User login
Team recommends changes to trial guidelines
Researchers have recommended changes to international guidelines used in the development of clinical trials.
The group’s goal is to improve the use of patient-reported outcomes (PROs)—feedback from patients about how the clinical trial has affected their overall health and quality of life.
“Patient-reported outcome data from clinical trials can provide valuable evidence to inform shared-decision making, pharmaceutical labeling claims, clinical guidelines, and health policy,” said Melanie Calvert, PhD, of the University of Birmingham in Edgbaston, Birmingham, England.
“However, clinical trial protocols often lack important information regarding the collection of quality of life and symptom data. Working in collaboration with international stakeholders, we have developed consensus-based, PRO-specific protocol guidance to help ensure high-quality data to inform patient-centered care.”
Dr Calvert and her colleagues published the guidance in JAMA.
The guidance is known as the SPIRIT-PRO Extension. The SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) statement, which was published in 2013, was intended to improve trial protocols by providing evidence-based recommendations for the minimum set of items to be addressed.
To add a PRO component to SPIRIT, Dr Calvert and her colleagues conducted a systematic review of existing PRO-specific protocol guidance. A taskforce used this data to compile a list of potential PRO-specific protocol items.
The review revealed 162 PRO-specific protocol recommendations, and the task force reduced this to 56. After 138 international stakeholders and 99 Delphi panelists weighed in, the final recommendations were agreed upon by 29 participants at a consensus meeting.
The final recommendations include 11 extensions and 5 elaborations to the SPIRIT checklist. The recommendations focus on PRO-specific issues relating to objectives, eligibility criteria, data collection, monitoring, and other aspects of trials.
“While this guidance has been developed for trials where PROs are a primary or key secondary outcome, we are actively encouraging protocol writers to consider use of this guidance in all trials or clinical research studies where PROs are collected,” Dr Calvert said.
“The guidance does not aim to be prescriptive, but instead to encourage and facilitate careful planning of PRO components of trials and thereby improve PRO trial design, which we hope will help staff and patients understand the rationale for PRO assessment, improve PRO data completeness and quality, facilitate high-quality analysis and reporting, and ultimately improve the quality of the global PRO evidence base.” ![]()
Researchers have recommended changes to international guidelines used in the development of clinical trials.
The group’s goal is to improve the use of patient-reported outcomes (PROs)—feedback from patients about how the clinical trial has affected their overall health and quality of life.
“Patient-reported outcome data from clinical trials can provide valuable evidence to inform shared-decision making, pharmaceutical labeling claims, clinical guidelines, and health policy,” said Melanie Calvert, PhD, of the University of Birmingham in Edgbaston, Birmingham, England.
“However, clinical trial protocols often lack important information regarding the collection of quality of life and symptom data. Working in collaboration with international stakeholders, we have developed consensus-based, PRO-specific protocol guidance to help ensure high-quality data to inform patient-centered care.”
Dr Calvert and her colleagues published the guidance in JAMA.
The guidance is known as the SPIRIT-PRO Extension. The SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) statement, which was published in 2013, was intended to improve trial protocols by providing evidence-based recommendations for the minimum set of items to be addressed.
To add a PRO component to SPIRIT, Dr Calvert and her colleagues conducted a systematic review of existing PRO-specific protocol guidance. A taskforce used this data to compile a list of potential PRO-specific protocol items.
The review revealed 162 PRO-specific protocol recommendations, and the task force reduced this to 56. After 138 international stakeholders and 99 Delphi panelists weighed in, the final recommendations were agreed upon by 29 participants at a consensus meeting.
The final recommendations include 11 extensions and 5 elaborations to the SPIRIT checklist. The recommendations focus on PRO-specific issues relating to objectives, eligibility criteria, data collection, monitoring, and other aspects of trials.
“While this guidance has been developed for trials where PROs are a primary or key secondary outcome, we are actively encouraging protocol writers to consider use of this guidance in all trials or clinical research studies where PROs are collected,” Dr Calvert said.
“The guidance does not aim to be prescriptive, but instead to encourage and facilitate careful planning of PRO components of trials and thereby improve PRO trial design, which we hope will help staff and patients understand the rationale for PRO assessment, improve PRO data completeness and quality, facilitate high-quality analysis and reporting, and ultimately improve the quality of the global PRO evidence base.” ![]()
Researchers have recommended changes to international guidelines used in the development of clinical trials.
The group’s goal is to improve the use of patient-reported outcomes (PROs)—feedback from patients about how the clinical trial has affected their overall health and quality of life.
“Patient-reported outcome data from clinical trials can provide valuable evidence to inform shared-decision making, pharmaceutical labeling claims, clinical guidelines, and health policy,” said Melanie Calvert, PhD, of the University of Birmingham in Edgbaston, Birmingham, England.
“However, clinical trial protocols often lack important information regarding the collection of quality of life and symptom data. Working in collaboration with international stakeholders, we have developed consensus-based, PRO-specific protocol guidance to help ensure high-quality data to inform patient-centered care.”
Dr Calvert and her colleagues published the guidance in JAMA.
The guidance is known as the SPIRIT-PRO Extension. The SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) statement, which was published in 2013, was intended to improve trial protocols by providing evidence-based recommendations for the minimum set of items to be addressed.
To add a PRO component to SPIRIT, Dr Calvert and her colleagues conducted a systematic review of existing PRO-specific protocol guidance. A taskforce used this data to compile a list of potential PRO-specific protocol items.
The review revealed 162 PRO-specific protocol recommendations, and the task force reduced this to 56. After 138 international stakeholders and 99 Delphi panelists weighed in, the final recommendations were agreed upon by 29 participants at a consensus meeting.
The final recommendations include 11 extensions and 5 elaborations to the SPIRIT checklist. The recommendations focus on PRO-specific issues relating to objectives, eligibility criteria, data collection, monitoring, and other aspects of trials.
“While this guidance has been developed for trials where PROs are a primary or key secondary outcome, we are actively encouraging protocol writers to consider use of this guidance in all trials or clinical research studies where PROs are collected,” Dr Calvert said.
“The guidance does not aim to be prescriptive, but instead to encourage and facilitate careful planning of PRO components of trials and thereby improve PRO trial design, which we hope will help staff and patients understand the rationale for PRO assessment, improve PRO data completeness and quality, facilitate high-quality analysis and reporting, and ultimately improve the quality of the global PRO evidence base.” ![]()
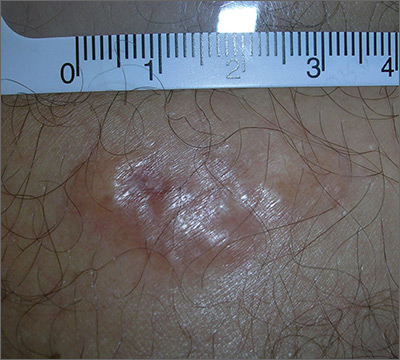
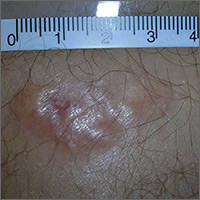
Painful growing lesion
The biopsy results indicated that the lesion was a dermatofibrosarcoma protuberans (DFSP)—a malignant fibrotic tumor of the skin and subcutaneous tissues. Note that the shiny surface and multilobular appearance can be characteristic of DFSP, but not all DFSPs present this way. Some may just present as a growing, firm, subcutaneous painful tumor.
DFSPs resemble a large irregular dermatofibroma, but a dermatofibroma is not a precursor to this. A DFSP is a separate malignant tumor, and not the result of a dermatofibroma that is growing out of control. While DFSPs typically do not metastasize, they can be locally aggressive, with a high recurrence rate after standard surgery. Because of this, the FP referred the patient to a Mohs surgeon to perform the excision. Mohs surgery allowed the surgeon to look at all margins of the lesion under a microscope to get the highest possible cure rate.
Two years later, this patient had no signs of regrowth. The most important lesson with this case was that one should not rely on incomplete biopsy sampling, especially when there is continued growth and symptoms that suggest an undiagnosed malignancy.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Usatine R. Dermatofibroma. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 935-939.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The biopsy results indicated that the lesion was a dermatofibrosarcoma protuberans (DFSP)—a malignant fibrotic tumor of the skin and subcutaneous tissues. Note that the shiny surface and multilobular appearance can be characteristic of DFSP, but not all DFSPs present this way. Some may just present as a growing, firm, subcutaneous painful tumor.
DFSPs resemble a large irregular dermatofibroma, but a dermatofibroma is not a precursor to this. A DFSP is a separate malignant tumor, and not the result of a dermatofibroma that is growing out of control. While DFSPs typically do not metastasize, they can be locally aggressive, with a high recurrence rate after standard surgery. Because of this, the FP referred the patient to a Mohs surgeon to perform the excision. Mohs surgery allowed the surgeon to look at all margins of the lesion under a microscope to get the highest possible cure rate.
Two years later, this patient had no signs of regrowth. The most important lesson with this case was that one should not rely on incomplete biopsy sampling, especially when there is continued growth and symptoms that suggest an undiagnosed malignancy.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Usatine R. Dermatofibroma. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 935-939.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The biopsy results indicated that the lesion was a dermatofibrosarcoma protuberans (DFSP)—a malignant fibrotic tumor of the skin and subcutaneous tissues. Note that the shiny surface and multilobular appearance can be characteristic of DFSP, but not all DFSPs present this way. Some may just present as a growing, firm, subcutaneous painful tumor.
DFSPs resemble a large irregular dermatofibroma, but a dermatofibroma is not a precursor to this. A DFSP is a separate malignant tumor, and not the result of a dermatofibroma that is growing out of control. While DFSPs typically do not metastasize, they can be locally aggressive, with a high recurrence rate after standard surgery. Because of this, the FP referred the patient to a Mohs surgeon to perform the excision. Mohs surgery allowed the surgeon to look at all margins of the lesion under a microscope to get the highest possible cure rate.
Two years later, this patient had no signs of regrowth. The most important lesson with this case was that one should not rely on incomplete biopsy sampling, especially when there is continued growth and symptoms that suggest an undiagnosed malignancy.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Usatine R. Dermatofibroma. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 935-939.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Could This Lesion Be Deadly?
ANSWER
The correct diagnosis is blue nevus (BN; choice “d”).
Some BNs can mimic melanomas (choice “a”), and vice versa. These cases often have to be sent to consultants, and sometimes even they can’t agree. For this reason, there is a low threshold for removal if there’s any question of change or family history of melanoma—which were missing in this case.
Kaposi sarcoma (KS; choice “b”) is a type of cancer caused by human herpesvirus-8 that affects the inner lining of blood vessels and manifests with dark macules or nodules. But outside the context of HIV/AIDS, KS is quite rare.
This lesion could have been an angioma (choice “c”), but its history and dark blue color made that diagnosis unlikely. In younger patients, angiomas are typically bright red. Only later, in the sixth and seventh decades of life, do they take on a dark appearance.
DISCUSSION
BNs are benign melanocytic nevi that typically appear on the trunk or upper extremities during the second or third decade of life. There are several varieties, the most common of which (and the type seen in this case) is Jadassohn-Tieche. The steel-blue color and planar surface of this patient’s lesion are typical.
Histologically, BNs are composed mostly of pigmented melanocytes that congregate deep in the dermis (unlike normal melanocytes, which typically line the dermo-epidermal junction). While these melanocytes are usually brown, they take on a bluish hue when they develop on a deeper level of skin—a phenomenon known as the Tyndall effect.
It can be challenging to differentiate a BN from a melanoma, even with a microscope. Sometimes the answer is to treat the lesion as though it were a melanoma by excising it with wide margins.
In this case, given the benign appearance and history, the decision was made to leave it alone unless it changes. Excision might have been a good option, but the patient’s type IV skin and the lesion’s location made scarring a likely possibility.
ANSWER
The correct diagnosis is blue nevus (BN; choice “d”).
Some BNs can mimic melanomas (choice “a”), and vice versa. These cases often have to be sent to consultants, and sometimes even they can’t agree. For this reason, there is a low threshold for removal if there’s any question of change or family history of melanoma—which were missing in this case.
Kaposi sarcoma (KS; choice “b”) is a type of cancer caused by human herpesvirus-8 that affects the inner lining of blood vessels and manifests with dark macules or nodules. But outside the context of HIV/AIDS, KS is quite rare.
This lesion could have been an angioma (choice “c”), but its history and dark blue color made that diagnosis unlikely. In younger patients, angiomas are typically bright red. Only later, in the sixth and seventh decades of life, do they take on a dark appearance.
DISCUSSION
BNs are benign melanocytic nevi that typically appear on the trunk or upper extremities during the second or third decade of life. There are several varieties, the most common of which (and the type seen in this case) is Jadassohn-Tieche. The steel-blue color and planar surface of this patient’s lesion are typical.
Histologically, BNs are composed mostly of pigmented melanocytes that congregate deep in the dermis (unlike normal melanocytes, which typically line the dermo-epidermal junction). While these melanocytes are usually brown, they take on a bluish hue when they develop on a deeper level of skin—a phenomenon known as the Tyndall effect.
It can be challenging to differentiate a BN from a melanoma, even with a microscope. Sometimes the answer is to treat the lesion as though it were a melanoma by excising it with wide margins.
In this case, given the benign appearance and history, the decision was made to leave it alone unless it changes. Excision might have been a good option, but the patient’s type IV skin and the lesion’s location made scarring a likely possibility.
ANSWER
The correct diagnosis is blue nevus (BN; choice “d”).
Some BNs can mimic melanomas (choice “a”), and vice versa. These cases often have to be sent to consultants, and sometimes even they can’t agree. For this reason, there is a low threshold for removal if there’s any question of change or family history of melanoma—which were missing in this case.
Kaposi sarcoma (KS; choice “b”) is a type of cancer caused by human herpesvirus-8 that affects the inner lining of blood vessels and manifests with dark macules or nodules. But outside the context of HIV/AIDS, KS is quite rare.
This lesion could have been an angioma (choice “c”), but its history and dark blue color made that diagnosis unlikely. In younger patients, angiomas are typically bright red. Only later, in the sixth and seventh decades of life, do they take on a dark appearance.
DISCUSSION
BNs are benign melanocytic nevi that typically appear on the trunk or upper extremities during the second or third decade of life. There are several varieties, the most common of which (and the type seen in this case) is Jadassohn-Tieche. The steel-blue color and planar surface of this patient’s lesion are typical.
Histologically, BNs are composed mostly of pigmented melanocytes that congregate deep in the dermis (unlike normal melanocytes, which typically line the dermo-epidermal junction). While these melanocytes are usually brown, they take on a bluish hue when they develop on a deeper level of skin—a phenomenon known as the Tyndall effect.
It can be challenging to differentiate a BN from a melanoma, even with a microscope. Sometimes the answer is to treat the lesion as though it were a melanoma by excising it with wide margins.
In this case, given the benign appearance and history, the decision was made to leave it alone unless it changes. Excision might have been a good option, but the patient’s type IV skin and the lesion’s location made scarring a likely possibility.
For years, this 24-year-old woman has had an asymptomatic lesion on her upper arm. Aside from growing a bit, as she has, it has remained basically unchanged.
The bluish black, intradermal, planar nodule is located on the lateral right deltoid. Barely palpable, the 7-mm lesion is neither tender nor particularly firm.
The patient is otherwise healthy. Her type IV skin shows little evidence of sun damage.
Survey highlights challenges in Asian American stroke patients
LOS ANGELES – A large survey of Asian Americans suggests that the group experiences more severe ischemic strokes and is less likely to receive intravenous tissue plasminogen activator (tPA) than do white patients, among other discrepancies. The research found that whites had declining stroke severity between 2004 and 2016, but there was no change in Asian Americans.
The research encompasses all self-identified Asian Americans in the Get-with-the-Guidelines stroke database, which is a voluntary stroke quality improvement program begun by the American Heart Association in 2003. The analysis included 64,337 Asian Americans and 1,707,962 white Americans at 2,171 hospitals nationwide that participated in the program during 2004-2016.
“I think the most important finding is that they’re not getting as much tPA and having more tPA complications, such as bleeding more. I think it gives it an urgency that maybe was lacking, an urgency that we really need to address this issue by finding innovative ways to reach Asian Americans, to educate them about stroke. We need to find culturally appropriate ways to reach out to Asian populations,” said Dr. Song, who is a vascular neurologist at Rush Medical College, Chicago.
Dr. Song is working on small-scale interventions that are culturally tailored for Asian populations. “I think the way to approach any insular community is to work from within, so that’s my goal,” Dr. Song said.
One particular finding suggested a need for better education among Asian American communities. Asian Americans were less likely than whites to report a clinical history of having heightened levels of low-density lipoproteins. “They didn’t know that they had high cholesterol, but they had a higher LDL [cholesterol levels] than Caucasians on average,” said Dr. Song. The mean LDL cholesterol value was 101 mg/dL in Asian Americans, compared with 95 mg/dL in whites, which was statistically significant.
White patients had higher rates of atrial fibrillation (21.2% vs. 16.0%), coronary artery disease (27.8% vs. 17.5%), and stenosis (4.7% vs. 2.0%), while Asian Americans were more prone to diabetes (38.0% vs. 29.2%).
Severe strokes (National Institutes of Health stroke score of 16 or greater) were more common among Asian Americans (odds ratio, 1.35; P less than .0001). After adjustment for stroke severity, the researchers found that Asian Americans were less likely to receive tPA (OR, 0.90; P less than .0001) and more likely to experience symptomatic intracerebral hemorrhage within 36 hours of receiving tPA (OR, 1.23; P = .003). “I think that may have something to do with the pathophysiology of Asian stroke that we don’t quite understand yet, but we can see there is a problem,” Dr. Song said.
Although in-hospital mortality was initially higher among Asian Americans, this trend switched after researchers corrected for stroke severity, leading to a better outcome for Asian Americans (OR, 0.95; P = .008). Some quality of care measures also favored Asian Americans, including receipt of stroke education (OR, 1.08; P = .02), receipt of IV tPA within 60 minutes of arrival (OR, 1.14; P = .0006), LDL cholesterol documentation (OR, 1.19; P less than .0001), and receipt of intensive statin therapy (OR, 1.15; P less than .0001). However, Asian Americans were less likely to receive a CT scan within 25 minutes of arrival (OR, 0.92; P less than .0001).
Between 2004 and 2016, both groups benefited from similar improvements, but there were differences. In 2016, a stroke in a white patient was less likely to be severe than in 2004 (OR, 0.97; P less than .0001), while there was no change in Asian Americans (OR, 1.00; P = .95).
The study is limited by the fact that the database is voluntary, which could lead to selection bias, and all Asian Americans are combined into one group. “One can argue that South Asian stroke is not the same as Japanese stroke or stroke in the Philippines,” Dr. Song said. Still, the findings suggest problems that need to be addressed. “I think it highlights the problem that Asian ischemic stroke patients don’t do as well, they bleed more, and they receive less tPA. I think that’s a big deal.”
The study received no specific funding. Dr. Song reported having no financial disclosures.
SOURCE: Song S et al. ISC 2018, Abstract TMP75.
LOS ANGELES – A large survey of Asian Americans suggests that the group experiences more severe ischemic strokes and is less likely to receive intravenous tissue plasminogen activator (tPA) than do white patients, among other discrepancies. The research found that whites had declining stroke severity between 2004 and 2016, but there was no change in Asian Americans.
The research encompasses all self-identified Asian Americans in the Get-with-the-Guidelines stroke database, which is a voluntary stroke quality improvement program begun by the American Heart Association in 2003. The analysis included 64,337 Asian Americans and 1,707,962 white Americans at 2,171 hospitals nationwide that participated in the program during 2004-2016.
“I think the most important finding is that they’re not getting as much tPA and having more tPA complications, such as bleeding more. I think it gives it an urgency that maybe was lacking, an urgency that we really need to address this issue by finding innovative ways to reach Asian Americans, to educate them about stroke. We need to find culturally appropriate ways to reach out to Asian populations,” said Dr. Song, who is a vascular neurologist at Rush Medical College, Chicago.
Dr. Song is working on small-scale interventions that are culturally tailored for Asian populations. “I think the way to approach any insular community is to work from within, so that’s my goal,” Dr. Song said.
One particular finding suggested a need for better education among Asian American communities. Asian Americans were less likely than whites to report a clinical history of having heightened levels of low-density lipoproteins. “They didn’t know that they had high cholesterol, but they had a higher LDL [cholesterol levels] than Caucasians on average,” said Dr. Song. The mean LDL cholesterol value was 101 mg/dL in Asian Americans, compared with 95 mg/dL in whites, which was statistically significant.
White patients had higher rates of atrial fibrillation (21.2% vs. 16.0%), coronary artery disease (27.8% vs. 17.5%), and stenosis (4.7% vs. 2.0%), while Asian Americans were more prone to diabetes (38.0% vs. 29.2%).
Severe strokes (National Institutes of Health stroke score of 16 or greater) were more common among Asian Americans (odds ratio, 1.35; P less than .0001). After adjustment for stroke severity, the researchers found that Asian Americans were less likely to receive tPA (OR, 0.90; P less than .0001) and more likely to experience symptomatic intracerebral hemorrhage within 36 hours of receiving tPA (OR, 1.23; P = .003). “I think that may have something to do with the pathophysiology of Asian stroke that we don’t quite understand yet, but we can see there is a problem,” Dr. Song said.
Although in-hospital mortality was initially higher among Asian Americans, this trend switched after researchers corrected for stroke severity, leading to a better outcome for Asian Americans (OR, 0.95; P = .008). Some quality of care measures also favored Asian Americans, including receipt of stroke education (OR, 1.08; P = .02), receipt of IV tPA within 60 minutes of arrival (OR, 1.14; P = .0006), LDL cholesterol documentation (OR, 1.19; P less than .0001), and receipt of intensive statin therapy (OR, 1.15; P less than .0001). However, Asian Americans were less likely to receive a CT scan within 25 minutes of arrival (OR, 0.92; P less than .0001).
Between 2004 and 2016, both groups benefited from similar improvements, but there were differences. In 2016, a stroke in a white patient was less likely to be severe than in 2004 (OR, 0.97; P less than .0001), while there was no change in Asian Americans (OR, 1.00; P = .95).
The study is limited by the fact that the database is voluntary, which could lead to selection bias, and all Asian Americans are combined into one group. “One can argue that South Asian stroke is not the same as Japanese stroke or stroke in the Philippines,” Dr. Song said. Still, the findings suggest problems that need to be addressed. “I think it highlights the problem that Asian ischemic stroke patients don’t do as well, they bleed more, and they receive less tPA. I think that’s a big deal.”
The study received no specific funding. Dr. Song reported having no financial disclosures.
SOURCE: Song S et al. ISC 2018, Abstract TMP75.
LOS ANGELES – A large survey of Asian Americans suggests that the group experiences more severe ischemic strokes and is less likely to receive intravenous tissue plasminogen activator (tPA) than do white patients, among other discrepancies. The research found that whites had declining stroke severity between 2004 and 2016, but there was no change in Asian Americans.
The research encompasses all self-identified Asian Americans in the Get-with-the-Guidelines stroke database, which is a voluntary stroke quality improvement program begun by the American Heart Association in 2003. The analysis included 64,337 Asian Americans and 1,707,962 white Americans at 2,171 hospitals nationwide that participated in the program during 2004-2016.
“I think the most important finding is that they’re not getting as much tPA and having more tPA complications, such as bleeding more. I think it gives it an urgency that maybe was lacking, an urgency that we really need to address this issue by finding innovative ways to reach Asian Americans, to educate them about stroke. We need to find culturally appropriate ways to reach out to Asian populations,” said Dr. Song, who is a vascular neurologist at Rush Medical College, Chicago.
Dr. Song is working on small-scale interventions that are culturally tailored for Asian populations. “I think the way to approach any insular community is to work from within, so that’s my goal,” Dr. Song said.
One particular finding suggested a need for better education among Asian American communities. Asian Americans were less likely than whites to report a clinical history of having heightened levels of low-density lipoproteins. “They didn’t know that they had high cholesterol, but they had a higher LDL [cholesterol levels] than Caucasians on average,” said Dr. Song. The mean LDL cholesterol value was 101 mg/dL in Asian Americans, compared with 95 mg/dL in whites, which was statistically significant.
White patients had higher rates of atrial fibrillation (21.2% vs. 16.0%), coronary artery disease (27.8% vs. 17.5%), and stenosis (4.7% vs. 2.0%), while Asian Americans were more prone to diabetes (38.0% vs. 29.2%).
Severe strokes (National Institutes of Health stroke score of 16 or greater) were more common among Asian Americans (odds ratio, 1.35; P less than .0001). After adjustment for stroke severity, the researchers found that Asian Americans were less likely to receive tPA (OR, 0.90; P less than .0001) and more likely to experience symptomatic intracerebral hemorrhage within 36 hours of receiving tPA (OR, 1.23; P = .003). “I think that may have something to do with the pathophysiology of Asian stroke that we don’t quite understand yet, but we can see there is a problem,” Dr. Song said.
Although in-hospital mortality was initially higher among Asian Americans, this trend switched after researchers corrected for stroke severity, leading to a better outcome for Asian Americans (OR, 0.95; P = .008). Some quality of care measures also favored Asian Americans, including receipt of stroke education (OR, 1.08; P = .02), receipt of IV tPA within 60 minutes of arrival (OR, 1.14; P = .0006), LDL cholesterol documentation (OR, 1.19; P less than .0001), and receipt of intensive statin therapy (OR, 1.15; P less than .0001). However, Asian Americans were less likely to receive a CT scan within 25 minutes of arrival (OR, 0.92; P less than .0001).
Between 2004 and 2016, both groups benefited from similar improvements, but there were differences. In 2016, a stroke in a white patient was less likely to be severe than in 2004 (OR, 0.97; P less than .0001), while there was no change in Asian Americans (OR, 1.00; P = .95).
The study is limited by the fact that the database is voluntary, which could lead to selection bias, and all Asian Americans are combined into one group. “One can argue that South Asian stroke is not the same as Japanese stroke or stroke in the Philippines,” Dr. Song said. Still, the findings suggest problems that need to be addressed. “I think it highlights the problem that Asian ischemic stroke patients don’t do as well, they bleed more, and they receive less tPA. I think that’s a big deal.”
The study received no specific funding. Dr. Song reported having no financial disclosures.
SOURCE: Song S et al. ISC 2018, Abstract TMP75.
REPORTING FROM ISC 2018
Key clinical point: Asian stroke patients don’t do as well as whites on some outcomes and quality measures.
Major finding: Asian Americans had a 35% higher frequency of severe stroke.
Data source: Retrospective analysis (n = 1,772,299).
Disclosures: The study received no specific funding. Dr. Song reported having no financial disclosures.
Source: Song S et al. ISC 2018, Abstract TMP75.
Duodenoscope redesign prompts voluntary recall by Pentax
Pentax has issued a voluntary recall for Pentax ED-3490TK duodenoscopes because of infections associated with reprocessed duodenoscopes, and the Food and Drug Administration has cleared the 510(k) to improve the device. The new design will, it is hoped, improve cleaning and disinfection for these devices.
“Reducing infections associated with duodenoscopes remains a top priority for the FDA, and we believe the new design changes to the Pentax duodenoscope will make these devices easier to clean and high-level disinfect to help enhance their safety,” said Suzanne Schwartz, MD, associate director for science and strategic partnerships at the FDA’s Center for Devices and Radiological Health. “We will continue to encourage new innovations for these devices to protect public health while enabling patients to have continued access to minimally invasive, life-saving endoscopy procedures.”
. In one study, even after double high-level disinfection or standard high-level disinfection followed by ethylene oxide gas sterilization, duodenoscopes had similar rates of contamination. These contamination events were associated with outbreaks of carbapenem-resistant Enterobacteriaceae infections. One of the culprits behind residual contamination may be the presence of biofilms, which are notoriously difficult to clean with standard disinfection methods.
Prior to the clearance of the elevator channel sealing mechanism, the first duodenoscope with a disposable distal cap was introduced, the Pentax ED34-i10T. The use of a disposable tip for the duodenoscope is meant to decrease the risk of future infections associated with these devices. The use of a disposable tip also improves cleaning and reprocessing of the duodenoscopes.
The FDA continues to work with manufacturers to improve the safety of duodenscopes and other reusable medical devices to protect patients from bacterial infections.
Pentax has issued a voluntary recall for Pentax ED-3490TK duodenoscopes because of infections associated with reprocessed duodenoscopes, and the Food and Drug Administration has cleared the 510(k) to improve the device. The new design will, it is hoped, improve cleaning and disinfection for these devices.
“Reducing infections associated with duodenoscopes remains a top priority for the FDA, and we believe the new design changes to the Pentax duodenoscope will make these devices easier to clean and high-level disinfect to help enhance their safety,” said Suzanne Schwartz, MD, associate director for science and strategic partnerships at the FDA’s Center for Devices and Radiological Health. “We will continue to encourage new innovations for these devices to protect public health while enabling patients to have continued access to minimally invasive, life-saving endoscopy procedures.”
. In one study, even after double high-level disinfection or standard high-level disinfection followed by ethylene oxide gas sterilization, duodenoscopes had similar rates of contamination. These contamination events were associated with outbreaks of carbapenem-resistant Enterobacteriaceae infections. One of the culprits behind residual contamination may be the presence of biofilms, which are notoriously difficult to clean with standard disinfection methods.
Prior to the clearance of the elevator channel sealing mechanism, the first duodenoscope with a disposable distal cap was introduced, the Pentax ED34-i10T. The use of a disposable tip for the duodenoscope is meant to decrease the risk of future infections associated with these devices. The use of a disposable tip also improves cleaning and reprocessing of the duodenoscopes.
The FDA continues to work with manufacturers to improve the safety of duodenscopes and other reusable medical devices to protect patients from bacterial infections.
Pentax has issued a voluntary recall for Pentax ED-3490TK duodenoscopes because of infections associated with reprocessed duodenoscopes, and the Food and Drug Administration has cleared the 510(k) to improve the device. The new design will, it is hoped, improve cleaning and disinfection for these devices.
“Reducing infections associated with duodenoscopes remains a top priority for the FDA, and we believe the new design changes to the Pentax duodenoscope will make these devices easier to clean and high-level disinfect to help enhance their safety,” said Suzanne Schwartz, MD, associate director for science and strategic partnerships at the FDA’s Center for Devices and Radiological Health. “We will continue to encourage new innovations for these devices to protect public health while enabling patients to have continued access to minimally invasive, life-saving endoscopy procedures.”
. In one study, even after double high-level disinfection or standard high-level disinfection followed by ethylene oxide gas sterilization, duodenoscopes had similar rates of contamination. These contamination events were associated with outbreaks of carbapenem-resistant Enterobacteriaceae infections. One of the culprits behind residual contamination may be the presence of biofilms, which are notoriously difficult to clean with standard disinfection methods.
Prior to the clearance of the elevator channel sealing mechanism, the first duodenoscope with a disposable distal cap was introduced, the Pentax ED34-i10T. The use of a disposable tip for the duodenoscope is meant to decrease the risk of future infections associated with these devices. The use of a disposable tip also improves cleaning and reprocessing of the duodenoscopes.
The FDA continues to work with manufacturers to improve the safety of duodenscopes and other reusable medical devices to protect patients from bacterial infections.
FDA approves complete combo tablet for HIV
The Food and Drug Administration announced that it has approved Symfi Lo tablets, a fixed-dose combination product containing efavirenz (400 mg), lamivudine (300 mg), and tenofovir disoproxil fumarate (300 mg, equivalent to 245 mg of tenofovir disoproxil). The tablets are indicated as a complete regimen for treating HIV-1 in adults and in pediatric patients weighing at least 35 kg.
The recommended dose is one tablet taken daily by mouth on an empty stomach, preferably at bedtime, as that may improve the tolerability of nervous system symptoms, according to an email release by the FDA Office of Health and Constituent Affairs.
The most common adverse reactions (in more than 5% of patients taking Symfi Lo) were rash and dizziness. The warnings and precautions contained in the label are: lactic acidosis/severe hepatomegaly with steatosis; new-onset or worsening renal impairment; and serious psychiatric symptoms, such as severe depression and suicidal ideation.
The FDA approval was primarily based upon the results of two randomized trials: Study 903 and Encore-1.
SOURCE: FDA email release and full label with prescribing information.
The Food and Drug Administration announced that it has approved Symfi Lo tablets, a fixed-dose combination product containing efavirenz (400 mg), lamivudine (300 mg), and tenofovir disoproxil fumarate (300 mg, equivalent to 245 mg of tenofovir disoproxil). The tablets are indicated as a complete regimen for treating HIV-1 in adults and in pediatric patients weighing at least 35 kg.
The recommended dose is one tablet taken daily by mouth on an empty stomach, preferably at bedtime, as that may improve the tolerability of nervous system symptoms, according to an email release by the FDA Office of Health and Constituent Affairs.
The most common adverse reactions (in more than 5% of patients taking Symfi Lo) were rash and dizziness. The warnings and precautions contained in the label are: lactic acidosis/severe hepatomegaly with steatosis; new-onset or worsening renal impairment; and serious psychiatric symptoms, such as severe depression and suicidal ideation.
The FDA approval was primarily based upon the results of two randomized trials: Study 903 and Encore-1.
SOURCE: FDA email release and full label with prescribing information.
The Food and Drug Administration announced that it has approved Symfi Lo tablets, a fixed-dose combination product containing efavirenz (400 mg), lamivudine (300 mg), and tenofovir disoproxil fumarate (300 mg, equivalent to 245 mg of tenofovir disoproxil). The tablets are indicated as a complete regimen for treating HIV-1 in adults and in pediatric patients weighing at least 35 kg.
The recommended dose is one tablet taken daily by mouth on an empty stomach, preferably at bedtime, as that may improve the tolerability of nervous system symptoms, according to an email release by the FDA Office of Health and Constituent Affairs.
The most common adverse reactions (in more than 5% of patients taking Symfi Lo) were rash and dizziness. The warnings and precautions contained in the label are: lactic acidosis/severe hepatomegaly with steatosis; new-onset or worsening renal impairment; and serious psychiatric symptoms, such as severe depression and suicidal ideation.
The FDA approval was primarily based upon the results of two randomized trials: Study 903 and Encore-1.
SOURCE: FDA email release and full label with prescribing information.
Pulmonary Fibrosis Foundation offers trial-finding app
The Pulmonary Fibrosis Foundation (PFF) has begun offering a tool to help patients navigate through more than 100 clinical trials aimed at advancing the treatment of pulmonary fibrosis, according to a statement from the Foundation.
The platform includes trials for patients with idiopathic pulmonary fibrosis.
“Before this, it was pretty impossible to search for clinical trials,” noted Bill Burke, a PF patient and support group leader from Williamsburg, Va., in the statement.
The tool, which is available both on the PFF website and as a free app, draws on information from ClinicalTrials.gov.
The Foundation intends for the new tool to give patients “a voice in their care process,” according to the statement.
“We want to empower patients to actively participate in identifying clinical trials,” said Harold R. Collard, MD, senior medical adviser of research and development for the Pulmonary Fibrosis Foundation.
More information about the trial finder is available on the PFF’s website.
The Pulmonary Fibrosis Foundation (PFF) has begun offering a tool to help patients navigate through more than 100 clinical trials aimed at advancing the treatment of pulmonary fibrosis, according to a statement from the Foundation.
The platform includes trials for patients with idiopathic pulmonary fibrosis.
“Before this, it was pretty impossible to search for clinical trials,” noted Bill Burke, a PF patient and support group leader from Williamsburg, Va., in the statement.
The tool, which is available both on the PFF website and as a free app, draws on information from ClinicalTrials.gov.
The Foundation intends for the new tool to give patients “a voice in their care process,” according to the statement.
“We want to empower patients to actively participate in identifying clinical trials,” said Harold R. Collard, MD, senior medical adviser of research and development for the Pulmonary Fibrosis Foundation.
More information about the trial finder is available on the PFF’s website.
The Pulmonary Fibrosis Foundation (PFF) has begun offering a tool to help patients navigate through more than 100 clinical trials aimed at advancing the treatment of pulmonary fibrosis, according to a statement from the Foundation.
The platform includes trials for patients with idiopathic pulmonary fibrosis.
“Before this, it was pretty impossible to search for clinical trials,” noted Bill Burke, a PF patient and support group leader from Williamsburg, Va., in the statement.
The tool, which is available both on the PFF website and as a free app, draws on information from ClinicalTrials.gov.
The Foundation intends for the new tool to give patients “a voice in their care process,” according to the statement.
“We want to empower patients to actively participate in identifying clinical trials,” said Harold R. Collard, MD, senior medical adviser of research and development for the Pulmonary Fibrosis Foundation.
More information about the trial finder is available on the PFF’s website.
Mogamulizumab active in HTLV-1–associated myelopathy
Mogamulizumab reduced the number of HTLV-1–infected cells and levels of inflammatory markers in patients with HTLV-1–associated myelopathy–tropical spastic paraparesis (HAM-TSP) in a recently reported phase 1-2a study.
Treatment with the anti-CCR4 monoclonal antibody also reduced spasticity and motor disability in some patients with HAM-TSP, according to results published in the New England Journal of Medicine.
Rash was the main side effect of treatment, but the 21-patient trial was “too small and too short to evaluate the clinical safety of mogamulizumab,” said lead author Tomoo Sato, MD, PhD, of the Department of Rare Diseases Research, St. Marianna University, Kawasaki, Japan, and colleagues.
HAM-TSP is a chronic, progressive, and debilitating neuroinflammatory disorder primarily seen in equatorial regions, according to the National Institute of Neurological Disorders and Stroke.
HTVL-1 infects primarily CCR4+ T cells, and mogamulizumab is an anti-CCR4 antibody that targets those affected cells, according to Dr. Sato and colleagues.
Current treatment for HAM-TSP is typically focused on suppressing inflammation with glucocorticoid or interferon-alpha rather than attacking infected cells and reducing proviral load.
The current investigator-initiated study included 21 patients with glucocorticoid-refractory HAM-TSP. In phase 1 of the study, patients received a single intravenous infusion of mogamulizumab and were observed for 85 days. Of those patients, 19 continued to phase 2a and received infusions at 8- or 12-week intervals for a total of 24 weeks.
Treatment resulted in a reduction of 64.9% (95% confidence interval, 51.7-78.1) in proviral load in peripheral-blood mononuclear cells by day 15 postinfusion. Reductions in inflammatory markers at day 29 were also reported, including a 37.3% decrease in CXCL10 levels and a 21.0% decrease in neopterin levels.
In all, 79% of patients had a reduction in spasticity, and 32% had decreased motor disability after treatment.
“The clinical effects appeared very quickly, long before any changes in the markers of inflammation in the central nervous system,” the investigators said.
Grade 1 or 2 rash was seen in 48% of patients, while lymphopenia and leukopenia were seen in 33% of patients each.
A phase 2 study is ongoing to evaluate the long-term safety and efficacy of mogamulizumab in patients with HAM-TSP.
The study was supported by the Japan Agency for Medical Research and Development and by the Ministry of Health, Labor, and Welfare. Two of the coauthors reported patents related to treating HTLV-I–related myelopathy.
SOURCE: Sato T et al. N Engl J Med. 2018;378:529-38.
Mogamulizumab reduced the number of HTLV-1–infected cells and levels of inflammatory markers in patients with HTLV-1–associated myelopathy–tropical spastic paraparesis (HAM-TSP) in a recently reported phase 1-2a study.
Treatment with the anti-CCR4 monoclonal antibody also reduced spasticity and motor disability in some patients with HAM-TSP, according to results published in the New England Journal of Medicine.
Rash was the main side effect of treatment, but the 21-patient trial was “too small and too short to evaluate the clinical safety of mogamulizumab,” said lead author Tomoo Sato, MD, PhD, of the Department of Rare Diseases Research, St. Marianna University, Kawasaki, Japan, and colleagues.
HAM-TSP is a chronic, progressive, and debilitating neuroinflammatory disorder primarily seen in equatorial regions, according to the National Institute of Neurological Disorders and Stroke.
HTVL-1 infects primarily CCR4+ T cells, and mogamulizumab is an anti-CCR4 antibody that targets those affected cells, according to Dr. Sato and colleagues.
Current treatment for HAM-TSP is typically focused on suppressing inflammation with glucocorticoid or interferon-alpha rather than attacking infected cells and reducing proviral load.
The current investigator-initiated study included 21 patients with glucocorticoid-refractory HAM-TSP. In phase 1 of the study, patients received a single intravenous infusion of mogamulizumab and were observed for 85 days. Of those patients, 19 continued to phase 2a and received infusions at 8- or 12-week intervals for a total of 24 weeks.
Treatment resulted in a reduction of 64.9% (95% confidence interval, 51.7-78.1) in proviral load in peripheral-blood mononuclear cells by day 15 postinfusion. Reductions in inflammatory markers at day 29 were also reported, including a 37.3% decrease in CXCL10 levels and a 21.0% decrease in neopterin levels.
In all, 79% of patients had a reduction in spasticity, and 32% had decreased motor disability after treatment.
“The clinical effects appeared very quickly, long before any changes in the markers of inflammation in the central nervous system,” the investigators said.
Grade 1 or 2 rash was seen in 48% of patients, while lymphopenia and leukopenia were seen in 33% of patients each.
A phase 2 study is ongoing to evaluate the long-term safety and efficacy of mogamulizumab in patients with HAM-TSP.
The study was supported by the Japan Agency for Medical Research and Development and by the Ministry of Health, Labor, and Welfare. Two of the coauthors reported patents related to treating HTLV-I–related myelopathy.
SOURCE: Sato T et al. N Engl J Med. 2018;378:529-38.
Mogamulizumab reduced the number of HTLV-1–infected cells and levels of inflammatory markers in patients with HTLV-1–associated myelopathy–tropical spastic paraparesis (HAM-TSP) in a recently reported phase 1-2a study.
Treatment with the anti-CCR4 monoclonal antibody also reduced spasticity and motor disability in some patients with HAM-TSP, according to results published in the New England Journal of Medicine.
Rash was the main side effect of treatment, but the 21-patient trial was “too small and too short to evaluate the clinical safety of mogamulizumab,” said lead author Tomoo Sato, MD, PhD, of the Department of Rare Diseases Research, St. Marianna University, Kawasaki, Japan, and colleagues.
HAM-TSP is a chronic, progressive, and debilitating neuroinflammatory disorder primarily seen in equatorial regions, according to the National Institute of Neurological Disorders and Stroke.
HTVL-1 infects primarily CCR4+ T cells, and mogamulizumab is an anti-CCR4 antibody that targets those affected cells, according to Dr. Sato and colleagues.
Current treatment for HAM-TSP is typically focused on suppressing inflammation with glucocorticoid or interferon-alpha rather than attacking infected cells and reducing proviral load.
The current investigator-initiated study included 21 patients with glucocorticoid-refractory HAM-TSP. In phase 1 of the study, patients received a single intravenous infusion of mogamulizumab and were observed for 85 days. Of those patients, 19 continued to phase 2a and received infusions at 8- or 12-week intervals for a total of 24 weeks.
Treatment resulted in a reduction of 64.9% (95% confidence interval, 51.7-78.1) in proviral load in peripheral-blood mononuclear cells by day 15 postinfusion. Reductions in inflammatory markers at day 29 were also reported, including a 37.3% decrease in CXCL10 levels and a 21.0% decrease in neopterin levels.
In all, 79% of patients had a reduction in spasticity, and 32% had decreased motor disability after treatment.
“The clinical effects appeared very quickly, long before any changes in the markers of inflammation in the central nervous system,” the investigators said.
Grade 1 or 2 rash was seen in 48% of patients, while lymphopenia and leukopenia were seen in 33% of patients each.
A phase 2 study is ongoing to evaluate the long-term safety and efficacy of mogamulizumab in patients with HAM-TSP.
The study was supported by the Japan Agency for Medical Research and Development and by the Ministry of Health, Labor, and Welfare. Two of the coauthors reported patents related to treating HTLV-I–related myelopathy.
SOURCE: Sato T et al. N Engl J Med. 2018;378:529-38.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Investigators reported a 64.9% reduction in proviral load by day 15 postinfusion and reductions in inflammatory markers by day 29.
Study details: An investigator-initiated, uncontrolled phase 1-2a study of mogamulizumab in 21 patients with glucocorticoid-refractory HAM-TSP.
Disclosures: The study was supported by the Japan Agency for Medical Research and Development and by the Ministry of Health, Labor, and Welfare. Two of the coauthors reported patents related to treating HTLV-I–related myelopathy.
Source: Sato T et al. N Engl J Med. 2018;378:529-38.
High patient activation linked to clinical remission in IBD
LAS VEGAS – results from a longitudinal analysis suggest.
“Patient activation is defined as understanding one’s role in the health care process and having the knowledge, skills, and confidence to manage one’s health,” Edward L. Barnes, MD, MPH, said at the Crohn’s & Colitis Congress, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. “It emphasizes an individual’s willingness to take independent actions and manage their own health care. In many chronic conditions, higher levels of patient activation have been linked to improved health outcomes, better patient experiences related to health care, higher quality of life scores, and lower overall health care costs.”
A 13-question survey known as the Patient Activation Measure from Insignia Health can be used to assess patient activation (Health Serv Res 2005;4096 Pt 1:1918-30). This measure is scored from zero to 100 and allows the categorization of individuals into four levels of activation. In level 1, the patient believes an active role is important. In level 2, the patient has the confidence and knowledge to take action. In level 3 the patient takes action, and in level 4, the patient stays the course during stress.
Dr. Barnes and his associates set out to evaluate the demographic and clinical characteristics associated with higher patient activation in patients with IBD. A secondary aim was to determine whether higher levels of patient care are associated with decreased frequency of relapse or flare. They performed a prospective cohort study of individuals who participated in the Crohn’s and Colitis Foundation’s Partners Internet cohort. Consecutive participants who completed a Partners survey between June 2, 2016, and Jan. 5, 2017, were asked to complete the Patient Activation Measure as an optional module. Clinical remission was defined via the short Crohn’s Disease Activity Index (a score of 150 or lower) and the Simple Clinical Colitis Activity Index (a score of 2 or less).
High patient activation was defined as level 3 or level 4 on the Patient Activation Measure, and multivariable logistic regression was used to evaluate predictors of patient activation level and the relationship between level of patient activation and clinical remission. All covariates included in the multivariable analyses were identified a priori based on prior association with patient activation or clinical disease activity in IBD.
The survey was administered to 1,486 participants. Of these, 1,082 (73%) completed follow-up surveys, including assessments of disease activity. The mean age of respondents was 44 years, 74% were female, 5% were nonwhite, and 77% reported their highest education level as college or graduate school. The mean disease duration was 14.4 years.
Patients with less than a 12th grade education were significantly associated with a decreased odds of having patient activation (adjusted odds ratio 0.25 [95% confidence interval, 0.07-0.94]). Although nonsignificant after adjustment for potential confounders, nonwhite race was also associated with decreased odds of high patient activation (aOR 0.64). Meanwhile, there was a trend among those who graduated from college or graduate school in predicting high patient activation level (aOR of 1.44 and 1.36, respectively).
After adjustment for race, educational status, time since diagnosis, smoking status, and history of IBD-related surgery among patients with Crohn’s disease, patients with higher patient activation were more likely to be in clinical remission at follow-up for both Crohn’s disease (71% vs. 62%; aOR of 1.60 [95% CI, 1.00-2.57], P = .05) and ulcerative colitis (54% vs. 34%; aOR 2.23, respectively; [95% CI, 1.15-4.19], P = .01).
Dr. Barnes acknowledged certain limitations of the study, including the fact that study participants comprised a voluntary, Internet-based cohort. “Participants may exhibit higher levels of patient activation than the general population of patients with IBD,” he said. “There may be an overrepresentation of college graduates in this sample, and the racial and ethnic makeup of this cohort may be different from that of a clinic-based population or the general population of patients with IBD.” He added that there might be unmeasured confounders in the relationship between patient activation and remission that the researchers could not assess.
“Patient activation appears to impact the disease course in patients with CD [Crohn’s disease] and UC [ulcerative colitis],” Dr. Barnes concluded. “The effect of patient activation on the disease course may be larger in UC than in CD. Efforts to improve patient activation in patients with IBD may have the ability to ultimately improve clinical outcomes.”
He reported having no financial disclosures.
*This story was updated on 3/26.
SOURCE: Barnes EL et al. Crohn’s & Colitis Congress, Clinical Abstract 12.
LAS VEGAS – results from a longitudinal analysis suggest.
“Patient activation is defined as understanding one’s role in the health care process and having the knowledge, skills, and confidence to manage one’s health,” Edward L. Barnes, MD, MPH, said at the Crohn’s & Colitis Congress, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. “It emphasizes an individual’s willingness to take independent actions and manage their own health care. In many chronic conditions, higher levels of patient activation have been linked to improved health outcomes, better patient experiences related to health care, higher quality of life scores, and lower overall health care costs.”
A 13-question survey known as the Patient Activation Measure from Insignia Health can be used to assess patient activation (Health Serv Res 2005;4096 Pt 1:1918-30). This measure is scored from zero to 100 and allows the categorization of individuals into four levels of activation. In level 1, the patient believes an active role is important. In level 2, the patient has the confidence and knowledge to take action. In level 3 the patient takes action, and in level 4, the patient stays the course during stress.
Dr. Barnes and his associates set out to evaluate the demographic and clinical characteristics associated with higher patient activation in patients with IBD. A secondary aim was to determine whether higher levels of patient care are associated with decreased frequency of relapse or flare. They performed a prospective cohort study of individuals who participated in the Crohn’s and Colitis Foundation’s Partners Internet cohort. Consecutive participants who completed a Partners survey between June 2, 2016, and Jan. 5, 2017, were asked to complete the Patient Activation Measure as an optional module. Clinical remission was defined via the short Crohn’s Disease Activity Index (a score of 150 or lower) and the Simple Clinical Colitis Activity Index (a score of 2 or less).
High patient activation was defined as level 3 or level 4 on the Patient Activation Measure, and multivariable logistic regression was used to evaluate predictors of patient activation level and the relationship between level of patient activation and clinical remission. All covariates included in the multivariable analyses were identified a priori based on prior association with patient activation or clinical disease activity in IBD.
The survey was administered to 1,486 participants. Of these, 1,082 (73%) completed follow-up surveys, including assessments of disease activity. The mean age of respondents was 44 years, 74% were female, 5% were nonwhite, and 77% reported their highest education level as college or graduate school. The mean disease duration was 14.4 years.
Patients with less than a 12th grade education were significantly associated with a decreased odds of having patient activation (adjusted odds ratio 0.25 [95% confidence interval, 0.07-0.94]). Although nonsignificant after adjustment for potential confounders, nonwhite race was also associated with decreased odds of high patient activation (aOR 0.64). Meanwhile, there was a trend among those who graduated from college or graduate school in predicting high patient activation level (aOR of 1.44 and 1.36, respectively).
After adjustment for race, educational status, time since diagnosis, smoking status, and history of IBD-related surgery among patients with Crohn’s disease, patients with higher patient activation were more likely to be in clinical remission at follow-up for both Crohn’s disease (71% vs. 62%; aOR of 1.60 [95% CI, 1.00-2.57], P = .05) and ulcerative colitis (54% vs. 34%; aOR 2.23, respectively; [95% CI, 1.15-4.19], P = .01).
Dr. Barnes acknowledged certain limitations of the study, including the fact that study participants comprised a voluntary, Internet-based cohort. “Participants may exhibit higher levels of patient activation than the general population of patients with IBD,” he said. “There may be an overrepresentation of college graduates in this sample, and the racial and ethnic makeup of this cohort may be different from that of a clinic-based population or the general population of patients with IBD.” He added that there might be unmeasured confounders in the relationship between patient activation and remission that the researchers could not assess.
“Patient activation appears to impact the disease course in patients with CD [Crohn’s disease] and UC [ulcerative colitis],” Dr. Barnes concluded. “The effect of patient activation on the disease course may be larger in UC than in CD. Efforts to improve patient activation in patients with IBD may have the ability to ultimately improve clinical outcomes.”
He reported having no financial disclosures.
*This story was updated on 3/26.
SOURCE: Barnes EL et al. Crohn’s & Colitis Congress, Clinical Abstract 12.
LAS VEGAS – results from a longitudinal analysis suggest.
“Patient activation is defined as understanding one’s role in the health care process and having the knowledge, skills, and confidence to manage one’s health,” Edward L. Barnes, MD, MPH, said at the Crohn’s & Colitis Congress, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. “It emphasizes an individual’s willingness to take independent actions and manage their own health care. In many chronic conditions, higher levels of patient activation have been linked to improved health outcomes, better patient experiences related to health care, higher quality of life scores, and lower overall health care costs.”
A 13-question survey known as the Patient Activation Measure from Insignia Health can be used to assess patient activation (Health Serv Res 2005;4096 Pt 1:1918-30). This measure is scored from zero to 100 and allows the categorization of individuals into four levels of activation. In level 1, the patient believes an active role is important. In level 2, the patient has the confidence and knowledge to take action. In level 3 the patient takes action, and in level 4, the patient stays the course during stress.
Dr. Barnes and his associates set out to evaluate the demographic and clinical characteristics associated with higher patient activation in patients with IBD. A secondary aim was to determine whether higher levels of patient care are associated with decreased frequency of relapse or flare. They performed a prospective cohort study of individuals who participated in the Crohn’s and Colitis Foundation’s Partners Internet cohort. Consecutive participants who completed a Partners survey between June 2, 2016, and Jan. 5, 2017, were asked to complete the Patient Activation Measure as an optional module. Clinical remission was defined via the short Crohn’s Disease Activity Index (a score of 150 or lower) and the Simple Clinical Colitis Activity Index (a score of 2 or less).
High patient activation was defined as level 3 or level 4 on the Patient Activation Measure, and multivariable logistic regression was used to evaluate predictors of patient activation level and the relationship between level of patient activation and clinical remission. All covariates included in the multivariable analyses were identified a priori based on prior association with patient activation or clinical disease activity in IBD.
The survey was administered to 1,486 participants. Of these, 1,082 (73%) completed follow-up surveys, including assessments of disease activity. The mean age of respondents was 44 years, 74% were female, 5% were nonwhite, and 77% reported their highest education level as college or graduate school. The mean disease duration was 14.4 years.
Patients with less than a 12th grade education were significantly associated with a decreased odds of having patient activation (adjusted odds ratio 0.25 [95% confidence interval, 0.07-0.94]). Although nonsignificant after adjustment for potential confounders, nonwhite race was also associated with decreased odds of high patient activation (aOR 0.64). Meanwhile, there was a trend among those who graduated from college or graduate school in predicting high patient activation level (aOR of 1.44 and 1.36, respectively).
After adjustment for race, educational status, time since diagnosis, smoking status, and history of IBD-related surgery among patients with Crohn’s disease, patients with higher patient activation were more likely to be in clinical remission at follow-up for both Crohn’s disease (71% vs. 62%; aOR of 1.60 [95% CI, 1.00-2.57], P = .05) and ulcerative colitis (54% vs. 34%; aOR 2.23, respectively; [95% CI, 1.15-4.19], P = .01).
Dr. Barnes acknowledged certain limitations of the study, including the fact that study participants comprised a voluntary, Internet-based cohort. “Participants may exhibit higher levels of patient activation than the general population of patients with IBD,” he said. “There may be an overrepresentation of college graduates in this sample, and the racial and ethnic makeup of this cohort may be different from that of a clinic-based population or the general population of patients with IBD.” He added that there might be unmeasured confounders in the relationship between patient activation and remission that the researchers could not assess.
“Patient activation appears to impact the disease course in patients with CD [Crohn’s disease] and UC [ulcerative colitis],” Dr. Barnes concluded. “The effect of patient activation on the disease course may be larger in UC than in CD. Efforts to improve patient activation in patients with IBD may have the ability to ultimately improve clinical outcomes.”
He reported having no financial disclosures.
*This story was updated on 3/26.
SOURCE: Barnes EL et al. Crohn’s & Colitis Congress, Clinical Abstract 12.
REPORTING FROM THE CROHN’S & COLITIS CONGRESS
Key clinical point: Patient activation appears to impact the disease course in IBD patients.
Major finding: Individuals with higher patient activation were more likely to be in clinical remission at follow-up for both CD and UC (adjusted OR of 1.60 vs. adjusted OR of 2.23, respectively).
Study details: Responses from 1,082 IBD patients who participated in the Crohn’s and Colitis Foundation’s Partners Internet cohort.
Disclosures: Dr. Barnes reported having no financial disclosures.
Source: Barnes EL et al. Crohn’s & Colitis Congress, Clinical Abstract 12.
On-label stent use looks safe in intracranial atherosclerotic disease
LOS ANGELES – A postmarketing study of the Wingspan stent shows that the safety of the device in the treatment of intracranial atherosclerotic disease (ICAD) is good enough to be a reasonable alternative to medical management in these patients, but only if the device is used on label.
The results may reassure some interventionalists who were alarmed by results from the Stenting versus Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial (N Engl J Med. 2011;365:993-1003), which showed a 30-day rate of stroke or death of 14.7%. The new study showed a frequency of 2.6%, so long as the device was used on label. Off-label use yielded a frequency of 23.9%.
Up to 10% of strokes in the United States result from ICAD, and in China the rate is an estimated 20%-46%. The condition can also be treated medically. Early trials of the Wingspan device showed initial success with complication rates of 4.5%-6.2%, but the SAMMPRIS trial, which directly compared stenting to aggressive medical management, showed superior outcomes with medical treatment. The 30-day rate of stroke or death of 14.7% was too high to compete with medical therapy, which included aspirin 325 mg per day, clopidogrel 75 mg per day for 90 days after enrollment, and management of primary and secondary risk factors.
Dr. Alexander believes that the SAMMPRIS trial did not employ favorable patient selection. “ICAD is variable. Some patients present with hemodynamic compromise, where stenting is probably beneficial. Some present with embolic stroke, and some present with small-vessel perforator strokes that are unlikely to be responsive to stenting and better treated with medical therapy. All these patients were grouped together” in SAMMPRIS, said Dr. Alexander, who is director of the Neurovascular Center and endovascular surgery at Cedars-Sinai in Los Angeles.
The SAMMPRIS findings put a damper on stenting, and use of the procedure has dropped at many U.S. hospitals. But studies conducted prior to SAMMPRIS had shown much lower periprocedural morbidity, and those studies looked at on-label use of stenting. SAMMPRIS was off label.
Now the WEAVE study, which was an Food and Drug Administration–mandated, postmarketing surveillance study of Stryker’s Wingspan stent, suggests that the off-label use of the system in the SAMMPRIS trial may explain the poor results. SAMMPRIS had attempted to extend the approved boundaries of stenting by treating patients who presented with transient ischemic attacks only, patients who had failed medical therapy, and patients who had experienced a stroke in the past 7 days. In fact, about half of the patients were treated within 7 days of the previous event, sometimes within 24 hours.
Previous studies had shown that risk factors for poor outcomes included stenting within 10 days of the last event, stenting a posterior circulation target lesion, stenting presentations other than stroke, and sites with a low patient volume for stenting. Patient selection is vital to success, according to Dr. Alexander. Patients with hemodynamic compromise are good candidates for stenting, while those with perforator stroke alone are better off with medical therapy. Embolic stroke patients are candidates for either approach.
WEAVE looked at 152 consecutive patients treated on label at 24 institutions. The primary analysis group consisted of patients with a 70%-99% stenotic intracranial atherosclerotic lesion who were refractory to medical treatment, 22-80 years of age, and had a modified Rankin Scale (mRS) score of 3 or less at baseline. The treatment was performed at least 7 days after the last stroke. Finally, patients had to have experienced two or more strokes. This last requirement presented a problem for recruitment, according to Dr. Alexander. “That was never a criterion for any of the [previous] trials, so it’s not clear why FDA added that. That made it very difficult to enroll for this trial – to have patients who had two or more strokes and still had a functional mRS score,” he said.
The study protocol aimed for a frequency of 6.6% for periprocedural morbidity, defined as a stroke or death within 72 hours.
An interim analysis at 100 patients showed that the periprocedural morbidity frequency was below 4%, which met the agency’s requirement and allowed the trial to be halted once the trial enrolled 150 on-label patients. The total number of on-label patients reached 152, and the researchers analyzed the results from another 46 patients who were treated with stenting despite not meeting the study’s inclusion criteria, and these patients were considered to be off-label use. The final analysis showed that the on-label group had a periprocedural morbidity of 2.6%, compared with 23.9% in the off-label group (P = .0001).
The most glaring difference in the patient populations was that half of the off-label group received the stent within 7 days of experiencing a stroke. What might be the reason for worse outcomes when stenting is performed within 7 days? “There’s speculation that the plaques might be hot, and those patients might have a higher thrombotic risk with putting a foreign body in the vessel, or there’s capillary instability, so reperfusing a vessel that has a 99% stenosis has a higher risk for reperfusion hemorrhage,” Dr. Alexander said.
Experience may also be a factor. Interventionalists participating in the WEAVE trial had performed a stent using Wingspan an average of 37 times before the study began, compared with a mean of 10 cases for physicians in the SAMMPRIS trial. Those who had performed over 50 procedures had no periprocedural morbidity outcomes at all.
The study was funded by Stryker Neurovascular. Dr. Alexander has consulted for Stryker.
SOURCE: Alexander M et al. ISC 2018 Abstract LB13.
LOS ANGELES – A postmarketing study of the Wingspan stent shows that the safety of the device in the treatment of intracranial atherosclerotic disease (ICAD) is good enough to be a reasonable alternative to medical management in these patients, but only if the device is used on label.
The results may reassure some interventionalists who were alarmed by results from the Stenting versus Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial (N Engl J Med. 2011;365:993-1003), which showed a 30-day rate of stroke or death of 14.7%. The new study showed a frequency of 2.6%, so long as the device was used on label. Off-label use yielded a frequency of 23.9%.
Up to 10% of strokes in the United States result from ICAD, and in China the rate is an estimated 20%-46%. The condition can also be treated medically. Early trials of the Wingspan device showed initial success with complication rates of 4.5%-6.2%, but the SAMMPRIS trial, which directly compared stenting to aggressive medical management, showed superior outcomes with medical treatment. The 30-day rate of stroke or death of 14.7% was too high to compete with medical therapy, which included aspirin 325 mg per day, clopidogrel 75 mg per day for 90 days after enrollment, and management of primary and secondary risk factors.
Dr. Alexander believes that the SAMMPRIS trial did not employ favorable patient selection. “ICAD is variable. Some patients present with hemodynamic compromise, where stenting is probably beneficial. Some present with embolic stroke, and some present with small-vessel perforator strokes that are unlikely to be responsive to stenting and better treated with medical therapy. All these patients were grouped together” in SAMMPRIS, said Dr. Alexander, who is director of the Neurovascular Center and endovascular surgery at Cedars-Sinai in Los Angeles.
The SAMMPRIS findings put a damper on stenting, and use of the procedure has dropped at many U.S. hospitals. But studies conducted prior to SAMMPRIS had shown much lower periprocedural morbidity, and those studies looked at on-label use of stenting. SAMMPRIS was off label.
Now the WEAVE study, which was an Food and Drug Administration–mandated, postmarketing surveillance study of Stryker’s Wingspan stent, suggests that the off-label use of the system in the SAMMPRIS trial may explain the poor results. SAMMPRIS had attempted to extend the approved boundaries of stenting by treating patients who presented with transient ischemic attacks only, patients who had failed medical therapy, and patients who had experienced a stroke in the past 7 days. In fact, about half of the patients were treated within 7 days of the previous event, sometimes within 24 hours.
Previous studies had shown that risk factors for poor outcomes included stenting within 10 days of the last event, stenting a posterior circulation target lesion, stenting presentations other than stroke, and sites with a low patient volume for stenting. Patient selection is vital to success, according to Dr. Alexander. Patients with hemodynamic compromise are good candidates for stenting, while those with perforator stroke alone are better off with medical therapy. Embolic stroke patients are candidates for either approach.
WEAVE looked at 152 consecutive patients treated on label at 24 institutions. The primary analysis group consisted of patients with a 70%-99% stenotic intracranial atherosclerotic lesion who were refractory to medical treatment, 22-80 years of age, and had a modified Rankin Scale (mRS) score of 3 or less at baseline. The treatment was performed at least 7 days after the last stroke. Finally, patients had to have experienced two or more strokes. This last requirement presented a problem for recruitment, according to Dr. Alexander. “That was never a criterion for any of the [previous] trials, so it’s not clear why FDA added that. That made it very difficult to enroll for this trial – to have patients who had two or more strokes and still had a functional mRS score,” he said.
The study protocol aimed for a frequency of 6.6% for periprocedural morbidity, defined as a stroke or death within 72 hours.
An interim analysis at 100 patients showed that the periprocedural morbidity frequency was below 4%, which met the agency’s requirement and allowed the trial to be halted once the trial enrolled 150 on-label patients. The total number of on-label patients reached 152, and the researchers analyzed the results from another 46 patients who were treated with stenting despite not meeting the study’s inclusion criteria, and these patients were considered to be off-label use. The final analysis showed that the on-label group had a periprocedural morbidity of 2.6%, compared with 23.9% in the off-label group (P = .0001).
The most glaring difference in the patient populations was that half of the off-label group received the stent within 7 days of experiencing a stroke. What might be the reason for worse outcomes when stenting is performed within 7 days? “There’s speculation that the plaques might be hot, and those patients might have a higher thrombotic risk with putting a foreign body in the vessel, or there’s capillary instability, so reperfusing a vessel that has a 99% stenosis has a higher risk for reperfusion hemorrhage,” Dr. Alexander said.
Experience may also be a factor. Interventionalists participating in the WEAVE trial had performed a stent using Wingspan an average of 37 times before the study began, compared with a mean of 10 cases for physicians in the SAMMPRIS trial. Those who had performed over 50 procedures had no periprocedural morbidity outcomes at all.
The study was funded by Stryker Neurovascular. Dr. Alexander has consulted for Stryker.
SOURCE: Alexander M et al. ISC 2018 Abstract LB13.
LOS ANGELES – A postmarketing study of the Wingspan stent shows that the safety of the device in the treatment of intracranial atherosclerotic disease (ICAD) is good enough to be a reasonable alternative to medical management in these patients, but only if the device is used on label.
The results may reassure some interventionalists who were alarmed by results from the Stenting versus Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial (N Engl J Med. 2011;365:993-1003), which showed a 30-day rate of stroke or death of 14.7%. The new study showed a frequency of 2.6%, so long as the device was used on label. Off-label use yielded a frequency of 23.9%.
Up to 10% of strokes in the United States result from ICAD, and in China the rate is an estimated 20%-46%. The condition can also be treated medically. Early trials of the Wingspan device showed initial success with complication rates of 4.5%-6.2%, but the SAMMPRIS trial, which directly compared stenting to aggressive medical management, showed superior outcomes with medical treatment. The 30-day rate of stroke or death of 14.7% was too high to compete with medical therapy, which included aspirin 325 mg per day, clopidogrel 75 mg per day for 90 days after enrollment, and management of primary and secondary risk factors.
Dr. Alexander believes that the SAMMPRIS trial did not employ favorable patient selection. “ICAD is variable. Some patients present with hemodynamic compromise, where stenting is probably beneficial. Some present with embolic stroke, and some present with small-vessel perforator strokes that are unlikely to be responsive to stenting and better treated with medical therapy. All these patients were grouped together” in SAMMPRIS, said Dr. Alexander, who is director of the Neurovascular Center and endovascular surgery at Cedars-Sinai in Los Angeles.
The SAMMPRIS findings put a damper on stenting, and use of the procedure has dropped at many U.S. hospitals. But studies conducted prior to SAMMPRIS had shown much lower periprocedural morbidity, and those studies looked at on-label use of stenting. SAMMPRIS was off label.
Now the WEAVE study, which was an Food and Drug Administration–mandated, postmarketing surveillance study of Stryker’s Wingspan stent, suggests that the off-label use of the system in the SAMMPRIS trial may explain the poor results. SAMMPRIS had attempted to extend the approved boundaries of stenting by treating patients who presented with transient ischemic attacks only, patients who had failed medical therapy, and patients who had experienced a stroke in the past 7 days. In fact, about half of the patients were treated within 7 days of the previous event, sometimes within 24 hours.
Previous studies had shown that risk factors for poor outcomes included stenting within 10 days of the last event, stenting a posterior circulation target lesion, stenting presentations other than stroke, and sites with a low patient volume for stenting. Patient selection is vital to success, according to Dr. Alexander. Patients with hemodynamic compromise are good candidates for stenting, while those with perforator stroke alone are better off with medical therapy. Embolic stroke patients are candidates for either approach.
WEAVE looked at 152 consecutive patients treated on label at 24 institutions. The primary analysis group consisted of patients with a 70%-99% stenotic intracranial atherosclerotic lesion who were refractory to medical treatment, 22-80 years of age, and had a modified Rankin Scale (mRS) score of 3 or less at baseline. The treatment was performed at least 7 days after the last stroke. Finally, patients had to have experienced two or more strokes. This last requirement presented a problem for recruitment, according to Dr. Alexander. “That was never a criterion for any of the [previous] trials, so it’s not clear why FDA added that. That made it very difficult to enroll for this trial – to have patients who had two or more strokes and still had a functional mRS score,” he said.
The study protocol aimed for a frequency of 6.6% for periprocedural morbidity, defined as a stroke or death within 72 hours.
An interim analysis at 100 patients showed that the periprocedural morbidity frequency was below 4%, which met the agency’s requirement and allowed the trial to be halted once the trial enrolled 150 on-label patients. The total number of on-label patients reached 152, and the researchers analyzed the results from another 46 patients who were treated with stenting despite not meeting the study’s inclusion criteria, and these patients were considered to be off-label use. The final analysis showed that the on-label group had a periprocedural morbidity of 2.6%, compared with 23.9% in the off-label group (P = .0001).
The most glaring difference in the patient populations was that half of the off-label group received the stent within 7 days of experiencing a stroke. What might be the reason for worse outcomes when stenting is performed within 7 days? “There’s speculation that the plaques might be hot, and those patients might have a higher thrombotic risk with putting a foreign body in the vessel, or there’s capillary instability, so reperfusing a vessel that has a 99% stenosis has a higher risk for reperfusion hemorrhage,” Dr. Alexander said.
Experience may also be a factor. Interventionalists participating in the WEAVE trial had performed a stent using Wingspan an average of 37 times before the study began, compared with a mean of 10 cases for physicians in the SAMMPRIS trial. Those who had performed over 50 procedures had no periprocedural morbidity outcomes at all.
The study was funded by Stryker Neurovascular. Dr. Alexander has consulted for Stryker.
SOURCE: Alexander M et al. ISC 2018 Abstract LB13.
REPORTING FROM ISC 2018
Key clinical point:
Major finding: On-label 72-hour death and stroke rate was 2.6%, compared with 23.9% off label.
Data source: Postmarketing analysis of 198 consecutive patients.
Disclosures: The study was funded by Stryker Neurovascular. Dr. Alexander has consulted for Stryker.
Source: Alexander M et al. ISC 2018 Abstract LB13.