User login
Levetiracetam increased time between seizures for infants with epilepsy
, a multicenter, prospective, observational study has shown.
“Our findings suggest that levetiracetam has superior effectiveness compared with phenobarbital as initial monotherapy for nonsyndromic epilepsy in infants,” wrote Zachary Grinspan, MD, director of the pediatric epilepsy program at Cornell University, New York, and his colleagues. “We estimate that for every 100 infants with epilepsy treated with levetiracetam instead of phenobarbital, 44 infants would be free from monotherapy failure instead of 16.”
To evaluate the effectiveness of levetiracetam vs. phenobarbital, Dr. Grinspan and his colleagues developed the Early Life Epilepsy Study, a multicenter, prospective, observational investigation of 155 children with nonsyndromic epilepsy. Patient information for this study was obtained from medical records and was collected from March 1, 2012, through April 30, 2015. All of the patients in the study were observed in the first 3 years of life.
Of the 155 children included in the analysis for this study, 117 were treated with levetiracetam and 38 with phenobarbital. There were some differences between the groups. Children treated with levetiracetam were, on average, 2 months older at seizure onset than were those in the phenobarbital group (5.2 months vs. 3.0 months; P less than .001). Infants treated with levetiracetam also tended to begin treatment further from the time of their first seizure and exhibited less developmental delay at the time of epilepsy diagnosis. There were some other differences of possible clinical importance (developmental structural brain abnormalities, head circumference) that did not reach statistical significance.
Freedom from monotherapy failure was greater in the levetiracetam group (47 [40.2%] vs. 6 [15.8%]; P = .01; odds ratio, 3.6; 95% confidence interval, 1.5-10). Overall, the researchers concluded that levetiracetam was superior to phenobarbital for nonsyndromic epilepsy in pediatric patients (OR, 4.2; 95% CI, 1.1-16; number needed to treat, 3.5 [95% CI, 1.7-60]).
Outcome information was missing for more infants treated with levetiracetam than for those treated with phenobarbital, which could have skewed the analyses, Dr. Grinspan and his associates said. The nature of nonsyndromic epilepsy also makes it difficult to study because of the intricate genetic interactions that can influence the disorder.
Although this study provides information that could potentially benefit infantile epilepsy patients, the investigators said that more work must be done on the topic.
“A prospective clinical trial is needed. Levetiracetam and phenobarbital are both commonly used for infantile-onset epilepsy, indicating community equipoise regarding their relative effectiveness,” they wrote. “However, the effect size in our analysis was surprisingly large (number needed to treat, 3.5), suggesting that a change in practice could meaningfully improve outcomes.”
The investigators reported receiving grants and fees and consulting with a range of institutions, and the complete list can be found on the JAMA Pediatrics website. This study was funded by the Pediatric Epilepsy Research Foundation.
SOURCE: Grinspan Z et al. JAMA Pediatr. 2018 Feb 12. doi: 10.1001/jamapediatrics.2017.5211.
, a multicenter, prospective, observational study has shown.
“Our findings suggest that levetiracetam has superior effectiveness compared with phenobarbital as initial monotherapy for nonsyndromic epilepsy in infants,” wrote Zachary Grinspan, MD, director of the pediatric epilepsy program at Cornell University, New York, and his colleagues. “We estimate that for every 100 infants with epilepsy treated with levetiracetam instead of phenobarbital, 44 infants would be free from monotherapy failure instead of 16.”
To evaluate the effectiveness of levetiracetam vs. phenobarbital, Dr. Grinspan and his colleagues developed the Early Life Epilepsy Study, a multicenter, prospective, observational investigation of 155 children with nonsyndromic epilepsy. Patient information for this study was obtained from medical records and was collected from March 1, 2012, through April 30, 2015. All of the patients in the study were observed in the first 3 years of life.
Of the 155 children included in the analysis for this study, 117 were treated with levetiracetam and 38 with phenobarbital. There were some differences between the groups. Children treated with levetiracetam were, on average, 2 months older at seizure onset than were those in the phenobarbital group (5.2 months vs. 3.0 months; P less than .001). Infants treated with levetiracetam also tended to begin treatment further from the time of their first seizure and exhibited less developmental delay at the time of epilepsy diagnosis. There were some other differences of possible clinical importance (developmental structural brain abnormalities, head circumference) that did not reach statistical significance.
Freedom from monotherapy failure was greater in the levetiracetam group (47 [40.2%] vs. 6 [15.8%]; P = .01; odds ratio, 3.6; 95% confidence interval, 1.5-10). Overall, the researchers concluded that levetiracetam was superior to phenobarbital for nonsyndromic epilepsy in pediatric patients (OR, 4.2; 95% CI, 1.1-16; number needed to treat, 3.5 [95% CI, 1.7-60]).
Outcome information was missing for more infants treated with levetiracetam than for those treated with phenobarbital, which could have skewed the analyses, Dr. Grinspan and his associates said. The nature of nonsyndromic epilepsy also makes it difficult to study because of the intricate genetic interactions that can influence the disorder.
Although this study provides information that could potentially benefit infantile epilepsy patients, the investigators said that more work must be done on the topic.
“A prospective clinical trial is needed. Levetiracetam and phenobarbital are both commonly used for infantile-onset epilepsy, indicating community equipoise regarding their relative effectiveness,” they wrote. “However, the effect size in our analysis was surprisingly large (number needed to treat, 3.5), suggesting that a change in practice could meaningfully improve outcomes.”
The investigators reported receiving grants and fees and consulting with a range of institutions, and the complete list can be found on the JAMA Pediatrics website. This study was funded by the Pediatric Epilepsy Research Foundation.
SOURCE: Grinspan Z et al. JAMA Pediatr. 2018 Feb 12. doi: 10.1001/jamapediatrics.2017.5211.
, a multicenter, prospective, observational study has shown.
“Our findings suggest that levetiracetam has superior effectiveness compared with phenobarbital as initial monotherapy for nonsyndromic epilepsy in infants,” wrote Zachary Grinspan, MD, director of the pediatric epilepsy program at Cornell University, New York, and his colleagues. “We estimate that for every 100 infants with epilepsy treated with levetiracetam instead of phenobarbital, 44 infants would be free from monotherapy failure instead of 16.”
To evaluate the effectiveness of levetiracetam vs. phenobarbital, Dr. Grinspan and his colleagues developed the Early Life Epilepsy Study, a multicenter, prospective, observational investigation of 155 children with nonsyndromic epilepsy. Patient information for this study was obtained from medical records and was collected from March 1, 2012, through April 30, 2015. All of the patients in the study were observed in the first 3 years of life.
Of the 155 children included in the analysis for this study, 117 were treated with levetiracetam and 38 with phenobarbital. There were some differences between the groups. Children treated with levetiracetam were, on average, 2 months older at seizure onset than were those in the phenobarbital group (5.2 months vs. 3.0 months; P less than .001). Infants treated with levetiracetam also tended to begin treatment further from the time of their first seizure and exhibited less developmental delay at the time of epilepsy diagnosis. There were some other differences of possible clinical importance (developmental structural brain abnormalities, head circumference) that did not reach statistical significance.
Freedom from monotherapy failure was greater in the levetiracetam group (47 [40.2%] vs. 6 [15.8%]; P = .01; odds ratio, 3.6; 95% confidence interval, 1.5-10). Overall, the researchers concluded that levetiracetam was superior to phenobarbital for nonsyndromic epilepsy in pediatric patients (OR, 4.2; 95% CI, 1.1-16; number needed to treat, 3.5 [95% CI, 1.7-60]).
Outcome information was missing for more infants treated with levetiracetam than for those treated with phenobarbital, which could have skewed the analyses, Dr. Grinspan and his associates said. The nature of nonsyndromic epilepsy also makes it difficult to study because of the intricate genetic interactions that can influence the disorder.
Although this study provides information that could potentially benefit infantile epilepsy patients, the investigators said that more work must be done on the topic.
“A prospective clinical trial is needed. Levetiracetam and phenobarbital are both commonly used for infantile-onset epilepsy, indicating community equipoise regarding their relative effectiveness,” they wrote. “However, the effect size in our analysis was surprisingly large (number needed to treat, 3.5), suggesting that a change in practice could meaningfully improve outcomes.”
The investigators reported receiving grants and fees and consulting with a range of institutions, and the complete list can be found on the JAMA Pediatrics website. This study was funded by the Pediatric Epilepsy Research Foundation.
SOURCE: Grinspan Z et al. JAMA Pediatr. 2018 Feb 12. doi: 10.1001/jamapediatrics.2017.5211.
FROM JAMA PEDIATRICS
Key clinical point: Levetiracetam gave infants with nonsyndromic epilepsy greater freedom from seizure.
Major finding: Freedom from monotherapy failure was greater with levetiracetam than with phenobarbital (40.2% vs. 15.8%).
Study details: A multicenter, prospective, observational study of 155 children with nonsyndromic epilepsy.
Disclosures: The investigators reported receiving grants and fees and consulting with a range of institutions, and the complete list can be found on the JAMA Pediatrics website. This study was funded by the Pediatric Epilepsy Research Foundation.
Source: Grinspan Z et al. JAMA Pediatr. 2018 Feb 12. doi: 10.1001/jamapediatrics.2017.5211.
Rivaroxaban versus warfarin in mild acute ischemic stroke secondary to atrial fibrillation
Clinical question: Is rivaroxaban as effective and safe as warfarin immediately following minor acute ischemic stroke from atrial fibrillation?
Background: There is uncertainty regarding the best approach to anticoagulation acutely after ischemic stroke secondary to atrial fibrillation. To reduce the risk of intracranial hemorrhage, many physicians start aspirin immediately and delay initiating warfarin until days to weeks later. With their more predictable and rapid anticoagulant effect with potentially lower risk of intracranial hemorrhage, direct oral anticoagulants such as rivaroxaban are an attractive possible alternative to warfarin in the acute setting.
Study design: Multicenter, randomized, open-label superiority trial with blinded outcome assessment.
Setting: Fourteen academic hospitals in South Korea.
Synopsis: One hundred eighty-three patients with mild acute (within 5 days) ischemic stroke secondary to nonvalvular atrial fibrillation were randomized to immediately initiate either rivaroxaban or warfarin. The primary outcome (composite of new ischemic lesion or new intracranial hemorrhage on MRI at 4 weeks) occurred at similar frequency between groups (49.5% versus 54.5%, P = .49). Rates of adverse events were comparable in each group. Median hospitalization length was shorter in those randomized to rivaroxaban (4.0 versus 6.0 days, P less than .001). Limitations include a radiographic primary outcome that captured many asymptomatic lesions, homogenous study population, and lack of a delayed anticoagulation group.
Bottom line: In patients with mild acute stroke from nonvalvular atrial fibrillation, rivaroxaban and warfarin demonstrated comparable efficacy and safety. More study is needed to determine the optimal anticoagulation strategy in acute stroke.
Citation: Hong K-S et al. Rivaroxaban vs. warfarin sodium in the ultra-early period after atrial fibrillation-related mild ischemic stroke: A randomized clinical trial. JAMA Neurol. 2017; 74(10):1206-15.
Dr. Kanjee is a hospitalist, Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Clinical question: Is rivaroxaban as effective and safe as warfarin immediately following minor acute ischemic stroke from atrial fibrillation?
Background: There is uncertainty regarding the best approach to anticoagulation acutely after ischemic stroke secondary to atrial fibrillation. To reduce the risk of intracranial hemorrhage, many physicians start aspirin immediately and delay initiating warfarin until days to weeks later. With their more predictable and rapid anticoagulant effect with potentially lower risk of intracranial hemorrhage, direct oral anticoagulants such as rivaroxaban are an attractive possible alternative to warfarin in the acute setting.
Study design: Multicenter, randomized, open-label superiority trial with blinded outcome assessment.
Setting: Fourteen academic hospitals in South Korea.
Synopsis: One hundred eighty-three patients with mild acute (within 5 days) ischemic stroke secondary to nonvalvular atrial fibrillation were randomized to immediately initiate either rivaroxaban or warfarin. The primary outcome (composite of new ischemic lesion or new intracranial hemorrhage on MRI at 4 weeks) occurred at similar frequency between groups (49.5% versus 54.5%, P = .49). Rates of adverse events were comparable in each group. Median hospitalization length was shorter in those randomized to rivaroxaban (4.0 versus 6.0 days, P less than .001). Limitations include a radiographic primary outcome that captured many asymptomatic lesions, homogenous study population, and lack of a delayed anticoagulation group.
Bottom line: In patients with mild acute stroke from nonvalvular atrial fibrillation, rivaroxaban and warfarin demonstrated comparable efficacy and safety. More study is needed to determine the optimal anticoagulation strategy in acute stroke.
Citation: Hong K-S et al. Rivaroxaban vs. warfarin sodium in the ultra-early period after atrial fibrillation-related mild ischemic stroke: A randomized clinical trial. JAMA Neurol. 2017; 74(10):1206-15.
Dr. Kanjee is a hospitalist, Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Clinical question: Is rivaroxaban as effective and safe as warfarin immediately following minor acute ischemic stroke from atrial fibrillation?
Background: There is uncertainty regarding the best approach to anticoagulation acutely after ischemic stroke secondary to atrial fibrillation. To reduce the risk of intracranial hemorrhage, many physicians start aspirin immediately and delay initiating warfarin until days to weeks later. With their more predictable and rapid anticoagulant effect with potentially lower risk of intracranial hemorrhage, direct oral anticoagulants such as rivaroxaban are an attractive possible alternative to warfarin in the acute setting.
Study design: Multicenter, randomized, open-label superiority trial with blinded outcome assessment.
Setting: Fourteen academic hospitals in South Korea.
Synopsis: One hundred eighty-three patients with mild acute (within 5 days) ischemic stroke secondary to nonvalvular atrial fibrillation were randomized to immediately initiate either rivaroxaban or warfarin. The primary outcome (composite of new ischemic lesion or new intracranial hemorrhage on MRI at 4 weeks) occurred at similar frequency between groups (49.5% versus 54.5%, P = .49). Rates of adverse events were comparable in each group. Median hospitalization length was shorter in those randomized to rivaroxaban (4.0 versus 6.0 days, P less than .001). Limitations include a radiographic primary outcome that captured many asymptomatic lesions, homogenous study population, and lack of a delayed anticoagulation group.
Bottom line: In patients with mild acute stroke from nonvalvular atrial fibrillation, rivaroxaban and warfarin demonstrated comparable efficacy and safety. More study is needed to determine the optimal anticoagulation strategy in acute stroke.
Citation: Hong K-S et al. Rivaroxaban vs. warfarin sodium in the ultra-early period after atrial fibrillation-related mild ischemic stroke: A randomized clinical trial. JAMA Neurol. 2017; 74(10):1206-15.
Dr. Kanjee is a hospitalist, Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Webinar on Medicare Reimbursement is Thursday; Still Time to Avoid ’17 Penalties
The SVS Patient Safety Organization and the SVS Quality and Performance Measures Committee (QPMC) will hold a webinar at 8 p.m. Eastern Standard Time, Thursday, Feb. 15. It will help unravel the new Quality Payment Program (QPP) under Medicare, including what surgeons still can do for 2017 to avoid reimbursement penalties. Learn more here and register here.
The SVS Patient Safety Organization and the SVS Quality and Performance Measures Committee (QPMC) will hold a webinar at 8 p.m. Eastern Standard Time, Thursday, Feb. 15. It will help unravel the new Quality Payment Program (QPP) under Medicare, including what surgeons still can do for 2017 to avoid reimbursement penalties. Learn more here and register here.
The SVS Patient Safety Organization and the SVS Quality and Performance Measures Committee (QPMC) will hold a webinar at 8 p.m. Eastern Standard Time, Thursday, Feb. 15. It will help unravel the new Quality Payment Program (QPP) under Medicare, including what surgeons still can do for 2017 to avoid reimbursement penalties. Learn more here and register here.
Rheumatologists target prior authorization, Stark Law as barriers to competition
Onerous prior authorization rules, Stark Law, and other issues are creating barriers to health care choice and competition, the American College of Rheumatology recently told the Department of Health & Human Services.
The agency recently issued an informal request for information seeking “input from the public on the extent to which existing state and federal laws, regulations, guidance, requirements, and policies limit choice and competition across all health care markets, and the identification of actions that states or the federal government could take to support the development and operations of a health care system that provides high-quality care at affordable prices for the American people.”
“We believe this is a waste of valuable resources that results in delays in care and does not add value to health care delivery,” the ACR said in its Jan. 25 reply to the request for information. The ACR offered a series of recommendations to make the use of prior authorization by the Centers for Medicare & Medicaid Services more efficient.
Among the requested changes are:
• CMS should require all Medicare Advantage and Part D prescription drug plans to publicly disclose in a searchable electronic format to both patients and physicians all drugs and medical services that are subject to coverage restrictions (prior authorization, step therapy, formulary restrictions, quantity limits), and provide this information to vendors to be displayed in electronic health records.
• CMS should ensure that all utilization management requirements are based on accurate and up-to-date, publicly available clinical criteria and never cost alone.
• CMS should ensure that any “peer-to-peer” reviews utilize physicians from the same specialty/subspecialty as the ordering physician.
• CMS should restrict prior authorization requirements to “outlier” providers whose prescribing or ordering patterns differ significantly from their peers after adjusting for patient mix.
• CMS should not allow Part B services to be subject to prior authorization requirements because this would increase physician time spent on administrative tasks and reduce availability for patient care.
In addition, the ACR expressed concern about the consolidated pharmacy benefit managers market, noting that two PBMs cover more than 170 million Americans, and urged the HHS to “consider policies that require PBMs to be more transparent about their payment practices, including transparency around the true cost of prescription drugs.”
In the area of the Stark Law, the ACR called for the HHS to waive the prohibitions “for physicians seeking to develop and operate alternative payment models (APMs) as was provided to accountable care organizations in the Affordable Care Act. We also recommend removing the ‘volume or value’ prohibition in Stark policy so that physician practices can incentivize physicians to abide by best practices and succeed in new value-based alternative payment models.”
The ACR asked the CMS not to alter policy on assigning unique J-codes to biosimilars, as the unique codes allow for better monitoring of effectiveness and ensure adequate pharmacovigilance.
The physician organization also called for the removal of antitrust exemptions to insurance companies to give “the federal government the ability to intervene in places where insurance monopolies exist or develop.”
One request made – the removal of Part B drug payments from being adjusted by scoring in the Merit-based Incentive Payment System in the Quality Payment Program created by MACRA – was addressed in legislation passed by Congress and signed into law Feb. 9 by President Trump that provided short-term funding for the government and funding and other policy changes in the health care space, among other issues.
Onerous prior authorization rules, Stark Law, and other issues are creating barriers to health care choice and competition, the American College of Rheumatology recently told the Department of Health & Human Services.
The agency recently issued an informal request for information seeking “input from the public on the extent to which existing state and federal laws, regulations, guidance, requirements, and policies limit choice and competition across all health care markets, and the identification of actions that states or the federal government could take to support the development and operations of a health care system that provides high-quality care at affordable prices for the American people.”
“We believe this is a waste of valuable resources that results in delays in care and does not add value to health care delivery,” the ACR said in its Jan. 25 reply to the request for information. The ACR offered a series of recommendations to make the use of prior authorization by the Centers for Medicare & Medicaid Services more efficient.
Among the requested changes are:
• CMS should require all Medicare Advantage and Part D prescription drug plans to publicly disclose in a searchable electronic format to both patients and physicians all drugs and medical services that are subject to coverage restrictions (prior authorization, step therapy, formulary restrictions, quantity limits), and provide this information to vendors to be displayed in electronic health records.
• CMS should ensure that all utilization management requirements are based on accurate and up-to-date, publicly available clinical criteria and never cost alone.
• CMS should ensure that any “peer-to-peer” reviews utilize physicians from the same specialty/subspecialty as the ordering physician.
• CMS should restrict prior authorization requirements to “outlier” providers whose prescribing or ordering patterns differ significantly from their peers after adjusting for patient mix.
• CMS should not allow Part B services to be subject to prior authorization requirements because this would increase physician time spent on administrative tasks and reduce availability for patient care.
In addition, the ACR expressed concern about the consolidated pharmacy benefit managers market, noting that two PBMs cover more than 170 million Americans, and urged the HHS to “consider policies that require PBMs to be more transparent about their payment practices, including transparency around the true cost of prescription drugs.”
In the area of the Stark Law, the ACR called for the HHS to waive the prohibitions “for physicians seeking to develop and operate alternative payment models (APMs) as was provided to accountable care organizations in the Affordable Care Act. We also recommend removing the ‘volume or value’ prohibition in Stark policy so that physician practices can incentivize physicians to abide by best practices and succeed in new value-based alternative payment models.”
The ACR asked the CMS not to alter policy on assigning unique J-codes to biosimilars, as the unique codes allow for better monitoring of effectiveness and ensure adequate pharmacovigilance.
The physician organization also called for the removal of antitrust exemptions to insurance companies to give “the federal government the ability to intervene in places where insurance monopolies exist or develop.”
One request made – the removal of Part B drug payments from being adjusted by scoring in the Merit-based Incentive Payment System in the Quality Payment Program created by MACRA – was addressed in legislation passed by Congress and signed into law Feb. 9 by President Trump that provided short-term funding for the government and funding and other policy changes in the health care space, among other issues.
Onerous prior authorization rules, Stark Law, and other issues are creating barriers to health care choice and competition, the American College of Rheumatology recently told the Department of Health & Human Services.
The agency recently issued an informal request for information seeking “input from the public on the extent to which existing state and federal laws, regulations, guidance, requirements, and policies limit choice and competition across all health care markets, and the identification of actions that states or the federal government could take to support the development and operations of a health care system that provides high-quality care at affordable prices for the American people.”
“We believe this is a waste of valuable resources that results in delays in care and does not add value to health care delivery,” the ACR said in its Jan. 25 reply to the request for information. The ACR offered a series of recommendations to make the use of prior authorization by the Centers for Medicare & Medicaid Services more efficient.
Among the requested changes are:
• CMS should require all Medicare Advantage and Part D prescription drug plans to publicly disclose in a searchable electronic format to both patients and physicians all drugs and medical services that are subject to coverage restrictions (prior authorization, step therapy, formulary restrictions, quantity limits), and provide this information to vendors to be displayed in electronic health records.
• CMS should ensure that all utilization management requirements are based on accurate and up-to-date, publicly available clinical criteria and never cost alone.
• CMS should ensure that any “peer-to-peer” reviews utilize physicians from the same specialty/subspecialty as the ordering physician.
• CMS should restrict prior authorization requirements to “outlier” providers whose prescribing or ordering patterns differ significantly from their peers after adjusting for patient mix.
• CMS should not allow Part B services to be subject to prior authorization requirements because this would increase physician time spent on administrative tasks and reduce availability for patient care.
In addition, the ACR expressed concern about the consolidated pharmacy benefit managers market, noting that two PBMs cover more than 170 million Americans, and urged the HHS to “consider policies that require PBMs to be more transparent about their payment practices, including transparency around the true cost of prescription drugs.”
In the area of the Stark Law, the ACR called for the HHS to waive the prohibitions “for physicians seeking to develop and operate alternative payment models (APMs) as was provided to accountable care organizations in the Affordable Care Act. We also recommend removing the ‘volume or value’ prohibition in Stark policy so that physician practices can incentivize physicians to abide by best practices and succeed in new value-based alternative payment models.”
The ACR asked the CMS not to alter policy on assigning unique J-codes to biosimilars, as the unique codes allow for better monitoring of effectiveness and ensure adequate pharmacovigilance.
The physician organization also called for the removal of antitrust exemptions to insurance companies to give “the federal government the ability to intervene in places where insurance monopolies exist or develop.”
One request made – the removal of Part B drug payments from being adjusted by scoring in the Merit-based Incentive Payment System in the Quality Payment Program created by MACRA – was addressed in legislation passed by Congress and signed into law Feb. 9 by President Trump that provided short-term funding for the government and funding and other policy changes in the health care space, among other issues.
It takes a missile to focus your mind
I was one of about 600 dermatologists sitting in a lecture hall during a meeting in Maui when our muted smartphones suddenly started howling in unison. A text message popped up stating, “BALLISTIC MISSILE THREAT INBOUND TO HAWAII. SEEK IMMEDIATE SHELTER. THIS IS NOT A DRILL.” We all assumed a nuclear weapon from North Korea was headed our way.
The lecture was interrupted. The confused and concerned attendees milled around. The immediate response was largely “this can’t be real.” Meanwhile, the text alarm went off again transmitting the same message. The hotel intercom repeated the message and warned us to get inside the ballroom.
Among the attendees was David Cohen, trained in disaster preparedness. He ran up to his room, filled his bathtub, and put his mattress against the glass window. Richard Winkelman went down to the beach where he could at least witness what was about to annihilate him. Dirk Elston speculated the missile’s target was Kauai, where nuclear weapons are siloed. My wife was confident that the missiles would be intercepted, similar to the way they are under the Israeli missile defense system, and shot down before they hit any target.
And so, we mostly waited. The 30 minutes of largely silent confusion gave me a unique opportunity for self-reflection. I realized that many of my issues are petty and that I am insignificant in the scheme of things. I have never felt so helpless in my life. My mind entered a sort of fugue state, and I looked back on my life and realized it had been a darn good ride. I thought about my teenage children. I realized I had many, many things to be grateful for. I felt a huge burden lift off my shoulders and felt like I was floating about an inch above the floor. As I stared into nothingness, I internally reprioritized objectives. I was ready to die.
Then, just as suddenly and unpredictably as it arrived, the alert was called off. The imminent attack was no more than the mistaken push of a button.
But the event remains a defining moment for me. All the small battles that make up a life and career will continue, but I can no longer take them as seriously, and I will now take more time to reflect. I think I will get closer to God. I will try to be a better man, a better father, and a better citizen. It changed me, I think for the better.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
I was one of about 600 dermatologists sitting in a lecture hall during a meeting in Maui when our muted smartphones suddenly started howling in unison. A text message popped up stating, “BALLISTIC MISSILE THREAT INBOUND TO HAWAII. SEEK IMMEDIATE SHELTER. THIS IS NOT A DRILL.” We all assumed a nuclear weapon from North Korea was headed our way.
The lecture was interrupted. The confused and concerned attendees milled around. The immediate response was largely “this can’t be real.” Meanwhile, the text alarm went off again transmitting the same message. The hotel intercom repeated the message and warned us to get inside the ballroom.
Among the attendees was David Cohen, trained in disaster preparedness. He ran up to his room, filled his bathtub, and put his mattress against the glass window. Richard Winkelman went down to the beach where he could at least witness what was about to annihilate him. Dirk Elston speculated the missile’s target was Kauai, where nuclear weapons are siloed. My wife was confident that the missiles would be intercepted, similar to the way they are under the Israeli missile defense system, and shot down before they hit any target.
And so, we mostly waited. The 30 minutes of largely silent confusion gave me a unique opportunity for self-reflection. I realized that many of my issues are petty and that I am insignificant in the scheme of things. I have never felt so helpless in my life. My mind entered a sort of fugue state, and I looked back on my life and realized it had been a darn good ride. I thought about my teenage children. I realized I had many, many things to be grateful for. I felt a huge burden lift off my shoulders and felt like I was floating about an inch above the floor. As I stared into nothingness, I internally reprioritized objectives. I was ready to die.
Then, just as suddenly and unpredictably as it arrived, the alert was called off. The imminent attack was no more than the mistaken push of a button.
But the event remains a defining moment for me. All the small battles that make up a life and career will continue, but I can no longer take them as seriously, and I will now take more time to reflect. I think I will get closer to God. I will try to be a better man, a better father, and a better citizen. It changed me, I think for the better.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
I was one of about 600 dermatologists sitting in a lecture hall during a meeting in Maui when our muted smartphones suddenly started howling in unison. A text message popped up stating, “BALLISTIC MISSILE THREAT INBOUND TO HAWAII. SEEK IMMEDIATE SHELTER. THIS IS NOT A DRILL.” We all assumed a nuclear weapon from North Korea was headed our way.
The lecture was interrupted. The confused and concerned attendees milled around. The immediate response was largely “this can’t be real.” Meanwhile, the text alarm went off again transmitting the same message. The hotel intercom repeated the message and warned us to get inside the ballroom.
Among the attendees was David Cohen, trained in disaster preparedness. He ran up to his room, filled his bathtub, and put his mattress against the glass window. Richard Winkelman went down to the beach where he could at least witness what was about to annihilate him. Dirk Elston speculated the missile’s target was Kauai, where nuclear weapons are siloed. My wife was confident that the missiles would be intercepted, similar to the way they are under the Israeli missile defense system, and shot down before they hit any target.
And so, we mostly waited. The 30 minutes of largely silent confusion gave me a unique opportunity for self-reflection. I realized that many of my issues are petty and that I am insignificant in the scheme of things. I have never felt so helpless in my life. My mind entered a sort of fugue state, and I looked back on my life and realized it had been a darn good ride. I thought about my teenage children. I realized I had many, many things to be grateful for. I felt a huge burden lift off my shoulders and felt like I was floating about an inch above the floor. As I stared into nothingness, I internally reprioritized objectives. I was ready to die.
Then, just as suddenly and unpredictably as it arrived, the alert was called off. The imminent attack was no more than the mistaken push of a button.
But the event remains a defining moment for me. All the small battles that make up a life and career will continue, but I can no longer take them as seriously, and I will now take more time to reflect. I think I will get closer to God. I will try to be a better man, a better father, and a better citizen. It changed me, I think for the better.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Choosing noninvasive tightening treatments wisely
We all have one priority with all of our facial rejuvenation patients: Having happy, satisfied patients. With this in mind, I find I am torn by the armamentarium of noninvasive tightening devices to choose from. What are the critical factors in choosing a platform for your practice? Most practices look at pain, downtime, cost, and the number of treatments necessary to reach the expected outcome.
The treatment options are varied and include radio-frequency, ultrasound, and fractional resurfacing. There are numerous devices on the market that deliver energy into the dermis thereby causing collagen contraction and neocollagenesis. In my experience, the more “invasive” procedures or surgical tissue-tightening procedures provide the most reliable and immediate results. The radio-frequency and ultrasound devices that are “noninvasive” have little down-time, but multiple treatments are often needed and have inconsistent outcomes.
The technology for noninvasive modalities has improved over the last decade, but there are still no longterm clinical data, and results are highly varied. The difference in protocols and outcomes depends on proper patient selection, method of energy delivery, and sequential treatments.
We believe the optimal way to utilize these devices is as a combination approach with other procedures to optimize skin tightening and improvement in tone and texture. Tissue-tightening devices should be used with fractional ablative or nonablative resurfacing, fillers, and toxins. Often, we recommend starting with fillers and resurfacing treatments first to get the immediate “wow” factor and achieve immediate patient satisfaction. If patients want to then add skin tightening, this can be useful as an adjunct treatment and can even be used as a maintenance approach once per year. Actinic damage is also highly predictive of the degree of tissue laxity. Treating both the dermis and epidermis together delivers more immediate results. Using a fractional resurfacing device provides tissue tightening, improved skin color, decreased discoloration, and a reduction in the number of brown spots and freckles. Patients usually only need one to two treatments, there is minimal downtime, and satisfaction is very high.
In my practice, I choose fractional resurfacing treatments first. If patients want additional tissue tightening, radio-frequency is used as an adjunct treatment. This keeps costs lower, patients happier, and results more attainable.
When choosing devices for my practice, I follow a simple mantra: highest satisfaction per patient dollar spent. Happy patients build trust and integrity for the provider and practice. Don’t just buy a device because others are using it, and don’t just recommend a device because you have it.
Dr. Lily Talakoub and Dr. Naissan Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They have no relevant disclosures.
We all have one priority with all of our facial rejuvenation patients: Having happy, satisfied patients. With this in mind, I find I am torn by the armamentarium of noninvasive tightening devices to choose from. What are the critical factors in choosing a platform for your practice? Most practices look at pain, downtime, cost, and the number of treatments necessary to reach the expected outcome.
The treatment options are varied and include radio-frequency, ultrasound, and fractional resurfacing. There are numerous devices on the market that deliver energy into the dermis thereby causing collagen contraction and neocollagenesis. In my experience, the more “invasive” procedures or surgical tissue-tightening procedures provide the most reliable and immediate results. The radio-frequency and ultrasound devices that are “noninvasive” have little down-time, but multiple treatments are often needed and have inconsistent outcomes.
The technology for noninvasive modalities has improved over the last decade, but there are still no longterm clinical data, and results are highly varied. The difference in protocols and outcomes depends on proper patient selection, method of energy delivery, and sequential treatments.
We believe the optimal way to utilize these devices is as a combination approach with other procedures to optimize skin tightening and improvement in tone and texture. Tissue-tightening devices should be used with fractional ablative or nonablative resurfacing, fillers, and toxins. Often, we recommend starting with fillers and resurfacing treatments first to get the immediate “wow” factor and achieve immediate patient satisfaction. If patients want to then add skin tightening, this can be useful as an adjunct treatment and can even be used as a maintenance approach once per year. Actinic damage is also highly predictive of the degree of tissue laxity. Treating both the dermis and epidermis together delivers more immediate results. Using a fractional resurfacing device provides tissue tightening, improved skin color, decreased discoloration, and a reduction in the number of brown spots and freckles. Patients usually only need one to two treatments, there is minimal downtime, and satisfaction is very high.
In my practice, I choose fractional resurfacing treatments first. If patients want additional tissue tightening, radio-frequency is used as an adjunct treatment. This keeps costs lower, patients happier, and results more attainable.
When choosing devices for my practice, I follow a simple mantra: highest satisfaction per patient dollar spent. Happy patients build trust and integrity for the provider and practice. Don’t just buy a device because others are using it, and don’t just recommend a device because you have it.
Dr. Lily Talakoub and Dr. Naissan Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They have no relevant disclosures.
We all have one priority with all of our facial rejuvenation patients: Having happy, satisfied patients. With this in mind, I find I am torn by the armamentarium of noninvasive tightening devices to choose from. What are the critical factors in choosing a platform for your practice? Most practices look at pain, downtime, cost, and the number of treatments necessary to reach the expected outcome.
The treatment options are varied and include radio-frequency, ultrasound, and fractional resurfacing. There are numerous devices on the market that deliver energy into the dermis thereby causing collagen contraction and neocollagenesis. In my experience, the more “invasive” procedures or surgical tissue-tightening procedures provide the most reliable and immediate results. The radio-frequency and ultrasound devices that are “noninvasive” have little down-time, but multiple treatments are often needed and have inconsistent outcomes.
The technology for noninvasive modalities has improved over the last decade, but there are still no longterm clinical data, and results are highly varied. The difference in protocols and outcomes depends on proper patient selection, method of energy delivery, and sequential treatments.
We believe the optimal way to utilize these devices is as a combination approach with other procedures to optimize skin tightening and improvement in tone and texture. Tissue-tightening devices should be used with fractional ablative or nonablative resurfacing, fillers, and toxins. Often, we recommend starting with fillers and resurfacing treatments first to get the immediate “wow” factor and achieve immediate patient satisfaction. If patients want to then add skin tightening, this can be useful as an adjunct treatment and can even be used as a maintenance approach once per year. Actinic damage is also highly predictive of the degree of tissue laxity. Treating both the dermis and epidermis together delivers more immediate results. Using a fractional resurfacing device provides tissue tightening, improved skin color, decreased discoloration, and a reduction in the number of brown spots and freckles. Patients usually only need one to two treatments, there is minimal downtime, and satisfaction is very high.
In my practice, I choose fractional resurfacing treatments first. If patients want additional tissue tightening, radio-frequency is used as an adjunct treatment. This keeps costs lower, patients happier, and results more attainable.
When choosing devices for my practice, I follow a simple mantra: highest satisfaction per patient dollar spent. Happy patients build trust and integrity for the provider and practice. Don’t just buy a device because others are using it, and don’t just recommend a device because you have it.
Dr. Lily Talakoub and Dr. Naissan Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They have no relevant disclosures.
MDedge Daily News: The P word and the flu
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Is it time to use the P word about influenza? Your Medicare Part B drug pay earns a reprieve, a pregnancy-friendly psoriasis biologic is coming, and it’s good news, bad news for melanoma.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Is it time to use the P word about influenza? Your Medicare Part B drug pay earns a reprieve, a pregnancy-friendly psoriasis biologic is coming, and it’s good news, bad news for melanoma.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Is it time to use the P word about influenza? Your Medicare Part B drug pay earns a reprieve, a pregnancy-friendly psoriasis biologic is coming, and it’s good news, bad news for melanoma.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Patient Views of Discharge and a Novel e-Tool to Improve Transition from the Hospital
From the Mayo Clinic, Rochester, MN.
Abstract
- Objective: To elicit patient perceptions of a computer tablet (“e-Board”) used to display information relevant to hospital discharge and to gather patients’ expectations and perceptions regarding hospital discharge.
- Methods: Adult patients discharged from 1 of 3 medical-surgical, noncardiac monitored units of a 1265-bed, academic, tertiary care hospital were interviewed during patient focus groups. Reviewer pairs performed qualitative analysis of focus group transcripts and identified key themes, which were grouped into categories.
- Results: Patients felt a novel e-Board could help with the discharge process. They identified coordination of discharge, communication about discharge, ramifications of unexpected admissions, and interpersonal interactions during admission as the most significant issues around discharge.
- Conclusions: Focus groups elicit actionable information from patients about hospital discharge. Using this information, e-tools may help to design a patient-centered discharge process.
Key words: hospitals; patient satisfaction; focus groups; acute inpatient care.
Transition from the hospital to home represents a critical time for patients after acute illness, and support of patients and their care partners can help decrease consequences of poor care transitions, such as readmissions [1]. Focused discharge planning may improve outcomes and increase patient satisfaction [2], which is a key metric in hospital value-based purchasing programs, which tie Hospital Consumer Assessment of Healthcare Providers and Services (HCAHPS) survey scores to reimbursement. Although patient experience surveys explore several categories of patient satisfaction, HCAHPS may not reveal readily actionable opportunities that would allow clinicians to improve patient experience. Conducting focus groups and interviews can help discern patients’ perceptions and provide patient-centered opportunities to improve hospital discharge processes. Recent studies using these methodologies have revealed patients’ perceptions of barriers to inter-professional collaboration during discharge [3] and their desires and expectations of, as well as suggestions for improvement of, hospitalization [4].
Care transition bundles have been developed to facilitate the process of transitioning home [1,5], but none include e-health tools to help facilitate the discharge process. A study group leveraged available software at our institution to create a bedside “e-Board,” addressing opportunities that surfaced during previous patient focus groups regarding our institution’s discharge process. The software tools were loaded onto a tablet computer (Apple iPad; Cupertino, CA) and included displays of the patient’s physician and nurse, with estimated time of team bedside rounds; day and time of anticipated discharge; display of discharge medications; and a screening tool, I-MOVE, to assess mobility prior to return to independent living [6].
We conducted focus groups to gather patients’ insights for incorporation into a bedside e-health tool for discharge and into our hospital’s current discharge process. The primary objective of the current study was to elicit patient and family perceptions of a bedside e-Board, created to display information regarding discharge. Our secondary objective was to learn about patient expectations and perceptions regarding the hospital discharge process.
Methods
Setting
The study setting was 3 medical-surgical, non-cardiac monitored units of a 1265-bed, academic, tertiary care hospital in Rochester, MN. The study was considered a minimal risk study by the center’s institutional review board.
Participants
Patients aged 18 years or older discharged from 1 of the 3 study units during 2012–2013 were eligible to participate. Patients were excluded if they were not discharged home or to assisted living, were clinic employees, retirees or dependents of clinic employees, were hospitalized longer than 6 months prior to study entry, lived further than 60 miles from the town of Rochester, could not travel, or did not sign research consent.
There were 975 patients who met inclusion criteria. The institution’s survey research center randomly selected 300 eligible patients and contacted them by letter after discharge. The letter was followed up with a telephone call and verbal consent was obtained if the patient expressed interest in participation. Of the 17 patients who gave consent, 12 patients participated in focus group interviews.
E-Board Development
Prior focus group discussions facilitated by our institution’s marketing department (Mr. Kent Seltman, personal communication) explored patients’ perceptions of the discharge process from the institution’s primary hospital. The opportunities for improvement that surfaced during these focus groups included identifying the date of discharge, communication about the time of discharge, and discharging the patient at the identified time, not several hours later. The study group leveraged software available at our institution to create a bedside e-Board that could possibly mitigate these issues by improving communication about discharge. The software tools were loaded onto a tablet computer for patients to use as a resource during their admission. These tools included:
- A photo display of the patient’s nurse and physician, with estimated time of bedside rounds
- A display of the day and time of anticipated discharge. Providing anticipated day and time of discharge has been found to be an achievable goal for internal medicine and surgical services [7].
- A medication display, named the “Durable Display at Discharge,” previously found to improve patient understanding of prescribed medications [8]
- A display of a mobility tool, I-MOVE, designed to screen for debility that could prevent patients’ return to independent living [6].
Focus Groups
Facilitated interviews were conducted on 2 consecutive days in March 2014. Participants were divided into a focus group of 5 to 6 participants if they were functionally independent, or dyads of patient and care partner if they were functionally dependent. Interviews were both video- and audiotaped.
A trained facilitator led 1.5-hour sessions with each focus group. The sessions began with introductions and guidelines by which the focus groups were conducted, including explanations of the video and audio recording equipment, and a request for participants to speak one person at a time to facilitate recording. Discussions were carried out in 2 parts, guided by a facilitator script ( available from the authors). First, participants were asked to share their experiences regarding planning for discharge and the information they received leading up to their planned day and time of hospital discharge. Second, participants were shown a prototype of the e-Board. Participants were asked to reflect as to whether they had received similar information when they had been hospitalized, whether that information was helpful or useful, what information they did not receive that would have been helpful, how information was given, and whether information displayed via an e-Board would be better or worse than the ways they received information while in the hospital.
Data Analysis
Three teams, each comprised of 2 reviewers, met to analyze the video and audio recordings of each focus group. Unfortunately, the video files from the dyad interviews were not recoverable after the recorded sessions, and thus those groups were excluded from the study. Reviewers met prior to analyzing the focus group video and audio recordings to review the qualitative analysis protocol developed by the research team [9] (protocol available froom the authors). The teams then independently reviewed the video recordings and transcripts of the focus groups. The reviewer teams observed the focus group recordings and identified (1) themes regarding perceptions of the bedside e-Board and (2) experiences and perceptions around discharge. The protocol helped reviewer teams create a classification structure by identifying the key themes, which were then combined to create categories. The reviewer teams then compared their classification structures and by incorporating the most frequently identified categories, built a relational model of discharge perceptions.
Results
Eleven patients participated in 2 focus groups, one group of 5 patients and the other of 6 patients. Patient participants included 6 females and 5 males ranging in age from 22 to 84 years.
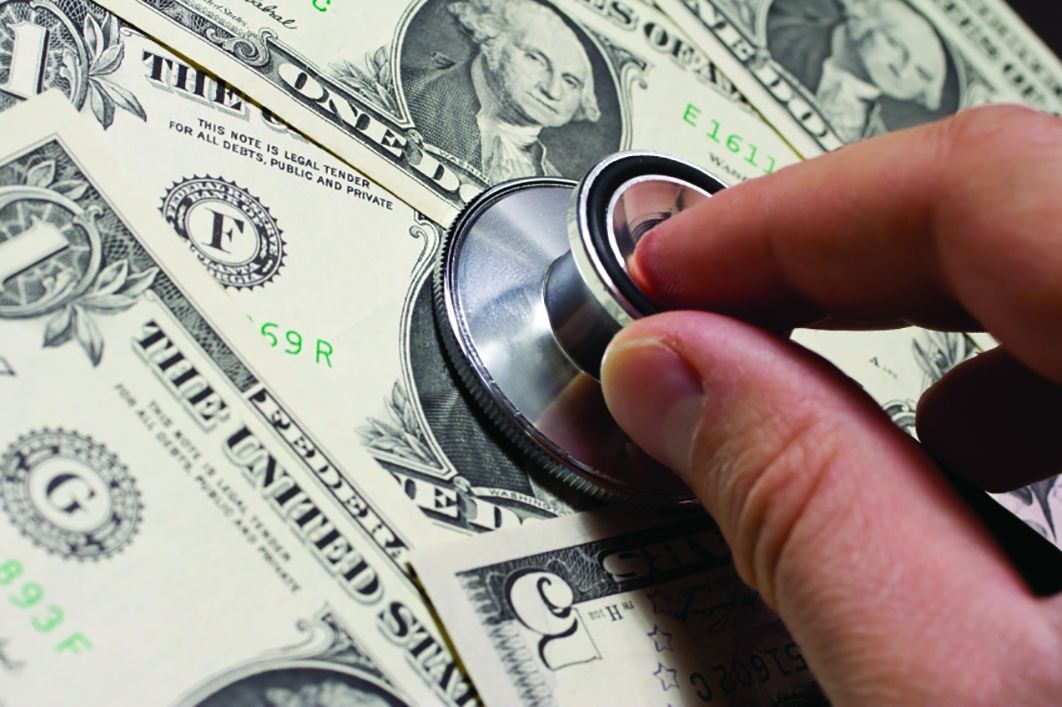
Using the qualitative analysis protocol, review teams grouped key themes from the focus group discussions about discharge into 4 categories. The categories, with themes listed below and representative patient comments in the Table, were
- Coordination and timing of discharge
- Giving patients the opportunity to prepare for discussion with clinician teams
- Communicating the specific time of discharge
- Internal collaboration of inter-professional teams
- Preparing for transition out of the hospital
2. Communication
- Patient inclusion in care discussions
- Discharge summary delay and/or completeness
- Education at the time of discharge
3. Ramifications of the unexpected and unknown
- Increased stress and frustration due to inability to plan, fear of the unknown, and lack of information
4. Interpersonal interactions
- Both favorable and unfavorable interactions caused an emotional response that impacts perceptions of hospitalization and discharge
The reviewers also analyzed patients’ comments regarding the bedside e-Board. The medication display (“Durable Display at Discharge,” Figure 1) was universally considered to be the most relevant and best-liked of the 4 elements tested. The visual display of medications and their purpose were commonly referenced as the most positive aspects of the display, and patients and caregivers were readily able to generate multiple potential uses for the display. Several mentioned that the information on the medication display were so desirable and necessary that if not supplied by the hospital, they hand-crafted such reminder displays at home.
The display of the care team and rounding time was perceived as helpful in allowing patients and family members to coordinate schedules with family members or care partners who may wish to be present during rounds. Patients also favorably reviewed the discharge day and time display, although multiple comments were made that this information is only helpful if it is accurate. Discussion around discharge time evoked the most emotions of topics discussed and patients expressed frustration with the inaccuracy of discharge time communicated to them on the day of discharge. Elaborating on this sentiment, a patient specified, “I prefer they don’t tell me a time at all until they know for sure”, and another shared that, “there is only going to be frustration with that if you say 4 pm and it ends up being 7 pm.”
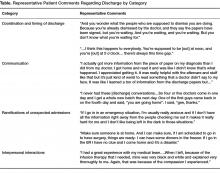
It was difficult for patients to see how the I-MOVE assessment (Figure 2) would apply to their discharge planning. They perceived I-MOVE as a tool for clinicians. One exception was a patient who had on a previous admission undergone heart surgery. She explained to the other patients that in such debilitated conditions, mobility independence assessments were important and commonly done.
Patients voiced some skepticism and concerns regarding the e-Board, including expense, privacy, security, and cleanliness. One patient observed the tablet was “more current than a printed piece of paper. It’s more up to date.” Other patients, however, questioned the process required to update information and wondered how much electronic displays added compared to the dry-erase board already in each patient’s room with which they were more familiar. They also voiced concern that the tablet would replace face-to-face interactions with their care teams. A patient shared that, “if we don’t have the conversation and we just get it through this, then I would hate that…you want to be able to give your input.”
Discussion
In this study, we used available software to create a bedside e-Board that addressed opportunities for patient-centered improvement in our institution’s discharge process. Patients felt that 3 of 4 software tools on the tablet could enhance the discharge experience. Additionally, we explored patients’ expectations and perceptions of our hospital discharge process.
Key information to inform our current discharge process was divulged by our patients during focus groups. Patients conveyed that the only time that matters to them is the time they get to walk out the door of the hospital, and that general statements (eg, “You’ll probably be going home today.”) create anxiety and dissatisfaction. Since family and care partners need to manage hospital discharge in combination with regular activities of daily life (eg, work schedules, child care), un-communicated changes to the discharge time are very difficult to accommodate and should be discussed in advance. Further, acknowledging the disruption of hospital admission to patients’, their families’, and care partners’ daily lives, as well as being mindful of the impact of interpersonal interactions with patients, remind clinicians of the impact hospitalization has on patients.
Focus group discussions revealed that an ideal patient-centered discharge process would include active patient participation, clear communication regarding the discharge process, especially changes in the specific discharge date and time, and education regarding discharge summary instructions. Further, patients voiced that the unexpected nature of admissions can be very disruptive to patients’ lives and that interpersonal interactions during admission cause emotional responses in patients that influence their perceptions of hospitalization.
Comments regarding poor coordination and communication of internal processes, opportunities to improve collaboration within and across care teams, and need to improve communication with patients regarding timing of discharge and plan of care are consistent with recent studies that used focus groups to explore patient perceptions and expectations around discharge [3,4]. The ramifications of unexpected admissions and the emotional responses patients expressed regarding interpersonal interactions during admission have not been reported by others conducting patient focus groups.
The unexpected nature of many admissions, and the uncertainty of the day-to-day activities during hospitalization, caused patients anxiety and stress. These emotions perhaps heightened their response and memories of both favorable and unfavorable interpersonal interactions. These memories left lasting impressions on patients and care teams may help alleviate anxiety and stress by providing consistency and routine such as rounding at the same time daily, and communicating this time with patients. In this regard, the e-Board was helpful in communicating the patients’ care team and their planned rounding time.
Regarding the ability of e-tools to facilitate information sharing and planning for discharge, patients felt that the display of medications would have been most beneficial when thinking about post-discharge care. They perceived a display of discharge date and time estimate display as very useful to coordinate the activities around physically leaving the hospital, but based on their experiences did not find anticipated discharge times to be believable.
Patients’ perceptions of the tool were assessed after a recent hospitalization, and our data would have been strengthened had patients and their care partners used the e-Board during the actual admission. On the other hand, post-discharge, patients had time to reflect on opportunities for improving their recent admission and had insight into gaps in their discharge that the tool could potentially fill. Because we were unable to access video recordings from our dyad groups, which led us to exclude these participants, we lost care partners’ perceptions of the e-Board and discharge process. Care partners likely have different perceptions of discharge processes compared to patients, and their insight would have augmented our findings.
Several patients observed that the e-Board presented much of the same information that was filled out by care teams on the in-room dry erase boards and questioned whether the tablet was needed. These observations provide future opportunity for studies comparing display of discharge information on in-room dry-erase boards to an electronic tablet display. E-tools have shown some benefit when used for patient self-monitoring [10], to increase patient engagement [11,12], or to improve patient education [12]. Computer tablets may be most useful when used in these manners, compared to information display.
Focus groups provide patient-provided information that is readily actionable, and this work presents patient insight into discharge processes elicited through focus groups. Patients discussed their perceptions of an e-tool that might address patient-identified opportunities to improve the discharge process. Future work in this area will explore e-tools, and how best to leverage their functionality to design a patient-centered discharge process.
Acknowledgments: Our thanks to Mr. Thomas J. (Tripp) Welch for the original suggestion of this study design, and to Ms. Heidi Miller and Ms. Lizann Williams for their invaluable contributions to this work. A special thanks to our exceptional colleagues of the Mayo Clinic Department of Medicine Clinical Research Office Clinical Trials Unit for their efforts in executing this study, and to the study participants who participated in this research, without whom this project would not have been possible.
Corresponding author: Deanne Kashiwagi, MD, MS, 200 First Street SW, Rochester, MN 55902, [email protected].
Financial disclosures: None.
1. Coleman EA, Parry C, Chalmers S, et al. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med 2006;166:1822–8.
2. Goncalves-Bradley DC, Lannin NA, Clemson LM, et al. Discharge planning from hospital. Cochrane Database Syst Rev 2016;1:CD000313.
3. Pinelli V, Stuckey HL, Gonzalo JD. Exploring challenges in the patient’s discharge process from the internal medicine service: A qualitative study of patients’ and providers’ perceptions. J Interprof Care 2017:1–9.
4. Neeman, N, Quinn K, Shoeb M, et al. Postdischarge focus groups to improve the hospital experience. Am J Med Qual 2013;28:536–8.
5. Jack, BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med 2009;150:178–87.
6. Manning, DM, Keller AS, Frank DL. Home alone: assessing mobility independence before discharge. J Hosp Med 2009;4:252–4.
7. Manning, DM, Tammel KJ, Blegen RN, et al. In-room display of day and time patient is anticipated to leave hospital: a “discharge appointment.” J Hosp Med 2007;2:13–6.
8. Manning, DM, O’Meara JG, Williams AR, et al. 3D: a tool for medication discharge education. Qual Saf Health Care 2007;16:71–6.
9. Vaismoradi M, Turunen H, Bondas T. Content analysis and thematic analysis: implications for conducting a qualitative descriptive study. Nurs Health Sci 2013;15:398–405.
10. Kampmeijer, R, Pavlova M, Tambor M, et al. The use of e-health and m-health tools in health promotion and primary prevention among older adults: a systematic literature review. BMC Health Serv Res 2016;16 Suppl 5:290.
11. Vawdrey, DK, Wilcox LG, Collins SA, et al. A tablet computer application for patients to participate in their hospital care. AMIA Annu Symp Proc 2011:1428–35.
12. Greysen, SR, Khanna RR, Jacolbia R, et al. Tablet computers for hospitalized patients: a pilot study to improve inpatient engagement. J Hosp Med 2014;9:396–9.
From the Mayo Clinic, Rochester, MN.
Abstract
- Objective: To elicit patient perceptions of a computer tablet (“e-Board”) used to display information relevant to hospital discharge and to gather patients’ expectations and perceptions regarding hospital discharge.
- Methods: Adult patients discharged from 1 of 3 medical-surgical, noncardiac monitored units of a 1265-bed, academic, tertiary care hospital were interviewed during patient focus groups. Reviewer pairs performed qualitative analysis of focus group transcripts and identified key themes, which were grouped into categories.
- Results: Patients felt a novel e-Board could help with the discharge process. They identified coordination of discharge, communication about discharge, ramifications of unexpected admissions, and interpersonal interactions during admission as the most significant issues around discharge.
- Conclusions: Focus groups elicit actionable information from patients about hospital discharge. Using this information, e-tools may help to design a patient-centered discharge process.
Key words: hospitals; patient satisfaction; focus groups; acute inpatient care.
Transition from the hospital to home represents a critical time for patients after acute illness, and support of patients and their care partners can help decrease consequences of poor care transitions, such as readmissions [1]. Focused discharge planning may improve outcomes and increase patient satisfaction [2], which is a key metric in hospital value-based purchasing programs, which tie Hospital Consumer Assessment of Healthcare Providers and Services (HCAHPS) survey scores to reimbursement. Although patient experience surveys explore several categories of patient satisfaction, HCAHPS may not reveal readily actionable opportunities that would allow clinicians to improve patient experience. Conducting focus groups and interviews can help discern patients’ perceptions and provide patient-centered opportunities to improve hospital discharge processes. Recent studies using these methodologies have revealed patients’ perceptions of barriers to inter-professional collaboration during discharge [3] and their desires and expectations of, as well as suggestions for improvement of, hospitalization [4].
Care transition bundles have been developed to facilitate the process of transitioning home [1,5], but none include e-health tools to help facilitate the discharge process. A study group leveraged available software at our institution to create a bedside “e-Board,” addressing opportunities that surfaced during previous patient focus groups regarding our institution’s discharge process. The software tools were loaded onto a tablet computer (Apple iPad; Cupertino, CA) and included displays of the patient’s physician and nurse, with estimated time of team bedside rounds; day and time of anticipated discharge; display of discharge medications; and a screening tool, I-MOVE, to assess mobility prior to return to independent living [6].
We conducted focus groups to gather patients’ insights for incorporation into a bedside e-health tool for discharge and into our hospital’s current discharge process. The primary objective of the current study was to elicit patient and family perceptions of a bedside e-Board, created to display information regarding discharge. Our secondary objective was to learn about patient expectations and perceptions regarding the hospital discharge process.
Methods
Setting
The study setting was 3 medical-surgical, non-cardiac monitored units of a 1265-bed, academic, tertiary care hospital in Rochester, MN. The study was considered a minimal risk study by the center’s institutional review board.
Participants
Patients aged 18 years or older discharged from 1 of the 3 study units during 2012–2013 were eligible to participate. Patients were excluded if they were not discharged home or to assisted living, were clinic employees, retirees or dependents of clinic employees, were hospitalized longer than 6 months prior to study entry, lived further than 60 miles from the town of Rochester, could not travel, or did not sign research consent.
There were 975 patients who met inclusion criteria. The institution’s survey research center randomly selected 300 eligible patients and contacted them by letter after discharge. The letter was followed up with a telephone call and verbal consent was obtained if the patient expressed interest in participation. Of the 17 patients who gave consent, 12 patients participated in focus group interviews.
E-Board Development
Prior focus group discussions facilitated by our institution’s marketing department (Mr. Kent Seltman, personal communication) explored patients’ perceptions of the discharge process from the institution’s primary hospital. The opportunities for improvement that surfaced during these focus groups included identifying the date of discharge, communication about the time of discharge, and discharging the patient at the identified time, not several hours later. The study group leveraged software available at our institution to create a bedside e-Board that could possibly mitigate these issues by improving communication about discharge. The software tools were loaded onto a tablet computer for patients to use as a resource during their admission. These tools included:
- A photo display of the patient’s nurse and physician, with estimated time of bedside rounds
- A display of the day and time of anticipated discharge. Providing anticipated day and time of discharge has been found to be an achievable goal for internal medicine and surgical services [7].
- A medication display, named the “Durable Display at Discharge,” previously found to improve patient understanding of prescribed medications [8]
- A display of a mobility tool, I-MOVE, designed to screen for debility that could prevent patients’ return to independent living [6].
Focus Groups
Facilitated interviews were conducted on 2 consecutive days in March 2014. Participants were divided into a focus group of 5 to 6 participants if they were functionally independent, or dyads of patient and care partner if they were functionally dependent. Interviews were both video- and audiotaped.
A trained facilitator led 1.5-hour sessions with each focus group. The sessions began with introductions and guidelines by which the focus groups were conducted, including explanations of the video and audio recording equipment, and a request for participants to speak one person at a time to facilitate recording. Discussions were carried out in 2 parts, guided by a facilitator script ( available from the authors). First, participants were asked to share their experiences regarding planning for discharge and the information they received leading up to their planned day and time of hospital discharge. Second, participants were shown a prototype of the e-Board. Participants were asked to reflect as to whether they had received similar information when they had been hospitalized, whether that information was helpful or useful, what information they did not receive that would have been helpful, how information was given, and whether information displayed via an e-Board would be better or worse than the ways they received information while in the hospital.
Data Analysis
Three teams, each comprised of 2 reviewers, met to analyze the video and audio recordings of each focus group. Unfortunately, the video files from the dyad interviews were not recoverable after the recorded sessions, and thus those groups were excluded from the study. Reviewers met prior to analyzing the focus group video and audio recordings to review the qualitative analysis protocol developed by the research team [9] (protocol available froom the authors). The teams then independently reviewed the video recordings and transcripts of the focus groups. The reviewer teams observed the focus group recordings and identified (1) themes regarding perceptions of the bedside e-Board and (2) experiences and perceptions around discharge. The protocol helped reviewer teams create a classification structure by identifying the key themes, which were then combined to create categories. The reviewer teams then compared their classification structures and by incorporating the most frequently identified categories, built a relational model of discharge perceptions.
Results
Eleven patients participated in 2 focus groups, one group of 5 patients and the other of 6 patients. Patient participants included 6 females and 5 males ranging in age from 22 to 84 years.
Using the qualitative analysis protocol, review teams grouped key themes from the focus group discussions about discharge into 4 categories. The categories, with themes listed below and representative patient comments in the Table, were
- Coordination and timing of discharge
- Giving patients the opportunity to prepare for discussion with clinician teams
- Communicating the specific time of discharge
- Internal collaboration of inter-professional teams
- Preparing for transition out of the hospital
2. Communication
- Patient inclusion in care discussions
- Discharge summary delay and/or completeness
- Education at the time of discharge
3. Ramifications of the unexpected and unknown
- Increased stress and frustration due to inability to plan, fear of the unknown, and lack of information
4. Interpersonal interactions
- Both favorable and unfavorable interactions caused an emotional response that impacts perceptions of hospitalization and discharge
The reviewers also analyzed patients’ comments regarding the bedside e-Board. The medication display (“Durable Display at Discharge,” Figure 1) was universally considered to be the most relevant and best-liked of the 4 elements tested. The visual display of medications and their purpose were commonly referenced as the most positive aspects of the display, and patients and caregivers were readily able to generate multiple potential uses for the display. Several mentioned that the information on the medication display were so desirable and necessary that if not supplied by the hospital, they hand-crafted such reminder displays at home.
The display of the care team and rounding time was perceived as helpful in allowing patients and family members to coordinate schedules with family members or care partners who may wish to be present during rounds. Patients also favorably reviewed the discharge day and time display, although multiple comments were made that this information is only helpful if it is accurate. Discussion around discharge time evoked the most emotions of topics discussed and patients expressed frustration with the inaccuracy of discharge time communicated to them on the day of discharge. Elaborating on this sentiment, a patient specified, “I prefer they don’t tell me a time at all until they know for sure”, and another shared that, “there is only going to be frustration with that if you say 4 pm and it ends up being 7 pm.”
It was difficult for patients to see how the I-MOVE assessment (Figure 2) would apply to their discharge planning. They perceived I-MOVE as a tool for clinicians. One exception was a patient who had on a previous admission undergone heart surgery. She explained to the other patients that in such debilitated conditions, mobility independence assessments were important and commonly done.
Patients voiced some skepticism and concerns regarding the e-Board, including expense, privacy, security, and cleanliness. One patient observed the tablet was “more current than a printed piece of paper. It’s more up to date.” Other patients, however, questioned the process required to update information and wondered how much electronic displays added compared to the dry-erase board already in each patient’s room with which they were more familiar. They also voiced concern that the tablet would replace face-to-face interactions with their care teams. A patient shared that, “if we don’t have the conversation and we just get it through this, then I would hate that…you want to be able to give your input.”
Discussion
In this study, we used available software to create a bedside e-Board that addressed opportunities for patient-centered improvement in our institution’s discharge process. Patients felt that 3 of 4 software tools on the tablet could enhance the discharge experience. Additionally, we explored patients’ expectations and perceptions of our hospital discharge process.
Key information to inform our current discharge process was divulged by our patients during focus groups. Patients conveyed that the only time that matters to them is the time they get to walk out the door of the hospital, and that general statements (eg, “You’ll probably be going home today.”) create anxiety and dissatisfaction. Since family and care partners need to manage hospital discharge in combination with regular activities of daily life (eg, work schedules, child care), un-communicated changes to the discharge time are very difficult to accommodate and should be discussed in advance. Further, acknowledging the disruption of hospital admission to patients’, their families’, and care partners’ daily lives, as well as being mindful of the impact of interpersonal interactions with patients, remind clinicians of the impact hospitalization has on patients.
Focus group discussions revealed that an ideal patient-centered discharge process would include active patient participation, clear communication regarding the discharge process, especially changes in the specific discharge date and time, and education regarding discharge summary instructions. Further, patients voiced that the unexpected nature of admissions can be very disruptive to patients’ lives and that interpersonal interactions during admission cause emotional responses in patients that influence their perceptions of hospitalization.
Comments regarding poor coordination and communication of internal processes, opportunities to improve collaboration within and across care teams, and need to improve communication with patients regarding timing of discharge and plan of care are consistent with recent studies that used focus groups to explore patient perceptions and expectations around discharge [3,4]. The ramifications of unexpected admissions and the emotional responses patients expressed regarding interpersonal interactions during admission have not been reported by others conducting patient focus groups.
The unexpected nature of many admissions, and the uncertainty of the day-to-day activities during hospitalization, caused patients anxiety and stress. These emotions perhaps heightened their response and memories of both favorable and unfavorable interpersonal interactions. These memories left lasting impressions on patients and care teams may help alleviate anxiety and stress by providing consistency and routine such as rounding at the same time daily, and communicating this time with patients. In this regard, the e-Board was helpful in communicating the patients’ care team and their planned rounding time.
Regarding the ability of e-tools to facilitate information sharing and planning for discharge, patients felt that the display of medications would have been most beneficial when thinking about post-discharge care. They perceived a display of discharge date and time estimate display as very useful to coordinate the activities around physically leaving the hospital, but based on their experiences did not find anticipated discharge times to be believable.
Patients’ perceptions of the tool were assessed after a recent hospitalization, and our data would have been strengthened had patients and their care partners used the e-Board during the actual admission. On the other hand, post-discharge, patients had time to reflect on opportunities for improving their recent admission and had insight into gaps in their discharge that the tool could potentially fill. Because we were unable to access video recordings from our dyad groups, which led us to exclude these participants, we lost care partners’ perceptions of the e-Board and discharge process. Care partners likely have different perceptions of discharge processes compared to patients, and their insight would have augmented our findings.
Several patients observed that the e-Board presented much of the same information that was filled out by care teams on the in-room dry erase boards and questioned whether the tablet was needed. These observations provide future opportunity for studies comparing display of discharge information on in-room dry-erase boards to an electronic tablet display. E-tools have shown some benefit when used for patient self-monitoring [10], to increase patient engagement [11,12], or to improve patient education [12]. Computer tablets may be most useful when used in these manners, compared to information display.
Focus groups provide patient-provided information that is readily actionable, and this work presents patient insight into discharge processes elicited through focus groups. Patients discussed their perceptions of an e-tool that might address patient-identified opportunities to improve the discharge process. Future work in this area will explore e-tools, and how best to leverage their functionality to design a patient-centered discharge process.
Acknowledgments: Our thanks to Mr. Thomas J. (Tripp) Welch for the original suggestion of this study design, and to Ms. Heidi Miller and Ms. Lizann Williams for their invaluable contributions to this work. A special thanks to our exceptional colleagues of the Mayo Clinic Department of Medicine Clinical Research Office Clinical Trials Unit for their efforts in executing this study, and to the study participants who participated in this research, without whom this project would not have been possible.
Corresponding author: Deanne Kashiwagi, MD, MS, 200 First Street SW, Rochester, MN 55902, [email protected].
Financial disclosures: None.
From the Mayo Clinic, Rochester, MN.
Abstract
- Objective: To elicit patient perceptions of a computer tablet (“e-Board”) used to display information relevant to hospital discharge and to gather patients’ expectations and perceptions regarding hospital discharge.
- Methods: Adult patients discharged from 1 of 3 medical-surgical, noncardiac monitored units of a 1265-bed, academic, tertiary care hospital were interviewed during patient focus groups. Reviewer pairs performed qualitative analysis of focus group transcripts and identified key themes, which were grouped into categories.
- Results: Patients felt a novel e-Board could help with the discharge process. They identified coordination of discharge, communication about discharge, ramifications of unexpected admissions, and interpersonal interactions during admission as the most significant issues around discharge.
- Conclusions: Focus groups elicit actionable information from patients about hospital discharge. Using this information, e-tools may help to design a patient-centered discharge process.
Key words: hospitals; patient satisfaction; focus groups; acute inpatient care.
Transition from the hospital to home represents a critical time for patients after acute illness, and support of patients and their care partners can help decrease consequences of poor care transitions, such as readmissions [1]. Focused discharge planning may improve outcomes and increase patient satisfaction [2], which is a key metric in hospital value-based purchasing programs, which tie Hospital Consumer Assessment of Healthcare Providers and Services (HCAHPS) survey scores to reimbursement. Although patient experience surveys explore several categories of patient satisfaction, HCAHPS may not reveal readily actionable opportunities that would allow clinicians to improve patient experience. Conducting focus groups and interviews can help discern patients’ perceptions and provide patient-centered opportunities to improve hospital discharge processes. Recent studies using these methodologies have revealed patients’ perceptions of barriers to inter-professional collaboration during discharge [3] and their desires and expectations of, as well as suggestions for improvement of, hospitalization [4].
Care transition bundles have been developed to facilitate the process of transitioning home [1,5], but none include e-health tools to help facilitate the discharge process. A study group leveraged available software at our institution to create a bedside “e-Board,” addressing opportunities that surfaced during previous patient focus groups regarding our institution’s discharge process. The software tools were loaded onto a tablet computer (Apple iPad; Cupertino, CA) and included displays of the patient’s physician and nurse, with estimated time of team bedside rounds; day and time of anticipated discharge; display of discharge medications; and a screening tool, I-MOVE, to assess mobility prior to return to independent living [6].
We conducted focus groups to gather patients’ insights for incorporation into a bedside e-health tool for discharge and into our hospital’s current discharge process. The primary objective of the current study was to elicit patient and family perceptions of a bedside e-Board, created to display information regarding discharge. Our secondary objective was to learn about patient expectations and perceptions regarding the hospital discharge process.
Methods
Setting
The study setting was 3 medical-surgical, non-cardiac monitored units of a 1265-bed, academic, tertiary care hospital in Rochester, MN. The study was considered a minimal risk study by the center’s institutional review board.
Participants
Patients aged 18 years or older discharged from 1 of the 3 study units during 2012–2013 were eligible to participate. Patients were excluded if they were not discharged home or to assisted living, were clinic employees, retirees or dependents of clinic employees, were hospitalized longer than 6 months prior to study entry, lived further than 60 miles from the town of Rochester, could not travel, or did not sign research consent.
There were 975 patients who met inclusion criteria. The institution’s survey research center randomly selected 300 eligible patients and contacted them by letter after discharge. The letter was followed up with a telephone call and verbal consent was obtained if the patient expressed interest in participation. Of the 17 patients who gave consent, 12 patients participated in focus group interviews.
E-Board Development
Prior focus group discussions facilitated by our institution’s marketing department (Mr. Kent Seltman, personal communication) explored patients’ perceptions of the discharge process from the institution’s primary hospital. The opportunities for improvement that surfaced during these focus groups included identifying the date of discharge, communication about the time of discharge, and discharging the patient at the identified time, not several hours later. The study group leveraged software available at our institution to create a bedside e-Board that could possibly mitigate these issues by improving communication about discharge. The software tools were loaded onto a tablet computer for patients to use as a resource during their admission. These tools included:
- A photo display of the patient’s nurse and physician, with estimated time of bedside rounds
- A display of the day and time of anticipated discharge. Providing anticipated day and time of discharge has been found to be an achievable goal for internal medicine and surgical services [7].
- A medication display, named the “Durable Display at Discharge,” previously found to improve patient understanding of prescribed medications [8]
- A display of a mobility tool, I-MOVE, designed to screen for debility that could prevent patients’ return to independent living [6].
Focus Groups
Facilitated interviews were conducted on 2 consecutive days in March 2014. Participants were divided into a focus group of 5 to 6 participants if they were functionally independent, or dyads of patient and care partner if they were functionally dependent. Interviews were both video- and audiotaped.
A trained facilitator led 1.5-hour sessions with each focus group. The sessions began with introductions and guidelines by which the focus groups were conducted, including explanations of the video and audio recording equipment, and a request for participants to speak one person at a time to facilitate recording. Discussions were carried out in 2 parts, guided by a facilitator script ( available from the authors). First, participants were asked to share their experiences regarding planning for discharge and the information they received leading up to their planned day and time of hospital discharge. Second, participants were shown a prototype of the e-Board. Participants were asked to reflect as to whether they had received similar information when they had been hospitalized, whether that information was helpful or useful, what information they did not receive that would have been helpful, how information was given, and whether information displayed via an e-Board would be better or worse than the ways they received information while in the hospital.
Data Analysis
Three teams, each comprised of 2 reviewers, met to analyze the video and audio recordings of each focus group. Unfortunately, the video files from the dyad interviews were not recoverable after the recorded sessions, and thus those groups were excluded from the study. Reviewers met prior to analyzing the focus group video and audio recordings to review the qualitative analysis protocol developed by the research team [9] (protocol available froom the authors). The teams then independently reviewed the video recordings and transcripts of the focus groups. The reviewer teams observed the focus group recordings and identified (1) themes regarding perceptions of the bedside e-Board and (2) experiences and perceptions around discharge. The protocol helped reviewer teams create a classification structure by identifying the key themes, which were then combined to create categories. The reviewer teams then compared their classification structures and by incorporating the most frequently identified categories, built a relational model of discharge perceptions.
Results
Eleven patients participated in 2 focus groups, one group of 5 patients and the other of 6 patients. Patient participants included 6 females and 5 males ranging in age from 22 to 84 years.
Using the qualitative analysis protocol, review teams grouped key themes from the focus group discussions about discharge into 4 categories. The categories, with themes listed below and representative patient comments in the Table, were
- Coordination and timing of discharge
- Giving patients the opportunity to prepare for discussion with clinician teams
- Communicating the specific time of discharge
- Internal collaboration of inter-professional teams
- Preparing for transition out of the hospital
2. Communication
- Patient inclusion in care discussions
- Discharge summary delay and/or completeness
- Education at the time of discharge
3. Ramifications of the unexpected and unknown
- Increased stress and frustration due to inability to plan, fear of the unknown, and lack of information
4. Interpersonal interactions
- Both favorable and unfavorable interactions caused an emotional response that impacts perceptions of hospitalization and discharge
The reviewers also analyzed patients’ comments regarding the bedside e-Board. The medication display (“Durable Display at Discharge,” Figure 1) was universally considered to be the most relevant and best-liked of the 4 elements tested. The visual display of medications and their purpose were commonly referenced as the most positive aspects of the display, and patients and caregivers were readily able to generate multiple potential uses for the display. Several mentioned that the information on the medication display were so desirable and necessary that if not supplied by the hospital, they hand-crafted such reminder displays at home.
The display of the care team and rounding time was perceived as helpful in allowing patients and family members to coordinate schedules with family members or care partners who may wish to be present during rounds. Patients also favorably reviewed the discharge day and time display, although multiple comments were made that this information is only helpful if it is accurate. Discussion around discharge time evoked the most emotions of topics discussed and patients expressed frustration with the inaccuracy of discharge time communicated to them on the day of discharge. Elaborating on this sentiment, a patient specified, “I prefer they don’t tell me a time at all until they know for sure”, and another shared that, “there is only going to be frustration with that if you say 4 pm and it ends up being 7 pm.”
It was difficult for patients to see how the I-MOVE assessment (Figure 2) would apply to their discharge planning. They perceived I-MOVE as a tool for clinicians. One exception was a patient who had on a previous admission undergone heart surgery. She explained to the other patients that in such debilitated conditions, mobility independence assessments were important and commonly done.
Patients voiced some skepticism and concerns regarding the e-Board, including expense, privacy, security, and cleanliness. One patient observed the tablet was “more current than a printed piece of paper. It’s more up to date.” Other patients, however, questioned the process required to update information and wondered how much electronic displays added compared to the dry-erase board already in each patient’s room with which they were more familiar. They also voiced concern that the tablet would replace face-to-face interactions with their care teams. A patient shared that, “if we don’t have the conversation and we just get it through this, then I would hate that…you want to be able to give your input.”
Discussion
In this study, we used available software to create a bedside e-Board that addressed opportunities for patient-centered improvement in our institution’s discharge process. Patients felt that 3 of 4 software tools on the tablet could enhance the discharge experience. Additionally, we explored patients’ expectations and perceptions of our hospital discharge process.
Key information to inform our current discharge process was divulged by our patients during focus groups. Patients conveyed that the only time that matters to them is the time they get to walk out the door of the hospital, and that general statements (eg, “You’ll probably be going home today.”) create anxiety and dissatisfaction. Since family and care partners need to manage hospital discharge in combination with regular activities of daily life (eg, work schedules, child care), un-communicated changes to the discharge time are very difficult to accommodate and should be discussed in advance. Further, acknowledging the disruption of hospital admission to patients’, their families’, and care partners’ daily lives, as well as being mindful of the impact of interpersonal interactions with patients, remind clinicians of the impact hospitalization has on patients.
Focus group discussions revealed that an ideal patient-centered discharge process would include active patient participation, clear communication regarding the discharge process, especially changes in the specific discharge date and time, and education regarding discharge summary instructions. Further, patients voiced that the unexpected nature of admissions can be very disruptive to patients’ lives and that interpersonal interactions during admission cause emotional responses in patients that influence their perceptions of hospitalization.
Comments regarding poor coordination and communication of internal processes, opportunities to improve collaboration within and across care teams, and need to improve communication with patients regarding timing of discharge and plan of care are consistent with recent studies that used focus groups to explore patient perceptions and expectations around discharge [3,4]. The ramifications of unexpected admissions and the emotional responses patients expressed regarding interpersonal interactions during admission have not been reported by others conducting patient focus groups.
The unexpected nature of many admissions, and the uncertainty of the day-to-day activities during hospitalization, caused patients anxiety and stress. These emotions perhaps heightened their response and memories of both favorable and unfavorable interpersonal interactions. These memories left lasting impressions on patients and care teams may help alleviate anxiety and stress by providing consistency and routine such as rounding at the same time daily, and communicating this time with patients. In this regard, the e-Board was helpful in communicating the patients’ care team and their planned rounding time.
Regarding the ability of e-tools to facilitate information sharing and planning for discharge, patients felt that the display of medications would have been most beneficial when thinking about post-discharge care. They perceived a display of discharge date and time estimate display as very useful to coordinate the activities around physically leaving the hospital, but based on their experiences did not find anticipated discharge times to be believable.
Patients’ perceptions of the tool were assessed after a recent hospitalization, and our data would have been strengthened had patients and their care partners used the e-Board during the actual admission. On the other hand, post-discharge, patients had time to reflect on opportunities for improving their recent admission and had insight into gaps in their discharge that the tool could potentially fill. Because we were unable to access video recordings from our dyad groups, which led us to exclude these participants, we lost care partners’ perceptions of the e-Board and discharge process. Care partners likely have different perceptions of discharge processes compared to patients, and their insight would have augmented our findings.
Several patients observed that the e-Board presented much of the same information that was filled out by care teams on the in-room dry erase boards and questioned whether the tablet was needed. These observations provide future opportunity for studies comparing display of discharge information on in-room dry-erase boards to an electronic tablet display. E-tools have shown some benefit when used for patient self-monitoring [10], to increase patient engagement [11,12], or to improve patient education [12]. Computer tablets may be most useful when used in these manners, compared to information display.
Focus groups provide patient-provided information that is readily actionable, and this work presents patient insight into discharge processes elicited through focus groups. Patients discussed their perceptions of an e-tool that might address patient-identified opportunities to improve the discharge process. Future work in this area will explore e-tools, and how best to leverage their functionality to design a patient-centered discharge process.
Acknowledgments: Our thanks to Mr. Thomas J. (Tripp) Welch for the original suggestion of this study design, and to Ms. Heidi Miller and Ms. Lizann Williams for their invaluable contributions to this work. A special thanks to our exceptional colleagues of the Mayo Clinic Department of Medicine Clinical Research Office Clinical Trials Unit for their efforts in executing this study, and to the study participants who participated in this research, without whom this project would not have been possible.
Corresponding author: Deanne Kashiwagi, MD, MS, 200 First Street SW, Rochester, MN 55902, [email protected].
Financial disclosures: None.
1. Coleman EA, Parry C, Chalmers S, et al. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med 2006;166:1822–8.
2. Goncalves-Bradley DC, Lannin NA, Clemson LM, et al. Discharge planning from hospital. Cochrane Database Syst Rev 2016;1:CD000313.
3. Pinelli V, Stuckey HL, Gonzalo JD. Exploring challenges in the patient’s discharge process from the internal medicine service: A qualitative study of patients’ and providers’ perceptions. J Interprof Care 2017:1–9.
4. Neeman, N, Quinn K, Shoeb M, et al. Postdischarge focus groups to improve the hospital experience. Am J Med Qual 2013;28:536–8.
5. Jack, BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med 2009;150:178–87.
6. Manning, DM, Keller AS, Frank DL. Home alone: assessing mobility independence before discharge. J Hosp Med 2009;4:252–4.
7. Manning, DM, Tammel KJ, Blegen RN, et al. In-room display of day and time patient is anticipated to leave hospital: a “discharge appointment.” J Hosp Med 2007;2:13–6.
8. Manning, DM, O’Meara JG, Williams AR, et al. 3D: a tool for medication discharge education. Qual Saf Health Care 2007;16:71–6.
9. Vaismoradi M, Turunen H, Bondas T. Content analysis and thematic analysis: implications for conducting a qualitative descriptive study. Nurs Health Sci 2013;15:398–405.
10. Kampmeijer, R, Pavlova M, Tambor M, et al. The use of e-health and m-health tools in health promotion and primary prevention among older adults: a systematic literature review. BMC Health Serv Res 2016;16 Suppl 5:290.
11. Vawdrey, DK, Wilcox LG, Collins SA, et al. A tablet computer application for patients to participate in their hospital care. AMIA Annu Symp Proc 2011:1428–35.
12. Greysen, SR, Khanna RR, Jacolbia R, et al. Tablet computers for hospitalized patients: a pilot study to improve inpatient engagement. J Hosp Med 2014;9:396–9.
1. Coleman EA, Parry C, Chalmers S, et al. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med 2006;166:1822–8.
2. Goncalves-Bradley DC, Lannin NA, Clemson LM, et al. Discharge planning from hospital. Cochrane Database Syst Rev 2016;1:CD000313.
3. Pinelli V, Stuckey HL, Gonzalo JD. Exploring challenges in the patient’s discharge process from the internal medicine service: A qualitative study of patients’ and providers’ perceptions. J Interprof Care 2017:1–9.
4. Neeman, N, Quinn K, Shoeb M, et al. Postdischarge focus groups to improve the hospital experience. Am J Med Qual 2013;28:536–8.
5. Jack, BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med 2009;150:178–87.
6. Manning, DM, Keller AS, Frank DL. Home alone: assessing mobility independence before discharge. J Hosp Med 2009;4:252–4.
7. Manning, DM, Tammel KJ, Blegen RN, et al. In-room display of day and time patient is anticipated to leave hospital: a “discharge appointment.” J Hosp Med 2007;2:13–6.
8. Manning, DM, O’Meara JG, Williams AR, et al. 3D: a tool for medication discharge education. Qual Saf Health Care 2007;16:71–6.
9. Vaismoradi M, Turunen H, Bondas T. Content analysis and thematic analysis: implications for conducting a qualitative descriptive study. Nurs Health Sci 2013;15:398–405.
10. Kampmeijer, R, Pavlova M, Tambor M, et al. The use of e-health and m-health tools in health promotion and primary prevention among older adults: a systematic literature review. BMC Health Serv Res 2016;16 Suppl 5:290.
11. Vawdrey, DK, Wilcox LG, Collins SA, et al. A tablet computer application for patients to participate in their hospital care. AMIA Annu Symp Proc 2011:1428–35.
12. Greysen, SR, Khanna RR, Jacolbia R, et al. Tablet computers for hospitalized patients: a pilot study to improve inpatient engagement. J Hosp Med 2014;9:396–9.
Endobronchial Valves for Severe Emphysema
Study Overview
Objective. To evaluate the efficacy and safety of Zephyr endobronchial valves (EBVs) in patients with heterogeneous emphysema and absence of collateral ventilation.
Design. Multicenter, randomized, nonblinded clinical trial.
Setting and participants. This study was conducted at 17 sites across Europe between 2014 and 2016. Patients with severe emphysema who were ex-smokers and ≥ 40 years old were recruited. Key inclusion criteria were post-bronchodilator FEV1 between 15%–45% predicted despite optimal medical management, total lung capacity greater than 100% predicted, residual volume ≥ 180% predicted, and a 6-minute walk distance of between 150 and 450 meters. Heterogenous emphysema was defined as a greater than 10% difference in destruction score between target and ipsilateral lobes as measured by high-resolution CT. All eligible patients underwent Chartis pulmonary assessment (Pulmonx, Redwood City, CA) assessment to determine the presence of collateral ventilation between the target and adjacent lobes, and patients with collateral ventilation were excluded.
Intervention. Patients were randomized 2:1 to either EBV plus standard of care (intervention) or standard of care alone (control) by blocked design and concealed envelopes. The EBV group underwent immediate placement of Zephyr EBVs with the intention of complete lobar occlusion.
Main outcome measures. The primary outcome at 3 months post-procedure was the percentage of subjects with FEV1 improvement from baseline of 12% or greater. Changes in FEV1, residual volume, 6-minute walk distance, St. George’s Respiratory Questionnaire score and modified Medical Research Council score were assessed at 3 and 6 months and target lobe volume reduction on chest CT at 3 months.
Main results. 97 subjects were randomized to the intervention (n = 65) or control group (n = 32). At 3 months, 55.4% of intervention and 6.5% of control subjects had an FEV1 improvement of 12% or more (P < 0.001). Improvements were maintained at 6 months: intervention, 56.3%, versus control, 3.2% (P < 0.001), with a mean ± SD change in FEV1% at 6 months of 20.7 ± 29.6% and –8.6 ± 13.0%, respectively. A total of 89.8% of intervention subjects had target lobe volume reduction greater than or equal to 350 mL (mean, 1.09 ± 0.62 L; P < 0.001). The differences in outcomes between the intervention and control groups were statistically significant, with the following measured differences: residual volume, –700 m; 6-minute walk distance, +78.7 m; St. George’s Respiratory Questionnaire score, –6.5 points; modified Medical Research Council dyspnea score, –0.6 points; and BODE (body mass index, airflow obstruction, dyspnea, and exercise capacity) index, –1.8 points (all P < 0.05). Pneumothorax was the most common adverse event, occurring in 19 of 65 (29.2%) of intervention subjects.
Conclusion. Endobronchial valve treatment in hyperinflated patients with heterogeneous emphysema without collateral ventilation resulted in clinically meaningful benefits in lung function, dyspnea, exercise tolerance and quality of life, with an acceptable safety profile.
Commentary
Patients with severe emphysema are difficult to manage. Optimal medical management is required to maintain their lung function and quality of life, with combination bronchodilators (long-acting beta 2 agonists, long-acting anticholinergics, and inhaled corticosteroids), roflumilast (selective phosphodiesterase-4 inhibitors), oral corticosteroids or macrolide antibiotics when indicated, long-term oxygen, and noninvasive ventilator support. Palliative team care consultation and support, adequate nutritional support, influenza and pneumococcal vaccination, and pulmonary rehabilitation/graded exercise training are important aspects of emphysema treatment [1].
To help patients with severe emphysema who experience further decline despite intensive medical management, a lung volume reduction strategy was devised. In 2003, the NETT trial was conducted [2]. In this study, lung volume reduction surgery was performed in 608 patients, who were followed for 29 months. This study revealed a lack of survival benefit with significant immediate postoperative mortality and complication rate. Despite this disappointing result, a subgroup of patients (upper-lobe predominant disease and low baseline exercise capacity) had a statistically significant mortality benefit from surgery.
Since then, many have sought to determine a less invasive method of lung volume reduction. So far, one-way endobronchial valves, self-activating coils, and targeted destruction and remodeling of emphysematous lung with vapor or sealant methods have been studied. Several studies have examined the efficacy and safety of coils, with reasonable improvement of 6-minute walk distance and FEV1; however, complications including death, pneumothorax and pneumonia were noted. Vapor ablation (STEP-UP trial) [3] and lung sealant [4] were also attempted in order to achieve lung volume reduction, but increased infection was problematic. The 2017 GOLD guidelines suggested lung volume reduction by endobronchial one-way valve or lung coils as interventional bronchoscopic options for lung volume reduction [1].
Two types of endobronchial valves have been introduced to date: the intra bronchial valve, developed by Olympus, and the Zephyr valve by Pulmonx. Endobronchial valves are deployed to the bronchi via bronchoscopic guidance, and limit airflow to the portions of the lung distal to the valve while allowing mucus and air movement in the proximal direction. The VENT study, the largest endobronchial valve trial using the Zephyr valve, was published in 2010 [5]. This study demonstrated the efficacy of endobronchial valve treatment, especially in patients with heterogeneous emphysema and complete interlobar fissures as opposed to homogeneous emphysema and incomplete interlobar fissures. Subsequent studies demonstrated the importance of absence of collateral ventilation, measured by the Chartis system, when considering endobronchial valves [6].
The current study by Kemp et al is the first multicenter randomized endobronchial valve trial conducted in Europe. The study was able to demonstrate remarkable improvement in FEV1 (mean 140 mL decrease vs 90 mL increase) and 6-minute walk distance (mean +36.2 meter vs –42.5 meter) after endobronchial valve treatment in severe emphysema patients. The amount of volume reduction was reaching up to 2 liters. Patients in the control group were given the opportunity to receive endobronchial valve after the 6 months study follow-up period and 30 out of 32 patients opted for the endobronchial valve treatment. The authors concluded that the endobronchial valve therapy resulted in clinically meaningful benefits in lung function, dyspnea, exercise tolerance and quality of life with an acceptable safety profile.
It is notable that the authors included only selected patients, limited to those with presence of heterogeneous emphysema, absence of collateral ventilation, low risk of COPD exacerbation or infection, and patients who were likely able to tolerate pneumothorax. Despite this, 13 patients developed pneumothorax and death occurred in 1 patient, leading to a significantly longer average length of hospital stay in the treatment group. Although this rate of complications is not higher than prior endobronchial valve studies, it is important to note when broadly applying the outcomes of this study to patient care. Lack of long-term follow-up and the nonblinded study design also limit the strength of this study.
Applications for Clinical Practice
Many patients suffer from emphysema. Among them, severe emphysema is the most difficult to manage. It is important to incorporate optimal medical management including bronchodilators, palliative care, oxygen therapy, pulmonary rehabilitation and non-invasive ventilation options. When patients with severe emphysema continue to decline or seek further improvement in their care, and when they meet the specific criteria for lung volume reduction, endobronchial valve therapy should be considered an option and physicians should refer them to appropriate centers. However, the risk of complications, such as pneumothorax, still remains high.
—Minkyung Kwon, MD, Pulmonary and Critical Care Medicine, Mayo Clinic Florida, Jacksonville, FL, and Joel Roberson, MD, Department of Radiology, William Beaumont Hospital, Royal Oak, MI
1. The Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017.
2. Weinmann GG, Chiang YP, Sheingold S. The National Emphysema Treatment Trial (NETT): a study in agency collaboration. Proc Am Thorac Soc 2008;5:381–4.
3. Herth FJ, Valipour A, Shah PL, et al. Segmental volume reduction using thermal vapour ablation in patients with severe emphysema: 6-month results of the multicentre, parallel-group, open-label, randomised controlled STEP-UP trial. Lancet Respir Med 2016;4:185–93.
4. Come CE, Kramer MR, Dransfield MT, et al. A randomised trial of lung sealant versus medical therapy for advanced emphysema. Eur Respir J 2015;46:651–62.
5. Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010;363:1233–44.
6. Klooster K, ten Hacken NH, Hartman JE, et al. Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med 2015;373:2325–35.
Study Overview
Objective. To evaluate the efficacy and safety of Zephyr endobronchial valves (EBVs) in patients with heterogeneous emphysema and absence of collateral ventilation.
Design. Multicenter, randomized, nonblinded clinical trial.
Setting and participants. This study was conducted at 17 sites across Europe between 2014 and 2016. Patients with severe emphysema who were ex-smokers and ≥ 40 years old were recruited. Key inclusion criteria were post-bronchodilator FEV1 between 15%–45% predicted despite optimal medical management, total lung capacity greater than 100% predicted, residual volume ≥ 180% predicted, and a 6-minute walk distance of between 150 and 450 meters. Heterogenous emphysema was defined as a greater than 10% difference in destruction score between target and ipsilateral lobes as measured by high-resolution CT. All eligible patients underwent Chartis pulmonary assessment (Pulmonx, Redwood City, CA) assessment to determine the presence of collateral ventilation between the target and adjacent lobes, and patients with collateral ventilation were excluded.
Intervention. Patients were randomized 2:1 to either EBV plus standard of care (intervention) or standard of care alone (control) by blocked design and concealed envelopes. The EBV group underwent immediate placement of Zephyr EBVs with the intention of complete lobar occlusion.
Main outcome measures. The primary outcome at 3 months post-procedure was the percentage of subjects with FEV1 improvement from baseline of 12% or greater. Changes in FEV1, residual volume, 6-minute walk distance, St. George’s Respiratory Questionnaire score and modified Medical Research Council score were assessed at 3 and 6 months and target lobe volume reduction on chest CT at 3 months.
Main results. 97 subjects were randomized to the intervention (n = 65) or control group (n = 32). At 3 months, 55.4% of intervention and 6.5% of control subjects had an FEV1 improvement of 12% or more (P < 0.001). Improvements were maintained at 6 months: intervention, 56.3%, versus control, 3.2% (P < 0.001), with a mean ± SD change in FEV1% at 6 months of 20.7 ± 29.6% and –8.6 ± 13.0%, respectively. A total of 89.8% of intervention subjects had target lobe volume reduction greater than or equal to 350 mL (mean, 1.09 ± 0.62 L; P < 0.001). The differences in outcomes between the intervention and control groups were statistically significant, with the following measured differences: residual volume, –700 m; 6-minute walk distance, +78.7 m; St. George’s Respiratory Questionnaire score, –6.5 points; modified Medical Research Council dyspnea score, –0.6 points; and BODE (body mass index, airflow obstruction, dyspnea, and exercise capacity) index, –1.8 points (all P < 0.05). Pneumothorax was the most common adverse event, occurring in 19 of 65 (29.2%) of intervention subjects.
Conclusion. Endobronchial valve treatment in hyperinflated patients with heterogeneous emphysema without collateral ventilation resulted in clinically meaningful benefits in lung function, dyspnea, exercise tolerance and quality of life, with an acceptable safety profile.
Commentary
Patients with severe emphysema are difficult to manage. Optimal medical management is required to maintain their lung function and quality of life, with combination bronchodilators (long-acting beta 2 agonists, long-acting anticholinergics, and inhaled corticosteroids), roflumilast (selective phosphodiesterase-4 inhibitors), oral corticosteroids or macrolide antibiotics when indicated, long-term oxygen, and noninvasive ventilator support. Palliative team care consultation and support, adequate nutritional support, influenza and pneumococcal vaccination, and pulmonary rehabilitation/graded exercise training are important aspects of emphysema treatment [1].
To help patients with severe emphysema who experience further decline despite intensive medical management, a lung volume reduction strategy was devised. In 2003, the NETT trial was conducted [2]. In this study, lung volume reduction surgery was performed in 608 patients, who were followed for 29 months. This study revealed a lack of survival benefit with significant immediate postoperative mortality and complication rate. Despite this disappointing result, a subgroup of patients (upper-lobe predominant disease and low baseline exercise capacity) had a statistically significant mortality benefit from surgery.
Since then, many have sought to determine a less invasive method of lung volume reduction. So far, one-way endobronchial valves, self-activating coils, and targeted destruction and remodeling of emphysematous lung with vapor or sealant methods have been studied. Several studies have examined the efficacy and safety of coils, with reasonable improvement of 6-minute walk distance and FEV1; however, complications including death, pneumothorax and pneumonia were noted. Vapor ablation (STEP-UP trial) [3] and lung sealant [4] were also attempted in order to achieve lung volume reduction, but increased infection was problematic. The 2017 GOLD guidelines suggested lung volume reduction by endobronchial one-way valve or lung coils as interventional bronchoscopic options for lung volume reduction [1].
Two types of endobronchial valves have been introduced to date: the intra bronchial valve, developed by Olympus, and the Zephyr valve by Pulmonx. Endobronchial valves are deployed to the bronchi via bronchoscopic guidance, and limit airflow to the portions of the lung distal to the valve while allowing mucus and air movement in the proximal direction. The VENT study, the largest endobronchial valve trial using the Zephyr valve, was published in 2010 [5]. This study demonstrated the efficacy of endobronchial valve treatment, especially in patients with heterogeneous emphysema and complete interlobar fissures as opposed to homogeneous emphysema and incomplete interlobar fissures. Subsequent studies demonstrated the importance of absence of collateral ventilation, measured by the Chartis system, when considering endobronchial valves [6].
The current study by Kemp et al is the first multicenter randomized endobronchial valve trial conducted in Europe. The study was able to demonstrate remarkable improvement in FEV1 (mean 140 mL decrease vs 90 mL increase) and 6-minute walk distance (mean +36.2 meter vs –42.5 meter) after endobronchial valve treatment in severe emphysema patients. The amount of volume reduction was reaching up to 2 liters. Patients in the control group were given the opportunity to receive endobronchial valve after the 6 months study follow-up period and 30 out of 32 patients opted for the endobronchial valve treatment. The authors concluded that the endobronchial valve therapy resulted in clinically meaningful benefits in lung function, dyspnea, exercise tolerance and quality of life with an acceptable safety profile.
It is notable that the authors included only selected patients, limited to those with presence of heterogeneous emphysema, absence of collateral ventilation, low risk of COPD exacerbation or infection, and patients who were likely able to tolerate pneumothorax. Despite this, 13 patients developed pneumothorax and death occurred in 1 patient, leading to a significantly longer average length of hospital stay in the treatment group. Although this rate of complications is not higher than prior endobronchial valve studies, it is important to note when broadly applying the outcomes of this study to patient care. Lack of long-term follow-up and the nonblinded study design also limit the strength of this study.
Applications for Clinical Practice
Many patients suffer from emphysema. Among them, severe emphysema is the most difficult to manage. It is important to incorporate optimal medical management including bronchodilators, palliative care, oxygen therapy, pulmonary rehabilitation and non-invasive ventilation options. When patients with severe emphysema continue to decline or seek further improvement in their care, and when they meet the specific criteria for lung volume reduction, endobronchial valve therapy should be considered an option and physicians should refer them to appropriate centers. However, the risk of complications, such as pneumothorax, still remains high.
—Minkyung Kwon, MD, Pulmonary and Critical Care Medicine, Mayo Clinic Florida, Jacksonville, FL, and Joel Roberson, MD, Department of Radiology, William Beaumont Hospital, Royal Oak, MI
Study Overview
Objective. To evaluate the efficacy and safety of Zephyr endobronchial valves (EBVs) in patients with heterogeneous emphysema and absence of collateral ventilation.
Design. Multicenter, randomized, nonblinded clinical trial.
Setting and participants. This study was conducted at 17 sites across Europe between 2014 and 2016. Patients with severe emphysema who were ex-smokers and ≥ 40 years old were recruited. Key inclusion criteria were post-bronchodilator FEV1 between 15%–45% predicted despite optimal medical management, total lung capacity greater than 100% predicted, residual volume ≥ 180% predicted, and a 6-minute walk distance of between 150 and 450 meters. Heterogenous emphysema was defined as a greater than 10% difference in destruction score between target and ipsilateral lobes as measured by high-resolution CT. All eligible patients underwent Chartis pulmonary assessment (Pulmonx, Redwood City, CA) assessment to determine the presence of collateral ventilation between the target and adjacent lobes, and patients with collateral ventilation were excluded.
Intervention. Patients were randomized 2:1 to either EBV plus standard of care (intervention) or standard of care alone (control) by blocked design and concealed envelopes. The EBV group underwent immediate placement of Zephyr EBVs with the intention of complete lobar occlusion.
Main outcome measures. The primary outcome at 3 months post-procedure was the percentage of subjects with FEV1 improvement from baseline of 12% or greater. Changes in FEV1, residual volume, 6-minute walk distance, St. George’s Respiratory Questionnaire score and modified Medical Research Council score were assessed at 3 and 6 months and target lobe volume reduction on chest CT at 3 months.
Main results. 97 subjects were randomized to the intervention (n = 65) or control group (n = 32). At 3 months, 55.4% of intervention and 6.5% of control subjects had an FEV1 improvement of 12% or more (P < 0.001). Improvements were maintained at 6 months: intervention, 56.3%, versus control, 3.2% (P < 0.001), with a mean ± SD change in FEV1% at 6 months of 20.7 ± 29.6% and –8.6 ± 13.0%, respectively. A total of 89.8% of intervention subjects had target lobe volume reduction greater than or equal to 350 mL (mean, 1.09 ± 0.62 L; P < 0.001). The differences in outcomes between the intervention and control groups were statistically significant, with the following measured differences: residual volume, –700 m; 6-minute walk distance, +78.7 m; St. George’s Respiratory Questionnaire score, –6.5 points; modified Medical Research Council dyspnea score, –0.6 points; and BODE (body mass index, airflow obstruction, dyspnea, and exercise capacity) index, –1.8 points (all P < 0.05). Pneumothorax was the most common adverse event, occurring in 19 of 65 (29.2%) of intervention subjects.
Conclusion. Endobronchial valve treatment in hyperinflated patients with heterogeneous emphysema without collateral ventilation resulted in clinically meaningful benefits in lung function, dyspnea, exercise tolerance and quality of life, with an acceptable safety profile.
Commentary
Patients with severe emphysema are difficult to manage. Optimal medical management is required to maintain their lung function and quality of life, with combination bronchodilators (long-acting beta 2 agonists, long-acting anticholinergics, and inhaled corticosteroids), roflumilast (selective phosphodiesterase-4 inhibitors), oral corticosteroids or macrolide antibiotics when indicated, long-term oxygen, and noninvasive ventilator support. Palliative team care consultation and support, adequate nutritional support, influenza and pneumococcal vaccination, and pulmonary rehabilitation/graded exercise training are important aspects of emphysema treatment [1].
To help patients with severe emphysema who experience further decline despite intensive medical management, a lung volume reduction strategy was devised. In 2003, the NETT trial was conducted [2]. In this study, lung volume reduction surgery was performed in 608 patients, who were followed for 29 months. This study revealed a lack of survival benefit with significant immediate postoperative mortality and complication rate. Despite this disappointing result, a subgroup of patients (upper-lobe predominant disease and low baseline exercise capacity) had a statistically significant mortality benefit from surgery.
Since then, many have sought to determine a less invasive method of lung volume reduction. So far, one-way endobronchial valves, self-activating coils, and targeted destruction and remodeling of emphysematous lung with vapor or sealant methods have been studied. Several studies have examined the efficacy and safety of coils, with reasonable improvement of 6-minute walk distance and FEV1; however, complications including death, pneumothorax and pneumonia were noted. Vapor ablation (STEP-UP trial) [3] and lung sealant [4] were also attempted in order to achieve lung volume reduction, but increased infection was problematic. The 2017 GOLD guidelines suggested lung volume reduction by endobronchial one-way valve or lung coils as interventional bronchoscopic options for lung volume reduction [1].
Two types of endobronchial valves have been introduced to date: the intra bronchial valve, developed by Olympus, and the Zephyr valve by Pulmonx. Endobronchial valves are deployed to the bronchi via bronchoscopic guidance, and limit airflow to the portions of the lung distal to the valve while allowing mucus and air movement in the proximal direction. The VENT study, the largest endobronchial valve trial using the Zephyr valve, was published in 2010 [5]. This study demonstrated the efficacy of endobronchial valve treatment, especially in patients with heterogeneous emphysema and complete interlobar fissures as opposed to homogeneous emphysema and incomplete interlobar fissures. Subsequent studies demonstrated the importance of absence of collateral ventilation, measured by the Chartis system, when considering endobronchial valves [6].
The current study by Kemp et al is the first multicenter randomized endobronchial valve trial conducted in Europe. The study was able to demonstrate remarkable improvement in FEV1 (mean 140 mL decrease vs 90 mL increase) and 6-minute walk distance (mean +36.2 meter vs –42.5 meter) after endobronchial valve treatment in severe emphysema patients. The amount of volume reduction was reaching up to 2 liters. Patients in the control group were given the opportunity to receive endobronchial valve after the 6 months study follow-up period and 30 out of 32 patients opted for the endobronchial valve treatment. The authors concluded that the endobronchial valve therapy resulted in clinically meaningful benefits in lung function, dyspnea, exercise tolerance and quality of life with an acceptable safety profile.
It is notable that the authors included only selected patients, limited to those with presence of heterogeneous emphysema, absence of collateral ventilation, low risk of COPD exacerbation or infection, and patients who were likely able to tolerate pneumothorax. Despite this, 13 patients developed pneumothorax and death occurred in 1 patient, leading to a significantly longer average length of hospital stay in the treatment group. Although this rate of complications is not higher than prior endobronchial valve studies, it is important to note when broadly applying the outcomes of this study to patient care. Lack of long-term follow-up and the nonblinded study design also limit the strength of this study.
Applications for Clinical Practice
Many patients suffer from emphysema. Among them, severe emphysema is the most difficult to manage. It is important to incorporate optimal medical management including bronchodilators, palliative care, oxygen therapy, pulmonary rehabilitation and non-invasive ventilation options. When patients with severe emphysema continue to decline or seek further improvement in their care, and when they meet the specific criteria for lung volume reduction, endobronchial valve therapy should be considered an option and physicians should refer them to appropriate centers. However, the risk of complications, such as pneumothorax, still remains high.
—Minkyung Kwon, MD, Pulmonary and Critical Care Medicine, Mayo Clinic Florida, Jacksonville, FL, and Joel Roberson, MD, Department of Radiology, William Beaumont Hospital, Royal Oak, MI
1. The Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017.
2. Weinmann GG, Chiang YP, Sheingold S. The National Emphysema Treatment Trial (NETT): a study in agency collaboration. Proc Am Thorac Soc 2008;5:381–4.
3. Herth FJ, Valipour A, Shah PL, et al. Segmental volume reduction using thermal vapour ablation in patients with severe emphysema: 6-month results of the multicentre, parallel-group, open-label, randomised controlled STEP-UP trial. Lancet Respir Med 2016;4:185–93.
4. Come CE, Kramer MR, Dransfield MT, et al. A randomised trial of lung sealant versus medical therapy for advanced emphysema. Eur Respir J 2015;46:651–62.
5. Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010;363:1233–44.
6. Klooster K, ten Hacken NH, Hartman JE, et al. Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med 2015;373:2325–35.
1. The Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017.
2. Weinmann GG, Chiang YP, Sheingold S. The National Emphysema Treatment Trial (NETT): a study in agency collaboration. Proc Am Thorac Soc 2008;5:381–4.
3. Herth FJ, Valipour A, Shah PL, et al. Segmental volume reduction using thermal vapour ablation in patients with severe emphysema: 6-month results of the multicentre, parallel-group, open-label, randomised controlled STEP-UP trial. Lancet Respir Med 2016;4:185–93.
4. Come CE, Kramer MR, Dransfield MT, et al. A randomised trial of lung sealant versus medical therapy for advanced emphysema. Eur Respir J 2015;46:651–62.
5. Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010;363:1233–44.
6. Klooster K, ten Hacken NH, Hartman JE, et al. Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med 2015;373:2325–35.
Management of Community-Acquired Pneumonia in Adults
From the University of North Dakota School of Medicine & Health Sciences, Fargo, ND.
Abstract
- Objective: To review the management of community-acquired pneumonia (CAP) in adults.
- Methods: Review of the literature.
- Results: Approximately 4 to 5 million cases of CAP are diagnosed in the United States annually, accounting for significant morbidity and mortality. While numerous studies have previously shown pneumococcus to be the most common causative pathogen, the 2015 EPIC study found that in nearly two-thirds of patients with CAP who required hospitalization, no pathogen was detected. Symptoms and signs of respiratory tract infection are useful in helping to diagnose pneumonia; however, they are less sensitive than chest imaging studies. Laboratory tests used in diagnosing pneumonia include sputum Gram stain and culture, blood culture, urinary antigen, polymerase chain reaction, and biologic markers. In empiric treatment of CAP, both the typical and atypical pathogens should be targeted. Influenza vaccine and pneumococcal polysaccharide and conjugate vaccines should be administered as recommended by the CDC to reduce risk of CAP.
- Conclusion: CAP is a common illness with high rates of morbidity and mortality. Treatment is for the most part empirical; diagnostic testing can be used to identify the causative organism and guide pathogen-specific therapy.
Key words: community-acquired pneumonia; adults; management; vaccines.
Despite advances in medical science, pneumonia remains a major cause of morbidity and mortality. In 2014, 50,620 patients in the United States died from the disease [1]. Pneumonia can be classified as community-acquired, hospital-acquired, or ventilator-associated. Another category, healthcare-associated pneumonia, was included in an earlier American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) guideline but was removed from the 2016 guideline because there was no clear evidence that patients diagnosed with healthcare-associated pneumonia were at higher risk for harboring multidrug-resistant pathogens [2]. In this article, we review the epidemiology, microbiology, predisposing factors, diagnosis, treatment, and prevention of community-acquired pneumonia (CAP).
Definition and Epidemiology
CAP is defined as an acute infection of the lungs that develops in patients who have not been hospitalized recently and have not had regular exposure to the health care system [3]. A previously ambulatory patient who is diagnosed with pneumonia within 48 hours after admission also meets the criteria for CAP. Approximately 4 to 5 million cases of CAP are diagnosed in the United States annually [4]. About 25% of CAP patients require hospitalization, and about 5% to 10% of these patients are admitted to the intensive care unit (ICU) [5]. In-hospital mortality is considerable (~10% in population-based studies) [6] and 30-day mortality was found to be as high as 23% in a review by File and Marrie [7]. CAP also confers a high risk of long-term morbidity and mortality compared with the general population who have never had CAP, irrespective of age [8].
Causative Organisms
Numerous microorganisms can cause CAP. Common causes and less common causes are delineated in Table 1.
Predisposing Factors
Most people diagnosed with CAP have one or more predisposing factors [12,13] (Table 2).
Clinical Signs and Symptoms
Symptoms of CAP include fever, chills, rigors, fatigue, anorexia, diaphoresis, dyspnea, cough (with or without sputum production), and pleuritic chest pain. There is no individual symptom or cluster of symptoms that can absolutely differentiate pneumonia from other acute respiratory diseases, including upper and lower respiratory infections. However, if a patient presents with the constellation of symptoms of fever ≥ 1000F (37.80C), productive cough, and tachycardia, it is more suggestive of pneumonia [14]. Abnormal vital signs include fever, hypothermia, tachypnea, tachycardia, and oxygen desaturation. Auscultation of the chest reveals crackles or other adventitious breath sounds. Elderly patients with pneumonia report a significantly lower number of both respiratory and nonrespiratory symptoms compared with younger patients. Clinicians should be aware of this phenomenon so it does not lead to delayed diagnosis and treatment [15].
Imaging Evaluation
The presence of a pulmonary consolidation or an infiltrate on chest radiograph is required to diagnose CAP, and a chest radiograph should be obtained when CAP is suspected [16]. It should be noted that there is no pattern of radiographic abnormalities reliable enough to differentiate infectious pneumonia from noninfectious causes [17].
There are case reports and case series demonstrating false-negative plain chest radiographs existing in dehydrated patients [18] or in neutropenic state. However, animal studies have shown that dogs challenged with pneumococcus showed abnormal pulmonary shadow, suggestive of pneumonia, regardless of hydration status [19]. There is also no reliable scientific evidence to support the notion that severe neutropenia can cause false-negative radiographs because of the inability to develop an acute inflammatory reaction in the lungs [20].
A chest CT scan is more sensitive than a plain chest radiograph in detecting pneumonia. Therefore, a chest CT should be performed in a patient with negative plain chest radiograph when pneumonia is still highly suspected [21]. A chest CT scan is also more sensitive in detecting cavitation, adenopathy, interstitial disease and empyema. It also has the advantage of better defining anatomical changes than plain films [22].
Because improvement of pulmonary opacities in patients with CAP lags behind clinical improvement, repeating chest imaging studies is not recommended in patients who demonstrate clinical improvement. Sometimes clearing of pulmonary infiltrate or consolidation can take 6 weeks or longer [23].
Laboratory Evaluation
Generally the etiologic agent of CAP cannot be determined solely on the basis of clinical signs and symptoms or imaging studies. Although routine microbiological testing for patients suspicious for CAP is not necessary for empirical treatment, by determining the etiologic agent of the pneumonia, a clinician will be able to narrow the antibiotics from a broad-spectrum empirical regimen to specific pathogen-directed therapy. Determination of certain etiologic agents causing the pneumonia can have important public health implications (eg, Mycobacterium tuberculosis and influenza virus) [24].
Sputum Gram Stain and Culture
Sputum Gram stain is an inexpensive test that may identify pathogens that cause CAP (eg, S. pneumonia and Haemophilus influenzae). A quality specimen is required. A sputum sample must contain > 25 neutrophils and < 10 squamous epithelial cells/low power field on Gram stain to be considered suitable for culture.
The sensitivity and specificity of sputum Gram stain and culture are highly variable in different clinical settings (eg, outpatient setting, nursing home, ICU). Reed et al’s meta-analysis of patients diagnosed with CAP in the United States showed the sensitivity and specificity of sputum Gram stain (compared with sputum culture) ranged from 15% to 100% and 11% to 100%, respectively [24]. In cases of proven bacteremic pneumococcal pneumonia, positive cultures from sputum samples were positive less than 50% of the time [25].
For patients who cannot provide sputum samples or are intubated, a deep-suction aspirate or bronchoalveolar lavage through a bronchoscopic procedure might be necessary to obtain pulmonary secretion for Gram stain and culture. Besides bacterial culture, sputum samples can also be sent for fungal and mycobacterial cultures and acid-fast stain if deemed clinically necessary.
Blood Culture
Because the positivity rate of blood culture in patients who are suspected to have pneumonia but not exposed to antimicrobial agents is disappointingly low (5%–14%), blood cultures are no longer recommended in patients hospitalized for CAP. Another reason for not recommending blood culture is positive culture rarely leads to changes in antibiotic regimen in patients without underlying diseases [26]. However, high-risk patients, including patients with severe CAP or in immunocompromised patients (eg, patients with neutropenia, asplenia or complement deficiencies) should have a blood culture done [24].
A multinational study published in 2008 examined 125 patients with pneumococcal bacteremic CAP versus 1847 patients with non-bacteremic CAP [27]. Analysis of the data demonstrated no association of pneumococcal bacteremic CAP and time to clinical stability, length of hospital stay, all-cause mortality or CAP-related mortality. The authors concluded that pneumococcal bacteremia does not increase the risk of poor outcomes in patients with CAP compared to non-bacteremic patients, and the presence of pneumococcal bacteremia should not deter de-escalation of therapy in clinically stable patients.
Urinary Antigen Tests
Urinary antigen tests may assist clinicians in narrowing antibiotic therapy when test results are positive. There are 2 U.S. Food and Drug Administration–approved tests available to clinicians for detecting pneumococcal and Legionella antigen in urine. The test for Legionella pneumophila detects disease due to serogroup 1 only, which accounts for 80% of community-acquired Legionnaires disease. The sensitivity and specificity of the Legionella urine antigen test are 90% and 99%, respectively. The pneumococcal urine antigen test is less sensitive and specific than the Legionella urine antigen test (sensitivity 80% and specificity > 90%) [28,29].
Advantages of the urinary antigen tests are that they are easily performed, results are available in less than an hour if done in-house, and results are not affected by prior exposure to antibiotics. However, the tests do not meet Clinical Laboratory Improvements Amendments criteria for waiver and must be performed by a technician in the laboratory.
Polymerase Chain Reaction
There are several FDA-approved polymerase chain reaction (PCR) tests commercially available to assist clinicians in diagnosing pneumonia. PCR test of nasopharyngeal swabs for diagnosing influenza have become standard in many medical U.S. facilities. The great advantage of using PCR to diagnose influenza is its high sensitivity and specificity and rapid turnaround time. PCR can also be used to detect Legionella species, S. pneumonia, Mycoplasma pneumoniae, Chlamydophila pneumonia and mycobacterial species [24].
One limitation of using PCR tests on respiratory specimens is that specimens can be contaminated with oral or upper airway flora, so the results must be interpreted with caution, bearing in mind that some of the pathogens isolated may be colonizers of the oral or upper airway flora [30].
Biologic Markers
Two biologic markers—procalcitonin and C-reactive protein (CRP)—can be used in conjunction with history, physical examination, laboratory tests and imaging studies to assist in the diagnosis and treatment of CAP [24]. Procalcitonin is a peptide precursor of the hormone calcitonin that is released by parenchymal cells into the bloodstream resulting in increased serum level in patients with bacterial infections. In contrast, there is no remarkable proclacitonin level increase with viral or noninfectious inflammation. The reference value of procalcitonin in the blood of an adult individual without infection or inflammation is < 0.15 ng/mL. In the blood, procalcitonin has a half-life of 25 to 30 hours. The quantitative immunoluminometric method (LUMI test, Brahms PCT, Berlin, Germany ) is the preferred test to use because of its high sensitivity [31].
A 2012 Cochrane meta-analysis that involved 4221 patients with acute respiratory infections (with half of the patients diagnosed with CAP) from 14 prospective trials found the use of procalcitonin test for antibiotic use significantly decreased median antibiotic exposure from 8 to 4 days without an increase in treatment failure, mortality rates in any clinical setting (eg, outpatient clinic, emergency room), or length of hospitalization [32]. A prospective study conducted in France on 100 ICU patients showed that increased procalcitonin from day 1 to day 3 has a poor prognosis factor for severe CAP whereas decreasing procalcitonin levels is associated with a favorable outcome [33].
CRP is an acute phase protein produced by the liver. CRP level in the blood increases in response to acute infection or inflammation. Use of CRP in assisting diagnosis and guiding treatment of CAP is more limited in part due to its poor specificity. A prospective study conducted on 168 consecutive patients presented with cough showed that a CRP > 40 mg/L had a sensitivity and specificity of 70% and 90%, respectively [34].
T reatment
Site of Care Decision
For patients with CAP, the clinician must decide whether the patient will be treated in an outpatient or inpatient setting, and for those in the inpatient setting, whether they can safely be treated on the general medical ward or should be the ICU. Two common scoring systems that can be used to aid the clinician in determining severity of the infection and guiding site-of-care decisions are the Pneumonia Severity Index (PSI) and CURB-65 scores.
The PSI score uses 20 different parameters, including comorbidities, laboratory parameters and radiographic findings to stratify patients into 5 mortality risk classes [35]. On the basis of associated mortality rates, it has been suggested that risk class I and II patients should be treated as outpatients, risk class III patients should be treated in an observation unit or with a short hospitalization, and risk class IV and V patients should be treated as inpatients [35].
The CURB-65 method of risk stratification is based on 5 clinical parameters: confusion, urea level, respiratory rate, systolic blood pressure and age ≥ 65 (Table 3) [36].
Patients with CURB-65 scores of 4 or 5 are considered to have severe pneumonia and admission to the ICU should be considered. Aside from the CURB-65 score, anyone requiring vasopressor support or mechanical ventilation merits admission to the ICU [16]. IDSA/ATS guidelines also recommend the use of “minor criteria” for making ICU admission decisions; these include respiratory rate ≥ 30 breaths / minute, PaO2 fraction ≤ 250, multilobar infiltrates, confusion, blood urea nitrogen ≥ 20 mg/dL, leukopenia, thrombocytopenia, hypothermia and hypotension [16]. These factors are associated with increased mortality due to CAP and admission to an ICU is indicated if 3 of the minor criteria for severe CAP are present.
Similar to CURB-65, another clinical calculator that can be used for assessing severity of CAP is SMART-COP [39]. This scoring system uses 8 weighted criteria to predict which patients will require intensive respiratory or vasopressor support. SMART-COP has a sensitivity of 79% and specificity 64% in predicting ICU admission, whereas CURB-65 had a pooled sensitivity of 57.2% and specificity of 77.2% [40].
Antibiotic Therapy
Antibiotics are the mainstay of treatment for CAP, with the majority of patients with CAP treated empirically taking into account the site of care, likely pathogen, and antimicrobial resistance issues. Patients with pneumonia who are treated as outpatients usually respond well to empiric antibiotic treatment and a causative pathogen is not usually sought. Patients who are hospitalized for treatment of CAP usually receive empiric antibiotic on admission. Once the etiology has been determined by microbiologic or serologic means, antimicrobial therapy should be adjusted accordingly. As noted previously, a CDC study found that the burden of viral etiologies was higher than previously thought, with rhinovirus and influenza accounting for 15% of cases and S. pneumoniae for only 5% [9]. This study highlighted the fact that despite advances in molecular techniques, most patients with pneumonia have no pathogen identified [9]. Given the lack of discernable pathogens in the majority of cases, unless a nonbacterial etiology is found patients should continue to be treated with antibiotics.
Outpatients without comorbidities or risk factors for drug-resistant S. pneumoniae (Table 4)
As previously mentioned, antibiotic therapy is typically empiric; neither clinical features nor radiographic features are sufficient to include or exclude infectious etiologies. Epidemiologic risk factors should be considered and, in certain cases, expanded antimicrobial coverage to include those entities; for example, treatment of anaerobes in the setting of lung abscess and antipseudomonal antibiotics for patients with bronchiectasis.
Of concern in the treatment of CAP is the increased prevalence of antimicrobial resistance among S. pneumoniae. The IDSA guidelines report that drug-resistant S. pneumoniae is more common in persons aged < 2 or > 65 years, and those with ß-lactam therapy within the previous 3 months, alcoholism, medical comorbidities, immunosuppressive illness or therapy, or exposure to a child who attends a day care center [16].
S. aureus should be considered during influenza outbreaks, with either vancomycin or linezolid being the recommended agents in the setting of methicillin-resistant S. aureus (MRSA). In a study comparing vancomycin versus linezolid for nosocomial pneumonia, the all-cause 60-day mortality was similar for both agents [41]. Datpomycin is another agent used against MRSA; however, its use in the setting of pneumonia is not indicated as daptomycin binds to surfactant, yielding it ineffective in the treatment of pneumonia [42]. Ceftaroline is a newer cephalosporin with activity against MRSA; its role in treatment of community-acquired MRSA pneumonia has not been fully elucidated, but it appears to be a useful agent for this indication [43,44].
A summary of recommended empiric antibiotic therapy is presented in Table 5.
Antibiotic Therapy for Selected Pathogens
S. pneumoniae
Patients with pneumococcal pneumonia who have penicillin-susceptible strains can be treated with intravenous penicillin (2 or 3 million units every 4 hours) or ceftriaxone. Once a patient meets criteria of stability, they can then be transitioned to oral penicillin, amoxicillin, or clarithromycin. Those with strains with reduced susceptibility can still be treated with penicillin but at a higher dose (4 million units IV every 4 hours) or a third-generation cephalosporin. Those whose pneumococcal pneumonia is complicated by bacteremia will benefit from dual therapy if severely ill, requiring ICU monitoring. Those not severely ill can be treated with monotherapy [46].
S. aureus
S. aureus is more commonly associated with hospital-acquired pneumonia but may also be seen during the influenza season and in those with severe necrotizing CAP. Both linezolid and vancomycin can be used to treat MRSA CAP. As noted above, ceftaroline has activity against MRSA and is approved for treatment of CAP, but is not approved by the FDA for MRSA CAP treatment. Similarly, tigecycline is approved for CAP and has activity against MRSA, but is not approved for MRSA CAP. Moreover, the FDA has warned of increased risk of death with tigecycline and has a black box warning to that effect [47].
Legionella
Treatment of legionellosis can be achieved with tetracyclines, macrolides, or fluoroquinolones. For nonimmunosuppressed patients with mild pneumonia, any of the listed antibiotics is considered appropriate. However, patients with severe infection or those with immunosuppression should be treated with either levofloxacin or azithromycin for 7 to 10 days [48].
C. pneumoniae
As with other atypical organisms, C. pneumoniae can be treated with doxycycline, a macrolide, or respiratory fluoroquinolones. However, length of therapy varies by regimen used; whereas treating with doxycycline 100 mg twice daily generally requires 14–21 days, moxifloxacin 400 mg daily only requires 10 days [49].
M. pneumoniae
As with C. pneumoniae, length of therapy of M. pneumoniae varies by antimicrobial used. Shortest courses are seen with the use of macrolides for 5 days, whereas 14 days is considered standard for doxycycline or a respiratory fluoroquinolone [50]. It should be noted that there has been increasingly documented resistance to macrolides, with known resistance of 8.2% in the United States [51].
Duration of Treatment
Most patients with CAP respond within 72 hours to appropriate therapy. IDSA/ATS guidelines recommend that patients be treated for a minimum of 5 days, and before discontinuing antibiotics patients should be afebrile a minimum of 48-72 hours and be clinically stable (Table 6) [16].
Hospitalized patients do not need to be monitored for an additional day once they have reached clinical stability (Table 6), are able to maintain oral intake, and have normal mentation, provided that other comorbidities are stable and social needs have been met [16]. Patients discharged from the hospital with instability have higher risk of readmission or death [55].
Transition to Oral Therapy
IDSA/ATS guidelines [16] recommend that patients should be transitioned from IV to oral antibiotics when they are improving clinically, have stable vital signs, and are able to ingest food/fluids and medications.
Management of Nonresponders
Although the majority of patients respond to antibiotics within 72 hours, treatment failure occurs in up to 15% of patients [45]. Nonresponding pneumonia is generally seen in 2 patterns: worsening of clinical status despite empiric antibiotics OR delay in achieving clinical stability as defined in Table 5 after 72 hours of treatment [13]. Risk factors associated with nonresponding pneumonia [56] are:
- Radiographic: multilobar infiltrates, pleural effusion, cavitation
- Bacteriologic: MRSA, gram-negative or Legionella pneumonia
- Severity index: PSI > 90
- Pharmacologic: incorrect antibiotic choice based on susceptibility
Patients with acute deterioration of clinical status will prompt transfer to a higher level of care and may require mechanical ventilator support. In those with delay in achieving clinical stability, question centers on whether the same antibiotics can be continued while doing further radiographic/microbiologic workup and/or changing antibiotics.
History should be reviewed with particular attention to exposures, travel history, and microbiologic and radiographic data. Clinicians should recall that viral causes account for up to 20% of pneumonias and there are also noninfectious causes that can mimic pyogenic infections [57]. If adequate initial cultures were not obtained, they should be obtained; however, care must be taken in reviewing new sets of cultures while on antibiotics as they may reveal colonization selected out by antibiotics and not a true pathogen. If repeat evaluation is unrevealing, then further evaluation with CT scan and bronchoscopy with bronchoalveolar lavage and biopsy is warranted. CT scans can show pleural effusions, bronchial obstructions or pattern suggestive of cryptogenic pneumonia. A bronchoscopy might yield a microbiologic diagnosis and with biopsy can also evaluate for noninfectious causes.
As with other infections, if escalation of antibiotics is undertaken, clinicians should be mindful to ensure that efforts are being made to elucidate the reason for nonresponse. To simply broaden antimicrobial therapy without attempts at establishing a microbiologic or radiographic cause for nonresponse may lead to inappropriate treatment recurrence of infection. Aside from patients who have bacteremic pneumococcal pneumonia in an ICU setting, there are no published reports pointing to superiority of combination antibiotics [46].
Other Treatment
Because of the inflammatory response associated with pneumonia, several agents have been evaluated as adjunctive treatment of pneumonia to decrease this inflammatory state; namely, steroids, macrolide antibiotics and statins. To date, only the use of steroids (methylprednisolone 0.5 mg/kg every 12 hours for 5 days) in those with severe CAP and high initial anti-inflammatory response (CRP > 150) was shown to decrease treatment failure, decreased risk of ARDS, possibly reduce length of stay, duration of intravenous antibiotics and clinical stability, without effect on mortality or adverse side effects [58,59].
Other adjunctive methods have not been found to have significant impact [16].
Prevention of Pneumonia
Prevention of pneumococcal pneumonia is twofold: prevention of infection caused by S. pneumoniae and prevention of influenza infection. As influenza infection is a risk factor for bacterial infection, specifically with S. pneumoniae, influenza vaccination can prevent bacterial pneumonia [60]. In their most recent recommendations, the CDC continues to recommend routine influenza vaccination for all persons aged greater than 6 months, unless otherwise contraindicated [61].
There are 2 vaccines for prevention of pneumococcal disease: the pneumococcal polysaccharide vaccine (PPSV23) and a conjugate vaccine (PCV13). Following vaccination with PPSV23, 80% of adults develop antibodies against at least 18 of the 23 serotypes [62]. Despite this response, PPSV23 is reported to be protective against invasive pneumococcal infection; yet there is no consensus regarding PPSV23 leading to decreased rates of pneumonia [63]. On the other hand, PCV13 vaccination was associated with prevention of both invasive disease and community-acquired pneumonia in adults 65 years or older [64]. The CDC recommends that all children aged 2 or under receive PCV13, whereas those aged 65 or older should receive PCV13 followed by a dose of PPSV23 [65]. The dose of PPSV23 should be given ≥1 year following the dose of PCV13 [66].Persons < 65 years of age with immunocompromising and certain other conditions should also receive vaccination [67] (Table 7). Full details, many scenarios, and timing of vaccinations can be found at www.cdc.gov/vaccines/schedules/downloads/adult/adult-schedule.pdf.
Cigarette smoking increases the risk of respiratory infections as evidenced by smokers accounting for almost half of all patients with invasive pneumococcal disease [11]. As this is a modifiable risk factor it should be a goal of a comprehensive approach towards prevention of pneumonia.
Summary
CAP remains a leading cause of hospitalization and death in the 21st century. Traditionally, pneumococcus has been considered the major pathogen causing CAP; however, the 2015 EPIC study found that in only 5% of patients diagnosed with CAP was S. pneumoniae detected. Despite the new findings, it is still recommended that empiric treatment for CAP target common typical bacteria (pneumococcus, H. influenzae, Moraxella catarrhalis) and atypical bacteria (M. pneumonia, C. pneumoniae, L. pneumophila).
Because diagnosing pneumonia through history and clinical examination is less than 50% sensitive, a chest imaging study (a plain chest radiograph or a chest CT scan) is usually required to make the diagnosis. Laboratory tests, such as sputum Gram stain/culture, blood culture, urinary antigen tests, PCR test, procalcitonin, and CRP are important adjunctive diagnostic modalities to assist in the diagnosis and management of CAP. However, no single test is sensitive and specific enough to be a stand-alone test. They should be used in conjunction with history, physical examination, and imaging studies. Because vaccination (PPSV23, PCV13, and influenza vaccine) remains the most effective tool in preventing the development of CAP, clinicians, should strive for 100% vaccination rates in appropriate persons.
Corresponding author: Tze Shein Lo, MD, University of North Dakota, 1919 Elm Street, Fargo, ND 58102, [email protected].
Financial disclosures: None.
Author contributions: drafting of article, PM, TSL; critical revision of the article, PM, TSL.
1. Centers for Disease Control and Prevention. National Center for Health Statistics. FastStats - Pneumonia. Accessed 6 Oct 2016 at www.cdc.gov/nchs/fastats/pneumonia.htm.
2. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016;63:e61-e111.
3. Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med 2014;371:1619–28.
4. Mandell LA. Epidemiology and etiology of community-acquired pneumonia. Infect Dis
5. Hoare Z, Lim WS. Pneumonia: update on diagnosis and management. BMJ 2006;332:1077–9.
6. Johnstone J, Marrie TJ, Eurich DT, Majumdar SR. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med 2007;167:1938–43
7. File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med 2010;122:130–41.
8. Eurich DT, Marrie TJ, Minhas-Sandhu JK, Majumdar SR. Ten-year mortality after community-acquired pneumonia. a prospective cohort. Am J Respir Crit Care Med 2015;192:597-604.
9. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015;373:415–27.
10. Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med 2013;369:155–63.
11. Nuorti JP, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med 2000;342:681–9.
12. Almirall J, Serra-Prat M, Bolíbar I, BalassoV. Risk factors for community-acquired pneumonia in adults: a systemic review of observational studies. Respiration 2017;94:299–311.
13. Janoff EM.Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s principles and practice of infectioius diseases. 8th ed. Philadelphia: Sauders; 2015: 2310–27.
14. Diehr P, Wood RW, Bushyhead J, et al. Prediction of pneumonia in outpatients with acute cough--a statistical approach. J Chronic Dis 1984;37:215–25.
15. Metlay JP, Schulz R, Li YH, et al. Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch Intern Med 1997;157:1453–9.
16. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44 Suppl 2:S27–72.
17. Jartti A, Rauvala E, Kauma H, et al. Chest imaging findings in hospitalized patients with H1N1 influenza. Acta Radiol 2011;52:297–304.
18. Basi SK, Marrie TJ, Huang JQ, Majumdar SR. Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med 2004;117:305–11.
19. Caldwell A, Glauser FL, Smith WR, et al. The effects of dehydration on the radiologic and pathologic appearance of experimental canine segmental pneumonia. Am Rev Respir Dis 1975;112:651–6.
20. Bartlett JG. Pneumonia. In: Barlett JG, editor. Management of respiratory tract infections. Philadelphia: Lippincott, Williams & Wilkins; 2001: 1–122.
21. Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med 2015;192:974–82.
22. Wheeler JH, Fishman EK. Computed tomography in the management of chest infections: current status. Clin Infect Dis 1996;23:232–40.
23. Chesnutt MP. Pulmonary disorders. In: Papadakis MM, editor. Current medical diagnosis and treatment. New York: McGraw-Hill; 2016: 242–320.
24. Mandell LW. Pneumonia. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 188–201.
25. Reed WW, Byrd GS, Gates RH Jr, et al. Sputum gram’s stain in community-acquired pneumococcal pneumonia. A meta-analysis. West J Med 1996;165:197–204.
26. Chalasani NP, Valdecanas MA, Gopal AK, et al. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined underlying risks. Chest 1995;108:932–6.
27. Bordon J, Peyrani P, Brock GN, et al. The presence of pneumococcal bacteremia does not influence clinical outcomes in patients with community-acquired pneumonia: results from the Community-Acquired Pneumonia Organization (CAPO) International Cohort study. Chest 2008;133:618–24.
28. Helbig JH, Uldum SA, Bernander S, et al. Clinical utility of urinary antigen detection for diagnosis of community-acquired, travel-associated, and nosocomial legionnaires’ disease. J Clin Microbiol 2003;41:838–40.
29. Smith MD, Derrington P, Evans R, et al. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol 2003;41:2810–3.
30. Johansson N, Kalin M, Tiveljung-Lindell A, et al. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis 2010;50:202–9.
31. Gilbert DN. Procalcitonin as a biomarker in respiratory tract infection. Clin Infect Dis 2011;52 Suppl 4:S346–50.
32. Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev 2012;(9):CD007498.
33. Boussekey N, Leroy O, Alfandari S, et al. Procalcitonin kinetics in the prognosis of severe community-acquired pneumonia. Intensive Care Med 2006;32:469–72.
34. Flanders SA, Stein J, Shochat G, et al. Performance of a bedside C-reactive protein test in the diagnosis of community-acquired pneumonia in adults with acute cough. Am J Med 2004;116:529–35.
35. Fine MJ, et al A prediction rule to identify low-risk patients with community-acquired pneumonia.N Engl J Med.1997;336:243-50.
36. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003;58:377–82.
37. Arnold FW, Ramirez JA, McDonald LC, Xia EL. Hospitalization for community-acquired pneumonia: the pneumonia severity index vs clinical judgment. Chest 2003;124:121–4.
38. Aujesky D, McCausland JB, Whittle J, et al. Reasons why emergency department providers do not rely on the pneumonia severity index to determine the initial site of treatment for patients with pneumonia. Clin Infect Dis 2009;49:e100–8.
39. Charles PG, Wolfe R, Whitby M, et al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis 2008;47:375–84.
40. Marti C, Garin N, Grosgurin O, et al. Prediction of severe community-acquired pneumonia: a systematic review and meta-analysis. Crit Care 2012;16:R141.
41. Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 2012;54:621–9.
42. Silverman JA, Mortin LI, Vanpraagh AD, et al. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis 2005;191:2149–52.
43. El Hajj MS, Turgeon RD, Wilby KJ. Ceftaroline fosamil for community-acquired pneumonia and skin and skin structure infections: a systematic review. Int J Clin Pharm 2017 Jan 5.
44. Taboada M, Melnick D, Iaconis JP, et al. Ceftaroline fosamil versus ceftriaxone for the treatment of community-acquired pneumonia: individual patient data meta-analysis of randomized controlled trials. J Antimicrob Chemother 2016;71:862–70.
45. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011;52:285–92.
46. Baddour LM, Yu VL, Klugman KP, et al. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med 2004;170:440–4.
47. FDA Drug Safety Communication: FDA warns of increased risk of death with IV antibacterial Tygacil (tigecycline) and approves new boxed warning [Internet]. 15 Jan 2016. Available at www.fda.gov/Drugs/DrugSafety/ucm369580.htm.
48. Edelstein PR, CR. Legionnaires’ Disease and Pontiac Fever. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 2633.
49. Hammerschlag MR, Kohlhoff SA, Gaydos, CA. Chlamydia pneumoniae. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 2174.
50. Holzman RS, MS. Mycoplasma pneumoniae and Atypical Pneumonia. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 2183.
51. Yamada M, Buller R, Bledsoe S, Storch GA. Rising rates of macrolide-resistant Mycoplasma pneumoniae in the central United States. Pediatr Infect Dis J 2012;31:409–10.
52. Yi SH, Hatfield KM, Baggs J, et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis 2017 Nov 6.
53. Hayashi Y, Paterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis 2011;52:1232–40.
54. Akram AR, Chalmers JD, Taylor JK, et al. An evaluation of clinical stability criteria to predict hospital course in community-acquired pneumonia. Clin Microbiol Infect 2013;19:1174–80.
55. Halm EA, Fine MJ, Kapoor WN, et al. Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia. Arch Intern Med 2002;162:1278–84.
56. Roson B, Carratala J, Fernandez-Sabe N, et al. Causes and factors associated with early failure in hospitalized patients with community-acquired pneumonia. Arch Intern Med 2004;164:502–8.
57. El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med 2003;167:1650–4.
58. Wan YD, Sun TW, Liu ZQ, et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: a systematic review and meta-analysis. Chest 2016;149:209–19.
59. Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 2015;313:677–86.
60. McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev 2006;19:571–82.
61. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines. MMWR Recomm Rep 2016;65:1–54.
62. Rubins JB, Alter M, Loch J, Janoff EN. Determination of antibody responses of elderly adults to all 23 capsular polysaccharides after pneumococcal vaccination. Infect Immun 1999;67:5979–84.
63. Centers for Disease Control. Vaccines and preventable diseases [Internet]. 22 Nov 2016. Available at www.cdc.gov/vaccines/vpd/pneumo/hcp/about-vaccine.html.
64. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015;372:1114–25.
65. Centers for Disease Control. Recommended adult immunization schedule -- United States -- 2016 [Internet]. 2016. Available at www.cdc.gov/vaccines/schedules/downloads/adult/adult-schedule.pdf.
66. Kobayashi M, Bennett NM, Gierke R, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2015;64:944–7.
67. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012;61:816–9.
68. Aujesky D, Auble TE, Yealy DM, et al. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med 2005;118:384–92.
From the University of North Dakota School of Medicine & Health Sciences, Fargo, ND.
Abstract
- Objective: To review the management of community-acquired pneumonia (CAP) in adults.
- Methods: Review of the literature.
- Results: Approximately 4 to 5 million cases of CAP are diagnosed in the United States annually, accounting for significant morbidity and mortality. While numerous studies have previously shown pneumococcus to be the most common causative pathogen, the 2015 EPIC study found that in nearly two-thirds of patients with CAP who required hospitalization, no pathogen was detected. Symptoms and signs of respiratory tract infection are useful in helping to diagnose pneumonia; however, they are less sensitive than chest imaging studies. Laboratory tests used in diagnosing pneumonia include sputum Gram stain and culture, blood culture, urinary antigen, polymerase chain reaction, and biologic markers. In empiric treatment of CAP, both the typical and atypical pathogens should be targeted. Influenza vaccine and pneumococcal polysaccharide and conjugate vaccines should be administered as recommended by the CDC to reduce risk of CAP.
- Conclusion: CAP is a common illness with high rates of morbidity and mortality. Treatment is for the most part empirical; diagnostic testing can be used to identify the causative organism and guide pathogen-specific therapy.
Key words: community-acquired pneumonia; adults; management; vaccines.
Despite advances in medical science, pneumonia remains a major cause of morbidity and mortality. In 2014, 50,620 patients in the United States died from the disease [1]. Pneumonia can be classified as community-acquired, hospital-acquired, or ventilator-associated. Another category, healthcare-associated pneumonia, was included in an earlier American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) guideline but was removed from the 2016 guideline because there was no clear evidence that patients diagnosed with healthcare-associated pneumonia were at higher risk for harboring multidrug-resistant pathogens [2]. In this article, we review the epidemiology, microbiology, predisposing factors, diagnosis, treatment, and prevention of community-acquired pneumonia (CAP).
Definition and Epidemiology
CAP is defined as an acute infection of the lungs that develops in patients who have not been hospitalized recently and have not had regular exposure to the health care system [3]. A previously ambulatory patient who is diagnosed with pneumonia within 48 hours after admission also meets the criteria for CAP. Approximately 4 to 5 million cases of CAP are diagnosed in the United States annually [4]. About 25% of CAP patients require hospitalization, and about 5% to 10% of these patients are admitted to the intensive care unit (ICU) [5]. In-hospital mortality is considerable (~10% in population-based studies) [6] and 30-day mortality was found to be as high as 23% in a review by File and Marrie [7]. CAP also confers a high risk of long-term morbidity and mortality compared with the general population who have never had CAP, irrespective of age [8].
Causative Organisms
Numerous microorganisms can cause CAP. Common causes and less common causes are delineated in Table 1.
Predisposing Factors
Most people diagnosed with CAP have one or more predisposing factors [12,13] (Table 2).
Clinical Signs and Symptoms
Symptoms of CAP include fever, chills, rigors, fatigue, anorexia, diaphoresis, dyspnea, cough (with or without sputum production), and pleuritic chest pain. There is no individual symptom or cluster of symptoms that can absolutely differentiate pneumonia from other acute respiratory diseases, including upper and lower respiratory infections. However, if a patient presents with the constellation of symptoms of fever ≥ 1000F (37.80C), productive cough, and tachycardia, it is more suggestive of pneumonia [14]. Abnormal vital signs include fever, hypothermia, tachypnea, tachycardia, and oxygen desaturation. Auscultation of the chest reveals crackles or other adventitious breath sounds. Elderly patients with pneumonia report a significantly lower number of both respiratory and nonrespiratory symptoms compared with younger patients. Clinicians should be aware of this phenomenon so it does not lead to delayed diagnosis and treatment [15].
Imaging Evaluation
The presence of a pulmonary consolidation or an infiltrate on chest radiograph is required to diagnose CAP, and a chest radiograph should be obtained when CAP is suspected [16]. It should be noted that there is no pattern of radiographic abnormalities reliable enough to differentiate infectious pneumonia from noninfectious causes [17].
There are case reports and case series demonstrating false-negative plain chest radiographs existing in dehydrated patients [18] or in neutropenic state. However, animal studies have shown that dogs challenged with pneumococcus showed abnormal pulmonary shadow, suggestive of pneumonia, regardless of hydration status [19]. There is also no reliable scientific evidence to support the notion that severe neutropenia can cause false-negative radiographs because of the inability to develop an acute inflammatory reaction in the lungs [20].
A chest CT scan is more sensitive than a plain chest radiograph in detecting pneumonia. Therefore, a chest CT should be performed in a patient with negative plain chest radiograph when pneumonia is still highly suspected [21]. A chest CT scan is also more sensitive in detecting cavitation, adenopathy, interstitial disease and empyema. It also has the advantage of better defining anatomical changes than plain films [22].
Because improvement of pulmonary opacities in patients with CAP lags behind clinical improvement, repeating chest imaging studies is not recommended in patients who demonstrate clinical improvement. Sometimes clearing of pulmonary infiltrate or consolidation can take 6 weeks or longer [23].
Laboratory Evaluation
Generally the etiologic agent of CAP cannot be determined solely on the basis of clinical signs and symptoms or imaging studies. Although routine microbiological testing for patients suspicious for CAP is not necessary for empirical treatment, by determining the etiologic agent of the pneumonia, a clinician will be able to narrow the antibiotics from a broad-spectrum empirical regimen to specific pathogen-directed therapy. Determination of certain etiologic agents causing the pneumonia can have important public health implications (eg, Mycobacterium tuberculosis and influenza virus) [24].
Sputum Gram Stain and Culture
Sputum Gram stain is an inexpensive test that may identify pathogens that cause CAP (eg, S. pneumonia and Haemophilus influenzae). A quality specimen is required. A sputum sample must contain > 25 neutrophils and < 10 squamous epithelial cells/low power field on Gram stain to be considered suitable for culture.
The sensitivity and specificity of sputum Gram stain and culture are highly variable in different clinical settings (eg, outpatient setting, nursing home, ICU). Reed et al’s meta-analysis of patients diagnosed with CAP in the United States showed the sensitivity and specificity of sputum Gram stain (compared with sputum culture) ranged from 15% to 100% and 11% to 100%, respectively [24]. In cases of proven bacteremic pneumococcal pneumonia, positive cultures from sputum samples were positive less than 50% of the time [25].
For patients who cannot provide sputum samples or are intubated, a deep-suction aspirate or bronchoalveolar lavage through a bronchoscopic procedure might be necessary to obtain pulmonary secretion for Gram stain and culture. Besides bacterial culture, sputum samples can also be sent for fungal and mycobacterial cultures and acid-fast stain if deemed clinically necessary.
Blood Culture
Because the positivity rate of blood culture in patients who are suspected to have pneumonia but not exposed to antimicrobial agents is disappointingly low (5%–14%), blood cultures are no longer recommended in patients hospitalized for CAP. Another reason for not recommending blood culture is positive culture rarely leads to changes in antibiotic regimen in patients without underlying diseases [26]. However, high-risk patients, including patients with severe CAP or in immunocompromised patients (eg, patients with neutropenia, asplenia or complement deficiencies) should have a blood culture done [24].
A multinational study published in 2008 examined 125 patients with pneumococcal bacteremic CAP versus 1847 patients with non-bacteremic CAP [27]. Analysis of the data demonstrated no association of pneumococcal bacteremic CAP and time to clinical stability, length of hospital stay, all-cause mortality or CAP-related mortality. The authors concluded that pneumococcal bacteremia does not increase the risk of poor outcomes in patients with CAP compared to non-bacteremic patients, and the presence of pneumococcal bacteremia should not deter de-escalation of therapy in clinically stable patients.
Urinary Antigen Tests
Urinary antigen tests may assist clinicians in narrowing antibiotic therapy when test results are positive. There are 2 U.S. Food and Drug Administration–approved tests available to clinicians for detecting pneumococcal and Legionella antigen in urine. The test for Legionella pneumophila detects disease due to serogroup 1 only, which accounts for 80% of community-acquired Legionnaires disease. The sensitivity and specificity of the Legionella urine antigen test are 90% and 99%, respectively. The pneumococcal urine antigen test is less sensitive and specific than the Legionella urine antigen test (sensitivity 80% and specificity > 90%) [28,29].
Advantages of the urinary antigen tests are that they are easily performed, results are available in less than an hour if done in-house, and results are not affected by prior exposure to antibiotics. However, the tests do not meet Clinical Laboratory Improvements Amendments criteria for waiver and must be performed by a technician in the laboratory.
Polymerase Chain Reaction
There are several FDA-approved polymerase chain reaction (PCR) tests commercially available to assist clinicians in diagnosing pneumonia. PCR test of nasopharyngeal swabs for diagnosing influenza have become standard in many medical U.S. facilities. The great advantage of using PCR to diagnose influenza is its high sensitivity and specificity and rapid turnaround time. PCR can also be used to detect Legionella species, S. pneumonia, Mycoplasma pneumoniae, Chlamydophila pneumonia and mycobacterial species [24].
One limitation of using PCR tests on respiratory specimens is that specimens can be contaminated with oral or upper airway flora, so the results must be interpreted with caution, bearing in mind that some of the pathogens isolated may be colonizers of the oral or upper airway flora [30].
Biologic Markers
Two biologic markers—procalcitonin and C-reactive protein (CRP)—can be used in conjunction with history, physical examination, laboratory tests and imaging studies to assist in the diagnosis and treatment of CAP [24]. Procalcitonin is a peptide precursor of the hormone calcitonin that is released by parenchymal cells into the bloodstream resulting in increased serum level in patients with bacterial infections. In contrast, there is no remarkable proclacitonin level increase with viral or noninfectious inflammation. The reference value of procalcitonin in the blood of an adult individual without infection or inflammation is < 0.15 ng/mL. In the blood, procalcitonin has a half-life of 25 to 30 hours. The quantitative immunoluminometric method (LUMI test, Brahms PCT, Berlin, Germany ) is the preferred test to use because of its high sensitivity [31].
A 2012 Cochrane meta-analysis that involved 4221 patients with acute respiratory infections (with half of the patients diagnosed with CAP) from 14 prospective trials found the use of procalcitonin test for antibiotic use significantly decreased median antibiotic exposure from 8 to 4 days without an increase in treatment failure, mortality rates in any clinical setting (eg, outpatient clinic, emergency room), or length of hospitalization [32]. A prospective study conducted in France on 100 ICU patients showed that increased procalcitonin from day 1 to day 3 has a poor prognosis factor for severe CAP whereas decreasing procalcitonin levels is associated with a favorable outcome [33].
CRP is an acute phase protein produced by the liver. CRP level in the blood increases in response to acute infection or inflammation. Use of CRP in assisting diagnosis and guiding treatment of CAP is more limited in part due to its poor specificity. A prospective study conducted on 168 consecutive patients presented with cough showed that a CRP > 40 mg/L had a sensitivity and specificity of 70% and 90%, respectively [34].
T reatment
Site of Care Decision
For patients with CAP, the clinician must decide whether the patient will be treated in an outpatient or inpatient setting, and for those in the inpatient setting, whether they can safely be treated on the general medical ward or should be the ICU. Two common scoring systems that can be used to aid the clinician in determining severity of the infection and guiding site-of-care decisions are the Pneumonia Severity Index (PSI) and CURB-65 scores.
The PSI score uses 20 different parameters, including comorbidities, laboratory parameters and radiographic findings to stratify patients into 5 mortality risk classes [35]. On the basis of associated mortality rates, it has been suggested that risk class I and II patients should be treated as outpatients, risk class III patients should be treated in an observation unit or with a short hospitalization, and risk class IV and V patients should be treated as inpatients [35].
The CURB-65 method of risk stratification is based on 5 clinical parameters: confusion, urea level, respiratory rate, systolic blood pressure and age ≥ 65 (Table 3) [36].
Patients with CURB-65 scores of 4 or 5 are considered to have severe pneumonia and admission to the ICU should be considered. Aside from the CURB-65 score, anyone requiring vasopressor support or mechanical ventilation merits admission to the ICU [16]. IDSA/ATS guidelines also recommend the use of “minor criteria” for making ICU admission decisions; these include respiratory rate ≥ 30 breaths / minute, PaO2 fraction ≤ 250, multilobar infiltrates, confusion, blood urea nitrogen ≥ 20 mg/dL, leukopenia, thrombocytopenia, hypothermia and hypotension [16]. These factors are associated with increased mortality due to CAP and admission to an ICU is indicated if 3 of the minor criteria for severe CAP are present.
Similar to CURB-65, another clinical calculator that can be used for assessing severity of CAP is SMART-COP [39]. This scoring system uses 8 weighted criteria to predict which patients will require intensive respiratory or vasopressor support. SMART-COP has a sensitivity of 79% and specificity 64% in predicting ICU admission, whereas CURB-65 had a pooled sensitivity of 57.2% and specificity of 77.2% [40].
Antibiotic Therapy
Antibiotics are the mainstay of treatment for CAP, with the majority of patients with CAP treated empirically taking into account the site of care, likely pathogen, and antimicrobial resistance issues. Patients with pneumonia who are treated as outpatients usually respond well to empiric antibiotic treatment and a causative pathogen is not usually sought. Patients who are hospitalized for treatment of CAP usually receive empiric antibiotic on admission. Once the etiology has been determined by microbiologic or serologic means, antimicrobial therapy should be adjusted accordingly. As noted previously, a CDC study found that the burden of viral etiologies was higher than previously thought, with rhinovirus and influenza accounting for 15% of cases and S. pneumoniae for only 5% [9]. This study highlighted the fact that despite advances in molecular techniques, most patients with pneumonia have no pathogen identified [9]. Given the lack of discernable pathogens in the majority of cases, unless a nonbacterial etiology is found patients should continue to be treated with antibiotics.
Outpatients without comorbidities or risk factors for drug-resistant S. pneumoniae (Table 4)
As previously mentioned, antibiotic therapy is typically empiric; neither clinical features nor radiographic features are sufficient to include or exclude infectious etiologies. Epidemiologic risk factors should be considered and, in certain cases, expanded antimicrobial coverage to include those entities; for example, treatment of anaerobes in the setting of lung abscess and antipseudomonal antibiotics for patients with bronchiectasis.
Of concern in the treatment of CAP is the increased prevalence of antimicrobial resistance among S. pneumoniae. The IDSA guidelines report that drug-resistant S. pneumoniae is more common in persons aged < 2 or > 65 years, and those with ß-lactam therapy within the previous 3 months, alcoholism, medical comorbidities, immunosuppressive illness or therapy, or exposure to a child who attends a day care center [16].
S. aureus should be considered during influenza outbreaks, with either vancomycin or linezolid being the recommended agents in the setting of methicillin-resistant S. aureus (MRSA). In a study comparing vancomycin versus linezolid for nosocomial pneumonia, the all-cause 60-day mortality was similar for both agents [41]. Datpomycin is another agent used against MRSA; however, its use in the setting of pneumonia is not indicated as daptomycin binds to surfactant, yielding it ineffective in the treatment of pneumonia [42]. Ceftaroline is a newer cephalosporin with activity against MRSA; its role in treatment of community-acquired MRSA pneumonia has not been fully elucidated, but it appears to be a useful agent for this indication [43,44].
A summary of recommended empiric antibiotic therapy is presented in Table 5.
Antibiotic Therapy for Selected Pathogens
S. pneumoniae
Patients with pneumococcal pneumonia who have penicillin-susceptible strains can be treated with intravenous penicillin (2 or 3 million units every 4 hours) or ceftriaxone. Once a patient meets criteria of stability, they can then be transitioned to oral penicillin, amoxicillin, or clarithromycin. Those with strains with reduced susceptibility can still be treated with penicillin but at a higher dose (4 million units IV every 4 hours) or a third-generation cephalosporin. Those whose pneumococcal pneumonia is complicated by bacteremia will benefit from dual therapy if severely ill, requiring ICU monitoring. Those not severely ill can be treated with monotherapy [46].
S. aureus
S. aureus is more commonly associated with hospital-acquired pneumonia but may also be seen during the influenza season and in those with severe necrotizing CAP. Both linezolid and vancomycin can be used to treat MRSA CAP. As noted above, ceftaroline has activity against MRSA and is approved for treatment of CAP, but is not approved by the FDA for MRSA CAP treatment. Similarly, tigecycline is approved for CAP and has activity against MRSA, but is not approved for MRSA CAP. Moreover, the FDA has warned of increased risk of death with tigecycline and has a black box warning to that effect [47].
Legionella
Treatment of legionellosis can be achieved with tetracyclines, macrolides, or fluoroquinolones. For nonimmunosuppressed patients with mild pneumonia, any of the listed antibiotics is considered appropriate. However, patients with severe infection or those with immunosuppression should be treated with either levofloxacin or azithromycin for 7 to 10 days [48].
C. pneumoniae
As with other atypical organisms, C. pneumoniae can be treated with doxycycline, a macrolide, or respiratory fluoroquinolones. However, length of therapy varies by regimen used; whereas treating with doxycycline 100 mg twice daily generally requires 14–21 days, moxifloxacin 400 mg daily only requires 10 days [49].
M. pneumoniae
As with C. pneumoniae, length of therapy of M. pneumoniae varies by antimicrobial used. Shortest courses are seen with the use of macrolides for 5 days, whereas 14 days is considered standard for doxycycline or a respiratory fluoroquinolone [50]. It should be noted that there has been increasingly documented resistance to macrolides, with known resistance of 8.2% in the United States [51].
Duration of Treatment
Most patients with CAP respond within 72 hours to appropriate therapy. IDSA/ATS guidelines recommend that patients be treated for a minimum of 5 days, and before discontinuing antibiotics patients should be afebrile a minimum of 48-72 hours and be clinically stable (Table 6) [16].
Hospitalized patients do not need to be monitored for an additional day once they have reached clinical stability (Table 6), are able to maintain oral intake, and have normal mentation, provided that other comorbidities are stable and social needs have been met [16]. Patients discharged from the hospital with instability have higher risk of readmission or death [55].
Transition to Oral Therapy
IDSA/ATS guidelines [16] recommend that patients should be transitioned from IV to oral antibiotics when they are improving clinically, have stable vital signs, and are able to ingest food/fluids and medications.
Management of Nonresponders
Although the majority of patients respond to antibiotics within 72 hours, treatment failure occurs in up to 15% of patients [45]. Nonresponding pneumonia is generally seen in 2 patterns: worsening of clinical status despite empiric antibiotics OR delay in achieving clinical stability as defined in Table 5 after 72 hours of treatment [13]. Risk factors associated with nonresponding pneumonia [56] are:
- Radiographic: multilobar infiltrates, pleural effusion, cavitation
- Bacteriologic: MRSA, gram-negative or Legionella pneumonia
- Severity index: PSI > 90
- Pharmacologic: incorrect antibiotic choice based on susceptibility
Patients with acute deterioration of clinical status will prompt transfer to a higher level of care and may require mechanical ventilator support. In those with delay in achieving clinical stability, question centers on whether the same antibiotics can be continued while doing further radiographic/microbiologic workup and/or changing antibiotics.
History should be reviewed with particular attention to exposures, travel history, and microbiologic and radiographic data. Clinicians should recall that viral causes account for up to 20% of pneumonias and there are also noninfectious causes that can mimic pyogenic infections [57]. If adequate initial cultures were not obtained, they should be obtained; however, care must be taken in reviewing new sets of cultures while on antibiotics as they may reveal colonization selected out by antibiotics and not a true pathogen. If repeat evaluation is unrevealing, then further evaluation with CT scan and bronchoscopy with bronchoalveolar lavage and biopsy is warranted. CT scans can show pleural effusions, bronchial obstructions or pattern suggestive of cryptogenic pneumonia. A bronchoscopy might yield a microbiologic diagnosis and with biopsy can also evaluate for noninfectious causes.
As with other infections, if escalation of antibiotics is undertaken, clinicians should be mindful to ensure that efforts are being made to elucidate the reason for nonresponse. To simply broaden antimicrobial therapy without attempts at establishing a microbiologic or radiographic cause for nonresponse may lead to inappropriate treatment recurrence of infection. Aside from patients who have bacteremic pneumococcal pneumonia in an ICU setting, there are no published reports pointing to superiority of combination antibiotics [46].
Other Treatment
Because of the inflammatory response associated with pneumonia, several agents have been evaluated as adjunctive treatment of pneumonia to decrease this inflammatory state; namely, steroids, macrolide antibiotics and statins. To date, only the use of steroids (methylprednisolone 0.5 mg/kg every 12 hours for 5 days) in those with severe CAP and high initial anti-inflammatory response (CRP > 150) was shown to decrease treatment failure, decreased risk of ARDS, possibly reduce length of stay, duration of intravenous antibiotics and clinical stability, without effect on mortality or adverse side effects [58,59].
Other adjunctive methods have not been found to have significant impact [16].
Prevention of Pneumonia
Prevention of pneumococcal pneumonia is twofold: prevention of infection caused by S. pneumoniae and prevention of influenza infection. As influenza infection is a risk factor for bacterial infection, specifically with S. pneumoniae, influenza vaccination can prevent bacterial pneumonia [60]. In their most recent recommendations, the CDC continues to recommend routine influenza vaccination for all persons aged greater than 6 months, unless otherwise contraindicated [61].
There are 2 vaccines for prevention of pneumococcal disease: the pneumococcal polysaccharide vaccine (PPSV23) and a conjugate vaccine (PCV13). Following vaccination with PPSV23, 80% of adults develop antibodies against at least 18 of the 23 serotypes [62]. Despite this response, PPSV23 is reported to be protective against invasive pneumococcal infection; yet there is no consensus regarding PPSV23 leading to decreased rates of pneumonia [63]. On the other hand, PCV13 vaccination was associated with prevention of both invasive disease and community-acquired pneumonia in adults 65 years or older [64]. The CDC recommends that all children aged 2 or under receive PCV13, whereas those aged 65 or older should receive PCV13 followed by a dose of PPSV23 [65]. The dose of PPSV23 should be given ≥1 year following the dose of PCV13 [66].Persons < 65 years of age with immunocompromising and certain other conditions should also receive vaccination [67] (Table 7). Full details, many scenarios, and timing of vaccinations can be found at www.cdc.gov/vaccines/schedules/downloads/adult/adult-schedule.pdf.
Cigarette smoking increases the risk of respiratory infections as evidenced by smokers accounting for almost half of all patients with invasive pneumococcal disease [11]. As this is a modifiable risk factor it should be a goal of a comprehensive approach towards prevention of pneumonia.
Summary
CAP remains a leading cause of hospitalization and death in the 21st century. Traditionally, pneumococcus has been considered the major pathogen causing CAP; however, the 2015 EPIC study found that in only 5% of patients diagnosed with CAP was S. pneumoniae detected. Despite the new findings, it is still recommended that empiric treatment for CAP target common typical bacteria (pneumococcus, H. influenzae, Moraxella catarrhalis) and atypical bacteria (M. pneumonia, C. pneumoniae, L. pneumophila).
Because diagnosing pneumonia through history and clinical examination is less than 50% sensitive, a chest imaging study (a plain chest radiograph or a chest CT scan) is usually required to make the diagnosis. Laboratory tests, such as sputum Gram stain/culture, blood culture, urinary antigen tests, PCR test, procalcitonin, and CRP are important adjunctive diagnostic modalities to assist in the diagnosis and management of CAP. However, no single test is sensitive and specific enough to be a stand-alone test. They should be used in conjunction with history, physical examination, and imaging studies. Because vaccination (PPSV23, PCV13, and influenza vaccine) remains the most effective tool in preventing the development of CAP, clinicians, should strive for 100% vaccination rates in appropriate persons.
Corresponding author: Tze Shein Lo, MD, University of North Dakota, 1919 Elm Street, Fargo, ND 58102, [email protected].
Financial disclosures: None.
Author contributions: drafting of article, PM, TSL; critical revision of the article, PM, TSL.
From the University of North Dakota School of Medicine & Health Sciences, Fargo, ND.
Abstract
- Objective: To review the management of community-acquired pneumonia (CAP) in adults.
- Methods: Review of the literature.
- Results: Approximately 4 to 5 million cases of CAP are diagnosed in the United States annually, accounting for significant morbidity and mortality. While numerous studies have previously shown pneumococcus to be the most common causative pathogen, the 2015 EPIC study found that in nearly two-thirds of patients with CAP who required hospitalization, no pathogen was detected. Symptoms and signs of respiratory tract infection are useful in helping to diagnose pneumonia; however, they are less sensitive than chest imaging studies. Laboratory tests used in diagnosing pneumonia include sputum Gram stain and culture, blood culture, urinary antigen, polymerase chain reaction, and biologic markers. In empiric treatment of CAP, both the typical and atypical pathogens should be targeted. Influenza vaccine and pneumococcal polysaccharide and conjugate vaccines should be administered as recommended by the CDC to reduce risk of CAP.
- Conclusion: CAP is a common illness with high rates of morbidity and mortality. Treatment is for the most part empirical; diagnostic testing can be used to identify the causative organism and guide pathogen-specific therapy.
Key words: community-acquired pneumonia; adults; management; vaccines.
Despite advances in medical science, pneumonia remains a major cause of morbidity and mortality. In 2014, 50,620 patients in the United States died from the disease [1]. Pneumonia can be classified as community-acquired, hospital-acquired, or ventilator-associated. Another category, healthcare-associated pneumonia, was included in an earlier American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) guideline but was removed from the 2016 guideline because there was no clear evidence that patients diagnosed with healthcare-associated pneumonia were at higher risk for harboring multidrug-resistant pathogens [2]. In this article, we review the epidemiology, microbiology, predisposing factors, diagnosis, treatment, and prevention of community-acquired pneumonia (CAP).
Definition and Epidemiology
CAP is defined as an acute infection of the lungs that develops in patients who have not been hospitalized recently and have not had regular exposure to the health care system [3]. A previously ambulatory patient who is diagnosed with pneumonia within 48 hours after admission also meets the criteria for CAP. Approximately 4 to 5 million cases of CAP are diagnosed in the United States annually [4]. About 25% of CAP patients require hospitalization, and about 5% to 10% of these patients are admitted to the intensive care unit (ICU) [5]. In-hospital mortality is considerable (~10% in population-based studies) [6] and 30-day mortality was found to be as high as 23% in a review by File and Marrie [7]. CAP also confers a high risk of long-term morbidity and mortality compared with the general population who have never had CAP, irrespective of age [8].
Causative Organisms
Numerous microorganisms can cause CAP. Common causes and less common causes are delineated in Table 1.
Predisposing Factors
Most people diagnosed with CAP have one or more predisposing factors [12,13] (Table 2).
Clinical Signs and Symptoms
Symptoms of CAP include fever, chills, rigors, fatigue, anorexia, diaphoresis, dyspnea, cough (with or without sputum production), and pleuritic chest pain. There is no individual symptom or cluster of symptoms that can absolutely differentiate pneumonia from other acute respiratory diseases, including upper and lower respiratory infections. However, if a patient presents with the constellation of symptoms of fever ≥ 1000F (37.80C), productive cough, and tachycardia, it is more suggestive of pneumonia [14]. Abnormal vital signs include fever, hypothermia, tachypnea, tachycardia, and oxygen desaturation. Auscultation of the chest reveals crackles or other adventitious breath sounds. Elderly patients with pneumonia report a significantly lower number of both respiratory and nonrespiratory symptoms compared with younger patients. Clinicians should be aware of this phenomenon so it does not lead to delayed diagnosis and treatment [15].
Imaging Evaluation
The presence of a pulmonary consolidation or an infiltrate on chest radiograph is required to diagnose CAP, and a chest radiograph should be obtained when CAP is suspected [16]. It should be noted that there is no pattern of radiographic abnormalities reliable enough to differentiate infectious pneumonia from noninfectious causes [17].
There are case reports and case series demonstrating false-negative plain chest radiographs existing in dehydrated patients [18] or in neutropenic state. However, animal studies have shown that dogs challenged with pneumococcus showed abnormal pulmonary shadow, suggestive of pneumonia, regardless of hydration status [19]. There is also no reliable scientific evidence to support the notion that severe neutropenia can cause false-negative radiographs because of the inability to develop an acute inflammatory reaction in the lungs [20].
A chest CT scan is more sensitive than a plain chest radiograph in detecting pneumonia. Therefore, a chest CT should be performed in a patient with negative plain chest radiograph when pneumonia is still highly suspected [21]. A chest CT scan is also more sensitive in detecting cavitation, adenopathy, interstitial disease and empyema. It also has the advantage of better defining anatomical changes than plain films [22].
Because improvement of pulmonary opacities in patients with CAP lags behind clinical improvement, repeating chest imaging studies is not recommended in patients who demonstrate clinical improvement. Sometimes clearing of pulmonary infiltrate or consolidation can take 6 weeks or longer [23].
Laboratory Evaluation
Generally the etiologic agent of CAP cannot be determined solely on the basis of clinical signs and symptoms or imaging studies. Although routine microbiological testing for patients suspicious for CAP is not necessary for empirical treatment, by determining the etiologic agent of the pneumonia, a clinician will be able to narrow the antibiotics from a broad-spectrum empirical regimen to specific pathogen-directed therapy. Determination of certain etiologic agents causing the pneumonia can have important public health implications (eg, Mycobacterium tuberculosis and influenza virus) [24].
Sputum Gram Stain and Culture
Sputum Gram stain is an inexpensive test that may identify pathogens that cause CAP (eg, S. pneumonia and Haemophilus influenzae). A quality specimen is required. A sputum sample must contain > 25 neutrophils and < 10 squamous epithelial cells/low power field on Gram stain to be considered suitable for culture.
The sensitivity and specificity of sputum Gram stain and culture are highly variable in different clinical settings (eg, outpatient setting, nursing home, ICU). Reed et al’s meta-analysis of patients diagnosed with CAP in the United States showed the sensitivity and specificity of sputum Gram stain (compared with sputum culture) ranged from 15% to 100% and 11% to 100%, respectively [24]. In cases of proven bacteremic pneumococcal pneumonia, positive cultures from sputum samples were positive less than 50% of the time [25].
For patients who cannot provide sputum samples or are intubated, a deep-suction aspirate or bronchoalveolar lavage through a bronchoscopic procedure might be necessary to obtain pulmonary secretion for Gram stain and culture. Besides bacterial culture, sputum samples can also be sent for fungal and mycobacterial cultures and acid-fast stain if deemed clinically necessary.
Blood Culture
Because the positivity rate of blood culture in patients who are suspected to have pneumonia but not exposed to antimicrobial agents is disappointingly low (5%–14%), blood cultures are no longer recommended in patients hospitalized for CAP. Another reason for not recommending blood culture is positive culture rarely leads to changes in antibiotic regimen in patients without underlying diseases [26]. However, high-risk patients, including patients with severe CAP or in immunocompromised patients (eg, patients with neutropenia, asplenia or complement deficiencies) should have a blood culture done [24].
A multinational study published in 2008 examined 125 patients with pneumococcal bacteremic CAP versus 1847 patients with non-bacteremic CAP [27]. Analysis of the data demonstrated no association of pneumococcal bacteremic CAP and time to clinical stability, length of hospital stay, all-cause mortality or CAP-related mortality. The authors concluded that pneumococcal bacteremia does not increase the risk of poor outcomes in patients with CAP compared to non-bacteremic patients, and the presence of pneumococcal bacteremia should not deter de-escalation of therapy in clinically stable patients.
Urinary Antigen Tests
Urinary antigen tests may assist clinicians in narrowing antibiotic therapy when test results are positive. There are 2 U.S. Food and Drug Administration–approved tests available to clinicians for detecting pneumococcal and Legionella antigen in urine. The test for Legionella pneumophila detects disease due to serogroup 1 only, which accounts for 80% of community-acquired Legionnaires disease. The sensitivity and specificity of the Legionella urine antigen test are 90% and 99%, respectively. The pneumococcal urine antigen test is less sensitive and specific than the Legionella urine antigen test (sensitivity 80% and specificity > 90%) [28,29].
Advantages of the urinary antigen tests are that they are easily performed, results are available in less than an hour if done in-house, and results are not affected by prior exposure to antibiotics. However, the tests do not meet Clinical Laboratory Improvements Amendments criteria for waiver and must be performed by a technician in the laboratory.
Polymerase Chain Reaction
There are several FDA-approved polymerase chain reaction (PCR) tests commercially available to assist clinicians in diagnosing pneumonia. PCR test of nasopharyngeal swabs for diagnosing influenza have become standard in many medical U.S. facilities. The great advantage of using PCR to diagnose influenza is its high sensitivity and specificity and rapid turnaround time. PCR can also be used to detect Legionella species, S. pneumonia, Mycoplasma pneumoniae, Chlamydophila pneumonia and mycobacterial species [24].
One limitation of using PCR tests on respiratory specimens is that specimens can be contaminated with oral or upper airway flora, so the results must be interpreted with caution, bearing in mind that some of the pathogens isolated may be colonizers of the oral or upper airway flora [30].
Biologic Markers
Two biologic markers—procalcitonin and C-reactive protein (CRP)—can be used in conjunction with history, physical examination, laboratory tests and imaging studies to assist in the diagnosis and treatment of CAP [24]. Procalcitonin is a peptide precursor of the hormone calcitonin that is released by parenchymal cells into the bloodstream resulting in increased serum level in patients with bacterial infections. In contrast, there is no remarkable proclacitonin level increase with viral or noninfectious inflammation. The reference value of procalcitonin in the blood of an adult individual without infection or inflammation is < 0.15 ng/mL. In the blood, procalcitonin has a half-life of 25 to 30 hours. The quantitative immunoluminometric method (LUMI test, Brahms PCT, Berlin, Germany ) is the preferred test to use because of its high sensitivity [31].
A 2012 Cochrane meta-analysis that involved 4221 patients with acute respiratory infections (with half of the patients diagnosed with CAP) from 14 prospective trials found the use of procalcitonin test for antibiotic use significantly decreased median antibiotic exposure from 8 to 4 days without an increase in treatment failure, mortality rates in any clinical setting (eg, outpatient clinic, emergency room), or length of hospitalization [32]. A prospective study conducted in France on 100 ICU patients showed that increased procalcitonin from day 1 to day 3 has a poor prognosis factor for severe CAP whereas decreasing procalcitonin levels is associated with a favorable outcome [33].
CRP is an acute phase protein produced by the liver. CRP level in the blood increases in response to acute infection or inflammation. Use of CRP in assisting diagnosis and guiding treatment of CAP is more limited in part due to its poor specificity. A prospective study conducted on 168 consecutive patients presented with cough showed that a CRP > 40 mg/L had a sensitivity and specificity of 70% and 90%, respectively [34].
T reatment
Site of Care Decision
For patients with CAP, the clinician must decide whether the patient will be treated in an outpatient or inpatient setting, and for those in the inpatient setting, whether they can safely be treated on the general medical ward or should be the ICU. Two common scoring systems that can be used to aid the clinician in determining severity of the infection and guiding site-of-care decisions are the Pneumonia Severity Index (PSI) and CURB-65 scores.
The PSI score uses 20 different parameters, including comorbidities, laboratory parameters and radiographic findings to stratify patients into 5 mortality risk classes [35]. On the basis of associated mortality rates, it has been suggested that risk class I and II patients should be treated as outpatients, risk class III patients should be treated in an observation unit or with a short hospitalization, and risk class IV and V patients should be treated as inpatients [35].
The CURB-65 method of risk stratification is based on 5 clinical parameters: confusion, urea level, respiratory rate, systolic blood pressure and age ≥ 65 (Table 3) [36].
Patients with CURB-65 scores of 4 or 5 are considered to have severe pneumonia and admission to the ICU should be considered. Aside from the CURB-65 score, anyone requiring vasopressor support or mechanical ventilation merits admission to the ICU [16]. IDSA/ATS guidelines also recommend the use of “minor criteria” for making ICU admission decisions; these include respiratory rate ≥ 30 breaths / minute, PaO2 fraction ≤ 250, multilobar infiltrates, confusion, blood urea nitrogen ≥ 20 mg/dL, leukopenia, thrombocytopenia, hypothermia and hypotension [16]. These factors are associated with increased mortality due to CAP and admission to an ICU is indicated if 3 of the minor criteria for severe CAP are present.
Similar to CURB-65, another clinical calculator that can be used for assessing severity of CAP is SMART-COP [39]. This scoring system uses 8 weighted criteria to predict which patients will require intensive respiratory or vasopressor support. SMART-COP has a sensitivity of 79% and specificity 64% in predicting ICU admission, whereas CURB-65 had a pooled sensitivity of 57.2% and specificity of 77.2% [40].
Antibiotic Therapy
Antibiotics are the mainstay of treatment for CAP, with the majority of patients with CAP treated empirically taking into account the site of care, likely pathogen, and antimicrobial resistance issues. Patients with pneumonia who are treated as outpatients usually respond well to empiric antibiotic treatment and a causative pathogen is not usually sought. Patients who are hospitalized for treatment of CAP usually receive empiric antibiotic on admission. Once the etiology has been determined by microbiologic or serologic means, antimicrobial therapy should be adjusted accordingly. As noted previously, a CDC study found that the burden of viral etiologies was higher than previously thought, with rhinovirus and influenza accounting for 15% of cases and S. pneumoniae for only 5% [9]. This study highlighted the fact that despite advances in molecular techniques, most patients with pneumonia have no pathogen identified [9]. Given the lack of discernable pathogens in the majority of cases, unless a nonbacterial etiology is found patients should continue to be treated with antibiotics.
Outpatients without comorbidities or risk factors for drug-resistant S. pneumoniae (Table 4)
As previously mentioned, antibiotic therapy is typically empiric; neither clinical features nor radiographic features are sufficient to include or exclude infectious etiologies. Epidemiologic risk factors should be considered and, in certain cases, expanded antimicrobial coverage to include those entities; for example, treatment of anaerobes in the setting of lung abscess and antipseudomonal antibiotics for patients with bronchiectasis.
Of concern in the treatment of CAP is the increased prevalence of antimicrobial resistance among S. pneumoniae. The IDSA guidelines report that drug-resistant S. pneumoniae is more common in persons aged < 2 or > 65 years, and those with ß-lactam therapy within the previous 3 months, alcoholism, medical comorbidities, immunosuppressive illness or therapy, or exposure to a child who attends a day care center [16].
S. aureus should be considered during influenza outbreaks, with either vancomycin or linezolid being the recommended agents in the setting of methicillin-resistant S. aureus (MRSA). In a study comparing vancomycin versus linezolid for nosocomial pneumonia, the all-cause 60-day mortality was similar for both agents [41]. Datpomycin is another agent used against MRSA; however, its use in the setting of pneumonia is not indicated as daptomycin binds to surfactant, yielding it ineffective in the treatment of pneumonia [42]. Ceftaroline is a newer cephalosporin with activity against MRSA; its role in treatment of community-acquired MRSA pneumonia has not been fully elucidated, but it appears to be a useful agent for this indication [43,44].
A summary of recommended empiric antibiotic therapy is presented in Table 5.
Antibiotic Therapy for Selected Pathogens
S. pneumoniae
Patients with pneumococcal pneumonia who have penicillin-susceptible strains can be treated with intravenous penicillin (2 or 3 million units every 4 hours) or ceftriaxone. Once a patient meets criteria of stability, they can then be transitioned to oral penicillin, amoxicillin, or clarithromycin. Those with strains with reduced susceptibility can still be treated with penicillin but at a higher dose (4 million units IV every 4 hours) or a third-generation cephalosporin. Those whose pneumococcal pneumonia is complicated by bacteremia will benefit from dual therapy if severely ill, requiring ICU monitoring. Those not severely ill can be treated with monotherapy [46].
S. aureus
S. aureus is more commonly associated with hospital-acquired pneumonia but may also be seen during the influenza season and in those with severe necrotizing CAP. Both linezolid and vancomycin can be used to treat MRSA CAP. As noted above, ceftaroline has activity against MRSA and is approved for treatment of CAP, but is not approved by the FDA for MRSA CAP treatment. Similarly, tigecycline is approved for CAP and has activity against MRSA, but is not approved for MRSA CAP. Moreover, the FDA has warned of increased risk of death with tigecycline and has a black box warning to that effect [47].
Legionella
Treatment of legionellosis can be achieved with tetracyclines, macrolides, or fluoroquinolones. For nonimmunosuppressed patients with mild pneumonia, any of the listed antibiotics is considered appropriate. However, patients with severe infection or those with immunosuppression should be treated with either levofloxacin or azithromycin for 7 to 10 days [48].
C. pneumoniae
As with other atypical organisms, C. pneumoniae can be treated with doxycycline, a macrolide, or respiratory fluoroquinolones. However, length of therapy varies by regimen used; whereas treating with doxycycline 100 mg twice daily generally requires 14–21 days, moxifloxacin 400 mg daily only requires 10 days [49].
M. pneumoniae
As with C. pneumoniae, length of therapy of M. pneumoniae varies by antimicrobial used. Shortest courses are seen with the use of macrolides for 5 days, whereas 14 days is considered standard for doxycycline or a respiratory fluoroquinolone [50]. It should be noted that there has been increasingly documented resistance to macrolides, with known resistance of 8.2% in the United States [51].
Duration of Treatment
Most patients with CAP respond within 72 hours to appropriate therapy. IDSA/ATS guidelines recommend that patients be treated for a minimum of 5 days, and before discontinuing antibiotics patients should be afebrile a minimum of 48-72 hours and be clinically stable (Table 6) [16].
Hospitalized patients do not need to be monitored for an additional day once they have reached clinical stability (Table 6), are able to maintain oral intake, and have normal mentation, provided that other comorbidities are stable and social needs have been met [16]. Patients discharged from the hospital with instability have higher risk of readmission or death [55].
Transition to Oral Therapy
IDSA/ATS guidelines [16] recommend that patients should be transitioned from IV to oral antibiotics when they are improving clinically, have stable vital signs, and are able to ingest food/fluids and medications.
Management of Nonresponders
Although the majority of patients respond to antibiotics within 72 hours, treatment failure occurs in up to 15% of patients [45]. Nonresponding pneumonia is generally seen in 2 patterns: worsening of clinical status despite empiric antibiotics OR delay in achieving clinical stability as defined in Table 5 after 72 hours of treatment [13]. Risk factors associated with nonresponding pneumonia [56] are:
- Radiographic: multilobar infiltrates, pleural effusion, cavitation
- Bacteriologic: MRSA, gram-negative or Legionella pneumonia
- Severity index: PSI > 90
- Pharmacologic: incorrect antibiotic choice based on susceptibility
Patients with acute deterioration of clinical status will prompt transfer to a higher level of care and may require mechanical ventilator support. In those with delay in achieving clinical stability, question centers on whether the same antibiotics can be continued while doing further radiographic/microbiologic workup and/or changing antibiotics.
History should be reviewed with particular attention to exposures, travel history, and microbiologic and radiographic data. Clinicians should recall that viral causes account for up to 20% of pneumonias and there are also noninfectious causes that can mimic pyogenic infections [57]. If adequate initial cultures were not obtained, they should be obtained; however, care must be taken in reviewing new sets of cultures while on antibiotics as they may reveal colonization selected out by antibiotics and not a true pathogen. If repeat evaluation is unrevealing, then further evaluation with CT scan and bronchoscopy with bronchoalveolar lavage and biopsy is warranted. CT scans can show pleural effusions, bronchial obstructions or pattern suggestive of cryptogenic pneumonia. A bronchoscopy might yield a microbiologic diagnosis and with biopsy can also evaluate for noninfectious causes.
As with other infections, if escalation of antibiotics is undertaken, clinicians should be mindful to ensure that efforts are being made to elucidate the reason for nonresponse. To simply broaden antimicrobial therapy without attempts at establishing a microbiologic or radiographic cause for nonresponse may lead to inappropriate treatment recurrence of infection. Aside from patients who have bacteremic pneumococcal pneumonia in an ICU setting, there are no published reports pointing to superiority of combination antibiotics [46].
Other Treatment
Because of the inflammatory response associated with pneumonia, several agents have been evaluated as adjunctive treatment of pneumonia to decrease this inflammatory state; namely, steroids, macrolide antibiotics and statins. To date, only the use of steroids (methylprednisolone 0.5 mg/kg every 12 hours for 5 days) in those with severe CAP and high initial anti-inflammatory response (CRP > 150) was shown to decrease treatment failure, decreased risk of ARDS, possibly reduce length of stay, duration of intravenous antibiotics and clinical stability, without effect on mortality or adverse side effects [58,59].
Other adjunctive methods have not been found to have significant impact [16].
Prevention of Pneumonia
Prevention of pneumococcal pneumonia is twofold: prevention of infection caused by S. pneumoniae and prevention of influenza infection. As influenza infection is a risk factor for bacterial infection, specifically with S. pneumoniae, influenza vaccination can prevent bacterial pneumonia [60]. In their most recent recommendations, the CDC continues to recommend routine influenza vaccination for all persons aged greater than 6 months, unless otherwise contraindicated [61].
There are 2 vaccines for prevention of pneumococcal disease: the pneumococcal polysaccharide vaccine (PPSV23) and a conjugate vaccine (PCV13). Following vaccination with PPSV23, 80% of adults develop antibodies against at least 18 of the 23 serotypes [62]. Despite this response, PPSV23 is reported to be protective against invasive pneumococcal infection; yet there is no consensus regarding PPSV23 leading to decreased rates of pneumonia [63]. On the other hand, PCV13 vaccination was associated with prevention of both invasive disease and community-acquired pneumonia in adults 65 years or older [64]. The CDC recommends that all children aged 2 or under receive PCV13, whereas those aged 65 or older should receive PCV13 followed by a dose of PPSV23 [65]. The dose of PPSV23 should be given ≥1 year following the dose of PCV13 [66].Persons < 65 years of age with immunocompromising and certain other conditions should also receive vaccination [67] (Table 7). Full details, many scenarios, and timing of vaccinations can be found at www.cdc.gov/vaccines/schedules/downloads/adult/adult-schedule.pdf.
Cigarette smoking increases the risk of respiratory infections as evidenced by smokers accounting for almost half of all patients with invasive pneumococcal disease [11]. As this is a modifiable risk factor it should be a goal of a comprehensive approach towards prevention of pneumonia.
Summary
CAP remains a leading cause of hospitalization and death in the 21st century. Traditionally, pneumococcus has been considered the major pathogen causing CAP; however, the 2015 EPIC study found that in only 5% of patients diagnosed with CAP was S. pneumoniae detected. Despite the new findings, it is still recommended that empiric treatment for CAP target common typical bacteria (pneumococcus, H. influenzae, Moraxella catarrhalis) and atypical bacteria (M. pneumonia, C. pneumoniae, L. pneumophila).
Because diagnosing pneumonia through history and clinical examination is less than 50% sensitive, a chest imaging study (a plain chest radiograph or a chest CT scan) is usually required to make the diagnosis. Laboratory tests, such as sputum Gram stain/culture, blood culture, urinary antigen tests, PCR test, procalcitonin, and CRP are important adjunctive diagnostic modalities to assist in the diagnosis and management of CAP. However, no single test is sensitive and specific enough to be a stand-alone test. They should be used in conjunction with history, physical examination, and imaging studies. Because vaccination (PPSV23, PCV13, and influenza vaccine) remains the most effective tool in preventing the development of CAP, clinicians, should strive for 100% vaccination rates in appropriate persons.
Corresponding author: Tze Shein Lo, MD, University of North Dakota, 1919 Elm Street, Fargo, ND 58102, [email protected].
Financial disclosures: None.
Author contributions: drafting of article, PM, TSL; critical revision of the article, PM, TSL.
1. Centers for Disease Control and Prevention. National Center for Health Statistics. FastStats - Pneumonia. Accessed 6 Oct 2016 at www.cdc.gov/nchs/fastats/pneumonia.htm.
2. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016;63:e61-e111.
3. Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med 2014;371:1619–28.
4. Mandell LA. Epidemiology and etiology of community-acquired pneumonia. Infect Dis
5. Hoare Z, Lim WS. Pneumonia: update on diagnosis and management. BMJ 2006;332:1077–9.
6. Johnstone J, Marrie TJ, Eurich DT, Majumdar SR. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med 2007;167:1938–43
7. File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med 2010;122:130–41.
8. Eurich DT, Marrie TJ, Minhas-Sandhu JK, Majumdar SR. Ten-year mortality after community-acquired pneumonia. a prospective cohort. Am J Respir Crit Care Med 2015;192:597-604.
9. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015;373:415–27.
10. Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med 2013;369:155–63.
11. Nuorti JP, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med 2000;342:681–9.
12. Almirall J, Serra-Prat M, Bolíbar I, BalassoV. Risk factors for community-acquired pneumonia in adults: a systemic review of observational studies. Respiration 2017;94:299–311.
13. Janoff EM.Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s principles and practice of infectioius diseases. 8th ed. Philadelphia: Sauders; 2015: 2310–27.
14. Diehr P, Wood RW, Bushyhead J, et al. Prediction of pneumonia in outpatients with acute cough--a statistical approach. J Chronic Dis 1984;37:215–25.
15. Metlay JP, Schulz R, Li YH, et al. Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch Intern Med 1997;157:1453–9.
16. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44 Suppl 2:S27–72.
17. Jartti A, Rauvala E, Kauma H, et al. Chest imaging findings in hospitalized patients with H1N1 influenza. Acta Radiol 2011;52:297–304.
18. Basi SK, Marrie TJ, Huang JQ, Majumdar SR. Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med 2004;117:305–11.
19. Caldwell A, Glauser FL, Smith WR, et al. The effects of dehydration on the radiologic and pathologic appearance of experimental canine segmental pneumonia. Am Rev Respir Dis 1975;112:651–6.
20. Bartlett JG. Pneumonia. In: Barlett JG, editor. Management of respiratory tract infections. Philadelphia: Lippincott, Williams & Wilkins; 2001: 1–122.
21. Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med 2015;192:974–82.
22. Wheeler JH, Fishman EK. Computed tomography in the management of chest infections: current status. Clin Infect Dis 1996;23:232–40.
23. Chesnutt MP. Pulmonary disorders. In: Papadakis MM, editor. Current medical diagnosis and treatment. New York: McGraw-Hill; 2016: 242–320.
24. Mandell LW. Pneumonia. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 188–201.
25. Reed WW, Byrd GS, Gates RH Jr, et al. Sputum gram’s stain in community-acquired pneumococcal pneumonia. A meta-analysis. West J Med 1996;165:197–204.
26. Chalasani NP, Valdecanas MA, Gopal AK, et al. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined underlying risks. Chest 1995;108:932–6.
27. Bordon J, Peyrani P, Brock GN, et al. The presence of pneumococcal bacteremia does not influence clinical outcomes in patients with community-acquired pneumonia: results from the Community-Acquired Pneumonia Organization (CAPO) International Cohort study. Chest 2008;133:618–24.
28. Helbig JH, Uldum SA, Bernander S, et al. Clinical utility of urinary antigen detection for diagnosis of community-acquired, travel-associated, and nosocomial legionnaires’ disease. J Clin Microbiol 2003;41:838–40.
29. Smith MD, Derrington P, Evans R, et al. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol 2003;41:2810–3.
30. Johansson N, Kalin M, Tiveljung-Lindell A, et al. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis 2010;50:202–9.
31. Gilbert DN. Procalcitonin as a biomarker in respiratory tract infection. Clin Infect Dis 2011;52 Suppl 4:S346–50.
32. Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev 2012;(9):CD007498.
33. Boussekey N, Leroy O, Alfandari S, et al. Procalcitonin kinetics in the prognosis of severe community-acquired pneumonia. Intensive Care Med 2006;32:469–72.
34. Flanders SA, Stein J, Shochat G, et al. Performance of a bedside C-reactive protein test in the diagnosis of community-acquired pneumonia in adults with acute cough. Am J Med 2004;116:529–35.
35. Fine MJ, et al A prediction rule to identify low-risk patients with community-acquired pneumonia.N Engl J Med.1997;336:243-50.
36. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003;58:377–82.
37. Arnold FW, Ramirez JA, McDonald LC, Xia EL. Hospitalization for community-acquired pneumonia: the pneumonia severity index vs clinical judgment. Chest 2003;124:121–4.
38. Aujesky D, McCausland JB, Whittle J, et al. Reasons why emergency department providers do not rely on the pneumonia severity index to determine the initial site of treatment for patients with pneumonia. Clin Infect Dis 2009;49:e100–8.
39. Charles PG, Wolfe R, Whitby M, et al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis 2008;47:375–84.
40. Marti C, Garin N, Grosgurin O, et al. Prediction of severe community-acquired pneumonia: a systematic review and meta-analysis. Crit Care 2012;16:R141.
41. Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 2012;54:621–9.
42. Silverman JA, Mortin LI, Vanpraagh AD, et al. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis 2005;191:2149–52.
43. El Hajj MS, Turgeon RD, Wilby KJ. Ceftaroline fosamil for community-acquired pneumonia and skin and skin structure infections: a systematic review. Int J Clin Pharm 2017 Jan 5.
44. Taboada M, Melnick D, Iaconis JP, et al. Ceftaroline fosamil versus ceftriaxone for the treatment of community-acquired pneumonia: individual patient data meta-analysis of randomized controlled trials. J Antimicrob Chemother 2016;71:862–70.
45. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011;52:285–92.
46. Baddour LM, Yu VL, Klugman KP, et al. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med 2004;170:440–4.
47. FDA Drug Safety Communication: FDA warns of increased risk of death with IV antibacterial Tygacil (tigecycline) and approves new boxed warning [Internet]. 15 Jan 2016. Available at www.fda.gov/Drugs/DrugSafety/ucm369580.htm.
48. Edelstein PR, CR. Legionnaires’ Disease and Pontiac Fever. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 2633.
49. Hammerschlag MR, Kohlhoff SA, Gaydos, CA. Chlamydia pneumoniae. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 2174.
50. Holzman RS, MS. Mycoplasma pneumoniae and Atypical Pneumonia. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 2183.
51. Yamada M, Buller R, Bledsoe S, Storch GA. Rising rates of macrolide-resistant Mycoplasma pneumoniae in the central United States. Pediatr Infect Dis J 2012;31:409–10.
52. Yi SH, Hatfield KM, Baggs J, et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis 2017 Nov 6.
53. Hayashi Y, Paterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis 2011;52:1232–40.
54. Akram AR, Chalmers JD, Taylor JK, et al. An evaluation of clinical stability criteria to predict hospital course in community-acquired pneumonia. Clin Microbiol Infect 2013;19:1174–80.
55. Halm EA, Fine MJ, Kapoor WN, et al. Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia. Arch Intern Med 2002;162:1278–84.
56. Roson B, Carratala J, Fernandez-Sabe N, et al. Causes and factors associated with early failure in hospitalized patients with community-acquired pneumonia. Arch Intern Med 2004;164:502–8.
57. El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med 2003;167:1650–4.
58. Wan YD, Sun TW, Liu ZQ, et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: a systematic review and meta-analysis. Chest 2016;149:209–19.
59. Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 2015;313:677–86.
60. McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev 2006;19:571–82.
61. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines. MMWR Recomm Rep 2016;65:1–54.
62. Rubins JB, Alter M, Loch J, Janoff EN. Determination of antibody responses of elderly adults to all 23 capsular polysaccharides after pneumococcal vaccination. Infect Immun 1999;67:5979–84.
63. Centers for Disease Control. Vaccines and preventable diseases [Internet]. 22 Nov 2016. Available at www.cdc.gov/vaccines/vpd/pneumo/hcp/about-vaccine.html.
64. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015;372:1114–25.
65. Centers for Disease Control. Recommended adult immunization schedule -- United States -- 2016 [Internet]. 2016. Available at www.cdc.gov/vaccines/schedules/downloads/adult/adult-schedule.pdf.
66. Kobayashi M, Bennett NM, Gierke R, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2015;64:944–7.
67. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012;61:816–9.
68. Aujesky D, Auble TE, Yealy DM, et al. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med 2005;118:384–92.
1. Centers for Disease Control and Prevention. National Center for Health Statistics. FastStats - Pneumonia. Accessed 6 Oct 2016 at www.cdc.gov/nchs/fastats/pneumonia.htm.
2. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016;63:e61-e111.
3. Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med 2014;371:1619–28.
4. Mandell LA. Epidemiology and etiology of community-acquired pneumonia. Infect Dis
5. Hoare Z, Lim WS. Pneumonia: update on diagnosis and management. BMJ 2006;332:1077–9.
6. Johnstone J, Marrie TJ, Eurich DT, Majumdar SR. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med 2007;167:1938–43
7. File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med 2010;122:130–41.
8. Eurich DT, Marrie TJ, Minhas-Sandhu JK, Majumdar SR. Ten-year mortality after community-acquired pneumonia. a prospective cohort. Am J Respir Crit Care Med 2015;192:597-604.
9. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015;373:415–27.
10. Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med 2013;369:155–63.
11. Nuorti JP, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med 2000;342:681–9.
12. Almirall J, Serra-Prat M, Bolíbar I, BalassoV. Risk factors for community-acquired pneumonia in adults: a systemic review of observational studies. Respiration 2017;94:299–311.
13. Janoff EM.Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s principles and practice of infectioius diseases. 8th ed. Philadelphia: Sauders; 2015: 2310–27.
14. Diehr P, Wood RW, Bushyhead J, et al. Prediction of pneumonia in outpatients with acute cough--a statistical approach. J Chronic Dis 1984;37:215–25.
15. Metlay JP, Schulz R, Li YH, et al. Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch Intern Med 1997;157:1453–9.
16. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44 Suppl 2:S27–72.
17. Jartti A, Rauvala E, Kauma H, et al. Chest imaging findings in hospitalized patients with H1N1 influenza. Acta Radiol 2011;52:297–304.
18. Basi SK, Marrie TJ, Huang JQ, Majumdar SR. Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med 2004;117:305–11.
19. Caldwell A, Glauser FL, Smith WR, et al. The effects of dehydration on the radiologic and pathologic appearance of experimental canine segmental pneumonia. Am Rev Respir Dis 1975;112:651–6.
20. Bartlett JG. Pneumonia. In: Barlett JG, editor. Management of respiratory tract infections. Philadelphia: Lippincott, Williams & Wilkins; 2001: 1–122.
21. Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med 2015;192:974–82.
22. Wheeler JH, Fishman EK. Computed tomography in the management of chest infections: current status. Clin Infect Dis 1996;23:232–40.
23. Chesnutt MP. Pulmonary disorders. In: Papadakis MM, editor. Current medical diagnosis and treatment. New York: McGraw-Hill; 2016: 242–320.
24. Mandell LW. Pneumonia. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 188–201.
25. Reed WW, Byrd GS, Gates RH Jr, et al. Sputum gram’s stain in community-acquired pneumococcal pneumonia. A meta-analysis. West J Med 1996;165:197–204.
26. Chalasani NP, Valdecanas MA, Gopal AK, et al. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined underlying risks. Chest 1995;108:932–6.
27. Bordon J, Peyrani P, Brock GN, et al. The presence of pneumococcal bacteremia does not influence clinical outcomes in patients with community-acquired pneumonia: results from the Community-Acquired Pneumonia Organization (CAPO) International Cohort study. Chest 2008;133:618–24.
28. Helbig JH, Uldum SA, Bernander S, et al. Clinical utility of urinary antigen detection for diagnosis of community-acquired, travel-associated, and nosocomial legionnaires’ disease. J Clin Microbiol 2003;41:838–40.
29. Smith MD, Derrington P, Evans R, et al. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol 2003;41:2810–3.
30. Johansson N, Kalin M, Tiveljung-Lindell A, et al. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis 2010;50:202–9.
31. Gilbert DN. Procalcitonin as a biomarker in respiratory tract infection. Clin Infect Dis 2011;52 Suppl 4:S346–50.
32. Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev 2012;(9):CD007498.
33. Boussekey N, Leroy O, Alfandari S, et al. Procalcitonin kinetics in the prognosis of severe community-acquired pneumonia. Intensive Care Med 2006;32:469–72.
34. Flanders SA, Stein J, Shochat G, et al. Performance of a bedside C-reactive protein test in the diagnosis of community-acquired pneumonia in adults with acute cough. Am J Med 2004;116:529–35.
35. Fine MJ, et al A prediction rule to identify low-risk patients with community-acquired pneumonia.N Engl J Med.1997;336:243-50.
36. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003;58:377–82.
37. Arnold FW, Ramirez JA, McDonald LC, Xia EL. Hospitalization for community-acquired pneumonia: the pneumonia severity index vs clinical judgment. Chest 2003;124:121–4.
38. Aujesky D, McCausland JB, Whittle J, et al. Reasons why emergency department providers do not rely on the pneumonia severity index to determine the initial site of treatment for patients with pneumonia. Clin Infect Dis 2009;49:e100–8.
39. Charles PG, Wolfe R, Whitby M, et al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis 2008;47:375–84.
40. Marti C, Garin N, Grosgurin O, et al. Prediction of severe community-acquired pneumonia: a systematic review and meta-analysis. Crit Care 2012;16:R141.
41. Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 2012;54:621–9.
42. Silverman JA, Mortin LI, Vanpraagh AD, et al. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis 2005;191:2149–52.
43. El Hajj MS, Turgeon RD, Wilby KJ. Ceftaroline fosamil for community-acquired pneumonia and skin and skin structure infections: a systematic review. Int J Clin Pharm 2017 Jan 5.
44. Taboada M, Melnick D, Iaconis JP, et al. Ceftaroline fosamil versus ceftriaxone for the treatment of community-acquired pneumonia: individual patient data meta-analysis of randomized controlled trials. J Antimicrob Chemother 2016;71:862–70.
45. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011;52:285–92.
46. Baddour LM, Yu VL, Klugman KP, et al. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med 2004;170:440–4.
47. FDA Drug Safety Communication: FDA warns of increased risk of death with IV antibacterial Tygacil (tigecycline) and approves new boxed warning [Internet]. 15 Jan 2016. Available at www.fda.gov/Drugs/DrugSafety/ucm369580.htm.
48. Edelstein PR, CR. Legionnaires’ Disease and Pontiac Fever. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 2633.
49. Hammerschlag MR, Kohlhoff SA, Gaydos, CA. Chlamydia pneumoniae. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 2174.
50. Holzman RS, MS. Mycoplasma pneumoniae and Atypical Pneumonia. In: Kasper DF, editor. Harrison’s infectious diseases. 1st ed. New York: McGraw-Hill; 2010: 2183.
51. Yamada M, Buller R, Bledsoe S, Storch GA. Rising rates of macrolide-resistant Mycoplasma pneumoniae in the central United States. Pediatr Infect Dis J 2012;31:409–10.
52. Yi SH, Hatfield KM, Baggs J, et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis 2017 Nov 6.
53. Hayashi Y, Paterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis 2011;52:1232–40.
54. Akram AR, Chalmers JD, Taylor JK, et al. An evaluation of clinical stability criteria to predict hospital course in community-acquired pneumonia. Clin Microbiol Infect 2013;19:1174–80.
55. Halm EA, Fine MJ, Kapoor WN, et al. Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia. Arch Intern Med 2002;162:1278–84.
56. Roson B, Carratala J, Fernandez-Sabe N, et al. Causes and factors associated with early failure in hospitalized patients with community-acquired pneumonia. Arch Intern Med 2004;164:502–8.
57. El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med 2003;167:1650–4.
58. Wan YD, Sun TW, Liu ZQ, et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: a systematic review and meta-analysis. Chest 2016;149:209–19.
59. Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 2015;313:677–86.
60. McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev 2006;19:571–82.
61. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines. MMWR Recomm Rep 2016;65:1–54.
62. Rubins JB, Alter M, Loch J, Janoff EN. Determination of antibody responses of elderly adults to all 23 capsular polysaccharides after pneumococcal vaccination. Infect Immun 1999;67:5979–84.
63. Centers for Disease Control. Vaccines and preventable diseases [Internet]. 22 Nov 2016. Available at www.cdc.gov/vaccines/vpd/pneumo/hcp/about-vaccine.html.
64. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015;372:1114–25.
65. Centers for Disease Control. Recommended adult immunization schedule -- United States -- 2016 [Internet]. 2016. Available at www.cdc.gov/vaccines/schedules/downloads/adult/adult-schedule.pdf.
66. Kobayashi M, Bennett NM, Gierke R, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2015;64:944–7.
67. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012;61:816–9.
68. Aujesky D, Auble TE, Yealy DM, et al. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med 2005;118:384–92.