User login
Adding hypertension in pregnancy doesn’t refine ASCVD risk prediction tool
ANAHEIM, CALIF. – The first-ever study to examine the clinical utility of incorporating a history of hypertensive disorders of pregnancy into the American College of Cardiology/American Heart Association Atherosclerotic Cardiovascular Disease Risk Calculator in an effort to better delineate risk in women failed to show any added benefit.
But that doesn’t mean such a history is without value in daily clinical practice, Jennifer J. Stuart, ScD, asserted at the American Heart Association scientific sessions.
“While we did not demonstrate that hypertensive disorders of pregnancy improved cardiovascular risk discrimination in women, we do still believe – and I still believe – that hypertensive disorders of pregnancy remain an important risk marker for cardiovascular disease in women,” said Dr. Stuart of the Harvard School of Public Health, Boston.
“In this latest analysis we’ve demonstrated over a fourfold increased risk of chronic hypertension in these women in the first 5 years after delivery. So we do see increased risk very soon after delivery, and it persists for decades. And hypertensive disorders of pregnancy as a risk marker does offer practical advantages: ease of ascertainment, low cost, and availability earlier in life,” the researcher noted.
Her study hypothesis was that adding hypertensive disorders of pregnancy – that is, gestational hypertension and preeclampsia – and parity to the current ACC/AHA ASCVD Risk Calculator would enhance the tool’s predictive tool’s power in women.
“We wanted to know if a history of hypertensive disorders of pregnancy can be leveraged to capture women currently being missed in screening,” she explained.
Her expectation that this would be the case was based on solid evidence that hypertensive disorders of pregnancy, which occur in 10%-15% of all pregnancies, are associated with subsequent increased risk of cardiovascular events. For example, a meta-analysis of published studies involving nearly 3.5 million women, including almost 200,000 with a history of preeclampsia, showed that preeclampsia was associated with a 2.16-fold increased risk of ischemic heart disease during 11.7 years of follow-up (BMJ. 2007 Nov 10;335[7627]:974). This meta-analysis was among the evidence that persuaded the AHA in 2011 to formally add hypertensive disorders of pregnancy to the list of risk factors for cardiovascular disease in women (Circulation. 2011 Mar 22;123[11]:1243-62).
Dr. Stuart also incorporated parity in her investigational revised ASCVD risk model, because parity has been shown to be an independent risk factor of cardiovascular disease in a relationship described by a J-shaped curve.
She put the souped-up risk prediction model to the test by separately applying it and the current version to data from the longitudinal prospective Nurses’ Health Study II. For this analysis, nearly 68,000 female registered nurses were followed for new diseases every 2 years from 1989, when they were aged 25-42, through 2013. She and her coinvestigators applied the two 10-year risk-prediction models to the overall cohort and again separately to women at aged 40-49 and 50-59.
The bottom line: This might be because the nurses comprise a relatively low-risk population. Also, even though hypertensive disorders of pregnancy are associated with 10-year cardiovascular disease risk independent of the established risk factors, there may be enough overlap that the standard risk factors sufficiently capture the risk.
“It may be that the information on history of hypertensive disorders of pregnancy should be ascertained in the 20s and 30s, rather than waiting until age 40 or later, when we generally apply cardiovascular risk scores in practice. That [earlier assessment] may be a really important and valuable opportunity to identify these women at high risk and utilize primary prevention,” according to Dr. Stuart.
What’s next? “I’m certainly interested in educating these women about their increased risk. This is not something that’s done consistently across the country and across practices, and we really believe this is an important message for women to get when they deliver these pregnancies,” she added.
Dr. Stuart reported having no financial conflicts of interest regarding her study.
SOURCE: Stuart JJ. AHA Scientific Sessions.
ANAHEIM, CALIF. – The first-ever study to examine the clinical utility of incorporating a history of hypertensive disorders of pregnancy into the American College of Cardiology/American Heart Association Atherosclerotic Cardiovascular Disease Risk Calculator in an effort to better delineate risk in women failed to show any added benefit.
But that doesn’t mean such a history is without value in daily clinical practice, Jennifer J. Stuart, ScD, asserted at the American Heart Association scientific sessions.
“While we did not demonstrate that hypertensive disorders of pregnancy improved cardiovascular risk discrimination in women, we do still believe – and I still believe – that hypertensive disorders of pregnancy remain an important risk marker for cardiovascular disease in women,” said Dr. Stuart of the Harvard School of Public Health, Boston.
“In this latest analysis we’ve demonstrated over a fourfold increased risk of chronic hypertension in these women in the first 5 years after delivery. So we do see increased risk very soon after delivery, and it persists for decades. And hypertensive disorders of pregnancy as a risk marker does offer practical advantages: ease of ascertainment, low cost, and availability earlier in life,” the researcher noted.
Her study hypothesis was that adding hypertensive disorders of pregnancy – that is, gestational hypertension and preeclampsia – and parity to the current ACC/AHA ASCVD Risk Calculator would enhance the tool’s predictive tool’s power in women.
“We wanted to know if a history of hypertensive disorders of pregnancy can be leveraged to capture women currently being missed in screening,” she explained.
Her expectation that this would be the case was based on solid evidence that hypertensive disorders of pregnancy, which occur in 10%-15% of all pregnancies, are associated with subsequent increased risk of cardiovascular events. For example, a meta-analysis of published studies involving nearly 3.5 million women, including almost 200,000 with a history of preeclampsia, showed that preeclampsia was associated with a 2.16-fold increased risk of ischemic heart disease during 11.7 years of follow-up (BMJ. 2007 Nov 10;335[7627]:974). This meta-analysis was among the evidence that persuaded the AHA in 2011 to formally add hypertensive disorders of pregnancy to the list of risk factors for cardiovascular disease in women (Circulation. 2011 Mar 22;123[11]:1243-62).
Dr. Stuart also incorporated parity in her investigational revised ASCVD risk model, because parity has been shown to be an independent risk factor of cardiovascular disease in a relationship described by a J-shaped curve.
She put the souped-up risk prediction model to the test by separately applying it and the current version to data from the longitudinal prospective Nurses’ Health Study II. For this analysis, nearly 68,000 female registered nurses were followed for new diseases every 2 years from 1989, when they were aged 25-42, through 2013. She and her coinvestigators applied the two 10-year risk-prediction models to the overall cohort and again separately to women at aged 40-49 and 50-59.
The bottom line: This might be because the nurses comprise a relatively low-risk population. Also, even though hypertensive disorders of pregnancy are associated with 10-year cardiovascular disease risk independent of the established risk factors, there may be enough overlap that the standard risk factors sufficiently capture the risk.
“It may be that the information on history of hypertensive disorders of pregnancy should be ascertained in the 20s and 30s, rather than waiting until age 40 or later, when we generally apply cardiovascular risk scores in practice. That [earlier assessment] may be a really important and valuable opportunity to identify these women at high risk and utilize primary prevention,” according to Dr. Stuart.
What’s next? “I’m certainly interested in educating these women about their increased risk. This is not something that’s done consistently across the country and across practices, and we really believe this is an important message for women to get when they deliver these pregnancies,” she added.
Dr. Stuart reported having no financial conflicts of interest regarding her study.
SOURCE: Stuart JJ. AHA Scientific Sessions.
ANAHEIM, CALIF. – The first-ever study to examine the clinical utility of incorporating a history of hypertensive disorders of pregnancy into the American College of Cardiology/American Heart Association Atherosclerotic Cardiovascular Disease Risk Calculator in an effort to better delineate risk in women failed to show any added benefit.
But that doesn’t mean such a history is without value in daily clinical practice, Jennifer J. Stuart, ScD, asserted at the American Heart Association scientific sessions.
“While we did not demonstrate that hypertensive disorders of pregnancy improved cardiovascular risk discrimination in women, we do still believe – and I still believe – that hypertensive disorders of pregnancy remain an important risk marker for cardiovascular disease in women,” said Dr. Stuart of the Harvard School of Public Health, Boston.
“In this latest analysis we’ve demonstrated over a fourfold increased risk of chronic hypertension in these women in the first 5 years after delivery. So we do see increased risk very soon after delivery, and it persists for decades. And hypertensive disorders of pregnancy as a risk marker does offer practical advantages: ease of ascertainment, low cost, and availability earlier in life,” the researcher noted.
Her study hypothesis was that adding hypertensive disorders of pregnancy – that is, gestational hypertension and preeclampsia – and parity to the current ACC/AHA ASCVD Risk Calculator would enhance the tool’s predictive tool’s power in women.
“We wanted to know if a history of hypertensive disorders of pregnancy can be leveraged to capture women currently being missed in screening,” she explained.
Her expectation that this would be the case was based on solid evidence that hypertensive disorders of pregnancy, which occur in 10%-15% of all pregnancies, are associated with subsequent increased risk of cardiovascular events. For example, a meta-analysis of published studies involving nearly 3.5 million women, including almost 200,000 with a history of preeclampsia, showed that preeclampsia was associated with a 2.16-fold increased risk of ischemic heart disease during 11.7 years of follow-up (BMJ. 2007 Nov 10;335[7627]:974). This meta-analysis was among the evidence that persuaded the AHA in 2011 to formally add hypertensive disorders of pregnancy to the list of risk factors for cardiovascular disease in women (Circulation. 2011 Mar 22;123[11]:1243-62).
Dr. Stuart also incorporated parity in her investigational revised ASCVD risk model, because parity has been shown to be an independent risk factor of cardiovascular disease in a relationship described by a J-shaped curve.
She put the souped-up risk prediction model to the test by separately applying it and the current version to data from the longitudinal prospective Nurses’ Health Study II. For this analysis, nearly 68,000 female registered nurses were followed for new diseases every 2 years from 1989, when they were aged 25-42, through 2013. She and her coinvestigators applied the two 10-year risk-prediction models to the overall cohort and again separately to women at aged 40-49 and 50-59.
The bottom line: This might be because the nurses comprise a relatively low-risk population. Also, even though hypertensive disorders of pregnancy are associated with 10-year cardiovascular disease risk independent of the established risk factors, there may be enough overlap that the standard risk factors sufficiently capture the risk.
“It may be that the information on history of hypertensive disorders of pregnancy should be ascertained in the 20s and 30s, rather than waiting until age 40 or later, when we generally apply cardiovascular risk scores in practice. That [earlier assessment] may be a really important and valuable opportunity to identify these women at high risk and utilize primary prevention,” according to Dr. Stuart.
What’s next? “I’m certainly interested in educating these women about their increased risk. This is not something that’s done consistently across the country and across practices, and we really believe this is an important message for women to get when they deliver these pregnancies,” she added.
Dr. Stuart reported having no financial conflicts of interest regarding her study.
SOURCE: Stuart JJ. AHA Scientific Sessions.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: The ACC/AHA Atherosclerotic Cardiovascular Disease Risk Calculator doesn’t do a better job of predicting 10-year risk in women when a history of hypertensive disorders of pregnancy is added into the formula.
Major finding: Incorporating a woman’s history of hypertensive disorders of pregnancy into the Atherosclerotic Cardiovascular Disease Risk Calculator doesn’t improve the tool’s accuracy in predicting 10-year risk.
Study details: An analysis of data from the longitudinal, prospective Nurses’ Health Study II.
Disclosures: The study presenter reported having no financial conflicts of interest.
Source: Stuart JJ. AHA Scientific Sessions.
Study finds AD accounts for hundreds of thousands of annual ED visits
SAN DIEGO – A new study finds that primary diagnoses of atopic dermatitis (AD) are made hundreds of thousands of times in United States emergency departments each year.
The numbers appear to be rising along with costs, researchers reported, and there are signs of disparities, with poorer people more likely to have an ED visit with a primary diagnosis of AD. The study was presented in a poster at the annual meeting of the American Academy of Dermatology.
He and his coauthor, Lauren Kwa, also with the department of dermatology at Northwestern, conducted the analysis to better understand the role of AD in emergency care. “Many AD patients experience severe, unpredictable flares and worsening chronic disease that warrant urgent treatment,” Dr. Silverberg said. “However, patients typically don’t have instant access to outpatient dermatological care and may be forced to turn to the urgent care setting.”
Indeed, he noted, “previous U.S. population–based studies showed that people with AD have higher odds of [ED] utilization than the rest of the population.”
He and Ms. Kwa examined 2006-2012 data from the Nationwide Emergency Department Sample, which includes information on about 20% of all emergency visits in the United States.
During that period, there were 1.86 million ED visits with a primary diagnosis of AD. The annual weighted prevalence of primary diagnoses of AD stayed fairly stable through the period, ranging from 2,589 to 2,769 per 1 million visits. However, the weighted prevalence of secondary AD diagnoses grew steadily from 1,227 per 1 million visits in 2006 to 1,533 per 1 million visits in 2012.
The researchers estimated that the total cost of annual costs of AD-related ED visits grew from $86.9 million in 2006 to $172.8 million in 2012 (P less than .05).
The study also linked primary diagnoses of AD to having Medicaid insurance or being uninsured, being poorer, or visiting hospitals in “micropolitan” areas (small urban communities such as Bozeman, Mont., and Durango, Colo.).
The study did not examine what medications were prescribed in the ED. However, Dr. Silverberg said, “my anecdotal experience has been that many AD patients are prescribed systemic steroids by nondermatologists in the [ED] setting. While these are rapidly effective, they typically have short-lived efficacy and result in rebound flares upon cessation. Patients are rarely counseled on appropriate skin care techniques or given long-term treatment approaches in the [ED] setting, which fails to achieve adequate long-term disease control.”
What’s next? “We are now studying how AD severity, disease course, and treatment impact [ED] utilization for AD,” Dr. Silverberg said.
No specific study funding was reported. He and Ms. Kwa report no relevant disclosures.
SOURCE: Silverberg, J et al. Poster 7021.
SAN DIEGO – A new study finds that primary diagnoses of atopic dermatitis (AD) are made hundreds of thousands of times in United States emergency departments each year.
The numbers appear to be rising along with costs, researchers reported, and there are signs of disparities, with poorer people more likely to have an ED visit with a primary diagnosis of AD. The study was presented in a poster at the annual meeting of the American Academy of Dermatology.
He and his coauthor, Lauren Kwa, also with the department of dermatology at Northwestern, conducted the analysis to better understand the role of AD in emergency care. “Many AD patients experience severe, unpredictable flares and worsening chronic disease that warrant urgent treatment,” Dr. Silverberg said. “However, patients typically don’t have instant access to outpatient dermatological care and may be forced to turn to the urgent care setting.”
Indeed, he noted, “previous U.S. population–based studies showed that people with AD have higher odds of [ED] utilization than the rest of the population.”
He and Ms. Kwa examined 2006-2012 data from the Nationwide Emergency Department Sample, which includes information on about 20% of all emergency visits in the United States.
During that period, there were 1.86 million ED visits with a primary diagnosis of AD. The annual weighted prevalence of primary diagnoses of AD stayed fairly stable through the period, ranging from 2,589 to 2,769 per 1 million visits. However, the weighted prevalence of secondary AD diagnoses grew steadily from 1,227 per 1 million visits in 2006 to 1,533 per 1 million visits in 2012.
The researchers estimated that the total cost of annual costs of AD-related ED visits grew from $86.9 million in 2006 to $172.8 million in 2012 (P less than .05).
The study also linked primary diagnoses of AD to having Medicaid insurance or being uninsured, being poorer, or visiting hospitals in “micropolitan” areas (small urban communities such as Bozeman, Mont., and Durango, Colo.).
The study did not examine what medications were prescribed in the ED. However, Dr. Silverberg said, “my anecdotal experience has been that many AD patients are prescribed systemic steroids by nondermatologists in the [ED] setting. While these are rapidly effective, they typically have short-lived efficacy and result in rebound flares upon cessation. Patients are rarely counseled on appropriate skin care techniques or given long-term treatment approaches in the [ED] setting, which fails to achieve adequate long-term disease control.”
What’s next? “We are now studying how AD severity, disease course, and treatment impact [ED] utilization for AD,” Dr. Silverberg said.
No specific study funding was reported. He and Ms. Kwa report no relevant disclosures.
SOURCE: Silverberg, J et al. Poster 7021.
SAN DIEGO – A new study finds that primary diagnoses of atopic dermatitis (AD) are made hundreds of thousands of times in United States emergency departments each year.
The numbers appear to be rising along with costs, researchers reported, and there are signs of disparities, with poorer people more likely to have an ED visit with a primary diagnosis of AD. The study was presented in a poster at the annual meeting of the American Academy of Dermatology.
He and his coauthor, Lauren Kwa, also with the department of dermatology at Northwestern, conducted the analysis to better understand the role of AD in emergency care. “Many AD patients experience severe, unpredictable flares and worsening chronic disease that warrant urgent treatment,” Dr. Silverberg said. “However, patients typically don’t have instant access to outpatient dermatological care and may be forced to turn to the urgent care setting.”
Indeed, he noted, “previous U.S. population–based studies showed that people with AD have higher odds of [ED] utilization than the rest of the population.”
He and Ms. Kwa examined 2006-2012 data from the Nationwide Emergency Department Sample, which includes information on about 20% of all emergency visits in the United States.
During that period, there were 1.86 million ED visits with a primary diagnosis of AD. The annual weighted prevalence of primary diagnoses of AD stayed fairly stable through the period, ranging from 2,589 to 2,769 per 1 million visits. However, the weighted prevalence of secondary AD diagnoses grew steadily from 1,227 per 1 million visits in 2006 to 1,533 per 1 million visits in 2012.
The researchers estimated that the total cost of annual costs of AD-related ED visits grew from $86.9 million in 2006 to $172.8 million in 2012 (P less than .05).
The study also linked primary diagnoses of AD to having Medicaid insurance or being uninsured, being poorer, or visiting hospitals in “micropolitan” areas (small urban communities such as Bozeman, Mont., and Durango, Colo.).
The study did not examine what medications were prescribed in the ED. However, Dr. Silverberg said, “my anecdotal experience has been that many AD patients are prescribed systemic steroids by nondermatologists in the [ED] setting. While these are rapidly effective, they typically have short-lived efficacy and result in rebound flares upon cessation. Patients are rarely counseled on appropriate skin care techniques or given long-term treatment approaches in the [ED] setting, which fails to achieve adequate long-term disease control.”
What’s next? “We are now studying how AD severity, disease course, and treatment impact [ED] utilization for AD,” Dr. Silverberg said.
No specific study funding was reported. He and Ms. Kwa report no relevant disclosures.
SOURCE: Silverberg, J et al. Poster 7021.
REPORTING FROM AAD 18
Key clinical point: ED visits for atopic dermatitis are common, and their numbers are growing.
Major finding: An estimated 1.86 million ED visits in the United States from 2006 to 2012 were linked to a primary diagnosis of AD.
Study details: Analysis of data from the Nationwide Emergency Department Sample for 2006-2016.
Disclosures: No study funding was reported. The authors had no relevant disclosures.
Source: Silverberg J et al. Poster 7021.
Mast cell synovitis: potential target in RA?
MAUI, HAWAII – Could mast cell-rich synovitis become a novel target in patients with early rheumatoid arthritis?
Investigators at the University of London believe so. In a small but provocative study presented at the 2017 annual meeting of the American College of Rheumatology, they demonstrated that these mast cells are not an innocent bystander in joint inflammation; rather, they play an active albeit still incompletely understood role in the synovial inflammatory infiltrate and in disease progression, Orrin M. Troum, MD, said at the 2018 Rheumatology Winter Clinical Symposium.
Treatment with synthetic DMARDs was partially effective, both clinically and at the cellular level. Mean mast cell density fell from 14.2/mm2 at baseline to 8.2/mm2 at 6 months. But synovial mast cells were still present at 6 months in 9 of 20 patients. Those nine patients had significantly higher DAS28 scores – a mean of 4.08 versus 2.41 in the synovial mast cell–negative group – as well as higher synovitis scores. Disease remission as defined by a DAS28 score below 2.6 was achieved in only 2 of 9 patients with persistent mast cell-rich synovitis, as compared with 8 of 11 mast cell-negative patients.
Moreover, four of the nine patients with persistent mast cell-rich synovitis after 6 months of synthetic DMARDs had synovial lymphoid aggregates of CD20-positive B cells and/or CD138-positive plasma cells; in contrast, none of the 11 synovial mast cell-negative patients did, noted Dr. Troum, a rheumatologist at the University of Southern California, Los Angeles, who is in private practice in Santa Monica, Calif.
In theory, if a baseline synovial tissue immune cell profile could be identified that’s predictive of high-level responsiveness to synthetic DMARDs or, conversely, nonresponsiveness, tissue specimens could be used to stratify patients with early RA to different initial treatment strategies. The problem with that is few rheumatologists in the United States – and indeed worldwide – are proficient at performing ultrasound-guided synovial biopsies, according to Dr. Troum.
His description of the London study prompted a question as to whether any of the biologics used in rheumatology can inhibit mast cells. Arthur Kavanaugh, MD, symposium director, said the only agent that comes to mind is imatinib (Gleevec), a tyrosine kinase inhibitor used in the treatment of aggressive systemic mastocytosis as well as for certain leukemias.
“It has a ton of toxicity, though,” said Dr. Kavanaugh, professor of medicine and director of the Center for Innovative Therapy in the division of rheumatology, allergy, and immunology at the University of California, San Diego.
Dr. Troum reported having no financial conflicts regarding his presentation.
MAUI, HAWAII – Could mast cell-rich synovitis become a novel target in patients with early rheumatoid arthritis?
Investigators at the University of London believe so. In a small but provocative study presented at the 2017 annual meeting of the American College of Rheumatology, they demonstrated that these mast cells are not an innocent bystander in joint inflammation; rather, they play an active albeit still incompletely understood role in the synovial inflammatory infiltrate and in disease progression, Orrin M. Troum, MD, said at the 2018 Rheumatology Winter Clinical Symposium.
Treatment with synthetic DMARDs was partially effective, both clinically and at the cellular level. Mean mast cell density fell from 14.2/mm2 at baseline to 8.2/mm2 at 6 months. But synovial mast cells were still present at 6 months in 9 of 20 patients. Those nine patients had significantly higher DAS28 scores – a mean of 4.08 versus 2.41 in the synovial mast cell–negative group – as well as higher synovitis scores. Disease remission as defined by a DAS28 score below 2.6 was achieved in only 2 of 9 patients with persistent mast cell-rich synovitis, as compared with 8 of 11 mast cell-negative patients.
Moreover, four of the nine patients with persistent mast cell-rich synovitis after 6 months of synthetic DMARDs had synovial lymphoid aggregates of CD20-positive B cells and/or CD138-positive plasma cells; in contrast, none of the 11 synovial mast cell-negative patients did, noted Dr. Troum, a rheumatologist at the University of Southern California, Los Angeles, who is in private practice in Santa Monica, Calif.
In theory, if a baseline synovial tissue immune cell profile could be identified that’s predictive of high-level responsiveness to synthetic DMARDs or, conversely, nonresponsiveness, tissue specimens could be used to stratify patients with early RA to different initial treatment strategies. The problem with that is few rheumatologists in the United States – and indeed worldwide – are proficient at performing ultrasound-guided synovial biopsies, according to Dr. Troum.
His description of the London study prompted a question as to whether any of the biologics used in rheumatology can inhibit mast cells. Arthur Kavanaugh, MD, symposium director, said the only agent that comes to mind is imatinib (Gleevec), a tyrosine kinase inhibitor used in the treatment of aggressive systemic mastocytosis as well as for certain leukemias.
“It has a ton of toxicity, though,” said Dr. Kavanaugh, professor of medicine and director of the Center for Innovative Therapy in the division of rheumatology, allergy, and immunology at the University of California, San Diego.
Dr. Troum reported having no financial conflicts regarding his presentation.
MAUI, HAWAII – Could mast cell-rich synovitis become a novel target in patients with early rheumatoid arthritis?
Investigators at the University of London believe so. In a small but provocative study presented at the 2017 annual meeting of the American College of Rheumatology, they demonstrated that these mast cells are not an innocent bystander in joint inflammation; rather, they play an active albeit still incompletely understood role in the synovial inflammatory infiltrate and in disease progression, Orrin M. Troum, MD, said at the 2018 Rheumatology Winter Clinical Symposium.
Treatment with synthetic DMARDs was partially effective, both clinically and at the cellular level. Mean mast cell density fell from 14.2/mm2 at baseline to 8.2/mm2 at 6 months. But synovial mast cells were still present at 6 months in 9 of 20 patients. Those nine patients had significantly higher DAS28 scores – a mean of 4.08 versus 2.41 in the synovial mast cell–negative group – as well as higher synovitis scores. Disease remission as defined by a DAS28 score below 2.6 was achieved in only 2 of 9 patients with persistent mast cell-rich synovitis, as compared with 8 of 11 mast cell-negative patients.
Moreover, four of the nine patients with persistent mast cell-rich synovitis after 6 months of synthetic DMARDs had synovial lymphoid aggregates of CD20-positive B cells and/or CD138-positive plasma cells; in contrast, none of the 11 synovial mast cell-negative patients did, noted Dr. Troum, a rheumatologist at the University of Southern California, Los Angeles, who is in private practice in Santa Monica, Calif.
In theory, if a baseline synovial tissue immune cell profile could be identified that’s predictive of high-level responsiveness to synthetic DMARDs or, conversely, nonresponsiveness, tissue specimens could be used to stratify patients with early RA to different initial treatment strategies. The problem with that is few rheumatologists in the United States – and indeed worldwide – are proficient at performing ultrasound-guided synovial biopsies, according to Dr. Troum.
His description of the London study prompted a question as to whether any of the biologics used in rheumatology can inhibit mast cells. Arthur Kavanaugh, MD, symposium director, said the only agent that comes to mind is imatinib (Gleevec), a tyrosine kinase inhibitor used in the treatment of aggressive systemic mastocytosis as well as for certain leukemias.
“It has a ton of toxicity, though,” said Dr. Kavanaugh, professor of medicine and director of the Center for Innovative Therapy in the division of rheumatology, allergy, and immunology at the University of California, San Diego.
Dr. Troum reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM RWCS 2018
Collaboration, consultation part of AAP teen depression guidelines update
The updated information includes recommendations on collaborative care, practice preparation, establishing networks of referrals, and much more.
“These guidelines were developed for PC clinicians who are in a position to identify and assist youth with depression in their practice settings,” they said. The guidelines apply to individuals aged 10-21 years, and support universal depression screening for those aged 12 and older.
Known as the Guidelines for Adolescent Depression in Primary Care (GLAD-PC), they consist of two parts: Practice Preparation, Identification, Assessment, and Initial Management, with Dr. Zuckerbrot as the lead author, and Treatment and Ongoing Management, led by Amy H. Cheung, MD, of the University of Toronto. They were published online in Pediatrics.
“It has been over 10 years since the [last] guidelines were published and they are supposed to be updated every 5,” Dr. Zuckerbrot said in an interview. “Given the new evidence on screening, psychopharmacology, and collaborative care, the guidelines needed to be revised. The USPSTF [United States Preventive Services Task Force ] and the AAP had already supported universal adolescent depression screening, and these guidelines are finally aligned with those positions.
“Different parts of the guidelines will be the go-to for different pediatricians, depending on where they are in their delivery of mental health care,” she explained. “Some may need help with practice preparation while others may need advice on screening; others may already be prescribing and may need advice on ongoing treatment and follow-up. I think there is something for everyone.”
Implementation of the guidelines is difficult in a short visit, Dr. Zuckerbrot acknowledged. “In addition, pediatricians may not have been well trained in the management of adolescent depression during their residencies.” However, the guidelines discuss both “real teams to support the pediatricians in their efforts, as well as virtual teams when staffing is limited.
“The guidelines advise that pediatricians learn about child psychiatry primary care consultation programs in their state and make use of those free telephone consultation programs.” The guidelines also discuss strategies for collaborative or integrative care, she said.
Part I
Part I of the guidelines, “Practice Preparation, Identification, Assessment, and Initial Management,” includes several recommendations for each topic.
For practice preparation, the guidelines recommend that clinicians seek training in the assessment, diagnosis, and treatment of depression, and that they establish a network of referrals and mental health resources in their communities. This network may include not only health professionals, but also current patients and families who are managing teen depression. If available, state-wide or regional child and adolescent psychiatry consultation programs can be included.
The identification and surveillance section of the guidelines calls for screening all patients aged 12 years and older for depression each year, using a formal screening tool on paper or online. The screening could occur at an annual wellness visit or any other medical visit, such as a sports physical. A second recommendation calls for identifying patients at increased risk for depression because of factors such as personal history, family history, substance use, other psychiatric disorders, frequent somatic complaints, or trauma, and monitoring these individuals regularly for signs of depression using a formal screening tool.
The assessment and diagnosis section states that assessment should include interviews with the patients alone as well as with their families or caregivers, and should include screening teens for functional impairment.
Primary care physicians should evaluate for depression not only if an adolescent tests positive on a screening tool, but also in children who present with any emotional problem as the chief complaint, and in those in whom depression is highly suspected even if they test negative on a formal screening tool, the guidelines state.
The three recommendations for initial management of depression in the primary care setting are educating patients and families about depression; developing a treatment plan (if the primary care clinician has had appropriate training) and setting specific treatment goals in areas of functioning such as at home, with peers, and at school; and developing a safety plan that includes restricting access to weapons or other means of self-harm, according to the guidelines.
Part II
Part II of the recommendations, “Treatment and Ongoing Management,” discusses options for managing depression in the primary care setting and utilizing outside resources.
The treatment recommendations emphasize the use of integrated models, if possible. “There is a growing recognition that complex chronic conditions, such as depression, are most successfully managed with proactive, multidisciplinary, patient-centered care teams,” Dr. Cheung and her associates said.
The recommendation for cases of mild depression calls for a period of “active support and monitoring” for 6-8 weeks before reassessing if the teen shows no improvement. By contrast, for cases of moderate to severe depression or cases with evidence of substance abuse or other psychoses, the recommendation calls for potential consultation with a mental health specialist and a discussion of the roles primary and specialty care will play in treatment. The guidelines include a flow chart for PC physicians to follow.
The guidelines suggest that PC clinicians recommend “scientifically tested and proven treatments,” such as psychotherapies, cognitive behavioral therapy (CBT) or interpersonal psychotherapy for adolescents, and/or antidepressant treatment, such as SSRIs, whenever possible and appropriate. It is important to monitor teens on antidepressants regularly to identify adverse events.
Recommendations for the ongoing management of teens with depression in the primary care setting include regular tracking of progress, reassessment if the teen shows no improvement in 6-8 weeks, and consultation with a mental health professional for those who show only partial improvement after exhausting primary care diagnostic and treatment options. Assessment of depressive symptoms is not the only thing to track. Functioning at home, school, and among peers also is important.
The final treatment recommendation is for active support of a depressed teen’s referral to mental health if necessary for best management and sharing care if possible, with an understanding of the roles of the primary and specialty clinicians, the guidelines state.
The guidelines project was funded by the Resource for Advancing Children’s Health Institute and the Bell Canada Chair in Adolescent Mood and Anxiety Disorders.
Dr. Cheung and Dr. Zuckerbrot receive book royalties. Dr Zuckerbrot works for child and adolescent psychiatry for primary care (CAP-PC), now a regional provider for Project TEACH in New York State, and she is on the steering committee as well as faculty for the REACH Institute; both of these institutions are described in the guidelines. Peter S. Jensen, MD, has received royalties from Random House, Oxford University Press, and APPI Inc. He is a part owner of a consulting company, CATCH Services LLC. He is the chief executive officer and president of a nonprofit organization, the Resource for Advancing Children’s Health Institute, but receives no compensation. The other authors indicated they have no financial relationships relevant to the guidelines.
“Mental health disorders have become one of the new morbidities in pediatric care,” Karalyn Kinsella, MD, said in an interview. “With one in five patients having depression, it is an illness that must be within our domain to identify and treat. I think the guidelines will make providers feel more confident in making a diagnosis and providing initial treatment. For those that do not feel comfortable, hopefully the guidelines will encourage them to seek training.
The take-home message for general pediatricians is that a standardized screening tool makes identifying depression relatively easy. “We have been using the PHQ-9 [Patient Health Questionnaire-9] in my office for several years, and it is very easy to administer and score, and is billable,” said Dr. Kinsella. “It can take some practice to tease out some typical teen behaviors, especially on the sleep and fatigue questions, but it provides an opportunity to open up discussion with the teen.
“Treatment [of depression] can be more complicated and time consuming, but rewarding and invaluable to the patient,” she emphasized. “Many states now have psychiatrists available by phone consultation to aid in management of medication. The key is establishing a list of quality counselors for referrals. With those supports and frequent follow-up, pediatricians can play a key role in the treatment of this prevalent and important illness that affects our patients.”
Dr. Kinsella is a pediatrician in Cheshire, Conn., and a member of the Pediatric News editorial advisory board. She was asked to comment on the new AAP teen depression guidelines.
“Mental health disorders have become one of the new morbidities in pediatric care,” Karalyn Kinsella, MD, said in an interview. “With one in five patients having depression, it is an illness that must be within our domain to identify and treat. I think the guidelines will make providers feel more confident in making a diagnosis and providing initial treatment. For those that do not feel comfortable, hopefully the guidelines will encourage them to seek training.
The take-home message for general pediatricians is that a standardized screening tool makes identifying depression relatively easy. “We have been using the PHQ-9 [Patient Health Questionnaire-9] in my office for several years, and it is very easy to administer and score, and is billable,” said Dr. Kinsella. “It can take some practice to tease out some typical teen behaviors, especially on the sleep and fatigue questions, but it provides an opportunity to open up discussion with the teen.
“Treatment [of depression] can be more complicated and time consuming, but rewarding and invaluable to the patient,” she emphasized. “Many states now have psychiatrists available by phone consultation to aid in management of medication. The key is establishing a list of quality counselors for referrals. With those supports and frequent follow-up, pediatricians can play a key role in the treatment of this prevalent and important illness that affects our patients.”
Dr. Kinsella is a pediatrician in Cheshire, Conn., and a member of the Pediatric News editorial advisory board. She was asked to comment on the new AAP teen depression guidelines.
“Mental health disorders have become one of the new morbidities in pediatric care,” Karalyn Kinsella, MD, said in an interview. “With one in five patients having depression, it is an illness that must be within our domain to identify and treat. I think the guidelines will make providers feel more confident in making a diagnosis and providing initial treatment. For those that do not feel comfortable, hopefully the guidelines will encourage them to seek training.
The take-home message for general pediatricians is that a standardized screening tool makes identifying depression relatively easy. “We have been using the PHQ-9 [Patient Health Questionnaire-9] in my office for several years, and it is very easy to administer and score, and is billable,” said Dr. Kinsella. “It can take some practice to tease out some typical teen behaviors, especially on the sleep and fatigue questions, but it provides an opportunity to open up discussion with the teen.
“Treatment [of depression] can be more complicated and time consuming, but rewarding and invaluable to the patient,” she emphasized. “Many states now have psychiatrists available by phone consultation to aid in management of medication. The key is establishing a list of quality counselors for referrals. With those supports and frequent follow-up, pediatricians can play a key role in the treatment of this prevalent and important illness that affects our patients.”
Dr. Kinsella is a pediatrician in Cheshire, Conn., and a member of the Pediatric News editorial advisory board. She was asked to comment on the new AAP teen depression guidelines.
The updated information includes recommendations on collaborative care, practice preparation, establishing networks of referrals, and much more.
“These guidelines were developed for PC clinicians who are in a position to identify and assist youth with depression in their practice settings,” they said. The guidelines apply to individuals aged 10-21 years, and support universal depression screening for those aged 12 and older.
Known as the Guidelines for Adolescent Depression in Primary Care (GLAD-PC), they consist of two parts: Practice Preparation, Identification, Assessment, and Initial Management, with Dr. Zuckerbrot as the lead author, and Treatment and Ongoing Management, led by Amy H. Cheung, MD, of the University of Toronto. They were published online in Pediatrics.
“It has been over 10 years since the [last] guidelines were published and they are supposed to be updated every 5,” Dr. Zuckerbrot said in an interview. “Given the new evidence on screening, psychopharmacology, and collaborative care, the guidelines needed to be revised. The USPSTF [United States Preventive Services Task Force ] and the AAP had already supported universal adolescent depression screening, and these guidelines are finally aligned with those positions.
“Different parts of the guidelines will be the go-to for different pediatricians, depending on where they are in their delivery of mental health care,” she explained. “Some may need help with practice preparation while others may need advice on screening; others may already be prescribing and may need advice on ongoing treatment and follow-up. I think there is something for everyone.”
Implementation of the guidelines is difficult in a short visit, Dr. Zuckerbrot acknowledged. “In addition, pediatricians may not have been well trained in the management of adolescent depression during their residencies.” However, the guidelines discuss both “real teams to support the pediatricians in their efforts, as well as virtual teams when staffing is limited.
“The guidelines advise that pediatricians learn about child psychiatry primary care consultation programs in their state and make use of those free telephone consultation programs.” The guidelines also discuss strategies for collaborative or integrative care, she said.
Part I
Part I of the guidelines, “Practice Preparation, Identification, Assessment, and Initial Management,” includes several recommendations for each topic.
For practice preparation, the guidelines recommend that clinicians seek training in the assessment, diagnosis, and treatment of depression, and that they establish a network of referrals and mental health resources in their communities. This network may include not only health professionals, but also current patients and families who are managing teen depression. If available, state-wide or regional child and adolescent psychiatry consultation programs can be included.
The identification and surveillance section of the guidelines calls for screening all patients aged 12 years and older for depression each year, using a formal screening tool on paper or online. The screening could occur at an annual wellness visit or any other medical visit, such as a sports physical. A second recommendation calls for identifying patients at increased risk for depression because of factors such as personal history, family history, substance use, other psychiatric disorders, frequent somatic complaints, or trauma, and monitoring these individuals regularly for signs of depression using a formal screening tool.
The assessment and diagnosis section states that assessment should include interviews with the patients alone as well as with their families or caregivers, and should include screening teens for functional impairment.
Primary care physicians should evaluate for depression not only if an adolescent tests positive on a screening tool, but also in children who present with any emotional problem as the chief complaint, and in those in whom depression is highly suspected even if they test negative on a formal screening tool, the guidelines state.
The three recommendations for initial management of depression in the primary care setting are educating patients and families about depression; developing a treatment plan (if the primary care clinician has had appropriate training) and setting specific treatment goals in areas of functioning such as at home, with peers, and at school; and developing a safety plan that includes restricting access to weapons or other means of self-harm, according to the guidelines.
Part II
Part II of the recommendations, “Treatment and Ongoing Management,” discusses options for managing depression in the primary care setting and utilizing outside resources.
The treatment recommendations emphasize the use of integrated models, if possible. “There is a growing recognition that complex chronic conditions, such as depression, are most successfully managed with proactive, multidisciplinary, patient-centered care teams,” Dr. Cheung and her associates said.
The recommendation for cases of mild depression calls for a period of “active support and monitoring” for 6-8 weeks before reassessing if the teen shows no improvement. By contrast, for cases of moderate to severe depression or cases with evidence of substance abuse or other psychoses, the recommendation calls for potential consultation with a mental health specialist and a discussion of the roles primary and specialty care will play in treatment. The guidelines include a flow chart for PC physicians to follow.
The guidelines suggest that PC clinicians recommend “scientifically tested and proven treatments,” such as psychotherapies, cognitive behavioral therapy (CBT) or interpersonal psychotherapy for adolescents, and/or antidepressant treatment, such as SSRIs, whenever possible and appropriate. It is important to monitor teens on antidepressants regularly to identify adverse events.
Recommendations for the ongoing management of teens with depression in the primary care setting include regular tracking of progress, reassessment if the teen shows no improvement in 6-8 weeks, and consultation with a mental health professional for those who show only partial improvement after exhausting primary care diagnostic and treatment options. Assessment of depressive symptoms is not the only thing to track. Functioning at home, school, and among peers also is important.
The final treatment recommendation is for active support of a depressed teen’s referral to mental health if necessary for best management and sharing care if possible, with an understanding of the roles of the primary and specialty clinicians, the guidelines state.
The guidelines project was funded by the Resource for Advancing Children’s Health Institute and the Bell Canada Chair in Adolescent Mood and Anxiety Disorders.
Dr. Cheung and Dr. Zuckerbrot receive book royalties. Dr Zuckerbrot works for child and adolescent psychiatry for primary care (CAP-PC), now a regional provider for Project TEACH in New York State, and she is on the steering committee as well as faculty for the REACH Institute; both of these institutions are described in the guidelines. Peter S. Jensen, MD, has received royalties from Random House, Oxford University Press, and APPI Inc. He is a part owner of a consulting company, CATCH Services LLC. He is the chief executive officer and president of a nonprofit organization, the Resource for Advancing Children’s Health Institute, but receives no compensation. The other authors indicated they have no financial relationships relevant to the guidelines.
The updated information includes recommendations on collaborative care, practice preparation, establishing networks of referrals, and much more.
“These guidelines were developed for PC clinicians who are in a position to identify and assist youth with depression in their practice settings,” they said. The guidelines apply to individuals aged 10-21 years, and support universal depression screening for those aged 12 and older.
Known as the Guidelines for Adolescent Depression in Primary Care (GLAD-PC), they consist of two parts: Practice Preparation, Identification, Assessment, and Initial Management, with Dr. Zuckerbrot as the lead author, and Treatment and Ongoing Management, led by Amy H. Cheung, MD, of the University of Toronto. They were published online in Pediatrics.
“It has been over 10 years since the [last] guidelines were published and they are supposed to be updated every 5,” Dr. Zuckerbrot said in an interview. “Given the new evidence on screening, psychopharmacology, and collaborative care, the guidelines needed to be revised. The USPSTF [United States Preventive Services Task Force ] and the AAP had already supported universal adolescent depression screening, and these guidelines are finally aligned with those positions.
“Different parts of the guidelines will be the go-to for different pediatricians, depending on where they are in their delivery of mental health care,” she explained. “Some may need help with practice preparation while others may need advice on screening; others may already be prescribing and may need advice on ongoing treatment and follow-up. I think there is something for everyone.”
Implementation of the guidelines is difficult in a short visit, Dr. Zuckerbrot acknowledged. “In addition, pediatricians may not have been well trained in the management of adolescent depression during their residencies.” However, the guidelines discuss both “real teams to support the pediatricians in their efforts, as well as virtual teams when staffing is limited.
“The guidelines advise that pediatricians learn about child psychiatry primary care consultation programs in their state and make use of those free telephone consultation programs.” The guidelines also discuss strategies for collaborative or integrative care, she said.
Part I
Part I of the guidelines, “Practice Preparation, Identification, Assessment, and Initial Management,” includes several recommendations for each topic.
For practice preparation, the guidelines recommend that clinicians seek training in the assessment, diagnosis, and treatment of depression, and that they establish a network of referrals and mental health resources in their communities. This network may include not only health professionals, but also current patients and families who are managing teen depression. If available, state-wide or regional child and adolescent psychiatry consultation programs can be included.
The identification and surveillance section of the guidelines calls for screening all patients aged 12 years and older for depression each year, using a formal screening tool on paper or online. The screening could occur at an annual wellness visit or any other medical visit, such as a sports physical. A second recommendation calls for identifying patients at increased risk for depression because of factors such as personal history, family history, substance use, other psychiatric disorders, frequent somatic complaints, or trauma, and monitoring these individuals regularly for signs of depression using a formal screening tool.
The assessment and diagnosis section states that assessment should include interviews with the patients alone as well as with their families or caregivers, and should include screening teens for functional impairment.
Primary care physicians should evaluate for depression not only if an adolescent tests positive on a screening tool, but also in children who present with any emotional problem as the chief complaint, and in those in whom depression is highly suspected even if they test negative on a formal screening tool, the guidelines state.
The three recommendations for initial management of depression in the primary care setting are educating patients and families about depression; developing a treatment plan (if the primary care clinician has had appropriate training) and setting specific treatment goals in areas of functioning such as at home, with peers, and at school; and developing a safety plan that includes restricting access to weapons or other means of self-harm, according to the guidelines.
Part II
Part II of the recommendations, “Treatment and Ongoing Management,” discusses options for managing depression in the primary care setting and utilizing outside resources.
The treatment recommendations emphasize the use of integrated models, if possible. “There is a growing recognition that complex chronic conditions, such as depression, are most successfully managed with proactive, multidisciplinary, patient-centered care teams,” Dr. Cheung and her associates said.
The recommendation for cases of mild depression calls for a period of “active support and monitoring” for 6-8 weeks before reassessing if the teen shows no improvement. By contrast, for cases of moderate to severe depression or cases with evidence of substance abuse or other psychoses, the recommendation calls for potential consultation with a mental health specialist and a discussion of the roles primary and specialty care will play in treatment. The guidelines include a flow chart for PC physicians to follow.
The guidelines suggest that PC clinicians recommend “scientifically tested and proven treatments,” such as psychotherapies, cognitive behavioral therapy (CBT) or interpersonal psychotherapy for adolescents, and/or antidepressant treatment, such as SSRIs, whenever possible and appropriate. It is important to monitor teens on antidepressants regularly to identify adverse events.
Recommendations for the ongoing management of teens with depression in the primary care setting include regular tracking of progress, reassessment if the teen shows no improvement in 6-8 weeks, and consultation with a mental health professional for those who show only partial improvement after exhausting primary care diagnostic and treatment options. Assessment of depressive symptoms is not the only thing to track. Functioning at home, school, and among peers also is important.
The final treatment recommendation is for active support of a depressed teen’s referral to mental health if necessary for best management and sharing care if possible, with an understanding of the roles of the primary and specialty clinicians, the guidelines state.
The guidelines project was funded by the Resource for Advancing Children’s Health Institute and the Bell Canada Chair in Adolescent Mood and Anxiety Disorders.
Dr. Cheung and Dr. Zuckerbrot receive book royalties. Dr Zuckerbrot works for child and adolescent psychiatry for primary care (CAP-PC), now a regional provider for Project TEACH in New York State, and she is on the steering committee as well as faculty for the REACH Institute; both of these institutions are described in the guidelines. Peter S. Jensen, MD, has received royalties from Random House, Oxford University Press, and APPI Inc. He is a part owner of a consulting company, CATCH Services LLC. He is the chief executive officer and president of a nonprofit organization, the Resource for Advancing Children’s Health Institute, but receives no compensation. The other authors indicated they have no financial relationships relevant to the guidelines.
FROM PEDIATRICS
When to worry about congenital melanocytic nevi
KAUAI, HAWAII – according to Jennifer Huang, MD, a pediatric dermatologist at Boston Children’s Hospital.
Two or more nevi increase the risk of CNS involvement, which in turn increases the risk of malignant conversion by more than 16-fold.
Among the studies she cited was a 2017 literature review of 448 children with congenital nevi, 10 of whom developed melanoma: It arose in the skin in 2, the brain in 6, and an unknown location in 2. All 10 children were born with two or more nevi, and not all of them had large or giant nevi, which is a known risk factor for malignant conversion (Br J Dermatol. 2017 May;176[5]:1131-43).
“If the scanning brain MRI is normal, [children] might not have congenital melanocytic nevus syndrome, and would be at low risk for melanoma,” Dr. Huang said. “If it’s abnormal, they might be at high risk for melanoma.” In the 2017 study, the odds ratio for melanoma with an abnormal MRI was 16.7 (P = .001).
Both melanocytes and neuronal cells arise from the embryonic neural crest, which explains the link between congenital nevi and brain lesions. Almost all congenital nevi are associated with early postzygotic mutations in the NRAS gene, and it’s possible the mutations affect other neural crest cell lines, including in the CNS, she said.
It’s also important to remember that childhood melanoma often doesn’t follow the ABCDE (asymmetry, border irregularity, color not uniform, diameter greater than 6 mm, and evolving) signs of melanoma common in adults.
In a retrospective study of 70 children with melanoma or ambiguous melanocytic tumors, 40% of pubertal subjects and 60% of prepubertal participants did not meet conventional adult ABCDE criteria. The majority of cases were raised, even in color, less than 6 mm across, symmetric, and de novo (J Am Acad Dermatol. 2013 Jun;68[6]:913-25).
It turns out that rapid evolution in size, shape, and color is the number one, unifying factor in childhood melanomas. Other key clues include raised lesions with uniform color or no pigmentation at all. A modified ABCDE for pediatric melanoma has been proposed: amelanotic, bump/bleeding, color uniform, diameter variable, de novo, and evolution.
“The lesson to learn is not to ignore the traditional ABCDEs of melanoma, but to recognize that pediatric melanoma may present with different clinical characteristics, and to incorporate this awareness into our practice,” Dr. Huang said.
She did not have any disclosures. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – according to Jennifer Huang, MD, a pediatric dermatologist at Boston Children’s Hospital.
Two or more nevi increase the risk of CNS involvement, which in turn increases the risk of malignant conversion by more than 16-fold.
Among the studies she cited was a 2017 literature review of 448 children with congenital nevi, 10 of whom developed melanoma: It arose in the skin in 2, the brain in 6, and an unknown location in 2. All 10 children were born with two or more nevi, and not all of them had large or giant nevi, which is a known risk factor for malignant conversion (Br J Dermatol. 2017 May;176[5]:1131-43).
“If the scanning brain MRI is normal, [children] might not have congenital melanocytic nevus syndrome, and would be at low risk for melanoma,” Dr. Huang said. “If it’s abnormal, they might be at high risk for melanoma.” In the 2017 study, the odds ratio for melanoma with an abnormal MRI was 16.7 (P = .001).
Both melanocytes and neuronal cells arise from the embryonic neural crest, which explains the link between congenital nevi and brain lesions. Almost all congenital nevi are associated with early postzygotic mutations in the NRAS gene, and it’s possible the mutations affect other neural crest cell lines, including in the CNS, she said.
It’s also important to remember that childhood melanoma often doesn’t follow the ABCDE (asymmetry, border irregularity, color not uniform, diameter greater than 6 mm, and evolving) signs of melanoma common in adults.
In a retrospective study of 70 children with melanoma or ambiguous melanocytic tumors, 40% of pubertal subjects and 60% of prepubertal participants did not meet conventional adult ABCDE criteria. The majority of cases were raised, even in color, less than 6 mm across, symmetric, and de novo (J Am Acad Dermatol. 2013 Jun;68[6]:913-25).
It turns out that rapid evolution in size, shape, and color is the number one, unifying factor in childhood melanomas. Other key clues include raised lesions with uniform color or no pigmentation at all. A modified ABCDE for pediatric melanoma has been proposed: amelanotic, bump/bleeding, color uniform, diameter variable, de novo, and evolution.
“The lesson to learn is not to ignore the traditional ABCDEs of melanoma, but to recognize that pediatric melanoma may present with different clinical characteristics, and to incorporate this awareness into our practice,” Dr. Huang said.
She did not have any disclosures. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – according to Jennifer Huang, MD, a pediatric dermatologist at Boston Children’s Hospital.
Two or more nevi increase the risk of CNS involvement, which in turn increases the risk of malignant conversion by more than 16-fold.
Among the studies she cited was a 2017 literature review of 448 children with congenital nevi, 10 of whom developed melanoma: It arose in the skin in 2, the brain in 6, and an unknown location in 2. All 10 children were born with two or more nevi, and not all of them had large or giant nevi, which is a known risk factor for malignant conversion (Br J Dermatol. 2017 May;176[5]:1131-43).
“If the scanning brain MRI is normal, [children] might not have congenital melanocytic nevus syndrome, and would be at low risk for melanoma,” Dr. Huang said. “If it’s abnormal, they might be at high risk for melanoma.” In the 2017 study, the odds ratio for melanoma with an abnormal MRI was 16.7 (P = .001).
Both melanocytes and neuronal cells arise from the embryonic neural crest, which explains the link between congenital nevi and brain lesions. Almost all congenital nevi are associated with early postzygotic mutations in the NRAS gene, and it’s possible the mutations affect other neural crest cell lines, including in the CNS, she said.
It’s also important to remember that childhood melanoma often doesn’t follow the ABCDE (asymmetry, border irregularity, color not uniform, diameter greater than 6 mm, and evolving) signs of melanoma common in adults.
In a retrospective study of 70 children with melanoma or ambiguous melanocytic tumors, 40% of pubertal subjects and 60% of prepubertal participants did not meet conventional adult ABCDE criteria. The majority of cases were raised, even in color, less than 6 mm across, symmetric, and de novo (J Am Acad Dermatol. 2013 Jun;68[6]:913-25).
It turns out that rapid evolution in size, shape, and color is the number one, unifying factor in childhood melanomas. Other key clues include raised lesions with uniform color or no pigmentation at all. A modified ABCDE for pediatric melanoma has been proposed: amelanotic, bump/bleeding, color uniform, diameter variable, de novo, and evolution.
“The lesson to learn is not to ignore the traditional ABCDEs of melanoma, but to recognize that pediatric melanoma may present with different clinical characteristics, and to incorporate this awareness into our practice,” Dr. Huang said.
She did not have any disclosures. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
200 cardiovascular drugs now in development
, according to Pharmaceutical Research and Manufacturers of America.
One of those 42 treatments is “a nonviral gene therapy that targets a tissue repair and regeneration pathway in the body,” PhRMA noted. A total of 200 medicines, including those for lipid disorders and hypertension, were either in clinical trials or undergoing regulatory review as of Jan. 24, 2018, the group reported. Some treatments are being considered for more than one condition.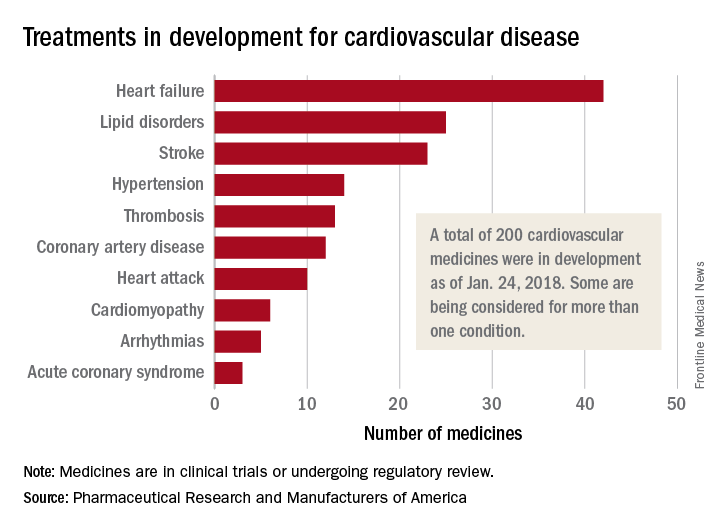
, according to Pharmaceutical Research and Manufacturers of America.
One of those 42 treatments is “a nonviral gene therapy that targets a tissue repair and regeneration pathway in the body,” PhRMA noted. A total of 200 medicines, including those for lipid disorders and hypertension, were either in clinical trials or undergoing regulatory review as of Jan. 24, 2018, the group reported. Some treatments are being considered for more than one condition.
, according to Pharmaceutical Research and Manufacturers of America.
One of those 42 treatments is “a nonviral gene therapy that targets a tissue repair and regeneration pathway in the body,” PhRMA noted. A total of 200 medicines, including those for lipid disorders and hypertension, were either in clinical trials or undergoing regulatory review as of Jan. 24, 2018, the group reported. Some treatments are being considered for more than one condition.
MDedge Daily News: Moving toward a universal flu vaccine
Federal scientists advance a plan for a universal flu vaccine, making health care affordable has broad support, some encouraging news about proton pump inhibitors and myocardial infarction, and how obesity affects cardiovascular risk.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Federal scientists advance a plan for a universal flu vaccine, making health care affordable has broad support, some encouraging news about proton pump inhibitors and myocardial infarction, and how obesity affects cardiovascular risk.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Federal scientists advance a plan for a universal flu vaccine, making health care affordable has broad support, some encouraging news about proton pump inhibitors and myocardial infarction, and how obesity affects cardiovascular risk.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Listen to the MDedge Daily News podcast for all the details on today’s top news.
HSCT approach provides ‘excellent’ survival in FA
SALT LAKE CITY—A “risk-adjusted” approach leads to “excellent” survival in patients with Fanconi anemia (FA) undergoing alternative donor hematopoietic stem cell transplant (HSCT), according to a speaker at the 2018 BMT Tandem Meetings.
All FA patients who received personalized doses of busulfan in place of total body irradiation (TBI) were alive and disease-free after undergoing HSCT for bone marrow failure or myelodysplastic syndrome (MDS).
None of the patients developed graft-vs-host disease (GVHD), and the most common toxicity was viral infection.
Parinda A. Mehta, MD, of Cincinnati Children’s Hospital Medical Center in Ohio, presented these results at this year’s BMT Tandem Meetings as abstract 109.*
“We all know that inherent chemotherapy and radiation sensitivity makes transplant for patients with Fanconi anemia quite challenging,” Dr Mehta began. “In our recently published, prospective, multi-institutional study, we showed excellent outcomes of alternative donor transplant in patients with Fanconi anemia without using radiation.”
“In that study,** TBI was replaced by pharmacokinetically adjusted busulfan. It proved that, yes, we can do alternative donor transplant successfully without radiation by showing an overall survival of 80% for a total of 45 patients. We were quite ecstatic to see these numbers.”
The study also showed that younger patients fared better with this regimen, and younger patients did best with the lowest dose of busulfan tested (0.6 mg/kg vs 0.8 to 1.0 mg/kg). In addition, patients who underwent HSCT for bone marrow failure had better outcomes than patients who had MDS.
This led Dr Mehta and her colleagues to hypothesize that adjusting busulfan dosing based on a patient’s age and disease status at HSCT could minimize toxicity and improve outcomes.
Patients
The researchers tested their theory in 22 FA patients. They had a median age of 7 (range, 4-27), and most (n=13) were female.
Twelve patients had pancytopenia, 6 had severe single-lineage cytopenia, 3 had low-grade MDS, and 1 patient had acute myeloid leukemia (AML).
Eighteen patients had a history of transfusions, and 3 had a history of androgen use.
Treatment
The preparative regimen consisted of 4 doses of busulfan (every 12 hours on day -7 to -6), followed by cyclophosphamide at 10 mg/kg/day (on day -5 to -2), fludarabine at 35 mg/m2/day (on day -5 to -2), and rabbit antithymocyte globulin at 2.5 mg/kg/day (on day -5 to -2).
Busulfan doses were adjusted according to age and disease status.
Children (age 18 and younger) with bone marrow failure received busulfan at 0.6 to 0.8 mg/kg. Children with MDS/AML received busulfan at 0.8 to 1.0 mg/kg. Adults (19 and older) received the lowest dose of busulfan—0.4 mg/kg—regardless of disease status.
“At the first sight, this will look counterintuitive . . . ,” Dr Mehta said. “However, based on our previous experience, in general and also from results of our previous study, this was specifically designed to avoid upfront TRM [transplant-related mortality] for these adult patients.”
All 22 patients received CD34-selected, T-cell-depleted peripheral blood stem cells from unrelated donors. Eleven patients received a fully matched graft (10/10), 8 patients had a 9/10 match, and 3 had an 8/10 match.
The median number of CD34+ cells/kg was 23.9 x 106 (range, 4.9-76.6), and the median number of CD3 cells/kg was 1 x 104 (range, 0.003-3.1).
T-cell depletion was the only GVHD prophylaxis used.
Patients with MDS/AML could receive azacitidine at day 42 after HSCT, an option intended to prevent relapse in these patients.
Toxicity
There were no cases of acute or chronic GVHD.
Toxicities included infections (n=24), oral mucositis (n=14), hyperbilirubinemia (n=2), pulmonary hemorrhage (n=1), and sinusoidal obstruction syndrome (n=1).
There were 20 viral infections, 4 bacterial infections, and no fungal infections. Viral infections included BK virus (n=7), cytomegalovirus (n=6), Epstein-Barr virus (n=6), and adenovirus (n=1).
Dr Mehta noted that viral infections are “not unexpected in a T-cell-depleted graft setting.”
“Because we know this complication [can occur], and we worry about our patients, one of the things that, in recent years, we have done is, we manufacture viral-specific CTLs [cytotoxic T lymphocytes] for all of these patients ahead of time whenever possible,” she said.
“To give you an example, 19 out of these 20 patients’ viral infections—or rather, viremias—are completely under control with the use of either antivirals or donor-specific CTLs, including a third-party CTL in one of the patients.”
Response and survival
All 22 patients engrafted. The median time to neutrophil engraftment was 9 days (range, 8-10), and the median time to platelet engraftment was 16 days (range, 11-40).
Twenty-one of the 22 patients (95%) were alive and disease-free at last follow-up. The median follow-up was 21 months (range, 6-44).
The single AML patient achieved remission but died of post-transplant lymphoproliferative disorder (PTLD) on day 202 after HSCT. Dr Mehta said this was due to partial loss of follow-up and noncompliance with medical recommendations during PTLD treatment.
The AML patient also had “significant upfront toxicity” but “recovered very nicely,” according to Dr Mehta. He had severe mucositis, herpetic stomatitis, and sinusoidal obstruction syndrome that responded to defibrotide.
“Overall, we are quite excited to see 95% overall survival for this cohort and conclude that the current risk-adjusted approach leads to excellent overall survival and disease-free survival in patients undergoing alternative donor transplant either for marrow failure or MDS/AML,” Dr Mehta said.
“Enrollment is ongoing, and we hope to see continued success in patients with MDS/AML as well as in adult patients.”
*Data in the abstract differ from the presentation.
**Mehta PA et al. Radiation-free, alternative donor HCT for Fanconi anemia patients: results from a prospective multi-institutional study. Blood 2017; doi: https://doi.org/10.1182/blood-2016-09-743112.
SALT LAKE CITY—A “risk-adjusted” approach leads to “excellent” survival in patients with Fanconi anemia (FA) undergoing alternative donor hematopoietic stem cell transplant (HSCT), according to a speaker at the 2018 BMT Tandem Meetings.
All FA patients who received personalized doses of busulfan in place of total body irradiation (TBI) were alive and disease-free after undergoing HSCT for bone marrow failure or myelodysplastic syndrome (MDS).
None of the patients developed graft-vs-host disease (GVHD), and the most common toxicity was viral infection.
Parinda A. Mehta, MD, of Cincinnati Children’s Hospital Medical Center in Ohio, presented these results at this year’s BMT Tandem Meetings as abstract 109.*
“We all know that inherent chemotherapy and radiation sensitivity makes transplant for patients with Fanconi anemia quite challenging,” Dr Mehta began. “In our recently published, prospective, multi-institutional study, we showed excellent outcomes of alternative donor transplant in patients with Fanconi anemia without using radiation.”
“In that study,** TBI was replaced by pharmacokinetically adjusted busulfan. It proved that, yes, we can do alternative donor transplant successfully without radiation by showing an overall survival of 80% for a total of 45 patients. We were quite ecstatic to see these numbers.”
The study also showed that younger patients fared better with this regimen, and younger patients did best with the lowest dose of busulfan tested (0.6 mg/kg vs 0.8 to 1.0 mg/kg). In addition, patients who underwent HSCT for bone marrow failure had better outcomes than patients who had MDS.
This led Dr Mehta and her colleagues to hypothesize that adjusting busulfan dosing based on a patient’s age and disease status at HSCT could minimize toxicity and improve outcomes.
Patients
The researchers tested their theory in 22 FA patients. They had a median age of 7 (range, 4-27), and most (n=13) were female.
Twelve patients had pancytopenia, 6 had severe single-lineage cytopenia, 3 had low-grade MDS, and 1 patient had acute myeloid leukemia (AML).
Eighteen patients had a history of transfusions, and 3 had a history of androgen use.
Treatment
The preparative regimen consisted of 4 doses of busulfan (every 12 hours on day -7 to -6), followed by cyclophosphamide at 10 mg/kg/day (on day -5 to -2), fludarabine at 35 mg/m2/day (on day -5 to -2), and rabbit antithymocyte globulin at 2.5 mg/kg/day (on day -5 to -2).
Busulfan doses were adjusted according to age and disease status.
Children (age 18 and younger) with bone marrow failure received busulfan at 0.6 to 0.8 mg/kg. Children with MDS/AML received busulfan at 0.8 to 1.0 mg/kg. Adults (19 and older) received the lowest dose of busulfan—0.4 mg/kg—regardless of disease status.
“At the first sight, this will look counterintuitive . . . ,” Dr Mehta said. “However, based on our previous experience, in general and also from results of our previous study, this was specifically designed to avoid upfront TRM [transplant-related mortality] for these adult patients.”
All 22 patients received CD34-selected, T-cell-depleted peripheral blood stem cells from unrelated donors. Eleven patients received a fully matched graft (10/10), 8 patients had a 9/10 match, and 3 had an 8/10 match.
The median number of CD34+ cells/kg was 23.9 x 106 (range, 4.9-76.6), and the median number of CD3 cells/kg was 1 x 104 (range, 0.003-3.1).
T-cell depletion was the only GVHD prophylaxis used.
Patients with MDS/AML could receive azacitidine at day 42 after HSCT, an option intended to prevent relapse in these patients.
Toxicity
There were no cases of acute or chronic GVHD.
Toxicities included infections (n=24), oral mucositis (n=14), hyperbilirubinemia (n=2), pulmonary hemorrhage (n=1), and sinusoidal obstruction syndrome (n=1).
There were 20 viral infections, 4 bacterial infections, and no fungal infections. Viral infections included BK virus (n=7), cytomegalovirus (n=6), Epstein-Barr virus (n=6), and adenovirus (n=1).
Dr Mehta noted that viral infections are “not unexpected in a T-cell-depleted graft setting.”
“Because we know this complication [can occur], and we worry about our patients, one of the things that, in recent years, we have done is, we manufacture viral-specific CTLs [cytotoxic T lymphocytes] for all of these patients ahead of time whenever possible,” she said.
“To give you an example, 19 out of these 20 patients’ viral infections—or rather, viremias—are completely under control with the use of either antivirals or donor-specific CTLs, including a third-party CTL in one of the patients.”
Response and survival
All 22 patients engrafted. The median time to neutrophil engraftment was 9 days (range, 8-10), and the median time to platelet engraftment was 16 days (range, 11-40).
Twenty-one of the 22 patients (95%) were alive and disease-free at last follow-up. The median follow-up was 21 months (range, 6-44).
The single AML patient achieved remission but died of post-transplant lymphoproliferative disorder (PTLD) on day 202 after HSCT. Dr Mehta said this was due to partial loss of follow-up and noncompliance with medical recommendations during PTLD treatment.
The AML patient also had “significant upfront toxicity” but “recovered very nicely,” according to Dr Mehta. He had severe mucositis, herpetic stomatitis, and sinusoidal obstruction syndrome that responded to defibrotide.
“Overall, we are quite excited to see 95% overall survival for this cohort and conclude that the current risk-adjusted approach leads to excellent overall survival and disease-free survival in patients undergoing alternative donor transplant either for marrow failure or MDS/AML,” Dr Mehta said.
“Enrollment is ongoing, and we hope to see continued success in patients with MDS/AML as well as in adult patients.”
*Data in the abstract differ from the presentation.
**Mehta PA et al. Radiation-free, alternative donor HCT for Fanconi anemia patients: results from a prospective multi-institutional study. Blood 2017; doi: https://doi.org/10.1182/blood-2016-09-743112.
SALT LAKE CITY—A “risk-adjusted” approach leads to “excellent” survival in patients with Fanconi anemia (FA) undergoing alternative donor hematopoietic stem cell transplant (HSCT), according to a speaker at the 2018 BMT Tandem Meetings.
All FA patients who received personalized doses of busulfan in place of total body irradiation (TBI) were alive and disease-free after undergoing HSCT for bone marrow failure or myelodysplastic syndrome (MDS).
None of the patients developed graft-vs-host disease (GVHD), and the most common toxicity was viral infection.
Parinda A. Mehta, MD, of Cincinnati Children’s Hospital Medical Center in Ohio, presented these results at this year’s BMT Tandem Meetings as abstract 109.*
“We all know that inherent chemotherapy and radiation sensitivity makes transplant for patients with Fanconi anemia quite challenging,” Dr Mehta began. “In our recently published, prospective, multi-institutional study, we showed excellent outcomes of alternative donor transplant in patients with Fanconi anemia without using radiation.”
“In that study,** TBI was replaced by pharmacokinetically adjusted busulfan. It proved that, yes, we can do alternative donor transplant successfully without radiation by showing an overall survival of 80% for a total of 45 patients. We were quite ecstatic to see these numbers.”
The study also showed that younger patients fared better with this regimen, and younger patients did best with the lowest dose of busulfan tested (0.6 mg/kg vs 0.8 to 1.0 mg/kg). In addition, patients who underwent HSCT for bone marrow failure had better outcomes than patients who had MDS.
This led Dr Mehta and her colleagues to hypothesize that adjusting busulfan dosing based on a patient’s age and disease status at HSCT could minimize toxicity and improve outcomes.
Patients
The researchers tested their theory in 22 FA patients. They had a median age of 7 (range, 4-27), and most (n=13) were female.
Twelve patients had pancytopenia, 6 had severe single-lineage cytopenia, 3 had low-grade MDS, and 1 patient had acute myeloid leukemia (AML).
Eighteen patients had a history of transfusions, and 3 had a history of androgen use.
Treatment
The preparative regimen consisted of 4 doses of busulfan (every 12 hours on day -7 to -6), followed by cyclophosphamide at 10 mg/kg/day (on day -5 to -2), fludarabine at 35 mg/m2/day (on day -5 to -2), and rabbit antithymocyte globulin at 2.5 mg/kg/day (on day -5 to -2).
Busulfan doses were adjusted according to age and disease status.
Children (age 18 and younger) with bone marrow failure received busulfan at 0.6 to 0.8 mg/kg. Children with MDS/AML received busulfan at 0.8 to 1.0 mg/kg. Adults (19 and older) received the lowest dose of busulfan—0.4 mg/kg—regardless of disease status.
“At the first sight, this will look counterintuitive . . . ,” Dr Mehta said. “However, based on our previous experience, in general and also from results of our previous study, this was specifically designed to avoid upfront TRM [transplant-related mortality] for these adult patients.”
All 22 patients received CD34-selected, T-cell-depleted peripheral blood stem cells from unrelated donors. Eleven patients received a fully matched graft (10/10), 8 patients had a 9/10 match, and 3 had an 8/10 match.
The median number of CD34+ cells/kg was 23.9 x 106 (range, 4.9-76.6), and the median number of CD3 cells/kg was 1 x 104 (range, 0.003-3.1).
T-cell depletion was the only GVHD prophylaxis used.
Patients with MDS/AML could receive azacitidine at day 42 after HSCT, an option intended to prevent relapse in these patients.
Toxicity
There were no cases of acute or chronic GVHD.
Toxicities included infections (n=24), oral mucositis (n=14), hyperbilirubinemia (n=2), pulmonary hemorrhage (n=1), and sinusoidal obstruction syndrome (n=1).
There were 20 viral infections, 4 bacterial infections, and no fungal infections. Viral infections included BK virus (n=7), cytomegalovirus (n=6), Epstein-Barr virus (n=6), and adenovirus (n=1).
Dr Mehta noted that viral infections are “not unexpected in a T-cell-depleted graft setting.”
“Because we know this complication [can occur], and we worry about our patients, one of the things that, in recent years, we have done is, we manufacture viral-specific CTLs [cytotoxic T lymphocytes] for all of these patients ahead of time whenever possible,” she said.
“To give you an example, 19 out of these 20 patients’ viral infections—or rather, viremias—are completely under control with the use of either antivirals or donor-specific CTLs, including a third-party CTL in one of the patients.”
Response and survival
All 22 patients engrafted. The median time to neutrophil engraftment was 9 days (range, 8-10), and the median time to platelet engraftment was 16 days (range, 11-40).
Twenty-one of the 22 patients (95%) were alive and disease-free at last follow-up. The median follow-up was 21 months (range, 6-44).
The single AML patient achieved remission but died of post-transplant lymphoproliferative disorder (PTLD) on day 202 after HSCT. Dr Mehta said this was due to partial loss of follow-up and noncompliance with medical recommendations during PTLD treatment.
The AML patient also had “significant upfront toxicity” but “recovered very nicely,” according to Dr Mehta. He had severe mucositis, herpetic stomatitis, and sinusoidal obstruction syndrome that responded to defibrotide.
“Overall, we are quite excited to see 95% overall survival for this cohort and conclude that the current risk-adjusted approach leads to excellent overall survival and disease-free survival in patients undergoing alternative donor transplant either for marrow failure or MDS/AML,” Dr Mehta said.
“Enrollment is ongoing, and we hope to see continued success in patients with MDS/AML as well as in adult patients.”
*Data in the abstract differ from the presentation.
**Mehta PA et al. Radiation-free, alternative donor HCT for Fanconi anemia patients: results from a prospective multi-institutional study. Blood 2017; doi: https://doi.org/10.1182/blood-2016-09-743112.
EC approves emicizumab for hemophilia A with inhibitors
The European Commission (EC) has granted marketing authorization for emicizumab (Hemlibra®), a bispecific factor IXa- and factor X-directed antibody.
This means emicizumab is approved for use in the European Union for routine prophylaxis of bleeding episodes in patients of all ages who have hemophilia A and factor VIII inhibitors.
The recommended dose of emicizumab is 3 mg/kg once a week for the first 4 weeks, followed by 1.5 mg/kg once a week.
Emicizumab is designed to bring together factor IXa and factor X, proteins required to activate the natural coagulation cascade and restore the blood clotting process for patients with hemophilia A.
Emicizumab was created by Chugai Pharmaceutical Co., Ltd. and is being co-developed by Chugai, Roche, and Genentech.
The EC’s decision to authorize marketing of emicizumab is based on results from a pair of phase 3 studies—HAVEN 1 and HAVEN 2.
Results from HAVEN 1 were published in NEJM and presented at the 26th ISTH Congress in July 2017. Updated results from HAVEN 2 were presented at the 2017 ASH Annual Meeting in December.
HAVEN 1
This study enrolled 109 patients (age 12 and older) with hemophilia A and factor VIII inhibitors who were previously treated with bypassing agents (BPAs) on-demand or as prophylaxis.
The patients were randomized to receive emicizumab prophylaxis or no prophylaxis. On-demand treatment of breakthrough bleeds with BPAs was allowed.
There was a significant reduction in treated bleeds of 87% with emicizumab prophylaxis compared to no prophylaxis (95% CI: 72.3; 94.3, P<0.0001). And there was an 80% reduction in all bleeds with emicizumab (95% CI: 62.5; 89.8, P<0.0001).
Adverse events (AEs) occurring in at least 5% of patients treated with emicizumab were local injection site reactions, headache, fatigue, upper respiratory tract infection, and arthralgia.
Two patients experienced thromboembolic events (TEs), and 3 had thrombotic microangiopathy (TMA) while receiving emicizumab prophylaxis and more than 100 u/kg/day of activated prothrombin complex concentrate, on average, for 24 hours or more before the event. Two of these patients had also received recombinant factor VIIa.
Neither TE required anticoagulation therapy, and 1 patient restarted emicizumab. The cases of TMA observed were transient, and 1 patient restarted emicizumab.
HAVEN 2
In this single-arm trial, researchers evaluated emicizumab prophylaxis in 60 patients, ages 1 to 17, who had hemophilia A with factor VIII inhibitors.
The efficacy analysis included 57 patients who were younger than 12. The 3 older patients were only included in the safety analysis.
Of the 57 patients, 64.9% had 0 bleeds, 94.7% had 0 treated bleeds, and 98.2% had 0 treated spontaneous bleeds and 0 treated joint bleeds. None of the patients had treated target joint bleeds.
Forty patients had a total of 201 AEs. The most common of these were viral upper respiratory tract infections (16.7%) and injection site reactions (16.7%).
There were no TEs or TMA events, and none of the patients tested positive for anti-drug antibodies. None of the 7 serious AEs in this trial were considered treatment-related.
The European Commission (EC) has granted marketing authorization for emicizumab (Hemlibra®), a bispecific factor IXa- and factor X-directed antibody.
This means emicizumab is approved for use in the European Union for routine prophylaxis of bleeding episodes in patients of all ages who have hemophilia A and factor VIII inhibitors.
The recommended dose of emicizumab is 3 mg/kg once a week for the first 4 weeks, followed by 1.5 mg/kg once a week.
Emicizumab is designed to bring together factor IXa and factor X, proteins required to activate the natural coagulation cascade and restore the blood clotting process for patients with hemophilia A.
Emicizumab was created by Chugai Pharmaceutical Co., Ltd. and is being co-developed by Chugai, Roche, and Genentech.
The EC’s decision to authorize marketing of emicizumab is based on results from a pair of phase 3 studies—HAVEN 1 and HAVEN 2.
Results from HAVEN 1 were published in NEJM and presented at the 26th ISTH Congress in July 2017. Updated results from HAVEN 2 were presented at the 2017 ASH Annual Meeting in December.
HAVEN 1
This study enrolled 109 patients (age 12 and older) with hemophilia A and factor VIII inhibitors who were previously treated with bypassing agents (BPAs) on-demand or as prophylaxis.
The patients were randomized to receive emicizumab prophylaxis or no prophylaxis. On-demand treatment of breakthrough bleeds with BPAs was allowed.
There was a significant reduction in treated bleeds of 87% with emicizumab prophylaxis compared to no prophylaxis (95% CI: 72.3; 94.3, P<0.0001). And there was an 80% reduction in all bleeds with emicizumab (95% CI: 62.5; 89.8, P<0.0001).
Adverse events (AEs) occurring in at least 5% of patients treated with emicizumab were local injection site reactions, headache, fatigue, upper respiratory tract infection, and arthralgia.
Two patients experienced thromboembolic events (TEs), and 3 had thrombotic microangiopathy (TMA) while receiving emicizumab prophylaxis and more than 100 u/kg/day of activated prothrombin complex concentrate, on average, for 24 hours or more before the event. Two of these patients had also received recombinant factor VIIa.
Neither TE required anticoagulation therapy, and 1 patient restarted emicizumab. The cases of TMA observed were transient, and 1 patient restarted emicizumab.
HAVEN 2
In this single-arm trial, researchers evaluated emicizumab prophylaxis in 60 patients, ages 1 to 17, who had hemophilia A with factor VIII inhibitors.
The efficacy analysis included 57 patients who were younger than 12. The 3 older patients were only included in the safety analysis.
Of the 57 patients, 64.9% had 0 bleeds, 94.7% had 0 treated bleeds, and 98.2% had 0 treated spontaneous bleeds and 0 treated joint bleeds. None of the patients had treated target joint bleeds.
Forty patients had a total of 201 AEs. The most common of these were viral upper respiratory tract infections (16.7%) and injection site reactions (16.7%).
There were no TEs or TMA events, and none of the patients tested positive for anti-drug antibodies. None of the 7 serious AEs in this trial were considered treatment-related.
The European Commission (EC) has granted marketing authorization for emicizumab (Hemlibra®), a bispecific factor IXa- and factor X-directed antibody.
This means emicizumab is approved for use in the European Union for routine prophylaxis of bleeding episodes in patients of all ages who have hemophilia A and factor VIII inhibitors.
The recommended dose of emicizumab is 3 mg/kg once a week for the first 4 weeks, followed by 1.5 mg/kg once a week.
Emicizumab is designed to bring together factor IXa and factor X, proteins required to activate the natural coagulation cascade and restore the blood clotting process for patients with hemophilia A.
Emicizumab was created by Chugai Pharmaceutical Co., Ltd. and is being co-developed by Chugai, Roche, and Genentech.
The EC’s decision to authorize marketing of emicizumab is based on results from a pair of phase 3 studies—HAVEN 1 and HAVEN 2.
Results from HAVEN 1 were published in NEJM and presented at the 26th ISTH Congress in July 2017. Updated results from HAVEN 2 were presented at the 2017 ASH Annual Meeting in December.
HAVEN 1
This study enrolled 109 patients (age 12 and older) with hemophilia A and factor VIII inhibitors who were previously treated with bypassing agents (BPAs) on-demand or as prophylaxis.
The patients were randomized to receive emicizumab prophylaxis or no prophylaxis. On-demand treatment of breakthrough bleeds with BPAs was allowed.
There was a significant reduction in treated bleeds of 87% with emicizumab prophylaxis compared to no prophylaxis (95% CI: 72.3; 94.3, P<0.0001). And there was an 80% reduction in all bleeds with emicizumab (95% CI: 62.5; 89.8, P<0.0001).
Adverse events (AEs) occurring in at least 5% of patients treated with emicizumab were local injection site reactions, headache, fatigue, upper respiratory tract infection, and arthralgia.
Two patients experienced thromboembolic events (TEs), and 3 had thrombotic microangiopathy (TMA) while receiving emicizumab prophylaxis and more than 100 u/kg/day of activated prothrombin complex concentrate, on average, for 24 hours or more before the event. Two of these patients had also received recombinant factor VIIa.
Neither TE required anticoagulation therapy, and 1 patient restarted emicizumab. The cases of TMA observed were transient, and 1 patient restarted emicizumab.
HAVEN 2
In this single-arm trial, researchers evaluated emicizumab prophylaxis in 60 patients, ages 1 to 17, who had hemophilia A with factor VIII inhibitors.
The efficacy analysis included 57 patients who were younger than 12. The 3 older patients were only included in the safety analysis.
Of the 57 patients, 64.9% had 0 bleeds, 94.7% had 0 treated bleeds, and 98.2% had 0 treated spontaneous bleeds and 0 treated joint bleeds. None of the patients had treated target joint bleeds.
Forty patients had a total of 201 AEs. The most common of these were viral upper respiratory tract infections (16.7%) and injection site reactions (16.7%).
There were no TEs or TMA events, and none of the patients tested positive for anti-drug antibodies. None of the 7 serious AEs in this trial were considered treatment-related.
Use of RBC, plasma transfusions may be declining in US
A study of US hospital discharges revealed a decrease in the use of red blood cell (RBC) and plasma transfusions—but not platelet transfusions—in recent years.
Researchers examined a sample of US hospital inpatient discharges from 1993 to 2014.
Overall, platelet, plasma, and RBC transfusions increased from 1993 to 2010.
However, from 2011 to 2014, there was a significant decrease in both plasma and RBC transfusions. Platelet transfusions remained stable over that period.
Aaron A. R. Tobian, MD, PhD, of Johns Hopkins University in Baltimore, Maryland, and his colleagues reported these findings in a letter to JAMA.
The researchers analyzed data from the National Inpatient Sample, which uses a stratified probability sample of 20% of all inpatient discharges in the US.
The team looked at transfusion trends from 1993 to 2014 but focused on trends from 2011 to 2014 because there was an inflection point in RBC transfusion in 2011. They used multivariable Poisson regression to estimate adjusted risk ratios (aRRs) comparing the risk of transfusion in 2011 and 2014.
The researchers found that RBC transfusions decreased from 6.8% in 2011 to 5.7% in 2014 (aRR=0.83; 95% confidence interval [CI], 0.78-0.88; P<0.001).
Plasma transfusions decreased from 1.0% to 0.87% (aRR=0.87; 95% CI, 0.80-0.95; P=0.003). And platelet transfusions remained stable (aRR=0.99; 95% CI, 0.89-1.10; P=0.93).
The researchers also conducted subgroup analyses to explore trends in RBC transfusion. They found that, from 2011 to 2014, there were significant reductions in RBC transfusions regardless of patients’ sex, race/ethnicity, risk severity, payer type, and admission type.
However, there was no significant reduction in RBC transfusions among patients younger than 18 years of age or in private investor–owned hospitals.
The researchers said the diagnostic coding used for this study was carried out primarily for billing purposes, and it was not possible to verify its accuracy. They also noted that the study only covered inpatient transfusions, so the results may not be generalizable to outpatient transfusions.
A study of US hospital discharges revealed a decrease in the use of red blood cell (RBC) and plasma transfusions—but not platelet transfusions—in recent years.
Researchers examined a sample of US hospital inpatient discharges from 1993 to 2014.
Overall, platelet, plasma, and RBC transfusions increased from 1993 to 2010.
However, from 2011 to 2014, there was a significant decrease in both plasma and RBC transfusions. Platelet transfusions remained stable over that period.
Aaron A. R. Tobian, MD, PhD, of Johns Hopkins University in Baltimore, Maryland, and his colleagues reported these findings in a letter to JAMA.
The researchers analyzed data from the National Inpatient Sample, which uses a stratified probability sample of 20% of all inpatient discharges in the US.
The team looked at transfusion trends from 1993 to 2014 but focused on trends from 2011 to 2014 because there was an inflection point in RBC transfusion in 2011. They used multivariable Poisson regression to estimate adjusted risk ratios (aRRs) comparing the risk of transfusion in 2011 and 2014.
The researchers found that RBC transfusions decreased from 6.8% in 2011 to 5.7% in 2014 (aRR=0.83; 95% confidence interval [CI], 0.78-0.88; P<0.001).
Plasma transfusions decreased from 1.0% to 0.87% (aRR=0.87; 95% CI, 0.80-0.95; P=0.003). And platelet transfusions remained stable (aRR=0.99; 95% CI, 0.89-1.10; P=0.93).
The researchers also conducted subgroup analyses to explore trends in RBC transfusion. They found that, from 2011 to 2014, there were significant reductions in RBC transfusions regardless of patients’ sex, race/ethnicity, risk severity, payer type, and admission type.
However, there was no significant reduction in RBC transfusions among patients younger than 18 years of age or in private investor–owned hospitals.
The researchers said the diagnostic coding used for this study was carried out primarily for billing purposes, and it was not possible to verify its accuracy. They also noted that the study only covered inpatient transfusions, so the results may not be generalizable to outpatient transfusions.
A study of US hospital discharges revealed a decrease in the use of red blood cell (RBC) and plasma transfusions—but not platelet transfusions—in recent years.
Researchers examined a sample of US hospital inpatient discharges from 1993 to 2014.
Overall, platelet, plasma, and RBC transfusions increased from 1993 to 2010.
However, from 2011 to 2014, there was a significant decrease in both plasma and RBC transfusions. Platelet transfusions remained stable over that period.
Aaron A. R. Tobian, MD, PhD, of Johns Hopkins University in Baltimore, Maryland, and his colleagues reported these findings in a letter to JAMA.
The researchers analyzed data from the National Inpatient Sample, which uses a stratified probability sample of 20% of all inpatient discharges in the US.
The team looked at transfusion trends from 1993 to 2014 but focused on trends from 2011 to 2014 because there was an inflection point in RBC transfusion in 2011. They used multivariable Poisson regression to estimate adjusted risk ratios (aRRs) comparing the risk of transfusion in 2011 and 2014.
The researchers found that RBC transfusions decreased from 6.8% in 2011 to 5.7% in 2014 (aRR=0.83; 95% confidence interval [CI], 0.78-0.88; P<0.001).
Plasma transfusions decreased from 1.0% to 0.87% (aRR=0.87; 95% CI, 0.80-0.95; P=0.003). And platelet transfusions remained stable (aRR=0.99; 95% CI, 0.89-1.10; P=0.93).
The researchers also conducted subgroup analyses to explore trends in RBC transfusion. They found that, from 2011 to 2014, there were significant reductions in RBC transfusions regardless of patients’ sex, race/ethnicity, risk severity, payer type, and admission type.
However, there was no significant reduction in RBC transfusions among patients younger than 18 years of age or in private investor–owned hospitals.
The researchers said the diagnostic coding used for this study was carried out primarily for billing purposes, and it was not possible to verify its accuracy. They also noted that the study only covered inpatient transfusions, so the results may not be generalizable to outpatient transfusions.















