User login
Psychosocial factors and treatment satisfaction after radical prostatectomy
More than 164,690 men are expected to be diagnosed with prostate cancer in the United States in 2018.1 Men with prostate cancer face not only stress associated with the diagnosis but also decisional conflict regarding different treatment options.2 Most men diagnosed with clinically localized prostate cancer receive 1 or more of the following treatments: radical prostatectomy, external-beam radiation therapy, and/or brachytherapy, all of which are associated with posttreatment urological or sexual side effects including bowel, urinary, or erectile dysfunction.3-5 Men who choose active surveillance may experience increased anxiety associated with the constant vigilance and monitoring of their tumor status along with the uncertainty of not definitively removing or radiating their prostate.6 In addition to direct functional limitations of sexual and urological side effects, treatment can also lead to secondary psychosocial effects, including depression, self-blame, embarrassment, guilt, lower masculine self-esteem, increased reticence to participate socially or engage in sexual activity, and relationship distress.7-9 Therefore, health-related quality of life (HRQoL) and treatment satisfaction are important for this population.
Urological and sexual side effects of prostate cancer treatments are often a primary focus during treatment decision making between patients and providers. However, little prospective empirical data exist regarding the role of HRQoL and other nonurological physical and psychosocial outcomes on overall treatment satisfaction. The purpose of this study was to prospectively evaluate the role of both urological and nonurological outcomes on overall treatment satisfaction in men diagnosed with prostate cancer. We hypothesize that such an understanding can help describe changes in physical and psychosocial factors that are important to men beyond traditional urological outcomes, including their association with overall treatment satisfaction.
Methods
This was a prospective longitudinal assessment of patients from the Department of Urology at Northwestern University’s Feinberg School of Medicine in Chicago. Patients were eligible if they met the following inclusion criteria: they had been diagnosed with clinically localized or locally advanced prostate cancer; they had not yet received a primary treatment (eg, surgery, radiation, active surveillance) before their baseline assessment; they were 18 years or older; and they were able to read, write, speak, and understand English. Patients were excluded if they had a physical debilitation that would make participation not feasible or would create undue hardship, or if they had a history of diagnosed severe mental illness or hospitalization for chronic psychiatric reasons, as identified by referring physicians.
Eligible participants were approached before their treatment decision (if any). Patient enrollment occurred in 2 ways. For patients invited to participate during their clinic visit, the research assistant explained the study and obtained written informed consent for interested patients. A unique user identification and password was created for each patient, and they practiced using the touch screen computer while the research assistant observed and provided guidance as needed. When the patients were ready to start their pretreatment online interview, they completed the questionnaires by themselves. For patients who were invited to participate but were not scheduled to return in the foreseeable future, enrollment was carried out differently. In those cases, participating physicians contacted eligible patients who were not scheduled for a visit and informed them of the study opportunity. Interested patients were contacted by the research assistant who provided them with the study website address, which directed them to the online consent form. After a patient had completed the consent form, he was prompted to self-register. He received a unique user identification and password that could be used to complete the baseline assessment and subsequent assessments. However, for interested patients who did not have access to a computer or Internet connection, the research assistant provided them with paper consent forms and paper versions of all study assessments. After participants had completed the baseline assessment, the research assistant provided them with a written schedule of future assessments, which were expected to occur at 1 month posttreatment, 3 months posttreatment, 6 months posttreatment, and 12 months posttreatment.
For all follow-up appointments, participants could complete assessments either at clinic visits or from home using a secure online assessment platform called Assessment Center.10 The research assistant used a patient log to track participants and their progress in the study, which included study number, patient name (or initials), registration date, date of birth, sex, and timeline of completed or future assessments. The research assistant called or emailed participants (depending on patient preference) about a week before each of their follow-up assessments to facilitate adherence. If the participant did not log into the system by the target day, the research assistant contacted him the following day (target day +1) with a phone or email reminder to log into the system and complete the assessments. If the participant did not log in by midnight 1 day after the target day, the research assistant attempted to contact him one last time (target day +2) with either a reminder to log into the system or to ascertain his status that might be related to his noncompletion. Overall, a participant was called or e-mailed 1 to 3 times to remind him of his assessment. If he was unresponsive after 3 attempts, he was recorded as having withdrawn for an unknown reason.
At baseline and each follow-up time point, study participants completed a battery of patient-reported outcome measures, with most coming from the Patient-Reported Outcomes Measurement Information System (PROMIS)11 and the Surgical Outcomes Measurement System (SOMS).12 PROMIS is a National Institutes of Health (NIH) funded measurement system that has helped standardize and improve self-reported assessment of health status, symptoms, side effects, and different aspects of HRQoL, including physical, emotional, cognitive, and social health. SOMS is a suite of patient-reported outcome measures assessing important aspects of HRQoL after surgery. It was developed with feedback from surgeons, postoperative patients, and surgical nurses. PROMIS items were directly incorporated into numerous SOMS measures to facilitate easier comparisons and score crosswalks across measures and patient populations. In addition to PROMIS and SOMS measures, we also administered several well-known instruments of urological and sexual function, including the International Index of Erectile Function (IIEF) and American Urological Association Symptom Score Index (AUASS).13,14
Outcome measures were compared across sociodemographic and clinical variables at each time point using t tests for numerical variables (age) and with chi-square or Fisher exact tests for categorical variables; those variables with significant differences were used as covariates in statistical models. To examine differences in patient-reported scores over time, we used repeated measures analysis of covariance with general linear modeling methods. We used Pearson correlation coefficients to evaluate for correlations between quality-of-life outcomes and treatment satisfaction.
Not all participants completed each of the follow-up surveys, and reasons for dropout were prospectively documented. Most participants elected surgical resection as their primary treatment compared with the fewer than 10% of patients who chose radiation or chemotherapy as their primary treatment and about 20% of men who chose active surveillance after their initial diagnosis. Therefore, our analysis focused on patients who elected surgical resection. For comparison purposes, we included the HRQoL results from active surveillance patients.
Results
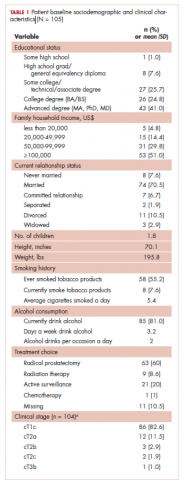
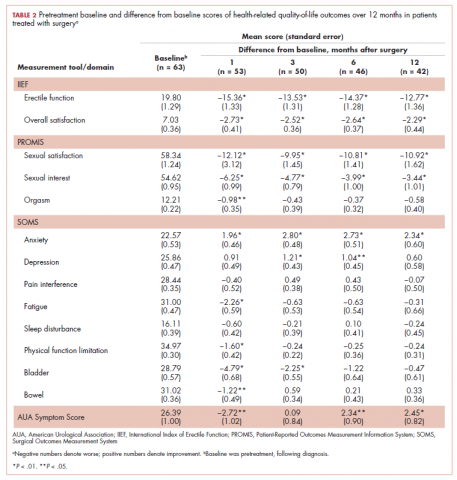
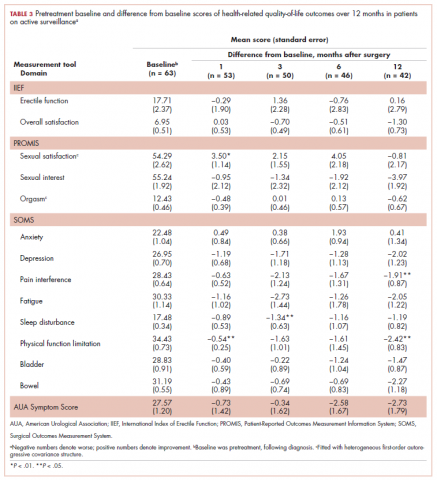
A total of 105 patients diagnosed with prostate cancer were enrolled in the study. Response rates decreased throughout the study (n = 75 at 1 month; n = 71 at 3 months; n = 64 at 6 months; n = 54 at 12 months). Sociodemographic and clinical characteristics of participants are shown in Table 1. The mean change from pretreatment (baseline) scores for each measure in patients treated with surgery is shown in Table 2, and the mean change from pretreatment scores in patients who elected active surveillance is shown in Table 3 (in both tables, a negative score denotes worsened function, and a positive change denotes improvement).
After surgery, patients reported significantly lower erectile function and sexual satisfaction scores. These included statistically significant decreases for IIEF Erectile Function, IIEF Overall Satisfaction, PROMIS Sexual Satisfaction, PROMIS Sexual Interest, and PROMIS Orgasm. In patients treated with surgery, there were significant improvements in anxiety observed for patients at each follow-up time, whereas significantly worse bladder problems were observed on SOMS Bladder at 1 and 3 months but returned to baseline by 12 months after surgery. AUASS was worse at 1 month but significantly improved at 6 and 12 months. Fatigue scores significantly worsened at 1 month but were no longer significant at 6 and 12 months. Physical Function was worsened at 1 month but not throughout the rest of the study. Bowel Problems (SOMS) were significantly worse at 1 month, but changes became nonsignificant on subsequent assessments. The only 2 domains that did not demonstrate any significant changes o
In active surveillance patients, sexual function domains were generally unchanged over the course of the study. However, unlike treated patients, there was no significant improvement in anxiety, depression, pain, fatigue, or sleep. In fact, most of these domains demonstrated worsened functioning, although these were not statistically significant. Urinary domains generally remained unchanged.
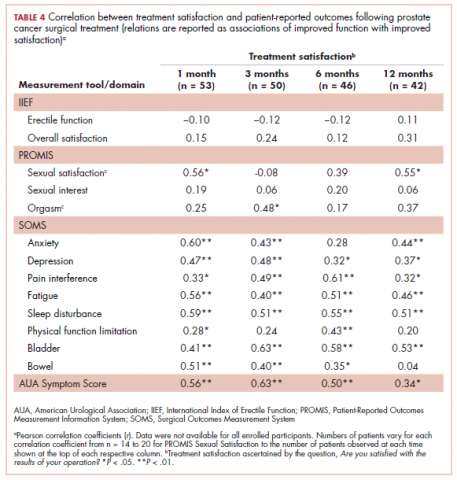
Pearson correlation coefficients between HRQoL measures and overall treatment satisfaction (assessed by the question, Are you satisfied with the results of your operation?) at each follow-up time point in patients treated with surgery are shown in Table 4. Relations between treatment satisfaction and sexual outcomes were generally statistically insignificant (r, .08-.56). However, sleep disturbance, depression, pain interference, fatigue, embarrassment, and bladder problems all demonstrated statistically significant positive associations with treatment satisfaction, with coefficients ranging from small to medium in magnitude (r, .32-.61). Other outcomes such as anxiety, physical function, and bowel problems demonstrated small to medium statistically significant associations with treatment satisfaction (r, .04-.60) but not at every time point. We performed t tests to examine treatment satisfaction in patients with detectable initial posttreatment prostate-specific antigen (PSA; >0.01 ng/mL). We found no difference in treatment satisfaction between patients with detectable PSA values and those with undetectable PSA at each time point.
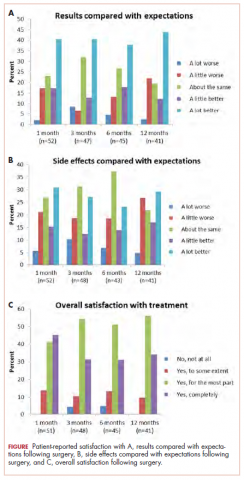
When the patients were asked, Compared with what you expected, how do you rate the results of your operation?, most of those treated with surgery reported that the results of their operation were better than they had expected (Figure 1A; p. e137). More than 75% of the patients had results that were as expected or better than expected. When asked, Compared with what you expected, how do you rate your side effects of the operation?, almost 70% of patients reported side effects no worse than expected (Figure 1B). When asked, Are you satisfied with the results of your operation?, most patients reported that overall, they were satisfied with the results of their operation (Figure 1C).
At 12 months, none of the patients reported overall dissatisfaction with their treatment choice. More than 90% of patients were mostly or completely satisfied with the results of their operation.
Discussion
This prospective study assessed the HRQoL from pretreatment through 12 months posttreatment in men diagnosed with clinically localized prostate cancer that had been treated with surgery. Although the indicators of sexual function significantly decreased over time, they were not meaningfully associated with overall treatment satisfaction. Instead, a host of other factors, including psychosocial (eg, anxiety, depression, body image dissatisfaction, embarrassment), nonurological physical symptoms (pain interference, physical function, sleep disturbance, fatigue), and bladder problems, were significantly related to overall treatment satisfaction. Although this may not be surprising in other clinical oncology paradigms, the sheer surfeit of focus and attention on sexual function has overshadowed aspects of HRQoL that many men report are important to them, despite worsened sexual function outcomes.
Understanding potential treatment-related changes in HRQoL can be challenging for men when choosing providers and different therapeutic options. The increasing complexity of treatment in prostate cancer has created an opportunity to not only understand efficacy on cancer control but also focus on meaningful patient-reported outcomes. Hospitals and medical groups are increasingly aware of the importance of improving the patient care experience. Objective measures of patient satisfaction for health care providers, such as the Press-Ganey and Net Promoter score, exist to measure and improve patient experience. In prostate cancer, clinicians and large groups, including governmental agencies such as the US Preventive Services Task Force, have often focused on declines in urinary and erectile function15 without considering the full impact of prostate cancer treatment on global HRQoL. Our study was a prospective, longitudinal, self-reported examination of the impact, positive and negative, of prostate cancer treatment over a 12-month period.
Numerous studies have documented the treatment-related side effects of erectile, urinary, and bowel dysfunction in patients treated for prostate cancer, which may occur after definitive local therapies.5,16-18 The present study shows a similar impact on urinary, bowel, and erectile domains after treatment. Although erectile function scores remained lower through the course of the 12-month study, bowel and bladder domains returned to baseline by month 12. Unlike other studies, we also examined psychosocial and nonurological aspects of prostate cancer treatment. We found that there was a measurable and significant positive impact on other HRQoL measurements such as decreased anxiety. Despite a variety of declines across HRQoL domains, most patients reported that their results were largely as they had expected, and their side effects were the same or better than they had expected. No patient in the cohort reported being dissatisfied with his overall treatment, and more than 90% of patients were mostly or completely satisfied with their treatment choice. This highlights the point that while sexual and other urological domains of HRQoL are important, impairments in these areas do not necessarily reflect how many patients perceive success or satisfaction with their treatment choice. We also showed correlations between treatment satisfaction and improvement in sleep, anxiety, depression, and fatigue. It is worth noting that although there were decreases in the erectile and sexual function domains after treatment, those factors were not correlated with overall treatment satisfaction. Those factors may not routinely be assessed before, during, and after treatment for prostate cancer in most clinical encounters. However, because they were strongly associated with satisfaction with treatment outcomes in this study, identification in impairments may lead to opportunities to intervene and improve the patient experience. Therefore, important “teachable moments” may be missed (for both patients and providers) during treatment decision-making encounters if other factors beyond sexual and urological outcomes are not adequately considered and addressed. Furthermore, the results of our study may help clinicians counsel patients on their expectations for their recovery after surgery and identify particular issues related to HRQoL to pay close attention to in follow-up visits.
Strengths of our study include its prospective nature, which allowed evaluation of HRQoL outcomes at multiple time points throughout the first year after treatment. In addition, we used existing patient-reported outcome tools validated by the NIH to assess changes in HRQoL. PROMIS is an NIH-supported tool that can be leveraged in the pre- and posttreatment periods to identify patients who have impairments with HRQoL. It can provide clinicians with a unique opportunity to detect and intervene in setbacks and side effects to improve patient satisfaction and HRQoL.
Limitations of the current study include that most patients selected surgery for their treatment choice and that not all patients completed all longitudinal questionnaires, although this is expected in longitudinal studies of this nature. Although all the patients were approached and encouraged to participate, many did not participate and were not captured. In addition, not all patients completed end-of-study surveys. These factors may have biased our results because of unmeasurable factors related to nonparticipation or dropout. Our study encompassed the preoperative period up to 12 months postoperatively, which may fail to identify improvements or declines in HRQoL that may occur more than 12 months postoperatively, particularly related to continence and erectile function. The participants were enrolled by 6 surgeons, and we were not able to standardize the preoperative counseling either preoperatively or postoperatively, which may have biased our results. Finally, our study population consisted of predominantly white, married men of higher socioeconomic status; therefore, our results may not be generalizable to newly diagnosed prostate cancer patients overall.
Conclusions
By using validated self-administered questionnaires, we found that despite decreased sexual and urinary function, patients treated for prostate cancer were satisfied with their treatment choice. Correlates to higher patient satisfaction included decreased anxiety, depression, fatigue, and sleep disturbances.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7‐30.
2. Berry DL, Ellis WJ, Woods NF, Schwien C, Mullen KH, Yang C. Treatment decision-making by men with localized prostate cancer: the influence of personal factors. Urol Oncol. 2003;21(2):93-100.
3. Dubbelman YD, Dohle GR, Schröder FH. Sexual function before and after radical retropubic prostatectomy: a systematic review of prognostic indicators for a successful outcome. Eur Urol. 2006;50(4):711-718; discussion 718-720.
4. McCullough AR. Sexual dysfunction after radical prostatectomy. Rev Urol. 2005;7(2 suppl):S3-S10.
5. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250-1261.
6. Latini DM, Hart SL, Knight SJ, et al. The relationship between anxiety and time to treatment for patients with prostate cancer on surveillance. J Urol. 2007;178(3, pt 1):826-831; discussion 831-832.
7. Meyer JP, Gillatt DA, Lockyer R, Macdonagh R. The effect of erectile dysfunction on the quality of life of men after radical prostatectomy. BJU Int. 2003;92(9):929-931.
8. Casey RG, Corcoran NM, Goldenberg SL. Quality of life issues in men undergoing androgen deprivation therapy: a review. Asian J Androl. 2012;14(2):226-231.
9. Segrin C, Badger TA, Harrington J. Interdependent psychological quality of life in dyads adjusting to prostate cancer. Health Psychol. 2012;31(1):70-79.
10. Gershon RC, Rothrock N, Hanrahan R, Bass M, Cella D. The use of PROMIS and assessment center to deliver patient-reported outcome measures in clinical research. J Appl Meas. 2010;11(3):304-314.
11. Cella D, Yount S, Rothrock N, et al. The patient-reported outcomes measurement information system (PROMIS): progress of an NIH roadmap cooperative group during its first two years. Med Care. 2007;45(5 suppl 1):S3-S11.
12. Zapf M, Denham W, Barrera E, et al. Patient-centered outcomes after laparoscopic cholecystectomy. Surg Endosc. 2013;27(12):4491-4498.
13. Barry MJ, Fowler FJ Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549-1557; discussion 1564.
14. Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822-830.
15. United States Preventive Services Task Force. Final update summary: prostate cancer: screening. http:// www.uspreventiveservicestaskforce.org/Page/ Document/UpdateSummaryFinal/prostate-cancer-screening. Updated July 2015. Accessed April 14, 2017
16. Litwin MS, Gore JL, Kwan L, et al. Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer. Cancer. 2007;109(11):2239-2247.
17. Miwa S, Mizokami A, Konaka H, et al. Prospective longitudinal comparative study of health-related quality of life and treatment satisfaction in patients treated with hormone therapy, radical retropubic prostatectomy, and high or low dose rate brachytherapy for prostate cancer. Prostate Int. 2013;1(3):117-124.
18. Miller DC, Sanda MG, Dunn RL et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23(12):2772-2780.
More than 164,690 men are expected to be diagnosed with prostate cancer in the United States in 2018.1 Men with prostate cancer face not only stress associated with the diagnosis but also decisional conflict regarding different treatment options.2 Most men diagnosed with clinically localized prostate cancer receive 1 or more of the following treatments: radical prostatectomy, external-beam radiation therapy, and/or brachytherapy, all of which are associated with posttreatment urological or sexual side effects including bowel, urinary, or erectile dysfunction.3-5 Men who choose active surveillance may experience increased anxiety associated with the constant vigilance and monitoring of their tumor status along with the uncertainty of not definitively removing or radiating their prostate.6 In addition to direct functional limitations of sexual and urological side effects, treatment can also lead to secondary psychosocial effects, including depression, self-blame, embarrassment, guilt, lower masculine self-esteem, increased reticence to participate socially or engage in sexual activity, and relationship distress.7-9 Therefore, health-related quality of life (HRQoL) and treatment satisfaction are important for this population.
Urological and sexual side effects of prostate cancer treatments are often a primary focus during treatment decision making between patients and providers. However, little prospective empirical data exist regarding the role of HRQoL and other nonurological physical and psychosocial outcomes on overall treatment satisfaction. The purpose of this study was to prospectively evaluate the role of both urological and nonurological outcomes on overall treatment satisfaction in men diagnosed with prostate cancer. We hypothesize that such an understanding can help describe changes in physical and psychosocial factors that are important to men beyond traditional urological outcomes, including their association with overall treatment satisfaction.
Methods
This was a prospective longitudinal assessment of patients from the Department of Urology at Northwestern University’s Feinberg School of Medicine in Chicago. Patients were eligible if they met the following inclusion criteria: they had been diagnosed with clinically localized or locally advanced prostate cancer; they had not yet received a primary treatment (eg, surgery, radiation, active surveillance) before their baseline assessment; they were 18 years or older; and they were able to read, write, speak, and understand English. Patients were excluded if they had a physical debilitation that would make participation not feasible or would create undue hardship, or if they had a history of diagnosed severe mental illness or hospitalization for chronic psychiatric reasons, as identified by referring physicians.
Eligible participants were approached before their treatment decision (if any). Patient enrollment occurred in 2 ways. For patients invited to participate during their clinic visit, the research assistant explained the study and obtained written informed consent for interested patients. A unique user identification and password was created for each patient, and they practiced using the touch screen computer while the research assistant observed and provided guidance as needed. When the patients were ready to start their pretreatment online interview, they completed the questionnaires by themselves. For patients who were invited to participate but were not scheduled to return in the foreseeable future, enrollment was carried out differently. In those cases, participating physicians contacted eligible patients who were not scheduled for a visit and informed them of the study opportunity. Interested patients were contacted by the research assistant who provided them with the study website address, which directed them to the online consent form. After a patient had completed the consent form, he was prompted to self-register. He received a unique user identification and password that could be used to complete the baseline assessment and subsequent assessments. However, for interested patients who did not have access to a computer or Internet connection, the research assistant provided them with paper consent forms and paper versions of all study assessments. After participants had completed the baseline assessment, the research assistant provided them with a written schedule of future assessments, which were expected to occur at 1 month posttreatment, 3 months posttreatment, 6 months posttreatment, and 12 months posttreatment.
For all follow-up appointments, participants could complete assessments either at clinic visits or from home using a secure online assessment platform called Assessment Center.10 The research assistant used a patient log to track participants and their progress in the study, which included study number, patient name (or initials), registration date, date of birth, sex, and timeline of completed or future assessments. The research assistant called or emailed participants (depending on patient preference) about a week before each of their follow-up assessments to facilitate adherence. If the participant did not log into the system by the target day, the research assistant contacted him the following day (target day +1) with a phone or email reminder to log into the system and complete the assessments. If the participant did not log in by midnight 1 day after the target day, the research assistant attempted to contact him one last time (target day +2) with either a reminder to log into the system or to ascertain his status that might be related to his noncompletion. Overall, a participant was called or e-mailed 1 to 3 times to remind him of his assessment. If he was unresponsive after 3 attempts, he was recorded as having withdrawn for an unknown reason.
At baseline and each follow-up time point, study participants completed a battery of patient-reported outcome measures, with most coming from the Patient-Reported Outcomes Measurement Information System (PROMIS)11 and the Surgical Outcomes Measurement System (SOMS).12 PROMIS is a National Institutes of Health (NIH) funded measurement system that has helped standardize and improve self-reported assessment of health status, symptoms, side effects, and different aspects of HRQoL, including physical, emotional, cognitive, and social health. SOMS is a suite of patient-reported outcome measures assessing important aspects of HRQoL after surgery. It was developed with feedback from surgeons, postoperative patients, and surgical nurses. PROMIS items were directly incorporated into numerous SOMS measures to facilitate easier comparisons and score crosswalks across measures and patient populations. In addition to PROMIS and SOMS measures, we also administered several well-known instruments of urological and sexual function, including the International Index of Erectile Function (IIEF) and American Urological Association Symptom Score Index (AUASS).13,14
Outcome measures were compared across sociodemographic and clinical variables at each time point using t tests for numerical variables (age) and with chi-square or Fisher exact tests for categorical variables; those variables with significant differences were used as covariates in statistical models. To examine differences in patient-reported scores over time, we used repeated measures analysis of covariance with general linear modeling methods. We used Pearson correlation coefficients to evaluate for correlations between quality-of-life outcomes and treatment satisfaction.
Not all participants completed each of the follow-up surveys, and reasons for dropout were prospectively documented. Most participants elected surgical resection as their primary treatment compared with the fewer than 10% of patients who chose radiation or chemotherapy as their primary treatment and about 20% of men who chose active surveillance after their initial diagnosis. Therefore, our analysis focused on patients who elected surgical resection. For comparison purposes, we included the HRQoL results from active surveillance patients.
Results
A total of 105 patients diagnosed with prostate cancer were enrolled in the study. Response rates decreased throughout the study (n = 75 at 1 month; n = 71 at 3 months; n = 64 at 6 months; n = 54 at 12 months). Sociodemographic and clinical characteristics of participants are shown in Table 1. The mean change from pretreatment (baseline) scores for each measure in patients treated with surgery is shown in Table 2, and the mean change from pretreatment scores in patients who elected active surveillance is shown in Table 3 (in both tables, a negative score denotes worsened function, and a positive change denotes improvement).
After surgery, patients reported significantly lower erectile function and sexual satisfaction scores. These included statistically significant decreases for IIEF Erectile Function, IIEF Overall Satisfaction, PROMIS Sexual Satisfaction, PROMIS Sexual Interest, and PROMIS Orgasm. In patients treated with surgery, there were significant improvements in anxiety observed for patients at each follow-up time, whereas significantly worse bladder problems were observed on SOMS Bladder at 1 and 3 months but returned to baseline by 12 months after surgery. AUASS was worse at 1 month but significantly improved at 6 and 12 months. Fatigue scores significantly worsened at 1 month but were no longer significant at 6 and 12 months. Physical Function was worsened at 1 month but not throughout the rest of the study. Bowel Problems (SOMS) were significantly worse at 1 month, but changes became nonsignificant on subsequent assessments. The only 2 domains that did not demonstrate any significant changes o
In active surveillance patients, sexual function domains were generally unchanged over the course of the study. However, unlike treated patients, there was no significant improvement in anxiety, depression, pain, fatigue, or sleep. In fact, most of these domains demonstrated worsened functioning, although these were not statistically significant. Urinary domains generally remained unchanged.
Pearson correlation coefficients between HRQoL measures and overall treatment satisfaction (assessed by the question, Are you satisfied with the results of your operation?) at each follow-up time point in patients treated with surgery are shown in Table 4. Relations between treatment satisfaction and sexual outcomes were generally statistically insignificant (r, .08-.56). However, sleep disturbance, depression, pain interference, fatigue, embarrassment, and bladder problems all demonstrated statistically significant positive associations with treatment satisfaction, with coefficients ranging from small to medium in magnitude (r, .32-.61). Other outcomes such as anxiety, physical function, and bowel problems demonstrated small to medium statistically significant associations with treatment satisfaction (r, .04-.60) but not at every time point. We performed t tests to examine treatment satisfaction in patients with detectable initial posttreatment prostate-specific antigen (PSA; >0.01 ng/mL). We found no difference in treatment satisfaction between patients with detectable PSA values and those with undetectable PSA at each time point.
When the patients were asked, Compared with what you expected, how do you rate the results of your operation?, most of those treated with surgery reported that the results of their operation were better than they had expected (Figure 1A; p. e137). More than 75% of the patients had results that were as expected or better than expected. When asked, Compared with what you expected, how do you rate your side effects of the operation?, almost 70% of patients reported side effects no worse than expected (Figure 1B). When asked, Are you satisfied with the results of your operation?, most patients reported that overall, they were satisfied with the results of their operation (Figure 1C).
At 12 months, none of the patients reported overall dissatisfaction with their treatment choice. More than 90% of patients were mostly or completely satisfied with the results of their operation.
Discussion
This prospective study assessed the HRQoL from pretreatment through 12 months posttreatment in men diagnosed with clinically localized prostate cancer that had been treated with surgery. Although the indicators of sexual function significantly decreased over time, they were not meaningfully associated with overall treatment satisfaction. Instead, a host of other factors, including psychosocial (eg, anxiety, depression, body image dissatisfaction, embarrassment), nonurological physical symptoms (pain interference, physical function, sleep disturbance, fatigue), and bladder problems, were significantly related to overall treatment satisfaction. Although this may not be surprising in other clinical oncology paradigms, the sheer surfeit of focus and attention on sexual function has overshadowed aspects of HRQoL that many men report are important to them, despite worsened sexual function outcomes.
Understanding potential treatment-related changes in HRQoL can be challenging for men when choosing providers and different therapeutic options. The increasing complexity of treatment in prostate cancer has created an opportunity to not only understand efficacy on cancer control but also focus on meaningful patient-reported outcomes. Hospitals and medical groups are increasingly aware of the importance of improving the patient care experience. Objective measures of patient satisfaction for health care providers, such as the Press-Ganey and Net Promoter score, exist to measure and improve patient experience. In prostate cancer, clinicians and large groups, including governmental agencies such as the US Preventive Services Task Force, have often focused on declines in urinary and erectile function15 without considering the full impact of prostate cancer treatment on global HRQoL. Our study was a prospective, longitudinal, self-reported examination of the impact, positive and negative, of prostate cancer treatment over a 12-month period.
Numerous studies have documented the treatment-related side effects of erectile, urinary, and bowel dysfunction in patients treated for prostate cancer, which may occur after definitive local therapies.5,16-18 The present study shows a similar impact on urinary, bowel, and erectile domains after treatment. Although erectile function scores remained lower through the course of the 12-month study, bowel and bladder domains returned to baseline by month 12. Unlike other studies, we also examined psychosocial and nonurological aspects of prostate cancer treatment. We found that there was a measurable and significant positive impact on other HRQoL measurements such as decreased anxiety. Despite a variety of declines across HRQoL domains, most patients reported that their results were largely as they had expected, and their side effects were the same or better than they had expected. No patient in the cohort reported being dissatisfied with his overall treatment, and more than 90% of patients were mostly or completely satisfied with their treatment choice. This highlights the point that while sexual and other urological domains of HRQoL are important, impairments in these areas do not necessarily reflect how many patients perceive success or satisfaction with their treatment choice. We also showed correlations between treatment satisfaction and improvement in sleep, anxiety, depression, and fatigue. It is worth noting that although there were decreases in the erectile and sexual function domains after treatment, those factors were not correlated with overall treatment satisfaction. Those factors may not routinely be assessed before, during, and after treatment for prostate cancer in most clinical encounters. However, because they were strongly associated with satisfaction with treatment outcomes in this study, identification in impairments may lead to opportunities to intervene and improve the patient experience. Therefore, important “teachable moments” may be missed (for both patients and providers) during treatment decision-making encounters if other factors beyond sexual and urological outcomes are not adequately considered and addressed. Furthermore, the results of our study may help clinicians counsel patients on their expectations for their recovery after surgery and identify particular issues related to HRQoL to pay close attention to in follow-up visits.
Strengths of our study include its prospective nature, which allowed evaluation of HRQoL outcomes at multiple time points throughout the first year after treatment. In addition, we used existing patient-reported outcome tools validated by the NIH to assess changes in HRQoL. PROMIS is an NIH-supported tool that can be leveraged in the pre- and posttreatment periods to identify patients who have impairments with HRQoL. It can provide clinicians with a unique opportunity to detect and intervene in setbacks and side effects to improve patient satisfaction and HRQoL.
Limitations of the current study include that most patients selected surgery for their treatment choice and that not all patients completed all longitudinal questionnaires, although this is expected in longitudinal studies of this nature. Although all the patients were approached and encouraged to participate, many did not participate and were not captured. In addition, not all patients completed end-of-study surveys. These factors may have biased our results because of unmeasurable factors related to nonparticipation or dropout. Our study encompassed the preoperative period up to 12 months postoperatively, which may fail to identify improvements or declines in HRQoL that may occur more than 12 months postoperatively, particularly related to continence and erectile function. The participants were enrolled by 6 surgeons, and we were not able to standardize the preoperative counseling either preoperatively or postoperatively, which may have biased our results. Finally, our study population consisted of predominantly white, married men of higher socioeconomic status; therefore, our results may not be generalizable to newly diagnosed prostate cancer patients overall.
Conclusions
By using validated self-administered questionnaires, we found that despite decreased sexual and urinary function, patients treated for prostate cancer were satisfied with their treatment choice. Correlates to higher patient satisfaction included decreased anxiety, depression, fatigue, and sleep disturbances.
More than 164,690 men are expected to be diagnosed with prostate cancer in the United States in 2018.1 Men with prostate cancer face not only stress associated with the diagnosis but also decisional conflict regarding different treatment options.2 Most men diagnosed with clinically localized prostate cancer receive 1 or more of the following treatments: radical prostatectomy, external-beam radiation therapy, and/or brachytherapy, all of which are associated with posttreatment urological or sexual side effects including bowel, urinary, or erectile dysfunction.3-5 Men who choose active surveillance may experience increased anxiety associated with the constant vigilance and monitoring of their tumor status along with the uncertainty of not definitively removing or radiating their prostate.6 In addition to direct functional limitations of sexual and urological side effects, treatment can also lead to secondary psychosocial effects, including depression, self-blame, embarrassment, guilt, lower masculine self-esteem, increased reticence to participate socially or engage in sexual activity, and relationship distress.7-9 Therefore, health-related quality of life (HRQoL) and treatment satisfaction are important for this population.
Urological and sexual side effects of prostate cancer treatments are often a primary focus during treatment decision making between patients and providers. However, little prospective empirical data exist regarding the role of HRQoL and other nonurological physical and psychosocial outcomes on overall treatment satisfaction. The purpose of this study was to prospectively evaluate the role of both urological and nonurological outcomes on overall treatment satisfaction in men diagnosed with prostate cancer. We hypothesize that such an understanding can help describe changes in physical and psychosocial factors that are important to men beyond traditional urological outcomes, including their association with overall treatment satisfaction.
Methods
This was a prospective longitudinal assessment of patients from the Department of Urology at Northwestern University’s Feinberg School of Medicine in Chicago. Patients were eligible if they met the following inclusion criteria: they had been diagnosed with clinically localized or locally advanced prostate cancer; they had not yet received a primary treatment (eg, surgery, radiation, active surveillance) before their baseline assessment; they were 18 years or older; and they were able to read, write, speak, and understand English. Patients were excluded if they had a physical debilitation that would make participation not feasible or would create undue hardship, or if they had a history of diagnosed severe mental illness or hospitalization for chronic psychiatric reasons, as identified by referring physicians.
Eligible participants were approached before their treatment decision (if any). Patient enrollment occurred in 2 ways. For patients invited to participate during their clinic visit, the research assistant explained the study and obtained written informed consent for interested patients. A unique user identification and password was created for each patient, and they practiced using the touch screen computer while the research assistant observed and provided guidance as needed. When the patients were ready to start their pretreatment online interview, they completed the questionnaires by themselves. For patients who were invited to participate but were not scheduled to return in the foreseeable future, enrollment was carried out differently. In those cases, participating physicians contacted eligible patients who were not scheduled for a visit and informed them of the study opportunity. Interested patients were contacted by the research assistant who provided them with the study website address, which directed them to the online consent form. After a patient had completed the consent form, he was prompted to self-register. He received a unique user identification and password that could be used to complete the baseline assessment and subsequent assessments. However, for interested patients who did not have access to a computer or Internet connection, the research assistant provided them with paper consent forms and paper versions of all study assessments. After participants had completed the baseline assessment, the research assistant provided them with a written schedule of future assessments, which were expected to occur at 1 month posttreatment, 3 months posttreatment, 6 months posttreatment, and 12 months posttreatment.
For all follow-up appointments, participants could complete assessments either at clinic visits or from home using a secure online assessment platform called Assessment Center.10 The research assistant used a patient log to track participants and their progress in the study, which included study number, patient name (or initials), registration date, date of birth, sex, and timeline of completed or future assessments. The research assistant called or emailed participants (depending on patient preference) about a week before each of their follow-up assessments to facilitate adherence. If the participant did not log into the system by the target day, the research assistant contacted him the following day (target day +1) with a phone or email reminder to log into the system and complete the assessments. If the participant did not log in by midnight 1 day after the target day, the research assistant attempted to contact him one last time (target day +2) with either a reminder to log into the system or to ascertain his status that might be related to his noncompletion. Overall, a participant was called or e-mailed 1 to 3 times to remind him of his assessment. If he was unresponsive after 3 attempts, he was recorded as having withdrawn for an unknown reason.
At baseline and each follow-up time point, study participants completed a battery of patient-reported outcome measures, with most coming from the Patient-Reported Outcomes Measurement Information System (PROMIS)11 and the Surgical Outcomes Measurement System (SOMS).12 PROMIS is a National Institutes of Health (NIH) funded measurement system that has helped standardize and improve self-reported assessment of health status, symptoms, side effects, and different aspects of HRQoL, including physical, emotional, cognitive, and social health. SOMS is a suite of patient-reported outcome measures assessing important aspects of HRQoL after surgery. It was developed with feedback from surgeons, postoperative patients, and surgical nurses. PROMIS items were directly incorporated into numerous SOMS measures to facilitate easier comparisons and score crosswalks across measures and patient populations. In addition to PROMIS and SOMS measures, we also administered several well-known instruments of urological and sexual function, including the International Index of Erectile Function (IIEF) and American Urological Association Symptom Score Index (AUASS).13,14
Outcome measures were compared across sociodemographic and clinical variables at each time point using t tests for numerical variables (age) and with chi-square or Fisher exact tests for categorical variables; those variables with significant differences were used as covariates in statistical models. To examine differences in patient-reported scores over time, we used repeated measures analysis of covariance with general linear modeling methods. We used Pearson correlation coefficients to evaluate for correlations between quality-of-life outcomes and treatment satisfaction.
Not all participants completed each of the follow-up surveys, and reasons for dropout were prospectively documented. Most participants elected surgical resection as their primary treatment compared with the fewer than 10% of patients who chose radiation or chemotherapy as their primary treatment and about 20% of men who chose active surveillance after their initial diagnosis. Therefore, our analysis focused on patients who elected surgical resection. For comparison purposes, we included the HRQoL results from active surveillance patients.
Results
A total of 105 patients diagnosed with prostate cancer were enrolled in the study. Response rates decreased throughout the study (n = 75 at 1 month; n = 71 at 3 months; n = 64 at 6 months; n = 54 at 12 months). Sociodemographic and clinical characteristics of participants are shown in Table 1. The mean change from pretreatment (baseline) scores for each measure in patients treated with surgery is shown in Table 2, and the mean change from pretreatment scores in patients who elected active surveillance is shown in Table 3 (in both tables, a negative score denotes worsened function, and a positive change denotes improvement).
After surgery, patients reported significantly lower erectile function and sexual satisfaction scores. These included statistically significant decreases for IIEF Erectile Function, IIEF Overall Satisfaction, PROMIS Sexual Satisfaction, PROMIS Sexual Interest, and PROMIS Orgasm. In patients treated with surgery, there were significant improvements in anxiety observed for patients at each follow-up time, whereas significantly worse bladder problems were observed on SOMS Bladder at 1 and 3 months but returned to baseline by 12 months after surgery. AUASS was worse at 1 month but significantly improved at 6 and 12 months. Fatigue scores significantly worsened at 1 month but were no longer significant at 6 and 12 months. Physical Function was worsened at 1 month but not throughout the rest of the study. Bowel Problems (SOMS) were significantly worse at 1 month, but changes became nonsignificant on subsequent assessments. The only 2 domains that did not demonstrate any significant changes o
In active surveillance patients, sexual function domains were generally unchanged over the course of the study. However, unlike treated patients, there was no significant improvement in anxiety, depression, pain, fatigue, or sleep. In fact, most of these domains demonstrated worsened functioning, although these were not statistically significant. Urinary domains generally remained unchanged.
Pearson correlation coefficients between HRQoL measures and overall treatment satisfaction (assessed by the question, Are you satisfied with the results of your operation?) at each follow-up time point in patients treated with surgery are shown in Table 4. Relations between treatment satisfaction and sexual outcomes were generally statistically insignificant (r, .08-.56). However, sleep disturbance, depression, pain interference, fatigue, embarrassment, and bladder problems all demonstrated statistically significant positive associations with treatment satisfaction, with coefficients ranging from small to medium in magnitude (r, .32-.61). Other outcomes such as anxiety, physical function, and bowel problems demonstrated small to medium statistically significant associations with treatment satisfaction (r, .04-.60) but not at every time point. We performed t tests to examine treatment satisfaction in patients with detectable initial posttreatment prostate-specific antigen (PSA; >0.01 ng/mL). We found no difference in treatment satisfaction between patients with detectable PSA values and those with undetectable PSA at each time point.
When the patients were asked, Compared with what you expected, how do you rate the results of your operation?, most of those treated with surgery reported that the results of their operation were better than they had expected (Figure 1A; p. e137). More than 75% of the patients had results that were as expected or better than expected. When asked, Compared with what you expected, how do you rate your side effects of the operation?, almost 70% of patients reported side effects no worse than expected (Figure 1B). When asked, Are you satisfied with the results of your operation?, most patients reported that overall, they were satisfied with the results of their operation (Figure 1C).
At 12 months, none of the patients reported overall dissatisfaction with their treatment choice. More than 90% of patients were mostly or completely satisfied with the results of their operation.
Discussion
This prospective study assessed the HRQoL from pretreatment through 12 months posttreatment in men diagnosed with clinically localized prostate cancer that had been treated with surgery. Although the indicators of sexual function significantly decreased over time, they were not meaningfully associated with overall treatment satisfaction. Instead, a host of other factors, including psychosocial (eg, anxiety, depression, body image dissatisfaction, embarrassment), nonurological physical symptoms (pain interference, physical function, sleep disturbance, fatigue), and bladder problems, were significantly related to overall treatment satisfaction. Although this may not be surprising in other clinical oncology paradigms, the sheer surfeit of focus and attention on sexual function has overshadowed aspects of HRQoL that many men report are important to them, despite worsened sexual function outcomes.
Understanding potential treatment-related changes in HRQoL can be challenging for men when choosing providers and different therapeutic options. The increasing complexity of treatment in prostate cancer has created an opportunity to not only understand efficacy on cancer control but also focus on meaningful patient-reported outcomes. Hospitals and medical groups are increasingly aware of the importance of improving the patient care experience. Objective measures of patient satisfaction for health care providers, such as the Press-Ganey and Net Promoter score, exist to measure and improve patient experience. In prostate cancer, clinicians and large groups, including governmental agencies such as the US Preventive Services Task Force, have often focused on declines in urinary and erectile function15 without considering the full impact of prostate cancer treatment on global HRQoL. Our study was a prospective, longitudinal, self-reported examination of the impact, positive and negative, of prostate cancer treatment over a 12-month period.
Numerous studies have documented the treatment-related side effects of erectile, urinary, and bowel dysfunction in patients treated for prostate cancer, which may occur after definitive local therapies.5,16-18 The present study shows a similar impact on urinary, bowel, and erectile domains after treatment. Although erectile function scores remained lower through the course of the 12-month study, bowel and bladder domains returned to baseline by month 12. Unlike other studies, we also examined psychosocial and nonurological aspects of prostate cancer treatment. We found that there was a measurable and significant positive impact on other HRQoL measurements such as decreased anxiety. Despite a variety of declines across HRQoL domains, most patients reported that their results were largely as they had expected, and their side effects were the same or better than they had expected. No patient in the cohort reported being dissatisfied with his overall treatment, and more than 90% of patients were mostly or completely satisfied with their treatment choice. This highlights the point that while sexual and other urological domains of HRQoL are important, impairments in these areas do not necessarily reflect how many patients perceive success or satisfaction with their treatment choice. We also showed correlations between treatment satisfaction and improvement in sleep, anxiety, depression, and fatigue. It is worth noting that although there were decreases in the erectile and sexual function domains after treatment, those factors were not correlated with overall treatment satisfaction. Those factors may not routinely be assessed before, during, and after treatment for prostate cancer in most clinical encounters. However, because they were strongly associated with satisfaction with treatment outcomes in this study, identification in impairments may lead to opportunities to intervene and improve the patient experience. Therefore, important “teachable moments” may be missed (for both patients and providers) during treatment decision-making encounters if other factors beyond sexual and urological outcomes are not adequately considered and addressed. Furthermore, the results of our study may help clinicians counsel patients on their expectations for their recovery after surgery and identify particular issues related to HRQoL to pay close attention to in follow-up visits.
Strengths of our study include its prospective nature, which allowed evaluation of HRQoL outcomes at multiple time points throughout the first year after treatment. In addition, we used existing patient-reported outcome tools validated by the NIH to assess changes in HRQoL. PROMIS is an NIH-supported tool that can be leveraged in the pre- and posttreatment periods to identify patients who have impairments with HRQoL. It can provide clinicians with a unique opportunity to detect and intervene in setbacks and side effects to improve patient satisfaction and HRQoL.
Limitations of the current study include that most patients selected surgery for their treatment choice and that not all patients completed all longitudinal questionnaires, although this is expected in longitudinal studies of this nature. Although all the patients were approached and encouraged to participate, many did not participate and were not captured. In addition, not all patients completed end-of-study surveys. These factors may have biased our results because of unmeasurable factors related to nonparticipation or dropout. Our study encompassed the preoperative period up to 12 months postoperatively, which may fail to identify improvements or declines in HRQoL that may occur more than 12 months postoperatively, particularly related to continence and erectile function. The participants were enrolled by 6 surgeons, and we were not able to standardize the preoperative counseling either preoperatively or postoperatively, which may have biased our results. Finally, our study population consisted of predominantly white, married men of higher socioeconomic status; therefore, our results may not be generalizable to newly diagnosed prostate cancer patients overall.
Conclusions
By using validated self-administered questionnaires, we found that despite decreased sexual and urinary function, patients treated for prostate cancer were satisfied with their treatment choice. Correlates to higher patient satisfaction included decreased anxiety, depression, fatigue, and sleep disturbances.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7‐30.
2. Berry DL, Ellis WJ, Woods NF, Schwien C, Mullen KH, Yang C. Treatment decision-making by men with localized prostate cancer: the influence of personal factors. Urol Oncol. 2003;21(2):93-100.
3. Dubbelman YD, Dohle GR, Schröder FH. Sexual function before and after radical retropubic prostatectomy: a systematic review of prognostic indicators for a successful outcome. Eur Urol. 2006;50(4):711-718; discussion 718-720.
4. McCullough AR. Sexual dysfunction after radical prostatectomy. Rev Urol. 2005;7(2 suppl):S3-S10.
5. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250-1261.
6. Latini DM, Hart SL, Knight SJ, et al. The relationship between anxiety and time to treatment for patients with prostate cancer on surveillance. J Urol. 2007;178(3, pt 1):826-831; discussion 831-832.
7. Meyer JP, Gillatt DA, Lockyer R, Macdonagh R. The effect of erectile dysfunction on the quality of life of men after radical prostatectomy. BJU Int. 2003;92(9):929-931.
8. Casey RG, Corcoran NM, Goldenberg SL. Quality of life issues in men undergoing androgen deprivation therapy: a review. Asian J Androl. 2012;14(2):226-231.
9. Segrin C, Badger TA, Harrington J. Interdependent psychological quality of life in dyads adjusting to prostate cancer. Health Psychol. 2012;31(1):70-79.
10. Gershon RC, Rothrock N, Hanrahan R, Bass M, Cella D. The use of PROMIS and assessment center to deliver patient-reported outcome measures in clinical research. J Appl Meas. 2010;11(3):304-314.
11. Cella D, Yount S, Rothrock N, et al. The patient-reported outcomes measurement information system (PROMIS): progress of an NIH roadmap cooperative group during its first two years. Med Care. 2007;45(5 suppl 1):S3-S11.
12. Zapf M, Denham W, Barrera E, et al. Patient-centered outcomes after laparoscopic cholecystectomy. Surg Endosc. 2013;27(12):4491-4498.
13. Barry MJ, Fowler FJ Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549-1557; discussion 1564.
14. Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822-830.
15. United States Preventive Services Task Force. Final update summary: prostate cancer: screening. http:// www.uspreventiveservicestaskforce.org/Page/ Document/UpdateSummaryFinal/prostate-cancer-screening. Updated July 2015. Accessed April 14, 2017
16. Litwin MS, Gore JL, Kwan L, et al. Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer. Cancer. 2007;109(11):2239-2247.
17. Miwa S, Mizokami A, Konaka H, et al. Prospective longitudinal comparative study of health-related quality of life and treatment satisfaction in patients treated with hormone therapy, radical retropubic prostatectomy, and high or low dose rate brachytherapy for prostate cancer. Prostate Int. 2013;1(3):117-124.
18. Miller DC, Sanda MG, Dunn RL et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23(12):2772-2780.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7‐30.
2. Berry DL, Ellis WJ, Woods NF, Schwien C, Mullen KH, Yang C. Treatment decision-making by men with localized prostate cancer: the influence of personal factors. Urol Oncol. 2003;21(2):93-100.
3. Dubbelman YD, Dohle GR, Schröder FH. Sexual function before and after radical retropubic prostatectomy: a systematic review of prognostic indicators for a successful outcome. Eur Urol. 2006;50(4):711-718; discussion 718-720.
4. McCullough AR. Sexual dysfunction after radical prostatectomy. Rev Urol. 2005;7(2 suppl):S3-S10.
5. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250-1261.
6. Latini DM, Hart SL, Knight SJ, et al. The relationship between anxiety and time to treatment for patients with prostate cancer on surveillance. J Urol. 2007;178(3, pt 1):826-831; discussion 831-832.
7. Meyer JP, Gillatt DA, Lockyer R, Macdonagh R. The effect of erectile dysfunction on the quality of life of men after radical prostatectomy. BJU Int. 2003;92(9):929-931.
8. Casey RG, Corcoran NM, Goldenberg SL. Quality of life issues in men undergoing androgen deprivation therapy: a review. Asian J Androl. 2012;14(2):226-231.
9. Segrin C, Badger TA, Harrington J. Interdependent psychological quality of life in dyads adjusting to prostate cancer. Health Psychol. 2012;31(1):70-79.
10. Gershon RC, Rothrock N, Hanrahan R, Bass M, Cella D. The use of PROMIS and assessment center to deliver patient-reported outcome measures in clinical research. J Appl Meas. 2010;11(3):304-314.
11. Cella D, Yount S, Rothrock N, et al. The patient-reported outcomes measurement information system (PROMIS): progress of an NIH roadmap cooperative group during its first two years. Med Care. 2007;45(5 suppl 1):S3-S11.
12. Zapf M, Denham W, Barrera E, et al. Patient-centered outcomes after laparoscopic cholecystectomy. Surg Endosc. 2013;27(12):4491-4498.
13. Barry MJ, Fowler FJ Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549-1557; discussion 1564.
14. Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822-830.
15. United States Preventive Services Task Force. Final update summary: prostate cancer: screening. http:// www.uspreventiveservicestaskforce.org/Page/ Document/UpdateSummaryFinal/prostate-cancer-screening. Updated July 2015. Accessed April 14, 2017
16. Litwin MS, Gore JL, Kwan L, et al. Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer. Cancer. 2007;109(11):2239-2247.
17. Miwa S, Mizokami A, Konaka H, et al. Prospective longitudinal comparative study of health-related quality of life and treatment satisfaction in patients treated with hormone therapy, radical retropubic prostatectomy, and high or low dose rate brachytherapy for prostate cancer. Prostate Int. 2013;1(3):117-124.
18. Miller DC, Sanda MG, Dunn RL et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23(12):2772-2780.
ACIP votes to recommend new strains for the 2018-2019 flu vaccine
Thirteen members of the Advisory Committee on Immunization Practices (ACIP) voted to approve the influenza vaccine recommendations for 2018-2019, while one member abstained from voting at the summer ACIP meeting.
The 2018-2019 recommendation maintains the core recommendation that influenza vaccines should be administered to all persons 6 months or older who have no contraindications.
FluMist Quadrivalent (LAIV4) also is being updated for the 2018-2019 season. At the February meeting of ACIP, the committee approved language that providers may provide any licensed, age-appropriate influenza vaccine, and LAIV4 is considered in this set of vaccine options.
Prior to this approval, there was a discussion of the safety of the 2017-2018 vaccine. For many of the available vaccines, there were no new safety concerns raised from reports during the flu season. Monitoring during the 2018-2019 will yield more safety monitoring data concerning pregnancy and influenza vaccinations and anaphylaxis in persons with an egg allergy.
The committee’s recommendations must be approved by the Centers for Disease Control and Prevention’s director before they are considered official recommendations.
Thirteen members of the Advisory Committee on Immunization Practices (ACIP) voted to approve the influenza vaccine recommendations for 2018-2019, while one member abstained from voting at the summer ACIP meeting.
The 2018-2019 recommendation maintains the core recommendation that influenza vaccines should be administered to all persons 6 months or older who have no contraindications.
FluMist Quadrivalent (LAIV4) also is being updated for the 2018-2019 season. At the February meeting of ACIP, the committee approved language that providers may provide any licensed, age-appropriate influenza vaccine, and LAIV4 is considered in this set of vaccine options.
Prior to this approval, there was a discussion of the safety of the 2017-2018 vaccine. For many of the available vaccines, there were no new safety concerns raised from reports during the flu season. Monitoring during the 2018-2019 will yield more safety monitoring data concerning pregnancy and influenza vaccinations and anaphylaxis in persons with an egg allergy.
The committee’s recommendations must be approved by the Centers for Disease Control and Prevention’s director before they are considered official recommendations.
Thirteen members of the Advisory Committee on Immunization Practices (ACIP) voted to approve the influenza vaccine recommendations for 2018-2019, while one member abstained from voting at the summer ACIP meeting.
The 2018-2019 recommendation maintains the core recommendation that influenza vaccines should be administered to all persons 6 months or older who have no contraindications.
FluMist Quadrivalent (LAIV4) also is being updated for the 2018-2019 season. At the February meeting of ACIP, the committee approved language that providers may provide any licensed, age-appropriate influenza vaccine, and LAIV4 is considered in this set of vaccine options.
Prior to this approval, there was a discussion of the safety of the 2017-2018 vaccine. For many of the available vaccines, there were no new safety concerns raised from reports during the flu season. Monitoring during the 2018-2019 will yield more safety monitoring data concerning pregnancy and influenza vaccinations and anaphylaxis in persons with an egg allergy.
The committee’s recommendations must be approved by the Centers for Disease Control and Prevention’s director before they are considered official recommendations.
REPORTING FROM AN ACIP MEETING
Preview of ADA/EASD statement on hyperglycemia
A move toward more individualized treatment of hyperglycemia is coming in the next American Diabetes Association/European Association for the Study of Diabetes Consensus Report, according to John B. Buse, MD, PhD, cochair of the committee writing the new consensus statement.
He will present a draft of the statement on the management of hyperglycemia in type 2 diabetes at the ADA’s annual scientific sessions in Orlando.
When finalized – after revisions based on comments and feedback from diabetes care providers – clinical researchers, patient groups, payers, regulators, and stakeholders – the statement will update the last revision, issued in 2015.
“We are taking a new look at hyperglycemia based on the many studies conducted since 2014, particularly the cardiovascular outcomes trials,” Dr. Buse, the Verne S. Caviness Distinguished Professor in the division of endocrinology and metabolism and chief of endocrinology at the University of North Carolina, Chapel Hill, said in a statement.
But it’s a good bet that ADA scientific sessions attendees will see a move toward more specific recommendations based on patient characteristics and fewer one-size-fits-all recommendations. Specific characteristics like obesity, cardiovascular disease, and chronic kidney disease will likely be addressed in the new consensus statement.
One aspect of patient care that will see more attention in the ultimate statement is personalized care. “We will certainly highlight the need to individualize all aspects of care in a patient-centered way, taking into account both specific patient attributes and preferences,” Dr. Buse said.
The draft statement will be presented on Tuesday, June 26, at 8:00 a.m., so it may be worth staying for that last day of the meeting.
The final draft of the new statement will be released in October at the EASD annual meeting in Berlin, noted Dr. Buse, also director of the diabetes center at the university.
A move toward more individualized treatment of hyperglycemia is coming in the next American Diabetes Association/European Association for the Study of Diabetes Consensus Report, according to John B. Buse, MD, PhD, cochair of the committee writing the new consensus statement.
He will present a draft of the statement on the management of hyperglycemia in type 2 diabetes at the ADA’s annual scientific sessions in Orlando.
When finalized – after revisions based on comments and feedback from diabetes care providers – clinical researchers, patient groups, payers, regulators, and stakeholders – the statement will update the last revision, issued in 2015.
“We are taking a new look at hyperglycemia based on the many studies conducted since 2014, particularly the cardiovascular outcomes trials,” Dr. Buse, the Verne S. Caviness Distinguished Professor in the division of endocrinology and metabolism and chief of endocrinology at the University of North Carolina, Chapel Hill, said in a statement.
But it’s a good bet that ADA scientific sessions attendees will see a move toward more specific recommendations based on patient characteristics and fewer one-size-fits-all recommendations. Specific characteristics like obesity, cardiovascular disease, and chronic kidney disease will likely be addressed in the new consensus statement.
One aspect of patient care that will see more attention in the ultimate statement is personalized care. “We will certainly highlight the need to individualize all aspects of care in a patient-centered way, taking into account both specific patient attributes and preferences,” Dr. Buse said.
The draft statement will be presented on Tuesday, June 26, at 8:00 a.m., so it may be worth staying for that last day of the meeting.
The final draft of the new statement will be released in October at the EASD annual meeting in Berlin, noted Dr. Buse, also director of the diabetes center at the university.
A move toward more individualized treatment of hyperglycemia is coming in the next American Diabetes Association/European Association for the Study of Diabetes Consensus Report, according to John B. Buse, MD, PhD, cochair of the committee writing the new consensus statement.
He will present a draft of the statement on the management of hyperglycemia in type 2 diabetes at the ADA’s annual scientific sessions in Orlando.
When finalized – after revisions based on comments and feedback from diabetes care providers – clinical researchers, patient groups, payers, regulators, and stakeholders – the statement will update the last revision, issued in 2015.
“We are taking a new look at hyperglycemia based on the many studies conducted since 2014, particularly the cardiovascular outcomes trials,” Dr. Buse, the Verne S. Caviness Distinguished Professor in the division of endocrinology and metabolism and chief of endocrinology at the University of North Carolina, Chapel Hill, said in a statement.
But it’s a good bet that ADA scientific sessions attendees will see a move toward more specific recommendations based on patient characteristics and fewer one-size-fits-all recommendations. Specific characteristics like obesity, cardiovascular disease, and chronic kidney disease will likely be addressed in the new consensus statement.
One aspect of patient care that will see more attention in the ultimate statement is personalized care. “We will certainly highlight the need to individualize all aspects of care in a patient-centered way, taking into account both specific patient attributes and preferences,” Dr. Buse said.
The draft statement will be presented on Tuesday, June 26, at 8:00 a.m., so it may be worth staying for that last day of the meeting.
The final draft of the new statement will be released in October at the EASD annual meeting in Berlin, noted Dr. Buse, also director of the diabetes center at the university.
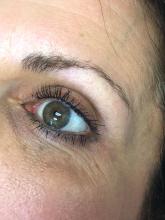
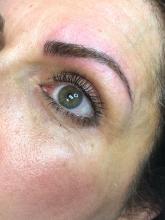
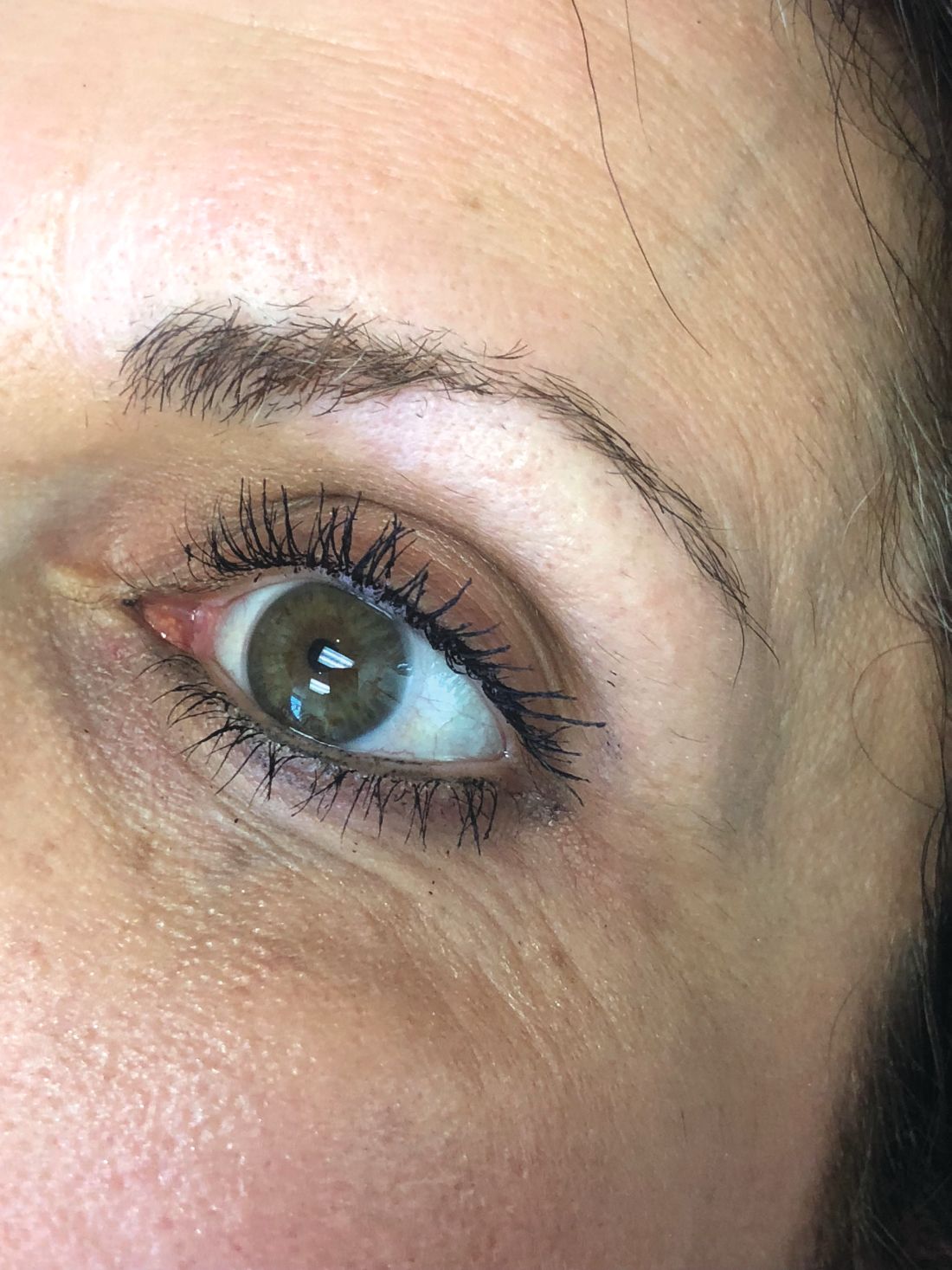
The magic of microblading
The use of permanent cosmetics dates back thousands of years in history. and has rapidly become one of the most popular cosmetic procedures in the United States. However, it has not completely replaced traditional eyebrow micropigmentation techniques: Many people may not be candidates for microblading because of how the pigment is manually deposited in the skin through tiny “tears” in the skin with this procedure.
The use of microblading has increased exponentially since 2015, as reflected by the millions of searches on popular social media sites. With the increase in the popularity and volume of tattoo artists performing these procedures, there has also been an increase in side effects and complications from microblading provided by poorly trained and unlicensed “artists,” a problem facilitated by the absence of adequate training requirements and/or regulatory oversight in many states.
Microblading is a revolutionary technique that can transform the lives of patients with hypotrichosis of the eyebrows, trichotillomania, eyebrow loss due to internal disease (such as thyroid disease), chemotherapy-induced eyebrow loss, or alopecia – or simply those seeking it for cosmetic improvement. The art of shaping the eyebrow depends on the natural growth of the brow (if any), facial symmetry, and meticulous measurement and mapping of the brow position based on facial landmarks and bone structure. The color of pigment selection is based on Fitzpatrick skin type and skin color undertones.
While dermatologists usually do not perform microblading, we may see patients with these complications. Practitioners treating patients who have had eyebrow microblading should also be aware of how to prevent premature fading of the eyebrow tattoo pigment. Tattooed eyebrows should be covered with petroleum jelly prior to the use of alpha hydroxy acids, vitamin C, chemical peels, hydroquinone, or retinols because these preparations can fade the pigment rapidly even if applied far from the microblading site. Any UV exposure, heat (such as steam from a facial), LED light exposure, or radio frequency can fade the pigment and exacerbate postinflammatory hyperpigmentation. Patients who have a history of hypertrophic scarring or keloids or are using isotretinoin concurrently should avoid microblading entirely. Resurfacing lasers and intense pulsed-light lasers should be used with caution as these aesthetic procedures will cause fading of the eyebrow pigment even if applied at a considerable distance from the eyebrow. Microbladed eyebrows should be covered with 20% zinc oxide paste prior to the use of any intense pulsed-light or resurfacing lasers.
The pigment used in eyebrow colors also may be composed of a mixture of iron oxide pigments, which should not be removed with traditional Q-switched lasers, with which not only is there potential for the pigment to darken but also postinflammatory hyper- or hypopigmentation to occur as well. Hairs can be singed, and the light absorbed by the pigment chromophore in the hair follicle can permanently damage the follicle, leading to hair loss in the area.
Despite the absolute precision and aggressive safety precautions needed for microblading, there are wide state-to-state variations in training and regulatory oversight. Infectious diseases, poor treatment outcomes, and unsterile conditions are just a few of the horrific consequences of unlicensed and untrained tattoo artists. Regulations should be imposed in every state to protect consumers and prevent serious medical complications related to microblading.
Like other cosmetic treatments, cheaper is never better.
Dr. Talakoub and Dr. Wesley and are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. They had no relevant disclosures. Write to them at [email protected]. This column was written with the help and professional expertise of Emily Joy, a cosmetic tattoo artist and the founder of Dollistic in McLean.
The use of permanent cosmetics dates back thousands of years in history. and has rapidly become one of the most popular cosmetic procedures in the United States. However, it has not completely replaced traditional eyebrow micropigmentation techniques: Many people may not be candidates for microblading because of how the pigment is manually deposited in the skin through tiny “tears” in the skin with this procedure.
The use of microblading has increased exponentially since 2015, as reflected by the millions of searches on popular social media sites. With the increase in the popularity and volume of tattoo artists performing these procedures, there has also been an increase in side effects and complications from microblading provided by poorly trained and unlicensed “artists,” a problem facilitated by the absence of adequate training requirements and/or regulatory oversight in many states.
Microblading is a revolutionary technique that can transform the lives of patients with hypotrichosis of the eyebrows, trichotillomania, eyebrow loss due to internal disease (such as thyroid disease), chemotherapy-induced eyebrow loss, or alopecia – or simply those seeking it for cosmetic improvement. The art of shaping the eyebrow depends on the natural growth of the brow (if any), facial symmetry, and meticulous measurement and mapping of the brow position based on facial landmarks and bone structure. The color of pigment selection is based on Fitzpatrick skin type and skin color undertones.
While dermatologists usually do not perform microblading, we may see patients with these complications. Practitioners treating patients who have had eyebrow microblading should also be aware of how to prevent premature fading of the eyebrow tattoo pigment. Tattooed eyebrows should be covered with petroleum jelly prior to the use of alpha hydroxy acids, vitamin C, chemical peels, hydroquinone, or retinols because these preparations can fade the pigment rapidly even if applied far from the microblading site. Any UV exposure, heat (such as steam from a facial), LED light exposure, or radio frequency can fade the pigment and exacerbate postinflammatory hyperpigmentation. Patients who have a history of hypertrophic scarring or keloids or are using isotretinoin concurrently should avoid microblading entirely. Resurfacing lasers and intense pulsed-light lasers should be used with caution as these aesthetic procedures will cause fading of the eyebrow pigment even if applied at a considerable distance from the eyebrow. Microbladed eyebrows should be covered with 20% zinc oxide paste prior to the use of any intense pulsed-light or resurfacing lasers.
The pigment used in eyebrow colors also may be composed of a mixture of iron oxide pigments, which should not be removed with traditional Q-switched lasers, with which not only is there potential for the pigment to darken but also postinflammatory hyper- or hypopigmentation to occur as well. Hairs can be singed, and the light absorbed by the pigment chromophore in the hair follicle can permanently damage the follicle, leading to hair loss in the area.
Despite the absolute precision and aggressive safety precautions needed for microblading, there are wide state-to-state variations in training and regulatory oversight. Infectious diseases, poor treatment outcomes, and unsterile conditions are just a few of the horrific consequences of unlicensed and untrained tattoo artists. Regulations should be imposed in every state to protect consumers and prevent serious medical complications related to microblading.
Like other cosmetic treatments, cheaper is never better.
Dr. Talakoub and Dr. Wesley and are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. They had no relevant disclosures. Write to them at [email protected]. This column was written with the help and professional expertise of Emily Joy, a cosmetic tattoo artist and the founder of Dollistic in McLean.
The use of permanent cosmetics dates back thousands of years in history. and has rapidly become one of the most popular cosmetic procedures in the United States. However, it has not completely replaced traditional eyebrow micropigmentation techniques: Many people may not be candidates for microblading because of how the pigment is manually deposited in the skin through tiny “tears” in the skin with this procedure.
The use of microblading has increased exponentially since 2015, as reflected by the millions of searches on popular social media sites. With the increase in the popularity and volume of tattoo artists performing these procedures, there has also been an increase in side effects and complications from microblading provided by poorly trained and unlicensed “artists,” a problem facilitated by the absence of adequate training requirements and/or regulatory oversight in many states.
Microblading is a revolutionary technique that can transform the lives of patients with hypotrichosis of the eyebrows, trichotillomania, eyebrow loss due to internal disease (such as thyroid disease), chemotherapy-induced eyebrow loss, or alopecia – or simply those seeking it for cosmetic improvement. The art of shaping the eyebrow depends on the natural growth of the brow (if any), facial symmetry, and meticulous measurement and mapping of the brow position based on facial landmarks and bone structure. The color of pigment selection is based on Fitzpatrick skin type and skin color undertones.
While dermatologists usually do not perform microblading, we may see patients with these complications. Practitioners treating patients who have had eyebrow microblading should also be aware of how to prevent premature fading of the eyebrow tattoo pigment. Tattooed eyebrows should be covered with petroleum jelly prior to the use of alpha hydroxy acids, vitamin C, chemical peels, hydroquinone, or retinols because these preparations can fade the pigment rapidly even if applied far from the microblading site. Any UV exposure, heat (such as steam from a facial), LED light exposure, or radio frequency can fade the pigment and exacerbate postinflammatory hyperpigmentation. Patients who have a history of hypertrophic scarring or keloids or are using isotretinoin concurrently should avoid microblading entirely. Resurfacing lasers and intense pulsed-light lasers should be used with caution as these aesthetic procedures will cause fading of the eyebrow pigment even if applied at a considerable distance from the eyebrow. Microbladed eyebrows should be covered with 20% zinc oxide paste prior to the use of any intense pulsed-light or resurfacing lasers.
The pigment used in eyebrow colors also may be composed of a mixture of iron oxide pigments, which should not be removed with traditional Q-switched lasers, with which not only is there potential for the pigment to darken but also postinflammatory hyper- or hypopigmentation to occur as well. Hairs can be singed, and the light absorbed by the pigment chromophore in the hair follicle can permanently damage the follicle, leading to hair loss in the area.
Despite the absolute precision and aggressive safety precautions needed for microblading, there are wide state-to-state variations in training and regulatory oversight. Infectious diseases, poor treatment outcomes, and unsterile conditions are just a few of the horrific consequences of unlicensed and untrained tattoo artists. Regulations should be imposed in every state to protect consumers and prevent serious medical complications related to microblading.
Like other cosmetic treatments, cheaper is never better.
Dr. Talakoub and Dr. Wesley and are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. They had no relevant disclosures. Write to them at [email protected]. This column was written with the help and professional expertise of Emily Joy, a cosmetic tattoo artist and the founder of Dollistic in McLean.
Effective Management and Counseling of Patients with Recurrent Bacterial Vaginosis
Click Here to Read Supplement.
Bacterial vaginosis (BV) affects women worldwide and recurrent BV can be a frustrating condition for both patients and providers. In this new supplement, expert Khady Diouf, MD, discusses her treatment approach and suggested counseling for patients with recurrent BV.
Click Here to Read Supplement.
Bacterial vaginosis (BV) affects women worldwide and recurrent BV can be a frustrating condition for both patients and providers. In this new supplement, expert Khady Diouf, MD, discusses her treatment approach and suggested counseling for patients with recurrent BV.
Click Here to Read Supplement.
Bacterial vaginosis (BV) affects women worldwide and recurrent BV can be a frustrating condition for both patients and providers. In this new supplement, expert Khady Diouf, MD, discusses her treatment approach and suggested counseling for patients with recurrent BV.
Preterm infant GER is a normal phenomenon
Treatment of gastroesophageal reflux (GER) in preterm infants with traditional treatments, such as body positioning, and newer treatments with pharmacologic agents appear to be ineffective, and pharmacologic agents in particular may cause significant harm, according to a clinical report by the American Academy of Pediatrics Committee on Fetus and Newborn.
“I think that probably the most important point for any physician, including neonatologists, is that the committee concluded on the basis of the evidence that Eric Eichenwald, MD, lead author of the committee’s clinical report and chief of neonatology at Children’s Hospital of Philadelphia, said in an interview. “So really the bottom line of the clinical report is watchful waiting, conservative management, and patience is the most important approach to a baby that you think is suffering from reflux.”
Pharmacologic management
The committee members focused on four categories of pharmacologic interventions in their report in Pediatrics.
Prokinetic (promotility) agents, such as metoclopramide, domperidone, and erythromycin, are widely used in treating symptoms of GER in older infants and appear to improve gastric emptying, reduce regurgitation, and enhance lower esophageal sphincter tone, but they do not appear to reduce GER symptoms in preterm infants. In addition to not being effective in these infants, there is also a potential for significant adverse events, including cardiac arrhythmia and neurologic side effects. Another common pharmacologic treatment is the use of sodium alginate in combination with sodium bicarbonate. In the presence of gastric acid, sodium alginate precipitates as a gel that forms a physical barrier that protects the gastric mucosa. When sodium bicarbonate is added, a carbon dioxide foam forms that is less harmful to the esophagus than GER-related fluids. While this combination treatment has reduced the number of acidic GER exposures and esophageal acid exposure in preterm infants in small studies, the long-term safety has not been evaluated in this populations.
Histamine2 (H2) blockers, like famotidine and ranitidine, also are commonly prescribed to treat preterm infant gastroesophageal reflux. H2 blockers compete with H2 for the histamine receptors of the parietal cells, which causes a decrease in hydrochloric acid and a subsequent increase in intragastric pH. These are often prescribed on the premise that GER symptoms are secondary to acid reflux in the lower esophagus, but there is no research on the efficacy of H2 blockers on the symptom profile of GER in preterm infants. This class of drugs also has been linked with an increased risk of necrotizing enterocolitis and a higher incidence of late-onset infections and death. This is thought to be caused by alteration of the intestinal microbiome, according to the clinical report.
Proton pump inhibitors (PPIs) are another treatment for reducing acid secretion by the parietal cells, but are largely ineffective in relieving clinical signs of GER in preterm infants. PPIs also have been associated with a higher risk of bacterial overgrowth, gastroenteritis, and community-acquired pneumonia in older children. It is theorized that, because of the acid mitigating effects of PPIs, they will have the potential for adverse effects similar to those seen with H2 blockers, although this has not been investigated.
Traditional treatments
Dr. Eichenwald also was quick to point out that even traditional methods of treating preterm infant GER are not particularly effective.
“Some of the conservative approaches that have been advocated include head-up position and different ways of side-lying to enhance emptying of the stomach after feeding. And none of those have been shown to reduce clinically appreciated signs of reflux in preterm infants. If anything – in term babies – some of those positions have been shown to increase the amount of reflux,” he said in an interview.
“I think that the other important point to make about this is that there are many signs that clinicians attribute to reflux in preterm babies, which include wakefulness, irritability, arching after a feeding. And none of those behaviors have been shown to be associated with reflux when it’s critically examined using either a pH Probe or multichannel impedance monitoring. And therefore the treatments to try to decrease reflux don’t really have an effect on those behaviors either.”
Parental concern
Treating a pediatric issue is not as simple as diagnosis and treatment. Often, parents are justifiably concerned about their children. Dr. Eichenwald sees educating parents as an important facet of treating GER in preterm infants.
“Quite honestly I think that there’s some projection on the part of adults who say, ‘I know how I feel when I have heartburn, which is the adult equivalent of reflux, and the baby must be experiencing the same thing, and that’s why they’re acting uncomfortable,’ ” suggested Dr. Eichenwald. “I think that it’s important for clinicians to educate families that a lot of the signs that we typically have attributed to gastroesophageal reflux are not really related to it.”
With both traditional and pharmacological interventions failing to treat preterm infant GER, Dr. Eichenwald believes that the most effective treatment could be patiently waiting. “I think that the important thing to stress is that reflux is a normal physiologic phenomenon. It rarely causes pathology in preterm infants, and therefore, in treating it, you’re not treating any pathology. You should just be patient and it will likely just go away on its own.”
Dr. Eichenwald has no potential conflicts of interest or external funding to report.
SOURCE: Eichenwald E et al. Pediatrics. 2018 June. doi: 10.1542/peds.2018-1061 .
Treatment of gastroesophageal reflux (GER) in preterm infants with traditional treatments, such as body positioning, and newer treatments with pharmacologic agents appear to be ineffective, and pharmacologic agents in particular may cause significant harm, according to a clinical report by the American Academy of Pediatrics Committee on Fetus and Newborn.
“I think that probably the most important point for any physician, including neonatologists, is that the committee concluded on the basis of the evidence that Eric Eichenwald, MD, lead author of the committee’s clinical report and chief of neonatology at Children’s Hospital of Philadelphia, said in an interview. “So really the bottom line of the clinical report is watchful waiting, conservative management, and patience is the most important approach to a baby that you think is suffering from reflux.”
Pharmacologic management
The committee members focused on four categories of pharmacologic interventions in their report in Pediatrics.
Prokinetic (promotility) agents, such as metoclopramide, domperidone, and erythromycin, are widely used in treating symptoms of GER in older infants and appear to improve gastric emptying, reduce regurgitation, and enhance lower esophageal sphincter tone, but they do not appear to reduce GER symptoms in preterm infants. In addition to not being effective in these infants, there is also a potential for significant adverse events, including cardiac arrhythmia and neurologic side effects. Another common pharmacologic treatment is the use of sodium alginate in combination with sodium bicarbonate. In the presence of gastric acid, sodium alginate precipitates as a gel that forms a physical barrier that protects the gastric mucosa. When sodium bicarbonate is added, a carbon dioxide foam forms that is less harmful to the esophagus than GER-related fluids. While this combination treatment has reduced the number of acidic GER exposures and esophageal acid exposure in preterm infants in small studies, the long-term safety has not been evaluated in this populations.
Histamine2 (H2) blockers, like famotidine and ranitidine, also are commonly prescribed to treat preterm infant gastroesophageal reflux. H2 blockers compete with H2 for the histamine receptors of the parietal cells, which causes a decrease in hydrochloric acid and a subsequent increase in intragastric pH. These are often prescribed on the premise that GER symptoms are secondary to acid reflux in the lower esophagus, but there is no research on the efficacy of H2 blockers on the symptom profile of GER in preterm infants. This class of drugs also has been linked with an increased risk of necrotizing enterocolitis and a higher incidence of late-onset infections and death. This is thought to be caused by alteration of the intestinal microbiome, according to the clinical report.
Proton pump inhibitors (PPIs) are another treatment for reducing acid secretion by the parietal cells, but are largely ineffective in relieving clinical signs of GER in preterm infants. PPIs also have been associated with a higher risk of bacterial overgrowth, gastroenteritis, and community-acquired pneumonia in older children. It is theorized that, because of the acid mitigating effects of PPIs, they will have the potential for adverse effects similar to those seen with H2 blockers, although this has not been investigated.
Traditional treatments
Dr. Eichenwald also was quick to point out that even traditional methods of treating preterm infant GER are not particularly effective.
“Some of the conservative approaches that have been advocated include head-up position and different ways of side-lying to enhance emptying of the stomach after feeding. And none of those have been shown to reduce clinically appreciated signs of reflux in preterm infants. If anything – in term babies – some of those positions have been shown to increase the amount of reflux,” he said in an interview.
“I think that the other important point to make about this is that there are many signs that clinicians attribute to reflux in preterm babies, which include wakefulness, irritability, arching after a feeding. And none of those behaviors have been shown to be associated with reflux when it’s critically examined using either a pH Probe or multichannel impedance monitoring. And therefore the treatments to try to decrease reflux don’t really have an effect on those behaviors either.”
Parental concern
Treating a pediatric issue is not as simple as diagnosis and treatment. Often, parents are justifiably concerned about their children. Dr. Eichenwald sees educating parents as an important facet of treating GER in preterm infants.
“Quite honestly I think that there’s some projection on the part of adults who say, ‘I know how I feel when I have heartburn, which is the adult equivalent of reflux, and the baby must be experiencing the same thing, and that’s why they’re acting uncomfortable,’ ” suggested Dr. Eichenwald. “I think that it’s important for clinicians to educate families that a lot of the signs that we typically have attributed to gastroesophageal reflux are not really related to it.”
With both traditional and pharmacological interventions failing to treat preterm infant GER, Dr. Eichenwald believes that the most effective treatment could be patiently waiting. “I think that the important thing to stress is that reflux is a normal physiologic phenomenon. It rarely causes pathology in preterm infants, and therefore, in treating it, you’re not treating any pathology. You should just be patient and it will likely just go away on its own.”
Dr. Eichenwald has no potential conflicts of interest or external funding to report.
SOURCE: Eichenwald E et al. Pediatrics. 2018 June. doi: 10.1542/peds.2018-1061 .
Treatment of gastroesophageal reflux (GER) in preterm infants with traditional treatments, such as body positioning, and newer treatments with pharmacologic agents appear to be ineffective, and pharmacologic agents in particular may cause significant harm, according to a clinical report by the American Academy of Pediatrics Committee on Fetus and Newborn.
“I think that probably the most important point for any physician, including neonatologists, is that the committee concluded on the basis of the evidence that Eric Eichenwald, MD, lead author of the committee’s clinical report and chief of neonatology at Children’s Hospital of Philadelphia, said in an interview. “So really the bottom line of the clinical report is watchful waiting, conservative management, and patience is the most important approach to a baby that you think is suffering from reflux.”
Pharmacologic management
The committee members focused on four categories of pharmacologic interventions in their report in Pediatrics.
Prokinetic (promotility) agents, such as metoclopramide, domperidone, and erythromycin, are widely used in treating symptoms of GER in older infants and appear to improve gastric emptying, reduce regurgitation, and enhance lower esophageal sphincter tone, but they do not appear to reduce GER symptoms in preterm infants. In addition to not being effective in these infants, there is also a potential for significant adverse events, including cardiac arrhythmia and neurologic side effects. Another common pharmacologic treatment is the use of sodium alginate in combination with sodium bicarbonate. In the presence of gastric acid, sodium alginate precipitates as a gel that forms a physical barrier that protects the gastric mucosa. When sodium bicarbonate is added, a carbon dioxide foam forms that is less harmful to the esophagus than GER-related fluids. While this combination treatment has reduced the number of acidic GER exposures and esophageal acid exposure in preterm infants in small studies, the long-term safety has not been evaluated in this populations.
Histamine2 (H2) blockers, like famotidine and ranitidine, also are commonly prescribed to treat preterm infant gastroesophageal reflux. H2 blockers compete with H2 for the histamine receptors of the parietal cells, which causes a decrease in hydrochloric acid and a subsequent increase in intragastric pH. These are often prescribed on the premise that GER symptoms are secondary to acid reflux in the lower esophagus, but there is no research on the efficacy of H2 blockers on the symptom profile of GER in preterm infants. This class of drugs also has been linked with an increased risk of necrotizing enterocolitis and a higher incidence of late-onset infections and death. This is thought to be caused by alteration of the intestinal microbiome, according to the clinical report.
Proton pump inhibitors (PPIs) are another treatment for reducing acid secretion by the parietal cells, but are largely ineffective in relieving clinical signs of GER in preterm infants. PPIs also have been associated with a higher risk of bacterial overgrowth, gastroenteritis, and community-acquired pneumonia in older children. It is theorized that, because of the acid mitigating effects of PPIs, they will have the potential for adverse effects similar to those seen with H2 blockers, although this has not been investigated.
Traditional treatments
Dr. Eichenwald also was quick to point out that even traditional methods of treating preterm infant GER are not particularly effective.
“Some of the conservative approaches that have been advocated include head-up position and different ways of side-lying to enhance emptying of the stomach after feeding. And none of those have been shown to reduce clinically appreciated signs of reflux in preterm infants. If anything – in term babies – some of those positions have been shown to increase the amount of reflux,” he said in an interview.
“I think that the other important point to make about this is that there are many signs that clinicians attribute to reflux in preterm babies, which include wakefulness, irritability, arching after a feeding. And none of those behaviors have been shown to be associated with reflux when it’s critically examined using either a pH Probe or multichannel impedance monitoring. And therefore the treatments to try to decrease reflux don’t really have an effect on those behaviors either.”
Parental concern
Treating a pediatric issue is not as simple as diagnosis and treatment. Often, parents are justifiably concerned about their children. Dr. Eichenwald sees educating parents as an important facet of treating GER in preterm infants.
“Quite honestly I think that there’s some projection on the part of adults who say, ‘I know how I feel when I have heartburn, which is the adult equivalent of reflux, and the baby must be experiencing the same thing, and that’s why they’re acting uncomfortable,’ ” suggested Dr. Eichenwald. “I think that it’s important for clinicians to educate families that a lot of the signs that we typically have attributed to gastroesophageal reflux are not really related to it.”
With both traditional and pharmacological interventions failing to treat preterm infant GER, Dr. Eichenwald believes that the most effective treatment could be patiently waiting. “I think that the important thing to stress is that reflux is a normal physiologic phenomenon. It rarely causes pathology in preterm infants, and therefore, in treating it, you’re not treating any pathology. You should just be patient and it will likely just go away on its own.”
Dr. Eichenwald has no potential conflicts of interest or external funding to report.
SOURCE: Eichenwald E et al. Pediatrics. 2018 June. doi: 10.1542/peds.2018-1061 .
FROM PEDIATRICS
Impulse control disorders in Parkinson’s patients may be higher than thought
Nearly half of patients with Parkinson’s disease who were taking dopamine agonist treatment experienced impulse control disorders over a follow-up of 5 years, according to recently published results of a longitudinal study.
The 5-year cumulative incidence of impulse control disorders was approximately 45% in the study, which included 411 patients with a high prevalence of dopamine agonist use and disease duration of 5 years or less at baseline.
There was a strong association between dopamine agonist use and impulse control disorders in the study, which was conducted by Jean-Christophe Corvol, MD, of Publique Hôpitaux de Paris and his coinvestigators.
Impulse disorders increased in incidence with both duration and dose of dopamine agonists and resolved progressively after discontinuation of those agents, the investigators reported online June 20 in Neurology. The investigators used item 1.6 of part I of the Movement Disorder Society Unified Parkinson’s Disease Rating Scale to determine the presence of an impulse control disorder.
“Given the high cumulative incidence of impulse control disorders in patients with Parkinson’s disease, these adverse effects should be carefully monitored in patients ever treated with dopamine agonists,” Dr. Corvol and his coauthors wrote.
The results came from the ongoing Drug Interaction With Genes in Parkinson’s Disease (DIGPD) study, a longitudinal cohort study including Parkinson’s disease patients consecutively recruited between 2009 and 2013 at eight French hospitals. All patients had no more than 5 years of disease duration at recruitment, and follow-up included annual evaluations by movement disorder specialists.
At baseline, the majority of patients (302, or 73.5%) had taken dopamine agonists within the past 12 months.
Over the course of 5 years, the prevalence of impulse control disorders increased from 19.7% at baseline to 32.8%, Dr. Corvol and his colleagues reported.
Among 306 patients with no impulse control disorders at baseline, 94 developed one, for a 5-year cumulative incidence of 46.1%, they added. Only 4 of the 94 new cases occurred in patients who never used dopamine agonists.
Dopamine agonist use in the previous 12 months was associated with a 2.23-fold higher prevalence of impulse control disorders (P less than .001), with prevalence increasing along with average daily dose and cumulative dose duration over that time period, according to the investigators.
These findings suggests tools are needed to screen for impulse control disorders and identify high-risk patients, they said.
“Further studies are needed to understand the mechanisms involved in the relation between [dopamine agonists] and [impulse control disorders], in particular the role of apathy, anxiety, and depression,” they added.
The study was funded by grants from the French Ministry of Health and from the French Drug Agency. Dr. Corvol and many of his colleagues reported financial disclosures with many pharmaceutical companies.
SOURCE: Corvol J-C et al. Neurology. 2018 Jun 20. doi: 10.1212/WNL.0000000000005816.
Data from the study by Dr. Corvol and colleagues are robust and suggest neurologists may be “missing the boat or even harming patients” by underestimating the adverse effects associated with dopamine agonists, the authors of an editorial wrote.
The 5-year incidence of impulse control disorders approaching 50% suggests they are even more common than previously reported, according to editorial authors Laura S. Boylan, MD, and Vladimir S. Kostic, MD, PhD.Compulsive gambling, shopping, eating, sexual behaviors and other impulse control disorders at their worst can ruin finances, disrupt families, and have legal implications, Dr. Boylan and Dr. Kostic said in their editorial.
Neurologists are “often uncomfortable” with psychiatric disorders, they added, even though they are the ones managing movement disorder medications.
There is an absence of high-quality evidence on how to treat impulse control disorders, though one common approach, switching to levodopa, is in the wheelhouse of neurologists. However, “levodopaphobia” persists in some circles despite evidence debunking the notion that the medication is neurotoxic, according to Dr. Boylan and Dr. Kostic.
“Practice change in medicine, as in other areas, can be like turning a cruise ship,” they wrote, “but this study may help give a little push to the boat and, we hope, promote further basic and clinical research on nonmotor aspects of PD and other movement disorders.”
Dr. Boylan is with Essentia Health, Duluth, Minn., Albany-Stratton VA Medical Center, Albany, N.Y., and Bellevue Hospital/New York University. Dr. Kosticis with the Institute of Neurology CCS, School of Medicine University of Belgrade (Serbia). Dr. Kostic reported receiving speaker honoraria from Novartis, Teva, and Salveo. Their editorial accompanied Dr. Corvol and colleagues’ report (Neurology. 2018 Jun 20. doi: 10.1212/WNL.0000000000005806 ).
Data from the study by Dr. Corvol and colleagues are robust and suggest neurologists may be “missing the boat or even harming patients” by underestimating the adverse effects associated with dopamine agonists, the authors of an editorial wrote.
The 5-year incidence of impulse control disorders approaching 50% suggests they are even more common than previously reported, according to editorial authors Laura S. Boylan, MD, and Vladimir S. Kostic, MD, PhD.Compulsive gambling, shopping, eating, sexual behaviors and other impulse control disorders at their worst can ruin finances, disrupt families, and have legal implications, Dr. Boylan and Dr. Kostic said in their editorial.
Neurologists are “often uncomfortable” with psychiatric disorders, they added, even though they are the ones managing movement disorder medications.
There is an absence of high-quality evidence on how to treat impulse control disorders, though one common approach, switching to levodopa, is in the wheelhouse of neurologists. However, “levodopaphobia” persists in some circles despite evidence debunking the notion that the medication is neurotoxic, according to Dr. Boylan and Dr. Kostic.
“Practice change in medicine, as in other areas, can be like turning a cruise ship,” they wrote, “but this study may help give a little push to the boat and, we hope, promote further basic and clinical research on nonmotor aspects of PD and other movement disorders.”
Dr. Boylan is with Essentia Health, Duluth, Minn., Albany-Stratton VA Medical Center, Albany, N.Y., and Bellevue Hospital/New York University. Dr. Kosticis with the Institute of Neurology CCS, School of Medicine University of Belgrade (Serbia). Dr. Kostic reported receiving speaker honoraria from Novartis, Teva, and Salveo. Their editorial accompanied Dr. Corvol and colleagues’ report (Neurology. 2018 Jun 20. doi: 10.1212/WNL.0000000000005806 ).
Data from the study by Dr. Corvol and colleagues are robust and suggest neurologists may be “missing the boat or even harming patients” by underestimating the adverse effects associated with dopamine agonists, the authors of an editorial wrote.
The 5-year incidence of impulse control disorders approaching 50% suggests they are even more common than previously reported, according to editorial authors Laura S. Boylan, MD, and Vladimir S. Kostic, MD, PhD.Compulsive gambling, shopping, eating, sexual behaviors and other impulse control disorders at their worst can ruin finances, disrupt families, and have legal implications, Dr. Boylan and Dr. Kostic said in their editorial.
Neurologists are “often uncomfortable” with psychiatric disorders, they added, even though they are the ones managing movement disorder medications.
There is an absence of high-quality evidence on how to treat impulse control disorders, though one common approach, switching to levodopa, is in the wheelhouse of neurologists. However, “levodopaphobia” persists in some circles despite evidence debunking the notion that the medication is neurotoxic, according to Dr. Boylan and Dr. Kostic.
“Practice change in medicine, as in other areas, can be like turning a cruise ship,” they wrote, “but this study may help give a little push to the boat and, we hope, promote further basic and clinical research on nonmotor aspects of PD and other movement disorders.”
Dr. Boylan is with Essentia Health, Duluth, Minn., Albany-Stratton VA Medical Center, Albany, N.Y., and Bellevue Hospital/New York University. Dr. Kosticis with the Institute of Neurology CCS, School of Medicine University of Belgrade (Serbia). Dr. Kostic reported receiving speaker honoraria from Novartis, Teva, and Salveo. Their editorial accompanied Dr. Corvol and colleagues’ report (Neurology. 2018 Jun 20. doi: 10.1212/WNL.0000000000005806 ).
Nearly half of patients with Parkinson’s disease who were taking dopamine agonist treatment experienced impulse control disorders over a follow-up of 5 years, according to recently published results of a longitudinal study.
The 5-year cumulative incidence of impulse control disorders was approximately 45% in the study, which included 411 patients with a high prevalence of dopamine agonist use and disease duration of 5 years or less at baseline.
There was a strong association between dopamine agonist use and impulse control disorders in the study, which was conducted by Jean-Christophe Corvol, MD, of Publique Hôpitaux de Paris and his coinvestigators.
Impulse disorders increased in incidence with both duration and dose of dopamine agonists and resolved progressively after discontinuation of those agents, the investigators reported online June 20 in Neurology. The investigators used item 1.6 of part I of the Movement Disorder Society Unified Parkinson’s Disease Rating Scale to determine the presence of an impulse control disorder.
“Given the high cumulative incidence of impulse control disorders in patients with Parkinson’s disease, these adverse effects should be carefully monitored in patients ever treated with dopamine agonists,” Dr. Corvol and his coauthors wrote.
The results came from the ongoing Drug Interaction With Genes in Parkinson’s Disease (DIGPD) study, a longitudinal cohort study including Parkinson’s disease patients consecutively recruited between 2009 and 2013 at eight French hospitals. All patients had no more than 5 years of disease duration at recruitment, and follow-up included annual evaluations by movement disorder specialists.
At baseline, the majority of patients (302, or 73.5%) had taken dopamine agonists within the past 12 months.
Over the course of 5 years, the prevalence of impulse control disorders increased from 19.7% at baseline to 32.8%, Dr. Corvol and his colleagues reported.
Among 306 patients with no impulse control disorders at baseline, 94 developed one, for a 5-year cumulative incidence of 46.1%, they added. Only 4 of the 94 new cases occurred in patients who never used dopamine agonists.
Dopamine agonist use in the previous 12 months was associated with a 2.23-fold higher prevalence of impulse control disorders (P less than .001), with prevalence increasing along with average daily dose and cumulative dose duration over that time period, according to the investigators.
These findings suggests tools are needed to screen for impulse control disorders and identify high-risk patients, they said.
“Further studies are needed to understand the mechanisms involved in the relation between [dopamine agonists] and [impulse control disorders], in particular the role of apathy, anxiety, and depression,” they added.
The study was funded by grants from the French Ministry of Health and from the French Drug Agency. Dr. Corvol and many of his colleagues reported financial disclosures with many pharmaceutical companies.
SOURCE: Corvol J-C et al. Neurology. 2018 Jun 20. doi: 10.1212/WNL.0000000000005816.
Nearly half of patients with Parkinson’s disease who were taking dopamine agonist treatment experienced impulse control disorders over a follow-up of 5 years, according to recently published results of a longitudinal study.
The 5-year cumulative incidence of impulse control disorders was approximately 45% in the study, which included 411 patients with a high prevalence of dopamine agonist use and disease duration of 5 years or less at baseline.
There was a strong association between dopamine agonist use and impulse control disorders in the study, which was conducted by Jean-Christophe Corvol, MD, of Publique Hôpitaux de Paris and his coinvestigators.
Impulse disorders increased in incidence with both duration and dose of dopamine agonists and resolved progressively after discontinuation of those agents, the investigators reported online June 20 in Neurology. The investigators used item 1.6 of part I of the Movement Disorder Society Unified Parkinson’s Disease Rating Scale to determine the presence of an impulse control disorder.
“Given the high cumulative incidence of impulse control disorders in patients with Parkinson’s disease, these adverse effects should be carefully monitored in patients ever treated with dopamine agonists,” Dr. Corvol and his coauthors wrote.
The results came from the ongoing Drug Interaction With Genes in Parkinson’s Disease (DIGPD) study, a longitudinal cohort study including Parkinson’s disease patients consecutively recruited between 2009 and 2013 at eight French hospitals. All patients had no more than 5 years of disease duration at recruitment, and follow-up included annual evaluations by movement disorder specialists.
At baseline, the majority of patients (302, or 73.5%) had taken dopamine agonists within the past 12 months.
Over the course of 5 years, the prevalence of impulse control disorders increased from 19.7% at baseline to 32.8%, Dr. Corvol and his colleagues reported.
Among 306 patients with no impulse control disorders at baseline, 94 developed one, for a 5-year cumulative incidence of 46.1%, they added. Only 4 of the 94 new cases occurred in patients who never used dopamine agonists.
Dopamine agonist use in the previous 12 months was associated with a 2.23-fold higher prevalence of impulse control disorders (P less than .001), with prevalence increasing along with average daily dose and cumulative dose duration over that time period, according to the investigators.
These findings suggests tools are needed to screen for impulse control disorders and identify high-risk patients, they said.
“Further studies are needed to understand the mechanisms involved in the relation between [dopamine agonists] and [impulse control disorders], in particular the role of apathy, anxiety, and depression,” they added.
The study was funded by grants from the French Ministry of Health and from the French Drug Agency. Dr. Corvol and many of his colleagues reported financial disclosures with many pharmaceutical companies.
SOURCE: Corvol J-C et al. Neurology. 2018 Jun 20. doi: 10.1212/WNL.0000000000005816.
FROM NEUROLOGY
Key clinical point: Nearly half of Parkinson’s disease patients reported having an impulse control disorder during a 5-year period, the great majority of whom were receiving dopamine agonist treatment.
Major finding: The 5-year cumulative incidence of impulse control disorders was approximately 45%, with increased risk correlating with dose and duration of dopamine agonist treatment.
Study details: Analysis of a multicenter, longitudinal cohort including 5 years of follow-up on 411 consecutive patients with Parkinson’s disease and a disease duration of 5 years or less at baseline.
Disclosures: The study was funded by grants from the French Ministry of Health and from the French Drug Agency. Many of the authors reported financial disclosures with many pharmaceutical companies.
Source: Corvol J-C et al. Neurology. 2018 Jun 20. doi: 10.1212/WNL.0000000000005816.
Neurology Board Review: Epilepsy
Click here to read Neurology Board Review: Epilepsy
Neurology Board Review: Epilepsy is a resource developed by leading clinical educators for studying for board certification and maintenance of certification exams.
After reading the article, Click Here to Access the Board Review Questions
About the Authors
Shavonne L. Massey, MD
Clinical Instructor
Departments of Neurology and Pediatrics
Children’s Hospital of Philadelphia
University of Pennsylvania
Philadelphia, Pennsylvania
Hannah C. Glass, MDCM, MAS
Associate Professor
Departments of Neurology, Pediatrics, and
Epidemiology & Biostatistics
University of California, San Francisco
San Francisco, California
Click here to read Neurology Board Review: Epilepsy
After reading the article, Click Here to Access the Board Review Questions
Click here to read Neurology Board Review: Epilepsy
Neurology Board Review: Epilepsy is a resource developed by leading clinical educators for studying for board certification and maintenance of certification exams.
After reading the article, Click Here to Access the Board Review Questions
About the Authors
Shavonne L. Massey, MD
Clinical Instructor
Departments of Neurology and Pediatrics
Children’s Hospital of Philadelphia
University of Pennsylvania
Philadelphia, Pennsylvania
Hannah C. Glass, MDCM, MAS
Associate Professor
Departments of Neurology, Pediatrics, and
Epidemiology & Biostatistics
University of California, San Francisco
San Francisco, California
Click here to read Neurology Board Review: Epilepsy
After reading the article, Click Here to Access the Board Review Questions
Click here to read Neurology Board Review: Epilepsy
Neurology Board Review: Epilepsy is a resource developed by leading clinical educators for studying for board certification and maintenance of certification exams.
After reading the article, Click Here to Access the Board Review Questions
About the Authors
Shavonne L. Massey, MD
Clinical Instructor
Departments of Neurology and Pediatrics
Children’s Hospital of Philadelphia
University of Pennsylvania
Philadelphia, Pennsylvania
Hannah C. Glass, MDCM, MAS
Associate Professor
Departments of Neurology, Pediatrics, and
Epidemiology & Biostatistics
University of California, San Francisco
San Francisco, California
Click here to read Neurology Board Review: Epilepsy
After reading the article, Click Here to Access the Board Review Questions
Clinical trials to look for at ADA 2018
More than 2,000 abstracts will be presented at the annual scientific sessions of the American Diabetes Association in Orlando, from basic science studies to clinical trials. Maureen A. Gannon, PhD, who chairs the Scientific Sessions Meeting Planning Committee, highlighted several as being the most relevant to clinical practice.
TEDDY at 13
VADT at 15
Final follow-up data from Veterans Administration Diabetes Trial will be presented on Sunday, June 24, at 4:30 p.m. The trial randomized nearly 2,000 military veterans with poor glycemic control to a mean of 5.6 years of intensive glycemic therapy versus standard treatment, with a goal of lowering HbA1c below 8%.
RISE
Restoring Insulin Secretion (RISE) comprises three intervention trials, two in adults and one in adolescents. The trials are studying whether aggressive glucose lowering will lead to recovery of beta-cell function can be sustained after withdrawal of treatment. Initial results from the adolescent trial will be reported on Monday, June 25, at 2:15 p.m.
SGLT inhibition in type 1 diabetes
Presenters in this session, on Tuesday, June 26 at 10:15 a.m., will provide trial results an insights on a regulatory pathway for sodium-glucose cotransporter (SGLT)-1 and -2 inhibitors in type 1 diabetes patients. Julio Rosenstock, MD, who will present the latest data on empagliflozin from the EASE (Empagliflozin as Adjunctive to InSulin thErapy) trial program, said, “This symposium brings together the lead investigators from the three major competitors that are pursuing approval of a SGLT inhibitor for type 1 diabetes. They will report top-level data that will eventually be submitted to regulators.”
DIY technology
This symposium on Saturday at 1:45 pm, The Diabetes Do-It-Yourself Revolution, will explore the evolving, DIY revolution in diabetes, in which patients are upending traditional treatment pathways and closing their own insulin delivery loop.
“I’m excited about the variety we have in the program this year,” said Dr. Gannon, professor of medicine in the division of diabetes, endocrinology and metabolism; molecular physiology and biophysics; and cell and developmental biology at Vanderbilt University, Nashville, Tenn. “This is the place for cutting-edge information for anybody who is involved in diabetes research or patient care.”
More than 2,000 abstracts will be presented at the annual scientific sessions of the American Diabetes Association in Orlando, from basic science studies to clinical trials. Maureen A. Gannon, PhD, who chairs the Scientific Sessions Meeting Planning Committee, highlighted several as being the most relevant to clinical practice.
TEDDY at 13
VADT at 15
Final follow-up data from Veterans Administration Diabetes Trial will be presented on Sunday, June 24, at 4:30 p.m. The trial randomized nearly 2,000 military veterans with poor glycemic control to a mean of 5.6 years of intensive glycemic therapy versus standard treatment, with a goal of lowering HbA1c below 8%.
RISE
Restoring Insulin Secretion (RISE) comprises three intervention trials, two in adults and one in adolescents. The trials are studying whether aggressive glucose lowering will lead to recovery of beta-cell function can be sustained after withdrawal of treatment. Initial results from the adolescent trial will be reported on Monday, June 25, at 2:15 p.m.
SGLT inhibition in type 1 diabetes
Presenters in this session, on Tuesday, June 26 at 10:15 a.m., will provide trial results an insights on a regulatory pathway for sodium-glucose cotransporter (SGLT)-1 and -2 inhibitors in type 1 diabetes patients. Julio Rosenstock, MD, who will present the latest data on empagliflozin from the EASE (Empagliflozin as Adjunctive to InSulin thErapy) trial program, said, “This symposium brings together the lead investigators from the three major competitors that are pursuing approval of a SGLT inhibitor for type 1 diabetes. They will report top-level data that will eventually be submitted to regulators.”
DIY technology
This symposium on Saturday at 1:45 pm, The Diabetes Do-It-Yourself Revolution, will explore the evolving, DIY revolution in diabetes, in which patients are upending traditional treatment pathways and closing their own insulin delivery loop.
“I’m excited about the variety we have in the program this year,” said Dr. Gannon, professor of medicine in the division of diabetes, endocrinology and metabolism; molecular physiology and biophysics; and cell and developmental biology at Vanderbilt University, Nashville, Tenn. “This is the place for cutting-edge information for anybody who is involved in diabetes research or patient care.”
More than 2,000 abstracts will be presented at the annual scientific sessions of the American Diabetes Association in Orlando, from basic science studies to clinical trials. Maureen A. Gannon, PhD, who chairs the Scientific Sessions Meeting Planning Committee, highlighted several as being the most relevant to clinical practice.
TEDDY at 13
VADT at 15
Final follow-up data from Veterans Administration Diabetes Trial will be presented on Sunday, June 24, at 4:30 p.m. The trial randomized nearly 2,000 military veterans with poor glycemic control to a mean of 5.6 years of intensive glycemic therapy versus standard treatment, with a goal of lowering HbA1c below 8%.
RISE
Restoring Insulin Secretion (RISE) comprises three intervention trials, two in adults and one in adolescents. The trials are studying whether aggressive glucose lowering will lead to recovery of beta-cell function can be sustained after withdrawal of treatment. Initial results from the adolescent trial will be reported on Monday, June 25, at 2:15 p.m.
SGLT inhibition in type 1 diabetes
Presenters in this session, on Tuesday, June 26 at 10:15 a.m., will provide trial results an insights on a regulatory pathway for sodium-glucose cotransporter (SGLT)-1 and -2 inhibitors in type 1 diabetes patients. Julio Rosenstock, MD, who will present the latest data on empagliflozin from the EASE (Empagliflozin as Adjunctive to InSulin thErapy) trial program, said, “This symposium brings together the lead investigators from the three major competitors that are pursuing approval of a SGLT inhibitor for type 1 diabetes. They will report top-level data that will eventually be submitted to regulators.”
DIY technology
This symposium on Saturday at 1:45 pm, The Diabetes Do-It-Yourself Revolution, will explore the evolving, DIY revolution in diabetes, in which patients are upending traditional treatment pathways and closing their own insulin delivery loop.
“I’m excited about the variety we have in the program this year,” said Dr. Gannon, professor of medicine in the division of diabetes, endocrinology and metabolism; molecular physiology and biophysics; and cell and developmental biology at Vanderbilt University, Nashville, Tenn. “This is the place for cutting-edge information for anybody who is involved in diabetes research or patient care.”
Free Composite Serratus Anterior-Latissimus-Rib Flaps for Acute One-Stage Reconstruction of Gustilo IIIB Tibia Fractures
ABSTRACT
Gustilo IIIB injuries of the tibia with segmental bone loss continue to be a difficult reconstructive problem. The serratus anterior-latissimus-rib (SALR) composite flap consists of bone and muscle; this flap can provide soft tissue coverage and vascularized bone in a single surgical procedure. The purpose of this study is to describe the use of the SALR flap for the treatment of a large open tibia fracture with segmental bone loss, with a specific focus on postoperative complications, limb salvage, and time to union.
We reviewed the medical records of patients undergoing an SALR flap (n = 5) for the treatment of Gustilo Type IIIB tibia fractures within 1 month of injury. We compared the mechanism of injury, injury severity score, time from injury to free tissue transfer, complications, and time to radiographic and clinical union.
All patients were male, with a mean age of 25 years. On average, patients underwent free tissue transfer within 1 week of injury. The average time to radiographic union was 7 months. Two patients underwent reoperation. There were no graft failures.
Free SALR flaps can be a useful option for the treatment of high-energy tibia fractures with extensive soft tissue and bone loss. These flaps provide immediate osseous and soft tissue reconstruction with an acceptable complication profile.
Reconstruction of the lower extremity following Gustilo’s grade IIIB injuries is difficult due to loss of both combined soft tissue and segmental bone loss. Since these injuries necessitate the need for soft tissue flap coverage along with vascularized bone grafting, free fibula flaps have classically been used for reconstruction.1-3 In the setting of bilateral lower extremity injury, the contralateral fibula is often not appropriate to harvest and transfer; therefore, other sources of vascularized bone grafts must be utilized including vascularized iliac crest and rib.1-5 The vascularized iliac crest graft is insufficient to provide the bony reconstruction of bone defects >6 cm to 7 cm and does not have a reliable skin paddle.4 In contrast, free composite serratus anterior-latissimus-rib (SALR) flaps can provide both long segments of vascularized bone and abundant soft tissue coverage for large segmental defects.1-5
Continue to: Free fibula grafts have been considered...
Free fibula grafts have been considered the gold standard for the reconstruction of large (>6 cm) bone defects.6 In cases of “mangled extremities,” bone defects are associated with large soft tissue defects, which require either single-stage surgery consisting of 2 separate free flaps (ie, free fibula and free latissimus) or a 2-stage procedure where the soft tissue reconstruction precedes the bone reconstruction.2,7-9 Unlike free fibula and latissimus flaps, composite SALR flaps provide both osseous reconstruction and soft tissue in 1 flap supplied by a single vascular pedicle; unfortunately, outcomes using this flap for large Gustilo IIIB injuries are limited.1-5 The purpose of this study is to examine the use of free composite SALR flaps for soft tissue coverage in cases of Gustilo IIIB injuries with large soft tissue and bony deficits. This study specifically examines time to union, need for reoperation, and graft failure following the use of these flaps.
MATERIALS AND METHODS
Following approval from our Institutional Review Board, we retrospectively reviewed the medical records of patients undergoing a free composite SALR flap (n = 5) for the treatment of a severe open tibia fracture within 1 month of injury. All patients sustained open injuries classified as IIIB on the Gustilo-Anderson scale.10 Medical records were examined for the mechanism of injury (MOI), injury severity score (ISS), time from injury to free tissue transfer, medical comorbidities, surgical complications, and time to radiographic and clinical union. Radiographic union was determined by the presence of bridging bone on 3 of 4 of cortices on plain film radiographs.
All patients were male (n = 5), with a mean age of 25 years (range, 19-30 years) at the time of injury (Table).
Table. Demographics and Outcomes of Patients Undergoing Free Tissue Transfer
| Free Serratus Anterior-Latissimus-Rib Flaps |
Age (Mean ± SEM) | 23 ± 2 years |
Males | 5 |
Females | 0 |
Tobacco Use | 2 |
Body Mass Index (Mean ± SEM) | 26.2 ± 0.9 kg/m2 |
Injury Severity Score (Mean ± SEM) | 18 ± 5 |
Time to Tissue Transfer (Mean ± SEM) | 1 ± 0.3 weeks |
Time to Boney Union (Mean ± SEM) | 7 ± 0.7 months |
Time Non-Weight-Bearing (Mean ± SEM) | 5 ± 0.5 months |
The MOI included motorcycle collisions (n = 2), pedestrian struck by car (n = 1), motor vehicle collisions (n = 1), and direct blow to the leg (n = 1). The mean ISS of the cohort was 18 (range, 10-34) (Table). On average, patients underwent free tissue transfer within 1 week (range, 3 days to 2 weeks) from the time of injury. Patients in this cohort were followed clinically for a mean of 4 years (range, 1-6 years) after surgery. Patients were non-weight-bearing for an average of 5 months (range, 4-6 months) following their reconstructions.
RESULTS
All flaps survived. The mean time to radiographic and clinical union was 7 months (range, 6-9 months). Two patients underwent reoperation. One patient underwent a bone grafting procedure for a delayed union at 6 months postoperative, and 1 patient underwent irrigation and débridement of superficial soft tissue infection. Donor site complications occurred in 2 patients, including chronic rib pain (n = 1) and a pleural effusion requiring drainage (n = 1). At the last follow-up, all ribs had incorporated, and all patients were weight-bearing as tolerated on the limb.
CASE EXAMPLE
A 22-year-old male smoker was transferred to our facility after a motor vehicle accident with bilateral tibia fractures, 1 closed and 1 open with significant bone loss (Figures 1A, 1B).
Continue to: Surgical Technique...
SURGICAL TECHNIQUE
The patient is placed in the lateral decubitus position during the procedure. A 2-team approach is used for dissection of the flap and preparation of recipient vessels to decrease operative time. A J-shaped incision is started on the chest at the mid-axillary line and extended just over the fifth and sixth rib. The incision can be made to fall into the intermammary crease in a woman to hide the scar. The dissection begins by exposing the anterior border of the latissimus muscle (Figure 2A). Next, the latissimus is dissected to reveal the thoracodorsal vessels (Figure 2B). At this level, the thoracodorsal vessel can be traced into the axilla. The branch going into the fifth, sixth, and lower slips of the serratus are dissected. The long thoracic nerve and the thoracodorsal nerve are preserved during the dissection (Figure 2C). The fifth, sixth, and seventh slips of the serratus are preferentially included in the dissection while leaving the most superior slips of the serratus to preserve scapular stability. Dissection begins by identifying 2 adjacent rib sections of the fifth and sixth or sixth and seventh ribs. The defect in the lower extremity determines the length of rib harvested. The serratus slips are then divided anteriorly over the chest wall. The dissection is extended to the intercostal spaces of the fourth and fifth ribs. The supraperiosteal dissection is performed at the anterior margin of the rib (Figure 2D).
Continue to: Following the surgical procedure...
Following the surgical procedure, patients are made non-weight-bearing on the operative extremity until signs of healing are apparent on radiographs. In this case, at the patients’ last follow-up visit, the skin graft was healed, and there was solid fusion of the rib/tibia junction (Figures 4B, 4C).
DISCUSSION
High-energy open injuries to the lower extremities are devastating injuries, with a high rate of late amputation and poor functional outcomes.11-13 Vascularized bone grafting provides both essential osteoinductive and osteoconductive properties to segmental bone defects in areas with inadequate soft tissue coverage, particularly in the setting of >6 cm of bone loss.4,14 The results of this study show that acute reconstruction of the lower limb with a composite vascularized SALR graft is a reliable procedure with an acceptable complication profile.
The timing of soft tissue coverage should be performed as soon as the patient is medically stable enough to undergo a reconstructive procedure, ideally within 7 to 10 days; and this timetable has been shown to decrease rates of infection and free flap failure.15-19 Early coverage provides both control of the soft-tissue envelope and reduces the risk of losing bone.1 Unlike the timing of coverage, the staging of the procedure is controversial. Proponents of the 2-stage free tissue (soft tissue followed by bony flap) transfer feel that although the tissue may not be infected at the time of coverage, it is contaminated with bacteria at the time of bone reconstruction, and as such is at high risk for both infection and complications.20 Unlike 2-stage procedures, single-stage coverage provides immediate soft tissue coverage, as well as bony support. This reduces the time to bony union and negates the need for repeated surgery in a mangled extremity where secondary surgery is complicated by both scar tissue and altered anatomy.1,2 Furthermore, it has been shown that there is no difference in the rates of infection when performing a single-stage compared with a 2-stage procedure.9 In this study, SALR flaps were typically performed within 2 weeks following an injury as a single procedure. We feel this resulted in the low number of complications in the SALR group.
Unlike free fibulas, rib flaps are easily pedicled with an associated soft-tissue flap due to their blood supply, making them ideal for 1-stage reconstruction. The rib has a dual blood supply: 1 from the posterior intercostal artery, and the other, an abundant periosteal blood supply, from the serratus anterior muscle.4 The blood supply to the serratus anterior comes from the thoracodorsal artery, and usually provides 14 cm of a large-caliber pedicle, making it a reliable flap for soft tissue reconstruction.21,22 Another unique feature of the blood supply to this flap is the amount of soft tissue available for both harvest and transfer; larger portions of serratus muscle and latissimus muscle can be harvested if necessary to cover the soft tissue defect.4
Comminuted tibias with segmental bone loss are difficult to manage since they are associated with bony as well as soft tissue defects.1,12,13,23 These injuries are ideal candidates for a single-stage reconstruction using a vascularized SALR flap. In our series, the use of an SALR flap resulted ultimately in a 100% union and limb salvage rate, with no flap failures and a low complication profile. Unlike the SALR, free fibular flaps must be transferred along with a separate latissimus dorsi flap to provide enough soft tissue coverage necessary for reconstructing large Gustilo IIIB injuries, which could increase the risk of flap failure. Since ribs are composed of membranous bone and have a similar cross-sectional area to both metacarpal and metatarsals, there are concerns regarding the biomechanical properties of ribs for weight-bearing.4,22,24-26 To compensate for this relatively small cross-sectional area, 2 ribs (either consecutive or alternative) are frequently harvested.1,4,5,23 Previous studies examining the use of ribs for bony reconstruction have frequently supplemented the rib reconstruction to the tibia using screws and external fixation alone.1,4,5,23 In our series, all SALR grafts were supported with the use of an intramedullary nail (n = 3) or locked plating (n = 1). The use of this supplemental fixation of the SALR graft allowed our patients to return to full weight-bearing (mean, 6 months) much earlier than the length of time cited in previous reports (12 months) examining these injuries.1,4,5,23
Continue to: There are several limitations...
There are several limitations to this study. The small sample size and retrospective nature of the study limits the amount of data we are able to collect from the medical record and places obvious constraints on the analysis. Although all these procedures were performed at 1 institution, multiple providers were involved in the reconstruction of these injuries, and there is no standard protocol for their treatment. Similarly, although other forms of extremity reconstruction were used during this time period, there was no standard protocol that could serve as a comparator for patients who underwent an SALR compared with other reconstructive procedures.
Overall, SALR grafts provide an excellent option for 1-stage reconstruction of severe, open lower extremity injuries. In this series we noted a 100% graft success rate with an acceptable complication profile.
This paper will be judged for the Resident Writer’s Award.
1. Yazar S, Lin CH, Wei FC. One-stage reconstruction of composite bone and soft-tissue defects in traumatic lower extremities. Plast Reconstr Surg. 2004;114(6):1457-1466. doi:10.1097/01.PRS.0000138811.88807.65.
2. Lin CH, Wei FC, Chen HC, Chuang DC. Outcome comparison in traumatic lower-extremity reconstruction by using various composite vascularized bone transplantation. Plast Reconstr Surg. 1999;104(4):984-992. doi:10.1097/00006534-199909040-00013.
3. Tu YK, Yen CY, Yeh WL, Wang IC, Wang KC, Ueng SW. Reconstruction of posttraumatic long bone defect with free vascularized bone graft: good outcome in 48 patients with 6 years' follow-up. Acta Orthopaedica Scandinavica. 2001;72(4):359-364. doi:10.1080/000164701753542014.
4. Lin CH, Wei FC, Levin LS, Su JI, Fan KF, Yeh WL, Hsu DT. Free composite serratus anterior and rib flaps for tibial composite bone and soft-tissue defect. Plast Reconstr Surg. 1997;99(6):1656-1665. Doi:10.1097/00006534-199705000-00028.
5. Georgescu AV, Ignatiadis I, Ileana M, Irina C, Filip A, Olariu R. Long-term results after muscle-rib flap transfer for reconstruction of composite limb defects. Microsurgery. 2011;31(3):218-222. doi:10.1002/micr.20857.
6. Moran CG, Wood MB. Vascularized bone autografts. Orthop Rev. 1993;22(2):187-197. doi:10.1097/01241398-199307000-00031.
7. Banic A, Hertel R. Double vascularized fibulas for reconstruction of large tibial defects. J Reconstr Microsurg. 1993;9(6):421-428. doi:10.1055/s-2007-1006751.
8. Malizos KN, Nunley JA, Goldner RD, Urbaniak JR, Harrelson JM. Free vascularized fibula in traumatic long bone defects and in limb salvaging following tumor resection: comparative study. Microsurgery. 1993;14(6):368-374. doi:10.1002/micr.1920140603.
9. Peat BG, Liggins DF. Microvascular soft tissue reconstruction for acute tibial fractures--late complications and the role of bone grafting. Ann Plast Surg. 1990;24(6):517-520.
10. Gustilo RB, Anderson JT. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976;58(4):453-458.
11. Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma. 1984;24(8):742-746. doi:10.1097/00005373-198408000-00009.
12. Bosse MJ, MacKenzie EJ, Kellam JF, et al. An analysis of outcomes of reconstruction or amputation after leg-threatening injuries. NEJM. 2002;347(24):1924-1931. doi:10.1056/NEJMoa012604.
13. MacKenzie EJ, Bosse MJ, Pollak AN, et al. Long-term persistence of disability following severe lower-limb trauma. Results of a seven-year follow-up. J Bone Joint Surg Am. 2005;87(8):1801-1809. doi:10.2106/JBJS.E.00032.
14. Bieber EJ, Wood MB. Bone reconstruction. Clin Plast Surg. 1986;13(4):645-655.
15. Melvin JS, Dombroski DG, Torbert JT, Kovach SJ, Esterhai JL, Mehta S. Open tibial shaft fractures: II. Definitive management and limb salvage. J Am Acad Orthop Surg. 2010;18(2):108-117. doi:10.5435/00124635-201002000-00005.
16. Godina M. Early microsurgical reconstruction of complex trauma of the extremities. Plast Reconstr Surg. 1986;78(3):285-292. doi:10.1055/s-2006-944324.
17. Gopal S, Majumder S, Batchelor AG, Knight SL, De Boer P, Smith RM. Fix and flap: the radical orthopaedic and plastic treatment of severe open fractures of the tibia. J Bone Joint Surg Br. 2000;82(7):959-966. doi:10.1302/0301-620X.82B7.0820959.
18. Fischer MD, Gustilo RB, Varecka TF. The timing of flap coverage, bone-grafting, and intramedullary nailing in patients who have a fracture of the tibial shaft with extensive soft-tissue injury. J Bone Joint Surg Am. 1991;73(9):1316-1322. doi:10.2106/00004623-199173090-00005.
19. Tielinen L, Lindahl JE, Tukiainen EJ. Acute unreamed intramedullary nailing and soft tissue reconstruction with muscle flaps for the treatment of severe open tibial shaft fractures. Injury. 2007;38(8):906-912. doi:10.1016/j.injury.2007.02.052.
20. Yaremchuk MJ, Brumback RJ, Manson PN, Burgess AR, Poka A, Weiland AJ. Acute and definitive management of traumatic osteocutaneous defects of the lower extremity. Plast Reconstr Surg. 1987;80(1):1-14. doi:10.1097/00006534-198707000-00002.
21. Ueng WN, Chuang CC, Shih CH. Double-rib composite free transfer to reconstruct a single-spared lower extremity defect. J Trauma. 1995;38(2):210-212.
22. Bruck JC, Bier J, Kistler D. The serratus anterior osteocutaneous free flap. J Reconstr Microsurg. 1990;6(3):209-213. doi:10.1055/s-2007-1006820.
23. Lin CH, Yazar S. Revisiting the serratus anterior rib flap for composite tibial defects. Plast Reconstr Surg. 2004;114(7):1871-1877. doi:10.1097/01.PRS.0000142767.13493.63.
24. Hui KC, Zhang F, Lineaweaver WC, Moon W, Buncke GM, Buncke HJ. Serratus anterior-rib composite flap: anatomic studies and clinical application to hand reconstruction. Ann Plast Surg. 1999;42(2):132-136. doi:10.1097/00000637-199902000-00004.
25. Buncke HJ, Furnas DW, Gordon L, Achauer BM. Free osteocutaneous flap from a rib to the tibia. Plast Reconstr Surg. 1977;59(6):799-804. doi:10.1097/00006534-197706000-00002.
26. Nusbickel FR, Dell PC, Mcandrew MP, Moore MM. Vascularized autografts for reconstruction of skeletal defects following lower extremity trauma. A review. Clin Orthop Relat Res. 1989;(243):65-70.
ABSTRACT
Gustilo IIIB injuries of the tibia with segmental bone loss continue to be a difficult reconstructive problem. The serratus anterior-latissimus-rib (SALR) composite flap consists of bone and muscle; this flap can provide soft tissue coverage and vascularized bone in a single surgical procedure. The purpose of this study is to describe the use of the SALR flap for the treatment of a large open tibia fracture with segmental bone loss, with a specific focus on postoperative complications, limb salvage, and time to union.
We reviewed the medical records of patients undergoing an SALR flap (n = 5) for the treatment of Gustilo Type IIIB tibia fractures within 1 month of injury. We compared the mechanism of injury, injury severity score, time from injury to free tissue transfer, complications, and time to radiographic and clinical union.
All patients were male, with a mean age of 25 years. On average, patients underwent free tissue transfer within 1 week of injury. The average time to radiographic union was 7 months. Two patients underwent reoperation. There were no graft failures.
Free SALR flaps can be a useful option for the treatment of high-energy tibia fractures with extensive soft tissue and bone loss. These flaps provide immediate osseous and soft tissue reconstruction with an acceptable complication profile.
Reconstruction of the lower extremity following Gustilo’s grade IIIB injuries is difficult due to loss of both combined soft tissue and segmental bone loss. Since these injuries necessitate the need for soft tissue flap coverage along with vascularized bone grafting, free fibula flaps have classically been used for reconstruction.1-3 In the setting of bilateral lower extremity injury, the contralateral fibula is often not appropriate to harvest and transfer; therefore, other sources of vascularized bone grafts must be utilized including vascularized iliac crest and rib.1-5 The vascularized iliac crest graft is insufficient to provide the bony reconstruction of bone defects >6 cm to 7 cm and does not have a reliable skin paddle.4 In contrast, free composite serratus anterior-latissimus-rib (SALR) flaps can provide both long segments of vascularized bone and abundant soft tissue coverage for large segmental defects.1-5
Continue to: Free fibula grafts have been considered...
Free fibula grafts have been considered the gold standard for the reconstruction of large (>6 cm) bone defects.6 In cases of “mangled extremities,” bone defects are associated with large soft tissue defects, which require either single-stage surgery consisting of 2 separate free flaps (ie, free fibula and free latissimus) or a 2-stage procedure where the soft tissue reconstruction precedes the bone reconstruction.2,7-9 Unlike free fibula and latissimus flaps, composite SALR flaps provide both osseous reconstruction and soft tissue in 1 flap supplied by a single vascular pedicle; unfortunately, outcomes using this flap for large Gustilo IIIB injuries are limited.1-5 The purpose of this study is to examine the use of free composite SALR flaps for soft tissue coverage in cases of Gustilo IIIB injuries with large soft tissue and bony deficits. This study specifically examines time to union, need for reoperation, and graft failure following the use of these flaps.
MATERIALS AND METHODS
Following approval from our Institutional Review Board, we retrospectively reviewed the medical records of patients undergoing a free composite SALR flap (n = 5) for the treatment of a severe open tibia fracture within 1 month of injury. All patients sustained open injuries classified as IIIB on the Gustilo-Anderson scale.10 Medical records were examined for the mechanism of injury (MOI), injury severity score (ISS), time from injury to free tissue transfer, medical comorbidities, surgical complications, and time to radiographic and clinical union. Radiographic union was determined by the presence of bridging bone on 3 of 4 of cortices on plain film radiographs.
All patients were male (n = 5), with a mean age of 25 years (range, 19-30 years) at the time of injury (Table).
Table. Demographics and Outcomes of Patients Undergoing Free Tissue Transfer
| Free Serratus Anterior-Latissimus-Rib Flaps |
Age (Mean ± SEM) | 23 ± 2 years |
Males | 5 |
Females | 0 |
Tobacco Use | 2 |
Body Mass Index (Mean ± SEM) | 26.2 ± 0.9 kg/m2 |
Injury Severity Score (Mean ± SEM) | 18 ± 5 |
Time to Tissue Transfer (Mean ± SEM) | 1 ± 0.3 weeks |
Time to Boney Union (Mean ± SEM) | 7 ± 0.7 months |
Time Non-Weight-Bearing (Mean ± SEM) | 5 ± 0.5 months |
The MOI included motorcycle collisions (n = 2), pedestrian struck by car (n = 1), motor vehicle collisions (n = 1), and direct blow to the leg (n = 1). The mean ISS of the cohort was 18 (range, 10-34) (Table). On average, patients underwent free tissue transfer within 1 week (range, 3 days to 2 weeks) from the time of injury. Patients in this cohort were followed clinically for a mean of 4 years (range, 1-6 years) after surgery. Patients were non-weight-bearing for an average of 5 months (range, 4-6 months) following their reconstructions.
RESULTS
All flaps survived. The mean time to radiographic and clinical union was 7 months (range, 6-9 months). Two patients underwent reoperation. One patient underwent a bone grafting procedure for a delayed union at 6 months postoperative, and 1 patient underwent irrigation and débridement of superficial soft tissue infection. Donor site complications occurred in 2 patients, including chronic rib pain (n = 1) and a pleural effusion requiring drainage (n = 1). At the last follow-up, all ribs had incorporated, and all patients were weight-bearing as tolerated on the limb.
CASE EXAMPLE
A 22-year-old male smoker was transferred to our facility after a motor vehicle accident with bilateral tibia fractures, 1 closed and 1 open with significant bone loss (Figures 1A, 1B).
Continue to: Surgical Technique...
SURGICAL TECHNIQUE
The patient is placed in the lateral decubitus position during the procedure. A 2-team approach is used for dissection of the flap and preparation of recipient vessels to decrease operative time. A J-shaped incision is started on the chest at the mid-axillary line and extended just over the fifth and sixth rib. The incision can be made to fall into the intermammary crease in a woman to hide the scar. The dissection begins by exposing the anterior border of the latissimus muscle (Figure 2A). Next, the latissimus is dissected to reveal the thoracodorsal vessels (Figure 2B). At this level, the thoracodorsal vessel can be traced into the axilla. The branch going into the fifth, sixth, and lower slips of the serratus are dissected. The long thoracic nerve and the thoracodorsal nerve are preserved during the dissection (Figure 2C). The fifth, sixth, and seventh slips of the serratus are preferentially included in the dissection while leaving the most superior slips of the serratus to preserve scapular stability. Dissection begins by identifying 2 adjacent rib sections of the fifth and sixth or sixth and seventh ribs. The defect in the lower extremity determines the length of rib harvested. The serratus slips are then divided anteriorly over the chest wall. The dissection is extended to the intercostal spaces of the fourth and fifth ribs. The supraperiosteal dissection is performed at the anterior margin of the rib (Figure 2D).
Continue to: Following the surgical procedure...
Following the surgical procedure, patients are made non-weight-bearing on the operative extremity until signs of healing are apparent on radiographs. In this case, at the patients’ last follow-up visit, the skin graft was healed, and there was solid fusion of the rib/tibia junction (Figures 4B, 4C).
DISCUSSION
High-energy open injuries to the lower extremities are devastating injuries, with a high rate of late amputation and poor functional outcomes.11-13 Vascularized bone grafting provides both essential osteoinductive and osteoconductive properties to segmental bone defects in areas with inadequate soft tissue coverage, particularly in the setting of >6 cm of bone loss.4,14 The results of this study show that acute reconstruction of the lower limb with a composite vascularized SALR graft is a reliable procedure with an acceptable complication profile.
The timing of soft tissue coverage should be performed as soon as the patient is medically stable enough to undergo a reconstructive procedure, ideally within 7 to 10 days; and this timetable has been shown to decrease rates of infection and free flap failure.15-19 Early coverage provides both control of the soft-tissue envelope and reduces the risk of losing bone.1 Unlike the timing of coverage, the staging of the procedure is controversial. Proponents of the 2-stage free tissue (soft tissue followed by bony flap) transfer feel that although the tissue may not be infected at the time of coverage, it is contaminated with bacteria at the time of bone reconstruction, and as such is at high risk for both infection and complications.20 Unlike 2-stage procedures, single-stage coverage provides immediate soft tissue coverage, as well as bony support. This reduces the time to bony union and negates the need for repeated surgery in a mangled extremity where secondary surgery is complicated by both scar tissue and altered anatomy.1,2 Furthermore, it has been shown that there is no difference in the rates of infection when performing a single-stage compared with a 2-stage procedure.9 In this study, SALR flaps were typically performed within 2 weeks following an injury as a single procedure. We feel this resulted in the low number of complications in the SALR group.
Unlike free fibulas, rib flaps are easily pedicled with an associated soft-tissue flap due to their blood supply, making them ideal for 1-stage reconstruction. The rib has a dual blood supply: 1 from the posterior intercostal artery, and the other, an abundant periosteal blood supply, from the serratus anterior muscle.4 The blood supply to the serratus anterior comes from the thoracodorsal artery, and usually provides 14 cm of a large-caliber pedicle, making it a reliable flap for soft tissue reconstruction.21,22 Another unique feature of the blood supply to this flap is the amount of soft tissue available for both harvest and transfer; larger portions of serratus muscle and latissimus muscle can be harvested if necessary to cover the soft tissue defect.4
Comminuted tibias with segmental bone loss are difficult to manage since they are associated with bony as well as soft tissue defects.1,12,13,23 These injuries are ideal candidates for a single-stage reconstruction using a vascularized SALR flap. In our series, the use of an SALR flap resulted ultimately in a 100% union and limb salvage rate, with no flap failures and a low complication profile. Unlike the SALR, free fibular flaps must be transferred along with a separate latissimus dorsi flap to provide enough soft tissue coverage necessary for reconstructing large Gustilo IIIB injuries, which could increase the risk of flap failure. Since ribs are composed of membranous bone and have a similar cross-sectional area to both metacarpal and metatarsals, there are concerns regarding the biomechanical properties of ribs for weight-bearing.4,22,24-26 To compensate for this relatively small cross-sectional area, 2 ribs (either consecutive or alternative) are frequently harvested.1,4,5,23 Previous studies examining the use of ribs for bony reconstruction have frequently supplemented the rib reconstruction to the tibia using screws and external fixation alone.1,4,5,23 In our series, all SALR grafts were supported with the use of an intramedullary nail (n = 3) or locked plating (n = 1). The use of this supplemental fixation of the SALR graft allowed our patients to return to full weight-bearing (mean, 6 months) much earlier than the length of time cited in previous reports (12 months) examining these injuries.1,4,5,23
Continue to: There are several limitations...
There are several limitations to this study. The small sample size and retrospective nature of the study limits the amount of data we are able to collect from the medical record and places obvious constraints on the analysis. Although all these procedures were performed at 1 institution, multiple providers were involved in the reconstruction of these injuries, and there is no standard protocol for their treatment. Similarly, although other forms of extremity reconstruction were used during this time period, there was no standard protocol that could serve as a comparator for patients who underwent an SALR compared with other reconstructive procedures.
Overall, SALR grafts provide an excellent option for 1-stage reconstruction of severe, open lower extremity injuries. In this series we noted a 100% graft success rate with an acceptable complication profile.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Gustilo IIIB injuries of the tibia with segmental bone loss continue to be a difficult reconstructive problem. The serratus anterior-latissimus-rib (SALR) composite flap consists of bone and muscle; this flap can provide soft tissue coverage and vascularized bone in a single surgical procedure. The purpose of this study is to describe the use of the SALR flap for the treatment of a large open tibia fracture with segmental bone loss, with a specific focus on postoperative complications, limb salvage, and time to union.
We reviewed the medical records of patients undergoing an SALR flap (n = 5) for the treatment of Gustilo Type IIIB tibia fractures within 1 month of injury. We compared the mechanism of injury, injury severity score, time from injury to free tissue transfer, complications, and time to radiographic and clinical union.
All patients were male, with a mean age of 25 years. On average, patients underwent free tissue transfer within 1 week of injury. The average time to radiographic union was 7 months. Two patients underwent reoperation. There were no graft failures.
Free SALR flaps can be a useful option for the treatment of high-energy tibia fractures with extensive soft tissue and bone loss. These flaps provide immediate osseous and soft tissue reconstruction with an acceptable complication profile.
Reconstruction of the lower extremity following Gustilo’s grade IIIB injuries is difficult due to loss of both combined soft tissue and segmental bone loss. Since these injuries necessitate the need for soft tissue flap coverage along with vascularized bone grafting, free fibula flaps have classically been used for reconstruction.1-3 In the setting of bilateral lower extremity injury, the contralateral fibula is often not appropriate to harvest and transfer; therefore, other sources of vascularized bone grafts must be utilized including vascularized iliac crest and rib.1-5 The vascularized iliac crest graft is insufficient to provide the bony reconstruction of bone defects >6 cm to 7 cm and does not have a reliable skin paddle.4 In contrast, free composite serratus anterior-latissimus-rib (SALR) flaps can provide both long segments of vascularized bone and abundant soft tissue coverage for large segmental defects.1-5
Continue to: Free fibula grafts have been considered...
Free fibula grafts have been considered the gold standard for the reconstruction of large (>6 cm) bone defects.6 In cases of “mangled extremities,” bone defects are associated with large soft tissue defects, which require either single-stage surgery consisting of 2 separate free flaps (ie, free fibula and free latissimus) or a 2-stage procedure where the soft tissue reconstruction precedes the bone reconstruction.2,7-9 Unlike free fibula and latissimus flaps, composite SALR flaps provide both osseous reconstruction and soft tissue in 1 flap supplied by a single vascular pedicle; unfortunately, outcomes using this flap for large Gustilo IIIB injuries are limited.1-5 The purpose of this study is to examine the use of free composite SALR flaps for soft tissue coverage in cases of Gustilo IIIB injuries with large soft tissue and bony deficits. This study specifically examines time to union, need for reoperation, and graft failure following the use of these flaps.
MATERIALS AND METHODS
Following approval from our Institutional Review Board, we retrospectively reviewed the medical records of patients undergoing a free composite SALR flap (n = 5) for the treatment of a severe open tibia fracture within 1 month of injury. All patients sustained open injuries classified as IIIB on the Gustilo-Anderson scale.10 Medical records were examined for the mechanism of injury (MOI), injury severity score (ISS), time from injury to free tissue transfer, medical comorbidities, surgical complications, and time to radiographic and clinical union. Radiographic union was determined by the presence of bridging bone on 3 of 4 of cortices on plain film radiographs.
All patients were male (n = 5), with a mean age of 25 years (range, 19-30 years) at the time of injury (Table).
Table. Demographics and Outcomes of Patients Undergoing Free Tissue Transfer
| Free Serratus Anterior-Latissimus-Rib Flaps |
Age (Mean ± SEM) | 23 ± 2 years |
Males | 5 |
Females | 0 |
Tobacco Use | 2 |
Body Mass Index (Mean ± SEM) | 26.2 ± 0.9 kg/m2 |
Injury Severity Score (Mean ± SEM) | 18 ± 5 |
Time to Tissue Transfer (Mean ± SEM) | 1 ± 0.3 weeks |
Time to Boney Union (Mean ± SEM) | 7 ± 0.7 months |
Time Non-Weight-Bearing (Mean ± SEM) | 5 ± 0.5 months |
The MOI included motorcycle collisions (n = 2), pedestrian struck by car (n = 1), motor vehicle collisions (n = 1), and direct blow to the leg (n = 1). The mean ISS of the cohort was 18 (range, 10-34) (Table). On average, patients underwent free tissue transfer within 1 week (range, 3 days to 2 weeks) from the time of injury. Patients in this cohort were followed clinically for a mean of 4 years (range, 1-6 years) after surgery. Patients were non-weight-bearing for an average of 5 months (range, 4-6 months) following their reconstructions.
RESULTS
All flaps survived. The mean time to radiographic and clinical union was 7 months (range, 6-9 months). Two patients underwent reoperation. One patient underwent a bone grafting procedure for a delayed union at 6 months postoperative, and 1 patient underwent irrigation and débridement of superficial soft tissue infection. Donor site complications occurred in 2 patients, including chronic rib pain (n = 1) and a pleural effusion requiring drainage (n = 1). At the last follow-up, all ribs had incorporated, and all patients were weight-bearing as tolerated on the limb.
CASE EXAMPLE
A 22-year-old male smoker was transferred to our facility after a motor vehicle accident with bilateral tibia fractures, 1 closed and 1 open with significant bone loss (Figures 1A, 1B).
Continue to: Surgical Technique...
SURGICAL TECHNIQUE
The patient is placed in the lateral decubitus position during the procedure. A 2-team approach is used for dissection of the flap and preparation of recipient vessels to decrease operative time. A J-shaped incision is started on the chest at the mid-axillary line and extended just over the fifth and sixth rib. The incision can be made to fall into the intermammary crease in a woman to hide the scar. The dissection begins by exposing the anterior border of the latissimus muscle (Figure 2A). Next, the latissimus is dissected to reveal the thoracodorsal vessels (Figure 2B). At this level, the thoracodorsal vessel can be traced into the axilla. The branch going into the fifth, sixth, and lower slips of the serratus are dissected. The long thoracic nerve and the thoracodorsal nerve are preserved during the dissection (Figure 2C). The fifth, sixth, and seventh slips of the serratus are preferentially included in the dissection while leaving the most superior slips of the serratus to preserve scapular stability. Dissection begins by identifying 2 adjacent rib sections of the fifth and sixth or sixth and seventh ribs. The defect in the lower extremity determines the length of rib harvested. The serratus slips are then divided anteriorly over the chest wall. The dissection is extended to the intercostal spaces of the fourth and fifth ribs. The supraperiosteal dissection is performed at the anterior margin of the rib (Figure 2D).
Continue to: Following the surgical procedure...
Following the surgical procedure, patients are made non-weight-bearing on the operative extremity until signs of healing are apparent on radiographs. In this case, at the patients’ last follow-up visit, the skin graft was healed, and there was solid fusion of the rib/tibia junction (Figures 4B, 4C).
DISCUSSION
High-energy open injuries to the lower extremities are devastating injuries, with a high rate of late amputation and poor functional outcomes.11-13 Vascularized bone grafting provides both essential osteoinductive and osteoconductive properties to segmental bone defects in areas with inadequate soft tissue coverage, particularly in the setting of >6 cm of bone loss.4,14 The results of this study show that acute reconstruction of the lower limb with a composite vascularized SALR graft is a reliable procedure with an acceptable complication profile.
The timing of soft tissue coverage should be performed as soon as the patient is medically stable enough to undergo a reconstructive procedure, ideally within 7 to 10 days; and this timetable has been shown to decrease rates of infection and free flap failure.15-19 Early coverage provides both control of the soft-tissue envelope and reduces the risk of losing bone.1 Unlike the timing of coverage, the staging of the procedure is controversial. Proponents of the 2-stage free tissue (soft tissue followed by bony flap) transfer feel that although the tissue may not be infected at the time of coverage, it is contaminated with bacteria at the time of bone reconstruction, and as such is at high risk for both infection and complications.20 Unlike 2-stage procedures, single-stage coverage provides immediate soft tissue coverage, as well as bony support. This reduces the time to bony union and negates the need for repeated surgery in a mangled extremity where secondary surgery is complicated by both scar tissue and altered anatomy.1,2 Furthermore, it has been shown that there is no difference in the rates of infection when performing a single-stage compared with a 2-stage procedure.9 In this study, SALR flaps were typically performed within 2 weeks following an injury as a single procedure. We feel this resulted in the low number of complications in the SALR group.
Unlike free fibulas, rib flaps are easily pedicled with an associated soft-tissue flap due to their blood supply, making them ideal for 1-stage reconstruction. The rib has a dual blood supply: 1 from the posterior intercostal artery, and the other, an abundant periosteal blood supply, from the serratus anterior muscle.4 The blood supply to the serratus anterior comes from the thoracodorsal artery, and usually provides 14 cm of a large-caliber pedicle, making it a reliable flap for soft tissue reconstruction.21,22 Another unique feature of the blood supply to this flap is the amount of soft tissue available for both harvest and transfer; larger portions of serratus muscle and latissimus muscle can be harvested if necessary to cover the soft tissue defect.4
Comminuted tibias with segmental bone loss are difficult to manage since they are associated with bony as well as soft tissue defects.1,12,13,23 These injuries are ideal candidates for a single-stage reconstruction using a vascularized SALR flap. In our series, the use of an SALR flap resulted ultimately in a 100% union and limb salvage rate, with no flap failures and a low complication profile. Unlike the SALR, free fibular flaps must be transferred along with a separate latissimus dorsi flap to provide enough soft tissue coverage necessary for reconstructing large Gustilo IIIB injuries, which could increase the risk of flap failure. Since ribs are composed of membranous bone and have a similar cross-sectional area to both metacarpal and metatarsals, there are concerns regarding the biomechanical properties of ribs for weight-bearing.4,22,24-26 To compensate for this relatively small cross-sectional area, 2 ribs (either consecutive or alternative) are frequently harvested.1,4,5,23 Previous studies examining the use of ribs for bony reconstruction have frequently supplemented the rib reconstruction to the tibia using screws and external fixation alone.1,4,5,23 In our series, all SALR grafts were supported with the use of an intramedullary nail (n = 3) or locked plating (n = 1). The use of this supplemental fixation of the SALR graft allowed our patients to return to full weight-bearing (mean, 6 months) much earlier than the length of time cited in previous reports (12 months) examining these injuries.1,4,5,23
Continue to: There are several limitations...
There are several limitations to this study. The small sample size and retrospective nature of the study limits the amount of data we are able to collect from the medical record and places obvious constraints on the analysis. Although all these procedures were performed at 1 institution, multiple providers were involved in the reconstruction of these injuries, and there is no standard protocol for their treatment. Similarly, although other forms of extremity reconstruction were used during this time period, there was no standard protocol that could serve as a comparator for patients who underwent an SALR compared with other reconstructive procedures.
Overall, SALR grafts provide an excellent option for 1-stage reconstruction of severe, open lower extremity injuries. In this series we noted a 100% graft success rate with an acceptable complication profile.
This paper will be judged for the Resident Writer’s Award.
1. Yazar S, Lin CH, Wei FC. One-stage reconstruction of composite bone and soft-tissue defects in traumatic lower extremities. Plast Reconstr Surg. 2004;114(6):1457-1466. doi:10.1097/01.PRS.0000138811.88807.65.
2. Lin CH, Wei FC, Chen HC, Chuang DC. Outcome comparison in traumatic lower-extremity reconstruction by using various composite vascularized bone transplantation. Plast Reconstr Surg. 1999;104(4):984-992. doi:10.1097/00006534-199909040-00013.
3. Tu YK, Yen CY, Yeh WL, Wang IC, Wang KC, Ueng SW. Reconstruction of posttraumatic long bone defect with free vascularized bone graft: good outcome in 48 patients with 6 years' follow-up. Acta Orthopaedica Scandinavica. 2001;72(4):359-364. doi:10.1080/000164701753542014.
4. Lin CH, Wei FC, Levin LS, Su JI, Fan KF, Yeh WL, Hsu DT. Free composite serratus anterior and rib flaps for tibial composite bone and soft-tissue defect. Plast Reconstr Surg. 1997;99(6):1656-1665. Doi:10.1097/00006534-199705000-00028.
5. Georgescu AV, Ignatiadis I, Ileana M, Irina C, Filip A, Olariu R. Long-term results after muscle-rib flap transfer for reconstruction of composite limb defects. Microsurgery. 2011;31(3):218-222. doi:10.1002/micr.20857.
6. Moran CG, Wood MB. Vascularized bone autografts. Orthop Rev. 1993;22(2):187-197. doi:10.1097/01241398-199307000-00031.
7. Banic A, Hertel R. Double vascularized fibulas for reconstruction of large tibial defects. J Reconstr Microsurg. 1993;9(6):421-428. doi:10.1055/s-2007-1006751.
8. Malizos KN, Nunley JA, Goldner RD, Urbaniak JR, Harrelson JM. Free vascularized fibula in traumatic long bone defects and in limb salvaging following tumor resection: comparative study. Microsurgery. 1993;14(6):368-374. doi:10.1002/micr.1920140603.
9. Peat BG, Liggins DF. Microvascular soft tissue reconstruction for acute tibial fractures--late complications and the role of bone grafting. Ann Plast Surg. 1990;24(6):517-520.
10. Gustilo RB, Anderson JT. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976;58(4):453-458.
11. Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma. 1984;24(8):742-746. doi:10.1097/00005373-198408000-00009.
12. Bosse MJ, MacKenzie EJ, Kellam JF, et al. An analysis of outcomes of reconstruction or amputation after leg-threatening injuries. NEJM. 2002;347(24):1924-1931. doi:10.1056/NEJMoa012604.
13. MacKenzie EJ, Bosse MJ, Pollak AN, et al. Long-term persistence of disability following severe lower-limb trauma. Results of a seven-year follow-up. J Bone Joint Surg Am. 2005;87(8):1801-1809. doi:10.2106/JBJS.E.00032.
14. Bieber EJ, Wood MB. Bone reconstruction. Clin Plast Surg. 1986;13(4):645-655.
15. Melvin JS, Dombroski DG, Torbert JT, Kovach SJ, Esterhai JL, Mehta S. Open tibial shaft fractures: II. Definitive management and limb salvage. J Am Acad Orthop Surg. 2010;18(2):108-117. doi:10.5435/00124635-201002000-00005.
16. Godina M. Early microsurgical reconstruction of complex trauma of the extremities. Plast Reconstr Surg. 1986;78(3):285-292. doi:10.1055/s-2006-944324.
17. Gopal S, Majumder S, Batchelor AG, Knight SL, De Boer P, Smith RM. Fix and flap: the radical orthopaedic and plastic treatment of severe open fractures of the tibia. J Bone Joint Surg Br. 2000;82(7):959-966. doi:10.1302/0301-620X.82B7.0820959.
18. Fischer MD, Gustilo RB, Varecka TF. The timing of flap coverage, bone-grafting, and intramedullary nailing in patients who have a fracture of the tibial shaft with extensive soft-tissue injury. J Bone Joint Surg Am. 1991;73(9):1316-1322. doi:10.2106/00004623-199173090-00005.
19. Tielinen L, Lindahl JE, Tukiainen EJ. Acute unreamed intramedullary nailing and soft tissue reconstruction with muscle flaps for the treatment of severe open tibial shaft fractures. Injury. 2007;38(8):906-912. doi:10.1016/j.injury.2007.02.052.
20. Yaremchuk MJ, Brumback RJ, Manson PN, Burgess AR, Poka A, Weiland AJ. Acute and definitive management of traumatic osteocutaneous defects of the lower extremity. Plast Reconstr Surg. 1987;80(1):1-14. doi:10.1097/00006534-198707000-00002.
21. Ueng WN, Chuang CC, Shih CH. Double-rib composite free transfer to reconstruct a single-spared lower extremity defect. J Trauma. 1995;38(2):210-212.
22. Bruck JC, Bier J, Kistler D. The serratus anterior osteocutaneous free flap. J Reconstr Microsurg. 1990;6(3):209-213. doi:10.1055/s-2007-1006820.
23. Lin CH, Yazar S. Revisiting the serratus anterior rib flap for composite tibial defects. Plast Reconstr Surg. 2004;114(7):1871-1877. doi:10.1097/01.PRS.0000142767.13493.63.
24. Hui KC, Zhang F, Lineaweaver WC, Moon W, Buncke GM, Buncke HJ. Serratus anterior-rib composite flap: anatomic studies and clinical application to hand reconstruction. Ann Plast Surg. 1999;42(2):132-136. doi:10.1097/00000637-199902000-00004.
25. Buncke HJ, Furnas DW, Gordon L, Achauer BM. Free osteocutaneous flap from a rib to the tibia. Plast Reconstr Surg. 1977;59(6):799-804. doi:10.1097/00006534-197706000-00002.
26. Nusbickel FR, Dell PC, Mcandrew MP, Moore MM. Vascularized autografts for reconstruction of skeletal defects following lower extremity trauma. A review. Clin Orthop Relat Res. 1989;(243):65-70.
1. Yazar S, Lin CH, Wei FC. One-stage reconstruction of composite bone and soft-tissue defects in traumatic lower extremities. Plast Reconstr Surg. 2004;114(6):1457-1466. doi:10.1097/01.PRS.0000138811.88807.65.
2. Lin CH, Wei FC, Chen HC, Chuang DC. Outcome comparison in traumatic lower-extremity reconstruction by using various composite vascularized bone transplantation. Plast Reconstr Surg. 1999;104(4):984-992. doi:10.1097/00006534-199909040-00013.
3. Tu YK, Yen CY, Yeh WL, Wang IC, Wang KC, Ueng SW. Reconstruction of posttraumatic long bone defect with free vascularized bone graft: good outcome in 48 patients with 6 years' follow-up. Acta Orthopaedica Scandinavica. 2001;72(4):359-364. doi:10.1080/000164701753542014.
4. Lin CH, Wei FC, Levin LS, Su JI, Fan KF, Yeh WL, Hsu DT. Free composite serratus anterior and rib flaps for tibial composite bone and soft-tissue defect. Plast Reconstr Surg. 1997;99(6):1656-1665. Doi:10.1097/00006534-199705000-00028.
5. Georgescu AV, Ignatiadis I, Ileana M, Irina C, Filip A, Olariu R. Long-term results after muscle-rib flap transfer for reconstruction of composite limb defects. Microsurgery. 2011;31(3):218-222. doi:10.1002/micr.20857.
6. Moran CG, Wood MB. Vascularized bone autografts. Orthop Rev. 1993;22(2):187-197. doi:10.1097/01241398-199307000-00031.
7. Banic A, Hertel R. Double vascularized fibulas for reconstruction of large tibial defects. J Reconstr Microsurg. 1993;9(6):421-428. doi:10.1055/s-2007-1006751.
8. Malizos KN, Nunley JA, Goldner RD, Urbaniak JR, Harrelson JM. Free vascularized fibula in traumatic long bone defects and in limb salvaging following tumor resection: comparative study. Microsurgery. 1993;14(6):368-374. doi:10.1002/micr.1920140603.
9. Peat BG, Liggins DF. Microvascular soft tissue reconstruction for acute tibial fractures--late complications and the role of bone grafting. Ann Plast Surg. 1990;24(6):517-520.
10. Gustilo RB, Anderson JT. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976;58(4):453-458.
11. Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma. 1984;24(8):742-746. doi:10.1097/00005373-198408000-00009.
12. Bosse MJ, MacKenzie EJ, Kellam JF, et al. An analysis of outcomes of reconstruction or amputation after leg-threatening injuries. NEJM. 2002;347(24):1924-1931. doi:10.1056/NEJMoa012604.
13. MacKenzie EJ, Bosse MJ, Pollak AN, et al. Long-term persistence of disability following severe lower-limb trauma. Results of a seven-year follow-up. J Bone Joint Surg Am. 2005;87(8):1801-1809. doi:10.2106/JBJS.E.00032.
14. Bieber EJ, Wood MB. Bone reconstruction. Clin Plast Surg. 1986;13(4):645-655.
15. Melvin JS, Dombroski DG, Torbert JT, Kovach SJ, Esterhai JL, Mehta S. Open tibial shaft fractures: II. Definitive management and limb salvage. J Am Acad Orthop Surg. 2010;18(2):108-117. doi:10.5435/00124635-201002000-00005.
16. Godina M. Early microsurgical reconstruction of complex trauma of the extremities. Plast Reconstr Surg. 1986;78(3):285-292. doi:10.1055/s-2006-944324.
17. Gopal S, Majumder S, Batchelor AG, Knight SL, De Boer P, Smith RM. Fix and flap: the radical orthopaedic and plastic treatment of severe open fractures of the tibia. J Bone Joint Surg Br. 2000;82(7):959-966. doi:10.1302/0301-620X.82B7.0820959.
18. Fischer MD, Gustilo RB, Varecka TF. The timing of flap coverage, bone-grafting, and intramedullary nailing in patients who have a fracture of the tibial shaft with extensive soft-tissue injury. J Bone Joint Surg Am. 1991;73(9):1316-1322. doi:10.2106/00004623-199173090-00005.
19. Tielinen L, Lindahl JE, Tukiainen EJ. Acute unreamed intramedullary nailing and soft tissue reconstruction with muscle flaps for the treatment of severe open tibial shaft fractures. Injury. 2007;38(8):906-912. doi:10.1016/j.injury.2007.02.052.
20. Yaremchuk MJ, Brumback RJ, Manson PN, Burgess AR, Poka A, Weiland AJ. Acute and definitive management of traumatic osteocutaneous defects of the lower extremity. Plast Reconstr Surg. 1987;80(1):1-14. doi:10.1097/00006534-198707000-00002.
21. Ueng WN, Chuang CC, Shih CH. Double-rib composite free transfer to reconstruct a single-spared lower extremity defect. J Trauma. 1995;38(2):210-212.
22. Bruck JC, Bier J, Kistler D. The serratus anterior osteocutaneous free flap. J Reconstr Microsurg. 1990;6(3):209-213. doi:10.1055/s-2007-1006820.
23. Lin CH, Yazar S. Revisiting the serratus anterior rib flap for composite tibial defects. Plast Reconstr Surg. 2004;114(7):1871-1877. doi:10.1097/01.PRS.0000142767.13493.63.
24. Hui KC, Zhang F, Lineaweaver WC, Moon W, Buncke GM, Buncke HJ. Serratus anterior-rib composite flap: anatomic studies and clinical application to hand reconstruction. Ann Plast Surg. 1999;42(2):132-136. doi:10.1097/00000637-199902000-00004.
25. Buncke HJ, Furnas DW, Gordon L, Achauer BM. Free osteocutaneous flap from a rib to the tibia. Plast Reconstr Surg. 1977;59(6):799-804. doi:10.1097/00006534-197706000-00002.
26. Nusbickel FR, Dell PC, Mcandrew MP, Moore MM. Vascularized autografts for reconstruction of skeletal defects following lower extremity trauma. A review. Clin Orthop Relat Res. 1989;(243):65-70.
TAKE-HOME POINTS
- Gustilo IIIB injuries with segmental bone loss can be difficult to treat with conventional means.
- Vascularized bone grafts are beneficial for reconstructing bone defects >5 cm.
- The SALR composite flap consists of bone and muscle.
- This flap can provide soft tissue coverage and vascularized bone in a single surgical procedure.
- In this study, the use of the SALR composite flap was capable of healing large segmental bony defects at an average of 7 months.