User login
Digital Ischemia From Accidental Epinephrine Injection
Patients presenting to the ED with injuries due to accidental self-injection with an epinephrine pen typically receive treatment to alleviate symptoms and reduce the potential of digital ischemia leading to gangrene and loss of tissue and function. Although there is no consensus or set guidelines in the literature regarding the management protocol of such cases, many reports support pharmacological intervention. There are, however, other reports that advocate conservative, nonpharmaceutical management (eg, immersing the affected digit in warm water) or an observation-only approach.
We present the first case report in Saudi Arabia of digital ischemia due to accidental injection of an epinephrine autoinjector, along with a review of the literature and management recommendations.
Case
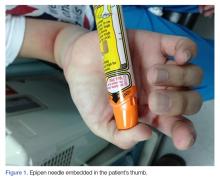
A 28-year-old woman presented to the ED in significant pain and discomfort 20 minutes after she accidentally injected the entire contents of her aunt’s epinephrine autoinjector (0.3 mg of 1:1000) into her right thumb. The patient, who was in significant pain and discomfort, stated that she was unable to remove the injector needle, which was firmly embedded in the bone of the palmer aspect of the distal phalanx in a manner similar to that of an intraosseous injection (Figure 1).
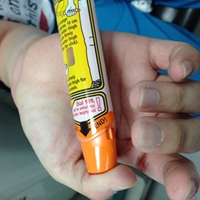
The patient’s vital signs and oxygen saturation on presentation were within normal limits. The emergency physician successfully removed the embedded needle through moderate countertraction. On examination, the patient’s right thumb was pale and cold, and had poor capillary refill (Figure 2). Due to concerns of the potential for digital tissue ischemia leading to tissue loss and gangrene, warm, moist compresses were applied to the affected thumb, followed by 2% topical nitroglycerin paste, after which the thumb was covered with an occlusive dressing. Since there was no improvement in circulation after 20 minutes, an infiltrate of 5 mg (0.5 mL of 10 mg/mL) of phentolamine (α-agonist) mixed with 2.5 mL of 2% lidocaine was injected at the puncture site and base of the right thumb.1 Hyperemia developed immediately at both injection sites, and the patient’s right thumb returned to a normal color and sensation 1 hour later, with a return to normal capillary refill. She remained in stable condition and was discharged home. Prior to discharge, the patient was educated on the proper handling and administration of an epinephrine autoinjector.
Discussion
Epinephrine is an ὰ- and β-adrenergic agonist that binds to the ὰ-adrenergic receptors of blood vessels, causing an increase in vascular resistance and vasoconstriction. Although the plasma half-life of epinephrine is approximately 2 to 3 minutes, subcutaneous or intramuscular injection resulting in local vasoconstriction may delay absorption; therefore, the effects of epinephrine may last much longer than its half-life.
The incidence of accidental injection from an epinephrine autoinjector is estimated to be 1 per 50,000 units dispensed.2 To date, there are no established treatment guidelines on managing cases of digital injection. An online PubMed and Google Scholar search of the literature found one systematic review,3 four observational studies,4-7 seven case series,8-14 and several case reports1,15-33 on the subject. Most of the patients in the published retrospective studies (71%) were treated conservatively with warming of the affected hand and observation, and the majority of patients in the case reports (87%) were treated pharmacologically, most commonly with topical nitroglycerin and phentolamine.1,3-34 All of the patients in both the retrospective studies and case reports had restoration of perfusion without necrosis, irrespective of treatment modality. However, patients who were managed conservatively or who were treated with topical nitroglycerin required a longer duration of stay in the ED, suffered from severe reperfusion pain, and in some cases, had a longer time to complete recovery (≥10 weeks).8
Pharmaceutical and Nonpharmaceutical Management
Phentolamine. Phentolamine is a nonselective ὰ-adrenergic antagonist that binds to ὰ1 and ὰ2 receptors of blood vessels, resulting in a decrease in peripheral vascular resistance and vasodilation. Phentolamine directly antagonizes the effect of epinephrine by blocking the ὰ-adrenergic receptors, which in our patient resulted in immediate return of digital circulation and full resolution of symptoms.
Topical Nitroglycerin. Nitroglycerin is a nitrate vasodilator that when metabolically converted to nitric oxide, results in smooth muscle relaxation, venodilation, and arteriodilation. Patients suffering from digital ischemia and vasoconstriction may be treated with topical nitroglycerin paste to reverse ischemia by causing smooth muscle relaxation of digital blood vessels. Conservative Management. As previously noted, not all cases of digital epinephrine injection are treated pharmacologically. Some patients are not treated, but kept in observation until the ischemic effects of epinephrine have resolved. Likewise, some patients are treated conservatively with warm water compresses or by fully immersing the affected digit in warm water to facilitate reversal of vasoconstriction and ischemia.3,8
Treatment Efficacy
In 2007, Fitzcharles-Bowe et al8 published a review of 59 cases of digital injection with high-dose epinephrine from 1989 to 2005. In this review, 32 of the 59 patients received no treatment, 25 patients received pharmacological treatment and in two patients, the treatment was unknown. Phentolamine was the most commonly used pharmacological agent (15 of 25 cases or 60%). Although none of the patients experienced digital necrosis, those treated with a local infiltration of phentolamine experienced a faster resolution of symptoms and normalization of perfusion. In 2004, Turner1 reported a case of a 10-year-old boy who was treated with phentolamine following an accidental injection of epinephrine into his left hand. While circulation returned to the affected digit within 5 minutes of receiving the phentolamine injection, the patient continued to experience reduced sensation in the digit 6 weeks later.8
Interestingly, one of the coauthors of the Fitzcharles-Bowe et al8 report intentionally injected three of the digits of his left hand (middle, ring, and small fingers) at the same time with high-dose epinephrine to carefully observe and document the outcomes. All three of the digits became very pale and cool, with decreased sensation. The author treated himself conservatively (observation-only). He experienced spontaneous return of circulation in two of the digits within 6 to 10 hours. Although there was some spontaneous return of circulation to the third digit after 13 hours, the author noted prolonged, intense reperfusion pain 4 hours after return of circulation. He also suffered from neuropraxia in the third digit, which did not fully resolve until 10 weeks after the injury.8
A review of the literature shows phentolamine to be a safe and effective treatment for patients presenting with digital ischemia, with no long-term adverse effects or complications. Moreover, phentolamine appears to be safe and effective for use in both adult and pediatric patients.3,8,35-38
Accidental Injection Prevention
Some of the cases of accidental epinephrine injection are due to user error. For example, a novice user may be holding the incorrect end of the injector in his or her hand when attempting to administer/deploy the device, resulting in premature dislodgement of the needle.39
Although, most of the autoinjector devices available today are user-friendly, we believe the addition of a safety feature such as a trigger or safety-lock may further help to reduce accidents. The European Medicines Agency recommends that all patients and caregivers receive training on the proper handling and administration of epinephrine autoinjectors, citing this as the most important factor to ensure successful use of an epinephrine autoinjector and reduce accidental injury.40 The patient in this case had not received any formal education or training regarding autoinjector use prior to this incident.
Safety of Lidocaine-Containing Epinephrine in Digital Anesthesia
Aside from cases of accidental digital epinephrine injection, clinicians have traditionally been taught to avoid using lidocaine with epinephrine for digital anesthesia. However, since the introduction of commercial lidocaine with epinephrine in 1948, there are no case reports of digital gangrene from commercially available lidocaine-epinephrine formulations.41,42 In a multicenter prospective study by Lalonde et al43 of 3,110 consecutive cases of elective injection of low-dose epinephrine in the hand, the authors concluded the likelihood of finger infarction is remote, particularly with possible phentolamine rescue therapy. Moreover, lidocaine-containing epinephrine (1%-2%) has a much lower concentration of epinephrine per mL of solution (5-10 mcg/mL) and appears to be safe for digital use.
Conclusion
This case describes the presentation and treatment of accidental digital injection of epinephrine, highlighting and supporting the benefits of local infiltration with phentolamine and observation until full recovery of perfusion. Local treatment with phentolamine not only facilitates recovery and return of capillary refill, but also shortens the duration of symptoms and alleviates vasoconstriction. In less severe cases, watchful waiting and observation may be appropriate and effective.
This case also underscores the importance of patient and caregiver education on the proper handling and administration of epinephrine autoinjectors to decrease the incidence of accidental injection.
1. Turner MJ. Accidental Epipen injection into a digit - the value of a Google search. Ann R Coll Surg Engl. 2004;86(3):218-219. doi:10.1308/003588404323043391.
2. McGovern SJ. Treatment of accidental digital injection of adrenaline from an auto-injector device. J Accid Emerg Med. 1997;14(6):379-380.
3. Wright M. Treatment after accidental injection with epinephrine autoinjector: a systematic review. J Allergy & Therapy. 2014;5(3):1000175. doi:10.4172/2155-6121.1000175.
4. Mrvos R, Anderson BD, Krenzelok EP. Accidental injection of epinephrine from an autoinjector: invasive treatment not always required. South Med J. 2002;95(3):318-320.
5. Muck AE, Bebarta VS, Borys DJ, Morgan DL. Six years of epinephrine digital injections: absence of significant local or systemic effects. Ann Emerg Med. 2010;56(3):270-274. doi:10.1016/j.annemergmed.2010.02.019.
6. Simons FE, Edwards ES, Read EJ Jr, Clark S, Liebelt EL. Voluntarily reported unintentional injections from epinephrine auto-injectors. J Allergy Clin Immunol. 2010;125(2):419-423. doi:10.1016/j.jaci.2009.10.056.
7. Blume-Odom CM, Scalzo AJ, Weber JA. EpiPen accidental injection-134 cases over 10 years. Clin Toxicol. 2010;48:651.
8. Fitzcharles-Bowe C, Denkler K, Lalonde D. Finger injection with high-dose (1:1,000) epinephrine: Does it cause finger necrosis and should it be treated? Hand. 2007;2(1):5-11. doi:10.1007/s11552-006-9012-4.
9. Velissariou I, Cottrell S, Berry K, Wilson B. Management of adrenaline (epinephrine) induced digital ischaemia in children after accidental injection from an EpiPen. Emerg Med J. 2004;21(3):387-388.
10. ElMaraghy MW, ElMaraghy AW, Evans HB. Digital adrenaline injection injuries: a case series and review. Can J Plast Surg. 1998;6:196-200.
11. Skorpinski EW, McGeady SJ, Yousef E. Two cases of accidental epinephrine injection into a finger. J Allergy Clin Immunol. 2006;117(2):463-464.
12. Nagaraj J, Reddy S, Murray R, Murphy N. Use of glyceryl trinitrate patches in the treatment of accidental digital injection of epinephrine from an autoinjector. Eur J Emerg Med. 2009;16(4):227-228. doi:10.1097/MEJ.0b013e328306f0ee.
13. Stier PA, Bogner MP, Webster K, Leikin JB, Burda A. Use of subcutaneous terbutaline to reverse peripheral ischemia. Am J Emerg Med. 1999;17(1):91-94.
14. Lee G, Thomas PC. Accidental digital injection of adrenaline from an autoinjector device. J Accid Emerg Med. 1998;15(4):287.
15. Baris S, Saricoban HE, Ak K, Ozdemir C. Papaverine chloride as a topical vasodilator in accidental injection of adrenaline into a digital finger. Allergy. 2011;66(11):1495-1496. doi:10.1111/j.1398-9995.2011.02664.x.
16. Buse K, Hein W, Drager N. Making Sense of Global Health Governance: A Policy Perspective. Basingstoke, England: Palgrave Macmillan UK; 2009.
17. Sherman SC. Digital Epipen® injection: a case of conservative management. J Emerg Med. 2011;41(6):672-674. doi:10.1016/j.jemermed.2009.07.027.
18. Janssen RL, Roeleveld-Versteegh AB, Wessels-Basten SJ, Hendriks T. [Auto-injection with epinephrine in the finger of a 5-year-old child]. Ned Tijdschr Geneeskd. 2008;152(17):1005-1008.
19. Singh T, Randhawa S, Khanna R. The EpiPen and the ischaemic finger. Eur J Emerg Med. 2007;14(4):222-223.
20. Barkhordarian AR, Wakelin SH, Paes TR. Accidental digital injection of adrenaline from an autoinjector device. Br J Dermatol. 2000;143(6):1359.
21. Deshmukh N, Tolland JT. Treatment of accidental epinephrine injection in a finger. J Emerg Med. 1989;7(4):408.
22. Hinterberger JW, Kintzi HE. Phentolamine reversal of epinephrine-induced digital vasospasm. How to save an ischemic finger. Arch Fam Med. 1994;3(2):193-195.
23. Peyko V, Cohen V, Jellinek-Cohen SP, Pearl-Davis M. Evaluation and treatment of accidental autoinjection of epinephrine. Am J Health Syst Pharm. 2013;70(9):778-781. doi:10.2146/ajhp120316.
24. Hardy SJ, Agostini DE. Accidental epinephrine auto-injector-induced digital ischemia reversed by phentolamine digital block. J Am Osteopath Assoc. 1995;95(6):377-378.
25. Kaspersen J, Vedsted P. [Accidental injection of adrenaline in a finger with EpiPen]. Ugeskr Laeger. 1998;160(45):6531-6532.
26. Schintler MV, Arbab E, Aberer W, Spendel S, Scharnagl E. Accidental perforating bone injury using the EpiPen autoinjection device. Allergy. 2005;60(2):259-260.
27. Khairalla E. Epinephrine-induced digital ischemia relieved by phentolamine. Plast Reconstr Surg. 2001;108(6):1831-1832.
28. Murali KS, Nayeem N. Accidental digital injection of adrenaline from an autoinjector device. J Accid Emerg Med. 1998;15(4):287.
29. Sellens C, Morrison L. Accidental injection of epinephrine by a child: a unique approach to treatment. CJEM. 1999;1(1):34-36.
30. Klemawesch P. Hyperbaric oxygen relieves severe digital ischaemia from accidental EpiPen injection. 2009 American Academy of Allergy, Asthma and Immunology Annual Meeting.
31. McCauley WA, Gerace RV, Scilley C. Treatment of accidental digital injection of epinephrine. Ann Emerg Med. 1991;20(6):665-668.
32. Mathez C, Favrat B, Staeger P. Management options for accidental injection of epinephrine from an autoinjector: a case report. J Med Case Rep. 2009;3:7268. doi:10.4076/1752-1947-3-7268.
33. Molony D. Adrenaline-induced digital ischaemia reversed with phentolamine. ANZ J Surg. 2006;76(12):1125-1126.
34. Carrascosa MF, Gallastegui-Menéndez A, Teja-Santamaría C, Caviedes JR. Accidental finger ischaemia induced by epinephrine autoinjector. BMJ Case Rep. 2013;2013. pii:bcr2013200783. doi:10.1136/bcr-2013-200783.
35. Patel R, Kumar H. Epinephrine induced digital ischemia after accidental injection from an auto-injector device. Indian Pediatr. 2013;50(2):247.
36. Xu J, Holt A. Use of Phentolamine in the treatment of Epipen induced digital ischemia. BMJ Case Rep. 2012;2012. doi:10.1136/bcr.12.2011.5450.
37. McNeil C, Copeland J. Accidental digital epinephrine injection: to treat or not to treat? Can Fam Physician. 2014;60(8):726-728.
38. Bodkin RP, Acquisto NM, Gunyan H, Wiegand TJ. Two cases of accidental injection of epinephrine into a digit treated with subcutaneous phentolamine injections. Case Rep Emerg Med. 2013;2013:586207. doi:10.1155/2013/586207.
39. Simons FE, Lieberman PL, Read EJ Jr, Edwards ES. Hazards of unintentional injection of epinephrine from autoinjectors: a systematic review. Ann Allergy Asthma Immunol. 2009;102(4):282-287. doi:10.1016/S1081-1206(10)60332-8.
40. European Medicines Agency. Better training tools recommended to support patients using adrenaline auto-injectors. European Medicines Agency, 2015.
41. Denkler K. A comprehensive review of epinephrine in the finger: to do or not to do.
42. Thomson CJ, Lalonde DH, Denkler KA, Feicht AJ. A critical look at the evidence for and against elective epinephrine use in the finger. Plast Reconstr Surg. 2007;119(1):260-266.
43. Lalonde D, Bell M, Benoit P, Sparkes G, Denkler K, Chang P. A multicenter prospective study of 3,110 consecutive cases of elective epinephrine use in the fingers and hand: the Dalhousie Project clinical phase. J Hand Surg Am. 2005;30(5):1061-1067. doi:10.1016/j.jhsa.2005.05.006.
Patients presenting to the ED with injuries due to accidental self-injection with an epinephrine pen typically receive treatment to alleviate symptoms and reduce the potential of digital ischemia leading to gangrene and loss of tissue and function. Although there is no consensus or set guidelines in the literature regarding the management protocol of such cases, many reports support pharmacological intervention. There are, however, other reports that advocate conservative, nonpharmaceutical management (eg, immersing the affected digit in warm water) or an observation-only approach.
We present the first case report in Saudi Arabia of digital ischemia due to accidental injection of an epinephrine autoinjector, along with a review of the literature and management recommendations.
Case
A 28-year-old woman presented to the ED in significant pain and discomfort 20 minutes after she accidentally injected the entire contents of her aunt’s epinephrine autoinjector (0.3 mg of 1:1000) into her right thumb. The patient, who was in significant pain and discomfort, stated that she was unable to remove the injector needle, which was firmly embedded in the bone of the palmer aspect of the distal phalanx in a manner similar to that of an intraosseous injection (Figure 1).
The patient’s vital signs and oxygen saturation on presentation were within normal limits. The emergency physician successfully removed the embedded needle through moderate countertraction. On examination, the patient’s right thumb was pale and cold, and had poor capillary refill (Figure 2). Due to concerns of the potential for digital tissue ischemia leading to tissue loss and gangrene, warm, moist compresses were applied to the affected thumb, followed by 2% topical nitroglycerin paste, after which the thumb was covered with an occlusive dressing. Since there was no improvement in circulation after 20 minutes, an infiltrate of 5 mg (0.5 mL of 10 mg/mL) of phentolamine (α-agonist) mixed with 2.5 mL of 2% lidocaine was injected at the puncture site and base of the right thumb.1 Hyperemia developed immediately at both injection sites, and the patient’s right thumb returned to a normal color and sensation 1 hour later, with a return to normal capillary refill. She remained in stable condition and was discharged home. Prior to discharge, the patient was educated on the proper handling and administration of an epinephrine autoinjector.
Discussion
Epinephrine is an ὰ- and β-adrenergic agonist that binds to the ὰ-adrenergic receptors of blood vessels, causing an increase in vascular resistance and vasoconstriction. Although the plasma half-life of epinephrine is approximately 2 to 3 minutes, subcutaneous or intramuscular injection resulting in local vasoconstriction may delay absorption; therefore, the effects of epinephrine may last much longer than its half-life.
The incidence of accidental injection from an epinephrine autoinjector is estimated to be 1 per 50,000 units dispensed.2 To date, there are no established treatment guidelines on managing cases of digital injection. An online PubMed and Google Scholar search of the literature found one systematic review,3 four observational studies,4-7 seven case series,8-14 and several case reports1,15-33 on the subject. Most of the patients in the published retrospective studies (71%) were treated conservatively with warming of the affected hand and observation, and the majority of patients in the case reports (87%) were treated pharmacologically, most commonly with topical nitroglycerin and phentolamine.1,3-34 All of the patients in both the retrospective studies and case reports had restoration of perfusion without necrosis, irrespective of treatment modality. However, patients who were managed conservatively or who were treated with topical nitroglycerin required a longer duration of stay in the ED, suffered from severe reperfusion pain, and in some cases, had a longer time to complete recovery (≥10 weeks).8
Pharmaceutical and Nonpharmaceutical Management
Phentolamine. Phentolamine is a nonselective ὰ-adrenergic antagonist that binds to ὰ1 and ὰ2 receptors of blood vessels, resulting in a decrease in peripheral vascular resistance and vasodilation. Phentolamine directly antagonizes the effect of epinephrine by blocking the ὰ-adrenergic receptors, which in our patient resulted in immediate return of digital circulation and full resolution of symptoms.
Topical Nitroglycerin. Nitroglycerin is a nitrate vasodilator that when metabolically converted to nitric oxide, results in smooth muscle relaxation, venodilation, and arteriodilation. Patients suffering from digital ischemia and vasoconstriction may be treated with topical nitroglycerin paste to reverse ischemia by causing smooth muscle relaxation of digital blood vessels. Conservative Management. As previously noted, not all cases of digital epinephrine injection are treated pharmacologically. Some patients are not treated, but kept in observation until the ischemic effects of epinephrine have resolved. Likewise, some patients are treated conservatively with warm water compresses or by fully immersing the affected digit in warm water to facilitate reversal of vasoconstriction and ischemia.3,8
Treatment Efficacy
In 2007, Fitzcharles-Bowe et al8 published a review of 59 cases of digital injection with high-dose epinephrine from 1989 to 2005. In this review, 32 of the 59 patients received no treatment, 25 patients received pharmacological treatment and in two patients, the treatment was unknown. Phentolamine was the most commonly used pharmacological agent (15 of 25 cases or 60%). Although none of the patients experienced digital necrosis, those treated with a local infiltration of phentolamine experienced a faster resolution of symptoms and normalization of perfusion. In 2004, Turner1 reported a case of a 10-year-old boy who was treated with phentolamine following an accidental injection of epinephrine into his left hand. While circulation returned to the affected digit within 5 minutes of receiving the phentolamine injection, the patient continued to experience reduced sensation in the digit 6 weeks later.8
Interestingly, one of the coauthors of the Fitzcharles-Bowe et al8 report intentionally injected three of the digits of his left hand (middle, ring, and small fingers) at the same time with high-dose epinephrine to carefully observe and document the outcomes. All three of the digits became very pale and cool, with decreased sensation. The author treated himself conservatively (observation-only). He experienced spontaneous return of circulation in two of the digits within 6 to 10 hours. Although there was some spontaneous return of circulation to the third digit after 13 hours, the author noted prolonged, intense reperfusion pain 4 hours after return of circulation. He also suffered from neuropraxia in the third digit, which did not fully resolve until 10 weeks after the injury.8
A review of the literature shows phentolamine to be a safe and effective treatment for patients presenting with digital ischemia, with no long-term adverse effects or complications. Moreover, phentolamine appears to be safe and effective for use in both adult and pediatric patients.3,8,35-38
Accidental Injection Prevention
Some of the cases of accidental epinephrine injection are due to user error. For example, a novice user may be holding the incorrect end of the injector in his or her hand when attempting to administer/deploy the device, resulting in premature dislodgement of the needle.39
Although, most of the autoinjector devices available today are user-friendly, we believe the addition of a safety feature such as a trigger or safety-lock may further help to reduce accidents. The European Medicines Agency recommends that all patients and caregivers receive training on the proper handling and administration of epinephrine autoinjectors, citing this as the most important factor to ensure successful use of an epinephrine autoinjector and reduce accidental injury.40 The patient in this case had not received any formal education or training regarding autoinjector use prior to this incident.
Safety of Lidocaine-Containing Epinephrine in Digital Anesthesia
Aside from cases of accidental digital epinephrine injection, clinicians have traditionally been taught to avoid using lidocaine with epinephrine for digital anesthesia. However, since the introduction of commercial lidocaine with epinephrine in 1948, there are no case reports of digital gangrene from commercially available lidocaine-epinephrine formulations.41,42 In a multicenter prospective study by Lalonde et al43 of 3,110 consecutive cases of elective injection of low-dose epinephrine in the hand, the authors concluded the likelihood of finger infarction is remote, particularly with possible phentolamine rescue therapy. Moreover, lidocaine-containing epinephrine (1%-2%) has a much lower concentration of epinephrine per mL of solution (5-10 mcg/mL) and appears to be safe for digital use.
Conclusion
This case describes the presentation and treatment of accidental digital injection of epinephrine, highlighting and supporting the benefits of local infiltration with phentolamine and observation until full recovery of perfusion. Local treatment with phentolamine not only facilitates recovery and return of capillary refill, but also shortens the duration of symptoms and alleviates vasoconstriction. In less severe cases, watchful waiting and observation may be appropriate and effective.
This case also underscores the importance of patient and caregiver education on the proper handling and administration of epinephrine autoinjectors to decrease the incidence of accidental injection.
Patients presenting to the ED with injuries due to accidental self-injection with an epinephrine pen typically receive treatment to alleviate symptoms and reduce the potential of digital ischemia leading to gangrene and loss of tissue and function. Although there is no consensus or set guidelines in the literature regarding the management protocol of such cases, many reports support pharmacological intervention. There are, however, other reports that advocate conservative, nonpharmaceutical management (eg, immersing the affected digit in warm water) or an observation-only approach.
We present the first case report in Saudi Arabia of digital ischemia due to accidental injection of an epinephrine autoinjector, along with a review of the literature and management recommendations.
Case
A 28-year-old woman presented to the ED in significant pain and discomfort 20 minutes after she accidentally injected the entire contents of her aunt’s epinephrine autoinjector (0.3 mg of 1:1000) into her right thumb. The patient, who was in significant pain and discomfort, stated that she was unable to remove the injector needle, which was firmly embedded in the bone of the palmer aspect of the distal phalanx in a manner similar to that of an intraosseous injection (Figure 1).
The patient’s vital signs and oxygen saturation on presentation were within normal limits. The emergency physician successfully removed the embedded needle through moderate countertraction. On examination, the patient’s right thumb was pale and cold, and had poor capillary refill (Figure 2). Due to concerns of the potential for digital tissue ischemia leading to tissue loss and gangrene, warm, moist compresses were applied to the affected thumb, followed by 2% topical nitroglycerin paste, after which the thumb was covered with an occlusive dressing. Since there was no improvement in circulation after 20 minutes, an infiltrate of 5 mg (0.5 mL of 10 mg/mL) of phentolamine (α-agonist) mixed with 2.5 mL of 2% lidocaine was injected at the puncture site and base of the right thumb.1 Hyperemia developed immediately at both injection sites, and the patient’s right thumb returned to a normal color and sensation 1 hour later, with a return to normal capillary refill. She remained in stable condition and was discharged home. Prior to discharge, the patient was educated on the proper handling and administration of an epinephrine autoinjector.
Discussion
Epinephrine is an ὰ- and β-adrenergic agonist that binds to the ὰ-adrenergic receptors of blood vessels, causing an increase in vascular resistance and vasoconstriction. Although the plasma half-life of epinephrine is approximately 2 to 3 minutes, subcutaneous or intramuscular injection resulting in local vasoconstriction may delay absorption; therefore, the effects of epinephrine may last much longer than its half-life.
The incidence of accidental injection from an epinephrine autoinjector is estimated to be 1 per 50,000 units dispensed.2 To date, there are no established treatment guidelines on managing cases of digital injection. An online PubMed and Google Scholar search of the literature found one systematic review,3 four observational studies,4-7 seven case series,8-14 and several case reports1,15-33 on the subject. Most of the patients in the published retrospective studies (71%) were treated conservatively with warming of the affected hand and observation, and the majority of patients in the case reports (87%) were treated pharmacologically, most commonly with topical nitroglycerin and phentolamine.1,3-34 All of the patients in both the retrospective studies and case reports had restoration of perfusion without necrosis, irrespective of treatment modality. However, patients who were managed conservatively or who were treated with topical nitroglycerin required a longer duration of stay in the ED, suffered from severe reperfusion pain, and in some cases, had a longer time to complete recovery (≥10 weeks).8
Pharmaceutical and Nonpharmaceutical Management
Phentolamine. Phentolamine is a nonselective ὰ-adrenergic antagonist that binds to ὰ1 and ὰ2 receptors of blood vessels, resulting in a decrease in peripheral vascular resistance and vasodilation. Phentolamine directly antagonizes the effect of epinephrine by blocking the ὰ-adrenergic receptors, which in our patient resulted in immediate return of digital circulation and full resolution of symptoms.
Topical Nitroglycerin. Nitroglycerin is a nitrate vasodilator that when metabolically converted to nitric oxide, results in smooth muscle relaxation, venodilation, and arteriodilation. Patients suffering from digital ischemia and vasoconstriction may be treated with topical nitroglycerin paste to reverse ischemia by causing smooth muscle relaxation of digital blood vessels. Conservative Management. As previously noted, not all cases of digital epinephrine injection are treated pharmacologically. Some patients are not treated, but kept in observation until the ischemic effects of epinephrine have resolved. Likewise, some patients are treated conservatively with warm water compresses or by fully immersing the affected digit in warm water to facilitate reversal of vasoconstriction and ischemia.3,8
Treatment Efficacy
In 2007, Fitzcharles-Bowe et al8 published a review of 59 cases of digital injection with high-dose epinephrine from 1989 to 2005. In this review, 32 of the 59 patients received no treatment, 25 patients received pharmacological treatment and in two patients, the treatment was unknown. Phentolamine was the most commonly used pharmacological agent (15 of 25 cases or 60%). Although none of the patients experienced digital necrosis, those treated with a local infiltration of phentolamine experienced a faster resolution of symptoms and normalization of perfusion. In 2004, Turner1 reported a case of a 10-year-old boy who was treated with phentolamine following an accidental injection of epinephrine into his left hand. While circulation returned to the affected digit within 5 minutes of receiving the phentolamine injection, the patient continued to experience reduced sensation in the digit 6 weeks later.8
Interestingly, one of the coauthors of the Fitzcharles-Bowe et al8 report intentionally injected three of the digits of his left hand (middle, ring, and small fingers) at the same time with high-dose epinephrine to carefully observe and document the outcomes. All three of the digits became very pale and cool, with decreased sensation. The author treated himself conservatively (observation-only). He experienced spontaneous return of circulation in two of the digits within 6 to 10 hours. Although there was some spontaneous return of circulation to the third digit after 13 hours, the author noted prolonged, intense reperfusion pain 4 hours after return of circulation. He also suffered from neuropraxia in the third digit, which did not fully resolve until 10 weeks after the injury.8
A review of the literature shows phentolamine to be a safe and effective treatment for patients presenting with digital ischemia, with no long-term adverse effects or complications. Moreover, phentolamine appears to be safe and effective for use in both adult and pediatric patients.3,8,35-38
Accidental Injection Prevention
Some of the cases of accidental epinephrine injection are due to user error. For example, a novice user may be holding the incorrect end of the injector in his or her hand when attempting to administer/deploy the device, resulting in premature dislodgement of the needle.39
Although, most of the autoinjector devices available today are user-friendly, we believe the addition of a safety feature such as a trigger or safety-lock may further help to reduce accidents. The European Medicines Agency recommends that all patients and caregivers receive training on the proper handling and administration of epinephrine autoinjectors, citing this as the most important factor to ensure successful use of an epinephrine autoinjector and reduce accidental injury.40 The patient in this case had not received any formal education or training regarding autoinjector use prior to this incident.
Safety of Lidocaine-Containing Epinephrine in Digital Anesthesia
Aside from cases of accidental digital epinephrine injection, clinicians have traditionally been taught to avoid using lidocaine with epinephrine for digital anesthesia. However, since the introduction of commercial lidocaine with epinephrine in 1948, there are no case reports of digital gangrene from commercially available lidocaine-epinephrine formulations.41,42 In a multicenter prospective study by Lalonde et al43 of 3,110 consecutive cases of elective injection of low-dose epinephrine in the hand, the authors concluded the likelihood of finger infarction is remote, particularly with possible phentolamine rescue therapy. Moreover, lidocaine-containing epinephrine (1%-2%) has a much lower concentration of epinephrine per mL of solution (5-10 mcg/mL) and appears to be safe for digital use.
Conclusion
This case describes the presentation and treatment of accidental digital injection of epinephrine, highlighting and supporting the benefits of local infiltration with phentolamine and observation until full recovery of perfusion. Local treatment with phentolamine not only facilitates recovery and return of capillary refill, but also shortens the duration of symptoms and alleviates vasoconstriction. In less severe cases, watchful waiting and observation may be appropriate and effective.
This case also underscores the importance of patient and caregiver education on the proper handling and administration of epinephrine autoinjectors to decrease the incidence of accidental injection.
1. Turner MJ. Accidental Epipen injection into a digit - the value of a Google search. Ann R Coll Surg Engl. 2004;86(3):218-219. doi:10.1308/003588404323043391.
2. McGovern SJ. Treatment of accidental digital injection of adrenaline from an auto-injector device. J Accid Emerg Med. 1997;14(6):379-380.
3. Wright M. Treatment after accidental injection with epinephrine autoinjector: a systematic review. J Allergy & Therapy. 2014;5(3):1000175. doi:10.4172/2155-6121.1000175.
4. Mrvos R, Anderson BD, Krenzelok EP. Accidental injection of epinephrine from an autoinjector: invasive treatment not always required. South Med J. 2002;95(3):318-320.
5. Muck AE, Bebarta VS, Borys DJ, Morgan DL. Six years of epinephrine digital injections: absence of significant local or systemic effects. Ann Emerg Med. 2010;56(3):270-274. doi:10.1016/j.annemergmed.2010.02.019.
6. Simons FE, Edwards ES, Read EJ Jr, Clark S, Liebelt EL. Voluntarily reported unintentional injections from epinephrine auto-injectors. J Allergy Clin Immunol. 2010;125(2):419-423. doi:10.1016/j.jaci.2009.10.056.
7. Blume-Odom CM, Scalzo AJ, Weber JA. EpiPen accidental injection-134 cases over 10 years. Clin Toxicol. 2010;48:651.
8. Fitzcharles-Bowe C, Denkler K, Lalonde D. Finger injection with high-dose (1:1,000) epinephrine: Does it cause finger necrosis and should it be treated? Hand. 2007;2(1):5-11. doi:10.1007/s11552-006-9012-4.
9. Velissariou I, Cottrell S, Berry K, Wilson B. Management of adrenaline (epinephrine) induced digital ischaemia in children after accidental injection from an EpiPen. Emerg Med J. 2004;21(3):387-388.
10. ElMaraghy MW, ElMaraghy AW, Evans HB. Digital adrenaline injection injuries: a case series and review. Can J Plast Surg. 1998;6:196-200.
11. Skorpinski EW, McGeady SJ, Yousef E. Two cases of accidental epinephrine injection into a finger. J Allergy Clin Immunol. 2006;117(2):463-464.
12. Nagaraj J, Reddy S, Murray R, Murphy N. Use of glyceryl trinitrate patches in the treatment of accidental digital injection of epinephrine from an autoinjector. Eur J Emerg Med. 2009;16(4):227-228. doi:10.1097/MEJ.0b013e328306f0ee.
13. Stier PA, Bogner MP, Webster K, Leikin JB, Burda A. Use of subcutaneous terbutaline to reverse peripheral ischemia. Am J Emerg Med. 1999;17(1):91-94.
14. Lee G, Thomas PC. Accidental digital injection of adrenaline from an autoinjector device. J Accid Emerg Med. 1998;15(4):287.
15. Baris S, Saricoban HE, Ak K, Ozdemir C. Papaverine chloride as a topical vasodilator in accidental injection of adrenaline into a digital finger. Allergy. 2011;66(11):1495-1496. doi:10.1111/j.1398-9995.2011.02664.x.
16. Buse K, Hein W, Drager N. Making Sense of Global Health Governance: A Policy Perspective. Basingstoke, England: Palgrave Macmillan UK; 2009.
17. Sherman SC. Digital Epipen® injection: a case of conservative management. J Emerg Med. 2011;41(6):672-674. doi:10.1016/j.jemermed.2009.07.027.
18. Janssen RL, Roeleveld-Versteegh AB, Wessels-Basten SJ, Hendriks T. [Auto-injection with epinephrine in the finger of a 5-year-old child]. Ned Tijdschr Geneeskd. 2008;152(17):1005-1008.
19. Singh T, Randhawa S, Khanna R. The EpiPen and the ischaemic finger. Eur J Emerg Med. 2007;14(4):222-223.
20. Barkhordarian AR, Wakelin SH, Paes TR. Accidental digital injection of adrenaline from an autoinjector device. Br J Dermatol. 2000;143(6):1359.
21. Deshmukh N, Tolland JT. Treatment of accidental epinephrine injection in a finger. J Emerg Med. 1989;7(4):408.
22. Hinterberger JW, Kintzi HE. Phentolamine reversal of epinephrine-induced digital vasospasm. How to save an ischemic finger. Arch Fam Med. 1994;3(2):193-195.
23. Peyko V, Cohen V, Jellinek-Cohen SP, Pearl-Davis M. Evaluation and treatment of accidental autoinjection of epinephrine. Am J Health Syst Pharm. 2013;70(9):778-781. doi:10.2146/ajhp120316.
24. Hardy SJ, Agostini DE. Accidental epinephrine auto-injector-induced digital ischemia reversed by phentolamine digital block. J Am Osteopath Assoc. 1995;95(6):377-378.
25. Kaspersen J, Vedsted P. [Accidental injection of adrenaline in a finger with EpiPen]. Ugeskr Laeger. 1998;160(45):6531-6532.
26. Schintler MV, Arbab E, Aberer W, Spendel S, Scharnagl E. Accidental perforating bone injury using the EpiPen autoinjection device. Allergy. 2005;60(2):259-260.
27. Khairalla E. Epinephrine-induced digital ischemia relieved by phentolamine. Plast Reconstr Surg. 2001;108(6):1831-1832.
28. Murali KS, Nayeem N. Accidental digital injection of adrenaline from an autoinjector device. J Accid Emerg Med. 1998;15(4):287.
29. Sellens C, Morrison L. Accidental injection of epinephrine by a child: a unique approach to treatment. CJEM. 1999;1(1):34-36.
30. Klemawesch P. Hyperbaric oxygen relieves severe digital ischaemia from accidental EpiPen injection. 2009 American Academy of Allergy, Asthma and Immunology Annual Meeting.
31. McCauley WA, Gerace RV, Scilley C. Treatment of accidental digital injection of epinephrine. Ann Emerg Med. 1991;20(6):665-668.
32. Mathez C, Favrat B, Staeger P. Management options for accidental injection of epinephrine from an autoinjector: a case report. J Med Case Rep. 2009;3:7268. doi:10.4076/1752-1947-3-7268.
33. Molony D. Adrenaline-induced digital ischaemia reversed with phentolamine. ANZ J Surg. 2006;76(12):1125-1126.
34. Carrascosa MF, Gallastegui-Menéndez A, Teja-Santamaría C, Caviedes JR. Accidental finger ischaemia induced by epinephrine autoinjector. BMJ Case Rep. 2013;2013. pii:bcr2013200783. doi:10.1136/bcr-2013-200783.
35. Patel R, Kumar H. Epinephrine induced digital ischemia after accidental injection from an auto-injector device. Indian Pediatr. 2013;50(2):247.
36. Xu J, Holt A. Use of Phentolamine in the treatment of Epipen induced digital ischemia. BMJ Case Rep. 2012;2012. doi:10.1136/bcr.12.2011.5450.
37. McNeil C, Copeland J. Accidental digital epinephrine injection: to treat or not to treat? Can Fam Physician. 2014;60(8):726-728.
38. Bodkin RP, Acquisto NM, Gunyan H, Wiegand TJ. Two cases of accidental injection of epinephrine into a digit treated with subcutaneous phentolamine injections. Case Rep Emerg Med. 2013;2013:586207. doi:10.1155/2013/586207.
39. Simons FE, Lieberman PL, Read EJ Jr, Edwards ES. Hazards of unintentional injection of epinephrine from autoinjectors: a systematic review. Ann Allergy Asthma Immunol. 2009;102(4):282-287. doi:10.1016/S1081-1206(10)60332-8.
40. European Medicines Agency. Better training tools recommended to support patients using adrenaline auto-injectors. European Medicines Agency, 2015.
41. Denkler K. A comprehensive review of epinephrine in the finger: to do or not to do.
42. Thomson CJ, Lalonde DH, Denkler KA, Feicht AJ. A critical look at the evidence for and against elective epinephrine use in the finger. Plast Reconstr Surg. 2007;119(1):260-266.
43. Lalonde D, Bell M, Benoit P, Sparkes G, Denkler K, Chang P. A multicenter prospective study of 3,110 consecutive cases of elective epinephrine use in the fingers and hand: the Dalhousie Project clinical phase. J Hand Surg Am. 2005;30(5):1061-1067. doi:10.1016/j.jhsa.2005.05.006.
1. Turner MJ. Accidental Epipen injection into a digit - the value of a Google search. Ann R Coll Surg Engl. 2004;86(3):218-219. doi:10.1308/003588404323043391.
2. McGovern SJ. Treatment of accidental digital injection of adrenaline from an auto-injector device. J Accid Emerg Med. 1997;14(6):379-380.
3. Wright M. Treatment after accidental injection with epinephrine autoinjector: a systematic review. J Allergy & Therapy. 2014;5(3):1000175. doi:10.4172/2155-6121.1000175.
4. Mrvos R, Anderson BD, Krenzelok EP. Accidental injection of epinephrine from an autoinjector: invasive treatment not always required. South Med J. 2002;95(3):318-320.
5. Muck AE, Bebarta VS, Borys DJ, Morgan DL. Six years of epinephrine digital injections: absence of significant local or systemic effects. Ann Emerg Med. 2010;56(3):270-274. doi:10.1016/j.annemergmed.2010.02.019.
6. Simons FE, Edwards ES, Read EJ Jr, Clark S, Liebelt EL. Voluntarily reported unintentional injections from epinephrine auto-injectors. J Allergy Clin Immunol. 2010;125(2):419-423. doi:10.1016/j.jaci.2009.10.056.
7. Blume-Odom CM, Scalzo AJ, Weber JA. EpiPen accidental injection-134 cases over 10 years. Clin Toxicol. 2010;48:651.
8. Fitzcharles-Bowe C, Denkler K, Lalonde D. Finger injection with high-dose (1:1,000) epinephrine: Does it cause finger necrosis and should it be treated? Hand. 2007;2(1):5-11. doi:10.1007/s11552-006-9012-4.
9. Velissariou I, Cottrell S, Berry K, Wilson B. Management of adrenaline (epinephrine) induced digital ischaemia in children after accidental injection from an EpiPen. Emerg Med J. 2004;21(3):387-388.
10. ElMaraghy MW, ElMaraghy AW, Evans HB. Digital adrenaline injection injuries: a case series and review. Can J Plast Surg. 1998;6:196-200.
11. Skorpinski EW, McGeady SJ, Yousef E. Two cases of accidental epinephrine injection into a finger. J Allergy Clin Immunol. 2006;117(2):463-464.
12. Nagaraj J, Reddy S, Murray R, Murphy N. Use of glyceryl trinitrate patches in the treatment of accidental digital injection of epinephrine from an autoinjector. Eur J Emerg Med. 2009;16(4):227-228. doi:10.1097/MEJ.0b013e328306f0ee.
13. Stier PA, Bogner MP, Webster K, Leikin JB, Burda A. Use of subcutaneous terbutaline to reverse peripheral ischemia. Am J Emerg Med. 1999;17(1):91-94.
14. Lee G, Thomas PC. Accidental digital injection of adrenaline from an autoinjector device. J Accid Emerg Med. 1998;15(4):287.
15. Baris S, Saricoban HE, Ak K, Ozdemir C. Papaverine chloride as a topical vasodilator in accidental injection of adrenaline into a digital finger. Allergy. 2011;66(11):1495-1496. doi:10.1111/j.1398-9995.2011.02664.x.
16. Buse K, Hein W, Drager N. Making Sense of Global Health Governance: A Policy Perspective. Basingstoke, England: Palgrave Macmillan UK; 2009.
17. Sherman SC. Digital Epipen® injection: a case of conservative management. J Emerg Med. 2011;41(6):672-674. doi:10.1016/j.jemermed.2009.07.027.
18. Janssen RL, Roeleveld-Versteegh AB, Wessels-Basten SJ, Hendriks T. [Auto-injection with epinephrine in the finger of a 5-year-old child]. Ned Tijdschr Geneeskd. 2008;152(17):1005-1008.
19. Singh T, Randhawa S, Khanna R. The EpiPen and the ischaemic finger. Eur J Emerg Med. 2007;14(4):222-223.
20. Barkhordarian AR, Wakelin SH, Paes TR. Accidental digital injection of adrenaline from an autoinjector device. Br J Dermatol. 2000;143(6):1359.
21. Deshmukh N, Tolland JT. Treatment of accidental epinephrine injection in a finger. J Emerg Med. 1989;7(4):408.
22. Hinterberger JW, Kintzi HE. Phentolamine reversal of epinephrine-induced digital vasospasm. How to save an ischemic finger. Arch Fam Med. 1994;3(2):193-195.
23. Peyko V, Cohen V, Jellinek-Cohen SP, Pearl-Davis M. Evaluation and treatment of accidental autoinjection of epinephrine. Am J Health Syst Pharm. 2013;70(9):778-781. doi:10.2146/ajhp120316.
24. Hardy SJ, Agostini DE. Accidental epinephrine auto-injector-induced digital ischemia reversed by phentolamine digital block. J Am Osteopath Assoc. 1995;95(6):377-378.
25. Kaspersen J, Vedsted P. [Accidental injection of adrenaline in a finger with EpiPen]. Ugeskr Laeger. 1998;160(45):6531-6532.
26. Schintler MV, Arbab E, Aberer W, Spendel S, Scharnagl E. Accidental perforating bone injury using the EpiPen autoinjection device. Allergy. 2005;60(2):259-260.
27. Khairalla E. Epinephrine-induced digital ischemia relieved by phentolamine. Plast Reconstr Surg. 2001;108(6):1831-1832.
28. Murali KS, Nayeem N. Accidental digital injection of adrenaline from an autoinjector device. J Accid Emerg Med. 1998;15(4):287.
29. Sellens C, Morrison L. Accidental injection of epinephrine by a child: a unique approach to treatment. CJEM. 1999;1(1):34-36.
30. Klemawesch P. Hyperbaric oxygen relieves severe digital ischaemia from accidental EpiPen injection. 2009 American Academy of Allergy, Asthma and Immunology Annual Meeting.
31. McCauley WA, Gerace RV, Scilley C. Treatment of accidental digital injection of epinephrine. Ann Emerg Med. 1991;20(6):665-668.
32. Mathez C, Favrat B, Staeger P. Management options for accidental injection of epinephrine from an autoinjector: a case report. J Med Case Rep. 2009;3:7268. doi:10.4076/1752-1947-3-7268.
33. Molony D. Adrenaline-induced digital ischaemia reversed with phentolamine. ANZ J Surg. 2006;76(12):1125-1126.
34. Carrascosa MF, Gallastegui-Menéndez A, Teja-Santamaría C, Caviedes JR. Accidental finger ischaemia induced by epinephrine autoinjector. BMJ Case Rep. 2013;2013. pii:bcr2013200783. doi:10.1136/bcr-2013-200783.
35. Patel R, Kumar H. Epinephrine induced digital ischemia after accidental injection from an auto-injector device. Indian Pediatr. 2013;50(2):247.
36. Xu J, Holt A. Use of Phentolamine in the treatment of Epipen induced digital ischemia. BMJ Case Rep. 2012;2012. doi:10.1136/bcr.12.2011.5450.
37. McNeil C, Copeland J. Accidental digital epinephrine injection: to treat or not to treat? Can Fam Physician. 2014;60(8):726-728.
38. Bodkin RP, Acquisto NM, Gunyan H, Wiegand TJ. Two cases of accidental injection of epinephrine into a digit treated with subcutaneous phentolamine injections. Case Rep Emerg Med. 2013;2013:586207. doi:10.1155/2013/586207.
39. Simons FE, Lieberman PL, Read EJ Jr, Edwards ES. Hazards of unintentional injection of epinephrine from autoinjectors: a systematic review. Ann Allergy Asthma Immunol. 2009;102(4):282-287. doi:10.1016/S1081-1206(10)60332-8.
40. European Medicines Agency. Better training tools recommended to support patients using adrenaline auto-injectors. European Medicines Agency, 2015.
41. Denkler K. A comprehensive review of epinephrine in the finger: to do or not to do.
42. Thomson CJ, Lalonde DH, Denkler KA, Feicht AJ. A critical look at the evidence for and against elective epinephrine use in the finger. Plast Reconstr Surg. 2007;119(1):260-266.
43. Lalonde D, Bell M, Benoit P, Sparkes G, Denkler K, Chang P. A multicenter prospective study of 3,110 consecutive cases of elective epinephrine use in the fingers and hand: the Dalhousie Project clinical phase. J Hand Surg Am. 2005;30(5):1061-1067. doi:10.1016/j.jhsa.2005.05.006.
Cognitive-behavioral therapy modified for maximum efficacy in the elderly
NEW YORK – For elderly individuals with depression exacerbated by physical limitations and personal losses, cognitive-behavioral therapy is a powerful tool for improving quality of life, according to the faculty of a workshop on this topic at the annual meeting of the American Psychiatric Association.
“The focus is on coping skills. It is about how to persevere in the face of adversity,” explained David A. Casey, MD, professor and chair of the department of psychiatry and behavioral sciences at University of Louisville (Ky.).
“It is not always a fair characterization, but CBT is often perceived as a strategy to address negative thoughts that are not real – but many of my elderly patients have losses and difficulties that are very real,” Dr. Casey said.
In the elderly who become increasingly isolated because of the loss of spouses, friends, and siblings while contending with medical problems that cause pain and limit activities, depression can engender withdrawal, a common coping mechanism, he said.
“Withdrawal may be an unexamined response to a sense of helplessness created by the problems of aging, but it can create a vicious cycle when depression contributes to lack of physical activity and further withdrawal,” explained Dr. Casey, who believes that mild cognitive impairment does not preclude the use of CBT.
CBT provides a “here-and-now” approach in which patients are reconnected to daily life by first identifying the activities that once provided pleasure or satisfaction and then developing a plan to reintroduce them into daily life. Except for its value in identifying activities meaningful to the patient, the history that preceded depression or psychological distress is less important than developing an immediate strategy to rebuilding an active life.
“Some patients are essentially immobilized by their withdrawal and convinced that their problems are unsolvable, but most will improve their quality of life through CBT,” he maintained.
There are data to support this contention, according to Jesse H. Wright III, MD, PhD, director of the Depression Center at the University of Louisville. He cited controlled studies demonstrating the efficacy of CBT relative to no CBT in relieving depression in the elderly.
“The evidence suggests that combining CBT with pharmacotherapy is better than either alone for managing depression in this age group,” Dr. Wright said.
In developing a therapeutic plan through CBT, patients are given assignments designed to develop participation in meaningful activities. These must be realistic within physical limitations and within the patient’s readiness to engage. Small steps toward a goal might be needed. At each therapeutic encounter, goals are set, and progress should be evaluated at the subsequent therapeutic encounter.
Dr. Casey cautioned. He said a rehearsal of the actions needed to achieve the assigned goals might be helpful before the patient leaves the treatment session. This allows the clinician to recognize and address potential obstacles, including practical issues, such as mobility, or psychological issues, such as fear of physical activities.
Developing persistence in the face of high levels of negativity can be a challenge not only for the patient but also for the physician. According to Dr. Casey, maintaining a positive attitude can be challenging after treating a series of highly withdrawn and discouraged patients. But he emphasized the need for a professional orientation, recognizing that incremental gains in patient well-being, not cure, should be considered a reasonable goal.
“If I can improve the patient’s quality of life, this is a significant success,” he said. He believes it is sometimes necessary to distract patients from potential problems to focus on expected benefits.
“Patients can have a view of their limitations that is accurate but unhelpful,” Dr. Casey said. The goal of CBT is to move the focus to strategies that can restore lost interest and pleasure in daily life.
Dr. Casey and Dr. Wright reported no potential conflicts of interest related to this topic.
NEW YORK – For elderly individuals with depression exacerbated by physical limitations and personal losses, cognitive-behavioral therapy is a powerful tool for improving quality of life, according to the faculty of a workshop on this topic at the annual meeting of the American Psychiatric Association.
“The focus is on coping skills. It is about how to persevere in the face of adversity,” explained David A. Casey, MD, professor and chair of the department of psychiatry and behavioral sciences at University of Louisville (Ky.).
“It is not always a fair characterization, but CBT is often perceived as a strategy to address negative thoughts that are not real – but many of my elderly patients have losses and difficulties that are very real,” Dr. Casey said.
In the elderly who become increasingly isolated because of the loss of spouses, friends, and siblings while contending with medical problems that cause pain and limit activities, depression can engender withdrawal, a common coping mechanism, he said.
“Withdrawal may be an unexamined response to a sense of helplessness created by the problems of aging, but it can create a vicious cycle when depression contributes to lack of physical activity and further withdrawal,” explained Dr. Casey, who believes that mild cognitive impairment does not preclude the use of CBT.
CBT provides a “here-and-now” approach in which patients are reconnected to daily life by first identifying the activities that once provided pleasure or satisfaction and then developing a plan to reintroduce them into daily life. Except for its value in identifying activities meaningful to the patient, the history that preceded depression or psychological distress is less important than developing an immediate strategy to rebuilding an active life.
“Some patients are essentially immobilized by their withdrawal and convinced that their problems are unsolvable, but most will improve their quality of life through CBT,” he maintained.
There are data to support this contention, according to Jesse H. Wright III, MD, PhD, director of the Depression Center at the University of Louisville. He cited controlled studies demonstrating the efficacy of CBT relative to no CBT in relieving depression in the elderly.
“The evidence suggests that combining CBT with pharmacotherapy is better than either alone for managing depression in this age group,” Dr. Wright said.
In developing a therapeutic plan through CBT, patients are given assignments designed to develop participation in meaningful activities. These must be realistic within physical limitations and within the patient’s readiness to engage. Small steps toward a goal might be needed. At each therapeutic encounter, goals are set, and progress should be evaluated at the subsequent therapeutic encounter.
Dr. Casey cautioned. He said a rehearsal of the actions needed to achieve the assigned goals might be helpful before the patient leaves the treatment session. This allows the clinician to recognize and address potential obstacles, including practical issues, such as mobility, or psychological issues, such as fear of physical activities.
Developing persistence in the face of high levels of negativity can be a challenge not only for the patient but also for the physician. According to Dr. Casey, maintaining a positive attitude can be challenging after treating a series of highly withdrawn and discouraged patients. But he emphasized the need for a professional orientation, recognizing that incremental gains in patient well-being, not cure, should be considered a reasonable goal.
“If I can improve the patient’s quality of life, this is a significant success,” he said. He believes it is sometimes necessary to distract patients from potential problems to focus on expected benefits.
“Patients can have a view of their limitations that is accurate but unhelpful,” Dr. Casey said. The goal of CBT is to move the focus to strategies that can restore lost interest and pleasure in daily life.
Dr. Casey and Dr. Wright reported no potential conflicts of interest related to this topic.
NEW YORK – For elderly individuals with depression exacerbated by physical limitations and personal losses, cognitive-behavioral therapy is a powerful tool for improving quality of life, according to the faculty of a workshop on this topic at the annual meeting of the American Psychiatric Association.
“The focus is on coping skills. It is about how to persevere in the face of adversity,” explained David A. Casey, MD, professor and chair of the department of psychiatry and behavioral sciences at University of Louisville (Ky.).
“It is not always a fair characterization, but CBT is often perceived as a strategy to address negative thoughts that are not real – but many of my elderly patients have losses and difficulties that are very real,” Dr. Casey said.
In the elderly who become increasingly isolated because of the loss of spouses, friends, and siblings while contending with medical problems that cause pain and limit activities, depression can engender withdrawal, a common coping mechanism, he said.
“Withdrawal may be an unexamined response to a sense of helplessness created by the problems of aging, but it can create a vicious cycle when depression contributes to lack of physical activity and further withdrawal,” explained Dr. Casey, who believes that mild cognitive impairment does not preclude the use of CBT.
CBT provides a “here-and-now” approach in which patients are reconnected to daily life by first identifying the activities that once provided pleasure or satisfaction and then developing a plan to reintroduce them into daily life. Except for its value in identifying activities meaningful to the patient, the history that preceded depression or psychological distress is less important than developing an immediate strategy to rebuilding an active life.
“Some patients are essentially immobilized by their withdrawal and convinced that their problems are unsolvable, but most will improve their quality of life through CBT,” he maintained.
There are data to support this contention, according to Jesse H. Wright III, MD, PhD, director of the Depression Center at the University of Louisville. He cited controlled studies demonstrating the efficacy of CBT relative to no CBT in relieving depression in the elderly.
“The evidence suggests that combining CBT with pharmacotherapy is better than either alone for managing depression in this age group,” Dr. Wright said.
In developing a therapeutic plan through CBT, patients are given assignments designed to develop participation in meaningful activities. These must be realistic within physical limitations and within the patient’s readiness to engage. Small steps toward a goal might be needed. At each therapeutic encounter, goals are set, and progress should be evaluated at the subsequent therapeutic encounter.
Dr. Casey cautioned. He said a rehearsal of the actions needed to achieve the assigned goals might be helpful before the patient leaves the treatment session. This allows the clinician to recognize and address potential obstacles, including practical issues, such as mobility, or psychological issues, such as fear of physical activities.
Developing persistence in the face of high levels of negativity can be a challenge not only for the patient but also for the physician. According to Dr. Casey, maintaining a positive attitude can be challenging after treating a series of highly withdrawn and discouraged patients. But he emphasized the need for a professional orientation, recognizing that incremental gains in patient well-being, not cure, should be considered a reasonable goal.
“If I can improve the patient’s quality of life, this is a significant success,” he said. He believes it is sometimes necessary to distract patients from potential problems to focus on expected benefits.
“Patients can have a view of their limitations that is accurate but unhelpful,” Dr. Casey said. The goal of CBT is to move the focus to strategies that can restore lost interest and pleasure in daily life.
Dr. Casey and Dr. Wright reported no potential conflicts of interest related to this topic.
EXPERT ANALYSIS FROM APA
Poor sleep tied to suicidal behaviors in college students
Poor sleep is associated with increased suicidal behaviors in college students – even when controlling for depression, a study of 1,700 students shows.
“Furthermore, findings suggest that some specific sleep components – shorter sleep duration, more frequent bad dreams, feeling too cold while sleeping, and greater sleep medication use – are particularly associated with increased suicidal behaviors in college students,” reported Stephen P. Becker, PhD, of the Cincinnati Children’s Hospital Center, and his associates.
The researchers recruited students from two universities. Most of the students (65%) were female, white (82%), and in their first year of college (63%). The participants’ sleep was assessed using the nine-item Pittsburgh Sleep Quality Index (PSQI), their depressive symptoms were assessed using the Depressive Anxiety Stress Scales-21, and their suicidal behavior was assessed using the Suicidal Behaviors Questionnaire-Revised (SBQ-R), which is a four-item, self-report measure.
About two-thirds of the students (64%) were found to have sleep problems (total PSQI score greater than 5), and 24% were found to have suicide risk (total SBQ-R score of at least 7). Of the students who were found to have suicide risk, 83% also had sleep problems.
Using regression analysis, Dr. Becker and his associates found that the odds of being classified with suicide risk were 6.5 times greater for students with depression and 2.7 times greater for those with sleep problems.
The results add to the literature suggesting that the researchers wrote.
SOURCE: Becker SP et al. J Psychiatr Res. 2018 Apr;99:123-8.
Poor sleep is associated with increased suicidal behaviors in college students – even when controlling for depression, a study of 1,700 students shows.
“Furthermore, findings suggest that some specific sleep components – shorter sleep duration, more frequent bad dreams, feeling too cold while sleeping, and greater sleep medication use – are particularly associated with increased suicidal behaviors in college students,” reported Stephen P. Becker, PhD, of the Cincinnati Children’s Hospital Center, and his associates.
The researchers recruited students from two universities. Most of the students (65%) were female, white (82%), and in their first year of college (63%). The participants’ sleep was assessed using the nine-item Pittsburgh Sleep Quality Index (PSQI), their depressive symptoms were assessed using the Depressive Anxiety Stress Scales-21, and their suicidal behavior was assessed using the Suicidal Behaviors Questionnaire-Revised (SBQ-R), which is a four-item, self-report measure.
About two-thirds of the students (64%) were found to have sleep problems (total PSQI score greater than 5), and 24% were found to have suicide risk (total SBQ-R score of at least 7). Of the students who were found to have suicide risk, 83% also had sleep problems.
Using regression analysis, Dr. Becker and his associates found that the odds of being classified with suicide risk were 6.5 times greater for students with depression and 2.7 times greater for those with sleep problems.
The results add to the literature suggesting that the researchers wrote.
SOURCE: Becker SP et al. J Psychiatr Res. 2018 Apr;99:123-8.
Poor sleep is associated with increased suicidal behaviors in college students – even when controlling for depression, a study of 1,700 students shows.
“Furthermore, findings suggest that some specific sleep components – shorter sleep duration, more frequent bad dreams, feeling too cold while sleeping, and greater sleep medication use – are particularly associated with increased suicidal behaviors in college students,” reported Stephen P. Becker, PhD, of the Cincinnati Children’s Hospital Center, and his associates.
The researchers recruited students from two universities. Most of the students (65%) were female, white (82%), and in their first year of college (63%). The participants’ sleep was assessed using the nine-item Pittsburgh Sleep Quality Index (PSQI), their depressive symptoms were assessed using the Depressive Anxiety Stress Scales-21, and their suicidal behavior was assessed using the Suicidal Behaviors Questionnaire-Revised (SBQ-R), which is a four-item, self-report measure.
About two-thirds of the students (64%) were found to have sleep problems (total PSQI score greater than 5), and 24% were found to have suicide risk (total SBQ-R score of at least 7). Of the students who were found to have suicide risk, 83% also had sleep problems.
Using regression analysis, Dr. Becker and his associates found that the odds of being classified with suicide risk were 6.5 times greater for students with depression and 2.7 times greater for those with sleep problems.
The results add to the literature suggesting that the researchers wrote.
SOURCE: Becker SP et al. J Psychiatr Res. 2018 Apr;99:123-8.
FROM THE JOURNAL OF PSYCHIATRIC RESEARCH
Elagolix shows long-term efficacy
AUSTIN, TEX. – A new treatment for endometriosis-related pain, Elagolix, showed evidence of being effective long term, according to a study presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Elagolix, an oral nonpeptide gonadotropin-releasing hormone (GnRH) antagonist, manufactured by AbbVie, would be the first treatment of its kind if approved by the Food and Drug Administration, and would fulfill a needed relief for a more tolerable approach to severe endometriosis patients, according to presenter Eric S. Surrey, MD, medical director at the Colorado Center of Reproductive Medicine, Lone Tree.
“There have been no new medications approved for a long time for systematic endometriosis and there is a huge gap because the current options are expensive, and they are often injectable drugs,” said Dr. Surrey in an interview. “This would be an oral agent, which would be fabulous because it allows for a lot of flexibility and for many patients this could be much less concerning than using something long acting.”
To test the long-term effects of Elagolix, investigators studied 570 women with moderate to severe endometriosis-related pain who had gathered to participate in a previous phase 3, randomized, placebo-controlled trial concerning the drug’s effectiveness.
In the two extension studies, all participants were given either a 150- or 200-mg dose of Elagolix.
Average age of each patient group was between 31 and 34 years, and all groups were majority white, with a mean length of time from surgical diagnosis ranging from 45.5 to 56.6 months.
Patient improvements in dysmenorrhea and nonmenstrual pelvic pain continued between the first 6 months and 12 months of treatment, with a decrease of 46%-77% in the overall number of analgesics taken per day.
After 12 months of consecutive treatment, patients given 150 mg of Elagolix saw mean dysmenorrhea scores improve by 49%-53% from baseline, and by 82% for those at 200 mg, with certain expected adverse events, according to Dr. Surrey.
One of the most common adverse events associated with Elagolix was hot flashes, an unsurprising finding for Dr. Surrey and his colleagues considering Elagolix is a drug that lowers estrogen levels. However, any hot flashes patients experienced during the trial were still better than those associated with current medications, according to Dr. Surrey.
“In this extension study nobody dropped out because of hot flashes in the additional 6-month extension time,” Dr. Surrey explained. “If you look at the gold standard drug for endometriosis now, which is a GnRH agonist, which are highly available and are either injectable or implants, [patients taking these drugs] can have very severe hot flashes that require additional medication to alleviate the hot flashes at the same time.”
Patients did also experience some loss in bone density; however, Dr. Surrey argues the frequency and level of these adverse events is still better than current treatment options. One patient was required to discontinue the trial for bone density loss.
Currently, Elagolix is under FDA priority review, and if approved will be the first oral endometriosis treatment approved in over a decade, according to Dr. Surrey.
Dr. Surrey and several coauthors receive financial support from AbbVie as consultants, board members, and/or employees. Dr. Surrey and Dr. Taylor receive additional support from companies including Pfizer, Bayer, and Obseva.
SOURCE: Surrey ES et al. ACOG 2018, Abstract 11OP.
Having had the opportunity to review Dr. Eric Surrey's abstract for this year's annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, entitled "Long-term Safety and Efficacy of Elagolix Treatment in Women With Endometriosis-associated Pain," I believe use of Elagolix, an oral nonpeptide gonadotropin-releasing hormone (GnRH) antagonist, is a much-needed advancement in the long-term treatment of endometriosis-related pain. The fact that it is an oral medication, thus, not requiring a monthly or 3-month injection as does Lupron Depot (leuprolide acetate), the most popular GnRH agonist in the United States, is advantageous both for the patient and the busy office staff.
While I certainly understand that it is easy to compare data regarding bone loss in the use of an oral antagonist, Elagolix, with historical data with the GnRH agonist and note a lessening of bone loss in the Elagolix patients, it would be interesting to compare bone loss in patients utilizing Elagolix with bone loss in those treated with GnRH-agonist plus add-back therapy. Many practitioners will utilize progesterone supplementation or estrogen/progesterone supplementation when using GnRH-agonist therapy to decrease this risk. Furthermore, it would be interesting, in the future, to evaluate the impact on efficacy and bone loss if progesterone and estrogen/progesterone add-back were utilized in Elagolix therapy.
While I certainly realize and deeply respect Dr. Surrey's vast experience as both a clinical researcher and clinician utilizing a GnRH-agonist regimen, I am curious as to the basis of Dr. Surrey's comments regarding less severe hot flashes in comparison to GnRH-agonist treatment. I am not aware of any head-to-head studies comparing hot flashes between GnRH agonists (in particular, leuprolide acetate) and Elagolix.
Without a side-by-side comparison utilizing a validated scoring system, I find it hard to accept this conclusion.
Nevertheless, after reviewing this study and Dr. Surrey's comments, I look forward to utilizing Elagolix in my practice for long-term treatment of endometriosis-related pain.
Charles Miller, MD, is a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL. He is a consultant and involved in research for AbbVie.
Having had the opportunity to review Dr. Eric Surrey's abstract for this year's annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, entitled "Long-term Safety and Efficacy of Elagolix Treatment in Women With Endometriosis-associated Pain," I believe use of Elagolix, an oral nonpeptide gonadotropin-releasing hormone (GnRH) antagonist, is a much-needed advancement in the long-term treatment of endometriosis-related pain. The fact that it is an oral medication, thus, not requiring a monthly or 3-month injection as does Lupron Depot (leuprolide acetate), the most popular GnRH agonist in the United States, is advantageous both for the patient and the busy office staff.
While I certainly understand that it is easy to compare data regarding bone loss in the use of an oral antagonist, Elagolix, with historical data with the GnRH agonist and note a lessening of bone loss in the Elagolix patients, it would be interesting to compare bone loss in patients utilizing Elagolix with bone loss in those treated with GnRH-agonist plus add-back therapy. Many practitioners will utilize progesterone supplementation or estrogen/progesterone supplementation when using GnRH-agonist therapy to decrease this risk. Furthermore, it would be interesting, in the future, to evaluate the impact on efficacy and bone loss if progesterone and estrogen/progesterone add-back were utilized in Elagolix therapy.
While I certainly realize and deeply respect Dr. Surrey's vast experience as both a clinical researcher and clinician utilizing a GnRH-agonist regimen, I am curious as to the basis of Dr. Surrey's comments regarding less severe hot flashes in comparison to GnRH-agonist treatment. I am not aware of any head-to-head studies comparing hot flashes between GnRH agonists (in particular, leuprolide acetate) and Elagolix.
Without a side-by-side comparison utilizing a validated scoring system, I find it hard to accept this conclusion.
Nevertheless, after reviewing this study and Dr. Surrey's comments, I look forward to utilizing Elagolix in my practice for long-term treatment of endometriosis-related pain.
Charles Miller, MD, is a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL. He is a consultant and involved in research for AbbVie.
Having had the opportunity to review Dr. Eric Surrey's abstract for this year's annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, entitled "Long-term Safety and Efficacy of Elagolix Treatment in Women With Endometriosis-associated Pain," I believe use of Elagolix, an oral nonpeptide gonadotropin-releasing hormone (GnRH) antagonist, is a much-needed advancement in the long-term treatment of endometriosis-related pain. The fact that it is an oral medication, thus, not requiring a monthly or 3-month injection as does Lupron Depot (leuprolide acetate), the most popular GnRH agonist in the United States, is advantageous both for the patient and the busy office staff.
While I certainly understand that it is easy to compare data regarding bone loss in the use of an oral antagonist, Elagolix, with historical data with the GnRH agonist and note a lessening of bone loss in the Elagolix patients, it would be interesting to compare bone loss in patients utilizing Elagolix with bone loss in those treated with GnRH-agonist plus add-back therapy. Many practitioners will utilize progesterone supplementation or estrogen/progesterone supplementation when using GnRH-agonist therapy to decrease this risk. Furthermore, it would be interesting, in the future, to evaluate the impact on efficacy and bone loss if progesterone and estrogen/progesterone add-back were utilized in Elagolix therapy.
While I certainly realize and deeply respect Dr. Surrey's vast experience as both a clinical researcher and clinician utilizing a GnRH-agonist regimen, I am curious as to the basis of Dr. Surrey's comments regarding less severe hot flashes in comparison to GnRH-agonist treatment. I am not aware of any head-to-head studies comparing hot flashes between GnRH agonists (in particular, leuprolide acetate) and Elagolix.
Without a side-by-side comparison utilizing a validated scoring system, I find it hard to accept this conclusion.
Nevertheless, after reviewing this study and Dr. Surrey's comments, I look forward to utilizing Elagolix in my practice for long-term treatment of endometriosis-related pain.
Charles Miller, MD, is a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL. He is a consultant and involved in research for AbbVie.
AUSTIN, TEX. – A new treatment for endometriosis-related pain, Elagolix, showed evidence of being effective long term, according to a study presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Elagolix, an oral nonpeptide gonadotropin-releasing hormone (GnRH) antagonist, manufactured by AbbVie, would be the first treatment of its kind if approved by the Food and Drug Administration, and would fulfill a needed relief for a more tolerable approach to severe endometriosis patients, according to presenter Eric S. Surrey, MD, medical director at the Colorado Center of Reproductive Medicine, Lone Tree.
“There have been no new medications approved for a long time for systematic endometriosis and there is a huge gap because the current options are expensive, and they are often injectable drugs,” said Dr. Surrey in an interview. “This would be an oral agent, which would be fabulous because it allows for a lot of flexibility and for many patients this could be much less concerning than using something long acting.”
To test the long-term effects of Elagolix, investigators studied 570 women with moderate to severe endometriosis-related pain who had gathered to participate in a previous phase 3, randomized, placebo-controlled trial concerning the drug’s effectiveness.
In the two extension studies, all participants were given either a 150- or 200-mg dose of Elagolix.
Average age of each patient group was between 31 and 34 years, and all groups were majority white, with a mean length of time from surgical diagnosis ranging from 45.5 to 56.6 months.
Patient improvements in dysmenorrhea and nonmenstrual pelvic pain continued between the first 6 months and 12 months of treatment, with a decrease of 46%-77% in the overall number of analgesics taken per day.
After 12 months of consecutive treatment, patients given 150 mg of Elagolix saw mean dysmenorrhea scores improve by 49%-53% from baseline, and by 82% for those at 200 mg, with certain expected adverse events, according to Dr. Surrey.
One of the most common adverse events associated with Elagolix was hot flashes, an unsurprising finding for Dr. Surrey and his colleagues considering Elagolix is a drug that lowers estrogen levels. However, any hot flashes patients experienced during the trial were still better than those associated with current medications, according to Dr. Surrey.
“In this extension study nobody dropped out because of hot flashes in the additional 6-month extension time,” Dr. Surrey explained. “If you look at the gold standard drug for endometriosis now, which is a GnRH agonist, which are highly available and are either injectable or implants, [patients taking these drugs] can have very severe hot flashes that require additional medication to alleviate the hot flashes at the same time.”
Patients did also experience some loss in bone density; however, Dr. Surrey argues the frequency and level of these adverse events is still better than current treatment options. One patient was required to discontinue the trial for bone density loss.
Currently, Elagolix is under FDA priority review, and if approved will be the first oral endometriosis treatment approved in over a decade, according to Dr. Surrey.
Dr. Surrey and several coauthors receive financial support from AbbVie as consultants, board members, and/or employees. Dr. Surrey and Dr. Taylor receive additional support from companies including Pfizer, Bayer, and Obseva.
SOURCE: Surrey ES et al. ACOG 2018, Abstract 11OP.
AUSTIN, TEX. – A new treatment for endometriosis-related pain, Elagolix, showed evidence of being effective long term, according to a study presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Elagolix, an oral nonpeptide gonadotropin-releasing hormone (GnRH) antagonist, manufactured by AbbVie, would be the first treatment of its kind if approved by the Food and Drug Administration, and would fulfill a needed relief for a more tolerable approach to severe endometriosis patients, according to presenter Eric S. Surrey, MD, medical director at the Colorado Center of Reproductive Medicine, Lone Tree.
“There have been no new medications approved for a long time for systematic endometriosis and there is a huge gap because the current options are expensive, and they are often injectable drugs,” said Dr. Surrey in an interview. “This would be an oral agent, which would be fabulous because it allows for a lot of flexibility and for many patients this could be much less concerning than using something long acting.”
To test the long-term effects of Elagolix, investigators studied 570 women with moderate to severe endometriosis-related pain who had gathered to participate in a previous phase 3, randomized, placebo-controlled trial concerning the drug’s effectiveness.
In the two extension studies, all participants were given either a 150- or 200-mg dose of Elagolix.
Average age of each patient group was between 31 and 34 years, and all groups were majority white, with a mean length of time from surgical diagnosis ranging from 45.5 to 56.6 months.
Patient improvements in dysmenorrhea and nonmenstrual pelvic pain continued between the first 6 months and 12 months of treatment, with a decrease of 46%-77% in the overall number of analgesics taken per day.
After 12 months of consecutive treatment, patients given 150 mg of Elagolix saw mean dysmenorrhea scores improve by 49%-53% from baseline, and by 82% for those at 200 mg, with certain expected adverse events, according to Dr. Surrey.
One of the most common adverse events associated with Elagolix was hot flashes, an unsurprising finding for Dr. Surrey and his colleagues considering Elagolix is a drug that lowers estrogen levels. However, any hot flashes patients experienced during the trial were still better than those associated with current medications, according to Dr. Surrey.
“In this extension study nobody dropped out because of hot flashes in the additional 6-month extension time,” Dr. Surrey explained. “If you look at the gold standard drug for endometriosis now, which is a GnRH agonist, which are highly available and are either injectable or implants, [patients taking these drugs] can have very severe hot flashes that require additional medication to alleviate the hot flashes at the same time.”
Patients did also experience some loss in bone density; however, Dr. Surrey argues the frequency and level of these adverse events is still better than current treatment options. One patient was required to discontinue the trial for bone density loss.
Currently, Elagolix is under FDA priority review, and if approved will be the first oral endometriosis treatment approved in over a decade, according to Dr. Surrey.
Dr. Surrey and several coauthors receive financial support from AbbVie as consultants, board members, and/or employees. Dr. Surrey and Dr. Taylor receive additional support from companies including Pfizer, Bayer, and Obseva.
SOURCE: Surrey ES et al. ACOG 2018, Abstract 11OP.
REPORTING FROM ACOG 2018
Key clinical point: New treatment for endometriosis-related pain shows long-term efficacy.
Major finding: Pain significantly decreased in test groups, compared with placebo (P less than .05).
Data source: A phase 3, randomized trial of 570 women with moderate to severe endometriosis.
Disclosures: Dr. Surrey and several coauthors receive financial support from AbbVie as consultants, board members, and/or employees. Dr. Surrey and Dr. Taylor receive additional support from companies including Pfizer, Bayer, and Obseva.
Source: Surrey ES et al. ACOG 2018, Abstract 11OP.
Which infants with invasive bacterial infections are at risk for adverse outcomes?
TORONTO – Among infants up to 60 days old with an invasive bacterial infection, adverse outcomes are associated with prematurity, ill appearance, and bacterial meningitis, a multicenter retrospective analysis found.
“Young infants are susceptible to serious bacterial infections, particularly when they’re less than 60 days of age,” Christopher Pruitt, MD, said at the annual Pediatric Academic Societies meeting. “Among these infants, bacteremia and bacterial meningitis, also referred to as invasive bacterial infections, are associated with higher rates of morbidity and mortality.”
The primary outcome measure was occurrence of an adverse clinical outcome within 30 days following the index ED visit. Adverse outcomes were defined as use of mechanical ventilation, vasoactive medications, any neurologic sequelae, and death. The researchers used a mixed-effects logistic regression model and retained covariates with a P value of less than .10. Covariates analyzed included age less than 28 days, prematurity, presence or absence of a complex chronic condition, presence of fever, ill appearance, bacterial meningitis, and concordant empiric antimicrobial therapy.
Of the 442 infants included in the final analysis, the majority (80%) had bacteremia, 14% had bacterial meningitis plus bacteremia, and 6% had bacterial meningitis only. “For purposes of this study, patients with bacterial meningitis with or without bacteremia were categorized as having bacterial meningitis,” Dr. Pruitt said. He and his associates found that 14.5% of infants had one or more adverse outcomes. Adverse outcomes occurred in 39% of infants with bacterial meningitis, compared with 8.2% of infants with isolated bacteremia. Need for mechanical ventilation, vasoactive medications, and neurologic disability also was more common among infants with bacterial meningitis than it was among children with isolated bacteremia. There were 10 deaths overall, which amounted to about 2% in both groups.
On multivariate analysis, the rate of adverse outcomes was significantly higher for patients with bacterial meningitis than it was for those with isolated bacteremia (adjusted odds ratio, 8.8), for premature versus term infants (AOR, 5.9), for infants who were ill appearing versus non-ill appearing (AOR, 3.9), and for infants with no fever versus those with fever (AOR, 2.4). No significant associations with 30-day adverse outcomes were seen in patients with a complex chronic condition, compared with those without a complex chronic condition (AOR, 2.0), nor in the those aged 29-60 days versus those younger than 29 days (15% vs. 14%, respectively; AOR 0.7).
“When looking at the most common scenario – a full-term infant without an ill appearance, and bacteremia as opposed to bacterial meningitis – 3 of these 219 infants, or 1.4%, had an adverse outcome,” said Dr. Pruitt, who cares for patients in the ED at Children’s of Alabama in Birmingham. “And there were no deaths.” He also reported that 12 infants with invasive bacterial infections were discharged from the index ED visit without antimicrobial treatment. All had bacteremia and none had an adverse outcome.
Dr. Pruitt acknowledged certain limitations of the study, including its retrospective design, that the outcomes were limited to 30 days, and the fact that the findings may not be generalizable to nontertiary settings. “Our findings have important implications for the care of infants with invasive bacterial infections,” he concluded. “In particular, the high rate of adverse outcomes for infants with bacterial meningitis can provide some context for clinicians in assessing the need for diagnostic evaluation for invasive bacterial infection and discussing testing and treatment with parents. Our findings may also help to inform inpatient management for hospitalized infants with invasive bacterial infections, as well as anticipatory guidance for parents, particularly around follow-up. Further prospective studies evaluating the long-term outcomes of infants with invasive bacterial infections are needed.”
The study was supported in part by a grant from the National Institutes of Health. Dr. Pruitt reported having no financial disclosures.
TORONTO – Among infants up to 60 days old with an invasive bacterial infection, adverse outcomes are associated with prematurity, ill appearance, and bacterial meningitis, a multicenter retrospective analysis found.
“Young infants are susceptible to serious bacterial infections, particularly when they’re less than 60 days of age,” Christopher Pruitt, MD, said at the annual Pediatric Academic Societies meeting. “Among these infants, bacteremia and bacterial meningitis, also referred to as invasive bacterial infections, are associated with higher rates of morbidity and mortality.”
The primary outcome measure was occurrence of an adverse clinical outcome within 30 days following the index ED visit. Adverse outcomes were defined as use of mechanical ventilation, vasoactive medications, any neurologic sequelae, and death. The researchers used a mixed-effects logistic regression model and retained covariates with a P value of less than .10. Covariates analyzed included age less than 28 days, prematurity, presence or absence of a complex chronic condition, presence of fever, ill appearance, bacterial meningitis, and concordant empiric antimicrobial therapy.
Of the 442 infants included in the final analysis, the majority (80%) had bacteremia, 14% had bacterial meningitis plus bacteremia, and 6% had bacterial meningitis only. “For purposes of this study, patients with bacterial meningitis with or without bacteremia were categorized as having bacterial meningitis,” Dr. Pruitt said. He and his associates found that 14.5% of infants had one or more adverse outcomes. Adverse outcomes occurred in 39% of infants with bacterial meningitis, compared with 8.2% of infants with isolated bacteremia. Need for mechanical ventilation, vasoactive medications, and neurologic disability also was more common among infants with bacterial meningitis than it was among children with isolated bacteremia. There were 10 deaths overall, which amounted to about 2% in both groups.
On multivariate analysis, the rate of adverse outcomes was significantly higher for patients with bacterial meningitis than it was for those with isolated bacteremia (adjusted odds ratio, 8.8), for premature versus term infants (AOR, 5.9), for infants who were ill appearing versus non-ill appearing (AOR, 3.9), and for infants with no fever versus those with fever (AOR, 2.4). No significant associations with 30-day adverse outcomes were seen in patients with a complex chronic condition, compared with those without a complex chronic condition (AOR, 2.0), nor in the those aged 29-60 days versus those younger than 29 days (15% vs. 14%, respectively; AOR 0.7).
“When looking at the most common scenario – a full-term infant without an ill appearance, and bacteremia as opposed to bacterial meningitis – 3 of these 219 infants, or 1.4%, had an adverse outcome,” said Dr. Pruitt, who cares for patients in the ED at Children’s of Alabama in Birmingham. “And there were no deaths.” He also reported that 12 infants with invasive bacterial infections were discharged from the index ED visit without antimicrobial treatment. All had bacteremia and none had an adverse outcome.
Dr. Pruitt acknowledged certain limitations of the study, including its retrospective design, that the outcomes were limited to 30 days, and the fact that the findings may not be generalizable to nontertiary settings. “Our findings have important implications for the care of infants with invasive bacterial infections,” he concluded. “In particular, the high rate of adverse outcomes for infants with bacterial meningitis can provide some context for clinicians in assessing the need for diagnostic evaluation for invasive bacterial infection and discussing testing and treatment with parents. Our findings may also help to inform inpatient management for hospitalized infants with invasive bacterial infections, as well as anticipatory guidance for parents, particularly around follow-up. Further prospective studies evaluating the long-term outcomes of infants with invasive bacterial infections are needed.”
The study was supported in part by a grant from the National Institutes of Health. Dr. Pruitt reported having no financial disclosures.
TORONTO – Among infants up to 60 days old with an invasive bacterial infection, adverse outcomes are associated with prematurity, ill appearance, and bacterial meningitis, a multicenter retrospective analysis found.
“Young infants are susceptible to serious bacterial infections, particularly when they’re less than 60 days of age,” Christopher Pruitt, MD, said at the annual Pediatric Academic Societies meeting. “Among these infants, bacteremia and bacterial meningitis, also referred to as invasive bacterial infections, are associated with higher rates of morbidity and mortality.”
The primary outcome measure was occurrence of an adverse clinical outcome within 30 days following the index ED visit. Adverse outcomes were defined as use of mechanical ventilation, vasoactive medications, any neurologic sequelae, and death. The researchers used a mixed-effects logistic regression model and retained covariates with a P value of less than .10. Covariates analyzed included age less than 28 days, prematurity, presence or absence of a complex chronic condition, presence of fever, ill appearance, bacterial meningitis, and concordant empiric antimicrobial therapy.
Of the 442 infants included in the final analysis, the majority (80%) had bacteremia, 14% had bacterial meningitis plus bacteremia, and 6% had bacterial meningitis only. “For purposes of this study, patients with bacterial meningitis with or without bacteremia were categorized as having bacterial meningitis,” Dr. Pruitt said. He and his associates found that 14.5% of infants had one or more adverse outcomes. Adverse outcomes occurred in 39% of infants with bacterial meningitis, compared with 8.2% of infants with isolated bacteremia. Need for mechanical ventilation, vasoactive medications, and neurologic disability also was more common among infants with bacterial meningitis than it was among children with isolated bacteremia. There were 10 deaths overall, which amounted to about 2% in both groups.
On multivariate analysis, the rate of adverse outcomes was significantly higher for patients with bacterial meningitis than it was for those with isolated bacteremia (adjusted odds ratio, 8.8), for premature versus term infants (AOR, 5.9), for infants who were ill appearing versus non-ill appearing (AOR, 3.9), and for infants with no fever versus those with fever (AOR, 2.4). No significant associations with 30-day adverse outcomes were seen in patients with a complex chronic condition, compared with those without a complex chronic condition (AOR, 2.0), nor in the those aged 29-60 days versus those younger than 29 days (15% vs. 14%, respectively; AOR 0.7).
“When looking at the most common scenario – a full-term infant without an ill appearance, and bacteremia as opposed to bacterial meningitis – 3 of these 219 infants, or 1.4%, had an adverse outcome,” said Dr. Pruitt, who cares for patients in the ED at Children’s of Alabama in Birmingham. “And there were no deaths.” He also reported that 12 infants with invasive bacterial infections were discharged from the index ED visit without antimicrobial treatment. All had bacteremia and none had an adverse outcome.
Dr. Pruitt acknowledged certain limitations of the study, including its retrospective design, that the outcomes were limited to 30 days, and the fact that the findings may not be generalizable to nontertiary settings. “Our findings have important implications for the care of infants with invasive bacterial infections,” he concluded. “In particular, the high rate of adverse outcomes for infants with bacterial meningitis can provide some context for clinicians in assessing the need for diagnostic evaluation for invasive bacterial infection and discussing testing and treatment with parents. Our findings may also help to inform inpatient management for hospitalized infants with invasive bacterial infections, as well as anticipatory guidance for parents, particularly around follow-up. Further prospective studies evaluating the long-term outcomes of infants with invasive bacterial infections are needed.”
The study was supported in part by a grant from the National Institutes of Health. Dr. Pruitt reported having no financial disclosures.
REPORTING FROM PAS 2018
Key clinical point:
Major finding: The rate of adverse outcomes was significantly higher for patients with bacterial meningitis versus those with isolated bacteremia (adjusted odds ratio, 8.8) and for premature versus term infants (AOR, 5.9).
Study details: A multicenter, retrospective review of 442 infants with invasive bacterial infections who were initially evaluated in the ED.
Disclosures: The study was supported in part by a grant from the National Institutes of Health. Dr. Pruitt reported having no financial disclosures.
Probiotics reduce the risk of Clostridium difficile –associated diarrhea in patients receiving antibiotics
Background: Antibiotic use is associated with an increased risk of C. difficile infection. Multiple studies have investigated the effects of probiotics in reducing the risk of C. difficile infection with varied results. This meta-analysis aims to assess the efficacy and safety of probiotics in reducing the risk of CDAD in patients taking antibiotics.
Study design: Meta-analysis.
Setting: A comprehensive electronic search for randomized, controlled trials investigating probiotics for prevention of CDAD or C. difficile infection were considered for inclusion. There were no language, publication status, or date limits applied.
Synopsis: This meta-analysis included 31 trials (8,672 participants) evaluating the relationship between probiotics and CDAD. The outcomes were pooled using a random effects model to calculate risk ratios and 95% confidence intervals. A complete case analysis suggested that probiotics reduce the risk of CDAD by 60% (1.5% vs. 4.0%; relative risk, 0.40; 95% confidence interval, 0.3-0.52), although a post-hoc subgroup analysis showed a statistically significant benefit only among patients with a high CDAD baseline risk (greater than 5%). Adverse events were assessed in 32 trials (8,305 participants), and the pooled analysis indicated that probiotic use reduced the risk of adverse events by 17% (RR, 0.83; 95% CI, 0.71-0.97).
Limitations to this meta-analysis include missing data from patients lost to follow-up and lack of success in testing all fecal samples. Lastly, that the strongest data for the beneficial effects of probiotics were demonstrated in patients with a high baseline risk of developing CDAD limits the study’s applicability to the general population.
Bottom line: Probiotic use in immunocompetent patients undergoing treatment with antibiotics decreases the incidence of CDAD without an increase in adverse events.
Citation: Goldenberg JZ et al. Probiotics for the prevention of Clostridium difficile–associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017. doi: 10.1002/14651858.CD006095.pub4.
Dr. Skinner is a hospitalist at Denver Health Medical Center and an assistant professor of medicine at the University of Colorado at Denver, Aurora.
Background: Antibiotic use is associated with an increased risk of C. difficile infection. Multiple studies have investigated the effects of probiotics in reducing the risk of C. difficile infection with varied results. This meta-analysis aims to assess the efficacy and safety of probiotics in reducing the risk of CDAD in patients taking antibiotics.
Study design: Meta-analysis.
Setting: A comprehensive electronic search for randomized, controlled trials investigating probiotics for prevention of CDAD or C. difficile infection were considered for inclusion. There were no language, publication status, or date limits applied.
Synopsis: This meta-analysis included 31 trials (8,672 participants) evaluating the relationship between probiotics and CDAD. The outcomes were pooled using a random effects model to calculate risk ratios and 95% confidence intervals. A complete case analysis suggested that probiotics reduce the risk of CDAD by 60% (1.5% vs. 4.0%; relative risk, 0.40; 95% confidence interval, 0.3-0.52), although a post-hoc subgroup analysis showed a statistically significant benefit only among patients with a high CDAD baseline risk (greater than 5%). Adverse events were assessed in 32 trials (8,305 participants), and the pooled analysis indicated that probiotic use reduced the risk of adverse events by 17% (RR, 0.83; 95% CI, 0.71-0.97).
Limitations to this meta-analysis include missing data from patients lost to follow-up and lack of success in testing all fecal samples. Lastly, that the strongest data for the beneficial effects of probiotics were demonstrated in patients with a high baseline risk of developing CDAD limits the study’s applicability to the general population.
Bottom line: Probiotic use in immunocompetent patients undergoing treatment with antibiotics decreases the incidence of CDAD without an increase in adverse events.
Citation: Goldenberg JZ et al. Probiotics for the prevention of Clostridium difficile–associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017. doi: 10.1002/14651858.CD006095.pub4.
Dr. Skinner is a hospitalist at Denver Health Medical Center and an assistant professor of medicine at the University of Colorado at Denver, Aurora.
Background: Antibiotic use is associated with an increased risk of C. difficile infection. Multiple studies have investigated the effects of probiotics in reducing the risk of C. difficile infection with varied results. This meta-analysis aims to assess the efficacy and safety of probiotics in reducing the risk of CDAD in patients taking antibiotics.
Study design: Meta-analysis.
Setting: A comprehensive electronic search for randomized, controlled trials investigating probiotics for prevention of CDAD or C. difficile infection were considered for inclusion. There were no language, publication status, or date limits applied.
Synopsis: This meta-analysis included 31 trials (8,672 participants) evaluating the relationship between probiotics and CDAD. The outcomes were pooled using a random effects model to calculate risk ratios and 95% confidence intervals. A complete case analysis suggested that probiotics reduce the risk of CDAD by 60% (1.5% vs. 4.0%; relative risk, 0.40; 95% confidence interval, 0.3-0.52), although a post-hoc subgroup analysis showed a statistically significant benefit only among patients with a high CDAD baseline risk (greater than 5%). Adverse events were assessed in 32 trials (8,305 participants), and the pooled analysis indicated that probiotic use reduced the risk of adverse events by 17% (RR, 0.83; 95% CI, 0.71-0.97).
Limitations to this meta-analysis include missing data from patients lost to follow-up and lack of success in testing all fecal samples. Lastly, that the strongest data for the beneficial effects of probiotics were demonstrated in patients with a high baseline risk of developing CDAD limits the study’s applicability to the general population.
Bottom line: Probiotic use in immunocompetent patients undergoing treatment with antibiotics decreases the incidence of CDAD without an increase in adverse events.
Citation: Goldenberg JZ et al. Probiotics for the prevention of Clostridium difficile–associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017. doi: 10.1002/14651858.CD006095.pub4.
Dr. Skinner is a hospitalist at Denver Health Medical Center and an assistant professor of medicine at the University of Colorado at Denver, Aurora.
Boston and beyond: Stay connected at #AACE2018
Come on. Everyone knows the best part of any conference is meeting up with colleagues, mentors, and friends. So here’s how to do it at (or away from) the annual meeting of the American Association of Clinical Endocrinologists in Boston on May 16-20.
No one is immune from the pull of colleagues past, present, and even future, not even the program chair Vin Tangpricha, MD, who gave the lowdown in an interview on the best venues for getting in touch.
- Thursday Night Chapter Receptions: After the first full day of the meeting, start in the room for your home chapter, then jump to your home state’s chapter, then your training chapter, and so on. This is the easiest and most exciting way at the meeting to connect with your web of colleagues, Dr. Tangpricha suggested enthusiastically. And don’t forget about AACE members from across the globe in the International Chapter Reception, in the biggest (and most fun, by some reports) party room by far. The receptions for all 22 domestic chapters and international members representing 102 countries will be held in the Sheraton Boston Hotel at 6 p.m.
- Wine and Cheese Reception: A Friday night tradition at AACE, from 4:30 p.m. to 6:30 p.m. in the exhibit hall, this offers a needed break at the end of the second full day of sessions to chat with colleagues, see exhibits, and check out posters.
- Women’s Luncheon: Thursday from 12 p.m. to 2:00 p.m. on Level C. In its fifth year, this luncheon has practically outgrown the meeting, with women entering endocrinology outpacing men. Good luck getting tickets; it’s always popular, Dr. Tangpricha said. SeanPavonePhot/Thinkstock
- College Breakfast With the Masters: This first-time-ever event welcomes and celebrates all past Masters of the American College of Endocrinology (MACEs). In this free, ticketed event on Saturday morning from 6:30 a.m. to 8 a.m., the largest assembly of MACEs ever gathered in the same room will be introduced to newer AACE fellows, who will then have the opportunity to meet with Masters and gather their insights on how to further their own careers in endocrinology, Dr. Tangpricha said.
- President’s Gala: This ticketed event open to members and family is always a great finale to the meeting, held Saturday night after the convocation, is one Dr. Tangpricha always looks forward to. This family-friendly event features food, libations, and live music.
- Social networking: Stay in touch with attendees and endocrinologists following the events and news from the meeting at #AACE2018, and with a Boston Strong–inspired hashtag of #ClinicalEndoStrong.
Come on. Everyone knows the best part of any conference is meeting up with colleagues, mentors, and friends. So here’s how to do it at (or away from) the annual meeting of the American Association of Clinical Endocrinologists in Boston on May 16-20.
No one is immune from the pull of colleagues past, present, and even future, not even the program chair Vin Tangpricha, MD, who gave the lowdown in an interview on the best venues for getting in touch.
- Thursday Night Chapter Receptions: After the first full day of the meeting, start in the room for your home chapter, then jump to your home state’s chapter, then your training chapter, and so on. This is the easiest and most exciting way at the meeting to connect with your web of colleagues, Dr. Tangpricha suggested enthusiastically. And don’t forget about AACE members from across the globe in the International Chapter Reception, in the biggest (and most fun, by some reports) party room by far. The receptions for all 22 domestic chapters and international members representing 102 countries will be held in the Sheraton Boston Hotel at 6 p.m.
- Wine and Cheese Reception: A Friday night tradition at AACE, from 4:30 p.m. to 6:30 p.m. in the exhibit hall, this offers a needed break at the end of the second full day of sessions to chat with colleagues, see exhibits, and check out posters.
- Women’s Luncheon: Thursday from 12 p.m. to 2:00 p.m. on Level C. In its fifth year, this luncheon has practically outgrown the meeting, with women entering endocrinology outpacing men. Good luck getting tickets; it’s always popular, Dr. Tangpricha said. SeanPavonePhot/Thinkstock
- College Breakfast With the Masters: This first-time-ever event welcomes and celebrates all past Masters of the American College of Endocrinology (MACEs). In this free, ticketed event on Saturday morning from 6:30 a.m. to 8 a.m., the largest assembly of MACEs ever gathered in the same room will be introduced to newer AACE fellows, who will then have the opportunity to meet with Masters and gather their insights on how to further their own careers in endocrinology, Dr. Tangpricha said.
- President’s Gala: This ticketed event open to members and family is always a great finale to the meeting, held Saturday night after the convocation, is one Dr. Tangpricha always looks forward to. This family-friendly event features food, libations, and live music.
- Social networking: Stay in touch with attendees and endocrinologists following the events and news from the meeting at #AACE2018, and with a Boston Strong–inspired hashtag of #ClinicalEndoStrong.
Come on. Everyone knows the best part of any conference is meeting up with colleagues, mentors, and friends. So here’s how to do it at (or away from) the annual meeting of the American Association of Clinical Endocrinologists in Boston on May 16-20.
No one is immune from the pull of colleagues past, present, and even future, not even the program chair Vin Tangpricha, MD, who gave the lowdown in an interview on the best venues for getting in touch.
- Thursday Night Chapter Receptions: After the first full day of the meeting, start in the room for your home chapter, then jump to your home state’s chapter, then your training chapter, and so on. This is the easiest and most exciting way at the meeting to connect with your web of colleagues, Dr. Tangpricha suggested enthusiastically. And don’t forget about AACE members from across the globe in the International Chapter Reception, in the biggest (and most fun, by some reports) party room by far. The receptions for all 22 domestic chapters and international members representing 102 countries will be held in the Sheraton Boston Hotel at 6 p.m.
- Wine and Cheese Reception: A Friday night tradition at AACE, from 4:30 p.m. to 6:30 p.m. in the exhibit hall, this offers a needed break at the end of the second full day of sessions to chat with colleagues, see exhibits, and check out posters.
- Women’s Luncheon: Thursday from 12 p.m. to 2:00 p.m. on Level C. In its fifth year, this luncheon has practically outgrown the meeting, with women entering endocrinology outpacing men. Good luck getting tickets; it’s always popular, Dr. Tangpricha said. SeanPavonePhot/Thinkstock
- College Breakfast With the Masters: This first-time-ever event welcomes and celebrates all past Masters of the American College of Endocrinology (MACEs). In this free, ticketed event on Saturday morning from 6:30 a.m. to 8 a.m., the largest assembly of MACEs ever gathered in the same room will be introduced to newer AACE fellows, who will then have the opportunity to meet with Masters and gather their insights on how to further their own careers in endocrinology, Dr. Tangpricha said.
- President’s Gala: This ticketed event open to members and family is always a great finale to the meeting, held Saturday night after the convocation, is one Dr. Tangpricha always looks forward to. This family-friendly event features food, libations, and live music.
- Social networking: Stay in touch with attendees and endocrinologists following the events and news from the meeting at #AACE2018, and with a Boston Strong–inspired hashtag of #ClinicalEndoStrong.
FROM AACE 2018
MDedge Daily News: Suicide prevention gets ‘standard care’ recommendations
Also in today’s edition of the Daily News Podcast, fremanezumab may be an effective episodic migraine treatment, e-cigarette usage has changed, and there is evidence that marketing perks increased opioid prescriptions. Listen to the MDedge Daily News Podcast for all the details ons today’s top news.
Also in today’s edition of the Daily News Podcast, fremanezumab may be an effective episodic migraine treatment, e-cigarette usage has changed, and there is evidence that marketing perks increased opioid prescriptions. Listen to the MDedge Daily News Podcast for all the details ons today’s top news.
Also in today’s edition of the Daily News Podcast, fremanezumab may be an effective episodic migraine treatment, e-cigarette usage has changed, and there is evidence that marketing perks increased opioid prescriptions. Listen to the MDedge Daily News Podcast for all the details ons today’s top news.
Approach assesses imminent suicide risk
Lorenzo Norris, MD, editor in chief of MDedge Psychiatry, sits down with Igor Galynker, MD, PhD, to talk about an evaluation model he and his team created aimed at assessing the risk of imminent suicide. Dr. Galynker also discusses ways clinicians can assess their own personal emotional responses to patients who are at risk.
Lorenzo Norris, MD, editor in chief of MDedge Psychiatry, sits down with Igor Galynker, MD, PhD, to talk about an evaluation model he and his team created aimed at assessing the risk of imminent suicide. Dr. Galynker also discusses ways clinicians can assess their own personal emotional responses to patients who are at risk.
Lorenzo Norris, MD, editor in chief of MDedge Psychiatry, sits down with Igor Galynker, MD, PhD, to talk about an evaluation model he and his team created aimed at assessing the risk of imminent suicide. Dr. Galynker also discusses ways clinicians can assess their own personal emotional responses to patients who are at risk.
A Forgotten Cause of Cardiac Tamponade
Purulent pericarditis is an infection within the pericardial space rarely seen in the modern antibiotic era. Most cases are secondary to another infectious process of bacterial, viral, fungal, or parasitic origin.1,2 Predisposing factors include malignancy, chronic kidney disease, immunosuppression, diabetes mellitus, and alcohol misuse disorder.1 Although purulent pericarditis has been described extensively in the literature, it is a challenging diagnosis if it is not initially considered within the differential diagnosis repertoire.1-4 Most authors agree that this may be because it has become an infrequent diagnosis.1,2 In addition, purulent pericarditis may have an atypical presentation when compared with a classic case of pericarditis.2,3 The authors believe that this forgotten entity will be revisited through this case.
Case Presentation
A 66-year-old-man was transferred to Veterans Affairs Caribbean Healthcare System (VACHS) from a community hospital with a diagnosis of community-acquired pneumonia (CAP) and bilateral pleural effusions. Four days prior to arrival at the community hospital, the patient had developed diffuse, watery diarrhea, which resolved in 3 days. After resolution of diarrhea, he began experiencing shortness of breath on exertion that progressed to onset at rest. The patient reported no fever, chills, nausea, vomiting, cough, or contact with others who were not healthy. He had a history of alcohol misuse without liver cirrhosis and reported no chronic diseases or use of medications. The patient had no history of tuberculosis exposure or pneumococcal vaccination, and had a negative interferon gamma release assay.
On admission to the community hospital, the patient was treated for CAP with ceftriaxone and azithromycin. On hospital day 3, the patient developed hypoxemia and an altered mental status. He was started on supplemental oxygen and transferred to the intensive care unit (ICU). Antibiotic therapy consequently was changed to levofloxacin and meropenem. However, no clinical improvement was noted on the following days.
On hospital day 7, the patient developed acute respiratory failure that required mechanical ventilation while being transferred to VACHS via air ambulance. His vital signs on arrival were the following: temperature, 97° F; heart rate, 86 beats/min; blood pressure, 103/61 mm Hg; respiratory rate, 14 breaths/min and SaO2 of 97%, measured while he breathed supplemental oxygen at an FiO2 of 0.4.
Hours after arrival, the patient developed sinus tachycardia and hypotension. A bedside 2D echocardiogram demonstrated a large pericardial effusion with diastolic collapse of the right atrium (Figure 2).
The patient’s clinical condition improved following drainage of pericardial fluid, with no further need for inotropic support. Antibiotic therapy was changed to vancomycin and meropenem. Initial microbiologic samples from pericardial fluid demonstrated Gram-positive diplococci, suggestive of Streptococcus pneumoniae (S pneumoniae) (Figure 4). Other diagnostic pericardial fluid test results included: WBC count 25,330 cmm, with 99% neutrophils and 1% lymphocytes; total protein, 3.8 mg/dL; glucose, < 2.0 mg/dL,LDH, > 2,500 U/L, potassium hydroxide preparation. The tests found no fungus, and the acid fast bacilli smear revealed no Bacillus. However, the pericardial fluid culture failed to demonstrate growth of any organism. Blood cultures also were negative.
The patient underwent anterior thoracotomy with partial pericardiectomy, and a pericardial tube was left in place connected to drainage. During the procedure, an abundant amount of fibrinous tissue was evacuated from the pericardial space (Figure 5).
The patient was extubated, pericardial and pleural tubes were removed, and he was transferred to the internal medicine ward 24 days after admission to the ICU. He received in-patient physical rehabilitation while completing a 6-week course of IV antibiotics (vancomycin and meropenem). After completion of therapy, the patient received the pneumococcal polysaccharide vaccination, and an echocardiography was repeated. No significant re-accumulation of pericardial effusion or constrictive pattern was evidenced. The patient was discharged to his out-of-state home, and follow-up was consequently lost.
Discussion
Purulent pericarditis is an infection localized within the pericardial space. Most cases are secondary to an infectious process elsewhere, which could be of bacterial, viral, fungal, or parasitic etiology.1 Five mechanisms could lead the infecting organism to infect the pericardial space; contiguous spread from intrathoracic site, hematogenous spread, extension from myocardial site, perforating injury or surgery, and extension from a subdiaphragmatic site.1 Predisposing factors for the development of this condition include malignancy, chronic kidney disease, immunosuppression, diabetes mellitus, and alcohol misuse. Pericarditis is an infection localized within the pericardial space.
Purulent pericarditis has become a rare entity in the antibiotic era.2 Prior to the development of antibiotics, most cases were secondary to S pneumoniae.1,2,5,6 As per Cilloniz and colleagues, about 40% to 50% of all cases of purulent pericarditis are caused by Gram-positive bacteria, mostly S pneumoniae.5 In this case study, bacterial culture did not reveal growth of an organism—most likely because the patient had received antibiotics elsewhere. However, Gram-positive cocci were seen within the initial pericardial aspirate. This organism was suspected to have spread contiguously from a pulmonary focus, which also led to pleural effusions.
Since the patient in this case study had no history of thoracic surgery, malignancy, or other immunosuppression, the patient’s history of alcohol misuse was the only predisposing factor for development of purulent pericarditis. Contrary to the common presentation of pericarditis, purulent pericarditis may not have the common clinical findings, such as chest pain, pericardial friction rub, and distended neck veins.2,3 Furthermore, according to Parikh and colleagues, about 35% of affected patients may have a normal electrocardiogram.2 Hence, the diagnosis of purulent pericarditis often is missed because the classic signs of pericarditis are often absent, and other nonspecific symptoms are attributed to initial underlying infection.7
A high index of suspicion is needed to diagnose purulent pericarditis. Once a diagnosis is made, initial treatment should consist of prompt drainage of pericardial fluid combined with systemic antibiotic therapy. Vancomycin and a third-generation cephalosporin may be started empirically until results of pericardial fluid cultures become available.3 Drainage can be achieved by pericardiocentesis, pericardiotomy, or pericardiectomy (partial or total).1 In cases of hemodynamic instability due to cardiac tamponade, sonographically guided pericardiocentesis should be undertaken and an indwelling pericardial catheter left in place.1 Although this is the simplest and fastest method of evacuation, it may not be effective when dealing with thick, fibrinous fluid. In such cases, intrapericardial fibrinolysis may be considered. This approach may be undertaken early in the process, after drainage insertion, or as salvage therapy, when there has been incomplete evacuation of purulent material or open surgical drainage is not available.
Streptokinase, urokinase, and tissue plasminogen activator have been used for intrapericadial fibrinolysis.1 However, there is no definite data on dosage or frequency at which these medications should be administered. No matter the therapeutic approach, effective drainage of the pericardial fluid is crucial to avoid the development of pericardial constriction. Constrictive pericarditis occurs when fibrosis and adhesions create a dense pericardium that encases the heart. This causes impaired ventricular filling that can lead eventually to heart failure.4 Pericardiectomy is the definitive treatment for constrictive pericarditis.
Conclusion
Although purulent pericarditis has become a rare diagnosis since the development of antibiotics, knowledge of how to identify it is essential since mortality reaches 100% if the diagnosis is missed.4 Even when the condition is promptly diagnosed and treated, mortality is 40%, mainly due to cardiac tamponade, septic shock, or constriction.1 The case presented here illustrates the clinical features associated with this condition. Knowing these features can translate in a successful patient outcome.
1. Ferreira dos Santos L, Moreira D, Ribeiro P, et al. Purulent pericarditis: a rare diagnosis [in Portuguese]. Rev Port Cardiol. 2013;32(9):721-727.
2. Parikh SV, Memon N, Echols M, Shah J, McGuire DK, Keeley EC. Purulent pericarditis: report of 2 cases and review of the literature. Medicine (Baltimore). 2009;88(1):52–65.
3. Go C, Asnis DS, Saltzman H. Pneumococcal pericarditis since 1980. Clin Infect Dis. 1998;27(5):1338-1340.
4. Wada A, Craft J, Mazzaferri EL. Purulent pericarditis leading to constriction. Cardiol Res. 2014;5(6):188-190.
5. Cillóniz C, Rangel E, Barlascini C, Piroddi IMG, Torres A, Nicolini A. Streptococcus pneumoniae-associated pneumonia complicated by purulent pericarditis: case series [in English, Portuguese]. J Bras Pneumol. 2015;41(4):389-394.
6. Saenz RE, Sanders CV, Aldridge KE, Patel MM. Purulent pericarditis with associated cardiac tamponade caused by a Streptococcus pneumoniae strain highly resistant to penicillin, cefotaxime, and ceftriaxone. Clin Infect Dis. 1998;26(3):762–763.
7. Sagristà-Sauleda J, Barrabés JA, Permanyer-Miralda G, Soler-Soler J. Purulent pericarditis: review of a 20-year experience in a general hospital. J Am Coll Cardiol. 1993; 22(6):1661-1665.
Purulent pericarditis is an infection within the pericardial space rarely seen in the modern antibiotic era. Most cases are secondary to another infectious process of bacterial, viral, fungal, or parasitic origin.1,2 Predisposing factors include malignancy, chronic kidney disease, immunosuppression, diabetes mellitus, and alcohol misuse disorder.1 Although purulent pericarditis has been described extensively in the literature, it is a challenging diagnosis if it is not initially considered within the differential diagnosis repertoire.1-4 Most authors agree that this may be because it has become an infrequent diagnosis.1,2 In addition, purulent pericarditis may have an atypical presentation when compared with a classic case of pericarditis.2,3 The authors believe that this forgotten entity will be revisited through this case.
Case Presentation
A 66-year-old-man was transferred to Veterans Affairs Caribbean Healthcare System (VACHS) from a community hospital with a diagnosis of community-acquired pneumonia (CAP) and bilateral pleural effusions. Four days prior to arrival at the community hospital, the patient had developed diffuse, watery diarrhea, which resolved in 3 days. After resolution of diarrhea, he began experiencing shortness of breath on exertion that progressed to onset at rest. The patient reported no fever, chills, nausea, vomiting, cough, or contact with others who were not healthy. He had a history of alcohol misuse without liver cirrhosis and reported no chronic diseases or use of medications. The patient had no history of tuberculosis exposure or pneumococcal vaccination, and had a negative interferon gamma release assay.
On admission to the community hospital, the patient was treated for CAP with ceftriaxone and azithromycin. On hospital day 3, the patient developed hypoxemia and an altered mental status. He was started on supplemental oxygen and transferred to the intensive care unit (ICU). Antibiotic therapy consequently was changed to levofloxacin and meropenem. However, no clinical improvement was noted on the following days.
On hospital day 7, the patient developed acute respiratory failure that required mechanical ventilation while being transferred to VACHS via air ambulance. His vital signs on arrival were the following: temperature, 97° F; heart rate, 86 beats/min; blood pressure, 103/61 mm Hg; respiratory rate, 14 breaths/min and SaO2 of 97%, measured while he breathed supplemental oxygen at an FiO2 of 0.4.
Hours after arrival, the patient developed sinus tachycardia and hypotension. A bedside 2D echocardiogram demonstrated a large pericardial effusion with diastolic collapse of the right atrium (Figure 2).
The patient’s clinical condition improved following drainage of pericardial fluid, with no further need for inotropic support. Antibiotic therapy was changed to vancomycin and meropenem. Initial microbiologic samples from pericardial fluid demonstrated Gram-positive diplococci, suggestive of Streptococcus pneumoniae (S pneumoniae) (Figure 4). Other diagnostic pericardial fluid test results included: WBC count 25,330 cmm, with 99% neutrophils and 1% lymphocytes; total protein, 3.8 mg/dL; glucose, < 2.0 mg/dL,LDH, > 2,500 U/L, potassium hydroxide preparation. The tests found no fungus, and the acid fast bacilli smear revealed no Bacillus. However, the pericardial fluid culture failed to demonstrate growth of any organism. Blood cultures also were negative.
The patient underwent anterior thoracotomy with partial pericardiectomy, and a pericardial tube was left in place connected to drainage. During the procedure, an abundant amount of fibrinous tissue was evacuated from the pericardial space (Figure 5).
The patient was extubated, pericardial and pleural tubes were removed, and he was transferred to the internal medicine ward 24 days after admission to the ICU. He received in-patient physical rehabilitation while completing a 6-week course of IV antibiotics (vancomycin and meropenem). After completion of therapy, the patient received the pneumococcal polysaccharide vaccination, and an echocardiography was repeated. No significant re-accumulation of pericardial effusion or constrictive pattern was evidenced. The patient was discharged to his out-of-state home, and follow-up was consequently lost.
Discussion
Purulent pericarditis is an infection localized within the pericardial space. Most cases are secondary to an infectious process elsewhere, which could be of bacterial, viral, fungal, or parasitic etiology.1 Five mechanisms could lead the infecting organism to infect the pericardial space; contiguous spread from intrathoracic site, hematogenous spread, extension from myocardial site, perforating injury or surgery, and extension from a subdiaphragmatic site.1 Predisposing factors for the development of this condition include malignancy, chronic kidney disease, immunosuppression, diabetes mellitus, and alcohol misuse. Pericarditis is an infection localized within the pericardial space.
Purulent pericarditis has become a rare entity in the antibiotic era.2 Prior to the development of antibiotics, most cases were secondary to S pneumoniae.1,2,5,6 As per Cilloniz and colleagues, about 40% to 50% of all cases of purulent pericarditis are caused by Gram-positive bacteria, mostly S pneumoniae.5 In this case study, bacterial culture did not reveal growth of an organism—most likely because the patient had received antibiotics elsewhere. However, Gram-positive cocci were seen within the initial pericardial aspirate. This organism was suspected to have spread contiguously from a pulmonary focus, which also led to pleural effusions.
Since the patient in this case study had no history of thoracic surgery, malignancy, or other immunosuppression, the patient’s history of alcohol misuse was the only predisposing factor for development of purulent pericarditis. Contrary to the common presentation of pericarditis, purulent pericarditis may not have the common clinical findings, such as chest pain, pericardial friction rub, and distended neck veins.2,3 Furthermore, according to Parikh and colleagues, about 35% of affected patients may have a normal electrocardiogram.2 Hence, the diagnosis of purulent pericarditis often is missed because the classic signs of pericarditis are often absent, and other nonspecific symptoms are attributed to initial underlying infection.7
A high index of suspicion is needed to diagnose purulent pericarditis. Once a diagnosis is made, initial treatment should consist of prompt drainage of pericardial fluid combined with systemic antibiotic therapy. Vancomycin and a third-generation cephalosporin may be started empirically until results of pericardial fluid cultures become available.3 Drainage can be achieved by pericardiocentesis, pericardiotomy, or pericardiectomy (partial or total).1 In cases of hemodynamic instability due to cardiac tamponade, sonographically guided pericardiocentesis should be undertaken and an indwelling pericardial catheter left in place.1 Although this is the simplest and fastest method of evacuation, it may not be effective when dealing with thick, fibrinous fluid. In such cases, intrapericardial fibrinolysis may be considered. This approach may be undertaken early in the process, after drainage insertion, or as salvage therapy, when there has been incomplete evacuation of purulent material or open surgical drainage is not available.
Streptokinase, urokinase, and tissue plasminogen activator have been used for intrapericadial fibrinolysis.1 However, there is no definite data on dosage or frequency at which these medications should be administered. No matter the therapeutic approach, effective drainage of the pericardial fluid is crucial to avoid the development of pericardial constriction. Constrictive pericarditis occurs when fibrosis and adhesions create a dense pericardium that encases the heart. This causes impaired ventricular filling that can lead eventually to heart failure.4 Pericardiectomy is the definitive treatment for constrictive pericarditis.
Conclusion
Although purulent pericarditis has become a rare diagnosis since the development of antibiotics, knowledge of how to identify it is essential since mortality reaches 100% if the diagnosis is missed.4 Even when the condition is promptly diagnosed and treated, mortality is 40%, mainly due to cardiac tamponade, septic shock, or constriction.1 The case presented here illustrates the clinical features associated with this condition. Knowing these features can translate in a successful patient outcome.
Purulent pericarditis is an infection within the pericardial space rarely seen in the modern antibiotic era. Most cases are secondary to another infectious process of bacterial, viral, fungal, or parasitic origin.1,2 Predisposing factors include malignancy, chronic kidney disease, immunosuppression, diabetes mellitus, and alcohol misuse disorder.1 Although purulent pericarditis has been described extensively in the literature, it is a challenging diagnosis if it is not initially considered within the differential diagnosis repertoire.1-4 Most authors agree that this may be because it has become an infrequent diagnosis.1,2 In addition, purulent pericarditis may have an atypical presentation when compared with a classic case of pericarditis.2,3 The authors believe that this forgotten entity will be revisited through this case.
Case Presentation
A 66-year-old-man was transferred to Veterans Affairs Caribbean Healthcare System (VACHS) from a community hospital with a diagnosis of community-acquired pneumonia (CAP) and bilateral pleural effusions. Four days prior to arrival at the community hospital, the patient had developed diffuse, watery diarrhea, which resolved in 3 days. After resolution of diarrhea, he began experiencing shortness of breath on exertion that progressed to onset at rest. The patient reported no fever, chills, nausea, vomiting, cough, or contact with others who were not healthy. He had a history of alcohol misuse without liver cirrhosis and reported no chronic diseases or use of medications. The patient had no history of tuberculosis exposure or pneumococcal vaccination, and had a negative interferon gamma release assay.
On admission to the community hospital, the patient was treated for CAP with ceftriaxone and azithromycin. On hospital day 3, the patient developed hypoxemia and an altered mental status. He was started on supplemental oxygen and transferred to the intensive care unit (ICU). Antibiotic therapy consequently was changed to levofloxacin and meropenem. However, no clinical improvement was noted on the following days.
On hospital day 7, the patient developed acute respiratory failure that required mechanical ventilation while being transferred to VACHS via air ambulance. His vital signs on arrival were the following: temperature, 97° F; heart rate, 86 beats/min; blood pressure, 103/61 mm Hg; respiratory rate, 14 breaths/min and SaO2 of 97%, measured while he breathed supplemental oxygen at an FiO2 of 0.4.
Hours after arrival, the patient developed sinus tachycardia and hypotension. A bedside 2D echocardiogram demonstrated a large pericardial effusion with diastolic collapse of the right atrium (Figure 2).
The patient’s clinical condition improved following drainage of pericardial fluid, with no further need for inotropic support. Antibiotic therapy was changed to vancomycin and meropenem. Initial microbiologic samples from pericardial fluid demonstrated Gram-positive diplococci, suggestive of Streptococcus pneumoniae (S pneumoniae) (Figure 4). Other diagnostic pericardial fluid test results included: WBC count 25,330 cmm, with 99% neutrophils and 1% lymphocytes; total protein, 3.8 mg/dL; glucose, < 2.0 mg/dL,LDH, > 2,500 U/L, potassium hydroxide preparation. The tests found no fungus, and the acid fast bacilli smear revealed no Bacillus. However, the pericardial fluid culture failed to demonstrate growth of any organism. Blood cultures also were negative.
The patient underwent anterior thoracotomy with partial pericardiectomy, and a pericardial tube was left in place connected to drainage. During the procedure, an abundant amount of fibrinous tissue was evacuated from the pericardial space (Figure 5).
The patient was extubated, pericardial and pleural tubes were removed, and he was transferred to the internal medicine ward 24 days after admission to the ICU. He received in-patient physical rehabilitation while completing a 6-week course of IV antibiotics (vancomycin and meropenem). After completion of therapy, the patient received the pneumococcal polysaccharide vaccination, and an echocardiography was repeated. No significant re-accumulation of pericardial effusion or constrictive pattern was evidenced. The patient was discharged to his out-of-state home, and follow-up was consequently lost.
Discussion
Purulent pericarditis is an infection localized within the pericardial space. Most cases are secondary to an infectious process elsewhere, which could be of bacterial, viral, fungal, or parasitic etiology.1 Five mechanisms could lead the infecting organism to infect the pericardial space; contiguous spread from intrathoracic site, hematogenous spread, extension from myocardial site, perforating injury or surgery, and extension from a subdiaphragmatic site.1 Predisposing factors for the development of this condition include malignancy, chronic kidney disease, immunosuppression, diabetes mellitus, and alcohol misuse. Pericarditis is an infection localized within the pericardial space.
Purulent pericarditis has become a rare entity in the antibiotic era.2 Prior to the development of antibiotics, most cases were secondary to S pneumoniae.1,2,5,6 As per Cilloniz and colleagues, about 40% to 50% of all cases of purulent pericarditis are caused by Gram-positive bacteria, mostly S pneumoniae.5 In this case study, bacterial culture did not reveal growth of an organism—most likely because the patient had received antibiotics elsewhere. However, Gram-positive cocci were seen within the initial pericardial aspirate. This organism was suspected to have spread contiguously from a pulmonary focus, which also led to pleural effusions.
Since the patient in this case study had no history of thoracic surgery, malignancy, or other immunosuppression, the patient’s history of alcohol misuse was the only predisposing factor for development of purulent pericarditis. Contrary to the common presentation of pericarditis, purulent pericarditis may not have the common clinical findings, such as chest pain, pericardial friction rub, and distended neck veins.2,3 Furthermore, according to Parikh and colleagues, about 35% of affected patients may have a normal electrocardiogram.2 Hence, the diagnosis of purulent pericarditis often is missed because the classic signs of pericarditis are often absent, and other nonspecific symptoms are attributed to initial underlying infection.7
A high index of suspicion is needed to diagnose purulent pericarditis. Once a diagnosis is made, initial treatment should consist of prompt drainage of pericardial fluid combined with systemic antibiotic therapy. Vancomycin and a third-generation cephalosporin may be started empirically until results of pericardial fluid cultures become available.3 Drainage can be achieved by pericardiocentesis, pericardiotomy, or pericardiectomy (partial or total).1 In cases of hemodynamic instability due to cardiac tamponade, sonographically guided pericardiocentesis should be undertaken and an indwelling pericardial catheter left in place.1 Although this is the simplest and fastest method of evacuation, it may not be effective when dealing with thick, fibrinous fluid. In such cases, intrapericardial fibrinolysis may be considered. This approach may be undertaken early in the process, after drainage insertion, or as salvage therapy, when there has been incomplete evacuation of purulent material or open surgical drainage is not available.
Streptokinase, urokinase, and tissue plasminogen activator have been used for intrapericadial fibrinolysis.1 However, there is no definite data on dosage or frequency at which these medications should be administered. No matter the therapeutic approach, effective drainage of the pericardial fluid is crucial to avoid the development of pericardial constriction. Constrictive pericarditis occurs when fibrosis and adhesions create a dense pericardium that encases the heart. This causes impaired ventricular filling that can lead eventually to heart failure.4 Pericardiectomy is the definitive treatment for constrictive pericarditis.
Conclusion
Although purulent pericarditis has become a rare diagnosis since the development of antibiotics, knowledge of how to identify it is essential since mortality reaches 100% if the diagnosis is missed.4 Even when the condition is promptly diagnosed and treated, mortality is 40%, mainly due to cardiac tamponade, septic shock, or constriction.1 The case presented here illustrates the clinical features associated with this condition. Knowing these features can translate in a successful patient outcome.
1. Ferreira dos Santos L, Moreira D, Ribeiro P, et al. Purulent pericarditis: a rare diagnosis [in Portuguese]. Rev Port Cardiol. 2013;32(9):721-727.
2. Parikh SV, Memon N, Echols M, Shah J, McGuire DK, Keeley EC. Purulent pericarditis: report of 2 cases and review of the literature. Medicine (Baltimore). 2009;88(1):52–65.
3. Go C, Asnis DS, Saltzman H. Pneumococcal pericarditis since 1980. Clin Infect Dis. 1998;27(5):1338-1340.
4. Wada A, Craft J, Mazzaferri EL. Purulent pericarditis leading to constriction. Cardiol Res. 2014;5(6):188-190.
5. Cillóniz C, Rangel E, Barlascini C, Piroddi IMG, Torres A, Nicolini A. Streptococcus pneumoniae-associated pneumonia complicated by purulent pericarditis: case series [in English, Portuguese]. J Bras Pneumol. 2015;41(4):389-394.
6. Saenz RE, Sanders CV, Aldridge KE, Patel MM. Purulent pericarditis with associated cardiac tamponade caused by a Streptococcus pneumoniae strain highly resistant to penicillin, cefotaxime, and ceftriaxone. Clin Infect Dis. 1998;26(3):762–763.
7. Sagristà-Sauleda J, Barrabés JA, Permanyer-Miralda G, Soler-Soler J. Purulent pericarditis: review of a 20-year experience in a general hospital. J Am Coll Cardiol. 1993; 22(6):1661-1665.
1. Ferreira dos Santos L, Moreira D, Ribeiro P, et al. Purulent pericarditis: a rare diagnosis [in Portuguese]. Rev Port Cardiol. 2013;32(9):721-727.
2. Parikh SV, Memon N, Echols M, Shah J, McGuire DK, Keeley EC. Purulent pericarditis: report of 2 cases and review of the literature. Medicine (Baltimore). 2009;88(1):52–65.
3. Go C, Asnis DS, Saltzman H. Pneumococcal pericarditis since 1980. Clin Infect Dis. 1998;27(5):1338-1340.
4. Wada A, Craft J, Mazzaferri EL. Purulent pericarditis leading to constriction. Cardiol Res. 2014;5(6):188-190.
5. Cillóniz C, Rangel E, Barlascini C, Piroddi IMG, Torres A, Nicolini A. Streptococcus pneumoniae-associated pneumonia complicated by purulent pericarditis: case series [in English, Portuguese]. J Bras Pneumol. 2015;41(4):389-394.
6. Saenz RE, Sanders CV, Aldridge KE, Patel MM. Purulent pericarditis with associated cardiac tamponade caused by a Streptococcus pneumoniae strain highly resistant to penicillin, cefotaxime, and ceftriaxone. Clin Infect Dis. 1998;26(3):762–763.
7. Sagristà-Sauleda J, Barrabés JA, Permanyer-Miralda G, Soler-Soler J. Purulent pericarditis: review of a 20-year experience in a general hospital. J Am Coll Cardiol. 1993; 22(6):1661-1665.