User login
What’s Eating You? Clothes Moths (Tineola Species)
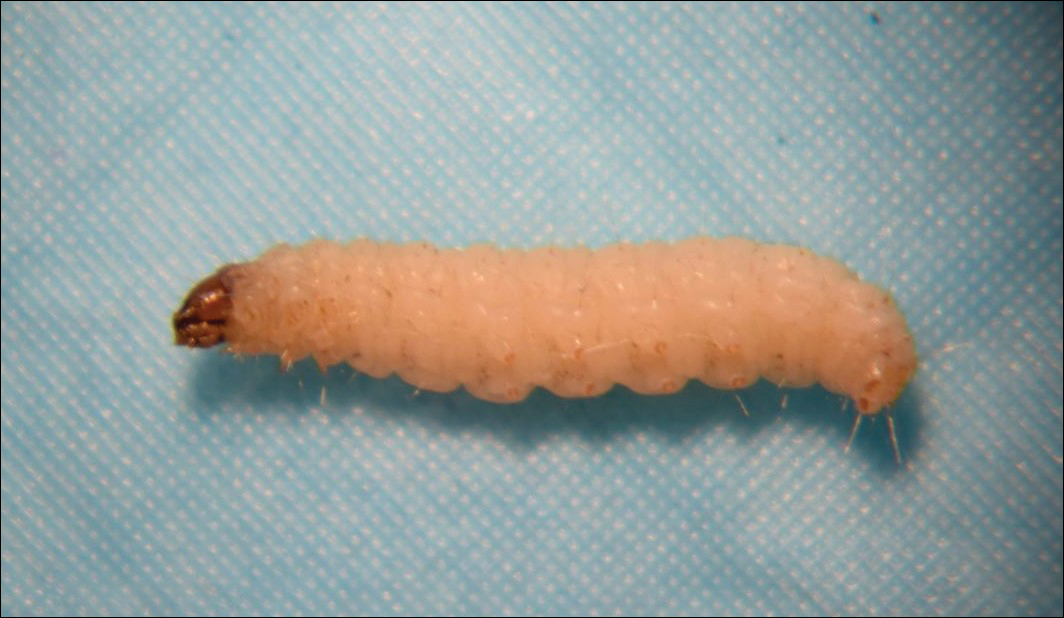
Clothes moths are common pests found inside buildings such as homes, stores, and museums. The most common species of importance include the webbing clothes moth (Tineola bisselliella)(Figure) and the casemaking clothes moth (Tineola pellionella). Both species target textiles such as wool, rugs, feathers, felts, hair, furs, and even grains.1,2 They avoid synthetics and plant materials such as cottons.1
Characteristics
Adult clothes moths extend 7 to 8 mm and are a golden (T bisselliella) to brown (T pellionella) color with fringed wings and a tuft on their heads.1,3 Adults do not eat; females die within a few days of laying eggs, while males live approximately 1 month. Once laid, eggs hatch within 4 to 10 days.1,3 The larvae (caterpillars) incur damage to clothes and other household goods. Fully mature larvae are 12- to 13-mm long, and the Tineola species have white- to cream-colored bodies with brown heads. The webbing clothes moth larva lacks ocelli (eyes), while the casemaking moth larva has a singular ocellus.1
Transmission
An infestation is evidenced by woolen items that have furrows or holes in them. Pheromone traps also can expose an active infestation.3 The webbing moth larvae can be found beneath a self-spun silken mat on the food source that offers the insect protection and camouflage while it eats; the mat collects frass (feces) and clothes particles.1,3,4 The casemaking moth larvae drag around a portable silken bag that takes on the color of the fabric being eaten and serves as a refuge when disturbed.1,3,4 Both adult and larval stages prefer low light conditions. The total time of development from caterpillar to adult varies depending on the temperature and humidity of the environment, but most clothes moths complete their life cycle within 1 to 3 months.1
Management of an Infestation
Multiple infestation treatment options exist should a patient present with a clothes moth infestation. Infested clothing articles or small blankets and rugs can be dry-cleaned or laundered. Any items not in use should be laundered before being sealed in airtight storage containers. Mothball vapor at appropriate concentrations is lethal to the moths, and when possible, clothing should be stored with mothballs or flakes at the concentration recommended by the manufacturer.4 Individuals should avoid application of household insecticides to clothing or bedding, which may be poisonous to people.1,4 Freezing, heating, and dry ice fumigation techniques also can be used to treat infested products.3 Cedarwood usually is insufficient to deter an infestation, as the oil vapor rarely reaches an effective concentration to repel or harm the insects.3,4 Strict housekeeping with attention to vacuuming carpets, baseboards, closets, and laundering all linens and furniture covers can further reduce an infestation.4 Clothes items can be set in the sunlight and brushed to help loosen the pests, as they dislike direct light and may fall from the garments.3 Dust insecticides also can be used per the manufacturer label to treat crevices and baseboards in an active area of infestation that may otherwise be difficult to clean.3 If an extensive infestation exists or larger items are infested, then a professional pest control agency should be employed for proper eradication.
Conclusion
Understanding the life cycle and basic biology of clothes moths and other common household pests will help the clinician identify an infestation and counsel patients if an insect is a true ectoparasite. Clothes moth larvae are not parasites but can be found on clothing and can be confused with myiasis or true parasites.
- Jacobs S. Clothes moth. Penn State Extension website. http://ento.psu.edu/extension/factsheets/clothes-moth. Updated January 2013. Accessed May 14, 2018.
- Querner P. Insect pests and integrated pest management in museums, libraries and historic buildings. Insects. 2015;6:595-607.
- Choe D-H. Pest notes: clothes moths (publication 7435). University of California Agriculture & Natural Resources website. http://ipm.ucanr.edu/PMG/PESTNOTES/pn7435.html. Updated March 2013. Accessed May 14, 2018.
- Potter M. Entfact-609: clothes moths. Entomology at the University of Kentucky website. https://entomology.ca.uky.edu/ef609. Updated October 2001. Accessed May 14, 2018.
Clothes moths are common pests found inside buildings such as homes, stores, and museums. The most common species of importance include the webbing clothes moth (Tineola bisselliella)(Figure) and the casemaking clothes moth (Tineola pellionella). Both species target textiles such as wool, rugs, feathers, felts, hair, furs, and even grains.1,2 They avoid synthetics and plant materials such as cottons.1

Characteristics
Adult clothes moths extend 7 to 8 mm and are a golden (T bisselliella) to brown (T pellionella) color with fringed wings and a tuft on their heads.1,3 Adults do not eat; females die within a few days of laying eggs, while males live approximately 1 month. Once laid, eggs hatch within 4 to 10 days.1,3 The larvae (caterpillars) incur damage to clothes and other household goods. Fully mature larvae are 12- to 13-mm long, and the Tineola species have white- to cream-colored bodies with brown heads. The webbing clothes moth larva lacks ocelli (eyes), while the casemaking moth larva has a singular ocellus.1
Transmission
An infestation is evidenced by woolen items that have furrows or holes in them. Pheromone traps also can expose an active infestation.3 The webbing moth larvae can be found beneath a self-spun silken mat on the food source that offers the insect protection and camouflage while it eats; the mat collects frass (feces) and clothes particles.1,3,4 The casemaking moth larvae drag around a portable silken bag that takes on the color of the fabric being eaten and serves as a refuge when disturbed.1,3,4 Both adult and larval stages prefer low light conditions. The total time of development from caterpillar to adult varies depending on the temperature and humidity of the environment, but most clothes moths complete their life cycle within 1 to 3 months.1
Management of an Infestation
Multiple infestation treatment options exist should a patient present with a clothes moth infestation. Infested clothing articles or small blankets and rugs can be dry-cleaned or laundered. Any items not in use should be laundered before being sealed in airtight storage containers. Mothball vapor at appropriate concentrations is lethal to the moths, and when possible, clothing should be stored with mothballs or flakes at the concentration recommended by the manufacturer.4 Individuals should avoid application of household insecticides to clothing or bedding, which may be poisonous to people.1,4 Freezing, heating, and dry ice fumigation techniques also can be used to treat infested products.3 Cedarwood usually is insufficient to deter an infestation, as the oil vapor rarely reaches an effective concentration to repel or harm the insects.3,4 Strict housekeeping with attention to vacuuming carpets, baseboards, closets, and laundering all linens and furniture covers can further reduce an infestation.4 Clothes items can be set in the sunlight and brushed to help loosen the pests, as they dislike direct light and may fall from the garments.3 Dust insecticides also can be used per the manufacturer label to treat crevices and baseboards in an active area of infestation that may otherwise be difficult to clean.3 If an extensive infestation exists or larger items are infested, then a professional pest control agency should be employed for proper eradication.
Conclusion
Understanding the life cycle and basic biology of clothes moths and other common household pests will help the clinician identify an infestation and counsel patients if an insect is a true ectoparasite. Clothes moth larvae are not parasites but can be found on clothing and can be confused with myiasis or true parasites.
Clothes moths are common pests found inside buildings such as homes, stores, and museums. The most common species of importance include the webbing clothes moth (Tineola bisselliella)(Figure) and the casemaking clothes moth (Tineola pellionella). Both species target textiles such as wool, rugs, feathers, felts, hair, furs, and even grains.1,2 They avoid synthetics and plant materials such as cottons.1

Characteristics
Adult clothes moths extend 7 to 8 mm and are a golden (T bisselliella) to brown (T pellionella) color with fringed wings and a tuft on their heads.1,3 Adults do not eat; females die within a few days of laying eggs, while males live approximately 1 month. Once laid, eggs hatch within 4 to 10 days.1,3 The larvae (caterpillars) incur damage to clothes and other household goods. Fully mature larvae are 12- to 13-mm long, and the Tineola species have white- to cream-colored bodies with brown heads. The webbing clothes moth larva lacks ocelli (eyes), while the casemaking moth larva has a singular ocellus.1
Transmission
An infestation is evidenced by woolen items that have furrows or holes in them. Pheromone traps also can expose an active infestation.3 The webbing moth larvae can be found beneath a self-spun silken mat on the food source that offers the insect protection and camouflage while it eats; the mat collects frass (feces) and clothes particles.1,3,4 The casemaking moth larvae drag around a portable silken bag that takes on the color of the fabric being eaten and serves as a refuge when disturbed.1,3,4 Both adult and larval stages prefer low light conditions. The total time of development from caterpillar to adult varies depending on the temperature and humidity of the environment, but most clothes moths complete their life cycle within 1 to 3 months.1
Management of an Infestation
Multiple infestation treatment options exist should a patient present with a clothes moth infestation. Infested clothing articles or small blankets and rugs can be dry-cleaned or laundered. Any items not in use should be laundered before being sealed in airtight storage containers. Mothball vapor at appropriate concentrations is lethal to the moths, and when possible, clothing should be stored with mothballs or flakes at the concentration recommended by the manufacturer.4 Individuals should avoid application of household insecticides to clothing or bedding, which may be poisonous to people.1,4 Freezing, heating, and dry ice fumigation techniques also can be used to treat infested products.3 Cedarwood usually is insufficient to deter an infestation, as the oil vapor rarely reaches an effective concentration to repel or harm the insects.3,4 Strict housekeeping with attention to vacuuming carpets, baseboards, closets, and laundering all linens and furniture covers can further reduce an infestation.4 Clothes items can be set in the sunlight and brushed to help loosen the pests, as they dislike direct light and may fall from the garments.3 Dust insecticides also can be used per the manufacturer label to treat crevices and baseboards in an active area of infestation that may otherwise be difficult to clean.3 If an extensive infestation exists or larger items are infested, then a professional pest control agency should be employed for proper eradication.
Conclusion
Understanding the life cycle and basic biology of clothes moths and other common household pests will help the clinician identify an infestation and counsel patients if an insect is a true ectoparasite. Clothes moth larvae are not parasites but can be found on clothing and can be confused with myiasis or true parasites.
- Jacobs S. Clothes moth. Penn State Extension website. http://ento.psu.edu/extension/factsheets/clothes-moth. Updated January 2013. Accessed May 14, 2018.
- Querner P. Insect pests and integrated pest management in museums, libraries and historic buildings. Insects. 2015;6:595-607.
- Choe D-H. Pest notes: clothes moths (publication 7435). University of California Agriculture & Natural Resources website. http://ipm.ucanr.edu/PMG/PESTNOTES/pn7435.html. Updated March 2013. Accessed May 14, 2018.
- Potter M. Entfact-609: clothes moths. Entomology at the University of Kentucky website. https://entomology.ca.uky.edu/ef609. Updated October 2001. Accessed May 14, 2018.
- Jacobs S. Clothes moth. Penn State Extension website. http://ento.psu.edu/extension/factsheets/clothes-moth. Updated January 2013. Accessed May 14, 2018.
- Querner P. Insect pests and integrated pest management in museums, libraries and historic buildings. Insects. 2015;6:595-607.
- Choe D-H. Pest notes: clothes moths (publication 7435). University of California Agriculture & Natural Resources website. http://ipm.ucanr.edu/PMG/PESTNOTES/pn7435.html. Updated March 2013. Accessed May 14, 2018.
- Potter M. Entfact-609: clothes moths. Entomology at the University of Kentucky website. https://entomology.ca.uky.edu/ef609. Updated October 2001. Accessed May 14, 2018.
Practice Points
- Clothes moth larvae are common household pests that may be misidentified as a parasitic infection such as myiasis when found on a person.
- Understanding the basic biology of clothes moths will help the clinician identify an infestation and appropriately counsel patients that clothes moths do not pose a considerable health risk.
Modifier -25 Victory, But the Battle Is Not Over
On February 23, 2018, Anthem Insurance Companies, Inc, announced the reversal of its proposed policy to reduce reimbursement for evaluation and management (E/M) services billed using modifier -25.1 This win for physicians was the result of a broad-based, multipronged advocacy campaign, and the American Academy of Dermatology Association (AADA) was critical to this victory.
Dermatology Took the Lead in Opposing Policy That Would Reduce Reimbursement
Dermatology was the first to trumpet the dangers of modifier -25 reduction policies and explain why other specialty societies should care. The AADA took the lead in assembling a coalition of physician groups to oppose the proposed policy by sharing language for opposition letters and meeting talking points with many societies outside of dermatology as well as producing the first draft for the American Medical Association (AMA) House of Delegates’ resolution that spurred action against Anthem’s proposed policy.2 Members of the AADA attended numerous conference calls and in-person meetings with health insurance officials to urge them to reverse the policy and helped coordinate opposition from state and national specialty societies. The influence and advocacy of the AMA was critical in reversing the proposed policy, but dermatology started the opposition and organized the players to bring the fight against Anthem.
AADA Continues to Fight
Despite the victory against Anthem, challenges to fair reimbursement of modifier -25 claims are ongoing. Two insurers recently announced implementation of new modifier -25 reduction policies.3,4 Moreover, 4 other insurers have existing modifier -25 reduction policies in place.5-8 The AADA has engaged, and will continue engaging, each of these insurers with the message that the ability to perform procedures and distinct E/M services on the same day is integral to efficient, patient-centered care of dermatologic diseases. The AADA argues that insurers’ rationale (overlapping indirect practice expenses) for payment reduction is improper and that appropriately documented modifier -25 claims should be reimbursed at 100% of allowable charges.
In addition to the existing insurer policies affecting modifier -25, Anthem has announced that it plans to conduct audits of modifier -25 claims with recoupments of inappropriate charges.1 Some Medicare Administrative Contractors also have cited modifier -25 as an area of concern and issued guidelines for reporting modifier -25, which frequently precede audits.9 The AADA is concerned that if Anthem follows through with its aggressive audits to recoup money, which can result in substantial take-backs, and finds a high error rate in modifier -25 claims, it also may consider revisiting its reduction policy. Moreover, it is likely that other insurers will use failed modifier -25 audits as an excuse to continue, expand, or adopt modifier -25 reduction policies. It is clear that blanket reduction in payment is much easier and less expensive for insurers than auditing medical records and not paying those who abuse modifier -25. As long as insurers are under financial pressure, modifier -25 will be a tempting target for reducing health care costs.
How to Use Modifier -25 Correctly
In addition to opposing inappropriate reimbursement of modifier -25, the AADA is committed to educating its members and insurers on the correct use and documentation of modifier -25. In December 2017, Mollie A. MacCormack, MD (Nashua, New Hampshire), and I led an educational webinar on modifier -25 for more than 1100 attendees.10 We discussed the performance standards and documentation requirements of modifier -25. It was clear that dermatologists were interested not only in the correct coding of modifier -25 claims but also avoiding the consequences of audits.
My peers frequently ask, “What can we [dermatologists] do to prepare for potential modifier -25 audits?” My advice is always, “Physician audit thyself.” I recommend self-auditing to make sure you and your practice are in compliance with modifier -25 documentation requirements. In a March 2017 Cutis column on modifier -25, I discussed what constitutes separate and distinct E/M services and what is included in a procedure’s global surgical package.11 A self-audit is as simple as pulling 10 to 20 medical records in which a same-day procedure and E/M service were billed. Cross out any information in the note included in the procedure’s global surgical package including history associated with establishing the diagnosis, physical examination of the procedure area(s), and discussion of treatment options. If complete documentation for an E/M is still present after removing the procedure and associated evaluation, you have passed the self-audit. If not, consider changing your coding to better reflect the documentation in your records. In my experience, insurance auditors are not physicians and often are not even medical professionals. Clear documentation and clear existence of a separate, distinct, and medically necessary E/M service is needed to succeed in a modifier -25 audit.
Final Thoughts
Dermatologists should rejoice that Anthem decided to rescind its modifier -25 policy. If this policy had gone into effect, the modifier -25 reduction would likely have spread to most other insurers as industry standard. This victory certainly shows what can be accomplished when organized medicine works together. Your state dermatology and medical societies, national societies, and the AMA collaborated on a critical existential threat to cost-effective and efficient patient care and won. These organizations deserve your membership and support as well as your thanks; however, our celebration must be short-lived, as there still are other insurers with modifier -25 reductions in place, and audits have been promised. We must continue to focus on proper use and documentation of modifier -25. This effort will not only help dermatologists decrease the risk of large recoupments from audits but also help the AADA continue to oppose inappropriate payment policies.
- Anthem Insurance Companies, Inc. Network Update. April 2018. https://www11.anthem.com/provider/ct/f5/s1/t0/pw_g334020.pdf?refer=ahpprovider&state=ct. Accessed May 25, 2018.
- American Academy of Dermatology, American Society for Dermatologic Surgery Association, American College of Mohs Surgery, American Society of Dermatopathology, Society for Investigative Dermatology. Opposition to reduced payment for the 25 modifier. https://www.ama-assn.org/sites/default/files/media-browser/public/hod/i17-808.pdf. Accessed May 14, 2018.
- We’re changing the payment policy when evaluation and management services are billed with surgery. Blue Cross Blue Shield of Michigan website. https://www.bcbsm.com/newsletter/therecord/2018/record_0418/Record_0418u.shtml. Published April 2018. Accessed May 14, 2018.
- Centene Corporation. Payment policy: problem oriented visits billed with surgical procedures. https://www.superiorhealthplan.com/content/dam/centene/Superior/Provider/PDFs/Problem%20Oriented%20Visits%20Billed
%20with%20Surgical%20Procedures%20(Ambetter%20MMP
%20and%20Medicare%20Advantage%20Only).pdf. Accessed May 14, 2018. - Blue Cross Blue Shield of Rhode Island. Payment Policy: Coding and Payment Guidelines. August 16, 2016. https://www.bcbsri.com/sites/default/files/polices/Coding-and-Payment-Guidelines-Oct2016.pdf. Accessed May 9, 2018.
- Modifier payment policy. Tufts Health Plan website. https://tuftshealthplan.com/documents/providers/payment-policies/modifier-payment-policy. Updated November 2017. Accessed May 14, 2018.
- Harvard Pilgrim Health Care. Payment Policies: Evaluation and Management. February 2018. https://www.harvardpilgrim.org/pls/portal/docs/PAGE/PROVIDERS/MANUALS/PAYMENT%20POLICIES/H-2%20EVALUATION-MGT_02
0118.PDF. Accessed May 25, 2018. - Updates to the policies on modifier 25 reporting and reimbursement. Independence Blue Cross website. http://provcomm.ibx.com/ProvComm/ProvComm.nsf/4bcc623b93e226638525792c00575962/bbe9e72728cc01e285258167005c629d!OpenDocument. Published July 24, 2017. Accessed May 14, 2018.
- Centers for Medicare & Medicaid Services. Payment for evaluation and management services provided during global period of surgery. MLN Matters. May 19, 2006. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/MM5025.pdf. Accessed May 14, 2018.
- Audits on modifier 25 are coming—complimentary live webinar. American Academy of Dermatology website. https://store.aad.org/products/11928. Accessed May 9, 2018.
- Rogers HW. One diagnosis and modifier -25: appropriate or audit target? Cutis. 2017;99:165-166.
On February 23, 2018, Anthem Insurance Companies, Inc, announced the reversal of its proposed policy to reduce reimbursement for evaluation and management (E/M) services billed using modifier -25.1 This win for physicians was the result of a broad-based, multipronged advocacy campaign, and the American Academy of Dermatology Association (AADA) was critical to this victory.
Dermatology Took the Lead in Opposing Policy That Would Reduce Reimbursement
Dermatology was the first to trumpet the dangers of modifier -25 reduction policies and explain why other specialty societies should care. The AADA took the lead in assembling a coalition of physician groups to oppose the proposed policy by sharing language for opposition letters and meeting talking points with many societies outside of dermatology as well as producing the first draft for the American Medical Association (AMA) House of Delegates’ resolution that spurred action against Anthem’s proposed policy.2 Members of the AADA attended numerous conference calls and in-person meetings with health insurance officials to urge them to reverse the policy and helped coordinate opposition from state and national specialty societies. The influence and advocacy of the AMA was critical in reversing the proposed policy, but dermatology started the opposition and organized the players to bring the fight against Anthem.
AADA Continues to Fight
Despite the victory against Anthem, challenges to fair reimbursement of modifier -25 claims are ongoing. Two insurers recently announced implementation of new modifier -25 reduction policies.3,4 Moreover, 4 other insurers have existing modifier -25 reduction policies in place.5-8 The AADA has engaged, and will continue engaging, each of these insurers with the message that the ability to perform procedures and distinct E/M services on the same day is integral to efficient, patient-centered care of dermatologic diseases. The AADA argues that insurers’ rationale (overlapping indirect practice expenses) for payment reduction is improper and that appropriately documented modifier -25 claims should be reimbursed at 100% of allowable charges.
In addition to the existing insurer policies affecting modifier -25, Anthem has announced that it plans to conduct audits of modifier -25 claims with recoupments of inappropriate charges.1 Some Medicare Administrative Contractors also have cited modifier -25 as an area of concern and issued guidelines for reporting modifier -25, which frequently precede audits.9 The AADA is concerned that if Anthem follows through with its aggressive audits to recoup money, which can result in substantial take-backs, and finds a high error rate in modifier -25 claims, it also may consider revisiting its reduction policy. Moreover, it is likely that other insurers will use failed modifier -25 audits as an excuse to continue, expand, or adopt modifier -25 reduction policies. It is clear that blanket reduction in payment is much easier and less expensive for insurers than auditing medical records and not paying those who abuse modifier -25. As long as insurers are under financial pressure, modifier -25 will be a tempting target for reducing health care costs.
How to Use Modifier -25 Correctly
In addition to opposing inappropriate reimbursement of modifier -25, the AADA is committed to educating its members and insurers on the correct use and documentation of modifier -25. In December 2017, Mollie A. MacCormack, MD (Nashua, New Hampshire), and I led an educational webinar on modifier -25 for more than 1100 attendees.10 We discussed the performance standards and documentation requirements of modifier -25. It was clear that dermatologists were interested not only in the correct coding of modifier -25 claims but also avoiding the consequences of audits.
My peers frequently ask, “What can we [dermatologists] do to prepare for potential modifier -25 audits?” My advice is always, “Physician audit thyself.” I recommend self-auditing to make sure you and your practice are in compliance with modifier -25 documentation requirements. In a March 2017 Cutis column on modifier -25, I discussed what constitutes separate and distinct E/M services and what is included in a procedure’s global surgical package.11 A self-audit is as simple as pulling 10 to 20 medical records in which a same-day procedure and E/M service were billed. Cross out any information in the note included in the procedure’s global surgical package including history associated with establishing the diagnosis, physical examination of the procedure area(s), and discussion of treatment options. If complete documentation for an E/M is still present after removing the procedure and associated evaluation, you have passed the self-audit. If not, consider changing your coding to better reflect the documentation in your records. In my experience, insurance auditors are not physicians and often are not even medical professionals. Clear documentation and clear existence of a separate, distinct, and medically necessary E/M service is needed to succeed in a modifier -25 audit.
Final Thoughts
Dermatologists should rejoice that Anthem decided to rescind its modifier -25 policy. If this policy had gone into effect, the modifier -25 reduction would likely have spread to most other insurers as industry standard. This victory certainly shows what can be accomplished when organized medicine works together. Your state dermatology and medical societies, national societies, and the AMA collaborated on a critical existential threat to cost-effective and efficient patient care and won. These organizations deserve your membership and support as well as your thanks; however, our celebration must be short-lived, as there still are other insurers with modifier -25 reductions in place, and audits have been promised. We must continue to focus on proper use and documentation of modifier -25. This effort will not only help dermatologists decrease the risk of large recoupments from audits but also help the AADA continue to oppose inappropriate payment policies.
On February 23, 2018, Anthem Insurance Companies, Inc, announced the reversal of its proposed policy to reduce reimbursement for evaluation and management (E/M) services billed using modifier -25.1 This win for physicians was the result of a broad-based, multipronged advocacy campaign, and the American Academy of Dermatology Association (AADA) was critical to this victory.
Dermatology Took the Lead in Opposing Policy That Would Reduce Reimbursement
Dermatology was the first to trumpet the dangers of modifier -25 reduction policies and explain why other specialty societies should care. The AADA took the lead in assembling a coalition of physician groups to oppose the proposed policy by sharing language for opposition letters and meeting talking points with many societies outside of dermatology as well as producing the first draft for the American Medical Association (AMA) House of Delegates’ resolution that spurred action against Anthem’s proposed policy.2 Members of the AADA attended numerous conference calls and in-person meetings with health insurance officials to urge them to reverse the policy and helped coordinate opposition from state and national specialty societies. The influence and advocacy of the AMA was critical in reversing the proposed policy, but dermatology started the opposition and organized the players to bring the fight against Anthem.
AADA Continues to Fight
Despite the victory against Anthem, challenges to fair reimbursement of modifier -25 claims are ongoing. Two insurers recently announced implementation of new modifier -25 reduction policies.3,4 Moreover, 4 other insurers have existing modifier -25 reduction policies in place.5-8 The AADA has engaged, and will continue engaging, each of these insurers with the message that the ability to perform procedures and distinct E/M services on the same day is integral to efficient, patient-centered care of dermatologic diseases. The AADA argues that insurers’ rationale (overlapping indirect practice expenses) for payment reduction is improper and that appropriately documented modifier -25 claims should be reimbursed at 100% of allowable charges.
In addition to the existing insurer policies affecting modifier -25, Anthem has announced that it plans to conduct audits of modifier -25 claims with recoupments of inappropriate charges.1 Some Medicare Administrative Contractors also have cited modifier -25 as an area of concern and issued guidelines for reporting modifier -25, which frequently precede audits.9 The AADA is concerned that if Anthem follows through with its aggressive audits to recoup money, which can result in substantial take-backs, and finds a high error rate in modifier -25 claims, it also may consider revisiting its reduction policy. Moreover, it is likely that other insurers will use failed modifier -25 audits as an excuse to continue, expand, or adopt modifier -25 reduction policies. It is clear that blanket reduction in payment is much easier and less expensive for insurers than auditing medical records and not paying those who abuse modifier -25. As long as insurers are under financial pressure, modifier -25 will be a tempting target for reducing health care costs.
How to Use Modifier -25 Correctly
In addition to opposing inappropriate reimbursement of modifier -25, the AADA is committed to educating its members and insurers on the correct use and documentation of modifier -25. In December 2017, Mollie A. MacCormack, MD (Nashua, New Hampshire), and I led an educational webinar on modifier -25 for more than 1100 attendees.10 We discussed the performance standards and documentation requirements of modifier -25. It was clear that dermatologists were interested not only in the correct coding of modifier -25 claims but also avoiding the consequences of audits.
My peers frequently ask, “What can we [dermatologists] do to prepare for potential modifier -25 audits?” My advice is always, “Physician audit thyself.” I recommend self-auditing to make sure you and your practice are in compliance with modifier -25 documentation requirements. In a March 2017 Cutis column on modifier -25, I discussed what constitutes separate and distinct E/M services and what is included in a procedure’s global surgical package.11 A self-audit is as simple as pulling 10 to 20 medical records in which a same-day procedure and E/M service were billed. Cross out any information in the note included in the procedure’s global surgical package including history associated with establishing the diagnosis, physical examination of the procedure area(s), and discussion of treatment options. If complete documentation for an E/M is still present after removing the procedure and associated evaluation, you have passed the self-audit. If not, consider changing your coding to better reflect the documentation in your records. In my experience, insurance auditors are not physicians and often are not even medical professionals. Clear documentation and clear existence of a separate, distinct, and medically necessary E/M service is needed to succeed in a modifier -25 audit.
Final Thoughts
Dermatologists should rejoice that Anthem decided to rescind its modifier -25 policy. If this policy had gone into effect, the modifier -25 reduction would likely have spread to most other insurers as industry standard. This victory certainly shows what can be accomplished when organized medicine works together. Your state dermatology and medical societies, national societies, and the AMA collaborated on a critical existential threat to cost-effective and efficient patient care and won. These organizations deserve your membership and support as well as your thanks; however, our celebration must be short-lived, as there still are other insurers with modifier -25 reductions in place, and audits have been promised. We must continue to focus on proper use and documentation of modifier -25. This effort will not only help dermatologists decrease the risk of large recoupments from audits but also help the AADA continue to oppose inappropriate payment policies.
- Anthem Insurance Companies, Inc. Network Update. April 2018. https://www11.anthem.com/provider/ct/f5/s1/t0/pw_g334020.pdf?refer=ahpprovider&state=ct. Accessed May 25, 2018.
- American Academy of Dermatology, American Society for Dermatologic Surgery Association, American College of Mohs Surgery, American Society of Dermatopathology, Society for Investigative Dermatology. Opposition to reduced payment for the 25 modifier. https://www.ama-assn.org/sites/default/files/media-browser/public/hod/i17-808.pdf. Accessed May 14, 2018.
- We’re changing the payment policy when evaluation and management services are billed with surgery. Blue Cross Blue Shield of Michigan website. https://www.bcbsm.com/newsletter/therecord/2018/record_0418/Record_0418u.shtml. Published April 2018. Accessed May 14, 2018.
- Centene Corporation. Payment policy: problem oriented visits billed with surgical procedures. https://www.superiorhealthplan.com/content/dam/centene/Superior/Provider/PDFs/Problem%20Oriented%20Visits%20Billed
%20with%20Surgical%20Procedures%20(Ambetter%20MMP
%20and%20Medicare%20Advantage%20Only).pdf. Accessed May 14, 2018. - Blue Cross Blue Shield of Rhode Island. Payment Policy: Coding and Payment Guidelines. August 16, 2016. https://www.bcbsri.com/sites/default/files/polices/Coding-and-Payment-Guidelines-Oct2016.pdf. Accessed May 9, 2018.
- Modifier payment policy. Tufts Health Plan website. https://tuftshealthplan.com/documents/providers/payment-policies/modifier-payment-policy. Updated November 2017. Accessed May 14, 2018.
- Harvard Pilgrim Health Care. Payment Policies: Evaluation and Management. February 2018. https://www.harvardpilgrim.org/pls/portal/docs/PAGE/PROVIDERS/MANUALS/PAYMENT%20POLICIES/H-2%20EVALUATION-MGT_02
0118.PDF. Accessed May 25, 2018. - Updates to the policies on modifier 25 reporting and reimbursement. Independence Blue Cross website. http://provcomm.ibx.com/ProvComm/ProvComm.nsf/4bcc623b93e226638525792c00575962/bbe9e72728cc01e285258167005c629d!OpenDocument. Published July 24, 2017. Accessed May 14, 2018.
- Centers for Medicare & Medicaid Services. Payment for evaluation and management services provided during global period of surgery. MLN Matters. May 19, 2006. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/MM5025.pdf. Accessed May 14, 2018.
- Audits on modifier 25 are coming—complimentary live webinar. American Academy of Dermatology website. https://store.aad.org/products/11928. Accessed May 9, 2018.
- Rogers HW. One diagnosis and modifier -25: appropriate or audit target? Cutis. 2017;99:165-166.
- Anthem Insurance Companies, Inc. Network Update. April 2018. https://www11.anthem.com/provider/ct/f5/s1/t0/pw_g334020.pdf?refer=ahpprovider&state=ct. Accessed May 25, 2018.
- American Academy of Dermatology, American Society for Dermatologic Surgery Association, American College of Mohs Surgery, American Society of Dermatopathology, Society for Investigative Dermatology. Opposition to reduced payment for the 25 modifier. https://www.ama-assn.org/sites/default/files/media-browser/public/hod/i17-808.pdf. Accessed May 14, 2018.
- We’re changing the payment policy when evaluation and management services are billed with surgery. Blue Cross Blue Shield of Michigan website. https://www.bcbsm.com/newsletter/therecord/2018/record_0418/Record_0418u.shtml. Published April 2018. Accessed May 14, 2018.
- Centene Corporation. Payment policy: problem oriented visits billed with surgical procedures. https://www.superiorhealthplan.com/content/dam/centene/Superior/Provider/PDFs/Problem%20Oriented%20Visits%20Billed
%20with%20Surgical%20Procedures%20(Ambetter%20MMP
%20and%20Medicare%20Advantage%20Only).pdf. Accessed May 14, 2018. - Blue Cross Blue Shield of Rhode Island. Payment Policy: Coding and Payment Guidelines. August 16, 2016. https://www.bcbsri.com/sites/default/files/polices/Coding-and-Payment-Guidelines-Oct2016.pdf. Accessed May 9, 2018.
- Modifier payment policy. Tufts Health Plan website. https://tuftshealthplan.com/documents/providers/payment-policies/modifier-payment-policy. Updated November 2017. Accessed May 14, 2018.
- Harvard Pilgrim Health Care. Payment Policies: Evaluation and Management. February 2018. https://www.harvardpilgrim.org/pls/portal/docs/PAGE/PROVIDERS/MANUALS/PAYMENT%20POLICIES/H-2%20EVALUATION-MGT_02
0118.PDF. Accessed May 25, 2018. - Updates to the policies on modifier 25 reporting and reimbursement. Independence Blue Cross website. http://provcomm.ibx.com/ProvComm/ProvComm.nsf/4bcc623b93e226638525792c00575962/bbe9e72728cc01e285258167005c629d!OpenDocument. Published July 24, 2017. Accessed May 14, 2018.
- Centers for Medicare & Medicaid Services. Payment for evaluation and management services provided during global period of surgery. MLN Matters. May 19, 2006. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/MM5025.pdf. Accessed May 14, 2018.
- Audits on modifier 25 are coming—complimentary live webinar. American Academy of Dermatology website. https://store.aad.org/products/11928. Accessed May 9, 2018.
- Rogers HW. One diagnosis and modifier -25: appropriate or audit target? Cutis. 2017;99:165-166.
Practice Points
- Insurers are increasingly targeting modifier -25 for audits and payment reductions.
- Physicians should understand modifier -25 documentation requirements and self-audit to ensure compliance.
Updated Guidelines on Peanut Allergy Prevention in Infants With Atopic Dermatitis
It has been said that “extraordinary claims require extraordinary evidence.”1 In the pursuit of evidence-based medicine, we are encouraged to follow a similar standard, with an emphasis on waiting for multiple studies with good-quality data and high levels of agreement before changing any aspect of our clinical practice. The ostensible purpose is that studies can be flawed, conclusions can be incorrect, or biases can be overlooked. In such cases, acting on questionable results could imperil patients. It is for this reason that so many review articles sometimes frustratingly seem to conclude that further evidence is needed.2
Based on this standard, recently published addendum guidelines from the National Institute of Allergy and Infectious Diseases for prevention of peanut allergy in the United States3 are somewhat striking in that they make fairly bold recommendations based on results from the 2015 Learning Early about Peanut Allergy (LEAP) study,4 a randomized trial evaluating early peanut introduction as a preventive strategy for peanut allergy. Of note, this study was not placebo controlled, was conducted at only 1 site in the United Kingdom, and only included 640 children, though the number of participants was admittedly large for this type of study.4 Arguably, the LEAP study alone does not provide enough evidence upon which to base what essentially amounts to an about-face in the official recommendations for prevention of peanut and other food allergies, which emphasized delayed introduction of high-risk foods, especially in high-risk individuals.5-7 To better understand this shift, we need to briefly explore the context of the addendum guidelines.
As many as one-third of pediatric patients with atopic dermatitis (AD) have food allergies, thus diet often is invoked by patients and providers alike as an underlying cause of the disease.8 Many patients in my practice are so focused on potential food allergies that actual treatment of the affected skin is marginalized and often dismissed as a stopgap that does not address the root of the problem. A 2004 study of 100 children with AD found that diet was manipulated by the parents in 75% of patients in an attempt to manage the disease.9
Patients are not the only ones who consider food allergies to be a driving force in AD. The medical literature indicates that this theory has existed for centuries; for instance, with regard to the relationship between diet and AD, the author of an article from 1830 quipped, “There is probably no subject in which more deeply rooted convictions have been held . . . than the connection between diet and disease, both as regards the causation and treatment of the latter . . .”10 More apropos perhaps is a statement from the 2010 National Institute of Allergy and Infectious Diseases guidelines on food allergy management, which noted that while the expert group “does not mean to imply that AD results from [food allergies], the role of [food allergies] in the pathogenesis and severity of this condition remains controversial.”11
Prior to the LEAP study, food allergy recommendations for clinical practice in the United Kingdom in 199812 and the United States in 200013 recommended excluding allergenic foods (eg, peanuts, tree nuts, soy, milk, eggs) from the diet in infants with a family history of atopy until 3 years of age. However, those recommendations did not seem to be working, when in fact just the opposite was happening. From 1997 until the LEAP study was conducted in 2015, the prevalence of peanut allergy more than quadrupled and became the leading cause of anaphylaxis and death related to food allergy.14 Additionally, study after study concluded that elimination diets did not seem to help most patients with AD.15 As is required in good scientific thinking, when a hypothesis is proven false, other approaches must be considered.
The idea arose that perhaps delaying introduction of allergenic foods was the opposite of the answer.4 The LEAP study tested the notion that peanut allergies are rare in countries where peanuts are introduced early and if telling families to delay introduction of peanuts in infants might actually be causing development of a peanut allergy, and the tests bore fruit. It was found that giving infants peanut-containing foods resulted in a more than 80% reduction in peanut allergy at 5 years of age (P<.001).4 What was perhaps even more interesting was the connection between AD and peanut allergy. An important idea articulated in the LEAP study is in some ways revolutionary: Rather than foods causing AD, it could be that “early environmental exposure (through the skin) to peanut may account for early sensitization, whereas early oral exposure may lead to immune tolerance.”4 This concept—that impaired eczematous skin may actually lead to the development of food allergies—turns the whole thing upside down.
What do these updated guidelines actually suggest? The first guideline focuses on infants with severe AD, egg allergy, or both, who therefore are thought to be at the highest risk for developing peanut allergy.3 Because of the higher baseline risk in this subgroup, measurement of the peanut-specific IgE (peanut sIgE) level, skin prick testing (SPT), or both is strongly recommended before introducing peanut protein into the diet. This testing can be performed by qualified providers as a screening measure, but if positive (≥0.35 kUA/L for peanut sIgE or >2 mm on the peanut SPT), referral to an allergy specialist is warranted. If these studies are negative, it is thought the likelihood of peanut allergy is low, and it is recommended that caregivers introduce age-appropriate peanut-containing foods (eg, peanut butter snack puffs, diluted peanut butter) as early as 4 to 6 months of age. The second guideline recommends that peanut-containing foods should be introduced into the diets of infants with mild or moderate AD at approximately 6 months of age without the need for prior screening via peanut sIgE or SPT. Lastly, the third guideline recommends that caregivers freely introduce peanut-containing foods together with other solid foods in infants without AD or food allergies in accordance with family preference.3
The results of the LEAP study are certainly exciting, and although the theoretical basis makes good scientific sense and the updated guidelines truly address an important and growing problem, there are several issues with this update that are worth considering. Given the constraints of the LEAP study, it certainly seems possible that the results will not be applicable to all populations or foods. More research is needed to ensure that this robust finding applies to other children and to explore the introduction of other allergenic foods, which the LEAP study investigators also emphasized.4
In fairness, the updated guidelines clearly state the quality of evidence of their recommendations and make it clear that expert opinion is playing a large role.3 For the first guideline regarding recommendations for those with severe AD and/or egg allergy, the quality of evidence is deemed moderate, while the contribution of expert opinion is listed as significant. For the second and third guidelines regarding recommendations for mild to moderate AD and those without AD, respectively, the quality of evidence is low and expert opinion is again listed as significant.3
Importantly, delineating severe AD from moderate disease—which is necessary because only severe AD warrants evaluation with peanut sIgE and/or SPT—can be difficult, as the distinction relies on a degree of subjectivity that may vary between specialists. Indeed, 2 publications suggest extending the definition of severe AD to include infants with early-onset AD (<3 months of age) and those with moderate AD not responding to treatment.16,17
Despite these reservations, the updated guidelines represent a breakthrough in understanding in an area truly in need of advancement. Although the evidence may not be exactly extraordinary, the context for these developments and our deeper understanding suggest that we do indeed live in extraordinary times.
- Encyclopaedia Galactica [television transcript]. Cosmos: A Personal Voyage. Public Broadcasting Service. December 14, 1980.
- Ezzo J, Bausell B, Moerman DE, et al. Reviewing the reviews: how strong is the evidence? how clear are the conclusions? Int J Technol Assess Health Care. 2001;17:457-466.
- Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases–sponsored expert panel.J Allergy Clin Immunol. 2017;139:29-44.
- Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
- Høst A, Koletzko B, Dreborg S, et al. Dietary products used in infants for treatment and prevention of food allergy. joint statement of the European Society for Paediatric Allergology and Clinical Immunology (ESPACI) Committee on Hypoallergenic Formulas and the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Committee on Nutrition. Arch Dis Child. 1999;81:80-84.
- American Academy of Pediatrics. Committee on Nutrition. hypoallergenic infant formulas. Pediatrics. 2000;106(2, pt 1):346-349.
- Fiocchi A, Assa’ad A, Bahna S; Adverse Reactions to Foods Committee; American College of Allergy, Asthma and Immunology. Food allergy and the introduction of solid foods to infants: a consensus document. Ann Allergy Asthma Immunol. 2006;97:10-20.
- Thompson MM, Hanifin JM. Effective therapy of childhood atopic dermatitis allays food allergy concerns. J Am Acad Dermatol. 2005;53(2 suppl 2):S214-S219.
- Johnston GA, Bilbao RM, Graham-Brown RA. The use of dietary manipulation by parents of children with atopic dermatitis. Br J Dermatol. 2004;150:1186-1189.
- Mackenzie S. The inaugural address on the advantages to be derived from the study of dermatology. BMJ. 1830;1:193-197.
- Boyce JA, Assa’ad A, Burks AW, et al; NIAID-Sponsored Expert Panel. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 suppl):S1-S58.
- Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment. Peanut Allergy. London, England: Department of Health; 1998.
- American Academy of Pediatrics Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics. 2000;106(2, pt 1):346-349.
- Gruchalla RS, Sampson HA. Preventing peanut allergy through early consumption—ready for prime time? N Engl J Med. 2015;372:875-877.
- Lim NR, Lohman ME, Lio PA. The role of elimination diets in atopic dermatitis: a comprehensive review. Pediatr Dermatol. 2017;34:516-527.
- Wong CC, Allen KJ, Orchard D. Changes to infant feeding guidelines: relevance to dermatologists. Australas J Dermatol. 2017;58:e171-e175.
- Martin PE, Eckert JK, Koplin JJ, et al. Which infants with eczema are at risk of food allergy? results from a population-based cohort. Clin Exp Allergy. 2015;45:255-264.
It has been said that “extraordinary claims require extraordinary evidence.”1 In the pursuit of evidence-based medicine, we are encouraged to follow a similar standard, with an emphasis on waiting for multiple studies with good-quality data and high levels of agreement before changing any aspect of our clinical practice. The ostensible purpose is that studies can be flawed, conclusions can be incorrect, or biases can be overlooked. In such cases, acting on questionable results could imperil patients. It is for this reason that so many review articles sometimes frustratingly seem to conclude that further evidence is needed.2
Based on this standard, recently published addendum guidelines from the National Institute of Allergy and Infectious Diseases for prevention of peanut allergy in the United States3 are somewhat striking in that they make fairly bold recommendations based on results from the 2015 Learning Early about Peanut Allergy (LEAP) study,4 a randomized trial evaluating early peanut introduction as a preventive strategy for peanut allergy. Of note, this study was not placebo controlled, was conducted at only 1 site in the United Kingdom, and only included 640 children, though the number of participants was admittedly large for this type of study.4 Arguably, the LEAP study alone does not provide enough evidence upon which to base what essentially amounts to an about-face in the official recommendations for prevention of peanut and other food allergies, which emphasized delayed introduction of high-risk foods, especially in high-risk individuals.5-7 To better understand this shift, we need to briefly explore the context of the addendum guidelines.
As many as one-third of pediatric patients with atopic dermatitis (AD) have food allergies, thus diet often is invoked by patients and providers alike as an underlying cause of the disease.8 Many patients in my practice are so focused on potential food allergies that actual treatment of the affected skin is marginalized and often dismissed as a stopgap that does not address the root of the problem. A 2004 study of 100 children with AD found that diet was manipulated by the parents in 75% of patients in an attempt to manage the disease.9
Patients are not the only ones who consider food allergies to be a driving force in AD. The medical literature indicates that this theory has existed for centuries; for instance, with regard to the relationship between diet and AD, the author of an article from 1830 quipped, “There is probably no subject in which more deeply rooted convictions have been held . . . than the connection between diet and disease, both as regards the causation and treatment of the latter . . .”10 More apropos perhaps is a statement from the 2010 National Institute of Allergy and Infectious Diseases guidelines on food allergy management, which noted that while the expert group “does not mean to imply that AD results from [food allergies], the role of [food allergies] in the pathogenesis and severity of this condition remains controversial.”11
Prior to the LEAP study, food allergy recommendations for clinical practice in the United Kingdom in 199812 and the United States in 200013 recommended excluding allergenic foods (eg, peanuts, tree nuts, soy, milk, eggs) from the diet in infants with a family history of atopy until 3 years of age. However, those recommendations did not seem to be working, when in fact just the opposite was happening. From 1997 until the LEAP study was conducted in 2015, the prevalence of peanut allergy more than quadrupled and became the leading cause of anaphylaxis and death related to food allergy.14 Additionally, study after study concluded that elimination diets did not seem to help most patients with AD.15 As is required in good scientific thinking, when a hypothesis is proven false, other approaches must be considered.
The idea arose that perhaps delaying introduction of allergenic foods was the opposite of the answer.4 The LEAP study tested the notion that peanut allergies are rare in countries where peanuts are introduced early and if telling families to delay introduction of peanuts in infants might actually be causing development of a peanut allergy, and the tests bore fruit. It was found that giving infants peanut-containing foods resulted in a more than 80% reduction in peanut allergy at 5 years of age (P<.001).4 What was perhaps even more interesting was the connection between AD and peanut allergy. An important idea articulated in the LEAP study is in some ways revolutionary: Rather than foods causing AD, it could be that “early environmental exposure (through the skin) to peanut may account for early sensitization, whereas early oral exposure may lead to immune tolerance.”4 This concept—that impaired eczematous skin may actually lead to the development of food allergies—turns the whole thing upside down.
What do these updated guidelines actually suggest? The first guideline focuses on infants with severe AD, egg allergy, or both, who therefore are thought to be at the highest risk for developing peanut allergy.3 Because of the higher baseline risk in this subgroup, measurement of the peanut-specific IgE (peanut sIgE) level, skin prick testing (SPT), or both is strongly recommended before introducing peanut protein into the diet. This testing can be performed by qualified providers as a screening measure, but if positive (≥0.35 kUA/L for peanut sIgE or >2 mm on the peanut SPT), referral to an allergy specialist is warranted. If these studies are negative, it is thought the likelihood of peanut allergy is low, and it is recommended that caregivers introduce age-appropriate peanut-containing foods (eg, peanut butter snack puffs, diluted peanut butter) as early as 4 to 6 months of age. The second guideline recommends that peanut-containing foods should be introduced into the diets of infants with mild or moderate AD at approximately 6 months of age without the need for prior screening via peanut sIgE or SPT. Lastly, the third guideline recommends that caregivers freely introduce peanut-containing foods together with other solid foods in infants without AD or food allergies in accordance with family preference.3
The results of the LEAP study are certainly exciting, and although the theoretical basis makes good scientific sense and the updated guidelines truly address an important and growing problem, there are several issues with this update that are worth considering. Given the constraints of the LEAP study, it certainly seems possible that the results will not be applicable to all populations or foods. More research is needed to ensure that this robust finding applies to other children and to explore the introduction of other allergenic foods, which the LEAP study investigators also emphasized.4
In fairness, the updated guidelines clearly state the quality of evidence of their recommendations and make it clear that expert opinion is playing a large role.3 For the first guideline regarding recommendations for those with severe AD and/or egg allergy, the quality of evidence is deemed moderate, while the contribution of expert opinion is listed as significant. For the second and third guidelines regarding recommendations for mild to moderate AD and those without AD, respectively, the quality of evidence is low and expert opinion is again listed as significant.3
Importantly, delineating severe AD from moderate disease—which is necessary because only severe AD warrants evaluation with peanut sIgE and/or SPT—can be difficult, as the distinction relies on a degree of subjectivity that may vary between specialists. Indeed, 2 publications suggest extending the definition of severe AD to include infants with early-onset AD (<3 months of age) and those with moderate AD not responding to treatment.16,17
Despite these reservations, the updated guidelines represent a breakthrough in understanding in an area truly in need of advancement. Although the evidence may not be exactly extraordinary, the context for these developments and our deeper understanding suggest that we do indeed live in extraordinary times.
It has been said that “extraordinary claims require extraordinary evidence.”1 In the pursuit of evidence-based medicine, we are encouraged to follow a similar standard, with an emphasis on waiting for multiple studies with good-quality data and high levels of agreement before changing any aspect of our clinical practice. The ostensible purpose is that studies can be flawed, conclusions can be incorrect, or biases can be overlooked. In such cases, acting on questionable results could imperil patients. It is for this reason that so many review articles sometimes frustratingly seem to conclude that further evidence is needed.2
Based on this standard, recently published addendum guidelines from the National Institute of Allergy and Infectious Diseases for prevention of peanut allergy in the United States3 are somewhat striking in that they make fairly bold recommendations based on results from the 2015 Learning Early about Peanut Allergy (LEAP) study,4 a randomized trial evaluating early peanut introduction as a preventive strategy for peanut allergy. Of note, this study was not placebo controlled, was conducted at only 1 site in the United Kingdom, and only included 640 children, though the number of participants was admittedly large for this type of study.4 Arguably, the LEAP study alone does not provide enough evidence upon which to base what essentially amounts to an about-face in the official recommendations for prevention of peanut and other food allergies, which emphasized delayed introduction of high-risk foods, especially in high-risk individuals.5-7 To better understand this shift, we need to briefly explore the context of the addendum guidelines.
As many as one-third of pediatric patients with atopic dermatitis (AD) have food allergies, thus diet often is invoked by patients and providers alike as an underlying cause of the disease.8 Many patients in my practice are so focused on potential food allergies that actual treatment of the affected skin is marginalized and often dismissed as a stopgap that does not address the root of the problem. A 2004 study of 100 children with AD found that diet was manipulated by the parents in 75% of patients in an attempt to manage the disease.9
Patients are not the only ones who consider food allergies to be a driving force in AD. The medical literature indicates that this theory has existed for centuries; for instance, with regard to the relationship between diet and AD, the author of an article from 1830 quipped, “There is probably no subject in which more deeply rooted convictions have been held . . . than the connection between diet and disease, both as regards the causation and treatment of the latter . . .”10 More apropos perhaps is a statement from the 2010 National Institute of Allergy and Infectious Diseases guidelines on food allergy management, which noted that while the expert group “does not mean to imply that AD results from [food allergies], the role of [food allergies] in the pathogenesis and severity of this condition remains controversial.”11
Prior to the LEAP study, food allergy recommendations for clinical practice in the United Kingdom in 199812 and the United States in 200013 recommended excluding allergenic foods (eg, peanuts, tree nuts, soy, milk, eggs) from the diet in infants with a family history of atopy until 3 years of age. However, those recommendations did not seem to be working, when in fact just the opposite was happening. From 1997 until the LEAP study was conducted in 2015, the prevalence of peanut allergy more than quadrupled and became the leading cause of anaphylaxis and death related to food allergy.14 Additionally, study after study concluded that elimination diets did not seem to help most patients with AD.15 As is required in good scientific thinking, when a hypothesis is proven false, other approaches must be considered.
The idea arose that perhaps delaying introduction of allergenic foods was the opposite of the answer.4 The LEAP study tested the notion that peanut allergies are rare in countries where peanuts are introduced early and if telling families to delay introduction of peanuts in infants might actually be causing development of a peanut allergy, and the tests bore fruit. It was found that giving infants peanut-containing foods resulted in a more than 80% reduction in peanut allergy at 5 years of age (P<.001).4 What was perhaps even more interesting was the connection between AD and peanut allergy. An important idea articulated in the LEAP study is in some ways revolutionary: Rather than foods causing AD, it could be that “early environmental exposure (through the skin) to peanut may account for early sensitization, whereas early oral exposure may lead to immune tolerance.”4 This concept—that impaired eczematous skin may actually lead to the development of food allergies—turns the whole thing upside down.
What do these updated guidelines actually suggest? The first guideline focuses on infants with severe AD, egg allergy, or both, who therefore are thought to be at the highest risk for developing peanut allergy.3 Because of the higher baseline risk in this subgroup, measurement of the peanut-specific IgE (peanut sIgE) level, skin prick testing (SPT), or both is strongly recommended before introducing peanut protein into the diet. This testing can be performed by qualified providers as a screening measure, but if positive (≥0.35 kUA/L for peanut sIgE or >2 mm on the peanut SPT), referral to an allergy specialist is warranted. If these studies are negative, it is thought the likelihood of peanut allergy is low, and it is recommended that caregivers introduce age-appropriate peanut-containing foods (eg, peanut butter snack puffs, diluted peanut butter) as early as 4 to 6 months of age. The second guideline recommends that peanut-containing foods should be introduced into the diets of infants with mild or moderate AD at approximately 6 months of age without the need for prior screening via peanut sIgE or SPT. Lastly, the third guideline recommends that caregivers freely introduce peanut-containing foods together with other solid foods in infants without AD or food allergies in accordance with family preference.3
The results of the LEAP study are certainly exciting, and although the theoretical basis makes good scientific sense and the updated guidelines truly address an important and growing problem, there are several issues with this update that are worth considering. Given the constraints of the LEAP study, it certainly seems possible that the results will not be applicable to all populations or foods. More research is needed to ensure that this robust finding applies to other children and to explore the introduction of other allergenic foods, which the LEAP study investigators also emphasized.4
In fairness, the updated guidelines clearly state the quality of evidence of their recommendations and make it clear that expert opinion is playing a large role.3 For the first guideline regarding recommendations for those with severe AD and/or egg allergy, the quality of evidence is deemed moderate, while the contribution of expert opinion is listed as significant. For the second and third guidelines regarding recommendations for mild to moderate AD and those without AD, respectively, the quality of evidence is low and expert opinion is again listed as significant.3
Importantly, delineating severe AD from moderate disease—which is necessary because only severe AD warrants evaluation with peanut sIgE and/or SPT—can be difficult, as the distinction relies on a degree of subjectivity that may vary between specialists. Indeed, 2 publications suggest extending the definition of severe AD to include infants with early-onset AD (<3 months of age) and those with moderate AD not responding to treatment.16,17
Despite these reservations, the updated guidelines represent a breakthrough in understanding in an area truly in need of advancement. Although the evidence may not be exactly extraordinary, the context for these developments and our deeper understanding suggest that we do indeed live in extraordinary times.
- Encyclopaedia Galactica [television transcript]. Cosmos: A Personal Voyage. Public Broadcasting Service. December 14, 1980.
- Ezzo J, Bausell B, Moerman DE, et al. Reviewing the reviews: how strong is the evidence? how clear are the conclusions? Int J Technol Assess Health Care. 2001;17:457-466.
- Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases–sponsored expert panel.J Allergy Clin Immunol. 2017;139:29-44.
- Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
- Høst A, Koletzko B, Dreborg S, et al. Dietary products used in infants for treatment and prevention of food allergy. joint statement of the European Society for Paediatric Allergology and Clinical Immunology (ESPACI) Committee on Hypoallergenic Formulas and the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Committee on Nutrition. Arch Dis Child. 1999;81:80-84.
- American Academy of Pediatrics. Committee on Nutrition. hypoallergenic infant formulas. Pediatrics. 2000;106(2, pt 1):346-349.
- Fiocchi A, Assa’ad A, Bahna S; Adverse Reactions to Foods Committee; American College of Allergy, Asthma and Immunology. Food allergy and the introduction of solid foods to infants: a consensus document. Ann Allergy Asthma Immunol. 2006;97:10-20.
- Thompson MM, Hanifin JM. Effective therapy of childhood atopic dermatitis allays food allergy concerns. J Am Acad Dermatol. 2005;53(2 suppl 2):S214-S219.
- Johnston GA, Bilbao RM, Graham-Brown RA. The use of dietary manipulation by parents of children with atopic dermatitis. Br J Dermatol. 2004;150:1186-1189.
- Mackenzie S. The inaugural address on the advantages to be derived from the study of dermatology. BMJ. 1830;1:193-197.
- Boyce JA, Assa’ad A, Burks AW, et al; NIAID-Sponsored Expert Panel. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 suppl):S1-S58.
- Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment. Peanut Allergy. London, England: Department of Health; 1998.
- American Academy of Pediatrics Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics. 2000;106(2, pt 1):346-349.
- Gruchalla RS, Sampson HA. Preventing peanut allergy through early consumption—ready for prime time? N Engl J Med. 2015;372:875-877.
- Lim NR, Lohman ME, Lio PA. The role of elimination diets in atopic dermatitis: a comprehensive review. Pediatr Dermatol. 2017;34:516-527.
- Wong CC, Allen KJ, Orchard D. Changes to infant feeding guidelines: relevance to dermatologists. Australas J Dermatol. 2017;58:e171-e175.
- Martin PE, Eckert JK, Koplin JJ, et al. Which infants with eczema are at risk of food allergy? results from a population-based cohort. Clin Exp Allergy. 2015;45:255-264.
- Encyclopaedia Galactica [television transcript]. Cosmos: A Personal Voyage. Public Broadcasting Service. December 14, 1980.
- Ezzo J, Bausell B, Moerman DE, et al. Reviewing the reviews: how strong is the evidence? how clear are the conclusions? Int J Technol Assess Health Care. 2001;17:457-466.
- Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases–sponsored expert panel.J Allergy Clin Immunol. 2017;139:29-44.
- Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
- Høst A, Koletzko B, Dreborg S, et al. Dietary products used in infants for treatment and prevention of food allergy. joint statement of the European Society for Paediatric Allergology and Clinical Immunology (ESPACI) Committee on Hypoallergenic Formulas and the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Committee on Nutrition. Arch Dis Child. 1999;81:80-84.
- American Academy of Pediatrics. Committee on Nutrition. hypoallergenic infant formulas. Pediatrics. 2000;106(2, pt 1):346-349.
- Fiocchi A, Assa’ad A, Bahna S; Adverse Reactions to Foods Committee; American College of Allergy, Asthma and Immunology. Food allergy and the introduction of solid foods to infants: a consensus document. Ann Allergy Asthma Immunol. 2006;97:10-20.
- Thompson MM, Hanifin JM. Effective therapy of childhood atopic dermatitis allays food allergy concerns. J Am Acad Dermatol. 2005;53(2 suppl 2):S214-S219.
- Johnston GA, Bilbao RM, Graham-Brown RA. The use of dietary manipulation by parents of children with atopic dermatitis. Br J Dermatol. 2004;150:1186-1189.
- Mackenzie S. The inaugural address on the advantages to be derived from the study of dermatology. BMJ. 1830;1:193-197.
- Boyce JA, Assa’ad A, Burks AW, et al; NIAID-Sponsored Expert Panel. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 suppl):S1-S58.
- Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment. Peanut Allergy. London, England: Department of Health; 1998.
- American Academy of Pediatrics Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics. 2000;106(2, pt 1):346-349.
- Gruchalla RS, Sampson HA. Preventing peanut allergy through early consumption—ready for prime time? N Engl J Med. 2015;372:875-877.
- Lim NR, Lohman ME, Lio PA. The role of elimination diets in atopic dermatitis: a comprehensive review. Pediatr Dermatol. 2017;34:516-527.
- Wong CC, Allen KJ, Orchard D. Changes to infant feeding guidelines: relevance to dermatologists. Australas J Dermatol. 2017;58:e171-e175.
- Martin PE, Eckert JK, Koplin JJ, et al. Which infants with eczema are at risk of food allergy? results from a population-based cohort. Clin Exp Allergy. 2015;45:255-264.
Statin effect in prostate cancer may be caused by reduced inflammation
SAN FRANCISCO – according to the results of a new genetic analysis of prostate cancers in men in the Health Professionals Follow Up Study.
A previous analysis from the Health Professionals Follow Up Study published in 2015 showed no difference in the risk of lethal prostate cancer for those who began using statins after diagnosis of their tumors.
A genetic analysis of these lethal cases revealed that patients taking long-term statins had a lower incidence of phosphatase and tensin homolog (PTEN)–null cancers, which are associated with worse outcomes. Integrating molecular and epidemiologic data, PTEN and PI3K (phosphatidylinositol) signaling and inflammation and immune activation appear to be two potential mechanisms contributing to this association.
Genetic analysis of normal prostate tissue also showed unique traits among statin users. “We found that the top ten pathways that came up were almost all involved in inflammation or immune activation, and we found those differences only in tumor and adjacent normal prostate tissue. We didn’t see any pathways that were differentially expressed by statin use within the tumor tissue itself,” said Emma Allott, PhD, who presented the research at a poster session at the annual meeting of the American Urological Association.
An association between statin use and improved survival was first described in a 2006 study based on results from the Health Professionals Follow Up Study. Since then, “we’ve been generating molecular data on cancers that developed in statin users and nonusers,” said Dr. Allott of the University of North Carolina, Chapel Hill.
Of the 5,792 prostate cancer diagnoses, 17% were advanced cases, defined as stage T3b or more, having spread to lymph nodes or metastasized, or lethal; 13% were lethal, 46% were positive for the ERG oncogene, and 14% were PTEN-null. “Statin use was associated with a lower risk of PTEN-null prostate cancer, so that seems to drive some of the reduced association with lethal disease,” said Dr. Allott.
There was no association between lethality and ERG-positive or ERG-negative status. Those who used statins for more than 5 years were less likely to have a PTEN-null tumor (hazard ratio, 0.42; 95% confidence interval, 0.20-0.90) but not more likely to have a PTEN-positive tumor (HR, 1.18; 95% CI, 0.95-1.46).
Compared with never users, long-term statin users also were less likely to have advanced prostate cancer (multivariate analysis, HR, 0.62; 95% CI, 0.45-0.85) as well as lethal prostate cancer (HR, 0.52; 95% CI, 0.35-0.78).
The researchers conducted a gene set enrichment analysis in statin users and found an enrichment of T-cell, B-cell, and PI3K signaling in tumor-adjacent normal prostate tissue, as well as other changes. “We think maybe there’s a microenvironment inflammation component to the mechanism through which statins are associated with lower risk of lethal prostate cancer,” said Dr. Allott.
The molecular data could identify patient subgroups that could benefit from statins. Dr. Allott said that is the goal, but it will take time. “That’s more obviously translatable to the clinic, but we don’t yet have enough data in this cohort to look at that.”
SOURCE: Allott E et al. AUA 2018, Abstract MP21-01.
SAN FRANCISCO – according to the results of a new genetic analysis of prostate cancers in men in the Health Professionals Follow Up Study.
A previous analysis from the Health Professionals Follow Up Study published in 2015 showed no difference in the risk of lethal prostate cancer for those who began using statins after diagnosis of their tumors.
A genetic analysis of these lethal cases revealed that patients taking long-term statins had a lower incidence of phosphatase and tensin homolog (PTEN)–null cancers, which are associated with worse outcomes. Integrating molecular and epidemiologic data, PTEN and PI3K (phosphatidylinositol) signaling and inflammation and immune activation appear to be two potential mechanisms contributing to this association.
Genetic analysis of normal prostate tissue also showed unique traits among statin users. “We found that the top ten pathways that came up were almost all involved in inflammation or immune activation, and we found those differences only in tumor and adjacent normal prostate tissue. We didn’t see any pathways that were differentially expressed by statin use within the tumor tissue itself,” said Emma Allott, PhD, who presented the research at a poster session at the annual meeting of the American Urological Association.
An association between statin use and improved survival was first described in a 2006 study based on results from the Health Professionals Follow Up Study. Since then, “we’ve been generating molecular data on cancers that developed in statin users and nonusers,” said Dr. Allott of the University of North Carolina, Chapel Hill.
Of the 5,792 prostate cancer diagnoses, 17% were advanced cases, defined as stage T3b or more, having spread to lymph nodes or metastasized, or lethal; 13% were lethal, 46% were positive for the ERG oncogene, and 14% were PTEN-null. “Statin use was associated with a lower risk of PTEN-null prostate cancer, so that seems to drive some of the reduced association with lethal disease,” said Dr. Allott.
There was no association between lethality and ERG-positive or ERG-negative status. Those who used statins for more than 5 years were less likely to have a PTEN-null tumor (hazard ratio, 0.42; 95% confidence interval, 0.20-0.90) but not more likely to have a PTEN-positive tumor (HR, 1.18; 95% CI, 0.95-1.46).
Compared with never users, long-term statin users also were less likely to have advanced prostate cancer (multivariate analysis, HR, 0.62; 95% CI, 0.45-0.85) as well as lethal prostate cancer (HR, 0.52; 95% CI, 0.35-0.78).
The researchers conducted a gene set enrichment analysis in statin users and found an enrichment of T-cell, B-cell, and PI3K signaling in tumor-adjacent normal prostate tissue, as well as other changes. “We think maybe there’s a microenvironment inflammation component to the mechanism through which statins are associated with lower risk of lethal prostate cancer,” said Dr. Allott.
The molecular data could identify patient subgroups that could benefit from statins. Dr. Allott said that is the goal, but it will take time. “That’s more obviously translatable to the clinic, but we don’t yet have enough data in this cohort to look at that.”
SOURCE: Allott E et al. AUA 2018, Abstract MP21-01.
SAN FRANCISCO – according to the results of a new genetic analysis of prostate cancers in men in the Health Professionals Follow Up Study.
A previous analysis from the Health Professionals Follow Up Study published in 2015 showed no difference in the risk of lethal prostate cancer for those who began using statins after diagnosis of their tumors.
A genetic analysis of these lethal cases revealed that patients taking long-term statins had a lower incidence of phosphatase and tensin homolog (PTEN)–null cancers, which are associated with worse outcomes. Integrating molecular and epidemiologic data, PTEN and PI3K (phosphatidylinositol) signaling and inflammation and immune activation appear to be two potential mechanisms contributing to this association.
Genetic analysis of normal prostate tissue also showed unique traits among statin users. “We found that the top ten pathways that came up were almost all involved in inflammation or immune activation, and we found those differences only in tumor and adjacent normal prostate tissue. We didn’t see any pathways that were differentially expressed by statin use within the tumor tissue itself,” said Emma Allott, PhD, who presented the research at a poster session at the annual meeting of the American Urological Association.
An association between statin use and improved survival was first described in a 2006 study based on results from the Health Professionals Follow Up Study. Since then, “we’ve been generating molecular data on cancers that developed in statin users and nonusers,” said Dr. Allott of the University of North Carolina, Chapel Hill.
Of the 5,792 prostate cancer diagnoses, 17% were advanced cases, defined as stage T3b or more, having spread to lymph nodes or metastasized, or lethal; 13% were lethal, 46% were positive for the ERG oncogene, and 14% were PTEN-null. “Statin use was associated with a lower risk of PTEN-null prostate cancer, so that seems to drive some of the reduced association with lethal disease,” said Dr. Allott.
There was no association between lethality and ERG-positive or ERG-negative status. Those who used statins for more than 5 years were less likely to have a PTEN-null tumor (hazard ratio, 0.42; 95% confidence interval, 0.20-0.90) but not more likely to have a PTEN-positive tumor (HR, 1.18; 95% CI, 0.95-1.46).
Compared with never users, long-term statin users also were less likely to have advanced prostate cancer (multivariate analysis, HR, 0.62; 95% CI, 0.45-0.85) as well as lethal prostate cancer (HR, 0.52; 95% CI, 0.35-0.78).
The researchers conducted a gene set enrichment analysis in statin users and found an enrichment of T-cell, B-cell, and PI3K signaling in tumor-adjacent normal prostate tissue, as well as other changes. “We think maybe there’s a microenvironment inflammation component to the mechanism through which statins are associated with lower risk of lethal prostate cancer,” said Dr. Allott.
The molecular data could identify patient subgroups that could benefit from statins. Dr. Allott said that is the goal, but it will take time. “That’s more obviously translatable to the clinic, but we don’t yet have enough data in this cohort to look at that.”
SOURCE: Allott E et al. AUA 2018, Abstract MP21-01.
REPORTING FROM THE AUA ANNUAL MEETING
Key clinical point: Researchers hope that genetic analyses could eventually point to prostate cancer patients who might benefit from statin use.
Major finding: Long-term statin users had lower odds of having a pTEN-null tumor (hazard ratio, 0.42), which is associated with worse outcomes.
Study details: Retrospective analysis of 5,792 diagnoses of prostate cancer among 44,076 men.
Disclosures: The study was funded by the Irish Cancer Society, the John Fitzpatrick Fellowship, and the National Cancer Institute. Dr. Allott reported no relevant financial relationships.
Source: Allott E et al. AUA 2018, Abstract MP21-01.
Verma unveils Medicaid scorecard but refuses to judge efforts
“This is about bringing a level of transparency and accountability to the Medicaid program that we have never had before,” said Seema Verma, administrator of the Centers for Medicare & Medicaid Services.
Yet in a meeting with reporters, Ms. Verma refused to discuss the findings in any detail or comment on any individual states that performed poorly or exceptionally.
“I will let you look at the data and make your own conclusions,” she told journalists a few minutes before the report was posted online.
When reporters pressed Ms. Verma to comment on the document, she refused to give an assessment of the Medicaid program, the federal-state health program for low-income residents. She has run Medicaid for the past 15 months.
“The idea here is to give you a sense of where states are on different areas,” she said. “The idea is to be used for best practices,” and it’s “an opportunity for us to identify” and have discussions with states that aren’t performing well.
Medicaid covers about 75 million people, about half of them children.
The report looked at how well states provide a wide variety of health services to children and adults. It also reviewed how quickly the federal government was approving state waiver requests to change their programs.
But not all states provided data for each service because sharing information was voluntary.
For example, half the states did not show how well they control Medicaid enrollees’ blood pressure.
The National Association of Medicaid Directors panned the scorecard. It acknowledged the need for a system to measure performance but said its members have concerns about its accuracy and usefulness.
“There are significant methodological issues with the underlying data, including completeness, timeliness, and quality,” the association said in a statement. It noted that most of the data come from 2015.
As expected, the data showed great variation in how states provide care, including immunizing teenagers and getting dental care to children. A big reason is that state Medicaid benefits and payments to doctors vary dramatically, the Medicaid directors said, so that “it will not be possible to make apples-to-apples comparisons between states.”
In her first public speech, Ms. Verma promised last November to release a Medicaid scorecard. She said states won’t immediately face any consequences for poor performance – but that could change.
“The data … begins to offer taxpayers insights into how their dollars are being spent and the impact those dollars have on health outcomes,” Ms. Verma said on June 4.
Sara Rosenbaum, a professor of health law and policy at George Washington University in Washington, who previously led a congressional advisory board on Medicaid, suggested that the information is still too incomplete to be of great value.
“It is amazing to me that in 2018 this is all we have when trying to understand how the nation’s largest insurer performs for its poorest and most vulnerable residents,” she said.
KHN’s coverage of children’s health care issues is supported in part by the Heising-Simons Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
“This is about bringing a level of transparency and accountability to the Medicaid program that we have never had before,” said Seema Verma, administrator of the Centers for Medicare & Medicaid Services.
Yet in a meeting with reporters, Ms. Verma refused to discuss the findings in any detail or comment on any individual states that performed poorly or exceptionally.
“I will let you look at the data and make your own conclusions,” she told journalists a few minutes before the report was posted online.
When reporters pressed Ms. Verma to comment on the document, she refused to give an assessment of the Medicaid program, the federal-state health program for low-income residents. She has run Medicaid for the past 15 months.
“The idea here is to give you a sense of where states are on different areas,” she said. “The idea is to be used for best practices,” and it’s “an opportunity for us to identify” and have discussions with states that aren’t performing well.
Medicaid covers about 75 million people, about half of them children.
The report looked at how well states provide a wide variety of health services to children and adults. It also reviewed how quickly the federal government was approving state waiver requests to change their programs.
But not all states provided data for each service because sharing information was voluntary.
For example, half the states did not show how well they control Medicaid enrollees’ blood pressure.
The National Association of Medicaid Directors panned the scorecard. It acknowledged the need for a system to measure performance but said its members have concerns about its accuracy and usefulness.
“There are significant methodological issues with the underlying data, including completeness, timeliness, and quality,” the association said in a statement. It noted that most of the data come from 2015.
As expected, the data showed great variation in how states provide care, including immunizing teenagers and getting dental care to children. A big reason is that state Medicaid benefits and payments to doctors vary dramatically, the Medicaid directors said, so that “it will not be possible to make apples-to-apples comparisons between states.”
In her first public speech, Ms. Verma promised last November to release a Medicaid scorecard. She said states won’t immediately face any consequences for poor performance – but that could change.
“The data … begins to offer taxpayers insights into how their dollars are being spent and the impact those dollars have on health outcomes,” Ms. Verma said on June 4.
Sara Rosenbaum, a professor of health law and policy at George Washington University in Washington, who previously led a congressional advisory board on Medicaid, suggested that the information is still too incomplete to be of great value.
“It is amazing to me that in 2018 this is all we have when trying to understand how the nation’s largest insurer performs for its poorest and most vulnerable residents,” she said.
KHN’s coverage of children’s health care issues is supported in part by the Heising-Simons Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
“This is about bringing a level of transparency and accountability to the Medicaid program that we have never had before,” said Seema Verma, administrator of the Centers for Medicare & Medicaid Services.
Yet in a meeting with reporters, Ms. Verma refused to discuss the findings in any detail or comment on any individual states that performed poorly or exceptionally.
“I will let you look at the data and make your own conclusions,” she told journalists a few minutes before the report was posted online.
When reporters pressed Ms. Verma to comment on the document, she refused to give an assessment of the Medicaid program, the federal-state health program for low-income residents. She has run Medicaid for the past 15 months.
“The idea here is to give you a sense of where states are on different areas,” she said. “The idea is to be used for best practices,” and it’s “an opportunity for us to identify” and have discussions with states that aren’t performing well.
Medicaid covers about 75 million people, about half of them children.
The report looked at how well states provide a wide variety of health services to children and adults. It also reviewed how quickly the federal government was approving state waiver requests to change their programs.
But not all states provided data for each service because sharing information was voluntary.
For example, half the states did not show how well they control Medicaid enrollees’ blood pressure.
The National Association of Medicaid Directors panned the scorecard. It acknowledged the need for a system to measure performance but said its members have concerns about its accuracy and usefulness.
“There are significant methodological issues with the underlying data, including completeness, timeliness, and quality,” the association said in a statement. It noted that most of the data come from 2015.
As expected, the data showed great variation in how states provide care, including immunizing teenagers and getting dental care to children. A big reason is that state Medicaid benefits and payments to doctors vary dramatically, the Medicaid directors said, so that “it will not be possible to make apples-to-apples comparisons between states.”
In her first public speech, Ms. Verma promised last November to release a Medicaid scorecard. She said states won’t immediately face any consequences for poor performance – but that could change.
“The data … begins to offer taxpayers insights into how their dollars are being spent and the impact those dollars have on health outcomes,” Ms. Verma said on June 4.
Sara Rosenbaum, a professor of health law and policy at George Washington University in Washington, who previously led a congressional advisory board on Medicaid, suggested that the information is still too incomplete to be of great value.
“It is amazing to me that in 2018 this is all we have when trying to understand how the nation’s largest insurer performs for its poorest and most vulnerable residents,” she said.
KHN’s coverage of children’s health care issues is supported in part by the Heising-Simons Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
FDA approves first biosimilar to pegfilgrastim
to decrease the chance of infection in patients with nonmyeloid cancer who are receiving myelosuppressive chemotherapy and are at risk of febrile neutropenia.
The approval is based on structural and functional characterization, animal study data, human pharmacokinetic and pharmacodynamic data, clinical immunogenicity data, and other clinical safety and effectiveness data that demonstrates pegfilgrastim-jmdb is biosimilar to pegfilgrastim, the FDA said in a statement.
The FDA warns that “patients with a history of serious allergic reactions to human granulocyte colony–stimulating factors such as pegfilgrastim or filgrastim products should not take pegfilgrastim-jmdb.”
This approval is part of the FDA’s efforts to “help promote competition that can reduce drug costs and promote access,” FDA commissioner Scott Gottlieb, MD, said in the statement. “This summer, we’ll release a comprehensive new plan to advance new policy efforts that promote biosimilar product development. Biologics represent some of the most clinically important, but also costliest products that patients use to promote their health. We want to make sure that the pathway for developing biosimilar versions of approved biologics is efficient and effective, so that patients benefit from competition to existing biologics once lawful intellectual property has lapsed on these products.”
Pegfilgrastim-jmdb will be marketed as Fulphila by Mylan GmbH.
to decrease the chance of infection in patients with nonmyeloid cancer who are receiving myelosuppressive chemotherapy and are at risk of febrile neutropenia.
The approval is based on structural and functional characterization, animal study data, human pharmacokinetic and pharmacodynamic data, clinical immunogenicity data, and other clinical safety and effectiveness data that demonstrates pegfilgrastim-jmdb is biosimilar to pegfilgrastim, the FDA said in a statement.
The FDA warns that “patients with a history of serious allergic reactions to human granulocyte colony–stimulating factors such as pegfilgrastim or filgrastim products should not take pegfilgrastim-jmdb.”
This approval is part of the FDA’s efforts to “help promote competition that can reduce drug costs and promote access,” FDA commissioner Scott Gottlieb, MD, said in the statement. “This summer, we’ll release a comprehensive new plan to advance new policy efforts that promote biosimilar product development. Biologics represent some of the most clinically important, but also costliest products that patients use to promote their health. We want to make sure that the pathway for developing biosimilar versions of approved biologics is efficient and effective, so that patients benefit from competition to existing biologics once lawful intellectual property has lapsed on these products.”
Pegfilgrastim-jmdb will be marketed as Fulphila by Mylan GmbH.
to decrease the chance of infection in patients with nonmyeloid cancer who are receiving myelosuppressive chemotherapy and are at risk of febrile neutropenia.
The approval is based on structural and functional characterization, animal study data, human pharmacokinetic and pharmacodynamic data, clinical immunogenicity data, and other clinical safety and effectiveness data that demonstrates pegfilgrastim-jmdb is biosimilar to pegfilgrastim, the FDA said in a statement.
The FDA warns that “patients with a history of serious allergic reactions to human granulocyte colony–stimulating factors such as pegfilgrastim or filgrastim products should not take pegfilgrastim-jmdb.”
This approval is part of the FDA’s efforts to “help promote competition that can reduce drug costs and promote access,” FDA commissioner Scott Gottlieb, MD, said in the statement. “This summer, we’ll release a comprehensive new plan to advance new policy efforts that promote biosimilar product development. Biologics represent some of the most clinically important, but also costliest products that patients use to promote their health. We want to make sure that the pathway for developing biosimilar versions of approved biologics is efficient and effective, so that patients benefit from competition to existing biologics once lawful intellectual property has lapsed on these products.”
Pegfilgrastim-jmdb will be marketed as Fulphila by Mylan GmbH.
TAVR for low-risk patients shines at 6 years in NOTION
PARIS – Follow-up data from a randomized trial of transcatheter versus surgical aortic valve replacement in low-surgical-risk patients with symptomatic severe aortic stenosis showed sustained superior hemodynamic valve performance and less structural valve deterioration in the transcatheter group through 6 years, Lars Sondergaard, MD, reported at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
Moreover, the rate of bioprosthetic valve failure as formally defined in a recent European consensus statement (Eur Heart J. 2017 Dec 1;38[45]:3382-90) was similarly low in the transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR) arms at about 7%, in the randomized study known as NOTION (Nordic Aortic Valve Intervention), added Dr. Sondergaard, professor of cardiology at the University of Copenhagen, who was a coauthor of the consensus statement.
“As we look to expand TAVR to younger patients with longer life expectancy, durability, of course, becomes much more important,” the cardiologist observed.
NOTION was a pioneering prospective, multicenter, nonblinded, randomized trial of first-generation TAVR technology. The 280 participants, average age 79 years, were truly a low-surgical-risk population, with a mean Society of Thoracic Surgeons risk score of 3%.
NOTION is a small trial, but the results at 6 years of a planned 10-year follow-up augur well for TAVR success in the large, ongoing, definitive, randomized trials of TAVR versus SAVR in low-risk patients. That’s because NOTION participants were treated in 2009-2013, when the self-expanding TAVR CoreValve was implanted on the basis of aortic annulus measurements obtained via echocardiography, which is considerably less accurate than CT, the standard practice today. For this reason, it’s highly unlikely that the larger, ongoing trials, including PARTNER 3 and the Medtronic Evolut Transcatheter Aortic Valve Replacement in Low Risk Patients trial, will experience moderate paravalvular leak rates anything like the 20.9% rate seen in the TAVR group in NOTION, where the SAVR group’s rate was just 1.5%.
“I’m sure quite a few of the NOTION patients would have a larger TAVR valve prosthesis if they were treated today,” according to Dr. Sondergaard.
Valve function
The rate of moderate hemodynamic structural valve deterioration through 6 years of follow-up was 3.6% in the TAVR arm, compared with 23.7% in the SAVR group. The rate of nonstructural valve deterioration was 54.0% with TAVR and 57.8% with SAVR, and there were no cases of moderate or severe aortic regurgitation in either group.
The mean aortic valve gradient in the TAVR group went from 44.9 mm Hg at baseline to 12.2 mm Hg at 3 months and to 14.7 mm Hg at 6 years. In the SAVR group, the figures were 44.9 mm Hg, 8.3 mm Hg, and 9.9 mm Hg, respectively.
The effective orifice area in the TAVR group improved from 0.74 cm2 at baseline to 1.37 cm2 at 3 months and to 1.16 cm2 at 6 years. With SAVR, the effective orifice area was 0.74 cm2 at baseline, 1.66 cm2 at 3 months, and 1.53 cm2 at 6 years.
Clinical outcomes
Through 6 years of follow-up, there were no cases of thrombosis in either study arm, and the rate of endocarditis was just under 6% in each group through 6 years. All-cause mortality through 6 years was 42.5% in the TAVR group, not significantly different from the 37.7% rate in the SAVR arm.
The valve-related death rate was 5.0% in the TAVR arm and similar at 3.7% with SAVR. Reintervention occurred in 2.2% of TAVR patients and in 0.7% of SAVR patients. Severe hemodynamic structural valve deterioration was documented in 0.7% of TAVR patients and 3.0% of SAVR patients. The overall rate of bioprosthetic valve failure – a composite of these three endpoints – was 7.5% with TAVR and similar at 6.7% with SAVR.
Session cochair Alain G. Cribier, MD, a TAVR pioneer who is professor of medicine and director of cardiology at Charles Nicolle Hospital, University of Rouen (France), declared, “This is extremely encouraging.”
However, discussant Corrado Tamburino, MD, was more circumspect.
“If you look, there’s a trend for increased all-cause mortality in the TAVR versus SAVR group at 6 years. This could be related to the big difference in paravalvular leak. Do you think paravalvular leak could have a real impact on mortality?” asked Dr. Tamburino, professor of cardiology at the University of Catania, Italy.
Not in the NOTION study, where they looked for but didn’t find any such association, according to Dr. Sondergaard.
“If you look at the survival curves from the beginning out to 6 years, you’ll see that the lines cross each other several times, so this small difference right now could just be random. I don’t think we can say there’s a higher mortality rate with TAVR for the time being,” he added.
The NOTION trial was funded by the Danish Heart Foundation. Dr. Sondergaard reported receiving research grants from and/or serving as a consultant to Abbott, Boston Scientific, Edwards Lifesciences, Medtronic, and Symetis.
PARIS – Follow-up data from a randomized trial of transcatheter versus surgical aortic valve replacement in low-surgical-risk patients with symptomatic severe aortic stenosis showed sustained superior hemodynamic valve performance and less structural valve deterioration in the transcatheter group through 6 years, Lars Sondergaard, MD, reported at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
Moreover, the rate of bioprosthetic valve failure as formally defined in a recent European consensus statement (Eur Heart J. 2017 Dec 1;38[45]:3382-90) was similarly low in the transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR) arms at about 7%, in the randomized study known as NOTION (Nordic Aortic Valve Intervention), added Dr. Sondergaard, professor of cardiology at the University of Copenhagen, who was a coauthor of the consensus statement.
“As we look to expand TAVR to younger patients with longer life expectancy, durability, of course, becomes much more important,” the cardiologist observed.
NOTION was a pioneering prospective, multicenter, nonblinded, randomized trial of first-generation TAVR technology. The 280 participants, average age 79 years, were truly a low-surgical-risk population, with a mean Society of Thoracic Surgeons risk score of 3%.
NOTION is a small trial, but the results at 6 years of a planned 10-year follow-up augur well for TAVR success in the large, ongoing, definitive, randomized trials of TAVR versus SAVR in low-risk patients. That’s because NOTION participants were treated in 2009-2013, when the self-expanding TAVR CoreValve was implanted on the basis of aortic annulus measurements obtained via echocardiography, which is considerably less accurate than CT, the standard practice today. For this reason, it’s highly unlikely that the larger, ongoing trials, including PARTNER 3 and the Medtronic Evolut Transcatheter Aortic Valve Replacement in Low Risk Patients trial, will experience moderate paravalvular leak rates anything like the 20.9% rate seen in the TAVR group in NOTION, where the SAVR group’s rate was just 1.5%.
“I’m sure quite a few of the NOTION patients would have a larger TAVR valve prosthesis if they were treated today,” according to Dr. Sondergaard.
Valve function
The rate of moderate hemodynamic structural valve deterioration through 6 years of follow-up was 3.6% in the TAVR arm, compared with 23.7% in the SAVR group. The rate of nonstructural valve deterioration was 54.0% with TAVR and 57.8% with SAVR, and there were no cases of moderate or severe aortic regurgitation in either group.
The mean aortic valve gradient in the TAVR group went from 44.9 mm Hg at baseline to 12.2 mm Hg at 3 months and to 14.7 mm Hg at 6 years. In the SAVR group, the figures were 44.9 mm Hg, 8.3 mm Hg, and 9.9 mm Hg, respectively.
The effective orifice area in the TAVR group improved from 0.74 cm2 at baseline to 1.37 cm2 at 3 months and to 1.16 cm2 at 6 years. With SAVR, the effective orifice area was 0.74 cm2 at baseline, 1.66 cm2 at 3 months, and 1.53 cm2 at 6 years.
Clinical outcomes
Through 6 years of follow-up, there were no cases of thrombosis in either study arm, and the rate of endocarditis was just under 6% in each group through 6 years. All-cause mortality through 6 years was 42.5% in the TAVR group, not significantly different from the 37.7% rate in the SAVR arm.
The valve-related death rate was 5.0% in the TAVR arm and similar at 3.7% with SAVR. Reintervention occurred in 2.2% of TAVR patients and in 0.7% of SAVR patients. Severe hemodynamic structural valve deterioration was documented in 0.7% of TAVR patients and 3.0% of SAVR patients. The overall rate of bioprosthetic valve failure – a composite of these three endpoints – was 7.5% with TAVR and similar at 6.7% with SAVR.
Session cochair Alain G. Cribier, MD, a TAVR pioneer who is professor of medicine and director of cardiology at Charles Nicolle Hospital, University of Rouen (France), declared, “This is extremely encouraging.”
However, discussant Corrado Tamburino, MD, was more circumspect.
“If you look, there’s a trend for increased all-cause mortality in the TAVR versus SAVR group at 6 years. This could be related to the big difference in paravalvular leak. Do you think paravalvular leak could have a real impact on mortality?” asked Dr. Tamburino, professor of cardiology at the University of Catania, Italy.
Not in the NOTION study, where they looked for but didn’t find any such association, according to Dr. Sondergaard.
“If you look at the survival curves from the beginning out to 6 years, you’ll see that the lines cross each other several times, so this small difference right now could just be random. I don’t think we can say there’s a higher mortality rate with TAVR for the time being,” he added.
The NOTION trial was funded by the Danish Heart Foundation. Dr. Sondergaard reported receiving research grants from and/or serving as a consultant to Abbott, Boston Scientific, Edwards Lifesciences, Medtronic, and Symetis.
PARIS – Follow-up data from a randomized trial of transcatheter versus surgical aortic valve replacement in low-surgical-risk patients with symptomatic severe aortic stenosis showed sustained superior hemodynamic valve performance and less structural valve deterioration in the transcatheter group through 6 years, Lars Sondergaard, MD, reported at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
Moreover, the rate of bioprosthetic valve failure as formally defined in a recent European consensus statement (Eur Heart J. 2017 Dec 1;38[45]:3382-90) was similarly low in the transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR) arms at about 7%, in the randomized study known as NOTION (Nordic Aortic Valve Intervention), added Dr. Sondergaard, professor of cardiology at the University of Copenhagen, who was a coauthor of the consensus statement.
“As we look to expand TAVR to younger patients with longer life expectancy, durability, of course, becomes much more important,” the cardiologist observed.
NOTION was a pioneering prospective, multicenter, nonblinded, randomized trial of first-generation TAVR technology. The 280 participants, average age 79 years, were truly a low-surgical-risk population, with a mean Society of Thoracic Surgeons risk score of 3%.
NOTION is a small trial, but the results at 6 years of a planned 10-year follow-up augur well for TAVR success in the large, ongoing, definitive, randomized trials of TAVR versus SAVR in low-risk patients. That’s because NOTION participants were treated in 2009-2013, when the self-expanding TAVR CoreValve was implanted on the basis of aortic annulus measurements obtained via echocardiography, which is considerably less accurate than CT, the standard practice today. For this reason, it’s highly unlikely that the larger, ongoing trials, including PARTNER 3 and the Medtronic Evolut Transcatheter Aortic Valve Replacement in Low Risk Patients trial, will experience moderate paravalvular leak rates anything like the 20.9% rate seen in the TAVR group in NOTION, where the SAVR group’s rate was just 1.5%.
“I’m sure quite a few of the NOTION patients would have a larger TAVR valve prosthesis if they were treated today,” according to Dr. Sondergaard.
Valve function
The rate of moderate hemodynamic structural valve deterioration through 6 years of follow-up was 3.6% in the TAVR arm, compared with 23.7% in the SAVR group. The rate of nonstructural valve deterioration was 54.0% with TAVR and 57.8% with SAVR, and there were no cases of moderate or severe aortic regurgitation in either group.
The mean aortic valve gradient in the TAVR group went from 44.9 mm Hg at baseline to 12.2 mm Hg at 3 months and to 14.7 mm Hg at 6 years. In the SAVR group, the figures were 44.9 mm Hg, 8.3 mm Hg, and 9.9 mm Hg, respectively.
The effective orifice area in the TAVR group improved from 0.74 cm2 at baseline to 1.37 cm2 at 3 months and to 1.16 cm2 at 6 years. With SAVR, the effective orifice area was 0.74 cm2 at baseline, 1.66 cm2 at 3 months, and 1.53 cm2 at 6 years.
Clinical outcomes
Through 6 years of follow-up, there were no cases of thrombosis in either study arm, and the rate of endocarditis was just under 6% in each group through 6 years. All-cause mortality through 6 years was 42.5% in the TAVR group, not significantly different from the 37.7% rate in the SAVR arm.
The valve-related death rate was 5.0% in the TAVR arm and similar at 3.7% with SAVR. Reintervention occurred in 2.2% of TAVR patients and in 0.7% of SAVR patients. Severe hemodynamic structural valve deterioration was documented in 0.7% of TAVR patients and 3.0% of SAVR patients. The overall rate of bioprosthetic valve failure – a composite of these three endpoints – was 7.5% with TAVR and similar at 6.7% with SAVR.
Session cochair Alain G. Cribier, MD, a TAVR pioneer who is professor of medicine and director of cardiology at Charles Nicolle Hospital, University of Rouen (France), declared, “This is extremely encouraging.”
However, discussant Corrado Tamburino, MD, was more circumspect.
“If you look, there’s a trend for increased all-cause mortality in the TAVR versus SAVR group at 6 years. This could be related to the big difference in paravalvular leak. Do you think paravalvular leak could have a real impact on mortality?” asked Dr. Tamburino, professor of cardiology at the University of Catania, Italy.
Not in the NOTION study, where they looked for but didn’t find any such association, according to Dr. Sondergaard.
“If you look at the survival curves from the beginning out to 6 years, you’ll see that the lines cross each other several times, so this small difference right now could just be random. I don’t think we can say there’s a higher mortality rate with TAVR for the time being,” he added.
The NOTION trial was funded by the Danish Heart Foundation. Dr. Sondergaard reported receiving research grants from and/or serving as a consultant to Abbott, Boston Scientific, Edwards Lifesciences, Medtronic, and Symetis.
REPORTING FROM EUROPCR 2018
Key clinical point: TAVR provided superior hemodynamic valve performance and less structural valve deterioration than SAVR in low-surgical-risk patients through 6 years of follow-up.
Major finding: The rate of moderate hemodynamic structural valve deterioration through 6 years was 3.6% with TAVR and 23.7% with SAVR.
Study details: An analysis of 6-year follow-up data from NOTION, a prospective, multicenter, randomized trial in 280 low-surgical-risk patients.
Disclosures: NOTION was funded by the Danish Heart Foundation. The presenter reported receiving research grants from and/or serving as a consultant to several medical device companies.
AMA: Opioid prescriptions down since 2013
Opioid prescriptions dropped by 22% over the last 5 years, with decreases seen in all 50 states, according to a new report from the American Medical Association’s opioid task force.
“The largest decrease in opioid prescriptions in 25 years reflects the fact that physicians and other health care professionals are increasingly judicious when prescribing opioids,” Patrice A. Harris, MD, chair of the task force, said in the report.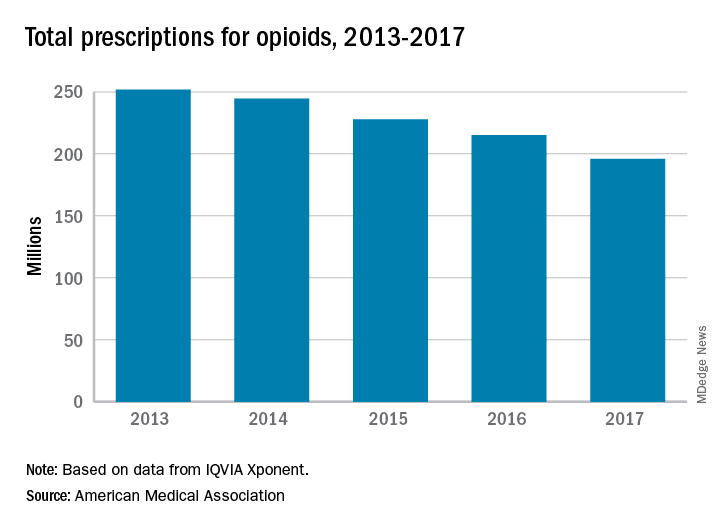
Other signs of progress against the opioid epidemic include the increasing number of health care professionals registered for prescription drug monitoring programs: It has more than tripled, from 472,000 in 2014 to 1.55 million in 2017. Furthermore, the number of naloxone prescriptions in 2017 had more than doubled, from about 3,500 per week to 8,000, the task force reported.
“Unfortunately, deaths related to heroin and illicit fentanyl, and to prescription opioids, continue to rise. We need well-designed initiatives that bring together public and private insurers, policymakers, public health infrastructure, and communities with the shared goal to improve access and coverage for comprehensive pain management and treatment for substance use disorders,” Dr. Harris said.
Opioid prescriptions dropped by 22% over the last 5 years, with decreases seen in all 50 states, according to a new report from the American Medical Association’s opioid task force.
“The largest decrease in opioid prescriptions in 25 years reflects the fact that physicians and other health care professionals are increasingly judicious when prescribing opioids,” Patrice A. Harris, MD, chair of the task force, said in the report.
Other signs of progress against the opioid epidemic include the increasing number of health care professionals registered for prescription drug monitoring programs: It has more than tripled, from 472,000 in 2014 to 1.55 million in 2017. Furthermore, the number of naloxone prescriptions in 2017 had more than doubled, from about 3,500 per week to 8,000, the task force reported.
“Unfortunately, deaths related to heroin and illicit fentanyl, and to prescription opioids, continue to rise. We need well-designed initiatives that bring together public and private insurers, policymakers, public health infrastructure, and communities with the shared goal to improve access and coverage for comprehensive pain management and treatment for substance use disorders,” Dr. Harris said.
Opioid prescriptions dropped by 22% over the last 5 years, with decreases seen in all 50 states, according to a new report from the American Medical Association’s opioid task force.
“The largest decrease in opioid prescriptions in 25 years reflects the fact that physicians and other health care professionals are increasingly judicious when prescribing opioids,” Patrice A. Harris, MD, chair of the task force, said in the report.
Other signs of progress against the opioid epidemic include the increasing number of health care professionals registered for prescription drug monitoring programs: It has more than tripled, from 472,000 in 2014 to 1.55 million in 2017. Furthermore, the number of naloxone prescriptions in 2017 had more than doubled, from about 3,500 per week to 8,000, the task force reported.
“Unfortunately, deaths related to heroin and illicit fentanyl, and to prescription opioids, continue to rise. We need well-designed initiatives that bring together public and private insurers, policymakers, public health infrastructure, and communities with the shared goal to improve access and coverage for comprehensive pain management and treatment for substance use disorders,” Dr. Harris said.
Checkpoint inhibitor shows promise in advanced squamous-cell carcinoma
An immune checkpoint inhibitor that targets the PD-1 receptor has shown “robust” efficacy among patients with advanced cutaneous squamous-cell carcinoma, according to researchers.
A combined phase 1/phase 2 study, published in the New England Journal of Medicine and presented simultaneously at the annual meeting of the American Society of Clinical Oncology, looked at the effect of monoclonal antibody cemiplimab in an expansion cohort of 26 patients with locally-advanced or metastatic cutaneous squamous-cell carcinoma who were not eligible for surgery. The phase 2 component involved 59 patients with metastatic disease.
Patients were treated with intravenous cemiplimab every 2 weeks for 48 weeks in the phase 1 study, and up to 96 weeks – or until unacceptable toxicity or disease progression – in the phase 2 study.
In the phase 1 study, researchers saw a response rate of 50% and a 65% rate of durable disease control, after a median follow-up of 11 months (1.1-17). The median time to response was 2.3 months, and more than half the patients (54%) who showed a response maintained that response past 6 months.
In the phase 2 study in patients with metastatic disease, 47% responded to the treatment – 24 patients showed a partial response and 4 showed a complete response. Of those who responded, 61% showed durable disease control after a median follow-up of 7.9 months.
The median time to response in this group of patients was 1.9 months, and 57% of those who did respond still showed a response at 6 months. However neither median progression-free survival nor median overall survival had been reached at the point of data cut-off.
The treatment showed similar effects in patients with regional and distant metastatic disease.
Advanced cutaneous squamous-cell carcinoma was thought to be an ideal target for immunotherapy because the high mutation burden in the tumor meant it would be sensitive to effector T cell attack, wrote Michael R. Migden, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his coauthors.
“In addition, the dramatically increased risk of cutaneous squamous-cell carcinoma among people with immunosuppression pointed to an important role for immune surveillance with this cancer,” the authors wrote.
In the phase 2 study, 29% of patients experienced a serious adverse event – including two cases of pneumonitis – and three patients (5%) discontinued treatment. There were three deaths due to adverse events: One patient died from pneumonia complications, one died in his sleep, and one patient died following hypercalcemia and deep vein thrombosis.
Aside from these, most adverse events were grade 1 or 2. Around one-quarter of patients experienced diarrhea (27%) or fatigue (24%), while the other most common adverse events were nausea (17%), constipation (15%) and rash (15%). The authors noted that these adverse events were similar to those seen in other PD-1 inhibitors.
“Our results are consistent with an emerging theme regarding the high efficacy of immune checkpoint blockade for the treatment of hypermutated cancers, since the mutation burden of cutaneous squamous-cell carcinoma is similar to that reported for advanced solid tumors with microsatellite instability,” the authors wrote.
Cemiplimab is now being tested in a phase 2 trial in patients with advanced basal cell carcinoma.
The study was supported by Regeneron Pharmaceuticals and Sanofi. Eight authors declared funding from Regeneron to conduct the trial. Ten authors were employees of Regeneron. Fifteen authors also declared funding and payments from pharmaceutical companies outside the submitted work. Four had nothing to disclose.
SOURCE: Migden M et al. NEJM, 2018; June 4. doi: 10.1056/NEJMoa1805131.
An immune checkpoint inhibitor that targets the PD-1 receptor has shown “robust” efficacy among patients with advanced cutaneous squamous-cell carcinoma, according to researchers.
A combined phase 1/phase 2 study, published in the New England Journal of Medicine and presented simultaneously at the annual meeting of the American Society of Clinical Oncology, looked at the effect of monoclonal antibody cemiplimab in an expansion cohort of 26 patients with locally-advanced or metastatic cutaneous squamous-cell carcinoma who were not eligible for surgery. The phase 2 component involved 59 patients with metastatic disease.
Patients were treated with intravenous cemiplimab every 2 weeks for 48 weeks in the phase 1 study, and up to 96 weeks – or until unacceptable toxicity or disease progression – in the phase 2 study.
In the phase 1 study, researchers saw a response rate of 50% and a 65% rate of durable disease control, after a median follow-up of 11 months (1.1-17). The median time to response was 2.3 months, and more than half the patients (54%) who showed a response maintained that response past 6 months.
In the phase 2 study in patients with metastatic disease, 47% responded to the treatment – 24 patients showed a partial response and 4 showed a complete response. Of those who responded, 61% showed durable disease control after a median follow-up of 7.9 months.
The median time to response in this group of patients was 1.9 months, and 57% of those who did respond still showed a response at 6 months. However neither median progression-free survival nor median overall survival had been reached at the point of data cut-off.
The treatment showed similar effects in patients with regional and distant metastatic disease.
Advanced cutaneous squamous-cell carcinoma was thought to be an ideal target for immunotherapy because the high mutation burden in the tumor meant it would be sensitive to effector T cell attack, wrote Michael R. Migden, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his coauthors.
“In addition, the dramatically increased risk of cutaneous squamous-cell carcinoma among people with immunosuppression pointed to an important role for immune surveillance with this cancer,” the authors wrote.
In the phase 2 study, 29% of patients experienced a serious adverse event – including two cases of pneumonitis – and three patients (5%) discontinued treatment. There were three deaths due to adverse events: One patient died from pneumonia complications, one died in his sleep, and one patient died following hypercalcemia and deep vein thrombosis.
Aside from these, most adverse events were grade 1 or 2. Around one-quarter of patients experienced diarrhea (27%) or fatigue (24%), while the other most common adverse events were nausea (17%), constipation (15%) and rash (15%). The authors noted that these adverse events were similar to those seen in other PD-1 inhibitors.
“Our results are consistent with an emerging theme regarding the high efficacy of immune checkpoint blockade for the treatment of hypermutated cancers, since the mutation burden of cutaneous squamous-cell carcinoma is similar to that reported for advanced solid tumors with microsatellite instability,” the authors wrote.
Cemiplimab is now being tested in a phase 2 trial in patients with advanced basal cell carcinoma.
The study was supported by Regeneron Pharmaceuticals and Sanofi. Eight authors declared funding from Regeneron to conduct the trial. Ten authors were employees of Regeneron. Fifteen authors also declared funding and payments from pharmaceutical companies outside the submitted work. Four had nothing to disclose.
SOURCE: Migden M et al. NEJM, 2018; June 4. doi: 10.1056/NEJMoa1805131.
An immune checkpoint inhibitor that targets the PD-1 receptor has shown “robust” efficacy among patients with advanced cutaneous squamous-cell carcinoma, according to researchers.
A combined phase 1/phase 2 study, published in the New England Journal of Medicine and presented simultaneously at the annual meeting of the American Society of Clinical Oncology, looked at the effect of monoclonal antibody cemiplimab in an expansion cohort of 26 patients with locally-advanced or metastatic cutaneous squamous-cell carcinoma who were not eligible for surgery. The phase 2 component involved 59 patients with metastatic disease.
Patients were treated with intravenous cemiplimab every 2 weeks for 48 weeks in the phase 1 study, and up to 96 weeks – or until unacceptable toxicity or disease progression – in the phase 2 study.
In the phase 1 study, researchers saw a response rate of 50% and a 65% rate of durable disease control, after a median follow-up of 11 months (1.1-17). The median time to response was 2.3 months, and more than half the patients (54%) who showed a response maintained that response past 6 months.
In the phase 2 study in patients with metastatic disease, 47% responded to the treatment – 24 patients showed a partial response and 4 showed a complete response. Of those who responded, 61% showed durable disease control after a median follow-up of 7.9 months.
The median time to response in this group of patients was 1.9 months, and 57% of those who did respond still showed a response at 6 months. However neither median progression-free survival nor median overall survival had been reached at the point of data cut-off.
The treatment showed similar effects in patients with regional and distant metastatic disease.
Advanced cutaneous squamous-cell carcinoma was thought to be an ideal target for immunotherapy because the high mutation burden in the tumor meant it would be sensitive to effector T cell attack, wrote Michael R. Migden, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his coauthors.
“In addition, the dramatically increased risk of cutaneous squamous-cell carcinoma among people with immunosuppression pointed to an important role for immune surveillance with this cancer,” the authors wrote.
In the phase 2 study, 29% of patients experienced a serious adverse event – including two cases of pneumonitis – and three patients (5%) discontinued treatment. There were three deaths due to adverse events: One patient died from pneumonia complications, one died in his sleep, and one patient died following hypercalcemia and deep vein thrombosis.
Aside from these, most adverse events were grade 1 or 2. Around one-quarter of patients experienced diarrhea (27%) or fatigue (24%), while the other most common adverse events were nausea (17%), constipation (15%) and rash (15%). The authors noted that these adverse events were similar to those seen in other PD-1 inhibitors.
“Our results are consistent with an emerging theme regarding the high efficacy of immune checkpoint blockade for the treatment of hypermutated cancers, since the mutation burden of cutaneous squamous-cell carcinoma is similar to that reported for advanced solid tumors with microsatellite instability,” the authors wrote.
Cemiplimab is now being tested in a phase 2 trial in patients with advanced basal cell carcinoma.
The study was supported by Regeneron Pharmaceuticals and Sanofi. Eight authors declared funding from Regeneron to conduct the trial. Ten authors were employees of Regeneron. Fifteen authors also declared funding and payments from pharmaceutical companies outside the submitted work. Four had nothing to disclose.
SOURCE: Migden M et al. NEJM, 2018; June 4. doi: 10.1056/NEJMoa1805131.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: PD-1 inhibitor cemiplimab shows significant response in advanced squamous-cell carcinoma.
Major finding: Around half of patients with advanced squamous-cell carcinoma responded to checkpoint inhibitor cemiplimab.
Study details: Phase 1 expanded cohort study of 26 patients with advanced cutaneous squamous-cell carcinoma and phase 2 study of 59 patients with metastatic squamous-cell carcinoma.
Disclosures: The study was supported by Regeneron Pharmaceuticals and Sanofi. Eight authors declared funding from Regeneron to conduct the trial. Ten authors were employees of Regeneron. Fifteen authors declared funding and payments from pharmaceutical companies outside the submitted work. Four had nothing to disclose.
Source: Migden M et al. N Engl J Med. 2018 June 4. doi: 10.1056/NEJMoa1805131.
FDA alerts clinicians to gastric balloon deaths
according to an alert from the Food and Drug Administration issued on June 4.
Seven of these deaths occurred in patients in the United States; four involved the ORBERA Intragastric Balloon System, and three involved the ReShape Integrated Dual Balloon System.
The FDA has approved updated labeling for the ORBERA and ReShape balloon systems in the United States. The labels contain more information about possible death associated with the use of these devices in the United States. The manufacturers’ sites, Apollo Endosurgery and ReShape Lifesciences, provide more details about the new labeling.
In a letter to health care providers, the FDA advised clinicians to educate bariatric surgery patients about the symptoms of complications from balloon procedures, including not only gastric perforation but also esophageal perforation, balloon deflation, gastrointestinal obstruction, and ulceration. In addition, the FDA reminded clinicians to monitor patients during the entire course of treatment for additional complications, including acute pancreatitis and spontaneous hyperinflation.
Any adverse events involving intragastric balloon systems should be reported to the FDA through MedWatch, the FDA Safety Information and Adverse Event Reporting program.
according to an alert from the Food and Drug Administration issued on June 4.
Seven of these deaths occurred in patients in the United States; four involved the ORBERA Intragastric Balloon System, and three involved the ReShape Integrated Dual Balloon System.
The FDA has approved updated labeling for the ORBERA and ReShape balloon systems in the United States. The labels contain more information about possible death associated with the use of these devices in the United States. The manufacturers’ sites, Apollo Endosurgery and ReShape Lifesciences, provide more details about the new labeling.
In a letter to health care providers, the FDA advised clinicians to educate bariatric surgery patients about the symptoms of complications from balloon procedures, including not only gastric perforation but also esophageal perforation, balloon deflation, gastrointestinal obstruction, and ulceration. In addition, the FDA reminded clinicians to monitor patients during the entire course of treatment for additional complications, including acute pancreatitis and spontaneous hyperinflation.
Any adverse events involving intragastric balloon systems should be reported to the FDA through MedWatch, the FDA Safety Information and Adverse Event Reporting program.
according to an alert from the Food and Drug Administration issued on June 4.
Seven of these deaths occurred in patients in the United States; four involved the ORBERA Intragastric Balloon System, and three involved the ReShape Integrated Dual Balloon System.
The FDA has approved updated labeling for the ORBERA and ReShape balloon systems in the United States. The labels contain more information about possible death associated with the use of these devices in the United States. The manufacturers’ sites, Apollo Endosurgery and ReShape Lifesciences, provide more details about the new labeling.
In a letter to health care providers, the FDA advised clinicians to educate bariatric surgery patients about the symptoms of complications from balloon procedures, including not only gastric perforation but also esophageal perforation, balloon deflation, gastrointestinal obstruction, and ulceration. In addition, the FDA reminded clinicians to monitor patients during the entire course of treatment for additional complications, including acute pancreatitis and spontaneous hyperinflation.
Any adverse events involving intragastric balloon systems should be reported to the FDA through MedWatch, the FDA Safety Information and Adverse Event Reporting program.






