User login
Boston Children’s Hospital named nation’s best
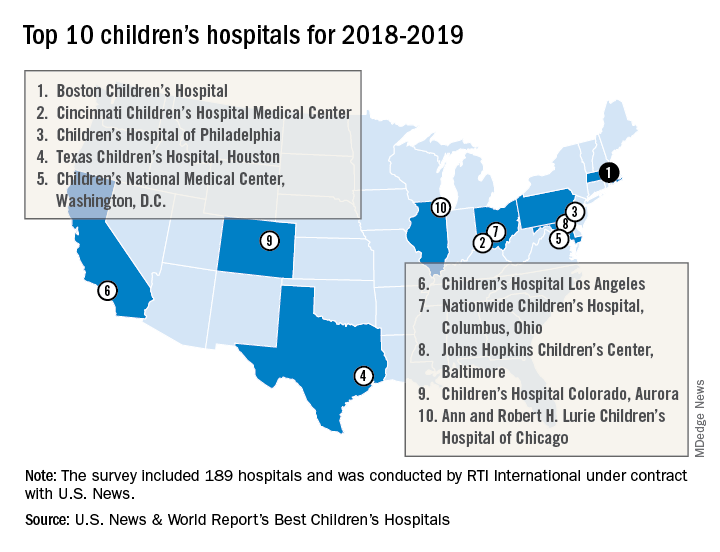
For the fourth consecutive year, Boston Children’s Hospital has been named the top children’s hospital by U.S. News & World Report.
The hospital finished among the top five in all 10 pediatric specialties included in the rankings: cancer (third), cardiology and heart surgery (second), diabetes and endocrinology (second), gastroenterology and GI surgery (second), neonatology (third), nephrology (first), neurology and neurosurgery (first), orthopedics (first), pulmonology (fourth), and urology (third), according to the 2018-2019 Best Children’s Hospitals rankings.
Of the 189 facilities that qualified for inclusion this year, 118 submitted sufficient data to be considered in at least 1 of the 10 specialties and 86 were ranked among the top 50 in at least 1 specialty. In addition, a survey of individuals conducted to establish the hospitals’ reputations – generally worth about 15% of a hospital’s score in each specialty – was completed by 4,165 physicians.
RTI International, a research and consulting firm, conducted the physician survey and produced the methodology and national rankings under contract with U.S. News.
For the fourth consecutive year, Boston Children’s Hospital has been named the top children’s hospital by U.S. News & World Report.
The hospital finished among the top five in all 10 pediatric specialties included in the rankings: cancer (third), cardiology and heart surgery (second), diabetes and endocrinology (second), gastroenterology and GI surgery (second), neonatology (third), nephrology (first), neurology and neurosurgery (first), orthopedics (first), pulmonology (fourth), and urology (third), according to the 2018-2019 Best Children’s Hospitals rankings.
Of the 189 facilities that qualified for inclusion this year, 118 submitted sufficient data to be considered in at least 1 of the 10 specialties and 86 were ranked among the top 50 in at least 1 specialty. In addition, a survey of individuals conducted to establish the hospitals’ reputations – generally worth about 15% of a hospital’s score in each specialty – was completed by 4,165 physicians.
RTI International, a research and consulting firm, conducted the physician survey and produced the methodology and national rankings under contract with U.S. News.
For the fourth consecutive year, Boston Children’s Hospital has been named the top children’s hospital by U.S. News & World Report.
The hospital finished among the top five in all 10 pediatric specialties included in the rankings: cancer (third), cardiology and heart surgery (second), diabetes and endocrinology (second), gastroenterology and GI surgery (second), neonatology (third), nephrology (first), neurology and neurosurgery (first), orthopedics (first), pulmonology (fourth), and urology (third), according to the 2018-2019 Best Children’s Hospitals rankings.
Of the 189 facilities that qualified for inclusion this year, 118 submitted sufficient data to be considered in at least 1 of the 10 specialties and 86 were ranked among the top 50 in at least 1 specialty. In addition, a survey of individuals conducted to establish the hospitals’ reputations – generally worth about 15% of a hospital’s score in each specialty – was completed by 4,165 physicians.
RTI International, a research and consulting firm, conducted the physician survey and produced the methodology and national rankings under contract with U.S. News.
Significant figures: The honesty in being precise
Physicists have strict rules about significant figures. Medical journals lack this professional discipline and it produces distortions that mislead readers.
Whenever you measure and report something in physics, the precision of the measurement is reflected in how the value is written. Writing a result with more digits implies that a higher precision was achieved. If that wasn’t the case, you are falsely claiming skill and accomplishment. You’ve entered the zone of post-truth.
This point was taught by my high school physics teacher, Mr. Gunnar Overgaard, may he rest in peace. Suppose we measured the length of the lab table with the meter stick. We repeated the action three times. We computed an average. Our table was 243.7 cm long. If we wrote 243.73 or 243.73333 we got a lower grade. Meter sticks only have markings of 0.1 cm. So the precision of the reported measurement should properly reflect that limitation.
Researchers in medicine seem to have skipped that lesson in physics lab. In medical journals, the default seems to be to report measurements with two decimal points, such as 16.67%, which is a gross distortion of the precision when I know that that really means 2 out of 12 patients had the finding.
This issue of precision came up recently in two papers published about the number of deaths caused by Hurricane Maria in Puerto Rico. The official death toll was 64. This number became a political hot potato when President Trump cited it as if it was evidence that he and the current local government had managed the emergency response better than George W. Bush did for Katrina.
On May 29, 2018, some researchers at the Harvard School of Public Health, a prestigious institution, published an article in The New England Journal of Medicine, a prestigious journal. You would presume that pair could report properly. The abstract said “This rate yielded a total of 4,645 excess deaths during this period (95% CI, 793 to 8,498).”1 Many newspapers published the number 4,645 in a headline. Most newspapers didn’t include all of the scientific mumbo jumbo about bias and confidence intervals.
However, the number 4,645 did not pass the sniff test at many newspapers, including the Washington Post. Their headline began “Harvard study estimates thousands died”2 and that story went on to clarify that “The Harvard study’s statistical analysis found that deaths related to the hurricane fell within a range of about 800 to more than 8,000.” That is one significant digit. Then the fact checkers went to work on it. They didn’t issue a Pinocchio score, but under a headline of “Did exactly 4,645 people die in Hurricane Maria? Nope”3 the fact checkers concluded that “it’s an egregious example of false precision to cite the ‘4,645’ number without explaining how fuzzy the number really is.”
The situation was compounded 3 days later when another news report had the Puerto Rico Department of Public Health putting the death toll at 1,397. Many assumptions go into determining what an excess death is. If the false precision makes it appear the scientists have a political agenda, it casts shade on whether the assumptions they made are objective and unbiased.
The result on social media was predictable. Outrage was expressed, as always. Lawsuits have been filed. The reputations of all scientists have been impugned. The implication is that, depending on your political polarization, you can choose the number 64, 1,000, 1,400, or 4,645 and any number is just as true as another. Worse, instead of focusing on the severity of the catastrophe and how we might have responded better then and better now and with better planning for the future, the debate has focused on alternative facts and fake scientific news. Thanks, Harvard.
So in the spirit of thinking globally but acting locally, what can I do? I love my editor. I have hinted before about how much easier it is to read, as well as more accurate scientifically, to round the numbers that we report. We've done it a few times recently, but now that the Washington Post has done it on a major news story, should this practice become the norm for journalism? If medical journal editors won't handle precision honestly, other journalists must step up. I'm distressed when I review an article that says 14.6% agreed and 79.2% strongly agreed and I know those percentages with 3 digits really mean 7/48 and 38/48, so they should be rounded to two significant figures. And isn’t it easier to read and comprehend if reporting that three treatment groups had positive findings of 4.25%, 12.08%, and 9.84% when rounded to 4%, 12%, and 10%?
Scientists using this false precision (and peer reviewers who allow it) need to be corrected. They are trying to sell their research as a Louis Vuitton handbag when we all know it is only a cheap knockoff.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected]
References
1. N Eng J Med. 2018 May 29. doi: 10.1056/NEJMsa1803972
2. “Harvard study estimates thousands died in Puerto Rico because of Hurricane Maria,” by Arelis R. Hernández and Laurie McGinley, The Washington Post, May 29, 2018.
3. “Did exactly 4,645 people die in Hurricane Maria? Nope.” by Glenn Kessler, The Washington Post, June 1, 2018.
Physicists have strict rules about significant figures. Medical journals lack this professional discipline and it produces distortions that mislead readers.
Whenever you measure and report something in physics, the precision of the measurement is reflected in how the value is written. Writing a result with more digits implies that a higher precision was achieved. If that wasn’t the case, you are falsely claiming skill and accomplishment. You’ve entered the zone of post-truth.
This point was taught by my high school physics teacher, Mr. Gunnar Overgaard, may he rest in peace. Suppose we measured the length of the lab table with the meter stick. We repeated the action three times. We computed an average. Our table was 243.7 cm long. If we wrote 243.73 or 243.73333 we got a lower grade. Meter sticks only have markings of 0.1 cm. So the precision of the reported measurement should properly reflect that limitation.
Researchers in medicine seem to have skipped that lesson in physics lab. In medical journals, the default seems to be to report measurements with two decimal points, such as 16.67%, which is a gross distortion of the precision when I know that that really means 2 out of 12 patients had the finding.
This issue of precision came up recently in two papers published about the number of deaths caused by Hurricane Maria in Puerto Rico. The official death toll was 64. This number became a political hot potato when President Trump cited it as if it was evidence that he and the current local government had managed the emergency response better than George W. Bush did for Katrina.
On May 29, 2018, some researchers at the Harvard School of Public Health, a prestigious institution, published an article in The New England Journal of Medicine, a prestigious journal. You would presume that pair could report properly. The abstract said “This rate yielded a total of 4,645 excess deaths during this period (95% CI, 793 to 8,498).”1 Many newspapers published the number 4,645 in a headline. Most newspapers didn’t include all of the scientific mumbo jumbo about bias and confidence intervals.
However, the number 4,645 did not pass the sniff test at many newspapers, including the Washington Post. Their headline began “Harvard study estimates thousands died”2 and that story went on to clarify that “The Harvard study’s statistical analysis found that deaths related to the hurricane fell within a range of about 800 to more than 8,000.” That is one significant digit. Then the fact checkers went to work on it. They didn’t issue a Pinocchio score, but under a headline of “Did exactly 4,645 people die in Hurricane Maria? Nope”3 the fact checkers concluded that “it’s an egregious example of false precision to cite the ‘4,645’ number without explaining how fuzzy the number really is.”
The situation was compounded 3 days later when another news report had the Puerto Rico Department of Public Health putting the death toll at 1,397. Many assumptions go into determining what an excess death is. If the false precision makes it appear the scientists have a political agenda, it casts shade on whether the assumptions they made are objective and unbiased.
The result on social media was predictable. Outrage was expressed, as always. Lawsuits have been filed. The reputations of all scientists have been impugned. The implication is that, depending on your political polarization, you can choose the number 64, 1,000, 1,400, or 4,645 and any number is just as true as another. Worse, instead of focusing on the severity of the catastrophe and how we might have responded better then and better now and with better planning for the future, the debate has focused on alternative facts and fake scientific news. Thanks, Harvard.
So in the spirit of thinking globally but acting locally, what can I do? I love my editor. I have hinted before about how much easier it is to read, as well as more accurate scientifically, to round the numbers that we report. We've done it a few times recently, but now that the Washington Post has done it on a major news story, should this practice become the norm for journalism? If medical journal editors won't handle precision honestly, other journalists must step up. I'm distressed when I review an article that says 14.6% agreed and 79.2% strongly agreed and I know those percentages with 3 digits really mean 7/48 and 38/48, so they should be rounded to two significant figures. And isn’t it easier to read and comprehend if reporting that three treatment groups had positive findings of 4.25%, 12.08%, and 9.84% when rounded to 4%, 12%, and 10%?
Scientists using this false precision (and peer reviewers who allow it) need to be corrected. They are trying to sell their research as a Louis Vuitton handbag when we all know it is only a cheap knockoff.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected]
References
1. N Eng J Med. 2018 May 29. doi: 10.1056/NEJMsa1803972
2. “Harvard study estimates thousands died in Puerto Rico because of Hurricane Maria,” by Arelis R. Hernández and Laurie McGinley, The Washington Post, May 29, 2018.
3. “Did exactly 4,645 people die in Hurricane Maria? Nope.” by Glenn Kessler, The Washington Post, June 1, 2018.
Physicists have strict rules about significant figures. Medical journals lack this professional discipline and it produces distortions that mislead readers.
Whenever you measure and report something in physics, the precision of the measurement is reflected in how the value is written. Writing a result with more digits implies that a higher precision was achieved. If that wasn’t the case, you are falsely claiming skill and accomplishment. You’ve entered the zone of post-truth.
This point was taught by my high school physics teacher, Mr. Gunnar Overgaard, may he rest in peace. Suppose we measured the length of the lab table with the meter stick. We repeated the action three times. We computed an average. Our table was 243.7 cm long. If we wrote 243.73 or 243.73333 we got a lower grade. Meter sticks only have markings of 0.1 cm. So the precision of the reported measurement should properly reflect that limitation.
Researchers in medicine seem to have skipped that lesson in physics lab. In medical journals, the default seems to be to report measurements with two decimal points, such as 16.67%, which is a gross distortion of the precision when I know that that really means 2 out of 12 patients had the finding.
This issue of precision came up recently in two papers published about the number of deaths caused by Hurricane Maria in Puerto Rico. The official death toll was 64. This number became a political hot potato when President Trump cited it as if it was evidence that he and the current local government had managed the emergency response better than George W. Bush did for Katrina.
On May 29, 2018, some researchers at the Harvard School of Public Health, a prestigious institution, published an article in The New England Journal of Medicine, a prestigious journal. You would presume that pair could report properly. The abstract said “This rate yielded a total of 4,645 excess deaths during this period (95% CI, 793 to 8,498).”1 Many newspapers published the number 4,645 in a headline. Most newspapers didn’t include all of the scientific mumbo jumbo about bias and confidence intervals.
However, the number 4,645 did not pass the sniff test at many newspapers, including the Washington Post. Their headline began “Harvard study estimates thousands died”2 and that story went on to clarify that “The Harvard study’s statistical analysis found that deaths related to the hurricane fell within a range of about 800 to more than 8,000.” That is one significant digit. Then the fact checkers went to work on it. They didn’t issue a Pinocchio score, but under a headline of “Did exactly 4,645 people die in Hurricane Maria? Nope”3 the fact checkers concluded that “it’s an egregious example of false precision to cite the ‘4,645’ number without explaining how fuzzy the number really is.”
The situation was compounded 3 days later when another news report had the Puerto Rico Department of Public Health putting the death toll at 1,397. Many assumptions go into determining what an excess death is. If the false precision makes it appear the scientists have a political agenda, it casts shade on whether the assumptions they made are objective and unbiased.
The result on social media was predictable. Outrage was expressed, as always. Lawsuits have been filed. The reputations of all scientists have been impugned. The implication is that, depending on your political polarization, you can choose the number 64, 1,000, 1,400, or 4,645 and any number is just as true as another. Worse, instead of focusing on the severity of the catastrophe and how we might have responded better then and better now and with better planning for the future, the debate has focused on alternative facts and fake scientific news. Thanks, Harvard.
So in the spirit of thinking globally but acting locally, what can I do? I love my editor. I have hinted before about how much easier it is to read, as well as more accurate scientifically, to round the numbers that we report. We've done it a few times recently, but now that the Washington Post has done it on a major news story, should this practice become the norm for journalism? If medical journal editors won't handle precision honestly, other journalists must step up. I'm distressed when I review an article that says 14.6% agreed and 79.2% strongly agreed and I know those percentages with 3 digits really mean 7/48 and 38/48, so they should be rounded to two significant figures. And isn’t it easier to read and comprehend if reporting that three treatment groups had positive findings of 4.25%, 12.08%, and 9.84% when rounded to 4%, 12%, and 10%?
Scientists using this false precision (and peer reviewers who allow it) need to be corrected. They are trying to sell their research as a Louis Vuitton handbag when we all know it is only a cheap knockoff.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected]
References
1. N Eng J Med. 2018 May 29. doi: 10.1056/NEJMsa1803972
2. “Harvard study estimates thousands died in Puerto Rico because of Hurricane Maria,” by Arelis R. Hernández and Laurie McGinley, The Washington Post, May 29, 2018.
3. “Did exactly 4,645 people die in Hurricane Maria? Nope.” by Glenn Kessler, The Washington Post, June 1, 2018.
How to Manage Diabetes While Keeping Costs Down
A cost-effective community program at Pennsylvania State University helped most participants change their behavior and significantly improve their HbA1c and blood pressure, according to a report in Preventing Chronic Disease.
The researchers for the extension program, Dining with Diabetes, collected data on 2,738 adults with type 2 diabetes or prediabetes and adult family members without diabetes. The program consisted of 4 weekly 2-hour classes and a follow-up class conducted 3 months later. The classes included hands-on food preparation, food tastings, and physical activity.
At the follow-up class, participants who completed the program had significant improvements in diabetes-related biomarkers. A greater percentage said they were confident they could keep their diabetes under control, compared with the number at baseline (67% v 58%). At baseline, most participants were adhering to medications; the researchers found no significant change in adherence.
Participants also increased the number of days per week on which they exercised for ≥ 20 minutes (from 2.9 to 3.4 days), and slightly increased the number of days on which they ate a variety of fruits and vegetables.
Nearly half of participants with baseline and follow-up measurements had a drop in HbA1c. At follow-up, 21% had a reduction large enough to lower their diabetes status. The changes translated to a 5.9% decrease in HbA1c for 27% of those who had uncontrolled diabetes at baseline. More than half (59%) had a drop in blood pressure, including 60% of those with uncontrolled diabetes.
The program, which was free to participants, cost Penn State Extension $407 per person. The researchers estimate that extending the program to half of the 1.3 million people with diabetes in Pennsylvania would save the state approximately $195 million at 1 year.
A cost-effective community program at Pennsylvania State University helped most participants change their behavior and significantly improve their HbA1c and blood pressure, according to a report in Preventing Chronic Disease.
The researchers for the extension program, Dining with Diabetes, collected data on 2,738 adults with type 2 diabetes or prediabetes and adult family members without diabetes. The program consisted of 4 weekly 2-hour classes and a follow-up class conducted 3 months later. The classes included hands-on food preparation, food tastings, and physical activity.
At the follow-up class, participants who completed the program had significant improvements in diabetes-related biomarkers. A greater percentage said they were confident they could keep their diabetes under control, compared with the number at baseline (67% v 58%). At baseline, most participants were adhering to medications; the researchers found no significant change in adherence.
Participants also increased the number of days per week on which they exercised for ≥ 20 minutes (from 2.9 to 3.4 days), and slightly increased the number of days on which they ate a variety of fruits and vegetables.
Nearly half of participants with baseline and follow-up measurements had a drop in HbA1c. At follow-up, 21% had a reduction large enough to lower their diabetes status. The changes translated to a 5.9% decrease in HbA1c for 27% of those who had uncontrolled diabetes at baseline. More than half (59%) had a drop in blood pressure, including 60% of those with uncontrolled diabetes.
The program, which was free to participants, cost Penn State Extension $407 per person. The researchers estimate that extending the program to half of the 1.3 million people with diabetes in Pennsylvania would save the state approximately $195 million at 1 year.
A cost-effective community program at Pennsylvania State University helped most participants change their behavior and significantly improve their HbA1c and blood pressure, according to a report in Preventing Chronic Disease.
The researchers for the extension program, Dining with Diabetes, collected data on 2,738 adults with type 2 diabetes or prediabetes and adult family members without diabetes. The program consisted of 4 weekly 2-hour classes and a follow-up class conducted 3 months later. The classes included hands-on food preparation, food tastings, and physical activity.
At the follow-up class, participants who completed the program had significant improvements in diabetes-related biomarkers. A greater percentage said they were confident they could keep their diabetes under control, compared with the number at baseline (67% v 58%). At baseline, most participants were adhering to medications; the researchers found no significant change in adherence.
Participants also increased the number of days per week on which they exercised for ≥ 20 minutes (from 2.9 to 3.4 days), and slightly increased the number of days on which they ate a variety of fruits and vegetables.
Nearly half of participants with baseline and follow-up measurements had a drop in HbA1c. At follow-up, 21% had a reduction large enough to lower their diabetes status. The changes translated to a 5.9% decrease in HbA1c for 27% of those who had uncontrolled diabetes at baseline. More than half (59%) had a drop in blood pressure, including 60% of those with uncontrolled diabetes.
The program, which was free to participants, cost Penn State Extension $407 per person. The researchers estimate that extending the program to half of the 1.3 million people with diabetes in Pennsylvania would save the state approximately $195 million at 1 year.
Acute Hepatitis E Superinfection Reactivates Chronic HBV
Many things can reactivate chronic hepatitis B virus (HBV) infection—withdrawal of antiviral therapy, pregnancy, and chemotherapy, to name a few. So when a patient with stable chronic HBV virus presented with significant hepatitis flare, clinicians from Beth Israel Deaconess Medical Center in Boston, Massachusetts, had a long list to check.
They first ruled out drug-associated hepatotoxicity and screened the patient for common causes of acute hepatitis. Beyond the HBV, the patient did not have other significant medical conditions, had not had close contact with anyone ill, and was not pregnant. Tests were negative for cytomegalovirus, Epstein-Barr syndrome, HIV, hepatitis A, C, and D. The patient tested negative for both antihepatitis E virus (HEV) IgM and IgG in a visit about 9 months before.
However, she reported regularly consuming pork, including a recent barbecue meal. Thus, the clinicians focused on HEV serology, which confirmed that she had an acute HEV infection.
Pigs act as a “natural reservoir” for HEV; contaminated meats and direct contact with animals are the most common causes of HEV human infection in industrialized countries. Recent data reveal the prevalence of HEV antibodies in the US is about 6%, illustrating that it is not as uncommon as it was thought to be. Although there was no direct evidence to confirm the source of her infection, it seemed likely due to the pork consumption.
The patient was started on tenofovir but stopped it 4 months later because she felt well. After a subsequent flare, “repeated counseling” persuaded the patient to start on entecavir, with successful viral suppression.
Hepatitis E superinfection on chronic HBV can contribute to significant morbidity and mortality, the clinicians say, particularly in patients with cirrhosis. Concurrent infection with another viral hepatitis should be considered in both immunodeficient and immunocompetent patients with chronic HBV reactivation.
Source:
Aslam A, Susheela A, Iriana S, Chan SS, Lau D. BMJ Case Rep. 2018;2018. pii: bcr-2017-223616.
doi: 10.1136/bcr-2017-223616.
Many things can reactivate chronic hepatitis B virus (HBV) infection—withdrawal of antiviral therapy, pregnancy, and chemotherapy, to name a few. So when a patient with stable chronic HBV virus presented with significant hepatitis flare, clinicians from Beth Israel Deaconess Medical Center in Boston, Massachusetts, had a long list to check.
They first ruled out drug-associated hepatotoxicity and screened the patient for common causes of acute hepatitis. Beyond the HBV, the patient did not have other significant medical conditions, had not had close contact with anyone ill, and was not pregnant. Tests were negative for cytomegalovirus, Epstein-Barr syndrome, HIV, hepatitis A, C, and D. The patient tested negative for both antihepatitis E virus (HEV) IgM and IgG in a visit about 9 months before.
However, she reported regularly consuming pork, including a recent barbecue meal. Thus, the clinicians focused on HEV serology, which confirmed that she had an acute HEV infection.
Pigs act as a “natural reservoir” for HEV; contaminated meats and direct contact with animals are the most common causes of HEV human infection in industrialized countries. Recent data reveal the prevalence of HEV antibodies in the US is about 6%, illustrating that it is not as uncommon as it was thought to be. Although there was no direct evidence to confirm the source of her infection, it seemed likely due to the pork consumption.
The patient was started on tenofovir but stopped it 4 months later because she felt well. After a subsequent flare, “repeated counseling” persuaded the patient to start on entecavir, with successful viral suppression.
Hepatitis E superinfection on chronic HBV can contribute to significant morbidity and mortality, the clinicians say, particularly in patients with cirrhosis. Concurrent infection with another viral hepatitis should be considered in both immunodeficient and immunocompetent patients with chronic HBV reactivation.
Source:
Aslam A, Susheela A, Iriana S, Chan SS, Lau D. BMJ Case Rep. 2018;2018. pii: bcr-2017-223616.
doi: 10.1136/bcr-2017-223616.
Many things can reactivate chronic hepatitis B virus (HBV) infection—withdrawal of antiviral therapy, pregnancy, and chemotherapy, to name a few. So when a patient with stable chronic HBV virus presented with significant hepatitis flare, clinicians from Beth Israel Deaconess Medical Center in Boston, Massachusetts, had a long list to check.
They first ruled out drug-associated hepatotoxicity and screened the patient for common causes of acute hepatitis. Beyond the HBV, the patient did not have other significant medical conditions, had not had close contact with anyone ill, and was not pregnant. Tests were negative for cytomegalovirus, Epstein-Barr syndrome, HIV, hepatitis A, C, and D. The patient tested negative for both antihepatitis E virus (HEV) IgM and IgG in a visit about 9 months before.
However, she reported regularly consuming pork, including a recent barbecue meal. Thus, the clinicians focused on HEV serology, which confirmed that she had an acute HEV infection.
Pigs act as a “natural reservoir” for HEV; contaminated meats and direct contact with animals are the most common causes of HEV human infection in industrialized countries. Recent data reveal the prevalence of HEV antibodies in the US is about 6%, illustrating that it is not as uncommon as it was thought to be. Although there was no direct evidence to confirm the source of her infection, it seemed likely due to the pork consumption.
The patient was started on tenofovir but stopped it 4 months later because she felt well. After a subsequent flare, “repeated counseling” persuaded the patient to start on entecavir, with successful viral suppression.
Hepatitis E superinfection on chronic HBV can contribute to significant morbidity and mortality, the clinicians say, particularly in patients with cirrhosis. Concurrent infection with another viral hepatitis should be considered in both immunodeficient and immunocompetent patients with chronic HBV reactivation.
Source:
Aslam A, Susheela A, Iriana S, Chan SS, Lau D. BMJ Case Rep. 2018;2018. pii: bcr-2017-223616.
doi: 10.1136/bcr-2017-223616.
Voxelotor benefits adolescents with SCD
STOCKHOLM—An ongoing phase 2 study suggests voxelotor (GBT440) can benefit adolescents with sickle cell disease (SCD).
In the HOPE-KIDS 1 study, voxelotor produced sustained improvements in hemoglobin levels and a reduction in clinical measures of hemolysis in a cohort of adolescents with SCD, most of whom were also receiving hydroxyurea (HU).
The most common adverse events (AEs) related to voxelotor were nausea, vomiting, headache, and rash.
These results were presented in a poster (abstract PF709) at the 23rd Congress of the European Hematology Association (EHA).
HOPE-KIDS 1 is sponsored by Global Blood Therapeutics, Inc.
In this study, researchers are evaluating voxelotor in SCD patients ages 6 to 17. In part A, researchers evaluated a 600 mg daily dose of voxelotor. In part B, they are testing voxelotor at daily doses of 900 mg and 1500 mg in patients ages 12 to 17.
At EHA, the researchers presented data on 25 patients who received voxelotor at 900 mg/day for 24 weeks in part B. Eighty-eight percent of the patients (n=22) were also taking HU.
The patients’ median age was 14 (range, 12-17), and 56% were male. Ninety-six percent (n=24) had the HbSS genotype.
Forty-eight percent of patients had 1 to 4 vaso-occlusive crises (VOCs) in the past year, 8% had more than 4 VOCs, and 44% had 0 VOCs.
At baseline, the median hemoglobin was 8.9 g/dL, the median fetal hemoglobin was 10.8 g/dL, and the median time-averaged mean of maximum velocity was 110 cm/s.
All 25 patients were dosed with voxelotor, and 22 completed 24 weeks of dosing. One patient withdrew consent, 1 was lost to follow-up, and 1 patient discontinued due to noncompliance.
Of the 22 patients who completed 24 weeks of voxelotor treatment, all but 3 were receiving concurrent HU.
Results
Voxelotor-related AEs occurring in at least 2 patients included nausea (12%, n=3), vomiting (8%, n=2), headache (8%, n=2), and rash (8%, n=2).
There was 1 case of grade 3 urticaria, which resolved and did not recur with continued dosing. There were no discontinuations of voxelotor due to AEs.
Patients experienced increased hemoglobin levels and improved clinical measures of hemolysis at 24 weeks, as evaluated by changes from baseline in hemoglobin, percent of reticulocytes, and percent of unconjugated bilirubin.
In all, 43% of patients (9/21) achieved a hemoglobin response (>1 g/dL) at 24 weeks. The median hemoglobin change from baseline was 0.7 g/dL, the median reduction in reticulocytes was 22.9%, and the median reduction in unconjugated bilirubin was 38.6%.
Sixty-two percent of patients (13/21) had a reduction in daily symptoms at 24 weeks, as assessed by total symptom scores (TSS). There was a 39% median reduction in TSS from baseline.
Fifty-five percent of patients (11/20) had a numerical decrease in transcranial doppler (TCD) flow at 24 weeks. Among hemoglobin responders (>1 g/dL), 88% (7/8) had a numerical decrease in TCD at 24 weeks.
“We continue to be encouraged by the results of the ongoing HOPE-KIDS 1 study, which are consistent with inhibition of HbS polymerization by voxelotor and support its ongoing clinical evaluation as a potential disease-modifying therapy for both adults and adolescents with SCD,” said Ted W. Love, MD, president and chief executive officer of Global Blood Therapeutics.
“Results to date support our ongoing development of voxelotor in a broad range of patients, including in our phase 3 HOPE study, which is also evaluating voxelotor at doses of 900 mg and 1500 mg per day in adolescents and adults. We continue to expect to announce top-line clinical data from part A of the HOPE study by the end of this quarter.”
STOCKHOLM—An ongoing phase 2 study suggests voxelotor (GBT440) can benefit adolescents with sickle cell disease (SCD).
In the HOPE-KIDS 1 study, voxelotor produced sustained improvements in hemoglobin levels and a reduction in clinical measures of hemolysis in a cohort of adolescents with SCD, most of whom were also receiving hydroxyurea (HU).
The most common adverse events (AEs) related to voxelotor were nausea, vomiting, headache, and rash.
These results were presented in a poster (abstract PF709) at the 23rd Congress of the European Hematology Association (EHA).
HOPE-KIDS 1 is sponsored by Global Blood Therapeutics, Inc.
In this study, researchers are evaluating voxelotor in SCD patients ages 6 to 17. In part A, researchers evaluated a 600 mg daily dose of voxelotor. In part B, they are testing voxelotor at daily doses of 900 mg and 1500 mg in patients ages 12 to 17.
At EHA, the researchers presented data on 25 patients who received voxelotor at 900 mg/day for 24 weeks in part B. Eighty-eight percent of the patients (n=22) were also taking HU.
The patients’ median age was 14 (range, 12-17), and 56% were male. Ninety-six percent (n=24) had the HbSS genotype.
Forty-eight percent of patients had 1 to 4 vaso-occlusive crises (VOCs) in the past year, 8% had more than 4 VOCs, and 44% had 0 VOCs.
At baseline, the median hemoglobin was 8.9 g/dL, the median fetal hemoglobin was 10.8 g/dL, and the median time-averaged mean of maximum velocity was 110 cm/s.
All 25 patients were dosed with voxelotor, and 22 completed 24 weeks of dosing. One patient withdrew consent, 1 was lost to follow-up, and 1 patient discontinued due to noncompliance.
Of the 22 patients who completed 24 weeks of voxelotor treatment, all but 3 were receiving concurrent HU.
Results
Voxelotor-related AEs occurring in at least 2 patients included nausea (12%, n=3), vomiting (8%, n=2), headache (8%, n=2), and rash (8%, n=2).
There was 1 case of grade 3 urticaria, which resolved and did not recur with continued dosing. There were no discontinuations of voxelotor due to AEs.
Patients experienced increased hemoglobin levels and improved clinical measures of hemolysis at 24 weeks, as evaluated by changes from baseline in hemoglobin, percent of reticulocytes, and percent of unconjugated bilirubin.
In all, 43% of patients (9/21) achieved a hemoglobin response (>1 g/dL) at 24 weeks. The median hemoglobin change from baseline was 0.7 g/dL, the median reduction in reticulocytes was 22.9%, and the median reduction in unconjugated bilirubin was 38.6%.
Sixty-two percent of patients (13/21) had a reduction in daily symptoms at 24 weeks, as assessed by total symptom scores (TSS). There was a 39% median reduction in TSS from baseline.
Fifty-five percent of patients (11/20) had a numerical decrease in transcranial doppler (TCD) flow at 24 weeks. Among hemoglobin responders (>1 g/dL), 88% (7/8) had a numerical decrease in TCD at 24 weeks.
“We continue to be encouraged by the results of the ongoing HOPE-KIDS 1 study, which are consistent with inhibition of HbS polymerization by voxelotor and support its ongoing clinical evaluation as a potential disease-modifying therapy for both adults and adolescents with SCD,” said Ted W. Love, MD, president and chief executive officer of Global Blood Therapeutics.
“Results to date support our ongoing development of voxelotor in a broad range of patients, including in our phase 3 HOPE study, which is also evaluating voxelotor at doses of 900 mg and 1500 mg per day in adolescents and adults. We continue to expect to announce top-line clinical data from part A of the HOPE study by the end of this quarter.”
STOCKHOLM—An ongoing phase 2 study suggests voxelotor (GBT440) can benefit adolescents with sickle cell disease (SCD).
In the HOPE-KIDS 1 study, voxelotor produced sustained improvements in hemoglobin levels and a reduction in clinical measures of hemolysis in a cohort of adolescents with SCD, most of whom were also receiving hydroxyurea (HU).
The most common adverse events (AEs) related to voxelotor were nausea, vomiting, headache, and rash.
These results were presented in a poster (abstract PF709) at the 23rd Congress of the European Hematology Association (EHA).
HOPE-KIDS 1 is sponsored by Global Blood Therapeutics, Inc.
In this study, researchers are evaluating voxelotor in SCD patients ages 6 to 17. In part A, researchers evaluated a 600 mg daily dose of voxelotor. In part B, they are testing voxelotor at daily doses of 900 mg and 1500 mg in patients ages 12 to 17.
At EHA, the researchers presented data on 25 patients who received voxelotor at 900 mg/day for 24 weeks in part B. Eighty-eight percent of the patients (n=22) were also taking HU.
The patients’ median age was 14 (range, 12-17), and 56% were male. Ninety-six percent (n=24) had the HbSS genotype.
Forty-eight percent of patients had 1 to 4 vaso-occlusive crises (VOCs) in the past year, 8% had more than 4 VOCs, and 44% had 0 VOCs.
At baseline, the median hemoglobin was 8.9 g/dL, the median fetal hemoglobin was 10.8 g/dL, and the median time-averaged mean of maximum velocity was 110 cm/s.
All 25 patients were dosed with voxelotor, and 22 completed 24 weeks of dosing. One patient withdrew consent, 1 was lost to follow-up, and 1 patient discontinued due to noncompliance.
Of the 22 patients who completed 24 weeks of voxelotor treatment, all but 3 were receiving concurrent HU.
Results
Voxelotor-related AEs occurring in at least 2 patients included nausea (12%, n=3), vomiting (8%, n=2), headache (8%, n=2), and rash (8%, n=2).
There was 1 case of grade 3 urticaria, which resolved and did not recur with continued dosing. There were no discontinuations of voxelotor due to AEs.
Patients experienced increased hemoglobin levels and improved clinical measures of hemolysis at 24 weeks, as evaluated by changes from baseline in hemoglobin, percent of reticulocytes, and percent of unconjugated bilirubin.
In all, 43% of patients (9/21) achieved a hemoglobin response (>1 g/dL) at 24 weeks. The median hemoglobin change from baseline was 0.7 g/dL, the median reduction in reticulocytes was 22.9%, and the median reduction in unconjugated bilirubin was 38.6%.
Sixty-two percent of patients (13/21) had a reduction in daily symptoms at 24 weeks, as assessed by total symptom scores (TSS). There was a 39% median reduction in TSS from baseline.
Fifty-five percent of patients (11/20) had a numerical decrease in transcranial doppler (TCD) flow at 24 weeks. Among hemoglobin responders (>1 g/dL), 88% (7/8) had a numerical decrease in TCD at 24 weeks.
“We continue to be encouraged by the results of the ongoing HOPE-KIDS 1 study, which are consistent with inhibition of HbS polymerization by voxelotor and support its ongoing clinical evaluation as a potential disease-modifying therapy for both adults and adolescents with SCD,” said Ted W. Love, MD, president and chief executive officer of Global Blood Therapeutics.
“Results to date support our ongoing development of voxelotor in a broad range of patients, including in our phase 3 HOPE study, which is also evaluating voxelotor at doses of 900 mg and 1500 mg per day in adolescents and adults. We continue to expect to announce top-line clinical data from part A of the HOPE study by the end of this quarter.”
Ibrutinib sNDA receives priority review
The US Food and Drug Administration (FDA) has accepted for priority review a supplemental new drug application (sNDA) for ibrutinib (Imbruvica®) to be used in combination with rituximab in patients with Waldenström’s macroglobulinemia (WM).
The FDA intends to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
Ibrutinib is a first-in-class Bruton’s tyrosine kinase inhibitor jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and Janssen Biotech, Inc.
Ibrutinib is already FDA-approved to treat chronic lymphocytic leukemia/small lymphocytic lymphoma, previously treated mantle cell lymphoma, previously treated marginal zone lymphoma, previously treated chronic graft-versus-host disease, and WM.
If the new sNDA is approved, the use of ibrutinib in WM will extend beyond its current approved use as a single agent.
Phase 3 trial
The sNDA for ibrutinib and rituximab in WM is supported by data from the phase 3 iNNOVATE study. Results from this trial were presented at the 2018 ASCO Annual Meeting (abstract 8003) and were simultaneously published in NEJM.
iNNOVATE is a placebo-controlled, double-blind, phase 3 study that enrolled 150 patients with relapsed/refractory and treatment-naïve WM.
All patients received rituximab at 375 mg/m2 with weekly infusions at weeks 1 to 4 and 17 to 20. They also received either ibrutinib (420 mg) or placebo once daily continuously until criteria for permanent discontinuation were met.
Overall response rates were significantly higher in the ibrutinib arm than the placebo arm—92% and 47%, respectively (P<0.0001). Complete response rates were 3% and 1%, respectively.
The median time to next treatment was not reached for the ibrutinib arm and was 18 months for the placebo arm (hazard ratio=0.096; P<0.0001). Of the patients randomized to ibrutinib plus rituximab, 75% continued on treatment at last follow-up.
The 30-month progression-free survival rates were 82% in the ibrutinib arm and 28% in the placebo arm. The median progression-free survival was not reached in the ibrutinib arm and was 20.3 months in the placebo arm (hazard ratio=0.20; P<0.0001).
The 30-month overall survival rates were 94% in the ibrutinib arm and 92% in the placebo arm.
Grade 3 or higher treatment-emergent adverse events (AEs) occurred in 60% of patients in the ibrutinib arm and 61% in the placebo arm.
Serious AEs occurred in 43% and 33% of patients, respectively. There were no fatal AEs in the ibrutinib arm and 3 in the rituximab arm.
Grade 3 or higher AEs that occurred more frequently in the ibrutinib arm than the placebo arm included atrial fibrillation (12% vs 1%) and hypertension (13% vs 4%).
AEs that occurred less frequently in the ibrutinib arm than the placebo arm included grade 3 or higher infusion reactions (1% vs 16%) and any-grade IgM flare (8% vs 47%).
The US Food and Drug Administration (FDA) has accepted for priority review a supplemental new drug application (sNDA) for ibrutinib (Imbruvica®) to be used in combination with rituximab in patients with Waldenström’s macroglobulinemia (WM).
The FDA intends to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
Ibrutinib is a first-in-class Bruton’s tyrosine kinase inhibitor jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and Janssen Biotech, Inc.
Ibrutinib is already FDA-approved to treat chronic lymphocytic leukemia/small lymphocytic lymphoma, previously treated mantle cell lymphoma, previously treated marginal zone lymphoma, previously treated chronic graft-versus-host disease, and WM.
If the new sNDA is approved, the use of ibrutinib in WM will extend beyond its current approved use as a single agent.
Phase 3 trial
The sNDA for ibrutinib and rituximab in WM is supported by data from the phase 3 iNNOVATE study. Results from this trial were presented at the 2018 ASCO Annual Meeting (abstract 8003) and were simultaneously published in NEJM.
iNNOVATE is a placebo-controlled, double-blind, phase 3 study that enrolled 150 patients with relapsed/refractory and treatment-naïve WM.
All patients received rituximab at 375 mg/m2 with weekly infusions at weeks 1 to 4 and 17 to 20. They also received either ibrutinib (420 mg) or placebo once daily continuously until criteria for permanent discontinuation were met.
Overall response rates were significantly higher in the ibrutinib arm than the placebo arm—92% and 47%, respectively (P<0.0001). Complete response rates were 3% and 1%, respectively.
The median time to next treatment was not reached for the ibrutinib arm and was 18 months for the placebo arm (hazard ratio=0.096; P<0.0001). Of the patients randomized to ibrutinib plus rituximab, 75% continued on treatment at last follow-up.
The 30-month progression-free survival rates were 82% in the ibrutinib arm and 28% in the placebo arm. The median progression-free survival was not reached in the ibrutinib arm and was 20.3 months in the placebo arm (hazard ratio=0.20; P<0.0001).
The 30-month overall survival rates were 94% in the ibrutinib arm and 92% in the placebo arm.
Grade 3 or higher treatment-emergent adverse events (AEs) occurred in 60% of patients in the ibrutinib arm and 61% in the placebo arm.
Serious AEs occurred in 43% and 33% of patients, respectively. There were no fatal AEs in the ibrutinib arm and 3 in the rituximab arm.
Grade 3 or higher AEs that occurred more frequently in the ibrutinib arm than the placebo arm included atrial fibrillation (12% vs 1%) and hypertension (13% vs 4%).
AEs that occurred less frequently in the ibrutinib arm than the placebo arm included grade 3 or higher infusion reactions (1% vs 16%) and any-grade IgM flare (8% vs 47%).
The US Food and Drug Administration (FDA) has accepted for priority review a supplemental new drug application (sNDA) for ibrutinib (Imbruvica®) to be used in combination with rituximab in patients with Waldenström’s macroglobulinemia (WM).
The FDA intends to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
Ibrutinib is a first-in-class Bruton’s tyrosine kinase inhibitor jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and Janssen Biotech, Inc.
Ibrutinib is already FDA-approved to treat chronic lymphocytic leukemia/small lymphocytic lymphoma, previously treated mantle cell lymphoma, previously treated marginal zone lymphoma, previously treated chronic graft-versus-host disease, and WM.
If the new sNDA is approved, the use of ibrutinib in WM will extend beyond its current approved use as a single agent.
Phase 3 trial
The sNDA for ibrutinib and rituximab in WM is supported by data from the phase 3 iNNOVATE study. Results from this trial were presented at the 2018 ASCO Annual Meeting (abstract 8003) and were simultaneously published in NEJM.
iNNOVATE is a placebo-controlled, double-blind, phase 3 study that enrolled 150 patients with relapsed/refractory and treatment-naïve WM.
All patients received rituximab at 375 mg/m2 with weekly infusions at weeks 1 to 4 and 17 to 20. They also received either ibrutinib (420 mg) or placebo once daily continuously until criteria for permanent discontinuation were met.
Overall response rates were significantly higher in the ibrutinib arm than the placebo arm—92% and 47%, respectively (P<0.0001). Complete response rates were 3% and 1%, respectively.
The median time to next treatment was not reached for the ibrutinib arm and was 18 months for the placebo arm (hazard ratio=0.096; P<0.0001). Of the patients randomized to ibrutinib plus rituximab, 75% continued on treatment at last follow-up.
The 30-month progression-free survival rates were 82% in the ibrutinib arm and 28% in the placebo arm. The median progression-free survival was not reached in the ibrutinib arm and was 20.3 months in the placebo arm (hazard ratio=0.20; P<0.0001).
The 30-month overall survival rates were 94% in the ibrutinib arm and 92% in the placebo arm.
Grade 3 or higher treatment-emergent adverse events (AEs) occurred in 60% of patients in the ibrutinib arm and 61% in the placebo arm.
Serious AEs occurred in 43% and 33% of patients, respectively. There were no fatal AEs in the ibrutinib arm and 3 in the rituximab arm.
Grade 3 or higher AEs that occurred more frequently in the ibrutinib arm than the placebo arm included atrial fibrillation (12% vs 1%) and hypertension (13% vs 4%).
AEs that occurred less frequently in the ibrutinib arm than the placebo arm included grade 3 or higher infusion reactions (1% vs 16%) and any-grade IgM flare (8% vs 47%).
Leading researcher in genetics, hematology dies at 84
George Stamatoyannopoulos, MD, who conducted important research into hemoglobinopathies, passed away this month at the age of 84.
Dr Stamatoyannopoulos’s research encompassed human genetics and hematology, including the structure and function of hemoglobinopathies, the genetics of thalassemia, and the molecular and cellular control of globin gene switching.
For much of his career, Dr Stamatoyannopoulos focused on the development of gene therapy for hemoglobinopathies and other disorders.
Dr Stamatoyannopoulos was born in Athens, Greece, on March 11, 1934. He entered medical school in Athens at age 17 and graduated at the top of his class 7 years later.
After his medical training, Dr Stamatoyannopoulos pursued thesis research on inherited blood disorders, particularly anemias.
Dr Stamatoyannopoulos performed the first large-scale molecular geographical survey of a genetic trait, which revealed the relationship between malaria and both thalassemia and sickle cell traits.
Dr Stamatoyannopoulos also discovered that fetal hemoglobin could ameliorate the effects of thalassemia. His theory that thalassemia and sickle cell anemia could be treated by re-activating fetal hemoglobin has underpinned decades of efforts to cure these disorders.
As a young investigator, Dr Stamatoyannopoulos expanded genetic studies of blood diseases in Greece, where his work drew the attention of medical genetics pioneer Arno Motulsky, MD.
Dr Motulsky recruited Dr Stamatoyannopoulos to the University of Washington (Seattle) in 1964. Dr Stamatoyannopoulos became a full professor there in 1973, founded the Markey Molecular Medicine Center, and was chief of medical genetics from 1989 to 2005.
Dr Stamatoyannopoulos organized the founding of the American Society of Gene and Cell Therapy. His contributions were recognized by establishment of the Stamatoyannopoulos lecture, the society’s highest award.
Dr Stamatoyannopoulos also served as president of the American Society of Hematology (1992) and president of the American Society of Gene and Cell Therapy (1996). He held leadership positions in other medical and scientific societies as well.
Dr Stamatoyannopoulos authored more than 420 scientific papers and 14 books, including The Molecular Basis of Blood Diseases.
Dr Stamatoyannopoulos died June 16, 2018. He is survived by his wife and collaborator Thalia Papayannopoulou, MD, (a professor of medicine and internationally recognized hematologist), 2 sons, and 3 grandchildren.
George Stamatoyannopoulos, MD, who conducted important research into hemoglobinopathies, passed away this month at the age of 84.
Dr Stamatoyannopoulos’s research encompassed human genetics and hematology, including the structure and function of hemoglobinopathies, the genetics of thalassemia, and the molecular and cellular control of globin gene switching.
For much of his career, Dr Stamatoyannopoulos focused on the development of gene therapy for hemoglobinopathies and other disorders.
Dr Stamatoyannopoulos was born in Athens, Greece, on March 11, 1934. He entered medical school in Athens at age 17 and graduated at the top of his class 7 years later.
After his medical training, Dr Stamatoyannopoulos pursued thesis research on inherited blood disorders, particularly anemias.
Dr Stamatoyannopoulos performed the first large-scale molecular geographical survey of a genetic trait, which revealed the relationship between malaria and both thalassemia and sickle cell traits.
Dr Stamatoyannopoulos also discovered that fetal hemoglobin could ameliorate the effects of thalassemia. His theory that thalassemia and sickle cell anemia could be treated by re-activating fetal hemoglobin has underpinned decades of efforts to cure these disorders.
As a young investigator, Dr Stamatoyannopoulos expanded genetic studies of blood diseases in Greece, where his work drew the attention of medical genetics pioneer Arno Motulsky, MD.
Dr Motulsky recruited Dr Stamatoyannopoulos to the University of Washington (Seattle) in 1964. Dr Stamatoyannopoulos became a full professor there in 1973, founded the Markey Molecular Medicine Center, and was chief of medical genetics from 1989 to 2005.
Dr Stamatoyannopoulos organized the founding of the American Society of Gene and Cell Therapy. His contributions were recognized by establishment of the Stamatoyannopoulos lecture, the society’s highest award.
Dr Stamatoyannopoulos also served as president of the American Society of Hematology (1992) and president of the American Society of Gene and Cell Therapy (1996). He held leadership positions in other medical and scientific societies as well.
Dr Stamatoyannopoulos authored more than 420 scientific papers and 14 books, including The Molecular Basis of Blood Diseases.
Dr Stamatoyannopoulos died June 16, 2018. He is survived by his wife and collaborator Thalia Papayannopoulou, MD, (a professor of medicine and internationally recognized hematologist), 2 sons, and 3 grandchildren.
George Stamatoyannopoulos, MD, who conducted important research into hemoglobinopathies, passed away this month at the age of 84.
Dr Stamatoyannopoulos’s research encompassed human genetics and hematology, including the structure and function of hemoglobinopathies, the genetics of thalassemia, and the molecular and cellular control of globin gene switching.
For much of his career, Dr Stamatoyannopoulos focused on the development of gene therapy for hemoglobinopathies and other disorders.
Dr Stamatoyannopoulos was born in Athens, Greece, on March 11, 1934. He entered medical school in Athens at age 17 and graduated at the top of his class 7 years later.
After his medical training, Dr Stamatoyannopoulos pursued thesis research on inherited blood disorders, particularly anemias.
Dr Stamatoyannopoulos performed the first large-scale molecular geographical survey of a genetic trait, which revealed the relationship between malaria and both thalassemia and sickle cell traits.
Dr Stamatoyannopoulos also discovered that fetal hemoglobin could ameliorate the effects of thalassemia. His theory that thalassemia and sickle cell anemia could be treated by re-activating fetal hemoglobin has underpinned decades of efforts to cure these disorders.
As a young investigator, Dr Stamatoyannopoulos expanded genetic studies of blood diseases in Greece, where his work drew the attention of medical genetics pioneer Arno Motulsky, MD.
Dr Motulsky recruited Dr Stamatoyannopoulos to the University of Washington (Seattle) in 1964. Dr Stamatoyannopoulos became a full professor there in 1973, founded the Markey Molecular Medicine Center, and was chief of medical genetics from 1989 to 2005.
Dr Stamatoyannopoulos organized the founding of the American Society of Gene and Cell Therapy. His contributions were recognized by establishment of the Stamatoyannopoulos lecture, the society’s highest award.
Dr Stamatoyannopoulos also served as president of the American Society of Hematology (1992) and president of the American Society of Gene and Cell Therapy (1996). He held leadership positions in other medical and scientific societies as well.
Dr Stamatoyannopoulos authored more than 420 scientific papers and 14 books, including The Molecular Basis of Blood Diseases.
Dr Stamatoyannopoulos died June 16, 2018. He is survived by his wife and collaborator Thalia Papayannopoulou, MD, (a professor of medicine and internationally recognized hematologist), 2 sons, and 3 grandchildren.
Checkpoint inhibitors in autoimmune disease: More flares, better cancer outcomes
AMSTERDAM – In patients with autoimmune diseases, cancer treatment with checkpoint inhibitor immunotherapy increases the risk of flares, but these flares are associated with improved cancer outcomes, according to a multicenter, retrospective study presented at the European Congress of Rheumatology.
“Survival was longer in patients who experienced a flare of their preexisting autoimmune disease or any other immune-related adverse event, but this gain was lost if an immunosuppressive therapy was used,” reported Alice Tison, a resident in rheumatology at the Centre Hospitalier Universitaire, Brest, France.
These were some of the mixed messages from this evaluation, which involved 112 patients with preexisting autoimmune disease (PAD) whose data were collected from 11 tertiary care centers in France. Of the cases of PAD represented, the majority involved joint diseases, including psoriatic arthritis (28%), rheumatoid arthritis (18%), and spondyloarthritis (4.5%). However, other types of PAD, including inflammatory bowel disease (13%), were included in the series.
Only 33% of the patients had active disease at the time that checkpoint inhibitor therapy was initiated, and only 21% were taking an immunosuppressive therapy for their disease. Of those on therapy, the majority were taking steroids, but about a third of those on therapy were taking a disease-modifying antirheumatic drug, such as methotrexate.
With the initiation of checkpoint inhibitors, which were offered primarily for the treatment of melanoma (59%) and non–small cell lung cancer (36%), 42% of patients with PAD developed a disease flare. Of these, 30% were considered severe. Other immune-related events not considered related to the underlying disease, such as colitis, were also observed but at rates not clearly different than those observed in patients without PAD.
The activity of checkpoint inhibitors did not appear to be different than that observed in non-PAD patients. For example, the overall response rate was 48% in those with melanoma and 54% in those with non–small cell lung cancer. After a median of 8 months of follow-up, the median progression-free survival was 12.4 months and 9.7 months for the two diseases, respectively. Median overall survival had not been reached in either disease.
However, those with a flare or another immune-related adverse event had significantly better progression-free survival (P = .016) and overall survival (P = .004) when compared with those who did not flare or have an immune-related adverse event. According to Ms. Tison, this has been reported before, but a more surprising finding was that the gain in progression-free survival and overall survival was lost in those treated with an immunosuppressive drug.
Even though non-PAD patients commonly receive steroids for immune-related adverse events such as colitis, the loss of benefit in PAD patients who received immunosuppressive therapies may be caused by, at least in part, cross-reactivity between tumor antigens and autoantigens, Ms. Tison speculated.
Ms. Tison was cautious in drawing conclusions about specific strategies to optimize benefits from checkpoint inhibitors in PAD based on this limited series of patients. However, she did suggest that discontinuation of immunosuppressive therapies prior to initiating checkpoint inhibitors may be prudent in PAD patients, particularly those with inactive disease.
Overall, she emphasized that checkpoint inhibitors “have revolutionized the management of several cancers” and should not be denied to PAD patients who are otherwise appropriate candidates. Although flares are common, more than half of PAD patients in this series did not flare and flares were mild to moderate in most of those who did.
“The response to checkpoint inhibitors in PAD patients is good,” Ms. Tison advised. For those who do flare, “we need prospective studies to understand which strategies provide a good balance of benefit to risk” for cancer immunotherapy and for the options to manage immune-related adverse events.
The study was not industry funded. Ms. Tison reported no potential conflicts of interest.
SOURCE: Tison A et al. Ann Rheum Dis. 2018;77(Suppl 2):147. EULAR Congress 2018, Abstract OP0196.
AMSTERDAM – In patients with autoimmune diseases, cancer treatment with checkpoint inhibitor immunotherapy increases the risk of flares, but these flares are associated with improved cancer outcomes, according to a multicenter, retrospective study presented at the European Congress of Rheumatology.
“Survival was longer in patients who experienced a flare of their preexisting autoimmune disease or any other immune-related adverse event, but this gain was lost if an immunosuppressive therapy was used,” reported Alice Tison, a resident in rheumatology at the Centre Hospitalier Universitaire, Brest, France.
These were some of the mixed messages from this evaluation, which involved 112 patients with preexisting autoimmune disease (PAD) whose data were collected from 11 tertiary care centers in France. Of the cases of PAD represented, the majority involved joint diseases, including psoriatic arthritis (28%), rheumatoid arthritis (18%), and spondyloarthritis (4.5%). However, other types of PAD, including inflammatory bowel disease (13%), were included in the series.
Only 33% of the patients had active disease at the time that checkpoint inhibitor therapy was initiated, and only 21% were taking an immunosuppressive therapy for their disease. Of those on therapy, the majority were taking steroids, but about a third of those on therapy were taking a disease-modifying antirheumatic drug, such as methotrexate.
With the initiation of checkpoint inhibitors, which were offered primarily for the treatment of melanoma (59%) and non–small cell lung cancer (36%), 42% of patients with PAD developed a disease flare. Of these, 30% were considered severe. Other immune-related events not considered related to the underlying disease, such as colitis, were also observed but at rates not clearly different than those observed in patients without PAD.
The activity of checkpoint inhibitors did not appear to be different than that observed in non-PAD patients. For example, the overall response rate was 48% in those with melanoma and 54% in those with non–small cell lung cancer. After a median of 8 months of follow-up, the median progression-free survival was 12.4 months and 9.7 months for the two diseases, respectively. Median overall survival had not been reached in either disease.
However, those with a flare or another immune-related adverse event had significantly better progression-free survival (P = .016) and overall survival (P = .004) when compared with those who did not flare or have an immune-related adverse event. According to Ms. Tison, this has been reported before, but a more surprising finding was that the gain in progression-free survival and overall survival was lost in those treated with an immunosuppressive drug.
Even though non-PAD patients commonly receive steroids for immune-related adverse events such as colitis, the loss of benefit in PAD patients who received immunosuppressive therapies may be caused by, at least in part, cross-reactivity between tumor antigens and autoantigens, Ms. Tison speculated.
Ms. Tison was cautious in drawing conclusions about specific strategies to optimize benefits from checkpoint inhibitors in PAD based on this limited series of patients. However, she did suggest that discontinuation of immunosuppressive therapies prior to initiating checkpoint inhibitors may be prudent in PAD patients, particularly those with inactive disease.
Overall, she emphasized that checkpoint inhibitors “have revolutionized the management of several cancers” and should not be denied to PAD patients who are otherwise appropriate candidates. Although flares are common, more than half of PAD patients in this series did not flare and flares were mild to moderate in most of those who did.
“The response to checkpoint inhibitors in PAD patients is good,” Ms. Tison advised. For those who do flare, “we need prospective studies to understand which strategies provide a good balance of benefit to risk” for cancer immunotherapy and for the options to manage immune-related adverse events.
The study was not industry funded. Ms. Tison reported no potential conflicts of interest.
SOURCE: Tison A et al. Ann Rheum Dis. 2018;77(Suppl 2):147. EULAR Congress 2018, Abstract OP0196.
AMSTERDAM – In patients with autoimmune diseases, cancer treatment with checkpoint inhibitor immunotherapy increases the risk of flares, but these flares are associated with improved cancer outcomes, according to a multicenter, retrospective study presented at the European Congress of Rheumatology.
“Survival was longer in patients who experienced a flare of their preexisting autoimmune disease or any other immune-related adverse event, but this gain was lost if an immunosuppressive therapy was used,” reported Alice Tison, a resident in rheumatology at the Centre Hospitalier Universitaire, Brest, France.
These were some of the mixed messages from this evaluation, which involved 112 patients with preexisting autoimmune disease (PAD) whose data were collected from 11 tertiary care centers in France. Of the cases of PAD represented, the majority involved joint diseases, including psoriatic arthritis (28%), rheumatoid arthritis (18%), and spondyloarthritis (4.5%). However, other types of PAD, including inflammatory bowel disease (13%), were included in the series.
Only 33% of the patients had active disease at the time that checkpoint inhibitor therapy was initiated, and only 21% were taking an immunosuppressive therapy for their disease. Of those on therapy, the majority were taking steroids, but about a third of those on therapy were taking a disease-modifying antirheumatic drug, such as methotrexate.
With the initiation of checkpoint inhibitors, which were offered primarily for the treatment of melanoma (59%) and non–small cell lung cancer (36%), 42% of patients with PAD developed a disease flare. Of these, 30% were considered severe. Other immune-related events not considered related to the underlying disease, such as colitis, were also observed but at rates not clearly different than those observed in patients without PAD.
The activity of checkpoint inhibitors did not appear to be different than that observed in non-PAD patients. For example, the overall response rate was 48% in those with melanoma and 54% in those with non–small cell lung cancer. After a median of 8 months of follow-up, the median progression-free survival was 12.4 months and 9.7 months for the two diseases, respectively. Median overall survival had not been reached in either disease.
However, those with a flare or another immune-related adverse event had significantly better progression-free survival (P = .016) and overall survival (P = .004) when compared with those who did not flare or have an immune-related adverse event. According to Ms. Tison, this has been reported before, but a more surprising finding was that the gain in progression-free survival and overall survival was lost in those treated with an immunosuppressive drug.
Even though non-PAD patients commonly receive steroids for immune-related adverse events such as colitis, the loss of benefit in PAD patients who received immunosuppressive therapies may be caused by, at least in part, cross-reactivity between tumor antigens and autoantigens, Ms. Tison speculated.
Ms. Tison was cautious in drawing conclusions about specific strategies to optimize benefits from checkpoint inhibitors in PAD based on this limited series of patients. However, she did suggest that discontinuation of immunosuppressive therapies prior to initiating checkpoint inhibitors may be prudent in PAD patients, particularly those with inactive disease.
Overall, she emphasized that checkpoint inhibitors “have revolutionized the management of several cancers” and should not be denied to PAD patients who are otherwise appropriate candidates. Although flares are common, more than half of PAD patients in this series did not flare and flares were mild to moderate in most of those who did.
“The response to checkpoint inhibitors in PAD patients is good,” Ms. Tison advised. For those who do flare, “we need prospective studies to understand which strategies provide a good balance of benefit to risk” for cancer immunotherapy and for the options to manage immune-related adverse events.
The study was not industry funded. Ms. Tison reported no potential conflicts of interest.
SOURCE: Tison A et al. Ann Rheum Dis. 2018;77(Suppl 2):147. EULAR Congress 2018, Abstract OP0196.
REPORTING FROM THE EULAR 2018 CONGRESS
Key clinical point: Cancer patients who take a checkpoint inhibitor and have a preexisting autoimmune disease were significantly more likely to have a disease flare but also a better cancer outcome than were those without preexisting disease.
Major finding: In those with a disease flare, progression-free and overall survival were significantly improved (P = .016 and P = .004, respectively).
Study details: Retrospective multicenter study.
Disclosures: The study was not industry funded. Ms. Tison reported no potential conflicts of interest.
Source: Tison A et al. Ann Rheum Dis. 2018;77(Suppl 2):147. EULAR Congress 2018, Abstract OP0196.
Data suggest harm outweighs benefit of opioids for musculoskeletal pain
AMSTERDAM – Opioids cannot be justified for the routine treatment for musculoskeletal pain because risks outweigh benefits, according to a detailed review of published studies presented at the European Congress of Rheumatology.
“There is very little evidence of benefit for the long-term management of nonmalignant pain, but very good evidence for harm,” reported Blair Smith, MD, head of the population health sciences division, University of Dundee (Scotland).
In the treatment of musculoskeletal pain, the goals are increased function and quality of life, rather than complete relief of pain, according to Dr. Smith. On this basis, opioids are not an appropriate routine therapy. He reported that pain relief is not well documented, while side effects such as sedation, dizziness, and constipation, are likely to be counterproductive to improved outcomes.
There is no absolute contraindication for opioids in the control of chronic musculoskeletal pain, but Dr. Smith’s summary of the data led him to conclude that they should be used judiciously and “only for carefully selected patients.”
Of the many studies he reviewed to draw this conclusion, one of the most recent was identified as the most persuasive. Published earlier this year, the SPACE study is “the first good-quality study of long-term opioid use” in patients with musculoskeletal complaints. It was negative.
“The pain intensity at the end of 12 months of treatment was slightly but significantly worse among those randomized to opioids,” reported Dr. Smith. “There was no difference in patient function, but there was an increased risk of adverse events.”
In the SPACE study, 240 patients with moderate to severe chronic back pain or hip or knee osteoarthritis were randomized to opioid or nonopioid pain management. In the nonopioid group, the first therapeutic step was acetaminophen, but medications could be changed, added, or adjusted within both groups to improve patient response.
At the end of 12 months, a lack of benefit on both pain control and functional improvement from opioids relative to nonopioid treatment was accompanied by a higher rate of adverse effects. This led the authors to conclude that opioids are not supported for musculoskeletal pain.
Not all the evidence argues against opioids for noncancer pain management, according to Dr. Smith, but he emphasized that those who support use of opioids do so for pain control only. They do not confirm an advantage for function and quality of life, which he suggested are the key endpoints. For example, a 2010 Cochrane review concluded from a systematic literature review that there is “weak evidence” for pain relief but inconclusive evidence of an improvement in functioning and quality of life.
Other investigators have drawn the same conclusion, according to Dr. Smith. He cited a statement from the International Association for Study of Pain that advises, “Caution should be used for prescribing opioids for chronic pain.” Although this statement was not specific to musculoskeletal pain, the IASP does specify that pain medications should be employed “to promote increased function and improved quality of life rather than complete relief of pain,” according to Dr. Smith.
Opioid prescriptions for chronic pain have been increasing in Europe as they have in the United States, but Dr. Smith indicated that opioids, if used at all, should be prescribed for very short periods and for very specific goals, particularly improvement in function.
“Probably most important for my [primary care] colleagues, patients prescribed opioids should be evaluated early and frequently to gauge benefit,” Dr. Smith said. Although he believes pain control is an important and worthwhile goal, it must be approached within the context of improved well-being rather than as an isolated endpoint.
SOURCE: Smith B et al. EULAR 2018, Abstract No. SP0073.
AMSTERDAM – Opioids cannot be justified for the routine treatment for musculoskeletal pain because risks outweigh benefits, according to a detailed review of published studies presented at the European Congress of Rheumatology.
“There is very little evidence of benefit for the long-term management of nonmalignant pain, but very good evidence for harm,” reported Blair Smith, MD, head of the population health sciences division, University of Dundee (Scotland).
In the treatment of musculoskeletal pain, the goals are increased function and quality of life, rather than complete relief of pain, according to Dr. Smith. On this basis, opioids are not an appropriate routine therapy. He reported that pain relief is not well documented, while side effects such as sedation, dizziness, and constipation, are likely to be counterproductive to improved outcomes.
There is no absolute contraindication for opioids in the control of chronic musculoskeletal pain, but Dr. Smith’s summary of the data led him to conclude that they should be used judiciously and “only for carefully selected patients.”
Of the many studies he reviewed to draw this conclusion, one of the most recent was identified as the most persuasive. Published earlier this year, the SPACE study is “the first good-quality study of long-term opioid use” in patients with musculoskeletal complaints. It was negative.
“The pain intensity at the end of 12 months of treatment was slightly but significantly worse among those randomized to opioids,” reported Dr. Smith. “There was no difference in patient function, but there was an increased risk of adverse events.”
In the SPACE study, 240 patients with moderate to severe chronic back pain or hip or knee osteoarthritis were randomized to opioid or nonopioid pain management. In the nonopioid group, the first therapeutic step was acetaminophen, but medications could be changed, added, or adjusted within both groups to improve patient response.
At the end of 12 months, a lack of benefit on both pain control and functional improvement from opioids relative to nonopioid treatment was accompanied by a higher rate of adverse effects. This led the authors to conclude that opioids are not supported for musculoskeletal pain.
Not all the evidence argues against opioids for noncancer pain management, according to Dr. Smith, but he emphasized that those who support use of opioids do so for pain control only. They do not confirm an advantage for function and quality of life, which he suggested are the key endpoints. For example, a 2010 Cochrane review concluded from a systematic literature review that there is “weak evidence” for pain relief but inconclusive evidence of an improvement in functioning and quality of life.
Other investigators have drawn the same conclusion, according to Dr. Smith. He cited a statement from the International Association for Study of Pain that advises, “Caution should be used for prescribing opioids for chronic pain.” Although this statement was not specific to musculoskeletal pain, the IASP does specify that pain medications should be employed “to promote increased function and improved quality of life rather than complete relief of pain,” according to Dr. Smith.
Opioid prescriptions for chronic pain have been increasing in Europe as they have in the United States, but Dr. Smith indicated that opioids, if used at all, should be prescribed for very short periods and for very specific goals, particularly improvement in function.
“Probably most important for my [primary care] colleagues, patients prescribed opioids should be evaluated early and frequently to gauge benefit,” Dr. Smith said. Although he believes pain control is an important and worthwhile goal, it must be approached within the context of improved well-being rather than as an isolated endpoint.
SOURCE: Smith B et al. EULAR 2018, Abstract No. SP0073.
AMSTERDAM – Opioids cannot be justified for the routine treatment for musculoskeletal pain because risks outweigh benefits, according to a detailed review of published studies presented at the European Congress of Rheumatology.
“There is very little evidence of benefit for the long-term management of nonmalignant pain, but very good evidence for harm,” reported Blair Smith, MD, head of the population health sciences division, University of Dundee (Scotland).
In the treatment of musculoskeletal pain, the goals are increased function and quality of life, rather than complete relief of pain, according to Dr. Smith. On this basis, opioids are not an appropriate routine therapy. He reported that pain relief is not well documented, while side effects such as sedation, dizziness, and constipation, are likely to be counterproductive to improved outcomes.
There is no absolute contraindication for opioids in the control of chronic musculoskeletal pain, but Dr. Smith’s summary of the data led him to conclude that they should be used judiciously and “only for carefully selected patients.”
Of the many studies he reviewed to draw this conclusion, one of the most recent was identified as the most persuasive. Published earlier this year, the SPACE study is “the first good-quality study of long-term opioid use” in patients with musculoskeletal complaints. It was negative.
“The pain intensity at the end of 12 months of treatment was slightly but significantly worse among those randomized to opioids,” reported Dr. Smith. “There was no difference in patient function, but there was an increased risk of adverse events.”
In the SPACE study, 240 patients with moderate to severe chronic back pain or hip or knee osteoarthritis were randomized to opioid or nonopioid pain management. In the nonopioid group, the first therapeutic step was acetaminophen, but medications could be changed, added, or adjusted within both groups to improve patient response.
At the end of 12 months, a lack of benefit on both pain control and functional improvement from opioids relative to nonopioid treatment was accompanied by a higher rate of adverse effects. This led the authors to conclude that opioids are not supported for musculoskeletal pain.
Not all the evidence argues against opioids for noncancer pain management, according to Dr. Smith, but he emphasized that those who support use of opioids do so for pain control only. They do not confirm an advantage for function and quality of life, which he suggested are the key endpoints. For example, a 2010 Cochrane review concluded from a systematic literature review that there is “weak evidence” for pain relief but inconclusive evidence of an improvement in functioning and quality of life.
Other investigators have drawn the same conclusion, according to Dr. Smith. He cited a statement from the International Association for Study of Pain that advises, “Caution should be used for prescribing opioids for chronic pain.” Although this statement was not specific to musculoskeletal pain, the IASP does specify that pain medications should be employed “to promote increased function and improved quality of life rather than complete relief of pain,” according to Dr. Smith.
Opioid prescriptions for chronic pain have been increasing in Europe as they have in the United States, but Dr. Smith indicated that opioids, if used at all, should be prescribed for very short periods and for very specific goals, particularly improvement in function.
“Probably most important for my [primary care] colleagues, patients prescribed opioids should be evaluated early and frequently to gauge benefit,” Dr. Smith said. Although he believes pain control is an important and worthwhile goal, it must be approached within the context of improved well-being rather than as an isolated endpoint.
SOURCE: Smith B et al. EULAR 2018, Abstract No. SP0073.
REPORTING FROM THE EULAR 2018 CONGRESS
Biologic efficacy differs in psoriatic arthritis by lymphocyte phenotype
AMSTERDAM – In patients with psoriatic arthritis (PsA), new evidence suggests selection of biologic disease-modifying antirheumatic drugs (bDMARDs) might be individualized by T-helper cell phenotype to improve disease control, according to the results of a study presented at the European Congress of Rheumatology.
“Our findings suggest a potential for precision medicine in patients with psoriatic arthritis,” reported Ippei Miyagawa, MD, of the University of Occupational and Environmental Health in Kitakyushu, Japan.
These phenotypes were employed to individualize therapy with the currently available targeted bDMARDs. Patients with a Th1-predominant phenotype received ustekinumab (Stelara), which blocks the p40 subunit of interleukin (IL)-12 and IL-23. Patients with a Th17-predominant phenotype received secukinumab (Cosentyx), which targets IL-17. Patients with the Th1/Th17-high phenotype received either secukinumab or a tumor necrosis factor inhibitor. Patients with the Th1/Th17-low phenotype received a TNF inhibitor.
The 26 patients whose bDMARD therapy was individualized were compared with 38 PsA patients who received bDMARDs selected according to EULAR recommendations. The groups were similar for baseline characteristics.
In both groups, there were significant decreases from baseline in essentially all clinical measures, including the Simplified Disease Activity Index, the Psoriasis Area and Severity Index, and the Patient Global Health Assessment. However, several disease markers suggested greater disease control in those receiving individualized therapy. For example, the Disease Activity Score in 28 joints using erythrocyte sedimentation rate (DAS28-ESR) at 6 months was 0.76 in the Th17-predominant group versus 1.32 in those on an unselected bDMARD therapy (P = .008).
As a proportion of lymphocytes, Th1-predominant cells greater than 1.2% and Th17-predominant cells greater than 1.5% appeared to be sensitive cutoffs for predicting response to ustekinumab and secukinumab, respectively, according to data presented by Dr. Miyagawa. Although the results in this small series of patients are considered preliminary, Dr. Miyagawa said, “We think that this research is the first step toward the future use of precision medicine in PsA.”
Larger studies are needed to verify that lymphocyte phenotyping is an effective and reproducible strategy for individualizing selection of bDMARDs, but Dr. Miyagawa acknowledged other practical barriers to routine clinical application of this strategy. In particular, he called flow cytometry, which was employed in this study to phenotype lymphocyte expression, “complicated” for routine clinical use. However, this study strongly suggests that lymphocyte expression is a predictor of response to the different bDMARDs now available for treatment of PsA.
“The bDMARDs effective in PsA have different targets and may not offer the same degree of efficacy in all patients. Our study suggests an approach to optimal drug selection,” he said.
The study was not industry funded. Dr. Miyagawa reported no relevant financial disclosures.
SOURCE: Miyagawa I et al. Ann Rheum Dis. 2018;77(Suppl 2):206-7. EULAR Congress 2018, Abstract OP0321.
AMSTERDAM – In patients with psoriatic arthritis (PsA), new evidence suggests selection of biologic disease-modifying antirheumatic drugs (bDMARDs) might be individualized by T-helper cell phenotype to improve disease control, according to the results of a study presented at the European Congress of Rheumatology.
“Our findings suggest a potential for precision medicine in patients with psoriatic arthritis,” reported Ippei Miyagawa, MD, of the University of Occupational and Environmental Health in Kitakyushu, Japan.
These phenotypes were employed to individualize therapy with the currently available targeted bDMARDs. Patients with a Th1-predominant phenotype received ustekinumab (Stelara), which blocks the p40 subunit of interleukin (IL)-12 and IL-23. Patients with a Th17-predominant phenotype received secukinumab (Cosentyx), which targets IL-17. Patients with the Th1/Th17-high phenotype received either secukinumab or a tumor necrosis factor inhibitor. Patients with the Th1/Th17-low phenotype received a TNF inhibitor.
The 26 patients whose bDMARD therapy was individualized were compared with 38 PsA patients who received bDMARDs selected according to EULAR recommendations. The groups were similar for baseline characteristics.
In both groups, there were significant decreases from baseline in essentially all clinical measures, including the Simplified Disease Activity Index, the Psoriasis Area and Severity Index, and the Patient Global Health Assessment. However, several disease markers suggested greater disease control in those receiving individualized therapy. For example, the Disease Activity Score in 28 joints using erythrocyte sedimentation rate (DAS28-ESR) at 6 months was 0.76 in the Th17-predominant group versus 1.32 in those on an unselected bDMARD therapy (P = .008).
As a proportion of lymphocytes, Th1-predominant cells greater than 1.2% and Th17-predominant cells greater than 1.5% appeared to be sensitive cutoffs for predicting response to ustekinumab and secukinumab, respectively, according to data presented by Dr. Miyagawa. Although the results in this small series of patients are considered preliminary, Dr. Miyagawa said, “We think that this research is the first step toward the future use of precision medicine in PsA.”
Larger studies are needed to verify that lymphocyte phenotyping is an effective and reproducible strategy for individualizing selection of bDMARDs, but Dr. Miyagawa acknowledged other practical barriers to routine clinical application of this strategy. In particular, he called flow cytometry, which was employed in this study to phenotype lymphocyte expression, “complicated” for routine clinical use. However, this study strongly suggests that lymphocyte expression is a predictor of response to the different bDMARDs now available for treatment of PsA.
“The bDMARDs effective in PsA have different targets and may not offer the same degree of efficacy in all patients. Our study suggests an approach to optimal drug selection,” he said.
The study was not industry funded. Dr. Miyagawa reported no relevant financial disclosures.
SOURCE: Miyagawa I et al. Ann Rheum Dis. 2018;77(Suppl 2):206-7. EULAR Congress 2018, Abstract OP0321.
AMSTERDAM – In patients with psoriatic arthritis (PsA), new evidence suggests selection of biologic disease-modifying antirheumatic drugs (bDMARDs) might be individualized by T-helper cell phenotype to improve disease control, according to the results of a study presented at the European Congress of Rheumatology.
“Our findings suggest a potential for precision medicine in patients with psoriatic arthritis,” reported Ippei Miyagawa, MD, of the University of Occupational and Environmental Health in Kitakyushu, Japan.
These phenotypes were employed to individualize therapy with the currently available targeted bDMARDs. Patients with a Th1-predominant phenotype received ustekinumab (Stelara), which blocks the p40 subunit of interleukin (IL)-12 and IL-23. Patients with a Th17-predominant phenotype received secukinumab (Cosentyx), which targets IL-17. Patients with the Th1/Th17-high phenotype received either secukinumab or a tumor necrosis factor inhibitor. Patients with the Th1/Th17-low phenotype received a TNF inhibitor.
The 26 patients whose bDMARD therapy was individualized were compared with 38 PsA patients who received bDMARDs selected according to EULAR recommendations. The groups were similar for baseline characteristics.
In both groups, there were significant decreases from baseline in essentially all clinical measures, including the Simplified Disease Activity Index, the Psoriasis Area and Severity Index, and the Patient Global Health Assessment. However, several disease markers suggested greater disease control in those receiving individualized therapy. For example, the Disease Activity Score in 28 joints using erythrocyte sedimentation rate (DAS28-ESR) at 6 months was 0.76 in the Th17-predominant group versus 1.32 in those on an unselected bDMARD therapy (P = .008).
As a proportion of lymphocytes, Th1-predominant cells greater than 1.2% and Th17-predominant cells greater than 1.5% appeared to be sensitive cutoffs for predicting response to ustekinumab and secukinumab, respectively, according to data presented by Dr. Miyagawa. Although the results in this small series of patients are considered preliminary, Dr. Miyagawa said, “We think that this research is the first step toward the future use of precision medicine in PsA.”
Larger studies are needed to verify that lymphocyte phenotyping is an effective and reproducible strategy for individualizing selection of bDMARDs, but Dr. Miyagawa acknowledged other practical barriers to routine clinical application of this strategy. In particular, he called flow cytometry, which was employed in this study to phenotype lymphocyte expression, “complicated” for routine clinical use. However, this study strongly suggests that lymphocyte expression is a predictor of response to the different bDMARDs now available for treatment of PsA.
“The bDMARDs effective in PsA have different targets and may not offer the same degree of efficacy in all patients. Our study suggests an approach to optimal drug selection,” he said.
The study was not industry funded. Dr. Miyagawa reported no relevant financial disclosures.
SOURCE: Miyagawa I et al. Ann Rheum Dis. 2018;77(Suppl 2):206-7. EULAR Congress 2018, Abstract OP0321.
REPORTING FROM THE EULAR 2018 CONGRESS
Key clinical point: Lymphocyte profile phenotypes appear to permit individualized biologic therapy in patients with PsA.
Major finding: Therapy individualized for Th17-predominant PsA produced a DAS28 score 0.56 points lower than did nonselected therapy (P = .008).
Study details: A prospective, nonrandomized study of 64 PsA patients with either individualized bDMARD therapy or bDMARDs selected according to EULAR recommendations.
Disclosures: The study was not industry funded. Dr. Miyagawa reported no relevant financial disclosures.
Source: Miyagawa I et al. Ann Rheum Dis. 2018;77(Suppl 2):206-7. EULAR Congress 2018, Abstract OP0321.