User login
Lung ultrasound predicts need for first-dose surfactant in neonates
Lung ultrasound score (LUS) is an effective means of predicting whether extremely preterm neonates undergoing continuous positive airway pressure (CPAP) treatment for respiratory distress syndrome (RDS) require surfactant, according to results of study published in Pediatrics.
Lucia De Martino, MD, of the division of pediatrics and neonatal critical care at the A. Béclère Medical Centre of the South Paris University Hospital and her associates enrolled 133 neonates of 30 weeks’ gestation or less born between 2015 and 2016. They designed the prospective diagnostic accuracy cohort study, which was conducted in an academic tertiary care referral neonatal ICU.
The first dose of surfactant was administered at a mean 4 hours of life. Those that required further treatment received a second dose of surfactant at a mean 28 hours of life. Each patient received a single lung ultrasound lasting an average of 3 minutes. In each case, the procedure was well tolerated.
In particular, the study demonstrated that LUS is able to accurately predict the need for a first dose and “reveals fair accuracy when it comes to predicting surfactant retreatment,” they observed. The authors speculate that using LUS to predict retreatment is somewhat less reliable because of either the lower number of patients requiring retreatment or reasons related to the biology of surfactant.
“A LUS cutoff value between 6 and 8 provides optimal sensitivity and specificity for predicting the need for the first surfactant dose, whereas a cutoff value of 10 predicts the need for surfactant retreatment,” Dr. De Martino and her colleagues advised.
Of key importance was the finding that LUS is of greatest value to preterm infants less than 34 weeks’ gestation; the authors observed that LUS had significantly lower diagnostic accuracy in infants who were either late term or term. They offered that this outcome was likely attributable to the homogeneous nature of preterm neonates, who are commonly affected by RDS and tend to present with a variety of respiratory disorders and surfactant injury to differing degrees.
At present, international guidelines only recommend surfactant replacement in cases where CPAP has failed, but administering surfactant within the first 2-3 hours of life is key to reducing bronchopulmonary dysplasia as well as the risk of death, they said.
Current surfactant replacement is determined solely by fraction of inspired oxygen cutoff levels, which can result in delayed or even unnecessary treatment. Because neonates who are extremely preterm benefit the most from treatment, “both situations are potentially harmful because late surfactant replacement is less efficacious and giving surfactant when it is not needed may be invasive and seems to increase lung inflammation in animal models,” Dr. De Martino and her associates cautioned.
The authors had no relevant financial disclosures.
SOURCE: De Martino L et al. Pediatrics. 2018. doi: 10.1542/peds.2018-0463.
Point-of-care ultrasound (POCUS) has been recognized for years for its value in assessing sick neonates, but a recent survey showed that less than one-third of U.S. neonatal-perinatal medicine programs actually use bedside ultrasound for health care diagnosis and management. Although its use historically has been confined to pediatric cardiology and radiology, it has gained more of a foothold in acute pediatric care settings, and its use in evaluating neonate lungs is a “relatively new and potentially revolutionary approach,” Maria V. Fraga, MD, and her associates wrote in an accompanying editorial.
A growing body of data over the past 2 decades is available to help radiologists and bedside providers to better understand the applications and limitations of POCUS. Findings in similar studies looking at the use of LUS in neonates “make the article by De Martino et al. so important,” Dr. Fraga and her associates emphasized. Dr. De Martino and her colleagues were able to use POCUS of the lung “to develop reliable predictive models for the need for surfactant treatment and re-dosing” in a group of preterm infants.
Although it would seem reasonable to expect the potential benefits of POCUS to have worldwide application, implementation is inconsistent. Clinicians in Australia, New Zealand, and Canada are trained and use POCUS daily, but this is not the case in other countries such as the United States. Concern over legal risks and training, as well as turf disputes with cardiology and radiology, the lack of clinicians actively using ultrasound, and scarce evidence showing benefit of its use could be to blame.
“The development of a POCUS program requires an accessible dedicated ultrasound machine kept in close proximity to clinical areas, a core group of interested clinicians, and a training and accreditation program with a commitment to continuing professional development,” advised Dr. Fraga and her associates.
“It is important to understand the limitation of bedside ultrasound, which should always be performed for a specific clinical purpose and to answer a clinical question and does not always mandate a full comprehensive study,” they added.
Dr. Fraga and her associates are affiliated with the department of pediatrics at the University of Pennsylvania, Philadelphia. There was no external funding and the authors had no relevant financial disclosures. These comments are adapted from an editorial accompanying the article by De Martino et al. (Pediatrics. 2018. doi: 10.1542/peds.2018-1621).
Point-of-care ultrasound (POCUS) has been recognized for years for its value in assessing sick neonates, but a recent survey showed that less than one-third of U.S. neonatal-perinatal medicine programs actually use bedside ultrasound for health care diagnosis and management. Although its use historically has been confined to pediatric cardiology and radiology, it has gained more of a foothold in acute pediatric care settings, and its use in evaluating neonate lungs is a “relatively new and potentially revolutionary approach,” Maria V. Fraga, MD, and her associates wrote in an accompanying editorial.
A growing body of data over the past 2 decades is available to help radiologists and bedside providers to better understand the applications and limitations of POCUS. Findings in similar studies looking at the use of LUS in neonates “make the article by De Martino et al. so important,” Dr. Fraga and her associates emphasized. Dr. De Martino and her colleagues were able to use POCUS of the lung “to develop reliable predictive models for the need for surfactant treatment and re-dosing” in a group of preterm infants.
Although it would seem reasonable to expect the potential benefits of POCUS to have worldwide application, implementation is inconsistent. Clinicians in Australia, New Zealand, and Canada are trained and use POCUS daily, but this is not the case in other countries such as the United States. Concern over legal risks and training, as well as turf disputes with cardiology and radiology, the lack of clinicians actively using ultrasound, and scarce evidence showing benefit of its use could be to blame.
“The development of a POCUS program requires an accessible dedicated ultrasound machine kept in close proximity to clinical areas, a core group of interested clinicians, and a training and accreditation program with a commitment to continuing professional development,” advised Dr. Fraga and her associates.
“It is important to understand the limitation of bedside ultrasound, which should always be performed for a specific clinical purpose and to answer a clinical question and does not always mandate a full comprehensive study,” they added.
Dr. Fraga and her associates are affiliated with the department of pediatrics at the University of Pennsylvania, Philadelphia. There was no external funding and the authors had no relevant financial disclosures. These comments are adapted from an editorial accompanying the article by De Martino et al. (Pediatrics. 2018. doi: 10.1542/peds.2018-1621).
Point-of-care ultrasound (POCUS) has been recognized for years for its value in assessing sick neonates, but a recent survey showed that less than one-third of U.S. neonatal-perinatal medicine programs actually use bedside ultrasound for health care diagnosis and management. Although its use historically has been confined to pediatric cardiology and radiology, it has gained more of a foothold in acute pediatric care settings, and its use in evaluating neonate lungs is a “relatively new and potentially revolutionary approach,” Maria V. Fraga, MD, and her associates wrote in an accompanying editorial.
A growing body of data over the past 2 decades is available to help radiologists and bedside providers to better understand the applications and limitations of POCUS. Findings in similar studies looking at the use of LUS in neonates “make the article by De Martino et al. so important,” Dr. Fraga and her associates emphasized. Dr. De Martino and her colleagues were able to use POCUS of the lung “to develop reliable predictive models for the need for surfactant treatment and re-dosing” in a group of preterm infants.
Although it would seem reasonable to expect the potential benefits of POCUS to have worldwide application, implementation is inconsistent. Clinicians in Australia, New Zealand, and Canada are trained and use POCUS daily, but this is not the case in other countries such as the United States. Concern over legal risks and training, as well as turf disputes with cardiology and radiology, the lack of clinicians actively using ultrasound, and scarce evidence showing benefit of its use could be to blame.
“The development of a POCUS program requires an accessible dedicated ultrasound machine kept in close proximity to clinical areas, a core group of interested clinicians, and a training and accreditation program with a commitment to continuing professional development,” advised Dr. Fraga and her associates.
“It is important to understand the limitation of bedside ultrasound, which should always be performed for a specific clinical purpose and to answer a clinical question and does not always mandate a full comprehensive study,” they added.
Dr. Fraga and her associates are affiliated with the department of pediatrics at the University of Pennsylvania, Philadelphia. There was no external funding and the authors had no relevant financial disclosures. These comments are adapted from an editorial accompanying the article by De Martino et al. (Pediatrics. 2018. doi: 10.1542/peds.2018-1621).
Lung ultrasound score (LUS) is an effective means of predicting whether extremely preterm neonates undergoing continuous positive airway pressure (CPAP) treatment for respiratory distress syndrome (RDS) require surfactant, according to results of study published in Pediatrics.
Lucia De Martino, MD, of the division of pediatrics and neonatal critical care at the A. Béclère Medical Centre of the South Paris University Hospital and her associates enrolled 133 neonates of 30 weeks’ gestation or less born between 2015 and 2016. They designed the prospective diagnostic accuracy cohort study, which was conducted in an academic tertiary care referral neonatal ICU.
The first dose of surfactant was administered at a mean 4 hours of life. Those that required further treatment received a second dose of surfactant at a mean 28 hours of life. Each patient received a single lung ultrasound lasting an average of 3 minutes. In each case, the procedure was well tolerated.
In particular, the study demonstrated that LUS is able to accurately predict the need for a first dose and “reveals fair accuracy when it comes to predicting surfactant retreatment,” they observed. The authors speculate that using LUS to predict retreatment is somewhat less reliable because of either the lower number of patients requiring retreatment or reasons related to the biology of surfactant.
“A LUS cutoff value between 6 and 8 provides optimal sensitivity and specificity for predicting the need for the first surfactant dose, whereas a cutoff value of 10 predicts the need for surfactant retreatment,” Dr. De Martino and her colleagues advised.
Of key importance was the finding that LUS is of greatest value to preterm infants less than 34 weeks’ gestation; the authors observed that LUS had significantly lower diagnostic accuracy in infants who were either late term or term. They offered that this outcome was likely attributable to the homogeneous nature of preterm neonates, who are commonly affected by RDS and tend to present with a variety of respiratory disorders and surfactant injury to differing degrees.
At present, international guidelines only recommend surfactant replacement in cases where CPAP has failed, but administering surfactant within the first 2-3 hours of life is key to reducing bronchopulmonary dysplasia as well as the risk of death, they said.
Current surfactant replacement is determined solely by fraction of inspired oxygen cutoff levels, which can result in delayed or even unnecessary treatment. Because neonates who are extremely preterm benefit the most from treatment, “both situations are potentially harmful because late surfactant replacement is less efficacious and giving surfactant when it is not needed may be invasive and seems to increase lung inflammation in animal models,” Dr. De Martino and her associates cautioned.
The authors had no relevant financial disclosures.
SOURCE: De Martino L et al. Pediatrics. 2018. doi: 10.1542/peds.2018-0463.
Lung ultrasound score (LUS) is an effective means of predicting whether extremely preterm neonates undergoing continuous positive airway pressure (CPAP) treatment for respiratory distress syndrome (RDS) require surfactant, according to results of study published in Pediatrics.
Lucia De Martino, MD, of the division of pediatrics and neonatal critical care at the A. Béclère Medical Centre of the South Paris University Hospital and her associates enrolled 133 neonates of 30 weeks’ gestation or less born between 2015 and 2016. They designed the prospective diagnostic accuracy cohort study, which was conducted in an academic tertiary care referral neonatal ICU.
The first dose of surfactant was administered at a mean 4 hours of life. Those that required further treatment received a second dose of surfactant at a mean 28 hours of life. Each patient received a single lung ultrasound lasting an average of 3 minutes. In each case, the procedure was well tolerated.
In particular, the study demonstrated that LUS is able to accurately predict the need for a first dose and “reveals fair accuracy when it comes to predicting surfactant retreatment,” they observed. The authors speculate that using LUS to predict retreatment is somewhat less reliable because of either the lower number of patients requiring retreatment or reasons related to the biology of surfactant.
“A LUS cutoff value between 6 and 8 provides optimal sensitivity and specificity for predicting the need for the first surfactant dose, whereas a cutoff value of 10 predicts the need for surfactant retreatment,” Dr. De Martino and her colleagues advised.
Of key importance was the finding that LUS is of greatest value to preterm infants less than 34 weeks’ gestation; the authors observed that LUS had significantly lower diagnostic accuracy in infants who were either late term or term. They offered that this outcome was likely attributable to the homogeneous nature of preterm neonates, who are commonly affected by RDS and tend to present with a variety of respiratory disorders and surfactant injury to differing degrees.
At present, international guidelines only recommend surfactant replacement in cases where CPAP has failed, but administering surfactant within the first 2-3 hours of life is key to reducing bronchopulmonary dysplasia as well as the risk of death, they said.
Current surfactant replacement is determined solely by fraction of inspired oxygen cutoff levels, which can result in delayed or even unnecessary treatment. Because neonates who are extremely preterm benefit the most from treatment, “both situations are potentially harmful because late surfactant replacement is less efficacious and giving surfactant when it is not needed may be invasive and seems to increase lung inflammation in animal models,” Dr. De Martino and her associates cautioned.
The authors had no relevant financial disclosures.
SOURCE: De Martino L et al. Pediatrics. 2018. doi: 10.1542/peds.2018-0463.
FROM PEDIATRICS
Key clinical point:
Major finding: A LUS cutoff value between 6 and 8 provides optimal sensitivity and specificity for predicting the need for the first surfactant dose.
Study details: Prospective cohort diagnostic accuracy study that included 133 infants.
Disclosures: The authors had no relevant financial disclosures.
Source: De Martino L et al. Pediatrics. 2018. doi: 10.1542/peds.2018-0463.
NIMH urged to shift priorities toward children’s mental health
ROCKVILLE, MD. – A researcher is calling for a major shift in funding and priorities within the National Institute of Mental Health to seize on ripe opportunities to better understand how social and environmental factors affect the development of children’s brains.
“This new agenda that I’m suggesting would prioritize child and family health over other populations,” Kimberly E. Hoagwood, PhD, of NYU Langone Health, said at a National Institute of Mental Health conference on mental health services research. “But if we want science to maximize the public health impact and we want our services implementation research to have the biggest impact, then I think we have to think about rebalancing the portfolio.”
Dr. Hoagwood made her argument during a well-received presentation at the meeting. In an interview afterward, Dr. Hoagwood confirmed that she’s advocating for a potential shifting of funds from basic neuroscience.
She previously argued a case for rebalancing priorities within mental health research in a paper published in the Journal of the American Academy of Child and Adolescent Psychiatry (2018 Jan;57[1]:10-3). In the paper, Dr. Hoagwood and her coauthors said the NIMH’s annual funding for child and adolescent services and intervention research decreased 42%, to $30.2 million, from fiscal 2005 to fiscal 2015.
“The NIMH made an explicit decision to invest in basic neuroscience in part because of concerns about the inadequacy of the diagnostic classification systems and limited understanding of the etiology of mental illness,” Dr. Hoagwood and her colleagues wrote in the paper. “This investment could well pay off in the future. However, at least 20% of children now suffer from mental health problems. They cannot be ignored.”
The share of NIMH’s annual budget dedicated to child and adolescent services and intervention research has hovered around 2%-3% in recent years, according to Dr. Hoagwood.
She argued that increased investment in child and adolescent services and intervention research is needed in part because of a flourishing atmosphere outside of the NIMH. At least two dozen notable initiatives looking at social and environmental factors are underway that could contribute greatly to the understanding of factors outside of genetics that influence early brain development, Dr. Hoagwood said.
Those initiatives include about a dozen Medicaid accountable care organizations that are identifying social risks such as poverty, homelessness, food insecurity, and unemployment. Efforts aimed at addressing the effects of poverty through the adoption of strategies such as living wage laws also are underway, she said.
Dr. Hoagwood highlighted the importance of work on the exposome, which the NIMH has described as a new approach to understanding the mechanisms by which environmental factors alter brain and behavior – starting from prenatal development. Dr. Hoagwood said this approach would collect samples to systematically monitor a range of broad-spectrum environmental exposures. She described it as the “complement to the genomic sequencing.”
She called for launching multisite studies to look at how such factors affect brain development, and where and how early interventions can improve children’s healthy development. Data from community efforts and some of those experiments might, at least initially, be “messy beyond belief,” she said.
“We have to not shy away from it. The genomic sequencing has not shied away from messy data,” Dr. Hoagwood said. “We don’t need to do that, either. We need new methods. We need small experiments of novel payment approaches. We need to use our data systems better.”
Dr. Hoagwood had no financial disclosures to report.
ROCKVILLE, MD. – A researcher is calling for a major shift in funding and priorities within the National Institute of Mental Health to seize on ripe opportunities to better understand how social and environmental factors affect the development of children’s brains.
“This new agenda that I’m suggesting would prioritize child and family health over other populations,” Kimberly E. Hoagwood, PhD, of NYU Langone Health, said at a National Institute of Mental Health conference on mental health services research. “But if we want science to maximize the public health impact and we want our services implementation research to have the biggest impact, then I think we have to think about rebalancing the portfolio.”
Dr. Hoagwood made her argument during a well-received presentation at the meeting. In an interview afterward, Dr. Hoagwood confirmed that she’s advocating for a potential shifting of funds from basic neuroscience.
She previously argued a case for rebalancing priorities within mental health research in a paper published in the Journal of the American Academy of Child and Adolescent Psychiatry (2018 Jan;57[1]:10-3). In the paper, Dr. Hoagwood and her coauthors said the NIMH’s annual funding for child and adolescent services and intervention research decreased 42%, to $30.2 million, from fiscal 2005 to fiscal 2015.
“The NIMH made an explicit decision to invest in basic neuroscience in part because of concerns about the inadequacy of the diagnostic classification systems and limited understanding of the etiology of mental illness,” Dr. Hoagwood and her colleagues wrote in the paper. “This investment could well pay off in the future. However, at least 20% of children now suffer from mental health problems. They cannot be ignored.”
The share of NIMH’s annual budget dedicated to child and adolescent services and intervention research has hovered around 2%-3% in recent years, according to Dr. Hoagwood.
She argued that increased investment in child and adolescent services and intervention research is needed in part because of a flourishing atmosphere outside of the NIMH. At least two dozen notable initiatives looking at social and environmental factors are underway that could contribute greatly to the understanding of factors outside of genetics that influence early brain development, Dr. Hoagwood said.
Those initiatives include about a dozen Medicaid accountable care organizations that are identifying social risks such as poverty, homelessness, food insecurity, and unemployment. Efforts aimed at addressing the effects of poverty through the adoption of strategies such as living wage laws also are underway, she said.
Dr. Hoagwood highlighted the importance of work on the exposome, which the NIMH has described as a new approach to understanding the mechanisms by which environmental factors alter brain and behavior – starting from prenatal development. Dr. Hoagwood said this approach would collect samples to systematically monitor a range of broad-spectrum environmental exposures. She described it as the “complement to the genomic sequencing.”
She called for launching multisite studies to look at how such factors affect brain development, and where and how early interventions can improve children’s healthy development. Data from community efforts and some of those experiments might, at least initially, be “messy beyond belief,” she said.
“We have to not shy away from it. The genomic sequencing has not shied away from messy data,” Dr. Hoagwood said. “We don’t need to do that, either. We need new methods. We need small experiments of novel payment approaches. We need to use our data systems better.”
Dr. Hoagwood had no financial disclosures to report.
ROCKVILLE, MD. – A researcher is calling for a major shift in funding and priorities within the National Institute of Mental Health to seize on ripe opportunities to better understand how social and environmental factors affect the development of children’s brains.
“This new agenda that I’m suggesting would prioritize child and family health over other populations,” Kimberly E. Hoagwood, PhD, of NYU Langone Health, said at a National Institute of Mental Health conference on mental health services research. “But if we want science to maximize the public health impact and we want our services implementation research to have the biggest impact, then I think we have to think about rebalancing the portfolio.”
Dr. Hoagwood made her argument during a well-received presentation at the meeting. In an interview afterward, Dr. Hoagwood confirmed that she’s advocating for a potential shifting of funds from basic neuroscience.
She previously argued a case for rebalancing priorities within mental health research in a paper published in the Journal of the American Academy of Child and Adolescent Psychiatry (2018 Jan;57[1]:10-3). In the paper, Dr. Hoagwood and her coauthors said the NIMH’s annual funding for child and adolescent services and intervention research decreased 42%, to $30.2 million, from fiscal 2005 to fiscal 2015.
“The NIMH made an explicit decision to invest in basic neuroscience in part because of concerns about the inadequacy of the diagnostic classification systems and limited understanding of the etiology of mental illness,” Dr. Hoagwood and her colleagues wrote in the paper. “This investment could well pay off in the future. However, at least 20% of children now suffer from mental health problems. They cannot be ignored.”
The share of NIMH’s annual budget dedicated to child and adolescent services and intervention research has hovered around 2%-3% in recent years, according to Dr. Hoagwood.
She argued that increased investment in child and adolescent services and intervention research is needed in part because of a flourishing atmosphere outside of the NIMH. At least two dozen notable initiatives looking at social and environmental factors are underway that could contribute greatly to the understanding of factors outside of genetics that influence early brain development, Dr. Hoagwood said.
Those initiatives include about a dozen Medicaid accountable care organizations that are identifying social risks such as poverty, homelessness, food insecurity, and unemployment. Efforts aimed at addressing the effects of poverty through the adoption of strategies such as living wage laws also are underway, she said.
Dr. Hoagwood highlighted the importance of work on the exposome, which the NIMH has described as a new approach to understanding the mechanisms by which environmental factors alter brain and behavior – starting from prenatal development. Dr. Hoagwood said this approach would collect samples to systematically monitor a range of broad-spectrum environmental exposures. She described it as the “complement to the genomic sequencing.”
She called for launching multisite studies to look at how such factors affect brain development, and where and how early interventions can improve children’s healthy development. Data from community efforts and some of those experiments might, at least initially, be “messy beyond belief,” she said.
“We have to not shy away from it. The genomic sequencing has not shied away from messy data,” Dr. Hoagwood said. “We don’t need to do that, either. We need new methods. We need small experiments of novel payment approaches. We need to use our data systems better.”
Dr. Hoagwood had no financial disclosures to report.
REPORTING FROM AN NIMH CONFERENCE
Sexual minorities seeking abortion report high levels of male violence
Pregnant lesbian and bisexual women who seek abortions are more likely than are their heterosexual counterparts to be the victims of violence by the men who impregnated them, a new study finds.
Rachel K. Jones, PhD, of the Guttmacher Institute, New York, and her associates also found that these sexual minority women, plus a group of individuals who described their sexual orientation as “something else,” were much more likely to report exposure to sexual and physical violence.
“No patient should be presumed to be heterosexual for any reason, including a pregnancy history. All pregnancies – like all patients – should be treated as unique and operating within the dynamic and interconnected circumstances of peoples’ lives, which may encompass differences in sexual orientation and exposure to violence,” the researchers wrote. Their report is in Obstetrics & Gynecology.
Previous research has suggested that nonheterosexual women are more likely than are straight women to become pregnant unintentionally. There also are signs suggesting that they have more abortions, too, although the findings are iffy, the study authors wrote.
For this study, Dr. Jones and her associates examined questionnaire answers of 8,380 women who responded to the Guttmacher Institute’s 2014 Abortion Patient Survey. All were undergoing abortions at 87 U.S. nonhospital facilities that performed 30 or more abortions each per year.
Of the sample, about 9% declined to describe their sexual orientation. Of the rest, 94% described themselves as heterosexual; of those, 41% were white, 28% were black, and 22% were Hispanic. Most were in their 20s, 47% were never married, and 48% had incomes below the federal poverty level.
Women also described themselves as bisexual (4%), “something else” (1%), and lesbian (0.4%). All these groups were more likely than were heterosexuals to be below the federal poverty level; more than half of the lesbian and bisexual respondents said they had previously given birth.
Fifteen percent of lesbians said their current pregnancy was caused by forced sex, compared with 1% of heterosexuals and 3% of bisexuals. (P less than .001).
Bisexuals (9%) and lesbians (33%) were more likely than were heterosexuals (4%) to say the men who impregnated them had physically abused them. The same was true for sexual abuse, which was reported by 7% of bisexuals, 35% of lesbians, and 2% of heterosexuals. After the researchers controlled for various factors including age and race, lesbians remained much more likely to report physical abuse, sexual abuse, and forced sex at the hands of the men who impregnated them (odds ratios = 15, 25, and 10, respectively, P less than .001).
“Exposure to physical and sexual violence was substantially higher among each of the sexual minority groups compared with their heterosexual counterparts, sometimes by a factor of 15 or more,” the study authors wrote. “We found that lesbian respondents had the highest levels of exposure to violence, perhaps because this population was more likely to have had sex with a man only in the context of forced sex.”
The researchers noted that their study has various limitations, such as low numbers of sexual minority women and the 4-year gap since the data were collected.
Still, Dr. Jones and her associates wrote, the study has strengths. “Health care providers, including those working in abortion settings, need to be aware that a proportion of their patient population identifies as something other than heterosexual,” they wrote.
The study was funded by the Susan Thompson Buffett Foundation with support from the National Institutes of Health via a grant to the Guttmacher Center for Population Research Innovation and Dissemination. The study authors reported no relevant financial disclosures.
SOURCE: Jones R et al. Obstet Gynecol. 2018 Sep;132(3):605-11.
Pregnant lesbian and bisexual women who seek abortions are more likely than are their heterosexual counterparts to be the victims of violence by the men who impregnated them, a new study finds.
Rachel K. Jones, PhD, of the Guttmacher Institute, New York, and her associates also found that these sexual minority women, plus a group of individuals who described their sexual orientation as “something else,” were much more likely to report exposure to sexual and physical violence.
“No patient should be presumed to be heterosexual for any reason, including a pregnancy history. All pregnancies – like all patients – should be treated as unique and operating within the dynamic and interconnected circumstances of peoples’ lives, which may encompass differences in sexual orientation and exposure to violence,” the researchers wrote. Their report is in Obstetrics & Gynecology.
Previous research has suggested that nonheterosexual women are more likely than are straight women to become pregnant unintentionally. There also are signs suggesting that they have more abortions, too, although the findings are iffy, the study authors wrote.
For this study, Dr. Jones and her associates examined questionnaire answers of 8,380 women who responded to the Guttmacher Institute’s 2014 Abortion Patient Survey. All were undergoing abortions at 87 U.S. nonhospital facilities that performed 30 or more abortions each per year.
Of the sample, about 9% declined to describe their sexual orientation. Of the rest, 94% described themselves as heterosexual; of those, 41% were white, 28% were black, and 22% were Hispanic. Most were in their 20s, 47% were never married, and 48% had incomes below the federal poverty level.
Women also described themselves as bisexual (4%), “something else” (1%), and lesbian (0.4%). All these groups were more likely than were heterosexuals to be below the federal poverty level; more than half of the lesbian and bisexual respondents said they had previously given birth.
Fifteen percent of lesbians said their current pregnancy was caused by forced sex, compared with 1% of heterosexuals and 3% of bisexuals. (P less than .001).
Bisexuals (9%) and lesbians (33%) were more likely than were heterosexuals (4%) to say the men who impregnated them had physically abused them. The same was true for sexual abuse, which was reported by 7% of bisexuals, 35% of lesbians, and 2% of heterosexuals. After the researchers controlled for various factors including age and race, lesbians remained much more likely to report physical abuse, sexual abuse, and forced sex at the hands of the men who impregnated them (odds ratios = 15, 25, and 10, respectively, P less than .001).
“Exposure to physical and sexual violence was substantially higher among each of the sexual minority groups compared with their heterosexual counterparts, sometimes by a factor of 15 or more,” the study authors wrote. “We found that lesbian respondents had the highest levels of exposure to violence, perhaps because this population was more likely to have had sex with a man only in the context of forced sex.”
The researchers noted that their study has various limitations, such as low numbers of sexual minority women and the 4-year gap since the data were collected.
Still, Dr. Jones and her associates wrote, the study has strengths. “Health care providers, including those working in abortion settings, need to be aware that a proportion of their patient population identifies as something other than heterosexual,” they wrote.
The study was funded by the Susan Thompson Buffett Foundation with support from the National Institutes of Health via a grant to the Guttmacher Center for Population Research Innovation and Dissemination. The study authors reported no relevant financial disclosures.
SOURCE: Jones R et al. Obstet Gynecol. 2018 Sep;132(3):605-11.
Pregnant lesbian and bisexual women who seek abortions are more likely than are their heterosexual counterparts to be the victims of violence by the men who impregnated them, a new study finds.
Rachel K. Jones, PhD, of the Guttmacher Institute, New York, and her associates also found that these sexual minority women, plus a group of individuals who described their sexual orientation as “something else,” were much more likely to report exposure to sexual and physical violence.
“No patient should be presumed to be heterosexual for any reason, including a pregnancy history. All pregnancies – like all patients – should be treated as unique and operating within the dynamic and interconnected circumstances of peoples’ lives, which may encompass differences in sexual orientation and exposure to violence,” the researchers wrote. Their report is in Obstetrics & Gynecology.
Previous research has suggested that nonheterosexual women are more likely than are straight women to become pregnant unintentionally. There also are signs suggesting that they have more abortions, too, although the findings are iffy, the study authors wrote.
For this study, Dr. Jones and her associates examined questionnaire answers of 8,380 women who responded to the Guttmacher Institute’s 2014 Abortion Patient Survey. All were undergoing abortions at 87 U.S. nonhospital facilities that performed 30 or more abortions each per year.
Of the sample, about 9% declined to describe their sexual orientation. Of the rest, 94% described themselves as heterosexual; of those, 41% were white, 28% were black, and 22% were Hispanic. Most were in their 20s, 47% were never married, and 48% had incomes below the federal poverty level.
Women also described themselves as bisexual (4%), “something else” (1%), and lesbian (0.4%). All these groups were more likely than were heterosexuals to be below the federal poverty level; more than half of the lesbian and bisexual respondents said they had previously given birth.
Fifteen percent of lesbians said their current pregnancy was caused by forced sex, compared with 1% of heterosexuals and 3% of bisexuals. (P less than .001).
Bisexuals (9%) and lesbians (33%) were more likely than were heterosexuals (4%) to say the men who impregnated them had physically abused them. The same was true for sexual abuse, which was reported by 7% of bisexuals, 35% of lesbians, and 2% of heterosexuals. After the researchers controlled for various factors including age and race, lesbians remained much more likely to report physical abuse, sexual abuse, and forced sex at the hands of the men who impregnated them (odds ratios = 15, 25, and 10, respectively, P less than .001).
“Exposure to physical and sexual violence was substantially higher among each of the sexual minority groups compared with their heterosexual counterparts, sometimes by a factor of 15 or more,” the study authors wrote. “We found that lesbian respondents had the highest levels of exposure to violence, perhaps because this population was more likely to have had sex with a man only in the context of forced sex.”
The researchers noted that their study has various limitations, such as low numbers of sexual minority women and the 4-year gap since the data were collected.
Still, Dr. Jones and her associates wrote, the study has strengths. “Health care providers, including those working in abortion settings, need to be aware that a proportion of their patient population identifies as something other than heterosexual,” they wrote.
The study was funded by the Susan Thompson Buffett Foundation with support from the National Institutes of Health via a grant to the Guttmacher Center for Population Research Innovation and Dissemination. The study authors reported no relevant financial disclosures.
SOURCE: Jones R et al. Obstet Gynecol. 2018 Sep;132(3):605-11.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point:
Major finding: Fifteen percent of lesbians said their current pregnancy was caused by forced sex, compared with 1% of heterosexuals (P less than .001), and 3% of bisexuals. Lesbians (33%) were more likely than were heterosexuals (4%) to say the man who impregnated them had physically and/or sexually abused them.
Study details: A 2014 survey of 8,380 women seeking abortions at 87 U.S. nonhospital facilities.
Disclosures: The study was funded by the Susan Thompson Buffett Foundation with support from the National Institutes of Health via a grant to the Guttmacher Center for Population Research Innovation and Dissemination. The study authors reported no relevant financial disclosures.
Source: Jones R et al. Obstet Gynecol. 2018 Sep;132(3):605-11.
Three Clinical Studies Demonstrating Safety and Efficacy of Treatment for Diarrhea-Predominant Irritable Bowel Syndrome (IBS-D) in Adults
In this supplement to Clinician Reviews, Christine Frissora, MD, provides an overview of the burdens that patients with IBS-D experience. Three clinical efficacy and safety studies surrounding an FDA-approved treatment are also examined.
Topics include:
- IBS-D diagnosis and treatment challenges
- The role of microbial imbalance and altered gut microbiota
- A treatment option for relief of IBS-D symptoms
XIFI.0273.USA.18
In this supplement to Clinician Reviews, Christine Frissora, MD, provides an overview of the burdens that patients with IBS-D experience. Three clinical efficacy and safety studies surrounding an FDA-approved treatment are also examined.
Topics include:
- IBS-D diagnosis and treatment challenges
- The role of microbial imbalance and altered gut microbiota
- A treatment option for relief of IBS-D symptoms
XIFI.0273.USA.18
In this supplement to Clinician Reviews, Christine Frissora, MD, provides an overview of the burdens that patients with IBS-D experience. Three clinical efficacy and safety studies surrounding an FDA-approved treatment are also examined.
Topics include:
- IBS-D diagnosis and treatment challenges
- The role of microbial imbalance and altered gut microbiota
- A treatment option for relief of IBS-D symptoms
XIFI.0273.USA.18
Three Clinical Studies Demonstrating Safety and Efficacy of Treatment for Diarrhea-Predominant Irritable Bowel Syndrome (IBS-D) in Adults
In this supplement to Family Practice News, Christine Frissora, MD, provides an overview of the burdens that patients with IBS-D experience. Three clinical efficacy and safety studies surrounding an FDA-approved treatment are also examined.
Topics include:
- IBS-D diagnosis and treatment challenges
- The role of microbial imbalance and altered gut microbiota
- A treatment option for relief of IBS-D symptoms
XIFI.0273.USA.18
In this supplement to Family Practice News, Christine Frissora, MD, provides an overview of the burdens that patients with IBS-D experience. Three clinical efficacy and safety studies surrounding an FDA-approved treatment are also examined.
Topics include:
- IBS-D diagnosis and treatment challenges
- The role of microbial imbalance and altered gut microbiota
- A treatment option for relief of IBS-D symptoms
XIFI.0273.USA.18
In this supplement to Family Practice News, Christine Frissora, MD, provides an overview of the burdens that patients with IBS-D experience. Three clinical efficacy and safety studies surrounding an FDA-approved treatment are also examined.
Topics include:
- IBS-D diagnosis and treatment challenges
- The role of microbial imbalance and altered gut microbiota
- A treatment option for relief of IBS-D symptoms
XIFI.0273.USA.18
Three Clinical Studies Demonstrating Safety and Efficacy of Treatment for Diarrhea-Predominant Irritable Bowel Syndrome (IBS-D) in Adults
In this supplement to GI & Hepatology news, Christine Frissora, MD, provides an overview of the burdens that patients with IBS-D experience. Three clinical efficacy and safety studies surrounding an FDA-approved treatment are also examined.
Topics include:
- IBS-D diagnosis and treatment challenges
- The role of microbial imbalance and altered gut microbiota
- A treatment option for relief of IBS-D symptoms
XIFI.0273.USA.18
In this supplement to GI & Hepatology news, Christine Frissora, MD, provides an overview of the burdens that patients with IBS-D experience. Three clinical efficacy and safety studies surrounding an FDA-approved treatment are also examined.
Topics include:
- IBS-D diagnosis and treatment challenges
- The role of microbial imbalance and altered gut microbiota
- A treatment option for relief of IBS-D symptoms
XIFI.0273.USA.18
In this supplement to GI & Hepatology news, Christine Frissora, MD, provides an overview of the burdens that patients with IBS-D experience. Three clinical efficacy and safety studies surrounding an FDA-approved treatment are also examined.
Topics include:
- IBS-D diagnosis and treatment challenges
- The role of microbial imbalance and altered gut microbiota
- A treatment option for relief of IBS-D symptoms
XIFI.0273.USA.18
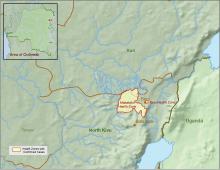
CDC supports Ebola response in DRC
The Centers for Disease Control and Prevention has been working with the Ministry of Health of the Democratic Republic of the Congo (DRC) on a new Ebola outbreak reported on Aug, 1, 2018, in North Kivu province.
“For the current outbreak, CDC has deployed experienced Ebola experts to DRC and the World Health Organization [WHO] to provide guidance on coordination of outbreak response, laboratory testing, disease contact tracing, infection control, and health communication,” according to a CDC press release.
The CDC’s online response also provides a Traveler’s Health notice of Watch Level 1 for the DRC, which advises standard precautions and avoiding infected individuals, but not an advisory against travel.
The Ebola virus associated with the current outbreak is Zaire ebolavirus, according to genetic testing by scientists in the DRC. This is the same species that caused an outbreak earlier this year in Equateur province in northwestern DRC, although differences between the genes of the viruses suggest the two outbreaks are not linked, according to the CDC media announcement.
As of Aug. 12, 2018, the following statistics were reported by the WHO on the outbreak:
- Confirmed cases: 30
- Probable cases: 27
- Total cases: 57
- Deaths: 41 (14 confirmed, 27 probable)
The outbreak is of particular concern because of the instability of the area, which hampers relief and quarantine efforts. The outbreak is in a part of the country identified by the U.S. State Department as a “restricted travel” zone due to armed conflict and violence targeting civilians, according to the CDC.
The Centers for Disease Control and Prevention has been working with the Ministry of Health of the Democratic Republic of the Congo (DRC) on a new Ebola outbreak reported on Aug, 1, 2018, in North Kivu province.
“For the current outbreak, CDC has deployed experienced Ebola experts to DRC and the World Health Organization [WHO] to provide guidance on coordination of outbreak response, laboratory testing, disease contact tracing, infection control, and health communication,” according to a CDC press release.
The CDC’s online response also provides a Traveler’s Health notice of Watch Level 1 for the DRC, which advises standard precautions and avoiding infected individuals, but not an advisory against travel.
The Ebola virus associated with the current outbreak is Zaire ebolavirus, according to genetic testing by scientists in the DRC. This is the same species that caused an outbreak earlier this year in Equateur province in northwestern DRC, although differences between the genes of the viruses suggest the two outbreaks are not linked, according to the CDC media announcement.
As of Aug. 12, 2018, the following statistics were reported by the WHO on the outbreak:
- Confirmed cases: 30
- Probable cases: 27
- Total cases: 57
- Deaths: 41 (14 confirmed, 27 probable)
The outbreak is of particular concern because of the instability of the area, which hampers relief and quarantine efforts. The outbreak is in a part of the country identified by the U.S. State Department as a “restricted travel” zone due to armed conflict and violence targeting civilians, according to the CDC.
The Centers for Disease Control and Prevention has been working with the Ministry of Health of the Democratic Republic of the Congo (DRC) on a new Ebola outbreak reported on Aug, 1, 2018, in North Kivu province.
“For the current outbreak, CDC has deployed experienced Ebola experts to DRC and the World Health Organization [WHO] to provide guidance on coordination of outbreak response, laboratory testing, disease contact tracing, infection control, and health communication,” according to a CDC press release.
The CDC’s online response also provides a Traveler’s Health notice of Watch Level 1 for the DRC, which advises standard precautions and avoiding infected individuals, but not an advisory against travel.
The Ebola virus associated with the current outbreak is Zaire ebolavirus, according to genetic testing by scientists in the DRC. This is the same species that caused an outbreak earlier this year in Equateur province in northwestern DRC, although differences between the genes of the viruses suggest the two outbreaks are not linked, according to the CDC media announcement.
As of Aug. 12, 2018, the following statistics were reported by the WHO on the outbreak:
- Confirmed cases: 30
- Probable cases: 27
- Total cases: 57
- Deaths: 41 (14 confirmed, 27 probable)
The outbreak is of particular concern because of the instability of the area, which hampers relief and quarantine efforts. The outbreak is in a part of the country identified by the U.S. State Department as a “restricted travel” zone due to armed conflict and violence targeting civilians, according to the CDC.
French warn of upsurge in pneumococcal meningitis
MALMO, SWEDEN – A French national study has documented a sharp increase in pneumococcal meningitis since 2015 in children under age 15 years.
The culprit has been identified as serotype 24F, which is not covered by the infant 13-valent conjugate pneumococcal vaccine (PCV13), Naim Ouldali, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
The rapid emergence of serotype 24F has been accompanied by a disturbing change in its penicillin susceptibility. Indeed, penicillin resistance was present in only 18% of serotype 24F isolates in France during 2000-2014, then jumped to 74% during 2015-2016, according to Dr. Ouldali of René Descartes University in Paris.
“PCV13 has strongly reduced the pneumococcal meningitis burden in children, but its benefit now seems to be jeopardized, at least in France. So serum 24F could become a major concern in the coming years because of its characteristics. And now the question is, is this emergence an epidemic phenomenon or not? And if it’s confirmed in future studies and in other countries, probably it should drive the development of next-generation PCV formulations,” he said.
Dr. Ouldali presented a population-based interrupted time-series analysis of a nationwide prospective survey conducted in France during 2001-2016. He noted that the Cochrane Collaboration has deemed this study design second only to the randomized controlled trial in terms of quality of evidence.
The study, which included 227 French pediatric wards and 168 microbiology departments, identified 1,778 children under age 15 years with pneumococcal meningitis. This is believed to be more than 60% of all cases that occurred in the country during the study years.
The purpose of the study was to determine the impact of implementation of routine PCV13 as part of the national vaccine strategy. Rates of PCV13 coverage in French children are very high: in excess of 90% during 2015 to 2016.
Implementation of PCV13 led to a dramatic 38% reduction in the monthly incidence of pneumococcal meningitis, from 0.12 cases per 100,000 children before PCV13 to a low of 0.07 cases per 100,000 in December 2014. But after that the rate rebounded sharply, by 2.3% per month during 2015-2016, to a high of 0.13 cases per 100,000 per month by the end of 2016. Drilling down into the data, Dr. Ouldali and his coinvestigators learned that the resurgence of pneumococcal meningitis was due largely to the emergence of serotype 24F.
“This serotype is of particular concern because of two characteristics: First, it is already known to have a high disease potential – one of the highest, along with serotype 12F – and second, this rapid emergence was accompanied by a change in its penicillin susceptibility,” he noted.
Most of the French rebound in pneumococcal meningitis has occurred in children under 2 years of age. Of note, German investigators also have recently reported a rebound in invasive pneumococcal disease in German children under 16 years of age. Non-PCV13 serotypes accounted for 84% of all invasive pneumococcal disease during 2015-2016, with serotypes 10A and 24F leading the way. As in France, most of the resurgence has involved children less than 2 years old. However, unlike in France, most of the German increase has been in nonmeningitis forms of invasive pneumococcal disease (Vaccine. 2018 Jan 25;36[4]:572-7).
In response to a question from a concerned audience member, Dr. Ouldali said that while the penicillin susceptibility of serotype 24F has taken a sharp turn for the worse, cephalosporin susceptibility has not.
“To date, we have not seen any cephalosporin-resistant strains. To date, there is no need to use vancomycin,” he said.
Dr. Ouldali said the next step he and his colleagues plan to take is to see if there is a clonal expansion or a particular underlying genetic pattern which could explain the explosive emergence of 24F.
The study was funded by a research grant from Pfizer and by the French Pediatric Infectious Diseases Group.
MALMO, SWEDEN – A French national study has documented a sharp increase in pneumococcal meningitis since 2015 in children under age 15 years.
The culprit has been identified as serotype 24F, which is not covered by the infant 13-valent conjugate pneumococcal vaccine (PCV13), Naim Ouldali, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
The rapid emergence of serotype 24F has been accompanied by a disturbing change in its penicillin susceptibility. Indeed, penicillin resistance was present in only 18% of serotype 24F isolates in France during 2000-2014, then jumped to 74% during 2015-2016, according to Dr. Ouldali of René Descartes University in Paris.
“PCV13 has strongly reduced the pneumococcal meningitis burden in children, but its benefit now seems to be jeopardized, at least in France. So serum 24F could become a major concern in the coming years because of its characteristics. And now the question is, is this emergence an epidemic phenomenon or not? And if it’s confirmed in future studies and in other countries, probably it should drive the development of next-generation PCV formulations,” he said.
Dr. Ouldali presented a population-based interrupted time-series analysis of a nationwide prospective survey conducted in France during 2001-2016. He noted that the Cochrane Collaboration has deemed this study design second only to the randomized controlled trial in terms of quality of evidence.
The study, which included 227 French pediatric wards and 168 microbiology departments, identified 1,778 children under age 15 years with pneumococcal meningitis. This is believed to be more than 60% of all cases that occurred in the country during the study years.
The purpose of the study was to determine the impact of implementation of routine PCV13 as part of the national vaccine strategy. Rates of PCV13 coverage in French children are very high: in excess of 90% during 2015 to 2016.
Implementation of PCV13 led to a dramatic 38% reduction in the monthly incidence of pneumococcal meningitis, from 0.12 cases per 100,000 children before PCV13 to a low of 0.07 cases per 100,000 in December 2014. But after that the rate rebounded sharply, by 2.3% per month during 2015-2016, to a high of 0.13 cases per 100,000 per month by the end of 2016. Drilling down into the data, Dr. Ouldali and his coinvestigators learned that the resurgence of pneumococcal meningitis was due largely to the emergence of serotype 24F.
“This serotype is of particular concern because of two characteristics: First, it is already known to have a high disease potential – one of the highest, along with serotype 12F – and second, this rapid emergence was accompanied by a change in its penicillin susceptibility,” he noted.
Most of the French rebound in pneumococcal meningitis has occurred in children under 2 years of age. Of note, German investigators also have recently reported a rebound in invasive pneumococcal disease in German children under 16 years of age. Non-PCV13 serotypes accounted for 84% of all invasive pneumococcal disease during 2015-2016, with serotypes 10A and 24F leading the way. As in France, most of the resurgence has involved children less than 2 years old. However, unlike in France, most of the German increase has been in nonmeningitis forms of invasive pneumococcal disease (Vaccine. 2018 Jan 25;36[4]:572-7).
In response to a question from a concerned audience member, Dr. Ouldali said that while the penicillin susceptibility of serotype 24F has taken a sharp turn for the worse, cephalosporin susceptibility has not.
“To date, we have not seen any cephalosporin-resistant strains. To date, there is no need to use vancomycin,” he said.
Dr. Ouldali said the next step he and his colleagues plan to take is to see if there is a clonal expansion or a particular underlying genetic pattern which could explain the explosive emergence of 24F.
The study was funded by a research grant from Pfizer and by the French Pediatric Infectious Diseases Group.
MALMO, SWEDEN – A French national study has documented a sharp increase in pneumococcal meningitis since 2015 in children under age 15 years.
The culprit has been identified as serotype 24F, which is not covered by the infant 13-valent conjugate pneumococcal vaccine (PCV13), Naim Ouldali, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
The rapid emergence of serotype 24F has been accompanied by a disturbing change in its penicillin susceptibility. Indeed, penicillin resistance was present in only 18% of serotype 24F isolates in France during 2000-2014, then jumped to 74% during 2015-2016, according to Dr. Ouldali of René Descartes University in Paris.
“PCV13 has strongly reduced the pneumococcal meningitis burden in children, but its benefit now seems to be jeopardized, at least in France. So serum 24F could become a major concern in the coming years because of its characteristics. And now the question is, is this emergence an epidemic phenomenon or not? And if it’s confirmed in future studies and in other countries, probably it should drive the development of next-generation PCV formulations,” he said.
Dr. Ouldali presented a population-based interrupted time-series analysis of a nationwide prospective survey conducted in France during 2001-2016. He noted that the Cochrane Collaboration has deemed this study design second only to the randomized controlled trial in terms of quality of evidence.
The study, which included 227 French pediatric wards and 168 microbiology departments, identified 1,778 children under age 15 years with pneumococcal meningitis. This is believed to be more than 60% of all cases that occurred in the country during the study years.
The purpose of the study was to determine the impact of implementation of routine PCV13 as part of the national vaccine strategy. Rates of PCV13 coverage in French children are very high: in excess of 90% during 2015 to 2016.
Implementation of PCV13 led to a dramatic 38% reduction in the monthly incidence of pneumococcal meningitis, from 0.12 cases per 100,000 children before PCV13 to a low of 0.07 cases per 100,000 in December 2014. But after that the rate rebounded sharply, by 2.3% per month during 2015-2016, to a high of 0.13 cases per 100,000 per month by the end of 2016. Drilling down into the data, Dr. Ouldali and his coinvestigators learned that the resurgence of pneumococcal meningitis was due largely to the emergence of serotype 24F.
“This serotype is of particular concern because of two characteristics: First, it is already known to have a high disease potential – one of the highest, along with serotype 12F – and second, this rapid emergence was accompanied by a change in its penicillin susceptibility,” he noted.
Most of the French rebound in pneumococcal meningitis has occurred in children under 2 years of age. Of note, German investigators also have recently reported a rebound in invasive pneumococcal disease in German children under 16 years of age. Non-PCV13 serotypes accounted for 84% of all invasive pneumococcal disease during 2015-2016, with serotypes 10A and 24F leading the way. As in France, most of the resurgence has involved children less than 2 years old. However, unlike in France, most of the German increase has been in nonmeningitis forms of invasive pneumococcal disease (Vaccine. 2018 Jan 25;36[4]:572-7).
In response to a question from a concerned audience member, Dr. Ouldali said that while the penicillin susceptibility of serotype 24F has taken a sharp turn for the worse, cephalosporin susceptibility has not.
“To date, we have not seen any cephalosporin-resistant strains. To date, there is no need to use vancomycin,” he said.
Dr. Ouldali said the next step he and his colleagues plan to take is to see if there is a clonal expansion or a particular underlying genetic pattern which could explain the explosive emergence of 24F.
The study was funded by a research grant from Pfizer and by the French Pediatric Infectious Diseases Group.
REPORTING FROM ESPID 2018
Key clinical point:
Major finding: The incidence of pneumococcal meningitis in French children jumped by 2.3% per month during 2015-2016.
Study details: This population-based interrupted time-series analysis included all 1,778 cases of pneumococcal meningitis in children under age 15 years during 2001-2016 in 227 French pediatric wards.
Disclosures: The study was funded by a grant from Pfizer and by the French Pediatric Infectious Diseases Group.
TBI linked to increased suicide risk
Traumatic brain injury might be associated with an increased risk of suicide, according to results published Aug. 14 in JAMA.
In a retrospective cohort study of 7,418,391 Danish individuals, including 34,529 who died by suicide, patients with medical contact for traumatic brain injury (TBI) had increased suicide risk, compared with the general population (adjusted incidence rate ratio [IRR] = 1.90; 95% confidence interval, 1.83-1.97).
Patients were aged 10 years or older, and follow-up was conducted from Jan. 1, 1980, until date of death, emigration from Denmark, or Dec. 31, 2014, whichever came first. Data were obtained from national registries, including the Danish Civil Registration System, the Database for Integrated Labour Market Research, the National Hospital Register, the Psychiatric Central Research Register, and the Cause of Death Register. Associations between the separate registries were possible because of unique identification numbers assigned to every resident of Denmark, wrote Trine Madsen, PhD, of the Danish Research Institute of Suicide Prevention at the Mental Health Centre Copenhagen, Capital Region of Denmark, and her coauthors.
TBI was recorded in the National Patient Register and was categorized into three types of injury: mild TBI (concussion), skull fracture without documented TBI, and severe TBI (head injury with evidence of structural brain injury). The number of medical contacts for distinct TBI events, accumulated number of days in hospital treatment, age at first TBI, and time since last medical contact for TBI also were assessed.
Data on psychiatric illness and nonfatal self-harm were obtained from the Psychiatric Central Research Register, because of their association with suicide. Data for deaths by suicide were obtained from the Cause of Death Register. Demographic data collected from other registries included sex, age, marital status, cohabitation status, education, and socioeconomic status. IRRs were calculated using adjusted Poisson regression models.
Of 7,418,391 residents of Denmark included in the follow-up period from 1980 to 2014; 567,823 had a TBI diagnosis. Dr. Madsen and her coauthors also found that 423,502 patients (5.7%) were diagnosed with mild TBI, 24,221 (0.3%) with skull fracture, and 120,100 (1.6%) with severe TBI. A total of 34,529 died by suicide.
Of those who died by suicide, 3,536 (10.2%) had a previous TBI diagnosis (2,701 with mild TBI, 174 with skull fracture, and 661 with severe TBI). The absolute rate of suicide in individuals with hospital contact for TBI was 40.6 per 100,000 person-years (95% CI, 39.2-41.9), compared with 19.9 per 100,000 person-years (95% CI, 19.7-20.1) in those with no hospital contact for TBI.
The fully adjusted analysis showed an IRR of 1.90 (95% CI, 1.83-1.97), as well as an increased risk of suicide by TBI severity. The absolute rate for mild TBI was 38.6 per 100,000 person-years (95% CI, 37.1-40.0) with an IRR of 1.81 (95% CI, 1.74-1.88); 42.4 per 100,000 person-years for skull fracture (95% CI, 36.1-48.7) with an IRR of 2.01 (95% CI, 1.73-2.34, P less than .001), and 50.8 per 100,000 person-years for severe TBI (95% CI, 46.9-54.6) with an IRR of 2.38 (95% CI, 2.20-2.58, P less than .001), compared with individuals with no TBI, the authors wrote.
Patients with a first medical contact between 16 and 20 years of age had the highest suicide risk, compared with individuals with no TBI (IRR, 3.01; 95% CI, 2.74-3.30). In addition, individuals who were diagnosed with a psychiatric illness after TBI had a higher risk of suicide than that of those with TBI only (IRR, 4.90; 95% CI, 4.55-5.29; P less than .001), as did those who had engaged in self-harm after TBI (IRR, 7.54; 95% CI, 6.91-8.22; P less than .001). Patients diagnosed with a psychological illness before their TBI had a higher risk of suicide than did those with TBI only (IRR, 2.32; 95% CI, 2.10-2.55; P less than .001), as did those who had engaged self-harm before TBI (IRR, 2.85; 95% CI, 2.53-3.19; P less than .001), the authors noted.
Dr. Madsen and her coauthors cited several limitations. One is that information was not available on which treatment patients with TBIs received. This information “would have been useful to estimate whether different treatment regimens or subsequent follow-up would have reduced the suicide risk,” they wrote.
“Traumatic brain injury is a major public health problem that has many serious consequences, including suicide,” Dr. Madsen and her colleagues wrote. Since falls and traffic accidents account for the largest share of TBIs, helmet use may be a useful protective measure, particularly for injuries related to bicycling and falls that occur at work, the researchers wrote.
“The high prevalence of TBI globally emphasizes the importance for preventing TBI in order to ameliorate its sequelae, such as increased suicide risk,” they concluded.
The study was funded in part by the Mental Health Services Capital Region Denmark and the Lundbeck Foundation. No other disclosures were reported.
SOURCE: Madsen T et al. JAMA. 2018 Aug 14;320(6):580-8.
The results of this study “add to a growing body of evidence pointing to traumatic brain injury (TBI) as an important risk factor for suicide,” Lee Goldstein, MD, PhD, and Ramon Diaz-Arrastia, MD, PhD, wrote in an editorial published with the study (JAMA. 2018 Aug 14;320:[6]:554-6).
The study also stimulates key questions for research, Dr. Goldstein and Dr. Diaz-Arrastia wrote. “How exactly do TBIs increase suicide risk?” they wrote. “What are the substrates and processes that causally link TBI, a highly heterogeneous condition, to a singular catastrophic outcome? The answers are undoubtedly multifactorial and complex.”
Nevertheless, they wrote, the study provides important insights into the “underappreciated relationship” between TBI and suicide, including evidence of a clinical “triad”: TBI history, recent injury, and more numerous post-injury medical contacts for TBI – that may serve as “red flags” for increased suicide risk. “Notably, the results of this study indicate that increased suicide risk is relevant across all TBI severity levels, including the far more common mild injuries. Clinicians, health care professionals, and mental health practitioners must take notice of this important information.”
Dr. Goldstein is affiliated with the department of psychiatry at Boston University and reported no conflicts of interest. Dr. Diaz-Arrastia is affiliated with the department of neurology at the University of Pennsylvania, Philadelphia, and also reported no conflicts of interest.
The results of this study “add to a growing body of evidence pointing to traumatic brain injury (TBI) as an important risk factor for suicide,” Lee Goldstein, MD, PhD, and Ramon Diaz-Arrastia, MD, PhD, wrote in an editorial published with the study (JAMA. 2018 Aug 14;320:[6]:554-6).
The study also stimulates key questions for research, Dr. Goldstein and Dr. Diaz-Arrastia wrote. “How exactly do TBIs increase suicide risk?” they wrote. “What are the substrates and processes that causally link TBI, a highly heterogeneous condition, to a singular catastrophic outcome? The answers are undoubtedly multifactorial and complex.”
Nevertheless, they wrote, the study provides important insights into the “underappreciated relationship” between TBI and suicide, including evidence of a clinical “triad”: TBI history, recent injury, and more numerous post-injury medical contacts for TBI – that may serve as “red flags” for increased suicide risk. “Notably, the results of this study indicate that increased suicide risk is relevant across all TBI severity levels, including the far more common mild injuries. Clinicians, health care professionals, and mental health practitioners must take notice of this important information.”
Dr. Goldstein is affiliated with the department of psychiatry at Boston University and reported no conflicts of interest. Dr. Diaz-Arrastia is affiliated with the department of neurology at the University of Pennsylvania, Philadelphia, and also reported no conflicts of interest.
The results of this study “add to a growing body of evidence pointing to traumatic brain injury (TBI) as an important risk factor for suicide,” Lee Goldstein, MD, PhD, and Ramon Diaz-Arrastia, MD, PhD, wrote in an editorial published with the study (JAMA. 2018 Aug 14;320:[6]:554-6).
The study also stimulates key questions for research, Dr. Goldstein and Dr. Diaz-Arrastia wrote. “How exactly do TBIs increase suicide risk?” they wrote. “What are the substrates and processes that causally link TBI, a highly heterogeneous condition, to a singular catastrophic outcome? The answers are undoubtedly multifactorial and complex.”
Nevertheless, they wrote, the study provides important insights into the “underappreciated relationship” between TBI and suicide, including evidence of a clinical “triad”: TBI history, recent injury, and more numerous post-injury medical contacts for TBI – that may serve as “red flags” for increased suicide risk. “Notably, the results of this study indicate that increased suicide risk is relevant across all TBI severity levels, including the far more common mild injuries. Clinicians, health care professionals, and mental health practitioners must take notice of this important information.”
Dr. Goldstein is affiliated with the department of psychiatry at Boston University and reported no conflicts of interest. Dr. Diaz-Arrastia is affiliated with the department of neurology at the University of Pennsylvania, Philadelphia, and also reported no conflicts of interest.
Traumatic brain injury might be associated with an increased risk of suicide, according to results published Aug. 14 in JAMA.
In a retrospective cohort study of 7,418,391 Danish individuals, including 34,529 who died by suicide, patients with medical contact for traumatic brain injury (TBI) had increased suicide risk, compared with the general population (adjusted incidence rate ratio [IRR] = 1.90; 95% confidence interval, 1.83-1.97).
Patients were aged 10 years or older, and follow-up was conducted from Jan. 1, 1980, until date of death, emigration from Denmark, or Dec. 31, 2014, whichever came first. Data were obtained from national registries, including the Danish Civil Registration System, the Database for Integrated Labour Market Research, the National Hospital Register, the Psychiatric Central Research Register, and the Cause of Death Register. Associations between the separate registries were possible because of unique identification numbers assigned to every resident of Denmark, wrote Trine Madsen, PhD, of the Danish Research Institute of Suicide Prevention at the Mental Health Centre Copenhagen, Capital Region of Denmark, and her coauthors.
TBI was recorded in the National Patient Register and was categorized into three types of injury: mild TBI (concussion), skull fracture without documented TBI, and severe TBI (head injury with evidence of structural brain injury). The number of medical contacts for distinct TBI events, accumulated number of days in hospital treatment, age at first TBI, and time since last medical contact for TBI also were assessed.
Data on psychiatric illness and nonfatal self-harm were obtained from the Psychiatric Central Research Register, because of their association with suicide. Data for deaths by suicide were obtained from the Cause of Death Register. Demographic data collected from other registries included sex, age, marital status, cohabitation status, education, and socioeconomic status. IRRs were calculated using adjusted Poisson regression models.
Of 7,418,391 residents of Denmark included in the follow-up period from 1980 to 2014; 567,823 had a TBI diagnosis. Dr. Madsen and her coauthors also found that 423,502 patients (5.7%) were diagnosed with mild TBI, 24,221 (0.3%) with skull fracture, and 120,100 (1.6%) with severe TBI. A total of 34,529 died by suicide.
Of those who died by suicide, 3,536 (10.2%) had a previous TBI diagnosis (2,701 with mild TBI, 174 with skull fracture, and 661 with severe TBI). The absolute rate of suicide in individuals with hospital contact for TBI was 40.6 per 100,000 person-years (95% CI, 39.2-41.9), compared with 19.9 per 100,000 person-years (95% CI, 19.7-20.1) in those with no hospital contact for TBI.
The fully adjusted analysis showed an IRR of 1.90 (95% CI, 1.83-1.97), as well as an increased risk of suicide by TBI severity. The absolute rate for mild TBI was 38.6 per 100,000 person-years (95% CI, 37.1-40.0) with an IRR of 1.81 (95% CI, 1.74-1.88); 42.4 per 100,000 person-years for skull fracture (95% CI, 36.1-48.7) with an IRR of 2.01 (95% CI, 1.73-2.34, P less than .001), and 50.8 per 100,000 person-years for severe TBI (95% CI, 46.9-54.6) with an IRR of 2.38 (95% CI, 2.20-2.58, P less than .001), compared with individuals with no TBI, the authors wrote.
Patients with a first medical contact between 16 and 20 years of age had the highest suicide risk, compared with individuals with no TBI (IRR, 3.01; 95% CI, 2.74-3.30). In addition, individuals who were diagnosed with a psychiatric illness after TBI had a higher risk of suicide than that of those with TBI only (IRR, 4.90; 95% CI, 4.55-5.29; P less than .001), as did those who had engaged in self-harm after TBI (IRR, 7.54; 95% CI, 6.91-8.22; P less than .001). Patients diagnosed with a psychological illness before their TBI had a higher risk of suicide than did those with TBI only (IRR, 2.32; 95% CI, 2.10-2.55; P less than .001), as did those who had engaged self-harm before TBI (IRR, 2.85; 95% CI, 2.53-3.19; P less than .001), the authors noted.
Dr. Madsen and her coauthors cited several limitations. One is that information was not available on which treatment patients with TBIs received. This information “would have been useful to estimate whether different treatment regimens or subsequent follow-up would have reduced the suicide risk,” they wrote.
“Traumatic brain injury is a major public health problem that has many serious consequences, including suicide,” Dr. Madsen and her colleagues wrote. Since falls and traffic accidents account for the largest share of TBIs, helmet use may be a useful protective measure, particularly for injuries related to bicycling and falls that occur at work, the researchers wrote.
“The high prevalence of TBI globally emphasizes the importance for preventing TBI in order to ameliorate its sequelae, such as increased suicide risk,” they concluded.
The study was funded in part by the Mental Health Services Capital Region Denmark and the Lundbeck Foundation. No other disclosures were reported.
SOURCE: Madsen T et al. JAMA. 2018 Aug 14;320(6):580-8.
Traumatic brain injury might be associated with an increased risk of suicide, according to results published Aug. 14 in JAMA.
In a retrospective cohort study of 7,418,391 Danish individuals, including 34,529 who died by suicide, patients with medical contact for traumatic brain injury (TBI) had increased suicide risk, compared with the general population (adjusted incidence rate ratio [IRR] = 1.90; 95% confidence interval, 1.83-1.97).
Patients were aged 10 years or older, and follow-up was conducted from Jan. 1, 1980, until date of death, emigration from Denmark, or Dec. 31, 2014, whichever came first. Data were obtained from national registries, including the Danish Civil Registration System, the Database for Integrated Labour Market Research, the National Hospital Register, the Psychiatric Central Research Register, and the Cause of Death Register. Associations between the separate registries were possible because of unique identification numbers assigned to every resident of Denmark, wrote Trine Madsen, PhD, of the Danish Research Institute of Suicide Prevention at the Mental Health Centre Copenhagen, Capital Region of Denmark, and her coauthors.
TBI was recorded in the National Patient Register and was categorized into three types of injury: mild TBI (concussion), skull fracture without documented TBI, and severe TBI (head injury with evidence of structural brain injury). The number of medical contacts for distinct TBI events, accumulated number of days in hospital treatment, age at first TBI, and time since last medical contact for TBI also were assessed.
Data on psychiatric illness and nonfatal self-harm were obtained from the Psychiatric Central Research Register, because of their association with suicide. Data for deaths by suicide were obtained from the Cause of Death Register. Demographic data collected from other registries included sex, age, marital status, cohabitation status, education, and socioeconomic status. IRRs were calculated using adjusted Poisson regression models.
Of 7,418,391 residents of Denmark included in the follow-up period from 1980 to 2014; 567,823 had a TBI diagnosis. Dr. Madsen and her coauthors also found that 423,502 patients (5.7%) were diagnosed with mild TBI, 24,221 (0.3%) with skull fracture, and 120,100 (1.6%) with severe TBI. A total of 34,529 died by suicide.
Of those who died by suicide, 3,536 (10.2%) had a previous TBI diagnosis (2,701 with mild TBI, 174 with skull fracture, and 661 with severe TBI). The absolute rate of suicide in individuals with hospital contact for TBI was 40.6 per 100,000 person-years (95% CI, 39.2-41.9), compared with 19.9 per 100,000 person-years (95% CI, 19.7-20.1) in those with no hospital contact for TBI.
The fully adjusted analysis showed an IRR of 1.90 (95% CI, 1.83-1.97), as well as an increased risk of suicide by TBI severity. The absolute rate for mild TBI was 38.6 per 100,000 person-years (95% CI, 37.1-40.0) with an IRR of 1.81 (95% CI, 1.74-1.88); 42.4 per 100,000 person-years for skull fracture (95% CI, 36.1-48.7) with an IRR of 2.01 (95% CI, 1.73-2.34, P less than .001), and 50.8 per 100,000 person-years for severe TBI (95% CI, 46.9-54.6) with an IRR of 2.38 (95% CI, 2.20-2.58, P less than .001), compared with individuals with no TBI, the authors wrote.
Patients with a first medical contact between 16 and 20 years of age had the highest suicide risk, compared with individuals with no TBI (IRR, 3.01; 95% CI, 2.74-3.30). In addition, individuals who were diagnosed with a psychiatric illness after TBI had a higher risk of suicide than that of those with TBI only (IRR, 4.90; 95% CI, 4.55-5.29; P less than .001), as did those who had engaged in self-harm after TBI (IRR, 7.54; 95% CI, 6.91-8.22; P less than .001). Patients diagnosed with a psychological illness before their TBI had a higher risk of suicide than did those with TBI only (IRR, 2.32; 95% CI, 2.10-2.55; P less than .001), as did those who had engaged self-harm before TBI (IRR, 2.85; 95% CI, 2.53-3.19; P less than .001), the authors noted.
Dr. Madsen and her coauthors cited several limitations. One is that information was not available on which treatment patients with TBIs received. This information “would have been useful to estimate whether different treatment regimens or subsequent follow-up would have reduced the suicide risk,” they wrote.
“Traumatic brain injury is a major public health problem that has many serious consequences, including suicide,” Dr. Madsen and her colleagues wrote. Since falls and traffic accidents account for the largest share of TBIs, helmet use may be a useful protective measure, particularly for injuries related to bicycling and falls that occur at work, the researchers wrote.
“The high prevalence of TBI globally emphasizes the importance for preventing TBI in order to ameliorate its sequelae, such as increased suicide risk,” they concluded.
The study was funded in part by the Mental Health Services Capital Region Denmark and the Lundbeck Foundation. No other disclosures were reported.
SOURCE: Madsen T et al. JAMA. 2018 Aug 14;320(6):580-8.
FROM JAMA
Key clinical point: Helmet use might be a useful protective measure against TBI, particularly for injuries related to bicycling and falls that occur at work.
Major finding: Patients with medical contact for TBI had increased suicide risk, compared with the general population (adjusted incidence rate ratio =1.90; 95% confidence interval, 1.83-1.97).
Study details: A retrospective cohort study of 7,418,391 Danish individuals, including 34,529 who died by suicide between 1980 and 2014.
Disclosures: The study was funded in part by the Mental Health Services Capital Region Denmark and the Lundbeck Foundation. No other disclosures were reported.
Source: Madsen T et al. JAMA. 2018 Aug 14;320:(6):580-8.
Long-acting beta2 agonists don’t impact cardiovascular risk factors
Neither heart rate nor blood pressure worsened under long-term use of , according to a post hoc pooled analysis published in Pulmonary Pharmacology & Therapeutics.
The study was conducted by Stefan Andreas, MD, department of cardiology and pneumology, University Medical Centre Göttingen, and Lung Clinic Immenhausen, Germany. The analysis evaluated data from four studies and included a total of 3,104 patients with moderate to very severe COPD, which was defined as Global Initiative for Chronic Obstructive Lung Disease stage 2-4. Patients were randomized to either once-daily olodaterol (5 or 10 mcg), twice-daily formoterol (12 mcg), or placebo. Heart rate and blood pressure were measured before and after dosing at baseline and at four time points during the study: 6 weeks, 12 weeks, 24 weeks, and 48 weeks.
At all time points, the increases seen in the placebo group were greater than seen in the treatment groups; both systolic and diastolic blood pressure showed either slight decreases from or similarities with those seen at baseline, depending on time point. Furthermore, short-term effects were seen around dosing, from before administration to after, although these changes were quantitatively small.
One limitation of the study is that it couldn’t include patients with unstable COPD because of safety reasons; this prevents the findings from being more broadly generalizable.
“These findings, in a large COPD database, speak against the potential negative cardiovascular effects of olodaterol, as well as those of formoterol,” the researchers concluded.
They reported personal fees from various industry entities, such as Novartis, AstraZeneca, and GlaxoSmithKline. Some also reported receiving personal fees from or working for Boehringer Ingelheim, which funded the work.
SOURCE: Andreas S et al. Pulm Pharmacol Ther. 2018 Aug 2. doi: 10.1016/j.pupt.2018.08.002.
Neither heart rate nor blood pressure worsened under long-term use of , according to a post hoc pooled analysis published in Pulmonary Pharmacology & Therapeutics.
The study was conducted by Stefan Andreas, MD, department of cardiology and pneumology, University Medical Centre Göttingen, and Lung Clinic Immenhausen, Germany. The analysis evaluated data from four studies and included a total of 3,104 patients with moderate to very severe COPD, which was defined as Global Initiative for Chronic Obstructive Lung Disease stage 2-4. Patients were randomized to either once-daily olodaterol (5 or 10 mcg), twice-daily formoterol (12 mcg), or placebo. Heart rate and blood pressure were measured before and after dosing at baseline and at four time points during the study: 6 weeks, 12 weeks, 24 weeks, and 48 weeks.
At all time points, the increases seen in the placebo group were greater than seen in the treatment groups; both systolic and diastolic blood pressure showed either slight decreases from or similarities with those seen at baseline, depending on time point. Furthermore, short-term effects were seen around dosing, from before administration to after, although these changes were quantitatively small.
One limitation of the study is that it couldn’t include patients with unstable COPD because of safety reasons; this prevents the findings from being more broadly generalizable.
“These findings, in a large COPD database, speak against the potential negative cardiovascular effects of olodaterol, as well as those of formoterol,” the researchers concluded.
They reported personal fees from various industry entities, such as Novartis, AstraZeneca, and GlaxoSmithKline. Some also reported receiving personal fees from or working for Boehringer Ingelheim, which funded the work.
SOURCE: Andreas S et al. Pulm Pharmacol Ther. 2018 Aug 2. doi: 10.1016/j.pupt.2018.08.002.
Neither heart rate nor blood pressure worsened under long-term use of , according to a post hoc pooled analysis published in Pulmonary Pharmacology & Therapeutics.
The study was conducted by Stefan Andreas, MD, department of cardiology and pneumology, University Medical Centre Göttingen, and Lung Clinic Immenhausen, Germany. The analysis evaluated data from four studies and included a total of 3,104 patients with moderate to very severe COPD, which was defined as Global Initiative for Chronic Obstructive Lung Disease stage 2-4. Patients were randomized to either once-daily olodaterol (5 or 10 mcg), twice-daily formoterol (12 mcg), or placebo. Heart rate and blood pressure were measured before and after dosing at baseline and at four time points during the study: 6 weeks, 12 weeks, 24 weeks, and 48 weeks.
At all time points, the increases seen in the placebo group were greater than seen in the treatment groups; both systolic and diastolic blood pressure showed either slight decreases from or similarities with those seen at baseline, depending on time point. Furthermore, short-term effects were seen around dosing, from before administration to after, although these changes were quantitatively small.
One limitation of the study is that it couldn’t include patients with unstable COPD because of safety reasons; this prevents the findings from being more broadly generalizable.
“These findings, in a large COPD database, speak against the potential negative cardiovascular effects of olodaterol, as well as those of formoterol,” the researchers concluded.
They reported personal fees from various industry entities, such as Novartis, AstraZeneca, and GlaxoSmithKline. Some also reported receiving personal fees from or working for Boehringer Ingelheim, which funded the work.
SOURCE: Andreas S et al. Pulm Pharmacol Ther. 2018 Aug 2. doi: 10.1016/j.pupt.2018.08.002.
FROM PULMONARY PHARMACOLOGY & THERAPEUTICS
Key clinical point: Olodaterol and formoterol had a minimal impact on cardiovascular factors.
Major finding: Patients who were randomized to once-daily olodaterol (5 or 10 mcg), twice-daily formoterol (12 mcg), or placebo showed little change in heart rate and blood pressure at 6, 12, 24, or 48 weeks.
Study details: Post hoc pooled analysis from four studies comprising a total of 3,104 patients with moderate to very severe COPD.
Disclosures: Investigators reported personal fees from various industry entities, such as Novartis, AstraZeneca, and GlaxoSmithKline. Some also reported receiving personal fees from or working for Boehringer Ingelheim.
Source: Andreas S et al. Pulm Pharmacol Ther. 2018 Aug 2. doi: 10.1016/j.pupt.2018.08.002.