they] don’t become too sick, then don’t have an opportunity to be treated even when the test results come back positive.”
— Susan London
QoL preserved with ribociclib-letrozole for advanced breast cancer
Key clinical point Patients who took ribociclib plus letrozole had less pain and no drop in QoL compared with letrozole alone. Major finding QoL was sustained and pain scores decreased when ribociclib was added to letrozole for patients with advanced breast cancer. Data source Double-blind, placebo-controlled phase 3 trial of letrozole plus ribociclib compared with letrozole plus placebo in 668 patients with advanced hormone receptor–positive, HER2-negative breast cancer. Disclosures Dr Verma reported financial relationships with Novartis, which markets ribociclib, and other firms.
Patients with advanced breast cancer whose aromatase inhibitor therapy was supplemented with a cycline-dependent kinase inhibitor had better PFS with no drop in quality of life (QoL). Health-related QoL for patients on the combination therapy was equivalent to that of patients on monotherapy in most aspects, but patients receiving both therapies had a sustained and clinically meaningful decrease in pain.
In addition, the time to definitive deterioration by 10% or more of the global health status/QoL scale score was similar between treatment arms (hazard ratio [HR], 0.944; 95% confidence interval, 0.720-1.237).
The MONALEESA-2 trial had previously shown that the CDK4/6 inhibitor ribociclib, when added to the aromatase inhibitor letrozole, significantly improved PFS for postmenopausal patients with hormone receptor–positive, HER2-negative advanced breast cancer, when compared with letrozole in combination with placebo.
Sunil Verma, MD, reported on health-related QoL and symptoms in the 2 arms of MONALEESA-2, showing change from baseline, time to a definitive 10% deterioration, and the mean scores for on-treatment time compared with end of treatment on the global health-status QoL subscale of the European Organization for Research and Treatment of Cancer's (EORTC's) 30-item core QoL questionnaire.
“During treatment, overall health-related quality of life was maintained from baseline and was similar in both arms,” said Dr Verma, the study’s first author. Changes during treatment were not statistically significant, and did not reach the predetermined threshold for a clinically meaningful difference. The effect of key symptoms such as fatigue, nausea, and vomiting on QoL was similar for patients receiving ribociclib or placebo, he said. Although symptom scores were slightly higher for patients in the active arm of the study, the results were not clinically significant.
Reporting validated, cancer-specific patient-reported outcomes from the trial, Dr Verma, professor and head of the department of oncology at the University of Calgary in Alberta, Canada, sought to “highlight the patient experience with a focus on health-related quality of life and symptoms,” he said during his presentation at the meeting. “A clinically meaningful – more than 5 points – improvement from baseline in pain score was maintained up to and including cycle 15 in the ribociclib plus letrozole arm.” The placebo arm had a mild, clinically insignificant improvement at most assessment points. For both treatment arms, pain scores increased a bit above baseline levels at the time of disease progression or end of therapy, he said.
Patients completed the EORTC 30-item core QoL questionnaire at their screening visit, and then every 8 weeks for the first 18 months. Then, they completed the questionnaires every 12 weeks until they experienced disease progression, died, were lost to follow-up or withdrew from the study, or stopped treatment.
Statistical analysis of the questionnaire results took into account the patients’ baseline scores, treatments received, and how they were stratified. Investigators assessed both statistical significance and the clinical meaningfulness of changes, defined as a change of 5-10 points.
In MONALEESA-2, 334 patients each were allocated to the ribociclib-letrozole arm and the placebo-letrozole arm. Patients in both arms, said Dr Verma, were very compliant with questionnaire completion. More than 90% of patients who were eligible completed questionnaire through cycle 19 of ribociclib or placebo. He explained that the overall numbers completing the questionnaire declined with time, as more patients had disease progression. Dr Verma said it is important to include those measures, because patients and their families care about “the quality of the time gained,” so patient-reported outcomes should be a part of risk-benefit assessments for new cancer therapies. “While delaying disease progression may help maintain patient quality of life, the addition of novel treatments to existing therapies can add toxicities, which may diminish quality of life,” he said.
— Kari Oakes
Ibrutinib and beyond: optimizing therapy in relapsed CLL
Key clinical point Ibrutinib had durable efficacy in relapsed CLL, and combinations with other targeted agents or with CAR-T cells are promising. Major finding Long-term PFS was better with ibrutinib than with ofatumumab (HR, 0.133). The overall response rate with the triplet of ibrutinib, ublituximab, and umbralisib was 100%. In all, 89% of patients achieved no evidence of disease in marrow when anti-CD19 CAR-T cells were added to ibrutinib. Data source An update of a phase 3 randomized trial among 391 patients with previously treated CLL or SLL (RESONATE). A phase 1/1b trial including 19 patients with mainly relapsed or refractory CLL or SLL. A pilot trial among 10 patients with previously treated, mainly higher-risk, CLL or SLL. Disclosures See article text.
Ibrutinib monotherapy
In the phase 3 randomized RESONATE trial, investigators compared ibrutinib with ofatumumab, an anti-CD20 antibody, among 391 patients with CLL or small lymphocytic lymphoma (SLL), with crossover allowed. Initial results favored ibrutinib.
Investigators led by John C Byrd, MD, director of the division of hematology at the Ohio State University Comprehensive Cancer Center in Columbus, reported updated data in a poster session at the meeting, now with a median 44 months of follow-up in the ibrutinib arm.
Median PFS was not reached with ibrutinib, compared with 8.1 months with ofatumumab (HR, 0.133). The 3-year rate of PFS for ibrutinib and ofatumumab was 59% and 3%, respectively.
The pattern was generally similar across patients stratified by cytogenetics (deletion of 17p, deletion of 11q, or neither), IGHV and TP53 mutation status, and previous lines of therapy, reported Dr Byrd. The 3-year overall survival rate for ibrutinib was 74%. In analyses adjusted for crossover, patients given the inhibitor had a markedly lower risk of death than did peers given the antibody (HR, 0.37). The overall response rate with ibrutinib was 91%. Although the rate of complete response increased with follow-up, it was still just 9%.
The most common grade 3 or worse adverse events were neutropenia (23%), pneumonia (17%), and anemia, thrombocytopenia, and hypertension (8% each). Major hemorrhage was reported in 2 patients (1%) in the ibrutinib group, and 3 (2%) in the ofatumumab group.
The investigators emphasized that these long-term results “show that extended treatment with ibrutinib is tolerable and continues to show sustained PFS in previously treated patients with CLL regardless of high-risk cytogenetics, adding that traditional poor prognostic factors for survival with chemoimmunotherapy, including del(17p) and del(11q), were not significant factors predictive of PFS outcomes with ibrutinib.
Funding and disclosures: Pharmacyclics funded the trial. Dr Byrd disclosed that he receives research funding from Pharmacyclics and other companies.
Ibrutinib plus ublituximab and umbralisib
Investigators led by Loretta J Nastoupil, MD, tested a triplet consisting of ibrutinib with ublituximab (another anti-CD20 antibody) and umbralisib (TGR-1202), an oral PI3 kinase-delta inhibitor, in a phase 1/1b trial of 38 patients with generally heavily pretreated leukemias and lymphomas, including 20 with CLL or SLL. Notably, 8 patients with CLL (50%) had a 17p or 11q deletion.
The median time on study was 11.1 months, reported Dr Nastoupil, of the department of lymphoma/myeloma at the University of Texas MD Anderson Cancer Center, Houston. The overall response rate for the 19 evaluable patients with CLL or SLL was 100% (complete response in 32%, partial response in 68%). The main grade 3 or 4 adverse events in the entire trial population were neutropenia (18%) and pyrexia (8%). Only two patients discontinued treatment because of adverse events.
“An expansion cohort is ongoing at the highest dose, and we will clearly need more patients treated and longer follow-up to really know the efficacy,” commented invited discussant Jennifer R Brown, MD, PhD, director of the Chronic Lymphocytic Leukemia Center at the Dana-Farber Cancer Institute in Boston.
Funding and disclosures: TG Therapeutics funded the trial. Dr Nastoupil has received honoraria and research funding from TG Therapeutics, and honoraria from Pharmacyclics.
Ibrutinib plus CAR-T cells
Investigators in a pilot trial led by Saar Gill, MD, PhD, of the hospital of the University of Pennsylvania in Philadelphia, tested the combination of ibrutinib with chimeric antigen receptor-T cells (CAR-T cells) against CD19, on the basis of preclinical evidence of synergy. The trial participants were 10 patients with CLL or SLL who had not achieved complete response with ibrutinib. All had a 17p deletion or a p53 mutation, or a complex karyotype, and some had a known ibrutinib resistance mutation.
At 3 months, 8 of 9 evaluable patients (89%) had no evidence of disease in bone marrow, reported Dr Gill. Seven patients (78%) achieved a complete or partial radiographic response in the spleen and lymph nodes. Overall, the treatment was well tolerated. One patient developed grade 4 tumor lysis syndrome, and two developed grade 3 cytokine release syndrome. But, none required anticytokine therapy.
Most patients remain on ibrutinib and are being monitored. In addition, the researchers plan to treat 25 more patients with the same combination.
Funding and disclosures: Novartis funded the trial. Dr Gill disclosed that he receives research funding from Novartis (institutional) and has patents for CAR-T cells for AML.
— Susan London
Pazopanib falls short as adjuvant therapy for high-risk RCC
Key clinical point Pazopanib is not efficacious for treating resected high-risk locally advanced RCC. Major finding Compared with placebo, pazopanib started at 600 mg daily did not significantly reduce the risk of DFS events (HR, 0.86; P = .16). Data source A phase 3 randomized controlled trial among 1,538 patients who had undergone nephrectomy for high-risk locally advanced RCC (PROTECT trial). Disclosures Novartis Oncology funded the trial. Dr Motzer disclosed that he is a consultant to Novartis and receives research funding from Novartis (institutional).
The antiangiogenic agent pazopanib is not efficacious when used as adjuvant therapy for resected renal cell carcinoma (RCC) with features that confer a high risk of recurrence, investigators in the PROTECT investigators reported at the meeting. “The trial did not meet its primary endpoint,” concluded lead investigator Robert J Motzer, MD, of Memorial Sloan-Kettering Cancer Center in New York. “Pazopanib is not recommended for adjuvant therapy following resection of locally advanced RCC.”
Adjuvant use of other agents in this class has yielded mixed results, Dr Motzer noted, citing the earlier ASSURE trial found that neither sunitinib nor sorafenib improved disease-free survival (DFS) or overall survival (Lancet 2016;387:2008-2016), and the S-TRAC trial that found that sunitinib improved DFS (N Engl J Med. 2016;375:2246-2254).
Pazopanib is an oral multitargeted tyrosine kinase inhibitor active against the vascular endothelial growth factor receptor (VEGFR). The PROTECT trial included 1,538 patients with resected pT2 (high grade), pT3, or greater nonmetastatic clear cell RCC, a highly vascular tumor typically reliant on aberrant signaling in the VEGF pathway. The patients were assigned evenly to receive pazopanib or placebo for 1 year. The starting dose was lowered from 800 mg daily to 600 mg daily (with escalation permitted) after 403 patients had been treated.
In intention-to-treat analysis among patients started on the 600-mg dose and having a median follow-up of about 31 months, DFS was better with pazopanib but not significantly so (HR, 0.86; P = .16). In secondary analyses, pazopanib had a significant DFS benefit in patients started on the 800-mg dose (HR, 0.69; P = .02) and among the entire trial population started on either dose (HR, 0.80; P = .01).
One possible explanation for the differing results seen with the 2 doses was the difference in follow-up, because the 800-mg group was treated earlier in the trial, said Dr Motzer. But with an additional year of blinded follow-up, the benefit in the 600-mg group diminished, but not in the 800-mg group.
Another possibility was the somewhat better performance of the placebo group used for comparison with the lower dose: the 3-year DFS rate with placebo was 64% for the 600-mg comparison but 56% for the 800-mg comparison. “One factor that could explain differences in the outcomes of the placebo groups includes unidentified patient demographic characteristics,” Dr Motzer added.
Overall survival was statistically indistinguishable between the pazopanib and placebo groups, regardless of dose. However, “the results are inconclusive as the data are not yet mature,” he said, with a definitive analysis planned for 2019.
Patients started on the 600-mg dose of pazopanib had a higher rate of grade 3 or 4 adverse events overall compared with those given placebo (60% vs 21%, respectively), driven in part by higher rates of hypertension and increased alanine aminotransferase levels. “Although the intent of modifying the protocol dose of pazopanib from 800 mg to 600 mg was to reduce the rate of discontinuation and improve the safety profile ... both cohorts had similar discontinuation rates and safety profiles,” Dr Motzer noted.
A QoL analysis for the 600-mg group using the 19-item Functional Assessment of Cancer Therapy Kidney Symptom Index showed values were consistently lower with the drug than with placebo during treatment, with a crossing of the threshold for a minimally important difference at week 8. Pharmacokinetic analyses from the trial, reported in a poster at the meeting, showed that in the group starting pazopanib at 600 mg, DFS was longer in patients who achieved higher drug trough concentrations at week 3 or 5.
— Susan London