User login
More deliveries now include opioid use disorder
according to the Centers for Disease Control and Prevention.
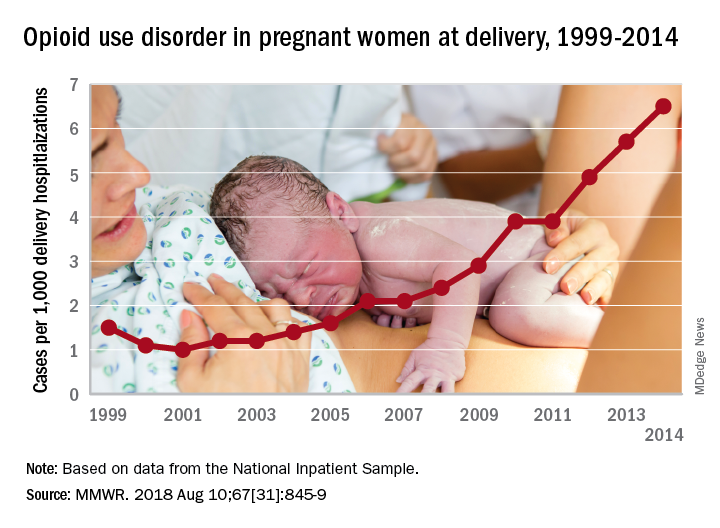
The national prevalence of opioid use disorder increased by 333% as it went from 1.5 cases per 1,000 delivery hospitalizations in 1999 to 6.5 cases per 1,000 in 2014. At the state level, there were significant increases in all 28 states with data available for at least 3 consecutive years during the study period, Sarah C. Haight, MPH, and her associates at the CDC in Atlanta said in the Morbidity and Mortality Weekly Report.
Average annual rate changes for those states ranged from a low of 0.01 per 1,000 delivery hospitalizations per year in California to 5.37 per year in Vermont, with the national rate change coming in at 0.39 per year. Of the 14 states with data available in 1999, Iowa had the lowest rate at 0.1 per 1,000 deliveries and Maryland had the highest at 8.2. In 2014, when data were available for 26 states and the District of Columbia, the highest rate was Vermont’s 48.6 per 1,000 deliveries and the lowest was 0.7 in Washington, D.C., the investigators reported.
Although “increasing trends might represent actual increases in prevalence or improved screening and diagnosis,” Ms. Haight and her associates added that “these estimates also correlate with state opioid prescribing rates in the general population. West Virginia, for example, had a prescribing rate estimated at 138 opioid prescriptions per 100 persons in 2012.”
“These findings illustrate the devastating impact of the opioid epidemic on families across the U.S., including on the very youngest,” said CDC Director Robert R. Redfield, MD. “Untreated opioid use disorder during pregnancy can lead to heartbreaking results. Each case represents a mother, a child, and a family in need of continued treatment and support.”
Data for the analysis came from the Agency for Healthcare Research and Quality’s National Inpatient Sample and State Inpatient Databases.
SOURCE: Haight SC et al. MMWR. 2018 Aug 10;67[31]:845-9.
according to the Centers for Disease Control and Prevention.

The national prevalence of opioid use disorder increased by 333% as it went from 1.5 cases per 1,000 delivery hospitalizations in 1999 to 6.5 cases per 1,000 in 2014. At the state level, there were significant increases in all 28 states with data available for at least 3 consecutive years during the study period, Sarah C. Haight, MPH, and her associates at the CDC in Atlanta said in the Morbidity and Mortality Weekly Report.
Average annual rate changes for those states ranged from a low of 0.01 per 1,000 delivery hospitalizations per year in California to 5.37 per year in Vermont, with the national rate change coming in at 0.39 per year. Of the 14 states with data available in 1999, Iowa had the lowest rate at 0.1 per 1,000 deliveries and Maryland had the highest at 8.2. In 2014, when data were available for 26 states and the District of Columbia, the highest rate was Vermont’s 48.6 per 1,000 deliveries and the lowest was 0.7 in Washington, D.C., the investigators reported.
Although “increasing trends might represent actual increases in prevalence or improved screening and diagnosis,” Ms. Haight and her associates added that “these estimates also correlate with state opioid prescribing rates in the general population. West Virginia, for example, had a prescribing rate estimated at 138 opioid prescriptions per 100 persons in 2012.”
“These findings illustrate the devastating impact of the opioid epidemic on families across the U.S., including on the very youngest,” said CDC Director Robert R. Redfield, MD. “Untreated opioid use disorder during pregnancy can lead to heartbreaking results. Each case represents a mother, a child, and a family in need of continued treatment and support.”
Data for the analysis came from the Agency for Healthcare Research and Quality’s National Inpatient Sample and State Inpatient Databases.
SOURCE: Haight SC et al. MMWR. 2018 Aug 10;67[31]:845-9.
according to the Centers for Disease Control and Prevention.

The national prevalence of opioid use disorder increased by 333% as it went from 1.5 cases per 1,000 delivery hospitalizations in 1999 to 6.5 cases per 1,000 in 2014. At the state level, there were significant increases in all 28 states with data available for at least 3 consecutive years during the study period, Sarah C. Haight, MPH, and her associates at the CDC in Atlanta said in the Morbidity and Mortality Weekly Report.
Average annual rate changes for those states ranged from a low of 0.01 per 1,000 delivery hospitalizations per year in California to 5.37 per year in Vermont, with the national rate change coming in at 0.39 per year. Of the 14 states with data available in 1999, Iowa had the lowest rate at 0.1 per 1,000 deliveries and Maryland had the highest at 8.2. In 2014, when data were available for 26 states and the District of Columbia, the highest rate was Vermont’s 48.6 per 1,000 deliveries and the lowest was 0.7 in Washington, D.C., the investigators reported.
Although “increasing trends might represent actual increases in prevalence or improved screening and diagnosis,” Ms. Haight and her associates added that “these estimates also correlate with state opioid prescribing rates in the general population. West Virginia, for example, had a prescribing rate estimated at 138 opioid prescriptions per 100 persons in 2012.”
“These findings illustrate the devastating impact of the opioid epidemic on families across the U.S., including on the very youngest,” said CDC Director Robert R. Redfield, MD. “Untreated opioid use disorder during pregnancy can lead to heartbreaking results. Each case represents a mother, a child, and a family in need of continued treatment and support.”
Data for the analysis came from the Agency for Healthcare Research and Quality’s National Inpatient Sample and State Inpatient Databases.
SOURCE: Haight SC et al. MMWR. 2018 Aug 10;67[31]:845-9.
FROM MMWR
What do you call a koala who is too sweet for its own good? Diabetic
SAN DIEGO – The 14-pound patient with the deep-pile complexion was lethargic, kept drinking a lot of water, and had a glucose level in the range of 600-700 mg/dL. He was nearly comatose by the time medical staff transferred him to a specialized facility.
The diagnosis: Diabetes. The treatment: Insulin. But multiple daily skin pricks were quite a challenge for Quincy the koala. After all, he requires up to 22 hours of shut-eye each day.
What to do? The veterinary staff at the San Diego Zoo turned to the experts – an endocrinologist and a manufacturer of continuous glucose monitors. Now, Quincy has his own CGM, and a medical team that is tracking his glucose levels in real time on their smartphones.
In fact, Athena Philis-Tsimikas, MD, of the Scripps Whittier Diabetes Institute, pulls out her phone and checks on him at least a couple times a day. She also gets alerts if his blood sugar drops too quickly.
“He is definitely another one of my patients,” she said in an interview. But he’s the only one who lives in trees and enjoys a nice eucalyptus smoothie.
Humans are hardly the only mammals who get diabetes
Veterinarians are quite familiar with diabetes. A wide variety of mammals from pigs and apes to horses and dolphins can develop an equivalent of the human condition. Dogs may be prescribed daily insulin shots, and cats even develop peripheral neuropathy and retinopathy like humans with diabetes.
So it’s not entirely surprising that a team at the Los Angeles Zoo diagnosed Quincy, a 3-year-old Queensland koala, with diabetes.
Quincy’s glucose levels should have been around 80-130 mg/dL, similar to the ideal levels in humans, said San Diego Zoo senior veterinarian Cora Singleton, DVM, in an interview. But tests prompted by his symptoms showed his levels were high, she said, and they stayed that way. According to her, that suggested he wasn’t just having a one-time elevation that animals can experience when they’re stressed.
Unfortunately, there are only a few scattered reports of diabetes in koalas, and “there’s not anything documented about treating a koala over a long term,” Dr. Singleton said. “We’re in uncharted territory here.”
So the Los Angeles Zoo sent Quincy down the California coast for more specialized treatment. The San Diego Zoo’s veterinary staff took in Quincy and treated him with glucose tests and insulin shots, Dr. Singleton said. “But we were looking a way for to get more information with less disturbance to Quincy.”
Someone mentioned the idea of a sensor. “We thought, ‘What a great idea,’” Dr. Singleton said. “It would be a way for us to get a lot of information and find out how his highs and lows are related.”
That’s when the team turned to local endocrinologist Dr. Tsimikas for a helping hand.
The key to koala calming: Eucalyptus smoothies
“They did reach out to us and asked what kind of sensors might be available. We connected them to Dexcom,” a CGM company that’s based in San Diego, Dr. Tsimikas said. “We knew the newest one was coming along and suggested they place that on him as a starting point.”
On June 1, a zoo team attached a Dexcom G6 Continuous Glucose Monitoring System to the koala’s side.
“He’s doing very well. He tolerates the CGM superbly,” Dr. Singleton said. And Quincy doesn’t react when sensors are applied, she said, although it helps that he gets to enjoy a eucalyptus smoothie during the procedure. “Put that in a big syringe, and he’ll volunteer for most anything,” she said.
Obesity can trigger diabetes in mammals other than humans. Could eucalyptus overindulgence explain Quincy’s case of diabetes? Nope.
According to Dr. Tsimikas, the ingredients of the eucalyptus smoothie are just pureed eucalyptus leaves that “go down fast and easy.” These naturally have a nice mix of carbohydrates, fat, and protein to better manage the koala’s sugars and other nutritional needs. If he is dropping his blood sugar values fast, there is another dextrose drink they give him in small amounts, which contains 5-10 g carbohydrates. This is enough to help bring his glucose values back up. It is similar to the treatment recommendations provided to humans with diabetes where they are told to take 15 g of carbohydrates such as honey, hard candies, or juice to prevent a severe hypoglycemic episode.
Dr. Singleton noted that Quincy appears to have the koala equivalent is type 1 diabetes mellitus (T1DM).
Dr. Tsimikas noted “We are not finding the typical antibodies that we find in human T1DM. Quincy is showing low insulin levels, which is why it more closely resembles T1DM. We will be doing further analysis and comparisons with nondiabetic koalas in the future to see if it can be better differentiated.
While he appears to have type 1 diabetes, it’s not clear why he developed it, Dr. Singleton said.
While Quincy is only 3 years old, he’s a full-fledged adult in koala terms. Koalas typically live up to their mid-teens, she said.
This speechless patient still manages to communicate
The San Diego Zoo’s veterinary staff is monitoring Quincy and trying to understand how his glucose levels and daily insulin shots affect him. His tiny size has ruled out use of an insulin pump: Although the insulin pumps have been getting smaller and lighter, they are still too large to attach to our tiny friend. Especially since he would need both the CGM device and the pump, there is not a lot of surface area on his body for attachment of all the devices, according to Dr. Tsimikas.
Since Quincy is so tiny, insulin doses must be minuscule to avoid sending him into hypoglycemia, Dr. Tsimikas said. She said the koala’s medical team is planning to try using a NovoPen Echo injector with a half-unit of insulin.
Dr. Singleton noted that for now, “he’s maintaining his body weight, and he has days when he feels spunky. Sometimes, when he knows it’s breakfast time, and he hears his caretakers coming up the doorway with his breakfast, he’ll be very active on his perch.”
But he has sluggish days, too, when he’ll try to sleep in. Dr. Singleton keeps an eye out for grogginess and signs of weakness and hypoglycemia or hyperglycemia like “a little wobble in his step.”
“The biggest thing I’ve learned from Quincy is the value of his particular nonverbal cues,” she said. “I’m starting to understand when he feels like his sugars are a little high or a little low. I imagine that doctors and parents have the same challenges with little patients, along with figuring out how you communicate that this is supposed to help them.”
Dr. Tsimikas agreed, noting that she sees similarities between Quincy and patients who are hospitalized and can’t easily communicate. Now, “we can track the folks who are on the CGM and intervene earlier than before,” said Dr. Tsimikas, who’s part of a clinical trial team testing CGM devices in two hospitals. “It’s almost like having another vital sign.
“It is only when we have all the data on all the other factors that can influence blood sugar, such as eating patterns, insulin dose and timing, and activity level that we can more accurately adjust the medical interventions.” This requires collaboration between all the groups involved in Quincy’s care. In koalas, the collaboration is with the veterinarian, koala zookeepers, dietitian, and the technology monitoring team. Whereas, for humans, we need parents, care providers, diabetes educators, dietitians, and physicians.
It’s not clear if Quincy will need his CGM for the rest of his life. If he’s stable on a specific insulin dose, Dr. Tsimikas said, he may not need it. But it sounds like eucalyptus smoothies will always be a vital part of his regimen.
In the name of thoroughness, take note that Quincy is not the first diabetic zoo animal whose care involved physicians from Scripps. “We have had several other consultations for animals with diabetes. Nearly 25 years ago, a roller-skating chimpanzee with diabetes was brought to the Scripps Whittier Institute labs for evaluation and treatment recommendations. A few years later, one of our medical directors, Alberto Hayek, MD, advised on the care of Lune, a diabetic baboon at the San Diego Zoo, for insulin management. This time we are making house calls to the zoo to treat Quincy in his home environment. Each animal experience offers opportunities to expand our knowledge about diabetes care and exchange approaches that we might not otherwise be aware of. This has been fun and rewarding. I am looking forward to seeing further outcomes from our interactions with Quincy,” according to Dr. Tsimikas.
Dr. Tsimikas reports that her center conducts research with Dexcom and Novo Nordisk. Dr. Singleton reports no relevant disclosures.
SAN DIEGO – The 14-pound patient with the deep-pile complexion was lethargic, kept drinking a lot of water, and had a glucose level in the range of 600-700 mg/dL. He was nearly comatose by the time medical staff transferred him to a specialized facility.
The diagnosis: Diabetes. The treatment: Insulin. But multiple daily skin pricks were quite a challenge for Quincy the koala. After all, he requires up to 22 hours of shut-eye each day.
What to do? The veterinary staff at the San Diego Zoo turned to the experts – an endocrinologist and a manufacturer of continuous glucose monitors. Now, Quincy has his own CGM, and a medical team that is tracking his glucose levels in real time on their smartphones.
In fact, Athena Philis-Tsimikas, MD, of the Scripps Whittier Diabetes Institute, pulls out her phone and checks on him at least a couple times a day. She also gets alerts if his blood sugar drops too quickly.
“He is definitely another one of my patients,” she said in an interview. But he’s the only one who lives in trees and enjoys a nice eucalyptus smoothie.
Humans are hardly the only mammals who get diabetes
Veterinarians are quite familiar with diabetes. A wide variety of mammals from pigs and apes to horses and dolphins can develop an equivalent of the human condition. Dogs may be prescribed daily insulin shots, and cats even develop peripheral neuropathy and retinopathy like humans with diabetes.
So it’s not entirely surprising that a team at the Los Angeles Zoo diagnosed Quincy, a 3-year-old Queensland koala, with diabetes.
Quincy’s glucose levels should have been around 80-130 mg/dL, similar to the ideal levels in humans, said San Diego Zoo senior veterinarian Cora Singleton, DVM, in an interview. But tests prompted by his symptoms showed his levels were high, she said, and they stayed that way. According to her, that suggested he wasn’t just having a one-time elevation that animals can experience when they’re stressed.
Unfortunately, there are only a few scattered reports of diabetes in koalas, and “there’s not anything documented about treating a koala over a long term,” Dr. Singleton said. “We’re in uncharted territory here.”
So the Los Angeles Zoo sent Quincy down the California coast for more specialized treatment. The San Diego Zoo’s veterinary staff took in Quincy and treated him with glucose tests and insulin shots, Dr. Singleton said. “But we were looking a way for to get more information with less disturbance to Quincy.”
Someone mentioned the idea of a sensor. “We thought, ‘What a great idea,’” Dr. Singleton said. “It would be a way for us to get a lot of information and find out how his highs and lows are related.”
That’s when the team turned to local endocrinologist Dr. Tsimikas for a helping hand.
The key to koala calming: Eucalyptus smoothies
“They did reach out to us and asked what kind of sensors might be available. We connected them to Dexcom,” a CGM company that’s based in San Diego, Dr. Tsimikas said. “We knew the newest one was coming along and suggested they place that on him as a starting point.”
On June 1, a zoo team attached a Dexcom G6 Continuous Glucose Monitoring System to the koala’s side.
“He’s doing very well. He tolerates the CGM superbly,” Dr. Singleton said. And Quincy doesn’t react when sensors are applied, she said, although it helps that he gets to enjoy a eucalyptus smoothie during the procedure. “Put that in a big syringe, and he’ll volunteer for most anything,” she said.
Obesity can trigger diabetes in mammals other than humans. Could eucalyptus overindulgence explain Quincy’s case of diabetes? Nope.
According to Dr. Tsimikas, the ingredients of the eucalyptus smoothie are just pureed eucalyptus leaves that “go down fast and easy.” These naturally have a nice mix of carbohydrates, fat, and protein to better manage the koala’s sugars and other nutritional needs. If he is dropping his blood sugar values fast, there is another dextrose drink they give him in small amounts, which contains 5-10 g carbohydrates. This is enough to help bring his glucose values back up. It is similar to the treatment recommendations provided to humans with diabetes where they are told to take 15 g of carbohydrates such as honey, hard candies, or juice to prevent a severe hypoglycemic episode.
Dr. Singleton noted that Quincy appears to have the koala equivalent is type 1 diabetes mellitus (T1DM).
Dr. Tsimikas noted “We are not finding the typical antibodies that we find in human T1DM. Quincy is showing low insulin levels, which is why it more closely resembles T1DM. We will be doing further analysis and comparisons with nondiabetic koalas in the future to see if it can be better differentiated.
While he appears to have type 1 diabetes, it’s not clear why he developed it, Dr. Singleton said.
While Quincy is only 3 years old, he’s a full-fledged adult in koala terms. Koalas typically live up to their mid-teens, she said.
This speechless patient still manages to communicate
The San Diego Zoo’s veterinary staff is monitoring Quincy and trying to understand how his glucose levels and daily insulin shots affect him. His tiny size has ruled out use of an insulin pump: Although the insulin pumps have been getting smaller and lighter, they are still too large to attach to our tiny friend. Especially since he would need both the CGM device and the pump, there is not a lot of surface area on his body for attachment of all the devices, according to Dr. Tsimikas.
Since Quincy is so tiny, insulin doses must be minuscule to avoid sending him into hypoglycemia, Dr. Tsimikas said. She said the koala’s medical team is planning to try using a NovoPen Echo injector with a half-unit of insulin.
Dr. Singleton noted that for now, “he’s maintaining his body weight, and he has days when he feels spunky. Sometimes, when he knows it’s breakfast time, and he hears his caretakers coming up the doorway with his breakfast, he’ll be very active on his perch.”
But he has sluggish days, too, when he’ll try to sleep in. Dr. Singleton keeps an eye out for grogginess and signs of weakness and hypoglycemia or hyperglycemia like “a little wobble in his step.”
“The biggest thing I’ve learned from Quincy is the value of his particular nonverbal cues,” she said. “I’m starting to understand when he feels like his sugars are a little high or a little low. I imagine that doctors and parents have the same challenges with little patients, along with figuring out how you communicate that this is supposed to help them.”
Dr. Tsimikas agreed, noting that she sees similarities between Quincy and patients who are hospitalized and can’t easily communicate. Now, “we can track the folks who are on the CGM and intervene earlier than before,” said Dr. Tsimikas, who’s part of a clinical trial team testing CGM devices in two hospitals. “It’s almost like having another vital sign.
“It is only when we have all the data on all the other factors that can influence blood sugar, such as eating patterns, insulin dose and timing, and activity level that we can more accurately adjust the medical interventions.” This requires collaboration between all the groups involved in Quincy’s care. In koalas, the collaboration is with the veterinarian, koala zookeepers, dietitian, and the technology monitoring team. Whereas, for humans, we need parents, care providers, diabetes educators, dietitians, and physicians.
It’s not clear if Quincy will need his CGM for the rest of his life. If he’s stable on a specific insulin dose, Dr. Tsimikas said, he may not need it. But it sounds like eucalyptus smoothies will always be a vital part of his regimen.
In the name of thoroughness, take note that Quincy is not the first diabetic zoo animal whose care involved physicians from Scripps. “We have had several other consultations for animals with diabetes. Nearly 25 years ago, a roller-skating chimpanzee with diabetes was brought to the Scripps Whittier Institute labs for evaluation and treatment recommendations. A few years later, one of our medical directors, Alberto Hayek, MD, advised on the care of Lune, a diabetic baboon at the San Diego Zoo, for insulin management. This time we are making house calls to the zoo to treat Quincy in his home environment. Each animal experience offers opportunities to expand our knowledge about diabetes care and exchange approaches that we might not otherwise be aware of. This has been fun and rewarding. I am looking forward to seeing further outcomes from our interactions with Quincy,” according to Dr. Tsimikas.
Dr. Tsimikas reports that her center conducts research with Dexcom and Novo Nordisk. Dr. Singleton reports no relevant disclosures.
SAN DIEGO – The 14-pound patient with the deep-pile complexion was lethargic, kept drinking a lot of water, and had a glucose level in the range of 600-700 mg/dL. He was nearly comatose by the time medical staff transferred him to a specialized facility.
The diagnosis: Diabetes. The treatment: Insulin. But multiple daily skin pricks were quite a challenge for Quincy the koala. After all, he requires up to 22 hours of shut-eye each day.
What to do? The veterinary staff at the San Diego Zoo turned to the experts – an endocrinologist and a manufacturer of continuous glucose monitors. Now, Quincy has his own CGM, and a medical team that is tracking his glucose levels in real time on their smartphones.
In fact, Athena Philis-Tsimikas, MD, of the Scripps Whittier Diabetes Institute, pulls out her phone and checks on him at least a couple times a day. She also gets alerts if his blood sugar drops too quickly.
“He is definitely another one of my patients,” she said in an interview. But he’s the only one who lives in trees and enjoys a nice eucalyptus smoothie.
Humans are hardly the only mammals who get diabetes
Veterinarians are quite familiar with diabetes. A wide variety of mammals from pigs and apes to horses and dolphins can develop an equivalent of the human condition. Dogs may be prescribed daily insulin shots, and cats even develop peripheral neuropathy and retinopathy like humans with diabetes.
So it’s not entirely surprising that a team at the Los Angeles Zoo diagnosed Quincy, a 3-year-old Queensland koala, with diabetes.
Quincy’s glucose levels should have been around 80-130 mg/dL, similar to the ideal levels in humans, said San Diego Zoo senior veterinarian Cora Singleton, DVM, in an interview. But tests prompted by his symptoms showed his levels were high, she said, and they stayed that way. According to her, that suggested he wasn’t just having a one-time elevation that animals can experience when they’re stressed.
Unfortunately, there are only a few scattered reports of diabetes in koalas, and “there’s not anything documented about treating a koala over a long term,” Dr. Singleton said. “We’re in uncharted territory here.”
So the Los Angeles Zoo sent Quincy down the California coast for more specialized treatment. The San Diego Zoo’s veterinary staff took in Quincy and treated him with glucose tests and insulin shots, Dr. Singleton said. “But we were looking a way for to get more information with less disturbance to Quincy.”
Someone mentioned the idea of a sensor. “We thought, ‘What a great idea,’” Dr. Singleton said. “It would be a way for us to get a lot of information and find out how his highs and lows are related.”
That’s when the team turned to local endocrinologist Dr. Tsimikas for a helping hand.
The key to koala calming: Eucalyptus smoothies
“They did reach out to us and asked what kind of sensors might be available. We connected them to Dexcom,” a CGM company that’s based in San Diego, Dr. Tsimikas said. “We knew the newest one was coming along and suggested they place that on him as a starting point.”
On June 1, a zoo team attached a Dexcom G6 Continuous Glucose Monitoring System to the koala’s side.
“He’s doing very well. He tolerates the CGM superbly,” Dr. Singleton said. And Quincy doesn’t react when sensors are applied, she said, although it helps that he gets to enjoy a eucalyptus smoothie during the procedure. “Put that in a big syringe, and he’ll volunteer for most anything,” she said.
Obesity can trigger diabetes in mammals other than humans. Could eucalyptus overindulgence explain Quincy’s case of diabetes? Nope.
According to Dr. Tsimikas, the ingredients of the eucalyptus smoothie are just pureed eucalyptus leaves that “go down fast and easy.” These naturally have a nice mix of carbohydrates, fat, and protein to better manage the koala’s sugars and other nutritional needs. If he is dropping his blood sugar values fast, there is another dextrose drink they give him in small amounts, which contains 5-10 g carbohydrates. This is enough to help bring his glucose values back up. It is similar to the treatment recommendations provided to humans with diabetes where they are told to take 15 g of carbohydrates such as honey, hard candies, or juice to prevent a severe hypoglycemic episode.
Dr. Singleton noted that Quincy appears to have the koala equivalent is type 1 diabetes mellitus (T1DM).
Dr. Tsimikas noted “We are not finding the typical antibodies that we find in human T1DM. Quincy is showing low insulin levels, which is why it more closely resembles T1DM. We will be doing further analysis and comparisons with nondiabetic koalas in the future to see if it can be better differentiated.
While he appears to have type 1 diabetes, it’s not clear why he developed it, Dr. Singleton said.
While Quincy is only 3 years old, he’s a full-fledged adult in koala terms. Koalas typically live up to their mid-teens, she said.
This speechless patient still manages to communicate
The San Diego Zoo’s veterinary staff is monitoring Quincy and trying to understand how his glucose levels and daily insulin shots affect him. His tiny size has ruled out use of an insulin pump: Although the insulin pumps have been getting smaller and lighter, they are still too large to attach to our tiny friend. Especially since he would need both the CGM device and the pump, there is not a lot of surface area on his body for attachment of all the devices, according to Dr. Tsimikas.
Since Quincy is so tiny, insulin doses must be minuscule to avoid sending him into hypoglycemia, Dr. Tsimikas said. She said the koala’s medical team is planning to try using a NovoPen Echo injector with a half-unit of insulin.
Dr. Singleton noted that for now, “he’s maintaining his body weight, and he has days when he feels spunky. Sometimes, when he knows it’s breakfast time, and he hears his caretakers coming up the doorway with his breakfast, he’ll be very active on his perch.”
But he has sluggish days, too, when he’ll try to sleep in. Dr. Singleton keeps an eye out for grogginess and signs of weakness and hypoglycemia or hyperglycemia like “a little wobble in his step.”
“The biggest thing I’ve learned from Quincy is the value of his particular nonverbal cues,” she said. “I’m starting to understand when he feels like his sugars are a little high or a little low. I imagine that doctors and parents have the same challenges with little patients, along with figuring out how you communicate that this is supposed to help them.”
Dr. Tsimikas agreed, noting that she sees similarities between Quincy and patients who are hospitalized and can’t easily communicate. Now, “we can track the folks who are on the CGM and intervene earlier than before,” said Dr. Tsimikas, who’s part of a clinical trial team testing CGM devices in two hospitals. “It’s almost like having another vital sign.
“It is only when we have all the data on all the other factors that can influence blood sugar, such as eating patterns, insulin dose and timing, and activity level that we can more accurately adjust the medical interventions.” This requires collaboration between all the groups involved in Quincy’s care. In koalas, the collaboration is with the veterinarian, koala zookeepers, dietitian, and the technology monitoring team. Whereas, for humans, we need parents, care providers, diabetes educators, dietitians, and physicians.
It’s not clear if Quincy will need his CGM for the rest of his life. If he’s stable on a specific insulin dose, Dr. Tsimikas said, he may not need it. But it sounds like eucalyptus smoothies will always be a vital part of his regimen.
In the name of thoroughness, take note that Quincy is not the first diabetic zoo animal whose care involved physicians from Scripps. “We have had several other consultations for animals with diabetes. Nearly 25 years ago, a roller-skating chimpanzee with diabetes was brought to the Scripps Whittier Institute labs for evaluation and treatment recommendations. A few years later, one of our medical directors, Alberto Hayek, MD, advised on the care of Lune, a diabetic baboon at the San Diego Zoo, for insulin management. This time we are making house calls to the zoo to treat Quincy in his home environment. Each animal experience offers opportunities to expand our knowledge about diabetes care and exchange approaches that we might not otherwise be aware of. This has been fun and rewarding. I am looking forward to seeing further outcomes from our interactions with Quincy,” according to Dr. Tsimikas.
Dr. Tsimikas reports that her center conducts research with Dexcom and Novo Nordisk. Dr. Singleton reports no relevant disclosures.
ASCO calls for expanding clinical trial eligibility
The American Society of Clinical Oncology and Friends of Cancer Research have submitted recommended language to the Food and Drug Administration for ways to expand eligibility criteria for cancer clinical trials.
The recommendations address five specific areas that were identified as most likely to restrict participation, but least likely to affect the safety of participants, and include minimum age requirements for trial enrollment, HIV/AIDS status, brain metastases, organ dysfunction, and prior and concurrent malignancies.
“Eligibility criteria ensure patient safety, but if they are overly strict, they can jeopardize accrual for clinical trials and reduce the ability to apply trial results to treating patients with cancer in clinical practice,” ASCO President Monica M. Bertagnolli, MD, said in a statement. “These guidance documents help trial sponsors understand how to modernize eligibility criteria and ensure that trial participants more accurately reflect the patients who will receive a drug after approval.”
The two organizations launched an effort to update clinical trial eligibility criteria in 2016 and published a joint statement in 2017. The letter to the FDA and the rationale and instructions for expanding eligibility criteria in each of the five areas can be found here on the Friends of Cancer Research website.
SOURCE: Friends of Cancer and ASCO letter to the FDA.
The American Society of Clinical Oncology and Friends of Cancer Research have submitted recommended language to the Food and Drug Administration for ways to expand eligibility criteria for cancer clinical trials.
The recommendations address five specific areas that were identified as most likely to restrict participation, but least likely to affect the safety of participants, and include minimum age requirements for trial enrollment, HIV/AIDS status, brain metastases, organ dysfunction, and prior and concurrent malignancies.
“Eligibility criteria ensure patient safety, but if they are overly strict, they can jeopardize accrual for clinical trials and reduce the ability to apply trial results to treating patients with cancer in clinical practice,” ASCO President Monica M. Bertagnolli, MD, said in a statement. “These guidance documents help trial sponsors understand how to modernize eligibility criteria and ensure that trial participants more accurately reflect the patients who will receive a drug after approval.”
The two organizations launched an effort to update clinical trial eligibility criteria in 2016 and published a joint statement in 2017. The letter to the FDA and the rationale and instructions for expanding eligibility criteria in each of the five areas can be found here on the Friends of Cancer Research website.
SOURCE: Friends of Cancer and ASCO letter to the FDA.
The American Society of Clinical Oncology and Friends of Cancer Research have submitted recommended language to the Food and Drug Administration for ways to expand eligibility criteria for cancer clinical trials.
The recommendations address five specific areas that were identified as most likely to restrict participation, but least likely to affect the safety of participants, and include minimum age requirements for trial enrollment, HIV/AIDS status, brain metastases, organ dysfunction, and prior and concurrent malignancies.
“Eligibility criteria ensure patient safety, but if they are overly strict, they can jeopardize accrual for clinical trials and reduce the ability to apply trial results to treating patients with cancer in clinical practice,” ASCO President Monica M. Bertagnolli, MD, said in a statement. “These guidance documents help trial sponsors understand how to modernize eligibility criteria and ensure that trial participants more accurately reflect the patients who will receive a drug after approval.”
The two organizations launched an effort to update clinical trial eligibility criteria in 2016 and published a joint statement in 2017. The letter to the FDA and the rationale and instructions for expanding eligibility criteria in each of the five areas can be found here on the Friends of Cancer Research website.
SOURCE: Friends of Cancer and ASCO letter to the FDA.
Key clinical point: ASCO and Friends of Cancer have submitted draft recommendations to the FDA for expanding cancer clinical trial participation.
Major finding: The organizations recommend addressing minimum age requirements, HIV/AIDS status, brain metastases, organ dysfunction, and prior and concurrent malignancies.
Data source: Draft guidance produced by ASCO and Friends of Cancer Research and submitted to the FDA.
Disclosures: Individual members of the working groups were not listed and conflicts of interest were not disclosed.
Source: Friends of Cancer and ASCO letter to the FDA.
Friable Erythema and Erosions on the Mouth
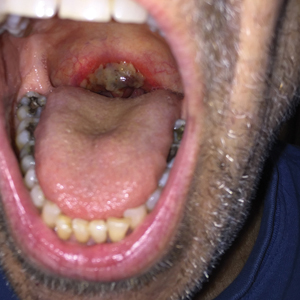
The Diagnosis: Radiation Mucositis
The patient was undergoing active radiation therapy for squamous cell carcinoma of the tongue, and according to the oncology team, the findings were in the precise location of radiation exposure. Radiation mucositis is a major and limiting side effect of radiation therapy for head and neck mucosal cancers, and symptom management is critical to ensure completion of the full radiation dose. Although infectious etiologies must be considered, the patient was already on prophylactic antiviral and antibacterial therapies. Moreover, the focal involvement with sparing of more mucosal tissue is atypical for most infections. Fixed drug reactions can present with localized mucosal and nonmucosal inflammation leading to erosion or ulceration. In this case, the only potential culprit was levofloxacin; however, it was initiated 2 days prior, and the patient never had reactions to this medication in the past.
Acute radiation mucositis is a transient but major limiting side effect of radiation therapy. The associated odynophagia, secondary infection, and reduced oral intake often can lead to diminished disease control secondary to treatment interruption and subsequent development of resistant tumor burden. Concurrent chemotherapy and alternated fractionation radiation therapy increase the incidence of mucositis. Trotti et al1 (n=6181) reported that severe mucositis (grades 3 to 4) was found in 56% of patients receiving altered fractionation radiation therapy compared to 34% of patients who received conventional radiation therapy. Other risk factors related to the development of acute radiation mucositis include associated chemotherapy, age (>65 years), poor oral hygiene, diabetes mellitus, and prior periodontal disease.2
Radiation causes direct cellular damage to keratinocytes, leading to ulceration and erythema, as well as keratinocyte stem cells, which interferes with the healing process. Typical symptoms of mucosal radiation injury may include erythema (asymptomatic or causing intolerance of warm foods) that develops at the end of the second week of radiation therapy, focal areas of desquamation that develops in week 3, and confluent mucositis that can further progress to ulceration and necrosis in weeks 4 to 5.2 The development of dysgeusia, which is estimated to occur in 67% of patients receiving radiotherapy and 76% of patients receiving combination therapy, also can contribute to nutritional difficulties and weight loss.3
Avoiding overtreatment by constraining radiation volume and limiting concurrent chemotherapy are important preventative measures. The mainstay for managing mucositis includes symptomatic relief with oral hygiene, topical agents, topical plus systemic analgesia, dietary changes, and treatment of associated infections. Benzydamine, a nonsteroidal anti-inflammatory drug, is not available in the United States but has been shown to effectively improve symptoms.4 Various formulations of topical anesthetics consisting of diphenhydramine with or without corticosteroids, antibiotics, and antifungals help alleviate symptoms of mucositis; however, no single formulation has been studied. Low-level laser therapy also has shown efficacy in managing symptoms of mucositis.5,6 For persistent odynophagia, systemic opioid therapy should be attempted to achieve uninterrupted radiation therapy. Severe mucositis requires balancing risks and benefits of interrupting treatment, as additional damage may cause permanent mucosal injury.
Our patient had adequate symptom control with benzocaine lozenges and a combination mouthwash containing diphenhydramine, nystatin, lidocaine, hydrocortisone, and tetracycline. He required only occasional doses of systemic oxycodone. After a 1-week hospital admission for treatment of the pneumonia, he resumed radiation therapy and completed a full 8-week radiation course.
- Trotti A, Bellm LA, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66:253-262.
- Mallick S, Benson R, Rath GK. Radiation induced oral mucositis: a review of current literature on prevention and management. Eur Arch Otorhinolaryngol. 2016;273:2285-2293.
- Hovan AJ, Williams PM, Stevenson-Moore P, et al; Dysgeusia Section, Oral Care Study Group, Multinational Association of Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO). A systematic review of dysgeusia induced by cancer therapies. Support Care Cancer. 2010;18:1081-1087.
- Epstein JB, Silverman S, Paggiarino DA, et al. Benzydamine HCl for prophylaxis of radiation‐induced oral mucositis. Cancer. 2001;92:875-885.
- Henke M, Alfonsi M, Foa P, et al. Palifermin decreases severe oral mucositis of patients undergoing postoperative radiochemotherapy for head and neck cancer: a randomized, placebo-controlled trial. J Clin Oncol. 2011;29:2815-2820.
- Bensadoun RJ, Nair RG. Low-level laser therapy in the prevention and treatment of cancer therapy-induced mucositis: 2012 state of the art based on literature review and meta-analysis. Curr Opin Oncol. 2012;24:363-370.
The Diagnosis: Radiation Mucositis
The patient was undergoing active radiation therapy for squamous cell carcinoma of the tongue, and according to the oncology team, the findings were in the precise location of radiation exposure. Radiation mucositis is a major and limiting side effect of radiation therapy for head and neck mucosal cancers, and symptom management is critical to ensure completion of the full radiation dose. Although infectious etiologies must be considered, the patient was already on prophylactic antiviral and antibacterial therapies. Moreover, the focal involvement with sparing of more mucosal tissue is atypical for most infections. Fixed drug reactions can present with localized mucosal and nonmucosal inflammation leading to erosion or ulceration. In this case, the only potential culprit was levofloxacin; however, it was initiated 2 days prior, and the patient never had reactions to this medication in the past.
Acute radiation mucositis is a transient but major limiting side effect of radiation therapy. The associated odynophagia, secondary infection, and reduced oral intake often can lead to diminished disease control secondary to treatment interruption and subsequent development of resistant tumor burden. Concurrent chemotherapy and alternated fractionation radiation therapy increase the incidence of mucositis. Trotti et al1 (n=6181) reported that severe mucositis (grades 3 to 4) was found in 56% of patients receiving altered fractionation radiation therapy compared to 34% of patients who received conventional radiation therapy. Other risk factors related to the development of acute radiation mucositis include associated chemotherapy, age (>65 years), poor oral hygiene, diabetes mellitus, and prior periodontal disease.2
Radiation causes direct cellular damage to keratinocytes, leading to ulceration and erythema, as well as keratinocyte stem cells, which interferes with the healing process. Typical symptoms of mucosal radiation injury may include erythema (asymptomatic or causing intolerance of warm foods) that develops at the end of the second week of radiation therapy, focal areas of desquamation that develops in week 3, and confluent mucositis that can further progress to ulceration and necrosis in weeks 4 to 5.2 The development of dysgeusia, which is estimated to occur in 67% of patients receiving radiotherapy and 76% of patients receiving combination therapy, also can contribute to nutritional difficulties and weight loss.3
Avoiding overtreatment by constraining radiation volume and limiting concurrent chemotherapy are important preventative measures. The mainstay for managing mucositis includes symptomatic relief with oral hygiene, topical agents, topical plus systemic analgesia, dietary changes, and treatment of associated infections. Benzydamine, a nonsteroidal anti-inflammatory drug, is not available in the United States but has been shown to effectively improve symptoms.4 Various formulations of topical anesthetics consisting of diphenhydramine with or without corticosteroids, antibiotics, and antifungals help alleviate symptoms of mucositis; however, no single formulation has been studied. Low-level laser therapy also has shown efficacy in managing symptoms of mucositis.5,6 For persistent odynophagia, systemic opioid therapy should be attempted to achieve uninterrupted radiation therapy. Severe mucositis requires balancing risks and benefits of interrupting treatment, as additional damage may cause permanent mucosal injury.
Our patient had adequate symptom control with benzocaine lozenges and a combination mouthwash containing diphenhydramine, nystatin, lidocaine, hydrocortisone, and tetracycline. He required only occasional doses of systemic oxycodone. After a 1-week hospital admission for treatment of the pneumonia, he resumed radiation therapy and completed a full 8-week radiation course.
The Diagnosis: Radiation Mucositis
The patient was undergoing active radiation therapy for squamous cell carcinoma of the tongue, and according to the oncology team, the findings were in the precise location of radiation exposure. Radiation mucositis is a major and limiting side effect of radiation therapy for head and neck mucosal cancers, and symptom management is critical to ensure completion of the full radiation dose. Although infectious etiologies must be considered, the patient was already on prophylactic antiviral and antibacterial therapies. Moreover, the focal involvement with sparing of more mucosal tissue is atypical for most infections. Fixed drug reactions can present with localized mucosal and nonmucosal inflammation leading to erosion or ulceration. In this case, the only potential culprit was levofloxacin; however, it was initiated 2 days prior, and the patient never had reactions to this medication in the past.
Acute radiation mucositis is a transient but major limiting side effect of radiation therapy. The associated odynophagia, secondary infection, and reduced oral intake often can lead to diminished disease control secondary to treatment interruption and subsequent development of resistant tumor burden. Concurrent chemotherapy and alternated fractionation radiation therapy increase the incidence of mucositis. Trotti et al1 (n=6181) reported that severe mucositis (grades 3 to 4) was found in 56% of patients receiving altered fractionation radiation therapy compared to 34% of patients who received conventional radiation therapy. Other risk factors related to the development of acute radiation mucositis include associated chemotherapy, age (>65 years), poor oral hygiene, diabetes mellitus, and prior periodontal disease.2
Radiation causes direct cellular damage to keratinocytes, leading to ulceration and erythema, as well as keratinocyte stem cells, which interferes with the healing process. Typical symptoms of mucosal radiation injury may include erythema (asymptomatic or causing intolerance of warm foods) that develops at the end of the second week of radiation therapy, focal areas of desquamation that develops in week 3, and confluent mucositis that can further progress to ulceration and necrosis in weeks 4 to 5.2 The development of dysgeusia, which is estimated to occur in 67% of patients receiving radiotherapy and 76% of patients receiving combination therapy, also can contribute to nutritional difficulties and weight loss.3
Avoiding overtreatment by constraining radiation volume and limiting concurrent chemotherapy are important preventative measures. The mainstay for managing mucositis includes symptomatic relief with oral hygiene, topical agents, topical plus systemic analgesia, dietary changes, and treatment of associated infections. Benzydamine, a nonsteroidal anti-inflammatory drug, is not available in the United States but has been shown to effectively improve symptoms.4 Various formulations of topical anesthetics consisting of diphenhydramine with or without corticosteroids, antibiotics, and antifungals help alleviate symptoms of mucositis; however, no single formulation has been studied. Low-level laser therapy also has shown efficacy in managing symptoms of mucositis.5,6 For persistent odynophagia, systemic opioid therapy should be attempted to achieve uninterrupted radiation therapy. Severe mucositis requires balancing risks and benefits of interrupting treatment, as additional damage may cause permanent mucosal injury.
Our patient had adequate symptom control with benzocaine lozenges and a combination mouthwash containing diphenhydramine, nystatin, lidocaine, hydrocortisone, and tetracycline. He required only occasional doses of systemic oxycodone. After a 1-week hospital admission for treatment of the pneumonia, he resumed radiation therapy and completed a full 8-week radiation course.
- Trotti A, Bellm LA, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66:253-262.
- Mallick S, Benson R, Rath GK. Radiation induced oral mucositis: a review of current literature on prevention and management. Eur Arch Otorhinolaryngol. 2016;273:2285-2293.
- Hovan AJ, Williams PM, Stevenson-Moore P, et al; Dysgeusia Section, Oral Care Study Group, Multinational Association of Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO). A systematic review of dysgeusia induced by cancer therapies. Support Care Cancer. 2010;18:1081-1087.
- Epstein JB, Silverman S, Paggiarino DA, et al. Benzydamine HCl for prophylaxis of radiation‐induced oral mucositis. Cancer. 2001;92:875-885.
- Henke M, Alfonsi M, Foa P, et al. Palifermin decreases severe oral mucositis of patients undergoing postoperative radiochemotherapy for head and neck cancer: a randomized, placebo-controlled trial. J Clin Oncol. 2011;29:2815-2820.
- Bensadoun RJ, Nair RG. Low-level laser therapy in the prevention and treatment of cancer therapy-induced mucositis: 2012 state of the art based on literature review and meta-analysis. Curr Opin Oncol. 2012;24:363-370.
- Trotti A, Bellm LA, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66:253-262.
- Mallick S, Benson R, Rath GK. Radiation induced oral mucositis: a review of current literature on prevention and management. Eur Arch Otorhinolaryngol. 2016;273:2285-2293.
- Hovan AJ, Williams PM, Stevenson-Moore P, et al; Dysgeusia Section, Oral Care Study Group, Multinational Association of Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO). A systematic review of dysgeusia induced by cancer therapies. Support Care Cancer. 2010;18:1081-1087.
- Epstein JB, Silverman S, Paggiarino DA, et al. Benzydamine HCl for prophylaxis of radiation‐induced oral mucositis. Cancer. 2001;92:875-885.
- Henke M, Alfonsi M, Foa P, et al. Palifermin decreases severe oral mucositis of patients undergoing postoperative radiochemotherapy for head and neck cancer: a randomized, placebo-controlled trial. J Clin Oncol. 2011;29:2815-2820.
- Bensadoun RJ, Nair RG. Low-level laser therapy in the prevention and treatment of cancer therapy-induced mucositis: 2012 state of the art based on literature review and meta-analysis. Curr Opin Oncol. 2012;24:363-370.
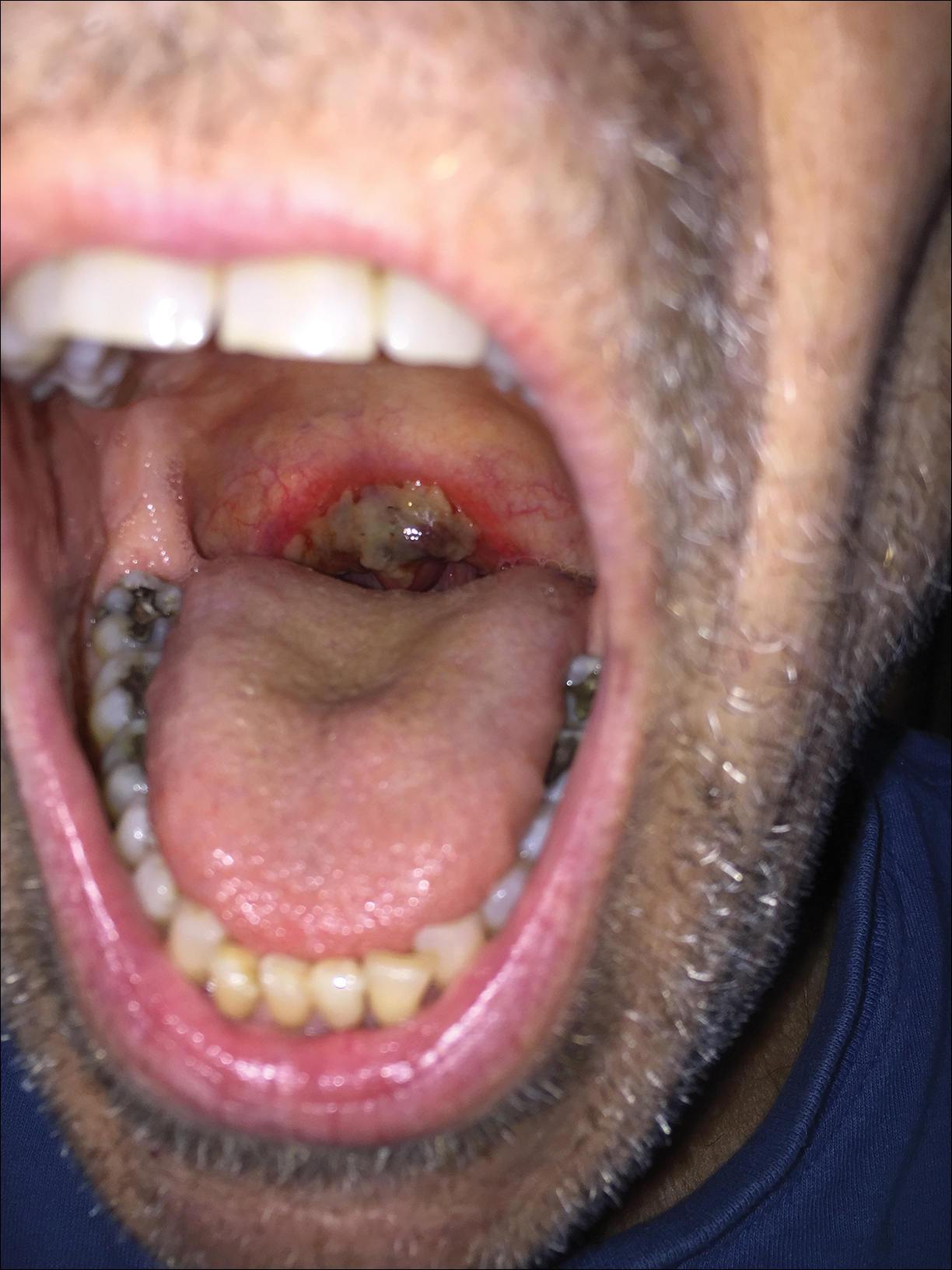
A 68-year-old man with squamous cell carcinoma of the tongue presented with a sore throat and odynophagia of 4 days' duration. At the time he was undergoing radiation therapy for the squamous cell carcinoma, and multiple myeloma was being actively treated with carfilzomib and pomalidomide. At the time of symptom onset he also was undergoing treatment with levofloxacin for community-acquired pneumonia. On day 2 of antibiotic therapy he noted pain with swallowing and an intolerance to warm foods. He was unaware of any new rash or lesions of the lips or mouth. He denied dysgeusia, changes in speech, bleeding, trauma, or recent smoking. He was taking prophylactic acyclovir and trimethoprim-sulfamethoxazole due to chemotherapy. Physical examination revealed a posterior oropharynx and uvula with well-defined friable erythema and erosions covered by white patches. There was no mucosal ulceration and no notable skin findings. The remainder of the physical examination was unremarkable.
Atrophodermalike Guttate Morphea
To the Editor:
Morphea, atrophoderma, guttate lichen sclerosus et atrophicus (LS&A), anetoderma, and their subtypes are inflammatory processes ultimately leading to dermal remodeling. We report a case of a scaly, hypopigmented, macular rash that clinically appeared as an entity along the morphea-atrophoderma spectrum and demonstrated unique histopathologic changes in both collagen and elastin confined to the upper reticular and papillary dermis. This case is a potentially rare variant representing a combination of clinical and microscopic findings.
A 29-year-old woman presented for an increasing number of white spots distributed on the trunk, arms, and legs. She denied local and systemic symptoms. The patient reported that she was stung by 100 wasps 23 years prior. Following the assault, her grandmother placed chewed tobacco leaves atop the painful erythematous wheals and flares. Upon resolution, hypopigmented macules and patches remained in their place. The patient denied associated symptoms or new lesions; she did not seek care at that time.
In her early 20s, the patient noted new, similarly distributed hypopigmented macules and patches without associated arthropod assault. She was treated by an outside dermatologist without result for presumed tinea versicolor. A follow-up superficial shave biopsy cited subtle psoriasiform dermatitis. Topical steroids did not improve the lesions. Her medical history also was remarkable for a reportedly unprovoked complete rotator cuff tear.
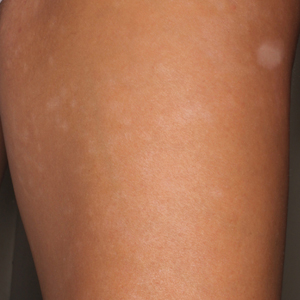
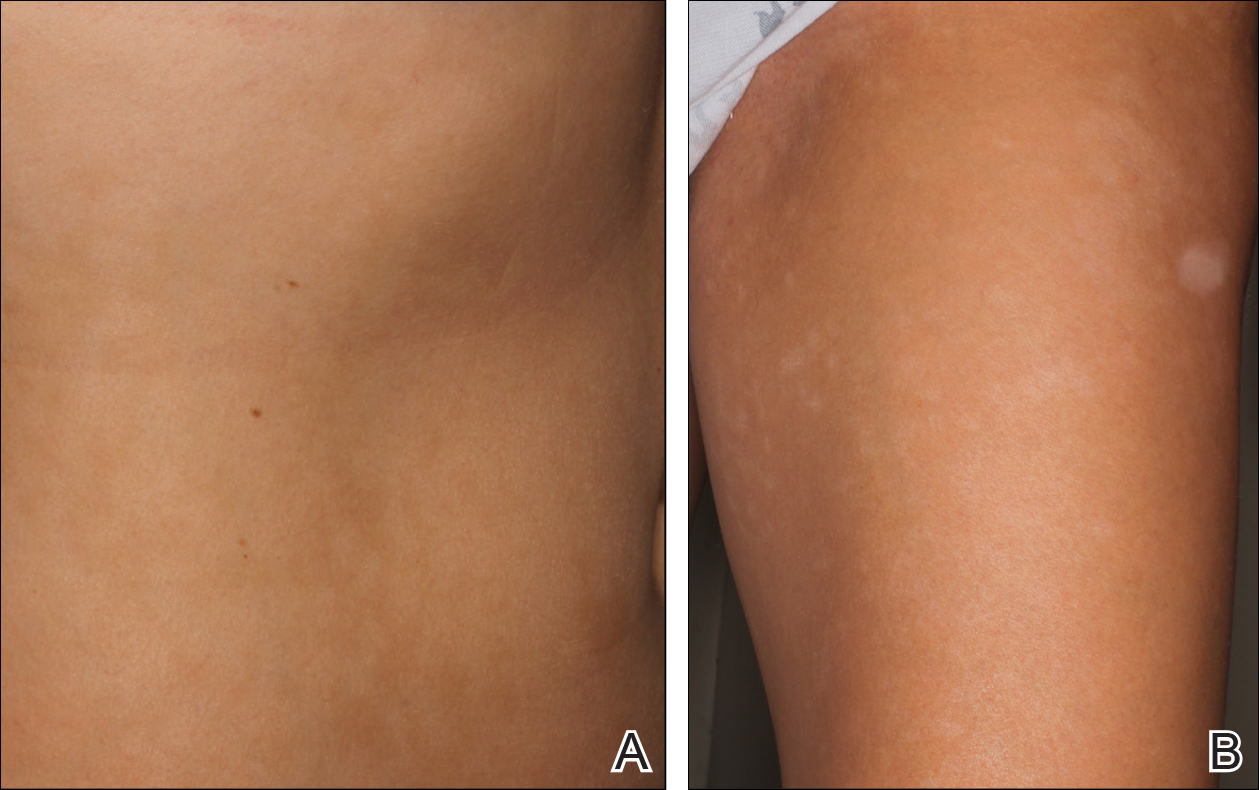
Physical examination revealed 0.5- to 2.0-cm, ill-defined, perifollicular and nonfollicular, slightly scaly macules and patches on the trunk, arms, and legs. There was no follicular plugging (Figure 1A). The hands, feet, face, and mucosal surfaces were spared. She had no family history of similar lesions. Although atrophic in appearance, a single lesion on the left thigh was palpably depressed (Figure 1B). Serology demonstrated a normal complete blood cell count and comprehensive metabolic panel, and negative Lyme titers. Light therapy and topical steroids failed to improve the lesions; calcipotriene cream 0.005% made the lesions erythematous and pruritic.
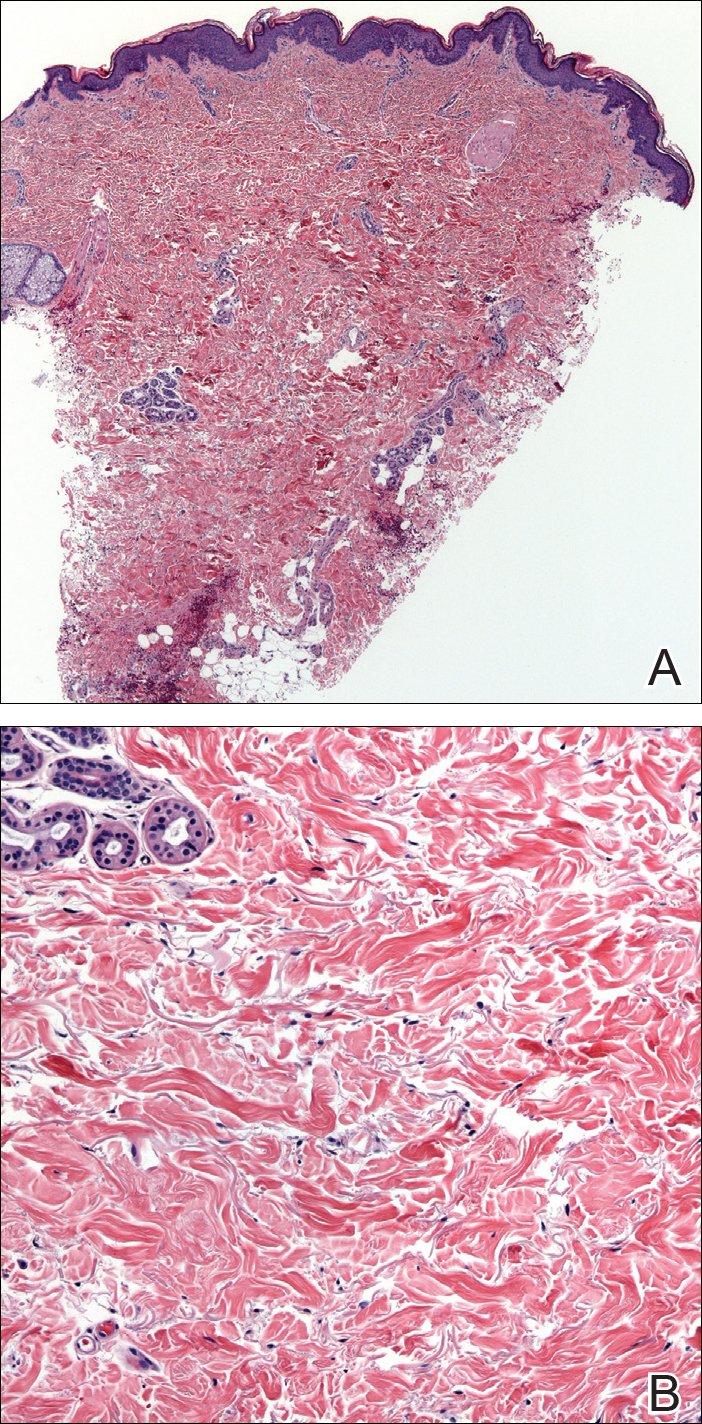
A biopsy from a flank lesion demonstrated a normal epithelium without thinning, a normal basal melanocyte population, and minimally effaced rete ridges. Thin collagen bundles were noted in the upper reticular and papillary dermis with associated fibroplasia (Figure 2). Verhoeff-van Gieson stain revealed decreased and fragmented elastin filaments in the same dermal distribution as the changed collagen (Figure 3). There was no evidence of primary inflammatory disease. The dermis was thinned. Periodic acid–Schiff stain confirmed the absence of hyphae and spores.
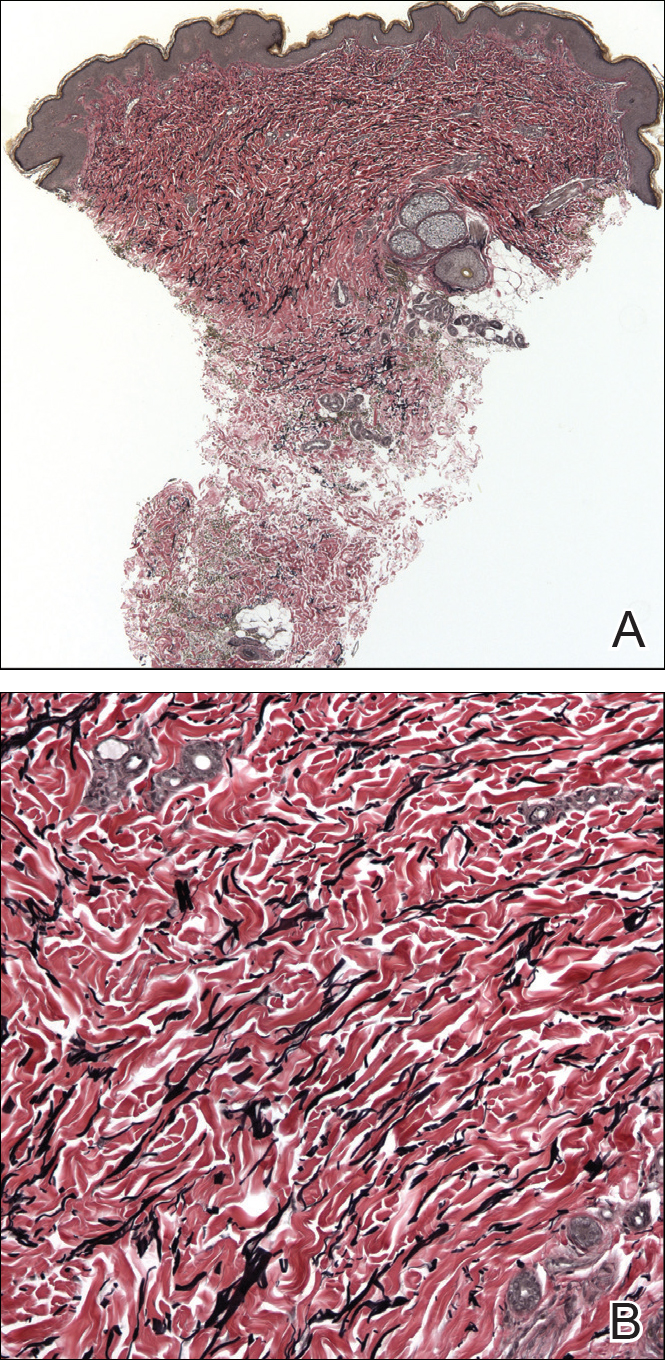
The relevant findings in our patient including the following: (1) onset of hypopigmented macules and patches following resolution of a toxic insult; (2) initially stable number of lesions that progressed in number but not size; (3) thinned collagen associated with fibroplasia in the upper reticular and papillary dermis; (4) decreased number and fragmentation of elastin filaments confined to the same region; (5) no congenital lesions or similar lesions in family members; and (6) a complete rotator cuff tear with no findings of a systemic connective-tissue disorder such as Ehlers-Danlos syndrome.
We performed a literature search of PubMed articles indexed for MEDLINE using combinations of the terms atrophic, hypopigmented, white, spot disease, confetti-like, guttate, macules, atrophoderma, morphea, anetoderma, elastin, and collagen to identify potentially similar reports of guttate hypopigmented macules demonstrating changes of the collagen and elastin in the papillary and upper reticular dermis. Some variants, namely atrophoderma of Pasini and Pierini (APP), guttate morphea, and superficial morphea, demonstrate similar clinical and histopathologic findings.
Findings similar to our case were documented in case reports of 2 women (aged 34 and 42 years)1 presenting with asymptomatic, atrophic, well-demarcated, shiny, hypopigmented macules over the trunk and upper extremities, which demonstrated a thinned epidermis with coarse hyalinized collagen bundles in the mid and lower dermis. There was upper and diffuse dermal elastolysis (patient 1 and patient 2, respectively).1 Our patient’s lesions were hypopigmented and atrophic in appearance but were slightly scaly and also involved the extremities. Distinct from these patient reports, histopathology from our case demonstrated thin packed collagen bundles and decreased fragmented elastin filaments confined to the upper reticular and papillary dermis.
Plaque morphea is the most common type of localized scleroderma.2 The subtype APP demonstrates round to ovoid, gray-brown depressions with cliff-drop borders. They may appear flesh colored or hypopigmented.3,4 These sclerodermoid lesions lack the violaceous border classic to morphea. Sclerosis and induration also are typically absent.5 Clinically, our patient’s macules resembled this entity. Histopathologically, APP shows normal epithelium with an increased basal layer pigmentation; preserved adnexal structures; and mid to lower dermal collagen edema, clumping, and homogenization.3,4 Elastic fibers classically are unchanged, with exceptions.6-11 Changes in the collagen and elastin of our patient were unlike those reported in APP, which occur in the mid to lower dermis.
Guttate morphea demonstrates small, pale, minimally indurated, coin-shaped lesions on the trunk. Histopathology reveals less sclerosis and more edema, resembling LS&A.12 The earliest descriptions of this entity describe 3 stages: ivory/chalk white, scaly, and atrophic. Follicular plugging (absent in this patient) and fine scale can exist at any stage.13,14 Flattened rete ridges mark an otherwise preserved epidermis; hyalinized collagen typically is superficial and demonstrates less sclerosis yet increased edema.12-14 Fewer elastic fibers typically are present compared to normal skin. Changes seen in this entity are more superficial, as with our patient, than classic scleroderma. However, classic edema was not found in our patient’s biopsy specimen.
Superficial morphea, occurring predominantly in females, presents with hyperpigmented or hypopigmented patches having minimal to no induration. The lesions typically are asymptomatic. Histopathologically, collagen deposition and inflammation are confined to the superficial dermis without homogenization associated with LS&A, findings that were consistent with this patient’s biopsy.15,16 However, similar to other morpheaform variants, elastic fibers are unchanged.15 Verhoeff-van Gieson stain of the biopsy (Figure 3) showed the decreased and fragmented elastin network in the upper reticular and papillary dermis, making this entity less compatible.
Guttate LS&A may present with interfollicular, bluish white macules or papules coalescing into patches or plaques. Lesions evolve to reveal atrophic thin skin with follicular plugging. Histology demonstrates a thinned epidermis with orthohypokeratosis marked by flattened rete ridges. The dermis reveals short hyalinized collagen fibrils with a loss of elastic fibers in the papillary and upper reticular dermis, giving a homogenized appearance. Early disease is marked by an inflammatory infiltrate.17 Most of these findings are consistent with our patient’s pathology, which was confined to the upper dermis. Lacking, however, were characteristic findings of LS&A, including upper dermal homogenization, near-total effacement of rete ridges, orthokeratosis, and vacuolar degeneration at the dermoepidermal junction. As such, this entity is less compatible.
Atrophoderma elastolyticum discretum has clinical features of atrophoderma with elastolytic histopathologic findings.1 Anetoderma presents with outpouchings of atrophic skin with a surrounding ring of normal tissue. Histopathologically, this entity shows normal collagen with elastolysis; there also is a decrease in desmosine, an elastin cross-linker.1,3 Neither the clinical nor histopathologic findings in this patient matched these 2 entities.
The reported chronologic association of these lesions with an arthropod assault raised suspicion to their association with toxic insult or postinflammatory changes. One study reported mechanical trauma, including insect bites, as a possible inciting factor of morphea.11 These data, gathered from patient surveys, reported trauma associated to lesion development.1,17 A review of the literature regarding atrophoderma, morphea, and LS&A failed to identify pathogenic changes seen in this patient following initial trauma. Moreover, although it is difficult to prove causality in the formation of the original hypopigmented spots, the development of identical spots in a similar distribution without further trauma suggests against these etiologies to fully explain her lesions. Nonetheless, circumstance makes it difficult to prove whether the original arthropod insult spurred a smoldering reactive process that caused the newer lesions.
Hereditary connective-tissue disorders also were considered in the differential diagnosis. Because of the patient’s history of an unprovoked complete rotator cuff tear, Ehlers-Danlos syndrome was considered; however, the remainder of her examination was normal, making a syndromic systemic disorder a less likely etiology.Because of the distinct clinical and histopathologic findings, this case may represent a rare and previously unreported variant of morphea. Clinically, these hypopigmented macules and patches exist somewhere along the morphea-atrophoderma spectrum. Histopathologic findings do not conform to prior reports. The name atrophodermalike guttate morphea may be an appropriate appellation. It is possible this presentation represents a variant of what dermatologists have referred to as white spot disease.18 We hope that this case may bring others to discussion, allowing for the identification of a more precise entity and etiology so that patients may receive more directed therapy.
- Aksoy B, Ustün H, Gulbahce R, et al. Confetti-like macular atrophy: a new entity? J Dermatol. 2009;36:592-597.
- Uitto J, Santa Cruz DJ, Bauer EA, et al. Morphea and lichen sclerosus et atrophicus. clinical and histopathologic studies in patients with combined features. J Am Acad Dermatol. 1980;3:271-279.
- Buechner SA, Rufli T. Atrophoderma of Pasini and Pierini. clinical and histopathologic findings and antibodies to Borrelia burgdorferi in thirty-four patients. J Am Acad Dermatol. 1994;30:441-446.
- Saleh Z, Abbas O, Dahdah MJ, et al. Atrophoderma of Pasini and Pierini: a clinical and histopathological study. J Cutan Pathol. 2008;35:1108-1114.
- Canizares O, Sachs PM, Jaimovich L, et al. Idiopathic atrophoderma of Pasini and Pierini. Arch Dermatol. 1958;77:42-58; discussion 58-60.
- Pullara TJ, Lober CW, Fenske NA. Idiopathic atrophoderma of Pasini and Pierini. Int J Dermatol. 1984;23:643-645.
- Jablonska S, Szczepanski A. Atrophoderma Pasini-Pierini: is it an entity? Dermatologica. 1962;125:226-242.
- Ang G, Hyde PM, Lee JB. Unilateral congenital linear atrophoderma of the leg. Pediatr Dermatol. 2005;22:350-354.
- Miteva L, Kadurina M. Unilateral idiopathic atrophoderma of Pasini and Pierini. Int J Dermatol. 2006;45:1391-1393.
- Kee CE, Brothers WS, New W. Idiopathic atrophoderma of Pasini and Pierini with coexistent morphea. a case report. Arch Dermatol. 1960;82:100-103.
- Zulian F, Athreya BH, Laxer R, et al. Juvenile localized scleroderma: clinical and epidemiological features in 750 children. an international study. Rheumatology. 2006;45:614-620.
- Winkelmann RK. Localized cutaneous scleroderma. Semin Dermatol. 1985;4:90-103.
- Dore SE. Two cases of morphoea guttata. Proc R Soc Med. 1918;11:26-28.
- Dore SE. Guttate morphoea. Proc R Soc Med. 1919;12:3-5.
- McNiff JM, Glusac EJ, Lazova RZ, et al. Morphea limited to the superficial reticular dermis: an underrecognized histologic phenomenon. Am J Dermatopathol. 1999;21:315-319.
- Jacobson L, Palazij R, Jaworsky C. Superficial morphea. J Am Acad Dermatol. 2003;49:323-325.
- Bolognia J, Jorizzo JL, Rapini RP, eds. Dermatology. 2nd ed. London, England: Mosby Elsevier; 2007.
- Bunch JL. White-spot disease (morphoea guttata). Proc R Soc Med. 1919;12:24-27.
To the Editor:
Morphea, atrophoderma, guttate lichen sclerosus et atrophicus (LS&A), anetoderma, and their subtypes are inflammatory processes ultimately leading to dermal remodeling. We report a case of a scaly, hypopigmented, macular rash that clinically appeared as an entity along the morphea-atrophoderma spectrum and demonstrated unique histopathologic changes in both collagen and elastin confined to the upper reticular and papillary dermis. This case is a potentially rare variant representing a combination of clinical and microscopic findings.
A 29-year-old woman presented for an increasing number of white spots distributed on the trunk, arms, and legs. She denied local and systemic symptoms. The patient reported that she was stung by 100 wasps 23 years prior. Following the assault, her grandmother placed chewed tobacco leaves atop the painful erythematous wheals and flares. Upon resolution, hypopigmented macules and patches remained in their place. The patient denied associated symptoms or new lesions; she did not seek care at that time.
In her early 20s, the patient noted new, similarly distributed hypopigmented macules and patches without associated arthropod assault. She was treated by an outside dermatologist without result for presumed tinea versicolor. A follow-up superficial shave biopsy cited subtle psoriasiform dermatitis. Topical steroids did not improve the lesions. Her medical history also was remarkable for a reportedly unprovoked complete rotator cuff tear.
Physical examination revealed 0.5- to 2.0-cm, ill-defined, perifollicular and nonfollicular, slightly scaly macules and patches on the trunk, arms, and legs. There was no follicular plugging (Figure 1A). The hands, feet, face, and mucosal surfaces were spared. She had no family history of similar lesions. Although atrophic in appearance, a single lesion on the left thigh was palpably depressed (Figure 1B). Serology demonstrated a normal complete blood cell count and comprehensive metabolic panel, and negative Lyme titers. Light therapy and topical steroids failed to improve the lesions; calcipotriene cream 0.005% made the lesions erythematous and pruritic.

A biopsy from a flank lesion demonstrated a normal epithelium without thinning, a normal basal melanocyte population, and minimally effaced rete ridges. Thin collagen bundles were noted in the upper reticular and papillary dermis with associated fibroplasia (Figure 2). Verhoeff-van Gieson stain revealed decreased and fragmented elastin filaments in the same dermal distribution as the changed collagen (Figure 3). There was no evidence of primary inflammatory disease. The dermis was thinned. Periodic acid–Schiff stain confirmed the absence of hyphae and spores.


The relevant findings in our patient including the following: (1) onset of hypopigmented macules and patches following resolution of a toxic insult; (2) initially stable number of lesions that progressed in number but not size; (3) thinned collagen associated with fibroplasia in the upper reticular and papillary dermis; (4) decreased number and fragmentation of elastin filaments confined to the same region; (5) no congenital lesions or similar lesions in family members; and (6) a complete rotator cuff tear with no findings of a systemic connective-tissue disorder such as Ehlers-Danlos syndrome.
We performed a literature search of PubMed articles indexed for MEDLINE using combinations of the terms atrophic, hypopigmented, white, spot disease, confetti-like, guttate, macules, atrophoderma, morphea, anetoderma, elastin, and collagen to identify potentially similar reports of guttate hypopigmented macules demonstrating changes of the collagen and elastin in the papillary and upper reticular dermis. Some variants, namely atrophoderma of Pasini and Pierini (APP), guttate morphea, and superficial morphea, demonstrate similar clinical and histopathologic findings.
Findings similar to our case were documented in case reports of 2 women (aged 34 and 42 years)1 presenting with asymptomatic, atrophic, well-demarcated, shiny, hypopigmented macules over the trunk and upper extremities, which demonstrated a thinned epidermis with coarse hyalinized collagen bundles in the mid and lower dermis. There was upper and diffuse dermal elastolysis (patient 1 and patient 2, respectively).1 Our patient’s lesions were hypopigmented and atrophic in appearance but were slightly scaly and also involved the extremities. Distinct from these patient reports, histopathology from our case demonstrated thin packed collagen bundles and decreased fragmented elastin filaments confined to the upper reticular and papillary dermis.
Plaque morphea is the most common type of localized scleroderma.2 The subtype APP demonstrates round to ovoid, gray-brown depressions with cliff-drop borders. They may appear flesh colored or hypopigmented.3,4 These sclerodermoid lesions lack the violaceous border classic to morphea. Sclerosis and induration also are typically absent.5 Clinically, our patient’s macules resembled this entity. Histopathologically, APP shows normal epithelium with an increased basal layer pigmentation; preserved adnexal structures; and mid to lower dermal collagen edema, clumping, and homogenization.3,4 Elastic fibers classically are unchanged, with exceptions.6-11 Changes in the collagen and elastin of our patient were unlike those reported in APP, which occur in the mid to lower dermis.
Guttate morphea demonstrates small, pale, minimally indurated, coin-shaped lesions on the trunk. Histopathology reveals less sclerosis and more edema, resembling LS&A.12 The earliest descriptions of this entity describe 3 stages: ivory/chalk white, scaly, and atrophic. Follicular plugging (absent in this patient) and fine scale can exist at any stage.13,14 Flattened rete ridges mark an otherwise preserved epidermis; hyalinized collagen typically is superficial and demonstrates less sclerosis yet increased edema.12-14 Fewer elastic fibers typically are present compared to normal skin. Changes seen in this entity are more superficial, as with our patient, than classic scleroderma. However, classic edema was not found in our patient’s biopsy specimen.
Superficial morphea, occurring predominantly in females, presents with hyperpigmented or hypopigmented patches having minimal to no induration. The lesions typically are asymptomatic. Histopathologically, collagen deposition and inflammation are confined to the superficial dermis without homogenization associated with LS&A, findings that were consistent with this patient’s biopsy.15,16 However, similar to other morpheaform variants, elastic fibers are unchanged.15 Verhoeff-van Gieson stain of the biopsy (Figure 3) showed the decreased and fragmented elastin network in the upper reticular and papillary dermis, making this entity less compatible.
Guttate LS&A may present with interfollicular, bluish white macules or papules coalescing into patches or plaques. Lesions evolve to reveal atrophic thin skin with follicular plugging. Histology demonstrates a thinned epidermis with orthohypokeratosis marked by flattened rete ridges. The dermis reveals short hyalinized collagen fibrils with a loss of elastic fibers in the papillary and upper reticular dermis, giving a homogenized appearance. Early disease is marked by an inflammatory infiltrate.17 Most of these findings are consistent with our patient’s pathology, which was confined to the upper dermis. Lacking, however, were characteristic findings of LS&A, including upper dermal homogenization, near-total effacement of rete ridges, orthokeratosis, and vacuolar degeneration at the dermoepidermal junction. As such, this entity is less compatible.
Atrophoderma elastolyticum discretum has clinical features of atrophoderma with elastolytic histopathologic findings.1 Anetoderma presents with outpouchings of atrophic skin with a surrounding ring of normal tissue. Histopathologically, this entity shows normal collagen with elastolysis; there also is a decrease in desmosine, an elastin cross-linker.1,3 Neither the clinical nor histopathologic findings in this patient matched these 2 entities.
The reported chronologic association of these lesions with an arthropod assault raised suspicion to their association with toxic insult or postinflammatory changes. One study reported mechanical trauma, including insect bites, as a possible inciting factor of morphea.11 These data, gathered from patient surveys, reported trauma associated to lesion development.1,17 A review of the literature regarding atrophoderma, morphea, and LS&A failed to identify pathogenic changes seen in this patient following initial trauma. Moreover, although it is difficult to prove causality in the formation of the original hypopigmented spots, the development of identical spots in a similar distribution without further trauma suggests against these etiologies to fully explain her lesions. Nonetheless, circumstance makes it difficult to prove whether the original arthropod insult spurred a smoldering reactive process that caused the newer lesions.
Hereditary connective-tissue disorders also were considered in the differential diagnosis. Because of the patient’s history of an unprovoked complete rotator cuff tear, Ehlers-Danlos syndrome was considered; however, the remainder of her examination was normal, making a syndromic systemic disorder a less likely etiology.Because of the distinct clinical and histopathologic findings, this case may represent a rare and previously unreported variant of morphea. Clinically, these hypopigmented macules and patches exist somewhere along the morphea-atrophoderma spectrum. Histopathologic findings do not conform to prior reports. The name atrophodermalike guttate morphea may be an appropriate appellation. It is possible this presentation represents a variant of what dermatologists have referred to as white spot disease.18 We hope that this case may bring others to discussion, allowing for the identification of a more precise entity and etiology so that patients may receive more directed therapy.
To the Editor:
Morphea, atrophoderma, guttate lichen sclerosus et atrophicus (LS&A), anetoderma, and their subtypes are inflammatory processes ultimately leading to dermal remodeling. We report a case of a scaly, hypopigmented, macular rash that clinically appeared as an entity along the morphea-atrophoderma spectrum and demonstrated unique histopathologic changes in both collagen and elastin confined to the upper reticular and papillary dermis. This case is a potentially rare variant representing a combination of clinical and microscopic findings.
A 29-year-old woman presented for an increasing number of white spots distributed on the trunk, arms, and legs. She denied local and systemic symptoms. The patient reported that she was stung by 100 wasps 23 years prior. Following the assault, her grandmother placed chewed tobacco leaves atop the painful erythematous wheals and flares. Upon resolution, hypopigmented macules and patches remained in their place. The patient denied associated symptoms or new lesions; she did not seek care at that time.
In her early 20s, the patient noted new, similarly distributed hypopigmented macules and patches without associated arthropod assault. She was treated by an outside dermatologist without result for presumed tinea versicolor. A follow-up superficial shave biopsy cited subtle psoriasiform dermatitis. Topical steroids did not improve the lesions. Her medical history also was remarkable for a reportedly unprovoked complete rotator cuff tear.
Physical examination revealed 0.5- to 2.0-cm, ill-defined, perifollicular and nonfollicular, slightly scaly macules and patches on the trunk, arms, and legs. There was no follicular plugging (Figure 1A). The hands, feet, face, and mucosal surfaces were spared. She had no family history of similar lesions. Although atrophic in appearance, a single lesion on the left thigh was palpably depressed (Figure 1B). Serology demonstrated a normal complete blood cell count and comprehensive metabolic panel, and negative Lyme titers. Light therapy and topical steroids failed to improve the lesions; calcipotriene cream 0.005% made the lesions erythematous and pruritic.

A biopsy from a flank lesion demonstrated a normal epithelium without thinning, a normal basal melanocyte population, and minimally effaced rete ridges. Thin collagen bundles were noted in the upper reticular and papillary dermis with associated fibroplasia (Figure 2). Verhoeff-van Gieson stain revealed decreased and fragmented elastin filaments in the same dermal distribution as the changed collagen (Figure 3). There was no evidence of primary inflammatory disease. The dermis was thinned. Periodic acid–Schiff stain confirmed the absence of hyphae and spores.


The relevant findings in our patient including the following: (1) onset of hypopigmented macules and patches following resolution of a toxic insult; (2) initially stable number of lesions that progressed in number but not size; (3) thinned collagen associated with fibroplasia in the upper reticular and papillary dermis; (4) decreased number and fragmentation of elastin filaments confined to the same region; (5) no congenital lesions or similar lesions in family members; and (6) a complete rotator cuff tear with no findings of a systemic connective-tissue disorder such as Ehlers-Danlos syndrome.
We performed a literature search of PubMed articles indexed for MEDLINE using combinations of the terms atrophic, hypopigmented, white, spot disease, confetti-like, guttate, macules, atrophoderma, morphea, anetoderma, elastin, and collagen to identify potentially similar reports of guttate hypopigmented macules demonstrating changes of the collagen and elastin in the papillary and upper reticular dermis. Some variants, namely atrophoderma of Pasini and Pierini (APP), guttate morphea, and superficial morphea, demonstrate similar clinical and histopathologic findings.
Findings similar to our case were documented in case reports of 2 women (aged 34 and 42 years)1 presenting with asymptomatic, atrophic, well-demarcated, shiny, hypopigmented macules over the trunk and upper extremities, which demonstrated a thinned epidermis with coarse hyalinized collagen bundles in the mid and lower dermis. There was upper and diffuse dermal elastolysis (patient 1 and patient 2, respectively).1 Our patient’s lesions were hypopigmented and atrophic in appearance but were slightly scaly and also involved the extremities. Distinct from these patient reports, histopathology from our case demonstrated thin packed collagen bundles and decreased fragmented elastin filaments confined to the upper reticular and papillary dermis.
Plaque morphea is the most common type of localized scleroderma.2 The subtype APP demonstrates round to ovoid, gray-brown depressions with cliff-drop borders. They may appear flesh colored or hypopigmented.3,4 These sclerodermoid lesions lack the violaceous border classic to morphea. Sclerosis and induration also are typically absent.5 Clinically, our patient’s macules resembled this entity. Histopathologically, APP shows normal epithelium with an increased basal layer pigmentation; preserved adnexal structures; and mid to lower dermal collagen edema, clumping, and homogenization.3,4 Elastic fibers classically are unchanged, with exceptions.6-11 Changes in the collagen and elastin of our patient were unlike those reported in APP, which occur in the mid to lower dermis.
Guttate morphea demonstrates small, pale, minimally indurated, coin-shaped lesions on the trunk. Histopathology reveals less sclerosis and more edema, resembling LS&A.12 The earliest descriptions of this entity describe 3 stages: ivory/chalk white, scaly, and atrophic. Follicular plugging (absent in this patient) and fine scale can exist at any stage.13,14 Flattened rete ridges mark an otherwise preserved epidermis; hyalinized collagen typically is superficial and demonstrates less sclerosis yet increased edema.12-14 Fewer elastic fibers typically are present compared to normal skin. Changes seen in this entity are more superficial, as with our patient, than classic scleroderma. However, classic edema was not found in our patient’s biopsy specimen.
Superficial morphea, occurring predominantly in females, presents with hyperpigmented or hypopigmented patches having minimal to no induration. The lesions typically are asymptomatic. Histopathologically, collagen deposition and inflammation are confined to the superficial dermis without homogenization associated with LS&A, findings that were consistent with this patient’s biopsy.15,16 However, similar to other morpheaform variants, elastic fibers are unchanged.15 Verhoeff-van Gieson stain of the biopsy (Figure 3) showed the decreased and fragmented elastin network in the upper reticular and papillary dermis, making this entity less compatible.
Guttate LS&A may present with interfollicular, bluish white macules or papules coalescing into patches or plaques. Lesions evolve to reveal atrophic thin skin with follicular plugging. Histology demonstrates a thinned epidermis with orthohypokeratosis marked by flattened rete ridges. The dermis reveals short hyalinized collagen fibrils with a loss of elastic fibers in the papillary and upper reticular dermis, giving a homogenized appearance. Early disease is marked by an inflammatory infiltrate.17 Most of these findings are consistent with our patient’s pathology, which was confined to the upper dermis. Lacking, however, were characteristic findings of LS&A, including upper dermal homogenization, near-total effacement of rete ridges, orthokeratosis, and vacuolar degeneration at the dermoepidermal junction. As such, this entity is less compatible.
Atrophoderma elastolyticum discretum has clinical features of atrophoderma with elastolytic histopathologic findings.1 Anetoderma presents with outpouchings of atrophic skin with a surrounding ring of normal tissue. Histopathologically, this entity shows normal collagen with elastolysis; there also is a decrease in desmosine, an elastin cross-linker.1,3 Neither the clinical nor histopathologic findings in this patient matched these 2 entities.
The reported chronologic association of these lesions with an arthropod assault raised suspicion to their association with toxic insult or postinflammatory changes. One study reported mechanical trauma, including insect bites, as a possible inciting factor of morphea.11 These data, gathered from patient surveys, reported trauma associated to lesion development.1,17 A review of the literature regarding atrophoderma, morphea, and LS&A failed to identify pathogenic changes seen in this patient following initial trauma. Moreover, although it is difficult to prove causality in the formation of the original hypopigmented spots, the development of identical spots in a similar distribution without further trauma suggests against these etiologies to fully explain her lesions. Nonetheless, circumstance makes it difficult to prove whether the original arthropod insult spurred a smoldering reactive process that caused the newer lesions.
Hereditary connective-tissue disorders also were considered in the differential diagnosis. Because of the patient’s history of an unprovoked complete rotator cuff tear, Ehlers-Danlos syndrome was considered; however, the remainder of her examination was normal, making a syndromic systemic disorder a less likely etiology.Because of the distinct clinical and histopathologic findings, this case may represent a rare and previously unreported variant of morphea. Clinically, these hypopigmented macules and patches exist somewhere along the morphea-atrophoderma spectrum. Histopathologic findings do not conform to prior reports. The name atrophodermalike guttate morphea may be an appropriate appellation. It is possible this presentation represents a variant of what dermatologists have referred to as white spot disease.18 We hope that this case may bring others to discussion, allowing for the identification of a more precise entity and etiology so that patients may receive more directed therapy.
- Aksoy B, Ustün H, Gulbahce R, et al. Confetti-like macular atrophy: a new entity? J Dermatol. 2009;36:592-597.
- Uitto J, Santa Cruz DJ, Bauer EA, et al. Morphea and lichen sclerosus et atrophicus. clinical and histopathologic studies in patients with combined features. J Am Acad Dermatol. 1980;3:271-279.
- Buechner SA, Rufli T. Atrophoderma of Pasini and Pierini. clinical and histopathologic findings and antibodies to Borrelia burgdorferi in thirty-four patients. J Am Acad Dermatol. 1994;30:441-446.
- Saleh Z, Abbas O, Dahdah MJ, et al. Atrophoderma of Pasini and Pierini: a clinical and histopathological study. J Cutan Pathol. 2008;35:1108-1114.
- Canizares O, Sachs PM, Jaimovich L, et al. Idiopathic atrophoderma of Pasini and Pierini. Arch Dermatol. 1958;77:42-58; discussion 58-60.
- Pullara TJ, Lober CW, Fenske NA. Idiopathic atrophoderma of Pasini and Pierini. Int J Dermatol. 1984;23:643-645.
- Jablonska S, Szczepanski A. Atrophoderma Pasini-Pierini: is it an entity? Dermatologica. 1962;125:226-242.
- Ang G, Hyde PM, Lee JB. Unilateral congenital linear atrophoderma of the leg. Pediatr Dermatol. 2005;22:350-354.
- Miteva L, Kadurina M. Unilateral idiopathic atrophoderma of Pasini and Pierini. Int J Dermatol. 2006;45:1391-1393.
- Kee CE, Brothers WS, New W. Idiopathic atrophoderma of Pasini and Pierini with coexistent morphea. a case report. Arch Dermatol. 1960;82:100-103.
- Zulian F, Athreya BH, Laxer R, et al. Juvenile localized scleroderma: clinical and epidemiological features in 750 children. an international study. Rheumatology. 2006;45:614-620.
- Winkelmann RK. Localized cutaneous scleroderma. Semin Dermatol. 1985;4:90-103.
- Dore SE. Two cases of morphoea guttata. Proc R Soc Med. 1918;11:26-28.
- Dore SE. Guttate morphoea. Proc R Soc Med. 1919;12:3-5.
- McNiff JM, Glusac EJ, Lazova RZ, et al. Morphea limited to the superficial reticular dermis: an underrecognized histologic phenomenon. Am J Dermatopathol. 1999;21:315-319.
- Jacobson L, Palazij R, Jaworsky C. Superficial morphea. J Am Acad Dermatol. 2003;49:323-325.
- Bolognia J, Jorizzo JL, Rapini RP, eds. Dermatology. 2nd ed. London, England: Mosby Elsevier; 2007.
- Bunch JL. White-spot disease (morphoea guttata). Proc R Soc Med. 1919;12:24-27.
- Aksoy B, Ustün H, Gulbahce R, et al. Confetti-like macular atrophy: a new entity? J Dermatol. 2009;36:592-597.
- Uitto J, Santa Cruz DJ, Bauer EA, et al. Morphea and lichen sclerosus et atrophicus. clinical and histopathologic studies in patients with combined features. J Am Acad Dermatol. 1980;3:271-279.
- Buechner SA, Rufli T. Atrophoderma of Pasini and Pierini. clinical and histopathologic findings and antibodies to Borrelia burgdorferi in thirty-four patients. J Am Acad Dermatol. 1994;30:441-446.
- Saleh Z, Abbas O, Dahdah MJ, et al. Atrophoderma of Pasini and Pierini: a clinical and histopathological study. J Cutan Pathol. 2008;35:1108-1114.
- Canizares O, Sachs PM, Jaimovich L, et al. Idiopathic atrophoderma of Pasini and Pierini. Arch Dermatol. 1958;77:42-58; discussion 58-60.
- Pullara TJ, Lober CW, Fenske NA. Idiopathic atrophoderma of Pasini and Pierini. Int J Dermatol. 1984;23:643-645.
- Jablonska S, Szczepanski A. Atrophoderma Pasini-Pierini: is it an entity? Dermatologica. 1962;125:226-242.
- Ang G, Hyde PM, Lee JB. Unilateral congenital linear atrophoderma of the leg. Pediatr Dermatol. 2005;22:350-354.
- Miteva L, Kadurina M. Unilateral idiopathic atrophoderma of Pasini and Pierini. Int J Dermatol. 2006;45:1391-1393.
- Kee CE, Brothers WS, New W. Idiopathic atrophoderma of Pasini and Pierini with coexistent morphea. a case report. Arch Dermatol. 1960;82:100-103.
- Zulian F, Athreya BH, Laxer R, et al. Juvenile localized scleroderma: clinical and epidemiological features in 750 children. an international study. Rheumatology. 2006;45:614-620.
- Winkelmann RK. Localized cutaneous scleroderma. Semin Dermatol. 1985;4:90-103.
- Dore SE. Two cases of morphoea guttata. Proc R Soc Med. 1918;11:26-28.
- Dore SE. Guttate morphoea. Proc R Soc Med. 1919;12:3-5.
- McNiff JM, Glusac EJ, Lazova RZ, et al. Morphea limited to the superficial reticular dermis: an underrecognized histologic phenomenon. Am J Dermatopathol. 1999;21:315-319.
- Jacobson L, Palazij R, Jaworsky C. Superficial morphea. J Am Acad Dermatol. 2003;49:323-325.
- Bolognia J, Jorizzo JL, Rapini RP, eds. Dermatology. 2nd ed. London, England: Mosby Elsevier; 2007.
- Bunch JL. White-spot disease (morphoea guttata). Proc R Soc Med. 1919;12:24-27.
Practice Points
- Atrophodermalike guttate morphea is a potentially underreported or undescribed entity consisting of a combination of clinicopathologic features.
- Widespread hypopigmented macules on the trunk and extremities marked by thinned collagen, fibroplasia, and altered fragmented elastin in the papillary dermis and upper reticular dermis are the key features.
- Atrophoderma, morphea, and lichen sclerosus et atrophicus should be ruled out during clinical workup.
Compounded topicals flagged on fears of Medicare fraud, risk
But a new report finds that officials are concerned about possible fraud and patient safety risks from products made at nearly a quarter of the pharmacies that fill the bulk of those prescriptions.
“Although some of this billing may be legitimate, all of these pharmacies warrant further scrutiny,” concludes the report from the Office of Inspector General for the Department of Health and Human Services.
In total, 547 pharmacies – nearly 23% of those that submit most of the bills to Medicare for making these creams – hit one or more of five red-flag markers set by investigators. Those included what the researchers called “extremely high” prices; large percentages of Medicare members getting identical drugs – 16 of the pharmacies billed for identical drugs for 200 or more customers; “greatly increased” year-over-year billing – 20 pharmacies increased their billing by more than 10,000%; or having a single medical provider writing more than 131 prescriptions. More than half of those pharmacies hit two or more measures – and 10 hit all five.
One Oregon pharmacy, for example, submitted claims for 91% of its customers. A pharmacy in New York submitted 5,342 prescriptions ordered by one podiatrist, while a Florida pharmacy saw its Medicare billing for such treatments go from $7,468 in 2015 to $1.8 million the following year.
Many of the pharmacies are clustered in four cities: Detroit, Houston, Los Angeles, and New York.
The report comes amid ongoing concern by Medicare officials about compounded drugs. In addition to questions like those raised in the report about overuse and pricing, safety has been a key issue in recent years. A meningitis outbreak in 2012 was linked to a Massachusetts pharmacy that did not maintain sterile conditions and sold tainted made-to-order injections that killed 64 Americans.
When done safely, pharmacy-made compounded drugs provide a legitimate option for patients whose medical needs can’t be met by commercially available products mass-produced by pharmaceutical companies. For example, a patient who can’t swallow a commercially available prescription pill might get a liquid version of a drug.
State boards of pharmacy generally oversee compounding pharmacies, and the drugs they produce are not considered approved by the Food and Drug Administration.
The new report focuses on concerns with compounded topical medications.
Medicare spending for such treatments has skyrocketed, rising more than 2,350%, from $13.2 million in 2010 to $323.5 million in 2016. Price hikes and an increase in the number of prescriptions written drove the increase, the report said.
It is not the first time the inspector general has looked at compounded drugs. A 2016 report found that overall spending on all types of compounded drugs – not just topical medications – rose sharply. The U.S. Postal Service inspector general and the Department of Defense also have raised concerns about rising spending and possible fraud for compounded drugs.
In response to those previous reports, the International Academy of Compounding Pharmacists, the industry’s trade group, has said that legitimately compounded drugs “can dramatically improve a patient’s quality of life,” noting that proper billing controls need to be in place. The inspector general’s report in 2016, it added, found that “such controls are not in place.”
This report, which the compounding trade group has not yet reviewed, focuses on topical drugs and a subset of the 15,290 pharmacies that provide at least one such prescription each year. It looked at billing records from the 2,388 pharmacies that do at least 10 such prescriptions a year – providing 93% of all compounded topical drugs paid for by Medicare.
Most of the prescriptions were for pain treatment, made from ingredients such as lidocaine or diclofenac sodium.
On average, those compounds were more expensive than noncompounded drugs with the same ingredients.
For example, Medicare paid an average of $751 per tube of compounded lidocaine, and $1,506 for the diclofenac, according to the inspector general’s report. Noncompounded tubes of those drugs averaged $445 and $128, respectively.
FDA Commissioner Scott Gottlieb, MD, recently outlined new efforts his agency is taking to oversee compounded drugs in the wake of legislation passed by Congress following the meningitis outbreak.
“The FDA is inspecting compounding facilities to assess whether drugs that are essentially copies of FDA-approved drugs are being compounded for patients” who could otherwise take a product sold commercially, he said in a statement issued on June 28.
Dr. Gottlieb also said the FDA plans to make more information available to patients and their doctors about compounded topical pain creams, including information about their effectiveness and any potential safety risks.
Not being effective is a safety risk, noted Miriam Anderson, a researcher with the inspector general’s office who helped write the report.
The report urged the Centers for Medicare & Medicaid Services to clarify some of its policies to emphasize that insurers can limit the use of compounded drugs by requiring prior authorization or other steps. The agency concurred with the recommendations, according to the report, including the need to “follow up on pharmacies with questionable Part D billing and the prescribers associated with these pharmacies.”
Ms. Anderson said the inspector general’s office is continuing to probe the issue.
“We will investigate a number of leads on specific pharmacies and prescribers who were identified as having these questionable patterns,” she said. “Whenever we see that kind of increase in spending, it raises concern about fraud, waste, and abuse.”
KHN’s coverage of prescription drug development, costs, and pricing is supported in part by the Laura and John Arnold Foundation.
Kaiser Health News (KHN) is a national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation which is not affiliated with Kaiser Permanente.
But a new report finds that officials are concerned about possible fraud and patient safety risks from products made at nearly a quarter of the pharmacies that fill the bulk of those prescriptions.
“Although some of this billing may be legitimate, all of these pharmacies warrant further scrutiny,” concludes the report from the Office of Inspector General for the Department of Health and Human Services.
In total, 547 pharmacies – nearly 23% of those that submit most of the bills to Medicare for making these creams – hit one or more of five red-flag markers set by investigators. Those included what the researchers called “extremely high” prices; large percentages of Medicare members getting identical drugs – 16 of the pharmacies billed for identical drugs for 200 or more customers; “greatly increased” year-over-year billing – 20 pharmacies increased their billing by more than 10,000%; or having a single medical provider writing more than 131 prescriptions. More than half of those pharmacies hit two or more measures – and 10 hit all five.
One Oregon pharmacy, for example, submitted claims for 91% of its customers. A pharmacy in New York submitted 5,342 prescriptions ordered by one podiatrist, while a Florida pharmacy saw its Medicare billing for such treatments go from $7,468 in 2015 to $1.8 million the following year.
Many of the pharmacies are clustered in four cities: Detroit, Houston, Los Angeles, and New York.
The report comes amid ongoing concern by Medicare officials about compounded drugs. In addition to questions like those raised in the report about overuse and pricing, safety has been a key issue in recent years. A meningitis outbreak in 2012 was linked to a Massachusetts pharmacy that did not maintain sterile conditions and sold tainted made-to-order injections that killed 64 Americans.
When done safely, pharmacy-made compounded drugs provide a legitimate option for patients whose medical needs can’t be met by commercially available products mass-produced by pharmaceutical companies. For example, a patient who can’t swallow a commercially available prescription pill might get a liquid version of a drug.
State boards of pharmacy generally oversee compounding pharmacies, and the drugs they produce are not considered approved by the Food and Drug Administration.
The new report focuses on concerns with compounded topical medications.
Medicare spending for such treatments has skyrocketed, rising more than 2,350%, from $13.2 million in 2010 to $323.5 million in 2016. Price hikes and an increase in the number of prescriptions written drove the increase, the report said.
It is not the first time the inspector general has looked at compounded drugs. A 2016 report found that overall spending on all types of compounded drugs – not just topical medications – rose sharply. The U.S. Postal Service inspector general and the Department of Defense also have raised concerns about rising spending and possible fraud for compounded drugs.
In response to those previous reports, the International Academy of Compounding Pharmacists, the industry’s trade group, has said that legitimately compounded drugs “can dramatically improve a patient’s quality of life,” noting that proper billing controls need to be in place. The inspector general’s report in 2016, it added, found that “such controls are not in place.”
This report, which the compounding trade group has not yet reviewed, focuses on topical drugs and a subset of the 15,290 pharmacies that provide at least one such prescription each year. It looked at billing records from the 2,388 pharmacies that do at least 10 such prescriptions a year – providing 93% of all compounded topical drugs paid for by Medicare.
Most of the prescriptions were for pain treatment, made from ingredients such as lidocaine or diclofenac sodium.
On average, those compounds were more expensive than noncompounded drugs with the same ingredients.
For example, Medicare paid an average of $751 per tube of compounded lidocaine, and $1,506 for the diclofenac, according to the inspector general’s report. Noncompounded tubes of those drugs averaged $445 and $128, respectively.
FDA Commissioner Scott Gottlieb, MD, recently outlined new efforts his agency is taking to oversee compounded drugs in the wake of legislation passed by Congress following the meningitis outbreak.
“The FDA is inspecting compounding facilities to assess whether drugs that are essentially copies of FDA-approved drugs are being compounded for patients” who could otherwise take a product sold commercially, he said in a statement issued on June 28.
Dr. Gottlieb also said the FDA plans to make more information available to patients and their doctors about compounded topical pain creams, including information about their effectiveness and any potential safety risks.
Not being effective is a safety risk, noted Miriam Anderson, a researcher with the inspector general’s office who helped write the report.
The report urged the Centers for Medicare & Medicaid Services to clarify some of its policies to emphasize that insurers can limit the use of compounded drugs by requiring prior authorization or other steps. The agency concurred with the recommendations, according to the report, including the need to “follow up on pharmacies with questionable Part D billing and the prescribers associated with these pharmacies.”
Ms. Anderson said the inspector general’s office is continuing to probe the issue.
“We will investigate a number of leads on specific pharmacies and prescribers who were identified as having these questionable patterns,” she said. “Whenever we see that kind of increase in spending, it raises concern about fraud, waste, and abuse.”
KHN’s coverage of prescription drug development, costs, and pricing is supported in part by the Laura and John Arnold Foundation.
Kaiser Health News (KHN) is a national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation which is not affiliated with Kaiser Permanente.
But a new report finds that officials are concerned about possible fraud and patient safety risks from products made at nearly a quarter of the pharmacies that fill the bulk of those prescriptions.
“Although some of this billing may be legitimate, all of these pharmacies warrant further scrutiny,” concludes the report from the Office of Inspector General for the Department of Health and Human Services.
In total, 547 pharmacies – nearly 23% of those that submit most of the bills to Medicare for making these creams – hit one or more of five red-flag markers set by investigators. Those included what the researchers called “extremely high” prices; large percentages of Medicare members getting identical drugs – 16 of the pharmacies billed for identical drugs for 200 or more customers; “greatly increased” year-over-year billing – 20 pharmacies increased their billing by more than 10,000%; or having a single medical provider writing more than 131 prescriptions. More than half of those pharmacies hit two or more measures – and 10 hit all five.
One Oregon pharmacy, for example, submitted claims for 91% of its customers. A pharmacy in New York submitted 5,342 prescriptions ordered by one podiatrist, while a Florida pharmacy saw its Medicare billing for such treatments go from $7,468 in 2015 to $1.8 million the following year.
Many of the pharmacies are clustered in four cities: Detroit, Houston, Los Angeles, and New York.
The report comes amid ongoing concern by Medicare officials about compounded drugs. In addition to questions like those raised in the report about overuse and pricing, safety has been a key issue in recent years. A meningitis outbreak in 2012 was linked to a Massachusetts pharmacy that did not maintain sterile conditions and sold tainted made-to-order injections that killed 64 Americans.
When done safely, pharmacy-made compounded drugs provide a legitimate option for patients whose medical needs can’t be met by commercially available products mass-produced by pharmaceutical companies. For example, a patient who can’t swallow a commercially available prescription pill might get a liquid version of a drug.
State boards of pharmacy generally oversee compounding pharmacies, and the drugs they produce are not considered approved by the Food and Drug Administration.
The new report focuses on concerns with compounded topical medications.
Medicare spending for such treatments has skyrocketed, rising more than 2,350%, from $13.2 million in 2010 to $323.5 million in 2016. Price hikes and an increase in the number of prescriptions written drove the increase, the report said.
It is not the first time the inspector general has looked at compounded drugs. A 2016 report found that overall spending on all types of compounded drugs – not just topical medications – rose sharply. The U.S. Postal Service inspector general and the Department of Defense also have raised concerns about rising spending and possible fraud for compounded drugs.
In response to those previous reports, the International Academy of Compounding Pharmacists, the industry’s trade group, has said that legitimately compounded drugs “can dramatically improve a patient’s quality of life,” noting that proper billing controls need to be in place. The inspector general’s report in 2016, it added, found that “such controls are not in place.”
This report, which the compounding trade group has not yet reviewed, focuses on topical drugs and a subset of the 15,290 pharmacies that provide at least one such prescription each year. It looked at billing records from the 2,388 pharmacies that do at least 10 such prescriptions a year – providing 93% of all compounded topical drugs paid for by Medicare.
Most of the prescriptions were for pain treatment, made from ingredients such as lidocaine or diclofenac sodium.
On average, those compounds were more expensive than noncompounded drugs with the same ingredients.
For example, Medicare paid an average of $751 per tube of compounded lidocaine, and $1,506 for the diclofenac, according to the inspector general’s report. Noncompounded tubes of those drugs averaged $445 and $128, respectively.
FDA Commissioner Scott Gottlieb, MD, recently outlined new efforts his agency is taking to oversee compounded drugs in the wake of legislation passed by Congress following the meningitis outbreak.
“The FDA is inspecting compounding facilities to assess whether drugs that are essentially copies of FDA-approved drugs are being compounded for patients” who could otherwise take a product sold commercially, he said in a statement issued on June 28.
Dr. Gottlieb also said the FDA plans to make more information available to patients and their doctors about compounded topical pain creams, including information about their effectiveness and any potential safety risks.
Not being effective is a safety risk, noted Miriam Anderson, a researcher with the inspector general’s office who helped write the report.
The report urged the Centers for Medicare & Medicaid Services to clarify some of its policies to emphasize that insurers can limit the use of compounded drugs by requiring prior authorization or other steps. The agency concurred with the recommendations, according to the report, including the need to “follow up on pharmacies with questionable Part D billing and the prescribers associated with these pharmacies.”
Ms. Anderson said the inspector general’s office is continuing to probe the issue.
“We will investigate a number of leads on specific pharmacies and prescribers who were identified as having these questionable patterns,” she said. “Whenever we see that kind of increase in spending, it raises concern about fraud, waste, and abuse.”
KHN’s coverage of prescription drug development, costs, and pricing is supported in part by the Laura and John Arnold Foundation.
Kaiser Health News (KHN) is a national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation which is not affiliated with Kaiser Permanente.
EGFR-mutant NSCLC may still respond to PD-1 blockade
PD-1 blockade should still be considered as a second-line approach in patients with EGFR-mutant non–small-cell lung cancer (NSCLC), as some may still respond to this therapy, investigators reported.
The researchers described a patient who was diagnosed with epidermal growth factor receptor (EGFR)-mutant NSCLC but who also showed high expression of PD-L1.
The 62-year-old woman first presented with a lung nodule and metastasis in the right hip, which were EGFR-mutant and had a PD-L1 tumor proportion score of 90%. She was treated with erlotinib – an EGFR tyrosine kinase inhibitor – and radiotherapy, according to the report, published in Annals of Oncology.
Two months later, she developed multiple new metastases in the chest wall. These also showed high PD-L1 expression, but no EGFR mutations, so she was treated with pembrolizumab as second-line therapy.
After the first course of treatment with pembrolizumab, the hip pain improved dramatically, and after three cycles the primary and metastatic lesions disappeared completely.
“Immunofluorescent analysis of both right ilium and chest wall lesions with an anti-EGFR antibody specific to Ex.19 del and an anti-PD-L1 antibody revealed the presence of distinct heterogeneity of EGFR-mutant clones and PD-L1 highly-expressing clones,” wrote Kei Kunimasa, MD, of Osaka International Cancer Institute, and coauthors.
Current clinical trials suggested that patients with EGFR mutations are likely to have a poor response to PD-1 blockade therapies for NSCLC, and the authors agreed that patients with EGFR mutations should be treated with EGFR tyrosine kinase inhibitors as first-line therapy.
“However, the present case suggested that shorter PFS in EGFR-TKI treatment for EGFR-mutant NSCLC patients with high PD-L1 TPS and without acquired T790M mutation could encourage us to try PD-1 blockade therapy as second line therapy,” they wrote.
The investigators suggested that certain clinical factors could identify patients with EGFR-mutant NSCLC who might respond well to PD-1 blockade, such as progression-free survival on EGFR tyrosine kinase inhibitor treatment, other targetable resistant mutations, tumor mutation burden, and PD-L1 expression.
Two authors reported grants and personal fees from pharmaceutical companies outside the submitted work. No other conflicts of interest were declared.
SOURCE: Kunimasa K et al. Ann Oncol, 2018 Aug 7. doi: 10.1093/annonc/mdy312.
PD-1 blockade should still be considered as a second-line approach in patients with EGFR-mutant non–small-cell lung cancer (NSCLC), as some may still respond to this therapy, investigators reported.
The researchers described a patient who was diagnosed with epidermal growth factor receptor (EGFR)-mutant NSCLC but who also showed high expression of PD-L1.
The 62-year-old woman first presented with a lung nodule and metastasis in the right hip, which were EGFR-mutant and had a PD-L1 tumor proportion score of 90%. She was treated with erlotinib – an EGFR tyrosine kinase inhibitor – and radiotherapy, according to the report, published in Annals of Oncology.
Two months later, she developed multiple new metastases in the chest wall. These also showed high PD-L1 expression, but no EGFR mutations, so she was treated with pembrolizumab as second-line therapy.
After the first course of treatment with pembrolizumab, the hip pain improved dramatically, and after three cycles the primary and metastatic lesions disappeared completely.
“Immunofluorescent analysis of both right ilium and chest wall lesions with an anti-EGFR antibody specific to Ex.19 del and an anti-PD-L1 antibody revealed the presence of distinct heterogeneity of EGFR-mutant clones and PD-L1 highly-expressing clones,” wrote Kei Kunimasa, MD, of Osaka International Cancer Institute, and coauthors.
Current clinical trials suggested that patients with EGFR mutations are likely to have a poor response to PD-1 blockade therapies for NSCLC, and the authors agreed that patients with EGFR mutations should be treated with EGFR tyrosine kinase inhibitors as first-line therapy.
“However, the present case suggested that shorter PFS in EGFR-TKI treatment for EGFR-mutant NSCLC patients with high PD-L1 TPS and without acquired T790M mutation could encourage us to try PD-1 blockade therapy as second line therapy,” they wrote.
The investigators suggested that certain clinical factors could identify patients with EGFR-mutant NSCLC who might respond well to PD-1 blockade, such as progression-free survival on EGFR tyrosine kinase inhibitor treatment, other targetable resistant mutations, tumor mutation burden, and PD-L1 expression.
Two authors reported grants and personal fees from pharmaceutical companies outside the submitted work. No other conflicts of interest were declared.
SOURCE: Kunimasa K et al. Ann Oncol, 2018 Aug 7. doi: 10.1093/annonc/mdy312.
PD-1 blockade should still be considered as a second-line approach in patients with EGFR-mutant non–small-cell lung cancer (NSCLC), as some may still respond to this therapy, investigators reported.
The researchers described a patient who was diagnosed with epidermal growth factor receptor (EGFR)-mutant NSCLC but who also showed high expression of PD-L1.
The 62-year-old woman first presented with a lung nodule and metastasis in the right hip, which were EGFR-mutant and had a PD-L1 tumor proportion score of 90%. She was treated with erlotinib – an EGFR tyrosine kinase inhibitor – and radiotherapy, according to the report, published in Annals of Oncology.
Two months later, she developed multiple new metastases in the chest wall. These also showed high PD-L1 expression, but no EGFR mutations, so she was treated with pembrolizumab as second-line therapy.
After the first course of treatment with pembrolizumab, the hip pain improved dramatically, and after three cycles the primary and metastatic lesions disappeared completely.
“Immunofluorescent analysis of both right ilium and chest wall lesions with an anti-EGFR antibody specific to Ex.19 del and an anti-PD-L1 antibody revealed the presence of distinct heterogeneity of EGFR-mutant clones and PD-L1 highly-expressing clones,” wrote Kei Kunimasa, MD, of Osaka International Cancer Institute, and coauthors.
Current clinical trials suggested that patients with EGFR mutations are likely to have a poor response to PD-1 blockade therapies for NSCLC, and the authors agreed that patients with EGFR mutations should be treated with EGFR tyrosine kinase inhibitors as first-line therapy.
“However, the present case suggested that shorter PFS in EGFR-TKI treatment for EGFR-mutant NSCLC patients with high PD-L1 TPS and without acquired T790M mutation could encourage us to try PD-1 blockade therapy as second line therapy,” they wrote.
The investigators suggested that certain clinical factors could identify patients with EGFR-mutant NSCLC who might respond well to PD-1 blockade, such as progression-free survival on EGFR tyrosine kinase inhibitor treatment, other targetable resistant mutations, tumor mutation burden, and PD-L1 expression.
Two authors reported grants and personal fees from pharmaceutical companies outside the submitted work. No other conflicts of interest were declared.
SOURCE: Kunimasa K et al. Ann Oncol, 2018 Aug 7. doi: 10.1093/annonc/mdy312.
FROM ANNALS OF ONCOLOGY
Key clinical point: Consider PD-1 blockade in EGFR-mutant non–small-cell lung cancer with high PD-L1 expression.Major finding: EGFR-mutant non–small-cell lung cancer may still respond to PD-1 blockade.
Study details: Case study.
Disclosures: Two authors reported grants and personal fees from pharmaceutical companies outside the submitted work. No other conflicts of interest were declared.
Source: Kunimasa K et al. Ann Oncol. 2018 Aug 7. doi: 10.1093/annonc/mdy312.
Rapid drug alteration a bust in metastatic GIST
CHICAGO – For patients with gastrointestinal stromal tumor (GIST) with KIT mutations conferring resistance to imatinib, a strategy of rapid alteration of drugs with complementary activity against KIT mutations is feasible but has thus far failed to yield significant clinical benefits, investigators said.
There were no objective responses among 12 patients treated continuously with 3 days of sunitinib (Sutent) followed by 4 days of regorafenib (Stivarga), and although 4 patients had stable disease in the short term, in each case the disease progressed within 16 weeks, reported Cesar Serrano-Garcia, MD, from the Dana-Farber Cancer Institute and Brigham and Women’s Hospital in Boston, and his colleagues.
“Drug exposure is critical to effectively target specific resistant subpopulations and low exposure may have contributed to the lack of efficacy in this cohort,” they wrote in a poster presented at the annual meeting of the American Society of Clinical Oncology.
The investigators noted that the main mechanism of resistance to imatinib (Gleevec) in GIST is polyclonal emergence of KIT secondary mutations. They then theorized that rapid alteration of sunitinib with regorafenib, which both have complementary activity against different KIT resistance mutations, could be a novel therapeutic strategy for controlling imatinib-resistant disease.
Both agents are active against KIT and platelet-derived growth factor receptor alpha (PDGFR-alpha). Sunitinib has stronger activity against ATP-binding pocket mutations, and regorafenib is more effective against activation loop oncoproteins, the investigators explained.
They conducted a phase Ib trial to evaluate the safety and preliminary efficacy of the strategy in patients with metastatic GIST that had advanced on therapy with all established protocols. The trial had a standard 3+3 design to determine the recommended phase 2 dose; a total of 14 patients were enrolled, but only 12 received one or more complete cycles.
The median patient age was 63.5%. Nine patients had Eastern Cooperative Oncology Group performance status of 0, and five had an ECOG status of 1. The patients had received a median of four prior lines of therapy, and all had received at least three lines.
The primary mutations were at KIT exon 11 in eight patients, exon 9 in five patients, and a KIT/PDGFR-alpha wild type in one patient.
Of the 12 patients who received one or more complete cycles, 7 were treated with sunitinib 37.5 mg daily for 3 days, followed by regorafenib 120 mg daily for 4 days. There were no dose-limiting toxicities in this group. The median number of cycles delivered was 2 (range 1-4).
The other five patients were treated with sunitinib at the same 37.5 mg daily dose for 3 days, followed immediately by regorafenib 160 mg daily for 4 days. There were two dose-limiting toxicities in this group, both grade 3 hypophosphatemia, one of which was refractory to phosphorous replacement.
Antitumor activity according to Response Evaluation Criteria in Solid Tumors version 1.1 included four cases of stable disease at the time of the efficacy analysis, and eight cases of disease progression. The median progression-free survival was 1.9 months. As noted before, there were no complete or partial responses among the 12 patients.
A pharmacokinetic profile at cycle 1 showed that neither drug reached its reported active blood drug levels.
The patients appeared to tolerate the treatment well, with grade 1 or 2 fatigue in all patients being the most common adverse events. Grade 3 or 4 events included hand-foot syndrome, hypertension, and hypophosphatemia in two patients each.
As noted, the authors acknowledged that low drug exposure levels may explain the lack of any responses in this cohort.
“Therapeutic strategies based on KIT inhibition remain crucial in GIST patients progressing to multiple lines,” they wrote.
The study was supported by an ASCO Young Investigator Award, Pfizer, and Bayer. Dr. Serrano-Garcia disclosed honoraria from Bayer, a consulting or advisory role for Deciphera, research funding from Bayer and Deciphera, and travel accommodations and expenses from Pfizer.
SOURCE: Serrano-Garcia C et al. ASCO 2018, Abstract 11510.
CHICAGO – For patients with gastrointestinal stromal tumor (GIST) with KIT mutations conferring resistance to imatinib, a strategy of rapid alteration of drugs with complementary activity against KIT mutations is feasible but has thus far failed to yield significant clinical benefits, investigators said.
There were no objective responses among 12 patients treated continuously with 3 days of sunitinib (Sutent) followed by 4 days of regorafenib (Stivarga), and although 4 patients had stable disease in the short term, in each case the disease progressed within 16 weeks, reported Cesar Serrano-Garcia, MD, from the Dana-Farber Cancer Institute and Brigham and Women’s Hospital in Boston, and his colleagues.
“Drug exposure is critical to effectively target specific resistant subpopulations and low exposure may have contributed to the lack of efficacy in this cohort,” they wrote in a poster presented at the annual meeting of the American Society of Clinical Oncology.
The investigators noted that the main mechanism of resistance to imatinib (Gleevec) in GIST is polyclonal emergence of KIT secondary mutations. They then theorized that rapid alteration of sunitinib with regorafenib, which both have complementary activity against different KIT resistance mutations, could be a novel therapeutic strategy for controlling imatinib-resistant disease.
Both agents are active against KIT and platelet-derived growth factor receptor alpha (PDGFR-alpha). Sunitinib has stronger activity against ATP-binding pocket mutations, and regorafenib is more effective against activation loop oncoproteins, the investigators explained.
They conducted a phase Ib trial to evaluate the safety and preliminary efficacy of the strategy in patients with metastatic GIST that had advanced on therapy with all established protocols. The trial had a standard 3+3 design to determine the recommended phase 2 dose; a total of 14 patients were enrolled, but only 12 received one or more complete cycles.
The median patient age was 63.5%. Nine patients had Eastern Cooperative Oncology Group performance status of 0, and five had an ECOG status of 1. The patients had received a median of four prior lines of therapy, and all had received at least three lines.
The primary mutations were at KIT exon 11 in eight patients, exon 9 in five patients, and a KIT/PDGFR-alpha wild type in one patient.
Of the 12 patients who received one or more complete cycles, 7 were treated with sunitinib 37.5 mg daily for 3 days, followed by regorafenib 120 mg daily for 4 days. There were no dose-limiting toxicities in this group. The median number of cycles delivered was 2 (range 1-4).
The other five patients were treated with sunitinib at the same 37.5 mg daily dose for 3 days, followed immediately by regorafenib 160 mg daily for 4 days. There were two dose-limiting toxicities in this group, both grade 3 hypophosphatemia, one of which was refractory to phosphorous replacement.
Antitumor activity according to Response Evaluation Criteria in Solid Tumors version 1.1 included four cases of stable disease at the time of the efficacy analysis, and eight cases of disease progression. The median progression-free survival was 1.9 months. As noted before, there were no complete or partial responses among the 12 patients.
A pharmacokinetic profile at cycle 1 showed that neither drug reached its reported active blood drug levels.
The patients appeared to tolerate the treatment well, with grade 1 or 2 fatigue in all patients being the most common adverse events. Grade 3 or 4 events included hand-foot syndrome, hypertension, and hypophosphatemia in two patients each.
As noted, the authors acknowledged that low drug exposure levels may explain the lack of any responses in this cohort.
“Therapeutic strategies based on KIT inhibition remain crucial in GIST patients progressing to multiple lines,” they wrote.
The study was supported by an ASCO Young Investigator Award, Pfizer, and Bayer. Dr. Serrano-Garcia disclosed honoraria from Bayer, a consulting or advisory role for Deciphera, research funding from Bayer and Deciphera, and travel accommodations and expenses from Pfizer.
SOURCE: Serrano-Garcia C et al. ASCO 2018, Abstract 11510.
CHICAGO – For patients with gastrointestinal stromal tumor (GIST) with KIT mutations conferring resistance to imatinib, a strategy of rapid alteration of drugs with complementary activity against KIT mutations is feasible but has thus far failed to yield significant clinical benefits, investigators said.
There were no objective responses among 12 patients treated continuously with 3 days of sunitinib (Sutent) followed by 4 days of regorafenib (Stivarga), and although 4 patients had stable disease in the short term, in each case the disease progressed within 16 weeks, reported Cesar Serrano-Garcia, MD, from the Dana-Farber Cancer Institute and Brigham and Women’s Hospital in Boston, and his colleagues.
“Drug exposure is critical to effectively target specific resistant subpopulations and low exposure may have contributed to the lack of efficacy in this cohort,” they wrote in a poster presented at the annual meeting of the American Society of Clinical Oncology.
The investigators noted that the main mechanism of resistance to imatinib (Gleevec) in GIST is polyclonal emergence of KIT secondary mutations. They then theorized that rapid alteration of sunitinib with regorafenib, which both have complementary activity against different KIT resistance mutations, could be a novel therapeutic strategy for controlling imatinib-resistant disease.
Both agents are active against KIT and platelet-derived growth factor receptor alpha (PDGFR-alpha). Sunitinib has stronger activity against ATP-binding pocket mutations, and regorafenib is more effective against activation loop oncoproteins, the investigators explained.
They conducted a phase Ib trial to evaluate the safety and preliminary efficacy of the strategy in patients with metastatic GIST that had advanced on therapy with all established protocols. The trial had a standard 3+3 design to determine the recommended phase 2 dose; a total of 14 patients were enrolled, but only 12 received one or more complete cycles.
The median patient age was 63.5%. Nine patients had Eastern Cooperative Oncology Group performance status of 0, and five had an ECOG status of 1. The patients had received a median of four prior lines of therapy, and all had received at least three lines.
The primary mutations were at KIT exon 11 in eight patients, exon 9 in five patients, and a KIT/PDGFR-alpha wild type in one patient.
Of the 12 patients who received one or more complete cycles, 7 were treated with sunitinib 37.5 mg daily for 3 days, followed by regorafenib 120 mg daily for 4 days. There were no dose-limiting toxicities in this group. The median number of cycles delivered was 2 (range 1-4).
The other five patients were treated with sunitinib at the same 37.5 mg daily dose for 3 days, followed immediately by regorafenib 160 mg daily for 4 days. There were two dose-limiting toxicities in this group, both grade 3 hypophosphatemia, one of which was refractory to phosphorous replacement.
Antitumor activity according to Response Evaluation Criteria in Solid Tumors version 1.1 included four cases of stable disease at the time of the efficacy analysis, and eight cases of disease progression. The median progression-free survival was 1.9 months. As noted before, there were no complete or partial responses among the 12 patients.
A pharmacokinetic profile at cycle 1 showed that neither drug reached its reported active blood drug levels.
The patients appeared to tolerate the treatment well, with grade 1 or 2 fatigue in all patients being the most common adverse events. Grade 3 or 4 events included hand-foot syndrome, hypertension, and hypophosphatemia in two patients each.
As noted, the authors acknowledged that low drug exposure levels may explain the lack of any responses in this cohort.
“Therapeutic strategies based on KIT inhibition remain crucial in GIST patients progressing to multiple lines,” they wrote.
The study was supported by an ASCO Young Investigator Award, Pfizer, and Bayer. Dr. Serrano-Garcia disclosed honoraria from Bayer, a consulting or advisory role for Deciphera, research funding from Bayer and Deciphera, and travel accommodations and expenses from Pfizer.
SOURCE: Serrano-Garcia C et al. ASCO 2018, Abstract 11510.
REPORTING FROM ASCO 2018
Key clinical point: The strategy of rapid alteration of drugs to overcome mutations conferring imatinib resistance in gastrointestinal stromal tumor (GIST) was feasible but ineffective.
Major finding: There were no objective responses among 12 patients treated with the strategy.
Study details: A phase Ib clinical trial in 12 patients with heavily pretreated metastatic GIST.
Disclosures: The study was supported by an ASCO Young Investigator Award, Pfizer, and Bayer. Dr. Serrano-Garcia disclosed honoraria from Bayer, a consulting or advisory role for Deciphera, research funding from Bayer and Deciphera, and travel accommodations and expenses from Pfizer.
Source: Serrano-Garcia C et al. ASCO 2018, Abstract 11510.
ctDNA profiles pre- and posttreatment KIT mutations in GIST
CHICAGO – Detection of circulating tumor DNA (ctDNA), aka “liquid biopsy,” may serve as a noninvasive marker for disease heterogeneity and aid in the assessment of clinical responses to therapy for patients with gastrointestinal stromal tumors (GIST), according to investigators.
In a phase 1 trial of the investigational agent DCC-2618, a pan-KIT/platelet-derived growth factor receptor alpha (PDGFRA) switch control inhibitor, identification of ctDNA by next-generation sequencing (NGS) was accomplished in the majority of patients, with findings that support the need for a broad-spectrum KIT inhibitor for patients with GIST resistant to imatinib (Gleevec), reported Suzanne George, MD, of Dana-Farber Cancer Institute in Boston, and her colleagues.
“This data demonstrates for the first time that the distribution of resistance mutations in KIT across exons 13, 14, 17, and 18 or a combination thereof is similar in 2nd, 3rd, and 4th-line patients,” they wrote in a poster presented at the annual meeting of the American Society of Clinical Oncology.
The dose-finding and escalation trial included baseline evaluations of KIT/PDGFRA mutations with both ctDNA and fresh tumor biopsy, and ctDNA measurements during treatment.
Biopsy detected 68 KIT mutations at baseline in 81 patients, and ctDNA detected 75 mutations in 95 patients. Some patients had multiple mutations within one exon.
An analysis of mutations by response showed that of 73 patients with detectable KIT mutations by ctDNA at baseline, 35 became KIT ctDNA negative during at least one treatment time point. Of this group, 8 had a partial response (PR) and 27 had stable disease (SD). In all, 57 of the 73 patients had a more than 50% reduction in KIT mutation allele frequency (MAF).
Some patients with stable disease remained KIT negative out to 60 weeks following the first DCC-2618 dose.
The investigators also looked at ctDNA at baseline (21 patients) and post treatment (20 patients) in those who had a PR as their best response. Ten of these patients had KIT mutations detected at baseline, and of this group, eight became KIT negative after treatment; one had no detectable mutations in one exon and one exon with an MAF less than .1%. No posttreatment samples were available for the remaining patient.
There were preliminary data suggesting that DCC-2618 in the second line could be more efficacious than sunitinib (Sutent) in the same setting, and that in KIT-driven GIST DCC-2618 may provide more benefit in the second line compared with the fourth or subsequent lines of therapy, the authors stated.
“The mutational profile of KIT in tumors and plasma at baseline in GIST patients supports the need for a broad spectrum KIT inhibitor in all post-imatinib lines of therapy,” they wrote.
The trial is supported by Deciphera Pharmaceuticals. Dr. George disclosed stock or other ownership in Abbott Laboratories and Abbvie, consulting/advising for AstraZeneca, Blueprint Medicines, and Deciphera, and institutional research funding from Ariad, Bayer, Blueprint Medicine, Deciphera, Novartis, and Pfizer.
SOURCE: George S et al. ASCO 2018. Abstract 11511.
CHICAGO – Detection of circulating tumor DNA (ctDNA), aka “liquid biopsy,” may serve as a noninvasive marker for disease heterogeneity and aid in the assessment of clinical responses to therapy for patients with gastrointestinal stromal tumors (GIST), according to investigators.
In a phase 1 trial of the investigational agent DCC-2618, a pan-KIT/platelet-derived growth factor receptor alpha (PDGFRA) switch control inhibitor, identification of ctDNA by next-generation sequencing (NGS) was accomplished in the majority of patients, with findings that support the need for a broad-spectrum KIT inhibitor for patients with GIST resistant to imatinib (Gleevec), reported Suzanne George, MD, of Dana-Farber Cancer Institute in Boston, and her colleagues.
“This data demonstrates for the first time that the distribution of resistance mutations in KIT across exons 13, 14, 17, and 18 or a combination thereof is similar in 2nd, 3rd, and 4th-line patients,” they wrote in a poster presented at the annual meeting of the American Society of Clinical Oncology.
The dose-finding and escalation trial included baseline evaluations of KIT/PDGFRA mutations with both ctDNA and fresh tumor biopsy, and ctDNA measurements during treatment.
Biopsy detected 68 KIT mutations at baseline in 81 patients, and ctDNA detected 75 mutations in 95 patients. Some patients had multiple mutations within one exon.
An analysis of mutations by response showed that of 73 patients with detectable KIT mutations by ctDNA at baseline, 35 became KIT ctDNA negative during at least one treatment time point. Of this group, 8 had a partial response (PR) and 27 had stable disease (SD). In all, 57 of the 73 patients had a more than 50% reduction in KIT mutation allele frequency (MAF).
Some patients with stable disease remained KIT negative out to 60 weeks following the first DCC-2618 dose.
The investigators also looked at ctDNA at baseline (21 patients) and post treatment (20 patients) in those who had a PR as their best response. Ten of these patients had KIT mutations detected at baseline, and of this group, eight became KIT negative after treatment; one had no detectable mutations in one exon and one exon with an MAF less than .1%. No posttreatment samples were available for the remaining patient.
There were preliminary data suggesting that DCC-2618 in the second line could be more efficacious than sunitinib (Sutent) in the same setting, and that in KIT-driven GIST DCC-2618 may provide more benefit in the second line compared with the fourth or subsequent lines of therapy, the authors stated.
“The mutational profile of KIT in tumors and plasma at baseline in GIST patients supports the need for a broad spectrum KIT inhibitor in all post-imatinib lines of therapy,” they wrote.
The trial is supported by Deciphera Pharmaceuticals. Dr. George disclosed stock or other ownership in Abbott Laboratories and Abbvie, consulting/advising for AstraZeneca, Blueprint Medicines, and Deciphera, and institutional research funding from Ariad, Bayer, Blueprint Medicine, Deciphera, Novartis, and Pfizer.
SOURCE: George S et al. ASCO 2018. Abstract 11511.
CHICAGO – Detection of circulating tumor DNA (ctDNA), aka “liquid biopsy,” may serve as a noninvasive marker for disease heterogeneity and aid in the assessment of clinical responses to therapy for patients with gastrointestinal stromal tumors (GIST), according to investigators.
In a phase 1 trial of the investigational agent DCC-2618, a pan-KIT/platelet-derived growth factor receptor alpha (PDGFRA) switch control inhibitor, identification of ctDNA by next-generation sequencing (NGS) was accomplished in the majority of patients, with findings that support the need for a broad-spectrum KIT inhibitor for patients with GIST resistant to imatinib (Gleevec), reported Suzanne George, MD, of Dana-Farber Cancer Institute in Boston, and her colleagues.
“This data demonstrates for the first time that the distribution of resistance mutations in KIT across exons 13, 14, 17, and 18 or a combination thereof is similar in 2nd, 3rd, and 4th-line patients,” they wrote in a poster presented at the annual meeting of the American Society of Clinical Oncology.
The dose-finding and escalation trial included baseline evaluations of KIT/PDGFRA mutations with both ctDNA and fresh tumor biopsy, and ctDNA measurements during treatment.
Biopsy detected 68 KIT mutations at baseline in 81 patients, and ctDNA detected 75 mutations in 95 patients. Some patients had multiple mutations within one exon.
An analysis of mutations by response showed that of 73 patients with detectable KIT mutations by ctDNA at baseline, 35 became KIT ctDNA negative during at least one treatment time point. Of this group, 8 had a partial response (PR) and 27 had stable disease (SD). In all, 57 of the 73 patients had a more than 50% reduction in KIT mutation allele frequency (MAF).
Some patients with stable disease remained KIT negative out to 60 weeks following the first DCC-2618 dose.
The investigators also looked at ctDNA at baseline (21 patients) and post treatment (20 patients) in those who had a PR as their best response. Ten of these patients had KIT mutations detected at baseline, and of this group, eight became KIT negative after treatment; one had no detectable mutations in one exon and one exon with an MAF less than .1%. No posttreatment samples were available for the remaining patient.
There were preliminary data suggesting that DCC-2618 in the second line could be more efficacious than sunitinib (Sutent) in the same setting, and that in KIT-driven GIST DCC-2618 may provide more benefit in the second line compared with the fourth or subsequent lines of therapy, the authors stated.
“The mutational profile of KIT in tumors and plasma at baseline in GIST patients supports the need for a broad spectrum KIT inhibitor in all post-imatinib lines of therapy,” they wrote.
The trial is supported by Deciphera Pharmaceuticals. Dr. George disclosed stock or other ownership in Abbott Laboratories and Abbvie, consulting/advising for AstraZeneca, Blueprint Medicines, and Deciphera, and institutional research funding from Ariad, Bayer, Blueprint Medicine, Deciphera, Novartis, and Pfizer.
SOURCE: George S et al. ASCO 2018. Abstract 11511.
REPORTING FROM ASCO 2018
Key clinical point: Circulating tumor DNA can be used for mutational profiling and responses assessment in patients with advanced imatinib-resistant GIST.
Major finding: Of 73 patients with detectable KIT mutations by ctDNA at baseline, 35 became KIT ctDNA negative during at least one treatment time point.
Study details: Subanalyses from a phase 1 trial of DCC-2618.
Disclosures: The trial is supported by Deciphera Pharmaceuticals. Dr. George disclosed stock or other ownership in Abbott Laboratories and Abbvie, consulting/advising for AstraZeneca, Blueprint Medicines, and Deciphera, and institutional research funding from Ariad, Bayer, Blueprint Medicine, Deciphera, Novartis, and Pfizer.
Source: George S et al. ASCO 2018. Abstract 11511.