User login
E-health app helps weight loss, QOL for African American breast cancer survivors
MONTREAL – African American breast cancer survivors who participated in fitness tracking and an online support program saw small but significant reductions in weight and improvement in quality of life, according to a new study.
Further, patients who reported a low baseline quality of life (QOL) achieved as much or more weight loss as did those whose QOL was initially high, said Jeanne Ferrante, MD, MPH, professor of family medicine and community health at Robert Wood Johnson Medical School, New Brunswick, N.J.
Overall, but not cancer-related, QOL improved during the 6 months of the study as well.
“Low quality of life at baseline was not a barrier to weight loss, and there’s the potential for weight loss to improve quality of life” in this group of cancer survivors, Dr. Ferrante said at the annual meeting of the North American Primary Care Research Group.
Although weight loss is known to improve functional status and QOL, few studies have examined these issues in African American breast cancer survivors, who may have more comorbidities and a greater risk for obesity compared with the general population, said Dr. Ferrante.
Dr. Ferrante and her coinvestigators hypothesized that QOL would be a predictor of weight loss, and that weight loss, in turn, would have a positive impact on QOL. They conducted a secondary data analysis of a trial of participants using a physical activity monitor alone (in this study, a Fitbit), compared with using the wrist-worn activity monitor together with an Internet program, SparkPeople, designed to provide information and support for increased activity and weight loss.
Eligible participants (n = 61) were African American women who had completed treatment for early stage (0-III) breast cancer, were aged 21-75 (mean 62) years, and had a body mass index of at least 25 kg/m2 (mean, 37; range 26-52). They had to be English speaking, and have Internet and smartphone access. Half the number of participants were retired, half were college graduates, and about a third were married.
One-third of the women reported that they had five or more chronic conditions at enrollment. The mean waist circumference at baseline was 45 inches, and the mean weight was 216 pounds. Patients who had bariatric surgery, had recently lost at least 5% of their body weight, or had limitations to exercise participation or other serious medical or psychiatric conditions were excluded.
To assess QOL, the investigators used the Quality of Life in Adult Cancer Survivors (Q-LACS) scale, which measures both generic and cancer-specific quality of life.
The women in the study also reported how many days out of the past 30 days their mental and their physical health was “not good.”
At baseline, the mean QOL was 108, generic quality of life was 70, and cancer-specific quality of life was 39; lower numbers are better on the scale. Patients reported that their mental health had not been good for 9 of the past 30 days, on average, and that their physical health had not been good for a mean of 6 of the past 30 days.
After 6 months (but not at 3 months), the mean improvement for overall QOL on the Q-LACS scale was –7 (P = .054). Generic QOL improved significantly at both 3 and 6 months (P = .051 and P = .017, respectively), but cancer-specific QOL did not change significantly.
The women saw no significant change over the 6 months in the number of “not good” mental and physical health days.
Waist circumference reduction was about a half inch at 3 months (–0.45 inches, not significant), with a drop at 6 months of 0.91 inches from baseline that met criteria for statistical significance. (P = .013).
The study’s limitations included its small sample size and relatively short duration, said Dr. Ferrante; however, the study continued for 12 months and those data are being analyzed now. Some bias may have been introduced by the need for Internet connection and a smartphone as well, she said.
The investigators are now piloting use of a premium version of the SparkPeople app that offers more customization and interaction with participants.
Dr. Ferrante reported no conflicts of interest.
[email protected]
On Twitter @karioakes
MONTREAL – African American breast cancer survivors who participated in fitness tracking and an online support program saw small but significant reductions in weight and improvement in quality of life, according to a new study.
Further, patients who reported a low baseline quality of life (QOL) achieved as much or more weight loss as did those whose QOL was initially high, said Jeanne Ferrante, MD, MPH, professor of family medicine and community health at Robert Wood Johnson Medical School, New Brunswick, N.J.
Overall, but not cancer-related, QOL improved during the 6 months of the study as well.
“Low quality of life at baseline was not a barrier to weight loss, and there’s the potential for weight loss to improve quality of life” in this group of cancer survivors, Dr. Ferrante said at the annual meeting of the North American Primary Care Research Group.
Although weight loss is known to improve functional status and QOL, few studies have examined these issues in African American breast cancer survivors, who may have more comorbidities and a greater risk for obesity compared with the general population, said Dr. Ferrante.
Dr. Ferrante and her coinvestigators hypothesized that QOL would be a predictor of weight loss, and that weight loss, in turn, would have a positive impact on QOL. They conducted a secondary data analysis of a trial of participants using a physical activity monitor alone (in this study, a Fitbit), compared with using the wrist-worn activity monitor together with an Internet program, SparkPeople, designed to provide information and support for increased activity and weight loss.
Eligible participants (n = 61) were African American women who had completed treatment for early stage (0-III) breast cancer, were aged 21-75 (mean 62) years, and had a body mass index of at least 25 kg/m2 (mean, 37; range 26-52). They had to be English speaking, and have Internet and smartphone access. Half the number of participants were retired, half were college graduates, and about a third were married.
One-third of the women reported that they had five or more chronic conditions at enrollment. The mean waist circumference at baseline was 45 inches, and the mean weight was 216 pounds. Patients who had bariatric surgery, had recently lost at least 5% of their body weight, or had limitations to exercise participation or other serious medical or psychiatric conditions were excluded.
To assess QOL, the investigators used the Quality of Life in Adult Cancer Survivors (Q-LACS) scale, which measures both generic and cancer-specific quality of life.
The women in the study also reported how many days out of the past 30 days their mental and their physical health was “not good.”
At baseline, the mean QOL was 108, generic quality of life was 70, and cancer-specific quality of life was 39; lower numbers are better on the scale. Patients reported that their mental health had not been good for 9 of the past 30 days, on average, and that their physical health had not been good for a mean of 6 of the past 30 days.
After 6 months (but not at 3 months), the mean improvement for overall QOL on the Q-LACS scale was –7 (P = .054). Generic QOL improved significantly at both 3 and 6 months (P = .051 and P = .017, respectively), but cancer-specific QOL did not change significantly.
The women saw no significant change over the 6 months in the number of “not good” mental and physical health days.
Waist circumference reduction was about a half inch at 3 months (–0.45 inches, not significant), with a drop at 6 months of 0.91 inches from baseline that met criteria for statistical significance. (P = .013).
The study’s limitations included its small sample size and relatively short duration, said Dr. Ferrante; however, the study continued for 12 months and those data are being analyzed now. Some bias may have been introduced by the need for Internet connection and a smartphone as well, she said.
The investigators are now piloting use of a premium version of the SparkPeople app that offers more customization and interaction with participants.
Dr. Ferrante reported no conflicts of interest.
[email protected]
On Twitter @karioakes
MONTREAL – African American breast cancer survivors who participated in fitness tracking and an online support program saw small but significant reductions in weight and improvement in quality of life, according to a new study.
Further, patients who reported a low baseline quality of life (QOL) achieved as much or more weight loss as did those whose QOL was initially high, said Jeanne Ferrante, MD, MPH, professor of family medicine and community health at Robert Wood Johnson Medical School, New Brunswick, N.J.
Overall, but not cancer-related, QOL improved during the 6 months of the study as well.
“Low quality of life at baseline was not a barrier to weight loss, and there’s the potential for weight loss to improve quality of life” in this group of cancer survivors, Dr. Ferrante said at the annual meeting of the North American Primary Care Research Group.
Although weight loss is known to improve functional status and QOL, few studies have examined these issues in African American breast cancer survivors, who may have more comorbidities and a greater risk for obesity compared with the general population, said Dr. Ferrante.
Dr. Ferrante and her coinvestigators hypothesized that QOL would be a predictor of weight loss, and that weight loss, in turn, would have a positive impact on QOL. They conducted a secondary data analysis of a trial of participants using a physical activity monitor alone (in this study, a Fitbit), compared with using the wrist-worn activity monitor together with an Internet program, SparkPeople, designed to provide information and support for increased activity and weight loss.
Eligible participants (n = 61) were African American women who had completed treatment for early stage (0-III) breast cancer, were aged 21-75 (mean 62) years, and had a body mass index of at least 25 kg/m2 (mean, 37; range 26-52). They had to be English speaking, and have Internet and smartphone access. Half the number of participants were retired, half were college graduates, and about a third were married.
One-third of the women reported that they had five or more chronic conditions at enrollment. The mean waist circumference at baseline was 45 inches, and the mean weight was 216 pounds. Patients who had bariatric surgery, had recently lost at least 5% of their body weight, or had limitations to exercise participation or other serious medical or psychiatric conditions were excluded.
To assess QOL, the investigators used the Quality of Life in Adult Cancer Survivors (Q-LACS) scale, which measures both generic and cancer-specific quality of life.
The women in the study also reported how many days out of the past 30 days their mental and their physical health was “not good.”
At baseline, the mean QOL was 108, generic quality of life was 70, and cancer-specific quality of life was 39; lower numbers are better on the scale. Patients reported that their mental health had not been good for 9 of the past 30 days, on average, and that their physical health had not been good for a mean of 6 of the past 30 days.
After 6 months (but not at 3 months), the mean improvement for overall QOL on the Q-LACS scale was –7 (P = .054). Generic QOL improved significantly at both 3 and 6 months (P = .051 and P = .017, respectively), but cancer-specific QOL did not change significantly.
The women saw no significant change over the 6 months in the number of “not good” mental and physical health days.
Waist circumference reduction was about a half inch at 3 months (–0.45 inches, not significant), with a drop at 6 months of 0.91 inches from baseline that met criteria for statistical significance. (P = .013).
The study’s limitations included its small sample size and relatively short duration, said Dr. Ferrante; however, the study continued for 12 months and those data are being analyzed now. Some bias may have been introduced by the need for Internet connection and a smartphone as well, she said.
The investigators are now piloting use of a premium version of the SparkPeople app that offers more customization and interaction with participants.
Dr. Ferrante reported no conflicts of interest.
[email protected]
On Twitter @karioakes
AT NAPCRG 2017
Key clinical point:
Major finding: Participants lost a mean 4.79 pounds from baseline (mean 2.14%, P less than .001).
Data source: Subanalysis from study of 61 African American survivors of early stage breast cancer, with BMI of 25 or higher.
Disclosures: Dr. Ferrante reported no conflicts of interest.
Lichen Planus Pemphigoides Treated With Ustekinumab
Case Report
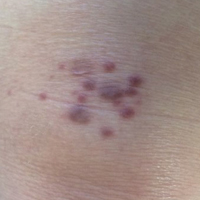
A 71-year-old woman presented with pink to violaceous, flat-topped, polygonal papules consistent with lichen planus (LP) on the volar wrists, extensor elbows, and bilateral lower legs of 3 years’ duration. She also had erythematous, violaceous, infiltrated plaques with microvesiculation on the bilateral thighs of several months’ duration (Figure 1). She reported pruritus, burning, and discomfort. Her medical history included type 2 diabetes mellitus, hypertension, and asthma with no history of skin rashes. A complete physical examination was performed. Age-appropriate screening for malignancy was negative. Hepatitis B and C antibody serologies were negative. Her medications at the time included risedronate and atenolol, which she had been taking for several years.
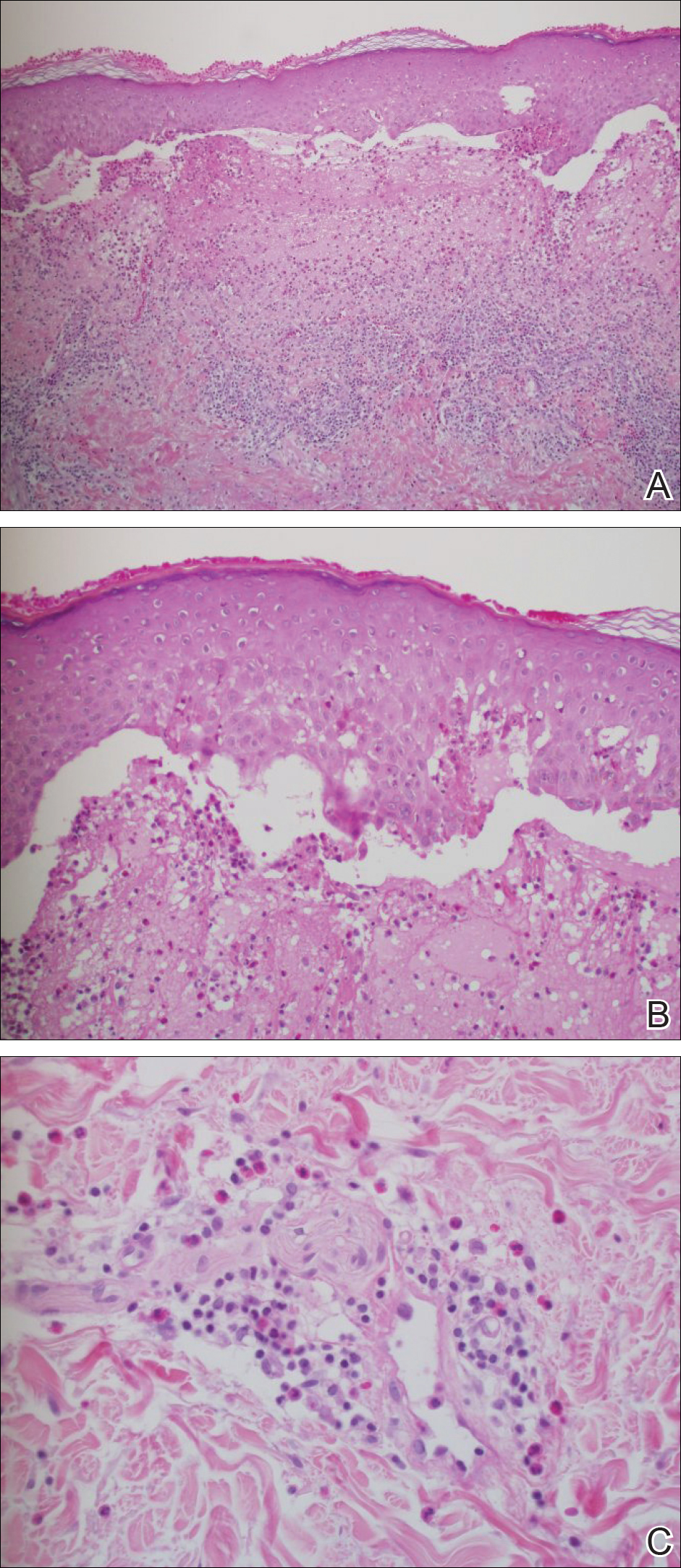
Punch biopsies from perilesional skin were submitted for hematoxylin and eosin staining and direct immunofluorescence (DIF). Histopathology showed a subepidermal blistering disease with tissue eosinophilia consistent with lichen planus pemphigoides (LPP)(Figure 2); direct immunofluorescence was positive for IgG, C3, and type IV collagen at the dermoepidermal junction. Serum BP180 was positive at 51 U/mL (reference range, <14 U/mL) and BP230 was negative. She was then started on tetracycline (500 mg twice daily), nicotinamide (500 mg twice daily), prednisone (5 mg daily), and dapsone (100 mg daily).
After 3 months without improvement, tetracycline and nicotinamide were discontinued, prednisone was increased to 10 mg daily, and dapsone was continued. A repeat biopsy was taken from a new area of involvement on the left lower leg, which revealed a psoriasiform dermatitis with interface changes. The DIF was positive for IgG and C3 along the basement membrane. A serum indirect immunofluorescence for BP180 also was positive.
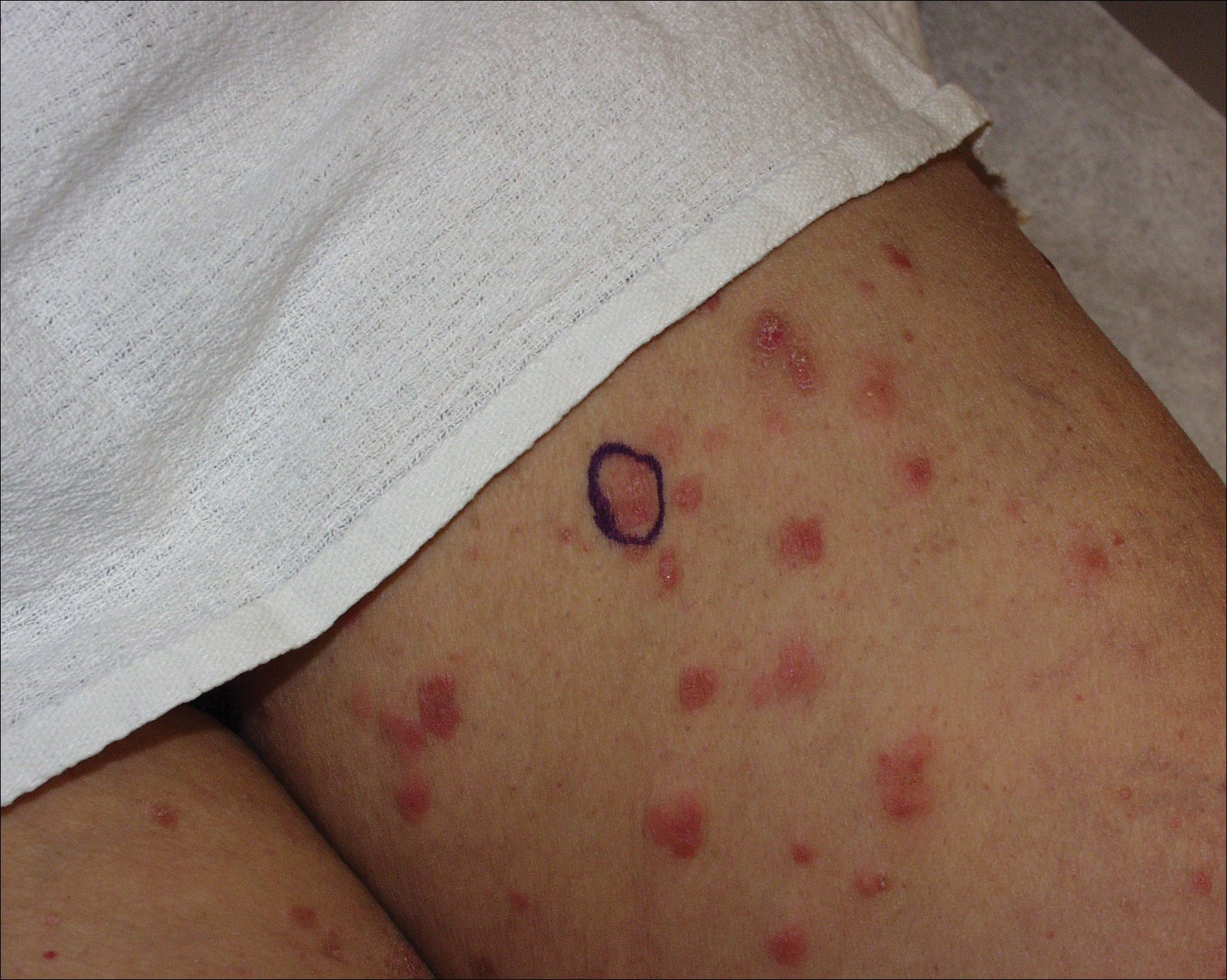
The patient developed mild hemolytic anemia on dapsone; the medication was eventually discontinued. Subsequent treatments included adequate trials of azathioprine, mycophenolate mofetil, and hydroxychloroquine. Azathioprine (150 mg daily) and hydroxychloroquine (400 mg daily) treatment failed. She initially improved on mycophenolate mofetil (500 mg in the morning and 1000 mg in the evening) with flattening of the papules on the arms and legs and decreased erythema. However, mycophenolate mofetil eventually lost its efficacy and was discontinued.
Because several medications failed (ie, tetracycline, nicotinamide, prednisone, dapsone, azathioprine, mycophenolate mofetil, hydroxychloroquine), she was started on ustekinumab (45 mg) initial loading dose by subcutaneous injection (patient’s weight, 63 kg). At 4 weeks, the patient was given the second subcutaneous injection of ustekinumab (45 mg). She experienced marked improvement with no new lesions. The prior lesions also had decreased in size and were only slightly pink. The prednisone dose was tapered to 5 mg daily.
She had near-complete resolution of the skin lesions 12 weeks after the second dose of ustekinumab. Since then, she has had some recrudescence of the papulosquamous lesions but no vesicles or bullae. With the exception of occasional scattered pink papules on the forearms, her condition greatly improved on ustekinumab. She is no longer taking any of the other medications with the exception of prednisone (down to 1 mg daily) with a plan to gradually taper completely off of it.
Comment
Clinical Presentation
Lichen planus pemphigoides is a rare autoimmune subepidermal blistering disease with few cases reported in the literature. It is considered a clinical variation of bullous pemphigoid (BP) or a coexistence of LP and BP.1,2 It is characterized by bullous lesions developing on LP papules as well as on clinically uninvolved areas of the skin. It has been reported that LPP is provoked by several medications including cinnarizine, captopril, ramipril, simvastatin, psoralen plus UVA, and antituberculous medications (eg, isoniazid, rifampin, ethambutol, pyrazinamide).1 Risedronate or atenolol have not been reported to cause LPP, LP, or BP; however, according to Litt,3 a lichenoid drug eruption has been associated with atenolol. Furthermore, some cases of LPP demonstrate overlapping characteristics with paraneoplastic pemphigus and have been associated with internal malignancy. Hamada et al4 described a case of LPP coupled with colon adenocarcinoma and numerous keratoacanthomas. The earliest depiction of the coexistence of a case of mainstream LP complicated by an extensive bullous eruption was by Kaposi5 in 1892. He coined the term lichen ruber pemphigoides.5
Compared to BP, LPP is believed to affect a younger age group and have a less serious clinical course. The mean age of onset of LPP is in the third to fourth decades of life, while BP typically presents in the sixth decade. When comparing the location of bullae in LPP versus BP, the lesions of LPP tend to occur on the limbs, while BP tends to occur on the trunk.6
Clinically, LPP is distinguished by the existence of bullous lesions developing atop of the lesions of LP as well as on normal skin, with the latter being more commonplace. A classic example of LPP is characterized by an initial episode of traditional LP lesions often having severe pruritus, with or without patches of erythema, with the sudden eruption of tense bullae. These bullae commonly appear on the extremities and can appear over the normal skin, erythematous patches, or preexisting papules.7 In the atypical clinical presentations of this dubious skin condition, the bullae may only be seen on the lesions of LP.8 There also could be a lichenoid erythrodermic manifestation of a bullous eruption.9
Oral lesions of LPP have been described but had not been studied immunopathologically until Allen et al10 portrayed a 59-year-old man with cutaneous and oral lesions of LPP. They performed biopsies on the oral lesions and examined them by routine light microscopy and immunofluorescent techniques. The fine keratotic striae on the anterior buccal mucosal lesions were clinically consistent with oral LP. Perilesional tissue in conjunction with ulceration of the posterior buccal mucosa demonstrated histologic and immunopathologic alterations consistent with BP.10
Histopathology
Histopathologically, the lesions of LP show a bandlike lymphohistiocytic infiltrate, colloid bodies in the dermis, irregular acanthosis with saw-toothed rete ridges, orthokeratosis, wedge-shaped hypergranulosis, and liquefaction degeneration of the basal layer. Direct immunofluorescence shows mainly IgM and C3 deposited on colloid bodies, fibrin, and fibrinogen.11 The histopathology of the bullous lesion of LPP depicts a subepidermal bulla with variable diffuse or sparse lymphohistiocytic infiltrate and frequent eosinophils with or without neutrophils in the upper dermis. The existence of C3 alone or with IgG along the dermoepidermal junction gives confirmation on DIF.7
Autoantibodies
The expression of IgG autoantibodies directed against the basement membrane zone distinguishes LPP from bullous LP.2 IgG autoantibodies to either one or both the 230-kDa and 180-kDa BP (type XVII collagen) antigens has been demonstrated with LPP.4,12-14 Hamada et al4 described a histologic pattern more consistent with paraneoplastic pemphigus. It has been suggested that injury to the basal cells in LP or damage due to other courses of therapy such as psoralen plus UVA unveil suppressed antigenic determinants or produce new antigens, leading to antibody development and production of BP.12,15
Zillikens et al2 performed a study to identify the target antigen of LPP autoantibodies. They used sera from patients with LPP (n=4) and stained the epidermal side of salt-split human skin in a configuration identical to BP sera. In BP, the autoimmune response is directed against BP180, a hemidesmosomal transmembrane collagenous glycoprotein. They demonstrated that sera from BP patients largely reacted with a set of 4 epitopes (MCW-0 through MCW-3) grouped within a 45 amino acid stretch of the major noncollagenous extracellular domain (NC16A) of BP180. By immunoblotting and enzyme-linked immunosorbent assay, LPP sera also were compellingly reactive with recombinant BP180 NC16A. Lichen planus pemphigoides epitopes were additionally mapped using a series of overlapping recombinant segments of the NC16A domain. The authors demonstrated that all LPP sera reacted with amino acids 46 through 59 of domain NC16A, a protein portion that was previously shown to be unreactive with BP sera. In addition, they showed that 2 LPP sera reacted with the immunodominant antigenic region related to BP. Furthermore, they identified a unique epitope within the BP180 NC16A domain—MCW-4—which was distinctively recognized by sera from patients with LPP.2
Pathogenesis
The pathogenesis of both LP and BP has been linked to multiple cytokines that induce apoptosis in basal keratinocytes. Implicated cytokines include IFN-γ, tumor necrosis factor α (TNF-α), IL-1, IL-6, and IL-8, as well as other apoptosis-related molecules, such as Fas/Apo-1 and Bcl-2 in LP.16-18 Soluble E-selectin, vascular endothelial growth factor, IL-1β, IL-8, IL-5, transforming growth factor β1, and TNF-α were found to be elevated in either blister fluid or sera of BP patients.15-17
Management
Lichen planus pemphigoides usually responds well to traditional therapies, with systemic steroids being the most efficacious treatment of extensive disease.12,13 Other options include tetracycline and nicotinamide, isotretinoin, dapsone, and immunosuppressive drugs such as systemic cortico-steroids.12 Demirçay et al12 described a patient with skin lesions that rapidly cleared after the administration of oral methylprednisolone (48 mg/d) and oral dapsone (100 mg/d). The methylprednisolone and dapsone were withdrawn after 12 and 16 weeks, respectively. There was no recurrence during the 1-year follow-up period.12 et al19 described a patient who was treated with pulsed intravenous corticosteroids and continued to develop new papular and vesicular skin lesions. However, when oral acitretin was added to the patient’s regimen, the skin lesions cleared.19 There are several case reports of the successful use of hydroxychloroquine in LP.20,21
Cutaneous, nail, and oral LP also can be treated with TNF-α inhibitors (eg, adalimumab, etanercept) with resolution of lesions.22-25 However, we have not been able to find any reports of treating LPP with biologic medications in a search of PubMed articles indexed for MEDLINE using the terms lichen planus pemphigoides and biologic treatments/therapies. Given the fact that TNF-α and other inflammatory cytokines are involved in the pathogenesis of BP and LP, it is feasible that they also may be involved in the pathogenesis of LPP.
In our patient with cutaneous LPP, we chose to use ustekinumab instead of a primary TNF-α inhibitor because ustekinumab indirectly blocks TNF-α, as well as other proinflammatory cytokines such as IFN-γ, IL-17, and IL-22, which also could have played a role in the patient’s disease. Our goal was to use ustekinumab as a potential corticosteroid-sparing agent. Ustekinumab greatly improved her skin condition and allowed us to discontinue other medications.
- Harting MS, Hsu S. Lichen planus pemphigoides: a case report and review of the literature. Dermatol Online J. 2006;12:10.
- Zillikens D, Caux F, Mascaro JM, et al. Autoantibodies in lichen planus pemphigoides react with a novel epitope within the C-terminal NC16A domain of BP180. J Invest Dermatol. 1999;113:117-121.
- Litt J. Litt’s Drug Eruptions and Reactions Manual. 18th Ed. London, England: Informa Healthcare; 2011.
- Hamada T, Fujimoto W, Okazaki F, et al. Lichen planus pemphigoides and multiple keratoacanthomas associated with colon adenocarcinoma. Br J Dermatol. 2004;151:252-254.
- Kaposi M. Lichen ruber pemphigoides. Arch Derm Syph. 1892;343-346.
- Swale VJ, Black MM, Bhogal BS. Lichen planus pemphigoides: two case reports. Clin Exp Dermatol. 1998;23:132-135.
- Okochi H, Nashiro K, Tsuchida T, et al. Lichen planus pemphigoides: case reports and results of immunofluorescence and immunoelectron microscopic study. J Am Acad Dermatol. 1990;22:626-631.
- Mendiratta V, Asati DP, Koranne RV. Lichen planus pemphigoides in an Indian female. Indian J Dermatol. 2005;50:224-226.
- Joly P, Tanasescu S, Wolkenstein P, et al. Lichenoid erythrodermic bullous pemphigoid of the African patient. J Am Acad Dermatol. 1998;39:691-697.
- Allen , , R. Lichen planus pemphigoides: report of a case with oral lesions. Oral Surg Oral Med Oral Pathol. 1987;63:184-188.
- Rapini RP. Practical Dermatopathology. Philadelphia, PA: Mosby Elsevier; 2005.
- Demirçay Z, Baykal C, Demirkesen C. Lichen planus pemphigoides: report of two cases. Int J Dermatol. 2001;40:757-759.
- Sakuma-Oyama Y, Powell AM, Albert S, et al. Lichen planus pemphigoides evolving into pemphigoid nodularis. Clin Exp Dermatol. 2004;28:613-616.
- Hsu S, Ghohestani RF, Uitto J. Lichen planus pemphigoides with IgG autoantibodies to the 180kd bullous pemphigoid antigen (type XVII collagen). J Am Acad Dermatol. 2000;42:136-141.
- Kuramoto N, Kishimoto S, Shibagaki R, et al. PUVA-induced lichen planus pemphigoides. Br J Dermatol. 2000;142:509-512.
- Ameglio F, D’Auria L, Cordiali-Fei P, et al. Bullous pemphigoid and pemphigus vulgaris: correlated behaviour of serum VEGF, sE-selectin and TNF-alpha levels. J Biol Regul Homeost Agents. 1997;11:148-153.
- Ameglio F, D’auria L, Bonifati C, et al. Cytokine pattern in blister fluid and serum of patients with bullous pemphigoid: relationships with disease intensity. Br J Dermatol. 1998;138:611-614.
- D’Auria L, Mussi A, Bonifati C, et al. Increased serum IL-6, TNF-alpha and IL-10 levels in patients with bullous pemphigoid: relationships with disease activity. J Eur Acad Dermatol Venereol. 1999;12:11-15.
- , ,, . Treatment of lichen planus pemphigoides with acitretin and pulsed corticosteroids. Hautarzt. 2003;54:268-273.
- Eisen D. Hydroxychloroquine sulfate (Plaquenil) improves oral lichen planus: an open trial. J Am Acad Dermatol. 1993;28:609-612.
- James WD, Berger T, Elston D. Andrews’ Diseases of the Skin. 11th ed. Philadelphia, PA: Mosby Elsevier; 2011.
- Holló P, Szakonyi J, Kiss D, et al. Successful treatment of lichen planus with adalimumab. Acta Derm Venereol. 2012;92:385-386.
- Yarom N. Etanercept for the management of oral lichen planus. Am J Clin Dermatol. 2007;8:121.
- Chao TJ. Adalimumab in the management of cutaneous and oral lichen planus. Cutis. 2009;84:325-328.
- Irla N, Schneiter T, Haneke E, et al. Nail lichen planus: successful treatment with etanercept. Case Rep Dermatol. 2010;2:173-176.
Case Report
A 71-year-old woman presented with pink to violaceous, flat-topped, polygonal papules consistent with lichen planus (LP) on the volar wrists, extensor elbows, and bilateral lower legs of 3 years’ duration. She also had erythematous, violaceous, infiltrated plaques with microvesiculation on the bilateral thighs of several months’ duration (Figure 1). She reported pruritus, burning, and discomfort. Her medical history included type 2 diabetes mellitus, hypertension, and asthma with no history of skin rashes. A complete physical examination was performed. Age-appropriate screening for malignancy was negative. Hepatitis B and C antibody serologies were negative. Her medications at the time included risedronate and atenolol, which she had been taking for several years.

Punch biopsies from perilesional skin were submitted for hematoxylin and eosin staining and direct immunofluorescence (DIF). Histopathology showed a subepidermal blistering disease with tissue eosinophilia consistent with lichen planus pemphigoides (LPP)(Figure 2); direct immunofluorescence was positive for IgG, C3, and type IV collagen at the dermoepidermal junction. Serum BP180 was positive at 51 U/mL (reference range, <14 U/mL) and BP230 was negative. She was then started on tetracycline (500 mg twice daily), nicotinamide (500 mg twice daily), prednisone (5 mg daily), and dapsone (100 mg daily).
After 3 months without improvement, tetracycline and nicotinamide were discontinued, prednisone was increased to 10 mg daily, and dapsone was continued. A repeat biopsy was taken from a new area of involvement on the left lower leg, which revealed a psoriasiform dermatitis with interface changes. The DIF was positive for IgG and C3 along the basement membrane. A serum indirect immunofluorescence for BP180 also was positive.

The patient developed mild hemolytic anemia on dapsone; the medication was eventually discontinued. Subsequent treatments included adequate trials of azathioprine, mycophenolate mofetil, and hydroxychloroquine. Azathioprine (150 mg daily) and hydroxychloroquine (400 mg daily) treatment failed. She initially improved on mycophenolate mofetil (500 mg in the morning and 1000 mg in the evening) with flattening of the papules on the arms and legs and decreased erythema. However, mycophenolate mofetil eventually lost its efficacy and was discontinued.
Because several medications failed (ie, tetracycline, nicotinamide, prednisone, dapsone, azathioprine, mycophenolate mofetil, hydroxychloroquine), she was started on ustekinumab (45 mg) initial loading dose by subcutaneous injection (patient’s weight, 63 kg). At 4 weeks, the patient was given the second subcutaneous injection of ustekinumab (45 mg). She experienced marked improvement with no new lesions. The prior lesions also had decreased in size and were only slightly pink. The prednisone dose was tapered to 5 mg daily.
She had near-complete resolution of the skin lesions 12 weeks after the second dose of ustekinumab. Since then, she has had some recrudescence of the papulosquamous lesions but no vesicles or bullae. With the exception of occasional scattered pink papules on the forearms, her condition greatly improved on ustekinumab. She is no longer taking any of the other medications with the exception of prednisone (down to 1 mg daily) with a plan to gradually taper completely off of it.
Comment
Clinical Presentation
Lichen planus pemphigoides is a rare autoimmune subepidermal blistering disease with few cases reported in the literature. It is considered a clinical variation of bullous pemphigoid (BP) or a coexistence of LP and BP.1,2 It is characterized by bullous lesions developing on LP papules as well as on clinically uninvolved areas of the skin. It has been reported that LPP is provoked by several medications including cinnarizine, captopril, ramipril, simvastatin, psoralen plus UVA, and antituberculous medications (eg, isoniazid, rifampin, ethambutol, pyrazinamide).1 Risedronate or atenolol have not been reported to cause LPP, LP, or BP; however, according to Litt,3 a lichenoid drug eruption has been associated with atenolol. Furthermore, some cases of LPP demonstrate overlapping characteristics with paraneoplastic pemphigus and have been associated with internal malignancy. Hamada et al4 described a case of LPP coupled with colon adenocarcinoma and numerous keratoacanthomas. The earliest depiction of the coexistence of a case of mainstream LP complicated by an extensive bullous eruption was by Kaposi5 in 1892. He coined the term lichen ruber pemphigoides.5
Compared to BP, LPP is believed to affect a younger age group and have a less serious clinical course. The mean age of onset of LPP is in the third to fourth decades of life, while BP typically presents in the sixth decade. When comparing the location of bullae in LPP versus BP, the lesions of LPP tend to occur on the limbs, while BP tends to occur on the trunk.6
Clinically, LPP is distinguished by the existence of bullous lesions developing atop of the lesions of LP as well as on normal skin, with the latter being more commonplace. A classic example of LPP is characterized by an initial episode of traditional LP lesions often having severe pruritus, with or without patches of erythema, with the sudden eruption of tense bullae. These bullae commonly appear on the extremities and can appear over the normal skin, erythematous patches, or preexisting papules.7 In the atypical clinical presentations of this dubious skin condition, the bullae may only be seen on the lesions of LP.8 There also could be a lichenoid erythrodermic manifestation of a bullous eruption.9
Oral lesions of LPP have been described but had not been studied immunopathologically until Allen et al10 portrayed a 59-year-old man with cutaneous and oral lesions of LPP. They performed biopsies on the oral lesions and examined them by routine light microscopy and immunofluorescent techniques. The fine keratotic striae on the anterior buccal mucosal lesions were clinically consistent with oral LP. Perilesional tissue in conjunction with ulceration of the posterior buccal mucosa demonstrated histologic and immunopathologic alterations consistent with BP.10
Histopathology
Histopathologically, the lesions of LP show a bandlike lymphohistiocytic infiltrate, colloid bodies in the dermis, irregular acanthosis with saw-toothed rete ridges, orthokeratosis, wedge-shaped hypergranulosis, and liquefaction degeneration of the basal layer. Direct immunofluorescence shows mainly IgM and C3 deposited on colloid bodies, fibrin, and fibrinogen.11 The histopathology of the bullous lesion of LPP depicts a subepidermal bulla with variable diffuse or sparse lymphohistiocytic infiltrate and frequent eosinophils with or without neutrophils in the upper dermis. The existence of C3 alone or with IgG along the dermoepidermal junction gives confirmation on DIF.7
Autoantibodies
The expression of IgG autoantibodies directed against the basement membrane zone distinguishes LPP from bullous LP.2 IgG autoantibodies to either one or both the 230-kDa and 180-kDa BP (type XVII collagen) antigens has been demonstrated with LPP.4,12-14 Hamada et al4 described a histologic pattern more consistent with paraneoplastic pemphigus. It has been suggested that injury to the basal cells in LP or damage due to other courses of therapy such as psoralen plus UVA unveil suppressed antigenic determinants or produce new antigens, leading to antibody development and production of BP.12,15
Zillikens et al2 performed a study to identify the target antigen of LPP autoantibodies. They used sera from patients with LPP (n=4) and stained the epidermal side of salt-split human skin in a configuration identical to BP sera. In BP, the autoimmune response is directed against BP180, a hemidesmosomal transmembrane collagenous glycoprotein. They demonstrated that sera from BP patients largely reacted with a set of 4 epitopes (MCW-0 through MCW-3) grouped within a 45 amino acid stretch of the major noncollagenous extracellular domain (NC16A) of BP180. By immunoblotting and enzyme-linked immunosorbent assay, LPP sera also were compellingly reactive with recombinant BP180 NC16A. Lichen planus pemphigoides epitopes were additionally mapped using a series of overlapping recombinant segments of the NC16A domain. The authors demonstrated that all LPP sera reacted with amino acids 46 through 59 of domain NC16A, a protein portion that was previously shown to be unreactive with BP sera. In addition, they showed that 2 LPP sera reacted with the immunodominant antigenic region related to BP. Furthermore, they identified a unique epitope within the BP180 NC16A domain—MCW-4—which was distinctively recognized by sera from patients with LPP.2
Pathogenesis
The pathogenesis of both LP and BP has been linked to multiple cytokines that induce apoptosis in basal keratinocytes. Implicated cytokines include IFN-γ, tumor necrosis factor α (TNF-α), IL-1, IL-6, and IL-8, as well as other apoptosis-related molecules, such as Fas/Apo-1 and Bcl-2 in LP.16-18 Soluble E-selectin, vascular endothelial growth factor, IL-1β, IL-8, IL-5, transforming growth factor β1, and TNF-α were found to be elevated in either blister fluid or sera of BP patients.15-17
Management
Lichen planus pemphigoides usually responds well to traditional therapies, with systemic steroids being the most efficacious treatment of extensive disease.12,13 Other options include tetracycline and nicotinamide, isotretinoin, dapsone, and immunosuppressive drugs such as systemic cortico-steroids.12 Demirçay et al12 described a patient with skin lesions that rapidly cleared after the administration of oral methylprednisolone (48 mg/d) and oral dapsone (100 mg/d). The methylprednisolone and dapsone were withdrawn after 12 and 16 weeks, respectively. There was no recurrence during the 1-year follow-up period.12 et al19 described a patient who was treated with pulsed intravenous corticosteroids and continued to develop new papular and vesicular skin lesions. However, when oral acitretin was added to the patient’s regimen, the skin lesions cleared.19 There are several case reports of the successful use of hydroxychloroquine in LP.20,21
Cutaneous, nail, and oral LP also can be treated with TNF-α inhibitors (eg, adalimumab, etanercept) with resolution of lesions.22-25 However, we have not been able to find any reports of treating LPP with biologic medications in a search of PubMed articles indexed for MEDLINE using the terms lichen planus pemphigoides and biologic treatments/therapies. Given the fact that TNF-α and other inflammatory cytokines are involved in the pathogenesis of BP and LP, it is feasible that they also may be involved in the pathogenesis of LPP.
In our patient with cutaneous LPP, we chose to use ustekinumab instead of a primary TNF-α inhibitor because ustekinumab indirectly blocks TNF-α, as well as other proinflammatory cytokines such as IFN-γ, IL-17, and IL-22, which also could have played a role in the patient’s disease. Our goal was to use ustekinumab as a potential corticosteroid-sparing agent. Ustekinumab greatly improved her skin condition and allowed us to discontinue other medications.
Case Report
A 71-year-old woman presented with pink to violaceous, flat-topped, polygonal papules consistent with lichen planus (LP) on the volar wrists, extensor elbows, and bilateral lower legs of 3 years’ duration. She also had erythematous, violaceous, infiltrated plaques with microvesiculation on the bilateral thighs of several months’ duration (Figure 1). She reported pruritus, burning, and discomfort. Her medical history included type 2 diabetes mellitus, hypertension, and asthma with no history of skin rashes. A complete physical examination was performed. Age-appropriate screening for malignancy was negative. Hepatitis B and C antibody serologies were negative. Her medications at the time included risedronate and atenolol, which she had been taking for several years.

Punch biopsies from perilesional skin were submitted for hematoxylin and eosin staining and direct immunofluorescence (DIF). Histopathology showed a subepidermal blistering disease with tissue eosinophilia consistent with lichen planus pemphigoides (LPP)(Figure 2); direct immunofluorescence was positive for IgG, C3, and type IV collagen at the dermoepidermal junction. Serum BP180 was positive at 51 U/mL (reference range, <14 U/mL) and BP230 was negative. She was then started on tetracycline (500 mg twice daily), nicotinamide (500 mg twice daily), prednisone (5 mg daily), and dapsone (100 mg daily).
After 3 months without improvement, tetracycline and nicotinamide were discontinued, prednisone was increased to 10 mg daily, and dapsone was continued. A repeat biopsy was taken from a new area of involvement on the left lower leg, which revealed a psoriasiform dermatitis with interface changes. The DIF was positive for IgG and C3 along the basement membrane. A serum indirect immunofluorescence for BP180 also was positive.

The patient developed mild hemolytic anemia on dapsone; the medication was eventually discontinued. Subsequent treatments included adequate trials of azathioprine, mycophenolate mofetil, and hydroxychloroquine. Azathioprine (150 mg daily) and hydroxychloroquine (400 mg daily) treatment failed. She initially improved on mycophenolate mofetil (500 mg in the morning and 1000 mg in the evening) with flattening of the papules on the arms and legs and decreased erythema. However, mycophenolate mofetil eventually lost its efficacy and was discontinued.
Because several medications failed (ie, tetracycline, nicotinamide, prednisone, dapsone, azathioprine, mycophenolate mofetil, hydroxychloroquine), she was started on ustekinumab (45 mg) initial loading dose by subcutaneous injection (patient’s weight, 63 kg). At 4 weeks, the patient was given the second subcutaneous injection of ustekinumab (45 mg). She experienced marked improvement with no new lesions. The prior lesions also had decreased in size and were only slightly pink. The prednisone dose was tapered to 5 mg daily.
She had near-complete resolution of the skin lesions 12 weeks after the second dose of ustekinumab. Since then, she has had some recrudescence of the papulosquamous lesions but no vesicles or bullae. With the exception of occasional scattered pink papules on the forearms, her condition greatly improved on ustekinumab. She is no longer taking any of the other medications with the exception of prednisone (down to 1 mg daily) with a plan to gradually taper completely off of it.
Comment
Clinical Presentation
Lichen planus pemphigoides is a rare autoimmune subepidermal blistering disease with few cases reported in the literature. It is considered a clinical variation of bullous pemphigoid (BP) or a coexistence of LP and BP.1,2 It is characterized by bullous lesions developing on LP papules as well as on clinically uninvolved areas of the skin. It has been reported that LPP is provoked by several medications including cinnarizine, captopril, ramipril, simvastatin, psoralen plus UVA, and antituberculous medications (eg, isoniazid, rifampin, ethambutol, pyrazinamide).1 Risedronate or atenolol have not been reported to cause LPP, LP, or BP; however, according to Litt,3 a lichenoid drug eruption has been associated with atenolol. Furthermore, some cases of LPP demonstrate overlapping characteristics with paraneoplastic pemphigus and have been associated with internal malignancy. Hamada et al4 described a case of LPP coupled with colon adenocarcinoma and numerous keratoacanthomas. The earliest depiction of the coexistence of a case of mainstream LP complicated by an extensive bullous eruption was by Kaposi5 in 1892. He coined the term lichen ruber pemphigoides.5
Compared to BP, LPP is believed to affect a younger age group and have a less serious clinical course. The mean age of onset of LPP is in the third to fourth decades of life, while BP typically presents in the sixth decade. When comparing the location of bullae in LPP versus BP, the lesions of LPP tend to occur on the limbs, while BP tends to occur on the trunk.6
Clinically, LPP is distinguished by the existence of bullous lesions developing atop of the lesions of LP as well as on normal skin, with the latter being more commonplace. A classic example of LPP is characterized by an initial episode of traditional LP lesions often having severe pruritus, with or without patches of erythema, with the sudden eruption of tense bullae. These bullae commonly appear on the extremities and can appear over the normal skin, erythematous patches, or preexisting papules.7 In the atypical clinical presentations of this dubious skin condition, the bullae may only be seen on the lesions of LP.8 There also could be a lichenoid erythrodermic manifestation of a bullous eruption.9
Oral lesions of LPP have been described but had not been studied immunopathologically until Allen et al10 portrayed a 59-year-old man with cutaneous and oral lesions of LPP. They performed biopsies on the oral lesions and examined them by routine light microscopy and immunofluorescent techniques. The fine keratotic striae on the anterior buccal mucosal lesions were clinically consistent with oral LP. Perilesional tissue in conjunction with ulceration of the posterior buccal mucosa demonstrated histologic and immunopathologic alterations consistent with BP.10
Histopathology
Histopathologically, the lesions of LP show a bandlike lymphohistiocytic infiltrate, colloid bodies in the dermis, irregular acanthosis with saw-toothed rete ridges, orthokeratosis, wedge-shaped hypergranulosis, and liquefaction degeneration of the basal layer. Direct immunofluorescence shows mainly IgM and C3 deposited on colloid bodies, fibrin, and fibrinogen.11 The histopathology of the bullous lesion of LPP depicts a subepidermal bulla with variable diffuse or sparse lymphohistiocytic infiltrate and frequent eosinophils with or without neutrophils in the upper dermis. The existence of C3 alone or with IgG along the dermoepidermal junction gives confirmation on DIF.7
Autoantibodies
The expression of IgG autoantibodies directed against the basement membrane zone distinguishes LPP from bullous LP.2 IgG autoantibodies to either one or both the 230-kDa and 180-kDa BP (type XVII collagen) antigens has been demonstrated with LPP.4,12-14 Hamada et al4 described a histologic pattern more consistent with paraneoplastic pemphigus. It has been suggested that injury to the basal cells in LP or damage due to other courses of therapy such as psoralen plus UVA unveil suppressed antigenic determinants or produce new antigens, leading to antibody development and production of BP.12,15
Zillikens et al2 performed a study to identify the target antigen of LPP autoantibodies. They used sera from patients with LPP (n=4) and stained the epidermal side of salt-split human skin in a configuration identical to BP sera. In BP, the autoimmune response is directed against BP180, a hemidesmosomal transmembrane collagenous glycoprotein. They demonstrated that sera from BP patients largely reacted with a set of 4 epitopes (MCW-0 through MCW-3) grouped within a 45 amino acid stretch of the major noncollagenous extracellular domain (NC16A) of BP180. By immunoblotting and enzyme-linked immunosorbent assay, LPP sera also were compellingly reactive with recombinant BP180 NC16A. Lichen planus pemphigoides epitopes were additionally mapped using a series of overlapping recombinant segments of the NC16A domain. The authors demonstrated that all LPP sera reacted with amino acids 46 through 59 of domain NC16A, a protein portion that was previously shown to be unreactive with BP sera. In addition, they showed that 2 LPP sera reacted with the immunodominant antigenic region related to BP. Furthermore, they identified a unique epitope within the BP180 NC16A domain—MCW-4—which was distinctively recognized by sera from patients with LPP.2
Pathogenesis
The pathogenesis of both LP and BP has been linked to multiple cytokines that induce apoptosis in basal keratinocytes. Implicated cytokines include IFN-γ, tumor necrosis factor α (TNF-α), IL-1, IL-6, and IL-8, as well as other apoptosis-related molecules, such as Fas/Apo-1 and Bcl-2 in LP.16-18 Soluble E-selectin, vascular endothelial growth factor, IL-1β, IL-8, IL-5, transforming growth factor β1, and TNF-α were found to be elevated in either blister fluid or sera of BP patients.15-17
Management
Lichen planus pemphigoides usually responds well to traditional therapies, with systemic steroids being the most efficacious treatment of extensive disease.12,13 Other options include tetracycline and nicotinamide, isotretinoin, dapsone, and immunosuppressive drugs such as systemic cortico-steroids.12 Demirçay et al12 described a patient with skin lesions that rapidly cleared after the administration of oral methylprednisolone (48 mg/d) and oral dapsone (100 mg/d). The methylprednisolone and dapsone were withdrawn after 12 and 16 weeks, respectively. There was no recurrence during the 1-year follow-up period.12 et al19 described a patient who was treated with pulsed intravenous corticosteroids and continued to develop new papular and vesicular skin lesions. However, when oral acitretin was added to the patient’s regimen, the skin lesions cleared.19 There are several case reports of the successful use of hydroxychloroquine in LP.20,21
Cutaneous, nail, and oral LP also can be treated with TNF-α inhibitors (eg, adalimumab, etanercept) with resolution of lesions.22-25 However, we have not been able to find any reports of treating LPP with biologic medications in a search of PubMed articles indexed for MEDLINE using the terms lichen planus pemphigoides and biologic treatments/therapies. Given the fact that TNF-α and other inflammatory cytokines are involved in the pathogenesis of BP and LP, it is feasible that they also may be involved in the pathogenesis of LPP.
In our patient with cutaneous LPP, we chose to use ustekinumab instead of a primary TNF-α inhibitor because ustekinumab indirectly blocks TNF-α, as well as other proinflammatory cytokines such as IFN-γ, IL-17, and IL-22, which also could have played a role in the patient’s disease. Our goal was to use ustekinumab as a potential corticosteroid-sparing agent. Ustekinumab greatly improved her skin condition and allowed us to discontinue other medications.
- Harting MS, Hsu S. Lichen planus pemphigoides: a case report and review of the literature. Dermatol Online J. 2006;12:10.
- Zillikens D, Caux F, Mascaro JM, et al. Autoantibodies in lichen planus pemphigoides react with a novel epitope within the C-terminal NC16A domain of BP180. J Invest Dermatol. 1999;113:117-121.
- Litt J. Litt’s Drug Eruptions and Reactions Manual. 18th Ed. London, England: Informa Healthcare; 2011.
- Hamada T, Fujimoto W, Okazaki F, et al. Lichen planus pemphigoides and multiple keratoacanthomas associated with colon adenocarcinoma. Br J Dermatol. 2004;151:252-254.
- Kaposi M. Lichen ruber pemphigoides. Arch Derm Syph. 1892;343-346.
- Swale VJ, Black MM, Bhogal BS. Lichen planus pemphigoides: two case reports. Clin Exp Dermatol. 1998;23:132-135.
- Okochi H, Nashiro K, Tsuchida T, et al. Lichen planus pemphigoides: case reports and results of immunofluorescence and immunoelectron microscopic study. J Am Acad Dermatol. 1990;22:626-631.
- Mendiratta V, Asati DP, Koranne RV. Lichen planus pemphigoides in an Indian female. Indian J Dermatol. 2005;50:224-226.
- Joly P, Tanasescu S, Wolkenstein P, et al. Lichenoid erythrodermic bullous pemphigoid of the African patient. J Am Acad Dermatol. 1998;39:691-697.
- Allen , , R. Lichen planus pemphigoides: report of a case with oral lesions. Oral Surg Oral Med Oral Pathol. 1987;63:184-188.
- Rapini RP. Practical Dermatopathology. Philadelphia, PA: Mosby Elsevier; 2005.
- Demirçay Z, Baykal C, Demirkesen C. Lichen planus pemphigoides: report of two cases. Int J Dermatol. 2001;40:757-759.
- Sakuma-Oyama Y, Powell AM, Albert S, et al. Lichen planus pemphigoides evolving into pemphigoid nodularis. Clin Exp Dermatol. 2004;28:613-616.
- Hsu S, Ghohestani RF, Uitto J. Lichen planus pemphigoides with IgG autoantibodies to the 180kd bullous pemphigoid antigen (type XVII collagen). J Am Acad Dermatol. 2000;42:136-141.
- Kuramoto N, Kishimoto S, Shibagaki R, et al. PUVA-induced lichen planus pemphigoides. Br J Dermatol. 2000;142:509-512.
- Ameglio F, D’Auria L, Cordiali-Fei P, et al. Bullous pemphigoid and pemphigus vulgaris: correlated behaviour of serum VEGF, sE-selectin and TNF-alpha levels. J Biol Regul Homeost Agents. 1997;11:148-153.
- Ameglio F, D’auria L, Bonifati C, et al. Cytokine pattern in blister fluid and serum of patients with bullous pemphigoid: relationships with disease intensity. Br J Dermatol. 1998;138:611-614.
- D’Auria L, Mussi A, Bonifati C, et al. Increased serum IL-6, TNF-alpha and IL-10 levels in patients with bullous pemphigoid: relationships with disease activity. J Eur Acad Dermatol Venereol. 1999;12:11-15.
- , ,, . Treatment of lichen planus pemphigoides with acitretin and pulsed corticosteroids. Hautarzt. 2003;54:268-273.
- Eisen D. Hydroxychloroquine sulfate (Plaquenil) improves oral lichen planus: an open trial. J Am Acad Dermatol. 1993;28:609-612.
- James WD, Berger T, Elston D. Andrews’ Diseases of the Skin. 11th ed. Philadelphia, PA: Mosby Elsevier; 2011.
- Holló P, Szakonyi J, Kiss D, et al. Successful treatment of lichen planus with adalimumab. Acta Derm Venereol. 2012;92:385-386.
- Yarom N. Etanercept for the management of oral lichen planus. Am J Clin Dermatol. 2007;8:121.
- Chao TJ. Adalimumab in the management of cutaneous and oral lichen planus. Cutis. 2009;84:325-328.
- Irla N, Schneiter T, Haneke E, et al. Nail lichen planus: successful treatment with etanercept. Case Rep Dermatol. 2010;2:173-176.
- Harting MS, Hsu S. Lichen planus pemphigoides: a case report and review of the literature. Dermatol Online J. 2006;12:10.
- Zillikens D, Caux F, Mascaro JM, et al. Autoantibodies in lichen planus pemphigoides react with a novel epitope within the C-terminal NC16A domain of BP180. J Invest Dermatol. 1999;113:117-121.
- Litt J. Litt’s Drug Eruptions and Reactions Manual. 18th Ed. London, England: Informa Healthcare; 2011.
- Hamada T, Fujimoto W, Okazaki F, et al. Lichen planus pemphigoides and multiple keratoacanthomas associated with colon adenocarcinoma. Br J Dermatol. 2004;151:252-254.
- Kaposi M. Lichen ruber pemphigoides. Arch Derm Syph. 1892;343-346.
- Swale VJ, Black MM, Bhogal BS. Lichen planus pemphigoides: two case reports. Clin Exp Dermatol. 1998;23:132-135.
- Okochi H, Nashiro K, Tsuchida T, et al. Lichen planus pemphigoides: case reports and results of immunofluorescence and immunoelectron microscopic study. J Am Acad Dermatol. 1990;22:626-631.
- Mendiratta V, Asati DP, Koranne RV. Lichen planus pemphigoides in an Indian female. Indian J Dermatol. 2005;50:224-226.
- Joly P, Tanasescu S, Wolkenstein P, et al. Lichenoid erythrodermic bullous pemphigoid of the African patient. J Am Acad Dermatol. 1998;39:691-697.
- Allen , , R. Lichen planus pemphigoides: report of a case with oral lesions. Oral Surg Oral Med Oral Pathol. 1987;63:184-188.
- Rapini RP. Practical Dermatopathology. Philadelphia, PA: Mosby Elsevier; 2005.
- Demirçay Z, Baykal C, Demirkesen C. Lichen planus pemphigoides: report of two cases. Int J Dermatol. 2001;40:757-759.
- Sakuma-Oyama Y, Powell AM, Albert S, et al. Lichen planus pemphigoides evolving into pemphigoid nodularis. Clin Exp Dermatol. 2004;28:613-616.
- Hsu S, Ghohestani RF, Uitto J. Lichen planus pemphigoides with IgG autoantibodies to the 180kd bullous pemphigoid antigen (type XVII collagen). J Am Acad Dermatol. 2000;42:136-141.
- Kuramoto N, Kishimoto S, Shibagaki R, et al. PUVA-induced lichen planus pemphigoides. Br J Dermatol. 2000;142:509-512.
- Ameglio F, D’Auria L, Cordiali-Fei P, et al. Bullous pemphigoid and pemphigus vulgaris: correlated behaviour of serum VEGF, sE-selectin and TNF-alpha levels. J Biol Regul Homeost Agents. 1997;11:148-153.
- Ameglio F, D’auria L, Bonifati C, et al. Cytokine pattern in blister fluid and serum of patients with bullous pemphigoid: relationships with disease intensity. Br J Dermatol. 1998;138:611-614.
- D’Auria L, Mussi A, Bonifati C, et al. Increased serum IL-6, TNF-alpha and IL-10 levels in patients with bullous pemphigoid: relationships with disease activity. J Eur Acad Dermatol Venereol. 1999;12:11-15.
- , ,, . Treatment of lichen planus pemphigoides with acitretin and pulsed corticosteroids. Hautarzt. 2003;54:268-273.
- Eisen D. Hydroxychloroquine sulfate (Plaquenil) improves oral lichen planus: an open trial. J Am Acad Dermatol. 1993;28:609-612.
- James WD, Berger T, Elston D. Andrews’ Diseases of the Skin. 11th ed. Philadelphia, PA: Mosby Elsevier; 2011.
- Holló P, Szakonyi J, Kiss D, et al. Successful treatment of lichen planus with adalimumab. Acta Derm Venereol. 2012;92:385-386.
- Yarom N. Etanercept for the management of oral lichen planus. Am J Clin Dermatol. 2007;8:121.
- Chao TJ. Adalimumab in the management of cutaneous and oral lichen planus. Cutis. 2009;84:325-328.
- Irla N, Schneiter T, Haneke E, et al. Nail lichen planus: successful treatment with etanercept. Case Rep Dermatol. 2010;2:173-176.
- Lichen planus pemphigoides (LPP) is a rare autoimmune subepidermal blistering disease with few cases reported in the literature.
- Because tumor necrosis factor 11α (TNF-11α) and other inflammatory cytokines are involved in the pathogenesis of bullous pemphigoid and lichen planus, it is feasible that they also may be involved in the pathogenesis of LPP.
- Ustekinumab may be used to treat LPP as a potential corticosteroid-sparing agent because it indirectly blocks TNF-α, as well as other proinflammatory cytokines such as IFN-γ, IL-17, and IL-22.
Phrenic-nerve stimulator maintains benefits for 18 months
TORONTO – The implanted phrenic-nerve stimulation device that received Food and Drug Administration marketing approval in October 2017 for treating central sleep apnea has now shown safety and efficacy out to 18 months of continuous use in 102 patients.
After 18 months of treatment with the Remede System, patients’ outcomes remained stable and patients continued to see the improvements they had experienced after 6 and 12 months of treatment. These improvements included significant average reductions from baseline in apnea-hypopnea index and central apnea index and significant increases in oxygenation and sleep quality, Andrew C. Kao, MD, said at the CHEST annual meeting.
“We were concerned that there would be a degradation of the benefit [over time]. We are very happy that the benefit was sustained,” said Dr. Kao, a heart failure cardiologist at Saint Luke’s Health System in Kansas City, Mo.
Dr. Kao did not report an 18-month follow-up for the study’s primary endpoint, the percentage of patients after 6 months on treatment who had at least a 50% reduction from baseline in their apnea-hypopnea index. His report focused on the 6-, 12-, and 18-month changes relative to baseline for five secondary outcomes: central sleep apnea index, apnea-hypopnea index, arousal index, oxygen desaturation index, and time spent in REM sleep. For all five of these outcomes, the 102 patients showed an average, statistically significant improvement compared with baseline after 6 months on treatment that persisted virtually unchanged at 12 and 18 months.
For example, average central sleep apnea index fell from 27 events/hour at baseline to 5 per hour at 6, 12, and 18 months. Average apnea-hypopnea index fell from 46 events/hour at baseline to about 25 per hour at 6, 12, and 18 months. The average percentage of sleep spent in REM sleep improved from 12% at baseline to about 15% at 6, 12, and 18 months.
During 18 months of treatment following device implantation, four of the 102 patients had a serious adverse event. One patient required lead repositioning to relieve discomfort and three had an interaction with an implanted cardiac device. The effects resolved in all four patients without long-term impact. An additional 16 patients had discomfort that required an unscheduled medical visit, but these were not classified as serious episodes, and in 14 of these patients the discomfort resolved.
The Remede System phrenic-nerve stimulator received FDA marketing approval for moderate to severe central sleep apnea based on 6-month efficacy and 12-month safety data (Lancet. 2016 Sept 3;388[10048]:974-82). The Pivotal Trial of the Remede System enrolled 151 patients with an apnea-hypopnea index of at least 20 events/hour, about half of whom had heart failure. All patients received a device implant: In the initial intervention group of 73 patients, researchers turned on the device 1 month after implantation, and in the 78 patients randomized to the initial control arm, the device remained off for the first 7 months and then went active. The researchers followed up with 46 patients drawn from both the original treatment arm and 56 patients from the original control arm, at which point the patients had been receiving 18 months of treatment.
The Remede System pivotal trial was sponsored by Respicardia, which markets the phrenic-verse stimulator. Dr. Kao’s institution, Saint Luke’s Health System, received grant support from Respicardia.
[email protected]
On Twitter @mitchelzoler
TORONTO – The implanted phrenic-nerve stimulation device that received Food and Drug Administration marketing approval in October 2017 for treating central sleep apnea has now shown safety and efficacy out to 18 months of continuous use in 102 patients.
After 18 months of treatment with the Remede System, patients’ outcomes remained stable and patients continued to see the improvements they had experienced after 6 and 12 months of treatment. These improvements included significant average reductions from baseline in apnea-hypopnea index and central apnea index and significant increases in oxygenation and sleep quality, Andrew C. Kao, MD, said at the CHEST annual meeting.
“We were concerned that there would be a degradation of the benefit [over time]. We are very happy that the benefit was sustained,” said Dr. Kao, a heart failure cardiologist at Saint Luke’s Health System in Kansas City, Mo.
Dr. Kao did not report an 18-month follow-up for the study’s primary endpoint, the percentage of patients after 6 months on treatment who had at least a 50% reduction from baseline in their apnea-hypopnea index. His report focused on the 6-, 12-, and 18-month changes relative to baseline for five secondary outcomes: central sleep apnea index, apnea-hypopnea index, arousal index, oxygen desaturation index, and time spent in REM sleep. For all five of these outcomes, the 102 patients showed an average, statistically significant improvement compared with baseline after 6 months on treatment that persisted virtually unchanged at 12 and 18 months.
For example, average central sleep apnea index fell from 27 events/hour at baseline to 5 per hour at 6, 12, and 18 months. Average apnea-hypopnea index fell from 46 events/hour at baseline to about 25 per hour at 6, 12, and 18 months. The average percentage of sleep spent in REM sleep improved from 12% at baseline to about 15% at 6, 12, and 18 months.
During 18 months of treatment following device implantation, four of the 102 patients had a serious adverse event. One patient required lead repositioning to relieve discomfort and three had an interaction with an implanted cardiac device. The effects resolved in all four patients without long-term impact. An additional 16 patients had discomfort that required an unscheduled medical visit, but these were not classified as serious episodes, and in 14 of these patients the discomfort resolved.
The Remede System phrenic-nerve stimulator received FDA marketing approval for moderate to severe central sleep apnea based on 6-month efficacy and 12-month safety data (Lancet. 2016 Sept 3;388[10048]:974-82). The Pivotal Trial of the Remede System enrolled 151 patients with an apnea-hypopnea index of at least 20 events/hour, about half of whom had heart failure. All patients received a device implant: In the initial intervention group of 73 patients, researchers turned on the device 1 month after implantation, and in the 78 patients randomized to the initial control arm, the device remained off for the first 7 months and then went active. The researchers followed up with 46 patients drawn from both the original treatment arm and 56 patients from the original control arm, at which point the patients had been receiving 18 months of treatment.
The Remede System pivotal trial was sponsored by Respicardia, which markets the phrenic-verse stimulator. Dr. Kao’s institution, Saint Luke’s Health System, received grant support from Respicardia.
[email protected]
On Twitter @mitchelzoler
TORONTO – The implanted phrenic-nerve stimulation device that received Food and Drug Administration marketing approval in October 2017 for treating central sleep apnea has now shown safety and efficacy out to 18 months of continuous use in 102 patients.
After 18 months of treatment with the Remede System, patients’ outcomes remained stable and patients continued to see the improvements they had experienced after 6 and 12 months of treatment. These improvements included significant average reductions from baseline in apnea-hypopnea index and central apnea index and significant increases in oxygenation and sleep quality, Andrew C. Kao, MD, said at the CHEST annual meeting.
“We were concerned that there would be a degradation of the benefit [over time]. We are very happy that the benefit was sustained,” said Dr. Kao, a heart failure cardiologist at Saint Luke’s Health System in Kansas City, Mo.
Dr. Kao did not report an 18-month follow-up for the study’s primary endpoint, the percentage of patients after 6 months on treatment who had at least a 50% reduction from baseline in their apnea-hypopnea index. His report focused on the 6-, 12-, and 18-month changes relative to baseline for five secondary outcomes: central sleep apnea index, apnea-hypopnea index, arousal index, oxygen desaturation index, and time spent in REM sleep. For all five of these outcomes, the 102 patients showed an average, statistically significant improvement compared with baseline after 6 months on treatment that persisted virtually unchanged at 12 and 18 months.
For example, average central sleep apnea index fell from 27 events/hour at baseline to 5 per hour at 6, 12, and 18 months. Average apnea-hypopnea index fell from 46 events/hour at baseline to about 25 per hour at 6, 12, and 18 months. The average percentage of sleep spent in REM sleep improved from 12% at baseline to about 15% at 6, 12, and 18 months.
During 18 months of treatment following device implantation, four of the 102 patients had a serious adverse event. One patient required lead repositioning to relieve discomfort and three had an interaction with an implanted cardiac device. The effects resolved in all four patients without long-term impact. An additional 16 patients had discomfort that required an unscheduled medical visit, but these were not classified as serious episodes, and in 14 of these patients the discomfort resolved.
The Remede System phrenic-nerve stimulator received FDA marketing approval for moderate to severe central sleep apnea based on 6-month efficacy and 12-month safety data (Lancet. 2016 Sept 3;388[10048]:974-82). The Pivotal Trial of the Remede System enrolled 151 patients with an apnea-hypopnea index of at least 20 events/hour, about half of whom had heart failure. All patients received a device implant: In the initial intervention group of 73 patients, researchers turned on the device 1 month after implantation, and in the 78 patients randomized to the initial control arm, the device remained off for the first 7 months and then went active. The researchers followed up with 46 patients drawn from both the original treatment arm and 56 patients from the original control arm, at which point the patients had been receiving 18 months of treatment.
The Remede System pivotal trial was sponsored by Respicardia, which markets the phrenic-verse stimulator. Dr. Kao’s institution, Saint Luke’s Health System, received grant support from Respicardia.
[email protected]
On Twitter @mitchelzoler
AT CHEST 2017
Key clinical point:
Major finding: Average central apnea index improved from 27 events/hour at baseline to 5 events/hour after 6, 12, and 18 months of treatment.
Data source: 102 patients enrolled in the Pivotal Trial of the remede System were followed for 18 months of treatment.
Disclosures: The remede System pivotal trial was sponsored by Respicardia, which markets the phrenic-verse stimulator. Dr. Kao’s institution, Saint Luke’s Health System, received grant support from Respicardia.
Potential postthyroidectomy quality improvement metrics arise from study
of U.S. hospitals, suggesting to the authors that these measures could be used for quality improvement metrics.
In the study, published Nov. 29 in JAMA Surgery, hospitals with significantly lower rates of hypocalcemia were more likely to conduct postoperative parathyroid hormone level measurement as well as to prescribe vitamin D, calcium supplements, or both. Hospitals with lower RLN injury rates more frequently used energy devices and intraoperative nerve monitoring.
“Causation cannot be proven by this, but the confidence that these practice parameters are important is high,” senior author Bruce Hall, MD, PhD, vice president and chief quality officer at BJC Healthcare, and professor of surgery at Washington University, St. Louis, said in an interview. Dr. Hall is consulting director for the American College of Surgeon’s National Surgical Quality Improvement Program (NSQIP), which provided data for the analysis (JAMA Surg. 2017 Nov 29. doi: 10.1001/jamasurg.2017.4593).
The researchers examined data from 14,540 patients who underwent thyroidectomies at 98 hospitals between Jan. 1, 2013, and Dec. 31, 2015. These included 13,242 operations at 96 hospitals with complete hypocalcemia data, 13,144 operations at 95 hospitals with complete RLN data, and 13,197 operations at 95 hospitals with complete hematoma data. The primary outcome was the 30-day incidence of hypocalcemia, RLN, and hematoma. The researchers also measured 30-day mortality, surgical site infections, and hospital readmissions.
A total of 3.3% of patients experienced clinically severe hypocalcemia (0.6% after partial thyroidectomy, 4.7% after total or subtotal thyroidectomy). Another 5.7% experienced RLN (4.2% after partial, 6.6% after total or subtotal). Hematoma occurred in 1.3% of cases, but there were no significant variations in rates of hematoma across participating institutions.
For hypocalcemia and RLN injury, there were hospital outliers both on the low end of complication rates and on the high end of complication rates, defined by odds ratios with 95% confidence ratios that were greater than 1 for high outliers, or lower than 1 for low outliers. There were no outliers with respect to hematoma, suggesting that it may not be a useful barometer of hospital performance.
With respect to hypocalcemia rates, four hospitals were low outliers, and seven were high. Eight hospitals were low outliers with respect to RLN injury, and 14 were high outliers.
In the analysis of postoperative hypocalcemia, both low and high outliers measured postoperative calcium with similar frequency (68.4% vs. 71.0%; P =.09). However, high performance outliers were more likely to prescribe postoperative calcium, vitamin D, or both (76.6% vs. 66.8%; P less than .001).
Among RLN outliers, intraoperative nerve monitoring was more common in the top performing hospitals (55.7% vs. 37.7%; P less than .001), as was the use of energy devices (69.1% vs. 55.2%; P less than .001).
There was one high outlier when it came to surgical site infections, and one high and one low outlier with respect to morbidity outcomes. There were no hospital readmission outliers.
No source of funding was disclosed. Dr. Liu and Dr. Hall reported having no financial disclosures.
of U.S. hospitals, suggesting to the authors that these measures could be used for quality improvement metrics.
In the study, published Nov. 29 in JAMA Surgery, hospitals with significantly lower rates of hypocalcemia were more likely to conduct postoperative parathyroid hormone level measurement as well as to prescribe vitamin D, calcium supplements, or both. Hospitals with lower RLN injury rates more frequently used energy devices and intraoperative nerve monitoring.
“Causation cannot be proven by this, but the confidence that these practice parameters are important is high,” senior author Bruce Hall, MD, PhD, vice president and chief quality officer at BJC Healthcare, and professor of surgery at Washington University, St. Louis, said in an interview. Dr. Hall is consulting director for the American College of Surgeon’s National Surgical Quality Improvement Program (NSQIP), which provided data for the analysis (JAMA Surg. 2017 Nov 29. doi: 10.1001/jamasurg.2017.4593).
The researchers examined data from 14,540 patients who underwent thyroidectomies at 98 hospitals between Jan. 1, 2013, and Dec. 31, 2015. These included 13,242 operations at 96 hospitals with complete hypocalcemia data, 13,144 operations at 95 hospitals with complete RLN data, and 13,197 operations at 95 hospitals with complete hematoma data. The primary outcome was the 30-day incidence of hypocalcemia, RLN, and hematoma. The researchers also measured 30-day mortality, surgical site infections, and hospital readmissions.
A total of 3.3% of patients experienced clinically severe hypocalcemia (0.6% after partial thyroidectomy, 4.7% after total or subtotal thyroidectomy). Another 5.7% experienced RLN (4.2% after partial, 6.6% after total or subtotal). Hematoma occurred in 1.3% of cases, but there were no significant variations in rates of hematoma across participating institutions.
For hypocalcemia and RLN injury, there were hospital outliers both on the low end of complication rates and on the high end of complication rates, defined by odds ratios with 95% confidence ratios that were greater than 1 for high outliers, or lower than 1 for low outliers. There were no outliers with respect to hematoma, suggesting that it may not be a useful barometer of hospital performance.
With respect to hypocalcemia rates, four hospitals were low outliers, and seven were high. Eight hospitals were low outliers with respect to RLN injury, and 14 were high outliers.
In the analysis of postoperative hypocalcemia, both low and high outliers measured postoperative calcium with similar frequency (68.4% vs. 71.0%; P =.09). However, high performance outliers were more likely to prescribe postoperative calcium, vitamin D, or both (76.6% vs. 66.8%; P less than .001).
Among RLN outliers, intraoperative nerve monitoring was more common in the top performing hospitals (55.7% vs. 37.7%; P less than .001), as was the use of energy devices (69.1% vs. 55.2%; P less than .001).
There was one high outlier when it came to surgical site infections, and one high and one low outlier with respect to morbidity outcomes. There were no hospital readmission outliers.
No source of funding was disclosed. Dr. Liu and Dr. Hall reported having no financial disclosures.
of U.S. hospitals, suggesting to the authors that these measures could be used for quality improvement metrics.
In the study, published Nov. 29 in JAMA Surgery, hospitals with significantly lower rates of hypocalcemia were more likely to conduct postoperative parathyroid hormone level measurement as well as to prescribe vitamin D, calcium supplements, or both. Hospitals with lower RLN injury rates more frequently used energy devices and intraoperative nerve monitoring.
“Causation cannot be proven by this, but the confidence that these practice parameters are important is high,” senior author Bruce Hall, MD, PhD, vice president and chief quality officer at BJC Healthcare, and professor of surgery at Washington University, St. Louis, said in an interview. Dr. Hall is consulting director for the American College of Surgeon’s National Surgical Quality Improvement Program (NSQIP), which provided data for the analysis (JAMA Surg. 2017 Nov 29. doi: 10.1001/jamasurg.2017.4593).
The researchers examined data from 14,540 patients who underwent thyroidectomies at 98 hospitals between Jan. 1, 2013, and Dec. 31, 2015. These included 13,242 operations at 96 hospitals with complete hypocalcemia data, 13,144 operations at 95 hospitals with complete RLN data, and 13,197 operations at 95 hospitals with complete hematoma data. The primary outcome was the 30-day incidence of hypocalcemia, RLN, and hematoma. The researchers also measured 30-day mortality, surgical site infections, and hospital readmissions.
A total of 3.3% of patients experienced clinically severe hypocalcemia (0.6% after partial thyroidectomy, 4.7% after total or subtotal thyroidectomy). Another 5.7% experienced RLN (4.2% after partial, 6.6% after total or subtotal). Hematoma occurred in 1.3% of cases, but there were no significant variations in rates of hematoma across participating institutions.
For hypocalcemia and RLN injury, there were hospital outliers both on the low end of complication rates and on the high end of complication rates, defined by odds ratios with 95% confidence ratios that were greater than 1 for high outliers, or lower than 1 for low outliers. There were no outliers with respect to hematoma, suggesting that it may not be a useful barometer of hospital performance.
With respect to hypocalcemia rates, four hospitals were low outliers, and seven were high. Eight hospitals were low outliers with respect to RLN injury, and 14 were high outliers.
In the analysis of postoperative hypocalcemia, both low and high outliers measured postoperative calcium with similar frequency (68.4% vs. 71.0%; P =.09). However, high performance outliers were more likely to prescribe postoperative calcium, vitamin D, or both (76.6% vs. 66.8%; P less than .001).
Among RLN outliers, intraoperative nerve monitoring was more common in the top performing hospitals (55.7% vs. 37.7%; P less than .001), as was the use of energy devices (69.1% vs. 55.2%; P less than .001).
There was one high outlier when it came to surgical site infections, and one high and one low outlier with respect to morbidity outcomes. There were no hospital readmission outliers.
No source of funding was disclosed. Dr. Liu and Dr. Hall reported having no financial disclosures.
FROM JAMA SURGERY
Key clinical point: Prescription of postoperative calcium, vitamin D, or both, and greater use of intraoperative nerve monitoring may lead to fewer adverse events after thyroidectomy.
Major finding: Both low and high outliers on 30-day rates of postoperative hypocalcemia measured postoperative calcium with similar frequency (68.4% vs. 71.0%; P =.09). However, high performance outliers were more likely to prescribe postoperative calcium, vitamin D, or both (76.6% vs. 66.8%; P less than .001).
Data source: Retrospective analysis of 14,540 patients at 98 hospitals in the American College of Surgeon’s National Surgical Quality Improvement Program.
Disclosures: No source of funding was disclosed. Dr. Liu and Dr. Hall reported having no financial disclosures.
Student Loan Burden and Its Impact on Career Decisions in Dermatology
Dermatology departments in the United States have been facing challenges in recruiting and retaining dermatologists for academic positions. Accordingly, a survey study reported that academic dermatologists were more likely than those in private practice to state that their institutions were recruiting new associates.1 Several factors could explain this phenomenon. Salary differences between jobs in academic and nonacademic settings may contribute to difficulty in recruiting dermatologists into academia, which is exacerbated by a theoretical shortage of dermatologists, leading to graduates who receive and accept private practice job offers.1,2 Furthermore, a large survey study reported that challenges unique to academic dermatologists include longer patient wait times in addition to responsibilities such as research, hospital consultations, medical writing, and teaching. These patterns raise concerns for the future of teaching institutions because academic dermatologists not only train future physicians but also conduct clinical and basic science research necessary to advance the field and improve patient care.2 Thus, it is important to evaluate the factors that affect career decisions in dermatology and to determine if these factors can be addressed. We hypothesized that student loan burden influences career plans in dermatology and that physicians are not fully educated on loan repayment options. The aims of this preliminary study were to explore the influence of student loan burden on career plans in dermatology and to determine if the Public Service Loan Forgiveness (PSLF) program could potentially encourage more dermatologists to consider academic careers.
Methods
The study aimed to investigate the factors that influence career decisions in dermatology and to assess attitudes toward the PSLF program as an option for student loan repayment. The target population included dermatology residents and attending physicians in the United States. Survey questions were adapted from a previously published study3 and were modified based on feedback from reviewers in the University of California (UC) Irvine department of dermatology. The survey was voluntary and did not collect identifying information. This study was granted exemption from oversight by the UC Irvine institutional review board.
Recruitment materials informed potential participants of the nature of the study and provided a hyperlink to the electronic survey. The UC Irvine department of dermatology emailed US dermatology residency program coordinators, requesting that they forward this study to residents and attending physicians in their programs.
Results
Demographics
The survey had 70 respondents including residents (56 [80.0%]) and attending physicians (14 [20.0%]). The mean age (SD) of the respondents was 32.4 (6.1) years, with 31 (44.3%) men and 39 (55.7%) women. The majority were married (38 [54.3%]) and did not have children (48 [68.6%]). Most respondents reported an annual household income of $200,000 or less (55 [78.6%]) and perceived a comfortable annual household income as greater than $200,000 (59 [84.3%])(Table 1).
Financing Medical Education
Most respondents currently had $200,000 or less in student loan debt (40 [57.1%]) and financed more than half of their medical education with student loans (53 [75.7%]). A large majority (61 [87.1%]) indicated that some portion of their medical education was funded by student loans.
Career Goals in Dermatology and the Influence of Student Loans
Respondents were asked to specify their career plans before versus after starting dermatology residency training (ie, current career plan). Prior to starting residency, 36 (51.4%) and 34 (48.6%) respondents indicated they were interested in private practice and academia, respectively. After starting residency, the number of respondents interested in private practice increased to 45 (64.3%), and the number of respondents interested in academia decreased to 25 (35.7%). Fifteen (21.4%) respondents changed career trajectories from academia to private practice, 6 (8.6%) changed from private practice to academia, and 49 (70.0%) did not change career goals.
The majority of respondents (39 [55.7%]) indicated that the amount of their student loan debt did not influence their career goals (Table 2); however, those with more than $200,000 in debt were more likely to state that student loans impacted their career goals compared to those with $200,000 or less in debt (70.0% [21/30] vs 25.0% [10/40]; P<.001).
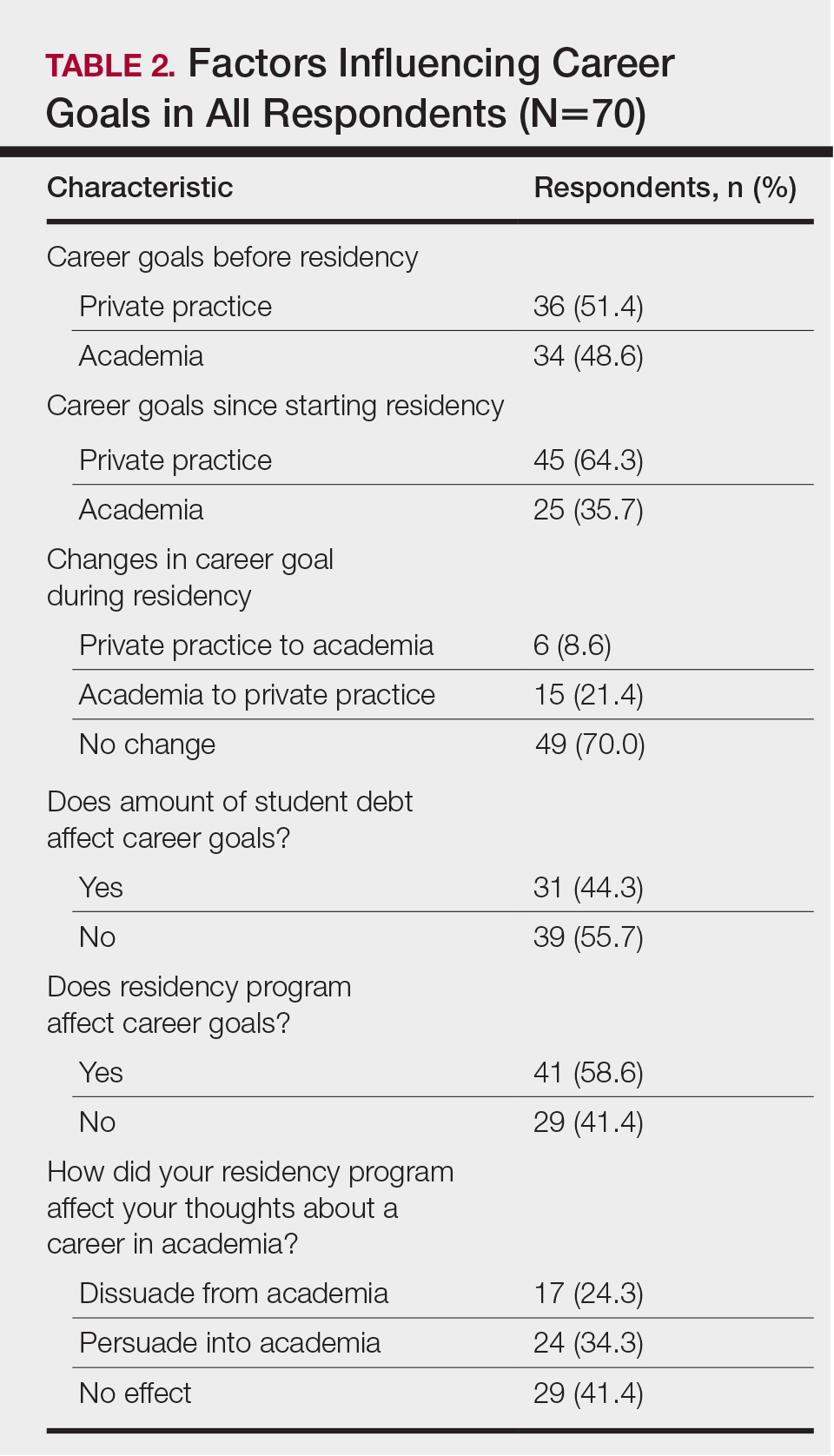
Comparison of Respondents Interested in Careers in Academia vs Private Practice
There were differences in financial circumstances between respondents interested in academia versus those interested in private practice. Compared to respondents interested in academia, those interested in private practice were more likely to have more than $200,000 in student loan debt (24 [53.3%] vs 6 [24.0%]; P<.05), have more than half of their education paid with student loans (38 [84.4%] vs 15 [60.0%]; P<.05), and state that student debt affected their career goals (28 [62.2%] vs 3 [12.0%]; P<.001)(Table 3). Demographic characteristics including gender, marital status, parental status, and current annual household income were not associated with a specific career goal.
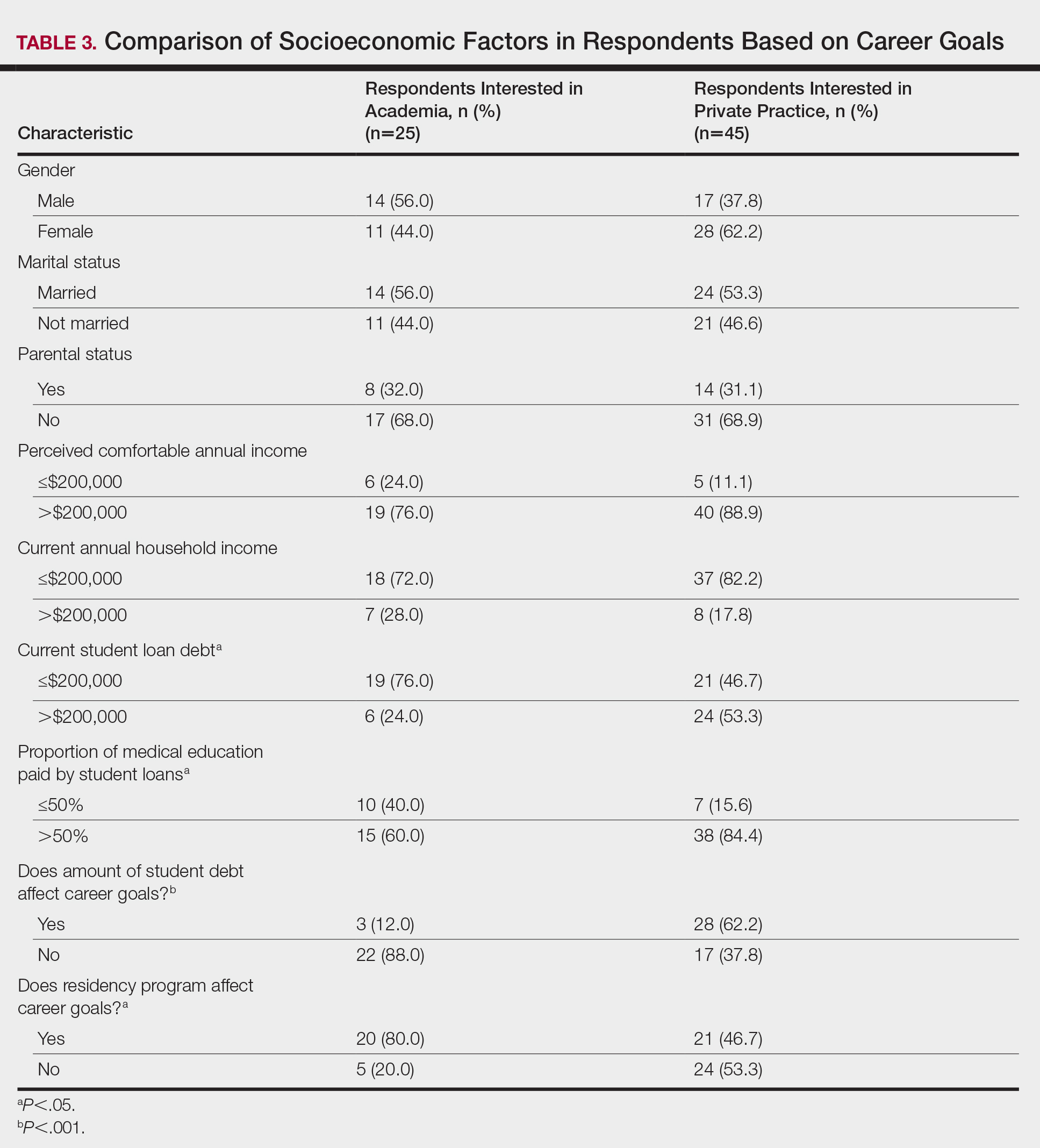
Subgroup analysis was performed on respondents who were initially interested in academic careers but subsequently decided to pursue private practice (n=15).
Residency program experience also may influence career trajectory. The majority (n=41 [58.6%]) of respondents indicated that their residency program experience affected their dermatology career goals. Of those, 41.7% and 58.5% stated that their residency program experiences dissuaded and persuaded them into academic positions, respectively. Those interested in academic dermatology were more likely to state that their residency program experience influenced their career goals (80.0% [20/25] vs 46.7% [21/45]; P<.05). Furthermore, those interested in academic positions responded with higher overall residency program satisfaction ratings on a scale of 1 to 10 (1 indicated the lowest satisfaction) than those interested in private practice, but the difference was not significant (mean [SD] score, 8.2 [2.3] vs 7.2 [1.9]; P=.07).
Respondents were asked to rate their interest in the following dermatology-related professional interests on a scale of 1 to 5 (1 indicated the least enjoyment): medical dermatology, dermatologic surgery, dermatopathology, cosmetics, and lasers. Those interested in private practice versus those interested in academic dermatology found more enjoyment in dermatologic surgery (mean [SD] score, 4.0 [0.8] vs 3.4 [1.3]; P<.05), cosmetics (3.4 [1.2] vs 2.6 [1.4]; P<.05), and lasers (3.7 [1.0] vs 2.8 [1.2]; P<.05)(Table 4).
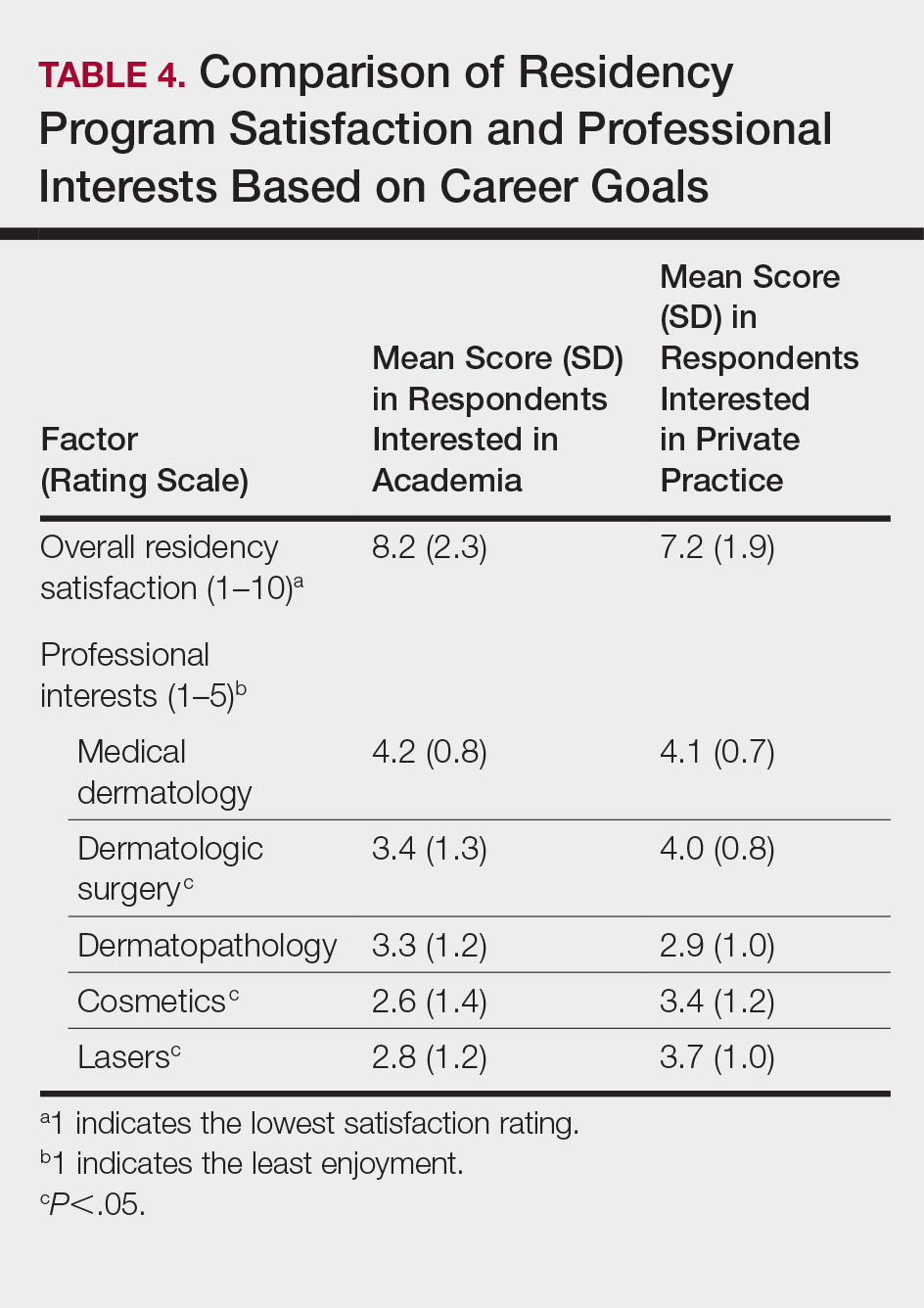
Respondents also were asked to select primary motivating factors for their career goals (ie, academia or private practice) and to indicate reasons for not choosing the alternative. The majority of those pursuing academia were motivated by opportunities to collaborate with colleagues (23 [92.0%]), teach and mentor (20 [80%]), and manage complex cases (17 [68.0%]). Most of the respondents who were pursing private practice were motivated by focus on patient care and clinician duties (36 [80.0%]), flexible work hours (35 [77.8%]), higher income (33 [73.3%]), and location flexibility (29 [64.4%]). Among those interested in academic dermatology, the top factors for disinterest in private practice were running a business (9 [36.0%]) and high patient turnover (9 [36.0%]). Most of those interested in private practice indicated that they were not interested in an academic position because of lower income (31 [68.9%]) and research duties (31 [68.9%])(Table 5).
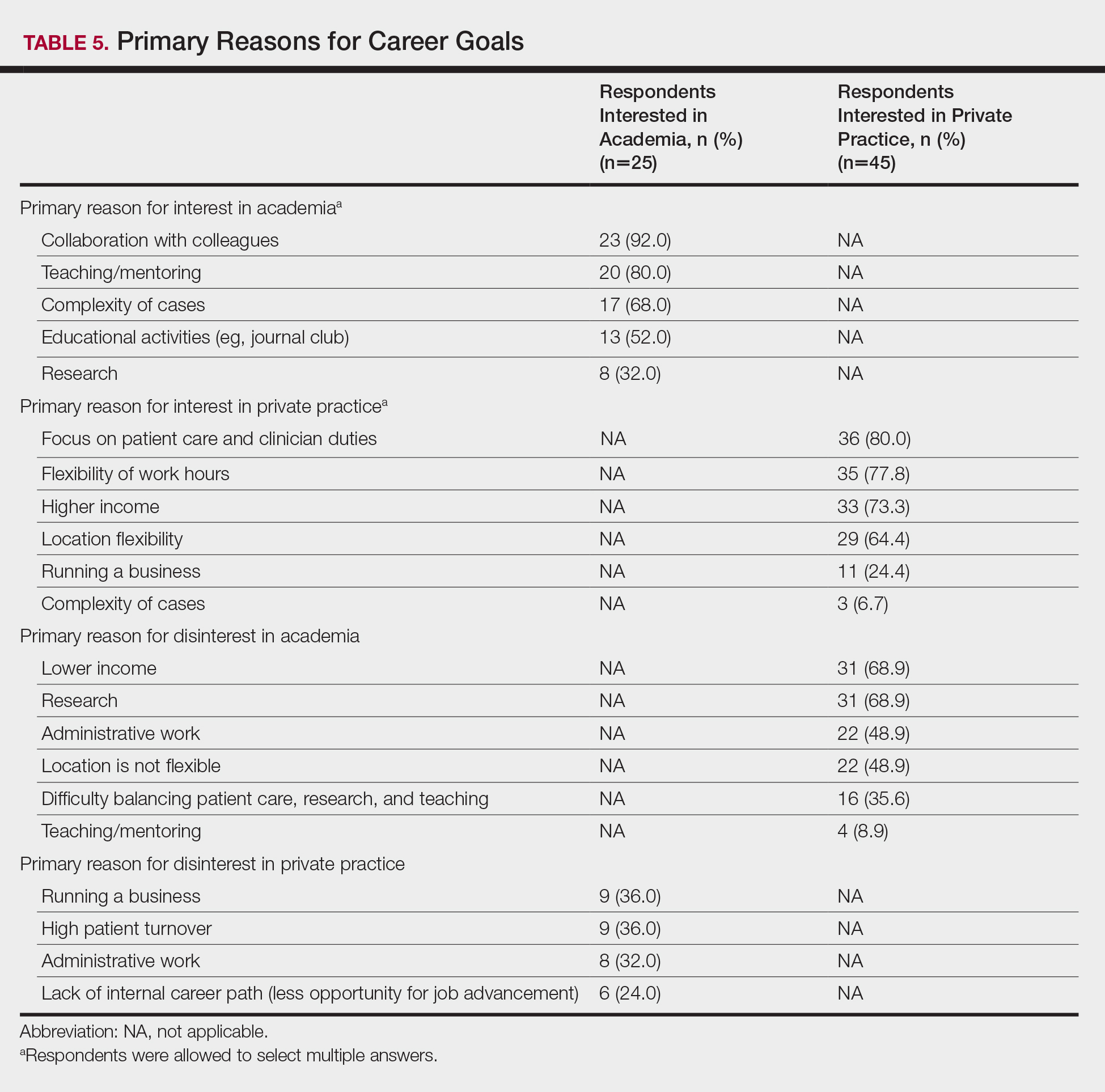
Awareness of and Attitudes Toward the PSLF Program
The majority of respondents were aware of PSLF (53 [75.7%]); however, only 1 respondent endorsed current plans to use PSLF for loan repayment. Respondents were asked how likely they would be to pursue an academic position if given the option to have their student loans forgiven by the PSLF program. Overall, 44.6% (n=25) of respondents indicated that this option would have no effect or would unlikely convince them to pursue an academic position, and 55.4% (n=31) of respondents indicated that they were somewhat likely, likely, or very likely to pursue academia if PSLF was an option. Of those who stated that they would consider enrolling in PSLF, 64.5% (20/31) of individuals were pursuing careers in private practice. Neither current student loan burden nor career goal was associated with likelihood of enrolling in the PSLF.
Comment
In 2015, 76% of medical school graduates in the United States accrued educational debt, with an average of $189,165, a number that has continued to increase over the years.4 In addition to the increasing cost of medical education, higher interest rates on federal student loans contribute to debt burden. Over the last 2 decades, some research has posited that debt may influence medical specialty selection, with most studies focusing on primary care.5-9 However, there is limited information on the effect of student loan debt on career decisions within dermatology.
The results of our study suggest that financial factors including income and amount of educational debt may influence career decisions in dermatology. There is a known income gap between academic and nonacademic settings.
The PSLF can potentially address this issue and be used as a recruiting tool for dermatology positions in academia. Under PSLF, borrowers can have the remainder of their loan balances forgiven after making 120 monthly payments while employed full time by public service employers, including some academic medical institutions. In our study, a large majority of respondents indicated that they are aware of the PSLF, and more than half said they would consider pursuing positions in academia if their loans could be forgiven through the program; however, when asked about plans for loan repayment, only 1 respondent endorsed current plans to enroll in PSLF. Thus, despite high interest in PSLF among the survey respondents, few had actual plans to use the service, suggesting that perhaps dermatologists are not provided enough information about PSLF to motivate enrollment. In the same way, almost a quarter of respondents were not familiar with the PSLF as a repayment option, further signifying that distribution of information about financial planning may be inadequate. If student loan burden is a notable factor in career decisions in dermatology, it is important that academic institutions provide sufficient information about repayment to encourage informed decisions. As such, it is possible that educating physicians about options such as PSLF can potentially recruit more dermatologists to academic positions.
Aside from financial reasons, residency program experience and differences in practices in academic and nonacademic settings may impact career trajectories. The majority of respondents stated their residency program experience influenced their career decisions; however, the majority of respondents did not change their minds about career goals since starting residency, suggesting that residency program experience may reinforce but not necessarily alter these choices. Interests in specific focuses within dermatology also may influence career decisions. This study suggests that those pursuing private practice positions are more interested in dermatologic surgery, lasers, and cosmetics.
In this study, we did not find an association between gender and career plans in dermatology. In 2013, more than 60% of dermatology resident physicians were female.12 However, a recent study suggested that women face challenges in academic dermatology, including a downtrend in the number of female investigators with grants from the National Institutes of Health.13
This preliminary study has several limitations. First, the small sample size limited generalizability to all dermatologists. Second, responder bias was possible, as those who have stronger opinions about this topic may have been more inclined to participate in this voluntary survey. Future studies with larger sample sizes are needed to further explore the factors that influence career decisions within dermatology and to determine if there are additional means to increase recruitment into academia.
Conclusion
It is recognized that there are challenges in recruiting dermatologists into academic positions. This study suggests that student loan burden influences career decisions in dermatology. Dermatologists may not be fully educated on options for student loan repayment. With increased awareness, the PSLF can potentially be used as a recruitment tool for positions in academic dermatology.
- Resneck JS Jr, Kimball AB. The dermatology workforce shortage. J Am Acad Dermatol. 2004;50:50-54.
- Resneck JS Jr, Tierney EP, Kimball AB. Challenges facing academic dermatology: survey data on the faculty workforce. J Am Acad Dermatol. 2006;54:211-216.
- Lanzon J, Edwards SP, Inglehart MR. Choosing academia versus private practice: factors affecting oral maxillofacial surgery residents’ career choices. J Oral Maxillofac Surg. 2012;70:1751-1761.
- AAMC Medical Student Education: Debt, Costs, and Loan Repayment Fact Card. https://members.aamc.org/eweb/upload/2016_Debt_Fact_Card.pdf. Published October 2016. Accessed November 18, 2017.
- Rosenblatt RA, Andrilla CH. The impact of U.S. medical students’ debt on their choice of primary care careers: an analysis of data from the 2002 medical school graduation questionnaire. Acad Med. 2005;80:815-819.
- Woodworth PA, Chang FC, Helmer SD. Debt and other influences on career choices among surgical and primary care residents in a community-based hospital system. Am J Surg. 2000;180:570-575; discussion 575-576.
- Phillips RL Jr, Dodoo MS, Petterson S, et al. Specialty and geographic distribution of the physician workforce: what influences medical student and resident choices? Robert Graham Center website. http://www.graham-center.org/dam/rgc/documents/publications-reports/monographs-books/Specialty-geography-compressed.pdf. Published March 2, 2009. Accessed November 17, 2017.
- Rosenthal MP, Marquette PA, Diamond JJ. Trends along the debt-income axis: implications for medical students’ selections of family practice careers. Acad Med. 1996;71:675-677.
- McDonald FS, West CP, Popkave C, et al. Educational debt and reported career plans among internal medicine residents. Ann Intern Med. 2008;149:416-420.
- Careers in Medicine. Association of American Medical Colleges website. https://www.aamc.org/cim/specialty/exploreoptions/list/us/336836/dermatology.html. Accessed November 18, 2017.
- Youngclaus JA, Koehler PA, Kotlikoff LJ, et al. Can medical students afford to choose primary care? an economic analysis of physician education debt repayment. Acad Med. 2013;88:16-25.
- Physician specialty data book 2014. Association of American Medical Colleges website. https://members.aamc.org/eweb/upload/Physician Specialty Databook 2014.pdf. Published November 2014. Updated June 3, 2015. Accessed November 17, 2017.
- Cheng MY, Sukhov A, Sultani H, et al. Trends in National Institutes of Health funding of principal investigators in dermatology research by academic degree and sex. JAMA Dermatol. 2016;152:883-888.
Dermatology departments in the United States have been facing challenges in recruiting and retaining dermatologists for academic positions. Accordingly, a survey study reported that academic dermatologists were more likely than those in private practice to state that their institutions were recruiting new associates.1 Several factors could explain this phenomenon. Salary differences between jobs in academic and nonacademic settings may contribute to difficulty in recruiting dermatologists into academia, which is exacerbated by a theoretical shortage of dermatologists, leading to graduates who receive and accept private practice job offers.1,2 Furthermore, a large survey study reported that challenges unique to academic dermatologists include longer patient wait times in addition to responsibilities such as research, hospital consultations, medical writing, and teaching. These patterns raise concerns for the future of teaching institutions because academic dermatologists not only train future physicians but also conduct clinical and basic science research necessary to advance the field and improve patient care.2 Thus, it is important to evaluate the factors that affect career decisions in dermatology and to determine if these factors can be addressed. We hypothesized that student loan burden influences career plans in dermatology and that physicians are not fully educated on loan repayment options. The aims of this preliminary study were to explore the influence of student loan burden on career plans in dermatology and to determine if the Public Service Loan Forgiveness (PSLF) program could potentially encourage more dermatologists to consider academic careers.
Methods
The study aimed to investigate the factors that influence career decisions in dermatology and to assess attitudes toward the PSLF program as an option for student loan repayment. The target population included dermatology residents and attending physicians in the United States. Survey questions were adapted from a previously published study3 and were modified based on feedback from reviewers in the University of California (UC) Irvine department of dermatology. The survey was voluntary and did not collect identifying information. This study was granted exemption from oversight by the UC Irvine institutional review board.
Recruitment materials informed potential participants of the nature of the study and provided a hyperlink to the electronic survey. The UC Irvine department of dermatology emailed US dermatology residency program coordinators, requesting that they forward this study to residents and attending physicians in their programs.
Results
Demographics
The survey had 70 respondents including residents (56 [80.0%]) and attending physicians (14 [20.0%]). The mean age (SD) of the respondents was 32.4 (6.1) years, with 31 (44.3%) men and 39 (55.7%) women. The majority were married (38 [54.3%]) and did not have children (48 [68.6%]). Most respondents reported an annual household income of $200,000 or less (55 [78.6%]) and perceived a comfortable annual household income as greater than $200,000 (59 [84.3%])(Table 1).

Financing Medical Education
Most respondents currently had $200,000 or less in student loan debt (40 [57.1%]) and financed more than half of their medical education with student loans (53 [75.7%]). A large majority (61 [87.1%]) indicated that some portion of their medical education was funded by student loans.
Career Goals in Dermatology and the Influence of Student Loans
Respondents were asked to specify their career plans before versus after starting dermatology residency training (ie, current career plan). Prior to starting residency, 36 (51.4%) and 34 (48.6%) respondents indicated they were interested in private practice and academia, respectively. After starting residency, the number of respondents interested in private practice increased to 45 (64.3%), and the number of respondents interested in academia decreased to 25 (35.7%). Fifteen (21.4%) respondents changed career trajectories from academia to private practice, 6 (8.6%) changed from private practice to academia, and 49 (70.0%) did not change career goals.
The majority of respondents (39 [55.7%]) indicated that the amount of their student loan debt did not influence their career goals (Table 2); however, those with more than $200,000 in debt were more likely to state that student loans impacted their career goals compared to those with $200,000 or less in debt (70.0% [21/30] vs 25.0% [10/40]; P<.001).

Comparison of Respondents Interested in Careers in Academia vs Private Practice
There were differences in financial circumstances between respondents interested in academia versus those interested in private practice. Compared to respondents interested in academia, those interested in private practice were more likely to have more than $200,000 in student loan debt (24 [53.3%] vs 6 [24.0%]; P<.05), have more than half of their education paid with student loans (38 [84.4%] vs 15 [60.0%]; P<.05), and state that student debt affected their career goals (28 [62.2%] vs 3 [12.0%]; P<.001)(Table 3). Demographic characteristics including gender, marital status, parental status, and current annual household income were not associated with a specific career goal.

Subgroup analysis was performed on respondents who were initially interested in academic careers but subsequently decided to pursue private practice (n=15).
Residency program experience also may influence career trajectory. The majority (n=41 [58.6%]) of respondents indicated that their residency program experience affected their dermatology career goals. Of those, 41.7% and 58.5% stated that their residency program experiences dissuaded and persuaded them into academic positions, respectively. Those interested in academic dermatology were more likely to state that their residency program experience influenced their career goals (80.0% [20/25] vs 46.7% [21/45]; P<.05). Furthermore, those interested in academic positions responded with higher overall residency program satisfaction ratings on a scale of 1 to 10 (1 indicated the lowest satisfaction) than those interested in private practice, but the difference was not significant (mean [SD] score, 8.2 [2.3] vs 7.2 [1.9]; P=.07).
Respondents were asked to rate their interest in the following dermatology-related professional interests on a scale of 1 to 5 (1 indicated the least enjoyment): medical dermatology, dermatologic surgery, dermatopathology, cosmetics, and lasers. Those interested in private practice versus those interested in academic dermatology found more enjoyment in dermatologic surgery (mean [SD] score, 4.0 [0.8] vs 3.4 [1.3]; P<.05), cosmetics (3.4 [1.2] vs 2.6 [1.4]; P<.05), and lasers (3.7 [1.0] vs 2.8 [1.2]; P<.05)(Table 4).

Respondents also were asked to select primary motivating factors for their career goals (ie, academia or private practice) and to indicate reasons for not choosing the alternative. The majority of those pursuing academia were motivated by opportunities to collaborate with colleagues (23 [92.0%]), teach and mentor (20 [80%]), and manage complex cases (17 [68.0%]). Most of the respondents who were pursing private practice were motivated by focus on patient care and clinician duties (36 [80.0%]), flexible work hours (35 [77.8%]), higher income (33 [73.3%]), and location flexibility (29 [64.4%]). Among those interested in academic dermatology, the top factors for disinterest in private practice were running a business (9 [36.0%]) and high patient turnover (9 [36.0%]). Most of those interested in private practice indicated that they were not interested in an academic position because of lower income (31 [68.9%]) and research duties (31 [68.9%])(Table 5).

Awareness of and Attitudes Toward the PSLF Program
The majority of respondents were aware of PSLF (53 [75.7%]); however, only 1 respondent endorsed current plans to use PSLF for loan repayment. Respondents were asked how likely they would be to pursue an academic position if given the option to have their student loans forgiven by the PSLF program. Overall, 44.6% (n=25) of respondents indicated that this option would have no effect or would unlikely convince them to pursue an academic position, and 55.4% (n=31) of respondents indicated that they were somewhat likely, likely, or very likely to pursue academia if PSLF was an option. Of those who stated that they would consider enrolling in PSLF, 64.5% (20/31) of individuals were pursuing careers in private practice. Neither current student loan burden nor career goal was associated with likelihood of enrolling in the PSLF.
Comment
In 2015, 76% of medical school graduates in the United States accrued educational debt, with an average of $189,165, a number that has continued to increase over the years.4 In addition to the increasing cost of medical education, higher interest rates on federal student loans contribute to debt burden. Over the last 2 decades, some research has posited that debt may influence medical specialty selection, with most studies focusing on primary care.5-9 However, there is limited information on the effect of student loan debt on career decisions within dermatology.
The results of our study suggest that financial factors including income and amount of educational debt may influence career decisions in dermatology. There is a known income gap between academic and nonacademic settings.
The PSLF can potentially address this issue and be used as a recruiting tool for dermatology positions in academia. Under PSLF, borrowers can have the remainder of their loan balances forgiven after making 120 monthly payments while employed full time by public service employers, including some academic medical institutions. In our study, a large majority of respondents indicated that they are aware of the PSLF, and more than half said they would consider pursuing positions in academia if their loans could be forgiven through the program; however, when asked about plans for loan repayment, only 1 respondent endorsed current plans to enroll in PSLF. Thus, despite high interest in PSLF among the survey respondents, few had actual plans to use the service, suggesting that perhaps dermatologists are not provided enough information about PSLF to motivate enrollment. In the same way, almost a quarter of respondents were not familiar with the PSLF as a repayment option, further signifying that distribution of information about financial planning may be inadequate. If student loan burden is a notable factor in career decisions in dermatology, it is important that academic institutions provide sufficient information about repayment to encourage informed decisions. As such, it is possible that educating physicians about options such as PSLF can potentially recruit more dermatologists to academic positions.
Aside from financial reasons, residency program experience and differences in practices in academic and nonacademic settings may impact career trajectories. The majority of respondents stated their residency program experience influenced their career decisions; however, the majority of respondents did not change their minds about career goals since starting residency, suggesting that residency program experience may reinforce but not necessarily alter these choices. Interests in specific focuses within dermatology also may influence career decisions. This study suggests that those pursuing private practice positions are more interested in dermatologic surgery, lasers, and cosmetics.
In this study, we did not find an association between gender and career plans in dermatology. In 2013, more than 60% of dermatology resident physicians were female.12 However, a recent study suggested that women face challenges in academic dermatology, including a downtrend in the number of female investigators with grants from the National Institutes of Health.13
This preliminary study has several limitations. First, the small sample size limited generalizability to all dermatologists. Second, responder bias was possible, as those who have stronger opinions about this topic may have been more inclined to participate in this voluntary survey. Future studies with larger sample sizes are needed to further explore the factors that influence career decisions within dermatology and to determine if there are additional means to increase recruitment into academia.
Conclusion
It is recognized that there are challenges in recruiting dermatologists into academic positions. This study suggests that student loan burden influences career decisions in dermatology. Dermatologists may not be fully educated on options for student loan repayment. With increased awareness, the PSLF can potentially be used as a recruitment tool for positions in academic dermatology.
Dermatology departments in the United States have been facing challenges in recruiting and retaining dermatologists for academic positions. Accordingly, a survey study reported that academic dermatologists were more likely than those in private practice to state that their institutions were recruiting new associates.1 Several factors could explain this phenomenon. Salary differences between jobs in academic and nonacademic settings may contribute to difficulty in recruiting dermatologists into academia, which is exacerbated by a theoretical shortage of dermatologists, leading to graduates who receive and accept private practice job offers.1,2 Furthermore, a large survey study reported that challenges unique to academic dermatologists include longer patient wait times in addition to responsibilities such as research, hospital consultations, medical writing, and teaching. These patterns raise concerns for the future of teaching institutions because academic dermatologists not only train future physicians but also conduct clinical and basic science research necessary to advance the field and improve patient care.2 Thus, it is important to evaluate the factors that affect career decisions in dermatology and to determine if these factors can be addressed. We hypothesized that student loan burden influences career plans in dermatology and that physicians are not fully educated on loan repayment options. The aims of this preliminary study were to explore the influence of student loan burden on career plans in dermatology and to determine if the Public Service Loan Forgiveness (PSLF) program could potentially encourage more dermatologists to consider academic careers.
Methods
The study aimed to investigate the factors that influence career decisions in dermatology and to assess attitudes toward the PSLF program as an option for student loan repayment. The target population included dermatology residents and attending physicians in the United States. Survey questions were adapted from a previously published study3 and were modified based on feedback from reviewers in the University of California (UC) Irvine department of dermatology. The survey was voluntary and did not collect identifying information. This study was granted exemption from oversight by the UC Irvine institutional review board.
Recruitment materials informed potential participants of the nature of the study and provided a hyperlink to the electronic survey. The UC Irvine department of dermatology emailed US dermatology residency program coordinators, requesting that they forward this study to residents and attending physicians in their programs.
Results
Demographics
The survey had 70 respondents including residents (56 [80.0%]) and attending physicians (14 [20.0%]). The mean age (SD) of the respondents was 32.4 (6.1) years, with 31 (44.3%) men and 39 (55.7%) women. The majority were married (38 [54.3%]) and did not have children (48 [68.6%]). Most respondents reported an annual household income of $200,000 or less (55 [78.6%]) and perceived a comfortable annual household income as greater than $200,000 (59 [84.3%])(Table 1).

Financing Medical Education
Most respondents currently had $200,000 or less in student loan debt (40 [57.1%]) and financed more than half of their medical education with student loans (53 [75.7%]). A large majority (61 [87.1%]) indicated that some portion of their medical education was funded by student loans.
Career Goals in Dermatology and the Influence of Student Loans
Respondents were asked to specify their career plans before versus after starting dermatology residency training (ie, current career plan). Prior to starting residency, 36 (51.4%) and 34 (48.6%) respondents indicated they were interested in private practice and academia, respectively. After starting residency, the number of respondents interested in private practice increased to 45 (64.3%), and the number of respondents interested in academia decreased to 25 (35.7%). Fifteen (21.4%) respondents changed career trajectories from academia to private practice, 6 (8.6%) changed from private practice to academia, and 49 (70.0%) did not change career goals.
The majority of respondents (39 [55.7%]) indicated that the amount of their student loan debt did not influence their career goals (Table 2); however, those with more than $200,000 in debt were more likely to state that student loans impacted their career goals compared to those with $200,000 or less in debt (70.0% [21/30] vs 25.0% [10/40]; P<.001).

Comparison of Respondents Interested in Careers in Academia vs Private Practice
There were differences in financial circumstances between respondents interested in academia versus those interested in private practice. Compared to respondents interested in academia, those interested in private practice were more likely to have more than $200,000 in student loan debt (24 [53.3%] vs 6 [24.0%]; P<.05), have more than half of their education paid with student loans (38 [84.4%] vs 15 [60.0%]; P<.05), and state that student debt affected their career goals (28 [62.2%] vs 3 [12.0%]; P<.001)(Table 3). Demographic characteristics including gender, marital status, parental status, and current annual household income were not associated with a specific career goal.

Subgroup analysis was performed on respondents who were initially interested in academic careers but subsequently decided to pursue private practice (n=15).
Residency program experience also may influence career trajectory. The majority (n=41 [58.6%]) of respondents indicated that their residency program experience affected their dermatology career goals. Of those, 41.7% and 58.5% stated that their residency program experiences dissuaded and persuaded them into academic positions, respectively. Those interested in academic dermatology were more likely to state that their residency program experience influenced their career goals (80.0% [20/25] vs 46.7% [21/45]; P<.05). Furthermore, those interested in academic positions responded with higher overall residency program satisfaction ratings on a scale of 1 to 10 (1 indicated the lowest satisfaction) than those interested in private practice, but the difference was not significant (mean [SD] score, 8.2 [2.3] vs 7.2 [1.9]; P=.07).
Respondents were asked to rate their interest in the following dermatology-related professional interests on a scale of 1 to 5 (1 indicated the least enjoyment): medical dermatology, dermatologic surgery, dermatopathology, cosmetics, and lasers. Those interested in private practice versus those interested in academic dermatology found more enjoyment in dermatologic surgery (mean [SD] score, 4.0 [0.8] vs 3.4 [1.3]; P<.05), cosmetics (3.4 [1.2] vs 2.6 [1.4]; P<.05), and lasers (3.7 [1.0] vs 2.8 [1.2]; P<.05)(Table 4).

Respondents also were asked to select primary motivating factors for their career goals (ie, academia or private practice) and to indicate reasons for not choosing the alternative. The majority of those pursuing academia were motivated by opportunities to collaborate with colleagues (23 [92.0%]), teach and mentor (20 [80%]), and manage complex cases (17 [68.0%]). Most of the respondents who were pursing private practice were motivated by focus on patient care and clinician duties (36 [80.0%]), flexible work hours (35 [77.8%]), higher income (33 [73.3%]), and location flexibility (29 [64.4%]). Among those interested in academic dermatology, the top factors for disinterest in private practice were running a business (9 [36.0%]) and high patient turnover (9 [36.0%]). Most of those interested in private practice indicated that they were not interested in an academic position because of lower income (31 [68.9%]) and research duties (31 [68.9%])(Table 5).

Awareness of and Attitudes Toward the PSLF Program
The majority of respondents were aware of PSLF (53 [75.7%]); however, only 1 respondent endorsed current plans to use PSLF for loan repayment. Respondents were asked how likely they would be to pursue an academic position if given the option to have their student loans forgiven by the PSLF program. Overall, 44.6% (n=25) of respondents indicated that this option would have no effect or would unlikely convince them to pursue an academic position, and 55.4% (n=31) of respondents indicated that they were somewhat likely, likely, or very likely to pursue academia if PSLF was an option. Of those who stated that they would consider enrolling in PSLF, 64.5% (20/31) of individuals were pursuing careers in private practice. Neither current student loan burden nor career goal was associated with likelihood of enrolling in the PSLF.
Comment
In 2015, 76% of medical school graduates in the United States accrued educational debt, with an average of $189,165, a number that has continued to increase over the years.4 In addition to the increasing cost of medical education, higher interest rates on federal student loans contribute to debt burden. Over the last 2 decades, some research has posited that debt may influence medical specialty selection, with most studies focusing on primary care.5-9 However, there is limited information on the effect of student loan debt on career decisions within dermatology.
The results of our study suggest that financial factors including income and amount of educational debt may influence career decisions in dermatology. There is a known income gap between academic and nonacademic settings.
The PSLF can potentially address this issue and be used as a recruiting tool for dermatology positions in academia. Under PSLF, borrowers can have the remainder of their loan balances forgiven after making 120 monthly payments while employed full time by public service employers, including some academic medical institutions. In our study, a large majority of respondents indicated that they are aware of the PSLF, and more than half said they would consider pursuing positions in academia if their loans could be forgiven through the program; however, when asked about plans for loan repayment, only 1 respondent endorsed current plans to enroll in PSLF. Thus, despite high interest in PSLF among the survey respondents, few had actual plans to use the service, suggesting that perhaps dermatologists are not provided enough information about PSLF to motivate enrollment. In the same way, almost a quarter of respondents were not familiar with the PSLF as a repayment option, further signifying that distribution of information about financial planning may be inadequate. If student loan burden is a notable factor in career decisions in dermatology, it is important that academic institutions provide sufficient information about repayment to encourage informed decisions. As such, it is possible that educating physicians about options such as PSLF can potentially recruit more dermatologists to academic positions.
Aside from financial reasons, residency program experience and differences in practices in academic and nonacademic settings may impact career trajectories. The majority of respondents stated their residency program experience influenced their career decisions; however, the majority of respondents did not change their minds about career goals since starting residency, suggesting that residency program experience may reinforce but not necessarily alter these choices. Interests in specific focuses within dermatology also may influence career decisions. This study suggests that those pursuing private practice positions are more interested in dermatologic surgery, lasers, and cosmetics.
In this study, we did not find an association between gender and career plans in dermatology. In 2013, more than 60% of dermatology resident physicians were female.12 However, a recent study suggested that women face challenges in academic dermatology, including a downtrend in the number of female investigators with grants from the National Institutes of Health.13
This preliminary study has several limitations. First, the small sample size limited generalizability to all dermatologists. Second, responder bias was possible, as those who have stronger opinions about this topic may have been more inclined to participate in this voluntary survey. Future studies with larger sample sizes are needed to further explore the factors that influence career decisions within dermatology and to determine if there are additional means to increase recruitment into academia.
Conclusion
It is recognized that there are challenges in recruiting dermatologists into academic positions. This study suggests that student loan burden influences career decisions in dermatology. Dermatologists may not be fully educated on options for student loan repayment. With increased awareness, the PSLF can potentially be used as a recruitment tool for positions in academic dermatology.
- Resneck JS Jr, Kimball AB. The dermatology workforce shortage. J Am Acad Dermatol. 2004;50:50-54.
- Resneck JS Jr, Tierney EP, Kimball AB. Challenges facing academic dermatology: survey data on the faculty workforce. J Am Acad Dermatol. 2006;54:211-216.
- Lanzon J, Edwards SP, Inglehart MR. Choosing academia versus private practice: factors affecting oral maxillofacial surgery residents’ career choices. J Oral Maxillofac Surg. 2012;70:1751-1761.
- AAMC Medical Student Education: Debt, Costs, and Loan Repayment Fact Card. https://members.aamc.org/eweb/upload/2016_Debt_Fact_Card.pdf. Published October 2016. Accessed November 18, 2017.
- Rosenblatt RA, Andrilla CH. The impact of U.S. medical students’ debt on their choice of primary care careers: an analysis of data from the 2002 medical school graduation questionnaire. Acad Med. 2005;80:815-819.
- Woodworth PA, Chang FC, Helmer SD. Debt and other influences on career choices among surgical and primary care residents in a community-based hospital system. Am J Surg. 2000;180:570-575; discussion 575-576.
- Phillips RL Jr, Dodoo MS, Petterson S, et al. Specialty and geographic distribution of the physician workforce: what influences medical student and resident choices? Robert Graham Center website. http://www.graham-center.org/dam/rgc/documents/publications-reports/monographs-books/Specialty-geography-compressed.pdf. Published March 2, 2009. Accessed November 17, 2017.
- Rosenthal MP, Marquette PA, Diamond JJ. Trends along the debt-income axis: implications for medical students’ selections of family practice careers. Acad Med. 1996;71:675-677.
- McDonald FS, West CP, Popkave C, et al. Educational debt and reported career plans among internal medicine residents. Ann Intern Med. 2008;149:416-420.
- Careers in Medicine. Association of American Medical Colleges website. https://www.aamc.org/cim/specialty/exploreoptions/list/us/336836/dermatology.html. Accessed November 18, 2017.
- Youngclaus JA, Koehler PA, Kotlikoff LJ, et al. Can medical students afford to choose primary care? an economic analysis of physician education debt repayment. Acad Med. 2013;88:16-25.
- Physician specialty data book 2014. Association of American Medical Colleges website. https://members.aamc.org/eweb/upload/Physician Specialty Databook 2014.pdf. Published November 2014. Updated June 3, 2015. Accessed November 17, 2017.
- Cheng MY, Sukhov A, Sultani H, et al. Trends in National Institutes of Health funding of principal investigators in dermatology research by academic degree and sex. JAMA Dermatol. 2016;152:883-888.
- Resneck JS Jr, Kimball AB. The dermatology workforce shortage. J Am Acad Dermatol. 2004;50:50-54.
- Resneck JS Jr, Tierney EP, Kimball AB. Challenges facing academic dermatology: survey data on the faculty workforce. J Am Acad Dermatol. 2006;54:211-216.
- Lanzon J, Edwards SP, Inglehart MR. Choosing academia versus private practice: factors affecting oral maxillofacial surgery residents’ career choices. J Oral Maxillofac Surg. 2012;70:1751-1761.
- AAMC Medical Student Education: Debt, Costs, and Loan Repayment Fact Card. https://members.aamc.org/eweb/upload/2016_Debt_Fact_Card.pdf. Published October 2016. Accessed November 18, 2017.
- Rosenblatt RA, Andrilla CH. The impact of U.S. medical students’ debt on their choice of primary care careers: an analysis of data from the 2002 medical school graduation questionnaire. Acad Med. 2005;80:815-819.
- Woodworth PA, Chang FC, Helmer SD. Debt and other influences on career choices among surgical and primary care residents in a community-based hospital system. Am J Surg. 2000;180:570-575; discussion 575-576.
- Phillips RL Jr, Dodoo MS, Petterson S, et al. Specialty and geographic distribution of the physician workforce: what influences medical student and resident choices? Robert Graham Center website. http://www.graham-center.org/dam/rgc/documents/publications-reports/monographs-books/Specialty-geography-compressed.pdf. Published March 2, 2009. Accessed November 17, 2017.
- Rosenthal MP, Marquette PA, Diamond JJ. Trends along the debt-income axis: implications for medical students’ selections of family practice careers. Acad Med. 1996;71:675-677.
- McDonald FS, West CP, Popkave C, et al. Educational debt and reported career plans among internal medicine residents. Ann Intern Med. 2008;149:416-420.
- Careers in Medicine. Association of American Medical Colleges website. https://www.aamc.org/cim/specialty/exploreoptions/list/us/336836/dermatology.html. Accessed November 18, 2017.
- Youngclaus JA, Koehler PA, Kotlikoff LJ, et al. Can medical students afford to choose primary care? an economic analysis of physician education debt repayment. Acad Med. 2013;88:16-25.
- Physician specialty data book 2014. Association of American Medical Colleges website. https://members.aamc.org/eweb/upload/Physician Specialty Databook 2014.pdf. Published November 2014. Updated June 3, 2015. Accessed November 17, 2017.
- Cheng MY, Sukhov A, Sultani H, et al. Trends in National Institutes of Health funding of principal investigators in dermatology research by academic degree and sex. JAMA Dermatol. 2016;152:883-888.
Practice Points
- Academic dermatology departments are facing challenges in recruiting physicians, raising concerns for the future of dermatology education and research.
- Large amounts of student loan burden may influence career plans in dermatology.
- Dermatologists may not be fully knowledgeable of loan repayment options; thus, education on this topic should be prioritized by dermatology training programs.
Multinucleate Cell Angiohistiocytoma
Multinucleate cell angiohistiocytoma (MCAH) is a rare benign, soft-tissue tumor first described in 1985 by Smith and Jones1 that presents clinically as erythematous to violaceous papules most commonly affecting females on the dorsal aspect of the hands and face.2 Multinucleate cell angiohistiocytoma is histologically characterized by vascular and histiocytic proliferations with dermal fibrosis. Few cases have been reported of lesions affecting the lower extremities. We report a case of MCAH affecting the legs.
Case Report
An 83-year-old white man with a history of basal cell carcinoma presented for evaluation of grouped, well-circumscribed, soft, red-violet, painless papules on the right anterior thigh that had been present for 8 months (Figure 1A). A review of symptoms was negative for immunologic, respiratory, and hematologic changes. The patient’s medical history also was remarkable for prostate cancer treated with radiation 18 years prior as well as right hip and left knee implants. The initial clinical impression was Kaposi sarcoma or a granulomatous disorder.
Histopathologic evaluation of a deep shave biopsy initially determined the lesion to be scar tissue without other pathologic findings. The patient returned to the clinic 12 months later for a complete skin examination given his history of skin cancer. Compared to clinical photographs taken a year prior, new violaceous papules were noted on the right thigh (Figure 1B) and left calf. Furthermore, there was no recurrence of the lesion at the prior biopsy site. Shave biopsies of the papules on the right thigh and left calf demonstrated similar histologic findings to each other. There was a mild increase in the number of small blood vessels in the superficial dermis (Figure 2A). A mild perivascular lymphocytic infiltrate surrounded some of these blood vessels. The endothelial cells had small nuclei with no evidence of nuclear pleomorphism. Careful examination of the interstitial dermis revealed scattered multinucleate cells with angulated cytoplasm (Figure 2B). Immunostaining for CD31 and human herpesvirus 8 were negative, excluding an infiltrative vascular tumor and Kaposi sarcoma, respectively. The diagnosis of MCAH was made based on the histopathologic findings.
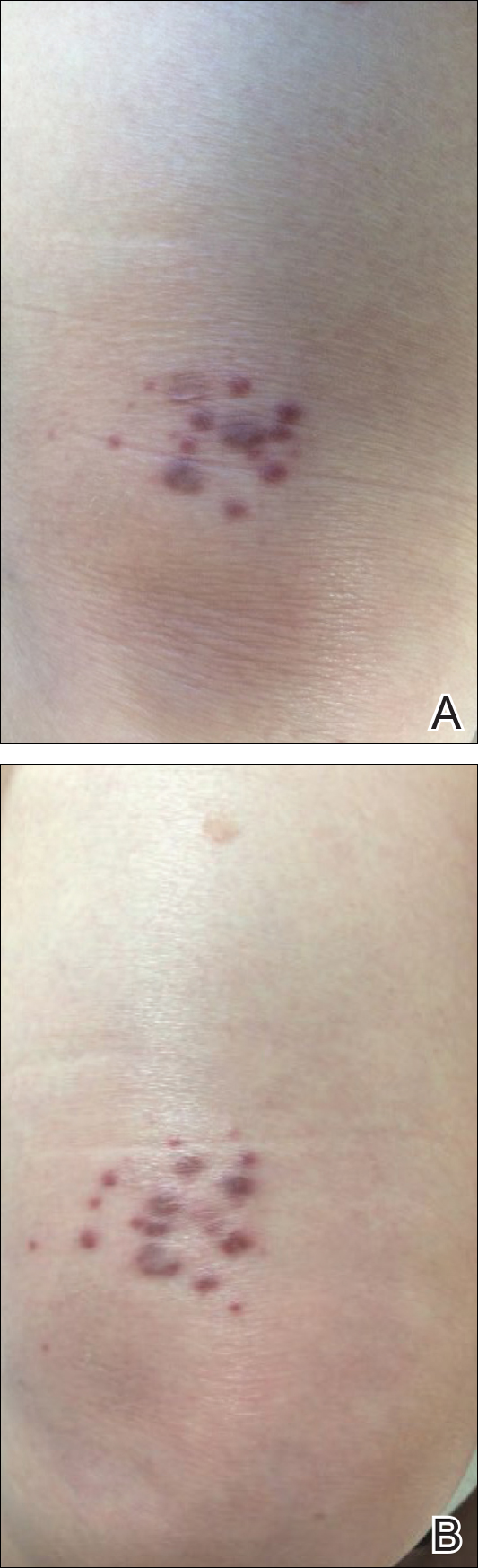
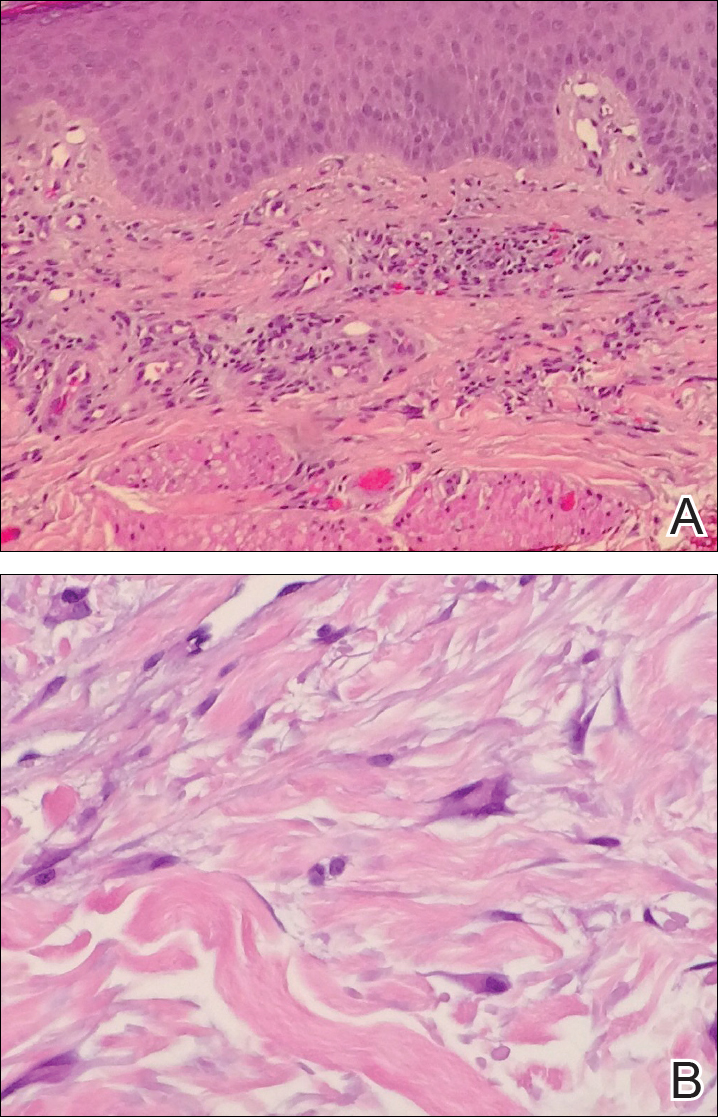
At 1-year follow-up, the condition was stable with no gross changes in the lesions based on prior photographs. Once again, there was no recurrence of the excised lesions at both biopsy sites.
Comment
Presentation
A systematic review of published reports determined that 79% of MCAH cases occur in females, with an average age of onset of 50.1 years.2 However, MCAH likely is underreported due to the overall lack of knowledge regarding this condition by physicians and pathologists. The hands and face are the most commonly affected areas, though other sites of involvement have been reported, including the lower extremities,3,4 oral mucosa and upper lip,5,6 and trunk,7,8 as well as generalized distribution.9-12 Additionally, 1 case presented as a single plaque on the trunk rather than having papular or nodular morphology.8 Multinucleate cell angiohistiocytoma lesions generally are asymptomatic, though pruritus may be present.13 The condition is regarded as benign, though a minority of cases have exhibited spontaneous resolution.14-16
Histopathology
Multinucleate cell angiohistiocytoma histology demonstrates full-thickness dermal microvessel proliferation and fibrosis with characteristic multinucleate giant cells.2,3 Vascular endothelial cells stained positive for CD68 in 60% of cases2 as well as the normal endothelial markers (ie, factor VIII, CD31, CD34). The multinucleate giant cells exhibit immunoreactivity for macrophage/histiocytic markers factor XIIIa and CD68.
Etiology
The pathogenesis of MCAH is not yet fully understood, but it is considered to be a benign vascular or fibrohistiocytic neoplasm.17 Calderaro et al18 described a series of 8 patients who developed MCAH either within a cutaneous neoplastic process or in conjunction with various cutaneous reactive conditions, including hidradenitis suppurativa and chronic radiation dermatitis, as well as overlying a bone prosthesis placed due to degenerative arthritis. These cases suggest that MCAH, or possibly a subset of the disease, is a reactive process.
Differential Diagnosis
The differential diagnosis for MCAH includes Kaposi sarcoma clinically and dermatofibroma and fibrous papules histologically. Sass et al21 determined the in vitro behavior of cultured MCAH cells to contrast markedly with Kaposi sarcoma–derived cells. Although Kaposi sarcoma–derived cells exhibited invasive behavior, cells isolated from MCAH lesions were less elongated and were unable to traverse basement membranes.
Treatment
Surgical excision or cryotherapy appear to be definitive treatments of MCAH; however, a number of cases have reported light and laser modalities as successful alternatives to excision. One case of MCAH affecting the face was treated with pulsed dye laser monotherapy.22 This modality allowed selective coagulation of the vascular structures in MCAH. At 8-month follow-up, the initial lesion was noted to be completely cleared, though similar lesions had recently appeared elsewhere on the face.22 Another case of MCAH affecting the leg was treated with pulsed dye laser and both topical and intralesional corticosteroid combination therapy. In this case, the lesion failed to respond to treatment, which may suggest that facial localization could influence response in pulsed dye laser treatment.3
Intense pulsed light also has been reported as a definitive treatment in 2 cases.2,13 Slight erythema and transient pruritus have been reported immediately following treatment. In this case, complete resolution with only residual hyperpigmentation was reported at 2-month follow-up, with no recurrence during 12 months of follow-up.13
Argon laser therapy has been used in 2 cases. After a single session, lesions were no longer palpable, with no scarring noted at 8 weeks follow-up.23 Lastly, 2 cases of MCAH have been successfully treated with the CO2 laser, with no relapse noted at 2.5- or 5-month follow-up, respectively.24
Conclusion
Multinucleate cell angiohistiocytoma is a rare and likely underdiagnosed dermatologic condition that is believed to be a reactive process. Characteristic histology of MCAH demonstrates microvascular proliferations of the dermis with multinucleate giant cells amidst a fibrous background. Although surgical excision is curative, there are reports in which laser and light therapies were used to effectively treat MCAH.
- Smith NP, Jones EW. Multinucleate cell angiohistiocytoma—a new entity. Br J Dermatol. 1985;113:15.
- Frew JW. Multinucleate cell angiohistiocytoma: clinicopathological correlation of 142 cases with insights into etiology and pathogenesis. Am J Dermatopathol. 2015;37:222-228.
- Applebaum DS, Shuja F, Hicks L, et al. Multinucleate cell angiohistiocytoma: a case report and review of the literature. Dermatol Online J. 2014;20:22610.
- Sagdeo A, Chu EY, Elenitsas R, et al. Multiple asymptomatic violaceous macules on the thigh. Multinucleate cell angiohistiocytoma (MCAH). JAMA Dermatol. 2013;149:357-363.
- Rawal YB, Anderson KM, Rawal SY. Multinucleate cell angiohistiocytoma: an uncommon mucosal tumour. Clin Exp Dermatol. 2009;34:333-336.
- Jones AC, Mullins D, Jimenez F. Multinucleate cell angiohistiocytoma of the upper lip. Oral Surg Oral Med Oral Pathol. 1994;78:743-747.
- Doshi-Chougule BN, Gust A, Mentzel T, et al. Multinucleate cell angiohistiocytoma with hypertrophic nerves. J Cutan Pathol. 2013;40:1048-1053.
- Issa AA, Lui H, Shapiro J, et al. Plaque-type multinucleate cell angiohistiocytoma. J Cutan Med Surg. 1998;3:112-114.
- Doane JA, Purdy K, Pasternak S. Generalized multinucleate cell angiohistiocytoma. J Cutan Med Surg. 2015;19:323-325.
- Marti N, Monteagudo C, Revert A, et al. Multiple papules on the trunk and extremities. generalized multinucleate cell angiohistiocytoma. Int J Dermatol. 2013;52:544-546.
- O’Blenes CA, Walsh NM, Green PJ, et al. Novel case of generalized multinucleate cell angiohistiocytoma. J Cutan Med Surg. 2010;14:178-180.
- Chang SN, Kim HS, Kim SC, et al. Generalized multinucleate cell angiohistiocytoma. J Am Acad Dermatol. 1996;35:320-322.
- Fernández-Jorge B, Del Pozo J, García-Silva J, et al. Multinucleate cell angiohistiocytoma: treatment using intense pulsed light. Dermatol Surg. 2009;35:1141-1143.
- Perez LP, Zulaica A, Rodriguez L, et al. Multinucleate cell angiohistiocytoma. report of five cases. J Cutan Pathol. 2006;33:349-352.
- Shapiro PE, Nova MP, Rosmarin LA, et al. Multinucleate cell angiohistiocytoma: a distinct entity diagnosable by clinical and histologic features. J Am Acad Dermatol. 1994;30:417-422.
- Jaconelli L, Kanitakis J, Ktiouet S, et al. Multinucleate cell angiohistiocytoma: report of three new cases and literature review. Dermatol Online J. 2009;15:4.
- Jones WE, Cerio R, Smith NP. Multinucleate cell angiohistiocytoma: an acquired vascular anomaly to be distinguished from Kaposi’s sarcoma. Br J Dermatol. 1990;122:651-663.
- Calderaro J, Rethers L, Ortonne N. Multinucleated cells angiohistiocytoma: a reactive lesion? Am J Dermatopathol. 2010;32:415-417.
- Cesinaro AM, Roncati L, Maiorana A. Estrogen receptor alpha overexpression in multinucleate cell angiohistiocytoma: new insights into the pathogenesis of a reactive process. Am J Dermatopathol. 2010;32:655-659.
- Losordo DW, Isner JM. Estrogen and angiogenesis: a review. Arterioscler Thromb Vasc Biol. 2001;21:6-12.
- Sass U, Noel JC, Andre J, et al. Multinucleate cell angiohistiocytoma: report of two cases with no evidence of human herpesvirus-8 infection. J Cutan Pathol. 2000;27:258-261.
- Richer V, Lui H. Facial multinucleate cell angiohistiocytoma: long-term remission with 585 nm pulsed dye laser. Clin Exp Dermatol. 2016;41:312-313.
- Kopera D, Smolle J, Kerl H. Multinucleate cell angiohistiocytoma: treatment with argon laser. Br J Dermatol. 1995;133:308-310.
- Väkevä L, Saksela O, Kariniemi AL. Multinucleate cell angiohistiocytoma: a report of four cases in Finland. Acta Derm Venereol. 2003;83:222-223.
Multinucleate cell angiohistiocytoma (MCAH) is a rare benign, soft-tissue tumor first described in 1985 by Smith and Jones1 that presents clinically as erythematous to violaceous papules most commonly affecting females on the dorsal aspect of the hands and face.2 Multinucleate cell angiohistiocytoma is histologically characterized by vascular and histiocytic proliferations with dermal fibrosis. Few cases have been reported of lesions affecting the lower extremities. We report a case of MCAH affecting the legs.
Case Report
An 83-year-old white man with a history of basal cell carcinoma presented for evaluation of grouped, well-circumscribed, soft, red-violet, painless papules on the right anterior thigh that had been present for 8 months (Figure 1A). A review of symptoms was negative for immunologic, respiratory, and hematologic changes. The patient’s medical history also was remarkable for prostate cancer treated with radiation 18 years prior as well as right hip and left knee implants. The initial clinical impression was Kaposi sarcoma or a granulomatous disorder.
Histopathologic evaluation of a deep shave biopsy initially determined the lesion to be scar tissue without other pathologic findings. The patient returned to the clinic 12 months later for a complete skin examination given his history of skin cancer. Compared to clinical photographs taken a year prior, new violaceous papules were noted on the right thigh (Figure 1B) and left calf. Furthermore, there was no recurrence of the lesion at the prior biopsy site. Shave biopsies of the papules on the right thigh and left calf demonstrated similar histologic findings to each other. There was a mild increase in the number of small blood vessels in the superficial dermis (Figure 2A). A mild perivascular lymphocytic infiltrate surrounded some of these blood vessels. The endothelial cells had small nuclei with no evidence of nuclear pleomorphism. Careful examination of the interstitial dermis revealed scattered multinucleate cells with angulated cytoplasm (Figure 2B). Immunostaining for CD31 and human herpesvirus 8 were negative, excluding an infiltrative vascular tumor and Kaposi sarcoma, respectively. The diagnosis of MCAH was made based on the histopathologic findings.


At 1-year follow-up, the condition was stable with no gross changes in the lesions based on prior photographs. Once again, there was no recurrence of the excised lesions at both biopsy sites.
Comment
Presentation
A systematic review of published reports determined that 79% of MCAH cases occur in females, with an average age of onset of 50.1 years.2 However, MCAH likely is underreported due to the overall lack of knowledge regarding this condition by physicians and pathologists. The hands and face are the most commonly affected areas, though other sites of involvement have been reported, including the lower extremities,3,4 oral mucosa and upper lip,5,6 and trunk,7,8 as well as generalized distribution.9-12 Additionally, 1 case presented as a single plaque on the trunk rather than having papular or nodular morphology.8 Multinucleate cell angiohistiocytoma lesions generally are asymptomatic, though pruritus may be present.13 The condition is regarded as benign, though a minority of cases have exhibited spontaneous resolution.14-16
Histopathology
Multinucleate cell angiohistiocytoma histology demonstrates full-thickness dermal microvessel proliferation and fibrosis with characteristic multinucleate giant cells.2,3 Vascular endothelial cells stained positive for CD68 in 60% of cases2 as well as the normal endothelial markers (ie, factor VIII, CD31, CD34). The multinucleate giant cells exhibit immunoreactivity for macrophage/histiocytic markers factor XIIIa and CD68.
Etiology
The pathogenesis of MCAH is not yet fully understood, but it is considered to be a benign vascular or fibrohistiocytic neoplasm.17 Calderaro et al18 described a series of 8 patients who developed MCAH either within a cutaneous neoplastic process or in conjunction with various cutaneous reactive conditions, including hidradenitis suppurativa and chronic radiation dermatitis, as well as overlying a bone prosthesis placed due to degenerative arthritis. These cases suggest that MCAH, or possibly a subset of the disease, is a reactive process.
Differential Diagnosis
The differential diagnosis for MCAH includes Kaposi sarcoma clinically and dermatofibroma and fibrous papules histologically. Sass et al21 determined the in vitro behavior of cultured MCAH cells to contrast markedly with Kaposi sarcoma–derived cells. Although Kaposi sarcoma–derived cells exhibited invasive behavior, cells isolated from MCAH lesions were less elongated and were unable to traverse basement membranes.
Treatment
Surgical excision or cryotherapy appear to be definitive treatments of MCAH; however, a number of cases have reported light and laser modalities as successful alternatives to excision. One case of MCAH affecting the face was treated with pulsed dye laser monotherapy.22 This modality allowed selective coagulation of the vascular structures in MCAH. At 8-month follow-up, the initial lesion was noted to be completely cleared, though similar lesions had recently appeared elsewhere on the face.22 Another case of MCAH affecting the leg was treated with pulsed dye laser and both topical and intralesional corticosteroid combination therapy. In this case, the lesion failed to respond to treatment, which may suggest that facial localization could influence response in pulsed dye laser treatment.3
Intense pulsed light also has been reported as a definitive treatment in 2 cases.2,13 Slight erythema and transient pruritus have been reported immediately following treatment. In this case, complete resolution with only residual hyperpigmentation was reported at 2-month follow-up, with no recurrence during 12 months of follow-up.13
Argon laser therapy has been used in 2 cases. After a single session, lesions were no longer palpable, with no scarring noted at 8 weeks follow-up.23 Lastly, 2 cases of MCAH have been successfully treated with the CO2 laser, with no relapse noted at 2.5- or 5-month follow-up, respectively.24
Conclusion
Multinucleate cell angiohistiocytoma is a rare and likely underdiagnosed dermatologic condition that is believed to be a reactive process. Characteristic histology of MCAH demonstrates microvascular proliferations of the dermis with multinucleate giant cells amidst a fibrous background. Although surgical excision is curative, there are reports in which laser and light therapies were used to effectively treat MCAH.
Multinucleate cell angiohistiocytoma (MCAH) is a rare benign, soft-tissue tumor first described in 1985 by Smith and Jones1 that presents clinically as erythematous to violaceous papules most commonly affecting females on the dorsal aspect of the hands and face.2 Multinucleate cell angiohistiocytoma is histologically characterized by vascular and histiocytic proliferations with dermal fibrosis. Few cases have been reported of lesions affecting the lower extremities. We report a case of MCAH affecting the legs.
Case Report
An 83-year-old white man with a history of basal cell carcinoma presented for evaluation of grouped, well-circumscribed, soft, red-violet, painless papules on the right anterior thigh that had been present for 8 months (Figure 1A). A review of symptoms was negative for immunologic, respiratory, and hematologic changes. The patient’s medical history also was remarkable for prostate cancer treated with radiation 18 years prior as well as right hip and left knee implants. The initial clinical impression was Kaposi sarcoma or a granulomatous disorder.
Histopathologic evaluation of a deep shave biopsy initially determined the lesion to be scar tissue without other pathologic findings. The patient returned to the clinic 12 months later for a complete skin examination given his history of skin cancer. Compared to clinical photographs taken a year prior, new violaceous papules were noted on the right thigh (Figure 1B) and left calf. Furthermore, there was no recurrence of the lesion at the prior biopsy site. Shave biopsies of the papules on the right thigh and left calf demonstrated similar histologic findings to each other. There was a mild increase in the number of small blood vessels in the superficial dermis (Figure 2A). A mild perivascular lymphocytic infiltrate surrounded some of these blood vessels. The endothelial cells had small nuclei with no evidence of nuclear pleomorphism. Careful examination of the interstitial dermis revealed scattered multinucleate cells with angulated cytoplasm (Figure 2B). Immunostaining for CD31 and human herpesvirus 8 were negative, excluding an infiltrative vascular tumor and Kaposi sarcoma, respectively. The diagnosis of MCAH was made based on the histopathologic findings.


At 1-year follow-up, the condition was stable with no gross changes in the lesions based on prior photographs. Once again, there was no recurrence of the excised lesions at both biopsy sites.
Comment
Presentation
A systematic review of published reports determined that 79% of MCAH cases occur in females, with an average age of onset of 50.1 years.2 However, MCAH likely is underreported due to the overall lack of knowledge regarding this condition by physicians and pathologists. The hands and face are the most commonly affected areas, though other sites of involvement have been reported, including the lower extremities,3,4 oral mucosa and upper lip,5,6 and trunk,7,8 as well as generalized distribution.9-12 Additionally, 1 case presented as a single plaque on the trunk rather than having papular or nodular morphology.8 Multinucleate cell angiohistiocytoma lesions generally are asymptomatic, though pruritus may be present.13 The condition is regarded as benign, though a minority of cases have exhibited spontaneous resolution.14-16
Histopathology
Multinucleate cell angiohistiocytoma histology demonstrates full-thickness dermal microvessel proliferation and fibrosis with characteristic multinucleate giant cells.2,3 Vascular endothelial cells stained positive for CD68 in 60% of cases2 as well as the normal endothelial markers (ie, factor VIII, CD31, CD34). The multinucleate giant cells exhibit immunoreactivity for macrophage/histiocytic markers factor XIIIa and CD68.
Etiology
The pathogenesis of MCAH is not yet fully understood, but it is considered to be a benign vascular or fibrohistiocytic neoplasm.17 Calderaro et al18 described a series of 8 patients who developed MCAH either within a cutaneous neoplastic process or in conjunction with various cutaneous reactive conditions, including hidradenitis suppurativa and chronic radiation dermatitis, as well as overlying a bone prosthesis placed due to degenerative arthritis. These cases suggest that MCAH, or possibly a subset of the disease, is a reactive process.
Differential Diagnosis
The differential diagnosis for MCAH includes Kaposi sarcoma clinically and dermatofibroma and fibrous papules histologically. Sass et al21 determined the in vitro behavior of cultured MCAH cells to contrast markedly with Kaposi sarcoma–derived cells. Although Kaposi sarcoma–derived cells exhibited invasive behavior, cells isolated from MCAH lesions were less elongated and were unable to traverse basement membranes.
Treatment
Surgical excision or cryotherapy appear to be definitive treatments of MCAH; however, a number of cases have reported light and laser modalities as successful alternatives to excision. One case of MCAH affecting the face was treated with pulsed dye laser monotherapy.22 This modality allowed selective coagulation of the vascular structures in MCAH. At 8-month follow-up, the initial lesion was noted to be completely cleared, though similar lesions had recently appeared elsewhere on the face.22 Another case of MCAH affecting the leg was treated with pulsed dye laser and both topical and intralesional corticosteroid combination therapy. In this case, the lesion failed to respond to treatment, which may suggest that facial localization could influence response in pulsed dye laser treatment.3
Intense pulsed light also has been reported as a definitive treatment in 2 cases.2,13 Slight erythema and transient pruritus have been reported immediately following treatment. In this case, complete resolution with only residual hyperpigmentation was reported at 2-month follow-up, with no recurrence during 12 months of follow-up.13
Argon laser therapy has been used in 2 cases. After a single session, lesions were no longer palpable, with no scarring noted at 8 weeks follow-up.23 Lastly, 2 cases of MCAH have been successfully treated with the CO2 laser, with no relapse noted at 2.5- or 5-month follow-up, respectively.24
Conclusion
Multinucleate cell angiohistiocytoma is a rare and likely underdiagnosed dermatologic condition that is believed to be a reactive process. Characteristic histology of MCAH demonstrates microvascular proliferations of the dermis with multinucleate giant cells amidst a fibrous background. Although surgical excision is curative, there are reports in which laser and light therapies were used to effectively treat MCAH.
- Smith NP, Jones EW. Multinucleate cell angiohistiocytoma—a new entity. Br J Dermatol. 1985;113:15.
- Frew JW. Multinucleate cell angiohistiocytoma: clinicopathological correlation of 142 cases with insights into etiology and pathogenesis. Am J Dermatopathol. 2015;37:222-228.
- Applebaum DS, Shuja F, Hicks L, et al. Multinucleate cell angiohistiocytoma: a case report and review of the literature. Dermatol Online J. 2014;20:22610.
- Sagdeo A, Chu EY, Elenitsas R, et al. Multiple asymptomatic violaceous macules on the thigh. Multinucleate cell angiohistiocytoma (MCAH). JAMA Dermatol. 2013;149:357-363.
- Rawal YB, Anderson KM, Rawal SY. Multinucleate cell angiohistiocytoma: an uncommon mucosal tumour. Clin Exp Dermatol. 2009;34:333-336.
- Jones AC, Mullins D, Jimenez F. Multinucleate cell angiohistiocytoma of the upper lip. Oral Surg Oral Med Oral Pathol. 1994;78:743-747.
- Doshi-Chougule BN, Gust A, Mentzel T, et al. Multinucleate cell angiohistiocytoma with hypertrophic nerves. J Cutan Pathol. 2013;40:1048-1053.
- Issa AA, Lui H, Shapiro J, et al. Plaque-type multinucleate cell angiohistiocytoma. J Cutan Med Surg. 1998;3:112-114.
- Doane JA, Purdy K, Pasternak S. Generalized multinucleate cell angiohistiocytoma. J Cutan Med Surg. 2015;19:323-325.
- Marti N, Monteagudo C, Revert A, et al. Multiple papules on the trunk and extremities. generalized multinucleate cell angiohistiocytoma. Int J Dermatol. 2013;52:544-546.
- O’Blenes CA, Walsh NM, Green PJ, et al. Novel case of generalized multinucleate cell angiohistiocytoma. J Cutan Med Surg. 2010;14:178-180.
- Chang SN, Kim HS, Kim SC, et al. Generalized multinucleate cell angiohistiocytoma. J Am Acad Dermatol. 1996;35:320-322.
- Fernández-Jorge B, Del Pozo J, García-Silva J, et al. Multinucleate cell angiohistiocytoma: treatment using intense pulsed light. Dermatol Surg. 2009;35:1141-1143.
- Perez LP, Zulaica A, Rodriguez L, et al. Multinucleate cell angiohistiocytoma. report of five cases. J Cutan Pathol. 2006;33:349-352.
- Shapiro PE, Nova MP, Rosmarin LA, et al. Multinucleate cell angiohistiocytoma: a distinct entity diagnosable by clinical and histologic features. J Am Acad Dermatol. 1994;30:417-422.
- Jaconelli L, Kanitakis J, Ktiouet S, et al. Multinucleate cell angiohistiocytoma: report of three new cases and literature review. Dermatol Online J. 2009;15:4.
- Jones WE, Cerio R, Smith NP. Multinucleate cell angiohistiocytoma: an acquired vascular anomaly to be distinguished from Kaposi’s sarcoma. Br J Dermatol. 1990;122:651-663.
- Calderaro J, Rethers L, Ortonne N. Multinucleated cells angiohistiocytoma: a reactive lesion? Am J Dermatopathol. 2010;32:415-417.
- Cesinaro AM, Roncati L, Maiorana A. Estrogen receptor alpha overexpression in multinucleate cell angiohistiocytoma: new insights into the pathogenesis of a reactive process. Am J Dermatopathol. 2010;32:655-659.
- Losordo DW, Isner JM. Estrogen and angiogenesis: a review. Arterioscler Thromb Vasc Biol. 2001;21:6-12.
- Sass U, Noel JC, Andre J, et al. Multinucleate cell angiohistiocytoma: report of two cases with no evidence of human herpesvirus-8 infection. J Cutan Pathol. 2000;27:258-261.
- Richer V, Lui H. Facial multinucleate cell angiohistiocytoma: long-term remission with 585 nm pulsed dye laser. Clin Exp Dermatol. 2016;41:312-313.
- Kopera D, Smolle J, Kerl H. Multinucleate cell angiohistiocytoma: treatment with argon laser. Br J Dermatol. 1995;133:308-310.
- Väkevä L, Saksela O, Kariniemi AL. Multinucleate cell angiohistiocytoma: a report of four cases in Finland. Acta Derm Venereol. 2003;83:222-223.
- Smith NP, Jones EW. Multinucleate cell angiohistiocytoma—a new entity. Br J Dermatol. 1985;113:15.
- Frew JW. Multinucleate cell angiohistiocytoma: clinicopathological correlation of 142 cases with insights into etiology and pathogenesis. Am J Dermatopathol. 2015;37:222-228.
- Applebaum DS, Shuja F, Hicks L, et al. Multinucleate cell angiohistiocytoma: a case report and review of the literature. Dermatol Online J. 2014;20:22610.
- Sagdeo A, Chu EY, Elenitsas R, et al. Multiple asymptomatic violaceous macules on the thigh. Multinucleate cell angiohistiocytoma (MCAH). JAMA Dermatol. 2013;149:357-363.
- Rawal YB, Anderson KM, Rawal SY. Multinucleate cell angiohistiocytoma: an uncommon mucosal tumour. Clin Exp Dermatol. 2009;34:333-336.
- Jones AC, Mullins D, Jimenez F. Multinucleate cell angiohistiocytoma of the upper lip. Oral Surg Oral Med Oral Pathol. 1994;78:743-747.
- Doshi-Chougule BN, Gust A, Mentzel T, et al. Multinucleate cell angiohistiocytoma with hypertrophic nerves. J Cutan Pathol. 2013;40:1048-1053.
- Issa AA, Lui H, Shapiro J, et al. Plaque-type multinucleate cell angiohistiocytoma. J Cutan Med Surg. 1998;3:112-114.
- Doane JA, Purdy K, Pasternak S. Generalized multinucleate cell angiohistiocytoma. J Cutan Med Surg. 2015;19:323-325.
- Marti N, Monteagudo C, Revert A, et al. Multiple papules on the trunk and extremities. generalized multinucleate cell angiohistiocytoma. Int J Dermatol. 2013;52:544-546.
- O’Blenes CA, Walsh NM, Green PJ, et al. Novel case of generalized multinucleate cell angiohistiocytoma. J Cutan Med Surg. 2010;14:178-180.
- Chang SN, Kim HS, Kim SC, et al. Generalized multinucleate cell angiohistiocytoma. J Am Acad Dermatol. 1996;35:320-322.
- Fernández-Jorge B, Del Pozo J, García-Silva J, et al. Multinucleate cell angiohistiocytoma: treatment using intense pulsed light. Dermatol Surg. 2009;35:1141-1143.
- Perez LP, Zulaica A, Rodriguez L, et al. Multinucleate cell angiohistiocytoma. report of five cases. J Cutan Pathol. 2006;33:349-352.
- Shapiro PE, Nova MP, Rosmarin LA, et al. Multinucleate cell angiohistiocytoma: a distinct entity diagnosable by clinical and histologic features. J Am Acad Dermatol. 1994;30:417-422.
- Jaconelli L, Kanitakis J, Ktiouet S, et al. Multinucleate cell angiohistiocytoma: report of three new cases and literature review. Dermatol Online J. 2009;15:4.
- Jones WE, Cerio R, Smith NP. Multinucleate cell angiohistiocytoma: an acquired vascular anomaly to be distinguished from Kaposi’s sarcoma. Br J Dermatol. 1990;122:651-663.
- Calderaro J, Rethers L, Ortonne N. Multinucleated cells angiohistiocytoma: a reactive lesion? Am J Dermatopathol. 2010;32:415-417.
- Cesinaro AM, Roncati L, Maiorana A. Estrogen receptor alpha overexpression in multinucleate cell angiohistiocytoma: new insights into the pathogenesis of a reactive process. Am J Dermatopathol. 2010;32:655-659.
- Losordo DW, Isner JM. Estrogen and angiogenesis: a review. Arterioscler Thromb Vasc Biol. 2001;21:6-12.
- Sass U, Noel JC, Andre J, et al. Multinucleate cell angiohistiocytoma: report of two cases with no evidence of human herpesvirus-8 infection. J Cutan Pathol. 2000;27:258-261.
- Richer V, Lui H. Facial multinucleate cell angiohistiocytoma: long-term remission with 585 nm pulsed dye laser. Clin Exp Dermatol. 2016;41:312-313.
- Kopera D, Smolle J, Kerl H. Multinucleate cell angiohistiocytoma: treatment with argon laser. Br J Dermatol. 1995;133:308-310.
- Väkevä L, Saksela O, Kariniemi AL. Multinucleate cell angiohistiocytoma: a report of four cases in Finland. Acta Derm Venereol. 2003;83:222-223.
Practice Points
- Multinucleate cell angiohistiocytoma (MCAH) is a rare underrecognized cutaneous tumor presenting as erythematous to violaceous papules.
- Although it clinically mimics Kaposi sarcoma, MCAH may be distinguished histopathologically by negative immunostaining for human herpesvirus 8.
- Surgical excision and laser therapies are definitive treatments for MCAH, which is a benign lesion.
MACRA Monday: Osteoarthritis assessment
If you haven’t started reporting quality data for the Merit-Based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
Measure #109: Osteoarthritis Function and Pain Assessment
This measure is aimed at capturing the percentage of visits that included an assessment of function and pain for patients with a diagnosis of osteoarthritis (OA) who are aged 21 years or older.
What you need to do: Perform an assessment of symptoms and functional status for patients with OA and document it in the medical record. Validated scales and questionnaires may be used but are not required.
Eligible cases include patients aged 21 years and older with a diagnosis of OA and a patient encounter during the performance period. Applicable codes include (CPT): 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, CPT II 1006F indicates that OA symptoms and functional status were assessed. Add the 8P modifier to CPT II 1006F if the assessment was not performed and the reason is not otherwise specified.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
- Those who enrolled in Medicare for the first time during a performance period.
- Those who have Medicare Part B allowed charges of $30,000 or less.
- Those who have 100 or fewer Medicare Part B patients.
- Those who are significantly participating in an Advanced Alternative Payment Model (APM).
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
If you haven’t started reporting quality data for the Merit-Based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
Measure #109: Osteoarthritis Function and Pain Assessment
This measure is aimed at capturing the percentage of visits that included an assessment of function and pain for patients with a diagnosis of osteoarthritis (OA) who are aged 21 years or older.
What you need to do: Perform an assessment of symptoms and functional status for patients with OA and document it in the medical record. Validated scales and questionnaires may be used but are not required.
Eligible cases include patients aged 21 years and older with a diagnosis of OA and a patient encounter during the performance period. Applicable codes include (CPT): 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, CPT II 1006F indicates that OA symptoms and functional status were assessed. Add the 8P modifier to CPT II 1006F if the assessment was not performed and the reason is not otherwise specified.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
- Those who enrolled in Medicare for the first time during a performance period.
- Those who have Medicare Part B allowed charges of $30,000 or less.
- Those who have 100 or fewer Medicare Part B patients.
- Those who are significantly participating in an Advanced Alternative Payment Model (APM).
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
If you haven’t started reporting quality data for the Merit-Based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
Measure #109: Osteoarthritis Function and Pain Assessment
This measure is aimed at capturing the percentage of visits that included an assessment of function and pain for patients with a diagnosis of osteoarthritis (OA) who are aged 21 years or older.
What you need to do: Perform an assessment of symptoms and functional status for patients with OA and document it in the medical record. Validated scales and questionnaires may be used but are not required.
Eligible cases include patients aged 21 years and older with a diagnosis of OA and a patient encounter during the performance period. Applicable codes include (CPT): 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, CPT II 1006F indicates that OA symptoms and functional status were assessed. Add the 8P modifier to CPT II 1006F if the assessment was not performed and the reason is not otherwise specified.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
- Those who enrolled in Medicare for the first time during a performance period.
- Those who have Medicare Part B allowed charges of $30,000 or less.
- Those who have 100 or fewer Medicare Part B patients.
- Those who are significantly participating in an Advanced Alternative Payment Model (APM).
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Antimalarial could aid treatment of ALL
An antimalarial drug and a BH3 mimetic have demonstrated promise for treating BCR-ABL-positive acute lymphoblastic leukemia (ALL), according to work published in Clinical Cancer Research.
Investigators found the widely used antimalarial dihydroartemisinin (DHA) sensitized BCR-ABL+ ALL to the BH3 mimetic navitoclax (formerly ABT-263).
The combination therapy had a synergistic effect on mouse and human BCR-ABL+ leukemic cell death and extended the lives of mice with BCR-ABL+ ALL.
“Survival rates for children and adults with this leukemia still lag, highlighting the urgent need for new therapies,” said study author Joseph Opferman, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“Our findings suggest that combining DHA with ABT-263 can significantly improve treatment response.”
As opposed to mice that received navitoclax alone, there was no evidence of navitoclax resistance in mice treated with navitoclax and DHA.
The investigators determined that DHA worked by repressing production of MCL-1, a protein that is elevated in many cancers and helps malignant cells resist BH3 mimetics.
“MCL-1 is widely recognized as an important survival molecule in many normal cell types as well as cancer,” Dr Opferman said. “MCL-1 inhibitors are in development, but none are currently available for treating patients.”
“And because MCL-1 is essential for proper functioning of many normal cell types, there is concern about potential toxicity. We sought to identify drugs that are available now to augment treatment of BCR-ABL+ ALL.”
The search for a drug to sensitize BCR-ABL+ ALL to navitoclax and related compounds led Dr Opferman and his colleagues to DHA. A drug screen showed that DHA killed BCR-ABL+ ALL cells from mice.
The investigators showed how DHA induced expression of the protein CHOP, which is a key regulator of the endoplasmic reticulum stress pathway in cells. CHOP expression triggered the stress pathway in BCR-ABL+ ALL cells from mice and led to the suppression of MCL-1.
“MCL-1 has a short half-life, so the cell’s MCL-1 stores are rapidly depleted if the protein’s translation is repressed,” said study author Amit Budhraja, PhD, a postdoctoral fellow in Dr Opferman’s lab.
Now, the investigators are studying the mechanism in human BCR-ABL+ leukemic cells as well as in other cancers.
“Identifying the mechanism will allow us to study the pathway in detail for other points to target for anticancer drug development,” Dr Opferman said. ![]()
An antimalarial drug and a BH3 mimetic have demonstrated promise for treating BCR-ABL-positive acute lymphoblastic leukemia (ALL), according to work published in Clinical Cancer Research.
Investigators found the widely used antimalarial dihydroartemisinin (DHA) sensitized BCR-ABL+ ALL to the BH3 mimetic navitoclax (formerly ABT-263).
The combination therapy had a synergistic effect on mouse and human BCR-ABL+ leukemic cell death and extended the lives of mice with BCR-ABL+ ALL.
“Survival rates for children and adults with this leukemia still lag, highlighting the urgent need for new therapies,” said study author Joseph Opferman, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“Our findings suggest that combining DHA with ABT-263 can significantly improve treatment response.”
As opposed to mice that received navitoclax alone, there was no evidence of navitoclax resistance in mice treated with navitoclax and DHA.
The investigators determined that DHA worked by repressing production of MCL-1, a protein that is elevated in many cancers and helps malignant cells resist BH3 mimetics.
“MCL-1 is widely recognized as an important survival molecule in many normal cell types as well as cancer,” Dr Opferman said. “MCL-1 inhibitors are in development, but none are currently available for treating patients.”
“And because MCL-1 is essential for proper functioning of many normal cell types, there is concern about potential toxicity. We sought to identify drugs that are available now to augment treatment of BCR-ABL+ ALL.”
The search for a drug to sensitize BCR-ABL+ ALL to navitoclax and related compounds led Dr Opferman and his colleagues to DHA. A drug screen showed that DHA killed BCR-ABL+ ALL cells from mice.
The investigators showed how DHA induced expression of the protein CHOP, which is a key regulator of the endoplasmic reticulum stress pathway in cells. CHOP expression triggered the stress pathway in BCR-ABL+ ALL cells from mice and led to the suppression of MCL-1.
“MCL-1 has a short half-life, so the cell’s MCL-1 stores are rapidly depleted if the protein’s translation is repressed,” said study author Amit Budhraja, PhD, a postdoctoral fellow in Dr Opferman’s lab.
Now, the investigators are studying the mechanism in human BCR-ABL+ leukemic cells as well as in other cancers.
“Identifying the mechanism will allow us to study the pathway in detail for other points to target for anticancer drug development,” Dr Opferman said. ![]()
An antimalarial drug and a BH3 mimetic have demonstrated promise for treating BCR-ABL-positive acute lymphoblastic leukemia (ALL), according to work published in Clinical Cancer Research.
Investigators found the widely used antimalarial dihydroartemisinin (DHA) sensitized BCR-ABL+ ALL to the BH3 mimetic navitoclax (formerly ABT-263).
The combination therapy had a synergistic effect on mouse and human BCR-ABL+ leukemic cell death and extended the lives of mice with BCR-ABL+ ALL.
“Survival rates for children and adults with this leukemia still lag, highlighting the urgent need for new therapies,” said study author Joseph Opferman, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“Our findings suggest that combining DHA with ABT-263 can significantly improve treatment response.”
As opposed to mice that received navitoclax alone, there was no evidence of navitoclax resistance in mice treated with navitoclax and DHA.
The investigators determined that DHA worked by repressing production of MCL-1, a protein that is elevated in many cancers and helps malignant cells resist BH3 mimetics.
“MCL-1 is widely recognized as an important survival molecule in many normal cell types as well as cancer,” Dr Opferman said. “MCL-1 inhibitors are in development, but none are currently available for treating patients.”
“And because MCL-1 is essential for proper functioning of many normal cell types, there is concern about potential toxicity. We sought to identify drugs that are available now to augment treatment of BCR-ABL+ ALL.”
The search for a drug to sensitize BCR-ABL+ ALL to navitoclax and related compounds led Dr Opferman and his colleagues to DHA. A drug screen showed that DHA killed BCR-ABL+ ALL cells from mice.
The investigators showed how DHA induced expression of the protein CHOP, which is a key regulator of the endoplasmic reticulum stress pathway in cells. CHOP expression triggered the stress pathway in BCR-ABL+ ALL cells from mice and led to the suppression of MCL-1.
“MCL-1 has a short half-life, so the cell’s MCL-1 stores are rapidly depleted if the protein’s translation is repressed,” said study author Amit Budhraja, PhD, a postdoctoral fellow in Dr Opferman’s lab.
Now, the investigators are studying the mechanism in human BCR-ABL+ leukemic cells as well as in other cancers.
“Identifying the mechanism will allow us to study the pathway in detail for other points to target for anticancer drug development,” Dr Opferman said. ![]()
Keeping Up With the … Clinicians?
This past week, I received “appointment reminder” text messages from two separate health care clinics requesting confirmation of my attendance—a practice that has become so commonplace, I didn’t think twice about it. But there was a time, not long ago, when this would’ve seemed like a scenario from the far-off future (like the flying cars on The Jetsons).
Times, they are a-changin’! Clinicians are becoming more electronically skilled and interested in connecting with patients in a more convenient, direct way. Accordingly, they are increasingly willing to embrace the world of social media—something health care institutions evaded for years and even discouraged employees from entering. Today, clinicians are starting to harness the potential of social media to increase health care awareness and improve access to care.
The popularity of social media has grown tremendously in recent years; 72% of US adults now use social media, up from 8% in 2005.1 Facebook—one of the most commonly used social networks, along with LinkedIn and Twitter—surpassed 1 billion users in the third quarter of 2012, making it the first to achieve this milestone.2
And that’s the beauty of social media: It’s a tool that’s all about speeding up and augmenting communication. Active users consider themselves part of a community and therefore tend to trust others within their social media “group.” As a result, patients—especially younger ones—are using social media to make health care decisions. They research and select their clinicians, hospitals, and even courses of treatment (for both themselves and their families) with input from this community.
Our patients are social media–savvy and expect us, as clinicians, to be equally skilled. Are we missing an opportunity by opting out of these networks? Or are we rightly avoiding a number of thorny legal and regulatory issues by staying “offline”?
Many clinicians believe that the dependability of social media can drive better quality of care. In fact, 60% of physicians are in favor of interacting with patients on social media for patient education, health monitoring, medication adherence, and promotion of behavioral change.4 And in a 2012 study, 56% of patients reported wanting clinicians to use “social media” (which included email) to set appointments, report diagnostic test results, send prescription notifications, provide health information, and answer questions.5
Social media provides a platform for the public, patients, and health care professionals to communicate about health issues and (possibly) improve health outcomes. It can be used for professional networking, education, organizational promotion, patient care, patient education, and public health programs.6 So, where’s the downside?
As use of social media increases, particularly in the health care industry, the risks for legal implications and noncompliance also rise. Numerous federal and state rules and regulations govern communication within the health care industry. One of the main challenges health care organizations face is the protection of the privacy of patient information (ie, HIPAA). To this end, organizations must exhibit that they are managing the activities of employees who have access to patient information (which includes social media use). Institutions planning to use social media also need to ensure that their electronic records are complete, secure, and tamper-proof for record retention and audit purposes. Noncompliance with health care regulations can not only damage the reputation of an institution, it can also impact the bottom line.
In addition, health care providers must consider legal issues related to patient privacy, litigation, and licensing before using social media. Federal and state privacy laws limit providers’ ability to interact with patients through social media, because anything that can be used to identify a patient
It’s no wonder some providers are leery of connecting with patients via social media. Many of them, however, have tested the waters by using social networks to connect with colleagues and peers, often sharing medical knowledge and opinions this way.
As social media has evolved, medically focused professional communities have been established. One example is SERMO, a popular, private, physician-only social network. SERMO (www.sermo.com/what-is-sermo/overview) is a virtual doctors’ lounge with more than 800,000 verified and credentialed physician members; it includes physicians from 150 countries, with plans for continued expansion. Another, the Medical Directors Forum (https://medicaldirectorsforum.com/site), is a verified, secure, closed-loop environment for peer-to-peer interaction between medical directors, which features discussion groups, calendar postings, and alerts. Finally, Doximity (www.doximity.com/review) is a social network for physicians, NPs, and PAs, which allows clinicians to call patients (with the physician’s office number displayed on caller ID), review pertinent medical articles, communicate with colleagues, and even fax and/or email documents. Additionally, the American Association of Nurse Practitioners (AANP), the American Academy of Physician Assistants (AAPA), and Clinician Reviews all have accounts on Facebook and Twitter, among other social media sites.

When it comes down to it, if used wisely, social media has the potential to promote individual and public health, as well as professional development and advancement. But carelessness can result in legal, ethical, and logistical issues, causing serious ramifications for the clinician—and possibly even the practice. So, which side of the social media debate are you on? Send your tips—or warnings—about integrating social media into your workplace to [email protected]
1. Antheunis ML, Tates K, Nieboer TE. Patients’ and health professionals’ use of social media in healthcare: motives, barriers, and expectations. Patient Educ Couns. 2013;92(3):426-431.
2. Kaplan AM, Haenlein M. Users of the world unite! The challenges and opportunities of social media. Bus Horiz. 2010;53:59-68.
3. Statistica. Number of Facebook users worldwide as of 3rd quarter 2017 (in millions). www.statista.com/statistics/264810/number-of-monthly-active-facebook-users-worldwide/. Accessed November 16, 2017.
4. Househ M. The use of social media in healthcare: organizational, clinic, and patient perspectives. Stud Health Technol Inform. 2013;183:244-248.
5. Fisher J, Clayton M. Who gives a tweet: assessing patients’ interest in the use of social media for health care. Worldviews on Evid Based Nurs. 2012;9(2):100-108.
6. Ventola CL. Social media and health care professionals: benefits, risks, and best practices. P T. 2014;39(7):491-499, 520.
This past week, I received “appointment reminder” text messages from two separate health care clinics requesting confirmation of my attendance—a practice that has become so commonplace, I didn’t think twice about it. But there was a time, not long ago, when this would’ve seemed like a scenario from the far-off future (like the flying cars on The Jetsons).
Times, they are a-changin’! Clinicians are becoming more electronically skilled and interested in connecting with patients in a more convenient, direct way. Accordingly, they are increasingly willing to embrace the world of social media—something health care institutions evaded for years and even discouraged employees from entering. Today, clinicians are starting to harness the potential of social media to increase health care awareness and improve access to care.
The popularity of social media has grown tremendously in recent years; 72% of US adults now use social media, up from 8% in 2005.1 Facebook—one of the most commonly used social networks, along with LinkedIn and Twitter—surpassed 1 billion users in the third quarter of 2012, making it the first to achieve this milestone.2
And that’s the beauty of social media: It’s a tool that’s all about speeding up and augmenting communication. Active users consider themselves part of a community and therefore tend to trust others within their social media “group.” As a result, patients—especially younger ones—are using social media to make health care decisions. They research and select their clinicians, hospitals, and even courses of treatment (for both themselves and their families) with input from this community.
Our patients are social media–savvy and expect us, as clinicians, to be equally skilled. Are we missing an opportunity by opting out of these networks? Or are we rightly avoiding a number of thorny legal and regulatory issues by staying “offline”?
Many clinicians believe that the dependability of social media can drive better quality of care. In fact, 60% of physicians are in favor of interacting with patients on social media for patient education, health monitoring, medication adherence, and promotion of behavioral change.4 And in a 2012 study, 56% of patients reported wanting clinicians to use “social media” (which included email) to set appointments, report diagnostic test results, send prescription notifications, provide health information, and answer questions.5
Social media provides a platform for the public, patients, and health care professionals to communicate about health issues and (possibly) improve health outcomes. It can be used for professional networking, education, organizational promotion, patient care, patient education, and public health programs.6 So, where’s the downside?
As use of social media increases, particularly in the health care industry, the risks for legal implications and noncompliance also rise. Numerous federal and state rules and regulations govern communication within the health care industry. One of the main challenges health care organizations face is the protection of the privacy of patient information (ie, HIPAA). To this end, organizations must exhibit that they are managing the activities of employees who have access to patient information (which includes social media use). Institutions planning to use social media also need to ensure that their electronic records are complete, secure, and tamper-proof for record retention and audit purposes. Noncompliance with health care regulations can not only damage the reputation of an institution, it can also impact the bottom line.
In addition, health care providers must consider legal issues related to patient privacy, litigation, and licensing before using social media. Federal and state privacy laws limit providers’ ability to interact with patients through social media, because anything that can be used to identify a patient

It’s no wonder some providers are leery of connecting with patients via social media. Many of them, however, have tested the waters by using social networks to connect with colleagues and peers, often sharing medical knowledge and opinions this way.
As social media has evolved, medically focused professional communities have been established. One example is SERMO, a popular, private, physician-only social network. SERMO (www.sermo.com/what-is-sermo/overview) is a virtual doctors’ lounge with more than 800,000 verified and credentialed physician members; it includes physicians from 150 countries, with plans for continued expansion. Another, the Medical Directors Forum (https://medicaldirectorsforum.com/site), is a verified, secure, closed-loop environment for peer-to-peer interaction between medical directors, which features discussion groups, calendar postings, and alerts. Finally, Doximity (www.doximity.com/review) is a social network for physicians, NPs, and PAs, which allows clinicians to call patients (with the physician’s office number displayed on caller ID), review pertinent medical articles, communicate with colleagues, and even fax and/or email documents. Additionally, the American Association of Nurse Practitioners (AANP), the American Academy of Physician Assistants (AAPA), and Clinician Reviews all have accounts on Facebook and Twitter, among other social media sites.

When it comes down to it, if used wisely, social media has the potential to promote individual and public health, as well as professional development and advancement. But carelessness can result in legal, ethical, and logistical issues, causing serious ramifications for the clinician—and possibly even the practice. So, which side of the social media debate are you on? Send your tips—or warnings—about integrating social media into your workplace to [email protected]
This past week, I received “appointment reminder” text messages from two separate health care clinics requesting confirmation of my attendance—a practice that has become so commonplace, I didn’t think twice about it. But there was a time, not long ago, when this would’ve seemed like a scenario from the far-off future (like the flying cars on The Jetsons).
Times, they are a-changin’! Clinicians are becoming more electronically skilled and interested in connecting with patients in a more convenient, direct way. Accordingly, they are increasingly willing to embrace the world of social media—something health care institutions evaded for years and even discouraged employees from entering. Today, clinicians are starting to harness the potential of social media to increase health care awareness and improve access to care.
The popularity of social media has grown tremendously in recent years; 72% of US adults now use social media, up from 8% in 2005.1 Facebook—one of the most commonly used social networks, along with LinkedIn and Twitter—surpassed 1 billion users in the third quarter of 2012, making it the first to achieve this milestone.2
And that’s the beauty of social media: It’s a tool that’s all about speeding up and augmenting communication. Active users consider themselves part of a community and therefore tend to trust others within their social media “group.” As a result, patients—especially younger ones—are using social media to make health care decisions. They research and select their clinicians, hospitals, and even courses of treatment (for both themselves and their families) with input from this community.
Our patients are social media–savvy and expect us, as clinicians, to be equally skilled. Are we missing an opportunity by opting out of these networks? Or are we rightly avoiding a number of thorny legal and regulatory issues by staying “offline”?
Many clinicians believe that the dependability of social media can drive better quality of care. In fact, 60% of physicians are in favor of interacting with patients on social media for patient education, health monitoring, medication adherence, and promotion of behavioral change.4 And in a 2012 study, 56% of patients reported wanting clinicians to use “social media” (which included email) to set appointments, report diagnostic test results, send prescription notifications, provide health information, and answer questions.5
Social media provides a platform for the public, patients, and health care professionals to communicate about health issues and (possibly) improve health outcomes. It can be used for professional networking, education, organizational promotion, patient care, patient education, and public health programs.6 So, where’s the downside?
As use of social media increases, particularly in the health care industry, the risks for legal implications and noncompliance also rise. Numerous federal and state rules and regulations govern communication within the health care industry. One of the main challenges health care organizations face is the protection of the privacy of patient information (ie, HIPAA). To this end, organizations must exhibit that they are managing the activities of employees who have access to patient information (which includes social media use). Institutions planning to use social media also need to ensure that their electronic records are complete, secure, and tamper-proof for record retention and audit purposes. Noncompliance with health care regulations can not only damage the reputation of an institution, it can also impact the bottom line.
In addition, health care providers must consider legal issues related to patient privacy, litigation, and licensing before using social media. Federal and state privacy laws limit providers’ ability to interact with patients through social media, because anything that can be used to identify a patient

It’s no wonder some providers are leery of connecting with patients via social media. Many of them, however, have tested the waters by using social networks to connect with colleagues and peers, often sharing medical knowledge and opinions this way.
As social media has evolved, medically focused professional communities have been established. One example is SERMO, a popular, private, physician-only social network. SERMO (www.sermo.com/what-is-sermo/overview) is a virtual doctors’ lounge with more than 800,000 verified and credentialed physician members; it includes physicians from 150 countries, with plans for continued expansion. Another, the Medical Directors Forum (https://medicaldirectorsforum.com/site), is a verified, secure, closed-loop environment for peer-to-peer interaction between medical directors, which features discussion groups, calendar postings, and alerts. Finally, Doximity (www.doximity.com/review) is a social network for physicians, NPs, and PAs, which allows clinicians to call patients (with the physician’s office number displayed on caller ID), review pertinent medical articles, communicate with colleagues, and even fax and/or email documents. Additionally, the American Association of Nurse Practitioners (AANP), the American Academy of Physician Assistants (AAPA), and Clinician Reviews all have accounts on Facebook and Twitter, among other social media sites.

When it comes down to it, if used wisely, social media has the potential to promote individual and public health, as well as professional development and advancement. But carelessness can result in legal, ethical, and logistical issues, causing serious ramifications for the clinician—and possibly even the practice. So, which side of the social media debate are you on? Send your tips—or warnings—about integrating social media into your workplace to [email protected]
1. Antheunis ML, Tates K, Nieboer TE. Patients’ and health professionals’ use of social media in healthcare: motives, barriers, and expectations. Patient Educ Couns. 2013;92(3):426-431.
2. Kaplan AM, Haenlein M. Users of the world unite! The challenges and opportunities of social media. Bus Horiz. 2010;53:59-68.
3. Statistica. Number of Facebook users worldwide as of 3rd quarter 2017 (in millions). www.statista.com/statistics/264810/number-of-monthly-active-facebook-users-worldwide/. Accessed November 16, 2017.
4. Househ M. The use of social media in healthcare: organizational, clinic, and patient perspectives. Stud Health Technol Inform. 2013;183:244-248.
5. Fisher J, Clayton M. Who gives a tweet: assessing patients’ interest in the use of social media for health care. Worldviews on Evid Based Nurs. 2012;9(2):100-108.
6. Ventola CL. Social media and health care professionals: benefits, risks, and best practices. P T. 2014;39(7):491-499, 520.
1. Antheunis ML, Tates K, Nieboer TE. Patients’ and health professionals’ use of social media in healthcare: motives, barriers, and expectations. Patient Educ Couns. 2013;92(3):426-431.
2. Kaplan AM, Haenlein M. Users of the world unite! The challenges and opportunities of social media. Bus Horiz. 2010;53:59-68.
3. Statistica. Number of Facebook users worldwide as of 3rd quarter 2017 (in millions). www.statista.com/statistics/264810/number-of-monthly-active-facebook-users-worldwide/. Accessed November 16, 2017.
4. Househ M. The use of social media in healthcare: organizational, clinic, and patient perspectives. Stud Health Technol Inform. 2013;183:244-248.
5. Fisher J, Clayton M. Who gives a tweet: assessing patients’ interest in the use of social media for health care. Worldviews on Evid Based Nurs. 2012;9(2):100-108.
6. Ventola CL. Social media and health care professionals: benefits, risks, and best practices. P T. 2014;39(7):491-499, 520.
Private practice’s freedom still outweighs its challenges for me
This past Thanksgiving weekend I left a message on my office machine that we were closed because my staff and I were camping in a tent outside a store to save $3 on socks on Black Friday.
Of course, I had the usual legal disclaimers about calling 911 for emergencies and how to reach the doctor on call, but the message was just silly.
This isn’t anything new for my practice. My patients are used to the occasional humor. Most of them probably expect it by now.
It’s been 17 years since I left a large group and opened up my solo operation, and I’m still here. I have no regrets. My little practice may be eclectic. I wear shorts to work. My secretary’s 2 year old is at work everyday, keeping us laughing. I get to leave goofy messages on office voice mail. But it suits me.
This doesn’t change my focus of trying to practice competent neurology. I don’t claim to be the world’s best doctor, but I hope I know what I’m doing. I may be trying to have some fun here, but that doesn’t mean I take this job any less seriously.
Although things may change, right now I just can’t see myself as part of a large institution. I know my patients. I see each of them myself. I try to understand their concerns and backgrounds so I can treat them appropriately. I don’t try to cram them through in 10 minutes while checking off electronic medical record boxes to document “meaningful use” requirements.
Of course, the flip side is that I don’t make as much money as I could. But as long as I can stay open and support my family, I don’t care. I came here to help in a way that’s meaningful to both my patients and myself, and I’ve found it.
In the November 2017 issue of Medscape Business of Medicine was an article about physicians choosing private practice. Dr. Richard May, a nephrologist in Ohio, said: “We’ve opted to stay with this model because we control our lives. We know the cost of doing that is that we are probably not making as much as we could if we were with a system, but we get up every morning feeling good about how we practice medicine. We’re happy answering to no one but ourselves and not feeling any pressure to meet patient-load quotas and hit monetary goals.”
And all I can say to that is: “Amen, brother.”
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
This past Thanksgiving weekend I left a message on my office machine that we were closed because my staff and I were camping in a tent outside a store to save $3 on socks on Black Friday.
Of course, I had the usual legal disclaimers about calling 911 for emergencies and how to reach the doctor on call, but the message was just silly.
This isn’t anything new for my practice. My patients are used to the occasional humor. Most of them probably expect it by now.
It’s been 17 years since I left a large group and opened up my solo operation, and I’m still here. I have no regrets. My little practice may be eclectic. I wear shorts to work. My secretary’s 2 year old is at work everyday, keeping us laughing. I get to leave goofy messages on office voice mail. But it suits me.
This doesn’t change my focus of trying to practice competent neurology. I don’t claim to be the world’s best doctor, but I hope I know what I’m doing. I may be trying to have some fun here, but that doesn’t mean I take this job any less seriously.
Although things may change, right now I just can’t see myself as part of a large institution. I know my patients. I see each of them myself. I try to understand their concerns and backgrounds so I can treat them appropriately. I don’t try to cram them through in 10 minutes while checking off electronic medical record boxes to document “meaningful use” requirements.
Of course, the flip side is that I don’t make as much money as I could. But as long as I can stay open and support my family, I don’t care. I came here to help in a way that’s meaningful to both my patients and myself, and I’ve found it.
In the November 2017 issue of Medscape Business of Medicine was an article about physicians choosing private practice. Dr. Richard May, a nephrologist in Ohio, said: “We’ve opted to stay with this model because we control our lives. We know the cost of doing that is that we are probably not making as much as we could if we were with a system, but we get up every morning feeling good about how we practice medicine. We’re happy answering to no one but ourselves and not feeling any pressure to meet patient-load quotas and hit monetary goals.”
And all I can say to that is: “Amen, brother.”
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
This past Thanksgiving weekend I left a message on my office machine that we were closed because my staff and I were camping in a tent outside a store to save $3 on socks on Black Friday.
Of course, I had the usual legal disclaimers about calling 911 for emergencies and how to reach the doctor on call, but the message was just silly.
This isn’t anything new for my practice. My patients are used to the occasional humor. Most of them probably expect it by now.
It’s been 17 years since I left a large group and opened up my solo operation, and I’m still here. I have no regrets. My little practice may be eclectic. I wear shorts to work. My secretary’s 2 year old is at work everyday, keeping us laughing. I get to leave goofy messages on office voice mail. But it suits me.
This doesn’t change my focus of trying to practice competent neurology. I don’t claim to be the world’s best doctor, but I hope I know what I’m doing. I may be trying to have some fun here, but that doesn’t mean I take this job any less seriously.
Although things may change, right now I just can’t see myself as part of a large institution. I know my patients. I see each of them myself. I try to understand their concerns and backgrounds so I can treat them appropriately. I don’t try to cram them through in 10 minutes while checking off electronic medical record boxes to document “meaningful use” requirements.
Of course, the flip side is that I don’t make as much money as I could. But as long as I can stay open and support my family, I don’t care. I came here to help in a way that’s meaningful to both my patients and myself, and I’ve found it.
In the November 2017 issue of Medscape Business of Medicine was an article about physicians choosing private practice. Dr. Richard May, a nephrologist in Ohio, said: “We’ve opted to stay with this model because we control our lives. We know the cost of doing that is that we are probably not making as much as we could if we were with a system, but we get up every morning feeling good about how we practice medicine. We’re happy answering to no one but ourselves and not feeling any pressure to meet patient-load quotas and hit monetary goals.”
And all I can say to that is: “Amen, brother.”
Dr. Block has a solo neurology practice in Scottsdale, Ariz.