User login
FDA approves new treatment for multidrug-resistant HIV
The Food and Drug Administration Feb. 6 approved ibalizumab-uiyk (Trogarzo) to treat multidrug-resistant HIV (MDR HIV) in adults, the agency announced.
“While most patients living with HIV can be successfully treated using a combination of two or more antiretroviral drugs, a small percentage of patients who have taken many HIV drugs in the past have multidrug-resistant HIV, limiting their treatment options and putting them at a high risk of HIV-related complications and progression to death,” Jeff Murray, MD, deputy director of the FDA Division of Antiviral Products, said in a statement. “Trogarzo is the first drug in a new class of antiretroviral medications that can provide significant benefit to patients who have run out of HIV treatment options. New treatment options may be able to improve their outcomes.”
Ibalizumab-uiyk, an HIV-1 inhibitor, was approved based on the results of a phase 3, single-arm study of 40 patients with MDR-HIV-1 who had high virus levels in their blood despite antiretroviral treatment. To be included in the study, all patients had to have received highly active antiretroviral therapy for at least 6 months prior. Over 24 weeks, patients were monitored to compare previous, infective treatments with ibalizumab-uiyk in conjunction with an optimized background regimen of antiretroviral drugs.
The majority of patients showed a significant decrease in their HIV-RNA levels 1 week after ibalizumab-uiyk was added to their previous drug regimens. After 24 weeks of treatment with ibalizumab-uiyk, in conjunction with other antiretroviral drugs, 43% of patients achieved HIV RNA suppression.
Ibalizumab-uiyk was granted Fast Track, Priority Review, and Breakthrough Therapy designations from the FDA. In addition, it was granted Orphan Drug designation, a program that encourages the development of drugs to treat rare diseases.
Ibalizumab-uiyk is administered once every 14 days in conjunction with other retroviral medications.
The most common adverse reactions to ibalizumab-uiyk were diarrhea, dizziness, nausea, and rash. Less common, and more severe, reactions were changes in the immune system.
Ibalizumab-uiyk will be marketed by Taimed Biologics USA.
The Food and Drug Administration Feb. 6 approved ibalizumab-uiyk (Trogarzo) to treat multidrug-resistant HIV (MDR HIV) in adults, the agency announced.
“While most patients living with HIV can be successfully treated using a combination of two or more antiretroviral drugs, a small percentage of patients who have taken many HIV drugs in the past have multidrug-resistant HIV, limiting their treatment options and putting them at a high risk of HIV-related complications and progression to death,” Jeff Murray, MD, deputy director of the FDA Division of Antiviral Products, said in a statement. “Trogarzo is the first drug in a new class of antiretroviral medications that can provide significant benefit to patients who have run out of HIV treatment options. New treatment options may be able to improve their outcomes.”
Ibalizumab-uiyk, an HIV-1 inhibitor, was approved based on the results of a phase 3, single-arm study of 40 patients with MDR-HIV-1 who had high virus levels in their blood despite antiretroviral treatment. To be included in the study, all patients had to have received highly active antiretroviral therapy for at least 6 months prior. Over 24 weeks, patients were monitored to compare previous, infective treatments with ibalizumab-uiyk in conjunction with an optimized background regimen of antiretroviral drugs.
The majority of patients showed a significant decrease in their HIV-RNA levels 1 week after ibalizumab-uiyk was added to their previous drug regimens. After 24 weeks of treatment with ibalizumab-uiyk, in conjunction with other antiretroviral drugs, 43% of patients achieved HIV RNA suppression.
Ibalizumab-uiyk was granted Fast Track, Priority Review, and Breakthrough Therapy designations from the FDA. In addition, it was granted Orphan Drug designation, a program that encourages the development of drugs to treat rare diseases.
Ibalizumab-uiyk is administered once every 14 days in conjunction with other retroviral medications.
The most common adverse reactions to ibalizumab-uiyk were diarrhea, dizziness, nausea, and rash. Less common, and more severe, reactions were changes in the immune system.
Ibalizumab-uiyk will be marketed by Taimed Biologics USA.
The Food and Drug Administration Feb. 6 approved ibalizumab-uiyk (Trogarzo) to treat multidrug-resistant HIV (MDR HIV) in adults, the agency announced.
“While most patients living with HIV can be successfully treated using a combination of two or more antiretroviral drugs, a small percentage of patients who have taken many HIV drugs in the past have multidrug-resistant HIV, limiting their treatment options and putting them at a high risk of HIV-related complications and progression to death,” Jeff Murray, MD, deputy director of the FDA Division of Antiviral Products, said in a statement. “Trogarzo is the first drug in a new class of antiretroviral medications that can provide significant benefit to patients who have run out of HIV treatment options. New treatment options may be able to improve their outcomes.”
Ibalizumab-uiyk, an HIV-1 inhibitor, was approved based on the results of a phase 3, single-arm study of 40 patients with MDR-HIV-1 who had high virus levels in their blood despite antiretroviral treatment. To be included in the study, all patients had to have received highly active antiretroviral therapy for at least 6 months prior. Over 24 weeks, patients were monitored to compare previous, infective treatments with ibalizumab-uiyk in conjunction with an optimized background regimen of antiretroviral drugs.
The majority of patients showed a significant decrease in their HIV-RNA levels 1 week after ibalizumab-uiyk was added to their previous drug regimens. After 24 weeks of treatment with ibalizumab-uiyk, in conjunction with other antiretroviral drugs, 43% of patients achieved HIV RNA suppression.
Ibalizumab-uiyk was granted Fast Track, Priority Review, and Breakthrough Therapy designations from the FDA. In addition, it was granted Orphan Drug designation, a program that encourages the development of drugs to treat rare diseases.
Ibalizumab-uiyk is administered once every 14 days in conjunction with other retroviral medications.
The most common adverse reactions to ibalizumab-uiyk were diarrhea, dizziness, nausea, and rash. Less common, and more severe, reactions were changes in the immune system.
Ibalizumab-uiyk will be marketed by Taimed Biologics USA.
Flu activity takes another turn for the better
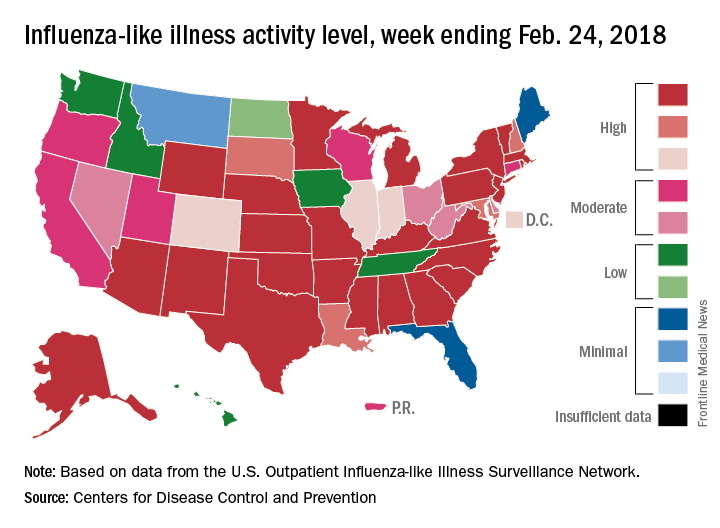
Outpatient influenza-like illness activity continues to drop, but pediatric deaths for 2017-2018 are already higher than either of the last two entire seasons, according to the Centers for Disease and Prevention.
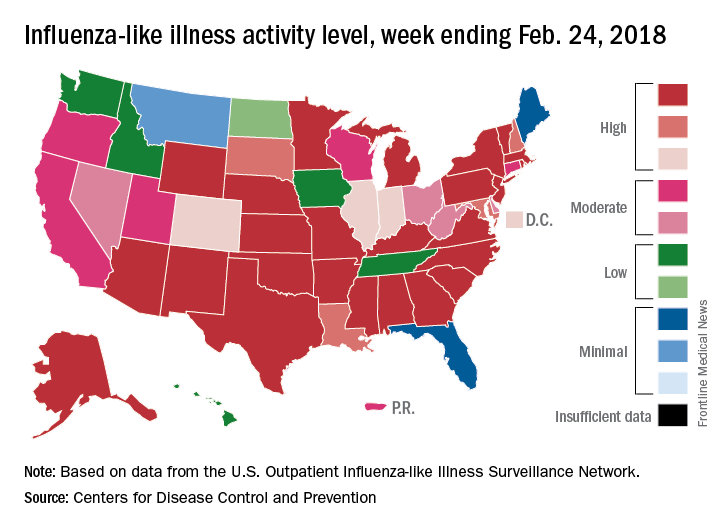
An additional 17 influenza-like illness-related (ILI) pediatric deaths were reported during the week ending Feb. 24, eight of which occurred in previous weeks. That brings the total to 114 for the 2017-2018 flu season so far, compared with 110 for the entire 2016-2017 season and 93 for the 2015-2016 season, the CDC reported Mar. 2.
The proportion of outpatient visits for ILI took another big drop, falling to 5.0% for the week, which was down from 6.4% the previous week and the seasonal high of 7.4% the 2 weeks before that (Feb. 10 and Feb. 3), CDC data show.
Flu-related hospitalizations, however, continued to rise to new highs, as the cumulative rate hit 81.7 per 100,000 population. In 2014-2015, the season with the highest number of hospitalizations since the CDC started keeping track, the cumulative rate for the corresponding week was 55.9 per 100,000, according to the CDC’s Fluview website.
The map of state-reported ILI activity shows that 25 states are at level 10 on the CDC’s 1-10 scale, which is down from 33 the week before. Eight other states and the District of Columbia were in the “high” range with activity at levels 8 and 9 for the week ending Feb. 24, the CDC said.
Outpatient influenza-like illness activity continues to drop, but pediatric deaths for 2017-2018 are already higher than either of the last two entire seasons, according to the Centers for Disease and Prevention.

An additional 17 influenza-like illness-related (ILI) pediatric deaths were reported during the week ending Feb. 24, eight of which occurred in previous weeks. That brings the total to 114 for the 2017-2018 flu season so far, compared with 110 for the entire 2016-2017 season and 93 for the 2015-2016 season, the CDC reported Mar. 2.
The proportion of outpatient visits for ILI took another big drop, falling to 5.0% for the week, which was down from 6.4% the previous week and the seasonal high of 7.4% the 2 weeks before that (Feb. 10 and Feb. 3), CDC data show.
Flu-related hospitalizations, however, continued to rise to new highs, as the cumulative rate hit 81.7 per 100,000 population. In 2014-2015, the season with the highest number of hospitalizations since the CDC started keeping track, the cumulative rate for the corresponding week was 55.9 per 100,000, according to the CDC’s Fluview website.
The map of state-reported ILI activity shows that 25 states are at level 10 on the CDC’s 1-10 scale, which is down from 33 the week before. Eight other states and the District of Columbia were in the “high” range with activity at levels 8 and 9 for the week ending Feb. 24, the CDC said.
Outpatient influenza-like illness activity continues to drop, but pediatric deaths for 2017-2018 are already higher than either of the last two entire seasons, according to the Centers for Disease and Prevention.

An additional 17 influenza-like illness-related (ILI) pediatric deaths were reported during the week ending Feb. 24, eight of which occurred in previous weeks. That brings the total to 114 for the 2017-2018 flu season so far, compared with 110 for the entire 2016-2017 season and 93 for the 2015-2016 season, the CDC reported Mar. 2.
The proportion of outpatient visits for ILI took another big drop, falling to 5.0% for the week, which was down from 6.4% the previous week and the seasonal high of 7.4% the 2 weeks before that (Feb. 10 and Feb. 3), CDC data show.
Flu-related hospitalizations, however, continued to rise to new highs, as the cumulative rate hit 81.7 per 100,000 population. In 2014-2015, the season with the highest number of hospitalizations since the CDC started keeping track, the cumulative rate for the corresponding week was 55.9 per 100,000, according to the CDC’s Fluview website.
The map of state-reported ILI activity shows that 25 states are at level 10 on the CDC’s 1-10 scale, which is down from 33 the week before. Eight other states and the District of Columbia were in the “high” range with activity at levels 8 and 9 for the week ending Feb. 24, the CDC said.
Opioid deaths in the ED increase nationally
Opioid-related deaths in emergency departments increased by approximately 30% across all regions of the United States between 2016 and 2017, according to the Centers for Disease Control and Prevention.
Analysis of 91 million ED visits from the CDC’s National Syndromic Surveillance Program and Enhanced State Opioid Overdose Surveillance database found significant increases in opioid overdose deaths in 16 states, reaching as high as 109% in Wisconsin and 106% in Delaware, CDC officials said during a press briefing.
“We are currently seeing the highest drug overdose death rate ever recorded in the United States, driven by prescription opioids and by illicit opioids such as heroin and illicitly manufactured fentanyl,” said Anne Schuchat, MD, acting CDC director. “In 2016, there were more than 63,000 drug overdose deaths, and more than 42,000 of those deaths involved an opioid.”
Of the 91 million visits, a total of 261,755 were suspected of opioid overdoses across both databases.
The greatest increase was seen in the Midwest region (69.7%), followed by the West (40.3%), Northeast (21.3%), Southwest (20.2%), and Southeast (14%).
Death rates rose across all demographics, regardless of sex or age.
While Delaware recorded some of the highest increases in deaths, Massachusetts, New Hampshire, and Rhode Island decreased, although not within statistical significance.
“These decreases may possibly be related to implementation of interventions, including expansion of access to medication-assisted treatment,” said Dr. Schuchat. “The decrease in Kentucky during this period of time may reflect some fluctuations in drug supply.”
In a comparison of urban and rural areas, large and medium metropolitan communities had the sharpest increase, at 45%.
To combat the rise in deaths, the CDC is encouraging an increase in naloxone distribution and training for first responders and community members.
The agency also recommends that local health departments begin using ED data to alert local communities when opioid-related deaths rise.
“This is a very difficult and fast-moving epidemic, and there are no easy solutions,” Dr. Schuchat said. [These data send] “a wake-up call about the need to improve what happens when patients leave the emergency department; all of us working together, government, public health, the medical community, law enforcement, and community members themselves can help fight this epidemic and save lives.”
SOURCE: Vivolo-Kantor AM et al. MMWR Morb Mortal Wkly Rep. 6 Mar 2018. doi: 10.15585/mmwr.mm6709e1.
Opioid-related deaths in emergency departments increased by approximately 30% across all regions of the United States between 2016 and 2017, according to the Centers for Disease Control and Prevention.
Analysis of 91 million ED visits from the CDC’s National Syndromic Surveillance Program and Enhanced State Opioid Overdose Surveillance database found significant increases in opioid overdose deaths in 16 states, reaching as high as 109% in Wisconsin and 106% in Delaware, CDC officials said during a press briefing.
“We are currently seeing the highest drug overdose death rate ever recorded in the United States, driven by prescription opioids and by illicit opioids such as heroin and illicitly manufactured fentanyl,” said Anne Schuchat, MD, acting CDC director. “In 2016, there were more than 63,000 drug overdose deaths, and more than 42,000 of those deaths involved an opioid.”
Of the 91 million visits, a total of 261,755 were suspected of opioid overdoses across both databases.
The greatest increase was seen in the Midwest region (69.7%), followed by the West (40.3%), Northeast (21.3%), Southwest (20.2%), and Southeast (14%).
Death rates rose across all demographics, regardless of sex or age.
While Delaware recorded some of the highest increases in deaths, Massachusetts, New Hampshire, and Rhode Island decreased, although not within statistical significance.
“These decreases may possibly be related to implementation of interventions, including expansion of access to medication-assisted treatment,” said Dr. Schuchat. “The decrease in Kentucky during this period of time may reflect some fluctuations in drug supply.”
In a comparison of urban and rural areas, large and medium metropolitan communities had the sharpest increase, at 45%.
To combat the rise in deaths, the CDC is encouraging an increase in naloxone distribution and training for first responders and community members.
The agency also recommends that local health departments begin using ED data to alert local communities when opioid-related deaths rise.
“This is a very difficult and fast-moving epidemic, and there are no easy solutions,” Dr. Schuchat said. [These data send] “a wake-up call about the need to improve what happens when patients leave the emergency department; all of us working together, government, public health, the medical community, law enforcement, and community members themselves can help fight this epidemic and save lives.”
SOURCE: Vivolo-Kantor AM et al. MMWR Morb Mortal Wkly Rep. 6 Mar 2018. doi: 10.15585/mmwr.mm6709e1.
Opioid-related deaths in emergency departments increased by approximately 30% across all regions of the United States between 2016 and 2017, according to the Centers for Disease Control and Prevention.
Analysis of 91 million ED visits from the CDC’s National Syndromic Surveillance Program and Enhanced State Opioid Overdose Surveillance database found significant increases in opioid overdose deaths in 16 states, reaching as high as 109% in Wisconsin and 106% in Delaware, CDC officials said during a press briefing.
“We are currently seeing the highest drug overdose death rate ever recorded in the United States, driven by prescription opioids and by illicit opioids such as heroin and illicitly manufactured fentanyl,” said Anne Schuchat, MD, acting CDC director. “In 2016, there were more than 63,000 drug overdose deaths, and more than 42,000 of those deaths involved an opioid.”
Of the 91 million visits, a total of 261,755 were suspected of opioid overdoses across both databases.
The greatest increase was seen in the Midwest region (69.7%), followed by the West (40.3%), Northeast (21.3%), Southwest (20.2%), and Southeast (14%).
Death rates rose across all demographics, regardless of sex or age.
While Delaware recorded some of the highest increases in deaths, Massachusetts, New Hampshire, and Rhode Island decreased, although not within statistical significance.
“These decreases may possibly be related to implementation of interventions, including expansion of access to medication-assisted treatment,” said Dr. Schuchat. “The decrease in Kentucky during this period of time may reflect some fluctuations in drug supply.”
In a comparison of urban and rural areas, large and medium metropolitan communities had the sharpest increase, at 45%.
To combat the rise in deaths, the CDC is encouraging an increase in naloxone distribution and training for first responders and community members.
The agency also recommends that local health departments begin using ED data to alert local communities when opioid-related deaths rise.
“This is a very difficult and fast-moving epidemic, and there are no easy solutions,” Dr. Schuchat said. [These data send] “a wake-up call about the need to improve what happens when patients leave the emergency department; all of us working together, government, public health, the medical community, law enforcement, and community members themselves can help fight this epidemic and save lives.”
SOURCE: Vivolo-Kantor AM et al. MMWR Morb Mortal Wkly Rep. 6 Mar 2018. doi: 10.15585/mmwr.mm6709e1.
FROM MMWR
Early childhood vaccines not associated with increased infection risk
There was no significant difference in vaccine antigen exposure through the first 23 months of life between children with non–vaccine-targeted infections and controls between 24 and 47 months of age, according to results published March 6 in JAMA.
This was determined in a nested, matched case-control study of 193 infection cases and 751 controls, in whom estimated mean cumulative vaccine antigen exposure was 240.6 for cases of non–vaccine-targeted infections, and 242.9 for controls, reported Jason M. Glanz, PhD, of Kaiser Permanente Colorado, Denver, and his coauthors. The between-group difference was –2.3 (P = .55), a nonsignificant difference.
Using data from the Centers for Disease Control and Prevention-funded Vaccine Safety Datalink (VSD), the investigators identified children born between Jan. 1, 2003, and Sep. 31, 2013. Exclusion criteria were not having at least two well-child visits before the first birthday, medical contraindications to vaccination, or receiving vaccines not recommended by the Advisory Committee on Immunization Practices. Eligible children were followed through age 47 months or until disenrollment from their health care organization, the authors said.
ICD-9 and ICD-10 codes were used to identify non–vaccine-targeted infections, including upper and lower respiratory infections, gastrointestinal infections, and other viral and bacterial infections from ages 24to 47 months. A medical record review was performed to confirm case status. Cases were included only if it was confirmed that the infection occurred, that it was an incident outcome, that the outcome was the primary reason for the medical visit, that the outcome occurred in the inpatient or emergency department setting, and that there was no evidence that the child was diagnosed as having a vaccine preventable disease (VPD) on the same day as the infection. Controls did not have a VPD or record of a non–vaccine-targeted infection prior to the index date, Dr. Glanz and his colleagues said.
Antigen exposure was measured as the number of immunogenic proteins and polysaccharides in each vaccine, and was estimated from birth through age 23 months in both groups. Cumulative antigen exposure was estimated by adding the number of antigens in each non–vaccine-targeted infection and controls.
Estimated mean cumulative vaccine antigen exposure was 240.6 for cases of non–vaccine-targeted infections, and 242.9 for controls, the authors reported. The matched odds ratio (mOR) for estimated cumulative antigen exposure through age 23 months was not significant in children with infections, compared with controls (mOR = 0.94; 95% confidence interval, 0.84-1.07). The estimated maximum single-day antigen exposure was not significantly associated with non–vaccine-targeted infection (mOR = 1.07; 95% CI, 0.81-1.41).
The findings of this study “did not reveal any beneficial or detrimental associations with estimated cumulative vaccine antigen exposure in young children with non–vaccine-targeted infections in ED and inpatient settings,” wrote Dr. Glanz and coauthors. In addition, the study “did not find evidence that multiple vaccine exposure was associated with the risk for non-targeted infectious diseases.”
The CDC funded the study. The authors reported receiving contracts, grants, and other funding from the CDC.
SOURCE: Glanz JM et al. JAMA. 2018;319(9):906-13.
These results provide “further reassurance about the safety of the U.S. child vaccination schedule,” said Sean T. O’Leary, MD, and Yvonne A. Maldonado, MD.
However, they added, more work must be done to strengthen the public’s trust and confidence in vaccines. Parents long have voiced concerns that vaccines might weaken their children’s immune systems.
“The small but vocal minority of anti-vaccine groups may not be satisfied by the evidence provided through VSD and other vaccine safety surveillance,” they wrote. “Simply providing scientific information and assuming parents will make the decision to vaccinate is not enough.
“Delivering evidence-based information to parents and clinicians in ways that inspire confidence in the robust and safe childhood immunization schedule is critical for maintaining the health of children,” they concluded.
Dr. O’Leary and Dr. Maldonado, both of the University of Colorado, Aurora, commented in an editorial accompanying the article by Glanz et al. (JAMA. 2018 Mar 6;319(9):870-1). Dr. Maldonado reported receiving personal fees for serving on a data and safety monitoring board for Pfizer. Dr. O’Leary reported no relevant financial disclosures.
These results provide “further reassurance about the safety of the U.S. child vaccination schedule,” said Sean T. O’Leary, MD, and Yvonne A. Maldonado, MD.
However, they added, more work must be done to strengthen the public’s trust and confidence in vaccines. Parents long have voiced concerns that vaccines might weaken their children’s immune systems.
“The small but vocal minority of anti-vaccine groups may not be satisfied by the evidence provided through VSD and other vaccine safety surveillance,” they wrote. “Simply providing scientific information and assuming parents will make the decision to vaccinate is not enough.
“Delivering evidence-based information to parents and clinicians in ways that inspire confidence in the robust and safe childhood immunization schedule is critical for maintaining the health of children,” they concluded.
Dr. O’Leary and Dr. Maldonado, both of the University of Colorado, Aurora, commented in an editorial accompanying the article by Glanz et al. (JAMA. 2018 Mar 6;319(9):870-1). Dr. Maldonado reported receiving personal fees for serving on a data and safety monitoring board for Pfizer. Dr. O’Leary reported no relevant financial disclosures.
These results provide “further reassurance about the safety of the U.S. child vaccination schedule,” said Sean T. O’Leary, MD, and Yvonne A. Maldonado, MD.
However, they added, more work must be done to strengthen the public’s trust and confidence in vaccines. Parents long have voiced concerns that vaccines might weaken their children’s immune systems.
“The small but vocal minority of anti-vaccine groups may not be satisfied by the evidence provided through VSD and other vaccine safety surveillance,” they wrote. “Simply providing scientific information and assuming parents will make the decision to vaccinate is not enough.
“Delivering evidence-based information to parents and clinicians in ways that inspire confidence in the robust and safe childhood immunization schedule is critical for maintaining the health of children,” they concluded.
Dr. O’Leary and Dr. Maldonado, both of the University of Colorado, Aurora, commented in an editorial accompanying the article by Glanz et al. (JAMA. 2018 Mar 6;319(9):870-1). Dr. Maldonado reported receiving personal fees for serving on a data and safety monitoring board for Pfizer. Dr. O’Leary reported no relevant financial disclosures.
There was no significant difference in vaccine antigen exposure through the first 23 months of life between children with non–vaccine-targeted infections and controls between 24 and 47 months of age, according to results published March 6 in JAMA.
This was determined in a nested, matched case-control study of 193 infection cases and 751 controls, in whom estimated mean cumulative vaccine antigen exposure was 240.6 for cases of non–vaccine-targeted infections, and 242.9 for controls, reported Jason M. Glanz, PhD, of Kaiser Permanente Colorado, Denver, and his coauthors. The between-group difference was –2.3 (P = .55), a nonsignificant difference.
Using data from the Centers for Disease Control and Prevention-funded Vaccine Safety Datalink (VSD), the investigators identified children born between Jan. 1, 2003, and Sep. 31, 2013. Exclusion criteria were not having at least two well-child visits before the first birthday, medical contraindications to vaccination, or receiving vaccines not recommended by the Advisory Committee on Immunization Practices. Eligible children were followed through age 47 months or until disenrollment from their health care organization, the authors said.
ICD-9 and ICD-10 codes were used to identify non–vaccine-targeted infections, including upper and lower respiratory infections, gastrointestinal infections, and other viral and bacterial infections from ages 24to 47 months. A medical record review was performed to confirm case status. Cases were included only if it was confirmed that the infection occurred, that it was an incident outcome, that the outcome was the primary reason for the medical visit, that the outcome occurred in the inpatient or emergency department setting, and that there was no evidence that the child was diagnosed as having a vaccine preventable disease (VPD) on the same day as the infection. Controls did not have a VPD or record of a non–vaccine-targeted infection prior to the index date, Dr. Glanz and his colleagues said.
Antigen exposure was measured as the number of immunogenic proteins and polysaccharides in each vaccine, and was estimated from birth through age 23 months in both groups. Cumulative antigen exposure was estimated by adding the number of antigens in each non–vaccine-targeted infection and controls.
Estimated mean cumulative vaccine antigen exposure was 240.6 for cases of non–vaccine-targeted infections, and 242.9 for controls, the authors reported. The matched odds ratio (mOR) for estimated cumulative antigen exposure through age 23 months was not significant in children with infections, compared with controls (mOR = 0.94; 95% confidence interval, 0.84-1.07). The estimated maximum single-day antigen exposure was not significantly associated with non–vaccine-targeted infection (mOR = 1.07; 95% CI, 0.81-1.41).
The findings of this study “did not reveal any beneficial or detrimental associations with estimated cumulative vaccine antigen exposure in young children with non–vaccine-targeted infections in ED and inpatient settings,” wrote Dr. Glanz and coauthors. In addition, the study “did not find evidence that multiple vaccine exposure was associated with the risk for non-targeted infectious diseases.”
The CDC funded the study. The authors reported receiving contracts, grants, and other funding from the CDC.
SOURCE: Glanz JM et al. JAMA. 2018;319(9):906-13.
There was no significant difference in vaccine antigen exposure through the first 23 months of life between children with non–vaccine-targeted infections and controls between 24 and 47 months of age, according to results published March 6 in JAMA.
This was determined in a nested, matched case-control study of 193 infection cases and 751 controls, in whom estimated mean cumulative vaccine antigen exposure was 240.6 for cases of non–vaccine-targeted infections, and 242.9 for controls, reported Jason M. Glanz, PhD, of Kaiser Permanente Colorado, Denver, and his coauthors. The between-group difference was –2.3 (P = .55), a nonsignificant difference.
Using data from the Centers for Disease Control and Prevention-funded Vaccine Safety Datalink (VSD), the investigators identified children born between Jan. 1, 2003, and Sep. 31, 2013. Exclusion criteria were not having at least two well-child visits before the first birthday, medical contraindications to vaccination, or receiving vaccines not recommended by the Advisory Committee on Immunization Practices. Eligible children were followed through age 47 months or until disenrollment from their health care organization, the authors said.
ICD-9 and ICD-10 codes were used to identify non–vaccine-targeted infections, including upper and lower respiratory infections, gastrointestinal infections, and other viral and bacterial infections from ages 24to 47 months. A medical record review was performed to confirm case status. Cases were included only if it was confirmed that the infection occurred, that it was an incident outcome, that the outcome was the primary reason for the medical visit, that the outcome occurred in the inpatient or emergency department setting, and that there was no evidence that the child was diagnosed as having a vaccine preventable disease (VPD) on the same day as the infection. Controls did not have a VPD or record of a non–vaccine-targeted infection prior to the index date, Dr. Glanz and his colleagues said.
Antigen exposure was measured as the number of immunogenic proteins and polysaccharides in each vaccine, and was estimated from birth through age 23 months in both groups. Cumulative antigen exposure was estimated by adding the number of antigens in each non–vaccine-targeted infection and controls.
Estimated mean cumulative vaccine antigen exposure was 240.6 for cases of non–vaccine-targeted infections, and 242.9 for controls, the authors reported. The matched odds ratio (mOR) for estimated cumulative antigen exposure through age 23 months was not significant in children with infections, compared with controls (mOR = 0.94; 95% confidence interval, 0.84-1.07). The estimated maximum single-day antigen exposure was not significantly associated with non–vaccine-targeted infection (mOR = 1.07; 95% CI, 0.81-1.41).
The findings of this study “did not reveal any beneficial or detrimental associations with estimated cumulative vaccine antigen exposure in young children with non–vaccine-targeted infections in ED and inpatient settings,” wrote Dr. Glanz and coauthors. In addition, the study “did not find evidence that multiple vaccine exposure was associated with the risk for non-targeted infectious diseases.”
The CDC funded the study. The authors reported receiving contracts, grants, and other funding from the CDC.
SOURCE: Glanz JM et al. JAMA. 2018;319(9):906-13.
FROM JAMA
Key clinical point: No significant difference was found in vaccine antigen exposure between controls and children with infectious diseases not targeted by vaccines.
Major finding: Estimated mean cumulative vaccine antigen exposure was 240.6 for cases and 242.9 for controls.
Study details: A matched case-control study of 944 patients enrolled in six integrated health care organizations as part of the Vaccine Safety Datalink (VSD).
Disclosures: The Centers for Disease Control and Prevention funded the study. The authors reported receiving contracts, grants, and other funding from the CDC.
Source: Glanz JM et al. JAMA. 2018;319(9):906-13.
FDA authorizes first direct-to-consumer BRCA1/2 test
The Food and Drug Administration has authorized the first direct-to-consumer (DTC) test to report on three specific BRCA1/BRCA2 breast cancer gene mutations.
Personal Genome Service Genetic Health Risk (GHR) Report for BRCA1/BRCA2 (Selected Variants) does not identify the most common BRCA1/2 mutations but rather the three most common in people of Ashkenazi (Eastern European) Jewish descent, the FDA said in a press statement.
The test, marketed by 23andMe, analyzes DNA from a self-collected saliva sample.
The three mutations identified by the test are present in about 2% of Ashkenazi Jewish women, but rarely in other ethnic populations. Any individual who takes the test may have other mutations in BRCA1 or BRCA2 genes, or other cancer-related gene mutations that are not detected by this test.
“This test provides information to certain individuals who may be at increased breast, ovarian, or prostate cancer risk and who might not otherwise get genetic screening and is a step forward in the availability of DTC genetic tests. But it has a lot of caveats,” Donald St. Pierre, acting director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health, said in the press statement. “While the detection of a BRCA mutation on this test does indicate an increased risk, only a small percentage of Americans carry one of these three mutations and most BRCA mutations that increase an individual’s risk are not detected by this test. The test should not be used as a substitute for seeing your doctor for cancer screenings or counseling on genetic and lifestyle factors that can increase or decrease cancer risk.”
The authorization was based on data provided by the company to indicate the test correctly identifies the three genetic variants in saliva samples and is reproducible. In addition, the company submitted data to demonstrate that the instructions are comprehensible and easy to follow.
The FDA cautions that consumers and health care professionals “should not use the test results to determine any treatments, including antihormone therapies and prophylactic removal of the breasts or ovaries.” Decisions should be made only after confirmatory testing and genetic counseling, they said.
The Food and Drug Administration has authorized the first direct-to-consumer (DTC) test to report on three specific BRCA1/BRCA2 breast cancer gene mutations.
Personal Genome Service Genetic Health Risk (GHR) Report for BRCA1/BRCA2 (Selected Variants) does not identify the most common BRCA1/2 mutations but rather the three most common in people of Ashkenazi (Eastern European) Jewish descent, the FDA said in a press statement.
The test, marketed by 23andMe, analyzes DNA from a self-collected saliva sample.
The three mutations identified by the test are present in about 2% of Ashkenazi Jewish women, but rarely in other ethnic populations. Any individual who takes the test may have other mutations in BRCA1 or BRCA2 genes, or other cancer-related gene mutations that are not detected by this test.
“This test provides information to certain individuals who may be at increased breast, ovarian, or prostate cancer risk and who might not otherwise get genetic screening and is a step forward in the availability of DTC genetic tests. But it has a lot of caveats,” Donald St. Pierre, acting director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health, said in the press statement. “While the detection of a BRCA mutation on this test does indicate an increased risk, only a small percentage of Americans carry one of these three mutations and most BRCA mutations that increase an individual’s risk are not detected by this test. The test should not be used as a substitute for seeing your doctor for cancer screenings or counseling on genetic and lifestyle factors that can increase or decrease cancer risk.”
The authorization was based on data provided by the company to indicate the test correctly identifies the three genetic variants in saliva samples and is reproducible. In addition, the company submitted data to demonstrate that the instructions are comprehensible and easy to follow.
The FDA cautions that consumers and health care professionals “should not use the test results to determine any treatments, including antihormone therapies and prophylactic removal of the breasts or ovaries.” Decisions should be made only after confirmatory testing and genetic counseling, they said.
The Food and Drug Administration has authorized the first direct-to-consumer (DTC) test to report on three specific BRCA1/BRCA2 breast cancer gene mutations.
Personal Genome Service Genetic Health Risk (GHR) Report for BRCA1/BRCA2 (Selected Variants) does not identify the most common BRCA1/2 mutations but rather the three most common in people of Ashkenazi (Eastern European) Jewish descent, the FDA said in a press statement.
The test, marketed by 23andMe, analyzes DNA from a self-collected saliva sample.
The three mutations identified by the test are present in about 2% of Ashkenazi Jewish women, but rarely in other ethnic populations. Any individual who takes the test may have other mutations in BRCA1 or BRCA2 genes, or other cancer-related gene mutations that are not detected by this test.
“This test provides information to certain individuals who may be at increased breast, ovarian, or prostate cancer risk and who might not otherwise get genetic screening and is a step forward in the availability of DTC genetic tests. But it has a lot of caveats,” Donald St. Pierre, acting director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health, said in the press statement. “While the detection of a BRCA mutation on this test does indicate an increased risk, only a small percentage of Americans carry one of these three mutations and most BRCA mutations that increase an individual’s risk are not detected by this test. The test should not be used as a substitute for seeing your doctor for cancer screenings or counseling on genetic and lifestyle factors that can increase or decrease cancer risk.”
The authorization was based on data provided by the company to indicate the test correctly identifies the three genetic variants in saliva samples and is reproducible. In addition, the company submitted data to demonstrate that the instructions are comprehensible and easy to follow.
The FDA cautions that consumers and health care professionals “should not use the test results to determine any treatments, including antihormone therapies and prophylactic removal of the breasts or ovaries.” Decisions should be made only after confirmatory testing and genetic counseling, they said.
Pediatric Psoriasis: An Interview With Nanette B. Silverberg, MD
What causes psoriasis in children?
Psoriasis is a chronic immune-mediated inflammatory skin disease with a genetic predisposition (Eichenfield et al). Similar to many inflammatory skin diseases, school-aged children have a greater predisposition before or in early adolescence. As with adult disease, pediatric psoriasis has a complex pathogenesis largely related to aberrant immune response to triggers such as infections (eg, streptococcal pharyngitis, perianal streptococcal dermatitis, upper respiratory viral infections), trauma (ie, Koebner phenomenon), stress, and obesity.
What are the emerging data and recommendations on screening for comorbidities in children with psoriasis?
Similar to psoriasis in adults, obesity and the metabolic syndrome are a true association with pediatric psoriasis that has been discussed in the literature (Eichenfield et al). Although many children with psoriasis have obesity as a potential comorbidity, the risk of cardiovascular comorbidities independent of obesity is high in pediatric psoriasis including elevated lipids, hypertension, polycystic ovaries, nonalcoholic liver disease, and elevated liver enzymes (Tollefson et al). Children with psoriasis have greater central obesity and adiposity, often accompanied by a family history of obesity. Interventions in this direction may be needed for long-term disease control and general health (Mercy and Paller). One target population is hospitalized children with psoriasis, particularly black and Hispanic children aged 0 to 9 years. This population has been identified to have a greater risk for obesity, diabetes mellitus, hypertension, arrhythmia, and valvular heart disease (Kwa et al). Therefore, it can be said that dermatologists can help to improve the overall health and lifestyle long-term in children with psoriasis.
Early-onset disease also is associated with greater risk for lifetime quality-of-life impairments including poor lifetime dermatology life quality index scores, depression and psoriasis-induced depression, social discrimination, sleep problems, and recreational drug usage (Kim et al).
How does psoriasis in children differ from adults?
Children have a variety of features that differ from adult disease. First, they are more likely to have an infectious trigger and therefore may have an identifiable treatable source. Second, they are more likely to have a family history of disease, with one-third having a relative with psoriasis, therefore, identifying the child at risk for long-standing disease. Third, children have far more visible head and neck disease, especially facial involvement including eyelids (Raychaudhuri and Gross), which increases the risk of bullying, social stigma, and negative effects on self-image. Of course, site is affected by age, and in infancy diaper dermatitis and inverse disease with maceration and overlying candidal diaper dermatitis can occur. Although children have less joint disease, it can be dramatic and crippling to the developing child.
What treatments are available for children?
In childhood, identification of precipitating infections such as streptococcal infection is ideal with appropriate intervention thereafter. Topical therapies are appropriate for limited disease with minimal disability; however, phototherapy and systemic agents can be used in pediatric psoriasis in extensive cases. Topical therapies can include corticosteroids, calcineurin inhibitors often used in sensitive skin such as the face and intertriginous areas, and calcipotriene (Eichenfield et al). Additional agents such as tar and salicylic acid can be used, with limitations on the latter due to risk for absorption in smaller children. Systemic interventions often are introduced after years of disease. A recent study identified practitioners with special interest in pediatric psoriasis and determined that systemic interventions were on average introduced 3 years after psoriasis was diagnosed and most commonly included methotrexate followed by etanercept, the latter having fewer gastrointestinal tract side effects. The panel found that usage of folic acid 6 days weekly minimized gastrointestinal tract side effects with methotrexate. Acitretin and cyclosporine were alternatives (Bronckers et al; Psoriasis Investigator Group [PsIG] of the Pediatric Dermatology Research Alliance and the European Working Group on Pediatric Psoriasis [EWGPP]).
Recently, dermatologists have become aware of the dramatic benefits of immune response modifiers and some biologics on pediatric psoriasis. In the setting of joint and skin involvement, I allow the rheumatologist to make the choice of agents for the child's best outcome. However, for pediatric and adolescent psoriasis, we now have 2 US Food and Drug Administration-approved agents and more rapid and thorough testing of adult-approved agents in children, with a hope of greater ability to modify disease course at a younger age, both now and in the future.
Which biologics are approved for the pediatric patient population?
Currently, in the United States 2 biologics have been approved: (1) etanercept, a fusion protein of tumor necrosis factor receptor extracellular domain linked to the Fc portion of human IgG, for moderate to severe plaque psoriasis in patients 4 years and older, and (2) ustekinumab, a human IgG1κ monoclonal antibody against the shared p40 subunit of the IL-12 and IL-23 cytokines, for moderate to severe plaque psoriasis in patients 12 years and older based on the encouraging data of the CADMUS trial (Kellen et al; Landells et al). In Europe, adalimumab has been approved as a first-line therapy in pediatric psoriasis (age ≥4 years), and etanercept (age ≥6 years) and ustekinumab (age ≥12 years) have been approved as second-line agents, all with grade A evidence, according to a recent Italian panel (Fortina et al). (A thorough review of the guidelines on screening, administration, and vaccination is available from Eichenfield et al.)
What treatments are in the pipeline?
In the United States we have clinical trials ongoing of adult-approved topical and immune response-modifying agents such as apremilast. These agents, as they become available and the data are gathered, will be added to what I refer to as our "pharmamentarium" of agents we can use to combat a difficult and disabling illness.
What gaps are there in the pediatric psoriasis research?
Currently, there is poor awareness that there is research for pediatric psoriasis, and there is a need for pediatric groups and the National Psoriasis Foundation to allow children, adolescents, and their families to know that clinical trials are available looking into newer, more targeted, and less immunosuppressive agents. There is new hope on the horizon!
Suggested Readings
Bronckers IMGJ, Seyger MMB, West DP, et al; Psoriasis Investigator Group (PsIG) of the Pediatric Dermatology Research Alliance and the European Working Group on Pediatric Psoriasis (EWGPP). Safety of systemic agents for the treatment of pediatric psoriasis. JAMA Dermatol. 2017;153:1147-1157.
Eichenfield LF, Paller AS, Tom WL, et al. Pediatric psoriasis: evolving perspectives [published online January 4, 2018]. Pediatr Dermatol. doi:10.1111/pde.13382.
Fortina AB, Bardazzi F, Berti S, et al. Treatment of severe psoriasis in children: recommendations of an Italian expert group [published online August 23, 2017]. Eur J Pediatr. 2017;176:1339-1354.
Kellen R, Silverberg NB, Lebwohl M. Efficacy and safety of ustekinumab in adolescents. Pediatric Health Med Ther. 2016;7:109-120.
Kim GE, Seidler E, Kimball AB. Effect of age at diagnosis on chronic quality of life and long-term outcomes of individuals with psoriasis [published online December 29, 2014]. Pediatr Dermatol. 2015;32:656-662.
Kwa L, Kwa MC, Silverberg JI. Cardiovascular comorbidities of pediatric psoriasis among hospitalized children in the United States. J Am Acad Dermatol. 2017;77:1023-1029.
Landells I, Marano C, Hsu MC, et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study [published online August 7, 2015]. J Am Acad Dermatol. 2015;73:594-603.
Mercy KM, Paller AS. The relationship between obesity and psoriasis in the pediatric population: implications and future directions. Cutis. 2013;92:107-109.
Raychaudhuri SP, Gross J. A comparative study of pediatric onset psoriasis with adult onset psoriasis. Pediatr Dermatol. 2000;17:174-178.
Tollefson MM, Van Houten HK, Asante D, et al. Association of psoriasis with comorbidity development in children with psoriasis [published online January 10, 2018]. JAMA Dermatol. doi:10.1001/jamadermatol.2017.5417.
What causes psoriasis in children?
Psoriasis is a chronic immune-mediated inflammatory skin disease with a genetic predisposition (Eichenfield et al). Similar to many inflammatory skin diseases, school-aged children have a greater predisposition before or in early adolescence. As with adult disease, pediatric psoriasis has a complex pathogenesis largely related to aberrant immune response to triggers such as infections (eg, streptococcal pharyngitis, perianal streptococcal dermatitis, upper respiratory viral infections), trauma (ie, Koebner phenomenon), stress, and obesity.
What are the emerging data and recommendations on screening for comorbidities in children with psoriasis?
Similar to psoriasis in adults, obesity and the metabolic syndrome are a true association with pediatric psoriasis that has been discussed in the literature (Eichenfield et al). Although many children with psoriasis have obesity as a potential comorbidity, the risk of cardiovascular comorbidities independent of obesity is high in pediatric psoriasis including elevated lipids, hypertension, polycystic ovaries, nonalcoholic liver disease, and elevated liver enzymes (Tollefson et al). Children with psoriasis have greater central obesity and adiposity, often accompanied by a family history of obesity. Interventions in this direction may be needed for long-term disease control and general health (Mercy and Paller). One target population is hospitalized children with psoriasis, particularly black and Hispanic children aged 0 to 9 years. This population has been identified to have a greater risk for obesity, diabetes mellitus, hypertension, arrhythmia, and valvular heart disease (Kwa et al). Therefore, it can be said that dermatologists can help to improve the overall health and lifestyle long-term in children with psoriasis.
Early-onset disease also is associated with greater risk for lifetime quality-of-life impairments including poor lifetime dermatology life quality index scores, depression and psoriasis-induced depression, social discrimination, sleep problems, and recreational drug usage (Kim et al).
How does psoriasis in children differ from adults?
Children have a variety of features that differ from adult disease. First, they are more likely to have an infectious trigger and therefore may have an identifiable treatable source. Second, they are more likely to have a family history of disease, with one-third having a relative with psoriasis, therefore, identifying the child at risk for long-standing disease. Third, children have far more visible head and neck disease, especially facial involvement including eyelids (Raychaudhuri and Gross), which increases the risk of bullying, social stigma, and negative effects on self-image. Of course, site is affected by age, and in infancy diaper dermatitis and inverse disease with maceration and overlying candidal diaper dermatitis can occur. Although children have less joint disease, it can be dramatic and crippling to the developing child.
What treatments are available for children?
In childhood, identification of precipitating infections such as streptococcal infection is ideal with appropriate intervention thereafter. Topical therapies are appropriate for limited disease with minimal disability; however, phototherapy and systemic agents can be used in pediatric psoriasis in extensive cases. Topical therapies can include corticosteroids, calcineurin inhibitors often used in sensitive skin such as the face and intertriginous areas, and calcipotriene (Eichenfield et al). Additional agents such as tar and salicylic acid can be used, with limitations on the latter due to risk for absorption in smaller children. Systemic interventions often are introduced after years of disease. A recent study identified practitioners with special interest in pediatric psoriasis and determined that systemic interventions were on average introduced 3 years after psoriasis was diagnosed and most commonly included methotrexate followed by etanercept, the latter having fewer gastrointestinal tract side effects. The panel found that usage of folic acid 6 days weekly minimized gastrointestinal tract side effects with methotrexate. Acitretin and cyclosporine were alternatives (Bronckers et al; Psoriasis Investigator Group [PsIG] of the Pediatric Dermatology Research Alliance and the European Working Group on Pediatric Psoriasis [EWGPP]).
Recently, dermatologists have become aware of the dramatic benefits of immune response modifiers and some biologics on pediatric psoriasis. In the setting of joint and skin involvement, I allow the rheumatologist to make the choice of agents for the child's best outcome. However, for pediatric and adolescent psoriasis, we now have 2 US Food and Drug Administration-approved agents and more rapid and thorough testing of adult-approved agents in children, with a hope of greater ability to modify disease course at a younger age, both now and in the future.
Which biologics are approved for the pediatric patient population?
Currently, in the United States 2 biologics have been approved: (1) etanercept, a fusion protein of tumor necrosis factor receptor extracellular domain linked to the Fc portion of human IgG, for moderate to severe plaque psoriasis in patients 4 years and older, and (2) ustekinumab, a human IgG1κ monoclonal antibody against the shared p40 subunit of the IL-12 and IL-23 cytokines, for moderate to severe plaque psoriasis in patients 12 years and older based on the encouraging data of the CADMUS trial (Kellen et al; Landells et al). In Europe, adalimumab has been approved as a first-line therapy in pediatric psoriasis (age ≥4 years), and etanercept (age ≥6 years) and ustekinumab (age ≥12 years) have been approved as second-line agents, all with grade A evidence, according to a recent Italian panel (Fortina et al). (A thorough review of the guidelines on screening, administration, and vaccination is available from Eichenfield et al.)
What treatments are in the pipeline?
In the United States we have clinical trials ongoing of adult-approved topical and immune response-modifying agents such as apremilast. These agents, as they become available and the data are gathered, will be added to what I refer to as our "pharmamentarium" of agents we can use to combat a difficult and disabling illness.
What gaps are there in the pediatric psoriasis research?
Currently, there is poor awareness that there is research for pediatric psoriasis, and there is a need for pediatric groups and the National Psoriasis Foundation to allow children, adolescents, and their families to know that clinical trials are available looking into newer, more targeted, and less immunosuppressive agents. There is new hope on the horizon!
Suggested Readings
Bronckers IMGJ, Seyger MMB, West DP, et al; Psoriasis Investigator Group (PsIG) of the Pediatric Dermatology Research Alliance and the European Working Group on Pediatric Psoriasis (EWGPP). Safety of systemic agents for the treatment of pediatric psoriasis. JAMA Dermatol. 2017;153:1147-1157.
Eichenfield LF, Paller AS, Tom WL, et al. Pediatric psoriasis: evolving perspectives [published online January 4, 2018]. Pediatr Dermatol. doi:10.1111/pde.13382.
Fortina AB, Bardazzi F, Berti S, et al. Treatment of severe psoriasis in children: recommendations of an Italian expert group [published online August 23, 2017]. Eur J Pediatr. 2017;176:1339-1354.
Kellen R, Silverberg NB, Lebwohl M. Efficacy and safety of ustekinumab in adolescents. Pediatric Health Med Ther. 2016;7:109-120.
Kim GE, Seidler E, Kimball AB. Effect of age at diagnosis on chronic quality of life and long-term outcomes of individuals with psoriasis [published online December 29, 2014]. Pediatr Dermatol. 2015;32:656-662.
Kwa L, Kwa MC, Silverberg JI. Cardiovascular comorbidities of pediatric psoriasis among hospitalized children in the United States. J Am Acad Dermatol. 2017;77:1023-1029.
Landells I, Marano C, Hsu MC, et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study [published online August 7, 2015]. J Am Acad Dermatol. 2015;73:594-603.
Mercy KM, Paller AS. The relationship between obesity and psoriasis in the pediatric population: implications and future directions. Cutis. 2013;92:107-109.
Raychaudhuri SP, Gross J. A comparative study of pediatric onset psoriasis with adult onset psoriasis. Pediatr Dermatol. 2000;17:174-178.
Tollefson MM, Van Houten HK, Asante D, et al. Association of psoriasis with comorbidity development in children with psoriasis [published online January 10, 2018]. JAMA Dermatol. doi:10.1001/jamadermatol.2017.5417.
What causes psoriasis in children?
Psoriasis is a chronic immune-mediated inflammatory skin disease with a genetic predisposition (Eichenfield et al). Similar to many inflammatory skin diseases, school-aged children have a greater predisposition before or in early adolescence. As with adult disease, pediatric psoriasis has a complex pathogenesis largely related to aberrant immune response to triggers such as infections (eg, streptococcal pharyngitis, perianal streptococcal dermatitis, upper respiratory viral infections), trauma (ie, Koebner phenomenon), stress, and obesity.
What are the emerging data and recommendations on screening for comorbidities in children with psoriasis?
Similar to psoriasis in adults, obesity and the metabolic syndrome are a true association with pediatric psoriasis that has been discussed in the literature (Eichenfield et al). Although many children with psoriasis have obesity as a potential comorbidity, the risk of cardiovascular comorbidities independent of obesity is high in pediatric psoriasis including elevated lipids, hypertension, polycystic ovaries, nonalcoholic liver disease, and elevated liver enzymes (Tollefson et al). Children with psoriasis have greater central obesity and adiposity, often accompanied by a family history of obesity. Interventions in this direction may be needed for long-term disease control and general health (Mercy and Paller). One target population is hospitalized children with psoriasis, particularly black and Hispanic children aged 0 to 9 years. This population has been identified to have a greater risk for obesity, diabetes mellitus, hypertension, arrhythmia, and valvular heart disease (Kwa et al). Therefore, it can be said that dermatologists can help to improve the overall health and lifestyle long-term in children with psoriasis.
Early-onset disease also is associated with greater risk for lifetime quality-of-life impairments including poor lifetime dermatology life quality index scores, depression and psoriasis-induced depression, social discrimination, sleep problems, and recreational drug usage (Kim et al).
How does psoriasis in children differ from adults?
Children have a variety of features that differ from adult disease. First, they are more likely to have an infectious trigger and therefore may have an identifiable treatable source. Second, they are more likely to have a family history of disease, with one-third having a relative with psoriasis, therefore, identifying the child at risk for long-standing disease. Third, children have far more visible head and neck disease, especially facial involvement including eyelids (Raychaudhuri and Gross), which increases the risk of bullying, social stigma, and negative effects on self-image. Of course, site is affected by age, and in infancy diaper dermatitis and inverse disease with maceration and overlying candidal diaper dermatitis can occur. Although children have less joint disease, it can be dramatic and crippling to the developing child.
What treatments are available for children?
In childhood, identification of precipitating infections such as streptococcal infection is ideal with appropriate intervention thereafter. Topical therapies are appropriate for limited disease with minimal disability; however, phototherapy and systemic agents can be used in pediatric psoriasis in extensive cases. Topical therapies can include corticosteroids, calcineurin inhibitors often used in sensitive skin such as the face and intertriginous areas, and calcipotriene (Eichenfield et al). Additional agents such as tar and salicylic acid can be used, with limitations on the latter due to risk for absorption in smaller children. Systemic interventions often are introduced after years of disease. A recent study identified practitioners with special interest in pediatric psoriasis and determined that systemic interventions were on average introduced 3 years after psoriasis was diagnosed and most commonly included methotrexate followed by etanercept, the latter having fewer gastrointestinal tract side effects. The panel found that usage of folic acid 6 days weekly minimized gastrointestinal tract side effects with methotrexate. Acitretin and cyclosporine were alternatives (Bronckers et al; Psoriasis Investigator Group [PsIG] of the Pediatric Dermatology Research Alliance and the European Working Group on Pediatric Psoriasis [EWGPP]).
Recently, dermatologists have become aware of the dramatic benefits of immune response modifiers and some biologics on pediatric psoriasis. In the setting of joint and skin involvement, I allow the rheumatologist to make the choice of agents for the child's best outcome. However, for pediatric and adolescent psoriasis, we now have 2 US Food and Drug Administration-approved agents and more rapid and thorough testing of adult-approved agents in children, with a hope of greater ability to modify disease course at a younger age, both now and in the future.
Which biologics are approved for the pediatric patient population?
Currently, in the United States 2 biologics have been approved: (1) etanercept, a fusion protein of tumor necrosis factor receptor extracellular domain linked to the Fc portion of human IgG, for moderate to severe plaque psoriasis in patients 4 years and older, and (2) ustekinumab, a human IgG1κ monoclonal antibody against the shared p40 subunit of the IL-12 and IL-23 cytokines, for moderate to severe plaque psoriasis in patients 12 years and older based on the encouraging data of the CADMUS trial (Kellen et al; Landells et al). In Europe, adalimumab has been approved as a first-line therapy in pediatric psoriasis (age ≥4 years), and etanercept (age ≥6 years) and ustekinumab (age ≥12 years) have been approved as second-line agents, all with grade A evidence, according to a recent Italian panel (Fortina et al). (A thorough review of the guidelines on screening, administration, and vaccination is available from Eichenfield et al.)
What treatments are in the pipeline?
In the United States we have clinical trials ongoing of adult-approved topical and immune response-modifying agents such as apremilast. These agents, as they become available and the data are gathered, will be added to what I refer to as our "pharmamentarium" of agents we can use to combat a difficult and disabling illness.
What gaps are there in the pediatric psoriasis research?
Currently, there is poor awareness that there is research for pediatric psoriasis, and there is a need for pediatric groups and the National Psoriasis Foundation to allow children, adolescents, and their families to know that clinical trials are available looking into newer, more targeted, and less immunosuppressive agents. There is new hope on the horizon!
Suggested Readings
Bronckers IMGJ, Seyger MMB, West DP, et al; Psoriasis Investigator Group (PsIG) of the Pediatric Dermatology Research Alliance and the European Working Group on Pediatric Psoriasis (EWGPP). Safety of systemic agents for the treatment of pediatric psoriasis. JAMA Dermatol. 2017;153:1147-1157.
Eichenfield LF, Paller AS, Tom WL, et al. Pediatric psoriasis: evolving perspectives [published online January 4, 2018]. Pediatr Dermatol. doi:10.1111/pde.13382.
Fortina AB, Bardazzi F, Berti S, et al. Treatment of severe psoriasis in children: recommendations of an Italian expert group [published online August 23, 2017]. Eur J Pediatr. 2017;176:1339-1354.
Kellen R, Silverberg NB, Lebwohl M. Efficacy and safety of ustekinumab in adolescents. Pediatric Health Med Ther. 2016;7:109-120.
Kim GE, Seidler E, Kimball AB. Effect of age at diagnosis on chronic quality of life and long-term outcomes of individuals with psoriasis [published online December 29, 2014]. Pediatr Dermatol. 2015;32:656-662.
Kwa L, Kwa MC, Silverberg JI. Cardiovascular comorbidities of pediatric psoriasis among hospitalized children in the United States. J Am Acad Dermatol. 2017;77:1023-1029.
Landells I, Marano C, Hsu MC, et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study [published online August 7, 2015]. J Am Acad Dermatol. 2015;73:594-603.
Mercy KM, Paller AS. The relationship between obesity and psoriasis in the pediatric population: implications and future directions. Cutis. 2013;92:107-109.
Raychaudhuri SP, Gross J. A comparative study of pediatric onset psoriasis with adult onset psoriasis. Pediatr Dermatol. 2000;17:174-178.
Tollefson MM, Van Houten HK, Asante D, et al. Association of psoriasis with comorbidity development in children with psoriasis [published online January 10, 2018]. JAMA Dermatol. doi:10.1001/jamadermatol.2017.5417.
Biologics and Systemic Therapies for Psoriasis: Treat the Patient, Not the Disease
What do patients need to know initially about psoriasis treatment?
It is important to set expectations with the patient based on the treatment selected, not only for patient satisfaction but to forge an enduring bond with the patient so he/she will trust you to guide the treatment plan if the first therapy does not work as well as anticipated. Because psoriasis is a longitudinal disease process, the patient-physician relationship should be, too. Certainly, these principles generally apply among all patient groups and demographics; however, one may take into account a few special circumstances when dealing with psoriasis. In a pediatric patient, I may try to see if topical therapy including calcipotriene can adequately treat the skin disease before pursuing systemic treatment. The rationale is 2-fold: (1) this patient would be committed to an extended period on immunomodulatory therapy if he/she truly requires it, and (2) some of the forms of psoriasis in children, such as guttate psoriasis, may be self-limited, so it is reasonable to see if it will persist before forging ahead with a long-term systemic medication. In patients with a recent history of cancer, I would likely choose an oral medication such as apremilast before a biologic; even though there are no real data to suggest biologics are associated with higher rates of solid-organ malignancy, most practitioners would err on the side of being more conservative. For patients with human immunodeficiency virus, the tendency is to use the agents with more data (eg, tumor necrosis factor α inhibitors) due to safety concerns with an immunomodulatory medication.
What are your go-to treatments?
I tend to be as aggressive as the patient wants to be with therapy. I regularly see patients in whom multiple systemic treatments have failed and a more creative regimen is needed, such as combining a biologic medication with an oral antipsoriatic treatment (eg, apremilast, acitretin). However, I do have patients with moderate to severe psoriasis who have not seen a dermatologist before. I do not find it necessary to have topical treatments fail before starting a biologic; after all, the sequelae of long-term topical steroid use are notable.
With the newer biologics on the market, such as the IL-17 and IL-23 inhibitors, the sky's the limit for psoriasis area and severity index clearance, but the true benefit is that these medications are much more targeted toward the pathogenesis of psoriasis. Unfortunately, we have to be mindful of insurance and formulary restrictions, but when faced with choosing a broad-acting immunomodulatory agent or a more specific/targeted immunomodulatory agent for an inflammatory disease, most dermatologists would choose the more targeted medication. The data support that the newer agents have better psoriasis area and severity index responses and a much greater proportion of clearance, but there is something to be said about biologics such as etanercept, adalimumab, and ustekinumab, which have been on the market for much longer and have shown durable response with a longer track record of safety and efficacy. Recent head-to-head comparisons can help guide treatment. For instance, patients who achieved suboptimal clearance on ustekinumab can safely and reasonably be switched to guselkumab based on the findings of the NAVIGATE study, which looked at this exact situation. More of these studies looking at specific prior treatment failures and improvement upon switching to a newer agent are needed to underscore the efficacy of these drugs and also to help argue for their placement on insurance formularies.
For a new patient with psoriasis, I will screen for psoriatic arthritis, look at involvement (eg, body surface area, individual plaque severity/thickness, locations such as scalp and extremities), and assess patient attitudes toward different treatments. Two patients with the exact same clinical appearance might have completely different strategies, one wanting to be as aggressive as possible to get rid of the psoriasis and the other not believing in systemic treatments and wanting to be as "natural" as possible.
For patients with only cutaneous involvement, the dosing frequency and efficacy of the newer IL-17 and IL-23 classes of medications are hard to beat. If a patient has notable psoriatic arthritis, I still tend to reach for a tumor necrosis factor α inhibitor first. For patients with limited involvement, especially those with scalp and/or palmoplantar psoriasis, I have found that apremilast works quite well. Apremilast, in general, would be a good first-step medication for patients wary of systemic therapy, and with its relatively benign side-effect profile, it has almost completely supplanted methotrexate in my practice. We also have a few newer topical medicines such as a calcipotriene 0.005%-betamethasone dipropionate 0.064% foam and a betamethasone dipropionate spray 0.05% that have proven useful, with more products in the pipeline.
How do you keep patients compliant with treatment?
Setting expectations is most important, and letting patients know what to expect from their first visit really helps to keep them satisfied with the plan and progress. Giving the patient a say in guiding the treatment and perhaps coming up with a rough treatment plan with a defined timeline also helps, such as starting with a topical regimen but moving on to an oral medicine if the topical does not work within 2 to 3 months, and then a biologic if oral therapy does not work well within 3 to 6 months. It is important not to push the patient to pursue a more aggressive therapy unless he/she wants to, otherwise the patient might not be compliant or may stop altogether.
What do you do if they refuse treatment?
If the patient is in your office, clearly he/she does want some help. Try to figure out what is at the root of the treatment refusal. Is the patient refusing topical steroids because he/she is afraid of them? Is the patient unable to stomach having to inject himself/herself? Finding the basis of their reticence may take more time, but we usually can find a mutually agreeable plan of action. Even if the first step is to watch and wait, you want the patient leaving your office knowing that if things do not progress as expected or get worse, they can have faith in you to come back and get more help.
What resources do you recommend to patients for more information?
The National Psoriasis Foundation is a great resource for patients. They have numerous outreach programs and a wealth of patient information. Also, the American Academy of Dermatology is a good resource, not just for patients but for providers; for example, the academy offers appeals letters that can be sent to insurance companies to try to advocate for a specific medication for patients.
Suggested Readings
Help patients appeal denial of psoriasis drugs. American Academy of Dermatology website. https://www.aad.org/members/publications/member-to-member/2017/jan-27-2017/help-patients-appeal-denial-of-psoriasis-drugs. Accessed February 9, 2018.
Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial [published online October 10, 2017]. Br J Dermatol. 2018;178:114-123.
What do patients need to know initially about psoriasis treatment?
It is important to set expectations with the patient based on the treatment selected, not only for patient satisfaction but to forge an enduring bond with the patient so he/she will trust you to guide the treatment plan if the first therapy does not work as well as anticipated. Because psoriasis is a longitudinal disease process, the patient-physician relationship should be, too. Certainly, these principles generally apply among all patient groups and demographics; however, one may take into account a few special circumstances when dealing with psoriasis. In a pediatric patient, I may try to see if topical therapy including calcipotriene can adequately treat the skin disease before pursuing systemic treatment. The rationale is 2-fold: (1) this patient would be committed to an extended period on immunomodulatory therapy if he/she truly requires it, and (2) some of the forms of psoriasis in children, such as guttate psoriasis, may be self-limited, so it is reasonable to see if it will persist before forging ahead with a long-term systemic medication. In patients with a recent history of cancer, I would likely choose an oral medication such as apremilast before a biologic; even though there are no real data to suggest biologics are associated with higher rates of solid-organ malignancy, most practitioners would err on the side of being more conservative. For patients with human immunodeficiency virus, the tendency is to use the agents with more data (eg, tumor necrosis factor α inhibitors) due to safety concerns with an immunomodulatory medication.
What are your go-to treatments?
I tend to be as aggressive as the patient wants to be with therapy. I regularly see patients in whom multiple systemic treatments have failed and a more creative regimen is needed, such as combining a biologic medication with an oral antipsoriatic treatment (eg, apremilast, acitretin). However, I do have patients with moderate to severe psoriasis who have not seen a dermatologist before. I do not find it necessary to have topical treatments fail before starting a biologic; after all, the sequelae of long-term topical steroid use are notable.
With the newer biologics on the market, such as the IL-17 and IL-23 inhibitors, the sky's the limit for psoriasis area and severity index clearance, but the true benefit is that these medications are much more targeted toward the pathogenesis of psoriasis. Unfortunately, we have to be mindful of insurance and formulary restrictions, but when faced with choosing a broad-acting immunomodulatory agent or a more specific/targeted immunomodulatory agent for an inflammatory disease, most dermatologists would choose the more targeted medication. The data support that the newer agents have better psoriasis area and severity index responses and a much greater proportion of clearance, but there is something to be said about biologics such as etanercept, adalimumab, and ustekinumab, which have been on the market for much longer and have shown durable response with a longer track record of safety and efficacy. Recent head-to-head comparisons can help guide treatment. For instance, patients who achieved suboptimal clearance on ustekinumab can safely and reasonably be switched to guselkumab based on the findings of the NAVIGATE study, which looked at this exact situation. More of these studies looking at specific prior treatment failures and improvement upon switching to a newer agent are needed to underscore the efficacy of these drugs and also to help argue for their placement on insurance formularies.
For a new patient with psoriasis, I will screen for psoriatic arthritis, look at involvement (eg, body surface area, individual plaque severity/thickness, locations such as scalp and extremities), and assess patient attitudes toward different treatments. Two patients with the exact same clinical appearance might have completely different strategies, one wanting to be as aggressive as possible to get rid of the psoriasis and the other not believing in systemic treatments and wanting to be as "natural" as possible.
For patients with only cutaneous involvement, the dosing frequency and efficacy of the newer IL-17 and IL-23 classes of medications are hard to beat. If a patient has notable psoriatic arthritis, I still tend to reach for a tumor necrosis factor α inhibitor first. For patients with limited involvement, especially those with scalp and/or palmoplantar psoriasis, I have found that apremilast works quite well. Apremilast, in general, would be a good first-step medication for patients wary of systemic therapy, and with its relatively benign side-effect profile, it has almost completely supplanted methotrexate in my practice. We also have a few newer topical medicines such as a calcipotriene 0.005%-betamethasone dipropionate 0.064% foam and a betamethasone dipropionate spray 0.05% that have proven useful, with more products in the pipeline.
How do you keep patients compliant with treatment?
Setting expectations is most important, and letting patients know what to expect from their first visit really helps to keep them satisfied with the plan and progress. Giving the patient a say in guiding the treatment and perhaps coming up with a rough treatment plan with a defined timeline also helps, such as starting with a topical regimen but moving on to an oral medicine if the topical does not work within 2 to 3 months, and then a biologic if oral therapy does not work well within 3 to 6 months. It is important not to push the patient to pursue a more aggressive therapy unless he/she wants to, otherwise the patient might not be compliant or may stop altogether.
What do you do if they refuse treatment?
If the patient is in your office, clearly he/she does want some help. Try to figure out what is at the root of the treatment refusal. Is the patient refusing topical steroids because he/she is afraid of them? Is the patient unable to stomach having to inject himself/herself? Finding the basis of their reticence may take more time, but we usually can find a mutually agreeable plan of action. Even if the first step is to watch and wait, you want the patient leaving your office knowing that if things do not progress as expected or get worse, they can have faith in you to come back and get more help.
What resources do you recommend to patients for more information?
The National Psoriasis Foundation is a great resource for patients. They have numerous outreach programs and a wealth of patient information. Also, the American Academy of Dermatology is a good resource, not just for patients but for providers; for example, the academy offers appeals letters that can be sent to insurance companies to try to advocate for a specific medication for patients.
Suggested Readings
Help patients appeal denial of psoriasis drugs. American Academy of Dermatology website. https://www.aad.org/members/publications/member-to-member/2017/jan-27-2017/help-patients-appeal-denial-of-psoriasis-drugs. Accessed February 9, 2018.
Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial [published online October 10, 2017]. Br J Dermatol. 2018;178:114-123.
What do patients need to know initially about psoriasis treatment?
It is important to set expectations with the patient based on the treatment selected, not only for patient satisfaction but to forge an enduring bond with the patient so he/she will trust you to guide the treatment plan if the first therapy does not work as well as anticipated. Because psoriasis is a longitudinal disease process, the patient-physician relationship should be, too. Certainly, these principles generally apply among all patient groups and demographics; however, one may take into account a few special circumstances when dealing with psoriasis. In a pediatric patient, I may try to see if topical therapy including calcipotriene can adequately treat the skin disease before pursuing systemic treatment. The rationale is 2-fold: (1) this patient would be committed to an extended period on immunomodulatory therapy if he/she truly requires it, and (2) some of the forms of psoriasis in children, such as guttate psoriasis, may be self-limited, so it is reasonable to see if it will persist before forging ahead with a long-term systemic medication. In patients with a recent history of cancer, I would likely choose an oral medication such as apremilast before a biologic; even though there are no real data to suggest biologics are associated with higher rates of solid-organ malignancy, most practitioners would err on the side of being more conservative. For patients with human immunodeficiency virus, the tendency is to use the agents with more data (eg, tumor necrosis factor α inhibitors) due to safety concerns with an immunomodulatory medication.
What are your go-to treatments?
I tend to be as aggressive as the patient wants to be with therapy. I regularly see patients in whom multiple systemic treatments have failed and a more creative regimen is needed, such as combining a biologic medication with an oral antipsoriatic treatment (eg, apremilast, acitretin). However, I do have patients with moderate to severe psoriasis who have not seen a dermatologist before. I do not find it necessary to have topical treatments fail before starting a biologic; after all, the sequelae of long-term topical steroid use are notable.
With the newer biologics on the market, such as the IL-17 and IL-23 inhibitors, the sky's the limit for psoriasis area and severity index clearance, but the true benefit is that these medications are much more targeted toward the pathogenesis of psoriasis. Unfortunately, we have to be mindful of insurance and formulary restrictions, but when faced with choosing a broad-acting immunomodulatory agent or a more specific/targeted immunomodulatory agent for an inflammatory disease, most dermatologists would choose the more targeted medication. The data support that the newer agents have better psoriasis area and severity index responses and a much greater proportion of clearance, but there is something to be said about biologics such as etanercept, adalimumab, and ustekinumab, which have been on the market for much longer and have shown durable response with a longer track record of safety and efficacy. Recent head-to-head comparisons can help guide treatment. For instance, patients who achieved suboptimal clearance on ustekinumab can safely and reasonably be switched to guselkumab based on the findings of the NAVIGATE study, which looked at this exact situation. More of these studies looking at specific prior treatment failures and improvement upon switching to a newer agent are needed to underscore the efficacy of these drugs and also to help argue for their placement on insurance formularies.
For a new patient with psoriasis, I will screen for psoriatic arthritis, look at involvement (eg, body surface area, individual plaque severity/thickness, locations such as scalp and extremities), and assess patient attitudes toward different treatments. Two patients with the exact same clinical appearance might have completely different strategies, one wanting to be as aggressive as possible to get rid of the psoriasis and the other not believing in systemic treatments and wanting to be as "natural" as possible.
For patients with only cutaneous involvement, the dosing frequency and efficacy of the newer IL-17 and IL-23 classes of medications are hard to beat. If a patient has notable psoriatic arthritis, I still tend to reach for a tumor necrosis factor α inhibitor first. For patients with limited involvement, especially those with scalp and/or palmoplantar psoriasis, I have found that apremilast works quite well. Apremilast, in general, would be a good first-step medication for patients wary of systemic therapy, and with its relatively benign side-effect profile, it has almost completely supplanted methotrexate in my practice. We also have a few newer topical medicines such as a calcipotriene 0.005%-betamethasone dipropionate 0.064% foam and a betamethasone dipropionate spray 0.05% that have proven useful, with more products in the pipeline.
How do you keep patients compliant with treatment?
Setting expectations is most important, and letting patients know what to expect from their first visit really helps to keep them satisfied with the plan and progress. Giving the patient a say in guiding the treatment and perhaps coming up with a rough treatment plan with a defined timeline also helps, such as starting with a topical regimen but moving on to an oral medicine if the topical does not work within 2 to 3 months, and then a biologic if oral therapy does not work well within 3 to 6 months. It is important not to push the patient to pursue a more aggressive therapy unless he/she wants to, otherwise the patient might not be compliant or may stop altogether.
What do you do if they refuse treatment?
If the patient is in your office, clearly he/she does want some help. Try to figure out what is at the root of the treatment refusal. Is the patient refusing topical steroids because he/she is afraid of them? Is the patient unable to stomach having to inject himself/herself? Finding the basis of their reticence may take more time, but we usually can find a mutually agreeable plan of action. Even if the first step is to watch and wait, you want the patient leaving your office knowing that if things do not progress as expected or get worse, they can have faith in you to come back and get more help.
What resources do you recommend to patients for more information?
The National Psoriasis Foundation is a great resource for patients. They have numerous outreach programs and a wealth of patient information. Also, the American Academy of Dermatology is a good resource, not just for patients but for providers; for example, the academy offers appeals letters that can be sent to insurance companies to try to advocate for a specific medication for patients.
Suggested Readings
Help patients appeal denial of psoriasis drugs. American Academy of Dermatology website. https://www.aad.org/members/publications/member-to-member/2017/jan-27-2017/help-patients-appeal-denial-of-psoriasis-drugs. Accessed February 9, 2018.
Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial [published online October 10, 2017]. Br J Dermatol. 2018;178:114-123.
Current Guidelines for Psoriasis Treatment: A Work in Progress
Psoriasis is a chronic autoinflammatory disorder affecting approximately 2% to 4% of the Western population.1 While there is no absolute cure for psoriasis, novel therapies allow for substantial reduction in symptoms and considerable improvement in quality of life (QoL). In the past few years, multiple treatment guidelines (recommendations based on evidence-based literature reviews) and consensus statements (a set of declarations determined and voted on by a panel of experts in the field) have been developed to guide physicians worldwide in treating psoriasis in the clinical setting (eTable).2-10
Because psoriasis is a complex disease with multiple comorbidities, applicability of these guidelines may be limited. Although some basic treatment algorithms exist, patient preference, disease severity, and other variables including comorbidities (eg, psoriatic arthritis [PsA], risk of major cardiac events, inflammatory bowel disease [IBD]), history of nonmelanoma skin cancer (NMSC), pregnancy and lactation, and specific contraindications to therapy (eg, renal failure, liver disease, active malignancy) should be considered. In this article, we summarize common themes across existing guidelines and consensus statements for the treatment of psoriasis and highlight areas where there is consistent agreement or lack of sufficient information.
Disease Severity and Treatment Outcomes
There currently are no consensus definitions for mild, moderate, and severe psoriasis, but several consensus statements have attempted to standardize grading systems based on objective values, such as body surface area (BSA) and psoriasis area and severity index (PASI)(a scoring system used to grade the degree of redness, thickness, and scaling of psoriasis plaques), as well as subjective QoL measures.2,6 Although classification of disease severity varies, mild psoriasis generally is characterized as disease that can be managed with local and topical therapy, and moderate to severe psoriasis typically warrants consideration for escalated treatment with phototherapy or systemic agents.
Most definitions of disease severity in psoriasis reference 5% to 10% BSA involvement as a cutoff that should trigger consideration of systemic treatment; however, these criteria could result in undertreatment of patients with substantial disease. For example, patients who have limited BSA involvement but whose disease has a considerable impact on QoL, as well as those who have debilitating disease in localized areas (eg, palms, soles, scalp, nails) or substantial joint involvement may also be appropriate candidates for systemic treatment.5,8
Once therapy is initiated, patients should be evaluated for appropriate treatment response at dedicated intervals. While the time to maximum therapeutic benefit depends on the agent of choice, European guidelines recommend that patients be evaluated after an induction phase (typically 16–24 weeks) and define treatment success as either (1) at least 75% improvement in PASI or (2) at least 50% improvement in PASI and a Dermatology Quality of Life Index (DLQI) score of 5 or lower.6
Alternatively, the National Psoriasis Foundation (NPF) recommended BSA as the preferred outcome measure in a recent consensus statement and concluded that an outcome of 3% or less BSA involvement or improvement in BSA of 75% or more is considered a desirable treatment response.9 Additionally, the Medicare Merit-based Incentive Payment System (MIPS) guidelines for successful systemic treatment response include at least 1 of the following: (1) physician global assessment score of 2 or lower, (2) BSA involvement of less than 3%, (3) PASI score lower than 3, or (4) DLQI score of 5 or lower.10
Although an array of outcome measures have been utilized in clinical trials and proposed in psoriasis guidelines and consensus statements, BSA is typically a manageable measure of treatment response in a clinical setting; however, DLQI should also be assessed if possible, particularly in patients with debilitating localized disease.9
Treatment Options
Because topical treatment regimens can be arduous and typically do not result in sustained clearance, patient expectations should be ascertained prior to initiation of therapy. Topical corticosteroids often can be used as monotherapy in patients with mild psoriasis.3 Topical vitamin D analogues and retinoids also can be effective; however, combined use of these agents with topical steroids should be considered to increase efficacy, and combination formulations can be prescribed to simplify application and improve adherence.
Treatment with UVB or psoralen plus UVA phototherapy is recommended for patients with moderate to severe psoriasis as well as in those who have had minimal response to topical therapy.4 Targeted phototherapy with an excimer laser can be used in patients with BSA involvement of less than 10%.
Methotrexate (MTX), cyclosporine, and acitretin are the most commonly prescribed systemic medications for severe psoriasis in the United States.5 Despite the risk for hepatotoxicity, MTX appears to have the best combined safety and efficacy profile in terms of serious adverse events compared to other systemic agents.11 Guidelines for MTX monitoring, especially with regard to when to do a liver biopsy, have been substantially liberalized over time, and the recommended interval for biopsy has been extended by years; biopsy was previously recommended after a cumulative MTX dose of 1 to 1.5 g, but guidelines now suggest biopsy after 3.5 to 4 g in low-risk patients.5 While abnormally elevated liver function tests during treatment with MTX may necessitate liver biopsy, the use of transient elastography and a panel of serum biomarkers for liver function also can be used to monitor noninvasively for hepatotoxicity before biopsy is considered; these recommendations are likely to be incorporated into newer guidelines in development.12 Methotrexate has demonstrated safety and increased efficacy when used in combination with biologic agents such as adalimumab, etanercept, infliximab, and secukinumab7 and has been studied in combination with many biologics indicated for PsA.13
Due to a considerable risk of glomerulosclerosis, cyclosporine is approved for a maximum of 1 year of continuous treatment of psoriasis in the United States and2 years in Europe.5,7 Cyclosporine is best used as induction therapy in psoriasis patients with severe disease who are seeking faster abatement of symptoms.
Acitretin is another systemic treatment option, although efficacy of this agent is dose dependent. Higher dosing often is limited due to lower tolerability.5
Given that many insurance formularies primarily cover traditional systemic therapies and that MTX and phototherapy are generally well tolerated and cost effective, patients may need to be treated with traditional agents before escalating to biologics. Prior to starting treatment with any biologic, patients should typically be screened for tuberculosis (TB), human immunodeficiency virus infection, and immunization for, exposure to, and/or infection with hepatitis B and C virus, and any other active infections. In patients who do not demonstrate hepatitis immunity, the hepatitis B vaccine should be administered prior to starting treatment with a biologic.14 In psoriasis patients with latent TB, 2 months of treatment should be completed before initiating biologic therapy8; once a biologic has been initiated, all patients should be screened annually for TB.
European guidelines for biologic treatment recommend that complete blood count and liver and renal function be evaluated at baseline, at months 1 and 3 of treatment, and then every 3 to 6 months thereafter while on the biologic agent.7 These recommendations are more stringent than those indicated in regulatory labeling and, based on the continual accumulation of data regarding the safety of these agents, some investigators have argued that laboratory testing might not be necessary at all.15
Treatment in Special Populations
Psoriasis patients often present with comorbidities or a complicated medical history, which can make it challenging to decide which therapy is most suitable. Patients with comorbid diseases (eg, PsA, risk of major cardiac event, IBD) or a history of NMSC and those who are pregnant or are lactating require special considerations to ensure treatment safety and efficacy.
Tumor necrosis factor α (TNF-α) and IL-17 inhibitors are used in the treatment of joint disorders and should be considered in patients with PsA. IL-23/IL-12 inhibition appears to have less benefit in patients with PsA, but studies on IL-23 inhibition (p19 antibodies) alone are ongoing.16 It has been reported that TNF-α inhibition may be beneficial in patients at risk for major cardiac events.8,17 In patients with IBD, IL-17 inhibitors should be avoided because they may exacerbate the condition; however, TNF-α and IL-23/IL-12 inhibition have shown to be safe in patients with IBD and many agents in these classes are approved by the US Food and Drug Administration for use in this population.18,19
Although biologics may increase the risk of developing NMSC20 and should generally be avoided in patients with any active malignancy, specific guidelines for screening and initiation of treatment in patients with a history of cancer are not clearly outlined. Prior to initiating systemic therapy in any patient, a careful medical history should be obtained. These agents often are not prescribed in patients with a history of cancer until remission has been established for at least 5 years, with the exception of patients with a history of treated NMSC.8 Annual skin monitoring for NMSC should be undertaken for psoriasis patients on most immunomodulating systemic therapies.
Recommendations for biologic treatment in psoriasis patients who are pregnant or lactating also are limited. European guidelines have noted pregnancy as an absolute contraindication to treatment with biologics,7but the regulatory guidance has recently changed for some agents, so this recommendation also may evolve.21 British8 and US5 guidelines do not consider pregnancy a contraindication for treatment with biologics.
Information on the safety of TNF-α antagonists during pregnancy comes primarily from use in patients with IBD and rheumatologic disease. To date, reports on the incidence of congenital malformations have been generally reassuring. Because IgG antibodies are actively transferred across the placenta in the late-second or the third trimesters, neonates born to mothers on biologic treatments may have high levels of some biologic drugs at birth. As a result, live vaccination should be avoided in neonates whose mothers were treated with IgG-based biologics.
Changing Treatment Agents
Patients may need to stop and change treatment agents due to ineffectiveness, personal preference, or worsening disease. When transitioning from any systemic or biologic agent to another (other than MTX), the British Association of Dermatologists recommends a washout period of at least 1 month before initiating a new therapy.8 Most guidelines do not define parameters for therapy escalation when patients fail multiple systemic agents, so physicians should use clinical judgment along with consideration of patient preference and comorbidity profile to ascertain which agent is most appropriate.
Conclusion
Keeping psoriasis treatment guidelines updated can be difficult, especially as new therapeutic options for psoriasis and treatment regimens rapidly evolve. Regulatory recommendations also vary worldwide, but most guidelines are reasonably consistent without being overly prescriptive, appropriately allowing for flexibility for application in clinical practice. Nonetheless, physicians should keep in mind new or changing guidelines while tailoring psoriasis treatment recommendations to best suit their individual patients.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTty (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence [published online September 27, 2012]. J Invest Dermatol. 2013;133:377-385.
- Pariser DM, Bagel J, Gelfand JM, et al. National Psoriasis Foundation clinical consensus on disease severity. Arch Dermatol. 2007;143:239-242.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
- Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303:1-10.
- Nast A, Gisondi P, Ormerod AD, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris—update 2015—short version—EDF in cooperation with EADV and IPC [published online October 9, 2015]. J Eur Acad Dermatol Venereol. 2015;29:2277-2294.
- Smith CH, Jabbar-Lopez ZK, Yiu ZZ, et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol. 2017;177:628-636.
- Armstrong AW, Siegel MP, Bagel J, et al. From the medical board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Quality ID #410: psoriasis: clinical response to oral systemic or biologic medications—national quality strategy domain: person and caregiver-centered experience and outcomes. Centers for Medicare and Medicaid Services website. https://www.cms.gov/Medicare/Quality-Payment-Program/Resource-Library/2018-Resources.html. Accessed February 27, 2018.
- Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database of Syst Rev. 2017;12:CD011535.
- Lynch M, Higgins E, McCormick PA, et al. The use of transient elastography and FibroTest for monitoring hepatotoxicity in patients receiving methotrexate for psoriasis. JAMA Dermatol. 2014;150:856-862.
- Behrens F, Canete J, Olivieri I, et al. Tumor necrosis factor inhibitor monotherapy versus combination with MTX in the treatment of PsA: a systemic review of the literature. Rheumatology. 2015;54:915-926.
- Karadağ Ö, Kaşifoğlu T, Özer B, et al. Viral hepatitis screening guideline before biological drug use in rheumatic patients. Eur J Rheumatol. 2016;3:25-28.
- Ahn CS, Dothard EH, Garner ML, et al. To test or not to test? an updated evidence-based assessment of the value of screening and monitoring tests when using systemic biologic agents to treat psoriasis and psoriatic arthritis. J Am Acad Dermatol. 2015;73:420-428.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator–controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Wu JJ, Guérin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Humira [package insert]. North Chicago, IL: Abbott Laboratories; 2011.
- Stelara [package insert]. Bloomington, IN: Janssen Biotech, Inc; 2016.
- Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56:2886-2895.
- Cimzia [package insert]. UCB, Inc: Smyrna, GA; 2016.
Psoriasis is a chronic autoinflammatory disorder affecting approximately 2% to 4% of the Western population.1 While there is no absolute cure for psoriasis, novel therapies allow for substantial reduction in symptoms and considerable improvement in quality of life (QoL). In the past few years, multiple treatment guidelines (recommendations based on evidence-based literature reviews) and consensus statements (a set of declarations determined and voted on by a panel of experts in the field) have been developed to guide physicians worldwide in treating psoriasis in the clinical setting (eTable).2-10

Because psoriasis is a complex disease with multiple comorbidities, applicability of these guidelines may be limited. Although some basic treatment algorithms exist, patient preference, disease severity, and other variables including comorbidities (eg, psoriatic arthritis [PsA], risk of major cardiac events, inflammatory bowel disease [IBD]), history of nonmelanoma skin cancer (NMSC), pregnancy and lactation, and specific contraindications to therapy (eg, renal failure, liver disease, active malignancy) should be considered. In this article, we summarize common themes across existing guidelines and consensus statements for the treatment of psoriasis and highlight areas where there is consistent agreement or lack of sufficient information.
Disease Severity and Treatment Outcomes
There currently are no consensus definitions for mild, moderate, and severe psoriasis, but several consensus statements have attempted to standardize grading systems based on objective values, such as body surface area (BSA) and psoriasis area and severity index (PASI)(a scoring system used to grade the degree of redness, thickness, and scaling of psoriasis plaques), as well as subjective QoL measures.2,6 Although classification of disease severity varies, mild psoriasis generally is characterized as disease that can be managed with local and topical therapy, and moderate to severe psoriasis typically warrants consideration for escalated treatment with phototherapy or systemic agents.
Most definitions of disease severity in psoriasis reference 5% to 10% BSA involvement as a cutoff that should trigger consideration of systemic treatment; however, these criteria could result in undertreatment of patients with substantial disease. For example, patients who have limited BSA involvement but whose disease has a considerable impact on QoL, as well as those who have debilitating disease in localized areas (eg, palms, soles, scalp, nails) or substantial joint involvement may also be appropriate candidates for systemic treatment.5,8
Once therapy is initiated, patients should be evaluated for appropriate treatment response at dedicated intervals. While the time to maximum therapeutic benefit depends on the agent of choice, European guidelines recommend that patients be evaluated after an induction phase (typically 16–24 weeks) and define treatment success as either (1) at least 75% improvement in PASI or (2) at least 50% improvement in PASI and a Dermatology Quality of Life Index (DLQI) score of 5 or lower.6
Alternatively, the National Psoriasis Foundation (NPF) recommended BSA as the preferred outcome measure in a recent consensus statement and concluded that an outcome of 3% or less BSA involvement or improvement in BSA of 75% or more is considered a desirable treatment response.9 Additionally, the Medicare Merit-based Incentive Payment System (MIPS) guidelines for successful systemic treatment response include at least 1 of the following: (1) physician global assessment score of 2 or lower, (2) BSA involvement of less than 3%, (3) PASI score lower than 3, or (4) DLQI score of 5 or lower.10
Although an array of outcome measures have been utilized in clinical trials and proposed in psoriasis guidelines and consensus statements, BSA is typically a manageable measure of treatment response in a clinical setting; however, DLQI should also be assessed if possible, particularly in patients with debilitating localized disease.9
Treatment Options
Because topical treatment regimens can be arduous and typically do not result in sustained clearance, patient expectations should be ascertained prior to initiation of therapy. Topical corticosteroids often can be used as monotherapy in patients with mild psoriasis.3 Topical vitamin D analogues and retinoids also can be effective; however, combined use of these agents with topical steroids should be considered to increase efficacy, and combination formulations can be prescribed to simplify application and improve adherence.
Treatment with UVB or psoralen plus UVA phototherapy is recommended for patients with moderate to severe psoriasis as well as in those who have had minimal response to topical therapy.4 Targeted phototherapy with an excimer laser can be used in patients with BSA involvement of less than 10%.
Methotrexate (MTX), cyclosporine, and acitretin are the most commonly prescribed systemic medications for severe psoriasis in the United States.5 Despite the risk for hepatotoxicity, MTX appears to have the best combined safety and efficacy profile in terms of serious adverse events compared to other systemic agents.11 Guidelines for MTX monitoring, especially with regard to when to do a liver biopsy, have been substantially liberalized over time, and the recommended interval for biopsy has been extended by years; biopsy was previously recommended after a cumulative MTX dose of 1 to 1.5 g, but guidelines now suggest biopsy after 3.5 to 4 g in low-risk patients.5 While abnormally elevated liver function tests during treatment with MTX may necessitate liver biopsy, the use of transient elastography and a panel of serum biomarkers for liver function also can be used to monitor noninvasively for hepatotoxicity before biopsy is considered; these recommendations are likely to be incorporated into newer guidelines in development.12 Methotrexate has demonstrated safety and increased efficacy when used in combination with biologic agents such as adalimumab, etanercept, infliximab, and secukinumab7 and has been studied in combination with many biologics indicated for PsA.13
Due to a considerable risk of glomerulosclerosis, cyclosporine is approved for a maximum of 1 year of continuous treatment of psoriasis in the United States and2 years in Europe.5,7 Cyclosporine is best used as induction therapy in psoriasis patients with severe disease who are seeking faster abatement of symptoms.
Acitretin is another systemic treatment option, although efficacy of this agent is dose dependent. Higher dosing often is limited due to lower tolerability.5
Given that many insurance formularies primarily cover traditional systemic therapies and that MTX and phototherapy are generally well tolerated and cost effective, patients may need to be treated with traditional agents before escalating to biologics. Prior to starting treatment with any biologic, patients should typically be screened for tuberculosis (TB), human immunodeficiency virus infection, and immunization for, exposure to, and/or infection with hepatitis B and C virus, and any other active infections. In patients who do not demonstrate hepatitis immunity, the hepatitis B vaccine should be administered prior to starting treatment with a biologic.14 In psoriasis patients with latent TB, 2 months of treatment should be completed before initiating biologic therapy8; once a biologic has been initiated, all patients should be screened annually for TB.
European guidelines for biologic treatment recommend that complete blood count and liver and renal function be evaluated at baseline, at months 1 and 3 of treatment, and then every 3 to 6 months thereafter while on the biologic agent.7 These recommendations are more stringent than those indicated in regulatory labeling and, based on the continual accumulation of data regarding the safety of these agents, some investigators have argued that laboratory testing might not be necessary at all.15
Treatment in Special Populations
Psoriasis patients often present with comorbidities or a complicated medical history, which can make it challenging to decide which therapy is most suitable. Patients with comorbid diseases (eg, PsA, risk of major cardiac event, IBD) or a history of NMSC and those who are pregnant or are lactating require special considerations to ensure treatment safety and efficacy.
Tumor necrosis factor α (TNF-α) and IL-17 inhibitors are used in the treatment of joint disorders and should be considered in patients with PsA. IL-23/IL-12 inhibition appears to have less benefit in patients with PsA, but studies on IL-23 inhibition (p19 antibodies) alone are ongoing.16 It has been reported that TNF-α inhibition may be beneficial in patients at risk for major cardiac events.8,17 In patients with IBD, IL-17 inhibitors should be avoided because they may exacerbate the condition; however, TNF-α and IL-23/IL-12 inhibition have shown to be safe in patients with IBD and many agents in these classes are approved by the US Food and Drug Administration for use in this population.18,19
Although biologics may increase the risk of developing NMSC20 and should generally be avoided in patients with any active malignancy, specific guidelines for screening and initiation of treatment in patients with a history of cancer are not clearly outlined. Prior to initiating systemic therapy in any patient, a careful medical history should be obtained. These agents often are not prescribed in patients with a history of cancer until remission has been established for at least 5 years, with the exception of patients with a history of treated NMSC.8 Annual skin monitoring for NMSC should be undertaken for psoriasis patients on most immunomodulating systemic therapies.
Recommendations for biologic treatment in psoriasis patients who are pregnant or lactating also are limited. European guidelines have noted pregnancy as an absolute contraindication to treatment with biologics,7but the regulatory guidance has recently changed for some agents, so this recommendation also may evolve.21 British8 and US5 guidelines do not consider pregnancy a contraindication for treatment with biologics.
Information on the safety of TNF-α antagonists during pregnancy comes primarily from use in patients with IBD and rheumatologic disease. To date, reports on the incidence of congenital malformations have been generally reassuring. Because IgG antibodies are actively transferred across the placenta in the late-second or the third trimesters, neonates born to mothers on biologic treatments may have high levels of some biologic drugs at birth. As a result, live vaccination should be avoided in neonates whose mothers were treated with IgG-based biologics.
Changing Treatment Agents
Patients may need to stop and change treatment agents due to ineffectiveness, personal preference, or worsening disease. When transitioning from any systemic or biologic agent to another (other than MTX), the British Association of Dermatologists recommends a washout period of at least 1 month before initiating a new therapy.8 Most guidelines do not define parameters for therapy escalation when patients fail multiple systemic agents, so physicians should use clinical judgment along with consideration of patient preference and comorbidity profile to ascertain which agent is most appropriate.
Conclusion
Keeping psoriasis treatment guidelines updated can be difficult, especially as new therapeutic options for psoriasis and treatment regimens rapidly evolve. Regulatory recommendations also vary worldwide, but most guidelines are reasonably consistent without being overly prescriptive, appropriately allowing for flexibility for application in clinical practice. Nonetheless, physicians should keep in mind new or changing guidelines while tailoring psoriasis treatment recommendations to best suit their individual patients.
Psoriasis is a chronic autoinflammatory disorder affecting approximately 2% to 4% of the Western population.1 While there is no absolute cure for psoriasis, novel therapies allow for substantial reduction in symptoms and considerable improvement in quality of life (QoL). In the past few years, multiple treatment guidelines (recommendations based on evidence-based literature reviews) and consensus statements (a set of declarations determined and voted on by a panel of experts in the field) have been developed to guide physicians worldwide in treating psoriasis in the clinical setting (eTable).2-10

Because psoriasis is a complex disease with multiple comorbidities, applicability of these guidelines may be limited. Although some basic treatment algorithms exist, patient preference, disease severity, and other variables including comorbidities (eg, psoriatic arthritis [PsA], risk of major cardiac events, inflammatory bowel disease [IBD]), history of nonmelanoma skin cancer (NMSC), pregnancy and lactation, and specific contraindications to therapy (eg, renal failure, liver disease, active malignancy) should be considered. In this article, we summarize common themes across existing guidelines and consensus statements for the treatment of psoriasis and highlight areas where there is consistent agreement or lack of sufficient information.
Disease Severity and Treatment Outcomes
There currently are no consensus definitions for mild, moderate, and severe psoriasis, but several consensus statements have attempted to standardize grading systems based on objective values, such as body surface area (BSA) and psoriasis area and severity index (PASI)(a scoring system used to grade the degree of redness, thickness, and scaling of psoriasis plaques), as well as subjective QoL measures.2,6 Although classification of disease severity varies, mild psoriasis generally is characterized as disease that can be managed with local and topical therapy, and moderate to severe psoriasis typically warrants consideration for escalated treatment with phototherapy or systemic agents.
Most definitions of disease severity in psoriasis reference 5% to 10% BSA involvement as a cutoff that should trigger consideration of systemic treatment; however, these criteria could result in undertreatment of patients with substantial disease. For example, patients who have limited BSA involvement but whose disease has a considerable impact on QoL, as well as those who have debilitating disease in localized areas (eg, palms, soles, scalp, nails) or substantial joint involvement may also be appropriate candidates for systemic treatment.5,8
Once therapy is initiated, patients should be evaluated for appropriate treatment response at dedicated intervals. While the time to maximum therapeutic benefit depends on the agent of choice, European guidelines recommend that patients be evaluated after an induction phase (typically 16–24 weeks) and define treatment success as either (1) at least 75% improvement in PASI or (2) at least 50% improvement in PASI and a Dermatology Quality of Life Index (DLQI) score of 5 or lower.6
Alternatively, the National Psoriasis Foundation (NPF) recommended BSA as the preferred outcome measure in a recent consensus statement and concluded that an outcome of 3% or less BSA involvement or improvement in BSA of 75% or more is considered a desirable treatment response.9 Additionally, the Medicare Merit-based Incentive Payment System (MIPS) guidelines for successful systemic treatment response include at least 1 of the following: (1) physician global assessment score of 2 or lower, (2) BSA involvement of less than 3%, (3) PASI score lower than 3, or (4) DLQI score of 5 or lower.10
Although an array of outcome measures have been utilized in clinical trials and proposed in psoriasis guidelines and consensus statements, BSA is typically a manageable measure of treatment response in a clinical setting; however, DLQI should also be assessed if possible, particularly in patients with debilitating localized disease.9
Treatment Options
Because topical treatment regimens can be arduous and typically do not result in sustained clearance, patient expectations should be ascertained prior to initiation of therapy. Topical corticosteroids often can be used as monotherapy in patients with mild psoriasis.3 Topical vitamin D analogues and retinoids also can be effective; however, combined use of these agents with topical steroids should be considered to increase efficacy, and combination formulations can be prescribed to simplify application and improve adherence.
Treatment with UVB or psoralen plus UVA phototherapy is recommended for patients with moderate to severe psoriasis as well as in those who have had minimal response to topical therapy.4 Targeted phototherapy with an excimer laser can be used in patients with BSA involvement of less than 10%.
Methotrexate (MTX), cyclosporine, and acitretin are the most commonly prescribed systemic medications for severe psoriasis in the United States.5 Despite the risk for hepatotoxicity, MTX appears to have the best combined safety and efficacy profile in terms of serious adverse events compared to other systemic agents.11 Guidelines for MTX monitoring, especially with regard to when to do a liver biopsy, have been substantially liberalized over time, and the recommended interval for biopsy has been extended by years; biopsy was previously recommended after a cumulative MTX dose of 1 to 1.5 g, but guidelines now suggest biopsy after 3.5 to 4 g in low-risk patients.5 While abnormally elevated liver function tests during treatment with MTX may necessitate liver biopsy, the use of transient elastography and a panel of serum biomarkers for liver function also can be used to monitor noninvasively for hepatotoxicity before biopsy is considered; these recommendations are likely to be incorporated into newer guidelines in development.12 Methotrexate has demonstrated safety and increased efficacy when used in combination with biologic agents such as adalimumab, etanercept, infliximab, and secukinumab7 and has been studied in combination with many biologics indicated for PsA.13
Due to a considerable risk of glomerulosclerosis, cyclosporine is approved for a maximum of 1 year of continuous treatment of psoriasis in the United States and2 years in Europe.5,7 Cyclosporine is best used as induction therapy in psoriasis patients with severe disease who are seeking faster abatement of symptoms.
Acitretin is another systemic treatment option, although efficacy of this agent is dose dependent. Higher dosing often is limited due to lower tolerability.5
Given that many insurance formularies primarily cover traditional systemic therapies and that MTX and phototherapy are generally well tolerated and cost effective, patients may need to be treated with traditional agents before escalating to biologics. Prior to starting treatment with any biologic, patients should typically be screened for tuberculosis (TB), human immunodeficiency virus infection, and immunization for, exposure to, and/or infection with hepatitis B and C virus, and any other active infections. In patients who do not demonstrate hepatitis immunity, the hepatitis B vaccine should be administered prior to starting treatment with a biologic.14 In psoriasis patients with latent TB, 2 months of treatment should be completed before initiating biologic therapy8; once a biologic has been initiated, all patients should be screened annually for TB.
European guidelines for biologic treatment recommend that complete blood count and liver and renal function be evaluated at baseline, at months 1 and 3 of treatment, and then every 3 to 6 months thereafter while on the biologic agent.7 These recommendations are more stringent than those indicated in regulatory labeling and, based on the continual accumulation of data regarding the safety of these agents, some investigators have argued that laboratory testing might not be necessary at all.15
Treatment in Special Populations
Psoriasis patients often present with comorbidities or a complicated medical history, which can make it challenging to decide which therapy is most suitable. Patients with comorbid diseases (eg, PsA, risk of major cardiac event, IBD) or a history of NMSC and those who are pregnant or are lactating require special considerations to ensure treatment safety and efficacy.
Tumor necrosis factor α (TNF-α) and IL-17 inhibitors are used in the treatment of joint disorders and should be considered in patients with PsA. IL-23/IL-12 inhibition appears to have less benefit in patients with PsA, but studies on IL-23 inhibition (p19 antibodies) alone are ongoing.16 It has been reported that TNF-α inhibition may be beneficial in patients at risk for major cardiac events.8,17 In patients with IBD, IL-17 inhibitors should be avoided because they may exacerbate the condition; however, TNF-α and IL-23/IL-12 inhibition have shown to be safe in patients with IBD and many agents in these classes are approved by the US Food and Drug Administration for use in this population.18,19
Although biologics may increase the risk of developing NMSC20 and should generally be avoided in patients with any active malignancy, specific guidelines for screening and initiation of treatment in patients with a history of cancer are not clearly outlined. Prior to initiating systemic therapy in any patient, a careful medical history should be obtained. These agents often are not prescribed in patients with a history of cancer until remission has been established for at least 5 years, with the exception of patients with a history of treated NMSC.8 Annual skin monitoring for NMSC should be undertaken for psoriasis patients on most immunomodulating systemic therapies.
Recommendations for biologic treatment in psoriasis patients who are pregnant or lactating also are limited. European guidelines have noted pregnancy as an absolute contraindication to treatment with biologics,7but the regulatory guidance has recently changed for some agents, so this recommendation also may evolve.21 British8 and US5 guidelines do not consider pregnancy a contraindication for treatment with biologics.
Information on the safety of TNF-α antagonists during pregnancy comes primarily from use in patients with IBD and rheumatologic disease. To date, reports on the incidence of congenital malformations have been generally reassuring. Because IgG antibodies are actively transferred across the placenta in the late-second or the third trimesters, neonates born to mothers on biologic treatments may have high levels of some biologic drugs at birth. As a result, live vaccination should be avoided in neonates whose mothers were treated with IgG-based biologics.
Changing Treatment Agents
Patients may need to stop and change treatment agents due to ineffectiveness, personal preference, or worsening disease. When transitioning from any systemic or biologic agent to another (other than MTX), the British Association of Dermatologists recommends a washout period of at least 1 month before initiating a new therapy.8 Most guidelines do not define parameters for therapy escalation when patients fail multiple systemic agents, so physicians should use clinical judgment along with consideration of patient preference and comorbidity profile to ascertain which agent is most appropriate.
Conclusion
Keeping psoriasis treatment guidelines updated can be difficult, especially as new therapeutic options for psoriasis and treatment regimens rapidly evolve. Regulatory recommendations also vary worldwide, but most guidelines are reasonably consistent without being overly prescriptive, appropriately allowing for flexibility for application in clinical practice. Nonetheless, physicians should keep in mind new or changing guidelines while tailoring psoriasis treatment recommendations to best suit their individual patients.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTty (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence [published online September 27, 2012]. J Invest Dermatol. 2013;133:377-385.
- Pariser DM, Bagel J, Gelfand JM, et al. National Psoriasis Foundation clinical consensus on disease severity. Arch Dermatol. 2007;143:239-242.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
- Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303:1-10.
- Nast A, Gisondi P, Ormerod AD, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris—update 2015—short version—EDF in cooperation with EADV and IPC [published online October 9, 2015]. J Eur Acad Dermatol Venereol. 2015;29:2277-2294.
- Smith CH, Jabbar-Lopez ZK, Yiu ZZ, et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol. 2017;177:628-636.
- Armstrong AW, Siegel MP, Bagel J, et al. From the medical board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Quality ID #410: psoriasis: clinical response to oral systemic or biologic medications—national quality strategy domain: person and caregiver-centered experience and outcomes. Centers for Medicare and Medicaid Services website. https://www.cms.gov/Medicare/Quality-Payment-Program/Resource-Library/2018-Resources.html. Accessed February 27, 2018.
- Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database of Syst Rev. 2017;12:CD011535.
- Lynch M, Higgins E, McCormick PA, et al. The use of transient elastography and FibroTest for monitoring hepatotoxicity in patients receiving methotrexate for psoriasis. JAMA Dermatol. 2014;150:856-862.
- Behrens F, Canete J, Olivieri I, et al. Tumor necrosis factor inhibitor monotherapy versus combination with MTX in the treatment of PsA: a systemic review of the literature. Rheumatology. 2015;54:915-926.
- Karadağ Ö, Kaşifoğlu T, Özer B, et al. Viral hepatitis screening guideline before biological drug use in rheumatic patients. Eur J Rheumatol. 2016;3:25-28.
- Ahn CS, Dothard EH, Garner ML, et al. To test or not to test? an updated evidence-based assessment of the value of screening and monitoring tests when using systemic biologic agents to treat psoriasis and psoriatic arthritis. J Am Acad Dermatol. 2015;73:420-428.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator–controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Wu JJ, Guérin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Humira [package insert]. North Chicago, IL: Abbott Laboratories; 2011.
- Stelara [package insert]. Bloomington, IN: Janssen Biotech, Inc; 2016.
- Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56:2886-2895.
- Cimzia [package insert]. UCB, Inc: Smyrna, GA; 2016.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTty (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence [published online September 27, 2012]. J Invest Dermatol. 2013;133:377-385.
- Pariser DM, Bagel J, Gelfand JM, et al. National Psoriasis Foundation clinical consensus on disease severity. Arch Dermatol. 2007;143:239-242.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
- Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303:1-10.
- Nast A, Gisondi P, Ormerod AD, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris—update 2015—short version—EDF in cooperation with EADV and IPC [published online October 9, 2015]. J Eur Acad Dermatol Venereol. 2015;29:2277-2294.
- Smith CH, Jabbar-Lopez ZK, Yiu ZZ, et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol. 2017;177:628-636.
- Armstrong AW, Siegel MP, Bagel J, et al. From the medical board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Quality ID #410: psoriasis: clinical response to oral systemic or biologic medications—national quality strategy domain: person and caregiver-centered experience and outcomes. Centers for Medicare and Medicaid Services website. https://www.cms.gov/Medicare/Quality-Payment-Program/Resource-Library/2018-Resources.html. Accessed February 27, 2018.
- Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database of Syst Rev. 2017;12:CD011535.
- Lynch M, Higgins E, McCormick PA, et al. The use of transient elastography and FibroTest for monitoring hepatotoxicity in patients receiving methotrexate for psoriasis. JAMA Dermatol. 2014;150:856-862.
- Behrens F, Canete J, Olivieri I, et al. Tumor necrosis factor inhibitor monotherapy versus combination with MTX in the treatment of PsA: a systemic review of the literature. Rheumatology. 2015;54:915-926.
- Karadağ Ö, Kaşifoğlu T, Özer B, et al. Viral hepatitis screening guideline before biological drug use in rheumatic patients. Eur J Rheumatol. 2016;3:25-28.
- Ahn CS, Dothard EH, Garner ML, et al. To test or not to test? an updated evidence-based assessment of the value of screening and monitoring tests when using systemic biologic agents to treat psoriasis and psoriatic arthritis. J Am Acad Dermatol. 2015;73:420-428.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator–controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Wu JJ, Guérin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Humira [package insert]. North Chicago, IL: Abbott Laboratories; 2011.
- Stelara [package insert]. Bloomington, IN: Janssen Biotech, Inc; 2016.
- Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56:2886-2895.
- Cimzia [package insert]. UCB, Inc: Smyrna, GA; 2016.
Practice Points
- Guidelines and consensus statements for psoriasis treatment are generally but not always consistent.
- As guidelines evolve, individual patient preferences, disease severity, and comorbid conditions remain important considerations when selecting treatment agents for psoriasis.
- More frequent updates to psoriasis treatment guidelines are becoming increasingly important given the rapid changes in the field.
PICU, hospital admissions up due to opioid ingestion
Hospitalization and pediatric ICU admission rates for pediatric opioid-related ingestion are increasing, along with hospitalization costs, according to a retrospective cohort study.
“In this study, we demonstrate a significant and steady increase in the diagnosis of opioid ingestion and poisoning across all age groups in U.S. children’s hospitals from 2004 to 2015,” wrote Jason Kane, MD, of the University of Chicago, and his associates. “Not only did the absolute number of opioid-related admissions increase but the rate of both hospital and PICU [pediatric ICU] admissions increased as well.”
Using the Pediatric Health Information System database, the research team performed a retrospective cohort study of children aged 1-17 years who had been admitted to a PICU between Jan. 1, 2004, and Sep. 30, 2015. For statistical analysis, the years were grouped into separate epochs: 2004-2007, 2008-2011, and 2012-2015.
Of the 4,175,624 admissions to 31 different children’s hospitals around the United States, 3,647 (0.09%) were due to opioid-related conditions. Across the three epochs of the study, the number of opioid-related hospitalizations more than doubled from 797 to 1,504 and concurrently increased the rate of hospital admissions from 6.7 per 10,000 in 2004 to 10.9 per 10,000 in 2015 (P less than .001).
Similar to the trends in overall hospital admissions and hospital admission rates, admission to the PICU and PICU admission rates also increased. Of the 3,647 children admitted for opioid-related issues, 1,564 (43%) were subsequently admitted to the PICU. PICU admission rates also increased from 25 to 36 per 10,000 admissions (P less than .001).While the majority of opioid-related hospitalizations are associated with children aged 12-17 years, children under the age of 6 years accounted for one-third of these hospitalizations. Many PICU admissions are severe enough to warrant mechanical ventilator support (37%, P less than .001) and vasopressors (20%, P less than .001).
The opioids ingested prior to hospital admission varied between age groups, with 20% (243 of 1,249) patients aged 1-5 years ingesting methadone, compared with 10% (218 of 2,223) of patients aged 12-17 years. Heroin was much more common in this group, accounting for 4.4% (99 of 2,223) of patient hospitalizations.
In addition to the human cost of pediatric hospital admissions, there is a significant economic cost on the health care system. The median cost for PICU admission was $4,931. Although these costs have been dropping for the better part of a decade ($6,523 in 2004-2007 to $4,552 in 2012-2015, P less than .001), it still represents a substantial problem. In addition, admission rates are increasing, which will only place a heavier burden on the health care system, according to Dr. Kane and his associates.
Perhaps one positive point from this study is that although hospitalizations and intensive care rates have gone up, mortality decreased over time from 2.8% in 2004-2007 to 1.3% in 2012-2015.
A possible limitation of the data in this study is that it provides data from subjects whose data is accessible to the researcher, rather than those strategically selected. In addition, referral bias may reduce the ability to generalize the information to non–tertiary care children’s hospitals.
“The current U.S. opioid crisis is negatively impacting pediatric patients as the rate of hospitalization and PICU care for the ingestion of opioids by children continues to increase over time,” wrote Dr. Kane and his associates. “Current efforts to reduce prescription opioid use in adults have not curtailed the incidence of pediatric opioid ingestion, and additional efforts are needed to reduce preventable opioid exposure in children.”
This study had no external funding. Dr. Allison H. Bartlett has served as a consultant member of the CVS Caremark National Pharmacy and Therapeutics Committee. All other authors had no relevant financial disclosures to report.
SOURCE: Kane JM et al. Pediatrics. 2018 Mar 5;141(4):e20173335.
The opioid crisis in the United States is staggering. As of 2016, an estimated 2.4 million Americans were considered to have an opioid use disorder, either from prescription drug misuse or heroin addiction. This number includes 0.6% of adolescents (12- to 17-year-olds) and 1.1% of young adults (18- to 25-year-olds). And 33,000 Americans died from opioid overdose in 2015. Despite the best attempts to control the supply of drugs and increase access to treatment, overdose deaths have doubled in the past 10 years. While the overdose death rate has plateaued among children under the age of 18, and misuse rates have dropped among 12th graders, opioid-related hospitalizations are increasing in preschool-age children and adolescents.
Prior to the work of Kane et al., little was known about critical care resource usage among pediatric patients admitted to pediatric ICUs across the country. They found that hospitalization rates were up, with over one-third of patients requiring mechanical ventilation and about 20% needing vasopressors. Perhaps one of the most important findings is that methadone accounted for nearly 20% of opioids ingested, displaying how adults being treated for their own opioid use disorder can put the children they live with at risk.
As the opioid crisis has worsened and overdoses have increased, the Council of Economic Advisers attempted to measure the societal costs of opioid overdoses using the “value of a statistical life” analytic method. This considers activities other than just lost work productivity and earnings, such as volunteering and raising a family. Using the value of a statistical life method, the Council determined that the true cost to society was nearly $504 billion, which included both fatal and nonfatal overdoses, and is approximately 2.8% of the 2015 U.S. gross domestic product.
Clearly, opioid abuse is both an emotional and financial burden to individual families and society as a whole. Pediatricians must help combat the ongoing opioid crisis in this country by addressing the needs of pediatric patients.
Sheryl A. Ryan, MD, is a pediatrician at Penn State Health Children’s Hospital, Milton S. Hershey Medical Center in Hershey, Pa. She wrote this commentary to the article by Kane et al. (Pediatrics. 2018 Mar. 5;41(4):e20174129). There was no external funding for this commentary, and Dr. Ryan said she had no relevant financial disclosures.
The opioid crisis in the United States is staggering. As of 2016, an estimated 2.4 million Americans were considered to have an opioid use disorder, either from prescription drug misuse or heroin addiction. This number includes 0.6% of adolescents (12- to 17-year-olds) and 1.1% of young adults (18- to 25-year-olds). And 33,000 Americans died from opioid overdose in 2015. Despite the best attempts to control the supply of drugs and increase access to treatment, overdose deaths have doubled in the past 10 years. While the overdose death rate has plateaued among children under the age of 18, and misuse rates have dropped among 12th graders, opioid-related hospitalizations are increasing in preschool-age children and adolescents.
Prior to the work of Kane et al., little was known about critical care resource usage among pediatric patients admitted to pediatric ICUs across the country. They found that hospitalization rates were up, with over one-third of patients requiring mechanical ventilation and about 20% needing vasopressors. Perhaps one of the most important findings is that methadone accounted for nearly 20% of opioids ingested, displaying how adults being treated for their own opioid use disorder can put the children they live with at risk.
As the opioid crisis has worsened and overdoses have increased, the Council of Economic Advisers attempted to measure the societal costs of opioid overdoses using the “value of a statistical life” analytic method. This considers activities other than just lost work productivity and earnings, such as volunteering and raising a family. Using the value of a statistical life method, the Council determined that the true cost to society was nearly $504 billion, which included both fatal and nonfatal overdoses, and is approximately 2.8% of the 2015 U.S. gross domestic product.
Clearly, opioid abuse is both an emotional and financial burden to individual families and society as a whole. Pediatricians must help combat the ongoing opioid crisis in this country by addressing the needs of pediatric patients.
Sheryl A. Ryan, MD, is a pediatrician at Penn State Health Children’s Hospital, Milton S. Hershey Medical Center in Hershey, Pa. She wrote this commentary to the article by Kane et al. (Pediatrics. 2018 Mar. 5;41(4):e20174129). There was no external funding for this commentary, and Dr. Ryan said she had no relevant financial disclosures.
The opioid crisis in the United States is staggering. As of 2016, an estimated 2.4 million Americans were considered to have an opioid use disorder, either from prescription drug misuse or heroin addiction. This number includes 0.6% of adolescents (12- to 17-year-olds) and 1.1% of young adults (18- to 25-year-olds). And 33,000 Americans died from opioid overdose in 2015. Despite the best attempts to control the supply of drugs and increase access to treatment, overdose deaths have doubled in the past 10 years. While the overdose death rate has plateaued among children under the age of 18, and misuse rates have dropped among 12th graders, opioid-related hospitalizations are increasing in preschool-age children and adolescents.
Prior to the work of Kane et al., little was known about critical care resource usage among pediatric patients admitted to pediatric ICUs across the country. They found that hospitalization rates were up, with over one-third of patients requiring mechanical ventilation and about 20% needing vasopressors. Perhaps one of the most important findings is that methadone accounted for nearly 20% of opioids ingested, displaying how adults being treated for their own opioid use disorder can put the children they live with at risk.
As the opioid crisis has worsened and overdoses have increased, the Council of Economic Advisers attempted to measure the societal costs of opioid overdoses using the “value of a statistical life” analytic method. This considers activities other than just lost work productivity and earnings, such as volunteering and raising a family. Using the value of a statistical life method, the Council determined that the true cost to society was nearly $504 billion, which included both fatal and nonfatal overdoses, and is approximately 2.8% of the 2015 U.S. gross domestic product.
Clearly, opioid abuse is both an emotional and financial burden to individual families and society as a whole. Pediatricians must help combat the ongoing opioid crisis in this country by addressing the needs of pediatric patients.
Sheryl A. Ryan, MD, is a pediatrician at Penn State Health Children’s Hospital, Milton S. Hershey Medical Center in Hershey, Pa. She wrote this commentary to the article by Kane et al. (Pediatrics. 2018 Mar. 5;41(4):e20174129). There was no external funding for this commentary, and Dr. Ryan said she had no relevant financial disclosures.
Hospitalization and pediatric ICU admission rates for pediatric opioid-related ingestion are increasing, along with hospitalization costs, according to a retrospective cohort study.
“In this study, we demonstrate a significant and steady increase in the diagnosis of opioid ingestion and poisoning across all age groups in U.S. children’s hospitals from 2004 to 2015,” wrote Jason Kane, MD, of the University of Chicago, and his associates. “Not only did the absolute number of opioid-related admissions increase but the rate of both hospital and PICU [pediatric ICU] admissions increased as well.”
Using the Pediatric Health Information System database, the research team performed a retrospective cohort study of children aged 1-17 years who had been admitted to a PICU between Jan. 1, 2004, and Sep. 30, 2015. For statistical analysis, the years were grouped into separate epochs: 2004-2007, 2008-2011, and 2012-2015.
Of the 4,175,624 admissions to 31 different children’s hospitals around the United States, 3,647 (0.09%) were due to opioid-related conditions. Across the three epochs of the study, the number of opioid-related hospitalizations more than doubled from 797 to 1,504 and concurrently increased the rate of hospital admissions from 6.7 per 10,000 in 2004 to 10.9 per 10,000 in 2015 (P less than .001).
Similar to the trends in overall hospital admissions and hospital admission rates, admission to the PICU and PICU admission rates also increased. Of the 3,647 children admitted for opioid-related issues, 1,564 (43%) were subsequently admitted to the PICU. PICU admission rates also increased from 25 to 36 per 10,000 admissions (P less than .001).While the majority of opioid-related hospitalizations are associated with children aged 12-17 years, children under the age of 6 years accounted for one-third of these hospitalizations. Many PICU admissions are severe enough to warrant mechanical ventilator support (37%, P less than .001) and vasopressors (20%, P less than .001).
The opioids ingested prior to hospital admission varied between age groups, with 20% (243 of 1,249) patients aged 1-5 years ingesting methadone, compared with 10% (218 of 2,223) of patients aged 12-17 years. Heroin was much more common in this group, accounting for 4.4% (99 of 2,223) of patient hospitalizations.
In addition to the human cost of pediatric hospital admissions, there is a significant economic cost on the health care system. The median cost for PICU admission was $4,931. Although these costs have been dropping for the better part of a decade ($6,523 in 2004-2007 to $4,552 in 2012-2015, P less than .001), it still represents a substantial problem. In addition, admission rates are increasing, which will only place a heavier burden on the health care system, according to Dr. Kane and his associates.
Perhaps one positive point from this study is that although hospitalizations and intensive care rates have gone up, mortality decreased over time from 2.8% in 2004-2007 to 1.3% in 2012-2015.
A possible limitation of the data in this study is that it provides data from subjects whose data is accessible to the researcher, rather than those strategically selected. In addition, referral bias may reduce the ability to generalize the information to non–tertiary care children’s hospitals.
“The current U.S. opioid crisis is negatively impacting pediatric patients as the rate of hospitalization and PICU care for the ingestion of opioids by children continues to increase over time,” wrote Dr. Kane and his associates. “Current efforts to reduce prescription opioid use in adults have not curtailed the incidence of pediatric opioid ingestion, and additional efforts are needed to reduce preventable opioid exposure in children.”
This study had no external funding. Dr. Allison H. Bartlett has served as a consultant member of the CVS Caremark National Pharmacy and Therapeutics Committee. All other authors had no relevant financial disclosures to report.
SOURCE: Kane JM et al. Pediatrics. 2018 Mar 5;141(4):e20173335.
Hospitalization and pediatric ICU admission rates for pediatric opioid-related ingestion are increasing, along with hospitalization costs, according to a retrospective cohort study.
“In this study, we demonstrate a significant and steady increase in the diagnosis of opioid ingestion and poisoning across all age groups in U.S. children’s hospitals from 2004 to 2015,” wrote Jason Kane, MD, of the University of Chicago, and his associates. “Not only did the absolute number of opioid-related admissions increase but the rate of both hospital and PICU [pediatric ICU] admissions increased as well.”
Using the Pediatric Health Information System database, the research team performed a retrospective cohort study of children aged 1-17 years who had been admitted to a PICU between Jan. 1, 2004, and Sep. 30, 2015. For statistical analysis, the years were grouped into separate epochs: 2004-2007, 2008-2011, and 2012-2015.
Of the 4,175,624 admissions to 31 different children’s hospitals around the United States, 3,647 (0.09%) were due to opioid-related conditions. Across the three epochs of the study, the number of opioid-related hospitalizations more than doubled from 797 to 1,504 and concurrently increased the rate of hospital admissions from 6.7 per 10,000 in 2004 to 10.9 per 10,000 in 2015 (P less than .001).
Similar to the trends in overall hospital admissions and hospital admission rates, admission to the PICU and PICU admission rates also increased. Of the 3,647 children admitted for opioid-related issues, 1,564 (43%) were subsequently admitted to the PICU. PICU admission rates also increased from 25 to 36 per 10,000 admissions (P less than .001).While the majority of opioid-related hospitalizations are associated with children aged 12-17 years, children under the age of 6 years accounted for one-third of these hospitalizations. Many PICU admissions are severe enough to warrant mechanical ventilator support (37%, P less than .001) and vasopressors (20%, P less than .001).
The opioids ingested prior to hospital admission varied between age groups, with 20% (243 of 1,249) patients aged 1-5 years ingesting methadone, compared with 10% (218 of 2,223) of patients aged 12-17 years. Heroin was much more common in this group, accounting for 4.4% (99 of 2,223) of patient hospitalizations.
In addition to the human cost of pediatric hospital admissions, there is a significant economic cost on the health care system. The median cost for PICU admission was $4,931. Although these costs have been dropping for the better part of a decade ($6,523 in 2004-2007 to $4,552 in 2012-2015, P less than .001), it still represents a substantial problem. In addition, admission rates are increasing, which will only place a heavier burden on the health care system, according to Dr. Kane and his associates.
Perhaps one positive point from this study is that although hospitalizations and intensive care rates have gone up, mortality decreased over time from 2.8% in 2004-2007 to 1.3% in 2012-2015.
A possible limitation of the data in this study is that it provides data from subjects whose data is accessible to the researcher, rather than those strategically selected. In addition, referral bias may reduce the ability to generalize the information to non–tertiary care children’s hospitals.
“The current U.S. opioid crisis is negatively impacting pediatric patients as the rate of hospitalization and PICU care for the ingestion of opioids by children continues to increase over time,” wrote Dr. Kane and his associates. “Current efforts to reduce prescription opioid use in adults have not curtailed the incidence of pediatric opioid ingestion, and additional efforts are needed to reduce preventable opioid exposure in children.”
This study had no external funding. Dr. Allison H. Bartlett has served as a consultant member of the CVS Caremark National Pharmacy and Therapeutics Committee. All other authors had no relevant financial disclosures to report.
SOURCE: Kane JM et al. Pediatrics. 2018 Mar 5;141(4):e20173335.
FROM PEDIATRICS
Key clinical point: The rate of hospitalizations and pediatric ICU admissions are up due to opioid ingestion.
Major finding: Over 40% of pediatric patients admitted to hospitals required PICU care.
Study details: A retrospective cohort study of children aged 1-17 years who were admitted to a PICU between Jan. 1, 2004, and Sep. 30, 2015.
Disclosures: This study had no external funding. Dr. Allison H. Bartlett has served as a consultant member of the CVS Caremark National Pharmacy and Therapeutics Committee. All other authors had no relevant financial disclosures to report.
Source: Kane JM et al. Pediatrics. 2018 Mar. 5;141(4):e20173335.
GCLAM therapy shows promise for relapsed/refractory AML
ATLANTA – GCLAM – the combined use of granulocyte colony-stimulating factor (G-CSF), cladribine, high-dose cytarabine, and mitoxantrone – was well tolerated and had potent antileukemia activity in a phase 1/2 trial of adults with relapsed/refractory acute myeloid leukemia or high-grade myeloid neoplasms.
Of 40 patients who were treated with GCLAM (with mitoxantrone at the maximum tolerated dose of 16 mg/m2 per day as established in phase 1 of the trial), 11 achieved a complete response (CR), and 13 achieved a complete response with incomplete blood count recovery (CRi), for an overall response rate of 60%, Anna B. Halpern, MD, reported at the annual meeting of the American Society of Hematology.
“Nine of the 11 CR patients and 11 of 13 with CRis were negative for minimal residual disease, for an overall MRD-negative CR rate of 23%,” said Dr. Halpern of the University of Washington and the Fred Hutchinson Cancer Research Center, both in Seattle.
Resistant disease occurred in 11 patients, she noted.
Median overall survival was 11.5 months, and the treatment-related mortality (TRM) rate was 5%.
Overall, 21 of 40 patients went to transplant, with a 49% 1-year survival rate, she said.
The patients had a median age of 63 years. Thirty-four had acute myeloid leukemia (AML), and 6 had high-grade myelodysplastic syndrome; 28 had secondary disease. Nineteen had primary refractory disease, 21 had relapsed disease after a median initial CR duration of 12 months, and 7 had prior allogeneic transplant. The median TRM score for all patients was 2.0, indicating a low risk for treatment-related mortality.
“Cytogenetics distribution, based on Medical Research Council criteria, was as expected,” she added.
At the mitoxantrone maximum tolerated dose of 16 mg/m2 per day – which was established during phase 1 in 26 patients in whom higher dose levels (18 mg/m2 per day) led to dose-limiting encephalopathy and cardiogenic shock – the most common grade 3 or greater adverse events included neutropenic fever, infectious complications, and hypoxia during therapy. This was largely attributed to volume overload and infection, Dr. Halpern said.
“Although three patients did have decreased ejection fraction in cycle 2, this was largely in the setting of sepsis, making the etiology difficult to ascribe to the anthracycline versus sepsis physiology,” she noted.
The median times to an absolute neutrophil count of 500/mcL or greater and platelet count of 50,000/mcL or greater were 29 days each, she noted.
A multivariable analysis controlling for baseline prognostic features showed that the mitoxantrone dose of 16 mg/m2 per day was associated with significantly better overall survival, compared with a dose of 10 mg/m2 per day used in a historical cohort according to standard GCLAM dosing (hazard ratio for death, 0.45). Additionally, more of those receiving a dose of 16 mg/m2 per day went on to transplant (52% vs. 37%), she said. The overall response rate was also higher with the 16-mg/m2 dose, but the difference was not statistically significant (odds ratio, 1.87).
“Further, the outcomes appear to be as good or better with GCLAM with mitoxantrone at 16 mg/m2 compared to other salvage regimens used at our institution, including decitabine priming plus mitoxantrone, etoposide, and cytarabine [d/MEC] and G-CSF with clofarabine and high-dose cytarabine [GCLAC],” she said, noting that the examination is currently ongoing in a larger sample.
The initial analysis, however, showed that, after controlling for age, cytogenetic risk, first CR duration, and prior hematopoietic cell transplant, overall response and overall survival rates were better with GCLAM than with d/MEC (OR, 3.23; HR for death, 0.64) and that the overall response rate was similar between GCLAM and GCLAC (OR, 1.75), she said.
The findings are encouraging because outcomes with standard chemotherapies for relapsed/refractory myeloid neoplasms are poor, with complete remission rates rarely exceeding 15%-20%, Dr. Halpern said.
The current study was undertaken based on promising results from a previous phase 2 study in poor-risk relapsed/refractory AML, which also showed encouraging activity with GCLAM and based on data suggesting benefit with escalated doses of anthracyclines in AML, she explained.
Patients 18 years and older were eligible if they had adequate organ function and a TRM score of 6.9 or lower, which corresponds to a predicted 28-day mortality of no more than 6.9% with standard induction chemotherapy. Those with uncontrolled infection or concomitant illness with expected survival of less than 1 year were excluded.
The phase 1 dose escalation involved cohorts of 6-12 patients who were assigned to receive mitoxantrone dose levels of 12, 14, 16, or 18 mg/m2 per day on days 1-3. The doses of the remaining drugs in the combination were fixed at 300 mcg or 480 mcg of G-CSF on days 0-5, 5 mg/m2 of cladribine on days 1-5, and 2 mg/m2 of cytarabine on days 1-5.
“All patients received GCLAM induction at their assigned mitoxantrone dose level. If CR wasn’t achieved with cycle 1, a second identical course of GCLAM was given,” Dr. Halpern explained, noting that patients with resistant disease after 2 cycles were taken off the study.
If CR or CRi was achieved within 1-2 cycles of induction, up to 4 cycles of consolidation with G-CLA (mitoxantrone omitted) were allowed, and responders could proceed with transplant at any time.
In phase 2, patients received the maximum tolerated dose of mitoxantrone (16mg/m2 per day), as defined in phase 1.
“Relapsed and refractory AML and high grade myeloid neoplasms are a challenging disease to treat. With an overall response rate of 60%, this regimen showed efficacy in a heavily pretreated patient population,” Dr. Halpern said. “And many of the responders were able to go on to receive a stem cell transplant, the only known curative option in this situation.”
A follow-up study is currently exploring the relative value of decitabine priming followed by GCLAM in this setting, she said.
Dr. Halpern reported having no relevant financial disclosures.
SOURCE: Halpern AB et al. ASH 2017, Abstract 149
ATLANTA – GCLAM – the combined use of granulocyte colony-stimulating factor (G-CSF), cladribine, high-dose cytarabine, and mitoxantrone – was well tolerated and had potent antileukemia activity in a phase 1/2 trial of adults with relapsed/refractory acute myeloid leukemia or high-grade myeloid neoplasms.
Of 40 patients who were treated with GCLAM (with mitoxantrone at the maximum tolerated dose of 16 mg/m2 per day as established in phase 1 of the trial), 11 achieved a complete response (CR), and 13 achieved a complete response with incomplete blood count recovery (CRi), for an overall response rate of 60%, Anna B. Halpern, MD, reported at the annual meeting of the American Society of Hematology.
“Nine of the 11 CR patients and 11 of 13 with CRis were negative for minimal residual disease, for an overall MRD-negative CR rate of 23%,” said Dr. Halpern of the University of Washington and the Fred Hutchinson Cancer Research Center, both in Seattle.
Resistant disease occurred in 11 patients, she noted.
Median overall survival was 11.5 months, and the treatment-related mortality (TRM) rate was 5%.
Overall, 21 of 40 patients went to transplant, with a 49% 1-year survival rate, she said.
The patients had a median age of 63 years. Thirty-four had acute myeloid leukemia (AML), and 6 had high-grade myelodysplastic syndrome; 28 had secondary disease. Nineteen had primary refractory disease, 21 had relapsed disease after a median initial CR duration of 12 months, and 7 had prior allogeneic transplant. The median TRM score for all patients was 2.0, indicating a low risk for treatment-related mortality.
“Cytogenetics distribution, based on Medical Research Council criteria, was as expected,” she added.
At the mitoxantrone maximum tolerated dose of 16 mg/m2 per day – which was established during phase 1 in 26 patients in whom higher dose levels (18 mg/m2 per day) led to dose-limiting encephalopathy and cardiogenic shock – the most common grade 3 or greater adverse events included neutropenic fever, infectious complications, and hypoxia during therapy. This was largely attributed to volume overload and infection, Dr. Halpern said.
“Although three patients did have decreased ejection fraction in cycle 2, this was largely in the setting of sepsis, making the etiology difficult to ascribe to the anthracycline versus sepsis physiology,” she noted.
The median times to an absolute neutrophil count of 500/mcL or greater and platelet count of 50,000/mcL or greater were 29 days each, she noted.
A multivariable analysis controlling for baseline prognostic features showed that the mitoxantrone dose of 16 mg/m2 per day was associated with significantly better overall survival, compared with a dose of 10 mg/m2 per day used in a historical cohort according to standard GCLAM dosing (hazard ratio for death, 0.45). Additionally, more of those receiving a dose of 16 mg/m2 per day went on to transplant (52% vs. 37%), she said. The overall response rate was also higher with the 16-mg/m2 dose, but the difference was not statistically significant (odds ratio, 1.87).
“Further, the outcomes appear to be as good or better with GCLAM with mitoxantrone at 16 mg/m2 compared to other salvage regimens used at our institution, including decitabine priming plus mitoxantrone, etoposide, and cytarabine [d/MEC] and G-CSF with clofarabine and high-dose cytarabine [GCLAC],” she said, noting that the examination is currently ongoing in a larger sample.
The initial analysis, however, showed that, after controlling for age, cytogenetic risk, first CR duration, and prior hematopoietic cell transplant, overall response and overall survival rates were better with GCLAM than with d/MEC (OR, 3.23; HR for death, 0.64) and that the overall response rate was similar between GCLAM and GCLAC (OR, 1.75), she said.
The findings are encouraging because outcomes with standard chemotherapies for relapsed/refractory myeloid neoplasms are poor, with complete remission rates rarely exceeding 15%-20%, Dr. Halpern said.
The current study was undertaken based on promising results from a previous phase 2 study in poor-risk relapsed/refractory AML, which also showed encouraging activity with GCLAM and based on data suggesting benefit with escalated doses of anthracyclines in AML, she explained.
Patients 18 years and older were eligible if they had adequate organ function and a TRM score of 6.9 or lower, which corresponds to a predicted 28-day mortality of no more than 6.9% with standard induction chemotherapy. Those with uncontrolled infection or concomitant illness with expected survival of less than 1 year were excluded.
The phase 1 dose escalation involved cohorts of 6-12 patients who were assigned to receive mitoxantrone dose levels of 12, 14, 16, or 18 mg/m2 per day on days 1-3. The doses of the remaining drugs in the combination were fixed at 300 mcg or 480 mcg of G-CSF on days 0-5, 5 mg/m2 of cladribine on days 1-5, and 2 mg/m2 of cytarabine on days 1-5.
“All patients received GCLAM induction at their assigned mitoxantrone dose level. If CR wasn’t achieved with cycle 1, a second identical course of GCLAM was given,” Dr. Halpern explained, noting that patients with resistant disease after 2 cycles were taken off the study.
If CR or CRi was achieved within 1-2 cycles of induction, up to 4 cycles of consolidation with G-CLA (mitoxantrone omitted) were allowed, and responders could proceed with transplant at any time.
In phase 2, patients received the maximum tolerated dose of mitoxantrone (16mg/m2 per day), as defined in phase 1.
“Relapsed and refractory AML and high grade myeloid neoplasms are a challenging disease to treat. With an overall response rate of 60%, this regimen showed efficacy in a heavily pretreated patient population,” Dr. Halpern said. “And many of the responders were able to go on to receive a stem cell transplant, the only known curative option in this situation.”
A follow-up study is currently exploring the relative value of decitabine priming followed by GCLAM in this setting, she said.
Dr. Halpern reported having no relevant financial disclosures.
SOURCE: Halpern AB et al. ASH 2017, Abstract 149
ATLANTA – GCLAM – the combined use of granulocyte colony-stimulating factor (G-CSF), cladribine, high-dose cytarabine, and mitoxantrone – was well tolerated and had potent antileukemia activity in a phase 1/2 trial of adults with relapsed/refractory acute myeloid leukemia or high-grade myeloid neoplasms.
Of 40 patients who were treated with GCLAM (with mitoxantrone at the maximum tolerated dose of 16 mg/m2 per day as established in phase 1 of the trial), 11 achieved a complete response (CR), and 13 achieved a complete response with incomplete blood count recovery (CRi), for an overall response rate of 60%, Anna B. Halpern, MD, reported at the annual meeting of the American Society of Hematology.
“Nine of the 11 CR patients and 11 of 13 with CRis were negative for minimal residual disease, for an overall MRD-negative CR rate of 23%,” said Dr. Halpern of the University of Washington and the Fred Hutchinson Cancer Research Center, both in Seattle.
Resistant disease occurred in 11 patients, she noted.
Median overall survival was 11.5 months, and the treatment-related mortality (TRM) rate was 5%.
Overall, 21 of 40 patients went to transplant, with a 49% 1-year survival rate, she said.
The patients had a median age of 63 years. Thirty-four had acute myeloid leukemia (AML), and 6 had high-grade myelodysplastic syndrome; 28 had secondary disease. Nineteen had primary refractory disease, 21 had relapsed disease after a median initial CR duration of 12 months, and 7 had prior allogeneic transplant. The median TRM score for all patients was 2.0, indicating a low risk for treatment-related mortality.
“Cytogenetics distribution, based on Medical Research Council criteria, was as expected,” she added.
At the mitoxantrone maximum tolerated dose of 16 mg/m2 per day – which was established during phase 1 in 26 patients in whom higher dose levels (18 mg/m2 per day) led to dose-limiting encephalopathy and cardiogenic shock – the most common grade 3 or greater adverse events included neutropenic fever, infectious complications, and hypoxia during therapy. This was largely attributed to volume overload and infection, Dr. Halpern said.
“Although three patients did have decreased ejection fraction in cycle 2, this was largely in the setting of sepsis, making the etiology difficult to ascribe to the anthracycline versus sepsis physiology,” she noted.
The median times to an absolute neutrophil count of 500/mcL or greater and platelet count of 50,000/mcL or greater were 29 days each, she noted.
A multivariable analysis controlling for baseline prognostic features showed that the mitoxantrone dose of 16 mg/m2 per day was associated with significantly better overall survival, compared with a dose of 10 mg/m2 per day used in a historical cohort according to standard GCLAM dosing (hazard ratio for death, 0.45). Additionally, more of those receiving a dose of 16 mg/m2 per day went on to transplant (52% vs. 37%), she said. The overall response rate was also higher with the 16-mg/m2 dose, but the difference was not statistically significant (odds ratio, 1.87).
“Further, the outcomes appear to be as good or better with GCLAM with mitoxantrone at 16 mg/m2 compared to other salvage regimens used at our institution, including decitabine priming plus mitoxantrone, etoposide, and cytarabine [d/MEC] and G-CSF with clofarabine and high-dose cytarabine [GCLAC],” she said, noting that the examination is currently ongoing in a larger sample.
The initial analysis, however, showed that, after controlling for age, cytogenetic risk, first CR duration, and prior hematopoietic cell transplant, overall response and overall survival rates were better with GCLAM than with d/MEC (OR, 3.23; HR for death, 0.64) and that the overall response rate was similar between GCLAM and GCLAC (OR, 1.75), she said.
The findings are encouraging because outcomes with standard chemotherapies for relapsed/refractory myeloid neoplasms are poor, with complete remission rates rarely exceeding 15%-20%, Dr. Halpern said.
The current study was undertaken based on promising results from a previous phase 2 study in poor-risk relapsed/refractory AML, which also showed encouraging activity with GCLAM and based on data suggesting benefit with escalated doses of anthracyclines in AML, she explained.
Patients 18 years and older were eligible if they had adequate organ function and a TRM score of 6.9 or lower, which corresponds to a predicted 28-day mortality of no more than 6.9% with standard induction chemotherapy. Those with uncontrolled infection or concomitant illness with expected survival of less than 1 year were excluded.
The phase 1 dose escalation involved cohorts of 6-12 patients who were assigned to receive mitoxantrone dose levels of 12, 14, 16, or 18 mg/m2 per day on days 1-3. The doses of the remaining drugs in the combination were fixed at 300 mcg or 480 mcg of G-CSF on days 0-5, 5 mg/m2 of cladribine on days 1-5, and 2 mg/m2 of cytarabine on days 1-5.
“All patients received GCLAM induction at their assigned mitoxantrone dose level. If CR wasn’t achieved with cycle 1, a second identical course of GCLAM was given,” Dr. Halpern explained, noting that patients with resistant disease after 2 cycles were taken off the study.
If CR or CRi was achieved within 1-2 cycles of induction, up to 4 cycles of consolidation with G-CLA (mitoxantrone omitted) were allowed, and responders could proceed with transplant at any time.
In phase 2, patients received the maximum tolerated dose of mitoxantrone (16mg/m2 per day), as defined in phase 1.
“Relapsed and refractory AML and high grade myeloid neoplasms are a challenging disease to treat. With an overall response rate of 60%, this regimen showed efficacy in a heavily pretreated patient population,” Dr. Halpern said. “And many of the responders were able to go on to receive a stem cell transplant, the only known curative option in this situation.”
A follow-up study is currently exploring the relative value of decitabine priming followed by GCLAM in this setting, she said.
Dr. Halpern reported having no relevant financial disclosures.
SOURCE: Halpern AB et al. ASH 2017, Abstract 149
REPORTING FROM ASH 2017
Key clinical point: GCLAM was well tolerated and had potent antileukemia activity in a phase 1/2 trial.
Major finding: The overall response rate was 60%.
Study details: A phase 1/2 study of 40 patients.
Disclosures: Dr. Halpern reported having no financial disclosures.
Source: Halpern AB et al. ASH 2017, Abstract 149.