User login
Continuation, complication rates similar for implants, IUDs
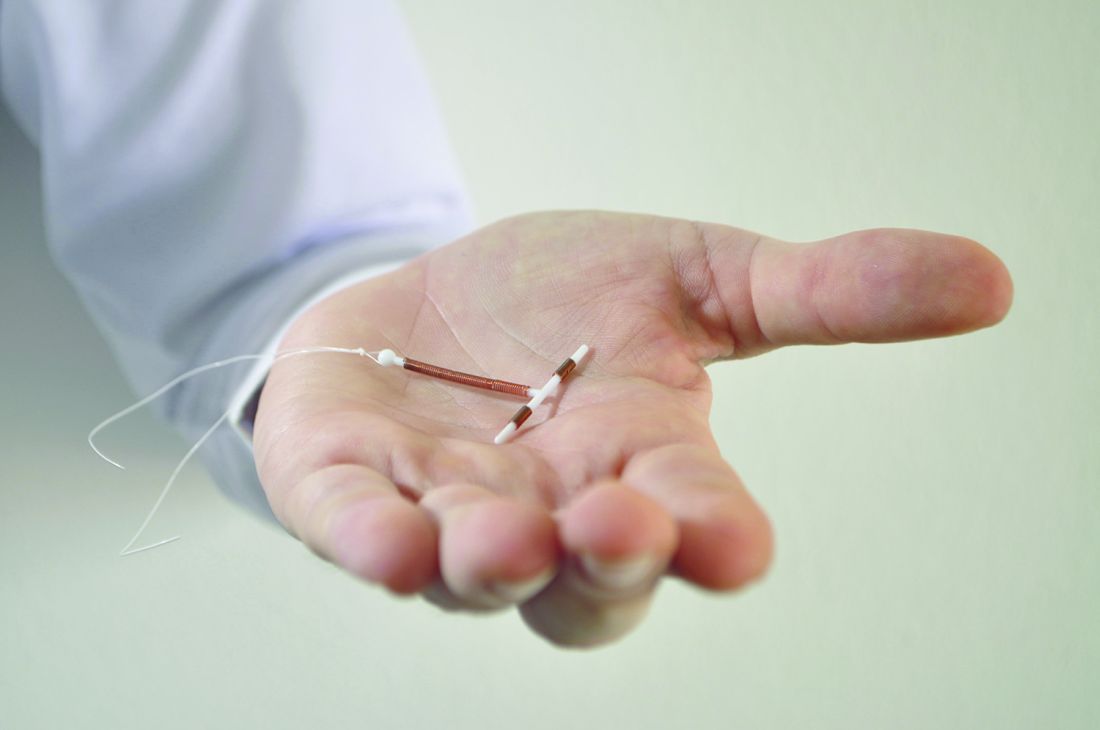
A new study of Medicaid-covered women who sought long-acting reversible contraception via subdermal etonogestrel implants or IUDs found that levels of continuation at 1 year were high, and complication rates were low.
There was little difference between the groups on both fronts.
“This study demonstrates high rates of continued use of subdermal and intrauterine contraceptives by Medicaid clients, particularly among adolescents, and therefore, interventions that increase adolescent access to these contraceptives are likely to be cost effective from the payer perspective,” the study authors wrote in Contraception.
The study, led by Max J. Romano, MD, MPH, of MedStar Franklin Square Medical Center in Baltimore, retrospectively examined the medical claims of 3,305 women treated via MedStar Family Choice, a Medicaid managed-choice insurer.
The women, who lived in Maryland and the District of Columbia, were aged 15-44 years when they underwent contraceptive treatment during 2012-2015. Of the women, 1,335 had subdermal etonogestrel implants inserted, and 1,970 had IUDs inserted. Researchers followed the women for a mean 344 days.
The implant users were younger than the IUD group (mean age, 25 years vs. 28 years; P = less than .001), and women under age 20 years were more than more than twice as likely to get implants than IUDs (71% vs. 29%).
Women older than 20 years were more likely to get IUDs than implants, with those older than 25 preferring them by a ratio of 70% to 30%.
After researchers controlled for such factors as age group and year of insertion, they found that implant recipients were slightly more likely to still have the contraception tool in place at 1 year: 81% (implants) vs. IUDs (77%; P = .01). It’s not clear why women had their implants or IUDs removed.
Claims for complications were similar between the groups (8% for implants, 7% for IUDs), and researchers didn’t find a statistically significant difference after they controlled for various factors. Dysfunctional uterine bleeding was the most common complication, followed by excessive or frequent menstruation.
Few women reported pregnancy among the implant (0.82%) and IUD (0.86%) groups, and there was no statistically significant difference between the groups.
The researchers reported that their study has various limitations: The insurance claims information may provide misleading information about minor complications, and it doesn’t include details about abortions. The claims also may not report IUD expulsions and self-removals.
The authors also noted that they didn’t control for factors like socioeconomic status, psychological conditions, and multimorbidity.
MedStar Family Choice provided study funding. Dr. Romano and Loral Patchen, PhD, disclosed receiving free CME trainings and insertion certification regarding the long-acting contraceptive devices discussed in the study from Merck, Bayer, and Teva. Dr. Patchen disclosed serving on the Diclegis speakers bureau.
A new study of Medicaid-covered women who sought long-acting reversible contraception via subdermal etonogestrel implants or IUDs found that levels of continuation at 1 year were high, and complication rates were low.
There was little difference between the groups on both fronts.
“This study demonstrates high rates of continued use of subdermal and intrauterine contraceptives by Medicaid clients, particularly among adolescents, and therefore, interventions that increase adolescent access to these contraceptives are likely to be cost effective from the payer perspective,” the study authors wrote in Contraception.
The study, led by Max J. Romano, MD, MPH, of MedStar Franklin Square Medical Center in Baltimore, retrospectively examined the medical claims of 3,305 women treated via MedStar Family Choice, a Medicaid managed-choice insurer.
The women, who lived in Maryland and the District of Columbia, were aged 15-44 years when they underwent contraceptive treatment during 2012-2015. Of the women, 1,335 had subdermal etonogestrel implants inserted, and 1,970 had IUDs inserted. Researchers followed the women for a mean 344 days.
The implant users were younger than the IUD group (mean age, 25 years vs. 28 years; P = less than .001), and women under age 20 years were more than more than twice as likely to get implants than IUDs (71% vs. 29%).
Women older than 20 years were more likely to get IUDs than implants, with those older than 25 preferring them by a ratio of 70% to 30%.
After researchers controlled for such factors as age group and year of insertion, they found that implant recipients were slightly more likely to still have the contraception tool in place at 1 year: 81% (implants) vs. IUDs (77%; P = .01). It’s not clear why women had their implants or IUDs removed.
Claims for complications were similar between the groups (8% for implants, 7% for IUDs), and researchers didn’t find a statistically significant difference after they controlled for various factors. Dysfunctional uterine bleeding was the most common complication, followed by excessive or frequent menstruation.
Few women reported pregnancy among the implant (0.82%) and IUD (0.86%) groups, and there was no statistically significant difference between the groups.
The researchers reported that their study has various limitations: The insurance claims information may provide misleading information about minor complications, and it doesn’t include details about abortions. The claims also may not report IUD expulsions and self-removals.
The authors also noted that they didn’t control for factors like socioeconomic status, psychological conditions, and multimorbidity.
MedStar Family Choice provided study funding. Dr. Romano and Loral Patchen, PhD, disclosed receiving free CME trainings and insertion certification regarding the long-acting contraceptive devices discussed in the study from Merck, Bayer, and Teva. Dr. Patchen disclosed serving on the Diclegis speakers bureau.
A new study of Medicaid-covered women who sought long-acting reversible contraception via subdermal etonogestrel implants or IUDs found that levels of continuation at 1 year were high, and complication rates were low.
There was little difference between the groups on both fronts.
“This study demonstrates high rates of continued use of subdermal and intrauterine contraceptives by Medicaid clients, particularly among adolescents, and therefore, interventions that increase adolescent access to these contraceptives are likely to be cost effective from the payer perspective,” the study authors wrote in Contraception.
The study, led by Max J. Romano, MD, MPH, of MedStar Franklin Square Medical Center in Baltimore, retrospectively examined the medical claims of 3,305 women treated via MedStar Family Choice, a Medicaid managed-choice insurer.
The women, who lived in Maryland and the District of Columbia, were aged 15-44 years when they underwent contraceptive treatment during 2012-2015. Of the women, 1,335 had subdermal etonogestrel implants inserted, and 1,970 had IUDs inserted. Researchers followed the women for a mean 344 days.
The implant users were younger than the IUD group (mean age, 25 years vs. 28 years; P = less than .001), and women under age 20 years were more than more than twice as likely to get implants than IUDs (71% vs. 29%).
Women older than 20 years were more likely to get IUDs than implants, with those older than 25 preferring them by a ratio of 70% to 30%.
After researchers controlled for such factors as age group and year of insertion, they found that implant recipients were slightly more likely to still have the contraception tool in place at 1 year: 81% (implants) vs. IUDs (77%; P = .01). It’s not clear why women had their implants or IUDs removed.
Claims for complications were similar between the groups (8% for implants, 7% for IUDs), and researchers didn’t find a statistically significant difference after they controlled for various factors. Dysfunctional uterine bleeding was the most common complication, followed by excessive or frequent menstruation.
Few women reported pregnancy among the implant (0.82%) and IUD (0.86%) groups, and there was no statistically significant difference between the groups.
The researchers reported that their study has various limitations: The insurance claims information may provide misleading information about minor complications, and it doesn’t include details about abortions. The claims also may not report IUD expulsions and self-removals.
The authors also noted that they didn’t control for factors like socioeconomic status, psychological conditions, and multimorbidity.
MedStar Family Choice provided study funding. Dr. Romano and Loral Patchen, PhD, disclosed receiving free CME trainings and insertion certification regarding the long-acting contraceptive devices discussed in the study from Merck, Bayer, and Teva. Dr. Patchen disclosed serving on the Diclegis speakers bureau.
FROM CONTRACEPTION
Key clinical point:
Major finding: For implants and IUDS, respectively, 1-year continuation rates were similar (adjusted 81% vs. 77%, P = .01), as were complication rates (unadjusted 8% for implants, 7% for IUDs).
Study details: Retrospective analysis of 3,305 Medicaid-covered women treated with the two types of contraceptives during 2012-2015 in the District of Columbia and Maryland.
Disclosures: MedStar Family Choice provided study funding. Dr. Romano and Loral Patchen, PhD, disclosed receiving free CME trainings and insertion certification regarding the long-acting contraceptive devices discussed in the study from Merck, Bayer, and Teva. Dr. Patchen discloses serving on the Diclegis speakers bureau.
Source: Romano MJ et al. Contraception. 2018 Aug;98:125-9.
New IUD expelled less often after C-section than older device
that’s inserted the same way, according to results of a Turkish study.
The study authors, led by Ceren Unal, MD, of Zeynep Kamil Women’s and Children’s Disease Training and Research Hospital, Istanbul, wrote that the new device, the frameless copper-releasing Gyn-CS IUD, “could be a major advance, potentially suitable for general use due to the ease and safety of the insertion procedure,” which requires limited training.
According to the study authors, most IUDs are retained in the uterus, and most have a T-shape configuration. Some are inserted immediately following expulsion of the placenta at birth, although techniques “are far from being optimal,” and devices are frequently displaced and sometimes expelled.
The newly developed Gyn-CS IUD, a redesigned version of a previous model, has an alternative “anchor” design.
The investigators tracked 140 pregnant women – 106 who underwent elective cesarean section and 34 who underwent emergency cesarean section – who had the Gyn-CS or the TCu380A IUD inserted shortly after they gave birth. Their median age was around 30 years , all were white, and all were married.
The TCu380A IUD, a decades-old model that’s been recommended by the World Health Organization, and the Gyn-CS IUD were each inserted in 70 women. The researchers then followed them for 3 months. Three women (one in the Gyn-CS IUD group and two in the TCu380A IUD group) were lost to follow-up.
In total, 61 of 70 IUDs (88%) remained in place in the Gyn-CS group, and 54 of 70 (79%) in the TCu380A group (P = .30). One (1%) Gyn-CS IUD was expelled , possibly because it was incorrectly anchored, and eight (11%) TCu380A IUDs were expelled (P = .04). There were equal numbers of medical removals (four) and nonmedical removals (two) in the two groups, and one “other medical removal” in the Gyn-CS IUD group.
“The very low expulsion rate of Gyn-CS, compared with TCu380A, is a very strong argument in favor of the anchored IUD, preventing expulsion and displacement,” Dr. Unal and colleagues reported in Contraception.
They noted that the study has a limited 3-month tracking period, but they also pointed out that most expulsions inserted post partum occurred in the initial 6 weeks.
The low expulsion rate of the Gyn-CS device “will prevent more women becoming pregnant too soon which constitutes an important safety issue during future pregnancy,” the authors wrote. “The device, preferably its high-load 10-year version, could also interest many women as a reversible alternative for tubal sterilization. Additional clinical experience with the Gyn-CS IUD is, therefore, urgently warranted.”
No study funding is reported, although Contrel Research provided the Gyn-CS devices at no charge. The study authors reported no relevant disclosures.
that’s inserted the same way, according to results of a Turkish study.
The study authors, led by Ceren Unal, MD, of Zeynep Kamil Women’s and Children’s Disease Training and Research Hospital, Istanbul, wrote that the new device, the frameless copper-releasing Gyn-CS IUD, “could be a major advance, potentially suitable for general use due to the ease and safety of the insertion procedure,” which requires limited training.
According to the study authors, most IUDs are retained in the uterus, and most have a T-shape configuration. Some are inserted immediately following expulsion of the placenta at birth, although techniques “are far from being optimal,” and devices are frequently displaced and sometimes expelled.
The newly developed Gyn-CS IUD, a redesigned version of a previous model, has an alternative “anchor” design.
The investigators tracked 140 pregnant women – 106 who underwent elective cesarean section and 34 who underwent emergency cesarean section – who had the Gyn-CS or the TCu380A IUD inserted shortly after they gave birth. Their median age was around 30 years , all were white, and all were married.
The TCu380A IUD, a decades-old model that’s been recommended by the World Health Organization, and the Gyn-CS IUD were each inserted in 70 women. The researchers then followed them for 3 months. Three women (one in the Gyn-CS IUD group and two in the TCu380A IUD group) were lost to follow-up.
In total, 61 of 70 IUDs (88%) remained in place in the Gyn-CS group, and 54 of 70 (79%) in the TCu380A group (P = .30). One (1%) Gyn-CS IUD was expelled , possibly because it was incorrectly anchored, and eight (11%) TCu380A IUDs were expelled (P = .04). There were equal numbers of medical removals (four) and nonmedical removals (two) in the two groups, and one “other medical removal” in the Gyn-CS IUD group.
“The very low expulsion rate of Gyn-CS, compared with TCu380A, is a very strong argument in favor of the anchored IUD, preventing expulsion and displacement,” Dr. Unal and colleagues reported in Contraception.
They noted that the study has a limited 3-month tracking period, but they also pointed out that most expulsions inserted post partum occurred in the initial 6 weeks.
The low expulsion rate of the Gyn-CS device “will prevent more women becoming pregnant too soon which constitutes an important safety issue during future pregnancy,” the authors wrote. “The device, preferably its high-load 10-year version, could also interest many women as a reversible alternative for tubal sterilization. Additional clinical experience with the Gyn-CS IUD is, therefore, urgently warranted.”
No study funding is reported, although Contrel Research provided the Gyn-CS devices at no charge. The study authors reported no relevant disclosures.
that’s inserted the same way, according to results of a Turkish study.
The study authors, led by Ceren Unal, MD, of Zeynep Kamil Women’s and Children’s Disease Training and Research Hospital, Istanbul, wrote that the new device, the frameless copper-releasing Gyn-CS IUD, “could be a major advance, potentially suitable for general use due to the ease and safety of the insertion procedure,” which requires limited training.
According to the study authors, most IUDs are retained in the uterus, and most have a T-shape configuration. Some are inserted immediately following expulsion of the placenta at birth, although techniques “are far from being optimal,” and devices are frequently displaced and sometimes expelled.
The newly developed Gyn-CS IUD, a redesigned version of a previous model, has an alternative “anchor” design.
The investigators tracked 140 pregnant women – 106 who underwent elective cesarean section and 34 who underwent emergency cesarean section – who had the Gyn-CS or the TCu380A IUD inserted shortly after they gave birth. Their median age was around 30 years , all were white, and all were married.
The TCu380A IUD, a decades-old model that’s been recommended by the World Health Organization, and the Gyn-CS IUD were each inserted in 70 women. The researchers then followed them for 3 months. Three women (one in the Gyn-CS IUD group and two in the TCu380A IUD group) were lost to follow-up.
In total, 61 of 70 IUDs (88%) remained in place in the Gyn-CS group, and 54 of 70 (79%) in the TCu380A group (P = .30). One (1%) Gyn-CS IUD was expelled , possibly because it was incorrectly anchored, and eight (11%) TCu380A IUDs were expelled (P = .04). There were equal numbers of medical removals (four) and nonmedical removals (two) in the two groups, and one “other medical removal” in the Gyn-CS IUD group.
“The very low expulsion rate of Gyn-CS, compared with TCu380A, is a very strong argument in favor of the anchored IUD, preventing expulsion and displacement,” Dr. Unal and colleagues reported in Contraception.
They noted that the study has a limited 3-month tracking period, but they also pointed out that most expulsions inserted post partum occurred in the initial 6 weeks.
The low expulsion rate of the Gyn-CS device “will prevent more women becoming pregnant too soon which constitutes an important safety issue during future pregnancy,” the authors wrote. “The device, preferably its high-load 10-year version, could also interest many women as a reversible alternative for tubal sterilization. Additional clinical experience with the Gyn-CS IUD is, therefore, urgently warranted.”
No study funding is reported, although Contrel Research provided the Gyn-CS devices at no charge. The study authors reported no relevant disclosures.
FROM CONTRACEPTION
Key clinical point: A newly developed IUD appears to be expelled less frequently than an older device after insertion following cesarean section births.
Major finding: 61 of 70 IUDs (88%) remained in place in women who received the frameless copper-releasing Gyn-CS device, while 54 of 70 (79%) did so in those who received the TCu380A device (P = .30).
Study details: Randomized trial of 140 women who underwent cesarean section followed by insertion of one of the two IUD types (n = 70 for each).
Disclosures: No study funding is reported, although Contrel Research provided the Gyn-CS devices at no charge. The study authors reported no relevant disclosures.
Source: Unal C et al. Contraception. 2018 Aug;98:135-40.
FDA approves first mobile app for contraceptive use
The Food and Drug Administration has approved marketing of the first medical mobile application that can be used as a contraceptive, the agency announced in a written statement.
The app, called Natural Cycles, contains an algorithm that calculates the days of the month women are likely to be fertile, based on daily body temperature readings and menstrual cycle information, and is intended for premenopausal women aged 18 years and older. App users are instructed to take their daily body temperatures with a basal body thermometer and enter the information into the app. The more-sensitive basal body thermometer can detect temperature elevations during ovulation, and the app will display a warning on days when users are most fertile. During these days, women should either abstain from sex or use protection.
In clinical studies comprising more than 15,000 women, Natural Cycles had a “perfect use” failure rate of 1.8% and a “normal use” failure rate of 6.5%. This compares favorably with other forms of contraception and birth control: Male condoms have a perfect use failure rate of 2.0% and a normal use failure rate of 18.0%, and combined oral contraceptives have a perfect use failure rate of 0.3% and a normal use failure rate of 9.0%, according to the Association of Reproductive Care Professionals.
“Consumers are increasingly using digital health technologies to inform their everyday health decisions, and this new app can provide an effective method of contraception if it’s used carefully and correctly,” Terri Cornelison, MD, PhD, assistant director for the health of women in the FDA’s Center for Devices and Radiological Health, said in the statement.
The app should not be used by women with a condition in which a pregnancy would present risk to the mother or fetus or by women who are using a birth control or hormonal treatment that inhibits ovulation. Natural Cycles does not protect against sexually transmitted infections.
The Food and Drug Administration has approved marketing of the first medical mobile application that can be used as a contraceptive, the agency announced in a written statement.
The app, called Natural Cycles, contains an algorithm that calculates the days of the month women are likely to be fertile, based on daily body temperature readings and menstrual cycle information, and is intended for premenopausal women aged 18 years and older. App users are instructed to take their daily body temperatures with a basal body thermometer and enter the information into the app. The more-sensitive basal body thermometer can detect temperature elevations during ovulation, and the app will display a warning on days when users are most fertile. During these days, women should either abstain from sex or use protection.
In clinical studies comprising more than 15,000 women, Natural Cycles had a “perfect use” failure rate of 1.8% and a “normal use” failure rate of 6.5%. This compares favorably with other forms of contraception and birth control: Male condoms have a perfect use failure rate of 2.0% and a normal use failure rate of 18.0%, and combined oral contraceptives have a perfect use failure rate of 0.3% and a normal use failure rate of 9.0%, according to the Association of Reproductive Care Professionals.
“Consumers are increasingly using digital health technologies to inform their everyday health decisions, and this new app can provide an effective method of contraception if it’s used carefully and correctly,” Terri Cornelison, MD, PhD, assistant director for the health of women in the FDA’s Center for Devices and Radiological Health, said in the statement.
The app should not be used by women with a condition in which a pregnancy would present risk to the mother or fetus or by women who are using a birth control or hormonal treatment that inhibits ovulation. Natural Cycles does not protect against sexually transmitted infections.
The Food and Drug Administration has approved marketing of the first medical mobile application that can be used as a contraceptive, the agency announced in a written statement.
The app, called Natural Cycles, contains an algorithm that calculates the days of the month women are likely to be fertile, based on daily body temperature readings and menstrual cycle information, and is intended for premenopausal women aged 18 years and older. App users are instructed to take their daily body temperatures with a basal body thermometer and enter the information into the app. The more-sensitive basal body thermometer can detect temperature elevations during ovulation, and the app will display a warning on days when users are most fertile. During these days, women should either abstain from sex or use protection.
In clinical studies comprising more than 15,000 women, Natural Cycles had a “perfect use” failure rate of 1.8% and a “normal use” failure rate of 6.5%. This compares favorably with other forms of contraception and birth control: Male condoms have a perfect use failure rate of 2.0% and a normal use failure rate of 18.0%, and combined oral contraceptives have a perfect use failure rate of 0.3% and a normal use failure rate of 9.0%, according to the Association of Reproductive Care Professionals.
“Consumers are increasingly using digital health technologies to inform their everyday health decisions, and this new app can provide an effective method of contraception if it’s used carefully and correctly,” Terri Cornelison, MD, PhD, assistant director for the health of women in the FDA’s Center for Devices and Radiological Health, said in the statement.
The app should not be used by women with a condition in which a pregnancy would present risk to the mother or fetus or by women who are using a birth control or hormonal treatment that inhibits ovulation. Natural Cycles does not protect against sexually transmitted infections.
Adjuvant chemotherapy benefits high-risk sarcoma patients
CHICAGO – Patients with high-risk soft-tissue sarcomas identified by patient data and a risk calculator had significantly better overall and disease-free survival when they were treated with adjuvant doxorubicin and ifosfamide, a retrospective analysis from a randomized clinical trial showed.
The findings suggest that future clinical trials for sarcoma therapies may need to focus on specific risk categories, investigators said.
Among 290 patients with soft tissues sarcomas (STS) of the trunk wall or extremities, adjuvant chemotherapy with doxorubicin, ifosfamide, mesna, and lenograstim more than halved the risk of death for patients determined by the nomogram to have a low probability of 10-year overall survival, reported Sandro Pasquali, MD, from the Fondazione IRCCS Istituto Nazionale dei Tumori in Milan, and his colleagues.
“These findings interpret conflicting results of randomized controlled trials on perioperative chemotherapy in STS showing that inclusion of low-risk tumors have diluted the effect of chemotherapy leading to negative results and small study effect in meta-analysis,” they wrote in a poster presented at the annual meeting of the American Society of Clinical Oncology.
The clinical trial in question, EORTC-STBSG 62931, results of which were published in 2012 in The Lancet Oncology, was technically a failure, because it did not show a significant benefit of adjuvant chemotherapy vs. observation in patients with STS.
To see whether adjuvant chemotherapy may have benefited select patients, the investigators calculated 10-year probabilities of overall survival (P-OS) for 290 patients with STS of the trunk wall or extremities out of the total trial cohort of 351 patients. The P-OS for each of three categories – low (51% or less), intermediate (52%-66%), and high (67% or greater) – was calculated using individual patient data and the freely available smartphone-based nomogram Sarculator (available in the Apple App Store and Google Play).
They calculated disease-free survival at the study median follow-up of 8 years.
The tumor histologies included malignant fibrous histiocytoma/undifferentiated pleomorphic sarcoma, liposarcoma, leiomyosarcoma, rhabdomyosarcoma, angiosarcoma, synovial sarcoma, malignant peripheral nerve sheath tumor, and others.
A total of 52 patients were in the low P-OS group, including 24 treated with observation, and 28 with adjuvant chemotherapy. Respective numbers for the intermediate and high P-OS categories were 34/34, and 90/80.
The investigators found that for patients in the low P-OS group, adjuvant chemotherapy cut the risk of death by slightly more than half, with a hazard ratio of 0.46 (P = .033). In contrast, there were no significant differences in the risk of death for patients at either intermediate or high probability of 10 year OS (HR, 1.00 and 1.08, respectively; P values not significant).
Similarly, adjuvant chemotherapy cut the risk of disease progression by the same amount, with an HR of 0.46 (P = .021), whereas there was no additional benefit among patients at either intermediate or high probability of 10-year OS (HR 0.74 and 0.90; P values were not significant).
The absolute risk reduction for adjuvant chemotherapy was 21% (8-yr disease-free survival of 34% for adjuvant chemotherapy vs. 13% for observation), with a number needed to treated of 4.76.
The study was supported by the European Organization for Research and Treatment of Cancer. Dr. Pasquali reported having no conflicts of interest.
SOURCE: Pasquali S et al. ASCO 2018, Abstract 115118.
CHICAGO – Patients with high-risk soft-tissue sarcomas identified by patient data and a risk calculator had significantly better overall and disease-free survival when they were treated with adjuvant doxorubicin and ifosfamide, a retrospective analysis from a randomized clinical trial showed.
The findings suggest that future clinical trials for sarcoma therapies may need to focus on specific risk categories, investigators said.
Among 290 patients with soft tissues sarcomas (STS) of the trunk wall or extremities, adjuvant chemotherapy with doxorubicin, ifosfamide, mesna, and lenograstim more than halved the risk of death for patients determined by the nomogram to have a low probability of 10-year overall survival, reported Sandro Pasquali, MD, from the Fondazione IRCCS Istituto Nazionale dei Tumori in Milan, and his colleagues.
“These findings interpret conflicting results of randomized controlled trials on perioperative chemotherapy in STS showing that inclusion of low-risk tumors have diluted the effect of chemotherapy leading to negative results and small study effect in meta-analysis,” they wrote in a poster presented at the annual meeting of the American Society of Clinical Oncology.
The clinical trial in question, EORTC-STBSG 62931, results of which were published in 2012 in The Lancet Oncology, was technically a failure, because it did not show a significant benefit of adjuvant chemotherapy vs. observation in patients with STS.
To see whether adjuvant chemotherapy may have benefited select patients, the investigators calculated 10-year probabilities of overall survival (P-OS) for 290 patients with STS of the trunk wall or extremities out of the total trial cohort of 351 patients. The P-OS for each of three categories – low (51% or less), intermediate (52%-66%), and high (67% or greater) – was calculated using individual patient data and the freely available smartphone-based nomogram Sarculator (available in the Apple App Store and Google Play).
They calculated disease-free survival at the study median follow-up of 8 years.
The tumor histologies included malignant fibrous histiocytoma/undifferentiated pleomorphic sarcoma, liposarcoma, leiomyosarcoma, rhabdomyosarcoma, angiosarcoma, synovial sarcoma, malignant peripheral nerve sheath tumor, and others.
A total of 52 patients were in the low P-OS group, including 24 treated with observation, and 28 with adjuvant chemotherapy. Respective numbers for the intermediate and high P-OS categories were 34/34, and 90/80.
The investigators found that for patients in the low P-OS group, adjuvant chemotherapy cut the risk of death by slightly more than half, with a hazard ratio of 0.46 (P = .033). In contrast, there were no significant differences in the risk of death for patients at either intermediate or high probability of 10 year OS (HR, 1.00 and 1.08, respectively; P values not significant).
Similarly, adjuvant chemotherapy cut the risk of disease progression by the same amount, with an HR of 0.46 (P = .021), whereas there was no additional benefit among patients at either intermediate or high probability of 10-year OS (HR 0.74 and 0.90; P values were not significant).
The absolute risk reduction for adjuvant chemotherapy was 21% (8-yr disease-free survival of 34% for adjuvant chemotherapy vs. 13% for observation), with a number needed to treated of 4.76.
The study was supported by the European Organization for Research and Treatment of Cancer. Dr. Pasquali reported having no conflicts of interest.
SOURCE: Pasquali S et al. ASCO 2018, Abstract 115118.
CHICAGO – Patients with high-risk soft-tissue sarcomas identified by patient data and a risk calculator had significantly better overall and disease-free survival when they were treated with adjuvant doxorubicin and ifosfamide, a retrospective analysis from a randomized clinical trial showed.
The findings suggest that future clinical trials for sarcoma therapies may need to focus on specific risk categories, investigators said.
Among 290 patients with soft tissues sarcomas (STS) of the trunk wall or extremities, adjuvant chemotherapy with doxorubicin, ifosfamide, mesna, and lenograstim more than halved the risk of death for patients determined by the nomogram to have a low probability of 10-year overall survival, reported Sandro Pasquali, MD, from the Fondazione IRCCS Istituto Nazionale dei Tumori in Milan, and his colleagues.
“These findings interpret conflicting results of randomized controlled trials on perioperative chemotherapy in STS showing that inclusion of low-risk tumors have diluted the effect of chemotherapy leading to negative results and small study effect in meta-analysis,” they wrote in a poster presented at the annual meeting of the American Society of Clinical Oncology.
The clinical trial in question, EORTC-STBSG 62931, results of which were published in 2012 in The Lancet Oncology, was technically a failure, because it did not show a significant benefit of adjuvant chemotherapy vs. observation in patients with STS.
To see whether adjuvant chemotherapy may have benefited select patients, the investigators calculated 10-year probabilities of overall survival (P-OS) for 290 patients with STS of the trunk wall or extremities out of the total trial cohort of 351 patients. The P-OS for each of three categories – low (51% or less), intermediate (52%-66%), and high (67% or greater) – was calculated using individual patient data and the freely available smartphone-based nomogram Sarculator (available in the Apple App Store and Google Play).
They calculated disease-free survival at the study median follow-up of 8 years.
The tumor histologies included malignant fibrous histiocytoma/undifferentiated pleomorphic sarcoma, liposarcoma, leiomyosarcoma, rhabdomyosarcoma, angiosarcoma, synovial sarcoma, malignant peripheral nerve sheath tumor, and others.
A total of 52 patients were in the low P-OS group, including 24 treated with observation, and 28 with adjuvant chemotherapy. Respective numbers for the intermediate and high P-OS categories were 34/34, and 90/80.
The investigators found that for patients in the low P-OS group, adjuvant chemotherapy cut the risk of death by slightly more than half, with a hazard ratio of 0.46 (P = .033). In contrast, there were no significant differences in the risk of death for patients at either intermediate or high probability of 10 year OS (HR, 1.00 and 1.08, respectively; P values not significant).
Similarly, adjuvant chemotherapy cut the risk of disease progression by the same amount, with an HR of 0.46 (P = .021), whereas there was no additional benefit among patients at either intermediate or high probability of 10-year OS (HR 0.74 and 0.90; P values were not significant).
The absolute risk reduction for adjuvant chemotherapy was 21% (8-yr disease-free survival of 34% for adjuvant chemotherapy vs. 13% for observation), with a number needed to treated of 4.76.
The study was supported by the European Organization for Research and Treatment of Cancer. Dr. Pasquali reported having no conflicts of interest.
SOURCE: Pasquali S et al. ASCO 2018, Abstract 115118.
REPORTING FROM ASCO 2018
Key clinical point: Patients with high-risk soft-tissue sarcomas benefit from adjuvant chemotherapy.
Major finding: Hazard ratios for death and disease progression in patients with a low probability of 10-year overall survival were 0.46 for each.
Study details: Retrospective analysis of a randomized phase 3 trial comparing adjuvant chemotherapy with observation in 290 patients with soft tissue sarcomas of the trunk wall or extremities.
Disclosures: The study was supported by the European Organisation for Research and Treatment of Cancer. Dr. Pasquali reported having no conflicts of interest.
Source: Pasquali S et al. ASCO 2018, Abstract 115118.
Sonic hedgehog inhibitors have mixed efficacy for advanced BCC
For patients with locally advanced or metastatic basal cell carcinoma, Sonic hedgehog inhibitors (SSHi) are effective but are associated with primarily partial responses, and the two Food and Drug Administration–approved agents have significant toxicities, results of a systematic review and meta-analysis indicated.
Data on patients with metastatic or locally advanced basal cell carcinoma (BCC) treated with either vismodegib (Erivedge) or sonidegib (Odomzo) showed that the two agents had roughly similar overall response rates (ORR). Vismodegib, however, had a significantly higher rate of complete responses (CR) in patients with locally advanced disease, reported Pingxing Xie, MD, PhD, and Philippe Lefrançois, MD, PhD, from McGill University, Montreal.
“Common side effects of SHHi therapy tend to incapacitate patients, leading to high discontinuation rates. Over 25% of patients stopped treatment due to side effects. Most side effects are reversible after therapy cessation, except cases of persistent alopecia have been reported,” they wrote in the Journal of the American Academy of Dermatology.
The authors conducted the review to evaluate SHHi as a class and to get a better idea of the efficacy and safety of each agent in the class. They searched the literature to identify all studies using SHHi to treat BCC with vismodegib, sonidegib, itraconazole, or the investigational compound TAK-441, and identified 14 studies focused on vismodegib, 2 on sonidegib, and 1 each on itraconazole and TAK-441.
Of the 18 studies, data from 16 were pooled in fixed-effects linear models to analyze efficacy. The pooled ORR for all patients was 59.6%, “indicating that most patients receiving SHHi achieve at least a partial response.”
Combined ORR results showed a rate of 61.9% for vismodegib, 55.2% for sonidegib, 50% for itraconazole, and 20% for TAK-441, although data for the latter two agents were limited.
In studies looking at locally advanced and metastatic BCC separately, vismodegib was numerically better but statistically similar to sonidegib for locally advanced disease (ORR, 68.8% vs. 56.6%). However, vismodegib was significantly superior to sonidegib for patients with metastatic BCC (ORR, 39.7% vs. 14.7%; P = .007).
The pooled CR for all patients was 23.5%, and there were no CRs with either itraconazole or TAK-441. The combined CR rate for vismodegib was 28% (P = .012). The combined CR rate for sonidegib was just 8.9% and was not statistically significant, the investigators found.
In subgroup analyses for locally advanced BCC, the CR rate for vismodegib was 30.9% for vismodegib (P = .012), “meaning that many patients can expect cure.” In contrast, only 3% of patients treated for locally advanced disease had a CR with sonidegib. The difference between the drugs in this subpopulation was significant (P less than .0001).
Neither drug produced significant CRs in patients with metastatic melanoma, and the pooled clinical benefit rate (all patients with stable disease or better) was 94.9%, with rates similar among all four drugs.
Pooled prevalences of adverse events showed a 67.1% prevalence of muscle spasms, 54.1% prevalence of dysgeusia, and a 57.7% prevalence of alopecia. The proportions of side effects were similar between vismodegib and sonidegib, but sonidegib was associated with a higher prevalence of upper gastrointestinal tract distress than vismodegib.
The study was partially funded by the Canadian Dermatology Foundation. The authors reported having no conflicts of interest.
SOURCE: Xie P et al. J Am Acad Dermatol. 2018 Jul 9. doi: 10.1016/j.jaad.2018.07.004.
For patients with locally advanced or metastatic basal cell carcinoma, Sonic hedgehog inhibitors (SSHi) are effective but are associated with primarily partial responses, and the two Food and Drug Administration–approved agents have significant toxicities, results of a systematic review and meta-analysis indicated.
Data on patients with metastatic or locally advanced basal cell carcinoma (BCC) treated with either vismodegib (Erivedge) or sonidegib (Odomzo) showed that the two agents had roughly similar overall response rates (ORR). Vismodegib, however, had a significantly higher rate of complete responses (CR) in patients with locally advanced disease, reported Pingxing Xie, MD, PhD, and Philippe Lefrançois, MD, PhD, from McGill University, Montreal.
“Common side effects of SHHi therapy tend to incapacitate patients, leading to high discontinuation rates. Over 25% of patients stopped treatment due to side effects. Most side effects are reversible after therapy cessation, except cases of persistent alopecia have been reported,” they wrote in the Journal of the American Academy of Dermatology.
The authors conducted the review to evaluate SHHi as a class and to get a better idea of the efficacy and safety of each agent in the class. They searched the literature to identify all studies using SHHi to treat BCC with vismodegib, sonidegib, itraconazole, or the investigational compound TAK-441, and identified 14 studies focused on vismodegib, 2 on sonidegib, and 1 each on itraconazole and TAK-441.
Of the 18 studies, data from 16 were pooled in fixed-effects linear models to analyze efficacy. The pooled ORR for all patients was 59.6%, “indicating that most patients receiving SHHi achieve at least a partial response.”
Combined ORR results showed a rate of 61.9% for vismodegib, 55.2% for sonidegib, 50% for itraconazole, and 20% for TAK-441, although data for the latter two agents were limited.
In studies looking at locally advanced and metastatic BCC separately, vismodegib was numerically better but statistically similar to sonidegib for locally advanced disease (ORR, 68.8% vs. 56.6%). However, vismodegib was significantly superior to sonidegib for patients with metastatic BCC (ORR, 39.7% vs. 14.7%; P = .007).
The pooled CR for all patients was 23.5%, and there were no CRs with either itraconazole or TAK-441. The combined CR rate for vismodegib was 28% (P = .012). The combined CR rate for sonidegib was just 8.9% and was not statistically significant, the investigators found.
In subgroup analyses for locally advanced BCC, the CR rate for vismodegib was 30.9% for vismodegib (P = .012), “meaning that many patients can expect cure.” In contrast, only 3% of patients treated for locally advanced disease had a CR with sonidegib. The difference between the drugs in this subpopulation was significant (P less than .0001).
Neither drug produced significant CRs in patients with metastatic melanoma, and the pooled clinical benefit rate (all patients with stable disease or better) was 94.9%, with rates similar among all four drugs.
Pooled prevalences of adverse events showed a 67.1% prevalence of muscle spasms, 54.1% prevalence of dysgeusia, and a 57.7% prevalence of alopecia. The proportions of side effects were similar between vismodegib and sonidegib, but sonidegib was associated with a higher prevalence of upper gastrointestinal tract distress than vismodegib.
The study was partially funded by the Canadian Dermatology Foundation. The authors reported having no conflicts of interest.
SOURCE: Xie P et al. J Am Acad Dermatol. 2018 Jul 9. doi: 10.1016/j.jaad.2018.07.004.
For patients with locally advanced or metastatic basal cell carcinoma, Sonic hedgehog inhibitors (SSHi) are effective but are associated with primarily partial responses, and the two Food and Drug Administration–approved agents have significant toxicities, results of a systematic review and meta-analysis indicated.
Data on patients with metastatic or locally advanced basal cell carcinoma (BCC) treated with either vismodegib (Erivedge) or sonidegib (Odomzo) showed that the two agents had roughly similar overall response rates (ORR). Vismodegib, however, had a significantly higher rate of complete responses (CR) in patients with locally advanced disease, reported Pingxing Xie, MD, PhD, and Philippe Lefrançois, MD, PhD, from McGill University, Montreal.
“Common side effects of SHHi therapy tend to incapacitate patients, leading to high discontinuation rates. Over 25% of patients stopped treatment due to side effects. Most side effects are reversible after therapy cessation, except cases of persistent alopecia have been reported,” they wrote in the Journal of the American Academy of Dermatology.
The authors conducted the review to evaluate SHHi as a class and to get a better idea of the efficacy and safety of each agent in the class. They searched the literature to identify all studies using SHHi to treat BCC with vismodegib, sonidegib, itraconazole, or the investigational compound TAK-441, and identified 14 studies focused on vismodegib, 2 on sonidegib, and 1 each on itraconazole and TAK-441.
Of the 18 studies, data from 16 were pooled in fixed-effects linear models to analyze efficacy. The pooled ORR for all patients was 59.6%, “indicating that most patients receiving SHHi achieve at least a partial response.”
Combined ORR results showed a rate of 61.9% for vismodegib, 55.2% for sonidegib, 50% for itraconazole, and 20% for TAK-441, although data for the latter two agents were limited.
In studies looking at locally advanced and metastatic BCC separately, vismodegib was numerically better but statistically similar to sonidegib for locally advanced disease (ORR, 68.8% vs. 56.6%). However, vismodegib was significantly superior to sonidegib for patients with metastatic BCC (ORR, 39.7% vs. 14.7%; P = .007).
The pooled CR for all patients was 23.5%, and there were no CRs with either itraconazole or TAK-441. The combined CR rate for vismodegib was 28% (P = .012). The combined CR rate for sonidegib was just 8.9% and was not statistically significant, the investigators found.
In subgroup analyses for locally advanced BCC, the CR rate for vismodegib was 30.9% for vismodegib (P = .012), “meaning that many patients can expect cure.” In contrast, only 3% of patients treated for locally advanced disease had a CR with sonidegib. The difference between the drugs in this subpopulation was significant (P less than .0001).
Neither drug produced significant CRs in patients with metastatic melanoma, and the pooled clinical benefit rate (all patients with stable disease or better) was 94.9%, with rates similar among all four drugs.
Pooled prevalences of adverse events showed a 67.1% prevalence of muscle spasms, 54.1% prevalence of dysgeusia, and a 57.7% prevalence of alopecia. The proportions of side effects were similar between vismodegib and sonidegib, but sonidegib was associated with a higher prevalence of upper gastrointestinal tract distress than vismodegib.
The study was partially funded by the Canadian Dermatology Foundation. The authors reported having no conflicts of interest.
SOURCE: Xie P et al. J Am Acad Dermatol. 2018 Jul 9. doi: 10.1016/j.jaad.2018.07.004.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: Sonic hedgehog inhibitors (SHHi) have mixed efficacy against metastatic or locally advanced basal cell carcinoma (BCC).
Major finding: Combined overall response rate results showed a rate of 61.9% for vismodegib, 55.2% for sonidegib, 50% for itraconazole, and 20% for TAK-441.
Study details: A systematic review and meta-analysis of studies looking at SHHi in patients with BCC.
Disclosures: The study was partially funded by the Canadian Dermatology Foundation. The authors reported having no conflicts of interest.
Source: Xie P et al. J Am Acad Dermatol. 2018 Jul 9. doi:10.1016/j.jaad.2018.07.004.
Groups release guidelines for CAR T treatment in children
emphasize the need for a flexible approach to detect early signs of serious complications for younger patients treated with this emerging class of medicines.
Researchers at the University of Texas MD Anderson Cancer Center, Houston, and the Pediatric Acute Lung Injury and Sepsis Investigators Network (PALISI) developed the guidelines, which were published in Nature Reviews Clinical Oncology. The recommendations build on the guidelines for more general use of these medicines from MD Anderson’s CARTOX Program, which Nature Reviews Clinical Oncology published in 2017.
Among the chief concerns with this new class of medicines are cytokine-release syndrome (CRS) and CAR T cell-related encephalopathy syndrome (CRES), according to Kris Michael Mahadeo, MD MPH, of the MD Anderson Cancer Center and his coauthors of the new paper.
Some of the tools used for older patients in screening for complications with CAR T drugs don’t work as well with younger ones, Dr. Mahadeo said in an interview. For instance, at MD Anderson, a handwriting sample is used to monitor patients for CAR T cell-related encephalopathy syndrome, which has symptoms of confusion and delirium. Patients provide a baseline handwriting sample of a single sentence that’s scanned into the medical record, and then they are asked to write this again during their time in the hospital, he said. But this tool may not work for children too young to write well.
The new guidelines suggest using the Cornell Assessment of Pediatric Delirium (CAPD) or to evaluate a child’s mental state, asking questions about eye contact, and level of awareness and mood, Dr. Mahadeo said. An alternative for patients aged 12 years and older with greater cognitive ability is the CARTOX-10 grading system.
“The nurses who spent most of the day with these patients will observe them over their shift and kind of get an idea of what was normal and answer a series of questions” through the CAPD tool, which is already used in ICUs, Dr. Mahadeo said. “It takes into consideration both the nurses’ perception and the parents, or whoever is at the bedside with the child. So that if they have a concern, it gives them a point that actually escalates things upward.”
The newly published recommendations also remind physicians and others caring for young patients to pay attention to these reports.
“Parent and/or caregiver concerns should be addressed because early signs or symptoms of CRS can be subtle and best recognized by those who know the child best,” Dr. Mahadeo and his colleagues wrote in a summary of key recommendations in the paper.
The recommendations also noted a need for close monitoring for complications such as hypotension, hypocalcemia, and catheter-related pain in young patients who require a leukapheresis catheter for cell collection. Infant and younger children “might not verbalize these symptoms,” according to the researchers.
Other recommendations include:
- Obtaining the child’s assent when appropriate, with psychological services often aiding in this goal. Dr. Mahadeo and his colleagues recommend considering “age-appropriate advance directives.”
- Maintaining high vigilance for sinus tachycardia as an early sign of CRS, using age-specific normal range or baseline values.
- Giving pediatric dosing of tocilizumab, with patients weighing less than 30 kg receiving 12 mg/kg, and those weighing 30 kg or greater receiving 8 mg/kg.
- Considering participation with a prospective collaboration with intensive-care registries that could allow accurate data entry of cell-therapy variables into the Center for International Blood and Marrow Transplant Research registry by cell-therapy programs.
The Food and Drug Administration approved the first two CAR T-cell therapies in the United States in 2017: Novartis’ tisagenlecleucel (Kymriah) for children and young adults with B-cell precursor acute lymphoblastic leukemia and later for adults with large B-cell lymphoma; and axicabtagene ciloleucel (Yescarta), sold by Gilead, for adults with large B-cell lymphoma. The therapies involve reengineering a patient’s T cells such that they recognize the threat of cancer, and then introducing them back into the body. The European Medicines Agency’s Committee for Medicinal Products for Human Use in June recommended granting marketing authorization to these drugs.
In the new pediatric guidelines, Dr. Mahadeo and his colleagues noted the use of CAR T-cell therapies for treatment of solid tumors and other malignancies in children already “is being explored.” “Moreover, consideration of earlier or upfront use of CAR T-cell therapy might spare patients the acute and long-term toxicities associated with traditional chemotherapy and/or radiation regimens,” they wrote.
There’s been great interest in learning how to most safely use the CAR T cell therapies, said Helen Heslop, MD, of Baylor College of Medicine.
She pointed to a 2014 publication in the journal Blood from Daniel W. Lee and his colleagues as an earlier example of this research. By now, cancer centers will have worked out their own procedures for pediatric use of CAR T therapies, hewing to standards set by the Foundation for the Accreditation of Cellular Therapy (FACT), Dr. Heslop said.
Dr. Heslop also stressed the role of the FDA in requiring risk evaluation and management strategy programs for these drugs. All of this, including the new guidelines from Dr. Mahadeo and his colleagues, is part of a growing body of research into safe use of CAR T therapies, Dr. Heslop said.
“It’s an active area of research,” she said. “Most centers will look at all of it and then develop what works best in their own individual center for providing the best care for the patients.”
The newly published guidelines could prove an “important contribution” to managing the risk of CAR T therapies, Phyllis I. Warkentin, MD, chief medical officer for FACT, said in an interview, while stressing that they were not more or less important than other similar efforts. Physicians learning how to use the CAR T therapies may welcome new input, as most of what’s been published has been about adults, she said.
“You don’t have the luxury of a lot of time to be learning on the job, so to speak,” with CAR T therapies, she said. “Many of the toxicities are fairly severe and fairly sudden.”
Dr. Heslop has been on advisory board for Gilead and Novartis. Dr. Warkentin and Dr. Mahadeo each reported having no financial disclosures. Other authors of the guidelines paper reported a patent with applications in the field of gene-modified T cell therapy for cancer, as well as financial ties to Cellectis, NexImmune, Torque Pharma, Kite Pharma (a Gilead company), Poseida Therapeutics, Celgene, Novartis, and Unum Therapeutics.
SOURCE: Mahadeo KM et al. Nat Rev Clin Oncol. 2018 Aug 6. doi: 10.1038/s41571-018-0075-2.
emphasize the need for a flexible approach to detect early signs of serious complications for younger patients treated with this emerging class of medicines.
Researchers at the University of Texas MD Anderson Cancer Center, Houston, and the Pediatric Acute Lung Injury and Sepsis Investigators Network (PALISI) developed the guidelines, which were published in Nature Reviews Clinical Oncology. The recommendations build on the guidelines for more general use of these medicines from MD Anderson’s CARTOX Program, which Nature Reviews Clinical Oncology published in 2017.
Among the chief concerns with this new class of medicines are cytokine-release syndrome (CRS) and CAR T cell-related encephalopathy syndrome (CRES), according to Kris Michael Mahadeo, MD MPH, of the MD Anderson Cancer Center and his coauthors of the new paper.
Some of the tools used for older patients in screening for complications with CAR T drugs don’t work as well with younger ones, Dr. Mahadeo said in an interview. For instance, at MD Anderson, a handwriting sample is used to monitor patients for CAR T cell-related encephalopathy syndrome, which has symptoms of confusion and delirium. Patients provide a baseline handwriting sample of a single sentence that’s scanned into the medical record, and then they are asked to write this again during their time in the hospital, he said. But this tool may not work for children too young to write well.
The new guidelines suggest using the Cornell Assessment of Pediatric Delirium (CAPD) or to evaluate a child’s mental state, asking questions about eye contact, and level of awareness and mood, Dr. Mahadeo said. An alternative for patients aged 12 years and older with greater cognitive ability is the CARTOX-10 grading system.
“The nurses who spent most of the day with these patients will observe them over their shift and kind of get an idea of what was normal and answer a series of questions” through the CAPD tool, which is already used in ICUs, Dr. Mahadeo said. “It takes into consideration both the nurses’ perception and the parents, or whoever is at the bedside with the child. So that if they have a concern, it gives them a point that actually escalates things upward.”
The newly published recommendations also remind physicians and others caring for young patients to pay attention to these reports.
“Parent and/or caregiver concerns should be addressed because early signs or symptoms of CRS can be subtle and best recognized by those who know the child best,” Dr. Mahadeo and his colleagues wrote in a summary of key recommendations in the paper.
The recommendations also noted a need for close monitoring for complications such as hypotension, hypocalcemia, and catheter-related pain in young patients who require a leukapheresis catheter for cell collection. Infant and younger children “might not verbalize these symptoms,” according to the researchers.
Other recommendations include:
- Obtaining the child’s assent when appropriate, with psychological services often aiding in this goal. Dr. Mahadeo and his colleagues recommend considering “age-appropriate advance directives.”
- Maintaining high vigilance for sinus tachycardia as an early sign of CRS, using age-specific normal range or baseline values.
- Giving pediatric dosing of tocilizumab, with patients weighing less than 30 kg receiving 12 mg/kg, and those weighing 30 kg or greater receiving 8 mg/kg.
- Considering participation with a prospective collaboration with intensive-care registries that could allow accurate data entry of cell-therapy variables into the Center for International Blood and Marrow Transplant Research registry by cell-therapy programs.
The Food and Drug Administration approved the first two CAR T-cell therapies in the United States in 2017: Novartis’ tisagenlecleucel (Kymriah) for children and young adults with B-cell precursor acute lymphoblastic leukemia and later for adults with large B-cell lymphoma; and axicabtagene ciloleucel (Yescarta), sold by Gilead, for adults with large B-cell lymphoma. The therapies involve reengineering a patient’s T cells such that they recognize the threat of cancer, and then introducing them back into the body. The European Medicines Agency’s Committee for Medicinal Products for Human Use in June recommended granting marketing authorization to these drugs.
In the new pediatric guidelines, Dr. Mahadeo and his colleagues noted the use of CAR T-cell therapies for treatment of solid tumors and other malignancies in children already “is being explored.” “Moreover, consideration of earlier or upfront use of CAR T-cell therapy might spare patients the acute and long-term toxicities associated with traditional chemotherapy and/or radiation regimens,” they wrote.
There’s been great interest in learning how to most safely use the CAR T cell therapies, said Helen Heslop, MD, of Baylor College of Medicine.
She pointed to a 2014 publication in the journal Blood from Daniel W. Lee and his colleagues as an earlier example of this research. By now, cancer centers will have worked out their own procedures for pediatric use of CAR T therapies, hewing to standards set by the Foundation for the Accreditation of Cellular Therapy (FACT), Dr. Heslop said.
Dr. Heslop also stressed the role of the FDA in requiring risk evaluation and management strategy programs for these drugs. All of this, including the new guidelines from Dr. Mahadeo and his colleagues, is part of a growing body of research into safe use of CAR T therapies, Dr. Heslop said.
“It’s an active area of research,” she said. “Most centers will look at all of it and then develop what works best in their own individual center for providing the best care for the patients.”
The newly published guidelines could prove an “important contribution” to managing the risk of CAR T therapies, Phyllis I. Warkentin, MD, chief medical officer for FACT, said in an interview, while stressing that they were not more or less important than other similar efforts. Physicians learning how to use the CAR T therapies may welcome new input, as most of what’s been published has been about adults, she said.
“You don’t have the luxury of a lot of time to be learning on the job, so to speak,” with CAR T therapies, she said. “Many of the toxicities are fairly severe and fairly sudden.”
Dr. Heslop has been on advisory board for Gilead and Novartis. Dr. Warkentin and Dr. Mahadeo each reported having no financial disclosures. Other authors of the guidelines paper reported a patent with applications in the field of gene-modified T cell therapy for cancer, as well as financial ties to Cellectis, NexImmune, Torque Pharma, Kite Pharma (a Gilead company), Poseida Therapeutics, Celgene, Novartis, and Unum Therapeutics.
SOURCE: Mahadeo KM et al. Nat Rev Clin Oncol. 2018 Aug 6. doi: 10.1038/s41571-018-0075-2.
emphasize the need for a flexible approach to detect early signs of serious complications for younger patients treated with this emerging class of medicines.
Researchers at the University of Texas MD Anderson Cancer Center, Houston, and the Pediatric Acute Lung Injury and Sepsis Investigators Network (PALISI) developed the guidelines, which were published in Nature Reviews Clinical Oncology. The recommendations build on the guidelines for more general use of these medicines from MD Anderson’s CARTOX Program, which Nature Reviews Clinical Oncology published in 2017.
Among the chief concerns with this new class of medicines are cytokine-release syndrome (CRS) and CAR T cell-related encephalopathy syndrome (CRES), according to Kris Michael Mahadeo, MD MPH, of the MD Anderson Cancer Center and his coauthors of the new paper.
Some of the tools used for older patients in screening for complications with CAR T drugs don’t work as well with younger ones, Dr. Mahadeo said in an interview. For instance, at MD Anderson, a handwriting sample is used to monitor patients for CAR T cell-related encephalopathy syndrome, which has symptoms of confusion and delirium. Patients provide a baseline handwriting sample of a single sentence that’s scanned into the medical record, and then they are asked to write this again during their time in the hospital, he said. But this tool may not work for children too young to write well.
The new guidelines suggest using the Cornell Assessment of Pediatric Delirium (CAPD) or to evaluate a child’s mental state, asking questions about eye contact, and level of awareness and mood, Dr. Mahadeo said. An alternative for patients aged 12 years and older with greater cognitive ability is the CARTOX-10 grading system.
“The nurses who spent most of the day with these patients will observe them over their shift and kind of get an idea of what was normal and answer a series of questions” through the CAPD tool, which is already used in ICUs, Dr. Mahadeo said. “It takes into consideration both the nurses’ perception and the parents, or whoever is at the bedside with the child. So that if they have a concern, it gives them a point that actually escalates things upward.”
The newly published recommendations also remind physicians and others caring for young patients to pay attention to these reports.
“Parent and/or caregiver concerns should be addressed because early signs or symptoms of CRS can be subtle and best recognized by those who know the child best,” Dr. Mahadeo and his colleagues wrote in a summary of key recommendations in the paper.
The recommendations also noted a need for close monitoring for complications such as hypotension, hypocalcemia, and catheter-related pain in young patients who require a leukapheresis catheter for cell collection. Infant and younger children “might not verbalize these symptoms,” according to the researchers.
Other recommendations include:
- Obtaining the child’s assent when appropriate, with psychological services often aiding in this goal. Dr. Mahadeo and his colleagues recommend considering “age-appropriate advance directives.”
- Maintaining high vigilance for sinus tachycardia as an early sign of CRS, using age-specific normal range or baseline values.
- Giving pediatric dosing of tocilizumab, with patients weighing less than 30 kg receiving 12 mg/kg, and those weighing 30 kg or greater receiving 8 mg/kg.
- Considering participation with a prospective collaboration with intensive-care registries that could allow accurate data entry of cell-therapy variables into the Center for International Blood and Marrow Transplant Research registry by cell-therapy programs.
The Food and Drug Administration approved the first two CAR T-cell therapies in the United States in 2017: Novartis’ tisagenlecleucel (Kymriah) for children and young adults with B-cell precursor acute lymphoblastic leukemia and later for adults with large B-cell lymphoma; and axicabtagene ciloleucel (Yescarta), sold by Gilead, for adults with large B-cell lymphoma. The therapies involve reengineering a patient’s T cells such that they recognize the threat of cancer, and then introducing them back into the body. The European Medicines Agency’s Committee for Medicinal Products for Human Use in June recommended granting marketing authorization to these drugs.
In the new pediatric guidelines, Dr. Mahadeo and his colleagues noted the use of CAR T-cell therapies for treatment of solid tumors and other malignancies in children already “is being explored.” “Moreover, consideration of earlier or upfront use of CAR T-cell therapy might spare patients the acute and long-term toxicities associated with traditional chemotherapy and/or radiation regimens,” they wrote.
There’s been great interest in learning how to most safely use the CAR T cell therapies, said Helen Heslop, MD, of Baylor College of Medicine.
She pointed to a 2014 publication in the journal Blood from Daniel W. Lee and his colleagues as an earlier example of this research. By now, cancer centers will have worked out their own procedures for pediatric use of CAR T therapies, hewing to standards set by the Foundation for the Accreditation of Cellular Therapy (FACT), Dr. Heslop said.
Dr. Heslop also stressed the role of the FDA in requiring risk evaluation and management strategy programs for these drugs. All of this, including the new guidelines from Dr. Mahadeo and his colleagues, is part of a growing body of research into safe use of CAR T therapies, Dr. Heslop said.
“It’s an active area of research,” she said. “Most centers will look at all of it and then develop what works best in their own individual center for providing the best care for the patients.”
The newly published guidelines could prove an “important contribution” to managing the risk of CAR T therapies, Phyllis I. Warkentin, MD, chief medical officer for FACT, said in an interview, while stressing that they were not more or less important than other similar efforts. Physicians learning how to use the CAR T therapies may welcome new input, as most of what’s been published has been about adults, she said.
“You don’t have the luxury of a lot of time to be learning on the job, so to speak,” with CAR T therapies, she said. “Many of the toxicities are fairly severe and fairly sudden.”
Dr. Heslop has been on advisory board for Gilead and Novartis. Dr. Warkentin and Dr. Mahadeo each reported having no financial disclosures. Other authors of the guidelines paper reported a patent with applications in the field of gene-modified T cell therapy for cancer, as well as financial ties to Cellectis, NexImmune, Torque Pharma, Kite Pharma (a Gilead company), Poseida Therapeutics, Celgene, Novartis, and Unum Therapeutics.
SOURCE: Mahadeo KM et al. Nat Rev Clin Oncol. 2018 Aug 6. doi: 10.1038/s41571-018-0075-2.
FROM NATURE REVIEWS CLINICAL ONCOLOGY
Key clinical point: Multidisciplinary approach aids in managing CAR T-cell therapy’s severe potential toxicities in children.
Major finding: The guideline calls for pediatric dosing of tocilizumab, with patients weighing less than 30 kg receiving 12 mg/kg, and those weighing 30 kg or greater receiving 8 mg/kg.
Study details: Consensus guidelines on the care of children receiving CAR T-cell therapy from the Pediatric Acute Lung Injury and Sepsis Investigators and the MD Anderson Cancer Center CARTOX program.
Disclosures: Dr. Mahadeo reported having no financial disclosures. Other coauthors reported a patent with applications in the field of gene-modified T cell therapy for cancer, as well as financial ties to Cellectis, NexImmune, Torque Pharma, Kite Pharma (a Gilead company), Poseida Therapeutics, Celgene, Novartis, and Unum Therapeutics.
Source: Mahadeo KM et al. Nat Rev Clin Oncol. 2018 Aug 6. doi: 10.1038/s41571-018-0075-2.
On-demand and daily PrEP look similar for HIV prevention
AMSTERDAM – Episodic, “on-demand” pre-exposure prophylaxis to prevent transmission of HIV was as effective as daily pre-exposure prophylaxis in a prospective, real-world study of more than 1,100 men who have sex with men in the Paris region.
The results showed very similar performance and complete prevention of new HIV infections in both groups, data that “strengthen the case for on-demand prophylaxis as an alternative to daily PrEP [pre-exposure prophylaxis],” Jean-Michel Molina, MD, said at the 22nd International AIDS Conference.
The evidence for efficacy of on-demand PrEP, used starting a day before through a day after unprotected sex by a man uninfected by HIV, to prevent potential infection “is now good enough for clinicians to counsel people that it is equally effective as daily PrEP and the person can decide” which regimen to use, he said in an interview. “In the future, we’ll use both strategies. We want to make PrEP easy for people to use. We want alternatives so that more people use PrEP, ” said Dr. Molina, professor of infectious diseases at the University of Paris and head of infectious diseases at Saint-Louis Hospital in Paris.
However the study focused on men who have sex with men (MSM) and so the efficacy of on-demand PrEP is not proven for preventing HIV infection during heterosexual sex, including protecting women, Dr. Molina cautioned.
The on-demand PrEP regimen tested by Dr. Molina and his associates uses the same combination of 300 mg tenofovir (Viread) and 200 mg emtricitabine (Emtriva) – formulated into a single pill and marketed as Truvada – as used for daily PrEP.
For on-demand use patients take two of these pills 2-24 hours prior to their anticipated sexual activity and then 1 pill every 24 hours until the sexual activity stops, with a final pill 24 hours following the last sex event. This translates into an ideal regimen of at least 4 pills total.
The researchers first documented the efficacy of on-demand PrEP in a placebo-controlled study with 400 randomized people that showed the on-demand regimen reduced the rate of new HIV infection by 86%, compared with placebo, among MSM who had unprotected sex (N Engl J Med. 2015 Dec 3;373[23]:2237-46). They designed the current, open-label study, ANRS-PREVENIR (Prevention of HIV in Île-de-France), to further examine the efficacy of and adherence to an on-demand PrEP regimen in a real-world setting and to compare it with daily PrEP.
The study began in May 2017, with a goal of enrolling 3,000 HIV-negative MSM. As of early July 2018, the study had enrolled 1,628 people including 1,102 who had been followed for at least 3 months and for an average of 7 months, including 124 followed for at least 1 year. Participants had a choice of using PrEP in either an on-demand or daily regimen and were allowed to switch from one regimen to the other. At enrollment, 55% opted for the on-demand regimen, and during subsequent months the fraction of participants using an on-demand regimen remained at about half. Based on self-reported use of the regimen, 96% of the men who had opted for on-demand PrEP used it “correctly,” defined as taking at least one pill within 24 hours of their sex event and at least one pill within 24 hours after the event, a rate that was identical to the “correct” usage by participants who opted for daily PrEP. The only substantial difference in PrEP use between the two subgroups was that 19% of men in the on-demand group did not use PrEP prior to at least one sex event, compared with 2% of men in the daily subgroup.
“We need to better understand” why so many participants in the on-demand group failed to use PrEP, admitted Dr. Molina. He speculated that these failures to use PrEP occurred because participants had sex with partners whom they knew were not infected with HIV.
During follow-up, the researchers identified no one who became HIV infected during 949 person-years of follow-up, a level of protection from PrEP that Dr. Molina called “remarkable.” Based on the epidemiology of HIV in the Paris region he estimated that use of PrEP by the participants in the study had prevented what might have otherwise been 85 new HIV infections. During follow-up, participants completely stopped using either daily or on-demand PrEP at a rate of 1.5 discontinuations per 100 person-years.
The incidence of adverse events was similar in the two arms of the study, with no discontinuations because of adverse events, and a 4-5 per 100 person-years incidence of grade 3 or 4 adverse events and an incidence of 2 per 100 person-years of grade 3 or 4 laboratory abnormalities. “The drugs are very well tolerated,” Dr. Molina noted, and a potential reduction in adverse events is not a reason for someone to choose on-demand PrEP instead of daily use. The primary difference is a potentially smaller pill burden: People using on-demand PrEP take on average about half the number of pills as someone on daily PrEP, which could lower the cost of prophylaxis in countries or settings where the person on PrEP has financial responsibility for the drugs. This was not an issue in the current study as the pills are available to people at no charge under the French health coverage system, Dr. Molina noted.
“On-demand PrEP is a viable option. For some people on-demand makes sense,” commented Patrick Sullivan, PhD, a professor of epidemiology at Emory University in Atlanta. It can potentially reduce drug costs, an issue for many Americans, Dr. Sullivan noted in an interview. So far, however, no reported studies have documented the efficacy of on-demand PrEP in a U.S. population, he said.
On-demand PrEP has received endorsement for use by selected people at high risk for HIV infection by public health authorities in the European Union, the United Kingdom, France, Canada, and Australia, Dr. Molina said, and in late July this strategy also received endorsement in revised HIV treatment and prevention recommendations issued by the International Antiviral Society–USA (JAMA. 2018 July 24/31;320[4]:379-96).
ANRS-PREVENIR received no commercial funding. Dr. Molina has been an advisor to Gilead, Merck, Teva, and Viiv and has received research fundings from Gilead.
SOURCE: Molina J-M et al. AIDS 2018, Abstract 13278.
AMSTERDAM – Episodic, “on-demand” pre-exposure prophylaxis to prevent transmission of HIV was as effective as daily pre-exposure prophylaxis in a prospective, real-world study of more than 1,100 men who have sex with men in the Paris region.
The results showed very similar performance and complete prevention of new HIV infections in both groups, data that “strengthen the case for on-demand prophylaxis as an alternative to daily PrEP [pre-exposure prophylaxis],” Jean-Michel Molina, MD, said at the 22nd International AIDS Conference.
The evidence for efficacy of on-demand PrEP, used starting a day before through a day after unprotected sex by a man uninfected by HIV, to prevent potential infection “is now good enough for clinicians to counsel people that it is equally effective as daily PrEP and the person can decide” which regimen to use, he said in an interview. “In the future, we’ll use both strategies. We want to make PrEP easy for people to use. We want alternatives so that more people use PrEP, ” said Dr. Molina, professor of infectious diseases at the University of Paris and head of infectious diseases at Saint-Louis Hospital in Paris.
However the study focused on men who have sex with men (MSM) and so the efficacy of on-demand PrEP is not proven for preventing HIV infection during heterosexual sex, including protecting women, Dr. Molina cautioned.
The on-demand PrEP regimen tested by Dr. Molina and his associates uses the same combination of 300 mg tenofovir (Viread) and 200 mg emtricitabine (Emtriva) – formulated into a single pill and marketed as Truvada – as used for daily PrEP.
For on-demand use patients take two of these pills 2-24 hours prior to their anticipated sexual activity and then 1 pill every 24 hours until the sexual activity stops, with a final pill 24 hours following the last sex event. This translates into an ideal regimen of at least 4 pills total.
The researchers first documented the efficacy of on-demand PrEP in a placebo-controlled study with 400 randomized people that showed the on-demand regimen reduced the rate of new HIV infection by 86%, compared with placebo, among MSM who had unprotected sex (N Engl J Med. 2015 Dec 3;373[23]:2237-46). They designed the current, open-label study, ANRS-PREVENIR (Prevention of HIV in Île-de-France), to further examine the efficacy of and adherence to an on-demand PrEP regimen in a real-world setting and to compare it with daily PrEP.
The study began in May 2017, with a goal of enrolling 3,000 HIV-negative MSM. As of early July 2018, the study had enrolled 1,628 people including 1,102 who had been followed for at least 3 months and for an average of 7 months, including 124 followed for at least 1 year. Participants had a choice of using PrEP in either an on-demand or daily regimen and were allowed to switch from one regimen to the other. At enrollment, 55% opted for the on-demand regimen, and during subsequent months the fraction of participants using an on-demand regimen remained at about half. Based on self-reported use of the regimen, 96% of the men who had opted for on-demand PrEP used it “correctly,” defined as taking at least one pill within 24 hours of their sex event and at least one pill within 24 hours after the event, a rate that was identical to the “correct” usage by participants who opted for daily PrEP. The only substantial difference in PrEP use between the two subgroups was that 19% of men in the on-demand group did not use PrEP prior to at least one sex event, compared with 2% of men in the daily subgroup.
“We need to better understand” why so many participants in the on-demand group failed to use PrEP, admitted Dr. Molina. He speculated that these failures to use PrEP occurred because participants had sex with partners whom they knew were not infected with HIV.
During follow-up, the researchers identified no one who became HIV infected during 949 person-years of follow-up, a level of protection from PrEP that Dr. Molina called “remarkable.” Based on the epidemiology of HIV in the Paris region he estimated that use of PrEP by the participants in the study had prevented what might have otherwise been 85 new HIV infections. During follow-up, participants completely stopped using either daily or on-demand PrEP at a rate of 1.5 discontinuations per 100 person-years.
The incidence of adverse events was similar in the two arms of the study, with no discontinuations because of adverse events, and a 4-5 per 100 person-years incidence of grade 3 or 4 adverse events and an incidence of 2 per 100 person-years of grade 3 or 4 laboratory abnormalities. “The drugs are very well tolerated,” Dr. Molina noted, and a potential reduction in adverse events is not a reason for someone to choose on-demand PrEP instead of daily use. The primary difference is a potentially smaller pill burden: People using on-demand PrEP take on average about half the number of pills as someone on daily PrEP, which could lower the cost of prophylaxis in countries or settings where the person on PrEP has financial responsibility for the drugs. This was not an issue in the current study as the pills are available to people at no charge under the French health coverage system, Dr. Molina noted.
“On-demand PrEP is a viable option. For some people on-demand makes sense,” commented Patrick Sullivan, PhD, a professor of epidemiology at Emory University in Atlanta. It can potentially reduce drug costs, an issue for many Americans, Dr. Sullivan noted in an interview. So far, however, no reported studies have documented the efficacy of on-demand PrEP in a U.S. population, he said.
On-demand PrEP has received endorsement for use by selected people at high risk for HIV infection by public health authorities in the European Union, the United Kingdom, France, Canada, and Australia, Dr. Molina said, and in late July this strategy also received endorsement in revised HIV treatment and prevention recommendations issued by the International Antiviral Society–USA (JAMA. 2018 July 24/31;320[4]:379-96).
ANRS-PREVENIR received no commercial funding. Dr. Molina has been an advisor to Gilead, Merck, Teva, and Viiv and has received research fundings from Gilead.
SOURCE: Molina J-M et al. AIDS 2018, Abstract 13278.
AMSTERDAM – Episodic, “on-demand” pre-exposure prophylaxis to prevent transmission of HIV was as effective as daily pre-exposure prophylaxis in a prospective, real-world study of more than 1,100 men who have sex with men in the Paris region.
The results showed very similar performance and complete prevention of new HIV infections in both groups, data that “strengthen the case for on-demand prophylaxis as an alternative to daily PrEP [pre-exposure prophylaxis],” Jean-Michel Molina, MD, said at the 22nd International AIDS Conference.
The evidence for efficacy of on-demand PrEP, used starting a day before through a day after unprotected sex by a man uninfected by HIV, to prevent potential infection “is now good enough for clinicians to counsel people that it is equally effective as daily PrEP and the person can decide” which regimen to use, he said in an interview. “In the future, we’ll use both strategies. We want to make PrEP easy for people to use. We want alternatives so that more people use PrEP, ” said Dr. Molina, professor of infectious diseases at the University of Paris and head of infectious diseases at Saint-Louis Hospital in Paris.
However the study focused on men who have sex with men (MSM) and so the efficacy of on-demand PrEP is not proven for preventing HIV infection during heterosexual sex, including protecting women, Dr. Molina cautioned.
The on-demand PrEP regimen tested by Dr. Molina and his associates uses the same combination of 300 mg tenofovir (Viread) and 200 mg emtricitabine (Emtriva) – formulated into a single pill and marketed as Truvada – as used for daily PrEP.
For on-demand use patients take two of these pills 2-24 hours prior to their anticipated sexual activity and then 1 pill every 24 hours until the sexual activity stops, with a final pill 24 hours following the last sex event. This translates into an ideal regimen of at least 4 pills total.
The researchers first documented the efficacy of on-demand PrEP in a placebo-controlled study with 400 randomized people that showed the on-demand regimen reduced the rate of new HIV infection by 86%, compared with placebo, among MSM who had unprotected sex (N Engl J Med. 2015 Dec 3;373[23]:2237-46). They designed the current, open-label study, ANRS-PREVENIR (Prevention of HIV in Île-de-France), to further examine the efficacy of and adherence to an on-demand PrEP regimen in a real-world setting and to compare it with daily PrEP.
The study began in May 2017, with a goal of enrolling 3,000 HIV-negative MSM. As of early July 2018, the study had enrolled 1,628 people including 1,102 who had been followed for at least 3 months and for an average of 7 months, including 124 followed for at least 1 year. Participants had a choice of using PrEP in either an on-demand or daily regimen and were allowed to switch from one regimen to the other. At enrollment, 55% opted for the on-demand regimen, and during subsequent months the fraction of participants using an on-demand regimen remained at about half. Based on self-reported use of the regimen, 96% of the men who had opted for on-demand PrEP used it “correctly,” defined as taking at least one pill within 24 hours of their sex event and at least one pill within 24 hours after the event, a rate that was identical to the “correct” usage by participants who opted for daily PrEP. The only substantial difference in PrEP use between the two subgroups was that 19% of men in the on-demand group did not use PrEP prior to at least one sex event, compared with 2% of men in the daily subgroup.
“We need to better understand” why so many participants in the on-demand group failed to use PrEP, admitted Dr. Molina. He speculated that these failures to use PrEP occurred because participants had sex with partners whom they knew were not infected with HIV.
During follow-up, the researchers identified no one who became HIV infected during 949 person-years of follow-up, a level of protection from PrEP that Dr. Molina called “remarkable.” Based on the epidemiology of HIV in the Paris region he estimated that use of PrEP by the participants in the study had prevented what might have otherwise been 85 new HIV infections. During follow-up, participants completely stopped using either daily or on-demand PrEP at a rate of 1.5 discontinuations per 100 person-years.
The incidence of adverse events was similar in the two arms of the study, with no discontinuations because of adverse events, and a 4-5 per 100 person-years incidence of grade 3 or 4 adverse events and an incidence of 2 per 100 person-years of grade 3 or 4 laboratory abnormalities. “The drugs are very well tolerated,” Dr. Molina noted, and a potential reduction in adverse events is not a reason for someone to choose on-demand PrEP instead of daily use. The primary difference is a potentially smaller pill burden: People using on-demand PrEP take on average about half the number of pills as someone on daily PrEP, which could lower the cost of prophylaxis in countries or settings where the person on PrEP has financial responsibility for the drugs. This was not an issue in the current study as the pills are available to people at no charge under the French health coverage system, Dr. Molina noted.
“On-demand PrEP is a viable option. For some people on-demand makes sense,” commented Patrick Sullivan, PhD, a professor of epidemiology at Emory University in Atlanta. It can potentially reduce drug costs, an issue for many Americans, Dr. Sullivan noted in an interview. So far, however, no reported studies have documented the efficacy of on-demand PrEP in a U.S. population, he said.
On-demand PrEP has received endorsement for use by selected people at high risk for HIV infection by public health authorities in the European Union, the United Kingdom, France, Canada, and Australia, Dr. Molina said, and in late July this strategy also received endorsement in revised HIV treatment and prevention recommendations issued by the International Antiviral Society–USA (JAMA. 2018 July 24/31;320[4]:379-96).
ANRS-PREVENIR received no commercial funding. Dr. Molina has been an advisor to Gilead, Merck, Teva, and Viiv and has received research fundings from Gilead.
SOURCE: Molina J-M et al. AIDS 2018, Abstract 13278.
REPORTING FROM AIDS 2018
Key clinical point:
Major finding: Men using on-demand PrEP had no HIV infections during an average 7 months of follow-up.
Study details: ANRS-PREVENIR, a prospective, multicenter, open-label study with 1,102 people followed for at least 3 months.
Disclosures: ANRS-PREVENIR received no commercial funding. Dr. Molina has been an advisor to Gilead, Merck, Teva, and Viiv and has received research fundings from Gilead.
Source: Molina J-M et al. AIDS 2018, Abstract 13278.
CMS pushes ACOs to take on more risk
Accountable care organizations (ACOs) are playing it too safe, according to the Centers for Medicare & Medicaid Services, which wants to change the rules and push more ACOs into taking on more financial risk – with a little flexibility added in.
“ACOs were designed to move Medicare away from fee for service by encouraging providers to find efficiencies and innovative ways to deliver high-quality care to their patients while reducing costs, giving them the flexibility they need to focus on health outcomes over process,” CMS Administrator Seema Verma said during an Aug. 9 press conference.
That’s not what is happening now, Ms. Verma said. Under the current program, physicians and other health care professionals can participate in an ACO that takes on upside risk only – that is, to share in any of the savings it is able to generate – for up to 6 years before having to take on downside risk to return payment to the government if the ACO fails to hit spending targets.
“This environment has created a perverse incentive leading to the shocking present day reality that, after 6 years, over 82% of shared saving program ACOs are in an upside-only track, meaning that these ACOs have no incentive at all to reduce health care cost while improving outcomes as they were intended,” she said. “The program has not lived up to the accountability part of their name.”
In the aggregate, ACOs that only take on upside risk generally are spending more money and not generating the savings that ACOs in a two-sided risk arrangement are, she added.
To get more health care professionals into two-sided risk arrangements, the CMS released a proposed rule Aug. 9 that caps ACO participation in an upside risk–only arrangement for 2 years before having to migrate to a two-sided risk arrangement. To sweeten the pot, the CMS proposes to allow more flexibility for innovation and encouraging patients to maintain their health.
“On top of [antikickback law] waivers they already receive, we are proposing to allow ACOs that are taking risk to give incentive payments in order to reward patients for taking steps to achieve good health, such as gift cards for patients who receive necessary primary and preventive care,” she said, adding that ACOs who take on downside risk also will be eligible to receive payments for providing telehealth services.
ACOs also will need to adopt the 2015 edition of certified EHR technology and the CMS also will be looking to streamline quality measures, according to the proposal.
The CMS is extending current ACO contracts for 6 months, with the new rules, if finalized, going into effect in the middle of 2019.
The proposal also simplifies the ACO program by offering two tracks, as detailed in a blog post penned by Administrator Verma that appeared Aug. 9 in Health Affairs.
The Basic track “would feature a glide path for taking risk,” she wrote. “It would begin with up to 2 years of upside-only risk and then gradually transition in years 3, 4, and 5 to increasing levels of performance risk, concluding in year 5 at a level of risk that meets the standard to qualify as an advanced alternative payment model [APM]” under the Quality Payment Program. Those entering the enhanced track, taking on two-sided risk immediately, would need to meet the standard of an APM immediately.
The proposal also calls for more transparency and would require providers to alert Medicare beneficiaries that services are being provided in the context of an ACO and to explain what that means for their care.
Spending benchmarks would continue to be calculated using both regional and national spending trends. Program integrity also will be enhanced “by holding ACOs in two-sided models accountable for losses even if they exit midway through a performance year, and by authorizing termination of ACOs with multiple years of poor financial performance,” Ms. Verma wrote.
The Pathways to Success proposed rule was slated to be published in the Federal Register on Aug. 17. Comments are being accepted at regulations.gov until Oct. 16.
Accountable care organizations (ACOs) are playing it too safe, according to the Centers for Medicare & Medicaid Services, which wants to change the rules and push more ACOs into taking on more financial risk – with a little flexibility added in.
“ACOs were designed to move Medicare away from fee for service by encouraging providers to find efficiencies and innovative ways to deliver high-quality care to their patients while reducing costs, giving them the flexibility they need to focus on health outcomes over process,” CMS Administrator Seema Verma said during an Aug. 9 press conference.
That’s not what is happening now, Ms. Verma said. Under the current program, physicians and other health care professionals can participate in an ACO that takes on upside risk only – that is, to share in any of the savings it is able to generate – for up to 6 years before having to take on downside risk to return payment to the government if the ACO fails to hit spending targets.
“This environment has created a perverse incentive leading to the shocking present day reality that, after 6 years, over 82% of shared saving program ACOs are in an upside-only track, meaning that these ACOs have no incentive at all to reduce health care cost while improving outcomes as they were intended,” she said. “The program has not lived up to the accountability part of their name.”
In the aggregate, ACOs that only take on upside risk generally are spending more money and not generating the savings that ACOs in a two-sided risk arrangement are, she added.
To get more health care professionals into two-sided risk arrangements, the CMS released a proposed rule Aug. 9 that caps ACO participation in an upside risk–only arrangement for 2 years before having to migrate to a two-sided risk arrangement. To sweeten the pot, the CMS proposes to allow more flexibility for innovation and encouraging patients to maintain their health.
“On top of [antikickback law] waivers they already receive, we are proposing to allow ACOs that are taking risk to give incentive payments in order to reward patients for taking steps to achieve good health, such as gift cards for patients who receive necessary primary and preventive care,” she said, adding that ACOs who take on downside risk also will be eligible to receive payments for providing telehealth services.
ACOs also will need to adopt the 2015 edition of certified EHR technology and the CMS also will be looking to streamline quality measures, according to the proposal.
The CMS is extending current ACO contracts for 6 months, with the new rules, if finalized, going into effect in the middle of 2019.
The proposal also simplifies the ACO program by offering two tracks, as detailed in a blog post penned by Administrator Verma that appeared Aug. 9 in Health Affairs.
The Basic track “would feature a glide path for taking risk,” she wrote. “It would begin with up to 2 years of upside-only risk and then gradually transition in years 3, 4, and 5 to increasing levels of performance risk, concluding in year 5 at a level of risk that meets the standard to qualify as an advanced alternative payment model [APM]” under the Quality Payment Program. Those entering the enhanced track, taking on two-sided risk immediately, would need to meet the standard of an APM immediately.
The proposal also calls for more transparency and would require providers to alert Medicare beneficiaries that services are being provided in the context of an ACO and to explain what that means for their care.
Spending benchmarks would continue to be calculated using both regional and national spending trends. Program integrity also will be enhanced “by holding ACOs in two-sided models accountable for losses even if they exit midway through a performance year, and by authorizing termination of ACOs with multiple years of poor financial performance,” Ms. Verma wrote.
The Pathways to Success proposed rule was slated to be published in the Federal Register on Aug. 17. Comments are being accepted at regulations.gov until Oct. 16.
Accountable care organizations (ACOs) are playing it too safe, according to the Centers for Medicare & Medicaid Services, which wants to change the rules and push more ACOs into taking on more financial risk – with a little flexibility added in.
“ACOs were designed to move Medicare away from fee for service by encouraging providers to find efficiencies and innovative ways to deliver high-quality care to their patients while reducing costs, giving them the flexibility they need to focus on health outcomes over process,” CMS Administrator Seema Verma said during an Aug. 9 press conference.
That’s not what is happening now, Ms. Verma said. Under the current program, physicians and other health care professionals can participate in an ACO that takes on upside risk only – that is, to share in any of the savings it is able to generate – for up to 6 years before having to take on downside risk to return payment to the government if the ACO fails to hit spending targets.
“This environment has created a perverse incentive leading to the shocking present day reality that, after 6 years, over 82% of shared saving program ACOs are in an upside-only track, meaning that these ACOs have no incentive at all to reduce health care cost while improving outcomes as they were intended,” she said. “The program has not lived up to the accountability part of their name.”
In the aggregate, ACOs that only take on upside risk generally are spending more money and not generating the savings that ACOs in a two-sided risk arrangement are, she added.
To get more health care professionals into two-sided risk arrangements, the CMS released a proposed rule Aug. 9 that caps ACO participation in an upside risk–only arrangement for 2 years before having to migrate to a two-sided risk arrangement. To sweeten the pot, the CMS proposes to allow more flexibility for innovation and encouraging patients to maintain their health.
“On top of [antikickback law] waivers they already receive, we are proposing to allow ACOs that are taking risk to give incentive payments in order to reward patients for taking steps to achieve good health, such as gift cards for patients who receive necessary primary and preventive care,” she said, adding that ACOs who take on downside risk also will be eligible to receive payments for providing telehealth services.
ACOs also will need to adopt the 2015 edition of certified EHR technology and the CMS also will be looking to streamline quality measures, according to the proposal.
The CMS is extending current ACO contracts for 6 months, with the new rules, if finalized, going into effect in the middle of 2019.
The proposal also simplifies the ACO program by offering two tracks, as detailed in a blog post penned by Administrator Verma that appeared Aug. 9 in Health Affairs.
The Basic track “would feature a glide path for taking risk,” she wrote. “It would begin with up to 2 years of upside-only risk and then gradually transition in years 3, 4, and 5 to increasing levels of performance risk, concluding in year 5 at a level of risk that meets the standard to qualify as an advanced alternative payment model [APM]” under the Quality Payment Program. Those entering the enhanced track, taking on two-sided risk immediately, would need to meet the standard of an APM immediately.
The proposal also calls for more transparency and would require providers to alert Medicare beneficiaries that services are being provided in the context of an ACO and to explain what that means for their care.
Spending benchmarks would continue to be calculated using both regional and national spending trends. Program integrity also will be enhanced “by holding ACOs in two-sided models accountable for losses even if they exit midway through a performance year, and by authorizing termination of ACOs with multiple years of poor financial performance,” Ms. Verma wrote.
The Pathways to Success proposed rule was slated to be published in the Federal Register on Aug. 17. Comments are being accepted at regulations.gov until Oct. 16.
Autotransplant is linked to higher AML, MDS risk
Patients undergoing autologous hematopoietic cell transplantation for lymphoma or plasma cell myeloma have 10-100 times the risk of acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) seen in the general population, according to a retrospective cohort study.
The elevated risk also exceeds that of similar patients largely untreated with autotransplant.
Exposure to DNA-damaging drugs and ionizing radiation – both used in autotransplant – is known to increase risk of these treatment-related myeloid neoplasms, according to Tomas Radivoyevitch, PhD, of the Cleveland Clinic Foundation and his colleagues. Concern about this complication has been growing as long-term survivorship after transplant improves.
The investigators analyzed data reported to the Center for International Blood and Marrow Transplant Research. Analyses were based on 9,028 patients undergoing autotransplant during 1995-2010 for Hodgkin lymphoma (916 patients), non-Hodgkin lymphoma (3,546 patients), or plasma cell myeloma (4,566 patients). Their median duration of follow-up was 90 months, 110 months, and 97 months, respectively.
Overall, 3.7% of the cohort developed AML or MDS after their transplant. More aggressive transplantation protocols increased the likelihood of this outcome: Risk was higher for patients with Hodgkin lymphoma who received conditioning with total body radiation versus chemotherapy alone (hazard ratio, 4.0); patients with non-Hodgkin lymphoma who received conditioning with total body radiation (HR, 1.7) or with busulfan and melphalan or cyclophosphamide (HR, 1.8) versus the BEAM regimen; patients with non-Hodgkin lymphoma or plasma cell myeloma who received three or more lines of chemotherapy versus just one line (HR, 1.9 and 1.8, respectively); and patients with non-Hodgkin lymphoma who underwent transplantation in 2005-2010 versus 1995-1999 (HR, 2.1).
Patients reported to Surveillance, Epidemiology and End Results (SEER) database with the same lymphoma and plasma cell myeloma diagnoses, few of whom underwent autotransplant, had risks of AML and MDS that were 5-10 times higher than the background level in the population. But the study autotransplant cohort had a risk of AML that was 10-50 times higher, and a relative risk of MDS that was roughly 100 times higher than the background level.
“These increases may be related to exposure to high doses of DNA-damaging drugs given for the autotransplant, but this hypothesis can only be tested in a prospective study,” Dr. Radivoyevitch and his coinvestigators wrote.
The reason for the greater elevation of MDS risk, compared with AML risk, is unknown. “One possible explanation is that many cases of MDS evolve to AML, and that earlier diagnosis from increased posttransplant surveillance resulted in a deficiency of AML,” they wrote. “A second is based on steeper MDS versus AML incidences versus age … and the possibility that transplantation recipient marrow ages (i.e., marrow biological ages) are perhaps decades older than calendar ages.”
The Center for International Blood and Marrow Transplant Research is supported by several U.S. government agencies and numerous pharmaceutical companies. The authors reported that they had no relevant conflicts of interest.
SOURCE: Radivoyevitch T et al. Leuk Res. 2018 Jul 19. pii: S0145-2126(18)30160-7.
Patients undergoing autologous hematopoietic cell transplantation for lymphoma or plasma cell myeloma have 10-100 times the risk of acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) seen in the general population, according to a retrospective cohort study.
The elevated risk also exceeds that of similar patients largely untreated with autotransplant.
Exposure to DNA-damaging drugs and ionizing radiation – both used in autotransplant – is known to increase risk of these treatment-related myeloid neoplasms, according to Tomas Radivoyevitch, PhD, of the Cleveland Clinic Foundation and his colleagues. Concern about this complication has been growing as long-term survivorship after transplant improves.
The investigators analyzed data reported to the Center for International Blood and Marrow Transplant Research. Analyses were based on 9,028 patients undergoing autotransplant during 1995-2010 for Hodgkin lymphoma (916 patients), non-Hodgkin lymphoma (3,546 patients), or plasma cell myeloma (4,566 patients). Their median duration of follow-up was 90 months, 110 months, and 97 months, respectively.
Overall, 3.7% of the cohort developed AML or MDS after their transplant. More aggressive transplantation protocols increased the likelihood of this outcome: Risk was higher for patients with Hodgkin lymphoma who received conditioning with total body radiation versus chemotherapy alone (hazard ratio, 4.0); patients with non-Hodgkin lymphoma who received conditioning with total body radiation (HR, 1.7) or with busulfan and melphalan or cyclophosphamide (HR, 1.8) versus the BEAM regimen; patients with non-Hodgkin lymphoma or plasma cell myeloma who received three or more lines of chemotherapy versus just one line (HR, 1.9 and 1.8, respectively); and patients with non-Hodgkin lymphoma who underwent transplantation in 2005-2010 versus 1995-1999 (HR, 2.1).
Patients reported to Surveillance, Epidemiology and End Results (SEER) database with the same lymphoma and plasma cell myeloma diagnoses, few of whom underwent autotransplant, had risks of AML and MDS that were 5-10 times higher than the background level in the population. But the study autotransplant cohort had a risk of AML that was 10-50 times higher, and a relative risk of MDS that was roughly 100 times higher than the background level.
“These increases may be related to exposure to high doses of DNA-damaging drugs given for the autotransplant, but this hypothesis can only be tested in a prospective study,” Dr. Radivoyevitch and his coinvestigators wrote.
The reason for the greater elevation of MDS risk, compared with AML risk, is unknown. “One possible explanation is that many cases of MDS evolve to AML, and that earlier diagnosis from increased posttransplant surveillance resulted in a deficiency of AML,” they wrote. “A second is based on steeper MDS versus AML incidences versus age … and the possibility that transplantation recipient marrow ages (i.e., marrow biological ages) are perhaps decades older than calendar ages.”
The Center for International Blood and Marrow Transplant Research is supported by several U.S. government agencies and numerous pharmaceutical companies. The authors reported that they had no relevant conflicts of interest.
SOURCE: Radivoyevitch T et al. Leuk Res. 2018 Jul 19. pii: S0145-2126(18)30160-7.
Patients undergoing autologous hematopoietic cell transplantation for lymphoma or plasma cell myeloma have 10-100 times the risk of acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) seen in the general population, according to a retrospective cohort study.
The elevated risk also exceeds that of similar patients largely untreated with autotransplant.
Exposure to DNA-damaging drugs and ionizing radiation – both used in autotransplant – is known to increase risk of these treatment-related myeloid neoplasms, according to Tomas Radivoyevitch, PhD, of the Cleveland Clinic Foundation and his colleagues. Concern about this complication has been growing as long-term survivorship after transplant improves.
The investigators analyzed data reported to the Center for International Blood and Marrow Transplant Research. Analyses were based on 9,028 patients undergoing autotransplant during 1995-2010 for Hodgkin lymphoma (916 patients), non-Hodgkin lymphoma (3,546 patients), or plasma cell myeloma (4,566 patients). Their median duration of follow-up was 90 months, 110 months, and 97 months, respectively.
Overall, 3.7% of the cohort developed AML or MDS after their transplant. More aggressive transplantation protocols increased the likelihood of this outcome: Risk was higher for patients with Hodgkin lymphoma who received conditioning with total body radiation versus chemotherapy alone (hazard ratio, 4.0); patients with non-Hodgkin lymphoma who received conditioning with total body radiation (HR, 1.7) or with busulfan and melphalan or cyclophosphamide (HR, 1.8) versus the BEAM regimen; patients with non-Hodgkin lymphoma or plasma cell myeloma who received three or more lines of chemotherapy versus just one line (HR, 1.9 and 1.8, respectively); and patients with non-Hodgkin lymphoma who underwent transplantation in 2005-2010 versus 1995-1999 (HR, 2.1).
Patients reported to Surveillance, Epidemiology and End Results (SEER) database with the same lymphoma and plasma cell myeloma diagnoses, few of whom underwent autotransplant, had risks of AML and MDS that were 5-10 times higher than the background level in the population. But the study autotransplant cohort had a risk of AML that was 10-50 times higher, and a relative risk of MDS that was roughly 100 times higher than the background level.
“These increases may be related to exposure to high doses of DNA-damaging drugs given for the autotransplant, but this hypothesis can only be tested in a prospective study,” Dr. Radivoyevitch and his coinvestigators wrote.
The reason for the greater elevation of MDS risk, compared with AML risk, is unknown. “One possible explanation is that many cases of MDS evolve to AML, and that earlier diagnosis from increased posttransplant surveillance resulted in a deficiency of AML,” they wrote. “A second is based on steeper MDS versus AML incidences versus age … and the possibility that transplantation recipient marrow ages (i.e., marrow biological ages) are perhaps decades older than calendar ages.”
The Center for International Blood and Marrow Transplant Research is supported by several U.S. government agencies and numerous pharmaceutical companies. The authors reported that they had no relevant conflicts of interest.
SOURCE: Radivoyevitch T et al. Leuk Res. 2018 Jul 19. pii: S0145-2126(18)30160-7.
FROM LEUKEMIA RESEARCH
Key clinical point:
Major finding: Patients undergoing autologous hematopoietic cell transplantation have risks for AML and MDS that are 10-100 times higher than those of the general population.
Study details: A retrospective cohort study of 9,028 patients undergoing hematopoietic cell autotransplant during 1995-2010 for Hodgkin lymphoma, non-Hodgkin lymphoma, or plasma cell myeloma.
Disclosures: The Center for International Blood and Marrow Transplant Research is supported by U.S. government agencies and numerous pharmaceutical companies. The authors reported that they have no relevant conflicts of interest.
Source: Radivoyevitch T et al. Leuk Res. 2018 Jul 19. pii: S0145-2126(18)30160-7.
Secondhand smoke drives ED visits for teens
Teens who were exposed to any type of and to have more such visits, compared with unexposed controls, based on data from more than 7,000 adolescents.
Approximately 35% of U.S. teens spent more than an hour exposed to secondhand smoke in a given week, wrote Ashley L. Merianos, PhD, of the University of Cincinnati and her colleagues.
In a study published in Pediatrics, the researchers conducted a secondary analysis of nonsmoking adolescents aged 12-17 years who had not been diagnosed with asthma and who were part of the PATH (Population Assessment of Tobacco and Health) study, a longitudinal cohort trial of tobacco use behavior and related health outcomes in adolescents and adults in the United States. The data were collected between Oct. 3, 2014, and Oct. 30, 2015. The researchers reviewed three main measures of tobacco smoke exposure (TSE): living with a smoker, being exposed to secondhand smoke at home, and being exposed to secondhand smoke for an hour or more in the past 7 days.
Overall, teens who lived with a smoker, had secondary exposure at home, or had at least 1 hour of TSE had significantly more emergency department and/or urgent care visits (mean ranged from 1.62 to 1.65), compared with unexposed peers (mean visits ranged from 1.42 to 1.48). Those who both lived with a smoker and had at least 1 hour of TSE exposure were significantly more likely to visit an ED or urgent care center.
In addition, teens who lived with a smoker, had secondary exposure at home, and had at least 1 hour of TSE were significantly more likely than were unexposed peers to report shortness of breath, difficulty exercising, wheezing during and after exercise, and a dry cough at night (P less than .001).
The researchers also assessed other health indicators, and found that teens with TSE exposure were significantly less likely than were unexposed peers to report very good or excellent health and were approximately 1.50 times more likely than unexposed peers to report missing school because of poor health.
The results were limited by several factors including the use of self-reports of TSE and parent reports of emergency or urgent care visits, and by the inclusion only of other public use variables in the PATH database, the researchers noted. But they adjusted for potentially confounding factors such as household income level, parent education, and health insurance status. However, “Because adolescents are high users of EDs and/or [urgent care] for primary care reasons, these venues are high-volume settings that should be used to offer interventions to adolescents with TSE and their families,” they said.
The researchers had no financial conflicts to disclose. The study was funded by the National Institutes of Health via the National Institute on Drug Abuse and the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
SOURCE: Merianos AL et al. Pediatrics 2018 Aug 6. doi: org/10.1542/peds.2018-0266.
Teens who were exposed to any type of and to have more such visits, compared with unexposed controls, based on data from more than 7,000 adolescents.
Approximately 35% of U.S. teens spent more than an hour exposed to secondhand smoke in a given week, wrote Ashley L. Merianos, PhD, of the University of Cincinnati and her colleagues.
In a study published in Pediatrics, the researchers conducted a secondary analysis of nonsmoking adolescents aged 12-17 years who had not been diagnosed with asthma and who were part of the PATH (Population Assessment of Tobacco and Health) study, a longitudinal cohort trial of tobacco use behavior and related health outcomes in adolescents and adults in the United States. The data were collected between Oct. 3, 2014, and Oct. 30, 2015. The researchers reviewed three main measures of tobacco smoke exposure (TSE): living with a smoker, being exposed to secondhand smoke at home, and being exposed to secondhand smoke for an hour or more in the past 7 days.
Overall, teens who lived with a smoker, had secondary exposure at home, or had at least 1 hour of TSE had significantly more emergency department and/or urgent care visits (mean ranged from 1.62 to 1.65), compared with unexposed peers (mean visits ranged from 1.42 to 1.48). Those who both lived with a smoker and had at least 1 hour of TSE exposure were significantly more likely to visit an ED or urgent care center.
In addition, teens who lived with a smoker, had secondary exposure at home, and had at least 1 hour of TSE were significantly more likely than were unexposed peers to report shortness of breath, difficulty exercising, wheezing during and after exercise, and a dry cough at night (P less than .001).
The researchers also assessed other health indicators, and found that teens with TSE exposure were significantly less likely than were unexposed peers to report very good or excellent health and were approximately 1.50 times more likely than unexposed peers to report missing school because of poor health.
The results were limited by several factors including the use of self-reports of TSE and parent reports of emergency or urgent care visits, and by the inclusion only of other public use variables in the PATH database, the researchers noted. But they adjusted for potentially confounding factors such as household income level, parent education, and health insurance status. However, “Because adolescents are high users of EDs and/or [urgent care] for primary care reasons, these venues are high-volume settings that should be used to offer interventions to adolescents with TSE and their families,” they said.
The researchers had no financial conflicts to disclose. The study was funded by the National Institutes of Health via the National Institute on Drug Abuse and the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
SOURCE: Merianos AL et al. Pediatrics 2018 Aug 6. doi: org/10.1542/peds.2018-0266.
Teens who were exposed to any type of and to have more such visits, compared with unexposed controls, based on data from more than 7,000 adolescents.
Approximately 35% of U.S. teens spent more than an hour exposed to secondhand smoke in a given week, wrote Ashley L. Merianos, PhD, of the University of Cincinnati and her colleagues.
In a study published in Pediatrics, the researchers conducted a secondary analysis of nonsmoking adolescents aged 12-17 years who had not been diagnosed with asthma and who were part of the PATH (Population Assessment of Tobacco and Health) study, a longitudinal cohort trial of tobacco use behavior and related health outcomes in adolescents and adults in the United States. The data were collected between Oct. 3, 2014, and Oct. 30, 2015. The researchers reviewed three main measures of tobacco smoke exposure (TSE): living with a smoker, being exposed to secondhand smoke at home, and being exposed to secondhand smoke for an hour or more in the past 7 days.
Overall, teens who lived with a smoker, had secondary exposure at home, or had at least 1 hour of TSE had significantly more emergency department and/or urgent care visits (mean ranged from 1.62 to 1.65), compared with unexposed peers (mean visits ranged from 1.42 to 1.48). Those who both lived with a smoker and had at least 1 hour of TSE exposure were significantly more likely to visit an ED or urgent care center.
In addition, teens who lived with a smoker, had secondary exposure at home, and had at least 1 hour of TSE were significantly more likely than were unexposed peers to report shortness of breath, difficulty exercising, wheezing during and after exercise, and a dry cough at night (P less than .001).
The researchers also assessed other health indicators, and found that teens with TSE exposure were significantly less likely than were unexposed peers to report very good or excellent health and were approximately 1.50 times more likely than unexposed peers to report missing school because of poor health.
The results were limited by several factors including the use of self-reports of TSE and parent reports of emergency or urgent care visits, and by the inclusion only of other public use variables in the PATH database, the researchers noted. But they adjusted for potentially confounding factors such as household income level, parent education, and health insurance status. However, “Because adolescents are high users of EDs and/or [urgent care] for primary care reasons, these venues are high-volume settings that should be used to offer interventions to adolescents with TSE and their families,” they said.
The researchers had no financial conflicts to disclose. The study was funded by the National Institutes of Health via the National Institute on Drug Abuse and the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
SOURCE: Merianos AL et al. Pediatrics 2018 Aug 6. doi: org/10.1542/peds.2018-0266.
FROM PEDIATRICS