User login
Exploding e-cigs can cause grievous injuries
With the increasing use of e-cigarettes among adolescents, explosions involving these devices are a growing concern because they can cause serious injuries.
One such incident, described by Elizabeth Ackley, MD, of the University of Colorado at Denver, Aurora, and her coauthors, involved a 17-year-old youth whose electronic nicotine-delivery systems (ENDS) exploded as he was about to take a puff. He presented with a burned left thumb with sensory loss, reduced motor control, and heavy bleeding. He underwent debridement, multiple antibiotic courses, and six operative procedures that ultimately led to removal of the lateral aspect of his thumb.
Dr. Ackley and her associates emphasized. This can occur with overheating, water exposure, excessive charging, improper charging with incompatible devices, contact with metallic objects such as keys or coins, or damage of the battery.
Mineral oil should be used for initial wound irrigation, the authors advise, and “surgical debridement is the definitive treatment for injuries and should remove any remaining alkaline material from tissues.” Delay use of water-based irrigation until after surgical debridement, and one report suggests probing the wound with litmus paper to ensure the pH is no longer alkaline prior to using water irrigation.
Although there are other talking points pediatricians may use when counseling teens about use of e-cigs and other tobacco products, the authors suggested that “the potential for major and disfiguring injury from ENDS explosions may be a more compelling talking point with teens instead of long-term or other nebulous adverse effects of ENDS and tobacco products.”
Read more about the subject at J Pediatr. 2018. doi: 10.1016/j.jpeds.2017.12.032.
With the increasing use of e-cigarettes among adolescents, explosions involving these devices are a growing concern because they can cause serious injuries.
One such incident, described by Elizabeth Ackley, MD, of the University of Colorado at Denver, Aurora, and her coauthors, involved a 17-year-old youth whose electronic nicotine-delivery systems (ENDS) exploded as he was about to take a puff. He presented with a burned left thumb with sensory loss, reduced motor control, and heavy bleeding. He underwent debridement, multiple antibiotic courses, and six operative procedures that ultimately led to removal of the lateral aspect of his thumb.
Dr. Ackley and her associates emphasized. This can occur with overheating, water exposure, excessive charging, improper charging with incompatible devices, contact with metallic objects such as keys or coins, or damage of the battery.
Mineral oil should be used for initial wound irrigation, the authors advise, and “surgical debridement is the definitive treatment for injuries and should remove any remaining alkaline material from tissues.” Delay use of water-based irrigation until after surgical debridement, and one report suggests probing the wound with litmus paper to ensure the pH is no longer alkaline prior to using water irrigation.
Although there are other talking points pediatricians may use when counseling teens about use of e-cigs and other tobacco products, the authors suggested that “the potential for major and disfiguring injury from ENDS explosions may be a more compelling talking point with teens instead of long-term or other nebulous adverse effects of ENDS and tobacco products.”
Read more about the subject at J Pediatr. 2018. doi: 10.1016/j.jpeds.2017.12.032.
With the increasing use of e-cigarettes among adolescents, explosions involving these devices are a growing concern because they can cause serious injuries.
One such incident, described by Elizabeth Ackley, MD, of the University of Colorado at Denver, Aurora, and her coauthors, involved a 17-year-old youth whose electronic nicotine-delivery systems (ENDS) exploded as he was about to take a puff. He presented with a burned left thumb with sensory loss, reduced motor control, and heavy bleeding. He underwent debridement, multiple antibiotic courses, and six operative procedures that ultimately led to removal of the lateral aspect of his thumb.
Dr. Ackley and her associates emphasized. This can occur with overheating, water exposure, excessive charging, improper charging with incompatible devices, contact with metallic objects such as keys or coins, or damage of the battery.
Mineral oil should be used for initial wound irrigation, the authors advise, and “surgical debridement is the definitive treatment for injuries and should remove any remaining alkaline material from tissues.” Delay use of water-based irrigation until after surgical debridement, and one report suggests probing the wound with litmus paper to ensure the pH is no longer alkaline prior to using water irrigation.
Although there are other talking points pediatricians may use when counseling teens about use of e-cigs and other tobacco products, the authors suggested that “the potential for major and disfiguring injury from ENDS explosions may be a more compelling talking point with teens instead of long-term or other nebulous adverse effects of ENDS and tobacco products.”
Read more about the subject at J Pediatr. 2018. doi: 10.1016/j.jpeds.2017.12.032.
This is what a flu pandemic looks like
For the week ending Feb. 3, 2018, the proportion of outpatient visits for influenza-like illness (ILI) was 7.7%, which would appear to equal the mark of 7.7% set in October of 2009. The earlier 7.7%, however, is rounded down from 7.715%, while the current mark is rounded up from 7.653%, data from the CDC’s Fluview website show.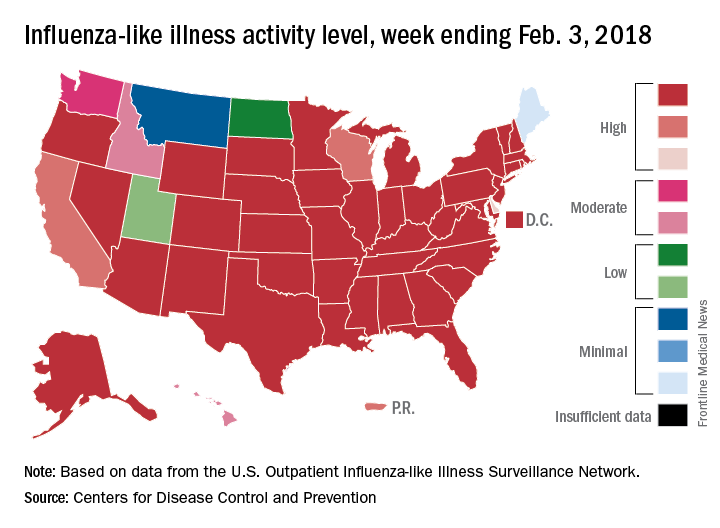
Deaths attributed to pneumonia and influenza were above the epidemic threshold set by the National Center for Health Statistics Mortality Surveillance system, acting CDC director Anne Schuchat, MD, said in a teleconference sponsored by the agency.
ILI activity was at level 10 on the CDC’s 1-10 scale in 41 states, compared with 34 the week before, and was categorized in the “high” range (levels 8-10) in another 3 states and Puerto Rico, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network. In California, which was noted as a possible bright spot last week by Dr. Schuchat because activity there had been decreasing, the ILI level went back up to level 9 after being at 7 the week before.
Flu-related hospitalizations are continuing to rise at a record clip, with the cumulative rate for the week of Feb. 3 at 59.9 per 100,000 population, the CDC reported. A total of 1 in 10 hospital-based deaths last week were related to influenza. At this point in the 2014-2015 flu season – which has the highest number of hospitalizations at 710,000 – the hospitalization rate was only 50.9 per 100,000 population.
There were 10 pediatric deaths reported for the week ending Feb. 3, although 9 occurred in previous weeks. There have been 63 flu-related deaths among children so far during the 2017-2018 season.
Dr. Schuchat continued to recommend members of the public to get a flu shot and to stay home if they are feeling sick.
“What could be mild symptoms for you could be deadly for someone else,” Dr. Schuchat said, adding that antiviral medications remain important. “Physicians do not have to wait for confirmatory flu testing. They should begin treatment with antiviral drugs immediately in they suspect they have a severely ill or a high risk patient.”
“Flu vaccines often have lower effectiveness against H3N1 viruses. However, some protection is better than none. The vaccine’s effectiveness against other flu viruses, like B and H1N1, is better. Because of the ongoing intensity of the flu season and the increasing circulation of influenza B and h1n1, we do continue to recommend vaccination even this late in the season.”
Dr. Schuchat stressed the importance of the pneumococcal pneumonia vaccine. “Flu can make people more vulnerable to secondary infections like bacterial pneumonia. We recommend people aged 65 and over get a pneumococcal pneumonia vaccine,” she said.
For the week ending Feb. 3, 2018, the proportion of outpatient visits for influenza-like illness (ILI) was 7.7%, which would appear to equal the mark of 7.7% set in October of 2009. The earlier 7.7%, however, is rounded down from 7.715%, while the current mark is rounded up from 7.653%, data from the CDC’s Fluview website show.
Deaths attributed to pneumonia and influenza were above the epidemic threshold set by the National Center for Health Statistics Mortality Surveillance system, acting CDC director Anne Schuchat, MD, said in a teleconference sponsored by the agency.
ILI activity was at level 10 on the CDC’s 1-10 scale in 41 states, compared with 34 the week before, and was categorized in the “high” range (levels 8-10) in another 3 states and Puerto Rico, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network. In California, which was noted as a possible bright spot last week by Dr. Schuchat because activity there had been decreasing, the ILI level went back up to level 9 after being at 7 the week before.
Flu-related hospitalizations are continuing to rise at a record clip, with the cumulative rate for the week of Feb. 3 at 59.9 per 100,000 population, the CDC reported. A total of 1 in 10 hospital-based deaths last week were related to influenza. At this point in the 2014-2015 flu season – which has the highest number of hospitalizations at 710,000 – the hospitalization rate was only 50.9 per 100,000 population.
There were 10 pediatric deaths reported for the week ending Feb. 3, although 9 occurred in previous weeks. There have been 63 flu-related deaths among children so far during the 2017-2018 season.
Dr. Schuchat continued to recommend members of the public to get a flu shot and to stay home if they are feeling sick.
“What could be mild symptoms for you could be deadly for someone else,” Dr. Schuchat said, adding that antiviral medications remain important. “Physicians do not have to wait for confirmatory flu testing. They should begin treatment with antiviral drugs immediately in they suspect they have a severely ill or a high risk patient.”
“Flu vaccines often have lower effectiveness against H3N1 viruses. However, some protection is better than none. The vaccine’s effectiveness against other flu viruses, like B and H1N1, is better. Because of the ongoing intensity of the flu season and the increasing circulation of influenza B and h1n1, we do continue to recommend vaccination even this late in the season.”
Dr. Schuchat stressed the importance of the pneumococcal pneumonia vaccine. “Flu can make people more vulnerable to secondary infections like bacterial pneumonia. We recommend people aged 65 and over get a pneumococcal pneumonia vaccine,” she said.
For the week ending Feb. 3, 2018, the proportion of outpatient visits for influenza-like illness (ILI) was 7.7%, which would appear to equal the mark of 7.7% set in October of 2009. The earlier 7.7%, however, is rounded down from 7.715%, while the current mark is rounded up from 7.653%, data from the CDC’s Fluview website show.
Deaths attributed to pneumonia and influenza were above the epidemic threshold set by the National Center for Health Statistics Mortality Surveillance system, acting CDC director Anne Schuchat, MD, said in a teleconference sponsored by the agency.
ILI activity was at level 10 on the CDC’s 1-10 scale in 41 states, compared with 34 the week before, and was categorized in the “high” range (levels 8-10) in another 3 states and Puerto Rico, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network. In California, which was noted as a possible bright spot last week by Dr. Schuchat because activity there had been decreasing, the ILI level went back up to level 9 after being at 7 the week before.
Flu-related hospitalizations are continuing to rise at a record clip, with the cumulative rate for the week of Feb. 3 at 59.9 per 100,000 population, the CDC reported. A total of 1 in 10 hospital-based deaths last week were related to influenza. At this point in the 2014-2015 flu season – which has the highest number of hospitalizations at 710,000 – the hospitalization rate was only 50.9 per 100,000 population.
There were 10 pediatric deaths reported for the week ending Feb. 3, although 9 occurred in previous weeks. There have been 63 flu-related deaths among children so far during the 2017-2018 season.
Dr. Schuchat continued to recommend members of the public to get a flu shot and to stay home if they are feeling sick.
“What could be mild symptoms for you could be deadly for someone else,” Dr. Schuchat said, adding that antiviral medications remain important. “Physicians do not have to wait for confirmatory flu testing. They should begin treatment with antiviral drugs immediately in they suspect they have a severely ill or a high risk patient.”
“Flu vaccines often have lower effectiveness against H3N1 viruses. However, some protection is better than none. The vaccine’s effectiveness against other flu viruses, like B and H1N1, is better. Because of the ongoing intensity of the flu season and the increasing circulation of influenza B and h1n1, we do continue to recommend vaccination even this late in the season.”
Dr. Schuchat stressed the importance of the pneumococcal pneumonia vaccine. “Flu can make people more vulnerable to secondary infections like bacterial pneumonia. We recommend people aged 65 and over get a pneumococcal pneumonia vaccine,” she said.
FROM A CDC TELECONFERENCE
Getting hematologic cancer drugs on the fast track
LA JOLLA, CALIF. – The words “rapid approval” and “Food and Drug Administration” rarely appear in the same sentence. But despite that perception, the pace of hematologic drug development has been accelerating over the last several years, according to an agency staffer.
“FDA is committed toward the expedited development of safe and effective therapies for serious and life-threatening diseases,” R. Angelo de Claro, MD, of the FDA’s Office of Hematology and Oncology Products said at the annual T-cell Lymphoma Forum. Dr. de Claro outlined his agency’s efforts to accelerate approval of drugs for treatment of T-cell malignancies.
Hematologic drug bonanza
In 2017 alone, the FDA approved 17 agents for new or expanded indications for hematologic malignancies, including brentuximab vedotin (Adcetris) for anaplastic large cell lymphoma (ALCL) and CD30-positive mycosis fungoides (MF).
Approval was based on a 56% objective response rate for brentuximab vedotin versus 12% for physician’s choice in a phase 3 trial (ALCANZA) of 131 patients with mycosis fungoides or primary cutaneous ALCL. All patients had received one prior systemic therapy and were randomized (1:1) to receive either brentuximab vedotin or the physician’s choice of methotrexate or bexarotene.
Dr. de Claro noted that in the ALCANZA trial, patients were required to have one or more biopsy samples with at least 10% CD30 expression, but among 184 patients with MF screened for the trial, 32% were ineligible because of less than 10% CD30 expression. The FDA therefore requested additional efficacy data for patients with MF with less than 10% CD30 expression and accepted data from two investigator-sponsored trials showing that 35 patients with MF expressing CD30 on 1%-9% of cells had a 31% overall response rate, whereas two patients with no CD30 expression did not have responses.
Who minds the store
Hematology products are under the aegis of the FDA’s Oncology Center of Excellence. Oversight includes benign hematology products, as well as products for hematologic cancers and hematologic support. Hematology and oncology toxicology is monitored by pharmacologists and toxicologists in a separate division, he explained.
“The Oncology Center of Excellence was formally launched in 2017 as part of the 21st century CURES Act. The mission of the Oncology Center of Excellence is to achieve patient-centered regulatory decision making through innovation and collaboration,” he said.
Getting the nod
To get approved, a new therapy requires “substantial” evidence of efficacy and safety. Regular approvals are based on either direct measures of clinical benefits – how a patient “feels, functions, or survives” – or a measure of the effect of a drug on an established surrogate endpoint.
For an accelerated approval, developers must be able to show evidence on either a surrogate or intermediate clinical endpoint that the agent is reasonably likely to offer a benefit and be a meaningful improvement over available therapies. Postapproval trials may be needed to verify the proposed benefits.
FDA accelerated approval programs include:
- Fast track. The pathway requires nonclinical or clinical data demonstrating the potential for addressing an unmet need.
- Breakthrough therapy. This pathway requires preliminary clinical evidence demonstrating substantial improvement over existing available therapies.
- Priority review. These are agents that, if approved, would provide significant improvements in safety or effectiveness.
- Accelerated approval. The drug must demonstrate an effect on an “endpoint reasonably likely to predict clinical benefit over available therapies.”
Dr. de Claro is employed by the FDA. The T-Cell Lymphoma Forum is held by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – The words “rapid approval” and “Food and Drug Administration” rarely appear in the same sentence. But despite that perception, the pace of hematologic drug development has been accelerating over the last several years, according to an agency staffer.
“FDA is committed toward the expedited development of safe and effective therapies for serious and life-threatening diseases,” R. Angelo de Claro, MD, of the FDA’s Office of Hematology and Oncology Products said at the annual T-cell Lymphoma Forum. Dr. de Claro outlined his agency’s efforts to accelerate approval of drugs for treatment of T-cell malignancies.
Hematologic drug bonanza
In 2017 alone, the FDA approved 17 agents for new or expanded indications for hematologic malignancies, including brentuximab vedotin (Adcetris) for anaplastic large cell lymphoma (ALCL) and CD30-positive mycosis fungoides (MF).
Approval was based on a 56% objective response rate for brentuximab vedotin versus 12% for physician’s choice in a phase 3 trial (ALCANZA) of 131 patients with mycosis fungoides or primary cutaneous ALCL. All patients had received one prior systemic therapy and were randomized (1:1) to receive either brentuximab vedotin or the physician’s choice of methotrexate or bexarotene.
Dr. de Claro noted that in the ALCANZA trial, patients were required to have one or more biopsy samples with at least 10% CD30 expression, but among 184 patients with MF screened for the trial, 32% were ineligible because of less than 10% CD30 expression. The FDA therefore requested additional efficacy data for patients with MF with less than 10% CD30 expression and accepted data from two investigator-sponsored trials showing that 35 patients with MF expressing CD30 on 1%-9% of cells had a 31% overall response rate, whereas two patients with no CD30 expression did not have responses.
Who minds the store
Hematology products are under the aegis of the FDA’s Oncology Center of Excellence. Oversight includes benign hematology products, as well as products for hematologic cancers and hematologic support. Hematology and oncology toxicology is monitored by pharmacologists and toxicologists in a separate division, he explained.
“The Oncology Center of Excellence was formally launched in 2017 as part of the 21st century CURES Act. The mission of the Oncology Center of Excellence is to achieve patient-centered regulatory decision making through innovation and collaboration,” he said.
Getting the nod
To get approved, a new therapy requires “substantial” evidence of efficacy and safety. Regular approvals are based on either direct measures of clinical benefits – how a patient “feels, functions, or survives” – or a measure of the effect of a drug on an established surrogate endpoint.
For an accelerated approval, developers must be able to show evidence on either a surrogate or intermediate clinical endpoint that the agent is reasonably likely to offer a benefit and be a meaningful improvement over available therapies. Postapproval trials may be needed to verify the proposed benefits.
FDA accelerated approval programs include:
- Fast track. The pathway requires nonclinical or clinical data demonstrating the potential for addressing an unmet need.
- Breakthrough therapy. This pathway requires preliminary clinical evidence demonstrating substantial improvement over existing available therapies.
- Priority review. These are agents that, if approved, would provide significant improvements in safety or effectiveness.
- Accelerated approval. The drug must demonstrate an effect on an “endpoint reasonably likely to predict clinical benefit over available therapies.”
Dr. de Claro is employed by the FDA. The T-Cell Lymphoma Forum is held by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – The words “rapid approval” and “Food and Drug Administration” rarely appear in the same sentence. But despite that perception, the pace of hematologic drug development has been accelerating over the last several years, according to an agency staffer.
“FDA is committed toward the expedited development of safe and effective therapies for serious and life-threatening diseases,” R. Angelo de Claro, MD, of the FDA’s Office of Hematology and Oncology Products said at the annual T-cell Lymphoma Forum. Dr. de Claro outlined his agency’s efforts to accelerate approval of drugs for treatment of T-cell malignancies.
Hematologic drug bonanza
In 2017 alone, the FDA approved 17 agents for new or expanded indications for hematologic malignancies, including brentuximab vedotin (Adcetris) for anaplastic large cell lymphoma (ALCL) and CD30-positive mycosis fungoides (MF).
Approval was based on a 56% objective response rate for brentuximab vedotin versus 12% for physician’s choice in a phase 3 trial (ALCANZA) of 131 patients with mycosis fungoides or primary cutaneous ALCL. All patients had received one prior systemic therapy and were randomized (1:1) to receive either brentuximab vedotin or the physician’s choice of methotrexate or bexarotene.
Dr. de Claro noted that in the ALCANZA trial, patients were required to have one or more biopsy samples with at least 10% CD30 expression, but among 184 patients with MF screened for the trial, 32% were ineligible because of less than 10% CD30 expression. The FDA therefore requested additional efficacy data for patients with MF with less than 10% CD30 expression and accepted data from two investigator-sponsored trials showing that 35 patients with MF expressing CD30 on 1%-9% of cells had a 31% overall response rate, whereas two patients with no CD30 expression did not have responses.
Who minds the store
Hematology products are under the aegis of the FDA’s Oncology Center of Excellence. Oversight includes benign hematology products, as well as products for hematologic cancers and hematologic support. Hematology and oncology toxicology is monitored by pharmacologists and toxicologists in a separate division, he explained.
“The Oncology Center of Excellence was formally launched in 2017 as part of the 21st century CURES Act. The mission of the Oncology Center of Excellence is to achieve patient-centered regulatory decision making through innovation and collaboration,” he said.
Getting the nod
To get approved, a new therapy requires “substantial” evidence of efficacy and safety. Regular approvals are based on either direct measures of clinical benefits – how a patient “feels, functions, or survives” – or a measure of the effect of a drug on an established surrogate endpoint.
For an accelerated approval, developers must be able to show evidence on either a surrogate or intermediate clinical endpoint that the agent is reasonably likely to offer a benefit and be a meaningful improvement over available therapies. Postapproval trials may be needed to verify the proposed benefits.
FDA accelerated approval programs include:
- Fast track. The pathway requires nonclinical or clinical data demonstrating the potential for addressing an unmet need.
- Breakthrough therapy. This pathway requires preliminary clinical evidence demonstrating substantial improvement over existing available therapies.
- Priority review. These are agents that, if approved, would provide significant improvements in safety or effectiveness.
- Accelerated approval. The drug must demonstrate an effect on an “endpoint reasonably likely to predict clinical benefit over available therapies.”
Dr. de Claro is employed by the FDA. The T-Cell Lymphoma Forum is held by Jonathan Wood & Associates, which is owned by the same company as this news organization.
EXPERT ANALYSIS FROM TCLF 2018
Hospitalist empathy associated with reduced patient anxiety
Clinical question: What effect does hospitalist empathy have on patient anxiety, ratings of physician communication, and duration of encounter?
Background: Physician empathy is associated with better patient-reported and medical outcomes in a number of settings. The effects of hospitalist empathy have been less well studied.
Study design: Observational study of audio recordings of hospitalist admission encounters.
Setting: General medical service at two urban hospitals within an academic medical center from August 2008 to March 2009.
Synopsis: Admission encounters (76 patients, 27 hospitalists) were recorded. Researchers detected negative emotional expressions from patients and characterized resultant physician replies as either empathic (“focuses toward further expression of emotion”), neutral (“focuses neither toward nor away from emotion”), or nonempathic (“focuses away from emotion”). Through use of regression models, response frequency was compared with change in pre/post-encounter patient anxiety, patient ratings of physician communication, and duration of encounter. Every additional empathic response was associated with a small decrease in anxiety, better ratings of physician communication, and no change in encounter duration. Nonempathic responses were associated with worse communication ratings. Limitations of the study include its observational nature, small sample size, exclusion of non–English-speaking patients, absence of data on nonverbal communication, and exclusively urban academic setting.
Bottom line: Empathic hospitalist responses during admission encounters are associated with reductions in patient anxiety and better ratings of physician communication without increases in encounter duration.
Citation: Weiss R et al. Associations of physician empathy with patient anxiety and ratings of communication in hospital admission encounters. J Hosp Med. 2017;12(10):805-10.
Dr. Kanjee is a hospitalist, Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Clinical question: What effect does hospitalist empathy have on patient anxiety, ratings of physician communication, and duration of encounter?
Background: Physician empathy is associated with better patient-reported and medical outcomes in a number of settings. The effects of hospitalist empathy have been less well studied.
Study design: Observational study of audio recordings of hospitalist admission encounters.
Setting: General medical service at two urban hospitals within an academic medical center from August 2008 to March 2009.
Synopsis: Admission encounters (76 patients, 27 hospitalists) were recorded. Researchers detected negative emotional expressions from patients and characterized resultant physician replies as either empathic (“focuses toward further expression of emotion”), neutral (“focuses neither toward nor away from emotion”), or nonempathic (“focuses away from emotion”). Through use of regression models, response frequency was compared with change in pre/post-encounter patient anxiety, patient ratings of physician communication, and duration of encounter. Every additional empathic response was associated with a small decrease in anxiety, better ratings of physician communication, and no change in encounter duration. Nonempathic responses were associated with worse communication ratings. Limitations of the study include its observational nature, small sample size, exclusion of non–English-speaking patients, absence of data on nonverbal communication, and exclusively urban academic setting.
Bottom line: Empathic hospitalist responses during admission encounters are associated with reductions in patient anxiety and better ratings of physician communication without increases in encounter duration.
Citation: Weiss R et al. Associations of physician empathy with patient anxiety and ratings of communication in hospital admission encounters. J Hosp Med. 2017;12(10):805-10.
Dr. Kanjee is a hospitalist, Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Clinical question: What effect does hospitalist empathy have on patient anxiety, ratings of physician communication, and duration of encounter?
Background: Physician empathy is associated with better patient-reported and medical outcomes in a number of settings. The effects of hospitalist empathy have been less well studied.
Study design: Observational study of audio recordings of hospitalist admission encounters.
Setting: General medical service at two urban hospitals within an academic medical center from August 2008 to March 2009.
Synopsis: Admission encounters (76 patients, 27 hospitalists) were recorded. Researchers detected negative emotional expressions from patients and characterized resultant physician replies as either empathic (“focuses toward further expression of emotion”), neutral (“focuses neither toward nor away from emotion”), or nonempathic (“focuses away from emotion”). Through use of regression models, response frequency was compared with change in pre/post-encounter patient anxiety, patient ratings of physician communication, and duration of encounter. Every additional empathic response was associated with a small decrease in anxiety, better ratings of physician communication, and no change in encounter duration. Nonempathic responses were associated with worse communication ratings. Limitations of the study include its observational nature, small sample size, exclusion of non–English-speaking patients, absence of data on nonverbal communication, and exclusively urban academic setting.
Bottom line: Empathic hospitalist responses during admission encounters are associated with reductions in patient anxiety and better ratings of physician communication without increases in encounter duration.
Citation: Weiss R et al. Associations of physician empathy with patient anxiety and ratings of communication in hospital admission encounters. J Hosp Med. 2017;12(10):805-10.
Dr. Kanjee is a hospitalist, Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Cerclage plus progesterone for preventing preterm births
DALLAS – Placing a cerclage conferred additional benefit over vaginal progesterone alone for women with extreme short cervixes and singleton pregnancies, in a recent study. Those who received rescue cerclage in addition to vaginal progesterone had a 92% overall reduction in spontaneous preterm birth rates, and infants had fewer neonatal ICU admissions and neonatal complications.
The single-center retrospective cohort study of 75 women with cervical length less than 10 cm who were receiving vaginal progesterone was presented by Christopher Enakpene, MD, at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Women who received rescue cerclage in addition to progesterone delivered at a mean 34.3 weeks gestational age, compared with 27.5 weeks for those receiving progesterone alone (P less than .001).
There were 13 neonatal ICU admissions for infants born to the 36 patients in the cerclage group, compared with 23 for the 39 women receiving progesterone alone (P = .009). And there were seven perinatal or neonatal deaths among the group who did not receive cerclage; no deaths occurred in the cerclage group.
“Premature cervical ripening plays a significant role in spontaneous preterm birth,” said Dr. Enakpene. “And studies have shown that, the shorter the cervix, the higher the risk of spontaneous preterm birth.”
Preterm birth, said Dr. Enakpene, is the top cause of neonatal morbidity and mortality both in the United States and worldwide; it is associated with 70% of neonatal deaths and half of all neurodevelopmental delay, he said.
At the University of Illinois at Chicago, where Dr. Enakpene is a maternal-fetal medicine fellow in the department of obstetrics and gynecology, all pregnant women are screened for cervical length by transvaginal ultrasound during their second trimester.
If screening reveals cervical length of less than 20 mm, the patient receives vaginal progesterone, “the current standard of care in singleton pregnancy with incidental short cervix,” said Dr. Enakpene. Vaginal progesterone can reduce the risk of spontaneous preterm birth by about half, he said.
At his facility, women with a short cervix then receive serial cervical length assessments every week or 2 until 24 weeks’ gestation, to identify women whose cervixes continue to shorten. For these women, it’s usually physician preference that determines whether cerclage is placed to reinforce the progressively shortening cervix, said Dr. Enakpene.
There had been previous retrospective work showing that cerclage was more effective at preventing spontaneous preterm birth than was doing nothing when a woman with a singleton pregnancy had a short cervix. However, Dr. Enakpene said that vaginal progesterone alone had not been compared with continuing progesterone and adding a cerclage.
During the study period, 310 women with cervical length of less than 20 mm were placed on progesterone. These women were included in the study cohort if, over the course of at least two cervical length measurements, their cervix shortened to less than 10 mm while they were on vaginal progesterone. They could not have indications that delivery was imminent when the 10 mm threshold was met, said Dr. Enakpene.
Women with known intrauterine fetal death, an undesired pregnancy, or any sign of intra-amniotic infection or fetal anomalies were excluded from the study.
A total of 75 women met final inclusion criteria, and received cerclage (n = 36), or not (n = 39) according to physician judgment. Demographic and pregnancy-specific characteristics were generally similar between the two groups.
In their statistical analysis, Dr. Enakpene and his colleagues used Kaplan-Meier survival analysis to look at pregnancy latency over time for women who did – or didn’t – receive a cerclage. There were significantly fewer spontaneous preterm births before 24, 28, and 34 weeks gestational age in the cerclage group (P less than .001 for all).
Dr. Enakpene acknowledged the study’s limitations, including its retrospective nature, the small sample size, and the fact that cerclage placement was done by the preference of the attending physician. However, this study was the first to examine rescue cerclage as add-on to vaginal progesterone, he said, adding, “Following this study, we are designing a prospective randomized interventional study to address this important topic.”
Dr. Enakpene reported that he had no conflicts of interest, and reported no outside sources of funding.
SOURCE: Enakpene C et al. Am J Obstet Gynecol. 2018 Jan;218:S72-S73.
DALLAS – Placing a cerclage conferred additional benefit over vaginal progesterone alone for women with extreme short cervixes and singleton pregnancies, in a recent study. Those who received rescue cerclage in addition to vaginal progesterone had a 92% overall reduction in spontaneous preterm birth rates, and infants had fewer neonatal ICU admissions and neonatal complications.
The single-center retrospective cohort study of 75 women with cervical length less than 10 cm who were receiving vaginal progesterone was presented by Christopher Enakpene, MD, at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Women who received rescue cerclage in addition to progesterone delivered at a mean 34.3 weeks gestational age, compared with 27.5 weeks for those receiving progesterone alone (P less than .001).
There were 13 neonatal ICU admissions for infants born to the 36 patients in the cerclage group, compared with 23 for the 39 women receiving progesterone alone (P = .009). And there were seven perinatal or neonatal deaths among the group who did not receive cerclage; no deaths occurred in the cerclage group.
“Premature cervical ripening plays a significant role in spontaneous preterm birth,” said Dr. Enakpene. “And studies have shown that, the shorter the cervix, the higher the risk of spontaneous preterm birth.”
Preterm birth, said Dr. Enakpene, is the top cause of neonatal morbidity and mortality both in the United States and worldwide; it is associated with 70% of neonatal deaths and half of all neurodevelopmental delay, he said.
At the University of Illinois at Chicago, where Dr. Enakpene is a maternal-fetal medicine fellow in the department of obstetrics and gynecology, all pregnant women are screened for cervical length by transvaginal ultrasound during their second trimester.
If screening reveals cervical length of less than 20 mm, the patient receives vaginal progesterone, “the current standard of care in singleton pregnancy with incidental short cervix,” said Dr. Enakpene. Vaginal progesterone can reduce the risk of spontaneous preterm birth by about half, he said.
At his facility, women with a short cervix then receive serial cervical length assessments every week or 2 until 24 weeks’ gestation, to identify women whose cervixes continue to shorten. For these women, it’s usually physician preference that determines whether cerclage is placed to reinforce the progressively shortening cervix, said Dr. Enakpene.
There had been previous retrospective work showing that cerclage was more effective at preventing spontaneous preterm birth than was doing nothing when a woman with a singleton pregnancy had a short cervix. However, Dr. Enakpene said that vaginal progesterone alone had not been compared with continuing progesterone and adding a cerclage.
During the study period, 310 women with cervical length of less than 20 mm were placed on progesterone. These women were included in the study cohort if, over the course of at least two cervical length measurements, their cervix shortened to less than 10 mm while they were on vaginal progesterone. They could not have indications that delivery was imminent when the 10 mm threshold was met, said Dr. Enakpene.
Women with known intrauterine fetal death, an undesired pregnancy, or any sign of intra-amniotic infection or fetal anomalies were excluded from the study.
A total of 75 women met final inclusion criteria, and received cerclage (n = 36), or not (n = 39) according to physician judgment. Demographic and pregnancy-specific characteristics were generally similar between the two groups.
In their statistical analysis, Dr. Enakpene and his colleagues used Kaplan-Meier survival analysis to look at pregnancy latency over time for women who did – or didn’t – receive a cerclage. There were significantly fewer spontaneous preterm births before 24, 28, and 34 weeks gestational age in the cerclage group (P less than .001 for all).
Dr. Enakpene acknowledged the study’s limitations, including its retrospective nature, the small sample size, and the fact that cerclage placement was done by the preference of the attending physician. However, this study was the first to examine rescue cerclage as add-on to vaginal progesterone, he said, adding, “Following this study, we are designing a prospective randomized interventional study to address this important topic.”
Dr. Enakpene reported that he had no conflicts of interest, and reported no outside sources of funding.
SOURCE: Enakpene C et al. Am J Obstet Gynecol. 2018 Jan;218:S72-S73.
DALLAS – Placing a cerclage conferred additional benefit over vaginal progesterone alone for women with extreme short cervixes and singleton pregnancies, in a recent study. Those who received rescue cerclage in addition to vaginal progesterone had a 92% overall reduction in spontaneous preterm birth rates, and infants had fewer neonatal ICU admissions and neonatal complications.
The single-center retrospective cohort study of 75 women with cervical length less than 10 cm who were receiving vaginal progesterone was presented by Christopher Enakpene, MD, at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Women who received rescue cerclage in addition to progesterone delivered at a mean 34.3 weeks gestational age, compared with 27.5 weeks for those receiving progesterone alone (P less than .001).
There were 13 neonatal ICU admissions for infants born to the 36 patients in the cerclage group, compared with 23 for the 39 women receiving progesterone alone (P = .009). And there were seven perinatal or neonatal deaths among the group who did not receive cerclage; no deaths occurred in the cerclage group.
“Premature cervical ripening plays a significant role in spontaneous preterm birth,” said Dr. Enakpene. “And studies have shown that, the shorter the cervix, the higher the risk of spontaneous preterm birth.”
Preterm birth, said Dr. Enakpene, is the top cause of neonatal morbidity and mortality both in the United States and worldwide; it is associated with 70% of neonatal deaths and half of all neurodevelopmental delay, he said.
At the University of Illinois at Chicago, where Dr. Enakpene is a maternal-fetal medicine fellow in the department of obstetrics and gynecology, all pregnant women are screened for cervical length by transvaginal ultrasound during their second trimester.
If screening reveals cervical length of less than 20 mm, the patient receives vaginal progesterone, “the current standard of care in singleton pregnancy with incidental short cervix,” said Dr. Enakpene. Vaginal progesterone can reduce the risk of spontaneous preterm birth by about half, he said.
At his facility, women with a short cervix then receive serial cervical length assessments every week or 2 until 24 weeks’ gestation, to identify women whose cervixes continue to shorten. For these women, it’s usually physician preference that determines whether cerclage is placed to reinforce the progressively shortening cervix, said Dr. Enakpene.
There had been previous retrospective work showing that cerclage was more effective at preventing spontaneous preterm birth than was doing nothing when a woman with a singleton pregnancy had a short cervix. However, Dr. Enakpene said that vaginal progesterone alone had not been compared with continuing progesterone and adding a cerclage.
During the study period, 310 women with cervical length of less than 20 mm were placed on progesterone. These women were included in the study cohort if, over the course of at least two cervical length measurements, their cervix shortened to less than 10 mm while they were on vaginal progesterone. They could not have indications that delivery was imminent when the 10 mm threshold was met, said Dr. Enakpene.
Women with known intrauterine fetal death, an undesired pregnancy, or any sign of intra-amniotic infection or fetal anomalies were excluded from the study.
A total of 75 women met final inclusion criteria, and received cerclage (n = 36), or not (n = 39) according to physician judgment. Demographic and pregnancy-specific characteristics were generally similar between the two groups.
In their statistical analysis, Dr. Enakpene and his colleagues used Kaplan-Meier survival analysis to look at pregnancy latency over time for women who did – or didn’t – receive a cerclage. There were significantly fewer spontaneous preterm births before 24, 28, and 34 weeks gestational age in the cerclage group (P less than .001 for all).
Dr. Enakpene acknowledged the study’s limitations, including its retrospective nature, the small sample size, and the fact that cerclage placement was done by the preference of the attending physician. However, this study was the first to examine rescue cerclage as add-on to vaginal progesterone, he said, adding, “Following this study, we are designing a prospective randomized interventional study to address this important topic.”
Dr. Enakpene reported that he had no conflicts of interest, and reported no outside sources of funding.
SOURCE: Enakpene C et al. Am J Obstet Gynecol. 2018 Jan;218:S72-S73.
REPORTING FROM THE PREGNANCY MEETING
Key clinical point: Women receiving rescue cerclage plus progesterone had fewer spontaneous preterm births.
Major finding: Those who also received cerclage delivered at 34.3 weeks gestational age, compared with 27.5 weeks for those on progesterone alone (P less than .001).
Study details: Retrospective cohort study of 75 women with singleton pregnancies and very short cervixes.
Disclosures: Dr. Enakpene reported no outside sources of funding and had no conflicts of interest.
Source: Enakpene C et al. Am J Obstet Gynecol. 2018 Jan;218:S72-S73.
Adil Harroud, MD
Katharina Eikermann-Haerter, MD
Jes Olesen, MD
States show large disparities in lung cancer mortality
, with the highest rate in West Virginia and the lowest in Utah.
Approximately 154,050 deaths from lung cancer – three times as many as any other cancer – are predicted for the year in the United States by the American Cancer Society in its Cancer Facts & Figures 2018, based on analysis of 2001-2015 data from the National Center for Health Statistics. That figure is down from the 155,870 predicted for 2017, as the most recent trend (2011-2015) in the death rate has been a decline of about 2.3% per year for women and 3.8% per year for men, the ACS noted.
The expected number of deaths for 2018, coupled with a current population estimate of nearly 326 million, works out to an expected death rate of 47.3 per a population of 100,000. The Census Bureau estimates for the state populations and the deaths projected by the ACS produce expected death rates of 80.8 per 100,000 for West Virginia and 15.2 for Utah. Kentucky’s rate of 79.3 is just behind West Virginia, but Colorado, the next-lowest state after Utah, has an estimated rate that’s almost twice as high at 28.5.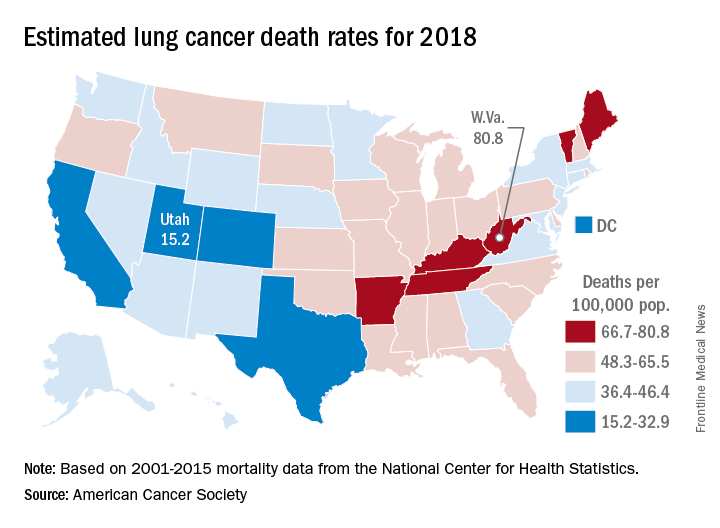
Nationally, death rates for lung cancer were 53.8 per 100,000 for males and 35.4 for females for 2011-2015, and incidence rates were 73 per 100,000 for males and 52.8 for females for 2010-2014, the ACS reported.
Among racial and ethnic groups, in men, the mortality was highest for those who were both non-Hispanic and black (66.9 per 100,00) during 2011-2015. Of the racial and ethnic groups of women for the same period, white women had the highest death rate (39). Hispanic/Latino men (26.4) and Hispanic/Latino women (13.3) had the lowest deaths rates, according to the report.
, with the highest rate in West Virginia and the lowest in Utah.
Approximately 154,050 deaths from lung cancer – three times as many as any other cancer – are predicted for the year in the United States by the American Cancer Society in its Cancer Facts & Figures 2018, based on analysis of 2001-2015 data from the National Center for Health Statistics. That figure is down from the 155,870 predicted for 2017, as the most recent trend (2011-2015) in the death rate has been a decline of about 2.3% per year for women and 3.8% per year for men, the ACS noted.
The expected number of deaths for 2018, coupled with a current population estimate of nearly 326 million, works out to an expected death rate of 47.3 per a population of 100,000. The Census Bureau estimates for the state populations and the deaths projected by the ACS produce expected death rates of 80.8 per 100,000 for West Virginia and 15.2 for Utah. Kentucky’s rate of 79.3 is just behind West Virginia, but Colorado, the next-lowest state after Utah, has an estimated rate that’s almost twice as high at 28.5.
Nationally, death rates for lung cancer were 53.8 per 100,000 for males and 35.4 for females for 2011-2015, and incidence rates were 73 per 100,000 for males and 52.8 for females for 2010-2014, the ACS reported.
Among racial and ethnic groups, in men, the mortality was highest for those who were both non-Hispanic and black (66.9 per 100,00) during 2011-2015. Of the racial and ethnic groups of women for the same period, white women had the highest death rate (39). Hispanic/Latino men (26.4) and Hispanic/Latino women (13.3) had the lowest deaths rates, according to the report.
, with the highest rate in West Virginia and the lowest in Utah.
Approximately 154,050 deaths from lung cancer – three times as many as any other cancer – are predicted for the year in the United States by the American Cancer Society in its Cancer Facts & Figures 2018, based on analysis of 2001-2015 data from the National Center for Health Statistics. That figure is down from the 155,870 predicted for 2017, as the most recent trend (2011-2015) in the death rate has been a decline of about 2.3% per year for women and 3.8% per year for men, the ACS noted.
The expected number of deaths for 2018, coupled with a current population estimate of nearly 326 million, works out to an expected death rate of 47.3 per a population of 100,000. The Census Bureau estimates for the state populations and the deaths projected by the ACS produce expected death rates of 80.8 per 100,000 for West Virginia and 15.2 for Utah. Kentucky’s rate of 79.3 is just behind West Virginia, but Colorado, the next-lowest state after Utah, has an estimated rate that’s almost twice as high at 28.5.
Nationally, death rates for lung cancer were 53.8 per 100,000 for males and 35.4 for females for 2011-2015, and incidence rates were 73 per 100,000 for males and 52.8 for females for 2010-2014, the ACS reported.
Among racial and ethnic groups, in men, the mortality was highest for those who were both non-Hispanic and black (66.9 per 100,00) during 2011-2015. Of the racial and ethnic groups of women for the same period, white women had the highest death rate (39). Hispanic/Latino men (26.4) and Hispanic/Latino women (13.3) had the lowest deaths rates, according to the report.









