User login
Recurrence of a small gastric gastrointestinal stromal tumor with high mitotic index
Gastrointestinal stromal tumor (GIST) is the most common soft tissue sarcoma of the gastrointestinal tract, usually arising from the interstitial cells of Cajal or similar cells in the outer wall of the gastrointestinal tract.1,2 Most GISTs have an activating mutation in KIT or platelet-derived growth factor receptor alpha (PDGFRA). Tumor size, mitotic rate, and anatomic site are the most common pathological features used to risk stratify GIST tumors.3-10 It is important to note when using such risk calculators that preoperative imatinib before determining tumor characteristics (such as mitoses per 50 high-power fields [hpf]) often changes the relevant parameters so that the same risk calculations may not apply. Tumors with a mitotic rate ≤5 mitoses per 50 hpf and a size ≤5 cm in greatest dimension have a lower recurrence rate after resection than tumors with a mitotic rate >5 mitoses per 50 hpf and a size >10 cm, and larger tumors can have a recurrence rate of up to 86%.11,12 Findings from a large observational study have suggested that the prognosis of gastric GIST in Korea and Japan may be more favorable compared with that in Western countries.13
The primary treatment of a localized primary GIST is surgical excision, but a cure is limited by recurrence.14,15 Imatinib is useful in the treatment of metastatic or recurrent GIST, and adjuvant treatment with imatinib after surgery has been shown to improve progression-free and overall survival in some cases.3,16-18 Responses to adjuvant imatinib depend on tumor sensitivity to the drug and the risk of recurrence. Drug sensitivity is largely dependent on the presence of mutations in KIT or PDGFRA.3,18 Recurrence risk is highly dependent on tumor size, tumor site, tumor rupture, and mitotic index.1,3,5,6,8,9,18,19 Findings on the use of gene expression patterns to predict recurrence risk have also been reported.20-27 However, recurrence risk is poorly understood for categories in which there are few cases with known outcomes, such as very small gastric GIST with a high mitotic index. For example, few cases of gastric GIST have been reported with a tumor size ≤2 cm, a mitotic rate >5 mitoses per 50 hpf, and adequate clinical follow-up. In such cases, it is difficult to assess the risk of recurrence.6 We report here the long-term outcome of a patient with a 1.8 cm gastric GIST with a mitotic index of 36 mitoses per 50 hpf and a KIT exon 11 mutation.
Case presentation and summary
A 69-year-old man presented with periumbilical and epigastric pain of 6-month duration. His medical history was notable for hyperlipidemia, hypertension, coronary angioplasty, and spinal surgery. He had a 40 pack-year smoking history and consumed 2 to 4 alcoholic drinks per day. The results of a physical examination were unremarkable. A computedtomographic (CT) scan showed no abnormalities. An esophagoduodenoscopy (EGD) revealed gastric ulcers. He was treated successfully with omeprazole 20 mg by mouth daily.
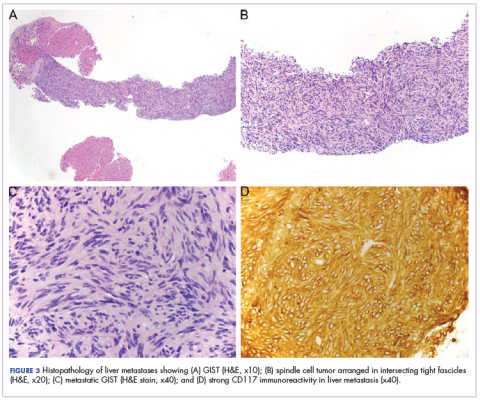
A month later, a follow-up EGD revealed a 1.8 × 1.5 cm submucosal mass 3 cm from the gastroesophageal junction. The patient underwent a fundus wedge resection, and a submucosal mass 1.8 cm in greatest dimension was removed. Pathologic examination revealed a GIST, spindle cell type, with a mitotic rate of 36 mitoses per 50 hpf with negative margins. Immunohistochemistry was positive for CD117. An exon 11 deletion (KVV558-560NV) was present in KIT. The patient’s risk of recurrence was unclear, and his follow-up included CT scans of the abdomen and pelvis every 3 to 4 months for the first 2 years, then every 6 months for the next 2.5 years.
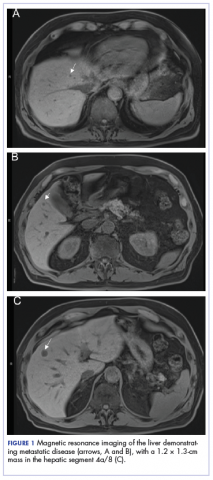
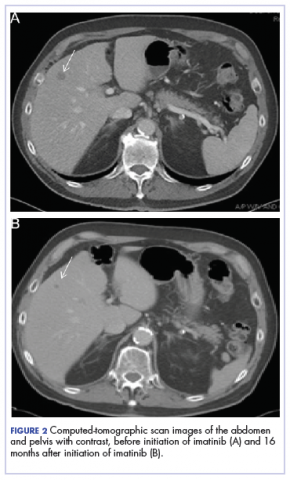
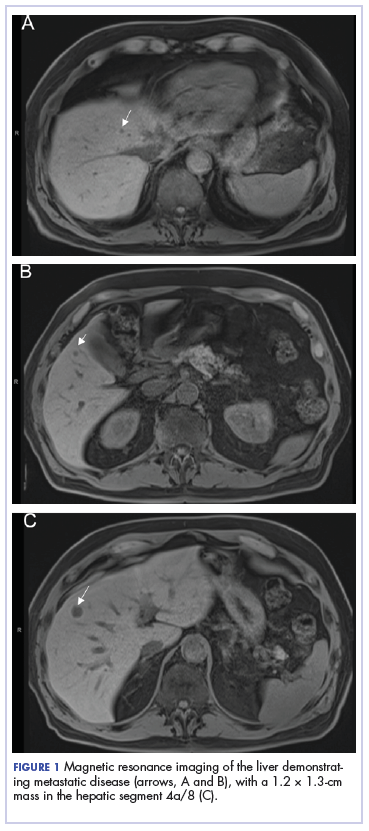
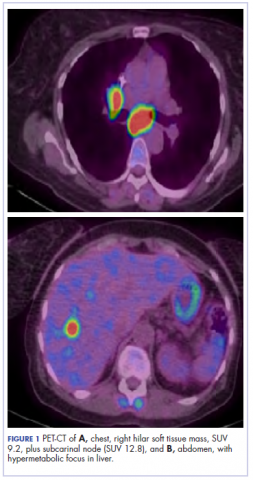
A CT scan about 3.5 years after primary resection revealed small nonspecific liver hypodensities that became more prominent during the next year. About 5 years after primary resection, magnetic resonance imaging (MRI) revealed several liver lesions, the largest of which measured at 1.3 cm in greatest dimension. The patient’s liver metastases were readily identified by MRI (Figure 1) and CT imaging (Figure 2A).
Discussion
Small gastric GISTs are sometimes found by endoscopy performed for unrelated reasons. Recent data suggest that the incidence of gastric GIST may be higher than previously thought. In a Japanese study of patients with gastric cancer in which 100 stomachs were systematically examined pathologically, 50 microscopic GISTs were found in 35 patients.28 Most small gastric GISTs have a low mitotic index. Few cases have been described with a high mitotic index. In a study of 1765 cases of GIST of the stomach, 8 patients had a tumor size less than 2 cm and a mitotic index greater than 5. Of those, only 6 patients had long-term follow-up, and 3 were alive without disease at 2, 17, and 20 years of follow-up.7 These limited data make it impossible to predict outcomes in patients with small gastric GIST with a high mitotic index.
For patients who are at high risk of recurrence after surgery, 3 years of adjuvant imatinib treatment compared with 1 year has been shown to improve overall survival and is the current standard of care.10,17 A study comparing 5 and 3 years of imatinib is ongoing to establish whether a longer period of adjuvant treatment is warranted. In patients with metastatic GIST, lifelong imatinib until lack of benefit is considered optimal treatment.10 All patients should undergo KIT mutation analysis. Those with the PDGFRA D842V mutation, SDH (succinate dehydrogenase) deficiency, or neurofibromatosis-related GIST should not receive adjuvant imatinib.
This case has several unusual features. The small tumor size with a very high mitotic rate is rare. Such cases have not been reported in large numbers and have therefore not been reliably incorporated into risk prediction algorithms. In addition, despite a high mitotic index, the tumor was not FDG avid on PET imaging. The diagnosis of GIST is strongly supported by the KIT mutation and response to imatinib. This particular KIT mutation in larger GISTs is associated with aggressive disease. The present case adds to the data on the biology of small gastric GISTs with a high mitotic index and suggests the mitotic index in these tumors may be a more important predictor than size.
Acknowledgment
The authors thank Michael Franklin, MS, for editorial assistance, and Sabrina Porter for media edits.
1. Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011;11(12):865-878.
2. Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577-580.
3. Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol. 2014;32(15):1563-1570.
4. Huang J, Zheng DL, Qin FS, et al. Genetic and epigenetic silencing of SCARA5 may contribute to human hepatocellular carcinoma by activating FAK signaling. J Clin Invest. 2010;120(1):223-241.
5. Joensuu H, Vehtari A, Riihimaki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13(3):265-274.
6. Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130(10):1466-1478.
7. Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29(1):52-68.
8. Patel S. Navigating risk stratification systems for the management of patients with GIST. Ann Surg Oncol. 2011;18(6):1698-1704.
9. Rossi S, Miceli R, Messerini L, et al. Natural history of imatinib-naive GISTs: a retrospective analysis of 929 cases with long-term follow-up and development of a survival nomogram based on mitotic index and size as continuous variables. Am J Surg Pathol. 2011;35(11):1646-1656.
10. National Comprehensive Cancer Network. Sarcoma. https://www.nccn.org. Accessed March 27, 2018.
11. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol. 2002;10(2):81-89.
12. Huang HY, Li CF, Huang WW, et al. A modification of NIH consensus criteria to better distinguish the highly lethal subset of primary localized gastrointestinal stromal tumors: a subdivision of the original high-risk group on the basis of outcome. Surgery. 2007;141(6):748-756.
13. Kim MC, Yook JH, Yang HK, et al. Long-term surgical outcome of 1057 gastric GISTs according to 7th UICC/AJCC TNM system: multicenter observational study From Korea and Japan. Medicine (Baltimore). 2015;94(41):e1526.
14. Casali PG, Blay JY; ESMO/CONTICANET/EUROBONET Consensus Panel of experts. Soft tissue sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v198-v203.
15. Joensuu H, DeMatteo RP. The management of gastrointestinal stromal tumors: a model for targeted and multidisciplinary therapy of malignancy. Annu Rev Med. 2012;63:247-258.
16. Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373(9669):1097-1104.
17. Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307(12):1265-1272.
18. Joensuu H, Rutkowski P, Nishida T, et al. KIT and PDGFRA mutations and the risk of GI stromal tumor recurrence. J Clin Oncol. 2015;33(6):634-642.
19. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33(5):459-465.
20. Antonescu CR, Viale A, Sarran L, et al. Gene expression in gastrointestinal stromal tumors is distinguished by KIT genotype and anatomic site. Clin Cancer Res. 2004;10(10):3282-3290.
21. Arne G, Kristiansson E, Nerman O, et al. Expression profiling of GIST: CD133 is associated with KIT exon 11 mutations, gastric location and poor prognosis. Int J Cancer. 2011;129(5):1149-1161.
22. Bertucci F, Finetti P, Ostrowski J, et al. Genomic Grade Index predicts postoperative clinical outcome of GIST. Br J Cancer. 2012;107(8):1433-1441.
23. Koon N, Schneider-Stock R, Sarlomo-Rikala M, et al. Molecular targets for tumour progression in gastrointestinal stromal tumours. Gut. 2004;53(2):235-240.
24. Lagarde P, Perot G, Kauffmann A, et al. Mitotic checkpoints and chromosome instability are strong predictors of clinical outcome in gastrointestinal stromal tumors. Clin Cancer Res. 2012;18(3):826-838.
25. Skubitz KM, Geschwind K, Xu WW, Koopmeiners JS, Skubitz AP. Gene expression identifies heterogeneity of metastatic behavior among gastrointestinal stromal tumors. J Transl Med. 2016;14:51.
26. Yamaguchi U, Nakayama R, Honda K, et al. Distinct gene expression-defined classes of gastrointestinal stromal tumor. J Clin Oncol. 2008;26(25):4100-4108.
27. Ylipaa A, Hunt KK, Yang J, et al. Integrative genomic characterization and a genomic staging system for gastrointestinal stromal tumors. Cancer. 2011;117(2):380-389.
28. Kawanowa K, Sakuma Y, Sakurai S, et al. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol. 2006;37(12):1527-1535.
Gastrointestinal stromal tumor (GIST) is the most common soft tissue sarcoma of the gastrointestinal tract, usually arising from the interstitial cells of Cajal or similar cells in the outer wall of the gastrointestinal tract.1,2 Most GISTs have an activating mutation in KIT or platelet-derived growth factor receptor alpha (PDGFRA). Tumor size, mitotic rate, and anatomic site are the most common pathological features used to risk stratify GIST tumors.3-10 It is important to note when using such risk calculators that preoperative imatinib before determining tumor characteristics (such as mitoses per 50 high-power fields [hpf]) often changes the relevant parameters so that the same risk calculations may not apply. Tumors with a mitotic rate ≤5 mitoses per 50 hpf and a size ≤5 cm in greatest dimension have a lower recurrence rate after resection than tumors with a mitotic rate >5 mitoses per 50 hpf and a size >10 cm, and larger tumors can have a recurrence rate of up to 86%.11,12 Findings from a large observational study have suggested that the prognosis of gastric GIST in Korea and Japan may be more favorable compared with that in Western countries.13
The primary treatment of a localized primary GIST is surgical excision, but a cure is limited by recurrence.14,15 Imatinib is useful in the treatment of metastatic or recurrent GIST, and adjuvant treatment with imatinib after surgery has been shown to improve progression-free and overall survival in some cases.3,16-18 Responses to adjuvant imatinib depend on tumor sensitivity to the drug and the risk of recurrence. Drug sensitivity is largely dependent on the presence of mutations in KIT or PDGFRA.3,18 Recurrence risk is highly dependent on tumor size, tumor site, tumor rupture, and mitotic index.1,3,5,6,8,9,18,19 Findings on the use of gene expression patterns to predict recurrence risk have also been reported.20-27 However, recurrence risk is poorly understood for categories in which there are few cases with known outcomes, such as very small gastric GIST with a high mitotic index. For example, few cases of gastric GIST have been reported with a tumor size ≤2 cm, a mitotic rate >5 mitoses per 50 hpf, and adequate clinical follow-up. In such cases, it is difficult to assess the risk of recurrence.6 We report here the long-term outcome of a patient with a 1.8 cm gastric GIST with a mitotic index of 36 mitoses per 50 hpf and a KIT exon 11 mutation.
Case presentation and summary
A 69-year-old man presented with periumbilical and epigastric pain of 6-month duration. His medical history was notable for hyperlipidemia, hypertension, coronary angioplasty, and spinal surgery. He had a 40 pack-year smoking history and consumed 2 to 4 alcoholic drinks per day. The results of a physical examination were unremarkable. A computedtomographic (CT) scan showed no abnormalities. An esophagoduodenoscopy (EGD) revealed gastric ulcers. He was treated successfully with omeprazole 20 mg by mouth daily.
A month later, a follow-up EGD revealed a 1.8 × 1.5 cm submucosal mass 3 cm from the gastroesophageal junction. The patient underwent a fundus wedge resection, and a submucosal mass 1.8 cm in greatest dimension was removed. Pathologic examination revealed a GIST, spindle cell type, with a mitotic rate of 36 mitoses per 50 hpf with negative margins. Immunohistochemistry was positive for CD117. An exon 11 deletion (KVV558-560NV) was present in KIT. The patient’s risk of recurrence was unclear, and his follow-up included CT scans of the abdomen and pelvis every 3 to 4 months for the first 2 years, then every 6 months for the next 2.5 years.
A CT scan about 3.5 years after primary resection revealed small nonspecific liver hypodensities that became more prominent during the next year. About 5 years after primary resection, magnetic resonance imaging (MRI) revealed several liver lesions, the largest of which measured at 1.3 cm in greatest dimension. The patient’s liver metastases were readily identified by MRI (Figure 1) and CT imaging (Figure 2A).
Discussion
Small gastric GISTs are sometimes found by endoscopy performed for unrelated reasons. Recent data suggest that the incidence of gastric GIST may be higher than previously thought. In a Japanese study of patients with gastric cancer in which 100 stomachs were systematically examined pathologically, 50 microscopic GISTs were found in 35 patients.28 Most small gastric GISTs have a low mitotic index. Few cases have been described with a high mitotic index. In a study of 1765 cases of GIST of the stomach, 8 patients had a tumor size less than 2 cm and a mitotic index greater than 5. Of those, only 6 patients had long-term follow-up, and 3 were alive without disease at 2, 17, and 20 years of follow-up.7 These limited data make it impossible to predict outcomes in patients with small gastric GIST with a high mitotic index.
For patients who are at high risk of recurrence after surgery, 3 years of adjuvant imatinib treatment compared with 1 year has been shown to improve overall survival and is the current standard of care.10,17 A study comparing 5 and 3 years of imatinib is ongoing to establish whether a longer period of adjuvant treatment is warranted. In patients with metastatic GIST, lifelong imatinib until lack of benefit is considered optimal treatment.10 All patients should undergo KIT mutation analysis. Those with the PDGFRA D842V mutation, SDH (succinate dehydrogenase) deficiency, or neurofibromatosis-related GIST should not receive adjuvant imatinib.
This case has several unusual features. The small tumor size with a very high mitotic rate is rare. Such cases have not been reported in large numbers and have therefore not been reliably incorporated into risk prediction algorithms. In addition, despite a high mitotic index, the tumor was not FDG avid on PET imaging. The diagnosis of GIST is strongly supported by the KIT mutation and response to imatinib. This particular KIT mutation in larger GISTs is associated with aggressive disease. The present case adds to the data on the biology of small gastric GISTs with a high mitotic index and suggests the mitotic index in these tumors may be a more important predictor than size.
Acknowledgment
The authors thank Michael Franklin, MS, for editorial assistance, and Sabrina Porter for media edits.
Gastrointestinal stromal tumor (GIST) is the most common soft tissue sarcoma of the gastrointestinal tract, usually arising from the interstitial cells of Cajal or similar cells in the outer wall of the gastrointestinal tract.1,2 Most GISTs have an activating mutation in KIT or platelet-derived growth factor receptor alpha (PDGFRA). Tumor size, mitotic rate, and anatomic site are the most common pathological features used to risk stratify GIST tumors.3-10 It is important to note when using such risk calculators that preoperative imatinib before determining tumor characteristics (such as mitoses per 50 high-power fields [hpf]) often changes the relevant parameters so that the same risk calculations may not apply. Tumors with a mitotic rate ≤5 mitoses per 50 hpf and a size ≤5 cm in greatest dimension have a lower recurrence rate after resection than tumors with a mitotic rate >5 mitoses per 50 hpf and a size >10 cm, and larger tumors can have a recurrence rate of up to 86%.11,12 Findings from a large observational study have suggested that the prognosis of gastric GIST in Korea and Japan may be more favorable compared with that in Western countries.13
The primary treatment of a localized primary GIST is surgical excision, but a cure is limited by recurrence.14,15 Imatinib is useful in the treatment of metastatic or recurrent GIST, and adjuvant treatment with imatinib after surgery has been shown to improve progression-free and overall survival in some cases.3,16-18 Responses to adjuvant imatinib depend on tumor sensitivity to the drug and the risk of recurrence. Drug sensitivity is largely dependent on the presence of mutations in KIT or PDGFRA.3,18 Recurrence risk is highly dependent on tumor size, tumor site, tumor rupture, and mitotic index.1,3,5,6,8,9,18,19 Findings on the use of gene expression patterns to predict recurrence risk have also been reported.20-27 However, recurrence risk is poorly understood for categories in which there are few cases with known outcomes, such as very small gastric GIST with a high mitotic index. For example, few cases of gastric GIST have been reported with a tumor size ≤2 cm, a mitotic rate >5 mitoses per 50 hpf, and adequate clinical follow-up. In such cases, it is difficult to assess the risk of recurrence.6 We report here the long-term outcome of a patient with a 1.8 cm gastric GIST with a mitotic index of 36 mitoses per 50 hpf and a KIT exon 11 mutation.
Case presentation and summary
A 69-year-old man presented with periumbilical and epigastric pain of 6-month duration. His medical history was notable for hyperlipidemia, hypertension, coronary angioplasty, and spinal surgery. He had a 40 pack-year smoking history and consumed 2 to 4 alcoholic drinks per day. The results of a physical examination were unremarkable. A computedtomographic (CT) scan showed no abnormalities. An esophagoduodenoscopy (EGD) revealed gastric ulcers. He was treated successfully with omeprazole 20 mg by mouth daily.
A month later, a follow-up EGD revealed a 1.8 × 1.5 cm submucosal mass 3 cm from the gastroesophageal junction. The patient underwent a fundus wedge resection, and a submucosal mass 1.8 cm in greatest dimension was removed. Pathologic examination revealed a GIST, spindle cell type, with a mitotic rate of 36 mitoses per 50 hpf with negative margins. Immunohistochemistry was positive for CD117. An exon 11 deletion (KVV558-560NV) was present in KIT. The patient’s risk of recurrence was unclear, and his follow-up included CT scans of the abdomen and pelvis every 3 to 4 months for the first 2 years, then every 6 months for the next 2.5 years.
A CT scan about 3.5 years after primary resection revealed small nonspecific liver hypodensities that became more prominent during the next year. About 5 years after primary resection, magnetic resonance imaging (MRI) revealed several liver lesions, the largest of which measured at 1.3 cm in greatest dimension. The patient’s liver metastases were readily identified by MRI (Figure 1) and CT imaging (Figure 2A).
Discussion
Small gastric GISTs are sometimes found by endoscopy performed for unrelated reasons. Recent data suggest that the incidence of gastric GIST may be higher than previously thought. In a Japanese study of patients with gastric cancer in which 100 stomachs were systematically examined pathologically, 50 microscopic GISTs were found in 35 patients.28 Most small gastric GISTs have a low mitotic index. Few cases have been described with a high mitotic index. In a study of 1765 cases of GIST of the stomach, 8 patients had a tumor size less than 2 cm and a mitotic index greater than 5. Of those, only 6 patients had long-term follow-up, and 3 were alive without disease at 2, 17, and 20 years of follow-up.7 These limited data make it impossible to predict outcomes in patients with small gastric GIST with a high mitotic index.
For patients who are at high risk of recurrence after surgery, 3 years of adjuvant imatinib treatment compared with 1 year has been shown to improve overall survival and is the current standard of care.10,17 A study comparing 5 and 3 years of imatinib is ongoing to establish whether a longer period of adjuvant treatment is warranted. In patients with metastatic GIST, lifelong imatinib until lack of benefit is considered optimal treatment.10 All patients should undergo KIT mutation analysis. Those with the PDGFRA D842V mutation, SDH (succinate dehydrogenase) deficiency, or neurofibromatosis-related GIST should not receive adjuvant imatinib.
This case has several unusual features. The small tumor size with a very high mitotic rate is rare. Such cases have not been reported in large numbers and have therefore not been reliably incorporated into risk prediction algorithms. In addition, despite a high mitotic index, the tumor was not FDG avid on PET imaging. The diagnosis of GIST is strongly supported by the KIT mutation and response to imatinib. This particular KIT mutation in larger GISTs is associated with aggressive disease. The present case adds to the data on the biology of small gastric GISTs with a high mitotic index and suggests the mitotic index in these tumors may be a more important predictor than size.
Acknowledgment
The authors thank Michael Franklin, MS, for editorial assistance, and Sabrina Porter for media edits.
1. Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011;11(12):865-878.
2. Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577-580.
3. Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol. 2014;32(15):1563-1570.
4. Huang J, Zheng DL, Qin FS, et al. Genetic and epigenetic silencing of SCARA5 may contribute to human hepatocellular carcinoma by activating FAK signaling. J Clin Invest. 2010;120(1):223-241.
5. Joensuu H, Vehtari A, Riihimaki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13(3):265-274.
6. Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130(10):1466-1478.
7. Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29(1):52-68.
8. Patel S. Navigating risk stratification systems for the management of patients with GIST. Ann Surg Oncol. 2011;18(6):1698-1704.
9. Rossi S, Miceli R, Messerini L, et al. Natural history of imatinib-naive GISTs: a retrospective analysis of 929 cases with long-term follow-up and development of a survival nomogram based on mitotic index and size as continuous variables. Am J Surg Pathol. 2011;35(11):1646-1656.
10. National Comprehensive Cancer Network. Sarcoma. https://www.nccn.org. Accessed March 27, 2018.
11. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol. 2002;10(2):81-89.
12. Huang HY, Li CF, Huang WW, et al. A modification of NIH consensus criteria to better distinguish the highly lethal subset of primary localized gastrointestinal stromal tumors: a subdivision of the original high-risk group on the basis of outcome. Surgery. 2007;141(6):748-756.
13. Kim MC, Yook JH, Yang HK, et al. Long-term surgical outcome of 1057 gastric GISTs according to 7th UICC/AJCC TNM system: multicenter observational study From Korea and Japan. Medicine (Baltimore). 2015;94(41):e1526.
14. Casali PG, Blay JY; ESMO/CONTICANET/EUROBONET Consensus Panel of experts. Soft tissue sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v198-v203.
15. Joensuu H, DeMatteo RP. The management of gastrointestinal stromal tumors: a model for targeted and multidisciplinary therapy of malignancy. Annu Rev Med. 2012;63:247-258.
16. Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373(9669):1097-1104.
17. Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307(12):1265-1272.
18. Joensuu H, Rutkowski P, Nishida T, et al. KIT and PDGFRA mutations and the risk of GI stromal tumor recurrence. J Clin Oncol. 2015;33(6):634-642.
19. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33(5):459-465.
20. Antonescu CR, Viale A, Sarran L, et al. Gene expression in gastrointestinal stromal tumors is distinguished by KIT genotype and anatomic site. Clin Cancer Res. 2004;10(10):3282-3290.
21. Arne G, Kristiansson E, Nerman O, et al. Expression profiling of GIST: CD133 is associated with KIT exon 11 mutations, gastric location and poor prognosis. Int J Cancer. 2011;129(5):1149-1161.
22. Bertucci F, Finetti P, Ostrowski J, et al. Genomic Grade Index predicts postoperative clinical outcome of GIST. Br J Cancer. 2012;107(8):1433-1441.
23. Koon N, Schneider-Stock R, Sarlomo-Rikala M, et al. Molecular targets for tumour progression in gastrointestinal stromal tumours. Gut. 2004;53(2):235-240.
24. Lagarde P, Perot G, Kauffmann A, et al. Mitotic checkpoints and chromosome instability are strong predictors of clinical outcome in gastrointestinal stromal tumors. Clin Cancer Res. 2012;18(3):826-838.
25. Skubitz KM, Geschwind K, Xu WW, Koopmeiners JS, Skubitz AP. Gene expression identifies heterogeneity of metastatic behavior among gastrointestinal stromal tumors. J Transl Med. 2016;14:51.
26. Yamaguchi U, Nakayama R, Honda K, et al. Distinct gene expression-defined classes of gastrointestinal stromal tumor. J Clin Oncol. 2008;26(25):4100-4108.
27. Ylipaa A, Hunt KK, Yang J, et al. Integrative genomic characterization and a genomic staging system for gastrointestinal stromal tumors. Cancer. 2011;117(2):380-389.
28. Kawanowa K, Sakuma Y, Sakurai S, et al. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol. 2006;37(12):1527-1535.
1. Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011;11(12):865-878.
2. Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577-580.
3. Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol. 2014;32(15):1563-1570.
4. Huang J, Zheng DL, Qin FS, et al. Genetic and epigenetic silencing of SCARA5 may contribute to human hepatocellular carcinoma by activating FAK signaling. J Clin Invest. 2010;120(1):223-241.
5. Joensuu H, Vehtari A, Riihimaki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13(3):265-274.
6. Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130(10):1466-1478.
7. Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29(1):52-68.
8. Patel S. Navigating risk stratification systems for the management of patients with GIST. Ann Surg Oncol. 2011;18(6):1698-1704.
9. Rossi S, Miceli R, Messerini L, et al. Natural history of imatinib-naive GISTs: a retrospective analysis of 929 cases with long-term follow-up and development of a survival nomogram based on mitotic index and size as continuous variables. Am J Surg Pathol. 2011;35(11):1646-1656.
10. National Comprehensive Cancer Network. Sarcoma. https://www.nccn.org. Accessed March 27, 2018.
11. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol. 2002;10(2):81-89.
12. Huang HY, Li CF, Huang WW, et al. A modification of NIH consensus criteria to better distinguish the highly lethal subset of primary localized gastrointestinal stromal tumors: a subdivision of the original high-risk group on the basis of outcome. Surgery. 2007;141(6):748-756.
13. Kim MC, Yook JH, Yang HK, et al. Long-term surgical outcome of 1057 gastric GISTs according to 7th UICC/AJCC TNM system: multicenter observational study From Korea and Japan. Medicine (Baltimore). 2015;94(41):e1526.
14. Casali PG, Blay JY; ESMO/CONTICANET/EUROBONET Consensus Panel of experts. Soft tissue sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v198-v203.
15. Joensuu H, DeMatteo RP. The management of gastrointestinal stromal tumors: a model for targeted and multidisciplinary therapy of malignancy. Annu Rev Med. 2012;63:247-258.
16. Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373(9669):1097-1104.
17. Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307(12):1265-1272.
18. Joensuu H, Rutkowski P, Nishida T, et al. KIT and PDGFRA mutations and the risk of GI stromal tumor recurrence. J Clin Oncol. 2015;33(6):634-642.
19. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33(5):459-465.
20. Antonescu CR, Viale A, Sarran L, et al. Gene expression in gastrointestinal stromal tumors is distinguished by KIT genotype and anatomic site. Clin Cancer Res. 2004;10(10):3282-3290.
21. Arne G, Kristiansson E, Nerman O, et al. Expression profiling of GIST: CD133 is associated with KIT exon 11 mutations, gastric location and poor prognosis. Int J Cancer. 2011;129(5):1149-1161.
22. Bertucci F, Finetti P, Ostrowski J, et al. Genomic Grade Index predicts postoperative clinical outcome of GIST. Br J Cancer. 2012;107(8):1433-1441.
23. Koon N, Schneider-Stock R, Sarlomo-Rikala M, et al. Molecular targets for tumour progression in gastrointestinal stromal tumours. Gut. 2004;53(2):235-240.
24. Lagarde P, Perot G, Kauffmann A, et al. Mitotic checkpoints and chromosome instability are strong predictors of clinical outcome in gastrointestinal stromal tumors. Clin Cancer Res. 2012;18(3):826-838.
25. Skubitz KM, Geschwind K, Xu WW, Koopmeiners JS, Skubitz AP. Gene expression identifies heterogeneity of metastatic behavior among gastrointestinal stromal tumors. J Transl Med. 2016;14:51.
26. Yamaguchi U, Nakayama R, Honda K, et al. Distinct gene expression-defined classes of gastrointestinal stromal tumor. J Clin Oncol. 2008;26(25):4100-4108.
27. Ylipaa A, Hunt KK, Yang J, et al. Integrative genomic characterization and a genomic staging system for gastrointestinal stromal tumors. Cancer. 2011;117(2):380-389.
28. Kawanowa K, Sakuma Y, Sakurai S, et al. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol. 2006;37(12):1527-1535.
Striking rash in a patient with lung cancer on a checkpoint inhibitor
Lung cancer remains the most common cause of cancer death in the United States and worldwide.1 Despite advances in the treatment of the disease and development of targeted therapy, the 5-year overall survival in stage IV non–small-cell lung cancer remains poor, ranging from 6% to 10%.2 More recently, checkpoint inhibitors have had a major impact on the treatment of lung cancer. Nivolumab was the first program cell death protein-1 (PD-1) inhibitor approved for malignant melanoma.3 In July 2015, it was approved as a second-line treatment of squamous cell carcinoma of the lung.4 Since then, the use of nivolumab has extended to other malignancies such as head and neck cancer, renal cell carcinoma, and the list continues to expand. In lung cancer, it demonstrated superior overall survival of 9 months, compared with 6 months with docetaxel.4 Other checkpoint inhibitors such as pembrolizumab5 and atezolizumab6 were subsequently developed, and are also used in the treatment of lung cancer.
Serious potential autoimmune complications arise in up to 30% of patients treated with PD-1 inhibitors. Dermatologic toxicity is the most common immune-related adverse event in these patients. In addition to vitiligo, most common is a reticular maculopapular rash on the trunk and extremities. Other adverse events, such as photosensitivity, alopecia, xerosis, and hair color changes, are reported less frequently.7 We report here a case of rash at an unusual location (auricular and periauricular) with skin exfoliation mimicking other common skin conditions such as eczema and psoriasis.
Case presentation and summary
A 57-year-old woman with a history of cerebrovascular accident with residual left lower-leg paresis presented for acute onset expressive aphasia in the absence of other constitutional or neurological findings. Magnetic resonance imaging of the brain showed a posterior, left parietal lobe lesion of 1.6 cm with intralesional hemorrhage and surrounding edema suggestive of brain metastasis. The patient had a 35 pack-year history of smoking. A staging work-up with computed-tomographic (CT) scans showed a spiculated enhancing nodule in the superior segment of the right lower lobe plus mediastinal adenopathy.
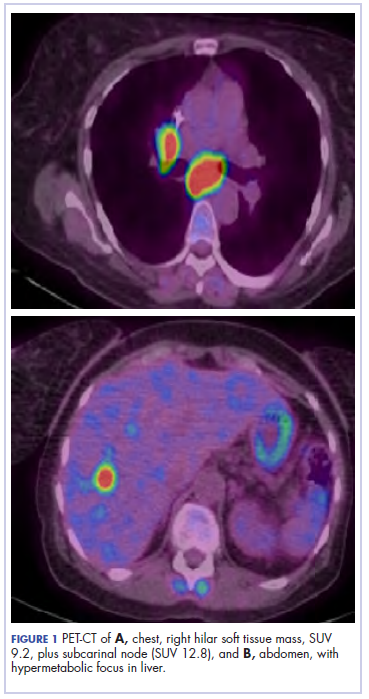
The patient underwent a CT-guided core biopsy of the spiculated nodule, which was found to be consistent with adenocarcinoma of the lung. It was negative for EGFR mutation or ALK rearrangement. She received stereotactic radiosurgery to the left posterior parietal lesion, and after completion of radiation, was started on systemic chemotherapy with cisplatin plus pemetrexed for adenocarcinoma of the lung. She received 4 cycles of chemotherapy. Repeat imaging with a PET-CT showed interval increase of the mediastinal hypermetabolic lymphadenopathy with new hypermetabolic pretracheal lymph nodes and interval development of multiple liver metastases in the right and left lobes of the liver (Figure 1). She was started on second-line therapy with nivolumab at a dose of 240 mg every 2 weeks. The treatment was complicated initially by new onset grade 2 papular pruritic rash after cycle 2 of therapy. The rash involved the upper and lower extremities, sparing the palms, soles, trunk, abdomen, and the back. It resolved with treatment delay and topical steroids.
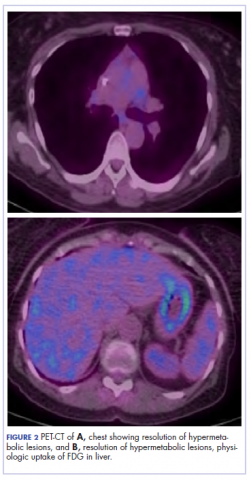
The patient resumed treatment with nivolumab after complete resolution of the rash. However, she developed grade 2 nephritis after cycle 5 with a creatinine level of 1.98 mg/dL (reference range, 0.6-1.2 mg/ dL). This was resolved after treatment with oral prednisone, at a starting dose of 1 mg/kg and tapered over 4 weeks. PET CT scans obtained after cycles 5 and 11 showed no metabolic activity in the mediastinum or the liver and markedly decreased uptake in the right lower lobe nodule, down to an SUV of 1.7 with no new nodules. An MRI of the brain was stable (Figure 2).
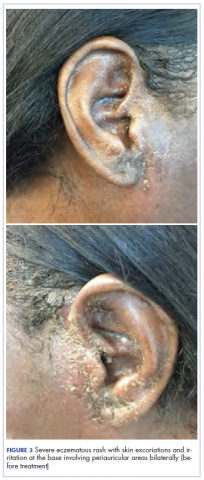
After cycle 16 of nivolumab, the patient developed a severe eczematous rash with excoriations at the base of both ears involving the periauricular and auricular areas bilaterally (Figure 3).
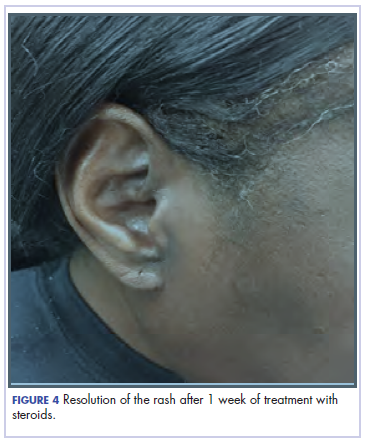
She completed 4 weeks of steroid therapy on a tapering schedule. Treatment with nivolumab was resumed afterward with no adverse autoimmune complications. At her last visit (25 months after initiating a PD-1 inhibitor), there was no clinical or radiologic evidence of lung cancer nor any of autoimmune adverse effects.
Discussion
Among multiple autoimmune complications, dermatologic toxicity is the most common immune-related adverse event, occuring in about 30% to 40% of patients7,8 and with an average onset of 3-4 weeks after initiating treatment with checkpoint inhibitors.9 In addition to vitiligo, the most common type of rash described is a reticular maculopapular rash on the trunk and extremities.10 Other findings, such as photosensitivity, alopecia, xerosis, and hair color changes, have been reported in smaller numbers. Skin exfoliation, as seen in the present case, has been reported in fewer than 1% of the cases.4 Perivascular lymphocytic infiltrates extending deep into the dermis are most likely to be seen if the lesions are biopsied. Both the location of the rash in our patient and its relapsing nature are rare and make it more interesting as it presents a diagnostic dilemma for treating physicians. Ear, nose, and throat surgeons are more likely to encounter such a complication with the expanded use of PD-1 and PD-ligand 1 inhibitors in advanced head and neck cancers. The differential diagnosis includes localized eczema, psoriatic rash, skin infection, or an autoimmune phenomenon.
The location of the rash was also of concern because there have been reports of autoimmune inner-ear disease related to immunotherapy.11 After the failure of treatment with empiric antibiotics and topical steroids, in addition to the development of a new rash on her abdomen, we concluded that this case might represent an unusual autoimmune skin complication. The resolution of the skin lesions in both locations (the ears and the abdomen) with the oral steroid therapy, supported our suspected diagnosis of autoimmune dermatitis.
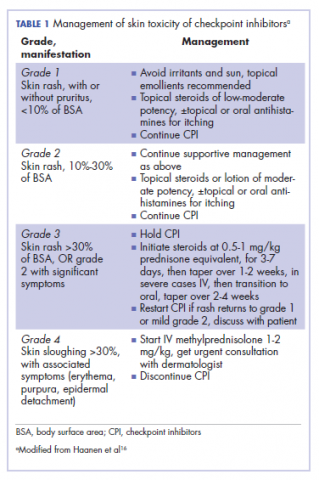
It is essential that these complications are detected early and misdiagnosis is avoided because timely treatment with steroids will prevent progression to more severe problems such as Steven-Johnson syndrome, toxic epidermal necrolysis,12 or extension into the inner ear.11This case is part of a growing spectrum of other unusual cases seen with immunotherapy treatment, such as erythema nodosum-like reactions,13 bullous dermatitis,14 and psoriasiform eruptions.15 It highlights the need for an awareness of expanding dermatologic complications from immunotherapy beyond the reported common manifestations. Established guidelines and algorithms for the management of immune-related dermatologic toxicity are available to assist the physician in treatment (Table 1).16 Skin biopsy should be considered if the diagnosis remains uncertain, although starting empiric treatment with steroids is a widely acceptable approach. Reassessing the skin rash in 48 hours to 1 week after treatment initiation is crucial because steroid-refractory cases will need additional immunosuppression. Early termination of steroids is associated with higher recurrence rate, therefore tapering steroids over 4 weeks is highly recommended before resuming treatment with checkpoint inhibitors.
In summary, increased awareness among health care professionals of the common and unusual complications of immunotherapy agents is important and essential in patient care. In addition to oncologists, head and neck surgeons, pulmonologists, urologists, dermatologists, and general internists will encounter patients with immunotherapy-related complications. Patient education should be emphasized to ensure prompt investigation and treatment of complications. Finally, it is not yet clear whether the development of autoimmune reactions predicts disease response to treatment. In a series of 134 patients with lung cancer, the occurrence of autoimmune adverse events correlated with improved survival.17 More research is needed to identify prognostic and predictive biomarkers for response to immunotherapy.
Conclusion
This pattern of autoimmune dermatitis localizing to the ears is rare (<1% of cases of dermatitis). Nevertheless, it raises the awareness for dermatologic complications of immunotherapy beyond the classical reported manifestations. Prompt diagnosis and treatment is essential to avoid serious complications such as Steven-Johnson syndrome, toxic epidermal necrolysis, and potentially damage to the inner ear.
1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87-108.
2. Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39-51.
3. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
4. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123-135.
5. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemo-therapy for PD- L1- positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823- 1833.
6. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255-265.
7. Collins LK, Chapman MS, Carter JB, Samie FH. Cutaneous adverse events of the immune checkpoint inhibitors. Curr Prob Cancer. 2017;41:125-128.
8. Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375.
9. Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-2697.
10. Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12-25.
11. Zibelman M, Pollak N, Olszanski AJ. Autoimmune inner ear disease in a melanoma patient treated with pembrolizumab. J Immunother Cancer. 2016;4:8.
12. Nayar N, Briscoe K, Penas PF. Toxic epidermal necrolysis-like reaction with severe satellite cell necrosis associated with nivolumab in a patient with ipilimumab refractory metastatic melanoma. J Immunother. 2016;39(3):149-152.
13. Tetzlaff MT, Jazaeri AA, Torres-Cabala CA, et al. Erythema nodosum-like panniculitis mimicking disease recurrence: a novel toxicity from immune checkpoint blockade therapy - report of 2 patients. J Cutan Pathol. 2017;44(12):1080-1086.
14. Naidoo J, Schindler K, Querfeld C, et al. Autoimmune bullous skin disorders with immune checkpoint inhibitors targeting PD-1 and PD-L1. Cancer Immunol Res. 2016;4(5):383-389.
15. Ohtsuka M, Miura T, Mori T, Ishikawa M, Yamamoto T. Occurrence of psoriasiform eruption during nivolumab therapy for primary oral mucosal melanoma. JAMA Dermatol. 2015;151(7):797-799.
16. Haanen JBAG, Carbonnel F, Robert C, et al; ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv119-iv142.
17. Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4(3):374-378.
Lung cancer remains the most common cause of cancer death in the United States and worldwide.1 Despite advances in the treatment of the disease and development of targeted therapy, the 5-year overall survival in stage IV non–small-cell lung cancer remains poor, ranging from 6% to 10%.2 More recently, checkpoint inhibitors have had a major impact on the treatment of lung cancer. Nivolumab was the first program cell death protein-1 (PD-1) inhibitor approved for malignant melanoma.3 In July 2015, it was approved as a second-line treatment of squamous cell carcinoma of the lung.4 Since then, the use of nivolumab has extended to other malignancies such as head and neck cancer, renal cell carcinoma, and the list continues to expand. In lung cancer, it demonstrated superior overall survival of 9 months, compared with 6 months with docetaxel.4 Other checkpoint inhibitors such as pembrolizumab5 and atezolizumab6 were subsequently developed, and are also used in the treatment of lung cancer.
Serious potential autoimmune complications arise in up to 30% of patients treated with PD-1 inhibitors. Dermatologic toxicity is the most common immune-related adverse event in these patients. In addition to vitiligo, most common is a reticular maculopapular rash on the trunk and extremities. Other adverse events, such as photosensitivity, alopecia, xerosis, and hair color changes, are reported less frequently.7 We report here a case of rash at an unusual location (auricular and periauricular) with skin exfoliation mimicking other common skin conditions such as eczema and psoriasis.
Case presentation and summary
A 57-year-old woman with a history of cerebrovascular accident with residual left lower-leg paresis presented for acute onset expressive aphasia in the absence of other constitutional or neurological findings. Magnetic resonance imaging of the brain showed a posterior, left parietal lobe lesion of 1.6 cm with intralesional hemorrhage and surrounding edema suggestive of brain metastasis. The patient had a 35 pack-year history of smoking. A staging work-up with computed-tomographic (CT) scans showed a spiculated enhancing nodule in the superior segment of the right lower lobe plus mediastinal adenopathy.
The patient underwent a CT-guided core biopsy of the spiculated nodule, which was found to be consistent with adenocarcinoma of the lung. It was negative for EGFR mutation or ALK rearrangement. She received stereotactic radiosurgery to the left posterior parietal lesion, and after completion of radiation, was started on systemic chemotherapy with cisplatin plus pemetrexed for adenocarcinoma of the lung. She received 4 cycles of chemotherapy. Repeat imaging with a PET-CT showed interval increase of the mediastinal hypermetabolic lymphadenopathy with new hypermetabolic pretracheal lymph nodes and interval development of multiple liver metastases in the right and left lobes of the liver (Figure 1). She was started on second-line therapy with nivolumab at a dose of 240 mg every 2 weeks. The treatment was complicated initially by new onset grade 2 papular pruritic rash after cycle 2 of therapy. The rash involved the upper and lower extremities, sparing the palms, soles, trunk, abdomen, and the back. It resolved with treatment delay and topical steroids.
The patient resumed treatment with nivolumab after complete resolution of the rash. However, she developed grade 2 nephritis after cycle 5 with a creatinine level of 1.98 mg/dL (reference range, 0.6-1.2 mg/ dL). This was resolved after treatment with oral prednisone, at a starting dose of 1 mg/kg and tapered over 4 weeks. PET CT scans obtained after cycles 5 and 11 showed no metabolic activity in the mediastinum or the liver and markedly decreased uptake in the right lower lobe nodule, down to an SUV of 1.7 with no new nodules. An MRI of the brain was stable (Figure 2).
After cycle 16 of nivolumab, the patient developed a severe eczematous rash with excoriations at the base of both ears involving the periauricular and auricular areas bilaterally (Figure 3).
She completed 4 weeks of steroid therapy on a tapering schedule. Treatment with nivolumab was resumed afterward with no adverse autoimmune complications. At her last visit (25 months after initiating a PD-1 inhibitor), there was no clinical or radiologic evidence of lung cancer nor any of autoimmune adverse effects.
Discussion
Among multiple autoimmune complications, dermatologic toxicity is the most common immune-related adverse event, occuring in about 30% to 40% of patients7,8 and with an average onset of 3-4 weeks after initiating treatment with checkpoint inhibitors.9 In addition to vitiligo, the most common type of rash described is a reticular maculopapular rash on the trunk and extremities.10 Other findings, such as photosensitivity, alopecia, xerosis, and hair color changes, have been reported in smaller numbers. Skin exfoliation, as seen in the present case, has been reported in fewer than 1% of the cases.4 Perivascular lymphocytic infiltrates extending deep into the dermis are most likely to be seen if the lesions are biopsied. Both the location of the rash in our patient and its relapsing nature are rare and make it more interesting as it presents a diagnostic dilemma for treating physicians. Ear, nose, and throat surgeons are more likely to encounter such a complication with the expanded use of PD-1 and PD-ligand 1 inhibitors in advanced head and neck cancers. The differential diagnosis includes localized eczema, psoriatic rash, skin infection, or an autoimmune phenomenon.
The location of the rash was also of concern because there have been reports of autoimmune inner-ear disease related to immunotherapy.11 After the failure of treatment with empiric antibiotics and topical steroids, in addition to the development of a new rash on her abdomen, we concluded that this case might represent an unusual autoimmune skin complication. The resolution of the skin lesions in both locations (the ears and the abdomen) with the oral steroid therapy, supported our suspected diagnosis of autoimmune dermatitis.
It is essential that these complications are detected early and misdiagnosis is avoided because timely treatment with steroids will prevent progression to more severe problems such as Steven-Johnson syndrome, toxic epidermal necrolysis,12 or extension into the inner ear.11This case is part of a growing spectrum of other unusual cases seen with immunotherapy treatment, such as erythema nodosum-like reactions,13 bullous dermatitis,14 and psoriasiform eruptions.15 It highlights the need for an awareness of expanding dermatologic complications from immunotherapy beyond the reported common manifestations. Established guidelines and algorithms for the management of immune-related dermatologic toxicity are available to assist the physician in treatment (Table 1).16 Skin biopsy should be considered if the diagnosis remains uncertain, although starting empiric treatment with steroids is a widely acceptable approach. Reassessing the skin rash in 48 hours to 1 week after treatment initiation is crucial because steroid-refractory cases will need additional immunosuppression. Early termination of steroids is associated with higher recurrence rate, therefore tapering steroids over 4 weeks is highly recommended before resuming treatment with checkpoint inhibitors.
In summary, increased awareness among health care professionals of the common and unusual complications of immunotherapy agents is important and essential in patient care. In addition to oncologists, head and neck surgeons, pulmonologists, urologists, dermatologists, and general internists will encounter patients with immunotherapy-related complications. Patient education should be emphasized to ensure prompt investigation and treatment of complications. Finally, it is not yet clear whether the development of autoimmune reactions predicts disease response to treatment. In a series of 134 patients with lung cancer, the occurrence of autoimmune adverse events correlated with improved survival.17 More research is needed to identify prognostic and predictive biomarkers for response to immunotherapy.
Conclusion
This pattern of autoimmune dermatitis localizing to the ears is rare (<1% of cases of dermatitis). Nevertheless, it raises the awareness for dermatologic complications of immunotherapy beyond the classical reported manifestations. Prompt diagnosis and treatment is essential to avoid serious complications such as Steven-Johnson syndrome, toxic epidermal necrolysis, and potentially damage to the inner ear.
Lung cancer remains the most common cause of cancer death in the United States and worldwide.1 Despite advances in the treatment of the disease and development of targeted therapy, the 5-year overall survival in stage IV non–small-cell lung cancer remains poor, ranging from 6% to 10%.2 More recently, checkpoint inhibitors have had a major impact on the treatment of lung cancer. Nivolumab was the first program cell death protein-1 (PD-1) inhibitor approved for malignant melanoma.3 In July 2015, it was approved as a second-line treatment of squamous cell carcinoma of the lung.4 Since then, the use of nivolumab has extended to other malignancies such as head and neck cancer, renal cell carcinoma, and the list continues to expand. In lung cancer, it demonstrated superior overall survival of 9 months, compared with 6 months with docetaxel.4 Other checkpoint inhibitors such as pembrolizumab5 and atezolizumab6 were subsequently developed, and are also used in the treatment of lung cancer.
Serious potential autoimmune complications arise in up to 30% of patients treated with PD-1 inhibitors. Dermatologic toxicity is the most common immune-related adverse event in these patients. In addition to vitiligo, most common is a reticular maculopapular rash on the trunk and extremities. Other adverse events, such as photosensitivity, alopecia, xerosis, and hair color changes, are reported less frequently.7 We report here a case of rash at an unusual location (auricular and periauricular) with skin exfoliation mimicking other common skin conditions such as eczema and psoriasis.
Case presentation and summary
A 57-year-old woman with a history of cerebrovascular accident with residual left lower-leg paresis presented for acute onset expressive aphasia in the absence of other constitutional or neurological findings. Magnetic resonance imaging of the brain showed a posterior, left parietal lobe lesion of 1.6 cm with intralesional hemorrhage and surrounding edema suggestive of brain metastasis. The patient had a 35 pack-year history of smoking. A staging work-up with computed-tomographic (CT) scans showed a spiculated enhancing nodule in the superior segment of the right lower lobe plus mediastinal adenopathy.
The patient underwent a CT-guided core biopsy of the spiculated nodule, which was found to be consistent with adenocarcinoma of the lung. It was negative for EGFR mutation or ALK rearrangement. She received stereotactic radiosurgery to the left posterior parietal lesion, and after completion of radiation, was started on systemic chemotherapy with cisplatin plus pemetrexed for adenocarcinoma of the lung. She received 4 cycles of chemotherapy. Repeat imaging with a PET-CT showed interval increase of the mediastinal hypermetabolic lymphadenopathy with new hypermetabolic pretracheal lymph nodes and interval development of multiple liver metastases in the right and left lobes of the liver (Figure 1). She was started on second-line therapy with nivolumab at a dose of 240 mg every 2 weeks. The treatment was complicated initially by new onset grade 2 papular pruritic rash after cycle 2 of therapy. The rash involved the upper and lower extremities, sparing the palms, soles, trunk, abdomen, and the back. It resolved with treatment delay and topical steroids.
The patient resumed treatment with nivolumab after complete resolution of the rash. However, she developed grade 2 nephritis after cycle 5 with a creatinine level of 1.98 mg/dL (reference range, 0.6-1.2 mg/ dL). This was resolved after treatment with oral prednisone, at a starting dose of 1 mg/kg and tapered over 4 weeks. PET CT scans obtained after cycles 5 and 11 showed no metabolic activity in the mediastinum or the liver and markedly decreased uptake in the right lower lobe nodule, down to an SUV of 1.7 with no new nodules. An MRI of the brain was stable (Figure 2).
After cycle 16 of nivolumab, the patient developed a severe eczematous rash with excoriations at the base of both ears involving the periauricular and auricular areas bilaterally (Figure 3).
She completed 4 weeks of steroid therapy on a tapering schedule. Treatment with nivolumab was resumed afterward with no adverse autoimmune complications. At her last visit (25 months after initiating a PD-1 inhibitor), there was no clinical or radiologic evidence of lung cancer nor any of autoimmune adverse effects.
Discussion
Among multiple autoimmune complications, dermatologic toxicity is the most common immune-related adverse event, occuring in about 30% to 40% of patients7,8 and with an average onset of 3-4 weeks after initiating treatment with checkpoint inhibitors.9 In addition to vitiligo, the most common type of rash described is a reticular maculopapular rash on the trunk and extremities.10 Other findings, such as photosensitivity, alopecia, xerosis, and hair color changes, have been reported in smaller numbers. Skin exfoliation, as seen in the present case, has been reported in fewer than 1% of the cases.4 Perivascular lymphocytic infiltrates extending deep into the dermis are most likely to be seen if the lesions are biopsied. Both the location of the rash in our patient and its relapsing nature are rare and make it more interesting as it presents a diagnostic dilemma for treating physicians. Ear, nose, and throat surgeons are more likely to encounter such a complication with the expanded use of PD-1 and PD-ligand 1 inhibitors in advanced head and neck cancers. The differential diagnosis includes localized eczema, psoriatic rash, skin infection, or an autoimmune phenomenon.
The location of the rash was also of concern because there have been reports of autoimmune inner-ear disease related to immunotherapy.11 After the failure of treatment with empiric antibiotics and topical steroids, in addition to the development of a new rash on her abdomen, we concluded that this case might represent an unusual autoimmune skin complication. The resolution of the skin lesions in both locations (the ears and the abdomen) with the oral steroid therapy, supported our suspected diagnosis of autoimmune dermatitis.
It is essential that these complications are detected early and misdiagnosis is avoided because timely treatment with steroids will prevent progression to more severe problems such as Steven-Johnson syndrome, toxic epidermal necrolysis,12 or extension into the inner ear.11This case is part of a growing spectrum of other unusual cases seen with immunotherapy treatment, such as erythema nodosum-like reactions,13 bullous dermatitis,14 and psoriasiform eruptions.15 It highlights the need for an awareness of expanding dermatologic complications from immunotherapy beyond the reported common manifestations. Established guidelines and algorithms for the management of immune-related dermatologic toxicity are available to assist the physician in treatment (Table 1).16 Skin biopsy should be considered if the diagnosis remains uncertain, although starting empiric treatment with steroids is a widely acceptable approach. Reassessing the skin rash in 48 hours to 1 week after treatment initiation is crucial because steroid-refractory cases will need additional immunosuppression. Early termination of steroids is associated with higher recurrence rate, therefore tapering steroids over 4 weeks is highly recommended before resuming treatment with checkpoint inhibitors.
In summary, increased awareness among health care professionals of the common and unusual complications of immunotherapy agents is important and essential in patient care. In addition to oncologists, head and neck surgeons, pulmonologists, urologists, dermatologists, and general internists will encounter patients with immunotherapy-related complications. Patient education should be emphasized to ensure prompt investigation and treatment of complications. Finally, it is not yet clear whether the development of autoimmune reactions predicts disease response to treatment. In a series of 134 patients with lung cancer, the occurrence of autoimmune adverse events correlated with improved survival.17 More research is needed to identify prognostic and predictive biomarkers for response to immunotherapy.
Conclusion
This pattern of autoimmune dermatitis localizing to the ears is rare (<1% of cases of dermatitis). Nevertheless, it raises the awareness for dermatologic complications of immunotherapy beyond the classical reported manifestations. Prompt diagnosis and treatment is essential to avoid serious complications such as Steven-Johnson syndrome, toxic epidermal necrolysis, and potentially damage to the inner ear.
1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87-108.
2. Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39-51.
3. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
4. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123-135.
5. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemo-therapy for PD- L1- positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823- 1833.
6. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255-265.
7. Collins LK, Chapman MS, Carter JB, Samie FH. Cutaneous adverse events of the immune checkpoint inhibitors. Curr Prob Cancer. 2017;41:125-128.
8. Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375.
9. Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-2697.
10. Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12-25.
11. Zibelman M, Pollak N, Olszanski AJ. Autoimmune inner ear disease in a melanoma patient treated with pembrolizumab. J Immunother Cancer. 2016;4:8.
12. Nayar N, Briscoe K, Penas PF. Toxic epidermal necrolysis-like reaction with severe satellite cell necrosis associated with nivolumab in a patient with ipilimumab refractory metastatic melanoma. J Immunother. 2016;39(3):149-152.
13. Tetzlaff MT, Jazaeri AA, Torres-Cabala CA, et al. Erythema nodosum-like panniculitis mimicking disease recurrence: a novel toxicity from immune checkpoint blockade therapy - report of 2 patients. J Cutan Pathol. 2017;44(12):1080-1086.
14. Naidoo J, Schindler K, Querfeld C, et al. Autoimmune bullous skin disorders with immune checkpoint inhibitors targeting PD-1 and PD-L1. Cancer Immunol Res. 2016;4(5):383-389.
15. Ohtsuka M, Miura T, Mori T, Ishikawa M, Yamamoto T. Occurrence of psoriasiform eruption during nivolumab therapy for primary oral mucosal melanoma. JAMA Dermatol. 2015;151(7):797-799.
16. Haanen JBAG, Carbonnel F, Robert C, et al; ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv119-iv142.
17. Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4(3):374-378.
1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87-108.
2. Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39-51.
3. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
4. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123-135.
5. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemo-therapy for PD- L1- positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823- 1833.
6. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255-265.
7. Collins LK, Chapman MS, Carter JB, Samie FH. Cutaneous adverse events of the immune checkpoint inhibitors. Curr Prob Cancer. 2017;41:125-128.
8. Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375.
9. Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-2697.
10. Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12-25.
11. Zibelman M, Pollak N, Olszanski AJ. Autoimmune inner ear disease in a melanoma patient treated with pembrolizumab. J Immunother Cancer. 2016;4:8.
12. Nayar N, Briscoe K, Penas PF. Toxic epidermal necrolysis-like reaction with severe satellite cell necrosis associated with nivolumab in a patient with ipilimumab refractory metastatic melanoma. J Immunother. 2016;39(3):149-152.
13. Tetzlaff MT, Jazaeri AA, Torres-Cabala CA, et al. Erythema nodosum-like panniculitis mimicking disease recurrence: a novel toxicity from immune checkpoint blockade therapy - report of 2 patients. J Cutan Pathol. 2017;44(12):1080-1086.
14. Naidoo J, Schindler K, Querfeld C, et al. Autoimmune bullous skin disorders with immune checkpoint inhibitors targeting PD-1 and PD-L1. Cancer Immunol Res. 2016;4(5):383-389.
15. Ohtsuka M, Miura T, Mori T, Ishikawa M, Yamamoto T. Occurrence of psoriasiform eruption during nivolumab therapy for primary oral mucosal melanoma. JAMA Dermatol. 2015;151(7):797-799.
16. Haanen JBAG, Carbonnel F, Robert C, et al; ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv119-iv142.
17. Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4(3):374-378.
Effective management of severe radiation dermatitis after head and neck radiotherapy
Head and neck cancer is among the most prevalent cancers in developing countries.1 Most of the patients in developing countries present in locally advanced stages, and radical radiation therapy with concurrent chemotherapy is the standard treatment.1 Radiation therapy is associated with radiation dermatitis, which causes severe symptoms in the patient and can lead to disruption of treatment, diminished rates of disease control rates, and impaired patient quality of life.2 The management of advanced radiation dermatitis is difficult and can cause consequential late morbidity to patients.2 We report here the rare case of a patient with locally advanced tonsil carcinoma who developed grade 3 radiation dermatitis while receiving radical chemoradiation. The patient’s radiation dermatitis was effectively managed with the use of a silver-containing antimicrobial dressing that yielded remarkable results, so the patient was able to resume and complete radiation therapy.
Case presentation and summary
A 48-year-old man was diagnosed with squamous cell carcinoma of the right tonsil, with bilateral neck nodes (Stage T4a N2c M0; The American Joint Committee on Cancer staging manual, 7th edition). In view of the locally advanced status of his disease, the patient was scheduled for radical radiation therapy at 70 Gy in 35 fractions over 7 weeks along with weekly chemotherapy (cisplatin 40 mg/m2). During the course of radiation therapy, the patient was monitored twice a week, and symptomatic care was done for radiation-therapy–induced toxicities.
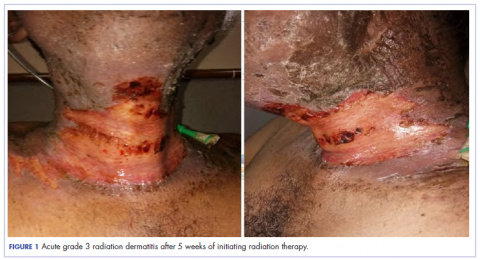
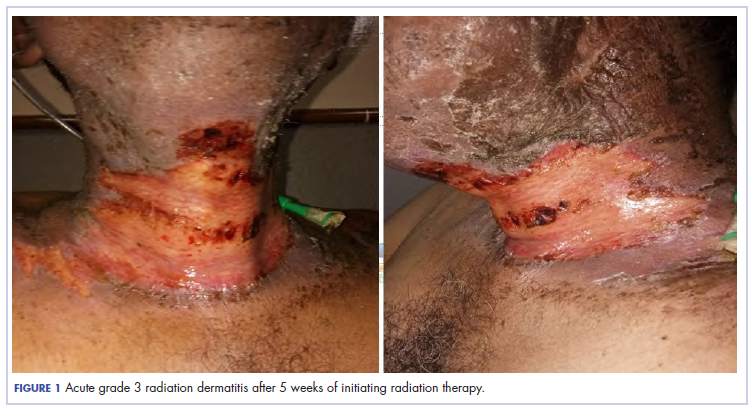
The patient presented with grade 3 radiation dermatitis after receiving 58 Gy in 29 fractions over 5 weeks (grade 0, no change; grades 3 and 4, severe change). The radiation dermatitis involved the anterior and bilateral neck with moist desquamation of the skin (Figure 1).
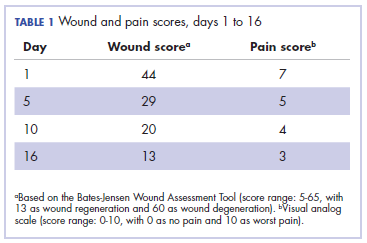
It was associated with severe pain, difficulty in swallowing, and oral mucositis. The patient was subsequently admitted to the hospital; radiation therapy was stopped, and treatment was initiated to ease the effects of the radiation dermatitis. Analgesics were administered for the pain, and adequate hydration and nutritional support was administered through a nasogastric tube. The patient’s score on the Bates-Jensen Wound Assessment Tool (BWAT) for monitoring wound status was 44, which falls in extreme severity status.
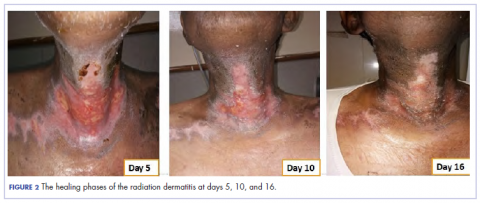
In view of the extreme severity status of the radiation dermatitis, after cleaning the wound with sterile water, we covered it with an antimicrobial dressing that contained silver salt (Mepilex AG; Mölnlycke Health Care, Norcross, GA). The dressing was changed regularly every 4 days. There was a gradual improvement in the radiation dermatitis (Figure 2).
Discussion
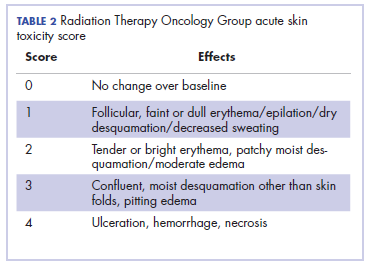
Head and neck cancer is one of the most common cancers in developing countries.1 Most patients present with locally advanced disease, so chemoradiation is the standard treatment in these patents. Radiation therapy is associated with acute and chronic toxicities. The common radiation therapy toxicities are directed at skin and mucosa, which leads to radiation dermatitis and radiation mucositis, respectively.2 These toxicities are graded as per the Radiation Therapy Oncology Group (RTOG) criteria (Table 2).3
Acute radiation dermatitis is radiation therapy dose-dependent and manifests within a few days to weeks after starting external beam radiation therapy. Its presentation varies in severity and gradually manifests as erythema, dry or moist desquamation, and ulceration when severe. These can cause severe symptoms in the patient, leading to frequent breaks in treatment, decreased rates of disease control, and impaired patient quality of life.2 Apart from RTOG grading, radiation dermatitis can also be scored using the BWAT. This tool has been validated across many studies to score initial wound status and monitor the subsequent status numerically.4 The radiation dermatitis of the index case was scored and monitored with both RTOG and BWAT scores.The management of advanced radiation dermatitis is difficult, and it causes consequential late morbidity in patients. A range of topical agents and dressings are used to treat radiation dermatitis, but there is minimal evidence to support their use.5 The Multinational Association for Supportive Care in Cancer treatment guidelines for prevention and treatment of radiation dermatitis have also concluded that there is a lack of sufficient evidence in the literature to support the superiority for any specific intervention.6 Management of radiation dermatitis varies among practitioners because of the inconclusive evidence for available treatment options.
The use of silver-based antimicrobial dressings has been reported in the literature in the prevention and treatment of radiation dermatitis, but with mixed results.7 Such dressings absorb exudate, maintain a moist environment that promotes wound healing, fight infection, and minimize the risk for maceration, according to the product information sheet.8 Clinical study findings have shown silver to be effective in fighting many different types of pathogens, including Methicillin-resistant Staphylococcus aureus and other drug-resistant bacteria.
Aquino-Parsons and colleagues studied 196 patients with breast cancer who were undergoing whole-breast radiation therapy.9 They showed that there was no benefit of silver-containing foam dressings for the prevention of acute grade 3 radiation dermatitis compared with patients who received standard skin care (with moisturizing cream, topical steroids, saline compress, and silver sulfadiazine cream). However, the incidence of itching in the last week of radiation and 1 week after treatment completion was lower among the patients who used the dressings.
Diggelmann and colleagues studied 24 patients with breast cancer who were undergoing radiation therapy.10 Each of the erythematous areas (n = 34) was randomly divided into 2 groups; 1 group was treated with Mepilex Lite dressing and the other with standard aqueous cream. There was a significant reduction in the severity of acute radiation dermatitis in the areas on which Mepilex Lite dressings were used compared with the areas on which standard aqueous cream was used.
The patient in the present case had severe grade 3 acute radiation dermatitis with a BWAT score indicative of extreme severity. After cleaning the wound with sterile water, instead of using the standard aqueous cream on the wounds, we used Mepilex AG, an antimicrobial dressing that contains silver salt. The results were remarkable (Figure 2 and Table 2). The patient was able to restart radiation therapy, and he completed his scheduled doses.
This case highlights the effectiveness of a silver-based antimicrobial dressing in the management of advanced and severe radiation dermatitis. Further large and randomized studies are needed to test the routine use of the dressing in the management of radiation dermatitis.
1. Simard EP, Torre LA, Jemal A. International trends in head and neck cancer incidence rates: differences by country, sex and anatomic site. Oral Oncol. 2014;50(5):387-403.
2. Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54(1):28-46.
3. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341-1346.
4. Harris C, Bates-Jensen B, Parslow N, Raizman R, Singh M, Ketchen R. Bates‐Jensen wound assessment tool: pictorial guide validation project. J Wound Ostomy Continence Nurs. 2010;37(3):253-259.
5. Lucey P, Zouzias C, Franco L, Chennupati SK, Kalnicki S, McLellan BN. Practice patterns for the prophylaxis and treatment of acute radiation dermatitis in the United States. Support Care Cancer. 2017;25(9):2857-2862.
6. Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21(10):2933-2948.
7. Vavassis P, Gelinas M, Chabot Tr J, Nguyen-Tân PF. Phase 2 study of silver leaf dressing for treatment of radiation-induced dermatitis in patients receiving radiotherapy to the head and neck. J Otolaryngology Head Neck Surg. 2008;37(1):124-129.
8. Mepilex Ag product information. Mölnlycke Health Care website. http://www.molnlycke.us/advanced-wound-care-products/antimicrobial-products/mepilex-ag/#confirm. Accessed May 3, 2018.
9. Aquino-Parsons C, Lomas S, Smith K, et al. Phase III study of silver leaf nylon dressing vs standard care for reduction of inframammary moist desquamation in patients undergoing adjuvant whole breast radiation therapy. J Med Imaging Radiat Sci. 2010;41(4):215-221.
10. Diggelmann KV, Zytkovicz AE, Tuaine JM, Bennett NC, Kelly LE, Herst PM. Mepilex Lite dressings for the management of radiation-induced erythema: a systematic inpatient controlled clinical trial. Br J Radiol. 2010;83(995):971-978.
Head and neck cancer is among the most prevalent cancers in developing countries.1 Most of the patients in developing countries present in locally advanced stages, and radical radiation therapy with concurrent chemotherapy is the standard treatment.1 Radiation therapy is associated with radiation dermatitis, which causes severe symptoms in the patient and can lead to disruption of treatment, diminished rates of disease control rates, and impaired patient quality of life.2 The management of advanced radiation dermatitis is difficult and can cause consequential late morbidity to patients.2 We report here the rare case of a patient with locally advanced tonsil carcinoma who developed grade 3 radiation dermatitis while receiving radical chemoradiation. The patient’s radiation dermatitis was effectively managed with the use of a silver-containing antimicrobial dressing that yielded remarkable results, so the patient was able to resume and complete radiation therapy.
Case presentation and summary
A 48-year-old man was diagnosed with squamous cell carcinoma of the right tonsil, with bilateral neck nodes (Stage T4a N2c M0; The American Joint Committee on Cancer staging manual, 7th edition). In view of the locally advanced status of his disease, the patient was scheduled for radical radiation therapy at 70 Gy in 35 fractions over 7 weeks along with weekly chemotherapy (cisplatin 40 mg/m2). During the course of radiation therapy, the patient was monitored twice a week, and symptomatic care was done for radiation-therapy–induced toxicities.
The patient presented with grade 3 radiation dermatitis after receiving 58 Gy in 29 fractions over 5 weeks (grade 0, no change; grades 3 and 4, severe change). The radiation dermatitis involved the anterior and bilateral neck with moist desquamation of the skin (Figure 1).
It was associated with severe pain, difficulty in swallowing, and oral mucositis. The patient was subsequently admitted to the hospital; radiation therapy was stopped, and treatment was initiated to ease the effects of the radiation dermatitis. Analgesics were administered for the pain, and adequate hydration and nutritional support was administered through a nasogastric tube. The patient’s score on the Bates-Jensen Wound Assessment Tool (BWAT) for monitoring wound status was 44, which falls in extreme severity status.
In view of the extreme severity status of the radiation dermatitis, after cleaning the wound with sterile water, we covered it with an antimicrobial dressing that contained silver salt (Mepilex AG; Mölnlycke Health Care, Norcross, GA). The dressing was changed regularly every 4 days. There was a gradual improvement in the radiation dermatitis (Figure 2).
Discussion
Head and neck cancer is one of the most common cancers in developing countries.1 Most patients present with locally advanced disease, so chemoradiation is the standard treatment in these patents. Radiation therapy is associated with acute and chronic toxicities. The common radiation therapy toxicities are directed at skin and mucosa, which leads to radiation dermatitis and radiation mucositis, respectively.2 These toxicities are graded as per the Radiation Therapy Oncology Group (RTOG) criteria (Table 2).3
Acute radiation dermatitis is radiation therapy dose-dependent and manifests within a few days to weeks after starting external beam radiation therapy. Its presentation varies in severity and gradually manifests as erythema, dry or moist desquamation, and ulceration when severe. These can cause severe symptoms in the patient, leading to frequent breaks in treatment, decreased rates of disease control, and impaired patient quality of life.2 Apart from RTOG grading, radiation dermatitis can also be scored using the BWAT. This tool has been validated across many studies to score initial wound status and monitor the subsequent status numerically.4 The radiation dermatitis of the index case was scored and monitored with both RTOG and BWAT scores.The management of advanced radiation dermatitis is difficult, and it causes consequential late morbidity in patients. A range of topical agents and dressings are used to treat radiation dermatitis, but there is minimal evidence to support their use.5 The Multinational Association for Supportive Care in Cancer treatment guidelines for prevention and treatment of radiation dermatitis have also concluded that there is a lack of sufficient evidence in the literature to support the superiority for any specific intervention.6 Management of radiation dermatitis varies among practitioners because of the inconclusive evidence for available treatment options.
The use of silver-based antimicrobial dressings has been reported in the literature in the prevention and treatment of radiation dermatitis, but with mixed results.7 Such dressings absorb exudate, maintain a moist environment that promotes wound healing, fight infection, and minimize the risk for maceration, according to the product information sheet.8 Clinical study findings have shown silver to be effective in fighting many different types of pathogens, including Methicillin-resistant Staphylococcus aureus and other drug-resistant bacteria.
Aquino-Parsons and colleagues studied 196 patients with breast cancer who were undergoing whole-breast radiation therapy.9 They showed that there was no benefit of silver-containing foam dressings for the prevention of acute grade 3 radiation dermatitis compared with patients who received standard skin care (with moisturizing cream, topical steroids, saline compress, and silver sulfadiazine cream). However, the incidence of itching in the last week of radiation and 1 week after treatment completion was lower among the patients who used the dressings.
Diggelmann and colleagues studied 24 patients with breast cancer who were undergoing radiation therapy.10 Each of the erythematous areas (n = 34) was randomly divided into 2 groups; 1 group was treated with Mepilex Lite dressing and the other with standard aqueous cream. There was a significant reduction in the severity of acute radiation dermatitis in the areas on which Mepilex Lite dressings were used compared with the areas on which standard aqueous cream was used.
The patient in the present case had severe grade 3 acute radiation dermatitis with a BWAT score indicative of extreme severity. After cleaning the wound with sterile water, instead of using the standard aqueous cream on the wounds, we used Mepilex AG, an antimicrobial dressing that contains silver salt. The results were remarkable (Figure 2 and Table 2). The patient was able to restart radiation therapy, and he completed his scheduled doses.
This case highlights the effectiveness of a silver-based antimicrobial dressing in the management of advanced and severe radiation dermatitis. Further large and randomized studies are needed to test the routine use of the dressing in the management of radiation dermatitis.
Head and neck cancer is among the most prevalent cancers in developing countries.1 Most of the patients in developing countries present in locally advanced stages, and radical radiation therapy with concurrent chemotherapy is the standard treatment.1 Radiation therapy is associated with radiation dermatitis, which causes severe symptoms in the patient and can lead to disruption of treatment, diminished rates of disease control rates, and impaired patient quality of life.2 The management of advanced radiation dermatitis is difficult and can cause consequential late morbidity to patients.2 We report here the rare case of a patient with locally advanced tonsil carcinoma who developed grade 3 radiation dermatitis while receiving radical chemoradiation. The patient’s radiation dermatitis was effectively managed with the use of a silver-containing antimicrobial dressing that yielded remarkable results, so the patient was able to resume and complete radiation therapy.
Case presentation and summary
A 48-year-old man was diagnosed with squamous cell carcinoma of the right tonsil, with bilateral neck nodes (Stage T4a N2c M0; The American Joint Committee on Cancer staging manual, 7th edition). In view of the locally advanced status of his disease, the patient was scheduled for radical radiation therapy at 70 Gy in 35 fractions over 7 weeks along with weekly chemotherapy (cisplatin 40 mg/m2). During the course of radiation therapy, the patient was monitored twice a week, and symptomatic care was done for radiation-therapy–induced toxicities.
The patient presented with grade 3 radiation dermatitis after receiving 58 Gy in 29 fractions over 5 weeks (grade 0, no change; grades 3 and 4, severe change). The radiation dermatitis involved the anterior and bilateral neck with moist desquamation of the skin (Figure 1).
It was associated with severe pain, difficulty in swallowing, and oral mucositis. The patient was subsequently admitted to the hospital; radiation therapy was stopped, and treatment was initiated to ease the effects of the radiation dermatitis. Analgesics were administered for the pain, and adequate hydration and nutritional support was administered through a nasogastric tube. The patient’s score on the Bates-Jensen Wound Assessment Tool (BWAT) for monitoring wound status was 44, which falls in extreme severity status.
In view of the extreme severity status of the radiation dermatitis, after cleaning the wound with sterile water, we covered it with an antimicrobial dressing that contained silver salt (Mepilex AG; Mölnlycke Health Care, Norcross, GA). The dressing was changed regularly every 4 days. There was a gradual improvement in the radiation dermatitis (Figure 2).
Discussion
Head and neck cancer is one of the most common cancers in developing countries.1 Most patients present with locally advanced disease, so chemoradiation is the standard treatment in these patents. Radiation therapy is associated with acute and chronic toxicities. The common radiation therapy toxicities are directed at skin and mucosa, which leads to radiation dermatitis and radiation mucositis, respectively.2 These toxicities are graded as per the Radiation Therapy Oncology Group (RTOG) criteria (Table 2).3
Acute radiation dermatitis is radiation therapy dose-dependent and manifests within a few days to weeks after starting external beam radiation therapy. Its presentation varies in severity and gradually manifests as erythema, dry or moist desquamation, and ulceration when severe. These can cause severe symptoms in the patient, leading to frequent breaks in treatment, decreased rates of disease control, and impaired patient quality of life.2 Apart from RTOG grading, radiation dermatitis can also be scored using the BWAT. This tool has been validated across many studies to score initial wound status and monitor the subsequent status numerically.4 The radiation dermatitis of the index case was scored and monitored with both RTOG and BWAT scores.The management of advanced radiation dermatitis is difficult, and it causes consequential late morbidity in patients. A range of topical agents and dressings are used to treat radiation dermatitis, but there is minimal evidence to support their use.5 The Multinational Association for Supportive Care in Cancer treatment guidelines for prevention and treatment of radiation dermatitis have also concluded that there is a lack of sufficient evidence in the literature to support the superiority for any specific intervention.6 Management of radiation dermatitis varies among practitioners because of the inconclusive evidence for available treatment options.
The use of silver-based antimicrobial dressings has been reported in the literature in the prevention and treatment of radiation dermatitis, but with mixed results.7 Such dressings absorb exudate, maintain a moist environment that promotes wound healing, fight infection, and minimize the risk for maceration, according to the product information sheet.8 Clinical study findings have shown silver to be effective in fighting many different types of pathogens, including Methicillin-resistant Staphylococcus aureus and other drug-resistant bacteria.
Aquino-Parsons and colleagues studied 196 patients with breast cancer who were undergoing whole-breast radiation therapy.9 They showed that there was no benefit of silver-containing foam dressings for the prevention of acute grade 3 radiation dermatitis compared with patients who received standard skin care (with moisturizing cream, topical steroids, saline compress, and silver sulfadiazine cream). However, the incidence of itching in the last week of radiation and 1 week after treatment completion was lower among the patients who used the dressings.
Diggelmann and colleagues studied 24 patients with breast cancer who were undergoing radiation therapy.10 Each of the erythematous areas (n = 34) was randomly divided into 2 groups; 1 group was treated with Mepilex Lite dressing and the other with standard aqueous cream. There was a significant reduction in the severity of acute radiation dermatitis in the areas on which Mepilex Lite dressings were used compared with the areas on which standard aqueous cream was used.
The patient in the present case had severe grade 3 acute radiation dermatitis with a BWAT score indicative of extreme severity. After cleaning the wound with sterile water, instead of using the standard aqueous cream on the wounds, we used Mepilex AG, an antimicrobial dressing that contains silver salt. The results were remarkable (Figure 2 and Table 2). The patient was able to restart radiation therapy, and he completed his scheduled doses.
This case highlights the effectiveness of a silver-based antimicrobial dressing in the management of advanced and severe radiation dermatitis. Further large and randomized studies are needed to test the routine use of the dressing in the management of radiation dermatitis.
1. Simard EP, Torre LA, Jemal A. International trends in head and neck cancer incidence rates: differences by country, sex and anatomic site. Oral Oncol. 2014;50(5):387-403.
2. Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54(1):28-46.
3. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341-1346.
4. Harris C, Bates-Jensen B, Parslow N, Raizman R, Singh M, Ketchen R. Bates‐Jensen wound assessment tool: pictorial guide validation project. J Wound Ostomy Continence Nurs. 2010;37(3):253-259.
5. Lucey P, Zouzias C, Franco L, Chennupati SK, Kalnicki S, McLellan BN. Practice patterns for the prophylaxis and treatment of acute radiation dermatitis in the United States. Support Care Cancer. 2017;25(9):2857-2862.
6. Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21(10):2933-2948.
7. Vavassis P, Gelinas M, Chabot Tr J, Nguyen-Tân PF. Phase 2 study of silver leaf dressing for treatment of radiation-induced dermatitis in patients receiving radiotherapy to the head and neck. J Otolaryngology Head Neck Surg. 2008;37(1):124-129.
8. Mepilex Ag product information. Mölnlycke Health Care website. http://www.molnlycke.us/advanced-wound-care-products/antimicrobial-products/mepilex-ag/#confirm. Accessed May 3, 2018.
9. Aquino-Parsons C, Lomas S, Smith K, et al. Phase III study of silver leaf nylon dressing vs standard care for reduction of inframammary moist desquamation in patients undergoing adjuvant whole breast radiation therapy. J Med Imaging Radiat Sci. 2010;41(4):215-221.
10. Diggelmann KV, Zytkovicz AE, Tuaine JM, Bennett NC, Kelly LE, Herst PM. Mepilex Lite dressings for the management of radiation-induced erythema: a systematic inpatient controlled clinical trial. Br J Radiol. 2010;83(995):971-978.
1. Simard EP, Torre LA, Jemal A. International trends in head and neck cancer incidence rates: differences by country, sex and anatomic site. Oral Oncol. 2014;50(5):387-403.
2. Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54(1):28-46.
3. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341-1346.
4. Harris C, Bates-Jensen B, Parslow N, Raizman R, Singh M, Ketchen R. Bates‐Jensen wound assessment tool: pictorial guide validation project. J Wound Ostomy Continence Nurs. 2010;37(3):253-259.
5. Lucey P, Zouzias C, Franco L, Chennupati SK, Kalnicki S, McLellan BN. Practice patterns for the prophylaxis and treatment of acute radiation dermatitis in the United States. Support Care Cancer. 2017;25(9):2857-2862.
6. Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21(10):2933-2948.
7. Vavassis P, Gelinas M, Chabot Tr J, Nguyen-Tân PF. Phase 2 study of silver leaf dressing for treatment of radiation-induced dermatitis in patients receiving radiotherapy to the head and neck. J Otolaryngology Head Neck Surg. 2008;37(1):124-129.
8. Mepilex Ag product information. Mölnlycke Health Care website. http://www.molnlycke.us/advanced-wound-care-products/antimicrobial-products/mepilex-ag/#confirm. Accessed May 3, 2018.
9. Aquino-Parsons C, Lomas S, Smith K, et al. Phase III study of silver leaf nylon dressing vs standard care for reduction of inframammary moist desquamation in patients undergoing adjuvant whole breast radiation therapy. J Med Imaging Radiat Sci. 2010;41(4):215-221.
10. Diggelmann KV, Zytkovicz AE, Tuaine JM, Bennett NC, Kelly LE, Herst PM. Mepilex Lite dressings for the management of radiation-induced erythema: a systematic inpatient controlled clinical trial. Br J Radiol. 2010;83(995):971-978.
The long-term effects of posttreatment exercise on pain in young women with breast cancer
Breast cancer is one of the most prevalent cancers in women worldwide, with more than 1 million new cases diagnosed annually.1 Prognosis for the disease has improved significantly, but 25% to 60% of women living with breast cancer experience some level of pain ranging from mild to severe, the nature of which can evolve from acute to chronic.2 Pre-, intra-, and post-treatment risk factors have been found to correlate with the development of acute and chronic pain and include young age, type of breast surgery (lumpectomy or total mastectomy), axillary node dissection, radiation therapy, and hormonal therapy.3-5 Chemotherapy, particularly anthracycline- and taxane-based regimens, has also been shown to induce pain, arthralgia, myalgia, and peripheral neuropathy during treatment.6 In particular, postradiation pain may result from subcutaneous fibrosis with fixation to underlying musculature and the development of fibrous flaps in the internal axilla.7 These tissue changes are commonly subclinical, occurring 4 to 12 months postradiation,8 and can progress undetected until pain and upper-limb disability develop.
The presence of persistent pain has a considerable impact on the quality of life in survivors of breast cancer: psychological distress is prevalent (anxiety, depression, worry, fear), the performance of daily activities is diminished (eg, bathing, dressing, preparing meals, shopping), and economic independence is compromised by the inability to work or reduced employment and income. These factors directly and indirectly contribute to an increase in the use of health care services.9,10
The management of pain is often characterized by pharmacologic-related treatment, such as the use of opioids and nonsteroidal anti-inflammatory medications, and nonpharmacologic-related treatment, such as exercise. Empirical evidence has shown that rehabilitative exercise programs, which commonly include a combination of resistance training and aerobic exercises, can effectively reduce pain in breast cancer survivors.10-12 Women living with breast cancer who are directed to rehabilitative exercise programs experience an improvement not only in pain levels but also in their ability to engage in activities of daily living, in their psychological health, and in their overall quality of life.13-15 However, despite evidence to support exercise programs to reduce pain related to breast cancer treatment, residual pain and upper-limb discomfort are common complaints in breast cancer survivors, and there is little focus on the duration of effectiveness of such programs for reducing pain after treatment for breast cancer. The objective of this study was to determine if an exercise program initiated postradiation would improve long-term pain levels in a carefully selected population of young women who were living with breast cancer and had no history of shoulder pathology or significant treatment complications.
Methods
Design
We used a pilot randomized control trial to compare the long-term effectiveness of a 12-week postradiation exercise program versus standard care on residual pain levels in young women (aged 18-45 years) living with breast cancer. The program was initiated 3 to 4 weeks postradiation to allow for acute inflammatory reactions to subside. Pain severity and interference were assessed using the Brief Pain Inventory-Short Form (BPI-SF), a tool for assessing cancer pain.16,17 Pain levels for isolated shoulder movements were also recorded on examination by a physical therapist. All measures were collected at 6 time points (T1-T6): postsurgery and preradiation (T1, baseline), postradiation and preintervention (T2), and 4 points during an 18-month period postradiation (T3-T6 at 3, 6, 12, and 18 months postradiation).
Sample
Young women living with breast cancer who met our eligibility criteria were identified from 2 clinics at the Jewish General Hospital – the Segal Cancer Center and the Department of Radiation Oncology in Montréal, Québec, Canada. Inclusion criteria included women with a diagnosis of stage I to stage III breast cancer, who were 18 to 45 years old, were scheduled for postoperative adjuvant radiation therapy, had an Eastern Cooperative Oncology Group Performance Status of 0 or 1 (normal ambulatory function, minimal symptoms), and who consented to participate in the study. Exclusion criteria included women with a metastatic (stage IV) diagnosis; significant musculoskeletal, cardiac, pulmonary, or metabolic comorbidities that would not allow for participation in physical activity; a previous breast cancer diagnosis with treatment to the ipsilateral or contralateral sides; postsurgical lymphedema; postsurgical capsulitis, tendonitis, or other shoulder inflammatory complications; and any contraindication to exercise. The recruitment goal was outlined as 50 patients per group; however, a protracted accrual time because of the stringent study criteria yielded a sample of 29 and 30 patients for the intervention and control groups, respectively, which was sufficient for significant testing of differences between the 2 study groups.18
Variables and measures
Clinical characteristics. We used standardized questions and chart review to document the participants’ clinical characteristics and to capture information on the following: the stage and subtype of breast cancer, hormonal and human epidermal growth factor receptors (HER2) (estrogen receptor, progesterone receptor, and HER2 status), extent of surgery (lumpectomy or total mastectomy), and other modalities of treatment (eg, chemotherapy, radiation therapy).
Pain assessment. The BPI-SF was used to assess participants’ cancer-related pain. Pain severity ranged from 0 (no pain), 1 to 4 (mild pain), 5 to 6 (moderate pain), to 7 to 10 (severe pain).18,19 The questionnaire also identifies the pain interference in daily activities using a Likert scale ranging from 0 (Does not interfere) to 10 (Completely interferes) in the following 7 domains or subscales: General Activity, Walking, Mood, Sleep, Work, Relations with Others, and Enjoyment of Life.16 For the purpose of this study, mean scores were tabulated using both pain intensity and interference scales.
Another important component of the BPI-SF instructs participants to localize pain by means of a body diagram. For purpose of analysis, 3 pain regions were established: shoulder girdle/chest wall on the affected side; neck and other upper extremity, including hand(s), forearm(s), wrist(s), and finger(s); and other regions, including abdominal discomfort, leg(s), hip(s), knee(s), ankle(s), lower back, and feet. In addition, pain levels on movement (Yes/No) were recorded for isolated shoulder flexion, abduction, and horizontal abduction (sitting and standing). The measurements were completed by a single physical therapist throughout the course of the study to minimize variance.
Procedure
The study protocol was approved by the Research Ethics Board at the Jewish General Hospital. Recruitment occurred from 2011 through 2015. The research was in accordance with the ethical standards of the responsible committee on human experimentation. Eligible women were recruited by the research coordinator who described the purpose, risks, and benefits of the study; advised on confidentiality, data collection, and intervention allocation procedures; and highlighted voluntary participation. The research coordinator addressed any concerns on the part of the participants before obtaining their written informed consent. Random allocation to the intervention and control groups was established using a web-based randomization plan generator (www.randomization.com). A single individual was responsible for the randomization process, and treatment assignments were revealed after each participant’s name had been entered. A physical therapist performed 6 sequential evaluations (T1-T6) at the time of participants’ medical follow-up appointments.
Intervention
The 12-week exercise intervention started 3 weeks postradiation and was composed of an initial 6-week program of low-level cardiovascular and resistance exercises that progressed to a set of more advanced exercises for the remaining 6 weeks. Participants were instructed to warm up for at least 10 minutes with a cardiovascular exercise of their choice (eg, a recumbent cross trainer, walking, or stairs) before doing a combined strength, endurance, and stretching exercise program for the upper body.20 The final portion of the exercise intervention included a period of light cool-down. Weight training resistance levels were based on a maximum 8 to 10 repetitions for strength and a maximum of 20 repetitions for endurance training exercises, which progressed gradually over the course of the 12-week exercise program to ensure participant safety.21,22 Participants in the intervention group were supervised at least once a week by an exercise physiologist at a center for oncology patients (Hope & Cope Wellness Centre), and patients were encouraged to perform the program at home 2 to 3 times a week. Those who were not able to exercise consistently at the center were provided with equipment and instructed on how to do the program safely at home.
By comparison, the control group received standard care, which included advice on the benefits of an active lifestyle, including exercise, but without a specific intervention. Participants were not restricted in their physical activity and/or sport participation levels, and their weekly activity levels were calculated using the Metabolic Equivalent of Task and recorded at each of the 6 time points.
Statistical analysis
Descriptive statistics were used to examine participant characteristics. The quantitative data collected through the BPI-SF measures were analyzed with JMP software (version 11.2; SAS Institute, Cary, NC). Continuous variables were tested for statistical significance (P ≤ .05) through the chi-square (categorical), analysis of variance, and nonparametric Wilcoxon tests. The analyses did not include missing data.
Results
A total of 59 young women were randomized into the intervention (n = 29) and control (n = 30) groups. Of those, 2 participants dropped out of the study because of family and time constraints, and 3 participants died, 2 from the control and 1 from the intervention group, after subsequently developing metastatic disease. Baseline data including comparative tumor characteristics, surgical interventions, and treatment interventions have been published in relation to other elements of this study.23,24 The participants had a mean age of 39.2 years (standard deviation [SD], 5.0). More than half of them had an invasive ductal carcinoma (69.5%) and were estrogen positive (78.0%), progesterone positive (74.6%), or HER2 positive (20.3%), whereas 10.2% were triple negative. Most of the participants had undergone breast-sparing procedures (86.4% lumpectomy), and 18.6% had a total mastectomy. By random chance, the intervention group had higher rates of total mastectomy (24.4% and 13.3%, respectively) and surgical reconstruction (12.2% and 6.7%, respectively) compared with the control group. Most of the women (71.2%) received chemotherapy, and all received radiation therapy. In the intervention group, 37.2% received radiation therapy localized to the axilla, and 88% received a boost of radiation to the surgical bed. Self-reported exercise diaries were returned by 15 of the 29 intervention participants, and training frequencies among them varied significantly (1-6 times a week).
The findings showed that there was little variance between the intervention and control groups in BPI-SF severity scores from T1 to T6, so the means and SDs of the BPI-SF scores were grouped at 6 time points (Table 1). There was no statistically significant difference between baseline measures at T1 (1.68; SD, 1.17) and measures at 18 months postintervention (T6: 1.46; SD, 1.37). At baseline, 87.7% of the women reported no pain (31.5%) or mild levels of pain (55.6%), and 13% reported moderate or severe pain. Over the duration of the study from T1 to T6, these primarily low levels of pain (BPI-SF, 0-4) remained consistent with a favorable shift toward having no pain (T1: 31.5%; T6: 24.4%). By 18 months postintervention, 95.7% of women reported no or mild pain, with 4.9% reporting moderate pain.
Similarly, there was little variance over time (T1-T6) and no statistically significant differences between the 2 groups in BPI-SF–measured levels of pain interference in daily activities (Table 2). Moreover, a domain analysis showed that there were no statistically significant differences in pain interference scores when comparing the type and extent of surgery (total mastectomy: 0.59 [1.17]; lumpectomy: 0.94 [1.96]). By chance – and not related directly to the objectives of this study – there was a statistically significant difference between the intervention and control groups in the interference of pain on the Enjoyment of Life domain in favor of the control group.
The sites of pain captured by the BPI-SF shed light on the preceding findings (Figure 1). At baseline (T1, postsurgery and preradiation), 37.0% of participants reported pain in the shoulder girdle–chest wall region, whereas 20.4% reported pain in the general neck–upper extremity region and 50% in other regions. Postradiation, shoulder girdle–chest wall pain was identified as the highest reported site of pain (49.1%; T2, postradiation and preintervention) and remained elevated at 3 months (T3) and 6 months (T4) postradiation (46.9% and 45.5%, respectively). At 12 and 18 months postradiation (T5 and T6), the principal focus of pain shifted once again to “other” regions at 30% and 32.5%, respectively, and the neck–upper extremity region at 10% and 15%, respectively. Shoulder girdle–chest wall pain concomitantly improved at those time points (15% and 25% respectively) but was not eliminated.
Pain levels recorded on physical examination for isolated shoulder range of movements were recently published,24 and they have been abbreviated and reproduced in this paper (Figure 2) to allow for a comparison of findings between the exercise intervention group and the control group to help determine the sensitivity of these tools for use in breast cancer patients. At baseline, pain levels with active movement were noted to be slightly greater in the intervention group for flexion and abduction.
Following the intervention, at 3 and 6 months postradiation (T3 and T4), the intervention group showed a steady decrease in pain levels in flexion and abduction, whereas the control group showed a 5-fold increase in pain with horizontal abduction. Furthermore, participants in the intervention group reported having no pain on movement 12 months postradiation (T5); however, recurrence of pain was apparent with all shoulder movements by 18 months postradiation (T6) in both the intervention and control groups.
Discussion
Previous studies have hypothesized that younger age (18-39 years), adjuvant radiotherapy, and axillary node dissection are risk factors for chronic pain in breast cancer survivors.22,25 Persistent pain is prevalent in 12% to 51% of breast cancer survivors, with up to one-third experiencing some pain more than 5 years after treatment,26,27 and our study outcomes concur with those findings. In our study, pain, as measured by the BPI-SF, was found to persist for most participants (75.6%) after the 18-month follow-up. The results of our trial showed that a 12-week exercise intervention administered postsurgery and postradiation had no statistically significant effect on long-term (18 months) pain severity and its interference in daily life. It is worth noting that body regions that had not been directly related to either surgical or radiation treatment for breast cancer were commonly identified as areas of pain but were not specifically targeted by our intervention. However, focusing on pain severity (BPI-SF), our findings suggest that the benefits of targeted upper-extremity exercise on pain in the intermediate time course of follow-up (T3, T4, and T5) was notable compared with the control group, which received standard care. The apparent recurrence of pain at 18 months in both groups was not anticipated and needs to be further investigated.
More specific objective assessments of pain on active shoulder movement identified distinct patterns of pain that could not be isolated using the BPI-SF alone. The incidence and localization of pain on movement differed between the population of women who received a specific exercise intervention and those who received standard care (Figure 2). Patterns of pain over time fluctuated in the control group, whereas the intervention group reported a linear decrease in pain. Residual pain on shoulder movement remained apparent in both groups at 18-months postradiation, but that finding was not reflected in the BPI-SF results. The literature supports our findings on persistent pain among breast cancer survivors,3,7,8,28-30 and in our study of young women carefully screened and excluded for pre-existent shoulder conditions or comorbid medical conditions, recurrent articular pain was nonetheless prevalent. It seems that unidentified or multiple factors may be part of the etiology of pain in this young adult cohort.
Although the BPI-SF is a generic measurement tool commonly used to assess and measure cancer patients’ pain levels, the lack of variance in our BPI-SF severity and interference outcomes over time (T1-T6) (Table 1, Table 2), the variety of “other” unrelated regions (Figure 1) identified by the BPI-SF, and the contrast in our findings on specific physical examination emphasize the potential limitations of this clinical tool.
Moreover, the BPI-SF has not been validated specifically for breast cancer. Harrington and colleagues have recommended using the BPI-SF to assess pain in women with breast cancer,31 but the use of a more multidimensional measurement tool that evaluates axillary, chest, trunk, and upper-limb pain may prove to be more valuable in this population.
Limitations
Recruitment of young adult women was difficult because of our stringent inclusion criteria, the long-term follow-up, and the relatively small population of breast cancer patients in this age demographic. Therefore, the duration of the recruitment phase, despite our having access to a specialized young adult and adolescent clinic in our institute, greatly surpassed the expectations we had when we designed the study. In addition, there remains an inherent bias in participants who accept participation in a study that includes exercise interventions. Potential participants who exercise regularly or have a positive inclination toward doing exercise are more likely to participate. Despite the prescription of a targeted 12-week upper-limb intervention in this study, the general activity levels of both groups may have had an impact on the significance of this study. In addition, the low adherence to the use of self-reported logs failed to capture the true compliance rates of our participants because their lack of tracking does not indicate failure to comply with the program. The use of weekly or biweekly telephone calls to monitor compliance rates of activity more vigilantly may be used in future studies.
Conclusions
Advances in clinical management of breast cancer have improved survival outcomes, and morbidity over recent years, yet symptoms such as pain remain prevalent in this population. The results of this study showed that a targeted, 12-week upper-limb exercise intervention postradiation transiently improved levels of shoulder pain without a concomitant impact on chronic pain or any positive influence on activities of daily living 18 months posttreatment. Furthermore, future studies should use a variety of measurement tools to evaluate trunk and upper-limb pain in women with breast cancer and investigate the optimal timing of postradiation exercise interventions.
Acknowledgments
The authors thank Hope & Cope, the CURE foundation, and the Jewish General Hospital Foundation/Weekend to End Breast Cancer for providing the financial resources needed to sustain this research study. They also thank the McGill Adolescent and Young Adult program for its continued support. Previous oral presentations of research Muanza TM, et al. Randomized clinical trial of a progressive exercise program for young women with breast cancer undergoing radiation therapy. Int J Radiat Oncol Biol Phys. 2015;93(3):s35-s36.
1. World Health Organization. Breast cancer: prevention and control. www.who.int/cancer/detection/breastcancer/en/. Updated 2017. Accessed September 16, 2016.
2. Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain. 2011;12(7):725-746.
3. Ernst MF, Voogd AC, Balder W, Klinkenbijl JH, Roukema JA. Early and late morbidity associated with axillary levels I-III dissection in breast cancer. J Surg Oncol. 2002;79(3):151-155; discussion 156.
4. Gulluoglu BM, Cingi A, Cakir T, Gercek A, Barlas A, Eti Z. Factors related to post-treatment chronic pain in breast cancer survivors: the interference of pain with life functions. Int J Fertil Womens Med. 2006;51(2):75-82.
5. Jung BF, Ahrendt GM, Oaklander AL, Dworkin RH. Neuropathic pain following breast cancer surgery: proposed classification and research update. Pain. 2003;104(1-2):1-13.
6. Saibil S, Fitzgerald B, Freedman OC, et al. Incidence of taxane-induced pain and distress in patients receiving chemotherapy for early-stage breast cancer: a retrospective, outcomes-based survey. Curr Oncol. 2010;17(4):42-47.
7. Tengrup I, Tennvall-Nittby L, Christiansson I, Laurin M. Arm morbidity after breast-conserving therapy for breast cancer. Acta Oncol. 2000;39(3):393-397.
8. Johansen J, Overgaard J, Blichert-Toft M, Overgaard M. Treatment of morbidity associated with the management of the axilla in breast-conserving therapy. Acta Oncol. 2000;39(3):349-354.
9. Mittmann N, Porter JM, Rangrej J, et al. Health system costs for stage-specific breast cancer: a population-based approach. Curr Oncol. 2014;21(6):281-293.
10. Page A. Keeping patients safe: transforming the work environment of nurses. Washington, DC: National Academies Press; 2004.
11. McNeely ML, Campbell K, Ospina M, et al. Exercise interventions for upper-limb dysfunction due to breast cancer treatment. Cochrane Database Syst Rev. 2010;(6):CD005211. doi:10.1002/14651858.CD005211.pub2
12. Wong P, Muanza T, Hijal T, et al. Effect of exercise in reducing breast and chest-wall pain in patients with breast cancer: a pilot study. Curr Oncol. 2012;19(3):e129-e135.
13. Fernández-Lao C, Cantarero-Villanueva I, Fernández-de-Las-Peñas C, del Moral-Ávila R, Castro-Sánchez AM, Arroyo-Morales M. Effectiveness of a multidimensional physical therapy program on pain, pressure hypersensitivity, and trigger points in breast cancer survivors: a randomized controlled clinical trial. Clin J Pain. 2012;28(2):113-121.
14. Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21(9):1660-1668.
15. Segal R, Evans W, Johnson D, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol. 2001;19(3):657-665.
16. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129-138.
17. Kumar SP. Utilization of Brief Pain Inventory as an assessment tool for pain in patients with cancer: a focused review. Indian J Palliat Care. 2011;17(2):108-115.
18. Van Voorhis CRW, Morgan BL. Understanding power and rules of thumb for determining sample sizes. Tutor Quant Methods Psychol. 2007;3(2):43-50.
19. Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277-284.
20. Lee TS, Kilbreath SL, Refshauge KM, Pendlebury SC, Beith JM, Lee MJ. Pectoral stretching program for women undergoing radiotherapy for breast cancer. Breast Cancer Res Treat. 2007;102(3):313-321.
21. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409-1426.
22. Pollock ML, Gaesser GA, Butcher JD, et al. ACSM position stand: the recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30(6):975-991.
23. Ibrahim M, Muanza T, Smirnow N, et al. Time course of upper limb function and return-to-work post-radiotherapy in young adults with breast cancer: a pilot randomized control trial on effects of targeted exercise program. J Cancer Surviv. 2017;11(6):791-799.
24. Ibrahim M, Muanza T, Smirnow N, et al. A pilot randomized controlled trial on the effects of a progressive exercise program on the range of motion and upper extremity grip strength in young adults with breast cancer. Clin Breast Cancer. 2018;18(1):e55-e64.
25. Gärtner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302(18):1985-1992.
26. Hayes SC, Johansson K, Stout NL, et al. Upper-body morbidity after breast cancer: incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of care. Cancer. 2012;118(suppl 8):2237-2249.
27. Kärki A, Simonen R, Mälkiä E, Selfe J. Impairments, activity limitations and participation restrictions 6 and 12 months after breast cancer operation. J Rehabil Med. 2005;37(3):180-188.
28. Katz J, Poleshuck EL, Andrus CH, et al. Risk factors for acute pain and its persistence following breast cancer surgery. Pain. 2005;119(1-3):16-25.
29. Tasmuth T, von Smitten K, Hietanen P, Kataja M, Kalso E. Pain and other symptoms after different treatment modalities of breast cancer. Ann Oncol. 1995;6(5):453-459.
30. Whelan TJ, Levine M, Julian J, Kirkbride P, Skingley P. The effects of radiation therapy on quality of life of women with breast carcinoma: results of a randomized trial. Ontario Clinical Oncology Group. Cancer. 2000;88(10):2260-2266.
31. Harrington S, Gilchrist L, Sander A. Breast cancer EDGE task force outcomes: clinical measures of pain. Rehabil Oncol. 2014;32(1):13-21.
Breast cancer is one of the most prevalent cancers in women worldwide, with more than 1 million new cases diagnosed annually.1 Prognosis for the disease has improved significantly, but 25% to 60% of women living with breast cancer experience some level of pain ranging from mild to severe, the nature of which can evolve from acute to chronic.2 Pre-, intra-, and post-treatment risk factors have been found to correlate with the development of acute and chronic pain and include young age, type of breast surgery (lumpectomy or total mastectomy), axillary node dissection, radiation therapy, and hormonal therapy.3-5 Chemotherapy, particularly anthracycline- and taxane-based regimens, has also been shown to induce pain, arthralgia, myalgia, and peripheral neuropathy during treatment.6 In particular, postradiation pain may result from subcutaneous fibrosis with fixation to underlying musculature and the development of fibrous flaps in the internal axilla.7 These tissue changes are commonly subclinical, occurring 4 to 12 months postradiation,8 and can progress undetected until pain and upper-limb disability develop.
The presence of persistent pain has a considerable impact on the quality of life in survivors of breast cancer: psychological distress is prevalent (anxiety, depression, worry, fear), the performance of daily activities is diminished (eg, bathing, dressing, preparing meals, shopping), and economic independence is compromised by the inability to work or reduced employment and income. These factors directly and indirectly contribute to an increase in the use of health care services.9,10
The management of pain is often characterized by pharmacologic-related treatment, such as the use of opioids and nonsteroidal anti-inflammatory medications, and nonpharmacologic-related treatment, such as exercise. Empirical evidence has shown that rehabilitative exercise programs, which commonly include a combination of resistance training and aerobic exercises, can effectively reduce pain in breast cancer survivors.10-12 Women living with breast cancer who are directed to rehabilitative exercise programs experience an improvement not only in pain levels but also in their ability to engage in activities of daily living, in their psychological health, and in their overall quality of life.13-15 However, despite evidence to support exercise programs to reduce pain related to breast cancer treatment, residual pain and upper-limb discomfort are common complaints in breast cancer survivors, and there is little focus on the duration of effectiveness of such programs for reducing pain after treatment for breast cancer. The objective of this study was to determine if an exercise program initiated postradiation would improve long-term pain levels in a carefully selected population of young women who were living with breast cancer and had no history of shoulder pathology or significant treatment complications.
Methods
Design
We used a pilot randomized control trial to compare the long-term effectiveness of a 12-week postradiation exercise program versus standard care on residual pain levels in young women (aged 18-45 years) living with breast cancer. The program was initiated 3 to 4 weeks postradiation to allow for acute inflammatory reactions to subside. Pain severity and interference were assessed using the Brief Pain Inventory-Short Form (BPI-SF), a tool for assessing cancer pain.16,17 Pain levels for isolated shoulder movements were also recorded on examination by a physical therapist. All measures were collected at 6 time points (T1-T6): postsurgery and preradiation (T1, baseline), postradiation and preintervention (T2), and 4 points during an 18-month period postradiation (T3-T6 at 3, 6, 12, and 18 months postradiation).
Sample
Young women living with breast cancer who met our eligibility criteria were identified from 2 clinics at the Jewish General Hospital – the Segal Cancer Center and the Department of Radiation Oncology in Montréal, Québec, Canada. Inclusion criteria included women with a diagnosis of stage I to stage III breast cancer, who were 18 to 45 years old, were scheduled for postoperative adjuvant radiation therapy, had an Eastern Cooperative Oncology Group Performance Status of 0 or 1 (normal ambulatory function, minimal symptoms), and who consented to participate in the study. Exclusion criteria included women with a metastatic (stage IV) diagnosis; significant musculoskeletal, cardiac, pulmonary, or metabolic comorbidities that would not allow for participation in physical activity; a previous breast cancer diagnosis with treatment to the ipsilateral or contralateral sides; postsurgical lymphedema; postsurgical capsulitis, tendonitis, or other shoulder inflammatory complications; and any contraindication to exercise. The recruitment goal was outlined as 50 patients per group; however, a protracted accrual time because of the stringent study criteria yielded a sample of 29 and 30 patients for the intervention and control groups, respectively, which was sufficient for significant testing of differences between the 2 study groups.18
Variables and measures
Clinical characteristics. We used standardized questions and chart review to document the participants’ clinical characteristics and to capture information on the following: the stage and subtype of breast cancer, hormonal and human epidermal growth factor receptors (HER2) (estrogen receptor, progesterone receptor, and HER2 status), extent of surgery (lumpectomy or total mastectomy), and other modalities of treatment (eg, chemotherapy, radiation therapy).
Pain assessment. The BPI-SF was used to assess participants’ cancer-related pain. Pain severity ranged from 0 (no pain), 1 to 4 (mild pain), 5 to 6 (moderate pain), to 7 to 10 (severe pain).18,19 The questionnaire also identifies the pain interference in daily activities using a Likert scale ranging from 0 (Does not interfere) to 10 (Completely interferes) in the following 7 domains or subscales: General Activity, Walking, Mood, Sleep, Work, Relations with Others, and Enjoyment of Life.16 For the purpose of this study, mean scores were tabulated using both pain intensity and interference scales.
Another important component of the BPI-SF instructs participants to localize pain by means of a body diagram. For purpose of analysis, 3 pain regions were established: shoulder girdle/chest wall on the affected side; neck and other upper extremity, including hand(s), forearm(s), wrist(s), and finger(s); and other regions, including abdominal discomfort, leg(s), hip(s), knee(s), ankle(s), lower back, and feet. In addition, pain levels on movement (Yes/No) were recorded for isolated shoulder flexion, abduction, and horizontal abduction (sitting and standing). The measurements were completed by a single physical therapist throughout the course of the study to minimize variance.
Procedure
The study protocol was approved by the Research Ethics Board at the Jewish General Hospital. Recruitment occurred from 2011 through 2015. The research was in accordance with the ethical standards of the responsible committee on human experimentation. Eligible women were recruited by the research coordinator who described the purpose, risks, and benefits of the study; advised on confidentiality, data collection, and intervention allocation procedures; and highlighted voluntary participation. The research coordinator addressed any concerns on the part of the participants before obtaining their written informed consent. Random allocation to the intervention and control groups was established using a web-based randomization plan generator (www.randomization.com). A single individual was responsible for the randomization process, and treatment assignments were revealed after each participant’s name had been entered. A physical therapist performed 6 sequential evaluations (T1-T6) at the time of participants’ medical follow-up appointments.
Intervention
The 12-week exercise intervention started 3 weeks postradiation and was composed of an initial 6-week program of low-level cardiovascular and resistance exercises that progressed to a set of more advanced exercises for the remaining 6 weeks. Participants were instructed to warm up for at least 10 minutes with a cardiovascular exercise of their choice (eg, a recumbent cross trainer, walking, or stairs) before doing a combined strength, endurance, and stretching exercise program for the upper body.20 The final portion of the exercise intervention included a period of light cool-down. Weight training resistance levels were based on a maximum 8 to 10 repetitions for strength and a maximum of 20 repetitions for endurance training exercises, which progressed gradually over the course of the 12-week exercise program to ensure participant safety.21,22 Participants in the intervention group were supervised at least once a week by an exercise physiologist at a center for oncology patients (Hope & Cope Wellness Centre), and patients were encouraged to perform the program at home 2 to 3 times a week. Those who were not able to exercise consistently at the center were provided with equipment and instructed on how to do the program safely at home.
By comparison, the control group received standard care, which included advice on the benefits of an active lifestyle, including exercise, but without a specific intervention. Participants were not restricted in their physical activity and/or sport participation levels, and their weekly activity levels were calculated using the Metabolic Equivalent of Task and recorded at each of the 6 time points.
Statistical analysis
Descriptive statistics were used to examine participant characteristics. The quantitative data collected through the BPI-SF measures were analyzed with JMP software (version 11.2; SAS Institute, Cary, NC). Continuous variables were tested for statistical significance (P ≤ .05) through the chi-square (categorical), analysis of variance, and nonparametric Wilcoxon tests. The analyses did not include missing data.
Results
A total of 59 young women were randomized into the intervention (n = 29) and control (n = 30) groups. Of those, 2 participants dropped out of the study because of family and time constraints, and 3 participants died, 2 from the control and 1 from the intervention group, after subsequently developing metastatic disease. Baseline data including comparative tumor characteristics, surgical interventions, and treatment interventions have been published in relation to other elements of this study.23,24 The participants had a mean age of 39.2 years (standard deviation [SD], 5.0). More than half of them had an invasive ductal carcinoma (69.5%) and were estrogen positive (78.0%), progesterone positive (74.6%), or HER2 positive (20.3%), whereas 10.2% were triple negative. Most of the participants had undergone breast-sparing procedures (86.4% lumpectomy), and 18.6% had a total mastectomy. By random chance, the intervention group had higher rates of total mastectomy (24.4% and 13.3%, respectively) and surgical reconstruction (12.2% and 6.7%, respectively) compared with the control group. Most of the women (71.2%) received chemotherapy, and all received radiation therapy. In the intervention group, 37.2% received radiation therapy localized to the axilla, and 88% received a boost of radiation to the surgical bed. Self-reported exercise diaries were returned by 15 of the 29 intervention participants, and training frequencies among them varied significantly (1-6 times a week).
The findings showed that there was little variance between the intervention and control groups in BPI-SF severity scores from T1 to T6, so the means and SDs of the BPI-SF scores were grouped at 6 time points (Table 1). There was no statistically significant difference between baseline measures at T1 (1.68; SD, 1.17) and measures at 18 months postintervention (T6: 1.46; SD, 1.37). At baseline, 87.7% of the women reported no pain (31.5%) or mild levels of pain (55.6%), and 13% reported moderate or severe pain. Over the duration of the study from T1 to T6, these primarily low levels of pain (BPI-SF, 0-4) remained consistent with a favorable shift toward having no pain (T1: 31.5%; T6: 24.4%). By 18 months postintervention, 95.7% of women reported no or mild pain, with 4.9% reporting moderate pain.
Similarly, there was little variance over time (T1-T6) and no statistically significant differences between the 2 groups in BPI-SF–measured levels of pain interference in daily activities (Table 2). Moreover, a domain analysis showed that there were no statistically significant differences in pain interference scores when comparing the type and extent of surgery (total mastectomy: 0.59 [1.17]; lumpectomy: 0.94 [1.96]). By chance – and not related directly to the objectives of this study – there was a statistically significant difference between the intervention and control groups in the interference of pain on the Enjoyment of Life domain in favor of the control group.
The sites of pain captured by the BPI-SF shed light on the preceding findings (Figure 1). At baseline (T1, postsurgery and preradiation), 37.0% of participants reported pain in the shoulder girdle–chest wall region, whereas 20.4% reported pain in the general neck–upper extremity region and 50% in other regions. Postradiation, shoulder girdle–chest wall pain was identified as the highest reported site of pain (49.1%; T2, postradiation and preintervention) and remained elevated at 3 months (T3) and 6 months (T4) postradiation (46.9% and 45.5%, respectively). At 12 and 18 months postradiation (T5 and T6), the principal focus of pain shifted once again to “other” regions at 30% and 32.5%, respectively, and the neck–upper extremity region at 10% and 15%, respectively. Shoulder girdle–chest wall pain concomitantly improved at those time points (15% and 25% respectively) but was not eliminated.
Pain levels recorded on physical examination for isolated shoulder range of movements were recently published,24 and they have been abbreviated and reproduced in this paper (Figure 2) to allow for a comparison of findings between the exercise intervention group and the control group to help determine the sensitivity of these tools for use in breast cancer patients. At baseline, pain levels with active movement were noted to be slightly greater in the intervention group for flexion and abduction.
Following the intervention, at 3 and 6 months postradiation (T3 and T4), the intervention group showed a steady decrease in pain levels in flexion and abduction, whereas the control group showed a 5-fold increase in pain with horizontal abduction. Furthermore, participants in the intervention group reported having no pain on movement 12 months postradiation (T5); however, recurrence of pain was apparent with all shoulder movements by 18 months postradiation (T6) in both the intervention and control groups.
Discussion
Previous studies have hypothesized that younger age (18-39 years), adjuvant radiotherapy, and axillary node dissection are risk factors for chronic pain in breast cancer survivors.22,25 Persistent pain is prevalent in 12% to 51% of breast cancer survivors, with up to one-third experiencing some pain more than 5 years after treatment,26,27 and our study outcomes concur with those findings. In our study, pain, as measured by the BPI-SF, was found to persist for most participants (75.6%) after the 18-month follow-up. The results of our trial showed that a 12-week exercise intervention administered postsurgery and postradiation had no statistically significant effect on long-term (18 months) pain severity and its interference in daily life. It is worth noting that body regions that had not been directly related to either surgical or radiation treatment for breast cancer were commonly identified as areas of pain but were not specifically targeted by our intervention. However, focusing on pain severity (BPI-SF), our findings suggest that the benefits of targeted upper-extremity exercise on pain in the intermediate time course of follow-up (T3, T4, and T5) was notable compared with the control group, which received standard care. The apparent recurrence of pain at 18 months in both groups was not anticipated and needs to be further investigated.
More specific objective assessments of pain on active shoulder movement identified distinct patterns of pain that could not be isolated using the BPI-SF alone. The incidence and localization of pain on movement differed between the population of women who received a specific exercise intervention and those who received standard care (Figure 2). Patterns of pain over time fluctuated in the control group, whereas the intervention group reported a linear decrease in pain. Residual pain on shoulder movement remained apparent in both groups at 18-months postradiation, but that finding was not reflected in the BPI-SF results. The literature supports our findings on persistent pain among breast cancer survivors,3,7,8,28-30 and in our study of young women carefully screened and excluded for pre-existent shoulder conditions or comorbid medical conditions, recurrent articular pain was nonetheless prevalent. It seems that unidentified or multiple factors may be part of the etiology of pain in this young adult cohort.
Although the BPI-SF is a generic measurement tool commonly used to assess and measure cancer patients’ pain levels, the lack of variance in our BPI-SF severity and interference outcomes over time (T1-T6) (Table 1, Table 2), the variety of “other” unrelated regions (Figure 1) identified by the BPI-SF, and the contrast in our findings on specific physical examination emphasize the potential limitations of this clinical tool.
Moreover, the BPI-SF has not been validated specifically for breast cancer. Harrington and colleagues have recommended using the BPI-SF to assess pain in women with breast cancer,31 but the use of a more multidimensional measurement tool that evaluates axillary, chest, trunk, and upper-limb pain may prove to be more valuable in this population.
Limitations
Recruitment of young adult women was difficult because of our stringent inclusion criteria, the long-term follow-up, and the relatively small population of breast cancer patients in this age demographic. Therefore, the duration of the recruitment phase, despite our having access to a specialized young adult and adolescent clinic in our institute, greatly surpassed the expectations we had when we designed the study. In addition, there remains an inherent bias in participants who accept participation in a study that includes exercise interventions. Potential participants who exercise regularly or have a positive inclination toward doing exercise are more likely to participate. Despite the prescription of a targeted 12-week upper-limb intervention in this study, the general activity levels of both groups may have had an impact on the significance of this study. In addition, the low adherence to the use of self-reported logs failed to capture the true compliance rates of our participants because their lack of tracking does not indicate failure to comply with the program. The use of weekly or biweekly telephone calls to monitor compliance rates of activity more vigilantly may be used in future studies.
Conclusions
Advances in clinical management of breast cancer have improved survival outcomes, and morbidity over recent years, yet symptoms such as pain remain prevalent in this population. The results of this study showed that a targeted, 12-week upper-limb exercise intervention postradiation transiently improved levels of shoulder pain without a concomitant impact on chronic pain or any positive influence on activities of daily living 18 months posttreatment. Furthermore, future studies should use a variety of measurement tools to evaluate trunk and upper-limb pain in women with breast cancer and investigate the optimal timing of postradiation exercise interventions.
Acknowledgments
The authors thank Hope & Cope, the CURE foundation, and the Jewish General Hospital Foundation/Weekend to End Breast Cancer for providing the financial resources needed to sustain this research study. They also thank the McGill Adolescent and Young Adult program for its continued support. Previous oral presentations of research Muanza TM, et al. Randomized clinical trial of a progressive exercise program for young women with breast cancer undergoing radiation therapy. Int J Radiat Oncol Biol Phys. 2015;93(3):s35-s36.
Breast cancer is one of the most prevalent cancers in women worldwide, with more than 1 million new cases diagnosed annually.1 Prognosis for the disease has improved significantly, but 25% to 60% of women living with breast cancer experience some level of pain ranging from mild to severe, the nature of which can evolve from acute to chronic.2 Pre-, intra-, and post-treatment risk factors have been found to correlate with the development of acute and chronic pain and include young age, type of breast surgery (lumpectomy or total mastectomy), axillary node dissection, radiation therapy, and hormonal therapy.3-5 Chemotherapy, particularly anthracycline- and taxane-based regimens, has also been shown to induce pain, arthralgia, myalgia, and peripheral neuropathy during treatment.6 In particular, postradiation pain may result from subcutaneous fibrosis with fixation to underlying musculature and the development of fibrous flaps in the internal axilla.7 These tissue changes are commonly subclinical, occurring 4 to 12 months postradiation,8 and can progress undetected until pain and upper-limb disability develop.
The presence of persistent pain has a considerable impact on the quality of life in survivors of breast cancer: psychological distress is prevalent (anxiety, depression, worry, fear), the performance of daily activities is diminished (eg, bathing, dressing, preparing meals, shopping), and economic independence is compromised by the inability to work or reduced employment and income. These factors directly and indirectly contribute to an increase in the use of health care services.9,10
The management of pain is often characterized by pharmacologic-related treatment, such as the use of opioids and nonsteroidal anti-inflammatory medications, and nonpharmacologic-related treatment, such as exercise. Empirical evidence has shown that rehabilitative exercise programs, which commonly include a combination of resistance training and aerobic exercises, can effectively reduce pain in breast cancer survivors.10-12 Women living with breast cancer who are directed to rehabilitative exercise programs experience an improvement not only in pain levels but also in their ability to engage in activities of daily living, in their psychological health, and in their overall quality of life.13-15 However, despite evidence to support exercise programs to reduce pain related to breast cancer treatment, residual pain and upper-limb discomfort are common complaints in breast cancer survivors, and there is little focus on the duration of effectiveness of such programs for reducing pain after treatment for breast cancer. The objective of this study was to determine if an exercise program initiated postradiation would improve long-term pain levels in a carefully selected population of young women who were living with breast cancer and had no history of shoulder pathology or significant treatment complications.
Methods
Design
We used a pilot randomized control trial to compare the long-term effectiveness of a 12-week postradiation exercise program versus standard care on residual pain levels in young women (aged 18-45 years) living with breast cancer. The program was initiated 3 to 4 weeks postradiation to allow for acute inflammatory reactions to subside. Pain severity and interference were assessed using the Brief Pain Inventory-Short Form (BPI-SF), a tool for assessing cancer pain.16,17 Pain levels for isolated shoulder movements were also recorded on examination by a physical therapist. All measures were collected at 6 time points (T1-T6): postsurgery and preradiation (T1, baseline), postradiation and preintervention (T2), and 4 points during an 18-month period postradiation (T3-T6 at 3, 6, 12, and 18 months postradiation).
Sample
Young women living with breast cancer who met our eligibility criteria were identified from 2 clinics at the Jewish General Hospital – the Segal Cancer Center and the Department of Radiation Oncology in Montréal, Québec, Canada. Inclusion criteria included women with a diagnosis of stage I to stage III breast cancer, who were 18 to 45 years old, were scheduled for postoperative adjuvant radiation therapy, had an Eastern Cooperative Oncology Group Performance Status of 0 or 1 (normal ambulatory function, minimal symptoms), and who consented to participate in the study. Exclusion criteria included women with a metastatic (stage IV) diagnosis; significant musculoskeletal, cardiac, pulmonary, or metabolic comorbidities that would not allow for participation in physical activity; a previous breast cancer diagnosis with treatment to the ipsilateral or contralateral sides; postsurgical lymphedema; postsurgical capsulitis, tendonitis, or other shoulder inflammatory complications; and any contraindication to exercise. The recruitment goal was outlined as 50 patients per group; however, a protracted accrual time because of the stringent study criteria yielded a sample of 29 and 30 patients for the intervention and control groups, respectively, which was sufficient for significant testing of differences between the 2 study groups.18
Variables and measures
Clinical characteristics. We used standardized questions and chart review to document the participants’ clinical characteristics and to capture information on the following: the stage and subtype of breast cancer, hormonal and human epidermal growth factor receptors (HER2) (estrogen receptor, progesterone receptor, and HER2 status), extent of surgery (lumpectomy or total mastectomy), and other modalities of treatment (eg, chemotherapy, radiation therapy).
Pain assessment. The BPI-SF was used to assess participants’ cancer-related pain. Pain severity ranged from 0 (no pain), 1 to 4 (mild pain), 5 to 6 (moderate pain), to 7 to 10 (severe pain).18,19 The questionnaire also identifies the pain interference in daily activities using a Likert scale ranging from 0 (Does not interfere) to 10 (Completely interferes) in the following 7 domains or subscales: General Activity, Walking, Mood, Sleep, Work, Relations with Others, and Enjoyment of Life.16 For the purpose of this study, mean scores were tabulated using both pain intensity and interference scales.
Another important component of the BPI-SF instructs participants to localize pain by means of a body diagram. For purpose of analysis, 3 pain regions were established: shoulder girdle/chest wall on the affected side; neck and other upper extremity, including hand(s), forearm(s), wrist(s), and finger(s); and other regions, including abdominal discomfort, leg(s), hip(s), knee(s), ankle(s), lower back, and feet. In addition, pain levels on movement (Yes/No) were recorded for isolated shoulder flexion, abduction, and horizontal abduction (sitting and standing). The measurements were completed by a single physical therapist throughout the course of the study to minimize variance.
Procedure
The study protocol was approved by the Research Ethics Board at the Jewish General Hospital. Recruitment occurred from 2011 through 2015. The research was in accordance with the ethical standards of the responsible committee on human experimentation. Eligible women were recruited by the research coordinator who described the purpose, risks, and benefits of the study; advised on confidentiality, data collection, and intervention allocation procedures; and highlighted voluntary participation. The research coordinator addressed any concerns on the part of the participants before obtaining their written informed consent. Random allocation to the intervention and control groups was established using a web-based randomization plan generator (www.randomization.com). A single individual was responsible for the randomization process, and treatment assignments were revealed after each participant’s name had been entered. A physical therapist performed 6 sequential evaluations (T1-T6) at the time of participants’ medical follow-up appointments.
Intervention
The 12-week exercise intervention started 3 weeks postradiation and was composed of an initial 6-week program of low-level cardiovascular and resistance exercises that progressed to a set of more advanced exercises for the remaining 6 weeks. Participants were instructed to warm up for at least 10 minutes with a cardiovascular exercise of their choice (eg, a recumbent cross trainer, walking, or stairs) before doing a combined strength, endurance, and stretching exercise program for the upper body.20 The final portion of the exercise intervention included a period of light cool-down. Weight training resistance levels were based on a maximum 8 to 10 repetitions for strength and a maximum of 20 repetitions for endurance training exercises, which progressed gradually over the course of the 12-week exercise program to ensure participant safety.21,22 Participants in the intervention group were supervised at least once a week by an exercise physiologist at a center for oncology patients (Hope & Cope Wellness Centre), and patients were encouraged to perform the program at home 2 to 3 times a week. Those who were not able to exercise consistently at the center were provided with equipment and instructed on how to do the program safely at home.
By comparison, the control group received standard care, which included advice on the benefits of an active lifestyle, including exercise, but without a specific intervention. Participants were not restricted in their physical activity and/or sport participation levels, and their weekly activity levels were calculated using the Metabolic Equivalent of Task and recorded at each of the 6 time points.
Statistical analysis
Descriptive statistics were used to examine participant characteristics. The quantitative data collected through the BPI-SF measures were analyzed with JMP software (version 11.2; SAS Institute, Cary, NC). Continuous variables were tested for statistical significance (P ≤ .05) through the chi-square (categorical), analysis of variance, and nonparametric Wilcoxon tests. The analyses did not include missing data.
Results
A total of 59 young women were randomized into the intervention (n = 29) and control (n = 30) groups. Of those, 2 participants dropped out of the study because of family and time constraints, and 3 participants died, 2 from the control and 1 from the intervention group, after subsequently developing metastatic disease. Baseline data including comparative tumor characteristics, surgical interventions, and treatment interventions have been published in relation to other elements of this study.23,24 The participants had a mean age of 39.2 years (standard deviation [SD], 5.0). More than half of them had an invasive ductal carcinoma (69.5%) and were estrogen positive (78.0%), progesterone positive (74.6%), or HER2 positive (20.3%), whereas 10.2% were triple negative. Most of the participants had undergone breast-sparing procedures (86.4% lumpectomy), and 18.6% had a total mastectomy. By random chance, the intervention group had higher rates of total mastectomy (24.4% and 13.3%, respectively) and surgical reconstruction (12.2% and 6.7%, respectively) compared with the control group. Most of the women (71.2%) received chemotherapy, and all received radiation therapy. In the intervention group, 37.2% received radiation therapy localized to the axilla, and 88% received a boost of radiation to the surgical bed. Self-reported exercise diaries were returned by 15 of the 29 intervention participants, and training frequencies among them varied significantly (1-6 times a week).
The findings showed that there was little variance between the intervention and control groups in BPI-SF severity scores from T1 to T6, so the means and SDs of the BPI-SF scores were grouped at 6 time points (Table 1). There was no statistically significant difference between baseline measures at T1 (1.68; SD, 1.17) and measures at 18 months postintervention (T6: 1.46; SD, 1.37). At baseline, 87.7% of the women reported no pain (31.5%) or mild levels of pain (55.6%), and 13% reported moderate or severe pain. Over the duration of the study from T1 to T6, these primarily low levels of pain (BPI-SF, 0-4) remained consistent with a favorable shift toward having no pain (T1: 31.5%; T6: 24.4%). By 18 months postintervention, 95.7% of women reported no or mild pain, with 4.9% reporting moderate pain.
Similarly, there was little variance over time (T1-T6) and no statistically significant differences between the 2 groups in BPI-SF–measured levels of pain interference in daily activities (Table 2). Moreover, a domain analysis showed that there were no statistically significant differences in pain interference scores when comparing the type and extent of surgery (total mastectomy: 0.59 [1.17]; lumpectomy: 0.94 [1.96]). By chance – and not related directly to the objectives of this study – there was a statistically significant difference between the intervention and control groups in the interference of pain on the Enjoyment of Life domain in favor of the control group.
The sites of pain captured by the BPI-SF shed light on the preceding findings (Figure 1). At baseline (T1, postsurgery and preradiation), 37.0% of participants reported pain in the shoulder girdle–chest wall region, whereas 20.4% reported pain in the general neck–upper extremity region and 50% in other regions. Postradiation, shoulder girdle–chest wall pain was identified as the highest reported site of pain (49.1%; T2, postradiation and preintervention) and remained elevated at 3 months (T3) and 6 months (T4) postradiation (46.9% and 45.5%, respectively). At 12 and 18 months postradiation (T5 and T6), the principal focus of pain shifted once again to “other” regions at 30% and 32.5%, respectively, and the neck–upper extremity region at 10% and 15%, respectively. Shoulder girdle–chest wall pain concomitantly improved at those time points (15% and 25% respectively) but was not eliminated.
Pain levels recorded on physical examination for isolated shoulder range of movements were recently published,24 and they have been abbreviated and reproduced in this paper (Figure 2) to allow for a comparison of findings between the exercise intervention group and the control group to help determine the sensitivity of these tools for use in breast cancer patients. At baseline, pain levels with active movement were noted to be slightly greater in the intervention group for flexion and abduction.
Following the intervention, at 3 and 6 months postradiation (T3 and T4), the intervention group showed a steady decrease in pain levels in flexion and abduction, whereas the control group showed a 5-fold increase in pain with horizontal abduction. Furthermore, participants in the intervention group reported having no pain on movement 12 months postradiation (T5); however, recurrence of pain was apparent with all shoulder movements by 18 months postradiation (T6) in both the intervention and control groups.
Discussion
Previous studies have hypothesized that younger age (18-39 years), adjuvant radiotherapy, and axillary node dissection are risk factors for chronic pain in breast cancer survivors.22,25 Persistent pain is prevalent in 12% to 51% of breast cancer survivors, with up to one-third experiencing some pain more than 5 years after treatment,26,27 and our study outcomes concur with those findings. In our study, pain, as measured by the BPI-SF, was found to persist for most participants (75.6%) after the 18-month follow-up. The results of our trial showed that a 12-week exercise intervention administered postsurgery and postradiation had no statistically significant effect on long-term (18 months) pain severity and its interference in daily life. It is worth noting that body regions that had not been directly related to either surgical or radiation treatment for breast cancer were commonly identified as areas of pain but were not specifically targeted by our intervention. However, focusing on pain severity (BPI-SF), our findings suggest that the benefits of targeted upper-extremity exercise on pain in the intermediate time course of follow-up (T3, T4, and T5) was notable compared with the control group, which received standard care. The apparent recurrence of pain at 18 months in both groups was not anticipated and needs to be further investigated.
More specific objective assessments of pain on active shoulder movement identified distinct patterns of pain that could not be isolated using the BPI-SF alone. The incidence and localization of pain on movement differed between the population of women who received a specific exercise intervention and those who received standard care (Figure 2). Patterns of pain over time fluctuated in the control group, whereas the intervention group reported a linear decrease in pain. Residual pain on shoulder movement remained apparent in both groups at 18-months postradiation, but that finding was not reflected in the BPI-SF results. The literature supports our findings on persistent pain among breast cancer survivors,3,7,8,28-30 and in our study of young women carefully screened and excluded for pre-existent shoulder conditions or comorbid medical conditions, recurrent articular pain was nonetheless prevalent. It seems that unidentified or multiple factors may be part of the etiology of pain in this young adult cohort.
Although the BPI-SF is a generic measurement tool commonly used to assess and measure cancer patients’ pain levels, the lack of variance in our BPI-SF severity and interference outcomes over time (T1-T6) (Table 1, Table 2), the variety of “other” unrelated regions (Figure 1) identified by the BPI-SF, and the contrast in our findings on specific physical examination emphasize the potential limitations of this clinical tool.
Moreover, the BPI-SF has not been validated specifically for breast cancer. Harrington and colleagues have recommended using the BPI-SF to assess pain in women with breast cancer,31 but the use of a more multidimensional measurement tool that evaluates axillary, chest, trunk, and upper-limb pain may prove to be more valuable in this population.
Limitations
Recruitment of young adult women was difficult because of our stringent inclusion criteria, the long-term follow-up, and the relatively small population of breast cancer patients in this age demographic. Therefore, the duration of the recruitment phase, despite our having access to a specialized young adult and adolescent clinic in our institute, greatly surpassed the expectations we had when we designed the study. In addition, there remains an inherent bias in participants who accept participation in a study that includes exercise interventions. Potential participants who exercise regularly or have a positive inclination toward doing exercise are more likely to participate. Despite the prescription of a targeted 12-week upper-limb intervention in this study, the general activity levels of both groups may have had an impact on the significance of this study. In addition, the low adherence to the use of self-reported logs failed to capture the true compliance rates of our participants because their lack of tracking does not indicate failure to comply with the program. The use of weekly or biweekly telephone calls to monitor compliance rates of activity more vigilantly may be used in future studies.
Conclusions
Advances in clinical management of breast cancer have improved survival outcomes, and morbidity over recent years, yet symptoms such as pain remain prevalent in this population. The results of this study showed that a targeted, 12-week upper-limb exercise intervention postradiation transiently improved levels of shoulder pain without a concomitant impact on chronic pain or any positive influence on activities of daily living 18 months posttreatment. Furthermore, future studies should use a variety of measurement tools to evaluate trunk and upper-limb pain in women with breast cancer and investigate the optimal timing of postradiation exercise interventions.
Acknowledgments
The authors thank Hope & Cope, the CURE foundation, and the Jewish General Hospital Foundation/Weekend to End Breast Cancer for providing the financial resources needed to sustain this research study. They also thank the McGill Adolescent and Young Adult program for its continued support. Previous oral presentations of research Muanza TM, et al. Randomized clinical trial of a progressive exercise program for young women with breast cancer undergoing radiation therapy. Int J Radiat Oncol Biol Phys. 2015;93(3):s35-s36.
1. World Health Organization. Breast cancer: prevention and control. www.who.int/cancer/detection/breastcancer/en/. Updated 2017. Accessed September 16, 2016.
2. Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain. 2011;12(7):725-746.
3. Ernst MF, Voogd AC, Balder W, Klinkenbijl JH, Roukema JA. Early and late morbidity associated with axillary levels I-III dissection in breast cancer. J Surg Oncol. 2002;79(3):151-155; discussion 156.
4. Gulluoglu BM, Cingi A, Cakir T, Gercek A, Barlas A, Eti Z. Factors related to post-treatment chronic pain in breast cancer survivors: the interference of pain with life functions. Int J Fertil Womens Med. 2006;51(2):75-82.
5. Jung BF, Ahrendt GM, Oaklander AL, Dworkin RH. Neuropathic pain following breast cancer surgery: proposed classification and research update. Pain. 2003;104(1-2):1-13.
6. Saibil S, Fitzgerald B, Freedman OC, et al. Incidence of taxane-induced pain and distress in patients receiving chemotherapy for early-stage breast cancer: a retrospective, outcomes-based survey. Curr Oncol. 2010;17(4):42-47.
7. Tengrup I, Tennvall-Nittby L, Christiansson I, Laurin M. Arm morbidity after breast-conserving therapy for breast cancer. Acta Oncol. 2000;39(3):393-397.
8. Johansen J, Overgaard J, Blichert-Toft M, Overgaard M. Treatment of morbidity associated with the management of the axilla in breast-conserving therapy. Acta Oncol. 2000;39(3):349-354.
9. Mittmann N, Porter JM, Rangrej J, et al. Health system costs for stage-specific breast cancer: a population-based approach. Curr Oncol. 2014;21(6):281-293.
10. Page A. Keeping patients safe: transforming the work environment of nurses. Washington, DC: National Academies Press; 2004.
11. McNeely ML, Campbell K, Ospina M, et al. Exercise interventions for upper-limb dysfunction due to breast cancer treatment. Cochrane Database Syst Rev. 2010;(6):CD005211. doi:10.1002/14651858.CD005211.pub2
12. Wong P, Muanza T, Hijal T, et al. Effect of exercise in reducing breast and chest-wall pain in patients with breast cancer: a pilot study. Curr Oncol. 2012;19(3):e129-e135.
13. Fernández-Lao C, Cantarero-Villanueva I, Fernández-de-Las-Peñas C, del Moral-Ávila R, Castro-Sánchez AM, Arroyo-Morales M. Effectiveness of a multidimensional physical therapy program on pain, pressure hypersensitivity, and trigger points in breast cancer survivors: a randomized controlled clinical trial. Clin J Pain. 2012;28(2):113-121.
14. Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21(9):1660-1668.
15. Segal R, Evans W, Johnson D, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol. 2001;19(3):657-665.
16. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129-138.
17. Kumar SP. Utilization of Brief Pain Inventory as an assessment tool for pain in patients with cancer: a focused review. Indian J Palliat Care. 2011;17(2):108-115.
18. Van Voorhis CRW, Morgan BL. Understanding power and rules of thumb for determining sample sizes. Tutor Quant Methods Psychol. 2007;3(2):43-50.
19. Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277-284.
20. Lee TS, Kilbreath SL, Refshauge KM, Pendlebury SC, Beith JM, Lee MJ. Pectoral stretching program for women undergoing radiotherapy for breast cancer. Breast Cancer Res Treat. 2007;102(3):313-321.
21. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409-1426.
22. Pollock ML, Gaesser GA, Butcher JD, et al. ACSM position stand: the recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30(6):975-991.
23. Ibrahim M, Muanza T, Smirnow N, et al. Time course of upper limb function and return-to-work post-radiotherapy in young adults with breast cancer: a pilot randomized control trial on effects of targeted exercise program. J Cancer Surviv. 2017;11(6):791-799.
24. Ibrahim M, Muanza T, Smirnow N, et al. A pilot randomized controlled trial on the effects of a progressive exercise program on the range of motion and upper extremity grip strength in young adults with breast cancer. Clin Breast Cancer. 2018;18(1):e55-e64.
25. Gärtner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302(18):1985-1992.
26. Hayes SC, Johansson K, Stout NL, et al. Upper-body morbidity after breast cancer: incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of care. Cancer. 2012;118(suppl 8):2237-2249.
27. Kärki A, Simonen R, Mälkiä E, Selfe J. Impairments, activity limitations and participation restrictions 6 and 12 months after breast cancer operation. J Rehabil Med. 2005;37(3):180-188.
28. Katz J, Poleshuck EL, Andrus CH, et al. Risk factors for acute pain and its persistence following breast cancer surgery. Pain. 2005;119(1-3):16-25.
29. Tasmuth T, von Smitten K, Hietanen P, Kataja M, Kalso E. Pain and other symptoms after different treatment modalities of breast cancer. Ann Oncol. 1995;6(5):453-459.
30. Whelan TJ, Levine M, Julian J, Kirkbride P, Skingley P. The effects of radiation therapy on quality of life of women with breast carcinoma: results of a randomized trial. Ontario Clinical Oncology Group. Cancer. 2000;88(10):2260-2266.
31. Harrington S, Gilchrist L, Sander A. Breast cancer EDGE task force outcomes: clinical measures of pain. Rehabil Oncol. 2014;32(1):13-21.
1. World Health Organization. Breast cancer: prevention and control. www.who.int/cancer/detection/breastcancer/en/. Updated 2017. Accessed September 16, 2016.
2. Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain. 2011;12(7):725-746.
3. Ernst MF, Voogd AC, Balder W, Klinkenbijl JH, Roukema JA. Early and late morbidity associated with axillary levels I-III dissection in breast cancer. J Surg Oncol. 2002;79(3):151-155; discussion 156.
4. Gulluoglu BM, Cingi A, Cakir T, Gercek A, Barlas A, Eti Z. Factors related to post-treatment chronic pain in breast cancer survivors: the interference of pain with life functions. Int J Fertil Womens Med. 2006;51(2):75-82.
5. Jung BF, Ahrendt GM, Oaklander AL, Dworkin RH. Neuropathic pain following breast cancer surgery: proposed classification and research update. Pain. 2003;104(1-2):1-13.
6. Saibil S, Fitzgerald B, Freedman OC, et al. Incidence of taxane-induced pain and distress in patients receiving chemotherapy for early-stage breast cancer: a retrospective, outcomes-based survey. Curr Oncol. 2010;17(4):42-47.
7. Tengrup I, Tennvall-Nittby L, Christiansson I, Laurin M. Arm morbidity after breast-conserving therapy for breast cancer. Acta Oncol. 2000;39(3):393-397.
8. Johansen J, Overgaard J, Blichert-Toft M, Overgaard M. Treatment of morbidity associated with the management of the axilla in breast-conserving therapy. Acta Oncol. 2000;39(3):349-354.
9. Mittmann N, Porter JM, Rangrej J, et al. Health system costs for stage-specific breast cancer: a population-based approach. Curr Oncol. 2014;21(6):281-293.
10. Page A. Keeping patients safe: transforming the work environment of nurses. Washington, DC: National Academies Press; 2004.
11. McNeely ML, Campbell K, Ospina M, et al. Exercise interventions for upper-limb dysfunction due to breast cancer treatment. Cochrane Database Syst Rev. 2010;(6):CD005211. doi:10.1002/14651858.CD005211.pub2
12. Wong P, Muanza T, Hijal T, et al. Effect of exercise in reducing breast and chest-wall pain in patients with breast cancer: a pilot study. Curr Oncol. 2012;19(3):e129-e135.
13. Fernández-Lao C, Cantarero-Villanueva I, Fernández-de-Las-Peñas C, del Moral-Ávila R, Castro-Sánchez AM, Arroyo-Morales M. Effectiveness of a multidimensional physical therapy program on pain, pressure hypersensitivity, and trigger points in breast cancer survivors: a randomized controlled clinical trial. Clin J Pain. 2012;28(2):113-121.
14. Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21(9):1660-1668.
15. Segal R, Evans W, Johnson D, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol. 2001;19(3):657-665.
16. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129-138.
17. Kumar SP. Utilization of Brief Pain Inventory as an assessment tool for pain in patients with cancer: a focused review. Indian J Palliat Care. 2011;17(2):108-115.
18. Van Voorhis CRW, Morgan BL. Understanding power and rules of thumb for determining sample sizes. Tutor Quant Methods Psychol. 2007;3(2):43-50.
19. Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277-284.
20. Lee TS, Kilbreath SL, Refshauge KM, Pendlebury SC, Beith JM, Lee MJ. Pectoral stretching program for women undergoing radiotherapy for breast cancer. Breast Cancer Res Treat. 2007;102(3):313-321.
21. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409-1426.
22. Pollock ML, Gaesser GA, Butcher JD, et al. ACSM position stand: the recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30(6):975-991.
23. Ibrahim M, Muanza T, Smirnow N, et al. Time course of upper limb function and return-to-work post-radiotherapy in young adults with breast cancer: a pilot randomized control trial on effects of targeted exercise program. J Cancer Surviv. 2017;11(6):791-799.
24. Ibrahim M, Muanza T, Smirnow N, et al. A pilot randomized controlled trial on the effects of a progressive exercise program on the range of motion and upper extremity grip strength in young adults with breast cancer. Clin Breast Cancer. 2018;18(1):e55-e64.
25. Gärtner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302(18):1985-1992.
26. Hayes SC, Johansson K, Stout NL, et al. Upper-body morbidity after breast cancer: incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of care. Cancer. 2012;118(suppl 8):2237-2249.
27. Kärki A, Simonen R, Mälkiä E, Selfe J. Impairments, activity limitations and participation restrictions 6 and 12 months after breast cancer operation. J Rehabil Med. 2005;37(3):180-188.
28. Katz J, Poleshuck EL, Andrus CH, et al. Risk factors for acute pain and its persistence following breast cancer surgery. Pain. 2005;119(1-3):16-25.
29. Tasmuth T, von Smitten K, Hietanen P, Kataja M, Kalso E. Pain and other symptoms after different treatment modalities of breast cancer. Ann Oncol. 1995;6(5):453-459.
30. Whelan TJ, Levine M, Julian J, Kirkbride P, Skingley P. The effects of radiation therapy on quality of life of women with breast carcinoma: results of a randomized trial. Ontario Clinical Oncology Group. Cancer. 2000;88(10):2260-2266.
31. Harrington S, Gilchrist L, Sander A. Breast cancer EDGE task force outcomes: clinical measures of pain. Rehabil Oncol. 2014;32(1):13-21.
Testicular Pain Leads to a Rare Diagnosis
A 26-year-old man presented to uroligy clinicians with right testicular pain and a right epididymal mass. It was a challenge to diagnose the cause—until he revealed some essential clues.
The differential diagnosis included testicular malignancy and lymphoma. However, tumor markers were within normal limits. Tests for HIV and syphilis were negative. The clinicians also considered granulomatous or chronic orchitis, but after treatment with nonsteroidal anti-inflammatory drugs, the pain and palpable epididymal mass had resolved. Then, follow-up testicular ultrasound images showed new diffuse heterogeneous hypoechoic lesions in the right testis.
The patient elected to have a right radical orchiectomy with sperm cryopreserved. He recovered well, and semen analysis did not show any abnormalities.
Pathology of the surgical specimen revealed necrotizing and nonnecrotizing granulomas. At this point, the patient recollected that he had developed cervical lymphadenopathy and oral ulcers several weeks after traveling to South America, 8 months before presenting with the testicular symptoms.
Combined with another clue—frequent exposure to cats during his South America trip—the patient’s symptoms now created a clearer picture. He had been diagnosed with toxoplasmosis at the time but had not received treatment because he was immunocompetent. The patient’s symptoms had resolved spontaneously, and he said he had been in his usual health between then and when he developed the testicular pain.
Based on this new information, the clinicians conducted immunohistochemical tests, which revealed isolated cysts about 20 µm in diameter, confirming a diagnosis of testicular toxoplasmosis. They started him on systemic toxoplasmosis treatment; he has been in good health since.
The clinicians note that toxoplasmosis is highly prevalent, infecting up to 30% of the world’s population. Cat feces is one source of infection with Toxoplasma gondii, which is typically asymptomatic. In immunocompetent patients, it tends to present as an acute infection that is benign and self-limited.
Only a few cases of testicular toxoplasmosis have been reported in the literature, and all have been in immunocompromised patients. Because this patient was immunocompetent, the case is unique, being the only one reported as yet. Given the immunocompetence, the clinicians say, the patient demonstrates the need for clinicians to have a high index of suspicion.
Source:
Wong V, Amarasekera C, Kundu S. BMJ Case Rep. 2018;2018. pii: bcr-2018-224962.
A 26-year-old man presented to uroligy clinicians with right testicular pain and a right epididymal mass. It was a challenge to diagnose the cause—until he revealed some essential clues.
The differential diagnosis included testicular malignancy and lymphoma. However, tumor markers were within normal limits. Tests for HIV and syphilis were negative. The clinicians also considered granulomatous or chronic orchitis, but after treatment with nonsteroidal anti-inflammatory drugs, the pain and palpable epididymal mass had resolved. Then, follow-up testicular ultrasound images showed new diffuse heterogeneous hypoechoic lesions in the right testis.
The patient elected to have a right radical orchiectomy with sperm cryopreserved. He recovered well, and semen analysis did not show any abnormalities.
Pathology of the surgical specimen revealed necrotizing and nonnecrotizing granulomas. At this point, the patient recollected that he had developed cervical lymphadenopathy and oral ulcers several weeks after traveling to South America, 8 months before presenting with the testicular symptoms.
Combined with another clue—frequent exposure to cats during his South America trip—the patient’s symptoms now created a clearer picture. He had been diagnosed with toxoplasmosis at the time but had not received treatment because he was immunocompetent. The patient’s symptoms had resolved spontaneously, and he said he had been in his usual health between then and when he developed the testicular pain.
Based on this new information, the clinicians conducted immunohistochemical tests, which revealed isolated cysts about 20 µm in diameter, confirming a diagnosis of testicular toxoplasmosis. They started him on systemic toxoplasmosis treatment; he has been in good health since.
The clinicians note that toxoplasmosis is highly prevalent, infecting up to 30% of the world’s population. Cat feces is one source of infection with Toxoplasma gondii, which is typically asymptomatic. In immunocompetent patients, it tends to present as an acute infection that is benign and self-limited.
Only a few cases of testicular toxoplasmosis have been reported in the literature, and all have been in immunocompromised patients. Because this patient was immunocompetent, the case is unique, being the only one reported as yet. Given the immunocompetence, the clinicians say, the patient demonstrates the need for clinicians to have a high index of suspicion.
Source:
Wong V, Amarasekera C, Kundu S. BMJ Case Rep. 2018;2018. pii: bcr-2018-224962.
A 26-year-old man presented to uroligy clinicians with right testicular pain and a right epididymal mass. It was a challenge to diagnose the cause—until he revealed some essential clues.
The differential diagnosis included testicular malignancy and lymphoma. However, tumor markers were within normal limits. Tests for HIV and syphilis were negative. The clinicians also considered granulomatous or chronic orchitis, but after treatment with nonsteroidal anti-inflammatory drugs, the pain and palpable epididymal mass had resolved. Then, follow-up testicular ultrasound images showed new diffuse heterogeneous hypoechoic lesions in the right testis.
The patient elected to have a right radical orchiectomy with sperm cryopreserved. He recovered well, and semen analysis did not show any abnormalities.
Pathology of the surgical specimen revealed necrotizing and nonnecrotizing granulomas. At this point, the patient recollected that he had developed cervical lymphadenopathy and oral ulcers several weeks after traveling to South America, 8 months before presenting with the testicular symptoms.
Combined with another clue—frequent exposure to cats during his South America trip—the patient’s symptoms now created a clearer picture. He had been diagnosed with toxoplasmosis at the time but had not received treatment because he was immunocompetent. The patient’s symptoms had resolved spontaneously, and he said he had been in his usual health between then and when he developed the testicular pain.
Based on this new information, the clinicians conducted immunohistochemical tests, which revealed isolated cysts about 20 µm in diameter, confirming a diagnosis of testicular toxoplasmosis. They started him on systemic toxoplasmosis treatment; he has been in good health since.
The clinicians note that toxoplasmosis is highly prevalent, infecting up to 30% of the world’s population. Cat feces is one source of infection with Toxoplasma gondii, which is typically asymptomatic. In immunocompetent patients, it tends to present as an acute infection that is benign and self-limited.
Only a few cases of testicular toxoplasmosis have been reported in the literature, and all have been in immunocompromised patients. Because this patient was immunocompetent, the case is unique, being the only one reported as yet. Given the immunocompetence, the clinicians say, the patient demonstrates the need for clinicians to have a high index of suspicion.
Source:
Wong V, Amarasekera C, Kundu S. BMJ Case Rep. 2018;2018. pii: bcr-2018-224962.
MAb doubles ORR, PFS in rel/ref MM
STOCKHOLM—Adding elotuzumab (E) to treatment with pomalidomide (P) and low-dose dexamethasone (d) can produce “clinically meaningful” results in patients with relapsed/refractory multiple myeloma (MM), according to an investigator for the ELOQUENT-3 trial.
In this phase 2 trial, patients who received EPd had double the overall response rate (ORR) and median progression-free survival (PFS) of patients who received Pd.
Additionally, adverse events (AEs) were comparable between the treatment arms.
Meletios Dimopoulos, MD, of National and Kapodistrian University of Athens in Greece, presented these results as a late-breaking abstract (LB2606) at the 23rd Congress of the European Hematology Association (EHA).
The research was sponsored by Bristol-Myers Squibb.
The ELOQUENT-3 trial enrolled MM patients who had refractory or relapsed and refractory MM. They had to have received lenalidomide and a proteasome inhibitor (PI).
The patients were randomized to receive EPd (n=60) or Pd (n=57) in 28-day cycles until disease progression or unacceptable toxicity.
Pomalidomide was given orally at 4 mg on days 1 to 21 of each cycle. In the Pd arm, dexamethasone was given as a 20 mg (for patients older than 75) or 40 mg (75 and younger) tablet weekly.
In the EPd arm, dexamethasone was split between oral (8 mg, 20 mg, or 40 mg tablets) and intravenous doses (8 mg or 28 mg).
Elotuzumab was given at 10 mg/kg intravenously weekly for the first 2 cycles and 20 mg/kg monthly from cycle 3 on.
Patient characteristics
The patients’ median age was 69 (range, 43-81) in the EPd arm and 66 (range, 36-81) in the Pd arm. They were a median of 4.8 years (EPd) or 4.4 years (Pd) from diagnosis.
The median number of prior therapies was 3 (range, 2-8) in both groups.
Ninety percent of patients in the EPd arm and 84% of those in the Pd arm were refractory to lenalidomide. Seventy-eight percent and 82%, respectively, were refractory to a PI. And 68% and 72%, respectively, were refractory to both lenalidomide and a PI.
Treatment duration
Dr Dimopoulos noted that twice as many patients remained on treatment with EPd compared to Pd at the time of database lock (February 21, 2018). Forty percent of EPd patients (n=24) and 20% of Pd patients (n=11) were still on treatment at that time.
Patients’ primary reason for treatment discontinuation was disease progression—43% of EPd recipients and 56% of Pd recipients. Two percent of EPd recipients and 4% of Pd recipients withdrew due to treatment-related toxicity. Four percent of patients in the Pd arm (and none in the EPd arm) withdrew due to maximum clinical benefit.
The median number of treatment cycles was 9 (range, 4-13) in the EPd arm and 5 (range, 3-10) in the Pd arm.
Efficacy
The ORR was 53% in the EPd arm and 26% in the Pd arm. The odds ratio was 3.25 (P=0.0029).
Eight percent of patients in the EPd arm had a complete response or stringent complete response, as did 2% of patients in the Pd arm.
The median duration of response was 8.3 months in the Pd arm and has not been reached in the EPd arm.
“Elotuzumab with pomalidomide and dexamethasone showed a significant and clinically meaningful 46% reduction in the risk of progression or death,” Dr Dimopoulos said.
The median PFS was 10.3 months with EPd and 4.7 months with Pd (hazard ratio=0.54, P=0.0078).
Although overall survival data are not yet mature, there was a trend favoring EPd over Pd (hazard ratio=0.62). There were 13 deaths in the EPd arm and 18 deaths in the Pd arm.
Safety
Dr Dimopoulos said AEs were comparable between the treatment arms. He pointed out that neutropenia was less common with EPd compared to Pd, despite similar pomalidomide dose intensity. And exposure-adjusted hematologic AEs and infections were lower with EPd than with Pd.
Ninety-seven percent of patients in the EPd arm and 95% in the Pd arm had at least 1 AE.
Grade 3-4 nonhematologic AEs (in the EPd and Pd arms, respectively) included constipation (2% and 0%), hyperglycemia (8% and 7%), bone pain (3% and 0%), dyspnea (3% and 2%), fatigue (0% and 4%), respiratory tract infection (0% and 2%), and upper respiratory tract infection (0% and 2%).
Grade 3-4 hematologic AEs (in the EPd and Pd arms, respectively) included anemia (10% and 20%), neutropenia (13% and 27%), thrombocytopenia (8% and 5%), and lymphopenia (8% and 2%).
Grade 3-4 AEs of special interest (in the EPd and Pd arms, respectively) included infections (13% and 22%), vascular disorders (3% and 0%), cardiac disorders (7% and 4%), and neoplasms (2% and 11%).
There were 5 grade 5 AEs in the EPd arm and 8 in the Pd arm.
In the EPd arm, grade 5 AEs included infection (n=3), cardiac failure, and general physical health deterioration.
In the Pd arm, grade 5 AEs included malignant neoplasm progression (n=4), infection, multiple organ failure and infection, myocardial infarction, and plasma cell myeloma.
STOCKHOLM—Adding elotuzumab (E) to treatment with pomalidomide (P) and low-dose dexamethasone (d) can produce “clinically meaningful” results in patients with relapsed/refractory multiple myeloma (MM), according to an investigator for the ELOQUENT-3 trial.
In this phase 2 trial, patients who received EPd had double the overall response rate (ORR) and median progression-free survival (PFS) of patients who received Pd.
Additionally, adverse events (AEs) were comparable between the treatment arms.
Meletios Dimopoulos, MD, of National and Kapodistrian University of Athens in Greece, presented these results as a late-breaking abstract (LB2606) at the 23rd Congress of the European Hematology Association (EHA).
The research was sponsored by Bristol-Myers Squibb.
The ELOQUENT-3 trial enrolled MM patients who had refractory or relapsed and refractory MM. They had to have received lenalidomide and a proteasome inhibitor (PI).
The patients were randomized to receive EPd (n=60) or Pd (n=57) in 28-day cycles until disease progression or unacceptable toxicity.
Pomalidomide was given orally at 4 mg on days 1 to 21 of each cycle. In the Pd arm, dexamethasone was given as a 20 mg (for patients older than 75) or 40 mg (75 and younger) tablet weekly.
In the EPd arm, dexamethasone was split between oral (8 mg, 20 mg, or 40 mg tablets) and intravenous doses (8 mg or 28 mg).
Elotuzumab was given at 10 mg/kg intravenously weekly for the first 2 cycles and 20 mg/kg monthly from cycle 3 on.
Patient characteristics
The patients’ median age was 69 (range, 43-81) in the EPd arm and 66 (range, 36-81) in the Pd arm. They were a median of 4.8 years (EPd) or 4.4 years (Pd) from diagnosis.
The median number of prior therapies was 3 (range, 2-8) in both groups.
Ninety percent of patients in the EPd arm and 84% of those in the Pd arm were refractory to lenalidomide. Seventy-eight percent and 82%, respectively, were refractory to a PI. And 68% and 72%, respectively, were refractory to both lenalidomide and a PI.
Treatment duration
Dr Dimopoulos noted that twice as many patients remained on treatment with EPd compared to Pd at the time of database lock (February 21, 2018). Forty percent of EPd patients (n=24) and 20% of Pd patients (n=11) were still on treatment at that time.
Patients’ primary reason for treatment discontinuation was disease progression—43% of EPd recipients and 56% of Pd recipients. Two percent of EPd recipients and 4% of Pd recipients withdrew due to treatment-related toxicity. Four percent of patients in the Pd arm (and none in the EPd arm) withdrew due to maximum clinical benefit.
The median number of treatment cycles was 9 (range, 4-13) in the EPd arm and 5 (range, 3-10) in the Pd arm.
Efficacy
The ORR was 53% in the EPd arm and 26% in the Pd arm. The odds ratio was 3.25 (P=0.0029).
Eight percent of patients in the EPd arm had a complete response or stringent complete response, as did 2% of patients in the Pd arm.
The median duration of response was 8.3 months in the Pd arm and has not been reached in the EPd arm.
“Elotuzumab with pomalidomide and dexamethasone showed a significant and clinically meaningful 46% reduction in the risk of progression or death,” Dr Dimopoulos said.
The median PFS was 10.3 months with EPd and 4.7 months with Pd (hazard ratio=0.54, P=0.0078).
Although overall survival data are not yet mature, there was a trend favoring EPd over Pd (hazard ratio=0.62). There were 13 deaths in the EPd arm and 18 deaths in the Pd arm.
Safety
Dr Dimopoulos said AEs were comparable between the treatment arms. He pointed out that neutropenia was less common with EPd compared to Pd, despite similar pomalidomide dose intensity. And exposure-adjusted hematologic AEs and infections were lower with EPd than with Pd.
Ninety-seven percent of patients in the EPd arm and 95% in the Pd arm had at least 1 AE.
Grade 3-4 nonhematologic AEs (in the EPd and Pd arms, respectively) included constipation (2% and 0%), hyperglycemia (8% and 7%), bone pain (3% and 0%), dyspnea (3% and 2%), fatigue (0% and 4%), respiratory tract infection (0% and 2%), and upper respiratory tract infection (0% and 2%).
Grade 3-4 hematologic AEs (in the EPd and Pd arms, respectively) included anemia (10% and 20%), neutropenia (13% and 27%), thrombocytopenia (8% and 5%), and lymphopenia (8% and 2%).
Grade 3-4 AEs of special interest (in the EPd and Pd arms, respectively) included infections (13% and 22%), vascular disorders (3% and 0%), cardiac disorders (7% and 4%), and neoplasms (2% and 11%).
There were 5 grade 5 AEs in the EPd arm and 8 in the Pd arm.
In the EPd arm, grade 5 AEs included infection (n=3), cardiac failure, and general physical health deterioration.
In the Pd arm, grade 5 AEs included malignant neoplasm progression (n=4), infection, multiple organ failure and infection, myocardial infarction, and plasma cell myeloma.
STOCKHOLM—Adding elotuzumab (E) to treatment with pomalidomide (P) and low-dose dexamethasone (d) can produce “clinically meaningful” results in patients with relapsed/refractory multiple myeloma (MM), according to an investigator for the ELOQUENT-3 trial.
In this phase 2 trial, patients who received EPd had double the overall response rate (ORR) and median progression-free survival (PFS) of patients who received Pd.
Additionally, adverse events (AEs) were comparable between the treatment arms.
Meletios Dimopoulos, MD, of National and Kapodistrian University of Athens in Greece, presented these results as a late-breaking abstract (LB2606) at the 23rd Congress of the European Hematology Association (EHA).
The research was sponsored by Bristol-Myers Squibb.
The ELOQUENT-3 trial enrolled MM patients who had refractory or relapsed and refractory MM. They had to have received lenalidomide and a proteasome inhibitor (PI).
The patients were randomized to receive EPd (n=60) or Pd (n=57) in 28-day cycles until disease progression or unacceptable toxicity.
Pomalidomide was given orally at 4 mg on days 1 to 21 of each cycle. In the Pd arm, dexamethasone was given as a 20 mg (for patients older than 75) or 40 mg (75 and younger) tablet weekly.
In the EPd arm, dexamethasone was split between oral (8 mg, 20 mg, or 40 mg tablets) and intravenous doses (8 mg or 28 mg).
Elotuzumab was given at 10 mg/kg intravenously weekly for the first 2 cycles and 20 mg/kg monthly from cycle 3 on.
Patient characteristics
The patients’ median age was 69 (range, 43-81) in the EPd arm and 66 (range, 36-81) in the Pd arm. They were a median of 4.8 years (EPd) or 4.4 years (Pd) from diagnosis.
The median number of prior therapies was 3 (range, 2-8) in both groups.
Ninety percent of patients in the EPd arm and 84% of those in the Pd arm were refractory to lenalidomide. Seventy-eight percent and 82%, respectively, were refractory to a PI. And 68% and 72%, respectively, were refractory to both lenalidomide and a PI.
Treatment duration
Dr Dimopoulos noted that twice as many patients remained on treatment with EPd compared to Pd at the time of database lock (February 21, 2018). Forty percent of EPd patients (n=24) and 20% of Pd patients (n=11) were still on treatment at that time.
Patients’ primary reason for treatment discontinuation was disease progression—43% of EPd recipients and 56% of Pd recipients. Two percent of EPd recipients and 4% of Pd recipients withdrew due to treatment-related toxicity. Four percent of patients in the Pd arm (and none in the EPd arm) withdrew due to maximum clinical benefit.
The median number of treatment cycles was 9 (range, 4-13) in the EPd arm and 5 (range, 3-10) in the Pd arm.
Efficacy
The ORR was 53% in the EPd arm and 26% in the Pd arm. The odds ratio was 3.25 (P=0.0029).
Eight percent of patients in the EPd arm had a complete response or stringent complete response, as did 2% of patients in the Pd arm.
The median duration of response was 8.3 months in the Pd arm and has not been reached in the EPd arm.
“Elotuzumab with pomalidomide and dexamethasone showed a significant and clinically meaningful 46% reduction in the risk of progression or death,” Dr Dimopoulos said.
The median PFS was 10.3 months with EPd and 4.7 months with Pd (hazard ratio=0.54, P=0.0078).
Although overall survival data are not yet mature, there was a trend favoring EPd over Pd (hazard ratio=0.62). There were 13 deaths in the EPd arm and 18 deaths in the Pd arm.
Safety
Dr Dimopoulos said AEs were comparable between the treatment arms. He pointed out that neutropenia was less common with EPd compared to Pd, despite similar pomalidomide dose intensity. And exposure-adjusted hematologic AEs and infections were lower with EPd than with Pd.
Ninety-seven percent of patients in the EPd arm and 95% in the Pd arm had at least 1 AE.
Grade 3-4 nonhematologic AEs (in the EPd and Pd arms, respectively) included constipation (2% and 0%), hyperglycemia (8% and 7%), bone pain (3% and 0%), dyspnea (3% and 2%), fatigue (0% and 4%), respiratory tract infection (0% and 2%), and upper respiratory tract infection (0% and 2%).
Grade 3-4 hematologic AEs (in the EPd and Pd arms, respectively) included anemia (10% and 20%), neutropenia (13% and 27%), thrombocytopenia (8% and 5%), and lymphopenia (8% and 2%).
Grade 3-4 AEs of special interest (in the EPd and Pd arms, respectively) included infections (13% and 22%), vascular disorders (3% and 0%), cardiac disorders (7% and 4%), and neoplasms (2% and 11%).
There were 5 grade 5 AEs in the EPd arm and 8 in the Pd arm.
In the EPd arm, grade 5 AEs included infection (n=3), cardiac failure, and general physical health deterioration.
In the Pd arm, grade 5 AEs included malignant neoplasm progression (n=4), infection, multiple organ failure and infection, myocardial infarction, and plasma cell myeloma.
Inhibitor exhibits activity in B- and T-cell NHLs
STOCKHOLM—The dual SYK/JAK inhibitor cerdulatinib has demonstrated efficacy in a phase 2 trial of patients with heavily pretreated B- and T-cell non-Hodgkin lymphomas (NHLs).
There were a few deaths due to sepsis or septic shock that were considered related to cerdulatinib, but investigators have taken steps to prevent additional deaths.
Cerdulatinib produced responses in patients with peripheral T-cell lymphoma (PTCL), cutaneous T-cell lymphoma (CTCL), chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), follicular lymphoma (FL), and other NHLs.
The 5 deaths due to sepsis or septic shock (3 concomitant with pneumonia) occurred early on in the trial, and dose reductions, monitoring, and antibiotic prophylaxis appeared to be effective in preventing additional deaths.
Results from this trial were presented in a poster (abstract PF437) at the 23rd Congress of the European Hematology Association (EHA).
The research was sponsored by Portola Pharmaceuticals, Inc.
The trial enrolled 114 patients. They had FL (grade 1-3A; n=39), PTCL (n=25), CTCL (n=5), CLL/SLL (n=28), other indolent NHLs (Waldenstrom’s macroglobulinemia and marginal zone lymphoma; n=12), or aggressive NHL (defined as diffuse large B-cell lymphoma [DLBCL], grade 3B FL, mantle cell lymphoma, and transformed NHL with relapsed disease; n=5).
The patients’ median age was 68 (range, 34-93), and 59% were male. The median number of prior treatment regimens was 3 (range, 1-13), and 37% of patients had refractory disease.
Patients received cerdulatinib at 25, 30, or 35 mg twice daily (BID). A total of 101 patients were evaluable as of May 4, 2018.
Response
The objective response rate (ORR) was 47% in the entire population. Thirteen patients achieved a complete response (CR), and 34 had a partial response (PR). Thirty-four patients remained on cerdulatinib at the data cut-off.
The ORR was 46% in the FL patients, with 3 patients achieving a CR and 13 achieving a PR. Thirteen FL patients remained on cerdulatinib.
In the CLL/SLL patients, the ORR was 61%. Two patients had a CR, and 15 had a PR. Four CLL/SLL patients remained on cerdulatinib.
In PTCL, the ORR was 35%. All 7 responders had a CR. Eleven PTCL patients remained on cerdulatinib.
Only 1 CTCL patient was evaluable, but this patient achieved a CR and remained on cerdulatinib.
The ORR was 42% for patients with other indolent NHLs, with 5 PRs and no CRs. Only 1 patient in this group remained on cerdulatinib.
For aggressive NHL, the ORR was 20%, with 1 PR and no CRs. None of the patients in this group stayed on cerdulatinib.
“Cerdulatinib continues to demonstrate promising results across a wide range of B- and T-cell malignancies, including early indications of the potential for durable responses,” said study investigator Paul Hamlin, MD, of Memorial Sloan Kettering Cancer Center in New York, New York.
“The new signals in relapsed/refractory PTCL and CTCL are particularly compelling when you consider the limited treatment options for patients that fail frontline therapy.”
Safety
Grade 3 or higher adverse events included lipase increase (18%), neutropenia (17%), pneumonia/lung infection (11%), diarrhea (8%), fatigue (6%), amylase increase (5%), sepsis/septic shock (4%), hypertension (4%), anemia (4%), thrombocytopenia (4%), and hypophosphatemia (4%).
The 5 deaths due to sepsis or septic shock (3 of which were concomitant with pneumonia) were considered related to cerdulatinib. Three of the deaths occurred in patients with CLL, 1 in a DLBCL patient, and 1 in an FL patient.
“One of the things we have seen [with CLL patients] is the background infection rate is quite a bit higher,” said John T. Curnutte, MD, PhD, interim co-president and head of research and development at Portola Pharmaceuticals.
“You see this with multiple other agents, so we were not particularly surprised to see [sepsis/septic shock in CLL].”
Dr Curnutte noted that grade 5 sepsis/septic shock tended to occur in patients who were pre-colonized and/or had high plasma levels of cerdulatinib.
So, to prevent these adverse events, the starting dose of cerdulatinib was lowered, investigators began monitoring patients’ plasma levels, and all patients began receiving antibiotic prophylaxis. (Previously, only CLL patients had received this prophylaxis.)
The investigators found that lowering the starting dose from 35 mg BID to 30 mg or even 25 mg BID reduced plasma levels.
“At the 35 mg dose, 1 out of every 4 patients showed accumulation of the drug,” Dr Curnutte said. “In general, the use of the 30 mg BID or step-down 25 mg BID did not result in accumulation of drug.”
Dr Curnutte also noted that enrollment of CLL/SLL patients is complete, and investigators would be “very careful” if the study were to be re-opened to these patients.
For now, Portola is focused on completing enrollment in other patient groups on this phase 2 trial. The company is also hoping to conduct a phase 3 trial of cerdulatinib in PTCL that could begin as early as the end of this year.
STOCKHOLM—The dual SYK/JAK inhibitor cerdulatinib has demonstrated efficacy in a phase 2 trial of patients with heavily pretreated B- and T-cell non-Hodgkin lymphomas (NHLs).
There were a few deaths due to sepsis or septic shock that were considered related to cerdulatinib, but investigators have taken steps to prevent additional deaths.
Cerdulatinib produced responses in patients with peripheral T-cell lymphoma (PTCL), cutaneous T-cell lymphoma (CTCL), chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), follicular lymphoma (FL), and other NHLs.
The 5 deaths due to sepsis or septic shock (3 concomitant with pneumonia) occurred early on in the trial, and dose reductions, monitoring, and antibiotic prophylaxis appeared to be effective in preventing additional deaths.
Results from this trial were presented in a poster (abstract PF437) at the 23rd Congress of the European Hematology Association (EHA).
The research was sponsored by Portola Pharmaceuticals, Inc.
The trial enrolled 114 patients. They had FL (grade 1-3A; n=39), PTCL (n=25), CTCL (n=5), CLL/SLL (n=28), other indolent NHLs (Waldenstrom’s macroglobulinemia and marginal zone lymphoma; n=12), or aggressive NHL (defined as diffuse large B-cell lymphoma [DLBCL], grade 3B FL, mantle cell lymphoma, and transformed NHL with relapsed disease; n=5).
The patients’ median age was 68 (range, 34-93), and 59% were male. The median number of prior treatment regimens was 3 (range, 1-13), and 37% of patients had refractory disease.
Patients received cerdulatinib at 25, 30, or 35 mg twice daily (BID). A total of 101 patients were evaluable as of May 4, 2018.
Response
The objective response rate (ORR) was 47% in the entire population. Thirteen patients achieved a complete response (CR), and 34 had a partial response (PR). Thirty-four patients remained on cerdulatinib at the data cut-off.
The ORR was 46% in the FL patients, with 3 patients achieving a CR and 13 achieving a PR. Thirteen FL patients remained on cerdulatinib.
In the CLL/SLL patients, the ORR was 61%. Two patients had a CR, and 15 had a PR. Four CLL/SLL patients remained on cerdulatinib.
In PTCL, the ORR was 35%. All 7 responders had a CR. Eleven PTCL patients remained on cerdulatinib.
Only 1 CTCL patient was evaluable, but this patient achieved a CR and remained on cerdulatinib.
The ORR was 42% for patients with other indolent NHLs, with 5 PRs and no CRs. Only 1 patient in this group remained on cerdulatinib.
For aggressive NHL, the ORR was 20%, with 1 PR and no CRs. None of the patients in this group stayed on cerdulatinib.
“Cerdulatinib continues to demonstrate promising results across a wide range of B- and T-cell malignancies, including early indications of the potential for durable responses,” said study investigator Paul Hamlin, MD, of Memorial Sloan Kettering Cancer Center in New York, New York.
“The new signals in relapsed/refractory PTCL and CTCL are particularly compelling when you consider the limited treatment options for patients that fail frontline therapy.”
Safety
Grade 3 or higher adverse events included lipase increase (18%), neutropenia (17%), pneumonia/lung infection (11%), diarrhea (8%), fatigue (6%), amylase increase (5%), sepsis/septic shock (4%), hypertension (4%), anemia (4%), thrombocytopenia (4%), and hypophosphatemia (4%).
The 5 deaths due to sepsis or septic shock (3 of which were concomitant with pneumonia) were considered related to cerdulatinib. Three of the deaths occurred in patients with CLL, 1 in a DLBCL patient, and 1 in an FL patient.
“One of the things we have seen [with CLL patients] is the background infection rate is quite a bit higher,” said John T. Curnutte, MD, PhD, interim co-president and head of research and development at Portola Pharmaceuticals.
“You see this with multiple other agents, so we were not particularly surprised to see [sepsis/septic shock in CLL].”
Dr Curnutte noted that grade 5 sepsis/septic shock tended to occur in patients who were pre-colonized and/or had high plasma levels of cerdulatinib.
So, to prevent these adverse events, the starting dose of cerdulatinib was lowered, investigators began monitoring patients’ plasma levels, and all patients began receiving antibiotic prophylaxis. (Previously, only CLL patients had received this prophylaxis.)
The investigators found that lowering the starting dose from 35 mg BID to 30 mg or even 25 mg BID reduced plasma levels.
“At the 35 mg dose, 1 out of every 4 patients showed accumulation of the drug,” Dr Curnutte said. “In general, the use of the 30 mg BID or step-down 25 mg BID did not result in accumulation of drug.”
Dr Curnutte also noted that enrollment of CLL/SLL patients is complete, and investigators would be “very careful” if the study were to be re-opened to these patients.
For now, Portola is focused on completing enrollment in other patient groups on this phase 2 trial. The company is also hoping to conduct a phase 3 trial of cerdulatinib in PTCL that could begin as early as the end of this year.
STOCKHOLM—The dual SYK/JAK inhibitor cerdulatinib has demonstrated efficacy in a phase 2 trial of patients with heavily pretreated B- and T-cell non-Hodgkin lymphomas (NHLs).
There were a few deaths due to sepsis or septic shock that were considered related to cerdulatinib, but investigators have taken steps to prevent additional deaths.
Cerdulatinib produced responses in patients with peripheral T-cell lymphoma (PTCL), cutaneous T-cell lymphoma (CTCL), chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), follicular lymphoma (FL), and other NHLs.
The 5 deaths due to sepsis or septic shock (3 concomitant with pneumonia) occurred early on in the trial, and dose reductions, monitoring, and antibiotic prophylaxis appeared to be effective in preventing additional deaths.
Results from this trial were presented in a poster (abstract PF437) at the 23rd Congress of the European Hematology Association (EHA).
The research was sponsored by Portola Pharmaceuticals, Inc.
The trial enrolled 114 patients. They had FL (grade 1-3A; n=39), PTCL (n=25), CTCL (n=5), CLL/SLL (n=28), other indolent NHLs (Waldenstrom’s macroglobulinemia and marginal zone lymphoma; n=12), or aggressive NHL (defined as diffuse large B-cell lymphoma [DLBCL], grade 3B FL, mantle cell lymphoma, and transformed NHL with relapsed disease; n=5).
The patients’ median age was 68 (range, 34-93), and 59% were male. The median number of prior treatment regimens was 3 (range, 1-13), and 37% of patients had refractory disease.
Patients received cerdulatinib at 25, 30, or 35 mg twice daily (BID). A total of 101 patients were evaluable as of May 4, 2018.
Response
The objective response rate (ORR) was 47% in the entire population. Thirteen patients achieved a complete response (CR), and 34 had a partial response (PR). Thirty-four patients remained on cerdulatinib at the data cut-off.
The ORR was 46% in the FL patients, with 3 patients achieving a CR and 13 achieving a PR. Thirteen FL patients remained on cerdulatinib.
In the CLL/SLL patients, the ORR was 61%. Two patients had a CR, and 15 had a PR. Four CLL/SLL patients remained on cerdulatinib.
In PTCL, the ORR was 35%. All 7 responders had a CR. Eleven PTCL patients remained on cerdulatinib.
Only 1 CTCL patient was evaluable, but this patient achieved a CR and remained on cerdulatinib.
The ORR was 42% for patients with other indolent NHLs, with 5 PRs and no CRs. Only 1 patient in this group remained on cerdulatinib.
For aggressive NHL, the ORR was 20%, with 1 PR and no CRs. None of the patients in this group stayed on cerdulatinib.
“Cerdulatinib continues to demonstrate promising results across a wide range of B- and T-cell malignancies, including early indications of the potential for durable responses,” said study investigator Paul Hamlin, MD, of Memorial Sloan Kettering Cancer Center in New York, New York.
“The new signals in relapsed/refractory PTCL and CTCL are particularly compelling when you consider the limited treatment options for patients that fail frontline therapy.”
Safety
Grade 3 or higher adverse events included lipase increase (18%), neutropenia (17%), pneumonia/lung infection (11%), diarrhea (8%), fatigue (6%), amylase increase (5%), sepsis/septic shock (4%), hypertension (4%), anemia (4%), thrombocytopenia (4%), and hypophosphatemia (4%).
The 5 deaths due to sepsis or septic shock (3 of which were concomitant with pneumonia) were considered related to cerdulatinib. Three of the deaths occurred in patients with CLL, 1 in a DLBCL patient, and 1 in an FL patient.
“One of the things we have seen [with CLL patients] is the background infection rate is quite a bit higher,” said John T. Curnutte, MD, PhD, interim co-president and head of research and development at Portola Pharmaceuticals.
“You see this with multiple other agents, so we were not particularly surprised to see [sepsis/septic shock in CLL].”
Dr Curnutte noted that grade 5 sepsis/septic shock tended to occur in patients who were pre-colonized and/or had high plasma levels of cerdulatinib.
So, to prevent these adverse events, the starting dose of cerdulatinib was lowered, investigators began monitoring patients’ plasma levels, and all patients began receiving antibiotic prophylaxis. (Previously, only CLL patients had received this prophylaxis.)
The investigators found that lowering the starting dose from 35 mg BID to 30 mg or even 25 mg BID reduced plasma levels.
“At the 35 mg dose, 1 out of every 4 patients showed accumulation of the drug,” Dr Curnutte said. “In general, the use of the 30 mg BID or step-down 25 mg BID did not result in accumulation of drug.”
Dr Curnutte also noted that enrollment of CLL/SLL patients is complete, and investigators would be “very careful” if the study were to be re-opened to these patients.
For now, Portola is focused on completing enrollment in other patient groups on this phase 2 trial. The company is also hoping to conduct a phase 3 trial of cerdulatinib in PTCL that could begin as early as the end of this year.
EC grants blinatumomab full approval
The European Commission (EC) has granted a full marketing authorization for blinatumomab (BLINCYTO®) as a treatment for adults with Philadelphia chromosome-negative (Ph-), relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).
The EC granted blinatumomab conditional authorization for this indication in 2015. Now, the drug has full authorization based on overall survival (OS) data from the phase 3 TOWER study.
This authorization is valid in all European Union and European Economic Area-European Free Trade Association states (Norway, Iceland, and Liechtenstein).
Blinatumomab is a bispecific CD19-directed CD3 T cell engager (BiTE®) immunotherapy construct. It binds to CD19 expressed on the surface of cells of B-lineage origin and CD3 expressed on the surface of effector T cells.
The TOWER study was a phase 3, randomized trial in which researchers compared blinatumomab to standard of care (SOC) chemotherapy in 405 adults with Ph-, relapsed/refractory B-cell precursor ALL.
Patients were randomized in a 2:1 ratio to receive blinatumomab (n=271) or investigator’s choice of SOC chemotherapy (n=134).
On the recommendation of an independent data monitoring committee, Amgen ended the study early for evidence of superior efficacy in the blinatumomab arm.
The median OS was 7.7 months in the blinatumomab arm and 4 months in the SOC arm (hazard ratio=0.71; P=0.012).
For patients treated in first salvage, the median OS was 11.1 months in the blinatumomab arm and 5.3 months in the SOC arm (hazard ratio=0.6).
Safety results in the blinatumomab arm were comparable to those seen in previous phase 2 studies.
These results were published in NEJM in March 2017.
The European Commission (EC) has granted a full marketing authorization for blinatumomab (BLINCYTO®) as a treatment for adults with Philadelphia chromosome-negative (Ph-), relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).
The EC granted blinatumomab conditional authorization for this indication in 2015. Now, the drug has full authorization based on overall survival (OS) data from the phase 3 TOWER study.
This authorization is valid in all European Union and European Economic Area-European Free Trade Association states (Norway, Iceland, and Liechtenstein).
Blinatumomab is a bispecific CD19-directed CD3 T cell engager (BiTE®) immunotherapy construct. It binds to CD19 expressed on the surface of cells of B-lineage origin and CD3 expressed on the surface of effector T cells.
The TOWER study was a phase 3, randomized trial in which researchers compared blinatumomab to standard of care (SOC) chemotherapy in 405 adults with Ph-, relapsed/refractory B-cell precursor ALL.
Patients were randomized in a 2:1 ratio to receive blinatumomab (n=271) or investigator’s choice of SOC chemotherapy (n=134).
On the recommendation of an independent data monitoring committee, Amgen ended the study early for evidence of superior efficacy in the blinatumomab arm.
The median OS was 7.7 months in the blinatumomab arm and 4 months in the SOC arm (hazard ratio=0.71; P=0.012).
For patients treated in first salvage, the median OS was 11.1 months in the blinatumomab arm and 5.3 months in the SOC arm (hazard ratio=0.6).
Safety results in the blinatumomab arm were comparable to those seen in previous phase 2 studies.
These results were published in NEJM in March 2017.
The European Commission (EC) has granted a full marketing authorization for blinatumomab (BLINCYTO®) as a treatment for adults with Philadelphia chromosome-negative (Ph-), relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).
The EC granted blinatumomab conditional authorization for this indication in 2015. Now, the drug has full authorization based on overall survival (OS) data from the phase 3 TOWER study.
This authorization is valid in all European Union and European Economic Area-European Free Trade Association states (Norway, Iceland, and Liechtenstein).
Blinatumomab is a bispecific CD19-directed CD3 T cell engager (BiTE®) immunotherapy construct. It binds to CD19 expressed on the surface of cells of B-lineage origin and CD3 expressed on the surface of effector T cells.
The TOWER study was a phase 3, randomized trial in which researchers compared blinatumomab to standard of care (SOC) chemotherapy in 405 adults with Ph-, relapsed/refractory B-cell precursor ALL.
Patients were randomized in a 2:1 ratio to receive blinatumomab (n=271) or investigator’s choice of SOC chemotherapy (n=134).
On the recommendation of an independent data monitoring committee, Amgen ended the study early for evidence of superior efficacy in the blinatumomab arm.
The median OS was 7.7 months in the blinatumomab arm and 4 months in the SOC arm (hazard ratio=0.71; P=0.012).
For patients treated in first salvage, the median OS was 11.1 months in the blinatumomab arm and 5.3 months in the SOC arm (hazard ratio=0.6).
Safety results in the blinatumomab arm were comparable to those seen in previous phase 2 studies.
These results were published in NEJM in March 2017.
Growth on scalp
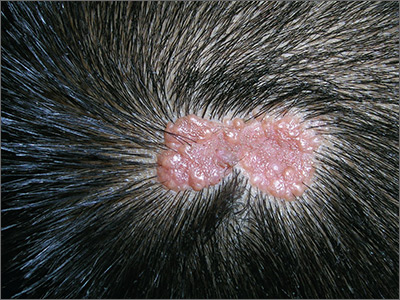
The family physician diagnosed a nevus sebaceous (NS) in this patient.
There are 3 stages of evolution paralleling the histologic differentiation of normal sebaceous glands:
- Infancy and young children. The lesion is smooth to slightly papillated, waxy, and hairless. (See Photo Rounds Friday, 6/15/18.)
- Puberty. Epidermal hyperplasia results in verrucous irregularity of the surface and coverage with numerous closely aggregated yellow-to-brown papules (this case).
- Development of secondary appendageal tumors. This occurs in 20% to 30% of patients. Most lesions are benign, but single (most commonly basal cell carcinoma) or multiple malignant tumors of both epidermal and adnexal origins may be seen. These malignancies are rarely seen in childhood.
In this case, a biopsy was not needed because the clinical picture was clear and no operative intervention was planned. When needed, a shave biopsy should provide adequate tissue for diagnosis because the pathology is epidermal and in the upper dermis. The NS need not be removed to prevent malignant transformation.
The FP explained that hair usually doesn’t grow where an NS is, and it was okay to proceed with observation only. He advised the patient’s father that if any changes were to occur, he would be happy to refer the child for surgical removal. The boy was not worried about the appearance of the NS and did not want to have surgery.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Epidermal nevus and nevus sebaceous. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine, 2nd ed. New York, NY: McGraw-Hill; 2013:958-962.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The family physician diagnosed a nevus sebaceous (NS) in this patient.
There are 3 stages of evolution paralleling the histologic differentiation of normal sebaceous glands:
- Infancy and young children. The lesion is smooth to slightly papillated, waxy, and hairless. (See Photo Rounds Friday, 6/15/18.)
- Puberty. Epidermal hyperplasia results in verrucous irregularity of the surface and coverage with numerous closely aggregated yellow-to-brown papules (this case).
- Development of secondary appendageal tumors. This occurs in 20% to 30% of patients. Most lesions are benign, but single (most commonly basal cell carcinoma) or multiple malignant tumors of both epidermal and adnexal origins may be seen. These malignancies are rarely seen in childhood.
In this case, a biopsy was not needed because the clinical picture was clear and no operative intervention was planned. When needed, a shave biopsy should provide adequate tissue for diagnosis because the pathology is epidermal and in the upper dermis. The NS need not be removed to prevent malignant transformation.
The FP explained that hair usually doesn’t grow where an NS is, and it was okay to proceed with observation only. He advised the patient’s father that if any changes were to occur, he would be happy to refer the child for surgical removal. The boy was not worried about the appearance of the NS and did not want to have surgery.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Epidermal nevus and nevus sebaceous. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine, 2nd ed. New York, NY: McGraw-Hill; 2013:958-962.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The family physician diagnosed a nevus sebaceous (NS) in this patient.
There are 3 stages of evolution paralleling the histologic differentiation of normal sebaceous glands:
- Infancy and young children. The lesion is smooth to slightly papillated, waxy, and hairless. (See Photo Rounds Friday, 6/15/18.)
- Puberty. Epidermal hyperplasia results in verrucous irregularity of the surface and coverage with numerous closely aggregated yellow-to-brown papules (this case).
- Development of secondary appendageal tumors. This occurs in 20% to 30% of patients. Most lesions are benign, but single (most commonly basal cell carcinoma) or multiple malignant tumors of both epidermal and adnexal origins may be seen. These malignancies are rarely seen in childhood.
In this case, a biopsy was not needed because the clinical picture was clear and no operative intervention was planned. When needed, a shave biopsy should provide adequate tissue for diagnosis because the pathology is epidermal and in the upper dermis. The NS need not be removed to prevent malignant transformation.
The FP explained that hair usually doesn’t grow where an NS is, and it was okay to proceed with observation only. He advised the patient’s father that if any changes were to occur, he would be happy to refer the child for surgical removal. The boy was not worried about the appearance of the NS and did not want to have surgery.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Epidermal nevus and nevus sebaceous. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine, 2nd ed. New York, NY: McGraw-Hill; 2013:958-962.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
The impact of inpatient rehabilitation on outcomes for patients with cancer
The American Cancer Society reports that 1.6 million people are diagnosed with cancer each year, of whom 78% are aged 55 years or older. The 5-year survival rate for cancer is 68%.1 Almost 15.5 million living Americans have been diagnosed with cancer.2 Many patients with cancer have difficulty walking and with activities of daily living. Patients with primary brain tumors or tumors metastatic to the brain may present with focal weakness or cognitive deficits similar to patients with stroke. Patients with tumors metastatic to the spine may have the same deficits as a patient with a traumatic spinal cord injury. Patients with metastasis to bone may have pathologic fractures of the hip or long bones. Patients may develop peripheral neuropathy associated with a paraneoplastic syndrome, chemotherapy, or critical illness neuropathy. Lehmann and colleagues evaluated 805 patients admitted to hospitals affiliated with the University of Washington Medical School with a diagnosis of cancer and found that 15% had difficulty walking and 20% had difficulty with activities of daily living.3
Many patients with cancer can benefit from inpatient rehabilitation.4,5 Study findings have shown that patients with impairments in function related to cancer are often not referred for rehabilitation. Among the reasons mentioned for that are that oncologists are more focused on treating the patients’ cancer than on their functional deficits and that specialists in rehabilitation medicine do not want to be involved with patients with complex medical problems. Rehabilitation facilities may not want to incur the costs associated with caring for patients with cancer.6
The present paper looks at the outcomes of 61 consecutive patients with cancer who were admitted to an inpatient rehabilitation facility (IRF) and received radiation therapy concurrent with rehabilitation. It compares the outcomes of the cancer patients with the outcomes of patients without cancer who were admitted with stroke or spinal cord injury, conditions more commonly treated at an IRF.
Methods
We reviewed electronic medical records of all patients with cancer admitted to the IRF from 2008 through 2013 who received radiation therapy while at the facility. We also reviewed the data of all patients without cancer admitted with a diagnosis of stroke in 2013 and all patients admitted with a diagnosis of traumatic spinal cord injury in 2012 and 2013. No patients were excluded from stroke and traumatic spinal cord injury groups.
We recorded the sex, age, diagnostic group, Functional Independence Measure (FIM) admission score, FIM discharge score, length of stay (LoS) in the IRF, place of discharge of each patient (eg, home, acute care, or subacute care), and calculated the FIM efficiency score (change in FIM/LoS) for each patient. The FIM is an instrument that has 18 items measuring mobility, participation in activities of daily living, ability to communicate, and cognitive function.7 Each item is scored from 1 to 7, with 1 denoting that the patient cannot perform the task and 7 that the activity can be performed independently. The minimum score is 18 (complete dependence), and the maximum score is 126 (independent function). Thirteen items compose the motor FIM score: eating, grooming, bathing, dressing upper body, dressing lower body, toileting, bladder management, management of bowel, transfer to bed or wheelchair, transfer to toilet, tub transfer, walking (or wheelchair use), and climbing stairs. Five items – comprehension, expression, social interaction, problem solving, and memory – compose the cognitive FIM score.
We used a 1-way analysis of variance to evaluate differences between age and cancer type, age and diagnostic group, admission FIM score and cancer type, discharge FIM score and cancer type, change in FIM and cancer type, LoS and cancer type, and LoS and diagnostic group. The Pearson chi-square test was used to test the goodness of fit between the place of disposition and diagnostic group. The paired t test was used to evaluate the improvement in FIM of the patients who were in the cancer groups. The Tukey Simultaneous Tests for Differences of Means was used to compare the FIM efficiency scores of the groups. A 2-sample t test was used to evaluate the factors associated with the need for transfer from the IRF to the acute medical service.
Results
The demographic characteristics of the patients in the study and the admission and discharge FIM scores are reported in Table 1. There were initially 62 cancer patients in the radiation group, which was further divided into 4 subgroups based on the site of the primary tumor or metastasis. In all, 23 had a primary malignant brain tumor and received radiation and temozolomide. Sixteen patients had malignancies metastatic to the brain, 15 patients had tumors metastatic to the spine, and 7 had tumors metastatic to the long bones. One patient had laryngeal cancer and was excluded from the study because we did not think that we could do an analysis of a group with only 1 patient. The final number of patients in the cancer group was therefore 61. There were 69 patients in the stroke group and 23 in the spinal cord injury group.
We report improvement in total FIM, motor FIM, and cognitive FIM scores and were able to identify all 18 of the items of the FIM score on 60 of the 61 patients in the cancer group. Improvement in total FIM of the 61 patients in the cancer groups was significant at P P P = .05. Just over 75% of the patients in the cancer group had sufficient enough improvement in their level of function that they were able to return to their homes (Table 1). The average FIM score at the time of discharge was 83.08. This was not significantly different than the level of function of patients discharged after stroke (87.52) or traumatic spinal cord injury (89.13).
The patients with primary brain tumors were younger than the patients with cancer metastatic to the brain (P = .013). The patients with a primary brain tumor had lower admission FIM scores than patients with tumors metastatic to the brain (P = .027). The patients with a primary brain tumor had a greater increase in FIM score than patients with metastasis to the brain (P = .043; Table 2). There was not a significant difference between these 2 groups in FIM score at discharge or in the likelihood of discharge to home (Table 1). The FIM efficiency score was 1.12 for the patients in the primary brain tumor group and .80 in those with metastasis to the brain. This difference was not significant P = .96.
There were 69 patients in the stroke group. We compared the 39 patients with primary or metastatic brain lesion to the stroke group. The patients with primary or metastatic cancer of the brain were younger than the patients with stroke, 60.4 years old versus 69.1 years old (P = .004). The patients in the combined cancer group had a higher admission FIM score compared with the stroke patients (68.4 vs 63.12; P = .05). The discharge FIM scores were 83.3 in the combined cancer group and 87.5 in the stroke group (Table 1). This difference was not significant, but the improvement in the combined cancer group (14.6) was less than the improvement in the stroke group (24.40; P = .002) (Table 3).
The average LoS in the IRF was 18.7 days in the combined cancer group and 16.8 days in the stroke group. This difference was not significant. An average of 82% of the patients in the primary tumor or brain metastasis group and 85.5% of the patients in the stroke group were discharged to home. This difference was not significant. The FIM efficiency score of the patients in the stroke group was 2.0. This was significantly greater than the score for the patients in the metastasis to the brain group (0.80; P = .044) but not significantly greater than the primary brain cancer group (1.19; P = .22).
There were 23 patients in the traumatic spinal cord injury group. A comparison of the patients with tumors metastatic to the spine and patients with traumatic spinal cord injury showed that the patients in the cancer group were older (60.27 and 42.70 years, respectively; P = .001). In all, 80% of patients with tumors metastatic to the spine were men. This was not significantly different from the percentage of men in the traumatic spinal cord injury group (82.6%; Table 1). The admission FIM score of the patients with cancer was 66.5 (standard deviation [SD], 13.3) and 58.03 (SD, 15.1) in the patients with a traumatic spinal cord injury (Table 1). The FIM score at discharge was 80.4 (SD, 19.1) in the patients with cancer and 89.1 (SD, 20.3) in the patients with a traumatic spinal cord injury (Table 1). Neither of these were statistically significant. The improvement in patients with cancer was 13.9 (SD, 12.2) and 31.1 (SD, 13.9) in the traumatic spinal cord injured patients. This difference was significant (P
The median LoS was 18.98 days in the cancer metastasis to spine group (interquartile range [IQR] is the 25th-75th percentile, 12-30 days). In the traumatic group the median LoS was 23 days (IQR, 16-50 days). This difference was not significant (P = .14 Mann-Whitney test). The mean FIM efficiency score was 1.46 in the traumatic spinal cord injury group and .78 in the group with cancer metastatic to the spine. This difference was not significant (P = .72). Sixty percent of the patients in the cancer group were discharged to home, and 87% of patients in the traumatic spinal cord group were discharged to home. This difference was not significant (P = .12; Fisher exact test).
As far as we can ascertain, this is the first paper that has looked at the outcomes of patients receiving rehabilitation concurrent with radiation of the long bones. The average improvement in FIM was 12.4 (Table 1). The LoS was 11.6 days, and the FIM efficiency was 1.25. In all, 71.4% made enough progress to go home.
Of the total number of cancer patients, 18% were transferred to the acute medical service of the hospital (Table 1). Neither age, sex, type of cancer, nor admission FIM score were associated with the need for transfer to acute hospital care. Change in FIM score was inversely associated with transfer to acute hospital care (P = .027). Patients whose function did not improve with rehabilitation were most likely to be transferred back to acute hospital care.
Discussion
Radiation therapy is considered a service that is provided to people who come for treatment as an outpatient. Caregivers may have difficulty transporting patients to radiation if the patient has deficits in mobility. This may be particularly true if the patient is heavy, the caregivers are frail, or perhaps if they live in rural settings where there is no wheelchair-accessible public transportation. There are many factors that help determine whether a patient with functional deficits can be discharged to his or her home. These include sex, age, marital status, family and/or community support, income, and insurance.8 The FIM is an instrument that indicates how much help a patient needs with mobility and self-care skills. It also correlates with the amount of time that caregivers must spend helping a patient.9 Study findings have shown that the FIM score is an important determinant of whether a patient can be discharged to home. The total FIM score is as useful as an analysis of the components of the FIM score in predicting whether a patient can return to the community.10,11 Reistetter and colleagues found a total FIM score of 78 to be the score that best separates patients who are likely to be able to go home and patients who are likely to need long-term care.11 Bottemiller and colleagues10 reported that 37% of patients with total discharge FIM scores of less than 40 were discharged to home. They reported that 62% of patients with FIM scores between 40 and 79 were discharged to home, and 88% of patients with scores of 80 or above were discharged to home.10 The goal in bringing patients to the IRF was to accept and treat patients with reasonable community support and potential to achieve a functional level compatible with discharge to the community. Most patients in each of the cancer groups were able to reach an FIM score of 78 to 80 and to be discharged to home.
Most of the patients in the cancer groups had underlying problems that are not considered curable. The primary goal was to enable the patients to have some time at home with their families before requiring readmission to a hospital or hospice care. Reasonable LoS and rate of progress are now expected or required by third-party payors and hospital administrators. Physicians at the Mayo Clinic have indicated that a rehabilitation service should aim for an FIM efficiency score of at least .6 points per day.10 The FIM efficiency of patients in each of the 4 cancer subgroups in this study was higher than this level.
J. Herbert Dietz, Jr was an early advocate of the need to provide comprehensive rehabilitation services for patients with cancer. He first described his work in 1969.12 Since that time, there have been many papers that have documented the benefits of IRF for patients with cancer. O’Toole and Golden have shown outcomes of a large series of patients from an IRF. They reported that at the time of admission, 14% of patients could ambulate, but at discharge, 80% could ambulate without hands-on assistance. They reported significant improvements in continence, FIM score, and score on the Karnofsky Performance Scale.13 Marciniak,14 Hunter,15 Shin,16 and Cole,17 and their respective colleagues have all shown that patients with many different types of cancer benefit from rehabilitation at the IRF level. Gallegos-Kearin and colleagues4 reported on the care of 115,570 patients admitted to IRF with cancer from 2002 to 2014. Patients had significant improvement in function, with more than 70% of patients discharged to home.4 Ng and colleagues studied a group of 200 patients who received IRF care and found there was significant improvement in function. Ninety-four percent of patients rated their stay as either extremely good or very good.5
Metastasis to the spine is a common problem. It is found in 30% of cancer patients at autopsy. The most common sources of metastasis to the spine are breast, lung, prostate, kidney, and thyroid.18 Multiple myeloma and lymphoma may also involve the spine. Several authors have shown that these patients benefit from inpatient rehabilitation. Mckinley and colleagues19 have noted that patients with metastasis to the spine make significant improvement with care at an IRF. Compared with patients with a traumatic spinal cord injury, the cancer patients had shorter LoS, smaller improvement in FIM, equal FIM efficiency (FIM gain/LoS), and equal success in making enough progress to be discharged to home.19 Eriks and colleagues showed that patients at an IRF in Amsterdam made significant improvement in function as measured by the Barthel’s Index.20 Tang .,21 and Parsch22 and their respective colleagues, Murray,23 and New24 and colleagues have published findings confirming that patients with spinal cord injury caused by metastasis to the spine make significant progress with inpatient rehabilitation programs. The present study adds to the literature by showing that patients with metastasis to the spine who are receiving radiation can make progress and be discharged to the community.
There are 24,000 new cases of primary malignant brain tumors in the United States each year.25 The incidence of metastatic cancer to the brain has been estimated to be 100,000 cases per year in the United States. The most common cancer sources are lung, breast, melanoma, kidney, and colon.26,27 The first study of patients admitted to an IRF for treatment of brain tumors was published in 1998 by Huang and colleagues28 who compared the outcomes of 63 patients with brain tumors with the outcomes of 63 patients with stroke. They reported that the patients with the brain tumors made significant improvement in function. There was not a significant difference between the 2 groups of patients in improvement in function, FIM efficiency, or success in discharging the patients to home.28 Greenberg29 and Bartolo30 and their respective colleagues compared the outcomes of patients admitted with brain tumors and patients with stroke and found that improvement in function and discharge to home was similar in the 2 groups. In 2000, Huang and his same colleagues31 compared a group of patients with brain tumors to a group of patients with traumatic brain injury. They found significant improvement in the function of the patients with brain tumors. Patients in the traumatic brain injury group made more progress but had longer LoS. FIM efficiency was not significantly different between the groups.31
Three papers have reported outcomes of patients who received radiation concurrent with inpatient rehabilitation. Tang and colleagues32 reported 63 patients, of whom 48% percent received radiation concurrent with rehabilitation. The patients who received radiation made significant gains in function, and more than 70% were discharged to home. There was no difference in the outcomes of the patients in the radiation and nonradiation groups.32 Marciniak33 and O’Dell34 and their colleagues also reported that patients with brain tumors that required radiation therapy can benefit from inpatient rehabilitation. The present paper is the fourth (with the largest patient group) to show that patients with primary and metastatic tumors to the brain can benefit from a program that provides radiation concurrent with inpatient rehabilitation. We have shown that patients can achieve functional levels and rates of discharge to home that are not significantly different from those of the most commonly admitted group of patients to IRF – patients with stroke.
In the present study, 18% of all of the cancer patients were transferred to medical services and/or acute hospital care (Table 1). This is consistent with a paper by Asher and colleagues35 who reported that 17.4% of patients at an IRF with a diagnosis of cancer required transfer back to medical service, and that low admission motor FIM score correlated with the likelihood of transfer back to medical service. In the present paper, the total admission FIM score was not related to the likelihood of return to medical service, although a lack of improvement in the FIM score did correlate with transfer to medical service.
All of the papers we reviewed found that appropriately selected patients with cancer make significant improvement in function with treatment at an IRF. Tang and colleagues have also shown that for patients with malignant brain tumors and metastasis to the spine, improvement in function correlates with increased survival.32 Our paper confirms that patients with primary malignant brain tumors, malignant tumors metastatic to the brain or spine, and tumors metastatic to long bones may benefit from rehabilitation concurrent with radiation. Rehabilitation units are traditionally associated with treating patients with stroke and spinal cord injury. The patients in our study had cancer and were receiving radiation therapy. They had significant improvement in function and FIM efficiency scores that are not below the threshold set as expected for care at an IRF. Most patients in our study achieved a functional level consistent with what is needed to go home.
There is a prospective payment or reimbursement system for rehabilitation units.36 The payments are based on the admitting diagnosis, the admission FIM score, the age of the patient, and comorbidities. There are 4 tiers for comorbidities with no additional payments for patients in tier 0 but with additional payments for patients with conditions that qualify for tiers 1 through 3. The highest payments are for patients in tier 1. Examples of conditions that can increase payment include morbid obesity, congestive heart failure, vocal cord paralysis, and the need for hemodialysis. There is no increased payment for provision of radiation therapy. There are no reports on the feasibility, in terms of finances, of providing radiation on an IRF. We asked the finance office of the Albany Medical Center to comment on the cost to the hospital of providing radiation therapy to patients on the rehabilitation unit. The hospital’s finance department reviewed available data and reported that the variable cost of providing radiation therapy is about 6.5% of the revenue collected from third-party payors for caring for patients who receive that service (personal communication from the finance office of Albany Medical Center to George Forrest, 2015). Our findings suggest that the Centers for Medicare & Medicaid Services should make an adjustment to the payment system to support the cost of providing radiation to patients at an IRF. Even under the current payment system, for a hospital that has the equipment and personnel to provide radiation treatments, the variable cost of 6.5% of revenue should not be an absolute barrier to providing this service.
Limitations
This study reports on the experience of only 1 facility. The number of patients in the radiation group is greater than the number of patients in any previous report of people receiving radiation at an IRF, but the statistician does not think it is large enough to allow statistical analysis of covariates such as age, sex, and comorbid conditions. In addition, we did not investigate all of the factors that influence the type of care patients are offered and their LoS, such as hospital policy, insurance coverage, income, and family structure.
Conclusions
Acute care medical units are now challenged to both reduce LoS and reduce the number of patients who are readmitted to the hospital. Rehabilitation units are challenged to maintain census, as government and private payors are shifting patients from acute rehabilitation units to subacute rehabilitation units. We found that patients with cancer who need radiation are a population of patients who are seen by payors as needing to be in a facility with excellent nursing, therapy, and comprehensive physician services. A comprehensive cancer care program within a rehabilitation unit can be a great benefit to the acute care services, the IRF, and, most importantly, patients and their families.
1. American Cancer Society. Cancer facts & figures 2016. Atlanta, GA: American Cancer Society; 2016.
2. National Cancer Institute: Office of cancer survivorship: statistics. https://cancercontrol.cancer.gov/ocs/statistics/statistics.html. Updated October 17, 2016. Accessed April 21, 2018.
3. Lehmann JF, DeLisa JA, Warren CG, deLateur BJ, Bryant PL, Nicholson CG. Cancer rehabilitation: assessment of need, development and evaluation of a model of care. Arch Phys Med Rehabil. 1978;59(9):410-419.
4. Gallegos-Kearin V, Knowlton SE, Goldstein R, et al. Outcome trends of adult cancer patients receiving inpatient rehabilitation: a 13-year review [published online Feb 21, 2018]. Am J Phys Med Rehabil. doi:10.1097/PHM.0000000000000911
5. Ng AH, Gupta E, Fontillas RC, et al. Patient-reported usefulness of acute cancer rehabilitation. PM R. 2017;9(11):1135-1143.
6. Cheville AL, Kornblith AB, Basford JR. An examination of the causes for the underutilization of rehabilitation services among people with advanced cancer. Am J Phys Med Rehabil. 2011;90(5 suppl 1):S27-S37.
7. Cohen ME, Marino RJ. The tools of disability outcomes research functional status measures. Arch Phys Med Rehabil. 2000;81(12 suppl 2):S21-S29.
8. Nguyen VQ, PrvuBettger J, Guerrier T, et al. Factors associated with discharge to home versus discharge to institutional care after inpatient stroke rehabilitation. Arch Phys Med Rehabil. 2015;96(7):1297-1303.
9. Forrest G, Schwam A, Cohen E. Time of care required by patients discharged from a rehabilitation unit. Am J Phys Med Rehabil. 2002;81(1):57-62.
10. Bottemiller KL, Bieber PL, Basford JR, Harris M. FIM scores, FIM efficiency and discharge following inpatient stroke rehabilitation. Rehabil Nurs. 2006;31(1):22-25.
11. Reistetter TA, Graham JE, Deutsch A, Granger CV, Markello S, Ottenbacher KJ. Utility of functional status for classifying community versus institutional discharges after inpatient rehabilitation for stroke. Arch Phys Med Rehabil. 2010;91(3):345-350.
12. Dietz JH Jr. Rehabilitation of the cancer patient. Med Clin North Am. 1969;53(3):607-624.
13. O'Toole DM, Golden AM. Evaluating cancer patients for rehabilitation potential. West J Med. 1991;155(4):384-387.
14. Marciniak CM, Sliwa JA, Spill G, Heinemann AW, Semik PE. Functional outcome following rehabilitation of the cancer patient. Arch Phys Med Rehabil. 1996;77(1):54-57.
15. Hunter EG, Baltisberger J. Functional outcomes by age for inpatient cancer rehabilitation: a retrospective chart review. J Appl Gerontol. 2013;32(4):443-456.
16. Shin KY, Guo Y, Konzen B, Fu J, Yadav R, Bruera E. Inpatient cancer rehabilitation: the experience of a national comprehensive cancer center. Am J Phys Med Rehabil. 2011;90(5 suppl 1):S63-S68.
17. Cole RP, Scialla S, Bednarz L. Functional recovery in cancer rehabilitation. Arch Phys Med Rehabil. 2000;81(5):623-627.
18. White AP, Kwon BK, Lindskog DM, Friedlaender GE, Grauer JN. Metastatic disease of the spine. J Am Acad Orthop Surg. 2006;14(11):587-598.
19. McKinley WO, Huang ME, Tewksbury MA. Neoplastic vs traumatic spinal cord injury: an inpatient rehabilitation comparison. Am J Phys Med Rehabil. 2000;79(2):138-144.
20. Eriks IE, Angenot EL, Lankhorst GJ. Epidural metastatic spinal cord compression: functional outcome and survival after inpatient rehabilitation. Spinal Cord. 2004;42(4):235-239.
21. Tang V, Harvey D, Park Dorsay J, Jiang S, Rathbone MP. Prognostic indicators in metastatic spinal cord compression: using functional independence measure and Tokuhashi scale to optimize rehabilitation planning. Spinal Cord. 2007;45(10):671-677.
22. Parsch D, Mikut R, Abel R. Postacute management of patients with spinal cord injury due to metastatic tumor disease: survival and efficacy of rehabilitation. Spinal Cord. 2003;41:205-210.
23. Murray PK. Functional outcome and survival in spinal cord injury secondary to neoplasia. Cancer. 1985;55:197-201.
24. New PW. Functional outcomes and disability after nontraumatic spinal cord injury rehabilitation: results from a retrospective study. Arch Phys Med Rehabil. 2005;86(2):250-261
25. Central Brain Tumor Registry of the United States: 2016 CBTRUS fact sheet. www.cbtrus.org/factsheet/factsheet.html. Updated 2017. Accessed May 28, 2016.
26. Memorial Sloan Kettering Cancer Center: Metastatic brain tumors & secondary brain cancer. https://www.mskcc.org/cancer-care/types/brain-tumors-metastatic. Updated 2018. Accessed April 21, 2018.
27. Bruckner JC, Brown PD, O'Neill BP, Meyer FB, Wetmore CJ, Uhm JH. Central nervous system tumors. Mayo Clin Proc. 2007;82(10):1271-1286.
28. Huang ME, Cifu DX, Keyser-Marcus L. Functional outcome after brain tumor and acute stroke: a comparative analysis. Arch Phys Med Rehabil. 1998;79(11):1386-1390.
29. Greenberg E, Treger I, Ring H. Rehabilitation outcomes in patients with brain tumors and acute stroke: comparative study of inpatient rehabilitation. Am J Phys Med Rehabil. 2006;85(7):568-573.
30. Bartolo M, Zucchella C, Pace A, et al. Early rehabilitation after surgery improves functional outcomes in inpatients with brain tumours. J Neurooncol. 2012;107(3);537-544.
31. Huang ME, Cifu DX, Keyser-Marcus L. Functional outcomes in patients with brain tumor after inpatient rehabilitation: comparison with traumatic brain injury. Am J Phys Med Rehabil. 2000;79(4):327-335.
32. Tang V, Rathbone M, Park Dorsay J, Jiang S, Harvey D. Rehabilitation in primary and metastatic brain tumours: impact of functional outcomes on survival. J Neurol. 2008;255(6):820-827.
33. Marciniak CM, Sliwa JA, Heinemann AW, Semik PE. Functional outcomes of persons with brain tumors after inpatient rehabilitation. Arch Phys Med Rehabil. 2001;82(4):457-463.
34. O'Dell MW, Barr K, Spanier D, Warnick RE. Functional outcome of inpatient rehabilitation in persons with brain tumors. Arch Phys Med Rehabil. 1998;79(12):1530-1534.
35. Asher A, Roberts PS, Bresee C, Zabel G, Riggs RV, Rogatko A. Transferring inpatient rehabilitation facility cancer patients back to acute care (TRIPBAC). PM R. 2014;6(9):808-813.
36. Centers for Medicare and Medicaid Services: Inpatient rehabilitation facilities. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/InpatientRehab.html. Published March 5, 2012. Accessed May 21, 2018.
The American Cancer Society reports that 1.6 million people are diagnosed with cancer each year, of whom 78% are aged 55 years or older. The 5-year survival rate for cancer is 68%.1 Almost 15.5 million living Americans have been diagnosed with cancer.2 Many patients with cancer have difficulty walking and with activities of daily living. Patients with primary brain tumors or tumors metastatic to the brain may present with focal weakness or cognitive deficits similar to patients with stroke. Patients with tumors metastatic to the spine may have the same deficits as a patient with a traumatic spinal cord injury. Patients with metastasis to bone may have pathologic fractures of the hip or long bones. Patients may develop peripheral neuropathy associated with a paraneoplastic syndrome, chemotherapy, or critical illness neuropathy. Lehmann and colleagues evaluated 805 patients admitted to hospitals affiliated with the University of Washington Medical School with a diagnosis of cancer and found that 15% had difficulty walking and 20% had difficulty with activities of daily living.3
Many patients with cancer can benefit from inpatient rehabilitation.4,5 Study findings have shown that patients with impairments in function related to cancer are often not referred for rehabilitation. Among the reasons mentioned for that are that oncologists are more focused on treating the patients’ cancer than on their functional deficits and that specialists in rehabilitation medicine do not want to be involved with patients with complex medical problems. Rehabilitation facilities may not want to incur the costs associated with caring for patients with cancer.6
The present paper looks at the outcomes of 61 consecutive patients with cancer who were admitted to an inpatient rehabilitation facility (IRF) and received radiation therapy concurrent with rehabilitation. It compares the outcomes of the cancer patients with the outcomes of patients without cancer who were admitted with stroke or spinal cord injury, conditions more commonly treated at an IRF.
Methods
We reviewed electronic medical records of all patients with cancer admitted to the IRF from 2008 through 2013 who received radiation therapy while at the facility. We also reviewed the data of all patients without cancer admitted with a diagnosis of stroke in 2013 and all patients admitted with a diagnosis of traumatic spinal cord injury in 2012 and 2013. No patients were excluded from stroke and traumatic spinal cord injury groups.
We recorded the sex, age, diagnostic group, Functional Independence Measure (FIM) admission score, FIM discharge score, length of stay (LoS) in the IRF, place of discharge of each patient (eg, home, acute care, or subacute care), and calculated the FIM efficiency score (change in FIM/LoS) for each patient. The FIM is an instrument that has 18 items measuring mobility, participation in activities of daily living, ability to communicate, and cognitive function.7 Each item is scored from 1 to 7, with 1 denoting that the patient cannot perform the task and 7 that the activity can be performed independently. The minimum score is 18 (complete dependence), and the maximum score is 126 (independent function). Thirteen items compose the motor FIM score: eating, grooming, bathing, dressing upper body, dressing lower body, toileting, bladder management, management of bowel, transfer to bed or wheelchair, transfer to toilet, tub transfer, walking (or wheelchair use), and climbing stairs. Five items – comprehension, expression, social interaction, problem solving, and memory – compose the cognitive FIM score.
We used a 1-way analysis of variance to evaluate differences between age and cancer type, age and diagnostic group, admission FIM score and cancer type, discharge FIM score and cancer type, change in FIM and cancer type, LoS and cancer type, and LoS and diagnostic group. The Pearson chi-square test was used to test the goodness of fit between the place of disposition and diagnostic group. The paired t test was used to evaluate the improvement in FIM of the patients who were in the cancer groups. The Tukey Simultaneous Tests for Differences of Means was used to compare the FIM efficiency scores of the groups. A 2-sample t test was used to evaluate the factors associated with the need for transfer from the IRF to the acute medical service.
Results
The demographic characteristics of the patients in the study and the admission and discharge FIM scores are reported in Table 1. There were initially 62 cancer patients in the radiation group, which was further divided into 4 subgroups based on the site of the primary tumor or metastasis. In all, 23 had a primary malignant brain tumor and received radiation and temozolomide. Sixteen patients had malignancies metastatic to the brain, 15 patients had tumors metastatic to the spine, and 7 had tumors metastatic to the long bones. One patient had laryngeal cancer and was excluded from the study because we did not think that we could do an analysis of a group with only 1 patient. The final number of patients in the cancer group was therefore 61. There were 69 patients in the stroke group and 23 in the spinal cord injury group.
We report improvement in total FIM, motor FIM, and cognitive FIM scores and were able to identify all 18 of the items of the FIM score on 60 of the 61 patients in the cancer group. Improvement in total FIM of the 61 patients in the cancer groups was significant at P P P = .05. Just over 75% of the patients in the cancer group had sufficient enough improvement in their level of function that they were able to return to their homes (Table 1). The average FIM score at the time of discharge was 83.08. This was not significantly different than the level of function of patients discharged after stroke (87.52) or traumatic spinal cord injury (89.13).
The patients with primary brain tumors were younger than the patients with cancer metastatic to the brain (P = .013). The patients with a primary brain tumor had lower admission FIM scores than patients with tumors metastatic to the brain (P = .027). The patients with a primary brain tumor had a greater increase in FIM score than patients with metastasis to the brain (P = .043; Table 2). There was not a significant difference between these 2 groups in FIM score at discharge or in the likelihood of discharge to home (Table 1). The FIM efficiency score was 1.12 for the patients in the primary brain tumor group and .80 in those with metastasis to the brain. This difference was not significant P = .96.
There were 69 patients in the stroke group. We compared the 39 patients with primary or metastatic brain lesion to the stroke group. The patients with primary or metastatic cancer of the brain were younger than the patients with stroke, 60.4 years old versus 69.1 years old (P = .004). The patients in the combined cancer group had a higher admission FIM score compared with the stroke patients (68.4 vs 63.12; P = .05). The discharge FIM scores were 83.3 in the combined cancer group and 87.5 in the stroke group (Table 1). This difference was not significant, but the improvement in the combined cancer group (14.6) was less than the improvement in the stroke group (24.40; P = .002) (Table 3).
The average LoS in the IRF was 18.7 days in the combined cancer group and 16.8 days in the stroke group. This difference was not significant. An average of 82% of the patients in the primary tumor or brain metastasis group and 85.5% of the patients in the stroke group were discharged to home. This difference was not significant. The FIM efficiency score of the patients in the stroke group was 2.0. This was significantly greater than the score for the patients in the metastasis to the brain group (0.80; P = .044) but not significantly greater than the primary brain cancer group (1.19; P = .22).
There were 23 patients in the traumatic spinal cord injury group. A comparison of the patients with tumors metastatic to the spine and patients with traumatic spinal cord injury showed that the patients in the cancer group were older (60.27 and 42.70 years, respectively; P = .001). In all, 80% of patients with tumors metastatic to the spine were men. This was not significantly different from the percentage of men in the traumatic spinal cord injury group (82.6%; Table 1). The admission FIM score of the patients with cancer was 66.5 (standard deviation [SD], 13.3) and 58.03 (SD, 15.1) in the patients with a traumatic spinal cord injury (Table 1). The FIM score at discharge was 80.4 (SD, 19.1) in the patients with cancer and 89.1 (SD, 20.3) in the patients with a traumatic spinal cord injury (Table 1). Neither of these were statistically significant. The improvement in patients with cancer was 13.9 (SD, 12.2) and 31.1 (SD, 13.9) in the traumatic spinal cord injured patients. This difference was significant (P
The median LoS was 18.98 days in the cancer metastasis to spine group (interquartile range [IQR] is the 25th-75th percentile, 12-30 days). In the traumatic group the median LoS was 23 days (IQR, 16-50 days). This difference was not significant (P = .14 Mann-Whitney test). The mean FIM efficiency score was 1.46 in the traumatic spinal cord injury group and .78 in the group with cancer metastatic to the spine. This difference was not significant (P = .72). Sixty percent of the patients in the cancer group were discharged to home, and 87% of patients in the traumatic spinal cord group were discharged to home. This difference was not significant (P = .12; Fisher exact test).
As far as we can ascertain, this is the first paper that has looked at the outcomes of patients receiving rehabilitation concurrent with radiation of the long bones. The average improvement in FIM was 12.4 (Table 1). The LoS was 11.6 days, and the FIM efficiency was 1.25. In all, 71.4% made enough progress to go home.
Of the total number of cancer patients, 18% were transferred to the acute medical service of the hospital (Table 1). Neither age, sex, type of cancer, nor admission FIM score were associated with the need for transfer to acute hospital care. Change in FIM score was inversely associated with transfer to acute hospital care (P = .027). Patients whose function did not improve with rehabilitation were most likely to be transferred back to acute hospital care.
Discussion
Radiation therapy is considered a service that is provided to people who come for treatment as an outpatient. Caregivers may have difficulty transporting patients to radiation if the patient has deficits in mobility. This may be particularly true if the patient is heavy, the caregivers are frail, or perhaps if they live in rural settings where there is no wheelchair-accessible public transportation. There are many factors that help determine whether a patient with functional deficits can be discharged to his or her home. These include sex, age, marital status, family and/or community support, income, and insurance.8 The FIM is an instrument that indicates how much help a patient needs with mobility and self-care skills. It also correlates with the amount of time that caregivers must spend helping a patient.9 Study findings have shown that the FIM score is an important determinant of whether a patient can be discharged to home. The total FIM score is as useful as an analysis of the components of the FIM score in predicting whether a patient can return to the community.10,11 Reistetter and colleagues found a total FIM score of 78 to be the score that best separates patients who are likely to be able to go home and patients who are likely to need long-term care.11 Bottemiller and colleagues10 reported that 37% of patients with total discharge FIM scores of less than 40 were discharged to home. They reported that 62% of patients with FIM scores between 40 and 79 were discharged to home, and 88% of patients with scores of 80 or above were discharged to home.10 The goal in bringing patients to the IRF was to accept and treat patients with reasonable community support and potential to achieve a functional level compatible with discharge to the community. Most patients in each of the cancer groups were able to reach an FIM score of 78 to 80 and to be discharged to home.
Most of the patients in the cancer groups had underlying problems that are not considered curable. The primary goal was to enable the patients to have some time at home with their families before requiring readmission to a hospital or hospice care. Reasonable LoS and rate of progress are now expected or required by third-party payors and hospital administrators. Physicians at the Mayo Clinic have indicated that a rehabilitation service should aim for an FIM efficiency score of at least .6 points per day.10 The FIM efficiency of patients in each of the 4 cancer subgroups in this study was higher than this level.
J. Herbert Dietz, Jr was an early advocate of the need to provide comprehensive rehabilitation services for patients with cancer. He first described his work in 1969.12 Since that time, there have been many papers that have documented the benefits of IRF for patients with cancer. O’Toole and Golden have shown outcomes of a large series of patients from an IRF. They reported that at the time of admission, 14% of patients could ambulate, but at discharge, 80% could ambulate without hands-on assistance. They reported significant improvements in continence, FIM score, and score on the Karnofsky Performance Scale.13 Marciniak,14 Hunter,15 Shin,16 and Cole,17 and their respective colleagues have all shown that patients with many different types of cancer benefit from rehabilitation at the IRF level. Gallegos-Kearin and colleagues4 reported on the care of 115,570 patients admitted to IRF with cancer from 2002 to 2014. Patients had significant improvement in function, with more than 70% of patients discharged to home.4 Ng and colleagues studied a group of 200 patients who received IRF care and found there was significant improvement in function. Ninety-four percent of patients rated their stay as either extremely good or very good.5
Metastasis to the spine is a common problem. It is found in 30% of cancer patients at autopsy. The most common sources of metastasis to the spine are breast, lung, prostate, kidney, and thyroid.18 Multiple myeloma and lymphoma may also involve the spine. Several authors have shown that these patients benefit from inpatient rehabilitation. Mckinley and colleagues19 have noted that patients with metastasis to the spine make significant improvement with care at an IRF. Compared with patients with a traumatic spinal cord injury, the cancer patients had shorter LoS, smaller improvement in FIM, equal FIM efficiency (FIM gain/LoS), and equal success in making enough progress to be discharged to home.19 Eriks and colleagues showed that patients at an IRF in Amsterdam made significant improvement in function as measured by the Barthel’s Index.20 Tang .,21 and Parsch22 and their respective colleagues, Murray,23 and New24 and colleagues have published findings confirming that patients with spinal cord injury caused by metastasis to the spine make significant progress with inpatient rehabilitation programs. The present study adds to the literature by showing that patients with metastasis to the spine who are receiving radiation can make progress and be discharged to the community.
There are 24,000 new cases of primary malignant brain tumors in the United States each year.25 The incidence of metastatic cancer to the brain has been estimated to be 100,000 cases per year in the United States. The most common cancer sources are lung, breast, melanoma, kidney, and colon.26,27 The first study of patients admitted to an IRF for treatment of brain tumors was published in 1998 by Huang and colleagues28 who compared the outcomes of 63 patients with brain tumors with the outcomes of 63 patients with stroke. They reported that the patients with the brain tumors made significant improvement in function. There was not a significant difference between the 2 groups of patients in improvement in function, FIM efficiency, or success in discharging the patients to home.28 Greenberg29 and Bartolo30 and their respective colleagues compared the outcomes of patients admitted with brain tumors and patients with stroke and found that improvement in function and discharge to home was similar in the 2 groups. In 2000, Huang and his same colleagues31 compared a group of patients with brain tumors to a group of patients with traumatic brain injury. They found significant improvement in the function of the patients with brain tumors. Patients in the traumatic brain injury group made more progress but had longer LoS. FIM efficiency was not significantly different between the groups.31
Three papers have reported outcomes of patients who received radiation concurrent with inpatient rehabilitation. Tang and colleagues32 reported 63 patients, of whom 48% percent received radiation concurrent with rehabilitation. The patients who received radiation made significant gains in function, and more than 70% were discharged to home. There was no difference in the outcomes of the patients in the radiation and nonradiation groups.32 Marciniak33 and O’Dell34 and their colleagues also reported that patients with brain tumors that required radiation therapy can benefit from inpatient rehabilitation. The present paper is the fourth (with the largest patient group) to show that patients with primary and metastatic tumors to the brain can benefit from a program that provides radiation concurrent with inpatient rehabilitation. We have shown that patients can achieve functional levels and rates of discharge to home that are not significantly different from those of the most commonly admitted group of patients to IRF – patients with stroke.
In the present study, 18% of all of the cancer patients were transferred to medical services and/or acute hospital care (Table 1). This is consistent with a paper by Asher and colleagues35 who reported that 17.4% of patients at an IRF with a diagnosis of cancer required transfer back to medical service, and that low admission motor FIM score correlated with the likelihood of transfer back to medical service. In the present paper, the total admission FIM score was not related to the likelihood of return to medical service, although a lack of improvement in the FIM score did correlate with transfer to medical service.
All of the papers we reviewed found that appropriately selected patients with cancer make significant improvement in function with treatment at an IRF. Tang and colleagues have also shown that for patients with malignant brain tumors and metastasis to the spine, improvement in function correlates with increased survival.32 Our paper confirms that patients with primary malignant brain tumors, malignant tumors metastatic to the brain or spine, and tumors metastatic to long bones may benefit from rehabilitation concurrent with radiation. Rehabilitation units are traditionally associated with treating patients with stroke and spinal cord injury. The patients in our study had cancer and were receiving radiation therapy. They had significant improvement in function and FIM efficiency scores that are not below the threshold set as expected for care at an IRF. Most patients in our study achieved a functional level consistent with what is needed to go home.
There is a prospective payment or reimbursement system for rehabilitation units.36 The payments are based on the admitting diagnosis, the admission FIM score, the age of the patient, and comorbidities. There are 4 tiers for comorbidities with no additional payments for patients in tier 0 but with additional payments for patients with conditions that qualify for tiers 1 through 3. The highest payments are for patients in tier 1. Examples of conditions that can increase payment include morbid obesity, congestive heart failure, vocal cord paralysis, and the need for hemodialysis. There is no increased payment for provision of radiation therapy. There are no reports on the feasibility, in terms of finances, of providing radiation on an IRF. We asked the finance office of the Albany Medical Center to comment on the cost to the hospital of providing radiation therapy to patients on the rehabilitation unit. The hospital’s finance department reviewed available data and reported that the variable cost of providing radiation therapy is about 6.5% of the revenue collected from third-party payors for caring for patients who receive that service (personal communication from the finance office of Albany Medical Center to George Forrest, 2015). Our findings suggest that the Centers for Medicare & Medicaid Services should make an adjustment to the payment system to support the cost of providing radiation to patients at an IRF. Even under the current payment system, for a hospital that has the equipment and personnel to provide radiation treatments, the variable cost of 6.5% of revenue should not be an absolute barrier to providing this service.
Limitations
This study reports on the experience of only 1 facility. The number of patients in the radiation group is greater than the number of patients in any previous report of people receiving radiation at an IRF, but the statistician does not think it is large enough to allow statistical analysis of covariates such as age, sex, and comorbid conditions. In addition, we did not investigate all of the factors that influence the type of care patients are offered and their LoS, such as hospital policy, insurance coverage, income, and family structure.
Conclusions
Acute care medical units are now challenged to both reduce LoS and reduce the number of patients who are readmitted to the hospital. Rehabilitation units are challenged to maintain census, as government and private payors are shifting patients from acute rehabilitation units to subacute rehabilitation units. We found that patients with cancer who need radiation are a population of patients who are seen by payors as needing to be in a facility with excellent nursing, therapy, and comprehensive physician services. A comprehensive cancer care program within a rehabilitation unit can be a great benefit to the acute care services, the IRF, and, most importantly, patients and their families.
The American Cancer Society reports that 1.6 million people are diagnosed with cancer each year, of whom 78% are aged 55 years or older. The 5-year survival rate for cancer is 68%.1 Almost 15.5 million living Americans have been diagnosed with cancer.2 Many patients with cancer have difficulty walking and with activities of daily living. Patients with primary brain tumors or tumors metastatic to the brain may present with focal weakness or cognitive deficits similar to patients with stroke. Patients with tumors metastatic to the spine may have the same deficits as a patient with a traumatic spinal cord injury. Patients with metastasis to bone may have pathologic fractures of the hip or long bones. Patients may develop peripheral neuropathy associated with a paraneoplastic syndrome, chemotherapy, or critical illness neuropathy. Lehmann and colleagues evaluated 805 patients admitted to hospitals affiliated with the University of Washington Medical School with a diagnosis of cancer and found that 15% had difficulty walking and 20% had difficulty with activities of daily living.3
Many patients with cancer can benefit from inpatient rehabilitation.4,5 Study findings have shown that patients with impairments in function related to cancer are often not referred for rehabilitation. Among the reasons mentioned for that are that oncologists are more focused on treating the patients’ cancer than on their functional deficits and that specialists in rehabilitation medicine do not want to be involved with patients with complex medical problems. Rehabilitation facilities may not want to incur the costs associated with caring for patients with cancer.6
The present paper looks at the outcomes of 61 consecutive patients with cancer who were admitted to an inpatient rehabilitation facility (IRF) and received radiation therapy concurrent with rehabilitation. It compares the outcomes of the cancer patients with the outcomes of patients without cancer who were admitted with stroke or spinal cord injury, conditions more commonly treated at an IRF.
Methods
We reviewed electronic medical records of all patients with cancer admitted to the IRF from 2008 through 2013 who received radiation therapy while at the facility. We also reviewed the data of all patients without cancer admitted with a diagnosis of stroke in 2013 and all patients admitted with a diagnosis of traumatic spinal cord injury in 2012 and 2013. No patients were excluded from stroke and traumatic spinal cord injury groups.
We recorded the sex, age, diagnostic group, Functional Independence Measure (FIM) admission score, FIM discharge score, length of stay (LoS) in the IRF, place of discharge of each patient (eg, home, acute care, or subacute care), and calculated the FIM efficiency score (change in FIM/LoS) for each patient. The FIM is an instrument that has 18 items measuring mobility, participation in activities of daily living, ability to communicate, and cognitive function.7 Each item is scored from 1 to 7, with 1 denoting that the patient cannot perform the task and 7 that the activity can be performed independently. The minimum score is 18 (complete dependence), and the maximum score is 126 (independent function). Thirteen items compose the motor FIM score: eating, grooming, bathing, dressing upper body, dressing lower body, toileting, bladder management, management of bowel, transfer to bed or wheelchair, transfer to toilet, tub transfer, walking (or wheelchair use), and climbing stairs. Five items – comprehension, expression, social interaction, problem solving, and memory – compose the cognitive FIM score.
We used a 1-way analysis of variance to evaluate differences between age and cancer type, age and diagnostic group, admission FIM score and cancer type, discharge FIM score and cancer type, change in FIM and cancer type, LoS and cancer type, and LoS and diagnostic group. The Pearson chi-square test was used to test the goodness of fit between the place of disposition and diagnostic group. The paired t test was used to evaluate the improvement in FIM of the patients who were in the cancer groups. The Tukey Simultaneous Tests for Differences of Means was used to compare the FIM efficiency scores of the groups. A 2-sample t test was used to evaluate the factors associated with the need for transfer from the IRF to the acute medical service.
Results
The demographic characteristics of the patients in the study and the admission and discharge FIM scores are reported in Table 1. There were initially 62 cancer patients in the radiation group, which was further divided into 4 subgroups based on the site of the primary tumor or metastasis. In all, 23 had a primary malignant brain tumor and received radiation and temozolomide. Sixteen patients had malignancies metastatic to the brain, 15 patients had tumors metastatic to the spine, and 7 had tumors metastatic to the long bones. One patient had laryngeal cancer and was excluded from the study because we did not think that we could do an analysis of a group with only 1 patient. The final number of patients in the cancer group was therefore 61. There were 69 patients in the stroke group and 23 in the spinal cord injury group.
We report improvement in total FIM, motor FIM, and cognitive FIM scores and were able to identify all 18 of the items of the FIM score on 60 of the 61 patients in the cancer group. Improvement in total FIM of the 61 patients in the cancer groups was significant at P P P = .05. Just over 75% of the patients in the cancer group had sufficient enough improvement in their level of function that they were able to return to their homes (Table 1). The average FIM score at the time of discharge was 83.08. This was not significantly different than the level of function of patients discharged after stroke (87.52) or traumatic spinal cord injury (89.13).
The patients with primary brain tumors were younger than the patients with cancer metastatic to the brain (P = .013). The patients with a primary brain tumor had lower admission FIM scores than patients with tumors metastatic to the brain (P = .027). The patients with a primary brain tumor had a greater increase in FIM score than patients with metastasis to the brain (P = .043; Table 2). There was not a significant difference between these 2 groups in FIM score at discharge or in the likelihood of discharge to home (Table 1). The FIM efficiency score was 1.12 for the patients in the primary brain tumor group and .80 in those with metastasis to the brain. This difference was not significant P = .96.
There were 69 patients in the stroke group. We compared the 39 patients with primary or metastatic brain lesion to the stroke group. The patients with primary or metastatic cancer of the brain were younger than the patients with stroke, 60.4 years old versus 69.1 years old (P = .004). The patients in the combined cancer group had a higher admission FIM score compared with the stroke patients (68.4 vs 63.12; P = .05). The discharge FIM scores were 83.3 in the combined cancer group and 87.5 in the stroke group (Table 1). This difference was not significant, but the improvement in the combined cancer group (14.6) was less than the improvement in the stroke group (24.40; P = .002) (Table 3).
The average LoS in the IRF was 18.7 days in the combined cancer group and 16.8 days in the stroke group. This difference was not significant. An average of 82% of the patients in the primary tumor or brain metastasis group and 85.5% of the patients in the stroke group were discharged to home. This difference was not significant. The FIM efficiency score of the patients in the stroke group was 2.0. This was significantly greater than the score for the patients in the metastasis to the brain group (0.80; P = .044) but not significantly greater than the primary brain cancer group (1.19; P = .22).
There were 23 patients in the traumatic spinal cord injury group. A comparison of the patients with tumors metastatic to the spine and patients with traumatic spinal cord injury showed that the patients in the cancer group were older (60.27 and 42.70 years, respectively; P = .001). In all, 80% of patients with tumors metastatic to the spine were men. This was not significantly different from the percentage of men in the traumatic spinal cord injury group (82.6%; Table 1). The admission FIM score of the patients with cancer was 66.5 (standard deviation [SD], 13.3) and 58.03 (SD, 15.1) in the patients with a traumatic spinal cord injury (Table 1). The FIM score at discharge was 80.4 (SD, 19.1) in the patients with cancer and 89.1 (SD, 20.3) in the patients with a traumatic spinal cord injury (Table 1). Neither of these were statistically significant. The improvement in patients with cancer was 13.9 (SD, 12.2) and 31.1 (SD, 13.9) in the traumatic spinal cord injured patients. This difference was significant (P
The median LoS was 18.98 days in the cancer metastasis to spine group (interquartile range [IQR] is the 25th-75th percentile, 12-30 days). In the traumatic group the median LoS was 23 days (IQR, 16-50 days). This difference was not significant (P = .14 Mann-Whitney test). The mean FIM efficiency score was 1.46 in the traumatic spinal cord injury group and .78 in the group with cancer metastatic to the spine. This difference was not significant (P = .72). Sixty percent of the patients in the cancer group were discharged to home, and 87% of patients in the traumatic spinal cord group were discharged to home. This difference was not significant (P = .12; Fisher exact test).
As far as we can ascertain, this is the first paper that has looked at the outcomes of patients receiving rehabilitation concurrent with radiation of the long bones. The average improvement in FIM was 12.4 (Table 1). The LoS was 11.6 days, and the FIM efficiency was 1.25. In all, 71.4% made enough progress to go home.
Of the total number of cancer patients, 18% were transferred to the acute medical service of the hospital (Table 1). Neither age, sex, type of cancer, nor admission FIM score were associated with the need for transfer to acute hospital care. Change in FIM score was inversely associated with transfer to acute hospital care (P = .027). Patients whose function did not improve with rehabilitation were most likely to be transferred back to acute hospital care.
Discussion
Radiation therapy is considered a service that is provided to people who come for treatment as an outpatient. Caregivers may have difficulty transporting patients to radiation if the patient has deficits in mobility. This may be particularly true if the patient is heavy, the caregivers are frail, or perhaps if they live in rural settings where there is no wheelchair-accessible public transportation. There are many factors that help determine whether a patient with functional deficits can be discharged to his or her home. These include sex, age, marital status, family and/or community support, income, and insurance.8 The FIM is an instrument that indicates how much help a patient needs with mobility and self-care skills. It also correlates with the amount of time that caregivers must spend helping a patient.9 Study findings have shown that the FIM score is an important determinant of whether a patient can be discharged to home. The total FIM score is as useful as an analysis of the components of the FIM score in predicting whether a patient can return to the community.10,11 Reistetter and colleagues found a total FIM score of 78 to be the score that best separates patients who are likely to be able to go home and patients who are likely to need long-term care.11 Bottemiller and colleagues10 reported that 37% of patients with total discharge FIM scores of less than 40 were discharged to home. They reported that 62% of patients with FIM scores between 40 and 79 were discharged to home, and 88% of patients with scores of 80 or above were discharged to home.10 The goal in bringing patients to the IRF was to accept and treat patients with reasonable community support and potential to achieve a functional level compatible with discharge to the community. Most patients in each of the cancer groups were able to reach an FIM score of 78 to 80 and to be discharged to home.
Most of the patients in the cancer groups had underlying problems that are not considered curable. The primary goal was to enable the patients to have some time at home with their families before requiring readmission to a hospital or hospice care. Reasonable LoS and rate of progress are now expected or required by third-party payors and hospital administrators. Physicians at the Mayo Clinic have indicated that a rehabilitation service should aim for an FIM efficiency score of at least .6 points per day.10 The FIM efficiency of patients in each of the 4 cancer subgroups in this study was higher than this level.
J. Herbert Dietz, Jr was an early advocate of the need to provide comprehensive rehabilitation services for patients with cancer. He first described his work in 1969.12 Since that time, there have been many papers that have documented the benefits of IRF for patients with cancer. O’Toole and Golden have shown outcomes of a large series of patients from an IRF. They reported that at the time of admission, 14% of patients could ambulate, but at discharge, 80% could ambulate without hands-on assistance. They reported significant improvements in continence, FIM score, and score on the Karnofsky Performance Scale.13 Marciniak,14 Hunter,15 Shin,16 and Cole,17 and their respective colleagues have all shown that patients with many different types of cancer benefit from rehabilitation at the IRF level. Gallegos-Kearin and colleagues4 reported on the care of 115,570 patients admitted to IRF with cancer from 2002 to 2014. Patients had significant improvement in function, with more than 70% of patients discharged to home.4 Ng and colleagues studied a group of 200 patients who received IRF care and found there was significant improvement in function. Ninety-four percent of patients rated their stay as either extremely good or very good.5
Metastasis to the spine is a common problem. It is found in 30% of cancer patients at autopsy. The most common sources of metastasis to the spine are breast, lung, prostate, kidney, and thyroid.18 Multiple myeloma and lymphoma may also involve the spine. Several authors have shown that these patients benefit from inpatient rehabilitation. Mckinley and colleagues19 have noted that patients with metastasis to the spine make significant improvement with care at an IRF. Compared with patients with a traumatic spinal cord injury, the cancer patients had shorter LoS, smaller improvement in FIM, equal FIM efficiency (FIM gain/LoS), and equal success in making enough progress to be discharged to home.19 Eriks and colleagues showed that patients at an IRF in Amsterdam made significant improvement in function as measured by the Barthel’s Index.20 Tang .,21 and Parsch22 and their respective colleagues, Murray,23 and New24 and colleagues have published findings confirming that patients with spinal cord injury caused by metastasis to the spine make significant progress with inpatient rehabilitation programs. The present study adds to the literature by showing that patients with metastasis to the spine who are receiving radiation can make progress and be discharged to the community.
There are 24,000 new cases of primary malignant brain tumors in the United States each year.25 The incidence of metastatic cancer to the brain has been estimated to be 100,000 cases per year in the United States. The most common cancer sources are lung, breast, melanoma, kidney, and colon.26,27 The first study of patients admitted to an IRF for treatment of brain tumors was published in 1998 by Huang and colleagues28 who compared the outcomes of 63 patients with brain tumors with the outcomes of 63 patients with stroke. They reported that the patients with the brain tumors made significant improvement in function. There was not a significant difference between the 2 groups of patients in improvement in function, FIM efficiency, or success in discharging the patients to home.28 Greenberg29 and Bartolo30 and their respective colleagues compared the outcomes of patients admitted with brain tumors and patients with stroke and found that improvement in function and discharge to home was similar in the 2 groups. In 2000, Huang and his same colleagues31 compared a group of patients with brain tumors to a group of patients with traumatic brain injury. They found significant improvement in the function of the patients with brain tumors. Patients in the traumatic brain injury group made more progress but had longer LoS. FIM efficiency was not significantly different between the groups.31
Three papers have reported outcomes of patients who received radiation concurrent with inpatient rehabilitation. Tang and colleagues32 reported 63 patients, of whom 48% percent received radiation concurrent with rehabilitation. The patients who received radiation made significant gains in function, and more than 70% were discharged to home. There was no difference in the outcomes of the patients in the radiation and nonradiation groups.32 Marciniak33 and O’Dell34 and their colleagues also reported that patients with brain tumors that required radiation therapy can benefit from inpatient rehabilitation. The present paper is the fourth (with the largest patient group) to show that patients with primary and metastatic tumors to the brain can benefit from a program that provides radiation concurrent with inpatient rehabilitation. We have shown that patients can achieve functional levels and rates of discharge to home that are not significantly different from those of the most commonly admitted group of patients to IRF – patients with stroke.
In the present study, 18% of all of the cancer patients were transferred to medical services and/or acute hospital care (Table 1). This is consistent with a paper by Asher and colleagues35 who reported that 17.4% of patients at an IRF with a diagnosis of cancer required transfer back to medical service, and that low admission motor FIM score correlated with the likelihood of transfer back to medical service. In the present paper, the total admission FIM score was not related to the likelihood of return to medical service, although a lack of improvement in the FIM score did correlate with transfer to medical service.
All of the papers we reviewed found that appropriately selected patients with cancer make significant improvement in function with treatment at an IRF. Tang and colleagues have also shown that for patients with malignant brain tumors and metastasis to the spine, improvement in function correlates with increased survival.32 Our paper confirms that patients with primary malignant brain tumors, malignant tumors metastatic to the brain or spine, and tumors metastatic to long bones may benefit from rehabilitation concurrent with radiation. Rehabilitation units are traditionally associated with treating patients with stroke and spinal cord injury. The patients in our study had cancer and were receiving radiation therapy. They had significant improvement in function and FIM efficiency scores that are not below the threshold set as expected for care at an IRF. Most patients in our study achieved a functional level consistent with what is needed to go home.
There is a prospective payment or reimbursement system for rehabilitation units.36 The payments are based on the admitting diagnosis, the admission FIM score, the age of the patient, and comorbidities. There are 4 tiers for comorbidities with no additional payments for patients in tier 0 but with additional payments for patients with conditions that qualify for tiers 1 through 3. The highest payments are for patients in tier 1. Examples of conditions that can increase payment include morbid obesity, congestive heart failure, vocal cord paralysis, and the need for hemodialysis. There is no increased payment for provision of radiation therapy. There are no reports on the feasibility, in terms of finances, of providing radiation on an IRF. We asked the finance office of the Albany Medical Center to comment on the cost to the hospital of providing radiation therapy to patients on the rehabilitation unit. The hospital’s finance department reviewed available data and reported that the variable cost of providing radiation therapy is about 6.5% of the revenue collected from third-party payors for caring for patients who receive that service (personal communication from the finance office of Albany Medical Center to George Forrest, 2015). Our findings suggest that the Centers for Medicare & Medicaid Services should make an adjustment to the payment system to support the cost of providing radiation to patients at an IRF. Even under the current payment system, for a hospital that has the equipment and personnel to provide radiation treatments, the variable cost of 6.5% of revenue should not be an absolute barrier to providing this service.
Limitations
This study reports on the experience of only 1 facility. The number of patients in the radiation group is greater than the number of patients in any previous report of people receiving radiation at an IRF, but the statistician does not think it is large enough to allow statistical analysis of covariates such as age, sex, and comorbid conditions. In addition, we did not investigate all of the factors that influence the type of care patients are offered and their LoS, such as hospital policy, insurance coverage, income, and family structure.
Conclusions
Acute care medical units are now challenged to both reduce LoS and reduce the number of patients who are readmitted to the hospital. Rehabilitation units are challenged to maintain census, as government and private payors are shifting patients from acute rehabilitation units to subacute rehabilitation units. We found that patients with cancer who need radiation are a population of patients who are seen by payors as needing to be in a facility with excellent nursing, therapy, and comprehensive physician services. A comprehensive cancer care program within a rehabilitation unit can be a great benefit to the acute care services, the IRF, and, most importantly, patients and their families.
1. American Cancer Society. Cancer facts & figures 2016. Atlanta, GA: American Cancer Society; 2016.
2. National Cancer Institute: Office of cancer survivorship: statistics. https://cancercontrol.cancer.gov/ocs/statistics/statistics.html. Updated October 17, 2016. Accessed April 21, 2018.
3. Lehmann JF, DeLisa JA, Warren CG, deLateur BJ, Bryant PL, Nicholson CG. Cancer rehabilitation: assessment of need, development and evaluation of a model of care. Arch Phys Med Rehabil. 1978;59(9):410-419.
4. Gallegos-Kearin V, Knowlton SE, Goldstein R, et al. Outcome trends of adult cancer patients receiving inpatient rehabilitation: a 13-year review [published online Feb 21, 2018]. Am J Phys Med Rehabil. doi:10.1097/PHM.0000000000000911
5. Ng AH, Gupta E, Fontillas RC, et al. Patient-reported usefulness of acute cancer rehabilitation. PM R. 2017;9(11):1135-1143.
6. Cheville AL, Kornblith AB, Basford JR. An examination of the causes for the underutilization of rehabilitation services among people with advanced cancer. Am J Phys Med Rehabil. 2011;90(5 suppl 1):S27-S37.
7. Cohen ME, Marino RJ. The tools of disability outcomes research functional status measures. Arch Phys Med Rehabil. 2000;81(12 suppl 2):S21-S29.
8. Nguyen VQ, PrvuBettger J, Guerrier T, et al. Factors associated with discharge to home versus discharge to institutional care after inpatient stroke rehabilitation. Arch Phys Med Rehabil. 2015;96(7):1297-1303.
9. Forrest G, Schwam A, Cohen E. Time of care required by patients discharged from a rehabilitation unit. Am J Phys Med Rehabil. 2002;81(1):57-62.
10. Bottemiller KL, Bieber PL, Basford JR, Harris M. FIM scores, FIM efficiency and discharge following inpatient stroke rehabilitation. Rehabil Nurs. 2006;31(1):22-25.
11. Reistetter TA, Graham JE, Deutsch A, Granger CV, Markello S, Ottenbacher KJ. Utility of functional status for classifying community versus institutional discharges after inpatient rehabilitation for stroke. Arch Phys Med Rehabil. 2010;91(3):345-350.
12. Dietz JH Jr. Rehabilitation of the cancer patient. Med Clin North Am. 1969;53(3):607-624.
13. O'Toole DM, Golden AM. Evaluating cancer patients for rehabilitation potential. West J Med. 1991;155(4):384-387.
14. Marciniak CM, Sliwa JA, Spill G, Heinemann AW, Semik PE. Functional outcome following rehabilitation of the cancer patient. Arch Phys Med Rehabil. 1996;77(1):54-57.
15. Hunter EG, Baltisberger J. Functional outcomes by age for inpatient cancer rehabilitation: a retrospective chart review. J Appl Gerontol. 2013;32(4):443-456.
16. Shin KY, Guo Y, Konzen B, Fu J, Yadav R, Bruera E. Inpatient cancer rehabilitation: the experience of a national comprehensive cancer center. Am J Phys Med Rehabil. 2011;90(5 suppl 1):S63-S68.
17. Cole RP, Scialla S, Bednarz L. Functional recovery in cancer rehabilitation. Arch Phys Med Rehabil. 2000;81(5):623-627.
18. White AP, Kwon BK, Lindskog DM, Friedlaender GE, Grauer JN. Metastatic disease of the spine. J Am Acad Orthop Surg. 2006;14(11):587-598.
19. McKinley WO, Huang ME, Tewksbury MA. Neoplastic vs traumatic spinal cord injury: an inpatient rehabilitation comparison. Am J Phys Med Rehabil. 2000;79(2):138-144.
20. Eriks IE, Angenot EL, Lankhorst GJ. Epidural metastatic spinal cord compression: functional outcome and survival after inpatient rehabilitation. Spinal Cord. 2004;42(4):235-239.
21. Tang V, Harvey D, Park Dorsay J, Jiang S, Rathbone MP. Prognostic indicators in metastatic spinal cord compression: using functional independence measure and Tokuhashi scale to optimize rehabilitation planning. Spinal Cord. 2007;45(10):671-677.
22. Parsch D, Mikut R, Abel R. Postacute management of patients with spinal cord injury due to metastatic tumor disease: survival and efficacy of rehabilitation. Spinal Cord. 2003;41:205-210.
23. Murray PK. Functional outcome and survival in spinal cord injury secondary to neoplasia. Cancer. 1985;55:197-201.
24. New PW. Functional outcomes and disability after nontraumatic spinal cord injury rehabilitation: results from a retrospective study. Arch Phys Med Rehabil. 2005;86(2):250-261
25. Central Brain Tumor Registry of the United States: 2016 CBTRUS fact sheet. www.cbtrus.org/factsheet/factsheet.html. Updated 2017. Accessed May 28, 2016.
26. Memorial Sloan Kettering Cancer Center: Metastatic brain tumors & secondary brain cancer. https://www.mskcc.org/cancer-care/types/brain-tumors-metastatic. Updated 2018. Accessed April 21, 2018.
27. Bruckner JC, Brown PD, O'Neill BP, Meyer FB, Wetmore CJ, Uhm JH. Central nervous system tumors. Mayo Clin Proc. 2007;82(10):1271-1286.
28. Huang ME, Cifu DX, Keyser-Marcus L. Functional outcome after brain tumor and acute stroke: a comparative analysis. Arch Phys Med Rehabil. 1998;79(11):1386-1390.
29. Greenberg E, Treger I, Ring H. Rehabilitation outcomes in patients with brain tumors and acute stroke: comparative study of inpatient rehabilitation. Am J Phys Med Rehabil. 2006;85(7):568-573.
30. Bartolo M, Zucchella C, Pace A, et al. Early rehabilitation after surgery improves functional outcomes in inpatients with brain tumours. J Neurooncol. 2012;107(3);537-544.
31. Huang ME, Cifu DX, Keyser-Marcus L. Functional outcomes in patients with brain tumor after inpatient rehabilitation: comparison with traumatic brain injury. Am J Phys Med Rehabil. 2000;79(4):327-335.
32. Tang V, Rathbone M, Park Dorsay J, Jiang S, Harvey D. Rehabilitation in primary and metastatic brain tumours: impact of functional outcomes on survival. J Neurol. 2008;255(6):820-827.
33. Marciniak CM, Sliwa JA, Heinemann AW, Semik PE. Functional outcomes of persons with brain tumors after inpatient rehabilitation. Arch Phys Med Rehabil. 2001;82(4):457-463.
34. O'Dell MW, Barr K, Spanier D, Warnick RE. Functional outcome of inpatient rehabilitation in persons with brain tumors. Arch Phys Med Rehabil. 1998;79(12):1530-1534.
35. Asher A, Roberts PS, Bresee C, Zabel G, Riggs RV, Rogatko A. Transferring inpatient rehabilitation facility cancer patients back to acute care (TRIPBAC). PM R. 2014;6(9):808-813.
36. Centers for Medicare and Medicaid Services: Inpatient rehabilitation facilities. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/InpatientRehab.html. Published March 5, 2012. Accessed May 21, 2018.
1. American Cancer Society. Cancer facts & figures 2016. Atlanta, GA: American Cancer Society; 2016.
2. National Cancer Institute: Office of cancer survivorship: statistics. https://cancercontrol.cancer.gov/ocs/statistics/statistics.html. Updated October 17, 2016. Accessed April 21, 2018.
3. Lehmann JF, DeLisa JA, Warren CG, deLateur BJ, Bryant PL, Nicholson CG. Cancer rehabilitation: assessment of need, development and evaluation of a model of care. Arch Phys Med Rehabil. 1978;59(9):410-419.
4. Gallegos-Kearin V, Knowlton SE, Goldstein R, et al. Outcome trends of adult cancer patients receiving inpatient rehabilitation: a 13-year review [published online Feb 21, 2018]. Am J Phys Med Rehabil. doi:10.1097/PHM.0000000000000911
5. Ng AH, Gupta E, Fontillas RC, et al. Patient-reported usefulness of acute cancer rehabilitation. PM R. 2017;9(11):1135-1143.
6. Cheville AL, Kornblith AB, Basford JR. An examination of the causes for the underutilization of rehabilitation services among people with advanced cancer. Am J Phys Med Rehabil. 2011;90(5 suppl 1):S27-S37.
7. Cohen ME, Marino RJ. The tools of disability outcomes research functional status measures. Arch Phys Med Rehabil. 2000;81(12 suppl 2):S21-S29.
8. Nguyen VQ, PrvuBettger J, Guerrier T, et al. Factors associated with discharge to home versus discharge to institutional care after inpatient stroke rehabilitation. Arch Phys Med Rehabil. 2015;96(7):1297-1303.
9. Forrest G, Schwam A, Cohen E. Time of care required by patients discharged from a rehabilitation unit. Am J Phys Med Rehabil. 2002;81(1):57-62.
10. Bottemiller KL, Bieber PL, Basford JR, Harris M. FIM scores, FIM efficiency and discharge following inpatient stroke rehabilitation. Rehabil Nurs. 2006;31(1):22-25.
11. Reistetter TA, Graham JE, Deutsch A, Granger CV, Markello S, Ottenbacher KJ. Utility of functional status for classifying community versus institutional discharges after inpatient rehabilitation for stroke. Arch Phys Med Rehabil. 2010;91(3):345-350.
12. Dietz JH Jr. Rehabilitation of the cancer patient. Med Clin North Am. 1969;53(3):607-624.
13. O'Toole DM, Golden AM. Evaluating cancer patients for rehabilitation potential. West J Med. 1991;155(4):384-387.
14. Marciniak CM, Sliwa JA, Spill G, Heinemann AW, Semik PE. Functional outcome following rehabilitation of the cancer patient. Arch Phys Med Rehabil. 1996;77(1):54-57.
15. Hunter EG, Baltisberger J. Functional outcomes by age for inpatient cancer rehabilitation: a retrospective chart review. J Appl Gerontol. 2013;32(4):443-456.
16. Shin KY, Guo Y, Konzen B, Fu J, Yadav R, Bruera E. Inpatient cancer rehabilitation: the experience of a national comprehensive cancer center. Am J Phys Med Rehabil. 2011;90(5 suppl 1):S63-S68.
17. Cole RP, Scialla S, Bednarz L. Functional recovery in cancer rehabilitation. Arch Phys Med Rehabil. 2000;81(5):623-627.
18. White AP, Kwon BK, Lindskog DM, Friedlaender GE, Grauer JN. Metastatic disease of the spine. J Am Acad Orthop Surg. 2006;14(11):587-598.
19. McKinley WO, Huang ME, Tewksbury MA. Neoplastic vs traumatic spinal cord injury: an inpatient rehabilitation comparison. Am J Phys Med Rehabil. 2000;79(2):138-144.
20. Eriks IE, Angenot EL, Lankhorst GJ. Epidural metastatic spinal cord compression: functional outcome and survival after inpatient rehabilitation. Spinal Cord. 2004;42(4):235-239.
21. Tang V, Harvey D, Park Dorsay J, Jiang S, Rathbone MP. Prognostic indicators in metastatic spinal cord compression: using functional independence measure and Tokuhashi scale to optimize rehabilitation planning. Spinal Cord. 2007;45(10):671-677.
22. Parsch D, Mikut R, Abel R. Postacute management of patients with spinal cord injury due to metastatic tumor disease: survival and efficacy of rehabilitation. Spinal Cord. 2003;41:205-210.
23. Murray PK. Functional outcome and survival in spinal cord injury secondary to neoplasia. Cancer. 1985;55:197-201.
24. New PW. Functional outcomes and disability after nontraumatic spinal cord injury rehabilitation: results from a retrospective study. Arch Phys Med Rehabil. 2005;86(2):250-261
25. Central Brain Tumor Registry of the United States: 2016 CBTRUS fact sheet. www.cbtrus.org/factsheet/factsheet.html. Updated 2017. Accessed May 28, 2016.
26. Memorial Sloan Kettering Cancer Center: Metastatic brain tumors & secondary brain cancer. https://www.mskcc.org/cancer-care/types/brain-tumors-metastatic. Updated 2018. Accessed April 21, 2018.
27. Bruckner JC, Brown PD, O'Neill BP, Meyer FB, Wetmore CJ, Uhm JH. Central nervous system tumors. Mayo Clin Proc. 2007;82(10):1271-1286.
28. Huang ME, Cifu DX, Keyser-Marcus L. Functional outcome after brain tumor and acute stroke: a comparative analysis. Arch Phys Med Rehabil. 1998;79(11):1386-1390.
29. Greenberg E, Treger I, Ring H. Rehabilitation outcomes in patients with brain tumors and acute stroke: comparative study of inpatient rehabilitation. Am J Phys Med Rehabil. 2006;85(7):568-573.
30. Bartolo M, Zucchella C, Pace A, et al. Early rehabilitation after surgery improves functional outcomes in inpatients with brain tumours. J Neurooncol. 2012;107(3);537-544.
31. Huang ME, Cifu DX, Keyser-Marcus L. Functional outcomes in patients with brain tumor after inpatient rehabilitation: comparison with traumatic brain injury. Am J Phys Med Rehabil. 2000;79(4):327-335.
32. Tang V, Rathbone M, Park Dorsay J, Jiang S, Harvey D. Rehabilitation in primary and metastatic brain tumours: impact of functional outcomes on survival. J Neurol. 2008;255(6):820-827.
33. Marciniak CM, Sliwa JA, Heinemann AW, Semik PE. Functional outcomes of persons with brain tumors after inpatient rehabilitation. Arch Phys Med Rehabil. 2001;82(4):457-463.
34. O'Dell MW, Barr K, Spanier D, Warnick RE. Functional outcome of inpatient rehabilitation in persons with brain tumors. Arch Phys Med Rehabil. 1998;79(12):1530-1534.
35. Asher A, Roberts PS, Bresee C, Zabel G, Riggs RV, Rogatko A. Transferring inpatient rehabilitation facility cancer patients back to acute care (TRIPBAC). PM R. 2014;6(9):808-813.
36. Centers for Medicare and Medicaid Services: Inpatient rehabilitation facilities. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/InpatientRehab.html. Published March 5, 2012. Accessed May 21, 2018.