User login
Statin use cuts risks in compensated cirrhosis
For patients with compensated cirrhosis, statin therapy was associated with about a 46% decrease in the risk of hepatic decompensation and mortality and with a 27% drop in the risk of portal hypertension and variceal bleeding, according to moderate-quality evidence from a systematic review and meta-analysis of 13 studies.
Low-quality data also suggested that statins might help protect against the progression of noncirrhotic chronic liver disease, said Rebecca G. Kim of the University of California at San Diego and her associates. “Large, pragmatic randomized controlled trials in patients with compensated cirrhosis are required to confirm these observations,” they wrote in the October issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2017.04.039).
Prior studies have reported mixed findings on how statin therapy affects chronic liver disease. For their review, Ms. Kim and her associates searched MEDLINE, Embase, the Cochrane Central Register of Controlled Trials, Cochrane Database and Systematic Reviews, Scopus, Web of Science, and PubMed for randomized controlled trials or cohort studies published through March 25, 2017. They identified 10 cohort studies and three randomized controlled trials of adults with fibrosis without cirrhosis, compensated cirrhosis, or decompensated cirrhosis that evaluated statin exposure and reported associations between exposure and outcomes related to cirrhosis. They excluded case-control studies, cross-sectional studies, and studies that focused only on the relationship between statin use and the risk of hepatocellular carcinoma.
The resulting data set included 121,058 patients with chronic liver diseases, of whom 85% had chronic hepatitis C virus infection. A total of 46% of patients were exposed to statins, which appeared to reduce their risk of hepatic decompensation, variceal bleeding, and mortality. Among 87 such patients in five studies, statin use was associated with a 46% decrease in the risk of hepatic decompensation and death, with risk ratios of 0.54 (95% confidence intervals, 0.46-0.62 and 0.47-0.61, respectively). Statin use also was associated with a 27% lower risk of variceal bleeding or progression of portal hypertension, based on an analysis of 110 events in 236 patients from three trials (RR, 0.73; 95% CI, 0.59-0.91). Finally, statin use also was associated with a 58% lower risk of fibrosis progression or cirrhosis in patients with noncirrhotic chronic liver disease, but the 95% CIs for the risk estimate did not reach statistical significance (0.16-1.11).
Source: American Gastroenterological Association
Most studies lacked data on dose and duration of statin exposure, the researchers said. However, four cohort studies reported dose-dependent effects that were most pronounced after more than a year of treatment. “Similarly, several different types of statins were studied, and observed effects were assumed to be class-specific effects,” the reviewers wrote. “However, it is possible that lipophilic and lipophobic statins may have differential efficacy in decreasing fibrosis progression.”
Together, these findings support prior studies suggesting that statin therapy is safe and can potentially reduce the risk of hepatocellular carcinoma in this patient population, they concluded. Statins “may potentially improve patient-relevant outcomes in patients with chronic liver diseases and improve survival without significant additional costs.”
The reviewers acknowledged the American Gastroenterological Association Foundation, a T. Franklin Williams Scholarship Award, the National Institutes of Health, and the National Library of Medicine. They reported having no relevant conflicts of interest.
This story was updated on 9/13/2017.
The main mechanism in the development of cirrhosis in patients with chronic liver disease (CLD) is increased hepatic fibrogenesis. The initial consequence of cirrhosis is portal hypertension, which is the main driver of decompensation (defined as the presence of ascites, variceal hemorrhage, or encephalopathy).
Statins are widely used for reducing cholesterol levels and cardiovascular risk. However, statins ameliorate endothelial dysfunction and have additional antifibrotic, anti-inflammatory, and antithrombotic properties, all of them of potential benefit in preventing progression of CLD/cirrhosis. In fact, statins have been shown to reduce portal pressure in cirrhosis.
In a meta-analysis of 13 studies, Kim et al. demonstrated that statin use is associated with a 58% lower risk of developing cirrhosis/fibrosis progression in patients with CLD (not statistically significant), while in patients with compensated cirrhosis of any etiology, statin use was associated with a statistically significant 46% lower risk of developing decompensation and death.
Most studies in the meta-analysis were observational/retrospective. Although the authors jointly analyzed three randomized controlled trials, only one of the trials looked at clinical outcomes. This important double-blind, placebo-controlled study in patients with recent variceal hemorrhage showed a significantly lower mortality in patients randomized to simvastatin.
Therefore, although the evidence is not yet sufficient to recommend the widespread use of statins in patients with CLD/cirrhosis, providers should not avoid using statins in patients with CLD/cirrhosis who otherwise need them. In fact, they should actively look for indications that would justify their use.
Guadalupe Garcia-Tsao, MD, is professor of medicine at Yale University, chief of digestive diseases at the VA-CT Healthcare System, and director of the clinical core of the Yale Liver Center, New Haven, Conn. She had no conflicts of interest.
The main mechanism in the development of cirrhosis in patients with chronic liver disease (CLD) is increased hepatic fibrogenesis. The initial consequence of cirrhosis is portal hypertension, which is the main driver of decompensation (defined as the presence of ascites, variceal hemorrhage, or encephalopathy).
Statins are widely used for reducing cholesterol levels and cardiovascular risk. However, statins ameliorate endothelial dysfunction and have additional antifibrotic, anti-inflammatory, and antithrombotic properties, all of them of potential benefit in preventing progression of CLD/cirrhosis. In fact, statins have been shown to reduce portal pressure in cirrhosis.
In a meta-analysis of 13 studies, Kim et al. demonstrated that statin use is associated with a 58% lower risk of developing cirrhosis/fibrosis progression in patients with CLD (not statistically significant), while in patients with compensated cirrhosis of any etiology, statin use was associated with a statistically significant 46% lower risk of developing decompensation and death.
Most studies in the meta-analysis were observational/retrospective. Although the authors jointly analyzed three randomized controlled trials, only one of the trials looked at clinical outcomes. This important double-blind, placebo-controlled study in patients with recent variceal hemorrhage showed a significantly lower mortality in patients randomized to simvastatin.
Therefore, although the evidence is not yet sufficient to recommend the widespread use of statins in patients with CLD/cirrhosis, providers should not avoid using statins in patients with CLD/cirrhosis who otherwise need them. In fact, they should actively look for indications that would justify their use.
Guadalupe Garcia-Tsao, MD, is professor of medicine at Yale University, chief of digestive diseases at the VA-CT Healthcare System, and director of the clinical core of the Yale Liver Center, New Haven, Conn. She had no conflicts of interest.
The main mechanism in the development of cirrhosis in patients with chronic liver disease (CLD) is increased hepatic fibrogenesis. The initial consequence of cirrhosis is portal hypertension, which is the main driver of decompensation (defined as the presence of ascites, variceal hemorrhage, or encephalopathy).
Statins are widely used for reducing cholesterol levels and cardiovascular risk. However, statins ameliorate endothelial dysfunction and have additional antifibrotic, anti-inflammatory, and antithrombotic properties, all of them of potential benefit in preventing progression of CLD/cirrhosis. In fact, statins have been shown to reduce portal pressure in cirrhosis.
In a meta-analysis of 13 studies, Kim et al. demonstrated that statin use is associated with a 58% lower risk of developing cirrhosis/fibrosis progression in patients with CLD (not statistically significant), while in patients with compensated cirrhosis of any etiology, statin use was associated with a statistically significant 46% lower risk of developing decompensation and death.
Most studies in the meta-analysis were observational/retrospective. Although the authors jointly analyzed three randomized controlled trials, only one of the trials looked at clinical outcomes. This important double-blind, placebo-controlled study in patients with recent variceal hemorrhage showed a significantly lower mortality in patients randomized to simvastatin.
Therefore, although the evidence is not yet sufficient to recommend the widespread use of statins in patients with CLD/cirrhosis, providers should not avoid using statins in patients with CLD/cirrhosis who otherwise need them. In fact, they should actively look for indications that would justify their use.
Guadalupe Garcia-Tsao, MD, is professor of medicine at Yale University, chief of digestive diseases at the VA-CT Healthcare System, and director of the clinical core of the Yale Liver Center, New Haven, Conn. She had no conflicts of interest.
For patients with compensated cirrhosis, statin therapy was associated with about a 46% decrease in the risk of hepatic decompensation and mortality and with a 27% drop in the risk of portal hypertension and variceal bleeding, according to moderate-quality evidence from a systematic review and meta-analysis of 13 studies.
Low-quality data also suggested that statins might help protect against the progression of noncirrhotic chronic liver disease, said Rebecca G. Kim of the University of California at San Diego and her associates. “Large, pragmatic randomized controlled trials in patients with compensated cirrhosis are required to confirm these observations,” they wrote in the October issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2017.04.039).
Prior studies have reported mixed findings on how statin therapy affects chronic liver disease. For their review, Ms. Kim and her associates searched MEDLINE, Embase, the Cochrane Central Register of Controlled Trials, Cochrane Database and Systematic Reviews, Scopus, Web of Science, and PubMed for randomized controlled trials or cohort studies published through March 25, 2017. They identified 10 cohort studies and three randomized controlled trials of adults with fibrosis without cirrhosis, compensated cirrhosis, or decompensated cirrhosis that evaluated statin exposure and reported associations between exposure and outcomes related to cirrhosis. They excluded case-control studies, cross-sectional studies, and studies that focused only on the relationship between statin use and the risk of hepatocellular carcinoma.
The resulting data set included 121,058 patients with chronic liver diseases, of whom 85% had chronic hepatitis C virus infection. A total of 46% of patients were exposed to statins, which appeared to reduce their risk of hepatic decompensation, variceal bleeding, and mortality. Among 87 such patients in five studies, statin use was associated with a 46% decrease in the risk of hepatic decompensation and death, with risk ratios of 0.54 (95% confidence intervals, 0.46-0.62 and 0.47-0.61, respectively). Statin use also was associated with a 27% lower risk of variceal bleeding or progression of portal hypertension, based on an analysis of 110 events in 236 patients from three trials (RR, 0.73; 95% CI, 0.59-0.91). Finally, statin use also was associated with a 58% lower risk of fibrosis progression or cirrhosis in patients with noncirrhotic chronic liver disease, but the 95% CIs for the risk estimate did not reach statistical significance (0.16-1.11).
Source: American Gastroenterological Association
Most studies lacked data on dose and duration of statin exposure, the researchers said. However, four cohort studies reported dose-dependent effects that were most pronounced after more than a year of treatment. “Similarly, several different types of statins were studied, and observed effects were assumed to be class-specific effects,” the reviewers wrote. “However, it is possible that lipophilic and lipophobic statins may have differential efficacy in decreasing fibrosis progression.”
Together, these findings support prior studies suggesting that statin therapy is safe and can potentially reduce the risk of hepatocellular carcinoma in this patient population, they concluded. Statins “may potentially improve patient-relevant outcomes in patients with chronic liver diseases and improve survival without significant additional costs.”
The reviewers acknowledged the American Gastroenterological Association Foundation, a T. Franklin Williams Scholarship Award, the National Institutes of Health, and the National Library of Medicine. They reported having no relevant conflicts of interest.
This story was updated on 9/13/2017.
For patients with compensated cirrhosis, statin therapy was associated with about a 46% decrease in the risk of hepatic decompensation and mortality and with a 27% drop in the risk of portal hypertension and variceal bleeding, according to moderate-quality evidence from a systematic review and meta-analysis of 13 studies.
Low-quality data also suggested that statins might help protect against the progression of noncirrhotic chronic liver disease, said Rebecca G. Kim of the University of California at San Diego and her associates. “Large, pragmatic randomized controlled trials in patients with compensated cirrhosis are required to confirm these observations,” they wrote in the October issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2017.04.039).
Prior studies have reported mixed findings on how statin therapy affects chronic liver disease. For their review, Ms. Kim and her associates searched MEDLINE, Embase, the Cochrane Central Register of Controlled Trials, Cochrane Database and Systematic Reviews, Scopus, Web of Science, and PubMed for randomized controlled trials or cohort studies published through March 25, 2017. They identified 10 cohort studies and three randomized controlled trials of adults with fibrosis without cirrhosis, compensated cirrhosis, or decompensated cirrhosis that evaluated statin exposure and reported associations between exposure and outcomes related to cirrhosis. They excluded case-control studies, cross-sectional studies, and studies that focused only on the relationship between statin use and the risk of hepatocellular carcinoma.
The resulting data set included 121,058 patients with chronic liver diseases, of whom 85% had chronic hepatitis C virus infection. A total of 46% of patients were exposed to statins, which appeared to reduce their risk of hepatic decompensation, variceal bleeding, and mortality. Among 87 such patients in five studies, statin use was associated with a 46% decrease in the risk of hepatic decompensation and death, with risk ratios of 0.54 (95% confidence intervals, 0.46-0.62 and 0.47-0.61, respectively). Statin use also was associated with a 27% lower risk of variceal bleeding or progression of portal hypertension, based on an analysis of 110 events in 236 patients from three trials (RR, 0.73; 95% CI, 0.59-0.91). Finally, statin use also was associated with a 58% lower risk of fibrosis progression or cirrhosis in patients with noncirrhotic chronic liver disease, but the 95% CIs for the risk estimate did not reach statistical significance (0.16-1.11).
Source: American Gastroenterological Association
Most studies lacked data on dose and duration of statin exposure, the researchers said. However, four cohort studies reported dose-dependent effects that were most pronounced after more than a year of treatment. “Similarly, several different types of statins were studied, and observed effects were assumed to be class-specific effects,” the reviewers wrote. “However, it is possible that lipophilic and lipophobic statins may have differential efficacy in decreasing fibrosis progression.”
Together, these findings support prior studies suggesting that statin therapy is safe and can potentially reduce the risk of hepatocellular carcinoma in this patient population, they concluded. Statins “may potentially improve patient-relevant outcomes in patients with chronic liver diseases and improve survival without significant additional costs.”
The reviewers acknowledged the American Gastroenterological Association Foundation, a T. Franklin Williams Scholarship Award, the National Institutes of Health, and the National Library of Medicine. They reported having no relevant conflicts of interest.
This story was updated on 9/13/2017.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Statin therapy was associated with a significantly lower risk of hepatic decompensation and death in patients with compensated cirrhosis.
Major finding: Statin therapy was associated with a 46% decrease in the risk of both hepatic decompensation and mortality (risk ratios, 0.54) and with a 27% drop in the risk of portal hypertension and variceal bleeding (RR, 0.73).
Data source: A systematic review and meta-analysis of 10 cohort studies and three randomized controlled trials (121,058 patients).
Disclosures: The reviewers acknowledged the American Gastroenterological Association Foundation, a T. Franklin Williams Scholarship Award, the National Institutes of Health, and the National Library of Medicine. They reported having no relevant conflicts of interest.
Check children’s eyes early for best corrections
or its risk factors with the goal of improving visual acuity, publishing its final recommendation statement and evidence summary online Sept. 5 in JAMA.
A review of the latest evidence supports a B recommendation for vision screening at least once in children aged 3-5 years, but the evidence is insufficient to determine the balance of risks and benefits for vision screening in children younger than 3 years (meriting an I statement from the USPSTF). The recommendation updates the 2011 USPSTF recommendation, which also recommended vision screening for children aged 3-5 years with a B recommendation.
“The prevalence of amblyopia, strabismus, and anisometropia ranges from 1% to 6% among children younger than 6 years in the United States,” which can lead to permanent vision loss if left untreated, chair and corresponding author David C. Grossman, MD, of Kaiser Permanente Washington Health Research Institute, Seattle, and colleagues noted in the recommendation statement (JAMA. 2017;318:836-44).
The USPSTF found “inadequate evidence that treatment reduced the incidence of long-term amblyopia or improved school performance, functioning, or quality of life.” However, the USPSTF concluded that the harms of screening and treating preschool children for amblyopia and its risk factors were small, and that treatment improved visual acuity, “which is likely to result in permanent improvements throughout life.”
The benefits of early treatment were characterized as moderate because of the risk of permanent, uncorrectable vision loss associated with untreated amblyopia, “and the benefits of screening and treatment can be experienced over a child’s lifetime,” the researchers said.
The evidence report accompanying the recommendations contained data from 40 studies with 34,709 participants, and addressed issues including the benefits of screening, accuracy of vision screening tests, and the potential harms and benefits of treatments including eye patches and glasses (JAMA. 2017;318:845-58).
“Studies directly evaluating the effectiveness of screening were limited and do not establish whether vision screening in preschool children is better than no screening,” Daniel E. Jonas, MD, of RTI International–University of North Carolina at Chapel Hill Evidence-based Practice Center and his colleagues wrote in the evidence report.
Therefore, the Task Force called for additional research while recommending at least one screening.
The study was funded by the Agency for Healthcare Research and Quality. The researchers had no financial conflicts to disclose.
Read the complete recommendations online at http://www.uspreventiveservicestaskforce.org.
or its risk factors with the goal of improving visual acuity, publishing its final recommendation statement and evidence summary online Sept. 5 in JAMA.
A review of the latest evidence supports a B recommendation for vision screening at least once in children aged 3-5 years, but the evidence is insufficient to determine the balance of risks and benefits for vision screening in children younger than 3 years (meriting an I statement from the USPSTF). The recommendation updates the 2011 USPSTF recommendation, which also recommended vision screening for children aged 3-5 years with a B recommendation.
“The prevalence of amblyopia, strabismus, and anisometropia ranges from 1% to 6% among children younger than 6 years in the United States,” which can lead to permanent vision loss if left untreated, chair and corresponding author David C. Grossman, MD, of Kaiser Permanente Washington Health Research Institute, Seattle, and colleagues noted in the recommendation statement (JAMA. 2017;318:836-44).
The USPSTF found “inadequate evidence that treatment reduced the incidence of long-term amblyopia or improved school performance, functioning, or quality of life.” However, the USPSTF concluded that the harms of screening and treating preschool children for amblyopia and its risk factors were small, and that treatment improved visual acuity, “which is likely to result in permanent improvements throughout life.”
The benefits of early treatment were characterized as moderate because of the risk of permanent, uncorrectable vision loss associated with untreated amblyopia, “and the benefits of screening and treatment can be experienced over a child’s lifetime,” the researchers said.
The evidence report accompanying the recommendations contained data from 40 studies with 34,709 participants, and addressed issues including the benefits of screening, accuracy of vision screening tests, and the potential harms and benefits of treatments including eye patches and glasses (JAMA. 2017;318:845-58).
“Studies directly evaluating the effectiveness of screening were limited and do not establish whether vision screening in preschool children is better than no screening,” Daniel E. Jonas, MD, of RTI International–University of North Carolina at Chapel Hill Evidence-based Practice Center and his colleagues wrote in the evidence report.
Therefore, the Task Force called for additional research while recommending at least one screening.
The study was funded by the Agency for Healthcare Research and Quality. The researchers had no financial conflicts to disclose.
Read the complete recommendations online at http://www.uspreventiveservicestaskforce.org.
or its risk factors with the goal of improving visual acuity, publishing its final recommendation statement and evidence summary online Sept. 5 in JAMA.
A review of the latest evidence supports a B recommendation for vision screening at least once in children aged 3-5 years, but the evidence is insufficient to determine the balance of risks and benefits for vision screening in children younger than 3 years (meriting an I statement from the USPSTF). The recommendation updates the 2011 USPSTF recommendation, which also recommended vision screening for children aged 3-5 years with a B recommendation.
“The prevalence of amblyopia, strabismus, and anisometropia ranges from 1% to 6% among children younger than 6 years in the United States,” which can lead to permanent vision loss if left untreated, chair and corresponding author David C. Grossman, MD, of Kaiser Permanente Washington Health Research Institute, Seattle, and colleagues noted in the recommendation statement (JAMA. 2017;318:836-44).
The USPSTF found “inadequate evidence that treatment reduced the incidence of long-term amblyopia or improved school performance, functioning, or quality of life.” However, the USPSTF concluded that the harms of screening and treating preschool children for amblyopia and its risk factors were small, and that treatment improved visual acuity, “which is likely to result in permanent improvements throughout life.”
The benefits of early treatment were characterized as moderate because of the risk of permanent, uncorrectable vision loss associated with untreated amblyopia, “and the benefits of screening and treatment can be experienced over a child’s lifetime,” the researchers said.
The evidence report accompanying the recommendations contained data from 40 studies with 34,709 participants, and addressed issues including the benefits of screening, accuracy of vision screening tests, and the potential harms and benefits of treatments including eye patches and glasses (JAMA. 2017;318:845-58).
“Studies directly evaluating the effectiveness of screening were limited and do not establish whether vision screening in preschool children is better than no screening,” Daniel E. Jonas, MD, of RTI International–University of North Carolina at Chapel Hill Evidence-based Practice Center and his colleagues wrote in the evidence report.
Therefore, the Task Force called for additional research while recommending at least one screening.
The study was funded by the Agency for Healthcare Research and Quality. The researchers had no financial conflicts to disclose.
Read the complete recommendations online at http://www.uspreventiveservicestaskforce.org.
FROM JAMA
Q&A: CDC director Brenda Fitzgerald stresses ‘science and service’
Brenda Fitzgerald, MD, an ob.gyn. and most recently the public health commissioner for Georgia, was named the 17th director of the Centers for Disease Control and Prevention in July.
Dr. Fitzgerald comes to the CDC after 6 years as commissioner and state health officer for the Georgia Department of Public Health. She also has been a health care policy adviser to former House Speaker Newt Gingrich (R-Ga.) and ran for Congress herself in the 1990s. In addition to practicing medicine for 3 decades, she has served on the board and as president of the Georgia Obstetrical and Gynecological Society.
Question: What are you looking forward to as the new CDC director, and what are some of your top goals during your tenure?
Answer: The federal government holds responsibility to provide for the common defense. And CDC provides the common defense of the country against health threats. That’s why CDC’s mission of saving lives and protecting people means so much to me. As a doctor and a mother, I want to do everything I can to protect our future generations and keep them safe, whether it be from infectious or chronic diseases, natural or man-made disasters.
We are going to continue a legacy of protecting Americans from the things that we’ve been fighting for a long time, like heart disease and cancer, and from newer things like opioid overdose and the Zika virus. And we’ll continue to prepare for the next health threat, whatever it may be.
Q: How does your experience as an ob.gyn. shape your approach to this job?
A: There are two things that are important at CDC: science and service. CDC researchers are at the forefront of scientific research – and also provide a service by using scientific discoveries to improve health. That is the same approach I used as a practicing ob.gyn.: I brought the clinical lens that looked at the science, and then my work with my patients emphasized the service part.
As a practicing ob.gyn., it was my responsibility to address the questions and concerns of my patients. At CDC, I am just as committed to making science-based decisions and working with our experts to make sure doctors and patients have the state-of-the-art health information they need. I said when I went from being a clinician to being the state health official for Georgia that I went from treating one patient at a time to treating 10 million at a time. Now that’s changed to over 300 million.
Even though I am now CDC director and protecting the health of millions of people, my experiences as an ob.gyn. for 3 decades will forever shape how I work to improve the health of individual people and patients.
Q: What are the next steps for the agency in terms of its response to Zika virus?
A: Zika is the first infectious disease in 50 years that has been linked to birth defects. We know that the virus attacks neural tissue. We know that about 10% of babies are born with severe birth defects – and some babies born apparently normal later turned out to have neurologic problems, like blindness or deafness. And we suspect that there may be other problems we don’t know about yet.
I’m particularly concerned about developmental delays, as we know that early brain development is key to health and achievement for a child’s future. We are still learning more about Zika every day. It’s crucial that we – the health care and public health communities – work together and remain vigilant to ensure these babies receive the care they need. What Zika has taught us is that we need to establish a pregnancy and infant registry that can flag patterns of concerning health issues so that we can work together with health officials and clinicians to get ahead of emerging health threats.
Q: How can clinicians stay up to date on the rapidly changing guidance during an outbreak like Zika?
A: During outbreaks like Zika, public health officials are continually updating guidance so clinicians can get the latest information. You can check the latest CDC guidance about testing and follow-up care for pregnant women at www.cdc.gov/zika/hc-providers. Health care providers can also contact their state, local, or territorial health department to ensure the appropriate tests are ordered and interpreted correctly. CDC also maintains a 24/7 Zika consultation service for health officials and healthcare providers caring for pregnant women with possible Zika exposure.
Q: What steps will you take to decrease maternal mortality and to understand why this number is rising?
A: As an obstetrician, survival of mothers and babies is paramount to me. It’s so basic: mothers shouldn’t die. Sadly, about 700 women die each year in the U.S. as a result of pregnancy or delivery complications.
We know the leading causes of maternal mortality: cardiovascular disease, infections or sepsis, hemorrhage, and cardiomyopathy. We also know that an increasing number of pregnant women in the U.S. have chronic health conditions such as high blood pressure, diabetes, or heart disease that puts them at risk of pregnancy complications or death.
CDC is committed to preventing pregnancy-related deaths and ensuring the best possible birth outcomes. We do this by conducting surveillance, supporting states in developing recommendations, and working with partners to promote evidence-based recommendations and best practices. State-based initiatives are important allies in bringing together OBs and the public health sector to show the importance of statewide efforts to reduce maternal mortality. There has been some exciting work done by the California Maternal Quality Care Collaborative, which has created a series of toolkits designed to help health care providers with pregnancy or childbirth complications. CMQCC’s work is inspiring other states to follow suit.
The best coalitions are between ob.gyns., public health, and hospital associations. For example, AWHONN’s [Association of Women’s Health, Obstetric and Neonatal Nurses] Postpartum Hemorrhage initiative in Georgia, New Jersey, and the District of Columbia aimed to improve the treatment of obstetric hemorrhage. Even though 54% to 93% of maternal hemorrhage-related deaths are preventable with improved clinical response, not one single hospital in Georgia or New Jersey had implemented all the recommended preparedness elements for postpartum hemorrhage events. We can save mothers’ lives – we know what to do. It just takes all of us – ob.gyns., public health and hospital associations – leading the way to make sure it happens.
Q: How can we increase HPV vaccination?
A: The latest news is that more girls and boys are getting the HPV vaccine. New CDC data showed that 60% of teens aged 13-17 had received one or more doses of HPV vaccine in 2016 – an increase of 4% from 2015. Plus, the latest statistics show that HPV vaccination has led to significant drops in HPV infections: HPV-related cancers and genital warts dropped by 71% among teen girls and 61% among young women. Still, too many children aren’t completing the HPV vaccine series, leaving them vulnerable to cancers caused by HPV infection.
Providers can help increase HPV vaccination rates by using every patient visit to review vaccination histories, providing strong clinical recommendations and education to parents for HPV and other recommended vaccines, and implementing systems to minimize missed opportunities.
[email protected]
On Twitter @legal_med
Brenda Fitzgerald, MD, an ob.gyn. and most recently the public health commissioner for Georgia, was named the 17th director of the Centers for Disease Control and Prevention in July.
Dr. Fitzgerald comes to the CDC after 6 years as commissioner and state health officer for the Georgia Department of Public Health. She also has been a health care policy adviser to former House Speaker Newt Gingrich (R-Ga.) and ran for Congress herself in the 1990s. In addition to practicing medicine for 3 decades, she has served on the board and as president of the Georgia Obstetrical and Gynecological Society.
Question: What are you looking forward to as the new CDC director, and what are some of your top goals during your tenure?
Answer: The federal government holds responsibility to provide for the common defense. And CDC provides the common defense of the country against health threats. That’s why CDC’s mission of saving lives and protecting people means so much to me. As a doctor and a mother, I want to do everything I can to protect our future generations and keep them safe, whether it be from infectious or chronic diseases, natural or man-made disasters.
We are going to continue a legacy of protecting Americans from the things that we’ve been fighting for a long time, like heart disease and cancer, and from newer things like opioid overdose and the Zika virus. And we’ll continue to prepare for the next health threat, whatever it may be.
Q: How does your experience as an ob.gyn. shape your approach to this job?
A: There are two things that are important at CDC: science and service. CDC researchers are at the forefront of scientific research – and also provide a service by using scientific discoveries to improve health. That is the same approach I used as a practicing ob.gyn.: I brought the clinical lens that looked at the science, and then my work with my patients emphasized the service part.
As a practicing ob.gyn., it was my responsibility to address the questions and concerns of my patients. At CDC, I am just as committed to making science-based decisions and working with our experts to make sure doctors and patients have the state-of-the-art health information they need. I said when I went from being a clinician to being the state health official for Georgia that I went from treating one patient at a time to treating 10 million at a time. Now that’s changed to over 300 million.
Even though I am now CDC director and protecting the health of millions of people, my experiences as an ob.gyn. for 3 decades will forever shape how I work to improve the health of individual people and patients.
Q: What are the next steps for the agency in terms of its response to Zika virus?
A: Zika is the first infectious disease in 50 years that has been linked to birth defects. We know that the virus attacks neural tissue. We know that about 10% of babies are born with severe birth defects – and some babies born apparently normal later turned out to have neurologic problems, like blindness or deafness. And we suspect that there may be other problems we don’t know about yet.
I’m particularly concerned about developmental delays, as we know that early brain development is key to health and achievement for a child’s future. We are still learning more about Zika every day. It’s crucial that we – the health care and public health communities – work together and remain vigilant to ensure these babies receive the care they need. What Zika has taught us is that we need to establish a pregnancy and infant registry that can flag patterns of concerning health issues so that we can work together with health officials and clinicians to get ahead of emerging health threats.
Q: How can clinicians stay up to date on the rapidly changing guidance during an outbreak like Zika?
A: During outbreaks like Zika, public health officials are continually updating guidance so clinicians can get the latest information. You can check the latest CDC guidance about testing and follow-up care for pregnant women at www.cdc.gov/zika/hc-providers. Health care providers can also contact their state, local, or territorial health department to ensure the appropriate tests are ordered and interpreted correctly. CDC also maintains a 24/7 Zika consultation service for health officials and healthcare providers caring for pregnant women with possible Zika exposure.
Q: What steps will you take to decrease maternal mortality and to understand why this number is rising?
A: As an obstetrician, survival of mothers and babies is paramount to me. It’s so basic: mothers shouldn’t die. Sadly, about 700 women die each year in the U.S. as a result of pregnancy or delivery complications.
We know the leading causes of maternal mortality: cardiovascular disease, infections or sepsis, hemorrhage, and cardiomyopathy. We also know that an increasing number of pregnant women in the U.S. have chronic health conditions such as high blood pressure, diabetes, or heart disease that puts them at risk of pregnancy complications or death.
CDC is committed to preventing pregnancy-related deaths and ensuring the best possible birth outcomes. We do this by conducting surveillance, supporting states in developing recommendations, and working with partners to promote evidence-based recommendations and best practices. State-based initiatives are important allies in bringing together OBs and the public health sector to show the importance of statewide efforts to reduce maternal mortality. There has been some exciting work done by the California Maternal Quality Care Collaborative, which has created a series of toolkits designed to help health care providers with pregnancy or childbirth complications. CMQCC’s work is inspiring other states to follow suit.
The best coalitions are between ob.gyns., public health, and hospital associations. For example, AWHONN’s [Association of Women’s Health, Obstetric and Neonatal Nurses] Postpartum Hemorrhage initiative in Georgia, New Jersey, and the District of Columbia aimed to improve the treatment of obstetric hemorrhage. Even though 54% to 93% of maternal hemorrhage-related deaths are preventable with improved clinical response, not one single hospital in Georgia or New Jersey had implemented all the recommended preparedness elements for postpartum hemorrhage events. We can save mothers’ lives – we know what to do. It just takes all of us – ob.gyns., public health and hospital associations – leading the way to make sure it happens.
Q: How can we increase HPV vaccination?
A: The latest news is that more girls and boys are getting the HPV vaccine. New CDC data showed that 60% of teens aged 13-17 had received one or more doses of HPV vaccine in 2016 – an increase of 4% from 2015. Plus, the latest statistics show that HPV vaccination has led to significant drops in HPV infections: HPV-related cancers and genital warts dropped by 71% among teen girls and 61% among young women. Still, too many children aren’t completing the HPV vaccine series, leaving them vulnerable to cancers caused by HPV infection.
Providers can help increase HPV vaccination rates by using every patient visit to review vaccination histories, providing strong clinical recommendations and education to parents for HPV and other recommended vaccines, and implementing systems to minimize missed opportunities.
[email protected]
On Twitter @legal_med
Brenda Fitzgerald, MD, an ob.gyn. and most recently the public health commissioner for Georgia, was named the 17th director of the Centers for Disease Control and Prevention in July.
Dr. Fitzgerald comes to the CDC after 6 years as commissioner and state health officer for the Georgia Department of Public Health. She also has been a health care policy adviser to former House Speaker Newt Gingrich (R-Ga.) and ran for Congress herself in the 1990s. In addition to practicing medicine for 3 decades, she has served on the board and as president of the Georgia Obstetrical and Gynecological Society.
Question: What are you looking forward to as the new CDC director, and what are some of your top goals during your tenure?
Answer: The federal government holds responsibility to provide for the common defense. And CDC provides the common defense of the country against health threats. That’s why CDC’s mission of saving lives and protecting people means so much to me. As a doctor and a mother, I want to do everything I can to protect our future generations and keep them safe, whether it be from infectious or chronic diseases, natural or man-made disasters.
We are going to continue a legacy of protecting Americans from the things that we’ve been fighting for a long time, like heart disease and cancer, and from newer things like opioid overdose and the Zika virus. And we’ll continue to prepare for the next health threat, whatever it may be.
Q: How does your experience as an ob.gyn. shape your approach to this job?
A: There are two things that are important at CDC: science and service. CDC researchers are at the forefront of scientific research – and also provide a service by using scientific discoveries to improve health. That is the same approach I used as a practicing ob.gyn.: I brought the clinical lens that looked at the science, and then my work with my patients emphasized the service part.
As a practicing ob.gyn., it was my responsibility to address the questions and concerns of my patients. At CDC, I am just as committed to making science-based decisions and working with our experts to make sure doctors and patients have the state-of-the-art health information they need. I said when I went from being a clinician to being the state health official for Georgia that I went from treating one patient at a time to treating 10 million at a time. Now that’s changed to over 300 million.
Even though I am now CDC director and protecting the health of millions of people, my experiences as an ob.gyn. for 3 decades will forever shape how I work to improve the health of individual people and patients.
Q: What are the next steps for the agency in terms of its response to Zika virus?
A: Zika is the first infectious disease in 50 years that has been linked to birth defects. We know that the virus attacks neural tissue. We know that about 10% of babies are born with severe birth defects – and some babies born apparently normal later turned out to have neurologic problems, like blindness or deafness. And we suspect that there may be other problems we don’t know about yet.
I’m particularly concerned about developmental delays, as we know that early brain development is key to health and achievement for a child’s future. We are still learning more about Zika every day. It’s crucial that we – the health care and public health communities – work together and remain vigilant to ensure these babies receive the care they need. What Zika has taught us is that we need to establish a pregnancy and infant registry that can flag patterns of concerning health issues so that we can work together with health officials and clinicians to get ahead of emerging health threats.
Q: How can clinicians stay up to date on the rapidly changing guidance during an outbreak like Zika?
A: During outbreaks like Zika, public health officials are continually updating guidance so clinicians can get the latest information. You can check the latest CDC guidance about testing and follow-up care for pregnant women at www.cdc.gov/zika/hc-providers. Health care providers can also contact their state, local, or territorial health department to ensure the appropriate tests are ordered and interpreted correctly. CDC also maintains a 24/7 Zika consultation service for health officials and healthcare providers caring for pregnant women with possible Zika exposure.
Q: What steps will you take to decrease maternal mortality and to understand why this number is rising?
A: As an obstetrician, survival of mothers and babies is paramount to me. It’s so basic: mothers shouldn’t die. Sadly, about 700 women die each year in the U.S. as a result of pregnancy or delivery complications.
We know the leading causes of maternal mortality: cardiovascular disease, infections or sepsis, hemorrhage, and cardiomyopathy. We also know that an increasing number of pregnant women in the U.S. have chronic health conditions such as high blood pressure, diabetes, or heart disease that puts them at risk of pregnancy complications or death.
CDC is committed to preventing pregnancy-related deaths and ensuring the best possible birth outcomes. We do this by conducting surveillance, supporting states in developing recommendations, and working with partners to promote evidence-based recommendations and best practices. State-based initiatives are important allies in bringing together OBs and the public health sector to show the importance of statewide efforts to reduce maternal mortality. There has been some exciting work done by the California Maternal Quality Care Collaborative, which has created a series of toolkits designed to help health care providers with pregnancy or childbirth complications. CMQCC’s work is inspiring other states to follow suit.
The best coalitions are between ob.gyns., public health, and hospital associations. For example, AWHONN’s [Association of Women’s Health, Obstetric and Neonatal Nurses] Postpartum Hemorrhage initiative in Georgia, New Jersey, and the District of Columbia aimed to improve the treatment of obstetric hemorrhage. Even though 54% to 93% of maternal hemorrhage-related deaths are preventable with improved clinical response, not one single hospital in Georgia or New Jersey had implemented all the recommended preparedness elements for postpartum hemorrhage events. We can save mothers’ lives – we know what to do. It just takes all of us – ob.gyns., public health and hospital associations – leading the way to make sure it happens.
Q: How can we increase HPV vaccination?
A: The latest news is that more girls and boys are getting the HPV vaccine. New CDC data showed that 60% of teens aged 13-17 had received one or more doses of HPV vaccine in 2016 – an increase of 4% from 2015. Plus, the latest statistics show that HPV vaccination has led to significant drops in HPV infections: HPV-related cancers and genital warts dropped by 71% among teen girls and 61% among young women. Still, too many children aren’t completing the HPV vaccine series, leaving them vulnerable to cancers caused by HPV infection.
Providers can help increase HPV vaccination rates by using every patient visit to review vaccination histories, providing strong clinical recommendations and education to parents for HPV and other recommended vaccines, and implementing systems to minimize missed opportunities.
[email protected]
On Twitter @legal_med
Enhanced disinfection of duodenoscopes did not reduce contamination
Duodenoscopes had similar rates of contamination after double high-level disinfection, standard high-level disinfection, or standard high-level disinfection followed by ethylene oxide gas sterilization, a randomized, prospective study of 516 bacterial cultures of 18 duodenoscopes showed.
“Our results do not support the routine use of double high-level disinfection or ethylene oxide sterilization for duodenoscope reprocessing,” wrote Graham M. Snyder, MD, of Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, and his associates. They stopped the study after 3 months because none of the duodenoscopes cultured multidrug-resistant organisms, the primary endpoint. “[We] found that in the nonoutbreak setting, duodenoscope contamination by multidrug-resistant organisms is extremely uncommon,” they wrote in the October issue of Gastroenterology (doi: 10.1053/j.gastro.2017.06.052). However, 16% of duodenoscopes cultured at least one colony-forming unit (CFU) after either standard high-level or double high-level disinfection, and 23% of duodenoscopes produced at least one CFU despite standard high-level disinfection followed by ethylene gas sterilization (P = .2), the investigators reported.
Outbreaks of carbapenem-resistant Enterobacteriaceae infections have been traced to duodenoscopes, even though they were reprocessed according to manufacturer instructions. In 2015, the Food and Drug Administration responded by warning that the design of duodenoscopes might preclude effective cleaning. Reasons for residual contamination remain uncertain, but biofilms, which are notoriously resistant to standard disinfection methods, might be a culprit, Dr. Snyder and his associates noted. Accordingly, some experts have suggested repeating the reprocessing cycle or adding ethylene oxide sterilization, but these measures are costly, time intensive, and not widely available. Furthermore, their efficacy “has never been systematically studied in a nonoutbreak setting,” the researchers wrote.
In response, they studied 516 cultures of elevator mechanisms and working channels from 18 reprocessed duodenoscopes (Olympus, model TJF-Q180). Immediately after use, each duodenoscope was manually wiped with enzymatic solution (EmPower), and then was manually reprocessed within an hour before undergoing automated reprocessing (System 83 Plus 9) with ortho-phthalaldehyde disinfectant (MetriCide OPA Plus) followed by ethanol flush. One-third of the duodenoscopes were randomly assigned to undergo double high-level disinfection with two automated reprocessing cycles, and another third underwent standard high-level disinfection followed by ethylene oxide gas sterilization (Steri-Vac sterilizer/aerator). All instruments were stored by hanging them vertically in an unventilated cabinet.
Multidrug-resistant organisms were cultured from 3% of rectal swabs and duodenal aspirates, but not from any of the cultures of duodenoscopes. Therefore, the study was stopped for futility. The enhanced disinfection methods failed to prevent contamination, compared with standard high-level disinfection, the researchers noted. Ten or more CFUs grew in 2% of duodenoscopes that underwent standard high-level disinfection, 4% of those that underwent double high-level disinfection, and 4% of those that underwent high-level disinfection followed by ethylene oxide sterilization (P = .4).
“There is no consensus on what parts of the standard high-level disinfection process should be repeated,” the investigators wrote. “It is uncertain if the addition of a second cycle of manual reprocessing might have improved the effectiveness of double high-level disinfection.”
Funders included the American Society for Gastrointestinal Endoscopy and Beth Israel Deaconess Medical Center. The investigators reported having no conflicts of interest.
Duodenoscopes had similar rates of contamination after double high-level disinfection, standard high-level disinfection, or standard high-level disinfection followed by ethylene oxide gas sterilization, a randomized, prospective study of 516 bacterial cultures of 18 duodenoscopes showed.
“Our results do not support the routine use of double high-level disinfection or ethylene oxide sterilization for duodenoscope reprocessing,” wrote Graham M. Snyder, MD, of Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, and his associates. They stopped the study after 3 months because none of the duodenoscopes cultured multidrug-resistant organisms, the primary endpoint. “[We] found that in the nonoutbreak setting, duodenoscope contamination by multidrug-resistant organisms is extremely uncommon,” they wrote in the October issue of Gastroenterology (doi: 10.1053/j.gastro.2017.06.052). However, 16% of duodenoscopes cultured at least one colony-forming unit (CFU) after either standard high-level or double high-level disinfection, and 23% of duodenoscopes produced at least one CFU despite standard high-level disinfection followed by ethylene gas sterilization (P = .2), the investigators reported.
Outbreaks of carbapenem-resistant Enterobacteriaceae infections have been traced to duodenoscopes, even though they were reprocessed according to manufacturer instructions. In 2015, the Food and Drug Administration responded by warning that the design of duodenoscopes might preclude effective cleaning. Reasons for residual contamination remain uncertain, but biofilms, which are notoriously resistant to standard disinfection methods, might be a culprit, Dr. Snyder and his associates noted. Accordingly, some experts have suggested repeating the reprocessing cycle or adding ethylene oxide sterilization, but these measures are costly, time intensive, and not widely available. Furthermore, their efficacy “has never been systematically studied in a nonoutbreak setting,” the researchers wrote.
In response, they studied 516 cultures of elevator mechanisms and working channels from 18 reprocessed duodenoscopes (Olympus, model TJF-Q180). Immediately after use, each duodenoscope was manually wiped with enzymatic solution (EmPower), and then was manually reprocessed within an hour before undergoing automated reprocessing (System 83 Plus 9) with ortho-phthalaldehyde disinfectant (MetriCide OPA Plus) followed by ethanol flush. One-third of the duodenoscopes were randomly assigned to undergo double high-level disinfection with two automated reprocessing cycles, and another third underwent standard high-level disinfection followed by ethylene oxide gas sterilization (Steri-Vac sterilizer/aerator). All instruments were stored by hanging them vertically in an unventilated cabinet.
Multidrug-resistant organisms were cultured from 3% of rectal swabs and duodenal aspirates, but not from any of the cultures of duodenoscopes. Therefore, the study was stopped for futility. The enhanced disinfection methods failed to prevent contamination, compared with standard high-level disinfection, the researchers noted. Ten or more CFUs grew in 2% of duodenoscopes that underwent standard high-level disinfection, 4% of those that underwent double high-level disinfection, and 4% of those that underwent high-level disinfection followed by ethylene oxide sterilization (P = .4).
“There is no consensus on what parts of the standard high-level disinfection process should be repeated,” the investigators wrote. “It is uncertain if the addition of a second cycle of manual reprocessing might have improved the effectiveness of double high-level disinfection.”
Funders included the American Society for Gastrointestinal Endoscopy and Beth Israel Deaconess Medical Center. The investigators reported having no conflicts of interest.
Duodenoscopes had similar rates of contamination after double high-level disinfection, standard high-level disinfection, or standard high-level disinfection followed by ethylene oxide gas sterilization, a randomized, prospective study of 516 bacterial cultures of 18 duodenoscopes showed.
“Our results do not support the routine use of double high-level disinfection or ethylene oxide sterilization for duodenoscope reprocessing,” wrote Graham M. Snyder, MD, of Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, and his associates. They stopped the study after 3 months because none of the duodenoscopes cultured multidrug-resistant organisms, the primary endpoint. “[We] found that in the nonoutbreak setting, duodenoscope contamination by multidrug-resistant organisms is extremely uncommon,” they wrote in the October issue of Gastroenterology (doi: 10.1053/j.gastro.2017.06.052). However, 16% of duodenoscopes cultured at least one colony-forming unit (CFU) after either standard high-level or double high-level disinfection, and 23% of duodenoscopes produced at least one CFU despite standard high-level disinfection followed by ethylene gas sterilization (P = .2), the investigators reported.
Outbreaks of carbapenem-resistant Enterobacteriaceae infections have been traced to duodenoscopes, even though they were reprocessed according to manufacturer instructions. In 2015, the Food and Drug Administration responded by warning that the design of duodenoscopes might preclude effective cleaning. Reasons for residual contamination remain uncertain, but biofilms, which are notoriously resistant to standard disinfection methods, might be a culprit, Dr. Snyder and his associates noted. Accordingly, some experts have suggested repeating the reprocessing cycle or adding ethylene oxide sterilization, but these measures are costly, time intensive, and not widely available. Furthermore, their efficacy “has never been systematically studied in a nonoutbreak setting,” the researchers wrote.
In response, they studied 516 cultures of elevator mechanisms and working channels from 18 reprocessed duodenoscopes (Olympus, model TJF-Q180). Immediately after use, each duodenoscope was manually wiped with enzymatic solution (EmPower), and then was manually reprocessed within an hour before undergoing automated reprocessing (System 83 Plus 9) with ortho-phthalaldehyde disinfectant (MetriCide OPA Plus) followed by ethanol flush. One-third of the duodenoscopes were randomly assigned to undergo double high-level disinfection with two automated reprocessing cycles, and another third underwent standard high-level disinfection followed by ethylene oxide gas sterilization (Steri-Vac sterilizer/aerator). All instruments were stored by hanging them vertically in an unventilated cabinet.
Multidrug-resistant organisms were cultured from 3% of rectal swabs and duodenal aspirates, but not from any of the cultures of duodenoscopes. Therefore, the study was stopped for futility. The enhanced disinfection methods failed to prevent contamination, compared with standard high-level disinfection, the researchers noted. Ten or more CFUs grew in 2% of duodenoscopes that underwent standard high-level disinfection, 4% of those that underwent double high-level disinfection, and 4% of those that underwent high-level disinfection followed by ethylene oxide sterilization (P = .4).
“There is no consensus on what parts of the standard high-level disinfection process should be repeated,” the investigators wrote. “It is uncertain if the addition of a second cycle of manual reprocessing might have improved the effectiveness of double high-level disinfection.”
Funders included the American Society for Gastrointestinal Endoscopy and Beth Israel Deaconess Medical Center. The investigators reported having no conflicts of interest.
FROM GASTROENTEROLOGY
Key clinical point: Enhanced disinfection of duodenoscopes did not provide additional protection against contamination.
Major finding: No cultures were positive for multidrug-resistant organisms, but 16% of duodenoscopes had at least one colony-forming unit despite standard high-level disinfection or double high-level disinfection. Standard high-level disinfection followed by ethylene oxide gas failed to sterilize 23% of duodenoscopes (P = .2).
Data source: A single-center, prospective randomized study of 516 cultures of 18 duodenoscopes.
Disclosures: Funders included the American Society for Gastrointestinal Endoscopy and Beth Israel Deaconess Medical Center. The investigators reported having no conflicts of interest.
Light alcohol use did not affect liver fibrosis progression in HIV/HCV-coinfected women
Women coinfected with HIV and hepatitis C virus who drank light and moderate amounts of alcohol did not experience significant progression of liver fibrosis, compared with those who drank heavily, results from a large cohort study found.
“Although heavy alcohol use is clearly detrimental to the health of patients with CHC [chronic hepatitis C], it is unclear whether consumption of smaller quantities of alcohol impact fibrosis progression,” researchers led by Erin M. Kelly, MD, wrote in a study published online Aug. 16 in Clinical Infectious Diseases (doi: 10.1093/cid/cix716). “Many patients with CHC consume alcohol and are unable or unwilling to completely abstain. Some studies have suggested a linear dose-response relationship for fibrosis progression even at lower quantities, while others have not clearly demonstrated a risk for fibrosis progression below 20-50 g of alcohol per day. HIV/HCV [hepatitis C virus]-coinfected patients have accelerated fibrosis progression, compared with HCV monoinfected individuals. Whether regular consumption of small quantities of alcohol further increase the rate of fibrosis progression is unknown.”
At baseline, the mean age of study participants was 40 years, their mean body mass index was 26 kg/m2, 17% had diabetes, and 11% had significant fibrosis, defined as a FIB-4 index of greater than 3.25. Nearly half (46%) reported no alcohol use; 26.8% reported light use; 7.1%, moderate use; and 19.7%, heavy use. The median FIB-4 scores at entry were similar between groups. On multivariable analysis, no significant difference in fibrosis progression was observed in abstainers, compared with those who reported light and moderate alcohol use (0.004 and 0.006 FIB-4 units/year, respectively). On the other hand, those who reported very heavy drinking showed significant fibrosis acceleration, compared with abstainers (0.25 FIB-4 units/year), while those who reported drinking 8-14 drinks per week showed minimal acceleration of fibrosis progression (0.04 FIB-4 units/year).
“Of interest, despite WIHS research clinicians recommending limiting or avoiding alcohol at semiannual visits, most women who consumed alcohol at WIHS entry continued to have periods of alcohol use in follow-up,” Dr. Kelly and her associates wrote. “This suggests that, despite being enrolled in a long-term observational cohort study, patients did not change their drinking behaviors, limiting any potential bias in changing behaviors due to participation in a research study.”
The investigators acknowledged certain limitations of the study, including the fact that while current alcohol use was captured at study entry and at follow-up, lifetime alcohol exposure was not collected. “Women categorized as abstinent may have had a prior history of alcohol use,” they noted. “Some of these women may represent ‘sick abstainers’ that have ceased alcohol consumption due to the severity of their liver disease. This may explain the finding that entry fibrosis scores were similar between groups, when one would expect heavy users to have higher fibrosis scores, as compared to abstinent patients.”
The study was supported by the National Institute of Allergy and Infectious Diseases, with additional cofunding from the National Institute of Child Health and Human Development; the National Cancer Institute; the National Institute on Drug Abuse; the National Institute on Mental Health; and the University of California, San Francisco, Liver Center Biostatistics Core. The researchers reported having no financial disclosures.
This study highlights the importance of assessing alcohol consumption during routine practice (for example, with the Alcohol Use Disorders Identification Test) to classify patients’ level of use. HIV/HCV-coinfected women should be counseled to minimize alcohol consumption, and any patient with evidence of advanced hepatic fibrosis/cirrhosis should avoid alcohol use, given that the risk of liver complications, such as decompensated cirrhosis and hepatocellular carcinoma, associated with light or moderate use remains unknown in this group. However, some may be unable or unwilling to completely abstain from alcohol because of mental health or substance use disorders. This study suggests that light or moderate alcohol use by coinfected women is not associated with accelerated liver fibrosis progression, as measured by changes in the FIB-4 score.
Future studies should determine the effects of light and moderate alcohol consumption on changes in other noninvasive measures of liver fibrosis, such as transient elastography, and on rates of liver complications, such as hepatic decompensation and hepatocellular carcinoma, in HIV/HCV-coinfected men and women to confirm this study’s findings. Additional research also is needed to evaluate the effects of alcohol use categories on adherence to direct-acting antiviral therapy and HCV treatment response to examine whether these outcomes differ by HIV status and sex. These data will further help inform whether there is a “safe” level of alcohol intake in HIV/HCV patients.
This text is taken from a commentary published online Aug. 16 in Clinical Infectious Diseases (doi: 10.1093/cid/cix720). Vincent Lo Re III, MD, MSCE, is with the Center for Clinical Epidemiology and Biostatistics at the University of Pennsylvania, Philadelphia. He disclosed having received research grant support from the University of Pennsylvania, the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and AstraZeneca.
This study highlights the importance of assessing alcohol consumption during routine practice (for example, with the Alcohol Use Disorders Identification Test) to classify patients’ level of use. HIV/HCV-coinfected women should be counseled to minimize alcohol consumption, and any patient with evidence of advanced hepatic fibrosis/cirrhosis should avoid alcohol use, given that the risk of liver complications, such as decompensated cirrhosis and hepatocellular carcinoma, associated with light or moderate use remains unknown in this group. However, some may be unable or unwilling to completely abstain from alcohol because of mental health or substance use disorders. This study suggests that light or moderate alcohol use by coinfected women is not associated with accelerated liver fibrosis progression, as measured by changes in the FIB-4 score.
Future studies should determine the effects of light and moderate alcohol consumption on changes in other noninvasive measures of liver fibrosis, such as transient elastography, and on rates of liver complications, such as hepatic decompensation and hepatocellular carcinoma, in HIV/HCV-coinfected men and women to confirm this study’s findings. Additional research also is needed to evaluate the effects of alcohol use categories on adherence to direct-acting antiviral therapy and HCV treatment response to examine whether these outcomes differ by HIV status and sex. These data will further help inform whether there is a “safe” level of alcohol intake in HIV/HCV patients.
This text is taken from a commentary published online Aug. 16 in Clinical Infectious Diseases (doi: 10.1093/cid/cix720). Vincent Lo Re III, MD, MSCE, is with the Center for Clinical Epidemiology and Biostatistics at the University of Pennsylvania, Philadelphia. He disclosed having received research grant support from the University of Pennsylvania, the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and AstraZeneca.
This study highlights the importance of assessing alcohol consumption during routine practice (for example, with the Alcohol Use Disorders Identification Test) to classify patients’ level of use. HIV/HCV-coinfected women should be counseled to minimize alcohol consumption, and any patient with evidence of advanced hepatic fibrosis/cirrhosis should avoid alcohol use, given that the risk of liver complications, such as decompensated cirrhosis and hepatocellular carcinoma, associated with light or moderate use remains unknown in this group. However, some may be unable or unwilling to completely abstain from alcohol because of mental health or substance use disorders. This study suggests that light or moderate alcohol use by coinfected women is not associated with accelerated liver fibrosis progression, as measured by changes in the FIB-4 score.
Future studies should determine the effects of light and moderate alcohol consumption on changes in other noninvasive measures of liver fibrosis, such as transient elastography, and on rates of liver complications, such as hepatic decompensation and hepatocellular carcinoma, in HIV/HCV-coinfected men and women to confirm this study’s findings. Additional research also is needed to evaluate the effects of alcohol use categories on adherence to direct-acting antiviral therapy and HCV treatment response to examine whether these outcomes differ by HIV status and sex. These data will further help inform whether there is a “safe” level of alcohol intake in HIV/HCV patients.
This text is taken from a commentary published online Aug. 16 in Clinical Infectious Diseases (doi: 10.1093/cid/cix720). Vincent Lo Re III, MD, MSCE, is with the Center for Clinical Epidemiology and Biostatistics at the University of Pennsylvania, Philadelphia. He disclosed having received research grant support from the University of Pennsylvania, the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and AstraZeneca.
Women coinfected with HIV and hepatitis C virus who drank light and moderate amounts of alcohol did not experience significant progression of liver fibrosis, compared with those who drank heavily, results from a large cohort study found.
“Although heavy alcohol use is clearly detrimental to the health of patients with CHC [chronic hepatitis C], it is unclear whether consumption of smaller quantities of alcohol impact fibrosis progression,” researchers led by Erin M. Kelly, MD, wrote in a study published online Aug. 16 in Clinical Infectious Diseases (doi: 10.1093/cid/cix716). “Many patients with CHC consume alcohol and are unable or unwilling to completely abstain. Some studies have suggested a linear dose-response relationship for fibrosis progression even at lower quantities, while others have not clearly demonstrated a risk for fibrosis progression below 20-50 g of alcohol per day. HIV/HCV [hepatitis C virus]-coinfected patients have accelerated fibrosis progression, compared with HCV monoinfected individuals. Whether regular consumption of small quantities of alcohol further increase the rate of fibrosis progression is unknown.”
At baseline, the mean age of study participants was 40 years, their mean body mass index was 26 kg/m2, 17% had diabetes, and 11% had significant fibrosis, defined as a FIB-4 index of greater than 3.25. Nearly half (46%) reported no alcohol use; 26.8% reported light use; 7.1%, moderate use; and 19.7%, heavy use. The median FIB-4 scores at entry were similar between groups. On multivariable analysis, no significant difference in fibrosis progression was observed in abstainers, compared with those who reported light and moderate alcohol use (0.004 and 0.006 FIB-4 units/year, respectively). On the other hand, those who reported very heavy drinking showed significant fibrosis acceleration, compared with abstainers (0.25 FIB-4 units/year), while those who reported drinking 8-14 drinks per week showed minimal acceleration of fibrosis progression (0.04 FIB-4 units/year).
“Of interest, despite WIHS research clinicians recommending limiting or avoiding alcohol at semiannual visits, most women who consumed alcohol at WIHS entry continued to have periods of alcohol use in follow-up,” Dr. Kelly and her associates wrote. “This suggests that, despite being enrolled in a long-term observational cohort study, patients did not change their drinking behaviors, limiting any potential bias in changing behaviors due to participation in a research study.”
The investigators acknowledged certain limitations of the study, including the fact that while current alcohol use was captured at study entry and at follow-up, lifetime alcohol exposure was not collected. “Women categorized as abstinent may have had a prior history of alcohol use,” they noted. “Some of these women may represent ‘sick abstainers’ that have ceased alcohol consumption due to the severity of their liver disease. This may explain the finding that entry fibrosis scores were similar between groups, when one would expect heavy users to have higher fibrosis scores, as compared to abstinent patients.”
The study was supported by the National Institute of Allergy and Infectious Diseases, with additional cofunding from the National Institute of Child Health and Human Development; the National Cancer Institute; the National Institute on Drug Abuse; the National Institute on Mental Health; and the University of California, San Francisco, Liver Center Biostatistics Core. The researchers reported having no financial disclosures.
Women coinfected with HIV and hepatitis C virus who drank light and moderate amounts of alcohol did not experience significant progression of liver fibrosis, compared with those who drank heavily, results from a large cohort study found.
“Although heavy alcohol use is clearly detrimental to the health of patients with CHC [chronic hepatitis C], it is unclear whether consumption of smaller quantities of alcohol impact fibrosis progression,” researchers led by Erin M. Kelly, MD, wrote in a study published online Aug. 16 in Clinical Infectious Diseases (doi: 10.1093/cid/cix716). “Many patients with CHC consume alcohol and are unable or unwilling to completely abstain. Some studies have suggested a linear dose-response relationship for fibrosis progression even at lower quantities, while others have not clearly demonstrated a risk for fibrosis progression below 20-50 g of alcohol per day. HIV/HCV [hepatitis C virus]-coinfected patients have accelerated fibrosis progression, compared with HCV monoinfected individuals. Whether regular consumption of small quantities of alcohol further increase the rate of fibrosis progression is unknown.”
At baseline, the mean age of study participants was 40 years, their mean body mass index was 26 kg/m2, 17% had diabetes, and 11% had significant fibrosis, defined as a FIB-4 index of greater than 3.25. Nearly half (46%) reported no alcohol use; 26.8% reported light use; 7.1%, moderate use; and 19.7%, heavy use. The median FIB-4 scores at entry were similar between groups. On multivariable analysis, no significant difference in fibrosis progression was observed in abstainers, compared with those who reported light and moderate alcohol use (0.004 and 0.006 FIB-4 units/year, respectively). On the other hand, those who reported very heavy drinking showed significant fibrosis acceleration, compared with abstainers (0.25 FIB-4 units/year), while those who reported drinking 8-14 drinks per week showed minimal acceleration of fibrosis progression (0.04 FIB-4 units/year).
“Of interest, despite WIHS research clinicians recommending limiting or avoiding alcohol at semiannual visits, most women who consumed alcohol at WIHS entry continued to have periods of alcohol use in follow-up,” Dr. Kelly and her associates wrote. “This suggests that, despite being enrolled in a long-term observational cohort study, patients did not change their drinking behaviors, limiting any potential bias in changing behaviors due to participation in a research study.”
The investigators acknowledged certain limitations of the study, including the fact that while current alcohol use was captured at study entry and at follow-up, lifetime alcohol exposure was not collected. “Women categorized as abstinent may have had a prior history of alcohol use,” they noted. “Some of these women may represent ‘sick abstainers’ that have ceased alcohol consumption due to the severity of their liver disease. This may explain the finding that entry fibrosis scores were similar between groups, when one would expect heavy users to have higher fibrosis scores, as compared to abstinent patients.”
The study was supported by the National Institute of Allergy and Infectious Diseases, with additional cofunding from the National Institute of Child Health and Human Development; the National Cancer Institute; the National Institute on Drug Abuse; the National Institute on Mental Health; and the University of California, San Francisco, Liver Center Biostatistics Core. The researchers reported having no financial disclosures.
FROM CLINICAL INFECTIOUS DISEASES
Key clinical point:
Major finding: On multivariable analysis, no significant difference in fibrosis progression was observed in abstainers, compared with those who reported light and moderate alcohol use (0.004 and 0.006 FIB-4 units/year, respectively).
Data source: A cohort study of 686 participants in the multicenter Women’s Interagency HIV Study.
Disclosures: The study was supported by the National Institute of Allergy and Infectious Diseases, with additional cofunding from the National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute on Drug Abuse, the National Institute on Mental Health, and the UCSF Liver Center Biostatistics Core. The researchers reported having no financial disclosures.
Pembrolizumab, nivolumab linked to 3% rate of neurologic events
Three percent of patients developed immune-related adverse neurologic events within 12 months of receiving nivolumab or pembrolizumab, according to the results of a single-center retrospective study.
These syndromes included myopathy, axonal thoracolumbar polyradiculopathy, severe demyelinating length-dependent peripheral neuropathy with axonal loss, a facial diplegic variant of Guillain-Barré syndrome, asymmetric vasculitic neuropathy, cerebellar ataxia with dysarthria, autoimmune retinopathy, bilateral internuclear ophthalmoplegia, and headache, reported Justin C. Kao, MD, of Mayo Clinic, Rochester, Minn., and his coinvestigators. Most patients improved after stopping treatment and starting corticosteroids, but one patient developed necrotizing myopathy and died after withdrawal of ventilator support.
Nivolumab and pembrolizumab, which inhibit the programmed death–1 (PD-1) receptor, are approved for treating metastatic melanoma, non–small-cell lung cancer, renal cell carcinoma, Hodgkin lymphoma, head and neck cancers, and urothelial carcinoma. In response to a surge in reports of neurologic events associated with anti–PD-1 therapy, the investigators searched the Mayo Clinic pharmacy database and identified 347 patients treated with pembrolizumab or nivolumab between 2014 and 2016. Ten patients (2.9%) developed neurologic complications within 12 months of anti–PD-1 exposure, including eight men and two women. The median age was 71 years. None of their neurologic symptoms could be directly attributed to other treatments or to metastatic disease. Most had mild to moderate disability, with modified Rankin Scale (mRS) scores of 2, and symptom severity peaked between 1 day and more than 3 months after starting anti–PD-1 treatment (JAMA Neurol. 2017 Sep 5. doi: 10.1001/jamaneurol.2017.1912).
Stopping anti–PD-1 treatment and starting high-dose corticosteroids led to substantial neurologic improvements (mRS scores, 0-3), except in the case of fatal necrotizing myopathy, the researchers said. That patient, who was receiving pembrolizumab for stage 4 melanoma, developed extraocular, bulbar, and proximal limb girdle weakness that worsened over a period of 3 weeks and did not respond to prednisone (80 mg daily) or to three sessions of plasmapheresis.
If a patient on anti–PD-1 therapy develops neurologic symptoms, clinicians should promptly stop treatment and pursue a full work-up, including electrodiagnostic studies and consideration of muscle or nerve biopsy to clarify underlying pathophysiologic mechanisms, the researchers said. “If the clinical examination demonstrates severe clinical deficits at onset or worsens despite medication discontinuation, additional immune suppressant treatment should be considered,” they said. They recommended prednisone (1 mg/kg) with a taper over a 1-month period. Intravenous immunoglobulin therapy or plasma exchange may be warranted if patients continue to worsen, they said.
The investigators did not report external funding sources. Mr. Kao had no disclosures. Two coinvestigators disclosed ties to the American Association of Neuromuscular & Electrodiagnostic Medicine, the American Academy of Neurology, the Continuum: Lifelong Learning in Neurology, Ionis Pharmaceuticals, Alnylam, and Oxford University Press. The remaining coinvestigators reported having no conflicts of interest.
Neurologic symptoms have been and continue to be one of the most common reasons for admission to a cancer center. Neurotoxic chemotherapy, direct invasion of cancer, and other neurologic complications of treatment contribute to the substantial cross talk between oncologists and neurologists. Over the past 5 years, oncology has witnessed an explosion of new immunotherapeutics that are revolutionizing drug development and patient care in oncology today. In contrast to traditional chemotherapy, which targets rapidly dividing cancer cells and can lead to adverse effects in other organs with rapid cell turnover, immunotherapies target and activate the immune system, potentially leading to a wide range of inflammatory and immune-mediated adverse events, including those in the nervous system.
Only 5 of the 10 patients described by Kao et al. experienced nonneurologic immune-related adverse events, suggesting that neurologic complications may be the only defining symptom of an immune-related reaction. Consultation calls from the cancer center are all too familiar for neurologists, and this pattern appears likely to persist in the era of immunotherapy. The horizon of new checkpoint targets continues to expand, and combination therapies are beginning to emerge. Neurologists and oncologists need to be aware of the important checkpoints ahead in patient care.
Roy E. Strowd III, MD, is with the section on hematology and oncology, department of neurology and internal medicine, Wake Forest University, Winston-Salem, N.C. He reported having no conflicts of interest. These comments are excerpted from his editorial (JAMA Neurol. 2017 Sep 5. doi: 10.1001/jamaneurol.2017.1916).
Neurologic symptoms have been and continue to be one of the most common reasons for admission to a cancer center. Neurotoxic chemotherapy, direct invasion of cancer, and other neurologic complications of treatment contribute to the substantial cross talk between oncologists and neurologists. Over the past 5 years, oncology has witnessed an explosion of new immunotherapeutics that are revolutionizing drug development and patient care in oncology today. In contrast to traditional chemotherapy, which targets rapidly dividing cancer cells and can lead to adverse effects in other organs with rapid cell turnover, immunotherapies target and activate the immune system, potentially leading to a wide range of inflammatory and immune-mediated adverse events, including those in the nervous system.
Only 5 of the 10 patients described by Kao et al. experienced nonneurologic immune-related adverse events, suggesting that neurologic complications may be the only defining symptom of an immune-related reaction. Consultation calls from the cancer center are all too familiar for neurologists, and this pattern appears likely to persist in the era of immunotherapy. The horizon of new checkpoint targets continues to expand, and combination therapies are beginning to emerge. Neurologists and oncologists need to be aware of the important checkpoints ahead in patient care.
Roy E. Strowd III, MD, is with the section on hematology and oncology, department of neurology and internal medicine, Wake Forest University, Winston-Salem, N.C. He reported having no conflicts of interest. These comments are excerpted from his editorial (JAMA Neurol. 2017 Sep 5. doi: 10.1001/jamaneurol.2017.1916).
Neurologic symptoms have been and continue to be one of the most common reasons for admission to a cancer center. Neurotoxic chemotherapy, direct invasion of cancer, and other neurologic complications of treatment contribute to the substantial cross talk between oncologists and neurologists. Over the past 5 years, oncology has witnessed an explosion of new immunotherapeutics that are revolutionizing drug development and patient care in oncology today. In contrast to traditional chemotherapy, which targets rapidly dividing cancer cells and can lead to adverse effects in other organs with rapid cell turnover, immunotherapies target and activate the immune system, potentially leading to a wide range of inflammatory and immune-mediated adverse events, including those in the nervous system.
Only 5 of the 10 patients described by Kao et al. experienced nonneurologic immune-related adverse events, suggesting that neurologic complications may be the only defining symptom of an immune-related reaction. Consultation calls from the cancer center are all too familiar for neurologists, and this pattern appears likely to persist in the era of immunotherapy. The horizon of new checkpoint targets continues to expand, and combination therapies are beginning to emerge. Neurologists and oncologists need to be aware of the important checkpoints ahead in patient care.
Roy E. Strowd III, MD, is with the section on hematology and oncology, department of neurology and internal medicine, Wake Forest University, Winston-Salem, N.C. He reported having no conflicts of interest. These comments are excerpted from his editorial (JAMA Neurol. 2017 Sep 5. doi: 10.1001/jamaneurol.2017.1916).
Three percent of patients developed immune-related adverse neurologic events within 12 months of receiving nivolumab or pembrolizumab, according to the results of a single-center retrospective study.
These syndromes included myopathy, axonal thoracolumbar polyradiculopathy, severe demyelinating length-dependent peripheral neuropathy with axonal loss, a facial diplegic variant of Guillain-Barré syndrome, asymmetric vasculitic neuropathy, cerebellar ataxia with dysarthria, autoimmune retinopathy, bilateral internuclear ophthalmoplegia, and headache, reported Justin C. Kao, MD, of Mayo Clinic, Rochester, Minn., and his coinvestigators. Most patients improved after stopping treatment and starting corticosteroids, but one patient developed necrotizing myopathy and died after withdrawal of ventilator support.
Nivolumab and pembrolizumab, which inhibit the programmed death–1 (PD-1) receptor, are approved for treating metastatic melanoma, non–small-cell lung cancer, renal cell carcinoma, Hodgkin lymphoma, head and neck cancers, and urothelial carcinoma. In response to a surge in reports of neurologic events associated with anti–PD-1 therapy, the investigators searched the Mayo Clinic pharmacy database and identified 347 patients treated with pembrolizumab or nivolumab between 2014 and 2016. Ten patients (2.9%) developed neurologic complications within 12 months of anti–PD-1 exposure, including eight men and two women. The median age was 71 years. None of their neurologic symptoms could be directly attributed to other treatments or to metastatic disease. Most had mild to moderate disability, with modified Rankin Scale (mRS) scores of 2, and symptom severity peaked between 1 day and more than 3 months after starting anti–PD-1 treatment (JAMA Neurol. 2017 Sep 5. doi: 10.1001/jamaneurol.2017.1912).
Stopping anti–PD-1 treatment and starting high-dose corticosteroids led to substantial neurologic improvements (mRS scores, 0-3), except in the case of fatal necrotizing myopathy, the researchers said. That patient, who was receiving pembrolizumab for stage 4 melanoma, developed extraocular, bulbar, and proximal limb girdle weakness that worsened over a period of 3 weeks and did not respond to prednisone (80 mg daily) or to three sessions of plasmapheresis.
If a patient on anti–PD-1 therapy develops neurologic symptoms, clinicians should promptly stop treatment and pursue a full work-up, including electrodiagnostic studies and consideration of muscle or nerve biopsy to clarify underlying pathophysiologic mechanisms, the researchers said. “If the clinical examination demonstrates severe clinical deficits at onset or worsens despite medication discontinuation, additional immune suppressant treatment should be considered,” they said. They recommended prednisone (1 mg/kg) with a taper over a 1-month period. Intravenous immunoglobulin therapy or plasma exchange may be warranted if patients continue to worsen, they said.
The investigators did not report external funding sources. Mr. Kao had no disclosures. Two coinvestigators disclosed ties to the American Association of Neuromuscular & Electrodiagnostic Medicine, the American Academy of Neurology, the Continuum: Lifelong Learning in Neurology, Ionis Pharmaceuticals, Alnylam, and Oxford University Press. The remaining coinvestigators reported having no conflicts of interest.
Three percent of patients developed immune-related adverse neurologic events within 12 months of receiving nivolumab or pembrolizumab, according to the results of a single-center retrospective study.
These syndromes included myopathy, axonal thoracolumbar polyradiculopathy, severe demyelinating length-dependent peripheral neuropathy with axonal loss, a facial diplegic variant of Guillain-Barré syndrome, asymmetric vasculitic neuropathy, cerebellar ataxia with dysarthria, autoimmune retinopathy, bilateral internuclear ophthalmoplegia, and headache, reported Justin C. Kao, MD, of Mayo Clinic, Rochester, Minn., and his coinvestigators. Most patients improved after stopping treatment and starting corticosteroids, but one patient developed necrotizing myopathy and died after withdrawal of ventilator support.
Nivolumab and pembrolizumab, which inhibit the programmed death–1 (PD-1) receptor, are approved for treating metastatic melanoma, non–small-cell lung cancer, renal cell carcinoma, Hodgkin lymphoma, head and neck cancers, and urothelial carcinoma. In response to a surge in reports of neurologic events associated with anti–PD-1 therapy, the investigators searched the Mayo Clinic pharmacy database and identified 347 patients treated with pembrolizumab or nivolumab between 2014 and 2016. Ten patients (2.9%) developed neurologic complications within 12 months of anti–PD-1 exposure, including eight men and two women. The median age was 71 years. None of their neurologic symptoms could be directly attributed to other treatments or to metastatic disease. Most had mild to moderate disability, with modified Rankin Scale (mRS) scores of 2, and symptom severity peaked between 1 day and more than 3 months after starting anti–PD-1 treatment (JAMA Neurol. 2017 Sep 5. doi: 10.1001/jamaneurol.2017.1912).
Stopping anti–PD-1 treatment and starting high-dose corticosteroids led to substantial neurologic improvements (mRS scores, 0-3), except in the case of fatal necrotizing myopathy, the researchers said. That patient, who was receiving pembrolizumab for stage 4 melanoma, developed extraocular, bulbar, and proximal limb girdle weakness that worsened over a period of 3 weeks and did not respond to prednisone (80 mg daily) or to three sessions of plasmapheresis.
If a patient on anti–PD-1 therapy develops neurologic symptoms, clinicians should promptly stop treatment and pursue a full work-up, including electrodiagnostic studies and consideration of muscle or nerve biopsy to clarify underlying pathophysiologic mechanisms, the researchers said. “If the clinical examination demonstrates severe clinical deficits at onset or worsens despite medication discontinuation, additional immune suppressant treatment should be considered,” they said. They recommended prednisone (1 mg/kg) with a taper over a 1-month period. Intravenous immunoglobulin therapy or plasma exchange may be warranted if patients continue to worsen, they said.
The investigators did not report external funding sources. Mr. Kao had no disclosures. Two coinvestigators disclosed ties to the American Association of Neuromuscular & Electrodiagnostic Medicine, the American Academy of Neurology, the Continuum: Lifelong Learning in Neurology, Ionis Pharmaceuticals, Alnylam, and Oxford University Press. The remaining coinvestigators reported having no conflicts of interest.
FROM JAMA NEUROLOGY
Key clinical point: Watch for immune-related adverse effects of nivolumab and pembrolizumab.
Major finding: Ten of 347 patients (2.9%) developed subacute neurologic immune-related adverse events, typically neuromuscular syndromes.
Data source: A single-center, retrospective cohort study of 347 patients who received pembrolizumab or nivolumab for metastatic melanoma or solid tumors.
Disclosures: The investigators did not report external funding sources. Mr. Kao had no disclosures. Two coinvestigators disclosed ties to the American Association of Neuromuscular & Electrodiagnostic Medicine, the American Academy of Neurology, the Continuum: Lifelong Learning in Neurology, Ionis Pharmaceuticals, Alnylam, and Oxford University Press. The remaining coinvestigators reported having no conflicts of interest.
Exenatide improved motor function in Parkinson’s patients with off-medication symptoms
An anti-diabetes drug significantly improved motor function in patients with Parkinson’s disease who had off-medication symptoms despite dopaminergic therapy in a phase 2 trial.
Patients taking exenatide (Byetta), an agonist of the GLP-1 receptor, experienced a mean 2.5-point improvement in the part 3 motor score on the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) over 48 weeks, compared with a 1-point decline in patients taking placebo, Dilan Athauda, MBBS, and his colleagues reported (Lancet. 2017 Aug 3. doi: 10.1016/S0140-6736[17]31585-4).
The mechanism of action is unclear, the investigators noted. Dopamine transporter scanning with [123I]FP-CIT single photon emission CT (DaTscan) revealed a tantalizing hint of neuroprotection, as the rate of decline in dopaminergic neurons seemed to be slightly reduced among those taking the medication. However, it’s also possible that exenatide somehow altered the pharmacokinetics of levodopa and other dopaminergic drugs, making them more effective, Dr. Athauda and his associates said.
Still, the double-blinded study’s positive results are encouraging, and they replicate those of the team’s 2013 open-label trial (J Clin Invest. 2013 Jun 3;123[6]:2730-6), they asserted.
“Whether this drug acts as a novel symptomatic agent, influences compensatory responses or behaviors, or has neuroprotective effects on underlying pathology is unclear, but there is a strong indication that GLP-1 receptor agonists may have a useful role in future treatment of Parkinson’s disease,” the investigators wrote.
The study randomized 62 patients who had Parkinson’s with off-medication motor symptoms to weekly injections of either placebo or 2 mg subcutaneous exenatide for 48 weeks. A 12-week washout period followed. Despite randomization, there were some important baseline differences between the groups. Those taking exenatide were older (62 vs. 58 years) and had a higher score on the part 3 motor score of the MDS-UPDRS, the study’s primary endpoint (32.8 vs. 27.1). Exenatide users were also taking a lower mean dopaminergic drug dose (mean 774 mg vs. 826 mg levodopa equivalent).
Patients were assessed in clinic every 12 weeks, not only for the primary endpoint of dyskinesia off-medication, but for cognition, quality of life, mood, and nonmotor symptoms. All assessments were done in the morning, after at least 8 hours off levodopa or 36 hours off long-acting dopaminergic drugs.
Exenatide’s benefit in off-medication dyskinesias was apparent after the first 12 weeks of treatment, Dr. Athauda and his coauthors noted. The MDS-UPDRS score had decreased from 32.8 to 30.2 in the active group, and increased from 27.1 to 27.6 in the placebo group. Those taking exenatide held steady at that improvement for the entire 48 weeks, ending at 30.3 (2.3 points below baseline). Those taking placebo continued to decline, ending at 28.8 (1.7 points above baseline). The adjusted between-group difference was 4.3 points, in favor of exenatide (P = .0026).
At 60 weeks, after the 12-week washout period, patients who took exenatide were still doing better, reaching an adjusted between-group difference of –3.5 (P-= .0318).
However, off-medication dyskinesia was the only improvement noted in the trial. Exenatide did not affect any secondary endpoints, including any sections of the on-medication MDS-UPDRS.
The investigators noted that, during the 60 weeks, mean levodopa equivalent dosage increased more in the active group than in the placebo group (132 vs. 112 mg). This brought the active group up much closer to the placebo group’s dose than had been observed at baseline (906 vs. 942 mg).
Exenatide was generally well tolerated, with the exception of a mean 2.6-kg weight loss among those taking it. This was likely related to an increased incidence of gastrointestinal side effects. Weight returned to normal during the washout period.
There were three drop-outs, two in the placebo arm because of worsening anxiety and worsening dyskinesia and one in the exenatide arm because of asymptomatic hyperamylasemia.
The investigators also measured dopamine transporter availability via DaTscan to assess exenatide’s potential impact on dopaminergic neurons. Although areas of decreased binding declined in both groups, the exenatide group showed a signal of reduced rate of decline in the right and left putamen.
“However,” the authors noted, “because this signal was detectable only at uncorrected height thresholds of P = .0034 or less, these data would benefit from larger confirmatory studies or studies of patients at an earlier disease stage when the rate of change of DaTscan uptake is greater, making group differences more readily detectable.”
It won’t be easy to discover how exenatide exerts its benefit, the authors said. They pointed to a robust compendium of preclinical data suggesting that the drug reduces inflammation, promotes mitochondrial biogenesis, exerts neurotrophic effects, stimulates neurogenesis, and restores neuronal insulin signaling.
“Whether some or all of these mechanisms contributed to the clinical effects in our study cannot be definitively established, but one or several of these mechanisms could have acted in synergy to promote cell survival, preserve compensatory responses, and prevent maladaptive responses.”
The Michael J. Fox Foundation for Parkinson’s Research funded the study. Dr. Athauda had no financial disclosures but several of his coauthors disclosed relationships with pharmaceutical companies.
[email protected]
On Twitter @alz_gal
The EXENATIDE-PD trial is an exciting peek into a potential new mechanism in treating Parkinson’s, but it must be viewed cautiously.
The baseline between-group differences are concerning, and although the authors tried to adjust for this discrepancy, a confounding effect for differences in concomitant dopaminergic therapy during the trial cannot be excluded.
It is also puzzling that only off-medication dyskinesias improved without any on-medication improvements or other benefits. The 12-week washout period also might have been too short to eliminate potentially long-lasting symptomatic effects of exenatide.
The DaTscan results are not completely reliable in this analysis because it has previously been shown that GLP-1 receptor stimulation in rodents inhibits the ability of cocaine to increase extracellular dopamine concentrations, which is associated with increased DAT surface expression in the forebrain lateral septum. If present in human beings, such a pharmacological mechanism could potentially account for the symptomatic motor effects of exenatide in Parkinson’s disease.
Nevertheless, the MDS-UPDRS part 3 improvements at 12 weeks do suggest that exenatide has symptomatic motor effects. It’s just not clear how the drug exerts those effects. Other potential symptomatic pharmacological mechanisms of exenatide could include improved functioning in surviving dopaminergic neurons or modified pharmacokinetics of dopaminergic treatments.
Whether exenatide acts as a novel symptomatic agent or has neuroprotective effects on the underlying Parkinson’s disease pathology remains unclear, but this study opens up a new therapeutic avenue in treatment of Parkinson’s disease.
Werner Poewe, MD, is professor of neurology and director of the department of neurology at Innsbruck (Austria) Medical University. Klaus Seppi, MD, is assistant professor of neurology there. Both reported a variety of financial relationships with companies that make drugs for Parkinson’s. Their comments are taken from an editorial accompanying the EXENATIDE-PD trial report (Lancet. 2017 Aug 3. doi: 10.1016/S0140-6736[17]32101-3).
The EXENATIDE-PD trial is an exciting peek into a potential new mechanism in treating Parkinson’s, but it must be viewed cautiously.
The baseline between-group differences are concerning, and although the authors tried to adjust for this discrepancy, a confounding effect for differences in concomitant dopaminergic therapy during the trial cannot be excluded.
It is also puzzling that only off-medication dyskinesias improved without any on-medication improvements or other benefits. The 12-week washout period also might have been too short to eliminate potentially long-lasting symptomatic effects of exenatide.
The DaTscan results are not completely reliable in this analysis because it has previously been shown that GLP-1 receptor stimulation in rodents inhibits the ability of cocaine to increase extracellular dopamine concentrations, which is associated with increased DAT surface expression in the forebrain lateral septum. If present in human beings, such a pharmacological mechanism could potentially account for the symptomatic motor effects of exenatide in Parkinson’s disease.
Nevertheless, the MDS-UPDRS part 3 improvements at 12 weeks do suggest that exenatide has symptomatic motor effects. It’s just not clear how the drug exerts those effects. Other potential symptomatic pharmacological mechanisms of exenatide could include improved functioning in surviving dopaminergic neurons or modified pharmacokinetics of dopaminergic treatments.
Whether exenatide acts as a novel symptomatic agent or has neuroprotective effects on the underlying Parkinson’s disease pathology remains unclear, but this study opens up a new therapeutic avenue in treatment of Parkinson’s disease.
Werner Poewe, MD, is professor of neurology and director of the department of neurology at Innsbruck (Austria) Medical University. Klaus Seppi, MD, is assistant professor of neurology there. Both reported a variety of financial relationships with companies that make drugs for Parkinson’s. Their comments are taken from an editorial accompanying the EXENATIDE-PD trial report (Lancet. 2017 Aug 3. doi: 10.1016/S0140-6736[17]32101-3).
The EXENATIDE-PD trial is an exciting peek into a potential new mechanism in treating Parkinson’s, but it must be viewed cautiously.
The baseline between-group differences are concerning, and although the authors tried to adjust for this discrepancy, a confounding effect for differences in concomitant dopaminergic therapy during the trial cannot be excluded.
It is also puzzling that only off-medication dyskinesias improved without any on-medication improvements or other benefits. The 12-week washout period also might have been too short to eliminate potentially long-lasting symptomatic effects of exenatide.
The DaTscan results are not completely reliable in this analysis because it has previously been shown that GLP-1 receptor stimulation in rodents inhibits the ability of cocaine to increase extracellular dopamine concentrations, which is associated with increased DAT surface expression in the forebrain lateral septum. If present in human beings, such a pharmacological mechanism could potentially account for the symptomatic motor effects of exenatide in Parkinson’s disease.
Nevertheless, the MDS-UPDRS part 3 improvements at 12 weeks do suggest that exenatide has symptomatic motor effects. It’s just not clear how the drug exerts those effects. Other potential symptomatic pharmacological mechanisms of exenatide could include improved functioning in surviving dopaminergic neurons or modified pharmacokinetics of dopaminergic treatments.
Whether exenatide acts as a novel symptomatic agent or has neuroprotective effects on the underlying Parkinson’s disease pathology remains unclear, but this study opens up a new therapeutic avenue in treatment of Parkinson’s disease.
Werner Poewe, MD, is professor of neurology and director of the department of neurology at Innsbruck (Austria) Medical University. Klaus Seppi, MD, is assistant professor of neurology there. Both reported a variety of financial relationships with companies that make drugs for Parkinson’s. Their comments are taken from an editorial accompanying the EXENATIDE-PD trial report (Lancet. 2017 Aug 3. doi: 10.1016/S0140-6736[17]32101-3).
An anti-diabetes drug significantly improved motor function in patients with Parkinson’s disease who had off-medication symptoms despite dopaminergic therapy in a phase 2 trial.
Patients taking exenatide (Byetta), an agonist of the GLP-1 receptor, experienced a mean 2.5-point improvement in the part 3 motor score on the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) over 48 weeks, compared with a 1-point decline in patients taking placebo, Dilan Athauda, MBBS, and his colleagues reported (Lancet. 2017 Aug 3. doi: 10.1016/S0140-6736[17]31585-4).
The mechanism of action is unclear, the investigators noted. Dopamine transporter scanning with [123I]FP-CIT single photon emission CT (DaTscan) revealed a tantalizing hint of neuroprotection, as the rate of decline in dopaminergic neurons seemed to be slightly reduced among those taking the medication. However, it’s also possible that exenatide somehow altered the pharmacokinetics of levodopa and other dopaminergic drugs, making them more effective, Dr. Athauda and his associates said.
Still, the double-blinded study’s positive results are encouraging, and they replicate those of the team’s 2013 open-label trial (J Clin Invest. 2013 Jun 3;123[6]:2730-6), they asserted.
“Whether this drug acts as a novel symptomatic agent, influences compensatory responses or behaviors, or has neuroprotective effects on underlying pathology is unclear, but there is a strong indication that GLP-1 receptor agonists may have a useful role in future treatment of Parkinson’s disease,” the investigators wrote.
The study randomized 62 patients who had Parkinson’s with off-medication motor symptoms to weekly injections of either placebo or 2 mg subcutaneous exenatide for 48 weeks. A 12-week washout period followed. Despite randomization, there were some important baseline differences between the groups. Those taking exenatide were older (62 vs. 58 years) and had a higher score on the part 3 motor score of the MDS-UPDRS, the study’s primary endpoint (32.8 vs. 27.1). Exenatide users were also taking a lower mean dopaminergic drug dose (mean 774 mg vs. 826 mg levodopa equivalent).
Patients were assessed in clinic every 12 weeks, not only for the primary endpoint of dyskinesia off-medication, but for cognition, quality of life, mood, and nonmotor symptoms. All assessments were done in the morning, after at least 8 hours off levodopa or 36 hours off long-acting dopaminergic drugs.
Exenatide’s benefit in off-medication dyskinesias was apparent after the first 12 weeks of treatment, Dr. Athauda and his coauthors noted. The MDS-UPDRS score had decreased from 32.8 to 30.2 in the active group, and increased from 27.1 to 27.6 in the placebo group. Those taking exenatide held steady at that improvement for the entire 48 weeks, ending at 30.3 (2.3 points below baseline). Those taking placebo continued to decline, ending at 28.8 (1.7 points above baseline). The adjusted between-group difference was 4.3 points, in favor of exenatide (P = .0026).
At 60 weeks, after the 12-week washout period, patients who took exenatide were still doing better, reaching an adjusted between-group difference of –3.5 (P-= .0318).
However, off-medication dyskinesia was the only improvement noted in the trial. Exenatide did not affect any secondary endpoints, including any sections of the on-medication MDS-UPDRS.
The investigators noted that, during the 60 weeks, mean levodopa equivalent dosage increased more in the active group than in the placebo group (132 vs. 112 mg). This brought the active group up much closer to the placebo group’s dose than had been observed at baseline (906 vs. 942 mg).
Exenatide was generally well tolerated, with the exception of a mean 2.6-kg weight loss among those taking it. This was likely related to an increased incidence of gastrointestinal side effects. Weight returned to normal during the washout period.
There were three drop-outs, two in the placebo arm because of worsening anxiety and worsening dyskinesia and one in the exenatide arm because of asymptomatic hyperamylasemia.
The investigators also measured dopamine transporter availability via DaTscan to assess exenatide’s potential impact on dopaminergic neurons. Although areas of decreased binding declined in both groups, the exenatide group showed a signal of reduced rate of decline in the right and left putamen.
“However,” the authors noted, “because this signal was detectable only at uncorrected height thresholds of P = .0034 or less, these data would benefit from larger confirmatory studies or studies of patients at an earlier disease stage when the rate of change of DaTscan uptake is greater, making group differences more readily detectable.”
It won’t be easy to discover how exenatide exerts its benefit, the authors said. They pointed to a robust compendium of preclinical data suggesting that the drug reduces inflammation, promotes mitochondrial biogenesis, exerts neurotrophic effects, stimulates neurogenesis, and restores neuronal insulin signaling.
“Whether some or all of these mechanisms contributed to the clinical effects in our study cannot be definitively established, but one or several of these mechanisms could have acted in synergy to promote cell survival, preserve compensatory responses, and prevent maladaptive responses.”
The Michael J. Fox Foundation for Parkinson’s Research funded the study. Dr. Athauda had no financial disclosures but several of his coauthors disclosed relationships with pharmaceutical companies.
[email protected]
On Twitter @alz_gal
An anti-diabetes drug significantly improved motor function in patients with Parkinson’s disease who had off-medication symptoms despite dopaminergic therapy in a phase 2 trial.
Patients taking exenatide (Byetta), an agonist of the GLP-1 receptor, experienced a mean 2.5-point improvement in the part 3 motor score on the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) over 48 weeks, compared with a 1-point decline in patients taking placebo, Dilan Athauda, MBBS, and his colleagues reported (Lancet. 2017 Aug 3. doi: 10.1016/S0140-6736[17]31585-4).
The mechanism of action is unclear, the investigators noted. Dopamine transporter scanning with [123I]FP-CIT single photon emission CT (DaTscan) revealed a tantalizing hint of neuroprotection, as the rate of decline in dopaminergic neurons seemed to be slightly reduced among those taking the medication. However, it’s also possible that exenatide somehow altered the pharmacokinetics of levodopa and other dopaminergic drugs, making them more effective, Dr. Athauda and his associates said.
Still, the double-blinded study’s positive results are encouraging, and they replicate those of the team’s 2013 open-label trial (J Clin Invest. 2013 Jun 3;123[6]:2730-6), they asserted.
“Whether this drug acts as a novel symptomatic agent, influences compensatory responses or behaviors, or has neuroprotective effects on underlying pathology is unclear, but there is a strong indication that GLP-1 receptor agonists may have a useful role in future treatment of Parkinson’s disease,” the investigators wrote.
The study randomized 62 patients who had Parkinson’s with off-medication motor symptoms to weekly injections of either placebo or 2 mg subcutaneous exenatide for 48 weeks. A 12-week washout period followed. Despite randomization, there were some important baseline differences between the groups. Those taking exenatide were older (62 vs. 58 years) and had a higher score on the part 3 motor score of the MDS-UPDRS, the study’s primary endpoint (32.8 vs. 27.1). Exenatide users were also taking a lower mean dopaminergic drug dose (mean 774 mg vs. 826 mg levodopa equivalent).
Patients were assessed in clinic every 12 weeks, not only for the primary endpoint of dyskinesia off-medication, but for cognition, quality of life, mood, and nonmotor symptoms. All assessments were done in the morning, after at least 8 hours off levodopa or 36 hours off long-acting dopaminergic drugs.
Exenatide’s benefit in off-medication dyskinesias was apparent after the first 12 weeks of treatment, Dr. Athauda and his coauthors noted. The MDS-UPDRS score had decreased from 32.8 to 30.2 in the active group, and increased from 27.1 to 27.6 in the placebo group. Those taking exenatide held steady at that improvement for the entire 48 weeks, ending at 30.3 (2.3 points below baseline). Those taking placebo continued to decline, ending at 28.8 (1.7 points above baseline). The adjusted between-group difference was 4.3 points, in favor of exenatide (P = .0026).
At 60 weeks, after the 12-week washout period, patients who took exenatide were still doing better, reaching an adjusted between-group difference of –3.5 (P-= .0318).
However, off-medication dyskinesia was the only improvement noted in the trial. Exenatide did not affect any secondary endpoints, including any sections of the on-medication MDS-UPDRS.
The investigators noted that, during the 60 weeks, mean levodopa equivalent dosage increased more in the active group than in the placebo group (132 vs. 112 mg). This brought the active group up much closer to the placebo group’s dose than had been observed at baseline (906 vs. 942 mg).
Exenatide was generally well tolerated, with the exception of a mean 2.6-kg weight loss among those taking it. This was likely related to an increased incidence of gastrointestinal side effects. Weight returned to normal during the washout period.
There were three drop-outs, two in the placebo arm because of worsening anxiety and worsening dyskinesia and one in the exenatide arm because of asymptomatic hyperamylasemia.
The investigators also measured dopamine transporter availability via DaTscan to assess exenatide’s potential impact on dopaminergic neurons. Although areas of decreased binding declined in both groups, the exenatide group showed a signal of reduced rate of decline in the right and left putamen.
“However,” the authors noted, “because this signal was detectable only at uncorrected height thresholds of P = .0034 or less, these data would benefit from larger confirmatory studies or studies of patients at an earlier disease stage when the rate of change of DaTscan uptake is greater, making group differences more readily detectable.”
It won’t be easy to discover how exenatide exerts its benefit, the authors said. They pointed to a robust compendium of preclinical data suggesting that the drug reduces inflammation, promotes mitochondrial biogenesis, exerts neurotrophic effects, stimulates neurogenesis, and restores neuronal insulin signaling.
“Whether some or all of these mechanisms contributed to the clinical effects in our study cannot be definitively established, but one or several of these mechanisms could have acted in synergy to promote cell survival, preserve compensatory responses, and prevent maladaptive responses.”
The Michael J. Fox Foundation for Parkinson’s Research funded the study. Dr. Athauda had no financial disclosures but several of his coauthors disclosed relationships with pharmaceutical companies.
[email protected]
On Twitter @alz_gal
FROM THE LANCET
Key clinical point:
Major finding: After 48 weeks, those taking the drug had a 4.3-point advantage over those taking placebo on the Movement Disorders Society Unified Parkinson’s Disease Rating Scale part 3 motor score.
Data source: The phase 2, double-blind, randomized, placebo-controlled study comprised 62 patients with moderate Parkinson’s.
Disclosures: The Michael J. Fox Foundation for Parkinson’s Research funded the study. Dr. Athauda had no financial disclosures; several of his coauthors disclosed relationships with pharmaceutical companies.
Adoption of robotic-assisted surgery uneven across specialties
Robotic-assisted laparoscopy is on the rise but its spread is uneven across specialties and procedures, findings of a large national study of surgical technology show.
The trend favoring robotic-assisted surgery is especially apparent for urologic, gynecologic, and endocrinologic procedures, according to a study of data drawn from the Healthcare Cost and Utilization Project Nationwide Inpatient Sample (HCUP-NIS) conducted by Yen-Yi Juo, MD, of George Washington University, Washington, and his colleagues (Surg Endosc. 2017 Aug 25. doi: 10.1007/s00464-017-5822-4).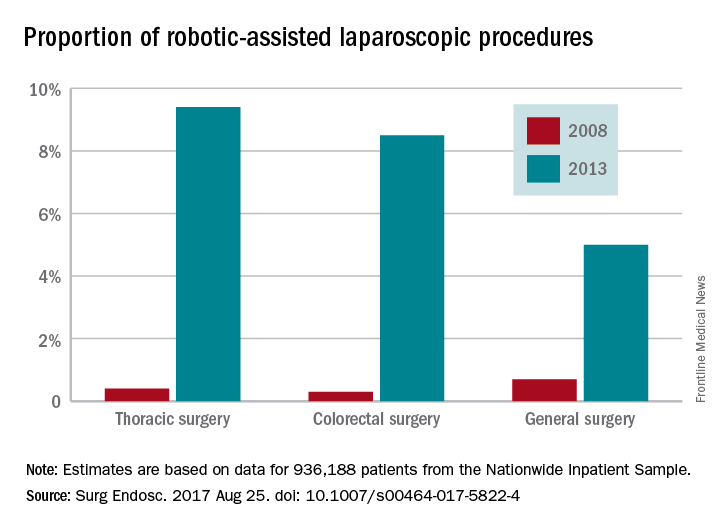
While robotic-assisted surgery is gradually increasing, conventional laparoscopic procedures are declining, the data showed. The case volume of all traditional laparoscopic surgeries decreased by 39.4% between 2009 and 2013 while robotic-assisted laparoscopic procedures increased by 250% over a 6-year period, although the total number of robotic-assisted surgeries is still comparatively small. The study found that the total number of traditional laparoscopic procedures decreased from 956,304 to 737,615 from 2009 to 2013. From mid-2008 to mid-2013, robotic-assisted laparoscopic procedures increased from 17,720 to 33,530.
Patient characteristics such as age, sex, income level, type of insurance, presence of comorbidity, and type of hospital were all significant predictors of whether robotic-assisted surgery would be utilized during a laparoscopic procedure, study findings suggest. Additionally, 5 of the top 10 most common robotic-assisted procedures are performed by urologists.
The investigators noted that there were limitations on the study due to the lack of data on operative indication, disease severity, and postoperative complications in the HCUP-NIS database. In addition, “surgeons are known to preferentially select ‘safer’ patients during the initial adoption of new technology; this may lead to an overestimation of robotic procedure’s clinical benefit in our simple outcome measure.”
The investigators concluded that “although robotic-assisted technology has seen progressive increase in prevalence, its adoption is conspicuously uneven across specialties and procedures. ... The next step in this body of work is to identify specific perceived technical advantages by surgeons working in certain anatomic areas in order to optimize target procedures for the uptake of robotic technology in the future.”
The investigators reported no conflicts of interest.
Robotic-assisted laparoscopy is on the rise but its spread is uneven across specialties and procedures, findings of a large national study of surgical technology show.
The trend favoring robotic-assisted surgery is especially apparent for urologic, gynecologic, and endocrinologic procedures, according to a study of data drawn from the Healthcare Cost and Utilization Project Nationwide Inpatient Sample (HCUP-NIS) conducted by Yen-Yi Juo, MD, of George Washington University, Washington, and his colleagues (Surg Endosc. 2017 Aug 25. doi: 10.1007/s00464-017-5822-4).
While robotic-assisted surgery is gradually increasing, conventional laparoscopic procedures are declining, the data showed. The case volume of all traditional laparoscopic surgeries decreased by 39.4% between 2009 and 2013 while robotic-assisted laparoscopic procedures increased by 250% over a 6-year period, although the total number of robotic-assisted surgeries is still comparatively small. The study found that the total number of traditional laparoscopic procedures decreased from 956,304 to 737,615 from 2009 to 2013. From mid-2008 to mid-2013, robotic-assisted laparoscopic procedures increased from 17,720 to 33,530.
Patient characteristics such as age, sex, income level, type of insurance, presence of comorbidity, and type of hospital were all significant predictors of whether robotic-assisted surgery would be utilized during a laparoscopic procedure, study findings suggest. Additionally, 5 of the top 10 most common robotic-assisted procedures are performed by urologists.
The investigators noted that there were limitations on the study due to the lack of data on operative indication, disease severity, and postoperative complications in the HCUP-NIS database. In addition, “surgeons are known to preferentially select ‘safer’ patients during the initial adoption of new technology; this may lead to an overestimation of robotic procedure’s clinical benefit in our simple outcome measure.”
The investigators concluded that “although robotic-assisted technology has seen progressive increase in prevalence, its adoption is conspicuously uneven across specialties and procedures. ... The next step in this body of work is to identify specific perceived technical advantages by surgeons working in certain anatomic areas in order to optimize target procedures for the uptake of robotic technology in the future.”
The investigators reported no conflicts of interest.
Robotic-assisted laparoscopy is on the rise but its spread is uneven across specialties and procedures, findings of a large national study of surgical technology show.
The trend favoring robotic-assisted surgery is especially apparent for urologic, gynecologic, and endocrinologic procedures, according to a study of data drawn from the Healthcare Cost and Utilization Project Nationwide Inpatient Sample (HCUP-NIS) conducted by Yen-Yi Juo, MD, of George Washington University, Washington, and his colleagues (Surg Endosc. 2017 Aug 25. doi: 10.1007/s00464-017-5822-4).
While robotic-assisted surgery is gradually increasing, conventional laparoscopic procedures are declining, the data showed. The case volume of all traditional laparoscopic surgeries decreased by 39.4% between 2009 and 2013 while robotic-assisted laparoscopic procedures increased by 250% over a 6-year period, although the total number of robotic-assisted surgeries is still comparatively small. The study found that the total number of traditional laparoscopic procedures decreased from 956,304 to 737,615 from 2009 to 2013. From mid-2008 to mid-2013, robotic-assisted laparoscopic procedures increased from 17,720 to 33,530.
Patient characteristics such as age, sex, income level, type of insurance, presence of comorbidity, and type of hospital were all significant predictors of whether robotic-assisted surgery would be utilized during a laparoscopic procedure, study findings suggest. Additionally, 5 of the top 10 most common robotic-assisted procedures are performed by urologists.
The investigators noted that there were limitations on the study due to the lack of data on operative indication, disease severity, and postoperative complications in the HCUP-NIS database. In addition, “surgeons are known to preferentially select ‘safer’ patients during the initial adoption of new technology; this may lead to an overestimation of robotic procedure’s clinical benefit in our simple outcome measure.”
The investigators concluded that “although robotic-assisted technology has seen progressive increase in prevalence, its adoption is conspicuously uneven across specialties and procedures. ... The next step in this body of work is to identify specific perceived technical advantages by surgeons working in certain anatomic areas in order to optimize target procedures for the uptake of robotic technology in the future.”
The investigators reported no conflicts of interest.
FROM SURGICAL ENDOSCOPY
Key clinical point:
Major finding: Procedures performed with robotic assistance increased from 6.8% to 17% over a 5-year period.
Data source: Analysis of data from 936,188 patients from the Healthcare Cost and Utilization Project Nationwide Inpatient Sample database.
Disclosures: Investigators reported no conflicts of interest.
In California, medical vaccine exemptions tripled after personal belief exemption ban
The proportion of California kindergartners with medical exemptions from vaccination tripled after the state eliminated personal belief exemptions, a study has shown.
Furthermore, California counties that previously had the highest rates of personal belief exemptions now have the highest rates of medical exemptions, Paul L. Delamater, PhD, of the University of North Carolina at Chapel Hill, and his associates reported in a research letter in JAMA. Such trends undermine California Senate Bill 277, will “limit [the law’s] long-term benefits, if sustained,” and could lead to outbreaks of vaccine-preventable diseases in the near future, the researchers warned.
Beginning in fall 2016, SB 277 prohibited unvaccinated kindergartners from matriculating at a public or private school in California without a medical exemption from vaccination, having eliminated the personal belief exemption from the state’s school-entry vaccine requirements. But the new law also gave physicians broader discretion to grant the medical exemptions, prompting concerns that “vaccine-hesitant” parents might successfully obtain medical exemptions in lieu of personal belief exemptions. To explore that possibility, the researchers analyzed data from the California state health department on kindergarten enrollment, vaccination, and vaccination exemptions. These data covered about 95% of California kindergartners, Dr. Delamater and his associates noted (JAMA. 2017;318[9]:863-4).
At the county level, rates of medical exemptions strongly correlated with historic rates of personal belief exemptions (P less than .001). Thus, counties with the highest historic rates of personal belief exemptions had the largest increases in rates of medical exemptions in 2016, that is, a change of between –1.00% and 3.38% of kindergartners. Furthermore, vaccination rates among all elementary school-aged children in California are even lower because SB 277 permitted children who previously entered kindergarten with personal belief exemptions to continue attending school without receiving vaccines until seventh grade. “Because the largest increases in [medical exemption] percentage occurred in regions with high past–[personal belief exemption] use, portions of California may remain susceptible to vaccine-preventable disease outbreaks in the near future,” Dr. Delamater and his associates concluded.
The investigators reported having no conflicts of interest.
The proportion of California kindergartners with medical exemptions from vaccination tripled after the state eliminated personal belief exemptions, a study has shown.
Furthermore, California counties that previously had the highest rates of personal belief exemptions now have the highest rates of medical exemptions, Paul L. Delamater, PhD, of the University of North Carolina at Chapel Hill, and his associates reported in a research letter in JAMA. Such trends undermine California Senate Bill 277, will “limit [the law’s] long-term benefits, if sustained,” and could lead to outbreaks of vaccine-preventable diseases in the near future, the researchers warned.
Beginning in fall 2016, SB 277 prohibited unvaccinated kindergartners from matriculating at a public or private school in California without a medical exemption from vaccination, having eliminated the personal belief exemption from the state’s school-entry vaccine requirements. But the new law also gave physicians broader discretion to grant the medical exemptions, prompting concerns that “vaccine-hesitant” parents might successfully obtain medical exemptions in lieu of personal belief exemptions. To explore that possibility, the researchers analyzed data from the California state health department on kindergarten enrollment, vaccination, and vaccination exemptions. These data covered about 95% of California kindergartners, Dr. Delamater and his associates noted (JAMA. 2017;318[9]:863-4).
At the county level, rates of medical exemptions strongly correlated with historic rates of personal belief exemptions (P less than .001). Thus, counties with the highest historic rates of personal belief exemptions had the largest increases in rates of medical exemptions in 2016, that is, a change of between –1.00% and 3.38% of kindergartners. Furthermore, vaccination rates among all elementary school-aged children in California are even lower because SB 277 permitted children who previously entered kindergarten with personal belief exemptions to continue attending school without receiving vaccines until seventh grade. “Because the largest increases in [medical exemption] percentage occurred in regions with high past–[personal belief exemption] use, portions of California may remain susceptible to vaccine-preventable disease outbreaks in the near future,” Dr. Delamater and his associates concluded.
The investigators reported having no conflicts of interest.
The proportion of California kindergartners with medical exemptions from vaccination tripled after the state eliminated personal belief exemptions, a study has shown.
Furthermore, California counties that previously had the highest rates of personal belief exemptions now have the highest rates of medical exemptions, Paul L. Delamater, PhD, of the University of North Carolina at Chapel Hill, and his associates reported in a research letter in JAMA. Such trends undermine California Senate Bill 277, will “limit [the law’s] long-term benefits, if sustained,” and could lead to outbreaks of vaccine-preventable diseases in the near future, the researchers warned.
Beginning in fall 2016, SB 277 prohibited unvaccinated kindergartners from matriculating at a public or private school in California without a medical exemption from vaccination, having eliminated the personal belief exemption from the state’s school-entry vaccine requirements. But the new law also gave physicians broader discretion to grant the medical exemptions, prompting concerns that “vaccine-hesitant” parents might successfully obtain medical exemptions in lieu of personal belief exemptions. To explore that possibility, the researchers analyzed data from the California state health department on kindergarten enrollment, vaccination, and vaccination exemptions. These data covered about 95% of California kindergartners, Dr. Delamater and his associates noted (JAMA. 2017;318[9]:863-4).
At the county level, rates of medical exemptions strongly correlated with historic rates of personal belief exemptions (P less than .001). Thus, counties with the highest historic rates of personal belief exemptions had the largest increases in rates of medical exemptions in 2016, that is, a change of between –1.00% and 3.38% of kindergartners. Furthermore, vaccination rates among all elementary school-aged children in California are even lower because SB 277 permitted children who previously entered kindergarten with personal belief exemptions to continue attending school without receiving vaccines until seventh grade. “Because the largest increases in [medical exemption] percentage occurred in regions with high past–[personal belief exemption] use, portions of California may remain susceptible to vaccine-preventable disease outbreaks in the near future,” Dr. Delamater and his associates concluded.
The investigators reported having no conflicts of interest.
FROM JAMA
Key clinical point:
Major finding: In 2016, 0.51% of California kindergartners had medical exemptions, a threefold rise from 2015.
Data source: An analysis of reportable state health department data from 2001 to 2016.
Disclosures: The investigators reported having no conflicts of interest.
The Disease for Which There Is No Cure and Not Enough Conversation
If I simply let the title of this column stand alone, I suspect most readers of Federal Practitioner would fill in the blank with diseases, such as cancer, HIV, or even devastating genetic conditions, just as I would if presented with the statement without explication.
I read the sentence several weeks ago on a website for caregivers of patients with dementia while browsing for quite a different purpose, and it has haunted me ever since. As a consultation psychiatrist who has spent my career as a VA hospitalist, I am well aware of the sad reality of dementia, but against the backdrop of the aging veteran population, the poignancy of the human tragedy overwhelmed me.
Almost every day on the medical and surgical wards of the VA hospital where I have worked for nearly 2 decades, I see an aging veteran population. There are days when the average age of inpatients is pushing 70 years, and there are many patients in their 80s and 90s. The statistics show that my facility is by no means unique in the VA. Data from the American Community Survey Profile of veterans in 2015 indicate that the median age of veterans is 64 years whereas that of nonveterans is 41.1 The survey emphasized that this age factor has a rippling effect on many other demographic parameters, such as disability, income, and employment, all, in turn, impact the epidemiology of health and illness.1
It is not just age that increases the likelihood that a veteran will develop dementia: Research has identified several aspects of military service that raise the risk of being diagnosed with major neurocognitive disorder, the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition designation for dementia. Many families, patients, and even a few health care professionals may not realize that major neurocognitive disorder is the new neuropsychiatric term for dementia.
Also, many health care professionals do not realize that dementia is the sixth leading cause of death in the U.S.2 Traumatic brain injury, posttraumatic stress disorder, and depression are identified as potential contributors to a higher incidence of dementia in service men and women often with onset at an earlier age.3 Given the prevalence of these comorbidities in persons who were in the military, the VA and DoD will face the medical and psychosocial challenges of providing not only clinical treatment, but also a range of social services for military personnel and veterans. Indeed, federal institutions like the GRECC (Geriatric Research Education and Clinical Center) already are engaged in cutting edge research, delivering high-quality medical treatment, and specialized geriatric and dementia care education and support.
Despite these impressive efforts, too often families ask me 2 crucial questions when a patient is already at a moderate or severe stage of the disease: Is there a cure, and will they get better with or without treatment? This lack of knowledge and understanding is by no means confined to federal health care.
A 2015 report from the Alzheimer’s Association found that 45% of patients with Alzheimer disease or their caregivers were not told about the diagnosis by the doctor.2 Doctors reported that they were more likely to have informed the family of a cancer diagnosis at least in part because they felt there were treatments available and in some cases a cure.
Families ask these questions of me and other health care professionals in the hope of finding guidance. Often the veteran has been hospitalized after behavioral disturbances or wandering have made it impossible to care for the loved elder at home. The family is faced with a double blow: learning the patient has an incurable terminal disease and having to make the decision to place a grandmother or father in a nursing facility. Granted this woeful decision may have to be made even when the family has been fully informed at the time of diagnosis, but it is more distressing when the decision is needed immediately based on safety.
Husbands and wives of 50 years or more and adult children, graying themselves, often ask the second question about improvement. Although treatments exist that can help relieve symptoms and slow progression temporarily, the inexorable and tragic course of the wiping away of memory cannot be reversed or halted.
Not surprisingly, practitioners avoid telling patients and families about a dementia diagnosis because those conversations are painful and difficult. However, the news is much less agonizing to hear when there is time to enjoy the good days that remain and to make arrangements for finances and families. For these important reasons, VA emphasizes shared decision making as the cornerstone of geriatric care. Yet there can be no shared decisions without the compassionate and truthful telling about the diagnosis and the prognosis.
1. U.S. Department of Veterans Affairs National Ce- nter for Veterans Analytics and Statistics. Profile of veterans: 2015 data from the American Community Survey. https://www.va.gov/vetdata/docs/Specia lReports/Profile_of_Veterans_2015.pdf. Published March 2017. Accessed August 22, 2017.
2. Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015;11(3):332-384.
3. Weiner MW, Friedl KE, Pacifico A, et al. Military risk factors for Alzheimer’s disease. Alzheimers Dement. 2013;9(4):445-451.
If I simply let the title of this column stand alone, I suspect most readers of Federal Practitioner would fill in the blank with diseases, such as cancer, HIV, or even devastating genetic conditions, just as I would if presented with the statement without explication.
I read the sentence several weeks ago on a website for caregivers of patients with dementia while browsing for quite a different purpose, and it has haunted me ever since. As a consultation psychiatrist who has spent my career as a VA hospitalist, I am well aware of the sad reality of dementia, but against the backdrop of the aging veteran population, the poignancy of the human tragedy overwhelmed me.
Almost every day on the medical and surgical wards of the VA hospital where I have worked for nearly 2 decades, I see an aging veteran population. There are days when the average age of inpatients is pushing 70 years, and there are many patients in their 80s and 90s. The statistics show that my facility is by no means unique in the VA. Data from the American Community Survey Profile of veterans in 2015 indicate that the median age of veterans is 64 years whereas that of nonveterans is 41.1 The survey emphasized that this age factor has a rippling effect on many other demographic parameters, such as disability, income, and employment, all, in turn, impact the epidemiology of health and illness.1
It is not just age that increases the likelihood that a veteran will develop dementia: Research has identified several aspects of military service that raise the risk of being diagnosed with major neurocognitive disorder, the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition designation for dementia. Many families, patients, and even a few health care professionals may not realize that major neurocognitive disorder is the new neuropsychiatric term for dementia.
Also, many health care professionals do not realize that dementia is the sixth leading cause of death in the U.S.2 Traumatic brain injury, posttraumatic stress disorder, and depression are identified as potential contributors to a higher incidence of dementia in service men and women often with onset at an earlier age.3 Given the prevalence of these comorbidities in persons who were in the military, the VA and DoD will face the medical and psychosocial challenges of providing not only clinical treatment, but also a range of social services for military personnel and veterans. Indeed, federal institutions like the GRECC (Geriatric Research Education and Clinical Center) already are engaged in cutting edge research, delivering high-quality medical treatment, and specialized geriatric and dementia care education and support.
Despite these impressive efforts, too often families ask me 2 crucial questions when a patient is already at a moderate or severe stage of the disease: Is there a cure, and will they get better with or without treatment? This lack of knowledge and understanding is by no means confined to federal health care.
A 2015 report from the Alzheimer’s Association found that 45% of patients with Alzheimer disease or their caregivers were not told about the diagnosis by the doctor.2 Doctors reported that they were more likely to have informed the family of a cancer diagnosis at least in part because they felt there were treatments available and in some cases a cure.
Families ask these questions of me and other health care professionals in the hope of finding guidance. Often the veteran has been hospitalized after behavioral disturbances or wandering have made it impossible to care for the loved elder at home. The family is faced with a double blow: learning the patient has an incurable terminal disease and having to make the decision to place a grandmother or father in a nursing facility. Granted this woeful decision may have to be made even when the family has been fully informed at the time of diagnosis, but it is more distressing when the decision is needed immediately based on safety.
Husbands and wives of 50 years or more and adult children, graying themselves, often ask the second question about improvement. Although treatments exist that can help relieve symptoms and slow progression temporarily, the inexorable and tragic course of the wiping away of memory cannot be reversed or halted.
Not surprisingly, practitioners avoid telling patients and families about a dementia diagnosis because those conversations are painful and difficult. However, the news is much less agonizing to hear when there is time to enjoy the good days that remain and to make arrangements for finances and families. For these important reasons, VA emphasizes shared decision making as the cornerstone of geriatric care. Yet there can be no shared decisions without the compassionate and truthful telling about the diagnosis and the prognosis.
If I simply let the title of this column stand alone, I suspect most readers of Federal Practitioner would fill in the blank with diseases, such as cancer, HIV, or even devastating genetic conditions, just as I would if presented with the statement without explication.
I read the sentence several weeks ago on a website for caregivers of patients with dementia while browsing for quite a different purpose, and it has haunted me ever since. As a consultation psychiatrist who has spent my career as a VA hospitalist, I am well aware of the sad reality of dementia, but against the backdrop of the aging veteran population, the poignancy of the human tragedy overwhelmed me.
Almost every day on the medical and surgical wards of the VA hospital where I have worked for nearly 2 decades, I see an aging veteran population. There are days when the average age of inpatients is pushing 70 years, and there are many patients in their 80s and 90s. The statistics show that my facility is by no means unique in the VA. Data from the American Community Survey Profile of veterans in 2015 indicate that the median age of veterans is 64 years whereas that of nonveterans is 41.1 The survey emphasized that this age factor has a rippling effect on many other demographic parameters, such as disability, income, and employment, all, in turn, impact the epidemiology of health and illness.1
It is not just age that increases the likelihood that a veteran will develop dementia: Research has identified several aspects of military service that raise the risk of being diagnosed with major neurocognitive disorder, the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition designation for dementia. Many families, patients, and even a few health care professionals may not realize that major neurocognitive disorder is the new neuropsychiatric term for dementia.
Also, many health care professionals do not realize that dementia is the sixth leading cause of death in the U.S.2 Traumatic brain injury, posttraumatic stress disorder, and depression are identified as potential contributors to a higher incidence of dementia in service men and women often with onset at an earlier age.3 Given the prevalence of these comorbidities in persons who were in the military, the VA and DoD will face the medical and psychosocial challenges of providing not only clinical treatment, but also a range of social services for military personnel and veterans. Indeed, federal institutions like the GRECC (Geriatric Research Education and Clinical Center) already are engaged in cutting edge research, delivering high-quality medical treatment, and specialized geriatric and dementia care education and support.
Despite these impressive efforts, too often families ask me 2 crucial questions when a patient is already at a moderate or severe stage of the disease: Is there a cure, and will they get better with or without treatment? This lack of knowledge and understanding is by no means confined to federal health care.
A 2015 report from the Alzheimer’s Association found that 45% of patients with Alzheimer disease or their caregivers were not told about the diagnosis by the doctor.2 Doctors reported that they were more likely to have informed the family of a cancer diagnosis at least in part because they felt there were treatments available and in some cases a cure.
Families ask these questions of me and other health care professionals in the hope of finding guidance. Often the veteran has been hospitalized after behavioral disturbances or wandering have made it impossible to care for the loved elder at home. The family is faced with a double blow: learning the patient has an incurable terminal disease and having to make the decision to place a grandmother or father in a nursing facility. Granted this woeful decision may have to be made even when the family has been fully informed at the time of diagnosis, but it is more distressing when the decision is needed immediately based on safety.
Husbands and wives of 50 years or more and adult children, graying themselves, often ask the second question about improvement. Although treatments exist that can help relieve symptoms and slow progression temporarily, the inexorable and tragic course of the wiping away of memory cannot be reversed or halted.
Not surprisingly, practitioners avoid telling patients and families about a dementia diagnosis because those conversations are painful and difficult. However, the news is much less agonizing to hear when there is time to enjoy the good days that remain and to make arrangements for finances and families. For these important reasons, VA emphasizes shared decision making as the cornerstone of geriatric care. Yet there can be no shared decisions without the compassionate and truthful telling about the diagnosis and the prognosis.
1. U.S. Department of Veterans Affairs National Ce- nter for Veterans Analytics and Statistics. Profile of veterans: 2015 data from the American Community Survey. https://www.va.gov/vetdata/docs/Specia lReports/Profile_of_Veterans_2015.pdf. Published March 2017. Accessed August 22, 2017.
2. Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015;11(3):332-384.
3. Weiner MW, Friedl KE, Pacifico A, et al. Military risk factors for Alzheimer’s disease. Alzheimers Dement. 2013;9(4):445-451.
1. U.S. Department of Veterans Affairs National Ce- nter for Veterans Analytics and Statistics. Profile of veterans: 2015 data from the American Community Survey. https://www.va.gov/vetdata/docs/Specia lReports/Profile_of_Veterans_2015.pdf. Published March 2017. Accessed August 22, 2017.
2. Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015;11(3):332-384.
3. Weiner MW, Friedl KE, Pacifico A, et al. Military risk factors for Alzheimer’s disease. Alzheimers Dement. 2013;9(4):445-451.