User login
Emerging Therapies In Psoriasis: A Systematic Review
Psoriasis is a chronic, autoimmune-mediated disease estimated to affect 2.8% of the US population.1 The pathogenesis of psoriasis is thought to involve a complex process triggered by a combination of genetic and environmental factors that induce tumor necrosis factor (TNF) α secretion by keratinocytes, which in turn activates dendritic cells. Activated dendritic cells produce IL-23, leading to helper T cell (TH17) differentiation.2,3 TH17 cells secrete IL-17A, which has been shown to promote psoriatic skin changes.4 Therefore, TNF-α, IL-23, and IL-17A have been recognized as key targets for psoriasis therapy.
The newest biologic agents targeting IL-17–mediated pathways include ixekizumab, brodalumab, and bimekizumab. Secukinumab, the first US Food and Drug Administration (FDA)–approved IL-17 inhibitor, has been available since 2015 and therefore is not included in this review. IL-23 inhibitors that are FDA approved or being evaluated in clinical trials include guselkumab, tildrakizumab, and risankizumab. In addition, certolizumab pegol, a TNF-α inhibitor, is being studied for use in psoriasis.
METHODS
We reviewed the published results of phase 3 clinical trials for ixekizumab, brodalumab, bimekizumab, guselkumab, tildrakizumab, risankizumab, and certolizumab pegol. We performed an English-language literature search (January 1, 2012 to October 15, 2017) of articles indexed for PubMed/MEDLINE using the following combinations of keywords: IL-23 and psoriasis; IL-17 and psoriasis; tumor necrosis factor and psoriasis; [drug name] and psoriasis. If data from phase 3 clinical trials were not yet available, data from phase 2 clinical trials were incorporated in our analysis. We also reviewed citations within articles to identify relevant sources.
RESULTS
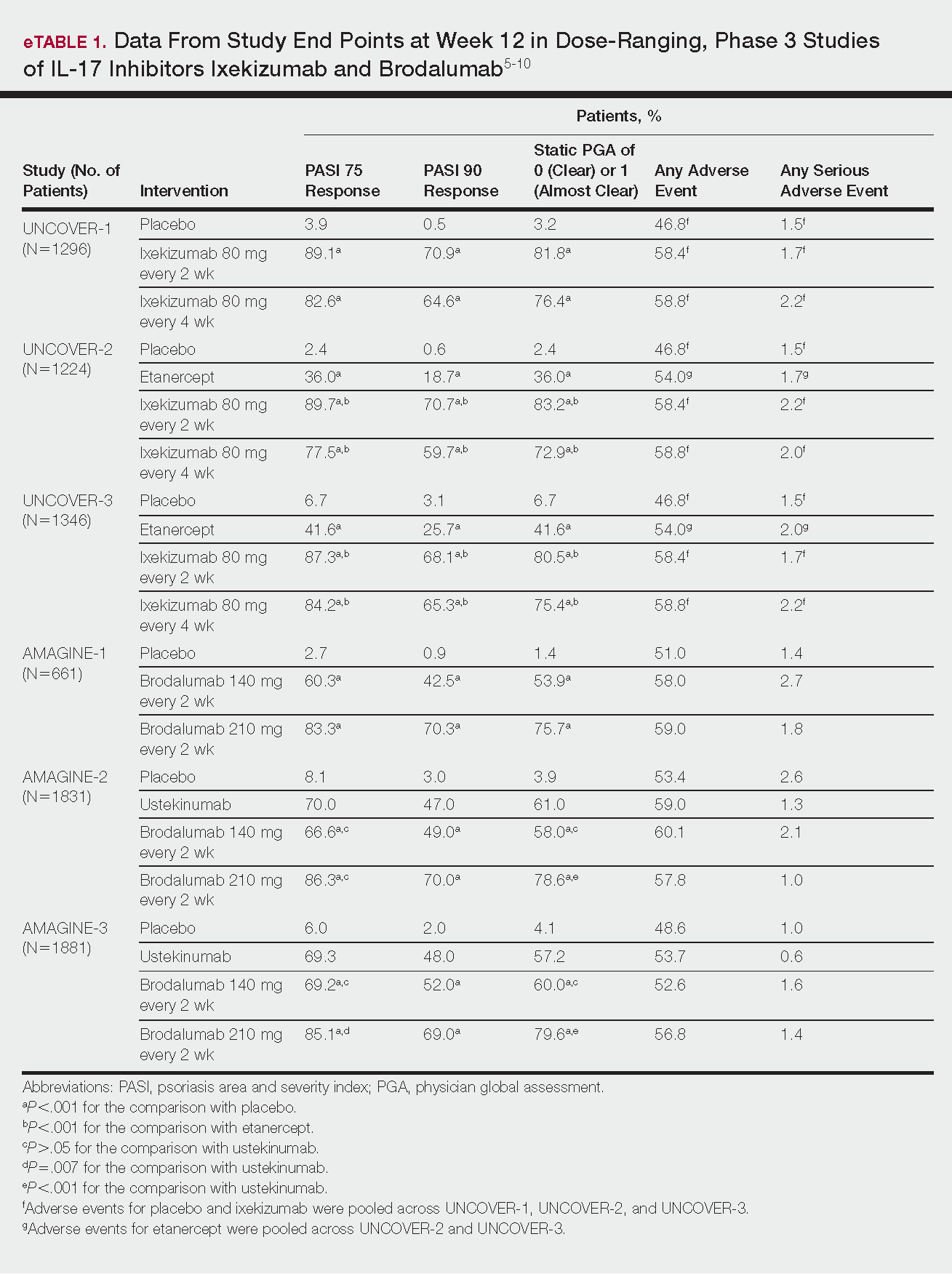
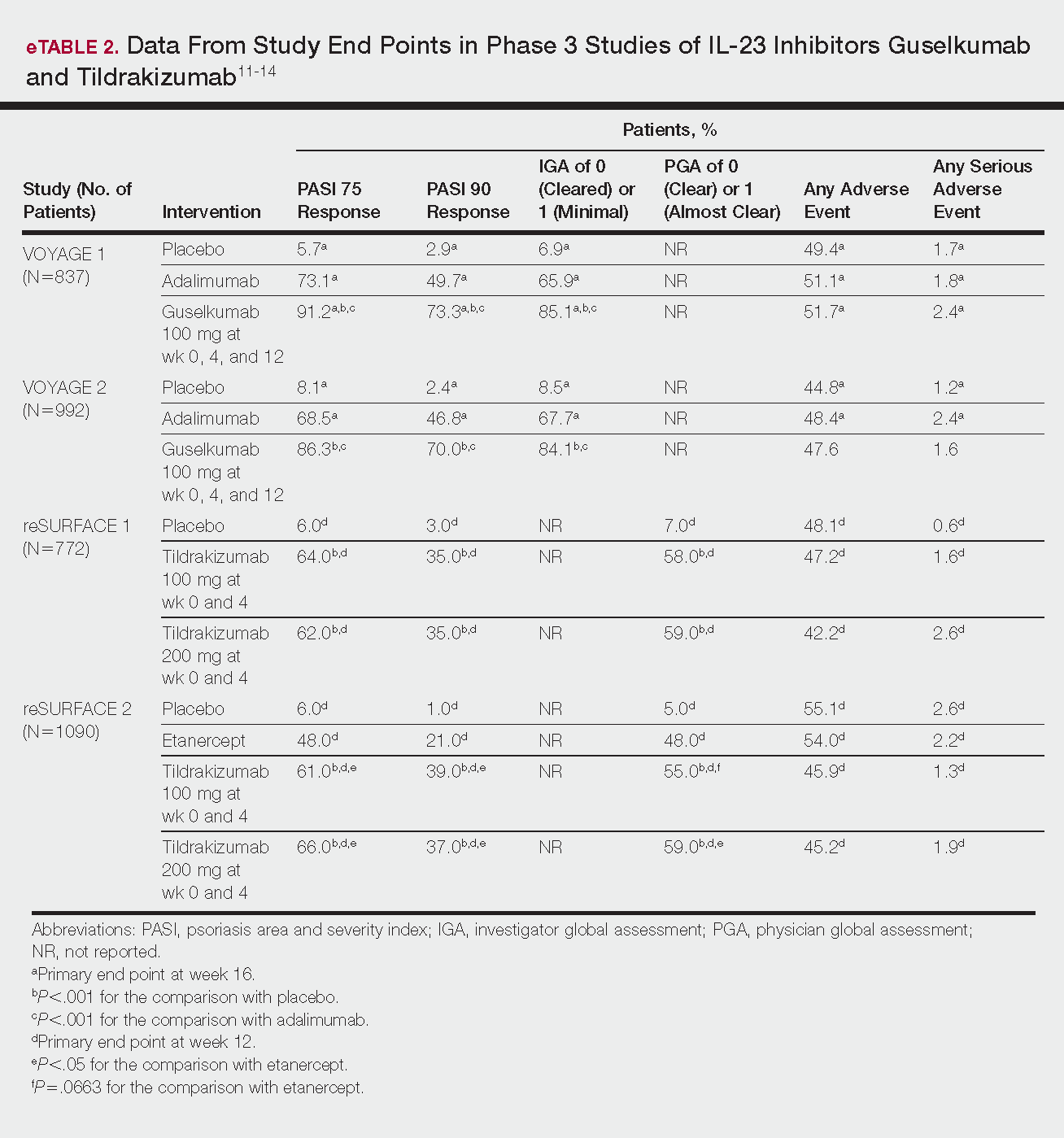
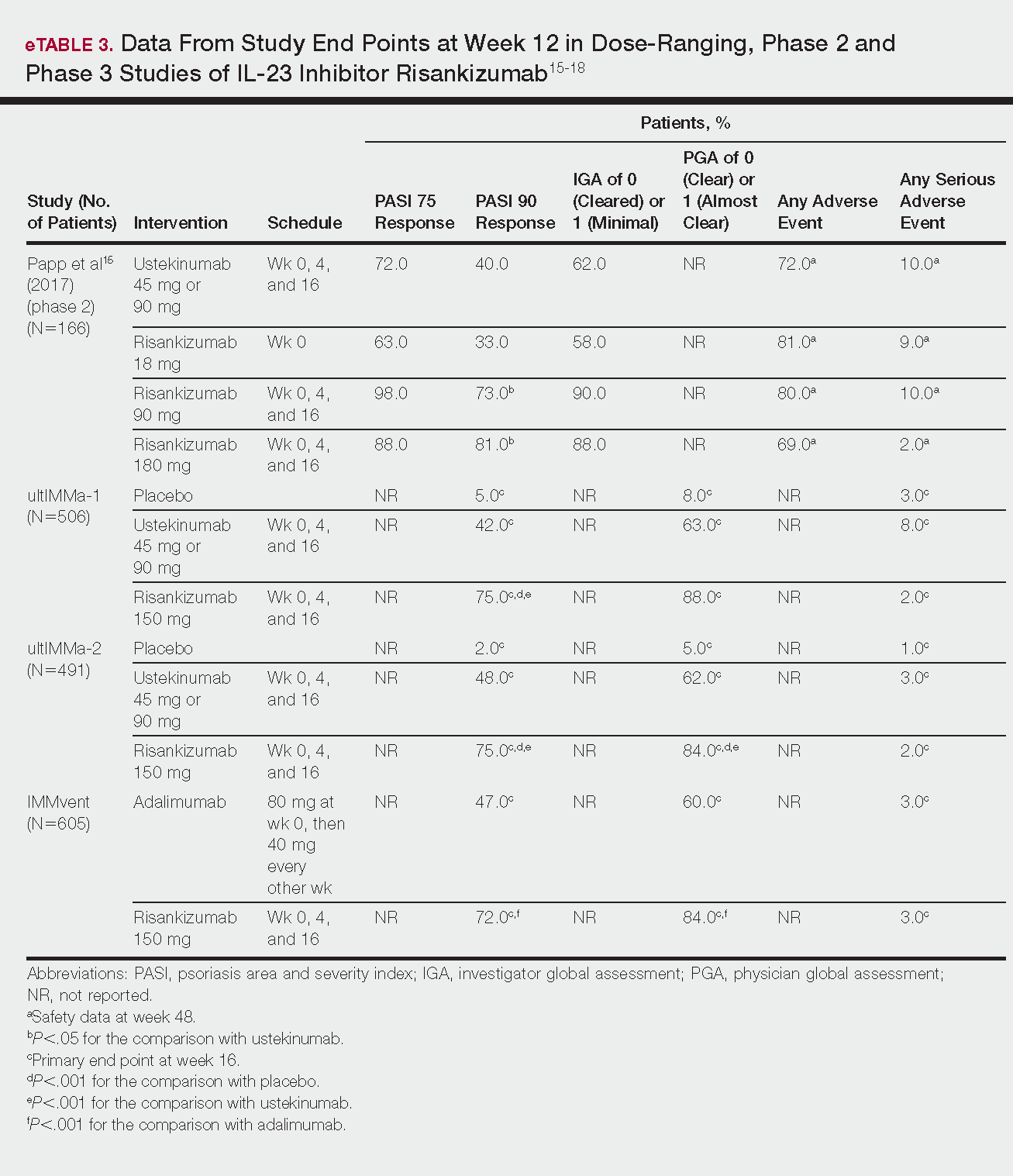
Phase 3 clinical trial design, efficacy, and adverse events (AEs) for ixekizumab and brodalumab are reported in eTable 15-10 and for guselkumab and tildrakizumab in eTable 2.11-14 Phase 2 clinical trial design, efficacy, and AEs are presented for risankizumab in eTable 315-18 and for certolizumab pegol in eTable 4.17,19 No published clinical trial data were found for bimekizumab.
IL-17 Inhibitors
Ixekizumab
This recombinant, high-affinity IgG4κ antibody selectively binds and neutralizes IL-17A.5,6 Three phase 3 clinical trials—UNCOVER-1, UNCOVER-2, and UNCOVER-3—evaluated ixekizumab for moderate to severe plaque psoriasis.7
The 3 UNCOVER trials were randomized, double-blind, phase 3 trials of 1296, 1224, and 1346 patients, respectively, assigned to a placebo group; a group treated with ixekizumab 80 mg every 2 weeks; and a group treated with ixekizumab 80 mg every 4 weeks. Both ixekizumab groups received a loading dose of 160 mg at week 0.5,6 UNCOVER-2 and UNCOVER-3 also included a comparator group of patients on etanercept 50 mg.5 Co-primary end points included the percentage of patients reaching a psoriasis area and severity index (PASI) of 75 and with a static physician global assessment (PGA) score of clear (0) or almost clear (1) at week 12.5,6
Ixekizumab achieved greater efficacy than placebo: 89.1%, 89.7%, and 87.3% of patients achieved PASI 75 in the every 2-week dosing group, and 82.6%, 77.5% and 84.2% achieved PASI 75 in the every 4-week dosing group in UNCOVER-1, UNCOVER-2, and UNCOVER-3, respectively (P<.001 for both treatment arms compared to placebo in all trials). The percentage of patients achieving a static PGA score of 0 or 1 also was higher in the ixekizumab groups in the 2-week and 4-week dosing groups in all UNCOVER trials—81.8% and 76.4% in UNCOVER-1, 83.2% and 72.9% in UNCOVER-2, and 80.5% and 75.4% in UNCOVER-3—compared to 3.2%, 2.4%, and 6.7% in the placebo groups of the 3 trials (P<.001 for both ixekizumab groups compared to placebo in all trials).5,6 Ixekizumab also was found to be more effective than etanercept for both co-primary end points in both UNCOVER-2 and UNCOVER-3 (eTable 1).5
Safety data for all UNCOVER trials were pooled and reported.6 At week 12 the rate of at least 1 AE was 58.4% in patients on ixekizumab every 2 weeks and 58.8% in patients on ixekizumab every 4 weeks compared to 54.0% in the etanercept group in UNCOVER-2 and UNCOVER-3 and 46.8% in the placebo group. At week 12, 72 nonfatal serious AEs were reported: 12 in the placebo group, 14 in the etanercept group, 20 in the ixekizumab every 2 weeks group, and 26 in the ixekizumab every 4 weeks group.6
The most common AE across all groups was nasopharyngitis. Overall, infections were more frequent in patients treated with ixekizumab than in patients treated with placebo or etanercept. Specifically, oral candidiasis occurred more frequently in the ixekizumab groups, with a higher rate in the 2-week dosing group than in the 4-week dosing group.6 Two myocardial infarctions (MIs) occurred: 1 in the etanercept group and 1 in the placebo group.5
Brodalumab
This human monoclonal antibody binds to IL-17ra.8,9 Three double-blind, placebo-controlled, phase 3 trials—AMAGINE-1, AMAGINE-2, and AMAGINE-3—evaluated its use for plaque psoriasis.10
In AMAGINE-1 (N=661), patients were randomized to receive brodalumab 140 mg or 210 mg (every 2 weeks for 12 weeks), or placebo.8 In AMAGINE-2 (N=1831) and AMAGINE-3 (N=1881), patients were randomized to receive brodalumab 140 mg or 210 mg (every 2 weeks for 12 weeks), ustekinumab 45 mg or 90 mg by weight (at weeks 0 and 4, then every 12 weeks thereafter), or placebo. In all trials, patients on brodalumab received a dose at week 0 and week 1. Co-primary end points were PASI 75 and a static PGA score of 0 or 1 at 12 weeks compared to placebo and to ustekinumab (in AMAGINE-2 and AMAGINE-3 only).8
At week 12, 83.3%, 86.3%, and 85.1% of patients on brodalumab 210 mg, and 60.3%, 66.6%, and 69.2% of patients on brodalumab 140 mg, achieved PASI 75 in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively, compared to 2.7%, 8.1%, and 6.0% in the placebo groups (P<.001 between both brodalumab groups and placebo in all trials).8 Both brodalumab groups were noninferior but not significantly superior to ustekinumab, which achieved a PASI 75 of 70.0% in AMAGINE-2 and 69.3% in AMAGINE-3. The PASI 90 rate was higher, however, in both brodalumab groups compared to ustekinumab but significance was not reported (eTable 1).9 For both brodalumab groups, significantly more patients achieved a static PGA value of 0 or 1 compared to placebo (P<.001 across all trials). However, only the brodalumab 210-mg group achieved a significantly higher rate of static PGA 0 or 1 compared to ustekinumab in AMAGINE-2 and AMAGINE-3 (P<.001).9
After 12 weeks, the percentage of patients reporting at least 1 AE was 59.0%, 57.8%, and 56.8% in the brodalumab 210-mg group in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively; 58.0%, 60.1%, and 52.6% in the brodalumab 140-mg group; and 51.0%, 53.4%, and 48.6% in the placebo group. Patients taking ustekinumab had an AE rate of 59.0% in AMAGINE-2 and 53.7% in AMAGINE-3. The most common AE was nasopharyngitis, followed by upper respiratory infection (URI) and headache across all trials.8,9 Serious AEs were rare: 10 in AMAGINE-1, 31 in AMAGINE-2, and 24 in AMAGINE-3 across all groups. One death occurred from stroke in the brodalumab 210-mg group in AMAGINE-2.9
IL-23 Inhibitors
Guselkumab
This drug is a human IgG1κ antibody that binds to the p19 subunit of IL-23, thereby inhibiting IL-23 signaling.11,12 Guselkumab was approved by the FDA in July 2017 for moderate to severe plaque psoriasis.13
VOYAGE 1 and VOYAGE 2 were phase 3, double-blind, placebo- and active comparator–controlled trials of 837 and 992 patients, respectively, randomized to receive adalimumab (80 mg at week 0 and 40 mg at week 1, then at 40 mg every 2 weeks thereafter), guselkumab 100 mg at weeks 0, 4, and 12, or placebo.11 Co-primary end points for both trials were the percentage of patients reaching PASI 90 and an investigator global assessment (IGA) score of cleared (0) or minimal (1) at week 16.11
By week 16 of both trials, PASI 90 values were statistically superior for guselkumab (VOYAGE 1, 73.3%; VOYAGE 2, 70.0%) compared to adalimumab (VOYAGE 1, 49.7%; VOYAGE 2, 46.8%) and placebo (VOYAGE 1, 2.9%; VOYAGE 2, 2.4%)(P<.001). Moreover, patients on guselkumab achieved a higher rate of IGA values of 0 and 1 at week 12 (85.1% in VOYAGE 1 and 84.1% in VOYAGE 2) than patients on adalimumab (65.9% in VOYAGE 1 and 67.7% in VOYAGE 2) and placebo (6.9% in VOYAGE 1 and 8.5% in VOYAGE 2)(P<.001).11,12
The frequency of AEs was comparable across all groups in both trials.11,12 During the 16-week treatment period, 51.7% and 47.6% of the guselkumab groups in VOYAGE 1 and VOYAGE 2, respectively; 51.1% and 48.4% of the adalimumab groups; and 49.4% and 44.8% of the placebo groups reported at least 1 AE. The most common AEs in all groups were nasopharyngitis, headache, and URI.11,12
Serious AEs also occurred at similar rates: 2.4% and 1.6% in the guselkumab group in VOYAGE 1 and VOYAGE 2, respectively; 2.4% and 1.8% in the adalimumab group; and 1.7% and 1.2% in the placebo group.11,12 One case of malignancy occurred in the VOYAGE 1 trial: basal cell carcinoma in the guselkumab group.11 Three major cardiovascular events occurred across both trials: 1 MI in the guselkumab group in each trial and 1 MI in the adalimumab group in VOYAGE 1.11,12
Tildrakizumab
A high-affinity, humanized IgG1κ antibody, tildrakizumab targets the p19 subunit of IL-23. As of February 2018, 2 double-blind, randomized phase 3 trials have studied tildrakizumab with published results: reSURFACE 1 and reSURFACE 2.14
reSURFACE 1 (N=772) and reSURFACE 2 (N=1090) randomized patients to receive tildrakizumab 100 or 200 mg (at weeks 0 and 4), etanercept 50 mg (twice weekly) for 12 weeks (reSURFACE 2 only), or placebo. Co-primary end points were the percentage of patients achieving PASI 75 and the percentage of patients achieving a PGA score of 0 or 1 at week 12.14
In reSURFACE 1, significantly more patients receiving tildrakizumab attained PASI 75 at week 12 compared to placebo: 200 mg, 62.0%; 100 mg, 64.0%; and placebo, 6.0% (P<.001 for tildrakizumab groups compared to placebo). Moreover, significantly proportionally more patients received a PGA score of 0 or 1 compared to placebo: 100 mg, 59%; 200 mg, 58.0%; placebo, 7.0% (P<.001 for both tildrakizumab groups compared to placebo).14
In reSURFACE 2, significantly more patients receiving tildrakizumab achieved PASI 75 compared to etanercept and placebo at week 12: 200 mg, 66.0%; 100mg, 61.0%; etanercept, 48.0%; placebo, 6.0% (P<.001 for both tildrakizumab groups compared to placebo; P<.05 for both tildrakizumab groups compared to etanercept). Additionally, significantly more patients in the tildrakizumab groups experienced a PGA score of 0 or 1 at week 12 compared to placebo: 200 mg, 59%; 100 mg, 55.0%; placebo, 5% (P<.001 for both tildrakizumab groups compared to placebo).14
Adverse events were reported at a similar rate across all groups. For reSURFACE 1 and reSURFACE 2, at least 1 AE by week 12 was reported by 42.2% and 45.2% of patients in the 200-mg group; 47.2% and 45.9% in the 100-mg group; and 48.1% and 55.1% in the placebo groups.14The most common AEs were nasopharyngitis, URI (reSURFACE 1), and erythema at the injection site (reSURFACE 2). One case of serious infection was reported in each of the tildrakizumab groups: 1 case of drug-related hypersensitivity reaction in the 200-mg group, and 1 major cardiovascular event in the 100-mg group of reSURFACE 1. There was 1 serious AE in reSURFACE 2 that led to death in which the cause was undetermined.14
Risankizumab
This humanized IgG1 antibody binds the p19 unit of IL-23.15,16 The drug is undergoing 3 phase 3 trials—ultIMMa-1, ultIMMa-2, and IMMvent—for which only preliminary data have been published and are reported here.16,17 There is 1 phase 2 randomized, dose-ranging trial with published data.15
ultIMMa-1 and ultIMMa-2 comprised 506 and 491 patients, respectively, randomized to receive risankizumab (150 mg at weeks 0, 4, and 16), ustekinumab (45 mg or 90 mg, by weight, at weeks 0, 4, and 16), or placebo. Co-primary end points were PASI 90 and a PGA score of 0 or 1 at week 16.17
In ultIMMa-1 and ultIMMa-2, 75.0% and 75.0% of patients on risankizumab 150 mg achieved PASI 90 compared to 42.0% and 48.0% on ustekinumab and 5.0% and 2.0% on placebo at 16 weeks (P<.001 between both placebo and ustekinumab in both trials).17 In both trials, patients receiving risankizumab achieved higher rates of a static PGA score of 0 or 1 (88.0% and 84.0%) compared to ustekinumab (63.0% and 62.0%) and placebo (8.0% and 5.0%) at 16 weeks (P<.001 for both trials).18
At week 16, 2.0% of patients on risankizumab reported a serious AE in both trials, compared to 8.0% and 3.0% of patients on ustekinumab and 3.0% and 1.0% on placebo. No new safety concerns were noted.17
In the phase 3 IMMvent trial, 605 patients were randomized to receive risankizumab (150 mg at weeks 0, 4, and 16) or adalimumab (80 mg at week 0, 40 mg at week 1, then 40 mg every 2 weeks). Co-primary end points were PASI 90 and a static PGA score of 0 or 1 at week 16.17
In IMMvent, risankizumab was significantly more effective than adalimumab for PASI 75 (risankizumab, 72.0%; adalimumab, 47.0%) and a static PGA score of 0 or 1 (risankizumab 84.0%; adalimumab, 60.0%) (P<.001 risankizumab compared to adalimumab for both end points).17
At week 16, serious AEs were reported in 3.0% of patients on risankizumab and 3.0% of patients on adalimumab. One patient receiving risankizumab died of an acute MI during the treatment phase.17
TNF Inhibitor
Certolizumab Pegol
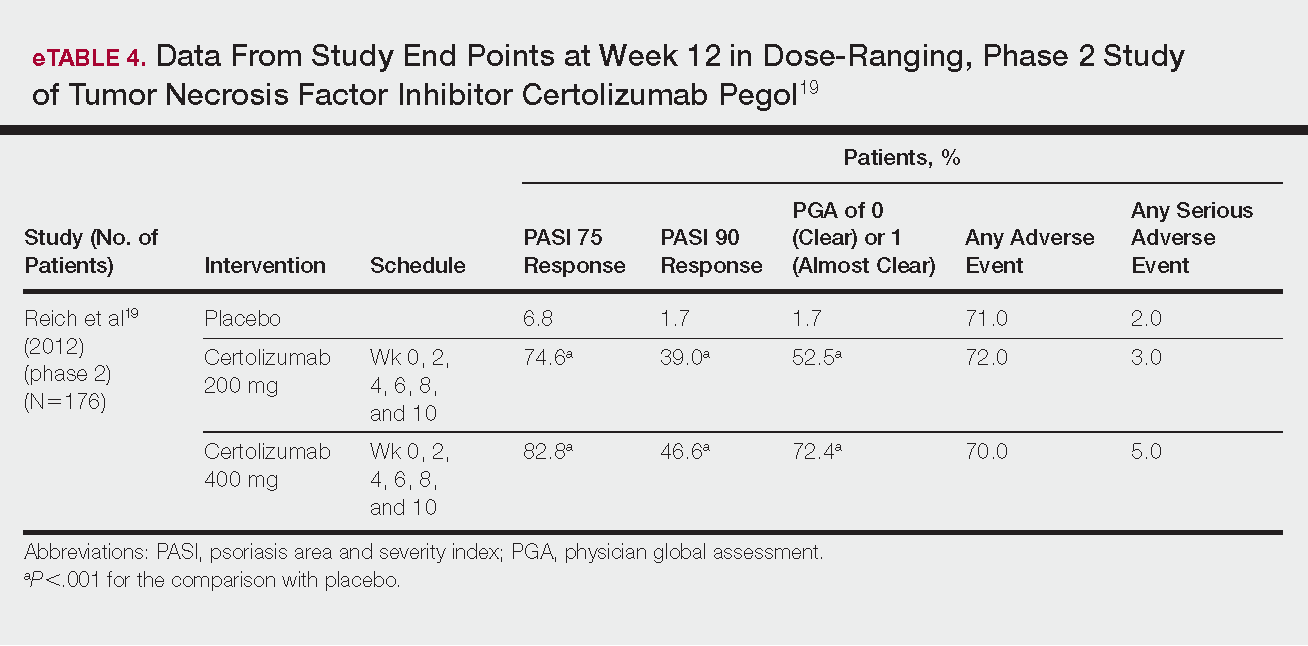
Certolizumab pegol is a human PEGylated anti-TNF agent. In vitro studies have shown that certolizumab binds to soluble and membrane-bound TNF.19 Unlike other TNF inhibitors, certolizumab pegol is a Fab‘ portion of anti-TNF conjugated to a molecule of polyethylene glycol.19 The drug is approved in the United States for treating psoriatic arthritis, Crohn disease, and rheumatoid arthritis; its potential for treating psoriasis has been confirmed. Results of 1 phase 2 trial have been published19; data from 3 phase 3 trials are forthcoming.
This randomized, placebo-controlled, double-blind phase 2 study comprised 176 patients who received certolizumab 200 mg, certolizumab 400 mg, or placebo. The dosing schedule was 400 mg at week 0, followed by either 200 or 400 mg every other week until week 10. Co-primary end points were PASI 75 and a PGA score of 0 or 1 at week 12.19
Certolizumab was significantly more effective than placebo at week 12: 74.6% of the 200-mg group and 82.8% of the 400-mg group achieved PASI 75 compared to 6.8% of the placebo group (P<.001). Certolizumab also performed better for the PGA score: 52.5% and 72.4% of patients attained a score of 0 or 1 in the 200-mg and 400-mg groups compared to 1.7% in the placebo group.19
Adverse events were reported equally across all groups: 72% of patients in the 200-mg group, 70% in the 400-mg group, and 71% in the placebo group reported at least 1 AE, most commonly nasopharyngitis, headache, and pruritis.19
COMMENT
With the development of new insights into the pathogenesis of psoriasis, therapies that are targeted toward key cytokines may contribute to improved management of the disease. The results of these clinical trials demonstrate numerous promising options for psoriatic patients.
IL-17 Inhibitors Ixekizumab and Brodalumab
When comparing these 2 biologics, it is important to consider that these studies were not performed head to head, thereby inhibiting direct comparisons. Moreover, dosage ranges of the investigative drugs were not identical, which also makes comparisons challenging. However, when looking at the highest dosages of ixekizumab and brodalumab, results indicate that ixekizumab may be slightly more effective than brodalumab based on the percentage of patients who achieved a PASI 75 and a static PGA score of 0 or 1 (eTable 1).
Phase 3 trials have shown ixekizumab to maintain efficacy over 60 weeks of treatment.6 Ixekizumab also has been shown to alleviate other symptoms of psoriasis, such as itching, pain, and nail involvement.20,21 Furthermore, ixekizumab appears to be equally effective in patients with or without prior exposure to biologics22; therefore, ixekizumab may benefit patients who have not experienced success with other biologics.
Across the UNCOVER trials, 11 cases of inflammatory bowel disease were reported in patients receiving ixekizumab (ulcerative colitis in 7; Crohn disease in 4)6; it appears that at least 3 of these cases were new diagnoses. In light of a study suggesting that IL-17A might have a protective function in the intestine,23 these findings may have important clinical implications and require follow-up studies.
Brodalumab also has been shown to maintain efficacy and acceptable safety for as long as 120 weeks.24 In the extension period of the AMAGINE-1 trial, patients who experienced a return of disease during a withdrawal period recaptured static PGA success with re-treatment for 12 weeks (re-treatment was successful in 97% of those given a dosage of 210 mg and in 84% of those given 140 mg).8
Furthermore, phase 2 trials also have shown that brodalumab is effective in patients with a history of biologic use.25 Across all AMAGINE trials, only 1 case of Crohn disease was reported in a patient taking brodalumab.9 There are concerns about depression, despite data from AMAGINE-1 stating patients on brodalumab actually had greater improvements in Hospital Anxiety and Depression Scale scores after 12 weeks of treatment (P<.001) for both brodalumab 140 mg and 210 mg compared to placebo.8 Regardless, brodalumab has a black-box warning for suicidal ideation and behavior, and availability is restricted through a Risk Evaluation and Mitigation Strategy (REMS) program.26
Bimekizumab
Although no phase 2 or phase 3 clinical trial data have been published for bimekizumab (phase 2 trials are underway), it has been shown in a phase 1 trial to be effective for psoriasis. Bimekizumab also is unique; it is the first dual inhibitor of IL-17A and IL-17F.18
IL-23 Inhibitors Guselkumab, Tildrakizumab, and Risankizumab
Making comparisons among the IL-23 inhibitors also is difficult; studies were not head-to-head comparison trials, and the VOYAGE and reSURFACE studies used different time points for primary end points. Furthermore, only phase 2 trial data are available for risankizumab. Despite these limitations, results of these trials suggest that guselkumab and risankizumab may be slightly more efficacious than tildrakizumab. However, future studies, including head-to-head studies, would ultimately provide further information on how these agents compare.
Guselkumab was shown to remain efficacious at 48 weeks, though patients on maintenance dosing had better results than those who were re-treated.12 Moreover, guselkumab was found to be effective in hard-to-treat areas, such as the scalp,11 and in patients who did not respond to adalimumab. Guselkumab may therefore benefit patients who have experienced limited clinical improvement on other biologics.12
Tildrakizumab was shown to improve PASI 75 and PGA scores through week 28 of treatment. Moreover, a higher percentage of patients taking tildrakizumab scored 0 or 1 on the dermatology life quality index, suggesting that the drug improves quality of life.14 No specific safety concerns arose in either reSURFACE trial; however, long-term studies are needed for further evaluation.
Risankizumab appears to be a promising new therapy based on phase 2 trial results. Improvements also were seen in dermatology life quality index scores, scalp and fingernail symptoms, and palmoplantar psoriasis.15 Of note, neutralizing antidrug antibodies were found in 3 patients during this study,15 which may present potential problems for long-term efficacy. However, preliminary data from 3 phase 3 trials—ultIMMa-1, ultIMMa-2, and IMMvent—are promising.17
CONCLUSION
Advances in the understanding of psoriasis have led to new targeted therapies. Ongoing clinical trials have shown encouraging results for treating physical and psychological symptoms of psoriasis. The findings of these trials support the idea that therapies targeting IL-23, specifically its p19 subunit, are effective against psoriasis while sparing IL-12. Long-term data from open-label extension studies would help guide clinical recommendations regarding the safety profiles of these agents and determine their long-term utility.
- Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(suppl 2):ii18-ii23; discussion, ii24, ii25.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Amin M, Darji K, No DJ, et al. Review of phase III trial data on IL-23 inhibitors tildrakizumab and guselkumab for psoriasis. J Eur Acad Dermatol Venereol. 2017;31:1627-1632.
- Arican O, Aral M, Sasmaz S, et al. Levels of TNF-alpha, IFN-gamma, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:273-279.
- Griffiths CE, Reich K, Lebwohl M, et al; UNCOVER-2 and UNCOVER-3 investigators. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Gordon KB, Blauvelt A, Papp KA, et al; UNCOVER-1 study group, UNCOVER-2 study group, UNCOVER-3 study group. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- FDA approves new psoriasis drug Taltz [news release]. Silver Spring, MD: US Food and Drug Administration; March 22, 2016. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm491872.htm. Accessed January 29, 2018.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286.
- Lebwohl M, Strober B, Mentor A, et al. Phase 3 studies comparing brodalumab with ustekinumab for psoriasis. N Engl J Med. 2015;373:1318-1328.
- FDA approves new psoriasis drug [news release]. Silver Spring, MD: US Food and Drug Administration; February 15, 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm541981.htm. Accessed January 29, 2018.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate-to-severe plaque psoriasis: results from the phase III, double-blinded placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Janssen announces U.S. FDA approval of Tremfya™ (guselkumab) for the treatment of moderate to severe plaque psoriasis [news release]. Horsham, PA: Johnson & Johnson; July 13, 2017. https://www.jnj.com/media-center/press-releases/janssen-announces-us-fda-approval-of-tremfya-guselkumab-for-the-treatment-of-moderate-to-severe-plaque-psoriasis. Accessed January 29, 2018.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE1 and reSURFACE 2): results from two randomized controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Risankizumab. AbbVie Inc website. https://www.abbvie.com/our-science/pipeline/risankizumab.html. Accessed January 29, 2018.
- Risankizumab meets all co-primary and ranked secondary endpoints, achieving significantly greater efficacy versus standard biologic therapies in three pivotal phase 3 psoriasis studies [news release]. North Chicago, IL: AbbVie Inc; October 26, 2017. https://news.abbvie.com/news/risankizumab-meets-all-co-primary-and-ranked-secondary-endpoints-achieving-significantly-greater-efficacy-versus-standard-biologic-therapies-in-three-pivotal-phase-3-psoriasis-studies.htm. Accessed January 29, 2018.
- Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83:991-1001.
- Reich K, Ortonne JP, Gottlieb AB, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab‘ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167:180-190.
- Kimball AB, Luger T, Gottlieb A, et al. Impact of ixekizumab on psoriasis itch severity and other psoriasis symptoms: results from 3 phase III psoriasis clinical trials. J Am Acad Dermatol. 2016;75:1156-1161.
- Dennehy EB, Zhang L, Amato D, et al. Ixekizumab is effective in subjects with moderate to severe plaque psoriasis with significant nail involvement: results from UNCOVER 3. J Drugs Dermatol. 2016;15:958-961.
- Gottlieb AB, Lacour JP, Korman N, et al. Treatment outcomes with ixekizumab in patients with moderate-to-severe psoriasis who have not received prior biological therapies: an integrated analysis of two phase III randomized studies. J Eur Acad Dermatol Venereol. 2017;31:679-685.
- Hueber W, Sands BE, Lewitsky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693-1700.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183-1190.
- Papp K, Menter A, Strober B, et al. Efficacy and safety of brodalumab in subpopulations of patients with difficult-to-treat moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2015;72:436-439.
- SILIQ [package insert]. Thousand Oaks, CA: Amgen, Inc; 2017.
Psoriasis is a chronic, autoimmune-mediated disease estimated to affect 2.8% of the US population.1 The pathogenesis of psoriasis is thought to involve a complex process triggered by a combination of genetic and environmental factors that induce tumor necrosis factor (TNF) α secretion by keratinocytes, which in turn activates dendritic cells. Activated dendritic cells produce IL-23, leading to helper T cell (TH17) differentiation.2,3 TH17 cells secrete IL-17A, which has been shown to promote psoriatic skin changes.4 Therefore, TNF-α, IL-23, and IL-17A have been recognized as key targets for psoriasis therapy.
The newest biologic agents targeting IL-17–mediated pathways include ixekizumab, brodalumab, and bimekizumab. Secukinumab, the first US Food and Drug Administration (FDA)–approved IL-17 inhibitor, has been available since 2015 and therefore is not included in this review. IL-23 inhibitors that are FDA approved or being evaluated in clinical trials include guselkumab, tildrakizumab, and risankizumab. In addition, certolizumab pegol, a TNF-α inhibitor, is being studied for use in psoriasis.
METHODS
We reviewed the published results of phase 3 clinical trials for ixekizumab, brodalumab, bimekizumab, guselkumab, tildrakizumab, risankizumab, and certolizumab pegol. We performed an English-language literature search (January 1, 2012 to October 15, 2017) of articles indexed for PubMed/MEDLINE using the following combinations of keywords: IL-23 and psoriasis; IL-17 and psoriasis; tumor necrosis factor and psoriasis; [drug name] and psoriasis. If data from phase 3 clinical trials were not yet available, data from phase 2 clinical trials were incorporated in our analysis. We also reviewed citations within articles to identify relevant sources.
RESULTS
Phase 3 clinical trial design, efficacy, and adverse events (AEs) for ixekizumab and brodalumab are reported in eTable 15-10 and for guselkumab and tildrakizumab in eTable 2.11-14 Phase 2 clinical trial design, efficacy, and AEs are presented for risankizumab in eTable 315-18 and for certolizumab pegol in eTable 4.17,19 No published clinical trial data were found for bimekizumab.




IL-17 Inhibitors
Ixekizumab
This recombinant, high-affinity IgG4κ antibody selectively binds and neutralizes IL-17A.5,6 Three phase 3 clinical trials—UNCOVER-1, UNCOVER-2, and UNCOVER-3—evaluated ixekizumab for moderate to severe plaque psoriasis.7
The 3 UNCOVER trials were randomized, double-blind, phase 3 trials of 1296, 1224, and 1346 patients, respectively, assigned to a placebo group; a group treated with ixekizumab 80 mg every 2 weeks; and a group treated with ixekizumab 80 mg every 4 weeks. Both ixekizumab groups received a loading dose of 160 mg at week 0.5,6 UNCOVER-2 and UNCOVER-3 also included a comparator group of patients on etanercept 50 mg.5 Co-primary end points included the percentage of patients reaching a psoriasis area and severity index (PASI) of 75 and with a static physician global assessment (PGA) score of clear (0) or almost clear (1) at week 12.5,6
Ixekizumab achieved greater efficacy than placebo: 89.1%, 89.7%, and 87.3% of patients achieved PASI 75 in the every 2-week dosing group, and 82.6%, 77.5% and 84.2% achieved PASI 75 in the every 4-week dosing group in UNCOVER-1, UNCOVER-2, and UNCOVER-3, respectively (P<.001 for both treatment arms compared to placebo in all trials). The percentage of patients achieving a static PGA score of 0 or 1 also was higher in the ixekizumab groups in the 2-week and 4-week dosing groups in all UNCOVER trials—81.8% and 76.4% in UNCOVER-1, 83.2% and 72.9% in UNCOVER-2, and 80.5% and 75.4% in UNCOVER-3—compared to 3.2%, 2.4%, and 6.7% in the placebo groups of the 3 trials (P<.001 for both ixekizumab groups compared to placebo in all trials).5,6 Ixekizumab also was found to be more effective than etanercept for both co-primary end points in both UNCOVER-2 and UNCOVER-3 (eTable 1).5
Safety data for all UNCOVER trials were pooled and reported.6 At week 12 the rate of at least 1 AE was 58.4% in patients on ixekizumab every 2 weeks and 58.8% in patients on ixekizumab every 4 weeks compared to 54.0% in the etanercept group in UNCOVER-2 and UNCOVER-3 and 46.8% in the placebo group. At week 12, 72 nonfatal serious AEs were reported: 12 in the placebo group, 14 in the etanercept group, 20 in the ixekizumab every 2 weeks group, and 26 in the ixekizumab every 4 weeks group.6
The most common AE across all groups was nasopharyngitis. Overall, infections were more frequent in patients treated with ixekizumab than in patients treated with placebo or etanercept. Specifically, oral candidiasis occurred more frequently in the ixekizumab groups, with a higher rate in the 2-week dosing group than in the 4-week dosing group.6 Two myocardial infarctions (MIs) occurred: 1 in the etanercept group and 1 in the placebo group.5
Brodalumab
This human monoclonal antibody binds to IL-17ra.8,9 Three double-blind, placebo-controlled, phase 3 trials—AMAGINE-1, AMAGINE-2, and AMAGINE-3—evaluated its use for plaque psoriasis.10
In AMAGINE-1 (N=661), patients were randomized to receive brodalumab 140 mg or 210 mg (every 2 weeks for 12 weeks), or placebo.8 In AMAGINE-2 (N=1831) and AMAGINE-3 (N=1881), patients were randomized to receive brodalumab 140 mg or 210 mg (every 2 weeks for 12 weeks), ustekinumab 45 mg or 90 mg by weight (at weeks 0 and 4, then every 12 weeks thereafter), or placebo. In all trials, patients on brodalumab received a dose at week 0 and week 1. Co-primary end points were PASI 75 and a static PGA score of 0 or 1 at 12 weeks compared to placebo and to ustekinumab (in AMAGINE-2 and AMAGINE-3 only).8
At week 12, 83.3%, 86.3%, and 85.1% of patients on brodalumab 210 mg, and 60.3%, 66.6%, and 69.2% of patients on brodalumab 140 mg, achieved PASI 75 in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively, compared to 2.7%, 8.1%, and 6.0% in the placebo groups (P<.001 between both brodalumab groups and placebo in all trials).8 Both brodalumab groups were noninferior but not significantly superior to ustekinumab, which achieved a PASI 75 of 70.0% in AMAGINE-2 and 69.3% in AMAGINE-3. The PASI 90 rate was higher, however, in both brodalumab groups compared to ustekinumab but significance was not reported (eTable 1).9 For both brodalumab groups, significantly more patients achieved a static PGA value of 0 or 1 compared to placebo (P<.001 across all trials). However, only the brodalumab 210-mg group achieved a significantly higher rate of static PGA 0 or 1 compared to ustekinumab in AMAGINE-2 and AMAGINE-3 (P<.001).9
After 12 weeks, the percentage of patients reporting at least 1 AE was 59.0%, 57.8%, and 56.8% in the brodalumab 210-mg group in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively; 58.0%, 60.1%, and 52.6% in the brodalumab 140-mg group; and 51.0%, 53.4%, and 48.6% in the placebo group. Patients taking ustekinumab had an AE rate of 59.0% in AMAGINE-2 and 53.7% in AMAGINE-3. The most common AE was nasopharyngitis, followed by upper respiratory infection (URI) and headache across all trials.8,9 Serious AEs were rare: 10 in AMAGINE-1, 31 in AMAGINE-2, and 24 in AMAGINE-3 across all groups. One death occurred from stroke in the brodalumab 210-mg group in AMAGINE-2.9
IL-23 Inhibitors
Guselkumab
This drug is a human IgG1κ antibody that binds to the p19 subunit of IL-23, thereby inhibiting IL-23 signaling.11,12 Guselkumab was approved by the FDA in July 2017 for moderate to severe plaque psoriasis.13
VOYAGE 1 and VOYAGE 2 were phase 3, double-blind, placebo- and active comparator–controlled trials of 837 and 992 patients, respectively, randomized to receive adalimumab (80 mg at week 0 and 40 mg at week 1, then at 40 mg every 2 weeks thereafter), guselkumab 100 mg at weeks 0, 4, and 12, or placebo.11 Co-primary end points for both trials were the percentage of patients reaching PASI 90 and an investigator global assessment (IGA) score of cleared (0) or minimal (1) at week 16.11
By week 16 of both trials, PASI 90 values were statistically superior for guselkumab (VOYAGE 1, 73.3%; VOYAGE 2, 70.0%) compared to adalimumab (VOYAGE 1, 49.7%; VOYAGE 2, 46.8%) and placebo (VOYAGE 1, 2.9%; VOYAGE 2, 2.4%)(P<.001). Moreover, patients on guselkumab achieved a higher rate of IGA values of 0 and 1 at week 12 (85.1% in VOYAGE 1 and 84.1% in VOYAGE 2) than patients on adalimumab (65.9% in VOYAGE 1 and 67.7% in VOYAGE 2) and placebo (6.9% in VOYAGE 1 and 8.5% in VOYAGE 2)(P<.001).11,12
The frequency of AEs was comparable across all groups in both trials.11,12 During the 16-week treatment period, 51.7% and 47.6% of the guselkumab groups in VOYAGE 1 and VOYAGE 2, respectively; 51.1% and 48.4% of the adalimumab groups; and 49.4% and 44.8% of the placebo groups reported at least 1 AE. The most common AEs in all groups were nasopharyngitis, headache, and URI.11,12
Serious AEs also occurred at similar rates: 2.4% and 1.6% in the guselkumab group in VOYAGE 1 and VOYAGE 2, respectively; 2.4% and 1.8% in the adalimumab group; and 1.7% and 1.2% in the placebo group.11,12 One case of malignancy occurred in the VOYAGE 1 trial: basal cell carcinoma in the guselkumab group.11 Three major cardiovascular events occurred across both trials: 1 MI in the guselkumab group in each trial and 1 MI in the adalimumab group in VOYAGE 1.11,12
Tildrakizumab
A high-affinity, humanized IgG1κ antibody, tildrakizumab targets the p19 subunit of IL-23. As of February 2018, 2 double-blind, randomized phase 3 trials have studied tildrakizumab with published results: reSURFACE 1 and reSURFACE 2.14
reSURFACE 1 (N=772) and reSURFACE 2 (N=1090) randomized patients to receive tildrakizumab 100 or 200 mg (at weeks 0 and 4), etanercept 50 mg (twice weekly) for 12 weeks (reSURFACE 2 only), or placebo. Co-primary end points were the percentage of patients achieving PASI 75 and the percentage of patients achieving a PGA score of 0 or 1 at week 12.14
In reSURFACE 1, significantly more patients receiving tildrakizumab attained PASI 75 at week 12 compared to placebo: 200 mg, 62.0%; 100 mg, 64.0%; and placebo, 6.0% (P<.001 for tildrakizumab groups compared to placebo). Moreover, significantly proportionally more patients received a PGA score of 0 or 1 compared to placebo: 100 mg, 59%; 200 mg, 58.0%; placebo, 7.0% (P<.001 for both tildrakizumab groups compared to placebo).14
In reSURFACE 2, significantly more patients receiving tildrakizumab achieved PASI 75 compared to etanercept and placebo at week 12: 200 mg, 66.0%; 100mg, 61.0%; etanercept, 48.0%; placebo, 6.0% (P<.001 for both tildrakizumab groups compared to placebo; P<.05 for both tildrakizumab groups compared to etanercept). Additionally, significantly more patients in the tildrakizumab groups experienced a PGA score of 0 or 1 at week 12 compared to placebo: 200 mg, 59%; 100 mg, 55.0%; placebo, 5% (P<.001 for both tildrakizumab groups compared to placebo).14
Adverse events were reported at a similar rate across all groups. For reSURFACE 1 and reSURFACE 2, at least 1 AE by week 12 was reported by 42.2% and 45.2% of patients in the 200-mg group; 47.2% and 45.9% in the 100-mg group; and 48.1% and 55.1% in the placebo groups.14The most common AEs were nasopharyngitis, URI (reSURFACE 1), and erythema at the injection site (reSURFACE 2). One case of serious infection was reported in each of the tildrakizumab groups: 1 case of drug-related hypersensitivity reaction in the 200-mg group, and 1 major cardiovascular event in the 100-mg group of reSURFACE 1. There was 1 serious AE in reSURFACE 2 that led to death in which the cause was undetermined.14
Risankizumab
This humanized IgG1 antibody binds the p19 unit of IL-23.15,16 The drug is undergoing 3 phase 3 trials—ultIMMa-1, ultIMMa-2, and IMMvent—for which only preliminary data have been published and are reported here.16,17 There is 1 phase 2 randomized, dose-ranging trial with published data.15
ultIMMa-1 and ultIMMa-2 comprised 506 and 491 patients, respectively, randomized to receive risankizumab (150 mg at weeks 0, 4, and 16), ustekinumab (45 mg or 90 mg, by weight, at weeks 0, 4, and 16), or placebo. Co-primary end points were PASI 90 and a PGA score of 0 or 1 at week 16.17
In ultIMMa-1 and ultIMMa-2, 75.0% and 75.0% of patients on risankizumab 150 mg achieved PASI 90 compared to 42.0% and 48.0% on ustekinumab and 5.0% and 2.0% on placebo at 16 weeks (P<.001 between both placebo and ustekinumab in both trials).17 In both trials, patients receiving risankizumab achieved higher rates of a static PGA score of 0 or 1 (88.0% and 84.0%) compared to ustekinumab (63.0% and 62.0%) and placebo (8.0% and 5.0%) at 16 weeks (P<.001 for both trials).18
At week 16, 2.0% of patients on risankizumab reported a serious AE in both trials, compared to 8.0% and 3.0% of patients on ustekinumab and 3.0% and 1.0% on placebo. No new safety concerns were noted.17
In the phase 3 IMMvent trial, 605 patients were randomized to receive risankizumab (150 mg at weeks 0, 4, and 16) or adalimumab (80 mg at week 0, 40 mg at week 1, then 40 mg every 2 weeks). Co-primary end points were PASI 90 and a static PGA score of 0 or 1 at week 16.17
In IMMvent, risankizumab was significantly more effective than adalimumab for PASI 75 (risankizumab, 72.0%; adalimumab, 47.0%) and a static PGA score of 0 or 1 (risankizumab 84.0%; adalimumab, 60.0%) (P<.001 risankizumab compared to adalimumab for both end points).17
At week 16, serious AEs were reported in 3.0% of patients on risankizumab and 3.0% of patients on adalimumab. One patient receiving risankizumab died of an acute MI during the treatment phase.17
TNF Inhibitor
Certolizumab Pegol
Certolizumab pegol is a human PEGylated anti-TNF agent. In vitro studies have shown that certolizumab binds to soluble and membrane-bound TNF.19 Unlike other TNF inhibitors, certolizumab pegol is a Fab‘ portion of anti-TNF conjugated to a molecule of polyethylene glycol.19 The drug is approved in the United States for treating psoriatic arthritis, Crohn disease, and rheumatoid arthritis; its potential for treating psoriasis has been confirmed. Results of 1 phase 2 trial have been published19; data from 3 phase 3 trials are forthcoming.
This randomized, placebo-controlled, double-blind phase 2 study comprised 176 patients who received certolizumab 200 mg, certolizumab 400 mg, or placebo. The dosing schedule was 400 mg at week 0, followed by either 200 or 400 mg every other week until week 10. Co-primary end points were PASI 75 and a PGA score of 0 or 1 at week 12.19
Certolizumab was significantly more effective than placebo at week 12: 74.6% of the 200-mg group and 82.8% of the 400-mg group achieved PASI 75 compared to 6.8% of the placebo group (P<.001). Certolizumab also performed better for the PGA score: 52.5% and 72.4% of patients attained a score of 0 or 1 in the 200-mg and 400-mg groups compared to 1.7% in the placebo group.19
Adverse events were reported equally across all groups: 72% of patients in the 200-mg group, 70% in the 400-mg group, and 71% in the placebo group reported at least 1 AE, most commonly nasopharyngitis, headache, and pruritis.19
COMMENT
With the development of new insights into the pathogenesis of psoriasis, therapies that are targeted toward key cytokines may contribute to improved management of the disease. The results of these clinical trials demonstrate numerous promising options for psoriatic patients.
IL-17 Inhibitors Ixekizumab and Brodalumab
When comparing these 2 biologics, it is important to consider that these studies were not performed head to head, thereby inhibiting direct comparisons. Moreover, dosage ranges of the investigative drugs were not identical, which also makes comparisons challenging. However, when looking at the highest dosages of ixekizumab and brodalumab, results indicate that ixekizumab may be slightly more effective than brodalumab based on the percentage of patients who achieved a PASI 75 and a static PGA score of 0 or 1 (eTable 1).
Phase 3 trials have shown ixekizumab to maintain efficacy over 60 weeks of treatment.6 Ixekizumab also has been shown to alleviate other symptoms of psoriasis, such as itching, pain, and nail involvement.20,21 Furthermore, ixekizumab appears to be equally effective in patients with or without prior exposure to biologics22; therefore, ixekizumab may benefit patients who have not experienced success with other biologics.
Across the UNCOVER trials, 11 cases of inflammatory bowel disease were reported in patients receiving ixekizumab (ulcerative colitis in 7; Crohn disease in 4)6; it appears that at least 3 of these cases were new diagnoses. In light of a study suggesting that IL-17A might have a protective function in the intestine,23 these findings may have important clinical implications and require follow-up studies.
Brodalumab also has been shown to maintain efficacy and acceptable safety for as long as 120 weeks.24 In the extension period of the AMAGINE-1 trial, patients who experienced a return of disease during a withdrawal period recaptured static PGA success with re-treatment for 12 weeks (re-treatment was successful in 97% of those given a dosage of 210 mg and in 84% of those given 140 mg).8
Furthermore, phase 2 trials also have shown that brodalumab is effective in patients with a history of biologic use.25 Across all AMAGINE trials, only 1 case of Crohn disease was reported in a patient taking brodalumab.9 There are concerns about depression, despite data from AMAGINE-1 stating patients on brodalumab actually had greater improvements in Hospital Anxiety and Depression Scale scores after 12 weeks of treatment (P<.001) for both brodalumab 140 mg and 210 mg compared to placebo.8 Regardless, brodalumab has a black-box warning for suicidal ideation and behavior, and availability is restricted through a Risk Evaluation and Mitigation Strategy (REMS) program.26
Bimekizumab
Although no phase 2 or phase 3 clinical trial data have been published for bimekizumab (phase 2 trials are underway), it has been shown in a phase 1 trial to be effective for psoriasis. Bimekizumab also is unique; it is the first dual inhibitor of IL-17A and IL-17F.18
IL-23 Inhibitors Guselkumab, Tildrakizumab, and Risankizumab
Making comparisons among the IL-23 inhibitors also is difficult; studies were not head-to-head comparison trials, and the VOYAGE and reSURFACE studies used different time points for primary end points. Furthermore, only phase 2 trial data are available for risankizumab. Despite these limitations, results of these trials suggest that guselkumab and risankizumab may be slightly more efficacious than tildrakizumab. However, future studies, including head-to-head studies, would ultimately provide further information on how these agents compare.
Guselkumab was shown to remain efficacious at 48 weeks, though patients on maintenance dosing had better results than those who were re-treated.12 Moreover, guselkumab was found to be effective in hard-to-treat areas, such as the scalp,11 and in patients who did not respond to adalimumab. Guselkumab may therefore benefit patients who have experienced limited clinical improvement on other biologics.12
Tildrakizumab was shown to improve PASI 75 and PGA scores through week 28 of treatment. Moreover, a higher percentage of patients taking tildrakizumab scored 0 or 1 on the dermatology life quality index, suggesting that the drug improves quality of life.14 No specific safety concerns arose in either reSURFACE trial; however, long-term studies are needed for further evaluation.
Risankizumab appears to be a promising new therapy based on phase 2 trial results. Improvements also were seen in dermatology life quality index scores, scalp and fingernail symptoms, and palmoplantar psoriasis.15 Of note, neutralizing antidrug antibodies were found in 3 patients during this study,15 which may present potential problems for long-term efficacy. However, preliminary data from 3 phase 3 trials—ultIMMa-1, ultIMMa-2, and IMMvent—are promising.17
CONCLUSION
Advances in the understanding of psoriasis have led to new targeted therapies. Ongoing clinical trials have shown encouraging results for treating physical and psychological symptoms of psoriasis. The findings of these trials support the idea that therapies targeting IL-23, specifically its p19 subunit, are effective against psoriasis while sparing IL-12. Long-term data from open-label extension studies would help guide clinical recommendations regarding the safety profiles of these agents and determine their long-term utility.
Psoriasis is a chronic, autoimmune-mediated disease estimated to affect 2.8% of the US population.1 The pathogenesis of psoriasis is thought to involve a complex process triggered by a combination of genetic and environmental factors that induce tumor necrosis factor (TNF) α secretion by keratinocytes, which in turn activates dendritic cells. Activated dendritic cells produce IL-23, leading to helper T cell (TH17) differentiation.2,3 TH17 cells secrete IL-17A, which has been shown to promote psoriatic skin changes.4 Therefore, TNF-α, IL-23, and IL-17A have been recognized as key targets for psoriasis therapy.
The newest biologic agents targeting IL-17–mediated pathways include ixekizumab, brodalumab, and bimekizumab. Secukinumab, the first US Food and Drug Administration (FDA)–approved IL-17 inhibitor, has been available since 2015 and therefore is not included in this review. IL-23 inhibitors that are FDA approved or being evaluated in clinical trials include guselkumab, tildrakizumab, and risankizumab. In addition, certolizumab pegol, a TNF-α inhibitor, is being studied for use in psoriasis.
METHODS
We reviewed the published results of phase 3 clinical trials for ixekizumab, brodalumab, bimekizumab, guselkumab, tildrakizumab, risankizumab, and certolizumab pegol. We performed an English-language literature search (January 1, 2012 to October 15, 2017) of articles indexed for PubMed/MEDLINE using the following combinations of keywords: IL-23 and psoriasis; IL-17 and psoriasis; tumor necrosis factor and psoriasis; [drug name] and psoriasis. If data from phase 3 clinical trials were not yet available, data from phase 2 clinical trials were incorporated in our analysis. We also reviewed citations within articles to identify relevant sources.
RESULTS
Phase 3 clinical trial design, efficacy, and adverse events (AEs) for ixekizumab and brodalumab are reported in eTable 15-10 and for guselkumab and tildrakizumab in eTable 2.11-14 Phase 2 clinical trial design, efficacy, and AEs are presented for risankizumab in eTable 315-18 and for certolizumab pegol in eTable 4.17,19 No published clinical trial data were found for bimekizumab.




IL-17 Inhibitors
Ixekizumab
This recombinant, high-affinity IgG4κ antibody selectively binds and neutralizes IL-17A.5,6 Three phase 3 clinical trials—UNCOVER-1, UNCOVER-2, and UNCOVER-3—evaluated ixekizumab for moderate to severe plaque psoriasis.7
The 3 UNCOVER trials were randomized, double-blind, phase 3 trials of 1296, 1224, and 1346 patients, respectively, assigned to a placebo group; a group treated with ixekizumab 80 mg every 2 weeks; and a group treated with ixekizumab 80 mg every 4 weeks. Both ixekizumab groups received a loading dose of 160 mg at week 0.5,6 UNCOVER-2 and UNCOVER-3 also included a comparator group of patients on etanercept 50 mg.5 Co-primary end points included the percentage of patients reaching a psoriasis area and severity index (PASI) of 75 and with a static physician global assessment (PGA) score of clear (0) or almost clear (1) at week 12.5,6
Ixekizumab achieved greater efficacy than placebo: 89.1%, 89.7%, and 87.3% of patients achieved PASI 75 in the every 2-week dosing group, and 82.6%, 77.5% and 84.2% achieved PASI 75 in the every 4-week dosing group in UNCOVER-1, UNCOVER-2, and UNCOVER-3, respectively (P<.001 for both treatment arms compared to placebo in all trials). The percentage of patients achieving a static PGA score of 0 or 1 also was higher in the ixekizumab groups in the 2-week and 4-week dosing groups in all UNCOVER trials—81.8% and 76.4% in UNCOVER-1, 83.2% and 72.9% in UNCOVER-2, and 80.5% and 75.4% in UNCOVER-3—compared to 3.2%, 2.4%, and 6.7% in the placebo groups of the 3 trials (P<.001 for both ixekizumab groups compared to placebo in all trials).5,6 Ixekizumab also was found to be more effective than etanercept for both co-primary end points in both UNCOVER-2 and UNCOVER-3 (eTable 1).5
Safety data for all UNCOVER trials were pooled and reported.6 At week 12 the rate of at least 1 AE was 58.4% in patients on ixekizumab every 2 weeks and 58.8% in patients on ixekizumab every 4 weeks compared to 54.0% in the etanercept group in UNCOVER-2 and UNCOVER-3 and 46.8% in the placebo group. At week 12, 72 nonfatal serious AEs were reported: 12 in the placebo group, 14 in the etanercept group, 20 in the ixekizumab every 2 weeks group, and 26 in the ixekizumab every 4 weeks group.6
The most common AE across all groups was nasopharyngitis. Overall, infections were more frequent in patients treated with ixekizumab than in patients treated with placebo or etanercept. Specifically, oral candidiasis occurred more frequently in the ixekizumab groups, with a higher rate in the 2-week dosing group than in the 4-week dosing group.6 Two myocardial infarctions (MIs) occurred: 1 in the etanercept group and 1 in the placebo group.5
Brodalumab
This human monoclonal antibody binds to IL-17ra.8,9 Three double-blind, placebo-controlled, phase 3 trials—AMAGINE-1, AMAGINE-2, and AMAGINE-3—evaluated its use for plaque psoriasis.10
In AMAGINE-1 (N=661), patients were randomized to receive brodalumab 140 mg or 210 mg (every 2 weeks for 12 weeks), or placebo.8 In AMAGINE-2 (N=1831) and AMAGINE-3 (N=1881), patients were randomized to receive brodalumab 140 mg or 210 mg (every 2 weeks for 12 weeks), ustekinumab 45 mg or 90 mg by weight (at weeks 0 and 4, then every 12 weeks thereafter), or placebo. In all trials, patients on brodalumab received a dose at week 0 and week 1. Co-primary end points were PASI 75 and a static PGA score of 0 or 1 at 12 weeks compared to placebo and to ustekinumab (in AMAGINE-2 and AMAGINE-3 only).8
At week 12, 83.3%, 86.3%, and 85.1% of patients on brodalumab 210 mg, and 60.3%, 66.6%, and 69.2% of patients on brodalumab 140 mg, achieved PASI 75 in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively, compared to 2.7%, 8.1%, and 6.0% in the placebo groups (P<.001 between both brodalumab groups and placebo in all trials).8 Both brodalumab groups were noninferior but not significantly superior to ustekinumab, which achieved a PASI 75 of 70.0% in AMAGINE-2 and 69.3% in AMAGINE-3. The PASI 90 rate was higher, however, in both brodalumab groups compared to ustekinumab but significance was not reported (eTable 1).9 For both brodalumab groups, significantly more patients achieved a static PGA value of 0 or 1 compared to placebo (P<.001 across all trials). However, only the brodalumab 210-mg group achieved a significantly higher rate of static PGA 0 or 1 compared to ustekinumab in AMAGINE-2 and AMAGINE-3 (P<.001).9
After 12 weeks, the percentage of patients reporting at least 1 AE was 59.0%, 57.8%, and 56.8% in the brodalumab 210-mg group in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively; 58.0%, 60.1%, and 52.6% in the brodalumab 140-mg group; and 51.0%, 53.4%, and 48.6% in the placebo group. Patients taking ustekinumab had an AE rate of 59.0% in AMAGINE-2 and 53.7% in AMAGINE-3. The most common AE was nasopharyngitis, followed by upper respiratory infection (URI) and headache across all trials.8,9 Serious AEs were rare: 10 in AMAGINE-1, 31 in AMAGINE-2, and 24 in AMAGINE-3 across all groups. One death occurred from stroke in the brodalumab 210-mg group in AMAGINE-2.9
IL-23 Inhibitors
Guselkumab
This drug is a human IgG1κ antibody that binds to the p19 subunit of IL-23, thereby inhibiting IL-23 signaling.11,12 Guselkumab was approved by the FDA in July 2017 for moderate to severe plaque psoriasis.13
VOYAGE 1 and VOYAGE 2 were phase 3, double-blind, placebo- and active comparator–controlled trials of 837 and 992 patients, respectively, randomized to receive adalimumab (80 mg at week 0 and 40 mg at week 1, then at 40 mg every 2 weeks thereafter), guselkumab 100 mg at weeks 0, 4, and 12, or placebo.11 Co-primary end points for both trials were the percentage of patients reaching PASI 90 and an investigator global assessment (IGA) score of cleared (0) or minimal (1) at week 16.11
By week 16 of both trials, PASI 90 values were statistically superior for guselkumab (VOYAGE 1, 73.3%; VOYAGE 2, 70.0%) compared to adalimumab (VOYAGE 1, 49.7%; VOYAGE 2, 46.8%) and placebo (VOYAGE 1, 2.9%; VOYAGE 2, 2.4%)(P<.001). Moreover, patients on guselkumab achieved a higher rate of IGA values of 0 and 1 at week 12 (85.1% in VOYAGE 1 and 84.1% in VOYAGE 2) than patients on adalimumab (65.9% in VOYAGE 1 and 67.7% in VOYAGE 2) and placebo (6.9% in VOYAGE 1 and 8.5% in VOYAGE 2)(P<.001).11,12
The frequency of AEs was comparable across all groups in both trials.11,12 During the 16-week treatment period, 51.7% and 47.6% of the guselkumab groups in VOYAGE 1 and VOYAGE 2, respectively; 51.1% and 48.4% of the adalimumab groups; and 49.4% and 44.8% of the placebo groups reported at least 1 AE. The most common AEs in all groups were nasopharyngitis, headache, and URI.11,12
Serious AEs also occurred at similar rates: 2.4% and 1.6% in the guselkumab group in VOYAGE 1 and VOYAGE 2, respectively; 2.4% and 1.8% in the adalimumab group; and 1.7% and 1.2% in the placebo group.11,12 One case of malignancy occurred in the VOYAGE 1 trial: basal cell carcinoma in the guselkumab group.11 Three major cardiovascular events occurred across both trials: 1 MI in the guselkumab group in each trial and 1 MI in the adalimumab group in VOYAGE 1.11,12
Tildrakizumab
A high-affinity, humanized IgG1κ antibody, tildrakizumab targets the p19 subunit of IL-23. As of February 2018, 2 double-blind, randomized phase 3 trials have studied tildrakizumab with published results: reSURFACE 1 and reSURFACE 2.14
reSURFACE 1 (N=772) and reSURFACE 2 (N=1090) randomized patients to receive tildrakizumab 100 or 200 mg (at weeks 0 and 4), etanercept 50 mg (twice weekly) for 12 weeks (reSURFACE 2 only), or placebo. Co-primary end points were the percentage of patients achieving PASI 75 and the percentage of patients achieving a PGA score of 0 or 1 at week 12.14
In reSURFACE 1, significantly more patients receiving tildrakizumab attained PASI 75 at week 12 compared to placebo: 200 mg, 62.0%; 100 mg, 64.0%; and placebo, 6.0% (P<.001 for tildrakizumab groups compared to placebo). Moreover, significantly proportionally more patients received a PGA score of 0 or 1 compared to placebo: 100 mg, 59%; 200 mg, 58.0%; placebo, 7.0% (P<.001 for both tildrakizumab groups compared to placebo).14
In reSURFACE 2, significantly more patients receiving tildrakizumab achieved PASI 75 compared to etanercept and placebo at week 12: 200 mg, 66.0%; 100mg, 61.0%; etanercept, 48.0%; placebo, 6.0% (P<.001 for both tildrakizumab groups compared to placebo; P<.05 for both tildrakizumab groups compared to etanercept). Additionally, significantly more patients in the tildrakizumab groups experienced a PGA score of 0 or 1 at week 12 compared to placebo: 200 mg, 59%; 100 mg, 55.0%; placebo, 5% (P<.001 for both tildrakizumab groups compared to placebo).14
Adverse events were reported at a similar rate across all groups. For reSURFACE 1 and reSURFACE 2, at least 1 AE by week 12 was reported by 42.2% and 45.2% of patients in the 200-mg group; 47.2% and 45.9% in the 100-mg group; and 48.1% and 55.1% in the placebo groups.14The most common AEs were nasopharyngitis, URI (reSURFACE 1), and erythema at the injection site (reSURFACE 2). One case of serious infection was reported in each of the tildrakizumab groups: 1 case of drug-related hypersensitivity reaction in the 200-mg group, and 1 major cardiovascular event in the 100-mg group of reSURFACE 1. There was 1 serious AE in reSURFACE 2 that led to death in which the cause was undetermined.14
Risankizumab
This humanized IgG1 antibody binds the p19 unit of IL-23.15,16 The drug is undergoing 3 phase 3 trials—ultIMMa-1, ultIMMa-2, and IMMvent—for which only preliminary data have been published and are reported here.16,17 There is 1 phase 2 randomized, dose-ranging trial with published data.15
ultIMMa-1 and ultIMMa-2 comprised 506 and 491 patients, respectively, randomized to receive risankizumab (150 mg at weeks 0, 4, and 16), ustekinumab (45 mg or 90 mg, by weight, at weeks 0, 4, and 16), or placebo. Co-primary end points were PASI 90 and a PGA score of 0 or 1 at week 16.17
In ultIMMa-1 and ultIMMa-2, 75.0% and 75.0% of patients on risankizumab 150 mg achieved PASI 90 compared to 42.0% and 48.0% on ustekinumab and 5.0% and 2.0% on placebo at 16 weeks (P<.001 between both placebo and ustekinumab in both trials).17 In both trials, patients receiving risankizumab achieved higher rates of a static PGA score of 0 or 1 (88.0% and 84.0%) compared to ustekinumab (63.0% and 62.0%) and placebo (8.0% and 5.0%) at 16 weeks (P<.001 for both trials).18
At week 16, 2.0% of patients on risankizumab reported a serious AE in both trials, compared to 8.0% and 3.0% of patients on ustekinumab and 3.0% and 1.0% on placebo. No new safety concerns were noted.17
In the phase 3 IMMvent trial, 605 patients were randomized to receive risankizumab (150 mg at weeks 0, 4, and 16) or adalimumab (80 mg at week 0, 40 mg at week 1, then 40 mg every 2 weeks). Co-primary end points were PASI 90 and a static PGA score of 0 or 1 at week 16.17
In IMMvent, risankizumab was significantly more effective than adalimumab for PASI 75 (risankizumab, 72.0%; adalimumab, 47.0%) and a static PGA score of 0 or 1 (risankizumab 84.0%; adalimumab, 60.0%) (P<.001 risankizumab compared to adalimumab for both end points).17
At week 16, serious AEs were reported in 3.0% of patients on risankizumab and 3.0% of patients on adalimumab. One patient receiving risankizumab died of an acute MI during the treatment phase.17
TNF Inhibitor
Certolizumab Pegol
Certolizumab pegol is a human PEGylated anti-TNF agent. In vitro studies have shown that certolizumab binds to soluble and membrane-bound TNF.19 Unlike other TNF inhibitors, certolizumab pegol is a Fab‘ portion of anti-TNF conjugated to a molecule of polyethylene glycol.19 The drug is approved in the United States for treating psoriatic arthritis, Crohn disease, and rheumatoid arthritis; its potential for treating psoriasis has been confirmed. Results of 1 phase 2 trial have been published19; data from 3 phase 3 trials are forthcoming.
This randomized, placebo-controlled, double-blind phase 2 study comprised 176 patients who received certolizumab 200 mg, certolizumab 400 mg, or placebo. The dosing schedule was 400 mg at week 0, followed by either 200 or 400 mg every other week until week 10. Co-primary end points were PASI 75 and a PGA score of 0 or 1 at week 12.19
Certolizumab was significantly more effective than placebo at week 12: 74.6% of the 200-mg group and 82.8% of the 400-mg group achieved PASI 75 compared to 6.8% of the placebo group (P<.001). Certolizumab also performed better for the PGA score: 52.5% and 72.4% of patients attained a score of 0 or 1 in the 200-mg and 400-mg groups compared to 1.7% in the placebo group.19
Adverse events were reported equally across all groups: 72% of patients in the 200-mg group, 70% in the 400-mg group, and 71% in the placebo group reported at least 1 AE, most commonly nasopharyngitis, headache, and pruritis.19
COMMENT
With the development of new insights into the pathogenesis of psoriasis, therapies that are targeted toward key cytokines may contribute to improved management of the disease. The results of these clinical trials demonstrate numerous promising options for psoriatic patients.
IL-17 Inhibitors Ixekizumab and Brodalumab
When comparing these 2 biologics, it is important to consider that these studies were not performed head to head, thereby inhibiting direct comparisons. Moreover, dosage ranges of the investigative drugs were not identical, which also makes comparisons challenging. However, when looking at the highest dosages of ixekizumab and brodalumab, results indicate that ixekizumab may be slightly more effective than brodalumab based on the percentage of patients who achieved a PASI 75 and a static PGA score of 0 or 1 (eTable 1).
Phase 3 trials have shown ixekizumab to maintain efficacy over 60 weeks of treatment.6 Ixekizumab also has been shown to alleviate other symptoms of psoriasis, such as itching, pain, and nail involvement.20,21 Furthermore, ixekizumab appears to be equally effective in patients with or without prior exposure to biologics22; therefore, ixekizumab may benefit patients who have not experienced success with other biologics.
Across the UNCOVER trials, 11 cases of inflammatory bowel disease were reported in patients receiving ixekizumab (ulcerative colitis in 7; Crohn disease in 4)6; it appears that at least 3 of these cases were new diagnoses. In light of a study suggesting that IL-17A might have a protective function in the intestine,23 these findings may have important clinical implications and require follow-up studies.
Brodalumab also has been shown to maintain efficacy and acceptable safety for as long as 120 weeks.24 In the extension period of the AMAGINE-1 trial, patients who experienced a return of disease during a withdrawal period recaptured static PGA success with re-treatment for 12 weeks (re-treatment was successful in 97% of those given a dosage of 210 mg and in 84% of those given 140 mg).8
Furthermore, phase 2 trials also have shown that brodalumab is effective in patients with a history of biologic use.25 Across all AMAGINE trials, only 1 case of Crohn disease was reported in a patient taking brodalumab.9 There are concerns about depression, despite data from AMAGINE-1 stating patients on brodalumab actually had greater improvements in Hospital Anxiety and Depression Scale scores after 12 weeks of treatment (P<.001) for both brodalumab 140 mg and 210 mg compared to placebo.8 Regardless, brodalumab has a black-box warning for suicidal ideation and behavior, and availability is restricted through a Risk Evaluation and Mitigation Strategy (REMS) program.26
Bimekizumab
Although no phase 2 or phase 3 clinical trial data have been published for bimekizumab (phase 2 trials are underway), it has been shown in a phase 1 trial to be effective for psoriasis. Bimekizumab also is unique; it is the first dual inhibitor of IL-17A and IL-17F.18
IL-23 Inhibitors Guselkumab, Tildrakizumab, and Risankizumab
Making comparisons among the IL-23 inhibitors also is difficult; studies were not head-to-head comparison trials, and the VOYAGE and reSURFACE studies used different time points for primary end points. Furthermore, only phase 2 trial data are available for risankizumab. Despite these limitations, results of these trials suggest that guselkumab and risankizumab may be slightly more efficacious than tildrakizumab. However, future studies, including head-to-head studies, would ultimately provide further information on how these agents compare.
Guselkumab was shown to remain efficacious at 48 weeks, though patients on maintenance dosing had better results than those who were re-treated.12 Moreover, guselkumab was found to be effective in hard-to-treat areas, such as the scalp,11 and in patients who did not respond to adalimumab. Guselkumab may therefore benefit patients who have experienced limited clinical improvement on other biologics.12
Tildrakizumab was shown to improve PASI 75 and PGA scores through week 28 of treatment. Moreover, a higher percentage of patients taking tildrakizumab scored 0 or 1 on the dermatology life quality index, suggesting that the drug improves quality of life.14 No specific safety concerns arose in either reSURFACE trial; however, long-term studies are needed for further evaluation.
Risankizumab appears to be a promising new therapy based on phase 2 trial results. Improvements also were seen in dermatology life quality index scores, scalp and fingernail symptoms, and palmoplantar psoriasis.15 Of note, neutralizing antidrug antibodies were found in 3 patients during this study,15 which may present potential problems for long-term efficacy. However, preliminary data from 3 phase 3 trials—ultIMMa-1, ultIMMa-2, and IMMvent—are promising.17
CONCLUSION
Advances in the understanding of psoriasis have led to new targeted therapies. Ongoing clinical trials have shown encouraging results for treating physical and psychological symptoms of psoriasis. The findings of these trials support the idea that therapies targeting IL-23, specifically its p19 subunit, are effective against psoriasis while sparing IL-12. Long-term data from open-label extension studies would help guide clinical recommendations regarding the safety profiles of these agents and determine their long-term utility.
- Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(suppl 2):ii18-ii23; discussion, ii24, ii25.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Amin M, Darji K, No DJ, et al. Review of phase III trial data on IL-23 inhibitors tildrakizumab and guselkumab for psoriasis. J Eur Acad Dermatol Venereol. 2017;31:1627-1632.
- Arican O, Aral M, Sasmaz S, et al. Levels of TNF-alpha, IFN-gamma, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:273-279.
- Griffiths CE, Reich K, Lebwohl M, et al; UNCOVER-2 and UNCOVER-3 investigators. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Gordon KB, Blauvelt A, Papp KA, et al; UNCOVER-1 study group, UNCOVER-2 study group, UNCOVER-3 study group. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- FDA approves new psoriasis drug Taltz [news release]. Silver Spring, MD: US Food and Drug Administration; March 22, 2016. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm491872.htm. Accessed January 29, 2018.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286.
- Lebwohl M, Strober B, Mentor A, et al. Phase 3 studies comparing brodalumab with ustekinumab for psoriasis. N Engl J Med. 2015;373:1318-1328.
- FDA approves new psoriasis drug [news release]. Silver Spring, MD: US Food and Drug Administration; February 15, 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm541981.htm. Accessed January 29, 2018.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate-to-severe plaque psoriasis: results from the phase III, double-blinded placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Janssen announces U.S. FDA approval of Tremfya™ (guselkumab) for the treatment of moderate to severe plaque psoriasis [news release]. Horsham, PA: Johnson & Johnson; July 13, 2017. https://www.jnj.com/media-center/press-releases/janssen-announces-us-fda-approval-of-tremfya-guselkumab-for-the-treatment-of-moderate-to-severe-plaque-psoriasis. Accessed January 29, 2018.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE1 and reSURFACE 2): results from two randomized controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Risankizumab. AbbVie Inc website. https://www.abbvie.com/our-science/pipeline/risankizumab.html. Accessed January 29, 2018.
- Risankizumab meets all co-primary and ranked secondary endpoints, achieving significantly greater efficacy versus standard biologic therapies in three pivotal phase 3 psoriasis studies [news release]. North Chicago, IL: AbbVie Inc; October 26, 2017. https://news.abbvie.com/news/risankizumab-meets-all-co-primary-and-ranked-secondary-endpoints-achieving-significantly-greater-efficacy-versus-standard-biologic-therapies-in-three-pivotal-phase-3-psoriasis-studies.htm. Accessed January 29, 2018.
- Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83:991-1001.
- Reich K, Ortonne JP, Gottlieb AB, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab‘ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167:180-190.
- Kimball AB, Luger T, Gottlieb A, et al. Impact of ixekizumab on psoriasis itch severity and other psoriasis symptoms: results from 3 phase III psoriasis clinical trials. J Am Acad Dermatol. 2016;75:1156-1161.
- Dennehy EB, Zhang L, Amato D, et al. Ixekizumab is effective in subjects with moderate to severe plaque psoriasis with significant nail involvement: results from UNCOVER 3. J Drugs Dermatol. 2016;15:958-961.
- Gottlieb AB, Lacour JP, Korman N, et al. Treatment outcomes with ixekizumab in patients with moderate-to-severe psoriasis who have not received prior biological therapies: an integrated analysis of two phase III randomized studies. J Eur Acad Dermatol Venereol. 2017;31:679-685.
- Hueber W, Sands BE, Lewitsky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693-1700.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183-1190.
- Papp K, Menter A, Strober B, et al. Efficacy and safety of brodalumab in subpopulations of patients with difficult-to-treat moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2015;72:436-439.
- SILIQ [package insert]. Thousand Oaks, CA: Amgen, Inc; 2017.
- Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(suppl 2):ii18-ii23; discussion, ii24, ii25.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Amin M, Darji K, No DJ, et al. Review of phase III trial data on IL-23 inhibitors tildrakizumab and guselkumab for psoriasis. J Eur Acad Dermatol Venereol. 2017;31:1627-1632.
- Arican O, Aral M, Sasmaz S, et al. Levels of TNF-alpha, IFN-gamma, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:273-279.
- Griffiths CE, Reich K, Lebwohl M, et al; UNCOVER-2 and UNCOVER-3 investigators. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Gordon KB, Blauvelt A, Papp KA, et al; UNCOVER-1 study group, UNCOVER-2 study group, UNCOVER-3 study group. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- FDA approves new psoriasis drug Taltz [news release]. Silver Spring, MD: US Food and Drug Administration; March 22, 2016. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm491872.htm. Accessed January 29, 2018.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286.
- Lebwohl M, Strober B, Mentor A, et al. Phase 3 studies comparing brodalumab with ustekinumab for psoriasis. N Engl J Med. 2015;373:1318-1328.
- FDA approves new psoriasis drug [news release]. Silver Spring, MD: US Food and Drug Administration; February 15, 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm541981.htm. Accessed January 29, 2018.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate-to-severe plaque psoriasis: results from the phase III, double-blinded placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Janssen announces U.S. FDA approval of Tremfya™ (guselkumab) for the treatment of moderate to severe plaque psoriasis [news release]. Horsham, PA: Johnson & Johnson; July 13, 2017. https://www.jnj.com/media-center/press-releases/janssen-announces-us-fda-approval-of-tremfya-guselkumab-for-the-treatment-of-moderate-to-severe-plaque-psoriasis. Accessed January 29, 2018.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE1 and reSURFACE 2): results from two randomized controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Risankizumab. AbbVie Inc website. https://www.abbvie.com/our-science/pipeline/risankizumab.html. Accessed January 29, 2018.
- Risankizumab meets all co-primary and ranked secondary endpoints, achieving significantly greater efficacy versus standard biologic therapies in three pivotal phase 3 psoriasis studies [news release]. North Chicago, IL: AbbVie Inc; October 26, 2017. https://news.abbvie.com/news/risankizumab-meets-all-co-primary-and-ranked-secondary-endpoints-achieving-significantly-greater-efficacy-versus-standard-biologic-therapies-in-three-pivotal-phase-3-psoriasis-studies.htm. Accessed January 29, 2018.
- Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83:991-1001.
- Reich K, Ortonne JP, Gottlieb AB, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab‘ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167:180-190.
- Kimball AB, Luger T, Gottlieb A, et al. Impact of ixekizumab on psoriasis itch severity and other psoriasis symptoms: results from 3 phase III psoriasis clinical trials. J Am Acad Dermatol. 2016;75:1156-1161.
- Dennehy EB, Zhang L, Amato D, et al. Ixekizumab is effective in subjects with moderate to severe plaque psoriasis with significant nail involvement: results from UNCOVER 3. J Drugs Dermatol. 2016;15:958-961.
- Gottlieb AB, Lacour JP, Korman N, et al. Treatment outcomes with ixekizumab in patients with moderate-to-severe psoriasis who have not received prior biological therapies: an integrated analysis of two phase III randomized studies. J Eur Acad Dermatol Venereol. 2017;31:679-685.
- Hueber W, Sands BE, Lewitsky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693-1700.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183-1190.
- Papp K, Menter A, Strober B, et al. Efficacy and safety of brodalumab in subpopulations of patients with difficult-to-treat moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2015;72:436-439.
- SILIQ [package insert]. Thousand Oaks, CA: Amgen, Inc; 2017.
Practice Points
- Tumor necrosis factor α, IL-23, and IL-17A are key targets for psoriasis therapy based on an understanding of the key role that these cytokines play in the pathophysiology of disease.
- The biologic agents secukinumab and ixekizumab are approved for use in the management of psoriasis. Other biologics—brodalumab, bimekizumab, guselkumab, tildrakizumab, risankizumab, and certolizumab pegol—have been (and some continue to be) the focus of phase 2 and phase 3 clinical trials.
- Findings of several of those trials support the idea that therapies targeting IL-23, specifically its p19 subunit, but that spare IL-12 are effective against psoriasis.
- Longer-term studies are needed to determine whether the agents reviewed here, including those approved for clinical use, are suitable for prolonged administration.
Pushing the Limits: Developing a New Standard of Care for Psoriasis
We are now in the midst of a second revolution in the care of patients with psoriasis. Since biologic therapies for psoriasis were first introduced in 2003 with the approval of alefacept, the psoriasis treatment paradigm has shifted and continues to evolve. Interestingly, the first 2 biologic agents approved for psoriasis, alefacept and efalizumab, are no longer on the market in the United States.
We certainly have made progress since the early days of psoriasis treatment. Over the years, we have come to understand the nature of psoriasis as a systemic inflammatory condition rather than as simply a skin disease. With this knowledge, we have continued to identify systemic comorbidities associated with psoriasis, including cardiovascular risk, diabetes, and metabolic syndrome. It is therefore the role of the dermatologist to serve as the gatekeeper for these individuals and help to screen for comorbidities of psoriasis, as well as provide appropriate counseling and referral.
Additionally, psoriasis therapies have been approved for new segments of the population. In 2016, the US Food and Drug Administration approved a supplemental biologics license application for use of etanercept in children aged 4 years and older with chronic moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Last year, the US Food and Drug Administration also approved an expanded indication for ustekinumab for the treatment of adolescents (aged 12 years and older) with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy.
Another treatment development included the approval of apremilast as a new oral therapeutic option for psoriasis patients. This agent, which is approved for both psoriasis and psoriatic arthritis, has become an attractive therapy for many patients who are new to systemic treatment. Many patients prefer an oral medication and like the fact that no routine laboratory monitoring is required. Often patients leave their dermatologist’s office with 2- to 4-weeks’ worth of samples and can begin their course immediately.
A treat-to-target approach also has been established for psoriasis. In 2016, the Medical Board of the National Psoriasis Foundation1 created specific treatment goals in order to make achieving clear or almost clear skin the new standard of care. A consensus-building study conducted among 25 psoriasis experts revealed that the most preferred instrument for evaluating disease severity was body surface area (BSA). The time at which most participants preferred to evaluate patient response after starting a new psoriasis therapy was 3 months, and an acceptable response at this timepoint was considered to be either BSA involvement of 3% or less or improvement in BSA involvement of 75% or more compared to baseline. The target response at 3 months after starting treatment was BSA involvement of 1% or less. During the maintenance period, evaluation every 6 months was most preferred, and the target response at every 6-month follow-up evaluation was BSA involvement of 1% or less.1 These standards enable and encourage both clinicians and patients to maximize their treatment success.
Over the past several years, a variety of new biologic agents also have come to the market, including 3 IL-17 inhibitors (ixekizumab, brodalumab, and secukinumab) and one IL-23 inhibitor (guselkumab). All of these agents have added new options to the armamentarium for psoriasis treatment and are highly effective. Overall, the clinical improvement and safety profiles for these agents are promising, and these new drugs may be equal to or more efficacious than the currently available therapeutic options for psoriasis treatment; however, long-term studies are still needed to further establish the safety and efficacy profiles for these biologic agents. Even more novel therapies are in development, as will be discussed by Lee et al2 in this issue.
It is the purpose of this special issue to review new standards of care for psoriasis in 2018. We hope that you find this issue enjoyable and informative.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis [published online November 28, 2016]. J Am Acad Dermatol. 2017;76:290-298.
- Lee EB, Amin M, Bhutani T, et al. Emerging therapies in psoriasis: a systematic review. Cutis. 2018;101(suppl 3):5-9.
We are now in the midst of a second revolution in the care of patients with psoriasis. Since biologic therapies for psoriasis were first introduced in 2003 with the approval of alefacept, the psoriasis treatment paradigm has shifted and continues to evolve. Interestingly, the first 2 biologic agents approved for psoriasis, alefacept and efalizumab, are no longer on the market in the United States.
We certainly have made progress since the early days of psoriasis treatment. Over the years, we have come to understand the nature of psoriasis as a systemic inflammatory condition rather than as simply a skin disease. With this knowledge, we have continued to identify systemic comorbidities associated with psoriasis, including cardiovascular risk, diabetes, and metabolic syndrome. It is therefore the role of the dermatologist to serve as the gatekeeper for these individuals and help to screen for comorbidities of psoriasis, as well as provide appropriate counseling and referral.
Additionally, psoriasis therapies have been approved for new segments of the population. In 2016, the US Food and Drug Administration approved a supplemental biologics license application for use of etanercept in children aged 4 years and older with chronic moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Last year, the US Food and Drug Administration also approved an expanded indication for ustekinumab for the treatment of adolescents (aged 12 years and older) with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy.
Another treatment development included the approval of apremilast as a new oral therapeutic option for psoriasis patients. This agent, which is approved for both psoriasis and psoriatic arthritis, has become an attractive therapy for many patients who are new to systemic treatment. Many patients prefer an oral medication and like the fact that no routine laboratory monitoring is required. Often patients leave their dermatologist’s office with 2- to 4-weeks’ worth of samples and can begin their course immediately.
A treat-to-target approach also has been established for psoriasis. In 2016, the Medical Board of the National Psoriasis Foundation1 created specific treatment goals in order to make achieving clear or almost clear skin the new standard of care. A consensus-building study conducted among 25 psoriasis experts revealed that the most preferred instrument for evaluating disease severity was body surface area (BSA). The time at which most participants preferred to evaluate patient response after starting a new psoriasis therapy was 3 months, and an acceptable response at this timepoint was considered to be either BSA involvement of 3% or less or improvement in BSA involvement of 75% or more compared to baseline. The target response at 3 months after starting treatment was BSA involvement of 1% or less. During the maintenance period, evaluation every 6 months was most preferred, and the target response at every 6-month follow-up evaluation was BSA involvement of 1% or less.1 These standards enable and encourage both clinicians and patients to maximize their treatment success.
Over the past several years, a variety of new biologic agents also have come to the market, including 3 IL-17 inhibitors (ixekizumab, brodalumab, and secukinumab) and one IL-23 inhibitor (guselkumab). All of these agents have added new options to the armamentarium for psoriasis treatment and are highly effective. Overall, the clinical improvement and safety profiles for these agents are promising, and these new drugs may be equal to or more efficacious than the currently available therapeutic options for psoriasis treatment; however, long-term studies are still needed to further establish the safety and efficacy profiles for these biologic agents. Even more novel therapies are in development, as will be discussed by Lee et al2 in this issue.
It is the purpose of this special issue to review new standards of care for psoriasis in 2018. We hope that you find this issue enjoyable and informative.
We are now in the midst of a second revolution in the care of patients with psoriasis. Since biologic therapies for psoriasis were first introduced in 2003 with the approval of alefacept, the psoriasis treatment paradigm has shifted and continues to evolve. Interestingly, the first 2 biologic agents approved for psoriasis, alefacept and efalizumab, are no longer on the market in the United States.
We certainly have made progress since the early days of psoriasis treatment. Over the years, we have come to understand the nature of psoriasis as a systemic inflammatory condition rather than as simply a skin disease. With this knowledge, we have continued to identify systemic comorbidities associated with psoriasis, including cardiovascular risk, diabetes, and metabolic syndrome. It is therefore the role of the dermatologist to serve as the gatekeeper for these individuals and help to screen for comorbidities of psoriasis, as well as provide appropriate counseling and referral.
Additionally, psoriasis therapies have been approved for new segments of the population. In 2016, the US Food and Drug Administration approved a supplemental biologics license application for use of etanercept in children aged 4 years and older with chronic moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Last year, the US Food and Drug Administration also approved an expanded indication for ustekinumab for the treatment of adolescents (aged 12 years and older) with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy.
Another treatment development included the approval of apremilast as a new oral therapeutic option for psoriasis patients. This agent, which is approved for both psoriasis and psoriatic arthritis, has become an attractive therapy for many patients who are new to systemic treatment. Many patients prefer an oral medication and like the fact that no routine laboratory monitoring is required. Often patients leave their dermatologist’s office with 2- to 4-weeks’ worth of samples and can begin their course immediately.
A treat-to-target approach also has been established for psoriasis. In 2016, the Medical Board of the National Psoriasis Foundation1 created specific treatment goals in order to make achieving clear or almost clear skin the new standard of care. A consensus-building study conducted among 25 psoriasis experts revealed that the most preferred instrument for evaluating disease severity was body surface area (BSA). The time at which most participants preferred to evaluate patient response after starting a new psoriasis therapy was 3 months, and an acceptable response at this timepoint was considered to be either BSA involvement of 3% or less or improvement in BSA involvement of 75% or more compared to baseline. The target response at 3 months after starting treatment was BSA involvement of 1% or less. During the maintenance period, evaluation every 6 months was most preferred, and the target response at every 6-month follow-up evaluation was BSA involvement of 1% or less.1 These standards enable and encourage both clinicians and patients to maximize their treatment success.
Over the past several years, a variety of new biologic agents also have come to the market, including 3 IL-17 inhibitors (ixekizumab, brodalumab, and secukinumab) and one IL-23 inhibitor (guselkumab). All of these agents have added new options to the armamentarium for psoriasis treatment and are highly effective. Overall, the clinical improvement and safety profiles for these agents are promising, and these new drugs may be equal to or more efficacious than the currently available therapeutic options for psoriasis treatment; however, long-term studies are still needed to further establish the safety and efficacy profiles for these biologic agents. Even more novel therapies are in development, as will be discussed by Lee et al2 in this issue.
It is the purpose of this special issue to review new standards of care for psoriasis in 2018. We hope that you find this issue enjoyable and informative.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis [published online November 28, 2016]. J Am Acad Dermatol. 2017;76:290-298.
- Lee EB, Amin M, Bhutani T, et al. Emerging therapies in psoriasis: a systematic review. Cutis. 2018;101(suppl 3):5-9.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis [published online November 28, 2016]. J Am Acad Dermatol. 2017;76:290-298.
- Lee EB, Amin M, Bhutani T, et al. Emerging therapies in psoriasis: a systematic review. Cutis. 2018;101(suppl 3):5-9.
Asymptomatic Subcutaneous Nodule on the Cheek
The Diagnosis: Lymphoepitheliomalike Carcinoma of the Skin
The term lymphoepitheliomalike carcinoma of the skin (LELCS) initially was proposed by Swanson et al1 in 1988 when they described 5 patients with cutaneous neoplasms histologically resembling nasopharyngeal carcinoma, also known as lymphoepithelioma. A PubMed search of articles indexed for MEDLINE using the term lymphoepitheliomalike carcinoma of the skin revealed over 60 cases of LELCS since 1988. However, unlike nasopharyngeal carcinoma, LELCS has not been associated with Epstein-Barr virus, with the exception of 1 known reported case.2 The clinical appearance of LELCS is nonspecific but usually presents as a flesh-colored to erythematous nodule, as was seen in the current case. Lesions commonly are found on the head and neck in middle-aged to elderly patients with a slight male predominance.2
On histology, LELCS is characterized by aggregations of large, atypical epithelioid cells surrounded by a dense lymphoplasmocytic infiltrate (right quiz image). The neoplasm tends to reside within the deep dermis and/or subcutis1 without appreciable epidermal involvement (left quiz image). The atypical epithelioid cells demonstrate positive immunoreactivity for cytokeratins (right quiz image inset), p40/p63, and epithelial membrane antigen,3 and the surrounding lymphocytic infiltrate stains positively for leukocyte common antigen. The tumor histogenesis still is unknown, although an epidermal origin has been suggested given its staining pattern.2 Other investigators have postulated on an adnexal origin, citing the tumor's dermal location along with case reports describing possible glandular, sebaceous, or follicular differentiation.2,4
Treatment for LELCS can include either standard surgical excision or Mohs micrographic surgery, with radiation reserved for lymph node involvement, tumor recurrence, or poor surgical candidates.2,3,5 With appropriate therapy, prognosis may be considered favorable. Data from 49 LELCS patients presenting from 1988 and 2008 showed that 36 (73.5%) had no evidence of recurrence after treatment with standard surgical excision, 4 (8.2%) had local recurrence, and 6 (12.2%) developed lymph node metastasis, which led to death in 1 (2.0%) patient.2
Given the histologic similarity of LELCS to nasopharyngeal carcinoma, it is important to rule out the possibility of cutaneous metastasis, which can be done by testing for Epstein-Barr virus and performing either computed tomography imaging or comprehensive laryngoscopic examination of the head and neck region. In the current case, the patient was referred for laryngoscopy, at which time no suspicious lesions were identified. He subsequently underwent treatment with Mohs micrographic surgery, and the tumor was cleared after 2 surgical stages. At 5-month follow-up, the patient continued to do well with no signs of clinical recurrence.
Cutaneous lymphadenoma may be included in the differential diagnosis for LELCS on histopathology. This neoplasm is characterized by a well-circumscribed dermal proliferation of basaloid tumor islands within a fibrotic stroma (Figure 1). The basaloid cells may display peripheral palisading, and lymphocytes often are seen infiltrating the tumor lobules and the surrounding stroma (Figure 1 inset). Clinically, cutaneous lymphadenomas are slowly growing nodules that typically occur in young to middle-aged patients,4,6 unlike LELCS, which is more commonly observed in middle-aged to elderly patients.2
The dense lymphocytic infiltrate seen in LELCS may obscure the neoplastic epithelioid cells and in doing so may mimic a lymphoproliferative disorder, such as lymphomatoid papulosis (LyP). Lymphomatoid papulosis is a chronic CD30+ lymphoproliferative disorder consisting of recurrent crops of self-resolving papulonodules occurring on the trunk, arms, and legs. The average age of onset is in the third to fourth decades of life. Histology is dependent on the subtype; type A, the most common subtype, displays a wedge-shaped dermal infiltrate consisting of small lymphocytes (Figure 2) admixed with larger CD30+ atypical lymphocytes with prominent nucleoli (Figure 2 inset).7 Bizarre, binucleated forms resembling Reed-Sternberg cells also may be observed along with hallmark cells, which contain a horseshoe-shaped nucleus. The presence of admixed neutrophils and eosinophils also are common in type A LyP, a feature that is not characteristic of LELCS. Moreover, the atypical cells in LyP would not stain positively for epithelial markers as they would in LELCS.
Rosai-Dorfman disease is a rare condition that usually presents with painless cervical lymphadenopathy, typically in the first and second decades of life. Skin involvement can be seen in a small subset of extranodal cases, but cutaneous involvement alone is uncommon. On histopathology, cutaneous lesions are characterized by a dense dermal infiltrate of atypical histiocytes with vesicular nuclei and pale cytoplasm admixed with inflammatory cells, including lymphocytes, neutrophils, and plasma cells (Figure 3). Intracytoplasmic inflammatory cells or emperipolesis often is appreciated (Figure 3 inset).8,9 The atypical histiocytes stain positively for S100 and negatively for CD1a.
Lymphoepitheliomalike carcinoma of the skin sometimes is considered to be a poorly differentiated, inflamed variant of squamous cell carcinoma (SCC).10 A number of features may allow distinction of a primary cutaneous SCC from LELCS; for instance, SCC is more likely to have an epidermal connection and at least focal signs of squamous differentiation,11 which can include the presence of poorly differentiated epithelial cells with mitoses (Figure 4), keratin pearls, dyskeratotic cells, or intercellular bridges.12 Moreover, SCCs have a more variable surrounding inflammatory infiltrate compared to LELCS.
- Swanson SA, Cooper PH, Mills SE, et al. Lymphoepithelioma-like carcinoma of the skin. Mod Pathol. 1988;1:359-365.
- Aoki R, Mitsui H, Harada K, et al. A case of lymphoepithelioma-like carcinoma of the skin associated with Epstein-Barr virus infection. J Am Acad Dermatol. 2010;62:681-684.
- Morteza Abedi S, Salama S, Alowami S. Lymphoepithelioma-like carcinoma of the skin: case report and approach to surgical pathology sign out. Rare Tumors. 2013;5:E47.
- Requena L, Sánchez Yus E, Jiménez E, et al. Lymphoepithelioma-like carcinoma of the skin: a light-microscopic and immunohistochemical study. J Cutan Pathol. 1994;21:541-548.
- Welch PQ, Williams SB, Foss RD, et al. Lymphoepithelioma-like carcinoma of head and neck skin: a systematic analysis of 11 cases and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:78-86.
- Santa Cruz DJ, Barr RJ, Headington JT. Cutaneous lymphadenoma. Am J Surg Pathol. 1991;15:101-110.
- Patterson JW. Cutaneous infiltrates--lymphomatous and leukemic. In: Patterson JW, Hosler GA, eds. Weedon's Skin Pathology. 4th ed. London, United Kingdom: Churchill Livingstone; 2016:1186-1189.
- Patterson JW. Cutaneous infiltrates--nonlymphoid. In: Patterson JW, Hosler GA, eds. Weedon's Skin Pathology. 4th ed. London, United Kingdom: Churchill Livingstone; 2016:1158.
- Skiljo M, Garcia-Lora E, Tercedor J, et al. Purely cutaneous Rosai-Dorfman disease. Dermatology. 1995;191:49-51.
- Wang G, Bordeaux JS, Rowe DJ, et al. Lymphoepithelioma-like carcinoma vs inflamed squamous cell carcinoma of the skin. JAMA Dermatol. 2014;150:1367-1368.
- Hall G, Duncan A, Azurdia R, et al. Lymphoepithelioma-like carcinoma of the skin: a case with lymph node metastases at presentation. Am J Dermatopathol. 2006;28:211-215.
- Lind AC, Breer WA, Wick MR. Lymphoepithelioma-like carcinoma of the skin with apparent origin in the epidermis--a pattern or an entity? a case report. Cancer. 1999;85:884-890.
The Diagnosis: Lymphoepitheliomalike Carcinoma of the Skin
The term lymphoepitheliomalike carcinoma of the skin (LELCS) initially was proposed by Swanson et al1 in 1988 when they described 5 patients with cutaneous neoplasms histologically resembling nasopharyngeal carcinoma, also known as lymphoepithelioma. A PubMed search of articles indexed for MEDLINE using the term lymphoepitheliomalike carcinoma of the skin revealed over 60 cases of LELCS since 1988. However, unlike nasopharyngeal carcinoma, LELCS has not been associated with Epstein-Barr virus, with the exception of 1 known reported case.2 The clinical appearance of LELCS is nonspecific but usually presents as a flesh-colored to erythematous nodule, as was seen in the current case. Lesions commonly are found on the head and neck in middle-aged to elderly patients with a slight male predominance.2
On histology, LELCS is characterized by aggregations of large, atypical epithelioid cells surrounded by a dense lymphoplasmocytic infiltrate (right quiz image). The neoplasm tends to reside within the deep dermis and/or subcutis1 without appreciable epidermal involvement (left quiz image). The atypical epithelioid cells demonstrate positive immunoreactivity for cytokeratins (right quiz image inset), p40/p63, and epithelial membrane antigen,3 and the surrounding lymphocytic infiltrate stains positively for leukocyte common antigen. The tumor histogenesis still is unknown, although an epidermal origin has been suggested given its staining pattern.2 Other investigators have postulated on an adnexal origin, citing the tumor's dermal location along with case reports describing possible glandular, sebaceous, or follicular differentiation.2,4
Treatment for LELCS can include either standard surgical excision or Mohs micrographic surgery, with radiation reserved for lymph node involvement, tumor recurrence, or poor surgical candidates.2,3,5 With appropriate therapy, prognosis may be considered favorable. Data from 49 LELCS patients presenting from 1988 and 2008 showed that 36 (73.5%) had no evidence of recurrence after treatment with standard surgical excision, 4 (8.2%) had local recurrence, and 6 (12.2%) developed lymph node metastasis, which led to death in 1 (2.0%) patient.2
Given the histologic similarity of LELCS to nasopharyngeal carcinoma, it is important to rule out the possibility of cutaneous metastasis, which can be done by testing for Epstein-Barr virus and performing either computed tomography imaging or comprehensive laryngoscopic examination of the head and neck region. In the current case, the patient was referred for laryngoscopy, at which time no suspicious lesions were identified. He subsequently underwent treatment with Mohs micrographic surgery, and the tumor was cleared after 2 surgical stages. At 5-month follow-up, the patient continued to do well with no signs of clinical recurrence.
Cutaneous lymphadenoma may be included in the differential diagnosis for LELCS on histopathology. This neoplasm is characterized by a well-circumscribed dermal proliferation of basaloid tumor islands within a fibrotic stroma (Figure 1). The basaloid cells may display peripheral palisading, and lymphocytes often are seen infiltrating the tumor lobules and the surrounding stroma (Figure 1 inset). Clinically, cutaneous lymphadenomas are slowly growing nodules that typically occur in young to middle-aged patients,4,6 unlike LELCS, which is more commonly observed in middle-aged to elderly patients.2

The dense lymphocytic infiltrate seen in LELCS may obscure the neoplastic epithelioid cells and in doing so may mimic a lymphoproliferative disorder, such as lymphomatoid papulosis (LyP). Lymphomatoid papulosis is a chronic CD30+ lymphoproliferative disorder consisting of recurrent crops of self-resolving papulonodules occurring on the trunk, arms, and legs. The average age of onset is in the third to fourth decades of life. Histology is dependent on the subtype; type A, the most common subtype, displays a wedge-shaped dermal infiltrate consisting of small lymphocytes (Figure 2) admixed with larger CD30+ atypical lymphocytes with prominent nucleoli (Figure 2 inset).7 Bizarre, binucleated forms resembling Reed-Sternberg cells also may be observed along with hallmark cells, which contain a horseshoe-shaped nucleus. The presence of admixed neutrophils and eosinophils also are common in type A LyP, a feature that is not characteristic of LELCS. Moreover, the atypical cells in LyP would not stain positively for epithelial markers as they would in LELCS.

Rosai-Dorfman disease is a rare condition that usually presents with painless cervical lymphadenopathy, typically in the first and second decades of life. Skin involvement can be seen in a small subset of extranodal cases, but cutaneous involvement alone is uncommon. On histopathology, cutaneous lesions are characterized by a dense dermal infiltrate of atypical histiocytes with vesicular nuclei and pale cytoplasm admixed with inflammatory cells, including lymphocytes, neutrophils, and plasma cells (Figure 3). Intracytoplasmic inflammatory cells or emperipolesis often is appreciated (Figure 3 inset).8,9 The atypical histiocytes stain positively for S100 and negatively for CD1a.

Lymphoepitheliomalike carcinoma of the skin sometimes is considered to be a poorly differentiated, inflamed variant of squamous cell carcinoma (SCC).10 A number of features may allow distinction of a primary cutaneous SCC from LELCS; for instance, SCC is more likely to have an epidermal connection and at least focal signs of squamous differentiation,11 which can include the presence of poorly differentiated epithelial cells with mitoses (Figure 4), keratin pearls, dyskeratotic cells, or intercellular bridges.12 Moreover, SCCs have a more variable surrounding inflammatory infiltrate compared to LELCS.

The Diagnosis: Lymphoepitheliomalike Carcinoma of the Skin
The term lymphoepitheliomalike carcinoma of the skin (LELCS) initially was proposed by Swanson et al1 in 1988 when they described 5 patients with cutaneous neoplasms histologically resembling nasopharyngeal carcinoma, also known as lymphoepithelioma. A PubMed search of articles indexed for MEDLINE using the term lymphoepitheliomalike carcinoma of the skin revealed over 60 cases of LELCS since 1988. However, unlike nasopharyngeal carcinoma, LELCS has not been associated with Epstein-Barr virus, with the exception of 1 known reported case.2 The clinical appearance of LELCS is nonspecific but usually presents as a flesh-colored to erythematous nodule, as was seen in the current case. Lesions commonly are found on the head and neck in middle-aged to elderly patients with a slight male predominance.2
On histology, LELCS is characterized by aggregations of large, atypical epithelioid cells surrounded by a dense lymphoplasmocytic infiltrate (right quiz image). The neoplasm tends to reside within the deep dermis and/or subcutis1 without appreciable epidermal involvement (left quiz image). The atypical epithelioid cells demonstrate positive immunoreactivity for cytokeratins (right quiz image inset), p40/p63, and epithelial membrane antigen,3 and the surrounding lymphocytic infiltrate stains positively for leukocyte common antigen. The tumor histogenesis still is unknown, although an epidermal origin has been suggested given its staining pattern.2 Other investigators have postulated on an adnexal origin, citing the tumor's dermal location along with case reports describing possible glandular, sebaceous, or follicular differentiation.2,4
Treatment for LELCS can include either standard surgical excision or Mohs micrographic surgery, with radiation reserved for lymph node involvement, tumor recurrence, or poor surgical candidates.2,3,5 With appropriate therapy, prognosis may be considered favorable. Data from 49 LELCS patients presenting from 1988 and 2008 showed that 36 (73.5%) had no evidence of recurrence after treatment with standard surgical excision, 4 (8.2%) had local recurrence, and 6 (12.2%) developed lymph node metastasis, which led to death in 1 (2.0%) patient.2
Given the histologic similarity of LELCS to nasopharyngeal carcinoma, it is important to rule out the possibility of cutaneous metastasis, which can be done by testing for Epstein-Barr virus and performing either computed tomography imaging or comprehensive laryngoscopic examination of the head and neck region. In the current case, the patient was referred for laryngoscopy, at which time no suspicious lesions were identified. He subsequently underwent treatment with Mohs micrographic surgery, and the tumor was cleared after 2 surgical stages. At 5-month follow-up, the patient continued to do well with no signs of clinical recurrence.
Cutaneous lymphadenoma may be included in the differential diagnosis for LELCS on histopathology. This neoplasm is characterized by a well-circumscribed dermal proliferation of basaloid tumor islands within a fibrotic stroma (Figure 1). The basaloid cells may display peripheral palisading, and lymphocytes often are seen infiltrating the tumor lobules and the surrounding stroma (Figure 1 inset). Clinically, cutaneous lymphadenomas are slowly growing nodules that typically occur in young to middle-aged patients,4,6 unlike LELCS, which is more commonly observed in middle-aged to elderly patients.2

The dense lymphocytic infiltrate seen in LELCS may obscure the neoplastic epithelioid cells and in doing so may mimic a lymphoproliferative disorder, such as lymphomatoid papulosis (LyP). Lymphomatoid papulosis is a chronic CD30+ lymphoproliferative disorder consisting of recurrent crops of self-resolving papulonodules occurring on the trunk, arms, and legs. The average age of onset is in the third to fourth decades of life. Histology is dependent on the subtype; type A, the most common subtype, displays a wedge-shaped dermal infiltrate consisting of small lymphocytes (Figure 2) admixed with larger CD30+ atypical lymphocytes with prominent nucleoli (Figure 2 inset).7 Bizarre, binucleated forms resembling Reed-Sternberg cells also may be observed along with hallmark cells, which contain a horseshoe-shaped nucleus. The presence of admixed neutrophils and eosinophils also are common in type A LyP, a feature that is not characteristic of LELCS. Moreover, the atypical cells in LyP would not stain positively for epithelial markers as they would in LELCS.

Rosai-Dorfman disease is a rare condition that usually presents with painless cervical lymphadenopathy, typically in the first and second decades of life. Skin involvement can be seen in a small subset of extranodal cases, but cutaneous involvement alone is uncommon. On histopathology, cutaneous lesions are characterized by a dense dermal infiltrate of atypical histiocytes with vesicular nuclei and pale cytoplasm admixed with inflammatory cells, including lymphocytes, neutrophils, and plasma cells (Figure 3). Intracytoplasmic inflammatory cells or emperipolesis often is appreciated (Figure 3 inset).8,9 The atypical histiocytes stain positively for S100 and negatively for CD1a.

Lymphoepitheliomalike carcinoma of the skin sometimes is considered to be a poorly differentiated, inflamed variant of squamous cell carcinoma (SCC).10 A number of features may allow distinction of a primary cutaneous SCC from LELCS; for instance, SCC is more likely to have an epidermal connection and at least focal signs of squamous differentiation,11 which can include the presence of poorly differentiated epithelial cells with mitoses (Figure 4), keratin pearls, dyskeratotic cells, or intercellular bridges.12 Moreover, SCCs have a more variable surrounding inflammatory infiltrate compared to LELCS.

- Swanson SA, Cooper PH, Mills SE, et al. Lymphoepithelioma-like carcinoma of the skin. Mod Pathol. 1988;1:359-365.
- Aoki R, Mitsui H, Harada K, et al. A case of lymphoepithelioma-like carcinoma of the skin associated with Epstein-Barr virus infection. J Am Acad Dermatol. 2010;62:681-684.
- Morteza Abedi S, Salama S, Alowami S. Lymphoepithelioma-like carcinoma of the skin: case report and approach to surgical pathology sign out. Rare Tumors. 2013;5:E47.
- Requena L, Sánchez Yus E, Jiménez E, et al. Lymphoepithelioma-like carcinoma of the skin: a light-microscopic and immunohistochemical study. J Cutan Pathol. 1994;21:541-548.
- Welch PQ, Williams SB, Foss RD, et al. Lymphoepithelioma-like carcinoma of head and neck skin: a systematic analysis of 11 cases and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:78-86.
- Santa Cruz DJ, Barr RJ, Headington JT. Cutaneous lymphadenoma. Am J Surg Pathol. 1991;15:101-110.
- Patterson JW. Cutaneous infiltrates--lymphomatous and leukemic. In: Patterson JW, Hosler GA, eds. Weedon's Skin Pathology. 4th ed. London, United Kingdom: Churchill Livingstone; 2016:1186-1189.
- Patterson JW. Cutaneous infiltrates--nonlymphoid. In: Patterson JW, Hosler GA, eds. Weedon's Skin Pathology. 4th ed. London, United Kingdom: Churchill Livingstone; 2016:1158.
- Skiljo M, Garcia-Lora E, Tercedor J, et al. Purely cutaneous Rosai-Dorfman disease. Dermatology. 1995;191:49-51.
- Wang G, Bordeaux JS, Rowe DJ, et al. Lymphoepithelioma-like carcinoma vs inflamed squamous cell carcinoma of the skin. JAMA Dermatol. 2014;150:1367-1368.
- Hall G, Duncan A, Azurdia R, et al. Lymphoepithelioma-like carcinoma of the skin: a case with lymph node metastases at presentation. Am J Dermatopathol. 2006;28:211-215.
- Lind AC, Breer WA, Wick MR. Lymphoepithelioma-like carcinoma of the skin with apparent origin in the epidermis--a pattern or an entity? a case report. Cancer. 1999;85:884-890.
- Swanson SA, Cooper PH, Mills SE, et al. Lymphoepithelioma-like carcinoma of the skin. Mod Pathol. 1988;1:359-365.
- Aoki R, Mitsui H, Harada K, et al. A case of lymphoepithelioma-like carcinoma of the skin associated with Epstein-Barr virus infection. J Am Acad Dermatol. 2010;62:681-684.
- Morteza Abedi S, Salama S, Alowami S. Lymphoepithelioma-like carcinoma of the skin: case report and approach to surgical pathology sign out. Rare Tumors. 2013;5:E47.
- Requena L, Sánchez Yus E, Jiménez E, et al. Lymphoepithelioma-like carcinoma of the skin: a light-microscopic and immunohistochemical study. J Cutan Pathol. 1994;21:541-548.
- Welch PQ, Williams SB, Foss RD, et al. Lymphoepithelioma-like carcinoma of head and neck skin: a systematic analysis of 11 cases and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:78-86.
- Santa Cruz DJ, Barr RJ, Headington JT. Cutaneous lymphadenoma. Am J Surg Pathol. 1991;15:101-110.
- Patterson JW. Cutaneous infiltrates--lymphomatous and leukemic. In: Patterson JW, Hosler GA, eds. Weedon's Skin Pathology. 4th ed. London, United Kingdom: Churchill Livingstone; 2016:1186-1189.
- Patterson JW. Cutaneous infiltrates--nonlymphoid. In: Patterson JW, Hosler GA, eds. Weedon's Skin Pathology. 4th ed. London, United Kingdom: Churchill Livingstone; 2016:1158.
- Skiljo M, Garcia-Lora E, Tercedor J, et al. Purely cutaneous Rosai-Dorfman disease. Dermatology. 1995;191:49-51.
- Wang G, Bordeaux JS, Rowe DJ, et al. Lymphoepithelioma-like carcinoma vs inflamed squamous cell carcinoma of the skin. JAMA Dermatol. 2014;150:1367-1368.
- Hall G, Duncan A, Azurdia R, et al. Lymphoepithelioma-like carcinoma of the skin: a case with lymph node metastases at presentation. Am J Dermatopathol. 2006;28:211-215.
- Lind AC, Breer WA, Wick MR. Lymphoepithelioma-like carcinoma of the skin with apparent origin in the epidermis--a pattern or an entity? a case report. Cancer. 1999;85:884-890.
An 81-year-old man with history of melanoma and nonmelanoma skin cancer presented with a subcutaneous nodule on the left cheek of 3 months' duration. The lesion was reportedly asymptomatic and measured 2.6×2.9 cm. A punch biopsy of the lesion was obtained for histopathologic evaluation.
Bullous Eruption in 2 Brothers
The Diagnosis: Bullous Scabies
Scabies infection is caused by the mite Sarcoptes scabiei var hominis. It is commonly transmitted via direct skin-to-skin contact.1 Classic manifestations include pruritus that worsens at night. It commonly presents with burrows and papules in the interdigital web spaces, as well as flexor surfaces of the wrists, elbows, axillae, buttocks, and genitalia. Pruritus occurs from infestation and delayed hypersensitivity reaction to mites. The recommended treatment of classic scabies is permethrin cream 5% for all occupants of the household and a repeat application for just the patients in 1 week. Posttreatment pruritus can last up to 3 weeks.2 At-risk populations include school-aged children and patients in long-term care facilities.
In our case, bullous lesions in a classic distribution with potassium hydroxide preparation of a scabietic mite (Figure) confirmed the diagnosis of bullous scabies. Treatment of bullous scabies is the same as classic scabies. Both patients were treated with 1 application of permethrin cream 5% before we evaluated them. We instructed to repeat application in 7 days for both boys and all family members.

Bullae may be secondary to hypersensitivity response3 or superinfection with Staphylococcus aureus causing bullous impetigo.4 Bullous scabies may present a diagnostic challenge and requires a high index of suspicion. Although childhood bullous pemphigoid can involve the palms and soles, patients usually present in infancy. Diagnoses such as dyshidrotic eczema and bullous tinea can present with pustules on the hands and feet; however, involvement of the genitalia would be uncommon.
- Chosidow O. Clinical practices. scabies. N Engl J Med. 2006;354:1718-1727.
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717-725.
- Ansarin H, Jalali MH, Mazloomi S, et al. Scabies presenting with bullous pemphigoid-like lesions. Dermatol Online J. 2006;12:19.
- Herman PS. Letter: scabies and bullae. JAMA. 1975;231:1134.
The Diagnosis: Bullous Scabies
Scabies infection is caused by the mite Sarcoptes scabiei var hominis. It is commonly transmitted via direct skin-to-skin contact.1 Classic manifestations include pruritus that worsens at night. It commonly presents with burrows and papules in the interdigital web spaces, as well as flexor surfaces of the wrists, elbows, axillae, buttocks, and genitalia. Pruritus occurs from infestation and delayed hypersensitivity reaction to mites. The recommended treatment of classic scabies is permethrin cream 5% for all occupants of the household and a repeat application for just the patients in 1 week. Posttreatment pruritus can last up to 3 weeks.2 At-risk populations include school-aged children and patients in long-term care facilities.
In our case, bullous lesions in a classic distribution with potassium hydroxide preparation of a scabietic mite (Figure) confirmed the diagnosis of bullous scabies. Treatment of bullous scabies is the same as classic scabies. Both patients were treated with 1 application of permethrin cream 5% before we evaluated them. We instructed to repeat application in 7 days for both boys and all family members.

Bullae may be secondary to hypersensitivity response3 or superinfection with Staphylococcus aureus causing bullous impetigo.4 Bullous scabies may present a diagnostic challenge and requires a high index of suspicion. Although childhood bullous pemphigoid can involve the palms and soles, patients usually present in infancy. Diagnoses such as dyshidrotic eczema and bullous tinea can present with pustules on the hands and feet; however, involvement of the genitalia would be uncommon.
The Diagnosis: Bullous Scabies
Scabies infection is caused by the mite Sarcoptes scabiei var hominis. It is commonly transmitted via direct skin-to-skin contact.1 Classic manifestations include pruritus that worsens at night. It commonly presents with burrows and papules in the interdigital web spaces, as well as flexor surfaces of the wrists, elbows, axillae, buttocks, and genitalia. Pruritus occurs from infestation and delayed hypersensitivity reaction to mites. The recommended treatment of classic scabies is permethrin cream 5% for all occupants of the household and a repeat application for just the patients in 1 week. Posttreatment pruritus can last up to 3 weeks.2 At-risk populations include school-aged children and patients in long-term care facilities.
In our case, bullous lesions in a classic distribution with potassium hydroxide preparation of a scabietic mite (Figure) confirmed the diagnosis of bullous scabies. Treatment of bullous scabies is the same as classic scabies. Both patients were treated with 1 application of permethrin cream 5% before we evaluated them. We instructed to repeat application in 7 days for both boys and all family members.

Bullae may be secondary to hypersensitivity response3 or superinfection with Staphylococcus aureus causing bullous impetigo.4 Bullous scabies may present a diagnostic challenge and requires a high index of suspicion. Although childhood bullous pemphigoid can involve the palms and soles, patients usually present in infancy. Diagnoses such as dyshidrotic eczema and bullous tinea can present with pustules on the hands and feet; however, involvement of the genitalia would be uncommon.
- Chosidow O. Clinical practices. scabies. N Engl J Med. 2006;354:1718-1727.
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717-725.
- Ansarin H, Jalali MH, Mazloomi S, et al. Scabies presenting with bullous pemphigoid-like lesions. Dermatol Online J. 2006;12:19.
- Herman PS. Letter: scabies and bullae. JAMA. 1975;231:1134.
- Chosidow O. Clinical practices. scabies. N Engl J Med. 2006;354:1718-1727.
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717-725.
- Ansarin H, Jalali MH, Mazloomi S, et al. Scabies presenting with bullous pemphigoid-like lesions. Dermatol Online J. 2006;12:19.
- Herman PS. Letter: scabies and bullae. JAMA. 1975;231:1134.
Brothers aged 7 and 8 years with a history of atopic dermatitis presented to the emergency department with similar diffuse pruritic eruptions of 1 week's duration. They previously were treated with permethrin cream 5% without improvement. Two days prior to presentation they developed painful pustules on the hands and feet. No other family members were affected. Physical examination revealed numerous yellow pustules and vesicles in the interdigital web spaces, elbows, and knees. Notably, the penis and scrotum also were involved in both brothers. A potassium hydroxide preparation of small pustules was obtained.
Hair tracks HIV antiretroviral adherence
BOSTON – Hair is the key to knowing if patients have been taking their HIV antiretrovirals. In fact, hair levels were the strongest independent predictor of virologic control in a major trial of HIV-positive, treatment-naive patients that compared atazanavir, darunavir, and raltegravir-based regimens. Virologic success was similar in all three arms, but the raltegravir regimen was better tolerated than the protease inhibitor arms (Ann Intern Med. 2014 Oct 7;161[7]:461-71).
Because patient self-reporting is notoriously unreliable, the investigators checked hair for adherence. The results for 599 participants followed for a median of 217 weeks were reported at the Conference on Retroviruses and Opportunistic Infections. Hair samples were collected at weeks 4, 8, 16, and then quarterly; concentrations of the three drugs were measured by liquid chromatography-tandem mass spectrometry.
Rates of virologic failure were 26%, 6%, and 3% for patients with hair levels in the lowest, middle, and highest tertiles, respectively. Lower hair antiretroviral (ARV) levels strongly predicted virologic failure (hazard ratio for every twofold decrease in hair level 1.69, 95% confidence interval, 1.43-2.04, P less than .001). Results were consistent across drugs and for each drug individually.
Patients with ARV hair levels in the lowest tertile were 6.8 times more likely to fail than were patients in the highest tertile. The actual level that was considered low depended on the drug.
Meanwhile, self-reported adherence – a median of 100% in each arm – barely correlated with actual ARV levels (Pearson’s r 0.15).
In the end, “hair levels were the strongest independent predictor of how you did,” said lead investigator Monica Gandhi, MD, associate chief of the Division of HIV, Infectious Diseases, and Global Medicine at the University of California, San Francisco, General Hospital.
Testing for hair levels is already a part of clinical care at UCSF, which has a hair analysis lab. In one case, a 21-year-old man seroconverted after saying he was taking pre-exposure prophylaxis (PrEP) perfectly. After a check of his hair, it turned out that he was, but had caught a drug-resistant virus. Another patient who seroconverted on PrEP turned out to have missed some doses a few months before.
Ideally, hair testing could be used early on to provide extra help to patients who prove to have trouble with adherence. Text messaging – as long as the doctor actually responds – is effective, but so is just bringing people in and asking them how they needed to be helped, Dr. Gandhi said.
All that’s required for testing is a small bit of hair from the back of the head, cut close to the scalp. It’s easy and quick, but even so, acceptance was only 55% in the trial. “Where it seems to not be accepted in this country is in men who have sex with men.” Sometimes patients worry about their hairstyle, but “it isn’t very disruptive because it’s a very small amount of hair,” she said.
Bleaching is the only hair treatment that seems to affect ARV levels, reducing them. Short hair is fine, but it keeps less of a record over time. In general, “hair levels are more helpful in PrEP than in treatment, because in treatment we need a real time point of care test” for adherence, Dr. Gandhi said; her team has come up with a urine screen for tenofovir that looks promising.
The mean age in the study was 38 years. About a third of the subjects were women, and a third were black. The findings were similar for men and women.
The drugs in the trial were provided by their manufacturers. Dr. Gandhi had no relevant disclosures.
SOURCE: Gandhi M et al. Abstract 24.
BOSTON – Hair is the key to knowing if patients have been taking their HIV antiretrovirals. In fact, hair levels were the strongest independent predictor of virologic control in a major trial of HIV-positive, treatment-naive patients that compared atazanavir, darunavir, and raltegravir-based regimens. Virologic success was similar in all three arms, but the raltegravir regimen was better tolerated than the protease inhibitor arms (Ann Intern Med. 2014 Oct 7;161[7]:461-71).
Because patient self-reporting is notoriously unreliable, the investigators checked hair for adherence. The results for 599 participants followed for a median of 217 weeks were reported at the Conference on Retroviruses and Opportunistic Infections. Hair samples were collected at weeks 4, 8, 16, and then quarterly; concentrations of the three drugs were measured by liquid chromatography-tandem mass spectrometry.
Rates of virologic failure were 26%, 6%, and 3% for patients with hair levels in the lowest, middle, and highest tertiles, respectively. Lower hair antiretroviral (ARV) levels strongly predicted virologic failure (hazard ratio for every twofold decrease in hair level 1.69, 95% confidence interval, 1.43-2.04, P less than .001). Results were consistent across drugs and for each drug individually.
Patients with ARV hair levels in the lowest tertile were 6.8 times more likely to fail than were patients in the highest tertile. The actual level that was considered low depended on the drug.
Meanwhile, self-reported adherence – a median of 100% in each arm – barely correlated with actual ARV levels (Pearson’s r 0.15).
In the end, “hair levels were the strongest independent predictor of how you did,” said lead investigator Monica Gandhi, MD, associate chief of the Division of HIV, Infectious Diseases, and Global Medicine at the University of California, San Francisco, General Hospital.
Testing for hair levels is already a part of clinical care at UCSF, which has a hair analysis lab. In one case, a 21-year-old man seroconverted after saying he was taking pre-exposure prophylaxis (PrEP) perfectly. After a check of his hair, it turned out that he was, but had caught a drug-resistant virus. Another patient who seroconverted on PrEP turned out to have missed some doses a few months before.
Ideally, hair testing could be used early on to provide extra help to patients who prove to have trouble with adherence. Text messaging – as long as the doctor actually responds – is effective, but so is just bringing people in and asking them how they needed to be helped, Dr. Gandhi said.
All that’s required for testing is a small bit of hair from the back of the head, cut close to the scalp. It’s easy and quick, but even so, acceptance was only 55% in the trial. “Where it seems to not be accepted in this country is in men who have sex with men.” Sometimes patients worry about their hairstyle, but “it isn’t very disruptive because it’s a very small amount of hair,” she said.
Bleaching is the only hair treatment that seems to affect ARV levels, reducing them. Short hair is fine, but it keeps less of a record over time. In general, “hair levels are more helpful in PrEP than in treatment, because in treatment we need a real time point of care test” for adherence, Dr. Gandhi said; her team has come up with a urine screen for tenofovir that looks promising.
The mean age in the study was 38 years. About a third of the subjects were women, and a third were black. The findings were similar for men and women.
The drugs in the trial were provided by their manufacturers. Dr. Gandhi had no relevant disclosures.
SOURCE: Gandhi M et al. Abstract 24.
BOSTON – Hair is the key to knowing if patients have been taking their HIV antiretrovirals. In fact, hair levels were the strongest independent predictor of virologic control in a major trial of HIV-positive, treatment-naive patients that compared atazanavir, darunavir, and raltegravir-based regimens. Virologic success was similar in all three arms, but the raltegravir regimen was better tolerated than the protease inhibitor arms (Ann Intern Med. 2014 Oct 7;161[7]:461-71).
Because patient self-reporting is notoriously unreliable, the investigators checked hair for adherence. The results for 599 participants followed for a median of 217 weeks were reported at the Conference on Retroviruses and Opportunistic Infections. Hair samples were collected at weeks 4, 8, 16, and then quarterly; concentrations of the three drugs were measured by liquid chromatography-tandem mass spectrometry.
Rates of virologic failure were 26%, 6%, and 3% for patients with hair levels in the lowest, middle, and highest tertiles, respectively. Lower hair antiretroviral (ARV) levels strongly predicted virologic failure (hazard ratio for every twofold decrease in hair level 1.69, 95% confidence interval, 1.43-2.04, P less than .001). Results were consistent across drugs and for each drug individually.
Patients with ARV hair levels in the lowest tertile were 6.8 times more likely to fail than were patients in the highest tertile. The actual level that was considered low depended on the drug.
Meanwhile, self-reported adherence – a median of 100% in each arm – barely correlated with actual ARV levels (Pearson’s r 0.15).
In the end, “hair levels were the strongest independent predictor of how you did,” said lead investigator Monica Gandhi, MD, associate chief of the Division of HIV, Infectious Diseases, and Global Medicine at the University of California, San Francisco, General Hospital.
Testing for hair levels is already a part of clinical care at UCSF, which has a hair analysis lab. In one case, a 21-year-old man seroconverted after saying he was taking pre-exposure prophylaxis (PrEP) perfectly. After a check of his hair, it turned out that he was, but had caught a drug-resistant virus. Another patient who seroconverted on PrEP turned out to have missed some doses a few months before.
Ideally, hair testing could be used early on to provide extra help to patients who prove to have trouble with adherence. Text messaging – as long as the doctor actually responds – is effective, but so is just bringing people in and asking them how they needed to be helped, Dr. Gandhi said.
All that’s required for testing is a small bit of hair from the back of the head, cut close to the scalp. It’s easy and quick, but even so, acceptance was only 55% in the trial. “Where it seems to not be accepted in this country is in men who have sex with men.” Sometimes patients worry about their hairstyle, but “it isn’t very disruptive because it’s a very small amount of hair,” she said.
Bleaching is the only hair treatment that seems to affect ARV levels, reducing them. Short hair is fine, but it keeps less of a record over time. In general, “hair levels are more helpful in PrEP than in treatment, because in treatment we need a real time point of care test” for adherence, Dr. Gandhi said; her team has come up with a urine screen for tenofovir that looks promising.
The mean age in the study was 38 years. About a third of the subjects were women, and a third were black. The findings were similar for men and women.
The drugs in the trial were provided by their manufacturers. Dr. Gandhi had no relevant disclosures.
SOURCE: Gandhi M et al. Abstract 24.
AT CROI
Key clinical point: Hair is a solid alternative to blood, urine, and self-report for HIV drug adherence.
Major finding: Virologic failure was 6,8 times more likely among subjects with antiretroviral hair levels in the lowest tertile, compared with those in the highest tertile.
Study details: Phase 3 trial with 599 subjects.
Disclosures: The drugs in the trial were provided by their manufacturers. The lead investigator had no relevant disclosures.
Source: Gandhi M et al. Abstract 24.
Risking it all on the miracle of teamwork
On Feb. 4, 2018, with his team narrowly leading the New England Patriots in Super Bowl 52, Philadelphia Eagles head coach Doug Pederson made an audacious 4th-and-goal call. At the suggestion of backup quarterback Nick Foles, Pederson chose to rely on his team’s ability to execute the “Philly Special.” This was a risky trick play that was rehearsed but never tested, and one which could prove disastrous unless executed just right. With 34 seconds left in the first half, the Eagles pulled it off. Foles caught the ball in the end zone, securing his team’s place in football history and becoming the first quarterback to both throw for and catch a touchdown in one Super Bowl. He was named MVP and led the team to its first NFL title in 58 years.
For those of us who call Philadelphia our home, Super Bowl 52 represented so much more than just a victory, it was a miracle. We have long endured the highs and lows of Philadelphia football, watching as year after year our hopes were dashed by coaches and players who showed such promise, yet demonstrated such disappointment. But this year everything changed.
True fans could sense something different in the weeks leading up to that cold February day in Minneapolis. As the Eagle’s chances of competing in the Super Bowl grew more and more possible, the narrative wasn’t about any star player or member of the coaching staff, but instead the story of an incredible team. Even after the injury of starting quarterback and football phenom Carson Wentz in week 14, players and fans never lost hope in the promise of victory. Finally, Philadelphia had the team that could, and would, pull off something that had heretofore seemed like only an impossible dream.
It occurs to us that physicians should find the story of the Philadelphia Eagles not only inspirational, but also aspirational, even more so after reading the original research published by Dr. Richard Young, et al. in the February issue of Family Medicine.1 In this article, Dr. Young and his colleagues observed physicians during 982 patient encounters. The group measured the total visit time, face-to-face time, non-face time, and EHR work time (before, during, and after patient hours). The results weren’t surprising: Physicians spend more time working in the EHR than they spend in face-to-face time with patients.
This study confirmed prior work done by Ardnt et al. published in the fall in Annals of Family Medicine,2 which demonstrated that “primary care physicians spend more than half their workday, nearly 6 hours, interacting with the EHR during and after clinic hours.” Sadly, despite improving technology, the chasm between interacting with computers and interacting with actual patients only seems to be widening. To preserve the sanctity of the physician-patient relationship, we are forced to consider a completely new approach to how we practice: team-based care.
Team-based care isn’t a new idea, but it is being embraced with new fervor in the era of electronic health records. This is because the blessing — and curse — of the EHR is the vast amount of information that can be stored and accessed while caring for patients. To take advantage of this, doctors have been forced to become the primary agents for data entry and retrieval, something that is nearly impossible to do effectively while performing the cognitive work of a highly educated clinician. Rather than allowing us to take better care of patients, EHRs seem to have a paradoxical effect, limiting “face-to-face” time and squelching our efforts to address anything outside the immediate issues at hand. To improve the experience for us and our patients, we need to begin to rely on others.
To start, consider how a team can help support your documentation. As we’ve written about before, scribe services can be a tremendous benefit but aren’t the only way to improve efficiency. Medical assistants and nursing staff need to be encouraged to operate at the top level of their license, documenting where allowable and even queueing up orders, medication refills, and preventative care interventions when appropriate. This can be tremendously useful during previsit planning and can ensure that nothing is missed during the patient encounter.
Team-based care can also extend far beyond the EHR. For example, care coordinators can be employed to focus on specific high- and rising-risk patient populations. These health care professionals (typically nurses) reach out directly to patients and review their care, and even schedule visits with patients independently of the physician. This establishes therapeutic relationships that have been shown to prevent disease exacerbations and hospital readmissions, greatly reducing the cost of care.
Some facilities are now also using scheduling advocates, charged with facilitating referrals, arranging specialist and diagnostic appointments, and following up with patients to make sure they’ve successfully navigated the health care landscape. Behavioral health specialists and clinical pharmacists are also making their way into physician practices to expand the scope of offerings and decompress the burden on physicians. While these all have an associated cost, changes in the way physicians get paid are making the extra support economical and often necessary to satisfy the requirements under risk-based and fee-for-value contracts. We also predict that practices that choose to eschew the team approach to care will lose a competitive advantage in a health care market that is becoming more and more consumer driven. Patients, as well as providers, see the benefits of highly effective care teams.
Following the Eagles’ dramatic Super Bowl victory, coach Doug Pederson addressed his team in the locker room with these words: “This is a team game. As we’ve said before, an individual can make a difference, but a team makes a miracle.” While we as physicians may easily become jaded by the “miracles” of modern medicine, our patients have not yet lost hope in our ability to deliver on the promise of victory. To meet their expectations, we need to acknowledge that we will no longer be able to make it into the end zone alone; instead — in the game of modern medicine — we’ll need a team to take us there.
- Young RA, Burge SK, Kumar KA, Wilson JM, Ortiz DF. A Time-Motion Study of Primary Care Physicians’ Work in the Electronic Health Record Era. Fam Med. 2018 February;50(2):91-9.
- Arndt BG, Beasley JW, Watkinson MD, Temte JL, Tuan W-J, Sinsky CA, Gilchrist VJ..Ann Fam Med. 2017 September/October;15(5)5:419-26.
Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Notte is a family physician and Associate Chief Medical Information Officer for Jefferson Health. Follow him on twitter (@doctornotte).
On Feb. 4, 2018, with his team narrowly leading the New England Patriots in Super Bowl 52, Philadelphia Eagles head coach Doug Pederson made an audacious 4th-and-goal call. At the suggestion of backup quarterback Nick Foles, Pederson chose to rely on his team’s ability to execute the “Philly Special.” This was a risky trick play that was rehearsed but never tested, and one which could prove disastrous unless executed just right. With 34 seconds left in the first half, the Eagles pulled it off. Foles caught the ball in the end zone, securing his team’s place in football history and becoming the first quarterback to both throw for and catch a touchdown in one Super Bowl. He was named MVP and led the team to its first NFL title in 58 years.
For those of us who call Philadelphia our home, Super Bowl 52 represented so much more than just a victory, it was a miracle. We have long endured the highs and lows of Philadelphia football, watching as year after year our hopes were dashed by coaches and players who showed such promise, yet demonstrated such disappointment. But this year everything changed.
True fans could sense something different in the weeks leading up to that cold February day in Minneapolis. As the Eagle’s chances of competing in the Super Bowl grew more and more possible, the narrative wasn’t about any star player or member of the coaching staff, but instead the story of an incredible team. Even after the injury of starting quarterback and football phenom Carson Wentz in week 14, players and fans never lost hope in the promise of victory. Finally, Philadelphia had the team that could, and would, pull off something that had heretofore seemed like only an impossible dream.
It occurs to us that physicians should find the story of the Philadelphia Eagles not only inspirational, but also aspirational, even more so after reading the original research published by Dr. Richard Young, et al. in the February issue of Family Medicine.1 In this article, Dr. Young and his colleagues observed physicians during 982 patient encounters. The group measured the total visit time, face-to-face time, non-face time, and EHR work time (before, during, and after patient hours). The results weren’t surprising: Physicians spend more time working in the EHR than they spend in face-to-face time with patients.
This study confirmed prior work done by Ardnt et al. published in the fall in Annals of Family Medicine,2 which demonstrated that “primary care physicians spend more than half their workday, nearly 6 hours, interacting with the EHR during and after clinic hours.” Sadly, despite improving technology, the chasm between interacting with computers and interacting with actual patients only seems to be widening. To preserve the sanctity of the physician-patient relationship, we are forced to consider a completely new approach to how we practice: team-based care.
Team-based care isn’t a new idea, but it is being embraced with new fervor in the era of electronic health records. This is because the blessing — and curse — of the EHR is the vast amount of information that can be stored and accessed while caring for patients. To take advantage of this, doctors have been forced to become the primary agents for data entry and retrieval, something that is nearly impossible to do effectively while performing the cognitive work of a highly educated clinician. Rather than allowing us to take better care of patients, EHRs seem to have a paradoxical effect, limiting “face-to-face” time and squelching our efforts to address anything outside the immediate issues at hand. To improve the experience for us and our patients, we need to begin to rely on others.
To start, consider how a team can help support your documentation. As we’ve written about before, scribe services can be a tremendous benefit but aren’t the only way to improve efficiency. Medical assistants and nursing staff need to be encouraged to operate at the top level of their license, documenting where allowable and even queueing up orders, medication refills, and preventative care interventions when appropriate. This can be tremendously useful during previsit planning and can ensure that nothing is missed during the patient encounter.
Team-based care can also extend far beyond the EHR. For example, care coordinators can be employed to focus on specific high- and rising-risk patient populations. These health care professionals (typically nurses) reach out directly to patients and review their care, and even schedule visits with patients independently of the physician. This establishes therapeutic relationships that have been shown to prevent disease exacerbations and hospital readmissions, greatly reducing the cost of care.
Some facilities are now also using scheduling advocates, charged with facilitating referrals, arranging specialist and diagnostic appointments, and following up with patients to make sure they’ve successfully navigated the health care landscape. Behavioral health specialists and clinical pharmacists are also making their way into physician practices to expand the scope of offerings and decompress the burden on physicians. While these all have an associated cost, changes in the way physicians get paid are making the extra support economical and often necessary to satisfy the requirements under risk-based and fee-for-value contracts. We also predict that practices that choose to eschew the team approach to care will lose a competitive advantage in a health care market that is becoming more and more consumer driven. Patients, as well as providers, see the benefits of highly effective care teams.
Following the Eagles’ dramatic Super Bowl victory, coach Doug Pederson addressed his team in the locker room with these words: “This is a team game. As we’ve said before, an individual can make a difference, but a team makes a miracle.” While we as physicians may easily become jaded by the “miracles” of modern medicine, our patients have not yet lost hope in our ability to deliver on the promise of victory. To meet their expectations, we need to acknowledge that we will no longer be able to make it into the end zone alone; instead — in the game of modern medicine — we’ll need a team to take us there.
- Young RA, Burge SK, Kumar KA, Wilson JM, Ortiz DF. A Time-Motion Study of Primary Care Physicians’ Work in the Electronic Health Record Era. Fam Med. 2018 February;50(2):91-9.
- Arndt BG, Beasley JW, Watkinson MD, Temte JL, Tuan W-J, Sinsky CA, Gilchrist VJ..Ann Fam Med. 2017 September/October;15(5)5:419-26.
Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Notte is a family physician and Associate Chief Medical Information Officer for Jefferson Health. Follow him on twitter (@doctornotte).
On Feb. 4, 2018, with his team narrowly leading the New England Patriots in Super Bowl 52, Philadelphia Eagles head coach Doug Pederson made an audacious 4th-and-goal call. At the suggestion of backup quarterback Nick Foles, Pederson chose to rely on his team’s ability to execute the “Philly Special.” This was a risky trick play that was rehearsed but never tested, and one which could prove disastrous unless executed just right. With 34 seconds left in the first half, the Eagles pulled it off. Foles caught the ball in the end zone, securing his team’s place in football history and becoming the first quarterback to both throw for and catch a touchdown in one Super Bowl. He was named MVP and led the team to its first NFL title in 58 years.
For those of us who call Philadelphia our home, Super Bowl 52 represented so much more than just a victory, it was a miracle. We have long endured the highs and lows of Philadelphia football, watching as year after year our hopes were dashed by coaches and players who showed such promise, yet demonstrated such disappointment. But this year everything changed.
True fans could sense something different in the weeks leading up to that cold February day in Minneapolis. As the Eagle’s chances of competing in the Super Bowl grew more and more possible, the narrative wasn’t about any star player or member of the coaching staff, but instead the story of an incredible team. Even after the injury of starting quarterback and football phenom Carson Wentz in week 14, players and fans never lost hope in the promise of victory. Finally, Philadelphia had the team that could, and would, pull off something that had heretofore seemed like only an impossible dream.
It occurs to us that physicians should find the story of the Philadelphia Eagles not only inspirational, but also aspirational, even more so after reading the original research published by Dr. Richard Young, et al. in the February issue of Family Medicine.1 In this article, Dr. Young and his colleagues observed physicians during 982 patient encounters. The group measured the total visit time, face-to-face time, non-face time, and EHR work time (before, during, and after patient hours). The results weren’t surprising: Physicians spend more time working in the EHR than they spend in face-to-face time with patients.
This study confirmed prior work done by Ardnt et al. published in the fall in Annals of Family Medicine,2 which demonstrated that “primary care physicians spend more than half their workday, nearly 6 hours, interacting with the EHR during and after clinic hours.” Sadly, despite improving technology, the chasm between interacting with computers and interacting with actual patients only seems to be widening. To preserve the sanctity of the physician-patient relationship, we are forced to consider a completely new approach to how we practice: team-based care.
Team-based care isn’t a new idea, but it is being embraced with new fervor in the era of electronic health records. This is because the blessing — and curse — of the EHR is the vast amount of information that can be stored and accessed while caring for patients. To take advantage of this, doctors have been forced to become the primary agents for data entry and retrieval, something that is nearly impossible to do effectively while performing the cognitive work of a highly educated clinician. Rather than allowing us to take better care of patients, EHRs seem to have a paradoxical effect, limiting “face-to-face” time and squelching our efforts to address anything outside the immediate issues at hand. To improve the experience for us and our patients, we need to begin to rely on others.
To start, consider how a team can help support your documentation. As we’ve written about before, scribe services can be a tremendous benefit but aren’t the only way to improve efficiency. Medical assistants and nursing staff need to be encouraged to operate at the top level of their license, documenting where allowable and even queueing up orders, medication refills, and preventative care interventions when appropriate. This can be tremendously useful during previsit planning and can ensure that nothing is missed during the patient encounter.
Team-based care can also extend far beyond the EHR. For example, care coordinators can be employed to focus on specific high- and rising-risk patient populations. These health care professionals (typically nurses) reach out directly to patients and review their care, and even schedule visits with patients independently of the physician. This establishes therapeutic relationships that have been shown to prevent disease exacerbations and hospital readmissions, greatly reducing the cost of care.
Some facilities are now also using scheduling advocates, charged with facilitating referrals, arranging specialist and diagnostic appointments, and following up with patients to make sure they’ve successfully navigated the health care landscape. Behavioral health specialists and clinical pharmacists are also making their way into physician practices to expand the scope of offerings and decompress the burden on physicians. While these all have an associated cost, changes in the way physicians get paid are making the extra support economical and often necessary to satisfy the requirements under risk-based and fee-for-value contracts. We also predict that practices that choose to eschew the team approach to care will lose a competitive advantage in a health care market that is becoming more and more consumer driven. Patients, as well as providers, see the benefits of highly effective care teams.
Following the Eagles’ dramatic Super Bowl victory, coach Doug Pederson addressed his team in the locker room with these words: “This is a team game. As we’ve said before, an individual can make a difference, but a team makes a miracle.” While we as physicians may easily become jaded by the “miracles” of modern medicine, our patients have not yet lost hope in our ability to deliver on the promise of victory. To meet their expectations, we need to acknowledge that we will no longer be able to make it into the end zone alone; instead — in the game of modern medicine — we’ll need a team to take us there.
- Young RA, Burge SK, Kumar KA, Wilson JM, Ortiz DF. A Time-Motion Study of Primary Care Physicians’ Work in the Electronic Health Record Era. Fam Med. 2018 February;50(2):91-9.
- Arndt BG, Beasley JW, Watkinson MD, Temte JL, Tuan W-J, Sinsky CA, Gilchrist VJ..Ann Fam Med. 2017 September/October;15(5)5:419-26.
Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Notte is a family physician and Associate Chief Medical Information Officer for Jefferson Health. Follow him on twitter (@doctornotte).
Invasive Penile Squamous Cell Carcinoma
Invasive penile cancer is a rare malignancy with considerable morbidity and mortality. The American Cancer Society estimates that there will be 2320 new cases of invasive penile cancer in the United States in 2018, of which primary penile squamous cell carcinoma (PSCC) represents the majority.1 In one study, the mean age at diagnosis was 60 years, with PSCC occurring only rarely in men younger than 35 years of age (estimated incidence, 0.01 cases per 100,000 individuals).2 Presentation to a physician generally occurs more than 1 year after initial onset of symptoms or clinical lesion(s). This delay in diagnosis and treatment often results in disease progression,3 which can have a devastating outcome.4 Therefore, physicians should maintain a high index of clinical suspicion for PSCC, particularly in young or middle-aged patients in whom presentation of PSCC is uncommon. The most commonly associated risk factors for PSCC include lack of circumcision (specifically during the neonatal period), high-risk human papillomavirus (HPV) infection, and tobacco use.5 Chronic alcoholism also has been linked to PSCC.6 It also is common in patients without health insurance.7 We report the case of a 27-year-old circumcised man who presented with invasive PSCC following a diagnosis of condyloma 8 years prior by an outside physician.
Case Report
A 27-year-old man presented for evaluation of persistent genital warts that had been diagnosed 8 years prior. His medical history was remarkable for intravenous drug use, active hepatitis C infection, tobacco smoking, chronic alcohol use, and mild asthma. Eight years prior to the current presentation, 7 lesions had developed on the penis and were diagnosed by an outside physician as condyloma, which was treated with cryotherapy and topical imiquimod. All of the lesions except for 1 responded to treatment. The residual lesion continued to grow until the size prompted him to contact his primary care physician, who referred him for dermatologic evaluation. The patient cited lack of health insurance as the primary reason he did not seek follow-up treatment after the initial evaluation and treatment 8 years prior.
Physical examination at the current presentation revealed a circumcised man with an asymptomatic, 2.6-cm, pink, friable, verrucous mass on the left lateral penile shaft (Figure 1) and otherwise unremarkable penile architecture. A clinically enlarged, nontender right inguinal lymph node was noted as well as subtle enlargement of a left inguinal lymph node. An excisional biopsy was performed with pathologic evaluation confirming a diagnosis of high-grade invasive squamous cell carcinoma (SCC) arising in the setting of squamous cell carcinoma in situ (Figure 2). Lymphovascular invasion was highlighted on cluster of differentiation 31 and podoplanin immunostaining (Figure 3). The patient was subsequently referred to urology and hematology-oncology specialists for further evaluation. Computed tomography (CT) of the abdomen and pelvis confirmed the contralaterally enlarged right inguinal lymph node discovered during physical examination and mildly enlarged ipsilateral inguinal, obturator, and external iliac nodes. Computed tomography–guided fine-needle aspiration of the right inguinal node confirmed the diagnosis of contralateral locoregional metastasis. Further evaluation with positron emission tomography/CT imaging revealed only a single metabolically active region confined to the right inguinal node. The patient’s history of active hepatitis C complicated proposed neoadjuvant chemotherapy regimens. Ultimately, after discussion with multiple surgical and oncologist specialties within our institution and others, a treatment plan was formulated. The patient underwent robotic laparoscopic bilateral pelvic and inguinal lymph node dissection and re-excision of the primary PSCC, with one of 15 right superficial inguinal nodes testing positive for tumor cells; the left superficial and bilateral deep inguinal lymph nodes were negative for SCC.
Repeat positron emission tomography/CT imaging at 6 months’ follow-up showed no evidence of active disease. On 1-year follow-up, a CT scan did not show any new or residual disease, but the patient continued to have edema of the bilateral legs, which began after lymph node dissection and was managed with physical therapy and compression stockings.
Comment
Prevalence
Penile cancer is rare in industrialized countries. Early detection is a critical factor for both overall survival and organ function. If successful interventions are to be made, physicians should be familiar with known risk factors as well as unusual presentations, such as lesions presenting in young circumcised men, as reported above. Similarly, tumors located on the shaft of the penis represent an uncommon location for tumor presentation, occurring in less than 5% of PSCC cases.8 Penile SCC most commonly develops as a solitary painless lesion on the glans, balanopreputial sulcus and/or prepuce.9 In our case, histopathology confirmed high-grade invasive SCC arising from squamous cell carcinoma in situ, an entity generally associated with older men with a 10% to 20% rate of progression into invasive SCC.9 Our patient denied any clinical change in the appearance of the tumor in the years prior to the current presentation, making it possible that the condyloma treated 8 years prior was squamous cell carcinoma in situ or PSCC. As many as 25% of premalignant lesions are mistaken for benign lesions, which can thus delay treatment and allow progression to malignancy.10
Diagnosis
Penile SCC often is etiologically subcategorized into 2 pathways based on HPV dependence or independence. Recent research suggests that this distinction often is difficult to make, and accurate laboratory and pathologic confirmation of HPV DNA, intact virions, and viral-related cutaneous changes is not always possible, leading to much speculation regarding the exact role of HPV in tumorigenesis.11 Cancers developing in the absence of HPV DNA often occur secondary to chronic inflammatory conditions such as lichen planus or lichen sclerosus. Human papillomavirus DNA has shown to be present in 70% to 100% of all SCC in situ of the penis11; therefore, the transformation of in situ disease to an invasive tumor in our patient most likely occurred via an HPV-dependent pathway. Viral carcinogenesis in the HPV-dependent pathway involves inactivation of host cell cycle regulatory proteins, specifically the retinoblastoma and p53 regulatory proteins by the viral oncoproteins E7 and E6, respectively.12,13 Human papillomavirus–dependent pathways are related to a patient’s age at first sexual intercourse, number of sexual partners, and history of condyloma and other sexually transmitted diseases.14,15 High-risk HPV types 16 and 18 are the most common viral types found in HPV related premalignant lesions, making it possible to decrease the incidence of PSCC with recently developed vaccines.16 Human papillomavirus vaccines have been shown to reduce the incidence of anal intraepithelial neoplasias and genital warts in men.17 While the effects of the HPV vaccine on reducing PSCC could not be assessed in the study due to low incidence of disease (both in the study population and in general), it is thought that HPV vaccination could potentially decrease the incidence of all PSCCs by one third, making it an important resource in the primary prevention of the disease.18
Management
Contemporary surgical management of PSCC has evolved from organ resection in toto for all PSCCs to a more conservative approach based upon tumor stage and grade. The standard margin for surgical resection of PSCC is 2 cm, a procedure often referred to as a partial penectomy. This remains the most common procedure for surgical resection of PSCC and has achieved good local control, with reported recurrence rates of 4% to 8%.19,20 Complication rates of the procedure are moderate one-third of patients experiencing compromise of sexual activity after surgery.21 With evidence that smaller resection margins may result in good local control and a lower incidence of postoperative functional impairment, resection margins of 5, 10, and 15 mm have been advocated for PSCCs of varying histologic grades and tumor stages.22-24 Treatment options for T1 and in situ tumors have expanded to include glansectomy, margin-controlled Mohs micrographic surgery, and ablative laser therapy for local disease control.5,20 More advanced tumors are still treated with partial or complete penectomy given the high risks for locoregional recurrence and distant spread.
Prognosis
The most important factor predicting survival in patients with PSCC is metastasis to inguinal lymph nodes. The 5-year survival rate for patients without nodal involvement is 85% to 100%, while those with pathologically positive lymph nodes have a 5-year survival rate of 15% to 45%.25 Once distant metastasis occurs, the mean time of survival is 7 to 10 months.26 Our patient presented with high-grade PSCC with histologic lymphovascular spread and palpable inguinal lymph nodes. When stratified with other similar cases at presentation, our patient was at a considerable risk for locoregional as well as distant metastasis. Management with regional nodal dissection with a plan for close observation (and deferment of chemotherapeutics) was based upon evaluations from multiple different medical specialties.
Conclusion
Invasive PSCC is rare in young circumcised adults, and a delay in diagnosis can lead to considerable morbidity and mortality. We present a case of invasive PSCC arising in the setting of squamous cell carcinoma in situ in an area previously treated with cryotherapy and imiquimod. Our patient’s young age, concurrent hepatitis C infection, and contralateral locoregional nodal metastasis made this a complex case, involving evaluation and treatment by multiple medical disciplines. This case highlights the importance of biopsy in any lesion recalcitrant to conventional modalities regardless of the patient’s age. Early detection and treatment of PSCC can prevent organ dysfunction, loss of organ, and even death.
- About penile cancer. American Cancer Society website. https://www.cancer.org/content/dam/CRC/PDF/Public/8783.00.pdf. Revised February 9, 2016. Accessed February 27, 2018.
- Barnholtz-Sloan JS, Maldonado JL, Pow-sang J, et al. Incidence trends in primary malignant penile cancer. Urol Oncol. 2007;25:361-367.
- Koifman L, Vides AJ, Koifman N, et al. Epidemiological aspects of penile cancer in Rio de Janeiro: evaluation of 230 cases. Int Braz J Urol. 2011;37:231-240.
- Kamat AM, Carpenter SM, Czerniak BA, et al. Metastatic penile cancer in a young Caucasian male: impact of delayed diagnosis. Urol Oncol. 2005;23:130-131.
- Deem S, Keane T, Bhavsar R, et al. Contemporary diagnosis and management of squamous cell carcinoma (SCC) of the penis. BJU Int. 2011;108:1378-1392.
- McIntyre M, Weiss A, Wahlquist A, et al. Penile cancer: an analysis of socioeconomic factors at a southeastern tertiary referral center. Can J Urol. 2011;18:5524-5528.
- Maden C, Sherman KJ, Beckmann AM, et al. History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J Natl Cancer Inst. 1993;85:19-24.
- Hernandez BY, Barnholtz-Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998-2003. Cancer. 2008;113(suppl 10):2883-2891.
- Ferrandiz-Pulido C, de Torres I, Garcia-Patos V. Penile squamous cell carcinoma. Actas Dermosifiliogr. 2012;103:478-487.
- Tietjen DN, Malek RS. Laser therapy of squamous cell dysplasia and carcinoma of the penis. Urology. 1998;52:559-565.
- Mannweiler S, Sygulla S, Winter E, et al. Two major pathways of penile carcinogenesis: HPV-induced penile cancers overexpress p16, HPV-negative cancers associated with dermatoses express p53, but lack p16 overexpression. J Am Acad Dermatol. 2013;69:73-81.
- Scheffner M, Werness BA, Huibregtse JM, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129-1136.
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76-79.
- Daling JR, Madeleine MM, Johnson LG, et al. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer. 2005;116:606-616.
- Bleeker MC, Heideman DA, Snijders PJ, et al. Penile cancer: epidemiology, pathogenesis and prevention. World J Urol. 2009;27:141-150.
- Shabbir M, Barod R, Hegarty PK, et al. Primary prevention and vaccination for penile cancer. Ther Adv Urol. 2013;5:161-169.
- Palefsky J, Giuliano A, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576-1585.
- Backes DM, Kurman RJ, Pimenta JM, et al. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20:449-457.
- Korets R, Koppie TM, Snyder ME, et al. Partial penectomy for patients with squamous cell carcinoma of the penis: the Memorial Sloan-Kettering experience. Ann Surg Oncol. 2007;14:3614-3619.
- Zukiwskyj M, Daly P, Chung E. Penile cancer and phallus preservation strategies: a review of current literature. BJU Int. 2013;112(suppl 2):21-26.
- Romero FR, Romero KR, Mattos MA, et al. Sexual function after partial penectomy for penile cancer. Urology. 2005;66:1292-1295.
- Minhas S, Kayes O, Hegarty P, et al. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int. 2005;96:1040-1043.
- Feldman AS, McDougal WS. Long-term outcome of excisional organ sparing surgery for carcinoma of the penis. J Urol. 2011;186:1303-1307.
- Philippou P, Shabbir M, Malone P, et al. Conservative surgery for squamous cell carcinoma of the penis: resection margins and long-term oncological control. J Urol. 2012;188:803-808.
- Brady KL, Mercurio MG, Brown MD. Malignant tumors of the penis. Dermatol Surg. 2013;39:527-547.
- Ornellas AA, Nobrega BL, Wei Kin Chin E, et al. Prognostic factors in invasive squamous cell carcinoma of the penis: analysis of 196 patients treated at the Brazilian National Cancer Institute. J Urol. 2008;180:1354-1359.
Invasive penile cancer is a rare malignancy with considerable morbidity and mortality. The American Cancer Society estimates that there will be 2320 new cases of invasive penile cancer in the United States in 2018, of which primary penile squamous cell carcinoma (PSCC) represents the majority.1 In one study, the mean age at diagnosis was 60 years, with PSCC occurring only rarely in men younger than 35 years of age (estimated incidence, 0.01 cases per 100,000 individuals).2 Presentation to a physician generally occurs more than 1 year after initial onset of symptoms or clinical lesion(s). This delay in diagnosis and treatment often results in disease progression,3 which can have a devastating outcome.4 Therefore, physicians should maintain a high index of clinical suspicion for PSCC, particularly in young or middle-aged patients in whom presentation of PSCC is uncommon. The most commonly associated risk factors for PSCC include lack of circumcision (specifically during the neonatal period), high-risk human papillomavirus (HPV) infection, and tobacco use.5 Chronic alcoholism also has been linked to PSCC.6 It also is common in patients without health insurance.7 We report the case of a 27-year-old circumcised man who presented with invasive PSCC following a diagnosis of condyloma 8 years prior by an outside physician.
Case Report
A 27-year-old man presented for evaluation of persistent genital warts that had been diagnosed 8 years prior. His medical history was remarkable for intravenous drug use, active hepatitis C infection, tobacco smoking, chronic alcohol use, and mild asthma. Eight years prior to the current presentation, 7 lesions had developed on the penis and were diagnosed by an outside physician as condyloma, which was treated with cryotherapy and topical imiquimod. All of the lesions except for 1 responded to treatment. The residual lesion continued to grow until the size prompted him to contact his primary care physician, who referred him for dermatologic evaluation. The patient cited lack of health insurance as the primary reason he did not seek follow-up treatment after the initial evaluation and treatment 8 years prior.
Physical examination at the current presentation revealed a circumcised man with an asymptomatic, 2.6-cm, pink, friable, verrucous mass on the left lateral penile shaft (Figure 1) and otherwise unremarkable penile architecture. A clinically enlarged, nontender right inguinal lymph node was noted as well as subtle enlargement of a left inguinal lymph node. An excisional biopsy was performed with pathologic evaluation confirming a diagnosis of high-grade invasive squamous cell carcinoma (SCC) arising in the setting of squamous cell carcinoma in situ (Figure 2). Lymphovascular invasion was highlighted on cluster of differentiation 31 and podoplanin immunostaining (Figure 3). The patient was subsequently referred to urology and hematology-oncology specialists for further evaluation. Computed tomography (CT) of the abdomen and pelvis confirmed the contralaterally enlarged right inguinal lymph node discovered during physical examination and mildly enlarged ipsilateral inguinal, obturator, and external iliac nodes. Computed tomography–guided fine-needle aspiration of the right inguinal node confirmed the diagnosis of contralateral locoregional metastasis. Further evaluation with positron emission tomography/CT imaging revealed only a single metabolically active region confined to the right inguinal node. The patient’s history of active hepatitis C complicated proposed neoadjuvant chemotherapy regimens. Ultimately, after discussion with multiple surgical and oncologist specialties within our institution and others, a treatment plan was formulated. The patient underwent robotic laparoscopic bilateral pelvic and inguinal lymph node dissection and re-excision of the primary PSCC, with one of 15 right superficial inguinal nodes testing positive for tumor cells; the left superficial and bilateral deep inguinal lymph nodes were negative for SCC.



Repeat positron emission tomography/CT imaging at 6 months’ follow-up showed no evidence of active disease. On 1-year follow-up, a CT scan did not show any new or residual disease, but the patient continued to have edema of the bilateral legs, which began after lymph node dissection and was managed with physical therapy and compression stockings.
Comment
Prevalence
Penile cancer is rare in industrialized countries. Early detection is a critical factor for both overall survival and organ function. If successful interventions are to be made, physicians should be familiar with known risk factors as well as unusual presentations, such as lesions presenting in young circumcised men, as reported above. Similarly, tumors located on the shaft of the penis represent an uncommon location for tumor presentation, occurring in less than 5% of PSCC cases.8 Penile SCC most commonly develops as a solitary painless lesion on the glans, balanopreputial sulcus and/or prepuce.9 In our case, histopathology confirmed high-grade invasive SCC arising from squamous cell carcinoma in situ, an entity generally associated with older men with a 10% to 20% rate of progression into invasive SCC.9 Our patient denied any clinical change in the appearance of the tumor in the years prior to the current presentation, making it possible that the condyloma treated 8 years prior was squamous cell carcinoma in situ or PSCC. As many as 25% of premalignant lesions are mistaken for benign lesions, which can thus delay treatment and allow progression to malignancy.10
Diagnosis
Penile SCC often is etiologically subcategorized into 2 pathways based on HPV dependence or independence. Recent research suggests that this distinction often is difficult to make, and accurate laboratory and pathologic confirmation of HPV DNA, intact virions, and viral-related cutaneous changes is not always possible, leading to much speculation regarding the exact role of HPV in tumorigenesis.11 Cancers developing in the absence of HPV DNA often occur secondary to chronic inflammatory conditions such as lichen planus or lichen sclerosus. Human papillomavirus DNA has shown to be present in 70% to 100% of all SCC in situ of the penis11; therefore, the transformation of in situ disease to an invasive tumor in our patient most likely occurred via an HPV-dependent pathway. Viral carcinogenesis in the HPV-dependent pathway involves inactivation of host cell cycle regulatory proteins, specifically the retinoblastoma and p53 regulatory proteins by the viral oncoproteins E7 and E6, respectively.12,13 Human papillomavirus–dependent pathways are related to a patient’s age at first sexual intercourse, number of sexual partners, and history of condyloma and other sexually transmitted diseases.14,15 High-risk HPV types 16 and 18 are the most common viral types found in HPV related premalignant lesions, making it possible to decrease the incidence of PSCC with recently developed vaccines.16 Human papillomavirus vaccines have been shown to reduce the incidence of anal intraepithelial neoplasias and genital warts in men.17 While the effects of the HPV vaccine on reducing PSCC could not be assessed in the study due to low incidence of disease (both in the study population and in general), it is thought that HPV vaccination could potentially decrease the incidence of all PSCCs by one third, making it an important resource in the primary prevention of the disease.18
Management
Contemporary surgical management of PSCC has evolved from organ resection in toto for all PSCCs to a more conservative approach based upon tumor stage and grade. The standard margin for surgical resection of PSCC is 2 cm, a procedure often referred to as a partial penectomy. This remains the most common procedure for surgical resection of PSCC and has achieved good local control, with reported recurrence rates of 4% to 8%.19,20 Complication rates of the procedure are moderate one-third of patients experiencing compromise of sexual activity after surgery.21 With evidence that smaller resection margins may result in good local control and a lower incidence of postoperative functional impairment, resection margins of 5, 10, and 15 mm have been advocated for PSCCs of varying histologic grades and tumor stages.22-24 Treatment options for T1 and in situ tumors have expanded to include glansectomy, margin-controlled Mohs micrographic surgery, and ablative laser therapy for local disease control.5,20 More advanced tumors are still treated with partial or complete penectomy given the high risks for locoregional recurrence and distant spread.
Prognosis
The most important factor predicting survival in patients with PSCC is metastasis to inguinal lymph nodes. The 5-year survival rate for patients without nodal involvement is 85% to 100%, while those with pathologically positive lymph nodes have a 5-year survival rate of 15% to 45%.25 Once distant metastasis occurs, the mean time of survival is 7 to 10 months.26 Our patient presented with high-grade PSCC with histologic lymphovascular spread and palpable inguinal lymph nodes. When stratified with other similar cases at presentation, our patient was at a considerable risk for locoregional as well as distant metastasis. Management with regional nodal dissection with a plan for close observation (and deferment of chemotherapeutics) was based upon evaluations from multiple different medical specialties.
Conclusion
Invasive PSCC is rare in young circumcised adults, and a delay in diagnosis can lead to considerable morbidity and mortality. We present a case of invasive PSCC arising in the setting of squamous cell carcinoma in situ in an area previously treated with cryotherapy and imiquimod. Our patient’s young age, concurrent hepatitis C infection, and contralateral locoregional nodal metastasis made this a complex case, involving evaluation and treatment by multiple medical disciplines. This case highlights the importance of biopsy in any lesion recalcitrant to conventional modalities regardless of the patient’s age. Early detection and treatment of PSCC can prevent organ dysfunction, loss of organ, and even death.
Invasive penile cancer is a rare malignancy with considerable morbidity and mortality. The American Cancer Society estimates that there will be 2320 new cases of invasive penile cancer in the United States in 2018, of which primary penile squamous cell carcinoma (PSCC) represents the majority.1 In one study, the mean age at diagnosis was 60 years, with PSCC occurring only rarely in men younger than 35 years of age (estimated incidence, 0.01 cases per 100,000 individuals).2 Presentation to a physician generally occurs more than 1 year after initial onset of symptoms or clinical lesion(s). This delay in diagnosis and treatment often results in disease progression,3 which can have a devastating outcome.4 Therefore, physicians should maintain a high index of clinical suspicion for PSCC, particularly in young or middle-aged patients in whom presentation of PSCC is uncommon. The most commonly associated risk factors for PSCC include lack of circumcision (specifically during the neonatal period), high-risk human papillomavirus (HPV) infection, and tobacco use.5 Chronic alcoholism also has been linked to PSCC.6 It also is common in patients without health insurance.7 We report the case of a 27-year-old circumcised man who presented with invasive PSCC following a diagnosis of condyloma 8 years prior by an outside physician.
Case Report
A 27-year-old man presented for evaluation of persistent genital warts that had been diagnosed 8 years prior. His medical history was remarkable for intravenous drug use, active hepatitis C infection, tobacco smoking, chronic alcohol use, and mild asthma. Eight years prior to the current presentation, 7 lesions had developed on the penis and were diagnosed by an outside physician as condyloma, which was treated with cryotherapy and topical imiquimod. All of the lesions except for 1 responded to treatment. The residual lesion continued to grow until the size prompted him to contact his primary care physician, who referred him for dermatologic evaluation. The patient cited lack of health insurance as the primary reason he did not seek follow-up treatment after the initial evaluation and treatment 8 years prior.
Physical examination at the current presentation revealed a circumcised man with an asymptomatic, 2.6-cm, pink, friable, verrucous mass on the left lateral penile shaft (Figure 1) and otherwise unremarkable penile architecture. A clinically enlarged, nontender right inguinal lymph node was noted as well as subtle enlargement of a left inguinal lymph node. An excisional biopsy was performed with pathologic evaluation confirming a diagnosis of high-grade invasive squamous cell carcinoma (SCC) arising in the setting of squamous cell carcinoma in situ (Figure 2). Lymphovascular invasion was highlighted on cluster of differentiation 31 and podoplanin immunostaining (Figure 3). The patient was subsequently referred to urology and hematology-oncology specialists for further evaluation. Computed tomography (CT) of the abdomen and pelvis confirmed the contralaterally enlarged right inguinal lymph node discovered during physical examination and mildly enlarged ipsilateral inguinal, obturator, and external iliac nodes. Computed tomography–guided fine-needle aspiration of the right inguinal node confirmed the diagnosis of contralateral locoregional metastasis. Further evaluation with positron emission tomography/CT imaging revealed only a single metabolically active region confined to the right inguinal node. The patient’s history of active hepatitis C complicated proposed neoadjuvant chemotherapy regimens. Ultimately, after discussion with multiple surgical and oncologist specialties within our institution and others, a treatment plan was formulated. The patient underwent robotic laparoscopic bilateral pelvic and inguinal lymph node dissection and re-excision of the primary PSCC, with one of 15 right superficial inguinal nodes testing positive for tumor cells; the left superficial and bilateral deep inguinal lymph nodes were negative for SCC.



Repeat positron emission tomography/CT imaging at 6 months’ follow-up showed no evidence of active disease. On 1-year follow-up, a CT scan did not show any new or residual disease, but the patient continued to have edema of the bilateral legs, which began after lymph node dissection and was managed with physical therapy and compression stockings.
Comment
Prevalence
Penile cancer is rare in industrialized countries. Early detection is a critical factor for both overall survival and organ function. If successful interventions are to be made, physicians should be familiar with known risk factors as well as unusual presentations, such as lesions presenting in young circumcised men, as reported above. Similarly, tumors located on the shaft of the penis represent an uncommon location for tumor presentation, occurring in less than 5% of PSCC cases.8 Penile SCC most commonly develops as a solitary painless lesion on the glans, balanopreputial sulcus and/or prepuce.9 In our case, histopathology confirmed high-grade invasive SCC arising from squamous cell carcinoma in situ, an entity generally associated with older men with a 10% to 20% rate of progression into invasive SCC.9 Our patient denied any clinical change in the appearance of the tumor in the years prior to the current presentation, making it possible that the condyloma treated 8 years prior was squamous cell carcinoma in situ or PSCC. As many as 25% of premalignant lesions are mistaken for benign lesions, which can thus delay treatment and allow progression to malignancy.10
Diagnosis
Penile SCC often is etiologically subcategorized into 2 pathways based on HPV dependence or independence. Recent research suggests that this distinction often is difficult to make, and accurate laboratory and pathologic confirmation of HPV DNA, intact virions, and viral-related cutaneous changes is not always possible, leading to much speculation regarding the exact role of HPV in tumorigenesis.11 Cancers developing in the absence of HPV DNA often occur secondary to chronic inflammatory conditions such as lichen planus or lichen sclerosus. Human papillomavirus DNA has shown to be present in 70% to 100% of all SCC in situ of the penis11; therefore, the transformation of in situ disease to an invasive tumor in our patient most likely occurred via an HPV-dependent pathway. Viral carcinogenesis in the HPV-dependent pathway involves inactivation of host cell cycle regulatory proteins, specifically the retinoblastoma and p53 regulatory proteins by the viral oncoproteins E7 and E6, respectively.12,13 Human papillomavirus–dependent pathways are related to a patient’s age at first sexual intercourse, number of sexual partners, and history of condyloma and other sexually transmitted diseases.14,15 High-risk HPV types 16 and 18 are the most common viral types found in HPV related premalignant lesions, making it possible to decrease the incidence of PSCC with recently developed vaccines.16 Human papillomavirus vaccines have been shown to reduce the incidence of anal intraepithelial neoplasias and genital warts in men.17 While the effects of the HPV vaccine on reducing PSCC could not be assessed in the study due to low incidence of disease (both in the study population and in general), it is thought that HPV vaccination could potentially decrease the incidence of all PSCCs by one third, making it an important resource in the primary prevention of the disease.18
Management
Contemporary surgical management of PSCC has evolved from organ resection in toto for all PSCCs to a more conservative approach based upon tumor stage and grade. The standard margin for surgical resection of PSCC is 2 cm, a procedure often referred to as a partial penectomy. This remains the most common procedure for surgical resection of PSCC and has achieved good local control, with reported recurrence rates of 4% to 8%.19,20 Complication rates of the procedure are moderate one-third of patients experiencing compromise of sexual activity after surgery.21 With evidence that smaller resection margins may result in good local control and a lower incidence of postoperative functional impairment, resection margins of 5, 10, and 15 mm have been advocated for PSCCs of varying histologic grades and tumor stages.22-24 Treatment options for T1 and in situ tumors have expanded to include glansectomy, margin-controlled Mohs micrographic surgery, and ablative laser therapy for local disease control.5,20 More advanced tumors are still treated with partial or complete penectomy given the high risks for locoregional recurrence and distant spread.
Prognosis
The most important factor predicting survival in patients with PSCC is metastasis to inguinal lymph nodes. The 5-year survival rate for patients without nodal involvement is 85% to 100%, while those with pathologically positive lymph nodes have a 5-year survival rate of 15% to 45%.25 Once distant metastasis occurs, the mean time of survival is 7 to 10 months.26 Our patient presented with high-grade PSCC with histologic lymphovascular spread and palpable inguinal lymph nodes. When stratified with other similar cases at presentation, our patient was at a considerable risk for locoregional as well as distant metastasis. Management with regional nodal dissection with a plan for close observation (and deferment of chemotherapeutics) was based upon evaluations from multiple different medical specialties.
Conclusion
Invasive PSCC is rare in young circumcised adults, and a delay in diagnosis can lead to considerable morbidity and mortality. We present a case of invasive PSCC arising in the setting of squamous cell carcinoma in situ in an area previously treated with cryotherapy and imiquimod. Our patient’s young age, concurrent hepatitis C infection, and contralateral locoregional nodal metastasis made this a complex case, involving evaluation and treatment by multiple medical disciplines. This case highlights the importance of biopsy in any lesion recalcitrant to conventional modalities regardless of the patient’s age. Early detection and treatment of PSCC can prevent organ dysfunction, loss of organ, and even death.
- About penile cancer. American Cancer Society website. https://www.cancer.org/content/dam/CRC/PDF/Public/8783.00.pdf. Revised February 9, 2016. Accessed February 27, 2018.
- Barnholtz-Sloan JS, Maldonado JL, Pow-sang J, et al. Incidence trends in primary malignant penile cancer. Urol Oncol. 2007;25:361-367.
- Koifman L, Vides AJ, Koifman N, et al. Epidemiological aspects of penile cancer in Rio de Janeiro: evaluation of 230 cases. Int Braz J Urol. 2011;37:231-240.
- Kamat AM, Carpenter SM, Czerniak BA, et al. Metastatic penile cancer in a young Caucasian male: impact of delayed diagnosis. Urol Oncol. 2005;23:130-131.
- Deem S, Keane T, Bhavsar R, et al. Contemporary diagnosis and management of squamous cell carcinoma (SCC) of the penis. BJU Int. 2011;108:1378-1392.
- McIntyre M, Weiss A, Wahlquist A, et al. Penile cancer: an analysis of socioeconomic factors at a southeastern tertiary referral center. Can J Urol. 2011;18:5524-5528.
- Maden C, Sherman KJ, Beckmann AM, et al. History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J Natl Cancer Inst. 1993;85:19-24.
- Hernandez BY, Barnholtz-Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998-2003. Cancer. 2008;113(suppl 10):2883-2891.
- Ferrandiz-Pulido C, de Torres I, Garcia-Patos V. Penile squamous cell carcinoma. Actas Dermosifiliogr. 2012;103:478-487.
- Tietjen DN, Malek RS. Laser therapy of squamous cell dysplasia and carcinoma of the penis. Urology. 1998;52:559-565.
- Mannweiler S, Sygulla S, Winter E, et al. Two major pathways of penile carcinogenesis: HPV-induced penile cancers overexpress p16, HPV-negative cancers associated with dermatoses express p53, but lack p16 overexpression. J Am Acad Dermatol. 2013;69:73-81.
- Scheffner M, Werness BA, Huibregtse JM, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129-1136.
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76-79.
- Daling JR, Madeleine MM, Johnson LG, et al. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer. 2005;116:606-616.
- Bleeker MC, Heideman DA, Snijders PJ, et al. Penile cancer: epidemiology, pathogenesis and prevention. World J Urol. 2009;27:141-150.
- Shabbir M, Barod R, Hegarty PK, et al. Primary prevention and vaccination for penile cancer. Ther Adv Urol. 2013;5:161-169.
- Palefsky J, Giuliano A, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576-1585.
- Backes DM, Kurman RJ, Pimenta JM, et al. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20:449-457.
- Korets R, Koppie TM, Snyder ME, et al. Partial penectomy for patients with squamous cell carcinoma of the penis: the Memorial Sloan-Kettering experience. Ann Surg Oncol. 2007;14:3614-3619.
- Zukiwskyj M, Daly P, Chung E. Penile cancer and phallus preservation strategies: a review of current literature. BJU Int. 2013;112(suppl 2):21-26.
- Romero FR, Romero KR, Mattos MA, et al. Sexual function after partial penectomy for penile cancer. Urology. 2005;66:1292-1295.
- Minhas S, Kayes O, Hegarty P, et al. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int. 2005;96:1040-1043.
- Feldman AS, McDougal WS. Long-term outcome of excisional organ sparing surgery for carcinoma of the penis. J Urol. 2011;186:1303-1307.
- Philippou P, Shabbir M, Malone P, et al. Conservative surgery for squamous cell carcinoma of the penis: resection margins and long-term oncological control. J Urol. 2012;188:803-808.
- Brady KL, Mercurio MG, Brown MD. Malignant tumors of the penis. Dermatol Surg. 2013;39:527-547.
- Ornellas AA, Nobrega BL, Wei Kin Chin E, et al. Prognostic factors in invasive squamous cell carcinoma of the penis: analysis of 196 patients treated at the Brazilian National Cancer Institute. J Urol. 2008;180:1354-1359.
- About penile cancer. American Cancer Society website. https://www.cancer.org/content/dam/CRC/PDF/Public/8783.00.pdf. Revised February 9, 2016. Accessed February 27, 2018.
- Barnholtz-Sloan JS, Maldonado JL, Pow-sang J, et al. Incidence trends in primary malignant penile cancer. Urol Oncol. 2007;25:361-367.
- Koifman L, Vides AJ, Koifman N, et al. Epidemiological aspects of penile cancer in Rio de Janeiro: evaluation of 230 cases. Int Braz J Urol. 2011;37:231-240.
- Kamat AM, Carpenter SM, Czerniak BA, et al. Metastatic penile cancer in a young Caucasian male: impact of delayed diagnosis. Urol Oncol. 2005;23:130-131.
- Deem S, Keane T, Bhavsar R, et al. Contemporary diagnosis and management of squamous cell carcinoma (SCC) of the penis. BJU Int. 2011;108:1378-1392.
- McIntyre M, Weiss A, Wahlquist A, et al. Penile cancer: an analysis of socioeconomic factors at a southeastern tertiary referral center. Can J Urol. 2011;18:5524-5528.
- Maden C, Sherman KJ, Beckmann AM, et al. History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J Natl Cancer Inst. 1993;85:19-24.
- Hernandez BY, Barnholtz-Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998-2003. Cancer. 2008;113(suppl 10):2883-2891.
- Ferrandiz-Pulido C, de Torres I, Garcia-Patos V. Penile squamous cell carcinoma. Actas Dermosifiliogr. 2012;103:478-487.
- Tietjen DN, Malek RS. Laser therapy of squamous cell dysplasia and carcinoma of the penis. Urology. 1998;52:559-565.
- Mannweiler S, Sygulla S, Winter E, et al. Two major pathways of penile carcinogenesis: HPV-induced penile cancers overexpress p16, HPV-negative cancers associated with dermatoses express p53, but lack p16 overexpression. J Am Acad Dermatol. 2013;69:73-81.
- Scheffner M, Werness BA, Huibregtse JM, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129-1136.
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76-79.
- Daling JR, Madeleine MM, Johnson LG, et al. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer. 2005;116:606-616.
- Bleeker MC, Heideman DA, Snijders PJ, et al. Penile cancer: epidemiology, pathogenesis and prevention. World J Urol. 2009;27:141-150.
- Shabbir M, Barod R, Hegarty PK, et al. Primary prevention and vaccination for penile cancer. Ther Adv Urol. 2013;5:161-169.
- Palefsky J, Giuliano A, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576-1585.
- Backes DM, Kurman RJ, Pimenta JM, et al. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20:449-457.
- Korets R, Koppie TM, Snyder ME, et al. Partial penectomy for patients with squamous cell carcinoma of the penis: the Memorial Sloan-Kettering experience. Ann Surg Oncol. 2007;14:3614-3619.
- Zukiwskyj M, Daly P, Chung E. Penile cancer and phallus preservation strategies: a review of current literature. BJU Int. 2013;112(suppl 2):21-26.
- Romero FR, Romero KR, Mattos MA, et al. Sexual function after partial penectomy for penile cancer. Urology. 2005;66:1292-1295.
- Minhas S, Kayes O, Hegarty P, et al. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int. 2005;96:1040-1043.
- Feldman AS, McDougal WS. Long-term outcome of excisional organ sparing surgery for carcinoma of the penis. J Urol. 2011;186:1303-1307.
- Philippou P, Shabbir M, Malone P, et al. Conservative surgery for squamous cell carcinoma of the penis: resection margins and long-term oncological control. J Urol. 2012;188:803-808.
- Brady KL, Mercurio MG, Brown MD. Malignant tumors of the penis. Dermatol Surg. 2013;39:527-547.
- Ornellas AA, Nobrega BL, Wei Kin Chin E, et al. Prognostic factors in invasive squamous cell carcinoma of the penis: analysis of 196 patients treated at the Brazilian National Cancer Institute. J Urol. 2008;180:1354-1359.
Practice Points
- Invasive penile squamous cell carcinoma (PSCC) is a rare malignancy with considerable morbidity and mortality that typically does not present in young men.
- Delayed or incorrect diagnosis of PSCC can have a devastating outcome; therefore, physicians should maintain a high index of clinical suspicion for PSCC in patients presenting with penile lesions, particularly in young or middle-aged patients.
Diffuse Cutaneous Breast Cancer Metastases Resembling Subcutaneous Nodules With No Surface Changes
Cutaneous metastases from solid tumors in general occur at a rate of about 1% per primary tumor.1 In breast cancer, cutaneous metastases occur at a rate of about 2.5% per primary tumor. Because of the high incidence of breast cancers relative to other internal malignancies, breast cancer accounts for almost 33% of all cutaneous metastases.2 Infiltrating ductal carcinoma accounts for almost 70% of cutaneous metastases from breast cancers, whereas lobular carcinoma accounts for about 15%.
Cutaneous metastases may be the first presenting sign of primary malignancy. In one retrospective study, 6% of breast carcinomas (N=992) initially presented with only skin manifestations.3 Clinical appearance can vary, but cutaneous metastases from breast adenocarcinomas often present as isolated dermal nodules with superficial discoloration or changes in texture. The most common location of cutaneous metastases is on the chest ipsilateral to the primary breast malignancy.4 We pre-sent a case of metastatic adenocarcinoma of the breast presenting with diffuse cutaneous nodules with no surface changes.
Case Report
A 64-year-old woman who was otherwise in good health presented to her primary care physician for evaluation of recent-onset fatigue. Laboratory testing revealed that she was mildly anemic with mild thrombocytopenia and lymphocytosis. She was referred to a hematologist, who ordered flow cytometry and cytogenetic testing. Blood abnormalities were not considered severe enough to warrant a bone marrow biopsy, and she was monitored clinically for the next 2 years.
Two years after the initial presentation, the primary care physician performed a breast examination that was unremarkable, but enlarged axillary lymph nodes up to 15 mm were discovered in the right breast during routine breast ultrasonography. Additionally, she noted that she had experienced unintentional weight loss of 10 lb over the past year. The hematologist suspected a low-grade lymphoma and performed a bone marrow biopsy. The immunohistochemistry of the bone marrow specimen was consistent with an estrogen receptor–positive, progesterone receptor–negative, human epidermal growth factor receptor 2–negative invasive lobular breast carcinoma, which was then confirmed in the right breast on magnetic resonance imaging. The patient denied any history of prior radiation treatment, but she disclosed a family history of breast cancer in her cousin.
Several weeks after the bone marrow biopsy, an oncologist found that the patient also had an abdominal mass and bone metastases of the primary breast cancer. Colonoscopy confirmed metastases to the colon that subsequently led to obstruction and ultimately required a right hemicolectomy. The patient’s oncologist started her on anastrozole, an aromatase inhibitor (AI), for treatment of the metastatic breast cancer and zoledronic acid, a bisphosphonate, along with calcium and vitamin D for the bone involvement.
Shortly after, during a routine annual skin examination, the patient’s dermatologist (H.T.N.) discovered 3 soft, fixed, subcutaneous-appearing nodules—one on the right chest that was 15 mm in diameter, one on the left mid back that was 7 mm, and one on the left upper anterior thigh that was 10 mm. They were discrete with well-defined borders but had only minimal elevation, making them difficult to detect clinically, especially without palpation. The nodules were not visibly apparent because they were flesh-colored with no surface discoloration or texture changes. The patient remembered that the lesions had appeared gradually several months prior, predating the breast cancer diagnosis, and were not associated with pain, itching, or burning, so she was not alarmed by their appearance and never sought medical attention. The dermatologist (H.T.N.) recommended a biopsy at the time of the skin examination, but the patient declined.
One year after the appearance of the first skin lesions, 14 more nodules (Figure 1) progressively erupted on the ipsilateral and contralateral chest (Figure 2A), axillae, arms, shoulders, back (Figure 2B), and thighs (Figure 2C). At this point, the dermatologists performed a punch biopsy on a lesion on the back to confirm the suspicion of cutaneous metastasis of the primary breast cancer. The biopsy showed interstitial dermal proliferation of atypical cells between collagen bundles and stained strongly positive for cytokeratin 7, an epithelial protein common in breast adenocarcinoma (Figure 3). Further immunohistochemical staining returned metastatic estrogen receptor–positive, progesterone receptor–negative, human epidermal growth factor receptor 2–negative invasive lobular breast carcinoma. Therefore, the markers for the cutaneous metastases were consistent with the markers for the original breast cancer.
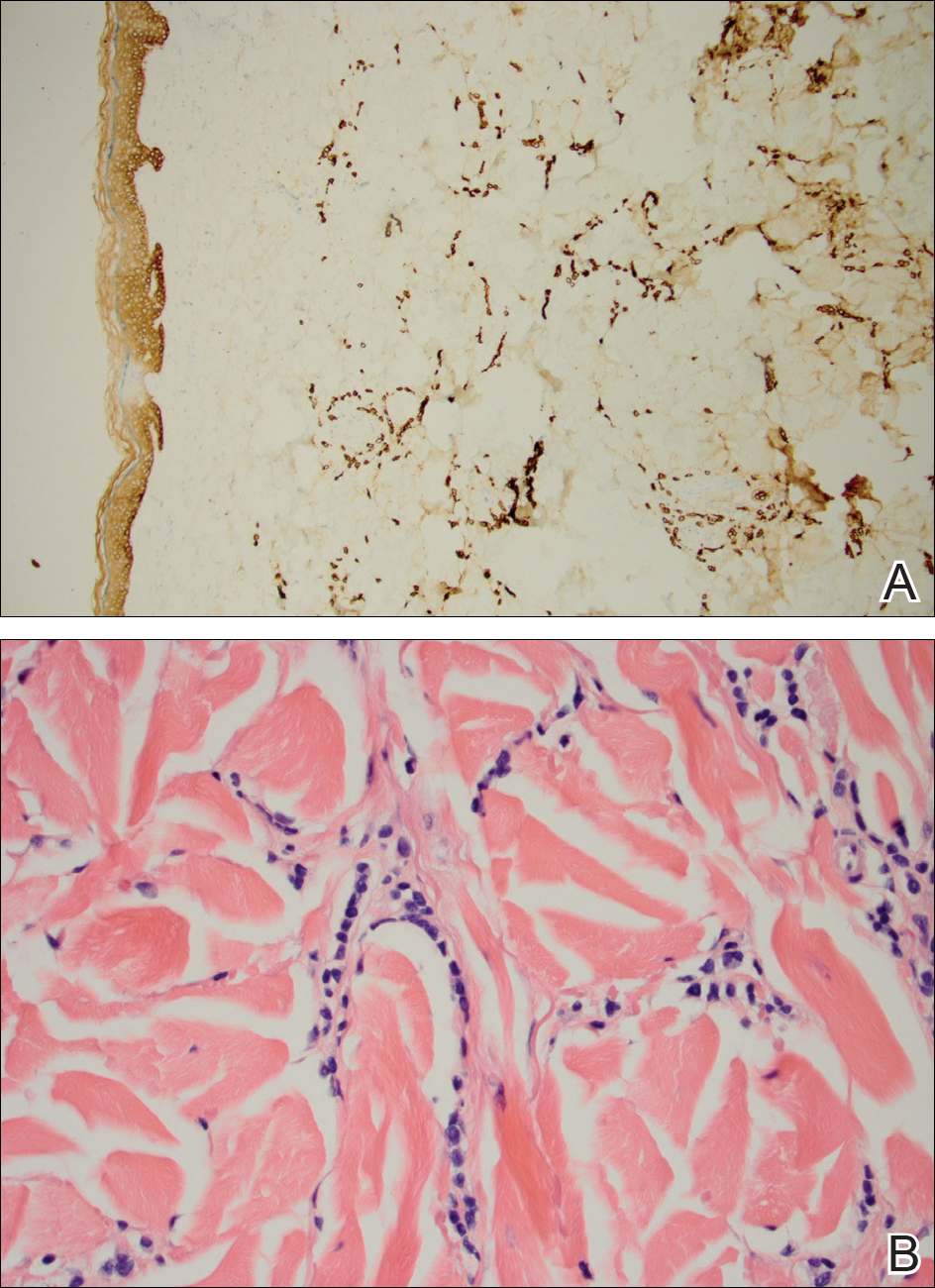
After 1 year of treatment with anastrozole, the patient’s internal metastases had not changed considerably, but the cutaneous metastases continued to grow—the lesion on the left thigh doubled from 10 to 20 mm in diameter, and new nodules developed on the chest, back, arms, and legs. One year and a half after the initial lesions were documented, several nodules had disappeared and several new ones appeared. The remaining nodules remained relatively constant in size.
After stopping anastrozole, the patient was enrolled in a research trial using bortezomib, a chemotherapeutic agent typically used for multiple myeloma, as well as fulvestrant, an estrogen receptor antagonist; however, because of continued progression of the metastatic cancer, the patient was removed from the trial and switched to the established regimen of everolimus, a chemotherapeutic agent, and exemestane, another AI. Everolimus eventually was stopped, but the patient continued on exemestane as monotherapy. In addition to development of pleural disease, the cutaneous metastases continued to progress. The patient did not receive any local treatment for her cutaneous metastases.
Comment
Typically, cutaneous metastases of breast cancer manifests as a 1- to 3-cm, asymptomatic, firm, pink to red-brown nodule on the chest ipsilateral to the primary tumor. There may be more than 1 nodule, and ulceration may be present.5,6 In addition to nodular metastases, which make up 47% of cases (N=305), other common presentations include alopecia neoplastica (12%), telangiectatic carcinoma (8%), melanomalike lesions (6%), carcinoma erysipeloides (6%), subungual lesions (5%), carcinoma en cuirasse (4%), and zosteriform metastases (4%).6
Although nodular metastases are the most common type of cutaneous breast cancer metastases, our case is unique in that the patient had soft nodules dispersed to both arms and legs, and the nodules had no surface changes. Although cutaneous metastases can present as flesh-colored nodules,7 they typically have an erythematous base, a slight change in coloration, or induration. Additionally, cutaneous metastases most often are few in number and appear in close proximity to the primary breast adenocarcinoma.8 Without the detection of a slight soft elevation on palpation, our patient’s nodules were practically indistinguishable from the normal skin.
Among common internal cancers, breast cancer is the most likely to metastasize to the skin at a rate of 2.42% per primary tumor (Table 1).1 Cutaneous metastases from lobular carcinomas are much rarer than those from ductal carcinomas.4 The metastases also are most often located locally on the chest ipsilateral to the primary malignancy. Distant metastases are relatively rare. In a review of 212 cases of breast cancer patients with skin metastases, only 9 had involvement of the legs and only 4 had involvement of the contralateral chest.4 Our patient had involvement of the ipsilateral chest, both arms and legs, and the contralateral chest.
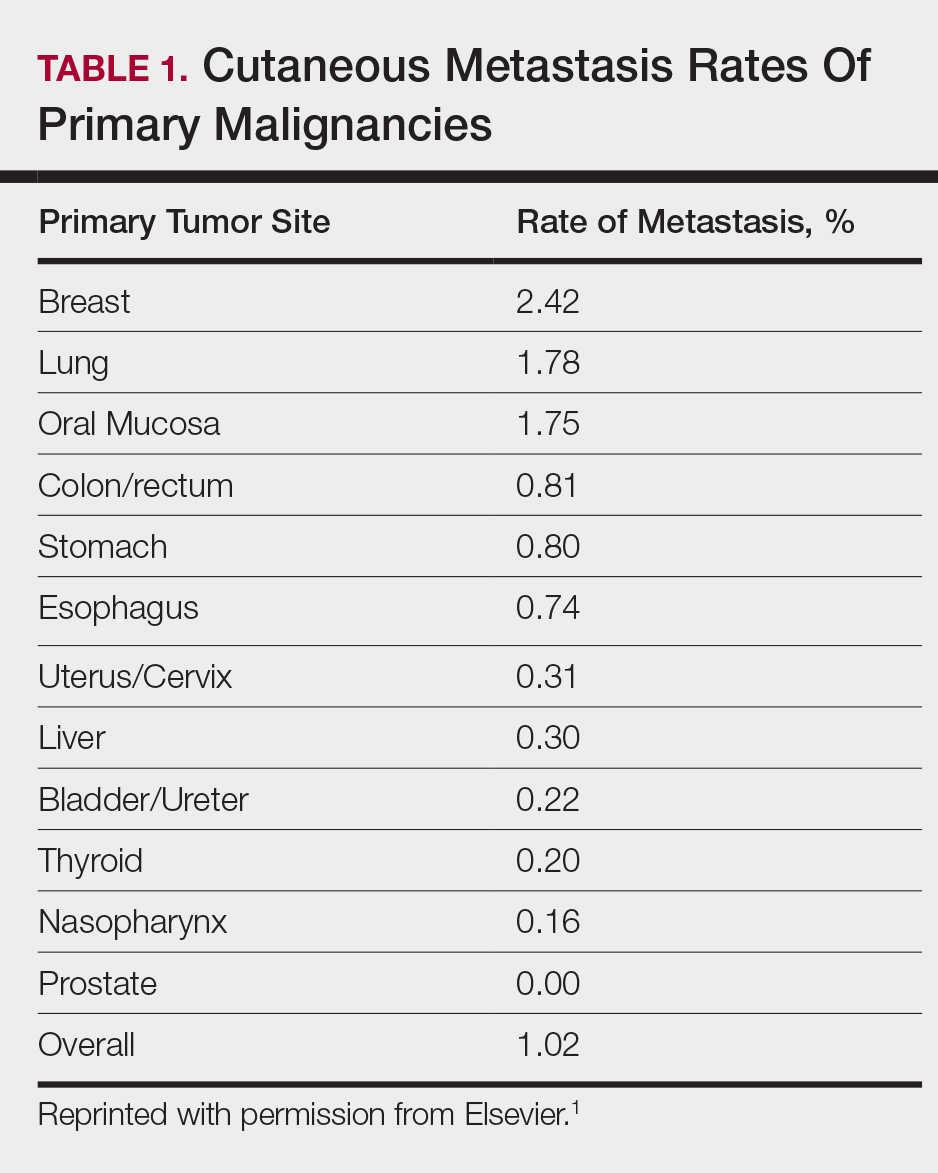
The 5-year relative survival rate for breast cancer patients varies based on the stage at diagnosis (99% in patients with localized cancer, 84% with regional lymph node involvement, 24% with distant metastases of any kind).9 In a study of 141 patients with cutaneous metastases in a Taiwanese medical center, Hu et al10 found that patients with breast cancer with only cutaneous metastases had a 5-year absolute survival rate of 38%. In the same study, patients with non–breast cancer metastasis including cutaneous metastasis had a 5-year survival rate of 15%.10 This data is summarized in Table 2.
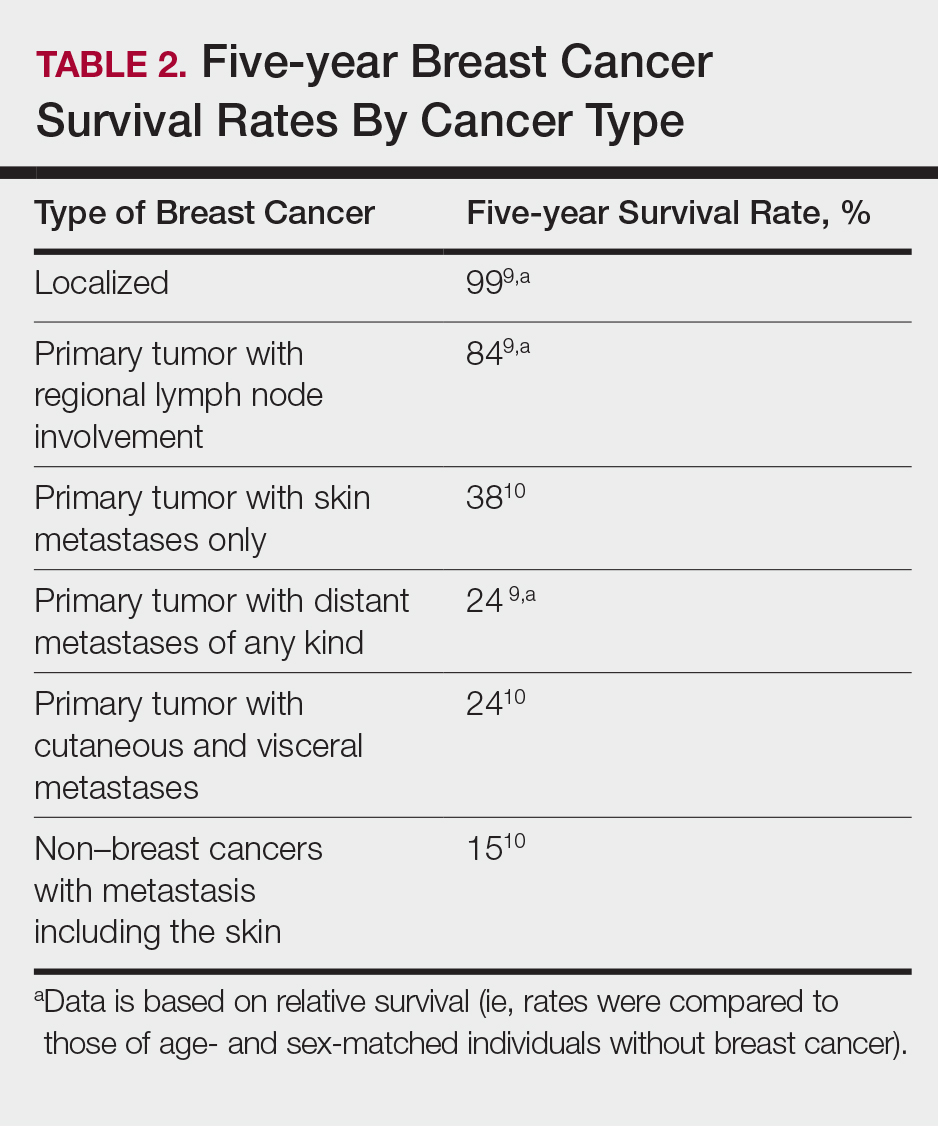
Breast cancer metastasis to soft tissue (eg, the skin) typically indicates a better prognosis than breast cancer metastasis to a visceral organ or bone. In a study of 439 patients with metastatic relapse after surgical resection of a primary breast cancer, those who had soft tissue metastases had a median survival period of 39 months, whereas those who had visceral or bone metastases had a median survival period of 13 and 28 months, respectively.11 Furthermore, cutaneous metastases from breast cancers do not necessarily indicate as poor a prognosis as skin metastases from other internal malignancies. Cutaneous metastases from other internal malignancies carry a relative risk of mortality of 4.3 compared to cutaneous metastases from breast cancer.10
Treatment of cutaneous metastases may be medically or cosmetically indicated. Standard treatments for cutaneous metastases from the breast include surgical excision, external beam radiotherapy, and systemic chemotherapy.6 While oncologists can use the response of cutaneous metastases to treatment as an indicator of systemic response to hormone therapy or chemotherapy,12 the response may be poorer due to the skin’s relatively weaker blood supply.13
Our patient was first prescribed anastrozole, an AI. For metastatic hormone receptor–positive breast cancer, AIs are a first-line therapy in postmenopausal women. In one meta-analysis, AIs showed greater improvement of survival rates relative to other endocrine therapies such as tamoxifen, an estrogen receptor antagonist (hazard ratio of 0.87).14 After stopping anastrozole, the patient was prescribed fulvestrant, another estrogen receptor antagonist, along with a trial drug. In a randomized, double-blind, placebo-controlled trial, fulvestrant was found to be an effective second-line treatment after anastrozole for hormone receptor–positive breast cancer in postmenopausal women.15 Our patient was then started on everolimus, a chemotherapeutic agent, and exemestane, another AI. After first-line treatment with anastrozole, this regimen also has been found to be an effective second-line treatment with improved progression-free survival.16 For the bone metastases, our patient was treated with zoledronic acid, a bisphosphonate. In a meta-analysis, bisphosphonates were found to reduce skeletal-related complications by a median of 28% in breast cancer patients with bone metastases.17
Some promising new local treatments for cutaneous breast metastases include topical imiquimod and electrochemotherapy. In a small study of 10 patients whose malignancies were refractory to radiotherapy, imiquimod achieved a partial response in 20% (2/10) of patients.18 In another study, 12 patients received electrochemotherapy involving electroporation (applying an electrical field to increase cell membrane permeability and thus increase drug uptake) followed by local administration of bleomycin, an antineoplastic agent. Seventy-five percent (9/12) of the patients received a complete response with disappearance of the metastases.19
This case report provides a rare presentation of diffuse nodular cutaneous metastases of breast adenocarcinoma with no surface changes. The subtle clinical findings in our patient demonstrate the spectrum of clinical manifestations for cutaneous metastases. Our case also serves to highlight the need for close inspection of the skin, including palpation in patients with a history of internal malignancy.
- Hu SC, Chen G, Wu C, et al. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60:379-387.
- Wong CY, Helm MA, Helm TN, et al. Patterns of skin metastases: a review of 25 years’ experience at a single cancer center. Int J Dermatol. 2014;53:56-60.
- Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma: a retrospective study of 7316 cancer patients. J Am Acad Dermatol. 1990;22:19-26.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2, part 1):228-236.
- Gan DEH, Teh YC, Ng CH, et al. Cutaneous metastases of breast cancer: a case report. Breast Case. 2012;1:23-36.
- De Giorgi V, Grazzini M, Alfaioli B, et al. Cutaneous manifestations of breast carcinoma. Dermatol Ther. 2010;23:581-589.
- Vano-Galvan S, Moreno-Martin P, Salguero I, et al. Cutaneous metastases of breast carcinoma: a case report. Cases J. 2009;2:71.
- Dacso M, Soldano AC, Talbott LB, et al. A solitary neck nodule as late evidence of recurrent lobular breast carcinoma. Case Rep Oncol. 2009;2:24-29.
- Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2010. Table 1.5 Age-Adjusted SEER Incidence and U.S. Death Rates and 5-Year Relative Survival (Percent) By Primary Cancer Site, Sex and Time Period. Bethesda, MD: National Cancer Institute; 2013. https://seer.cancer.gov/archive/csr/1975_2010/results_merged/topic_survival.pdf. Updated June 14, 2014. Accessed February 27, 2018.
- Hu SC, Chen GS, Lu YW, et al. Cutaneous metastases from different internal malignancies: a clinical and prognostic appraisal. J Eur Acad Dermatol Venereol. 2008;22:735-740.
- Insa A, Lluch A, Prosper F, et al. Prognostic factors predicting survival from first recurrence in patients with metastatic breast cancer: analysis of 439 patients. Breast Cancer Res Treat. 1999;56:67-78.
- Eisenhauer E, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247.
- Kamble R, Kumar L, Kochupillai V, et al. Cutaneous metastases of lung cancer. Postgrad Med J. 1995;71:741-743.
- Mauri D, Pavlidis N, Polyzos N, et al. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst. 2006;98:1285-1291.
- Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664-1670.
- Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N Engl J Med. 2012;366:520-529.
- Wong MH, Stockler M, Pavlakis N. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev. 2012;2:CD003474.
- Adams S, Kozhaya L, Martiniuk F, et al. Topical TLR7 agonist imiquimod can induce immune-mediated rejection of skin metastases in patients with breast cancer. Clin Cancer Res. 2012;18:6748-6757.
- Benevento R, Santoriello A, Perna G, et al. Electrochemotherapy of cutaneous metastastes from breast cancer in elderly patients: a preliminary report. BMC Surg. 2012;12(suppl 1):S6.
Cutaneous metastases from solid tumors in general occur at a rate of about 1% per primary tumor.1 In breast cancer, cutaneous metastases occur at a rate of about 2.5% per primary tumor. Because of the high incidence of breast cancers relative to other internal malignancies, breast cancer accounts for almost 33% of all cutaneous metastases.2 Infiltrating ductal carcinoma accounts for almost 70% of cutaneous metastases from breast cancers, whereas lobular carcinoma accounts for about 15%.
Cutaneous metastases may be the first presenting sign of primary malignancy. In one retrospective study, 6% of breast carcinomas (N=992) initially presented with only skin manifestations.3 Clinical appearance can vary, but cutaneous metastases from breast adenocarcinomas often present as isolated dermal nodules with superficial discoloration or changes in texture. The most common location of cutaneous metastases is on the chest ipsilateral to the primary breast malignancy.4 We pre-sent a case of metastatic adenocarcinoma of the breast presenting with diffuse cutaneous nodules with no surface changes.
Case Report
A 64-year-old woman who was otherwise in good health presented to her primary care physician for evaluation of recent-onset fatigue. Laboratory testing revealed that she was mildly anemic with mild thrombocytopenia and lymphocytosis. She was referred to a hematologist, who ordered flow cytometry and cytogenetic testing. Blood abnormalities were not considered severe enough to warrant a bone marrow biopsy, and she was monitored clinically for the next 2 years.
Two years after the initial presentation, the primary care physician performed a breast examination that was unremarkable, but enlarged axillary lymph nodes up to 15 mm were discovered in the right breast during routine breast ultrasonography. Additionally, she noted that she had experienced unintentional weight loss of 10 lb over the past year. The hematologist suspected a low-grade lymphoma and performed a bone marrow biopsy. The immunohistochemistry of the bone marrow specimen was consistent with an estrogen receptor–positive, progesterone receptor–negative, human epidermal growth factor receptor 2–negative invasive lobular breast carcinoma, which was then confirmed in the right breast on magnetic resonance imaging. The patient denied any history of prior radiation treatment, but she disclosed a family history of breast cancer in her cousin.
Several weeks after the bone marrow biopsy, an oncologist found that the patient also had an abdominal mass and bone metastases of the primary breast cancer. Colonoscopy confirmed metastases to the colon that subsequently led to obstruction and ultimately required a right hemicolectomy. The patient’s oncologist started her on anastrozole, an aromatase inhibitor (AI), for treatment of the metastatic breast cancer and zoledronic acid, a bisphosphonate, along with calcium and vitamin D for the bone involvement.
Shortly after, during a routine annual skin examination, the patient’s dermatologist (H.T.N.) discovered 3 soft, fixed, subcutaneous-appearing nodules—one on the right chest that was 15 mm in diameter, one on the left mid back that was 7 mm, and one on the left upper anterior thigh that was 10 mm. They were discrete with well-defined borders but had only minimal elevation, making them difficult to detect clinically, especially without palpation. The nodules were not visibly apparent because they were flesh-colored with no surface discoloration or texture changes. The patient remembered that the lesions had appeared gradually several months prior, predating the breast cancer diagnosis, and were not associated with pain, itching, or burning, so she was not alarmed by their appearance and never sought medical attention. The dermatologist (H.T.N.) recommended a biopsy at the time of the skin examination, but the patient declined.
One year after the appearance of the first skin lesions, 14 more nodules (Figure 1) progressively erupted on the ipsilateral and contralateral chest (Figure 2A), axillae, arms, shoulders, back (Figure 2B), and thighs (Figure 2C). At this point, the dermatologists performed a punch biopsy on a lesion on the back to confirm the suspicion of cutaneous metastasis of the primary breast cancer. The biopsy showed interstitial dermal proliferation of atypical cells between collagen bundles and stained strongly positive for cytokeratin 7, an epithelial protein common in breast adenocarcinoma (Figure 3). Further immunohistochemical staining returned metastatic estrogen receptor–positive, progesterone receptor–negative, human epidermal growth factor receptor 2–negative invasive lobular breast carcinoma. Therefore, the markers for the cutaneous metastases were consistent with the markers for the original breast cancer.



After 1 year of treatment with anastrozole, the patient’s internal metastases had not changed considerably, but the cutaneous metastases continued to grow—the lesion on the left thigh doubled from 10 to 20 mm in diameter, and new nodules developed on the chest, back, arms, and legs. One year and a half after the initial lesions were documented, several nodules had disappeared and several new ones appeared. The remaining nodules remained relatively constant in size.
After stopping anastrozole, the patient was enrolled in a research trial using bortezomib, a chemotherapeutic agent typically used for multiple myeloma, as well as fulvestrant, an estrogen receptor antagonist; however, because of continued progression of the metastatic cancer, the patient was removed from the trial and switched to the established regimen of everolimus, a chemotherapeutic agent, and exemestane, another AI. Everolimus eventually was stopped, but the patient continued on exemestane as monotherapy. In addition to development of pleural disease, the cutaneous metastases continued to progress. The patient did not receive any local treatment for her cutaneous metastases.
Comment
Typically, cutaneous metastases of breast cancer manifests as a 1- to 3-cm, asymptomatic, firm, pink to red-brown nodule on the chest ipsilateral to the primary tumor. There may be more than 1 nodule, and ulceration may be present.5,6 In addition to nodular metastases, which make up 47% of cases (N=305), other common presentations include alopecia neoplastica (12%), telangiectatic carcinoma (8%), melanomalike lesions (6%), carcinoma erysipeloides (6%), subungual lesions (5%), carcinoma en cuirasse (4%), and zosteriform metastases (4%).6
Although nodular metastases are the most common type of cutaneous breast cancer metastases, our case is unique in that the patient had soft nodules dispersed to both arms and legs, and the nodules had no surface changes. Although cutaneous metastases can present as flesh-colored nodules,7 they typically have an erythematous base, a slight change in coloration, or induration. Additionally, cutaneous metastases most often are few in number and appear in close proximity to the primary breast adenocarcinoma.8 Without the detection of a slight soft elevation on palpation, our patient’s nodules were practically indistinguishable from the normal skin.
Among common internal cancers, breast cancer is the most likely to metastasize to the skin at a rate of 2.42% per primary tumor (Table 1).1 Cutaneous metastases from lobular carcinomas are much rarer than those from ductal carcinomas.4 The metastases also are most often located locally on the chest ipsilateral to the primary malignancy. Distant metastases are relatively rare. In a review of 212 cases of breast cancer patients with skin metastases, only 9 had involvement of the legs and only 4 had involvement of the contralateral chest.4 Our patient had involvement of the ipsilateral chest, both arms and legs, and the contralateral chest.

The 5-year relative survival rate for breast cancer patients varies based on the stage at diagnosis (99% in patients with localized cancer, 84% with regional lymph node involvement, 24% with distant metastases of any kind).9 In a study of 141 patients with cutaneous metastases in a Taiwanese medical center, Hu et al10 found that patients with breast cancer with only cutaneous metastases had a 5-year absolute survival rate of 38%. In the same study, patients with non–breast cancer metastasis including cutaneous metastasis had a 5-year survival rate of 15%.10 This data is summarized in Table 2.

Breast cancer metastasis to soft tissue (eg, the skin) typically indicates a better prognosis than breast cancer metastasis to a visceral organ or bone. In a study of 439 patients with metastatic relapse after surgical resection of a primary breast cancer, those who had soft tissue metastases had a median survival period of 39 months, whereas those who had visceral or bone metastases had a median survival period of 13 and 28 months, respectively.11 Furthermore, cutaneous metastases from breast cancers do not necessarily indicate as poor a prognosis as skin metastases from other internal malignancies. Cutaneous metastases from other internal malignancies carry a relative risk of mortality of 4.3 compared to cutaneous metastases from breast cancer.10
Treatment of cutaneous metastases may be medically or cosmetically indicated. Standard treatments for cutaneous metastases from the breast include surgical excision, external beam radiotherapy, and systemic chemotherapy.6 While oncologists can use the response of cutaneous metastases to treatment as an indicator of systemic response to hormone therapy or chemotherapy,12 the response may be poorer due to the skin’s relatively weaker blood supply.13
Our patient was first prescribed anastrozole, an AI. For metastatic hormone receptor–positive breast cancer, AIs are a first-line therapy in postmenopausal women. In one meta-analysis, AIs showed greater improvement of survival rates relative to other endocrine therapies such as tamoxifen, an estrogen receptor antagonist (hazard ratio of 0.87).14 After stopping anastrozole, the patient was prescribed fulvestrant, another estrogen receptor antagonist, along with a trial drug. In a randomized, double-blind, placebo-controlled trial, fulvestrant was found to be an effective second-line treatment after anastrozole for hormone receptor–positive breast cancer in postmenopausal women.15 Our patient was then started on everolimus, a chemotherapeutic agent, and exemestane, another AI. After first-line treatment with anastrozole, this regimen also has been found to be an effective second-line treatment with improved progression-free survival.16 For the bone metastases, our patient was treated with zoledronic acid, a bisphosphonate. In a meta-analysis, bisphosphonates were found to reduce skeletal-related complications by a median of 28% in breast cancer patients with bone metastases.17
Some promising new local treatments for cutaneous breast metastases include topical imiquimod and electrochemotherapy. In a small study of 10 patients whose malignancies were refractory to radiotherapy, imiquimod achieved a partial response in 20% (2/10) of patients.18 In another study, 12 patients received electrochemotherapy involving electroporation (applying an electrical field to increase cell membrane permeability and thus increase drug uptake) followed by local administration of bleomycin, an antineoplastic agent. Seventy-five percent (9/12) of the patients received a complete response with disappearance of the metastases.19
This case report provides a rare presentation of diffuse nodular cutaneous metastases of breast adenocarcinoma with no surface changes. The subtle clinical findings in our patient demonstrate the spectrum of clinical manifestations for cutaneous metastases. Our case also serves to highlight the need for close inspection of the skin, including palpation in patients with a history of internal malignancy.
Cutaneous metastases from solid tumors in general occur at a rate of about 1% per primary tumor.1 In breast cancer, cutaneous metastases occur at a rate of about 2.5% per primary tumor. Because of the high incidence of breast cancers relative to other internal malignancies, breast cancer accounts for almost 33% of all cutaneous metastases.2 Infiltrating ductal carcinoma accounts for almost 70% of cutaneous metastases from breast cancers, whereas lobular carcinoma accounts for about 15%.
Cutaneous metastases may be the first presenting sign of primary malignancy. In one retrospective study, 6% of breast carcinomas (N=992) initially presented with only skin manifestations.3 Clinical appearance can vary, but cutaneous metastases from breast adenocarcinomas often present as isolated dermal nodules with superficial discoloration or changes in texture. The most common location of cutaneous metastases is on the chest ipsilateral to the primary breast malignancy.4 We pre-sent a case of metastatic adenocarcinoma of the breast presenting with diffuse cutaneous nodules with no surface changes.
Case Report
A 64-year-old woman who was otherwise in good health presented to her primary care physician for evaluation of recent-onset fatigue. Laboratory testing revealed that she was mildly anemic with mild thrombocytopenia and lymphocytosis. She was referred to a hematologist, who ordered flow cytometry and cytogenetic testing. Blood abnormalities were not considered severe enough to warrant a bone marrow biopsy, and she was monitored clinically for the next 2 years.
Two years after the initial presentation, the primary care physician performed a breast examination that was unremarkable, but enlarged axillary lymph nodes up to 15 mm were discovered in the right breast during routine breast ultrasonography. Additionally, she noted that she had experienced unintentional weight loss of 10 lb over the past year. The hematologist suspected a low-grade lymphoma and performed a bone marrow biopsy. The immunohistochemistry of the bone marrow specimen was consistent with an estrogen receptor–positive, progesterone receptor–negative, human epidermal growth factor receptor 2–negative invasive lobular breast carcinoma, which was then confirmed in the right breast on magnetic resonance imaging. The patient denied any history of prior radiation treatment, but she disclosed a family history of breast cancer in her cousin.
Several weeks after the bone marrow biopsy, an oncologist found that the patient also had an abdominal mass and bone metastases of the primary breast cancer. Colonoscopy confirmed metastases to the colon that subsequently led to obstruction and ultimately required a right hemicolectomy. The patient’s oncologist started her on anastrozole, an aromatase inhibitor (AI), for treatment of the metastatic breast cancer and zoledronic acid, a bisphosphonate, along with calcium and vitamin D for the bone involvement.
Shortly after, during a routine annual skin examination, the patient’s dermatologist (H.T.N.) discovered 3 soft, fixed, subcutaneous-appearing nodules—one on the right chest that was 15 mm in diameter, one on the left mid back that was 7 mm, and one on the left upper anterior thigh that was 10 mm. They were discrete with well-defined borders but had only minimal elevation, making them difficult to detect clinically, especially without palpation. The nodules were not visibly apparent because they were flesh-colored with no surface discoloration or texture changes. The patient remembered that the lesions had appeared gradually several months prior, predating the breast cancer diagnosis, and were not associated with pain, itching, or burning, so she was not alarmed by their appearance and never sought medical attention. The dermatologist (H.T.N.) recommended a biopsy at the time of the skin examination, but the patient declined.
One year after the appearance of the first skin lesions, 14 more nodules (Figure 1) progressively erupted on the ipsilateral and contralateral chest (Figure 2A), axillae, arms, shoulders, back (Figure 2B), and thighs (Figure 2C). At this point, the dermatologists performed a punch biopsy on a lesion on the back to confirm the suspicion of cutaneous metastasis of the primary breast cancer. The biopsy showed interstitial dermal proliferation of atypical cells between collagen bundles and stained strongly positive for cytokeratin 7, an epithelial protein common in breast adenocarcinoma (Figure 3). Further immunohistochemical staining returned metastatic estrogen receptor–positive, progesterone receptor–negative, human epidermal growth factor receptor 2–negative invasive lobular breast carcinoma. Therefore, the markers for the cutaneous metastases were consistent with the markers for the original breast cancer.



After 1 year of treatment with anastrozole, the patient’s internal metastases had not changed considerably, but the cutaneous metastases continued to grow—the lesion on the left thigh doubled from 10 to 20 mm in diameter, and new nodules developed on the chest, back, arms, and legs. One year and a half after the initial lesions were documented, several nodules had disappeared and several new ones appeared. The remaining nodules remained relatively constant in size.
After stopping anastrozole, the patient was enrolled in a research trial using bortezomib, a chemotherapeutic agent typically used for multiple myeloma, as well as fulvestrant, an estrogen receptor antagonist; however, because of continued progression of the metastatic cancer, the patient was removed from the trial and switched to the established regimen of everolimus, a chemotherapeutic agent, and exemestane, another AI. Everolimus eventually was stopped, but the patient continued on exemestane as monotherapy. In addition to development of pleural disease, the cutaneous metastases continued to progress. The patient did not receive any local treatment for her cutaneous metastases.
Comment
Typically, cutaneous metastases of breast cancer manifests as a 1- to 3-cm, asymptomatic, firm, pink to red-brown nodule on the chest ipsilateral to the primary tumor. There may be more than 1 nodule, and ulceration may be present.5,6 In addition to nodular metastases, which make up 47% of cases (N=305), other common presentations include alopecia neoplastica (12%), telangiectatic carcinoma (8%), melanomalike lesions (6%), carcinoma erysipeloides (6%), subungual lesions (5%), carcinoma en cuirasse (4%), and zosteriform metastases (4%).6
Although nodular metastases are the most common type of cutaneous breast cancer metastases, our case is unique in that the patient had soft nodules dispersed to both arms and legs, and the nodules had no surface changes. Although cutaneous metastases can present as flesh-colored nodules,7 they typically have an erythematous base, a slight change in coloration, or induration. Additionally, cutaneous metastases most often are few in number and appear in close proximity to the primary breast adenocarcinoma.8 Without the detection of a slight soft elevation on palpation, our patient’s nodules were practically indistinguishable from the normal skin.
Among common internal cancers, breast cancer is the most likely to metastasize to the skin at a rate of 2.42% per primary tumor (Table 1).1 Cutaneous metastases from lobular carcinomas are much rarer than those from ductal carcinomas.4 The metastases also are most often located locally on the chest ipsilateral to the primary malignancy. Distant metastases are relatively rare. In a review of 212 cases of breast cancer patients with skin metastases, only 9 had involvement of the legs and only 4 had involvement of the contralateral chest.4 Our patient had involvement of the ipsilateral chest, both arms and legs, and the contralateral chest.

The 5-year relative survival rate for breast cancer patients varies based on the stage at diagnosis (99% in patients with localized cancer, 84% with regional lymph node involvement, 24% with distant metastases of any kind).9 In a study of 141 patients with cutaneous metastases in a Taiwanese medical center, Hu et al10 found that patients with breast cancer with only cutaneous metastases had a 5-year absolute survival rate of 38%. In the same study, patients with non–breast cancer metastasis including cutaneous metastasis had a 5-year survival rate of 15%.10 This data is summarized in Table 2.

Breast cancer metastasis to soft tissue (eg, the skin) typically indicates a better prognosis than breast cancer metastasis to a visceral organ or bone. In a study of 439 patients with metastatic relapse after surgical resection of a primary breast cancer, those who had soft tissue metastases had a median survival period of 39 months, whereas those who had visceral or bone metastases had a median survival period of 13 and 28 months, respectively.11 Furthermore, cutaneous metastases from breast cancers do not necessarily indicate as poor a prognosis as skin metastases from other internal malignancies. Cutaneous metastases from other internal malignancies carry a relative risk of mortality of 4.3 compared to cutaneous metastases from breast cancer.10
Treatment of cutaneous metastases may be medically or cosmetically indicated. Standard treatments for cutaneous metastases from the breast include surgical excision, external beam radiotherapy, and systemic chemotherapy.6 While oncologists can use the response of cutaneous metastases to treatment as an indicator of systemic response to hormone therapy or chemotherapy,12 the response may be poorer due to the skin’s relatively weaker blood supply.13
Our patient was first prescribed anastrozole, an AI. For metastatic hormone receptor–positive breast cancer, AIs are a first-line therapy in postmenopausal women. In one meta-analysis, AIs showed greater improvement of survival rates relative to other endocrine therapies such as tamoxifen, an estrogen receptor antagonist (hazard ratio of 0.87).14 After stopping anastrozole, the patient was prescribed fulvestrant, another estrogen receptor antagonist, along with a trial drug. In a randomized, double-blind, placebo-controlled trial, fulvestrant was found to be an effective second-line treatment after anastrozole for hormone receptor–positive breast cancer in postmenopausal women.15 Our patient was then started on everolimus, a chemotherapeutic agent, and exemestane, another AI. After first-line treatment with anastrozole, this regimen also has been found to be an effective second-line treatment with improved progression-free survival.16 For the bone metastases, our patient was treated with zoledronic acid, a bisphosphonate. In a meta-analysis, bisphosphonates were found to reduce skeletal-related complications by a median of 28% in breast cancer patients with bone metastases.17
Some promising new local treatments for cutaneous breast metastases include topical imiquimod and electrochemotherapy. In a small study of 10 patients whose malignancies were refractory to radiotherapy, imiquimod achieved a partial response in 20% (2/10) of patients.18 In another study, 12 patients received electrochemotherapy involving electroporation (applying an electrical field to increase cell membrane permeability and thus increase drug uptake) followed by local administration of bleomycin, an antineoplastic agent. Seventy-five percent (9/12) of the patients received a complete response with disappearance of the metastases.19
This case report provides a rare presentation of diffuse nodular cutaneous metastases of breast adenocarcinoma with no surface changes. The subtle clinical findings in our patient demonstrate the spectrum of clinical manifestations for cutaneous metastases. Our case also serves to highlight the need for close inspection of the skin, including palpation in patients with a history of internal malignancy.
- Hu SC, Chen G, Wu C, et al. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60:379-387.
- Wong CY, Helm MA, Helm TN, et al. Patterns of skin metastases: a review of 25 years’ experience at a single cancer center. Int J Dermatol. 2014;53:56-60.
- Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma: a retrospective study of 7316 cancer patients. J Am Acad Dermatol. 1990;22:19-26.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2, part 1):228-236.
- Gan DEH, Teh YC, Ng CH, et al. Cutaneous metastases of breast cancer: a case report. Breast Case. 2012;1:23-36.
- De Giorgi V, Grazzini M, Alfaioli B, et al. Cutaneous manifestations of breast carcinoma. Dermatol Ther. 2010;23:581-589.
- Vano-Galvan S, Moreno-Martin P, Salguero I, et al. Cutaneous metastases of breast carcinoma: a case report. Cases J. 2009;2:71.
- Dacso M, Soldano AC, Talbott LB, et al. A solitary neck nodule as late evidence of recurrent lobular breast carcinoma. Case Rep Oncol. 2009;2:24-29.
- Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2010. Table 1.5 Age-Adjusted SEER Incidence and U.S. Death Rates and 5-Year Relative Survival (Percent) By Primary Cancer Site, Sex and Time Period. Bethesda, MD: National Cancer Institute; 2013. https://seer.cancer.gov/archive/csr/1975_2010/results_merged/topic_survival.pdf. Updated June 14, 2014. Accessed February 27, 2018.
- Hu SC, Chen GS, Lu YW, et al. Cutaneous metastases from different internal malignancies: a clinical and prognostic appraisal. J Eur Acad Dermatol Venereol. 2008;22:735-740.
- Insa A, Lluch A, Prosper F, et al. Prognostic factors predicting survival from first recurrence in patients with metastatic breast cancer: analysis of 439 patients. Breast Cancer Res Treat. 1999;56:67-78.
- Eisenhauer E, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247.
- Kamble R, Kumar L, Kochupillai V, et al. Cutaneous metastases of lung cancer. Postgrad Med J. 1995;71:741-743.
- Mauri D, Pavlidis N, Polyzos N, et al. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst. 2006;98:1285-1291.
- Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664-1670.
- Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N Engl J Med. 2012;366:520-529.
- Wong MH, Stockler M, Pavlakis N. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev. 2012;2:CD003474.
- Adams S, Kozhaya L, Martiniuk F, et al. Topical TLR7 agonist imiquimod can induce immune-mediated rejection of skin metastases in patients with breast cancer. Clin Cancer Res. 2012;18:6748-6757.
- Benevento R, Santoriello A, Perna G, et al. Electrochemotherapy of cutaneous metastastes from breast cancer in elderly patients: a preliminary report. BMC Surg. 2012;12(suppl 1):S6.
- Hu SC, Chen G, Wu C, et al. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60:379-387.
- Wong CY, Helm MA, Helm TN, et al. Patterns of skin metastases: a review of 25 years’ experience at a single cancer center. Int J Dermatol. 2014;53:56-60.
- Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma: a retrospective study of 7316 cancer patients. J Am Acad Dermatol. 1990;22:19-26.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2, part 1):228-236.
- Gan DEH, Teh YC, Ng CH, et al. Cutaneous metastases of breast cancer: a case report. Breast Case. 2012;1:23-36.
- De Giorgi V, Grazzini M, Alfaioli B, et al. Cutaneous manifestations of breast carcinoma. Dermatol Ther. 2010;23:581-589.
- Vano-Galvan S, Moreno-Martin P, Salguero I, et al. Cutaneous metastases of breast carcinoma: a case report. Cases J. 2009;2:71.
- Dacso M, Soldano AC, Talbott LB, et al. A solitary neck nodule as late evidence of recurrent lobular breast carcinoma. Case Rep Oncol. 2009;2:24-29.
- Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2010. Table 1.5 Age-Adjusted SEER Incidence and U.S. Death Rates and 5-Year Relative Survival (Percent) By Primary Cancer Site, Sex and Time Period. Bethesda, MD: National Cancer Institute; 2013. https://seer.cancer.gov/archive/csr/1975_2010/results_merged/topic_survival.pdf. Updated June 14, 2014. Accessed February 27, 2018.
- Hu SC, Chen GS, Lu YW, et al. Cutaneous metastases from different internal malignancies: a clinical and prognostic appraisal. J Eur Acad Dermatol Venereol. 2008;22:735-740.
- Insa A, Lluch A, Prosper F, et al. Prognostic factors predicting survival from first recurrence in patients with metastatic breast cancer: analysis of 439 patients. Breast Cancer Res Treat. 1999;56:67-78.
- Eisenhauer E, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247.
- Kamble R, Kumar L, Kochupillai V, et al. Cutaneous metastases of lung cancer. Postgrad Med J. 1995;71:741-743.
- Mauri D, Pavlidis N, Polyzos N, et al. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst. 2006;98:1285-1291.
- Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664-1670.
- Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N Engl J Med. 2012;366:520-529.
- Wong MH, Stockler M, Pavlakis N. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev. 2012;2:CD003474.
- Adams S, Kozhaya L, Martiniuk F, et al. Topical TLR7 agonist imiquimod can induce immune-mediated rejection of skin metastases in patients with breast cancer. Clin Cancer Res. 2012;18:6748-6757.
- Benevento R, Santoriello A, Perna G, et al. Electrochemotherapy of cutaneous metastastes from breast cancer in elderly patients: a preliminary report. BMC Surg. 2012;12(suppl 1):S6.
Practice Points
- Although breast cancer has the highest rate of cutaneous metastasis among internal malignancies, cutaneous metastases occur in only a small minority of breast cancer patients.
- Cutaneous metastases from breast cancer typically do not carry as poor a prognosis as those in other internal malignancies.
- The clinical presentation of cutaneous metastases from breast cancer can be varied. In our patient, the metastases were subtle and resembled subcutaneous nodules lacking surface changes, thus making them best detectable by palpation.
- While oncologists can use the response of cutaneous metastases to treatment as an indicator of systemic response, the cutaneous response may be poorer due to the skin’s relatively weaker blood supply.
Deepithelialized Flaps and Grafts: Applications in Dermatologic Surgery
Deepithelialized flaps and grafts have been widely used by reconstructive surgeons in a diverse range of medical specialties since the early 20th century. 1 These reconstructive modalities have more recently been applied to dermatologic surgery. Deepithelialized flaps and grafts involve removal of the epidermis from the dermis for a variety of surgical purposes. Although these techniques play an important role in dermatologic surgery, reports of application of deepithelialized flaps and grafts in the dermatology literature is limited. This article includes a presentation of the applications of deepithelialized flaps and grafts in procedural dermatology.
DEEPITHELIALIZATION TECHNIQUES
There are a variety of techniques for deepithelialization, although sharp deepithelialization generally is preferred by dermatologic surgeons. The scalpel technique can be accomplished by making an intradermal incision with a No. 15 blade. Traction is an essential component of the deepthelialization process and facilitates sharp removal of the epidermis and superficial dermis in an even plane. The peeling orange technique, which has been described in reduction mammoplasty, is a variant of the scalpel technique used for creating a large area of deepithelialized tissue.2 A No. 10 blade is used to make multiple partial-thickness intradermal incisions 1 to 2 cm apart along the pedicle. Traction facilitates rapid deepithelialization of the skin strips on the pedicle. A sharp curette is an alternative option for sharply removing the epithelium from a small area. Electric dermatome, laser, and electrocautery techniques for deepithelialization also can be considered.2,3
APPLICATION OF DEEPITHELIALIZED FLAPS
Deepithelialized flaps may be considered for single-stage reconstruction with tunneled interpolation flaps, reconstruction requiring contour preservation, and reconstruction involving free margins.4-17
Reconstruction With Single-Stage Tunneled Interpolated Flaps
Alar Base
A partially deepithelialized tunneled interpolated flap is an elegant reconstructive option for defects involving the upper cutaneous lip and alar base. The flap is elevated from the ipsilateral nasolabial fold, deepithelialized proximally, and tunneled under the intact portion of the cutaneous upper lip and ala. The flap is then deepithelialized superiorly to bolster the alar base and inset at the recipient site.4
Nasal Ala
The tunneled interpolated flap is useful for reconstruction of defects of the nasal ala. A flap with a superior deepithelialized pedicle and an anticipated inferior Burow triangle is designed along the axis of the nasolabial fold. The inferior Burow triangle and central flap are elevated at the level of the superficial subcutaneous fat and the pedicle is dissected. The donor and recipient sites are widely undermined, and the flap and pedicle pass through the tunnel. The donor site is closed primarily, the inferior Burow triangle is trimmed, and the flap is sutured into the defect.5 This flap allows for preservation of free margins and favorable placement of incision lines. Furthermore, pincushioning of the flap helps to recreate the rounded shape of the lateral ala.6
Nasal Tip
Nasal tip defects can be repaired with a retroangular flap, centered on the angular artery. The flap is elevated along the axis of the nasolabial fold, deepithelialized at its proximal base, and transferred through a subcutaneous tunnel to the nasal tip. The angular artery is ligated at the inferior aspect of the flap.7
Nasal Sidewall
A deepithelialized tunneled interpolated forehead flap, similar to the classic paramedian forehead flap, can be used to reconstruct nasal sidewall defects. A flap is elevated on the contralateral forehead and the proximal portion is deepithelialized. A tunnel is then bluntly dissected just above the periosteum, and the flap is introduced into the defect through the tunnel and inset. This flap has the advantages of being a single-stage procedure, restoring volume to the defect area, and maintaining excellent vascular supply.8
Eyelid
A tunneled interpolated forehead flap also can be used to repair medial canthal defects and for anterior lamellar repair of lower eyelid defects. In a study of 9 patients receiving a tunneled interpolated forehead flap in these anatomic locations, all flaps demonstrated viability, protection of the globe, and preservation of the concave architecture of the medial canthus.9
Earlobe
Earlobe defects may be repaired with a pull-through interpolated preauricular flap. A flap is elevated superiorly in the preauricular region and the proximal aspect of the flap is deepithelialized. The flap is pulled through a tunnel and inset at the anterior earlobe defect. The donor site is closed primarily.10,11
Concha
Reconstruction of anterior conchal defects with exposed cartilage can be accomplished with a pull-through interpolated postauricular flap based on the auriculomastoid fossa. The postauricular flap is elevated, the base is deepithelialized, an incision is made in the medial aspect of the defect, and the flap is moved through a tunnel between the posterior and anterior surfaces of the ear. The flap is secured to the anterior surface of the concha.12
Reconstruction Requiring Contour Preservation
Central Face
The hinge flap is optimal for reconstruction of deep central facial defects (Figure 1). The hinge flap is planned at a site contiguous with a margin of the defect and can include the dermis, subcutaneous tissue, muscle, or a combination of these. The desired tissue is folded over on the pedicle to fill the defect. Cutaneous coverage is accomplished through a primary closure, separate flap, or skin graft. In addition to restoring contour and therefore the cosmetic subunit, the hinge flap is performed in a single stage, resists wound contracture, and provides a well-vascularized wound bed resulting in a low incidence of graft failure.13,14 Muscular hinge flaps have been described for reconstruction of forehead defects with exposed bone based on the frontalis muscle.15
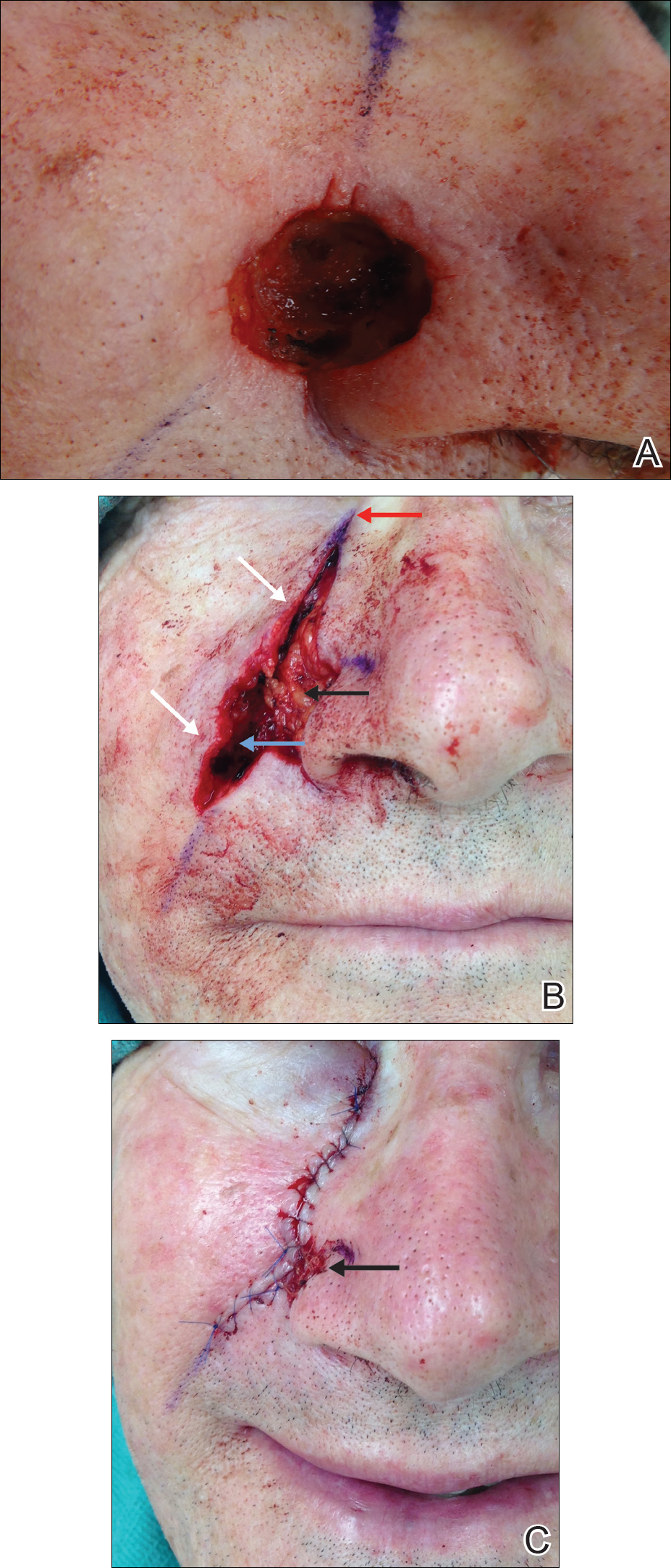
Lower Lip
A variant of a V-Y advancement flap has been described for reconstruction of defects greater than one-third the length of the lower lip. The top of the “V” is deepithelialized and the flap is advanced such that the top of the “V” abuts the inferior border of the defect. The “V” flap is inset at its advanced position, converting the “V”-shaped wound into a “Y.” An overlying buccal mucosal graft provides reconstruction of the lower red lip and labial mucosa.16
Helix of the Ear
Large defects of the scapha and helix of the ear can be reconstructed with the use of a staged interpolated postauricular flap. The postauricular flap is elevated into a subcutaneous plane. A full-thickness incision is made medial to the helical rim, and the flap is tunneled through and sutured into place. The pedicle is later divided, and the distal aspect of the flap is deepithelialized and inset into the helical rim for volume restoration.17
Reconstruction Involving Free Margins
Nasal Ala
For large defects involving the upper cutaneous lip with adjacent alar base involvement, a partially deepithelialized V-Y flap is a useful reconstructive option (Figure 2).
Infraorbital Region
A deepithelialized variant of a V-Y advancement flap can be used for closure of infraorbital defects. The limbs of the V-Y flap are deepithelialized and anchored to the medial and lateral canthal tendons or periosteum. Ectropion prevention is the primary advantage of this flap.18
APPLICATION OF DEEPITHELIALIZED GRAFTS
Deepithelialized grafts may be considered for volume replacement, reconstruction requiring contour preservation, and restoration of mechanical integrity in areas of high mechanical tension.3,19-21
Reconstruction Requiring Contour Preservation
Deepithelialized grafts are used to improve depressed nasal scars and restore volume in deep nasal wounds. One method involves deepithelialization of 2 postauricular punch biopsies. An 18-gauge needle is used to make a small hole in the depressed nasal scar, the dermal grafts are inserted, and the defect is closed primarily.19 Dermal grafts may be harvested from excess full-thickness skin grafts (FTSGs) or dog-ear tissue. When used under flaps, the dermal graft is trimmed to the size of the defect. When used under FTSGs, thin dermal graft strips are placed in a gridlike pattern to allow for revascularization. A study of 15 patients with contour deformities reconstructed with dermal graft insertions demonstrated that 14 (94%) patients had no significant complications and improvement of scar depression was achieved.20
Reconstruction in Areas of High Mechanical Tension
Plantar Foot
A combined dermal and full-thickness sandwich graft has been described for reconstruction of plantar foot defects.3 The graft is created by obtaining a FTSG twice the size of the wound defect and deepithelializing half of the graft. The graft is then defatted and the deepithelialized portion is folded beneath the other half, allowing the papillary dermis to make contact with the wound surface.
Scalp
Dermal graft reconstruction for scalp defects may be accomplished with a split-thickness skin flap. The flap is harvested using an electronic dermatome that ensures the proximal aspect is still attached to adjacent skin. The dermis is removed from the area underneath the back-folded split-thickness skin flap. The dermal graft is meshed and sutured into the recipient site. The split-thickness skin flap is replaced over the donor site. Meshed reversed dermal grafts have excellent survival rates, even with direct placement on bone without periosteum. Querings et al21 reported graft survival with no complications in 19 of 21 (90.4%) patients undergoing scalp or plantar sole reconstruction.
CONCLUSION
With the widespread adoption of the fresh-tissue technique for Mohs micrographic surgery and the establishment of the American Society for Dermatologic Surgery in 1970, the depth and scope of techniques used by dermatologic surgeons has dramatically expanded. Although the use of dermal flaps and grafts is not as widespread in dermatology as other reconstructive techniques, their unique advantages should be considered. Deepithelialized flaps and grafts should be considered when the following reconstructive goals are desired: (1) conversion of a 2-stage interpolation flap to a single-stage tunneled flap, (2) contour and cosmetic subunit preservation of deep defects through volume augmentation, (3) reconstruction in areas of high mechanical tension, and (4) free margin preservation. The multiple applications of deepithelialized flaps and grafts as described in this review demonstrate their continued applicability in dermatologic surgery.
- Straatsma CR. Use of the dermal graft in the repairs of small saddle defects of the nose. Arch Otolaryngol. 1932;16:506-509.
- Cydeli A, Hunter J. Peeling orange: rapid deepithelialization in reduction mammoplasty. J Aesthet Surg. 2004;24:580-581.
- Bechara F, Sand M, Radenhausen M, et al. Erbium:YAG laser-assisted preparation of a combined dermal/full thickness sandwich skin graft. Dermatol Surg. 2006;32:353-358.
- Cook JL. Tunneled and transposed island flaps in facial reconstructive surgery. Dermatol Surg. 2014;40(suppl 9):S16-S29.
- Krishnan RS, Clark DP. Tunneled transposition flap for reconstruction of defects of the nasal ala. Dermatol Surg. 2007;33:1496-1501.
- Mahlberg M. Tunneled melolabial pedicle flap for small but deep lateral alar rim defect. Dermatol Surg. 2013;39:1527-1529.
- Ascari-Raccagni A, Balderi U. The retroangular flap used in the surgery of nasal tip defects. Dermatol Surg. 2004;30:1131-1137.
- Hollmig ST, Leach BC, Cook J. Single-staged interpolation flaps in facial reconstruction. Dermatol Surg. 2014;40(suppl 9):S62-S70.
- Mombaerts I, Gillis A. The tunneled forehead flap in medial canthal and eyelid reconstruction. Dermatol Surg. 2010:36:1118-1125.
- Wang SQ, Goldberg LH, Kimyah-Asadi A. Tunneled island pedicle flap for an earlobe defect. Dermatol Surg. 2007;33:835-838.
- Hatoko M, Kuwahara M, Shiba A, et al. Earlobe reconstruction using a subcutaneous island pedicle flap after resection of “earlobe keloid.” Dermatol Surg. 1998;24:257-261.
- Alder N, Ad-El D, Azaria R. Reconstruction of nonhelical auricular defects with local flaps. Dermatol Surg. 2008;34:501-507.
- Fader DJ, Wang TS, Johnson TM. Nasal reconstruction utilizing a muscle hinge flap with overlying FTSG. J Am Acad Dermatol. 2000;43:837-840.
- Braun MA, Cook J. Hinge flaps in facial reconstruction. Dermatol Surg. 2007;33:213-221.
- Salmon PL, Mortimer NL, Hill SE. Muscular hinge flaps: utility and technique in facial reconstructive surgery. Dermatol Surg. 2010;36:227-234.
- Seo Y, Song S, Choi Y, et al. A lower lip reconstruction. Dermatol Surg. 2015;41:505-507.
- Malone CH, Wagner RF. Partially de-epithelialized postauricular flap for ear reconstruction. J Am Acad Dermatol. 2015;73:E219-E220.
- Yildrim S, Akoz T, Akan M, et al. Nasolabial V-Y advancement for closure of the midface defects. Dermatol Surg. 2001;27:656-662.
- Jensen DJ, Cohen JL. Nasal tip revision using a dermal graft. Dermatol Surg. 2014;40:1140-1142.
- Meyers S, Rohrer T. Use of dermal grafts in reconstructing deep nasal defects and shaping the ala nasi. Dermatol Surg. 2001;27:300-305.
- Querings K, Bachter D, Balda B. Meshed reversed dermal graft in patients with surgical defects of sole and scalp: technique and long-term results. Dermatol Surg. 2002;28:122-126.
Deepithelialized flaps and grafts have been widely used by reconstructive surgeons in a diverse range of medical specialties since the early 20th century. 1 These reconstructive modalities have more recently been applied to dermatologic surgery. Deepithelialized flaps and grafts involve removal of the epidermis from the dermis for a variety of surgical purposes. Although these techniques play an important role in dermatologic surgery, reports of application of deepithelialized flaps and grafts in the dermatology literature is limited. This article includes a presentation of the applications of deepithelialized flaps and grafts in procedural dermatology.
DEEPITHELIALIZATION TECHNIQUES
There are a variety of techniques for deepithelialization, although sharp deepithelialization generally is preferred by dermatologic surgeons. The scalpel technique can be accomplished by making an intradermal incision with a No. 15 blade. Traction is an essential component of the deepthelialization process and facilitates sharp removal of the epidermis and superficial dermis in an even plane. The peeling orange technique, which has been described in reduction mammoplasty, is a variant of the scalpel technique used for creating a large area of deepithelialized tissue.2 A No. 10 blade is used to make multiple partial-thickness intradermal incisions 1 to 2 cm apart along the pedicle. Traction facilitates rapid deepithelialization of the skin strips on the pedicle. A sharp curette is an alternative option for sharply removing the epithelium from a small area. Electric dermatome, laser, and electrocautery techniques for deepithelialization also can be considered.2,3
APPLICATION OF DEEPITHELIALIZED FLAPS
Deepithelialized flaps may be considered for single-stage reconstruction with tunneled interpolation flaps, reconstruction requiring contour preservation, and reconstruction involving free margins.4-17
Reconstruction With Single-Stage Tunneled Interpolated Flaps
Alar Base
A partially deepithelialized tunneled interpolated flap is an elegant reconstructive option for defects involving the upper cutaneous lip and alar base. The flap is elevated from the ipsilateral nasolabial fold, deepithelialized proximally, and tunneled under the intact portion of the cutaneous upper lip and ala. The flap is then deepithelialized superiorly to bolster the alar base and inset at the recipient site.4
Nasal Ala
The tunneled interpolated flap is useful for reconstruction of defects of the nasal ala. A flap with a superior deepithelialized pedicle and an anticipated inferior Burow triangle is designed along the axis of the nasolabial fold. The inferior Burow triangle and central flap are elevated at the level of the superficial subcutaneous fat and the pedicle is dissected. The donor and recipient sites are widely undermined, and the flap and pedicle pass through the tunnel. The donor site is closed primarily, the inferior Burow triangle is trimmed, and the flap is sutured into the defect.5 This flap allows for preservation of free margins and favorable placement of incision lines. Furthermore, pincushioning of the flap helps to recreate the rounded shape of the lateral ala.6
Nasal Tip
Nasal tip defects can be repaired with a retroangular flap, centered on the angular artery. The flap is elevated along the axis of the nasolabial fold, deepithelialized at its proximal base, and transferred through a subcutaneous tunnel to the nasal tip. The angular artery is ligated at the inferior aspect of the flap.7
Nasal Sidewall
A deepithelialized tunneled interpolated forehead flap, similar to the classic paramedian forehead flap, can be used to reconstruct nasal sidewall defects. A flap is elevated on the contralateral forehead and the proximal portion is deepithelialized. A tunnel is then bluntly dissected just above the periosteum, and the flap is introduced into the defect through the tunnel and inset. This flap has the advantages of being a single-stage procedure, restoring volume to the defect area, and maintaining excellent vascular supply.8
Eyelid
A tunneled interpolated forehead flap also can be used to repair medial canthal defects and for anterior lamellar repair of lower eyelid defects. In a study of 9 patients receiving a tunneled interpolated forehead flap in these anatomic locations, all flaps demonstrated viability, protection of the globe, and preservation of the concave architecture of the medial canthus.9
Earlobe
Earlobe defects may be repaired with a pull-through interpolated preauricular flap. A flap is elevated superiorly in the preauricular region and the proximal aspect of the flap is deepithelialized. The flap is pulled through a tunnel and inset at the anterior earlobe defect. The donor site is closed primarily.10,11
Concha
Reconstruction of anterior conchal defects with exposed cartilage can be accomplished with a pull-through interpolated postauricular flap based on the auriculomastoid fossa. The postauricular flap is elevated, the base is deepithelialized, an incision is made in the medial aspect of the defect, and the flap is moved through a tunnel between the posterior and anterior surfaces of the ear. The flap is secured to the anterior surface of the concha.12
Reconstruction Requiring Contour Preservation
Central Face
The hinge flap is optimal for reconstruction of deep central facial defects (Figure 1). The hinge flap is planned at a site contiguous with a margin of the defect and can include the dermis, subcutaneous tissue, muscle, or a combination of these. The desired tissue is folded over on the pedicle to fill the defect. Cutaneous coverage is accomplished through a primary closure, separate flap, or skin graft. In addition to restoring contour and therefore the cosmetic subunit, the hinge flap is performed in a single stage, resists wound contracture, and provides a well-vascularized wound bed resulting in a low incidence of graft failure.13,14 Muscular hinge flaps have been described for reconstruction of forehead defects with exposed bone based on the frontalis muscle.15

Lower Lip
A variant of a V-Y advancement flap has been described for reconstruction of defects greater than one-third the length of the lower lip. The top of the “V” is deepithelialized and the flap is advanced such that the top of the “V” abuts the inferior border of the defect. The “V” flap is inset at its advanced position, converting the “V”-shaped wound into a “Y.” An overlying buccal mucosal graft provides reconstruction of the lower red lip and labial mucosa.16
Helix of the Ear
Large defects of the scapha and helix of the ear can be reconstructed with the use of a staged interpolated postauricular flap. The postauricular flap is elevated into a subcutaneous plane. A full-thickness incision is made medial to the helical rim, and the flap is tunneled through and sutured into place. The pedicle is later divided, and the distal aspect of the flap is deepithelialized and inset into the helical rim for volume restoration.17
Reconstruction Involving Free Margins
Nasal Ala
For large defects involving the upper cutaneous lip with adjacent alar base involvement, a partially deepithelialized V-Y flap is a useful reconstructive option (Figure 2).

Infraorbital Region
A deepithelialized variant of a V-Y advancement flap can be used for closure of infraorbital defects. The limbs of the V-Y flap are deepithelialized and anchored to the medial and lateral canthal tendons or periosteum. Ectropion prevention is the primary advantage of this flap.18
APPLICATION OF DEEPITHELIALIZED GRAFTS
Deepithelialized grafts may be considered for volume replacement, reconstruction requiring contour preservation, and restoration of mechanical integrity in areas of high mechanical tension.3,19-21
Reconstruction Requiring Contour Preservation
Deepithelialized grafts are used to improve depressed nasal scars and restore volume in deep nasal wounds. One method involves deepithelialization of 2 postauricular punch biopsies. An 18-gauge needle is used to make a small hole in the depressed nasal scar, the dermal grafts are inserted, and the defect is closed primarily.19 Dermal grafts may be harvested from excess full-thickness skin grafts (FTSGs) or dog-ear tissue. When used under flaps, the dermal graft is trimmed to the size of the defect. When used under FTSGs, thin dermal graft strips are placed in a gridlike pattern to allow for revascularization. A study of 15 patients with contour deformities reconstructed with dermal graft insertions demonstrated that 14 (94%) patients had no significant complications and improvement of scar depression was achieved.20
Reconstruction in Areas of High Mechanical Tension
Plantar Foot
A combined dermal and full-thickness sandwich graft has been described for reconstruction of plantar foot defects.3 The graft is created by obtaining a FTSG twice the size of the wound defect and deepithelializing half of the graft. The graft is then defatted and the deepithelialized portion is folded beneath the other half, allowing the papillary dermis to make contact with the wound surface.
Scalp
Dermal graft reconstruction for scalp defects may be accomplished with a split-thickness skin flap. The flap is harvested using an electronic dermatome that ensures the proximal aspect is still attached to adjacent skin. The dermis is removed from the area underneath the back-folded split-thickness skin flap. The dermal graft is meshed and sutured into the recipient site. The split-thickness skin flap is replaced over the donor site. Meshed reversed dermal grafts have excellent survival rates, even with direct placement on bone without periosteum. Querings et al21 reported graft survival with no complications in 19 of 21 (90.4%) patients undergoing scalp or plantar sole reconstruction.
CONCLUSION
With the widespread adoption of the fresh-tissue technique for Mohs micrographic surgery and the establishment of the American Society for Dermatologic Surgery in 1970, the depth and scope of techniques used by dermatologic surgeons has dramatically expanded. Although the use of dermal flaps and grafts is not as widespread in dermatology as other reconstructive techniques, their unique advantages should be considered. Deepithelialized flaps and grafts should be considered when the following reconstructive goals are desired: (1) conversion of a 2-stage interpolation flap to a single-stage tunneled flap, (2) contour and cosmetic subunit preservation of deep defects through volume augmentation, (3) reconstruction in areas of high mechanical tension, and (4) free margin preservation. The multiple applications of deepithelialized flaps and grafts as described in this review demonstrate their continued applicability in dermatologic surgery.
Deepithelialized flaps and grafts have been widely used by reconstructive surgeons in a diverse range of medical specialties since the early 20th century. 1 These reconstructive modalities have more recently been applied to dermatologic surgery. Deepithelialized flaps and grafts involve removal of the epidermis from the dermis for a variety of surgical purposes. Although these techniques play an important role in dermatologic surgery, reports of application of deepithelialized flaps and grafts in the dermatology literature is limited. This article includes a presentation of the applications of deepithelialized flaps and grafts in procedural dermatology.
DEEPITHELIALIZATION TECHNIQUES
There are a variety of techniques for deepithelialization, although sharp deepithelialization generally is preferred by dermatologic surgeons. The scalpel technique can be accomplished by making an intradermal incision with a No. 15 blade. Traction is an essential component of the deepthelialization process and facilitates sharp removal of the epidermis and superficial dermis in an even plane. The peeling orange technique, which has been described in reduction mammoplasty, is a variant of the scalpel technique used for creating a large area of deepithelialized tissue.2 A No. 10 blade is used to make multiple partial-thickness intradermal incisions 1 to 2 cm apart along the pedicle. Traction facilitates rapid deepithelialization of the skin strips on the pedicle. A sharp curette is an alternative option for sharply removing the epithelium from a small area. Electric dermatome, laser, and electrocautery techniques for deepithelialization also can be considered.2,3
APPLICATION OF DEEPITHELIALIZED FLAPS
Deepithelialized flaps may be considered for single-stage reconstruction with tunneled interpolation flaps, reconstruction requiring contour preservation, and reconstruction involving free margins.4-17
Reconstruction With Single-Stage Tunneled Interpolated Flaps
Alar Base
A partially deepithelialized tunneled interpolated flap is an elegant reconstructive option for defects involving the upper cutaneous lip and alar base. The flap is elevated from the ipsilateral nasolabial fold, deepithelialized proximally, and tunneled under the intact portion of the cutaneous upper lip and ala. The flap is then deepithelialized superiorly to bolster the alar base and inset at the recipient site.4
Nasal Ala
The tunneled interpolated flap is useful for reconstruction of defects of the nasal ala. A flap with a superior deepithelialized pedicle and an anticipated inferior Burow triangle is designed along the axis of the nasolabial fold. The inferior Burow triangle and central flap are elevated at the level of the superficial subcutaneous fat and the pedicle is dissected. The donor and recipient sites are widely undermined, and the flap and pedicle pass through the tunnel. The donor site is closed primarily, the inferior Burow triangle is trimmed, and the flap is sutured into the defect.5 This flap allows for preservation of free margins and favorable placement of incision lines. Furthermore, pincushioning of the flap helps to recreate the rounded shape of the lateral ala.6
Nasal Tip
Nasal tip defects can be repaired with a retroangular flap, centered on the angular artery. The flap is elevated along the axis of the nasolabial fold, deepithelialized at its proximal base, and transferred through a subcutaneous tunnel to the nasal tip. The angular artery is ligated at the inferior aspect of the flap.7
Nasal Sidewall
A deepithelialized tunneled interpolated forehead flap, similar to the classic paramedian forehead flap, can be used to reconstruct nasal sidewall defects. A flap is elevated on the contralateral forehead and the proximal portion is deepithelialized. A tunnel is then bluntly dissected just above the periosteum, and the flap is introduced into the defect through the tunnel and inset. This flap has the advantages of being a single-stage procedure, restoring volume to the defect area, and maintaining excellent vascular supply.8
Eyelid
A tunneled interpolated forehead flap also can be used to repair medial canthal defects and for anterior lamellar repair of lower eyelid defects. In a study of 9 patients receiving a tunneled interpolated forehead flap in these anatomic locations, all flaps demonstrated viability, protection of the globe, and preservation of the concave architecture of the medial canthus.9
Earlobe
Earlobe defects may be repaired with a pull-through interpolated preauricular flap. A flap is elevated superiorly in the preauricular region and the proximal aspect of the flap is deepithelialized. The flap is pulled through a tunnel and inset at the anterior earlobe defect. The donor site is closed primarily.10,11
Concha
Reconstruction of anterior conchal defects with exposed cartilage can be accomplished with a pull-through interpolated postauricular flap based on the auriculomastoid fossa. The postauricular flap is elevated, the base is deepithelialized, an incision is made in the medial aspect of the defect, and the flap is moved through a tunnel between the posterior and anterior surfaces of the ear. The flap is secured to the anterior surface of the concha.12
Reconstruction Requiring Contour Preservation
Central Face
The hinge flap is optimal for reconstruction of deep central facial defects (Figure 1). The hinge flap is planned at a site contiguous with a margin of the defect and can include the dermis, subcutaneous tissue, muscle, or a combination of these. The desired tissue is folded over on the pedicle to fill the defect. Cutaneous coverage is accomplished through a primary closure, separate flap, or skin graft. In addition to restoring contour and therefore the cosmetic subunit, the hinge flap is performed in a single stage, resists wound contracture, and provides a well-vascularized wound bed resulting in a low incidence of graft failure.13,14 Muscular hinge flaps have been described for reconstruction of forehead defects with exposed bone based on the frontalis muscle.15

Lower Lip
A variant of a V-Y advancement flap has been described for reconstruction of defects greater than one-third the length of the lower lip. The top of the “V” is deepithelialized and the flap is advanced such that the top of the “V” abuts the inferior border of the defect. The “V” flap is inset at its advanced position, converting the “V”-shaped wound into a “Y.” An overlying buccal mucosal graft provides reconstruction of the lower red lip and labial mucosa.16
Helix of the Ear
Large defects of the scapha and helix of the ear can be reconstructed with the use of a staged interpolated postauricular flap. The postauricular flap is elevated into a subcutaneous plane. A full-thickness incision is made medial to the helical rim, and the flap is tunneled through and sutured into place. The pedicle is later divided, and the distal aspect of the flap is deepithelialized and inset into the helical rim for volume restoration.17
Reconstruction Involving Free Margins
Nasal Ala
For large defects involving the upper cutaneous lip with adjacent alar base involvement, a partially deepithelialized V-Y flap is a useful reconstructive option (Figure 2).

Infraorbital Region
A deepithelialized variant of a V-Y advancement flap can be used for closure of infraorbital defects. The limbs of the V-Y flap are deepithelialized and anchored to the medial and lateral canthal tendons or periosteum. Ectropion prevention is the primary advantage of this flap.18
APPLICATION OF DEEPITHELIALIZED GRAFTS
Deepithelialized grafts may be considered for volume replacement, reconstruction requiring contour preservation, and restoration of mechanical integrity in areas of high mechanical tension.3,19-21
Reconstruction Requiring Contour Preservation
Deepithelialized grafts are used to improve depressed nasal scars and restore volume in deep nasal wounds. One method involves deepithelialization of 2 postauricular punch biopsies. An 18-gauge needle is used to make a small hole in the depressed nasal scar, the dermal grafts are inserted, and the defect is closed primarily.19 Dermal grafts may be harvested from excess full-thickness skin grafts (FTSGs) or dog-ear tissue. When used under flaps, the dermal graft is trimmed to the size of the defect. When used under FTSGs, thin dermal graft strips are placed in a gridlike pattern to allow for revascularization. A study of 15 patients with contour deformities reconstructed with dermal graft insertions demonstrated that 14 (94%) patients had no significant complications and improvement of scar depression was achieved.20
Reconstruction in Areas of High Mechanical Tension
Plantar Foot
A combined dermal and full-thickness sandwich graft has been described for reconstruction of plantar foot defects.3 The graft is created by obtaining a FTSG twice the size of the wound defect and deepithelializing half of the graft. The graft is then defatted and the deepithelialized portion is folded beneath the other half, allowing the papillary dermis to make contact with the wound surface.
Scalp
Dermal graft reconstruction for scalp defects may be accomplished with a split-thickness skin flap. The flap is harvested using an electronic dermatome that ensures the proximal aspect is still attached to adjacent skin. The dermis is removed from the area underneath the back-folded split-thickness skin flap. The dermal graft is meshed and sutured into the recipient site. The split-thickness skin flap is replaced over the donor site. Meshed reversed dermal grafts have excellent survival rates, even with direct placement on bone without periosteum. Querings et al21 reported graft survival with no complications in 19 of 21 (90.4%) patients undergoing scalp or plantar sole reconstruction.
CONCLUSION
With the widespread adoption of the fresh-tissue technique for Mohs micrographic surgery and the establishment of the American Society for Dermatologic Surgery in 1970, the depth and scope of techniques used by dermatologic surgeons has dramatically expanded. Although the use of dermal flaps and grafts is not as widespread in dermatology as other reconstructive techniques, their unique advantages should be considered. Deepithelialized flaps and grafts should be considered when the following reconstructive goals are desired: (1) conversion of a 2-stage interpolation flap to a single-stage tunneled flap, (2) contour and cosmetic subunit preservation of deep defects through volume augmentation, (3) reconstruction in areas of high mechanical tension, and (4) free margin preservation. The multiple applications of deepithelialized flaps and grafts as described in this review demonstrate their continued applicability in dermatologic surgery.
- Straatsma CR. Use of the dermal graft in the repairs of small saddle defects of the nose. Arch Otolaryngol. 1932;16:506-509.
- Cydeli A, Hunter J. Peeling orange: rapid deepithelialization in reduction mammoplasty. J Aesthet Surg. 2004;24:580-581.
- Bechara F, Sand M, Radenhausen M, et al. Erbium:YAG laser-assisted preparation of a combined dermal/full thickness sandwich skin graft. Dermatol Surg. 2006;32:353-358.
- Cook JL. Tunneled and transposed island flaps in facial reconstructive surgery. Dermatol Surg. 2014;40(suppl 9):S16-S29.
- Krishnan RS, Clark DP. Tunneled transposition flap for reconstruction of defects of the nasal ala. Dermatol Surg. 2007;33:1496-1501.
- Mahlberg M. Tunneled melolabial pedicle flap for small but deep lateral alar rim defect. Dermatol Surg. 2013;39:1527-1529.
- Ascari-Raccagni A, Balderi U. The retroangular flap used in the surgery of nasal tip defects. Dermatol Surg. 2004;30:1131-1137.
- Hollmig ST, Leach BC, Cook J. Single-staged interpolation flaps in facial reconstruction. Dermatol Surg. 2014;40(suppl 9):S62-S70.
- Mombaerts I, Gillis A. The tunneled forehead flap in medial canthal and eyelid reconstruction. Dermatol Surg. 2010:36:1118-1125.
- Wang SQ, Goldberg LH, Kimyah-Asadi A. Tunneled island pedicle flap for an earlobe defect. Dermatol Surg. 2007;33:835-838.
- Hatoko M, Kuwahara M, Shiba A, et al. Earlobe reconstruction using a subcutaneous island pedicle flap after resection of “earlobe keloid.” Dermatol Surg. 1998;24:257-261.
- Alder N, Ad-El D, Azaria R. Reconstruction of nonhelical auricular defects with local flaps. Dermatol Surg. 2008;34:501-507.
- Fader DJ, Wang TS, Johnson TM. Nasal reconstruction utilizing a muscle hinge flap with overlying FTSG. J Am Acad Dermatol. 2000;43:837-840.
- Braun MA, Cook J. Hinge flaps in facial reconstruction. Dermatol Surg. 2007;33:213-221.
- Salmon PL, Mortimer NL, Hill SE. Muscular hinge flaps: utility and technique in facial reconstructive surgery. Dermatol Surg. 2010;36:227-234.
- Seo Y, Song S, Choi Y, et al. A lower lip reconstruction. Dermatol Surg. 2015;41:505-507.
- Malone CH, Wagner RF. Partially de-epithelialized postauricular flap for ear reconstruction. J Am Acad Dermatol. 2015;73:E219-E220.
- Yildrim S, Akoz T, Akan M, et al. Nasolabial V-Y advancement for closure of the midface defects. Dermatol Surg. 2001;27:656-662.
- Jensen DJ, Cohen JL. Nasal tip revision using a dermal graft. Dermatol Surg. 2014;40:1140-1142.
- Meyers S, Rohrer T. Use of dermal grafts in reconstructing deep nasal defects and shaping the ala nasi. Dermatol Surg. 2001;27:300-305.
- Querings K, Bachter D, Balda B. Meshed reversed dermal graft in patients with surgical defects of sole and scalp: technique and long-term results. Dermatol Surg. 2002;28:122-126.
- Straatsma CR. Use of the dermal graft in the repairs of small saddle defects of the nose. Arch Otolaryngol. 1932;16:506-509.
- Cydeli A, Hunter J. Peeling orange: rapid deepithelialization in reduction mammoplasty. J Aesthet Surg. 2004;24:580-581.
- Bechara F, Sand M, Radenhausen M, et al. Erbium:YAG laser-assisted preparation of a combined dermal/full thickness sandwich skin graft. Dermatol Surg. 2006;32:353-358.
- Cook JL. Tunneled and transposed island flaps in facial reconstructive surgery. Dermatol Surg. 2014;40(suppl 9):S16-S29.
- Krishnan RS, Clark DP. Tunneled transposition flap for reconstruction of defects of the nasal ala. Dermatol Surg. 2007;33:1496-1501.
- Mahlberg M. Tunneled melolabial pedicle flap for small but deep lateral alar rim defect. Dermatol Surg. 2013;39:1527-1529.
- Ascari-Raccagni A, Balderi U. The retroangular flap used in the surgery of nasal tip defects. Dermatol Surg. 2004;30:1131-1137.
- Hollmig ST, Leach BC, Cook J. Single-staged interpolation flaps in facial reconstruction. Dermatol Surg. 2014;40(suppl 9):S62-S70.
- Mombaerts I, Gillis A. The tunneled forehead flap in medial canthal and eyelid reconstruction. Dermatol Surg. 2010:36:1118-1125.
- Wang SQ, Goldberg LH, Kimyah-Asadi A. Tunneled island pedicle flap for an earlobe defect. Dermatol Surg. 2007;33:835-838.
- Hatoko M, Kuwahara M, Shiba A, et al. Earlobe reconstruction using a subcutaneous island pedicle flap after resection of “earlobe keloid.” Dermatol Surg. 1998;24:257-261.
- Alder N, Ad-El D, Azaria R. Reconstruction of nonhelical auricular defects with local flaps. Dermatol Surg. 2008;34:501-507.
- Fader DJ, Wang TS, Johnson TM. Nasal reconstruction utilizing a muscle hinge flap with overlying FTSG. J Am Acad Dermatol. 2000;43:837-840.
- Braun MA, Cook J. Hinge flaps in facial reconstruction. Dermatol Surg. 2007;33:213-221.
- Salmon PL, Mortimer NL, Hill SE. Muscular hinge flaps: utility and technique in facial reconstructive surgery. Dermatol Surg. 2010;36:227-234.
- Seo Y, Song S, Choi Y, et al. A lower lip reconstruction. Dermatol Surg. 2015;41:505-507.
- Malone CH, Wagner RF. Partially de-epithelialized postauricular flap for ear reconstruction. J Am Acad Dermatol. 2015;73:E219-E220.
- Yildrim S, Akoz T, Akan M, et al. Nasolabial V-Y advancement for closure of the midface defects. Dermatol Surg. 2001;27:656-662.
- Jensen DJ, Cohen JL. Nasal tip revision using a dermal graft. Dermatol Surg. 2014;40:1140-1142.
- Meyers S, Rohrer T. Use of dermal grafts in reconstructing deep nasal defects and shaping the ala nasi. Dermatol Surg. 2001;27:300-305.
- Querings K, Bachter D, Balda B. Meshed reversed dermal graft in patients with surgical defects of sole and scalp: technique and long-term results. Dermatol Surg. 2002;28:122-126.
Practice Points
- Deepithelialized flaps should be considered for single-stage reconstruction with tunneled interpolation flaps, reconstruction requiring contour preservation, and reconstruction involving free margins.
- Deepithelialized grafts may be considered for volume replacement, reconstruction requiring contour preservation, and reconstruction in areas of high mechanical tension.
MS Medication Withdrawn Because of Safety Concerns
Citing concerns about safety, Biogen and AbbVie announced March 2 that they will be withdrawing daclizumab (Zinbryta) from worldwide markets. Daclizumab has known risks, so it was usually prescribed only for people with relapsing multiple sclerosis who had tried two or more other medications that hadn’t worked well enough.
Reports of inflammatory encephalitis and meningoencephalitis led the European Medicines Agency to initiate an Article 20 referral procedure. In such referrals, a medicine or class of medicines are scientifically assessed because of concerns over safety or quality.
However, Biogen and AbbVie concluded that, because of the complex nature of these reports and how few patients were taking daclizumab, it would be difficult to characterize the nature of the medication’s harms and benefits, so the companies instead have decided to withdraw the medication from the market.
Patients taking daclizumab should contact their health care providers. More information can be found in the press release.
—Christopher Palmer
Citing concerns about safety, Biogen and AbbVie announced March 2 that they will be withdrawing daclizumab (Zinbryta) from worldwide markets. Daclizumab has known risks, so it was usually prescribed only for people with relapsing multiple sclerosis who had tried two or more other medications that hadn’t worked well enough.
Reports of inflammatory encephalitis and meningoencephalitis led the European Medicines Agency to initiate an Article 20 referral procedure. In such referrals, a medicine or class of medicines are scientifically assessed because of concerns over safety or quality.
However, Biogen and AbbVie concluded that, because of the complex nature of these reports and how few patients were taking daclizumab, it would be difficult to characterize the nature of the medication’s harms and benefits, so the companies instead have decided to withdraw the medication from the market.
Patients taking daclizumab should contact their health care providers. More information can be found in the press release.
—Christopher Palmer
Citing concerns about safety, Biogen and AbbVie announced March 2 that they will be withdrawing daclizumab (Zinbryta) from worldwide markets. Daclizumab has known risks, so it was usually prescribed only for people with relapsing multiple sclerosis who had tried two or more other medications that hadn’t worked well enough.
Reports of inflammatory encephalitis and meningoencephalitis led the European Medicines Agency to initiate an Article 20 referral procedure. In such referrals, a medicine or class of medicines are scientifically assessed because of concerns over safety or quality.
However, Biogen and AbbVie concluded that, because of the complex nature of these reports and how few patients were taking daclizumab, it would be difficult to characterize the nature of the medication’s harms and benefits, so the companies instead have decided to withdraw the medication from the market.
Patients taking daclizumab should contact their health care providers. More information can be found in the press release.
—Christopher Palmer