User login
High patient activation linked to clinical remission in IBD
LAS VEGAS – results from a longitudinal analysis suggest.
“Patient activation is defined as understanding one’s role in the health care process and having the knowledge, skills, and confidence to manage one’s health,” Edward L. Barnes, MD, MPH, said at the Crohn’s & Colitis Congress, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. “It emphasizes an individual’s willingness to take independent actions and manage their own health care. In many chronic conditions, higher levels of patient activation have been linked to improved health outcomes, better patient experiences related to health care, higher quality of life scores, and lower overall health care costs.”
A 13-question survey known as the Patient Activation Measure from Insignia Health can be used to assess patient activation (Health Serv Res 2005;4096 Pt 1:1918-30). This measure is scored from zero to 100 and allows the categorization of individuals into four levels of activation. In level 1, the patient believes an active role is important. In level 2, the patient has the confidence and knowledge to take action. In level 3 the patient takes action, and in level 4, the patient stays the course during stress.
Dr. Barnes and his associates set out to evaluate the demographic and clinical characteristics associated with higher patient activation in patients with IBD. A secondary aim was to determine whether higher levels of patient care are associated with decreased frequency of relapse or flare. They performed a prospective cohort study of individuals who participated in the Crohn’s and Colitis Foundation’s Partners Internet cohort. Consecutive participants who completed a Partners survey between June 2, 2016, and Jan. 5, 2017, were asked to complete the Patient Activation Measure as an optional module. Clinical remission was defined via the short Crohn’s Disease Activity Index (a score of 150 or lower) and the Simple Clinical Colitis Activity Index (a score of 2 or less).
High patient activation was defined as level 3 or level 4 on the Patient Activation Measure, and multivariable logistic regression was used to evaluate predictors of patient activation level and the relationship between level of patient activation and clinical remission. All covariates included in the multivariable analyses were identified a priori based on prior association with patient activation or clinical disease activity in IBD.
The survey was administered to 1,486 participants. Of these, 1,082 (73%) completed follow-up surveys, including assessments of disease activity. The mean age of respondents was 44 years, 74% were female, 5% were nonwhite, and 77% reported their highest education level as college or graduate school. The mean disease duration was 14.4 years.
Patients with less than a 12th grade education were significantly associated with a decreased odds of having patient activation (adjusted odds ratio 0.25 [95% confidence interval, 0.07-0.94]). Although nonsignificant after adjustment for potential confounders, nonwhite race was also associated with decreased odds of high patient activation (aOR 0.64). Meanwhile, there was a trend among those who graduated from college or graduate school in predicting high patient activation level (aOR of 1.44 and 1.36, respectively).
After adjustment for race, educational status, time since diagnosis, smoking status, and history of IBD-related surgery among patients with Crohn’s disease, patients with higher patient activation were more likely to be in clinical remission at follow-up for both Crohn’s disease (71% vs. 62%; aOR of 1.60 [95% CI, 1.00-2.57], P = .05) and ulcerative colitis (54% vs. 34%; aOR 2.23, respectively; [95% CI, 1.15-4.19], P = .01).
Dr. Barnes acknowledged certain limitations of the study, including the fact that study participants comprised a voluntary, Internet-based cohort. “Participants may exhibit higher levels of patient activation than the general population of patients with IBD,” he said. “There may be an overrepresentation of college graduates in this sample, and the racial and ethnic makeup of this cohort may be different from that of a clinic-based population or the general population of patients with IBD.” He added that there might be unmeasured confounders in the relationship between patient activation and remission that the researchers could not assess.
“Patient activation appears to impact the disease course in patients with CD [Crohn’s disease] and UC [ulcerative colitis],” Dr. Barnes concluded. “The effect of patient activation on the disease course may be larger in UC than in CD. Efforts to improve patient activation in patients with IBD may have the ability to ultimately improve clinical outcomes.”
He reported having no financial disclosures.
*This story was updated on 3/26.
SOURCE: Barnes EL et al. Crohn’s & Colitis Congress, Clinical Abstract 12.
LAS VEGAS – results from a longitudinal analysis suggest.
“Patient activation is defined as understanding one’s role in the health care process and having the knowledge, skills, and confidence to manage one’s health,” Edward L. Barnes, MD, MPH, said at the Crohn’s & Colitis Congress, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. “It emphasizes an individual’s willingness to take independent actions and manage their own health care. In many chronic conditions, higher levels of patient activation have been linked to improved health outcomes, better patient experiences related to health care, higher quality of life scores, and lower overall health care costs.”
A 13-question survey known as the Patient Activation Measure from Insignia Health can be used to assess patient activation (Health Serv Res 2005;4096 Pt 1:1918-30). This measure is scored from zero to 100 and allows the categorization of individuals into four levels of activation. In level 1, the patient believes an active role is important. In level 2, the patient has the confidence and knowledge to take action. In level 3 the patient takes action, and in level 4, the patient stays the course during stress.
Dr. Barnes and his associates set out to evaluate the demographic and clinical characteristics associated with higher patient activation in patients with IBD. A secondary aim was to determine whether higher levels of patient care are associated with decreased frequency of relapse or flare. They performed a prospective cohort study of individuals who participated in the Crohn’s and Colitis Foundation’s Partners Internet cohort. Consecutive participants who completed a Partners survey between June 2, 2016, and Jan. 5, 2017, were asked to complete the Patient Activation Measure as an optional module. Clinical remission was defined via the short Crohn’s Disease Activity Index (a score of 150 or lower) and the Simple Clinical Colitis Activity Index (a score of 2 or less).
High patient activation was defined as level 3 or level 4 on the Patient Activation Measure, and multivariable logistic regression was used to evaluate predictors of patient activation level and the relationship between level of patient activation and clinical remission. All covariates included in the multivariable analyses were identified a priori based on prior association with patient activation or clinical disease activity in IBD.
The survey was administered to 1,486 participants. Of these, 1,082 (73%) completed follow-up surveys, including assessments of disease activity. The mean age of respondents was 44 years, 74% were female, 5% were nonwhite, and 77% reported their highest education level as college or graduate school. The mean disease duration was 14.4 years.
Patients with less than a 12th grade education were significantly associated with a decreased odds of having patient activation (adjusted odds ratio 0.25 [95% confidence interval, 0.07-0.94]). Although nonsignificant after adjustment for potential confounders, nonwhite race was also associated with decreased odds of high patient activation (aOR 0.64). Meanwhile, there was a trend among those who graduated from college or graduate school in predicting high patient activation level (aOR of 1.44 and 1.36, respectively).
After adjustment for race, educational status, time since diagnosis, smoking status, and history of IBD-related surgery among patients with Crohn’s disease, patients with higher patient activation were more likely to be in clinical remission at follow-up for both Crohn’s disease (71% vs. 62%; aOR of 1.60 [95% CI, 1.00-2.57], P = .05) and ulcerative colitis (54% vs. 34%; aOR 2.23, respectively; [95% CI, 1.15-4.19], P = .01).
Dr. Barnes acknowledged certain limitations of the study, including the fact that study participants comprised a voluntary, Internet-based cohort. “Participants may exhibit higher levels of patient activation than the general population of patients with IBD,” he said. “There may be an overrepresentation of college graduates in this sample, and the racial and ethnic makeup of this cohort may be different from that of a clinic-based population or the general population of patients with IBD.” He added that there might be unmeasured confounders in the relationship between patient activation and remission that the researchers could not assess.
“Patient activation appears to impact the disease course in patients with CD [Crohn’s disease] and UC [ulcerative colitis],” Dr. Barnes concluded. “The effect of patient activation on the disease course may be larger in UC than in CD. Efforts to improve patient activation in patients with IBD may have the ability to ultimately improve clinical outcomes.”
He reported having no financial disclosures.
*This story was updated on 3/26.
SOURCE: Barnes EL et al. Crohn’s & Colitis Congress, Clinical Abstract 12.
LAS VEGAS – results from a longitudinal analysis suggest.
“Patient activation is defined as understanding one’s role in the health care process and having the knowledge, skills, and confidence to manage one’s health,” Edward L. Barnes, MD, MPH, said at the Crohn’s & Colitis Congress, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. “It emphasizes an individual’s willingness to take independent actions and manage their own health care. In many chronic conditions, higher levels of patient activation have been linked to improved health outcomes, better patient experiences related to health care, higher quality of life scores, and lower overall health care costs.”
A 13-question survey known as the Patient Activation Measure from Insignia Health can be used to assess patient activation (Health Serv Res 2005;4096 Pt 1:1918-30). This measure is scored from zero to 100 and allows the categorization of individuals into four levels of activation. In level 1, the patient believes an active role is important. In level 2, the patient has the confidence and knowledge to take action. In level 3 the patient takes action, and in level 4, the patient stays the course during stress.
Dr. Barnes and his associates set out to evaluate the demographic and clinical characteristics associated with higher patient activation in patients with IBD. A secondary aim was to determine whether higher levels of patient care are associated with decreased frequency of relapse or flare. They performed a prospective cohort study of individuals who participated in the Crohn’s and Colitis Foundation’s Partners Internet cohort. Consecutive participants who completed a Partners survey between June 2, 2016, and Jan. 5, 2017, were asked to complete the Patient Activation Measure as an optional module. Clinical remission was defined via the short Crohn’s Disease Activity Index (a score of 150 or lower) and the Simple Clinical Colitis Activity Index (a score of 2 or less).
High patient activation was defined as level 3 or level 4 on the Patient Activation Measure, and multivariable logistic regression was used to evaluate predictors of patient activation level and the relationship between level of patient activation and clinical remission. All covariates included in the multivariable analyses were identified a priori based on prior association with patient activation or clinical disease activity in IBD.
The survey was administered to 1,486 participants. Of these, 1,082 (73%) completed follow-up surveys, including assessments of disease activity. The mean age of respondents was 44 years, 74% were female, 5% were nonwhite, and 77% reported their highest education level as college or graduate school. The mean disease duration was 14.4 years.
Patients with less than a 12th grade education were significantly associated with a decreased odds of having patient activation (adjusted odds ratio 0.25 [95% confidence interval, 0.07-0.94]). Although nonsignificant after adjustment for potential confounders, nonwhite race was also associated with decreased odds of high patient activation (aOR 0.64). Meanwhile, there was a trend among those who graduated from college or graduate school in predicting high patient activation level (aOR of 1.44 and 1.36, respectively).
After adjustment for race, educational status, time since diagnosis, smoking status, and history of IBD-related surgery among patients with Crohn’s disease, patients with higher patient activation were more likely to be in clinical remission at follow-up for both Crohn’s disease (71% vs. 62%; aOR of 1.60 [95% CI, 1.00-2.57], P = .05) and ulcerative colitis (54% vs. 34%; aOR 2.23, respectively; [95% CI, 1.15-4.19], P = .01).
Dr. Barnes acknowledged certain limitations of the study, including the fact that study participants comprised a voluntary, Internet-based cohort. “Participants may exhibit higher levels of patient activation than the general population of patients with IBD,” he said. “There may be an overrepresentation of college graduates in this sample, and the racial and ethnic makeup of this cohort may be different from that of a clinic-based population or the general population of patients with IBD.” He added that there might be unmeasured confounders in the relationship between patient activation and remission that the researchers could not assess.
“Patient activation appears to impact the disease course in patients with CD [Crohn’s disease] and UC [ulcerative colitis],” Dr. Barnes concluded. “The effect of patient activation on the disease course may be larger in UC than in CD. Efforts to improve patient activation in patients with IBD may have the ability to ultimately improve clinical outcomes.”
He reported having no financial disclosures.
*This story was updated on 3/26.
SOURCE: Barnes EL et al. Crohn’s & Colitis Congress, Clinical Abstract 12.
REPORTING FROM THE CROHN’S & COLITIS CONGRESS
Key clinical point: Patient activation appears to impact the disease course in IBD patients.
Major finding: Individuals with higher patient activation were more likely to be in clinical remission at follow-up for both CD and UC (adjusted OR of 1.60 vs. adjusted OR of 2.23, respectively).
Study details: Responses from 1,082 IBD patients who participated in the Crohn’s and Colitis Foundation’s Partners Internet cohort.
Disclosures: Dr. Barnes reported having no financial disclosures.
Source: Barnes EL et al. Crohn’s & Colitis Congress, Clinical Abstract 12.
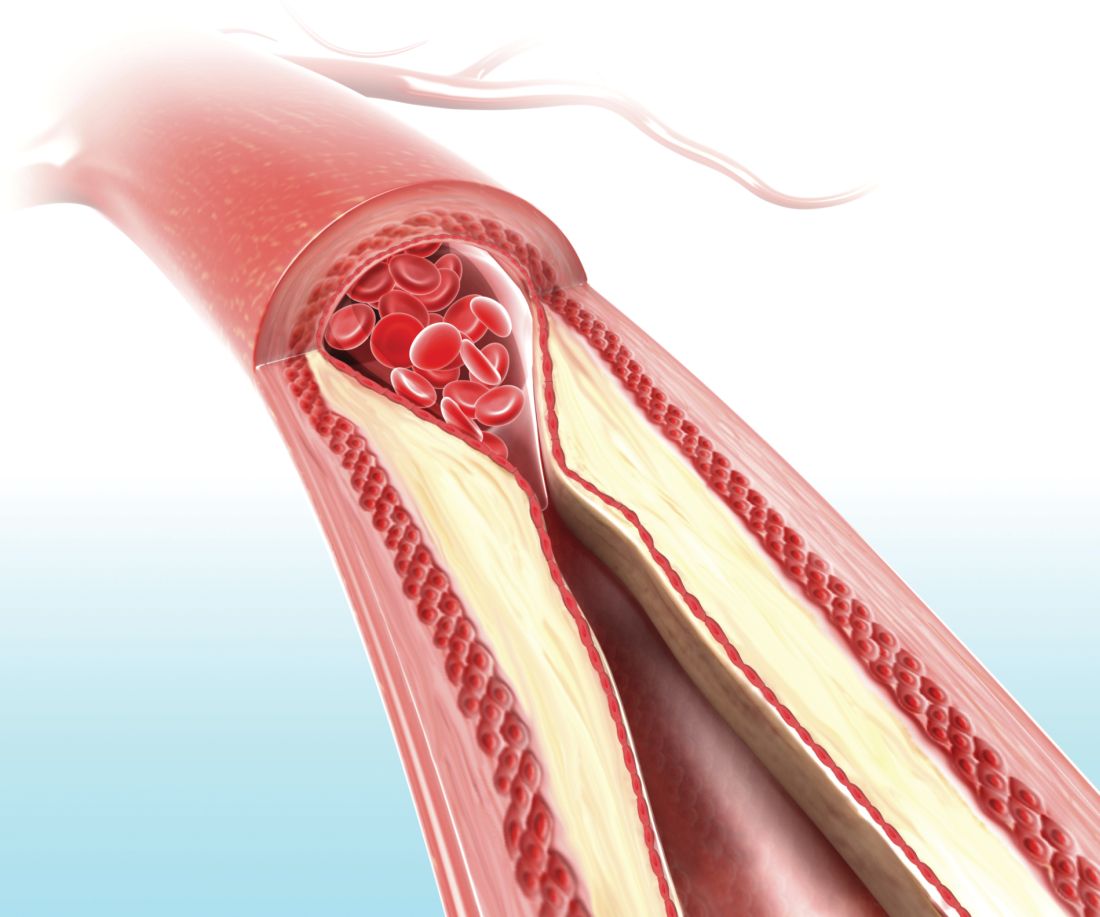
On-label stent use looks safe in intracranial atherosclerotic disease
LOS ANGELES – A postmarketing study of the Wingspan stent shows that the safety of the device in the treatment of intracranial atherosclerotic disease (ICAD) is good enough to be a reasonable alternative to medical management in these patients, but only if the device is used on label.
The results may reassure some interventionalists who were alarmed by results from the Stenting versus Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial (N Engl J Med. 2011;365:993-1003), which showed a 30-day rate of stroke or death of 14.7%. The new study showed a frequency of 2.6%, so long as the device was used on label. Off-label use yielded a frequency of 23.9%.
Up to 10% of strokes in the United States result from ICAD, and in China the rate is an estimated 20%-46%. The condition can also be treated medically. Early trials of the Wingspan device showed initial success with complication rates of 4.5%-6.2%, but the SAMMPRIS trial, which directly compared stenting to aggressive medical management, showed superior outcomes with medical treatment. The 30-day rate of stroke or death of 14.7% was too high to compete with medical therapy, which included aspirin 325 mg per day, clopidogrel 75 mg per day for 90 days after enrollment, and management of primary and secondary risk factors.
Dr. Alexander believes that the SAMMPRIS trial did not employ favorable patient selection. “ICAD is variable. Some patients present with hemodynamic compromise, where stenting is probably beneficial. Some present with embolic stroke, and some present with small-vessel perforator strokes that are unlikely to be responsive to stenting and better treated with medical therapy. All these patients were grouped together” in SAMMPRIS, said Dr. Alexander, who is director of the Neurovascular Center and endovascular surgery at Cedars-Sinai in Los Angeles.
The SAMMPRIS findings put a damper on stenting, and use of the procedure has dropped at many U.S. hospitals. But studies conducted prior to SAMMPRIS had shown much lower periprocedural morbidity, and those studies looked at on-label use of stenting. SAMMPRIS was off label.
Now the WEAVE study, which was an Food and Drug Administration–mandated, postmarketing surveillance study of Stryker’s Wingspan stent, suggests that the off-label use of the system in the SAMMPRIS trial may explain the poor results. SAMMPRIS had attempted to extend the approved boundaries of stenting by treating patients who presented with transient ischemic attacks only, patients who had failed medical therapy, and patients who had experienced a stroke in the past 7 days. In fact, about half of the patients were treated within 7 days of the previous event, sometimes within 24 hours.
Previous studies had shown that risk factors for poor outcomes included stenting within 10 days of the last event, stenting a posterior circulation target lesion, stenting presentations other than stroke, and sites with a low patient volume for stenting. Patient selection is vital to success, according to Dr. Alexander. Patients with hemodynamic compromise are good candidates for stenting, while those with perforator stroke alone are better off with medical therapy. Embolic stroke patients are candidates for either approach.
WEAVE looked at 152 consecutive patients treated on label at 24 institutions. The primary analysis group consisted of patients with a 70%-99% stenotic intracranial atherosclerotic lesion who were refractory to medical treatment, 22-80 years of age, and had a modified Rankin Scale (mRS) score of 3 or less at baseline. The treatment was performed at least 7 days after the last stroke. Finally, patients had to have experienced two or more strokes. This last requirement presented a problem for recruitment, according to Dr. Alexander. “That was never a criterion for any of the [previous] trials, so it’s not clear why FDA added that. That made it very difficult to enroll for this trial – to have patients who had two or more strokes and still had a functional mRS score,” he said.
The study protocol aimed for a frequency of 6.6% for periprocedural morbidity, defined as a stroke or death within 72 hours.
An interim analysis at 100 patients showed that the periprocedural morbidity frequency was below 4%, which met the agency’s requirement and allowed the trial to be halted once the trial enrolled 150 on-label patients. The total number of on-label patients reached 152, and the researchers analyzed the results from another 46 patients who were treated with stenting despite not meeting the study’s inclusion criteria, and these patients were considered to be off-label use. The final analysis showed that the on-label group had a periprocedural morbidity of 2.6%, compared with 23.9% in the off-label group (P = .0001).
The most glaring difference in the patient populations was that half of the off-label group received the stent within 7 days of experiencing a stroke. What might be the reason for worse outcomes when stenting is performed within 7 days? “There’s speculation that the plaques might be hot, and those patients might have a higher thrombotic risk with putting a foreign body in the vessel, or there’s capillary instability, so reperfusing a vessel that has a 99% stenosis has a higher risk for reperfusion hemorrhage,” Dr. Alexander said.
Experience may also be a factor. Interventionalists participating in the WEAVE trial had performed a stent using Wingspan an average of 37 times before the study began, compared with a mean of 10 cases for physicians in the SAMMPRIS trial. Those who had performed over 50 procedures had no periprocedural morbidity outcomes at all.
The study was funded by Stryker Neurovascular. Dr. Alexander has consulted for Stryker.
SOURCE: Alexander M et al. ISC 2018 Abstract LB13.
LOS ANGELES – A postmarketing study of the Wingspan stent shows that the safety of the device in the treatment of intracranial atherosclerotic disease (ICAD) is good enough to be a reasonable alternative to medical management in these patients, but only if the device is used on label.
The results may reassure some interventionalists who were alarmed by results from the Stenting versus Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial (N Engl J Med. 2011;365:993-1003), which showed a 30-day rate of stroke or death of 14.7%. The new study showed a frequency of 2.6%, so long as the device was used on label. Off-label use yielded a frequency of 23.9%.
Up to 10% of strokes in the United States result from ICAD, and in China the rate is an estimated 20%-46%. The condition can also be treated medically. Early trials of the Wingspan device showed initial success with complication rates of 4.5%-6.2%, but the SAMMPRIS trial, which directly compared stenting to aggressive medical management, showed superior outcomes with medical treatment. The 30-day rate of stroke or death of 14.7% was too high to compete with medical therapy, which included aspirin 325 mg per day, clopidogrel 75 mg per day for 90 days after enrollment, and management of primary and secondary risk factors.
Dr. Alexander believes that the SAMMPRIS trial did not employ favorable patient selection. “ICAD is variable. Some patients present with hemodynamic compromise, where stenting is probably beneficial. Some present with embolic stroke, and some present with small-vessel perforator strokes that are unlikely to be responsive to stenting and better treated with medical therapy. All these patients were grouped together” in SAMMPRIS, said Dr. Alexander, who is director of the Neurovascular Center and endovascular surgery at Cedars-Sinai in Los Angeles.
The SAMMPRIS findings put a damper on stenting, and use of the procedure has dropped at many U.S. hospitals. But studies conducted prior to SAMMPRIS had shown much lower periprocedural morbidity, and those studies looked at on-label use of stenting. SAMMPRIS was off label.
Now the WEAVE study, which was an Food and Drug Administration–mandated, postmarketing surveillance study of Stryker’s Wingspan stent, suggests that the off-label use of the system in the SAMMPRIS trial may explain the poor results. SAMMPRIS had attempted to extend the approved boundaries of stenting by treating patients who presented with transient ischemic attacks only, patients who had failed medical therapy, and patients who had experienced a stroke in the past 7 days. In fact, about half of the patients were treated within 7 days of the previous event, sometimes within 24 hours.
Previous studies had shown that risk factors for poor outcomes included stenting within 10 days of the last event, stenting a posterior circulation target lesion, stenting presentations other than stroke, and sites with a low patient volume for stenting. Patient selection is vital to success, according to Dr. Alexander. Patients with hemodynamic compromise are good candidates for stenting, while those with perforator stroke alone are better off with medical therapy. Embolic stroke patients are candidates for either approach.
WEAVE looked at 152 consecutive patients treated on label at 24 institutions. The primary analysis group consisted of patients with a 70%-99% stenotic intracranial atherosclerotic lesion who were refractory to medical treatment, 22-80 years of age, and had a modified Rankin Scale (mRS) score of 3 or less at baseline. The treatment was performed at least 7 days after the last stroke. Finally, patients had to have experienced two or more strokes. This last requirement presented a problem for recruitment, according to Dr. Alexander. “That was never a criterion for any of the [previous] trials, so it’s not clear why FDA added that. That made it very difficult to enroll for this trial – to have patients who had two or more strokes and still had a functional mRS score,” he said.
The study protocol aimed for a frequency of 6.6% for periprocedural morbidity, defined as a stroke or death within 72 hours.
An interim analysis at 100 patients showed that the periprocedural morbidity frequency was below 4%, which met the agency’s requirement and allowed the trial to be halted once the trial enrolled 150 on-label patients. The total number of on-label patients reached 152, and the researchers analyzed the results from another 46 patients who were treated with stenting despite not meeting the study’s inclusion criteria, and these patients were considered to be off-label use. The final analysis showed that the on-label group had a periprocedural morbidity of 2.6%, compared with 23.9% in the off-label group (P = .0001).
The most glaring difference in the patient populations was that half of the off-label group received the stent within 7 days of experiencing a stroke. What might be the reason for worse outcomes when stenting is performed within 7 days? “There’s speculation that the plaques might be hot, and those patients might have a higher thrombotic risk with putting a foreign body in the vessel, or there’s capillary instability, so reperfusing a vessel that has a 99% stenosis has a higher risk for reperfusion hemorrhage,” Dr. Alexander said.
Experience may also be a factor. Interventionalists participating in the WEAVE trial had performed a stent using Wingspan an average of 37 times before the study began, compared with a mean of 10 cases for physicians in the SAMMPRIS trial. Those who had performed over 50 procedures had no periprocedural morbidity outcomes at all.
The study was funded by Stryker Neurovascular. Dr. Alexander has consulted for Stryker.
SOURCE: Alexander M et al. ISC 2018 Abstract LB13.
LOS ANGELES – A postmarketing study of the Wingspan stent shows that the safety of the device in the treatment of intracranial atherosclerotic disease (ICAD) is good enough to be a reasonable alternative to medical management in these patients, but only if the device is used on label.
The results may reassure some interventionalists who were alarmed by results from the Stenting versus Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial (N Engl J Med. 2011;365:993-1003), which showed a 30-day rate of stroke or death of 14.7%. The new study showed a frequency of 2.6%, so long as the device was used on label. Off-label use yielded a frequency of 23.9%.
Up to 10% of strokes in the United States result from ICAD, and in China the rate is an estimated 20%-46%. The condition can also be treated medically. Early trials of the Wingspan device showed initial success with complication rates of 4.5%-6.2%, but the SAMMPRIS trial, which directly compared stenting to aggressive medical management, showed superior outcomes with medical treatment. The 30-day rate of stroke or death of 14.7% was too high to compete with medical therapy, which included aspirin 325 mg per day, clopidogrel 75 mg per day for 90 days after enrollment, and management of primary and secondary risk factors.
Dr. Alexander believes that the SAMMPRIS trial did not employ favorable patient selection. “ICAD is variable. Some patients present with hemodynamic compromise, where stenting is probably beneficial. Some present with embolic stroke, and some present with small-vessel perforator strokes that are unlikely to be responsive to stenting and better treated with medical therapy. All these patients were grouped together” in SAMMPRIS, said Dr. Alexander, who is director of the Neurovascular Center and endovascular surgery at Cedars-Sinai in Los Angeles.
The SAMMPRIS findings put a damper on stenting, and use of the procedure has dropped at many U.S. hospitals. But studies conducted prior to SAMMPRIS had shown much lower periprocedural morbidity, and those studies looked at on-label use of stenting. SAMMPRIS was off label.
Now the WEAVE study, which was an Food and Drug Administration–mandated, postmarketing surveillance study of Stryker’s Wingspan stent, suggests that the off-label use of the system in the SAMMPRIS trial may explain the poor results. SAMMPRIS had attempted to extend the approved boundaries of stenting by treating patients who presented with transient ischemic attacks only, patients who had failed medical therapy, and patients who had experienced a stroke in the past 7 days. In fact, about half of the patients were treated within 7 days of the previous event, sometimes within 24 hours.
Previous studies had shown that risk factors for poor outcomes included stenting within 10 days of the last event, stenting a posterior circulation target lesion, stenting presentations other than stroke, and sites with a low patient volume for stenting. Patient selection is vital to success, according to Dr. Alexander. Patients with hemodynamic compromise are good candidates for stenting, while those with perforator stroke alone are better off with medical therapy. Embolic stroke patients are candidates for either approach.
WEAVE looked at 152 consecutive patients treated on label at 24 institutions. The primary analysis group consisted of patients with a 70%-99% stenotic intracranial atherosclerotic lesion who were refractory to medical treatment, 22-80 years of age, and had a modified Rankin Scale (mRS) score of 3 or less at baseline. The treatment was performed at least 7 days after the last stroke. Finally, patients had to have experienced two or more strokes. This last requirement presented a problem for recruitment, according to Dr. Alexander. “That was never a criterion for any of the [previous] trials, so it’s not clear why FDA added that. That made it very difficult to enroll for this trial – to have patients who had two or more strokes and still had a functional mRS score,” he said.
The study protocol aimed for a frequency of 6.6% for periprocedural morbidity, defined as a stroke or death within 72 hours.
An interim analysis at 100 patients showed that the periprocedural morbidity frequency was below 4%, which met the agency’s requirement and allowed the trial to be halted once the trial enrolled 150 on-label patients. The total number of on-label patients reached 152, and the researchers analyzed the results from another 46 patients who were treated with stenting despite not meeting the study’s inclusion criteria, and these patients were considered to be off-label use. The final analysis showed that the on-label group had a periprocedural morbidity of 2.6%, compared with 23.9% in the off-label group (P = .0001).
The most glaring difference in the patient populations was that half of the off-label group received the stent within 7 days of experiencing a stroke. What might be the reason for worse outcomes when stenting is performed within 7 days? “There’s speculation that the plaques might be hot, and those patients might have a higher thrombotic risk with putting a foreign body in the vessel, or there’s capillary instability, so reperfusing a vessel that has a 99% stenosis has a higher risk for reperfusion hemorrhage,” Dr. Alexander said.
Experience may also be a factor. Interventionalists participating in the WEAVE trial had performed a stent using Wingspan an average of 37 times before the study began, compared with a mean of 10 cases for physicians in the SAMMPRIS trial. Those who had performed over 50 procedures had no periprocedural morbidity outcomes at all.
The study was funded by Stryker Neurovascular. Dr. Alexander has consulted for Stryker.
SOURCE: Alexander M et al. ISC 2018 Abstract LB13.
REPORTING FROM ISC 2018
Key clinical point:
Major finding: On-label 72-hour death and stroke rate was 2.6%, compared with 23.9% off label.
Data source: Postmarketing analysis of 198 consecutive patients.
Disclosures: The study was funded by Stryker Neurovascular. Dr. Alexander has consulted for Stryker.
Source: Alexander M et al. ISC 2018 Abstract LB13.
Hernia repair patients had less pain with lightweight mesh
and less discomfort at 1 year in a group of patients who had lightweight mesh, compared with patients who had heavyweight mesh, in a multicenter, randomized clinical trial.
Martin Rutegård, MD, of Umeå (Sweden) University, and his research associates reported that 3%-10% of all hernia surgeries “result in severe or moderately severe pain for more than a year after hernia surgery, which may have a significant impact on social activities, sex life, and quality of life. ... Interest in the use of lightweight meshes in groin hernia repair has increased in recent years, as it is assumed that this type of mesh may cause less discomfort and chronic pain.”
Patients were followed for 1-3 years and given questionnaires to report their outcomes. Patients were all male and were close in weight (mean body mass index, 25.2 kg/m2 in the lightweight-mesh group and 25.3 in the heavyweight-mesh group), age (59 and 58, respectively), and American Society of Anaesthesiologists classification of their hernia defect.
Of a total of 363 patients, 185 patients were randomized to the lightweight-mesh group and 178 patients to the heavyweight group. Investigators found that there were significant differences concerning awareness of a groin lump and groin discomfort, favoring the lightweight group 1 year after surgery. A total of 6% of the lightweight group reported the groin lump awareness at 1 year, vs. 18% of the heavyweight group. Groin discomfort was reported by 18% of the lightweight group and 28% of the heavyweight group.
After 1 year, that difference subsided. In terms of discomfort, the investigators found no statistically significant or clinically relevant differences between types of mesh, with 263/288 patients (91.3%) reporting improvement after 12 months, 19/288 patients (6.6%) experiencing no change, and 6/288 patients (2.1%) having worsened.
Additionally, there was no statistically significant difference in quality of life as measured by the EuroQol five dimensions (EQ-5D) between the different mesh groups. It was noted that all the patients had a statistically significantly better quality of life postoperatively from day 11 and onward, compared with before surgery. In addition, the investigators did not detect a significant difference between the mesh groups in their reported sexual life after surgery at 4 and 12 months subsequent to the operation.
The recurrence rate at the follow-up visit and clinical examination was 2.4% and equal between both groups.
The study was limited by possible bias of the expertise-based design and also some missing data, especially with regard to sexual life after surgery.
The study was funded by the Västerbotten County Council, VISARE NORR Fund, and Northern Country Councils Regional Federation. The investigators reported no conflicts of interest.
SOURCE: M. Rutegård et al. Hernia 2018 Jan 20. doi: 10.1007/s10029-018-1734-z.
and less discomfort at 1 year in a group of patients who had lightweight mesh, compared with patients who had heavyweight mesh, in a multicenter, randomized clinical trial.
Martin Rutegård, MD, of Umeå (Sweden) University, and his research associates reported that 3%-10% of all hernia surgeries “result in severe or moderately severe pain for more than a year after hernia surgery, which may have a significant impact on social activities, sex life, and quality of life. ... Interest in the use of lightweight meshes in groin hernia repair has increased in recent years, as it is assumed that this type of mesh may cause less discomfort and chronic pain.”
Patients were followed for 1-3 years and given questionnaires to report their outcomes. Patients were all male and were close in weight (mean body mass index, 25.2 kg/m2 in the lightweight-mesh group and 25.3 in the heavyweight-mesh group), age (59 and 58, respectively), and American Society of Anaesthesiologists classification of their hernia defect.
Of a total of 363 patients, 185 patients were randomized to the lightweight-mesh group and 178 patients to the heavyweight group. Investigators found that there were significant differences concerning awareness of a groin lump and groin discomfort, favoring the lightweight group 1 year after surgery. A total of 6% of the lightweight group reported the groin lump awareness at 1 year, vs. 18% of the heavyweight group. Groin discomfort was reported by 18% of the lightweight group and 28% of the heavyweight group.
After 1 year, that difference subsided. In terms of discomfort, the investigators found no statistically significant or clinically relevant differences between types of mesh, with 263/288 patients (91.3%) reporting improvement after 12 months, 19/288 patients (6.6%) experiencing no change, and 6/288 patients (2.1%) having worsened.
Additionally, there was no statistically significant difference in quality of life as measured by the EuroQol five dimensions (EQ-5D) between the different mesh groups. It was noted that all the patients had a statistically significantly better quality of life postoperatively from day 11 and onward, compared with before surgery. In addition, the investigators did not detect a significant difference between the mesh groups in their reported sexual life after surgery at 4 and 12 months subsequent to the operation.
The recurrence rate at the follow-up visit and clinical examination was 2.4% and equal between both groups.
The study was limited by possible bias of the expertise-based design and also some missing data, especially with regard to sexual life after surgery.
The study was funded by the Västerbotten County Council, VISARE NORR Fund, and Northern Country Councils Regional Federation. The investigators reported no conflicts of interest.
SOURCE: M. Rutegård et al. Hernia 2018 Jan 20. doi: 10.1007/s10029-018-1734-z.
and less discomfort at 1 year in a group of patients who had lightweight mesh, compared with patients who had heavyweight mesh, in a multicenter, randomized clinical trial.
Martin Rutegård, MD, of Umeå (Sweden) University, and his research associates reported that 3%-10% of all hernia surgeries “result in severe or moderately severe pain for more than a year after hernia surgery, which may have a significant impact on social activities, sex life, and quality of life. ... Interest in the use of lightweight meshes in groin hernia repair has increased in recent years, as it is assumed that this type of mesh may cause less discomfort and chronic pain.”
Patients were followed for 1-3 years and given questionnaires to report their outcomes. Patients were all male and were close in weight (mean body mass index, 25.2 kg/m2 in the lightweight-mesh group and 25.3 in the heavyweight-mesh group), age (59 and 58, respectively), and American Society of Anaesthesiologists classification of their hernia defect.
Of a total of 363 patients, 185 patients were randomized to the lightweight-mesh group and 178 patients to the heavyweight group. Investigators found that there were significant differences concerning awareness of a groin lump and groin discomfort, favoring the lightweight group 1 year after surgery. A total of 6% of the lightweight group reported the groin lump awareness at 1 year, vs. 18% of the heavyweight group. Groin discomfort was reported by 18% of the lightweight group and 28% of the heavyweight group.
After 1 year, that difference subsided. In terms of discomfort, the investigators found no statistically significant or clinically relevant differences between types of mesh, with 263/288 patients (91.3%) reporting improvement after 12 months, 19/288 patients (6.6%) experiencing no change, and 6/288 patients (2.1%) having worsened.
Additionally, there was no statistically significant difference in quality of life as measured by the EuroQol five dimensions (EQ-5D) between the different mesh groups. It was noted that all the patients had a statistically significantly better quality of life postoperatively from day 11 and onward, compared with before surgery. In addition, the investigators did not detect a significant difference between the mesh groups in their reported sexual life after surgery at 4 and 12 months subsequent to the operation.
The recurrence rate at the follow-up visit and clinical examination was 2.4% and equal between both groups.
The study was limited by possible bias of the expertise-based design and also some missing data, especially with regard to sexual life after surgery.
The study was funded by the Västerbotten County Council, VISARE NORR Fund, and Northern Country Councils Regional Federation. The investigators reported no conflicts of interest.
SOURCE: M. Rutegård et al. Hernia 2018 Jan 20. doi: 10.1007/s10029-018-1734-z.
FROM HERNIA
Key clinical point: The weight of mesh used for inguinal hernia repair did not impact outcomes.
Major finding: A total of 6% of the lightweight group reported groin lump awareness at 1 year, vs. 18% of the heavyweight group.
Study details: A randomized, multicenter study of 363 patients.
Disclosures: The study was funded by the Västerbotten County Council, VISARE NORR Fund, and Northern Country Councils Regional Federation. The investigators reported no conflicts of interest.
Source: Rutegård M et al. Hernia. 2018 Jan 20. doi: 10.1007/s10029-018-1734-z.
VIDEO: The skinny on patch testing
KAUAI, HAWAII – .
That’s sometimes the assumption, but it’s incorrect, according to Mark Davis, MD, chair of the department of dermatology at the Mayo Clinic, Rochester, Minn. Tixocortol is the marker for topical steroid allergy in many series of patch tests, but there is research showing that it is a marker for one class of topical steroids, and “there’s substantial literature saying that if you’re only reacting to tixocortol pivalate, it should be safe to use other classes of topical steroids,” he said.
It’s also important to remember that skin patch tests need to be checked on day 5, not just day 3; it’s the only way to differentiate a true skin allergy from mere skin irritation, and it does matter.
Dr. Davis explained those issues and more – including what to do with minor reactions and how to use the T.R.U.E. TEST kit – in an interview filled with pearls at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Meanwhile, during a presentation at the meeting, he noted two newer options to help allergic patients find skin care products they won’t react to: the Mayo Clinic’s SkinSAFE database and the Contact Allergen Management Program from the American Contact Dermatitis Society.
Dr. Davis had no disclosures.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – .
That’s sometimes the assumption, but it’s incorrect, according to Mark Davis, MD, chair of the department of dermatology at the Mayo Clinic, Rochester, Minn. Tixocortol is the marker for topical steroid allergy in many series of patch tests, but there is research showing that it is a marker for one class of topical steroids, and “there’s substantial literature saying that if you’re only reacting to tixocortol pivalate, it should be safe to use other classes of topical steroids,” he said.
It’s also important to remember that skin patch tests need to be checked on day 5, not just day 3; it’s the only way to differentiate a true skin allergy from mere skin irritation, and it does matter.
Dr. Davis explained those issues and more – including what to do with minor reactions and how to use the T.R.U.E. TEST kit – in an interview filled with pearls at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Meanwhile, during a presentation at the meeting, he noted two newer options to help allergic patients find skin care products they won’t react to: the Mayo Clinic’s SkinSAFE database and the Contact Allergen Management Program from the American Contact Dermatitis Society.
Dr. Davis had no disclosures.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – .
That’s sometimes the assumption, but it’s incorrect, according to Mark Davis, MD, chair of the department of dermatology at the Mayo Clinic, Rochester, Minn. Tixocortol is the marker for topical steroid allergy in many series of patch tests, but there is research showing that it is a marker for one class of topical steroids, and “there’s substantial literature saying that if you’re only reacting to tixocortol pivalate, it should be safe to use other classes of topical steroids,” he said.
It’s also important to remember that skin patch tests need to be checked on day 5, not just day 3; it’s the only way to differentiate a true skin allergy from mere skin irritation, and it does matter.
Dr. Davis explained those issues and more – including what to do with minor reactions and how to use the T.R.U.E. TEST kit – in an interview filled with pearls at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Meanwhile, during a presentation at the meeting, he noted two newer options to help allergic patients find skin care products they won’t react to: the Mayo Clinic’s SkinSAFE database and the Contact Allergen Management Program from the American Contact Dermatitis Society.
Dr. Davis had no disclosures.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
VIDEO: Advanced practice providers take on many roles in MS care
SAN DIEGO – A new survey suggests that advanced practice providers who care for multiple sclerosis patients are highly satisfied with their jobs, which encompass a wide range of responsibilities.
Researchers received survey responses from 215 nurse practitioners and 395 physician assistants who answered Web questionnaires in 2016 and 2017. Of those who care for multiple sclerosis (MS) patients, 92.5% and 77.8% respectively said they provide at least 9 of 11 services, such as direct care, supportive services, and care coordination.
“Nurse practitioners in particular are providing lots of different services from diagnosis to education to symptom management,” said Michael T. Halpern, MD, PhD, MPH, of Temple University, Philadelphia. “Physician assistants are also providing a diverse range of MS services, but not as frequently as nurse practitioners.”
Dr. Halpern was the presenting author of the study reporting the survey results. He spoke in a video interview at ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis, where the study findings were presented.
Advanced practice providers also reported high levels of job satisfaction. About 80% in both groups reported being very or extremely satisfied with their careers and with their colleagues; 90% of nurse practitioners reported being very or extremely satisfied by their relationships with patients, as did 86% of physician assistants.
The providers “appear to really enjoy working with individuals with MS,” Dr. Halpern said. But he cautioned that there’s a need for additional training for these providers; some respondents said their lack of knowledge was a hindrance to care.
The study was funded by the National Multiple Sclerosis Society. Dr. Halpern reported no relevant disclosures.
SAN DIEGO – A new survey suggests that advanced practice providers who care for multiple sclerosis patients are highly satisfied with their jobs, which encompass a wide range of responsibilities.
Researchers received survey responses from 215 nurse practitioners and 395 physician assistants who answered Web questionnaires in 2016 and 2017. Of those who care for multiple sclerosis (MS) patients, 92.5% and 77.8% respectively said they provide at least 9 of 11 services, such as direct care, supportive services, and care coordination.
“Nurse practitioners in particular are providing lots of different services from diagnosis to education to symptom management,” said Michael T. Halpern, MD, PhD, MPH, of Temple University, Philadelphia. “Physician assistants are also providing a diverse range of MS services, but not as frequently as nurse practitioners.”
Dr. Halpern was the presenting author of the study reporting the survey results. He spoke in a video interview at ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis, where the study findings were presented.
Advanced practice providers also reported high levels of job satisfaction. About 80% in both groups reported being very or extremely satisfied with their careers and with their colleagues; 90% of nurse practitioners reported being very or extremely satisfied by their relationships with patients, as did 86% of physician assistants.
The providers “appear to really enjoy working with individuals with MS,” Dr. Halpern said. But he cautioned that there’s a need for additional training for these providers; some respondents said their lack of knowledge was a hindrance to care.
The study was funded by the National Multiple Sclerosis Society. Dr. Halpern reported no relevant disclosures.
SAN DIEGO – A new survey suggests that advanced practice providers who care for multiple sclerosis patients are highly satisfied with their jobs, which encompass a wide range of responsibilities.
Researchers received survey responses from 215 nurse practitioners and 395 physician assistants who answered Web questionnaires in 2016 and 2017. Of those who care for multiple sclerosis (MS) patients, 92.5% and 77.8% respectively said they provide at least 9 of 11 services, such as direct care, supportive services, and care coordination.
“Nurse practitioners in particular are providing lots of different services from diagnosis to education to symptom management,” said Michael T. Halpern, MD, PhD, MPH, of Temple University, Philadelphia. “Physician assistants are also providing a diverse range of MS services, but not as frequently as nurse practitioners.”
Dr. Halpern was the presenting author of the study reporting the survey results. He spoke in a video interview at ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis, where the study findings were presented.
Advanced practice providers also reported high levels of job satisfaction. About 80% in both groups reported being very or extremely satisfied with their careers and with their colleagues; 90% of nurse practitioners reported being very or extremely satisfied by their relationships with patients, as did 86% of physician assistants.
The providers “appear to really enjoy working with individuals with MS,” Dr. Halpern said. But he cautioned that there’s a need for additional training for these providers; some respondents said their lack of knowledge was a hindrance to care.
The study was funded by the National Multiple Sclerosis Society. Dr. Halpern reported no relevant disclosures.
REPORTING FROM ACTRIMS FORUM 2018
REPROVE: Ceftazidime-avibactam noninferior to meropenem for nosocomial pneumonia
Ceftazidime-avibactam was noninferior to meropenem for nosocomial pneumonia including ventilator-associated pneumonia from gram-negative organisms, results from the REPROVE trial demonstrated.
Nosocomial or hospital-acquired pneumonia is a common hospital-acquired infection associated with increased cost and mortality. Further, nosocomial pneumonia is associated with gram-negative pathogens such as Pseudomonas aeruginosa and Enterobacteriaceae that may carry extended-spectrum beta-lactamases and carbapenemase, thereby limiting the treatment options. However, ceftazidime-avibactam has both antipseudomonal and extended beta-lactamase coverage for multidrug-resistant gram-negative infections, and may provide an alternative to meropenem.
Antoni Torres, MD, of the University of Barcelona and his colleagues sought to compare the safety and efficacy of ceftazidime-avibactam to meropenem in patients with nosocomial and ventilator-associated pneumonia. The REPROVE study was a phase 3, double-blind, noninferiority trial performed at 136 centers in 23 countries. Patients were randomly assigned 1:1 to receive either ceftazidime-avibactam (500-2,000 mg every 8 hours) or meropenem (1,000 mg every 8 hours) with adjustment as needed for renal function.
Participants included in the study were 18-90 years of age with nosocomial pneumonia as evidenced by pneumonia 48 hours or more after admission or within 7 days after discharge from an inpatient facility. Patients with ventilator-associated pneumonia had lung infection within 48 hours of intubation and mechanical ventilation. Sputum culture and gram stains were obtained within 48 hours before randomization, and patients were excluded for evidence of gram-positive–only pathogens or those not expected to respond to meropenem or ceftazidime-avibactam.
The study involved a safety population (808 patients), a clinically modified intention-to-treat population (726), and a clinically evaluable population (527). The intention-to-treat population demonstrated a predominance of Klebsiella pneumoniae (37%), and Pseudomonas aeruginosa (30%); 28% of the intention-to-treat population were identified as not susceptible to ceftazidime.
Overall, the clinically modified intention-to-treat group demonstrated a clinical cure rate of 68.8% (245/356) in the ceftazidime-avibactam and 73.0% (270/370) for the meropenem group (difference, –4.2%; 95% confidence interval, –10.8 to 2.5). The evaluable population demonstrated a clinical cure rate of 77.4% (199/257) in the ceftazidime-avibactam group and 78.1% (211/270) in the meropenem group (–0.7%; 95% CI, –7.9 to 6.4).
The all-cause mortality rate was similar between groups at the test-of-cure date and at day 28. The clinically modified intention-to-treat population demonstrated a mortality of 8.1% vs. 6.8% at the test-of-cure date and 8.4% vs. 7.3% at day 28 for ceftazidime-avibactam and meropenem, respectively.
Adverse events were noted in 75% vs. 74% of patients in the ceftazidime-avibactam groups and meropenem groups, respectively. Most adverse events were rated as mild to moderate and deemed likely unrelated to the treatment.
However, serious adverse events occurred in 19% (n = 75) in the ceftazidime-avibactam group and 13% (n = 54) in the meropenem group. Four serious adverse events were thought to be possibly related to the study drug ceftazidime-avibactam and included diarrhea, acute coronary syndrome, subacute hepatic failure, and abnormal liver function test results. The authors noted the adverse events in the trial were consistent and detected no new safety concerns for ceftazidime-avibactam.
Limitations of the study included an inability to establish the optimal duration of treatment for nosocomial pneumonia treated with meropenem or ceftazidime-avibactam.
“Our results show noninferiority for the treatment of nosocomial pneumonia caused by ceftazidime-nonsusceptible or ceftazidime-susceptible gram-negative aerobic pathogens,” the authors concluded.
The study was initially funded by AstraZeneca until the rights to ceftazidime-avibactam were acquired by Pfizer. Multiple authors reported financial relationships with AstraZeneca including grant funding, employment, and shareholding.
SOURCE: Torres A et al. Lancet Infect Dis. 2017. doi: 10.1016/S1473-3099(17)30747-8.
Ceftazidime-avibactam was noninferior to meropenem for nosocomial pneumonia including ventilator-associated pneumonia from gram-negative organisms, results from the REPROVE trial demonstrated.
Nosocomial or hospital-acquired pneumonia is a common hospital-acquired infection associated with increased cost and mortality. Further, nosocomial pneumonia is associated with gram-negative pathogens such as Pseudomonas aeruginosa and Enterobacteriaceae that may carry extended-spectrum beta-lactamases and carbapenemase, thereby limiting the treatment options. However, ceftazidime-avibactam has both antipseudomonal and extended beta-lactamase coverage for multidrug-resistant gram-negative infections, and may provide an alternative to meropenem.
Antoni Torres, MD, of the University of Barcelona and his colleagues sought to compare the safety and efficacy of ceftazidime-avibactam to meropenem in patients with nosocomial and ventilator-associated pneumonia. The REPROVE study was a phase 3, double-blind, noninferiority trial performed at 136 centers in 23 countries. Patients were randomly assigned 1:1 to receive either ceftazidime-avibactam (500-2,000 mg every 8 hours) or meropenem (1,000 mg every 8 hours) with adjustment as needed for renal function.
Participants included in the study were 18-90 years of age with nosocomial pneumonia as evidenced by pneumonia 48 hours or more after admission or within 7 days after discharge from an inpatient facility. Patients with ventilator-associated pneumonia had lung infection within 48 hours of intubation and mechanical ventilation. Sputum culture and gram stains were obtained within 48 hours before randomization, and patients were excluded for evidence of gram-positive–only pathogens or those not expected to respond to meropenem or ceftazidime-avibactam.
The study involved a safety population (808 patients), a clinically modified intention-to-treat population (726), and a clinically evaluable population (527). The intention-to-treat population demonstrated a predominance of Klebsiella pneumoniae (37%), and Pseudomonas aeruginosa (30%); 28% of the intention-to-treat population were identified as not susceptible to ceftazidime.
Overall, the clinically modified intention-to-treat group demonstrated a clinical cure rate of 68.8% (245/356) in the ceftazidime-avibactam and 73.0% (270/370) for the meropenem group (difference, –4.2%; 95% confidence interval, –10.8 to 2.5). The evaluable population demonstrated a clinical cure rate of 77.4% (199/257) in the ceftazidime-avibactam group and 78.1% (211/270) in the meropenem group (–0.7%; 95% CI, –7.9 to 6.4).
The all-cause mortality rate was similar between groups at the test-of-cure date and at day 28. The clinically modified intention-to-treat population demonstrated a mortality of 8.1% vs. 6.8% at the test-of-cure date and 8.4% vs. 7.3% at day 28 for ceftazidime-avibactam and meropenem, respectively.
Adverse events were noted in 75% vs. 74% of patients in the ceftazidime-avibactam groups and meropenem groups, respectively. Most adverse events were rated as mild to moderate and deemed likely unrelated to the treatment.
However, serious adverse events occurred in 19% (n = 75) in the ceftazidime-avibactam group and 13% (n = 54) in the meropenem group. Four serious adverse events were thought to be possibly related to the study drug ceftazidime-avibactam and included diarrhea, acute coronary syndrome, subacute hepatic failure, and abnormal liver function test results. The authors noted the adverse events in the trial were consistent and detected no new safety concerns for ceftazidime-avibactam.
Limitations of the study included an inability to establish the optimal duration of treatment for nosocomial pneumonia treated with meropenem or ceftazidime-avibactam.
“Our results show noninferiority for the treatment of nosocomial pneumonia caused by ceftazidime-nonsusceptible or ceftazidime-susceptible gram-negative aerobic pathogens,” the authors concluded.
The study was initially funded by AstraZeneca until the rights to ceftazidime-avibactam were acquired by Pfizer. Multiple authors reported financial relationships with AstraZeneca including grant funding, employment, and shareholding.
SOURCE: Torres A et al. Lancet Infect Dis. 2017. doi: 10.1016/S1473-3099(17)30747-8.
Ceftazidime-avibactam was noninferior to meropenem for nosocomial pneumonia including ventilator-associated pneumonia from gram-negative organisms, results from the REPROVE trial demonstrated.
Nosocomial or hospital-acquired pneumonia is a common hospital-acquired infection associated with increased cost and mortality. Further, nosocomial pneumonia is associated with gram-negative pathogens such as Pseudomonas aeruginosa and Enterobacteriaceae that may carry extended-spectrum beta-lactamases and carbapenemase, thereby limiting the treatment options. However, ceftazidime-avibactam has both antipseudomonal and extended beta-lactamase coverage for multidrug-resistant gram-negative infections, and may provide an alternative to meropenem.
Antoni Torres, MD, of the University of Barcelona and his colleagues sought to compare the safety and efficacy of ceftazidime-avibactam to meropenem in patients with nosocomial and ventilator-associated pneumonia. The REPROVE study was a phase 3, double-blind, noninferiority trial performed at 136 centers in 23 countries. Patients were randomly assigned 1:1 to receive either ceftazidime-avibactam (500-2,000 mg every 8 hours) or meropenem (1,000 mg every 8 hours) with adjustment as needed for renal function.
Participants included in the study were 18-90 years of age with nosocomial pneumonia as evidenced by pneumonia 48 hours or more after admission or within 7 days after discharge from an inpatient facility. Patients with ventilator-associated pneumonia had lung infection within 48 hours of intubation and mechanical ventilation. Sputum culture and gram stains were obtained within 48 hours before randomization, and patients were excluded for evidence of gram-positive–only pathogens or those not expected to respond to meropenem or ceftazidime-avibactam.
The study involved a safety population (808 patients), a clinically modified intention-to-treat population (726), and a clinically evaluable population (527). The intention-to-treat population demonstrated a predominance of Klebsiella pneumoniae (37%), and Pseudomonas aeruginosa (30%); 28% of the intention-to-treat population were identified as not susceptible to ceftazidime.
Overall, the clinically modified intention-to-treat group demonstrated a clinical cure rate of 68.8% (245/356) in the ceftazidime-avibactam and 73.0% (270/370) for the meropenem group (difference, –4.2%; 95% confidence interval, –10.8 to 2.5). The evaluable population demonstrated a clinical cure rate of 77.4% (199/257) in the ceftazidime-avibactam group and 78.1% (211/270) in the meropenem group (–0.7%; 95% CI, –7.9 to 6.4).
The all-cause mortality rate was similar between groups at the test-of-cure date and at day 28. The clinically modified intention-to-treat population demonstrated a mortality of 8.1% vs. 6.8% at the test-of-cure date and 8.4% vs. 7.3% at day 28 for ceftazidime-avibactam and meropenem, respectively.
Adverse events were noted in 75% vs. 74% of patients in the ceftazidime-avibactam groups and meropenem groups, respectively. Most adverse events were rated as mild to moderate and deemed likely unrelated to the treatment.
However, serious adverse events occurred in 19% (n = 75) in the ceftazidime-avibactam group and 13% (n = 54) in the meropenem group. Four serious adverse events were thought to be possibly related to the study drug ceftazidime-avibactam and included diarrhea, acute coronary syndrome, subacute hepatic failure, and abnormal liver function test results. The authors noted the adverse events in the trial were consistent and detected no new safety concerns for ceftazidime-avibactam.
Limitations of the study included an inability to establish the optimal duration of treatment for nosocomial pneumonia treated with meropenem or ceftazidime-avibactam.
“Our results show noninferiority for the treatment of nosocomial pneumonia caused by ceftazidime-nonsusceptible or ceftazidime-susceptible gram-negative aerobic pathogens,” the authors concluded.
The study was initially funded by AstraZeneca until the rights to ceftazidime-avibactam were acquired by Pfizer. Multiple authors reported financial relationships with AstraZeneca including grant funding, employment, and shareholding.
SOURCE: Torres A et al. Lancet Infect Dis. 2017. doi: 10.1016/S1473-3099(17)30747-8.
FROM THE LANCET INFECTIOUS DISEASES
Key clinical point: Ceftazidime-avibactam was noninferior to meropenem for nosocomial pneumonia.
Major finding: The clinically modified intention-to-treat group demonstrated clinical cure rates of 69% and 73% in the ceftazidime-avibactam vs. the meropenem group, respectively.
Data source: A phase 3, double-blind, noninferiority trial performed at 136 centers in 23 countries.
Disclosures: The study was initially funded by AstraZeneca until the rights to ceftazidime-avibactam were acquired by Pfizer. Multiple authors reported financial relationships with AstraZeneca including grant funding, employment, and shareholding.
Source: Torres A et al. Lancet Infect Dis. 2017. doi: 10.1016/S1473-3099(17)30747-8.
‘Real-world evidence’ used to compare agents for relapsing-remitting MS
SAN DIEGO – Delayed-release dimethyl fumarate did not show any differences versus fingolimod in relapse rate over a 1-year, “real-world” study of patients with relapsing-remitting multiple sclerosis, but a significantly greater proportion of patients taking delayed-release dimethyl fumarate achieved relapse-free status and a lower annualized relapsed rate, compared with patients on glatiramer acetate
“There is a need for real-world data that compares the effectiveness of the growing number of MS [multiple sclerosis] treatment options,” Christophe Hotermans, MD, vice president of Global Medical Therapeutic Areas at Boston-based Biogen, said in an interview during ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “These results were consistent with previous analyses of efficacy of these treatments in people with relapsing-remitting MS, which showed no significant differences in efficacy between delayed-release dimethyl fumarate [DMF, Tecfidera] versus fingolimod [FTY, Gilenya] and greater efficacy with dimethyl fumarate, compared with glatiramer acetate [GA].”
The findings come from EFFECT (Observational Study to Characterize Real-world Clinical Outcomes With Relapsing-remitting Multiple Sclerosis), a multicenter, international, retrospective, single-time-point medical record review study comparing the effectiveness of DMF vs. other disease-modifying therapies, including FTY and GA in patients with relapsing-remitting MS.
Endpoints included the Kaplan-Meier estimated proportion of patients who relapsed at 12 months and annualized relapse rate. Baseline covariates were used in estimating propensity scores. The data were divided into four strata using quartiles of propensity scores. After assessing for balance in baseline covariates between the treatment groups, Kaplan-Meier estimates of relapse and estimates of treatment effects were pooled across the four strata.
At the meeting, Jinny Min, PharmD, a medical postdoctoral research fellow at Biogen, reported results from 816 DMF patients, 781 FTY patients, and 1,042 GA patients. In the trimmed analysis set, the estimated proportion of DMF and FTY patients who relapsed at 12 months after treatment initiation was 12% vs. 13%, respectively (hazard ratio, 1.07, P = .693; the adjusted rate ratio for annualized relapse was 1.09, P = .617). In the analysis of DMF vs. GA patients, the estimated proportion of DMF patients that relapsed at 12 months was 12% vs. 21%, respectively (HR, 0.71), which represented a significant decrease of 29% (P less than .02). The adjusted rate ratio for annualized relapse was 0.69, representing a significant decrease of 31% (P less than .01).
“We hope that these data help health care providers and people living with MS as they consider their treatment options,” Dr. Hotermans said. “The limitations of this study are similar to those that would be present in other retrospective studies that utilize real-world data. However, we worked to mitigate many of those limitations through a propensity-score estimation approach to adjust for confounders. An additional limitation that is inherent to the study design (retrospective chart review) is that patients’ medical history, MS disease, treatment history, and relapse history were limited to the information available in the medical records.”
The study was supported by Biogen, which markets DMF. Dr. Hotermans and Dr. Min are employees of the company.
SOURCE: Min J et al. ACTRIMS Forum 2018, Abstract P016.
SAN DIEGO – Delayed-release dimethyl fumarate did not show any differences versus fingolimod in relapse rate over a 1-year, “real-world” study of patients with relapsing-remitting multiple sclerosis, but a significantly greater proportion of patients taking delayed-release dimethyl fumarate achieved relapse-free status and a lower annualized relapsed rate, compared with patients on glatiramer acetate
“There is a need for real-world data that compares the effectiveness of the growing number of MS [multiple sclerosis] treatment options,” Christophe Hotermans, MD, vice president of Global Medical Therapeutic Areas at Boston-based Biogen, said in an interview during ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “These results were consistent with previous analyses of efficacy of these treatments in people with relapsing-remitting MS, which showed no significant differences in efficacy between delayed-release dimethyl fumarate [DMF, Tecfidera] versus fingolimod [FTY, Gilenya] and greater efficacy with dimethyl fumarate, compared with glatiramer acetate [GA].”
The findings come from EFFECT (Observational Study to Characterize Real-world Clinical Outcomes With Relapsing-remitting Multiple Sclerosis), a multicenter, international, retrospective, single-time-point medical record review study comparing the effectiveness of DMF vs. other disease-modifying therapies, including FTY and GA in patients with relapsing-remitting MS.
Endpoints included the Kaplan-Meier estimated proportion of patients who relapsed at 12 months and annualized relapse rate. Baseline covariates were used in estimating propensity scores. The data were divided into four strata using quartiles of propensity scores. After assessing for balance in baseline covariates between the treatment groups, Kaplan-Meier estimates of relapse and estimates of treatment effects were pooled across the four strata.
At the meeting, Jinny Min, PharmD, a medical postdoctoral research fellow at Biogen, reported results from 816 DMF patients, 781 FTY patients, and 1,042 GA patients. In the trimmed analysis set, the estimated proportion of DMF and FTY patients who relapsed at 12 months after treatment initiation was 12% vs. 13%, respectively (hazard ratio, 1.07, P = .693; the adjusted rate ratio for annualized relapse was 1.09, P = .617). In the analysis of DMF vs. GA patients, the estimated proportion of DMF patients that relapsed at 12 months was 12% vs. 21%, respectively (HR, 0.71), which represented a significant decrease of 29% (P less than .02). The adjusted rate ratio for annualized relapse was 0.69, representing a significant decrease of 31% (P less than .01).
“We hope that these data help health care providers and people living with MS as they consider their treatment options,” Dr. Hotermans said. “The limitations of this study are similar to those that would be present in other retrospective studies that utilize real-world data. However, we worked to mitigate many of those limitations through a propensity-score estimation approach to adjust for confounders. An additional limitation that is inherent to the study design (retrospective chart review) is that patients’ medical history, MS disease, treatment history, and relapse history were limited to the information available in the medical records.”
The study was supported by Biogen, which markets DMF. Dr. Hotermans and Dr. Min are employees of the company.
SOURCE: Min J et al. ACTRIMS Forum 2018, Abstract P016.
SAN DIEGO – Delayed-release dimethyl fumarate did not show any differences versus fingolimod in relapse rate over a 1-year, “real-world” study of patients with relapsing-remitting multiple sclerosis, but a significantly greater proportion of patients taking delayed-release dimethyl fumarate achieved relapse-free status and a lower annualized relapsed rate, compared with patients on glatiramer acetate
“There is a need for real-world data that compares the effectiveness of the growing number of MS [multiple sclerosis] treatment options,” Christophe Hotermans, MD, vice president of Global Medical Therapeutic Areas at Boston-based Biogen, said in an interview during ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “These results were consistent with previous analyses of efficacy of these treatments in people with relapsing-remitting MS, which showed no significant differences in efficacy between delayed-release dimethyl fumarate [DMF, Tecfidera] versus fingolimod [FTY, Gilenya] and greater efficacy with dimethyl fumarate, compared with glatiramer acetate [GA].”
The findings come from EFFECT (Observational Study to Characterize Real-world Clinical Outcomes With Relapsing-remitting Multiple Sclerosis), a multicenter, international, retrospective, single-time-point medical record review study comparing the effectiveness of DMF vs. other disease-modifying therapies, including FTY and GA in patients with relapsing-remitting MS.
Endpoints included the Kaplan-Meier estimated proportion of patients who relapsed at 12 months and annualized relapse rate. Baseline covariates were used in estimating propensity scores. The data were divided into four strata using quartiles of propensity scores. After assessing for balance in baseline covariates between the treatment groups, Kaplan-Meier estimates of relapse and estimates of treatment effects were pooled across the four strata.
At the meeting, Jinny Min, PharmD, a medical postdoctoral research fellow at Biogen, reported results from 816 DMF patients, 781 FTY patients, and 1,042 GA patients. In the trimmed analysis set, the estimated proportion of DMF and FTY patients who relapsed at 12 months after treatment initiation was 12% vs. 13%, respectively (hazard ratio, 1.07, P = .693; the adjusted rate ratio for annualized relapse was 1.09, P = .617). In the analysis of DMF vs. GA patients, the estimated proportion of DMF patients that relapsed at 12 months was 12% vs. 21%, respectively (HR, 0.71), which represented a significant decrease of 29% (P less than .02). The adjusted rate ratio for annualized relapse was 0.69, representing a significant decrease of 31% (P less than .01).
“We hope that these data help health care providers and people living with MS as they consider their treatment options,” Dr. Hotermans said. “The limitations of this study are similar to those that would be present in other retrospective studies that utilize real-world data. However, we worked to mitigate many of those limitations through a propensity-score estimation approach to adjust for confounders. An additional limitation that is inherent to the study design (retrospective chart review) is that patients’ medical history, MS disease, treatment history, and relapse history were limited to the information available in the medical records.”
The study was supported by Biogen, which markets DMF. Dr. Hotermans and Dr. Min are employees of the company.
SOURCE: Min J et al. ACTRIMS Forum 2018, Abstract P016.
REPORTING FROM ACTRIMS FORUM 2018
Key clinical point:
Major finding: Delayed-release dimethyl fumarate had a 29% lower risk of relapse during the 12-month period vs. glatiramer acetate.
Study details: Results from a multicenter study of 816 delayed-release dimethyl fumarate patients, 781 fingolimod patients, and 1,042 glatiramer acetate patients with relapsing-remitting MS.
Disclosures: The study was supported by Biogen, which markets delayed-release dimethyl fumarate. Dr. Hotermans and Dr. Min are employees of the company.
Source: Min J et al. ACTRIMS Forum 2018, Abstract P016.
Macrophage activation syndrome’s impact in childhood SLE felt mostly early
Nearly 10% of children with systemic lupus erythematosus (SLE) developed macrophage activation syndrome (MAS) at some point during a mean follow-up time of more than 3 years at one center, and most were concomitantly diagnosed with the syndrome.
Although the investigators from the University of Toronto reported significantly higher mortality among patients with MAS, most cases were successfully treated with corticosteroids, and no relapses were observed during follow-up.
MAS was first identified in patients with juvenile idiopathic arthritis and is most well known as a complication of that broadly named disease, but data on outcomes and disease course in SLE patients are limited, first author Roberto Ezequiel Borgia, MD, and his colleagues wrote in their report in Arthritis & Rheumatology.
The researchers identified 403 children with SLE seen at the Hospital for Sick Children in Toronto during 2002-2012. Overall, 38 patients (9%) had MAS; of those patients, 68% received a MAS diagnosis within 7 days of the SLE diagnosis – termed “concomitant” diagnosis – while another 29% received a MAS diagnosis within 180 days of their SLE diagnosis.
The researchers explained that “since there are no validated nor universally accepted diagnostic criteria for MAS in SLE, the definition of MAS was based on the treating pediatric rheumatologist’s expert opinion at the time of the initial presentation.” The most common presenting feature of MAS was fever (100%), followed by generalized lymphadenopathy (24%), hepatomegaly (18%), CNS dysfunction secondary to MAS (18%), hemorrhage (13%), and splenomegaly (10%).
The average age of the children at diagnosis was nearly 14 years, and 79% were female. The average follow-up was 3.5 years. There were no significant differences in the demographic features of children with and without MAS nor were there any in variables used to assess lupus outcomes, which included immunosuppressive drug use, average daily corticosteroid dose (18.3 mg/day with MAS vs. 18.6 mg/day without MAS), and the number of pediatric ICU visits (incidence rate ratio for MAS vs. non-MAS, 1.60 [95% CI, 0.74-3.18]).
Mortality was significantly higher in children with MAS, compared with those without MAS (5.3% vs. 0.3%; P = .02), although the overall number of deaths in the cohort was small (n = 3). Apart from the “acute illness which was associated with 2 deaths secondary to MAS,” the investigators said that they “did not find any significant differences in the number of deaths or damage accrual between the cohorts, including overall SLICC [Systemic Lupus International Collaborating Clinics] damage score or any specific damage feature within the score.”
The study findings were limited by several factors including the lack of validated MAS criteria for children with SLE and a lack of follow-up data on the patients beyond 18 years of age, the researchers said.
The results suggest that MAS remains a life-threatening complication in children with SLE and should be considered an important cause of mortality for them, but “if the initial presentation does not result in death, the long-term outcome seem[s] to be comparable to those without MAS,” the investigators wrote.
The researchers had no financial conflicts to disclose.
SOURCE: Borgia R et al. Arthritis Rheumatol. 2018 Jan 17. doi: 10.1002/art.40417
As we learn more about the role of macrophage activation syndrome (MAS), a secondary form of hemophagocytic lymphohistiocytosis in rheumatic diseases, it has become clear that patients may develop this syndrome in a variety of settings. The most common presentation of MAS is in association with systemic onset juvenile idiopathic arthritis, but is has been described in other forms of childhood rheumatic diseases, including other types of juvenile idiopathic arthritis, lupus, mixed connective tissue disease, Kawasaki disease, and sarcoidosis. Study of secondary MAS has led to suggested diagnostic criteria; however, those criteria are very similar to the presentation of adult and childhood systemic lupus with cytopenias, hepatitis, and coagulopathy.
The work by Borgia et al. encourages us to look for evidence of MAS in our lupus patients as it allows us to identify patients at risk for poor outcomes and to provide interventions to reduce those risks.
Marisa S. Klein-Gitelman, MD , is a professor of pediatrics at Northwestern University, Chicago, and is a pediatric rheumatologist at the Ann & Robert H. Lurie Children’s Hospital of Chicago. She has no relevant disclosures.
As we learn more about the role of macrophage activation syndrome (MAS), a secondary form of hemophagocytic lymphohistiocytosis in rheumatic diseases, it has become clear that patients may develop this syndrome in a variety of settings. The most common presentation of MAS is in association with systemic onset juvenile idiopathic arthritis, but is has been described in other forms of childhood rheumatic diseases, including other types of juvenile idiopathic arthritis, lupus, mixed connective tissue disease, Kawasaki disease, and sarcoidosis. Study of secondary MAS has led to suggested diagnostic criteria; however, those criteria are very similar to the presentation of adult and childhood systemic lupus with cytopenias, hepatitis, and coagulopathy.
The work by Borgia et al. encourages us to look for evidence of MAS in our lupus patients as it allows us to identify patients at risk for poor outcomes and to provide interventions to reduce those risks.
Marisa S. Klein-Gitelman, MD , is a professor of pediatrics at Northwestern University, Chicago, and is a pediatric rheumatologist at the Ann & Robert H. Lurie Children’s Hospital of Chicago. She has no relevant disclosures.
As we learn more about the role of macrophage activation syndrome (MAS), a secondary form of hemophagocytic lymphohistiocytosis in rheumatic diseases, it has become clear that patients may develop this syndrome in a variety of settings. The most common presentation of MAS is in association with systemic onset juvenile idiopathic arthritis, but is has been described in other forms of childhood rheumatic diseases, including other types of juvenile idiopathic arthritis, lupus, mixed connective tissue disease, Kawasaki disease, and sarcoidosis. Study of secondary MAS has led to suggested diagnostic criteria; however, those criteria are very similar to the presentation of adult and childhood systemic lupus with cytopenias, hepatitis, and coagulopathy.
The work by Borgia et al. encourages us to look for evidence of MAS in our lupus patients as it allows us to identify patients at risk for poor outcomes and to provide interventions to reduce those risks.
Marisa S. Klein-Gitelman, MD , is a professor of pediatrics at Northwestern University, Chicago, and is a pediatric rheumatologist at the Ann & Robert H. Lurie Children’s Hospital of Chicago. She has no relevant disclosures.
Nearly 10% of children with systemic lupus erythematosus (SLE) developed macrophage activation syndrome (MAS) at some point during a mean follow-up time of more than 3 years at one center, and most were concomitantly diagnosed with the syndrome.
Although the investigators from the University of Toronto reported significantly higher mortality among patients with MAS, most cases were successfully treated with corticosteroids, and no relapses were observed during follow-up.
MAS was first identified in patients with juvenile idiopathic arthritis and is most well known as a complication of that broadly named disease, but data on outcomes and disease course in SLE patients are limited, first author Roberto Ezequiel Borgia, MD, and his colleagues wrote in their report in Arthritis & Rheumatology.
The researchers identified 403 children with SLE seen at the Hospital for Sick Children in Toronto during 2002-2012. Overall, 38 patients (9%) had MAS; of those patients, 68% received a MAS diagnosis within 7 days of the SLE diagnosis – termed “concomitant” diagnosis – while another 29% received a MAS diagnosis within 180 days of their SLE diagnosis.
The researchers explained that “since there are no validated nor universally accepted diagnostic criteria for MAS in SLE, the definition of MAS was based on the treating pediatric rheumatologist’s expert opinion at the time of the initial presentation.” The most common presenting feature of MAS was fever (100%), followed by generalized lymphadenopathy (24%), hepatomegaly (18%), CNS dysfunction secondary to MAS (18%), hemorrhage (13%), and splenomegaly (10%).
The average age of the children at diagnosis was nearly 14 years, and 79% were female. The average follow-up was 3.5 years. There were no significant differences in the demographic features of children with and without MAS nor were there any in variables used to assess lupus outcomes, which included immunosuppressive drug use, average daily corticosteroid dose (18.3 mg/day with MAS vs. 18.6 mg/day without MAS), and the number of pediatric ICU visits (incidence rate ratio for MAS vs. non-MAS, 1.60 [95% CI, 0.74-3.18]).
Mortality was significantly higher in children with MAS, compared with those without MAS (5.3% vs. 0.3%; P = .02), although the overall number of deaths in the cohort was small (n = 3). Apart from the “acute illness which was associated with 2 deaths secondary to MAS,” the investigators said that they “did not find any significant differences in the number of deaths or damage accrual between the cohorts, including overall SLICC [Systemic Lupus International Collaborating Clinics] damage score or any specific damage feature within the score.”
The study findings were limited by several factors including the lack of validated MAS criteria for children with SLE and a lack of follow-up data on the patients beyond 18 years of age, the researchers said.
The results suggest that MAS remains a life-threatening complication in children with SLE and should be considered an important cause of mortality for them, but “if the initial presentation does not result in death, the long-term outcome seem[s] to be comparable to those without MAS,” the investigators wrote.
The researchers had no financial conflicts to disclose.
SOURCE: Borgia R et al. Arthritis Rheumatol. 2018 Jan 17. doi: 10.1002/art.40417
Nearly 10% of children with systemic lupus erythematosus (SLE) developed macrophage activation syndrome (MAS) at some point during a mean follow-up time of more than 3 years at one center, and most were concomitantly diagnosed with the syndrome.
Although the investigators from the University of Toronto reported significantly higher mortality among patients with MAS, most cases were successfully treated with corticosteroids, and no relapses were observed during follow-up.
MAS was first identified in patients with juvenile idiopathic arthritis and is most well known as a complication of that broadly named disease, but data on outcomes and disease course in SLE patients are limited, first author Roberto Ezequiel Borgia, MD, and his colleagues wrote in their report in Arthritis & Rheumatology.
The researchers identified 403 children with SLE seen at the Hospital for Sick Children in Toronto during 2002-2012. Overall, 38 patients (9%) had MAS; of those patients, 68% received a MAS diagnosis within 7 days of the SLE diagnosis – termed “concomitant” diagnosis – while another 29% received a MAS diagnosis within 180 days of their SLE diagnosis.
The researchers explained that “since there are no validated nor universally accepted diagnostic criteria for MAS in SLE, the definition of MAS was based on the treating pediatric rheumatologist’s expert opinion at the time of the initial presentation.” The most common presenting feature of MAS was fever (100%), followed by generalized lymphadenopathy (24%), hepatomegaly (18%), CNS dysfunction secondary to MAS (18%), hemorrhage (13%), and splenomegaly (10%).
The average age of the children at diagnosis was nearly 14 years, and 79% were female. The average follow-up was 3.5 years. There were no significant differences in the demographic features of children with and without MAS nor were there any in variables used to assess lupus outcomes, which included immunosuppressive drug use, average daily corticosteroid dose (18.3 mg/day with MAS vs. 18.6 mg/day without MAS), and the number of pediatric ICU visits (incidence rate ratio for MAS vs. non-MAS, 1.60 [95% CI, 0.74-3.18]).
Mortality was significantly higher in children with MAS, compared with those without MAS (5.3% vs. 0.3%; P = .02), although the overall number of deaths in the cohort was small (n = 3). Apart from the “acute illness which was associated with 2 deaths secondary to MAS,” the investigators said that they “did not find any significant differences in the number of deaths or damage accrual between the cohorts, including overall SLICC [Systemic Lupus International Collaborating Clinics] damage score or any specific damage feature within the score.”
The study findings were limited by several factors including the lack of validated MAS criteria for children with SLE and a lack of follow-up data on the patients beyond 18 years of age, the researchers said.
The results suggest that MAS remains a life-threatening complication in children with SLE and should be considered an important cause of mortality for them, but “if the initial presentation does not result in death, the long-term outcome seem[s] to be comparable to those without MAS,” the investigators wrote.
The researchers had no financial conflicts to disclose.
SOURCE: Borgia R et al. Arthritis Rheumatol. 2018 Jan 17. doi: 10.1002/art.40417
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: Nearly 10% of children with SLE developed MAS at some point during a mean follow-up time of more than 3 years, but many outcomes were the same in patients with and without MAS.
Major finding: Mortality was 5.3% in children with MAS, compared with 0.3% in those without MAS (P = .02), over a 3.5-year follow-up period.
Study details: The data come from 403 children with SLE seen at a single center during 2002-2012.
Disclosures: The researchers had no financial conflicts to disclose.
Source: Borgia R et al. Arthritis Rheumatol. 2018 Jan 17. doi: 10.1002/art.40417.
Study seeks optimal duration of second stage of labor
, according to researchers.
The researchers performed a retrospective analysis of more than 103,000 pregnancies from the Consortium on Safe Labor, a study of electronic medical records from 12 U.S. sites from 2002 to 2008, according to a study published in Obstetrics & Gynecology.
The duration of the second stage was calculated from the time of 10-cm cervical dilation to the time of birth. The researchers stratified the results into nulliparous women who did or did not receive an epidural, and multiparous women who did or did not receive an epidural.
For nulliparous women, rates of spontaneous vaginal birth (rather than operative vaginal birth or cesarean delivery) without morbidity increased slightly in the second half-hour over the first, then decreased. Rates decreased steadily for multiparous women. Both decreases occurred regardless of epidural status. Deliveries with morbidity varied, to as high as a 12.3% likelihood that a nulliparous woman with an epidural would deliver with maternal or neonatal morbidity or mortality between 3 hours’ and 6 hours’ second-stage duration.
The researchers noted the various society recommendations for when to diagnose second-stage arrest, but concluded: “In our study, we did not observe an inflection at a particular hour mark. ... Ultimately the willingness to accept a certain percentage risk of morbidity to achieve vaginal delivery is up to the woman and clinician.”
The authors reported having no disclosures.
SOURCE: Grantz KL et al. Obstet Gynecol. 2018 Feb;131(2):345-53.
, according to researchers.
The researchers performed a retrospective analysis of more than 103,000 pregnancies from the Consortium on Safe Labor, a study of electronic medical records from 12 U.S. sites from 2002 to 2008, according to a study published in Obstetrics & Gynecology.
The duration of the second stage was calculated from the time of 10-cm cervical dilation to the time of birth. The researchers stratified the results into nulliparous women who did or did not receive an epidural, and multiparous women who did or did not receive an epidural.
For nulliparous women, rates of spontaneous vaginal birth (rather than operative vaginal birth or cesarean delivery) without morbidity increased slightly in the second half-hour over the first, then decreased. Rates decreased steadily for multiparous women. Both decreases occurred regardless of epidural status. Deliveries with morbidity varied, to as high as a 12.3% likelihood that a nulliparous woman with an epidural would deliver with maternal or neonatal morbidity or mortality between 3 hours’ and 6 hours’ second-stage duration.
The researchers noted the various society recommendations for when to diagnose second-stage arrest, but concluded: “In our study, we did not observe an inflection at a particular hour mark. ... Ultimately the willingness to accept a certain percentage risk of morbidity to achieve vaginal delivery is up to the woman and clinician.”
The authors reported having no disclosures.
SOURCE: Grantz KL et al. Obstet Gynecol. 2018 Feb;131(2):345-53.
, according to researchers.
The researchers performed a retrospective analysis of more than 103,000 pregnancies from the Consortium on Safe Labor, a study of electronic medical records from 12 U.S. sites from 2002 to 2008, according to a study published in Obstetrics & Gynecology.
The duration of the second stage was calculated from the time of 10-cm cervical dilation to the time of birth. The researchers stratified the results into nulliparous women who did or did not receive an epidural, and multiparous women who did or did not receive an epidural.
For nulliparous women, rates of spontaneous vaginal birth (rather than operative vaginal birth or cesarean delivery) without morbidity increased slightly in the second half-hour over the first, then decreased. Rates decreased steadily for multiparous women. Both decreases occurred regardless of epidural status. Deliveries with morbidity varied, to as high as a 12.3% likelihood that a nulliparous woman with an epidural would deliver with maternal or neonatal morbidity or mortality between 3 hours’ and 6 hours’ second-stage duration.
The researchers noted the various society recommendations for when to diagnose second-stage arrest, but concluded: “In our study, we did not observe an inflection at a particular hour mark. ... Ultimately the willingness to accept a certain percentage risk of morbidity to achieve vaginal delivery is up to the woman and clinician.”
The authors reported having no disclosures.
SOURCE: Grantz KL et al. Obstet Gynecol. 2018 Feb;131(2):345-53.
FROM OBSTETRICS & GYNECOLOGY
Treatment of Biliary Tract Cancers
Introduction
Biliary tract carcinoma (BTC) is th
Epidemiology
In the United States, BTC is rare and accounts for approximately 4% of all gastrointestinal malignancies, with an estimated 6000 to 7000 cases of carcinoma of the gallbladder and 3000 to 4000 cases of carcinoma of the bile duct diagnosed annually.4 Among women, there is a 26-fold variation in BTC mortality worldwide, ranging from 0.8 deaths per 100,000 in South Africa to 21.2 per 100,000 in Chile.1,5 Interestingly, for American Indians in New Mexico, gallbladder cancer mortality rates (8.9 per 100,000) surpass those for breast and pancreatic cancers.6 The incidence of anatomical cholangiocarcinoma subtypes also varies regionally, reflecting disparities in genetic and environmental predisposing factors.2,7 In a large, single-center study in the United States, intrahepatic cholangiocarcinoma accounted for less than 10% of cases, perihilar accounted for 50%, and distal accounted for the remaining 40%.8 Importantly, intrahepatic cholangiocarcinoma is the second most common primary malignancy of the liver, and its incidence seems to be rising in many western countries. In the United States, there has been an estimated 128% rise over the past 40 years.4,9
BTC is associated with high mortality rates.10 Median overall survival (OS) for cholangiocarcinoma is 20 to 28 months and 5-year survival is around 25%.10 Most cholangiocarcinomas are diagnosed at advanced stages with unresectable tumors.10 Furthermore, outcomes following resection with curative intent are poor—median disease-free survival (DFS) of 12 to 36 months has been reported.11,12 Patients with intrahepatic disease have a better prognosis when compared with patients who have extrahepatic tumors.12 Gallbladder cancer, likewise, carries a poor overall prognosis; median OS is 32 months and 5-year survival is as low as 13%.6
Risk factors for BTC include intrinsic and extrinsic elements.6 Incidence of BTC increases with age, and diagnosis typically occurs in the sixth to eighth decade of life.5,6,13 In contrast to gallbladder cancer, the incidence of cholangiocarcinoma is slightly higher in men.9 Obesity, diabetes, and consumption of sweetened drinks also increase the risk for BTC.14–16 Cholelithiasis is the most prevalent risk factor for gallbladder cancer, and the risk is greater for larger stones.5 Around 1 in 5 patients with porcelain gallbladder will develop gallbladder carcinoma.17 Primary sclerosing cholangitis (PSC), chronic calculi of the bile duct, choledochal cysts, cirrhosis, hepatitis C, and liver fluke infections are well established risk factors for cholangiocarcinoma.7,12,18 PSC is one of the best described entities among these predisposing conditions. Lifetime prevalence of cholangiocarcinoma among patients with PSC ranges from 5% to 10%.18,19 These patients also present at a younger age; in one series, the median age at diagnosis for BTC arising from PSC was 39 years.18 It is important to recognize, however, that in most patients diagnosed with cholangiocarcinoma, no predisposing factors are identified.8
Diagnosis
Clinical Presentation
Clinical presentation of BTC depends upon anatomic location.20 Patients with early invasive gallbladder cancer are most often asymptomatic.21 When symptoms occur, they may be nonspecific and mimic cholelithiasis.21 The most common clinical presentations include jaundice, weight loss, and abdominal pain.21 Prior to widespread availability of imaging studies, the preoperative diagnosis rate for gallbladder cancer was as low as 10%.22 However, the accuracy of computed tomography (CT) has changed this scenario, with sensitivity ranging from 73% to 87% and specificity from 88% to 100%.21 As a result of its silent clinical character, cholangiocarcinoma is frequently difficult to diagnose.23 Perihilar and distal cholangiocarcinoma characteristically present with signs of biliary obstruction, and imaging and laboratory data can corroborate the presence of cholestasis.24 On examination, patients with extrahepatic cholangiocarcinoma may present with jaundice, hepatomegaly, and a palpable right upper quadrant mass.25 A palpable gallbladder (Courvoisier sign) can also be present.25 Intrahepatic cholangiocarcinoma presents differently, and patients are less likely to be jaundiced.23 Typical clinical features are nonspecific and include dull right upper quadrant pain, weight loss, and an elevated alkaline phosphatase level.23 Alternatively, asymptomatic patients can present with incidentally detected lesions, when imaging is obtained as part of the workup for other causes or during screening for hepatocellular carcinoma in patients with viral hepatitis or cirrhosis.23,26 Uncommonly, BTC patients present because of signs or symptoms related to metastatic disease or evidence of metastatic disease on imaging.
Pathology and Grading
The majority of BTCs are adenocarcinomas, corresponding to 90% of cholangiocarcinomas and 99% of gallbladder cancers.27,28 They are graded as well, moderately, or poorly differentiated.2 Adenosquamous and squamous cell carcinoma are responsible for most of the remaining cases.2,29 Cholangiocarcinomas are divided into 3 types, defined by the Liver Cancer Study Group of Japan: (1) mass-forming, (2) periductal-infiltrating, and (3) intraductal-growing.30,31 Mass-forming intrahepatic cholangiocarcinomas are characterized morphologically by a homogeneous gray-yellow mass with frequent satellite nodules and irregular but well-defined margins.17,30 Central necrosis and fibrosis are also common.30 In the periductal-infiltrating type, tumor typically grows along the bile duct wall without mass formation, resulting in concentric mural thickening and proximal biliary dilation.30 Intraductal-growing papillary cholangiocarcinoma is characterized by the presence of intraluminal papillary or tubular polypoid tumors of the intra- or extrahepatic bile ducts, with partial obstruction and proximal biliary dilation.30
Cholangiocarcinoma
Case Presentation
A previously healthy 59-year-old man presents to his primary care physician with a 3-month history of dull right upper quadrant pain associated with weight loss. The patient is markedly cachectic and abdominal examination reveals upper quadrant tenderness. Laboratory exams are significant for elevated alkaline phosphatase (500 U/L; reference range 45–115 U/L), cancer antigen 19-9 (CA 19-9, 73 U/mL; reference range ≤ 37 U/mL), and carcinoembryonic antigen (CEA , 20 ng/mL; reference range for nonsmokers ≤ 3.0 ng/mL). Aspartate aminotransferase, alanine aminotransferase, total bilirubin, and coagulation studies are within normal range. Ultrasound demonstrates a homogeneous mass with irregular borders in the right lobe of the liver. Triphasic contrast-enhanced CT scan demonstrates a tumor with ragged rim enhancement at the periphery, and portal venous phase shows gradual centripetal enhancement of the tumor with capsular retraction. No abdominal lymph nodes or extrahepatic tumors are noted (Figure 1, Image A).
- What are the next diagnostic steps?
The most critical differential diagnosis of solid liver mass in patients without cirrhosis is cholangiocarcinoma and metastases from another primary site.32 Alternatively, when an intrahepatic lesion is noted on an imaging study in the setting of cirrhosis, the next diagnostic step is differentiation between cholangiocarcinoma and hepatocellular carcinoma (HCC).32 Triphasic contrast-enhanced CT and dynamic magnetic resonance imaging (MRI) are key diagnostic procedures.32,33 In the appropriate setting, classical imaging features in the arterial phase with washout in portal venous or delayed phase can be diagnostic of HCC and may obviate the need for a biopsy (Figure 2).
Typical radiographic features of cholangiocarcinoma include a hypodense hepatic lesion that can be either well-defined or infiltrative and is frequently associated with biliary dilatation (Figure 1, Image A).33 The dense fibrotic nature of the tumor may cause capsular retraction, which is seen in up to 20% of cases.17 This finding is highly suggestive of cholangiocarcinoma and is rarely present in HCC.33 Following contrast administration, there is peripheral (rim) enhancement throughout both arterial and venous phases.32–34 However, these classic features were present in only 70% of cases in one study.35 Although intrahepatic cholangiocarcinomas are most commonly hypovascular, small mass-forming intrahepatic cholangiocarcinomas can often be arterially hyperenhancing and mimic HCC.33 Tumor enhancement on delayed CT imaging has been correlated with survival. Asayama et al demonstrated that tumors that exhibited delayed enhancement on CT in more than two-thirds of their volume were associated with a worse prognosis.36
Patients without cirrhosis who present with a localized lesion of the liver should undergo extensive evaluation for a primary cancer site.37 CT of the chest, abdomen, and pelvis with contrast should be obtained.37 Additionally, mammogram and endoscopic evaluation with esophagogastroduodenoscopy (EGD) and colonoscopy should be included in the work-up.37
Preoperative tumor markers are also included in the work-up. All patients with a solid liver lesion should have serum alpha-fetoprotein (AFP) levels checked. AFP is a serum glycoprotein recognized as a marker for HCC and is reported to detect preclinical HCC.38 However, serum concentrations are normal in up to 40% of small HCCs.38 Although no specific marker for cholangiocarcinoma has yet been identified, the presence of certain tumor markers in the serum of patients may be of diagnostic value, especially in patients with PSC. CA 19-9 and CEA are the best studied. Elevated levels of CA 19-9 prior to treatment are associated with a poorer prognosis, and CA 19-9 concentrations greater than 1000 U/mL are consistent with advanced disease.39,40 One large series evaluated the diagnostic value of serum CEA levels in 333 patients with PSC, 13% of whom were diagnosed with cholangiocarcinoma.34 A serum CEA level greater than 5.2 ng/mL had a sensitivity of 68.0% and specificity of 81.5%.38
If a biopsy is obtained, appropriate immunohistochemistry (IHC) can facilitate the diagnosis. BTC is strongly positive for CK-7 and CK-19.41 CK-7 positivity is not specific and is also common among metastatic cancers of the lung and breast; therefore, in some cases cholangiocarcinoma may be a diagnosis of exclusion. Immunostaining for monoclonal CEA is diffusely positive in up to 75% of cases.41 An IHC panel consisting of Hep Par-1, arginase-1, monoclonal CEA, CK-7, CK-20, TTF-1, MOC-31, and CDX-2 has been proposed to optimize the differential diagnosis of HCC, metastatic adenocarcinoma, and cholangiocarcinoma.41
Case Continued
CT of the chest, abdomen, and pelvis reveals no concerns for metastasis and no evidence of primary cancer elsewhere. EGD and colonoscopy are clear. AFP levels are within normal limits (2 ng/mL). Biopsy is performed and demonstrates adenocarcinoma. IHC studies demonstrate cells positive for monoclonal CEA, CK-7, CK-19, and MOC-31, and negative for Napsin A, TTF-1, and CK-20.
- How is cholangiocarcinoma staged and classified?
The purpose of the staging system is to provide information on prognosis and guidance for therapy. Prognostic factors and the therapeutic approaches for BTC differ depending upon their location in the biliary tree. Accordingly, TNM classification systems for intrahepatic, hilar, and distal cholangiocarcinoma and gallbladder cancer have been separated (Table 1 and Table 2).23
The Bismuth-Corlette classification is used to further classify perihilar cholangiocarcinoma according to patterns of hepatic duct involvement. Type I tumors are located below the confluence of the left and right hepatic ducts.42 Type II reach the confluence of the hepatic ducts.42 Type III occlude the common hepatic duct and either the right or left hepatic duct (IIIa and IIIb, respectively).42 Finally, type IV are multicentric, or involve the confluence and both the right and left hepatic ducts.42 Tumors that involve the common hepatic duct bifurcation are named Klatskin tumors.42
- What is the first-line treatment for localized cholangiocarcinomas?
Surgical resection is the only potentially curative treatment for localized cholangiocarcinoma, although fewer than 20% of patients are suitable for curative treatment, due to the presence of advanced disease at diagnosis.43,44 Available evidence supports the recommendation that resection with negative margins, regardless of extent, should be the goal of therapy for patients with potentially resectable disease.44 Extensive hepatic resections are often necessary to achieve clear margins since the majority of patients present with large masses. Substantial evidence corroborates that R0 resection is associated with better survival, whereas the benefit of wide compared to narrow (< 5–10 mm) margins is unclear.45 A recent analysis of 96 patients suggests that the proximal resection margin has more prognostic implications than distal margins.45
Surgical options and resectability criteria depend upon tumor location. Extent of tumor in the bile duct is one of the most important factors that determine resectability.17 Although multifocal liver tumors (including satellite lesions), lymph node metastases to the porta hepatis, and distant metastases are considered relative contraindications to surgery, surgical approaches can be considered in selected patients.43 Patient selection for surgery is facilitated by careful preoperative staging, which may include laparoscopy. Laparoscopic staging prior to resection may prevent unnecessary laparotomy in 30% to 45% of patients.42,46
- Is there a role for adjuvant treatment?
Recurrence following complete resection is a primary limitation for cure in BTC, which provides a rationale for the use of adjuvant therapy.47,48 In a sample of 79 patients with extrahepatic cholangiocarcinoma who underwent curative resection, the cumulative recurrence rate after 4 years was 56%.47 Initial recurrence at a distant site occurs in 40% to 50% of patients.48
Lymphovascular and perineural invasion, lymph node metastasis, and tumor size ≥ 5 cm have been reported as independent predictors of recurrence and mortality following resection.49 A 2017 meta-analysis which included 30 studies involving more than 22,499 patients reported a 41% reduction in the risk of death with adjuvant chemotherapy, which translated to a mean OS benefit of 4 months in an unselected population.49 Moreover, this study revealed inferior OS in patients given adjuvant radiation therapy (RT) in combination with chemotherapy.49 These results are in line with the previous meta-analysis by Horgan et al, which demonstrated that adjuvant RT seems to benefit only patients with R1 resections, with a possible detrimental effect in R0 disease.50 Therefore, adjuvant chemoradiation cannot be viewed as a standard practice following R0 resection, and should be reserved for those patients with positive margins (R1/ 2) to reduce local progression.
In the phase 3 BILCAP trial presented at ASCO 2017, 447 patients with completely resected cholangiocarcinoma or gallbladder cancer with adequate biliary drainage and Eastern Cooperative Oncology Group (ECOG) performance score ≤ 2 were randomly assigned to observation or capecitabine (1250 mg/m2 twice daily for days 1–14 every 21 days for 8 cycles).51 Surgical treatment achieved R0 resection in 62% of patients and 46% were node-negative. Median OS was 51 months for the capecitabine group and 36 months for the control arm (hazard ratio [HR] 0.80, 95% CI 0.63 to 1.04, P = 0.097). Analyses with adjustment for nodal status, grade of disease, and gender indicated a HR of 0.71 (P < 0.01). Median DFS was 25 months versus 18 months favoring the capecitabine group, and rates of grade 3 or 4 toxicity were less than anticipated. Following the results of this trial, adjuvant capecitabine should become the new standard of care.
- What is the treatment for locally advanced cholangiocarcinoma?
The optimal approach to patients with locally advanced unresectable cholangiocarcinoma has not been established. The prognosis for patients with either locally unresectable or locally recurrent disease is typically measured in months. Goals of palliative therapy are relief of symptoms and improvement in quality of life, and there is no role for surgical debulking.
Liver transplantation is a potentially curative option for selected patients with hilar or intrahepatic cholangiocarcinoma. Patients with lymph node-negative, non-disseminated, locally advanced hilar cholangiocarcinomas have 5-year survival rates ranging from 25% to 42% following transplantation.52 Retrospective data suggests that neoadjuvant chemoradiation followed by liver transplantation is highly effective for selected patients with hilar cholangiocarcinoma.52 However, these results require confirmation from prospective clinical evidence. It is important to recognize that liver transplantation plays no role in the management of distal cholangiocarcinoma or gallbladder cancer.
Rarely, patients with borderline resectable intrahepatic cholangiocarcinoma will have a sufficient response to chemotherapy to permit later resection, and, in such cases, starting with chemotherapy and then restaging to evaluate resectability is appropriate.54 A single-center, retrospective analysis including 186 patients by Le Roy et al evaluated survival in patients with locally advanced, unresectable intrahepatic cholangiocarcinoma who received primary chemotherapy, followed by surgery in those with secondary resectability.54 After a median of 6 cycles of chemotherapy, 53% of patients achieved resectability and underwent surgery with curative intent. These patients had similar short- and long-term results compared to patients with initially resectable intrahepatic cholangiocarcinoma who had surgery alone, with median OS reaching 24 months.54
Ablative radiotherapy is an additional option for localized inoperable intrahepatic cholangiocarcinoma. Tao and colleagues evaluated 79 consecutive patients with inoperable intrahepatic cholangiocarcinoma treated with definitive RT.55 Median tumor size was 7.9 cm and 89% of patients received chemotherapy before RT. Median OS was 30 months and 3-year OS was 44%. Radiation dose was the single most important prognostic factor, and higher doses correlated with improved local control and OS. A biologic equivalent dose (BED) greater than 80.5 Gy was identified as an ablative dose of RT for large intrahepatic cholangiocarcinomas. The 3-year OS for patients receiving BED greater than 80.5 Gy was 73% versus 38% for those receiving lower doses.
Case Continued
The patient is deemed to have resectable disease and undergoes surgical resection followed by adjuvant capecitabine for 8 cycles. Unfortunately, after 1 year, follow-up imaging identifies bilateral enlarging lung nodules. Biopsy is performed and confirms metastatic cholangiocarcinoma.
- What is the treatment for metastatic BTC?
The prognosis of patients with advanced BTC is poor and OS for those undergoing supportive care alone is short. A benefit of chemotherapy over best supportive care for cholangiocarcinoma was demonstrated in an early phase 3 trial that randomly assigned 90 patients with advanced pancreatic or biliary cancer (37 with bile duct cancer) to receive either fluorouracil (FU) -based systemic chemotherapy or best supportive care. Results showed that chemotherapy significantly improved OS (6 months versus 2.5 months).56 Chemotherapy is also beneficial for patients with unresectable gallbladder cancer. In a single-center randomized study including 81 patients with unresectable gallbladder cancer, gemcitabine and oxaliplatin (GEMOX) improved progression-free survival (PFS) and OS compared to best supportive care.57 Treatment for metastatic cholangiocarcinoma and gallbladder cancer follows the same algorithm.
In 2010, cisplatin plus gemcitabine was established as a reference regimen for first-line therapy by the ABC-02 study, in which 410 patients with locally advanced or metastatic bile duct, gallbladder, or ampullary cancer were randomly assigned to 6 courses of cisplatin (25 mg/m2) plus gemcitabine (1000 mg/m2 on days 1 and 8, every 21 days) or gemcitabine alone (1000 mg/m2 days 1, 8, 15, every 28 days).58 OS was significantly greater with combination therapy (11.7 versus 8.1 months), and PFS also favored the combination arm (8 versus 5 months). Toxicity was comparable in both groups, with the exception of significantly higher rates of grade 3 or 4 neutropenia with gemcitabine plus cisplatin (25% versus 17%), and higher rates of grade 3 or 4 abnormal liver function with gemcitabine alone (27% versus 17%). Most quality-of-life scales showed a trend favoring combined therapy.58 A smaller, identically designed Japanese phase 3 randomized trial achieved similar results, demonstrating greater OS with cisplatin plus gemcitabine compared to gemcitabine alone (11.2 versus 7.7 months).59
The gemcitabine plus cisplatin combination has not been directly compared with other gemcitabine combinations in phase 3 trials. A pooled analysis of 104 trials of a variety of chemotherapy regimens in advanced biliary cancer concluded that the gemcitabine plus cisplatin regimen offered the highest rates of objective response and tumor control compared with either gemcitabine-free or cisplatin-free regimens.60 However, this did not translate into significant benefit in terms of either time to tumor progression or median OS. It is important to note that this analysis did not include results of the subsequent ABC-02 trial.
There is no standard treatment for patients with cholangiocarcinoma for whom first-line gemcitabine-based therapy fails. There are no completed prospective phase 3 trials supporting the use of second-line chemotherapy after failure of first-line chemotherapy in BTC, and the selection of candidates for second-line therapy as well as the optimal regimen are not established.61 The ongoing phase 2 multicenter ABC-06 trial is evaluating oxaliplatin plus short-term infusional FU and leucovorin (FOLFOX) versus best supportive care for second-line therapy. In a systematic review including 23 studies (14 phase 2 clinical trials and 9 retrospective studies) with 761 patients with BTC, the median OS was 7.2 months.
The optimal selection of candidates for second-line chemotherapy is not established. Two independent studies suggest that patients who have a good performance status (0 or 1), disease control with the first-line chemotherapy, low CA 19-9 level, and possibly previous surgery on their primary tumor, have the longest survival with second-line chemotherapy. However, whether these characteristics predict for chemotherapy responsiveness or more favorable biologic behavior is not clear.62,63 No particular regimen has proved superior to any other, and the choice of second-line regimen remains empiric.
For patients with adequate performance status, examples of other conventional chemotherapy regimens with demonstrated activity that could be considered for second-line therapy include: FOLFOX or capecitabine, gemcitabine plus capecitabine, capecitabine plus cisplatin, or irinotecan plus short-term infusional FU and leucovorin (FOLFIRI) with or without bevacizumab.64 For selected patients, second-line molecularly targeted therapy using erlotinib plus bevacizumab may be considered. However, this regimen is very costly.64 Examples of other regimens with demonstrated activity in phase 2 trials include GEMOX, gemcitabine plus fluoropyrimidine, and fluoropyrimidine plus oxaliplatin or cisplatin.64
There is promising data from studies of targeted therapy for specific molecular subgroups. A recent phase 2 trial evaluated the activity of BGJ398, an orally bioavailable, selective, ATP-competitive pan inhibitor of human fibroblast growth factor receptor (FGFR) kinase, in patients with FGFR-altered advanced cholangiocarcinoma.65 The overall response rate was 14.8% (18.8% FGFR2 fusions only) and disease control rate was 75.4% (83.3% FGFR2 fusions only). All responsive tumors contained FGFR2 fusions. Adverse events were manageable, and grade 3 or 4 treatment-related adverse events occurred in 25 patients (41%). Those included hyperphosphatemia, stomatitis, and palmar-plantar erythrodysesthesia. Javle and colleagues also identified HER2/neu blockade as a promising treatment strategy for gallbladder cancer patients with this gene amplification.66 This retrospective analysis included 9 patients with gallbladder cancer and 5 patients with cholangiocarcinoma who received HER2/neu-directed therapy (trastuzumab, lapatinib, or pertuzumab). In the gallbladder cancer group, HER2/neu gene amplification or overexpression was detected in 8 cases. These patients experienced disease stability (n = 3), partial response (n = 4), or complete response (n = 1) with HER2/neu–directed therapy. Median duration of response was 40 weeks. The cholangiocarcinoma cases treated in this series had no radiological responses despite HER2/neu mutations or amplification.
Gallbladder Cancer
Case Presentation
A 57-year-old woman from Chile presents with a 3-week history of progressive right upper quadrant abdominal pain. She denies nausea, vomiting, dysphagia, odynophagia, alterations in bowel habits, fever, or jaundice. Her past medical history is significant for obesity and hypertension. She has no history of smoking, alcohol, or illicit drug use. Laboratory studies show marked leukocytosis (23,800/µL) with neutrophilia (91%). Liver function test results are within normal limits. Ultrasound of the abdomen reveals gallbladder wall thickening and cholelithiasis.
The patient undergoes an uneventful laparoscopic cholecystectomy and is discharged from the hospital after 48 hours. Pathology report reveals a moderately differentiated adenocarcinoma of the gallbladder invading the perimuscular connective tissue (T2). No lymph nodes are identified in the specimen.
- What is the appropriate surgical management of gallbladder cancer?
Gallbladder cancer can be diagnosed preoperatively or can be found incidentally by intraoperative or pathological findings. In one large series, gallbladder cancer was incidentally found during 0.25% of laparoscopic cholecystectomies.67
For patients who are diagnosed with previously unsuspected gallbladder cancer by pathology findings, the extent of tumor invasion (T stage) indicates the need for re-resection (Figure 3).64
Alternatively, when gallbladder cancer is documented or suspected preoperatively, adequate imaging is important to identify patients with absolute contraindications to resection. Contraindications to surgery include metastasis, extensive involvement of the hepatoduodenal ligament, encasement of major vessels, and involvement of celiac, peripancreatic, periduodenal, or superior mesenteric nodes.72 Notwithstanding, retrospective series suggest individual patients may benefit, and surgical indications in advanced disease should be determined on an individual basis.73 Staging imaging should be obtained using multiphasic contrast-enhanced CT or MRI of the chest, abdomen, and pelvis. PET-scan can be used in selected cases where metastatic disease is suspected.64 Laparoscopic diagnostic staging should be considered prior to resection.64 This procedure can identify previously unknown contraindications to tumor resection in as much as 23% of patients, and the yield is significantly higher in locally advanced tumors.73
Patients with a diagnosis of potentially resectable, localized gallbladder cancer should be offered definitive surgery. Extended cholecystectomy is recommended for patients stage T2 or above. This procedure involves wedge resection of the gallbladder bed or a segmentectomy IVb/V and lymph node dissection, which should include the cystic duct, common bile duct, posterior superior pancreaticoduodenal lymph nodes, and those around the hepatoduodenal ligament.72 Bile duct excision should be performed if there is malignant involvement.64
Conclusion
BTCs are anatomically and clinically heterogeneous tumors. Prognostic factors and therapeutic approaches for BTCs differ depending upon their location in the biliary tree and, accordingly, TNM classification systems for intrahepatic, hilar, and distal cholangiocarcinoma and gallbladder cancer have been separated. Surgical resection is the only potentially curative treatment for localized BTC. However, recurrence following complete resection is a primary limitation for cure, which provides a rationale for the use of adjuvant therapy. The prognosis of patients with advanced BTC is poor and OS for those undergoing supportive care alone is short. Multiple randomized clinical trials have demonstrated a benefit of chemotherapy for metastatic disease. For patients with adequate performance status, second-line therapy can be considered, and data from studies that evaluated targeted therapy for specific molecular subgroups is promising.
1. Goldstein D, Lemech C, Valle J. New molecular and immunotherapeutic approaches in biliary cancer. ESMO Open 2017;2(Suppl 1):e000152.
2. Rizvi S, Khan SA, Hallemeier CL, et al. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol 2017 Oct 10. doi: 10.1038/nrclinonc.2017.157.
3. Hezel AF, Zhu AX. Systemic therapy for biliary tract cancers. Oncologist 2008;13:415–23.
4. U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999-2014 Incidence and Mortality Web-based Report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2017.
5. Torre LA, Siegel RL, Islami F, et al. Worldwide burden of and trends in mortality from gallbladder and other biliary tract cancers. Clin Gastroenterol Hepatol 2017 Aug 18. doi: 10.1016/j.cgh.2017.08.017.
6. Lau CSM, Zywot A, Mahendraraj K, Chamberlain CS. Gallbladder carcinoma in the United States: a population based clinical outcomes study involving 22,343 patients from the Surveillance, Epidemiology, and End Result Database (1973–2013). HPB Surg 2017;2017:1532835. doi:10.1155/2017/1532835.
7. Hughes T, O’Connor T, Techasen A, et al. Opisthorchiasis and cholangiocarcinoma in Southeast Asia: an unresolved problem. Int J Gen Med 2017;10:227–37.
8. DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg 2007;245:755–62.
9. Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-year trends in cholangiocarcinoma incidence in the U.S.: intrahepatic disease on the rise. Oncologist 2016;21:594–9.
10. Yao KJ, Jabbour S, Parekh N, et al. Increasing mortality in the United States from cholangiocarcinoma: an analysis of the National Center for Health Statistics Database. BMC Gastroenterol 2016;16:117.
11. Choi SB, Kim KS, Choi JY, et al. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol 2009;16:3048–56.
12. Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg 2008;248:84–96.
13. Duffy A, Capanu M, Abou-Alfa GK, et al. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC). J Surg Oncol 2008;98:485–9.
14. Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer — viewpoint of the IARC Working Group. N Engl J Med 2016;375:794–8.
15. Chen J, Han Y, Xu C, et al. Effect of type 2 diabetes mellitus on the risk for hepatocellular carcinoma in chronic liver diseases. Eur J Cancer Prev 2015;24:89–99.
16. Larsson SC, Giovannucci EL, Wolk A. Sweetened beverage consumption and risk of biliary tract and gallbladder cancer in a prospective study. J Natl Cancer Inst 2016;108: doi: 10.1093/jnci/djw125.
17. Gore RM. Biliary tract neoplasms: diagnosis and staging. Cancer Imaging 2007;7(Special Issue A):S15–23.
18. Broome U, Olsson R, Lööf L, et al. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut 1996;38:610–5.
19. Burak K, Angulo P, Pasha T, et al. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol 2004;99:523–6.
20. Rodrigues J, Diehl DL. Cholangiocarcinoma: clinical manifestations and diagnosis. Tech Gastrointest Endosc 2016;18:75–82.
21. Mitchell CH, Johnson PT, Fishman EK, et al. Features suggestive of gallbladder malignancy. J Comput Assist Tomogr 2014;38:235–41.
22. Beltz WR, Condon RE. Primary carcinoma of the gallbladder. Ann Surg 1974;180:180–4.
23. Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol 2011;8:512–22.
24. Patel T. Cholangiocarcinoma—controversies and challenges. Nat Rev Gastroenterol Hepatol 2011;8:189–200.
25. Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg 1996;224:463–73.
26. Bartella I, Dufour JF. Clinical diagnosis and staging of intrahepatic cholangiocarcinoma. J Gastrointestin Liver Dis 2015;24:481-9.
27. Yamaguchi K, Enjoji M. Carcinoma of the gallbladder: a clinicopathology of 103 patients and a newly proposed staging. Cancer 1988;62:1425–32.
28. Esposito I, Schirmacher P. Pathological aspects of cholangiocarcinoma. HPB. 2008;10:83–6.
29. Silva VWK, Askan G, Daniel TD, et al. Biliary carcinomas: pathology and the role of DNA mismatch repair deficiency. Chin Clin Oncol 2016;5:62.
30. Chung YE, Kim MJ, Park YN, et al. Varying appearances of cholangiocarcinoma: radiologic-pathologic correlation. Radiographics 2009;29:683–700.
31. Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepatobiliary Pancreat Surg 2003;10:288–91.
32. Rao PN. Nodule in liver: investigations, differential diagnosis and follow-up. J Clin Exp Hepatol 2014;4(Suppl 3):S57–62.
33. Kim TK, Lee E, Jang HJ. Imaging findings of mimickers of hepatocellular carcinoma. Clin Mol Hepatol 2015;21:326–43.
34. Hennedige TP, Neo WT, Venkatesh SK. Imaging of malignancies of the biliary tract- an update. Cancer Imaging 2014;14:14.
35. Kim SH, Lee CH, Kim BH, et al. Typical and atypical imaging findings of intrahepatic cholangiocarcinoma using gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging. J Comput Assist Tomogr 2012;36:704–9.
36. Asayama Y, Yoshimitsu K, Irie H, et al. Delayed-phase dynamic CT enhancement as a prognostic factor for mass-forming intrahepatic cholangiocarcinoma. Radiology 2006;238:150–5.
37. National Comprehensive Cancer Network. Cancer of unknown primary. www.nccn.org/professionals/physician_gls/pdf/bone.pdf. Accessed 1 Dec 2017.
38. Kefeli A, Basyigit S, Yeniova AO. Diagnosis of hepatocellular carcinoma. In: Abdeldayem HM, ed. Updates in liver cancer. London: InTech; 2017.
39. Bergquist JR, Ivanics T, Storlie CB, et al. Implications of CA19-9 elevation for survival, staging, and treatment sequencing in intrahepatic cholangiocarcinoma: A national cohort analysis. J Surg Oncol 2016;114:475–82.
40. Chung YJ, Choi DW, Choi SH, et al. Prognostic factors following surgical resection of distal bile duct cancer. J Korean Surg Soc 2013;85:212–8.
41. Lau SK, Prakash S, Geller SA, Alsabeh R. Comparative immunohistochemical profile of hepatocellular carcinoma, cholangiocarcinoma, and metastatic adenocarcinoma. Hum Pathol 2002;33:1175–81.
42. Paul A, Kaiser GM, Molmenti EP, et al. Klatskin tumors and the accuracy of the Bismuth-Corlette classification. Am Surg 2011;77:1695–9.
43. Cannavale A, Santoni M, Gazzetti M, et al. Updated management of malignant biliary tract tumors: an illustrative review. J Vasc Interv Radiol 2016;27:1056–69.
44. Matsuo K, Rocha FG, Ito K, et al. The Blumgart preoperative staging system for hilar cholangiocarcinoma: analysis of resectability and outcomes in 380 patients. J Am Coll Surg 2012;215:343–55.
45. Yoo T, Park SJ, Han SS, et al. Proximal resection margins: more prognostic than distal resection margins in patients undergoing hilar cholangiocarcinoma resection. Cancer Res Treat 2017 Nov 16; doi.org/10.4143/crt.2017.320.
46. Joseph S, Connor S, Garden OJ. Staging laparoscopy for cholangiocarcinoma. HPB 2008;10:116–9.
47. Jarnagin WR, Ruo L, Little SA, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer 2003;98:1689–700.
48. Kobayashi A, Miwa S, Nakata T, Miyagawa S. Disease recurrence patterns after R0 resection of hilar cholangiocarcinoma. Br J Surg 2010;97:56–64.
49. Ghidini M, Tomasello G, Botticelli A, et al. Adjuvant chemotherapy for resected biliary tract cancers: a systematic review and meta-analysis. HPB 2017;19:741–8.
50. Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol 2012;30:1934–40.
51. Primrose JN, Fox R, Palmer DH, et al. Adjuvant capecitabine for biliary tract cancer: the BILCAP randomized study [abstract]. J Clin Oncol 2017 35:15_suppl:4006-4006.
52. Darwish Murad S, Kim WR, Darnois DM, et al. Efficacy of neoadjuvant chemoradiation followed by liver transplantation for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology 2012;143:88–98.
53. Sapisochin G, Facciuto M, Rubbia-Brandt L, et al. Liver transplantation for “very early” intrahepatic cholangiocarcinoma: International retrospective study supporting a prospective assessment. Hepatology 2016;64:1178–88.
54. Le Roy B, Gelli M, Pittau G, et al. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br J Surg 2017 Aug 31. doi: 10.1002/bjs.10641.
55. Tao R, Krishnan S, Bhosale PR, et al. Ablative radiotherapy doses lead to a substantial prolongation of survival in patients with inoperable intrahepatic cholangiocarcinoma: a retrospective dose response analysis. J Clin Oncol 2016;34:219–26.
56. Glimelius B, Hoffman K, SjÓdén PO, et al. 555 Palliative chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Eur J Cancer 1995;31:S118.
57. Sharma A, Dwary AD, Mohanti BK, et al. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J Clin Oncol 2010;28:4581–6.
58. Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273–81.
59. Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer 2010;103:469–74.
60. Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer 2007;96:896–902.
61. Lamarca A, Hubner RA, David Ryder W, Valle JW. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol 2014;25:2328–38.
62. Brieau B, Dahan L, De Rycke Y, et al. Second-line chemotherapy for advanced biliary tract cancer after failure of the gemcitabine-platinum combination: A large multicenter study by the Association des Gastro-Entérologues Oncologues. Cancer 2015;121:3290–7.
63. Fornaro L, Cereda S, Aprile G, et al. Multivariate prognostic factors analysis for second-line chemotherapy in advanced biliary tract cancer. Br J Cancer 2014;110:2165–9.
64. National Comprehensive Cancer Network. Hepatobiliary cancer. www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. Accessed 12 Nov 2017.
65. Javle M, Lowery M, Shroff RT, et al. Phase II study of BGJ398 in patients with FGFR-altered advanced cholangiocarcinoma. J Clin Oncol 2017 Nov 28;JCO2017755009.
66. Javle M, Churi C, Kang HC, et al. HER2/neu-directed therapy for biliary tract cancer. J Hematol Oncol 2015;8:58.
67. Konstantinidis IT, Deshpande V, Genevay M, et al. Trends in presentation and survival for gallbladder cancer during a period of more than 4 decades: a single-institution experience. Arch Surg 2009;144:441–47.
68. Singh S, Agarwal AK. Gallbladder cancer: the role of laparoscopy and radical resection. Ann Surg 2009;250:494–5.
69. Kapoor VK, Haribhakti SP. Extended cholecystectomy for carcinoma of the gall bladder. Trop Gastroenterol 1995;16:74–5.
70. Ethun CG, Postlewait LM, Le N, et al. Association of optimal time Interval to re-resection for incidental gallbladder cancer with overall survival: a multi-Institution analysis from the US extrahepatic biliary malignancy consortium. JAMA Surg 2017;152:143–9.
71. Goetze TO, Paolucci V. Benefits of reoperation of T2 and more advanced incidental gallbladder carcinoma: analysis of the German registry. Ann Surg 2008;247:104–8.
72. Nishio H, Nagino M, Ebata T, et al. Aggressive surgery for stage IV gallbladder carcinoma; what are the contraindications? J Hepatobiliary Pancreat Surg 2007;14:351–7.
73. Agarwal AK, Kalayarasan R, Javed A, et al. The role of staging laparoscopy in primary gallbladder cancer--an analysis of 409 patients: a prospective study to evaluate the role of staging laparoscopy in the management of gallbladder cancer. Ann Surg 2013;258:318–23.
Introduction
Biliary tract carcinoma (BTC) is th
Epidemiology
In the United States, BTC is rare and accounts for approximately 4% of all gastrointestinal malignancies, with an estimated 6000 to 7000 cases of carcinoma of the gallbladder and 3000 to 4000 cases of carcinoma of the bile duct diagnosed annually.4 Among women, there is a 26-fold variation in BTC mortality worldwide, ranging from 0.8 deaths per 100,000 in South Africa to 21.2 per 100,000 in Chile.1,5 Interestingly, for American Indians in New Mexico, gallbladder cancer mortality rates (8.9 per 100,000) surpass those for breast and pancreatic cancers.6 The incidence of anatomical cholangiocarcinoma subtypes also varies regionally, reflecting disparities in genetic and environmental predisposing factors.2,7 In a large, single-center study in the United States, intrahepatic cholangiocarcinoma accounted for less than 10% of cases, perihilar accounted for 50%, and distal accounted for the remaining 40%.8 Importantly, intrahepatic cholangiocarcinoma is the second most common primary malignancy of the liver, and its incidence seems to be rising in many western countries. In the United States, there has been an estimated 128% rise over the past 40 years.4,9
BTC is associated with high mortality rates.10 Median overall survival (OS) for cholangiocarcinoma is 20 to 28 months and 5-year survival is around 25%.10 Most cholangiocarcinomas are diagnosed at advanced stages with unresectable tumors.10 Furthermore, outcomes following resection with curative intent are poor—median disease-free survival (DFS) of 12 to 36 months has been reported.11,12 Patients with intrahepatic disease have a better prognosis when compared with patients who have extrahepatic tumors.12 Gallbladder cancer, likewise, carries a poor overall prognosis; median OS is 32 months and 5-year survival is as low as 13%.6
Risk factors for BTC include intrinsic and extrinsic elements.6 Incidence of BTC increases with age, and diagnosis typically occurs in the sixth to eighth decade of life.5,6,13 In contrast to gallbladder cancer, the incidence of cholangiocarcinoma is slightly higher in men.9 Obesity, diabetes, and consumption of sweetened drinks also increase the risk for BTC.14–16 Cholelithiasis is the most prevalent risk factor for gallbladder cancer, and the risk is greater for larger stones.5 Around 1 in 5 patients with porcelain gallbladder will develop gallbladder carcinoma.17 Primary sclerosing cholangitis (PSC), chronic calculi of the bile duct, choledochal cysts, cirrhosis, hepatitis C, and liver fluke infections are well established risk factors for cholangiocarcinoma.7,12,18 PSC is one of the best described entities among these predisposing conditions. Lifetime prevalence of cholangiocarcinoma among patients with PSC ranges from 5% to 10%.18,19 These patients also present at a younger age; in one series, the median age at diagnosis for BTC arising from PSC was 39 years.18 It is important to recognize, however, that in most patients diagnosed with cholangiocarcinoma, no predisposing factors are identified.8
Diagnosis
Clinical Presentation
Clinical presentation of BTC depends upon anatomic location.20 Patients with early invasive gallbladder cancer are most often asymptomatic.21 When symptoms occur, they may be nonspecific and mimic cholelithiasis.21 The most common clinical presentations include jaundice, weight loss, and abdominal pain.21 Prior to widespread availability of imaging studies, the preoperative diagnosis rate for gallbladder cancer was as low as 10%.22 However, the accuracy of computed tomography (CT) has changed this scenario, with sensitivity ranging from 73% to 87% and specificity from 88% to 100%.21 As a result of its silent clinical character, cholangiocarcinoma is frequently difficult to diagnose.23 Perihilar and distal cholangiocarcinoma characteristically present with signs of biliary obstruction, and imaging and laboratory data can corroborate the presence of cholestasis.24 On examination, patients with extrahepatic cholangiocarcinoma may present with jaundice, hepatomegaly, and a palpable right upper quadrant mass.25 A palpable gallbladder (Courvoisier sign) can also be present.25 Intrahepatic cholangiocarcinoma presents differently, and patients are less likely to be jaundiced.23 Typical clinical features are nonspecific and include dull right upper quadrant pain, weight loss, and an elevated alkaline phosphatase level.23 Alternatively, asymptomatic patients can present with incidentally detected lesions, when imaging is obtained as part of the workup for other causes or during screening for hepatocellular carcinoma in patients with viral hepatitis or cirrhosis.23,26 Uncommonly, BTC patients present because of signs or symptoms related to metastatic disease or evidence of metastatic disease on imaging.
Pathology and Grading
The majority of BTCs are adenocarcinomas, corresponding to 90% of cholangiocarcinomas and 99% of gallbladder cancers.27,28 They are graded as well, moderately, or poorly differentiated.2 Adenosquamous and squamous cell carcinoma are responsible for most of the remaining cases.2,29 Cholangiocarcinomas are divided into 3 types, defined by the Liver Cancer Study Group of Japan: (1) mass-forming, (2) periductal-infiltrating, and (3) intraductal-growing.30,31 Mass-forming intrahepatic cholangiocarcinomas are characterized morphologically by a homogeneous gray-yellow mass with frequent satellite nodules and irregular but well-defined margins.17,30 Central necrosis and fibrosis are also common.30 In the periductal-infiltrating type, tumor typically grows along the bile duct wall without mass formation, resulting in concentric mural thickening and proximal biliary dilation.30 Intraductal-growing papillary cholangiocarcinoma is characterized by the presence of intraluminal papillary or tubular polypoid tumors of the intra- or extrahepatic bile ducts, with partial obstruction and proximal biliary dilation.30
Cholangiocarcinoma
Case Presentation
A previously healthy 59-year-old man presents to his primary care physician with a 3-month history of dull right upper quadrant pain associated with weight loss. The patient is markedly cachectic and abdominal examination reveals upper quadrant tenderness. Laboratory exams are significant for elevated alkaline phosphatase (500 U/L; reference range 45–115 U/L), cancer antigen 19-9 (CA 19-9, 73 U/mL; reference range ≤ 37 U/mL), and carcinoembryonic antigen (CEA , 20 ng/mL; reference range for nonsmokers ≤ 3.0 ng/mL). Aspartate aminotransferase, alanine aminotransferase, total bilirubin, and coagulation studies are within normal range. Ultrasound demonstrates a homogeneous mass with irregular borders in the right lobe of the liver. Triphasic contrast-enhanced CT scan demonstrates a tumor with ragged rim enhancement at the periphery, and portal venous phase shows gradual centripetal enhancement of the tumor with capsular retraction. No abdominal lymph nodes or extrahepatic tumors are noted (Figure 1, Image A).
- What are the next diagnostic steps?
The most critical differential diagnosis of solid liver mass in patients without cirrhosis is cholangiocarcinoma and metastases from another primary site.32 Alternatively, when an intrahepatic lesion is noted on an imaging study in the setting of cirrhosis, the next diagnostic step is differentiation between cholangiocarcinoma and hepatocellular carcinoma (HCC).32 Triphasic contrast-enhanced CT and dynamic magnetic resonance imaging (MRI) are key diagnostic procedures.32,33 In the appropriate setting, classical imaging features in the arterial phase with washout in portal venous or delayed phase can be diagnostic of HCC and may obviate the need for a biopsy (Figure 2).
Typical radiographic features of cholangiocarcinoma include a hypodense hepatic lesion that can be either well-defined or infiltrative and is frequently associated with biliary dilatation (Figure 1, Image A).33 The dense fibrotic nature of the tumor may cause capsular retraction, which is seen in up to 20% of cases.17 This finding is highly suggestive of cholangiocarcinoma and is rarely present in HCC.33 Following contrast administration, there is peripheral (rim) enhancement throughout both arterial and venous phases.32–34 However, these classic features were present in only 70% of cases in one study.35 Although intrahepatic cholangiocarcinomas are most commonly hypovascular, small mass-forming intrahepatic cholangiocarcinomas can often be arterially hyperenhancing and mimic HCC.33 Tumor enhancement on delayed CT imaging has been correlated with survival. Asayama et al demonstrated that tumors that exhibited delayed enhancement on CT in more than two-thirds of their volume were associated with a worse prognosis.36
Patients without cirrhosis who present with a localized lesion of the liver should undergo extensive evaluation for a primary cancer site.37 CT of the chest, abdomen, and pelvis with contrast should be obtained.37 Additionally, mammogram and endoscopic evaluation with esophagogastroduodenoscopy (EGD) and colonoscopy should be included in the work-up.37
Preoperative tumor markers are also included in the work-up. All patients with a solid liver lesion should have serum alpha-fetoprotein (AFP) levels checked. AFP is a serum glycoprotein recognized as a marker for HCC and is reported to detect preclinical HCC.38 However, serum concentrations are normal in up to 40% of small HCCs.38 Although no specific marker for cholangiocarcinoma has yet been identified, the presence of certain tumor markers in the serum of patients may be of diagnostic value, especially in patients with PSC. CA 19-9 and CEA are the best studied. Elevated levels of CA 19-9 prior to treatment are associated with a poorer prognosis, and CA 19-9 concentrations greater than 1000 U/mL are consistent with advanced disease.39,40 One large series evaluated the diagnostic value of serum CEA levels in 333 patients with PSC, 13% of whom were diagnosed with cholangiocarcinoma.34 A serum CEA level greater than 5.2 ng/mL had a sensitivity of 68.0% and specificity of 81.5%.38
If a biopsy is obtained, appropriate immunohistochemistry (IHC) can facilitate the diagnosis. BTC is strongly positive for CK-7 and CK-19.41 CK-7 positivity is not specific and is also common among metastatic cancers of the lung and breast; therefore, in some cases cholangiocarcinoma may be a diagnosis of exclusion. Immunostaining for monoclonal CEA is diffusely positive in up to 75% of cases.41 An IHC panel consisting of Hep Par-1, arginase-1, monoclonal CEA, CK-7, CK-20, TTF-1, MOC-31, and CDX-2 has been proposed to optimize the differential diagnosis of HCC, metastatic adenocarcinoma, and cholangiocarcinoma.41
Case Continued
CT of the chest, abdomen, and pelvis reveals no concerns for metastasis and no evidence of primary cancer elsewhere. EGD and colonoscopy are clear. AFP levels are within normal limits (2 ng/mL). Biopsy is performed and demonstrates adenocarcinoma. IHC studies demonstrate cells positive for monoclonal CEA, CK-7, CK-19, and MOC-31, and negative for Napsin A, TTF-1, and CK-20.
- How is cholangiocarcinoma staged and classified?
The purpose of the staging system is to provide information on prognosis and guidance for therapy. Prognostic factors and the therapeutic approaches for BTC differ depending upon their location in the biliary tree. Accordingly, TNM classification systems for intrahepatic, hilar, and distal cholangiocarcinoma and gallbladder cancer have been separated (Table 1 and Table 2).23
The Bismuth-Corlette classification is used to further classify perihilar cholangiocarcinoma according to patterns of hepatic duct involvement. Type I tumors are located below the confluence of the left and right hepatic ducts.42 Type II reach the confluence of the hepatic ducts.42 Type III occlude the common hepatic duct and either the right or left hepatic duct (IIIa and IIIb, respectively).42 Finally, type IV are multicentric, or involve the confluence and both the right and left hepatic ducts.42 Tumors that involve the common hepatic duct bifurcation are named Klatskin tumors.42
- What is the first-line treatment for localized cholangiocarcinomas?
Surgical resection is the only potentially curative treatment for localized cholangiocarcinoma, although fewer than 20% of patients are suitable for curative treatment, due to the presence of advanced disease at diagnosis.43,44 Available evidence supports the recommendation that resection with negative margins, regardless of extent, should be the goal of therapy for patients with potentially resectable disease.44 Extensive hepatic resections are often necessary to achieve clear margins since the majority of patients present with large masses. Substantial evidence corroborates that R0 resection is associated with better survival, whereas the benefit of wide compared to narrow (< 5–10 mm) margins is unclear.45 A recent analysis of 96 patients suggests that the proximal resection margin has more prognostic implications than distal margins.45
Surgical options and resectability criteria depend upon tumor location. Extent of tumor in the bile duct is one of the most important factors that determine resectability.17 Although multifocal liver tumors (including satellite lesions), lymph node metastases to the porta hepatis, and distant metastases are considered relative contraindications to surgery, surgical approaches can be considered in selected patients.43 Patient selection for surgery is facilitated by careful preoperative staging, which may include laparoscopy. Laparoscopic staging prior to resection may prevent unnecessary laparotomy in 30% to 45% of patients.42,46
- Is there a role for adjuvant treatment?
Recurrence following complete resection is a primary limitation for cure in BTC, which provides a rationale for the use of adjuvant therapy.47,48 In a sample of 79 patients with extrahepatic cholangiocarcinoma who underwent curative resection, the cumulative recurrence rate after 4 years was 56%.47 Initial recurrence at a distant site occurs in 40% to 50% of patients.48
Lymphovascular and perineural invasion, lymph node metastasis, and tumor size ≥ 5 cm have been reported as independent predictors of recurrence and mortality following resection.49 A 2017 meta-analysis which included 30 studies involving more than 22,499 patients reported a 41% reduction in the risk of death with adjuvant chemotherapy, which translated to a mean OS benefit of 4 months in an unselected population.49 Moreover, this study revealed inferior OS in patients given adjuvant radiation therapy (RT) in combination with chemotherapy.49 These results are in line with the previous meta-analysis by Horgan et al, which demonstrated that adjuvant RT seems to benefit only patients with R1 resections, with a possible detrimental effect in R0 disease.50 Therefore, adjuvant chemoradiation cannot be viewed as a standard practice following R0 resection, and should be reserved for those patients with positive margins (R1/ 2) to reduce local progression.
In the phase 3 BILCAP trial presented at ASCO 2017, 447 patients with completely resected cholangiocarcinoma or gallbladder cancer with adequate biliary drainage and Eastern Cooperative Oncology Group (ECOG) performance score ≤ 2 were randomly assigned to observation or capecitabine (1250 mg/m2 twice daily for days 1–14 every 21 days for 8 cycles).51 Surgical treatment achieved R0 resection in 62% of patients and 46% were node-negative. Median OS was 51 months for the capecitabine group and 36 months for the control arm (hazard ratio [HR] 0.80, 95% CI 0.63 to 1.04, P = 0.097). Analyses with adjustment for nodal status, grade of disease, and gender indicated a HR of 0.71 (P < 0.01). Median DFS was 25 months versus 18 months favoring the capecitabine group, and rates of grade 3 or 4 toxicity were less than anticipated. Following the results of this trial, adjuvant capecitabine should become the new standard of care.
- What is the treatment for locally advanced cholangiocarcinoma?
The optimal approach to patients with locally advanced unresectable cholangiocarcinoma has not been established. The prognosis for patients with either locally unresectable or locally recurrent disease is typically measured in months. Goals of palliative therapy are relief of symptoms and improvement in quality of life, and there is no role for surgical debulking.
Liver transplantation is a potentially curative option for selected patients with hilar or intrahepatic cholangiocarcinoma. Patients with lymph node-negative, non-disseminated, locally advanced hilar cholangiocarcinomas have 5-year survival rates ranging from 25% to 42% following transplantation.52 Retrospective data suggests that neoadjuvant chemoradiation followed by liver transplantation is highly effective for selected patients with hilar cholangiocarcinoma.52 However, these results require confirmation from prospective clinical evidence. It is important to recognize that liver transplantation plays no role in the management of distal cholangiocarcinoma or gallbladder cancer.
Rarely, patients with borderline resectable intrahepatic cholangiocarcinoma will have a sufficient response to chemotherapy to permit later resection, and, in such cases, starting with chemotherapy and then restaging to evaluate resectability is appropriate.54 A single-center, retrospective analysis including 186 patients by Le Roy et al evaluated survival in patients with locally advanced, unresectable intrahepatic cholangiocarcinoma who received primary chemotherapy, followed by surgery in those with secondary resectability.54 After a median of 6 cycles of chemotherapy, 53% of patients achieved resectability and underwent surgery with curative intent. These patients had similar short- and long-term results compared to patients with initially resectable intrahepatic cholangiocarcinoma who had surgery alone, with median OS reaching 24 months.54
Ablative radiotherapy is an additional option for localized inoperable intrahepatic cholangiocarcinoma. Tao and colleagues evaluated 79 consecutive patients with inoperable intrahepatic cholangiocarcinoma treated with definitive RT.55 Median tumor size was 7.9 cm and 89% of patients received chemotherapy before RT. Median OS was 30 months and 3-year OS was 44%. Radiation dose was the single most important prognostic factor, and higher doses correlated with improved local control and OS. A biologic equivalent dose (BED) greater than 80.5 Gy was identified as an ablative dose of RT for large intrahepatic cholangiocarcinomas. The 3-year OS for patients receiving BED greater than 80.5 Gy was 73% versus 38% for those receiving lower doses.
Case Continued
The patient is deemed to have resectable disease and undergoes surgical resection followed by adjuvant capecitabine for 8 cycles. Unfortunately, after 1 year, follow-up imaging identifies bilateral enlarging lung nodules. Biopsy is performed and confirms metastatic cholangiocarcinoma.
- What is the treatment for metastatic BTC?
The prognosis of patients with advanced BTC is poor and OS for those undergoing supportive care alone is short. A benefit of chemotherapy over best supportive care for cholangiocarcinoma was demonstrated in an early phase 3 trial that randomly assigned 90 patients with advanced pancreatic or biliary cancer (37 with bile duct cancer) to receive either fluorouracil (FU) -based systemic chemotherapy or best supportive care. Results showed that chemotherapy significantly improved OS (6 months versus 2.5 months).56 Chemotherapy is also beneficial for patients with unresectable gallbladder cancer. In a single-center randomized study including 81 patients with unresectable gallbladder cancer, gemcitabine and oxaliplatin (GEMOX) improved progression-free survival (PFS) and OS compared to best supportive care.57 Treatment for metastatic cholangiocarcinoma and gallbladder cancer follows the same algorithm.
In 2010, cisplatin plus gemcitabine was established as a reference regimen for first-line therapy by the ABC-02 study, in which 410 patients with locally advanced or metastatic bile duct, gallbladder, or ampullary cancer were randomly assigned to 6 courses of cisplatin (25 mg/m2) plus gemcitabine (1000 mg/m2 on days 1 and 8, every 21 days) or gemcitabine alone (1000 mg/m2 days 1, 8, 15, every 28 days).58 OS was significantly greater with combination therapy (11.7 versus 8.1 months), and PFS also favored the combination arm (8 versus 5 months). Toxicity was comparable in both groups, with the exception of significantly higher rates of grade 3 or 4 neutropenia with gemcitabine plus cisplatin (25% versus 17%), and higher rates of grade 3 or 4 abnormal liver function with gemcitabine alone (27% versus 17%). Most quality-of-life scales showed a trend favoring combined therapy.58 A smaller, identically designed Japanese phase 3 randomized trial achieved similar results, demonstrating greater OS with cisplatin plus gemcitabine compared to gemcitabine alone (11.2 versus 7.7 months).59
The gemcitabine plus cisplatin combination has not been directly compared with other gemcitabine combinations in phase 3 trials. A pooled analysis of 104 trials of a variety of chemotherapy regimens in advanced biliary cancer concluded that the gemcitabine plus cisplatin regimen offered the highest rates of objective response and tumor control compared with either gemcitabine-free or cisplatin-free regimens.60 However, this did not translate into significant benefit in terms of either time to tumor progression or median OS. It is important to note that this analysis did not include results of the subsequent ABC-02 trial.
There is no standard treatment for patients with cholangiocarcinoma for whom first-line gemcitabine-based therapy fails. There are no completed prospective phase 3 trials supporting the use of second-line chemotherapy after failure of first-line chemotherapy in BTC, and the selection of candidates for second-line therapy as well as the optimal regimen are not established.61 The ongoing phase 2 multicenter ABC-06 trial is evaluating oxaliplatin plus short-term infusional FU and leucovorin (FOLFOX) versus best supportive care for second-line therapy. In a systematic review including 23 studies (14 phase 2 clinical trials and 9 retrospective studies) with 761 patients with BTC, the median OS was 7.2 months.
The optimal selection of candidates for second-line chemotherapy is not established. Two independent studies suggest that patients who have a good performance status (0 or 1), disease control with the first-line chemotherapy, low CA 19-9 level, and possibly previous surgery on their primary tumor, have the longest survival with second-line chemotherapy. However, whether these characteristics predict for chemotherapy responsiveness or more favorable biologic behavior is not clear.62,63 No particular regimen has proved superior to any other, and the choice of second-line regimen remains empiric.
For patients with adequate performance status, examples of other conventional chemotherapy regimens with demonstrated activity that could be considered for second-line therapy include: FOLFOX or capecitabine, gemcitabine plus capecitabine, capecitabine plus cisplatin, or irinotecan plus short-term infusional FU and leucovorin (FOLFIRI) with or without bevacizumab.64 For selected patients, second-line molecularly targeted therapy using erlotinib plus bevacizumab may be considered. However, this regimen is very costly.64 Examples of other regimens with demonstrated activity in phase 2 trials include GEMOX, gemcitabine plus fluoropyrimidine, and fluoropyrimidine plus oxaliplatin or cisplatin.64
There is promising data from studies of targeted therapy for specific molecular subgroups. A recent phase 2 trial evaluated the activity of BGJ398, an orally bioavailable, selective, ATP-competitive pan inhibitor of human fibroblast growth factor receptor (FGFR) kinase, in patients with FGFR-altered advanced cholangiocarcinoma.65 The overall response rate was 14.8% (18.8% FGFR2 fusions only) and disease control rate was 75.4% (83.3% FGFR2 fusions only). All responsive tumors contained FGFR2 fusions. Adverse events were manageable, and grade 3 or 4 treatment-related adverse events occurred in 25 patients (41%). Those included hyperphosphatemia, stomatitis, and palmar-plantar erythrodysesthesia. Javle and colleagues also identified HER2/neu blockade as a promising treatment strategy for gallbladder cancer patients with this gene amplification.66 This retrospective analysis included 9 patients with gallbladder cancer and 5 patients with cholangiocarcinoma who received HER2/neu-directed therapy (trastuzumab, lapatinib, or pertuzumab). In the gallbladder cancer group, HER2/neu gene amplification or overexpression was detected in 8 cases. These patients experienced disease stability (n = 3), partial response (n = 4), or complete response (n = 1) with HER2/neu–directed therapy. Median duration of response was 40 weeks. The cholangiocarcinoma cases treated in this series had no radiological responses despite HER2/neu mutations or amplification.
Gallbladder Cancer
Case Presentation
A 57-year-old woman from Chile presents with a 3-week history of progressive right upper quadrant abdominal pain. She denies nausea, vomiting, dysphagia, odynophagia, alterations in bowel habits, fever, or jaundice. Her past medical history is significant for obesity and hypertension. She has no history of smoking, alcohol, or illicit drug use. Laboratory studies show marked leukocytosis (23,800/µL) with neutrophilia (91%). Liver function test results are within normal limits. Ultrasound of the abdomen reveals gallbladder wall thickening and cholelithiasis.
The patient undergoes an uneventful laparoscopic cholecystectomy and is discharged from the hospital after 48 hours. Pathology report reveals a moderately differentiated adenocarcinoma of the gallbladder invading the perimuscular connective tissue (T2). No lymph nodes are identified in the specimen.
- What is the appropriate surgical management of gallbladder cancer?
Gallbladder cancer can be diagnosed preoperatively or can be found incidentally by intraoperative or pathological findings. In one large series, gallbladder cancer was incidentally found during 0.25% of laparoscopic cholecystectomies.67
For patients who are diagnosed with previously unsuspected gallbladder cancer by pathology findings, the extent of tumor invasion (T stage) indicates the need for re-resection (Figure 3).64
Alternatively, when gallbladder cancer is documented or suspected preoperatively, adequate imaging is important to identify patients with absolute contraindications to resection. Contraindications to surgery include metastasis, extensive involvement of the hepatoduodenal ligament, encasement of major vessels, and involvement of celiac, peripancreatic, periduodenal, or superior mesenteric nodes.72 Notwithstanding, retrospective series suggest individual patients may benefit, and surgical indications in advanced disease should be determined on an individual basis.73 Staging imaging should be obtained using multiphasic contrast-enhanced CT or MRI of the chest, abdomen, and pelvis. PET-scan can be used in selected cases where metastatic disease is suspected.64 Laparoscopic diagnostic staging should be considered prior to resection.64 This procedure can identify previously unknown contraindications to tumor resection in as much as 23% of patients, and the yield is significantly higher in locally advanced tumors.73
Patients with a diagnosis of potentially resectable, localized gallbladder cancer should be offered definitive surgery. Extended cholecystectomy is recommended for patients stage T2 or above. This procedure involves wedge resection of the gallbladder bed or a segmentectomy IVb/V and lymph node dissection, which should include the cystic duct, common bile duct, posterior superior pancreaticoduodenal lymph nodes, and those around the hepatoduodenal ligament.72 Bile duct excision should be performed if there is malignant involvement.64
Conclusion
BTCs are anatomically and clinically heterogeneous tumors. Prognostic factors and therapeutic approaches for BTCs differ depending upon their location in the biliary tree and, accordingly, TNM classification systems for intrahepatic, hilar, and distal cholangiocarcinoma and gallbladder cancer have been separated. Surgical resection is the only potentially curative treatment for localized BTC. However, recurrence following complete resection is a primary limitation for cure, which provides a rationale for the use of adjuvant therapy. The prognosis of patients with advanced BTC is poor and OS for those undergoing supportive care alone is short. Multiple randomized clinical trials have demonstrated a benefit of chemotherapy for metastatic disease. For patients with adequate performance status, second-line therapy can be considered, and data from studies that evaluated targeted therapy for specific molecular subgroups is promising.
Introduction
Biliary tract carcinoma (BTC) is th
Epidemiology
In the United States, BTC is rare and accounts for approximately 4% of all gastrointestinal malignancies, with an estimated 6000 to 7000 cases of carcinoma of the gallbladder and 3000 to 4000 cases of carcinoma of the bile duct diagnosed annually.4 Among women, there is a 26-fold variation in BTC mortality worldwide, ranging from 0.8 deaths per 100,000 in South Africa to 21.2 per 100,000 in Chile.1,5 Interestingly, for American Indians in New Mexico, gallbladder cancer mortality rates (8.9 per 100,000) surpass those for breast and pancreatic cancers.6 The incidence of anatomical cholangiocarcinoma subtypes also varies regionally, reflecting disparities in genetic and environmental predisposing factors.2,7 In a large, single-center study in the United States, intrahepatic cholangiocarcinoma accounted for less than 10% of cases, perihilar accounted for 50%, and distal accounted for the remaining 40%.8 Importantly, intrahepatic cholangiocarcinoma is the second most common primary malignancy of the liver, and its incidence seems to be rising in many western countries. In the United States, there has been an estimated 128% rise over the past 40 years.4,9
BTC is associated with high mortality rates.10 Median overall survival (OS) for cholangiocarcinoma is 20 to 28 months and 5-year survival is around 25%.10 Most cholangiocarcinomas are diagnosed at advanced stages with unresectable tumors.10 Furthermore, outcomes following resection with curative intent are poor—median disease-free survival (DFS) of 12 to 36 months has been reported.11,12 Patients with intrahepatic disease have a better prognosis when compared with patients who have extrahepatic tumors.12 Gallbladder cancer, likewise, carries a poor overall prognosis; median OS is 32 months and 5-year survival is as low as 13%.6
Risk factors for BTC include intrinsic and extrinsic elements.6 Incidence of BTC increases with age, and diagnosis typically occurs in the sixth to eighth decade of life.5,6,13 In contrast to gallbladder cancer, the incidence of cholangiocarcinoma is slightly higher in men.9 Obesity, diabetes, and consumption of sweetened drinks also increase the risk for BTC.14–16 Cholelithiasis is the most prevalent risk factor for gallbladder cancer, and the risk is greater for larger stones.5 Around 1 in 5 patients with porcelain gallbladder will develop gallbladder carcinoma.17 Primary sclerosing cholangitis (PSC), chronic calculi of the bile duct, choledochal cysts, cirrhosis, hepatitis C, and liver fluke infections are well established risk factors for cholangiocarcinoma.7,12,18 PSC is one of the best described entities among these predisposing conditions. Lifetime prevalence of cholangiocarcinoma among patients with PSC ranges from 5% to 10%.18,19 These patients also present at a younger age; in one series, the median age at diagnosis for BTC arising from PSC was 39 years.18 It is important to recognize, however, that in most patients diagnosed with cholangiocarcinoma, no predisposing factors are identified.8
Diagnosis
Clinical Presentation
Clinical presentation of BTC depends upon anatomic location.20 Patients with early invasive gallbladder cancer are most often asymptomatic.21 When symptoms occur, they may be nonspecific and mimic cholelithiasis.21 The most common clinical presentations include jaundice, weight loss, and abdominal pain.21 Prior to widespread availability of imaging studies, the preoperative diagnosis rate for gallbladder cancer was as low as 10%.22 However, the accuracy of computed tomography (CT) has changed this scenario, with sensitivity ranging from 73% to 87% and specificity from 88% to 100%.21 As a result of its silent clinical character, cholangiocarcinoma is frequently difficult to diagnose.23 Perihilar and distal cholangiocarcinoma characteristically present with signs of biliary obstruction, and imaging and laboratory data can corroborate the presence of cholestasis.24 On examination, patients with extrahepatic cholangiocarcinoma may present with jaundice, hepatomegaly, and a palpable right upper quadrant mass.25 A palpable gallbladder (Courvoisier sign) can also be present.25 Intrahepatic cholangiocarcinoma presents differently, and patients are less likely to be jaundiced.23 Typical clinical features are nonspecific and include dull right upper quadrant pain, weight loss, and an elevated alkaline phosphatase level.23 Alternatively, asymptomatic patients can present with incidentally detected lesions, when imaging is obtained as part of the workup for other causes or during screening for hepatocellular carcinoma in patients with viral hepatitis or cirrhosis.23,26 Uncommonly, BTC patients present because of signs or symptoms related to metastatic disease or evidence of metastatic disease on imaging.
Pathology and Grading
The majority of BTCs are adenocarcinomas, corresponding to 90% of cholangiocarcinomas and 99% of gallbladder cancers.27,28 They are graded as well, moderately, or poorly differentiated.2 Adenosquamous and squamous cell carcinoma are responsible for most of the remaining cases.2,29 Cholangiocarcinomas are divided into 3 types, defined by the Liver Cancer Study Group of Japan: (1) mass-forming, (2) periductal-infiltrating, and (3) intraductal-growing.30,31 Mass-forming intrahepatic cholangiocarcinomas are characterized morphologically by a homogeneous gray-yellow mass with frequent satellite nodules and irregular but well-defined margins.17,30 Central necrosis and fibrosis are also common.30 In the periductal-infiltrating type, tumor typically grows along the bile duct wall without mass formation, resulting in concentric mural thickening and proximal biliary dilation.30 Intraductal-growing papillary cholangiocarcinoma is characterized by the presence of intraluminal papillary or tubular polypoid tumors of the intra- or extrahepatic bile ducts, with partial obstruction and proximal biliary dilation.30
Cholangiocarcinoma
Case Presentation
A previously healthy 59-year-old man presents to his primary care physician with a 3-month history of dull right upper quadrant pain associated with weight loss. The patient is markedly cachectic and abdominal examination reveals upper quadrant tenderness. Laboratory exams are significant for elevated alkaline phosphatase (500 U/L; reference range 45–115 U/L), cancer antigen 19-9 (CA 19-9, 73 U/mL; reference range ≤ 37 U/mL), and carcinoembryonic antigen (CEA , 20 ng/mL; reference range for nonsmokers ≤ 3.0 ng/mL). Aspartate aminotransferase, alanine aminotransferase, total bilirubin, and coagulation studies are within normal range. Ultrasound demonstrates a homogeneous mass with irregular borders in the right lobe of the liver. Triphasic contrast-enhanced CT scan demonstrates a tumor with ragged rim enhancement at the periphery, and portal venous phase shows gradual centripetal enhancement of the tumor with capsular retraction. No abdominal lymph nodes or extrahepatic tumors are noted (Figure 1, Image A).
- What are the next diagnostic steps?
The most critical differential diagnosis of solid liver mass in patients without cirrhosis is cholangiocarcinoma and metastases from another primary site.32 Alternatively, when an intrahepatic lesion is noted on an imaging study in the setting of cirrhosis, the next diagnostic step is differentiation between cholangiocarcinoma and hepatocellular carcinoma (HCC).32 Triphasic contrast-enhanced CT and dynamic magnetic resonance imaging (MRI) are key diagnostic procedures.32,33 In the appropriate setting, classical imaging features in the arterial phase with washout in portal venous or delayed phase can be diagnostic of HCC and may obviate the need for a biopsy (Figure 2).
Typical radiographic features of cholangiocarcinoma include a hypodense hepatic lesion that can be either well-defined or infiltrative and is frequently associated with biliary dilatation (Figure 1, Image A).33 The dense fibrotic nature of the tumor may cause capsular retraction, which is seen in up to 20% of cases.17 This finding is highly suggestive of cholangiocarcinoma and is rarely present in HCC.33 Following contrast administration, there is peripheral (rim) enhancement throughout both arterial and venous phases.32–34 However, these classic features were present in only 70% of cases in one study.35 Although intrahepatic cholangiocarcinomas are most commonly hypovascular, small mass-forming intrahepatic cholangiocarcinomas can often be arterially hyperenhancing and mimic HCC.33 Tumor enhancement on delayed CT imaging has been correlated with survival. Asayama et al demonstrated that tumors that exhibited delayed enhancement on CT in more than two-thirds of their volume were associated with a worse prognosis.36
Patients without cirrhosis who present with a localized lesion of the liver should undergo extensive evaluation for a primary cancer site.37 CT of the chest, abdomen, and pelvis with contrast should be obtained.37 Additionally, mammogram and endoscopic evaluation with esophagogastroduodenoscopy (EGD) and colonoscopy should be included in the work-up.37
Preoperative tumor markers are also included in the work-up. All patients with a solid liver lesion should have serum alpha-fetoprotein (AFP) levels checked. AFP is a serum glycoprotein recognized as a marker for HCC and is reported to detect preclinical HCC.38 However, serum concentrations are normal in up to 40% of small HCCs.38 Although no specific marker for cholangiocarcinoma has yet been identified, the presence of certain tumor markers in the serum of patients may be of diagnostic value, especially in patients with PSC. CA 19-9 and CEA are the best studied. Elevated levels of CA 19-9 prior to treatment are associated with a poorer prognosis, and CA 19-9 concentrations greater than 1000 U/mL are consistent with advanced disease.39,40 One large series evaluated the diagnostic value of serum CEA levels in 333 patients with PSC, 13% of whom were diagnosed with cholangiocarcinoma.34 A serum CEA level greater than 5.2 ng/mL had a sensitivity of 68.0% and specificity of 81.5%.38
If a biopsy is obtained, appropriate immunohistochemistry (IHC) can facilitate the diagnosis. BTC is strongly positive for CK-7 and CK-19.41 CK-7 positivity is not specific and is also common among metastatic cancers of the lung and breast; therefore, in some cases cholangiocarcinoma may be a diagnosis of exclusion. Immunostaining for monoclonal CEA is diffusely positive in up to 75% of cases.41 An IHC panel consisting of Hep Par-1, arginase-1, monoclonal CEA, CK-7, CK-20, TTF-1, MOC-31, and CDX-2 has been proposed to optimize the differential diagnosis of HCC, metastatic adenocarcinoma, and cholangiocarcinoma.41
Case Continued
CT of the chest, abdomen, and pelvis reveals no concerns for metastasis and no evidence of primary cancer elsewhere. EGD and colonoscopy are clear. AFP levels are within normal limits (2 ng/mL). Biopsy is performed and demonstrates adenocarcinoma. IHC studies demonstrate cells positive for monoclonal CEA, CK-7, CK-19, and MOC-31, and negative for Napsin A, TTF-1, and CK-20.
- How is cholangiocarcinoma staged and classified?
The purpose of the staging system is to provide information on prognosis and guidance for therapy. Prognostic factors and the therapeutic approaches for BTC differ depending upon their location in the biliary tree. Accordingly, TNM classification systems for intrahepatic, hilar, and distal cholangiocarcinoma and gallbladder cancer have been separated (Table 1 and Table 2).23
The Bismuth-Corlette classification is used to further classify perihilar cholangiocarcinoma according to patterns of hepatic duct involvement. Type I tumors are located below the confluence of the left and right hepatic ducts.42 Type II reach the confluence of the hepatic ducts.42 Type III occlude the common hepatic duct and either the right or left hepatic duct (IIIa and IIIb, respectively).42 Finally, type IV are multicentric, or involve the confluence and both the right and left hepatic ducts.42 Tumors that involve the common hepatic duct bifurcation are named Klatskin tumors.42
- What is the first-line treatment for localized cholangiocarcinomas?
Surgical resection is the only potentially curative treatment for localized cholangiocarcinoma, although fewer than 20% of patients are suitable for curative treatment, due to the presence of advanced disease at diagnosis.43,44 Available evidence supports the recommendation that resection with negative margins, regardless of extent, should be the goal of therapy for patients with potentially resectable disease.44 Extensive hepatic resections are often necessary to achieve clear margins since the majority of patients present with large masses. Substantial evidence corroborates that R0 resection is associated with better survival, whereas the benefit of wide compared to narrow (< 5–10 mm) margins is unclear.45 A recent analysis of 96 patients suggests that the proximal resection margin has more prognostic implications than distal margins.45
Surgical options and resectability criteria depend upon tumor location. Extent of tumor in the bile duct is one of the most important factors that determine resectability.17 Although multifocal liver tumors (including satellite lesions), lymph node metastases to the porta hepatis, and distant metastases are considered relative contraindications to surgery, surgical approaches can be considered in selected patients.43 Patient selection for surgery is facilitated by careful preoperative staging, which may include laparoscopy. Laparoscopic staging prior to resection may prevent unnecessary laparotomy in 30% to 45% of patients.42,46
- Is there a role for adjuvant treatment?
Recurrence following complete resection is a primary limitation for cure in BTC, which provides a rationale for the use of adjuvant therapy.47,48 In a sample of 79 patients with extrahepatic cholangiocarcinoma who underwent curative resection, the cumulative recurrence rate after 4 years was 56%.47 Initial recurrence at a distant site occurs in 40% to 50% of patients.48
Lymphovascular and perineural invasion, lymph node metastasis, and tumor size ≥ 5 cm have been reported as independent predictors of recurrence and mortality following resection.49 A 2017 meta-analysis which included 30 studies involving more than 22,499 patients reported a 41% reduction in the risk of death with adjuvant chemotherapy, which translated to a mean OS benefit of 4 months in an unselected population.49 Moreover, this study revealed inferior OS in patients given adjuvant radiation therapy (RT) in combination with chemotherapy.49 These results are in line with the previous meta-analysis by Horgan et al, which demonstrated that adjuvant RT seems to benefit only patients with R1 resections, with a possible detrimental effect in R0 disease.50 Therefore, adjuvant chemoradiation cannot be viewed as a standard practice following R0 resection, and should be reserved for those patients with positive margins (R1/ 2) to reduce local progression.
In the phase 3 BILCAP trial presented at ASCO 2017, 447 patients with completely resected cholangiocarcinoma or gallbladder cancer with adequate biliary drainage and Eastern Cooperative Oncology Group (ECOG) performance score ≤ 2 were randomly assigned to observation or capecitabine (1250 mg/m2 twice daily for days 1–14 every 21 days for 8 cycles).51 Surgical treatment achieved R0 resection in 62% of patients and 46% were node-negative. Median OS was 51 months for the capecitabine group and 36 months for the control arm (hazard ratio [HR] 0.80, 95% CI 0.63 to 1.04, P = 0.097). Analyses with adjustment for nodal status, grade of disease, and gender indicated a HR of 0.71 (P < 0.01). Median DFS was 25 months versus 18 months favoring the capecitabine group, and rates of grade 3 or 4 toxicity were less than anticipated. Following the results of this trial, adjuvant capecitabine should become the new standard of care.
- What is the treatment for locally advanced cholangiocarcinoma?
The optimal approach to patients with locally advanced unresectable cholangiocarcinoma has not been established. The prognosis for patients with either locally unresectable or locally recurrent disease is typically measured in months. Goals of palliative therapy are relief of symptoms and improvement in quality of life, and there is no role for surgical debulking.
Liver transplantation is a potentially curative option for selected patients with hilar or intrahepatic cholangiocarcinoma. Patients with lymph node-negative, non-disseminated, locally advanced hilar cholangiocarcinomas have 5-year survival rates ranging from 25% to 42% following transplantation.52 Retrospective data suggests that neoadjuvant chemoradiation followed by liver transplantation is highly effective for selected patients with hilar cholangiocarcinoma.52 However, these results require confirmation from prospective clinical evidence. It is important to recognize that liver transplantation plays no role in the management of distal cholangiocarcinoma or gallbladder cancer.
Rarely, patients with borderline resectable intrahepatic cholangiocarcinoma will have a sufficient response to chemotherapy to permit later resection, and, in such cases, starting with chemotherapy and then restaging to evaluate resectability is appropriate.54 A single-center, retrospective analysis including 186 patients by Le Roy et al evaluated survival in patients with locally advanced, unresectable intrahepatic cholangiocarcinoma who received primary chemotherapy, followed by surgery in those with secondary resectability.54 After a median of 6 cycles of chemotherapy, 53% of patients achieved resectability and underwent surgery with curative intent. These patients had similar short- and long-term results compared to patients with initially resectable intrahepatic cholangiocarcinoma who had surgery alone, with median OS reaching 24 months.54
Ablative radiotherapy is an additional option for localized inoperable intrahepatic cholangiocarcinoma. Tao and colleagues evaluated 79 consecutive patients with inoperable intrahepatic cholangiocarcinoma treated with definitive RT.55 Median tumor size was 7.9 cm and 89% of patients received chemotherapy before RT. Median OS was 30 months and 3-year OS was 44%. Radiation dose was the single most important prognostic factor, and higher doses correlated with improved local control and OS. A biologic equivalent dose (BED) greater than 80.5 Gy was identified as an ablative dose of RT for large intrahepatic cholangiocarcinomas. The 3-year OS for patients receiving BED greater than 80.5 Gy was 73% versus 38% for those receiving lower doses.
Case Continued
The patient is deemed to have resectable disease and undergoes surgical resection followed by adjuvant capecitabine for 8 cycles. Unfortunately, after 1 year, follow-up imaging identifies bilateral enlarging lung nodules. Biopsy is performed and confirms metastatic cholangiocarcinoma.
- What is the treatment for metastatic BTC?
The prognosis of patients with advanced BTC is poor and OS for those undergoing supportive care alone is short. A benefit of chemotherapy over best supportive care for cholangiocarcinoma was demonstrated in an early phase 3 trial that randomly assigned 90 patients with advanced pancreatic or biliary cancer (37 with bile duct cancer) to receive either fluorouracil (FU) -based systemic chemotherapy or best supportive care. Results showed that chemotherapy significantly improved OS (6 months versus 2.5 months).56 Chemotherapy is also beneficial for patients with unresectable gallbladder cancer. In a single-center randomized study including 81 patients with unresectable gallbladder cancer, gemcitabine and oxaliplatin (GEMOX) improved progression-free survival (PFS) and OS compared to best supportive care.57 Treatment for metastatic cholangiocarcinoma and gallbladder cancer follows the same algorithm.
In 2010, cisplatin plus gemcitabine was established as a reference regimen for first-line therapy by the ABC-02 study, in which 410 patients with locally advanced or metastatic bile duct, gallbladder, or ampullary cancer were randomly assigned to 6 courses of cisplatin (25 mg/m2) plus gemcitabine (1000 mg/m2 on days 1 and 8, every 21 days) or gemcitabine alone (1000 mg/m2 days 1, 8, 15, every 28 days).58 OS was significantly greater with combination therapy (11.7 versus 8.1 months), and PFS also favored the combination arm (8 versus 5 months). Toxicity was comparable in both groups, with the exception of significantly higher rates of grade 3 or 4 neutropenia with gemcitabine plus cisplatin (25% versus 17%), and higher rates of grade 3 or 4 abnormal liver function with gemcitabine alone (27% versus 17%). Most quality-of-life scales showed a trend favoring combined therapy.58 A smaller, identically designed Japanese phase 3 randomized trial achieved similar results, demonstrating greater OS with cisplatin plus gemcitabine compared to gemcitabine alone (11.2 versus 7.7 months).59
The gemcitabine plus cisplatin combination has not been directly compared with other gemcitabine combinations in phase 3 trials. A pooled analysis of 104 trials of a variety of chemotherapy regimens in advanced biliary cancer concluded that the gemcitabine plus cisplatin regimen offered the highest rates of objective response and tumor control compared with either gemcitabine-free or cisplatin-free regimens.60 However, this did not translate into significant benefit in terms of either time to tumor progression or median OS. It is important to note that this analysis did not include results of the subsequent ABC-02 trial.
There is no standard treatment for patients with cholangiocarcinoma for whom first-line gemcitabine-based therapy fails. There are no completed prospective phase 3 trials supporting the use of second-line chemotherapy after failure of first-line chemotherapy in BTC, and the selection of candidates for second-line therapy as well as the optimal regimen are not established.61 The ongoing phase 2 multicenter ABC-06 trial is evaluating oxaliplatin plus short-term infusional FU and leucovorin (FOLFOX) versus best supportive care for second-line therapy. In a systematic review including 23 studies (14 phase 2 clinical trials and 9 retrospective studies) with 761 patients with BTC, the median OS was 7.2 months.
The optimal selection of candidates for second-line chemotherapy is not established. Two independent studies suggest that patients who have a good performance status (0 or 1), disease control with the first-line chemotherapy, low CA 19-9 level, and possibly previous surgery on their primary tumor, have the longest survival with second-line chemotherapy. However, whether these characteristics predict for chemotherapy responsiveness or more favorable biologic behavior is not clear.62,63 No particular regimen has proved superior to any other, and the choice of second-line regimen remains empiric.
For patients with adequate performance status, examples of other conventional chemotherapy regimens with demonstrated activity that could be considered for second-line therapy include: FOLFOX or capecitabine, gemcitabine plus capecitabine, capecitabine plus cisplatin, or irinotecan plus short-term infusional FU and leucovorin (FOLFIRI) with or without bevacizumab.64 For selected patients, second-line molecularly targeted therapy using erlotinib plus bevacizumab may be considered. However, this regimen is very costly.64 Examples of other regimens with demonstrated activity in phase 2 trials include GEMOX, gemcitabine plus fluoropyrimidine, and fluoropyrimidine plus oxaliplatin or cisplatin.64
There is promising data from studies of targeted therapy for specific molecular subgroups. A recent phase 2 trial evaluated the activity of BGJ398, an orally bioavailable, selective, ATP-competitive pan inhibitor of human fibroblast growth factor receptor (FGFR) kinase, in patients with FGFR-altered advanced cholangiocarcinoma.65 The overall response rate was 14.8% (18.8% FGFR2 fusions only) and disease control rate was 75.4% (83.3% FGFR2 fusions only). All responsive tumors contained FGFR2 fusions. Adverse events were manageable, and grade 3 or 4 treatment-related adverse events occurred in 25 patients (41%). Those included hyperphosphatemia, stomatitis, and palmar-plantar erythrodysesthesia. Javle and colleagues also identified HER2/neu blockade as a promising treatment strategy for gallbladder cancer patients with this gene amplification.66 This retrospective analysis included 9 patients with gallbladder cancer and 5 patients with cholangiocarcinoma who received HER2/neu-directed therapy (trastuzumab, lapatinib, or pertuzumab). In the gallbladder cancer group, HER2/neu gene amplification or overexpression was detected in 8 cases. These patients experienced disease stability (n = 3), partial response (n = 4), or complete response (n = 1) with HER2/neu–directed therapy. Median duration of response was 40 weeks. The cholangiocarcinoma cases treated in this series had no radiological responses despite HER2/neu mutations or amplification.
Gallbladder Cancer
Case Presentation
A 57-year-old woman from Chile presents with a 3-week history of progressive right upper quadrant abdominal pain. She denies nausea, vomiting, dysphagia, odynophagia, alterations in bowel habits, fever, or jaundice. Her past medical history is significant for obesity and hypertension. She has no history of smoking, alcohol, or illicit drug use. Laboratory studies show marked leukocytosis (23,800/µL) with neutrophilia (91%). Liver function test results are within normal limits. Ultrasound of the abdomen reveals gallbladder wall thickening and cholelithiasis.
The patient undergoes an uneventful laparoscopic cholecystectomy and is discharged from the hospital after 48 hours. Pathology report reveals a moderately differentiated adenocarcinoma of the gallbladder invading the perimuscular connective tissue (T2). No lymph nodes are identified in the specimen.
- What is the appropriate surgical management of gallbladder cancer?
Gallbladder cancer can be diagnosed preoperatively or can be found incidentally by intraoperative or pathological findings. In one large series, gallbladder cancer was incidentally found during 0.25% of laparoscopic cholecystectomies.67
For patients who are diagnosed with previously unsuspected gallbladder cancer by pathology findings, the extent of tumor invasion (T stage) indicates the need for re-resection (Figure 3).64
Alternatively, when gallbladder cancer is documented or suspected preoperatively, adequate imaging is important to identify patients with absolute contraindications to resection. Contraindications to surgery include metastasis, extensive involvement of the hepatoduodenal ligament, encasement of major vessels, and involvement of celiac, peripancreatic, periduodenal, or superior mesenteric nodes.72 Notwithstanding, retrospective series suggest individual patients may benefit, and surgical indications in advanced disease should be determined on an individual basis.73 Staging imaging should be obtained using multiphasic contrast-enhanced CT or MRI of the chest, abdomen, and pelvis. PET-scan can be used in selected cases where metastatic disease is suspected.64 Laparoscopic diagnostic staging should be considered prior to resection.64 This procedure can identify previously unknown contraindications to tumor resection in as much as 23% of patients, and the yield is significantly higher in locally advanced tumors.73
Patients with a diagnosis of potentially resectable, localized gallbladder cancer should be offered definitive surgery. Extended cholecystectomy is recommended for patients stage T2 or above. This procedure involves wedge resection of the gallbladder bed or a segmentectomy IVb/V and lymph node dissection, which should include the cystic duct, common bile duct, posterior superior pancreaticoduodenal lymph nodes, and those around the hepatoduodenal ligament.72 Bile duct excision should be performed if there is malignant involvement.64
Conclusion
BTCs are anatomically and clinically heterogeneous tumors. Prognostic factors and therapeutic approaches for BTCs differ depending upon their location in the biliary tree and, accordingly, TNM classification systems for intrahepatic, hilar, and distal cholangiocarcinoma and gallbladder cancer have been separated. Surgical resection is the only potentially curative treatment for localized BTC. However, recurrence following complete resection is a primary limitation for cure, which provides a rationale for the use of adjuvant therapy. The prognosis of patients with advanced BTC is poor and OS for those undergoing supportive care alone is short. Multiple randomized clinical trials have demonstrated a benefit of chemotherapy for metastatic disease. For patients with adequate performance status, second-line therapy can be considered, and data from studies that evaluated targeted therapy for specific molecular subgroups is promising.
1. Goldstein D, Lemech C, Valle J. New molecular and immunotherapeutic approaches in biliary cancer. ESMO Open 2017;2(Suppl 1):e000152.
2. Rizvi S, Khan SA, Hallemeier CL, et al. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol 2017 Oct 10. doi: 10.1038/nrclinonc.2017.157.
3. Hezel AF, Zhu AX. Systemic therapy for biliary tract cancers. Oncologist 2008;13:415–23.
4. U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999-2014 Incidence and Mortality Web-based Report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2017.
5. Torre LA, Siegel RL, Islami F, et al. Worldwide burden of and trends in mortality from gallbladder and other biliary tract cancers. Clin Gastroenterol Hepatol 2017 Aug 18. doi: 10.1016/j.cgh.2017.08.017.
6. Lau CSM, Zywot A, Mahendraraj K, Chamberlain CS. Gallbladder carcinoma in the United States: a population based clinical outcomes study involving 22,343 patients from the Surveillance, Epidemiology, and End Result Database (1973–2013). HPB Surg 2017;2017:1532835. doi:10.1155/2017/1532835.
7. Hughes T, O’Connor T, Techasen A, et al. Opisthorchiasis and cholangiocarcinoma in Southeast Asia: an unresolved problem. Int J Gen Med 2017;10:227–37.
8. DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg 2007;245:755–62.
9. Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-year trends in cholangiocarcinoma incidence in the U.S.: intrahepatic disease on the rise. Oncologist 2016;21:594–9.
10. Yao KJ, Jabbour S, Parekh N, et al. Increasing mortality in the United States from cholangiocarcinoma: an analysis of the National Center for Health Statistics Database. BMC Gastroenterol 2016;16:117.
11. Choi SB, Kim KS, Choi JY, et al. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol 2009;16:3048–56.
12. Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg 2008;248:84–96.
13. Duffy A, Capanu M, Abou-Alfa GK, et al. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC). J Surg Oncol 2008;98:485–9.
14. Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer — viewpoint of the IARC Working Group. N Engl J Med 2016;375:794–8.
15. Chen J, Han Y, Xu C, et al. Effect of type 2 diabetes mellitus on the risk for hepatocellular carcinoma in chronic liver diseases. Eur J Cancer Prev 2015;24:89–99.
16. Larsson SC, Giovannucci EL, Wolk A. Sweetened beverage consumption and risk of biliary tract and gallbladder cancer in a prospective study. J Natl Cancer Inst 2016;108: doi: 10.1093/jnci/djw125.
17. Gore RM. Biliary tract neoplasms: diagnosis and staging. Cancer Imaging 2007;7(Special Issue A):S15–23.
18. Broome U, Olsson R, Lööf L, et al. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut 1996;38:610–5.
19. Burak K, Angulo P, Pasha T, et al. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol 2004;99:523–6.
20. Rodrigues J, Diehl DL. Cholangiocarcinoma: clinical manifestations and diagnosis. Tech Gastrointest Endosc 2016;18:75–82.
21. Mitchell CH, Johnson PT, Fishman EK, et al. Features suggestive of gallbladder malignancy. J Comput Assist Tomogr 2014;38:235–41.
22. Beltz WR, Condon RE. Primary carcinoma of the gallbladder. Ann Surg 1974;180:180–4.
23. Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol 2011;8:512–22.
24. Patel T. Cholangiocarcinoma—controversies and challenges. Nat Rev Gastroenterol Hepatol 2011;8:189–200.
25. Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg 1996;224:463–73.
26. Bartella I, Dufour JF. Clinical diagnosis and staging of intrahepatic cholangiocarcinoma. J Gastrointestin Liver Dis 2015;24:481-9.
27. Yamaguchi K, Enjoji M. Carcinoma of the gallbladder: a clinicopathology of 103 patients and a newly proposed staging. Cancer 1988;62:1425–32.
28. Esposito I, Schirmacher P. Pathological aspects of cholangiocarcinoma. HPB. 2008;10:83–6.
29. Silva VWK, Askan G, Daniel TD, et al. Biliary carcinomas: pathology and the role of DNA mismatch repair deficiency. Chin Clin Oncol 2016;5:62.
30. Chung YE, Kim MJ, Park YN, et al. Varying appearances of cholangiocarcinoma: radiologic-pathologic correlation. Radiographics 2009;29:683–700.
31. Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepatobiliary Pancreat Surg 2003;10:288–91.
32. Rao PN. Nodule in liver: investigations, differential diagnosis and follow-up. J Clin Exp Hepatol 2014;4(Suppl 3):S57–62.
33. Kim TK, Lee E, Jang HJ. Imaging findings of mimickers of hepatocellular carcinoma. Clin Mol Hepatol 2015;21:326–43.
34. Hennedige TP, Neo WT, Venkatesh SK. Imaging of malignancies of the biliary tract- an update. Cancer Imaging 2014;14:14.
35. Kim SH, Lee CH, Kim BH, et al. Typical and atypical imaging findings of intrahepatic cholangiocarcinoma using gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging. J Comput Assist Tomogr 2012;36:704–9.
36. Asayama Y, Yoshimitsu K, Irie H, et al. Delayed-phase dynamic CT enhancement as a prognostic factor for mass-forming intrahepatic cholangiocarcinoma. Radiology 2006;238:150–5.
37. National Comprehensive Cancer Network. Cancer of unknown primary. www.nccn.org/professionals/physician_gls/pdf/bone.pdf. Accessed 1 Dec 2017.
38. Kefeli A, Basyigit S, Yeniova AO. Diagnosis of hepatocellular carcinoma. In: Abdeldayem HM, ed. Updates in liver cancer. London: InTech; 2017.
39. Bergquist JR, Ivanics T, Storlie CB, et al. Implications of CA19-9 elevation for survival, staging, and treatment sequencing in intrahepatic cholangiocarcinoma: A national cohort analysis. J Surg Oncol 2016;114:475–82.
40. Chung YJ, Choi DW, Choi SH, et al. Prognostic factors following surgical resection of distal bile duct cancer. J Korean Surg Soc 2013;85:212–8.
41. Lau SK, Prakash S, Geller SA, Alsabeh R. Comparative immunohistochemical profile of hepatocellular carcinoma, cholangiocarcinoma, and metastatic adenocarcinoma. Hum Pathol 2002;33:1175–81.
42. Paul A, Kaiser GM, Molmenti EP, et al. Klatskin tumors and the accuracy of the Bismuth-Corlette classification. Am Surg 2011;77:1695–9.
43. Cannavale A, Santoni M, Gazzetti M, et al. Updated management of malignant biliary tract tumors: an illustrative review. J Vasc Interv Radiol 2016;27:1056–69.
44. Matsuo K, Rocha FG, Ito K, et al. The Blumgart preoperative staging system for hilar cholangiocarcinoma: analysis of resectability and outcomes in 380 patients. J Am Coll Surg 2012;215:343–55.
45. Yoo T, Park SJ, Han SS, et al. Proximal resection margins: more prognostic than distal resection margins in patients undergoing hilar cholangiocarcinoma resection. Cancer Res Treat 2017 Nov 16; doi.org/10.4143/crt.2017.320.
46. Joseph S, Connor S, Garden OJ. Staging laparoscopy for cholangiocarcinoma. HPB 2008;10:116–9.
47. Jarnagin WR, Ruo L, Little SA, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer 2003;98:1689–700.
48. Kobayashi A, Miwa S, Nakata T, Miyagawa S. Disease recurrence patterns after R0 resection of hilar cholangiocarcinoma. Br J Surg 2010;97:56–64.
49. Ghidini M, Tomasello G, Botticelli A, et al. Adjuvant chemotherapy for resected biliary tract cancers: a systematic review and meta-analysis. HPB 2017;19:741–8.
50. Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol 2012;30:1934–40.
51. Primrose JN, Fox R, Palmer DH, et al. Adjuvant capecitabine for biliary tract cancer: the BILCAP randomized study [abstract]. J Clin Oncol 2017 35:15_suppl:4006-4006.
52. Darwish Murad S, Kim WR, Darnois DM, et al. Efficacy of neoadjuvant chemoradiation followed by liver transplantation for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology 2012;143:88–98.
53. Sapisochin G, Facciuto M, Rubbia-Brandt L, et al. Liver transplantation for “very early” intrahepatic cholangiocarcinoma: International retrospective study supporting a prospective assessment. Hepatology 2016;64:1178–88.
54. Le Roy B, Gelli M, Pittau G, et al. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br J Surg 2017 Aug 31. doi: 10.1002/bjs.10641.
55. Tao R, Krishnan S, Bhosale PR, et al. Ablative radiotherapy doses lead to a substantial prolongation of survival in patients with inoperable intrahepatic cholangiocarcinoma: a retrospective dose response analysis. J Clin Oncol 2016;34:219–26.
56. Glimelius B, Hoffman K, SjÓdén PO, et al. 555 Palliative chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Eur J Cancer 1995;31:S118.
57. Sharma A, Dwary AD, Mohanti BK, et al. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J Clin Oncol 2010;28:4581–6.
58. Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273–81.
59. Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer 2010;103:469–74.
60. Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer 2007;96:896–902.
61. Lamarca A, Hubner RA, David Ryder W, Valle JW. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol 2014;25:2328–38.
62. Brieau B, Dahan L, De Rycke Y, et al. Second-line chemotherapy for advanced biliary tract cancer after failure of the gemcitabine-platinum combination: A large multicenter study by the Association des Gastro-Entérologues Oncologues. Cancer 2015;121:3290–7.
63. Fornaro L, Cereda S, Aprile G, et al. Multivariate prognostic factors analysis for second-line chemotherapy in advanced biliary tract cancer. Br J Cancer 2014;110:2165–9.
64. National Comprehensive Cancer Network. Hepatobiliary cancer. www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. Accessed 12 Nov 2017.
65. Javle M, Lowery M, Shroff RT, et al. Phase II study of BGJ398 in patients with FGFR-altered advanced cholangiocarcinoma. J Clin Oncol 2017 Nov 28;JCO2017755009.
66. Javle M, Churi C, Kang HC, et al. HER2/neu-directed therapy for biliary tract cancer. J Hematol Oncol 2015;8:58.
67. Konstantinidis IT, Deshpande V, Genevay M, et al. Trends in presentation and survival for gallbladder cancer during a period of more than 4 decades: a single-institution experience. Arch Surg 2009;144:441–47.
68. Singh S, Agarwal AK. Gallbladder cancer: the role of laparoscopy and radical resection. Ann Surg 2009;250:494–5.
69. Kapoor VK, Haribhakti SP. Extended cholecystectomy for carcinoma of the gall bladder. Trop Gastroenterol 1995;16:74–5.
70. Ethun CG, Postlewait LM, Le N, et al. Association of optimal time Interval to re-resection for incidental gallbladder cancer with overall survival: a multi-Institution analysis from the US extrahepatic biliary malignancy consortium. JAMA Surg 2017;152:143–9.
71. Goetze TO, Paolucci V. Benefits of reoperation of T2 and more advanced incidental gallbladder carcinoma: analysis of the German registry. Ann Surg 2008;247:104–8.
72. Nishio H, Nagino M, Ebata T, et al. Aggressive surgery for stage IV gallbladder carcinoma; what are the contraindications? J Hepatobiliary Pancreat Surg 2007;14:351–7.
73. Agarwal AK, Kalayarasan R, Javed A, et al. The role of staging laparoscopy in primary gallbladder cancer--an analysis of 409 patients: a prospective study to evaluate the role of staging laparoscopy in the management of gallbladder cancer. Ann Surg 2013;258:318–23.
1. Goldstein D, Lemech C, Valle J. New molecular and immunotherapeutic approaches in biliary cancer. ESMO Open 2017;2(Suppl 1):e000152.
2. Rizvi S, Khan SA, Hallemeier CL, et al. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol 2017 Oct 10. doi: 10.1038/nrclinonc.2017.157.
3. Hezel AF, Zhu AX. Systemic therapy for biliary tract cancers. Oncologist 2008;13:415–23.
4. U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999-2014 Incidence and Mortality Web-based Report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2017.
5. Torre LA, Siegel RL, Islami F, et al. Worldwide burden of and trends in mortality from gallbladder and other biliary tract cancers. Clin Gastroenterol Hepatol 2017 Aug 18. doi: 10.1016/j.cgh.2017.08.017.
6. Lau CSM, Zywot A, Mahendraraj K, Chamberlain CS. Gallbladder carcinoma in the United States: a population based clinical outcomes study involving 22,343 patients from the Surveillance, Epidemiology, and End Result Database (1973–2013). HPB Surg 2017;2017:1532835. doi:10.1155/2017/1532835.
7. Hughes T, O’Connor T, Techasen A, et al. Opisthorchiasis and cholangiocarcinoma in Southeast Asia: an unresolved problem. Int J Gen Med 2017;10:227–37.
8. DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg 2007;245:755–62.
9. Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-year trends in cholangiocarcinoma incidence in the U.S.: intrahepatic disease on the rise. Oncologist 2016;21:594–9.
10. Yao KJ, Jabbour S, Parekh N, et al. Increasing mortality in the United States from cholangiocarcinoma: an analysis of the National Center for Health Statistics Database. BMC Gastroenterol 2016;16:117.
11. Choi SB, Kim KS, Choi JY, et al. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol 2009;16:3048–56.
12. Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg 2008;248:84–96.
13. Duffy A, Capanu M, Abou-Alfa GK, et al. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC). J Surg Oncol 2008;98:485–9.
14. Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer — viewpoint of the IARC Working Group. N Engl J Med 2016;375:794–8.
15. Chen J, Han Y, Xu C, et al. Effect of type 2 diabetes mellitus on the risk for hepatocellular carcinoma in chronic liver diseases. Eur J Cancer Prev 2015;24:89–99.
16. Larsson SC, Giovannucci EL, Wolk A. Sweetened beverage consumption and risk of biliary tract and gallbladder cancer in a prospective study. J Natl Cancer Inst 2016;108: doi: 10.1093/jnci/djw125.
17. Gore RM. Biliary tract neoplasms: diagnosis and staging. Cancer Imaging 2007;7(Special Issue A):S15–23.
18. Broome U, Olsson R, Lööf L, et al. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut 1996;38:610–5.
19. Burak K, Angulo P, Pasha T, et al. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol 2004;99:523–6.
20. Rodrigues J, Diehl DL. Cholangiocarcinoma: clinical manifestations and diagnosis. Tech Gastrointest Endosc 2016;18:75–82.
21. Mitchell CH, Johnson PT, Fishman EK, et al. Features suggestive of gallbladder malignancy. J Comput Assist Tomogr 2014;38:235–41.
22. Beltz WR, Condon RE. Primary carcinoma of the gallbladder. Ann Surg 1974;180:180–4.
23. Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol 2011;8:512–22.
24. Patel T. Cholangiocarcinoma—controversies and challenges. Nat Rev Gastroenterol Hepatol 2011;8:189–200.
25. Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg 1996;224:463–73.
26. Bartella I, Dufour JF. Clinical diagnosis and staging of intrahepatic cholangiocarcinoma. J Gastrointestin Liver Dis 2015;24:481-9.
27. Yamaguchi K, Enjoji M. Carcinoma of the gallbladder: a clinicopathology of 103 patients and a newly proposed staging. Cancer 1988;62:1425–32.
28. Esposito I, Schirmacher P. Pathological aspects of cholangiocarcinoma. HPB. 2008;10:83–6.
29. Silva VWK, Askan G, Daniel TD, et al. Biliary carcinomas: pathology and the role of DNA mismatch repair deficiency. Chin Clin Oncol 2016;5:62.
30. Chung YE, Kim MJ, Park YN, et al. Varying appearances of cholangiocarcinoma: radiologic-pathologic correlation. Radiographics 2009;29:683–700.
31. Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepatobiliary Pancreat Surg 2003;10:288–91.
32. Rao PN. Nodule in liver: investigations, differential diagnosis and follow-up. J Clin Exp Hepatol 2014;4(Suppl 3):S57–62.
33. Kim TK, Lee E, Jang HJ. Imaging findings of mimickers of hepatocellular carcinoma. Clin Mol Hepatol 2015;21:326–43.
34. Hennedige TP, Neo WT, Venkatesh SK. Imaging of malignancies of the biliary tract- an update. Cancer Imaging 2014;14:14.
35. Kim SH, Lee CH, Kim BH, et al. Typical and atypical imaging findings of intrahepatic cholangiocarcinoma using gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging. J Comput Assist Tomogr 2012;36:704–9.
36. Asayama Y, Yoshimitsu K, Irie H, et al. Delayed-phase dynamic CT enhancement as a prognostic factor for mass-forming intrahepatic cholangiocarcinoma. Radiology 2006;238:150–5.
37. National Comprehensive Cancer Network. Cancer of unknown primary. www.nccn.org/professionals/physician_gls/pdf/bone.pdf. Accessed 1 Dec 2017.
38. Kefeli A, Basyigit S, Yeniova AO. Diagnosis of hepatocellular carcinoma. In: Abdeldayem HM, ed. Updates in liver cancer. London: InTech; 2017.
39. Bergquist JR, Ivanics T, Storlie CB, et al. Implications of CA19-9 elevation for survival, staging, and treatment sequencing in intrahepatic cholangiocarcinoma: A national cohort analysis. J Surg Oncol 2016;114:475–82.
40. Chung YJ, Choi DW, Choi SH, et al. Prognostic factors following surgical resection of distal bile duct cancer. J Korean Surg Soc 2013;85:212–8.
41. Lau SK, Prakash S, Geller SA, Alsabeh R. Comparative immunohistochemical profile of hepatocellular carcinoma, cholangiocarcinoma, and metastatic adenocarcinoma. Hum Pathol 2002;33:1175–81.
42. Paul A, Kaiser GM, Molmenti EP, et al. Klatskin tumors and the accuracy of the Bismuth-Corlette classification. Am Surg 2011;77:1695–9.
43. Cannavale A, Santoni M, Gazzetti M, et al. Updated management of malignant biliary tract tumors: an illustrative review. J Vasc Interv Radiol 2016;27:1056–69.
44. Matsuo K, Rocha FG, Ito K, et al. The Blumgart preoperative staging system for hilar cholangiocarcinoma: analysis of resectability and outcomes in 380 patients. J Am Coll Surg 2012;215:343–55.
45. Yoo T, Park SJ, Han SS, et al. Proximal resection margins: more prognostic than distal resection margins in patients undergoing hilar cholangiocarcinoma resection. Cancer Res Treat 2017 Nov 16; doi.org/10.4143/crt.2017.320.
46. Joseph S, Connor S, Garden OJ. Staging laparoscopy for cholangiocarcinoma. HPB 2008;10:116–9.
47. Jarnagin WR, Ruo L, Little SA, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer 2003;98:1689–700.
48. Kobayashi A, Miwa S, Nakata T, Miyagawa S. Disease recurrence patterns after R0 resection of hilar cholangiocarcinoma. Br J Surg 2010;97:56–64.
49. Ghidini M, Tomasello G, Botticelli A, et al. Adjuvant chemotherapy for resected biliary tract cancers: a systematic review and meta-analysis. HPB 2017;19:741–8.
50. Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol 2012;30:1934–40.
51. Primrose JN, Fox R, Palmer DH, et al. Adjuvant capecitabine for biliary tract cancer: the BILCAP randomized study [abstract]. J Clin Oncol 2017 35:15_suppl:4006-4006.
52. Darwish Murad S, Kim WR, Darnois DM, et al. Efficacy of neoadjuvant chemoradiation followed by liver transplantation for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology 2012;143:88–98.
53. Sapisochin G, Facciuto M, Rubbia-Brandt L, et al. Liver transplantation for “very early” intrahepatic cholangiocarcinoma: International retrospective study supporting a prospective assessment. Hepatology 2016;64:1178–88.
54. Le Roy B, Gelli M, Pittau G, et al. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br J Surg 2017 Aug 31. doi: 10.1002/bjs.10641.
55. Tao R, Krishnan S, Bhosale PR, et al. Ablative radiotherapy doses lead to a substantial prolongation of survival in patients with inoperable intrahepatic cholangiocarcinoma: a retrospective dose response analysis. J Clin Oncol 2016;34:219–26.
56. Glimelius B, Hoffman K, SjÓdén PO, et al. 555 Palliative chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Eur J Cancer 1995;31:S118.
57. Sharma A, Dwary AD, Mohanti BK, et al. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J Clin Oncol 2010;28:4581–6.
58. Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273–81.
59. Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer 2010;103:469–74.
60. Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer 2007;96:896–902.
61. Lamarca A, Hubner RA, David Ryder W, Valle JW. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol 2014;25:2328–38.
62. Brieau B, Dahan L, De Rycke Y, et al. Second-line chemotherapy for advanced biliary tract cancer after failure of the gemcitabine-platinum combination: A large multicenter study by the Association des Gastro-Entérologues Oncologues. Cancer 2015;121:3290–7.
63. Fornaro L, Cereda S, Aprile G, et al. Multivariate prognostic factors analysis for second-line chemotherapy in advanced biliary tract cancer. Br J Cancer 2014;110:2165–9.
64. National Comprehensive Cancer Network. Hepatobiliary cancer. www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. Accessed 12 Nov 2017.
65. Javle M, Lowery M, Shroff RT, et al. Phase II study of BGJ398 in patients with FGFR-altered advanced cholangiocarcinoma. J Clin Oncol 2017 Nov 28;JCO2017755009.
66. Javle M, Churi C, Kang HC, et al. HER2/neu-directed therapy for biliary tract cancer. J Hematol Oncol 2015;8:58.
67. Konstantinidis IT, Deshpande V, Genevay M, et al. Trends in presentation and survival for gallbladder cancer during a period of more than 4 decades: a single-institution experience. Arch Surg 2009;144:441–47.
68. Singh S, Agarwal AK. Gallbladder cancer: the role of laparoscopy and radical resection. Ann Surg 2009;250:494–5.
69. Kapoor VK, Haribhakti SP. Extended cholecystectomy for carcinoma of the gall bladder. Trop Gastroenterol 1995;16:74–5.
70. Ethun CG, Postlewait LM, Le N, et al. Association of optimal time Interval to re-resection for incidental gallbladder cancer with overall survival: a multi-Institution analysis from the US extrahepatic biliary malignancy consortium. JAMA Surg 2017;152:143–9.
71. Goetze TO, Paolucci V. Benefits of reoperation of T2 and more advanced incidental gallbladder carcinoma: analysis of the German registry. Ann Surg 2008;247:104–8.
72. Nishio H, Nagino M, Ebata T, et al. Aggressive surgery for stage IV gallbladder carcinoma; what are the contraindications? J Hepatobiliary Pancreat Surg 2007;14:351–7.
73. Agarwal AK, Kalayarasan R, Javed A, et al. The role of staging laparoscopy in primary gallbladder cancer--an analysis of 409 patients: a prospective study to evaluate the role of staging laparoscopy in the management of gallbladder cancer. Ann Surg 2013;258:318–23.