User login
CHEST Annual Meeting Highlights 2017
- Sepsis response team not beneficial
- Outcomes better for patients with H1N1 vaccination
- Lung recovery high after ECMO in near-fatal asthma
- Robotic-assisted pulmonary lobectomy removes large tumors
- Aspirin responsiveness improved in some with obstructive sleep apnea
- Solriamfetol improves excessive sleepiness in OSA patients
- Acute kidney injury linked with doubled inpatient VTEs
- Alarm reductions don’t improve ICU response times
- ARDS incidence is declining. Is it a preventable syndrome?
- Balanced crystalloids protect kidney better than saline
- Omalizumab helps asthma COPD overlap patients
- Nebulized glycopyrrolate improves lung function in COPD
- Nebulized LABA safe for long-term use in COPD
- IGRA preferred test for latent TB diagnosis
- Only half of appropriate COPD patients get long-acting bronchodilators
- Rapid influenza test obviates empiric antivirals
- Remimazolam surpasses midazolam
- Revised guidelines raise lung cancer screening age ceiling
- Ultrathin bronchoscopy plus radial EBUS unreliable at making diagnoses
- Phrenic-nerve stimulator maintains benefits for 18 months
- Cardiogenic shock boosts PAH readmissions 10-fold
- Evidence mounts for pulmonary embolism benefit from catheter thrombolysis
- Tracheobronchial tree size changes may predict IPF outcomes
- Shorter walk test predicts IPF outcomes
- Comparing arterial ratios may aid risk assessment in IPF
- FDA’s standards for approving generics are questioned
- Live Streaming at CHEST 2017
- Sepsis response team not beneficial
- Outcomes better for patients with H1N1 vaccination
- Lung recovery high after ECMO in near-fatal asthma
- Robotic-assisted pulmonary lobectomy removes large tumors
- Aspirin responsiveness improved in some with obstructive sleep apnea
- Solriamfetol improves excessive sleepiness in OSA patients
- Acute kidney injury linked with doubled inpatient VTEs
- Alarm reductions don’t improve ICU response times
- ARDS incidence is declining. Is it a preventable syndrome?
- Balanced crystalloids protect kidney better than saline
- Omalizumab helps asthma COPD overlap patients
- Nebulized glycopyrrolate improves lung function in COPD
- Nebulized LABA safe for long-term use in COPD
- IGRA preferred test for latent TB diagnosis
- Only half of appropriate COPD patients get long-acting bronchodilators
- Rapid influenza test obviates empiric antivirals
- Remimazolam surpasses midazolam
- Revised guidelines raise lung cancer screening age ceiling
- Ultrathin bronchoscopy plus radial EBUS unreliable at making diagnoses
- Phrenic-nerve stimulator maintains benefits for 18 months
- Cardiogenic shock boosts PAH readmissions 10-fold
- Evidence mounts for pulmonary embolism benefit from catheter thrombolysis
- Tracheobronchial tree size changes may predict IPF outcomes
- Shorter walk test predicts IPF outcomes
- Comparing arterial ratios may aid risk assessment in IPF
- FDA’s standards for approving generics are questioned
- Live Streaming at CHEST 2017
- Sepsis response team not beneficial
- Outcomes better for patients with H1N1 vaccination
- Lung recovery high after ECMO in near-fatal asthma
- Robotic-assisted pulmonary lobectomy removes large tumors
- Aspirin responsiveness improved in some with obstructive sleep apnea
- Solriamfetol improves excessive sleepiness in OSA patients
- Acute kidney injury linked with doubled inpatient VTEs
- Alarm reductions don’t improve ICU response times
- ARDS incidence is declining. Is it a preventable syndrome?
- Balanced crystalloids protect kidney better than saline
- Omalizumab helps asthma COPD overlap patients
- Nebulized glycopyrrolate improves lung function in COPD
- Nebulized LABA safe for long-term use in COPD
- IGRA preferred test for latent TB diagnosis
- Only half of appropriate COPD patients get long-acting bronchodilators
- Rapid influenza test obviates empiric antivirals
- Remimazolam surpasses midazolam
- Revised guidelines raise lung cancer screening age ceiling
- Ultrathin bronchoscopy plus radial EBUS unreliable at making diagnoses
- Phrenic-nerve stimulator maintains benefits for 18 months
- Cardiogenic shock boosts PAH readmissions 10-fold
- Evidence mounts for pulmonary embolism benefit from catheter thrombolysis
- Tracheobronchial tree size changes may predict IPF outcomes
- Shorter walk test predicts IPF outcomes
- Comparing arterial ratios may aid risk assessment in IPF
- FDA’s standards for approving generics are questioned
- Live Streaming at CHEST 2017
Interventions ‘key’ when ADHD, conduct disorder, and delinquency overlap
LAS VEGAS – The overlap of ADHD, conduct disorder, substance use disorder, and criminality likely reflect related underlying mechanisms, which may elucidate different developmental pathways of offending.
“Early interventions are key,” Praveen R. Kambam, MD, said at an annual psychopharmacology update held by the Nevada Psychiatric Association.
According to Dr. Kambam, a clinical and forensic psychiatrist at the University of California, Los Angeles, ADHD is overrepresented in correctional settings worldwide, especially the hyperactive-impulsive subtype. “In juvenile settings, ADHD rates are 3-4 times higher than rates in the general population,” he said. “If you combine juvenile and adult prison populations worldwide, the rates are about 2-5 times higher than the general population.”
The risks are increased for comorbid oppositional defiant disorder (ODD) and conduct disorder. In fact, ADHD and conduct disorder co-occur in about 50% of cases. In girls, the prevalence rate of conduct disorder is steady at 0.8% around age 5 years and increases to 2.8% around age 15 years, while in boys, conduct disorder is steady at 2.1% around age 5 years and rises to 5.5% at age 15 years.
According to a literature review of 18 prospective studies, 13 retrospective studies, and four reviews, individuals with ADHD plus or minus conduct disorder had an increased the risk of antisocial personality disorder, and those with ADHD plus conduct disorder had an increased risk of criminality (J Atten Disord. 2016;20[10]:815-24). “So it’s a subtle difference, where antisocial personality disorder and criminality are slightly different,” Dr. Kambam said. “It could be that the diagnostic criteria are catching the same thing. However, the added [conduct disorder] suggests that there may be subpopulations that are vulnerable.”
He went on to note that individuals with ADHD and delinquency tend to have more learning problems, poor academic achievement, peer relationship problems, and risk of social rejection, while individuals with oppositional defiant disorder and delinquency tend to have peer relationship problems, a negative parent-child relationship, and increased risk of developing conduct disorder.
ADHD is associated with alcohol and drug use in adulthood and nicotine use in adolescence. “Comorbidity between ADHD and ODD/[conduct disorder] is robustly related to substance outcomes,” Dr. Kambam said. “However, both initiation and continuation of substance use disorder are more likely when ADHD symptoms are present, even when controlling for ODD/[conduct disorder]. As for substance use disorder [SUD] and delinquency, the onset of delinquency is more likely in children with onset of SUD by age 11, and SUDs are closely linked with criminality in both juveniles and adults.”
Comorbidity of SUD with conduct disorder and ADHD likely reflects multifactorial mechanisms, he said, such as inherent novelty seeking or school failure leading to association with antisocial peers. Risk factors for chronic offending include early onset of criminal behaviors, ADHD plus conduct disorder, and ODD. ADHD has an independent yet weaker relationship with antisocial behaviors as well, while ADHD, conduct disorder, and SUD are independently associated with increased recidivism.
Environmental factors for chronic offending include the home environment, peer response, parenting skills, and in utero exposures and perinatal complications. “Whether ADHD develops into more severe conduct problems depends considerably on exposure to potentiating environmental factors,” Dr. Kambam said. “The converse is also true: Low-risk environments promote desistance from this pathway in impulsive boys.” He added that the chronic offenders/criminality pathway likely stems from underlying mechanisms, such as impulsivity, low self-control, and executive dysfunction.
If left untreated, ADHD is associated with poor academic and employment outcomes, SUDs, depression, bipolar disorder, suicide attempts, vehicular accidents, and use of mental health services. “The economic costs are estimated to be $42.5 billion annually, so it has a large impact,” he said.
Limited evidence exists to support pharmacological treatments for conduct disorder, although stimulants/alpha-agonists, antipsychotics, lithium, and mood stabilizers may offer some benefit for target symptoms. “Most of the treatment data center around multisystemic therapy, including behavioral modification/parent management training, and functional family training,” Dr. Kambam said. “Treating disruptive behavior disorders and SUDs are
likely to reduce criminality and recidivism, particularly if started early. There are many beneficial economic impacts. Think about the cost of having youth detained in the criminal justice systems. In Los Angeles County, that cost is about $230,000 per year per kid. That money can probably be better spent somewhere else.”
Numerous studies show that the nonmedical use of stimulants ranges from 25%-40%. “They’re mostly used to enhance academic and/or work performance, but some are used for euphoric effect,” he said. “Individuals in college and just out of college seem to be at the highest risk. There is a strong relationship between [conduct disorder]/[antisocial personality disorder] or SUDs and nonmedical use.”
Treatment with stimulants in correctional settings is controversial. “Some say try after failure of nonstimulants, while others say never use them due to substance abuse, misuse, intimidation of patients to surrender medication, and security/costs,” Dr. Kambam said. “The protocol for ADHD treatment in Massachusetts prisons calls for use of nonstimulants first, followed by ‘crushable’ stimulants if indicated.” The methylphenidate patch and lisdexamfetamine also can be effective in the incarcerated population.
Dr. Kambam reported having no financial disclosures.
SOURCE: Kambam PR. NPA 2018.
LAS VEGAS – The overlap of ADHD, conduct disorder, substance use disorder, and criminality likely reflect related underlying mechanisms, which may elucidate different developmental pathways of offending.
“Early interventions are key,” Praveen R. Kambam, MD, said at an annual psychopharmacology update held by the Nevada Psychiatric Association.
According to Dr. Kambam, a clinical and forensic psychiatrist at the University of California, Los Angeles, ADHD is overrepresented in correctional settings worldwide, especially the hyperactive-impulsive subtype. “In juvenile settings, ADHD rates are 3-4 times higher than rates in the general population,” he said. “If you combine juvenile and adult prison populations worldwide, the rates are about 2-5 times higher than the general population.”
The risks are increased for comorbid oppositional defiant disorder (ODD) and conduct disorder. In fact, ADHD and conduct disorder co-occur in about 50% of cases. In girls, the prevalence rate of conduct disorder is steady at 0.8% around age 5 years and increases to 2.8% around age 15 years, while in boys, conduct disorder is steady at 2.1% around age 5 years and rises to 5.5% at age 15 years.
According to a literature review of 18 prospective studies, 13 retrospective studies, and four reviews, individuals with ADHD plus or minus conduct disorder had an increased the risk of antisocial personality disorder, and those with ADHD plus conduct disorder had an increased risk of criminality (J Atten Disord. 2016;20[10]:815-24). “So it’s a subtle difference, where antisocial personality disorder and criminality are slightly different,” Dr. Kambam said. “It could be that the diagnostic criteria are catching the same thing. However, the added [conduct disorder] suggests that there may be subpopulations that are vulnerable.”
He went on to note that individuals with ADHD and delinquency tend to have more learning problems, poor academic achievement, peer relationship problems, and risk of social rejection, while individuals with oppositional defiant disorder and delinquency tend to have peer relationship problems, a negative parent-child relationship, and increased risk of developing conduct disorder.
ADHD is associated with alcohol and drug use in adulthood and nicotine use in adolescence. “Comorbidity between ADHD and ODD/[conduct disorder] is robustly related to substance outcomes,” Dr. Kambam said. “However, both initiation and continuation of substance use disorder are more likely when ADHD symptoms are present, even when controlling for ODD/[conduct disorder]. As for substance use disorder [SUD] and delinquency, the onset of delinquency is more likely in children with onset of SUD by age 11, and SUDs are closely linked with criminality in both juveniles and adults.”
Comorbidity of SUD with conduct disorder and ADHD likely reflects multifactorial mechanisms, he said, such as inherent novelty seeking or school failure leading to association with antisocial peers. Risk factors for chronic offending include early onset of criminal behaviors, ADHD plus conduct disorder, and ODD. ADHD has an independent yet weaker relationship with antisocial behaviors as well, while ADHD, conduct disorder, and SUD are independently associated with increased recidivism.
Environmental factors for chronic offending include the home environment, peer response, parenting skills, and in utero exposures and perinatal complications. “Whether ADHD develops into more severe conduct problems depends considerably on exposure to potentiating environmental factors,” Dr. Kambam said. “The converse is also true: Low-risk environments promote desistance from this pathway in impulsive boys.” He added that the chronic offenders/criminality pathway likely stems from underlying mechanisms, such as impulsivity, low self-control, and executive dysfunction.
If left untreated, ADHD is associated with poor academic and employment outcomes, SUDs, depression, bipolar disorder, suicide attempts, vehicular accidents, and use of mental health services. “The economic costs are estimated to be $42.5 billion annually, so it has a large impact,” he said.
Limited evidence exists to support pharmacological treatments for conduct disorder, although stimulants/alpha-agonists, antipsychotics, lithium, and mood stabilizers may offer some benefit for target symptoms. “Most of the treatment data center around multisystemic therapy, including behavioral modification/parent management training, and functional family training,” Dr. Kambam said. “Treating disruptive behavior disorders and SUDs are
likely to reduce criminality and recidivism, particularly if started early. There are many beneficial economic impacts. Think about the cost of having youth detained in the criminal justice systems. In Los Angeles County, that cost is about $230,000 per year per kid. That money can probably be better spent somewhere else.”
Numerous studies show that the nonmedical use of stimulants ranges from 25%-40%. “They’re mostly used to enhance academic and/or work performance, but some are used for euphoric effect,” he said. “Individuals in college and just out of college seem to be at the highest risk. There is a strong relationship between [conduct disorder]/[antisocial personality disorder] or SUDs and nonmedical use.”
Treatment with stimulants in correctional settings is controversial. “Some say try after failure of nonstimulants, while others say never use them due to substance abuse, misuse, intimidation of patients to surrender medication, and security/costs,” Dr. Kambam said. “The protocol for ADHD treatment in Massachusetts prisons calls for use of nonstimulants first, followed by ‘crushable’ stimulants if indicated.” The methylphenidate patch and lisdexamfetamine also can be effective in the incarcerated population.
Dr. Kambam reported having no financial disclosures.
SOURCE: Kambam PR. NPA 2018.
LAS VEGAS – The overlap of ADHD, conduct disorder, substance use disorder, and criminality likely reflect related underlying mechanisms, which may elucidate different developmental pathways of offending.
“Early interventions are key,” Praveen R. Kambam, MD, said at an annual psychopharmacology update held by the Nevada Psychiatric Association.
According to Dr. Kambam, a clinical and forensic psychiatrist at the University of California, Los Angeles, ADHD is overrepresented in correctional settings worldwide, especially the hyperactive-impulsive subtype. “In juvenile settings, ADHD rates are 3-4 times higher than rates in the general population,” he said. “If you combine juvenile and adult prison populations worldwide, the rates are about 2-5 times higher than the general population.”
The risks are increased for comorbid oppositional defiant disorder (ODD) and conduct disorder. In fact, ADHD and conduct disorder co-occur in about 50% of cases. In girls, the prevalence rate of conduct disorder is steady at 0.8% around age 5 years and increases to 2.8% around age 15 years, while in boys, conduct disorder is steady at 2.1% around age 5 years and rises to 5.5% at age 15 years.
According to a literature review of 18 prospective studies, 13 retrospective studies, and four reviews, individuals with ADHD plus or minus conduct disorder had an increased the risk of antisocial personality disorder, and those with ADHD plus conduct disorder had an increased risk of criminality (J Atten Disord. 2016;20[10]:815-24). “So it’s a subtle difference, where antisocial personality disorder and criminality are slightly different,” Dr. Kambam said. “It could be that the diagnostic criteria are catching the same thing. However, the added [conduct disorder] suggests that there may be subpopulations that are vulnerable.”
He went on to note that individuals with ADHD and delinquency tend to have more learning problems, poor academic achievement, peer relationship problems, and risk of social rejection, while individuals with oppositional defiant disorder and delinquency tend to have peer relationship problems, a negative parent-child relationship, and increased risk of developing conduct disorder.
ADHD is associated with alcohol and drug use in adulthood and nicotine use in adolescence. “Comorbidity between ADHD and ODD/[conduct disorder] is robustly related to substance outcomes,” Dr. Kambam said. “However, both initiation and continuation of substance use disorder are more likely when ADHD symptoms are present, even when controlling for ODD/[conduct disorder]. As for substance use disorder [SUD] and delinquency, the onset of delinquency is more likely in children with onset of SUD by age 11, and SUDs are closely linked with criminality in both juveniles and adults.”
Comorbidity of SUD with conduct disorder and ADHD likely reflects multifactorial mechanisms, he said, such as inherent novelty seeking or school failure leading to association with antisocial peers. Risk factors for chronic offending include early onset of criminal behaviors, ADHD plus conduct disorder, and ODD. ADHD has an independent yet weaker relationship with antisocial behaviors as well, while ADHD, conduct disorder, and SUD are independently associated with increased recidivism.
Environmental factors for chronic offending include the home environment, peer response, parenting skills, and in utero exposures and perinatal complications. “Whether ADHD develops into more severe conduct problems depends considerably on exposure to potentiating environmental factors,” Dr. Kambam said. “The converse is also true: Low-risk environments promote desistance from this pathway in impulsive boys.” He added that the chronic offenders/criminality pathway likely stems from underlying mechanisms, such as impulsivity, low self-control, and executive dysfunction.
If left untreated, ADHD is associated with poor academic and employment outcomes, SUDs, depression, bipolar disorder, suicide attempts, vehicular accidents, and use of mental health services. “The economic costs are estimated to be $42.5 billion annually, so it has a large impact,” he said.
Limited evidence exists to support pharmacological treatments for conduct disorder, although stimulants/alpha-agonists, antipsychotics, lithium, and mood stabilizers may offer some benefit for target symptoms. “Most of the treatment data center around multisystemic therapy, including behavioral modification/parent management training, and functional family training,” Dr. Kambam said. “Treating disruptive behavior disorders and SUDs are
likely to reduce criminality and recidivism, particularly if started early. There are many beneficial economic impacts. Think about the cost of having youth detained in the criminal justice systems. In Los Angeles County, that cost is about $230,000 per year per kid. That money can probably be better spent somewhere else.”
Numerous studies show that the nonmedical use of stimulants ranges from 25%-40%. “They’re mostly used to enhance academic and/or work performance, but some are used for euphoric effect,” he said. “Individuals in college and just out of college seem to be at the highest risk. There is a strong relationship between [conduct disorder]/[antisocial personality disorder] or SUDs and nonmedical use.”
Treatment with stimulants in correctional settings is controversial. “Some say try after failure of nonstimulants, while others say never use them due to substance abuse, misuse, intimidation of patients to surrender medication, and security/costs,” Dr. Kambam said. “The protocol for ADHD treatment in Massachusetts prisons calls for use of nonstimulants first, followed by ‘crushable’ stimulants if indicated.” The methylphenidate patch and lisdexamfetamine also can be effective in the incarcerated population.
Dr. Kambam reported having no financial disclosures.
SOURCE: Kambam PR. NPA 2018.
REPORTING FROM NPA 2018
Avelumab safety compares with other checkpoint inhibitors
The immune checkpoint inhibitor avelumab (Bavencio), targeted against programmed cell death protein 1 and its ligand (PD1/PD-L1), appears to be well tolerated with a manageable safety profile, pooled data from two clinical trials suggest.
Of the 1,738 patients enrolled in the phase 1 JAVELIN solid tumor trial and the phase 2 JAVELIN Merkel 200 trial, 1,164 (67%) had a treatment-related adverse event (TRAE), and 177 (10.2%) had grade 3 or greater TRAEs. Grade 3 or greater immune-related adverse events (irAEs) occurred in just 2.2% of patients, reported Karen Kelly, MD, from the University of California, Davis, and her colleagues.
“Although conclusions drawn from cross-study comparisons should be made with caution, and to the best of our knowledge the number of pan-tumor clinical studies of [immune checkpoint inhibitor] monotherapy is limited, this analysis of a large population of patients across a broad scope of tumor types suggests that avelumab was associated with an incidence of irAEs that is consistent with that of other ICIs,” they wrote in Cancer.
Adverse events common with other immune checkpoint inhibitors include low-grade fatigue, pruritus, and rash, as well as serious irAEs, including high-grade pneumonitis and autoimmune-like side effects, the authors noted.
To characterize the adverse event profile of avelumab, they reviewed safety data on 1,650 patients enrolled in the solid tumor trial and 88 enrolled in the Merkel cell carcinoma trial, which included all patients in the trial who had received at least one dose of avelumab monotherapy by the cutoff date.
At the time of the analysis, 287 patients (16.5%) were continuing treatment, and 1,451 had discontinued therapy, largely because of disease progression.
Nearly all patients – 1,697 (97.6%) – had at least one adverse event of any grade or cause.
Four patients died from what investigators determined were TRAEs, including autoimmune hepatitis with peritoneal metastases and ascites in a patient with gastric cancer, liver metastases and acute liver failure in a patient with metastatic breast cancer, respiratory distress in a patient with breast cancer and multiple comorbidities, and treatment-related pneumonitis with ongoing Clostridium difficile colitis and diverticulitis not related to study treatment in a patient with urothelial carcinoma.
An additional 59 patients (3.4%) died from adverse events not deemed to be treatment-related, and 104 patient (6%) died from unknown or undocumented causes.
Any grade of irAE occurred in 247 patients (14.2%) and were grade 3 or greater in 39 (2.2%). Management of irAEs included systemic corticosteroids and nonsteroidal immunosuppressants.
In all, 439 patients (25.3%) had infusion-related reactions, which were treated generally with systemic corticosteroid. The protocol of the solid tumor trial was amended later to include diphenhydramine and acetaminophen before the first avelumab infusion as prophylaxis.
The study was sponsored by Merck and part of an alliance between Merck and Pfizer. Dr. Kelly reported no conflicts of interest. Multiple coauthors reported research funding, consulting fees, honoraria, or other consideration from various companies, and several coauthors are Merck employees.
SOURCE: Kelly K et al. Cancer. 2018 Feb 22. doi: 10.1002/cncr.31293.
The immune checkpoint inhibitor avelumab (Bavencio), targeted against programmed cell death protein 1 and its ligand (PD1/PD-L1), appears to be well tolerated with a manageable safety profile, pooled data from two clinical trials suggest.
Of the 1,738 patients enrolled in the phase 1 JAVELIN solid tumor trial and the phase 2 JAVELIN Merkel 200 trial, 1,164 (67%) had a treatment-related adverse event (TRAE), and 177 (10.2%) had grade 3 or greater TRAEs. Grade 3 or greater immune-related adverse events (irAEs) occurred in just 2.2% of patients, reported Karen Kelly, MD, from the University of California, Davis, and her colleagues.
“Although conclusions drawn from cross-study comparisons should be made with caution, and to the best of our knowledge the number of pan-tumor clinical studies of [immune checkpoint inhibitor] monotherapy is limited, this analysis of a large population of patients across a broad scope of tumor types suggests that avelumab was associated with an incidence of irAEs that is consistent with that of other ICIs,” they wrote in Cancer.
Adverse events common with other immune checkpoint inhibitors include low-grade fatigue, pruritus, and rash, as well as serious irAEs, including high-grade pneumonitis and autoimmune-like side effects, the authors noted.
To characterize the adverse event profile of avelumab, they reviewed safety data on 1,650 patients enrolled in the solid tumor trial and 88 enrolled in the Merkel cell carcinoma trial, which included all patients in the trial who had received at least one dose of avelumab monotherapy by the cutoff date.
At the time of the analysis, 287 patients (16.5%) were continuing treatment, and 1,451 had discontinued therapy, largely because of disease progression.
Nearly all patients – 1,697 (97.6%) – had at least one adverse event of any grade or cause.
Four patients died from what investigators determined were TRAEs, including autoimmune hepatitis with peritoneal metastases and ascites in a patient with gastric cancer, liver metastases and acute liver failure in a patient with metastatic breast cancer, respiratory distress in a patient with breast cancer and multiple comorbidities, and treatment-related pneumonitis with ongoing Clostridium difficile colitis and diverticulitis not related to study treatment in a patient with urothelial carcinoma.
An additional 59 patients (3.4%) died from adverse events not deemed to be treatment-related, and 104 patient (6%) died from unknown or undocumented causes.
Any grade of irAE occurred in 247 patients (14.2%) and were grade 3 or greater in 39 (2.2%). Management of irAEs included systemic corticosteroids and nonsteroidal immunosuppressants.
In all, 439 patients (25.3%) had infusion-related reactions, which were treated generally with systemic corticosteroid. The protocol of the solid tumor trial was amended later to include diphenhydramine and acetaminophen before the first avelumab infusion as prophylaxis.
The study was sponsored by Merck and part of an alliance between Merck and Pfizer. Dr. Kelly reported no conflicts of interest. Multiple coauthors reported research funding, consulting fees, honoraria, or other consideration from various companies, and several coauthors are Merck employees.
SOURCE: Kelly K et al. Cancer. 2018 Feb 22. doi: 10.1002/cncr.31293.
The immune checkpoint inhibitor avelumab (Bavencio), targeted against programmed cell death protein 1 and its ligand (PD1/PD-L1), appears to be well tolerated with a manageable safety profile, pooled data from two clinical trials suggest.
Of the 1,738 patients enrolled in the phase 1 JAVELIN solid tumor trial and the phase 2 JAVELIN Merkel 200 trial, 1,164 (67%) had a treatment-related adverse event (TRAE), and 177 (10.2%) had grade 3 or greater TRAEs. Grade 3 or greater immune-related adverse events (irAEs) occurred in just 2.2% of patients, reported Karen Kelly, MD, from the University of California, Davis, and her colleagues.
“Although conclusions drawn from cross-study comparisons should be made with caution, and to the best of our knowledge the number of pan-tumor clinical studies of [immune checkpoint inhibitor] monotherapy is limited, this analysis of a large population of patients across a broad scope of tumor types suggests that avelumab was associated with an incidence of irAEs that is consistent with that of other ICIs,” they wrote in Cancer.
Adverse events common with other immune checkpoint inhibitors include low-grade fatigue, pruritus, and rash, as well as serious irAEs, including high-grade pneumonitis and autoimmune-like side effects, the authors noted.
To characterize the adverse event profile of avelumab, they reviewed safety data on 1,650 patients enrolled in the solid tumor trial and 88 enrolled in the Merkel cell carcinoma trial, which included all patients in the trial who had received at least one dose of avelumab monotherapy by the cutoff date.
At the time of the analysis, 287 patients (16.5%) were continuing treatment, and 1,451 had discontinued therapy, largely because of disease progression.
Nearly all patients – 1,697 (97.6%) – had at least one adverse event of any grade or cause.
Four patients died from what investigators determined were TRAEs, including autoimmune hepatitis with peritoneal metastases and ascites in a patient with gastric cancer, liver metastases and acute liver failure in a patient with metastatic breast cancer, respiratory distress in a patient with breast cancer and multiple comorbidities, and treatment-related pneumonitis with ongoing Clostridium difficile colitis and diverticulitis not related to study treatment in a patient with urothelial carcinoma.
An additional 59 patients (3.4%) died from adverse events not deemed to be treatment-related, and 104 patient (6%) died from unknown or undocumented causes.
Any grade of irAE occurred in 247 patients (14.2%) and were grade 3 or greater in 39 (2.2%). Management of irAEs included systemic corticosteroids and nonsteroidal immunosuppressants.
In all, 439 patients (25.3%) had infusion-related reactions, which were treated generally with systemic corticosteroid. The protocol of the solid tumor trial was amended later to include diphenhydramine and acetaminophen before the first avelumab infusion as prophylaxis.
The study was sponsored by Merck and part of an alliance between Merck and Pfizer. Dr. Kelly reported no conflicts of interest. Multiple coauthors reported research funding, consulting fees, honoraria, or other consideration from various companies, and several coauthors are Merck employees.
SOURCE: Kelly K et al. Cancer. 2018 Feb 22. doi: 10.1002/cncr.31293.
FROM CANCER
Key clinical point: The immune checkpoint inhibitor avelumab appears to be well tolerated with a manageable safety profile.
Major finding: In all, 67% of patients had a treatment-related adverse event, and 10.2% had ones that were grade 3 or greater.
Study details: Safety analysis of pooled data on 1,738 patients treated with avelumab in a phase 1 and a phase 2 clinical trial.
Disclosures: The study was sponsored by Merck and part of an alliance between Merck and Pfizer. Dr. Kelly reported no conflicts of interest. Multiple coauthors reported research funding, consulting fees, honoraria, or other consideration from various companies, and several coauthors are Merck employees.
Source: Kelly K et al. Cancer. 2018 Feb 22. doi: 10.1002/cncr.31293.
Immunotherapy regimen influences inflammatory arthritis presentation
Variations in the clinical presentation of immunotherapy-induced inflammatory arthritis is partly explained by which treatment regimen was used to treat the cancer, a single-center study suggests.
While immune checkpoint inhibitors (ICI) have revolutionized the field of oncology, their use for an ever-widening range of indications had created an increasing population of patients referred to rheumatologists for the management of immune-related adverse events (IrAEs), according to Laura C. Cappelli, MD, and her colleagues at John Hopkins University, Baltimore.
Well-established guidelines exist for managing adverse events such as colitis and pneumonitis, but there are only preliminary guidelines for evaluating and treating immunotherapy-induced inflammatory arthritis (IA). “This may stem from a lack of consistent reporting of rheumatologic IrAEs in clinical trials, the non–life threatening nature of [inflammatory arthritis], or lack of recognition of musculoskeletal symptoms by treating providers,” they wrote in Seminars in Arthritis and Rheumatism.
Clinical trials have reported ranges of arthralgia in 1%-43% of patients treated with ICIs, but no accurate estimate of the incidence of IA exists.
The researchers noted that treating patients with ICI-induced IA is complicated by a history of active or recently treated cancer and concerns over using immunosuppression in the context of ICI therapy.
They set out to evaluate the clinical presentations of 30 patients seen in their clinic with ICI-induced IA. Patients were a median of 59 years old and 12 (40%) were female. Tumor types included metastatic melanoma, non–small cell lung cancer, small cell lung cancer, colorectal cancer, Hodgkin lymphoma, cutaneous lymphoma, renal cell carcinoma, duodenal carcinoma, Merkel cell carcinoma, cutaneous basal cell carcinoma, and cutaneous squamous cell carcinoma.
Sixteen patients were treated with anti–programmed cell death protein 1 (PD-1)/programmed death ligand 1 monotherapy, and 14 were treated with combination anti–CTLA-4/PD-1 therapy.
Patients on combination therapy were significantly younger (7.5 years, P = 0.01) and were more likely to have metastatic melanoma as their underlying cancer.
Patients who received combination therapy were more likely to present first with knee IA (n = 10) and none had small joint involvement. In contrast, initial small joint involvement was more common in the monotherapy group (n = 6).
C-reactive protein levels were significantly higher in the combination therapy group (4mg/dL vs. 0.5mg/dL, P = 0.03). Only monotherapy patients were positive for anti–citrullinated peptide antibodies, rheumatoid factor, or antinuclear antibodies.
Most of the patients in the study had an additional IrAE, with colitis being the most common (n=10), followed by thyroid disease, pneumonitis, and rash. Patients on PD-1 or programmed death ligand 1 monotherapy were more likely to have IA as their first IrAE.
The research team noted that the median time to symptom onset was 5 months after ICI initiation.
Diagnosis of IA following patient-reported symptoms was an average of 5.2 months, with a significant difference in lag time to diagnosis depending on initial joint presentation. For example, patients with initial small joint involvement had a 10 month longer lag time to IA diagnosis than those with knees as the initial joint involved.
In terms of treatment, 24 patients were treated with systemic corticosteroids and 10 required additional immunosuppression. The need for corticosteroids did not differ by ICI treatment regimen, but those treated with combination therapy were more likely to require additional immunosuppression (P = 0.02).
Tumor necrosis factor inhibitors with or without methotrexate were prescribed for seven patients. All of the patients had a clinical improvement in their arthritis symptoms. Four had a complete tumor response at the time of tumor necrosis factor inhibitor initiation with none having tumor progression.
The three patients treated with methotrexate monotherapy had a complete or sustained partial tumor response to ICI therapy and their cancer did not develop during IA management follow-up.
The authors went on to look at the persistence of IA after cessation of therapy in a subset of 21 patients. They found that 18 of these patients still had IA symptoms months after stopping treatment. They suggested that the delay in diagnosis and treatment seen in their study might explain the finding.
The study provides “critical information, not just for rheumatologists as they try to recognize subgroups in ICI-induced IA and diagnose patients with this new entity, but also for oncology providers who are usually first to encounter patients with ICI-induced IA and subsequently refer patients to rheumatology,” Dr. Cappelli and colleagues wrote.
The experience so far with using immunosuppression in ICI-induced IA “has been reassuring in terms of cancer outcomes, but more studies are needed to confirm this finding,” they concluded.
SOURCE: Cappelli LC et al. Semin Arthritis Rheum. doi: 10.1016/j.semarthrit. 2018.02.011.
Variations in the clinical presentation of immunotherapy-induced inflammatory arthritis is partly explained by which treatment regimen was used to treat the cancer, a single-center study suggests.
While immune checkpoint inhibitors (ICI) have revolutionized the field of oncology, their use for an ever-widening range of indications had created an increasing population of patients referred to rheumatologists for the management of immune-related adverse events (IrAEs), according to Laura C. Cappelli, MD, and her colleagues at John Hopkins University, Baltimore.
Well-established guidelines exist for managing adverse events such as colitis and pneumonitis, but there are only preliminary guidelines for evaluating and treating immunotherapy-induced inflammatory arthritis (IA). “This may stem from a lack of consistent reporting of rheumatologic IrAEs in clinical trials, the non–life threatening nature of [inflammatory arthritis], or lack of recognition of musculoskeletal symptoms by treating providers,” they wrote in Seminars in Arthritis and Rheumatism.
Clinical trials have reported ranges of arthralgia in 1%-43% of patients treated with ICIs, but no accurate estimate of the incidence of IA exists.
The researchers noted that treating patients with ICI-induced IA is complicated by a history of active or recently treated cancer and concerns over using immunosuppression in the context of ICI therapy.
They set out to evaluate the clinical presentations of 30 patients seen in their clinic with ICI-induced IA. Patients were a median of 59 years old and 12 (40%) were female. Tumor types included metastatic melanoma, non–small cell lung cancer, small cell lung cancer, colorectal cancer, Hodgkin lymphoma, cutaneous lymphoma, renal cell carcinoma, duodenal carcinoma, Merkel cell carcinoma, cutaneous basal cell carcinoma, and cutaneous squamous cell carcinoma.
Sixteen patients were treated with anti–programmed cell death protein 1 (PD-1)/programmed death ligand 1 monotherapy, and 14 were treated with combination anti–CTLA-4/PD-1 therapy.
Patients on combination therapy were significantly younger (7.5 years, P = 0.01) and were more likely to have metastatic melanoma as their underlying cancer.
Patients who received combination therapy were more likely to present first with knee IA (n = 10) and none had small joint involvement. In contrast, initial small joint involvement was more common in the monotherapy group (n = 6).
C-reactive protein levels were significantly higher in the combination therapy group (4mg/dL vs. 0.5mg/dL, P = 0.03). Only monotherapy patients were positive for anti–citrullinated peptide antibodies, rheumatoid factor, or antinuclear antibodies.
Most of the patients in the study had an additional IrAE, with colitis being the most common (n=10), followed by thyroid disease, pneumonitis, and rash. Patients on PD-1 or programmed death ligand 1 monotherapy were more likely to have IA as their first IrAE.
The research team noted that the median time to symptom onset was 5 months after ICI initiation.
Diagnosis of IA following patient-reported symptoms was an average of 5.2 months, with a significant difference in lag time to diagnosis depending on initial joint presentation. For example, patients with initial small joint involvement had a 10 month longer lag time to IA diagnosis than those with knees as the initial joint involved.
In terms of treatment, 24 patients were treated with systemic corticosteroids and 10 required additional immunosuppression. The need for corticosteroids did not differ by ICI treatment regimen, but those treated with combination therapy were more likely to require additional immunosuppression (P = 0.02).
Tumor necrosis factor inhibitors with or without methotrexate were prescribed for seven patients. All of the patients had a clinical improvement in their arthritis symptoms. Four had a complete tumor response at the time of tumor necrosis factor inhibitor initiation with none having tumor progression.
The three patients treated with methotrexate monotherapy had a complete or sustained partial tumor response to ICI therapy and their cancer did not develop during IA management follow-up.
The authors went on to look at the persistence of IA after cessation of therapy in a subset of 21 patients. They found that 18 of these patients still had IA symptoms months after stopping treatment. They suggested that the delay in diagnosis and treatment seen in their study might explain the finding.
The study provides “critical information, not just for rheumatologists as they try to recognize subgroups in ICI-induced IA and diagnose patients with this new entity, but also for oncology providers who are usually first to encounter patients with ICI-induced IA and subsequently refer patients to rheumatology,” Dr. Cappelli and colleagues wrote.
The experience so far with using immunosuppression in ICI-induced IA “has been reassuring in terms of cancer outcomes, but more studies are needed to confirm this finding,” they concluded.
SOURCE: Cappelli LC et al. Semin Arthritis Rheum. doi: 10.1016/j.semarthrit. 2018.02.011.
Variations in the clinical presentation of immunotherapy-induced inflammatory arthritis is partly explained by which treatment regimen was used to treat the cancer, a single-center study suggests.
While immune checkpoint inhibitors (ICI) have revolutionized the field of oncology, their use for an ever-widening range of indications had created an increasing population of patients referred to rheumatologists for the management of immune-related adverse events (IrAEs), according to Laura C. Cappelli, MD, and her colleagues at John Hopkins University, Baltimore.
Well-established guidelines exist for managing adverse events such as colitis and pneumonitis, but there are only preliminary guidelines for evaluating and treating immunotherapy-induced inflammatory arthritis (IA). “This may stem from a lack of consistent reporting of rheumatologic IrAEs in clinical trials, the non–life threatening nature of [inflammatory arthritis], or lack of recognition of musculoskeletal symptoms by treating providers,” they wrote in Seminars in Arthritis and Rheumatism.
Clinical trials have reported ranges of arthralgia in 1%-43% of patients treated with ICIs, but no accurate estimate of the incidence of IA exists.
The researchers noted that treating patients with ICI-induced IA is complicated by a history of active or recently treated cancer and concerns over using immunosuppression in the context of ICI therapy.
They set out to evaluate the clinical presentations of 30 patients seen in their clinic with ICI-induced IA. Patients were a median of 59 years old and 12 (40%) were female. Tumor types included metastatic melanoma, non–small cell lung cancer, small cell lung cancer, colorectal cancer, Hodgkin lymphoma, cutaneous lymphoma, renal cell carcinoma, duodenal carcinoma, Merkel cell carcinoma, cutaneous basal cell carcinoma, and cutaneous squamous cell carcinoma.
Sixteen patients were treated with anti–programmed cell death protein 1 (PD-1)/programmed death ligand 1 monotherapy, and 14 were treated with combination anti–CTLA-4/PD-1 therapy.
Patients on combination therapy were significantly younger (7.5 years, P = 0.01) and were more likely to have metastatic melanoma as their underlying cancer.
Patients who received combination therapy were more likely to present first with knee IA (n = 10) and none had small joint involvement. In contrast, initial small joint involvement was more common in the monotherapy group (n = 6).
C-reactive protein levels were significantly higher in the combination therapy group (4mg/dL vs. 0.5mg/dL, P = 0.03). Only monotherapy patients were positive for anti–citrullinated peptide antibodies, rheumatoid factor, or antinuclear antibodies.
Most of the patients in the study had an additional IrAE, with colitis being the most common (n=10), followed by thyroid disease, pneumonitis, and rash. Patients on PD-1 or programmed death ligand 1 monotherapy were more likely to have IA as their first IrAE.
The research team noted that the median time to symptom onset was 5 months after ICI initiation.
Diagnosis of IA following patient-reported symptoms was an average of 5.2 months, with a significant difference in lag time to diagnosis depending on initial joint presentation. For example, patients with initial small joint involvement had a 10 month longer lag time to IA diagnosis than those with knees as the initial joint involved.
In terms of treatment, 24 patients were treated with systemic corticosteroids and 10 required additional immunosuppression. The need for corticosteroids did not differ by ICI treatment regimen, but those treated with combination therapy were more likely to require additional immunosuppression (P = 0.02).
Tumor necrosis factor inhibitors with or without methotrexate were prescribed for seven patients. All of the patients had a clinical improvement in their arthritis symptoms. Four had a complete tumor response at the time of tumor necrosis factor inhibitor initiation with none having tumor progression.
The three patients treated with methotrexate monotherapy had a complete or sustained partial tumor response to ICI therapy and their cancer did not develop during IA management follow-up.
The authors went on to look at the persistence of IA after cessation of therapy in a subset of 21 patients. They found that 18 of these patients still had IA symptoms months after stopping treatment. They suggested that the delay in diagnosis and treatment seen in their study might explain the finding.
The study provides “critical information, not just for rheumatologists as they try to recognize subgroups in ICI-induced IA and diagnose patients with this new entity, but also for oncology providers who are usually first to encounter patients with ICI-induced IA and subsequently refer patients to rheumatology,” Dr. Cappelli and colleagues wrote.
The experience so far with using immunosuppression in ICI-induced IA “has been reassuring in terms of cancer outcomes, but more studies are needed to confirm this finding,” they concluded.
SOURCE: Cappelli LC et al. Semin Arthritis Rheum. doi: 10.1016/j.semarthrit. 2018.02.011.
FROM SEMINARS IN ARTHRITIS AND RHEUMATISM
Key clinical point: The clinical features of patients with immunotherapy-induced inflammatory arthritis differ according to the treatment regimen used.
Major findings: Combination immune checkpoint inhibitor therapy was associated with higher C-reactive protein levels and a higher likelihood of having a large joint affected first.
Study details: A single-center, retrospective cohort study of 30 patients with rheumatologist-confirmed inflammatory arthritis after receiving immune checkpoint inhibitor therapy.
Disclosures: The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Disease and the Jerome L. Greene Foundation.
Source: Cappelli LC et al. Semin Arthritis Rheum. doi: 10.1016/j.semarthrit. 2018.02.011.
The Top 5 Habits/Tips of a Highly Successful Emergency Physician
Define It, Engage, Listen, Organize, and Closure. There, You Have It!
When I was asked to give this lecture at the 2017 American College of Emergency Physicians’ (ACEP) Scientific Assembly, I literally snorted and looked over my shoulder. What? Moi give this lecture? Am I a successful emergency physician (EP)? Well, of course I am as the first emergency medicine (EM) residency-trained female professor at the University of Colorado, chair of the meetings subcommittee for the ACEP Educational Committee, and director on the American Board of Emergency Medicine.
What I have learned is that the first step to being successful is to define your personal barriers and self-defeating behaviors, and to identify and define your personal self-defeating behaviors—eg, perfectionism, procrastination, self-doubt?
My own personal self-defeating behavior is most certainly “imposter syndrome,” which is very common among professionals. I first heard about imposter syndrome on my very first day of medical school, and this behavior still follows me today. I have written some short blurbs on this topic because I want others to know they are not alone. Highly successful people have this syndrome, and it can be very debilitating. What is imposter syndrome? According to Sandberg, “Despite being high achievers, even experts in their fields, women can’t seem to shake the sense that it is only a matter of time until they are found out for who they really are- impostors with limited skills or abilities.”1 Valerie Young, an internationally recognized expert on the subject, categorized imposter syndrome into five subgroups or habits: (1) the perfectionist; (2) the superwoman/man; (3) the natural genius; (4) the rugged individualist; and (5) the expert. In her book, The Secret Thoughts of Successful Women: Why Capable People Suffer From the Imposter Syndrome and How to Thrive in Spite of It, Young builds on decades of research studying fraudulent feelings among high achievers.2
Identify Your Self-Defeating Behaviors and Write Them Down
Regarding the top five subgroups/habits proposed by Young unfortunately, there is no evidence-based literature on imposter syndrome. Most information consists of anecdotal reports from highly successful people. When you read about successful habits from such individuals, they include such things as: efficiency; bring your A-game; embrace communication; personal wellness; and most importantly, growth mentality.3 You have most likely heard phrases such as, “Don’t touch a piece of paper more than once.” The “touch it once” philosophy maybe is efficient, but I’m not sure about it being a successful habit.4 The following is my list of the top five principles that I have used to guide my career.
Define
What is success to you? Are you thinking about your whole career, or just a successful shift or a successful triathlon? Do you want to win the triathlon or just finish it? Or, do you want to be a department chair? Define and set your goal(s), and make sure to reach and stretch yourself to the best of your abilities to attain your goal. To achieve your goal, you must force yourself out of your comfort zone. If you do not reach for something, chances are it is not going to drop in your lap.
When I attended my very first ACEP Scientific Assembly as a newly minted EM residency-trained EP, I thought the lectures were a bit too basic and needed to be at a higher level of knowledge. I decided I really wanted to be a part of that process. I defined my personal challenge as improving the ACEP educational content level, and I set my goal as getting on that committee. Your goal may be quite different—eg, maybe you wish to become the medical director of an ED, a residency program director, or an officer on your hospital’s medical staff. Regardless of your goal, the first step is to decide and define what it is that you desire.
Engage
After you have defined and set your goal, the first steps to attaining it are to get started on the road you’ve chosen by showing up at relevant meetings, events; being present, engaging, and demonstrating curiosity. Maybe you will have an interesting journey!
I can’t stress enough how important it is to just show up. Sometimes, you will find that you start in one direction and get pushed in another. One of the first steps I took to getting on the ACEP education committee was to ask other ACEP members and colleagues how to do so. Most told me that the education committee was a very highly regarded one and that perhaps I should start by getting on any ACEP committee—or even better, start with a section. A respected friend in the “know” suggested that I choose an ACEP committee/section of which I had high interest, and to just show up to one of the meetings. I have found this advice to be true for most of life, whether it’s your hospital medical staff, local medical society, or state specialty society, or another professional organization—just show up.
Listen
When you do show up and attend a meeting or event, sit and listen to what others have to say, and when a task comes up with which you think you could be of assistance, step up and volunteer to help. When you are involved in a project or task, or are just listening, always keep an open mind—maybe your agenda is not exactly the same as other members of the organization/committee, but you will learn and gain important experience by being open to the thoughts and opinions of others.
When you step up and offer your assistance, you should make sure you volunteer for something that interests you. In general, to do a good job, the subject matter needs to be of interest to you, and the greater the interest, the more likely you are to be successful at completing the task. It also helps to make sure what you volunteer to do is attainable and realistic.
Organize/Action
After you’ve volunteered and committed yourself to a project, always be a productive member of the group. Do what you say you are going to do, and do it on time. These two simple things, completing your assignment/fulfilling your commitment and doing so on time, will set you apart from the pack. Do not be surprised when the reward for such an accomplishment is a request for you to do more, or take on leadership responsibilities.
Regarding my own personal journey, after I found out who served on the education committee. I started to set down some of the groundwork of networking, showing interest in the committee, and letting committee members know that I was very interested in their group and capable of helping in attaining their goals. Five years after taking these first steps to become involved in the group, I was appointed to serve on the meeting subcommittee of the ACEP education committee. This is the group that sets the curriculum and speakers for the Annual ACEP Scientific Assembly. I had made it! Then, after 8 years on the committee, I was appointed chair and worked hard to bring the meeting to Denver, Colorado, my home town. I pushed hard to reduce the length of many of the 50-minute lectures to 25 minutes, and also added some “rapid-fire” lectures to the curriculum.
Failures
On the path to attaining your goals, you will often encounter failure. It is important to keep in mind that if you never fail, then you probably are not reaching high or far enough. For example, I once wanted my institution to be more integrated at the affiliated University’s campus. I had defined this as my goal. To reach it, when the annual election for the medical school faculty senate came along, I had as many of my faculty colleagues vote for me as secretary, the lowest faculty position available. To my shock, I got elected! The problem was, as the secretary, I was supposed to be present at all of the monthly meetings and actually take notes. Not only did I not know who any of the individuals speaking at these meetings were, but I could only make approximately 50% of the meetings due to scheduling conflicts and other commitments. It is my own shame for not doing my homework and learning the roles and responsibilities of the secretarial position. I had the definition of success as a vague one: I engaged but did not really have an attainable goal. After 3 months, I had to go to the dean and admit I had made a mistake and was not capable of performing the duty of secretary. Although, the dean understood and thanked me for my honesty, this was a humbling experience for me and one that also reflected poorly on my department.
However, we are all human and we do make mistakes. By acknowledging our mistakes and shortcomings, reflecting on why they happened, and learning how to handle and do things differently in the future is all part of the journey to success.
Closure
Did I find all of the time and work I put in over the years to be where I am now worth it to me personally? Was I successful? Yes on both counts! It was one long journey. In addition to the long-term journey, I also choose short ones. For example, I want a successful shift, which I now define as sitting down at least 50% of the time when taking a patient’s history. I also want to be engaged with my patients. Remember, the key to being a successful EP is to set goals, whether they are long-term, short-term, major, or minor. So, reach, define, engage, listen, organize, and attain closure. Expect and be ready for some failures—these are steps on the path to success.
1. Sandberg S. Lean In: Women, Work, and the Will to Lead. New York, NY: Alfred A Knopf; 2013.
2. Young V. The Secret Thoughts of Successful Women: Why Capable People Suffer from the Impostor Syndrome and How to Thrive in Spite of It. 1st ed. New York, NY: Crown Business; 2011.
3. Silverman M. Step it up: 5 habits of successful EPs. Emergency Physicians Monthly Web site. http://epmonthly.com/article/step-it-up-5-habits-of-successful-eps/. Published December 31, 2014. Accessed January 3, 2018.
4. Sexton Z. The “touch it once” principle that will skyrocket your personal efficiency. Asian Efficiency Web site. http://www.asianefficiency.com/mindsets/touch-it-once-productivity-principle/. Accessed February 18, 2018.
Define It, Engage, Listen, Organize, and Closure. There, You Have It!
When I was asked to give this lecture at the 2017 American College of Emergency Physicians’ (ACEP) Scientific Assembly, I literally snorted and looked over my shoulder. What? Moi give this lecture? Am I a successful emergency physician (EP)? Well, of course I am as the first emergency medicine (EM) residency-trained female professor at the University of Colorado, chair of the meetings subcommittee for the ACEP Educational Committee, and director on the American Board of Emergency Medicine.
What I have learned is that the first step to being successful is to define your personal barriers and self-defeating behaviors, and to identify and define your personal self-defeating behaviors—eg, perfectionism, procrastination, self-doubt?
My own personal self-defeating behavior is most certainly “imposter syndrome,” which is very common among professionals. I first heard about imposter syndrome on my very first day of medical school, and this behavior still follows me today. I have written some short blurbs on this topic because I want others to know they are not alone. Highly successful people have this syndrome, and it can be very debilitating. What is imposter syndrome? According to Sandberg, “Despite being high achievers, even experts in their fields, women can’t seem to shake the sense that it is only a matter of time until they are found out for who they really are- impostors with limited skills or abilities.”1 Valerie Young, an internationally recognized expert on the subject, categorized imposter syndrome into five subgroups or habits: (1) the perfectionist; (2) the superwoman/man; (3) the natural genius; (4) the rugged individualist; and (5) the expert. In her book, The Secret Thoughts of Successful Women: Why Capable People Suffer From the Imposter Syndrome and How to Thrive in Spite of It, Young builds on decades of research studying fraudulent feelings among high achievers.2
Identify Your Self-Defeating Behaviors and Write Them Down
Regarding the top five subgroups/habits proposed by Young unfortunately, there is no evidence-based literature on imposter syndrome. Most information consists of anecdotal reports from highly successful people. When you read about successful habits from such individuals, they include such things as: efficiency; bring your A-game; embrace communication; personal wellness; and most importantly, growth mentality.3 You have most likely heard phrases such as, “Don’t touch a piece of paper more than once.” The “touch it once” philosophy maybe is efficient, but I’m not sure about it being a successful habit.4 The following is my list of the top five principles that I have used to guide my career.
Define
What is success to you? Are you thinking about your whole career, or just a successful shift or a successful triathlon? Do you want to win the triathlon or just finish it? Or, do you want to be a department chair? Define and set your goal(s), and make sure to reach and stretch yourself to the best of your abilities to attain your goal. To achieve your goal, you must force yourself out of your comfort zone. If you do not reach for something, chances are it is not going to drop in your lap.
When I attended my very first ACEP Scientific Assembly as a newly minted EM residency-trained EP, I thought the lectures were a bit too basic and needed to be at a higher level of knowledge. I decided I really wanted to be a part of that process. I defined my personal challenge as improving the ACEP educational content level, and I set my goal as getting on that committee. Your goal may be quite different—eg, maybe you wish to become the medical director of an ED, a residency program director, or an officer on your hospital’s medical staff. Regardless of your goal, the first step is to decide and define what it is that you desire.
Engage
After you have defined and set your goal, the first steps to attaining it are to get started on the road you’ve chosen by showing up at relevant meetings, events; being present, engaging, and demonstrating curiosity. Maybe you will have an interesting journey!
I can’t stress enough how important it is to just show up. Sometimes, you will find that you start in one direction and get pushed in another. One of the first steps I took to getting on the ACEP education committee was to ask other ACEP members and colleagues how to do so. Most told me that the education committee was a very highly regarded one and that perhaps I should start by getting on any ACEP committee—or even better, start with a section. A respected friend in the “know” suggested that I choose an ACEP committee/section of which I had high interest, and to just show up to one of the meetings. I have found this advice to be true for most of life, whether it’s your hospital medical staff, local medical society, or state specialty society, or another professional organization—just show up.
Listen
When you do show up and attend a meeting or event, sit and listen to what others have to say, and when a task comes up with which you think you could be of assistance, step up and volunteer to help. When you are involved in a project or task, or are just listening, always keep an open mind—maybe your agenda is not exactly the same as other members of the organization/committee, but you will learn and gain important experience by being open to the thoughts and opinions of others.
When you step up and offer your assistance, you should make sure you volunteer for something that interests you. In general, to do a good job, the subject matter needs to be of interest to you, and the greater the interest, the more likely you are to be successful at completing the task. It also helps to make sure what you volunteer to do is attainable and realistic.
Organize/Action
After you’ve volunteered and committed yourself to a project, always be a productive member of the group. Do what you say you are going to do, and do it on time. These two simple things, completing your assignment/fulfilling your commitment and doing so on time, will set you apart from the pack. Do not be surprised when the reward for such an accomplishment is a request for you to do more, or take on leadership responsibilities.
Regarding my own personal journey, after I found out who served on the education committee. I started to set down some of the groundwork of networking, showing interest in the committee, and letting committee members know that I was very interested in their group and capable of helping in attaining their goals. Five years after taking these first steps to become involved in the group, I was appointed to serve on the meeting subcommittee of the ACEP education committee. This is the group that sets the curriculum and speakers for the Annual ACEP Scientific Assembly. I had made it! Then, after 8 years on the committee, I was appointed chair and worked hard to bring the meeting to Denver, Colorado, my home town. I pushed hard to reduce the length of many of the 50-minute lectures to 25 minutes, and also added some “rapid-fire” lectures to the curriculum.
Failures
On the path to attaining your goals, you will often encounter failure. It is important to keep in mind that if you never fail, then you probably are not reaching high or far enough. For example, I once wanted my institution to be more integrated at the affiliated University’s campus. I had defined this as my goal. To reach it, when the annual election for the medical school faculty senate came along, I had as many of my faculty colleagues vote for me as secretary, the lowest faculty position available. To my shock, I got elected! The problem was, as the secretary, I was supposed to be present at all of the monthly meetings and actually take notes. Not only did I not know who any of the individuals speaking at these meetings were, but I could only make approximately 50% of the meetings due to scheduling conflicts and other commitments. It is my own shame for not doing my homework and learning the roles and responsibilities of the secretarial position. I had the definition of success as a vague one: I engaged but did not really have an attainable goal. After 3 months, I had to go to the dean and admit I had made a mistake and was not capable of performing the duty of secretary. Although, the dean understood and thanked me for my honesty, this was a humbling experience for me and one that also reflected poorly on my department.
However, we are all human and we do make mistakes. By acknowledging our mistakes and shortcomings, reflecting on why they happened, and learning how to handle and do things differently in the future is all part of the journey to success.
Closure
Did I find all of the time and work I put in over the years to be where I am now worth it to me personally? Was I successful? Yes on both counts! It was one long journey. In addition to the long-term journey, I also choose short ones. For example, I want a successful shift, which I now define as sitting down at least 50% of the time when taking a patient’s history. I also want to be engaged with my patients. Remember, the key to being a successful EP is to set goals, whether they are long-term, short-term, major, or minor. So, reach, define, engage, listen, organize, and attain closure. Expect and be ready for some failures—these are steps on the path to success.
Define It, Engage, Listen, Organize, and Closure. There, You Have It!
When I was asked to give this lecture at the 2017 American College of Emergency Physicians’ (ACEP) Scientific Assembly, I literally snorted and looked over my shoulder. What? Moi give this lecture? Am I a successful emergency physician (EP)? Well, of course I am as the first emergency medicine (EM) residency-trained female professor at the University of Colorado, chair of the meetings subcommittee for the ACEP Educational Committee, and director on the American Board of Emergency Medicine.
What I have learned is that the first step to being successful is to define your personal barriers and self-defeating behaviors, and to identify and define your personal self-defeating behaviors—eg, perfectionism, procrastination, self-doubt?
My own personal self-defeating behavior is most certainly “imposter syndrome,” which is very common among professionals. I first heard about imposter syndrome on my very first day of medical school, and this behavior still follows me today. I have written some short blurbs on this topic because I want others to know they are not alone. Highly successful people have this syndrome, and it can be very debilitating. What is imposter syndrome? According to Sandberg, “Despite being high achievers, even experts in their fields, women can’t seem to shake the sense that it is only a matter of time until they are found out for who they really are- impostors with limited skills or abilities.”1 Valerie Young, an internationally recognized expert on the subject, categorized imposter syndrome into five subgroups or habits: (1) the perfectionist; (2) the superwoman/man; (3) the natural genius; (4) the rugged individualist; and (5) the expert. In her book, The Secret Thoughts of Successful Women: Why Capable People Suffer From the Imposter Syndrome and How to Thrive in Spite of It, Young builds on decades of research studying fraudulent feelings among high achievers.2
Identify Your Self-Defeating Behaviors and Write Them Down
Regarding the top five subgroups/habits proposed by Young unfortunately, there is no evidence-based literature on imposter syndrome. Most information consists of anecdotal reports from highly successful people. When you read about successful habits from such individuals, they include such things as: efficiency; bring your A-game; embrace communication; personal wellness; and most importantly, growth mentality.3 You have most likely heard phrases such as, “Don’t touch a piece of paper more than once.” The “touch it once” philosophy maybe is efficient, but I’m not sure about it being a successful habit.4 The following is my list of the top five principles that I have used to guide my career.
Define
What is success to you? Are you thinking about your whole career, or just a successful shift or a successful triathlon? Do you want to win the triathlon or just finish it? Or, do you want to be a department chair? Define and set your goal(s), and make sure to reach and stretch yourself to the best of your abilities to attain your goal. To achieve your goal, you must force yourself out of your comfort zone. If you do not reach for something, chances are it is not going to drop in your lap.
When I attended my very first ACEP Scientific Assembly as a newly minted EM residency-trained EP, I thought the lectures were a bit too basic and needed to be at a higher level of knowledge. I decided I really wanted to be a part of that process. I defined my personal challenge as improving the ACEP educational content level, and I set my goal as getting on that committee. Your goal may be quite different—eg, maybe you wish to become the medical director of an ED, a residency program director, or an officer on your hospital’s medical staff. Regardless of your goal, the first step is to decide and define what it is that you desire.
Engage
After you have defined and set your goal, the first steps to attaining it are to get started on the road you’ve chosen by showing up at relevant meetings, events; being present, engaging, and demonstrating curiosity. Maybe you will have an interesting journey!
I can’t stress enough how important it is to just show up. Sometimes, you will find that you start in one direction and get pushed in another. One of the first steps I took to getting on the ACEP education committee was to ask other ACEP members and colleagues how to do so. Most told me that the education committee was a very highly regarded one and that perhaps I should start by getting on any ACEP committee—or even better, start with a section. A respected friend in the “know” suggested that I choose an ACEP committee/section of which I had high interest, and to just show up to one of the meetings. I have found this advice to be true for most of life, whether it’s your hospital medical staff, local medical society, or state specialty society, or another professional organization—just show up.
Listen
When you do show up and attend a meeting or event, sit and listen to what others have to say, and when a task comes up with which you think you could be of assistance, step up and volunteer to help. When you are involved in a project or task, or are just listening, always keep an open mind—maybe your agenda is not exactly the same as other members of the organization/committee, but you will learn and gain important experience by being open to the thoughts and opinions of others.
When you step up and offer your assistance, you should make sure you volunteer for something that interests you. In general, to do a good job, the subject matter needs to be of interest to you, and the greater the interest, the more likely you are to be successful at completing the task. It also helps to make sure what you volunteer to do is attainable and realistic.
Organize/Action
After you’ve volunteered and committed yourself to a project, always be a productive member of the group. Do what you say you are going to do, and do it on time. These two simple things, completing your assignment/fulfilling your commitment and doing so on time, will set you apart from the pack. Do not be surprised when the reward for such an accomplishment is a request for you to do more, or take on leadership responsibilities.
Regarding my own personal journey, after I found out who served on the education committee. I started to set down some of the groundwork of networking, showing interest in the committee, and letting committee members know that I was very interested in their group and capable of helping in attaining their goals. Five years after taking these first steps to become involved in the group, I was appointed to serve on the meeting subcommittee of the ACEP education committee. This is the group that sets the curriculum and speakers for the Annual ACEP Scientific Assembly. I had made it! Then, after 8 years on the committee, I was appointed chair and worked hard to bring the meeting to Denver, Colorado, my home town. I pushed hard to reduce the length of many of the 50-minute lectures to 25 minutes, and also added some “rapid-fire” lectures to the curriculum.
Failures
On the path to attaining your goals, you will often encounter failure. It is important to keep in mind that if you never fail, then you probably are not reaching high or far enough. For example, I once wanted my institution to be more integrated at the affiliated University’s campus. I had defined this as my goal. To reach it, when the annual election for the medical school faculty senate came along, I had as many of my faculty colleagues vote for me as secretary, the lowest faculty position available. To my shock, I got elected! The problem was, as the secretary, I was supposed to be present at all of the monthly meetings and actually take notes. Not only did I not know who any of the individuals speaking at these meetings were, but I could only make approximately 50% of the meetings due to scheduling conflicts and other commitments. It is my own shame for not doing my homework and learning the roles and responsibilities of the secretarial position. I had the definition of success as a vague one: I engaged but did not really have an attainable goal. After 3 months, I had to go to the dean and admit I had made a mistake and was not capable of performing the duty of secretary. Although, the dean understood and thanked me for my honesty, this was a humbling experience for me and one that also reflected poorly on my department.
However, we are all human and we do make mistakes. By acknowledging our mistakes and shortcomings, reflecting on why they happened, and learning how to handle and do things differently in the future is all part of the journey to success.
Closure
Did I find all of the time and work I put in over the years to be where I am now worth it to me personally? Was I successful? Yes on both counts! It was one long journey. In addition to the long-term journey, I also choose short ones. For example, I want a successful shift, which I now define as sitting down at least 50% of the time when taking a patient’s history. I also want to be engaged with my patients. Remember, the key to being a successful EP is to set goals, whether they are long-term, short-term, major, or minor. So, reach, define, engage, listen, organize, and attain closure. Expect and be ready for some failures—these are steps on the path to success.
1. Sandberg S. Lean In: Women, Work, and the Will to Lead. New York, NY: Alfred A Knopf; 2013.
2. Young V. The Secret Thoughts of Successful Women: Why Capable People Suffer from the Impostor Syndrome and How to Thrive in Spite of It. 1st ed. New York, NY: Crown Business; 2011.
3. Silverman M. Step it up: 5 habits of successful EPs. Emergency Physicians Monthly Web site. http://epmonthly.com/article/step-it-up-5-habits-of-successful-eps/. Published December 31, 2014. Accessed January 3, 2018.
4. Sexton Z. The “touch it once” principle that will skyrocket your personal efficiency. Asian Efficiency Web site. http://www.asianefficiency.com/mindsets/touch-it-once-productivity-principle/. Accessed February 18, 2018.
1. Sandberg S. Lean In: Women, Work, and the Will to Lead. New York, NY: Alfred A Knopf; 2013.
2. Young V. The Secret Thoughts of Successful Women: Why Capable People Suffer from the Impostor Syndrome and How to Thrive in Spite of It. 1st ed. New York, NY: Crown Business; 2011.
3. Silverman M. Step it up: 5 habits of successful EPs. Emergency Physicians Monthly Web site. http://epmonthly.com/article/step-it-up-5-habits-of-successful-eps/. Published December 31, 2014. Accessed January 3, 2018.
4. Sexton Z. The “touch it once” principle that will skyrocket your personal efficiency. Asian Efficiency Web site. http://www.asianefficiency.com/mindsets/touch-it-once-productivity-principle/. Accessed February 18, 2018.
App collects allergy symptoms in real time
ORLANDO – Use of an app combining patient-reported symptoms with local environmental triggers led patients to take action to improve their health, Penny Jones, PhD, reported at the joint congress of the American Academy of Allergy, Asthma, and Immunology and the World Asthma Organization.
AirRater is a smartphone app and data collection network that includes information on air particulates, daily pollen and fungi counts, temperature, and planned burn locations. Patients enter their respiratory symptoms, which are correlated with local environmental conditions, according to Dr. Jones, a postdoctoral fellow at the University of Tasmania (Australia) in Hobart.
Most of the environmental data are gathered from government agencies; however, researchers collect pollen and fungi counts at their own stations.
Patients do not see the environmental data until they’ve logged in their symptoms so that their reports aren’t biased by that information, Dr. Jones said, adding that the app also sends notifications when pollen and pollutant levels are high.
“It’s an environmental monitoring system coupled with a smartphone app designed to help people with allergies and asthma make better decisions around their health,” Dr. Jones said.
The AirRater network and app are now operating in both Tasmania and Canberra, Australia.
There are more than 6,000 users, and data from surveys show that it is having an effect, Dr. Jones said. About 40% of users said they have changed their behavior in some way because of information provided by the app, including staying indoors, taking preventive medication, or speaking with their doctors.“It does appear that people are generally finding it a useful tool,” she said.
In a pilot study, researchers found that several environmental triggers were significantly correlated with exacerbation of patient symptoms, including maximum temperature (P < .001), particulate pollution (P < .001), relative humidity (P = .01), birch pollen (P = .006), and cypress pollen (P = .004).
Researchers plan to expand use of the network and app to other parts of Australia and are working to refine the understanding of aerobiological symptom drivers through DNA analysis of airborne particles. Their goal is to be able to identify personalized drivers of sensitivities, she said.
“We’ll keep working on this,” Dr. Jones said. “But we think that certainly has promise.”
The investigators reported no financial conflicts of interest, and the study had no outside funding.
SOURCE: Jones P et al. AAAAI/WAO Joint Congress, Abstract 270.
ORLANDO – Use of an app combining patient-reported symptoms with local environmental triggers led patients to take action to improve their health, Penny Jones, PhD, reported at the joint congress of the American Academy of Allergy, Asthma, and Immunology and the World Asthma Organization.
AirRater is a smartphone app and data collection network that includes information on air particulates, daily pollen and fungi counts, temperature, and planned burn locations. Patients enter their respiratory symptoms, which are correlated with local environmental conditions, according to Dr. Jones, a postdoctoral fellow at the University of Tasmania (Australia) in Hobart.
Most of the environmental data are gathered from government agencies; however, researchers collect pollen and fungi counts at their own stations.
Patients do not see the environmental data until they’ve logged in their symptoms so that their reports aren’t biased by that information, Dr. Jones said, adding that the app also sends notifications when pollen and pollutant levels are high.
“It’s an environmental monitoring system coupled with a smartphone app designed to help people with allergies and asthma make better decisions around their health,” Dr. Jones said.
The AirRater network and app are now operating in both Tasmania and Canberra, Australia.
There are more than 6,000 users, and data from surveys show that it is having an effect, Dr. Jones said. About 40% of users said they have changed their behavior in some way because of information provided by the app, including staying indoors, taking preventive medication, or speaking with their doctors.“It does appear that people are generally finding it a useful tool,” she said.
In a pilot study, researchers found that several environmental triggers were significantly correlated with exacerbation of patient symptoms, including maximum temperature (P < .001), particulate pollution (P < .001), relative humidity (P = .01), birch pollen (P = .006), and cypress pollen (P = .004).
Researchers plan to expand use of the network and app to other parts of Australia and are working to refine the understanding of aerobiological symptom drivers through DNA analysis of airborne particles. Their goal is to be able to identify personalized drivers of sensitivities, she said.
“We’ll keep working on this,” Dr. Jones said. “But we think that certainly has promise.”
The investigators reported no financial conflicts of interest, and the study had no outside funding.
SOURCE: Jones P et al. AAAAI/WAO Joint Congress, Abstract 270.
ORLANDO – Use of an app combining patient-reported symptoms with local environmental triggers led patients to take action to improve their health, Penny Jones, PhD, reported at the joint congress of the American Academy of Allergy, Asthma, and Immunology and the World Asthma Organization.
AirRater is a smartphone app and data collection network that includes information on air particulates, daily pollen and fungi counts, temperature, and planned burn locations. Patients enter their respiratory symptoms, which are correlated with local environmental conditions, according to Dr. Jones, a postdoctoral fellow at the University of Tasmania (Australia) in Hobart.
Most of the environmental data are gathered from government agencies; however, researchers collect pollen and fungi counts at their own stations.
Patients do not see the environmental data until they’ve logged in their symptoms so that their reports aren’t biased by that information, Dr. Jones said, adding that the app also sends notifications when pollen and pollutant levels are high.
“It’s an environmental monitoring system coupled with a smartphone app designed to help people with allergies and asthma make better decisions around their health,” Dr. Jones said.
The AirRater network and app are now operating in both Tasmania and Canberra, Australia.
There are more than 6,000 users, and data from surveys show that it is having an effect, Dr. Jones said. About 40% of users said they have changed their behavior in some way because of information provided by the app, including staying indoors, taking preventive medication, or speaking with their doctors.“It does appear that people are generally finding it a useful tool,” she said.
In a pilot study, researchers found that several environmental triggers were significantly correlated with exacerbation of patient symptoms, including maximum temperature (P < .001), particulate pollution (P < .001), relative humidity (P = .01), birch pollen (P = .006), and cypress pollen (P = .004).
Researchers plan to expand use of the network and app to other parts of Australia and are working to refine the understanding of aerobiological symptom drivers through DNA analysis of airborne particles. Their goal is to be able to identify personalized drivers of sensitivities, she said.
“We’ll keep working on this,” Dr. Jones said. “But we think that certainly has promise.”
The investigators reported no financial conflicts of interest, and the study had no outside funding.
SOURCE: Jones P et al. AAAAI/WAO Joint Congress, Abstract 270.
REPORTING FROM THE AAAAI/WAO JOINT CONGRESS
Key clinical point: A smartphone app has been developed to collect patient allergy and asthma symptoms and fuse that information with environmental data to find correlations.
Major finding: About 40% of app users said information provided by the app prompted them to take preventive action.
Study details: A survey of 6,000 app users and a retrospective study correlating reported allergy and asthma symptoms with real-time environmental data.
Disclosures: The investigators reported no financial conflicts of interest, and the study had no outside funding.
Source: Jones P et al. AAAAI/WAO Joint Congress, Abstract 270.
AHA and ASA Publish New Ischemic Stroke Guidelines
LOS ANGELES—The American Heart Association (AHA) and the American Stroke Association (ASA) updated their guidelines for the early management of patients with acute ischemic stroke. In contrast with the previous guidelines, the new guidelines address the comprehensive management of patients when they are hospitalized, including the initiation of treatments to prevent further stroke that are usually instituted within the first two weeks, said William J. Powers, MD, Chair of Neurology at the University of North Carolina School of Medicine in Chapel Hill, and chair of the guidelines writing group. The guidelines were presented at the International Stroke Conference 2018 and published online ahead of print January 24 in Stroke.
The new guidelines supersede the 2013 guidelines and subsequent updates and were created for all healthcare providers who care for patients with acute ischemic stroke, said Dr. Powers. The new guidelines do not address children or clots in the veins, he added.
Prehospital Care
The new guidelines strongly recommend that each region in the country create systems in which patients receive emergency treatment in small hospitals and are rapidly moved to large hospitals for more comprehensive therapy. “We want the patients who do have a stroke to get to the hospital as fast as possible. This means some kind of screening in the field by emergency medical services…. They need to go to the closest hospital that can adequately evaluate them and give them IV alteplase if they are eligible for it,” said Dr. Powers.
IV and Intra-Arterial Therapies
IV alteplase remains the first-line treatment for patients with acute ischemic stroke. “Everyone who is eligible for this should get it, and this should not be delayed to determine if they are eligible for other treatment,” said Dr. Powers. The new criteria recommend IV alteplase treatment within four and a half hours of acute ischemic stroke onset for an increased number of eligible patients. New data suggest that patients with mild stroke also benefit from IV alteplase within the three-hour-to-four-and-a-half-hour treatment window.
The guidelines also cite evidence for performing a mechanical thrombectomy. The guidelines recommend using eligibility criteria derived from clinical trials to select patients. For those patients who can be treated within six hours or less, eligibility criteria are derived from five trials published in 2015. DAWN and DEFUSE 3 trial eligibility criteria are recommended to select patients for thrombectomy from six to 24 hours. DEFUSE 3 treated patients within six to 16 hours after onset, and the DAWN trial treated patients within six to 24 hours after onset.
In addition, the document’s revised blood pressure guidelines acknowledge that few data can support the choice of effective blood pressure treatment in patients with acute ischemic stroke. Understanding this limitation is important for avoiding overtreatment in patients with high blood pressure, said Dr. Powers.
The new guidelines also provide updated recommendations for deep vein thrombosis prophylaxis. Blood thinners have been advocated as the most effective way to prevent this complication, but the new recommendations state that intermittent pneumatic compression is the best preventive measure.
Diagnostic Tests
Finally, the new guidelines examined the benefits of diagnostic tests and concluded that routinely performing multiple diagnostic tests in every stroke patient is not good medical practice. Not only is this practice expensive, but there are no data to indicate that such indiscriminate testing “will improve overall patient outcome. It actually can lead to further testing and things that could adversely affect patient outcomes,” said Dr. Powers. “We made recommendations that diagnostic testing be individualized … and restricted to answering those questions that will lead to a treatment change of proven benefit.”
—Erica Tricarico
Suggested Reading
Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018 Jan 24 [Epub ahead of print].
LOS ANGELES—The American Heart Association (AHA) and the American Stroke Association (ASA) updated their guidelines for the early management of patients with acute ischemic stroke. In contrast with the previous guidelines, the new guidelines address the comprehensive management of patients when they are hospitalized, including the initiation of treatments to prevent further stroke that are usually instituted within the first two weeks, said William J. Powers, MD, Chair of Neurology at the University of North Carolina School of Medicine in Chapel Hill, and chair of the guidelines writing group. The guidelines were presented at the International Stroke Conference 2018 and published online ahead of print January 24 in Stroke.
The new guidelines supersede the 2013 guidelines and subsequent updates and were created for all healthcare providers who care for patients with acute ischemic stroke, said Dr. Powers. The new guidelines do not address children or clots in the veins, he added.
Prehospital Care
The new guidelines strongly recommend that each region in the country create systems in which patients receive emergency treatment in small hospitals and are rapidly moved to large hospitals for more comprehensive therapy. “We want the patients who do have a stroke to get to the hospital as fast as possible. This means some kind of screening in the field by emergency medical services…. They need to go to the closest hospital that can adequately evaluate them and give them IV alteplase if they are eligible for it,” said Dr. Powers.
IV and Intra-Arterial Therapies
IV alteplase remains the first-line treatment for patients with acute ischemic stroke. “Everyone who is eligible for this should get it, and this should not be delayed to determine if they are eligible for other treatment,” said Dr. Powers. The new criteria recommend IV alteplase treatment within four and a half hours of acute ischemic stroke onset for an increased number of eligible patients. New data suggest that patients with mild stroke also benefit from IV alteplase within the three-hour-to-four-and-a-half-hour treatment window.
The guidelines also cite evidence for performing a mechanical thrombectomy. The guidelines recommend using eligibility criteria derived from clinical trials to select patients. For those patients who can be treated within six hours or less, eligibility criteria are derived from five trials published in 2015. DAWN and DEFUSE 3 trial eligibility criteria are recommended to select patients for thrombectomy from six to 24 hours. DEFUSE 3 treated patients within six to 16 hours after onset, and the DAWN trial treated patients within six to 24 hours after onset.
In addition, the document’s revised blood pressure guidelines acknowledge that few data can support the choice of effective blood pressure treatment in patients with acute ischemic stroke. Understanding this limitation is important for avoiding overtreatment in patients with high blood pressure, said Dr. Powers.
The new guidelines also provide updated recommendations for deep vein thrombosis prophylaxis. Blood thinners have been advocated as the most effective way to prevent this complication, but the new recommendations state that intermittent pneumatic compression is the best preventive measure.
Diagnostic Tests
Finally, the new guidelines examined the benefits of diagnostic tests and concluded that routinely performing multiple diagnostic tests in every stroke patient is not good medical practice. Not only is this practice expensive, but there are no data to indicate that such indiscriminate testing “will improve overall patient outcome. It actually can lead to further testing and things that could adversely affect patient outcomes,” said Dr. Powers. “We made recommendations that diagnostic testing be individualized … and restricted to answering those questions that will lead to a treatment change of proven benefit.”
—Erica Tricarico
Suggested Reading
Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018 Jan 24 [Epub ahead of print].
LOS ANGELES—The American Heart Association (AHA) and the American Stroke Association (ASA) updated their guidelines for the early management of patients with acute ischemic stroke. In contrast with the previous guidelines, the new guidelines address the comprehensive management of patients when they are hospitalized, including the initiation of treatments to prevent further stroke that are usually instituted within the first two weeks, said William J. Powers, MD, Chair of Neurology at the University of North Carolina School of Medicine in Chapel Hill, and chair of the guidelines writing group. The guidelines were presented at the International Stroke Conference 2018 and published online ahead of print January 24 in Stroke.
The new guidelines supersede the 2013 guidelines and subsequent updates and were created for all healthcare providers who care for patients with acute ischemic stroke, said Dr. Powers. The new guidelines do not address children or clots in the veins, he added.
Prehospital Care
The new guidelines strongly recommend that each region in the country create systems in which patients receive emergency treatment in small hospitals and are rapidly moved to large hospitals for more comprehensive therapy. “We want the patients who do have a stroke to get to the hospital as fast as possible. This means some kind of screening in the field by emergency medical services…. They need to go to the closest hospital that can adequately evaluate them and give them IV alteplase if they are eligible for it,” said Dr. Powers.
IV and Intra-Arterial Therapies
IV alteplase remains the first-line treatment for patients with acute ischemic stroke. “Everyone who is eligible for this should get it, and this should not be delayed to determine if they are eligible for other treatment,” said Dr. Powers. The new criteria recommend IV alteplase treatment within four and a half hours of acute ischemic stroke onset for an increased number of eligible patients. New data suggest that patients with mild stroke also benefit from IV alteplase within the three-hour-to-four-and-a-half-hour treatment window.
The guidelines also cite evidence for performing a mechanical thrombectomy. The guidelines recommend using eligibility criteria derived from clinical trials to select patients. For those patients who can be treated within six hours or less, eligibility criteria are derived from five trials published in 2015. DAWN and DEFUSE 3 trial eligibility criteria are recommended to select patients for thrombectomy from six to 24 hours. DEFUSE 3 treated patients within six to 16 hours after onset, and the DAWN trial treated patients within six to 24 hours after onset.
In addition, the document’s revised blood pressure guidelines acknowledge that few data can support the choice of effective blood pressure treatment in patients with acute ischemic stroke. Understanding this limitation is important for avoiding overtreatment in patients with high blood pressure, said Dr. Powers.
The new guidelines also provide updated recommendations for deep vein thrombosis prophylaxis. Blood thinners have been advocated as the most effective way to prevent this complication, but the new recommendations state that intermittent pneumatic compression is the best preventive measure.
Diagnostic Tests
Finally, the new guidelines examined the benefits of diagnostic tests and concluded that routinely performing multiple diagnostic tests in every stroke patient is not good medical practice. Not only is this practice expensive, but there are no data to indicate that such indiscriminate testing “will improve overall patient outcome. It actually can lead to further testing and things that could adversely affect patient outcomes,” said Dr. Powers. “We made recommendations that diagnostic testing be individualized … and restricted to answering those questions that will lead to a treatment change of proven benefit.”
—Erica Tricarico
Suggested Reading
Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018 Jan 24 [Epub ahead of print].
‘Modified rush’ immunotherapy delivers good results
ORLANDO – Patients with allergic rhinitis undergoing allergen immunotherapy achieved the goal of their monthly maintenance dose at a 20% higher rate when they underwent went a “modified rush” protocol, compared with those who received the therapy with the much slower conventional approach.
The findings offer proof that the faster approach is safe, more effective, and should be more widely used even though many allergists continue to be hesitant to do so, according to Christine Schafer, MD, clinical assistant professor at Michigan State University, East Lansing and a private practice allergist in Grand Rapids, Mich.
“The beauty of it is you’ve consolidated 3 months into a day,” said Dr. Schafer, who added that patients also feel better faster. “They don’t have to have this going every week for 3 months not feeling any better.”
Dr. Schafer and her colleagues reviewed a year’s worth of results for patients undergoing conventional immunotherapy or a “modified rush” protocol in 2014. They found that the conventional approach produced a 64.5% success rate, compared with an 84.4% success rate for those doing the faster protocol (P less than .001).
Typically, allergen immunotherapy takes 6 months, with patients making weekly visits with gradually increasing doses. The weekly visits can be a burden, and many patients drop out. The modified rush protocol seems to be easier for patients to accomplish, Dr. Schafer said at the joint congress of the American Academy of Allergy, Asthma, and Immunology and the World Asthma Organization.
The study compared 231 patients on the faster protocol with 392 treated with the traditional approach.
On the “modified rush” protocol, patients aged 12-15 years took preventive medications – 20 mg of prednisone and 150 mg of ranitidine 2 days before their appointment and again the day after. In addition, they took 10 mg of cetirizine and 5 mg of montelukast 2 days before their appointment. Patients also took those same medications the morning of the treatment.
For patients 16 and older, the dose of prednisone was 30 mg and the dose of montelukast was 10 mg.
The protocol consisted of eight shots, starting at a more diluted dose than the conventional protocol, as an additional safety precaution. The shots were given first every 15 minutes, then every 30 minutes or an hour as the dose increases. Once the treatment was over, the dilution was 1:10, a milestone not reached until 3 months under the conventional approach.
An hour or two after the treatment, all patients received 20 mg of prednisone.
Patients resumed the normal conventional protocol after the first visit.
Reactions were rare under the “modified rush” protocol, with 6 patients experiencing flushing but nothing else, 21 with grade 1 reactions, 3 with grade 2, and no reactions worse than that. Five patients needed epinephrine.
Dr. Schafer said the approach may hold appeal because, even though it’s accelerated, there is a slower lead-in to the maintenance dose.
“If you ask allergists, ‘Do you rush?’ they’ll say, ‘No, no, no, I don’t rush. It’s too risky.’” she said. “Hence, modified rush.”
SOURCE: Schafer C et al. AAAAI/WAO Joint Congress abstract 520.
ORLANDO – Patients with allergic rhinitis undergoing allergen immunotherapy achieved the goal of their monthly maintenance dose at a 20% higher rate when they underwent went a “modified rush” protocol, compared with those who received the therapy with the much slower conventional approach.
The findings offer proof that the faster approach is safe, more effective, and should be more widely used even though many allergists continue to be hesitant to do so, according to Christine Schafer, MD, clinical assistant professor at Michigan State University, East Lansing and a private practice allergist in Grand Rapids, Mich.
“The beauty of it is you’ve consolidated 3 months into a day,” said Dr. Schafer, who added that patients also feel better faster. “They don’t have to have this going every week for 3 months not feeling any better.”
Dr. Schafer and her colleagues reviewed a year’s worth of results for patients undergoing conventional immunotherapy or a “modified rush” protocol in 2014. They found that the conventional approach produced a 64.5% success rate, compared with an 84.4% success rate for those doing the faster protocol (P less than .001).
Typically, allergen immunotherapy takes 6 months, with patients making weekly visits with gradually increasing doses. The weekly visits can be a burden, and many patients drop out. The modified rush protocol seems to be easier for patients to accomplish, Dr. Schafer said at the joint congress of the American Academy of Allergy, Asthma, and Immunology and the World Asthma Organization.
The study compared 231 patients on the faster protocol with 392 treated with the traditional approach.
On the “modified rush” protocol, patients aged 12-15 years took preventive medications – 20 mg of prednisone and 150 mg of ranitidine 2 days before their appointment and again the day after. In addition, they took 10 mg of cetirizine and 5 mg of montelukast 2 days before their appointment. Patients also took those same medications the morning of the treatment.
For patients 16 and older, the dose of prednisone was 30 mg and the dose of montelukast was 10 mg.
The protocol consisted of eight shots, starting at a more diluted dose than the conventional protocol, as an additional safety precaution. The shots were given first every 15 minutes, then every 30 minutes or an hour as the dose increases. Once the treatment was over, the dilution was 1:10, a milestone not reached until 3 months under the conventional approach.
An hour or two after the treatment, all patients received 20 mg of prednisone.
Patients resumed the normal conventional protocol after the first visit.
Reactions were rare under the “modified rush” protocol, with 6 patients experiencing flushing but nothing else, 21 with grade 1 reactions, 3 with grade 2, and no reactions worse than that. Five patients needed epinephrine.
Dr. Schafer said the approach may hold appeal because, even though it’s accelerated, there is a slower lead-in to the maintenance dose.
“If you ask allergists, ‘Do you rush?’ they’ll say, ‘No, no, no, I don’t rush. It’s too risky.’” she said. “Hence, modified rush.”
SOURCE: Schafer C et al. AAAAI/WAO Joint Congress abstract 520.
ORLANDO – Patients with allergic rhinitis undergoing allergen immunotherapy achieved the goal of their monthly maintenance dose at a 20% higher rate when they underwent went a “modified rush” protocol, compared with those who received the therapy with the much slower conventional approach.
The findings offer proof that the faster approach is safe, more effective, and should be more widely used even though many allergists continue to be hesitant to do so, according to Christine Schafer, MD, clinical assistant professor at Michigan State University, East Lansing and a private practice allergist in Grand Rapids, Mich.
“The beauty of it is you’ve consolidated 3 months into a day,” said Dr. Schafer, who added that patients also feel better faster. “They don’t have to have this going every week for 3 months not feeling any better.”
Dr. Schafer and her colleagues reviewed a year’s worth of results for patients undergoing conventional immunotherapy or a “modified rush” protocol in 2014. They found that the conventional approach produced a 64.5% success rate, compared with an 84.4% success rate for those doing the faster protocol (P less than .001).
Typically, allergen immunotherapy takes 6 months, with patients making weekly visits with gradually increasing doses. The weekly visits can be a burden, and many patients drop out. The modified rush protocol seems to be easier for patients to accomplish, Dr. Schafer said at the joint congress of the American Academy of Allergy, Asthma, and Immunology and the World Asthma Organization.
The study compared 231 patients on the faster protocol with 392 treated with the traditional approach.
On the “modified rush” protocol, patients aged 12-15 years took preventive medications – 20 mg of prednisone and 150 mg of ranitidine 2 days before their appointment and again the day after. In addition, they took 10 mg of cetirizine and 5 mg of montelukast 2 days before their appointment. Patients also took those same medications the morning of the treatment.
For patients 16 and older, the dose of prednisone was 30 mg and the dose of montelukast was 10 mg.
The protocol consisted of eight shots, starting at a more diluted dose than the conventional protocol, as an additional safety precaution. The shots were given first every 15 minutes, then every 30 minutes or an hour as the dose increases. Once the treatment was over, the dilution was 1:10, a milestone not reached until 3 months under the conventional approach.
An hour or two after the treatment, all patients received 20 mg of prednisone.
Patients resumed the normal conventional protocol after the first visit.
Reactions were rare under the “modified rush” protocol, with 6 patients experiencing flushing but nothing else, 21 with grade 1 reactions, 3 with grade 2, and no reactions worse than that. Five patients needed epinephrine.
Dr. Schafer said the approach may hold appeal because, even though it’s accelerated, there is a slower lead-in to the maintenance dose.
“If you ask allergists, ‘Do you rush?’ they’ll say, ‘No, no, no, I don’t rush. It’s too risky.’” she said. “Hence, modified rush.”
SOURCE: Schafer C et al. AAAAI/WAO Joint Congress abstract 520.
REPORTING FROM AAAAI/WAO JOINT CONGRESS
Key clinical point: Completing an immunotherapy regimen on a ‘modified rush’ basis leads to better adherence.
Major finding: A total of 84.4% completed the ‘modified rush’ protocol, vs. 65.4% for the conventional protocol.
Study details: Retrospective chart review of 623 patients who received allergen immunotherapy via the conventional, 6-month approach or a modified, faster approach.
Disclosures: The investigators reported no conflicts of interest.
Source: Schafer C et al. AAAAI/WAO Joint Congress abstract 520.
Managing Glenoid Bone Deficiency—The Augment Experience in Anatomic and Reverse Shoulder Arthroplasty
ABSTRACT
Glenoid bone deficiency in the setting of shoulder replacement surgery is far more common than originally reported. The frequency and severity of the glenoid defects are noted to be more common and severe with the advent of computer-assisted surgery. The results of an anatomic total shoulder arthroplasty (aTSA) with glenoid deficiency have been reported to be inferior to aTSA patients without a glenoid deficiency. Options for treating the glenoid deficiency include eccentric reaming, bone grafting, and the use of augmented glenoid components. The purpose of this article is to present the indications, technique, and results of augmented glenoids for both aTSA and reverse TSA (RTSA).
Augments for both aTSA and RTSA are viable options. They preserve subchondral bone at the same time as optimizing the joint line without the need for bone grafts. Complications, revisions and results are as good as compared to shoulder arthroplasties without glenoid wear.
Continue to: Glenoid bone deficiency...
Glenoid bone deficiency in arthritic or cuff-deficient shoulder has been reported in up to 50% of shoulder defect cases.1,2 The type and severity of glenoid deformities vary depending on the underlying pathology and time of manifestation. Osteoarthritis with bone loss typically results in posterior or posterior inferior glenoid wear and is commonly classified as Walch types B1 or B2 (biconcave). In cases of severe erosion, B3 classification has been proposed; in this classification, bone loss becomes extremely severe, progressing to resemble a type C glenoid. Unlike primary osteoarthritis, inflammatory arthropathy more commonly causes central loss of glenoid bone (Walch A2). With the rotator cuff insufficiency, superior migration of the humeral head occurs. As these conditions progress, cuff tear arthropathy (CTA) changes result in superior or posterior-superior bone loss.1 Anterior bone loss (type D) will be rarely encountered due to recurrent anterior instability.3
Classically, with anatomic total shoulder arthroplasty (aTSA), the surgeon considers several options for managing glenoid deficiencies. The most commonly employed technique involves eccentrically reaming the glenoid and correcting the deformity. This procedure is relatively easy but features significant drawbacks, such as sacrificing the subchondral bone, medializing the glenohumeral joint line, and secondarily shrinking the glenoid surface area. Other options include structural bone grafting behind the glenoid component. Most anatomic prosthetic glenoids prove to be unsuitable for fixation of structural bone graft. Therefore, the graft is first internally fixed, followed by placement of the glenoid component. Cement, which is commonly used for glenoid fixation, may potentially inhibit bone-graft healing. Reports using this technique documented high radiographic failure rate of up to 40% at midterm follow-up.4 Although leaving the glenoid component retroverted may be considered, surgeons should develop awareness of the possibility of peg penetration of the anterior glenoid neck. Additionally, retroversion in excess of 5°may increase the risk of recurrent posterior subluxation, resulting in early glenoid loosening.5-7 Results of aTSA under significant glenoid deficiency are inferior to those of aTSA patients without glenoid deficiency.8 Such findings have been extremely inferior in patients with significant glenoid wear, prompting numerous surgeons to abandon aTSA in this population in favor of reverse TSA (RTSA) due to improved bony fixation.
In 2010, augmented anatomic glenoids were first introduced as a wedge (Exactech) and as a step shortly thereafter (DePuy Synthes; Figures 1A-1C). More recently, hemi-wedges have been introduced (Wright Medical Group). Augments have gained popularity due to improved range of motion vs reverse shoulder arthroplasty (RSA). However, debates remain regarding the use of posteriorly augmented components in the setting of posterior glenoid bone loss.8 Augments serve as another viable option for handling glenoid bone deficiency in aTSA.
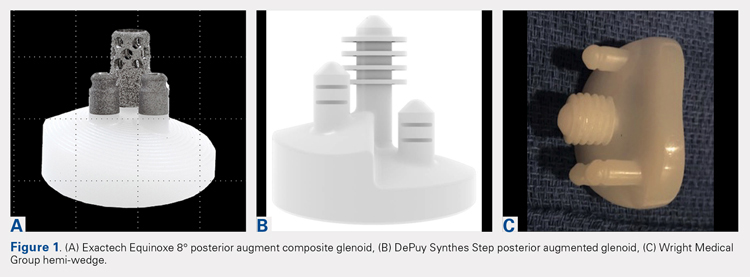
Glenoid bone loss in RTSA presents similar options to aTSA. However, screw fixation of the glenoid component offers several distinct advantages. Baseplate fixation can readily be used with bone grafting and with a highly anticipated success rate. With multiple screw options, 100% support of the baseplate is not mandatory. Although bony increase offset RSAs (BIO-RSAs) have shown success, augmentation with allograft or autograft increases operative time and relies on osseous integration for long-term implant success.9 Metal augmented baseplates were first introduced in 2011 (Exactech) as a means of managing glenoid bone loss without structural grafting. Although initial results have been encouraging, additional studies are needed to assess the longevity of these implants (Figures 1A-1C).
aTSA AUGMENTS
aTSA augments were introduced as a means of correcting acquired glenoid bone deficiency, restoring native glenoid version, correcting humeral subluxation, and preserving the native subchondral bone. Compared with glenoid bone grafting, augmented glenoid components decrease operative time, allow for a technically easier operation, and require no bone healing for clinical success. Early and midterm results are encouraging, showing similar findings comparable to those of aTSA in non-glenoid deficient shoulders.10-12
Continue to: INDICATIONS
INDICATIONS
Indications and limitations for augmented aTSA glenoids remain incompletely defined. The most common indication for an augmented aTSA is osteoarthritis with a B2 glenoid. We recommend augments in the occurrence of any indication of significant eccentric glenoid wear. With the expertise of surgeons, deformities of up to 20° to 25° of deformity can be readily handled with good predictability. More severe deformities can be managed with augmented aTSA components, but early failure rates may be high. The most severe acquired deformities remain best managed with RTSA. Currently, we prefer RTSA when glenoid bone loss exceeds 25°. With the widespread availability of computed tomography (CT) scans with 3-dimensional (3-D) reconstruction, glenoid bone defects are increasingly recognized. When correcting deformity, surgeons should strive to limit residual retroversion to a maximum of 5°.13 Preoperative planning software and computer-assisted surgery (ExactechGPS) may allow surgeons to better define the limits of augmented glenoid fixation prior to the date of surgery. We routinely utilize computer-guided glenoid preparation to control glenoid version to within 5° of neutral position.
The differences between B3 and a true type C glenoid must be recognized. Although B3 glenoids may still be a candidate for an augmented anatomic glenoid component, type C glenoids are not. Developmental abnormalities of type C glenoid occur simultaneously with humeral deformities, including medialized posterior rotator cuff musculature. Correction of the joint line to neutral version may not replicate the non-diseased state of a dysplastic type shoulder. Davis and colleagues14 have proposed treating these patients by leaving both the humerus and glenoid in their native version without correction.
TECHNIQUE
The implant that we have the most experience with is an 8° full-wedge augmented glenoid component. Such an implant is typically utilized for B2 glenoids. We recommend that a high-quality CT scan be performed for preoperative planning. As a general rule, the starting point often lies close to the ridge of B2 glenoid and more anterior than the apparent glenoid center, which is viewed intraoperatively due to asymmetric posterior wear. Full-wedge component is utilized to ream the ridge separating the neo and paleoglenoids to create a flat surface. This condition is best achieved by drilling a pilot hole at the planned glenoid central peg position to prevent the reamer from sliding anteriorly during reaming. Glenoid preparation begins with the smallest reamer until the ridge has been flattened, and the reamer makes full contact with the glenoid. The reamer diameter is then increased based on glenoid size. Slightly downsizing the glenoid implant will require less reaming to achieve full backside support. Once the glenoid is properly reamed, the central and peripheral peg holes are drilled using the appropriate guides. Holes are then dried, and all-polyethylene or composite glenoid component (either partially or completely cemented) is installed using favored cementing techniques. The advantage of composite glenoid component is that the central cage allows for bone ingrowth and may potentially improve long-term implant survival. Press fit of the central cage requires no waiting time for glenoid cement hardening before proceeding to the humerus. When placing an augmented component, adequate glenoid exposure is imperative to allow in-line placement and appropriate seating of the component without impingement on adjacent retractors.
When using the step-augmented glenoid, the paleoglenoid is prepared in a similar fashion to a standard aTSA. Once the paleoglenoid has been reamed to a neutral position, a protector plate is placed onto the paleoglenoid. and a step-cut saw is used to prepare the posterior stepped bone cut. Peripheral pegs are then drilled, and the component is installed in routine fashion. When using hemi-wedge augments, the paleoglenoid is again prepared in a similar fashion as a standard glenoid component over a cannulated guidewire. The neoglenoid is subsequently prepared using a specialized angled reamer with a positive stop to prevent over-reaming. These glenoid implants improve rotational force neutralization given the absence of flat back against the glenoid. All 3 designs preserve bone when compared with eccentric reaming alone,15 with the half-augmented wedge preserving the most bone.
Table 1. Results of Various Augmented Glenoid Components in Anatomic Total Shoulder
Arthroplasty
| Augment | American Shoulder and Elbow Surgeons Score | Constant Score | Active Forward Flexion | Active External Rotation | ||||||||||||||||
8° cage (N = 21) |
|
|
|
|
| |||||||||||||||
All-polyethylene 8° (N = 45) |
|
|
|
|
| |||||||||||||||
All-polyethylene 16° (N = 7) |
|
|
|
|
|
RESULTS
In our institution, we first used all-polyethylene posteriorly augmented glenoid components in 2010. Between 2010 and 2015, 45 patients received an 8° all-polyethylene posterior augment, and 7 patients received a 16° augment. In 2015, we transitioned to the composite caged posterior augment. All patients in our database who received an augmented glenoid component experienced improvement in active forward elevation, external rotation, American Shoulder and Elbow Surgeons (ASES), and Constant scores (Table 1). Minimum follow-up was 1 year for patients receiving both an 8° cage (mean, 1.48 years) and an 8° all-polyethylene augment (mean, 3.18 years). Figures 2A-2C show a patient with significant posterior glenoid wear and humeral head subluxation treated with an 8° wedge composite posterior augment glenoid 3 years postoperative.
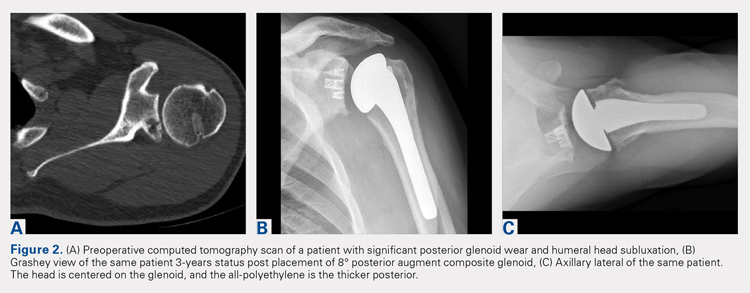
Continue to: COMPLICATIONS
COMPLICATIONS
Two complications developed in the group undergoing composite cage augment. One patient experienced glenoid loosening after a motor vehicle accident. Another patient sustained significant intraoperative tuberosity avulsion during implantation of the humeral component, requiring a change of implant and tuberosity fixation. Although no complications were noted in the 8° all-polyethylene group, 3 patients in the 16° augment group sustained complications. One of these patients suffered a cardiac event that was unrelated to the implant. Two complications in this group were both related to loosening of the glenoid component, requiring subsequent revision.
DISCUSSION
The first report on augmented aTSA was published in 2008, and it involved a 5° augmented, anatomic glenoid.12 One study was based on a small series of augments; the poor results led the reporting surgeons to subsequently abandon the implant.12 This early design produced a correction on the articular side of the implant rather than the pathologic bony side. By performing such correction, the component pegs remained anteriorly oriented, placing the component at risk of perforation through the anterior glenoid neck. All current augment designs feature pegs that are oriented down the glenoid vault, with corrections occurring on the bony surface. This condition requires 2 different axes for reaming the glenoid and drilling the pegs. This approach allows the pegs to be directed down the glenoid neck, and is a far superior solution to neutralizing shear forces when compared with the implants used in the 1990s.
Early to midterm results of modern aTSA augments have been extremely encouraging with low revision rates. The main concern of recurrent posterior subluxation has been rarely reported. The concerns over glenoid loosening due to high shear forces, similarly, have not been described to date. However, surgeons should remain cautious, as longer-term follow-up remains unavailable.
The main advantage of aTSA augments is their capacity to preserve bone compared with eccentric reaming and better long-term stability. Each of the augment designs requires varying amounts of bone removal. Through biomechanics and using finite element analysis, the 3 augment types act differently, with no design demonstrating remarkable biomechanical superiority.6 Favorito and colleagues16 performed a retrospective review of 22 patients who underwent aTSA using an all-polyethylene, posteriorly augmented, and stepped glenoid component for posterior bone loss. At an average follow-up of 36 months, all patients experienced improvements in active forward elevation, external rotation, visual analog scale, Short Form-36 Physical Component Summary, and Western Ontario Osteoarthritis of the Shoulder scores. The authors noted that 2 patients (9%) experienced complications: 1 with an anterior dislocation and the other with recurrent posterior instability requiring revision. Sandow and Schutz17 reported the preliminary results of 10 patients who underwent aTSA using trabecular metal augment with a minimum of 2-year follow-up. All patients received either a 15° or 30° posterior, metal-backed augment for severe glenoid bone loss (Walch grade B2 or C). At a minimum of 2-year follow-up, all patients received correction to within 10° of neutral glenoid version, without any complications nor implant failures.
Regardless of augment design, all current components restore the native glenoid version, improving the length and subsequent tension of rotator cuff musculature. Similarly, re-centering the humeral head decreases the forces on the glenoid and allows for optimal function with decreasing loss of vital subchondral bone.
Continue to: RTSA AUGMENTS
RTSA AUGMENTS
Similar to anatomic augments, metal augments were introduced for use with RTSA in 2011. Unlike anatomic augments, those for RTSA were manufactured with metal. Given the difference in bony wear patterns in patients requiring RTSA, augments were available in a number of configurations. With CTA, wear is most commonly superior. Leaving a superiorly inclined baseplate must be avoided due to risks of notching, loosening, and early failure. However, correcting this tilt will require significant reaming of the inferior glenoid. A superior augment is ideally suited for this bone-loss pattern. If the glenoid is retroverted significantly, difficulty can also arise during glenoid preparation and baseplate placement. Posterior augments may ease this aspect of the procedure. Posterior augments feature the additional benefits of tensioning any remaining posterior rotator cuff, minimizing posterior inferior impingement, and technically easing the operation.18 As we improve our awareness of glenoid orientation using computer navigation, a posterior-superior augmented implant is commonly needed to simultaneously optimize the baseplate position and to minimize reaming (Figure 3). The posterior-superior augmented baseplate has become the most commonly used baseplate augment of choice in 90% of our RTSA cases that require an augment.

INDICATIONS
Augmented RTSA baseplates are indicated when adequate backside contact cannot be achieved with eccentric reaming, thus compromising potential fixation. In our practice, we preferably use augments at <50% contact with the backside of the baseplate. Excessive superior inclination is observed in a CTA setting, commonly indicating the use of superior augments. Similarly, severe primary osteoarthritis may contain elements of posterior bone loss, leading to increased retroversion, which is where we use posterior augments. When patients exhibit combined deformities, or when the surgeon wishes to tension the posterior rotator cuff, a posterior-superior augmented glenoid baseplate is used. For extremely severe defects, we have combined bone grafting and augments. In patients with a highly deficient glenoid but good quality of the remaining bone stock, an augment allows for better contact with less reaming although it is not fully supported when compared with a non-augmented baseplate. Bone grafts can function similarly, but the autograft humeral head is not constantly present in revision situations and requires increased operative time to allow for precision carpentry. Additionally, the success of BIO-RSA requires healing of bone graft on the native glenoid to support the baseplate.19 Jones and colleagues9 compared metal augmented RTSA with BIO-RSA and presented equivalent results.
To minimize reaming and to obtain appropriately inferior inclination, we have discovered preoperative templating and intraoperative, computer-guided glenoid preparation to be extremely valuable (ExactechGPS). These tools allow appropriate assessment of augments and for minimal bone removal when preparing the glenoid.
TECHNIQUE
When using an augment, a fine-cut CT scan is highly recommended to aid in surgery planning. We also find 3-D reconstructions to be helpful. Preoperative planning software also allows surgeons to maximize fixation of implant within the glenoid vault. The starting point for reaming is planned based on CT. Some surgeons using augments perform minimal or no reaming at all, electing to remove the remaining cartilage with a Cobb elevator. Different reaming and drilling axes are used when using augments. In cases of severe glenoid deformity and unavailability of computer assistance, a guide wire with inferior inclination can be installed based on CT scan. Penetration of this wire down the glenoid neck can be palpated and compared with the preoperative plan. We generally prefer at least 24 mm of bone containment for the central cage. Once the surgeon is satisfied with the placement of the wire, the appropriate augment guide is placed, followed by a second guide wire. This second wire acts as the reaming axis. The first wire is removed, and the glenoid is reamed with a cannulated reamer. Once reaming is completed, the original wire is replaced in the same hole and trajectory, and the reaming wire is removed. The first wire is then drilled with a cannulated drill for the central cage. The augmented baseplate is then impacted into place, and screw fixation is performed. Again, intraoperative computer guidance allows for precision screw placement with maximal bone attachment.
Table 2. Results of Reverse Total Shoulder Arthroplasty Augmented Baseplates
| Augment | American Shoulder and Elbow Surgeons Score | Constant Score | Active Forward Flexion | Active External Rotation | ||||||||||||||||
Superior (N = 22) |
|
|
|
|
| |||||||||||||||
Posterior (N = 50) |
|
|
|
|
| |||||||||||||||
Posterosuperior (N = 67) |
|
|
|
|
|
RESULTS
Based on our experience, glenoid augments for RTSA have performed well at short- and mid-term follow-up. From October 2011 to July 2016, 139 patients undergoing RTSA received a posterior, superior, or posterior-superior augmented glenoid baseplate. All groups demonstrated improvements in functional outcome measures, including Constant, ASES, Shoulder Pain and Disability Index, and Simple Shoulder Test scores compared with baseline values (Table 2). The posterior-superior augment group experienced the most significant improvement in active forward flexion and external rotation, whereas the posterior augment group experienced the most significant improvement in ASES and Constant scores. Figures 4A-4C displays the radiographs of a patient with significant glenoid wear treated with a posterior-superior augment RTSA.
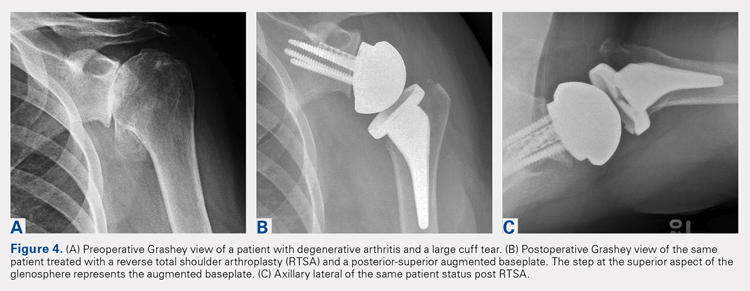
Continue to: COMPLICATIONS
COMPLICATIONS
In the superior augment group, 3 patients (13%) sustained 5 complications. One patient sustained 3 separate episodes of instability, eventually requiring revision of prosthesis. In the posterior augment group, 4 patients (8%) sustained complications. Two of the 4 patients presented postoperative humeral fractures related to traumatic events, whereas another patient sustained an intraoperative tuberosity fracture. The last complication in this group involved a postoperative draining wound that was treated with oral antibiotics.
Nine complications developed in the posterior-superior augment group (13%); these complications included aseptic baseplate loosening (5), glenoid fracture (1), humeral fracture (1), acromial stress fracture (1), and cerebrovascular accident (1).
DISCUSSION
As the use of augments in RTSA is relatively new, significantly scarce data exist regarding their outcomes and longevity. A few studies have focused on the short-term outcomes of these augments. Jones and colleagues9 performed a retrospective review of 80 patients who underwent RTSA and required either a structural bone graft or an augmented glenoid baseplate.9 They observed that although all patients showed improvements in pain, range of motion, and functional scores, the structural bone graft group incurred a 14.6% complication rate compared with none observed in the augment group. Additionally, Jones and colleagues9 noted that the augmented baseplate group exhibited a significantly lower rate of scapular notching compared with the bone-graft group (10% vs 18.5%) at similar follow-up intervals. A separate study by Wright and colleagues18 compared posterior vs superior augmented baseplates in RTSA. The posterior augment group demonstrated lower rates of scapular notching (6.3% vs 14.3%) and showed more significant improvements in Constant, ASES, and active forward elevation measures, compared with the superior augment group.
As more manufacturers develop augments for RTSA, and as ExactechGPS uses become more widespread, the use of RTSA baseplate augments will continually grow. Custom implants using massive metal augments are now also being introduced. Although currently too expensive for most cases, as technology drives the cost down, every patient may receive customized augmented implants in the future.
The advantages of augmented baseplate designs include minimized reaming and notching, improved tension of the remaining rotator cuff, and decreased operating room time. The disadvantages include increased cost and lack of mid- or long-term clinical data. The concerns with baseplate loosening with augments in RTSA are much less than those with augments for aTSA due to the outstanding baseplate fixation that can be achieved in RTSA.
Continue to: CONLCLUSION
CONCLUSION
Augments offer an excellent tool for surgeons performing both aTSA and RTSA with glenoid bone loss. Use of augments will become more common as more manufacturers develop them. Although clinical results fall short in full midterm, they have been positive for both augmented RTSA and aTSA. Concerns arise when performing augmented aTSA, as an upper limit of correction has not been defined with regard to component failure. Currently, no data support the maximum amount of correction that can be achieved. In our current practice, we face difficulty in correcting more than 25° of version in young active patients with aTSA augment. Beyond this point, we perform a RTSA with an augment. In older patients or low-demand patients, we only correct minor deformities (<20°) with an aTSA augment, opting instead for an augmented RTSA due to the lower midterm failure rates observed with this implant.
1. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. J Bone Joint Surg Br. 2004;86(3):388-395. doi:10.1302/0301-620X.86B3.
2. Churchill RS, Spencer Jr EE, Fehringer EV. Quantification of B2 glenoid morphology in total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(8):1212-1217. doi:10.1016/j.jse.2015.01.007.
3. Bercik MJ, Kruse K, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-1606. doi:10.1016/j.jse.2016.03.010.
4. Klika BJ, Wooten CW, Sperling JW, et al. Structural bone grafting for glenoid deficiency in primary total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):1066-1072. doi:10.1016/j.jse.2013.09.017.
5. Franklin JL, Barrett WP, Jackins SE, Matsen FA 3rd. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3(1):39-46.
6. Hermida JC, Flores-Hernandez C, Hoenecke HR, D’Lima DD. Augmented wedge-shaped glenoid component for the correction of glenoid retroversion: a finite element analysis. J Shoulder Elbow Surg. 2014;23(3):347-354. doi:10.1016/j.jse.2013.06.008.
7. Ho JC, Sabesan VJ, Iannotti JP. Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg Am. 2013;95(12):e82. doi:10.2106/JBJS.L.00336.
8. Denard PJ, Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22(11):1589-1598. doi:10.1016/j.jse.2013.06.017.
9. Jones RB, Wright TW, Roche CP. Bone grafting the glenoid versus use of augmented glenoid baseplates with reverse shoulder arthroplasty. Bull Hosp Jt Dis (2013). 2015;73(suppl 1):S129-S135.
10. Hsu JE, Ricchetti ET, Huffman GR, Iannotti JP, Glaser DL. Addressing glenoid bone deficiency and asymmetric posterior erosion in shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(9):1298-1308. doi:10.1016/j.jse.2013.04.014.
11. Cil A, Sperling JW, Cofield RH. Nonstandard glenoid components for bone deficiencies in shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):e149-e157. doi:10.1016/j.jse.2013.09.023.
12. Rice RS, Sperling JW, Miletti J, Schleck C, Cofield RH. Augmented glenoid component for bone deficiency in shoulder arthroplasty. Clin Orthop Relat Res. 2008;466(3):579-583. doi:10.1007/s11999-007-0104-4.
13. Sabesan V, Callanan M, Sharma V, Iannotti JP. Correction of acquired glenoid bone loss in osteoarthritis with a standard versus an augmented glenoid component. J Shoulder Elbow Surg. 2014;23(7):964-973. doi:10.1016/j.jse.2013.09.019.
14. Davis DE, Acevedo D, Williams A, Williams G. Total shoulder arthroplasty using an inlay mini-glenoid component for glenoid deficiency: a 2-year follow-up of 9 shoulders in 7 patients. J Shoulder Elbow Surg. 2016;25(8):1354-1361. doi:10.1016/j.jse.2015.12.010.
15. Kersten AD, Flores-Hernandez C, Hoenecke HR, D'Lima DD. Posterior augmented glenoid designs preserve more bone in biconcave glenoids. J Shoulder Elbow Surg. 2015;24(7):1135-1141. doi:10.1016/j.jse.2014.12.007.
16. Favorito PJ, Freed RJ, Passanise AM, Brown MJ. Total shoulder arthroplasty for glenohumeral arthritis associated with posterior glenoid bone loss: results of an all-polyethylene, posteriorly augmented glenoid component. J Shoulder Elbow Surg. 2016;25(10):1681-1689. doi:10.1016/j.jse.2016.02.020.
17. Sandow M, Schutz C. Total shoulder arthroplasty using trabecular metal augments to address glenoid retroversion: the preliminary result of 10 patients with minimum 2-year follow-up. J Shoulder Elbow Surg. 2016;25(4):598-607. doi:10.1016/j.jse.2016.01.001.
18. Wright TW, Roche CP, Wright L, Flurin PH, Crosby LA, Zuckerman JD. Reverse shoulder arthroplasty augments for glenoid wear: A comparison of posterior augments to superior augments. Bull Hosp Jt Dis. 2015;73(suppl 1):S124-S128.
19. Boileau P, Morin-Salvo N, Gauci MO, et al. Angled BIO-RSA (bony-increased offset-reverse shoulder arthroplasty): a solution for the management glenoid bone loss and erosion. J Shoulder Elbow Surg. 2017;26(12):2133-2142. doi:10.1016/j.jse.2017.05.024.
ABSTRACT
Glenoid bone deficiency in the setting of shoulder replacement surgery is far more common than originally reported. The frequency and severity of the glenoid defects are noted to be more common and severe with the advent of computer-assisted surgery. The results of an anatomic total shoulder arthroplasty (aTSA) with glenoid deficiency have been reported to be inferior to aTSA patients without a glenoid deficiency. Options for treating the glenoid deficiency include eccentric reaming, bone grafting, and the use of augmented glenoid components. The purpose of this article is to present the indications, technique, and results of augmented glenoids for both aTSA and reverse TSA (RTSA).
Augments for both aTSA and RTSA are viable options. They preserve subchondral bone at the same time as optimizing the joint line without the need for bone grafts. Complications, revisions and results are as good as compared to shoulder arthroplasties without glenoid wear.
Continue to: Glenoid bone deficiency...
Glenoid bone deficiency in arthritic or cuff-deficient shoulder has been reported in up to 50% of shoulder defect cases.1,2 The type and severity of glenoid deformities vary depending on the underlying pathology and time of manifestation. Osteoarthritis with bone loss typically results in posterior or posterior inferior glenoid wear and is commonly classified as Walch types B1 or B2 (biconcave). In cases of severe erosion, B3 classification has been proposed; in this classification, bone loss becomes extremely severe, progressing to resemble a type C glenoid. Unlike primary osteoarthritis, inflammatory arthropathy more commonly causes central loss of glenoid bone (Walch A2). With the rotator cuff insufficiency, superior migration of the humeral head occurs. As these conditions progress, cuff tear arthropathy (CTA) changes result in superior or posterior-superior bone loss.1 Anterior bone loss (type D) will be rarely encountered due to recurrent anterior instability.3
Classically, with anatomic total shoulder arthroplasty (aTSA), the surgeon considers several options for managing glenoid deficiencies. The most commonly employed technique involves eccentrically reaming the glenoid and correcting the deformity. This procedure is relatively easy but features significant drawbacks, such as sacrificing the subchondral bone, medializing the glenohumeral joint line, and secondarily shrinking the glenoid surface area. Other options include structural bone grafting behind the glenoid component. Most anatomic prosthetic glenoids prove to be unsuitable for fixation of structural bone graft. Therefore, the graft is first internally fixed, followed by placement of the glenoid component. Cement, which is commonly used for glenoid fixation, may potentially inhibit bone-graft healing. Reports using this technique documented high radiographic failure rate of up to 40% at midterm follow-up.4 Although leaving the glenoid component retroverted may be considered, surgeons should develop awareness of the possibility of peg penetration of the anterior glenoid neck. Additionally, retroversion in excess of 5°may increase the risk of recurrent posterior subluxation, resulting in early glenoid loosening.5-7 Results of aTSA under significant glenoid deficiency are inferior to those of aTSA patients without glenoid deficiency.8 Such findings have been extremely inferior in patients with significant glenoid wear, prompting numerous surgeons to abandon aTSA in this population in favor of reverse TSA (RTSA) due to improved bony fixation.
In 2010, augmented anatomic glenoids were first introduced as a wedge (Exactech) and as a step shortly thereafter (DePuy Synthes; Figures 1A-1C). More recently, hemi-wedges have been introduced (Wright Medical Group). Augments have gained popularity due to improved range of motion vs reverse shoulder arthroplasty (RSA). However, debates remain regarding the use of posteriorly augmented components in the setting of posterior glenoid bone loss.8 Augments serve as another viable option for handling glenoid bone deficiency in aTSA.

Glenoid bone loss in RTSA presents similar options to aTSA. However, screw fixation of the glenoid component offers several distinct advantages. Baseplate fixation can readily be used with bone grafting and with a highly anticipated success rate. With multiple screw options, 100% support of the baseplate is not mandatory. Although bony increase offset RSAs (BIO-RSAs) have shown success, augmentation with allograft or autograft increases operative time and relies on osseous integration for long-term implant success.9 Metal augmented baseplates were first introduced in 2011 (Exactech) as a means of managing glenoid bone loss without structural grafting. Although initial results have been encouraging, additional studies are needed to assess the longevity of these implants (Figures 1A-1C).
aTSA AUGMENTS
aTSA augments were introduced as a means of correcting acquired glenoid bone deficiency, restoring native glenoid version, correcting humeral subluxation, and preserving the native subchondral bone. Compared with glenoid bone grafting, augmented glenoid components decrease operative time, allow for a technically easier operation, and require no bone healing for clinical success. Early and midterm results are encouraging, showing similar findings comparable to those of aTSA in non-glenoid deficient shoulders.10-12
Continue to: INDICATIONS
INDICATIONS
Indications and limitations for augmented aTSA glenoids remain incompletely defined. The most common indication for an augmented aTSA is osteoarthritis with a B2 glenoid. We recommend augments in the occurrence of any indication of significant eccentric glenoid wear. With the expertise of surgeons, deformities of up to 20° to 25° of deformity can be readily handled with good predictability. More severe deformities can be managed with augmented aTSA components, but early failure rates may be high. The most severe acquired deformities remain best managed with RTSA. Currently, we prefer RTSA when glenoid bone loss exceeds 25°. With the widespread availability of computed tomography (CT) scans with 3-dimensional (3-D) reconstruction, glenoid bone defects are increasingly recognized. When correcting deformity, surgeons should strive to limit residual retroversion to a maximum of 5°.13 Preoperative planning software and computer-assisted surgery (ExactechGPS) may allow surgeons to better define the limits of augmented glenoid fixation prior to the date of surgery. We routinely utilize computer-guided glenoid preparation to control glenoid version to within 5° of neutral position.
The differences between B3 and a true type C glenoid must be recognized. Although B3 glenoids may still be a candidate for an augmented anatomic glenoid component, type C glenoids are not. Developmental abnormalities of type C glenoid occur simultaneously with humeral deformities, including medialized posterior rotator cuff musculature. Correction of the joint line to neutral version may not replicate the non-diseased state of a dysplastic type shoulder. Davis and colleagues14 have proposed treating these patients by leaving both the humerus and glenoid in their native version without correction.
TECHNIQUE
The implant that we have the most experience with is an 8° full-wedge augmented glenoid component. Such an implant is typically utilized for B2 glenoids. We recommend that a high-quality CT scan be performed for preoperative planning. As a general rule, the starting point often lies close to the ridge of B2 glenoid and more anterior than the apparent glenoid center, which is viewed intraoperatively due to asymmetric posterior wear. Full-wedge component is utilized to ream the ridge separating the neo and paleoglenoids to create a flat surface. This condition is best achieved by drilling a pilot hole at the planned glenoid central peg position to prevent the reamer from sliding anteriorly during reaming. Glenoid preparation begins with the smallest reamer until the ridge has been flattened, and the reamer makes full contact with the glenoid. The reamer diameter is then increased based on glenoid size. Slightly downsizing the glenoid implant will require less reaming to achieve full backside support. Once the glenoid is properly reamed, the central and peripheral peg holes are drilled using the appropriate guides. Holes are then dried, and all-polyethylene or composite glenoid component (either partially or completely cemented) is installed using favored cementing techniques. The advantage of composite glenoid component is that the central cage allows for bone ingrowth and may potentially improve long-term implant survival. Press fit of the central cage requires no waiting time for glenoid cement hardening before proceeding to the humerus. When placing an augmented component, adequate glenoid exposure is imperative to allow in-line placement and appropriate seating of the component without impingement on adjacent retractors.
When using the step-augmented glenoid, the paleoglenoid is prepared in a similar fashion to a standard aTSA. Once the paleoglenoid has been reamed to a neutral position, a protector plate is placed onto the paleoglenoid. and a step-cut saw is used to prepare the posterior stepped bone cut. Peripheral pegs are then drilled, and the component is installed in routine fashion. When using hemi-wedge augments, the paleoglenoid is again prepared in a similar fashion as a standard glenoid component over a cannulated guidewire. The neoglenoid is subsequently prepared using a specialized angled reamer with a positive stop to prevent over-reaming. These glenoid implants improve rotational force neutralization given the absence of flat back against the glenoid. All 3 designs preserve bone when compared with eccentric reaming alone,15 with the half-augmented wedge preserving the most bone.
Table 1. Results of Various Augmented Glenoid Components in Anatomic Total Shoulder
Arthroplasty
| Augment | American Shoulder and Elbow Surgeons Score | Constant Score | Active Forward Flexion | Active External Rotation | ||||||||||||||||
8° cage (N = 21) |
|
|
|
|
| |||||||||||||||
All-polyethylene 8° (N = 45) |
|
|
|
|
| |||||||||||||||
All-polyethylene 16° (N = 7) |
|
|
|
|
|
RESULTS
In our institution, we first used all-polyethylene posteriorly augmented glenoid components in 2010. Between 2010 and 2015, 45 patients received an 8° all-polyethylene posterior augment, and 7 patients received a 16° augment. In 2015, we transitioned to the composite caged posterior augment. All patients in our database who received an augmented glenoid component experienced improvement in active forward elevation, external rotation, American Shoulder and Elbow Surgeons (ASES), and Constant scores (Table 1). Minimum follow-up was 1 year for patients receiving both an 8° cage (mean, 1.48 years) and an 8° all-polyethylene augment (mean, 3.18 years). Figures 2A-2C show a patient with significant posterior glenoid wear and humeral head subluxation treated with an 8° wedge composite posterior augment glenoid 3 years postoperative.

Continue to: COMPLICATIONS
COMPLICATIONS
Two complications developed in the group undergoing composite cage augment. One patient experienced glenoid loosening after a motor vehicle accident. Another patient sustained significant intraoperative tuberosity avulsion during implantation of the humeral component, requiring a change of implant and tuberosity fixation. Although no complications were noted in the 8° all-polyethylene group, 3 patients in the 16° augment group sustained complications. One of these patients suffered a cardiac event that was unrelated to the implant. Two complications in this group were both related to loosening of the glenoid component, requiring subsequent revision.
DISCUSSION
The first report on augmented aTSA was published in 2008, and it involved a 5° augmented, anatomic glenoid.12 One study was based on a small series of augments; the poor results led the reporting surgeons to subsequently abandon the implant.12 This early design produced a correction on the articular side of the implant rather than the pathologic bony side. By performing such correction, the component pegs remained anteriorly oriented, placing the component at risk of perforation through the anterior glenoid neck. All current augment designs feature pegs that are oriented down the glenoid vault, with corrections occurring on the bony surface. This condition requires 2 different axes for reaming the glenoid and drilling the pegs. This approach allows the pegs to be directed down the glenoid neck, and is a far superior solution to neutralizing shear forces when compared with the implants used in the 1990s.
Early to midterm results of modern aTSA augments have been extremely encouraging with low revision rates. The main concern of recurrent posterior subluxation has been rarely reported. The concerns over glenoid loosening due to high shear forces, similarly, have not been described to date. However, surgeons should remain cautious, as longer-term follow-up remains unavailable.
The main advantage of aTSA augments is their capacity to preserve bone compared with eccentric reaming and better long-term stability. Each of the augment designs requires varying amounts of bone removal. Through biomechanics and using finite element analysis, the 3 augment types act differently, with no design demonstrating remarkable biomechanical superiority.6 Favorito and colleagues16 performed a retrospective review of 22 patients who underwent aTSA using an all-polyethylene, posteriorly augmented, and stepped glenoid component for posterior bone loss. At an average follow-up of 36 months, all patients experienced improvements in active forward elevation, external rotation, visual analog scale, Short Form-36 Physical Component Summary, and Western Ontario Osteoarthritis of the Shoulder scores. The authors noted that 2 patients (9%) experienced complications: 1 with an anterior dislocation and the other with recurrent posterior instability requiring revision. Sandow and Schutz17 reported the preliminary results of 10 patients who underwent aTSA using trabecular metal augment with a minimum of 2-year follow-up. All patients received either a 15° or 30° posterior, metal-backed augment for severe glenoid bone loss (Walch grade B2 or C). At a minimum of 2-year follow-up, all patients received correction to within 10° of neutral glenoid version, without any complications nor implant failures.
Regardless of augment design, all current components restore the native glenoid version, improving the length and subsequent tension of rotator cuff musculature. Similarly, re-centering the humeral head decreases the forces on the glenoid and allows for optimal function with decreasing loss of vital subchondral bone.
Continue to: RTSA AUGMENTS
RTSA AUGMENTS
Similar to anatomic augments, metal augments were introduced for use with RTSA in 2011. Unlike anatomic augments, those for RTSA were manufactured with metal. Given the difference in bony wear patterns in patients requiring RTSA, augments were available in a number of configurations. With CTA, wear is most commonly superior. Leaving a superiorly inclined baseplate must be avoided due to risks of notching, loosening, and early failure. However, correcting this tilt will require significant reaming of the inferior glenoid. A superior augment is ideally suited for this bone-loss pattern. If the glenoid is retroverted significantly, difficulty can also arise during glenoid preparation and baseplate placement. Posterior augments may ease this aspect of the procedure. Posterior augments feature the additional benefits of tensioning any remaining posterior rotator cuff, minimizing posterior inferior impingement, and technically easing the operation.18 As we improve our awareness of glenoid orientation using computer navigation, a posterior-superior augmented implant is commonly needed to simultaneously optimize the baseplate position and to minimize reaming (Figure 3). The posterior-superior augmented baseplate has become the most commonly used baseplate augment of choice in 90% of our RTSA cases that require an augment.

INDICATIONS
Augmented RTSA baseplates are indicated when adequate backside contact cannot be achieved with eccentric reaming, thus compromising potential fixation. In our practice, we preferably use augments at <50% contact with the backside of the baseplate. Excessive superior inclination is observed in a CTA setting, commonly indicating the use of superior augments. Similarly, severe primary osteoarthritis may contain elements of posterior bone loss, leading to increased retroversion, which is where we use posterior augments. When patients exhibit combined deformities, or when the surgeon wishes to tension the posterior rotator cuff, a posterior-superior augmented glenoid baseplate is used. For extremely severe defects, we have combined bone grafting and augments. In patients with a highly deficient glenoid but good quality of the remaining bone stock, an augment allows for better contact with less reaming although it is not fully supported when compared with a non-augmented baseplate. Bone grafts can function similarly, but the autograft humeral head is not constantly present in revision situations and requires increased operative time to allow for precision carpentry. Additionally, the success of BIO-RSA requires healing of bone graft on the native glenoid to support the baseplate.19 Jones and colleagues9 compared metal augmented RTSA with BIO-RSA and presented equivalent results.
To minimize reaming and to obtain appropriately inferior inclination, we have discovered preoperative templating and intraoperative, computer-guided glenoid preparation to be extremely valuable (ExactechGPS). These tools allow appropriate assessment of augments and for minimal bone removal when preparing the glenoid.
TECHNIQUE
When using an augment, a fine-cut CT scan is highly recommended to aid in surgery planning. We also find 3-D reconstructions to be helpful. Preoperative planning software also allows surgeons to maximize fixation of implant within the glenoid vault. The starting point for reaming is planned based on CT. Some surgeons using augments perform minimal or no reaming at all, electing to remove the remaining cartilage with a Cobb elevator. Different reaming and drilling axes are used when using augments. In cases of severe glenoid deformity and unavailability of computer assistance, a guide wire with inferior inclination can be installed based on CT scan. Penetration of this wire down the glenoid neck can be palpated and compared with the preoperative plan. We generally prefer at least 24 mm of bone containment for the central cage. Once the surgeon is satisfied with the placement of the wire, the appropriate augment guide is placed, followed by a second guide wire. This second wire acts as the reaming axis. The first wire is removed, and the glenoid is reamed with a cannulated reamer. Once reaming is completed, the original wire is replaced in the same hole and trajectory, and the reaming wire is removed. The first wire is then drilled with a cannulated drill for the central cage. The augmented baseplate is then impacted into place, and screw fixation is performed. Again, intraoperative computer guidance allows for precision screw placement with maximal bone attachment.
Table 2. Results of Reverse Total Shoulder Arthroplasty Augmented Baseplates
| Augment | American Shoulder and Elbow Surgeons Score | Constant Score | Active Forward Flexion | Active External Rotation | ||||||||||||||||
Superior (N = 22) |
|
|
|
|
| |||||||||||||||
Posterior (N = 50) |
|
|
|
|
| |||||||||||||||
Posterosuperior (N = 67) |
|
|
|
|
|
RESULTS
Based on our experience, glenoid augments for RTSA have performed well at short- and mid-term follow-up. From October 2011 to July 2016, 139 patients undergoing RTSA received a posterior, superior, or posterior-superior augmented glenoid baseplate. All groups demonstrated improvements in functional outcome measures, including Constant, ASES, Shoulder Pain and Disability Index, and Simple Shoulder Test scores compared with baseline values (Table 2). The posterior-superior augment group experienced the most significant improvement in active forward flexion and external rotation, whereas the posterior augment group experienced the most significant improvement in ASES and Constant scores. Figures 4A-4C displays the radiographs of a patient with significant glenoid wear treated with a posterior-superior augment RTSA.

Continue to: COMPLICATIONS
COMPLICATIONS
In the superior augment group, 3 patients (13%) sustained 5 complications. One patient sustained 3 separate episodes of instability, eventually requiring revision of prosthesis. In the posterior augment group, 4 patients (8%) sustained complications. Two of the 4 patients presented postoperative humeral fractures related to traumatic events, whereas another patient sustained an intraoperative tuberosity fracture. The last complication in this group involved a postoperative draining wound that was treated with oral antibiotics.
Nine complications developed in the posterior-superior augment group (13%); these complications included aseptic baseplate loosening (5), glenoid fracture (1), humeral fracture (1), acromial stress fracture (1), and cerebrovascular accident (1).
DISCUSSION
As the use of augments in RTSA is relatively new, significantly scarce data exist regarding their outcomes and longevity. A few studies have focused on the short-term outcomes of these augments. Jones and colleagues9 performed a retrospective review of 80 patients who underwent RTSA and required either a structural bone graft or an augmented glenoid baseplate.9 They observed that although all patients showed improvements in pain, range of motion, and functional scores, the structural bone graft group incurred a 14.6% complication rate compared with none observed in the augment group. Additionally, Jones and colleagues9 noted that the augmented baseplate group exhibited a significantly lower rate of scapular notching compared with the bone-graft group (10% vs 18.5%) at similar follow-up intervals. A separate study by Wright and colleagues18 compared posterior vs superior augmented baseplates in RTSA. The posterior augment group demonstrated lower rates of scapular notching (6.3% vs 14.3%) and showed more significant improvements in Constant, ASES, and active forward elevation measures, compared with the superior augment group.
As more manufacturers develop augments for RTSA, and as ExactechGPS uses become more widespread, the use of RTSA baseplate augments will continually grow. Custom implants using massive metal augments are now also being introduced. Although currently too expensive for most cases, as technology drives the cost down, every patient may receive customized augmented implants in the future.
The advantages of augmented baseplate designs include minimized reaming and notching, improved tension of the remaining rotator cuff, and decreased operating room time. The disadvantages include increased cost and lack of mid- or long-term clinical data. The concerns with baseplate loosening with augments in RTSA are much less than those with augments for aTSA due to the outstanding baseplate fixation that can be achieved in RTSA.
Continue to: CONLCLUSION
CONCLUSION
Augments offer an excellent tool for surgeons performing both aTSA and RTSA with glenoid bone loss. Use of augments will become more common as more manufacturers develop them. Although clinical results fall short in full midterm, they have been positive for both augmented RTSA and aTSA. Concerns arise when performing augmented aTSA, as an upper limit of correction has not been defined with regard to component failure. Currently, no data support the maximum amount of correction that can be achieved. In our current practice, we face difficulty in correcting more than 25° of version in young active patients with aTSA augment. Beyond this point, we perform a RTSA with an augment. In older patients or low-demand patients, we only correct minor deformities (<20°) with an aTSA augment, opting instead for an augmented RTSA due to the lower midterm failure rates observed with this implant.
ABSTRACT
Glenoid bone deficiency in the setting of shoulder replacement surgery is far more common than originally reported. The frequency and severity of the glenoid defects are noted to be more common and severe with the advent of computer-assisted surgery. The results of an anatomic total shoulder arthroplasty (aTSA) with glenoid deficiency have been reported to be inferior to aTSA patients without a glenoid deficiency. Options for treating the glenoid deficiency include eccentric reaming, bone grafting, and the use of augmented glenoid components. The purpose of this article is to present the indications, technique, and results of augmented glenoids for both aTSA and reverse TSA (RTSA).
Augments for both aTSA and RTSA are viable options. They preserve subchondral bone at the same time as optimizing the joint line without the need for bone grafts. Complications, revisions and results are as good as compared to shoulder arthroplasties without glenoid wear.
Continue to: Glenoid bone deficiency...
Glenoid bone deficiency in arthritic or cuff-deficient shoulder has been reported in up to 50% of shoulder defect cases.1,2 The type and severity of glenoid deformities vary depending on the underlying pathology and time of manifestation. Osteoarthritis with bone loss typically results in posterior or posterior inferior glenoid wear and is commonly classified as Walch types B1 or B2 (biconcave). In cases of severe erosion, B3 classification has been proposed; in this classification, bone loss becomes extremely severe, progressing to resemble a type C glenoid. Unlike primary osteoarthritis, inflammatory arthropathy more commonly causes central loss of glenoid bone (Walch A2). With the rotator cuff insufficiency, superior migration of the humeral head occurs. As these conditions progress, cuff tear arthropathy (CTA) changes result in superior or posterior-superior bone loss.1 Anterior bone loss (type D) will be rarely encountered due to recurrent anterior instability.3
Classically, with anatomic total shoulder arthroplasty (aTSA), the surgeon considers several options for managing glenoid deficiencies. The most commonly employed technique involves eccentrically reaming the glenoid and correcting the deformity. This procedure is relatively easy but features significant drawbacks, such as sacrificing the subchondral bone, medializing the glenohumeral joint line, and secondarily shrinking the glenoid surface area. Other options include structural bone grafting behind the glenoid component. Most anatomic prosthetic glenoids prove to be unsuitable for fixation of structural bone graft. Therefore, the graft is first internally fixed, followed by placement of the glenoid component. Cement, which is commonly used for glenoid fixation, may potentially inhibit bone-graft healing. Reports using this technique documented high radiographic failure rate of up to 40% at midterm follow-up.4 Although leaving the glenoid component retroverted may be considered, surgeons should develop awareness of the possibility of peg penetration of the anterior glenoid neck. Additionally, retroversion in excess of 5°may increase the risk of recurrent posterior subluxation, resulting in early glenoid loosening.5-7 Results of aTSA under significant glenoid deficiency are inferior to those of aTSA patients without glenoid deficiency.8 Such findings have been extremely inferior in patients with significant glenoid wear, prompting numerous surgeons to abandon aTSA in this population in favor of reverse TSA (RTSA) due to improved bony fixation.
In 2010, augmented anatomic glenoids were first introduced as a wedge (Exactech) and as a step shortly thereafter (DePuy Synthes; Figures 1A-1C). More recently, hemi-wedges have been introduced (Wright Medical Group). Augments have gained popularity due to improved range of motion vs reverse shoulder arthroplasty (RSA). However, debates remain regarding the use of posteriorly augmented components in the setting of posterior glenoid bone loss.8 Augments serve as another viable option for handling glenoid bone deficiency in aTSA.

Glenoid bone loss in RTSA presents similar options to aTSA. However, screw fixation of the glenoid component offers several distinct advantages. Baseplate fixation can readily be used with bone grafting and with a highly anticipated success rate. With multiple screw options, 100% support of the baseplate is not mandatory. Although bony increase offset RSAs (BIO-RSAs) have shown success, augmentation with allograft or autograft increases operative time and relies on osseous integration for long-term implant success.9 Metal augmented baseplates were first introduced in 2011 (Exactech) as a means of managing glenoid bone loss without structural grafting. Although initial results have been encouraging, additional studies are needed to assess the longevity of these implants (Figures 1A-1C).
aTSA AUGMENTS
aTSA augments were introduced as a means of correcting acquired glenoid bone deficiency, restoring native glenoid version, correcting humeral subluxation, and preserving the native subchondral bone. Compared with glenoid bone grafting, augmented glenoid components decrease operative time, allow for a technically easier operation, and require no bone healing for clinical success. Early and midterm results are encouraging, showing similar findings comparable to those of aTSA in non-glenoid deficient shoulders.10-12
Continue to: INDICATIONS
INDICATIONS
Indications and limitations for augmented aTSA glenoids remain incompletely defined. The most common indication for an augmented aTSA is osteoarthritis with a B2 glenoid. We recommend augments in the occurrence of any indication of significant eccentric glenoid wear. With the expertise of surgeons, deformities of up to 20° to 25° of deformity can be readily handled with good predictability. More severe deformities can be managed with augmented aTSA components, but early failure rates may be high. The most severe acquired deformities remain best managed with RTSA. Currently, we prefer RTSA when glenoid bone loss exceeds 25°. With the widespread availability of computed tomography (CT) scans with 3-dimensional (3-D) reconstruction, glenoid bone defects are increasingly recognized. When correcting deformity, surgeons should strive to limit residual retroversion to a maximum of 5°.13 Preoperative planning software and computer-assisted surgery (ExactechGPS) may allow surgeons to better define the limits of augmented glenoid fixation prior to the date of surgery. We routinely utilize computer-guided glenoid preparation to control glenoid version to within 5° of neutral position.
The differences between B3 and a true type C glenoid must be recognized. Although B3 glenoids may still be a candidate for an augmented anatomic glenoid component, type C glenoids are not. Developmental abnormalities of type C glenoid occur simultaneously with humeral deformities, including medialized posterior rotator cuff musculature. Correction of the joint line to neutral version may not replicate the non-diseased state of a dysplastic type shoulder. Davis and colleagues14 have proposed treating these patients by leaving both the humerus and glenoid in their native version without correction.
TECHNIQUE
The implant that we have the most experience with is an 8° full-wedge augmented glenoid component. Such an implant is typically utilized for B2 glenoids. We recommend that a high-quality CT scan be performed for preoperative planning. As a general rule, the starting point often lies close to the ridge of B2 glenoid and more anterior than the apparent glenoid center, which is viewed intraoperatively due to asymmetric posterior wear. Full-wedge component is utilized to ream the ridge separating the neo and paleoglenoids to create a flat surface. This condition is best achieved by drilling a pilot hole at the planned glenoid central peg position to prevent the reamer from sliding anteriorly during reaming. Glenoid preparation begins with the smallest reamer until the ridge has been flattened, and the reamer makes full contact with the glenoid. The reamer diameter is then increased based on glenoid size. Slightly downsizing the glenoid implant will require less reaming to achieve full backside support. Once the glenoid is properly reamed, the central and peripheral peg holes are drilled using the appropriate guides. Holes are then dried, and all-polyethylene or composite glenoid component (either partially or completely cemented) is installed using favored cementing techniques. The advantage of composite glenoid component is that the central cage allows for bone ingrowth and may potentially improve long-term implant survival. Press fit of the central cage requires no waiting time for glenoid cement hardening before proceeding to the humerus. When placing an augmented component, adequate glenoid exposure is imperative to allow in-line placement and appropriate seating of the component without impingement on adjacent retractors.
When using the step-augmented glenoid, the paleoglenoid is prepared in a similar fashion to a standard aTSA. Once the paleoglenoid has been reamed to a neutral position, a protector plate is placed onto the paleoglenoid. and a step-cut saw is used to prepare the posterior stepped bone cut. Peripheral pegs are then drilled, and the component is installed in routine fashion. When using hemi-wedge augments, the paleoglenoid is again prepared in a similar fashion as a standard glenoid component over a cannulated guidewire. The neoglenoid is subsequently prepared using a specialized angled reamer with a positive stop to prevent over-reaming. These glenoid implants improve rotational force neutralization given the absence of flat back against the glenoid. All 3 designs preserve bone when compared with eccentric reaming alone,15 with the half-augmented wedge preserving the most bone.
Table 1. Results of Various Augmented Glenoid Components in Anatomic Total Shoulder
Arthroplasty
| Augment | American Shoulder and Elbow Surgeons Score | Constant Score | Active Forward Flexion | Active External Rotation | ||||||||||||||||
8° cage (N = 21) |
|
|
|
|
| |||||||||||||||
All-polyethylene 8° (N = 45) |
|
|
|
|
| |||||||||||||||
All-polyethylene 16° (N = 7) |
|
|
|
|
|
RESULTS
In our institution, we first used all-polyethylene posteriorly augmented glenoid components in 2010. Between 2010 and 2015, 45 patients received an 8° all-polyethylene posterior augment, and 7 patients received a 16° augment. In 2015, we transitioned to the composite caged posterior augment. All patients in our database who received an augmented glenoid component experienced improvement in active forward elevation, external rotation, American Shoulder and Elbow Surgeons (ASES), and Constant scores (Table 1). Minimum follow-up was 1 year for patients receiving both an 8° cage (mean, 1.48 years) and an 8° all-polyethylene augment (mean, 3.18 years). Figures 2A-2C show a patient with significant posterior glenoid wear and humeral head subluxation treated with an 8° wedge composite posterior augment glenoid 3 years postoperative.

Continue to: COMPLICATIONS
COMPLICATIONS
Two complications developed in the group undergoing composite cage augment. One patient experienced glenoid loosening after a motor vehicle accident. Another patient sustained significant intraoperative tuberosity avulsion during implantation of the humeral component, requiring a change of implant and tuberosity fixation. Although no complications were noted in the 8° all-polyethylene group, 3 patients in the 16° augment group sustained complications. One of these patients suffered a cardiac event that was unrelated to the implant. Two complications in this group were both related to loosening of the glenoid component, requiring subsequent revision.
DISCUSSION
The first report on augmented aTSA was published in 2008, and it involved a 5° augmented, anatomic glenoid.12 One study was based on a small series of augments; the poor results led the reporting surgeons to subsequently abandon the implant.12 This early design produced a correction on the articular side of the implant rather than the pathologic bony side. By performing such correction, the component pegs remained anteriorly oriented, placing the component at risk of perforation through the anterior glenoid neck. All current augment designs feature pegs that are oriented down the glenoid vault, with corrections occurring on the bony surface. This condition requires 2 different axes for reaming the glenoid and drilling the pegs. This approach allows the pegs to be directed down the glenoid neck, and is a far superior solution to neutralizing shear forces when compared with the implants used in the 1990s.
Early to midterm results of modern aTSA augments have been extremely encouraging with low revision rates. The main concern of recurrent posterior subluxation has been rarely reported. The concerns over glenoid loosening due to high shear forces, similarly, have not been described to date. However, surgeons should remain cautious, as longer-term follow-up remains unavailable.
The main advantage of aTSA augments is their capacity to preserve bone compared with eccentric reaming and better long-term stability. Each of the augment designs requires varying amounts of bone removal. Through biomechanics and using finite element analysis, the 3 augment types act differently, with no design demonstrating remarkable biomechanical superiority.6 Favorito and colleagues16 performed a retrospective review of 22 patients who underwent aTSA using an all-polyethylene, posteriorly augmented, and stepped glenoid component for posterior bone loss. At an average follow-up of 36 months, all patients experienced improvements in active forward elevation, external rotation, visual analog scale, Short Form-36 Physical Component Summary, and Western Ontario Osteoarthritis of the Shoulder scores. The authors noted that 2 patients (9%) experienced complications: 1 with an anterior dislocation and the other with recurrent posterior instability requiring revision. Sandow and Schutz17 reported the preliminary results of 10 patients who underwent aTSA using trabecular metal augment with a minimum of 2-year follow-up. All patients received either a 15° or 30° posterior, metal-backed augment for severe glenoid bone loss (Walch grade B2 or C). At a minimum of 2-year follow-up, all patients received correction to within 10° of neutral glenoid version, without any complications nor implant failures.
Regardless of augment design, all current components restore the native glenoid version, improving the length and subsequent tension of rotator cuff musculature. Similarly, re-centering the humeral head decreases the forces on the glenoid and allows for optimal function with decreasing loss of vital subchondral bone.
Continue to: RTSA AUGMENTS
RTSA AUGMENTS
Similar to anatomic augments, metal augments were introduced for use with RTSA in 2011. Unlike anatomic augments, those for RTSA were manufactured with metal. Given the difference in bony wear patterns in patients requiring RTSA, augments were available in a number of configurations. With CTA, wear is most commonly superior. Leaving a superiorly inclined baseplate must be avoided due to risks of notching, loosening, and early failure. However, correcting this tilt will require significant reaming of the inferior glenoid. A superior augment is ideally suited for this bone-loss pattern. If the glenoid is retroverted significantly, difficulty can also arise during glenoid preparation and baseplate placement. Posterior augments may ease this aspect of the procedure. Posterior augments feature the additional benefits of tensioning any remaining posterior rotator cuff, minimizing posterior inferior impingement, and technically easing the operation.18 As we improve our awareness of glenoid orientation using computer navigation, a posterior-superior augmented implant is commonly needed to simultaneously optimize the baseplate position and to minimize reaming (Figure 3). The posterior-superior augmented baseplate has become the most commonly used baseplate augment of choice in 90% of our RTSA cases that require an augment.

INDICATIONS
Augmented RTSA baseplates are indicated when adequate backside contact cannot be achieved with eccentric reaming, thus compromising potential fixation. In our practice, we preferably use augments at <50% contact with the backside of the baseplate. Excessive superior inclination is observed in a CTA setting, commonly indicating the use of superior augments. Similarly, severe primary osteoarthritis may contain elements of posterior bone loss, leading to increased retroversion, which is where we use posterior augments. When patients exhibit combined deformities, or when the surgeon wishes to tension the posterior rotator cuff, a posterior-superior augmented glenoid baseplate is used. For extremely severe defects, we have combined bone grafting and augments. In patients with a highly deficient glenoid but good quality of the remaining bone stock, an augment allows for better contact with less reaming although it is not fully supported when compared with a non-augmented baseplate. Bone grafts can function similarly, but the autograft humeral head is not constantly present in revision situations and requires increased operative time to allow for precision carpentry. Additionally, the success of BIO-RSA requires healing of bone graft on the native glenoid to support the baseplate.19 Jones and colleagues9 compared metal augmented RTSA with BIO-RSA and presented equivalent results.
To minimize reaming and to obtain appropriately inferior inclination, we have discovered preoperative templating and intraoperative, computer-guided glenoid preparation to be extremely valuable (ExactechGPS). These tools allow appropriate assessment of augments and for minimal bone removal when preparing the glenoid.
TECHNIQUE
When using an augment, a fine-cut CT scan is highly recommended to aid in surgery planning. We also find 3-D reconstructions to be helpful. Preoperative planning software also allows surgeons to maximize fixation of implant within the glenoid vault. The starting point for reaming is planned based on CT. Some surgeons using augments perform minimal or no reaming at all, electing to remove the remaining cartilage with a Cobb elevator. Different reaming and drilling axes are used when using augments. In cases of severe glenoid deformity and unavailability of computer assistance, a guide wire with inferior inclination can be installed based on CT scan. Penetration of this wire down the glenoid neck can be palpated and compared with the preoperative plan. We generally prefer at least 24 mm of bone containment for the central cage. Once the surgeon is satisfied with the placement of the wire, the appropriate augment guide is placed, followed by a second guide wire. This second wire acts as the reaming axis. The first wire is removed, and the glenoid is reamed with a cannulated reamer. Once reaming is completed, the original wire is replaced in the same hole and trajectory, and the reaming wire is removed. The first wire is then drilled with a cannulated drill for the central cage. The augmented baseplate is then impacted into place, and screw fixation is performed. Again, intraoperative computer guidance allows for precision screw placement with maximal bone attachment.
Table 2. Results of Reverse Total Shoulder Arthroplasty Augmented Baseplates
| Augment | American Shoulder and Elbow Surgeons Score | Constant Score | Active Forward Flexion | Active External Rotation | ||||||||||||||||
Superior (N = 22) |
|
|
|
|
| |||||||||||||||
Posterior (N = 50) |
|
|
|
|
| |||||||||||||||
Posterosuperior (N = 67) |
|
|
|
|
|
RESULTS
Based on our experience, glenoid augments for RTSA have performed well at short- and mid-term follow-up. From October 2011 to July 2016, 139 patients undergoing RTSA received a posterior, superior, or posterior-superior augmented glenoid baseplate. All groups demonstrated improvements in functional outcome measures, including Constant, ASES, Shoulder Pain and Disability Index, and Simple Shoulder Test scores compared with baseline values (Table 2). The posterior-superior augment group experienced the most significant improvement in active forward flexion and external rotation, whereas the posterior augment group experienced the most significant improvement in ASES and Constant scores. Figures 4A-4C displays the radiographs of a patient with significant glenoid wear treated with a posterior-superior augment RTSA.

Continue to: COMPLICATIONS
COMPLICATIONS
In the superior augment group, 3 patients (13%) sustained 5 complications. One patient sustained 3 separate episodes of instability, eventually requiring revision of prosthesis. In the posterior augment group, 4 patients (8%) sustained complications. Two of the 4 patients presented postoperative humeral fractures related to traumatic events, whereas another patient sustained an intraoperative tuberosity fracture. The last complication in this group involved a postoperative draining wound that was treated with oral antibiotics.
Nine complications developed in the posterior-superior augment group (13%); these complications included aseptic baseplate loosening (5), glenoid fracture (1), humeral fracture (1), acromial stress fracture (1), and cerebrovascular accident (1).
DISCUSSION
As the use of augments in RTSA is relatively new, significantly scarce data exist regarding their outcomes and longevity. A few studies have focused on the short-term outcomes of these augments. Jones and colleagues9 performed a retrospective review of 80 patients who underwent RTSA and required either a structural bone graft or an augmented glenoid baseplate.9 They observed that although all patients showed improvements in pain, range of motion, and functional scores, the structural bone graft group incurred a 14.6% complication rate compared with none observed in the augment group. Additionally, Jones and colleagues9 noted that the augmented baseplate group exhibited a significantly lower rate of scapular notching compared with the bone-graft group (10% vs 18.5%) at similar follow-up intervals. A separate study by Wright and colleagues18 compared posterior vs superior augmented baseplates in RTSA. The posterior augment group demonstrated lower rates of scapular notching (6.3% vs 14.3%) and showed more significant improvements in Constant, ASES, and active forward elevation measures, compared with the superior augment group.
As more manufacturers develop augments for RTSA, and as ExactechGPS uses become more widespread, the use of RTSA baseplate augments will continually grow. Custom implants using massive metal augments are now also being introduced. Although currently too expensive for most cases, as technology drives the cost down, every patient may receive customized augmented implants in the future.
The advantages of augmented baseplate designs include minimized reaming and notching, improved tension of the remaining rotator cuff, and decreased operating room time. The disadvantages include increased cost and lack of mid- or long-term clinical data. The concerns with baseplate loosening with augments in RTSA are much less than those with augments for aTSA due to the outstanding baseplate fixation that can be achieved in RTSA.
Continue to: CONLCLUSION
CONCLUSION
Augments offer an excellent tool for surgeons performing both aTSA and RTSA with glenoid bone loss. Use of augments will become more common as more manufacturers develop them. Although clinical results fall short in full midterm, they have been positive for both augmented RTSA and aTSA. Concerns arise when performing augmented aTSA, as an upper limit of correction has not been defined with regard to component failure. Currently, no data support the maximum amount of correction that can be achieved. In our current practice, we face difficulty in correcting more than 25° of version in young active patients with aTSA augment. Beyond this point, we perform a RTSA with an augment. In older patients or low-demand patients, we only correct minor deformities (<20°) with an aTSA augment, opting instead for an augmented RTSA due to the lower midterm failure rates observed with this implant.
1. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. J Bone Joint Surg Br. 2004;86(3):388-395. doi:10.1302/0301-620X.86B3.
2. Churchill RS, Spencer Jr EE, Fehringer EV. Quantification of B2 glenoid morphology in total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(8):1212-1217. doi:10.1016/j.jse.2015.01.007.
3. Bercik MJ, Kruse K, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-1606. doi:10.1016/j.jse.2016.03.010.
4. Klika BJ, Wooten CW, Sperling JW, et al. Structural bone grafting for glenoid deficiency in primary total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):1066-1072. doi:10.1016/j.jse.2013.09.017.
5. Franklin JL, Barrett WP, Jackins SE, Matsen FA 3rd. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3(1):39-46.
6. Hermida JC, Flores-Hernandez C, Hoenecke HR, D’Lima DD. Augmented wedge-shaped glenoid component for the correction of glenoid retroversion: a finite element analysis. J Shoulder Elbow Surg. 2014;23(3):347-354. doi:10.1016/j.jse.2013.06.008.
7. Ho JC, Sabesan VJ, Iannotti JP. Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg Am. 2013;95(12):e82. doi:10.2106/JBJS.L.00336.
8. Denard PJ, Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22(11):1589-1598. doi:10.1016/j.jse.2013.06.017.
9. Jones RB, Wright TW, Roche CP. Bone grafting the glenoid versus use of augmented glenoid baseplates with reverse shoulder arthroplasty. Bull Hosp Jt Dis (2013). 2015;73(suppl 1):S129-S135.
10. Hsu JE, Ricchetti ET, Huffman GR, Iannotti JP, Glaser DL. Addressing glenoid bone deficiency and asymmetric posterior erosion in shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(9):1298-1308. doi:10.1016/j.jse.2013.04.014.
11. Cil A, Sperling JW, Cofield RH. Nonstandard glenoid components for bone deficiencies in shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):e149-e157. doi:10.1016/j.jse.2013.09.023.
12. Rice RS, Sperling JW, Miletti J, Schleck C, Cofield RH. Augmented glenoid component for bone deficiency in shoulder arthroplasty. Clin Orthop Relat Res. 2008;466(3):579-583. doi:10.1007/s11999-007-0104-4.
13. Sabesan V, Callanan M, Sharma V, Iannotti JP. Correction of acquired glenoid bone loss in osteoarthritis with a standard versus an augmented glenoid component. J Shoulder Elbow Surg. 2014;23(7):964-973. doi:10.1016/j.jse.2013.09.019.
14. Davis DE, Acevedo D, Williams A, Williams G. Total shoulder arthroplasty using an inlay mini-glenoid component for glenoid deficiency: a 2-year follow-up of 9 shoulders in 7 patients. J Shoulder Elbow Surg. 2016;25(8):1354-1361. doi:10.1016/j.jse.2015.12.010.
15. Kersten AD, Flores-Hernandez C, Hoenecke HR, D'Lima DD. Posterior augmented glenoid designs preserve more bone in biconcave glenoids. J Shoulder Elbow Surg. 2015;24(7):1135-1141. doi:10.1016/j.jse.2014.12.007.
16. Favorito PJ, Freed RJ, Passanise AM, Brown MJ. Total shoulder arthroplasty for glenohumeral arthritis associated with posterior glenoid bone loss: results of an all-polyethylene, posteriorly augmented glenoid component. J Shoulder Elbow Surg. 2016;25(10):1681-1689. doi:10.1016/j.jse.2016.02.020.
17. Sandow M, Schutz C. Total shoulder arthroplasty using trabecular metal augments to address glenoid retroversion: the preliminary result of 10 patients with minimum 2-year follow-up. J Shoulder Elbow Surg. 2016;25(4):598-607. doi:10.1016/j.jse.2016.01.001.
18. Wright TW, Roche CP, Wright L, Flurin PH, Crosby LA, Zuckerman JD. Reverse shoulder arthroplasty augments for glenoid wear: A comparison of posterior augments to superior augments. Bull Hosp Jt Dis. 2015;73(suppl 1):S124-S128.
19. Boileau P, Morin-Salvo N, Gauci MO, et al. Angled BIO-RSA (bony-increased offset-reverse shoulder arthroplasty): a solution for the management glenoid bone loss and erosion. J Shoulder Elbow Surg. 2017;26(12):2133-2142. doi:10.1016/j.jse.2017.05.024.
1. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. J Bone Joint Surg Br. 2004;86(3):388-395. doi:10.1302/0301-620X.86B3.
2. Churchill RS, Spencer Jr EE, Fehringer EV. Quantification of B2 glenoid morphology in total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(8):1212-1217. doi:10.1016/j.jse.2015.01.007.
3. Bercik MJ, Kruse K, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-1606. doi:10.1016/j.jse.2016.03.010.
4. Klika BJ, Wooten CW, Sperling JW, et al. Structural bone grafting for glenoid deficiency in primary total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):1066-1072. doi:10.1016/j.jse.2013.09.017.
5. Franklin JL, Barrett WP, Jackins SE, Matsen FA 3rd. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3(1):39-46.
6. Hermida JC, Flores-Hernandez C, Hoenecke HR, D’Lima DD. Augmented wedge-shaped glenoid component for the correction of glenoid retroversion: a finite element analysis. J Shoulder Elbow Surg. 2014;23(3):347-354. doi:10.1016/j.jse.2013.06.008.
7. Ho JC, Sabesan VJ, Iannotti JP. Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg Am. 2013;95(12):e82. doi:10.2106/JBJS.L.00336.
8. Denard PJ, Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22(11):1589-1598. doi:10.1016/j.jse.2013.06.017.
9. Jones RB, Wright TW, Roche CP. Bone grafting the glenoid versus use of augmented glenoid baseplates with reverse shoulder arthroplasty. Bull Hosp Jt Dis (2013). 2015;73(suppl 1):S129-S135.
10. Hsu JE, Ricchetti ET, Huffman GR, Iannotti JP, Glaser DL. Addressing glenoid bone deficiency and asymmetric posterior erosion in shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(9):1298-1308. doi:10.1016/j.jse.2013.04.014.
11. Cil A, Sperling JW, Cofield RH. Nonstandard glenoid components for bone deficiencies in shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):e149-e157. doi:10.1016/j.jse.2013.09.023.
12. Rice RS, Sperling JW, Miletti J, Schleck C, Cofield RH. Augmented glenoid component for bone deficiency in shoulder arthroplasty. Clin Orthop Relat Res. 2008;466(3):579-583. doi:10.1007/s11999-007-0104-4.
13. Sabesan V, Callanan M, Sharma V, Iannotti JP. Correction of acquired glenoid bone loss in osteoarthritis with a standard versus an augmented glenoid component. J Shoulder Elbow Surg. 2014;23(7):964-973. doi:10.1016/j.jse.2013.09.019.
14. Davis DE, Acevedo D, Williams A, Williams G. Total shoulder arthroplasty using an inlay mini-glenoid component for glenoid deficiency: a 2-year follow-up of 9 shoulders in 7 patients. J Shoulder Elbow Surg. 2016;25(8):1354-1361. doi:10.1016/j.jse.2015.12.010.
15. Kersten AD, Flores-Hernandez C, Hoenecke HR, D'Lima DD. Posterior augmented glenoid designs preserve more bone in biconcave glenoids. J Shoulder Elbow Surg. 2015;24(7):1135-1141. doi:10.1016/j.jse.2014.12.007.
16. Favorito PJ, Freed RJ, Passanise AM, Brown MJ. Total shoulder arthroplasty for glenohumeral arthritis associated with posterior glenoid bone loss: results of an all-polyethylene, posteriorly augmented glenoid component. J Shoulder Elbow Surg. 2016;25(10):1681-1689. doi:10.1016/j.jse.2016.02.020.
17. Sandow M, Schutz C. Total shoulder arthroplasty using trabecular metal augments to address glenoid retroversion: the preliminary result of 10 patients with minimum 2-year follow-up. J Shoulder Elbow Surg. 2016;25(4):598-607. doi:10.1016/j.jse.2016.01.001.
18. Wright TW, Roche CP, Wright L, Flurin PH, Crosby LA, Zuckerman JD. Reverse shoulder arthroplasty augments for glenoid wear: A comparison of posterior augments to superior augments. Bull Hosp Jt Dis. 2015;73(suppl 1):S124-S128.
19. Boileau P, Morin-Salvo N, Gauci MO, et al. Angled BIO-RSA (bony-increased offset-reverse shoulder arthroplasty): a solution for the management glenoid bone loss and erosion. J Shoulder Elbow Surg. 2017;26(12):2133-2142. doi:10.1016/j.jse.2017.05.024.
TAKE-HOME POINTS
- Glenoid defects are very common.
- Options for treating glenoid defects include eccentric reaming, bone grafting, and augmented glenoids.
- As computer-assisted surgery use becomes more widespread the use of augments in both TSA and RTSA will become very common.
- Subchondral bone is precious and cannot be replaced once reamed away. Eccentric glenoids introduce a mechanism to minimize reaming and preserve this precious bone.
- On short-term to midterm follow-up augments perform at least as well if not better than non-augmented glenoid components with complication rate and revisions likewise similar.
MDedge Daily News: FDA warns on new compounding woes
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The FDA MedWatch program warns physicians not to use medications compounded by a particular pharmacy, while the agency’s advisers recommend swapping in two new strains to next year’s influenza vaccine. Also, researchers look at aspirin to prevent dementia in women with type 2 diabetes.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The FDA MedWatch program warns physicians not to use medications compounded by a particular pharmacy, while the agency’s advisers recommend swapping in two new strains to next year’s influenza vaccine. Also, researchers look at aspirin to prevent dementia in women with type 2 diabetes.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The FDA MedWatch program warns physicians not to use medications compounded by a particular pharmacy, while the agency’s advisers recommend swapping in two new strains to next year’s influenza vaccine. Also, researchers look at aspirin to prevent dementia in women with type 2 diabetes.
Listen to the MDedge Daily News podcast for all the details on today’s top news.