User login
No-shows
When a patient fails to show up for his appointment, your reaction may run the gamut from elation to anger or land somewhere on the spectrum between concern and self-doubt. If you are overbooked and running behind with a waiting room that looks like a bus station at rush hour, an unexpectedly unfilled appointment slot can provide a much needed but all too brief respite. However, if the patient who no-shows is someone whom you have been worried about, you may wonder if he has slipped further into a debilitating depression. Or maybe he found a physician that he prefers?
If you keep your finger on the economic pulse of your practice, you know that the empty slot created when a patient no-shows is valuable time that is not generating any income. Your practice administrator may have sent a practice-wide email expressing concern about what she feels is an unacceptably high and economically unsustainable no-show rate. She already may have replaced your antiquated system using postcards and personal phone call reminders with preprogrammed emails and robo-calls.
If despite these high tech targeted reminders your no-show rate continues to be unacceptably high, the problem may be with how and when your office schedules appointments. When a parent or older patient calls with what she feels is an urgent or time-sensitive complaint, is she offered an appointment that satisfies her sense of urgency? She may agree to make an appointment but as soon as she hangs up may begin searching for another source of care and neglect to cancel the appointment with you when she finds a more timely response.
On the other hand, the patient’s problem may have resolved itself. With this in mind, I asked our receptionists to not make next-day appointments for a child with ear pain if for whatever reason the child was unable to come in for a same-day appointment. I knew from experience that ear pain often resolved and appointments weren’t kept or parents would cancel at the last minute. However, we guaranteed that if the child’s pain persisted we would see them immediately in the morning.
You may be muttering to yourself that you can’t possibly give every patient an appointment as soon as they would like to be seen. True. But aren’t there some patients who could be well served by a quick same-day appointment to allay their fear and sketch out a starting point for diagnosis and management at a later visit? A skillful and calming appointment secretary or nurse may be able to provide the same level of reassurance. But sometimes a short office visit is a more effective and efficient way to depressurize the situation and avoid a longer appointment that has a high likelihood of being no-showed or canceled.
Finally, are you or other members of your group in the habit of making follow-up appointments for problems that probably don’t require follow up? Most patients have an excellent sense when a follow-up appointment is unnecessary and are likely to cancel at the last minute or no-show. They may have had more than one experience in which they took off time from work and traveled 20 miles for a 3-minute visit that didn’t seem worth the effort. A quick phone call or two from you or your staff may be a better way to make sure things are going in the right direction and avoid the cost and frustration of a no-show.
The bottom line is that no-shows happen but when appointments are thoughtfully made the patients are more likely to keep them.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
When a patient fails to show up for his appointment, your reaction may run the gamut from elation to anger or land somewhere on the spectrum between concern and self-doubt. If you are overbooked and running behind with a waiting room that looks like a bus station at rush hour, an unexpectedly unfilled appointment slot can provide a much needed but all too brief respite. However, if the patient who no-shows is someone whom you have been worried about, you may wonder if he has slipped further into a debilitating depression. Or maybe he found a physician that he prefers?
If you keep your finger on the economic pulse of your practice, you know that the empty slot created when a patient no-shows is valuable time that is not generating any income. Your practice administrator may have sent a practice-wide email expressing concern about what she feels is an unacceptably high and economically unsustainable no-show rate. She already may have replaced your antiquated system using postcards and personal phone call reminders with preprogrammed emails and robo-calls.
If despite these high tech targeted reminders your no-show rate continues to be unacceptably high, the problem may be with how and when your office schedules appointments. When a parent or older patient calls with what she feels is an urgent or time-sensitive complaint, is she offered an appointment that satisfies her sense of urgency? She may agree to make an appointment but as soon as she hangs up may begin searching for another source of care and neglect to cancel the appointment with you when she finds a more timely response.
On the other hand, the patient’s problem may have resolved itself. With this in mind, I asked our receptionists to not make next-day appointments for a child with ear pain if for whatever reason the child was unable to come in for a same-day appointment. I knew from experience that ear pain often resolved and appointments weren’t kept or parents would cancel at the last minute. However, we guaranteed that if the child’s pain persisted we would see them immediately in the morning.
You may be muttering to yourself that you can’t possibly give every patient an appointment as soon as they would like to be seen. True. But aren’t there some patients who could be well served by a quick same-day appointment to allay their fear and sketch out a starting point for diagnosis and management at a later visit? A skillful and calming appointment secretary or nurse may be able to provide the same level of reassurance. But sometimes a short office visit is a more effective and efficient way to depressurize the situation and avoid a longer appointment that has a high likelihood of being no-showed or canceled.
Finally, are you or other members of your group in the habit of making follow-up appointments for problems that probably don’t require follow up? Most patients have an excellent sense when a follow-up appointment is unnecessary and are likely to cancel at the last minute or no-show. They may have had more than one experience in which they took off time from work and traveled 20 miles for a 3-minute visit that didn’t seem worth the effort. A quick phone call or two from you or your staff may be a better way to make sure things are going in the right direction and avoid the cost and frustration of a no-show.
The bottom line is that no-shows happen but when appointments are thoughtfully made the patients are more likely to keep them.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
When a patient fails to show up for his appointment, your reaction may run the gamut from elation to anger or land somewhere on the spectrum between concern and self-doubt. If you are overbooked and running behind with a waiting room that looks like a bus station at rush hour, an unexpectedly unfilled appointment slot can provide a much needed but all too brief respite. However, if the patient who no-shows is someone whom you have been worried about, you may wonder if he has slipped further into a debilitating depression. Or maybe he found a physician that he prefers?
If you keep your finger on the economic pulse of your practice, you know that the empty slot created when a patient no-shows is valuable time that is not generating any income. Your practice administrator may have sent a practice-wide email expressing concern about what she feels is an unacceptably high and economically unsustainable no-show rate. She already may have replaced your antiquated system using postcards and personal phone call reminders with preprogrammed emails and robo-calls.
If despite these high tech targeted reminders your no-show rate continues to be unacceptably high, the problem may be with how and when your office schedules appointments. When a parent or older patient calls with what she feels is an urgent or time-sensitive complaint, is she offered an appointment that satisfies her sense of urgency? She may agree to make an appointment but as soon as she hangs up may begin searching for another source of care and neglect to cancel the appointment with you when she finds a more timely response.
On the other hand, the patient’s problem may have resolved itself. With this in mind, I asked our receptionists to not make next-day appointments for a child with ear pain if for whatever reason the child was unable to come in for a same-day appointment. I knew from experience that ear pain often resolved and appointments weren’t kept or parents would cancel at the last minute. However, we guaranteed that if the child’s pain persisted we would see them immediately in the morning.
You may be muttering to yourself that you can’t possibly give every patient an appointment as soon as they would like to be seen. True. But aren’t there some patients who could be well served by a quick same-day appointment to allay their fear and sketch out a starting point for diagnosis and management at a later visit? A skillful and calming appointment secretary or nurse may be able to provide the same level of reassurance. But sometimes a short office visit is a more effective and efficient way to depressurize the situation and avoid a longer appointment that has a high likelihood of being no-showed or canceled.
Finally, are you or other members of your group in the habit of making follow-up appointments for problems that probably don’t require follow up? Most patients have an excellent sense when a follow-up appointment is unnecessary and are likely to cancel at the last minute or no-show. They may have had more than one experience in which they took off time from work and traveled 20 miles for a 3-minute visit that didn’t seem worth the effort. A quick phone call or two from you or your staff may be a better way to make sure things are going in the right direction and avoid the cost and frustration of a no-show.
The bottom line is that no-shows happen but when appointments are thoughtfully made the patients are more likely to keep them.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Intraperitoneal chemo missed main endpoint, yet may benefit gastric cancer
Intraperitoneal chemotherapy may have clinical benefits over standard treatment in gastric cancer patients with peritoneal metastasis, exploratory analyses of a randomized clinical trial suggest.
Although it failed to demonstrate a statistical overall survival advantage versus standard chemotherapy in the primary analysis of the phase 3 trial, intraperitoneal treatment had a significantly longer overall survival in an analysis adjusted for presence of ascites, study investigators reported.
In addition, the 3-year overall survival rate was significantly higher in the intraperitoneal arm of the trial, according to Hironori Ishigami, MD, PhD, of the University of Tokyo, and coinvestigators.
“Considering the results of these analyses, the efficacy of the IP [intraperitoneal] regimen seems underestimated by the primary analysis,” Dr. Ishigami and coauthors wrote in the Journal of Clinical Oncology.
The main culprits were an imbalance in ascites between arms, and crossover from standard therapy to the intraperitoneal arm, they added.
The phase 3 trial included 183 patients with gastric cancer and peritoneal metastases who had less than 2 months of prior chemotherapy. They were randomly assigned to receive either intraperitoneal and intravenous paclitaxel plus S-1 (tegafur/gimeracil/oteracil) or standard S-1 plus cisplatin.
Median survival was 17.7 months in the intraperitoneal arm, and 15.2 months in the standard-treatment arm (hazard ratio, 0.72; P = .08), Dr. Ishigami and associates reported.
That difference reached statistical significance in a sensitivity analysis adjusted for baseline ascites, investigators said (HR, 0.59; P = .008). In addition, the 3-year overall survival rate reached 21.9% in the intraperitoneal arm, versus 6.0% for standard treatment.
Based on these possible clinical benefits, further investigation is warranted, authors said. One single-arm trial evaluating the intraperitoneal regimen is underway in Japan now and has enrolled 111 patients. Several other trials recently completed, ongoing, or planned are also evaluating intraperitoneal treatment plus other systemic therapy, they wrote.
“The results of these trials are awaited to confirm the efficacy of intraperitoneal therapy for gastric cancer,” Dr. Ishigami and associates said.
The study was supported in part by Sawai Pharmaceutical. Dr. Ishigami reported disclosures related to Taiho Pharmaceutical and Chugai Pharma.
SOURCE: Ishigami H et al. J Clin Oncol. 2018 May 10. doi: 10.1200/JCO.2018.77.8613.
Intraperitoneal chemotherapy may have clinical benefits over standard treatment in gastric cancer patients with peritoneal metastasis, exploratory analyses of a randomized clinical trial suggest.
Although it failed to demonstrate a statistical overall survival advantage versus standard chemotherapy in the primary analysis of the phase 3 trial, intraperitoneal treatment had a significantly longer overall survival in an analysis adjusted for presence of ascites, study investigators reported.
In addition, the 3-year overall survival rate was significantly higher in the intraperitoneal arm of the trial, according to Hironori Ishigami, MD, PhD, of the University of Tokyo, and coinvestigators.
“Considering the results of these analyses, the efficacy of the IP [intraperitoneal] regimen seems underestimated by the primary analysis,” Dr. Ishigami and coauthors wrote in the Journal of Clinical Oncology.
The main culprits were an imbalance in ascites between arms, and crossover from standard therapy to the intraperitoneal arm, they added.
The phase 3 trial included 183 patients with gastric cancer and peritoneal metastases who had less than 2 months of prior chemotherapy. They were randomly assigned to receive either intraperitoneal and intravenous paclitaxel plus S-1 (tegafur/gimeracil/oteracil) or standard S-1 plus cisplatin.
Median survival was 17.7 months in the intraperitoneal arm, and 15.2 months in the standard-treatment arm (hazard ratio, 0.72; P = .08), Dr. Ishigami and associates reported.
That difference reached statistical significance in a sensitivity analysis adjusted for baseline ascites, investigators said (HR, 0.59; P = .008). In addition, the 3-year overall survival rate reached 21.9% in the intraperitoneal arm, versus 6.0% for standard treatment.
Based on these possible clinical benefits, further investigation is warranted, authors said. One single-arm trial evaluating the intraperitoneal regimen is underway in Japan now and has enrolled 111 patients. Several other trials recently completed, ongoing, or planned are also evaluating intraperitoneal treatment plus other systemic therapy, they wrote.
“The results of these trials are awaited to confirm the efficacy of intraperitoneal therapy for gastric cancer,” Dr. Ishigami and associates said.
The study was supported in part by Sawai Pharmaceutical. Dr. Ishigami reported disclosures related to Taiho Pharmaceutical and Chugai Pharma.
SOURCE: Ishigami H et al. J Clin Oncol. 2018 May 10. doi: 10.1200/JCO.2018.77.8613.
Intraperitoneal chemotherapy may have clinical benefits over standard treatment in gastric cancer patients with peritoneal metastasis, exploratory analyses of a randomized clinical trial suggest.
Although it failed to demonstrate a statistical overall survival advantage versus standard chemotherapy in the primary analysis of the phase 3 trial, intraperitoneal treatment had a significantly longer overall survival in an analysis adjusted for presence of ascites, study investigators reported.
In addition, the 3-year overall survival rate was significantly higher in the intraperitoneal arm of the trial, according to Hironori Ishigami, MD, PhD, of the University of Tokyo, and coinvestigators.
“Considering the results of these analyses, the efficacy of the IP [intraperitoneal] regimen seems underestimated by the primary analysis,” Dr. Ishigami and coauthors wrote in the Journal of Clinical Oncology.
The main culprits were an imbalance in ascites between arms, and crossover from standard therapy to the intraperitoneal arm, they added.
The phase 3 trial included 183 patients with gastric cancer and peritoneal metastases who had less than 2 months of prior chemotherapy. They were randomly assigned to receive either intraperitoneal and intravenous paclitaxel plus S-1 (tegafur/gimeracil/oteracil) or standard S-1 plus cisplatin.
Median survival was 17.7 months in the intraperitoneal arm, and 15.2 months in the standard-treatment arm (hazard ratio, 0.72; P = .08), Dr. Ishigami and associates reported.
That difference reached statistical significance in a sensitivity analysis adjusted for baseline ascites, investigators said (HR, 0.59; P = .008). In addition, the 3-year overall survival rate reached 21.9% in the intraperitoneal arm, versus 6.0% for standard treatment.
Based on these possible clinical benefits, further investigation is warranted, authors said. One single-arm trial evaluating the intraperitoneal regimen is underway in Japan now and has enrolled 111 patients. Several other trials recently completed, ongoing, or planned are also evaluating intraperitoneal treatment plus other systemic therapy, they wrote.
“The results of these trials are awaited to confirm the efficacy of intraperitoneal therapy for gastric cancer,” Dr. Ishigami and associates said.
The study was supported in part by Sawai Pharmaceutical. Dr. Ishigami reported disclosures related to Taiho Pharmaceutical and Chugai Pharma.
SOURCE: Ishigami H et al. J Clin Oncol. 2018 May 10. doi: 10.1200/JCO.2018.77.8613.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Intraperitoneal chemotherapy failed to show statistical superiority to standard chemotherapy in patients with gastric cancer and peritoneal metastases, but exploratory analyses suggested a benefit.
Major finding: For overall survival, the hazard ratio was 0.72 (P = .08) in the primary analysis, and 0.59 (P = .008) in an analysis adjusting for baseline ascites.
Study details: A randomized, phase 3 trial including 183 patients with gastric cancer with peritoneal metastasis with less than 2 months of prior chemotherapy.
Disclosures: The study was supported in part by Sawai Pharmaceutical. Authors reported disclosures related to Taiho Pharmaceutical, Chugai Pharma, and other entities.
Source: Ishigami H et al. J Clin Oncol. 2018 May 10. doi: 10.1200/JCO.2018.77.8613.
Pediatric cancers are on the rise
PITTSBURGH – The incidence of many pediatric cancers are on the rise, and the increase is occurring in nearly all demographic groups studied, according to the latest data from the U.S. Centers for Disease Control and Prevention.
Pediatric cancers that increased significantly in incidence from 2001 through 2014, compared with previous time periods, include thyroid carcinoma, hepatic tumors, lymphomas, renal tumors, and brain tumors. Other cancer types remained unchanged, except malignant melanoma, which saw a significant decline in incidence over the same period, reported David A. Siegel, MD, of the Epidemic Intelligence Service at the CDC in Atlanta.
Recent studies of trends in pediatric cancer have either used data from before 2010 or covered less than a third of the U.S. population, the investigators noted.
To get a more accurate estimate of current trends, the investigators relied on the United States Cancer Statistics, which combines data from the Surveillance, Epidemiology, and End Results (SEER) program and the National Program of Cancer Registries. Together, the combined databases cover 100% of the U.S. population.
Dr. Siegel and his colleagues looked at cancer incidence rates and trends among individuals younger than 20 years of age from across 48 states from 2001 to 2014 – Mississippi, Nevada, and the District of Columbia were not included.
They used a joinpoint regression method to calculate average annual percent change (AAPC) in rates, then stratified rates and trends by sex, age, and race/ethnicity; location; economic status; and cancer type.
During the 14-year period of the study, there were a total of 196,200 incident cases of pediatric cancer, for an overall cancer incidence rate of 173 per million. The pediatric cancer with the highest incident rate was leukemia of any type (45.6 per million), brain tumors (30.8), and lymphomas (26.0).
Incidence rates were highest among males, patients from infancy through age 4, non-Hispanic whites, children who live in the Northeast region, those who live in the wealthiest counties, and those who live in urban/metropolitan counties. The overall pediatric cancer incidence rate increased, with an AAPC of 0.7 (95% confidence interval, 0.5-0.8).
“Rates increased in each stratum of sex, age, and race/ethnicity (except non-Hispanic American Indian/Alaska Native), region, economic status, and rural/urban classification,” the investigators wrote.
Cancers with significantly increased AAPC included thyroid carcinomas (AAPC, 4.8), hepatic tumors (2.5), lymphomas (1.7), renal tumors (0.6), and brain tumors (all types, 0.4).
There were no significant changes in the incidence of either germ cell cancer, retinoblastoma, leukemia, neuroblastoma, soft-tissue sarcomas, or bone tumors.
The only significant decrease over the study period was in the incidence of melanoma in children (–2.6).
“Possible causes of increasing rates might include changes in diagnostic, coding, and reporting standards, increased detection, population-based changes (such as increasing obesity), and environmental exposures,” they wrote.
Public health campaigns about the dangers of UV exposure and promoting the use of sunscreens may account for the decline in the incidence of malignant melanoma, they suggested.
The study was supported by the CDC. Dr. Siegel and coauthors are CDC employees. They reported having no conflicts of interest.
SOURCE: Siegel DA et al. ASPHO 2018, Abstract 605.
PITTSBURGH – The incidence of many pediatric cancers are on the rise, and the increase is occurring in nearly all demographic groups studied, according to the latest data from the U.S. Centers for Disease Control and Prevention.
Pediatric cancers that increased significantly in incidence from 2001 through 2014, compared with previous time periods, include thyroid carcinoma, hepatic tumors, lymphomas, renal tumors, and brain tumors. Other cancer types remained unchanged, except malignant melanoma, which saw a significant decline in incidence over the same period, reported David A. Siegel, MD, of the Epidemic Intelligence Service at the CDC in Atlanta.
Recent studies of trends in pediatric cancer have either used data from before 2010 or covered less than a third of the U.S. population, the investigators noted.
To get a more accurate estimate of current trends, the investigators relied on the United States Cancer Statistics, which combines data from the Surveillance, Epidemiology, and End Results (SEER) program and the National Program of Cancer Registries. Together, the combined databases cover 100% of the U.S. population.
Dr. Siegel and his colleagues looked at cancer incidence rates and trends among individuals younger than 20 years of age from across 48 states from 2001 to 2014 – Mississippi, Nevada, and the District of Columbia were not included.
They used a joinpoint regression method to calculate average annual percent change (AAPC) in rates, then stratified rates and trends by sex, age, and race/ethnicity; location; economic status; and cancer type.
During the 14-year period of the study, there were a total of 196,200 incident cases of pediatric cancer, for an overall cancer incidence rate of 173 per million. The pediatric cancer with the highest incident rate was leukemia of any type (45.6 per million), brain tumors (30.8), and lymphomas (26.0).
Incidence rates were highest among males, patients from infancy through age 4, non-Hispanic whites, children who live in the Northeast region, those who live in the wealthiest counties, and those who live in urban/metropolitan counties. The overall pediatric cancer incidence rate increased, with an AAPC of 0.7 (95% confidence interval, 0.5-0.8).
“Rates increased in each stratum of sex, age, and race/ethnicity (except non-Hispanic American Indian/Alaska Native), region, economic status, and rural/urban classification,” the investigators wrote.
Cancers with significantly increased AAPC included thyroid carcinomas (AAPC, 4.8), hepatic tumors (2.5), lymphomas (1.7), renal tumors (0.6), and brain tumors (all types, 0.4).
There were no significant changes in the incidence of either germ cell cancer, retinoblastoma, leukemia, neuroblastoma, soft-tissue sarcomas, or bone tumors.
The only significant decrease over the study period was in the incidence of melanoma in children (–2.6).
“Possible causes of increasing rates might include changes in diagnostic, coding, and reporting standards, increased detection, population-based changes (such as increasing obesity), and environmental exposures,” they wrote.
Public health campaigns about the dangers of UV exposure and promoting the use of sunscreens may account for the decline in the incidence of malignant melanoma, they suggested.
The study was supported by the CDC. Dr. Siegel and coauthors are CDC employees. They reported having no conflicts of interest.
SOURCE: Siegel DA et al. ASPHO 2018, Abstract 605.
PITTSBURGH – The incidence of many pediatric cancers are on the rise, and the increase is occurring in nearly all demographic groups studied, according to the latest data from the U.S. Centers for Disease Control and Prevention.
Pediatric cancers that increased significantly in incidence from 2001 through 2014, compared with previous time periods, include thyroid carcinoma, hepatic tumors, lymphomas, renal tumors, and brain tumors. Other cancer types remained unchanged, except malignant melanoma, which saw a significant decline in incidence over the same period, reported David A. Siegel, MD, of the Epidemic Intelligence Service at the CDC in Atlanta.
Recent studies of trends in pediatric cancer have either used data from before 2010 or covered less than a third of the U.S. population, the investigators noted.
To get a more accurate estimate of current trends, the investigators relied on the United States Cancer Statistics, which combines data from the Surveillance, Epidemiology, and End Results (SEER) program and the National Program of Cancer Registries. Together, the combined databases cover 100% of the U.S. population.
Dr. Siegel and his colleagues looked at cancer incidence rates and trends among individuals younger than 20 years of age from across 48 states from 2001 to 2014 – Mississippi, Nevada, and the District of Columbia were not included.
They used a joinpoint regression method to calculate average annual percent change (AAPC) in rates, then stratified rates and trends by sex, age, and race/ethnicity; location; economic status; and cancer type.
During the 14-year period of the study, there were a total of 196,200 incident cases of pediatric cancer, for an overall cancer incidence rate of 173 per million. The pediatric cancer with the highest incident rate was leukemia of any type (45.6 per million), brain tumors (30.8), and lymphomas (26.0).
Incidence rates were highest among males, patients from infancy through age 4, non-Hispanic whites, children who live in the Northeast region, those who live in the wealthiest counties, and those who live in urban/metropolitan counties. The overall pediatric cancer incidence rate increased, with an AAPC of 0.7 (95% confidence interval, 0.5-0.8).
“Rates increased in each stratum of sex, age, and race/ethnicity (except non-Hispanic American Indian/Alaska Native), region, economic status, and rural/urban classification,” the investigators wrote.
Cancers with significantly increased AAPC included thyroid carcinomas (AAPC, 4.8), hepatic tumors (2.5), lymphomas (1.7), renal tumors (0.6), and brain tumors (all types, 0.4).
There were no significant changes in the incidence of either germ cell cancer, retinoblastoma, leukemia, neuroblastoma, soft-tissue sarcomas, or bone tumors.
The only significant decrease over the study period was in the incidence of melanoma in children (–2.6).
“Possible causes of increasing rates might include changes in diagnostic, coding, and reporting standards, increased detection, population-based changes (such as increasing obesity), and environmental exposures,” they wrote.
Public health campaigns about the dangers of UV exposure and promoting the use of sunscreens may account for the decline in the incidence of malignant melanoma, they suggested.
The study was supported by the CDC. Dr. Siegel and coauthors are CDC employees. They reported having no conflicts of interest.
SOURCE: Siegel DA et al. ASPHO 2018, Abstract 605.
REPORTING FROM ASPHO 2018
Key clinical point: Major finding: From 2001 to 2014, there were 196,200 incident cases of pediatric cancer for an overall cancer incidence rate of 173 per 1 million.
Study details: A review of data from the United States Cancer Statistics for children under age 20.
Disclosures: The CDC supported the study. Dr. Siegel and his coauthors are CDC employees. They reported having no conflicts of interest.
Source: Siegel DA et al. ASPHO 2018, Abstract 605.
Simple postural exercises may reduce depressive symptoms
NEW YORK – Postural exercises that take only a few minutes but are performed several times a day produced large reductions in symptoms of depression and increased the rate of remission at 12 weeks, according to results of a randomized study presented at the annual meeting of the American Psychiatric Association.
“These were very marked symptom improvements seen at the end of 12 weeks in those randomized to the exercises when compared to the controls who received antidepressants alone,” reported Martin Furman, MD, a researcher in psychiatry at the University of Maimónides, Buenos Aires.
The patients in this study all continued on their baseline SSRI dose but were randomized to start postural exercises or to remain on an SSRI alone. The two sets of exercises, which took only a couple of minutes, were repeated four to six times per day with at least 2 hours of separation.
The equilibrium exercises involved raised arms with flexing of first one lower limb then the other. Each flex was maintained for 15 seconds. In a separate exercise, patients were instructed to hold a pencil between the teeth and smile for 1 minute, a technique that activates facial skin afferents, according to Dr. Furman.
“In previous studies, posture and balance have been linked to a reduction in anxiety and moderate depression,” Dr. Furman explained. “Feedback of the muscular and facial skin afferents has been associated with modulation of neural activity within the central circuit of emotions.”
Much of this work was pioneered by Tomás Ortiz Alonso, MD, PhD, director of the department of psychiatry at Universidad Complutense de Madrid, according to Dr. Furman. While working to complete his PhD, Dr. Furman is collaborating with Dr. Ortiz Alonso.
Patients participating in the study were evaluated with the Beck Depression Inventory (BDI) and the 17-item Hamilton Depression Rating Scale (HDRS-17) at baseline and the end of 12 weeks. Response for both scales was defined as at least a 50% score reduction from baseline. Remission of depression was defined as HDRS-17 score of less than 7.
At 12 weeks, 86% of those in the experimental arm versus 28.5% of those in the control arm achieved a response on the HDRS-17 tool. For the BDI tool, the response rates were 71.5% and 19%, respectively. The remission rates as defined by HDRS-17 score of less than 7 were 66.6% and 19%. All differences were statistically significant (P less than .05).
“The exercises are simple and take very little time,” said Dr. Furman, who reported that compliance was good in the study arm according to patient self-report.
There are numerous published studies suggesting that exercise improves mood in a variety of circumstances, including the treatment of depression, and Dr. Furman said these exercises are not physically demanding. If larger studies validate the results of this initial trial, this might prove to be a highly cost-effective tool for depression treatment.
“Further studies are needed to determine whether these exercises are most effective as adjunctive treatment to pharmacotherapy or can be used alone without medications,” he said.
In addition to larger efficacy studies, Dr. Furman reported that efforts to understand the mechanism of benefit are being considered, such as studies with functional MRI or EEG to evaluate the effect of the exercises on brain activity.
“We are excited about these results. ,” he said.
Dr. Furman reports no potential conflicts of interest related to this topic.
NEW YORK – Postural exercises that take only a few minutes but are performed several times a day produced large reductions in symptoms of depression and increased the rate of remission at 12 weeks, according to results of a randomized study presented at the annual meeting of the American Psychiatric Association.
“These were very marked symptom improvements seen at the end of 12 weeks in those randomized to the exercises when compared to the controls who received antidepressants alone,” reported Martin Furman, MD, a researcher in psychiatry at the University of Maimónides, Buenos Aires.
The patients in this study all continued on their baseline SSRI dose but were randomized to start postural exercises or to remain on an SSRI alone. The two sets of exercises, which took only a couple of minutes, were repeated four to six times per day with at least 2 hours of separation.
The equilibrium exercises involved raised arms with flexing of first one lower limb then the other. Each flex was maintained for 15 seconds. In a separate exercise, patients were instructed to hold a pencil between the teeth and smile for 1 minute, a technique that activates facial skin afferents, according to Dr. Furman.
“In previous studies, posture and balance have been linked to a reduction in anxiety and moderate depression,” Dr. Furman explained. “Feedback of the muscular and facial skin afferents has been associated with modulation of neural activity within the central circuit of emotions.”
Much of this work was pioneered by Tomás Ortiz Alonso, MD, PhD, director of the department of psychiatry at Universidad Complutense de Madrid, according to Dr. Furman. While working to complete his PhD, Dr. Furman is collaborating with Dr. Ortiz Alonso.
Patients participating in the study were evaluated with the Beck Depression Inventory (BDI) and the 17-item Hamilton Depression Rating Scale (HDRS-17) at baseline and the end of 12 weeks. Response for both scales was defined as at least a 50% score reduction from baseline. Remission of depression was defined as HDRS-17 score of less than 7.
At 12 weeks, 86% of those in the experimental arm versus 28.5% of those in the control arm achieved a response on the HDRS-17 tool. For the BDI tool, the response rates were 71.5% and 19%, respectively. The remission rates as defined by HDRS-17 score of less than 7 were 66.6% and 19%. All differences were statistically significant (P less than .05).
“The exercises are simple and take very little time,” said Dr. Furman, who reported that compliance was good in the study arm according to patient self-report.
There are numerous published studies suggesting that exercise improves mood in a variety of circumstances, including the treatment of depression, and Dr. Furman said these exercises are not physically demanding. If larger studies validate the results of this initial trial, this might prove to be a highly cost-effective tool for depression treatment.
“Further studies are needed to determine whether these exercises are most effective as adjunctive treatment to pharmacotherapy or can be used alone without medications,” he said.
In addition to larger efficacy studies, Dr. Furman reported that efforts to understand the mechanism of benefit are being considered, such as studies with functional MRI or EEG to evaluate the effect of the exercises on brain activity.
“We are excited about these results. ,” he said.
Dr. Furman reports no potential conflicts of interest related to this topic.
NEW YORK – Postural exercises that take only a few minutes but are performed several times a day produced large reductions in symptoms of depression and increased the rate of remission at 12 weeks, according to results of a randomized study presented at the annual meeting of the American Psychiatric Association.
“These were very marked symptom improvements seen at the end of 12 weeks in those randomized to the exercises when compared to the controls who received antidepressants alone,” reported Martin Furman, MD, a researcher in psychiatry at the University of Maimónides, Buenos Aires.
The patients in this study all continued on their baseline SSRI dose but were randomized to start postural exercises or to remain on an SSRI alone. The two sets of exercises, which took only a couple of minutes, were repeated four to six times per day with at least 2 hours of separation.
The equilibrium exercises involved raised arms with flexing of first one lower limb then the other. Each flex was maintained for 15 seconds. In a separate exercise, patients were instructed to hold a pencil between the teeth and smile for 1 minute, a technique that activates facial skin afferents, according to Dr. Furman.
“In previous studies, posture and balance have been linked to a reduction in anxiety and moderate depression,” Dr. Furman explained. “Feedback of the muscular and facial skin afferents has been associated with modulation of neural activity within the central circuit of emotions.”
Much of this work was pioneered by Tomás Ortiz Alonso, MD, PhD, director of the department of psychiatry at Universidad Complutense de Madrid, according to Dr. Furman. While working to complete his PhD, Dr. Furman is collaborating with Dr. Ortiz Alonso.
Patients participating in the study were evaluated with the Beck Depression Inventory (BDI) and the 17-item Hamilton Depression Rating Scale (HDRS-17) at baseline and the end of 12 weeks. Response for both scales was defined as at least a 50% score reduction from baseline. Remission of depression was defined as HDRS-17 score of less than 7.
At 12 weeks, 86% of those in the experimental arm versus 28.5% of those in the control arm achieved a response on the HDRS-17 tool. For the BDI tool, the response rates were 71.5% and 19%, respectively. The remission rates as defined by HDRS-17 score of less than 7 were 66.6% and 19%. All differences were statistically significant (P less than .05).
“The exercises are simple and take very little time,” said Dr. Furman, who reported that compliance was good in the study arm according to patient self-report.
There are numerous published studies suggesting that exercise improves mood in a variety of circumstances, including the treatment of depression, and Dr. Furman said these exercises are not physically demanding. If larger studies validate the results of this initial trial, this might prove to be a highly cost-effective tool for depression treatment.
“Further studies are needed to determine whether these exercises are most effective as adjunctive treatment to pharmacotherapy or can be used alone without medications,” he said.
In addition to larger efficacy studies, Dr. Furman reported that efforts to understand the mechanism of benefit are being considered, such as studies with functional MRI or EEG to evaluate the effect of the exercises on brain activity.
“We are excited about these results. ,” he said.
Dr. Furman reports no potential conflicts of interest related to this topic.
REPORTING FROM APA
Key clinical point: In patients with major depression, a simple set of daily postural exercises appears effective for improving symptom control.
Major finding: After 12 weeks, 67% of those performing exercises versus 19% of controls (P less than .05) met criteria for depression remission.
Study details: Randomized, controlled trial of 42 patients who met DSM-5 criteria for major depressive disorder.
Disclosures: Dr. Furman reports no potential conflicts of interest related to this topic.
Americans are getting more sleep
an analysis of data from the American Time Use Survey (ATUS) has suggested.
Many people living in the United States habitually sleep less than the recommended 7-9 hours each day. “Experimental studies have demonstrated that both acute total and chronic partial sleep restriction in healthy adults are associated with physiological changes that can be considered precursors of manifest diseases (e.g. decreased insulin sensitivity),” noted Mathias Basner, MD, PhD, and David F. Dinges, PhD, both of the division of sleep and chronobiology at the University of Pennsylvania, Philadelphia, in their paper, which was published in the journal Sleep.
This new study is the first to have demonstrated that large parts of the U.S. population significantly increased their sleep between 2003 and 2016.
The investigators analyzed ATUS responses from 181,335 Americans aged 15 years and older; respondents included in the analysis were not active in the military or residing in institutions such as nursing homes or prisons. In 15- to 20-minute computer-assisted telephone interviews, the survey participants reported the activities they performed over a 24-hour period on a minute-by-minute basis. In-depth analyses only included groups “that showed a significant increase in sleep duration across survey years either on weekdays or weekends (or both): employed respondents, full-time students, and retirees.”
Using this data from ATUS, Dr. Basner and Dr. Dinges found that on workdays the prevalence of people who were sleeping 7 hours or less a day decreased by 0.44% per year (P less than .0001), while the percentage people who were sleeping more than 9 hours a day increased by 0.48% per year (P less than .0001).
Overall, respondents’ sleep increased by an average of 1.40 minutes during a weekday and 0.83 minutes during a weekend day every year.
These findings will be welcome news for organizations, such as the American Academy of Sleep Medicine, the Sleep Research Society, and the Centers for Disease Control and Prevention, that have been campaigning for years to increase sleep time among Americans.
The researchers also observed that the percentage of respondents in short sleep duration categories decreased significantly, and the percentage of respondents in long sleep duration categories increased significantly across survey years. One of the “most pronounced changes” occurred in the size of the group of patients receiving 6-7 hours of sleep. This group decreased by 0.23% per year. The biggest change was seen in the category of patients receiving 9-10 hours of sleep, which increased by 0.24% per year.
“[The] change in sleep duration across survey years on weekdays can mostly be explained by respondents going to bed earlier at night and, to a lesser degree, by getting up later in the morning,” the researchers said. “On weekends/holidays, ‘time to bed’ shifted significantly to earlier bed times by 1.1 min/year across survey years, which was comparable to the shift observed on weekdays.”
Study participants aged 18-24 slept the most, with hours slept having “decreased with increasing age.” On weekdays, adults aged 45-54 years slept the least, and on weekends, adults aged 55-64 years got the least shuteye. Hispanic, Asian, and black respondents slept more than white and “other race/ethnicity” survey participants. The researchers also found that women overall got more sleep than men.
Dr. Basner and Dr. Dinges expressed optimism about Americans’ ongoing battle against chronic sleep deficiency. “These findings presented here suggest that we are on the right track ... even if there is still a long way to go,” they said.
The authors reported no conflicts of interest.
David Schulman, MD, FCCP, comments: For more than fifteen years, we have had evidence that sleep deprivation is not only associated with increased accident risk, but also other common causes of mortality, including cardiovascular disease and cancer. Despite this knowledge, decades of trending in sleep patterns of the United States population have continued to show declines in total sleep time, which have been attributed to multiple factors, including longer work hours and the pervasive use of electronics, which can serve both as a distraction from sleep and a contributor to circadian dysrhythmia.
While our work in improving public awareness of sleep deprivation is far from done, perhaps this study is the first sign that a new day is dawning on improved sleep health for the country.
David Schulman, MD, FCCP, comments: For more than fifteen years, we have had evidence that sleep deprivation is not only associated with increased accident risk, but also other common causes of mortality, including cardiovascular disease and cancer. Despite this knowledge, decades of trending in sleep patterns of the United States population have continued to show declines in total sleep time, which have been attributed to multiple factors, including longer work hours and the pervasive use of electronics, which can serve both as a distraction from sleep and a contributor to circadian dysrhythmia.
While our work in improving public awareness of sleep deprivation is far from done, perhaps this study is the first sign that a new day is dawning on improved sleep health for the country.
David Schulman, MD, FCCP, comments: For more than fifteen years, we have had evidence that sleep deprivation is not only associated with increased accident risk, but also other common causes of mortality, including cardiovascular disease and cancer. Despite this knowledge, decades of trending in sleep patterns of the United States population have continued to show declines in total sleep time, which have been attributed to multiple factors, including longer work hours and the pervasive use of electronics, which can serve both as a distraction from sleep and a contributor to circadian dysrhythmia.
While our work in improving public awareness of sleep deprivation is far from done, perhaps this study is the first sign that a new day is dawning on improved sleep health for the country.
an analysis of data from the American Time Use Survey (ATUS) has suggested.
Many people living in the United States habitually sleep less than the recommended 7-9 hours each day. “Experimental studies have demonstrated that both acute total and chronic partial sleep restriction in healthy adults are associated with physiological changes that can be considered precursors of manifest diseases (e.g. decreased insulin sensitivity),” noted Mathias Basner, MD, PhD, and David F. Dinges, PhD, both of the division of sleep and chronobiology at the University of Pennsylvania, Philadelphia, in their paper, which was published in the journal Sleep.
This new study is the first to have demonstrated that large parts of the U.S. population significantly increased their sleep between 2003 and 2016.
The investigators analyzed ATUS responses from 181,335 Americans aged 15 years and older; respondents included in the analysis were not active in the military or residing in institutions such as nursing homes or prisons. In 15- to 20-minute computer-assisted telephone interviews, the survey participants reported the activities they performed over a 24-hour period on a minute-by-minute basis. In-depth analyses only included groups “that showed a significant increase in sleep duration across survey years either on weekdays or weekends (or both): employed respondents, full-time students, and retirees.”
Using this data from ATUS, Dr. Basner and Dr. Dinges found that on workdays the prevalence of people who were sleeping 7 hours or less a day decreased by 0.44% per year (P less than .0001), while the percentage people who were sleeping more than 9 hours a day increased by 0.48% per year (P less than .0001).
Overall, respondents’ sleep increased by an average of 1.40 minutes during a weekday and 0.83 minutes during a weekend day every year.
These findings will be welcome news for organizations, such as the American Academy of Sleep Medicine, the Sleep Research Society, and the Centers for Disease Control and Prevention, that have been campaigning for years to increase sleep time among Americans.
The researchers also observed that the percentage of respondents in short sleep duration categories decreased significantly, and the percentage of respondents in long sleep duration categories increased significantly across survey years. One of the “most pronounced changes” occurred in the size of the group of patients receiving 6-7 hours of sleep. This group decreased by 0.23% per year. The biggest change was seen in the category of patients receiving 9-10 hours of sleep, which increased by 0.24% per year.
“[The] change in sleep duration across survey years on weekdays can mostly be explained by respondents going to bed earlier at night and, to a lesser degree, by getting up later in the morning,” the researchers said. “On weekends/holidays, ‘time to bed’ shifted significantly to earlier bed times by 1.1 min/year across survey years, which was comparable to the shift observed on weekdays.”
Study participants aged 18-24 slept the most, with hours slept having “decreased with increasing age.” On weekdays, adults aged 45-54 years slept the least, and on weekends, adults aged 55-64 years got the least shuteye. Hispanic, Asian, and black respondents slept more than white and “other race/ethnicity” survey participants. The researchers also found that women overall got more sleep than men.
Dr. Basner and Dr. Dinges expressed optimism about Americans’ ongoing battle against chronic sleep deficiency. “These findings presented here suggest that we are on the right track ... even if there is still a long way to go,” they said.
The authors reported no conflicts of interest.
an analysis of data from the American Time Use Survey (ATUS) has suggested.
Many people living in the United States habitually sleep less than the recommended 7-9 hours each day. “Experimental studies have demonstrated that both acute total and chronic partial sleep restriction in healthy adults are associated with physiological changes that can be considered precursors of manifest diseases (e.g. decreased insulin sensitivity),” noted Mathias Basner, MD, PhD, and David F. Dinges, PhD, both of the division of sleep and chronobiology at the University of Pennsylvania, Philadelphia, in their paper, which was published in the journal Sleep.
This new study is the first to have demonstrated that large parts of the U.S. population significantly increased their sleep between 2003 and 2016.
The investigators analyzed ATUS responses from 181,335 Americans aged 15 years and older; respondents included in the analysis were not active in the military or residing in institutions such as nursing homes or prisons. In 15- to 20-minute computer-assisted telephone interviews, the survey participants reported the activities they performed over a 24-hour period on a minute-by-minute basis. In-depth analyses only included groups “that showed a significant increase in sleep duration across survey years either on weekdays or weekends (or both): employed respondents, full-time students, and retirees.”
Using this data from ATUS, Dr. Basner and Dr. Dinges found that on workdays the prevalence of people who were sleeping 7 hours or less a day decreased by 0.44% per year (P less than .0001), while the percentage people who were sleeping more than 9 hours a day increased by 0.48% per year (P less than .0001).
Overall, respondents’ sleep increased by an average of 1.40 minutes during a weekday and 0.83 minutes during a weekend day every year.
These findings will be welcome news for organizations, such as the American Academy of Sleep Medicine, the Sleep Research Society, and the Centers for Disease Control and Prevention, that have been campaigning for years to increase sleep time among Americans.
The researchers also observed that the percentage of respondents in short sleep duration categories decreased significantly, and the percentage of respondents in long sleep duration categories increased significantly across survey years. One of the “most pronounced changes” occurred in the size of the group of patients receiving 6-7 hours of sleep. This group decreased by 0.23% per year. The biggest change was seen in the category of patients receiving 9-10 hours of sleep, which increased by 0.24% per year.
“[The] change in sleep duration across survey years on weekdays can mostly be explained by respondents going to bed earlier at night and, to a lesser degree, by getting up later in the morning,” the researchers said. “On weekends/holidays, ‘time to bed’ shifted significantly to earlier bed times by 1.1 min/year across survey years, which was comparable to the shift observed on weekdays.”
Study participants aged 18-24 slept the most, with hours slept having “decreased with increasing age.” On weekdays, adults aged 45-54 years slept the least, and on weekends, adults aged 55-64 years got the least shuteye. Hispanic, Asian, and black respondents slept more than white and “other race/ethnicity” survey participants. The researchers also found that women overall got more sleep than men.
Dr. Basner and Dr. Dinges expressed optimism about Americans’ ongoing battle against chronic sleep deficiency. “These findings presented here suggest that we are on the right track ... even if there is still a long way to go,” they said.
The authors reported no conflicts of interest.
FROM SLEEP
Key clinical point: The amount of time people in the United States spend sleeping has increased.
Major finding: On workdays, the prevalence of people who were sleeping 7 hours or less a day decreased by 0.44% per year, and the percentage of people who were sleeping more than 9 hours a day increased by 0.48% per year.
Study details: An analysis of survey responses from 181,335 Americans over 2003-2016.
Disclosures: The authors reported no conflicts of interest.
Source: Basner M et al. Sleep. 2018 Apr 1;41(4):1-16.
Multiple solid tumors targeted by concept CAR T
PITTSBURGH – Call it the CAR of the future – an investigational chimeric antigen receptor–T cell construct targeted against an antigen highly expressed on pediatric solid tumors has shown promising efficacy in preclinical studies.
Investigators found that the antigen, labeled B7-H3, was expressed on 84% of microarrays of pediatric solid tumors. More importantly, a single dose of CAR targeted to B7-H3 caused complete regression of osteosarcoma and Ewing sarcoma xenografts and improved survival over an untransduced, CD19-targeted CAR in mice, Robbie Majzner, MD, reported at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Dr. Majzner was the recipient of an ASPHO young investigator award for his team’s research into developing a CAR T that could be as effective against solid tumors as other CAR Ts have been against hematologic malignancies such as acute lymphoblastic leukemia.
Solid tumors are more challenging to target than leukemias or lymphomas because of the small number of antigens expressed on most pediatric tumors, he said.
“Over 95% of tumors have a very low rate of mutations, which means that they have very few neoantigens which the immune system can recognize in order to attack,” he said.
In the Children’s Oncology Group ADVL1412 trial, single-agent immunotherapy with the anti–programmed death protein 1 (PD-1) inhibitor nivolumab (Opdivo) showed no evidence of efficacy against either Ewing sarcoma, osteosarcoma, rhabdomyosarcoma, or measurable neuroblastoma. PD–ligand 1 was found to be expressed in only a few of the 43 tumors studied, suggesting that checkpoint inhibitor therapy is unlikely to work in these solid tumors, he said.
In contrast, B7-H3 is highly expressed on many different pediatric solid tumors, including rhabdomyosarcoma (95% of tumors stained), Ewing sarcoma (89%), Wilms tumor (100%), neuroblastoma (82%), ganglioneuroblastoma and ganglioneuroma (53%), medulloblastoma (96%), glioblastoma multiforme (84%), and diffuse intrinsic pontine glioma (100%).
To see whether CAR T therapy might have better efficacy than checkpoint inhibitors in this population, the investigators created a B7-H3 CAR using the B7-H3 tumor–specific monoclonal antibody MGA271, which has been shown to be safe in both adults and children in early clinical trials.
In human tumor xenograft models of osteosarcoma, all mice who received a single dose of the B7-H3 CAR survived at least 70 days after tumor engraftment, whereas all control mice, who received the CD19 CAR, died by day 60 (P = .0067). Similarly, in a model of Ewing sarcoma, all mice treated with B7-H3 survived at least 100 days, whereas all controls were dead by day 50 (P = .0015).
The B7-H3 construct also showed good activity against a model of medulloblastoma, showing that it was capable of crossing the blood-brain barrier.
Since B7-H3 has been reported to be expressed on both myeloid and lymphoid leukemia cells, the investigators also tested the CAR against a murine model of leukemia generated by injection of K562, a well-characterized line of myeloid leukemia cells.
“While we found some increase in survival in the mice that received the B7-H3 CAR T cells, compared to mice that received untransduced CAR T cells, this clearly is not as effective as in our solid tumor models,” Dr. Majzner said.
Going back to the cell line, they discovered that expression of B7-H3 was considerably lower in the K562 cells than in either the osteosarcoma or medulloblastoma cell lines used in their other models.
They found that both in vitro and in vivo, high levels of B7-H3 expression were necessary to provoke the immune system into releasing cytokines necessary for an adequate antitumor response.
The investigators are currently planning clinical trials using the B7-H3 CAR T-cell construct in patients with solid tumors.
The work is supported by the Sarcoma Alliance for Research through Collaboration, the St. Baldrick’s Foundation, and Stand Up to Cancer. Dr. Majzner reported having no financial disclosures.
SOURCE: Majzner RG et al. ASPHO 2018, Abstract #PS2003.
PITTSBURGH – Call it the CAR of the future – an investigational chimeric antigen receptor–T cell construct targeted against an antigen highly expressed on pediatric solid tumors has shown promising efficacy in preclinical studies.
Investigators found that the antigen, labeled B7-H3, was expressed on 84% of microarrays of pediatric solid tumors. More importantly, a single dose of CAR targeted to B7-H3 caused complete regression of osteosarcoma and Ewing sarcoma xenografts and improved survival over an untransduced, CD19-targeted CAR in mice, Robbie Majzner, MD, reported at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Dr. Majzner was the recipient of an ASPHO young investigator award for his team’s research into developing a CAR T that could be as effective against solid tumors as other CAR Ts have been against hematologic malignancies such as acute lymphoblastic leukemia.
Solid tumors are more challenging to target than leukemias or lymphomas because of the small number of antigens expressed on most pediatric tumors, he said.
“Over 95% of tumors have a very low rate of mutations, which means that they have very few neoantigens which the immune system can recognize in order to attack,” he said.
In the Children’s Oncology Group ADVL1412 trial, single-agent immunotherapy with the anti–programmed death protein 1 (PD-1) inhibitor nivolumab (Opdivo) showed no evidence of efficacy against either Ewing sarcoma, osteosarcoma, rhabdomyosarcoma, or measurable neuroblastoma. PD–ligand 1 was found to be expressed in only a few of the 43 tumors studied, suggesting that checkpoint inhibitor therapy is unlikely to work in these solid tumors, he said.
In contrast, B7-H3 is highly expressed on many different pediatric solid tumors, including rhabdomyosarcoma (95% of tumors stained), Ewing sarcoma (89%), Wilms tumor (100%), neuroblastoma (82%), ganglioneuroblastoma and ganglioneuroma (53%), medulloblastoma (96%), glioblastoma multiforme (84%), and diffuse intrinsic pontine glioma (100%).
To see whether CAR T therapy might have better efficacy than checkpoint inhibitors in this population, the investigators created a B7-H3 CAR using the B7-H3 tumor–specific monoclonal antibody MGA271, which has been shown to be safe in both adults and children in early clinical trials.
In human tumor xenograft models of osteosarcoma, all mice who received a single dose of the B7-H3 CAR survived at least 70 days after tumor engraftment, whereas all control mice, who received the CD19 CAR, died by day 60 (P = .0067). Similarly, in a model of Ewing sarcoma, all mice treated with B7-H3 survived at least 100 days, whereas all controls were dead by day 50 (P = .0015).
The B7-H3 construct also showed good activity against a model of medulloblastoma, showing that it was capable of crossing the blood-brain barrier.
Since B7-H3 has been reported to be expressed on both myeloid and lymphoid leukemia cells, the investigators also tested the CAR against a murine model of leukemia generated by injection of K562, a well-characterized line of myeloid leukemia cells.
“While we found some increase in survival in the mice that received the B7-H3 CAR T cells, compared to mice that received untransduced CAR T cells, this clearly is not as effective as in our solid tumor models,” Dr. Majzner said.
Going back to the cell line, they discovered that expression of B7-H3 was considerably lower in the K562 cells than in either the osteosarcoma or medulloblastoma cell lines used in their other models.
They found that both in vitro and in vivo, high levels of B7-H3 expression were necessary to provoke the immune system into releasing cytokines necessary for an adequate antitumor response.
The investigators are currently planning clinical trials using the B7-H3 CAR T-cell construct in patients with solid tumors.
The work is supported by the Sarcoma Alliance for Research through Collaboration, the St. Baldrick’s Foundation, and Stand Up to Cancer. Dr. Majzner reported having no financial disclosures.
SOURCE: Majzner RG et al. ASPHO 2018, Abstract #PS2003.
PITTSBURGH – Call it the CAR of the future – an investigational chimeric antigen receptor–T cell construct targeted against an antigen highly expressed on pediatric solid tumors has shown promising efficacy in preclinical studies.
Investigators found that the antigen, labeled B7-H3, was expressed on 84% of microarrays of pediatric solid tumors. More importantly, a single dose of CAR targeted to B7-H3 caused complete regression of osteosarcoma and Ewing sarcoma xenografts and improved survival over an untransduced, CD19-targeted CAR in mice, Robbie Majzner, MD, reported at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Dr. Majzner was the recipient of an ASPHO young investigator award for his team’s research into developing a CAR T that could be as effective against solid tumors as other CAR Ts have been against hematologic malignancies such as acute lymphoblastic leukemia.
Solid tumors are more challenging to target than leukemias or lymphomas because of the small number of antigens expressed on most pediatric tumors, he said.
“Over 95% of tumors have a very low rate of mutations, which means that they have very few neoantigens which the immune system can recognize in order to attack,” he said.
In the Children’s Oncology Group ADVL1412 trial, single-agent immunotherapy with the anti–programmed death protein 1 (PD-1) inhibitor nivolumab (Opdivo) showed no evidence of efficacy against either Ewing sarcoma, osteosarcoma, rhabdomyosarcoma, or measurable neuroblastoma. PD–ligand 1 was found to be expressed in only a few of the 43 tumors studied, suggesting that checkpoint inhibitor therapy is unlikely to work in these solid tumors, he said.
In contrast, B7-H3 is highly expressed on many different pediatric solid tumors, including rhabdomyosarcoma (95% of tumors stained), Ewing sarcoma (89%), Wilms tumor (100%), neuroblastoma (82%), ganglioneuroblastoma and ganglioneuroma (53%), medulloblastoma (96%), glioblastoma multiforme (84%), and diffuse intrinsic pontine glioma (100%).
To see whether CAR T therapy might have better efficacy than checkpoint inhibitors in this population, the investigators created a B7-H3 CAR using the B7-H3 tumor–specific monoclonal antibody MGA271, which has been shown to be safe in both adults and children in early clinical trials.
In human tumor xenograft models of osteosarcoma, all mice who received a single dose of the B7-H3 CAR survived at least 70 days after tumor engraftment, whereas all control mice, who received the CD19 CAR, died by day 60 (P = .0067). Similarly, in a model of Ewing sarcoma, all mice treated with B7-H3 survived at least 100 days, whereas all controls were dead by day 50 (P = .0015).
The B7-H3 construct also showed good activity against a model of medulloblastoma, showing that it was capable of crossing the blood-brain barrier.
Since B7-H3 has been reported to be expressed on both myeloid and lymphoid leukemia cells, the investigators also tested the CAR against a murine model of leukemia generated by injection of K562, a well-characterized line of myeloid leukemia cells.
“While we found some increase in survival in the mice that received the B7-H3 CAR T cells, compared to mice that received untransduced CAR T cells, this clearly is not as effective as in our solid tumor models,” Dr. Majzner said.
Going back to the cell line, they discovered that expression of B7-H3 was considerably lower in the K562 cells than in either the osteosarcoma or medulloblastoma cell lines used in their other models.
They found that both in vitro and in vivo, high levels of B7-H3 expression were necessary to provoke the immune system into releasing cytokines necessary for an adequate antitumor response.
The investigators are currently planning clinical trials using the B7-H3 CAR T-cell construct in patients with solid tumors.
The work is supported by the Sarcoma Alliance for Research through Collaboration, the St. Baldrick’s Foundation, and Stand Up to Cancer. Dr. Majzner reported having no financial disclosures.
SOURCE: Majzner RG et al. ASPHO 2018, Abstract #PS2003.
REPORTING FROM ASPHO 2018
Key clinical point:
Major finding: A single dose of the B7-H3 CAR caused complete regression of osteosarcoma and Ewing sarcoma xenografts and extended survival in mice.
Study details: Preclinical research.
Disclosures: The work is supported by the Sarcoma Alliance for Research through Collaboration, St. Baldrick’s Foundation, and Stand Up to Cancer. Dr. Majzner reported having no financial disclosures.
Source: Majzner RG et al. ASPHO 2018, Abstract #PS2003.
Myelin antibody predicts ADEM relapse
LOS ANGELES – There’s substantially higher risk of relapse and epilepsy after acute disseminated encephalomyelitis when children present with serum antibodies against myelin oligodendrocyte glycoprotein, according to British investigators.
“Traditionally, we told parents that ADEM [acute disseminated encephalomyelitis] is typically monophasic. I do tell parents now that the risk of relapse is higher if we see” myelin oligodendrocyte glycoprotein antibodies (MOG-Ab), said senior investigator Yael Hacohen, MBBS, DPhil, a pediatric neurology lecturer at University College London.
There was also a strong trend for relapsing disease when children presented with seizures, and an increased risk of post-ADEM epilepsy with oligoclonal bands on cerebrospinal fluid analysis, a marker of inflammation.
ADEM is an acute CNS demyelinating disorder primarily affecting young children, often after upper respiratory tract infections and occasionally after measles, mumps, and rubella vaccination. Signs can include limb weakness, stumbling, and coma. Many children recover without incident, but some don’t.
There’s been an increasing number of reports of children – and adults – presenting with antibodies against MOG, a glycoprotein on the outermost layer of the myelin sheath. Its exact function is unknown, but antibodies have been found in a number of inflammatory CNS conditions, and its role in pathogenesis is being explored. Testing is available for clinical use, but it isn’t standardized. For now, titer levels aren’t being used to guide treatment at University College London, Dr. Hacohen said at the American Academy of Neurology annual meeting.
The team reviewed 74 children with ADEM who presented at three pediatric neurology centers during 2005-2017, at a median age of 4.5 years. There were about equal numbers of boys and girls, and all had MRI abnormalities consistent with ADEM. Fifty children (68%) were MOG-ab positive.
Twenty-seven antibody-positive children (54%) relapsed, versus 3 of the 24 negative children (13%). Relapse was almost six times more likely with MOB-Ab (95% confidence interval [CI], 1.8-19.7; P = .002). The overall relapse rate of 42% (31/74) was higher than in previous studies, probably because of longer follow-up, lasting years in some cases.
Sixteen children (22%) presented with seizures, which nearly tripled the risk of relapse, although the finding wasn’t statistically significant (95% CI, 0.9-9.2; P = .06). There was a trend toward more seizures at onset in the MOG-Ab group.
Twelve children (16.2%) developed post-ADEM epilepsy, all but one MOG-Ab positive. The median time to seizure onset was 3 months. All of the children remained on antiepileptic medications at 2-year follow-up.
Oligoclonal bands were found in 8 of 37 children tested (22%), and also markedly increased the risk of post-ADEM seizures (odds ratio, 8.7; 95% CI, 1.5-54; P = .01).
“The majority of the children that we’ve looked at remain MOG-Ab positive. There may be a trend in antibody titers going down, but overall we didn’t find titers clinically useful. I know two children where titers went down. They relapsed,” and the titers went “up again, so we don’t’ really use them clinically for treatment,” Dr. Hacohen said.
“I think there is more to do” when it comes to optimizing ADEM management. “It’s a very heterogeneous [condition, and] I don’t want to put [everyone] on immunosuppression for years. I’ve got one patient who had an event, and 7 years later had a second event, and then was back to normal in a week.” On the other hand, “10%-20% of our patients do quite poorly and relapse on all treatments,” she said.
The investigators didn’t have any disclosures and there was no industry funding for the work.
SOURCE: Rossor T et al. Neurology. 2018 Apr 90(15 Suppl.):S35.004.
LOS ANGELES – There’s substantially higher risk of relapse and epilepsy after acute disseminated encephalomyelitis when children present with serum antibodies against myelin oligodendrocyte glycoprotein, according to British investigators.
“Traditionally, we told parents that ADEM [acute disseminated encephalomyelitis] is typically monophasic. I do tell parents now that the risk of relapse is higher if we see” myelin oligodendrocyte glycoprotein antibodies (MOG-Ab), said senior investigator Yael Hacohen, MBBS, DPhil, a pediatric neurology lecturer at University College London.
There was also a strong trend for relapsing disease when children presented with seizures, and an increased risk of post-ADEM epilepsy with oligoclonal bands on cerebrospinal fluid analysis, a marker of inflammation.
ADEM is an acute CNS demyelinating disorder primarily affecting young children, often after upper respiratory tract infections and occasionally after measles, mumps, and rubella vaccination. Signs can include limb weakness, stumbling, and coma. Many children recover without incident, but some don’t.
There’s been an increasing number of reports of children – and adults – presenting with antibodies against MOG, a glycoprotein on the outermost layer of the myelin sheath. Its exact function is unknown, but antibodies have been found in a number of inflammatory CNS conditions, and its role in pathogenesis is being explored. Testing is available for clinical use, but it isn’t standardized. For now, titer levels aren’t being used to guide treatment at University College London, Dr. Hacohen said at the American Academy of Neurology annual meeting.
The team reviewed 74 children with ADEM who presented at three pediatric neurology centers during 2005-2017, at a median age of 4.5 years. There were about equal numbers of boys and girls, and all had MRI abnormalities consistent with ADEM. Fifty children (68%) were MOG-ab positive.
Twenty-seven antibody-positive children (54%) relapsed, versus 3 of the 24 negative children (13%). Relapse was almost six times more likely with MOB-Ab (95% confidence interval [CI], 1.8-19.7; P = .002). The overall relapse rate of 42% (31/74) was higher than in previous studies, probably because of longer follow-up, lasting years in some cases.
Sixteen children (22%) presented with seizures, which nearly tripled the risk of relapse, although the finding wasn’t statistically significant (95% CI, 0.9-9.2; P = .06). There was a trend toward more seizures at onset in the MOG-Ab group.
Twelve children (16.2%) developed post-ADEM epilepsy, all but one MOG-Ab positive. The median time to seizure onset was 3 months. All of the children remained on antiepileptic medications at 2-year follow-up.
Oligoclonal bands were found in 8 of 37 children tested (22%), and also markedly increased the risk of post-ADEM seizures (odds ratio, 8.7; 95% CI, 1.5-54; P = .01).
“The majority of the children that we’ve looked at remain MOG-Ab positive. There may be a trend in antibody titers going down, but overall we didn’t find titers clinically useful. I know two children where titers went down. They relapsed,” and the titers went “up again, so we don’t’ really use them clinically for treatment,” Dr. Hacohen said.
“I think there is more to do” when it comes to optimizing ADEM management. “It’s a very heterogeneous [condition, and] I don’t want to put [everyone] on immunosuppression for years. I’ve got one patient who had an event, and 7 years later had a second event, and then was back to normal in a week.” On the other hand, “10%-20% of our patients do quite poorly and relapse on all treatments,” she said.
The investigators didn’t have any disclosures and there was no industry funding for the work.
SOURCE: Rossor T et al. Neurology. 2018 Apr 90(15 Suppl.):S35.004.
LOS ANGELES – There’s substantially higher risk of relapse and epilepsy after acute disseminated encephalomyelitis when children present with serum antibodies against myelin oligodendrocyte glycoprotein, according to British investigators.
“Traditionally, we told parents that ADEM [acute disseminated encephalomyelitis] is typically monophasic. I do tell parents now that the risk of relapse is higher if we see” myelin oligodendrocyte glycoprotein antibodies (MOG-Ab), said senior investigator Yael Hacohen, MBBS, DPhil, a pediatric neurology lecturer at University College London.
There was also a strong trend for relapsing disease when children presented with seizures, and an increased risk of post-ADEM epilepsy with oligoclonal bands on cerebrospinal fluid analysis, a marker of inflammation.
ADEM is an acute CNS demyelinating disorder primarily affecting young children, often after upper respiratory tract infections and occasionally after measles, mumps, and rubella vaccination. Signs can include limb weakness, stumbling, and coma. Many children recover without incident, but some don’t.
There’s been an increasing number of reports of children – and adults – presenting with antibodies against MOG, a glycoprotein on the outermost layer of the myelin sheath. Its exact function is unknown, but antibodies have been found in a number of inflammatory CNS conditions, and its role in pathogenesis is being explored. Testing is available for clinical use, but it isn’t standardized. For now, titer levels aren’t being used to guide treatment at University College London, Dr. Hacohen said at the American Academy of Neurology annual meeting.
The team reviewed 74 children with ADEM who presented at three pediatric neurology centers during 2005-2017, at a median age of 4.5 years. There were about equal numbers of boys and girls, and all had MRI abnormalities consistent with ADEM. Fifty children (68%) were MOG-ab positive.
Twenty-seven antibody-positive children (54%) relapsed, versus 3 of the 24 negative children (13%). Relapse was almost six times more likely with MOB-Ab (95% confidence interval [CI], 1.8-19.7; P = .002). The overall relapse rate of 42% (31/74) was higher than in previous studies, probably because of longer follow-up, lasting years in some cases.
Sixteen children (22%) presented with seizures, which nearly tripled the risk of relapse, although the finding wasn’t statistically significant (95% CI, 0.9-9.2; P = .06). There was a trend toward more seizures at onset in the MOG-Ab group.
Twelve children (16.2%) developed post-ADEM epilepsy, all but one MOG-Ab positive. The median time to seizure onset was 3 months. All of the children remained on antiepileptic medications at 2-year follow-up.
Oligoclonal bands were found in 8 of 37 children tested (22%), and also markedly increased the risk of post-ADEM seizures (odds ratio, 8.7; 95% CI, 1.5-54; P = .01).
“The majority of the children that we’ve looked at remain MOG-Ab positive. There may be a trend in antibody titers going down, but overall we didn’t find titers clinically useful. I know two children where titers went down. They relapsed,” and the titers went “up again, so we don’t’ really use them clinically for treatment,” Dr. Hacohen said.
“I think there is more to do” when it comes to optimizing ADEM management. “It’s a very heterogeneous [condition, and] I don’t want to put [everyone] on immunosuppression for years. I’ve got one patient who had an event, and 7 years later had a second event, and then was back to normal in a week.” On the other hand, “10%-20% of our patients do quite poorly and relapse on all treatments,” she said.
The investigators didn’t have any disclosures and there was no industry funding for the work.
SOURCE: Rossor T et al. Neurology. 2018 Apr 90(15 Suppl.):S35.004.
REPORTING FROM AAN 2018
Key clinical point: Myelin oligodendrocyte glycoprotein antibodies help to identify children who will have relapsing ADEM.
Major finding: Twenty-seven out of 50 antibody-positive children (54%) relapsed, versus 3 of 24 negative children (13%).
Study details: Review of 74 children with ADEM
Disclosures: There was no industry funding, and the investigators had no disclosures.
Source: Rossor T et al. Neurology. 2018 Apr 90(15 Suppl.):S35.004.
Diabetes spending topped $101 billion in 2013
according to investigators from the University of Washington, Seattle.
The largest share of personal health spending on diabetes in 2013 went for prescribed retail pharmaceuticals, which tallied over $58 billion. That was followed by ambulatory care at $24 billion, inpatient care at just under $10 billion, nursing home care at $9 billion, and emergency department care at $0.4 billion, Ellen Squires and her associates said in Diabetes Care.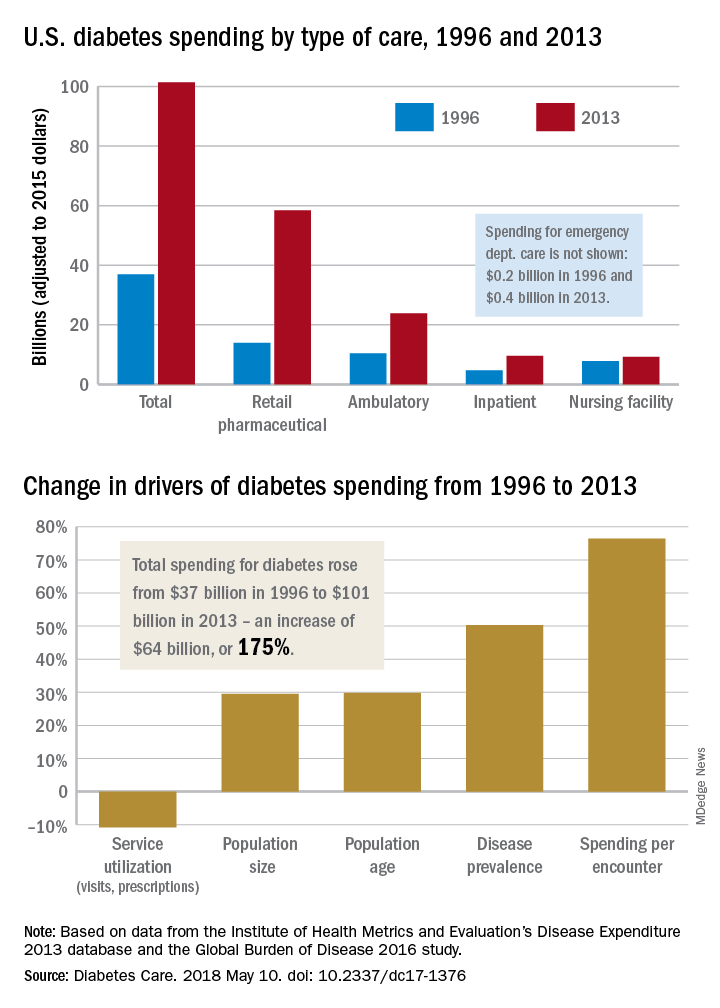
“The rate of increase in pharmaceutical spending was especially drastic from 2008 to 2013, and research suggests that these upward trends have continued in more recent years,” Ms. Squires and her associates wrote.
The analysis used data from the Institute for Health Metrics and Evaluation’s Disease Expenditure 2013 database and the Global Burden of Disease 2016 study. The current study was funded by the Peterson Center on Healthcare and the National Institute on Aging. One investigator receives research support from Medtronic Diabetes and is a consultant for Abbott Diabetes Care, Bigfoot Biomedical, Adocia, and Roche. No other relevant conflicts of interest were reported.
SOURCE: Squires E et al. Diabetes Care. 2018 May 10. doi: 10.2337/dc17-1376.
according to investigators from the University of Washington, Seattle.
The largest share of personal health spending on diabetes in 2013 went for prescribed retail pharmaceuticals, which tallied over $58 billion. That was followed by ambulatory care at $24 billion, inpatient care at just under $10 billion, nursing home care at $9 billion, and emergency department care at $0.4 billion, Ellen Squires and her associates said in Diabetes Care.
“The rate of increase in pharmaceutical spending was especially drastic from 2008 to 2013, and research suggests that these upward trends have continued in more recent years,” Ms. Squires and her associates wrote.
The analysis used data from the Institute for Health Metrics and Evaluation’s Disease Expenditure 2013 database and the Global Burden of Disease 2016 study. The current study was funded by the Peterson Center on Healthcare and the National Institute on Aging. One investigator receives research support from Medtronic Diabetes and is a consultant for Abbott Diabetes Care, Bigfoot Biomedical, Adocia, and Roche. No other relevant conflicts of interest were reported.
SOURCE: Squires E et al. Diabetes Care. 2018 May 10. doi: 10.2337/dc17-1376.
according to investigators from the University of Washington, Seattle.
The largest share of personal health spending on diabetes in 2013 went for prescribed retail pharmaceuticals, which tallied over $58 billion. That was followed by ambulatory care at $24 billion, inpatient care at just under $10 billion, nursing home care at $9 billion, and emergency department care at $0.4 billion, Ellen Squires and her associates said in Diabetes Care.
“The rate of increase in pharmaceutical spending was especially drastic from 2008 to 2013, and research suggests that these upward trends have continued in more recent years,” Ms. Squires and her associates wrote.
The analysis used data from the Institute for Health Metrics and Evaluation’s Disease Expenditure 2013 database and the Global Burden of Disease 2016 study. The current study was funded by the Peterson Center on Healthcare and the National Institute on Aging. One investigator receives research support from Medtronic Diabetes and is a consultant for Abbott Diabetes Care, Bigfoot Biomedical, Adocia, and Roche. No other relevant conflicts of interest were reported.
SOURCE: Squires E et al. Diabetes Care. 2018 May 10. doi: 10.2337/dc17-1376.
FROM DIABETES CARE
FDA: PrEP indication updated to include adolescents at risk of HIV infection
The following is the text of an announcement by the U.S. Food and Drug Administration regarding a revision in the Truvada label to expand the PrEP indication to include at-risk adolescents. The new label change has not yet been posted.
The Food and Drug Administration approved revisions to the Truvada (emtricitabine and tenofovir disoproxil fumarate) labeling to expand the Pre-Exposure Prophylaxis (PrEP) indication to include adolescents weighing at least 35 kg who are at risk of HIV-1 acquisition. The major labeling changes with respect to this expanded indication are summarized below. In addition, Section 8 was reformatted per the Pregnancy and Lactation Labeling Rule (PLLR) and includes updated information specific to the use of Truvada for PrEP during pregnancy and breastfeeding. Other sections of labeling were reformatted for consistency with current and best labeling practices, as well as with labeling for other HIV fixed-dose combination products.
Indications and usage
1.2 HIV-1 pre-exposure prophylaxis (PrEP)
Truvada is indicated in combination with safer sex practices for pre-exposure prophylaxis (PrEP) to reduce the risk of sexually acquired HIV-1 in at-risk adults and adolescents weighing at least 35 kg. Individuals must have a negative HIV-1 test immediately prior to initiating Truvada for HIV-1 PrEP.
If clinical symptoms consistent with acute viral infection are present and recent (less than 1 month) exposures are suspected, delay starting PrEP for at least one month and reconfirm HIV-1 status or use a test cleared by the FDA as an aid in the diagnosis of HIV-1 infection, including acute or primary HIV-1 infection
When considering Truvada for HIV-1 PrEP, factors that help to identify individuals at risk may include:
– has partner(s) known to be HIV-1 infected, or
– engages in sexual activity within a high prevalence area or social network and has additional risk factors for HIV-1 acquisition, such as:
- inconsistent or no condom use.
- diagnosis of sexually transmitted infections.
- exchange of sex for commodities (such as money, food, shelter, or drugs).
- use of illicit drugs or alcohol dependence.
- incarceration.
- partner(s) of unknown HIV-1 status with any of the factors listed above.
Dosage and administration
2.1 Testing prior to initiation of Truvada for treatment of HIV-1 infection or for HIV-1 PrEP
Prior to or when initiating Truvada, test patients for hepatitis B virus infection [see Warnings and Precautions (5.1)].
Prior to initiation and during use of Truvada, on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose and urine protein in all patients. In patients with chronic kidney disease, also assess serum phosphorus
2.2 HIV-1 screening for individuals receiving Truvada for HIV-1 PrEP
Screen all patients for HIV-1 infection before initiating Truvada for HIV-1 PrEP and at least once every 3 months while taking Truvada
2.5 Recommended dosage for HIV-1 PrEP
The dosage of Truvada in HIV-1 uninfected adults and adolescents weighing at least 35 kg is one tablet (containing 200 mg of FTC and 300 mg of TDF) once daily taken orally with or without food.
6.0 Adverse reactions
Clinical trials in adolescent subjects
In a single-arm, open-label clinical trial (ATN113), in which 67 HIV-1 uninfected adolescent (15 to 18 years of age) men who have sex with men received Truvada once daily for HIV-1 PrEP, the safety profile of Truvada was similar to that observed in adults. Median duration to exposure of Truvada was 47 weeks.
In the ATN113 trial, median BMD increased from baseline to Week 48, +2.58% for lumbar spine and +0.72% for total body. One subject had significant (greater than or equal to 4%) total body BMD loss at Week 24. Median changes from baseline BMD Z-scores were 0.0 for lumbar spine and −0.2 for total body at Week 48. Three subjects showed a worsening (change from greater than −2 to less than or equal to −2) from baseline in their lumbar spine or total body BMD Z-scores at Week 24 or 48. Interpretation of these data, however, may be limited by the low rate of adherence to Truvada by Week 48.
8.4 Pediatric use
HIV-1 PrEP
The safety and effectiveness of Truvada for HIV-1 PrEP in at-risk adolescents weighing at least 35 kg is supported by data from adequate and well-controlled studies of Truvada for HIV-1 PrEP in adults with additional data from safety and pharmacokinetic studies in previously conducted trials with the individual drug products, FTC and TDF, in HIV-1 infected adults and pediatric subjects.
Safety, adherence, and resistance were evaluated in a single-arm, open-label clinical trial (ATN113) in which 67 HIV-1 uninfected at-risk adolescent men who have sex with men received Truvada once daily for HIV-1 PrEP. The mean age of subjects was 17 years (range, 15-18 years); 46% were Hispanic, 52% black, and 37% white. The safety profile of Truvada in ATN113 was similar to that observed in the adult HIV-1 PrEP trials.
In the ATN113 trial, HIV-1 seroconversion occurred in three subjects. Tenofovir diphosphate levels in dried blood spot assays indicate that these subjects had poor adherence. No tenofovir- or FTC-associated HIV-1 resistance substitutions were detected in virus isolated from the three subjects who seroconverted.
Adherence to study drug, as demonstrated by tenofovir diphosphate levels in dried blood spot assays, declined markedly after Week 12 once subjects switched from monthly to quarterly visits, suggesting that adolescents may benefit from more frequent visits and counseling.
12.0 Clinical pharmacology
HIV-1 PrEP
The pharmacokinetic data for tenofovir and FTC following administration of Truvada in HIV-1 uninfected adolescents weighing 35 kg and above are not available. The dosage recommendations of Truvada for HIV-1 PrEP in this population are based on safety and adherence data from the ATN113 trial [see Use in Specific Populations (8.4)] and known pharmacokinetic information in HIV-infected adolescents taking TDF and FTC for treatment.
ResistanceATN113 Trial
In ATN113, a clinical trial of HIV-1 seronegative adolescent subjects [see Use in Specific Populations (8.4)], no amino acid substitutions associated with resistance to FTC or TDF were detected at the time of seroconversion from any of the 3 subjects who became infected with HIV-1 during the trial. All 3 subjects who seroconverted were nonadherent to the recommended Truvada dosage.
The updated label will soon be available at drugs@fda or DailyMed.
The following is the text of an announcement by the U.S. Food and Drug Administration regarding a revision in the Truvada label to expand the PrEP indication to include at-risk adolescents. The new label change has not yet been posted.
The Food and Drug Administration approved revisions to the Truvada (emtricitabine and tenofovir disoproxil fumarate) labeling to expand the Pre-Exposure Prophylaxis (PrEP) indication to include adolescents weighing at least 35 kg who are at risk of HIV-1 acquisition. The major labeling changes with respect to this expanded indication are summarized below. In addition, Section 8 was reformatted per the Pregnancy and Lactation Labeling Rule (PLLR) and includes updated information specific to the use of Truvada for PrEP during pregnancy and breastfeeding. Other sections of labeling were reformatted for consistency with current and best labeling practices, as well as with labeling for other HIV fixed-dose combination products.
Indications and usage
1.2 HIV-1 pre-exposure prophylaxis (PrEP)
Truvada is indicated in combination with safer sex practices for pre-exposure prophylaxis (PrEP) to reduce the risk of sexually acquired HIV-1 in at-risk adults and adolescents weighing at least 35 kg. Individuals must have a negative HIV-1 test immediately prior to initiating Truvada for HIV-1 PrEP.
If clinical symptoms consistent with acute viral infection are present and recent (less than 1 month) exposures are suspected, delay starting PrEP for at least one month and reconfirm HIV-1 status or use a test cleared by the FDA as an aid in the diagnosis of HIV-1 infection, including acute or primary HIV-1 infection
When considering Truvada for HIV-1 PrEP, factors that help to identify individuals at risk may include:
– has partner(s) known to be HIV-1 infected, or
– engages in sexual activity within a high prevalence area or social network and has additional risk factors for HIV-1 acquisition, such as:
- inconsistent or no condom use.
- diagnosis of sexually transmitted infections.
- exchange of sex for commodities (such as money, food, shelter, or drugs).
- use of illicit drugs or alcohol dependence.
- incarceration.
- partner(s) of unknown HIV-1 status with any of the factors listed above.
Dosage and administration
2.1 Testing prior to initiation of Truvada for treatment of HIV-1 infection or for HIV-1 PrEP
Prior to or when initiating Truvada, test patients for hepatitis B virus infection [see Warnings and Precautions (5.1)].
Prior to initiation and during use of Truvada, on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose and urine protein in all patients. In patients with chronic kidney disease, also assess serum phosphorus
2.2 HIV-1 screening for individuals receiving Truvada for HIV-1 PrEP
Screen all patients for HIV-1 infection before initiating Truvada for HIV-1 PrEP and at least once every 3 months while taking Truvada
2.5 Recommended dosage for HIV-1 PrEP
The dosage of Truvada in HIV-1 uninfected adults and adolescents weighing at least 35 kg is one tablet (containing 200 mg of FTC and 300 mg of TDF) once daily taken orally with or without food.
6.0 Adverse reactions
Clinical trials in adolescent subjects
In a single-arm, open-label clinical trial (ATN113), in which 67 HIV-1 uninfected adolescent (15 to 18 years of age) men who have sex with men received Truvada once daily for HIV-1 PrEP, the safety profile of Truvada was similar to that observed in adults. Median duration to exposure of Truvada was 47 weeks.
In the ATN113 trial, median BMD increased from baseline to Week 48, +2.58% for lumbar spine and +0.72% for total body. One subject had significant (greater than or equal to 4%) total body BMD loss at Week 24. Median changes from baseline BMD Z-scores were 0.0 for lumbar spine and −0.2 for total body at Week 48. Three subjects showed a worsening (change from greater than −2 to less than or equal to −2) from baseline in their lumbar spine or total body BMD Z-scores at Week 24 or 48. Interpretation of these data, however, may be limited by the low rate of adherence to Truvada by Week 48.
8.4 Pediatric use
HIV-1 PrEP
The safety and effectiveness of Truvada for HIV-1 PrEP in at-risk adolescents weighing at least 35 kg is supported by data from adequate and well-controlled studies of Truvada for HIV-1 PrEP in adults with additional data from safety and pharmacokinetic studies in previously conducted trials with the individual drug products, FTC and TDF, in HIV-1 infected adults and pediatric subjects.
Safety, adherence, and resistance were evaluated in a single-arm, open-label clinical trial (ATN113) in which 67 HIV-1 uninfected at-risk adolescent men who have sex with men received Truvada once daily for HIV-1 PrEP. The mean age of subjects was 17 years (range, 15-18 years); 46% were Hispanic, 52% black, and 37% white. The safety profile of Truvada in ATN113 was similar to that observed in the adult HIV-1 PrEP trials.
In the ATN113 trial, HIV-1 seroconversion occurred in three subjects. Tenofovir diphosphate levels in dried blood spot assays indicate that these subjects had poor adherence. No tenofovir- or FTC-associated HIV-1 resistance substitutions were detected in virus isolated from the three subjects who seroconverted.
Adherence to study drug, as demonstrated by tenofovir diphosphate levels in dried blood spot assays, declined markedly after Week 12 once subjects switched from monthly to quarterly visits, suggesting that adolescents may benefit from more frequent visits and counseling.
12.0 Clinical pharmacology
HIV-1 PrEP
The pharmacokinetic data for tenofovir and FTC following administration of Truvada in HIV-1 uninfected adolescents weighing 35 kg and above are not available. The dosage recommendations of Truvada for HIV-1 PrEP in this population are based on safety and adherence data from the ATN113 trial [see Use in Specific Populations (8.4)] and known pharmacokinetic information in HIV-infected adolescents taking TDF and FTC for treatment.
ResistanceATN113 Trial
In ATN113, a clinical trial of HIV-1 seronegative adolescent subjects [see Use in Specific Populations (8.4)], no amino acid substitutions associated with resistance to FTC or TDF were detected at the time of seroconversion from any of the 3 subjects who became infected with HIV-1 during the trial. All 3 subjects who seroconverted were nonadherent to the recommended Truvada dosage.
The updated label will soon be available at drugs@fda or DailyMed.
The following is the text of an announcement by the U.S. Food and Drug Administration regarding a revision in the Truvada label to expand the PrEP indication to include at-risk adolescents. The new label change has not yet been posted.
The Food and Drug Administration approved revisions to the Truvada (emtricitabine and tenofovir disoproxil fumarate) labeling to expand the Pre-Exposure Prophylaxis (PrEP) indication to include adolescents weighing at least 35 kg who are at risk of HIV-1 acquisition. The major labeling changes with respect to this expanded indication are summarized below. In addition, Section 8 was reformatted per the Pregnancy and Lactation Labeling Rule (PLLR) and includes updated information specific to the use of Truvada for PrEP during pregnancy and breastfeeding. Other sections of labeling were reformatted for consistency with current and best labeling practices, as well as with labeling for other HIV fixed-dose combination products.
Indications and usage
1.2 HIV-1 pre-exposure prophylaxis (PrEP)
Truvada is indicated in combination with safer sex practices for pre-exposure prophylaxis (PrEP) to reduce the risk of sexually acquired HIV-1 in at-risk adults and adolescents weighing at least 35 kg. Individuals must have a negative HIV-1 test immediately prior to initiating Truvada for HIV-1 PrEP.
If clinical symptoms consistent with acute viral infection are present and recent (less than 1 month) exposures are suspected, delay starting PrEP for at least one month and reconfirm HIV-1 status or use a test cleared by the FDA as an aid in the diagnosis of HIV-1 infection, including acute or primary HIV-1 infection
When considering Truvada for HIV-1 PrEP, factors that help to identify individuals at risk may include:
– has partner(s) known to be HIV-1 infected, or
– engages in sexual activity within a high prevalence area or social network and has additional risk factors for HIV-1 acquisition, such as:
- inconsistent or no condom use.
- diagnosis of sexually transmitted infections.
- exchange of sex for commodities (such as money, food, shelter, or drugs).
- use of illicit drugs or alcohol dependence.
- incarceration.
- partner(s) of unknown HIV-1 status with any of the factors listed above.
Dosage and administration
2.1 Testing prior to initiation of Truvada for treatment of HIV-1 infection or for HIV-1 PrEP
Prior to or when initiating Truvada, test patients for hepatitis B virus infection [see Warnings and Precautions (5.1)].
Prior to initiation and during use of Truvada, on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose and urine protein in all patients. In patients with chronic kidney disease, also assess serum phosphorus
2.2 HIV-1 screening for individuals receiving Truvada for HIV-1 PrEP
Screen all patients for HIV-1 infection before initiating Truvada for HIV-1 PrEP and at least once every 3 months while taking Truvada
2.5 Recommended dosage for HIV-1 PrEP
The dosage of Truvada in HIV-1 uninfected adults and adolescents weighing at least 35 kg is one tablet (containing 200 mg of FTC and 300 mg of TDF) once daily taken orally with or without food.
6.0 Adverse reactions
Clinical trials in adolescent subjects
In a single-arm, open-label clinical trial (ATN113), in which 67 HIV-1 uninfected adolescent (15 to 18 years of age) men who have sex with men received Truvada once daily for HIV-1 PrEP, the safety profile of Truvada was similar to that observed in adults. Median duration to exposure of Truvada was 47 weeks.
In the ATN113 trial, median BMD increased from baseline to Week 48, +2.58% for lumbar spine and +0.72% for total body. One subject had significant (greater than or equal to 4%) total body BMD loss at Week 24. Median changes from baseline BMD Z-scores were 0.0 for lumbar spine and −0.2 for total body at Week 48. Three subjects showed a worsening (change from greater than −2 to less than or equal to −2) from baseline in their lumbar spine or total body BMD Z-scores at Week 24 or 48. Interpretation of these data, however, may be limited by the low rate of adherence to Truvada by Week 48.
8.4 Pediatric use
HIV-1 PrEP
The safety and effectiveness of Truvada for HIV-1 PrEP in at-risk adolescents weighing at least 35 kg is supported by data from adequate and well-controlled studies of Truvada for HIV-1 PrEP in adults with additional data from safety and pharmacokinetic studies in previously conducted trials with the individual drug products, FTC and TDF, in HIV-1 infected adults and pediatric subjects.
Safety, adherence, and resistance were evaluated in a single-arm, open-label clinical trial (ATN113) in which 67 HIV-1 uninfected at-risk adolescent men who have sex with men received Truvada once daily for HIV-1 PrEP. The mean age of subjects was 17 years (range, 15-18 years); 46% were Hispanic, 52% black, and 37% white. The safety profile of Truvada in ATN113 was similar to that observed in the adult HIV-1 PrEP trials.
In the ATN113 trial, HIV-1 seroconversion occurred in three subjects. Tenofovir diphosphate levels in dried blood spot assays indicate that these subjects had poor adherence. No tenofovir- or FTC-associated HIV-1 resistance substitutions were detected in virus isolated from the three subjects who seroconverted.
Adherence to study drug, as demonstrated by tenofovir diphosphate levels in dried blood spot assays, declined markedly after Week 12 once subjects switched from monthly to quarterly visits, suggesting that adolescents may benefit from more frequent visits and counseling.
12.0 Clinical pharmacology
HIV-1 PrEP
The pharmacokinetic data for tenofovir and FTC following administration of Truvada in HIV-1 uninfected adolescents weighing 35 kg and above are not available. The dosage recommendations of Truvada for HIV-1 PrEP in this population are based on safety and adherence data from the ATN113 trial [see Use in Specific Populations (8.4)] and known pharmacokinetic information in HIV-infected adolescents taking TDF and FTC for treatment.
ResistanceATN113 Trial
In ATN113, a clinical trial of HIV-1 seronegative adolescent subjects [see Use in Specific Populations (8.4)], no amino acid substitutions associated with resistance to FTC or TDF were detected at the time of seroconversion from any of the 3 subjects who became infected with HIV-1 during the trial. All 3 subjects who seroconverted were nonadherent to the recommended Truvada dosage.
The updated label will soon be available at drugs@fda or DailyMed.
Study supports observation for select cases of porcelain gallbladder
Some adults with porcelain gallbladder may be eligible to forgo prophylactic cholecystectomy, suggest the results of a single-center retrospective study.
Over 1.7 years of median follow-up (range, 0 to 12.7 years), the observational group had no detected gallbladder malignancies and 4% developed adverse events versus 13% in the prophylactic cholecystectomy group (P = .15), wrote Haley DesJardins and her associates at Tufts University, Boston. The report was published in the Journal of the American College of Surgery.
The findings “still raise concern about an association between gallbladder wall calcifications and gallbladder malignancies, and therefore still suggest the need for cholecystectomy in the young, healthy, or symptomatic patient,” the researchers wrote. Nonetheless, surveillance for patients “who are poor surgical candidates is a reasonable approach, with a low risk of malignancy over a limited time frame.”
The investigators suggest that surgeons consider intervention when symptoms and workup points to gallbladder malignancy. But consider avoiding prophylactic cholecystectomy in patients with “limited life expectancy and significant comorbidities,” they emphasized. “Based on the results of this study, the act of prophylactic cholecystectomy for every single patient with gallbladder wall calcifications seems obsolete.”
The study comprised 113 patients with porcelain gallbladder diagnosed between 2004 and 2016. Radiographic reviews identified 70 definite cases and 43 “highly probable” cases. In all, 90 patients started out with observation only, of whom 26% with abdominal pain did not have cholecystectomy because of “significant comorbidities.” Four patients (4.4%) in the observational group subsequently underwent cholecystectomy for biliary colic, as part of liver transplantation, or for prophylactic reasons. None developed complications. In all, 11% developed new gallstones on follow-up imaging and 8% showed progression from focal to diffuse porcelain bladder, the researchers said. None developed gallbladder malignancy during 1.7 years of median follow-up.
Histopathologies of the operative group identified two cases of gallbladder malignancy, of which one was detected on initial imaging. “This patient had a mass at the gallbladder infundibulum extending into the hepatic duct bifurcation,” the researchers explained. “It was not entirely evident whether the resected adenocarcinoma was originating from the gallbladder or from the bile duct. For the purpose of this study, this patient was listed as [having] gallbladder cancer.” The second case consisted of metastatic squamous cell gallbladder carcinoma.
The investigators concluded that “while it is seemingly very reasonable to observe asymptomatic patients with limited life expectancy and significant comorbidities, the decision to proceed with prophylactic cholecystectomy versus observation remains in the hands of the treating physician and patient; especially since absolute criteria or cut-offs cannot be defined at this point.”
No external funding sources were reported. The researchers reported having no conflicts of interest.
SOURCE: DesJardins H et al. J Am Coll Surg. 2018 Apr 22. doi: 10.1016/j.jamcollsurg.2017.11.026.
Some adults with porcelain gallbladder may be eligible to forgo prophylactic cholecystectomy, suggest the results of a single-center retrospective study.
Over 1.7 years of median follow-up (range, 0 to 12.7 years), the observational group had no detected gallbladder malignancies and 4% developed adverse events versus 13% in the prophylactic cholecystectomy group (P = .15), wrote Haley DesJardins and her associates at Tufts University, Boston. The report was published in the Journal of the American College of Surgery.
The findings “still raise concern about an association between gallbladder wall calcifications and gallbladder malignancies, and therefore still suggest the need for cholecystectomy in the young, healthy, or symptomatic patient,” the researchers wrote. Nonetheless, surveillance for patients “who are poor surgical candidates is a reasonable approach, with a low risk of malignancy over a limited time frame.”
The investigators suggest that surgeons consider intervention when symptoms and workup points to gallbladder malignancy. But consider avoiding prophylactic cholecystectomy in patients with “limited life expectancy and significant comorbidities,” they emphasized. “Based on the results of this study, the act of prophylactic cholecystectomy for every single patient with gallbladder wall calcifications seems obsolete.”
The study comprised 113 patients with porcelain gallbladder diagnosed between 2004 and 2016. Radiographic reviews identified 70 definite cases and 43 “highly probable” cases. In all, 90 patients started out with observation only, of whom 26% with abdominal pain did not have cholecystectomy because of “significant comorbidities.” Four patients (4.4%) in the observational group subsequently underwent cholecystectomy for biliary colic, as part of liver transplantation, or for prophylactic reasons. None developed complications. In all, 11% developed new gallstones on follow-up imaging and 8% showed progression from focal to diffuse porcelain bladder, the researchers said. None developed gallbladder malignancy during 1.7 years of median follow-up.
Histopathologies of the operative group identified two cases of gallbladder malignancy, of which one was detected on initial imaging. “This patient had a mass at the gallbladder infundibulum extending into the hepatic duct bifurcation,” the researchers explained. “It was not entirely evident whether the resected adenocarcinoma was originating from the gallbladder or from the bile duct. For the purpose of this study, this patient was listed as [having] gallbladder cancer.” The second case consisted of metastatic squamous cell gallbladder carcinoma.
The investigators concluded that “while it is seemingly very reasonable to observe asymptomatic patients with limited life expectancy and significant comorbidities, the decision to proceed with prophylactic cholecystectomy versus observation remains in the hands of the treating physician and patient; especially since absolute criteria or cut-offs cannot be defined at this point.”
No external funding sources were reported. The researchers reported having no conflicts of interest.
SOURCE: DesJardins H et al. J Am Coll Surg. 2018 Apr 22. doi: 10.1016/j.jamcollsurg.2017.11.026.
Some adults with porcelain gallbladder may be eligible to forgo prophylactic cholecystectomy, suggest the results of a single-center retrospective study.
Over 1.7 years of median follow-up (range, 0 to 12.7 years), the observational group had no detected gallbladder malignancies and 4% developed adverse events versus 13% in the prophylactic cholecystectomy group (P = .15), wrote Haley DesJardins and her associates at Tufts University, Boston. The report was published in the Journal of the American College of Surgery.
The findings “still raise concern about an association between gallbladder wall calcifications and gallbladder malignancies, and therefore still suggest the need for cholecystectomy in the young, healthy, or symptomatic patient,” the researchers wrote. Nonetheless, surveillance for patients “who are poor surgical candidates is a reasonable approach, with a low risk of malignancy over a limited time frame.”
The investigators suggest that surgeons consider intervention when symptoms and workup points to gallbladder malignancy. But consider avoiding prophylactic cholecystectomy in patients with “limited life expectancy and significant comorbidities,” they emphasized. “Based on the results of this study, the act of prophylactic cholecystectomy for every single patient with gallbladder wall calcifications seems obsolete.”
The study comprised 113 patients with porcelain gallbladder diagnosed between 2004 and 2016. Radiographic reviews identified 70 definite cases and 43 “highly probable” cases. In all, 90 patients started out with observation only, of whom 26% with abdominal pain did not have cholecystectomy because of “significant comorbidities.” Four patients (4.4%) in the observational group subsequently underwent cholecystectomy for biliary colic, as part of liver transplantation, or for prophylactic reasons. None developed complications. In all, 11% developed new gallstones on follow-up imaging and 8% showed progression from focal to diffuse porcelain bladder, the researchers said. None developed gallbladder malignancy during 1.7 years of median follow-up.
Histopathologies of the operative group identified two cases of gallbladder malignancy, of which one was detected on initial imaging. “This patient had a mass at the gallbladder infundibulum extending into the hepatic duct bifurcation,” the researchers explained. “It was not entirely evident whether the resected adenocarcinoma was originating from the gallbladder or from the bile duct. For the purpose of this study, this patient was listed as [having] gallbladder cancer.” The second case consisted of metastatic squamous cell gallbladder carcinoma.
The investigators concluded that “while it is seemingly very reasonable to observe asymptomatic patients with limited life expectancy and significant comorbidities, the decision to proceed with prophylactic cholecystectomy versus observation remains in the hands of the treating physician and patient; especially since absolute criteria or cut-offs cannot be defined at this point.”
No external funding sources were reported. The researchers reported having no conflicts of interest.
SOURCE: DesJardins H et al. J Am Coll Surg. 2018 Apr 22. doi: 10.1016/j.jamcollsurg.2017.11.026.
FROM JOURNAL OF THE AMERICAN COLLEGE OF SURGERY
Key clinical point: Observation is an option for select patients with porcelain gallbladder .
Major finding: Rates of adverse events were 4% with observation and 13% with surgery (P = .15).
Study details: Single-center retrospective cohort study of 113 patients.
Disclosures: No external funding sources were reported. The researchers reported having no conflicts of interest.
Source: DesJardins H et al. J Am Coll Surg. 2018 Apr 22. doi: 10.1016/j.jamcollsurg.2017.11.026.