User login
Marijuana’s perceived approval ratings on the rise
Parents’ disapproval of marijuana use has dropped since 1979 – at least that’s what their teenage children say, according to results of the 2017 Monitoring the Future survey.
The approximately 13,500 12th graders involved in the 2017 survey believe that their parents are much less likely to disapprove of marijuana use, compared with the students who responded to the survey during 1976-1979. At that time, 15% of the 12th graders said that their parents would not disapprove of using marijuana once or twice, but by 2017 the number had made a statistically significant rise to 23%, Richard A. Miech, PhD, and his associates said in their report on the 2017 survey.
Perceived approval of occasional marijuana use, which had garnered only an 8% share of respondents in 1976-1979, was up to a significantly higher 17% in 2017, and regular use went from 4% to 13%, said Dr. Miech and his associates, of the University of Michigan Institute for Social Research, Ann Arbor.
Parents’ increased acceptance of marijuana, as perceived by the 12th-grade students, was not matched for other substances. Disapproval for smoking one or more packs of cigarettes a day, for example, climbed from 89% in 1976-1979 to 92% in 2017, while disapproval of weekend binge drinking rose just a bit, going from 85% to 86%, they said.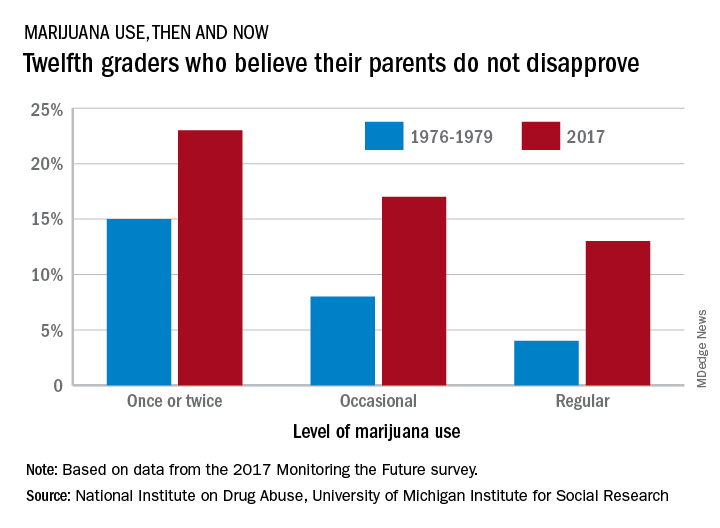
Measures of parental disapproval were reintroduced into the survey in 2017 after being removed in 1979 – the survey began in 1975 – “because students’ responses varied little over time and across drugs,” Dr. Miech and his associates noted. “Today’s parents of 12th graders have more experience with drug use than did parents in the late 1970s [and] population attitudes toward marijuana use across all ages are becoming more lenient,” they wrote.
The 2017 edition of the annual survey, which is funded by the National Institute on Drug Abuse, is based on reports from almost 44,000 students in 8th, 10th, and 12th grade in 360 public and private secondary schools across the country.
Parents’ disapproval of marijuana use has dropped since 1979 – at least that’s what their teenage children say, according to results of the 2017 Monitoring the Future survey.
The approximately 13,500 12th graders involved in the 2017 survey believe that their parents are much less likely to disapprove of marijuana use, compared with the students who responded to the survey during 1976-1979. At that time, 15% of the 12th graders said that their parents would not disapprove of using marijuana once or twice, but by 2017 the number had made a statistically significant rise to 23%, Richard A. Miech, PhD, and his associates said in their report on the 2017 survey.
Perceived approval of occasional marijuana use, which had garnered only an 8% share of respondents in 1976-1979, was up to a significantly higher 17% in 2017, and regular use went from 4% to 13%, said Dr. Miech and his associates, of the University of Michigan Institute for Social Research, Ann Arbor.
Parents’ increased acceptance of marijuana, as perceived by the 12th-grade students, was not matched for other substances. Disapproval for smoking one or more packs of cigarettes a day, for example, climbed from 89% in 1976-1979 to 92% in 2017, while disapproval of weekend binge drinking rose just a bit, going from 85% to 86%, they said.
Measures of parental disapproval were reintroduced into the survey in 2017 after being removed in 1979 – the survey began in 1975 – “because students’ responses varied little over time and across drugs,” Dr. Miech and his associates noted. “Today’s parents of 12th graders have more experience with drug use than did parents in the late 1970s [and] population attitudes toward marijuana use across all ages are becoming more lenient,” they wrote.
The 2017 edition of the annual survey, which is funded by the National Institute on Drug Abuse, is based on reports from almost 44,000 students in 8th, 10th, and 12th grade in 360 public and private secondary schools across the country.
Parents’ disapproval of marijuana use has dropped since 1979 – at least that’s what their teenage children say, according to results of the 2017 Monitoring the Future survey.
The approximately 13,500 12th graders involved in the 2017 survey believe that their parents are much less likely to disapprove of marijuana use, compared with the students who responded to the survey during 1976-1979. At that time, 15% of the 12th graders said that their parents would not disapprove of using marijuana once or twice, but by 2017 the number had made a statistically significant rise to 23%, Richard A. Miech, PhD, and his associates said in their report on the 2017 survey.
Perceived approval of occasional marijuana use, which had garnered only an 8% share of respondents in 1976-1979, was up to a significantly higher 17% in 2017, and regular use went from 4% to 13%, said Dr. Miech and his associates, of the University of Michigan Institute for Social Research, Ann Arbor.
Parents’ increased acceptance of marijuana, as perceived by the 12th-grade students, was not matched for other substances. Disapproval for smoking one or more packs of cigarettes a day, for example, climbed from 89% in 1976-1979 to 92% in 2017, while disapproval of weekend binge drinking rose just a bit, going from 85% to 86%, they said.
Measures of parental disapproval were reintroduced into the survey in 2017 after being removed in 1979 – the survey began in 1975 – “because students’ responses varied little over time and across drugs,” Dr. Miech and his associates noted. “Today’s parents of 12th graders have more experience with drug use than did parents in the late 1970s [and] population attitudes toward marijuana use across all ages are becoming more lenient,” they wrote.
The 2017 edition of the annual survey, which is funded by the National Institute on Drug Abuse, is based on reports from almost 44,000 students in 8th, 10th, and 12th grade in 360 public and private secondary schools across the country.
Two agents could take AML therapy in new directions
CHICAGO—Two agents targeting novel pathways in myeloid malignancies—mivebresib and bencentinib—are showing promise in early studies, according to a speaker at the 2018 ASCO Annual Meeting.
“Both BET and AXL inhibition appear to be new and exciting targets in myeloid malignancies,” said Alice S. Mims, MD, and both have produced responses as single agents.
Dr Mims, of Ohio State University Wexner Medical Center in Columbus, made these observations in a poster discussion presentation that included commentary on the two agents.
Mivebresib (ABBV-075), an inhibitor of bromodomain and extra terminal (BET) proteins, yielded some responses in relapsed/refractory acute myeloid leukemia (AML) patients in a first-in-human study presented at the meeting (abstract 7019*).
Bemcentinib (BGB324), a first-in class selective inhibitor of the AXL tyrosine kinase, also showed activity in preliminary results of a study including patients with relapsed/refractory disease (abstract 7020*).
“It will be important to know individual patient characteristics to determine the potential response predictors,” Dr Mims said.
Mivebresib (NCT02391480)
Mivebresib is the subject of an ongoing phase 1 dose-escalation study in which 23 patients have been treated. That includes 12 who received the BET inhibitor as monotherapy, and 11 who got it in combination with the BCL-2 inhibitor venetoclax, which is indicated in CLL and has breakthrough therapy designation for AML.
Investigators observed responses in 3 of 17 evaluable patients (17.6%), including 1 complete remission with incomplete blood count recovery in a patient on mivebresib monotherapy, plus 1 partial response and 1 patient achieving a morphologic leukemia-free state with the combination.
The most common grade 3/4 treatment-emergent adverse events included anemia in 52%, thrombocytopenia in 44%, and febrile neutropenia in 26% of patients, with no dose-limiting toxicities noted as of this report.
Bemcentinib (NCT02488408)
Bemcentinib is being evaluated in a phase 1/2 trial including patients with relapsed/refractory AML and myelodysplastic syndromes (MDS).
For 32 patients treated so far, 3 patients achieved a complete remission, including 1 AML and 2 MDS patients.
In addition, 3 patients achieved partial response, including 1 MDS and 2 AML patients.
Treatment with bemcentinib was generally well-tolerated, and most adverse events were mild or moderate, investigators reported in their poster.
Pre-treatment levels of soluble AXL were lower in responders compared with non-responders, investigators also noted.
“Soluble AXL levels may be a predictive biomarker for AXL inhibition, but further assessment is necessary,” Dr Mims said.
*Data presented at the meeting differ from the abstracts.
CHICAGO—Two agents targeting novel pathways in myeloid malignancies—mivebresib and bencentinib—are showing promise in early studies, according to a speaker at the 2018 ASCO Annual Meeting.
“Both BET and AXL inhibition appear to be new and exciting targets in myeloid malignancies,” said Alice S. Mims, MD, and both have produced responses as single agents.
Dr Mims, of Ohio State University Wexner Medical Center in Columbus, made these observations in a poster discussion presentation that included commentary on the two agents.
Mivebresib (ABBV-075), an inhibitor of bromodomain and extra terminal (BET) proteins, yielded some responses in relapsed/refractory acute myeloid leukemia (AML) patients in a first-in-human study presented at the meeting (abstract 7019*).
Bemcentinib (BGB324), a first-in class selective inhibitor of the AXL tyrosine kinase, also showed activity in preliminary results of a study including patients with relapsed/refractory disease (abstract 7020*).
“It will be important to know individual patient characteristics to determine the potential response predictors,” Dr Mims said.
Mivebresib (NCT02391480)
Mivebresib is the subject of an ongoing phase 1 dose-escalation study in which 23 patients have been treated. That includes 12 who received the BET inhibitor as monotherapy, and 11 who got it in combination with the BCL-2 inhibitor venetoclax, which is indicated in CLL and has breakthrough therapy designation for AML.
Investigators observed responses in 3 of 17 evaluable patients (17.6%), including 1 complete remission with incomplete blood count recovery in a patient on mivebresib monotherapy, plus 1 partial response and 1 patient achieving a morphologic leukemia-free state with the combination.
The most common grade 3/4 treatment-emergent adverse events included anemia in 52%, thrombocytopenia in 44%, and febrile neutropenia in 26% of patients, with no dose-limiting toxicities noted as of this report.
Bemcentinib (NCT02488408)
Bemcentinib is being evaluated in a phase 1/2 trial including patients with relapsed/refractory AML and myelodysplastic syndromes (MDS).
For 32 patients treated so far, 3 patients achieved a complete remission, including 1 AML and 2 MDS patients.
In addition, 3 patients achieved partial response, including 1 MDS and 2 AML patients.
Treatment with bemcentinib was generally well-tolerated, and most adverse events were mild or moderate, investigators reported in their poster.
Pre-treatment levels of soluble AXL were lower in responders compared with non-responders, investigators also noted.
“Soluble AXL levels may be a predictive biomarker for AXL inhibition, but further assessment is necessary,” Dr Mims said.
*Data presented at the meeting differ from the abstracts.
CHICAGO—Two agents targeting novel pathways in myeloid malignancies—mivebresib and bencentinib—are showing promise in early studies, according to a speaker at the 2018 ASCO Annual Meeting.
“Both BET and AXL inhibition appear to be new and exciting targets in myeloid malignancies,” said Alice S. Mims, MD, and both have produced responses as single agents.
Dr Mims, of Ohio State University Wexner Medical Center in Columbus, made these observations in a poster discussion presentation that included commentary on the two agents.
Mivebresib (ABBV-075), an inhibitor of bromodomain and extra terminal (BET) proteins, yielded some responses in relapsed/refractory acute myeloid leukemia (AML) patients in a first-in-human study presented at the meeting (abstract 7019*).
Bemcentinib (BGB324), a first-in class selective inhibitor of the AXL tyrosine kinase, also showed activity in preliminary results of a study including patients with relapsed/refractory disease (abstract 7020*).
“It will be important to know individual patient characteristics to determine the potential response predictors,” Dr Mims said.
Mivebresib (NCT02391480)
Mivebresib is the subject of an ongoing phase 1 dose-escalation study in which 23 patients have been treated. That includes 12 who received the BET inhibitor as monotherapy, and 11 who got it in combination with the BCL-2 inhibitor venetoclax, which is indicated in CLL and has breakthrough therapy designation for AML.
Investigators observed responses in 3 of 17 evaluable patients (17.6%), including 1 complete remission with incomplete blood count recovery in a patient on mivebresib monotherapy, plus 1 partial response and 1 patient achieving a morphologic leukemia-free state with the combination.
The most common grade 3/4 treatment-emergent adverse events included anemia in 52%, thrombocytopenia in 44%, and febrile neutropenia in 26% of patients, with no dose-limiting toxicities noted as of this report.
Bemcentinib (NCT02488408)
Bemcentinib is being evaluated in a phase 1/2 trial including patients with relapsed/refractory AML and myelodysplastic syndromes (MDS).
For 32 patients treated so far, 3 patients achieved a complete remission, including 1 AML and 2 MDS patients.
In addition, 3 patients achieved partial response, including 1 MDS and 2 AML patients.
Treatment with bemcentinib was generally well-tolerated, and most adverse events were mild or moderate, investigators reported in their poster.
Pre-treatment levels of soluble AXL were lower in responders compared with non-responders, investigators also noted.
“Soluble AXL levels may be a predictive biomarker for AXL inhibition, but further assessment is necessary,” Dr Mims said.
*Data presented at the meeting differ from the abstracts.
Emicizumab granted priority review for hemophilia A without inhibitors
The US Food and Drug Administration (FDA) has granted priority review for emicizumab (Hemlibra®) for adults and children with hemophilia A without factor VIII inhibitors.
Earlier this year, the agency awarded emicizumab breakthrough therapy designation for the same population.
Emicizumab is a bispecific factor IXa- and factor X-directed antibody approved by the FDA for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adults and children who have hemophilia A with factor VIII inhibitors.
The FDA based its decision to grant emicizumab priority review on the phase 3 HAVEN 3 study, results of which were presented recently at the World Federation of Hemophilia congress.
In HAVEN 3, emicizumab demonstrated a 68% reduction (P<0.0001) in treated bleeds based on an intra-patient comparison in patients who were previously enrolled in a prospective non-interventional study.
According to Genentech, co-developer of the drug, this makes emicizumab the first medicine to show superior efficacy to prior treatment with factor VIII prophylaxis, the current standard of care for people with hemophilia A without factor VIII inhibitors.
About HAVEN 3
The randomized, multicenter, open-label trial evaluated prophylaxis versus no prophylaxis in patients without factor VIII inhibitors.
The study included 152 patients 12 years or older who were previously treated with factor VIII therapy on-demand or as prophylaxis.
Patients previously treated with on-demand factor VIII were randomized in a 2:2:1 fashion to 1 of 3 treatment groups:
- Arm A received emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 1.5 mg/kg/wk until the end of study.
- Arm B received emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 3 mg/kg/2wks for at least 24 weeks.
- Arm C received no prophylaxis
Patients previously treated prophylactically with factor VIII were enrolled in Arm D and received emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 1.5 mg/kg/wk until the end of study.
The protocol permitted episodic treatment of breakthrough bleeds with factor VIII therapy.
Patients in the prophylaxis groups achieved a 96% (P<0.0001) and 97% (P<0.0001) reduction in treated bleeds, respectively, compared to those who received no prophylaxis.
Additionally, 55.6% of patients treated weekly and 60% treated every 2 weeks had no treated bleeds. In contrast, 0% in the prophylaxis group achieved zero treated bleeds.
Investigators observed no unexpected or serious adverse events (AEs), no thrombotic events, and no cases of thrombotic microangiopathy.
The most common AEs occurring in 5% or more of patients were injection site reactions, arthralgia, nasopharyngitis, headache, upper respiratory tract infection, and influenza.
The FDA is expected to make a decision regarding approval by October 4.
The US Food and Drug Administration (FDA) has granted priority review for emicizumab (Hemlibra®) for adults and children with hemophilia A without factor VIII inhibitors.
Earlier this year, the agency awarded emicizumab breakthrough therapy designation for the same population.
Emicizumab is a bispecific factor IXa- and factor X-directed antibody approved by the FDA for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adults and children who have hemophilia A with factor VIII inhibitors.
The FDA based its decision to grant emicizumab priority review on the phase 3 HAVEN 3 study, results of which were presented recently at the World Federation of Hemophilia congress.
In HAVEN 3, emicizumab demonstrated a 68% reduction (P<0.0001) in treated bleeds based on an intra-patient comparison in patients who were previously enrolled in a prospective non-interventional study.
According to Genentech, co-developer of the drug, this makes emicizumab the first medicine to show superior efficacy to prior treatment with factor VIII prophylaxis, the current standard of care for people with hemophilia A without factor VIII inhibitors.
About HAVEN 3
The randomized, multicenter, open-label trial evaluated prophylaxis versus no prophylaxis in patients without factor VIII inhibitors.
The study included 152 patients 12 years or older who were previously treated with factor VIII therapy on-demand or as prophylaxis.
Patients previously treated with on-demand factor VIII were randomized in a 2:2:1 fashion to 1 of 3 treatment groups:
- Arm A received emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 1.5 mg/kg/wk until the end of study.
- Arm B received emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 3 mg/kg/2wks for at least 24 weeks.
- Arm C received no prophylaxis
Patients previously treated prophylactically with factor VIII were enrolled in Arm D and received emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 1.5 mg/kg/wk until the end of study.
The protocol permitted episodic treatment of breakthrough bleeds with factor VIII therapy.
Patients in the prophylaxis groups achieved a 96% (P<0.0001) and 97% (P<0.0001) reduction in treated bleeds, respectively, compared to those who received no prophylaxis.
Additionally, 55.6% of patients treated weekly and 60% treated every 2 weeks had no treated bleeds. In contrast, 0% in the prophylaxis group achieved zero treated bleeds.
Investigators observed no unexpected or serious adverse events (AEs), no thrombotic events, and no cases of thrombotic microangiopathy.
The most common AEs occurring in 5% or more of patients were injection site reactions, arthralgia, nasopharyngitis, headache, upper respiratory tract infection, and influenza.
The FDA is expected to make a decision regarding approval by October 4.
The US Food and Drug Administration (FDA) has granted priority review for emicizumab (Hemlibra®) for adults and children with hemophilia A without factor VIII inhibitors.
Earlier this year, the agency awarded emicizumab breakthrough therapy designation for the same population.
Emicizumab is a bispecific factor IXa- and factor X-directed antibody approved by the FDA for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adults and children who have hemophilia A with factor VIII inhibitors.
The FDA based its decision to grant emicizumab priority review on the phase 3 HAVEN 3 study, results of which were presented recently at the World Federation of Hemophilia congress.
In HAVEN 3, emicizumab demonstrated a 68% reduction (P<0.0001) in treated bleeds based on an intra-patient comparison in patients who were previously enrolled in a prospective non-interventional study.
According to Genentech, co-developer of the drug, this makes emicizumab the first medicine to show superior efficacy to prior treatment with factor VIII prophylaxis, the current standard of care for people with hemophilia A without factor VIII inhibitors.
About HAVEN 3
The randomized, multicenter, open-label trial evaluated prophylaxis versus no prophylaxis in patients without factor VIII inhibitors.
The study included 152 patients 12 years or older who were previously treated with factor VIII therapy on-demand or as prophylaxis.
Patients previously treated with on-demand factor VIII were randomized in a 2:2:1 fashion to 1 of 3 treatment groups:
- Arm A received emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 1.5 mg/kg/wk until the end of study.
- Arm B received emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 3 mg/kg/2wks for at least 24 weeks.
- Arm C received no prophylaxis
Patients previously treated prophylactically with factor VIII were enrolled in Arm D and received emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 1.5 mg/kg/wk until the end of study.
The protocol permitted episodic treatment of breakthrough bleeds with factor VIII therapy.
Patients in the prophylaxis groups achieved a 96% (P<0.0001) and 97% (P<0.0001) reduction in treated bleeds, respectively, compared to those who received no prophylaxis.
Additionally, 55.6% of patients treated weekly and 60% treated every 2 weeks had no treated bleeds. In contrast, 0% in the prophylaxis group achieved zero treated bleeds.
Investigators observed no unexpected or serious adverse events (AEs), no thrombotic events, and no cases of thrombotic microangiopathy.
The most common AEs occurring in 5% or more of patients were injection site reactions, arthralgia, nasopharyngitis, headache, upper respiratory tract infection, and influenza.
The FDA is expected to make a decision regarding approval by October 4.
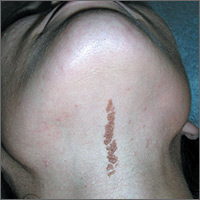
Growth on neck
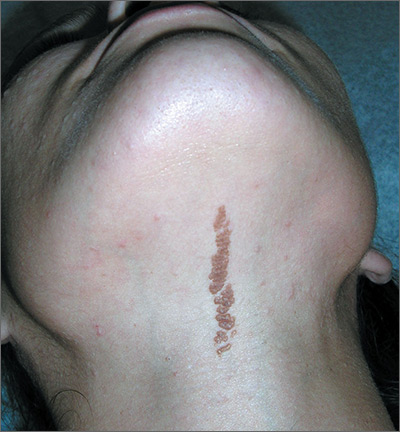
The FP recognized the lesion as a linear epidermal nevus.
Epidermal nevi (EN) are congenital hamartomas of ectodermal origin that are uncommon (occurring in < 1% of newborns and children), sporadic, and usually present at birth, although they can appear in early childhood. EN are associated with disorders of the eye, nervous system, and musculoskeletal system in 10% to 30% of patients.
EN are linear, round or oblong, well circumscribed, elevated, and flat topped. EN are often yellow-tan to dark brown in color, with a surface that is uniformly velvety or warty. They most commonly occur on the head and neck, although they can occur on the trunk and proximal extremities.
The FP determined that the patient had no neurological, musculoskeletal, or vision problems that could be associated with a linear epidermal nevus syndrome and reassured the patient and his mother that the nevus was not dangerous and did not need to be removed.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Epidermal nevus and nevus sebaceous. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine, 2nd ed. New York, NY: McGraw-Hill; 2013:958-962.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP recognized the lesion as a linear epidermal nevus.
Epidermal nevi (EN) are congenital hamartomas of ectodermal origin that are uncommon (occurring in < 1% of newborns and children), sporadic, and usually present at birth, although they can appear in early childhood. EN are associated with disorders of the eye, nervous system, and musculoskeletal system in 10% to 30% of patients.
EN are linear, round or oblong, well circumscribed, elevated, and flat topped. EN are often yellow-tan to dark brown in color, with a surface that is uniformly velvety or warty. They most commonly occur on the head and neck, although they can occur on the trunk and proximal extremities.
The FP determined that the patient had no neurological, musculoskeletal, or vision problems that could be associated with a linear epidermal nevus syndrome and reassured the patient and his mother that the nevus was not dangerous and did not need to be removed.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Epidermal nevus and nevus sebaceous. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine, 2nd ed. New York, NY: McGraw-Hill; 2013:958-962.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP recognized the lesion as a linear epidermal nevus.
Epidermal nevi (EN) are congenital hamartomas of ectodermal origin that are uncommon (occurring in < 1% of newborns and children), sporadic, and usually present at birth, although they can appear in early childhood. EN are associated with disorders of the eye, nervous system, and musculoskeletal system in 10% to 30% of patients.
EN are linear, round or oblong, well circumscribed, elevated, and flat topped. EN are often yellow-tan to dark brown in color, with a surface that is uniformly velvety or warty. They most commonly occur on the head and neck, although they can occur on the trunk and proximal extremities.
The FP determined that the patient had no neurological, musculoskeletal, or vision problems that could be associated with a linear epidermal nevus syndrome and reassured the patient and his mother that the nevus was not dangerous and did not need to be removed.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Epidermal nevus and nevus sebaceous. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine, 2nd ed. New York, NY: McGraw-Hill; 2013:958-962.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Bezafibrate shows promise as second-line option for PBC
Nearly one-third of patients with primary biliary cholangitis treated with bezafibrate showed clinical improvement after 24 months, according to data from a randomized trial of 100 adults.
Ursodeoxycholic acid remains the standard first-line therapy for primary biliary cholangitis (PBC), but many patients have an incomplete response to the treatment, and consequently their long-term survival is limited, wrote Christophe Corpechot, MD, of Sorbonne University, Paris, and his colleagues. PBC is also known as primary biliary cirrhosis.
In the BEZURSO trial (Bezafibrate in Combination with Ursodeoxycholic Acid in Primary Biliary Cirrhosis), published in the New England Journal of Medicine, the researchers randomized 100 primary PBC patients with an inadequate response to ursodeoxycholic acid to receive 400 mg per day of bezafibrate or a placebo for 24 months. Inadequate response was defined as “a serum level of alkaline phosphatase or aspartate aminotransferase more than 1.5 times the upper limit of the normal range or an abnormal total bilirubin level, assessed after at least 6 months of treatment with ursodeoxycholic acid,” the researchers said.
Baseline demographics were not significantly different between the groups. The average age of the patients was 53 years, and 95% were white women.
After 24 months, 31% of the patients in the treatment group met the primary outcome, which was the achievement of normal levels of alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, total bilirubin, and albumin, plus a normal prothrombin index. By contrast, none of the patients in the placebo group achieved the primary outcome.
In particular, bezafibrate patients showed a 60% reduction in alkaline phosphatase levels from baseline to 3 months, and a 14% decrease in total bilirubin from baseline during the course of the study.
Clinical outcomes were similar between the groups; 20% of the bezafibrate group and 18% of the placebo group developed portal hypertension, and two patients in each group developed liver complications. No deaths occurred in either group during the study. Approximately half of the patients in each group reported adverse events. Serious adverse events occurred in 14 bezafibrate patients and 12 placebo patients.
The findings were limited by the small study population, which prevented assessment of bezafibrate on liver transplantation and death, and by the limited histologic data to look at the impact on liver fibrosis and hepatic inflammation, the researchers said.
However, the results support the use of bezafibrate as an add-on to ursodeoxycholic acid in PBC patients, and merit larger, longer studies, they noted.
The study was supported by the Programme Hospitalier de Recherche Clinique 2010, Ministry of Health, and Arrow Génériques. Dr. Corpechot disclosed relationships with companies including Intercept France, Inventiva Pharma, and GlaxoSmithKline.
SOURCE: Corpechot C et al. N Engl J Med. 2018 June 6. doi: 10.1056/NEJMoa1714519.
The BEZURSO study findings “merit cautious excitement,” Elizabeth J. Carey, MD, wrote in an editorial.
“This pivotal trial effectively doubles the limited options for second-line therapy of primary biliary cholangitis,” she said.
Approximately 40% of primary biliary cholangitis patients fail to respond adequately to ursodeoxycholic acid, the first-line therapy, and they remain at risk for progression of liver disease and liver failure, wrote Dr. Carey. Bezafibrate is the first drug to generate improvement in these patients not only in measures of biochemical markers, but also measures of fibrosis and disease symptoms, she said. Patient reports of reduced itching and lower levels of fatigue are worth noting, although they were not the primary outcomes, said Dr. Carey.
“Improvement in patient-reported outcomes prompts the question of whether there is a role for the use of bezafibrate for the management of fatigue or pruritus, even in patients who have a biochemical response to ursodeoxycholic acid,” she noted (N Engl J Med. 2018 June 6. doi: 10.1056/NEJMe1804945).
Despite the promising results, challenges remain for primary biliary cholangitis patients, as approximately 70% did not meet the primary outcome, and those with more severe disease were less likely to respond, Dr. Carey said. However, she added, any agent “that both delays disease progression and alleviates symptoms is a potential boon for patients with the debilitating symptoms of primary biliary cholangitis.”
Dr. Carey is affiliated with the Mayo Clinic in Phoenix, Ariz. Disclosure forms provided by the author are available at NEJM.org.
The BEZURSO study findings “merit cautious excitement,” Elizabeth J. Carey, MD, wrote in an editorial.
“This pivotal trial effectively doubles the limited options for second-line therapy of primary biliary cholangitis,” she said.
Approximately 40% of primary biliary cholangitis patients fail to respond adequately to ursodeoxycholic acid, the first-line therapy, and they remain at risk for progression of liver disease and liver failure, wrote Dr. Carey. Bezafibrate is the first drug to generate improvement in these patients not only in measures of biochemical markers, but also measures of fibrosis and disease symptoms, she said. Patient reports of reduced itching and lower levels of fatigue are worth noting, although they were not the primary outcomes, said Dr. Carey.
“Improvement in patient-reported outcomes prompts the question of whether there is a role for the use of bezafibrate for the management of fatigue or pruritus, even in patients who have a biochemical response to ursodeoxycholic acid,” she noted (N Engl J Med. 2018 June 6. doi: 10.1056/NEJMe1804945).
Despite the promising results, challenges remain for primary biliary cholangitis patients, as approximately 70% did not meet the primary outcome, and those with more severe disease were less likely to respond, Dr. Carey said. However, she added, any agent “that both delays disease progression and alleviates symptoms is a potential boon for patients with the debilitating symptoms of primary biliary cholangitis.”
Dr. Carey is affiliated with the Mayo Clinic in Phoenix, Ariz. Disclosure forms provided by the author are available at NEJM.org.
The BEZURSO study findings “merit cautious excitement,” Elizabeth J. Carey, MD, wrote in an editorial.
“This pivotal trial effectively doubles the limited options for second-line therapy of primary biliary cholangitis,” she said.
Approximately 40% of primary biliary cholangitis patients fail to respond adequately to ursodeoxycholic acid, the first-line therapy, and they remain at risk for progression of liver disease and liver failure, wrote Dr. Carey. Bezafibrate is the first drug to generate improvement in these patients not only in measures of biochemical markers, but also measures of fibrosis and disease symptoms, she said. Patient reports of reduced itching and lower levels of fatigue are worth noting, although they were not the primary outcomes, said Dr. Carey.
“Improvement in patient-reported outcomes prompts the question of whether there is a role for the use of bezafibrate for the management of fatigue or pruritus, even in patients who have a biochemical response to ursodeoxycholic acid,” she noted (N Engl J Med. 2018 June 6. doi: 10.1056/NEJMe1804945).
Despite the promising results, challenges remain for primary biliary cholangitis patients, as approximately 70% did not meet the primary outcome, and those with more severe disease were less likely to respond, Dr. Carey said. However, she added, any agent “that both delays disease progression and alleviates symptoms is a potential boon for patients with the debilitating symptoms of primary biliary cholangitis.”
Dr. Carey is affiliated with the Mayo Clinic in Phoenix, Ariz. Disclosure forms provided by the author are available at NEJM.org.
Nearly one-third of patients with primary biliary cholangitis treated with bezafibrate showed clinical improvement after 24 months, according to data from a randomized trial of 100 adults.
Ursodeoxycholic acid remains the standard first-line therapy for primary biliary cholangitis (PBC), but many patients have an incomplete response to the treatment, and consequently their long-term survival is limited, wrote Christophe Corpechot, MD, of Sorbonne University, Paris, and his colleagues. PBC is also known as primary biliary cirrhosis.
In the BEZURSO trial (Bezafibrate in Combination with Ursodeoxycholic Acid in Primary Biliary Cirrhosis), published in the New England Journal of Medicine, the researchers randomized 100 primary PBC patients with an inadequate response to ursodeoxycholic acid to receive 400 mg per day of bezafibrate or a placebo for 24 months. Inadequate response was defined as “a serum level of alkaline phosphatase or aspartate aminotransferase more than 1.5 times the upper limit of the normal range or an abnormal total bilirubin level, assessed after at least 6 months of treatment with ursodeoxycholic acid,” the researchers said.
Baseline demographics were not significantly different between the groups. The average age of the patients was 53 years, and 95% were white women.
After 24 months, 31% of the patients in the treatment group met the primary outcome, which was the achievement of normal levels of alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, total bilirubin, and albumin, plus a normal prothrombin index. By contrast, none of the patients in the placebo group achieved the primary outcome.
In particular, bezafibrate patients showed a 60% reduction in alkaline phosphatase levels from baseline to 3 months, and a 14% decrease in total bilirubin from baseline during the course of the study.
Clinical outcomes were similar between the groups; 20% of the bezafibrate group and 18% of the placebo group developed portal hypertension, and two patients in each group developed liver complications. No deaths occurred in either group during the study. Approximately half of the patients in each group reported adverse events. Serious adverse events occurred in 14 bezafibrate patients and 12 placebo patients.
The findings were limited by the small study population, which prevented assessment of bezafibrate on liver transplantation and death, and by the limited histologic data to look at the impact on liver fibrosis and hepatic inflammation, the researchers said.
However, the results support the use of bezafibrate as an add-on to ursodeoxycholic acid in PBC patients, and merit larger, longer studies, they noted.
The study was supported by the Programme Hospitalier de Recherche Clinique 2010, Ministry of Health, and Arrow Génériques. Dr. Corpechot disclosed relationships with companies including Intercept France, Inventiva Pharma, and GlaxoSmithKline.
SOURCE: Corpechot C et al. N Engl J Med. 2018 June 6. doi: 10.1056/NEJMoa1714519.
Nearly one-third of patients with primary biliary cholangitis treated with bezafibrate showed clinical improvement after 24 months, according to data from a randomized trial of 100 adults.
Ursodeoxycholic acid remains the standard first-line therapy for primary biliary cholangitis (PBC), but many patients have an incomplete response to the treatment, and consequently their long-term survival is limited, wrote Christophe Corpechot, MD, of Sorbonne University, Paris, and his colleagues. PBC is also known as primary biliary cirrhosis.
In the BEZURSO trial (Bezafibrate in Combination with Ursodeoxycholic Acid in Primary Biliary Cirrhosis), published in the New England Journal of Medicine, the researchers randomized 100 primary PBC patients with an inadequate response to ursodeoxycholic acid to receive 400 mg per day of bezafibrate or a placebo for 24 months. Inadequate response was defined as “a serum level of alkaline phosphatase or aspartate aminotransferase more than 1.5 times the upper limit of the normal range or an abnormal total bilirubin level, assessed after at least 6 months of treatment with ursodeoxycholic acid,” the researchers said.
Baseline demographics were not significantly different between the groups. The average age of the patients was 53 years, and 95% were white women.
After 24 months, 31% of the patients in the treatment group met the primary outcome, which was the achievement of normal levels of alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, total bilirubin, and albumin, plus a normal prothrombin index. By contrast, none of the patients in the placebo group achieved the primary outcome.
In particular, bezafibrate patients showed a 60% reduction in alkaline phosphatase levels from baseline to 3 months, and a 14% decrease in total bilirubin from baseline during the course of the study.
Clinical outcomes were similar between the groups; 20% of the bezafibrate group and 18% of the placebo group developed portal hypertension, and two patients in each group developed liver complications. No deaths occurred in either group during the study. Approximately half of the patients in each group reported adverse events. Serious adverse events occurred in 14 bezafibrate patients and 12 placebo patients.
The findings were limited by the small study population, which prevented assessment of bezafibrate on liver transplantation and death, and by the limited histologic data to look at the impact on liver fibrosis and hepatic inflammation, the researchers said.
However, the results support the use of bezafibrate as an add-on to ursodeoxycholic acid in PBC patients, and merit larger, longer studies, they noted.
The study was supported by the Programme Hospitalier de Recherche Clinique 2010, Ministry of Health, and Arrow Génériques. Dr. Corpechot disclosed relationships with companies including Intercept France, Inventiva Pharma, and GlaxoSmithKline.
SOURCE: Corpechot C et al. N Engl J Med. 2018 June 6. doi: 10.1056/NEJMoa1714519.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Primary biliary cholangitis patients who took bezafibrate showed decreases in alkaline phosphatase levels and total bilirubin.
Major finding: A total of 31% of patients who took bezafibrate achieved normal levels of disease biomarkers after 24 months compared with 0% of placebo patients.
Study details: The data come from a double-blind, placebo-controlled trial of 100 adults with primary biliary cholangitis at 21 medical centers in France.
Disclosures: Programme Hospitalier de Recherche Clinique 2010 (Ministry of Health) and Arrow Génériques supported the study. Dr. Corpechot disclosed relationships with companies including Intercept France, Inventiva Pharma, and GlaxoSmithKline.
Source: Corpechot C et al. N Engl J Med. 2018 June 6. doi: 10.1056/NEJMoa1714519.
Mifepristone, then misoprostol is best in early pregnancy loss
In a randomized trial of women with early pregnancy loss, pretreatment with mifepristone before misoprostol was superior to misoprostol alone at achieving gestational sac expulsion by the time of the first follow-up visit without additional intervention.
“Women generally prefer active management; the ability to have control over the management of miscarriage may relieve some of the emotional burden that accompanies first trimester pregnancy loss,” Courtney A. Schreiber, MD, of the University of Pennsylvania, Philadelphia, and her coauthors wrote in the New England Journal of Medicine. But misoprostol (Cytotec) alone for women with a closed cervical os can require a second dose or intervention.
The trial enrolled 300 participants. Each had an ultrasound showing a nonviable intrauterine pregnancy of 5-12 weeks’ gestation. Women with an incomplete or inevitable abortion (that is, the absence of a gestational sac, an open cervical os, or both) were excluded, as misoprostol alone is effective for management of that diagnosis.
After randomization, 149 participants received 200 mg of oral mifepristone (Mifeprex), with 800 mcg misoprostol administered approximately 24 hours later. The other 151 participants received the standard 800 mcg dose of misoprostol alone. In both groups, the misoprostol was self-administered vaginally at home by inserting four 200-mcg tablets.
Follow-up came 24 hours to 4 days after misoprostol administration. The primary outcome was a gestational sac expulsion by the time of this follow-up, and no additional surgical or medical intervention within 30 days. If the gestational sac was present at follow-up, participants chose either expectant management, surgical management, or a second misoprostol dose.
The primary outcome was achieved in 124 of 148 women (83.8%; 95% confidence interval, 76.8-89.3) in the mifepristone-pretreatment group and in 100 of 149 women (67.1%; 95% CI, 59.0-74.6) in the misoprostol-alone group for a relative risk of 1.25 (95% CI, 1.09 to 1.43). Two women were lost to follow-up and one who was declared ineligible because of a possible ectopic pregnancy.
At 30 days’ follow-up, the cumulative rate of gestational sac expulsion with up to two doses of misoprostol was 91.2% (95% CI, 85.4-95.2) in the mifepristone-pretreatment group and 75.8% (95% CI, 68.2%-82.5%) in the misoprostol-alone group. Also by 30 days’ follow-up, 13 women in the mifepristone-pretreatment group and 35 women in the misoprostol-alone group had undergone uterine aspiration (relative risk, 0.37; 95% CI, 0.21-0.68).
Serious adverse events were rare in both groups, and both groups had matching mean scores for bleeding intensity and pain.
“Pretreatment with mifepristone followed by treatment with misoprostol resulted in a significantly higher rate of complete gestational sac expulsion by approximately 2 days after treatment ... [and] a significantly lower rate of uterine aspiration than misoprostol use alone,” wrote Dr. Schreiber and her coauthors. Patient satisfaction was similar between the two groups (89.4% vs. 87.4%, respectively, described their experience overall as either “good” or “neutral”).
The trial was funded by the National Institute of Child Health and Human Development. Two coauthors reported grants from the National Institutes of Health during the study and another reported personal fees from Danco Laboratories, which markets mifepristone, outside the submitted work.
SOURCE: Schreiber CA et al. N Engl J Med. 2018;378:2161-70.
The results of this study provide strong evidence that the sequential regimen of mifepristone followed by misoprostol is safe and superior to misoprostol alone in achieving treatment success and avoiding an aspiration procedure, wrote Carolyn L. Westhoff, MD, in an editorial accompanying the article.
In addition to its greater efficacy, the mifepristone treatment is quicker, which is more desirable for patients and reduces costs, inconvenience, and patient anxiety. Some women still will need prompt access to aspiration.
The mifepristone-pretreatment regimen should be the standard of care, Dr. Westhoff writes, but access to mifepristone is limited by the FDA’s Risk Evaluation and Mitigation Strategy restriction, which requires that the oral drug be taken in the doctor’s office rather than obtained at a retail pharmacy. “Extensive clinical experience with mifepristone indicates that there is no need for such restrictions,” she wrote.
Carolyn L. Westhoff, MD, is a professor of epidemiology and population and family health at Columbia University in New York. Her remarks are adapted from an accompanying editorial (N Engl J Med. 2018;378:2232-3). She reported personal fees from Planned Parenthood, Bayer, Agile Therapeutics, Cooper Surgical, Allergan, Elsevier, and personal fees and nonfinancial support from Merck.
The results of this study provide strong evidence that the sequential regimen of mifepristone followed by misoprostol is safe and superior to misoprostol alone in achieving treatment success and avoiding an aspiration procedure, wrote Carolyn L. Westhoff, MD, in an editorial accompanying the article.
In addition to its greater efficacy, the mifepristone treatment is quicker, which is more desirable for patients and reduces costs, inconvenience, and patient anxiety. Some women still will need prompt access to aspiration.
The mifepristone-pretreatment regimen should be the standard of care, Dr. Westhoff writes, but access to mifepristone is limited by the FDA’s Risk Evaluation and Mitigation Strategy restriction, which requires that the oral drug be taken in the doctor’s office rather than obtained at a retail pharmacy. “Extensive clinical experience with mifepristone indicates that there is no need for such restrictions,” she wrote.
Carolyn L. Westhoff, MD, is a professor of epidemiology and population and family health at Columbia University in New York. Her remarks are adapted from an accompanying editorial (N Engl J Med. 2018;378:2232-3). She reported personal fees from Planned Parenthood, Bayer, Agile Therapeutics, Cooper Surgical, Allergan, Elsevier, and personal fees and nonfinancial support from Merck.
The results of this study provide strong evidence that the sequential regimen of mifepristone followed by misoprostol is safe and superior to misoprostol alone in achieving treatment success and avoiding an aspiration procedure, wrote Carolyn L. Westhoff, MD, in an editorial accompanying the article.
In addition to its greater efficacy, the mifepristone treatment is quicker, which is more desirable for patients and reduces costs, inconvenience, and patient anxiety. Some women still will need prompt access to aspiration.
The mifepristone-pretreatment regimen should be the standard of care, Dr. Westhoff writes, but access to mifepristone is limited by the FDA’s Risk Evaluation and Mitigation Strategy restriction, which requires that the oral drug be taken in the doctor’s office rather than obtained at a retail pharmacy. “Extensive clinical experience with mifepristone indicates that there is no need for such restrictions,” she wrote.
Carolyn L. Westhoff, MD, is a professor of epidemiology and population and family health at Columbia University in New York. Her remarks are adapted from an accompanying editorial (N Engl J Med. 2018;378:2232-3). She reported personal fees from Planned Parenthood, Bayer, Agile Therapeutics, Cooper Surgical, Allergan, Elsevier, and personal fees and nonfinancial support from Merck.
In a randomized trial of women with early pregnancy loss, pretreatment with mifepristone before misoprostol was superior to misoprostol alone at achieving gestational sac expulsion by the time of the first follow-up visit without additional intervention.
“Women generally prefer active management; the ability to have control over the management of miscarriage may relieve some of the emotional burden that accompanies first trimester pregnancy loss,” Courtney A. Schreiber, MD, of the University of Pennsylvania, Philadelphia, and her coauthors wrote in the New England Journal of Medicine. But misoprostol (Cytotec) alone for women with a closed cervical os can require a second dose or intervention.
The trial enrolled 300 participants. Each had an ultrasound showing a nonviable intrauterine pregnancy of 5-12 weeks’ gestation. Women with an incomplete or inevitable abortion (that is, the absence of a gestational sac, an open cervical os, or both) were excluded, as misoprostol alone is effective for management of that diagnosis.
After randomization, 149 participants received 200 mg of oral mifepristone (Mifeprex), with 800 mcg misoprostol administered approximately 24 hours later. The other 151 participants received the standard 800 mcg dose of misoprostol alone. In both groups, the misoprostol was self-administered vaginally at home by inserting four 200-mcg tablets.
Follow-up came 24 hours to 4 days after misoprostol administration. The primary outcome was a gestational sac expulsion by the time of this follow-up, and no additional surgical or medical intervention within 30 days. If the gestational sac was present at follow-up, participants chose either expectant management, surgical management, or a second misoprostol dose.
The primary outcome was achieved in 124 of 148 women (83.8%; 95% confidence interval, 76.8-89.3) in the mifepristone-pretreatment group and in 100 of 149 women (67.1%; 95% CI, 59.0-74.6) in the misoprostol-alone group for a relative risk of 1.25 (95% CI, 1.09 to 1.43). Two women were lost to follow-up and one who was declared ineligible because of a possible ectopic pregnancy.
At 30 days’ follow-up, the cumulative rate of gestational sac expulsion with up to two doses of misoprostol was 91.2% (95% CI, 85.4-95.2) in the mifepristone-pretreatment group and 75.8% (95% CI, 68.2%-82.5%) in the misoprostol-alone group. Also by 30 days’ follow-up, 13 women in the mifepristone-pretreatment group and 35 women in the misoprostol-alone group had undergone uterine aspiration (relative risk, 0.37; 95% CI, 0.21-0.68).
Serious adverse events were rare in both groups, and both groups had matching mean scores for bleeding intensity and pain.
“Pretreatment with mifepristone followed by treatment with misoprostol resulted in a significantly higher rate of complete gestational sac expulsion by approximately 2 days after treatment ... [and] a significantly lower rate of uterine aspiration than misoprostol use alone,” wrote Dr. Schreiber and her coauthors. Patient satisfaction was similar between the two groups (89.4% vs. 87.4%, respectively, described their experience overall as either “good” or “neutral”).
The trial was funded by the National Institute of Child Health and Human Development. Two coauthors reported grants from the National Institutes of Health during the study and another reported personal fees from Danco Laboratories, which markets mifepristone, outside the submitted work.
SOURCE: Schreiber CA et al. N Engl J Med. 2018;378:2161-70.
In a randomized trial of women with early pregnancy loss, pretreatment with mifepristone before misoprostol was superior to misoprostol alone at achieving gestational sac expulsion by the time of the first follow-up visit without additional intervention.
“Women generally prefer active management; the ability to have control over the management of miscarriage may relieve some of the emotional burden that accompanies first trimester pregnancy loss,” Courtney A. Schreiber, MD, of the University of Pennsylvania, Philadelphia, and her coauthors wrote in the New England Journal of Medicine. But misoprostol (Cytotec) alone for women with a closed cervical os can require a second dose or intervention.
The trial enrolled 300 participants. Each had an ultrasound showing a nonviable intrauterine pregnancy of 5-12 weeks’ gestation. Women with an incomplete or inevitable abortion (that is, the absence of a gestational sac, an open cervical os, or both) were excluded, as misoprostol alone is effective for management of that diagnosis.
After randomization, 149 participants received 200 mg of oral mifepristone (Mifeprex), with 800 mcg misoprostol administered approximately 24 hours later. The other 151 participants received the standard 800 mcg dose of misoprostol alone. In both groups, the misoprostol was self-administered vaginally at home by inserting four 200-mcg tablets.
Follow-up came 24 hours to 4 days after misoprostol administration. The primary outcome was a gestational sac expulsion by the time of this follow-up, and no additional surgical or medical intervention within 30 days. If the gestational sac was present at follow-up, participants chose either expectant management, surgical management, or a second misoprostol dose.
The primary outcome was achieved in 124 of 148 women (83.8%; 95% confidence interval, 76.8-89.3) in the mifepristone-pretreatment group and in 100 of 149 women (67.1%; 95% CI, 59.0-74.6) in the misoprostol-alone group for a relative risk of 1.25 (95% CI, 1.09 to 1.43). Two women were lost to follow-up and one who was declared ineligible because of a possible ectopic pregnancy.
At 30 days’ follow-up, the cumulative rate of gestational sac expulsion with up to two doses of misoprostol was 91.2% (95% CI, 85.4-95.2) in the mifepristone-pretreatment group and 75.8% (95% CI, 68.2%-82.5%) in the misoprostol-alone group. Also by 30 days’ follow-up, 13 women in the mifepristone-pretreatment group and 35 women in the misoprostol-alone group had undergone uterine aspiration (relative risk, 0.37; 95% CI, 0.21-0.68).
Serious adverse events were rare in both groups, and both groups had matching mean scores for bleeding intensity and pain.
“Pretreatment with mifepristone followed by treatment with misoprostol resulted in a significantly higher rate of complete gestational sac expulsion by approximately 2 days after treatment ... [and] a significantly lower rate of uterine aspiration than misoprostol use alone,” wrote Dr. Schreiber and her coauthors. Patient satisfaction was similar between the two groups (89.4% vs. 87.4%, respectively, described their experience overall as either “good” or “neutral”).
The trial was funded by the National Institute of Child Health and Human Development. Two coauthors reported grants from the National Institutes of Health during the study and another reported personal fees from Danco Laboratories, which markets mifepristone, outside the submitted work.
SOURCE: Schreiber CA et al. N Engl J Med. 2018;378:2161-70.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Gestational sac expulsion was achieved in 83.8% of the mifepristone pretreatment group and in 67.1% of the misoprostol-only group.
Study details: A randomized trial of 300 women who experienced early pregnancy loss.
Disclosures: The trial was funded by the National Institute of Child Health and Human Development. Two coauthors reported grants from the National Institutes of Health during the study and another reported personal fees from Danco Laboratories, outside the submitted work.
Source: Schreiber CA et al. N Engl J Med 2018;378:2161-70.
Neurofilament Light Concentration Indicates Repetitive Concussive TBI
The biomarker can distinguish between patients with short and long durations of postconcussion syndrome.
LOS ANGELES—Plasma levels of neurofilament light indicate repetitive concussive traumatic brain injury (TBI) and axonal injury, according to data presented at the 70th Annual Meeting of the American Academy of Neurology. Differences in this biomarker are detectable for more than a year after the insult. Furthermore, plasma levels of neurofilament light reflect CSF levels of neurofilament light and may identify patients at increased risk of persistent postconcussion syndrome (PCS), according to the researchers.
In a previous study of boxers, Pashtun Shahim, MD, PhD, researcher at the University of Gothenburg in Sweden, and colleagues found that CSF levels of neurofilament light increased more after a bout than did other potential biomarkers such as total tau, phosphorylated tau, and amyloid beta. They also observed that concentrations of serum neurofilament light were higher in boxers with severe head impact, compared with those with mild head impact. In a study of hockey players, Dr. Shahim and colleagues found that neurofilament light measured in the acute setting could distinguish those who would have a prolonged return to play from those who would have a quick return to play.
A Study of Professional Athletes
In their latest study, Dr. Shahim and colleagues sought to determine whether professional athletes with PCS resulting from repetitive concussive TBI have elevated CSF and plasma neurofilament light and tau, compared with controls. They also investigated whether elevated concentrations of these biomarkers are associated with persistent PCS.
Between September 2013 and September 2017, the investigators enrolled 31 athletes (ie, professional players of hockey and soccer) with PCS resulting from repetitive concussive TBI, 48 athletes with concussion but without PCS, and 30 control athletes into their study. Participants underwent CSF and plasma biomarker assessments, responded to the Rivermead Post-Concussion Symptoms Questionnaire (RPQ), and underwent structural brain MRI. The population’s median time since the last concussion was 1.5 years. The three groups were matched on sport.
Biomarker Indicated Duration of PCS
Dr. Shahim and colleagues found “a tight correlation between plasma concentrations of neurofilament [light] and CSF neurofilament [light], while there was no correlation between plasma tau and CSF tau.”
Plasma neurofilament light concentrations were significantly increased in participants with concussion and those with PCS, compared with controls. The concentration of plasma neurofilament light was higher in patients with concussion but without PCS, compared with the PCS group. This finding was “expected, as we are comparing acute cases of concussion versus chronic cases,” said Dr. Shahim
When they examined only participants with PCS, athletes who had had PCS for more than one year had higher concentrations of neurofilament light, compared with athletes who had had PCS for less than one year. The latter returned to play, while the former resigned, said Dr. Shahim. In addition, plasma neurofilament light concentration correlated with RPQ scores and lifetime number of concussions.
Levels of tau were lower among participants with PCS than among control athletes, and the researchers found no correlation between tau level and duration of PCS. Levels of tau also had no association with RPQ scores.
Dr. Shahim and colleagues next examined whether these biomarkers had prognostic value. They found that CSF neurofilament concentrations could distinguish between participants who had had PCS for less than a year and those who had had PCS for more than a year (area under the curve [AUC], 0.86). Plasma neurofilament light distinguished between these groups with an AUC of 0.85. Neither CSF tau nor plasma tau reliably indicated duration of PCS, however. In addition, the investigators found no correlation between CSF and plasma tau concentrations from the same individual. This finding casts doubt on the hypothesis that tau concentrations reflect axonal injury, said Dr. Shahim.
—Erik Greb
Shahim P, Tegner Y, Marklund N, et al. Neurofilament light and tau as blood biomarkers for sports-related concussion. Neurology. 2018;90(20):e1780-e1788.
Shahim P, Zetterberg H, Tegner Y, Blennow K. Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology. 2017;88(19):1788-1794.
The biomarker can distinguish between patients with short and long durations of postconcussion syndrome.
The biomarker can distinguish between patients with short and long durations of postconcussion syndrome.
LOS ANGELES—Plasma levels of neurofilament light indicate repetitive concussive traumatic brain injury (TBI) and axonal injury, according to data presented at the 70th Annual Meeting of the American Academy of Neurology. Differences in this biomarker are detectable for more than a year after the insult. Furthermore, plasma levels of neurofilament light reflect CSF levels of neurofilament light and may identify patients at increased risk of persistent postconcussion syndrome (PCS), according to the researchers.
In a previous study of boxers, Pashtun Shahim, MD, PhD, researcher at the University of Gothenburg in Sweden, and colleagues found that CSF levels of neurofilament light increased more after a bout than did other potential biomarkers such as total tau, phosphorylated tau, and amyloid beta. They also observed that concentrations of serum neurofilament light were higher in boxers with severe head impact, compared with those with mild head impact. In a study of hockey players, Dr. Shahim and colleagues found that neurofilament light measured in the acute setting could distinguish those who would have a prolonged return to play from those who would have a quick return to play.
A Study of Professional Athletes
In their latest study, Dr. Shahim and colleagues sought to determine whether professional athletes with PCS resulting from repetitive concussive TBI have elevated CSF and plasma neurofilament light and tau, compared with controls. They also investigated whether elevated concentrations of these biomarkers are associated with persistent PCS.
Between September 2013 and September 2017, the investigators enrolled 31 athletes (ie, professional players of hockey and soccer) with PCS resulting from repetitive concussive TBI, 48 athletes with concussion but without PCS, and 30 control athletes into their study. Participants underwent CSF and plasma biomarker assessments, responded to the Rivermead Post-Concussion Symptoms Questionnaire (RPQ), and underwent structural brain MRI. The population’s median time since the last concussion was 1.5 years. The three groups were matched on sport.
Biomarker Indicated Duration of PCS
Dr. Shahim and colleagues found “a tight correlation between plasma concentrations of neurofilament [light] and CSF neurofilament [light], while there was no correlation between plasma tau and CSF tau.”
Plasma neurofilament light concentrations were significantly increased in participants with concussion and those with PCS, compared with controls. The concentration of plasma neurofilament light was higher in patients with concussion but without PCS, compared with the PCS group. This finding was “expected, as we are comparing acute cases of concussion versus chronic cases,” said Dr. Shahim
When they examined only participants with PCS, athletes who had had PCS for more than one year had higher concentrations of neurofilament light, compared with athletes who had had PCS for less than one year. The latter returned to play, while the former resigned, said Dr. Shahim. In addition, plasma neurofilament light concentration correlated with RPQ scores and lifetime number of concussions.
Levels of tau were lower among participants with PCS than among control athletes, and the researchers found no correlation between tau level and duration of PCS. Levels of tau also had no association with RPQ scores.
Dr. Shahim and colleagues next examined whether these biomarkers had prognostic value. They found that CSF neurofilament concentrations could distinguish between participants who had had PCS for less than a year and those who had had PCS for more than a year (area under the curve [AUC], 0.86). Plasma neurofilament light distinguished between these groups with an AUC of 0.85. Neither CSF tau nor plasma tau reliably indicated duration of PCS, however. In addition, the investigators found no correlation between CSF and plasma tau concentrations from the same individual. This finding casts doubt on the hypothesis that tau concentrations reflect axonal injury, said Dr. Shahim.
—Erik Greb
Shahim P, Tegner Y, Marklund N, et al. Neurofilament light and tau as blood biomarkers for sports-related concussion. Neurology. 2018;90(20):e1780-e1788.
Shahim P, Zetterberg H, Tegner Y, Blennow K. Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology. 2017;88(19):1788-1794.
LOS ANGELES—Plasma levels of neurofilament light indicate repetitive concussive traumatic brain injury (TBI) and axonal injury, according to data presented at the 70th Annual Meeting of the American Academy of Neurology. Differences in this biomarker are detectable for more than a year after the insult. Furthermore, plasma levels of neurofilament light reflect CSF levels of neurofilament light and may identify patients at increased risk of persistent postconcussion syndrome (PCS), according to the researchers.
In a previous study of boxers, Pashtun Shahim, MD, PhD, researcher at the University of Gothenburg in Sweden, and colleagues found that CSF levels of neurofilament light increased more after a bout than did other potential biomarkers such as total tau, phosphorylated tau, and amyloid beta. They also observed that concentrations of serum neurofilament light were higher in boxers with severe head impact, compared with those with mild head impact. In a study of hockey players, Dr. Shahim and colleagues found that neurofilament light measured in the acute setting could distinguish those who would have a prolonged return to play from those who would have a quick return to play.
A Study of Professional Athletes
In their latest study, Dr. Shahim and colleagues sought to determine whether professional athletes with PCS resulting from repetitive concussive TBI have elevated CSF and plasma neurofilament light and tau, compared with controls. They also investigated whether elevated concentrations of these biomarkers are associated with persistent PCS.
Between September 2013 and September 2017, the investigators enrolled 31 athletes (ie, professional players of hockey and soccer) with PCS resulting from repetitive concussive TBI, 48 athletes with concussion but without PCS, and 30 control athletes into their study. Participants underwent CSF and plasma biomarker assessments, responded to the Rivermead Post-Concussion Symptoms Questionnaire (RPQ), and underwent structural brain MRI. The population’s median time since the last concussion was 1.5 years. The three groups were matched on sport.
Biomarker Indicated Duration of PCS
Dr. Shahim and colleagues found “a tight correlation between plasma concentrations of neurofilament [light] and CSF neurofilament [light], while there was no correlation between plasma tau and CSF tau.”
Plasma neurofilament light concentrations were significantly increased in participants with concussion and those with PCS, compared with controls. The concentration of plasma neurofilament light was higher in patients with concussion but without PCS, compared with the PCS group. This finding was “expected, as we are comparing acute cases of concussion versus chronic cases,” said Dr. Shahim
When they examined only participants with PCS, athletes who had had PCS for more than one year had higher concentrations of neurofilament light, compared with athletes who had had PCS for less than one year. The latter returned to play, while the former resigned, said Dr. Shahim. In addition, plasma neurofilament light concentration correlated with RPQ scores and lifetime number of concussions.
Levels of tau were lower among participants with PCS than among control athletes, and the researchers found no correlation between tau level and duration of PCS. Levels of tau also had no association with RPQ scores.
Dr. Shahim and colleagues next examined whether these biomarkers had prognostic value. They found that CSF neurofilament concentrations could distinguish between participants who had had PCS for less than a year and those who had had PCS for more than a year (area under the curve [AUC], 0.86). Plasma neurofilament light distinguished between these groups with an AUC of 0.85. Neither CSF tau nor plasma tau reliably indicated duration of PCS, however. In addition, the investigators found no correlation between CSF and plasma tau concentrations from the same individual. This finding casts doubt on the hypothesis that tau concentrations reflect axonal injury, said Dr. Shahim.
—Erik Greb
Shahim P, Tegner Y, Marklund N, et al. Neurofilament light and tau as blood biomarkers for sports-related concussion. Neurology. 2018;90(20):e1780-e1788.
Shahim P, Zetterberg H, Tegner Y, Blennow K. Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology. 2017;88(19):1788-1794.
H&N cancer may be undertreated in women
CHICAGO – Sex disparities in the treatment of head and neck cancer may be leading to poorer outcomes for women, according to a retrospective registry-based cohort study of 884 patients reported at annual meeting of the American Society of Clinical Oncology.
“The treatment of head and neck cancer often requires intensive treatment that can have lasting side effects,” senior study author Jed A. Katzel, MD, a medical oncologist at Kaiser Permanente in Santa Clara, Calif., said in a press briefing. “Our goal was to review data from a large group of patients in Northern California to determine which patients are most likely to benefit from aggressive therapy, while minimizing toxicity for those likely to die from competing events.”
The reasons for the observed sex disparities are not known, according to Dr. Katzel. However, they may include patient preferences, physician practices, and the higher proportion among men of oropharynx tumors, as those tumors are more commonly associated with human papillomavirus (HPV), which carries a more favorable prognosis.
“Further investigation is needed to determine if there is an actual difference in treatment and outcomes for women, compared with men,” he said. “To this end, we have planned a chart-by-chart review, as well as a prospective analysis that will be performed in the currently enrolling NRG HN004 clinical trial.”
“The outcome of this study was very surprising to us, the idea that there are disparities in both the treatment that women receive relative to men, but also in the rate of death from head and neck cancer for women compared to men,” commented ASCO Expert Joshua Jones, MD, MA, who is also a radiation oncologist at the Perelman Center for Advanced Medicine, University of Pennsylvania, Philadelphia.
Dr. Katzel and his colleagues used the Kaiser Permanente Northern California registry to identify patients with stage II to IVB head and neck cancer diagnosed during 2000-2015.
Analyses were based on 223 women and 661 men, relative numbers that are not surprising given the known demographics of this cancer. Oropharyngeal tumors accounted for 38% of the cancers in the former, but 55% in the latter. (HPV status was not directly ascertained.)
The rate of receipt of intensive chemotherapy was 35% for women and 46% for men (adjusted odds ratio, 0.68; 95% CI, 0.48-0.98; P = .006). Similarly, the rate of receipt of radiation therapy was 60% for women and 70% for men (AOR, 0.79; 95% CI, 0.56-1.11; P = .008). Receipt of surgery was similar for the sexes.
The investigators analyzed deaths according to type using a GCE model that controlled for age, sex, tumor site, and Charlson Comorbidity Index. “The GCE model essentially describes the degree to which cancer is the patient’s problem,” Dr. Katzel explained.
Results showed that both women and men were more likely to die from cancer than from other causes; however, the ratio was 7 for women, compared with just 3.8 for men, a difference translating to a relative hazard ratio of 1.92 (95% CI, 1.07-3.43).
In terms of potential confounding, there were only 19 noncancer deaths among the women studied, suggesting that they may have been more healthy than the men, which could have influenced the calculations, according to Dr. Katzel.
“This GCE model has been validated in head and neck cancer, but also in breast cancer, prostate cancer, and endometrial cancer, so we are using a validated model to do this evaluation,” he noted. “So I would say we are confident in our findings.”
Dr. Katzel disclosed that he had no relevant conflicts of interest. The study received funding from Kaiser Permanente Northern California Graduate Medical Education Department.
SOURCE: Park A et al. ASCO 2018 Abstract LBA6002.
CHICAGO – Sex disparities in the treatment of head and neck cancer may be leading to poorer outcomes for women, according to a retrospective registry-based cohort study of 884 patients reported at annual meeting of the American Society of Clinical Oncology.
“The treatment of head and neck cancer often requires intensive treatment that can have lasting side effects,” senior study author Jed A. Katzel, MD, a medical oncologist at Kaiser Permanente in Santa Clara, Calif., said in a press briefing. “Our goal was to review data from a large group of patients in Northern California to determine which patients are most likely to benefit from aggressive therapy, while minimizing toxicity for those likely to die from competing events.”
The reasons for the observed sex disparities are not known, according to Dr. Katzel. However, they may include patient preferences, physician practices, and the higher proportion among men of oropharynx tumors, as those tumors are more commonly associated with human papillomavirus (HPV), which carries a more favorable prognosis.
“Further investigation is needed to determine if there is an actual difference in treatment and outcomes for women, compared with men,” he said. “To this end, we have planned a chart-by-chart review, as well as a prospective analysis that will be performed in the currently enrolling NRG HN004 clinical trial.”
“The outcome of this study was very surprising to us, the idea that there are disparities in both the treatment that women receive relative to men, but also in the rate of death from head and neck cancer for women compared to men,” commented ASCO Expert Joshua Jones, MD, MA, who is also a radiation oncologist at the Perelman Center for Advanced Medicine, University of Pennsylvania, Philadelphia.
Dr. Katzel and his colleagues used the Kaiser Permanente Northern California registry to identify patients with stage II to IVB head and neck cancer diagnosed during 2000-2015.
Analyses were based on 223 women and 661 men, relative numbers that are not surprising given the known demographics of this cancer. Oropharyngeal tumors accounted for 38% of the cancers in the former, but 55% in the latter. (HPV status was not directly ascertained.)
The rate of receipt of intensive chemotherapy was 35% for women and 46% for men (adjusted odds ratio, 0.68; 95% CI, 0.48-0.98; P = .006). Similarly, the rate of receipt of radiation therapy was 60% for women and 70% for men (AOR, 0.79; 95% CI, 0.56-1.11; P = .008). Receipt of surgery was similar for the sexes.
The investigators analyzed deaths according to type using a GCE model that controlled for age, sex, tumor site, and Charlson Comorbidity Index. “The GCE model essentially describes the degree to which cancer is the patient’s problem,” Dr. Katzel explained.
Results showed that both women and men were more likely to die from cancer than from other causes; however, the ratio was 7 for women, compared with just 3.8 for men, a difference translating to a relative hazard ratio of 1.92 (95% CI, 1.07-3.43).
In terms of potential confounding, there were only 19 noncancer deaths among the women studied, suggesting that they may have been more healthy than the men, which could have influenced the calculations, according to Dr. Katzel.
“This GCE model has been validated in head and neck cancer, but also in breast cancer, prostate cancer, and endometrial cancer, so we are using a validated model to do this evaluation,” he noted. “So I would say we are confident in our findings.”
Dr. Katzel disclosed that he had no relevant conflicts of interest. The study received funding from Kaiser Permanente Northern California Graduate Medical Education Department.
SOURCE: Park A et al. ASCO 2018 Abstract LBA6002.
CHICAGO – Sex disparities in the treatment of head and neck cancer may be leading to poorer outcomes for women, according to a retrospective registry-based cohort study of 884 patients reported at annual meeting of the American Society of Clinical Oncology.
“The treatment of head and neck cancer often requires intensive treatment that can have lasting side effects,” senior study author Jed A. Katzel, MD, a medical oncologist at Kaiser Permanente in Santa Clara, Calif., said in a press briefing. “Our goal was to review data from a large group of patients in Northern California to determine which patients are most likely to benefit from aggressive therapy, while minimizing toxicity for those likely to die from competing events.”
The reasons for the observed sex disparities are not known, according to Dr. Katzel. However, they may include patient preferences, physician practices, and the higher proportion among men of oropharynx tumors, as those tumors are more commonly associated with human papillomavirus (HPV), which carries a more favorable prognosis.
“Further investigation is needed to determine if there is an actual difference in treatment and outcomes for women, compared with men,” he said. “To this end, we have planned a chart-by-chart review, as well as a prospective analysis that will be performed in the currently enrolling NRG HN004 clinical trial.”
“The outcome of this study was very surprising to us, the idea that there are disparities in both the treatment that women receive relative to men, but also in the rate of death from head and neck cancer for women compared to men,” commented ASCO Expert Joshua Jones, MD, MA, who is also a radiation oncologist at the Perelman Center for Advanced Medicine, University of Pennsylvania, Philadelphia.
Dr. Katzel and his colleagues used the Kaiser Permanente Northern California registry to identify patients with stage II to IVB head and neck cancer diagnosed during 2000-2015.
Analyses were based on 223 women and 661 men, relative numbers that are not surprising given the known demographics of this cancer. Oropharyngeal tumors accounted for 38% of the cancers in the former, but 55% in the latter. (HPV status was not directly ascertained.)
The rate of receipt of intensive chemotherapy was 35% for women and 46% for men (adjusted odds ratio, 0.68; 95% CI, 0.48-0.98; P = .006). Similarly, the rate of receipt of radiation therapy was 60% for women and 70% for men (AOR, 0.79; 95% CI, 0.56-1.11; P = .008). Receipt of surgery was similar for the sexes.
The investigators analyzed deaths according to type using a GCE model that controlled for age, sex, tumor site, and Charlson Comorbidity Index. “The GCE model essentially describes the degree to which cancer is the patient’s problem,” Dr. Katzel explained.
Results showed that both women and men were more likely to die from cancer than from other causes; however, the ratio was 7 for women, compared with just 3.8 for men, a difference translating to a relative hazard ratio of 1.92 (95% CI, 1.07-3.43).
In terms of potential confounding, there were only 19 noncancer deaths among the women studied, suggesting that they may have been more healthy than the men, which could have influenced the calculations, according to Dr. Katzel.
“This GCE model has been validated in head and neck cancer, but also in breast cancer, prostate cancer, and endometrial cancer, so we are using a validated model to do this evaluation,” he noted. “So I would say we are confident in our findings.”
Dr. Katzel disclosed that he had no relevant conflicts of interest. The study received funding from Kaiser Permanente Northern California Graduate Medical Education Department.
SOURCE: Park A et al. ASCO 2018 Abstract LBA6002.
REPORTING FROM ASCO 2018
Key clinical point: Women with head and neck cancer may be relatively undertreated and therefore are more likely to die from the disease.
Major finding: Compared with male counterparts, female patients had lower rates of receiving intensive chemotherapy (35% vs. 46%) and radiation therapy (60% vs. 70%) and a higher ratio of cancer to noncancer mortality (adjusted relative hazard ratio, 1.92).
Study details: Retrospective, registry-based, cohort study of 884 patients with stage II to IVB H&N cancer diagnosed during 2000-2015.
Disclosures: Dr. Katzel disclosed that he had no relevant conflicts of interest. The study received funding from Kaiser Permanente Northern California Graduate Medical Education Department.
Source: Park A et al. ASCO 2018, Abstract LBA6002.
Cutaneous lupus: Switching antimalarials can delay immunosuppressive therapy
ORLANDO – , according to Anthony Fernandez, MD, PhD, director of medical and inpatient dermatology at the Cleveland Clinic.
A switch to chloroquine, or adding quinacrine, might do the trick, saving at least some patients from having to move on to immunosuppressive therapy, Dr. Fernandez said at the International Conference on Cutaneous Lupus Erythematosus.
“What we are learning from the literature is that we can switch from one antimalarial to another. We need to think about this in our algorithms before reaching for potentially more toxic immunosuppressives,” Dr. Fernandez said.
As for quinacrine, about two-thirds of patients who fail hydroxychloroquine or chloroquine will have a positive response to quinacrine if added (Br J Dermatol. 2017 Jul;177[1]:188-96). “It’s important to remember that we are not adding any ocular toxicity” with quinacrine, he said.
Quinacrine does come with a major concern of its own: the risk of aplastic anemia. However, this seems to occur with doses higher than 100 mg/day, which are no longer recommended; there have been no reports of aplastic anemia in patients on 100 mg/day or less.
Quinacrine “is an underutilized antimalarial. I think a lot of people don’t know about it or know how to get it. It can be compounded into capsules for patients,” and for a reasonable price, at about $20 for a month supply at some pharmacies, Dr. Fernandez said.
Meanwhile, because of the risk of retinal toxicity with hydroxychloroquine, there’s been a shift in recent years from dosing up to 6.5 mg/kg per day of ideal body weight to a ceiling of 5 mg/kg per day of actual body weight (JAMA Ophthalmol. 2014 Dec;132[12]:1453-60), and a ceiling of 2.3 mg/kg per day actual body weight for chloroquine.
The idea was to prevent overdosing in people who are under their ideal body weight, but there have been concerns about the efficacy of the new dosing regimen in other patients. Dr. Fernandez has not seen evidence of this. “We do adhere to the new dosing recommendations” at the Cleveland Clinic, and “personally, I think we are seeing similar efficacy,” he said.
The most important risk factor for retinal toxicity is cumulative dose. The risk seems to be extremely low in the first 5 years, but increases afterwards. Most patients who develop retinal toxicity have taken a cumulative hydroxychloroquine dose of 1,000 g, equal to about 400 mg/d for 7 years. “The longer you are on the medicine, the higher your risk of developing retinal toxicity,” he noted.
Regardless of weight, it’s recommended to limit hydroxychloroquine to 400 mg daily and chloroquine to 250 mg daily, with a baseline ocular exam, and – barring any intervening problems – annual screening after 5 years.
In patients with highly active skin disease at baseline, Dr. Fernandez said he will sometimes start hydroxychloroquine higher than 400 mg initially but will bring patients back down to 400 mg after a few months.
Dr. Fernandez had no relevant disclosures.
ORLANDO – , according to Anthony Fernandez, MD, PhD, director of medical and inpatient dermatology at the Cleveland Clinic.
A switch to chloroquine, or adding quinacrine, might do the trick, saving at least some patients from having to move on to immunosuppressive therapy, Dr. Fernandez said at the International Conference on Cutaneous Lupus Erythematosus.
“What we are learning from the literature is that we can switch from one antimalarial to another. We need to think about this in our algorithms before reaching for potentially more toxic immunosuppressives,” Dr. Fernandez said.
As for quinacrine, about two-thirds of patients who fail hydroxychloroquine or chloroquine will have a positive response to quinacrine if added (Br J Dermatol. 2017 Jul;177[1]:188-96). “It’s important to remember that we are not adding any ocular toxicity” with quinacrine, he said.
Quinacrine does come with a major concern of its own: the risk of aplastic anemia. However, this seems to occur with doses higher than 100 mg/day, which are no longer recommended; there have been no reports of aplastic anemia in patients on 100 mg/day or less.
Quinacrine “is an underutilized antimalarial. I think a lot of people don’t know about it or know how to get it. It can be compounded into capsules for patients,” and for a reasonable price, at about $20 for a month supply at some pharmacies, Dr. Fernandez said.
Meanwhile, because of the risk of retinal toxicity with hydroxychloroquine, there’s been a shift in recent years from dosing up to 6.5 mg/kg per day of ideal body weight to a ceiling of 5 mg/kg per day of actual body weight (JAMA Ophthalmol. 2014 Dec;132[12]:1453-60), and a ceiling of 2.3 mg/kg per day actual body weight for chloroquine.
The idea was to prevent overdosing in people who are under their ideal body weight, but there have been concerns about the efficacy of the new dosing regimen in other patients. Dr. Fernandez has not seen evidence of this. “We do adhere to the new dosing recommendations” at the Cleveland Clinic, and “personally, I think we are seeing similar efficacy,” he said.
The most important risk factor for retinal toxicity is cumulative dose. The risk seems to be extremely low in the first 5 years, but increases afterwards. Most patients who develop retinal toxicity have taken a cumulative hydroxychloroquine dose of 1,000 g, equal to about 400 mg/d for 7 years. “The longer you are on the medicine, the higher your risk of developing retinal toxicity,” he noted.
Regardless of weight, it’s recommended to limit hydroxychloroquine to 400 mg daily and chloroquine to 250 mg daily, with a baseline ocular exam, and – barring any intervening problems – annual screening after 5 years.
In patients with highly active skin disease at baseline, Dr. Fernandez said he will sometimes start hydroxychloroquine higher than 400 mg initially but will bring patients back down to 400 mg after a few months.
Dr. Fernandez had no relevant disclosures.
ORLANDO – , according to Anthony Fernandez, MD, PhD, director of medical and inpatient dermatology at the Cleveland Clinic.
A switch to chloroquine, or adding quinacrine, might do the trick, saving at least some patients from having to move on to immunosuppressive therapy, Dr. Fernandez said at the International Conference on Cutaneous Lupus Erythematosus.
“What we are learning from the literature is that we can switch from one antimalarial to another. We need to think about this in our algorithms before reaching for potentially more toxic immunosuppressives,” Dr. Fernandez said.
As for quinacrine, about two-thirds of patients who fail hydroxychloroquine or chloroquine will have a positive response to quinacrine if added (Br J Dermatol. 2017 Jul;177[1]:188-96). “It’s important to remember that we are not adding any ocular toxicity” with quinacrine, he said.
Quinacrine does come with a major concern of its own: the risk of aplastic anemia. However, this seems to occur with doses higher than 100 mg/day, which are no longer recommended; there have been no reports of aplastic anemia in patients on 100 mg/day or less.
Quinacrine “is an underutilized antimalarial. I think a lot of people don’t know about it or know how to get it. It can be compounded into capsules for patients,” and for a reasonable price, at about $20 for a month supply at some pharmacies, Dr. Fernandez said.
Meanwhile, because of the risk of retinal toxicity with hydroxychloroquine, there’s been a shift in recent years from dosing up to 6.5 mg/kg per day of ideal body weight to a ceiling of 5 mg/kg per day of actual body weight (JAMA Ophthalmol. 2014 Dec;132[12]:1453-60), and a ceiling of 2.3 mg/kg per day actual body weight for chloroquine.
The idea was to prevent overdosing in people who are under their ideal body weight, but there have been concerns about the efficacy of the new dosing regimen in other patients. Dr. Fernandez has not seen evidence of this. “We do adhere to the new dosing recommendations” at the Cleveland Clinic, and “personally, I think we are seeing similar efficacy,” he said.
The most important risk factor for retinal toxicity is cumulative dose. The risk seems to be extremely low in the first 5 years, but increases afterwards. Most patients who develop retinal toxicity have taken a cumulative hydroxychloroquine dose of 1,000 g, equal to about 400 mg/d for 7 years. “The longer you are on the medicine, the higher your risk of developing retinal toxicity,” he noted.
Regardless of weight, it’s recommended to limit hydroxychloroquine to 400 mg daily and chloroquine to 250 mg daily, with a baseline ocular exam, and – barring any intervening problems – annual screening after 5 years.
In patients with highly active skin disease at baseline, Dr. Fernandez said he will sometimes start hydroxychloroquine higher than 400 mg initially but will bring patients back down to 400 mg after a few months.
Dr. Fernandez had no relevant disclosures.
EXPERT ANALYSIS FROM ICCLE 2018
Novel antibody shifts ‘eat me/don’t eat me’ balance in refractory NHL
CHICAGO – A first-in-class antibody targeting the macrophage checkpoint CD47 is a promising novel immunotherapy in non-Hodgkin lymphoma, according to Ranjana H. Advani, MD, of Stanford (Calif.) Cancer Institute.
Treatment with Hu5F9-G4 (5F9), an antibody designed to overcome the “don’t eat me” signal associated with CD47, produced “encouraging” antitumor activity in a phase 1b study of 22 patients, Dr. Advani said in an oral abstract presentation at the annual meeting of the American Society of Clinical Oncology.
“5F9 was well tolerated in combination with rituximab, with no maximum tolerated dose achieved,” said Dr. Advani, noting that there were complete remissions in 43% of the refractory follicular lymphoma patients and 33% of refractory diffuse large B-cell lymphoma patients in the phase 1b/2 study.
The antibody has an on-target anemia effect that occurs upon administration, but that was mitigated considerably by a priming and maintenance dosing approach, she added.
The study has demonstrated “excellent” response rates in a highly refractory patient population, said Caron A. Jacobson, MD, of Dana-Farber Cancer Institute and Harvard Medical School in Boston. “Targeting CD47 ... really helps to shift the balance from ‘don’t eat me’ to ‘eat me,’ ” Dr. Jacobson said at the meeting.
“Importantly, we saw very little toxicity in the study, with very few grade 4 adverse events and no immune-related adverse events,” she added.
Most adverse events were grade 1 or 2, with the most common being the expected on-target anemia associated with 5F9. Using an initial priming dose of 5F9 results in a “temporary and mild decline” in hemoglobin due to clearance of aged red blood cells, Dr. Advani said.
The objective response rate in the study was 50%, with efficacy observed in rituximab-refractory patients, Dr. Advani said. With a median follow-up of greater than 6 months, just 1 of 11 responders had progressed. The median duration of response was not reached, with the longest complete remission lasting more than 14 months.
5F9 is able to selectively eliminate cancer cells through blockade of CD47, while rituximab enhances 5F9’s activity via antibody-dependent cellular phagocytosis, according to Dr. Advani.
“CD47 blockade takes the foot off the brakes, while rituximab puts the foot on the accelerator, leading to maximal tumor phagocytosis,” she said.
The Food and Drug Administration recently granted 5F9 a fast track designation for both diffuse large B-cell lymphoma and follicular lymphoma. Phase 2 investigations of 5F9 in these lymphomas are ongoing, Dr. Advani said.
The trial is sponsored by Forty Seven. Dr. Advani reported research funding from Forty Seven, which is developing 5F9, as well as disclosures related to AstraZeneca, Bayer, Bristol-Myers Squibb, Cell Medica, Genentech/Roche, Gilead Sciences, Pharmacyclics, and Seattle Genetics, among others.
SOURCE: Advani RH et al. ASCO 2018, abstract 7504.
CHICAGO – A first-in-class antibody targeting the macrophage checkpoint CD47 is a promising novel immunotherapy in non-Hodgkin lymphoma, according to Ranjana H. Advani, MD, of Stanford (Calif.) Cancer Institute.
Treatment with Hu5F9-G4 (5F9), an antibody designed to overcome the “don’t eat me” signal associated with CD47, produced “encouraging” antitumor activity in a phase 1b study of 22 patients, Dr. Advani said in an oral abstract presentation at the annual meeting of the American Society of Clinical Oncology.
“5F9 was well tolerated in combination with rituximab, with no maximum tolerated dose achieved,” said Dr. Advani, noting that there were complete remissions in 43% of the refractory follicular lymphoma patients and 33% of refractory diffuse large B-cell lymphoma patients in the phase 1b/2 study.
The antibody has an on-target anemia effect that occurs upon administration, but that was mitigated considerably by a priming and maintenance dosing approach, she added.
The study has demonstrated “excellent” response rates in a highly refractory patient population, said Caron A. Jacobson, MD, of Dana-Farber Cancer Institute and Harvard Medical School in Boston. “Targeting CD47 ... really helps to shift the balance from ‘don’t eat me’ to ‘eat me,’ ” Dr. Jacobson said at the meeting.
“Importantly, we saw very little toxicity in the study, with very few grade 4 adverse events and no immune-related adverse events,” she added.
Most adverse events were grade 1 or 2, with the most common being the expected on-target anemia associated with 5F9. Using an initial priming dose of 5F9 results in a “temporary and mild decline” in hemoglobin due to clearance of aged red blood cells, Dr. Advani said.
The objective response rate in the study was 50%, with efficacy observed in rituximab-refractory patients, Dr. Advani said. With a median follow-up of greater than 6 months, just 1 of 11 responders had progressed. The median duration of response was not reached, with the longest complete remission lasting more than 14 months.
5F9 is able to selectively eliminate cancer cells through blockade of CD47, while rituximab enhances 5F9’s activity via antibody-dependent cellular phagocytosis, according to Dr. Advani.
“CD47 blockade takes the foot off the brakes, while rituximab puts the foot on the accelerator, leading to maximal tumor phagocytosis,” she said.
The Food and Drug Administration recently granted 5F9 a fast track designation for both diffuse large B-cell lymphoma and follicular lymphoma. Phase 2 investigations of 5F9 in these lymphomas are ongoing, Dr. Advani said.
The trial is sponsored by Forty Seven. Dr. Advani reported research funding from Forty Seven, which is developing 5F9, as well as disclosures related to AstraZeneca, Bayer, Bristol-Myers Squibb, Cell Medica, Genentech/Roche, Gilead Sciences, Pharmacyclics, and Seattle Genetics, among others.
SOURCE: Advani RH et al. ASCO 2018, abstract 7504.
CHICAGO – A first-in-class antibody targeting the macrophage checkpoint CD47 is a promising novel immunotherapy in non-Hodgkin lymphoma, according to Ranjana H. Advani, MD, of Stanford (Calif.) Cancer Institute.
Treatment with Hu5F9-G4 (5F9), an antibody designed to overcome the “don’t eat me” signal associated with CD47, produced “encouraging” antitumor activity in a phase 1b study of 22 patients, Dr. Advani said in an oral abstract presentation at the annual meeting of the American Society of Clinical Oncology.
“5F9 was well tolerated in combination with rituximab, with no maximum tolerated dose achieved,” said Dr. Advani, noting that there were complete remissions in 43% of the refractory follicular lymphoma patients and 33% of refractory diffuse large B-cell lymphoma patients in the phase 1b/2 study.
The antibody has an on-target anemia effect that occurs upon administration, but that was mitigated considerably by a priming and maintenance dosing approach, she added.
The study has demonstrated “excellent” response rates in a highly refractory patient population, said Caron A. Jacobson, MD, of Dana-Farber Cancer Institute and Harvard Medical School in Boston. “Targeting CD47 ... really helps to shift the balance from ‘don’t eat me’ to ‘eat me,’ ” Dr. Jacobson said at the meeting.
“Importantly, we saw very little toxicity in the study, with very few grade 4 adverse events and no immune-related adverse events,” she added.
Most adverse events were grade 1 or 2, with the most common being the expected on-target anemia associated with 5F9. Using an initial priming dose of 5F9 results in a “temporary and mild decline” in hemoglobin due to clearance of aged red blood cells, Dr. Advani said.
The objective response rate in the study was 50%, with efficacy observed in rituximab-refractory patients, Dr. Advani said. With a median follow-up of greater than 6 months, just 1 of 11 responders had progressed. The median duration of response was not reached, with the longest complete remission lasting more than 14 months.
5F9 is able to selectively eliminate cancer cells through blockade of CD47, while rituximab enhances 5F9’s activity via antibody-dependent cellular phagocytosis, according to Dr. Advani.
“CD47 blockade takes the foot off the brakes, while rituximab puts the foot on the accelerator, leading to maximal tumor phagocytosis,” she said.
The Food and Drug Administration recently granted 5F9 a fast track designation for both diffuse large B-cell lymphoma and follicular lymphoma. Phase 2 investigations of 5F9 in these lymphomas are ongoing, Dr. Advani said.
The trial is sponsored by Forty Seven. Dr. Advani reported research funding from Forty Seven, which is developing 5F9, as well as disclosures related to AstraZeneca, Bayer, Bristol-Myers Squibb, Cell Medica, Genentech/Roche, Gilead Sciences, Pharmacyclics, and Seattle Genetics, among others.
SOURCE: Advani RH et al. ASCO 2018, abstract 7504.
REPORTING FROM ASCO 2018
Key clinical point:
Major finding: Complete responses were seen in 43% of follicular lymphoma (FL) patients and 33% of diffuse large B-cell lymphoma (DLBCL) patients.
Study details: Initial reported results from a phase 1b/2 study of 7 patients with FL and 15 patients with DLBCL.
Disclosures: Forty Seven sponsored the trial. Dr. Advani reported research funding from Forty Seven, which is developing 5F9, as well as disclosures related to AstraZeneca, Bayer, Bristol-Myers Squibb, Cell Medica, Genentech/Roche, Gilead Sciences, Pharmacyclics, and Seattle Genetics, among others.
Source: Advani RH et al. ASCO 2018, abstract 7504.