User login
FDA approves first epoetin alfa biosimilar
The US Food and Drug Administration (FDA) has approved epoetin alfa-epbx (Retacrit), a biosimilar to epoetin alfa (Epogen/Procrit).
Epoetin alfa-epbx is approved for the treatment of anemia caused by chronic kidney disease, the use of zidovudine in patients with HIV infection, and myelosuppressive chemotherapy in patients who have a minimum of 2 additional months of planned chemotherapy.
Epoetin alfa-epbx is also approved for use before and after surgery to reduce the chance that red blood cell transfusions will be needed because of blood loss during elective, noncardiac, or nonvascular surgery.
As with epoetin alfa, the prescribing information for epoetin alfa-epbx contains a Boxed Warning noting that erythropoiesis-stimulating agents increase the risk of death, myocardial infarction, stroke, venous thromboembolism, thrombosis of vascular access, and tumor progression or recurrence.
The FDA granted approval of epoetin alfa-epbx to Hospira Inc., a Pfizer company.
The agency’s approval is based on a review of evidence that included structural and functional characterization, animal study data, human pharmacokinetic and pharmacodynamic data, clinical immunogenicity data, and other clinical safety and effectiveness data.
This evidence is available in an FDA briefing document on the biologics license application for epoetin alfa-epbx.
The US Food and Drug Administration (FDA) has approved epoetin alfa-epbx (Retacrit), a biosimilar to epoetin alfa (Epogen/Procrit).
Epoetin alfa-epbx is approved for the treatment of anemia caused by chronic kidney disease, the use of zidovudine in patients with HIV infection, and myelosuppressive chemotherapy in patients who have a minimum of 2 additional months of planned chemotherapy.
Epoetin alfa-epbx is also approved for use before and after surgery to reduce the chance that red blood cell transfusions will be needed because of blood loss during elective, noncardiac, or nonvascular surgery.
As with epoetin alfa, the prescribing information for epoetin alfa-epbx contains a Boxed Warning noting that erythropoiesis-stimulating agents increase the risk of death, myocardial infarction, stroke, venous thromboembolism, thrombosis of vascular access, and tumor progression or recurrence.
The FDA granted approval of epoetin alfa-epbx to Hospira Inc., a Pfizer company.
The agency’s approval is based on a review of evidence that included structural and functional characterization, animal study data, human pharmacokinetic and pharmacodynamic data, clinical immunogenicity data, and other clinical safety and effectiveness data.
This evidence is available in an FDA briefing document on the biologics license application for epoetin alfa-epbx.
The US Food and Drug Administration (FDA) has approved epoetin alfa-epbx (Retacrit), a biosimilar to epoetin alfa (Epogen/Procrit).
Epoetin alfa-epbx is approved for the treatment of anemia caused by chronic kidney disease, the use of zidovudine in patients with HIV infection, and myelosuppressive chemotherapy in patients who have a minimum of 2 additional months of planned chemotherapy.
Epoetin alfa-epbx is also approved for use before and after surgery to reduce the chance that red blood cell transfusions will be needed because of blood loss during elective, noncardiac, or nonvascular surgery.
As with epoetin alfa, the prescribing information for epoetin alfa-epbx contains a Boxed Warning noting that erythropoiesis-stimulating agents increase the risk of death, myocardial infarction, stroke, venous thromboembolism, thrombosis of vascular access, and tumor progression or recurrence.
The FDA granted approval of epoetin alfa-epbx to Hospira Inc., a Pfizer company.
The agency’s approval is based on a review of evidence that included structural and functional characterization, animal study data, human pharmacokinetic and pharmacodynamic data, clinical immunogenicity data, and other clinical safety and effectiveness data.
This evidence is available in an FDA briefing document on the biologics license application for epoetin alfa-epbx.
Team analyzes skin odor to detect malaria
Changes in skin odor can reveal malaria infection in patients with no external symptoms, according to research published in PNAS.
Researchers examined chemical compounds released from the skin of Kenyan children and discovered characteristic patterns in these compounds that identified patients with acute and asymptomatic malaria infections.
“Our previous work in a mouse model found that malaria infection altered the odors of infected mice in ways that made them more attractive to mosquitoes, particularly at a stage of infection where the transmissible stage of the parasite was present at high levels,” said study author Consuelo De Moraes, PhD, of ETH Zurich in Switzerland.
“We also found long-term changes in the odor profiles of infected mice. [So] we had reason to hope that similar changes in human odors might provide biomarkers that could be used for diagnosis.”
To test this theory, Dr De Moraes and her colleagues studied more than 400 Kenyan school children. The researchers collected blood samples as well as samples of volatile substances released from the subjects’ skin.
The team used the blood samples to test for malaria, first via light microscopy and an SD Bioline Rapid Diagnostic Test, then using polymerase chain reaction (PCR) methods to confirm the initial results.
There were 330 subjects who were clearly positive or negative for malaria, and there were 66 subjects who were positive by PCR but negative by microscopy. The researchers compared these findings to results from the skin tests.
To assess the subjects’ skin, the researchers placed each child’s foot and arm into sealed Teflon bags and passed an air current over the skin for about 1 hour. The air was then channeled through special filters that collected the volatile compounds.
Using gas chromatography and mass spectrometry, the researchers then determined the identity and quantity of each compound to generate odor profiles for infected and uninfected children.
Further analysis of these profiles revealed volatile biomarkers that enabled the researchers to accurately identify whether a child was infected with the malaria parasite. Even for asymptomatic infections, the detection rate was close to 100%.
“This high detection rate was encouraging,” Dr De Moraes said. “Initially, we weren’t sure which chemical compounds we should be looking for.”
The researchers noted that malaria infection does not create new chemical compounds, but it alters the amounts of compounds that are already present in the odors of healthy people.
Odor profiles were different for malaria-infected and uninfected subjects, but profiles were also different for patients with acute and asymptomatic infections.
The researchers hope the biomarkers they identified could be used to develop a new tool for the early detection of malaria.
“These new volatile biomarkers are an important first step,” said Mark Mescher, of ETH Zurich. “Now, someone needs to develop an application that can be used cheaply and reliably in the field.”
“In the near-term, our goal is to refine the current findings to find the most reliable and effective biomarkers we can. There is still a lot more work to be done to develop a practical diagnostic assay.”
Changes in skin odor can reveal malaria infection in patients with no external symptoms, according to research published in PNAS.
Researchers examined chemical compounds released from the skin of Kenyan children and discovered characteristic patterns in these compounds that identified patients with acute and asymptomatic malaria infections.
“Our previous work in a mouse model found that malaria infection altered the odors of infected mice in ways that made them more attractive to mosquitoes, particularly at a stage of infection where the transmissible stage of the parasite was present at high levels,” said study author Consuelo De Moraes, PhD, of ETH Zurich in Switzerland.
“We also found long-term changes in the odor profiles of infected mice. [So] we had reason to hope that similar changes in human odors might provide biomarkers that could be used for diagnosis.”
To test this theory, Dr De Moraes and her colleagues studied more than 400 Kenyan school children. The researchers collected blood samples as well as samples of volatile substances released from the subjects’ skin.
The team used the blood samples to test for malaria, first via light microscopy and an SD Bioline Rapid Diagnostic Test, then using polymerase chain reaction (PCR) methods to confirm the initial results.
There were 330 subjects who were clearly positive or negative for malaria, and there were 66 subjects who were positive by PCR but negative by microscopy. The researchers compared these findings to results from the skin tests.
To assess the subjects’ skin, the researchers placed each child’s foot and arm into sealed Teflon bags and passed an air current over the skin for about 1 hour. The air was then channeled through special filters that collected the volatile compounds.
Using gas chromatography and mass spectrometry, the researchers then determined the identity and quantity of each compound to generate odor profiles for infected and uninfected children.
Further analysis of these profiles revealed volatile biomarkers that enabled the researchers to accurately identify whether a child was infected with the malaria parasite. Even for asymptomatic infections, the detection rate was close to 100%.
“This high detection rate was encouraging,” Dr De Moraes said. “Initially, we weren’t sure which chemical compounds we should be looking for.”
The researchers noted that malaria infection does not create new chemical compounds, but it alters the amounts of compounds that are already present in the odors of healthy people.
Odor profiles were different for malaria-infected and uninfected subjects, but profiles were also different for patients with acute and asymptomatic infections.
The researchers hope the biomarkers they identified could be used to develop a new tool for the early detection of malaria.
“These new volatile biomarkers are an important first step,” said Mark Mescher, of ETH Zurich. “Now, someone needs to develop an application that can be used cheaply and reliably in the field.”
“In the near-term, our goal is to refine the current findings to find the most reliable and effective biomarkers we can. There is still a lot more work to be done to develop a practical diagnostic assay.”
Changes in skin odor can reveal malaria infection in patients with no external symptoms, according to research published in PNAS.
Researchers examined chemical compounds released from the skin of Kenyan children and discovered characteristic patterns in these compounds that identified patients with acute and asymptomatic malaria infections.
“Our previous work in a mouse model found that malaria infection altered the odors of infected mice in ways that made them more attractive to mosquitoes, particularly at a stage of infection where the transmissible stage of the parasite was present at high levels,” said study author Consuelo De Moraes, PhD, of ETH Zurich in Switzerland.
“We also found long-term changes in the odor profiles of infected mice. [So] we had reason to hope that similar changes in human odors might provide biomarkers that could be used for diagnosis.”
To test this theory, Dr De Moraes and her colleagues studied more than 400 Kenyan school children. The researchers collected blood samples as well as samples of volatile substances released from the subjects’ skin.
The team used the blood samples to test for malaria, first via light microscopy and an SD Bioline Rapid Diagnostic Test, then using polymerase chain reaction (PCR) methods to confirm the initial results.
There were 330 subjects who were clearly positive or negative for malaria, and there were 66 subjects who were positive by PCR but negative by microscopy. The researchers compared these findings to results from the skin tests.
To assess the subjects’ skin, the researchers placed each child’s foot and arm into sealed Teflon bags and passed an air current over the skin for about 1 hour. The air was then channeled through special filters that collected the volatile compounds.
Using gas chromatography and mass spectrometry, the researchers then determined the identity and quantity of each compound to generate odor profiles for infected and uninfected children.
Further analysis of these profiles revealed volatile biomarkers that enabled the researchers to accurately identify whether a child was infected with the malaria parasite. Even for asymptomatic infections, the detection rate was close to 100%.
“This high detection rate was encouraging,” Dr De Moraes said. “Initially, we weren’t sure which chemical compounds we should be looking for.”
The researchers noted that malaria infection does not create new chemical compounds, but it alters the amounts of compounds that are already present in the odors of healthy people.
Odor profiles were different for malaria-infected and uninfected subjects, but profiles were also different for patients with acute and asymptomatic infections.
The researchers hope the biomarkers they identified could be used to develop a new tool for the early detection of malaria.
“These new volatile biomarkers are an important first step,” said Mark Mescher, of ETH Zurich. “Now, someone needs to develop an application that can be used cheaply and reliably in the field.”
“In the near-term, our goal is to refine the current findings to find the most reliable and effective biomarkers we can. There is still a lot more work to be done to develop a practical diagnostic assay.”
Umbralisib has ‘distinct’ safety profile
Phase 1 trial results suggest umbralisib, a PI3Kδ/CK1ε inhibitor, can be safe and active in patients with relapsed or refractory B-cell malignancies.
Researchers said the safety profile of umbralisib “was distinct from that of other PI3Kδ inhibitors,” as it produced few immune-mediated adverse events (AEs).
Umbralisib also produced an objective response rate of 37% in the entire study cohort, 80% in patients with chronic lymphocytic leukemia (CLL), 53% in patients with follicular lymphoma (FL), and 31% in patients with diffuse large B-cell lymphoma (DLBCL).
These results were published in The Lancet Oncology. The study was sponsored by TG Therapeutics, Inc.
The trial enrolled 90 patients between January 17, 2013, and January 14, 2016.
There were 24 patients with CLL, 22 with FL, 16 with DLBCL, 11 with Hodgkin lymphoma, 6 with mantle cell lymphoma, 5 with marginal zone lymphoma, 3 with Waldenstrom’s macroglobulinemia, 2 with T-cell lymphoma, and 1 with hairy cell leukemia.
The median number of prior therapies was 3 (range, 2-5), and 49% of patients were refractory to previous therapy.
Treatment
Patients took umbralisib once daily in 28-day cycles until disease progression, unacceptable toxicity, or withdrawal of consent.
Initially, patients took the drug in a fasting state at doses of 50 mg, 100 mg, 200 mg, 400 mg, 800 mg, 1200 mg, or 1800 mg.
In April 2014, the researchers did a second dose-escalation with a micronized formulation of umbralisib, taken with food, at doses of 200 mg, 400 mg, 800 mg, 1200 mg, or 1800 mg.
In August, 2014, all patients who were still on the study transitioned to the 800 mg dose of the micronized formulation. This was the recommended phase 2 dose.
At the data cutoff in November 2016, 44 patients (49%) had received umbralisib for more than 6 cycles, and 23 (26%) had received the drug for more than 12 cycles. Thirteen patients (14%) were still taking umbralisib at the end of the study.
Most patients who stopped treatment did so because of disease progression (n=50, 56%) or AEs (n=9, 10%).
“We are pleased to have treated the first patient ever with umbralisib over 5 years ago and believe it has an important place in the treatment landscape for patients with hematologic malignancies,” said study author Howard A. Burris, MD, of the Sarah Cannon Research Institute in Nashville, Tennessee.
“Several patients from this phase 1 study are still on study today, approaching 5 years of continuous daily therapy, speaking to both the safety and efficacy profile of this unique agent.”
Safety
Dose-limiting toxicities (DLTs) occurred in 4 patients. One DLT was grade 3 maculopapular rash in a patient receiving the 800 mg dose of the initial formulation.
Another DLT was grade 3 hypokalemia in a patient receiving 1800 mg of the initial formulation. A third DLT was grade 3 fatigue, which occurred in 2 patients receiving 1800 mg of the micronized formulation.
Because of these toxicities, the maximum tolerated dose was 1200 mg of the micronized formulation.
The most common treatment-emergent AEs were diarrhea (43%), nausea (42%), and fatigue (31%). The most common grade 3/4 AEs were neutropenia (13%), anemia (9%), and thrombocytopenia (7%).
Serious AEs considered at least possibly related to umbralisib were pneumonia (3%), lung infection (1%), febrile neutropenia (1%), and colitis (2%).
Treatment discontinuation due to AEs considered at least possibly related to umbralisib occurred in 6 patients (7%). Two patients had grade 3 colitis, 2 had increased ALT/AST (grade 1 and grade 4), 1 had grade 2 diarrhea, and 1 had grade 3 fatigue.
There were no treatment-related deaths.
The researchers said the safety profile of umbralisib was distinct from that of other PI3Kδ inhibitors, as patients in this trial had fewer occurrences of autoimmune-like toxicities, such as colitis.
“Preclinically, umbralisib has a very unique profile, selectively inhibiting both PI3Kδ and CK1ε,” said study author Owen O’Connor, MD, PhD, of Columbia Presbyterian Medical Center in New York, New York.
“The clinical results in this paper support our thesis that the differentiated preclinical profile explains the differences seen in the clinic between umbralisib and the other PI3Kδ inhibitors.”
Response
The objective response rate was 37%, with 33 patients achieving a response and 3 patients having a complete response (CR).
Sixteen CLL patients responded (80%), all with partial responses (PRs). Four DLBCL patients responded (31%), all with PRs. And 9 FL patients responded (53%), 2 with CRs.
The remaining CR occurred in a Hodgkin lymphoma patient, and this was the only response in this patient group.
One patient with marginal zone lymphoma had a PR, as did 1 patient with mantle cell lymphoma. All other patients had stable disease or progressed.
The mean duration of response was 13.4 months in the CLL patients, 6.4 months in the DLBCL patients, and 9.3 months in the FL patients.
Phase 1 trial results suggest umbralisib, a PI3Kδ/CK1ε inhibitor, can be safe and active in patients with relapsed or refractory B-cell malignancies.
Researchers said the safety profile of umbralisib “was distinct from that of other PI3Kδ inhibitors,” as it produced few immune-mediated adverse events (AEs).
Umbralisib also produced an objective response rate of 37% in the entire study cohort, 80% in patients with chronic lymphocytic leukemia (CLL), 53% in patients with follicular lymphoma (FL), and 31% in patients with diffuse large B-cell lymphoma (DLBCL).
These results were published in The Lancet Oncology. The study was sponsored by TG Therapeutics, Inc.
The trial enrolled 90 patients between January 17, 2013, and January 14, 2016.
There were 24 patients with CLL, 22 with FL, 16 with DLBCL, 11 with Hodgkin lymphoma, 6 with mantle cell lymphoma, 5 with marginal zone lymphoma, 3 with Waldenstrom’s macroglobulinemia, 2 with T-cell lymphoma, and 1 with hairy cell leukemia.
The median number of prior therapies was 3 (range, 2-5), and 49% of patients were refractory to previous therapy.
Treatment
Patients took umbralisib once daily in 28-day cycles until disease progression, unacceptable toxicity, or withdrawal of consent.
Initially, patients took the drug in a fasting state at doses of 50 mg, 100 mg, 200 mg, 400 mg, 800 mg, 1200 mg, or 1800 mg.
In April 2014, the researchers did a second dose-escalation with a micronized formulation of umbralisib, taken with food, at doses of 200 mg, 400 mg, 800 mg, 1200 mg, or 1800 mg.
In August, 2014, all patients who were still on the study transitioned to the 800 mg dose of the micronized formulation. This was the recommended phase 2 dose.
At the data cutoff in November 2016, 44 patients (49%) had received umbralisib for more than 6 cycles, and 23 (26%) had received the drug for more than 12 cycles. Thirteen patients (14%) were still taking umbralisib at the end of the study.
Most patients who stopped treatment did so because of disease progression (n=50, 56%) or AEs (n=9, 10%).
“We are pleased to have treated the first patient ever with umbralisib over 5 years ago and believe it has an important place in the treatment landscape for patients with hematologic malignancies,” said study author Howard A. Burris, MD, of the Sarah Cannon Research Institute in Nashville, Tennessee.
“Several patients from this phase 1 study are still on study today, approaching 5 years of continuous daily therapy, speaking to both the safety and efficacy profile of this unique agent.”
Safety
Dose-limiting toxicities (DLTs) occurred in 4 patients. One DLT was grade 3 maculopapular rash in a patient receiving the 800 mg dose of the initial formulation.
Another DLT was grade 3 hypokalemia in a patient receiving 1800 mg of the initial formulation. A third DLT was grade 3 fatigue, which occurred in 2 patients receiving 1800 mg of the micronized formulation.
Because of these toxicities, the maximum tolerated dose was 1200 mg of the micronized formulation.
The most common treatment-emergent AEs were diarrhea (43%), nausea (42%), and fatigue (31%). The most common grade 3/4 AEs were neutropenia (13%), anemia (9%), and thrombocytopenia (7%).
Serious AEs considered at least possibly related to umbralisib were pneumonia (3%), lung infection (1%), febrile neutropenia (1%), and colitis (2%).
Treatment discontinuation due to AEs considered at least possibly related to umbralisib occurred in 6 patients (7%). Two patients had grade 3 colitis, 2 had increased ALT/AST (grade 1 and grade 4), 1 had grade 2 diarrhea, and 1 had grade 3 fatigue.
There were no treatment-related deaths.
The researchers said the safety profile of umbralisib was distinct from that of other PI3Kδ inhibitors, as patients in this trial had fewer occurrences of autoimmune-like toxicities, such as colitis.
“Preclinically, umbralisib has a very unique profile, selectively inhibiting both PI3Kδ and CK1ε,” said study author Owen O’Connor, MD, PhD, of Columbia Presbyterian Medical Center in New York, New York.
“The clinical results in this paper support our thesis that the differentiated preclinical profile explains the differences seen in the clinic between umbralisib and the other PI3Kδ inhibitors.”
Response
The objective response rate was 37%, with 33 patients achieving a response and 3 patients having a complete response (CR).
Sixteen CLL patients responded (80%), all with partial responses (PRs). Four DLBCL patients responded (31%), all with PRs. And 9 FL patients responded (53%), 2 with CRs.
The remaining CR occurred in a Hodgkin lymphoma patient, and this was the only response in this patient group.
One patient with marginal zone lymphoma had a PR, as did 1 patient with mantle cell lymphoma. All other patients had stable disease or progressed.
The mean duration of response was 13.4 months in the CLL patients, 6.4 months in the DLBCL patients, and 9.3 months in the FL patients.
Phase 1 trial results suggest umbralisib, a PI3Kδ/CK1ε inhibitor, can be safe and active in patients with relapsed or refractory B-cell malignancies.
Researchers said the safety profile of umbralisib “was distinct from that of other PI3Kδ inhibitors,” as it produced few immune-mediated adverse events (AEs).
Umbralisib also produced an objective response rate of 37% in the entire study cohort, 80% in patients with chronic lymphocytic leukemia (CLL), 53% in patients with follicular lymphoma (FL), and 31% in patients with diffuse large B-cell lymphoma (DLBCL).
These results were published in The Lancet Oncology. The study was sponsored by TG Therapeutics, Inc.
The trial enrolled 90 patients between January 17, 2013, and January 14, 2016.
There were 24 patients with CLL, 22 with FL, 16 with DLBCL, 11 with Hodgkin lymphoma, 6 with mantle cell lymphoma, 5 with marginal zone lymphoma, 3 with Waldenstrom’s macroglobulinemia, 2 with T-cell lymphoma, and 1 with hairy cell leukemia.
The median number of prior therapies was 3 (range, 2-5), and 49% of patients were refractory to previous therapy.
Treatment
Patients took umbralisib once daily in 28-day cycles until disease progression, unacceptable toxicity, or withdrawal of consent.
Initially, patients took the drug in a fasting state at doses of 50 mg, 100 mg, 200 mg, 400 mg, 800 mg, 1200 mg, or 1800 mg.
In April 2014, the researchers did a second dose-escalation with a micronized formulation of umbralisib, taken with food, at doses of 200 mg, 400 mg, 800 mg, 1200 mg, or 1800 mg.
In August, 2014, all patients who were still on the study transitioned to the 800 mg dose of the micronized formulation. This was the recommended phase 2 dose.
At the data cutoff in November 2016, 44 patients (49%) had received umbralisib for more than 6 cycles, and 23 (26%) had received the drug for more than 12 cycles. Thirteen patients (14%) were still taking umbralisib at the end of the study.
Most patients who stopped treatment did so because of disease progression (n=50, 56%) or AEs (n=9, 10%).
“We are pleased to have treated the first patient ever with umbralisib over 5 years ago and believe it has an important place in the treatment landscape for patients with hematologic malignancies,” said study author Howard A. Burris, MD, of the Sarah Cannon Research Institute in Nashville, Tennessee.
“Several patients from this phase 1 study are still on study today, approaching 5 years of continuous daily therapy, speaking to both the safety and efficacy profile of this unique agent.”
Safety
Dose-limiting toxicities (DLTs) occurred in 4 patients. One DLT was grade 3 maculopapular rash in a patient receiving the 800 mg dose of the initial formulation.
Another DLT was grade 3 hypokalemia in a patient receiving 1800 mg of the initial formulation. A third DLT was grade 3 fatigue, which occurred in 2 patients receiving 1800 mg of the micronized formulation.
Because of these toxicities, the maximum tolerated dose was 1200 mg of the micronized formulation.
The most common treatment-emergent AEs were diarrhea (43%), nausea (42%), and fatigue (31%). The most common grade 3/4 AEs were neutropenia (13%), anemia (9%), and thrombocytopenia (7%).
Serious AEs considered at least possibly related to umbralisib were pneumonia (3%), lung infection (1%), febrile neutropenia (1%), and colitis (2%).
Treatment discontinuation due to AEs considered at least possibly related to umbralisib occurred in 6 patients (7%). Two patients had grade 3 colitis, 2 had increased ALT/AST (grade 1 and grade 4), 1 had grade 2 diarrhea, and 1 had grade 3 fatigue.
There were no treatment-related deaths.
The researchers said the safety profile of umbralisib was distinct from that of other PI3Kδ inhibitors, as patients in this trial had fewer occurrences of autoimmune-like toxicities, such as colitis.
“Preclinically, umbralisib has a very unique profile, selectively inhibiting both PI3Kδ and CK1ε,” said study author Owen O’Connor, MD, PhD, of Columbia Presbyterian Medical Center in New York, New York.
“The clinical results in this paper support our thesis that the differentiated preclinical profile explains the differences seen in the clinic between umbralisib and the other PI3Kδ inhibitors.”
Response
The objective response rate was 37%, with 33 patients achieving a response and 3 patients having a complete response (CR).
Sixteen CLL patients responded (80%), all with partial responses (PRs). Four DLBCL patients responded (31%), all with PRs. And 9 FL patients responded (53%), 2 with CRs.
The remaining CR occurred in a Hodgkin lymphoma patient, and this was the only response in this patient group.
One patient with marginal zone lymphoma had a PR, as did 1 patient with mantle cell lymphoma. All other patients had stable disease or progressed.
The mean duration of response was 13.4 months in the CLL patients, 6.4 months in the DLBCL patients, and 9.3 months in the FL patients.
Adolescent suicidal ideation and attempts are on the rise
according to a retrospective analysis by Gregory Plemmons, MD, of Vanderbilt University, Nashville, Tenn., and his coinvestigators.
The researchers also found that suicidal ideation and suicide attempts occurred more often during the spring and fall than in the summer, coinciding with the academic school year, highlighting “the need for further research in the role that schools may play.”
The investigators distinguished three age groups corresponding with commonly accepted definitions of late childhood (5-11 years), early adolescence (12-14 years), and late adolescence (15-17 years). They also looked at differences according to patients’ race/ethnicity and sex, as well as month of the year of the admission.
There were increases in suicidal ideation or attempts across all three age groups, with 50% in late adolescence, 37% in early adolescence, and 13% in late childhood. They also found higher increases among non-Hispanic whites, compared with other races; nearly two-thirds of the suicidal ideation and suicide attempts were among girls.
Only 18.5% of total annual suicidal ideation and suicidal attempts occurred during summer months. Peaks were highest in fall and spring. “We underscore the need for future work to explore the relationship between school and suicidal ideation, recognizing that the role of academics is a complex one, and there may also be other additional influences at play regarding seasonality,” said Dr. Plemmons and his associates.
The investigators wrote that, although the reasons for these increasing trends among these age groups are not entirely clear, some have suggested the rise of cyberbullying and social media could be possible factors. This study and its data, though, “have important implications for exploring age- and sex-specific approaches to suicide screening and prevention interventions, as well as further research in examining causal factors for SI [suicidal ideation] and SA [suicide attempts],” they concluded.
SOURCE: Plemmons G et al. Pediatrics. 2018;141(6):e20172426.
according to a retrospective analysis by Gregory Plemmons, MD, of Vanderbilt University, Nashville, Tenn., and his coinvestigators.
The researchers also found that suicidal ideation and suicide attempts occurred more often during the spring and fall than in the summer, coinciding with the academic school year, highlighting “the need for further research in the role that schools may play.”
The investigators distinguished three age groups corresponding with commonly accepted definitions of late childhood (5-11 years), early adolescence (12-14 years), and late adolescence (15-17 years). They also looked at differences according to patients’ race/ethnicity and sex, as well as month of the year of the admission.
There were increases in suicidal ideation or attempts across all three age groups, with 50% in late adolescence, 37% in early adolescence, and 13% in late childhood. They also found higher increases among non-Hispanic whites, compared with other races; nearly two-thirds of the suicidal ideation and suicide attempts were among girls.
Only 18.5% of total annual suicidal ideation and suicidal attempts occurred during summer months. Peaks were highest in fall and spring. “We underscore the need for future work to explore the relationship between school and suicidal ideation, recognizing that the role of academics is a complex one, and there may also be other additional influences at play regarding seasonality,” said Dr. Plemmons and his associates.
The investigators wrote that, although the reasons for these increasing trends among these age groups are not entirely clear, some have suggested the rise of cyberbullying and social media could be possible factors. This study and its data, though, “have important implications for exploring age- and sex-specific approaches to suicide screening and prevention interventions, as well as further research in examining causal factors for SI [suicidal ideation] and SA [suicide attempts],” they concluded.
SOURCE: Plemmons G et al. Pediatrics. 2018;141(6):e20172426.
according to a retrospective analysis by Gregory Plemmons, MD, of Vanderbilt University, Nashville, Tenn., and his coinvestigators.
The researchers also found that suicidal ideation and suicide attempts occurred more often during the spring and fall than in the summer, coinciding with the academic school year, highlighting “the need for further research in the role that schools may play.”
The investigators distinguished three age groups corresponding with commonly accepted definitions of late childhood (5-11 years), early adolescence (12-14 years), and late adolescence (15-17 years). They also looked at differences according to patients’ race/ethnicity and sex, as well as month of the year of the admission.
There were increases in suicidal ideation or attempts across all three age groups, with 50% in late adolescence, 37% in early adolescence, and 13% in late childhood. They also found higher increases among non-Hispanic whites, compared with other races; nearly two-thirds of the suicidal ideation and suicide attempts were among girls.
Only 18.5% of total annual suicidal ideation and suicidal attempts occurred during summer months. Peaks were highest in fall and spring. “We underscore the need for future work to explore the relationship between school and suicidal ideation, recognizing that the role of academics is a complex one, and there may also be other additional influences at play regarding seasonality,” said Dr. Plemmons and his associates.
The investigators wrote that, although the reasons for these increasing trends among these age groups are not entirely clear, some have suggested the rise of cyberbullying and social media could be possible factors. This study and its data, though, “have important implications for exploring age- and sex-specific approaches to suicide screening and prevention interventions, as well as further research in examining causal factors for SI [suicidal ideation] and SA [suicide attempts],” they concluded.
SOURCE: Plemmons G et al. Pediatrics. 2018;141(6):e20172426.
FROM PEDIATRICS
At the AAPA conference? Visit Clinician Reviews at booth #917
We want to hear from you! If you’re in New Orleans for the AAPA conference, stop by the Clinician Reviews booth (#917) in the Exhibit Hall! We’re there from 9 am to 5 pm on Monday, May 21, and from 9 am to 2:30 pm on Tuesday, May 22.
At our booth, you can:
- Get a stamp for your Medical Pursuit card
- Meet our PA Editor-in-Chief, Randy D. Danielsen, PhD, PA, DFAAPA, on Monday (May 21) from 1 to 2:30 pm and Tuesday (May 22) from 11 am to 12:30 pm
- Talk to the editors about writing a clinical manuscript for publication
- Provide feedback on anything you’ve read in our print issue, on our website, or via our e-newsletters
We’re looking forward to chatting with you and learning how Clinician Reviews can be of continuing value to you in your practice!
We want to hear from you! If you’re in New Orleans for the AAPA conference, stop by the Clinician Reviews booth (#917) in the Exhibit Hall! We’re there from 9 am to 5 pm on Monday, May 21, and from 9 am to 2:30 pm on Tuesday, May 22.
At our booth, you can:
- Get a stamp for your Medical Pursuit card
- Meet our PA Editor-in-Chief, Randy D. Danielsen, PhD, PA, DFAAPA, on Monday (May 21) from 1 to 2:30 pm and Tuesday (May 22) from 11 am to 12:30 pm
- Talk to the editors about writing a clinical manuscript for publication
- Provide feedback on anything you’ve read in our print issue, on our website, or via our e-newsletters
We’re looking forward to chatting with you and learning how Clinician Reviews can be of continuing value to you in your practice!
We want to hear from you! If you’re in New Orleans for the AAPA conference, stop by the Clinician Reviews booth (#917) in the Exhibit Hall! We’re there from 9 am to 5 pm on Monday, May 21, and from 9 am to 2:30 pm on Tuesday, May 22.
At our booth, you can:
- Get a stamp for your Medical Pursuit card
- Meet our PA Editor-in-Chief, Randy D. Danielsen, PhD, PA, DFAAPA, on Monday (May 21) from 1 to 2:30 pm and Tuesday (May 22) from 11 am to 12:30 pm
- Talk to the editors about writing a clinical manuscript for publication
- Provide feedback on anything you’ve read in our print issue, on our website, or via our e-newsletters
We’re looking forward to chatting with you and learning how Clinician Reviews can be of continuing value to you in your practice!
All-Terrain, No Control
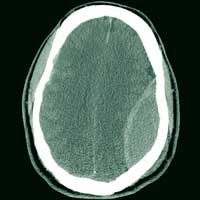
ANSWER
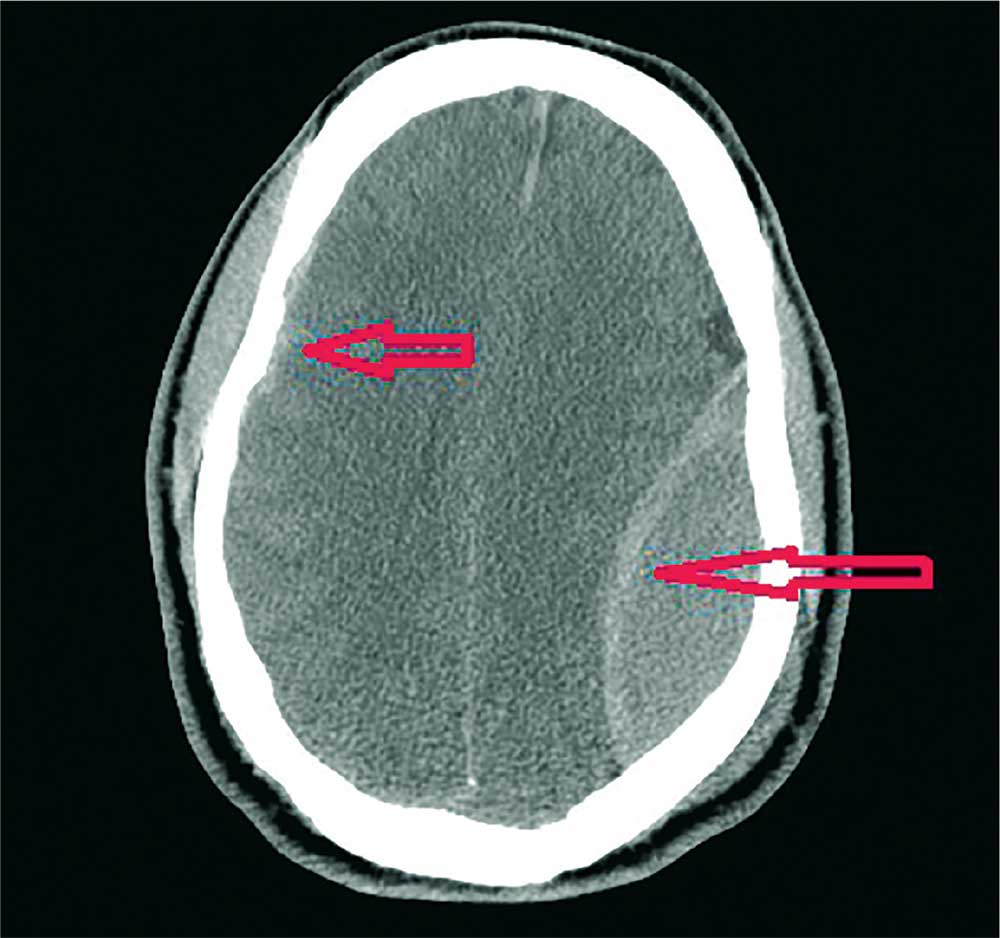
The image shows a large, convex hyperdensity within the left parietal region. This is a textbook image of an acute epidural hematoma. There is considerable mass effect and evidence of left-to-right shift. Windowing shows an underlying fracture, which is typically associated with these types of hemorrhages.
There is also evidence of a right-side concave hyperdensity, consistent with an acute subdural hematoma. Typically, this is referred to as a contrecoup injury.
The patient was transported to the operating room for an emergent left craniotomy for epidural evacuation; he recovered uneventfully.

ANSWER
The image shows a large, convex hyperdensity within the left parietal region. This is a textbook image of an acute epidural hematoma. There is considerable mass effect and evidence of left-to-right shift. Windowing shows an underlying fracture, which is typically associated with these types of hemorrhages.
There is also evidence of a right-side concave hyperdensity, consistent with an acute subdural hematoma. Typically, this is referred to as a contrecoup injury.
The patient was transported to the operating room for an emergent left craniotomy for epidural evacuation; he recovered uneventfully.

ANSWER
The image shows a large, convex hyperdensity within the left parietal region. This is a textbook image of an acute epidural hematoma. There is considerable mass effect and evidence of left-to-right shift. Windowing shows an underlying fracture, which is typically associated with these types of hemorrhages.
There is also evidence of a right-side concave hyperdensity, consistent with an acute subdural hematoma. Typically, this is referred to as a contrecoup injury.
The patient was transported to the operating room for an emergent left craniotomy for epidural evacuation; he recovered uneventfully.
A 40-year-old man is brought to the emergency department (ED) with a suspected intracranial hemorrhage after being thrown off an all-terrain vehicle. He was reportedly riding the vehicle without a helmet when he somehow lost control; the accident itself was unwitnessed.
En route to the ED, he was reportedly confused but hemodynamically stable, with a Glasgow Coma Scale score of 13-14. He lost consciousness while in the CT scanner, requiring emergent intubation for airway protection.
When you arrive to assess him, you note an intubated male with stable vital signs. The pupils display slight anisocoria but equally react. The patient withdraws in all four extremities secondary to pain, with slight posturing.
Noncontrast CT of the head is obtained, a static image from which is shown. What is your impression?
AACE 2018: A dream team of presenters
Boston is the location and inspiration for the featured presentations at the annual meeting of the American Association of Clinical Endocrinologists, program chair Vin Tangpricha, MD, PhD, said in an interview.
The program agenda for the congress, held May 16-20, boasts 143 speakers, 66 distinct clinical endocrinology educational sessions, and an opening plenary presentation featuring one of modern medicine’s most renowned diabetes and obesity researchers, according to a statement from the AACE.
New Dimensions in Insulin Action and Why They Are Important to Know
C. Ronald Kahn, MD, chief academic officer and head of Integrative Physiology and Metabolism at Joslin Diabetes Center in Boston, pioneered revolutionary work with insulin receptors and insulin resistance in diabetes and obesity. What makes his presentation on Thursday from 8:30 a.m. to 9:15 a.m. a must-see is its focus on the future: “Many new drugs are being developed based on the research on how insulin works. This lecture will be exciting to hear what is in the pipeline for drugs that manipulate insulin action,” Dr. Tangpricha said.
Cushing’s Syndrome
Beta-Cell Regeneration
The work of Andrew F. Stewart, MD, scientific director of the Mount Sinai Diabetes, Obesity and Metabolism Institute in New York, leads research into the basic mechanisms, prevention, and treatment of metabolic diseases. “In the past, we thought that there was a fixed number of beta cells in the body. However, recent research by Dr. Stewart’s group suggests that beta cells can be stimulated to grow. This is very exciting and can shape the future of how we take care of diabetes,” noted Dr. Tangpricha. The session is on Friday from 8:20 a.m. to 9:05 a.m.
New Insights Into Thyroid Hormone Action
On Thursday from 11:15 a.m. to 12:00 p.m., Anthony N. Hollenberg, MD, will present “an outstanding review on thyroid hormone and action and how this impacts patient care of those with thyroid disease,” Dr. Tangpricha said. Dr. Hollenberg is chief of the thyroid unit and the division of endocrinology, diabetes, and metabolism at Beth Israel Deaconess Medical Center in Boston.
Current and Evolving Approaches for Osteoporosis Treatment
Sundeep Khosla, MD, an expert on bone loss, will distill the myriad osteoporosis treatments into useful information that can inform your practice now. “There have been a number of drugs that have been released for the treatment of osteoporosis. We are now in an era where we can consider using drugs targeted for specific populations or specific combinations,” Dr. Tangpricha commented. This session is on Saturday at 8:15 a.m. to 9:00 a.m.
Boston is the location and inspiration for the featured presentations at the annual meeting of the American Association of Clinical Endocrinologists, program chair Vin Tangpricha, MD, PhD, said in an interview.
The program agenda for the congress, held May 16-20, boasts 143 speakers, 66 distinct clinical endocrinology educational sessions, and an opening plenary presentation featuring one of modern medicine’s most renowned diabetes and obesity researchers, according to a statement from the AACE.
New Dimensions in Insulin Action and Why They Are Important to Know
C. Ronald Kahn, MD, chief academic officer and head of Integrative Physiology and Metabolism at Joslin Diabetes Center in Boston, pioneered revolutionary work with insulin receptors and insulin resistance in diabetes and obesity. What makes his presentation on Thursday from 8:30 a.m. to 9:15 a.m. a must-see is its focus on the future: “Many new drugs are being developed based on the research on how insulin works. This lecture will be exciting to hear what is in the pipeline for drugs that manipulate insulin action,” Dr. Tangpricha said.
Cushing’s Syndrome
Beta-Cell Regeneration
The work of Andrew F. Stewart, MD, scientific director of the Mount Sinai Diabetes, Obesity and Metabolism Institute in New York, leads research into the basic mechanisms, prevention, and treatment of metabolic diseases. “In the past, we thought that there was a fixed number of beta cells in the body. However, recent research by Dr. Stewart’s group suggests that beta cells can be stimulated to grow. This is very exciting and can shape the future of how we take care of diabetes,” noted Dr. Tangpricha. The session is on Friday from 8:20 a.m. to 9:05 a.m.
New Insights Into Thyroid Hormone Action
On Thursday from 11:15 a.m. to 12:00 p.m., Anthony N. Hollenberg, MD, will present “an outstanding review on thyroid hormone and action and how this impacts patient care of those with thyroid disease,” Dr. Tangpricha said. Dr. Hollenberg is chief of the thyroid unit and the division of endocrinology, diabetes, and metabolism at Beth Israel Deaconess Medical Center in Boston.
Current and Evolving Approaches for Osteoporosis Treatment
Sundeep Khosla, MD, an expert on bone loss, will distill the myriad osteoporosis treatments into useful information that can inform your practice now. “There have been a number of drugs that have been released for the treatment of osteoporosis. We are now in an era where we can consider using drugs targeted for specific populations or specific combinations,” Dr. Tangpricha commented. This session is on Saturday at 8:15 a.m. to 9:00 a.m.
Boston is the location and inspiration for the featured presentations at the annual meeting of the American Association of Clinical Endocrinologists, program chair Vin Tangpricha, MD, PhD, said in an interview.
The program agenda for the congress, held May 16-20, boasts 143 speakers, 66 distinct clinical endocrinology educational sessions, and an opening plenary presentation featuring one of modern medicine’s most renowned diabetes and obesity researchers, according to a statement from the AACE.
New Dimensions in Insulin Action and Why They Are Important to Know
C. Ronald Kahn, MD, chief academic officer and head of Integrative Physiology and Metabolism at Joslin Diabetes Center in Boston, pioneered revolutionary work with insulin receptors and insulin resistance in diabetes and obesity. What makes his presentation on Thursday from 8:30 a.m. to 9:15 a.m. a must-see is its focus on the future: “Many new drugs are being developed based on the research on how insulin works. This lecture will be exciting to hear what is in the pipeline for drugs that manipulate insulin action,” Dr. Tangpricha said.
Cushing’s Syndrome
Beta-Cell Regeneration
The work of Andrew F. Stewart, MD, scientific director of the Mount Sinai Diabetes, Obesity and Metabolism Institute in New York, leads research into the basic mechanisms, prevention, and treatment of metabolic diseases. “In the past, we thought that there was a fixed number of beta cells in the body. However, recent research by Dr. Stewart’s group suggests that beta cells can be stimulated to grow. This is very exciting and can shape the future of how we take care of diabetes,” noted Dr. Tangpricha. The session is on Friday from 8:20 a.m. to 9:05 a.m.
New Insights Into Thyroid Hormone Action
On Thursday from 11:15 a.m. to 12:00 p.m., Anthony N. Hollenberg, MD, will present “an outstanding review on thyroid hormone and action and how this impacts patient care of those with thyroid disease,” Dr. Tangpricha said. Dr. Hollenberg is chief of the thyroid unit and the division of endocrinology, diabetes, and metabolism at Beth Israel Deaconess Medical Center in Boston.
Current and Evolving Approaches for Osteoporosis Treatment
Sundeep Khosla, MD, an expert on bone loss, will distill the myriad osteoporosis treatments into useful information that can inform your practice now. “There have been a number of drugs that have been released for the treatment of osteoporosis. We are now in an era where we can consider using drugs targeted for specific populations or specific combinations,” Dr. Tangpricha commented. This session is on Saturday at 8:15 a.m. to 9:00 a.m.
FROM AACE 2018
Circadian Dysfunction Linked with Migraine Severity
Circadian misalignment and delayed sleep timing are associated with higher migraine frequency and severity that was not better accounted for by the amount of sleep, according to a recent study. Twenty women with chronic migraine (CM) and 20 age‐matched healthy controls (HC) completed a protocol that included a 7-day sleep assessment at home using wrist actigraphy followed by a circadian phase assessment using salivary melatonin. Researchers compared CM vs HC on sleep parameters and circadian factors. Subsequently, they examined associations between dim‐light melatonin onset (DLMO), the midpoint of the sleep episode, and the phase angle (time from DLMO to sleep midpoint) with the number of migraine days per month and the migraine disability assessment scale (MIDAS). They found:
- CM and HC did not differ on measures of sleep or circadian phase.
- Within the CM group, more frequent migraine days per month was significantly correlated with DLMO (r = .49) and later sleep episode (r = .47).
- In addition, a greater phase angle (ie, circadian misalignment) was significantly correlated with more severe migraine‐related disability (r = .48).
- These relationships remained significant after adjusting for total sleep time.
Can Circadian dysregulation exacerbate migraines? [Published online ahead of print May 4, 2018]. Headache. doi:10.1111/head.13310.
Circadian misalignment and delayed sleep timing are associated with higher migraine frequency and severity that was not better accounted for by the amount of sleep, according to a recent study. Twenty women with chronic migraine (CM) and 20 age‐matched healthy controls (HC) completed a protocol that included a 7-day sleep assessment at home using wrist actigraphy followed by a circadian phase assessment using salivary melatonin. Researchers compared CM vs HC on sleep parameters and circadian factors. Subsequently, they examined associations between dim‐light melatonin onset (DLMO), the midpoint of the sleep episode, and the phase angle (time from DLMO to sleep midpoint) with the number of migraine days per month and the migraine disability assessment scale (MIDAS). They found:
- CM and HC did not differ on measures of sleep or circadian phase.
- Within the CM group, more frequent migraine days per month was significantly correlated with DLMO (r = .49) and later sleep episode (r = .47).
- In addition, a greater phase angle (ie, circadian misalignment) was significantly correlated with more severe migraine‐related disability (r = .48).
- These relationships remained significant after adjusting for total sleep time.
Can Circadian dysregulation exacerbate migraines? [Published online ahead of print May 4, 2018]. Headache. doi:10.1111/head.13310.
Circadian misalignment and delayed sleep timing are associated with higher migraine frequency and severity that was not better accounted for by the amount of sleep, according to a recent study. Twenty women with chronic migraine (CM) and 20 age‐matched healthy controls (HC) completed a protocol that included a 7-day sleep assessment at home using wrist actigraphy followed by a circadian phase assessment using salivary melatonin. Researchers compared CM vs HC on sleep parameters and circadian factors. Subsequently, they examined associations between dim‐light melatonin onset (DLMO), the midpoint of the sleep episode, and the phase angle (time from DLMO to sleep midpoint) with the number of migraine days per month and the migraine disability assessment scale (MIDAS). They found:
- CM and HC did not differ on measures of sleep or circadian phase.
- Within the CM group, more frequent migraine days per month was significantly correlated with DLMO (r = .49) and later sleep episode (r = .47).
- In addition, a greater phase angle (ie, circadian misalignment) was significantly correlated with more severe migraine‐related disability (r = .48).
- These relationships remained significant after adjusting for total sleep time.
Can Circadian dysregulation exacerbate migraines? [Published online ahead of print May 4, 2018]. Headache. doi:10.1111/head.13310.
Carotenoderma Associated With a Diet Rich in Red Palm Oil
To the Editor:
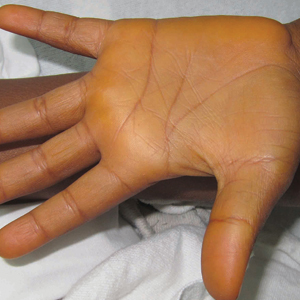
Carotenoderma is a cutaneous manifestation of elevated serum β-carotene levels and classically localizes to fatty tissues and areas rich in sweat glands. We present a case of carotenoderma associated with a diet rich in red palm oil, a common food additive in parts of the world outside of the United States.
A previously healthy 8-year-old boy who recently immigrated to the United States from Liberia was hospitalized for treatment of a febrile illness that subsequently was attributed to a viral syndrome. On physical examination by the dermatology department, the patient was noted to have marked orange discoloration on the palms and soles (Figure). Laboratory workup revealed elevated serum β-carotene levels of 809 μg/dL (reference range, 10–85 μg/dL). Testing of hemoglobin/hematocrit levels and liver, thyroid, and kidney function was normal, and systemic examination revealed no further abnormalities. Upon further inquiry by the dermatology department, the patient’s family reported frequent addition of red palm oil to all of the child’s meals. The patient subsequently was diagnosed with carotenoderma and was instructed to limit inclusion of red palm oil in his diet.

Red palm oil is a rich source of β-carotene and is commonly used outside the United States as a dietary supplement or food flavoring. Excessive consumption of red palm oil or other sources rich in carotenes can result in elevated serum carotene levels or hypercarotenemia. An elevation in serum β-carotene levels may be recognized from 4 to 7 weeks after starting a β-carotene–rich diet.1
While dietary consumption of carotenes is the most common cause of carotenoderma, others include kidney or liver disease, hyperlipidemia, porphyria, diabetes mellitus, hypothyroidism, and anorexia nervosa.2-4 Moreover, since carotenoids are enzymatically converted to vitamin A in the small intestine, a mutation of the gene of the conversion enzyme β-carotene 15,15’-monooxygenase 1 (BCMO1) also can cause be a rare cause of hypercarotenemia.3
Carotenoderma, the clinical cutaneous manifestation of hypercarotenemia, occurs as a result of β-carotene deposits in the skin when serum concentration exceeds 250 μg/dL. More specifically, β-carotene accumulates mainly in the lipid-rich stratum corneum as well as in sweat and sebum, which explains the localized discoloration in fatty tissues and areas rich in sweat glands (eg, nasolabial folds, palms, soles).3,4 The sclerae of the eyes are not affected by the surplus of β-carotene in carotenoderma, which helps distinguish it from jaundice.5
The differential diagnosis of yellow discoloration of the skin includes jaundice, encompassing the prehepatic, hepatocellular, and posthepatic categories.4 Also noteworthy in the differential diagnosis is lycopenemia, which occurs as a result of eating lycopene-rich foods (eg, tomatoes), resulting in a deeper orange-yellow pigmentation when compared to the cutaneous manifestation of hypercarotenemia.2,4,6 Several drugs also have been reported to induce yellow discoloration of the skin, including sunitinib,7 sorafenib,8 quinacrine, saffron supplements, santonin, fluorescein, 2,4-dinitrophenol, canthaxanthin, tetryl and picric acids, and acriflavine.2,4
Carotenoderma caused by a diet rich in β-carotene is a benign condition in which a diet low in β-carotene is implicated for treatment. Contrary to popular belief, vitamin A toxicity does not occur in the presence of a surplus of β-carotenes because the enzymatic conversion of β-carotene to vitamin A is strictly regulated.9 Although acknowledging the various causes of carotenoderma is important, a simple history and laboratory testing for elevated serum β-carotene levels can eliminate further unnecessary testing and allow for prompt recognition of the condition. Appropriate dietary modifications also may be warranted.
- Roe DA. Assessment of risk factors for carotenodermia and cutaneous signs of hypervitaminosis A in college-aged populations. Semin Dermatol. 1991;10:303-308.
- Manolios N, Samaras K. Hypercarotenaemia. Intern Med J. 2006;36:534.
- Wageesha ND, Ekanayake S, Jansz ER, et al. Studies on hypercarotenemia due to excessive ingestion of carrot, pumpkin and papaw [published online September 27, 2010]. Int J Food Sci Nutr. 2011;62:20-25.
- Maharshak N, Shapiro J, Trau H. Carotenoderma—a review of the current literature. Int J Dermatol. 2003;42:178-181.
- Maruani A, Labarthe F, Dupré T, et al. Hypercarotenaemia in an infant [in French]. Ann Dermatol Venereol. 2010;137:32-35.
- Shaw JA, Koti M. Clinical images. CMAJ. 2009;180:895.
- Vignand-Courtin C, Martin C, Le Beller C, et al. Cutaneous side effects associated with sunitinib: an analysis of 8 cases. Int J Clin Pharm. 2012;34:286-289.
- Dasanu CA, Alexandrescu DT, Dutcher J. Yellow skin discoloration associated with sorafenib use for treatment of metastatic renal cell carcinoma. South Med J. 2007;100:328-330.
- Lascari AD. Carotenemia. a review. Clin Pediatr (Phila). 1981;20:25-29.
To the Editor:
Carotenoderma is a cutaneous manifestation of elevated serum β-carotene levels and classically localizes to fatty tissues and areas rich in sweat glands. We present a case of carotenoderma associated with a diet rich in red palm oil, a common food additive in parts of the world outside of the United States.
A previously healthy 8-year-old boy who recently immigrated to the United States from Liberia was hospitalized for treatment of a febrile illness that subsequently was attributed to a viral syndrome. On physical examination by the dermatology department, the patient was noted to have marked orange discoloration on the palms and soles (Figure). Laboratory workup revealed elevated serum β-carotene levels of 809 μg/dL (reference range, 10–85 μg/dL). Testing of hemoglobin/hematocrit levels and liver, thyroid, and kidney function was normal, and systemic examination revealed no further abnormalities. Upon further inquiry by the dermatology department, the patient’s family reported frequent addition of red palm oil to all of the child’s meals. The patient subsequently was diagnosed with carotenoderma and was instructed to limit inclusion of red palm oil in his diet.

Red palm oil is a rich source of β-carotene and is commonly used outside the United States as a dietary supplement or food flavoring. Excessive consumption of red palm oil or other sources rich in carotenes can result in elevated serum carotene levels or hypercarotenemia. An elevation in serum β-carotene levels may be recognized from 4 to 7 weeks after starting a β-carotene–rich diet.1
While dietary consumption of carotenes is the most common cause of carotenoderma, others include kidney or liver disease, hyperlipidemia, porphyria, diabetes mellitus, hypothyroidism, and anorexia nervosa.2-4 Moreover, since carotenoids are enzymatically converted to vitamin A in the small intestine, a mutation of the gene of the conversion enzyme β-carotene 15,15’-monooxygenase 1 (BCMO1) also can cause be a rare cause of hypercarotenemia.3
Carotenoderma, the clinical cutaneous manifestation of hypercarotenemia, occurs as a result of β-carotene deposits in the skin when serum concentration exceeds 250 μg/dL. More specifically, β-carotene accumulates mainly in the lipid-rich stratum corneum as well as in sweat and sebum, which explains the localized discoloration in fatty tissues and areas rich in sweat glands (eg, nasolabial folds, palms, soles).3,4 The sclerae of the eyes are not affected by the surplus of β-carotene in carotenoderma, which helps distinguish it from jaundice.5
The differential diagnosis of yellow discoloration of the skin includes jaundice, encompassing the prehepatic, hepatocellular, and posthepatic categories.4 Also noteworthy in the differential diagnosis is lycopenemia, which occurs as a result of eating lycopene-rich foods (eg, tomatoes), resulting in a deeper orange-yellow pigmentation when compared to the cutaneous manifestation of hypercarotenemia.2,4,6 Several drugs also have been reported to induce yellow discoloration of the skin, including sunitinib,7 sorafenib,8 quinacrine, saffron supplements, santonin, fluorescein, 2,4-dinitrophenol, canthaxanthin, tetryl and picric acids, and acriflavine.2,4
Carotenoderma caused by a diet rich in β-carotene is a benign condition in which a diet low in β-carotene is implicated for treatment. Contrary to popular belief, vitamin A toxicity does not occur in the presence of a surplus of β-carotenes because the enzymatic conversion of β-carotene to vitamin A is strictly regulated.9 Although acknowledging the various causes of carotenoderma is important, a simple history and laboratory testing for elevated serum β-carotene levels can eliminate further unnecessary testing and allow for prompt recognition of the condition. Appropriate dietary modifications also may be warranted.
To the Editor:
Carotenoderma is a cutaneous manifestation of elevated serum β-carotene levels and classically localizes to fatty tissues and areas rich in sweat glands. We present a case of carotenoderma associated with a diet rich in red palm oil, a common food additive in parts of the world outside of the United States.
A previously healthy 8-year-old boy who recently immigrated to the United States from Liberia was hospitalized for treatment of a febrile illness that subsequently was attributed to a viral syndrome. On physical examination by the dermatology department, the patient was noted to have marked orange discoloration on the palms and soles (Figure). Laboratory workup revealed elevated serum β-carotene levels of 809 μg/dL (reference range, 10–85 μg/dL). Testing of hemoglobin/hematocrit levels and liver, thyroid, and kidney function was normal, and systemic examination revealed no further abnormalities. Upon further inquiry by the dermatology department, the patient’s family reported frequent addition of red palm oil to all of the child’s meals. The patient subsequently was diagnosed with carotenoderma and was instructed to limit inclusion of red palm oil in his diet.

Red palm oil is a rich source of β-carotene and is commonly used outside the United States as a dietary supplement or food flavoring. Excessive consumption of red palm oil or other sources rich in carotenes can result in elevated serum carotene levels or hypercarotenemia. An elevation in serum β-carotene levels may be recognized from 4 to 7 weeks after starting a β-carotene–rich diet.1
While dietary consumption of carotenes is the most common cause of carotenoderma, others include kidney or liver disease, hyperlipidemia, porphyria, diabetes mellitus, hypothyroidism, and anorexia nervosa.2-4 Moreover, since carotenoids are enzymatically converted to vitamin A in the small intestine, a mutation of the gene of the conversion enzyme β-carotene 15,15’-monooxygenase 1 (BCMO1) also can cause be a rare cause of hypercarotenemia.3
Carotenoderma, the clinical cutaneous manifestation of hypercarotenemia, occurs as a result of β-carotene deposits in the skin when serum concentration exceeds 250 μg/dL. More specifically, β-carotene accumulates mainly in the lipid-rich stratum corneum as well as in sweat and sebum, which explains the localized discoloration in fatty tissues and areas rich in sweat glands (eg, nasolabial folds, palms, soles).3,4 The sclerae of the eyes are not affected by the surplus of β-carotene in carotenoderma, which helps distinguish it from jaundice.5
The differential diagnosis of yellow discoloration of the skin includes jaundice, encompassing the prehepatic, hepatocellular, and posthepatic categories.4 Also noteworthy in the differential diagnosis is lycopenemia, which occurs as a result of eating lycopene-rich foods (eg, tomatoes), resulting in a deeper orange-yellow pigmentation when compared to the cutaneous manifestation of hypercarotenemia.2,4,6 Several drugs also have been reported to induce yellow discoloration of the skin, including sunitinib,7 sorafenib,8 quinacrine, saffron supplements, santonin, fluorescein, 2,4-dinitrophenol, canthaxanthin, tetryl and picric acids, and acriflavine.2,4
Carotenoderma caused by a diet rich in β-carotene is a benign condition in which a diet low in β-carotene is implicated for treatment. Contrary to popular belief, vitamin A toxicity does not occur in the presence of a surplus of β-carotenes because the enzymatic conversion of β-carotene to vitamin A is strictly regulated.9 Although acknowledging the various causes of carotenoderma is important, a simple history and laboratory testing for elevated serum β-carotene levels can eliminate further unnecessary testing and allow for prompt recognition of the condition. Appropriate dietary modifications also may be warranted.
- Roe DA. Assessment of risk factors for carotenodermia and cutaneous signs of hypervitaminosis A in college-aged populations. Semin Dermatol. 1991;10:303-308.
- Manolios N, Samaras K. Hypercarotenaemia. Intern Med J. 2006;36:534.
- Wageesha ND, Ekanayake S, Jansz ER, et al. Studies on hypercarotenemia due to excessive ingestion of carrot, pumpkin and papaw [published online September 27, 2010]. Int J Food Sci Nutr. 2011;62:20-25.
- Maharshak N, Shapiro J, Trau H. Carotenoderma—a review of the current literature. Int J Dermatol. 2003;42:178-181.
- Maruani A, Labarthe F, Dupré T, et al. Hypercarotenaemia in an infant [in French]. Ann Dermatol Venereol. 2010;137:32-35.
- Shaw JA, Koti M. Clinical images. CMAJ. 2009;180:895.
- Vignand-Courtin C, Martin C, Le Beller C, et al. Cutaneous side effects associated with sunitinib: an analysis of 8 cases. Int J Clin Pharm. 2012;34:286-289.
- Dasanu CA, Alexandrescu DT, Dutcher J. Yellow skin discoloration associated with sorafenib use for treatment of metastatic renal cell carcinoma. South Med J. 2007;100:328-330.
- Lascari AD. Carotenemia. a review. Clin Pediatr (Phila). 1981;20:25-29.
- Roe DA. Assessment of risk factors for carotenodermia and cutaneous signs of hypervitaminosis A in college-aged populations. Semin Dermatol. 1991;10:303-308.
- Manolios N, Samaras K. Hypercarotenaemia. Intern Med J. 2006;36:534.
- Wageesha ND, Ekanayake S, Jansz ER, et al. Studies on hypercarotenemia due to excessive ingestion of carrot, pumpkin and papaw [published online September 27, 2010]. Int J Food Sci Nutr. 2011;62:20-25.
- Maharshak N, Shapiro J, Trau H. Carotenoderma—a review of the current literature. Int J Dermatol. 2003;42:178-181.
- Maruani A, Labarthe F, Dupré T, et al. Hypercarotenaemia in an infant [in French]. Ann Dermatol Venereol. 2010;137:32-35.
- Shaw JA, Koti M. Clinical images. CMAJ. 2009;180:895.
- Vignand-Courtin C, Martin C, Le Beller C, et al. Cutaneous side effects associated with sunitinib: an analysis of 8 cases. Int J Clin Pharm. 2012;34:286-289.
- Dasanu CA, Alexandrescu DT, Dutcher J. Yellow skin discoloration associated with sorafenib use for treatment of metastatic renal cell carcinoma. South Med J. 2007;100:328-330.
- Lascari AD. Carotenemia. a review. Clin Pediatr (Phila). 1981;20:25-29.
Practice Points
- Carotenoderma is a cutaneous manifestation of elevated serum β-carotene levels and classically localizes to fatty tissues and areas rich in sweat glands.
- Carotenoderma caused by a diet rich in β-carotene is a benign condition in which a diet low in β-carotene is implicated for treatment.
How to harness value-based care codes
Many of you reading this column joined Medicare accountable care organizations (ACOs) sometime between 2011 and 2016. As the power of prevention, wellness, and the medical home model are starting to be realized and appreciated, those benefits may be swamped by two new Centers for Medicare and Medicaid Services value-based revenue streams that did not exist when many of you first joined your ACO.
The Medicare Access and CHIP Reauthorization Act (MACRA) was passed in 2015 and is just now being implemented. Value-based, fee-for-service payments started out rather modestly a few years ago as chronic care management codes, but they have exploded to include more than 20 codes, counting the new ones coming online in 2018. Let’s call them collectively value-based care codes, or VCCs.
Many practices are trying to understand and perform the basic requirements to avoid penalties under MACRA’s Merit-based Incentive Payment System (MIPS) program. Some primary care practices, however, see the upside potential and bonuses stacking up to 30% or more.
Did you know that even if you are in, let’s say, a basic Medicare Shared Savings Program ACO – the MSSP Track 1, with no exposure to risk – you get special treatment on reporting under MACRA as a MIPS Advanced Practice Model (APM)?
But more importantly, MACRA is a team game. Getting into an MSSP Track 1 is justified just to get practice for the care coordination you’ll need. Few physicians know that they are judged under MACRA MIPS for the total costs of their patients, not just their own costs. A primary care physician receives only up to 8% of the $10 million your patients consume on average. The best way to counter that is through an ACO.
Further, we are aware of ACOs that have chosen risk-taking Medicare models such as NextGen, even though they predict small losses. Those losses are because of the automatic 5% fee-for-service payment bump to its physicians for risk taking if they are in a MACRA Advanced Alternative Payment Model (AAPM).
There’s a wide range of primary care physicians who are seizing opportunities offered by VCCs.
A family physician friend of mine who practices in a rural area generated more than 50% of his revenue from value-based care coding last year. And he has personally generated more than $350,000 in additional annual revenue, not counting the revenue from additional medically necessary procedures revealed by this more proactive wellness assessment activity and early diagnoses.
On the other hand, because busy physicians have a hard time wading through all these regulations and implementing the required staff and technology changes, it is reported that only about 8% of physicians are employing even the chronic care management codes. And when they do, they only achieve an 18% eligible patient penetration. My friend has broken the code, so to speak; he has protocolized and templated the process, has happy patients, has an ongoing 93% penetration rate, and actually has more free time.
While you are busy saving lives, I have had the luxury of looking from a high level at these tectonic, value-based payment shifts. To me, it’s a no-brainer for a primary care physician to leverage their ACO to maximize all three revenue streams. Look at MACRA MIPS, MIPS-APM, and AAPM measures anew, and see how well they play into integrated care.
As quarterback of health care through the patient-centered medical home, you are in great position to drive substantial bonuses. Similarly, when one looks at VCCs, the ACO can: help you navigate through the paperwork, perform much of the required reporting, and select the highest value-adding initiatives to monitor and drive higher quality and shared savings for the ACO.
As readers know, we firmly believe that, to have sustained incentivization, every ACO needs to have a merit-based, shared savings distribution formula. Accordingly, your compensation should rise under MACRA, VCCs, and the ACO.
This shift to value care is hard. But your colleagues who have made these changes are enjoying practice as never before. Their professional and financial rewards have climbed. But, most important, their patients love it.
Mr. Bobbitt is head of the health law group at the Smith Anderson law firm in Raleigh, N.C. He is president of Value Health Partners, a health care strategic consulting company. He has years of experience assisting physicians form integrated delivery systems. He has spoken and written nationally to primary care physicians on the strategies and practicalities of forming or joining ACOs. This article is meant to be educational and does not constitute legal advice. For additional information, readers may contact the author at [email protected] or 919-821-6612.
Many of you reading this column joined Medicare accountable care organizations (ACOs) sometime between 2011 and 2016. As the power of prevention, wellness, and the medical home model are starting to be realized and appreciated, those benefits may be swamped by two new Centers for Medicare and Medicaid Services value-based revenue streams that did not exist when many of you first joined your ACO.
The Medicare Access and CHIP Reauthorization Act (MACRA) was passed in 2015 and is just now being implemented. Value-based, fee-for-service payments started out rather modestly a few years ago as chronic care management codes, but they have exploded to include more than 20 codes, counting the new ones coming online in 2018. Let’s call them collectively value-based care codes, or VCCs.
Many practices are trying to understand and perform the basic requirements to avoid penalties under MACRA’s Merit-based Incentive Payment System (MIPS) program. Some primary care practices, however, see the upside potential and bonuses stacking up to 30% or more.
Did you know that even if you are in, let’s say, a basic Medicare Shared Savings Program ACO – the MSSP Track 1, with no exposure to risk – you get special treatment on reporting under MACRA as a MIPS Advanced Practice Model (APM)?
But more importantly, MACRA is a team game. Getting into an MSSP Track 1 is justified just to get practice for the care coordination you’ll need. Few physicians know that they are judged under MACRA MIPS for the total costs of their patients, not just their own costs. A primary care physician receives only up to 8% of the $10 million your patients consume on average. The best way to counter that is through an ACO.
Further, we are aware of ACOs that have chosen risk-taking Medicare models such as NextGen, even though they predict small losses. Those losses are because of the automatic 5% fee-for-service payment bump to its physicians for risk taking if they are in a MACRA Advanced Alternative Payment Model (AAPM).
There’s a wide range of primary care physicians who are seizing opportunities offered by VCCs.
A family physician friend of mine who practices in a rural area generated more than 50% of his revenue from value-based care coding last year. And he has personally generated more than $350,000 in additional annual revenue, not counting the revenue from additional medically necessary procedures revealed by this more proactive wellness assessment activity and early diagnoses.
On the other hand, because busy physicians have a hard time wading through all these regulations and implementing the required staff and technology changes, it is reported that only about 8% of physicians are employing even the chronic care management codes. And when they do, they only achieve an 18% eligible patient penetration. My friend has broken the code, so to speak; he has protocolized and templated the process, has happy patients, has an ongoing 93% penetration rate, and actually has more free time.
While you are busy saving lives, I have had the luxury of looking from a high level at these tectonic, value-based payment shifts. To me, it’s a no-brainer for a primary care physician to leverage their ACO to maximize all three revenue streams. Look at MACRA MIPS, MIPS-APM, and AAPM measures anew, and see how well they play into integrated care.
As quarterback of health care through the patient-centered medical home, you are in great position to drive substantial bonuses. Similarly, when one looks at VCCs, the ACO can: help you navigate through the paperwork, perform much of the required reporting, and select the highest value-adding initiatives to monitor and drive higher quality and shared savings for the ACO.
As readers know, we firmly believe that, to have sustained incentivization, every ACO needs to have a merit-based, shared savings distribution formula. Accordingly, your compensation should rise under MACRA, VCCs, and the ACO.
This shift to value care is hard. But your colleagues who have made these changes are enjoying practice as never before. Their professional and financial rewards have climbed. But, most important, their patients love it.
Mr. Bobbitt is head of the health law group at the Smith Anderson law firm in Raleigh, N.C. He is president of Value Health Partners, a health care strategic consulting company. He has years of experience assisting physicians form integrated delivery systems. He has spoken and written nationally to primary care physicians on the strategies and practicalities of forming or joining ACOs. This article is meant to be educational and does not constitute legal advice. For additional information, readers may contact the author at [email protected] or 919-821-6612.
Many of you reading this column joined Medicare accountable care organizations (ACOs) sometime between 2011 and 2016. As the power of prevention, wellness, and the medical home model are starting to be realized and appreciated, those benefits may be swamped by two new Centers for Medicare and Medicaid Services value-based revenue streams that did not exist when many of you first joined your ACO.
The Medicare Access and CHIP Reauthorization Act (MACRA) was passed in 2015 and is just now being implemented. Value-based, fee-for-service payments started out rather modestly a few years ago as chronic care management codes, but they have exploded to include more than 20 codes, counting the new ones coming online in 2018. Let’s call them collectively value-based care codes, or VCCs.
Many practices are trying to understand and perform the basic requirements to avoid penalties under MACRA’s Merit-based Incentive Payment System (MIPS) program. Some primary care practices, however, see the upside potential and bonuses stacking up to 30% or more.
Did you know that even if you are in, let’s say, a basic Medicare Shared Savings Program ACO – the MSSP Track 1, with no exposure to risk – you get special treatment on reporting under MACRA as a MIPS Advanced Practice Model (APM)?
But more importantly, MACRA is a team game. Getting into an MSSP Track 1 is justified just to get practice for the care coordination you’ll need. Few physicians know that they are judged under MACRA MIPS for the total costs of their patients, not just their own costs. A primary care physician receives only up to 8% of the $10 million your patients consume on average. The best way to counter that is through an ACO.
Further, we are aware of ACOs that have chosen risk-taking Medicare models such as NextGen, even though they predict small losses. Those losses are because of the automatic 5% fee-for-service payment bump to its physicians for risk taking if they are in a MACRA Advanced Alternative Payment Model (AAPM).
There’s a wide range of primary care physicians who are seizing opportunities offered by VCCs.
A family physician friend of mine who practices in a rural area generated more than 50% of his revenue from value-based care coding last year. And he has personally generated more than $350,000 in additional annual revenue, not counting the revenue from additional medically necessary procedures revealed by this more proactive wellness assessment activity and early diagnoses.
On the other hand, because busy physicians have a hard time wading through all these regulations and implementing the required staff and technology changes, it is reported that only about 8% of physicians are employing even the chronic care management codes. And when they do, they only achieve an 18% eligible patient penetration. My friend has broken the code, so to speak; he has protocolized and templated the process, has happy patients, has an ongoing 93% penetration rate, and actually has more free time.
While you are busy saving lives, I have had the luxury of looking from a high level at these tectonic, value-based payment shifts. To me, it’s a no-brainer for a primary care physician to leverage their ACO to maximize all three revenue streams. Look at MACRA MIPS, MIPS-APM, and AAPM measures anew, and see how well they play into integrated care.
As quarterback of health care through the patient-centered medical home, you are in great position to drive substantial bonuses. Similarly, when one looks at VCCs, the ACO can: help you navigate through the paperwork, perform much of the required reporting, and select the highest value-adding initiatives to monitor and drive higher quality and shared savings for the ACO.
As readers know, we firmly believe that, to have sustained incentivization, every ACO needs to have a merit-based, shared savings distribution formula. Accordingly, your compensation should rise under MACRA, VCCs, and the ACO.
This shift to value care is hard. But your colleagues who have made these changes are enjoying practice as never before. Their professional and financial rewards have climbed. But, most important, their patients love it.
Mr. Bobbitt is head of the health law group at the Smith Anderson law firm in Raleigh, N.C. He is president of Value Health Partners, a health care strategic consulting company. He has years of experience assisting physicians form integrated delivery systems. He has spoken and written nationally to primary care physicians on the strategies and practicalities of forming or joining ACOs. This article is meant to be educational and does not constitute legal advice. For additional information, readers may contact the author at [email protected] or 919-821-6612.