User login
FDA approves new use for Zika test
The US Food and Drug Administration (FDA) has approved an additional use for the cobas Zika test.
The approval allows for the streamlined screening of multiple individual blood or plasma donations that have been pooled together.
The cobas Zika test is a qualitative in vitro nucleic acid screening test for the direct detection of Zika virus RNA in plasma specimens from blood donors.
The test is used with the cobas 6800/8800 systems, which are fully automated, high-volume systems that perform sample pooling, sample preparation (nucleic acid extraction and purification), and polymerase chain reaction amplification and detection.
Automated data management is performed by the cobas 6800/8800 software, which assigns a test result of non-reactive, reactive, or invalid.
Roche deployed the cobas Zika test in April 2016 under the FDA’s investigational new drug application protocol to screen blood donations collected in Puerto Rico.
This initial testing protocol enabled the reinstatement of blood services in Puerto Rico after concerns over high rates of Zika infection posed a threat to the blood supply.
The cobas Zika test received commercial approval from the FDA in October 2017, enabling routine use of the test to support individual donor screening efforts throughout Puerto Rico and the continental US.
“More than 6 million blood donations from the United States and Puerto Rico have been screened with the cobas Zika test since its initial release under the investigational new drug application protocol in 2016 and subsequent commercial approval in 2017,” said Uwe Oberlaender, head of Roche Molecular Diagnostics.
The US Food and Drug Administration (FDA) has approved an additional use for the cobas Zika test.
The approval allows for the streamlined screening of multiple individual blood or plasma donations that have been pooled together.
The cobas Zika test is a qualitative in vitro nucleic acid screening test for the direct detection of Zika virus RNA in plasma specimens from blood donors.
The test is used with the cobas 6800/8800 systems, which are fully automated, high-volume systems that perform sample pooling, sample preparation (nucleic acid extraction and purification), and polymerase chain reaction amplification and detection.
Automated data management is performed by the cobas 6800/8800 software, which assigns a test result of non-reactive, reactive, or invalid.
Roche deployed the cobas Zika test in April 2016 under the FDA’s investigational new drug application protocol to screen blood donations collected in Puerto Rico.
This initial testing protocol enabled the reinstatement of blood services in Puerto Rico after concerns over high rates of Zika infection posed a threat to the blood supply.
The cobas Zika test received commercial approval from the FDA in October 2017, enabling routine use of the test to support individual donor screening efforts throughout Puerto Rico and the continental US.
“More than 6 million blood donations from the United States and Puerto Rico have been screened with the cobas Zika test since its initial release under the investigational new drug application protocol in 2016 and subsequent commercial approval in 2017,” said Uwe Oberlaender, head of Roche Molecular Diagnostics.
The US Food and Drug Administration (FDA) has approved an additional use for the cobas Zika test.
The approval allows for the streamlined screening of multiple individual blood or plasma donations that have been pooled together.
The cobas Zika test is a qualitative in vitro nucleic acid screening test for the direct detection of Zika virus RNA in plasma specimens from blood donors.
The test is used with the cobas 6800/8800 systems, which are fully automated, high-volume systems that perform sample pooling, sample preparation (nucleic acid extraction and purification), and polymerase chain reaction amplification and detection.
Automated data management is performed by the cobas 6800/8800 software, which assigns a test result of non-reactive, reactive, or invalid.
Roche deployed the cobas Zika test in April 2016 under the FDA’s investigational new drug application protocol to screen blood donations collected in Puerto Rico.
This initial testing protocol enabled the reinstatement of blood services in Puerto Rico after concerns over high rates of Zika infection posed a threat to the blood supply.
The cobas Zika test received commercial approval from the FDA in October 2017, enabling routine use of the test to support individual donor screening efforts throughout Puerto Rico and the continental US.
“More than 6 million blood donations from the United States and Puerto Rico have been screened with the cobas Zika test since its initial release under the investigational new drug application protocol in 2016 and subsequent commercial approval in 2017,” said Uwe Oberlaender, head of Roche Molecular Diagnostics.
Study reveals gender imbalance in cancer research funding
A new study suggests male investigators in the UK receive more funding for cancer research than their female counterparts.
Researchers analyzed funding for more than 4000 studies and found that male primary investigators (PIs) were consistently awarded more funding.
The total investment value was 3.6 times greater for male PIs than for female PIs.
The mean award value was 1.6 times greater, and the median award value was 1.3 times greater for males.
Rifat Atun, MBBS, of Harvard T. H. Chan School of Public Health in Boston, Massachusetts, and his colleagues reported these findings in BMJ Open.
The researchers analyzed data on public and philanthropic cancer research funding awarded to UK institutions between 2000 and 2013.
From this data, the team identified 4186 eligible studies with a total investment value of £2.33 billion.
The researchers compared the total investment, number of awards, and mean and median award value between male and female PIs.
Male PIs were awarded 2890 (69%) grants with a total value of £1.8 billion (78%), while female PIs were awarded 1296 (31%) grants with a total value of £0.5 billion (22%).
The median award value was £252,647 (interquartile range, £127,343–£553,560) for men and £198,485 (interquartile range, £99,317–£382,650) for women.
The mean award value was £630,324 (standard deviation, £1,662,559) for men and £394,730 (standard deviation, £666,574) for women.
Dr Atun and his colleagues acknowledged that this study was dependent on the accuracy of original investment data from the funding bodies, and the team could not openly access data of private sector research funding nor obtain disaggregated data from Cancer Research UK, one of the largest funders of cancer research.
The researchers also noted that the gender discrepancies they observed “are likely multifactorial,” and the team was unable to “postulate the underlying mechanisms responsible.” Still, the data do suggest a “substantial” imbalance, which should be investigated.
A new study suggests male investigators in the UK receive more funding for cancer research than their female counterparts.
Researchers analyzed funding for more than 4000 studies and found that male primary investigators (PIs) were consistently awarded more funding.
The total investment value was 3.6 times greater for male PIs than for female PIs.
The mean award value was 1.6 times greater, and the median award value was 1.3 times greater for males.
Rifat Atun, MBBS, of Harvard T. H. Chan School of Public Health in Boston, Massachusetts, and his colleagues reported these findings in BMJ Open.
The researchers analyzed data on public and philanthropic cancer research funding awarded to UK institutions between 2000 and 2013.
From this data, the team identified 4186 eligible studies with a total investment value of £2.33 billion.
The researchers compared the total investment, number of awards, and mean and median award value between male and female PIs.
Male PIs were awarded 2890 (69%) grants with a total value of £1.8 billion (78%), while female PIs were awarded 1296 (31%) grants with a total value of £0.5 billion (22%).
The median award value was £252,647 (interquartile range, £127,343–£553,560) for men and £198,485 (interquartile range, £99,317–£382,650) for women.
The mean award value was £630,324 (standard deviation, £1,662,559) for men and £394,730 (standard deviation, £666,574) for women.
Dr Atun and his colleagues acknowledged that this study was dependent on the accuracy of original investment data from the funding bodies, and the team could not openly access data of private sector research funding nor obtain disaggregated data from Cancer Research UK, one of the largest funders of cancer research.
The researchers also noted that the gender discrepancies they observed “are likely multifactorial,” and the team was unable to “postulate the underlying mechanisms responsible.” Still, the data do suggest a “substantial” imbalance, which should be investigated.
A new study suggests male investigators in the UK receive more funding for cancer research than their female counterparts.
Researchers analyzed funding for more than 4000 studies and found that male primary investigators (PIs) were consistently awarded more funding.
The total investment value was 3.6 times greater for male PIs than for female PIs.
The mean award value was 1.6 times greater, and the median award value was 1.3 times greater for males.
Rifat Atun, MBBS, of Harvard T. H. Chan School of Public Health in Boston, Massachusetts, and his colleagues reported these findings in BMJ Open.
The researchers analyzed data on public and philanthropic cancer research funding awarded to UK institutions between 2000 and 2013.
From this data, the team identified 4186 eligible studies with a total investment value of £2.33 billion.
The researchers compared the total investment, number of awards, and mean and median award value between male and female PIs.
Male PIs were awarded 2890 (69%) grants with a total value of £1.8 billion (78%), while female PIs were awarded 1296 (31%) grants with a total value of £0.5 billion (22%).
The median award value was £252,647 (interquartile range, £127,343–£553,560) for men and £198,485 (interquartile range, £99,317–£382,650) for women.
The mean award value was £630,324 (standard deviation, £1,662,559) for men and £394,730 (standard deviation, £666,574) for women.
Dr Atun and his colleagues acknowledged that this study was dependent on the accuracy of original investment data from the funding bodies, and the team could not openly access data of private sector research funding nor obtain disaggregated data from Cancer Research UK, one of the largest funders of cancer research.
The researchers also noted that the gender discrepancies they observed “are likely multifactorial,” and the team was unable to “postulate the underlying mechanisms responsible.” Still, the data do suggest a “substantial” imbalance, which should be investigated.
NCCN releases guidelines for AML patients
The National Comprehensive Cancer Network (NCCN) has released guidelines for patients with acute myeloid leukemia (AML).
The guidelines are intended to help patients make better informed decision about their care.
The guidelines explain what AML is, describe testing procedures and treatment options, and provide tips to help patients choose the best treatment.
The guidelines also include a list of suggested questions for patients to bring up with their doctors, illustrations, and a glossary of key terms and acronyms.
“People with AML often aren’t aware that this isn’t a single disease but many different diseases that share fundamental characteristics,” said Martin Tallman, MD, a hematologic oncologist at Memorial Sloan Kettering Cancer Center in New York, New York, and vice-chair of the NCCN Guidelines Panel for AML.
“The good news is that the future for AML is getting a lot brighter. After 40 years without much progress, 4 new medications were just approved last year*, and more new treatment courses are in development as we speak.”
The guidelines are available for free on the NCCN website or via the NCCN Patient Guides for Cancer app. Printed versions of the guidelines can be purchased at Amazon.com.
NCCN also has guidelines for patients with acute lymphoblastic leukemia, chronic lymphocytic leukemia, chronic myelogenous leukemia, Hodgkin lymphoma, multiple myeloma, myelodysplastic syndromes, myeloproliferative neoplasms, non-Hodgkin lymphomas, and Waldenström’s macroglobulinemia, as well as solid tumor malignancies.
There are patient guidelines for adolescents and young adults with cancer and guidelines on distress/supportive care, and nausea and vomiting/supportive care as well.
*(1) Midostaurin (Rydapt) was approved for adults with newly diagnosed, FLT3-positive AML
(2) Gemtuzumab ozogamicin (Mylotarg) was approved for adults with newly diagnosed AML and patients age 2 and older with relapsed/refractory AML
(3) Liposomal daunorubicin/cytarabine (Vyxeos) was approved for AML with myelodysplasia-related changes and newly diagnosed, therapy-related AML
(4) Enasidenib (Idhifa) was approved for adults with relapsed/refractory AML and an IDH2 mutation.
The National Comprehensive Cancer Network (NCCN) has released guidelines for patients with acute myeloid leukemia (AML).
The guidelines are intended to help patients make better informed decision about their care.
The guidelines explain what AML is, describe testing procedures and treatment options, and provide tips to help patients choose the best treatment.
The guidelines also include a list of suggested questions for patients to bring up with their doctors, illustrations, and a glossary of key terms and acronyms.
“People with AML often aren’t aware that this isn’t a single disease but many different diseases that share fundamental characteristics,” said Martin Tallman, MD, a hematologic oncologist at Memorial Sloan Kettering Cancer Center in New York, New York, and vice-chair of the NCCN Guidelines Panel for AML.
“The good news is that the future for AML is getting a lot brighter. After 40 years without much progress, 4 new medications were just approved last year*, and more new treatment courses are in development as we speak.”
The guidelines are available for free on the NCCN website or via the NCCN Patient Guides for Cancer app. Printed versions of the guidelines can be purchased at Amazon.com.
NCCN also has guidelines for patients with acute lymphoblastic leukemia, chronic lymphocytic leukemia, chronic myelogenous leukemia, Hodgkin lymphoma, multiple myeloma, myelodysplastic syndromes, myeloproliferative neoplasms, non-Hodgkin lymphomas, and Waldenström’s macroglobulinemia, as well as solid tumor malignancies.
There are patient guidelines for adolescents and young adults with cancer and guidelines on distress/supportive care, and nausea and vomiting/supportive care as well.
*(1) Midostaurin (Rydapt) was approved for adults with newly diagnosed, FLT3-positive AML
(2) Gemtuzumab ozogamicin (Mylotarg) was approved for adults with newly diagnosed AML and patients age 2 and older with relapsed/refractory AML
(3) Liposomal daunorubicin/cytarabine (Vyxeos) was approved for AML with myelodysplasia-related changes and newly diagnosed, therapy-related AML
(4) Enasidenib (Idhifa) was approved for adults with relapsed/refractory AML and an IDH2 mutation.
The National Comprehensive Cancer Network (NCCN) has released guidelines for patients with acute myeloid leukemia (AML).
The guidelines are intended to help patients make better informed decision about their care.
The guidelines explain what AML is, describe testing procedures and treatment options, and provide tips to help patients choose the best treatment.
The guidelines also include a list of suggested questions for patients to bring up with their doctors, illustrations, and a glossary of key terms and acronyms.
“People with AML often aren’t aware that this isn’t a single disease but many different diseases that share fundamental characteristics,” said Martin Tallman, MD, a hematologic oncologist at Memorial Sloan Kettering Cancer Center in New York, New York, and vice-chair of the NCCN Guidelines Panel for AML.
“The good news is that the future for AML is getting a lot brighter. After 40 years without much progress, 4 new medications were just approved last year*, and more new treatment courses are in development as we speak.”
The guidelines are available for free on the NCCN website or via the NCCN Patient Guides for Cancer app. Printed versions of the guidelines can be purchased at Amazon.com.
NCCN also has guidelines for patients with acute lymphoblastic leukemia, chronic lymphocytic leukemia, chronic myelogenous leukemia, Hodgkin lymphoma, multiple myeloma, myelodysplastic syndromes, myeloproliferative neoplasms, non-Hodgkin lymphomas, and Waldenström’s macroglobulinemia, as well as solid tumor malignancies.
There are patient guidelines for adolescents and young adults with cancer and guidelines on distress/supportive care, and nausea and vomiting/supportive care as well.
*(1) Midostaurin (Rydapt) was approved for adults with newly diagnosed, FLT3-positive AML
(2) Gemtuzumab ozogamicin (Mylotarg) was approved for adults with newly diagnosed AML and patients age 2 and older with relapsed/refractory AML
(3) Liposomal daunorubicin/cytarabine (Vyxeos) was approved for AML with myelodysplasia-related changes and newly diagnosed, therapy-related AML
(4) Enasidenib (Idhifa) was approved for adults with relapsed/refractory AML and an IDH2 mutation.
Treating Migraines: It’s Different for Kids
A 15-year-old girl presents to your clinic with poorly controlled chronic migraines that prevent her from attending school three to four days per month. As part of her treatment regimen, you are considering migraine prevention strategies. Should you prescribe amitriptyline or topiramate?
Migraine headaches are the most common reason for headache presentation in pediatric neurology outpatient clinics, affecting 5% to 10% of the pediatric population worldwide.2 Current recommendations regarding prophylactic migraine therapy in childhood are based on consensus opinions.3,4 While the FDA has not approved any medications for migraine prevention in children younger than 12, surveys of pediatric headache specialists suggest that amitriptyline and topiramate are among the most commonly prescribed medications for childhood migraine prophylaxis.3,4
There is low-quality evidence from individual RCTs about the effectiveness of topiramate. A meta-analysis by El-Chammas and colleagues included three RCTs comparing topiramate to placebo for the prevention of episodic migraines (migraine headaches that occur < 15 x/mo) in a combined total of 283 children younger than 18.5 Topiramate demonstrated a nonclinically significant, but statistically significant, reduction of less than one headache per month (–0.71). This is based on moderate-quality evidence due to a high placebo response rate and study durations of only 12 weeks.5 The FDA has approved topiramate for migraine prevention in children ages 12 to 17.6
Adult guidelines. The findings described above are consistent with the most recent adult guidelines from the American Academy of Neurology and the American Headache Society.7 In a joint publication from 2012, these societies recommended both topiramate and amitriptyline for the prevention of migraines in adults based on high-quality (Level A) and medium-quality (Level B) evidence, respectively.7
STUDY SUMMARY
No better than placebo in children
A multicenter, double-blind RCT by Powers and colleagues compared the effectiveness of amitriptyline, topiramate, and placebo in the prevention of pediatric migraines.1 Target dosing for amitriptyline and topiramate was set at 1 mg/kg/d and 2 mg/kg/d, respectively. Titration toward these doses occurred over an eight-week period, based on reported adverse effects. Patients then continued their maximum tolerated dose for an additional 16 weeks.
Patients were predominantly white (70%), female (68%), and 8 to 17 years of age. They had at least four headache days over a prospective 28-day pretreatment period and a Pediatric Migraine Disability Assessment Scale (PedMIDAS) score of 11 to 139 (scores of 11-50 indicate mild-to-moderate disability; of > 50, severe disability).1,8 The primary endpoint consisted of at least a 50% relative reduction (RR) in the number of headache days in the final 28 days of the trial, compared to the 28-day pretherapy (baseline) period.1
The authors of the study included 328 patients in the primary efficacy analysis and randomly assigned them in a 2:2:1 ratio to receive either amitriptyline (132 patients), topiramate (130 patients), or placebo (66 patients). After 24 weeks of therapy, there was no significant difference between the amitriptyline, topiramate, and placebo groups in the primary endpoint (52% amitriptyline, 55% topiramate, 61% placebo; adjusted odds ratio [OR], 0.71 between amitriptyline and placebo; OR, 0.81 between topiramate and placebo; OR, 0.88 between amitriptyline and topiramate).
Continue to: There was also no difference...
There was also no difference in the secondary outcomes of absolute reduction in headache days and headache-related disability as determined by PedMIDAS. The study was stopped early for futility. Compared with placebo, amitriptyline significantly increased fatigue (number needed to harm [NNH], 8) and dry mouth (NNH, 9) and was associated with three serious adverse events of altered mood. Compared with placebo, topiramate significantly increased paresthesia (NNH, 4) and weight loss (NNH, 13) and was associated with one serious adverse event—a suicide attempt.1
WHAT’S NEW?
Higher-level evidence, lack of efficacy
This RCT provides new, higher-level evidence that demonstrates the lack of efficacy of amitriptyline and topiramate in the prevention of pediatric migraines. It also highlights the risk for increased adverse events with topiramate and amitriptyline.
Two of the three topiramate trials used in the older meta-analysis by El-Chammas and colleagues and this new RCT were included in an updated meta-analysis by Le and colleagues (total participants, 465) published in 2017.1,2,5 This newer meta-analysis found no statistical benefit associated with the use of topiramate over placebo. It demonstrated a nonsignificant decrease in the number of patients with at least a 50% relative reduction in headache frequency (risk ratio, 1.26) and in the overall number of headache days (mean difference, –0.77) in patients younger than 18.2 Both meta-analyses, however, showed an increase in the rate of adverse events in patients using topiramate versus placebo.2,5
CAVEATS
Is there a gender predominance?
El-Chammas and colleagues describe male pediatric patients as being the predominant pediatric gender with migraines.5 However, they do not quote an incidence rate or cite the reference for this statement. No other reference to gender predominance was noted in the literature. The current study, in addition to the total population of the meta-analysis by Le and colleagues, included women as the predominant patient population.1,2 Hopefully, future studies will help to delineate whether there is a gender predominance and, if so, whether the current treatment data apply to both genders.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
There are no barriers to implementing this recommendation immediately.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67 [4]:238-239, 241).
1. Powers SW, Coffey CS, Chamberlin LA, et al; for the CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017; 376:115-124.
2. Le K, Yu D, Wang J, et al. Is topiramate effective for migraine prevention in patients less than 18 years of age? A meta-analysis of randomized controlled trials. J Headache Pain. 2017;18:69.
3. Lewis D, Ashwal S, Hershey A, et al. Practice parameter: pharmacological treatment of migraine headache in children and adolescents: report of the American Academy of Neurology Quality Standards Subcommittee and the Practice Committee of the Child Neurology Society. Neurology. 2004;63:2215-2224.
4. Hershey AD. Current approaches to the diagnosis and management of paediatric migraine. Lancet Neurology. 2010;9:190-204.
5. El-Chammas K, Keyes J, Thompson N, et al. Pharmacologic treatment of pediatric headaches: a meta-analysis. JAMA Pediatr. 2013;167:250-258.
6. Qudexy XR. Highlights of prescribing information. www.accessdata.fda.gov/drugsatfda_docs/label/2017/205122s003s005lbl.pdf. Accessed April 6, 2018.
7. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
8. Hershey AD, Powers SW, Vockell AL, et al. PedMIDAS: development of a questionnaire to assess disability of migraines in children. Neurology. 2001;57:2034-2039.

A 15-year-old girl presents to your clinic with poorly controlled chronic migraines that prevent her from attending school three to four days per month. As part of her treatment regimen, you are considering migraine prevention strategies. Should you prescribe amitriptyline or topiramate?
Migraine headaches are the most common reason for headache presentation in pediatric neurology outpatient clinics, affecting 5% to 10% of the pediatric population worldwide.2 Current recommendations regarding prophylactic migraine therapy in childhood are based on consensus opinions.3,4 While the FDA has not approved any medications for migraine prevention in children younger than 12, surveys of pediatric headache specialists suggest that amitriptyline and topiramate are among the most commonly prescribed medications for childhood migraine prophylaxis.3,4
There is low-quality evidence from individual RCTs about the effectiveness of topiramate. A meta-analysis by El-Chammas and colleagues included three RCTs comparing topiramate to placebo for the prevention of episodic migraines (migraine headaches that occur < 15 x/mo) in a combined total of 283 children younger than 18.5 Topiramate demonstrated a nonclinically significant, but statistically significant, reduction of less than one headache per month (–0.71). This is based on moderate-quality evidence due to a high placebo response rate and study durations of only 12 weeks.5 The FDA has approved topiramate for migraine prevention in children ages 12 to 17.6
Adult guidelines. The findings described above are consistent with the most recent adult guidelines from the American Academy of Neurology and the American Headache Society.7 In a joint publication from 2012, these societies recommended both topiramate and amitriptyline for the prevention of migraines in adults based on high-quality (Level A) and medium-quality (Level B) evidence, respectively.7
STUDY SUMMARY
No better than placebo in children
A multicenter, double-blind RCT by Powers and colleagues compared the effectiveness of amitriptyline, topiramate, and placebo in the prevention of pediatric migraines.1 Target dosing for amitriptyline and topiramate was set at 1 mg/kg/d and 2 mg/kg/d, respectively. Titration toward these doses occurred over an eight-week period, based on reported adverse effects. Patients then continued their maximum tolerated dose for an additional 16 weeks.
Patients were predominantly white (70%), female (68%), and 8 to 17 years of age. They had at least four headache days over a prospective 28-day pretreatment period and a Pediatric Migraine Disability Assessment Scale (PedMIDAS) score of 11 to 139 (scores of 11-50 indicate mild-to-moderate disability; of > 50, severe disability).1,8 The primary endpoint consisted of at least a 50% relative reduction (RR) in the number of headache days in the final 28 days of the trial, compared to the 28-day pretherapy (baseline) period.1
The authors of the study included 328 patients in the primary efficacy analysis and randomly assigned them in a 2:2:1 ratio to receive either amitriptyline (132 patients), topiramate (130 patients), or placebo (66 patients). After 24 weeks of therapy, there was no significant difference between the amitriptyline, topiramate, and placebo groups in the primary endpoint (52% amitriptyline, 55% topiramate, 61% placebo; adjusted odds ratio [OR], 0.71 between amitriptyline and placebo; OR, 0.81 between topiramate and placebo; OR, 0.88 between amitriptyline and topiramate).
Continue to: There was also no difference...
There was also no difference in the secondary outcomes of absolute reduction in headache days and headache-related disability as determined by PedMIDAS. The study was stopped early for futility. Compared with placebo, amitriptyline significantly increased fatigue (number needed to harm [NNH], 8) and dry mouth (NNH, 9) and was associated with three serious adverse events of altered mood. Compared with placebo, topiramate significantly increased paresthesia (NNH, 4) and weight loss (NNH, 13) and was associated with one serious adverse event—a suicide attempt.1
WHAT’S NEW?
Higher-level evidence, lack of efficacy
This RCT provides new, higher-level evidence that demonstrates the lack of efficacy of amitriptyline and topiramate in the prevention of pediatric migraines. It also highlights the risk for increased adverse events with topiramate and amitriptyline.
Two of the three topiramate trials used in the older meta-analysis by El-Chammas and colleagues and this new RCT were included in an updated meta-analysis by Le and colleagues (total participants, 465) published in 2017.1,2,5 This newer meta-analysis found no statistical benefit associated with the use of topiramate over placebo. It demonstrated a nonsignificant decrease in the number of patients with at least a 50% relative reduction in headache frequency (risk ratio, 1.26) and in the overall number of headache days (mean difference, –0.77) in patients younger than 18.2 Both meta-analyses, however, showed an increase in the rate of adverse events in patients using topiramate versus placebo.2,5
CAVEATS
Is there a gender predominance?
El-Chammas and colleagues describe male pediatric patients as being the predominant pediatric gender with migraines.5 However, they do not quote an incidence rate or cite the reference for this statement. No other reference to gender predominance was noted in the literature. The current study, in addition to the total population of the meta-analysis by Le and colleagues, included women as the predominant patient population.1,2 Hopefully, future studies will help to delineate whether there is a gender predominance and, if so, whether the current treatment data apply to both genders.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
There are no barriers to implementing this recommendation immediately.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67 [4]:238-239, 241).

A 15-year-old girl presents to your clinic with poorly controlled chronic migraines that prevent her from attending school three to four days per month. As part of her treatment regimen, you are considering migraine prevention strategies. Should you prescribe amitriptyline or topiramate?
Migraine headaches are the most common reason for headache presentation in pediatric neurology outpatient clinics, affecting 5% to 10% of the pediatric population worldwide.2 Current recommendations regarding prophylactic migraine therapy in childhood are based on consensus opinions.3,4 While the FDA has not approved any medications for migraine prevention in children younger than 12, surveys of pediatric headache specialists suggest that amitriptyline and topiramate are among the most commonly prescribed medications for childhood migraine prophylaxis.3,4
There is low-quality evidence from individual RCTs about the effectiveness of topiramate. A meta-analysis by El-Chammas and colleagues included three RCTs comparing topiramate to placebo for the prevention of episodic migraines (migraine headaches that occur < 15 x/mo) in a combined total of 283 children younger than 18.5 Topiramate demonstrated a nonclinically significant, but statistically significant, reduction of less than one headache per month (–0.71). This is based on moderate-quality evidence due to a high placebo response rate and study durations of only 12 weeks.5 The FDA has approved topiramate for migraine prevention in children ages 12 to 17.6
Adult guidelines. The findings described above are consistent with the most recent adult guidelines from the American Academy of Neurology and the American Headache Society.7 In a joint publication from 2012, these societies recommended both topiramate and amitriptyline for the prevention of migraines in adults based on high-quality (Level A) and medium-quality (Level B) evidence, respectively.7
STUDY SUMMARY
No better than placebo in children
A multicenter, double-blind RCT by Powers and colleagues compared the effectiveness of amitriptyline, topiramate, and placebo in the prevention of pediatric migraines.1 Target dosing for amitriptyline and topiramate was set at 1 mg/kg/d and 2 mg/kg/d, respectively. Titration toward these doses occurred over an eight-week period, based on reported adverse effects. Patients then continued their maximum tolerated dose for an additional 16 weeks.
Patients were predominantly white (70%), female (68%), and 8 to 17 years of age. They had at least four headache days over a prospective 28-day pretreatment period and a Pediatric Migraine Disability Assessment Scale (PedMIDAS) score of 11 to 139 (scores of 11-50 indicate mild-to-moderate disability; of > 50, severe disability).1,8 The primary endpoint consisted of at least a 50% relative reduction (RR) in the number of headache days in the final 28 days of the trial, compared to the 28-day pretherapy (baseline) period.1
The authors of the study included 328 patients in the primary efficacy analysis and randomly assigned them in a 2:2:1 ratio to receive either amitriptyline (132 patients), topiramate (130 patients), or placebo (66 patients). After 24 weeks of therapy, there was no significant difference between the amitriptyline, topiramate, and placebo groups in the primary endpoint (52% amitriptyline, 55% topiramate, 61% placebo; adjusted odds ratio [OR], 0.71 between amitriptyline and placebo; OR, 0.81 between topiramate and placebo; OR, 0.88 between amitriptyline and topiramate).
Continue to: There was also no difference...
There was also no difference in the secondary outcomes of absolute reduction in headache days and headache-related disability as determined by PedMIDAS. The study was stopped early for futility. Compared with placebo, amitriptyline significantly increased fatigue (number needed to harm [NNH], 8) and dry mouth (NNH, 9) and was associated with three serious adverse events of altered mood. Compared with placebo, topiramate significantly increased paresthesia (NNH, 4) and weight loss (NNH, 13) and was associated with one serious adverse event—a suicide attempt.1
WHAT’S NEW?
Higher-level evidence, lack of efficacy
This RCT provides new, higher-level evidence that demonstrates the lack of efficacy of amitriptyline and topiramate in the prevention of pediatric migraines. It also highlights the risk for increased adverse events with topiramate and amitriptyline.
Two of the three topiramate trials used in the older meta-analysis by El-Chammas and colleagues and this new RCT were included in an updated meta-analysis by Le and colleagues (total participants, 465) published in 2017.1,2,5 This newer meta-analysis found no statistical benefit associated with the use of topiramate over placebo. It demonstrated a nonsignificant decrease in the number of patients with at least a 50% relative reduction in headache frequency (risk ratio, 1.26) and in the overall number of headache days (mean difference, –0.77) in patients younger than 18.2 Both meta-analyses, however, showed an increase in the rate of adverse events in patients using topiramate versus placebo.2,5
CAVEATS
Is there a gender predominance?
El-Chammas and colleagues describe male pediatric patients as being the predominant pediatric gender with migraines.5 However, they do not quote an incidence rate or cite the reference for this statement. No other reference to gender predominance was noted in the literature. The current study, in addition to the total population of the meta-analysis by Le and colleagues, included women as the predominant patient population.1,2 Hopefully, future studies will help to delineate whether there is a gender predominance and, if so, whether the current treatment data apply to both genders.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
There are no barriers to implementing this recommendation immediately.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67 [4]:238-239, 241).
1. Powers SW, Coffey CS, Chamberlin LA, et al; for the CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017; 376:115-124.
2. Le K, Yu D, Wang J, et al. Is topiramate effective for migraine prevention in patients less than 18 years of age? A meta-analysis of randomized controlled trials. J Headache Pain. 2017;18:69.
3. Lewis D, Ashwal S, Hershey A, et al. Practice parameter: pharmacological treatment of migraine headache in children and adolescents: report of the American Academy of Neurology Quality Standards Subcommittee and the Practice Committee of the Child Neurology Society. Neurology. 2004;63:2215-2224.
4. Hershey AD. Current approaches to the diagnosis and management of paediatric migraine. Lancet Neurology. 2010;9:190-204.
5. El-Chammas K, Keyes J, Thompson N, et al. Pharmacologic treatment of pediatric headaches: a meta-analysis. JAMA Pediatr. 2013;167:250-258.
6. Qudexy XR. Highlights of prescribing information. www.accessdata.fda.gov/drugsatfda_docs/label/2017/205122s003s005lbl.pdf. Accessed April 6, 2018.
7. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
8. Hershey AD, Powers SW, Vockell AL, et al. PedMIDAS: development of a questionnaire to assess disability of migraines in children. Neurology. 2001;57:2034-2039.
1. Powers SW, Coffey CS, Chamberlin LA, et al; for the CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017; 376:115-124.
2. Le K, Yu D, Wang J, et al. Is topiramate effective for migraine prevention in patients less than 18 years of age? A meta-analysis of randomized controlled trials. J Headache Pain. 2017;18:69.
3. Lewis D, Ashwal S, Hershey A, et al. Practice parameter: pharmacological treatment of migraine headache in children and adolescents: report of the American Academy of Neurology Quality Standards Subcommittee and the Practice Committee of the Child Neurology Society. Neurology. 2004;63:2215-2224.
4. Hershey AD. Current approaches to the diagnosis and management of paediatric migraine. Lancet Neurology. 2010;9:190-204.
5. El-Chammas K, Keyes J, Thompson N, et al. Pharmacologic treatment of pediatric headaches: a meta-analysis. JAMA Pediatr. 2013;167:250-258.
6. Qudexy XR. Highlights of prescribing information. www.accessdata.fda.gov/drugsatfda_docs/label/2017/205122s003s005lbl.pdf. Accessed April 6, 2018.
7. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
8. Hershey AD, Powers SW, Vockell AL, et al. PedMIDAS: development of a questionnaire to assess disability of migraines in children. Neurology. 2001;57:2034-2039.
Sarcoma dominance in uterine carcinosarcomas linked to decreased survival
Sarcoma dominance in uterine carcinosarcomas was associated with decreased survival among women with stages I-IV uterine carcinosarcomas who underwent primary surgery, according to Dr Koji Matsuo, MD, PhD, of the Keck School of Medicine, University of Southern California, Los Angeles, and his colleagues.
The researchers additionally found that adding radiotherapy to chemotherapy may be an effective postoperative strategy for these patients.
Uterine carcinosarcomas are rare, high-grade endometrial cancers that represent 5% of all endometrial cancers. Sarcoma dominance was defined as having more than a 50% sarcoma component in the uterine tumor. In this study, the sarcoma component was grouped as homologous (endometrial stromal sarcoma, leiomyosarcoma, fibrosarcoma, and undifferentiated sarcoma) or heterologous (rhabdomyosarcoma, osteosarcoma, chondrosarcoma, and liposarcoma) types
Among 1,192 cases of uterine carcinosarcomas identified in a secondary analysis of a multicenter retrospective study, 906 cases were available for histopathology slide review. Of those, 889 cases had evaluation for sarcoma dominance. The most common group was homologous sarcoma without sarcoma dominance (39.5%), followed by heterologous sarcoma with sarcoma dominance (21.3%), homologous sarcoma with sarcoma dominance (19.7%) and heterologous sarcoma with sarcoma non-dominance (19.6%), they reported in a study published online in Surgical Oncology https://doi.org/10.1016/j.suronc.2018.05.017
On univariate analysis, sarcoma dominance was associated with decreased progression-free survival (PFS) and cause-specific survival (CSS) in homologous cases (P less than 0.05) but not in heterologous cases. On multivariate models, both homologous and heterologous SD patterns remained independent prognostic factors for decreased PFS (adjusted-hazard ratio [HR] ranges: homologous/dominance 1.35-1.69, and heterologous/dominance 1.47-1.64) and CSS (adjusted-HR ranges: 1.52-1.84 and 1.66-1.81, respectively) compared to homologous/non-dominance (all, P less than 0.05).
In women with stage I-III disease, and tumors with sarcoma dominance, adding radiotherapy to chemotherapy was associated with improved PFS (adjusted-HR: homologous/dominance 0.49, and heterologous/dominance 0.45) and CSS (0.36 and 0.31, respectively) compared to chemotherapy alone (all, P less than 0.05); This association was not observed in women with tumors that lacked sarcoma dominance (all, P greater than 0.05), the researchers said.
Sarcoma dominance in uterine carcinosarcomas was associated with decreased survival among women with stages I-IV uterine carcinosarcomas who underwent primary surgery, according to Dr Koji Matsuo, MD, PhD, of the Keck School of Medicine, University of Southern California, Los Angeles, and his colleagues.
The researchers additionally found that adding radiotherapy to chemotherapy may be an effective postoperative strategy for these patients.
Uterine carcinosarcomas are rare, high-grade endometrial cancers that represent 5% of all endometrial cancers. Sarcoma dominance was defined as having more than a 50% sarcoma component in the uterine tumor. In this study, the sarcoma component was grouped as homologous (endometrial stromal sarcoma, leiomyosarcoma, fibrosarcoma, and undifferentiated sarcoma) or heterologous (rhabdomyosarcoma, osteosarcoma, chondrosarcoma, and liposarcoma) types
Among 1,192 cases of uterine carcinosarcomas identified in a secondary analysis of a multicenter retrospective study, 906 cases were available for histopathology slide review. Of those, 889 cases had evaluation for sarcoma dominance. The most common group was homologous sarcoma without sarcoma dominance (39.5%), followed by heterologous sarcoma with sarcoma dominance (21.3%), homologous sarcoma with sarcoma dominance (19.7%) and heterologous sarcoma with sarcoma non-dominance (19.6%), they reported in a study published online in Surgical Oncology https://doi.org/10.1016/j.suronc.2018.05.017
On univariate analysis, sarcoma dominance was associated with decreased progression-free survival (PFS) and cause-specific survival (CSS) in homologous cases (P less than 0.05) but not in heterologous cases. On multivariate models, both homologous and heterologous SD patterns remained independent prognostic factors for decreased PFS (adjusted-hazard ratio [HR] ranges: homologous/dominance 1.35-1.69, and heterologous/dominance 1.47-1.64) and CSS (adjusted-HR ranges: 1.52-1.84 and 1.66-1.81, respectively) compared to homologous/non-dominance (all, P less than 0.05).
In women with stage I-III disease, and tumors with sarcoma dominance, adding radiotherapy to chemotherapy was associated with improved PFS (adjusted-HR: homologous/dominance 0.49, and heterologous/dominance 0.45) and CSS (0.36 and 0.31, respectively) compared to chemotherapy alone (all, P less than 0.05); This association was not observed in women with tumors that lacked sarcoma dominance (all, P greater than 0.05), the researchers said.
Sarcoma dominance in uterine carcinosarcomas was associated with decreased survival among women with stages I-IV uterine carcinosarcomas who underwent primary surgery, according to Dr Koji Matsuo, MD, PhD, of the Keck School of Medicine, University of Southern California, Los Angeles, and his colleagues.
The researchers additionally found that adding radiotherapy to chemotherapy may be an effective postoperative strategy for these patients.
Uterine carcinosarcomas are rare, high-grade endometrial cancers that represent 5% of all endometrial cancers. Sarcoma dominance was defined as having more than a 50% sarcoma component in the uterine tumor. In this study, the sarcoma component was grouped as homologous (endometrial stromal sarcoma, leiomyosarcoma, fibrosarcoma, and undifferentiated sarcoma) or heterologous (rhabdomyosarcoma, osteosarcoma, chondrosarcoma, and liposarcoma) types
Among 1,192 cases of uterine carcinosarcomas identified in a secondary analysis of a multicenter retrospective study, 906 cases were available for histopathology slide review. Of those, 889 cases had evaluation for sarcoma dominance. The most common group was homologous sarcoma without sarcoma dominance (39.5%), followed by heterologous sarcoma with sarcoma dominance (21.3%), homologous sarcoma with sarcoma dominance (19.7%) and heterologous sarcoma with sarcoma non-dominance (19.6%), they reported in a study published online in Surgical Oncology https://doi.org/10.1016/j.suronc.2018.05.017
On univariate analysis, sarcoma dominance was associated with decreased progression-free survival (PFS) and cause-specific survival (CSS) in homologous cases (P less than 0.05) but not in heterologous cases. On multivariate models, both homologous and heterologous SD patterns remained independent prognostic factors for decreased PFS (adjusted-hazard ratio [HR] ranges: homologous/dominance 1.35-1.69, and heterologous/dominance 1.47-1.64) and CSS (adjusted-HR ranges: 1.52-1.84 and 1.66-1.81, respectively) compared to homologous/non-dominance (all, P less than 0.05).
In women with stage I-III disease, and tumors with sarcoma dominance, adding radiotherapy to chemotherapy was associated with improved PFS (adjusted-HR: homologous/dominance 0.49, and heterologous/dominance 0.45) and CSS (0.36 and 0.31, respectively) compared to chemotherapy alone (all, P less than 0.05); This association was not observed in women with tumors that lacked sarcoma dominance (all, P greater than 0.05), the researchers said.
FROM SURGICAL ONCOLOGY
FDA seeks comments on pediatric HIV product development
The U.S. Food and Drug Administration announced a draft guidance for industry entitled “Pediatric HIV Infection: Drug Development for Treatment.” (from birth to younger than 17 years of age).
According to the FDA announcement, the draft includes recommendations on when sponsors should initiate pediatric formulation development and when to begin pediatric studies to evaluate antiretroviral drug products for the treatment of HIV infection.
SOURCE: Federal Register May 14. Pediatric HIV Infection: Drug Development for Treatment; Draft Guidance for Industry; Availability.
The U.S. Food and Drug Administration announced a draft guidance for industry entitled “Pediatric HIV Infection: Drug Development for Treatment.” (from birth to younger than 17 years of age).
According to the FDA announcement, the draft includes recommendations on when sponsors should initiate pediatric formulation development and when to begin pediatric studies to evaluate antiretroviral drug products for the treatment of HIV infection.
SOURCE: Federal Register May 14. Pediatric HIV Infection: Drug Development for Treatment; Draft Guidance for Industry; Availability.
The U.S. Food and Drug Administration announced a draft guidance for industry entitled “Pediatric HIV Infection: Drug Development for Treatment.” (from birth to younger than 17 years of age).
According to the FDA announcement, the draft includes recommendations on when sponsors should initiate pediatric formulation development and when to begin pediatric studies to evaluate antiretroviral drug products for the treatment of HIV infection.
SOURCE: Federal Register May 14. Pediatric HIV Infection: Drug Development for Treatment; Draft Guidance for Industry; Availability.
Suicide prevention gets ‘standard care’ recommendations
WASHINGTON – The National Action Alliance for Suicide Prevention released in April 2018 what the organization said was the first set of “standard care” recommendations for suicide prevention in people with suicide risk.
Care for people with a suicide risk in the United States “is not working very well. Evidence-based tools exist to detect and manage suicidality, but they are new and infrequently used” by many clinicians, including those seeing suicidal patients in primary care, emergency, or hospital settings, said Michael F. Hogan, PhD, during a session on the new standard-care recommendations at the annual conference of the American Association of Suicidology.
The Action Alliance seeks to have the standard care recommendations widely disseminated and hopes the document will receive endorsement from other organizations, said Dr. Hogan, a health policy consultant in Delmar, N.Y., and a member of the eight-person panel that wrote the recommendations.
These recommendations specify interventions for caregivers in four separate settings: primary care, outpatient behavioral health care (mental health and substance use treatment settings), emergency departments, and behavioral health inpatient care (hospital-level psychiatric or addiction treatment). For each setting, the recommendations highlight one or more core approaches, and then specify standards for identification and assessment, safety planning, means reduction, and follow-up contacts.
For example, within the primary care setting, the recommendations say the goals are to identify suicide risk, enhance the safety for those at risk, refer for specialized care, and provide “caring contacts.” The specifications note that this is achieved with standardized screening and assessment instruments (the recommendations cite eight screening tool options and also suggest three different possible assessment tools); referral as appropriate; a brief safety-planning intervention (the recommendations list five options for this) that includes lethality means reduction along with follow-up to be sure that lethal means have been removed; arranging for rapid follow-up with a mental health professional; and follow-up contact by the primary care clinician within the next 48 hours.
According to Dr. Hogan, a motivation for releasing these recommendations has been the growing U.S. incidence of suicide, rising from 10.4 deaths/100,000 in 2000 to 13.3/100,000 in 2015, a 28% relative increase during a period when the rates of the top killers in the United States – cancer, heart disease, and stroke – were falling. Other telling statistics are that most people who die from suicide had seen a primary care provider during the year before death, and nearly half had seen a primary care provider during the month before their death.
But often the indicators of impending suicide are missed or not acted on. a misperception that contributes to a “failure to ask about suicide risk” on the part of health care professionals, the recommendations said. The document also highlighted the idea that, “most health care professionals are not aware of newly developed brief interventions for suicide, leading to the assumption that they should not ask about suicide because there is nothing practical that can be done in ordinary health care settings.”
One limitation of the recommendations is that they might be interpreted as “standard of care” for medicolegal purposes, warned Alan L. Berman, PhD, during the session’s discussion period. In addition, the evidence base for some of the recommended procedures is not very strong, such as risk stratification, said Dr. Berman, a clinical psychologist and former executive director of the American Association of Suicidology.
Dr. Hogan, Dr. Andrews, and Dr. Berman had no disclosures.
WASHINGTON – The National Action Alliance for Suicide Prevention released in April 2018 what the organization said was the first set of “standard care” recommendations for suicide prevention in people with suicide risk.
Care for people with a suicide risk in the United States “is not working very well. Evidence-based tools exist to detect and manage suicidality, but they are new and infrequently used” by many clinicians, including those seeing suicidal patients in primary care, emergency, or hospital settings, said Michael F. Hogan, PhD, during a session on the new standard-care recommendations at the annual conference of the American Association of Suicidology.
The Action Alliance seeks to have the standard care recommendations widely disseminated and hopes the document will receive endorsement from other organizations, said Dr. Hogan, a health policy consultant in Delmar, N.Y., and a member of the eight-person panel that wrote the recommendations.
These recommendations specify interventions for caregivers in four separate settings: primary care, outpatient behavioral health care (mental health and substance use treatment settings), emergency departments, and behavioral health inpatient care (hospital-level psychiatric or addiction treatment). For each setting, the recommendations highlight one or more core approaches, and then specify standards for identification and assessment, safety planning, means reduction, and follow-up contacts.
For example, within the primary care setting, the recommendations say the goals are to identify suicide risk, enhance the safety for those at risk, refer for specialized care, and provide “caring contacts.” The specifications note that this is achieved with standardized screening and assessment instruments (the recommendations cite eight screening tool options and also suggest three different possible assessment tools); referral as appropriate; a brief safety-planning intervention (the recommendations list five options for this) that includes lethality means reduction along with follow-up to be sure that lethal means have been removed; arranging for rapid follow-up with a mental health professional; and follow-up contact by the primary care clinician within the next 48 hours.
According to Dr. Hogan, a motivation for releasing these recommendations has been the growing U.S. incidence of suicide, rising from 10.4 deaths/100,000 in 2000 to 13.3/100,000 in 2015, a 28% relative increase during a period when the rates of the top killers in the United States – cancer, heart disease, and stroke – were falling. Other telling statistics are that most people who die from suicide had seen a primary care provider during the year before death, and nearly half had seen a primary care provider during the month before their death.
But often the indicators of impending suicide are missed or not acted on. a misperception that contributes to a “failure to ask about suicide risk” on the part of health care professionals, the recommendations said. The document also highlighted the idea that, “most health care professionals are not aware of newly developed brief interventions for suicide, leading to the assumption that they should not ask about suicide because there is nothing practical that can be done in ordinary health care settings.”
One limitation of the recommendations is that they might be interpreted as “standard of care” for medicolegal purposes, warned Alan L. Berman, PhD, during the session’s discussion period. In addition, the evidence base for some of the recommended procedures is not very strong, such as risk stratification, said Dr. Berman, a clinical psychologist and former executive director of the American Association of Suicidology.
Dr. Hogan, Dr. Andrews, and Dr. Berman had no disclosures.
WASHINGTON – The National Action Alliance for Suicide Prevention released in April 2018 what the organization said was the first set of “standard care” recommendations for suicide prevention in people with suicide risk.
Care for people with a suicide risk in the United States “is not working very well. Evidence-based tools exist to detect and manage suicidality, but they are new and infrequently used” by many clinicians, including those seeing suicidal patients in primary care, emergency, or hospital settings, said Michael F. Hogan, PhD, during a session on the new standard-care recommendations at the annual conference of the American Association of Suicidology.
The Action Alliance seeks to have the standard care recommendations widely disseminated and hopes the document will receive endorsement from other organizations, said Dr. Hogan, a health policy consultant in Delmar, N.Y., and a member of the eight-person panel that wrote the recommendations.
These recommendations specify interventions for caregivers in four separate settings: primary care, outpatient behavioral health care (mental health and substance use treatment settings), emergency departments, and behavioral health inpatient care (hospital-level psychiatric or addiction treatment). For each setting, the recommendations highlight one or more core approaches, and then specify standards for identification and assessment, safety planning, means reduction, and follow-up contacts.
For example, within the primary care setting, the recommendations say the goals are to identify suicide risk, enhance the safety for those at risk, refer for specialized care, and provide “caring contacts.” The specifications note that this is achieved with standardized screening and assessment instruments (the recommendations cite eight screening tool options and also suggest three different possible assessment tools); referral as appropriate; a brief safety-planning intervention (the recommendations list five options for this) that includes lethality means reduction along with follow-up to be sure that lethal means have been removed; arranging for rapid follow-up with a mental health professional; and follow-up contact by the primary care clinician within the next 48 hours.
According to Dr. Hogan, a motivation for releasing these recommendations has been the growing U.S. incidence of suicide, rising from 10.4 deaths/100,000 in 2000 to 13.3/100,000 in 2015, a 28% relative increase during a period when the rates of the top killers in the United States – cancer, heart disease, and stroke – were falling. Other telling statistics are that most people who die from suicide had seen a primary care provider during the year before death, and nearly half had seen a primary care provider during the month before their death.
But often the indicators of impending suicide are missed or not acted on. a misperception that contributes to a “failure to ask about suicide risk” on the part of health care professionals, the recommendations said. The document also highlighted the idea that, “most health care professionals are not aware of newly developed brief interventions for suicide, leading to the assumption that they should not ask about suicide because there is nothing practical that can be done in ordinary health care settings.”
One limitation of the recommendations is that they might be interpreted as “standard of care” for medicolegal purposes, warned Alan L. Berman, PhD, during the session’s discussion period. In addition, the evidence base for some of the recommended procedures is not very strong, such as risk stratification, said Dr. Berman, a clinical psychologist and former executive director of the American Association of Suicidology.
Dr. Hogan, Dr. Andrews, and Dr. Berman had no disclosures.
REPORTING FROM THE AAS ANNUAL CONFERENCE
FDA approves subcutaneous tocilizumab for polyarticular JIA
in patients aged 2 years and older, according to a statement released May 14 by the drug’s manufacturer, Genentech.
While a intravenous formulation of the treatment was approved in 2013, this new delivery method may help make this treatment more accessible to the approximately 30 in every 100,000 children affected by PJIA, according to the release.
Doses were determined based on weight. Patients under 30 kg received 162 mg of tocilizumab every 3 weeks, while those 30 kg and over received 162 mg tocilizumab every 2 weeks.
Overall, safety of the subcutaneous delivery method was consistent with the IV study, as was the efficacy of the drug, the company said. A total of 28.8% of patients reported injection-site reactions – all moderate – and 15.4% reported neutrophil counts below 1 x 109 per liter.Tocilizumab can be taken either by itself or with methotrexate.
in patients aged 2 years and older, according to a statement released May 14 by the drug’s manufacturer, Genentech.
While a intravenous formulation of the treatment was approved in 2013, this new delivery method may help make this treatment more accessible to the approximately 30 in every 100,000 children affected by PJIA, according to the release.
Doses were determined based on weight. Patients under 30 kg received 162 mg of tocilizumab every 3 weeks, while those 30 kg and over received 162 mg tocilizumab every 2 weeks.
Overall, safety of the subcutaneous delivery method was consistent with the IV study, as was the efficacy of the drug, the company said. A total of 28.8% of patients reported injection-site reactions – all moderate – and 15.4% reported neutrophil counts below 1 x 109 per liter.Tocilizumab can be taken either by itself or with methotrexate.
in patients aged 2 years and older, according to a statement released May 14 by the drug’s manufacturer, Genentech.
While a intravenous formulation of the treatment was approved in 2013, this new delivery method may help make this treatment more accessible to the approximately 30 in every 100,000 children affected by PJIA, according to the release.
Doses were determined based on weight. Patients under 30 kg received 162 mg of tocilizumab every 3 weeks, while those 30 kg and over received 162 mg tocilizumab every 2 weeks.
Overall, safety of the subcutaneous delivery method was consistent with the IV study, as was the efficacy of the drug, the company said. A total of 28.8% of patients reported injection-site reactions – all moderate – and 15.4% reported neutrophil counts below 1 x 109 per liter.Tocilizumab can be taken either by itself or with methotrexate.
Emergency Imaging: Femoral Pseudoaneurysm
Case
An 84-year-old man, who was a resident at a local nursing home, presented for evaluation after the nursing staff noticed an increasingly swollen mass on the patient’s left groin. The patient’s medical history was significant for bilateral aortofemoral graft surgery, dementia, hypertension, and severe peripheral artery disease (PAD). He was not on any anticoagulation or antiplatelet agents. Due to the patient’s dementia, he was unable to provide a history regarding the onset of the swelling or any other signs or symptoms.
On examination, the patient did not appear in distress. His son, who was the patient’s durable power of attorney, was likewise unable to provide a clear timeframe regarding onset of the mass. The patient had no recent history of trauma and had not undergone any recent medical procedures. Vital signs at presentation were: blood pressure, 110/70 mm Hg; heart rate, 84 beats/min; respiratory rate, 13 breaths/min; and temperature, 98.6°F. Oxygen saturation was 94% on room air.
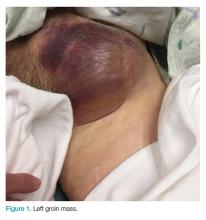
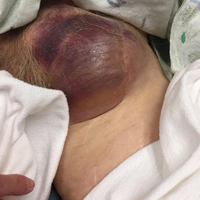
Clinical examination revealed a pulsatile, purple left groin mass and bruit. The mass was located around the left inguinal ligament and extended down the proximal, inner thigh (Figure 1). There was no drainage or lesions from the mass. Inspection of the patient’s hip demonstrated decreased adduction, limited by the mass; otherwise, there was normal range of motion. The dorsalis pedis and posterior tibial pulses were equal and intact, and the rest of the physical examination was unremarkable.
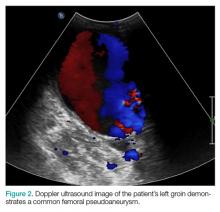
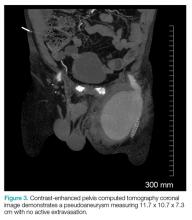
The patient tolerated the examination without focal signs of discomfort. A Doppler ultrasound revealed findings consistent with a common femoral pseudoaneurysm (PSA) (Figure 2). For better visualization and extension, a computed tomography angiogram (CTA) was obtained, which demonstrated a PSA measuring 11.7 x 10.7 x 7.3 cm; there was no active extravasation (Figure 3).
The patient was started on intravenous normal saline while vascular surgery services was consulted for management and repair. After a discussion with the son regarding the patient’s wishes, surgical intervention was refused and the patient was conservatively managed and transitioned to hospice care.
Discussion
A true aneurysm differs from a PSA in that true aneurysms involve all three layers of the vessel wall. A PSA consists partly of the vessel wall and partly of encapsulating fibrous tissue or surrounding tissue.
Etiology
Femoral artery PSAs can be iatrogenic, for example, develop following cardiac catheterization or at the anastomotic site of previous surgery.1 The incidence of diagnostic postcatheterization PSA ranges from 0.05% to 2%, whereas interventional postcatheterization PSA ranges from 2% to 6%.2
With the increasing number of peripheral coronary diagnostics and interventions, emergency physicians should include PSA in the differential diagnosis of patients with a recent or remote history of catheterization or bypass grafts. Less commonly, femoral PSAs are caused by non-surgical trauma or infection (ie, mycotic PSA). Patient risk factors for development of PSA include obesity, hypertension, PAD, and anticoagulation.3 Patients with femoral artery PSAs may present with a painful or painless pulsatile mass. Mass effect of the PSA can compress nearby neurovascular structures, leading to femoral neuropathies or limb edema secondary to venous obstruction.4 Complications of embolization or thrombosis can cause limb ischemia, neuropathy, and claudication, while rupture may present with a rapidly expanding groin hematoma. Additionally, sizeable PSAs can cause overlying skin necrosis.5
Imaging Studies
Diagnosis of a PSA can be made through Doppler ultrasound, which is the preferred imaging modality due to its accuracy, noninvasive nature, and low cost. Doppler ultrasound has been found to have a sensitivity of 94% and specificity of 97% in detecting PSAs. Additional imaging with CTA can provide further definition of vasculopathy.6 Treatment should be considered for patients with a symptomatic femoral PSA, a PSA measuring more than 3 cm, or patients who are on anticoagulation therapy. Studies have shown that observation-only and follow-up may be appropriate for patients with a PSA measuring less than 3 cm. A study by Toursarkissian et al7 found that the majority of PSAs smaller than 3 cm spontaneously resolved in a mean of 23 days without limb-threatening complications.
Treatment
Traditionally, open surgical repair techniques were the only treatment option for PSAs. However, in the early 1990s, the advent of new techniques such as stenting, coil insertion, ultrasound-guided compression, and ultrasound-guided thrombin injection, have developed as alternatives to open surgical repair; there has been variable success to these minimally invasive approaches.5,8
Ultrasound-Guided Compression. A conservative approach to treating PSAs, ultrasound-guided compression requires sustained compression by a skilled physician. This technique is associated with significant discomfort to the patient.5 Ultrasound-Guided Thrombin Injection. This technique is the treatment of choice for postcatheterization PSA. However, this intervention is contraindicated in patients who have concerning features such as an infected PSA, rapid expansion, skin necrosis, or signs of limb ischemia. Additionally, ultrasound-guided thrombin injection is not appropriate for use in patients with a PSA occurring at anastomosis of a synthetic graft and native artery.5
Conclusion
Based on our patient’s clinical presentation and history of aortofemoral bypass surgery, we suspected a femoral PSA. While the PSA noted in our patient was sizeable, imaging studies and clinical examination showed no sign of limb ischemia or rupture.
Femoral PSAs are usually iatrogenic in nature, typically developing shortly after catheterization or a previous bypass surgery. The most serious complication of a PSA is rupture, but a thorough examination of the distal extremity is warranted to assess for limb ischemia as well. Ultrasound imaging is considered the modality of choice based on its high sensitivity and sensitivity for detecting PSAs.
Small PSAs (<3 cm) can be managed medically, but larger PSAs (>3 cm) require treatment. Newer techniques, including stenting, coil insertion, ultrasound-guided compression, and ultrasound-guided thrombin injection are alternatives to open surgical repair of larger, uncomplicated PSAs. However, urgent open surgical repair is the only option when there is evidence of a ruptured PSA, ischemia, or skin necrosis.
1. Faggioli GL, Stella A, Gargiulo M, Tarantini S, D’Addato M, Ricotta JJ. Morphology of small aneurysms: definition and impact on risk of rupture. Am J Surg. 1994;168(2):131-135.
2. Hessel SJ, Adams DF, Abrams HL. Complications of angiography. Radiology. 1981;138(2):273-281. doi:10.1148/radiology.138.2.7455105.
3. Petrou E, Malakos I, Kampanarou S, Doulas N, Voudris V. Life-threatening rupture of a femoral pseudoaneurysm after cardiac catheterization. Open Cardiovasc Med J. 2016;10:201-204. doi:10.2174/1874192401610010201.
4. Mees B, Robinson D, Verhagen H, Chuen J. Non-aortic aneurysms—natural history and recommendations for referral and treatment. Aust Fam Physician. 2013;42(6):370-374.
5. Webber GW, Jang J, Gustavson S, Olin JW. Contemporary management of postcatheterization pseudoaneurysms. Circulation. 2007;115(20):2666-2674. doi:10.1161/CIRCULATIONAHA.106.681973.
6. Coughlin BF, Paushter DM. Peripheral pseudoaneurysms: evaluation with duplex US. Radiology. 1988;168(2):339-342. doi:10.1148/radiology.168.2.3293107.
7. Toursarkissian B, Allen BT, Petrinec D, et al. Spontaneous closure of selected iatrogenic pseudoaneurysms and arteriovenous fistulae. J Vasc Surg. 1997;25(5):803-809; discussion 808-809.
8. Corriere MA, Guzman RJ. True and false aneurysms of the femoral artery. Semin Vasc Surg. 2005;18(4):216-223. doi:10.1053/j.semvascsurg.2005.09.008.
Case
An 84-year-old man, who was a resident at a local nursing home, presented for evaluation after the nursing staff noticed an increasingly swollen mass on the patient’s left groin. The patient’s medical history was significant for bilateral aortofemoral graft surgery, dementia, hypertension, and severe peripheral artery disease (PAD). He was not on any anticoagulation or antiplatelet agents. Due to the patient’s dementia, he was unable to provide a history regarding the onset of the swelling or any other signs or symptoms.
On examination, the patient did not appear in distress. His son, who was the patient’s durable power of attorney, was likewise unable to provide a clear timeframe regarding onset of the mass. The patient had no recent history of trauma and had not undergone any recent medical procedures. Vital signs at presentation were: blood pressure, 110/70 mm Hg; heart rate, 84 beats/min; respiratory rate, 13 breaths/min; and temperature, 98.6°F. Oxygen saturation was 94% on room air.
Clinical examination revealed a pulsatile, purple left groin mass and bruit. The mass was located around the left inguinal ligament and extended down the proximal, inner thigh (Figure 1). There was no drainage or lesions from the mass. Inspection of the patient’s hip demonstrated decreased adduction, limited by the mass; otherwise, there was normal range of motion. The dorsalis pedis and posterior tibial pulses were equal and intact, and the rest of the physical examination was unremarkable.
The patient tolerated the examination without focal signs of discomfort. A Doppler ultrasound revealed findings consistent with a common femoral pseudoaneurysm (PSA) (Figure 2). For better visualization and extension, a computed tomography angiogram (CTA) was obtained, which demonstrated a PSA measuring 11.7 x 10.7 x 7.3 cm; there was no active extravasation (Figure 3).
The patient was started on intravenous normal saline while vascular surgery services was consulted for management and repair. After a discussion with the son regarding the patient’s wishes, surgical intervention was refused and the patient was conservatively managed and transitioned to hospice care.
Discussion
A true aneurysm differs from a PSA in that true aneurysms involve all three layers of the vessel wall. A PSA consists partly of the vessel wall and partly of encapsulating fibrous tissue or surrounding tissue.
Etiology
Femoral artery PSAs can be iatrogenic, for example, develop following cardiac catheterization or at the anastomotic site of previous surgery.1 The incidence of diagnostic postcatheterization PSA ranges from 0.05% to 2%, whereas interventional postcatheterization PSA ranges from 2% to 6%.2
With the increasing number of peripheral coronary diagnostics and interventions, emergency physicians should include PSA in the differential diagnosis of patients with a recent or remote history of catheterization or bypass grafts. Less commonly, femoral PSAs are caused by non-surgical trauma or infection (ie, mycotic PSA). Patient risk factors for development of PSA include obesity, hypertension, PAD, and anticoagulation.3 Patients with femoral artery PSAs may present with a painful or painless pulsatile mass. Mass effect of the PSA can compress nearby neurovascular structures, leading to femoral neuropathies or limb edema secondary to venous obstruction.4 Complications of embolization or thrombosis can cause limb ischemia, neuropathy, and claudication, while rupture may present with a rapidly expanding groin hematoma. Additionally, sizeable PSAs can cause overlying skin necrosis.5
Imaging Studies
Diagnosis of a PSA can be made through Doppler ultrasound, which is the preferred imaging modality due to its accuracy, noninvasive nature, and low cost. Doppler ultrasound has been found to have a sensitivity of 94% and specificity of 97% in detecting PSAs. Additional imaging with CTA can provide further definition of vasculopathy.6 Treatment should be considered for patients with a symptomatic femoral PSA, a PSA measuring more than 3 cm, or patients who are on anticoagulation therapy. Studies have shown that observation-only and follow-up may be appropriate for patients with a PSA measuring less than 3 cm. A study by Toursarkissian et al7 found that the majority of PSAs smaller than 3 cm spontaneously resolved in a mean of 23 days without limb-threatening complications.
Treatment
Traditionally, open surgical repair techniques were the only treatment option for PSAs. However, in the early 1990s, the advent of new techniques such as stenting, coil insertion, ultrasound-guided compression, and ultrasound-guided thrombin injection, have developed as alternatives to open surgical repair; there has been variable success to these minimally invasive approaches.5,8
Ultrasound-Guided Compression. A conservative approach to treating PSAs, ultrasound-guided compression requires sustained compression by a skilled physician. This technique is associated with significant discomfort to the patient.5 Ultrasound-Guided Thrombin Injection. This technique is the treatment of choice for postcatheterization PSA. However, this intervention is contraindicated in patients who have concerning features such as an infected PSA, rapid expansion, skin necrosis, or signs of limb ischemia. Additionally, ultrasound-guided thrombin injection is not appropriate for use in patients with a PSA occurring at anastomosis of a synthetic graft and native artery.5
Conclusion
Based on our patient’s clinical presentation and history of aortofemoral bypass surgery, we suspected a femoral PSA. While the PSA noted in our patient was sizeable, imaging studies and clinical examination showed no sign of limb ischemia or rupture.
Femoral PSAs are usually iatrogenic in nature, typically developing shortly after catheterization or a previous bypass surgery. The most serious complication of a PSA is rupture, but a thorough examination of the distal extremity is warranted to assess for limb ischemia as well. Ultrasound imaging is considered the modality of choice based on its high sensitivity and sensitivity for detecting PSAs.
Small PSAs (<3 cm) can be managed medically, but larger PSAs (>3 cm) require treatment. Newer techniques, including stenting, coil insertion, ultrasound-guided compression, and ultrasound-guided thrombin injection are alternatives to open surgical repair of larger, uncomplicated PSAs. However, urgent open surgical repair is the only option when there is evidence of a ruptured PSA, ischemia, or skin necrosis.
Case
An 84-year-old man, who was a resident at a local nursing home, presented for evaluation after the nursing staff noticed an increasingly swollen mass on the patient’s left groin. The patient’s medical history was significant for bilateral aortofemoral graft surgery, dementia, hypertension, and severe peripheral artery disease (PAD). He was not on any anticoagulation or antiplatelet agents. Due to the patient’s dementia, he was unable to provide a history regarding the onset of the swelling or any other signs or symptoms.
On examination, the patient did not appear in distress. His son, who was the patient’s durable power of attorney, was likewise unable to provide a clear timeframe regarding onset of the mass. The patient had no recent history of trauma and had not undergone any recent medical procedures. Vital signs at presentation were: blood pressure, 110/70 mm Hg; heart rate, 84 beats/min; respiratory rate, 13 breaths/min; and temperature, 98.6°F. Oxygen saturation was 94% on room air.
Clinical examination revealed a pulsatile, purple left groin mass and bruit. The mass was located around the left inguinal ligament and extended down the proximal, inner thigh (Figure 1). There was no drainage or lesions from the mass. Inspection of the patient’s hip demonstrated decreased adduction, limited by the mass; otherwise, there was normal range of motion. The dorsalis pedis and posterior tibial pulses were equal and intact, and the rest of the physical examination was unremarkable.
The patient tolerated the examination without focal signs of discomfort. A Doppler ultrasound revealed findings consistent with a common femoral pseudoaneurysm (PSA) (Figure 2). For better visualization and extension, a computed tomography angiogram (CTA) was obtained, which demonstrated a PSA measuring 11.7 x 10.7 x 7.3 cm; there was no active extravasation (Figure 3).
The patient was started on intravenous normal saline while vascular surgery services was consulted for management and repair. After a discussion with the son regarding the patient’s wishes, surgical intervention was refused and the patient was conservatively managed and transitioned to hospice care.
Discussion
A true aneurysm differs from a PSA in that true aneurysms involve all three layers of the vessel wall. A PSA consists partly of the vessel wall and partly of encapsulating fibrous tissue or surrounding tissue.
Etiology
Femoral artery PSAs can be iatrogenic, for example, develop following cardiac catheterization or at the anastomotic site of previous surgery.1 The incidence of diagnostic postcatheterization PSA ranges from 0.05% to 2%, whereas interventional postcatheterization PSA ranges from 2% to 6%.2
With the increasing number of peripheral coronary diagnostics and interventions, emergency physicians should include PSA in the differential diagnosis of patients with a recent or remote history of catheterization or bypass grafts. Less commonly, femoral PSAs are caused by non-surgical trauma or infection (ie, mycotic PSA). Patient risk factors for development of PSA include obesity, hypertension, PAD, and anticoagulation.3 Patients with femoral artery PSAs may present with a painful or painless pulsatile mass. Mass effect of the PSA can compress nearby neurovascular structures, leading to femoral neuropathies or limb edema secondary to venous obstruction.4 Complications of embolization or thrombosis can cause limb ischemia, neuropathy, and claudication, while rupture may present with a rapidly expanding groin hematoma. Additionally, sizeable PSAs can cause overlying skin necrosis.5
Imaging Studies
Diagnosis of a PSA can be made through Doppler ultrasound, which is the preferred imaging modality due to its accuracy, noninvasive nature, and low cost. Doppler ultrasound has been found to have a sensitivity of 94% and specificity of 97% in detecting PSAs. Additional imaging with CTA can provide further definition of vasculopathy.6 Treatment should be considered for patients with a symptomatic femoral PSA, a PSA measuring more than 3 cm, or patients who are on anticoagulation therapy. Studies have shown that observation-only and follow-up may be appropriate for patients with a PSA measuring less than 3 cm. A study by Toursarkissian et al7 found that the majority of PSAs smaller than 3 cm spontaneously resolved in a mean of 23 days without limb-threatening complications.
Treatment
Traditionally, open surgical repair techniques were the only treatment option for PSAs. However, in the early 1990s, the advent of new techniques such as stenting, coil insertion, ultrasound-guided compression, and ultrasound-guided thrombin injection, have developed as alternatives to open surgical repair; there has been variable success to these minimally invasive approaches.5,8
Ultrasound-Guided Compression. A conservative approach to treating PSAs, ultrasound-guided compression requires sustained compression by a skilled physician. This technique is associated with significant discomfort to the patient.5 Ultrasound-Guided Thrombin Injection. This technique is the treatment of choice for postcatheterization PSA. However, this intervention is contraindicated in patients who have concerning features such as an infected PSA, rapid expansion, skin necrosis, or signs of limb ischemia. Additionally, ultrasound-guided thrombin injection is not appropriate for use in patients with a PSA occurring at anastomosis of a synthetic graft and native artery.5
Conclusion
Based on our patient’s clinical presentation and history of aortofemoral bypass surgery, we suspected a femoral PSA. While the PSA noted in our patient was sizeable, imaging studies and clinical examination showed no sign of limb ischemia or rupture.
Femoral PSAs are usually iatrogenic in nature, typically developing shortly after catheterization or a previous bypass surgery. The most serious complication of a PSA is rupture, but a thorough examination of the distal extremity is warranted to assess for limb ischemia as well. Ultrasound imaging is considered the modality of choice based on its high sensitivity and sensitivity for detecting PSAs.
Small PSAs (<3 cm) can be managed medically, but larger PSAs (>3 cm) require treatment. Newer techniques, including stenting, coil insertion, ultrasound-guided compression, and ultrasound-guided thrombin injection are alternatives to open surgical repair of larger, uncomplicated PSAs. However, urgent open surgical repair is the only option when there is evidence of a ruptured PSA, ischemia, or skin necrosis.
1. Faggioli GL, Stella A, Gargiulo M, Tarantini S, D’Addato M, Ricotta JJ. Morphology of small aneurysms: definition and impact on risk of rupture. Am J Surg. 1994;168(2):131-135.
2. Hessel SJ, Adams DF, Abrams HL. Complications of angiography. Radiology. 1981;138(2):273-281. doi:10.1148/radiology.138.2.7455105.
3. Petrou E, Malakos I, Kampanarou S, Doulas N, Voudris V. Life-threatening rupture of a femoral pseudoaneurysm after cardiac catheterization. Open Cardiovasc Med J. 2016;10:201-204. doi:10.2174/1874192401610010201.
4. Mees B, Robinson D, Verhagen H, Chuen J. Non-aortic aneurysms—natural history and recommendations for referral and treatment. Aust Fam Physician. 2013;42(6):370-374.
5. Webber GW, Jang J, Gustavson S, Olin JW. Contemporary management of postcatheterization pseudoaneurysms. Circulation. 2007;115(20):2666-2674. doi:10.1161/CIRCULATIONAHA.106.681973.
6. Coughlin BF, Paushter DM. Peripheral pseudoaneurysms: evaluation with duplex US. Radiology. 1988;168(2):339-342. doi:10.1148/radiology.168.2.3293107.
7. Toursarkissian B, Allen BT, Petrinec D, et al. Spontaneous closure of selected iatrogenic pseudoaneurysms and arteriovenous fistulae. J Vasc Surg. 1997;25(5):803-809; discussion 808-809.
8. Corriere MA, Guzman RJ. True and false aneurysms of the femoral artery. Semin Vasc Surg. 2005;18(4):216-223. doi:10.1053/j.semvascsurg.2005.09.008.
1. Faggioli GL, Stella A, Gargiulo M, Tarantini S, D’Addato M, Ricotta JJ. Morphology of small aneurysms: definition and impact on risk of rupture. Am J Surg. 1994;168(2):131-135.
2. Hessel SJ, Adams DF, Abrams HL. Complications of angiography. Radiology. 1981;138(2):273-281. doi:10.1148/radiology.138.2.7455105.
3. Petrou E, Malakos I, Kampanarou S, Doulas N, Voudris V. Life-threatening rupture of a femoral pseudoaneurysm after cardiac catheterization. Open Cardiovasc Med J. 2016;10:201-204. doi:10.2174/1874192401610010201.
4. Mees B, Robinson D, Verhagen H, Chuen J. Non-aortic aneurysms—natural history and recommendations for referral and treatment. Aust Fam Physician. 2013;42(6):370-374.
5. Webber GW, Jang J, Gustavson S, Olin JW. Contemporary management of postcatheterization pseudoaneurysms. Circulation. 2007;115(20):2666-2674. doi:10.1161/CIRCULATIONAHA.106.681973.
6. Coughlin BF, Paushter DM. Peripheral pseudoaneurysms: evaluation with duplex US. Radiology. 1988;168(2):339-342. doi:10.1148/radiology.168.2.3293107.
7. Toursarkissian B, Allen BT, Petrinec D, et al. Spontaneous closure of selected iatrogenic pseudoaneurysms and arteriovenous fistulae. J Vasc Surg. 1997;25(5):803-809; discussion 808-809.
8. Corriere MA, Guzman RJ. True and false aneurysms of the femoral artery. Semin Vasc Surg. 2005;18(4):216-223. doi:10.1053/j.semvascsurg.2005.09.008.
Design limitations may have compromised DVT intervention trial
WASHINGTON – On the basis of a large randomized trial called ATTRACT, many clinicians have concluded that pharmacomechanical intervention is ineffective for preventing postthrombotic syndrome (PTS) in patients with deep venous thrombosis (DVT). But weaknesses in the study design challenge this conclusion, according to several experts in a DVT symposium at the 2018 Cardiovascular Research Technologies (CRT) meeting.
“The diagnosis and evaluation of DVT must be performed with IVUS [intravascular ultrasound], not with venography,” said Peter A. Soukas, MD, director of vascular medicine at Miriam Hospital in Providence, R.I. “You cannot know whether you successfully treated the clot if you cannot see it.”
“There were lots of limitations to that study. Here are some,” said Dr. Soukas, who then listed on a list of several considerations, including the fact that venograms – rather than IVUS, which Dr. Soukas labeled the “current gold standard” – were taken to evaluate procedure success. Another was that only half of patients had a moderate to severe DVT based on a Villalta score.
“If you look at the subgroup with a Villalta score of 10 or greater, the benefit [of pharmacomechanical intervention] was statistically significant,” he said.
In addition, the study enrolled a substantial number of patients with femoral-popliteal DVTs even though iliofemoral DVTs pose the greatest risk of postthrombotic syndrome. Dr. Soukas suggested these would have been a more appropriate focus of a study exploring the benefits of an intervention.
The limitations of the ATTRACT trial, which was conceived more than 5 years ago, have arisen primarily from advances in the field rather than problems with the design, Dr. Soukas explained. IVUS was not the preferred method for deep vein thrombosis evaluation then as it is now, and there have been several advances in current models of pharmacomechanical devices, which involve catheter-directed delivery of fibrinolytic therapy into the thrombus along with mechanical destruction of the clot.
Although further steps beyond clot lysis, such as stenting, were encouraged in ATTRACT to maintain venous patency, Dr. Soukas questioned whether these were employed sufficiently. For example, the rate of stenting in the experimental arm was 28%, a rate that “is not what we currently do” for patients at high risk of PTS, Dr. Soukas said.
In ATTRACT, major bleeding events were significantly higher in the experimental group (1.7% vs. 0.3%; P = .049). The authors cited this finding when they concluded that the experimental intervention was ineffective. Dr. Soukas acknowledged that bleeding risk is an important factor to consider, but he also emphasized the serious risks for failing to treat patients at high risk for PTS.
“PTS is devastating for patients, both functionally and economically,” Dr. Soukas said. He called the morbidity of deep vein thrombosis “staggering,” with in-hospital mortality in some series exceeding 10% and a risk of late development of postthrombotic syndrome persisting for up to 5 years. For those with proximal iliofemoral DVT, the PTS rate can reach 90%, about 15% of which can develop claudication with ulcerations, according to Dr. Soukas.
A large trial that was published in a prominent journal, ATTRACT has the potential to dissuade clinicians from considering pharmacomechanical intervention in high-risk patients who could benefit, Dr. Soukas said. Others speaking during the same symposium about advances in this field, such as John Fritz Angle, MD, director of the division of vascular and interventional radiology at the University of Virginia, Charlottesville, agreed with this assessment. Although other studies underway will reexamine this issue, there was consensus from several speakers at the CRT symposium that the results of ATTRACT should not preclude intervention in patients at high risk of PTS.
“I believe there is a role for DVT intervention for symptomatic patients with an extensive [proximal iliofemoral] clot provided they have a low bleeding risk,” Dr. Soukas said.
Dr. Soukas reported no potential conflicts of interest.
WASHINGTON – On the basis of a large randomized trial called ATTRACT, many clinicians have concluded that pharmacomechanical intervention is ineffective for preventing postthrombotic syndrome (PTS) in patients with deep venous thrombosis (DVT). But weaknesses in the study design challenge this conclusion, according to several experts in a DVT symposium at the 2018 Cardiovascular Research Technologies (CRT) meeting.
“The diagnosis and evaluation of DVT must be performed with IVUS [intravascular ultrasound], not with venography,” said Peter A. Soukas, MD, director of vascular medicine at Miriam Hospital in Providence, R.I. “You cannot know whether you successfully treated the clot if you cannot see it.”
“There were lots of limitations to that study. Here are some,” said Dr. Soukas, who then listed on a list of several considerations, including the fact that venograms – rather than IVUS, which Dr. Soukas labeled the “current gold standard” – were taken to evaluate procedure success. Another was that only half of patients had a moderate to severe DVT based on a Villalta score.
“If you look at the subgroup with a Villalta score of 10 or greater, the benefit [of pharmacomechanical intervention] was statistically significant,” he said.
In addition, the study enrolled a substantial number of patients with femoral-popliteal DVTs even though iliofemoral DVTs pose the greatest risk of postthrombotic syndrome. Dr. Soukas suggested these would have been a more appropriate focus of a study exploring the benefits of an intervention.
The limitations of the ATTRACT trial, which was conceived more than 5 years ago, have arisen primarily from advances in the field rather than problems with the design, Dr. Soukas explained. IVUS was not the preferred method for deep vein thrombosis evaluation then as it is now, and there have been several advances in current models of pharmacomechanical devices, which involve catheter-directed delivery of fibrinolytic therapy into the thrombus along with mechanical destruction of the clot.
Although further steps beyond clot lysis, such as stenting, were encouraged in ATTRACT to maintain venous patency, Dr. Soukas questioned whether these were employed sufficiently. For example, the rate of stenting in the experimental arm was 28%, a rate that “is not what we currently do” for patients at high risk of PTS, Dr. Soukas said.
In ATTRACT, major bleeding events were significantly higher in the experimental group (1.7% vs. 0.3%; P = .049). The authors cited this finding when they concluded that the experimental intervention was ineffective. Dr. Soukas acknowledged that bleeding risk is an important factor to consider, but he also emphasized the serious risks for failing to treat patients at high risk for PTS.
“PTS is devastating for patients, both functionally and economically,” Dr. Soukas said. He called the morbidity of deep vein thrombosis “staggering,” with in-hospital mortality in some series exceeding 10% and a risk of late development of postthrombotic syndrome persisting for up to 5 years. For those with proximal iliofemoral DVT, the PTS rate can reach 90%, about 15% of which can develop claudication with ulcerations, according to Dr. Soukas.
A large trial that was published in a prominent journal, ATTRACT has the potential to dissuade clinicians from considering pharmacomechanical intervention in high-risk patients who could benefit, Dr. Soukas said. Others speaking during the same symposium about advances in this field, such as John Fritz Angle, MD, director of the division of vascular and interventional radiology at the University of Virginia, Charlottesville, agreed with this assessment. Although other studies underway will reexamine this issue, there was consensus from several speakers at the CRT symposium that the results of ATTRACT should not preclude intervention in patients at high risk of PTS.
“I believe there is a role for DVT intervention for symptomatic patients with an extensive [proximal iliofemoral] clot provided they have a low bleeding risk,” Dr. Soukas said.
Dr. Soukas reported no potential conflicts of interest.
WASHINGTON – On the basis of a large randomized trial called ATTRACT, many clinicians have concluded that pharmacomechanical intervention is ineffective for preventing postthrombotic syndrome (PTS) in patients with deep venous thrombosis (DVT). But weaknesses in the study design challenge this conclusion, according to several experts in a DVT symposium at the 2018 Cardiovascular Research Technologies (CRT) meeting.
“The diagnosis and evaluation of DVT must be performed with IVUS [intravascular ultrasound], not with venography,” said Peter A. Soukas, MD, director of vascular medicine at Miriam Hospital in Providence, R.I. “You cannot know whether you successfully treated the clot if you cannot see it.”
“There were lots of limitations to that study. Here are some,” said Dr. Soukas, who then listed on a list of several considerations, including the fact that venograms – rather than IVUS, which Dr. Soukas labeled the “current gold standard” – were taken to evaluate procedure success. Another was that only half of patients had a moderate to severe DVT based on a Villalta score.
“If you look at the subgroup with a Villalta score of 10 or greater, the benefit [of pharmacomechanical intervention] was statistically significant,” he said.
In addition, the study enrolled a substantial number of patients with femoral-popliteal DVTs even though iliofemoral DVTs pose the greatest risk of postthrombotic syndrome. Dr. Soukas suggested these would have been a more appropriate focus of a study exploring the benefits of an intervention.
The limitations of the ATTRACT trial, which was conceived more than 5 years ago, have arisen primarily from advances in the field rather than problems with the design, Dr. Soukas explained. IVUS was not the preferred method for deep vein thrombosis evaluation then as it is now, and there have been several advances in current models of pharmacomechanical devices, which involve catheter-directed delivery of fibrinolytic therapy into the thrombus along with mechanical destruction of the clot.
Although further steps beyond clot lysis, such as stenting, were encouraged in ATTRACT to maintain venous patency, Dr. Soukas questioned whether these were employed sufficiently. For example, the rate of stenting in the experimental arm was 28%, a rate that “is not what we currently do” for patients at high risk of PTS, Dr. Soukas said.
In ATTRACT, major bleeding events were significantly higher in the experimental group (1.7% vs. 0.3%; P = .049). The authors cited this finding when they concluded that the experimental intervention was ineffective. Dr. Soukas acknowledged that bleeding risk is an important factor to consider, but he also emphasized the serious risks for failing to treat patients at high risk for PTS.
“PTS is devastating for patients, both functionally and economically,” Dr. Soukas said. He called the morbidity of deep vein thrombosis “staggering,” with in-hospital mortality in some series exceeding 10% and a risk of late development of postthrombotic syndrome persisting for up to 5 years. For those with proximal iliofemoral DVT, the PTS rate can reach 90%, about 15% of which can develop claudication with ulcerations, according to Dr. Soukas.
A large trial that was published in a prominent journal, ATTRACT has the potential to dissuade clinicians from considering pharmacomechanical intervention in high-risk patients who could benefit, Dr. Soukas said. Others speaking during the same symposium about advances in this field, such as John Fritz Angle, MD, director of the division of vascular and interventional radiology at the University of Virginia, Charlottesville, agreed with this assessment. Although other studies underway will reexamine this issue, there was consensus from several speakers at the CRT symposium that the results of ATTRACT should not preclude intervention in patients at high risk of PTS.
“I believe there is a role for DVT intervention for symptomatic patients with an extensive [proximal iliofemoral] clot provided they have a low bleeding risk,” Dr. Soukas said.
Dr. Soukas reported no potential conflicts of interest.
EXPERT ANALYSIS FROM THE 2018 CRT MEETING