User login
Is cancer immunotherapy more effective in men than women?
Cancer immunotherapy using checkpoint inhibitors may achieve greater mortality reductions in men than they do in women, new research has suggested.
In a meta-analysis and systematic review published in Lancet Oncology, researchers analyzed 20 randomized, controlled trials of immune checkpoint inhibitors that included detail on overall survival and patients’ sex; altogether, these studies involved 11,351 patients with advanced or metastatic cancers.
They found that while men treated with checkpoint inhibitors had a significant 28% reduced risk of death, compared with male controls, the survival benefit in women was smaller (14% reduced risk of death, compared with female controls).
Fabio Conforti, MD, from the European Institute of Oncology, Milan, and coauthors commented that the magnitude of the difference between the effect seen men and that in women was clinically significant.
“The pooled reduction of risk of death was double the size for male patients than for female patients – a difference that is similar to the size of the difference in survival benefit observed between patients with non–small cell lung cancer with PD-L1 positive (greater than 1%) tumors versus negative tumors, who were treated with anti-PD-1,” they wrote.
This difference between the benefit seen men and that in women was evident across all the subgroups in the study, which included subgroups based on cancer histotype, line of treatment, drugs used, and type of control.
However there was greater heterogeneity in the magnitude of the effect of checkpoint inhibitors on mortality in men than there was in women. The authors suggested this could be explained by the fact that the drugs have lower efficacy in women and this may therefore reduce the variability of results when compared with those in men.
The authors also looked at whether the studies that compared immunotherapies with nonimmunological therapies might show a different effect, but they still found a significantly higher benefit in men, compared with women.
The overall study population was two-thirds male and one-third female. The checkpoint inhibitors used were ipilimumab, tremelimumab, nivolumab, and pembrolizumab, and the trials were conducted in patients with melanoma, non–small cell lung cancer, head and neck cancer, renal cell carcinoma, urothelial tumors, gastric tumors, and mesothelioma.
Men have almost double the risk of mortality from cancer than do women, the authors said, with the greatest differences seen in melanoma, lung cancer, larynx cancer, esophagus cancer, and bladder cancer.
“This male-biased mortality is hypothesized to reflect differences not only in behavioral and biological factors, including causes of cancer and hormonal regulation, but also in the immune system.”
Despite this, sex is rarely taken into account when new therapeutic approaches are tested, the authors said.
They also commented on the fact that there was a relatively low number of women included in each trial, an issue that was recognized as far back as the 1990s as a major problem in medical trials.
“Our results further highlight this problem, showing clinically relevant differences in the efficacy of two important classes of immunological drugs, namely anti–CTLA-4 and anti–PD-1 antibodies, when compared with controls in male and female patients with advanced solid tumors,” they wrote.
They noted that they couldn’t exclude the possibility that the effect may be the result of other variables that were distributed differently between the sexes. However, they also qualified this by saying that variables known to affect the efficacy of immune checkpoint inhibitors, such as PD-L1 expression and mutation status, were not likely to explain the results.
Given their findings, the authors said a patient’s sex should be taken into account when weighing the risks and benefits of checkpoint inhibitors given the magnitude of benefit was sex-dependent. They also called for future immunotherapy studies to include more women.
No funding or conflicts of interest were declared.
SOURCE: Conforti F et al. Lancet Oncol. 2018 May 16. doi: 10.1016/S1470-2045(18)30261-4.
While cancer immunotherapy represents one of the most significant clinical advances in cancer treatment in the past decade, the basic but important clinical question about different effects between men and woman has not been addressed until now. The authors of this study are to be congratulated on such a comprehensive and well-conducted analysis, but the data does not completely support their final conclusion that checkpoint inhibitors benefit men more than women.
There are a large number of baseline characteristics of solid tumors that might differ between men and women and that have also been reported to impact the outcomes of patients treated with checkpoint inhibitors. Some of these may be lifestyle or behavioral characteristics – such as different smoking habits between men and women with non–small cell lung cancer – or differences in the distribution of oncogenic driver mutations between men and women.
We should therefore be cautious in jumping to conclusions and changing the current standard of care with respect to checkpoint inhibitors. In particular, we should not be denying treatment to women who are otherwise indicated for checkpoint inhibitors, based on these findings.
Omar Abdel-Rahman, MD, is from the clinical oncology department of the faculty of medicine at Ain Shams University in Cairo and from the Tom Baker Cancer Centre in Calgary. These comments are taken from an accompanying editorial (Lancet Oncol. 2018 May 16. doi: 10.1016/S1470-2045[18]30270-5.) No conflicts of interest were declared.
While cancer immunotherapy represents one of the most significant clinical advances in cancer treatment in the past decade, the basic but important clinical question about different effects between men and woman has not been addressed until now. The authors of this study are to be congratulated on such a comprehensive and well-conducted analysis, but the data does not completely support their final conclusion that checkpoint inhibitors benefit men more than women.
There are a large number of baseline characteristics of solid tumors that might differ between men and women and that have also been reported to impact the outcomes of patients treated with checkpoint inhibitors. Some of these may be lifestyle or behavioral characteristics – such as different smoking habits between men and women with non–small cell lung cancer – or differences in the distribution of oncogenic driver mutations between men and women.
We should therefore be cautious in jumping to conclusions and changing the current standard of care with respect to checkpoint inhibitors. In particular, we should not be denying treatment to women who are otherwise indicated for checkpoint inhibitors, based on these findings.
Omar Abdel-Rahman, MD, is from the clinical oncology department of the faculty of medicine at Ain Shams University in Cairo and from the Tom Baker Cancer Centre in Calgary. These comments are taken from an accompanying editorial (Lancet Oncol. 2018 May 16. doi: 10.1016/S1470-2045[18]30270-5.) No conflicts of interest were declared.
While cancer immunotherapy represents one of the most significant clinical advances in cancer treatment in the past decade, the basic but important clinical question about different effects between men and woman has not been addressed until now. The authors of this study are to be congratulated on such a comprehensive and well-conducted analysis, but the data does not completely support their final conclusion that checkpoint inhibitors benefit men more than women.
There are a large number of baseline characteristics of solid tumors that might differ between men and women and that have also been reported to impact the outcomes of patients treated with checkpoint inhibitors. Some of these may be lifestyle or behavioral characteristics – such as different smoking habits between men and women with non–small cell lung cancer – or differences in the distribution of oncogenic driver mutations between men and women.
We should therefore be cautious in jumping to conclusions and changing the current standard of care with respect to checkpoint inhibitors. In particular, we should not be denying treatment to women who are otherwise indicated for checkpoint inhibitors, based on these findings.
Omar Abdel-Rahman, MD, is from the clinical oncology department of the faculty of medicine at Ain Shams University in Cairo and from the Tom Baker Cancer Centre in Calgary. These comments are taken from an accompanying editorial (Lancet Oncol. 2018 May 16. doi: 10.1016/S1470-2045[18]30270-5.) No conflicts of interest were declared.
Cancer immunotherapy using checkpoint inhibitors may achieve greater mortality reductions in men than they do in women, new research has suggested.
In a meta-analysis and systematic review published in Lancet Oncology, researchers analyzed 20 randomized, controlled trials of immune checkpoint inhibitors that included detail on overall survival and patients’ sex; altogether, these studies involved 11,351 patients with advanced or metastatic cancers.
They found that while men treated with checkpoint inhibitors had a significant 28% reduced risk of death, compared with male controls, the survival benefit in women was smaller (14% reduced risk of death, compared with female controls).
Fabio Conforti, MD, from the European Institute of Oncology, Milan, and coauthors commented that the magnitude of the difference between the effect seen men and that in women was clinically significant.
“The pooled reduction of risk of death was double the size for male patients than for female patients – a difference that is similar to the size of the difference in survival benefit observed between patients with non–small cell lung cancer with PD-L1 positive (greater than 1%) tumors versus negative tumors, who were treated with anti-PD-1,” they wrote.
This difference between the benefit seen men and that in women was evident across all the subgroups in the study, which included subgroups based on cancer histotype, line of treatment, drugs used, and type of control.
However there was greater heterogeneity in the magnitude of the effect of checkpoint inhibitors on mortality in men than there was in women. The authors suggested this could be explained by the fact that the drugs have lower efficacy in women and this may therefore reduce the variability of results when compared with those in men.
The authors also looked at whether the studies that compared immunotherapies with nonimmunological therapies might show a different effect, but they still found a significantly higher benefit in men, compared with women.
The overall study population was two-thirds male and one-third female. The checkpoint inhibitors used were ipilimumab, tremelimumab, nivolumab, and pembrolizumab, and the trials were conducted in patients with melanoma, non–small cell lung cancer, head and neck cancer, renal cell carcinoma, urothelial tumors, gastric tumors, and mesothelioma.
Men have almost double the risk of mortality from cancer than do women, the authors said, with the greatest differences seen in melanoma, lung cancer, larynx cancer, esophagus cancer, and bladder cancer.
“This male-biased mortality is hypothesized to reflect differences not only in behavioral and biological factors, including causes of cancer and hormonal regulation, but also in the immune system.”
Despite this, sex is rarely taken into account when new therapeutic approaches are tested, the authors said.
They also commented on the fact that there was a relatively low number of women included in each trial, an issue that was recognized as far back as the 1990s as a major problem in medical trials.
“Our results further highlight this problem, showing clinically relevant differences in the efficacy of two important classes of immunological drugs, namely anti–CTLA-4 and anti–PD-1 antibodies, when compared with controls in male and female patients with advanced solid tumors,” they wrote.
They noted that they couldn’t exclude the possibility that the effect may be the result of other variables that were distributed differently between the sexes. However, they also qualified this by saying that variables known to affect the efficacy of immune checkpoint inhibitors, such as PD-L1 expression and mutation status, were not likely to explain the results.
Given their findings, the authors said a patient’s sex should be taken into account when weighing the risks and benefits of checkpoint inhibitors given the magnitude of benefit was sex-dependent. They also called for future immunotherapy studies to include more women.
No funding or conflicts of interest were declared.
SOURCE: Conforti F et al. Lancet Oncol. 2018 May 16. doi: 10.1016/S1470-2045(18)30261-4.
Cancer immunotherapy using checkpoint inhibitors may achieve greater mortality reductions in men than they do in women, new research has suggested.
In a meta-analysis and systematic review published in Lancet Oncology, researchers analyzed 20 randomized, controlled trials of immune checkpoint inhibitors that included detail on overall survival and patients’ sex; altogether, these studies involved 11,351 patients with advanced or metastatic cancers.
They found that while men treated with checkpoint inhibitors had a significant 28% reduced risk of death, compared with male controls, the survival benefit in women was smaller (14% reduced risk of death, compared with female controls).
Fabio Conforti, MD, from the European Institute of Oncology, Milan, and coauthors commented that the magnitude of the difference between the effect seen men and that in women was clinically significant.
“The pooled reduction of risk of death was double the size for male patients than for female patients – a difference that is similar to the size of the difference in survival benefit observed between patients with non–small cell lung cancer with PD-L1 positive (greater than 1%) tumors versus negative tumors, who were treated with anti-PD-1,” they wrote.
This difference between the benefit seen men and that in women was evident across all the subgroups in the study, which included subgroups based on cancer histotype, line of treatment, drugs used, and type of control.
However there was greater heterogeneity in the magnitude of the effect of checkpoint inhibitors on mortality in men than there was in women. The authors suggested this could be explained by the fact that the drugs have lower efficacy in women and this may therefore reduce the variability of results when compared with those in men.
The authors also looked at whether the studies that compared immunotherapies with nonimmunological therapies might show a different effect, but they still found a significantly higher benefit in men, compared with women.
The overall study population was two-thirds male and one-third female. The checkpoint inhibitors used were ipilimumab, tremelimumab, nivolumab, and pembrolizumab, and the trials were conducted in patients with melanoma, non–small cell lung cancer, head and neck cancer, renal cell carcinoma, urothelial tumors, gastric tumors, and mesothelioma.
Men have almost double the risk of mortality from cancer than do women, the authors said, with the greatest differences seen in melanoma, lung cancer, larynx cancer, esophagus cancer, and bladder cancer.
“This male-biased mortality is hypothesized to reflect differences not only in behavioral and biological factors, including causes of cancer and hormonal regulation, but also in the immune system.”
Despite this, sex is rarely taken into account when new therapeutic approaches are tested, the authors said.
They also commented on the fact that there was a relatively low number of women included in each trial, an issue that was recognized as far back as the 1990s as a major problem in medical trials.
“Our results further highlight this problem, showing clinically relevant differences in the efficacy of two important classes of immunological drugs, namely anti–CTLA-4 and anti–PD-1 antibodies, when compared with controls in male and female patients with advanced solid tumors,” they wrote.
They noted that they couldn’t exclude the possibility that the effect may be the result of other variables that were distributed differently between the sexes. However, they also qualified this by saying that variables known to affect the efficacy of immune checkpoint inhibitors, such as PD-L1 expression and mutation status, were not likely to explain the results.
Given their findings, the authors said a patient’s sex should be taken into account when weighing the risks and benefits of checkpoint inhibitors given the magnitude of benefit was sex-dependent. They also called for future immunotherapy studies to include more women.
No funding or conflicts of interest were declared.
SOURCE: Conforti F et al. Lancet Oncol. 2018 May 16. doi: 10.1016/S1470-2045(18)30261-4.
FROM LANCET ONCOLOGY
Key clinical point: Checkpoint inhibitors are linked with greater mortality reductions in men than in women.
Major finding: Checkpoint inhibitors are associated with a 28% reduction in cancer mortality in men and 14% in women.
Study details: Systematic review and meta-analysis of 20 randomized, controlled trials involving 11,351 patients.
Disclosures: No funding or conflicts of interest were declared.
Source: Conforti F et al. Lancet Oncol. 2018 May 16. doi: 10.1016/S1470-2045(18)30261-4.
As-needed budesonide-formoterol prevented exacerbations in mild asthma
according to the results of two large, double-blind, 52-week, randomized phase 3 trials.
In the SYGMA1 (Symbicort Given as Needed in Mild Asthma) trial, the regimen outperformed as-needed terbutaline in terms of asthma control (34.4% vs. 31.1% of weeks; P = .046) and exacerbations (rate ratio, 0.36; 95% confidence interval, 0.27-0.49). In the SYGMA2 study, it was noninferior to twice-daily budesonide for preventing severe exacerbations (RR, 0.97; upper one-sided 95% CI, 1.16). The findings were published in two reports in the New England Journal of Medicine.
Accordingly, they randomly assigned 3,849 patients aged 12 years and up who had mild persistent asthma (mean forced expiratory volume in 1 second [FEV1] before bronchodilator use, 84% of predicted value) to receive one of three regimens: twice-daily placebo plus terbutaline (0.5 mg) used as needed, twice-daily placebo plus budesonide-formoterol (200 mcg of budesonide and 6 mcg of formoterol) used as needed, or maintenance twice-daily budesonide (200 mcg) plus as-needed terbutaline (0.5 mg), all for 52 weeks.
In the final analysis of 3,836 patients, annual rates of severe exacerbations were 0.20 with terbutaline, significantly worse than with budesonide-formoterol (0.07) or maintenance budesonide (0.09). Using budesonide-formoterol (Symbicort) as needed improved the odds of having well-controlled asthma by about 14%, when compared with using terbutaline as needed (odds ratio, 1.14; 95% CI, 1.00-1.30; P = .046).
Although maintenance budesonide controlled asthma best (44.4% of weeks; OR vs. budesonide-formoterol, 0.64; 95% CI, 0.57-0.73), 21% of patients did not adhere to it, the researchers reported. “Patients are often more concerned [than their health care providers] about adverse effects of inhaled glucocorticoids, even when low inhaled doses are used,” they wrote. Notably, the budesonide-formoterol as-needed group received a median daily dose of only 57 mcg inhaled glucocorticoid, 17% of that received by the budesonide maintenance group.
In SYGMA2 (NCT02224157), 4,215 patients with mild persistent asthma aged 12 years and up were randomly assigned to receive either twice-daily placebo plus as-needed budesonide-formoterol or twice-daily maintenance budesonide plus as-needed terbutaline. Doses were the same as in the SYGMA1 trial. The regimens resembled each other in terms of severe exacerbations (annualized rates, 0.11 and 0.12, respectively) and time to first exacerbation, even though budesonide-formoterol patients received a 75% lower median daily dose of inhaled glucocorticoid, reported Eric D. Bateman, MD, of the University of Cape Town, South Africa, and his associates.
Results from both trials suggested that as-needed budesonide-formoterol provided better symptom control than did terbutaline but worse symptom control than did twice-daily budesonide. In SYGMA1, the change from baseline on the Asthma Control Questionnaire-5 (ACQ-5) favored budesonide-formoterol over terbutaline by an average of 0.15 units and similarly favored twice-daily budesonide over budesonide-formoterol. In SYGMA2, the budesonide maintenance group averaged 0.11 units greater improvement on the ACQ-5 and 0.10 better improvement on the standardized Asthma Quality of Life Questionnaire, compared with as-needed budesonide-formoterol recipients.
Finally, lung function assessments favored as-needed budesonide-formoterol over terbutaline but not over maintenance budesonide. SYGMA1, mean changes (from baseline) in FEV1 before bronchodilator use were 11.2 mL with terbutaline, 65.0 mL with budesonide-formoterol, and 119.3 mL with maintenance budesonide. In SYGMA2, these values were 104 mL with budesonide-formoterol and 136.6 mL with maintenance budesonide.
AstraZeneca provided funding. For SYGMA1, Dr. Byrne disclosed ties to AstraZeneca, Novartis, GlaxoSmithKline, Medimmune, and Genentech. For SYGMA2, Dr. Bateman disclosed ties to AstraZeneca, Novartis, Cipla, Vectura, Boehringer Ingelheim, and a number of other pharmaceutical companies.
SOURCES: O’Byrne PM et al. N Engl J Med. 2018;378(20):1865-76.Bateman ED et al. N Engl J Med. 2018;378(20):1877-87.
In the SYGMA1 and SYGMA2 trials, as-needed budesonide-formoterol (Symbicort) prevented exacerbations and loss of lung function, the two worst outcomes of poorly controlled asthma, concluded Stephen C. Lazarus, MD, in an editorial accompanying the studies in the New England Journal of Medicine.
“As-needed treatment was similar, or at least noninferior, to regular maintenance therapy with inhaled glucocorticoids with regard to the prevention of exacerbations, and exacerbations are the main contributor to loss of lung function, death, and cost,” wrote Dr. Lazarus.
Patients typically received only 17%-25% as much inhaled glucocorticoid as did those on maintenance budesonide, which would help prevent side effects and would make the regimen more acceptable to “glucocorticoid-averse patients,” he added. Another benefit to patients with mild persistent asthma using as-needed budesonide-formoterol instead of inhaled glucocorticoid maintenance therapy is that it would result in nearly $1 billion in cost savings in the United States yearly.
Budesonide-formoterol did not control symptoms as well as did maintenance budesonide, but patients might accept “occasional mild symptoms and inhaler use if it [freed] them from daily use of inhaled glucocorticoids while preventing loss of lung function and exacerbations,” he concluded. “For these patients, ‘Two out of three ain’t bad!’ ”
Dr. Lazarus is in the department of medicine and at the Cardiovascular Research Institute, University of California, San Francisco. He reported having no conflicts of interest. This comments are from his editorial (N Engl J Med. 2018 May 17. doi: 10.1056/NEJMe1802680).
In the SYGMA1 and SYGMA2 trials, as-needed budesonide-formoterol (Symbicort) prevented exacerbations and loss of lung function, the two worst outcomes of poorly controlled asthma, concluded Stephen C. Lazarus, MD, in an editorial accompanying the studies in the New England Journal of Medicine.
“As-needed treatment was similar, or at least noninferior, to regular maintenance therapy with inhaled glucocorticoids with regard to the prevention of exacerbations, and exacerbations are the main contributor to loss of lung function, death, and cost,” wrote Dr. Lazarus.
Patients typically received only 17%-25% as much inhaled glucocorticoid as did those on maintenance budesonide, which would help prevent side effects and would make the regimen more acceptable to “glucocorticoid-averse patients,” he added. Another benefit to patients with mild persistent asthma using as-needed budesonide-formoterol instead of inhaled glucocorticoid maintenance therapy is that it would result in nearly $1 billion in cost savings in the United States yearly.
Budesonide-formoterol did not control symptoms as well as did maintenance budesonide, but patients might accept “occasional mild symptoms and inhaler use if it [freed] them from daily use of inhaled glucocorticoids while preventing loss of lung function and exacerbations,” he concluded. “For these patients, ‘Two out of three ain’t bad!’ ”
Dr. Lazarus is in the department of medicine and at the Cardiovascular Research Institute, University of California, San Francisco. He reported having no conflicts of interest. This comments are from his editorial (N Engl J Med. 2018 May 17. doi: 10.1056/NEJMe1802680).
In the SYGMA1 and SYGMA2 trials, as-needed budesonide-formoterol (Symbicort) prevented exacerbations and loss of lung function, the two worst outcomes of poorly controlled asthma, concluded Stephen C. Lazarus, MD, in an editorial accompanying the studies in the New England Journal of Medicine.
“As-needed treatment was similar, or at least noninferior, to regular maintenance therapy with inhaled glucocorticoids with regard to the prevention of exacerbations, and exacerbations are the main contributor to loss of lung function, death, and cost,” wrote Dr. Lazarus.
Patients typically received only 17%-25% as much inhaled glucocorticoid as did those on maintenance budesonide, which would help prevent side effects and would make the regimen more acceptable to “glucocorticoid-averse patients,” he added. Another benefit to patients with mild persistent asthma using as-needed budesonide-formoterol instead of inhaled glucocorticoid maintenance therapy is that it would result in nearly $1 billion in cost savings in the United States yearly.
Budesonide-formoterol did not control symptoms as well as did maintenance budesonide, but patients might accept “occasional mild symptoms and inhaler use if it [freed] them from daily use of inhaled glucocorticoids while preventing loss of lung function and exacerbations,” he concluded. “For these patients, ‘Two out of three ain’t bad!’ ”
Dr. Lazarus is in the department of medicine and at the Cardiovascular Research Institute, University of California, San Francisco. He reported having no conflicts of interest. This comments are from his editorial (N Engl J Med. 2018 May 17. doi: 10.1056/NEJMe1802680).
according to the results of two large, double-blind, 52-week, randomized phase 3 trials.
In the SYGMA1 (Symbicort Given as Needed in Mild Asthma) trial, the regimen outperformed as-needed terbutaline in terms of asthma control (34.4% vs. 31.1% of weeks; P = .046) and exacerbations (rate ratio, 0.36; 95% confidence interval, 0.27-0.49). In the SYGMA2 study, it was noninferior to twice-daily budesonide for preventing severe exacerbations (RR, 0.97; upper one-sided 95% CI, 1.16). The findings were published in two reports in the New England Journal of Medicine.
Accordingly, they randomly assigned 3,849 patients aged 12 years and up who had mild persistent asthma (mean forced expiratory volume in 1 second [FEV1] before bronchodilator use, 84% of predicted value) to receive one of three regimens: twice-daily placebo plus terbutaline (0.5 mg) used as needed, twice-daily placebo plus budesonide-formoterol (200 mcg of budesonide and 6 mcg of formoterol) used as needed, or maintenance twice-daily budesonide (200 mcg) plus as-needed terbutaline (0.5 mg), all for 52 weeks.
In the final analysis of 3,836 patients, annual rates of severe exacerbations were 0.20 with terbutaline, significantly worse than with budesonide-formoterol (0.07) or maintenance budesonide (0.09). Using budesonide-formoterol (Symbicort) as needed improved the odds of having well-controlled asthma by about 14%, when compared with using terbutaline as needed (odds ratio, 1.14; 95% CI, 1.00-1.30; P = .046).
Although maintenance budesonide controlled asthma best (44.4% of weeks; OR vs. budesonide-formoterol, 0.64; 95% CI, 0.57-0.73), 21% of patients did not adhere to it, the researchers reported. “Patients are often more concerned [than their health care providers] about adverse effects of inhaled glucocorticoids, even when low inhaled doses are used,” they wrote. Notably, the budesonide-formoterol as-needed group received a median daily dose of only 57 mcg inhaled glucocorticoid, 17% of that received by the budesonide maintenance group.
In SYGMA2 (NCT02224157), 4,215 patients with mild persistent asthma aged 12 years and up were randomly assigned to receive either twice-daily placebo plus as-needed budesonide-formoterol or twice-daily maintenance budesonide plus as-needed terbutaline. Doses were the same as in the SYGMA1 trial. The regimens resembled each other in terms of severe exacerbations (annualized rates, 0.11 and 0.12, respectively) and time to first exacerbation, even though budesonide-formoterol patients received a 75% lower median daily dose of inhaled glucocorticoid, reported Eric D. Bateman, MD, of the University of Cape Town, South Africa, and his associates.
Results from both trials suggested that as-needed budesonide-formoterol provided better symptom control than did terbutaline but worse symptom control than did twice-daily budesonide. In SYGMA1, the change from baseline on the Asthma Control Questionnaire-5 (ACQ-5) favored budesonide-formoterol over terbutaline by an average of 0.15 units and similarly favored twice-daily budesonide over budesonide-formoterol. In SYGMA2, the budesonide maintenance group averaged 0.11 units greater improvement on the ACQ-5 and 0.10 better improvement on the standardized Asthma Quality of Life Questionnaire, compared with as-needed budesonide-formoterol recipients.
Finally, lung function assessments favored as-needed budesonide-formoterol over terbutaline but not over maintenance budesonide. SYGMA1, mean changes (from baseline) in FEV1 before bronchodilator use were 11.2 mL with terbutaline, 65.0 mL with budesonide-formoterol, and 119.3 mL with maintenance budesonide. In SYGMA2, these values were 104 mL with budesonide-formoterol and 136.6 mL with maintenance budesonide.
AstraZeneca provided funding. For SYGMA1, Dr. Byrne disclosed ties to AstraZeneca, Novartis, GlaxoSmithKline, Medimmune, and Genentech. For SYGMA2, Dr. Bateman disclosed ties to AstraZeneca, Novartis, Cipla, Vectura, Boehringer Ingelheim, and a number of other pharmaceutical companies.
SOURCES: O’Byrne PM et al. N Engl J Med. 2018;378(20):1865-76.Bateman ED et al. N Engl J Med. 2018;378(20):1877-87.
according to the results of two large, double-blind, 52-week, randomized phase 3 trials.
In the SYGMA1 (Symbicort Given as Needed in Mild Asthma) trial, the regimen outperformed as-needed terbutaline in terms of asthma control (34.4% vs. 31.1% of weeks; P = .046) and exacerbations (rate ratio, 0.36; 95% confidence interval, 0.27-0.49). In the SYGMA2 study, it was noninferior to twice-daily budesonide for preventing severe exacerbations (RR, 0.97; upper one-sided 95% CI, 1.16). The findings were published in two reports in the New England Journal of Medicine.
Accordingly, they randomly assigned 3,849 patients aged 12 years and up who had mild persistent asthma (mean forced expiratory volume in 1 second [FEV1] before bronchodilator use, 84% of predicted value) to receive one of three regimens: twice-daily placebo plus terbutaline (0.5 mg) used as needed, twice-daily placebo plus budesonide-formoterol (200 mcg of budesonide and 6 mcg of formoterol) used as needed, or maintenance twice-daily budesonide (200 mcg) plus as-needed terbutaline (0.5 mg), all for 52 weeks.
In the final analysis of 3,836 patients, annual rates of severe exacerbations were 0.20 with terbutaline, significantly worse than with budesonide-formoterol (0.07) or maintenance budesonide (0.09). Using budesonide-formoterol (Symbicort) as needed improved the odds of having well-controlled asthma by about 14%, when compared with using terbutaline as needed (odds ratio, 1.14; 95% CI, 1.00-1.30; P = .046).
Although maintenance budesonide controlled asthma best (44.4% of weeks; OR vs. budesonide-formoterol, 0.64; 95% CI, 0.57-0.73), 21% of patients did not adhere to it, the researchers reported. “Patients are often more concerned [than their health care providers] about adverse effects of inhaled glucocorticoids, even when low inhaled doses are used,” they wrote. Notably, the budesonide-formoterol as-needed group received a median daily dose of only 57 mcg inhaled glucocorticoid, 17% of that received by the budesonide maintenance group.
In SYGMA2 (NCT02224157), 4,215 patients with mild persistent asthma aged 12 years and up were randomly assigned to receive either twice-daily placebo plus as-needed budesonide-formoterol or twice-daily maintenance budesonide plus as-needed terbutaline. Doses were the same as in the SYGMA1 trial. The regimens resembled each other in terms of severe exacerbations (annualized rates, 0.11 and 0.12, respectively) and time to first exacerbation, even though budesonide-formoterol patients received a 75% lower median daily dose of inhaled glucocorticoid, reported Eric D. Bateman, MD, of the University of Cape Town, South Africa, and his associates.
Results from both trials suggested that as-needed budesonide-formoterol provided better symptom control than did terbutaline but worse symptom control than did twice-daily budesonide. In SYGMA1, the change from baseline on the Asthma Control Questionnaire-5 (ACQ-5) favored budesonide-formoterol over terbutaline by an average of 0.15 units and similarly favored twice-daily budesonide over budesonide-formoterol. In SYGMA2, the budesonide maintenance group averaged 0.11 units greater improvement on the ACQ-5 and 0.10 better improvement on the standardized Asthma Quality of Life Questionnaire, compared with as-needed budesonide-formoterol recipients.
Finally, lung function assessments favored as-needed budesonide-formoterol over terbutaline but not over maintenance budesonide. SYGMA1, mean changes (from baseline) in FEV1 before bronchodilator use were 11.2 mL with terbutaline, 65.0 mL with budesonide-formoterol, and 119.3 mL with maintenance budesonide. In SYGMA2, these values were 104 mL with budesonide-formoterol and 136.6 mL with maintenance budesonide.
AstraZeneca provided funding. For SYGMA1, Dr. Byrne disclosed ties to AstraZeneca, Novartis, GlaxoSmithKline, Medimmune, and Genentech. For SYGMA2, Dr. Bateman disclosed ties to AstraZeneca, Novartis, Cipla, Vectura, Boehringer Ingelheim, and a number of other pharmaceutical companies.
SOURCES: O’Byrne PM et al. N Engl J Med. 2018;378(20):1865-76.Bateman ED et al. N Engl J Med. 2018;378(20):1877-87.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: As-needed budesonide-formoterol (Symbicort) prevented exacerbations in patients with mild persistent asthma.
Major finding: In the SYGMA1 trial, the regimen outperformed as-needed terbutaline for asthma control (34.4% vs. 31.1% of weeks; P = .046) and exacerbations (RR, 0.36; 95% CI, 0.27-0.49). In SYGMA2, the regimen was noninferior to twice-daily budesonide for preventing severe exacerbations (RR, 0.97; upper one-sided 95% confidence limit, 1.16).
Study details: SYGMA1 and SYGMA2, randomized phase 3 trials of 8,012 patients aged 12 years and older with mild persistent asthma.
Disclosures: AstraZeneca provided funding. For SYGMA1, Dr. Byrne disclosed ties to AstraZeneca, Novartis, GlaxoSmithKline, Medimmune, and Genentech. For SYGMA2, Dr. Bateman disclosed ties to AstraZeneca, Novartis, Cipla, Vectura, Boehringer Ingelheim, and a number of other pharmaceutical companies.
Sources: O’Byrne PM et al. N Engl J Med. 2018 May 17;378(20);1865-76. Bateman ED et al. N Engl J Med. 2018 May 17;378(20):1877-87.
Adding bortezomib does not improve MCL outcomes
Bortezomib added to an alternating chemoimmunotherapy regimen did not improve time to treatment failure in patients with newly diagnosed mantle cell lymphoma (MCL), results of a phase 2 study have suggested.
Response rates and time to treatment failure were similar to what has been seen historically without the addition of bortezomib, according to study investigator Jorge E. Romaguera, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues.
The phase 2 study included 95 patients with newly diagnosed MCL treated with alternating cycles of bortezomib added to rituximab plus hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (BzR-hyperCVAD) and bortezomib added to rituximab plus high-dose methotrexate and high-dose cytarabine (BzR-MA).
Of 87 patients evaluable for response, alternating BzR-hyperCVAD/BzR-MA resulted in an overall response rate of 100% and a complete response rate of 82%, Dr. Romaguera and his colleagues reported in the journal Cancer. At a median follow-up of 44 months, median time to treatment failure was 55 months, and median overall survival had not yet been reached, according to the report.
Dr. Romaguera and his coauthors compared these results with those from a previous study of alternating R-hyperCVAD/R-MA, in which the median time to treatment failure was 56.4 months. “This suggests that the addition of bortezomib does not improve the outcome,” they wrote in the current report.
Although more follow-up is needed, the landscape of MCL treatment is changing quickly, they added. In particular, lenalidomide and ibrutinib, already approved for relapsed/refractory MCL, are now being evaluated as part of first-line MCL regimens. “These drugs will offer strategies of either consolidation or maintenance after induction and will hopefully help continue to improve the duration of the initial response and the overall outcome,” the researchers wrote.
In the current phase 2 study, the fact that 100% of patients achieved complete response suggested that relapses come from minimal residual disease, which “has clearly become a clinical factor for the outcomes of patients with MCL and will likely become the next endpoint,” they wrote.
The researchers reported having no financial disclosures related to the study, which was supported by Takeda Oncology.
SOURCE: Romaguera JE et al. Cancer. 2018 May 3. doi: 10.1002/cncr.31361.
Bortezomib added to an alternating chemoimmunotherapy regimen did not improve time to treatment failure in patients with newly diagnosed mantle cell lymphoma (MCL), results of a phase 2 study have suggested.
Response rates and time to treatment failure were similar to what has been seen historically without the addition of bortezomib, according to study investigator Jorge E. Romaguera, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues.
The phase 2 study included 95 patients with newly diagnosed MCL treated with alternating cycles of bortezomib added to rituximab plus hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (BzR-hyperCVAD) and bortezomib added to rituximab plus high-dose methotrexate and high-dose cytarabine (BzR-MA).
Of 87 patients evaluable for response, alternating BzR-hyperCVAD/BzR-MA resulted in an overall response rate of 100% and a complete response rate of 82%, Dr. Romaguera and his colleagues reported in the journal Cancer. At a median follow-up of 44 months, median time to treatment failure was 55 months, and median overall survival had not yet been reached, according to the report.
Dr. Romaguera and his coauthors compared these results with those from a previous study of alternating R-hyperCVAD/R-MA, in which the median time to treatment failure was 56.4 months. “This suggests that the addition of bortezomib does not improve the outcome,” they wrote in the current report.
Although more follow-up is needed, the landscape of MCL treatment is changing quickly, they added. In particular, lenalidomide and ibrutinib, already approved for relapsed/refractory MCL, are now being evaluated as part of first-line MCL regimens. “These drugs will offer strategies of either consolidation or maintenance after induction and will hopefully help continue to improve the duration of the initial response and the overall outcome,” the researchers wrote.
In the current phase 2 study, the fact that 100% of patients achieved complete response suggested that relapses come from minimal residual disease, which “has clearly become a clinical factor for the outcomes of patients with MCL and will likely become the next endpoint,” they wrote.
The researchers reported having no financial disclosures related to the study, which was supported by Takeda Oncology.
SOURCE: Romaguera JE et al. Cancer. 2018 May 3. doi: 10.1002/cncr.31361.
Bortezomib added to an alternating chemoimmunotherapy regimen did not improve time to treatment failure in patients with newly diagnosed mantle cell lymphoma (MCL), results of a phase 2 study have suggested.
Response rates and time to treatment failure were similar to what has been seen historically without the addition of bortezomib, according to study investigator Jorge E. Romaguera, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues.
The phase 2 study included 95 patients with newly diagnosed MCL treated with alternating cycles of bortezomib added to rituximab plus hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (BzR-hyperCVAD) and bortezomib added to rituximab plus high-dose methotrexate and high-dose cytarabine (BzR-MA).
Of 87 patients evaluable for response, alternating BzR-hyperCVAD/BzR-MA resulted in an overall response rate of 100% and a complete response rate of 82%, Dr. Romaguera and his colleagues reported in the journal Cancer. At a median follow-up of 44 months, median time to treatment failure was 55 months, and median overall survival had not yet been reached, according to the report.
Dr. Romaguera and his coauthors compared these results with those from a previous study of alternating R-hyperCVAD/R-MA, in which the median time to treatment failure was 56.4 months. “This suggests that the addition of bortezomib does not improve the outcome,” they wrote in the current report.
Although more follow-up is needed, the landscape of MCL treatment is changing quickly, they added. In particular, lenalidomide and ibrutinib, already approved for relapsed/refractory MCL, are now being evaluated as part of first-line MCL regimens. “These drugs will offer strategies of either consolidation or maintenance after induction and will hopefully help continue to improve the duration of the initial response and the overall outcome,” the researchers wrote.
In the current phase 2 study, the fact that 100% of patients achieved complete response suggested that relapses come from minimal residual disease, which “has clearly become a clinical factor for the outcomes of patients with MCL and will likely become the next endpoint,” they wrote.
The researchers reported having no financial disclosures related to the study, which was supported by Takeda Oncology.
SOURCE: Romaguera JE et al. Cancer. 2018 May 3. doi: 10.1002/cncr.31361.
FROM CANCER
Key clinical point:
Major finding: Rates of overall and complete response were 100% and 82%, respectively, while time to treatment failure was 55 months.
Study details: A phase 2 trial that included 95 patients treated with alternating cycles of bortezomib added to rituximab plus hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (BzR-hyperCVAD) and bortezomib added to rituximab plus high-dose methotrexate and high-dose cytarabine (BzR-MA).
Disclosures: The study was supported by Takeda Oncology. The researchers reported having no financial disclosures related to the study.
Source: Romaguera JE et al. Cancer. 2018 May 3. doi: 10.1002/cncr.31361
Understanding Pediatric Abdominal Migraine
Abdominal migraine is a common cause of chronic and recurrent abdominal pain in children, according to a recent review. It is characterized by paroxysms of moderate to severe abdominal pain that is midline, periumbilical, or diffuse in location and accompanied by other symptoms including headache, anorexia, nausea, vomiting, or pallor. Despite the presence of comprehensive diagnostic criteria, it continues to be an underdiagnosed entity. Key points include:
- The average age of diagnosis is 3 to 10 years with peak incidence at 7 years.
- Most of the patients have a personal or family history of migraine.
- Pathophysiology of the condition is believed to be similar to that of other functional gastrointestinal disorders and cephalic migraine; it is also well recognized as a type of pediatric migraine variant.
- A careful history, thorough physical examination, and use of well-defined, symptom-based guidelines are needed to make a diagnosis.
- Although it resolves completely in most of the patients, these patients have a strong propensity to develop migraine later in life.
- Nonpharmacologic treatment options, including avoidance of triggers, behavior therapy, and dietary modifications should be the initial line of management.
Pediatric abdominal migraine: current perspectives on a lesser known entity. [Published online ahead of print April 24, 2018]. Pediatric Health Med Ther. doi:10.2147%2FPHMT.S127210.
Abdominal migraine is a common cause of chronic and recurrent abdominal pain in children, according to a recent review. It is characterized by paroxysms of moderate to severe abdominal pain that is midline, periumbilical, or diffuse in location and accompanied by other symptoms including headache, anorexia, nausea, vomiting, or pallor. Despite the presence of comprehensive diagnostic criteria, it continues to be an underdiagnosed entity. Key points include:
- The average age of diagnosis is 3 to 10 years with peak incidence at 7 years.
- Most of the patients have a personal or family history of migraine.
- Pathophysiology of the condition is believed to be similar to that of other functional gastrointestinal disorders and cephalic migraine; it is also well recognized as a type of pediatric migraine variant.
- A careful history, thorough physical examination, and use of well-defined, symptom-based guidelines are needed to make a diagnosis.
- Although it resolves completely in most of the patients, these patients have a strong propensity to develop migraine later in life.
- Nonpharmacologic treatment options, including avoidance of triggers, behavior therapy, and dietary modifications should be the initial line of management.
Pediatric abdominal migraine: current perspectives on a lesser known entity. [Published online ahead of print April 24, 2018]. Pediatric Health Med Ther. doi:10.2147%2FPHMT.S127210.
Abdominal migraine is a common cause of chronic and recurrent abdominal pain in children, according to a recent review. It is characterized by paroxysms of moderate to severe abdominal pain that is midline, periumbilical, or diffuse in location and accompanied by other symptoms including headache, anorexia, nausea, vomiting, or pallor. Despite the presence of comprehensive diagnostic criteria, it continues to be an underdiagnosed entity. Key points include:
- The average age of diagnosis is 3 to 10 years with peak incidence at 7 years.
- Most of the patients have a personal or family history of migraine.
- Pathophysiology of the condition is believed to be similar to that of other functional gastrointestinal disorders and cephalic migraine; it is also well recognized as a type of pediatric migraine variant.
- A careful history, thorough physical examination, and use of well-defined, symptom-based guidelines are needed to make a diagnosis.
- Although it resolves completely in most of the patients, these patients have a strong propensity to develop migraine later in life.
- Nonpharmacologic treatment options, including avoidance of triggers, behavior therapy, and dietary modifications should be the initial line of management.
Pediatric abdominal migraine: current perspectives on a lesser known entity. [Published online ahead of print April 24, 2018]. Pediatric Health Med Ther. doi:10.2147%2FPHMT.S127210.
VIDEO: CASTLE-AF suggests atrial fibrillation burden better predicts outcomes
BOSTON – From the earliest days of using catheter ablation to treat atrial fibrillation (AF), in the 1990s, clinicians have defined ablation success based on whether patients had recurrence of their arrhythmia following treatment. New findings suggest that this standard was off, and that
The new study used data collected in the CASTLE-AF (Catheter Ablation vs. Standard Conventional Treatment in Patients With LV Dysfunction and AF) multicenter trial, which compared the efficacy of AF ablation with antiarrhythmic drug treatment in patients with heart failure for improving survival and freedom from hospitalization for heart failure. The trial’s primary finding showed that, in 363 randomized patients, AF ablation cut the primary adverse event rate by 38% relative to antiarrhythmic drug therapy (New Engl J Med. 2018 Feb 1;378[5]:417-27)
“There have been concerns about the high recurrence rate of AF following ablation,” with reported cumulative recurrence rates running as high as 80% by 5 years after ablation, noted Dr. Brachmann. “The news now is that recurrence alone doesn’t make a difference; we can still help patients” by reducing their AF burden, although he cautioned that this relationship has so far only been seen in patients with heart failure with reduced ejection fraction, the type of patients enrolled in CASTLE-AF.
“This information is very informative for clinicians counseling patients who undergo ablation. Ablation may not eliminate all of a patient’s AF, but it will substantially reduce it, and that’s associated with better outcomes,” commented Andrew D. Krahn, MD, professor and chief of cardiology at the University of British Columbia in Vancouver. “Early on using ablation, we had a curative approach and used ablation to ‘clip the wire.’ Now we have growing, objective evidence for ‘debulking’ the problem” working without the need to completely eliminate all AF episodes.
To run the post hoc analysis Dr. Brachmann and his associates categorized the 363 patients randomized in CASTLE-AF by the treatment they received during the study’s first 12 weeks: 150 patients underwent catheter ablation, and 210 received drug treatment, with three patients dropping out. Although this division of the patients diverged from the randomized subgroups, the ablated and drug-treated patients showed no significant differences when compared for several clinical parameters.
Ablation was significantly more effective than drug therapy for cutting atrial fibrillation burden, which started at an average of about 50% in all patients at baseline. AF burden fell to an average of about 10%-15% among the ablated patients when measured at several time points during follow-up, whereas AF burden remained at an average of about 50% or higher among the drug-treated patients.
A receiver operating characteristic analysis showed that change in AF burden after ablation produced a statistically significant 0.66 area-under-the-curve for the primary endpoint, which suggested that reduction in AF burden post ablation could account for about two-thirds of the drop in deaths and hospitalizations for heart failure. Among the nonablated patients the area-under-the-curve was an insignificant 0.49 showing that with drug treatment AF burden had no discernible relationship with outcomes.
One further observation in the new analysis was that a drop in AF burden was linked with improved outcomes regardless of whether or not a “blanking period” was imposed on the data. Researchers applied a 90-day blanking period after ablation when assessing the treatment’s efficacy to censor from the analysis recurrences that occurred soon after ablation. The need for a blanking period during the first 90 days “was put to rest” by this new analysis, Dr. Brachmann said.
CASTLE-AF was sponsored by Biotronik. Dr. Brachmann has been a consultant to and has received research funding from Biotronik and several other companies. Dr. Krahn has been a consultant to Medtronic and has received research support from Medtronic and Boston Scientific. Dr. Link had no disclosures.
SOURCE: Brachmann J et al. Heart Rhythm 2018, Abstract B-LBCT02-04.
This new analysis of data from the CASTLE-AF trial is exciting. It shows that, if we reduce the atrial fibrillation burden when we perform catheter ablation of atrial fibrillation in patients with heart failure, patients do better.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Until now, cardiac electrophysiologists who perform atrial fibrillation (AF) ablation have been too hard on themselves by counting as a failure every patient who develops an AF recurrence that lasts for 30 seconds or more. We know that patients who have a substantial drop in their AF burden after catheter ablation report feeling better even if they continue to have some AF events. When their AF burden drops substantially, patients are better able to work and perform activities of daily life. Many options, including noninvasive devices, are now available to monitor patients’ postablation change in AF burden.
We currently tell patients the success rates of catheter ablation on AF based on recurrence rates. Maybe we need to change our definition of success to a cut in AF burden. Based on these new findings, patients don’t need to be perfect after ablation, with absolutely no recurrences. I have patients who are very happy with their outcome after ablation who still have episodes. The success rate of catheter ablation for treating AF may be much better than we have thought.
Andrea M. Russo, MD , is professor and director of the electrophysiology and arrhythmia service at Cooper University Health Care in Camden, N.J. She made these comments during a press conference and in a video interview. She had no relevant disclosures.
This new analysis of data from the CASTLE-AF trial is exciting. It shows that, if we reduce the atrial fibrillation burden when we perform catheter ablation of atrial fibrillation in patients with heart failure, patients do better.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Until now, cardiac electrophysiologists who perform atrial fibrillation (AF) ablation have been too hard on themselves by counting as a failure every patient who develops an AF recurrence that lasts for 30 seconds or more. We know that patients who have a substantial drop in their AF burden after catheter ablation report feeling better even if they continue to have some AF events. When their AF burden drops substantially, patients are better able to work and perform activities of daily life. Many options, including noninvasive devices, are now available to monitor patients’ postablation change in AF burden.
We currently tell patients the success rates of catheter ablation on AF based on recurrence rates. Maybe we need to change our definition of success to a cut in AF burden. Based on these new findings, patients don’t need to be perfect after ablation, with absolutely no recurrences. I have patients who are very happy with their outcome after ablation who still have episodes. The success rate of catheter ablation for treating AF may be much better than we have thought.
Andrea M. Russo, MD , is professor and director of the electrophysiology and arrhythmia service at Cooper University Health Care in Camden, N.J. She made these comments during a press conference and in a video interview. She had no relevant disclosures.
This new analysis of data from the CASTLE-AF trial is exciting. It shows that, if we reduce the atrial fibrillation burden when we perform catheter ablation of atrial fibrillation in patients with heart failure, patients do better.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Until now, cardiac electrophysiologists who perform atrial fibrillation (AF) ablation have been too hard on themselves by counting as a failure every patient who develops an AF recurrence that lasts for 30 seconds or more. We know that patients who have a substantial drop in their AF burden after catheter ablation report feeling better even if they continue to have some AF events. When their AF burden drops substantially, patients are better able to work and perform activities of daily life. Many options, including noninvasive devices, are now available to monitor patients’ postablation change in AF burden.
We currently tell patients the success rates of catheter ablation on AF based on recurrence rates. Maybe we need to change our definition of success to a cut in AF burden. Based on these new findings, patients don’t need to be perfect after ablation, with absolutely no recurrences. I have patients who are very happy with their outcome after ablation who still have episodes. The success rate of catheter ablation for treating AF may be much better than we have thought.
Andrea M. Russo, MD , is professor and director of the electrophysiology and arrhythmia service at Cooper University Health Care in Camden, N.J. She made these comments during a press conference and in a video interview. She had no relevant disclosures.
BOSTON – From the earliest days of using catheter ablation to treat atrial fibrillation (AF), in the 1990s, clinicians have defined ablation success based on whether patients had recurrence of their arrhythmia following treatment. New findings suggest that this standard was off, and that
The new study used data collected in the CASTLE-AF (Catheter Ablation vs. Standard Conventional Treatment in Patients With LV Dysfunction and AF) multicenter trial, which compared the efficacy of AF ablation with antiarrhythmic drug treatment in patients with heart failure for improving survival and freedom from hospitalization for heart failure. The trial’s primary finding showed that, in 363 randomized patients, AF ablation cut the primary adverse event rate by 38% relative to antiarrhythmic drug therapy (New Engl J Med. 2018 Feb 1;378[5]:417-27)
“There have been concerns about the high recurrence rate of AF following ablation,” with reported cumulative recurrence rates running as high as 80% by 5 years after ablation, noted Dr. Brachmann. “The news now is that recurrence alone doesn’t make a difference; we can still help patients” by reducing their AF burden, although he cautioned that this relationship has so far only been seen in patients with heart failure with reduced ejection fraction, the type of patients enrolled in CASTLE-AF.
“This information is very informative for clinicians counseling patients who undergo ablation. Ablation may not eliminate all of a patient’s AF, but it will substantially reduce it, and that’s associated with better outcomes,” commented Andrew D. Krahn, MD, professor and chief of cardiology at the University of British Columbia in Vancouver. “Early on using ablation, we had a curative approach and used ablation to ‘clip the wire.’ Now we have growing, objective evidence for ‘debulking’ the problem” working without the need to completely eliminate all AF episodes.
To run the post hoc analysis Dr. Brachmann and his associates categorized the 363 patients randomized in CASTLE-AF by the treatment they received during the study’s first 12 weeks: 150 patients underwent catheter ablation, and 210 received drug treatment, with three patients dropping out. Although this division of the patients diverged from the randomized subgroups, the ablated and drug-treated patients showed no significant differences when compared for several clinical parameters.
Ablation was significantly more effective than drug therapy for cutting atrial fibrillation burden, which started at an average of about 50% in all patients at baseline. AF burden fell to an average of about 10%-15% among the ablated patients when measured at several time points during follow-up, whereas AF burden remained at an average of about 50% or higher among the drug-treated patients.
A receiver operating characteristic analysis showed that change in AF burden after ablation produced a statistically significant 0.66 area-under-the-curve for the primary endpoint, which suggested that reduction in AF burden post ablation could account for about two-thirds of the drop in deaths and hospitalizations for heart failure. Among the nonablated patients the area-under-the-curve was an insignificant 0.49 showing that with drug treatment AF burden had no discernible relationship with outcomes.
One further observation in the new analysis was that a drop in AF burden was linked with improved outcomes regardless of whether or not a “blanking period” was imposed on the data. Researchers applied a 90-day blanking period after ablation when assessing the treatment’s efficacy to censor from the analysis recurrences that occurred soon after ablation. The need for a blanking period during the first 90 days “was put to rest” by this new analysis, Dr. Brachmann said.
CASTLE-AF was sponsored by Biotronik. Dr. Brachmann has been a consultant to and has received research funding from Biotronik and several other companies. Dr. Krahn has been a consultant to Medtronic and has received research support from Medtronic and Boston Scientific. Dr. Link had no disclosures.
SOURCE: Brachmann J et al. Heart Rhythm 2018, Abstract B-LBCT02-04.
BOSTON – From the earliest days of using catheter ablation to treat atrial fibrillation (AF), in the 1990s, clinicians have defined ablation success based on whether patients had recurrence of their arrhythmia following treatment. New findings suggest that this standard was off, and that
The new study used data collected in the CASTLE-AF (Catheter Ablation vs. Standard Conventional Treatment in Patients With LV Dysfunction and AF) multicenter trial, which compared the efficacy of AF ablation with antiarrhythmic drug treatment in patients with heart failure for improving survival and freedom from hospitalization for heart failure. The trial’s primary finding showed that, in 363 randomized patients, AF ablation cut the primary adverse event rate by 38% relative to antiarrhythmic drug therapy (New Engl J Med. 2018 Feb 1;378[5]:417-27)
“There have been concerns about the high recurrence rate of AF following ablation,” with reported cumulative recurrence rates running as high as 80% by 5 years after ablation, noted Dr. Brachmann. “The news now is that recurrence alone doesn’t make a difference; we can still help patients” by reducing their AF burden, although he cautioned that this relationship has so far only been seen in patients with heart failure with reduced ejection fraction, the type of patients enrolled in CASTLE-AF.
“This information is very informative for clinicians counseling patients who undergo ablation. Ablation may not eliminate all of a patient’s AF, but it will substantially reduce it, and that’s associated with better outcomes,” commented Andrew D. Krahn, MD, professor and chief of cardiology at the University of British Columbia in Vancouver. “Early on using ablation, we had a curative approach and used ablation to ‘clip the wire.’ Now we have growing, objective evidence for ‘debulking’ the problem” working without the need to completely eliminate all AF episodes.
To run the post hoc analysis Dr. Brachmann and his associates categorized the 363 patients randomized in CASTLE-AF by the treatment they received during the study’s first 12 weeks: 150 patients underwent catheter ablation, and 210 received drug treatment, with three patients dropping out. Although this division of the patients diverged from the randomized subgroups, the ablated and drug-treated patients showed no significant differences when compared for several clinical parameters.
Ablation was significantly more effective than drug therapy for cutting atrial fibrillation burden, which started at an average of about 50% in all patients at baseline. AF burden fell to an average of about 10%-15% among the ablated patients when measured at several time points during follow-up, whereas AF burden remained at an average of about 50% or higher among the drug-treated patients.
A receiver operating characteristic analysis showed that change in AF burden after ablation produced a statistically significant 0.66 area-under-the-curve for the primary endpoint, which suggested that reduction in AF burden post ablation could account for about two-thirds of the drop in deaths and hospitalizations for heart failure. Among the nonablated patients the area-under-the-curve was an insignificant 0.49 showing that with drug treatment AF burden had no discernible relationship with outcomes.
One further observation in the new analysis was that a drop in AF burden was linked with improved outcomes regardless of whether or not a “blanking period” was imposed on the data. Researchers applied a 90-day blanking period after ablation when assessing the treatment’s efficacy to censor from the analysis recurrences that occurred soon after ablation. The need for a blanking period during the first 90 days “was put to rest” by this new analysis, Dr. Brachmann said.
CASTLE-AF was sponsored by Biotronik. Dr. Brachmann has been a consultant to and has received research funding from Biotronik and several other companies. Dr. Krahn has been a consultant to Medtronic and has received research support from Medtronic and Boston Scientific. Dr. Link had no disclosures.
SOURCE: Brachmann J et al. Heart Rhythm 2018, Abstract B-LBCT02-04.
REPORTING FROM HEART RHYTHM 2018
Key clinical point: Higher atrial fibrillation (AF) burden was more important than AF recurrence for predicting bad outcomes post ablation.
Major finding: Patients whose AF burden fell to 5% or less had about a threefold higher rate of good outcomes, compared with other patients.
Study details: Post hoc analysis of 360 patients with heart failure and AF enrolled in CASTLE-AF, a multicenter, randomized trial.
Disclosures: CASTLE-AF was sponsored by Biotronik. Dr. Brachmann has been a consultant to and has received research funding from Biotronik and several other companies. Dr. Krahn has been a consultant to Medtronic and has received research support from Medtronic and Boston Scientific. Dr. Link had no disclosures.
Source: Brachmann J et al. Heart Rhythm 2018, Abstract B-LBCT02-04.
Migraine History and Recovery from Concussion
Athletes with a pre-injury migraine history may be at an elevated risk for a protracted return to school after concussion, especially girls and women, according to a recent study. High school and collegiate athletes (n=1265; 42% female) who sustained a sport-related concussion were monitored by athletic trainers using a web-based surveillance system that collects information about concussion recovery. Researchers found:
- There were 117 athletes (9.2%) who reported a pre-injury migraine history.
- Athletes with a history of migraine took a median of 6 days to return to academics and 15.5 days to return to athletics, while those with no migraine history took a median of 5 days to return to academics and 14 days to return to athletics.
- There were no statistically significant differences in days to return to school or athletics between the groups.
- However, a lower percentage of athletes with a history of migraine had returned to school after 7 days, 14 days, and 21 days post-injury.
- Stratifying the analyses by sex showed that this effect was significant in girls and women with pre-existing migraines, but not boys and men with pre-existing migraines.
Pre-injury migraine history as a risk factor for prolonged return to school and sports following concussion. [Published online ahead of print May 5, 2018]. J Neurotrauma. doi:10.1089/neu.2017.5443.
Athletes with a pre-injury migraine history may be at an elevated risk for a protracted return to school after concussion, especially girls and women, according to a recent study. High school and collegiate athletes (n=1265; 42% female) who sustained a sport-related concussion were monitored by athletic trainers using a web-based surveillance system that collects information about concussion recovery. Researchers found:
- There were 117 athletes (9.2%) who reported a pre-injury migraine history.
- Athletes with a history of migraine took a median of 6 days to return to academics and 15.5 days to return to athletics, while those with no migraine history took a median of 5 days to return to academics and 14 days to return to athletics.
- There were no statistically significant differences in days to return to school or athletics between the groups.
- However, a lower percentage of athletes with a history of migraine had returned to school after 7 days, 14 days, and 21 days post-injury.
- Stratifying the analyses by sex showed that this effect was significant in girls and women with pre-existing migraines, but not boys and men with pre-existing migraines.
Pre-injury migraine history as a risk factor for prolonged return to school and sports following concussion. [Published online ahead of print May 5, 2018]. J Neurotrauma. doi:10.1089/neu.2017.5443.
Athletes with a pre-injury migraine history may be at an elevated risk for a protracted return to school after concussion, especially girls and women, according to a recent study. High school and collegiate athletes (n=1265; 42% female) who sustained a sport-related concussion were monitored by athletic trainers using a web-based surveillance system that collects information about concussion recovery. Researchers found:
- There were 117 athletes (9.2%) who reported a pre-injury migraine history.
- Athletes with a history of migraine took a median of 6 days to return to academics and 15.5 days to return to athletics, while those with no migraine history took a median of 5 days to return to academics and 14 days to return to athletics.
- There were no statistically significant differences in days to return to school or athletics between the groups.
- However, a lower percentage of athletes with a history of migraine had returned to school after 7 days, 14 days, and 21 days post-injury.
- Stratifying the analyses by sex showed that this effect was significant in girls and women with pre-existing migraines, but not boys and men with pre-existing migraines.
Pre-injury migraine history as a risk factor for prolonged return to school and sports following concussion. [Published online ahead of print May 5, 2018]. J Neurotrauma. doi:10.1089/neu.2017.5443.
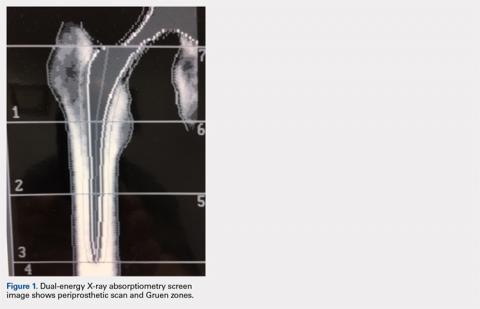
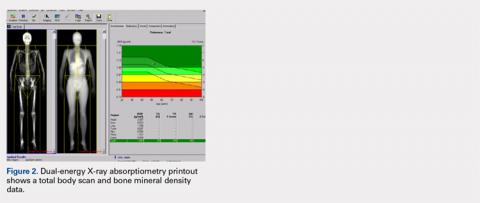
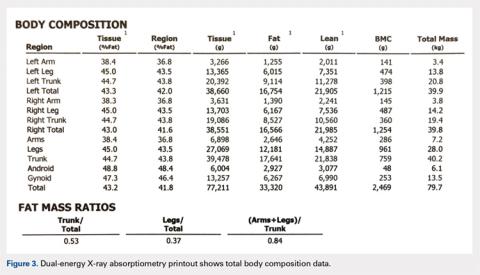
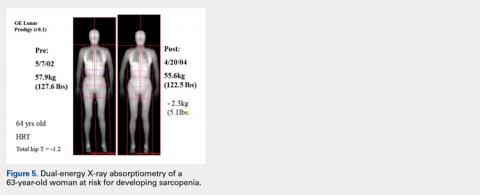
The Potential Value of Dual-Energy X-Ray Absorptiometry in Orthopedics
ABSTRACT
Dual-energy X-ray absorptiometry (DXA) is a well-established technology with an important and well-known role in measuring bone mineral density (BMD) for the purpose of determining fracture risk, diagnosing osteoporosis, and monitoring treatment efficacy. However, aside from the assessment of bone status, DXA is likely underutilized in the field of orthopedics, and most orthopedists may not be aware of the full capabilities of DXA, particularly with regard to total body scans and body composition assessment. For example, DXA would be a valuable tool for monitoring body composition after surgery where compensatory changes in the affected limb may lead to right-left asymmetry (eg, tracking lean mass change after knee surgery), rehabilitation regimens for athletes, congenital and metabolic disorders that affect the musculoskeletal system, or monitoring sarcopenia and frailty in the elderly. Furthermore, preoperative and postoperative regional scans can track BMD changes during healing or alert surgeons to impending problems such as loss of periprosthetic bone, which could lead to implant failure. This article discusses the capabilities of DXA and how this technology could be better used to the advantage of the attending orthopedist.
Dual-energy X-ray absorptiometry, abbreviated as “DXA,” (although usually abbreviated in older literature as “DEXA”) was first introduced in 1987 (Hologic QDR-1000 system, Hologic, Inc) and immediately made all previous forms of radiation-based bone mineral density (BMD) measurement systems obsolete.1 Since then, there have been many generations of the technology, with the main US manufacturers in 2017 being Hologic, Inc. and GE Lunar. There are 2 forms of DXA, peripheral systems (which usually measure BMD only in the radius, finger bones, or calcaneus) and central systems (which measure the radius, proximal femur [“hip”], lumbar spine, total body, and custom sites). The general principle of how DXA works is based on the differential attenuation of photons by bone, fat, and lean mass.2 The DXA technique uses a low- and high-energy X-ray beam produced by an X-ray tube. With the low-energy beam, attenuation by bone is greater than attenuation by soft tissue. With the high-energy beam, attenuation by bone and soft tissues are similar. The dual X-ray beams are passed through the body regions being scanned (usually posterioanteriorly), and the differential attenuation by bone and soft tissue is analyzed to produce BMD estimates. In addition, a high-quality image is produced to enable the operator of the DXA system to verify that the appropriate body region was scanned. It is important to realize that DXA is 2-dimensional (which is sometimes cited as a weakness of DXA), and the units of BMD are grams of mineral per centimeter squared (g/cm2).
Continue to: When assessing bone status...
When assessing bone status for the purpose of determining if a patient is normal, osteopenic, or osteoporotic, the skeletal sites (called regions of interest [ROI]) typically scanned are the proximal femur, lumbar spine, and radius. The BMD of the patient is then compared to a manufacturer-provided normative database of young adults (the logic being that the BMD in the young adult normative population represents maximal peak bone mass). Total body BMD and body composition can also be quantified (grams of lean and fat mass), and custom scans can be designed for other skeletal sites. Specifically, a patient’s BMD is compared to a database of sex- and age-adjusted normal values, and the deviation from normal is expressed as a T-score (the number of standard deviations the patient's BMD is above or below the average BMD of the young adult reference population) and Z-scores (the number of standard deviations a patient's BMD is above or below the average BMD of a sex- and age-matched reference population).3 The International Society for Clinical Densitometry (ISCD) has developed and published well-accepted guidelines used to assist in acquiring high-quality DXA scans and for the diagnosis of osteoporosis using BMD. The accuracy and, especially, the precision of DXA scans can be remarkable when they are performed by trained technologists, and thus, serial scans can be performed to monitor BMD and body composition changes with aging or in response to treatment.
Because of the nature of the scan mechanics and speed, the effective radiation dose with DXA is very low, expressed in microSieverts.4,5 Generally, the radiation exposure from a series of the lumbar spine, proximal femur, and distal radius is about the same as daily background radiation. Even total body scans present very low exposure due to the scan speed at which any 1 body part is exposed for only a fraction of a second.
BENEFITS OF USING DXA FOR THE ORTHEOPEDIST
At the time of this writing in 2018, the presumption could be made that most physicians in the specialties of internal medicine, rheumatology, endocrinology, radiology, and orthopedics were familiar with the capabilities of DXA to assess BMD for the purpose of diagnosing osteoporosis. However, DXA is likely underused for other purposes, as orthopedists may be unaware of the full capabilities of DXA. Printouts after a scan contain more information than simply BMD, and there are more features and applications of DXA that can potentially be useful to orthopedists.
BONE SIZE
Data from a DXA scan are expressed not only as g/cm2 (BMD) but also as total grams in the ROI (known as bone mineral content, abbreviated as BMC), and cm2 (area of the ROI). These data may appear on a separate page, being considered ancillary results. The latter 2 variables are rarely included on a report sent to a referring physician; therefore, awareness of their value is probably limited. However, there are instances where such information could be valuable when interpreting results, especially bone size.6,7 For example, on occasion, patients present with osteopenic lumbar vertebrate but larger than normal vertebral size (area). Many studies have shown that bone size is directly related to bone strength and thus fracture risk.8,9 Although an understudied phenomenon, large vertebral body size could be protective, counteracting a lower than optimal BMD. Further, because the area of the ROI is measured, it is possible to calculate the bone width (or measure directly with a ruler tool in the software if available) for the area measured. This is especially feasible for tubular bones such as the midshaft of the radius, or more specifically, the classic DXA ROI being the area approximately one third the length of the radius from the distal end, the radius 33% region (actually based on ulna length). Consequently, it is possible to use the width of the radius 33% ROI in addition to BMD and T-score when assessing fracture risk.
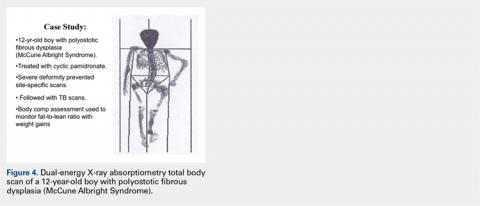
CASE STUDY
A 60-year-old man had a DXA series of the lumbar spine, proximal femur, and whole body. His total body T-score was 0.6 (normal), and his total proximal femur T-score was −0.8 (normal), but his lumbar spine vertebrae 2 to 4 T-score was −1.9. As the patient was osteopenic based on the lumbar spine T-score, some physicians may have initiated antiresorptive therapy, especially if other risk factors for fracture were present. Further examination of the ancillary results of the DXA scan revealed that the vertebral body height T-score was a remarkable 1.11 and 1.53 after adjustment for stature (automatic software calculation). These results suggested that the patient had vertebral bodies of above average size, which theoretically would be protective against fracture even though the BMD T-score was below normal. For this patient, this finding mitigated immediate concern about the lumbar spine T-score of −1.9. Although vertebral body size is not typically used in assessing fracture risk, it is useful information that could be factored into the decision to start treatment or watch for further change with aging.
Continue to: Case Series: Distal Radius Fractures...
CASE SERIES: DISTAL RADIUS FRACTURES
Table 1 summarizes the data comparing radius 33% ROI T-scores and ROI width in patients who fractured the contralateral radius and normal nonfractured controls.10
Table 1. Comparison of Radius Width at the 33% Region of Interest (ROI) and Bone Mineral Density T-Scores in Premenopausal Women With and Without Fractures
| 33% ROI T-score | Width of ROI, cm |
White women with distal radius fractures |
|
|
Premenopausal (<49 years), n = 36 | -0.2 + 0.9 | 1.22 + 0.11a |
Controls matched for race, age, BMIb |
|
|
Premenopausal (<49 years), n = 65 | -0.1 + 0.8 | 1.45 + 0.25 |
For premenopausal women with distal radius fractures, the width of the radius at the radius 33% ROI was significantly smaller than that in controls. However, there was no difference in T-scores between premenopausal women with distal radius fractures and controls. Thus, bone width more accurately identified women with fractures than T-scores based on BMD, and the orthopedist could use bone size in addition to BMD to predict fracture risk in a patient.
PREPARATION FOR SURGERY
For some procedures, there is potential benefit of assessing bone status prior to surgery. That is, determination of low BMD could potentially influence the type of hardware or fixation techniques used in surgery. Various studies have shown that poor bone quality and low BMD can impair purchase with various types of fixation.11-13 Low preoperative BMD has been shown to be related to high implant migration.14 Knowledge of BMD could influence the choice of screw type used or the type of implant metal (titanium vs cobalt chrome). Another example is predicting the risk of spine curvature progression in adolescent idiopathic scoliosis.15-17 It has been reported that low BMD is a risk factor for progression.15 Knowledge of BMD could potentially help with patient management strategies. For example, a patient with low BMD and vitamin D deficiency could be treated (vitamin D supplementation) prior to planning surgery in an effort to improve the low BMD.
PERIOPROSTHETIC BMD
It is possible to monitor changes in BMD around implants using the periprosthetic software application (this usually needs to be purchased separately from standard software that is installed with a system set-up). Dramatic loss of bone due to stress shielding after total hip arthroplasty (THA) can be a risk factor for implant migration or potentially outright failure of fixation or breakthrough. If bone loss occurs and is observed in the early stages, then antiresorptive treatment can be initiated to limit further loss.18,19 (Figure 1) shows the image from a periprosthetic scan.
Continue to: A 60-year-old, 215-lb man...
CASE REPORT
A 60-year-old, 215-lb man had a total hip replacement using a newly introduced cemented collared cobalt-chromium alloy femoral stem. A baseline periprosthetic DXA scan was performed 6 weeks postoperatively. Compared to baseline, the change in BMD in the Gruen zone 5 was −8.2%, +6.5%, +4.9%, and +9.46% at 3, 6, 12, and 24 months, respectively. In contrast, dramatic BMD loss was seen in Gruen zone 7 (calcar region): −33.2%, −40.8%, −37.1%, and −34.1% at 3, 6, 12, and 24 months, respectively. Similar findings in other patients led to discontinuation of use of this stem in favor of a collarless stem in which less BMD loss was seen in Gruen zone 7. Although additional technologist training is required and scans may not be reimbursable, for research purposes or for evaluating new component prototypes, the periprosthetic DXA scan capability can be useful.
Various other custom scans can be used to detect and quantify vertebral fractures (vertebral fracture assessment application), monitor healing of fractures by scanning through radiolucent cast materials, or for research purposes to assess BMD at unusual locations.21-23 Other new innovations, such as the ability to perform full-length scans of the femoral shaft and to quantify focal thickening of the lateral cortex to identify beaking, an abnormality associated with atypical femur fracture after long-term bisphosphonate use, continue to expand the utility of DXA. Using standard software, cadaver bones can be scanned prior to biomechanical testing for a variety of purposes, such as ensuring proper matching specimens in test groups. It has been reported that the common practice of using contralateral bone specimens can lead to bias, as the BMD can be significantly different in right and left bones from the same individual.9,24
TOTAL BODY BMD AND BODY COMPOSITION SCANS
Perhaps the least understood capability of DXA from our experience working with orthopedists is the ability to perform total body scans and to obtain not only total body and regional BMD but also body composition data, namely grams of lean and fat mass.25 Soft tissue (no bone pixels) is partitioned into fat and lean body mass by a calibration procedure (lean mass = total soft tissue –fat mass). DXA has become the standard for body composition assessment given the ease of data acquisition (a total body scan takes only a few minutes), accuracy, and precision of measurements. Compared with other methods (eg, skinfold thickness, bioelectrical impedance, and underwater weighing), it is the only method that gives regional values for fat mass, lean mass, and BMC (this allows the ability to compare left vs right sides).25-27 The ability to perform regional measurements cannot be overstated, as stable body weight belies potential changes with age and disease that relate to redistribution of fat and lean mass. It is not possible to identify, let alone track, such changes by measuring gross body weight on a scale or with BMI calculations. However, redistribution of fat and lean mass can be monitored in great detail using DXA. Figures 2 and 3 show the typical output from a DXA total body/body composition scan.
Total body scans with body composition analyses have many applications. For example, monitoring growth and development or treatment in patients with congenital deformity, metabolic bone disease, osteoporosis, and frailty; patients undergoing rehabilitation; and patients having surgery that could affect the use of a contralateral limb with potential hypertrophy or atrophy. Accurate assessment of percent body fat and fat distribution may help surgeons to improve risk stratification and surgical outcome.28-30 Fracture risk has been associated with muscle area.28 Simple measurements of quadriceps size underestimates atrophy, and total body composition can quantitate lean mass.30
In sports medicine, body composition assessments could be useful to monitor postoperative recovery and effectiveness of rehabilitation protocols after injury, effectiveness of conditioning and training programs, developmental changes due to sports participation, and for obtaining baseline assessment at the time of preseason physicals.27,31-34 In athletes, baseline status and morphological adaptations to training have traditionally been measured by anthropometry (eg, skinfold thickness, BMI, limb circumference, etc.), but DXA total body scanning allows for much more detailed assessments with the possibility of subregional quantitation. There is evidence for sports-specific body composition profiles and characteristic adaptations.27,31-34 Using DXA, adaptive changes as a result of training as well as changes and recovery after surgery or injury can be monitored. For example, quadriceps atrophy usually occurs to some extent after ACL repair, and bone mineral loss and muscle atrophy occur after a limb has been immobilized with a cast. DXA body composition assessment could be used to monitor leg lean mass after surgery for comparison with presurgery values or those of the contralateral noninjured side, or to track recovery of bone mineral and muscle after a cast is removed. Some technical sports, such as tennis and baseball pitching, are known to result in limb asymmetry; DXA body composition could be used to monitor development of right-left arm asymmetry in tennis players or baseball pitchers, and then measures could be taken to balance the asymmetry. Wrestlers and elite dancers are expected to maintain strict weight requirements, but diets are often poor, and as such, DXA body composition could be used to track the effects of dieting and training by comparing serial measurements to baseline to ensure that weight changes include preservation or gain of muscle mass.31
Continue to: For older patients...
For older patients being followed after orthopedic care, there is a growing concern about age-related loss of muscle mass, or sarcopenia, which can lead to functional impairment (eg, balance, gait, etc.), and physical disability leading to falling and increased risk of fracture.35-40 Even obese patients can be sarcopenic (a concept known as sarcopenic obesity), and their large body mass can mask the relative deficiency of lean mass.40 DXA total body scans can be used to monitor patients at risk for sarcopenia.
Finally, DXA total body composition scans are underused in the pediatric population. Given the low radiation burden, DXA can be used safely in children of all ages. In addition to the same uses as in adults for presurgical assessment, monitoring bone and soft-tissue changes after treatment and rehabilitation, scans can be used to monitor growth and development.41
CASE STUDY: MONITORING DEVELOPMENT AND TREATMENT
A 12-year-old boy with polyostotic fibrous dysplasia (McCune Albright Syndrome) was started on treatment with cyclic pamidronate to mitigate bone pain and reduce fracture risk. Use of DXA was planned to provide evidence of treatment efficacy by documenting increasing BMD. However, the severe skeletal deformity prevented standard site-specific DXA scans, and consequently, total body scans were effectively used to acquire the BMD data needed to monitor treatment (Figure 4).
CASE STUDY: AGE-RELATED SARCOPENIA
Figure 5 shows images of a 64-year-old woman who was followed after a distal radius fracture. A total body scan and body composition assessment was performed in 2002. At follow-up in 2004, total body weight seemed stable with only a seemingly benign 5.1-lb loss of weight, and the patient’s overall physical appearance was unchanged (Table 2).
Table 2. Age-Related Changes Potentially Leading to Sarcopenia
| Baseline, 2002 | Follow-up, 2004 | Change, % |
Body weight, kg | 57.9 (127.6 lb) | 55.6 (122.5 lb) | 4 |
BMI | 20.6 | 19.8 |
|
Total body fat, g | 13,619 | 13,390 | −1.7 |
Total body percent fat | 23.5 | 24.1 |
|
Total body lean, g | 42,038 | 39,949 | −5.0 |
Dual-energy X-ray absorptiometry scans were performed using a GE Lunar Prodigy system.
However, body composition assessment revealed a disproportionate loss of lean mass, with a resultant total percent body fat increase. This imbalance between the change in fat and lean mass could lead to clinical sarcopenia unless appropriate dietary and exercise measures are taken. Such subtle developing imbalances in body composition could only be quantitated using DXA total body scans.
Continue to: It is not uncommon...
CASE STUDY: WEIGHT CHANGE IN A RECREATIONAL ATHLETE
It is not uncommon to encounter patients who have substantial weight changes as a result of lifestyle changes, such as dieting. It is also possible that body weight remains stable, but variable changes occur in the amount and distribution of fat and lean mass. Combining exercise with dieting is more likely to be associated with preservation or gain of lean mass. Such a case is presented. After a knee injury, a club tennis player reported gaining 30 lb in the subsequent 12 months. She enrolled in a DXA study, and serial body composition assessments were performed as she started a diet program and exercised on a treadmill and stationary bike. Table 3 shows body composition changes from baseline.
Table 3. Body Composition Changes After Dieting and Exercise
|
|
| Total Body | ||
| Weight, lb | Body Mass Index | Bone Mineral Density, g/cm2 | Fat, g | Lean, g |
Baseline | 160 | 27.5 | 1.245 | 29,023 | 39,610 |
12-month follow-up | 148 | 25.4 | 1.230 | 22,581 | 41,979 |
Dual-energy X-ray absorptiometry scans were performed using a GE Lunar Prodigy system.
Although gross weight using a scale clearly showed progress in losing weight, it did not provide information about redistribution of fat and lean mass. The DXA body composition assessment showed that at follow up, there was a 22% decrease in total grams of fat and a 6% increase in lean mass (changes were uniform over different body regions). Her BMI still categorized her as being overweight; however, her body composition changes demonstrated that diet and exercise were producing positive results.
CONCLUSION
There are many ways in which DXA technology could provide orthopedists with valuable baseline and postoperative and post-treatment information about their patients. This technology could be used more effectively by orthopedists in both general clinical practice and research.
1. Miller PD. The history of bone densitometry. Bone. 2017;104:4-6 [Epub ahead of print].
2. Blake GM, Fogelman I. Technical principles of dual energy X ray absorptiometry. Semin Nucl Med. 1997;27(3):210-228.
3. Faulkner KG. The tale of the T-score: review and perspective. Osteoporo Int. 2005;16(4):347-352. doi:10.1007/s00198-004-1779-y.
4. Solomou G, Damilakis J. Radiation exposure in bone densitometry. Semin Musculoskelet Radiol. 2016;20(4):392-398. doi:10.1055/s-0036-1592430.
5. Adams J. Bone densitometry in children. Semin Musculoskelet Radiol. 2016;20(3):254-268. doi:10.1055/s-0036-1592369.
6. Duan Y, Parfitt AM, Seeman E. Vertebral bone mass, size, and volumetric density in women with spinal fractures. J Bone Miner Res. 1999;14(10):1796-1802. doi:10.1359/jbmr.1999.14.10.1796.
7. Szaulc P, Munoz F, Duboeuf F, Delmas PD. Low width of tubular bones is associated with increased risk of fragility fracture in elderly men–the MINOS study. Bone 2006;38(4):595-602. doi:10.1016/j.bone.2005.09.004.
8. Mi J, Li K, Zhao X, Zhao CQ, Li H, Zhao J. Vertebral body compressive strength evaluated by dual-energy x-ray absorptiometry and Hounsfield units in vitro. J Clin Densitom. 2018;21(1):148-153. doi:10.1016/j.jocd.2016.08.011.
9. Ambrose CG, Kiebzak GM, Sabonghy EP, et al. Biomechanical testing of cadaveric specimens: importance of bone mineral density assessment. Foot Ankle Int. 2002;23(9):850-855. doi:10.1177/107110070202300913.
10. Kiebzak G, Sassard WR. Smaller radius width in women with distal radius fractures compared to women without fractures. Cureus. 2017;9(12):e1950. doi:10.775/cureus.1950.
11. Krappinger D, Bizzotto N, Riedmann S, Kammerlander C, Hengg C, Kralinger FS. Predicting failure after surgical fixation of proximal humerus fractures. Injury 2011;42(11):1283-1288. doi:10.1016/j.injury.2011.01.017.
12. Suhm N, Hengg C, Schwyn R, Windolf M, Quarz V, Hänni M. Mechanical torque measurement predicts load to implant cut-out: a biomechanical study investigating DHS anchorage in femoral heads. Arch Orthop Trauma Surg. 2007;127(6):469-474. doi:10.1007/s00402-006-0265-8.
13. Persiani P, Ranaldi FM, Graci J, et al. Isolated olecranon fractures in children affected by osteogenesis imperfecta type I treated with single screw or tension band wiring system: outcomes and pitfalls in relation to bone mineral density. Medicine (Baltimore). 2017;96(20):e6766. doi:10.1097/MD.0000000000006766.
14. Andersen MR, Winther NS, Lind T, Schrøder HM, Flivik G, Petersen MM. Low preoperative BMD is related to high migration of tibia components in uncemented TKA–92 patients in a combined DEXA and RSA study with 2-year follow-up. J Arthroplasty. 2017;32(7):2141-2146. doi:10.1016/j.arth.2017.02.032.
15. Yip BH, Yu FW, Wang Z, et al. Prognostic value of bone mineral density on curve progression: A longitudinal cohort study of 513 girls with adolescent idiopathic scoliosis. Sci Rep. 2016;6:39220. doi:10.1038/srep39220.
16. Pourabbas Tahvildari B, Erfani MA, Nouraei H, Sadeghian M. Evaluation of bone mineral status in adolescent idiopathic scoliosis. Clin Orthop Surg. 2014;6(2):180-184. doi:10.4055/cios.2014.6.2.180.
17. Li XF, Li H, Liu ZD, Dai LY. Low bone mineral status in adolescent idiopathic scoliosis. Eur Spine J. 2008;17(11):1431-1440. doi:10.1007/s00586-008-0757-z.
18. Venesmaa PK, Kröger HP, Miettinen HJ, Jurvelin JS, Suomalainen OT, Alhava EM. Monitoring of periprosthetic BMD after uncemented total hip arthroplasty with dual-energy X-ray absorptiometry--a 3-year follow-up study. J Bone Miner Res. 2001;16(6):1056-1061. doi:10.1359/jbmr.2001.16.6.1056.
19. Arabmotlagh M, Pilz M, Warzecha J, Rauschmann M. Changes of femoral periprosthetic bone mineral density 6 years after treatment with alendronate following total hip arthroplasty J Orthop Res. 2009;27(2):183-188. doi:10.1002/jor.20748.
20. Gruen TA, McNeice GM, Amstutz HC. Modes of failure of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;(141):17-27.
21. Zeytinoglu M, Jain RK, Vokes TJ. Vertebral fracture assessment: Enhancing the diagnosis, prevention, and treatment of osteoporosis. Bone. 2017;104:54-65. doi:10.1016/j.bone.2017.03.004.
22. Kiebzak GM. Radiolucent casting tape allows for accurate measurement of forearm bone mineral density using dual-energy X-ray absorptiometry. J Clin Densitom. 1998;1(4):369-374.
23. Sung KH, Chung CY, Lee KM, et al. Correlation between central and peripheral bone mineral density around the elbow measured by dual-energy x-ray absorptiometry in healthy children and adolescents. J Clin Densitom. 2017;20(1):114-119. doi:10.1016/j.jocd.2016.04.007.
24. Hamdy R, Kiebzak GM, Seier E, Watts NB. The prevalence of significant left-right differences in hip bone mineral density. Osteoporos Int. 2006;17(12):1772-1780. doi:10.1007/s00198-006-0192-0.
25. Kelly TL, Berger N, Richardson TL. DXA body composition: Theory and practice. Appl Radiat Isot. 1998;49(5-6):511-513.
26. Kiebzak GM, Leamy LJ, Pierson LM, Nord RH, Zhang ZY. Measurement precision of body composition variables using the lunar DPX-L densitometer. J Clin Densitom. 2000;3(1):35-41.
27. Bilborough JC, Greenway k, Par D, Coutts AJ. The accuracy and precision of DXA for assessing body composition in team sport athletes. J Sports Sci. 2014;32(19):1821-1828. doi:10.1080/02640414.2014.926380.
28. Malkov S, Cawthon PM, Peters KW, et al. Health ABC Study. Hip fractures risk in older men and women associated with DXA-derived measures of thigh subcutaneous fat thickness, cross-sectional muscle area, and muscle density. J Bone Miner Res. 2015;30(8):1414-1421. doi:10.1002/jbmr.2469.
29. Arangio GA, Chen C, Klady M, Reed JF. Thigh muscle size and strength after anterior cruciate ligament reconstruction and rehabilitation. J Orthop Sports Phys Ther. 1997;26(5):238-245. doi:10.2519/jospt.1997.26.5.238.
30. Ledford CK, Millikan PD, Nickel BT, et al. Percent body fat Is more predictive of function after total joint arthroplasty than body mass index. J Bone Joint Surg. 2016;98(10):849-857. doi:10.2106/JBJS.15.00509.
31. Berlet G, Kiebzak GM, Dandar A, et al. Prospective analysis of body composition and SF36 profiles in professional dancers over a 7-month season: is there a correlation to injury? J Dance Med Sci. 2002;6(2):54-61.
32. Grant JA, Bedi A, Kurz J, Bancroft R, Gagnier JJ, Miller BS. Ability of preseason body composition and physical fitness to predict the risk of injury in male collegiate hockey players. Sports Health. 2015;7(1):45-51. doi:10.1177/1941738114540445.
33. Stewart AD, Hannan J. Subregional tissue morphometry in male athletes and controls using DXA. Int J Sport Nutr Exerc Metab. 2000;10(2):157-169. doi:10.1123/ijsnem.10.2.157.
34. Sannicandro I, Cofano G, Rosa RA, Piccinno A. Balance training exercises decrease lower-limb strength asymmetry in young tennis players. J Sports Sci Med. 2014;13(2):397-402.
35. Guglielmi G, Ponti F, Agostini M, Amadori M, Battista G, Bazzocchi A. The role of DXA in sarcopenia. Aging Clin Exp Res. 2016;28(6):1047-1060. doi:10.1007/s40520-016-0589-3.
36. Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159(4):413-421.
37. Landi F, Calvani R, Ortolani E, et al. The association between sarcopenia and functional outcomes among older patients with hip fracture undergoing in-hospital rehabilitation. Osteoporos Int. 2017;28(5):1569-1576. doi:10.1007/s00198-017-3929-z.
38. Roh YH, Noh JH, Gong HS, Baek GH. Effect of low appendicular lean mass, grip strength, and gait speed on the functional outcome after surgery for distal radius fractures. Arch Osteoporos. 2017;12(1):41. doi:10.1007/s11657-017-0335-2.
39. Miller MS, Callahan DM, Toth MJ. Skeletal muscle myofilament adaptations to aging, disease, and disuse and their effects on whole muscle performance in older adult humans. Front Physiol. 2014;5:369. doi:10.3389/fphys.2014.00369.
40. Waters DJ, Baumgartner RN. Sarcopenia and obesity. Clin Geriatr Med. 2011;27(3):401-421. doi:10.1016/j.cger.2011.03.007.
41. Bachrach LK, Gordon CM. Bone densitometry in children and adolescents. Pediatrics. 2016;138(4):e20162398. doi:10.1542/peds.2016-2398.
ABSTRACT
Dual-energy X-ray absorptiometry (DXA) is a well-established technology with an important and well-known role in measuring bone mineral density (BMD) for the purpose of determining fracture risk, diagnosing osteoporosis, and monitoring treatment efficacy. However, aside from the assessment of bone status, DXA is likely underutilized in the field of orthopedics, and most orthopedists may not be aware of the full capabilities of DXA, particularly with regard to total body scans and body composition assessment. For example, DXA would be a valuable tool for monitoring body composition after surgery where compensatory changes in the affected limb may lead to right-left asymmetry (eg, tracking lean mass change after knee surgery), rehabilitation regimens for athletes, congenital and metabolic disorders that affect the musculoskeletal system, or monitoring sarcopenia and frailty in the elderly. Furthermore, preoperative and postoperative regional scans can track BMD changes during healing or alert surgeons to impending problems such as loss of periprosthetic bone, which could lead to implant failure. This article discusses the capabilities of DXA and how this technology could be better used to the advantage of the attending orthopedist.
Dual-energy X-ray absorptiometry, abbreviated as “DXA,” (although usually abbreviated in older literature as “DEXA”) was first introduced in 1987 (Hologic QDR-1000 system, Hologic, Inc) and immediately made all previous forms of radiation-based bone mineral density (BMD) measurement systems obsolete.1 Since then, there have been many generations of the technology, with the main US manufacturers in 2017 being Hologic, Inc. and GE Lunar. There are 2 forms of DXA, peripheral systems (which usually measure BMD only in the radius, finger bones, or calcaneus) and central systems (which measure the radius, proximal femur [“hip”], lumbar spine, total body, and custom sites). The general principle of how DXA works is based on the differential attenuation of photons by bone, fat, and lean mass.2 The DXA technique uses a low- and high-energy X-ray beam produced by an X-ray tube. With the low-energy beam, attenuation by bone is greater than attenuation by soft tissue. With the high-energy beam, attenuation by bone and soft tissues are similar. The dual X-ray beams are passed through the body regions being scanned (usually posterioanteriorly), and the differential attenuation by bone and soft tissue is analyzed to produce BMD estimates. In addition, a high-quality image is produced to enable the operator of the DXA system to verify that the appropriate body region was scanned. It is important to realize that DXA is 2-dimensional (which is sometimes cited as a weakness of DXA), and the units of BMD are grams of mineral per centimeter squared (g/cm2).
Continue to: When assessing bone status...
When assessing bone status for the purpose of determining if a patient is normal, osteopenic, or osteoporotic, the skeletal sites (called regions of interest [ROI]) typically scanned are the proximal femur, lumbar spine, and radius. The BMD of the patient is then compared to a manufacturer-provided normative database of young adults (the logic being that the BMD in the young adult normative population represents maximal peak bone mass). Total body BMD and body composition can also be quantified (grams of lean and fat mass), and custom scans can be designed for other skeletal sites. Specifically, a patient’s BMD is compared to a database of sex- and age-adjusted normal values, and the deviation from normal is expressed as a T-score (the number of standard deviations the patient's BMD is above or below the average BMD of the young adult reference population) and Z-scores (the number of standard deviations a patient's BMD is above or below the average BMD of a sex- and age-matched reference population).3 The International Society for Clinical Densitometry (ISCD) has developed and published well-accepted guidelines used to assist in acquiring high-quality DXA scans and for the diagnosis of osteoporosis using BMD. The accuracy and, especially, the precision of DXA scans can be remarkable when they are performed by trained technologists, and thus, serial scans can be performed to monitor BMD and body composition changes with aging or in response to treatment.
Because of the nature of the scan mechanics and speed, the effective radiation dose with DXA is very low, expressed in microSieverts.4,5 Generally, the radiation exposure from a series of the lumbar spine, proximal femur, and distal radius is about the same as daily background radiation. Even total body scans present very low exposure due to the scan speed at which any 1 body part is exposed for only a fraction of a second.
BENEFITS OF USING DXA FOR THE ORTHEOPEDIST
At the time of this writing in 2018, the presumption could be made that most physicians in the specialties of internal medicine, rheumatology, endocrinology, radiology, and orthopedics were familiar with the capabilities of DXA to assess BMD for the purpose of diagnosing osteoporosis. However, DXA is likely underused for other purposes, as orthopedists may be unaware of the full capabilities of DXA. Printouts after a scan contain more information than simply BMD, and there are more features and applications of DXA that can potentially be useful to orthopedists.
BONE SIZE
Data from a DXA scan are expressed not only as g/cm2 (BMD) but also as total grams in the ROI (known as bone mineral content, abbreviated as BMC), and cm2 (area of the ROI). These data may appear on a separate page, being considered ancillary results. The latter 2 variables are rarely included on a report sent to a referring physician; therefore, awareness of their value is probably limited. However, there are instances where such information could be valuable when interpreting results, especially bone size.6,7 For example, on occasion, patients present with osteopenic lumbar vertebrate but larger than normal vertebral size (area). Many studies have shown that bone size is directly related to bone strength and thus fracture risk.8,9 Although an understudied phenomenon, large vertebral body size could be protective, counteracting a lower than optimal BMD. Further, because the area of the ROI is measured, it is possible to calculate the bone width (or measure directly with a ruler tool in the software if available) for the area measured. This is especially feasible for tubular bones such as the midshaft of the radius, or more specifically, the classic DXA ROI being the area approximately one third the length of the radius from the distal end, the radius 33% region (actually based on ulna length). Consequently, it is possible to use the width of the radius 33% ROI in addition to BMD and T-score when assessing fracture risk.
CASE STUDY
A 60-year-old man had a DXA series of the lumbar spine, proximal femur, and whole body. His total body T-score was 0.6 (normal), and his total proximal femur T-score was −0.8 (normal), but his lumbar spine vertebrae 2 to 4 T-score was −1.9. As the patient was osteopenic based on the lumbar spine T-score, some physicians may have initiated antiresorptive therapy, especially if other risk factors for fracture were present. Further examination of the ancillary results of the DXA scan revealed that the vertebral body height T-score was a remarkable 1.11 and 1.53 after adjustment for stature (automatic software calculation). These results suggested that the patient had vertebral bodies of above average size, which theoretically would be protective against fracture even though the BMD T-score was below normal. For this patient, this finding mitigated immediate concern about the lumbar spine T-score of −1.9. Although vertebral body size is not typically used in assessing fracture risk, it is useful information that could be factored into the decision to start treatment or watch for further change with aging.
Continue to: Case Series: Distal Radius Fractures...
CASE SERIES: DISTAL RADIUS FRACTURES
Table 1 summarizes the data comparing radius 33% ROI T-scores and ROI width in patients who fractured the contralateral radius and normal nonfractured controls.10
Table 1. Comparison of Radius Width at the 33% Region of Interest (ROI) and Bone Mineral Density T-Scores in Premenopausal Women With and Without Fractures
| 33% ROI T-score | Width of ROI, cm |
White women with distal radius fractures |
|
|
Premenopausal (<49 years), n = 36 | -0.2 + 0.9 | 1.22 + 0.11a |
Controls matched for race, age, BMIb |
|
|
Premenopausal (<49 years), n = 65 | -0.1 + 0.8 | 1.45 + 0.25 |
For premenopausal women with distal radius fractures, the width of the radius at the radius 33% ROI was significantly smaller than that in controls. However, there was no difference in T-scores between premenopausal women with distal radius fractures and controls. Thus, bone width more accurately identified women with fractures than T-scores based on BMD, and the orthopedist could use bone size in addition to BMD to predict fracture risk in a patient.
PREPARATION FOR SURGERY
For some procedures, there is potential benefit of assessing bone status prior to surgery. That is, determination of low BMD could potentially influence the type of hardware or fixation techniques used in surgery. Various studies have shown that poor bone quality and low BMD can impair purchase with various types of fixation.11-13 Low preoperative BMD has been shown to be related to high implant migration.14 Knowledge of BMD could influence the choice of screw type used or the type of implant metal (titanium vs cobalt chrome). Another example is predicting the risk of spine curvature progression in adolescent idiopathic scoliosis.15-17 It has been reported that low BMD is a risk factor for progression.15 Knowledge of BMD could potentially help with patient management strategies. For example, a patient with low BMD and vitamin D deficiency could be treated (vitamin D supplementation) prior to planning surgery in an effort to improve the low BMD.
PERIOPROSTHETIC BMD
It is possible to monitor changes in BMD around implants using the periprosthetic software application (this usually needs to be purchased separately from standard software that is installed with a system set-up). Dramatic loss of bone due to stress shielding after total hip arthroplasty (THA) can be a risk factor for implant migration or potentially outright failure of fixation or breakthrough. If bone loss occurs and is observed in the early stages, then antiresorptive treatment can be initiated to limit further loss.18,19 (Figure 1) shows the image from a periprosthetic scan.
Continue to: A 60-year-old, 215-lb man...
CASE REPORT
A 60-year-old, 215-lb man had a total hip replacement using a newly introduced cemented collared cobalt-chromium alloy femoral stem. A baseline periprosthetic DXA scan was performed 6 weeks postoperatively. Compared to baseline, the change in BMD in the Gruen zone 5 was −8.2%, +6.5%, +4.9%, and +9.46% at 3, 6, 12, and 24 months, respectively. In contrast, dramatic BMD loss was seen in Gruen zone 7 (calcar region): −33.2%, −40.8%, −37.1%, and −34.1% at 3, 6, 12, and 24 months, respectively. Similar findings in other patients led to discontinuation of use of this stem in favor of a collarless stem in which less BMD loss was seen in Gruen zone 7. Although additional technologist training is required and scans may not be reimbursable, for research purposes or for evaluating new component prototypes, the periprosthetic DXA scan capability can be useful.
Various other custom scans can be used to detect and quantify vertebral fractures (vertebral fracture assessment application), monitor healing of fractures by scanning through radiolucent cast materials, or for research purposes to assess BMD at unusual locations.21-23 Other new innovations, such as the ability to perform full-length scans of the femoral shaft and to quantify focal thickening of the lateral cortex to identify beaking, an abnormality associated with atypical femur fracture after long-term bisphosphonate use, continue to expand the utility of DXA. Using standard software, cadaver bones can be scanned prior to biomechanical testing for a variety of purposes, such as ensuring proper matching specimens in test groups. It has been reported that the common practice of using contralateral bone specimens can lead to bias, as the BMD can be significantly different in right and left bones from the same individual.9,24
TOTAL BODY BMD AND BODY COMPOSITION SCANS
Perhaps the least understood capability of DXA from our experience working with orthopedists is the ability to perform total body scans and to obtain not only total body and regional BMD but also body composition data, namely grams of lean and fat mass.25 Soft tissue (no bone pixels) is partitioned into fat and lean body mass by a calibration procedure (lean mass = total soft tissue –fat mass). DXA has become the standard for body composition assessment given the ease of data acquisition (a total body scan takes only a few minutes), accuracy, and precision of measurements. Compared with other methods (eg, skinfold thickness, bioelectrical impedance, and underwater weighing), it is the only method that gives regional values for fat mass, lean mass, and BMC (this allows the ability to compare left vs right sides).25-27 The ability to perform regional measurements cannot be overstated, as stable body weight belies potential changes with age and disease that relate to redistribution of fat and lean mass. It is not possible to identify, let alone track, such changes by measuring gross body weight on a scale or with BMI calculations. However, redistribution of fat and lean mass can be monitored in great detail using DXA. Figures 2 and 3 show the typical output from a DXA total body/body composition scan.
Total body scans with body composition analyses have many applications. For example, monitoring growth and development or treatment in patients with congenital deformity, metabolic bone disease, osteoporosis, and frailty; patients undergoing rehabilitation; and patients having surgery that could affect the use of a contralateral limb with potential hypertrophy or atrophy. Accurate assessment of percent body fat and fat distribution may help surgeons to improve risk stratification and surgical outcome.28-30 Fracture risk has been associated with muscle area.28 Simple measurements of quadriceps size underestimates atrophy, and total body composition can quantitate lean mass.30
In sports medicine, body composition assessments could be useful to monitor postoperative recovery and effectiveness of rehabilitation protocols after injury, effectiveness of conditioning and training programs, developmental changes due to sports participation, and for obtaining baseline assessment at the time of preseason physicals.27,31-34 In athletes, baseline status and morphological adaptations to training have traditionally been measured by anthropometry (eg, skinfold thickness, BMI, limb circumference, etc.), but DXA total body scanning allows for much more detailed assessments with the possibility of subregional quantitation. There is evidence for sports-specific body composition profiles and characteristic adaptations.27,31-34 Using DXA, adaptive changes as a result of training as well as changes and recovery after surgery or injury can be monitored. For example, quadriceps atrophy usually occurs to some extent after ACL repair, and bone mineral loss and muscle atrophy occur after a limb has been immobilized with a cast. DXA body composition assessment could be used to monitor leg lean mass after surgery for comparison with presurgery values or those of the contralateral noninjured side, or to track recovery of bone mineral and muscle after a cast is removed. Some technical sports, such as tennis and baseball pitching, are known to result in limb asymmetry; DXA body composition could be used to monitor development of right-left arm asymmetry in tennis players or baseball pitchers, and then measures could be taken to balance the asymmetry. Wrestlers and elite dancers are expected to maintain strict weight requirements, but diets are often poor, and as such, DXA body composition could be used to track the effects of dieting and training by comparing serial measurements to baseline to ensure that weight changes include preservation or gain of muscle mass.31
Continue to: For older patients...
For older patients being followed after orthopedic care, there is a growing concern about age-related loss of muscle mass, or sarcopenia, which can lead to functional impairment (eg, balance, gait, etc.), and physical disability leading to falling and increased risk of fracture.35-40 Even obese patients can be sarcopenic (a concept known as sarcopenic obesity), and their large body mass can mask the relative deficiency of lean mass.40 DXA total body scans can be used to monitor patients at risk for sarcopenia.
Finally, DXA total body composition scans are underused in the pediatric population. Given the low radiation burden, DXA can be used safely in children of all ages. In addition to the same uses as in adults for presurgical assessment, monitoring bone and soft-tissue changes after treatment and rehabilitation, scans can be used to monitor growth and development.41
CASE STUDY: MONITORING DEVELOPMENT AND TREATMENT
A 12-year-old boy with polyostotic fibrous dysplasia (McCune Albright Syndrome) was started on treatment with cyclic pamidronate to mitigate bone pain and reduce fracture risk. Use of DXA was planned to provide evidence of treatment efficacy by documenting increasing BMD. However, the severe skeletal deformity prevented standard site-specific DXA scans, and consequently, total body scans were effectively used to acquire the BMD data needed to monitor treatment (Figure 4).
CASE STUDY: AGE-RELATED SARCOPENIA
Figure 5 shows images of a 64-year-old woman who was followed after a distal radius fracture. A total body scan and body composition assessment was performed in 2002. At follow-up in 2004, total body weight seemed stable with only a seemingly benign 5.1-lb loss of weight, and the patient’s overall physical appearance was unchanged (Table 2).
Table 2. Age-Related Changes Potentially Leading to Sarcopenia
| Baseline, 2002 | Follow-up, 2004 | Change, % |
Body weight, kg | 57.9 (127.6 lb) | 55.6 (122.5 lb) | 4 |
BMI | 20.6 | 19.8 |
|
Total body fat, g | 13,619 | 13,390 | −1.7 |
Total body percent fat | 23.5 | 24.1 |
|
Total body lean, g | 42,038 | 39,949 | −5.0 |
Dual-energy X-ray absorptiometry scans were performed using a GE Lunar Prodigy system.
However, body composition assessment revealed a disproportionate loss of lean mass, with a resultant total percent body fat increase. This imbalance between the change in fat and lean mass could lead to clinical sarcopenia unless appropriate dietary and exercise measures are taken. Such subtle developing imbalances in body composition could only be quantitated using DXA total body scans.
Continue to: It is not uncommon...
CASE STUDY: WEIGHT CHANGE IN A RECREATIONAL ATHLETE
It is not uncommon to encounter patients who have substantial weight changes as a result of lifestyle changes, such as dieting. It is also possible that body weight remains stable, but variable changes occur in the amount and distribution of fat and lean mass. Combining exercise with dieting is more likely to be associated with preservation or gain of lean mass. Such a case is presented. After a knee injury, a club tennis player reported gaining 30 lb in the subsequent 12 months. She enrolled in a DXA study, and serial body composition assessments were performed as she started a diet program and exercised on a treadmill and stationary bike. Table 3 shows body composition changes from baseline.
Table 3. Body Composition Changes After Dieting and Exercise
|
|
| Total Body | ||
| Weight, lb | Body Mass Index | Bone Mineral Density, g/cm2 | Fat, g | Lean, g |
Baseline | 160 | 27.5 | 1.245 | 29,023 | 39,610 |
12-month follow-up | 148 | 25.4 | 1.230 | 22,581 | 41,979 |
Dual-energy X-ray absorptiometry scans were performed using a GE Lunar Prodigy system.
Although gross weight using a scale clearly showed progress in losing weight, it did not provide information about redistribution of fat and lean mass. The DXA body composition assessment showed that at follow up, there was a 22% decrease in total grams of fat and a 6% increase in lean mass (changes were uniform over different body regions). Her BMI still categorized her as being overweight; however, her body composition changes demonstrated that diet and exercise were producing positive results.
CONCLUSION
There are many ways in which DXA technology could provide orthopedists with valuable baseline and postoperative and post-treatment information about their patients. This technology could be used more effectively by orthopedists in both general clinical practice and research.
ABSTRACT
Dual-energy X-ray absorptiometry (DXA) is a well-established technology with an important and well-known role in measuring bone mineral density (BMD) for the purpose of determining fracture risk, diagnosing osteoporosis, and monitoring treatment efficacy. However, aside from the assessment of bone status, DXA is likely underutilized in the field of orthopedics, and most orthopedists may not be aware of the full capabilities of DXA, particularly with regard to total body scans and body composition assessment. For example, DXA would be a valuable tool for monitoring body composition after surgery where compensatory changes in the affected limb may lead to right-left asymmetry (eg, tracking lean mass change after knee surgery), rehabilitation regimens for athletes, congenital and metabolic disorders that affect the musculoskeletal system, or monitoring sarcopenia and frailty in the elderly. Furthermore, preoperative and postoperative regional scans can track BMD changes during healing or alert surgeons to impending problems such as loss of periprosthetic bone, which could lead to implant failure. This article discusses the capabilities of DXA and how this technology could be better used to the advantage of the attending orthopedist.
Dual-energy X-ray absorptiometry, abbreviated as “DXA,” (although usually abbreviated in older literature as “DEXA”) was first introduced in 1987 (Hologic QDR-1000 system, Hologic, Inc) and immediately made all previous forms of radiation-based bone mineral density (BMD) measurement systems obsolete.1 Since then, there have been many generations of the technology, with the main US manufacturers in 2017 being Hologic, Inc. and GE Lunar. There are 2 forms of DXA, peripheral systems (which usually measure BMD only in the radius, finger bones, or calcaneus) and central systems (which measure the radius, proximal femur [“hip”], lumbar spine, total body, and custom sites). The general principle of how DXA works is based on the differential attenuation of photons by bone, fat, and lean mass.2 The DXA technique uses a low- and high-energy X-ray beam produced by an X-ray tube. With the low-energy beam, attenuation by bone is greater than attenuation by soft tissue. With the high-energy beam, attenuation by bone and soft tissues are similar. The dual X-ray beams are passed through the body regions being scanned (usually posterioanteriorly), and the differential attenuation by bone and soft tissue is analyzed to produce BMD estimates. In addition, a high-quality image is produced to enable the operator of the DXA system to verify that the appropriate body region was scanned. It is important to realize that DXA is 2-dimensional (which is sometimes cited as a weakness of DXA), and the units of BMD are grams of mineral per centimeter squared (g/cm2).
Continue to: When assessing bone status...
When assessing bone status for the purpose of determining if a patient is normal, osteopenic, or osteoporotic, the skeletal sites (called regions of interest [ROI]) typically scanned are the proximal femur, lumbar spine, and radius. The BMD of the patient is then compared to a manufacturer-provided normative database of young adults (the logic being that the BMD in the young adult normative population represents maximal peak bone mass). Total body BMD and body composition can also be quantified (grams of lean and fat mass), and custom scans can be designed for other skeletal sites. Specifically, a patient’s BMD is compared to a database of sex- and age-adjusted normal values, and the deviation from normal is expressed as a T-score (the number of standard deviations the patient's BMD is above or below the average BMD of the young adult reference population) and Z-scores (the number of standard deviations a patient's BMD is above or below the average BMD of a sex- and age-matched reference population).3 The International Society for Clinical Densitometry (ISCD) has developed and published well-accepted guidelines used to assist in acquiring high-quality DXA scans and for the diagnosis of osteoporosis using BMD. The accuracy and, especially, the precision of DXA scans can be remarkable when they are performed by trained technologists, and thus, serial scans can be performed to monitor BMD and body composition changes with aging or in response to treatment.
Because of the nature of the scan mechanics and speed, the effective radiation dose with DXA is very low, expressed in microSieverts.4,5 Generally, the radiation exposure from a series of the lumbar spine, proximal femur, and distal radius is about the same as daily background radiation. Even total body scans present very low exposure due to the scan speed at which any 1 body part is exposed for only a fraction of a second.
BENEFITS OF USING DXA FOR THE ORTHEOPEDIST
At the time of this writing in 2018, the presumption could be made that most physicians in the specialties of internal medicine, rheumatology, endocrinology, radiology, and orthopedics were familiar with the capabilities of DXA to assess BMD for the purpose of diagnosing osteoporosis. However, DXA is likely underused for other purposes, as orthopedists may be unaware of the full capabilities of DXA. Printouts after a scan contain more information than simply BMD, and there are more features and applications of DXA that can potentially be useful to orthopedists.
BONE SIZE
Data from a DXA scan are expressed not only as g/cm2 (BMD) but also as total grams in the ROI (known as bone mineral content, abbreviated as BMC), and cm2 (area of the ROI). These data may appear on a separate page, being considered ancillary results. The latter 2 variables are rarely included on a report sent to a referring physician; therefore, awareness of their value is probably limited. However, there are instances where such information could be valuable when interpreting results, especially bone size.6,7 For example, on occasion, patients present with osteopenic lumbar vertebrate but larger than normal vertebral size (area). Many studies have shown that bone size is directly related to bone strength and thus fracture risk.8,9 Although an understudied phenomenon, large vertebral body size could be protective, counteracting a lower than optimal BMD. Further, because the area of the ROI is measured, it is possible to calculate the bone width (or measure directly with a ruler tool in the software if available) for the area measured. This is especially feasible for tubular bones such as the midshaft of the radius, or more specifically, the classic DXA ROI being the area approximately one third the length of the radius from the distal end, the radius 33% region (actually based on ulna length). Consequently, it is possible to use the width of the radius 33% ROI in addition to BMD and T-score when assessing fracture risk.
CASE STUDY
A 60-year-old man had a DXA series of the lumbar spine, proximal femur, and whole body. His total body T-score was 0.6 (normal), and his total proximal femur T-score was −0.8 (normal), but his lumbar spine vertebrae 2 to 4 T-score was −1.9. As the patient was osteopenic based on the lumbar spine T-score, some physicians may have initiated antiresorptive therapy, especially if other risk factors for fracture were present. Further examination of the ancillary results of the DXA scan revealed that the vertebral body height T-score was a remarkable 1.11 and 1.53 after adjustment for stature (automatic software calculation). These results suggested that the patient had vertebral bodies of above average size, which theoretically would be protective against fracture even though the BMD T-score was below normal. For this patient, this finding mitigated immediate concern about the lumbar spine T-score of −1.9. Although vertebral body size is not typically used in assessing fracture risk, it is useful information that could be factored into the decision to start treatment or watch for further change with aging.
Continue to: Case Series: Distal Radius Fractures...
CASE SERIES: DISTAL RADIUS FRACTURES
Table 1 summarizes the data comparing radius 33% ROI T-scores and ROI width in patients who fractured the contralateral radius and normal nonfractured controls.10
Table 1. Comparison of Radius Width at the 33% Region of Interest (ROI) and Bone Mineral Density T-Scores in Premenopausal Women With and Without Fractures
| 33% ROI T-score | Width of ROI, cm |
White women with distal radius fractures |
|
|
Premenopausal (<49 years), n = 36 | -0.2 + 0.9 | 1.22 + 0.11a |
Controls matched for race, age, BMIb |
|
|
Premenopausal (<49 years), n = 65 | -0.1 + 0.8 | 1.45 + 0.25 |
For premenopausal women with distal radius fractures, the width of the radius at the radius 33% ROI was significantly smaller than that in controls. However, there was no difference in T-scores between premenopausal women with distal radius fractures and controls. Thus, bone width more accurately identified women with fractures than T-scores based on BMD, and the orthopedist could use bone size in addition to BMD to predict fracture risk in a patient.
PREPARATION FOR SURGERY
For some procedures, there is potential benefit of assessing bone status prior to surgery. That is, determination of low BMD could potentially influence the type of hardware or fixation techniques used in surgery. Various studies have shown that poor bone quality and low BMD can impair purchase with various types of fixation.11-13 Low preoperative BMD has been shown to be related to high implant migration.14 Knowledge of BMD could influence the choice of screw type used or the type of implant metal (titanium vs cobalt chrome). Another example is predicting the risk of spine curvature progression in adolescent idiopathic scoliosis.15-17 It has been reported that low BMD is a risk factor for progression.15 Knowledge of BMD could potentially help with patient management strategies. For example, a patient with low BMD and vitamin D deficiency could be treated (vitamin D supplementation) prior to planning surgery in an effort to improve the low BMD.
PERIOPROSTHETIC BMD
It is possible to monitor changes in BMD around implants using the periprosthetic software application (this usually needs to be purchased separately from standard software that is installed with a system set-up). Dramatic loss of bone due to stress shielding after total hip arthroplasty (THA) can be a risk factor for implant migration or potentially outright failure of fixation or breakthrough. If bone loss occurs and is observed in the early stages, then antiresorptive treatment can be initiated to limit further loss.18,19 (Figure 1) shows the image from a periprosthetic scan.
Continue to: A 60-year-old, 215-lb man...
CASE REPORT
A 60-year-old, 215-lb man had a total hip replacement using a newly introduced cemented collared cobalt-chromium alloy femoral stem. A baseline periprosthetic DXA scan was performed 6 weeks postoperatively. Compared to baseline, the change in BMD in the Gruen zone 5 was −8.2%, +6.5%, +4.9%, and +9.46% at 3, 6, 12, and 24 months, respectively. In contrast, dramatic BMD loss was seen in Gruen zone 7 (calcar region): −33.2%, −40.8%, −37.1%, and −34.1% at 3, 6, 12, and 24 months, respectively. Similar findings in other patients led to discontinuation of use of this stem in favor of a collarless stem in which less BMD loss was seen in Gruen zone 7. Although additional technologist training is required and scans may not be reimbursable, for research purposes or for evaluating new component prototypes, the periprosthetic DXA scan capability can be useful.
Various other custom scans can be used to detect and quantify vertebral fractures (vertebral fracture assessment application), monitor healing of fractures by scanning through radiolucent cast materials, or for research purposes to assess BMD at unusual locations.21-23 Other new innovations, such as the ability to perform full-length scans of the femoral shaft and to quantify focal thickening of the lateral cortex to identify beaking, an abnormality associated with atypical femur fracture after long-term bisphosphonate use, continue to expand the utility of DXA. Using standard software, cadaver bones can be scanned prior to biomechanical testing for a variety of purposes, such as ensuring proper matching specimens in test groups. It has been reported that the common practice of using contralateral bone specimens can lead to bias, as the BMD can be significantly different in right and left bones from the same individual.9,24
TOTAL BODY BMD AND BODY COMPOSITION SCANS
Perhaps the least understood capability of DXA from our experience working with orthopedists is the ability to perform total body scans and to obtain not only total body and regional BMD but also body composition data, namely grams of lean and fat mass.25 Soft tissue (no bone pixels) is partitioned into fat and lean body mass by a calibration procedure (lean mass = total soft tissue –fat mass). DXA has become the standard for body composition assessment given the ease of data acquisition (a total body scan takes only a few minutes), accuracy, and precision of measurements. Compared with other methods (eg, skinfold thickness, bioelectrical impedance, and underwater weighing), it is the only method that gives regional values for fat mass, lean mass, and BMC (this allows the ability to compare left vs right sides).25-27 The ability to perform regional measurements cannot be overstated, as stable body weight belies potential changes with age and disease that relate to redistribution of fat and lean mass. It is not possible to identify, let alone track, such changes by measuring gross body weight on a scale or with BMI calculations. However, redistribution of fat and lean mass can be monitored in great detail using DXA. Figures 2 and 3 show the typical output from a DXA total body/body composition scan.
Total body scans with body composition analyses have many applications. For example, monitoring growth and development or treatment in patients with congenital deformity, metabolic bone disease, osteoporosis, and frailty; patients undergoing rehabilitation; and patients having surgery that could affect the use of a contralateral limb with potential hypertrophy or atrophy. Accurate assessment of percent body fat and fat distribution may help surgeons to improve risk stratification and surgical outcome.28-30 Fracture risk has been associated with muscle area.28 Simple measurements of quadriceps size underestimates atrophy, and total body composition can quantitate lean mass.30
In sports medicine, body composition assessments could be useful to monitor postoperative recovery and effectiveness of rehabilitation protocols after injury, effectiveness of conditioning and training programs, developmental changes due to sports participation, and for obtaining baseline assessment at the time of preseason physicals.27,31-34 In athletes, baseline status and morphological adaptations to training have traditionally been measured by anthropometry (eg, skinfold thickness, BMI, limb circumference, etc.), but DXA total body scanning allows for much more detailed assessments with the possibility of subregional quantitation. There is evidence for sports-specific body composition profiles and characteristic adaptations.27,31-34 Using DXA, adaptive changes as a result of training as well as changes and recovery after surgery or injury can be monitored. For example, quadriceps atrophy usually occurs to some extent after ACL repair, and bone mineral loss and muscle atrophy occur after a limb has been immobilized with a cast. DXA body composition assessment could be used to monitor leg lean mass after surgery for comparison with presurgery values or those of the contralateral noninjured side, or to track recovery of bone mineral and muscle after a cast is removed. Some technical sports, such as tennis and baseball pitching, are known to result in limb asymmetry; DXA body composition could be used to monitor development of right-left arm asymmetry in tennis players or baseball pitchers, and then measures could be taken to balance the asymmetry. Wrestlers and elite dancers are expected to maintain strict weight requirements, but diets are often poor, and as such, DXA body composition could be used to track the effects of dieting and training by comparing serial measurements to baseline to ensure that weight changes include preservation or gain of muscle mass.31
Continue to: For older patients...
For older patients being followed after orthopedic care, there is a growing concern about age-related loss of muscle mass, or sarcopenia, which can lead to functional impairment (eg, balance, gait, etc.), and physical disability leading to falling and increased risk of fracture.35-40 Even obese patients can be sarcopenic (a concept known as sarcopenic obesity), and their large body mass can mask the relative deficiency of lean mass.40 DXA total body scans can be used to monitor patients at risk for sarcopenia.
Finally, DXA total body composition scans are underused in the pediatric population. Given the low radiation burden, DXA can be used safely in children of all ages. In addition to the same uses as in adults for presurgical assessment, monitoring bone and soft-tissue changes after treatment and rehabilitation, scans can be used to monitor growth and development.41
CASE STUDY: MONITORING DEVELOPMENT AND TREATMENT
A 12-year-old boy with polyostotic fibrous dysplasia (McCune Albright Syndrome) was started on treatment with cyclic pamidronate to mitigate bone pain and reduce fracture risk. Use of DXA was planned to provide evidence of treatment efficacy by documenting increasing BMD. However, the severe skeletal deformity prevented standard site-specific DXA scans, and consequently, total body scans were effectively used to acquire the BMD data needed to monitor treatment (Figure 4).
CASE STUDY: AGE-RELATED SARCOPENIA
Figure 5 shows images of a 64-year-old woman who was followed after a distal radius fracture. A total body scan and body composition assessment was performed in 2002. At follow-up in 2004, total body weight seemed stable with only a seemingly benign 5.1-lb loss of weight, and the patient’s overall physical appearance was unchanged (Table 2).
Table 2. Age-Related Changes Potentially Leading to Sarcopenia
| Baseline, 2002 | Follow-up, 2004 | Change, % |
Body weight, kg | 57.9 (127.6 lb) | 55.6 (122.5 lb) | 4 |
BMI | 20.6 | 19.8 |
|
Total body fat, g | 13,619 | 13,390 | −1.7 |
Total body percent fat | 23.5 | 24.1 |
|
Total body lean, g | 42,038 | 39,949 | −5.0 |
Dual-energy X-ray absorptiometry scans were performed using a GE Lunar Prodigy system.
However, body composition assessment revealed a disproportionate loss of lean mass, with a resultant total percent body fat increase. This imbalance between the change in fat and lean mass could lead to clinical sarcopenia unless appropriate dietary and exercise measures are taken. Such subtle developing imbalances in body composition could only be quantitated using DXA total body scans.
Continue to: It is not uncommon...
CASE STUDY: WEIGHT CHANGE IN A RECREATIONAL ATHLETE
It is not uncommon to encounter patients who have substantial weight changes as a result of lifestyle changes, such as dieting. It is also possible that body weight remains stable, but variable changes occur in the amount and distribution of fat and lean mass. Combining exercise with dieting is more likely to be associated with preservation or gain of lean mass. Such a case is presented. After a knee injury, a club tennis player reported gaining 30 lb in the subsequent 12 months. She enrolled in a DXA study, and serial body composition assessments were performed as she started a diet program and exercised on a treadmill and stationary bike. Table 3 shows body composition changes from baseline.
Table 3. Body Composition Changes After Dieting and Exercise
|
|
| Total Body | ||
| Weight, lb | Body Mass Index | Bone Mineral Density, g/cm2 | Fat, g | Lean, g |
Baseline | 160 | 27.5 | 1.245 | 29,023 | 39,610 |
12-month follow-up | 148 | 25.4 | 1.230 | 22,581 | 41,979 |
Dual-energy X-ray absorptiometry scans were performed using a GE Lunar Prodigy system.
Although gross weight using a scale clearly showed progress in losing weight, it did not provide information about redistribution of fat and lean mass. The DXA body composition assessment showed that at follow up, there was a 22% decrease in total grams of fat and a 6% increase in lean mass (changes were uniform over different body regions). Her BMI still categorized her as being overweight; however, her body composition changes demonstrated that diet and exercise were producing positive results.
CONCLUSION
There are many ways in which DXA technology could provide orthopedists with valuable baseline and postoperative and post-treatment information about their patients. This technology could be used more effectively by orthopedists in both general clinical practice and research.
1. Miller PD. The history of bone densitometry. Bone. 2017;104:4-6 [Epub ahead of print].
2. Blake GM, Fogelman I. Technical principles of dual energy X ray absorptiometry. Semin Nucl Med. 1997;27(3):210-228.
3. Faulkner KG. The tale of the T-score: review and perspective. Osteoporo Int. 2005;16(4):347-352. doi:10.1007/s00198-004-1779-y.
4. Solomou G, Damilakis J. Radiation exposure in bone densitometry. Semin Musculoskelet Radiol. 2016;20(4):392-398. doi:10.1055/s-0036-1592430.
5. Adams J. Bone densitometry in children. Semin Musculoskelet Radiol. 2016;20(3):254-268. doi:10.1055/s-0036-1592369.
6. Duan Y, Parfitt AM, Seeman E. Vertebral bone mass, size, and volumetric density in women with spinal fractures. J Bone Miner Res. 1999;14(10):1796-1802. doi:10.1359/jbmr.1999.14.10.1796.
7. Szaulc P, Munoz F, Duboeuf F, Delmas PD. Low width of tubular bones is associated with increased risk of fragility fracture in elderly men–the MINOS study. Bone 2006;38(4):595-602. doi:10.1016/j.bone.2005.09.004.
8. Mi J, Li K, Zhao X, Zhao CQ, Li H, Zhao J. Vertebral body compressive strength evaluated by dual-energy x-ray absorptiometry and Hounsfield units in vitro. J Clin Densitom. 2018;21(1):148-153. doi:10.1016/j.jocd.2016.08.011.
9. Ambrose CG, Kiebzak GM, Sabonghy EP, et al. Biomechanical testing of cadaveric specimens: importance of bone mineral density assessment. Foot Ankle Int. 2002;23(9):850-855. doi:10.1177/107110070202300913.
10. Kiebzak G, Sassard WR. Smaller radius width in women with distal radius fractures compared to women without fractures. Cureus. 2017;9(12):e1950. doi:10.775/cureus.1950.
11. Krappinger D, Bizzotto N, Riedmann S, Kammerlander C, Hengg C, Kralinger FS. Predicting failure after surgical fixation of proximal humerus fractures. Injury 2011;42(11):1283-1288. doi:10.1016/j.injury.2011.01.017.
12. Suhm N, Hengg C, Schwyn R, Windolf M, Quarz V, Hänni M. Mechanical torque measurement predicts load to implant cut-out: a biomechanical study investigating DHS anchorage in femoral heads. Arch Orthop Trauma Surg. 2007;127(6):469-474. doi:10.1007/s00402-006-0265-8.
13. Persiani P, Ranaldi FM, Graci J, et al. Isolated olecranon fractures in children affected by osteogenesis imperfecta type I treated with single screw or tension band wiring system: outcomes and pitfalls in relation to bone mineral density. Medicine (Baltimore). 2017;96(20):e6766. doi:10.1097/MD.0000000000006766.
14. Andersen MR, Winther NS, Lind T, Schrøder HM, Flivik G, Petersen MM. Low preoperative BMD is related to high migration of tibia components in uncemented TKA–92 patients in a combined DEXA and RSA study with 2-year follow-up. J Arthroplasty. 2017;32(7):2141-2146. doi:10.1016/j.arth.2017.02.032.
15. Yip BH, Yu FW, Wang Z, et al. Prognostic value of bone mineral density on curve progression: A longitudinal cohort study of 513 girls with adolescent idiopathic scoliosis. Sci Rep. 2016;6:39220. doi:10.1038/srep39220.
16. Pourabbas Tahvildari B, Erfani MA, Nouraei H, Sadeghian M. Evaluation of bone mineral status in adolescent idiopathic scoliosis. Clin Orthop Surg. 2014;6(2):180-184. doi:10.4055/cios.2014.6.2.180.
17. Li XF, Li H, Liu ZD, Dai LY. Low bone mineral status in adolescent idiopathic scoliosis. Eur Spine J. 2008;17(11):1431-1440. doi:10.1007/s00586-008-0757-z.
18. Venesmaa PK, Kröger HP, Miettinen HJ, Jurvelin JS, Suomalainen OT, Alhava EM. Monitoring of periprosthetic BMD after uncemented total hip arthroplasty with dual-energy X-ray absorptiometry--a 3-year follow-up study. J Bone Miner Res. 2001;16(6):1056-1061. doi:10.1359/jbmr.2001.16.6.1056.
19. Arabmotlagh M, Pilz M, Warzecha J, Rauschmann M. Changes of femoral periprosthetic bone mineral density 6 years after treatment with alendronate following total hip arthroplasty J Orthop Res. 2009;27(2):183-188. doi:10.1002/jor.20748.
20. Gruen TA, McNeice GM, Amstutz HC. Modes of failure of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;(141):17-27.
21. Zeytinoglu M, Jain RK, Vokes TJ. Vertebral fracture assessment: Enhancing the diagnosis, prevention, and treatment of osteoporosis. Bone. 2017;104:54-65. doi:10.1016/j.bone.2017.03.004.
22. Kiebzak GM. Radiolucent casting tape allows for accurate measurement of forearm bone mineral density using dual-energy X-ray absorptiometry. J Clin Densitom. 1998;1(4):369-374.
23. Sung KH, Chung CY, Lee KM, et al. Correlation between central and peripheral bone mineral density around the elbow measured by dual-energy x-ray absorptiometry in healthy children and adolescents. J Clin Densitom. 2017;20(1):114-119. doi:10.1016/j.jocd.2016.04.007.
24. Hamdy R, Kiebzak GM, Seier E, Watts NB. The prevalence of significant left-right differences in hip bone mineral density. Osteoporos Int. 2006;17(12):1772-1780. doi:10.1007/s00198-006-0192-0.
25. Kelly TL, Berger N, Richardson TL. DXA body composition: Theory and practice. Appl Radiat Isot. 1998;49(5-6):511-513.
26. Kiebzak GM, Leamy LJ, Pierson LM, Nord RH, Zhang ZY. Measurement precision of body composition variables using the lunar DPX-L densitometer. J Clin Densitom. 2000;3(1):35-41.
27. Bilborough JC, Greenway k, Par D, Coutts AJ. The accuracy and precision of DXA for assessing body composition in team sport athletes. J Sports Sci. 2014;32(19):1821-1828. doi:10.1080/02640414.2014.926380.
28. Malkov S, Cawthon PM, Peters KW, et al. Health ABC Study. Hip fractures risk in older men and women associated with DXA-derived measures of thigh subcutaneous fat thickness, cross-sectional muscle area, and muscle density. J Bone Miner Res. 2015;30(8):1414-1421. doi:10.1002/jbmr.2469.
29. Arangio GA, Chen C, Klady M, Reed JF. Thigh muscle size and strength after anterior cruciate ligament reconstruction and rehabilitation. J Orthop Sports Phys Ther. 1997;26(5):238-245. doi:10.2519/jospt.1997.26.5.238.
30. Ledford CK, Millikan PD, Nickel BT, et al. Percent body fat Is more predictive of function after total joint arthroplasty than body mass index. J Bone Joint Surg. 2016;98(10):849-857. doi:10.2106/JBJS.15.00509.
31. Berlet G, Kiebzak GM, Dandar A, et al. Prospective analysis of body composition and SF36 profiles in professional dancers over a 7-month season: is there a correlation to injury? J Dance Med Sci. 2002;6(2):54-61.
32. Grant JA, Bedi A, Kurz J, Bancroft R, Gagnier JJ, Miller BS. Ability of preseason body composition and physical fitness to predict the risk of injury in male collegiate hockey players. Sports Health. 2015;7(1):45-51. doi:10.1177/1941738114540445.
33. Stewart AD, Hannan J. Subregional tissue morphometry in male athletes and controls using DXA. Int J Sport Nutr Exerc Metab. 2000;10(2):157-169. doi:10.1123/ijsnem.10.2.157.
34. Sannicandro I, Cofano G, Rosa RA, Piccinno A. Balance training exercises decrease lower-limb strength asymmetry in young tennis players. J Sports Sci Med. 2014;13(2):397-402.
35. Guglielmi G, Ponti F, Agostini M, Amadori M, Battista G, Bazzocchi A. The role of DXA in sarcopenia. Aging Clin Exp Res. 2016;28(6):1047-1060. doi:10.1007/s40520-016-0589-3.
36. Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159(4):413-421.
37. Landi F, Calvani R, Ortolani E, et al. The association between sarcopenia and functional outcomes among older patients with hip fracture undergoing in-hospital rehabilitation. Osteoporos Int. 2017;28(5):1569-1576. doi:10.1007/s00198-017-3929-z.
38. Roh YH, Noh JH, Gong HS, Baek GH. Effect of low appendicular lean mass, grip strength, and gait speed on the functional outcome after surgery for distal radius fractures. Arch Osteoporos. 2017;12(1):41. doi:10.1007/s11657-017-0335-2.
39. Miller MS, Callahan DM, Toth MJ. Skeletal muscle myofilament adaptations to aging, disease, and disuse and their effects on whole muscle performance in older adult humans. Front Physiol. 2014;5:369. doi:10.3389/fphys.2014.00369.
40. Waters DJ, Baumgartner RN. Sarcopenia and obesity. Clin Geriatr Med. 2011;27(3):401-421. doi:10.1016/j.cger.2011.03.007.
41. Bachrach LK, Gordon CM. Bone densitometry in children and adolescents. Pediatrics. 2016;138(4):e20162398. doi:10.1542/peds.2016-2398.
1. Miller PD. The history of bone densitometry. Bone. 2017;104:4-6 [Epub ahead of print].
2. Blake GM, Fogelman I. Technical principles of dual energy X ray absorptiometry. Semin Nucl Med. 1997;27(3):210-228.
3. Faulkner KG. The tale of the T-score: review and perspective. Osteoporo Int. 2005;16(4):347-352. doi:10.1007/s00198-004-1779-y.
4. Solomou G, Damilakis J. Radiation exposure in bone densitometry. Semin Musculoskelet Radiol. 2016;20(4):392-398. doi:10.1055/s-0036-1592430.
5. Adams J. Bone densitometry in children. Semin Musculoskelet Radiol. 2016;20(3):254-268. doi:10.1055/s-0036-1592369.
6. Duan Y, Parfitt AM, Seeman E. Vertebral bone mass, size, and volumetric density in women with spinal fractures. J Bone Miner Res. 1999;14(10):1796-1802. doi:10.1359/jbmr.1999.14.10.1796.
7. Szaulc P, Munoz F, Duboeuf F, Delmas PD. Low width of tubular bones is associated with increased risk of fragility fracture in elderly men–the MINOS study. Bone 2006;38(4):595-602. doi:10.1016/j.bone.2005.09.004.
8. Mi J, Li K, Zhao X, Zhao CQ, Li H, Zhao J. Vertebral body compressive strength evaluated by dual-energy x-ray absorptiometry and Hounsfield units in vitro. J Clin Densitom. 2018;21(1):148-153. doi:10.1016/j.jocd.2016.08.011.
9. Ambrose CG, Kiebzak GM, Sabonghy EP, et al. Biomechanical testing of cadaveric specimens: importance of bone mineral density assessment. Foot Ankle Int. 2002;23(9):850-855. doi:10.1177/107110070202300913.
10. Kiebzak G, Sassard WR. Smaller radius width in women with distal radius fractures compared to women without fractures. Cureus. 2017;9(12):e1950. doi:10.775/cureus.1950.
11. Krappinger D, Bizzotto N, Riedmann S, Kammerlander C, Hengg C, Kralinger FS. Predicting failure after surgical fixation of proximal humerus fractures. Injury 2011;42(11):1283-1288. doi:10.1016/j.injury.2011.01.017.
12. Suhm N, Hengg C, Schwyn R, Windolf M, Quarz V, Hänni M. Mechanical torque measurement predicts load to implant cut-out: a biomechanical study investigating DHS anchorage in femoral heads. Arch Orthop Trauma Surg. 2007;127(6):469-474. doi:10.1007/s00402-006-0265-8.
13. Persiani P, Ranaldi FM, Graci J, et al. Isolated olecranon fractures in children affected by osteogenesis imperfecta type I treated with single screw or tension band wiring system: outcomes and pitfalls in relation to bone mineral density. Medicine (Baltimore). 2017;96(20):e6766. doi:10.1097/MD.0000000000006766.
14. Andersen MR, Winther NS, Lind T, Schrøder HM, Flivik G, Petersen MM. Low preoperative BMD is related to high migration of tibia components in uncemented TKA–92 patients in a combined DEXA and RSA study with 2-year follow-up. J Arthroplasty. 2017;32(7):2141-2146. doi:10.1016/j.arth.2017.02.032.
15. Yip BH, Yu FW, Wang Z, et al. Prognostic value of bone mineral density on curve progression: A longitudinal cohort study of 513 girls with adolescent idiopathic scoliosis. Sci Rep. 2016;6:39220. doi:10.1038/srep39220.
16. Pourabbas Tahvildari B, Erfani MA, Nouraei H, Sadeghian M. Evaluation of bone mineral status in adolescent idiopathic scoliosis. Clin Orthop Surg. 2014;6(2):180-184. doi:10.4055/cios.2014.6.2.180.
17. Li XF, Li H, Liu ZD, Dai LY. Low bone mineral status in adolescent idiopathic scoliosis. Eur Spine J. 2008;17(11):1431-1440. doi:10.1007/s00586-008-0757-z.
18. Venesmaa PK, Kröger HP, Miettinen HJ, Jurvelin JS, Suomalainen OT, Alhava EM. Monitoring of periprosthetic BMD after uncemented total hip arthroplasty with dual-energy X-ray absorptiometry--a 3-year follow-up study. J Bone Miner Res. 2001;16(6):1056-1061. doi:10.1359/jbmr.2001.16.6.1056.
19. Arabmotlagh M, Pilz M, Warzecha J, Rauschmann M. Changes of femoral periprosthetic bone mineral density 6 years after treatment with alendronate following total hip arthroplasty J Orthop Res. 2009;27(2):183-188. doi:10.1002/jor.20748.
20. Gruen TA, McNeice GM, Amstutz HC. Modes of failure of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;(141):17-27.
21. Zeytinoglu M, Jain RK, Vokes TJ. Vertebral fracture assessment: Enhancing the diagnosis, prevention, and treatment of osteoporosis. Bone. 2017;104:54-65. doi:10.1016/j.bone.2017.03.004.
22. Kiebzak GM. Radiolucent casting tape allows for accurate measurement of forearm bone mineral density using dual-energy X-ray absorptiometry. J Clin Densitom. 1998;1(4):369-374.
23. Sung KH, Chung CY, Lee KM, et al. Correlation between central and peripheral bone mineral density around the elbow measured by dual-energy x-ray absorptiometry in healthy children and adolescents. J Clin Densitom. 2017;20(1):114-119. doi:10.1016/j.jocd.2016.04.007.
24. Hamdy R, Kiebzak GM, Seier E, Watts NB. The prevalence of significant left-right differences in hip bone mineral density. Osteoporos Int. 2006;17(12):1772-1780. doi:10.1007/s00198-006-0192-0.
25. Kelly TL, Berger N, Richardson TL. DXA body composition: Theory and practice. Appl Radiat Isot. 1998;49(5-6):511-513.
26. Kiebzak GM, Leamy LJ, Pierson LM, Nord RH, Zhang ZY. Measurement precision of body composition variables using the lunar DPX-L densitometer. J Clin Densitom. 2000;3(1):35-41.
27. Bilborough JC, Greenway k, Par D, Coutts AJ. The accuracy and precision of DXA for assessing body composition in team sport athletes. J Sports Sci. 2014;32(19):1821-1828. doi:10.1080/02640414.2014.926380.
28. Malkov S, Cawthon PM, Peters KW, et al. Health ABC Study. Hip fractures risk in older men and women associated with DXA-derived measures of thigh subcutaneous fat thickness, cross-sectional muscle area, and muscle density. J Bone Miner Res. 2015;30(8):1414-1421. doi:10.1002/jbmr.2469.
29. Arangio GA, Chen C, Klady M, Reed JF. Thigh muscle size and strength after anterior cruciate ligament reconstruction and rehabilitation. J Orthop Sports Phys Ther. 1997;26(5):238-245. doi:10.2519/jospt.1997.26.5.238.
30. Ledford CK, Millikan PD, Nickel BT, et al. Percent body fat Is more predictive of function after total joint arthroplasty than body mass index. J Bone Joint Surg. 2016;98(10):849-857. doi:10.2106/JBJS.15.00509.
31. Berlet G, Kiebzak GM, Dandar A, et al. Prospective analysis of body composition and SF36 profiles in professional dancers over a 7-month season: is there a correlation to injury? J Dance Med Sci. 2002;6(2):54-61.
32. Grant JA, Bedi A, Kurz J, Bancroft R, Gagnier JJ, Miller BS. Ability of preseason body composition and physical fitness to predict the risk of injury in male collegiate hockey players. Sports Health. 2015;7(1):45-51. doi:10.1177/1941738114540445.
33. Stewart AD, Hannan J. Subregional tissue morphometry in male athletes and controls using DXA. Int J Sport Nutr Exerc Metab. 2000;10(2):157-169. doi:10.1123/ijsnem.10.2.157.
34. Sannicandro I, Cofano G, Rosa RA, Piccinno A. Balance training exercises decrease lower-limb strength asymmetry in young tennis players. J Sports Sci Med. 2014;13(2):397-402.
35. Guglielmi G, Ponti F, Agostini M, Amadori M, Battista G, Bazzocchi A. The role of DXA in sarcopenia. Aging Clin Exp Res. 2016;28(6):1047-1060. doi:10.1007/s40520-016-0589-3.
36. Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159(4):413-421.
37. Landi F, Calvani R, Ortolani E, et al. The association between sarcopenia and functional outcomes among older patients with hip fracture undergoing in-hospital rehabilitation. Osteoporos Int. 2017;28(5):1569-1576. doi:10.1007/s00198-017-3929-z.
38. Roh YH, Noh JH, Gong HS, Baek GH. Effect of low appendicular lean mass, grip strength, and gait speed on the functional outcome after surgery for distal radius fractures. Arch Osteoporos. 2017;12(1):41. doi:10.1007/s11657-017-0335-2.
39. Miller MS, Callahan DM, Toth MJ. Skeletal muscle myofilament adaptations to aging, disease, and disuse and their effects on whole muscle performance in older adult humans. Front Physiol. 2014;5:369. doi:10.3389/fphys.2014.00369.
40. Waters DJ, Baumgartner RN. Sarcopenia and obesity. Clin Geriatr Med. 2011;27(3):401-421. doi:10.1016/j.cger.2011.03.007.
41. Bachrach LK, Gordon CM. Bone densitometry in children and adolescents. Pediatrics. 2016;138(4):e20162398. doi:10.1542/peds.2016-2398.
TAKE-HOME POINTS
- DXA is underutilized technology in orthopedics.
- More data ("ancillary data") are often available from a DXA scan then typically included in a standard report from a referral center.
- Most orthopedists are likely unaware of the detailed body composition data available with a total body scan.
- Preoperative DXA scans and knowledge of BMD may be informative when planning the type of fixation and implant metal to used.
- Serial follow-up body composition scans can be useful in monitoring the course of bone healing (mineralization) and soft tissue changes (fat and lean mass).
Cultural humility beats competence in psychotherapeutic settings
The emphasis on cultural context of diagnosis and treatment of psychiatric disorders was expanded in the DSM-5, but the commonly used term for this orientation, “cultural competence,” is potentially misleading, according to a panel of experienced clinicians participating in a workshop at the annual meeting of the American Psychiatric Association.
“I am not even sure how competent I am in my own culture,” said Richa Bhatia, MD, medical director of the Child and Adolescent OCD Institute at McLean Hospital, Belmont, Mass.. The remark was representative; like other panelists in a workshop developed by the Association of Women Psychiatrists, she de-emphasized the importance of becoming fluent in the specifics of a culture relative to simply being sensitive to variations in cultural landmarks and milestones.
However, she, like others, expressed concern about the label “competence.” “Cultural humility is really a much better term,” Dr. Olarte said. The reason is that Furthermore, it encourages clinicians to consider and manage their own prejudices, values, and biases in order to allow them to be effective in the therapeutic interaction.
In the DSM-5, a systematic outline is provided for eliciting culturally relevant information from the diagnostic interview and incorporating it into a therapeutic plan. Cultural competence is important for communication and for building patient trust, but the panelists uniformly agreed that it is not necessary to be fluent in the culture of the patient to be an effective clinician.
“Cultural identification is fluid, and patients have multiple identities,” said Lourdes M. Dominguez, MD, associate professor of psychiatry at Columbia University, New York. Recounting her work with first responders to the Sept. 11, 2001, World Trade Center attack, Dr. Dominguez offered care to police officers associated with a variety of cultures. In addition to different ethnicities and sexual orientations, this included the culture of law enforcement itself. The key for all patients was an ability to convey the message that the patient was being heard.
“The us-versus-them mentality in law enforcement limits the options when fellow officers are not providing the support they need,” Dr. Dominguez explained. “First, you need to win their trust.”
Familiarity with cultural milestones can be reassuring to patients, but Sherry P. Katz-Bearnot, MD, assistant clinical professor of psychiatry at Columbia University, cautioned that there is counterproductive underside to cultural competence. While recognizing the significance of cultural milestones, such as a bar mitvah or quinceañera, can be reassuring to patients, Dr. Katz-Bearnot emphasized that clinicians must remain sensitive to the personal responses to those events.
“If you know too much, there is a risk of glossing over the issues unique to the individual in front of you,” Dr. Katz-Bearnot said. She emphasized that those cultural landmarks do not necessarily mean the same thing to all members of a community. Sensitivity to personal issues trumps cultural familiarity.
The same statement could be made for delivering care to transgender patients, judging from a presentation by Courtney Saw, MD, a PGY3 resident in the department of psychiatry at the University of Pennsylvania, Philadelphia. Quoting a survey that found most transgender individuals consider health care professionals inadequately trained to manage their health issues, she stressed the importance of how questions are phrased.
“Every patient’s gender journey is unique,” Dr. Saw said. For example, specific questions about gender anatomy should be supplanted by open questions about gender transition, allowing patients to respond at their own level of comfort. This, according to Dr. Saw, is essential for “building a therapeutic collaboration.”
Embracing the concept of cultural humility, all of the panelists agreed that it is far less important to have cultural expertise than it is to be open, curious, and accepting. Sensitivity is more important than competence when specific cultural issues are relevant to treatment.
“We don’t have to have all the answers. We just need to be good at listening so that we can help patients work their way towards the answers,” Dr. Saw suggested. Others agreed.
Dr. Bhatia, Dr. Olarte, Dr. Dominguez, Dr. Katz-Bearnot, and Dr. Saw reported no potential conflicts of interest related to this topic.
The emphasis on cultural context of diagnosis and treatment of psychiatric disorders was expanded in the DSM-5, but the commonly used term for this orientation, “cultural competence,” is potentially misleading, according to a panel of experienced clinicians participating in a workshop at the annual meeting of the American Psychiatric Association.
“I am not even sure how competent I am in my own culture,” said Richa Bhatia, MD, medical director of the Child and Adolescent OCD Institute at McLean Hospital, Belmont, Mass.. The remark was representative; like other panelists in a workshop developed by the Association of Women Psychiatrists, she de-emphasized the importance of becoming fluent in the specifics of a culture relative to simply being sensitive to variations in cultural landmarks and milestones.
However, she, like others, expressed concern about the label “competence.” “Cultural humility is really a much better term,” Dr. Olarte said. The reason is that Furthermore, it encourages clinicians to consider and manage their own prejudices, values, and biases in order to allow them to be effective in the therapeutic interaction.
In the DSM-5, a systematic outline is provided for eliciting culturally relevant information from the diagnostic interview and incorporating it into a therapeutic plan. Cultural competence is important for communication and for building patient trust, but the panelists uniformly agreed that it is not necessary to be fluent in the culture of the patient to be an effective clinician.
“Cultural identification is fluid, and patients have multiple identities,” said Lourdes M. Dominguez, MD, associate professor of psychiatry at Columbia University, New York. Recounting her work with first responders to the Sept. 11, 2001, World Trade Center attack, Dr. Dominguez offered care to police officers associated with a variety of cultures. In addition to different ethnicities and sexual orientations, this included the culture of law enforcement itself. The key for all patients was an ability to convey the message that the patient was being heard.
“The us-versus-them mentality in law enforcement limits the options when fellow officers are not providing the support they need,” Dr. Dominguez explained. “First, you need to win their trust.”
Familiarity with cultural milestones can be reassuring to patients, but Sherry P. Katz-Bearnot, MD, assistant clinical professor of psychiatry at Columbia University, cautioned that there is counterproductive underside to cultural competence. While recognizing the significance of cultural milestones, such as a bar mitvah or quinceañera, can be reassuring to patients, Dr. Katz-Bearnot emphasized that clinicians must remain sensitive to the personal responses to those events.
“If you know too much, there is a risk of glossing over the issues unique to the individual in front of you,” Dr. Katz-Bearnot said. She emphasized that those cultural landmarks do not necessarily mean the same thing to all members of a community. Sensitivity to personal issues trumps cultural familiarity.
The same statement could be made for delivering care to transgender patients, judging from a presentation by Courtney Saw, MD, a PGY3 resident in the department of psychiatry at the University of Pennsylvania, Philadelphia. Quoting a survey that found most transgender individuals consider health care professionals inadequately trained to manage their health issues, she stressed the importance of how questions are phrased.
“Every patient’s gender journey is unique,” Dr. Saw said. For example, specific questions about gender anatomy should be supplanted by open questions about gender transition, allowing patients to respond at their own level of comfort. This, according to Dr. Saw, is essential for “building a therapeutic collaboration.”
Embracing the concept of cultural humility, all of the panelists agreed that it is far less important to have cultural expertise than it is to be open, curious, and accepting. Sensitivity is more important than competence when specific cultural issues are relevant to treatment.
“We don’t have to have all the answers. We just need to be good at listening so that we can help patients work their way towards the answers,” Dr. Saw suggested. Others agreed.
Dr. Bhatia, Dr. Olarte, Dr. Dominguez, Dr. Katz-Bearnot, and Dr. Saw reported no potential conflicts of interest related to this topic.
The emphasis on cultural context of diagnosis and treatment of psychiatric disorders was expanded in the DSM-5, but the commonly used term for this orientation, “cultural competence,” is potentially misleading, according to a panel of experienced clinicians participating in a workshop at the annual meeting of the American Psychiatric Association.
“I am not even sure how competent I am in my own culture,” said Richa Bhatia, MD, medical director of the Child and Adolescent OCD Institute at McLean Hospital, Belmont, Mass.. The remark was representative; like other panelists in a workshop developed by the Association of Women Psychiatrists, she de-emphasized the importance of becoming fluent in the specifics of a culture relative to simply being sensitive to variations in cultural landmarks and milestones.
However, she, like others, expressed concern about the label “competence.” “Cultural humility is really a much better term,” Dr. Olarte said. The reason is that Furthermore, it encourages clinicians to consider and manage their own prejudices, values, and biases in order to allow them to be effective in the therapeutic interaction.
In the DSM-5, a systematic outline is provided for eliciting culturally relevant information from the diagnostic interview and incorporating it into a therapeutic plan. Cultural competence is important for communication and for building patient trust, but the panelists uniformly agreed that it is not necessary to be fluent in the culture of the patient to be an effective clinician.
“Cultural identification is fluid, and patients have multiple identities,” said Lourdes M. Dominguez, MD, associate professor of psychiatry at Columbia University, New York. Recounting her work with first responders to the Sept. 11, 2001, World Trade Center attack, Dr. Dominguez offered care to police officers associated with a variety of cultures. In addition to different ethnicities and sexual orientations, this included the culture of law enforcement itself. The key for all patients was an ability to convey the message that the patient was being heard.
“The us-versus-them mentality in law enforcement limits the options when fellow officers are not providing the support they need,” Dr. Dominguez explained. “First, you need to win their trust.”
Familiarity with cultural milestones can be reassuring to patients, but Sherry P. Katz-Bearnot, MD, assistant clinical professor of psychiatry at Columbia University, cautioned that there is counterproductive underside to cultural competence. While recognizing the significance of cultural milestones, such as a bar mitvah or quinceañera, can be reassuring to patients, Dr. Katz-Bearnot emphasized that clinicians must remain sensitive to the personal responses to those events.
“If you know too much, there is a risk of glossing over the issues unique to the individual in front of you,” Dr. Katz-Bearnot said. She emphasized that those cultural landmarks do not necessarily mean the same thing to all members of a community. Sensitivity to personal issues trumps cultural familiarity.
The same statement could be made for delivering care to transgender patients, judging from a presentation by Courtney Saw, MD, a PGY3 resident in the department of psychiatry at the University of Pennsylvania, Philadelphia. Quoting a survey that found most transgender individuals consider health care professionals inadequately trained to manage their health issues, she stressed the importance of how questions are phrased.
“Every patient’s gender journey is unique,” Dr. Saw said. For example, specific questions about gender anatomy should be supplanted by open questions about gender transition, allowing patients to respond at their own level of comfort. This, according to Dr. Saw, is essential for “building a therapeutic collaboration.”
Embracing the concept of cultural humility, all of the panelists agreed that it is far less important to have cultural expertise than it is to be open, curious, and accepting. Sensitivity is more important than competence when specific cultural issues are relevant to treatment.
“We don’t have to have all the answers. We just need to be good at listening so that we can help patients work their way towards the answers,” Dr. Saw suggested. Others agreed.
Dr. Bhatia, Dr. Olarte, Dr. Dominguez, Dr. Katz-Bearnot, and Dr. Saw reported no potential conflicts of interest related to this topic.
EXPERT ANALYSIS FROM APA
Heart rate variability may be risk factor for depression, not a consequence
While some investigations have suggested that depression may lead to later unfavorable effects on heart rate variability, authors of a newly published study say they have found stronger evidence that the opposite is true.
Lower heart rate variability was independently associated with later increases in depressive symptoms, according to results of the longitudinal, twin difference study.
When researchers looked at the opposite direction, they found earlier depressive symptoms were associated with lower heart rate variability at follow-up in the study; however, investigators said those associations were not as robust and were largely explainable by antidepressant use.
That meant reduced heart rate variability is more likely a risk factor for depression, rather than a consequence, according to Minxuan Huang, ScM, of the department of epidemiology at Emory University Rollins School of Public Health, Atlanta, and his coinvestigators.
“These findings point to a central role of the autonomic nervous system in the regulation of mood and depression vulnerability,” they wrote in a report on the study appearing in JAMA Psychiatry.
The published analysis included 146 male twins, or 73 pairs, who participated in the national Vietnam Era Twin Registry.
Previous studies have linked depression to heart rate variability, a noninvasive index of cardiac autonomic nervous system regulation. However, these studies are not consistent on whether depression affects heart rate variability or vice versa, and the studies have been limited in their ability to assess the relationship between those two variables over time, the investigators said.
By contrast, the current study evaluated depression and heart rate variability at two time points: a baseline assessment conducted during 2002-2006 and a at a 7-year follow-up visit.
Investigators found consistent associations between heart rate variability on 24-hour electrocardiogram monitoring at baseline and scores on the Beck Depression Inventory-II at the 7-year follow-up, with beta coefficients ranging from –0.14 to –0.29, the report showed.
By contrast, the associations were less consistent between BDI-II score at the baseline visit and heart rate variability at follow-up, the investigators said. “These associations were largely explained by antidepressant use, which when added to the model, weakened the associations.”
These findings may help guide future research aimed at identifying individuals at a higher risk of later depression, the authors said, noting that treatment studies also are warranted. “Future interventions modulating autonomic nervous system regulation may be useful for the prevention and treatment of depression.” The study was supported by the National Institutes of Health. The Department of Veterans Affairs has supported the Vietnam Era Twin Registry. The researchers had no conflicts of interest.
SOURCE: Huang M et al. JAMA Psychiatry. 2018 May 16. doi: 10.1001/jamapsychiatry.2018.0747.
While some investigations have suggested that depression may lead to later unfavorable effects on heart rate variability, authors of a newly published study say they have found stronger evidence that the opposite is true.
Lower heart rate variability was independently associated with later increases in depressive symptoms, according to results of the longitudinal, twin difference study.
When researchers looked at the opposite direction, they found earlier depressive symptoms were associated with lower heart rate variability at follow-up in the study; however, investigators said those associations were not as robust and were largely explainable by antidepressant use.
That meant reduced heart rate variability is more likely a risk factor for depression, rather than a consequence, according to Minxuan Huang, ScM, of the department of epidemiology at Emory University Rollins School of Public Health, Atlanta, and his coinvestigators.
“These findings point to a central role of the autonomic nervous system in the regulation of mood and depression vulnerability,” they wrote in a report on the study appearing in JAMA Psychiatry.
The published analysis included 146 male twins, or 73 pairs, who participated in the national Vietnam Era Twin Registry.
Previous studies have linked depression to heart rate variability, a noninvasive index of cardiac autonomic nervous system regulation. However, these studies are not consistent on whether depression affects heart rate variability or vice versa, and the studies have been limited in their ability to assess the relationship between those two variables over time, the investigators said.
By contrast, the current study evaluated depression and heart rate variability at two time points: a baseline assessment conducted during 2002-2006 and a at a 7-year follow-up visit.
Investigators found consistent associations between heart rate variability on 24-hour electrocardiogram monitoring at baseline and scores on the Beck Depression Inventory-II at the 7-year follow-up, with beta coefficients ranging from –0.14 to –0.29, the report showed.
By contrast, the associations were less consistent between BDI-II score at the baseline visit and heart rate variability at follow-up, the investigators said. “These associations were largely explained by antidepressant use, which when added to the model, weakened the associations.”
These findings may help guide future research aimed at identifying individuals at a higher risk of later depression, the authors said, noting that treatment studies also are warranted. “Future interventions modulating autonomic nervous system regulation may be useful for the prevention and treatment of depression.” The study was supported by the National Institutes of Health. The Department of Veterans Affairs has supported the Vietnam Era Twin Registry. The researchers had no conflicts of interest.
SOURCE: Huang M et al. JAMA Psychiatry. 2018 May 16. doi: 10.1001/jamapsychiatry.2018.0747.
While some investigations have suggested that depression may lead to later unfavorable effects on heart rate variability, authors of a newly published study say they have found stronger evidence that the opposite is true.
Lower heart rate variability was independently associated with later increases in depressive symptoms, according to results of the longitudinal, twin difference study.
When researchers looked at the opposite direction, they found earlier depressive symptoms were associated with lower heart rate variability at follow-up in the study; however, investigators said those associations were not as robust and were largely explainable by antidepressant use.
That meant reduced heart rate variability is more likely a risk factor for depression, rather than a consequence, according to Minxuan Huang, ScM, of the department of epidemiology at Emory University Rollins School of Public Health, Atlanta, and his coinvestigators.
“These findings point to a central role of the autonomic nervous system in the regulation of mood and depression vulnerability,” they wrote in a report on the study appearing in JAMA Psychiatry.
The published analysis included 146 male twins, or 73 pairs, who participated in the national Vietnam Era Twin Registry.
Previous studies have linked depression to heart rate variability, a noninvasive index of cardiac autonomic nervous system regulation. However, these studies are not consistent on whether depression affects heart rate variability or vice versa, and the studies have been limited in their ability to assess the relationship between those two variables over time, the investigators said.
By contrast, the current study evaluated depression and heart rate variability at two time points: a baseline assessment conducted during 2002-2006 and a at a 7-year follow-up visit.
Investigators found consistent associations between heart rate variability on 24-hour electrocardiogram monitoring at baseline and scores on the Beck Depression Inventory-II at the 7-year follow-up, with beta coefficients ranging from –0.14 to –0.29, the report showed.
By contrast, the associations were less consistent between BDI-II score at the baseline visit and heart rate variability at follow-up, the investigators said. “These associations were largely explained by antidepressant use, which when added to the model, weakened the associations.”
These findings may help guide future research aimed at identifying individuals at a higher risk of later depression, the authors said, noting that treatment studies also are warranted. “Future interventions modulating autonomic nervous system regulation may be useful for the prevention and treatment of depression.” The study was supported by the National Institutes of Health. The Department of Veterans Affairs has supported the Vietnam Era Twin Registry. The researchers had no conflicts of interest.
SOURCE: Huang M et al. JAMA Psychiatry. 2018 May 16. doi: 10.1001/jamapsychiatry.2018.0747.
FROM JAMA PSYCHIATRY
Key clinical point:
Major finding: There were consistent associations between baseline heart rate variability and later depression scores (beta coefficients ranged from –0.14 to –0.29).
Study details: A longitudinal twin difference study including 166 individuals in the Vietnam Era Twin Registry, including baseline assessments conducted during 2002-2006 plus a 7-year follow-up visit.
Disclosures: The study was supported by the National Institutes of Health. The Department of Veterans Affairs has supported the Vietnam Era Twin Registry. Study authors had no conflicts of interest.
Source: Huang M et al. JAMA Psychiatry. 2018 May 16. doi: 10.1001/jamapsychiatry.2018.0747.
Two more and counting: Suicide in medical trainees
Like everyone in the arc of social media impact, I was shocked and terribly saddened by the recent suicides of two New York women in medicine – a final-year medical student on May 1 and a second-year resident on May 5. As a specialist in physician health, a former training director, a long-standing member of our institution’s medical student admissions committee, and the ombudsman for our medical students, I am finding these tragedies harder and harder to reconcile. Something isn’t working. But before I get to that, what follows is a bulleted list of some events of the past couple of weeks that may give a context for my statements and have informed my two recommendations.
- May 3, 2018: I give an invited GI grand rounds on stress, burnout, depression, and suicide in physicians. The residents are quiet and say nothing. Faculty members seem only concerned about preventing and eradicating burnout – and not that interested in anything more severe.
- May 5: A psychiatry resident from Melbourne arrives to spend 10 days with me to do an elective in physician health. As in the United States, there is a significant suicide death rate in medical students and residents Down Under. In the afternoon, I present a paper at the annual meeting of the American Academy of Psychodynamic Psychiatry and Psychoanalysis on the use of psychotherapy in treatment-resistant suicidal depression in physicians. There is increasing hope that this essential modality of care will return to the contemporary psychiatrist’s toolbox.
- May 6: At the annual meeting of the American Psychiatric Association in New York, I’m the discussant for powerful heartfelt papers of five psychiatrists (mostly early career psychiatrists and one resident) that talked about living with a psychiatric illness. The audience is huge, and we hear narratives about internal stigma, self-disclosure, external stigma, shunning, bullying, acceptance, rejection, alienation, connection, and love by peers and family. The authenticity and valor of the speakers create an atmosphere of safety, which enables psychiatrists in attendance from all over the world to share their personal stories – some at the microphone, some privately.
- May 7: Again at the APA, I chair and facilitate a workshop on physician suicide. We hear from four speakers, all women, who have lost a loved one to suicide – a husband, a father, a brother, a son – all doctors. Two of the speakers are psychiatrists. The stories are gripping, detailed, and tender. Yes, the atmosphere is very sad, but there is not a pall. We learn how these doctors lived, not just how they died. They all loved medicine; they were creative; they cared deeply; they suffered silently; and with shame, they lost hope. Again, a big audience of psychiatrists, many of whom share their own stories, that they, too, had lost a physician son, wife, or mother to suicide. Some of their deceased family members fell through the cracks and did not receive the life-saving care they deserved; some, fearing assaults to their medical license, hospital privileges, or insurance, refused to see anyone. They died untreated.
- May 8: Still at the APA, a psychiatrist colleague and I collaborate on a clinical case conference. Each of us describes losing a physician patient to suicide. We walk the attendees through the clinical details of assessment, treatment, and the aftermath of their deaths. We talk openly and frankly about our feelings, grief, outreach to colleagues and the family, and our own personal journeys of learning, growth, and healing. The clinician audience members give constructive feedback, and some share their own stories of losing patients to suicide. Like the day before, some psychiatrists are grieving the loss of a physician son or sibling to suicide. As mental health professionals, they suffer from an additional layer of failure and guilt that a loved one died “under their watch.”
- May 8: I rush across the Javits Center to catch the discussant for a concurrent symposium on physician burnout and depression. She foregoes any prepared remarks to share her previous 48 hours with the audience. She is the training director of the program that lost the second-year resident on May 5. She did not learn of the death until 24 hours later. We are all on the edge of our seats as we listen to this grieving, courageous woman, a seasoned psychiatrist and educator, who has been blindsided by this tragedy. She has not slept. She called all of her residents and broke the news personally as best she could. Aided by “After A Suicide: A Toolkit for Residency/Fellowship Programs” (American Foundation for Suicide Prevention), she and her colleagues instituted a plan of action and worked with administration and faculty. Her strength and commitment to the well-being of her trainees is palpable and magnanimous. When the session ends, many of us stand in line to give her a hug. It is a stark reminder of how many lives are affected when someone you know or care about takes his/her own life – and how, in the house of medicine, medical students and residents really are part of an institutional family.
- May 10: I facilitate a meeting of our 12 second-year residents, many of whom knew of or had met the resident who died. Almost everyone speaks, shares their feelings, poses questions, and calls for answers and change. There is disbelief, sadness, confusion, some guilt, and lots of anger. Also a feeling of disillusionment or paradox about the field of psychiatry: “Of all branches of medicine, shouldn’t residents who are struggling with psychiatric issues feel safe, protected, cared for in psychiatry?” There is also a feeling of lip service being paid to personal treatment, as in quoted statements: “By all means, get treatment for your issues, but don’t let it encroach on your duty hours” or “It’s good you’re getting help, but do you still have to go weekly?”
In the immediate aftermath of suicide, feelings run high, as they should. But rather than wait it out – and fearing a return to “business as usual” – let me make only two suggestions:
2. In psychiatry, we need to redouble our efforts in fighting the stigma attached to psychiatric illness in trainees. It is unconscionable that medical students and residents are dying of treatable disorders (I’ve never heard of a doctor dying of cancer who didn’t go to an oncologist at least once), yet too many are not availing themselves of services we provide – even when they’re free of charge or covered by insurance. And are we certain that, when they knock on our doors, we are providing them with state-of-the-art care? Is it possible that unrecognized internal stigma and shame deep within us might make us hesitant to help our trainees in their hour of need? Or cut corners? Or not get a second opinion? Very few psychiatrists on faculty of our medical schools divulge their personal experiences of depression, posttraumatic stress disorders, substance use disorders, and more (with the exception of being in therapy during residency, which is normative and isn’t stigmatized). Coming out is leveling, humane, and respectful – and it shrinks the power differential in the teaching dyad. It might even save a life.
Dr. Myers is a professor of clinical psychiatry at State University of New York, Brooklyn, and the author of “Why Physicians Die by Suicide: Lessons Learned From Their Families and Others Who Cared.”
Like everyone in the arc of social media impact, I was shocked and terribly saddened by the recent suicides of two New York women in medicine – a final-year medical student on May 1 and a second-year resident on May 5. As a specialist in physician health, a former training director, a long-standing member of our institution’s medical student admissions committee, and the ombudsman for our medical students, I am finding these tragedies harder and harder to reconcile. Something isn’t working. But before I get to that, what follows is a bulleted list of some events of the past couple of weeks that may give a context for my statements and have informed my two recommendations.
- May 3, 2018: I give an invited GI grand rounds on stress, burnout, depression, and suicide in physicians. The residents are quiet and say nothing. Faculty members seem only concerned about preventing and eradicating burnout – and not that interested in anything more severe.
- May 5: A psychiatry resident from Melbourne arrives to spend 10 days with me to do an elective in physician health. As in the United States, there is a significant suicide death rate in medical students and residents Down Under. In the afternoon, I present a paper at the annual meeting of the American Academy of Psychodynamic Psychiatry and Psychoanalysis on the use of psychotherapy in treatment-resistant suicidal depression in physicians. There is increasing hope that this essential modality of care will return to the contemporary psychiatrist’s toolbox.
- May 6: At the annual meeting of the American Psychiatric Association in New York, I’m the discussant for powerful heartfelt papers of five psychiatrists (mostly early career psychiatrists and one resident) that talked about living with a psychiatric illness. The audience is huge, and we hear narratives about internal stigma, self-disclosure, external stigma, shunning, bullying, acceptance, rejection, alienation, connection, and love by peers and family. The authenticity and valor of the speakers create an atmosphere of safety, which enables psychiatrists in attendance from all over the world to share their personal stories – some at the microphone, some privately.
- May 7: Again at the APA, I chair and facilitate a workshop on physician suicide. We hear from four speakers, all women, who have lost a loved one to suicide – a husband, a father, a brother, a son – all doctors. Two of the speakers are psychiatrists. The stories are gripping, detailed, and tender. Yes, the atmosphere is very sad, but there is not a pall. We learn how these doctors lived, not just how they died. They all loved medicine; they were creative; they cared deeply; they suffered silently; and with shame, they lost hope. Again, a big audience of psychiatrists, many of whom share their own stories, that they, too, had lost a physician son, wife, or mother to suicide. Some of their deceased family members fell through the cracks and did not receive the life-saving care they deserved; some, fearing assaults to their medical license, hospital privileges, or insurance, refused to see anyone. They died untreated.
- May 8: Still at the APA, a psychiatrist colleague and I collaborate on a clinical case conference. Each of us describes losing a physician patient to suicide. We walk the attendees through the clinical details of assessment, treatment, and the aftermath of their deaths. We talk openly and frankly about our feelings, grief, outreach to colleagues and the family, and our own personal journeys of learning, growth, and healing. The clinician audience members give constructive feedback, and some share their own stories of losing patients to suicide. Like the day before, some psychiatrists are grieving the loss of a physician son or sibling to suicide. As mental health professionals, they suffer from an additional layer of failure and guilt that a loved one died “under their watch.”
- May 8: I rush across the Javits Center to catch the discussant for a concurrent symposium on physician burnout and depression. She foregoes any prepared remarks to share her previous 48 hours with the audience. She is the training director of the program that lost the second-year resident on May 5. She did not learn of the death until 24 hours later. We are all on the edge of our seats as we listen to this grieving, courageous woman, a seasoned psychiatrist and educator, who has been blindsided by this tragedy. She has not slept. She called all of her residents and broke the news personally as best she could. Aided by “After A Suicide: A Toolkit for Residency/Fellowship Programs” (American Foundation for Suicide Prevention), she and her colleagues instituted a plan of action and worked with administration and faculty. Her strength and commitment to the well-being of her trainees is palpable and magnanimous. When the session ends, many of us stand in line to give her a hug. It is a stark reminder of how many lives are affected when someone you know or care about takes his/her own life – and how, in the house of medicine, medical students and residents really are part of an institutional family.
- May 10: I facilitate a meeting of our 12 second-year residents, many of whom knew of or had met the resident who died. Almost everyone speaks, shares their feelings, poses questions, and calls for answers and change. There is disbelief, sadness, confusion, some guilt, and lots of anger. Also a feeling of disillusionment or paradox about the field of psychiatry: “Of all branches of medicine, shouldn’t residents who are struggling with psychiatric issues feel safe, protected, cared for in psychiatry?” There is also a feeling of lip service being paid to personal treatment, as in quoted statements: “By all means, get treatment for your issues, but don’t let it encroach on your duty hours” or “It’s good you’re getting help, but do you still have to go weekly?”
In the immediate aftermath of suicide, feelings run high, as they should. But rather than wait it out – and fearing a return to “business as usual” – let me make only two suggestions:
2. In psychiatry, we need to redouble our efforts in fighting the stigma attached to psychiatric illness in trainees. It is unconscionable that medical students and residents are dying of treatable disorders (I’ve never heard of a doctor dying of cancer who didn’t go to an oncologist at least once), yet too many are not availing themselves of services we provide – even when they’re free of charge or covered by insurance. And are we certain that, when they knock on our doors, we are providing them with state-of-the-art care? Is it possible that unrecognized internal stigma and shame deep within us might make us hesitant to help our trainees in their hour of need? Or cut corners? Or not get a second opinion? Very few psychiatrists on faculty of our medical schools divulge their personal experiences of depression, posttraumatic stress disorders, substance use disorders, and more (with the exception of being in therapy during residency, which is normative and isn’t stigmatized). Coming out is leveling, humane, and respectful – and it shrinks the power differential in the teaching dyad. It might even save a life.
Dr. Myers is a professor of clinical psychiatry at State University of New York, Brooklyn, and the author of “Why Physicians Die by Suicide: Lessons Learned From Their Families and Others Who Cared.”
Like everyone in the arc of social media impact, I was shocked and terribly saddened by the recent suicides of two New York women in medicine – a final-year medical student on May 1 and a second-year resident on May 5. As a specialist in physician health, a former training director, a long-standing member of our institution’s medical student admissions committee, and the ombudsman for our medical students, I am finding these tragedies harder and harder to reconcile. Something isn’t working. But before I get to that, what follows is a bulleted list of some events of the past couple of weeks that may give a context for my statements and have informed my two recommendations.
- May 3, 2018: I give an invited GI grand rounds on stress, burnout, depression, and suicide in physicians. The residents are quiet and say nothing. Faculty members seem only concerned about preventing and eradicating burnout – and not that interested in anything more severe.
- May 5: A psychiatry resident from Melbourne arrives to spend 10 days with me to do an elective in physician health. As in the United States, there is a significant suicide death rate in medical students and residents Down Under. In the afternoon, I present a paper at the annual meeting of the American Academy of Psychodynamic Psychiatry and Psychoanalysis on the use of psychotherapy in treatment-resistant suicidal depression in physicians. There is increasing hope that this essential modality of care will return to the contemporary psychiatrist’s toolbox.
- May 6: At the annual meeting of the American Psychiatric Association in New York, I’m the discussant for powerful heartfelt papers of five psychiatrists (mostly early career psychiatrists and one resident) that talked about living with a psychiatric illness. The audience is huge, and we hear narratives about internal stigma, self-disclosure, external stigma, shunning, bullying, acceptance, rejection, alienation, connection, and love by peers and family. The authenticity and valor of the speakers create an atmosphere of safety, which enables psychiatrists in attendance from all over the world to share their personal stories – some at the microphone, some privately.
- May 7: Again at the APA, I chair and facilitate a workshop on physician suicide. We hear from four speakers, all women, who have lost a loved one to suicide – a husband, a father, a brother, a son – all doctors. Two of the speakers are psychiatrists. The stories are gripping, detailed, and tender. Yes, the atmosphere is very sad, but there is not a pall. We learn how these doctors lived, not just how they died. They all loved medicine; they were creative; they cared deeply; they suffered silently; and with shame, they lost hope. Again, a big audience of psychiatrists, many of whom share their own stories, that they, too, had lost a physician son, wife, or mother to suicide. Some of their deceased family members fell through the cracks and did not receive the life-saving care they deserved; some, fearing assaults to their medical license, hospital privileges, or insurance, refused to see anyone. They died untreated.
- May 8: Still at the APA, a psychiatrist colleague and I collaborate on a clinical case conference. Each of us describes losing a physician patient to suicide. We walk the attendees through the clinical details of assessment, treatment, and the aftermath of their deaths. We talk openly and frankly about our feelings, grief, outreach to colleagues and the family, and our own personal journeys of learning, growth, and healing. The clinician audience members give constructive feedback, and some share their own stories of losing patients to suicide. Like the day before, some psychiatrists are grieving the loss of a physician son or sibling to suicide. As mental health professionals, they suffer from an additional layer of failure and guilt that a loved one died “under their watch.”
- May 8: I rush across the Javits Center to catch the discussant for a concurrent symposium on physician burnout and depression. She foregoes any prepared remarks to share her previous 48 hours with the audience. She is the training director of the program that lost the second-year resident on May 5. She did not learn of the death until 24 hours later. We are all on the edge of our seats as we listen to this grieving, courageous woman, a seasoned psychiatrist and educator, who has been blindsided by this tragedy. She has not slept. She called all of her residents and broke the news personally as best she could. Aided by “After A Suicide: A Toolkit for Residency/Fellowship Programs” (American Foundation for Suicide Prevention), she and her colleagues instituted a plan of action and worked with administration and faculty. Her strength and commitment to the well-being of her trainees is palpable and magnanimous. When the session ends, many of us stand in line to give her a hug. It is a stark reminder of how many lives are affected when someone you know or care about takes his/her own life – and how, in the house of medicine, medical students and residents really are part of an institutional family.
- May 10: I facilitate a meeting of our 12 second-year residents, many of whom knew of or had met the resident who died. Almost everyone speaks, shares their feelings, poses questions, and calls for answers and change. There is disbelief, sadness, confusion, some guilt, and lots of anger. Also a feeling of disillusionment or paradox about the field of psychiatry: “Of all branches of medicine, shouldn’t residents who are struggling with psychiatric issues feel safe, protected, cared for in psychiatry?” There is also a feeling of lip service being paid to personal treatment, as in quoted statements: “By all means, get treatment for your issues, but don’t let it encroach on your duty hours” or “It’s good you’re getting help, but do you still have to go weekly?”
In the immediate aftermath of suicide, feelings run high, as they should. But rather than wait it out – and fearing a return to “business as usual” – let me make only two suggestions:
2. In psychiatry, we need to redouble our efforts in fighting the stigma attached to psychiatric illness in trainees. It is unconscionable that medical students and residents are dying of treatable disorders (I’ve never heard of a doctor dying of cancer who didn’t go to an oncologist at least once), yet too many are not availing themselves of services we provide – even when they’re free of charge or covered by insurance. And are we certain that, when they knock on our doors, we are providing them with state-of-the-art care? Is it possible that unrecognized internal stigma and shame deep within us might make us hesitant to help our trainees in their hour of need? Or cut corners? Or not get a second opinion? Very few psychiatrists on faculty of our medical schools divulge their personal experiences of depression, posttraumatic stress disorders, substance use disorders, and more (with the exception of being in therapy during residency, which is normative and isn’t stigmatized). Coming out is leveling, humane, and respectful – and it shrinks the power differential in the teaching dyad. It might even save a life.
Dr. Myers is a professor of clinical psychiatry at State University of New York, Brooklyn, and the author of “Why Physicians Die by Suicide: Lessons Learned From Their Families and Others Who Cared.”