User login
Trump support high in counties with chronic opioid use
Chronic use of prescription opioid drugs correlated with support for the Republican candidate in the 2016 U.S. presidential election, according to a cross-sectional analysis published in JAMA Network Open.
The mean Republican presidential vote was 60% in U.S. counties with significantly higher-than-average rates of prescriptions for prolonged opioid use, versus 39% for counties with significantly lower rates, according to the study, which was based on prescription data for Medicare Part D enrollees (JAMA Network Open. 2018;1(2):e180450).
Those findings suggest the importance of economic, cultural, and environmental factors in the opioid epidemic, according to study author James S. Goodwin, MD, of the University of Texas Medical Branch, Galveston, and co-authors.
“Public health policy directed at stemming the opioid epidemic must go beyond the medical model and incorporate socioenvironmental disadvantage factors and health behaviors into policy planning and implementation,” Dr. Goodwin and co-authors wrote.
The cross-sectional analysis included data for 3.76 million Medicare Part D enrollees. They looked specifically at the proportion of enrollees with chronic opioid use, which they defined as receipt of at least a 90-day supply in 1 year.
After adjusting for age, disability, and other factors, they found 693 out of 3,100 counties (22.4%) had rates of chronic opioid use higher than the mean, and 638 (20.6%) had rates lower than the mean. The correlation between opioid use rates and the presidential vote was 0.42 (P < .001), according to investigators.
They also published two county maps that they said shared some similar patterns. The first shows the proportion of older chronic opioid users by quintile, and the second shows Republican vote percentages, also by quintile. The correlation coefficient between those two rates was 0.32 (P < .001).
This study adds to the emerging literature on the relationships between health status and support of Donald Trump in the 2016 election, according to authors.
However, there were limitations to the study, they added. Of note, the presidential vote data was from 2016, but the information on prolonged opioid prescriptions was from 2015. Moreover, the voting data included all voters, while the opioid data came only from Medicare Part D enrollees, which represent about 72% of the full Medicare population.
One study author reported grants from the National Institute on Drug Abuse and Agency for Healthcare Research and Quality. Another reported membership in Physicians for Responsible Opioid Prescribing
SOURCE: Goodwin JS, et al. JAMA Network Open. 2018;1(2):e180450.
This article was updated 6/26/18.
, James Niels Rosenquist, MD, PhD, wrote in an editorial.
Public mental health is continually influencing economic and other societal forces, and in turn, being influenced by them, Dr. Rosenquist explained.
In the present study, opiate prescription rates were correlated with voting margins for Donald Trump in the 2016 presidential election using a “unique” data set based on Medicare Part D data, he said.
Studies such as these are good examples of how available data sources can be used creatively to test whether mental health trends such as opiate addiction might be correlated with “key outcomes such as elections,” Dr. Rosenquist wrote. “As elections are how political leaders are chosen in a democracy, arguments for focusing on mental health in this context may be particularly convincing to elected policy makers.”
James Niels Rosenquist, MD, PhD, is with the Center for Quantitative Health, Massachusetts General Hospital, Harvard Medical School, Boston. These comments are based on his editorial in JAMA Network Open (2018;1(2):e180451) . Dr. Rosenquist disclosed no relevant conflicts of interest.
, James Niels Rosenquist, MD, PhD, wrote in an editorial.
Public mental health is continually influencing economic and other societal forces, and in turn, being influenced by them, Dr. Rosenquist explained.
In the present study, opiate prescription rates were correlated with voting margins for Donald Trump in the 2016 presidential election using a “unique” data set based on Medicare Part D data, he said.
Studies such as these are good examples of how available data sources can be used creatively to test whether mental health trends such as opiate addiction might be correlated with “key outcomes such as elections,” Dr. Rosenquist wrote. “As elections are how political leaders are chosen in a democracy, arguments for focusing on mental health in this context may be particularly convincing to elected policy makers.”
James Niels Rosenquist, MD, PhD, is with the Center for Quantitative Health, Massachusetts General Hospital, Harvard Medical School, Boston. These comments are based on his editorial in JAMA Network Open (2018;1(2):e180451) . Dr. Rosenquist disclosed no relevant conflicts of interest.
, James Niels Rosenquist, MD, PhD, wrote in an editorial.
Public mental health is continually influencing economic and other societal forces, and in turn, being influenced by them, Dr. Rosenquist explained.
In the present study, opiate prescription rates were correlated with voting margins for Donald Trump in the 2016 presidential election using a “unique” data set based on Medicare Part D data, he said.
Studies such as these are good examples of how available data sources can be used creatively to test whether mental health trends such as opiate addiction might be correlated with “key outcomes such as elections,” Dr. Rosenquist wrote. “As elections are how political leaders are chosen in a democracy, arguments for focusing on mental health in this context may be particularly convincing to elected policy makers.”
James Niels Rosenquist, MD, PhD, is with the Center for Quantitative Health, Massachusetts General Hospital, Harvard Medical School, Boston. These comments are based on his editorial in JAMA Network Open (2018;1(2):e180451) . Dr. Rosenquist disclosed no relevant conflicts of interest.
Chronic use of prescription opioid drugs correlated with support for the Republican candidate in the 2016 U.S. presidential election, according to a cross-sectional analysis published in JAMA Network Open.
The mean Republican presidential vote was 60% in U.S. counties with significantly higher-than-average rates of prescriptions for prolonged opioid use, versus 39% for counties with significantly lower rates, according to the study, which was based on prescription data for Medicare Part D enrollees (JAMA Network Open. 2018;1(2):e180450).
Those findings suggest the importance of economic, cultural, and environmental factors in the opioid epidemic, according to study author James S. Goodwin, MD, of the University of Texas Medical Branch, Galveston, and co-authors.
“Public health policy directed at stemming the opioid epidemic must go beyond the medical model and incorporate socioenvironmental disadvantage factors and health behaviors into policy planning and implementation,” Dr. Goodwin and co-authors wrote.
The cross-sectional analysis included data for 3.76 million Medicare Part D enrollees. They looked specifically at the proportion of enrollees with chronic opioid use, which they defined as receipt of at least a 90-day supply in 1 year.
After adjusting for age, disability, and other factors, they found 693 out of 3,100 counties (22.4%) had rates of chronic opioid use higher than the mean, and 638 (20.6%) had rates lower than the mean. The correlation between opioid use rates and the presidential vote was 0.42 (P < .001), according to investigators.
They also published two county maps that they said shared some similar patterns. The first shows the proportion of older chronic opioid users by quintile, and the second shows Republican vote percentages, also by quintile. The correlation coefficient between those two rates was 0.32 (P < .001).
This study adds to the emerging literature on the relationships between health status and support of Donald Trump in the 2016 election, according to authors.
However, there were limitations to the study, they added. Of note, the presidential vote data was from 2016, but the information on prolonged opioid prescriptions was from 2015. Moreover, the voting data included all voters, while the opioid data came only from Medicare Part D enrollees, which represent about 72% of the full Medicare population.
One study author reported grants from the National Institute on Drug Abuse and Agency for Healthcare Research and Quality. Another reported membership in Physicians for Responsible Opioid Prescribing
SOURCE: Goodwin JS, et al. JAMA Network Open. 2018;1(2):e180450.
This article was updated 6/26/18.
Chronic use of prescription opioid drugs correlated with support for the Republican candidate in the 2016 U.S. presidential election, according to a cross-sectional analysis published in JAMA Network Open.
The mean Republican presidential vote was 60% in U.S. counties with significantly higher-than-average rates of prescriptions for prolonged opioid use, versus 39% for counties with significantly lower rates, according to the study, which was based on prescription data for Medicare Part D enrollees (JAMA Network Open. 2018;1(2):e180450).
Those findings suggest the importance of economic, cultural, and environmental factors in the opioid epidemic, according to study author James S. Goodwin, MD, of the University of Texas Medical Branch, Galveston, and co-authors.
“Public health policy directed at stemming the opioid epidemic must go beyond the medical model and incorporate socioenvironmental disadvantage factors and health behaviors into policy planning and implementation,” Dr. Goodwin and co-authors wrote.
The cross-sectional analysis included data for 3.76 million Medicare Part D enrollees. They looked specifically at the proportion of enrollees with chronic opioid use, which they defined as receipt of at least a 90-day supply in 1 year.
After adjusting for age, disability, and other factors, they found 693 out of 3,100 counties (22.4%) had rates of chronic opioid use higher than the mean, and 638 (20.6%) had rates lower than the mean. The correlation between opioid use rates and the presidential vote was 0.42 (P < .001), according to investigators.
They also published two county maps that they said shared some similar patterns. The first shows the proportion of older chronic opioid users by quintile, and the second shows Republican vote percentages, also by quintile. The correlation coefficient between those two rates was 0.32 (P < .001).
This study adds to the emerging literature on the relationships between health status and support of Donald Trump in the 2016 election, according to authors.
However, there were limitations to the study, they added. Of note, the presidential vote data was from 2016, but the information on prolonged opioid prescriptions was from 2015. Moreover, the voting data included all voters, while the opioid data came only from Medicare Part D enrollees, which represent about 72% of the full Medicare population.
One study author reported grants from the National Institute on Drug Abuse and Agency for Healthcare Research and Quality. Another reported membership in Physicians for Responsible Opioid Prescribing
SOURCE: Goodwin JS, et al. JAMA Network Open. 2018;1(2):e180450.
This article was updated 6/26/18.
FROM JAMA NETWORK OPEN
Key clinical point: Chronic use of prescription opioid drugs correlated with support for the Republican candidate in the 2016 U.S. presidential election.
Major finding: The mean Republican presidential vote was 60% in U.S. counties with significantly higher-than-average rates of prescriptions for prolonged opioid use, versus 39% for counties with significantly lower rates.
Study details: A retrospective, cross-sectional analysis including 3.76 million Medicare Part D enrollees.
Disclosures: One study author reported grants from the National Institute on Drug Abuse and Agency for Healthcare Research and Quality. Another reported membership in Physicians for Responsible Opioid Prescribing.
Source: Goodwin JS, et al. JAMA Network Open. 2018;1(2):e180450.
Average glucose, A1c discordance is common, highlights ADAG equation concerns
ORLANDO – Significant discordance exists between average glucose and hemoglobin A1c (HbA1c) measures in patients with certain comorbidities, according to findings from a retrospective chart review.
For example, there was a complete lack of correlation between average glucose (AG) and A1c measures in patients with advanced renal dysfunction and non-alcoholic fatty liver disease, (NAFLD) Jordan E. Perlman, MD, reported at the annual scientific sessions of the American Diabetes Association.
Unweighted averages of self-monitored blood glucose (SMBG) and continuous glucose monitor (CGM) readings were calculated based on downloads from 1,039 patients who had been prescribed insulin for diabetes mellitus between January 2011 and October 2016 and who had a comorbid condition proven or hypothesized to invalidate A1c, including anemia, chronic kidney disease (CKD), abnormal liver function tests (LFTs), and NAFLD. Predicted AG was also derived from paired A1c using the equation established by the A1c Derived Average Glucose (ADAG) Study Group in a 2013 re-analysis of its 2008 report, which excluded patients with comorbidities.
The averages calculated using downloads were then compared with the averages derived using the ADAG equation to assess concordance.
“The term ‘discordant’ refers to averages that differ by more than 15%,” Dr. Perlman explained.
She and her colleagues found that CGM, compared with SMBG, decreased the odds of discordance after controlling for diabetes type (odds ratio, 0.39).
Additionally, having type 2 vs. type 1 diabetes mellitus increased the odds of discordance, as did renal dysfunction.
“Having CKD stage 3b or worse increases the odds of ADAG discordance (OR, 2.04),” she said. “The relationship demonstrates statistical significance at a P value of 0.004. Unfortunately, we did not have enough patients to analyze stage 4 or 5 CKD alone.”
Poor linear correlation was clearly seen between AG and A1c in patients with NAFLD, she noted.
“The relationship doesn’t reach statistical significance, but the odds ratio of 1.6 is difficult to ignore. The wide confidence interval (0.67-3.58) leads us to believe that this particular analysis is probably underpowered,” Dr. Perlman said.
Factors assessed and found to have no significant effect on ADAG discordance included abnormal LFTs, age, body mass index, and hemoglobin, including by gender.
“These important data suggest that any patient on insulin who comes to diabetes clinic has an automatic 33.5% chance of mismatch between their A1c and average glucose, and this is before you know anything else about them. To go a step further, it seems excluding comorbidities doesn’t really improve the percent discordance,” she said, adding that this suggests comorbidities have less impact than previously thought. “This makes us wonder if maybe there is a problem with our test and not the person having the test.”
It remains unclear what is acceptable in terms of discordance, Dr. Perlman said, noting that using ADAG to interpret A1c yields a wide range of estimated AG.
“Comorbidities alone do not explain this variation,” she said. “Clinicians should not rely on A1c alone to make treatment decisions because it is unclear when discordance gains clinical relevance.”
This study is limited by the retrospective study design and a number of factors, such as the difficulties of confirming or excluding comorbidities based on a single encounter and the limitless potential for unestablished confounders of A1c and AG, Dr. Perlman noted.
Also, fingersticks inflate discordance.
“A better assessment of ADAG would be to measure only CGM averages in comorbities, though this may need to be a prospective trial as only 17% of our patients who have identified comorbidities use CGM,” she said.
Dr. Perlman concluded that fingersticks and CGM can provide important confirmation of A1c, but said this applies only at the population level and not to individual patients.
“For individual patients, any level of A1c can translate to a large range of average glucoses. We see this even in our concordant patients,” she said.
Further, while discordance is increased by some comorbidities, it also occurs absent of comorbidities at a rate of 28.7%.
she said.
Dr. Perlman reported having no disclosures. Senior author Irl B. Hirsch, MD, professor of medicine at the University of Washington, Seattle, disclosed financial relationships diabetes drug and device manufacturers Abbot, ADOCIA, Bigfoot Biomedical, Roche, and Medtronic MiniMed.
SOURCE: Perlman J et al., ADA 2018 Abstract 12-OR.
ORLANDO – Significant discordance exists between average glucose and hemoglobin A1c (HbA1c) measures in patients with certain comorbidities, according to findings from a retrospective chart review.
For example, there was a complete lack of correlation between average glucose (AG) and A1c measures in patients with advanced renal dysfunction and non-alcoholic fatty liver disease, (NAFLD) Jordan E. Perlman, MD, reported at the annual scientific sessions of the American Diabetes Association.
Unweighted averages of self-monitored blood glucose (SMBG) and continuous glucose monitor (CGM) readings were calculated based on downloads from 1,039 patients who had been prescribed insulin for diabetes mellitus between January 2011 and October 2016 and who had a comorbid condition proven or hypothesized to invalidate A1c, including anemia, chronic kidney disease (CKD), abnormal liver function tests (LFTs), and NAFLD. Predicted AG was also derived from paired A1c using the equation established by the A1c Derived Average Glucose (ADAG) Study Group in a 2013 re-analysis of its 2008 report, which excluded patients with comorbidities.
The averages calculated using downloads were then compared with the averages derived using the ADAG equation to assess concordance.
“The term ‘discordant’ refers to averages that differ by more than 15%,” Dr. Perlman explained.
She and her colleagues found that CGM, compared with SMBG, decreased the odds of discordance after controlling for diabetes type (odds ratio, 0.39).
Additionally, having type 2 vs. type 1 diabetes mellitus increased the odds of discordance, as did renal dysfunction.
“Having CKD stage 3b or worse increases the odds of ADAG discordance (OR, 2.04),” she said. “The relationship demonstrates statistical significance at a P value of 0.004. Unfortunately, we did not have enough patients to analyze stage 4 or 5 CKD alone.”
Poor linear correlation was clearly seen between AG and A1c in patients with NAFLD, she noted.
“The relationship doesn’t reach statistical significance, but the odds ratio of 1.6 is difficult to ignore. The wide confidence interval (0.67-3.58) leads us to believe that this particular analysis is probably underpowered,” Dr. Perlman said.
Factors assessed and found to have no significant effect on ADAG discordance included abnormal LFTs, age, body mass index, and hemoglobin, including by gender.
“These important data suggest that any patient on insulin who comes to diabetes clinic has an automatic 33.5% chance of mismatch between their A1c and average glucose, and this is before you know anything else about them. To go a step further, it seems excluding comorbidities doesn’t really improve the percent discordance,” she said, adding that this suggests comorbidities have less impact than previously thought. “This makes us wonder if maybe there is a problem with our test and not the person having the test.”
It remains unclear what is acceptable in terms of discordance, Dr. Perlman said, noting that using ADAG to interpret A1c yields a wide range of estimated AG.
“Comorbidities alone do not explain this variation,” she said. “Clinicians should not rely on A1c alone to make treatment decisions because it is unclear when discordance gains clinical relevance.”
This study is limited by the retrospective study design and a number of factors, such as the difficulties of confirming or excluding comorbidities based on a single encounter and the limitless potential for unestablished confounders of A1c and AG, Dr. Perlman noted.
Also, fingersticks inflate discordance.
“A better assessment of ADAG would be to measure only CGM averages in comorbities, though this may need to be a prospective trial as only 17% of our patients who have identified comorbidities use CGM,” she said.
Dr. Perlman concluded that fingersticks and CGM can provide important confirmation of A1c, but said this applies only at the population level and not to individual patients.
“For individual patients, any level of A1c can translate to a large range of average glucoses. We see this even in our concordant patients,” she said.
Further, while discordance is increased by some comorbidities, it also occurs absent of comorbidities at a rate of 28.7%.
she said.
Dr. Perlman reported having no disclosures. Senior author Irl B. Hirsch, MD, professor of medicine at the University of Washington, Seattle, disclosed financial relationships diabetes drug and device manufacturers Abbot, ADOCIA, Bigfoot Biomedical, Roche, and Medtronic MiniMed.
SOURCE: Perlman J et al., ADA 2018 Abstract 12-OR.
ORLANDO – Significant discordance exists between average glucose and hemoglobin A1c (HbA1c) measures in patients with certain comorbidities, according to findings from a retrospective chart review.
For example, there was a complete lack of correlation between average glucose (AG) and A1c measures in patients with advanced renal dysfunction and non-alcoholic fatty liver disease, (NAFLD) Jordan E. Perlman, MD, reported at the annual scientific sessions of the American Diabetes Association.
Unweighted averages of self-monitored blood glucose (SMBG) and continuous glucose monitor (CGM) readings were calculated based on downloads from 1,039 patients who had been prescribed insulin for diabetes mellitus between January 2011 and October 2016 and who had a comorbid condition proven or hypothesized to invalidate A1c, including anemia, chronic kidney disease (CKD), abnormal liver function tests (LFTs), and NAFLD. Predicted AG was also derived from paired A1c using the equation established by the A1c Derived Average Glucose (ADAG) Study Group in a 2013 re-analysis of its 2008 report, which excluded patients with comorbidities.
The averages calculated using downloads were then compared with the averages derived using the ADAG equation to assess concordance.
“The term ‘discordant’ refers to averages that differ by more than 15%,” Dr. Perlman explained.
She and her colleagues found that CGM, compared with SMBG, decreased the odds of discordance after controlling for diabetes type (odds ratio, 0.39).
Additionally, having type 2 vs. type 1 diabetes mellitus increased the odds of discordance, as did renal dysfunction.
“Having CKD stage 3b or worse increases the odds of ADAG discordance (OR, 2.04),” she said. “The relationship demonstrates statistical significance at a P value of 0.004. Unfortunately, we did not have enough patients to analyze stage 4 or 5 CKD alone.”
Poor linear correlation was clearly seen between AG and A1c in patients with NAFLD, she noted.
“The relationship doesn’t reach statistical significance, but the odds ratio of 1.6 is difficult to ignore. The wide confidence interval (0.67-3.58) leads us to believe that this particular analysis is probably underpowered,” Dr. Perlman said.
Factors assessed and found to have no significant effect on ADAG discordance included abnormal LFTs, age, body mass index, and hemoglobin, including by gender.
“These important data suggest that any patient on insulin who comes to diabetes clinic has an automatic 33.5% chance of mismatch between their A1c and average glucose, and this is before you know anything else about them. To go a step further, it seems excluding comorbidities doesn’t really improve the percent discordance,” she said, adding that this suggests comorbidities have less impact than previously thought. “This makes us wonder if maybe there is a problem with our test and not the person having the test.”
It remains unclear what is acceptable in terms of discordance, Dr. Perlman said, noting that using ADAG to interpret A1c yields a wide range of estimated AG.
“Comorbidities alone do not explain this variation,” she said. “Clinicians should not rely on A1c alone to make treatment decisions because it is unclear when discordance gains clinical relevance.”
This study is limited by the retrospective study design and a number of factors, such as the difficulties of confirming or excluding comorbidities based on a single encounter and the limitless potential for unestablished confounders of A1c and AG, Dr. Perlman noted.
Also, fingersticks inflate discordance.
“A better assessment of ADAG would be to measure only CGM averages in comorbities, though this may need to be a prospective trial as only 17% of our patients who have identified comorbidities use CGM,” she said.
Dr. Perlman concluded that fingersticks and CGM can provide important confirmation of A1c, but said this applies only at the population level and not to individual patients.
“For individual patients, any level of A1c can translate to a large range of average glucoses. We see this even in our concordant patients,” she said.
Further, while discordance is increased by some comorbidities, it also occurs absent of comorbidities at a rate of 28.7%.
she said.
Dr. Perlman reported having no disclosures. Senior author Irl B. Hirsch, MD, professor of medicine at the University of Washington, Seattle, disclosed financial relationships diabetes drug and device manufacturers Abbot, ADOCIA, Bigfoot Biomedical, Roche, and Medtronic MiniMed.
SOURCE: Perlman J et al., ADA 2018 Abstract 12-OR.
REPORTING FROM ADA 2018
Key clinical point: AG and A1c discordance is common, thus professional consensus regarding acceptable discordance is needed before ADAG equation use is broadened.
Major finding: CKD stage 3b or worse increases the odds of ADAG discordance (OR, 2.04).
Study details: A retrospective review of 1,039 patient charts.
Disclosures: Dr. Perlman reported having no disclosures. Senior author Irl B. Hirsch, MD, disclosed financial relationships diabetes drug and device manufacturers Abbot, ADOCIA, Bigfoot Biomedical, Roche, and Medtronic MiniMed.
Source: Perlman J et al. ADA 2018 Abstract 12-OR.
Patients going without as insulin prices skyrocket
ORLANDO – About a quarter of diabetes patients use less insulin than prescribed because they can’t afford it, and they have worse glycemic control because of it, according to a survey of patients at the Yale Diabetes Center in New Haven, Conn.
The soaring cost of insulin – especially analogues – has been in the news following a more than 300% increase from 2004-2018. The cash price for a 10 mL vial of insulin lispro (Humalog), for example, has climbed from $59 to $320. The American Diabetes Association recently released a white paper on the issue, citing a “lack of transparency throughout the insulin supply chain” that obscures the reasons for the surge. It’s working with other groups to ensure affordable access.
Six questions were key: In the past 12 months, did you, because of cost, use less insulin than prescribed; try to stretch out your insulin; take smaller doses of insulin than prescribed; stop insulin; not fill an insulin prescription; or not start insulin?
Fifty-one patients answered “yes” to at least one of those questions, signaling to investigators that they were using less insulin than prescribed because they couldn’t afford it. Compared with other patients, they were three times more likely to have HbA1c levels above 9%, controlling for age, sex, diabetes duration, and income (P = 0.03).
“One in four patients were using less of an essential medication because it costs too much for them to take the prescribed amount,” said investigator Darby Herkert, who participated in the study as an undergraduate at Yale. “It’s having a tangible health effect.”
The problem was greatest among people making less than $100,000 dollars a year, and was not associated with race or the type of diabetes they had. Employer health coverage was not protective, and patients who were covered by a mix of government and employer insurance were at greater risk of underuse, as were those who were unable to work.
The situation is probably common in the United States, Ms. Herkert noted at the American Diabetes Association scientific sessions meeting. “New Haven is a demographic microcosm of the U.S.”
“These results highlight an urgent need to address high insulin prices,” she said. This may be done through greater transparency in pricing, advocacy for patients who can’t afford their prescription, use of alternative insulin options for some patients, and assistance programs,” she said.
The work was funded in part by the National Institutes of Health. The investigators had no disclosures.
SOURCE: Herkert D et al. ADA 2018 abstract 2-OR
ORLANDO – About a quarter of diabetes patients use less insulin than prescribed because they can’t afford it, and they have worse glycemic control because of it, according to a survey of patients at the Yale Diabetes Center in New Haven, Conn.
The soaring cost of insulin – especially analogues – has been in the news following a more than 300% increase from 2004-2018. The cash price for a 10 mL vial of insulin lispro (Humalog), for example, has climbed from $59 to $320. The American Diabetes Association recently released a white paper on the issue, citing a “lack of transparency throughout the insulin supply chain” that obscures the reasons for the surge. It’s working with other groups to ensure affordable access.
Six questions were key: In the past 12 months, did you, because of cost, use less insulin than prescribed; try to stretch out your insulin; take smaller doses of insulin than prescribed; stop insulin; not fill an insulin prescription; or not start insulin?
Fifty-one patients answered “yes” to at least one of those questions, signaling to investigators that they were using less insulin than prescribed because they couldn’t afford it. Compared with other patients, they were three times more likely to have HbA1c levels above 9%, controlling for age, sex, diabetes duration, and income (P = 0.03).
“One in four patients were using less of an essential medication because it costs too much for them to take the prescribed amount,” said investigator Darby Herkert, who participated in the study as an undergraduate at Yale. “It’s having a tangible health effect.”
The problem was greatest among people making less than $100,000 dollars a year, and was not associated with race or the type of diabetes they had. Employer health coverage was not protective, and patients who were covered by a mix of government and employer insurance were at greater risk of underuse, as were those who were unable to work.
The situation is probably common in the United States, Ms. Herkert noted at the American Diabetes Association scientific sessions meeting. “New Haven is a demographic microcosm of the U.S.”
“These results highlight an urgent need to address high insulin prices,” she said. This may be done through greater transparency in pricing, advocacy for patients who can’t afford their prescription, use of alternative insulin options for some patients, and assistance programs,” she said.
The work was funded in part by the National Institutes of Health. The investigators had no disclosures.
SOURCE: Herkert D et al. ADA 2018 abstract 2-OR
ORLANDO – About a quarter of diabetes patients use less insulin than prescribed because they can’t afford it, and they have worse glycemic control because of it, according to a survey of patients at the Yale Diabetes Center in New Haven, Conn.
The soaring cost of insulin – especially analogues – has been in the news following a more than 300% increase from 2004-2018. The cash price for a 10 mL vial of insulin lispro (Humalog), for example, has climbed from $59 to $320. The American Diabetes Association recently released a white paper on the issue, citing a “lack of transparency throughout the insulin supply chain” that obscures the reasons for the surge. It’s working with other groups to ensure affordable access.
Six questions were key: In the past 12 months, did you, because of cost, use less insulin than prescribed; try to stretch out your insulin; take smaller doses of insulin than prescribed; stop insulin; not fill an insulin prescription; or not start insulin?
Fifty-one patients answered “yes” to at least one of those questions, signaling to investigators that they were using less insulin than prescribed because they couldn’t afford it. Compared with other patients, they were three times more likely to have HbA1c levels above 9%, controlling for age, sex, diabetes duration, and income (P = 0.03).
“One in four patients were using less of an essential medication because it costs too much for them to take the prescribed amount,” said investigator Darby Herkert, who participated in the study as an undergraduate at Yale. “It’s having a tangible health effect.”
The problem was greatest among people making less than $100,000 dollars a year, and was not associated with race or the type of diabetes they had. Employer health coverage was not protective, and patients who were covered by a mix of government and employer insurance were at greater risk of underuse, as were those who were unable to work.
The situation is probably common in the United States, Ms. Herkert noted at the American Diabetes Association scientific sessions meeting. “New Haven is a demographic microcosm of the U.S.”
“These results highlight an urgent need to address high insulin prices,” she said. This may be done through greater transparency in pricing, advocacy for patients who can’t afford their prescription, use of alternative insulin options for some patients, and assistance programs,” she said.
The work was funded in part by the National Institutes of Health. The investigators had no disclosures.
SOURCE: Herkert D et al. ADA 2018 abstract 2-OR
REPORTING FROM ADA 2018
Key clinical point: because of it.
Major finding: Compared with other patients, they were three times more likely to have hemoglobin A1c levels above 9%, controlling for age, sex, diabetes duration, and income (P = 0.03).
Study details: A single-center survey of 199 patients with type 1 or type 2 diabetes.
Disclosures: The work was funded in part by the National Institutes of Health. The investigators had no disclosures.
Source: Herkert D et al. ADA 2018, Abstract 2-OR
Nephrogenic Systemic Fibrosis in a Patient With Multiple Inflammatory Disorders
First described in 2000 in a case series of 15 patients, nephrogenic systemic fibrosis (NSF) is a rare scleroderma-like fibrosing skin condition associated with gadolinium exposure in end stage renal disease (ESRD).1 Patients with advanced chronic kidney disease (CKD) or ESRD are at the highest risk for this condition when exposed to gadolinium-based contrast dyes.
Nephrogenic systemic fibrosis is a devastating and rapidly progressive condition, making its prevention in at-risk populations of utmost importance. In this article, the authors describe a case of a patient who developed NSF in the setting of gadolinium exposure and multiple inflammatory dermatologic conditions. This case illustrates the possible role of a pro-inflammatory state in predisposing to NSF, which may help further elucidate its mechanism of action.
Case Presentation
A 61-year-old Hispanic male with a history of IV heroin use with ESRD secondary to membranous glomerulonephritis on hemodialysis and chronic hepatitis C infection presented to the West Los Angeles VAMC with fevers and night sweats that had persisted for 2 weeks. His physical examination was notable for diffuse tender palpable purpura and petechiae (including his palms and soles), altered mental status, and diffuse myoclonic jerks, which necessitated endotracheal intubation and mechanical ventilation for airway protection. Blood cultures were positive for methicillin-sensitive Staphylococcus aureus (MSSA). Laboratory results were notable for an elevated sedimentation rate of 53 mm/h (0-10 mm/h), C-reactive protein of 19.8 mg/L (< 0.744 mg/dL), and albumin of 1.2 g/dL (3.2-4.8 g/dL). An extensive rheumatologic workup was unrevealing, and a lumbar puncture was unremarkable. A biopsy of his skin lesions was consistent with leukocytoclastic vasculitis.
The patient’s prior hemodialysis access, a tunneled dialysis catheter in the right subclavian vein, was removed given concern for line infection and replaced with an internal jugular temporary hemodialysis line. Given his altered mental status and myoclonic jerks, the decision was made to pursue a magnetic resonance imaging (MRI) scan of the brain and spine with gadolinium contrast to evaluate for cerebral vasculitis and/or septic emboli to the brain.
The patient received 15 mL of gadoversetamide contrast in accordance with hospital imaging protocol. The MRI revealed only chronic ischemic changes. The patient underwent hemodialysis about 18 hours later. The patient was treated with a 6-week course of IV penicillin G. His altered mental status and myoclonic jerks resolved without intervention, and he was then discharged to an acute rehabilitation unit.
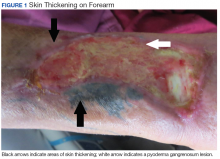
Eight weeks after his initial presentation the patient developed a purulent wound on his right forearm (Figure 1)
The patient was discharged to continue physical and occupational therapy to preserve his functional mobility, as no other treatment options were available.
Discussion
Nephrogenic systemic fibrosis is a poorly understood inflammatory condition that produces diffuse fibrosis of the skin. Typically, the disease begins with progressive skin induration of the extremities. Systemic involvement may occur, leading to fibrosis of skeletal muscle, fascia, and multiple organs. Flexion contractures may develop that limit physical function. Fibrosis can become apparent within days to months after exposure to gadolinium contrast.
Beyond renal insufficiency, it is unclear what other risk factors predispose patients to developing this condition. Only a minority of patients with CKD stages 1 through 4 will develop NSF on exposure to gadolinium contrast. However, the incidence of NSF among patients with CKD stage 5 who are exposed to gadolinium has been estimated to be about 13.4% in a prospective study involving 18 patients.2
In a 2015 meta-analysis by Zhang and colleagues, the only clear risk factor identified for the development of NSF, aside from gadolinium exposure, was severe renal insufficiency with a glomerular filtration rate of < 30 mL/min/1.75m2.3 Due to the limited number of patients identified with this disease, it is difficult to identify other risk factors associated with the development of NSF. Based on in vitro studies, it has been postulated that a pro-inflammatory state predisposes patients to develop NSF.4,5 The proposed mechanism for NSF involves extravasation of gadolinium in the setting of vascular endothelial permeability.5,6 Gadolinium then interacts with tissue macrophages, which induce the release of inflammatory cytokines and the secretion of smooth muscle actin by dermal fibroblasts.6,7
Treatment of NSF has been largely unsuccessful. Multiple modalities of treatment that included topical and oral steroids, immunosuppression, plasmapheresis, and ultraviolent therapy have been attempted, none of which have been proven to consistently limit progression of the disease.8 The most effective intervention is early physical therapy to preserve functionality and prevent contracture formation. For patients who are eligible, early renal transplantation may offer the best chance of improved mobility. In a case series review by Cuffy and colleagues, 5 of 6 patients who underwent renal transplantation after the development of NSF experienced softening of the involved skin, and 2 patients had improved mobility of joints.9
Conclusion
The case presented here illustrates a possible association between a pro-inflammatory state and the development of NSF. This patient had multiple inflammatory conditions, including MSSA bacteremia, leukocytoclastic vasculitis, and pyoderma gangrenosum (the latter 2 conditions were thought to be associated with his underlying chronic hepatitis C infection), which the authors believe predisposed him to endothelial permeability and risk for developing NSF. The risk of developing NSF in at-risk patients with each episode of gadolinium exposure is estimated around 2.4%, or an incidence of 4.3 cases per 1,000 patient-years, leading the American College of Radiologists to recommend against the administration of gadolinium-based contrast except in cases in which benefits clearly outweigh risks.10 However, an MRI with gadolinium contrast can offer high diagnostic yield in cases such as the one presented here in which a diagnosis remains elusive. Moreover, the use of linear gadolinium-based contrast agents such as gadoversetamide, as in this case, has been reported to be associated with higher incidence of NSF.5 Since this case, the West Los Angeles VAMC has switched to gadobutrol contrast for its MRI protocol, which has been purported to be a lower risk agent compared with that of linear gadolinium-based contrast agents (although several cases of NSF have been reported with gadobutrol in the literature).11
Providers weighing the decision to administer gadolinium contrast to patients with ESRD should discuss the risks and benefits thoroughly, especially in patients with preexisting inflammatory conditions. In addition, although it has not been shown to effectively reduce the risk of NSF after administration of gadolinium, hemodialysis is recommended 2 hours after contrast administration for individuals at risk (the study patient received hemodialysis approximately 18 hours after).12 Given the lack of effective treatment options for NSF, prevention is key. A deeper understanding of the pathophysiology of NSF and identification of its risk factors is paramount to the prevention of this devastating disease.
1. Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000;356(9234):1000-1001.
2. Todd DJ, Kagan A, Chibnik LB, Kay J. Cutaneous changes of nephrogenic systemic fibrosis. Arthritis Rheum. 2007;56(10):3433-3441.
3. Zhang B, Liang L, Chen W, Liang C, Zhang S. An updated study to determine association between gadolinium-based contrast agents and nephrogenic systemic fibrosis. PLoS One. 2015;10(6):e0129720.
4. Wermuth PJ, Del Galdo F, Jiménez SA. Induction of the expression of profibrotic cytokines and growth factors in normal human peripheral blood monocytes by gadolinium contrast agents. Arthritis Rheum. 2009;60(5):1508-1518.
5. Daftari Besheli L, Aran S, Shaqdan K, Kay J, Abujudeh H. Current status of nephrogenic systemic fibrosis. Clin Radiol. 2014;69(7):661-668.
6. Wagner B, Drel V, Gorin Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am J Physiol Renal Physiol. 2016;31(1):F1-F11.
7. Idée JM, Fretellier N, Robic C, Corot C. The role of gadolinium chelates in the mechanism of nephrogenic systemic fibrosis: a critical update. Crit Rev Toxicol. 2014;44(10):895-913.
8. Mendoza FA, Artlett CM, Sandorfi N, Latinis K, Piera-Velazquez S, Jimenez SA. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35(4):238-249.
9. Cuffy MC, Singh M, Formica R, et al. Renal transplantation for nephrogenic systemic fibrosis: a case report and review of the literature. Nephrol Dial Transplant. 2011;26(3):1099-1109.
10. Deo A, Fogel M, Cowper SE. Nephrogenic systemic fibrosis: a population study examining the relationship of disease development of gadolinium exposure. Clin J Am Soc Nephrol. 2007;2(2):264-267
11. Elmholdt TR, Jørgensen B, Ramsing M, Pedersen M, Olesen AB. Two cases of nephrogenic systemic fibrosis after exposure to the macrocyclic compound gadobutrol. NDT Plus. 2010;3(3):285-287.
12. Abu-Alfa AK. Nephrogenic systemic fibrosis and gadolinium-based contrast agents. Adv Chronic Kidney Dis. 2011;18(3);188-198.
First described in 2000 in a case series of 15 patients, nephrogenic systemic fibrosis (NSF) is a rare scleroderma-like fibrosing skin condition associated with gadolinium exposure in end stage renal disease (ESRD).1 Patients with advanced chronic kidney disease (CKD) or ESRD are at the highest risk for this condition when exposed to gadolinium-based contrast dyes.
Nephrogenic systemic fibrosis is a devastating and rapidly progressive condition, making its prevention in at-risk populations of utmost importance. In this article, the authors describe a case of a patient who developed NSF in the setting of gadolinium exposure and multiple inflammatory dermatologic conditions. This case illustrates the possible role of a pro-inflammatory state in predisposing to NSF, which may help further elucidate its mechanism of action.
Case Presentation
A 61-year-old Hispanic male with a history of IV heroin use with ESRD secondary to membranous glomerulonephritis on hemodialysis and chronic hepatitis C infection presented to the West Los Angeles VAMC with fevers and night sweats that had persisted for 2 weeks. His physical examination was notable for diffuse tender palpable purpura and petechiae (including his palms and soles), altered mental status, and diffuse myoclonic jerks, which necessitated endotracheal intubation and mechanical ventilation for airway protection. Blood cultures were positive for methicillin-sensitive Staphylococcus aureus (MSSA). Laboratory results were notable for an elevated sedimentation rate of 53 mm/h (0-10 mm/h), C-reactive protein of 19.8 mg/L (< 0.744 mg/dL), and albumin of 1.2 g/dL (3.2-4.8 g/dL). An extensive rheumatologic workup was unrevealing, and a lumbar puncture was unremarkable. A biopsy of his skin lesions was consistent with leukocytoclastic vasculitis.
The patient’s prior hemodialysis access, a tunneled dialysis catheter in the right subclavian vein, was removed given concern for line infection and replaced with an internal jugular temporary hemodialysis line. Given his altered mental status and myoclonic jerks, the decision was made to pursue a magnetic resonance imaging (MRI) scan of the brain and spine with gadolinium contrast to evaluate for cerebral vasculitis and/or septic emboli to the brain.
The patient received 15 mL of gadoversetamide contrast in accordance with hospital imaging protocol. The MRI revealed only chronic ischemic changes. The patient underwent hemodialysis about 18 hours later. The patient was treated with a 6-week course of IV penicillin G. His altered mental status and myoclonic jerks resolved without intervention, and he was then discharged to an acute rehabilitation unit.
Eight weeks after his initial presentation the patient developed a purulent wound on his right forearm (Figure 1)
The patient was discharged to continue physical and occupational therapy to preserve his functional mobility, as no other treatment options were available.
Discussion
Nephrogenic systemic fibrosis is a poorly understood inflammatory condition that produces diffuse fibrosis of the skin. Typically, the disease begins with progressive skin induration of the extremities. Systemic involvement may occur, leading to fibrosis of skeletal muscle, fascia, and multiple organs. Flexion contractures may develop that limit physical function. Fibrosis can become apparent within days to months after exposure to gadolinium contrast.
Beyond renal insufficiency, it is unclear what other risk factors predispose patients to developing this condition. Only a minority of patients with CKD stages 1 through 4 will develop NSF on exposure to gadolinium contrast. However, the incidence of NSF among patients with CKD stage 5 who are exposed to gadolinium has been estimated to be about 13.4% in a prospective study involving 18 patients.2
In a 2015 meta-analysis by Zhang and colleagues, the only clear risk factor identified for the development of NSF, aside from gadolinium exposure, was severe renal insufficiency with a glomerular filtration rate of < 30 mL/min/1.75m2.3 Due to the limited number of patients identified with this disease, it is difficult to identify other risk factors associated with the development of NSF. Based on in vitro studies, it has been postulated that a pro-inflammatory state predisposes patients to develop NSF.4,5 The proposed mechanism for NSF involves extravasation of gadolinium in the setting of vascular endothelial permeability.5,6 Gadolinium then interacts with tissue macrophages, which induce the release of inflammatory cytokines and the secretion of smooth muscle actin by dermal fibroblasts.6,7
Treatment of NSF has been largely unsuccessful. Multiple modalities of treatment that included topical and oral steroids, immunosuppression, plasmapheresis, and ultraviolent therapy have been attempted, none of which have been proven to consistently limit progression of the disease.8 The most effective intervention is early physical therapy to preserve functionality and prevent contracture formation. For patients who are eligible, early renal transplantation may offer the best chance of improved mobility. In a case series review by Cuffy and colleagues, 5 of 6 patients who underwent renal transplantation after the development of NSF experienced softening of the involved skin, and 2 patients had improved mobility of joints.9
Conclusion
The case presented here illustrates a possible association between a pro-inflammatory state and the development of NSF. This patient had multiple inflammatory conditions, including MSSA bacteremia, leukocytoclastic vasculitis, and pyoderma gangrenosum (the latter 2 conditions were thought to be associated with his underlying chronic hepatitis C infection), which the authors believe predisposed him to endothelial permeability and risk for developing NSF. The risk of developing NSF in at-risk patients with each episode of gadolinium exposure is estimated around 2.4%, or an incidence of 4.3 cases per 1,000 patient-years, leading the American College of Radiologists to recommend against the administration of gadolinium-based contrast except in cases in which benefits clearly outweigh risks.10 However, an MRI with gadolinium contrast can offer high diagnostic yield in cases such as the one presented here in which a diagnosis remains elusive. Moreover, the use of linear gadolinium-based contrast agents such as gadoversetamide, as in this case, has been reported to be associated with higher incidence of NSF.5 Since this case, the West Los Angeles VAMC has switched to gadobutrol contrast for its MRI protocol, which has been purported to be a lower risk agent compared with that of linear gadolinium-based contrast agents (although several cases of NSF have been reported with gadobutrol in the literature).11
Providers weighing the decision to administer gadolinium contrast to patients with ESRD should discuss the risks and benefits thoroughly, especially in patients with preexisting inflammatory conditions. In addition, although it has not been shown to effectively reduce the risk of NSF after administration of gadolinium, hemodialysis is recommended 2 hours after contrast administration for individuals at risk (the study patient received hemodialysis approximately 18 hours after).12 Given the lack of effective treatment options for NSF, prevention is key. A deeper understanding of the pathophysiology of NSF and identification of its risk factors is paramount to the prevention of this devastating disease.
First described in 2000 in a case series of 15 patients, nephrogenic systemic fibrosis (NSF) is a rare scleroderma-like fibrosing skin condition associated with gadolinium exposure in end stage renal disease (ESRD).1 Patients with advanced chronic kidney disease (CKD) or ESRD are at the highest risk for this condition when exposed to gadolinium-based contrast dyes.
Nephrogenic systemic fibrosis is a devastating and rapidly progressive condition, making its prevention in at-risk populations of utmost importance. In this article, the authors describe a case of a patient who developed NSF in the setting of gadolinium exposure and multiple inflammatory dermatologic conditions. This case illustrates the possible role of a pro-inflammatory state in predisposing to NSF, which may help further elucidate its mechanism of action.
Case Presentation
A 61-year-old Hispanic male with a history of IV heroin use with ESRD secondary to membranous glomerulonephritis on hemodialysis and chronic hepatitis C infection presented to the West Los Angeles VAMC with fevers and night sweats that had persisted for 2 weeks. His physical examination was notable for diffuse tender palpable purpura and petechiae (including his palms and soles), altered mental status, and diffuse myoclonic jerks, which necessitated endotracheal intubation and mechanical ventilation for airway protection. Blood cultures were positive for methicillin-sensitive Staphylococcus aureus (MSSA). Laboratory results were notable for an elevated sedimentation rate of 53 mm/h (0-10 mm/h), C-reactive protein of 19.8 mg/L (< 0.744 mg/dL), and albumin of 1.2 g/dL (3.2-4.8 g/dL). An extensive rheumatologic workup was unrevealing, and a lumbar puncture was unremarkable. A biopsy of his skin lesions was consistent with leukocytoclastic vasculitis.
The patient’s prior hemodialysis access, a tunneled dialysis catheter in the right subclavian vein, was removed given concern for line infection and replaced with an internal jugular temporary hemodialysis line. Given his altered mental status and myoclonic jerks, the decision was made to pursue a magnetic resonance imaging (MRI) scan of the brain and spine with gadolinium contrast to evaluate for cerebral vasculitis and/or septic emboli to the brain.
The patient received 15 mL of gadoversetamide contrast in accordance with hospital imaging protocol. The MRI revealed only chronic ischemic changes. The patient underwent hemodialysis about 18 hours later. The patient was treated with a 6-week course of IV penicillin G. His altered mental status and myoclonic jerks resolved without intervention, and he was then discharged to an acute rehabilitation unit.
Eight weeks after his initial presentation the patient developed a purulent wound on his right forearm (Figure 1)
The patient was discharged to continue physical and occupational therapy to preserve his functional mobility, as no other treatment options were available.
Discussion
Nephrogenic systemic fibrosis is a poorly understood inflammatory condition that produces diffuse fibrosis of the skin. Typically, the disease begins with progressive skin induration of the extremities. Systemic involvement may occur, leading to fibrosis of skeletal muscle, fascia, and multiple organs. Flexion contractures may develop that limit physical function. Fibrosis can become apparent within days to months after exposure to gadolinium contrast.
Beyond renal insufficiency, it is unclear what other risk factors predispose patients to developing this condition. Only a minority of patients with CKD stages 1 through 4 will develop NSF on exposure to gadolinium contrast. However, the incidence of NSF among patients with CKD stage 5 who are exposed to gadolinium has been estimated to be about 13.4% in a prospective study involving 18 patients.2
In a 2015 meta-analysis by Zhang and colleagues, the only clear risk factor identified for the development of NSF, aside from gadolinium exposure, was severe renal insufficiency with a glomerular filtration rate of < 30 mL/min/1.75m2.3 Due to the limited number of patients identified with this disease, it is difficult to identify other risk factors associated with the development of NSF. Based on in vitro studies, it has been postulated that a pro-inflammatory state predisposes patients to develop NSF.4,5 The proposed mechanism for NSF involves extravasation of gadolinium in the setting of vascular endothelial permeability.5,6 Gadolinium then interacts with tissue macrophages, which induce the release of inflammatory cytokines and the secretion of smooth muscle actin by dermal fibroblasts.6,7
Treatment of NSF has been largely unsuccessful. Multiple modalities of treatment that included topical and oral steroids, immunosuppression, plasmapheresis, and ultraviolent therapy have been attempted, none of which have been proven to consistently limit progression of the disease.8 The most effective intervention is early physical therapy to preserve functionality and prevent contracture formation. For patients who are eligible, early renal transplantation may offer the best chance of improved mobility. In a case series review by Cuffy and colleagues, 5 of 6 patients who underwent renal transplantation after the development of NSF experienced softening of the involved skin, and 2 patients had improved mobility of joints.9
Conclusion
The case presented here illustrates a possible association between a pro-inflammatory state and the development of NSF. This patient had multiple inflammatory conditions, including MSSA bacteremia, leukocytoclastic vasculitis, and pyoderma gangrenosum (the latter 2 conditions were thought to be associated with his underlying chronic hepatitis C infection), which the authors believe predisposed him to endothelial permeability and risk for developing NSF. The risk of developing NSF in at-risk patients with each episode of gadolinium exposure is estimated around 2.4%, or an incidence of 4.3 cases per 1,000 patient-years, leading the American College of Radiologists to recommend against the administration of gadolinium-based contrast except in cases in which benefits clearly outweigh risks.10 However, an MRI with gadolinium contrast can offer high diagnostic yield in cases such as the one presented here in which a diagnosis remains elusive. Moreover, the use of linear gadolinium-based contrast agents such as gadoversetamide, as in this case, has been reported to be associated with higher incidence of NSF.5 Since this case, the West Los Angeles VAMC has switched to gadobutrol contrast for its MRI protocol, which has been purported to be a lower risk agent compared with that of linear gadolinium-based contrast agents (although several cases of NSF have been reported with gadobutrol in the literature).11
Providers weighing the decision to administer gadolinium contrast to patients with ESRD should discuss the risks and benefits thoroughly, especially in patients with preexisting inflammatory conditions. In addition, although it has not been shown to effectively reduce the risk of NSF after administration of gadolinium, hemodialysis is recommended 2 hours after contrast administration for individuals at risk (the study patient received hemodialysis approximately 18 hours after).12 Given the lack of effective treatment options for NSF, prevention is key. A deeper understanding of the pathophysiology of NSF and identification of its risk factors is paramount to the prevention of this devastating disease.
1. Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000;356(9234):1000-1001.
2. Todd DJ, Kagan A, Chibnik LB, Kay J. Cutaneous changes of nephrogenic systemic fibrosis. Arthritis Rheum. 2007;56(10):3433-3441.
3. Zhang B, Liang L, Chen W, Liang C, Zhang S. An updated study to determine association between gadolinium-based contrast agents and nephrogenic systemic fibrosis. PLoS One. 2015;10(6):e0129720.
4. Wermuth PJ, Del Galdo F, Jiménez SA. Induction of the expression of profibrotic cytokines and growth factors in normal human peripheral blood monocytes by gadolinium contrast agents. Arthritis Rheum. 2009;60(5):1508-1518.
5. Daftari Besheli L, Aran S, Shaqdan K, Kay J, Abujudeh H. Current status of nephrogenic systemic fibrosis. Clin Radiol. 2014;69(7):661-668.
6. Wagner B, Drel V, Gorin Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am J Physiol Renal Physiol. 2016;31(1):F1-F11.
7. Idée JM, Fretellier N, Robic C, Corot C. The role of gadolinium chelates in the mechanism of nephrogenic systemic fibrosis: a critical update. Crit Rev Toxicol. 2014;44(10):895-913.
8. Mendoza FA, Artlett CM, Sandorfi N, Latinis K, Piera-Velazquez S, Jimenez SA. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35(4):238-249.
9. Cuffy MC, Singh M, Formica R, et al. Renal transplantation for nephrogenic systemic fibrosis: a case report and review of the literature. Nephrol Dial Transplant. 2011;26(3):1099-1109.
10. Deo A, Fogel M, Cowper SE. Nephrogenic systemic fibrosis: a population study examining the relationship of disease development of gadolinium exposure. Clin J Am Soc Nephrol. 2007;2(2):264-267
11. Elmholdt TR, Jørgensen B, Ramsing M, Pedersen M, Olesen AB. Two cases of nephrogenic systemic fibrosis after exposure to the macrocyclic compound gadobutrol. NDT Plus. 2010;3(3):285-287.
12. Abu-Alfa AK. Nephrogenic systemic fibrosis and gadolinium-based contrast agents. Adv Chronic Kidney Dis. 2011;18(3);188-198.
1. Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000;356(9234):1000-1001.
2. Todd DJ, Kagan A, Chibnik LB, Kay J. Cutaneous changes of nephrogenic systemic fibrosis. Arthritis Rheum. 2007;56(10):3433-3441.
3. Zhang B, Liang L, Chen W, Liang C, Zhang S. An updated study to determine association between gadolinium-based contrast agents and nephrogenic systemic fibrosis. PLoS One. 2015;10(6):e0129720.
4. Wermuth PJ, Del Galdo F, Jiménez SA. Induction of the expression of profibrotic cytokines and growth factors in normal human peripheral blood monocytes by gadolinium contrast agents. Arthritis Rheum. 2009;60(5):1508-1518.
5. Daftari Besheli L, Aran S, Shaqdan K, Kay J, Abujudeh H. Current status of nephrogenic systemic fibrosis. Clin Radiol. 2014;69(7):661-668.
6. Wagner B, Drel V, Gorin Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am J Physiol Renal Physiol. 2016;31(1):F1-F11.
7. Idée JM, Fretellier N, Robic C, Corot C. The role of gadolinium chelates in the mechanism of nephrogenic systemic fibrosis: a critical update. Crit Rev Toxicol. 2014;44(10):895-913.
8. Mendoza FA, Artlett CM, Sandorfi N, Latinis K, Piera-Velazquez S, Jimenez SA. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35(4):238-249.
9. Cuffy MC, Singh M, Formica R, et al. Renal transplantation for nephrogenic systemic fibrosis: a case report and review of the literature. Nephrol Dial Transplant. 2011;26(3):1099-1109.
10. Deo A, Fogel M, Cowper SE. Nephrogenic systemic fibrosis: a population study examining the relationship of disease development of gadolinium exposure. Clin J Am Soc Nephrol. 2007;2(2):264-267
11. Elmholdt TR, Jørgensen B, Ramsing M, Pedersen M, Olesen AB. Two cases of nephrogenic systemic fibrosis after exposure to the macrocyclic compound gadobutrol. NDT Plus. 2010;3(3):285-287.
12. Abu-Alfa AK. Nephrogenic systemic fibrosis and gadolinium-based contrast agents. Adv Chronic Kidney Dis. 2011;18(3);188-198.
Survey reveals patient perceptions of ITP
STOCKHOLM—A new survey has revealed patients’ perceptions of immune thrombocytopenia (ITP) and how the condition impacts their quality of life (QOL).
Patients reported delays in diagnosis, lack of support, severe fatigue, and impacts on both emotional well-being and their ability to work.
Interim results of this survey, the ITP World Impact Survey (I-WISh), were presented in a poster (abstract PF654) at the 23rd Congress of the European Hematology Association (EHA).
I-WISh is a cross-sectional survey of ITP patients developed by global ITP experts, patient groups, and Novartis.
Interim results of the survey included patients from 12 countries (Canada, China, Colombia, France, Germany, Italy, India, Japan, Spain, Turkey, UK, and US) who completed an online questionnaire beginning in January 2018.
As of May 14, 2018, 1400 adults (age 18 and older) had completed the survey. Sixty-five percent were female, and they had a mean age of 47.1 years. The patients’ mean length of time with ITP was 110 months.
Most patients (63%) reported a high score for their current health state (5 to 7 on the Likert scale), but 15% reported a low score (1-3).
Most patients were working full-time (45%) or part-time (16%) at the time of the survey. Nineteen percent were retired, 6% were homemakers, 4% were students, and 10% were not seeking employment, on long-term sick leave or disability, or “other.”
Diagnosis
Twenty-two percent of all patients (307/1400) felt they had a delay in their ITP diagnosis caused by waiting for additional tests (49%, 150/307) or referral to a specialist (37%, 114/307).
Three-quarters of patients with a perceived delay (229/307) were anxious throughout diagnosis.
And 66% of all patients (927/1400) wanted more support during their diagnosis.
Symptoms
Patients reported fatigue as one of the most severe symptoms at diagnosis (75%, 627/839) and at survey completion (66%, 480/722).
The other “most severe” symptoms at diagnosis were heavy menstrual bleeding (85%, 353/416) and anxiety surrounding unstable platelet count (78%, 382/487). The other “most severe” symptoms at survey completion were thrombosis (73%, 24/33) and anxiety surrounding unstable platelet count (66%, 284/431).
“Severe fatigue, in particular, was reported by many patients as the most difficult-to-manage symptom of ITP,” said study investigator Nichola Cooper, MD, of Hammersmith Hospital, Imperial College London, in the UK.
“This is an important message for healthcare providers treating patients with this rare disease. ITP is about more than bruising and risk of bleeding.”
QOL
Forty-four percent of respondents (611/1398) said ITP impacted their energy levels more than half the time, and 36% (501/1398) said ITP had a negative impact on their normal capacity to exercise more than half the time.
Half of all patients (697/1400) said ITP had a high impact on their emotional well-being.
Eighty-three percent (1157/1400) said they felt a stable and safe platelet count was important, 64% (900/1400) worried that their condition will get worse, and 63% (888/1400) were concerned that their platelet count changes for no apparent reason.
Thirty-seven percent of all patients (511/1400) had reduced their work hours because of ITP, 37% (522/1400) seriously considered reducing their hours, and 21% (294/1400) considered terminating their employment.
Thirty-five percent of patients (491/1400) said obtaining healthy blood counts was their most important treatment goal. Twenty-one percent (299/1400) said increasing their energy levels was most important, and 15% (203/1400) said reducing spontaneous bleeds/bruising was most important.
STOCKHOLM—A new survey has revealed patients’ perceptions of immune thrombocytopenia (ITP) and how the condition impacts their quality of life (QOL).
Patients reported delays in diagnosis, lack of support, severe fatigue, and impacts on both emotional well-being and their ability to work.
Interim results of this survey, the ITP World Impact Survey (I-WISh), were presented in a poster (abstract PF654) at the 23rd Congress of the European Hematology Association (EHA).
I-WISh is a cross-sectional survey of ITP patients developed by global ITP experts, patient groups, and Novartis.
Interim results of the survey included patients from 12 countries (Canada, China, Colombia, France, Germany, Italy, India, Japan, Spain, Turkey, UK, and US) who completed an online questionnaire beginning in January 2018.
As of May 14, 2018, 1400 adults (age 18 and older) had completed the survey. Sixty-five percent were female, and they had a mean age of 47.1 years. The patients’ mean length of time with ITP was 110 months.
Most patients (63%) reported a high score for their current health state (5 to 7 on the Likert scale), but 15% reported a low score (1-3).
Most patients were working full-time (45%) or part-time (16%) at the time of the survey. Nineteen percent were retired, 6% were homemakers, 4% were students, and 10% were not seeking employment, on long-term sick leave or disability, or “other.”
Diagnosis
Twenty-two percent of all patients (307/1400) felt they had a delay in their ITP diagnosis caused by waiting for additional tests (49%, 150/307) or referral to a specialist (37%, 114/307).
Three-quarters of patients with a perceived delay (229/307) were anxious throughout diagnosis.
And 66% of all patients (927/1400) wanted more support during their diagnosis.
Symptoms
Patients reported fatigue as one of the most severe symptoms at diagnosis (75%, 627/839) and at survey completion (66%, 480/722).
The other “most severe” symptoms at diagnosis were heavy menstrual bleeding (85%, 353/416) and anxiety surrounding unstable platelet count (78%, 382/487). The other “most severe” symptoms at survey completion were thrombosis (73%, 24/33) and anxiety surrounding unstable platelet count (66%, 284/431).
“Severe fatigue, in particular, was reported by many patients as the most difficult-to-manage symptom of ITP,” said study investigator Nichola Cooper, MD, of Hammersmith Hospital, Imperial College London, in the UK.
“This is an important message for healthcare providers treating patients with this rare disease. ITP is about more than bruising and risk of bleeding.”
QOL
Forty-four percent of respondents (611/1398) said ITP impacted their energy levels more than half the time, and 36% (501/1398) said ITP had a negative impact on their normal capacity to exercise more than half the time.
Half of all patients (697/1400) said ITP had a high impact on their emotional well-being.
Eighty-three percent (1157/1400) said they felt a stable and safe platelet count was important, 64% (900/1400) worried that their condition will get worse, and 63% (888/1400) were concerned that their platelet count changes for no apparent reason.
Thirty-seven percent of all patients (511/1400) had reduced their work hours because of ITP, 37% (522/1400) seriously considered reducing their hours, and 21% (294/1400) considered terminating their employment.
Thirty-five percent of patients (491/1400) said obtaining healthy blood counts was their most important treatment goal. Twenty-one percent (299/1400) said increasing their energy levels was most important, and 15% (203/1400) said reducing spontaneous bleeds/bruising was most important.
STOCKHOLM—A new survey has revealed patients’ perceptions of immune thrombocytopenia (ITP) and how the condition impacts their quality of life (QOL).
Patients reported delays in diagnosis, lack of support, severe fatigue, and impacts on both emotional well-being and their ability to work.
Interim results of this survey, the ITP World Impact Survey (I-WISh), were presented in a poster (abstract PF654) at the 23rd Congress of the European Hematology Association (EHA).
I-WISh is a cross-sectional survey of ITP patients developed by global ITP experts, patient groups, and Novartis.
Interim results of the survey included patients from 12 countries (Canada, China, Colombia, France, Germany, Italy, India, Japan, Spain, Turkey, UK, and US) who completed an online questionnaire beginning in January 2018.
As of May 14, 2018, 1400 adults (age 18 and older) had completed the survey. Sixty-five percent were female, and they had a mean age of 47.1 years. The patients’ mean length of time with ITP was 110 months.
Most patients (63%) reported a high score for their current health state (5 to 7 on the Likert scale), but 15% reported a low score (1-3).
Most patients were working full-time (45%) or part-time (16%) at the time of the survey. Nineteen percent were retired, 6% were homemakers, 4% were students, and 10% were not seeking employment, on long-term sick leave or disability, or “other.”
Diagnosis
Twenty-two percent of all patients (307/1400) felt they had a delay in their ITP diagnosis caused by waiting for additional tests (49%, 150/307) or referral to a specialist (37%, 114/307).
Three-quarters of patients with a perceived delay (229/307) were anxious throughout diagnosis.
And 66% of all patients (927/1400) wanted more support during their diagnosis.
Symptoms
Patients reported fatigue as one of the most severe symptoms at diagnosis (75%, 627/839) and at survey completion (66%, 480/722).
The other “most severe” symptoms at diagnosis were heavy menstrual bleeding (85%, 353/416) and anxiety surrounding unstable platelet count (78%, 382/487). The other “most severe” symptoms at survey completion were thrombosis (73%, 24/33) and anxiety surrounding unstable platelet count (66%, 284/431).
“Severe fatigue, in particular, was reported by many patients as the most difficult-to-manage symptom of ITP,” said study investigator Nichola Cooper, MD, of Hammersmith Hospital, Imperial College London, in the UK.
“This is an important message for healthcare providers treating patients with this rare disease. ITP is about more than bruising and risk of bleeding.”
QOL
Forty-four percent of respondents (611/1398) said ITP impacted their energy levels more than half the time, and 36% (501/1398) said ITP had a negative impact on their normal capacity to exercise more than half the time.
Half of all patients (697/1400) said ITP had a high impact on their emotional well-being.
Eighty-three percent (1157/1400) said they felt a stable and safe platelet count was important, 64% (900/1400) worried that their condition will get worse, and 63% (888/1400) were concerned that their platelet count changes for no apparent reason.
Thirty-seven percent of all patients (511/1400) had reduced their work hours because of ITP, 37% (522/1400) seriously considered reducing their hours, and 21% (294/1400) considered terminating their employment.
Thirty-five percent of patients (491/1400) said obtaining healthy blood counts was their most important treatment goal. Twenty-one percent (299/1400) said increasing their energy levels was most important, and 15% (203/1400) said reducing spontaneous bleeds/bruising was most important.
Family separations could lead to irreversible health outcomes
The recent crises of the separation of stressed children from their equally stressed parents at this country’s southern border raises the specter of emotional and cognitive reactions within these children – the negative ramifications of which will manifest themselves for years to come.
Stress is ubiquitous. Children experience stress in the normal everyday frustrations that come their way: going to day care for a few hours and leaving mommy, the tripping and falling as they learn to walk, a toy breaking. These stresses help a child develop the capacity for emotional regulation and are termed “tolerable stress.” There is, however, a big difference between tolerable stress and toxic stress.
When the stress reaction in response to perceived or actual danger is beyond tolerable levels by virtue of its quality, intensity, and longevity, it saps the body’s ability to rally or handle the trauma that is being faced because of depleted neurotransmitters that normally assist the body to fight or flee from danger. Unfortunately, that’s not the end of the story: As the stressor persists, it has the capacity to produce long-term alterations in the resilience of the brain and body of the young child. This change often is irreversible.
Scientists and the lay public have begun to actively discuss the impact of adverse childhood experiences and their causal link to irreversible negative adult health outcomes. We now know without a doubt the impact of toxic versus tolerable stress on the hypothalamic-pituitary-adrenal axis of the young child. We are aware that the brain of a young child is particularly vulnerable to stress during critical and sensitive periods of development and that downstream effects of early trauma show up as disorganized behavior and cognitive underperformance.
Development plays a central role in children’s behavioral response to separation from parents. Infants develop a sense of stranger anxiety and the primacy of one central figure between ages 8 and 10 months. The baby chooses the parent over strangers for comfort and care. Roughly between ages 3 and 4, a child develops an internal representation of the parent as the primary figure in their lives so that they can tolerate short periods of being away from the primary caregiver for, let’s say, half a day. They depend on the parent for all their physical and emotional needs, which includes the need for a stable, nurturing, and predictable presence.
Familiarity of the environment, family rituals, consistency of daily routines provided by the parent help neural pathways responsible for the biologic unfolding of developmental milestones. Only recently, the science of early brain development and the role of early childhood trauma on brain biology has caught up with the longitudinal observational studies of bereaved children who lost their parents under circumstances of acute stress (the blitzkrieg, the Yom Kippur War, the Hungarian orphans) followed by the chronic stress phase of no primary caregiver for months to years. These observational studies coupled by the emerging neuroscience of early brain development and trauma are powerful informants of what is tolerable stress for children and what is not.
As a psychiatrist and expert in early child development, I am concerned about these long-term effects on migrant children. ; frantically seeking the parent/comforting familiar caregiver. Gradually as that possibility fades, with their limited ability to verbalize needs, episodic weeping can give way to disorganized behavior, despondence, and finally apathy and regression of milestones and cognitive abilities already achieved. The separation is merely the proxy face of other disasters that are probably co-occurring for the child/family and multiplies the dose of the stress: loss of siblings, loss of familiar physical elements of the landscape, loss of adequate physical sustenance, loss of routine, loss of consistency, increased vulnerability for physical illness, and the list goes on.
By age 2 and above, children are more vocal in their desire for reunification and will weep inconsolably upon separation. At this time their bodies and the psychological lens through which they view the world is changing irrevocably. Stress hormones, noxious at high levels such as cortisol/adrenocorticotropic hormone and glutamate rise to levels that no longer respond to the traditional negative feedback loop of the body and reach levels that lead to cell death in areas of the brain responsible for modulating emotions that can permanently change brain architecture.
Psychologically, the child seeks out the parent by any means (crying, flailing, tantruming). When no positive outcome occurs, this is replaced by an unwillingness to engage with the world, or the child engages but by behavior that is aggressive, disorganized, and regressed. In their own way, young children’s mistrust of the world leads to a variety of behaviors that impede normal development: rejection of others, disengagement from social connections by quietly sitting in a corner, being unwilling to eat or sleeping fitfully.
As separation from the primary caregiver continues, there is a deepening sense of hopelessness. Generally, no amount of physical props (toys, playgrounds) without the human familiar faces can rectify the feeling of abandonment and hopelessness that children in these conditions face. Assuming they are no strangers to trauma experienced through the lives of their asylum-seeking parents, this separation refreshes all the wounds of days gone by. At least in those moments of initial trauma, they had the parent as a buffer. But with this new separation, they are left to emotionally fend for themselves with no tools to manage their emotions.
We have to deal with the aftereffects of so many natural disasters that cannot be avoided: earthquakes, tornadoes, tsunamis that displace children and families with horrific outcomes. Did we really need a man-made disaster that will irrevocably change the brains and behaviors of a generation of children who just happen to be on our southern borders because of an accident of birth? If parents abandoned their children this way it would be called child abuse. Deliberate policy decisions that have such dire effects on children also take on the label of child abuse.
Although images of my years as a young parent of three boys are turning a sepia color in faded memory, in my new role as a grandmother observing my son and his wife so in love with their 2-year-old reinforces how much mutual dependency of a child and parent on each other strengthens the very fabric of life. Children are our future and are the building blocks of a just and humane society.
As of this writing, the administration has vowed to end these separations. But how will children who have been separated be reunited with their families?
The education of decision makers about the far-reaching emotional and physical consequences on normative childhood development must be a top priority for all scientists and professionals interested in kids and families: I think that means all of us.
Dr. Sood is professor of psychiatry and pediatrics, and senior professor of child mental health policy at the Virginia Treatment Center at Virginia Commonwealth University, Richmond. She also is affiliated with the department of psychiatry at VCU and Children’s Hospital of Richmond.
The recent crises of the separation of stressed children from their equally stressed parents at this country’s southern border raises the specter of emotional and cognitive reactions within these children – the negative ramifications of which will manifest themselves for years to come.
Stress is ubiquitous. Children experience stress in the normal everyday frustrations that come their way: going to day care for a few hours and leaving mommy, the tripping and falling as they learn to walk, a toy breaking. These stresses help a child develop the capacity for emotional regulation and are termed “tolerable stress.” There is, however, a big difference between tolerable stress and toxic stress.
When the stress reaction in response to perceived or actual danger is beyond tolerable levels by virtue of its quality, intensity, and longevity, it saps the body’s ability to rally or handle the trauma that is being faced because of depleted neurotransmitters that normally assist the body to fight or flee from danger. Unfortunately, that’s not the end of the story: As the stressor persists, it has the capacity to produce long-term alterations in the resilience of the brain and body of the young child. This change often is irreversible.
Scientists and the lay public have begun to actively discuss the impact of adverse childhood experiences and their causal link to irreversible negative adult health outcomes. We now know without a doubt the impact of toxic versus tolerable stress on the hypothalamic-pituitary-adrenal axis of the young child. We are aware that the brain of a young child is particularly vulnerable to stress during critical and sensitive periods of development and that downstream effects of early trauma show up as disorganized behavior and cognitive underperformance.
Development plays a central role in children’s behavioral response to separation from parents. Infants develop a sense of stranger anxiety and the primacy of one central figure between ages 8 and 10 months. The baby chooses the parent over strangers for comfort and care. Roughly between ages 3 and 4, a child develops an internal representation of the parent as the primary figure in their lives so that they can tolerate short periods of being away from the primary caregiver for, let’s say, half a day. They depend on the parent for all their physical and emotional needs, which includes the need for a stable, nurturing, and predictable presence.
Familiarity of the environment, family rituals, consistency of daily routines provided by the parent help neural pathways responsible for the biologic unfolding of developmental milestones. Only recently, the science of early brain development and the role of early childhood trauma on brain biology has caught up with the longitudinal observational studies of bereaved children who lost their parents under circumstances of acute stress (the blitzkrieg, the Yom Kippur War, the Hungarian orphans) followed by the chronic stress phase of no primary caregiver for months to years. These observational studies coupled by the emerging neuroscience of early brain development and trauma are powerful informants of what is tolerable stress for children and what is not.
As a psychiatrist and expert in early child development, I am concerned about these long-term effects on migrant children. ; frantically seeking the parent/comforting familiar caregiver. Gradually as that possibility fades, with their limited ability to verbalize needs, episodic weeping can give way to disorganized behavior, despondence, and finally apathy and regression of milestones and cognitive abilities already achieved. The separation is merely the proxy face of other disasters that are probably co-occurring for the child/family and multiplies the dose of the stress: loss of siblings, loss of familiar physical elements of the landscape, loss of adequate physical sustenance, loss of routine, loss of consistency, increased vulnerability for physical illness, and the list goes on.
By age 2 and above, children are more vocal in their desire for reunification and will weep inconsolably upon separation. At this time their bodies and the psychological lens through which they view the world is changing irrevocably. Stress hormones, noxious at high levels such as cortisol/adrenocorticotropic hormone and glutamate rise to levels that no longer respond to the traditional negative feedback loop of the body and reach levels that lead to cell death in areas of the brain responsible for modulating emotions that can permanently change brain architecture.
Psychologically, the child seeks out the parent by any means (crying, flailing, tantruming). When no positive outcome occurs, this is replaced by an unwillingness to engage with the world, or the child engages but by behavior that is aggressive, disorganized, and regressed. In their own way, young children’s mistrust of the world leads to a variety of behaviors that impede normal development: rejection of others, disengagement from social connections by quietly sitting in a corner, being unwilling to eat or sleeping fitfully.
As separation from the primary caregiver continues, there is a deepening sense of hopelessness. Generally, no amount of physical props (toys, playgrounds) without the human familiar faces can rectify the feeling of abandonment and hopelessness that children in these conditions face. Assuming they are no strangers to trauma experienced through the lives of their asylum-seeking parents, this separation refreshes all the wounds of days gone by. At least in those moments of initial trauma, they had the parent as a buffer. But with this new separation, they are left to emotionally fend for themselves with no tools to manage their emotions.
We have to deal with the aftereffects of so many natural disasters that cannot be avoided: earthquakes, tornadoes, tsunamis that displace children and families with horrific outcomes. Did we really need a man-made disaster that will irrevocably change the brains and behaviors of a generation of children who just happen to be on our southern borders because of an accident of birth? If parents abandoned their children this way it would be called child abuse. Deliberate policy decisions that have such dire effects on children also take on the label of child abuse.
Although images of my years as a young parent of three boys are turning a sepia color in faded memory, in my new role as a grandmother observing my son and his wife so in love with their 2-year-old reinforces how much mutual dependency of a child and parent on each other strengthens the very fabric of life. Children are our future and are the building blocks of a just and humane society.
As of this writing, the administration has vowed to end these separations. But how will children who have been separated be reunited with their families?
The education of decision makers about the far-reaching emotional and physical consequences on normative childhood development must be a top priority for all scientists and professionals interested in kids and families: I think that means all of us.
Dr. Sood is professor of psychiatry and pediatrics, and senior professor of child mental health policy at the Virginia Treatment Center at Virginia Commonwealth University, Richmond. She also is affiliated with the department of psychiatry at VCU and Children’s Hospital of Richmond.
The recent crises of the separation of stressed children from their equally stressed parents at this country’s southern border raises the specter of emotional and cognitive reactions within these children – the negative ramifications of which will manifest themselves for years to come.
Stress is ubiquitous. Children experience stress in the normal everyday frustrations that come their way: going to day care for a few hours and leaving mommy, the tripping and falling as they learn to walk, a toy breaking. These stresses help a child develop the capacity for emotional regulation and are termed “tolerable stress.” There is, however, a big difference between tolerable stress and toxic stress.
When the stress reaction in response to perceived or actual danger is beyond tolerable levels by virtue of its quality, intensity, and longevity, it saps the body’s ability to rally or handle the trauma that is being faced because of depleted neurotransmitters that normally assist the body to fight or flee from danger. Unfortunately, that’s not the end of the story: As the stressor persists, it has the capacity to produce long-term alterations in the resilience of the brain and body of the young child. This change often is irreversible.
Scientists and the lay public have begun to actively discuss the impact of adverse childhood experiences and their causal link to irreversible negative adult health outcomes. We now know without a doubt the impact of toxic versus tolerable stress on the hypothalamic-pituitary-adrenal axis of the young child. We are aware that the brain of a young child is particularly vulnerable to stress during critical and sensitive periods of development and that downstream effects of early trauma show up as disorganized behavior and cognitive underperformance.
Development plays a central role in children’s behavioral response to separation from parents. Infants develop a sense of stranger anxiety and the primacy of one central figure between ages 8 and 10 months. The baby chooses the parent over strangers for comfort and care. Roughly between ages 3 and 4, a child develops an internal representation of the parent as the primary figure in their lives so that they can tolerate short periods of being away from the primary caregiver for, let’s say, half a day. They depend on the parent for all their physical and emotional needs, which includes the need for a stable, nurturing, and predictable presence.
Familiarity of the environment, family rituals, consistency of daily routines provided by the parent help neural pathways responsible for the biologic unfolding of developmental milestones. Only recently, the science of early brain development and the role of early childhood trauma on brain biology has caught up with the longitudinal observational studies of bereaved children who lost their parents under circumstances of acute stress (the blitzkrieg, the Yom Kippur War, the Hungarian orphans) followed by the chronic stress phase of no primary caregiver for months to years. These observational studies coupled by the emerging neuroscience of early brain development and trauma are powerful informants of what is tolerable stress for children and what is not.
As a psychiatrist and expert in early child development, I am concerned about these long-term effects on migrant children. ; frantically seeking the parent/comforting familiar caregiver. Gradually as that possibility fades, with their limited ability to verbalize needs, episodic weeping can give way to disorganized behavior, despondence, and finally apathy and regression of milestones and cognitive abilities already achieved. The separation is merely the proxy face of other disasters that are probably co-occurring for the child/family and multiplies the dose of the stress: loss of siblings, loss of familiar physical elements of the landscape, loss of adequate physical sustenance, loss of routine, loss of consistency, increased vulnerability for physical illness, and the list goes on.
By age 2 and above, children are more vocal in their desire for reunification and will weep inconsolably upon separation. At this time their bodies and the psychological lens through which they view the world is changing irrevocably. Stress hormones, noxious at high levels such as cortisol/adrenocorticotropic hormone and glutamate rise to levels that no longer respond to the traditional negative feedback loop of the body and reach levels that lead to cell death in areas of the brain responsible for modulating emotions that can permanently change brain architecture.
Psychologically, the child seeks out the parent by any means (crying, flailing, tantruming). When no positive outcome occurs, this is replaced by an unwillingness to engage with the world, or the child engages but by behavior that is aggressive, disorganized, and regressed. In their own way, young children’s mistrust of the world leads to a variety of behaviors that impede normal development: rejection of others, disengagement from social connections by quietly sitting in a corner, being unwilling to eat or sleeping fitfully.
As separation from the primary caregiver continues, there is a deepening sense of hopelessness. Generally, no amount of physical props (toys, playgrounds) without the human familiar faces can rectify the feeling of abandonment and hopelessness that children in these conditions face. Assuming they are no strangers to trauma experienced through the lives of their asylum-seeking parents, this separation refreshes all the wounds of days gone by. At least in those moments of initial trauma, they had the parent as a buffer. But with this new separation, they are left to emotionally fend for themselves with no tools to manage their emotions.
We have to deal with the aftereffects of so many natural disasters that cannot be avoided: earthquakes, tornadoes, tsunamis that displace children and families with horrific outcomes. Did we really need a man-made disaster that will irrevocably change the brains and behaviors of a generation of children who just happen to be on our southern borders because of an accident of birth? If parents abandoned their children this way it would be called child abuse. Deliberate policy decisions that have such dire effects on children also take on the label of child abuse.
Although images of my years as a young parent of three boys are turning a sepia color in faded memory, in my new role as a grandmother observing my son and his wife so in love with their 2-year-old reinforces how much mutual dependency of a child and parent on each other strengthens the very fabric of life. Children are our future and are the building blocks of a just and humane society.
As of this writing, the administration has vowed to end these separations. But how will children who have been separated be reunited with their families?
The education of decision makers about the far-reaching emotional and physical consequences on normative childhood development must be a top priority for all scientists and professionals interested in kids and families: I think that means all of us.
Dr. Sood is professor of psychiatry and pediatrics, and senior professor of child mental health policy at the Virginia Treatment Center at Virginia Commonwealth University, Richmond. She also is affiliated with the department of psychiatry at VCU and Children’s Hospital of Richmond.
Outcomes After Peripheral Nerve Block in Hip Arthroscopy
ABSTRACT
Pain control following hip arthroscopy presents a significant clinical challenge, with postoperative pain requiring considerable opioid use. Peripheral nerve blocks (PNBs) have emerged as one option to improve pain and limit the consequences of opioid use. The purpose of this study is to provide a comprehensive review of outcomes associated with PNB in hip arthroscopy. We hypothesize that the use of PNB in hip arthroscopy leads to improved outcomes and is associated with few complications. A systematic review of PubMed, Medline, Scopus, and Embase databases was conducted through January 2015 for English-language articles reporting outcome data, with 2 reviewers independently reviewing studies for inclusion. When available, similar outcomes were combined to generate frequency-weighted means. Six studies met the inclusion criteria for this review, reporting on 710 patients undergoing hip arthroscopy. The mean ages were 37.0 and 37.7 years for the PNB and comparator groups, respectively, with a reported total of 281 (40.5%) male and 412 (59.5%) female patients. Postoperative post-anesthesia care unit (PACU) pain was consistently reduced in the PNB group, with the use of a lower morphine equivalent dose and lower rates of inpatient admission, compared with that in the control groups. Postoperative nausea and/or vomiting as well as PACU discharge time showed mixed results. High satisfaction and few complications were reported. In conclusion, PNB is associated with reductions in postoperative pain, analgesic use, and the rate of inpatient admissions, though similar rates of nausea/vomiting and time to discharge were reported. Current PNB techniques are varied, and future research efforts should focus on examining which of these methods provides the optimal risk-benefit profile in hip arthroscopy.
Continue to: Hip arthroscopy has emerged...
Hip arthroscopy has emerged as a useful procedure in the diagnosis and treatment of hip pathology,1-8 experiencing a substantial rise in popularity in recent years, with the number of procedures growing by a factor of 18 from 1999 to 20099 and 25 from 2006 to 2013.10 Though hip arthroscopy is beneficial in many cases, marked postoperative pain has presented a substantial challenge, with patients requiring considerable doses of opiate-based medications in the post-anesthesia care unit (PACU).11,12 Increased narcotic use carries increased side effects, including postoperative nausea and vomiting,13 and poorly managed pain leads to increased unplanned admissions.14 Furthermore, patients with chronic hip pain and long-term opioid use may experience heightened and prolonged pain following the procedure, owing to medication tolerance and reduced opioid efficacy in this setting.15
Several pain control strategies have been employed in patients undergoing hip arthroscopy. General anesthesia16,17 and combined spinal epidural (CSE)18 are commonly used. However, such techniques rely heavily on opioids for postoperative pain control,11 and epidural anesthesia commonly requires adjunctive treatments (eg, neuromuscular blockade) to ensure muscle relaxation for joint distraction.19 One technique that has been employed recently is peripheral nerve block (PNB), which has been associated with a significant decrease in postoperative opioid use and nausea and vomiting.13,20 This method has proven successful in other fields of arthroscopy, including shoulder arthroscopy, in which it resulted in faster recovery, reduced opioid consumption,21 and demonstrated cost-effectiveness22 compared with general anesthesia and knee arthroscopy.23-26 As it is a relatively new field, little is known about the use of PNB in hip arthroscopy.
The goal of this systematic review was to comprehensively review the studies reporting on PNB in hip arthroscopy. We specifically focused on outcomes, including postoperative pain; analgesic use; nausea, vomiting, and antiemetic use; discharge time; inpatient admission; and patient satisfaction, as well as the complications associated with the use of PNB. Our knowledge of outcomes associated with PNB in hip arthroscopy is based on a few individual studies that have reported on small groups of patients using a variety of outcome measures and other findings. Furthermore, each of these studies commonly reflects the experience of an individual surgeon at a single institution and, when taken alone, may not be an accurate representation of the more general outcomes associated with PNB. A comprehensive review of such studies will provide surgeons, anesthesiologists, and patients with a better understanding of the anticipated outcomes of using PNB in hip arthroscopy. We hypothesize that the use of PNB in hip arthroscopy leads to improved outcomes and is associated with few complications.
MATERIALS AND METHODS
A systematic review of outcomes associated with PNB in hip arthroscopy was performed using the available English-language literature in accordance with the guidelines laid out by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement and included studies retrieved from the PubMed, Medline, Scopus, and Embase computerized literature databases. Searches were executed comprising all years from database inception through January 2015. Articles were retrieved by an electronic search of medical subject headings and keyword terms and their respective combinations (Table 1). The inclusion criteria for studies in this systematic review were studies that (1) were written in the English language and (2) reported explicit outcome data. The exclusion criteria were (1) review articles, meta-analyses, case reports, abstracts/conference papers, comments/letters, or technique articles without reported patient data and (2) basic research, biomechanics, or animal/cadaveric studies without reported patient data.
Table 1. Search Terms Entered to Identify English-Language Studies Through January 2015
Database | Search terms |
PubMed, Scopus | Keyword: (hip AND arthroscopy) AND (pain control OR pain management OR pain regimen OR nerve block OR spinal anesthesia OR regional anesthesia OR general anesthesia) |
Medline | MeSH (includes both MeSH terms and keywords): (Hip) AND (Arthroscopy) AND (“Pain Management” OR “Anesthesia, General” OR “Anesthesia” OR “Anesthesia, Inhalation”, OR “Balanced Anesthesia” OR “Anesthesia, Local” OR “Anesthesia, Spinal” OR “Anesthesia, Conduction” OR “Nerve Block”) |
Embase | MeSH (includes both MeSH terms and keywords): (Hip) AND (Arthroscopy) AND (“Pain Management” OR “General Anesthesia” OR “Anesthesia” OR “Inhalation Anesthesia”, OR “Balanced Anesthesia” OR “Local Anesthesia” OR “Spinal Anesthesia” OR “Regional Anesthesia” OR “Nerve Block”) |
The literature search strategy is outlined in the Figure. The initial title search yielded a subset of possible articles that were then further included or excluded on the basis of the contents of the article’s abstract, wherein articles were again selected on the basis of the aforementioned inclusion and exclusion criteria. Articles selected in both the title and abstract phases underwent full-text review, during which the full text of each qualifying article was reviewed. In addition, the reference sections from articles undergoing full-text review were scanned to identify any additional studies that had not been identified in the original literature search. Appropriate studies for final inclusion were then selected at this stage. The title, abstract, and full-text selection process were performed by 2 of the study authors (Dr. Steinhaus and Dr. Lynch), with any discrepancies being discussed and resolved by mutual agreement.
Continue to: For all 6 included studies...
For all 6 included studies,16-18,27-29 data were collected regarding the study specifics, patients included, and outcomes measured in the study. The journal of publication, type of study, level of evidence, and type of PNB, as well as the presence of a comparator group were noted (Table 2). Patient information included the number of patients at baseline and follow-up, mean age, gender, weight, height, body mass index, American Society of Anesthesiologists (ASA) status, and the specific procedures performed. In addition, data were collected on outcomes, including postoperative pain, as well as secondary outcomes and additional findings reported by the studies (Table 3). Where possible, weighted averages were calculated across all studies to obtain aggregate data.
RESULTS
STUDY INCLUSION
Six studies, all published between 2012 and 2014, were included in this systematic review (Table 2). Three studies involved lumbar plexus block, 2 studies involved femoral nerve block, and 1 study evaluated fascia iliaca block. Two studies used a control group of patients who received only general anesthesia (compared with the treatment group who received both general anesthesia and PNB); another study compared intravenous morphine with PNB; and 1 study compared CSE alone with PNB in addition to epidural.
DEMOGRAPHIC DATA
Demographic data from the included studies are presented in Table 2. In total, 710 and 549 patients were evaluated at baseline and final follow-up, respectively, which represents a follow-up rate of 77%. The frequency-weighted mean age of patients receiving PNB was 37.0 years compared with 37.7 years in the comparison groups, and the studies reported a total of 281 (40.5%) male and 412 (59.5%) female patients. The procedures performed were heterogeneously reported; therefore, totals were not tabulated, although the reported procedures included osteochondroplasty, labral débridement, labral and/or capsular repair, gluteus minimus repair, and synovectomy.
POSTOPERATIVE PAIN
Four studies reported on postoperative pain, and these data are presented in Table 3. In a retrospective study of patients receiving femoral nerve block in addition to general anesthesia, Dold and colleagues16 noted postoperative pain at 0, 15, 30, 45, and 60 minutes following arrival in the PACU, and discovered a statistically significantly lower level of pain at 60 minutes compared with inpatients receiving general anesthesia alone. YaDeau and colleagues18 found a significantly lower level of pain at rest in the PACU for those receiving CSE and lumbar plexus blockade compared with those receiving CSE alone. This significant difference did not persist at 24 hours or 6 months after the procedure, nor did it exist for pain with movement at any time point. Similarly, Schroeder and colleagues17 examined patients receiving general anesthesia and lumbar plexus block and found a significant reduction in pain immediately postoperatively in the PACU, though these effects disappeared the day following the procedure. Krych and colleagues27 also reported on postoperative pain in patients undergoing fascia iliaca blockade, although they did not include a comparator group. Outcome comparison between patients who received PNB and controls in the PACU and 1 day following the procedure are presented in Table 4.
ANALGESIC USE
Four studies reported on analgesic use after PNB, and these data are presented in Table 3. Dold and colleagues16 noted analgesic use intraoperatively, in the PACU, and in the surgical day care unit (SDCU). These authors found a significant reduction in morphine equivalent dose given in the operating room and in the PACU in the group receiving PNB, with a nonsignificant trend toward lower use of oxycodone in the SDCU. Schroeder and colleagues17 similarly reported significant reductions in morphine equivalent dose intraoperatively and in Phase I recovery for patients receiving PNB, and these differences disappeared in Phase II recovery as well as intraoperatively if the block dose was considered. In addition, these authors found a significant reduction in the use of fentanyl and hydromorphone in the operating room in the PNB group, as well as a significant reduction in the proportion of patients receiving ketorolac in the operating room or PACU. Finally, YaDeau and colleagues18 reported total analgesic usage in the PACU among PNB patients compared with those receiving CSE alone and showed a strong trend toward reduced use in the PNB group, although this difference was not significant (P = .051). PACU analgesic use is presented in Table 4.
Continue to: Postoperative nausea...
POSTOPERATIVE NAUSEA/VOMITING AND ANTIEMETIC USE
Five studies presented data on nausea, vomiting, or antiemetic use following PNB and are shown in Table 3. YaDeau and colleagues18 reported nausea among 34% of patients in the PNB group, compared with 20% in the control group, vomiting in 2% and 7%, respectively, and antiemetic use in 12% of both groups. Dold and colleagues16 identified a similar trend, with 41.1% of patients in the PNB group and 32.5% of patients in the control group experiencing postoperative nausea or vomiting, while Krych and colleagues27 noted only 10% of PNB patients with mild nausea and none requiring antiemetic use. In their study of patients receiving PNB, Schroeder and colleagues17 found a significant reduction in antiemetic use among PNB patients compared with those receiving general anesthesia alone. Similarly, Ward and colleagues29 noted a significant difference in postoperative nausea, with 10% of patients in the PNB group experiencing postoperative nausea compared with 75% of those in the comparator group who received intravenous morphine. The mean percentage of patients experiencing postoperative nausea and/or vomiting is shown in Table 4.
DISCHARGE TIME
Four studies presented data on discharge time from the PACU and are summarized in Table 3. Three of these studies included a comparator group. Both Dold and colleagues16 and YaDeau and colleagues18 reported an increase in the time to discharge for patients receiving PNB, although these differences were not significant. The study by Ward and colleagues,29 on the other hand, noted a significant reduction in the time to discharge for the PNB group. In addition to these studies, Krych and colleagues27 examined the time from skin closure to discharge for patients receiving PNB, noting a mean 199 minutes for the patients in their study. Mean times to discharge for the PNB and control groups are presented in Table 4.
INPATIENT ADMISSION
Four studies presented data on the proportion of study participants who were admitted as inpatients, and these data are shown in Table 3. Dold and colleagues16 reported no inpatient admissions in their PNB group compared with 5.0% for the control group (both cases of pain control), while YaDeau and colleagues18 found that 3 admissions occurred, with 2 in the control group (1 for oxygen desaturation and the other for intractable pain and nausea) and 1 from the PNB group (epidural spread and urinary retention). Two additional studies reported data on PNB groups alone. Krych and colleagues27 observed no overnight admissions in their study, while Nye and colleagues28 reported 1 readmission for bilateral leg numbness and weakness due to epidural spread, which resolved following discontinuation of the block. The mean proportion of inpatient admissions is presented in Table 4.
SATISFACTION
A total of 3 studies examined patient satisfaction, and these data are presented in Table 3. In their study, Ward and colleagues29 reported a significantly greater rate of satisfaction at 1 day postoperatively among the patients in the PNB group (90%) than among patients who received intravenous morphine (25%) (P < .0001). Similarly, YaDeau and colleagues18 noted greater satisfaction among the PNB group than among the control group, with PNB patients rating their satisfaction at a mean of 8.6 and control patients at a mean of 7.9 on a 10-point scale (0-10) 24 hours postoperatively, although this difference was not significant. Finally, Krych and colleagues27 found that 67% of patients were “very satisfied” and 33% were “satisfied”, based on a Likert scale.
COMPLICATIONS
Four studies presented data on complications, and these findings are summarized in Table 3. In their work, Nye and colleagues28 reported most extensively on complications associated with PNB. Overall, the authors found a rate of significant complications of 3.8%. In terms of specific complications, they noted local anesthetic systemic toxicity (0.9%), epidural spread (0.5%), sensory or motor deficits (9.4%), falls (0.5%), and catheter issues. In their study of patients receiving PNB and CSE, YaDeau and colleagues18 identified 1 patient in the PNB group with epidural spread and urinary retention, while they noted 1 case of oxygen desaturation and another case of intractable pain and nausea in the group receiving CSE alone, all 3 of which required inpatient admission. They found no permanent adverse events attributable to the PNB. In another study, Dold and colleagues16 observed no complications in patients receiving PNB compared with those in 2 admissions in the control group for inadequate pain control. Similarly, Krych and colleagues27 identified no complications in patients who received PNB in their study.
DISCUSSION
Hip arthroscopy has experienced a substantial gain in popularity in recent years, emerging as a beneficial technique for both the diagnosis and treatment of diverse hip pathologies in patients spanning a variety of demographics. Nevertheless, postoperative pain control, as well as medication side effects and unwanted patient admissions, present major challenges to the treating surgeon. As an adjuvant measure, peripheral nerve block represents one option to improve postoperative pain management, while at the same time addressing the adverse effects of considerable opioid use, which is commonly seen in these patients. Early experience with this method in hip arthroscopy was reported in a case series by Lee and colleagues.12 In an attempt to reduce postoperative pain, as well as limit the adverse effects and delay in discharge associated with considerable opioid use in the PACU, the authors used preoperative paravertebral blocks of L1 and L2 in 2 patients requiring hip arthroscopy with encouraging results. Since then, a number of studies have attempted the use of PNB in hip arthroscopy.16-18,27-29 However, we were unable to identify any prior reviews reporting on peripheral nerve blockade in hip arthroscopy, and thus this study is unique in providing a greater understanding of the outcomes associated with PNB use.
In general, we found that PNB was associated with improved outcomes. Based on the studies included in this review, there was a statistically significantly lower level of pain in the PACU for femoral nerve block (compared with general anesthesia alone)16 and lumbar plexus blockade (compared with general anesthesia17 and CSE18 alone). Nevertheless, these effects are likely short-lived, with differences disappearing the day following the procedure. In terms of analgesic use, 2 studies report significant reductions in analgesic use intraoperatively and in the PACU/Phase I recovery,16,17 with a third reporting a strong trend toward reduced analgesic use in the PACU (P = .051).18 Finally, we report fewer admissions for the PNB group, as well as high rates of satisfaction and few complications across these studies.
Continue to: Unlike these measures...
Unlike these measures, postoperative nausea, vomiting, and antiemetic use, as well as time to discharge, showed more mixed results. With regard to nausea/vomiting, 2 studies16,18 reported nonsignificantly increased rates in the PNB group, whereas others reported significant reductions in nausea/vomiting29 and in the proportion of patients receiving antiemetics.17 Similarly, mixed results were seen in terms of patient discharge time from the PACU. Two studies16,18 reported a nonsignificant increase in time to discharge for the PNB group, while another29 noted a significant reduction for the PNB group compared with those receiving intravenous morphine. These mixed results were surprising, as we expected reductions in opioid use to result in fewer instances of nausea/vomiting and a quicker time to discharge. The reasons underlying these findings are not clear, although it has been suggested that current discharge guidelines and clinical pathways limit the ability to take advantage of the accelerated timeline offered by regional anesthesia.16,30 As experience with PNB grows, our guidelines and pathways are likely to adapt to capitalize on these advantages, and future studies may show more reliable improvements in these measures.
While rare, the risk of bleeding requiring blood transfusion following hip arthroscopy is one of the most common complications of this procedure. Though the studies included in this review did not report on the need for transfusion, a recent study by Cvetanovich and colleagues10 used a national database and found that, of patients undergoing hip arthroscopy (n = 1338), 0.4% (n = 5) had bleeding requiring a transfusion, with 0.3% (n = 4) requiring return to the operating room, similar to an earlier study by Clarke and colleagues,31 who noted bleeding from the portal site in 0.4% of hip arthroscopy patients. In terms of risk factors, Cvetanovich and colleagues10 found that ASA class, older age, and prior cardiac surgery were significantly associated with minor and overall complications, whereas both regional anesthesia/monitored anesthesia care and alcohol consumption of >2 drinks a day were significantly associated with minor complications, including bleeding requiring transfusions. They noted, however, that these risk factors accounted for only 5% of the variance in complication rates, indicating that other unidentified variables better explained the variance in complication rates. These authors concluded that complications associated with hip arthroscopy are so rare that we may not be able to predict which risk factors or anesthesia types are more likely to cause them. Further characterization of bleeding following hip arthroscopy and its associated risk factors is a valuable area for future research.
LIMITATIONS
Our study contains a number of limitations. This review included studies whose level of evidence varied from I to IV; therefore, our study is limited by any bias or heterogeneity introduced in patient recruitment, selection, variability of technique, data collection, and analysis used in these studies. This heterogeneity is most apparent in the block types and comparator groups. Furthermore, several different outcome measures were reported across the 6 studies used in this review, which decreased the relevance of any one of these individual outcomes. Finally, given the limited data that currently exist for the use of PNB in hip arthroscopy, we are unable to note meaningful differences between various types of PNBs, such as differences in postoperative pain or other measures such as quadriceps weakness, which can accompany femoral nerve block.12 While it is important to read our work with these limitations in mind, this systematic review is, to our knowledge, the only comprehensive review to date of studies reporting on PNB in hip arthroscopy, providing clinicians and patients with a greater understanding of the associated outcomes across these studies.
CONCLUSION
This systematic review shows improved outcomes and few complications with PNB use in hip arthroscopy, with reductions in postoperative pain, analgesic use, and the rate of inpatient admissions. Although opioid use was reduced in these studies, we found similar rates of postoperative nausea/vomiting as well as similar time to discharge from the PACU, which may reflect our continued reliance on outdated discharge guidelines and clinical pathways. Current attempts to provide peripheral nerve blockade are quite varied, with studies targeting femoral nerve, fascia iliaca, L1/L2 paravertebral, and lumbar plexus blockade. Future research efforts with a large prospective trial investigating these techniques should focus on which of these PNBs presents the optimal risk-benefit profile for hip arthroscopy patients and thus appropriately address the clinical questions at hand.
This paper will be judged for the Resident Writer’s Award.
- Baber YF, Robinson AH, Villar RN. Is diagnostic arthroscopy of the hip worthwhile? A prospective review of 328 adults investigated for hip pain. J Bone Joint Surg Br. 1999;81:600-603.
- Byrd JW, Jones KS. Arthroscopic management of femoroacetabular impingement: minimum 2-year follow-up. Arthroscopy. 2011;27:1379-1388.
- Larson CM, Giveans MR. Arthroscopic management of femoroacetabular impingement: early outcomes measures. Arthroscopy. 2008;24:540-546.
- O'Leary JA, Berend K, Vail TP. The relationship between diagnosis and outcome in arthroscopy of the hip. Arthroscopy. 2001;17:181-188.
- Philippon M, Schenker M, Briggs K, Kuppersmith D. Femoroacetabular impingement in 45 professional athletes: associated pathologies and return to sport following arthroscopic decompression. Knee Surg Sports Traumatol Arthrosc. 2007;15:908-914.
- Potter BK, Freedman BA, Andersen RC, Bojescul JA, Kuklo TR, Murphy KP. Correlation of Short Form-36 and disability status with outcomes of arthroscopic acetabular labral debridement. Am J Sports Med. 2005;33:864-870.
- Robertson WJ, Kadrmas WR, Kelly BT. Arthroscopic management of labral tears in the hip: a systematic review of the literature. Clin Orthop Relat Res. 2007;455:88-92.
- Yusaf MA, Hame SL. Arthroscopy of the hip. Curr Sports Med Rep. 2008;7:269-274.
- Colvin AC, Harrast J, Harner C. Trends in hip arthroscopy. J Bone Joint Surg Am. 2012;94:e23.
- Cvetanovich GL, Chalmers PN, Levy DM, et al. Hip arthroscopy surgical volume trends and 30-day postoperative complications. Arthroscopy. 2016 Apr 8. [Epub before print].
- Baker JF, Byrne DP, Hunter K, Mulhall KJ. Post-operative opiate requirements after hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2011;19:1399-1402.
- Lee EM, Murphy KP, Ben-David B. Postoperative analgesia for hip arthroscopy: combined L1 and L2 paravertebral blocks. J Clin Anesth. 2008;20:462-465.
- Ganesh A, Rose JB, Wells L, et al. Continuous peripheral nerve blockade for inpatient and outpatient postoperative analgesia in children. Anesth Analg. 2007;105:1234-1242.
- Williams BA, Kentor ML, Vogt MT, et al. Femoral-sciatic nerve blocks for complex outpatient knee surgery are associated with less postoperative pain before same-day discharge: a review of 1,200 consecutive cases from the period 1996-1999. Anesthesiology. 2003;98:1206-1213.
- Zywiel MG, Stroh DA, Lee SY, Bonutti PM, Mont MA. Chronic opioid use prior to total knee arthroplasty. J Bone Joint Surg Am. 2011;93:1988-1993.
- Dold AP, Murnaghan L, Xing J, Abdallah FW, Brull R, Whelan DB. Preoperative femoral nerve block in hip arthroscopic surgery: a retrospective review of 108 consecutive cases. Am J Sports Med. 2014;42:144-149.
- Schroeder KM, Donnelly MJ, Anderson BM, Ford MP, Keene JS. The analgesic impact of preoperative lumbar plexus blocks for hip arthroscopy. A retrospective review. Hip Int. 2013;23:93-98.
- YaDeau JT, Tedore T, Goytizolo EA, et al. Lumbar plexus blockade reduces pain after hip arthroscopy: a prospective randomized controlled trial. Anesth Analg. 2012;115:968-972.
- Smart LR, Oetgen M, Noonan B, Medvecky M. Beginning hip arthroscopy: indications, positioning, portals, basic techniques, and complications. Arthroscopy. 2007;23:1348-1353.
- Stevens M, Harrison G, McGrail M. A modified fascia iliaca compartment block has significant morphine-sparing effect after total hip arthroplasty. Anaesth Intensive Care. 2007;35:949-952.
- Lehmann LJ, Loosen G, Weiss C, Schmittner MD. Interscalene plexus block versus general anaesthesia for shoulder surgery: a randomized controlled study. Eur J Orthop Surg Traumatol. 2015;25:255-261.
- Gonano C, Kettner SC, Ernstbrunner M, Schebesta K, Chiari A, Marhofer P. Comparison of economical aspects of interscalene brachial plexus blockade and general anaesthesia for arthroscopic shoulder surgery. Br J Anaesth. 2009;103:428-433.
- Hadzic A, Karaca PE, Hobeika P, et al. Peripheral nerve blocks result in superior recovery profile compared with general anesthesia in outpatient knee arthroscopy. Anesth Analg. 2005;100:976-981.
- Hsu LP, Oh S, Nuber GW, et al. Nerve block of the infrapatellar branch of the saphenous nerve in knee arthroscopy: a prospective, double-blinded, randomized, placebo-controlled trial. J Bone Joint Surg Am. 2013;95:1465-1472.
- Montes FR, Zarate E, Grueso R, et al. Comparison of spinal anesthesia with combined sciatic-femoral nerve block for outpatient knee arthroscopy. J Clin Anesth. 2008;20:415-420.
- Wulf H, Lowe J, Gnutzmann KH, Steinfeldt T. Femoral nerve block with ropivacaine or bupivacaine in day case anterior crucial ligament reconstruction. Acta Anaesthesiol Scand. 2010;54:414-420.
- Krych AJ, Baran S, Kuzma SA, Smith HM, Johnson RL, Levy BA. Utility of multimodal analgesia with fascia iliaca blockade for acute pain management following hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2014;22:843-847.
- Nye ZB, Horn JL, Crittenden W, Abrahams MS, Aziz MF. Ambulatory continuous posterior lumbar plexus blocks following hip arthroscopy: a review of 213 cases. J Clin Anesth. 2013;25:268-274.
- Ward JP, Albert DB, Altman R, Goldstein RY, Cuff G, Youm T. Are femoral nerve blocks effective for early postoperative pain management after hip arthroscopy? Arthroscopy. 2012;28:1064-1069.
- Liu SS, Strodtbeck WM, Richman JM, Wu CL. A comparison of regional versus general anesthesia for ambulatory anesthesia: a meta-analysis of randomized controlled trials. Anesth Analg. 2005;101:1634-1642.
- Clarke MT, Arora A, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop Relat Res. 2003;406:84-88.
ABSTRACT
Pain control following hip arthroscopy presents a significant clinical challenge, with postoperative pain requiring considerable opioid use. Peripheral nerve blocks (PNBs) have emerged as one option to improve pain and limit the consequences of opioid use. The purpose of this study is to provide a comprehensive review of outcomes associated with PNB in hip arthroscopy. We hypothesize that the use of PNB in hip arthroscopy leads to improved outcomes and is associated with few complications. A systematic review of PubMed, Medline, Scopus, and Embase databases was conducted through January 2015 for English-language articles reporting outcome data, with 2 reviewers independently reviewing studies for inclusion. When available, similar outcomes were combined to generate frequency-weighted means. Six studies met the inclusion criteria for this review, reporting on 710 patients undergoing hip arthroscopy. The mean ages were 37.0 and 37.7 years for the PNB and comparator groups, respectively, with a reported total of 281 (40.5%) male and 412 (59.5%) female patients. Postoperative post-anesthesia care unit (PACU) pain was consistently reduced in the PNB group, with the use of a lower morphine equivalent dose and lower rates of inpatient admission, compared with that in the control groups. Postoperative nausea and/or vomiting as well as PACU discharge time showed mixed results. High satisfaction and few complications were reported. In conclusion, PNB is associated with reductions in postoperative pain, analgesic use, and the rate of inpatient admissions, though similar rates of nausea/vomiting and time to discharge were reported. Current PNB techniques are varied, and future research efforts should focus on examining which of these methods provides the optimal risk-benefit profile in hip arthroscopy.
Continue to: Hip arthroscopy has emerged...
Hip arthroscopy has emerged as a useful procedure in the diagnosis and treatment of hip pathology,1-8 experiencing a substantial rise in popularity in recent years, with the number of procedures growing by a factor of 18 from 1999 to 20099 and 25 from 2006 to 2013.10 Though hip arthroscopy is beneficial in many cases, marked postoperative pain has presented a substantial challenge, with patients requiring considerable doses of opiate-based medications in the post-anesthesia care unit (PACU).11,12 Increased narcotic use carries increased side effects, including postoperative nausea and vomiting,13 and poorly managed pain leads to increased unplanned admissions.14 Furthermore, patients with chronic hip pain and long-term opioid use may experience heightened and prolonged pain following the procedure, owing to medication tolerance and reduced opioid efficacy in this setting.15
Several pain control strategies have been employed in patients undergoing hip arthroscopy. General anesthesia16,17 and combined spinal epidural (CSE)18 are commonly used. However, such techniques rely heavily on opioids for postoperative pain control,11 and epidural anesthesia commonly requires adjunctive treatments (eg, neuromuscular blockade) to ensure muscle relaxation for joint distraction.19 One technique that has been employed recently is peripheral nerve block (PNB), which has been associated with a significant decrease in postoperative opioid use and nausea and vomiting.13,20 This method has proven successful in other fields of arthroscopy, including shoulder arthroscopy, in which it resulted in faster recovery, reduced opioid consumption,21 and demonstrated cost-effectiveness22 compared with general anesthesia and knee arthroscopy.23-26 As it is a relatively new field, little is known about the use of PNB in hip arthroscopy.
The goal of this systematic review was to comprehensively review the studies reporting on PNB in hip arthroscopy. We specifically focused on outcomes, including postoperative pain; analgesic use; nausea, vomiting, and antiemetic use; discharge time; inpatient admission; and patient satisfaction, as well as the complications associated with the use of PNB. Our knowledge of outcomes associated with PNB in hip arthroscopy is based on a few individual studies that have reported on small groups of patients using a variety of outcome measures and other findings. Furthermore, each of these studies commonly reflects the experience of an individual surgeon at a single institution and, when taken alone, may not be an accurate representation of the more general outcomes associated with PNB. A comprehensive review of such studies will provide surgeons, anesthesiologists, and patients with a better understanding of the anticipated outcomes of using PNB in hip arthroscopy. We hypothesize that the use of PNB in hip arthroscopy leads to improved outcomes and is associated with few complications.
MATERIALS AND METHODS
A systematic review of outcomes associated with PNB in hip arthroscopy was performed using the available English-language literature in accordance with the guidelines laid out by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement and included studies retrieved from the PubMed, Medline, Scopus, and Embase computerized literature databases. Searches were executed comprising all years from database inception through January 2015. Articles were retrieved by an electronic search of medical subject headings and keyword terms and their respective combinations (Table 1). The inclusion criteria for studies in this systematic review were studies that (1) were written in the English language and (2) reported explicit outcome data. The exclusion criteria were (1) review articles, meta-analyses, case reports, abstracts/conference papers, comments/letters, or technique articles without reported patient data and (2) basic research, biomechanics, or animal/cadaveric studies without reported patient data.
Table 1. Search Terms Entered to Identify English-Language Studies Through January 2015
Database | Search terms |
PubMed, Scopus | Keyword: (hip AND arthroscopy) AND (pain control OR pain management OR pain regimen OR nerve block OR spinal anesthesia OR regional anesthesia OR general anesthesia) |
Medline | MeSH (includes both MeSH terms and keywords): (Hip) AND (Arthroscopy) AND (“Pain Management” OR “Anesthesia, General” OR “Anesthesia” OR “Anesthesia, Inhalation”, OR “Balanced Anesthesia” OR “Anesthesia, Local” OR “Anesthesia, Spinal” OR “Anesthesia, Conduction” OR “Nerve Block”) |
Embase | MeSH (includes both MeSH terms and keywords): (Hip) AND (Arthroscopy) AND (“Pain Management” OR “General Anesthesia” OR “Anesthesia” OR “Inhalation Anesthesia”, OR “Balanced Anesthesia” OR “Local Anesthesia” OR “Spinal Anesthesia” OR “Regional Anesthesia” OR “Nerve Block”) |
The literature search strategy is outlined in the Figure. The initial title search yielded a subset of possible articles that were then further included or excluded on the basis of the contents of the article’s abstract, wherein articles were again selected on the basis of the aforementioned inclusion and exclusion criteria. Articles selected in both the title and abstract phases underwent full-text review, during which the full text of each qualifying article was reviewed. In addition, the reference sections from articles undergoing full-text review were scanned to identify any additional studies that had not been identified in the original literature search. Appropriate studies for final inclusion were then selected at this stage. The title, abstract, and full-text selection process were performed by 2 of the study authors (Dr. Steinhaus and Dr. Lynch), with any discrepancies being discussed and resolved by mutual agreement.

Continue to: For all 6 included studies...
For all 6 included studies,16-18,27-29 data were collected regarding the study specifics, patients included, and outcomes measured in the study. The journal of publication, type of study, level of evidence, and type of PNB, as well as the presence of a comparator group were noted (Table 2). Patient information included the number of patients at baseline and follow-up, mean age, gender, weight, height, body mass index, American Society of Anesthesiologists (ASA) status, and the specific procedures performed. In addition, data were collected on outcomes, including postoperative pain, as well as secondary outcomes and additional findings reported by the studies (Table 3). Where possible, weighted averages were calculated across all studies to obtain aggregate data.
RESULTS
STUDY INCLUSION
Six studies, all published between 2012 and 2014, were included in this systematic review (Table 2). Three studies involved lumbar plexus block, 2 studies involved femoral nerve block, and 1 study evaluated fascia iliaca block. Two studies used a control group of patients who received only general anesthesia (compared with the treatment group who received both general anesthesia and PNB); another study compared intravenous morphine with PNB; and 1 study compared CSE alone with PNB in addition to epidural.
DEMOGRAPHIC DATA
Demographic data from the included studies are presented in Table 2. In total, 710 and 549 patients were evaluated at baseline and final follow-up, respectively, which represents a follow-up rate of 77%. The frequency-weighted mean age of patients receiving PNB was 37.0 years compared with 37.7 years in the comparison groups, and the studies reported a total of 281 (40.5%) male and 412 (59.5%) female patients. The procedures performed were heterogeneously reported; therefore, totals were not tabulated, although the reported procedures included osteochondroplasty, labral débridement, labral and/or capsular repair, gluteus minimus repair, and synovectomy.
POSTOPERATIVE PAIN
Four studies reported on postoperative pain, and these data are presented in Table 3. In a retrospective study of patients receiving femoral nerve block in addition to general anesthesia, Dold and colleagues16 noted postoperative pain at 0, 15, 30, 45, and 60 minutes following arrival in the PACU, and discovered a statistically significantly lower level of pain at 60 minutes compared with inpatients receiving general anesthesia alone. YaDeau and colleagues18 found a significantly lower level of pain at rest in the PACU for those receiving CSE and lumbar plexus blockade compared with those receiving CSE alone. This significant difference did not persist at 24 hours or 6 months after the procedure, nor did it exist for pain with movement at any time point. Similarly, Schroeder and colleagues17 examined patients receiving general anesthesia and lumbar plexus block and found a significant reduction in pain immediately postoperatively in the PACU, though these effects disappeared the day following the procedure. Krych and colleagues27 also reported on postoperative pain in patients undergoing fascia iliaca blockade, although they did not include a comparator group. Outcome comparison between patients who received PNB and controls in the PACU and 1 day following the procedure are presented in Table 4.
ANALGESIC USE
Four studies reported on analgesic use after PNB, and these data are presented in Table 3. Dold and colleagues16 noted analgesic use intraoperatively, in the PACU, and in the surgical day care unit (SDCU). These authors found a significant reduction in morphine equivalent dose given in the operating room and in the PACU in the group receiving PNB, with a nonsignificant trend toward lower use of oxycodone in the SDCU. Schroeder and colleagues17 similarly reported significant reductions in morphine equivalent dose intraoperatively and in Phase I recovery for patients receiving PNB, and these differences disappeared in Phase II recovery as well as intraoperatively if the block dose was considered. In addition, these authors found a significant reduction in the use of fentanyl and hydromorphone in the operating room in the PNB group, as well as a significant reduction in the proportion of patients receiving ketorolac in the operating room or PACU. Finally, YaDeau and colleagues18 reported total analgesic usage in the PACU among PNB patients compared with those receiving CSE alone and showed a strong trend toward reduced use in the PNB group, although this difference was not significant (P = .051). PACU analgesic use is presented in Table 4.
Continue to: Postoperative nausea...
POSTOPERATIVE NAUSEA/VOMITING AND ANTIEMETIC USE
Five studies presented data on nausea, vomiting, or antiemetic use following PNB and are shown in Table 3. YaDeau and colleagues18 reported nausea among 34% of patients in the PNB group, compared with 20% in the control group, vomiting in 2% and 7%, respectively, and antiemetic use in 12% of both groups. Dold and colleagues16 identified a similar trend, with 41.1% of patients in the PNB group and 32.5% of patients in the control group experiencing postoperative nausea or vomiting, while Krych and colleagues27 noted only 10% of PNB patients with mild nausea and none requiring antiemetic use. In their study of patients receiving PNB, Schroeder and colleagues17 found a significant reduction in antiemetic use among PNB patients compared with those receiving general anesthesia alone. Similarly, Ward and colleagues29 noted a significant difference in postoperative nausea, with 10% of patients in the PNB group experiencing postoperative nausea compared with 75% of those in the comparator group who received intravenous morphine. The mean percentage of patients experiencing postoperative nausea and/or vomiting is shown in Table 4.
DISCHARGE TIME
Four studies presented data on discharge time from the PACU and are summarized in Table 3. Three of these studies included a comparator group. Both Dold and colleagues16 and YaDeau and colleagues18 reported an increase in the time to discharge for patients receiving PNB, although these differences were not significant. The study by Ward and colleagues,29 on the other hand, noted a significant reduction in the time to discharge for the PNB group. In addition to these studies, Krych and colleagues27 examined the time from skin closure to discharge for patients receiving PNB, noting a mean 199 minutes for the patients in their study. Mean times to discharge for the PNB and control groups are presented in Table 4.
INPATIENT ADMISSION
Four studies presented data on the proportion of study participants who were admitted as inpatients, and these data are shown in Table 3. Dold and colleagues16 reported no inpatient admissions in their PNB group compared with 5.0% for the control group (both cases of pain control), while YaDeau and colleagues18 found that 3 admissions occurred, with 2 in the control group (1 for oxygen desaturation and the other for intractable pain and nausea) and 1 from the PNB group (epidural spread and urinary retention). Two additional studies reported data on PNB groups alone. Krych and colleagues27 observed no overnight admissions in their study, while Nye and colleagues28 reported 1 readmission for bilateral leg numbness and weakness due to epidural spread, which resolved following discontinuation of the block. The mean proportion of inpatient admissions is presented in Table 4.
SATISFACTION
A total of 3 studies examined patient satisfaction, and these data are presented in Table 3. In their study, Ward and colleagues29 reported a significantly greater rate of satisfaction at 1 day postoperatively among the patients in the PNB group (90%) than among patients who received intravenous morphine (25%) (P < .0001). Similarly, YaDeau and colleagues18 noted greater satisfaction among the PNB group than among the control group, with PNB patients rating their satisfaction at a mean of 8.6 and control patients at a mean of 7.9 on a 10-point scale (0-10) 24 hours postoperatively, although this difference was not significant. Finally, Krych and colleagues27 found that 67% of patients were “very satisfied” and 33% were “satisfied”, based on a Likert scale.
COMPLICATIONS
Four studies presented data on complications, and these findings are summarized in Table 3. In their work, Nye and colleagues28 reported most extensively on complications associated with PNB. Overall, the authors found a rate of significant complications of 3.8%. In terms of specific complications, they noted local anesthetic systemic toxicity (0.9%), epidural spread (0.5%), sensory or motor deficits (9.4%), falls (0.5%), and catheter issues. In their study of patients receiving PNB and CSE, YaDeau and colleagues18 identified 1 patient in the PNB group with epidural spread and urinary retention, while they noted 1 case of oxygen desaturation and another case of intractable pain and nausea in the group receiving CSE alone, all 3 of which required inpatient admission. They found no permanent adverse events attributable to the PNB. In another study, Dold and colleagues16 observed no complications in patients receiving PNB compared with those in 2 admissions in the control group for inadequate pain control. Similarly, Krych and colleagues27 identified no complications in patients who received PNB in their study.
DISCUSSION
Hip arthroscopy has experienced a substantial gain in popularity in recent years, emerging as a beneficial technique for both the diagnosis and treatment of diverse hip pathologies in patients spanning a variety of demographics. Nevertheless, postoperative pain control, as well as medication side effects and unwanted patient admissions, present major challenges to the treating surgeon. As an adjuvant measure, peripheral nerve block represents one option to improve postoperative pain management, while at the same time addressing the adverse effects of considerable opioid use, which is commonly seen in these patients. Early experience with this method in hip arthroscopy was reported in a case series by Lee and colleagues.12 In an attempt to reduce postoperative pain, as well as limit the adverse effects and delay in discharge associated with considerable opioid use in the PACU, the authors used preoperative paravertebral blocks of L1 and L2 in 2 patients requiring hip arthroscopy with encouraging results. Since then, a number of studies have attempted the use of PNB in hip arthroscopy.16-18,27-29 However, we were unable to identify any prior reviews reporting on peripheral nerve blockade in hip arthroscopy, and thus this study is unique in providing a greater understanding of the outcomes associated with PNB use.
In general, we found that PNB was associated with improved outcomes. Based on the studies included in this review, there was a statistically significantly lower level of pain in the PACU for femoral nerve block (compared with general anesthesia alone)16 and lumbar plexus blockade (compared with general anesthesia17 and CSE18 alone). Nevertheless, these effects are likely short-lived, with differences disappearing the day following the procedure. In terms of analgesic use, 2 studies report significant reductions in analgesic use intraoperatively and in the PACU/Phase I recovery,16,17 with a third reporting a strong trend toward reduced analgesic use in the PACU (P = .051).18 Finally, we report fewer admissions for the PNB group, as well as high rates of satisfaction and few complications across these studies.
Continue to: Unlike these measures...
Unlike these measures, postoperative nausea, vomiting, and antiemetic use, as well as time to discharge, showed more mixed results. With regard to nausea/vomiting, 2 studies16,18 reported nonsignificantly increased rates in the PNB group, whereas others reported significant reductions in nausea/vomiting29 and in the proportion of patients receiving antiemetics.17 Similarly, mixed results were seen in terms of patient discharge time from the PACU. Two studies16,18 reported a nonsignificant increase in time to discharge for the PNB group, while another29 noted a significant reduction for the PNB group compared with those receiving intravenous morphine. These mixed results were surprising, as we expected reductions in opioid use to result in fewer instances of nausea/vomiting and a quicker time to discharge. The reasons underlying these findings are not clear, although it has been suggested that current discharge guidelines and clinical pathways limit the ability to take advantage of the accelerated timeline offered by regional anesthesia.16,30 As experience with PNB grows, our guidelines and pathways are likely to adapt to capitalize on these advantages, and future studies may show more reliable improvements in these measures.
While rare, the risk of bleeding requiring blood transfusion following hip arthroscopy is one of the most common complications of this procedure. Though the studies included in this review did not report on the need for transfusion, a recent study by Cvetanovich and colleagues10 used a national database and found that, of patients undergoing hip arthroscopy (n = 1338), 0.4% (n = 5) had bleeding requiring a transfusion, with 0.3% (n = 4) requiring return to the operating room, similar to an earlier study by Clarke and colleagues,31 who noted bleeding from the portal site in 0.4% of hip arthroscopy patients. In terms of risk factors, Cvetanovich and colleagues10 found that ASA class, older age, and prior cardiac surgery were significantly associated with minor and overall complications, whereas both regional anesthesia/monitored anesthesia care and alcohol consumption of >2 drinks a day were significantly associated with minor complications, including bleeding requiring transfusions. They noted, however, that these risk factors accounted for only 5% of the variance in complication rates, indicating that other unidentified variables better explained the variance in complication rates. These authors concluded that complications associated with hip arthroscopy are so rare that we may not be able to predict which risk factors or anesthesia types are more likely to cause them. Further characterization of bleeding following hip arthroscopy and its associated risk factors is a valuable area for future research.
LIMITATIONS
Our study contains a number of limitations. This review included studies whose level of evidence varied from I to IV; therefore, our study is limited by any bias or heterogeneity introduced in patient recruitment, selection, variability of technique, data collection, and analysis used in these studies. This heterogeneity is most apparent in the block types and comparator groups. Furthermore, several different outcome measures were reported across the 6 studies used in this review, which decreased the relevance of any one of these individual outcomes. Finally, given the limited data that currently exist for the use of PNB in hip arthroscopy, we are unable to note meaningful differences between various types of PNBs, such as differences in postoperative pain or other measures such as quadriceps weakness, which can accompany femoral nerve block.12 While it is important to read our work with these limitations in mind, this systematic review is, to our knowledge, the only comprehensive review to date of studies reporting on PNB in hip arthroscopy, providing clinicians and patients with a greater understanding of the associated outcomes across these studies.
CONCLUSION
This systematic review shows improved outcomes and few complications with PNB use in hip arthroscopy, with reductions in postoperative pain, analgesic use, and the rate of inpatient admissions. Although opioid use was reduced in these studies, we found similar rates of postoperative nausea/vomiting as well as similar time to discharge from the PACU, which may reflect our continued reliance on outdated discharge guidelines and clinical pathways. Current attempts to provide peripheral nerve blockade are quite varied, with studies targeting femoral nerve, fascia iliaca, L1/L2 paravertebral, and lumbar plexus blockade. Future research efforts with a large prospective trial investigating these techniques should focus on which of these PNBs presents the optimal risk-benefit profile for hip arthroscopy patients and thus appropriately address the clinical questions at hand.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Pain control following hip arthroscopy presents a significant clinical challenge, with postoperative pain requiring considerable opioid use. Peripheral nerve blocks (PNBs) have emerged as one option to improve pain and limit the consequences of opioid use. The purpose of this study is to provide a comprehensive review of outcomes associated with PNB in hip arthroscopy. We hypothesize that the use of PNB in hip arthroscopy leads to improved outcomes and is associated with few complications. A systematic review of PubMed, Medline, Scopus, and Embase databases was conducted through January 2015 for English-language articles reporting outcome data, with 2 reviewers independently reviewing studies for inclusion. When available, similar outcomes were combined to generate frequency-weighted means. Six studies met the inclusion criteria for this review, reporting on 710 patients undergoing hip arthroscopy. The mean ages were 37.0 and 37.7 years for the PNB and comparator groups, respectively, with a reported total of 281 (40.5%) male and 412 (59.5%) female patients. Postoperative post-anesthesia care unit (PACU) pain was consistently reduced in the PNB group, with the use of a lower morphine equivalent dose and lower rates of inpatient admission, compared with that in the control groups. Postoperative nausea and/or vomiting as well as PACU discharge time showed mixed results. High satisfaction and few complications were reported. In conclusion, PNB is associated with reductions in postoperative pain, analgesic use, and the rate of inpatient admissions, though similar rates of nausea/vomiting and time to discharge were reported. Current PNB techniques are varied, and future research efforts should focus on examining which of these methods provides the optimal risk-benefit profile in hip arthroscopy.
Continue to: Hip arthroscopy has emerged...
Hip arthroscopy has emerged as a useful procedure in the diagnosis and treatment of hip pathology,1-8 experiencing a substantial rise in popularity in recent years, with the number of procedures growing by a factor of 18 from 1999 to 20099 and 25 from 2006 to 2013.10 Though hip arthroscopy is beneficial in many cases, marked postoperative pain has presented a substantial challenge, with patients requiring considerable doses of opiate-based medications in the post-anesthesia care unit (PACU).11,12 Increased narcotic use carries increased side effects, including postoperative nausea and vomiting,13 and poorly managed pain leads to increased unplanned admissions.14 Furthermore, patients with chronic hip pain and long-term opioid use may experience heightened and prolonged pain following the procedure, owing to medication tolerance and reduced opioid efficacy in this setting.15
Several pain control strategies have been employed in patients undergoing hip arthroscopy. General anesthesia16,17 and combined spinal epidural (CSE)18 are commonly used. However, such techniques rely heavily on opioids for postoperative pain control,11 and epidural anesthesia commonly requires adjunctive treatments (eg, neuromuscular blockade) to ensure muscle relaxation for joint distraction.19 One technique that has been employed recently is peripheral nerve block (PNB), which has been associated with a significant decrease in postoperative opioid use and nausea and vomiting.13,20 This method has proven successful in other fields of arthroscopy, including shoulder arthroscopy, in which it resulted in faster recovery, reduced opioid consumption,21 and demonstrated cost-effectiveness22 compared with general anesthesia and knee arthroscopy.23-26 As it is a relatively new field, little is known about the use of PNB in hip arthroscopy.
The goal of this systematic review was to comprehensively review the studies reporting on PNB in hip arthroscopy. We specifically focused on outcomes, including postoperative pain; analgesic use; nausea, vomiting, and antiemetic use; discharge time; inpatient admission; and patient satisfaction, as well as the complications associated with the use of PNB. Our knowledge of outcomes associated with PNB in hip arthroscopy is based on a few individual studies that have reported on small groups of patients using a variety of outcome measures and other findings. Furthermore, each of these studies commonly reflects the experience of an individual surgeon at a single institution and, when taken alone, may not be an accurate representation of the more general outcomes associated with PNB. A comprehensive review of such studies will provide surgeons, anesthesiologists, and patients with a better understanding of the anticipated outcomes of using PNB in hip arthroscopy. We hypothesize that the use of PNB in hip arthroscopy leads to improved outcomes and is associated with few complications.
MATERIALS AND METHODS
A systematic review of outcomes associated with PNB in hip arthroscopy was performed using the available English-language literature in accordance with the guidelines laid out by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement and included studies retrieved from the PubMed, Medline, Scopus, and Embase computerized literature databases. Searches were executed comprising all years from database inception through January 2015. Articles were retrieved by an electronic search of medical subject headings and keyword terms and their respective combinations (Table 1). The inclusion criteria for studies in this systematic review were studies that (1) were written in the English language and (2) reported explicit outcome data. The exclusion criteria were (1) review articles, meta-analyses, case reports, abstracts/conference papers, comments/letters, or technique articles without reported patient data and (2) basic research, biomechanics, or animal/cadaveric studies without reported patient data.
Table 1. Search Terms Entered to Identify English-Language Studies Through January 2015
Database | Search terms |
PubMed, Scopus | Keyword: (hip AND arthroscopy) AND (pain control OR pain management OR pain regimen OR nerve block OR spinal anesthesia OR regional anesthesia OR general anesthesia) |
Medline | MeSH (includes both MeSH terms and keywords): (Hip) AND (Arthroscopy) AND (“Pain Management” OR “Anesthesia, General” OR “Anesthesia” OR “Anesthesia, Inhalation”, OR “Balanced Anesthesia” OR “Anesthesia, Local” OR “Anesthesia, Spinal” OR “Anesthesia, Conduction” OR “Nerve Block”) |
Embase | MeSH (includes both MeSH terms and keywords): (Hip) AND (Arthroscopy) AND (“Pain Management” OR “General Anesthesia” OR “Anesthesia” OR “Inhalation Anesthesia”, OR “Balanced Anesthesia” OR “Local Anesthesia” OR “Spinal Anesthesia” OR “Regional Anesthesia” OR “Nerve Block”) |
The literature search strategy is outlined in the Figure. The initial title search yielded a subset of possible articles that were then further included or excluded on the basis of the contents of the article’s abstract, wherein articles were again selected on the basis of the aforementioned inclusion and exclusion criteria. Articles selected in both the title and abstract phases underwent full-text review, during which the full text of each qualifying article was reviewed. In addition, the reference sections from articles undergoing full-text review were scanned to identify any additional studies that had not been identified in the original literature search. Appropriate studies for final inclusion were then selected at this stage. The title, abstract, and full-text selection process were performed by 2 of the study authors (Dr. Steinhaus and Dr. Lynch), with any discrepancies being discussed and resolved by mutual agreement.

Continue to: For all 6 included studies...
For all 6 included studies,16-18,27-29 data were collected regarding the study specifics, patients included, and outcomes measured in the study. The journal of publication, type of study, level of evidence, and type of PNB, as well as the presence of a comparator group were noted (Table 2). Patient information included the number of patients at baseline and follow-up, mean age, gender, weight, height, body mass index, American Society of Anesthesiologists (ASA) status, and the specific procedures performed. In addition, data were collected on outcomes, including postoperative pain, as well as secondary outcomes and additional findings reported by the studies (Table 3). Where possible, weighted averages were calculated across all studies to obtain aggregate data.
RESULTS
STUDY INCLUSION
Six studies, all published between 2012 and 2014, were included in this systematic review (Table 2). Three studies involved lumbar plexus block, 2 studies involved femoral nerve block, and 1 study evaluated fascia iliaca block. Two studies used a control group of patients who received only general anesthesia (compared with the treatment group who received both general anesthesia and PNB); another study compared intravenous morphine with PNB; and 1 study compared CSE alone with PNB in addition to epidural.
DEMOGRAPHIC DATA
Demographic data from the included studies are presented in Table 2. In total, 710 and 549 patients were evaluated at baseline and final follow-up, respectively, which represents a follow-up rate of 77%. The frequency-weighted mean age of patients receiving PNB was 37.0 years compared with 37.7 years in the comparison groups, and the studies reported a total of 281 (40.5%) male and 412 (59.5%) female patients. The procedures performed were heterogeneously reported; therefore, totals were not tabulated, although the reported procedures included osteochondroplasty, labral débridement, labral and/or capsular repair, gluteus minimus repair, and synovectomy.
POSTOPERATIVE PAIN
Four studies reported on postoperative pain, and these data are presented in Table 3. In a retrospective study of patients receiving femoral nerve block in addition to general anesthesia, Dold and colleagues16 noted postoperative pain at 0, 15, 30, 45, and 60 minutes following arrival in the PACU, and discovered a statistically significantly lower level of pain at 60 minutes compared with inpatients receiving general anesthesia alone. YaDeau and colleagues18 found a significantly lower level of pain at rest in the PACU for those receiving CSE and lumbar plexus blockade compared with those receiving CSE alone. This significant difference did not persist at 24 hours or 6 months after the procedure, nor did it exist for pain with movement at any time point. Similarly, Schroeder and colleagues17 examined patients receiving general anesthesia and lumbar plexus block and found a significant reduction in pain immediately postoperatively in the PACU, though these effects disappeared the day following the procedure. Krych and colleagues27 also reported on postoperative pain in patients undergoing fascia iliaca blockade, although they did not include a comparator group. Outcome comparison between patients who received PNB and controls in the PACU and 1 day following the procedure are presented in Table 4.
ANALGESIC USE
Four studies reported on analgesic use after PNB, and these data are presented in Table 3. Dold and colleagues16 noted analgesic use intraoperatively, in the PACU, and in the surgical day care unit (SDCU). These authors found a significant reduction in morphine equivalent dose given in the operating room and in the PACU in the group receiving PNB, with a nonsignificant trend toward lower use of oxycodone in the SDCU. Schroeder and colleagues17 similarly reported significant reductions in morphine equivalent dose intraoperatively and in Phase I recovery for patients receiving PNB, and these differences disappeared in Phase II recovery as well as intraoperatively if the block dose was considered. In addition, these authors found a significant reduction in the use of fentanyl and hydromorphone in the operating room in the PNB group, as well as a significant reduction in the proportion of patients receiving ketorolac in the operating room or PACU. Finally, YaDeau and colleagues18 reported total analgesic usage in the PACU among PNB patients compared with those receiving CSE alone and showed a strong trend toward reduced use in the PNB group, although this difference was not significant (P = .051). PACU analgesic use is presented in Table 4.
Continue to: Postoperative nausea...
POSTOPERATIVE NAUSEA/VOMITING AND ANTIEMETIC USE
Five studies presented data on nausea, vomiting, or antiemetic use following PNB and are shown in Table 3. YaDeau and colleagues18 reported nausea among 34% of patients in the PNB group, compared with 20% in the control group, vomiting in 2% and 7%, respectively, and antiemetic use in 12% of both groups. Dold and colleagues16 identified a similar trend, with 41.1% of patients in the PNB group and 32.5% of patients in the control group experiencing postoperative nausea or vomiting, while Krych and colleagues27 noted only 10% of PNB patients with mild nausea and none requiring antiemetic use. In their study of patients receiving PNB, Schroeder and colleagues17 found a significant reduction in antiemetic use among PNB patients compared with those receiving general anesthesia alone. Similarly, Ward and colleagues29 noted a significant difference in postoperative nausea, with 10% of patients in the PNB group experiencing postoperative nausea compared with 75% of those in the comparator group who received intravenous morphine. The mean percentage of patients experiencing postoperative nausea and/or vomiting is shown in Table 4.
DISCHARGE TIME
Four studies presented data on discharge time from the PACU and are summarized in Table 3. Three of these studies included a comparator group. Both Dold and colleagues16 and YaDeau and colleagues18 reported an increase in the time to discharge for patients receiving PNB, although these differences were not significant. The study by Ward and colleagues,29 on the other hand, noted a significant reduction in the time to discharge for the PNB group. In addition to these studies, Krych and colleagues27 examined the time from skin closure to discharge for patients receiving PNB, noting a mean 199 minutes for the patients in their study. Mean times to discharge for the PNB and control groups are presented in Table 4.
INPATIENT ADMISSION
Four studies presented data on the proportion of study participants who were admitted as inpatients, and these data are shown in Table 3. Dold and colleagues16 reported no inpatient admissions in their PNB group compared with 5.0% for the control group (both cases of pain control), while YaDeau and colleagues18 found that 3 admissions occurred, with 2 in the control group (1 for oxygen desaturation and the other for intractable pain and nausea) and 1 from the PNB group (epidural spread and urinary retention). Two additional studies reported data on PNB groups alone. Krych and colleagues27 observed no overnight admissions in their study, while Nye and colleagues28 reported 1 readmission for bilateral leg numbness and weakness due to epidural spread, which resolved following discontinuation of the block. The mean proportion of inpatient admissions is presented in Table 4.
SATISFACTION
A total of 3 studies examined patient satisfaction, and these data are presented in Table 3. In their study, Ward and colleagues29 reported a significantly greater rate of satisfaction at 1 day postoperatively among the patients in the PNB group (90%) than among patients who received intravenous morphine (25%) (P < .0001). Similarly, YaDeau and colleagues18 noted greater satisfaction among the PNB group than among the control group, with PNB patients rating their satisfaction at a mean of 8.6 and control patients at a mean of 7.9 on a 10-point scale (0-10) 24 hours postoperatively, although this difference was not significant. Finally, Krych and colleagues27 found that 67% of patients were “very satisfied” and 33% were “satisfied”, based on a Likert scale.
COMPLICATIONS
Four studies presented data on complications, and these findings are summarized in Table 3. In their work, Nye and colleagues28 reported most extensively on complications associated with PNB. Overall, the authors found a rate of significant complications of 3.8%. In terms of specific complications, they noted local anesthetic systemic toxicity (0.9%), epidural spread (0.5%), sensory or motor deficits (9.4%), falls (0.5%), and catheter issues. In their study of patients receiving PNB and CSE, YaDeau and colleagues18 identified 1 patient in the PNB group with epidural spread and urinary retention, while they noted 1 case of oxygen desaturation and another case of intractable pain and nausea in the group receiving CSE alone, all 3 of which required inpatient admission. They found no permanent adverse events attributable to the PNB. In another study, Dold and colleagues16 observed no complications in patients receiving PNB compared with those in 2 admissions in the control group for inadequate pain control. Similarly, Krych and colleagues27 identified no complications in patients who received PNB in their study.
DISCUSSION
Hip arthroscopy has experienced a substantial gain in popularity in recent years, emerging as a beneficial technique for both the diagnosis and treatment of diverse hip pathologies in patients spanning a variety of demographics. Nevertheless, postoperative pain control, as well as medication side effects and unwanted patient admissions, present major challenges to the treating surgeon. As an adjuvant measure, peripheral nerve block represents one option to improve postoperative pain management, while at the same time addressing the adverse effects of considerable opioid use, which is commonly seen in these patients. Early experience with this method in hip arthroscopy was reported in a case series by Lee and colleagues.12 In an attempt to reduce postoperative pain, as well as limit the adverse effects and delay in discharge associated with considerable opioid use in the PACU, the authors used preoperative paravertebral blocks of L1 and L2 in 2 patients requiring hip arthroscopy with encouraging results. Since then, a number of studies have attempted the use of PNB in hip arthroscopy.16-18,27-29 However, we were unable to identify any prior reviews reporting on peripheral nerve blockade in hip arthroscopy, and thus this study is unique in providing a greater understanding of the outcomes associated with PNB use.
In general, we found that PNB was associated with improved outcomes. Based on the studies included in this review, there was a statistically significantly lower level of pain in the PACU for femoral nerve block (compared with general anesthesia alone)16 and lumbar plexus blockade (compared with general anesthesia17 and CSE18 alone). Nevertheless, these effects are likely short-lived, with differences disappearing the day following the procedure. In terms of analgesic use, 2 studies report significant reductions in analgesic use intraoperatively and in the PACU/Phase I recovery,16,17 with a third reporting a strong trend toward reduced analgesic use in the PACU (P = .051).18 Finally, we report fewer admissions for the PNB group, as well as high rates of satisfaction and few complications across these studies.
Continue to: Unlike these measures...
Unlike these measures, postoperative nausea, vomiting, and antiemetic use, as well as time to discharge, showed more mixed results. With regard to nausea/vomiting, 2 studies16,18 reported nonsignificantly increased rates in the PNB group, whereas others reported significant reductions in nausea/vomiting29 and in the proportion of patients receiving antiemetics.17 Similarly, mixed results were seen in terms of patient discharge time from the PACU. Two studies16,18 reported a nonsignificant increase in time to discharge for the PNB group, while another29 noted a significant reduction for the PNB group compared with those receiving intravenous morphine. These mixed results were surprising, as we expected reductions in opioid use to result in fewer instances of nausea/vomiting and a quicker time to discharge. The reasons underlying these findings are not clear, although it has been suggested that current discharge guidelines and clinical pathways limit the ability to take advantage of the accelerated timeline offered by regional anesthesia.16,30 As experience with PNB grows, our guidelines and pathways are likely to adapt to capitalize on these advantages, and future studies may show more reliable improvements in these measures.
While rare, the risk of bleeding requiring blood transfusion following hip arthroscopy is one of the most common complications of this procedure. Though the studies included in this review did not report on the need for transfusion, a recent study by Cvetanovich and colleagues10 used a national database and found that, of patients undergoing hip arthroscopy (n = 1338), 0.4% (n = 5) had bleeding requiring a transfusion, with 0.3% (n = 4) requiring return to the operating room, similar to an earlier study by Clarke and colleagues,31 who noted bleeding from the portal site in 0.4% of hip arthroscopy patients. In terms of risk factors, Cvetanovich and colleagues10 found that ASA class, older age, and prior cardiac surgery were significantly associated with minor and overall complications, whereas both regional anesthesia/monitored anesthesia care and alcohol consumption of >2 drinks a day were significantly associated with minor complications, including bleeding requiring transfusions. They noted, however, that these risk factors accounted for only 5% of the variance in complication rates, indicating that other unidentified variables better explained the variance in complication rates. These authors concluded that complications associated with hip arthroscopy are so rare that we may not be able to predict which risk factors or anesthesia types are more likely to cause them. Further characterization of bleeding following hip arthroscopy and its associated risk factors is a valuable area for future research.
LIMITATIONS
Our study contains a number of limitations. This review included studies whose level of evidence varied from I to IV; therefore, our study is limited by any bias or heterogeneity introduced in patient recruitment, selection, variability of technique, data collection, and analysis used in these studies. This heterogeneity is most apparent in the block types and comparator groups. Furthermore, several different outcome measures were reported across the 6 studies used in this review, which decreased the relevance of any one of these individual outcomes. Finally, given the limited data that currently exist for the use of PNB in hip arthroscopy, we are unable to note meaningful differences between various types of PNBs, such as differences in postoperative pain or other measures such as quadriceps weakness, which can accompany femoral nerve block.12 While it is important to read our work with these limitations in mind, this systematic review is, to our knowledge, the only comprehensive review to date of studies reporting on PNB in hip arthroscopy, providing clinicians and patients with a greater understanding of the associated outcomes across these studies.
CONCLUSION
This systematic review shows improved outcomes and few complications with PNB use in hip arthroscopy, with reductions in postoperative pain, analgesic use, and the rate of inpatient admissions. Although opioid use was reduced in these studies, we found similar rates of postoperative nausea/vomiting as well as similar time to discharge from the PACU, which may reflect our continued reliance on outdated discharge guidelines and clinical pathways. Current attempts to provide peripheral nerve blockade are quite varied, with studies targeting femoral nerve, fascia iliaca, L1/L2 paravertebral, and lumbar plexus blockade. Future research efforts with a large prospective trial investigating these techniques should focus on which of these PNBs presents the optimal risk-benefit profile for hip arthroscopy patients and thus appropriately address the clinical questions at hand.
This paper will be judged for the Resident Writer’s Award.
- Baber YF, Robinson AH, Villar RN. Is diagnostic arthroscopy of the hip worthwhile? A prospective review of 328 adults investigated for hip pain. J Bone Joint Surg Br. 1999;81:600-603.
- Byrd JW, Jones KS. Arthroscopic management of femoroacetabular impingement: minimum 2-year follow-up. Arthroscopy. 2011;27:1379-1388.
- Larson CM, Giveans MR. Arthroscopic management of femoroacetabular impingement: early outcomes measures. Arthroscopy. 2008;24:540-546.
- O'Leary JA, Berend K, Vail TP. The relationship between diagnosis and outcome in arthroscopy of the hip. Arthroscopy. 2001;17:181-188.
- Philippon M, Schenker M, Briggs K, Kuppersmith D. Femoroacetabular impingement in 45 professional athletes: associated pathologies and return to sport following arthroscopic decompression. Knee Surg Sports Traumatol Arthrosc. 2007;15:908-914.
- Potter BK, Freedman BA, Andersen RC, Bojescul JA, Kuklo TR, Murphy KP. Correlation of Short Form-36 and disability status with outcomes of arthroscopic acetabular labral debridement. Am J Sports Med. 2005;33:864-870.
- Robertson WJ, Kadrmas WR, Kelly BT. Arthroscopic management of labral tears in the hip: a systematic review of the literature. Clin Orthop Relat Res. 2007;455:88-92.
- Yusaf MA, Hame SL. Arthroscopy of the hip. Curr Sports Med Rep. 2008;7:269-274.
- Colvin AC, Harrast J, Harner C. Trends in hip arthroscopy. J Bone Joint Surg Am. 2012;94:e23.
- Cvetanovich GL, Chalmers PN, Levy DM, et al. Hip arthroscopy surgical volume trends and 30-day postoperative complications. Arthroscopy. 2016 Apr 8. [Epub before print].
- Baker JF, Byrne DP, Hunter K, Mulhall KJ. Post-operative opiate requirements after hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2011;19:1399-1402.
- Lee EM, Murphy KP, Ben-David B. Postoperative analgesia for hip arthroscopy: combined L1 and L2 paravertebral blocks. J Clin Anesth. 2008;20:462-465.
- Ganesh A, Rose JB, Wells L, et al. Continuous peripheral nerve blockade for inpatient and outpatient postoperative analgesia in children. Anesth Analg. 2007;105:1234-1242.
- Williams BA, Kentor ML, Vogt MT, et al. Femoral-sciatic nerve blocks for complex outpatient knee surgery are associated with less postoperative pain before same-day discharge: a review of 1,200 consecutive cases from the period 1996-1999. Anesthesiology. 2003;98:1206-1213.
- Zywiel MG, Stroh DA, Lee SY, Bonutti PM, Mont MA. Chronic opioid use prior to total knee arthroplasty. J Bone Joint Surg Am. 2011;93:1988-1993.
- Dold AP, Murnaghan L, Xing J, Abdallah FW, Brull R, Whelan DB. Preoperative femoral nerve block in hip arthroscopic surgery: a retrospective review of 108 consecutive cases. Am J Sports Med. 2014;42:144-149.
- Schroeder KM, Donnelly MJ, Anderson BM, Ford MP, Keene JS. The analgesic impact of preoperative lumbar plexus blocks for hip arthroscopy. A retrospective review. Hip Int. 2013;23:93-98.
- YaDeau JT, Tedore T, Goytizolo EA, et al. Lumbar plexus blockade reduces pain after hip arthroscopy: a prospective randomized controlled trial. Anesth Analg. 2012;115:968-972.
- Smart LR, Oetgen M, Noonan B, Medvecky M. Beginning hip arthroscopy: indications, positioning, portals, basic techniques, and complications. Arthroscopy. 2007;23:1348-1353.
- Stevens M, Harrison G, McGrail M. A modified fascia iliaca compartment block has significant morphine-sparing effect after total hip arthroplasty. Anaesth Intensive Care. 2007;35:949-952.
- Lehmann LJ, Loosen G, Weiss C, Schmittner MD. Interscalene plexus block versus general anaesthesia for shoulder surgery: a randomized controlled study. Eur J Orthop Surg Traumatol. 2015;25:255-261.
- Gonano C, Kettner SC, Ernstbrunner M, Schebesta K, Chiari A, Marhofer P. Comparison of economical aspects of interscalene brachial plexus blockade and general anaesthesia for arthroscopic shoulder surgery. Br J Anaesth. 2009;103:428-433.
- Hadzic A, Karaca PE, Hobeika P, et al. Peripheral nerve blocks result in superior recovery profile compared with general anesthesia in outpatient knee arthroscopy. Anesth Analg. 2005;100:976-981.
- Hsu LP, Oh S, Nuber GW, et al. Nerve block of the infrapatellar branch of the saphenous nerve in knee arthroscopy: a prospective, double-blinded, randomized, placebo-controlled trial. J Bone Joint Surg Am. 2013;95:1465-1472.
- Montes FR, Zarate E, Grueso R, et al. Comparison of spinal anesthesia with combined sciatic-femoral nerve block for outpatient knee arthroscopy. J Clin Anesth. 2008;20:415-420.
- Wulf H, Lowe J, Gnutzmann KH, Steinfeldt T. Femoral nerve block with ropivacaine or bupivacaine in day case anterior crucial ligament reconstruction. Acta Anaesthesiol Scand. 2010;54:414-420.
- Krych AJ, Baran S, Kuzma SA, Smith HM, Johnson RL, Levy BA. Utility of multimodal analgesia with fascia iliaca blockade for acute pain management following hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2014;22:843-847.
- Nye ZB, Horn JL, Crittenden W, Abrahams MS, Aziz MF. Ambulatory continuous posterior lumbar plexus blocks following hip arthroscopy: a review of 213 cases. J Clin Anesth. 2013;25:268-274.
- Ward JP, Albert DB, Altman R, Goldstein RY, Cuff G, Youm T. Are femoral nerve blocks effective for early postoperative pain management after hip arthroscopy? Arthroscopy. 2012;28:1064-1069.
- Liu SS, Strodtbeck WM, Richman JM, Wu CL. A comparison of regional versus general anesthesia for ambulatory anesthesia: a meta-analysis of randomized controlled trials. Anesth Analg. 2005;101:1634-1642.
- Clarke MT, Arora A, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop Relat Res. 2003;406:84-88.
- Baber YF, Robinson AH, Villar RN. Is diagnostic arthroscopy of the hip worthwhile? A prospective review of 328 adults investigated for hip pain. J Bone Joint Surg Br. 1999;81:600-603.
- Byrd JW, Jones KS. Arthroscopic management of femoroacetabular impingement: minimum 2-year follow-up. Arthroscopy. 2011;27:1379-1388.
- Larson CM, Giveans MR. Arthroscopic management of femoroacetabular impingement: early outcomes measures. Arthroscopy. 2008;24:540-546.
- O'Leary JA, Berend K, Vail TP. The relationship between diagnosis and outcome in arthroscopy of the hip. Arthroscopy. 2001;17:181-188.
- Philippon M, Schenker M, Briggs K, Kuppersmith D. Femoroacetabular impingement in 45 professional athletes: associated pathologies and return to sport following arthroscopic decompression. Knee Surg Sports Traumatol Arthrosc. 2007;15:908-914.
- Potter BK, Freedman BA, Andersen RC, Bojescul JA, Kuklo TR, Murphy KP. Correlation of Short Form-36 and disability status with outcomes of arthroscopic acetabular labral debridement. Am J Sports Med. 2005;33:864-870.
- Robertson WJ, Kadrmas WR, Kelly BT. Arthroscopic management of labral tears in the hip: a systematic review of the literature. Clin Orthop Relat Res. 2007;455:88-92.
- Yusaf MA, Hame SL. Arthroscopy of the hip. Curr Sports Med Rep. 2008;7:269-274.
- Colvin AC, Harrast J, Harner C. Trends in hip arthroscopy. J Bone Joint Surg Am. 2012;94:e23.
- Cvetanovich GL, Chalmers PN, Levy DM, et al. Hip arthroscopy surgical volume trends and 30-day postoperative complications. Arthroscopy. 2016 Apr 8. [Epub before print].
- Baker JF, Byrne DP, Hunter K, Mulhall KJ. Post-operative opiate requirements after hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2011;19:1399-1402.
- Lee EM, Murphy KP, Ben-David B. Postoperative analgesia for hip arthroscopy: combined L1 and L2 paravertebral blocks. J Clin Anesth. 2008;20:462-465.
- Ganesh A, Rose JB, Wells L, et al. Continuous peripheral nerve blockade for inpatient and outpatient postoperative analgesia in children. Anesth Analg. 2007;105:1234-1242.
- Williams BA, Kentor ML, Vogt MT, et al. Femoral-sciatic nerve blocks for complex outpatient knee surgery are associated with less postoperative pain before same-day discharge: a review of 1,200 consecutive cases from the period 1996-1999. Anesthesiology. 2003;98:1206-1213.
- Zywiel MG, Stroh DA, Lee SY, Bonutti PM, Mont MA. Chronic opioid use prior to total knee arthroplasty. J Bone Joint Surg Am. 2011;93:1988-1993.
- Dold AP, Murnaghan L, Xing J, Abdallah FW, Brull R, Whelan DB. Preoperative femoral nerve block in hip arthroscopic surgery: a retrospective review of 108 consecutive cases. Am J Sports Med. 2014;42:144-149.
- Schroeder KM, Donnelly MJ, Anderson BM, Ford MP, Keene JS. The analgesic impact of preoperative lumbar plexus blocks for hip arthroscopy. A retrospective review. Hip Int. 2013;23:93-98.
- YaDeau JT, Tedore T, Goytizolo EA, et al. Lumbar plexus blockade reduces pain after hip arthroscopy: a prospective randomized controlled trial. Anesth Analg. 2012;115:968-972.
- Smart LR, Oetgen M, Noonan B, Medvecky M. Beginning hip arthroscopy: indications, positioning, portals, basic techniques, and complications. Arthroscopy. 2007;23:1348-1353.
- Stevens M, Harrison G, McGrail M. A modified fascia iliaca compartment block has significant morphine-sparing effect after total hip arthroplasty. Anaesth Intensive Care. 2007;35:949-952.
- Lehmann LJ, Loosen G, Weiss C, Schmittner MD. Interscalene plexus block versus general anaesthesia for shoulder surgery: a randomized controlled study. Eur J Orthop Surg Traumatol. 2015;25:255-261.
- Gonano C, Kettner SC, Ernstbrunner M, Schebesta K, Chiari A, Marhofer P. Comparison of economical aspects of interscalene brachial plexus blockade and general anaesthesia for arthroscopic shoulder surgery. Br J Anaesth. 2009;103:428-433.
- Hadzic A, Karaca PE, Hobeika P, et al. Peripheral nerve blocks result in superior recovery profile compared with general anesthesia in outpatient knee arthroscopy. Anesth Analg. 2005;100:976-981.
- Hsu LP, Oh S, Nuber GW, et al. Nerve block of the infrapatellar branch of the saphenous nerve in knee arthroscopy: a prospective, double-blinded, randomized, placebo-controlled trial. J Bone Joint Surg Am. 2013;95:1465-1472.
- Montes FR, Zarate E, Grueso R, et al. Comparison of spinal anesthesia with combined sciatic-femoral nerve block for outpatient knee arthroscopy. J Clin Anesth. 2008;20:415-420.
- Wulf H, Lowe J, Gnutzmann KH, Steinfeldt T. Femoral nerve block with ropivacaine or bupivacaine in day case anterior crucial ligament reconstruction. Acta Anaesthesiol Scand. 2010;54:414-420.
- Krych AJ, Baran S, Kuzma SA, Smith HM, Johnson RL, Levy BA. Utility of multimodal analgesia with fascia iliaca blockade for acute pain management following hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2014;22:843-847.
- Nye ZB, Horn JL, Crittenden W, Abrahams MS, Aziz MF. Ambulatory continuous posterior lumbar plexus blocks following hip arthroscopy: a review of 213 cases. J Clin Anesth. 2013;25:268-274.
- Ward JP, Albert DB, Altman R, Goldstein RY, Cuff G, Youm T. Are femoral nerve blocks effective for early postoperative pain management after hip arthroscopy? Arthroscopy. 2012;28:1064-1069.
- Liu SS, Strodtbeck WM, Richman JM, Wu CL. A comparison of regional versus general anesthesia for ambulatory anesthesia: a meta-analysis of randomized controlled trials. Anesth Analg. 2005;101:1634-1642.
- Clarke MT, Arora A, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop Relat Res. 2003;406:84-88.
TAKE-HOME POINTS
- Postoperative PACU pain was consistently reduced in the PNB group.
- Patients with PNBs had lower postoperative pain medication requirements and lower rates of inpatient admission compared with controls.
- Similar rates of nausea/vomiting and time to discharge were reported for PNB patients and controls.
- PNBs are associated with high rates of satisfaction and few complications.
- Future research should focus on comparing across PNB techniques.
Higher BMI tied to lower breast cancer risk in women before menopause
Although obesity increases the risk of breast cancer in postmenopausal women, a large multicenter analysis has confirmed the opposite effect in premenopausal women.
The association had “a greater magnitude than previously shown and across the entire distribution of body mass index,” wrote Minouk J. Schoemaker, PhD, of the Institute of Cancer Research in London, with his associates, on behalf of the Premenopausal Breast Cancer Collaborative Group. The protective effect of adiposity was strongest during young adulthood (ages 18-24 years), when it spanned breast cancer subtypes. “Understanding the biological mechanisms underlying these associations could have important preventive potential,” they wrote in JAMA Oncology.
Prior studies have linked greater body fat with reduced risk of breast cancer in younger women, but the effect has not been well characterized. For this analysis, the investigators pooled data from 19 cohort studies that included a total of 758,592 premenopausal women; median age was 40.6 years (interquartile range, 35.2-45.5 years).
For each 5-unit increase in BMI, the estimated reduction in risk of breast cancer was 23% among women aged 18-24 years (hazard ratio, 0.77; 95% confidence interval, 0.73-0.80), 15% in women aged 25-34 years, 13% in women aged 35-44 years, and 12% in women aged 45-54 years. There was no BMI threshold for risk reduction: the inverse correlation existed even when women were not overweight. Risk also did vary significantly among subgroups stratified by other risk factors for breast cancer. Adiposity was more protective against estrogen receptor-positive and progesterone-receptor positive breast cancers and less protective against hormone receptor–negative breast cancers, which “implies a hormonal mechanism,” the investigators said. “Body mass index at ages 25-54 years was not consistently associated with triple-negative or hormone receptor–negative breast cancer overall.”
Funders included Breast Cancer Now, the Institute of Cancer Research, the National Institutes of Health, and many others. The researchers reported having no relevant conflicts of interest.
SOURCE: Schoemaker MJ et al. JAMA Oncol. 2018; Jun 21. doi: 10.1001/jamaoncol.2018.1771.
Although obesity increases the risk of breast cancer in postmenopausal women, a large multicenter analysis has confirmed the opposite effect in premenopausal women.
The association had “a greater magnitude than previously shown and across the entire distribution of body mass index,” wrote Minouk J. Schoemaker, PhD, of the Institute of Cancer Research in London, with his associates, on behalf of the Premenopausal Breast Cancer Collaborative Group. The protective effect of adiposity was strongest during young adulthood (ages 18-24 years), when it spanned breast cancer subtypes. “Understanding the biological mechanisms underlying these associations could have important preventive potential,” they wrote in JAMA Oncology.
Prior studies have linked greater body fat with reduced risk of breast cancer in younger women, but the effect has not been well characterized. For this analysis, the investigators pooled data from 19 cohort studies that included a total of 758,592 premenopausal women; median age was 40.6 years (interquartile range, 35.2-45.5 years).
For each 5-unit increase in BMI, the estimated reduction in risk of breast cancer was 23% among women aged 18-24 years (hazard ratio, 0.77; 95% confidence interval, 0.73-0.80), 15% in women aged 25-34 years, 13% in women aged 35-44 years, and 12% in women aged 45-54 years. There was no BMI threshold for risk reduction: the inverse correlation existed even when women were not overweight. Risk also did vary significantly among subgroups stratified by other risk factors for breast cancer. Adiposity was more protective against estrogen receptor-positive and progesterone-receptor positive breast cancers and less protective against hormone receptor–negative breast cancers, which “implies a hormonal mechanism,” the investigators said. “Body mass index at ages 25-54 years was not consistently associated with triple-negative or hormone receptor–negative breast cancer overall.”
Funders included Breast Cancer Now, the Institute of Cancer Research, the National Institutes of Health, and many others. The researchers reported having no relevant conflicts of interest.
SOURCE: Schoemaker MJ et al. JAMA Oncol. 2018; Jun 21. doi: 10.1001/jamaoncol.2018.1771.
Although obesity increases the risk of breast cancer in postmenopausal women, a large multicenter analysis has confirmed the opposite effect in premenopausal women.
The association had “a greater magnitude than previously shown and across the entire distribution of body mass index,” wrote Minouk J. Schoemaker, PhD, of the Institute of Cancer Research in London, with his associates, on behalf of the Premenopausal Breast Cancer Collaborative Group. The protective effect of adiposity was strongest during young adulthood (ages 18-24 years), when it spanned breast cancer subtypes. “Understanding the biological mechanisms underlying these associations could have important preventive potential,” they wrote in JAMA Oncology.
Prior studies have linked greater body fat with reduced risk of breast cancer in younger women, but the effect has not been well characterized. For this analysis, the investigators pooled data from 19 cohort studies that included a total of 758,592 premenopausal women; median age was 40.6 years (interquartile range, 35.2-45.5 years).
For each 5-unit increase in BMI, the estimated reduction in risk of breast cancer was 23% among women aged 18-24 years (hazard ratio, 0.77; 95% confidence interval, 0.73-0.80), 15% in women aged 25-34 years, 13% in women aged 35-44 years, and 12% in women aged 45-54 years. There was no BMI threshold for risk reduction: the inverse correlation existed even when women were not overweight. Risk also did vary significantly among subgroups stratified by other risk factors for breast cancer. Adiposity was more protective against estrogen receptor-positive and progesterone-receptor positive breast cancers and less protective against hormone receptor–negative breast cancers, which “implies a hormonal mechanism,” the investigators said. “Body mass index at ages 25-54 years was not consistently associated with triple-negative or hormone receptor–negative breast cancer overall.”
Funders included Breast Cancer Now, the Institute of Cancer Research, the National Institutes of Health, and many others. The researchers reported having no relevant conflicts of interest.
SOURCE: Schoemaker MJ et al. JAMA Oncol. 2018; Jun 21. doi: 10.1001/jamaoncol.2018.1771.
FROM JAMA ONCOLOGY
Key clinical point: In premenopausal women, adiposity inversely correlated with risk of breast cancer, and showed a stronger protective effect than previously documented.
Major finding: For each 5-unit increase in BMI, the estimated reduction in risk of breast cancer was 23% among women aged 18-24 years (hazard ratio, 0.77; 95% confidence interval, 0.73-0.80), 15% in women aged 25-34 years, 13% in women aged 35-44 years, and 12% in women aged 45-54 years.
Study details: Multicenter analysis of 19 cohort studies.
Disclosures: Funders included Breast Cancer Now, the Institute of Cancer Research, the National Institutes of Health, and many others. The researchers reported having no relevant conflicts of interest.
Source: Schoemaker MJ et al. JAMA Oncol. 2018; Jun 21. doi: 10.1001/jamaoncol.2018.1771.
Right to Try: Mission accomplished?
On May 30, 2018, President Trump signed into law the Trickett Wendler, Frank Mongiello, Jordan McLinn, and Matthew Bellina Right to Try Act of 2017, guaranteeing patients with terminal illnesses the right to seek access to investigational drugs which have passed at least phase 1 testing. As he signed the bipartisan-backed bill into law, the president said he believes it will ultimately save “hundreds of thousands” of lives, scoring an apparent win-win-win for the president, for Congress, and for patients. A critical appraisal of the law – and of the supposition that patients will reap great benefit from it – requires that we ask two questions: Does the law degrade measures originally put in place to protect patients, and does it actually improve access to potentially life-saving drugs?
As a hematologist with about 2 decades of experience conducting clinical research involving patients with incurable cancer, I want as many patients as possible to have access to promising new therapies. Clinical trials, as the sole means of determining a candidate drug’s safety and efficacy, are conducted in a systematic fashion, with phase 1 trials focusing predominantly on safety. As any experienced researcher is aware, however, a phase 1 trial only provides a basic sense of a drug’s safety. It would be unimaginable (and unethical) to conduct subsequent phase 2 and 3 trials without including rigorous ongoing monitoring for adverse effects.
A later study evaluating all nonpediatric phase 1 trials sponsored by the National Cancer Institute’s Cancer Therapy Evaluation Program between 2001 and 2012, which included more than 8,300 patients, found the therapy-related death rate to be similarly low – approximately 1%.2
It is distinctly possible that the same drugs used in the analyzed trials would have been more toxic to patients who, for one reason or another, would not have met enrollment criteria. It is impossible to precisely quantify the degree to which risk may be increased under the Right to Try law, but even if it were tripled or quadrupled, compared with these historical expectations, the absolute risk of treatment-related death remains low. It should be noted that the incidence of nonfatal grade 3-4 adverse events in the same analyses was 10%-20% so a similar proportional increase in these events would impact a larger number of patients. Overall, it is difficult to know the degree to which Right to Try impacts the safety of fragile patients with terminal illnesses.
The answer to the second question – Is access to life-saving drugs improved? – is much easier to answer: No.
One reason is that these patients already have the right to seek access to investigational drugs through the FDA’s Expanded Access Program. An overview of the Expanded Access Program reported that approximately 11,000 patients over a period of 10 years submitted requests for access to investigational drugs and that 99% of the requests were granted.3
Proponents of the Right to Try law argue that the new law somehow simplifies the request process, but it is difficult to understand exactly how. Further, in a position paper on the topic of Right to Try policy, Lisa Kearns and Alison Bateman-House, PhD, of New York University, wrote, “In the more than 2½ years since the first [individual state right to try] law was signed, there have been no documented cases of anyone receiving access, because of a right to try law, to an experimental product that would not have been available via the FDA’s Expanded Access Program.”4
Finally, and most importantly, neither the existing Expanded Access Program nor the newly enacted Right to Try law require that pharmaceutical companies actually provide the requested drugs. Industry leaders, in testimony before Congress, have cited patient safety, costs, drug supply, and negative impact on existing clinical trial accrual as reasons pharmaceutical companies commonly deny requests for access to investigational products.
The bottom line is that the Right to Try law really lacks teeth. Despite the favorable optics for the president and Congress, it is virtually certain that Right to Try will never save hundreds of thousands of lives, and the suggestion that it will is either disingenuous or uninformed. Hopefully, though, Congress’s passage of Right to Try is indicative of a more general interest in bipartisan cooperation to improve access to affordable, high-quality health care.
Dr. Zonder is a professor in the department of oncology at the Barbara Ann Karmanos Cancer Institute and Wayne State University in Detroit. He is the leader of the KCI Myeloma and Amyloidosis Team. He is also a member of the Hematology News Editorial Advisory Board. He reported having no relevant financial disclosures.
References
1. Roberts TG Jr. et al. JAMA. 2004 Nov 3;292(17):2130-40.
2. Fukada YK et al. J Clin Oncol. 2014 May 20. doi: 10.1200/jco.2014.32.15_suppl.2552.
3. Jarow JP et al. Ther Innov Regul Sci. 2017 Mar 1;51(2):177-9.
4. Kearns L et al. Ther Innov Regul Sci. 2017 Mar 1;51(2):170-6.
On May 30, 2018, President Trump signed into law the Trickett Wendler, Frank Mongiello, Jordan McLinn, and Matthew Bellina Right to Try Act of 2017, guaranteeing patients with terminal illnesses the right to seek access to investigational drugs which have passed at least phase 1 testing. As he signed the bipartisan-backed bill into law, the president said he believes it will ultimately save “hundreds of thousands” of lives, scoring an apparent win-win-win for the president, for Congress, and for patients. A critical appraisal of the law – and of the supposition that patients will reap great benefit from it – requires that we ask two questions: Does the law degrade measures originally put in place to protect patients, and does it actually improve access to potentially life-saving drugs?
As a hematologist with about 2 decades of experience conducting clinical research involving patients with incurable cancer, I want as many patients as possible to have access to promising new therapies. Clinical trials, as the sole means of determining a candidate drug’s safety and efficacy, are conducted in a systematic fashion, with phase 1 trials focusing predominantly on safety. As any experienced researcher is aware, however, a phase 1 trial only provides a basic sense of a drug’s safety. It would be unimaginable (and unethical) to conduct subsequent phase 2 and 3 trials without including rigorous ongoing monitoring for adverse effects.
A later study evaluating all nonpediatric phase 1 trials sponsored by the National Cancer Institute’s Cancer Therapy Evaluation Program between 2001 and 2012, which included more than 8,300 patients, found the therapy-related death rate to be similarly low – approximately 1%.2
It is distinctly possible that the same drugs used in the analyzed trials would have been more toxic to patients who, for one reason or another, would not have met enrollment criteria. It is impossible to precisely quantify the degree to which risk may be increased under the Right to Try law, but even if it were tripled or quadrupled, compared with these historical expectations, the absolute risk of treatment-related death remains low. It should be noted that the incidence of nonfatal grade 3-4 adverse events in the same analyses was 10%-20% so a similar proportional increase in these events would impact a larger number of patients. Overall, it is difficult to know the degree to which Right to Try impacts the safety of fragile patients with terminal illnesses.
The answer to the second question – Is access to life-saving drugs improved? – is much easier to answer: No.
One reason is that these patients already have the right to seek access to investigational drugs through the FDA’s Expanded Access Program. An overview of the Expanded Access Program reported that approximately 11,000 patients over a period of 10 years submitted requests for access to investigational drugs and that 99% of the requests were granted.3
Proponents of the Right to Try law argue that the new law somehow simplifies the request process, but it is difficult to understand exactly how. Further, in a position paper on the topic of Right to Try policy, Lisa Kearns and Alison Bateman-House, PhD, of New York University, wrote, “In the more than 2½ years since the first [individual state right to try] law was signed, there have been no documented cases of anyone receiving access, because of a right to try law, to an experimental product that would not have been available via the FDA’s Expanded Access Program.”4
Finally, and most importantly, neither the existing Expanded Access Program nor the newly enacted Right to Try law require that pharmaceutical companies actually provide the requested drugs. Industry leaders, in testimony before Congress, have cited patient safety, costs, drug supply, and negative impact on existing clinical trial accrual as reasons pharmaceutical companies commonly deny requests for access to investigational products.
The bottom line is that the Right to Try law really lacks teeth. Despite the favorable optics for the president and Congress, it is virtually certain that Right to Try will never save hundreds of thousands of lives, and the suggestion that it will is either disingenuous or uninformed. Hopefully, though, Congress’s passage of Right to Try is indicative of a more general interest in bipartisan cooperation to improve access to affordable, high-quality health care.
Dr. Zonder is a professor in the department of oncology at the Barbara Ann Karmanos Cancer Institute and Wayne State University in Detroit. He is the leader of the KCI Myeloma and Amyloidosis Team. He is also a member of the Hematology News Editorial Advisory Board. He reported having no relevant financial disclosures.
References
1. Roberts TG Jr. et al. JAMA. 2004 Nov 3;292(17):2130-40.
2. Fukada YK et al. J Clin Oncol. 2014 May 20. doi: 10.1200/jco.2014.32.15_suppl.2552.
3. Jarow JP et al. Ther Innov Regul Sci. 2017 Mar 1;51(2):177-9.
4. Kearns L et al. Ther Innov Regul Sci. 2017 Mar 1;51(2):170-6.
On May 30, 2018, President Trump signed into law the Trickett Wendler, Frank Mongiello, Jordan McLinn, and Matthew Bellina Right to Try Act of 2017, guaranteeing patients with terminal illnesses the right to seek access to investigational drugs which have passed at least phase 1 testing. As he signed the bipartisan-backed bill into law, the president said he believes it will ultimately save “hundreds of thousands” of lives, scoring an apparent win-win-win for the president, for Congress, and for patients. A critical appraisal of the law – and of the supposition that patients will reap great benefit from it – requires that we ask two questions: Does the law degrade measures originally put in place to protect patients, and does it actually improve access to potentially life-saving drugs?
As a hematologist with about 2 decades of experience conducting clinical research involving patients with incurable cancer, I want as many patients as possible to have access to promising new therapies. Clinical trials, as the sole means of determining a candidate drug’s safety and efficacy, are conducted in a systematic fashion, with phase 1 trials focusing predominantly on safety. As any experienced researcher is aware, however, a phase 1 trial only provides a basic sense of a drug’s safety. It would be unimaginable (and unethical) to conduct subsequent phase 2 and 3 trials without including rigorous ongoing monitoring for adverse effects.
A later study evaluating all nonpediatric phase 1 trials sponsored by the National Cancer Institute’s Cancer Therapy Evaluation Program between 2001 and 2012, which included more than 8,300 patients, found the therapy-related death rate to be similarly low – approximately 1%.2
It is distinctly possible that the same drugs used in the analyzed trials would have been more toxic to patients who, for one reason or another, would not have met enrollment criteria. It is impossible to precisely quantify the degree to which risk may be increased under the Right to Try law, but even if it were tripled or quadrupled, compared with these historical expectations, the absolute risk of treatment-related death remains low. It should be noted that the incidence of nonfatal grade 3-4 adverse events in the same analyses was 10%-20% so a similar proportional increase in these events would impact a larger number of patients. Overall, it is difficult to know the degree to which Right to Try impacts the safety of fragile patients with terminal illnesses.
The answer to the second question – Is access to life-saving drugs improved? – is much easier to answer: No.
One reason is that these patients already have the right to seek access to investigational drugs through the FDA’s Expanded Access Program. An overview of the Expanded Access Program reported that approximately 11,000 patients over a period of 10 years submitted requests for access to investigational drugs and that 99% of the requests were granted.3
Proponents of the Right to Try law argue that the new law somehow simplifies the request process, but it is difficult to understand exactly how. Further, in a position paper on the topic of Right to Try policy, Lisa Kearns and Alison Bateman-House, PhD, of New York University, wrote, “In the more than 2½ years since the first [individual state right to try] law was signed, there have been no documented cases of anyone receiving access, because of a right to try law, to an experimental product that would not have been available via the FDA’s Expanded Access Program.”4
Finally, and most importantly, neither the existing Expanded Access Program nor the newly enacted Right to Try law require that pharmaceutical companies actually provide the requested drugs. Industry leaders, in testimony before Congress, have cited patient safety, costs, drug supply, and negative impact on existing clinical trial accrual as reasons pharmaceutical companies commonly deny requests for access to investigational products.
The bottom line is that the Right to Try law really lacks teeth. Despite the favorable optics for the president and Congress, it is virtually certain that Right to Try will never save hundreds of thousands of lives, and the suggestion that it will is either disingenuous or uninformed. Hopefully, though, Congress’s passage of Right to Try is indicative of a more general interest in bipartisan cooperation to improve access to affordable, high-quality health care.
Dr. Zonder is a professor in the department of oncology at the Barbara Ann Karmanos Cancer Institute and Wayne State University in Detroit. He is the leader of the KCI Myeloma and Amyloidosis Team. He is also a member of the Hematology News Editorial Advisory Board. He reported having no relevant financial disclosures.
References
1. Roberts TG Jr. et al. JAMA. 2004 Nov 3;292(17):2130-40.
2. Fukada YK et al. J Clin Oncol. 2014 May 20. doi: 10.1200/jco.2014.32.15_suppl.2552.
3. Jarow JP et al. Ther Innov Regul Sci. 2017 Mar 1;51(2):177-9.
4. Kearns L et al. Ther Innov Regul Sci. 2017 Mar 1;51(2):170-6.
New SLE classification criteria reset disease definition
AMSTERDAM – The new systemic lupus erythematosus classification criteria of the American College of Rheumatology and the European League Against Rheumatism are based on a point system that will produce a “paradigm shift” in how the disease gets studied going forward, said Sindhu Johnson, MD, while presenting the latest version of the newly revised classification scheme at the European Congress of Rheumatology.
Until now, classification of systemic lupus erythematosus (SLE) was a yes-or-no decision, based on whether the patient had a minimum number of characteristic signs or symptoms. The new criteria, which are on track for formal endorsement before the end of 2018 by the two medical societies that sponsored the revision, instead use a point system that gives varying weight to each of the 22 criteria. A patient needs to score at least 10 points from these criteria, and all patients classified with SLE also must have an antinuclear antibody (ANA) titer of at least 1:80 on HEp-2 cells or an equivalent positive test. This means that the criteria also can define patients who just miss classification with SLE by meeting the ANA standard and by tallying 8 or 9 points, and the criteria also identify patients who far exceed the classification threshold by having the requisite ANA plus racking up as many as, perhaps, 20 or 30 points.
“This is a real research opportunity,” to follow patients who fall just short with 8 or 9 points to assess their longer-term prognosis, as well as to study whether “higher scores mean a higher risk for developing a bad outcome,” said Dr. Johnson, a rheumatologist at the University of Toronto and director of the Toronto Scleroderma Program. Other areas for future research with the new criteria include seeing how they work in various SLE subgroups, such as patients with renal-predominant disease or skin-predominant disease, and also seeing how they work in various ethnic populations.
“Diagnosis of lupus still falls within the realm of the treating physician,” but the classification criteria “inform our concept of the disease,” Dr. Johnson said in a video interview. “The new criteria allow for a shift in the way we think of the disease.”
For example, for the first time, the new criteria includes fever as a classification criterion, which receives 2 points if an infectious or other non-SLE cause can be discounted. Fever has recently been identified as a marker of early-stage SLE in at least some patients, and its addition to the classification criteria “adds a new dimension to how we think about the disease and allows us to distinguish early disease from mimicking diseases,” she explained. At the other end of the classification spectrum, a finding of class III or IV lupus nephritis on renal biopsy receives 10 points, and hence, this one finding plus having a high enough level of ANA leads to SLE classification regardless of whether the patient has any other signs or symptoms of the disease.
That’s because “85% of our experts said that they would feel confident classifying a patient as having lupus based only on a renal biopsy” and ANA positivity, said Dr. Johnson, who served as the ACR-appointed cochair of the criteria-writing panel along with a cochair selected by EULAR, Martin Aringer, MD, PhD, of the Technical University of Dresden (Germany). She cautioned that other levels of lupus nephritis, class II or V, confer only 8 points to the classification and so by themselves are not enough to label a person as having lupus.
During her presentation, Dr. Johnson cited the high levels of sensitivity and specificity that the new classification criteria demonstrated in a validation cohort of more than 1,000 cases and controls. In the validation analysis, the new criteria had a sensitivity of 96.12% and specificity of 94.43% for classifying SLE, giving the new criteria a better result on both these measures than either the 1997 ACR criteria (Arthritis Rheum. 1997 Sept;40[9]:1725) or the 2012 Systemic Lupus International Collaborating Clinics criteria (Arthritis Rheum. 2012 Aug;64[8]:2677-86).
The 22 criteria cluster into seven separate clinical domains and three different immunologic domains. The point values assigned to each criterion range from 2 to 10 points.
Dr. Johnson had no disclosures.
AMSTERDAM – The new systemic lupus erythematosus classification criteria of the American College of Rheumatology and the European League Against Rheumatism are based on a point system that will produce a “paradigm shift” in how the disease gets studied going forward, said Sindhu Johnson, MD, while presenting the latest version of the newly revised classification scheme at the European Congress of Rheumatology.
Until now, classification of systemic lupus erythematosus (SLE) was a yes-or-no decision, based on whether the patient had a minimum number of characteristic signs or symptoms. The new criteria, which are on track for formal endorsement before the end of 2018 by the two medical societies that sponsored the revision, instead use a point system that gives varying weight to each of the 22 criteria. A patient needs to score at least 10 points from these criteria, and all patients classified with SLE also must have an antinuclear antibody (ANA) titer of at least 1:80 on HEp-2 cells or an equivalent positive test. This means that the criteria also can define patients who just miss classification with SLE by meeting the ANA standard and by tallying 8 or 9 points, and the criteria also identify patients who far exceed the classification threshold by having the requisite ANA plus racking up as many as, perhaps, 20 or 30 points.
“This is a real research opportunity,” to follow patients who fall just short with 8 or 9 points to assess their longer-term prognosis, as well as to study whether “higher scores mean a higher risk for developing a bad outcome,” said Dr. Johnson, a rheumatologist at the University of Toronto and director of the Toronto Scleroderma Program. Other areas for future research with the new criteria include seeing how they work in various SLE subgroups, such as patients with renal-predominant disease or skin-predominant disease, and also seeing how they work in various ethnic populations.
“Diagnosis of lupus still falls within the realm of the treating physician,” but the classification criteria “inform our concept of the disease,” Dr. Johnson said in a video interview. “The new criteria allow for a shift in the way we think of the disease.”
For example, for the first time, the new criteria includes fever as a classification criterion, which receives 2 points if an infectious or other non-SLE cause can be discounted. Fever has recently been identified as a marker of early-stage SLE in at least some patients, and its addition to the classification criteria “adds a new dimension to how we think about the disease and allows us to distinguish early disease from mimicking diseases,” she explained. At the other end of the classification spectrum, a finding of class III or IV lupus nephritis on renal biopsy receives 10 points, and hence, this one finding plus having a high enough level of ANA leads to SLE classification regardless of whether the patient has any other signs or symptoms of the disease.
That’s because “85% of our experts said that they would feel confident classifying a patient as having lupus based only on a renal biopsy” and ANA positivity, said Dr. Johnson, who served as the ACR-appointed cochair of the criteria-writing panel along with a cochair selected by EULAR, Martin Aringer, MD, PhD, of the Technical University of Dresden (Germany). She cautioned that other levels of lupus nephritis, class II or V, confer only 8 points to the classification and so by themselves are not enough to label a person as having lupus.
During her presentation, Dr. Johnson cited the high levels of sensitivity and specificity that the new classification criteria demonstrated in a validation cohort of more than 1,000 cases and controls. In the validation analysis, the new criteria had a sensitivity of 96.12% and specificity of 94.43% for classifying SLE, giving the new criteria a better result on both these measures than either the 1997 ACR criteria (Arthritis Rheum. 1997 Sept;40[9]:1725) or the 2012 Systemic Lupus International Collaborating Clinics criteria (Arthritis Rheum. 2012 Aug;64[8]:2677-86).
The 22 criteria cluster into seven separate clinical domains and three different immunologic domains. The point values assigned to each criterion range from 2 to 10 points.
Dr. Johnson had no disclosures.
AMSTERDAM – The new systemic lupus erythematosus classification criteria of the American College of Rheumatology and the European League Against Rheumatism are based on a point system that will produce a “paradigm shift” in how the disease gets studied going forward, said Sindhu Johnson, MD, while presenting the latest version of the newly revised classification scheme at the European Congress of Rheumatology.
Until now, classification of systemic lupus erythematosus (SLE) was a yes-or-no decision, based on whether the patient had a minimum number of characteristic signs or symptoms. The new criteria, which are on track for formal endorsement before the end of 2018 by the two medical societies that sponsored the revision, instead use a point system that gives varying weight to each of the 22 criteria. A patient needs to score at least 10 points from these criteria, and all patients classified with SLE also must have an antinuclear antibody (ANA) titer of at least 1:80 on HEp-2 cells or an equivalent positive test. This means that the criteria also can define patients who just miss classification with SLE by meeting the ANA standard and by tallying 8 or 9 points, and the criteria also identify patients who far exceed the classification threshold by having the requisite ANA plus racking up as many as, perhaps, 20 or 30 points.
“This is a real research opportunity,” to follow patients who fall just short with 8 or 9 points to assess their longer-term prognosis, as well as to study whether “higher scores mean a higher risk for developing a bad outcome,” said Dr. Johnson, a rheumatologist at the University of Toronto and director of the Toronto Scleroderma Program. Other areas for future research with the new criteria include seeing how they work in various SLE subgroups, such as patients with renal-predominant disease or skin-predominant disease, and also seeing how they work in various ethnic populations.
“Diagnosis of lupus still falls within the realm of the treating physician,” but the classification criteria “inform our concept of the disease,” Dr. Johnson said in a video interview. “The new criteria allow for a shift in the way we think of the disease.”
For example, for the first time, the new criteria includes fever as a classification criterion, which receives 2 points if an infectious or other non-SLE cause can be discounted. Fever has recently been identified as a marker of early-stage SLE in at least some patients, and its addition to the classification criteria “adds a new dimension to how we think about the disease and allows us to distinguish early disease from mimicking diseases,” she explained. At the other end of the classification spectrum, a finding of class III or IV lupus nephritis on renal biopsy receives 10 points, and hence, this one finding plus having a high enough level of ANA leads to SLE classification regardless of whether the patient has any other signs or symptoms of the disease.
That’s because “85% of our experts said that they would feel confident classifying a patient as having lupus based only on a renal biopsy” and ANA positivity, said Dr. Johnson, who served as the ACR-appointed cochair of the criteria-writing panel along with a cochair selected by EULAR, Martin Aringer, MD, PhD, of the Technical University of Dresden (Germany). She cautioned that other levels of lupus nephritis, class II or V, confer only 8 points to the classification and so by themselves are not enough to label a person as having lupus.
During her presentation, Dr. Johnson cited the high levels of sensitivity and specificity that the new classification criteria demonstrated in a validation cohort of more than 1,000 cases and controls. In the validation analysis, the new criteria had a sensitivity of 96.12% and specificity of 94.43% for classifying SLE, giving the new criteria a better result on both these measures than either the 1997 ACR criteria (Arthritis Rheum. 1997 Sept;40[9]:1725) or the 2012 Systemic Lupus International Collaborating Clinics criteria (Arthritis Rheum. 2012 Aug;64[8]:2677-86).
The 22 criteria cluster into seven separate clinical domains and three different immunologic domains. The point values assigned to each criterion range from 2 to 10 points.
Dr. Johnson had no disclosures.
REPORTING FROM THE EULAR 2018 CONGRESS