User login
Suicide prevention gets ‘standard care’ recommendations
WASHINGTON – The National Action Alliance for Suicide Prevention released in April 2018 what the organization said was the first set of “standard care” recommendations for suicide prevention in people with suicide risk.
Care for people with a suicide risk in the United States “is not working very well. Evidence-based tools exist to detect and manage suicidality, but they are new and infrequently used” by many clinicians, including those seeing suicidal patients in primary care, emergency, or hospital settings, said Michael F. Hogan, PhD, during a session on the new standard-care recommendations at the annual conference of the American Association of Suicidology.
The Action Alliance seeks to have the standard care recommendations widely disseminated and hopes the document will receive endorsement from other organizations, said Dr. Hogan, a health policy consultant in Delmar, N.Y., and a member of the eight-person panel that wrote the recommendations.
These recommendations specify interventions for caregivers in four separate settings: primary care, outpatient behavioral health care (mental health and substance use treatment settings), emergency departments, and behavioral health inpatient care (hospital-level psychiatric or addiction treatment). For each setting, the recommendations highlight one or more core approaches, and then specify standards for identification and assessment, safety planning, means reduction, and follow-up contacts.
For example, within the primary care setting, the recommendations say the goals are to identify suicide risk, enhance the safety for those at risk, refer for specialized care, and provide “caring contacts.” The specifications note that this is achieved with standardized screening and assessment instruments (the recommendations cite eight screening tool options and also suggest three different possible assessment tools); referral as appropriate; a brief safety-planning intervention (the recommendations list five options for this) that includes lethality means reduction along with follow-up to be sure that lethal means have been removed; arranging for rapid follow-up with a mental health professional; and follow-up contact by the primary care clinician within the next 48 hours.
According to Dr. Hogan, a motivation for releasing these recommendations has been the growing U.S. incidence of suicide, rising from 10.4 deaths/100,000 in 2000 to 13.3/100,000 in 2015, a 28% relative increase during a period when the rates of the top killers in the United States – cancer, heart disease, and stroke – were falling. Other telling statistics are that most people who die from suicide had seen a primary care provider during the year before death, and nearly half had seen a primary care provider during the month before their death.
But often the indicators of impending suicide are missed or not acted on. a misperception that contributes to a “failure to ask about suicide risk” on the part of health care professionals, the recommendations said. The document also highlighted the idea that, “most health care professionals are not aware of newly developed brief interventions for suicide, leading to the assumption that they should not ask about suicide because there is nothing practical that can be done in ordinary health care settings.”
One limitation of the recommendations is that they might be interpreted as “standard of care” for medicolegal purposes, warned Alan L. Berman, PhD, during the session’s discussion period. In addition, the evidence base for some of the recommended procedures is not very strong, such as risk stratification, said Dr. Berman, a clinical psychologist and former executive director of the American Association of Suicidology.
Dr. Hogan, Dr. Andrews, and Dr. Berman had no disclosures.
WASHINGTON – The National Action Alliance for Suicide Prevention released in April 2018 what the organization said was the first set of “standard care” recommendations for suicide prevention in people with suicide risk.
Care for people with a suicide risk in the United States “is not working very well. Evidence-based tools exist to detect and manage suicidality, but they are new and infrequently used” by many clinicians, including those seeing suicidal patients in primary care, emergency, or hospital settings, said Michael F. Hogan, PhD, during a session on the new standard-care recommendations at the annual conference of the American Association of Suicidology.
The Action Alliance seeks to have the standard care recommendations widely disseminated and hopes the document will receive endorsement from other organizations, said Dr. Hogan, a health policy consultant in Delmar, N.Y., and a member of the eight-person panel that wrote the recommendations.
These recommendations specify interventions for caregivers in four separate settings: primary care, outpatient behavioral health care (mental health and substance use treatment settings), emergency departments, and behavioral health inpatient care (hospital-level psychiatric or addiction treatment). For each setting, the recommendations highlight one or more core approaches, and then specify standards for identification and assessment, safety planning, means reduction, and follow-up contacts.
For example, within the primary care setting, the recommendations say the goals are to identify suicide risk, enhance the safety for those at risk, refer for specialized care, and provide “caring contacts.” The specifications note that this is achieved with standardized screening and assessment instruments (the recommendations cite eight screening tool options and also suggest three different possible assessment tools); referral as appropriate; a brief safety-planning intervention (the recommendations list five options for this) that includes lethality means reduction along with follow-up to be sure that lethal means have been removed; arranging for rapid follow-up with a mental health professional; and follow-up contact by the primary care clinician within the next 48 hours.
According to Dr. Hogan, a motivation for releasing these recommendations has been the growing U.S. incidence of suicide, rising from 10.4 deaths/100,000 in 2000 to 13.3/100,000 in 2015, a 28% relative increase during a period when the rates of the top killers in the United States – cancer, heart disease, and stroke – were falling. Other telling statistics are that most people who die from suicide had seen a primary care provider during the year before death, and nearly half had seen a primary care provider during the month before their death.
But often the indicators of impending suicide are missed or not acted on. a misperception that contributes to a “failure to ask about suicide risk” on the part of health care professionals, the recommendations said. The document also highlighted the idea that, “most health care professionals are not aware of newly developed brief interventions for suicide, leading to the assumption that they should not ask about suicide because there is nothing practical that can be done in ordinary health care settings.”
One limitation of the recommendations is that they might be interpreted as “standard of care” for medicolegal purposes, warned Alan L. Berman, PhD, during the session’s discussion period. In addition, the evidence base for some of the recommended procedures is not very strong, such as risk stratification, said Dr. Berman, a clinical psychologist and former executive director of the American Association of Suicidology.
Dr. Hogan, Dr. Andrews, and Dr. Berman had no disclosures.
WASHINGTON – The National Action Alliance for Suicide Prevention released in April 2018 what the organization said was the first set of “standard care” recommendations for suicide prevention in people with suicide risk.
Care for people with a suicide risk in the United States “is not working very well. Evidence-based tools exist to detect and manage suicidality, but they are new and infrequently used” by many clinicians, including those seeing suicidal patients in primary care, emergency, or hospital settings, said Michael F. Hogan, PhD, during a session on the new standard-care recommendations at the annual conference of the American Association of Suicidology.
The Action Alliance seeks to have the standard care recommendations widely disseminated and hopes the document will receive endorsement from other organizations, said Dr. Hogan, a health policy consultant in Delmar, N.Y., and a member of the eight-person panel that wrote the recommendations.
These recommendations specify interventions for caregivers in four separate settings: primary care, outpatient behavioral health care (mental health and substance use treatment settings), emergency departments, and behavioral health inpatient care (hospital-level psychiatric or addiction treatment). For each setting, the recommendations highlight one or more core approaches, and then specify standards for identification and assessment, safety planning, means reduction, and follow-up contacts.
For example, within the primary care setting, the recommendations say the goals are to identify suicide risk, enhance the safety for those at risk, refer for specialized care, and provide “caring contacts.” The specifications note that this is achieved with standardized screening and assessment instruments (the recommendations cite eight screening tool options and also suggest three different possible assessment tools); referral as appropriate; a brief safety-planning intervention (the recommendations list five options for this) that includes lethality means reduction along with follow-up to be sure that lethal means have been removed; arranging for rapid follow-up with a mental health professional; and follow-up contact by the primary care clinician within the next 48 hours.
According to Dr. Hogan, a motivation for releasing these recommendations has been the growing U.S. incidence of suicide, rising from 10.4 deaths/100,000 in 2000 to 13.3/100,000 in 2015, a 28% relative increase during a period when the rates of the top killers in the United States – cancer, heart disease, and stroke – were falling. Other telling statistics are that most people who die from suicide had seen a primary care provider during the year before death, and nearly half had seen a primary care provider during the month before their death.
But often the indicators of impending suicide are missed or not acted on. a misperception that contributes to a “failure to ask about suicide risk” on the part of health care professionals, the recommendations said. The document also highlighted the idea that, “most health care professionals are not aware of newly developed brief interventions for suicide, leading to the assumption that they should not ask about suicide because there is nothing practical that can be done in ordinary health care settings.”
One limitation of the recommendations is that they might be interpreted as “standard of care” for medicolegal purposes, warned Alan L. Berman, PhD, during the session’s discussion period. In addition, the evidence base for some of the recommended procedures is not very strong, such as risk stratification, said Dr. Berman, a clinical psychologist and former executive director of the American Association of Suicidology.
Dr. Hogan, Dr. Andrews, and Dr. Berman had no disclosures.
REPORTING FROM THE AAS ANNUAL CONFERENCE
FDA approves subcutaneous tocilizumab for polyarticular JIA
in patients aged 2 years and older, according to a statement released May 14 by the drug’s manufacturer, Genentech.
While a intravenous formulation of the treatment was approved in 2013, this new delivery method may help make this treatment more accessible to the approximately 30 in every 100,000 children affected by PJIA, according to the release.
Doses were determined based on weight. Patients under 30 kg received 162 mg of tocilizumab every 3 weeks, while those 30 kg and over received 162 mg tocilizumab every 2 weeks.
Overall, safety of the subcutaneous delivery method was consistent with the IV study, as was the efficacy of the drug, the company said. A total of 28.8% of patients reported injection-site reactions – all moderate – and 15.4% reported neutrophil counts below 1 x 109 per liter.Tocilizumab can be taken either by itself or with methotrexate.
in patients aged 2 years and older, according to a statement released May 14 by the drug’s manufacturer, Genentech.
While a intravenous formulation of the treatment was approved in 2013, this new delivery method may help make this treatment more accessible to the approximately 30 in every 100,000 children affected by PJIA, according to the release.
Doses were determined based on weight. Patients under 30 kg received 162 mg of tocilizumab every 3 weeks, while those 30 kg and over received 162 mg tocilizumab every 2 weeks.
Overall, safety of the subcutaneous delivery method was consistent with the IV study, as was the efficacy of the drug, the company said. A total of 28.8% of patients reported injection-site reactions – all moderate – and 15.4% reported neutrophil counts below 1 x 109 per liter.Tocilizumab can be taken either by itself or with methotrexate.
in patients aged 2 years and older, according to a statement released May 14 by the drug’s manufacturer, Genentech.
While a intravenous formulation of the treatment was approved in 2013, this new delivery method may help make this treatment more accessible to the approximately 30 in every 100,000 children affected by PJIA, according to the release.
Doses were determined based on weight. Patients under 30 kg received 162 mg of tocilizumab every 3 weeks, while those 30 kg and over received 162 mg tocilizumab every 2 weeks.
Overall, safety of the subcutaneous delivery method was consistent with the IV study, as was the efficacy of the drug, the company said. A total of 28.8% of patients reported injection-site reactions – all moderate – and 15.4% reported neutrophil counts below 1 x 109 per liter.Tocilizumab can be taken either by itself or with methotrexate.
Emergency Imaging: Femoral Pseudoaneurysm
Case
An 84-year-old man, who was a resident at a local nursing home, presented for evaluation after the nursing staff noticed an increasingly swollen mass on the patient’s left groin. The patient’s medical history was significant for bilateral aortofemoral graft surgery, dementia, hypertension, and severe peripheral artery disease (PAD). He was not on any anticoagulation or antiplatelet agents. Due to the patient’s dementia, he was unable to provide a history regarding the onset of the swelling or any other signs or symptoms.
On examination, the patient did not appear in distress. His son, who was the patient’s durable power of attorney, was likewise unable to provide a clear timeframe regarding onset of the mass. The patient had no recent history of trauma and had not undergone any recent medical procedures. Vital signs at presentation were: blood pressure, 110/70 mm Hg; heart rate, 84 beats/min; respiratory rate, 13 breaths/min; and temperature, 98.6°F. Oxygen saturation was 94% on room air.
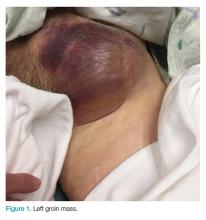
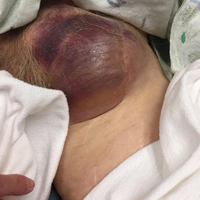
Clinical examination revealed a pulsatile, purple left groin mass and bruit. The mass was located around the left inguinal ligament and extended down the proximal, inner thigh (Figure 1). There was no drainage or lesions from the mass. Inspection of the patient’s hip demonstrated decreased adduction, limited by the mass; otherwise, there was normal range of motion. The dorsalis pedis and posterior tibial pulses were equal and intact, and the rest of the physical examination was unremarkable.
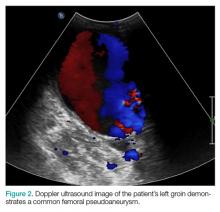
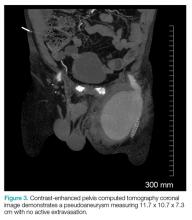
The patient tolerated the examination without focal signs of discomfort. A Doppler ultrasound revealed findings consistent with a common femoral pseudoaneurysm (PSA) (Figure 2). For better visualization and extension, a computed tomography angiogram (CTA) was obtained, which demonstrated a PSA measuring 11.7 x 10.7 x 7.3 cm; there was no active extravasation (Figure 3).
The patient was started on intravenous normal saline while vascular surgery services was consulted for management and repair. After a discussion with the son regarding the patient’s wishes, surgical intervention was refused and the patient was conservatively managed and transitioned to hospice care.
Discussion
A true aneurysm differs from a PSA in that true aneurysms involve all three layers of the vessel wall. A PSA consists partly of the vessel wall and partly of encapsulating fibrous tissue or surrounding tissue.
Etiology
Femoral artery PSAs can be iatrogenic, for example, develop following cardiac catheterization or at the anastomotic site of previous surgery.1 The incidence of diagnostic postcatheterization PSA ranges from 0.05% to 2%, whereas interventional postcatheterization PSA ranges from 2% to 6%.2
With the increasing number of peripheral coronary diagnostics and interventions, emergency physicians should include PSA in the differential diagnosis of patients with a recent or remote history of catheterization or bypass grafts. Less commonly, femoral PSAs are caused by non-surgical trauma or infection (ie, mycotic PSA). Patient risk factors for development of PSA include obesity, hypertension, PAD, and anticoagulation.3 Patients with femoral artery PSAs may present with a painful or painless pulsatile mass. Mass effect of the PSA can compress nearby neurovascular structures, leading to femoral neuropathies or limb edema secondary to venous obstruction.4 Complications of embolization or thrombosis can cause limb ischemia, neuropathy, and claudication, while rupture may present with a rapidly expanding groin hematoma. Additionally, sizeable PSAs can cause overlying skin necrosis.5
Imaging Studies
Diagnosis of a PSA can be made through Doppler ultrasound, which is the preferred imaging modality due to its accuracy, noninvasive nature, and low cost. Doppler ultrasound has been found to have a sensitivity of 94% and specificity of 97% in detecting PSAs. Additional imaging with CTA can provide further definition of vasculopathy.6 Treatment should be considered for patients with a symptomatic femoral PSA, a PSA measuring more than 3 cm, or patients who are on anticoagulation therapy. Studies have shown that observation-only and follow-up may be appropriate for patients with a PSA measuring less than 3 cm. A study by Toursarkissian et al7 found that the majority of PSAs smaller than 3 cm spontaneously resolved in a mean of 23 days without limb-threatening complications.
Treatment
Traditionally, open surgical repair techniques were the only treatment option for PSAs. However, in the early 1990s, the advent of new techniques such as stenting, coil insertion, ultrasound-guided compression, and ultrasound-guided thrombin injection, have developed as alternatives to open surgical repair; there has been variable success to these minimally invasive approaches.5,8
Ultrasound-Guided Compression. A conservative approach to treating PSAs, ultrasound-guided compression requires sustained compression by a skilled physician. This technique is associated with significant discomfort to the patient.5 Ultrasound-Guided Thrombin Injection. This technique is the treatment of choice for postcatheterization PSA. However, this intervention is contraindicated in patients who have concerning features such as an infected PSA, rapid expansion, skin necrosis, or signs of limb ischemia. Additionally, ultrasound-guided thrombin injection is not appropriate for use in patients with a PSA occurring at anastomosis of a synthetic graft and native artery.5
Conclusion
Based on our patient’s clinical presentation and history of aortofemoral bypass surgery, we suspected a femoral PSA. While the PSA noted in our patient was sizeable, imaging studies and clinical examination showed no sign of limb ischemia or rupture.
Femoral PSAs are usually iatrogenic in nature, typically developing shortly after catheterization or a previous bypass surgery. The most serious complication of a PSA is rupture, but a thorough examination of the distal extremity is warranted to assess for limb ischemia as well. Ultrasound imaging is considered the modality of choice based on its high sensitivity and sensitivity for detecting PSAs.
Small PSAs (<3 cm) can be managed medically, but larger PSAs (>3 cm) require treatment. Newer techniques, including stenting, coil insertion, ultrasound-guided compression, and ultrasound-guided thrombin injection are alternatives to open surgical repair of larger, uncomplicated PSAs. However, urgent open surgical repair is the only option when there is evidence of a ruptured PSA, ischemia, or skin necrosis.
1. Faggioli GL, Stella A, Gargiulo M, Tarantini S, D’Addato M, Ricotta JJ. Morphology of small aneurysms: definition and impact on risk of rupture. Am J Surg. 1994;168(2):131-135.
2. Hessel SJ, Adams DF, Abrams HL. Complications of angiography. Radiology. 1981;138(2):273-281. doi:10.1148/radiology.138.2.7455105.
3. Petrou E, Malakos I, Kampanarou S, Doulas N, Voudris V. Life-threatening rupture of a femoral pseudoaneurysm after cardiac catheterization. Open Cardiovasc Med J. 2016;10:201-204. doi:10.2174/1874192401610010201.
4. Mees B, Robinson D, Verhagen H, Chuen J. Non-aortic aneurysms—natural history and recommendations for referral and treatment. Aust Fam Physician. 2013;42(6):370-374.
5. Webber GW, Jang J, Gustavson S, Olin JW. Contemporary management of postcatheterization pseudoaneurysms. Circulation. 2007;115(20):2666-2674. doi:10.1161/CIRCULATIONAHA.106.681973.
6. Coughlin BF, Paushter DM. Peripheral pseudoaneurysms: evaluation with duplex US. Radiology. 1988;168(2):339-342. doi:10.1148/radiology.168.2.3293107.
7. Toursarkissian B, Allen BT, Petrinec D, et al. Spontaneous closure of selected iatrogenic pseudoaneurysms and arteriovenous fistulae. J Vasc Surg. 1997;25(5):803-809; discussion 808-809.
8. Corriere MA, Guzman RJ. True and false aneurysms of the femoral artery. Semin Vasc Surg. 2005;18(4):216-223. doi:10.1053/j.semvascsurg.2005.09.008.
Case
An 84-year-old man, who was a resident at a local nursing home, presented for evaluation after the nursing staff noticed an increasingly swollen mass on the patient’s left groin. The patient’s medical history was significant for bilateral aortofemoral graft surgery, dementia, hypertension, and severe peripheral artery disease (PAD). He was not on any anticoagulation or antiplatelet agents. Due to the patient’s dementia, he was unable to provide a history regarding the onset of the swelling or any other signs or symptoms.
On examination, the patient did not appear in distress. His son, who was the patient’s durable power of attorney, was likewise unable to provide a clear timeframe regarding onset of the mass. The patient had no recent history of trauma and had not undergone any recent medical procedures. Vital signs at presentation were: blood pressure, 110/70 mm Hg; heart rate, 84 beats/min; respiratory rate, 13 breaths/min; and temperature, 98.6°F. Oxygen saturation was 94% on room air.
Clinical examination revealed a pulsatile, purple left groin mass and bruit. The mass was located around the left inguinal ligament and extended down the proximal, inner thigh (Figure 1). There was no drainage or lesions from the mass. Inspection of the patient’s hip demonstrated decreased adduction, limited by the mass; otherwise, there was normal range of motion. The dorsalis pedis and posterior tibial pulses were equal and intact, and the rest of the physical examination was unremarkable.
The patient tolerated the examination without focal signs of discomfort. A Doppler ultrasound revealed findings consistent with a common femoral pseudoaneurysm (PSA) (Figure 2). For better visualization and extension, a computed tomography angiogram (CTA) was obtained, which demonstrated a PSA measuring 11.7 x 10.7 x 7.3 cm; there was no active extravasation (Figure 3).
The patient was started on intravenous normal saline while vascular surgery services was consulted for management and repair. After a discussion with the son regarding the patient’s wishes, surgical intervention was refused and the patient was conservatively managed and transitioned to hospice care.
Discussion
A true aneurysm differs from a PSA in that true aneurysms involve all three layers of the vessel wall. A PSA consists partly of the vessel wall and partly of encapsulating fibrous tissue or surrounding tissue.
Etiology
Femoral artery PSAs can be iatrogenic, for example, develop following cardiac catheterization or at the anastomotic site of previous surgery.1 The incidence of diagnostic postcatheterization PSA ranges from 0.05% to 2%, whereas interventional postcatheterization PSA ranges from 2% to 6%.2
With the increasing number of peripheral coronary diagnostics and interventions, emergency physicians should include PSA in the differential diagnosis of patients with a recent or remote history of catheterization or bypass grafts. Less commonly, femoral PSAs are caused by non-surgical trauma or infection (ie, mycotic PSA). Patient risk factors for development of PSA include obesity, hypertension, PAD, and anticoagulation.3 Patients with femoral artery PSAs may present with a painful or painless pulsatile mass. Mass effect of the PSA can compress nearby neurovascular structures, leading to femoral neuropathies or limb edema secondary to venous obstruction.4 Complications of embolization or thrombosis can cause limb ischemia, neuropathy, and claudication, while rupture may present with a rapidly expanding groin hematoma. Additionally, sizeable PSAs can cause overlying skin necrosis.5
Imaging Studies
Diagnosis of a PSA can be made through Doppler ultrasound, which is the preferred imaging modality due to its accuracy, noninvasive nature, and low cost. Doppler ultrasound has been found to have a sensitivity of 94% and specificity of 97% in detecting PSAs. Additional imaging with CTA can provide further definition of vasculopathy.6 Treatment should be considered for patients with a symptomatic femoral PSA, a PSA measuring more than 3 cm, or patients who are on anticoagulation therapy. Studies have shown that observation-only and follow-up may be appropriate for patients with a PSA measuring less than 3 cm. A study by Toursarkissian et al7 found that the majority of PSAs smaller than 3 cm spontaneously resolved in a mean of 23 days without limb-threatening complications.
Treatment
Traditionally, open surgical repair techniques were the only treatment option for PSAs. However, in the early 1990s, the advent of new techniques such as stenting, coil insertion, ultrasound-guided compression, and ultrasound-guided thrombin injection, have developed as alternatives to open surgical repair; there has been variable success to these minimally invasive approaches.5,8
Ultrasound-Guided Compression. A conservative approach to treating PSAs, ultrasound-guided compression requires sustained compression by a skilled physician. This technique is associated with significant discomfort to the patient.5 Ultrasound-Guided Thrombin Injection. This technique is the treatment of choice for postcatheterization PSA. However, this intervention is contraindicated in patients who have concerning features such as an infected PSA, rapid expansion, skin necrosis, or signs of limb ischemia. Additionally, ultrasound-guided thrombin injection is not appropriate for use in patients with a PSA occurring at anastomosis of a synthetic graft and native artery.5
Conclusion
Based on our patient’s clinical presentation and history of aortofemoral bypass surgery, we suspected a femoral PSA. While the PSA noted in our patient was sizeable, imaging studies and clinical examination showed no sign of limb ischemia or rupture.
Femoral PSAs are usually iatrogenic in nature, typically developing shortly after catheterization or a previous bypass surgery. The most serious complication of a PSA is rupture, but a thorough examination of the distal extremity is warranted to assess for limb ischemia as well. Ultrasound imaging is considered the modality of choice based on its high sensitivity and sensitivity for detecting PSAs.
Small PSAs (<3 cm) can be managed medically, but larger PSAs (>3 cm) require treatment. Newer techniques, including stenting, coil insertion, ultrasound-guided compression, and ultrasound-guided thrombin injection are alternatives to open surgical repair of larger, uncomplicated PSAs. However, urgent open surgical repair is the only option when there is evidence of a ruptured PSA, ischemia, or skin necrosis.
Case
An 84-year-old man, who was a resident at a local nursing home, presented for evaluation after the nursing staff noticed an increasingly swollen mass on the patient’s left groin. The patient’s medical history was significant for bilateral aortofemoral graft surgery, dementia, hypertension, and severe peripheral artery disease (PAD). He was not on any anticoagulation or antiplatelet agents. Due to the patient’s dementia, he was unable to provide a history regarding the onset of the swelling or any other signs or symptoms.
On examination, the patient did not appear in distress. His son, who was the patient’s durable power of attorney, was likewise unable to provide a clear timeframe regarding onset of the mass. The patient had no recent history of trauma and had not undergone any recent medical procedures. Vital signs at presentation were: blood pressure, 110/70 mm Hg; heart rate, 84 beats/min; respiratory rate, 13 breaths/min; and temperature, 98.6°F. Oxygen saturation was 94% on room air.
Clinical examination revealed a pulsatile, purple left groin mass and bruit. The mass was located around the left inguinal ligament and extended down the proximal, inner thigh (Figure 1). There was no drainage or lesions from the mass. Inspection of the patient’s hip demonstrated decreased adduction, limited by the mass; otherwise, there was normal range of motion. The dorsalis pedis and posterior tibial pulses were equal and intact, and the rest of the physical examination was unremarkable.
The patient tolerated the examination without focal signs of discomfort. A Doppler ultrasound revealed findings consistent with a common femoral pseudoaneurysm (PSA) (Figure 2). For better visualization and extension, a computed tomography angiogram (CTA) was obtained, which demonstrated a PSA measuring 11.7 x 10.7 x 7.3 cm; there was no active extravasation (Figure 3).
The patient was started on intravenous normal saline while vascular surgery services was consulted for management and repair. After a discussion with the son regarding the patient’s wishes, surgical intervention was refused and the patient was conservatively managed and transitioned to hospice care.
Discussion
A true aneurysm differs from a PSA in that true aneurysms involve all three layers of the vessel wall. A PSA consists partly of the vessel wall and partly of encapsulating fibrous tissue or surrounding tissue.
Etiology
Femoral artery PSAs can be iatrogenic, for example, develop following cardiac catheterization or at the anastomotic site of previous surgery.1 The incidence of diagnostic postcatheterization PSA ranges from 0.05% to 2%, whereas interventional postcatheterization PSA ranges from 2% to 6%.2
With the increasing number of peripheral coronary diagnostics and interventions, emergency physicians should include PSA in the differential diagnosis of patients with a recent or remote history of catheterization or bypass grafts. Less commonly, femoral PSAs are caused by non-surgical trauma or infection (ie, mycotic PSA). Patient risk factors for development of PSA include obesity, hypertension, PAD, and anticoagulation.3 Patients with femoral artery PSAs may present with a painful or painless pulsatile mass. Mass effect of the PSA can compress nearby neurovascular structures, leading to femoral neuropathies or limb edema secondary to venous obstruction.4 Complications of embolization or thrombosis can cause limb ischemia, neuropathy, and claudication, while rupture may present with a rapidly expanding groin hematoma. Additionally, sizeable PSAs can cause overlying skin necrosis.5
Imaging Studies
Diagnosis of a PSA can be made through Doppler ultrasound, which is the preferred imaging modality due to its accuracy, noninvasive nature, and low cost. Doppler ultrasound has been found to have a sensitivity of 94% and specificity of 97% in detecting PSAs. Additional imaging with CTA can provide further definition of vasculopathy.6 Treatment should be considered for patients with a symptomatic femoral PSA, a PSA measuring more than 3 cm, or patients who are on anticoagulation therapy. Studies have shown that observation-only and follow-up may be appropriate for patients with a PSA measuring less than 3 cm. A study by Toursarkissian et al7 found that the majority of PSAs smaller than 3 cm spontaneously resolved in a mean of 23 days without limb-threatening complications.
Treatment
Traditionally, open surgical repair techniques were the only treatment option for PSAs. However, in the early 1990s, the advent of new techniques such as stenting, coil insertion, ultrasound-guided compression, and ultrasound-guided thrombin injection, have developed as alternatives to open surgical repair; there has been variable success to these minimally invasive approaches.5,8
Ultrasound-Guided Compression. A conservative approach to treating PSAs, ultrasound-guided compression requires sustained compression by a skilled physician. This technique is associated with significant discomfort to the patient.5 Ultrasound-Guided Thrombin Injection. This technique is the treatment of choice for postcatheterization PSA. However, this intervention is contraindicated in patients who have concerning features such as an infected PSA, rapid expansion, skin necrosis, or signs of limb ischemia. Additionally, ultrasound-guided thrombin injection is not appropriate for use in patients with a PSA occurring at anastomosis of a synthetic graft and native artery.5
Conclusion
Based on our patient’s clinical presentation and history of aortofemoral bypass surgery, we suspected a femoral PSA. While the PSA noted in our patient was sizeable, imaging studies and clinical examination showed no sign of limb ischemia or rupture.
Femoral PSAs are usually iatrogenic in nature, typically developing shortly after catheterization or a previous bypass surgery. The most serious complication of a PSA is rupture, but a thorough examination of the distal extremity is warranted to assess for limb ischemia as well. Ultrasound imaging is considered the modality of choice based on its high sensitivity and sensitivity for detecting PSAs.
Small PSAs (<3 cm) can be managed medically, but larger PSAs (>3 cm) require treatment. Newer techniques, including stenting, coil insertion, ultrasound-guided compression, and ultrasound-guided thrombin injection are alternatives to open surgical repair of larger, uncomplicated PSAs. However, urgent open surgical repair is the only option when there is evidence of a ruptured PSA, ischemia, or skin necrosis.
1. Faggioli GL, Stella A, Gargiulo M, Tarantini S, D’Addato M, Ricotta JJ. Morphology of small aneurysms: definition and impact on risk of rupture. Am J Surg. 1994;168(2):131-135.
2. Hessel SJ, Adams DF, Abrams HL. Complications of angiography. Radiology. 1981;138(2):273-281. doi:10.1148/radiology.138.2.7455105.
3. Petrou E, Malakos I, Kampanarou S, Doulas N, Voudris V. Life-threatening rupture of a femoral pseudoaneurysm after cardiac catheterization. Open Cardiovasc Med J. 2016;10:201-204. doi:10.2174/1874192401610010201.
4. Mees B, Robinson D, Verhagen H, Chuen J. Non-aortic aneurysms—natural history and recommendations for referral and treatment. Aust Fam Physician. 2013;42(6):370-374.
5. Webber GW, Jang J, Gustavson S, Olin JW. Contemporary management of postcatheterization pseudoaneurysms. Circulation. 2007;115(20):2666-2674. doi:10.1161/CIRCULATIONAHA.106.681973.
6. Coughlin BF, Paushter DM. Peripheral pseudoaneurysms: evaluation with duplex US. Radiology. 1988;168(2):339-342. doi:10.1148/radiology.168.2.3293107.
7. Toursarkissian B, Allen BT, Petrinec D, et al. Spontaneous closure of selected iatrogenic pseudoaneurysms and arteriovenous fistulae. J Vasc Surg. 1997;25(5):803-809; discussion 808-809.
8. Corriere MA, Guzman RJ. True and false aneurysms of the femoral artery. Semin Vasc Surg. 2005;18(4):216-223. doi:10.1053/j.semvascsurg.2005.09.008.
1. Faggioli GL, Stella A, Gargiulo M, Tarantini S, D’Addato M, Ricotta JJ. Morphology of small aneurysms: definition and impact on risk of rupture. Am J Surg. 1994;168(2):131-135.
2. Hessel SJ, Adams DF, Abrams HL. Complications of angiography. Radiology. 1981;138(2):273-281. doi:10.1148/radiology.138.2.7455105.
3. Petrou E, Malakos I, Kampanarou S, Doulas N, Voudris V. Life-threatening rupture of a femoral pseudoaneurysm after cardiac catheterization. Open Cardiovasc Med J. 2016;10:201-204. doi:10.2174/1874192401610010201.
4. Mees B, Robinson D, Verhagen H, Chuen J. Non-aortic aneurysms—natural history and recommendations for referral and treatment. Aust Fam Physician. 2013;42(6):370-374.
5. Webber GW, Jang J, Gustavson S, Olin JW. Contemporary management of postcatheterization pseudoaneurysms. Circulation. 2007;115(20):2666-2674. doi:10.1161/CIRCULATIONAHA.106.681973.
6. Coughlin BF, Paushter DM. Peripheral pseudoaneurysms: evaluation with duplex US. Radiology. 1988;168(2):339-342. doi:10.1148/radiology.168.2.3293107.
7. Toursarkissian B, Allen BT, Petrinec D, et al. Spontaneous closure of selected iatrogenic pseudoaneurysms and arteriovenous fistulae. J Vasc Surg. 1997;25(5):803-809; discussion 808-809.
8. Corriere MA, Guzman RJ. True and false aneurysms of the femoral artery. Semin Vasc Surg. 2005;18(4):216-223. doi:10.1053/j.semvascsurg.2005.09.008.
Design limitations may have compromised DVT intervention trial
WASHINGTON – On the basis of a large randomized trial called ATTRACT, many clinicians have concluded that pharmacomechanical intervention is ineffective for preventing postthrombotic syndrome (PTS) in patients with deep venous thrombosis (DVT). But weaknesses in the study design challenge this conclusion, according to several experts in a DVT symposium at the 2018 Cardiovascular Research Technologies (CRT) meeting.
“The diagnosis and evaluation of DVT must be performed with IVUS [intravascular ultrasound], not with venography,” said Peter A. Soukas, MD, director of vascular medicine at Miriam Hospital in Providence, R.I. “You cannot know whether you successfully treated the clot if you cannot see it.”
“There were lots of limitations to that study. Here are some,” said Dr. Soukas, who then listed on a list of several considerations, including the fact that venograms – rather than IVUS, which Dr. Soukas labeled the “current gold standard” – were taken to evaluate procedure success. Another was that only half of patients had a moderate to severe DVT based on a Villalta score.
“If you look at the subgroup with a Villalta score of 10 or greater, the benefit [of pharmacomechanical intervention] was statistically significant,” he said.
In addition, the study enrolled a substantial number of patients with femoral-popliteal DVTs even though iliofemoral DVTs pose the greatest risk of postthrombotic syndrome. Dr. Soukas suggested these would have been a more appropriate focus of a study exploring the benefits of an intervention.
The limitations of the ATTRACT trial, which was conceived more than 5 years ago, have arisen primarily from advances in the field rather than problems with the design, Dr. Soukas explained. IVUS was not the preferred method for deep vein thrombosis evaluation then as it is now, and there have been several advances in current models of pharmacomechanical devices, which involve catheter-directed delivery of fibrinolytic therapy into the thrombus along with mechanical destruction of the clot.
Although further steps beyond clot lysis, such as stenting, were encouraged in ATTRACT to maintain venous patency, Dr. Soukas questioned whether these were employed sufficiently. For example, the rate of stenting in the experimental arm was 28%, a rate that “is not what we currently do” for patients at high risk of PTS, Dr. Soukas said.
In ATTRACT, major bleeding events were significantly higher in the experimental group (1.7% vs. 0.3%; P = .049). The authors cited this finding when they concluded that the experimental intervention was ineffective. Dr. Soukas acknowledged that bleeding risk is an important factor to consider, but he also emphasized the serious risks for failing to treat patients at high risk for PTS.
“PTS is devastating for patients, both functionally and economically,” Dr. Soukas said. He called the morbidity of deep vein thrombosis “staggering,” with in-hospital mortality in some series exceeding 10% and a risk of late development of postthrombotic syndrome persisting for up to 5 years. For those with proximal iliofemoral DVT, the PTS rate can reach 90%, about 15% of which can develop claudication with ulcerations, according to Dr. Soukas.
A large trial that was published in a prominent journal, ATTRACT has the potential to dissuade clinicians from considering pharmacomechanical intervention in high-risk patients who could benefit, Dr. Soukas said. Others speaking during the same symposium about advances in this field, such as John Fritz Angle, MD, director of the division of vascular and interventional radiology at the University of Virginia, Charlottesville, agreed with this assessment. Although other studies underway will reexamine this issue, there was consensus from several speakers at the CRT symposium that the results of ATTRACT should not preclude intervention in patients at high risk of PTS.
“I believe there is a role for DVT intervention for symptomatic patients with an extensive [proximal iliofemoral] clot provided they have a low bleeding risk,” Dr. Soukas said.
Dr. Soukas reported no potential conflicts of interest.
WASHINGTON – On the basis of a large randomized trial called ATTRACT, many clinicians have concluded that pharmacomechanical intervention is ineffective for preventing postthrombotic syndrome (PTS) in patients with deep venous thrombosis (DVT). But weaknesses in the study design challenge this conclusion, according to several experts in a DVT symposium at the 2018 Cardiovascular Research Technologies (CRT) meeting.
“The diagnosis and evaluation of DVT must be performed with IVUS [intravascular ultrasound], not with venography,” said Peter A. Soukas, MD, director of vascular medicine at Miriam Hospital in Providence, R.I. “You cannot know whether you successfully treated the clot if you cannot see it.”
“There were lots of limitations to that study. Here are some,” said Dr. Soukas, who then listed on a list of several considerations, including the fact that venograms – rather than IVUS, which Dr. Soukas labeled the “current gold standard” – were taken to evaluate procedure success. Another was that only half of patients had a moderate to severe DVT based on a Villalta score.
“If you look at the subgroup with a Villalta score of 10 or greater, the benefit [of pharmacomechanical intervention] was statistically significant,” he said.
In addition, the study enrolled a substantial number of patients with femoral-popliteal DVTs even though iliofemoral DVTs pose the greatest risk of postthrombotic syndrome. Dr. Soukas suggested these would have been a more appropriate focus of a study exploring the benefits of an intervention.
The limitations of the ATTRACT trial, which was conceived more than 5 years ago, have arisen primarily from advances in the field rather than problems with the design, Dr. Soukas explained. IVUS was not the preferred method for deep vein thrombosis evaluation then as it is now, and there have been several advances in current models of pharmacomechanical devices, which involve catheter-directed delivery of fibrinolytic therapy into the thrombus along with mechanical destruction of the clot.
Although further steps beyond clot lysis, such as stenting, were encouraged in ATTRACT to maintain venous patency, Dr. Soukas questioned whether these were employed sufficiently. For example, the rate of stenting in the experimental arm was 28%, a rate that “is not what we currently do” for patients at high risk of PTS, Dr. Soukas said.
In ATTRACT, major bleeding events were significantly higher in the experimental group (1.7% vs. 0.3%; P = .049). The authors cited this finding when they concluded that the experimental intervention was ineffective. Dr. Soukas acknowledged that bleeding risk is an important factor to consider, but he also emphasized the serious risks for failing to treat patients at high risk for PTS.
“PTS is devastating for patients, both functionally and economically,” Dr. Soukas said. He called the morbidity of deep vein thrombosis “staggering,” with in-hospital mortality in some series exceeding 10% and a risk of late development of postthrombotic syndrome persisting for up to 5 years. For those with proximal iliofemoral DVT, the PTS rate can reach 90%, about 15% of which can develop claudication with ulcerations, according to Dr. Soukas.
A large trial that was published in a prominent journal, ATTRACT has the potential to dissuade clinicians from considering pharmacomechanical intervention in high-risk patients who could benefit, Dr. Soukas said. Others speaking during the same symposium about advances in this field, such as John Fritz Angle, MD, director of the division of vascular and interventional radiology at the University of Virginia, Charlottesville, agreed with this assessment. Although other studies underway will reexamine this issue, there was consensus from several speakers at the CRT symposium that the results of ATTRACT should not preclude intervention in patients at high risk of PTS.
“I believe there is a role for DVT intervention for symptomatic patients with an extensive [proximal iliofemoral] clot provided they have a low bleeding risk,” Dr. Soukas said.
Dr. Soukas reported no potential conflicts of interest.
WASHINGTON – On the basis of a large randomized trial called ATTRACT, many clinicians have concluded that pharmacomechanical intervention is ineffective for preventing postthrombotic syndrome (PTS) in patients with deep venous thrombosis (DVT). But weaknesses in the study design challenge this conclusion, according to several experts in a DVT symposium at the 2018 Cardiovascular Research Technologies (CRT) meeting.
“The diagnosis and evaluation of DVT must be performed with IVUS [intravascular ultrasound], not with venography,” said Peter A. Soukas, MD, director of vascular medicine at Miriam Hospital in Providence, R.I. “You cannot know whether you successfully treated the clot if you cannot see it.”
“There were lots of limitations to that study. Here are some,” said Dr. Soukas, who then listed on a list of several considerations, including the fact that venograms – rather than IVUS, which Dr. Soukas labeled the “current gold standard” – were taken to evaluate procedure success. Another was that only half of patients had a moderate to severe DVT based on a Villalta score.
“If you look at the subgroup with a Villalta score of 10 or greater, the benefit [of pharmacomechanical intervention] was statistically significant,” he said.
In addition, the study enrolled a substantial number of patients with femoral-popliteal DVTs even though iliofemoral DVTs pose the greatest risk of postthrombotic syndrome. Dr. Soukas suggested these would have been a more appropriate focus of a study exploring the benefits of an intervention.
The limitations of the ATTRACT trial, which was conceived more than 5 years ago, have arisen primarily from advances in the field rather than problems with the design, Dr. Soukas explained. IVUS was not the preferred method for deep vein thrombosis evaluation then as it is now, and there have been several advances in current models of pharmacomechanical devices, which involve catheter-directed delivery of fibrinolytic therapy into the thrombus along with mechanical destruction of the clot.
Although further steps beyond clot lysis, such as stenting, were encouraged in ATTRACT to maintain venous patency, Dr. Soukas questioned whether these were employed sufficiently. For example, the rate of stenting in the experimental arm was 28%, a rate that “is not what we currently do” for patients at high risk of PTS, Dr. Soukas said.
In ATTRACT, major bleeding events were significantly higher in the experimental group (1.7% vs. 0.3%; P = .049). The authors cited this finding when they concluded that the experimental intervention was ineffective. Dr. Soukas acknowledged that bleeding risk is an important factor to consider, but he also emphasized the serious risks for failing to treat patients at high risk for PTS.
“PTS is devastating for patients, both functionally and economically,” Dr. Soukas said. He called the morbidity of deep vein thrombosis “staggering,” with in-hospital mortality in some series exceeding 10% and a risk of late development of postthrombotic syndrome persisting for up to 5 years. For those with proximal iliofemoral DVT, the PTS rate can reach 90%, about 15% of which can develop claudication with ulcerations, according to Dr. Soukas.
A large trial that was published in a prominent journal, ATTRACT has the potential to dissuade clinicians from considering pharmacomechanical intervention in high-risk patients who could benefit, Dr. Soukas said. Others speaking during the same symposium about advances in this field, such as John Fritz Angle, MD, director of the division of vascular and interventional radiology at the University of Virginia, Charlottesville, agreed with this assessment. Although other studies underway will reexamine this issue, there was consensus from several speakers at the CRT symposium that the results of ATTRACT should not preclude intervention in patients at high risk of PTS.
“I believe there is a role for DVT intervention for symptomatic patients with an extensive [proximal iliofemoral] clot provided they have a low bleeding risk,” Dr. Soukas said.
Dr. Soukas reported no potential conflicts of interest.
EXPERT ANALYSIS FROM THE 2018 CRT MEETING
Patients Who Die of SUDEP Largely Live Alone and Die Unwitnessed at Home
Patients whose fatality is attributed to sudden unexpected death in epilepsy (SUDEP) largely live alone; die unwitnessed at home at night, usually in the prone position; and have an indication of a preceding seizure, according to research published in the May issue of Epilepsia.
“Our results … highlight the difficulties in implementing preventive efforts that require immediate availability of another person to identify a seizure, to interact and correct body position, or to give pharmacologic emergency treatment,” said Olafur Sveinsson, a graduate student at the Karolinska Institute in Stockholm, and colleagues. “These obstacles need to be considered when strategies for SUDEP prevention are being developed.”
Previous case–control studies have identified a high frequency of tonic-clonic seizures, nocturnal seizures, and lack of nighttime supervision as risk factors for SUDEP, but mechanisms of SUDEP remain unclear. To analyze the circumstances of SUDEP and its incidence in relation to time of year, week, and day, Mr. Sveinsson and colleagues conducted a nationwide, population-based case series.
For their study, the investigators used the Swedish National Patient Registry to identify all persons that, at some point between 1998 and 2005, had an ICD-10 code for epilepsy and were alive on June 30, 2006. Eligible SUDEP cases were all deaths with epilepsy mentioned on the death certificate together with all individuals who died during 2008, irrespective of whether epilepsy was mentioned on the death certificate. Obvious non-SUDEP deaths such as those resulting from cancer, terminal illness, postmortem confirmed pneumonia, stroke, or myocardial infarction were excluded from further analysis.
SUDEP cases were divided into three subgroups based on the certainty of the diagnosis: definite SUDEP (when all clinical criteria were met and an autopsy revealed no alternate cause of death), probable SUDEP (when all clinical criteria were met, but no autopsy was performed), and possible SUDEP (when SUDEP could not be ruled out, but insufficient evidence was available regarding the circumstances of death, and no autopsy was performed). To identify SUDEP cases and related circumstances, investigators reviewed death certificates, medical charts, autopsy, and police records. Autopsied non-SUDEP deaths from the study population served as a reference. Researchers reviewed 3,166 deaths and identified 329 cases of SUDEP (37% were female). Of these cases, 167 were definite, 89 were probable, and 73 were possible. SUDEP cases were younger at death (50.8 years) than non-SUDEP deaths (73.3 years). Most SUDEP cases occurred at night (58%) and at home (91%), and 65% were found dead in bed. When documented, 70% were found in prone position, which may “facilitate SUDEP by compromising postictal ventilation,” said the authors.
Death was witnessed in 17% of SUDEP cases, and in 88% of these, a seizure was observed. In all, 71% of patients were living alone, and 14% shared a bedroom. Among the witnessed definite SUDEP patients, a tonic-clonic seizure was present in 95% of cases, compared with 21% in the autopsied non-SUDEP reference group, strengthening the notion that SUDEP in most cases is a seizure-related event, the researchers said.
Although sudden infant death syndrome (SIDS) and cardiac death have a higher incidence in the winter, the researchers did not find the same to be true in their SUDEP cohort. Furthermore, they did not find a preponderance for Mondays or morning hours, as reported for sudden cardiac death. The researchers did, however, find a clear diurnal variation, with the majority of cases dying during the night hours. Taken together, these findings prompted the researchers to conclude that the underlying mechanisms of SUDEP are different from those of SIDS and sudden cardiac death.
—Erica Tricarico
Suggested Reading
Sveinnson O, Andersson T, Carlsson S, Tomson T. Circumstances of SUDEP: a nationwide population-based case series. Epilepsia. 2018;59(5):1074-1082.
Patients whose fatality is attributed to sudden unexpected death in epilepsy (SUDEP) largely live alone; die unwitnessed at home at night, usually in the prone position; and have an indication of a preceding seizure, according to research published in the May issue of Epilepsia.
“Our results … highlight the difficulties in implementing preventive efforts that require immediate availability of another person to identify a seizure, to interact and correct body position, or to give pharmacologic emergency treatment,” said Olafur Sveinsson, a graduate student at the Karolinska Institute in Stockholm, and colleagues. “These obstacles need to be considered when strategies for SUDEP prevention are being developed.”
Previous case–control studies have identified a high frequency of tonic-clonic seizures, nocturnal seizures, and lack of nighttime supervision as risk factors for SUDEP, but mechanisms of SUDEP remain unclear. To analyze the circumstances of SUDEP and its incidence in relation to time of year, week, and day, Mr. Sveinsson and colleagues conducted a nationwide, population-based case series.
For their study, the investigators used the Swedish National Patient Registry to identify all persons that, at some point between 1998 and 2005, had an ICD-10 code for epilepsy and were alive on June 30, 2006. Eligible SUDEP cases were all deaths with epilepsy mentioned on the death certificate together with all individuals who died during 2008, irrespective of whether epilepsy was mentioned on the death certificate. Obvious non-SUDEP deaths such as those resulting from cancer, terminal illness, postmortem confirmed pneumonia, stroke, or myocardial infarction were excluded from further analysis.
SUDEP cases were divided into three subgroups based on the certainty of the diagnosis: definite SUDEP (when all clinical criteria were met and an autopsy revealed no alternate cause of death), probable SUDEP (when all clinical criteria were met, but no autopsy was performed), and possible SUDEP (when SUDEP could not be ruled out, but insufficient evidence was available regarding the circumstances of death, and no autopsy was performed). To identify SUDEP cases and related circumstances, investigators reviewed death certificates, medical charts, autopsy, and police records. Autopsied non-SUDEP deaths from the study population served as a reference. Researchers reviewed 3,166 deaths and identified 329 cases of SUDEP (37% were female). Of these cases, 167 were definite, 89 were probable, and 73 were possible. SUDEP cases were younger at death (50.8 years) than non-SUDEP deaths (73.3 years). Most SUDEP cases occurred at night (58%) and at home (91%), and 65% were found dead in bed. When documented, 70% were found in prone position, which may “facilitate SUDEP by compromising postictal ventilation,” said the authors.
Death was witnessed in 17% of SUDEP cases, and in 88% of these, a seizure was observed. In all, 71% of patients were living alone, and 14% shared a bedroom. Among the witnessed definite SUDEP patients, a tonic-clonic seizure was present in 95% of cases, compared with 21% in the autopsied non-SUDEP reference group, strengthening the notion that SUDEP in most cases is a seizure-related event, the researchers said.
Although sudden infant death syndrome (SIDS) and cardiac death have a higher incidence in the winter, the researchers did not find the same to be true in their SUDEP cohort. Furthermore, they did not find a preponderance for Mondays or morning hours, as reported for sudden cardiac death. The researchers did, however, find a clear diurnal variation, with the majority of cases dying during the night hours. Taken together, these findings prompted the researchers to conclude that the underlying mechanisms of SUDEP are different from those of SIDS and sudden cardiac death.
—Erica Tricarico
Suggested Reading
Sveinnson O, Andersson T, Carlsson S, Tomson T. Circumstances of SUDEP: a nationwide population-based case series. Epilepsia. 2018;59(5):1074-1082.
Patients whose fatality is attributed to sudden unexpected death in epilepsy (SUDEP) largely live alone; die unwitnessed at home at night, usually in the prone position; and have an indication of a preceding seizure, according to research published in the May issue of Epilepsia.
“Our results … highlight the difficulties in implementing preventive efforts that require immediate availability of another person to identify a seizure, to interact and correct body position, or to give pharmacologic emergency treatment,” said Olafur Sveinsson, a graduate student at the Karolinska Institute in Stockholm, and colleagues. “These obstacles need to be considered when strategies for SUDEP prevention are being developed.”
Previous case–control studies have identified a high frequency of tonic-clonic seizures, nocturnal seizures, and lack of nighttime supervision as risk factors for SUDEP, but mechanisms of SUDEP remain unclear. To analyze the circumstances of SUDEP and its incidence in relation to time of year, week, and day, Mr. Sveinsson and colleagues conducted a nationwide, population-based case series.
For their study, the investigators used the Swedish National Patient Registry to identify all persons that, at some point between 1998 and 2005, had an ICD-10 code for epilepsy and were alive on June 30, 2006. Eligible SUDEP cases were all deaths with epilepsy mentioned on the death certificate together with all individuals who died during 2008, irrespective of whether epilepsy was mentioned on the death certificate. Obvious non-SUDEP deaths such as those resulting from cancer, terminal illness, postmortem confirmed pneumonia, stroke, or myocardial infarction were excluded from further analysis.
SUDEP cases were divided into three subgroups based on the certainty of the diagnosis: definite SUDEP (when all clinical criteria were met and an autopsy revealed no alternate cause of death), probable SUDEP (when all clinical criteria were met, but no autopsy was performed), and possible SUDEP (when SUDEP could not be ruled out, but insufficient evidence was available regarding the circumstances of death, and no autopsy was performed). To identify SUDEP cases and related circumstances, investigators reviewed death certificates, medical charts, autopsy, and police records. Autopsied non-SUDEP deaths from the study population served as a reference. Researchers reviewed 3,166 deaths and identified 329 cases of SUDEP (37% were female). Of these cases, 167 were definite, 89 were probable, and 73 were possible. SUDEP cases were younger at death (50.8 years) than non-SUDEP deaths (73.3 years). Most SUDEP cases occurred at night (58%) and at home (91%), and 65% were found dead in bed. When documented, 70% were found in prone position, which may “facilitate SUDEP by compromising postictal ventilation,” said the authors.
Death was witnessed in 17% of SUDEP cases, and in 88% of these, a seizure was observed. In all, 71% of patients were living alone, and 14% shared a bedroom. Among the witnessed definite SUDEP patients, a tonic-clonic seizure was present in 95% of cases, compared with 21% in the autopsied non-SUDEP reference group, strengthening the notion that SUDEP in most cases is a seizure-related event, the researchers said.
Although sudden infant death syndrome (SIDS) and cardiac death have a higher incidence in the winter, the researchers did not find the same to be true in their SUDEP cohort. Furthermore, they did not find a preponderance for Mondays or morning hours, as reported for sudden cardiac death. The researchers did, however, find a clear diurnal variation, with the majority of cases dying during the night hours. Taken together, these findings prompted the researchers to conclude that the underlying mechanisms of SUDEP are different from those of SIDS and sudden cardiac death.
—Erica Tricarico
Suggested Reading
Sveinnson O, Andersson T, Carlsson S, Tomson T. Circumstances of SUDEP: a nationwide population-based case series. Epilepsia. 2018;59(5):1074-1082.
Novel initiative aims to combat resident burnout
TORONTO – Studies have demonstrated that up to 50% of medical residents meet criteria for burnout, but a new initiative aims to change that worrisome trend.
At the Pediatric Academic Societies meeting, Michael Dolinger, MD, shared initial results from ResiLIEnCE (Resident-led Initiative to Empower a Change in Culture and Promote Resilience), a curriculum that is being carried out at Cohen Children’s Medical Center, New York. “We know that medical residents are a prime target for work burnout,” said Dr. Dolinger, one of the center’s pediatric chief residents, in an interview. “We wanted to study what we can do to combat that burnout on a daily basis, a monthly basis, and a longitudinal basis. How specific can we get so it’s portable, and that other programs can adapt what we are doing to help reduce this burnout?”
Interventions were enacted during traditional pediatric resident work hours to improve attendance. These included a resident-led wellness committee with faculty leadership and wellness champions, a longitudinal noon conference lecture series on nutrition (with topics such as how to eat on a budget and quick meal options), financial health (with topics such as student loan repayment, budgeting on a resident’s salary, and retirement planning), mindfulness, and resiliency. Optional activities after work included personal fitness boot camps, a book club, a minority support group, and other peer interest groups. Maslach Burnout Inventories were distributed to residents before implementation of the curriculum and at 3-month intervals. Surveys at the completion of activities assessed the effectiveness of sessions.
A total of 100 pediatric residents were surveyed. Dr. Dolinger reported that before implementation of the curriculum, 41.0% of third-year residents admitted to “feeling burned out from my work” and to “feeling more callous since I took this job,” while 8.8% of rising first-year residents admitted to feeling burned out prior to starting residency. In addition, 3 months after the curriculum began, 48.0% of first-year, 23.5% of second-year, and 83.3% of third-year residents reported believing that residency interfered with their personal wellness.
Analysis of the curriculum’s impact is ongoing, but Dr. Dolinger reported that among those who attended a nutrition series, 80% of residents planned to eat healthier, while only 15% reported eating healthy prior to the session. Among those who attended a financial series, 50% of those who did not previously contribute to their retirement planned to do so. In addition, 80% of residents who attended a resident fitness workshop joined a local fitness center, compared with only 20% of residents prior. Among those who attended a lecture series on resiliency, 90% of residents indicated that they were able to reflect on a negative patient experience and learn something valuable.
“Hopefully this curriculum helps reduce the overall burnout in our residents over time, by increasing their aspects of well-being and promoting resilience for them individually,” Dr. Dolinger said.
The initiative was funded by the Association of Pediatric Program Directors via the Harvey Aiges Memorial Trainee Investigator Award. Dr. Dolinger reported having no financial disclosures.
TORONTO – Studies have demonstrated that up to 50% of medical residents meet criteria for burnout, but a new initiative aims to change that worrisome trend.
At the Pediatric Academic Societies meeting, Michael Dolinger, MD, shared initial results from ResiLIEnCE (Resident-led Initiative to Empower a Change in Culture and Promote Resilience), a curriculum that is being carried out at Cohen Children’s Medical Center, New York. “We know that medical residents are a prime target for work burnout,” said Dr. Dolinger, one of the center’s pediatric chief residents, in an interview. “We wanted to study what we can do to combat that burnout on a daily basis, a monthly basis, and a longitudinal basis. How specific can we get so it’s portable, and that other programs can adapt what we are doing to help reduce this burnout?”
Interventions were enacted during traditional pediatric resident work hours to improve attendance. These included a resident-led wellness committee with faculty leadership and wellness champions, a longitudinal noon conference lecture series on nutrition (with topics such as how to eat on a budget and quick meal options), financial health (with topics such as student loan repayment, budgeting on a resident’s salary, and retirement planning), mindfulness, and resiliency. Optional activities after work included personal fitness boot camps, a book club, a minority support group, and other peer interest groups. Maslach Burnout Inventories were distributed to residents before implementation of the curriculum and at 3-month intervals. Surveys at the completion of activities assessed the effectiveness of sessions.
A total of 100 pediatric residents were surveyed. Dr. Dolinger reported that before implementation of the curriculum, 41.0% of third-year residents admitted to “feeling burned out from my work” and to “feeling more callous since I took this job,” while 8.8% of rising first-year residents admitted to feeling burned out prior to starting residency. In addition, 3 months after the curriculum began, 48.0% of first-year, 23.5% of second-year, and 83.3% of third-year residents reported believing that residency interfered with their personal wellness.
Analysis of the curriculum’s impact is ongoing, but Dr. Dolinger reported that among those who attended a nutrition series, 80% of residents planned to eat healthier, while only 15% reported eating healthy prior to the session. Among those who attended a financial series, 50% of those who did not previously contribute to their retirement planned to do so. In addition, 80% of residents who attended a resident fitness workshop joined a local fitness center, compared with only 20% of residents prior. Among those who attended a lecture series on resiliency, 90% of residents indicated that they were able to reflect on a negative patient experience and learn something valuable.
“Hopefully this curriculum helps reduce the overall burnout in our residents over time, by increasing their aspects of well-being and promoting resilience for them individually,” Dr. Dolinger said.
The initiative was funded by the Association of Pediatric Program Directors via the Harvey Aiges Memorial Trainee Investigator Award. Dr. Dolinger reported having no financial disclosures.
TORONTO – Studies have demonstrated that up to 50% of medical residents meet criteria for burnout, but a new initiative aims to change that worrisome trend.
At the Pediatric Academic Societies meeting, Michael Dolinger, MD, shared initial results from ResiLIEnCE (Resident-led Initiative to Empower a Change in Culture and Promote Resilience), a curriculum that is being carried out at Cohen Children’s Medical Center, New York. “We know that medical residents are a prime target for work burnout,” said Dr. Dolinger, one of the center’s pediatric chief residents, in an interview. “We wanted to study what we can do to combat that burnout on a daily basis, a monthly basis, and a longitudinal basis. How specific can we get so it’s portable, and that other programs can adapt what we are doing to help reduce this burnout?”
Interventions were enacted during traditional pediatric resident work hours to improve attendance. These included a resident-led wellness committee with faculty leadership and wellness champions, a longitudinal noon conference lecture series on nutrition (with topics such as how to eat on a budget and quick meal options), financial health (with topics such as student loan repayment, budgeting on a resident’s salary, and retirement planning), mindfulness, and resiliency. Optional activities after work included personal fitness boot camps, a book club, a minority support group, and other peer interest groups. Maslach Burnout Inventories were distributed to residents before implementation of the curriculum and at 3-month intervals. Surveys at the completion of activities assessed the effectiveness of sessions.
A total of 100 pediatric residents were surveyed. Dr. Dolinger reported that before implementation of the curriculum, 41.0% of third-year residents admitted to “feeling burned out from my work” and to “feeling more callous since I took this job,” while 8.8% of rising first-year residents admitted to feeling burned out prior to starting residency. In addition, 3 months after the curriculum began, 48.0% of first-year, 23.5% of second-year, and 83.3% of third-year residents reported believing that residency interfered with their personal wellness.
Analysis of the curriculum’s impact is ongoing, but Dr. Dolinger reported that among those who attended a nutrition series, 80% of residents planned to eat healthier, while only 15% reported eating healthy prior to the session. Among those who attended a financial series, 50% of those who did not previously contribute to their retirement planned to do so. In addition, 80% of residents who attended a resident fitness workshop joined a local fitness center, compared with only 20% of residents prior. Among those who attended a lecture series on resiliency, 90% of residents indicated that they were able to reflect on a negative patient experience and learn something valuable.
“Hopefully this curriculum helps reduce the overall burnout in our residents over time, by increasing their aspects of well-being and promoting resilience for them individually,” Dr. Dolinger said.
The initiative was funded by the Association of Pediatric Program Directors via the Harvey Aiges Memorial Trainee Investigator Award. Dr. Dolinger reported having no financial disclosures.
REPORTING FROM PAS 2018
Key clinical point: Interventions targeted at specific aspects of resident well-being yielded tangible improvement in resident wellness behaviors.
Major finding: Among those who attended a nutrition series as part of the curriculum, 80% of residents planned to eat healthier, while only 15% reported eating healthy prior to the session.
Study details: A survey of 100 pediatric residents who took part in a Resident-led Initiative to Empower a Change in Culture and Promote Resilience.
Disclosures: The initiative was funded by the Association of Pediatric Program Directors via the Harvey Aiges Memorial Trainee Investigator Award. Dr. Dolinger reported having no financial disclosures.
Subcutaneous buprenorpine rivals sublingual for opioid use disorder
Long-acting subcutaneous doses of buprenorphine depot are an effective treatment option for opioid use disorder, results of a phase 3 study of 428 adults show.
Sublingual buprenorphine hydrochloride is a standard treatment for opioid use disorder (OUD), but challenges include poor medication adherence, potential for abuse, and accidental exposure to children, Michelle R. Lofwall, MD, of the University of Kentucky, Lexington, and her colleagues reported.
In a study published in JAMA Internal Medicine, Dr. Lofwall and her associates randomized treatment-seeking adults with moderate to severe opioid use disorder to subcutaneous buprenorphine depot weekly for 12 weeks followed by monthly for 12 weeks, or daily sublingual buprenorphine with naloxone for 24 weeks.
The proportion of opioid-negative urine samples was 35% in the subcutaneous buprenorphine depot group (1,347 of 3,834 samples) vs. 29% in the sublingual buprenorphine with naloxone group (1,099 of 3,870 samples) for a statistically significant difference of 6.7%. Urine samples were collected weekly for the first 12 weeks, and then at weeks 16, 20, and 24, reported Dr. Lofall, a psychiatrist and addiction medicine specialist, and her associates.
Patients in the sublingual buprenorphine with naloxone group received 4 mg of sublingual buprenorphine hydrochloride and naloxone hydrochloride at the start of the study, titrated to 16 mg/day. The average treatment dosage was 18-20 mg/day for sublingual buprenorphine with naloxone patients.
Patients in the subcutaneous buprenorphine depot group received 16 mg of subcutaneous buprenorphine in a weekly injection at the start of the study; monthly subcutaneous buprenorphine depot injections were 64, 96, 128, or 160 mg between weeks 12 and 24.
After initial titration, doses were flexible based on clinical judgment, the researchers noted, similar to the way in which patients would be managed in a clinical setting.
Adverse events were similar between the groups. The most common were injection-site pain, headache, constipation, nausea, and injection-site pruritus and erythema. Injection-site reactions were mild to moderate.
As a secondary outcome, the cumulative distribution function (CDF) in the subcutaneous buprenorphine depot group was statistically superior to the CDF found in the sublingual buprenorphine with naloxone in the percentage of opioid-negative results. “Cumulative distribution function values are an established endpoint used in early placebo-controlled, phase 3 clinical trials for OUD treatment,” Dr. Lofall and her associates wrote.
The study findings were limited by several factors, including an absence of assessment of patient adherence to sublingual medication and an inability to assess effectiveness vs. efficacy. However, the large size and diverse study population strengthen the results, which support the use of subcutaneous depot buprenorphine formulations for patients with OUD, the researchers noted.
“These formulations may also address potential limitations and concerns about daily dosing, including diversion, misuse, and accidental exposure of medication to children,” they said.
The study was supported in part by Braeburn Pharmaceuticals and the University of Kentucky, Lexington. Dr. Lofwall disclosed research funding and consulting fees from Braeburn Pharmaceuticals and Indivior.
SOURCE: Lofwall M et al. JAMA Intern Med. 2018 May 14. doi: 10.1001/jamainternmed.2018.1052.
Long-acting subcutaneous doses of buprenorphine depot are an effective treatment option for opioid use disorder, results of a phase 3 study of 428 adults show.
Sublingual buprenorphine hydrochloride is a standard treatment for opioid use disorder (OUD), but challenges include poor medication adherence, potential for abuse, and accidental exposure to children, Michelle R. Lofwall, MD, of the University of Kentucky, Lexington, and her colleagues reported.
In a study published in JAMA Internal Medicine, Dr. Lofwall and her associates randomized treatment-seeking adults with moderate to severe opioid use disorder to subcutaneous buprenorphine depot weekly for 12 weeks followed by monthly for 12 weeks, or daily sublingual buprenorphine with naloxone for 24 weeks.
The proportion of opioid-negative urine samples was 35% in the subcutaneous buprenorphine depot group (1,347 of 3,834 samples) vs. 29% in the sublingual buprenorphine with naloxone group (1,099 of 3,870 samples) for a statistically significant difference of 6.7%. Urine samples were collected weekly for the first 12 weeks, and then at weeks 16, 20, and 24, reported Dr. Lofall, a psychiatrist and addiction medicine specialist, and her associates.
Patients in the sublingual buprenorphine with naloxone group received 4 mg of sublingual buprenorphine hydrochloride and naloxone hydrochloride at the start of the study, titrated to 16 mg/day. The average treatment dosage was 18-20 mg/day for sublingual buprenorphine with naloxone patients.
Patients in the subcutaneous buprenorphine depot group received 16 mg of subcutaneous buprenorphine in a weekly injection at the start of the study; monthly subcutaneous buprenorphine depot injections were 64, 96, 128, or 160 mg between weeks 12 and 24.
After initial titration, doses were flexible based on clinical judgment, the researchers noted, similar to the way in which patients would be managed in a clinical setting.
Adverse events were similar between the groups. The most common were injection-site pain, headache, constipation, nausea, and injection-site pruritus and erythema. Injection-site reactions were mild to moderate.
As a secondary outcome, the cumulative distribution function (CDF) in the subcutaneous buprenorphine depot group was statistically superior to the CDF found in the sublingual buprenorphine with naloxone in the percentage of opioid-negative results. “Cumulative distribution function values are an established endpoint used in early placebo-controlled, phase 3 clinical trials for OUD treatment,” Dr. Lofall and her associates wrote.
The study findings were limited by several factors, including an absence of assessment of patient adherence to sublingual medication and an inability to assess effectiveness vs. efficacy. However, the large size and diverse study population strengthen the results, which support the use of subcutaneous depot buprenorphine formulations for patients with OUD, the researchers noted.
“These formulations may also address potential limitations and concerns about daily dosing, including diversion, misuse, and accidental exposure of medication to children,” they said.
The study was supported in part by Braeburn Pharmaceuticals and the University of Kentucky, Lexington. Dr. Lofwall disclosed research funding and consulting fees from Braeburn Pharmaceuticals and Indivior.
SOURCE: Lofwall M et al. JAMA Intern Med. 2018 May 14. doi: 10.1001/jamainternmed.2018.1052.
Long-acting subcutaneous doses of buprenorphine depot are an effective treatment option for opioid use disorder, results of a phase 3 study of 428 adults show.
Sublingual buprenorphine hydrochloride is a standard treatment for opioid use disorder (OUD), but challenges include poor medication adherence, potential for abuse, and accidental exposure to children, Michelle R. Lofwall, MD, of the University of Kentucky, Lexington, and her colleagues reported.
In a study published in JAMA Internal Medicine, Dr. Lofwall and her associates randomized treatment-seeking adults with moderate to severe opioid use disorder to subcutaneous buprenorphine depot weekly for 12 weeks followed by monthly for 12 weeks, or daily sublingual buprenorphine with naloxone for 24 weeks.
The proportion of opioid-negative urine samples was 35% in the subcutaneous buprenorphine depot group (1,347 of 3,834 samples) vs. 29% in the sublingual buprenorphine with naloxone group (1,099 of 3,870 samples) for a statistically significant difference of 6.7%. Urine samples were collected weekly for the first 12 weeks, and then at weeks 16, 20, and 24, reported Dr. Lofall, a psychiatrist and addiction medicine specialist, and her associates.
Patients in the sublingual buprenorphine with naloxone group received 4 mg of sublingual buprenorphine hydrochloride and naloxone hydrochloride at the start of the study, titrated to 16 mg/day. The average treatment dosage was 18-20 mg/day for sublingual buprenorphine with naloxone patients.
Patients in the subcutaneous buprenorphine depot group received 16 mg of subcutaneous buprenorphine in a weekly injection at the start of the study; monthly subcutaneous buprenorphine depot injections were 64, 96, 128, or 160 mg between weeks 12 and 24.
After initial titration, doses were flexible based on clinical judgment, the researchers noted, similar to the way in which patients would be managed in a clinical setting.
Adverse events were similar between the groups. The most common were injection-site pain, headache, constipation, nausea, and injection-site pruritus and erythema. Injection-site reactions were mild to moderate.
As a secondary outcome, the cumulative distribution function (CDF) in the subcutaneous buprenorphine depot group was statistically superior to the CDF found in the sublingual buprenorphine with naloxone in the percentage of opioid-negative results. “Cumulative distribution function values are an established endpoint used in early placebo-controlled, phase 3 clinical trials for OUD treatment,” Dr. Lofall and her associates wrote.
The study findings were limited by several factors, including an absence of assessment of patient adherence to sublingual medication and an inability to assess effectiveness vs. efficacy. However, the large size and diverse study population strengthen the results, which support the use of subcutaneous depot buprenorphine formulations for patients with OUD, the researchers noted.
“These formulations may also address potential limitations and concerns about daily dosing, including diversion, misuse, and accidental exposure of medication to children,” they said.
The study was supported in part by Braeburn Pharmaceuticals and the University of Kentucky, Lexington. Dr. Lofwall disclosed research funding and consulting fees from Braeburn Pharmaceuticals and Indivior.
SOURCE: Lofwall M et al. JAMA Intern Med. 2018 May 14. doi: 10.1001/jamainternmed.2018.1052.
FROM JAMA INTERNAL MEDICINE
Key clinical point: After 24 weeks, long-acting subcutaneous buprenorphine depot was noninferior to sublingual buprenorphine-naloxone for preventing opioid use.
Major finding: The proportion of opioid-negative urine samples was a statistically significant 6.7% higher in the subcutaneous group, compared with the sublingual group.
Study details: The data come from a randomized trial of 428 adults in treatment for opioid use disorder.
Disclosures: The study was supported in part by Braeburn Pharmaceuticals and the University of Kentucky, Lexington. Dr Lofwall disclosed research funding and consulting fees from Braeburn Pharmaceuticals and Indivior.
Source: Lofwall M et al. JAMA Intern Med. 2018 May 14; doi: 10.1001/jamainternmed.2018.1052.
Methotrexate-induced pulmonary fibrosis risk examined in 10-year study
LIVERPOOL, ENGLAND – A 10-year follow up of patients with inflammatory arthritis has shown that methotrexate does not appear to increase the risk of pulmonary fibrosis.
“As rheumatologists, it’s a really important message that methotrexate does not cause chronic pulmonary fibrosis and it should not be stopped because of pulmonary fibrosis,” Julie Dawson, MD, said in an interview at the British Society for Rheumatology annual conference. “It’s the rheumatoid arthritis. It’s not the methotrexate.”
Dr. Dawson, of St. Helens and Knowsley Teaching Hospitals NHS Trust, St. Helens, England, added that the current findings were consistent with her team’s prior research looking at earlier time periods. There was also no correlation between the duration or dose of methotrexate used and the development of the lung disease, she said.
“If anything, the suggestion is you’d be more symptomatic if you delay using methotrexate,” Dr. Dawson observed. If patients are not doing well on methotrexate, then perhaps adjusting therapy or changing to another drug would of course be the next step, but if patients are well controlled then “stopping it is the worst thing to do” for their arthritis, she said.
“This is of great clinical interest, and we can be reassured now about this, I think. This is really good, long-term data,” said Devesh Mewar, MD, of Royal Liverpool and Broadgreen University Hospitals NHS Trust, Liverpool, England, who was not involved in the research.
“We know that methotrexate is associated with a pneumonitis reaction, but there is no high-quality evidence that methotrexate is associated with a chronic pulmonary fibrosis” Dr. Dawson said, explaining the rationale for the current study she presented during a poster session. Previous studies considered data for up to 5 years, she added, so the aim of the current study, therefore, was to look at the longer-term effect of methotrexate use on the incidence of pulmonary fibrosis.
Data on 129 patients who had started treatment with methotrexate from 2004 to 2007 were analyzed, of whom 63 (49%) had stayed on methotrexate for 10 or more years. Most (82%) had been given methotrexate to treat rheumatoid arthritis (RA), with other indications including inflammatory arthritis (5.4%) and psoriatic arthritis (4.7%).
“Practice was different 10 years ago, so just 56% of patients commenced methotrexate within the first year of the diagnosis of rheumatoid arthritis,” Dr. Dawson reported.
Only four cases of symptomatic pulmonary fibrosis were seen, all in the RA patients, and three of these were in patients who had started methotrexate over 1 year after their diagnosis. The incidence of 3.8% seen in the study matches the expected incidence of pulmonary fibrosis in RA and was actually “at the lower end of the expected incidence,” Dr. Dawson said. Previous studies have suggested an incidence rate of RA-associated interstitial lung disease of about 3%-7%.
All of the pulmonary fibrosis cases had occurred in men and 75% were seropositive for rheumatoid factor. The mean duration of RA at the time of onset of pulmonary fibrosis was 7.8 years and the usual interstitial pattern of fibrosis was seen. The 125 patients without pulmonary fibrosis had taken methotrexate for a mean of 8 years at a mean final weekly dose of 16.3 mg, compared with a mean of 6 years at a mean dose of 18.1 mg per week in the 4 patients with pulmonary fibrosis.
One of the next steps is to look at cases where methotrexate has been stopped and the effects of that on pulmonary fibrosis and disease activity. In Dr. Dawson’s experience, stopping methotrexate just affects the management of the arthritis and had no difference to the progression of pulmonary fibrosis.
If patients start to experience any lung symptoms while continuing methotrexate, such as shortness of breath, then they would need to be assessed and undergo lung function tests to monitor their condition. Treating the fibrosis using an antifibrotic drug, such as pirfenidone, is something that might be possible in the future, but this needs investigation in inflammatory arthritis as the drug is currently only licensed for use in idiopathic cases.
This is something the British Rheumatoid Interstitial Lung network plans to investigate in a placebo-controlled study of RA patients with fibrotic lung disease. “We’re looking to see if antifibrotic agents are going to slow the disease as it does in idiopathic pulmonary fibrosis, which is obviously quite exciting when it’s such a hard condition to treat,” said Dr. Dawson, who will be one of the study’s investigators.
Dr. Dawson had no conflicts of interest to disclose. Dr. Mewar was not involved in the study and had nothing to disclose.
SOURCE: Dawson J et al. Rheumatology. 2018;57[Suppl. 3]:key075.470.
The subject of this retrospective study is of great interest. The authors point out that pulmonary fibrosis (as opposed to acute allergic reaction, which is extremely rare) is also extremely uncommon in patients using methotrexate over the long haul. Over 10 years, their data points to a 3.1% incidence of symptomatic pulmonary fibrosis.
The issue here is its generalizability. There were 63 patients who used methotrexate for 10 years or more and 88 who used it for 5 years or more, according to the poster. This must represent a highly selected population. For example, what percent of the total RA/psoriatic arthritis/”inflammatory arthritis” population do these patients represent, i.e., what is the denominator here? The authors stated that the 63 patients who stayed on methotrexate for 10 or more years represent 49% of the 129 patients on methotrexate overall in the study. This is a highly unusual datum, as most of the literature indicates that only 40% or less of patients stay on methotrexate for even 5 years. And this completely ignores the issue of adherence over this long a period; these patients must represent a truly minuscule percentage of the total if they actually stayed on methotrexate with even moderate adherence for 10 years.
Importantly, the authors point out that they had only four cases of symptomatic pulmonary fibrosis. Once more, this points to the highly selective group of patients seen, as this study does not examine patients with asymptomatic pulmonary fibrosis, including those with fibrosis on high-resolution CT of the lungs or chest film or evidence of abnormalities on pulmonary function tests, but who do not have sufficient symptoms ascribed to methotrexate to bring them to medical attention.
Daniel E. Furst, MD, is professor of rheumatology at the University of Washington, Seattle, who also is affiliated with the University of California, Los Angeles, and the University of Florence, Italy. He was not involved with the study.
The subject of this retrospective study is of great interest. The authors point out that pulmonary fibrosis (as opposed to acute allergic reaction, which is extremely rare) is also extremely uncommon in patients using methotrexate over the long haul. Over 10 years, their data points to a 3.1% incidence of symptomatic pulmonary fibrosis.
The issue here is its generalizability. There were 63 patients who used methotrexate for 10 years or more and 88 who used it for 5 years or more, according to the poster. This must represent a highly selected population. For example, what percent of the total RA/psoriatic arthritis/”inflammatory arthritis” population do these patients represent, i.e., what is the denominator here? The authors stated that the 63 patients who stayed on methotrexate for 10 or more years represent 49% of the 129 patients on methotrexate overall in the study. This is a highly unusual datum, as most of the literature indicates that only 40% or less of patients stay on methotrexate for even 5 years. And this completely ignores the issue of adherence over this long a period; these patients must represent a truly minuscule percentage of the total if they actually stayed on methotrexate with even moderate adherence for 10 years.
Importantly, the authors point out that they had only four cases of symptomatic pulmonary fibrosis. Once more, this points to the highly selective group of patients seen, as this study does not examine patients with asymptomatic pulmonary fibrosis, including those with fibrosis on high-resolution CT of the lungs or chest film or evidence of abnormalities on pulmonary function tests, but who do not have sufficient symptoms ascribed to methotrexate to bring them to medical attention.
Daniel E. Furst, MD, is professor of rheumatology at the University of Washington, Seattle, who also is affiliated with the University of California, Los Angeles, and the University of Florence, Italy. He was not involved with the study.
The subject of this retrospective study is of great interest. The authors point out that pulmonary fibrosis (as opposed to acute allergic reaction, which is extremely rare) is also extremely uncommon in patients using methotrexate over the long haul. Over 10 years, their data points to a 3.1% incidence of symptomatic pulmonary fibrosis.
The issue here is its generalizability. There were 63 patients who used methotrexate for 10 years or more and 88 who used it for 5 years or more, according to the poster. This must represent a highly selected population. For example, what percent of the total RA/psoriatic arthritis/”inflammatory arthritis” population do these patients represent, i.e., what is the denominator here? The authors stated that the 63 patients who stayed on methotrexate for 10 or more years represent 49% of the 129 patients on methotrexate overall in the study. This is a highly unusual datum, as most of the literature indicates that only 40% or less of patients stay on methotrexate for even 5 years. And this completely ignores the issue of adherence over this long a period; these patients must represent a truly minuscule percentage of the total if they actually stayed on methotrexate with even moderate adherence for 10 years.
Importantly, the authors point out that they had only four cases of symptomatic pulmonary fibrosis. Once more, this points to the highly selective group of patients seen, as this study does not examine patients with asymptomatic pulmonary fibrosis, including those with fibrosis on high-resolution CT of the lungs or chest film or evidence of abnormalities on pulmonary function tests, but who do not have sufficient symptoms ascribed to methotrexate to bring them to medical attention.
Daniel E. Furst, MD, is professor of rheumatology at the University of Washington, Seattle, who also is affiliated with the University of California, Los Angeles, and the University of Florence, Italy. He was not involved with the study.
LIVERPOOL, ENGLAND – A 10-year follow up of patients with inflammatory arthritis has shown that methotrexate does not appear to increase the risk of pulmonary fibrosis.
“As rheumatologists, it’s a really important message that methotrexate does not cause chronic pulmonary fibrosis and it should not be stopped because of pulmonary fibrosis,” Julie Dawson, MD, said in an interview at the British Society for Rheumatology annual conference. “It’s the rheumatoid arthritis. It’s not the methotrexate.”
Dr. Dawson, of St. Helens and Knowsley Teaching Hospitals NHS Trust, St. Helens, England, added that the current findings were consistent with her team’s prior research looking at earlier time periods. There was also no correlation between the duration or dose of methotrexate used and the development of the lung disease, she said.
“If anything, the suggestion is you’d be more symptomatic if you delay using methotrexate,” Dr. Dawson observed. If patients are not doing well on methotrexate, then perhaps adjusting therapy or changing to another drug would of course be the next step, but if patients are well controlled then “stopping it is the worst thing to do” for their arthritis, she said.
“This is of great clinical interest, and we can be reassured now about this, I think. This is really good, long-term data,” said Devesh Mewar, MD, of Royal Liverpool and Broadgreen University Hospitals NHS Trust, Liverpool, England, who was not involved in the research.
“We know that methotrexate is associated with a pneumonitis reaction, but there is no high-quality evidence that methotrexate is associated with a chronic pulmonary fibrosis” Dr. Dawson said, explaining the rationale for the current study she presented during a poster session. Previous studies considered data for up to 5 years, she added, so the aim of the current study, therefore, was to look at the longer-term effect of methotrexate use on the incidence of pulmonary fibrosis.
Data on 129 patients who had started treatment with methotrexate from 2004 to 2007 were analyzed, of whom 63 (49%) had stayed on methotrexate for 10 or more years. Most (82%) had been given methotrexate to treat rheumatoid arthritis (RA), with other indications including inflammatory arthritis (5.4%) and psoriatic arthritis (4.7%).
“Practice was different 10 years ago, so just 56% of patients commenced methotrexate within the first year of the diagnosis of rheumatoid arthritis,” Dr. Dawson reported.
Only four cases of symptomatic pulmonary fibrosis were seen, all in the RA patients, and three of these were in patients who had started methotrexate over 1 year after their diagnosis. The incidence of 3.8% seen in the study matches the expected incidence of pulmonary fibrosis in RA and was actually “at the lower end of the expected incidence,” Dr. Dawson said. Previous studies have suggested an incidence rate of RA-associated interstitial lung disease of about 3%-7%.
All of the pulmonary fibrosis cases had occurred in men and 75% were seropositive for rheumatoid factor. The mean duration of RA at the time of onset of pulmonary fibrosis was 7.8 years and the usual interstitial pattern of fibrosis was seen. The 125 patients without pulmonary fibrosis had taken methotrexate for a mean of 8 years at a mean final weekly dose of 16.3 mg, compared with a mean of 6 years at a mean dose of 18.1 mg per week in the 4 patients with pulmonary fibrosis.
One of the next steps is to look at cases where methotrexate has been stopped and the effects of that on pulmonary fibrosis and disease activity. In Dr. Dawson’s experience, stopping methotrexate just affects the management of the arthritis and had no difference to the progression of pulmonary fibrosis.
If patients start to experience any lung symptoms while continuing methotrexate, such as shortness of breath, then they would need to be assessed and undergo lung function tests to monitor their condition. Treating the fibrosis using an antifibrotic drug, such as pirfenidone, is something that might be possible in the future, but this needs investigation in inflammatory arthritis as the drug is currently only licensed for use in idiopathic cases.
This is something the British Rheumatoid Interstitial Lung network plans to investigate in a placebo-controlled study of RA patients with fibrotic lung disease. “We’re looking to see if antifibrotic agents are going to slow the disease as it does in idiopathic pulmonary fibrosis, which is obviously quite exciting when it’s such a hard condition to treat,” said Dr. Dawson, who will be one of the study’s investigators.
Dr. Dawson had no conflicts of interest to disclose. Dr. Mewar was not involved in the study and had nothing to disclose.
SOURCE: Dawson J et al. Rheumatology. 2018;57[Suppl. 3]:key075.470.
LIVERPOOL, ENGLAND – A 10-year follow up of patients with inflammatory arthritis has shown that methotrexate does not appear to increase the risk of pulmonary fibrosis.
“As rheumatologists, it’s a really important message that methotrexate does not cause chronic pulmonary fibrosis and it should not be stopped because of pulmonary fibrosis,” Julie Dawson, MD, said in an interview at the British Society for Rheumatology annual conference. “It’s the rheumatoid arthritis. It’s not the methotrexate.”
Dr. Dawson, of St. Helens and Knowsley Teaching Hospitals NHS Trust, St. Helens, England, added that the current findings were consistent with her team’s prior research looking at earlier time periods. There was also no correlation between the duration or dose of methotrexate used and the development of the lung disease, she said.
“If anything, the suggestion is you’d be more symptomatic if you delay using methotrexate,” Dr. Dawson observed. If patients are not doing well on methotrexate, then perhaps adjusting therapy or changing to another drug would of course be the next step, but if patients are well controlled then “stopping it is the worst thing to do” for their arthritis, she said.
“This is of great clinical interest, and we can be reassured now about this, I think. This is really good, long-term data,” said Devesh Mewar, MD, of Royal Liverpool and Broadgreen University Hospitals NHS Trust, Liverpool, England, who was not involved in the research.
“We know that methotrexate is associated with a pneumonitis reaction, but there is no high-quality evidence that methotrexate is associated with a chronic pulmonary fibrosis” Dr. Dawson said, explaining the rationale for the current study she presented during a poster session. Previous studies considered data for up to 5 years, she added, so the aim of the current study, therefore, was to look at the longer-term effect of methotrexate use on the incidence of pulmonary fibrosis.
Data on 129 patients who had started treatment with methotrexate from 2004 to 2007 were analyzed, of whom 63 (49%) had stayed on methotrexate for 10 or more years. Most (82%) had been given methotrexate to treat rheumatoid arthritis (RA), with other indications including inflammatory arthritis (5.4%) and psoriatic arthritis (4.7%).
“Practice was different 10 years ago, so just 56% of patients commenced methotrexate within the first year of the diagnosis of rheumatoid arthritis,” Dr. Dawson reported.
Only four cases of symptomatic pulmonary fibrosis were seen, all in the RA patients, and three of these were in patients who had started methotrexate over 1 year after their diagnosis. The incidence of 3.8% seen in the study matches the expected incidence of pulmonary fibrosis in RA and was actually “at the lower end of the expected incidence,” Dr. Dawson said. Previous studies have suggested an incidence rate of RA-associated interstitial lung disease of about 3%-7%.
All of the pulmonary fibrosis cases had occurred in men and 75% were seropositive for rheumatoid factor. The mean duration of RA at the time of onset of pulmonary fibrosis was 7.8 years and the usual interstitial pattern of fibrosis was seen. The 125 patients without pulmonary fibrosis had taken methotrexate for a mean of 8 years at a mean final weekly dose of 16.3 mg, compared with a mean of 6 years at a mean dose of 18.1 mg per week in the 4 patients with pulmonary fibrosis.
One of the next steps is to look at cases where methotrexate has been stopped and the effects of that on pulmonary fibrosis and disease activity. In Dr. Dawson’s experience, stopping methotrexate just affects the management of the arthritis and had no difference to the progression of pulmonary fibrosis.
If patients start to experience any lung symptoms while continuing methotrexate, such as shortness of breath, then they would need to be assessed and undergo lung function tests to monitor their condition. Treating the fibrosis using an antifibrotic drug, such as pirfenidone, is something that might be possible in the future, but this needs investigation in inflammatory arthritis as the drug is currently only licensed for use in idiopathic cases.
This is something the British Rheumatoid Interstitial Lung network plans to investigate in a placebo-controlled study of RA patients with fibrotic lung disease. “We’re looking to see if antifibrotic agents are going to slow the disease as it does in idiopathic pulmonary fibrosis, which is obviously quite exciting when it’s such a hard condition to treat,” said Dr. Dawson, who will be one of the study’s investigators.
Dr. Dawson had no conflicts of interest to disclose. Dr. Mewar was not involved in the study and had nothing to disclose.
SOURCE: Dawson J et al. Rheumatology. 2018;57[Suppl. 3]:key075.470.
REPORTING FROM RHEUMATOLOGY 2018
Key clinical point:
Major finding: At 10 years’ follow-up, four patients (3.1%) developed pulmonary fibrosis.
Study details: Retrospective analysis of 129 patients with inflammatory arthritis treated with methotrexate for up to 10 years.
Disclosures: Dr. Dawson had no conflicts of interest to disclose. Dr. Mewar was not involved in the study and had nothing to disclose.
Source: Dawson J et al. Rheumatology. 2018;57[Suppl. 3]:key075.470.
First, marijuana. Are magic mushrooms next?
In Oregon and Denver, where marijuana is legal for recreational use, activists are now pushing toward a psychedelic frontier: “magic mushrooms.”
“We don’t want individuals to lose their freedom over something that’s natural and has health benefits,” said Kevin Matthews, the campaign director of Denver for Psilocybin, the group working to decriminalize magic mushrooms in Colorado’s capital.
The recent failure of a nationally publicized campaign to decriminalize hallucinogenic mushrooms in California may not portend well for the psilocybin advocates in Oregon and Denver – though their initiatives are more limited than California’s.
The proposal in the Golden State would have decriminalized sales and transportation of magic mushrooms, not just possession. The proposed Denver measure would apply only to that city, while in Oregon mushroom use would be allowed only with the approval of a physician and under the supervision of a registered therapist.
None of the proposed initiatives envisions fully legalizing psilocybin mushrooms, which would allow the government to regulate and tax sales in a similar fashion to medical and recreational marijuana.
In Oregon, advocates face a steep climb to qualify their measure for the ballot, because such statewide initiatives typically require hiring paid signature gatherers, said William Lunch, a political analyst for Oregon Public Broadcasting and a former political science professor at Oregon State University.
Still, familiarity with recreational marijuana may have “softened up” voters and opponents of drug decriminalization, he said. Oregon legalized marijuana for recreational use in 2015, Colorado in 2012.
The Oregon and Denver activists, echoing Lunch, say they hope voters who already accepted pot would now feel comfortable decriminalizing personal use of magic mushrooms as well.
Taking mushrooms can lead to nausea, panic attacks and, rarely, paranoia and psychosis. But they generally are considered safer and less addictive than other illegal street drugs.
Even so, Paul Hutson, professor of pharmacy at the University of Wisconsin who has conducted psilocybin research, says he is wary of the drive for decriminalization. Psilocybin isn’t safe for some people – particularly those with paranoia or psychosis, he said.
“I reject the idea that that this is a natural progression from medical marijuana,” Hutson said, noting that the safety of pot is much better established. Mushrooms, he added, “are very, very potent medicines that are affecting your mind. In the proper setting, they’re safe, but in an uncontrolled fashion, I have grave concerns.”
Even psilocybin advocates share Hutson’s concerns. “It is such a powerful compound. People should take it very seriously when experimenting,” Matthews said.
These efforts to legitimize hallucinogenic mushrooms come at a time of renewed interest in the potential mental health benefits of psychedelics, including mushrooms, LSD and MDMA (known as ecstasy). Two small studies published in 2016 by researchers from Johns Hopkins University and New York University found that a single large dose of psilocybin, combined with psychotherapy, helped relieve depression and anxiety in cancer patients.
A British company backed by Silicon Valley investor Peter Thiel plans clinical studies in eight European countries to test the use of psilocybin for depression. Other research has examined the effectiveness of psilocybin in treating alcohol and tobacco addiction.
In California, the campaign to decriminalize psilocybin was always a long shot – even though the famously liberal state legalized possession of recreational marijuana in November 2016 and sales starting this year.
California ballot measures typically require nearly 366,000 signatures to qualify, and supporters usually have to spend between $1 million and $2 million to pay signature gatherers. A Monterey County couple leading the decriminalization campaign managed to collect more than 90,000 signatures for their proposal with the help of volunteers, but they halted their efforts late last month.
The initiative would have exempted Californians 21 and over from criminal penalties for possessing, selling, transporting or cultivating psilocybin mushrooms.
Possessing them is generally a misdemeanor under California law, but selling them is a felony. State statistics on psilocybin offenses are scarce, but few people are jailed for such crimes, according to an analysis by the California attorney general’s office.
“It’s not a reckless community,” said Kitty Merchant of Marina, Calif., who spearheaded the California psilocybin campaign alongside her husband, Kevin Saunders. “It’s experimentation with your mind and your thoughts. There’s a safeness to it. And there’s an intelligence to it.”
Merchant said she and Saunders, both medical marijuana advocates, spent about $20,000 of their own money on the campaign.
In Denver, Matthews and his pro-psilocybin colleagues want voters to pass a city ordinance eliminating criminal penalties for possessing, using or growing magic mushrooms. City officials have cleared the measure for signature gathering. Supporters need 5,000 signatures to get it on the ballot in November. Matthews said he has already lined up dozens of volunteer signature gatherers.
He said he has used mushrooms to help alleviate depression and other mental health problems. A big part of the decriminalization campaign, he said, is promoting responsible use.
Denver, a progressive city in a state that was the first to legalize recreational marijuana, “is a good testing place for this initiative nationwide,” Matthews said. Just getting it on the ballot, whether or not it passes, would be “a huge victory,” he added.
In Oregon, activists are proposing a measure for the 2020 ballot that would decriminalize psilocybin statewide for adults 21 and over who get approval from their doctors and agree to participate in a “psilocybin service.” The service would include a preparatory meeting with a therapist, one session of supervised mushroom use and a follow-up visit. Patients would be under the care of state-certified “Psilocybin Service Facilitators.”
Tom Eckert, a Portland, Ore.-based therapist who leads the psilocybin decriminalization campaign with his wife, Sheri, said the proposed limitations on psilocybin use are important.
“Psilocybin is generally safe, but it puts you in a vulnerable state of mind,” he said. “If you do it in the wrong setting, things can go sideways.”
This story was produced by Kaiser Health News, which publishes California Healthline, a service of the California Health Care Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
In Oregon and Denver, where marijuana is legal for recreational use, activists are now pushing toward a psychedelic frontier: “magic mushrooms.”
“We don’t want individuals to lose their freedom over something that’s natural and has health benefits,” said Kevin Matthews, the campaign director of Denver for Psilocybin, the group working to decriminalize magic mushrooms in Colorado’s capital.
The recent failure of a nationally publicized campaign to decriminalize hallucinogenic mushrooms in California may not portend well for the psilocybin advocates in Oregon and Denver – though their initiatives are more limited than California’s.
The proposal in the Golden State would have decriminalized sales and transportation of magic mushrooms, not just possession. The proposed Denver measure would apply only to that city, while in Oregon mushroom use would be allowed only with the approval of a physician and under the supervision of a registered therapist.
None of the proposed initiatives envisions fully legalizing psilocybin mushrooms, which would allow the government to regulate and tax sales in a similar fashion to medical and recreational marijuana.
In Oregon, advocates face a steep climb to qualify their measure for the ballot, because such statewide initiatives typically require hiring paid signature gatherers, said William Lunch, a political analyst for Oregon Public Broadcasting and a former political science professor at Oregon State University.
Still, familiarity with recreational marijuana may have “softened up” voters and opponents of drug decriminalization, he said. Oregon legalized marijuana for recreational use in 2015, Colorado in 2012.
The Oregon and Denver activists, echoing Lunch, say they hope voters who already accepted pot would now feel comfortable decriminalizing personal use of magic mushrooms as well.
Taking mushrooms can lead to nausea, panic attacks and, rarely, paranoia and psychosis. But they generally are considered safer and less addictive than other illegal street drugs.
Even so, Paul Hutson, professor of pharmacy at the University of Wisconsin who has conducted psilocybin research, says he is wary of the drive for decriminalization. Psilocybin isn’t safe for some people – particularly those with paranoia or psychosis, he said.
“I reject the idea that that this is a natural progression from medical marijuana,” Hutson said, noting that the safety of pot is much better established. Mushrooms, he added, “are very, very potent medicines that are affecting your mind. In the proper setting, they’re safe, but in an uncontrolled fashion, I have grave concerns.”
Even psilocybin advocates share Hutson’s concerns. “It is such a powerful compound. People should take it very seriously when experimenting,” Matthews said.
These efforts to legitimize hallucinogenic mushrooms come at a time of renewed interest in the potential mental health benefits of psychedelics, including mushrooms, LSD and MDMA (known as ecstasy). Two small studies published in 2016 by researchers from Johns Hopkins University and New York University found that a single large dose of psilocybin, combined with psychotherapy, helped relieve depression and anxiety in cancer patients.
A British company backed by Silicon Valley investor Peter Thiel plans clinical studies in eight European countries to test the use of psilocybin for depression. Other research has examined the effectiveness of psilocybin in treating alcohol and tobacco addiction.
In California, the campaign to decriminalize psilocybin was always a long shot – even though the famously liberal state legalized possession of recreational marijuana in November 2016 and sales starting this year.
California ballot measures typically require nearly 366,000 signatures to qualify, and supporters usually have to spend between $1 million and $2 million to pay signature gatherers. A Monterey County couple leading the decriminalization campaign managed to collect more than 90,000 signatures for their proposal with the help of volunteers, but they halted their efforts late last month.
The initiative would have exempted Californians 21 and over from criminal penalties for possessing, selling, transporting or cultivating psilocybin mushrooms.
Possessing them is generally a misdemeanor under California law, but selling them is a felony. State statistics on psilocybin offenses are scarce, but few people are jailed for such crimes, according to an analysis by the California attorney general’s office.
“It’s not a reckless community,” said Kitty Merchant of Marina, Calif., who spearheaded the California psilocybin campaign alongside her husband, Kevin Saunders. “It’s experimentation with your mind and your thoughts. There’s a safeness to it. And there’s an intelligence to it.”
Merchant said she and Saunders, both medical marijuana advocates, spent about $20,000 of their own money on the campaign.
In Denver, Matthews and his pro-psilocybin colleagues want voters to pass a city ordinance eliminating criminal penalties for possessing, using or growing magic mushrooms. City officials have cleared the measure for signature gathering. Supporters need 5,000 signatures to get it on the ballot in November. Matthews said he has already lined up dozens of volunteer signature gatherers.
He said he has used mushrooms to help alleviate depression and other mental health problems. A big part of the decriminalization campaign, he said, is promoting responsible use.
Denver, a progressive city in a state that was the first to legalize recreational marijuana, “is a good testing place for this initiative nationwide,” Matthews said. Just getting it on the ballot, whether or not it passes, would be “a huge victory,” he added.
In Oregon, activists are proposing a measure for the 2020 ballot that would decriminalize psilocybin statewide for adults 21 and over who get approval from their doctors and agree to participate in a “psilocybin service.” The service would include a preparatory meeting with a therapist, one session of supervised mushroom use and a follow-up visit. Patients would be under the care of state-certified “Psilocybin Service Facilitators.”
Tom Eckert, a Portland, Ore.-based therapist who leads the psilocybin decriminalization campaign with his wife, Sheri, said the proposed limitations on psilocybin use are important.
“Psilocybin is generally safe, but it puts you in a vulnerable state of mind,” he said. “If you do it in the wrong setting, things can go sideways.”
This story was produced by Kaiser Health News, which publishes California Healthline, a service of the California Health Care Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
In Oregon and Denver, where marijuana is legal for recreational use, activists are now pushing toward a psychedelic frontier: “magic mushrooms.”
“We don’t want individuals to lose their freedom over something that’s natural and has health benefits,” said Kevin Matthews, the campaign director of Denver for Psilocybin, the group working to decriminalize magic mushrooms in Colorado’s capital.
The recent failure of a nationally publicized campaign to decriminalize hallucinogenic mushrooms in California may not portend well for the psilocybin advocates in Oregon and Denver – though their initiatives are more limited than California’s.
The proposal in the Golden State would have decriminalized sales and transportation of magic mushrooms, not just possession. The proposed Denver measure would apply only to that city, while in Oregon mushroom use would be allowed only with the approval of a physician and under the supervision of a registered therapist.
None of the proposed initiatives envisions fully legalizing psilocybin mushrooms, which would allow the government to regulate and tax sales in a similar fashion to medical and recreational marijuana.
In Oregon, advocates face a steep climb to qualify their measure for the ballot, because such statewide initiatives typically require hiring paid signature gatherers, said William Lunch, a political analyst for Oregon Public Broadcasting and a former political science professor at Oregon State University.
Still, familiarity with recreational marijuana may have “softened up” voters and opponents of drug decriminalization, he said. Oregon legalized marijuana for recreational use in 2015, Colorado in 2012.
The Oregon and Denver activists, echoing Lunch, say they hope voters who already accepted pot would now feel comfortable decriminalizing personal use of magic mushrooms as well.
Taking mushrooms can lead to nausea, panic attacks and, rarely, paranoia and psychosis. But they generally are considered safer and less addictive than other illegal street drugs.
Even so, Paul Hutson, professor of pharmacy at the University of Wisconsin who has conducted psilocybin research, says he is wary of the drive for decriminalization. Psilocybin isn’t safe for some people – particularly those with paranoia or psychosis, he said.
“I reject the idea that that this is a natural progression from medical marijuana,” Hutson said, noting that the safety of pot is much better established. Mushrooms, he added, “are very, very potent medicines that are affecting your mind. In the proper setting, they’re safe, but in an uncontrolled fashion, I have grave concerns.”
Even psilocybin advocates share Hutson’s concerns. “It is such a powerful compound. People should take it very seriously when experimenting,” Matthews said.
These efforts to legitimize hallucinogenic mushrooms come at a time of renewed interest in the potential mental health benefits of psychedelics, including mushrooms, LSD and MDMA (known as ecstasy). Two small studies published in 2016 by researchers from Johns Hopkins University and New York University found that a single large dose of psilocybin, combined with psychotherapy, helped relieve depression and anxiety in cancer patients.
A British company backed by Silicon Valley investor Peter Thiel plans clinical studies in eight European countries to test the use of psilocybin for depression. Other research has examined the effectiveness of psilocybin in treating alcohol and tobacco addiction.
In California, the campaign to decriminalize psilocybin was always a long shot – even though the famously liberal state legalized possession of recreational marijuana in November 2016 and sales starting this year.
California ballot measures typically require nearly 366,000 signatures to qualify, and supporters usually have to spend between $1 million and $2 million to pay signature gatherers. A Monterey County couple leading the decriminalization campaign managed to collect more than 90,000 signatures for their proposal with the help of volunteers, but they halted their efforts late last month.
The initiative would have exempted Californians 21 and over from criminal penalties for possessing, selling, transporting or cultivating psilocybin mushrooms.
Possessing them is generally a misdemeanor under California law, but selling them is a felony. State statistics on psilocybin offenses are scarce, but few people are jailed for such crimes, according to an analysis by the California attorney general’s office.
“It’s not a reckless community,” said Kitty Merchant of Marina, Calif., who spearheaded the California psilocybin campaign alongside her husband, Kevin Saunders. “It’s experimentation with your mind and your thoughts. There’s a safeness to it. And there’s an intelligence to it.”
Merchant said she and Saunders, both medical marijuana advocates, spent about $20,000 of their own money on the campaign.
In Denver, Matthews and his pro-psilocybin colleagues want voters to pass a city ordinance eliminating criminal penalties for possessing, using or growing magic mushrooms. City officials have cleared the measure for signature gathering. Supporters need 5,000 signatures to get it on the ballot in November. Matthews said he has already lined up dozens of volunteer signature gatherers.
He said he has used mushrooms to help alleviate depression and other mental health problems. A big part of the decriminalization campaign, he said, is promoting responsible use.
Denver, a progressive city in a state that was the first to legalize recreational marijuana, “is a good testing place for this initiative nationwide,” Matthews said. Just getting it on the ballot, whether or not it passes, would be “a huge victory,” he added.
In Oregon, activists are proposing a measure for the 2020 ballot that would decriminalize psilocybin statewide for adults 21 and over who get approval from their doctors and agree to participate in a “psilocybin service.” The service would include a preparatory meeting with a therapist, one session of supervised mushroom use and a follow-up visit. Patients would be under the care of state-certified “Psilocybin Service Facilitators.”
Tom Eckert, a Portland, Ore.-based therapist who leads the psilocybin decriminalization campaign with his wife, Sheri, said the proposed limitations on psilocybin use are important.
“Psilocybin is generally safe, but it puts you in a vulnerable state of mind,” he said. “If you do it in the wrong setting, things can go sideways.”
This story was produced by Kaiser Health News, which publishes California Healthline, a service of the California Health Care Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Parkinson’s disease patients have impaired insulin secretion
LOS ANGELES – Parkinson’s disease patients with long-standing disease should be screened for glucose dysregulation and treated with insulin releasing drugs, instead of insulin sensitizers, when necessary, according to a French investigation that compared 50 patients with 50 healthy controls matched for age, sex, and body mass index.
The subjects underwent a 75-g oral glucose tolerance test. Glucose levels were higher in the Parkinson’s disease (PD) group from about 60 minutes through the end of the test at 180 minutes; the differences were statistically significant at 90 and 150 minutes. The total blood glucose area under the time curve (AUC) was significantly higher in the PD group (1,187 vs. 1,101 mmol.min l-1; P = .05).
In short, “PD patients had higher blood glucose following oral glucose intake without … the expected concomitant rise in insulin levels, suggesting an under-active insulin response. PD patients with advanced disease” – all the patients had had PD for more than 5 years – “have impaired blood glucose levels in response to oral glucose intake,” said lead investigator Ana Marques, MD, of the Université Clermont Auvergne in Clermont-Ferrand, France.
“Blood glucose dysregulation should be screened in PD patients with moderate to advanced disease. Insulin releasing drugs should possibly be preferred [over] insulin sensitizer drugs in PD patients with diabetes,” she said at the annual meeting of the American Academy of Neurology.
Higher blood glucose levels were also associated with longer PD duration, lower dopaminergic therapy doses, and higher degrees of dysautonomia. Mean PD duration in the study was about 8 years, and mean levodopa-equivalent dose 884 mg/d.
The findings add weight to the proposed and still somewhat controversial link between PD and diabetes. “Dysglycemia appears to be another nonmotor consequence of PD,” Dr. Marques said. Because insulin production “is modulated by the autonomic nervous system, the severity of dysautonomia in PD could be linked with blood glucose dysregulation. Sympathetic denervation might lead to beta-cell dysfunction.”
Subjects were 61 years old, on average; two-thirds were men. The mean BMI was about 25 kg/m2. Patients were excluded if they had a change in dopaminergic therapy in the previous month, previous deep brain stimulation, medications that would interfere with glucose metabolism, or diabetes, among other things.
PD patients had slightly higher fasting urine glucose (P = .02). They also had lower fasting plasma insulin, but the difference wasn’t statistically significant (P = .5). Dysglycemia was also associated with higher BMI. Male gender and higher levodopa-equivalent doses were protective.
“The association between dysglycemia and PD is bilateral. In many studies, PD enhances the risk, but dysglycemia and particularly diabetes have been reported to increase the risk of PD. It goes both ways. There’s a lot that remains to be understood,” Dr. Marques said.
There was no external funding, and the investigators had nothing to disclose.
SOURCE: Marques A et al. Neurology. 2018 Apr 90(15 Suppl.):S3.008
LOS ANGELES – Parkinson’s disease patients with long-standing disease should be screened for glucose dysregulation and treated with insulin releasing drugs, instead of insulin sensitizers, when necessary, according to a French investigation that compared 50 patients with 50 healthy controls matched for age, sex, and body mass index.
The subjects underwent a 75-g oral glucose tolerance test. Glucose levels were higher in the Parkinson’s disease (PD) group from about 60 minutes through the end of the test at 180 minutes; the differences were statistically significant at 90 and 150 minutes. The total blood glucose area under the time curve (AUC) was significantly higher in the PD group (1,187 vs. 1,101 mmol.min l-1; P = .05).
In short, “PD patients had higher blood glucose following oral glucose intake without … the expected concomitant rise in insulin levels, suggesting an under-active insulin response. PD patients with advanced disease” – all the patients had had PD for more than 5 years – “have impaired blood glucose levels in response to oral glucose intake,” said lead investigator Ana Marques, MD, of the Université Clermont Auvergne in Clermont-Ferrand, France.
“Blood glucose dysregulation should be screened in PD patients with moderate to advanced disease. Insulin releasing drugs should possibly be preferred [over] insulin sensitizer drugs in PD patients with diabetes,” she said at the annual meeting of the American Academy of Neurology.
Higher blood glucose levels were also associated with longer PD duration, lower dopaminergic therapy doses, and higher degrees of dysautonomia. Mean PD duration in the study was about 8 years, and mean levodopa-equivalent dose 884 mg/d.
The findings add weight to the proposed and still somewhat controversial link between PD and diabetes. “Dysglycemia appears to be another nonmotor consequence of PD,” Dr. Marques said. Because insulin production “is modulated by the autonomic nervous system, the severity of dysautonomia in PD could be linked with blood glucose dysregulation. Sympathetic denervation might lead to beta-cell dysfunction.”
Subjects were 61 years old, on average; two-thirds were men. The mean BMI was about 25 kg/m2. Patients were excluded if they had a change in dopaminergic therapy in the previous month, previous deep brain stimulation, medications that would interfere with glucose metabolism, or diabetes, among other things.
PD patients had slightly higher fasting urine glucose (P = .02). They also had lower fasting plasma insulin, but the difference wasn’t statistically significant (P = .5). Dysglycemia was also associated with higher BMI. Male gender and higher levodopa-equivalent doses were protective.
“The association between dysglycemia and PD is bilateral. In many studies, PD enhances the risk, but dysglycemia and particularly diabetes have been reported to increase the risk of PD. It goes both ways. There’s a lot that remains to be understood,” Dr. Marques said.
There was no external funding, and the investigators had nothing to disclose.
SOURCE: Marques A et al. Neurology. 2018 Apr 90(15 Suppl.):S3.008
LOS ANGELES – Parkinson’s disease patients with long-standing disease should be screened for glucose dysregulation and treated with insulin releasing drugs, instead of insulin sensitizers, when necessary, according to a French investigation that compared 50 patients with 50 healthy controls matched for age, sex, and body mass index.
The subjects underwent a 75-g oral glucose tolerance test. Glucose levels were higher in the Parkinson’s disease (PD) group from about 60 minutes through the end of the test at 180 minutes; the differences were statistically significant at 90 and 150 minutes. The total blood glucose area under the time curve (AUC) was significantly higher in the PD group (1,187 vs. 1,101 mmol.min l-1; P = .05).
In short, “PD patients had higher blood glucose following oral glucose intake without … the expected concomitant rise in insulin levels, suggesting an under-active insulin response. PD patients with advanced disease” – all the patients had had PD for more than 5 years – “have impaired blood glucose levels in response to oral glucose intake,” said lead investigator Ana Marques, MD, of the Université Clermont Auvergne in Clermont-Ferrand, France.
“Blood glucose dysregulation should be screened in PD patients with moderate to advanced disease. Insulin releasing drugs should possibly be preferred [over] insulin sensitizer drugs in PD patients with diabetes,” she said at the annual meeting of the American Academy of Neurology.
Higher blood glucose levels were also associated with longer PD duration, lower dopaminergic therapy doses, and higher degrees of dysautonomia. Mean PD duration in the study was about 8 years, and mean levodopa-equivalent dose 884 mg/d.
The findings add weight to the proposed and still somewhat controversial link between PD and diabetes. “Dysglycemia appears to be another nonmotor consequence of PD,” Dr. Marques said. Because insulin production “is modulated by the autonomic nervous system, the severity of dysautonomia in PD could be linked with blood glucose dysregulation. Sympathetic denervation might lead to beta-cell dysfunction.”
Subjects were 61 years old, on average; two-thirds were men. The mean BMI was about 25 kg/m2. Patients were excluded if they had a change in dopaminergic therapy in the previous month, previous deep brain stimulation, medications that would interfere with glucose metabolism, or diabetes, among other things.
PD patients had slightly higher fasting urine glucose (P = .02). They also had lower fasting plasma insulin, but the difference wasn’t statistically significant (P = .5). Dysglycemia was also associated with higher BMI. Male gender and higher levodopa-equivalent doses were protective.
“The association between dysglycemia and PD is bilateral. In many studies, PD enhances the risk, but dysglycemia and particularly diabetes have been reported to increase the risk of PD. It goes both ways. There’s a lot that remains to be understood,” Dr. Marques said.
There was no external funding, and the investigators had nothing to disclose.
SOURCE: Marques A et al. Neurology. 2018 Apr 90(15 Suppl.):S3.008
REPORTING FROM AAN 2018
Key clinical point: instead of insulin sensitizers, when necessary.
Major finding: The total blood glucose area under the time curve was significantly higher in the PD group after oral glucose challenge.
Study details: A study of 50 PD patients and 50 controls.
Disclosures: There was no external funding, and the investigators had nothing to disclose.
Source: Marques A et al. Neurology. 2018 Apr 90(15 Suppl.):S3.008