User login
ASCO 2018: Less is more as ‘tailoring’ takes on new meaning
A record-setting 40,000-plus oncology professionals attended this year’s annual meeting of the American Society of Clinical Oncology (ASCO) in Chicago. The outstanding education and scientific program, with the theme of Delivering Discoveries: Expanding the Reach of Precision Medicine, was planned and led by ASCO President Dr Bruce Johnson, professor and director of Thoracic Oncology at the Dana Farber Cancer Institute in Boston, and chaired by Sarah Cannon’s Dr David Spigel and Harvard’s Dr Ann Partridge. A recurring finding throughout the meeting was that “less is more” in several key areas of cancer therapy. From small molecules targeting driver mutations across various tumors to the application of immunotherapy in subsets of common cancers, it is clear that more patients are experiencing dramatic results from novel approaches.
A featured plenary session trial was TAILORx, a study of 10,273 women with hormone-receptor–positive, surgically resected breast cancer that had not spread to the lymph nodes, was less than 5 cm, and was not positive for the HER2 gene amplification. This clinical trial was sponsored by the NCI and initiated in 2006. It used the OncotypeDX genetic test to stratify patients into groups of low, intermediate, or high risk for recurrence. The low-risk patients received only hormonal therapy, and the high-risk patients were treated with hormonal therapy plus chemotherapy.
Dr Joseph Sparano, professor of Medicine and Women’s Health at the Albert Einstein College of Medicine in New York, presented the results from the group of 6,700 intermediate risk women who were randomized to receive hormonal therapy alone or in combination with chemotherapy. After 9 years of follow-up, 83.3% of the volunteers, as Dr Sparano appropriately referred to them, who were treated with hormonal therapy were still cancer free, compared with 84.3% of those who also received chemotherapy, demonstrating no statistical benefit for the addition of chemotherapy. Of note, breast cancer experts discussing the trial, including Dr Lisa Carey, professor of Breast Cancer Research at the UNC Lineberger Cancer Institute in Chapel Hill, urged that younger women, under the age of 50, with recurrence scores (RS) toward the higher end of the intermediate risk group (RS, 16-25) should still discuss and consider chemotherapy with their physicians. In summary, all patients fitting the study criteria with low (
These landmark and practice changing results mean that each year about 60,000 women in the United States will be spared the side effects of toxic drugs. These 10,273 study volunteers are true heroes to the women who will be diagnosed with breast cancer in coming years.
In the field of lung cancer, many new trial results using immunotherapy were presented, with the most talked about being single-agent pembrolizumab, a PD1 inhibitor, improving survival over traditional chemotherapy in patients with PD-L1 positive tumors, which comprise the majority of squamous cell and adenocarcinomas of the lung. Also in the plenary, Dr Gilberto Lopes of the Sylvester Cancer Center at the University of Miami, presented these results from the KEYNOTE-042 study. In patients with PD-L1 tumor proportion score (TPS) of >1%, the benefit in overall survival (OS) of pembrolizumab compared with chemotherapy was 16.7 versus 12.1 months, respectively (HR, 0.81). In those patients with a TPS of >20%, the OS benefit was 17.7 versus 1.0 months (HR, 0.77), and in the group with a TPS of >50%, the benefit was 20.0 versus 12.2 months (HR, 0.69). Overall, the quality of life and the occurrence of side effects were substantially better for those patients receiving immunotherapy alone. Other findings presented at the meeting demonstrated the benefit of adding immunotherapy to chemotherapy and of treating with combination immunotherapy (PD-1 and CTLA-4 inhibitors). Many options now exist, much work remains to be done, and accrual to clinical trials is more important than ever.
Another plenary session trial evaluated the benefit of performing a nephrectomy in patients with advanced or metastatic renal cell carcinoma (RCC), a long-held and practiced standard of care. Dr Arnaud Mejean of Paris Descartes University presented findings from the CARMENA trial, which randomized 450 patients with metastatic clear cell RCC to receive cytoreductive nephrectomy followed by sunitinib, or sunitinib alone. The OS results of 18.4 versus 13.9 months, respectively (HR, 0.89) favored sunitinib alone in this noninferiority analysis. Other endpoints lined up in favor of not removing the cancerous kidney, and the presenter and discussants were united in their opinion of the results and the resulting change in doing less surgery in these patients.
In a step away from less therapy, the European Pediatric Soft Tissue Sarcoma Study showed that adding 6 months of low-dose maintenance chemotherapy after standard intensive therapy improves survival in children with high-risk rhabdomyosarcoma. The addition of a vinorelbine and cyclophosphamide low-dose regimen improved 5-year disease-free survival from 69.8% to 77.6% (HR, 0.68) and OS from 73.7% to 86.5% (HR, 0.52) as presented by Dr Gianni Bisogno, University of Padovani, Italy. The maintenance regimen showed no increase in toxicity and actually fewer infections were noted.
In the area of molecular profiling, multiple studies at the meeting demonstrated the importance of assessing cancers for mutations as outstanding results were seen with therapies for NTRK, RET, ROS, and MSI-high driven tumors. In a debate on the role of molecular profiling, I had the opportunity to declare and support our position at Sarah Cannon that all patients with relapsed or metastatic cancers should have this testing performed. It will be through better understanding of the biology of these cancers that we will advance the field for all patients while sometimes finding a target or mutation that will dramatically change the life of a patient.
In keeping with the meeting’s theme, Delivering Discoveries: Expanding the Reach of Precision Medicine, the presentations and the discussions clearly demonstrated that through the use of precision medicine techniques such as prognostic gene assays and molecular profiling, patients can receive the best therapy, even “tailored” therapy, which may often actually be less therapy. It is an exciting time in cancer research, and I have never been more optimistic about the future of cancer treatment for our patients.
A record-setting 40,000-plus oncology professionals attended this year’s annual meeting of the American Society of Clinical Oncology (ASCO) in Chicago. The outstanding education and scientific program, with the theme of Delivering Discoveries: Expanding the Reach of Precision Medicine, was planned and led by ASCO President Dr Bruce Johnson, professor and director of Thoracic Oncology at the Dana Farber Cancer Institute in Boston, and chaired by Sarah Cannon’s Dr David Spigel and Harvard’s Dr Ann Partridge. A recurring finding throughout the meeting was that “less is more” in several key areas of cancer therapy. From small molecules targeting driver mutations across various tumors to the application of immunotherapy in subsets of common cancers, it is clear that more patients are experiencing dramatic results from novel approaches.
A featured plenary session trial was TAILORx, a study of 10,273 women with hormone-receptor–positive, surgically resected breast cancer that had not spread to the lymph nodes, was less than 5 cm, and was not positive for the HER2 gene amplification. This clinical trial was sponsored by the NCI and initiated in 2006. It used the OncotypeDX genetic test to stratify patients into groups of low, intermediate, or high risk for recurrence. The low-risk patients received only hormonal therapy, and the high-risk patients were treated with hormonal therapy plus chemotherapy.
Dr Joseph Sparano, professor of Medicine and Women’s Health at the Albert Einstein College of Medicine in New York, presented the results from the group of 6,700 intermediate risk women who were randomized to receive hormonal therapy alone or in combination with chemotherapy. After 9 years of follow-up, 83.3% of the volunteers, as Dr Sparano appropriately referred to them, who were treated with hormonal therapy were still cancer free, compared with 84.3% of those who also received chemotherapy, demonstrating no statistical benefit for the addition of chemotherapy. Of note, breast cancer experts discussing the trial, including Dr Lisa Carey, professor of Breast Cancer Research at the UNC Lineberger Cancer Institute in Chapel Hill, urged that younger women, under the age of 50, with recurrence scores (RS) toward the higher end of the intermediate risk group (RS, 16-25) should still discuss and consider chemotherapy with their physicians. In summary, all patients fitting the study criteria with low (
These landmark and practice changing results mean that each year about 60,000 women in the United States will be spared the side effects of toxic drugs. These 10,273 study volunteers are true heroes to the women who will be diagnosed with breast cancer in coming years.
In the field of lung cancer, many new trial results using immunotherapy were presented, with the most talked about being single-agent pembrolizumab, a PD1 inhibitor, improving survival over traditional chemotherapy in patients with PD-L1 positive tumors, which comprise the majority of squamous cell and adenocarcinomas of the lung. Also in the plenary, Dr Gilberto Lopes of the Sylvester Cancer Center at the University of Miami, presented these results from the KEYNOTE-042 study. In patients with PD-L1 tumor proportion score (TPS) of >1%, the benefit in overall survival (OS) of pembrolizumab compared with chemotherapy was 16.7 versus 12.1 months, respectively (HR, 0.81). In those patients with a TPS of >20%, the OS benefit was 17.7 versus 1.0 months (HR, 0.77), and in the group with a TPS of >50%, the benefit was 20.0 versus 12.2 months (HR, 0.69). Overall, the quality of life and the occurrence of side effects were substantially better for those patients receiving immunotherapy alone. Other findings presented at the meeting demonstrated the benefit of adding immunotherapy to chemotherapy and of treating with combination immunotherapy (PD-1 and CTLA-4 inhibitors). Many options now exist, much work remains to be done, and accrual to clinical trials is more important than ever.
Another plenary session trial evaluated the benefit of performing a nephrectomy in patients with advanced or metastatic renal cell carcinoma (RCC), a long-held and practiced standard of care. Dr Arnaud Mejean of Paris Descartes University presented findings from the CARMENA trial, which randomized 450 patients with metastatic clear cell RCC to receive cytoreductive nephrectomy followed by sunitinib, or sunitinib alone. The OS results of 18.4 versus 13.9 months, respectively (HR, 0.89) favored sunitinib alone in this noninferiority analysis. Other endpoints lined up in favor of not removing the cancerous kidney, and the presenter and discussants were united in their opinion of the results and the resulting change in doing less surgery in these patients.
In a step away from less therapy, the European Pediatric Soft Tissue Sarcoma Study showed that adding 6 months of low-dose maintenance chemotherapy after standard intensive therapy improves survival in children with high-risk rhabdomyosarcoma. The addition of a vinorelbine and cyclophosphamide low-dose regimen improved 5-year disease-free survival from 69.8% to 77.6% (HR, 0.68) and OS from 73.7% to 86.5% (HR, 0.52) as presented by Dr Gianni Bisogno, University of Padovani, Italy. The maintenance regimen showed no increase in toxicity and actually fewer infections were noted.
In the area of molecular profiling, multiple studies at the meeting demonstrated the importance of assessing cancers for mutations as outstanding results were seen with therapies for NTRK, RET, ROS, and MSI-high driven tumors. In a debate on the role of molecular profiling, I had the opportunity to declare and support our position at Sarah Cannon that all patients with relapsed or metastatic cancers should have this testing performed. It will be through better understanding of the biology of these cancers that we will advance the field for all patients while sometimes finding a target or mutation that will dramatically change the life of a patient.
In keeping with the meeting’s theme, Delivering Discoveries: Expanding the Reach of Precision Medicine, the presentations and the discussions clearly demonstrated that through the use of precision medicine techniques such as prognostic gene assays and molecular profiling, patients can receive the best therapy, even “tailored” therapy, which may often actually be less therapy. It is an exciting time in cancer research, and I have never been more optimistic about the future of cancer treatment for our patients.
A record-setting 40,000-plus oncology professionals attended this year’s annual meeting of the American Society of Clinical Oncology (ASCO) in Chicago. The outstanding education and scientific program, with the theme of Delivering Discoveries: Expanding the Reach of Precision Medicine, was planned and led by ASCO President Dr Bruce Johnson, professor and director of Thoracic Oncology at the Dana Farber Cancer Institute in Boston, and chaired by Sarah Cannon’s Dr David Spigel and Harvard’s Dr Ann Partridge. A recurring finding throughout the meeting was that “less is more” in several key areas of cancer therapy. From small molecules targeting driver mutations across various tumors to the application of immunotherapy in subsets of common cancers, it is clear that more patients are experiencing dramatic results from novel approaches.
A featured plenary session trial was TAILORx, a study of 10,273 women with hormone-receptor–positive, surgically resected breast cancer that had not spread to the lymph nodes, was less than 5 cm, and was not positive for the HER2 gene amplification. This clinical trial was sponsored by the NCI and initiated in 2006. It used the OncotypeDX genetic test to stratify patients into groups of low, intermediate, or high risk for recurrence. The low-risk patients received only hormonal therapy, and the high-risk patients were treated with hormonal therapy plus chemotherapy.
Dr Joseph Sparano, professor of Medicine and Women’s Health at the Albert Einstein College of Medicine in New York, presented the results from the group of 6,700 intermediate risk women who were randomized to receive hormonal therapy alone or in combination with chemotherapy. After 9 years of follow-up, 83.3% of the volunteers, as Dr Sparano appropriately referred to them, who were treated with hormonal therapy were still cancer free, compared with 84.3% of those who also received chemotherapy, demonstrating no statistical benefit for the addition of chemotherapy. Of note, breast cancer experts discussing the trial, including Dr Lisa Carey, professor of Breast Cancer Research at the UNC Lineberger Cancer Institute in Chapel Hill, urged that younger women, under the age of 50, with recurrence scores (RS) toward the higher end of the intermediate risk group (RS, 16-25) should still discuss and consider chemotherapy with their physicians. In summary, all patients fitting the study criteria with low (
These landmark and practice changing results mean that each year about 60,000 women in the United States will be spared the side effects of toxic drugs. These 10,273 study volunteers are true heroes to the women who will be diagnosed with breast cancer in coming years.
In the field of lung cancer, many new trial results using immunotherapy were presented, with the most talked about being single-agent pembrolizumab, a PD1 inhibitor, improving survival over traditional chemotherapy in patients with PD-L1 positive tumors, which comprise the majority of squamous cell and adenocarcinomas of the lung. Also in the plenary, Dr Gilberto Lopes of the Sylvester Cancer Center at the University of Miami, presented these results from the KEYNOTE-042 study. In patients with PD-L1 tumor proportion score (TPS) of >1%, the benefit in overall survival (OS) of pembrolizumab compared with chemotherapy was 16.7 versus 12.1 months, respectively (HR, 0.81). In those patients with a TPS of >20%, the OS benefit was 17.7 versus 1.0 months (HR, 0.77), and in the group with a TPS of >50%, the benefit was 20.0 versus 12.2 months (HR, 0.69). Overall, the quality of life and the occurrence of side effects were substantially better for those patients receiving immunotherapy alone. Other findings presented at the meeting demonstrated the benefit of adding immunotherapy to chemotherapy and of treating with combination immunotherapy (PD-1 and CTLA-4 inhibitors). Many options now exist, much work remains to be done, and accrual to clinical trials is more important than ever.
Another plenary session trial evaluated the benefit of performing a nephrectomy in patients with advanced or metastatic renal cell carcinoma (RCC), a long-held and practiced standard of care. Dr Arnaud Mejean of Paris Descartes University presented findings from the CARMENA trial, which randomized 450 patients with metastatic clear cell RCC to receive cytoreductive nephrectomy followed by sunitinib, or sunitinib alone. The OS results of 18.4 versus 13.9 months, respectively (HR, 0.89) favored sunitinib alone in this noninferiority analysis. Other endpoints lined up in favor of not removing the cancerous kidney, and the presenter and discussants were united in their opinion of the results and the resulting change in doing less surgery in these patients.
In a step away from less therapy, the European Pediatric Soft Tissue Sarcoma Study showed that adding 6 months of low-dose maintenance chemotherapy after standard intensive therapy improves survival in children with high-risk rhabdomyosarcoma. The addition of a vinorelbine and cyclophosphamide low-dose regimen improved 5-year disease-free survival from 69.8% to 77.6% (HR, 0.68) and OS from 73.7% to 86.5% (HR, 0.52) as presented by Dr Gianni Bisogno, University of Padovani, Italy. The maintenance regimen showed no increase in toxicity and actually fewer infections were noted.
In the area of molecular profiling, multiple studies at the meeting demonstrated the importance of assessing cancers for mutations as outstanding results were seen with therapies for NTRK, RET, ROS, and MSI-high driven tumors. In a debate on the role of molecular profiling, I had the opportunity to declare and support our position at Sarah Cannon that all patients with relapsed or metastatic cancers should have this testing performed. It will be through better understanding of the biology of these cancers that we will advance the field for all patients while sometimes finding a target or mutation that will dramatically change the life of a patient.
In keeping with the meeting’s theme, Delivering Discoveries: Expanding the Reach of Precision Medicine, the presentations and the discussions clearly demonstrated that through the use of precision medicine techniques such as prognostic gene assays and molecular profiling, patients can receive the best therapy, even “tailored” therapy, which may often actually be less therapy. It is an exciting time in cancer research, and I have never been more optimistic about the future of cancer treatment for our patients.
Tumor heterogeneity: a central foe in the war on cancer
A major challenge to effective cancer treatment is the astounding level of heterogeneity that tumors display on many different fronts. Here, we discuss how a deeper appreciation of this heterogeneity and its impact is driving research efforts to better understand and tackle it and a radical rethink of treatment paradigms.
A complex and dynamic disease
The nonuniformity of cancer has long been appreciated, reflected most visibly in the variation of response to the same treatment across patients with the same type of tumor (inter-tumor heterogeneity). The extent of tumor heterogeneity is being fully realized only now, with the advent of next-generation sequencing technologies. Even within the same tumor, there can be significant heterogeneity from cell to cell (intra-tumor heterogeneity), yielding substantial complexity in cancer.
Heterogeneity reveals itself on many different levels. Histologically speaking, tumors are composed of a nonhomogenous mass of cells that vary in type and number. In terms of their molecular make-up, there is substantial variation in the types of molecular alterations observed, all the way down to the single cell level. In even more abstract terms, beyond the cancer itself, the microenvironment in which it resides can be highly heterogeneous, composed of a plethora of different supportive and tumor-infiltrating normal cells.
Heterogeneity can manifest spatially, reflecting differences in the composition of the primary tumor and tumors at secondary sites or across regions of the same tumor mass and temporally, at different time points across a tumor’s natural history. Evocative of the second law of thermodynamics, cancers generally become more diverse and complex over time.1-3
A tale of 2 models
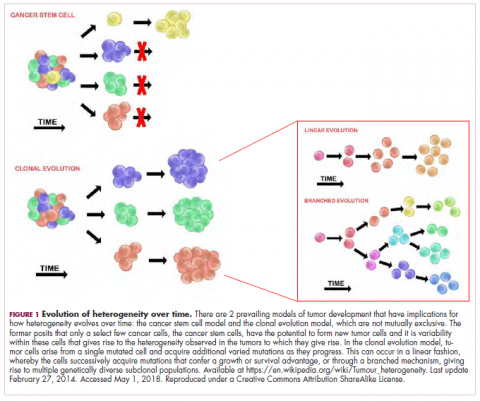
It is widely accepted that the transformation of a normal cell into a malignant one occurs with the acquisition of certain “hallmark” abilities, but there are myriad ways in which these can be attained.
The clonal evolution model
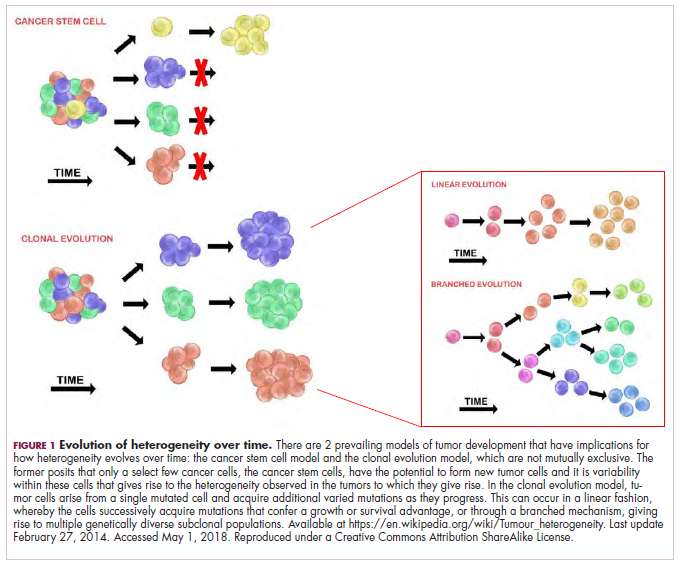
As cells divide, they randomly acquire mutations as a result of DNA damage. The clonal evolution model posits that cancer develops as the result of a multistep accumulation of a series of “driver” mutations that confer a promalignant advantage to the cell and ultimately fuel a cancerous hallmark.
This evolution can occur in a linear fashion, whereby the emergence of a new driver mutation conveys such a potent evolutionary advantage that it outcompetes all previous clones. There is limited evidence for linear evolution in most advanced human cancers; instead, they are thought to evolve predominantly through a process of branching evolution, in which multiple clones can diverge in parallel from a common ancestor through the acquisition of different driver mutations. This results in common clonal mutations that form the trunk of the cancer’s evolutionary tree and are shared by all cells and subclonal mutations, which make up the branches and differ from cell to cell.
More recently, several other mechanisms of clonal evolution have been proposed, including neutral evolution, a type of branching evolution in which there are no selective pressures and evolution occurs by random mutations occurring over time that lead to genetic drift, and punctuated evolution, in which there are short evolutionary bursts of hypermutation.4,5
The CSC model
This model posits that the ability to form and sustain a cancer is restricted to a single cell type – the cancer stem cells – which have the unique capacity for self-renewal and differentiation. Although the forces of evolution are still involved in this model, they act on a hierarchy of cells, with stem cells sitting at the top. A tumor is derived from a single stem cell that has acquired a mutation, and the heterogeneity observed results both from the differentiation and the accumulation of mutations in CSCs.
Accumulated experimental evidence suggests that these models are not mutually exclusive and that they can all contribute to heterogeneity in varied amounts across different tumor types. What is clear is that heterogeneity and evolution are intricately intertwined in cancer development.1,2,6
An unstable genome
Heterogeneity and evolution are fueled by genomic alterations and the genome instability that they foster. This genome instability can range from single base pair substitutions to a doubling of the entire genome and results from both exposure to exogenous mutagens (eg, chemicals and ultraviolet radiation) and genomic alterations that have an impact on important cellular processes (eg, DNA repair or replication).
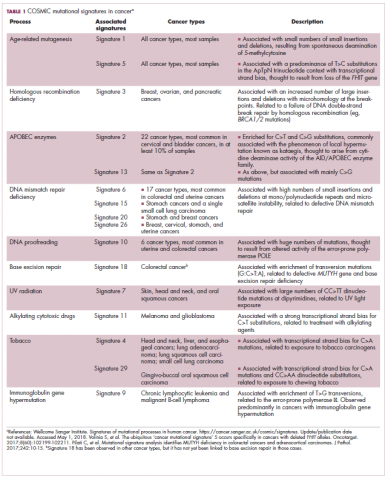
Among the most common causes of genome instability are mutations in the DNA mismatch repair pathway proteins or in the proofreading polymerase enzymes. Genome instability is often associated with unique mutational signatures – characteristic combinations of mutations that arose as the result of the specific biological processes underlying them.7
Genome-wide analyses have begun to reveal these mutational signatures across the spectrum of human cancers. The Wellcome Sanger Institute’s Catalogue of Somatic Mutations in Cancer (COSMIC) database has generated a set of 30 mutational signatures based on analysis of almost 11,000 exomes and more than 1,000 whole genomes spanning 40 different cancer types, some of which have been linked with specific mutagenic processes, such as tobacco, UV radiation, and DNA repair deficiency (Table 1).8
Fueling resistance
Arguably, heterogeneity presents one of the most significant barriers to effective cancer therapy, and this has become increasingly true in the era of personalized medicine in which targeted therapies take aim at specific molecular abnormalities.
It is vital that drugs target the truncal alterations that are present in all cancer cells to ensure that the entire cancer is eradicated. However, it is not always possible to target these alterations, for example, at the present time tumor suppressor proteins like p53 are not druggable.
Even when truncal alterations have been targeted successfully, such as epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) chromosomal rearrangements in non–small-cell lung cancer (NSCLC) and BRAF mutations in melanoma, the long-term efficacy of these drugs is almost invariably limited by the development of resistance.
Tumor heterogeneity and the clonal evolution it fuels are central drivers of resistance. Because tumors are dynamic and continue to evolve, anticancer treatments can act as a strong selective pressure and drive the emergence of drug-resistant subclones that allow the tumor to persist. In fact, study findings have revealed that small populations of resistant cells may be present before treatment. Thus, resistance may also occur as a result of the outgrowth of preexisting treatment-resistant cells that suddenly find that they acquire a survival advantage in the presence of a drug.1,6
Tackling heterogeneity
Despite extensive clinical documentation of the existence of heterogeneity and its underlying mechanisms across a range of tumor types, the development of novel clinical trial designs and therapeutic strategies that account for its effects have only recently begun to be explored.
For the most part, this was because of a lack of effective methods for evaluating intratumor heterogeneity. Multiregion biopsies, in which tissue derived from multiple different regions of a single tumor mass or from distinct cancerous lesions within the same patient, give a snapshot of tumor heterogeneity at a single point in time. The repeated longitudinal sampling required to gain a deeper appreciation of tumor heterogeneity over the course of tumor evolution is often not possible because of the morbidity associated with repeated surgical procedures.
Liquid biopsies, in which DNA sequencing can be performed on tumor components that are found circulating in the blood of cancer patients (including circulating tumor cells and cell-free circulating tumor DNA) have rapidly gained traction in the past several decades and offer an unprecedented opportunity for real-time assessment of evolving tumor heterogeneity.
They have proved to be highly sensitive and specific, with a high degree of concordance with tissue biopsy, they can identify both clonal and subclonal mutations, and they can detect resistance substantially earlier than radiographic imaging, which could permit earlier intervention.10,11 The first liquid biopsy-based companion diagnostic test was approved by the US Food and Drug Administration in 2016, for the detection of EGFR mutations associated with NSCLC.
Yet, even liquid biopsy alone is not able to fully dissect the extent of tumor heterogeneity, especially because it is limited in its ability to assess spatial heterogeneity. Truly effective assessment of tumor heterogeneity is likely to require a combination of liquid biopsy, carefully selected tumor tissue biopsies, imaging diagnostics, and biomarkers.
The ongoing TRACERx (Tracking cancer evolution through therapy [Rx]) trials are evaluating a combination of approaches to follow tumor evolution across the course of treatment. The study in NSCLC began in 2014 with a target enrollment of 842 patients and will follow patients over 6 years. Preliminary data from the first 100 patients were recently published and demonstrated that increased intratumor heterogeneity correlated with increased risk of recurrence or death.12
If patients consent, the TRACERx trials also feed into the PEACE (Posthumous evaluation of advanced cancer environment) trials, which are collecting postmortem biopsies to further evaluate tumor heterogeneity and evolution. TRACERx trials in several other cancer types are now also underway.
Cutting off the source
The main therapeutic strategies for overcoming tumor heterogeneity are focused on the mechanisms of resistance that it drives. It is becoming increasingly apparent that rationally designed combinations of drugs are likely to be required and might need to be administered early in the course of disease to prevent resistance.
However, according to mathematical modeling studies, combinations of at least 3 drugs may be necessary.13 In many cases, this is unlikely to be feasible owing to the unavailability of drugs for certain targets and issues of toxicity, as well as the high cost.
An alternative strategy is to use immunotherapy, because a single treatment can target multiple neoantigens simultaneously. Although immunotherapy has proved to be a highly effective treatment paradigm in multiple tumor types, resistance still arises through varied mechanisms with tumor heterogeneity at their core.14,15
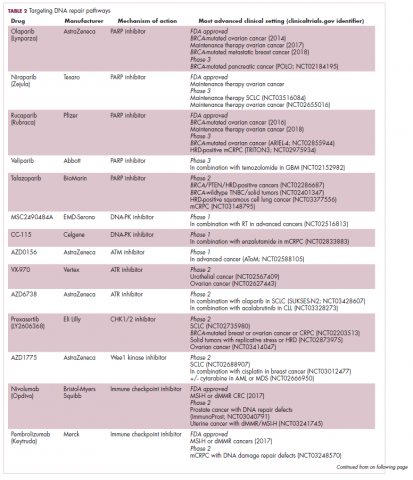
A promising avenue for drug development is to cut off the source of tumor heterogeneity – genomic instability and the mutagenic processes that foster it (Table 2). This is exemplified by the success of poly(ADP-ribose) polymerase (PARP) inhibitors in patients with breast cancer susceptibility (BRCA1/2) gene mutations.
Both germline and somatic mutations in the BRCA1/2 genes are observed in 10% to 15% of patients with ovarian cancer and a substantial number of patients with other types of cancer, including breast, pancreatic, and prostate cancers.16,17
These genes play a central role in the homologous recombination (HR) pathway of DNA repair, which repairs double-strand breaks in DNA. PARP inhibitors target a different DNA repair pathway, base excision repair, which repairs single-strand breaks. The use of PARP inhibitors in patients with BRCA1/2 mutations is designed to create irreparable damage to the DNA repair processes and drive an unsustainable level of genome instability that leads to cell death, whereas normal cells without HR deficiency can survive.18
A growing number of PARP inhibitors are now approved for use in the United States for the treatment of ovarian cancer. In January, olaparib became the first PARP inhibitor approved for patients with BRCA1/2-mutant breast cancer, based on data from the OlympiAD trial in which 302 patients were randomized to receive olaparib 300 mg twice daily or physician’s choice of chemotherapy. Olaparib improved progression-free survival from 4.2 months to 7.0 months (hazard ratio, 0.58; P = .0009), and the most common adverse events included anemia, nausea, fatigue, and vomiting.19
Tumors with other defects in HR have also shown susceptibility to PARP inhibition, shifting interest toward identifying and treating these tumors as a group, independent of histology – about a quarter of all tumors display HR deficiency.20 This novel strategy of targeting mutational processes across a range of tumor types has also been exploited in the development of immunotherapies.
Patients with defects in the mismatch repair (MMR) pathway and microsatellite instability (MSI) – multiple alterations in the length of microsatellite markers within the DNA – are more sensitive to immunotherapy, likely because they are predisposed to a high level of somatic mutations that can serve as neoantigens to provoke a strong anti-tumor immune response.
In 2017, 2 immune checkpoint inhibitors were approved for use in patients with MSI-high or defective MMR (dMMR) cancers. The indication for pembrolizumab (Keytruda) was independent of tumor histology, the first approval of its kind. It was based on the results of 5 clinical trials in which 149 patients with MSI-H or dMMR cancers were given pembrolizumab 200 mg every 3 weeks or 10 mg/kg every 2 weeks for a maximum of 24 months. The overall response rate was 39.6%, including 11 complete responses and 48 partial responses.21
A new paradigm
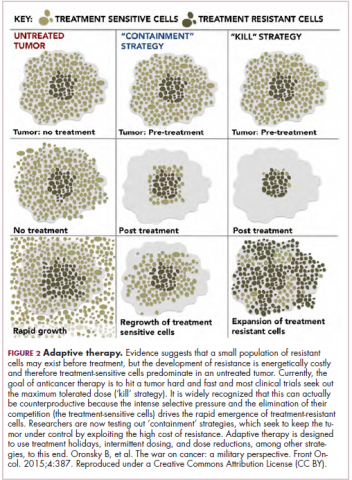
Treatment of a tumor is one of the major selective pressures that shapes its evolution and recent evidence has emerged that these selective pressures can be highly dynamic. Study findings have shown that there is a cost associated with evolution of resistant subclones and, if the selective pressure of therapy is removed, that cost may become too high, such that resistant subclones are then outcompeted by drug-sensitive ones. There have been reports of reversal of drug resistance when drug treatment is interrupted.
The current treatment paradigm is to try to eliminate tumors by hitting them hard and fast with the maximum tolerated dose (MTD) of a drug. However, there is increasing appreciation that this may be inadvertently fostering more rapid disease progression because it selects for the emergence of resistant cells and eliminates all their competitors (Figure 2).
This is driving a potential paradigm shift, in which researchers are applying concepts from evolutionary biology and the control of invasive species to the treatment of cancer. Instead of completely eliminating a cancer, a strategy of adaptive therapy could be used to set up competition between different subclones and keep tumor growth in check by exploiting the high cost of resistance.22
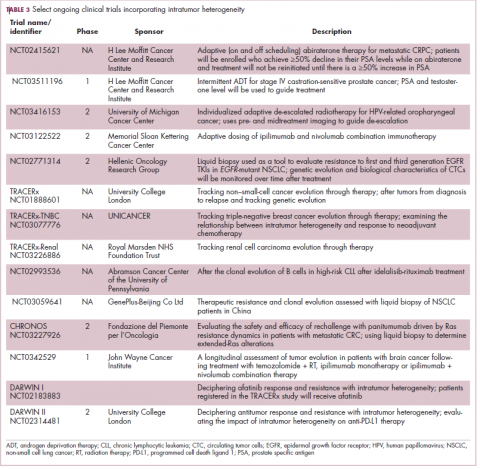
Adaptive therapy involves the use of treatment holidays, intermittent dosing schedules or reduced drug doses, rather than using the MTD. Adaptive therapy was tested recently in mice with triple-negative and estrogen receptor-positive breast cancer. The standard maximum dose of chemotherapy was compared with adaptive therapy with either reduced doses or skipped doses as the tumor responded. Tumor growth initially decreased with all 3 treatment scenarios, but then regrew when chemotherapy was stopped or doses were skipped. However, adaptive therapy with lower doses resulted in long-term stabilization of the tumor where treatment was eventually able to be withdrawn.23 Clinical trials of several different types of adaptive therapy strategies are ongoing (Table 3).
1. Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15(2):81-94.
2. Dzobo K, Senthebane DA, Thomford NE, Rowe A, Dandara C, Parker MI. Not everyone fits the mold: intratumor and intertumor heterogeneity and innovative cancer drug design and development. OMICS. 2018;22(1):17-34.
3. McGranahan N, Swanton C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell. 2017;168(4):613-628.
4. Davis A, Gao R, Navin N. Tumor evolution: linear, branching, neutral or punctuated? Biochim Biophys Acta. 2017;1867(2):151-161.
5. Amirouchene-Angelozzi N, Swanton C, Bardelli A. Tumor evolution as a therapeutic target. Cancer Discov. Published online first July 20, 2017. Accessed May 23, 2018. doi: 10.1158/2159-8290.CD-17-0343
6. Wu D, Wang DC, Cheng Y, et al. Roles of tumor heterogeneity in the development of drug resistance: a call for precision therapy. Semin Cancer Biol. 2017;42:13-19.
7. Ferguson LR, Chen H, Collins AR, et al. Genomic instability in human cancer: molecular insights and opportunities for therapeutic attack and prevention through diet and nutrition. Semin Cancer Biol. 2015;35(suppl):S5-S24.
8. Forbes SA, Beare D, Gunasekaran P, et al. COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015;43(Database issue):D805-811.
9. Rosenthal R, McGranahan N, Herrero J, Swanton C. Deciphering genetic intratumor heterogeneity and its impact on cancer evolution. Ann Rev Cancer Biol. 2017;1(1):223-240.
10. Esposito A, Criscitiello C, Locatelli M, Milano M, Curigliano G. Liquid biopsies for solid tumors: understanding tumor heterogeneity and real time monitoring of early resistance to targeted therapies. Pharmacol Ther. 2016;157:120-124.
11. Venesio T, Siravegna G, Bardelli A, Sapino A. Liquid biopsies for monitoring temporal genomic heterogeneity in breast and colon cancers. Pathobiology. 2018;85(1-2):146-154.
12. Jamal-Hanjani M, Wilson GA, McGranahan N, et al. Tracking the evolution of non–small-cell lung cancer. New Engl J Med. 2017;376(22):2109-2121.
13. Bozic I, Reiter JG, Allen B, et al. Evolutionary dynamics of cancer in response to targeted combination therapy. Elife. 2013;2:e00747.
14. Zugazagoitia J, Guedes C, Ponce S, Ferrer I, Molina-Pinelo S, Paz-Ares L. Current challenges in cancer treatment. Clin Ther. 2016;38(7):1551-1566.
15. Ventola CL. Cancer immunotherapy, Part 3: challenges and future trends. PT. 2017;42(8):514-521.
16. Cavanagh H, Rogers KMA. The role of BRCA1 and BRCA2 mutations in prostate, pancreatic and stomach cancers. Hered Cancer Clin Pract. 2015;13:16.
17. Moschetta M, George A, Kaye SB, Banerjee S. BRCA somatic mutations and epigenetic BRCA modifications in serous ovarian cancer. Ann Oncol. 2016;27(8):1449-1455.
18. Brown JS, O’Carrigan B, Jackson SP, Yap TA. Targeting DNA repair in cancer: beyond PARP inhibitors. Cancer Discov. 2017;7(1):20-37.
19. Robson M, Im S-A, Senkus E, et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. New England Journal of Medicine. 2017;377(6):523-533.
20. Williers H, Pfaffle HN, Zou L. Targeting homologous recombination repair in cancer: molecular targets and clinical applications. In: Kelley M, Fishel M, eds. DNA repair in cancer therapy. 2nd ed: Academic Press; 2016:119-160.
21. U.S. Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. 2017; https://www.fda.gov/Drugs/InformationOnDrugs/ ApprovedDrugs/ucm560040.htm. Accessed May 1st,, 2018.
22. Gallaher JA, Enriquez-Navas PM, Luddy KA, Gatenby RA, Anderson ARA. Adaptive Therapy For Heterogeneous Cancer: Exploiting Space And Trade-Offs In Drug Scheduling. bioRxiv. 2017.
23. Enriquez-Navas PM, Kam Y, Das T, et al. Exploiting evolutionary principles to prolong tumor control in preclinical models of breast cancer. Sci Transl Med. 2016;8(327):327ra24.
A major challenge to effective cancer treatment is the astounding level of heterogeneity that tumors display on many different fronts. Here, we discuss how a deeper appreciation of this heterogeneity and its impact is driving research efforts to better understand and tackle it and a radical rethink of treatment paradigms.
A complex and dynamic disease
The nonuniformity of cancer has long been appreciated, reflected most visibly in the variation of response to the same treatment across patients with the same type of tumor (inter-tumor heterogeneity). The extent of tumor heterogeneity is being fully realized only now, with the advent of next-generation sequencing technologies. Even within the same tumor, there can be significant heterogeneity from cell to cell (intra-tumor heterogeneity), yielding substantial complexity in cancer.
Heterogeneity reveals itself on many different levels. Histologically speaking, tumors are composed of a nonhomogenous mass of cells that vary in type and number. In terms of their molecular make-up, there is substantial variation in the types of molecular alterations observed, all the way down to the single cell level. In even more abstract terms, beyond the cancer itself, the microenvironment in which it resides can be highly heterogeneous, composed of a plethora of different supportive and tumor-infiltrating normal cells.
Heterogeneity can manifest spatially, reflecting differences in the composition of the primary tumor and tumors at secondary sites or across regions of the same tumor mass and temporally, at different time points across a tumor’s natural history. Evocative of the second law of thermodynamics, cancers generally become more diverse and complex over time.1-3
A tale of 2 models
It is widely accepted that the transformation of a normal cell into a malignant one occurs with the acquisition of certain “hallmark” abilities, but there are myriad ways in which these can be attained.
The clonal evolution model
As cells divide, they randomly acquire mutations as a result of DNA damage. The clonal evolution model posits that cancer develops as the result of a multistep accumulation of a series of “driver” mutations that confer a promalignant advantage to the cell and ultimately fuel a cancerous hallmark.
This evolution can occur in a linear fashion, whereby the emergence of a new driver mutation conveys such a potent evolutionary advantage that it outcompetes all previous clones. There is limited evidence for linear evolution in most advanced human cancers; instead, they are thought to evolve predominantly through a process of branching evolution, in which multiple clones can diverge in parallel from a common ancestor through the acquisition of different driver mutations. This results in common clonal mutations that form the trunk of the cancer’s evolutionary tree and are shared by all cells and subclonal mutations, which make up the branches and differ from cell to cell.
More recently, several other mechanisms of clonal evolution have been proposed, including neutral evolution, a type of branching evolution in which there are no selective pressures and evolution occurs by random mutations occurring over time that lead to genetic drift, and punctuated evolution, in which there are short evolutionary bursts of hypermutation.4,5
The CSC model
This model posits that the ability to form and sustain a cancer is restricted to a single cell type – the cancer stem cells – which have the unique capacity for self-renewal and differentiation. Although the forces of evolution are still involved in this model, they act on a hierarchy of cells, with stem cells sitting at the top. A tumor is derived from a single stem cell that has acquired a mutation, and the heterogeneity observed results both from the differentiation and the accumulation of mutations in CSCs.
Accumulated experimental evidence suggests that these models are not mutually exclusive and that they can all contribute to heterogeneity in varied amounts across different tumor types. What is clear is that heterogeneity and evolution are intricately intertwined in cancer development.1,2,6
An unstable genome
Heterogeneity and evolution are fueled by genomic alterations and the genome instability that they foster. This genome instability can range from single base pair substitutions to a doubling of the entire genome and results from both exposure to exogenous mutagens (eg, chemicals and ultraviolet radiation) and genomic alterations that have an impact on important cellular processes (eg, DNA repair or replication).
Among the most common causes of genome instability are mutations in the DNA mismatch repair pathway proteins or in the proofreading polymerase enzymes. Genome instability is often associated with unique mutational signatures – characteristic combinations of mutations that arose as the result of the specific biological processes underlying them.7
Genome-wide analyses have begun to reveal these mutational signatures across the spectrum of human cancers. The Wellcome Sanger Institute’s Catalogue of Somatic Mutations in Cancer (COSMIC) database has generated a set of 30 mutational signatures based on analysis of almost 11,000 exomes and more than 1,000 whole genomes spanning 40 different cancer types, some of which have been linked with specific mutagenic processes, such as tobacco, UV radiation, and DNA repair deficiency (Table 1).8
Fueling resistance
Arguably, heterogeneity presents one of the most significant barriers to effective cancer therapy, and this has become increasingly true in the era of personalized medicine in which targeted therapies take aim at specific molecular abnormalities.
It is vital that drugs target the truncal alterations that are present in all cancer cells to ensure that the entire cancer is eradicated. However, it is not always possible to target these alterations, for example, at the present time tumor suppressor proteins like p53 are not druggable.
Even when truncal alterations have been targeted successfully, such as epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) chromosomal rearrangements in non–small-cell lung cancer (NSCLC) and BRAF mutations in melanoma, the long-term efficacy of these drugs is almost invariably limited by the development of resistance.
Tumor heterogeneity and the clonal evolution it fuels are central drivers of resistance. Because tumors are dynamic and continue to evolve, anticancer treatments can act as a strong selective pressure and drive the emergence of drug-resistant subclones that allow the tumor to persist. In fact, study findings have revealed that small populations of resistant cells may be present before treatment. Thus, resistance may also occur as a result of the outgrowth of preexisting treatment-resistant cells that suddenly find that they acquire a survival advantage in the presence of a drug.1,6
Tackling heterogeneity
Despite extensive clinical documentation of the existence of heterogeneity and its underlying mechanisms across a range of tumor types, the development of novel clinical trial designs and therapeutic strategies that account for its effects have only recently begun to be explored.
For the most part, this was because of a lack of effective methods for evaluating intratumor heterogeneity. Multiregion biopsies, in which tissue derived from multiple different regions of a single tumor mass or from distinct cancerous lesions within the same patient, give a snapshot of tumor heterogeneity at a single point in time. The repeated longitudinal sampling required to gain a deeper appreciation of tumor heterogeneity over the course of tumor evolution is often not possible because of the morbidity associated with repeated surgical procedures.
Liquid biopsies, in which DNA sequencing can be performed on tumor components that are found circulating in the blood of cancer patients (including circulating tumor cells and cell-free circulating tumor DNA) have rapidly gained traction in the past several decades and offer an unprecedented opportunity for real-time assessment of evolving tumor heterogeneity.
They have proved to be highly sensitive and specific, with a high degree of concordance with tissue biopsy, they can identify both clonal and subclonal mutations, and they can detect resistance substantially earlier than radiographic imaging, which could permit earlier intervention.10,11 The first liquid biopsy-based companion diagnostic test was approved by the US Food and Drug Administration in 2016, for the detection of EGFR mutations associated with NSCLC.
Yet, even liquid biopsy alone is not able to fully dissect the extent of tumor heterogeneity, especially because it is limited in its ability to assess spatial heterogeneity. Truly effective assessment of tumor heterogeneity is likely to require a combination of liquid biopsy, carefully selected tumor tissue biopsies, imaging diagnostics, and biomarkers.
The ongoing TRACERx (Tracking cancer evolution through therapy [Rx]) trials are evaluating a combination of approaches to follow tumor evolution across the course of treatment. The study in NSCLC began in 2014 with a target enrollment of 842 patients and will follow patients over 6 years. Preliminary data from the first 100 patients were recently published and demonstrated that increased intratumor heterogeneity correlated with increased risk of recurrence or death.12
If patients consent, the TRACERx trials also feed into the PEACE (Posthumous evaluation of advanced cancer environment) trials, which are collecting postmortem biopsies to further evaluate tumor heterogeneity and evolution. TRACERx trials in several other cancer types are now also underway.
Cutting off the source
The main therapeutic strategies for overcoming tumor heterogeneity are focused on the mechanisms of resistance that it drives. It is becoming increasingly apparent that rationally designed combinations of drugs are likely to be required and might need to be administered early in the course of disease to prevent resistance.
However, according to mathematical modeling studies, combinations of at least 3 drugs may be necessary.13 In many cases, this is unlikely to be feasible owing to the unavailability of drugs for certain targets and issues of toxicity, as well as the high cost.
An alternative strategy is to use immunotherapy, because a single treatment can target multiple neoantigens simultaneously. Although immunotherapy has proved to be a highly effective treatment paradigm in multiple tumor types, resistance still arises through varied mechanisms with tumor heterogeneity at their core.14,15
A promising avenue for drug development is to cut off the source of tumor heterogeneity – genomic instability and the mutagenic processes that foster it (Table 2). This is exemplified by the success of poly(ADP-ribose) polymerase (PARP) inhibitors in patients with breast cancer susceptibility (BRCA1/2) gene mutations.
Both germline and somatic mutations in the BRCA1/2 genes are observed in 10% to 15% of patients with ovarian cancer and a substantial number of patients with other types of cancer, including breast, pancreatic, and prostate cancers.16,17
These genes play a central role in the homologous recombination (HR) pathway of DNA repair, which repairs double-strand breaks in DNA. PARP inhibitors target a different DNA repair pathway, base excision repair, which repairs single-strand breaks. The use of PARP inhibitors in patients with BRCA1/2 mutations is designed to create irreparable damage to the DNA repair processes and drive an unsustainable level of genome instability that leads to cell death, whereas normal cells without HR deficiency can survive.18
A growing number of PARP inhibitors are now approved for use in the United States for the treatment of ovarian cancer. In January, olaparib became the first PARP inhibitor approved for patients with BRCA1/2-mutant breast cancer, based on data from the OlympiAD trial in which 302 patients were randomized to receive olaparib 300 mg twice daily or physician’s choice of chemotherapy. Olaparib improved progression-free survival from 4.2 months to 7.0 months (hazard ratio, 0.58; P = .0009), and the most common adverse events included anemia, nausea, fatigue, and vomiting.19
Tumors with other defects in HR have also shown susceptibility to PARP inhibition, shifting interest toward identifying and treating these tumors as a group, independent of histology – about a quarter of all tumors display HR deficiency.20 This novel strategy of targeting mutational processes across a range of tumor types has also been exploited in the development of immunotherapies.
Patients with defects in the mismatch repair (MMR) pathway and microsatellite instability (MSI) – multiple alterations in the length of microsatellite markers within the DNA – are more sensitive to immunotherapy, likely because they are predisposed to a high level of somatic mutations that can serve as neoantigens to provoke a strong anti-tumor immune response.
In 2017, 2 immune checkpoint inhibitors were approved for use in patients with MSI-high or defective MMR (dMMR) cancers. The indication for pembrolizumab (Keytruda) was independent of tumor histology, the first approval of its kind. It was based on the results of 5 clinical trials in which 149 patients with MSI-H or dMMR cancers were given pembrolizumab 200 mg every 3 weeks or 10 mg/kg every 2 weeks for a maximum of 24 months. The overall response rate was 39.6%, including 11 complete responses and 48 partial responses.21
A new paradigm
Treatment of a tumor is one of the major selective pressures that shapes its evolution and recent evidence has emerged that these selective pressures can be highly dynamic. Study findings have shown that there is a cost associated with evolution of resistant subclones and, if the selective pressure of therapy is removed, that cost may become too high, such that resistant subclones are then outcompeted by drug-sensitive ones. There have been reports of reversal of drug resistance when drug treatment is interrupted.
The current treatment paradigm is to try to eliminate tumors by hitting them hard and fast with the maximum tolerated dose (MTD) of a drug. However, there is increasing appreciation that this may be inadvertently fostering more rapid disease progression because it selects for the emergence of resistant cells and eliminates all their competitors (Figure 2).
This is driving a potential paradigm shift, in which researchers are applying concepts from evolutionary biology and the control of invasive species to the treatment of cancer. Instead of completely eliminating a cancer, a strategy of adaptive therapy could be used to set up competition between different subclones and keep tumor growth in check by exploiting the high cost of resistance.22
Adaptive therapy involves the use of treatment holidays, intermittent dosing schedules or reduced drug doses, rather than using the MTD. Adaptive therapy was tested recently in mice with triple-negative and estrogen receptor-positive breast cancer. The standard maximum dose of chemotherapy was compared with adaptive therapy with either reduced doses or skipped doses as the tumor responded. Tumor growth initially decreased with all 3 treatment scenarios, but then regrew when chemotherapy was stopped or doses were skipped. However, adaptive therapy with lower doses resulted in long-term stabilization of the tumor where treatment was eventually able to be withdrawn.23 Clinical trials of several different types of adaptive therapy strategies are ongoing (Table 3).
A major challenge to effective cancer treatment is the astounding level of heterogeneity that tumors display on many different fronts. Here, we discuss how a deeper appreciation of this heterogeneity and its impact is driving research efforts to better understand and tackle it and a radical rethink of treatment paradigms.
A complex and dynamic disease
The nonuniformity of cancer has long been appreciated, reflected most visibly in the variation of response to the same treatment across patients with the same type of tumor (inter-tumor heterogeneity). The extent of tumor heterogeneity is being fully realized only now, with the advent of next-generation sequencing technologies. Even within the same tumor, there can be significant heterogeneity from cell to cell (intra-tumor heterogeneity), yielding substantial complexity in cancer.
Heterogeneity reveals itself on many different levels. Histologically speaking, tumors are composed of a nonhomogenous mass of cells that vary in type and number. In terms of their molecular make-up, there is substantial variation in the types of molecular alterations observed, all the way down to the single cell level. In even more abstract terms, beyond the cancer itself, the microenvironment in which it resides can be highly heterogeneous, composed of a plethora of different supportive and tumor-infiltrating normal cells.
Heterogeneity can manifest spatially, reflecting differences in the composition of the primary tumor and tumors at secondary sites or across regions of the same tumor mass and temporally, at different time points across a tumor’s natural history. Evocative of the second law of thermodynamics, cancers generally become more diverse and complex over time.1-3
A tale of 2 models
It is widely accepted that the transformation of a normal cell into a malignant one occurs with the acquisition of certain “hallmark” abilities, but there are myriad ways in which these can be attained.
The clonal evolution model
As cells divide, they randomly acquire mutations as a result of DNA damage. The clonal evolution model posits that cancer develops as the result of a multistep accumulation of a series of “driver” mutations that confer a promalignant advantage to the cell and ultimately fuel a cancerous hallmark.
This evolution can occur in a linear fashion, whereby the emergence of a new driver mutation conveys such a potent evolutionary advantage that it outcompetes all previous clones. There is limited evidence for linear evolution in most advanced human cancers; instead, they are thought to evolve predominantly through a process of branching evolution, in which multiple clones can diverge in parallel from a common ancestor through the acquisition of different driver mutations. This results in common clonal mutations that form the trunk of the cancer’s evolutionary tree and are shared by all cells and subclonal mutations, which make up the branches and differ from cell to cell.
More recently, several other mechanisms of clonal evolution have been proposed, including neutral evolution, a type of branching evolution in which there are no selective pressures and evolution occurs by random mutations occurring over time that lead to genetic drift, and punctuated evolution, in which there are short evolutionary bursts of hypermutation.4,5
The CSC model
This model posits that the ability to form and sustain a cancer is restricted to a single cell type – the cancer stem cells – which have the unique capacity for self-renewal and differentiation. Although the forces of evolution are still involved in this model, they act on a hierarchy of cells, with stem cells sitting at the top. A tumor is derived from a single stem cell that has acquired a mutation, and the heterogeneity observed results both from the differentiation and the accumulation of mutations in CSCs.
Accumulated experimental evidence suggests that these models are not mutually exclusive and that they can all contribute to heterogeneity in varied amounts across different tumor types. What is clear is that heterogeneity and evolution are intricately intertwined in cancer development.1,2,6
An unstable genome
Heterogeneity and evolution are fueled by genomic alterations and the genome instability that they foster. This genome instability can range from single base pair substitutions to a doubling of the entire genome and results from both exposure to exogenous mutagens (eg, chemicals and ultraviolet radiation) and genomic alterations that have an impact on important cellular processes (eg, DNA repair or replication).
Among the most common causes of genome instability are mutations in the DNA mismatch repair pathway proteins or in the proofreading polymerase enzymes. Genome instability is often associated with unique mutational signatures – characteristic combinations of mutations that arose as the result of the specific biological processes underlying them.7
Genome-wide analyses have begun to reveal these mutational signatures across the spectrum of human cancers. The Wellcome Sanger Institute’s Catalogue of Somatic Mutations in Cancer (COSMIC) database has generated a set of 30 mutational signatures based on analysis of almost 11,000 exomes and more than 1,000 whole genomes spanning 40 different cancer types, some of which have been linked with specific mutagenic processes, such as tobacco, UV radiation, and DNA repair deficiency (Table 1).8
Fueling resistance
Arguably, heterogeneity presents one of the most significant barriers to effective cancer therapy, and this has become increasingly true in the era of personalized medicine in which targeted therapies take aim at specific molecular abnormalities.
It is vital that drugs target the truncal alterations that are present in all cancer cells to ensure that the entire cancer is eradicated. However, it is not always possible to target these alterations, for example, at the present time tumor suppressor proteins like p53 are not druggable.
Even when truncal alterations have been targeted successfully, such as epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) chromosomal rearrangements in non–small-cell lung cancer (NSCLC) and BRAF mutations in melanoma, the long-term efficacy of these drugs is almost invariably limited by the development of resistance.
Tumor heterogeneity and the clonal evolution it fuels are central drivers of resistance. Because tumors are dynamic and continue to evolve, anticancer treatments can act as a strong selective pressure and drive the emergence of drug-resistant subclones that allow the tumor to persist. In fact, study findings have revealed that small populations of resistant cells may be present before treatment. Thus, resistance may also occur as a result of the outgrowth of preexisting treatment-resistant cells that suddenly find that they acquire a survival advantage in the presence of a drug.1,6
Tackling heterogeneity
Despite extensive clinical documentation of the existence of heterogeneity and its underlying mechanisms across a range of tumor types, the development of novel clinical trial designs and therapeutic strategies that account for its effects have only recently begun to be explored.
For the most part, this was because of a lack of effective methods for evaluating intratumor heterogeneity. Multiregion biopsies, in which tissue derived from multiple different regions of a single tumor mass or from distinct cancerous lesions within the same patient, give a snapshot of tumor heterogeneity at a single point in time. The repeated longitudinal sampling required to gain a deeper appreciation of tumor heterogeneity over the course of tumor evolution is often not possible because of the morbidity associated with repeated surgical procedures.
Liquid biopsies, in which DNA sequencing can be performed on tumor components that are found circulating in the blood of cancer patients (including circulating tumor cells and cell-free circulating tumor DNA) have rapidly gained traction in the past several decades and offer an unprecedented opportunity for real-time assessment of evolving tumor heterogeneity.
They have proved to be highly sensitive and specific, with a high degree of concordance with tissue biopsy, they can identify both clonal and subclonal mutations, and they can detect resistance substantially earlier than radiographic imaging, which could permit earlier intervention.10,11 The first liquid biopsy-based companion diagnostic test was approved by the US Food and Drug Administration in 2016, for the detection of EGFR mutations associated with NSCLC.
Yet, even liquid biopsy alone is not able to fully dissect the extent of tumor heterogeneity, especially because it is limited in its ability to assess spatial heterogeneity. Truly effective assessment of tumor heterogeneity is likely to require a combination of liquid biopsy, carefully selected tumor tissue biopsies, imaging diagnostics, and biomarkers.
The ongoing TRACERx (Tracking cancer evolution through therapy [Rx]) trials are evaluating a combination of approaches to follow tumor evolution across the course of treatment. The study in NSCLC began in 2014 with a target enrollment of 842 patients and will follow patients over 6 years. Preliminary data from the first 100 patients were recently published and demonstrated that increased intratumor heterogeneity correlated with increased risk of recurrence or death.12
If patients consent, the TRACERx trials also feed into the PEACE (Posthumous evaluation of advanced cancer environment) trials, which are collecting postmortem biopsies to further evaluate tumor heterogeneity and evolution. TRACERx trials in several other cancer types are now also underway.
Cutting off the source
The main therapeutic strategies for overcoming tumor heterogeneity are focused on the mechanisms of resistance that it drives. It is becoming increasingly apparent that rationally designed combinations of drugs are likely to be required and might need to be administered early in the course of disease to prevent resistance.
However, according to mathematical modeling studies, combinations of at least 3 drugs may be necessary.13 In many cases, this is unlikely to be feasible owing to the unavailability of drugs for certain targets and issues of toxicity, as well as the high cost.
An alternative strategy is to use immunotherapy, because a single treatment can target multiple neoantigens simultaneously. Although immunotherapy has proved to be a highly effective treatment paradigm in multiple tumor types, resistance still arises through varied mechanisms with tumor heterogeneity at their core.14,15
A promising avenue for drug development is to cut off the source of tumor heterogeneity – genomic instability and the mutagenic processes that foster it (Table 2). This is exemplified by the success of poly(ADP-ribose) polymerase (PARP) inhibitors in patients with breast cancer susceptibility (BRCA1/2) gene mutations.
Both germline and somatic mutations in the BRCA1/2 genes are observed in 10% to 15% of patients with ovarian cancer and a substantial number of patients with other types of cancer, including breast, pancreatic, and prostate cancers.16,17
These genes play a central role in the homologous recombination (HR) pathway of DNA repair, which repairs double-strand breaks in DNA. PARP inhibitors target a different DNA repair pathway, base excision repair, which repairs single-strand breaks. The use of PARP inhibitors in patients with BRCA1/2 mutations is designed to create irreparable damage to the DNA repair processes and drive an unsustainable level of genome instability that leads to cell death, whereas normal cells without HR deficiency can survive.18
A growing number of PARP inhibitors are now approved for use in the United States for the treatment of ovarian cancer. In January, olaparib became the first PARP inhibitor approved for patients with BRCA1/2-mutant breast cancer, based on data from the OlympiAD trial in which 302 patients were randomized to receive olaparib 300 mg twice daily or physician’s choice of chemotherapy. Olaparib improved progression-free survival from 4.2 months to 7.0 months (hazard ratio, 0.58; P = .0009), and the most common adverse events included anemia, nausea, fatigue, and vomiting.19
Tumors with other defects in HR have also shown susceptibility to PARP inhibition, shifting interest toward identifying and treating these tumors as a group, independent of histology – about a quarter of all tumors display HR deficiency.20 This novel strategy of targeting mutational processes across a range of tumor types has also been exploited in the development of immunotherapies.
Patients with defects in the mismatch repair (MMR) pathway and microsatellite instability (MSI) – multiple alterations in the length of microsatellite markers within the DNA – are more sensitive to immunotherapy, likely because they are predisposed to a high level of somatic mutations that can serve as neoantigens to provoke a strong anti-tumor immune response.
In 2017, 2 immune checkpoint inhibitors were approved for use in patients with MSI-high or defective MMR (dMMR) cancers. The indication for pembrolizumab (Keytruda) was independent of tumor histology, the first approval of its kind. It was based on the results of 5 clinical trials in which 149 patients with MSI-H or dMMR cancers were given pembrolizumab 200 mg every 3 weeks or 10 mg/kg every 2 weeks for a maximum of 24 months. The overall response rate was 39.6%, including 11 complete responses and 48 partial responses.21
A new paradigm
Treatment of a tumor is one of the major selective pressures that shapes its evolution and recent evidence has emerged that these selective pressures can be highly dynamic. Study findings have shown that there is a cost associated with evolution of resistant subclones and, if the selective pressure of therapy is removed, that cost may become too high, such that resistant subclones are then outcompeted by drug-sensitive ones. There have been reports of reversal of drug resistance when drug treatment is interrupted.
The current treatment paradigm is to try to eliminate tumors by hitting them hard and fast with the maximum tolerated dose (MTD) of a drug. However, there is increasing appreciation that this may be inadvertently fostering more rapid disease progression because it selects for the emergence of resistant cells and eliminates all their competitors (Figure 2).
This is driving a potential paradigm shift, in which researchers are applying concepts from evolutionary biology and the control of invasive species to the treatment of cancer. Instead of completely eliminating a cancer, a strategy of adaptive therapy could be used to set up competition between different subclones and keep tumor growth in check by exploiting the high cost of resistance.22
Adaptive therapy involves the use of treatment holidays, intermittent dosing schedules or reduced drug doses, rather than using the MTD. Adaptive therapy was tested recently in mice with triple-negative and estrogen receptor-positive breast cancer. The standard maximum dose of chemotherapy was compared with adaptive therapy with either reduced doses or skipped doses as the tumor responded. Tumor growth initially decreased with all 3 treatment scenarios, but then regrew when chemotherapy was stopped or doses were skipped. However, adaptive therapy with lower doses resulted in long-term stabilization of the tumor where treatment was eventually able to be withdrawn.23 Clinical trials of several different types of adaptive therapy strategies are ongoing (Table 3).
1. Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15(2):81-94.
2. Dzobo K, Senthebane DA, Thomford NE, Rowe A, Dandara C, Parker MI. Not everyone fits the mold: intratumor and intertumor heterogeneity and innovative cancer drug design and development. OMICS. 2018;22(1):17-34.
3. McGranahan N, Swanton C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell. 2017;168(4):613-628.
4. Davis A, Gao R, Navin N. Tumor evolution: linear, branching, neutral or punctuated? Biochim Biophys Acta. 2017;1867(2):151-161.
5. Amirouchene-Angelozzi N, Swanton C, Bardelli A. Tumor evolution as a therapeutic target. Cancer Discov. Published online first July 20, 2017. Accessed May 23, 2018. doi: 10.1158/2159-8290.CD-17-0343
6. Wu D, Wang DC, Cheng Y, et al. Roles of tumor heterogeneity in the development of drug resistance: a call for precision therapy. Semin Cancer Biol. 2017;42:13-19.
7. Ferguson LR, Chen H, Collins AR, et al. Genomic instability in human cancer: molecular insights and opportunities for therapeutic attack and prevention through diet and nutrition. Semin Cancer Biol. 2015;35(suppl):S5-S24.
8. Forbes SA, Beare D, Gunasekaran P, et al. COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015;43(Database issue):D805-811.
9. Rosenthal R, McGranahan N, Herrero J, Swanton C. Deciphering genetic intratumor heterogeneity and its impact on cancer evolution. Ann Rev Cancer Biol. 2017;1(1):223-240.
10. Esposito A, Criscitiello C, Locatelli M, Milano M, Curigliano G. Liquid biopsies for solid tumors: understanding tumor heterogeneity and real time monitoring of early resistance to targeted therapies. Pharmacol Ther. 2016;157:120-124.
11. Venesio T, Siravegna G, Bardelli A, Sapino A. Liquid biopsies for monitoring temporal genomic heterogeneity in breast and colon cancers. Pathobiology. 2018;85(1-2):146-154.
12. Jamal-Hanjani M, Wilson GA, McGranahan N, et al. Tracking the evolution of non–small-cell lung cancer. New Engl J Med. 2017;376(22):2109-2121.
13. Bozic I, Reiter JG, Allen B, et al. Evolutionary dynamics of cancer in response to targeted combination therapy. Elife. 2013;2:e00747.
14. Zugazagoitia J, Guedes C, Ponce S, Ferrer I, Molina-Pinelo S, Paz-Ares L. Current challenges in cancer treatment. Clin Ther. 2016;38(7):1551-1566.
15. Ventola CL. Cancer immunotherapy, Part 3: challenges and future trends. PT. 2017;42(8):514-521.
16. Cavanagh H, Rogers KMA. The role of BRCA1 and BRCA2 mutations in prostate, pancreatic and stomach cancers. Hered Cancer Clin Pract. 2015;13:16.
17. Moschetta M, George A, Kaye SB, Banerjee S. BRCA somatic mutations and epigenetic BRCA modifications in serous ovarian cancer. Ann Oncol. 2016;27(8):1449-1455.
18. Brown JS, O’Carrigan B, Jackson SP, Yap TA. Targeting DNA repair in cancer: beyond PARP inhibitors. Cancer Discov. 2017;7(1):20-37.
19. Robson M, Im S-A, Senkus E, et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. New England Journal of Medicine. 2017;377(6):523-533.
20. Williers H, Pfaffle HN, Zou L. Targeting homologous recombination repair in cancer: molecular targets and clinical applications. In: Kelley M, Fishel M, eds. DNA repair in cancer therapy. 2nd ed: Academic Press; 2016:119-160.
21. U.S. Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. 2017; https://www.fda.gov/Drugs/InformationOnDrugs/ ApprovedDrugs/ucm560040.htm. Accessed May 1st,, 2018.
22. Gallaher JA, Enriquez-Navas PM, Luddy KA, Gatenby RA, Anderson ARA. Adaptive Therapy For Heterogeneous Cancer: Exploiting Space And Trade-Offs In Drug Scheduling. bioRxiv. 2017.
23. Enriquez-Navas PM, Kam Y, Das T, et al. Exploiting evolutionary principles to prolong tumor control in preclinical models of breast cancer. Sci Transl Med. 2016;8(327):327ra24.
1. Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15(2):81-94.
2. Dzobo K, Senthebane DA, Thomford NE, Rowe A, Dandara C, Parker MI. Not everyone fits the mold: intratumor and intertumor heterogeneity and innovative cancer drug design and development. OMICS. 2018;22(1):17-34.
3. McGranahan N, Swanton C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell. 2017;168(4):613-628.
4. Davis A, Gao R, Navin N. Tumor evolution: linear, branching, neutral or punctuated? Biochim Biophys Acta. 2017;1867(2):151-161.
5. Amirouchene-Angelozzi N, Swanton C, Bardelli A. Tumor evolution as a therapeutic target. Cancer Discov. Published online first July 20, 2017. Accessed May 23, 2018. doi: 10.1158/2159-8290.CD-17-0343
6. Wu D, Wang DC, Cheng Y, et al. Roles of tumor heterogeneity in the development of drug resistance: a call for precision therapy. Semin Cancer Biol. 2017;42:13-19.
7. Ferguson LR, Chen H, Collins AR, et al. Genomic instability in human cancer: molecular insights and opportunities for therapeutic attack and prevention through diet and nutrition. Semin Cancer Biol. 2015;35(suppl):S5-S24.
8. Forbes SA, Beare D, Gunasekaran P, et al. COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015;43(Database issue):D805-811.
9. Rosenthal R, McGranahan N, Herrero J, Swanton C. Deciphering genetic intratumor heterogeneity and its impact on cancer evolution. Ann Rev Cancer Biol. 2017;1(1):223-240.
10. Esposito A, Criscitiello C, Locatelli M, Milano M, Curigliano G. Liquid biopsies for solid tumors: understanding tumor heterogeneity and real time monitoring of early resistance to targeted therapies. Pharmacol Ther. 2016;157:120-124.
11. Venesio T, Siravegna G, Bardelli A, Sapino A. Liquid biopsies for monitoring temporal genomic heterogeneity in breast and colon cancers. Pathobiology. 2018;85(1-2):146-154.
12. Jamal-Hanjani M, Wilson GA, McGranahan N, et al. Tracking the evolution of non–small-cell lung cancer. New Engl J Med. 2017;376(22):2109-2121.
13. Bozic I, Reiter JG, Allen B, et al. Evolutionary dynamics of cancer in response to targeted combination therapy. Elife. 2013;2:e00747.
14. Zugazagoitia J, Guedes C, Ponce S, Ferrer I, Molina-Pinelo S, Paz-Ares L. Current challenges in cancer treatment. Clin Ther. 2016;38(7):1551-1566.
15. Ventola CL. Cancer immunotherapy, Part 3: challenges and future trends. PT. 2017;42(8):514-521.
16. Cavanagh H, Rogers KMA. The role of BRCA1 and BRCA2 mutations in prostate, pancreatic and stomach cancers. Hered Cancer Clin Pract. 2015;13:16.
17. Moschetta M, George A, Kaye SB, Banerjee S. BRCA somatic mutations and epigenetic BRCA modifications in serous ovarian cancer. Ann Oncol. 2016;27(8):1449-1455.
18. Brown JS, O’Carrigan B, Jackson SP, Yap TA. Targeting DNA repair in cancer: beyond PARP inhibitors. Cancer Discov. 2017;7(1):20-37.
19. Robson M, Im S-A, Senkus E, et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. New England Journal of Medicine. 2017;377(6):523-533.
20. Williers H, Pfaffle HN, Zou L. Targeting homologous recombination repair in cancer: molecular targets and clinical applications. In: Kelley M, Fishel M, eds. DNA repair in cancer therapy. 2nd ed: Academic Press; 2016:119-160.
21. U.S. Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. 2017; https://www.fda.gov/Drugs/InformationOnDrugs/ ApprovedDrugs/ucm560040.htm. Accessed May 1st,, 2018.
22. Gallaher JA, Enriquez-Navas PM, Luddy KA, Gatenby RA, Anderson ARA. Adaptive Therapy For Heterogeneous Cancer: Exploiting Space And Trade-Offs In Drug Scheduling. bioRxiv. 2017.
23. Enriquez-Navas PM, Kam Y, Das T, et al. Exploiting evolutionary principles to prolong tumor control in preclinical models of breast cancer. Sci Transl Med. 2016;8(327):327ra24.
No strong evidence linking vitamin D levels and preeclampsia
Vitamin D status does not appear to have any effect on the risk of gestational hypertension or preeclampsia, regardless of a woman’s genetic risk profile for vitamin D deficiency.
Writing in the June 21 online edition of the BMJ, researchers reported the results of one- and two-sample mendelian randomization analyses of two pregnancy cohort studies and two case-control studies.
Overall, 7,389 women were included in the one-sample mendelian randomization analysis – 751 with gestational hypertension and 135 with preeclampsia. The two-sample analysis included 3,388 women with preeclampsia and 6,059 controls.
In a conventional multivariable analysis, researchers saw a 3% increase in the relative risk of preeclampsia for each 10% decrease in 25-hydroxyvitamin D levels. However, there was a doubling of risk in women whose 25-hydroxyvitamin D levels were below 25 nmol/L, compared with those with levels at or above 75 nmol/L, but no effect seen for gestational hypertension.
However, in the one-sample mendelian randomization analysis – using genetic risk score as an instrument – the authors saw no clear sign of a linear relationship between 25-hydroxyvitamin D levels and the risk of gestational hypertension or preeclampsia.
The two-sample mendelian randomization analysis showed an odds ratio for preeclampsia of 0.98 per 10% decrease in 25-hydroxyvitamin D level.
“We explored the association between the genetic instruments and intake of vitamin D supplements because, if women with lower genetically predicted 25-hydroxyvitamin D levels are more likely to take supplements, this could theoretically distort our findings,” wrote Maria C. Magnus, PhD, of the Medical Research Council Integrative Epidemiology Unit at the University of Bristol (England) and her coauthors.
They noted that the proportion of women taking vitamin D supplements during pregnancy differed between the two cohorts, which may have reflected cultural, socioeconomic, or policy difference.
The U.S. Institute of Medicine currently recommends that pregnant and lactating women have a dietary intake of 600 IU (15 mcg) of vitamin D per day.
While this study found no strong evidence to support a causal effect of vitamin D status on the risk of gestational hypertension or preeclampsia, the study’s authors suggested similar studies with larger numbers of women with preeclampsia were still needed to definitely establish this.
The study was supported by the European Union and the Research Council of Norway. One author declared funding from the pharmaceutical industry for unrelated research, and several authors declared funding from other institutions. No conflicts of interest were declared.
SOURCE: Magnus MC et al. BMJ. 2018 Jun 21. doi: 10.1136/bmj.k2167.
Vitamin D status does not appear to have any effect on the risk of gestational hypertension or preeclampsia, regardless of a woman’s genetic risk profile for vitamin D deficiency.
Writing in the June 21 online edition of the BMJ, researchers reported the results of one- and two-sample mendelian randomization analyses of two pregnancy cohort studies and two case-control studies.
Overall, 7,389 women were included in the one-sample mendelian randomization analysis – 751 with gestational hypertension and 135 with preeclampsia. The two-sample analysis included 3,388 women with preeclampsia and 6,059 controls.
In a conventional multivariable analysis, researchers saw a 3% increase in the relative risk of preeclampsia for each 10% decrease in 25-hydroxyvitamin D levels. However, there was a doubling of risk in women whose 25-hydroxyvitamin D levels were below 25 nmol/L, compared with those with levels at or above 75 nmol/L, but no effect seen for gestational hypertension.
However, in the one-sample mendelian randomization analysis – using genetic risk score as an instrument – the authors saw no clear sign of a linear relationship between 25-hydroxyvitamin D levels and the risk of gestational hypertension or preeclampsia.
The two-sample mendelian randomization analysis showed an odds ratio for preeclampsia of 0.98 per 10% decrease in 25-hydroxyvitamin D level.
“We explored the association between the genetic instruments and intake of vitamin D supplements because, if women with lower genetically predicted 25-hydroxyvitamin D levels are more likely to take supplements, this could theoretically distort our findings,” wrote Maria C. Magnus, PhD, of the Medical Research Council Integrative Epidemiology Unit at the University of Bristol (England) and her coauthors.
They noted that the proportion of women taking vitamin D supplements during pregnancy differed between the two cohorts, which may have reflected cultural, socioeconomic, or policy difference.
The U.S. Institute of Medicine currently recommends that pregnant and lactating women have a dietary intake of 600 IU (15 mcg) of vitamin D per day.
While this study found no strong evidence to support a causal effect of vitamin D status on the risk of gestational hypertension or preeclampsia, the study’s authors suggested similar studies with larger numbers of women with preeclampsia were still needed to definitely establish this.
The study was supported by the European Union and the Research Council of Norway. One author declared funding from the pharmaceutical industry for unrelated research, and several authors declared funding from other institutions. No conflicts of interest were declared.
SOURCE: Magnus MC et al. BMJ. 2018 Jun 21. doi: 10.1136/bmj.k2167.
Vitamin D status does not appear to have any effect on the risk of gestational hypertension or preeclampsia, regardless of a woman’s genetic risk profile for vitamin D deficiency.
Writing in the June 21 online edition of the BMJ, researchers reported the results of one- and two-sample mendelian randomization analyses of two pregnancy cohort studies and two case-control studies.
Overall, 7,389 women were included in the one-sample mendelian randomization analysis – 751 with gestational hypertension and 135 with preeclampsia. The two-sample analysis included 3,388 women with preeclampsia and 6,059 controls.
In a conventional multivariable analysis, researchers saw a 3% increase in the relative risk of preeclampsia for each 10% decrease in 25-hydroxyvitamin D levels. However, there was a doubling of risk in women whose 25-hydroxyvitamin D levels were below 25 nmol/L, compared with those with levels at or above 75 nmol/L, but no effect seen for gestational hypertension.
However, in the one-sample mendelian randomization analysis – using genetic risk score as an instrument – the authors saw no clear sign of a linear relationship between 25-hydroxyvitamin D levels and the risk of gestational hypertension or preeclampsia.
The two-sample mendelian randomization analysis showed an odds ratio for preeclampsia of 0.98 per 10% decrease in 25-hydroxyvitamin D level.
“We explored the association between the genetic instruments and intake of vitamin D supplements because, if women with lower genetically predicted 25-hydroxyvitamin D levels are more likely to take supplements, this could theoretically distort our findings,” wrote Maria C. Magnus, PhD, of the Medical Research Council Integrative Epidemiology Unit at the University of Bristol (England) and her coauthors.
They noted that the proportion of women taking vitamin D supplements during pregnancy differed between the two cohorts, which may have reflected cultural, socioeconomic, or policy difference.
The U.S. Institute of Medicine currently recommends that pregnant and lactating women have a dietary intake of 600 IU (15 mcg) of vitamin D per day.
While this study found no strong evidence to support a causal effect of vitamin D status on the risk of gestational hypertension or preeclampsia, the study’s authors suggested similar studies with larger numbers of women with preeclampsia were still needed to definitely establish this.
The study was supported by the European Union and the Research Council of Norway. One author declared funding from the pharmaceutical industry for unrelated research, and several authors declared funding from other institutions. No conflicts of interest were declared.
SOURCE: Magnus MC et al. BMJ. 2018 Jun 21. doi: 10.1136/bmj.k2167.
FROM THE BMJ
Key clinical point: No strong evidence linking vitamin D levels and preeclampsia risk.
Major finding: Women’s vitamin D status does not appear to affect their risk of preeclampsia.
Study details: Mendelian randomization analyses in 16,836 women.
Disclosures: The study was supported by the European Union and the Research Council of Norway. One author declared funding from the pharmaceutical industry for unrelated research, and several authors declared funding from other institutions. No conflicts of interest were declared.
Source: Magnus MC et al. BMJ. 2018 Jun 21. doi: 10.1136/bmj.k2167.
FDA okays fully implantable continuous glucose monitor/mobile app combo for diabetes
to transmit continuous information about blood glucose levels for people with diabetes.
The sensor-mobile app combo, called the Eversense Continuous Glucose Monitoring (CGM) system, is designed to supplant the need for frequent blood sampling to monitor blood glucose levels.
“The FDA is committed to advancing novel products that leverage digital technology to improve patient care,” said FDA commissioner Scott Gottlieb, MD, in the agency’s press release announcing the approval. The sensor, which is roughly 1.5 cm long, is coated with a material that fluoresces when exposed to glucose; the sensor uses the amount of light emitted to calculate blood glucose levels. Patients use an adhesive patch, changed daily, to attach a “smart” transmitter that overlies the area where the sensor is implanted. This rechargeable transmitter sends blood glucose levels to the mobile app every 5 minutes, and also powers the sensor.
The FDA’s approval was based on data from 125 patients with type 1 and type 2 diabetes who used the CGM system. The bulk of clinical data was acquired from PRECISE II, which enrolled 90 patients with type 1 and type 2 diabetes. When compared with levels returned from concurrently performed conventional home glucose monitoring, the CGM system achieved a mean absolute relative difference (MARD) of 8.8% (95% confidence interval, 8.1%-9.3%). This was less than the prespecified accuracy goal of 20% MARD (P less than .0001).
During the nonrandomized, blinded, prospective PRECISE II trial, 91% of the implanted sensors were functioning through the end of 90 days. A variation of the Eversense CGM, the Eversense CGM XL, has been approved for use up to 180 days in Europe.
The overall rate of serious adverse events among patients participating in the Eversense CGM trials was less than 1%. “The safety of this novel system will also be evaluated in a post-approval study,” wrote FDA officials in the press release.
In addition to adverse effects related to the outpatient procedure in which the glucose sensor is implanted subcutaneously, the FDA said that allergic reactions, ongoing pain, discomfort, scarring, and skin changes are possible with use of the CGM. Though the system sends frequent blood glucose measurements to the accompanying mobile app, missed alerts might still result in hypo- or hyperglycemia.
The Eversense CGM is marketed by Senseonics, which funded the studies underpinning approval.
to transmit continuous information about blood glucose levels for people with diabetes.
The sensor-mobile app combo, called the Eversense Continuous Glucose Monitoring (CGM) system, is designed to supplant the need for frequent blood sampling to monitor blood glucose levels.
“The FDA is committed to advancing novel products that leverage digital technology to improve patient care,” said FDA commissioner Scott Gottlieb, MD, in the agency’s press release announcing the approval. The sensor, which is roughly 1.5 cm long, is coated with a material that fluoresces when exposed to glucose; the sensor uses the amount of light emitted to calculate blood glucose levels. Patients use an adhesive patch, changed daily, to attach a “smart” transmitter that overlies the area where the sensor is implanted. This rechargeable transmitter sends blood glucose levels to the mobile app every 5 minutes, and also powers the sensor.
The FDA’s approval was based on data from 125 patients with type 1 and type 2 diabetes who used the CGM system. The bulk of clinical data was acquired from PRECISE II, which enrolled 90 patients with type 1 and type 2 diabetes. When compared with levels returned from concurrently performed conventional home glucose monitoring, the CGM system achieved a mean absolute relative difference (MARD) of 8.8% (95% confidence interval, 8.1%-9.3%). This was less than the prespecified accuracy goal of 20% MARD (P less than .0001).
During the nonrandomized, blinded, prospective PRECISE II trial, 91% of the implanted sensors were functioning through the end of 90 days. A variation of the Eversense CGM, the Eversense CGM XL, has been approved for use up to 180 days in Europe.
The overall rate of serious adverse events among patients participating in the Eversense CGM trials was less than 1%. “The safety of this novel system will also be evaluated in a post-approval study,” wrote FDA officials in the press release.
In addition to adverse effects related to the outpatient procedure in which the glucose sensor is implanted subcutaneously, the FDA said that allergic reactions, ongoing pain, discomfort, scarring, and skin changes are possible with use of the CGM. Though the system sends frequent blood glucose measurements to the accompanying mobile app, missed alerts might still result in hypo- or hyperglycemia.
The Eversense CGM is marketed by Senseonics, which funded the studies underpinning approval.
to transmit continuous information about blood glucose levels for people with diabetes.
The sensor-mobile app combo, called the Eversense Continuous Glucose Monitoring (CGM) system, is designed to supplant the need for frequent blood sampling to monitor blood glucose levels.
“The FDA is committed to advancing novel products that leverage digital technology to improve patient care,” said FDA commissioner Scott Gottlieb, MD, in the agency’s press release announcing the approval. The sensor, which is roughly 1.5 cm long, is coated with a material that fluoresces when exposed to glucose; the sensor uses the amount of light emitted to calculate blood glucose levels. Patients use an adhesive patch, changed daily, to attach a “smart” transmitter that overlies the area where the sensor is implanted. This rechargeable transmitter sends blood glucose levels to the mobile app every 5 minutes, and also powers the sensor.
The FDA’s approval was based on data from 125 patients with type 1 and type 2 diabetes who used the CGM system. The bulk of clinical data was acquired from PRECISE II, which enrolled 90 patients with type 1 and type 2 diabetes. When compared with levels returned from concurrently performed conventional home glucose monitoring, the CGM system achieved a mean absolute relative difference (MARD) of 8.8% (95% confidence interval, 8.1%-9.3%). This was less than the prespecified accuracy goal of 20% MARD (P less than .0001).
During the nonrandomized, blinded, prospective PRECISE II trial, 91% of the implanted sensors were functioning through the end of 90 days. A variation of the Eversense CGM, the Eversense CGM XL, has been approved for use up to 180 days in Europe.
The overall rate of serious adverse events among patients participating in the Eversense CGM trials was less than 1%. “The safety of this novel system will also be evaluated in a post-approval study,” wrote FDA officials in the press release.
In addition to adverse effects related to the outpatient procedure in which the glucose sensor is implanted subcutaneously, the FDA said that allergic reactions, ongoing pain, discomfort, scarring, and skin changes are possible with use of the CGM. Though the system sends frequent blood glucose measurements to the accompanying mobile app, missed alerts might still result in hypo- or hyperglycemia.
The Eversense CGM is marketed by Senseonics, which funded the studies underpinning approval.
U.S. immigration policy: What harms will persist?
The Trump policy of separating children and teenagers from their parents after crossing the U.S. border has been called un-American, immoral, cruel, and inhumane. The policy thankfully has been reversed or at least subject to delay. However, as I write today, 2,300 children and their parents are separated, not in contact, lost to each other, and with no clear plan on reunification. The ultimate outcome of immigration legislation and policy is unknown and mired in partisan politics. The policy hopefully has changed permanently, but what are the harms that will persist?
1. Many if not all of the 2,300 children taken from their parents to institutional settings will have suffered acute anxiety and despair. Following data gathered by René Spitz and John Bowlby 80 years ago, children forced to separate from their parents for long hospitalizations with limited visitation went through phases of protest, despair, and if repeated or lengthy separations, “detachment” that impaired their ability to form relationships.1
2. Many of these children have suffered traumas in their country of origin and through the journey to the U.S. border. Some of this traumatic experience was mitigated by being in the presence of their parent(s). Very likely some children have psychiatric and physical disorders that will add to the level of risk. The current trauma, forcible separation by armed guards into restrictive facilities, will compound or intensify the previous traumas without the benefit of parental support.
3. Will the harms persist? Likely this level of trauma has such a strong neurologic and psychological impact that many of the children will suffer from nightmares, depression, and persistent anxiety about trusting the safety of their setting. 2
4. The parents who are jailed, have had their children removed, and do not know where they are and aren’t able to talk to them have suffered a massive trauma. We all have lost sight of a child for a minute or two in a store or on the beach. Our anxiety is immediate, and if the separation is longer, we may remember those frightening minutes for the rest of our lives. How many immigrant parents will develop depression and posttraumatic stress disorder?
5. Guards were ordered to be the front-line implementers of the policy and must have been torn between their sworn duty and their inner knowledge that what they are doing is wrong. Hearing the children crying and calling for their parents must have elicited painful feelings of what it would have been like to have their own children taken away with no way to reach them or knowing where they were taken. Implementing this policy dehumanized them, and I believe made them feel guilty or unworthy.
6. Millions of immigrants – whether lawful, dreamers, or undocumented – must have felt fearful, powerless, and angry about this policy. Millions of their children must have been worried and lost a little bit of faith in their parents and in the United States.
7. Did U.S. citizens, many from immigrant roots, wonder if this could happen to them? How many children felt a little less secure? Was the anxiety higher for descendants of the U.S. citizens remembering the trauma of the World War II Japanese internment camps? Other descendants (like me) will remember quite vividly their mother’s story of being on the St. Louis steam ship and being turned away from the United States to face a high likelihood of death in Nazi Germany. A bit of fear will replace trust in and loyalty to the United States.
Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email him at [email protected].
References:
1. Dev Psychol. 1992;28:759-75.
2. www.cdc.gov/violenceprevention/acestudy/index.html
The Trump policy of separating children and teenagers from their parents after crossing the U.S. border has been called un-American, immoral, cruel, and inhumane. The policy thankfully has been reversed or at least subject to delay. However, as I write today, 2,300 children and their parents are separated, not in contact, lost to each other, and with no clear plan on reunification. The ultimate outcome of immigration legislation and policy is unknown and mired in partisan politics. The policy hopefully has changed permanently, but what are the harms that will persist?
1. Many if not all of the 2,300 children taken from their parents to institutional settings will have suffered acute anxiety and despair. Following data gathered by René Spitz and John Bowlby 80 years ago, children forced to separate from their parents for long hospitalizations with limited visitation went through phases of protest, despair, and if repeated or lengthy separations, “detachment” that impaired their ability to form relationships.1
2. Many of these children have suffered traumas in their country of origin and through the journey to the U.S. border. Some of this traumatic experience was mitigated by being in the presence of their parent(s). Very likely some children have psychiatric and physical disorders that will add to the level of risk. The current trauma, forcible separation by armed guards into restrictive facilities, will compound or intensify the previous traumas without the benefit of parental support.
3. Will the harms persist? Likely this level of trauma has such a strong neurologic and psychological impact that many of the children will suffer from nightmares, depression, and persistent anxiety about trusting the safety of their setting. 2
4. The parents who are jailed, have had their children removed, and do not know where they are and aren’t able to talk to them have suffered a massive trauma. We all have lost sight of a child for a minute or two in a store or on the beach. Our anxiety is immediate, and if the separation is longer, we may remember those frightening minutes for the rest of our lives. How many immigrant parents will develop depression and posttraumatic stress disorder?
5. Guards were ordered to be the front-line implementers of the policy and must have been torn between their sworn duty and their inner knowledge that what they are doing is wrong. Hearing the children crying and calling for their parents must have elicited painful feelings of what it would have been like to have their own children taken away with no way to reach them or knowing where they were taken. Implementing this policy dehumanized them, and I believe made them feel guilty or unworthy.
6. Millions of immigrants – whether lawful, dreamers, or undocumented – must have felt fearful, powerless, and angry about this policy. Millions of their children must have been worried and lost a little bit of faith in their parents and in the United States.
7. Did U.S. citizens, many from immigrant roots, wonder if this could happen to them? How many children felt a little less secure? Was the anxiety higher for descendants of the U.S. citizens remembering the trauma of the World War II Japanese internment camps? Other descendants (like me) will remember quite vividly their mother’s story of being on the St. Louis steam ship and being turned away from the United States to face a high likelihood of death in Nazi Germany. A bit of fear will replace trust in and loyalty to the United States.
Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email him at [email protected].
References:
1. Dev Psychol. 1992;28:759-75.
2. www.cdc.gov/violenceprevention/acestudy/index.html
The Trump policy of separating children and teenagers from their parents after crossing the U.S. border has been called un-American, immoral, cruel, and inhumane. The policy thankfully has been reversed or at least subject to delay. However, as I write today, 2,300 children and their parents are separated, not in contact, lost to each other, and with no clear plan on reunification. The ultimate outcome of immigration legislation and policy is unknown and mired in partisan politics. The policy hopefully has changed permanently, but what are the harms that will persist?
1. Many if not all of the 2,300 children taken from their parents to institutional settings will have suffered acute anxiety and despair. Following data gathered by René Spitz and John Bowlby 80 years ago, children forced to separate from their parents for long hospitalizations with limited visitation went through phases of protest, despair, and if repeated or lengthy separations, “detachment” that impaired their ability to form relationships.1
2. Many of these children have suffered traumas in their country of origin and through the journey to the U.S. border. Some of this traumatic experience was mitigated by being in the presence of their parent(s). Very likely some children have psychiatric and physical disorders that will add to the level of risk. The current trauma, forcible separation by armed guards into restrictive facilities, will compound or intensify the previous traumas without the benefit of parental support.
3. Will the harms persist? Likely this level of trauma has such a strong neurologic and psychological impact that many of the children will suffer from nightmares, depression, and persistent anxiety about trusting the safety of their setting. 2
4. The parents who are jailed, have had their children removed, and do not know where they are and aren’t able to talk to them have suffered a massive trauma. We all have lost sight of a child for a minute or two in a store or on the beach. Our anxiety is immediate, and if the separation is longer, we may remember those frightening minutes for the rest of our lives. How many immigrant parents will develop depression and posttraumatic stress disorder?
5. Guards were ordered to be the front-line implementers of the policy and must have been torn between their sworn duty and their inner knowledge that what they are doing is wrong. Hearing the children crying and calling for their parents must have elicited painful feelings of what it would have been like to have their own children taken away with no way to reach them or knowing where they were taken. Implementing this policy dehumanized them, and I believe made them feel guilty or unworthy.
6. Millions of immigrants – whether lawful, dreamers, or undocumented – must have felt fearful, powerless, and angry about this policy. Millions of their children must have been worried and lost a little bit of faith in their parents and in the United States.
7. Did U.S. citizens, many from immigrant roots, wonder if this could happen to them? How many children felt a little less secure? Was the anxiety higher for descendants of the U.S. citizens remembering the trauma of the World War II Japanese internment camps? Other descendants (like me) will remember quite vividly their mother’s story of being on the St. Louis steam ship and being turned away from the United States to face a high likelihood of death in Nazi Germany. A bit of fear will replace trust in and loyalty to the United States.
Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email him at [email protected].
References:
1. Dev Psychol. 1992;28:759-75.
2. www.cdc.gov/violenceprevention/acestudy/index.html
HHS’s Azar teases changes to APMs
“I am not sure that simply being in an alternative payment model, which was the metric the Obama administration used, is the one that I would find to be substantive and real in terms of transformation of our health care system,” Mr. Azar said June 20 at a forum hosted by the Washington Post.
The previous administration set a goal of having at least 50% of physician Medicare payments tied to quality by the end of this year. It’s first milestone of 30% by the end of 2016 was reached in March of that year.
The current administration may have had a tough time meeting the 50% goal because of changes it made to the Quality Payment Program exempted two-thirds of eligible clinicians from the Merit-Based Incentive Payment System track in 2018.
Mr. Azar said that he is working with the team at the Centers for Medicare & Medicaid Services to come up with a better way to determine whether paying for quality is effective.
“What I don’t want to do is have an approach where it’s a tag the base, hit a scorecard number,” he said. “We genuinely want to revolutionize how health care is paid for in this country in an outcome-based, health-based, non-procedure-, non-sickness-based way. We are working on that. We want to get to real concrete metrics.”
Mr. Azar also noted that the agency is working on “the concrete strategy for the Center for Medicare & Medicaid Innovation. That will also have dimensions for what we are doing within the fee-for-service program and Medicare Advantage around moving toward value-based payment arrangements.”
He praised the efforts of the Bush Administration and the Obama Administration as providing a good foundation for the transition to paying for quality and “we will build on that.”
“I am not sure that simply being in an alternative payment model, which was the metric the Obama administration used, is the one that I would find to be substantive and real in terms of transformation of our health care system,” Mr. Azar said June 20 at a forum hosted by the Washington Post.
The previous administration set a goal of having at least 50% of physician Medicare payments tied to quality by the end of this year. It’s first milestone of 30% by the end of 2016 was reached in March of that year.
The current administration may have had a tough time meeting the 50% goal because of changes it made to the Quality Payment Program exempted two-thirds of eligible clinicians from the Merit-Based Incentive Payment System track in 2018.
Mr. Azar said that he is working with the team at the Centers for Medicare & Medicaid Services to come up with a better way to determine whether paying for quality is effective.
“What I don’t want to do is have an approach where it’s a tag the base, hit a scorecard number,” he said. “We genuinely want to revolutionize how health care is paid for in this country in an outcome-based, health-based, non-procedure-, non-sickness-based way. We are working on that. We want to get to real concrete metrics.”
Mr. Azar also noted that the agency is working on “the concrete strategy for the Center for Medicare & Medicaid Innovation. That will also have dimensions for what we are doing within the fee-for-service program and Medicare Advantage around moving toward value-based payment arrangements.”
He praised the efforts of the Bush Administration and the Obama Administration as providing a good foundation for the transition to paying for quality and “we will build on that.”
“I am not sure that simply being in an alternative payment model, which was the metric the Obama administration used, is the one that I would find to be substantive and real in terms of transformation of our health care system,” Mr. Azar said June 20 at a forum hosted by the Washington Post.
The previous administration set a goal of having at least 50% of physician Medicare payments tied to quality by the end of this year. It’s first milestone of 30% by the end of 2016 was reached in March of that year.
The current administration may have had a tough time meeting the 50% goal because of changes it made to the Quality Payment Program exempted two-thirds of eligible clinicians from the Merit-Based Incentive Payment System track in 2018.
Mr. Azar said that he is working with the team at the Centers for Medicare & Medicaid Services to come up with a better way to determine whether paying for quality is effective.
“What I don’t want to do is have an approach where it’s a tag the base, hit a scorecard number,” he said. “We genuinely want to revolutionize how health care is paid for in this country in an outcome-based, health-based, non-procedure-, non-sickness-based way. We are working on that. We want to get to real concrete metrics.”
Mr. Azar also noted that the agency is working on “the concrete strategy for the Center for Medicare & Medicaid Innovation. That will also have dimensions for what we are doing within the fee-for-service program and Medicare Advantage around moving toward value-based payment arrangements.”
He praised the efforts of the Bush Administration and the Obama Administration as providing a good foundation for the transition to paying for quality and “we will build on that.”
Experts debate affordability of myeloma drugs at ASCO
CHICAGO – Are today’s myeloma drugs affordable? Two Mayo Clinic researchers agreed that costs are high but not whether the price is offset by the value.
“I don’t think there is any debate here. It’s like debating whether the Earth is flat or not,” S. Vincent Rajkumar, MD, of Mayo Clinic, Rochester, Minn., said during a debate at the annual meeting of the American Society of Clinical Oncology. “These drugs are expensive.”
“I would trust Dr. Rajkumar with my life if I were diagnosed with myeloma,” countered Rafael Fonseca, MD, of Mayo Clinic in Phoenix, Ariz., “But I think he’s wrong on drug economics.”
Dr. Rajkumar said the total lifetime costs to treat all patients diagnosed with multiple myeloma in 2017 were $22.4 billion, a “conservative estimate” that excluded hospital, infusion, laboratory, imaging, physician, nursing, and ancillary costs.
“Every single drug is expensive,” he said, referring to newer approved myeloma therapies that cost up to $192,000/year individually, and up to $590,000/year in triplet or quadruplet combination regimens, according to estimates he included in a related article he wrote for the 2018 ASCO Educational Book.
Of $50 billion spent in 2017 on cancer drugs, 80% of that spending was based on just 35 drugs, of which 6 were myeloma drugs – and myeloma is just 1% of all cancers. “Maybe it’s because of all the progress we’ve made in myeloma, but unless you think none of the other cancers should have the type of progress we have, this is not going to be affordable,” Dr. Rajkumar said.
Drugs approved by the Food and Drug Administration (FDA) in 2017 cost $100,000/year or more, with an average of $150,000/year, according to Dr. Rajkumar. He compared that with the average U.S. annual gross household income of $52,000, saying that the high price of drugs has contributed to compliance problems and medical bankruptcy.
While Dr. Fonseca agreed that drug prices are “skyrocketing,” he challenged the notion that the increases were not affordable in his presentation and an associated ASCO Educational Book article.
In his talk, Dr. Fonseca said the availability of new myeloma drugs has led to “astounding” improvements in overall survival, but today’s best drugs are still not good enough. “We cannot afford to stop innovation and the move forward as we are ever so close to curing a large fraction of myeloma patients,” he said.
The increasing cost of drugs has been offset by societal and health effects, Dr. Fonseca argued.
The war on cancer from 1988 to 2000 added 23 million additional life-years, which has equated to $1.9 trillion in social value for Americans, according to one analysis he cited. In one myeloma-specific study, investigators found myeloma drug costs increased from $36,607 in 2004 to $109,544 in 2009, but those increases were balanced out by $67,900 in health benefits.
Although the financial impact of myeloma on the individual patient can be significant, it’s not bankruptcies, but out-of-pocket costs such as copayments, that have the most direct effect on patients, Dr. Fonseca said. Research shows medical bankruptcies are not associated with drug copayments, he added, but rather other medical expenses, such as hospital and physician bills, along with loss of income and limited savings.
Dr. Rajkumar – unconvinced that myeloma drugs are currently affordable – urged action on several fronts, including value-based pricing or tying the price of a drug to how much value it produces.
The Medicare program has to be able to negotiate prices, he added, and patients should be allowed to reimport cancer drugs from other countries for personal use. He also pushed for more to be done to facilitate the entry of generics and biosimilars into the marketplace.
He also called for a relaxation of FDA regulations to lower drug development costs. “We have so many regulations so that every T is crossed and every I is dotted, to the point that it costs $30,000, $40,000 per patient to do a trial,” he said.
But Dr. Fonseca opposed market interference, saying that price controls would kill innovation.
“The patented drugs of today are the generics of the future, and absent innovation, we won’t have future generics,” he said in his presentation. “Price fixing kills innovation. ... So if we engage in that, today’s best is simply the best there is going to be.”
Dr. Rajkumar reported having no conflicts of interest. Dr. Fonseca reported consulting work with Amgen, Bristol-Myers Squibb, Celgene, Takeda Pharmaceutical, Bayer, Janssen, AbbVie, Pharmacyclics, Sanofi, Kite Pharma, and Juno Therapeutics, and scientific advisory board work with Adaptive Biotechnologies.
CHICAGO – Are today’s myeloma drugs affordable? Two Mayo Clinic researchers agreed that costs are high but not whether the price is offset by the value.
“I don’t think there is any debate here. It’s like debating whether the Earth is flat or not,” S. Vincent Rajkumar, MD, of Mayo Clinic, Rochester, Minn., said during a debate at the annual meeting of the American Society of Clinical Oncology. “These drugs are expensive.”
“I would trust Dr. Rajkumar with my life if I were diagnosed with myeloma,” countered Rafael Fonseca, MD, of Mayo Clinic in Phoenix, Ariz., “But I think he’s wrong on drug economics.”
Dr. Rajkumar said the total lifetime costs to treat all patients diagnosed with multiple myeloma in 2017 were $22.4 billion, a “conservative estimate” that excluded hospital, infusion, laboratory, imaging, physician, nursing, and ancillary costs.
“Every single drug is expensive,” he said, referring to newer approved myeloma therapies that cost up to $192,000/year individually, and up to $590,000/year in triplet or quadruplet combination regimens, according to estimates he included in a related article he wrote for the 2018 ASCO Educational Book.
Of $50 billion spent in 2017 on cancer drugs, 80% of that spending was based on just 35 drugs, of which 6 were myeloma drugs – and myeloma is just 1% of all cancers. “Maybe it’s because of all the progress we’ve made in myeloma, but unless you think none of the other cancers should have the type of progress we have, this is not going to be affordable,” Dr. Rajkumar said.
Drugs approved by the Food and Drug Administration (FDA) in 2017 cost $100,000/year or more, with an average of $150,000/year, according to Dr. Rajkumar. He compared that with the average U.S. annual gross household income of $52,000, saying that the high price of drugs has contributed to compliance problems and medical bankruptcy.
While Dr. Fonseca agreed that drug prices are “skyrocketing,” he challenged the notion that the increases were not affordable in his presentation and an associated ASCO Educational Book article.
In his talk, Dr. Fonseca said the availability of new myeloma drugs has led to “astounding” improvements in overall survival, but today’s best drugs are still not good enough. “We cannot afford to stop innovation and the move forward as we are ever so close to curing a large fraction of myeloma patients,” he said.
The increasing cost of drugs has been offset by societal and health effects, Dr. Fonseca argued.
The war on cancer from 1988 to 2000 added 23 million additional life-years, which has equated to $1.9 trillion in social value for Americans, according to one analysis he cited. In one myeloma-specific study, investigators found myeloma drug costs increased from $36,607 in 2004 to $109,544 in 2009, but those increases were balanced out by $67,900 in health benefits.
Although the financial impact of myeloma on the individual patient can be significant, it’s not bankruptcies, but out-of-pocket costs such as copayments, that have the most direct effect on patients, Dr. Fonseca said. Research shows medical bankruptcies are not associated with drug copayments, he added, but rather other medical expenses, such as hospital and physician bills, along with loss of income and limited savings.
Dr. Rajkumar – unconvinced that myeloma drugs are currently affordable – urged action on several fronts, including value-based pricing or tying the price of a drug to how much value it produces.
The Medicare program has to be able to negotiate prices, he added, and patients should be allowed to reimport cancer drugs from other countries for personal use. He also pushed for more to be done to facilitate the entry of generics and biosimilars into the marketplace.
He also called for a relaxation of FDA regulations to lower drug development costs. “We have so many regulations so that every T is crossed and every I is dotted, to the point that it costs $30,000, $40,000 per patient to do a trial,” he said.
But Dr. Fonseca opposed market interference, saying that price controls would kill innovation.
“The patented drugs of today are the generics of the future, and absent innovation, we won’t have future generics,” he said in his presentation. “Price fixing kills innovation. ... So if we engage in that, today’s best is simply the best there is going to be.”
Dr. Rajkumar reported having no conflicts of interest. Dr. Fonseca reported consulting work with Amgen, Bristol-Myers Squibb, Celgene, Takeda Pharmaceutical, Bayer, Janssen, AbbVie, Pharmacyclics, Sanofi, Kite Pharma, and Juno Therapeutics, and scientific advisory board work with Adaptive Biotechnologies.
CHICAGO – Are today’s myeloma drugs affordable? Two Mayo Clinic researchers agreed that costs are high but not whether the price is offset by the value.
“I don’t think there is any debate here. It’s like debating whether the Earth is flat or not,” S. Vincent Rajkumar, MD, of Mayo Clinic, Rochester, Minn., said during a debate at the annual meeting of the American Society of Clinical Oncology. “These drugs are expensive.”
“I would trust Dr. Rajkumar with my life if I were diagnosed with myeloma,” countered Rafael Fonseca, MD, of Mayo Clinic in Phoenix, Ariz., “But I think he’s wrong on drug economics.”
Dr. Rajkumar said the total lifetime costs to treat all patients diagnosed with multiple myeloma in 2017 were $22.4 billion, a “conservative estimate” that excluded hospital, infusion, laboratory, imaging, physician, nursing, and ancillary costs.
“Every single drug is expensive,” he said, referring to newer approved myeloma therapies that cost up to $192,000/year individually, and up to $590,000/year in triplet or quadruplet combination regimens, according to estimates he included in a related article he wrote for the 2018 ASCO Educational Book.
Of $50 billion spent in 2017 on cancer drugs, 80% of that spending was based on just 35 drugs, of which 6 were myeloma drugs – and myeloma is just 1% of all cancers. “Maybe it’s because of all the progress we’ve made in myeloma, but unless you think none of the other cancers should have the type of progress we have, this is not going to be affordable,” Dr. Rajkumar said.
Drugs approved by the Food and Drug Administration (FDA) in 2017 cost $100,000/year or more, with an average of $150,000/year, according to Dr. Rajkumar. He compared that with the average U.S. annual gross household income of $52,000, saying that the high price of drugs has contributed to compliance problems and medical bankruptcy.
While Dr. Fonseca agreed that drug prices are “skyrocketing,” he challenged the notion that the increases were not affordable in his presentation and an associated ASCO Educational Book article.
In his talk, Dr. Fonseca said the availability of new myeloma drugs has led to “astounding” improvements in overall survival, but today’s best drugs are still not good enough. “We cannot afford to stop innovation and the move forward as we are ever so close to curing a large fraction of myeloma patients,” he said.
The increasing cost of drugs has been offset by societal and health effects, Dr. Fonseca argued.
The war on cancer from 1988 to 2000 added 23 million additional life-years, which has equated to $1.9 trillion in social value for Americans, according to one analysis he cited. In one myeloma-specific study, investigators found myeloma drug costs increased from $36,607 in 2004 to $109,544 in 2009, but those increases were balanced out by $67,900 in health benefits.
Although the financial impact of myeloma on the individual patient can be significant, it’s not bankruptcies, but out-of-pocket costs such as copayments, that have the most direct effect on patients, Dr. Fonseca said. Research shows medical bankruptcies are not associated with drug copayments, he added, but rather other medical expenses, such as hospital and physician bills, along with loss of income and limited savings.
Dr. Rajkumar – unconvinced that myeloma drugs are currently affordable – urged action on several fronts, including value-based pricing or tying the price of a drug to how much value it produces.
The Medicare program has to be able to negotiate prices, he added, and patients should be allowed to reimport cancer drugs from other countries for personal use. He also pushed for more to be done to facilitate the entry of generics and biosimilars into the marketplace.
He also called for a relaxation of FDA regulations to lower drug development costs. “We have so many regulations so that every T is crossed and every I is dotted, to the point that it costs $30,000, $40,000 per patient to do a trial,” he said.
But Dr. Fonseca opposed market interference, saying that price controls would kill innovation.
“The patented drugs of today are the generics of the future, and absent innovation, we won’t have future generics,” he said in his presentation. “Price fixing kills innovation. ... So if we engage in that, today’s best is simply the best there is going to be.”
Dr. Rajkumar reported having no conflicts of interest. Dr. Fonseca reported consulting work with Amgen, Bristol-Myers Squibb, Celgene, Takeda Pharmaceutical, Bayer, Janssen, AbbVie, Pharmacyclics, Sanofi, Kite Pharma, and Juno Therapeutics, and scientific advisory board work with Adaptive Biotechnologies.
REPORTING FROM ASCO 2018
Could tackling maternal obesity prevent later CVD in offspring?
authors of a thematic literature review concluded.
Maternal obesity has been tied to the development of cardiovascular disease (CVD) and premature death in epidemiologic studies, the authors noted in the review.
One hypothesis, referred to as fetal programming, posits that in utero environmental factors may have adverse metabolic consequences in the offspring. Thus far, however, most evidence supporting this hypothesis has come from animal studies, they cautioned.
Nevertheless, endothelial cell dysfunction is a reversible process, offering a “window of opportunity” for intervention, according to authors Karolien Van De Maele and Inge Gies, MD, of the division of pediatric endocrinology at the University Hospital of Brussels and Roland Devlieger, MD, PhD, head of fetal maternal medicine at the University Hospitals Leuven (Belgium).
“The fundamental solution to break the vicious cycle seems [to be] an intervention before or in early pregnancy,” authors said in the journal Atherosclerosis.
Mary Norine Walsh, MD, immediate past president of the American College of Cardiology, agreed with the review article’s conclusion that more evidence would be needed to show that fetal programming is implicated in the associations between maternal obesity and long-term cardiovascular effects.
“As of right now, we cannot say the offspring of pregnant women have an increased risk of cardiovascular risk in later life due to ‘X’ because those studies haven’t been done yet,” Dr. Walsh said in an interview. “So I think it’s a really good framework to think about based on the animal work that’s been done, but we have yet to identify obesity in pregnant women as an independent risk factor for vascular disease in the offspring – we just have an association.”
On the other hand, it is known that obesity increases the risk of hypertension and diabetes in both pregnant and nonpregnant women, said Dr. Walsh, and that hypertensive disorders are a leading cause of maternal morbidity and mortality.
“I think it’s really important to recognize that maternal obesity puts a woman at significant risk, and we certainly can’t forget that in the process of thinking about the offspring,” said Dr. Walsh, medical director of the heart failure and cardiac transplantation program at St. Vincent Heart Center, Indianapolis.
In the recent review article in Atherosclerosis, Ms. Van De Maele and coauthors cited evidence linking maternal obesity to adverse outcomes in offspring from a 2013 report in the BMJ that included 28,540 women in Scotland and their 37,709 offspring.
In that study, after adjustment for maternal age, socioeconomic status, and other factors, offspring of mothers who had a body mass index greater than 30 kg/m2 had higher all-cause mortality (hazard ratio, 1.35; 95% confidence interval, 1.17-1.55) and increased risk of hospital admission for a cardiovascular event (HR, 1.29; 95% CI, 1.06-1.57), compared with those whose mothers had a healthy BMI.
“Evidence from animal models and emerging data from humans suggest that maternal obesity also creates an adverse in utero environment, with long-term ‘programmed’ detrimental effects for the offspring,” the authors of that BMJ report wrote at the time.
Ms. Van De Maele and her colleagues also cited animal studies, including several looking at offspring of animals fed with a maternal high-fat diet during pregnancy. In those studies, they said, investigators observed impaired endothelial cell relaxation, along with raised thickness of the intimal wall and increased vascular inflammatory marker expression.
“Raised leptin levels, secreted by the adipose tissue, inhibit the in vitro proliferation of smooth muscle cells and could impede the angiogenesis process in vivo, but this assumption needs scientific validation in humans,” they said in their review.
However, human studies are lacking, aside from the epidemiologic reports that “cannot be used to confirm or contradict” the fetal programming hypothesis, they said.
Meanwhile, an increasing body of evidence has suggested that stressors in critical periods of fetal development may lead to epigenetic alterations that could play a role in either up-regulating atherogenic genes or down-regulating enzymatic activities that guard against oxidative stress.
For example, cohort studies have shown differences in DNA methylation among offspring born before and after bariatric surgery in the mother, which has lent credence to the hypothesis that maternal obesity in pregnancy alters methylation patterns for those offspring, Ms. Van De Maele and her colleagues wrote.
Lifestyle changes in obese pregnant women may have an effect on adverse metabolic or cardiovascular outcomes in offspring, although results to date are inconclusive, they added.
Diet, exercise, or both during pregnancy may lower the risk of macrosomia, respiratory distress syndrome, or other neonatal outcomes, particularly in high-risk women, according to the conclusions of a 2015 Cochrane review that Ms. Van De Maele and her coauthors cited.
However, follow-up studies on offspring are scarce and have shown no clear effects on long-term metabolic profiles in offspring, likely because of insufficient follow-up time, they said in their review.
Ms. Van De Maele and her coauthors said they had no conflict of interest disclosures related to their manuscript.
SOURCE: Van De Maele K et al. Atherosclerosis. 2018 Jun. doi: 10.1016/j.atherosclerosis.2018.06.016.
authors of a thematic literature review concluded.
Maternal obesity has been tied to the development of cardiovascular disease (CVD) and premature death in epidemiologic studies, the authors noted in the review.
One hypothesis, referred to as fetal programming, posits that in utero environmental factors may have adverse metabolic consequences in the offspring. Thus far, however, most evidence supporting this hypothesis has come from animal studies, they cautioned.
Nevertheless, endothelial cell dysfunction is a reversible process, offering a “window of opportunity” for intervention, according to authors Karolien Van De Maele and Inge Gies, MD, of the division of pediatric endocrinology at the University Hospital of Brussels and Roland Devlieger, MD, PhD, head of fetal maternal medicine at the University Hospitals Leuven (Belgium).
“The fundamental solution to break the vicious cycle seems [to be] an intervention before or in early pregnancy,” authors said in the journal Atherosclerosis.
Mary Norine Walsh, MD, immediate past president of the American College of Cardiology, agreed with the review article’s conclusion that more evidence would be needed to show that fetal programming is implicated in the associations between maternal obesity and long-term cardiovascular effects.
“As of right now, we cannot say the offspring of pregnant women have an increased risk of cardiovascular risk in later life due to ‘X’ because those studies haven’t been done yet,” Dr. Walsh said in an interview. “So I think it’s a really good framework to think about based on the animal work that’s been done, but we have yet to identify obesity in pregnant women as an independent risk factor for vascular disease in the offspring – we just have an association.”
On the other hand, it is known that obesity increases the risk of hypertension and diabetes in both pregnant and nonpregnant women, said Dr. Walsh, and that hypertensive disorders are a leading cause of maternal morbidity and mortality.
“I think it’s really important to recognize that maternal obesity puts a woman at significant risk, and we certainly can’t forget that in the process of thinking about the offspring,” said Dr. Walsh, medical director of the heart failure and cardiac transplantation program at St. Vincent Heart Center, Indianapolis.
In the recent review article in Atherosclerosis, Ms. Van De Maele and coauthors cited evidence linking maternal obesity to adverse outcomes in offspring from a 2013 report in the BMJ that included 28,540 women in Scotland and their 37,709 offspring.
In that study, after adjustment for maternal age, socioeconomic status, and other factors, offspring of mothers who had a body mass index greater than 30 kg/m2 had higher all-cause mortality (hazard ratio, 1.35; 95% confidence interval, 1.17-1.55) and increased risk of hospital admission for a cardiovascular event (HR, 1.29; 95% CI, 1.06-1.57), compared with those whose mothers had a healthy BMI.
“Evidence from animal models and emerging data from humans suggest that maternal obesity also creates an adverse in utero environment, with long-term ‘programmed’ detrimental effects for the offspring,” the authors of that BMJ report wrote at the time.
Ms. Van De Maele and her colleagues also cited animal studies, including several looking at offspring of animals fed with a maternal high-fat diet during pregnancy. In those studies, they said, investigators observed impaired endothelial cell relaxation, along with raised thickness of the intimal wall and increased vascular inflammatory marker expression.
“Raised leptin levels, secreted by the adipose tissue, inhibit the in vitro proliferation of smooth muscle cells and could impede the angiogenesis process in vivo, but this assumption needs scientific validation in humans,” they said in their review.
However, human studies are lacking, aside from the epidemiologic reports that “cannot be used to confirm or contradict” the fetal programming hypothesis, they said.
Meanwhile, an increasing body of evidence has suggested that stressors in critical periods of fetal development may lead to epigenetic alterations that could play a role in either up-regulating atherogenic genes or down-regulating enzymatic activities that guard against oxidative stress.
For example, cohort studies have shown differences in DNA methylation among offspring born before and after bariatric surgery in the mother, which has lent credence to the hypothesis that maternal obesity in pregnancy alters methylation patterns for those offspring, Ms. Van De Maele and her colleagues wrote.
Lifestyle changes in obese pregnant women may have an effect on adverse metabolic or cardiovascular outcomes in offspring, although results to date are inconclusive, they added.
Diet, exercise, or both during pregnancy may lower the risk of macrosomia, respiratory distress syndrome, or other neonatal outcomes, particularly in high-risk women, according to the conclusions of a 2015 Cochrane review that Ms. Van De Maele and her coauthors cited.
However, follow-up studies on offspring are scarce and have shown no clear effects on long-term metabolic profiles in offspring, likely because of insufficient follow-up time, they said in their review.
Ms. Van De Maele and her coauthors said they had no conflict of interest disclosures related to their manuscript.
SOURCE: Van De Maele K et al. Atherosclerosis. 2018 Jun. doi: 10.1016/j.atherosclerosis.2018.06.016.
authors of a thematic literature review concluded.
Maternal obesity has been tied to the development of cardiovascular disease (CVD) and premature death in epidemiologic studies, the authors noted in the review.
One hypothesis, referred to as fetal programming, posits that in utero environmental factors may have adverse metabolic consequences in the offspring. Thus far, however, most evidence supporting this hypothesis has come from animal studies, they cautioned.
Nevertheless, endothelial cell dysfunction is a reversible process, offering a “window of opportunity” for intervention, according to authors Karolien Van De Maele and Inge Gies, MD, of the division of pediatric endocrinology at the University Hospital of Brussels and Roland Devlieger, MD, PhD, head of fetal maternal medicine at the University Hospitals Leuven (Belgium).
“The fundamental solution to break the vicious cycle seems [to be] an intervention before or in early pregnancy,” authors said in the journal Atherosclerosis.
Mary Norine Walsh, MD, immediate past president of the American College of Cardiology, agreed with the review article’s conclusion that more evidence would be needed to show that fetal programming is implicated in the associations between maternal obesity and long-term cardiovascular effects.
“As of right now, we cannot say the offspring of pregnant women have an increased risk of cardiovascular risk in later life due to ‘X’ because those studies haven’t been done yet,” Dr. Walsh said in an interview. “So I think it’s a really good framework to think about based on the animal work that’s been done, but we have yet to identify obesity in pregnant women as an independent risk factor for vascular disease in the offspring – we just have an association.”
On the other hand, it is known that obesity increases the risk of hypertension and diabetes in both pregnant and nonpregnant women, said Dr. Walsh, and that hypertensive disorders are a leading cause of maternal morbidity and mortality.
“I think it’s really important to recognize that maternal obesity puts a woman at significant risk, and we certainly can’t forget that in the process of thinking about the offspring,” said Dr. Walsh, medical director of the heart failure and cardiac transplantation program at St. Vincent Heart Center, Indianapolis.
In the recent review article in Atherosclerosis, Ms. Van De Maele and coauthors cited evidence linking maternal obesity to adverse outcomes in offspring from a 2013 report in the BMJ that included 28,540 women in Scotland and their 37,709 offspring.
In that study, after adjustment for maternal age, socioeconomic status, and other factors, offspring of mothers who had a body mass index greater than 30 kg/m2 had higher all-cause mortality (hazard ratio, 1.35; 95% confidence interval, 1.17-1.55) and increased risk of hospital admission for a cardiovascular event (HR, 1.29; 95% CI, 1.06-1.57), compared with those whose mothers had a healthy BMI.
“Evidence from animal models and emerging data from humans suggest that maternal obesity also creates an adverse in utero environment, with long-term ‘programmed’ detrimental effects for the offspring,” the authors of that BMJ report wrote at the time.
Ms. Van De Maele and her colleagues also cited animal studies, including several looking at offspring of animals fed with a maternal high-fat diet during pregnancy. In those studies, they said, investigators observed impaired endothelial cell relaxation, along with raised thickness of the intimal wall and increased vascular inflammatory marker expression.
“Raised leptin levels, secreted by the adipose tissue, inhibit the in vitro proliferation of smooth muscle cells and could impede the angiogenesis process in vivo, but this assumption needs scientific validation in humans,” they said in their review.
However, human studies are lacking, aside from the epidemiologic reports that “cannot be used to confirm or contradict” the fetal programming hypothesis, they said.
Meanwhile, an increasing body of evidence has suggested that stressors in critical periods of fetal development may lead to epigenetic alterations that could play a role in either up-regulating atherogenic genes or down-regulating enzymatic activities that guard against oxidative stress.
For example, cohort studies have shown differences in DNA methylation among offspring born before and after bariatric surgery in the mother, which has lent credence to the hypothesis that maternal obesity in pregnancy alters methylation patterns for those offspring, Ms. Van De Maele and her colleagues wrote.
Lifestyle changes in obese pregnant women may have an effect on adverse metabolic or cardiovascular outcomes in offspring, although results to date are inconclusive, they added.
Diet, exercise, or both during pregnancy may lower the risk of macrosomia, respiratory distress syndrome, or other neonatal outcomes, particularly in high-risk women, according to the conclusions of a 2015 Cochrane review that Ms. Van De Maele and her coauthors cited.
However, follow-up studies on offspring are scarce and have shown no clear effects on long-term metabolic profiles in offspring, likely because of insufficient follow-up time, they said in their review.
Ms. Van De Maele and her coauthors said they had no conflict of interest disclosures related to their manuscript.
SOURCE: Van De Maele K et al. Atherosclerosis. 2018 Jun. doi: 10.1016/j.atherosclerosis.2018.06.016.
FROM ATHEROSCLEROSIS
Quick Byte: PrEP advances
There are recent advances in preexposure prophylaxis, or PrEP, as a promising prevention option for HIV, according to a recent study.1
Reference
1. Desai M, Field N, Grant R, McCormack S. “Recent advances in pre-exposure prophylaxis for HIV.” BMJ. 2017;359:j5011.
There are recent advances in preexposure prophylaxis, or PrEP, as a promising prevention option for HIV, according to a recent study.1
Reference
1. Desai M, Field N, Grant R, McCormack S. “Recent advances in pre-exposure prophylaxis for HIV.” BMJ. 2017;359:j5011.
There are recent advances in preexposure prophylaxis, or PrEP, as a promising prevention option for HIV, according to a recent study.1
Reference
1. Desai M, Field N, Grant R, McCormack S. “Recent advances in pre-exposure prophylaxis for HIV.” BMJ. 2017;359:j5011.
Ethical violations scuttle NIH’s big alcohol study
A controversial study on abdominal aortic aneurysm screening; the importance of healthy lifestyle in diabetes; how NIH scientists corrupted a big alcohol study; and how cardiologists fare in starting salaries.
Listen to MDedge Cardiocast for all the details on the week’s top news.
A controversial study on abdominal aortic aneurysm screening; the importance of healthy lifestyle in diabetes; how NIH scientists corrupted a big alcohol study; and how cardiologists fare in starting salaries.
Listen to MDedge Cardiocast for all the details on the week’s top news.
A controversial study on abdominal aortic aneurysm screening; the importance of healthy lifestyle in diabetes; how NIH scientists corrupted a big alcohol study; and how cardiologists fare in starting salaries.
Listen to MDedge Cardiocast for all the details on the week’s top news.