User login
Low-dose CT fails to improve small cell lung cancer survival
Early had no significant impact on patient survival, based on data from a randomized trial.
Although early detection of small-cell lung cancer might improve outcomes for patients given the metastatic nature of the disease, previous studies on the value and impact of low-dose CT have been divergent, wrote Anish Thomas, MD, of the National Cancer Institute and his colleagues.
In a study published in Chest, the researchers reviewed characteristics of low-dose CT–detected small cell lung cancer (SCLC) in the randomized National Lung Screening Trial (NLST). The trial compared low-dose CT (LDCT) with chest radiography to assess patients at high risk for developing lung cancer. The study population included adults aged 55-74 years with a history of smoking of at least 30 pack-years, and former smokers who had quit within the past 15 years.
A total of 26,722 participants were randomized to the LDCT treatment arm with a median follow-up of 6.5 years; 143 SCLC cases and 926 non–small cell lung cancer (NSCLC) cases were identified in this group.
Of the 143 SCLC cases, 49 (34.2%) were screen detected, 15 (10.5%) were interval detected and 79 (55.2%) were not screened or were identified post screening.
A cancer was defined as screen detected if it was diagnosed within 1 year of a positive screening or diagnosed after a longer period but with no time gap between diagnostic procedures of more than 1 year; an interval cancer was diagnosed within a year of a negative screen, and non-screened cancers were those found in patients who received no NLST screening or other post screening.
Significantly more of the SCLC cases were at stage III or IV than the NSCLC cases (86% vs. 36%). Unfavorable stage III or IV lung cancers were identified in 80% of screen detected, 86% of interval detected, and 90% of nonscreened or postscreened cases.
In addition, 31 (63%) of the 49 screen-detected SCLC cases had at least one noncalcified nodule in the cancer lobe and 24 (49%) had a single NCN in the cancer lobe.
The 3-year cancer-specific survival rates were not significantly different for screen-detected, interval-detected, and nonscreened or postscreened cases (15.3%, 20.0%, and 13.8%, respectively).
“As expected, the majority of SCLC were late-stage cancers, but surprisingly the unfavorable stage distribution was present regardless of whether the cancer was screen-, interval- or post-screen detected,” the researchers noted.
The findings differed from the results of previous smaller studies showing more favorable stage distribution for SCLC cases detected by LDCT, but this difference may be accounted for by various factors including study populations that were smaller, younger, and lacking well-defined risk factors, the researchers said.
“Our findings underscore and provide further granularity to the premise that SCLC is a very aggressive neoplasm, and contrary to smaller prior studies, indicate that LDCT is an ineffective tool to screen for SCLC,” they said. “If early detection of SCLC were to be realistic, it would need to be detected before [lesions] are visible on LDCT,” they emphasized.
The study was supported in part by the National Cancer Institute. The researchers had no financial conflicts to disclose.
SOURCE: Thomas A et al. Chest. 2018 Aug 3. doi: 10.1016/j.chest.2018.07.029.
Early had no significant impact on patient survival, based on data from a randomized trial.
Although early detection of small-cell lung cancer might improve outcomes for patients given the metastatic nature of the disease, previous studies on the value and impact of low-dose CT have been divergent, wrote Anish Thomas, MD, of the National Cancer Institute and his colleagues.
In a study published in Chest, the researchers reviewed characteristics of low-dose CT–detected small cell lung cancer (SCLC) in the randomized National Lung Screening Trial (NLST). The trial compared low-dose CT (LDCT) with chest radiography to assess patients at high risk for developing lung cancer. The study population included adults aged 55-74 years with a history of smoking of at least 30 pack-years, and former smokers who had quit within the past 15 years.
A total of 26,722 participants were randomized to the LDCT treatment arm with a median follow-up of 6.5 years; 143 SCLC cases and 926 non–small cell lung cancer (NSCLC) cases were identified in this group.
Of the 143 SCLC cases, 49 (34.2%) were screen detected, 15 (10.5%) were interval detected and 79 (55.2%) were not screened or were identified post screening.
A cancer was defined as screen detected if it was diagnosed within 1 year of a positive screening or diagnosed after a longer period but with no time gap between diagnostic procedures of more than 1 year; an interval cancer was diagnosed within a year of a negative screen, and non-screened cancers were those found in patients who received no NLST screening or other post screening.
Significantly more of the SCLC cases were at stage III or IV than the NSCLC cases (86% vs. 36%). Unfavorable stage III or IV lung cancers were identified in 80% of screen detected, 86% of interval detected, and 90% of nonscreened or postscreened cases.
In addition, 31 (63%) of the 49 screen-detected SCLC cases had at least one noncalcified nodule in the cancer lobe and 24 (49%) had a single NCN in the cancer lobe.
The 3-year cancer-specific survival rates were not significantly different for screen-detected, interval-detected, and nonscreened or postscreened cases (15.3%, 20.0%, and 13.8%, respectively).
“As expected, the majority of SCLC were late-stage cancers, but surprisingly the unfavorable stage distribution was present regardless of whether the cancer was screen-, interval- or post-screen detected,” the researchers noted.
The findings differed from the results of previous smaller studies showing more favorable stage distribution for SCLC cases detected by LDCT, but this difference may be accounted for by various factors including study populations that were smaller, younger, and lacking well-defined risk factors, the researchers said.
“Our findings underscore and provide further granularity to the premise that SCLC is a very aggressive neoplasm, and contrary to smaller prior studies, indicate that LDCT is an ineffective tool to screen for SCLC,” they said. “If early detection of SCLC were to be realistic, it would need to be detected before [lesions] are visible on LDCT,” they emphasized.
The study was supported in part by the National Cancer Institute. The researchers had no financial conflicts to disclose.
SOURCE: Thomas A et al. Chest. 2018 Aug 3. doi: 10.1016/j.chest.2018.07.029.
Early had no significant impact on patient survival, based on data from a randomized trial.
Although early detection of small-cell lung cancer might improve outcomes for patients given the metastatic nature of the disease, previous studies on the value and impact of low-dose CT have been divergent, wrote Anish Thomas, MD, of the National Cancer Institute and his colleagues.
In a study published in Chest, the researchers reviewed characteristics of low-dose CT–detected small cell lung cancer (SCLC) in the randomized National Lung Screening Trial (NLST). The trial compared low-dose CT (LDCT) with chest radiography to assess patients at high risk for developing lung cancer. The study population included adults aged 55-74 years with a history of smoking of at least 30 pack-years, and former smokers who had quit within the past 15 years.
A total of 26,722 participants were randomized to the LDCT treatment arm with a median follow-up of 6.5 years; 143 SCLC cases and 926 non–small cell lung cancer (NSCLC) cases were identified in this group.
Of the 143 SCLC cases, 49 (34.2%) were screen detected, 15 (10.5%) were interval detected and 79 (55.2%) were not screened or were identified post screening.
A cancer was defined as screen detected if it was diagnosed within 1 year of a positive screening or diagnosed after a longer period but with no time gap between diagnostic procedures of more than 1 year; an interval cancer was diagnosed within a year of a negative screen, and non-screened cancers were those found in patients who received no NLST screening or other post screening.
Significantly more of the SCLC cases were at stage III or IV than the NSCLC cases (86% vs. 36%). Unfavorable stage III or IV lung cancers were identified in 80% of screen detected, 86% of interval detected, and 90% of nonscreened or postscreened cases.
In addition, 31 (63%) of the 49 screen-detected SCLC cases had at least one noncalcified nodule in the cancer lobe and 24 (49%) had a single NCN in the cancer lobe.
The 3-year cancer-specific survival rates were not significantly different for screen-detected, interval-detected, and nonscreened or postscreened cases (15.3%, 20.0%, and 13.8%, respectively).
“As expected, the majority of SCLC were late-stage cancers, but surprisingly the unfavorable stage distribution was present regardless of whether the cancer was screen-, interval- or post-screen detected,” the researchers noted.
The findings differed from the results of previous smaller studies showing more favorable stage distribution for SCLC cases detected by LDCT, but this difference may be accounted for by various factors including study populations that were smaller, younger, and lacking well-defined risk factors, the researchers said.
“Our findings underscore and provide further granularity to the premise that SCLC is a very aggressive neoplasm, and contrary to smaller prior studies, indicate that LDCT is an ineffective tool to screen for SCLC,” they said. “If early detection of SCLC were to be realistic, it would need to be detected before [lesions] are visible on LDCT,” they emphasized.
The study was supported in part by the National Cancer Institute. The researchers had no financial conflicts to disclose.
SOURCE: Thomas A et al. Chest. 2018 Aug 3. doi: 10.1016/j.chest.2018.07.029.
FROM CHEST
Key clinical point: No significant difference in survival occurred in SCLC cancer patients identified early by low-dose CT vs. those identified at interval or post screening.
Major finding: Unfavorable stage III or IV lung cancers were identified in 80% of screen-detected, 86% of interval-detected, and 90% of nonscreened or postscreened cases.
Study details: The data come from 143 small cell lung cancer cases identified in the randomized National Lung Screening Trial.
Disclosures: The study was supported in part by the National Cancer Institute. The researchers had no financial conflicts to disclose.
Source: Thomas A et al. Chest. 2018 Aug 3. doi: 10.1016/j.chest.2018.07.029.
Pediatricians doing good globally
Do the images of Syrian refugee children kindle a desire to leave the privileged suburban-dwelling patients in your practice for just a few weeks and donate your clinical skills to help the victims of political conflict? Or, are you looking for a way to give your post-retirement life some meaning by volunteering on a typhoon-ravaged island in Southeast Asia?
Before you ask your partners for a 1-month leave of absence or try to convince your spouse that camping out in a refugee camp would be a better way to spend this year’s travel budget, I urge you to read Pediatricians and Global Health: Opportunities and Considerations for Meaningful Engagement, published in Pediatrics. This lengthy and thorough report by the American Board of Pediatrics Global Health Task Force will give you pause and should serve as an important reality check before you run off to buy the plane tickets for your personal mission of mercy.
Gitanjli Arora, MD, of the Children’s Hospital of Los Angeles, and her coauthors point out that, just because there appears to be a need for people with clinical expertise does not mean that your little black bag of skills honed in the privileged bubble of a first-world country is going to be of much help. This question is one I have pondered on several occasions when I have been offered what might be called “medical voluntourism” opportunities.
If I have distinguished myself as a clinician, that skill has been based on my ability to listen and communicate. Dropped into a community where I have little or no language facility and cultural awareness, I would need to rely on my observational skills. While I pride myself on my ability to make a visual diagnosis, the truth is that my diagnostic successes based solely on observation are almost exclusively dermatologic ones. And, let me add, my familiarity with tropical skin rashes is severely limited. I’m sure I could learn but not in a stay as short as a month.
Taking a history and communicating a treatment plan would force me to rely on the skills of a translator. In settings dominated by upheaval, many of the presenting complaints are going to be the result of, or at least heavily colored by, the chaos induced by anxiety. The ability to sort out where the mind and body connect is difficult enough in a community in which I have spent 40 years and speak the language. Regardless of how much sympathy I feel for the victims of tragic events, my clinical skills would have little value in the short term. Even worse, I would probably just be in the way. As the Task Force points out, my presence could also be squandering local resources as on-site providers worked to get me up to speed.
The authors also explore the numerous other complicating factors that must be considered by those of us who feel the pull to help the victims of global strife and natural disaster. While the authors discourage physicians like you and me from “dropping in” to help, they offer an abundance of suggestions on how we can act globally and stay locally.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Do the images of Syrian refugee children kindle a desire to leave the privileged suburban-dwelling patients in your practice for just a few weeks and donate your clinical skills to help the victims of political conflict? Or, are you looking for a way to give your post-retirement life some meaning by volunteering on a typhoon-ravaged island in Southeast Asia?
Before you ask your partners for a 1-month leave of absence or try to convince your spouse that camping out in a refugee camp would be a better way to spend this year’s travel budget, I urge you to read Pediatricians and Global Health: Opportunities and Considerations for Meaningful Engagement, published in Pediatrics. This lengthy and thorough report by the American Board of Pediatrics Global Health Task Force will give you pause and should serve as an important reality check before you run off to buy the plane tickets for your personal mission of mercy.
Gitanjli Arora, MD, of the Children’s Hospital of Los Angeles, and her coauthors point out that, just because there appears to be a need for people with clinical expertise does not mean that your little black bag of skills honed in the privileged bubble of a first-world country is going to be of much help. This question is one I have pondered on several occasions when I have been offered what might be called “medical voluntourism” opportunities.
If I have distinguished myself as a clinician, that skill has been based on my ability to listen and communicate. Dropped into a community where I have little or no language facility and cultural awareness, I would need to rely on my observational skills. While I pride myself on my ability to make a visual diagnosis, the truth is that my diagnostic successes based solely on observation are almost exclusively dermatologic ones. And, let me add, my familiarity with tropical skin rashes is severely limited. I’m sure I could learn but not in a stay as short as a month.
Taking a history and communicating a treatment plan would force me to rely on the skills of a translator. In settings dominated by upheaval, many of the presenting complaints are going to be the result of, or at least heavily colored by, the chaos induced by anxiety. The ability to sort out where the mind and body connect is difficult enough in a community in which I have spent 40 years and speak the language. Regardless of how much sympathy I feel for the victims of tragic events, my clinical skills would have little value in the short term. Even worse, I would probably just be in the way. As the Task Force points out, my presence could also be squandering local resources as on-site providers worked to get me up to speed.
The authors also explore the numerous other complicating factors that must be considered by those of us who feel the pull to help the victims of global strife and natural disaster. While the authors discourage physicians like you and me from “dropping in” to help, they offer an abundance of suggestions on how we can act globally and stay locally.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Do the images of Syrian refugee children kindle a desire to leave the privileged suburban-dwelling patients in your practice for just a few weeks and donate your clinical skills to help the victims of political conflict? Or, are you looking for a way to give your post-retirement life some meaning by volunteering on a typhoon-ravaged island in Southeast Asia?
Before you ask your partners for a 1-month leave of absence or try to convince your spouse that camping out in a refugee camp would be a better way to spend this year’s travel budget, I urge you to read Pediatricians and Global Health: Opportunities and Considerations for Meaningful Engagement, published in Pediatrics. This lengthy and thorough report by the American Board of Pediatrics Global Health Task Force will give you pause and should serve as an important reality check before you run off to buy the plane tickets for your personal mission of mercy.
Gitanjli Arora, MD, of the Children’s Hospital of Los Angeles, and her coauthors point out that, just because there appears to be a need for people with clinical expertise does not mean that your little black bag of skills honed in the privileged bubble of a first-world country is going to be of much help. This question is one I have pondered on several occasions when I have been offered what might be called “medical voluntourism” opportunities.
If I have distinguished myself as a clinician, that skill has been based on my ability to listen and communicate. Dropped into a community where I have little or no language facility and cultural awareness, I would need to rely on my observational skills. While I pride myself on my ability to make a visual diagnosis, the truth is that my diagnostic successes based solely on observation are almost exclusively dermatologic ones. And, let me add, my familiarity with tropical skin rashes is severely limited. I’m sure I could learn but not in a stay as short as a month.
Taking a history and communicating a treatment plan would force me to rely on the skills of a translator. In settings dominated by upheaval, many of the presenting complaints are going to be the result of, or at least heavily colored by, the chaos induced by anxiety. The ability to sort out where the mind and body connect is difficult enough in a community in which I have spent 40 years and speak the language. Regardless of how much sympathy I feel for the victims of tragic events, my clinical skills would have little value in the short term. Even worse, I would probably just be in the way. As the Task Force points out, my presence could also be squandering local resources as on-site providers worked to get me up to speed.
The authors also explore the numerous other complicating factors that must be considered by those of us who feel the pull to help the victims of global strife and natural disaster. While the authors discourage physicians like you and me from “dropping in” to help, they offer an abundance of suggestions on how we can act globally and stay locally.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Employment practices liability insurance
No matter how complete your insurance portfolio, there is one policy – one you probably have never heard of – that you should definitely consider adding to it.
A while ago, I spoke with a dermatologist in California who experienced every employer’s nightmare: he fired an incompetent employee, who promptly sued him for wrongful termination and accused him of sexual harassment to boot. The charges were completely false, and the employee’s transgressions were well documented; but he was not insured against a suit of that type, and defending it would have been prohibitively expensive. He was forced to settle it for a significant sum of money.
Disasters like that are becoming more common. Plaintiffs’ attorneys know that most small businesses, including medical practices, are not insured against internal liability actions – and that settlements are cheaper than litigation.
Fortunately, there is a relatively inexpensive alternative: not covered by conventional liability insurance. These include wrongful termination, sexual harassment, discrimination, breach of employment contract, negligent hiring or evaluation, failure to promote, wrongful discipline, mismanagement of benefits, and the ever-popular “emotional distress.”
EPLI coverage would have permitted the California dermatologist to mount a proper defense against his employee’s groundless charges. In fact, there is a better than even chance that the lawsuit would have been dropped, or never filed to begin with.
Some liability carriers are beginning to cover some employee-related issues in “umbrella” policies, so before looking into EPLI, check your current coverage. Then, as with all insurance, you should shop around for the best price and carefully read the policies on your short list. All EPLI policies cover litigation against your practice and its owners by employees, but some cover only full-timers. Try to obtain the broadest coverage possible so that claims from part-time, temporary, and seasonal employees, and, if possible, even applicants for employment and former employees, also are covered.
You should also look for the most comprehensive policy in terms of coverage. Almost every EPLI policy covers the allegations mentioned above, but some offer a more comprehensive list of covered acts, such as invasion of privacy and defamation of character.
Also be aware of precisely what each policy does not cover. Most exclude punitive damages and court-imposed fines, as well as criminal acts, fraud, and other clearly illegal conduct. For example, you would not be covered if you fired an employee because he or she refused to falsify insurance claims.
Depending on where you practice, it may be necessary to ask an employment attorney to evaluate your individual EPLI needs. An underwriter cannot anticipate every eventuality for you, particularly if he or she does not live in your area and is not familiar with employment conditions in your community.
As with any liability policy, try to get a clause added that permits you to choose your own defense attorney. Better still, pick a specific attorney or firm that you trust and have that counsel named in an endorsement to the policy. Otherwise, the insurance carrier will select an attorney from its own panel who may not consider your interests a higher priority than those of the insurer itself.
If you must accept the insurer’s choice of counsel, you should find out whether that attorney is experienced in employment law, which is a very specialized area. And just as with your malpractice policy, you will want to maintain as much control as possible over the settlement of claims. Ideally, no claim should be settled without your express permission.
As with any insurance policy you buy, be sure to choose an established carrier with ample experience in the field and solid financial strength. A low premium is no bargain if the carrier is new to EPLI or goes broke.
Above all, as with any insurance policy, make sure that you can live with the claims definition and exclusions in the policy you choose, and seek advice if you are unsure what your specific needs are before signing on the dotted line.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
No matter how complete your insurance portfolio, there is one policy – one you probably have never heard of – that you should definitely consider adding to it.
A while ago, I spoke with a dermatologist in California who experienced every employer’s nightmare: he fired an incompetent employee, who promptly sued him for wrongful termination and accused him of sexual harassment to boot. The charges were completely false, and the employee’s transgressions were well documented; but he was not insured against a suit of that type, and defending it would have been prohibitively expensive. He was forced to settle it for a significant sum of money.
Disasters like that are becoming more common. Plaintiffs’ attorneys know that most small businesses, including medical practices, are not insured against internal liability actions – and that settlements are cheaper than litigation.
Fortunately, there is a relatively inexpensive alternative: not covered by conventional liability insurance. These include wrongful termination, sexual harassment, discrimination, breach of employment contract, negligent hiring or evaluation, failure to promote, wrongful discipline, mismanagement of benefits, and the ever-popular “emotional distress.”
EPLI coverage would have permitted the California dermatologist to mount a proper defense against his employee’s groundless charges. In fact, there is a better than even chance that the lawsuit would have been dropped, or never filed to begin with.
Some liability carriers are beginning to cover some employee-related issues in “umbrella” policies, so before looking into EPLI, check your current coverage. Then, as with all insurance, you should shop around for the best price and carefully read the policies on your short list. All EPLI policies cover litigation against your practice and its owners by employees, but some cover only full-timers. Try to obtain the broadest coverage possible so that claims from part-time, temporary, and seasonal employees, and, if possible, even applicants for employment and former employees, also are covered.
You should also look for the most comprehensive policy in terms of coverage. Almost every EPLI policy covers the allegations mentioned above, but some offer a more comprehensive list of covered acts, such as invasion of privacy and defamation of character.
Also be aware of precisely what each policy does not cover. Most exclude punitive damages and court-imposed fines, as well as criminal acts, fraud, and other clearly illegal conduct. For example, you would not be covered if you fired an employee because he or she refused to falsify insurance claims.
Depending on where you practice, it may be necessary to ask an employment attorney to evaluate your individual EPLI needs. An underwriter cannot anticipate every eventuality for you, particularly if he or she does not live in your area and is not familiar with employment conditions in your community.
As with any liability policy, try to get a clause added that permits you to choose your own defense attorney. Better still, pick a specific attorney or firm that you trust and have that counsel named in an endorsement to the policy. Otherwise, the insurance carrier will select an attorney from its own panel who may not consider your interests a higher priority than those of the insurer itself.
If you must accept the insurer’s choice of counsel, you should find out whether that attorney is experienced in employment law, which is a very specialized area. And just as with your malpractice policy, you will want to maintain as much control as possible over the settlement of claims. Ideally, no claim should be settled without your express permission.
As with any insurance policy you buy, be sure to choose an established carrier with ample experience in the field and solid financial strength. A low premium is no bargain if the carrier is new to EPLI or goes broke.
Above all, as with any insurance policy, make sure that you can live with the claims definition and exclusions in the policy you choose, and seek advice if you are unsure what your specific needs are before signing on the dotted line.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
No matter how complete your insurance portfolio, there is one policy – one you probably have never heard of – that you should definitely consider adding to it.
A while ago, I spoke with a dermatologist in California who experienced every employer’s nightmare: he fired an incompetent employee, who promptly sued him for wrongful termination and accused him of sexual harassment to boot. The charges were completely false, and the employee’s transgressions were well documented; but he was not insured against a suit of that type, and defending it would have been prohibitively expensive. He was forced to settle it for a significant sum of money.
Disasters like that are becoming more common. Plaintiffs’ attorneys know that most small businesses, including medical practices, are not insured against internal liability actions – and that settlements are cheaper than litigation.
Fortunately, there is a relatively inexpensive alternative: not covered by conventional liability insurance. These include wrongful termination, sexual harassment, discrimination, breach of employment contract, negligent hiring or evaluation, failure to promote, wrongful discipline, mismanagement of benefits, and the ever-popular “emotional distress.”
EPLI coverage would have permitted the California dermatologist to mount a proper defense against his employee’s groundless charges. In fact, there is a better than even chance that the lawsuit would have been dropped, or never filed to begin with.
Some liability carriers are beginning to cover some employee-related issues in “umbrella” policies, so before looking into EPLI, check your current coverage. Then, as with all insurance, you should shop around for the best price and carefully read the policies on your short list. All EPLI policies cover litigation against your practice and its owners by employees, but some cover only full-timers. Try to obtain the broadest coverage possible so that claims from part-time, temporary, and seasonal employees, and, if possible, even applicants for employment and former employees, also are covered.
You should also look for the most comprehensive policy in terms of coverage. Almost every EPLI policy covers the allegations mentioned above, but some offer a more comprehensive list of covered acts, such as invasion of privacy and defamation of character.
Also be aware of precisely what each policy does not cover. Most exclude punitive damages and court-imposed fines, as well as criminal acts, fraud, and other clearly illegal conduct. For example, you would not be covered if you fired an employee because he or she refused to falsify insurance claims.
Depending on where you practice, it may be necessary to ask an employment attorney to evaluate your individual EPLI needs. An underwriter cannot anticipate every eventuality for you, particularly if he or she does not live in your area and is not familiar with employment conditions in your community.
As with any liability policy, try to get a clause added that permits you to choose your own defense attorney. Better still, pick a specific attorney or firm that you trust and have that counsel named in an endorsement to the policy. Otherwise, the insurance carrier will select an attorney from its own panel who may not consider your interests a higher priority than those of the insurer itself.
If you must accept the insurer’s choice of counsel, you should find out whether that attorney is experienced in employment law, which is a very specialized area. And just as with your malpractice policy, you will want to maintain as much control as possible over the settlement of claims. Ideally, no claim should be settled without your express permission.
As with any insurance policy you buy, be sure to choose an established carrier with ample experience in the field and solid financial strength. A low premium is no bargain if the carrier is new to EPLI or goes broke.
Above all, as with any insurance policy, make sure that you can live with the claims definition and exclusions in the policy you choose, and seek advice if you are unsure what your specific needs are before signing on the dotted line.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Childhood obesity linked to severe dental infections
ATLANTA – Childhood obesity increases the risk of severe dental infections, according to a review presented at the Pediatric Hospital Medicine meeting.
Among 171 children admitted to Rady Children’s Hospital, San Diego, for infected cavities, obese children were almost four times more likely than others to require surgery, and five times more likely to have a tooth pulled.
The average cost for obese children was $13,000/day, versus $10,000/day for nonobese children, probably because of the greater need for surgery. Average length of stay was the same between the two groups, just under 2 days. The findings were statistically significant.
Obesity turns out to be “an important risk factor for invasive interventions for pediatric dental infections,” concluded study leader Michelle Edmunds, MD, a pediatric hospital medicine fellow at Rady.
Childhood obesity has been associated with cavities before, but it’s new information that it increases the severity of dental infections. The finding is something for pediatricians to be aware of and to use to encourage regular dental care. “Even if you are obese, if you are getting routine care, you should be able to have cavities fixed” before they get out of hand. “Unfortunately, a lot of the kids we see don’t get routine care,” Dr. Edmunds said.
The investigators couldn’t assess the role of diet because there wasn’t enough information about it in the medical records. It certainly must play a role, because soda and other junk foods increase the risk of both obesity and cavities.
Other factors also are likely at play. Obesity might affect the oral flora, and perhaps the balance of pathogens. It also seems to reduce healing and infection-fighting ability, so “there might be some immunocompromise that’s playing a role here,” Dr. Edmunds said.
The team compared 25 children up to 18 years old who were at or above the 95th percentile for body mass index – the study definition of obesity – to 146 children who were below that mark. They had all been admitted through the ED between July 2011 and June 2016 with dental abscesses, facial cellulitis, or other dental-associated infections. Eighty percent of the children were on Medicaid, which has, itself, been associated with less frequent visits to the dentist.
About 50% of the children were discharged home without a dental procedure. Among the rest, a quarter had incision and drainage, and a quarter had tooth extractions. Overall 72% (18) of obese children had surgery, usually extractions, versus 43% (62) of nonobese children.
There’s perhaps around 200,000 pediatric ED visits a year in the United States for dental problems. “We’ve had toddlers and kids who have never seen a dentist before; all of their teeth were rotten and had to be pulled out, every single tooth,” Dr. Edmunds said at the meeting sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
The mean age in the study was about 8 years. Nearly 60% of the subjects were boys, and a bit over 60% Hispanic. There were no statistical difference in demographics, prior antibiotic use, or cavity history between obese and nonobese children. Obese children were more likely to be on Medicaid, but not significantly so.
There was no industry funding for the work, and Dr. Edmunds didn’t have any disclosures.
ATLANTA – Childhood obesity increases the risk of severe dental infections, according to a review presented at the Pediatric Hospital Medicine meeting.
Among 171 children admitted to Rady Children’s Hospital, San Diego, for infected cavities, obese children were almost four times more likely than others to require surgery, and five times more likely to have a tooth pulled.
The average cost for obese children was $13,000/day, versus $10,000/day for nonobese children, probably because of the greater need for surgery. Average length of stay was the same between the two groups, just under 2 days. The findings were statistically significant.
Obesity turns out to be “an important risk factor for invasive interventions for pediatric dental infections,” concluded study leader Michelle Edmunds, MD, a pediatric hospital medicine fellow at Rady.
Childhood obesity has been associated with cavities before, but it’s new information that it increases the severity of dental infections. The finding is something for pediatricians to be aware of and to use to encourage regular dental care. “Even if you are obese, if you are getting routine care, you should be able to have cavities fixed” before they get out of hand. “Unfortunately, a lot of the kids we see don’t get routine care,” Dr. Edmunds said.
The investigators couldn’t assess the role of diet because there wasn’t enough information about it in the medical records. It certainly must play a role, because soda and other junk foods increase the risk of both obesity and cavities.
Other factors also are likely at play. Obesity might affect the oral flora, and perhaps the balance of pathogens. It also seems to reduce healing and infection-fighting ability, so “there might be some immunocompromise that’s playing a role here,” Dr. Edmunds said.
The team compared 25 children up to 18 years old who were at or above the 95th percentile for body mass index – the study definition of obesity – to 146 children who were below that mark. They had all been admitted through the ED between July 2011 and June 2016 with dental abscesses, facial cellulitis, or other dental-associated infections. Eighty percent of the children were on Medicaid, which has, itself, been associated with less frequent visits to the dentist.
About 50% of the children were discharged home without a dental procedure. Among the rest, a quarter had incision and drainage, and a quarter had tooth extractions. Overall 72% (18) of obese children had surgery, usually extractions, versus 43% (62) of nonobese children.
There’s perhaps around 200,000 pediatric ED visits a year in the United States for dental problems. “We’ve had toddlers and kids who have never seen a dentist before; all of their teeth were rotten and had to be pulled out, every single tooth,” Dr. Edmunds said at the meeting sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
The mean age in the study was about 8 years. Nearly 60% of the subjects were boys, and a bit over 60% Hispanic. There were no statistical difference in demographics, prior antibiotic use, or cavity history between obese and nonobese children. Obese children were more likely to be on Medicaid, but not significantly so.
There was no industry funding for the work, and Dr. Edmunds didn’t have any disclosures.
ATLANTA – Childhood obesity increases the risk of severe dental infections, according to a review presented at the Pediatric Hospital Medicine meeting.
Among 171 children admitted to Rady Children’s Hospital, San Diego, for infected cavities, obese children were almost four times more likely than others to require surgery, and five times more likely to have a tooth pulled.
The average cost for obese children was $13,000/day, versus $10,000/day for nonobese children, probably because of the greater need for surgery. Average length of stay was the same between the two groups, just under 2 days. The findings were statistically significant.
Obesity turns out to be “an important risk factor for invasive interventions for pediatric dental infections,” concluded study leader Michelle Edmunds, MD, a pediatric hospital medicine fellow at Rady.
Childhood obesity has been associated with cavities before, but it’s new information that it increases the severity of dental infections. The finding is something for pediatricians to be aware of and to use to encourage regular dental care. “Even if you are obese, if you are getting routine care, you should be able to have cavities fixed” before they get out of hand. “Unfortunately, a lot of the kids we see don’t get routine care,” Dr. Edmunds said.
The investigators couldn’t assess the role of diet because there wasn’t enough information about it in the medical records. It certainly must play a role, because soda and other junk foods increase the risk of both obesity and cavities.
Other factors also are likely at play. Obesity might affect the oral flora, and perhaps the balance of pathogens. It also seems to reduce healing and infection-fighting ability, so “there might be some immunocompromise that’s playing a role here,” Dr. Edmunds said.
The team compared 25 children up to 18 years old who were at or above the 95th percentile for body mass index – the study definition of obesity – to 146 children who were below that mark. They had all been admitted through the ED between July 2011 and June 2016 with dental abscesses, facial cellulitis, or other dental-associated infections. Eighty percent of the children were on Medicaid, which has, itself, been associated with less frequent visits to the dentist.
About 50% of the children were discharged home without a dental procedure. Among the rest, a quarter had incision and drainage, and a quarter had tooth extractions. Overall 72% (18) of obese children had surgery, usually extractions, versus 43% (62) of nonobese children.
There’s perhaps around 200,000 pediatric ED visits a year in the United States for dental problems. “We’ve had toddlers and kids who have never seen a dentist before; all of their teeth were rotten and had to be pulled out, every single tooth,” Dr. Edmunds said at the meeting sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
The mean age in the study was about 8 years. Nearly 60% of the subjects were boys, and a bit over 60% Hispanic. There were no statistical difference in demographics, prior antibiotic use, or cavity history between obese and nonobese children. Obese children were more likely to be on Medicaid, but not significantly so.
There was no industry funding for the work, and Dr. Edmunds didn’t have any disclosures.
REPORTING FROM PHM 2018
Key clinical point: Childhood obesity increases the risk of severe dental infections.
Major finding:
Study details: Review of 171 children admitted to Rady Children’s Hospital, San Diego, for infected cavities
Disclosures: There was no industry funding, and the presenter had no disclosures.
Caution urged over real-world bleeding risk with ibrutinib
The Bruton tyrosine kinase inhibitor ibrutinib has been linked to an almost 20-fold increased risk of major bleeding in blood cancer patients taking concomitant antiplatelet and anticoagulation therapy in a clinical setting.
Caution should be used when weighing the risks and benefits of ibrutinib for patients already taking antiplatelet or anticoagulation therapy, or both, wrote Paul R. Kunk, MD, of University of Virginia, Charlottesville, and his colleagues. Their report is in Clinical Lymphoma, Myeloma & Leukemia.
Ibrutinib had been associated with an increased risk of bleeding, albeit low, in the clinical trial setting but the authors suggested that this rate could be higher in everyday clinical practice.
“Much of the information [from clinical trials] on the bleeding risk with ibrutinib, included pooled analyses, was from patients exclusively treated in clinical trials with specific exclusion criteria. These criteria have generally excluded patients with significant comorbidities. However, these patients are seen in clinical practice,” the researchers wrote.
They conducted a review of patients attending their center and associated regional clinics between January 2012 and May 2016. They identified 70 patients, average age 72, who were taking ibrutinib for chronic lymphocytic leukemia (64%) and mantle cell lymphoma (27%), diffuse large B-cell lymphoma (4%), lymphoblastic lymphoma (3%), and Waldenström macroglobulinemia (1%).
The analysis showed that bleeding of any grade occurred in 56% of patients, mostly grade 1-2 bruising and epistaxis. However, major bleeding, defined as grade 3, occurred in 13 patients (19%), a figure that the authors noted was greater than the rate of around 7% reported by clinical trials.
Of these patients, seven were taking combined antiplatelet and anticoagulant therapy, four were taking antiplatelets alone, one was taking an anticoagulant agent alone, and one was taking only ibrutinib.
Univariate analysis showed that the factors associated with an increased risk of major bleeding included antiplatelet or anticoagulant medication, the combination of the two medications or interacting medications, anemia (hemoglobin less than 12 g/dL) and an elevated international normalized ratio (greater than 1.5).
However, in a multivariate analysis, only combined antiplatelet and anticoagulant use (hazard ratio, 20.0; 95% confidence interval, 2.1-200.0; P less than .01) and an elevated INR (HR, 4.6; 95% CI, 1.1-19.6; P less than .01) remained statistically significant.
The researchers said the risk of major bleeding in patients taking both antiplatelet and anticoagulant therapy was “unacceptably high” and “medications other than ibrutinib should be considered” in this patient population.
Overall, they said their findings confirmed “the increasingly recognized risk of major bleeding complications with ibrutinib compared with what was originally reported in the clinical trial setting.
“As ibrutinib increases in use, it is paramount to increase awareness of the known adverse events. This is especially important given the association of ibrutinib use with atrial fibrillation,” they wrote.
They noted that their trial was limited by the relatively small population size. Their finding that platelet count was not associated with bleeding risk was also “counterintuitive,” they noted.
SOURCE: Kunk PR et al. Clin Lymphoma Myeloma Leuk. 2018 Jul 15. doi: 10.1016/j.clml.2018.07.287.
The Bruton tyrosine kinase inhibitor ibrutinib has been linked to an almost 20-fold increased risk of major bleeding in blood cancer patients taking concomitant antiplatelet and anticoagulation therapy in a clinical setting.
Caution should be used when weighing the risks and benefits of ibrutinib for patients already taking antiplatelet or anticoagulation therapy, or both, wrote Paul R. Kunk, MD, of University of Virginia, Charlottesville, and his colleagues. Their report is in Clinical Lymphoma, Myeloma & Leukemia.
Ibrutinib had been associated with an increased risk of bleeding, albeit low, in the clinical trial setting but the authors suggested that this rate could be higher in everyday clinical practice.
“Much of the information [from clinical trials] on the bleeding risk with ibrutinib, included pooled analyses, was from patients exclusively treated in clinical trials with specific exclusion criteria. These criteria have generally excluded patients with significant comorbidities. However, these patients are seen in clinical practice,” the researchers wrote.
They conducted a review of patients attending their center and associated regional clinics between January 2012 and May 2016. They identified 70 patients, average age 72, who were taking ibrutinib for chronic lymphocytic leukemia (64%) and mantle cell lymphoma (27%), diffuse large B-cell lymphoma (4%), lymphoblastic lymphoma (3%), and Waldenström macroglobulinemia (1%).
The analysis showed that bleeding of any grade occurred in 56% of patients, mostly grade 1-2 bruising and epistaxis. However, major bleeding, defined as grade 3, occurred in 13 patients (19%), a figure that the authors noted was greater than the rate of around 7% reported by clinical trials.
Of these patients, seven were taking combined antiplatelet and anticoagulant therapy, four were taking antiplatelets alone, one was taking an anticoagulant agent alone, and one was taking only ibrutinib.
Univariate analysis showed that the factors associated with an increased risk of major bleeding included antiplatelet or anticoagulant medication, the combination of the two medications or interacting medications, anemia (hemoglobin less than 12 g/dL) and an elevated international normalized ratio (greater than 1.5).
However, in a multivariate analysis, only combined antiplatelet and anticoagulant use (hazard ratio, 20.0; 95% confidence interval, 2.1-200.0; P less than .01) and an elevated INR (HR, 4.6; 95% CI, 1.1-19.6; P less than .01) remained statistically significant.
The researchers said the risk of major bleeding in patients taking both antiplatelet and anticoagulant therapy was “unacceptably high” and “medications other than ibrutinib should be considered” in this patient population.
Overall, they said their findings confirmed “the increasingly recognized risk of major bleeding complications with ibrutinib compared with what was originally reported in the clinical trial setting.
“As ibrutinib increases in use, it is paramount to increase awareness of the known adverse events. This is especially important given the association of ibrutinib use with atrial fibrillation,” they wrote.
They noted that their trial was limited by the relatively small population size. Their finding that platelet count was not associated with bleeding risk was also “counterintuitive,” they noted.
SOURCE: Kunk PR et al. Clin Lymphoma Myeloma Leuk. 2018 Jul 15. doi: 10.1016/j.clml.2018.07.287.
The Bruton tyrosine kinase inhibitor ibrutinib has been linked to an almost 20-fold increased risk of major bleeding in blood cancer patients taking concomitant antiplatelet and anticoagulation therapy in a clinical setting.
Caution should be used when weighing the risks and benefits of ibrutinib for patients already taking antiplatelet or anticoagulation therapy, or both, wrote Paul R. Kunk, MD, of University of Virginia, Charlottesville, and his colleagues. Their report is in Clinical Lymphoma, Myeloma & Leukemia.
Ibrutinib had been associated with an increased risk of bleeding, albeit low, in the clinical trial setting but the authors suggested that this rate could be higher in everyday clinical practice.
“Much of the information [from clinical trials] on the bleeding risk with ibrutinib, included pooled analyses, was from patients exclusively treated in clinical trials with specific exclusion criteria. These criteria have generally excluded patients with significant comorbidities. However, these patients are seen in clinical practice,” the researchers wrote.
They conducted a review of patients attending their center and associated regional clinics between January 2012 and May 2016. They identified 70 patients, average age 72, who were taking ibrutinib for chronic lymphocytic leukemia (64%) and mantle cell lymphoma (27%), diffuse large B-cell lymphoma (4%), lymphoblastic lymphoma (3%), and Waldenström macroglobulinemia (1%).
The analysis showed that bleeding of any grade occurred in 56% of patients, mostly grade 1-2 bruising and epistaxis. However, major bleeding, defined as grade 3, occurred in 13 patients (19%), a figure that the authors noted was greater than the rate of around 7% reported by clinical trials.
Of these patients, seven were taking combined antiplatelet and anticoagulant therapy, four were taking antiplatelets alone, one was taking an anticoagulant agent alone, and one was taking only ibrutinib.
Univariate analysis showed that the factors associated with an increased risk of major bleeding included antiplatelet or anticoagulant medication, the combination of the two medications or interacting medications, anemia (hemoglobin less than 12 g/dL) and an elevated international normalized ratio (greater than 1.5).
However, in a multivariate analysis, only combined antiplatelet and anticoagulant use (hazard ratio, 20.0; 95% confidence interval, 2.1-200.0; P less than .01) and an elevated INR (HR, 4.6; 95% CI, 1.1-19.6; P less than .01) remained statistically significant.
The researchers said the risk of major bleeding in patients taking both antiplatelet and anticoagulant therapy was “unacceptably high” and “medications other than ibrutinib should be considered” in this patient population.
Overall, they said their findings confirmed “the increasingly recognized risk of major bleeding complications with ibrutinib compared with what was originally reported in the clinical trial setting.
“As ibrutinib increases in use, it is paramount to increase awareness of the known adverse events. This is especially important given the association of ibrutinib use with atrial fibrillation,” they wrote.
They noted that their trial was limited by the relatively small population size. Their finding that platelet count was not associated with bleeding risk was also “counterintuitive,” they noted.
SOURCE: Kunk PR et al. Clin Lymphoma Myeloma Leuk. 2018 Jul 15. doi: 10.1016/j.clml.2018.07.287.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Key clinical point: Clinicians should exercise caution when prescribing antiplatelet and anticoagulant medications in people taking the Bruton tyrosine kinase inhibitor ibrutinib.
Major finding: The use of both antiplatelet and anticoagulant therapy significantly increased the risk of a major bleed event (HR, 19.2; 95% CI, 2.3-166.7; P less than .01) in patients also taking ibrutinib.
Study details: A retrospective analysis of prescription data from 70 patients seen at a single U.S. cancer center and its regional clinics between January 2012 and May 2016.
Disclosures: Two of the authors reported receiving clinical trial support from Acerta and Abbvie.
Source: Kunk PR et al. Clin Lymphoma Myeloma Leuk. 2018 Jul 15. doi: 10.1016/j.clml.2018.07.287.
Make The Diagnosis - September 2018
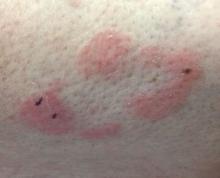
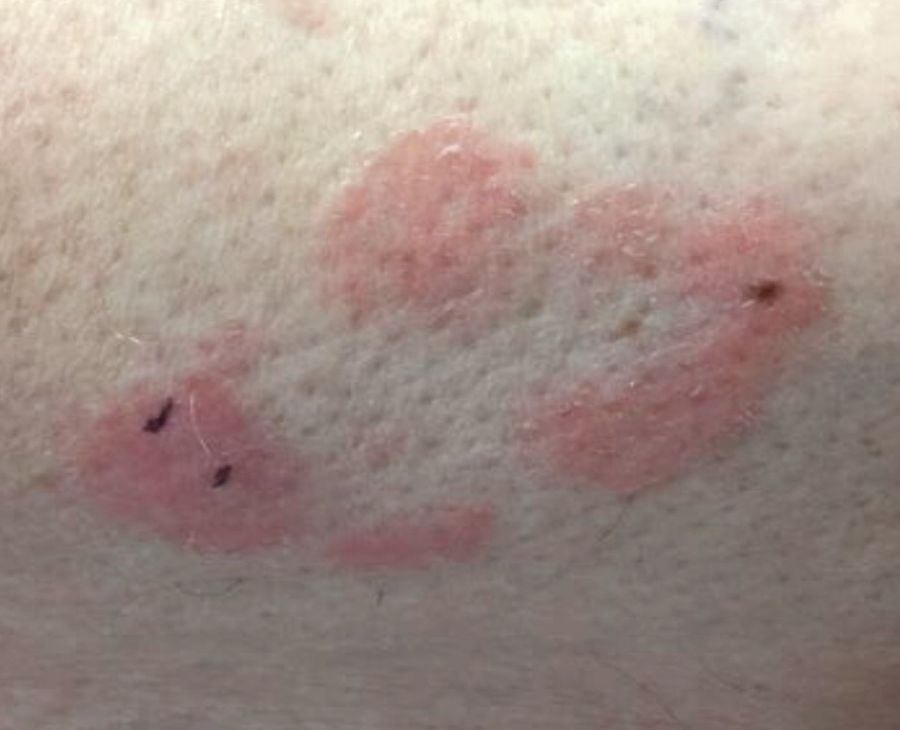
Some have postulated an infectious agent as the cause. Atopic dermatitis may confer an increased risk because of the chronic stimulation of T cells. Males are more commonly affected than females by a 2:1 ratio. A worse prognosis is associated with advanced age. Children and adolescents may be affected as well.
With mycosis fungoides, there are three main types of skin lesions: patch, plaque, and tumor. Patients will progress from patch to plaque to tumor stage in classic MF. Often, lesions begin as scaly, erythematous patches that resemble eczema. Because of the nonspecific nature of early lesions, the median duration from the onset of skin lesions to the diagnosis of MF is 4-6 years. Patch stage lesions may be pruritic or asymptomatic. Commonly, they present in non–sun-exposed areas, such as the buttocks. Annular, infiltrated, red-brown or violaceous plaques can develop, which represent malignant T-cell infiltration. Many patients never progress past the plaque stage. Tumor stage MF is more aggressive, with nodules that may undergo necrosis and ulceration.
The leukemic form of MF is Sézary syndrome. Patients present with pruritic erythroderma and lymphadenopathy. Nail dystrophy, scaling of palms and soles, and alopecia may be present. A peripheral blood smear reveals Sézary cells, which are large, hyperconvoluted lymphocytes. The count of Sézary cells is usually greater than 1000 cells/mm3.
Histology of early lesions may not be diagnostic for CTCL. Often, biopsies will be read as eczematous or psoriasiform for years before the diagnosis of MF is made. Classically, epidermotropism (single-cell exocytosis of lymphocytes into the epidermis) is present. Advanced stages may show a dense infiltrate of lymphocytes in the dermis. Groups of lymphocytes in the epidermis form Pautrier’s microabscesses. Mycosis cells may exhibit cerebriform nuclei. Neoplastic cells in MF are CD3+, CD4+, CD45RO+, CD8–. Tissue can be sent for T-cell gene rearrangement polymerase chain reaction. The presence of monoclonal T-cell gene receptor rearrangements can aid in the diagnosis of MF.
Treatment includes topical steroids, mechlorethamine (nitrogen mustard) or bexarotene gel, PUVA therapy, and narrow-band UVB light for limited and/or patch disease. Localized radiotherapy can be used for more resistant lesions. Topical therapies are preferred in the early stages in MF. Systemic treatments for patients who do not respond to local therapy, or in more advanced disease include methotrexate, interferon-alpha, oral bexarotene, denileukin diftitox, and combination chemotherapy. Photopheresis is reserved for erythrodermic disease.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
Some have postulated an infectious agent as the cause. Atopic dermatitis may confer an increased risk because of the chronic stimulation of T cells. Males are more commonly affected than females by a 2:1 ratio. A worse prognosis is associated with advanced age. Children and adolescents may be affected as well.
With mycosis fungoides, there are three main types of skin lesions: patch, plaque, and tumor. Patients will progress from patch to plaque to tumor stage in classic MF. Often, lesions begin as scaly, erythematous patches that resemble eczema. Because of the nonspecific nature of early lesions, the median duration from the onset of skin lesions to the diagnosis of MF is 4-6 years. Patch stage lesions may be pruritic or asymptomatic. Commonly, they present in non–sun-exposed areas, such as the buttocks. Annular, infiltrated, red-brown or violaceous plaques can develop, which represent malignant T-cell infiltration. Many patients never progress past the plaque stage. Tumor stage MF is more aggressive, with nodules that may undergo necrosis and ulceration.
The leukemic form of MF is Sézary syndrome. Patients present with pruritic erythroderma and lymphadenopathy. Nail dystrophy, scaling of palms and soles, and alopecia may be present. A peripheral blood smear reveals Sézary cells, which are large, hyperconvoluted lymphocytes. The count of Sézary cells is usually greater than 1000 cells/mm3.
Histology of early lesions may not be diagnostic for CTCL. Often, biopsies will be read as eczematous or psoriasiform for years before the diagnosis of MF is made. Classically, epidermotropism (single-cell exocytosis of lymphocytes into the epidermis) is present. Advanced stages may show a dense infiltrate of lymphocytes in the dermis. Groups of lymphocytes in the epidermis form Pautrier’s microabscesses. Mycosis cells may exhibit cerebriform nuclei. Neoplastic cells in MF are CD3+, CD4+, CD45RO+, CD8–. Tissue can be sent for T-cell gene rearrangement polymerase chain reaction. The presence of monoclonal T-cell gene receptor rearrangements can aid in the diagnosis of MF.
Treatment includes topical steroids, mechlorethamine (nitrogen mustard) or bexarotene gel, PUVA therapy, and narrow-band UVB light for limited and/or patch disease. Localized radiotherapy can be used for more resistant lesions. Topical therapies are preferred in the early stages in MF. Systemic treatments for patients who do not respond to local therapy, or in more advanced disease include methotrexate, interferon-alpha, oral bexarotene, denileukin diftitox, and combination chemotherapy. Photopheresis is reserved for erythrodermic disease.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
Some have postulated an infectious agent as the cause. Atopic dermatitis may confer an increased risk because of the chronic stimulation of T cells. Males are more commonly affected than females by a 2:1 ratio. A worse prognosis is associated with advanced age. Children and adolescents may be affected as well.
With mycosis fungoides, there are three main types of skin lesions: patch, plaque, and tumor. Patients will progress from patch to plaque to tumor stage in classic MF. Often, lesions begin as scaly, erythematous patches that resemble eczema. Because of the nonspecific nature of early lesions, the median duration from the onset of skin lesions to the diagnosis of MF is 4-6 years. Patch stage lesions may be pruritic or asymptomatic. Commonly, they present in non–sun-exposed areas, such as the buttocks. Annular, infiltrated, red-brown or violaceous plaques can develop, which represent malignant T-cell infiltration. Many patients never progress past the plaque stage. Tumor stage MF is more aggressive, with nodules that may undergo necrosis and ulceration.
The leukemic form of MF is Sézary syndrome. Patients present with pruritic erythroderma and lymphadenopathy. Nail dystrophy, scaling of palms and soles, and alopecia may be present. A peripheral blood smear reveals Sézary cells, which are large, hyperconvoluted lymphocytes. The count of Sézary cells is usually greater than 1000 cells/mm3.
Histology of early lesions may not be diagnostic for CTCL. Often, biopsies will be read as eczematous or psoriasiform for years before the diagnosis of MF is made. Classically, epidermotropism (single-cell exocytosis of lymphocytes into the epidermis) is present. Advanced stages may show a dense infiltrate of lymphocytes in the dermis. Groups of lymphocytes in the epidermis form Pautrier’s microabscesses. Mycosis cells may exhibit cerebriform nuclei. Neoplastic cells in MF are CD3+, CD4+, CD45RO+, CD8–. Tissue can be sent for T-cell gene rearrangement polymerase chain reaction. The presence of monoclonal T-cell gene receptor rearrangements can aid in the diagnosis of MF.
Treatment includes topical steroids, mechlorethamine (nitrogen mustard) or bexarotene gel, PUVA therapy, and narrow-band UVB light for limited and/or patch disease. Localized radiotherapy can be used for more resistant lesions. Topical therapies are preferred in the early stages in MF. Systemic treatments for patients who do not respond to local therapy, or in more advanced disease include methotrexate, interferon-alpha, oral bexarotene, denileukin diftitox, and combination chemotherapy. Photopheresis is reserved for erythrodermic disease.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
Crystal ball: The future of hospital medicine
Profound changes on the horizon
At HM18 in Orlando, the Society of Hospital Medicine’s CEO Larry Wellikson, MD, MHM, challenged our thinking by sharing a slide with the attendees that effectively and accurately captured the current environment. Today’s largest retailer, Amazon, owns no inventory; today’s largest taxi company, Uber, owns no cars; and today’s largest provider of accommodations, Airbnb, owns no real estate.
This powerful statement captures a transformative way of thinking, functioning, and thriving that has rapidly evolved over the past decade in the United States. And yet, health care fundamentally functions very similarly to how it did 10 years ago. I think we can all acknowledge that this is not a sustainable way to advance.
With megamergers dominating the health care landscape in 2017, the industry has become consolidated to weather the economic challenges ahead. Hospital contribution margins have been declining, forcing systems to critically evaluate how they deliver value-based care. In addition, the joining of forces between Amazon, Berkshire Hathaway, and JPMorgan further illustrates the pressures employers are experiencing with costs in the market.
What can we in hospital medicine do to proactively respond to, and shape, the evolving U.S. health care landscape?
If I had a crystal ball and could predict the future, I would say hospital medicine will be functioning very differently in 10 years to respond to today’s challenges.
The acute becomes more acute
When I started working as a hospitalist more than a decade ago, in a tertiary/quaternary academic medical center, the patients were severely ill with multiple comorbidities. Yet, in the span of 10 years, we care for many of those diagnoses in the ambulatory setting.
Reflecting on the severity of illness in my patients when I was recently on the medicine wards, I have to admit the patients now have a significantly higher burden of disease with twice as many comorbidities. As medicine has advanced and we have become more skilled at caring for patients, the acuity of patients has exponentially increased.
As this trend continues, hospitalists will need greater training in critical care components of hospital-based care. While we may comanage some of these patients with critical care, our skill sets need to intensify to address the growing needs of our patient population.
“Bread and butter” moves to lower-acuity settings and home
As our ability to manage patients advances, and the existing inpatient beds are occupied by sicker patients, the common hospital medicine diagnoses will move to skilled nursing facilities, long-term acute care settings, and ultimately home.
Delivery systems will have to create robust networks of home health and home services to actively manage patients with accountability. This provides an opportunity for hospitalists to manage acutely ill patients in less intense settings of care, and the emergence of telehealth will help facilitate this.
In a Feb. 6, 2018 article in JAMA – “Is it Time for a New Medical Specialty?” – Dr. Michael Nochomovitz and Dr. Rahul Sharma argue that, with rapid advances in technology and the establishment of telemedicine, a new specialty – the virtualist – will need to formally emerge (JAMA. 2018;319[5]:437-8. While telehealth has been successfully utilized for the delivery of acute care in remote regions, as well as the delivery of basic services for common diagnoses, it is not robustly and broadly integrated into all aspects of care delivery.
As we move from the hospital setting to less acute settings of care and home-based care, providers need specific training and skill sets in how to manage and deliver care without the patient in front of them. This includes knowledge of how to remotely manage acutely ill patients who are stable and do not require a hospitalization, as well as effectively managing day-to-day issues that arise with patients.
Translating our role in population health management
I have written previously about the expanding role of hospitalists in population health management. In addition to the transitions of care work that we are all involved in, hospitalists must actively partner with our ambulatory colleagues to identify and communicate key barriers to care.
Hospitalists are already instrumental in a number of institutions providing inpatient and ambulatory care for a select group of patients with high utilization. We have the ability to care for high utilizers and partner with ambulatory providers who can ensure we care for patients with high burdens of disease in the most appropriate settings of care. In the fall of 2018, SHM is convening a group of experts in population health to discuss the role of hospitalists in this area.
While I don’t have a crystal ball to predict the future, sadly, SHM is committed to proactively defining and advancing our specialty. I am confident that together we can find the solutions that will successfully advance us towards the future.
Dr. Afsar is president of the Society of Hospital Medicine, and chief ambulatory officer and chief medical officer for accountable care organizations at UC Irvine Health.
Profound changes on the horizon
Profound changes on the horizon
At HM18 in Orlando, the Society of Hospital Medicine’s CEO Larry Wellikson, MD, MHM, challenged our thinking by sharing a slide with the attendees that effectively and accurately captured the current environment. Today’s largest retailer, Amazon, owns no inventory; today’s largest taxi company, Uber, owns no cars; and today’s largest provider of accommodations, Airbnb, owns no real estate.
This powerful statement captures a transformative way of thinking, functioning, and thriving that has rapidly evolved over the past decade in the United States. And yet, health care fundamentally functions very similarly to how it did 10 years ago. I think we can all acknowledge that this is not a sustainable way to advance.
With megamergers dominating the health care landscape in 2017, the industry has become consolidated to weather the economic challenges ahead. Hospital contribution margins have been declining, forcing systems to critically evaluate how they deliver value-based care. In addition, the joining of forces between Amazon, Berkshire Hathaway, and JPMorgan further illustrates the pressures employers are experiencing with costs in the market.
What can we in hospital medicine do to proactively respond to, and shape, the evolving U.S. health care landscape?
If I had a crystal ball and could predict the future, I would say hospital medicine will be functioning very differently in 10 years to respond to today’s challenges.
The acute becomes more acute
When I started working as a hospitalist more than a decade ago, in a tertiary/quaternary academic medical center, the patients were severely ill with multiple comorbidities. Yet, in the span of 10 years, we care for many of those diagnoses in the ambulatory setting.
Reflecting on the severity of illness in my patients when I was recently on the medicine wards, I have to admit the patients now have a significantly higher burden of disease with twice as many comorbidities. As medicine has advanced and we have become more skilled at caring for patients, the acuity of patients has exponentially increased.
As this trend continues, hospitalists will need greater training in critical care components of hospital-based care. While we may comanage some of these patients with critical care, our skill sets need to intensify to address the growing needs of our patient population.
“Bread and butter” moves to lower-acuity settings and home
As our ability to manage patients advances, and the existing inpatient beds are occupied by sicker patients, the common hospital medicine diagnoses will move to skilled nursing facilities, long-term acute care settings, and ultimately home.
Delivery systems will have to create robust networks of home health and home services to actively manage patients with accountability. This provides an opportunity for hospitalists to manage acutely ill patients in less intense settings of care, and the emergence of telehealth will help facilitate this.
In a Feb. 6, 2018 article in JAMA – “Is it Time for a New Medical Specialty?” – Dr. Michael Nochomovitz and Dr. Rahul Sharma argue that, with rapid advances in technology and the establishment of telemedicine, a new specialty – the virtualist – will need to formally emerge (JAMA. 2018;319[5]:437-8. While telehealth has been successfully utilized for the delivery of acute care in remote regions, as well as the delivery of basic services for common diagnoses, it is not robustly and broadly integrated into all aspects of care delivery.
As we move from the hospital setting to less acute settings of care and home-based care, providers need specific training and skill sets in how to manage and deliver care without the patient in front of them. This includes knowledge of how to remotely manage acutely ill patients who are stable and do not require a hospitalization, as well as effectively managing day-to-day issues that arise with patients.
Translating our role in population health management
I have written previously about the expanding role of hospitalists in population health management. In addition to the transitions of care work that we are all involved in, hospitalists must actively partner with our ambulatory colleagues to identify and communicate key barriers to care.
Hospitalists are already instrumental in a number of institutions providing inpatient and ambulatory care for a select group of patients with high utilization. We have the ability to care for high utilizers and partner with ambulatory providers who can ensure we care for patients with high burdens of disease in the most appropriate settings of care. In the fall of 2018, SHM is convening a group of experts in population health to discuss the role of hospitalists in this area.
While I don’t have a crystal ball to predict the future, sadly, SHM is committed to proactively defining and advancing our specialty. I am confident that together we can find the solutions that will successfully advance us towards the future.
Dr. Afsar is president of the Society of Hospital Medicine, and chief ambulatory officer and chief medical officer for accountable care organizations at UC Irvine Health.
At HM18 in Orlando, the Society of Hospital Medicine’s CEO Larry Wellikson, MD, MHM, challenged our thinking by sharing a slide with the attendees that effectively and accurately captured the current environment. Today’s largest retailer, Amazon, owns no inventory; today’s largest taxi company, Uber, owns no cars; and today’s largest provider of accommodations, Airbnb, owns no real estate.
This powerful statement captures a transformative way of thinking, functioning, and thriving that has rapidly evolved over the past decade in the United States. And yet, health care fundamentally functions very similarly to how it did 10 years ago. I think we can all acknowledge that this is not a sustainable way to advance.
With megamergers dominating the health care landscape in 2017, the industry has become consolidated to weather the economic challenges ahead. Hospital contribution margins have been declining, forcing systems to critically evaluate how they deliver value-based care. In addition, the joining of forces between Amazon, Berkshire Hathaway, and JPMorgan further illustrates the pressures employers are experiencing with costs in the market.
What can we in hospital medicine do to proactively respond to, and shape, the evolving U.S. health care landscape?
If I had a crystal ball and could predict the future, I would say hospital medicine will be functioning very differently in 10 years to respond to today’s challenges.
The acute becomes more acute
When I started working as a hospitalist more than a decade ago, in a tertiary/quaternary academic medical center, the patients were severely ill with multiple comorbidities. Yet, in the span of 10 years, we care for many of those diagnoses in the ambulatory setting.
Reflecting on the severity of illness in my patients when I was recently on the medicine wards, I have to admit the patients now have a significantly higher burden of disease with twice as many comorbidities. As medicine has advanced and we have become more skilled at caring for patients, the acuity of patients has exponentially increased.
As this trend continues, hospitalists will need greater training in critical care components of hospital-based care. While we may comanage some of these patients with critical care, our skill sets need to intensify to address the growing needs of our patient population.
“Bread and butter” moves to lower-acuity settings and home
As our ability to manage patients advances, and the existing inpatient beds are occupied by sicker patients, the common hospital medicine diagnoses will move to skilled nursing facilities, long-term acute care settings, and ultimately home.
Delivery systems will have to create robust networks of home health and home services to actively manage patients with accountability. This provides an opportunity for hospitalists to manage acutely ill patients in less intense settings of care, and the emergence of telehealth will help facilitate this.
In a Feb. 6, 2018 article in JAMA – “Is it Time for a New Medical Specialty?” – Dr. Michael Nochomovitz and Dr. Rahul Sharma argue that, with rapid advances in technology and the establishment of telemedicine, a new specialty – the virtualist – will need to formally emerge (JAMA. 2018;319[5]:437-8. While telehealth has been successfully utilized for the delivery of acute care in remote regions, as well as the delivery of basic services for common diagnoses, it is not robustly and broadly integrated into all aspects of care delivery.
As we move from the hospital setting to less acute settings of care and home-based care, providers need specific training and skill sets in how to manage and deliver care without the patient in front of them. This includes knowledge of how to remotely manage acutely ill patients who are stable and do not require a hospitalization, as well as effectively managing day-to-day issues that arise with patients.
Translating our role in population health management
I have written previously about the expanding role of hospitalists in population health management. In addition to the transitions of care work that we are all involved in, hospitalists must actively partner with our ambulatory colleagues to identify and communicate key barriers to care.
Hospitalists are already instrumental in a number of institutions providing inpatient and ambulatory care for a select group of patients with high utilization. We have the ability to care for high utilizers and partner with ambulatory providers who can ensure we care for patients with high burdens of disease in the most appropriate settings of care. In the fall of 2018, SHM is convening a group of experts in population health to discuss the role of hospitalists in this area.
While I don’t have a crystal ball to predict the future, sadly, SHM is committed to proactively defining and advancing our specialty. I am confident that together we can find the solutions that will successfully advance us towards the future.
Dr. Afsar is president of the Society of Hospital Medicine, and chief ambulatory officer and chief medical officer for accountable care organizations at UC Irvine Health.
ADA underscores distinctions in youth, adult T1DM
Management of type 1 diabetes mellitus in children should include careful consideration of the unique features and challenges that differentiate it from T1DM in adults, according to a new position statement released by the American Diabetes Association.
The statement, published Aug. 10 in Diabetes Care, includes guidance on diagnosis, staging, screening, monitoring, treatment, nutrition, physical activity, and transition from pediatric to adult care.
With regard to diagnosis and staging, the recommendations emphasize the importance of distinguishing between T1DM, type 2 diabetes mellitus, and monogenic diabetes. It also asserts that a pediatric endocrinologist should be consulted before making a diagnosis when “isolated glycosuria or hyperglycemia is discovered in the setting of acute illness and in the absence of classic symptoms,” wrote Jane L. Chiang, MD, of McKinsey & Company and chief medical officer at Diasome Pharmaceuticals in Palo Alto, Calif., and coauthors.
The guidance also describes the three stages of type 1 diabetes development. Stage 1 is presymptomatic and features the presence of beta-cell autoimmunity. Stage 2, also presymptomatic, includes the presence of beta-cell autoimmunity with dysglycemia. Symptomatic disease from insulin deficiency begins in stage 3, and may include hyperglycemia, polyuria, polydipsia, weight loss, polyphagia, fatigue, and blurred vision. Perineal candidiasis is common in girls, and about one-third of cases present with diabetic ketoacidosis (DKA).
In patients with hyperglycemia symptoms, blood glucose, not hemoglobin A1c, should be used to diagnose acute onset of disease. Delays in diagnosis and insulin replacement therapy should be avoided and a definitive diagnosis made quickly, the authors added.
Because the current method of using HbA1c to diagnose diabetes was based on studies limited to adults, there is still debate over whether to use HbA1c to diagnose T1DM in children and adolescents, Dr. Chiang and colleagues noted. Additionally, physicians must take care to distinguish between diabetes types because of increased numbers of overweight children with T1DM, as well as frequent misdiagnosis of monogenic diabetes as T1DM.
The position statement emphasizes the importance of insulin therapy as treatment for children with T1DM and recommends that most patients should be treated with either multiple injections of prandial and basal insulin, or with continuous subcutaneous insulin infusion. HbA1c should be measured at 3-month intervals to assess glycemic control, with a target HbA1c of less than 7.5%, the authors said. Also covered are recommendations for blood glucose monitoring, blood and urine ketone monitoring, and continuous glucose monitoring.
The importance of integrating an exercise and nutrition plan is also highlighted in the guidance. In addition to monitoring carbohydrate and caloric intake with the help of a dietitian, 60 minutes of moderate to vigorous activity daily are recommended as an exercise goal. Steps should also be taken to prevent hypoglycemia during and after exercise, the authors added.
Measures must also be taken to anticipate and address the unique behavioral and social challenges that accompany diabetes management in developing adolescents, the authors said. Social and family issues, peer relationships, and disordered eating should all be considered, and, starting at age 12 years, patients should be allowed time to speak in confidentiality with their health care provider, Dr. Chiang and colleagues said.
Additionally, as adolescents assert increased independence and autonomy, independent disease management should be facilitated, and issues such as depression and risky behaviors discussed.
The guidelines also discuss the importance of following the Centers for Disease Control and Prevention immunization schedule, and monitoring growth and weight gain. Patients with T1DM and their caregivers should also be sufficiently educated on comorbidities such as diabetic ketoacidosis, hypoglycemia, retinopathy, dyslipidemia, autoimmune diseases, and other complications.
Supportive environments such as diabetes camps, as well as technological advances, may be effective tools in encouraging diabetes self-management. Though there is no “optimal transition age” for the shift from pediatric to adult care, ADA recommends that providers begin transition preparation in the early adolescent years, and provide counseling on diabetes self-management.
“An ineffective transition from pediatric to adult diabetes care may contribute to fragmentation of health care and increased risk for adverse outcomes,” the authors said. “An individualized approach to transition timing is recommended, prioritizing the developmental needs and preferences of the patient.”
The authors reported relationships with Diasome Pharmaceuticals and numerous other companies.
SOURCE: Chiang J et al. Diabetes Care. 2018 Jul. doi: 10.2337/dci18-0023.
Management of type 1 diabetes mellitus in children should include careful consideration of the unique features and challenges that differentiate it from T1DM in adults, according to a new position statement released by the American Diabetes Association.
The statement, published Aug. 10 in Diabetes Care, includes guidance on diagnosis, staging, screening, monitoring, treatment, nutrition, physical activity, and transition from pediatric to adult care.
With regard to diagnosis and staging, the recommendations emphasize the importance of distinguishing between T1DM, type 2 diabetes mellitus, and monogenic diabetes. It also asserts that a pediatric endocrinologist should be consulted before making a diagnosis when “isolated glycosuria or hyperglycemia is discovered in the setting of acute illness and in the absence of classic symptoms,” wrote Jane L. Chiang, MD, of McKinsey & Company and chief medical officer at Diasome Pharmaceuticals in Palo Alto, Calif., and coauthors.
The guidance also describes the three stages of type 1 diabetes development. Stage 1 is presymptomatic and features the presence of beta-cell autoimmunity. Stage 2, also presymptomatic, includes the presence of beta-cell autoimmunity with dysglycemia. Symptomatic disease from insulin deficiency begins in stage 3, and may include hyperglycemia, polyuria, polydipsia, weight loss, polyphagia, fatigue, and blurred vision. Perineal candidiasis is common in girls, and about one-third of cases present with diabetic ketoacidosis (DKA).
In patients with hyperglycemia symptoms, blood glucose, not hemoglobin A1c, should be used to diagnose acute onset of disease. Delays in diagnosis and insulin replacement therapy should be avoided and a definitive diagnosis made quickly, the authors added.
Because the current method of using HbA1c to diagnose diabetes was based on studies limited to adults, there is still debate over whether to use HbA1c to diagnose T1DM in children and adolescents, Dr. Chiang and colleagues noted. Additionally, physicians must take care to distinguish between diabetes types because of increased numbers of overweight children with T1DM, as well as frequent misdiagnosis of monogenic diabetes as T1DM.
The position statement emphasizes the importance of insulin therapy as treatment for children with T1DM and recommends that most patients should be treated with either multiple injections of prandial and basal insulin, or with continuous subcutaneous insulin infusion. HbA1c should be measured at 3-month intervals to assess glycemic control, with a target HbA1c of less than 7.5%, the authors said. Also covered are recommendations for blood glucose monitoring, blood and urine ketone monitoring, and continuous glucose monitoring.
The importance of integrating an exercise and nutrition plan is also highlighted in the guidance. In addition to monitoring carbohydrate and caloric intake with the help of a dietitian, 60 minutes of moderate to vigorous activity daily are recommended as an exercise goal. Steps should also be taken to prevent hypoglycemia during and after exercise, the authors added.
Measures must also be taken to anticipate and address the unique behavioral and social challenges that accompany diabetes management in developing adolescents, the authors said. Social and family issues, peer relationships, and disordered eating should all be considered, and, starting at age 12 years, patients should be allowed time to speak in confidentiality with their health care provider, Dr. Chiang and colleagues said.
Additionally, as adolescents assert increased independence and autonomy, independent disease management should be facilitated, and issues such as depression and risky behaviors discussed.
The guidelines also discuss the importance of following the Centers for Disease Control and Prevention immunization schedule, and monitoring growth and weight gain. Patients with T1DM and their caregivers should also be sufficiently educated on comorbidities such as diabetic ketoacidosis, hypoglycemia, retinopathy, dyslipidemia, autoimmune diseases, and other complications.
Supportive environments such as diabetes camps, as well as technological advances, may be effective tools in encouraging diabetes self-management. Though there is no “optimal transition age” for the shift from pediatric to adult care, ADA recommends that providers begin transition preparation in the early adolescent years, and provide counseling on diabetes self-management.
“An ineffective transition from pediatric to adult diabetes care may contribute to fragmentation of health care and increased risk for adverse outcomes,” the authors said. “An individualized approach to transition timing is recommended, prioritizing the developmental needs and preferences of the patient.”
The authors reported relationships with Diasome Pharmaceuticals and numerous other companies.
SOURCE: Chiang J et al. Diabetes Care. 2018 Jul. doi: 10.2337/dci18-0023.
Management of type 1 diabetes mellitus in children should include careful consideration of the unique features and challenges that differentiate it from T1DM in adults, according to a new position statement released by the American Diabetes Association.
The statement, published Aug. 10 in Diabetes Care, includes guidance on diagnosis, staging, screening, monitoring, treatment, nutrition, physical activity, and transition from pediatric to adult care.
With regard to diagnosis and staging, the recommendations emphasize the importance of distinguishing between T1DM, type 2 diabetes mellitus, and monogenic diabetes. It also asserts that a pediatric endocrinologist should be consulted before making a diagnosis when “isolated glycosuria or hyperglycemia is discovered in the setting of acute illness and in the absence of classic symptoms,” wrote Jane L. Chiang, MD, of McKinsey & Company and chief medical officer at Diasome Pharmaceuticals in Palo Alto, Calif., and coauthors.
The guidance also describes the three stages of type 1 diabetes development. Stage 1 is presymptomatic and features the presence of beta-cell autoimmunity. Stage 2, also presymptomatic, includes the presence of beta-cell autoimmunity with dysglycemia. Symptomatic disease from insulin deficiency begins in stage 3, and may include hyperglycemia, polyuria, polydipsia, weight loss, polyphagia, fatigue, and blurred vision. Perineal candidiasis is common in girls, and about one-third of cases present with diabetic ketoacidosis (DKA).
In patients with hyperglycemia symptoms, blood glucose, not hemoglobin A1c, should be used to diagnose acute onset of disease. Delays in diagnosis and insulin replacement therapy should be avoided and a definitive diagnosis made quickly, the authors added.
Because the current method of using HbA1c to diagnose diabetes was based on studies limited to adults, there is still debate over whether to use HbA1c to diagnose T1DM in children and adolescents, Dr. Chiang and colleagues noted. Additionally, physicians must take care to distinguish between diabetes types because of increased numbers of overweight children with T1DM, as well as frequent misdiagnosis of monogenic diabetes as T1DM.
The position statement emphasizes the importance of insulin therapy as treatment for children with T1DM and recommends that most patients should be treated with either multiple injections of prandial and basal insulin, or with continuous subcutaneous insulin infusion. HbA1c should be measured at 3-month intervals to assess glycemic control, with a target HbA1c of less than 7.5%, the authors said. Also covered are recommendations for blood glucose monitoring, blood and urine ketone monitoring, and continuous glucose monitoring.
The importance of integrating an exercise and nutrition plan is also highlighted in the guidance. In addition to monitoring carbohydrate and caloric intake with the help of a dietitian, 60 minutes of moderate to vigorous activity daily are recommended as an exercise goal. Steps should also be taken to prevent hypoglycemia during and after exercise, the authors added.
Measures must also be taken to anticipate and address the unique behavioral and social challenges that accompany diabetes management in developing adolescents, the authors said. Social and family issues, peer relationships, and disordered eating should all be considered, and, starting at age 12 years, patients should be allowed time to speak in confidentiality with their health care provider, Dr. Chiang and colleagues said.
Additionally, as adolescents assert increased independence and autonomy, independent disease management should be facilitated, and issues such as depression and risky behaviors discussed.
The guidelines also discuss the importance of following the Centers for Disease Control and Prevention immunization schedule, and monitoring growth and weight gain. Patients with T1DM and their caregivers should also be sufficiently educated on comorbidities such as diabetic ketoacidosis, hypoglycemia, retinopathy, dyslipidemia, autoimmune diseases, and other complications.
Supportive environments such as diabetes camps, as well as technological advances, may be effective tools in encouraging diabetes self-management. Though there is no “optimal transition age” for the shift from pediatric to adult care, ADA recommends that providers begin transition preparation in the early adolescent years, and provide counseling on diabetes self-management.
“An ineffective transition from pediatric to adult diabetes care may contribute to fragmentation of health care and increased risk for adverse outcomes,” the authors said. “An individualized approach to transition timing is recommended, prioritizing the developmental needs and preferences of the patient.”
The authors reported relationships with Diasome Pharmaceuticals and numerous other companies.
SOURCE: Chiang J et al. Diabetes Care. 2018 Jul. doi: 10.2337/dci18-0023.
FROM DIABETES CARE
Key clinical point: Management of type 1 diabetes in children and adolescents should take into account the unique challenges of disease management in that age group, and facilitate an effective transition to adult care.
Major finding: The position statement emphasizes the importance of insulin therapy as treatment for children with T1DM and the importance of integrating an exercise and nutrition plan.
Study details: An analysis of numerous diabetes studies and clinical trials.
Disclosures: The authors reported relationships with Diasome Pharmaceuticals and numerous other companies.
Source: Chiang J et al. Diabetes Care. 2018 Jul. doi: 10.2337/dci18-0023.
Variants in five genes signal TNBC risk
Women with germline pathogenic variants in five genes are at high risk for developing triple-negative breast cancer, and have a greater than 20% lifetime risk for breast cancer in general, results of a large study suggest.
Multigene testing of nearly 11,000 women with triple-negative breast cancer (TNBC; lacking estrogen, progesterone, and human epidermal growth factor receptors) showed that germline pathogenic variants in BARD1, BRCA1, BRCA2, PALB2, and RAD51D were associated with risk for clinical TNBC ranging from nearly sixfold to more than 16-fold higher than that of women without the genetic variants, reported Fergus J. Couch, PhD, of the Mayo Clinic, Rochester, Minn., and his colleagues.
“The results suggest that all TNBC patients should undergo multigene panel testing, regardless of age at diagnosis or family history of cancer, for improved cancer risk assessment and because of the ongoing development of targeted therapeutic approaches for TNBC patients with mutations in predisposition genes,” they wrote. Their report is in the Journal of the National Cancer Institute.
Although National Comprehensive Cancer Network guidelines recommend testing for the cancer predisposition genes BRCA1 and BRCA2 in women with a TNBC diagnosis at age 60 or younger or those with a family history of breast and/or ovarian cancer, the picture is less clear regarding genetic predisposition to TNBC, the authors noted.
“[R]ecommendations for testing of other genes are not fully established because the risks of TNBC associated with mutations in cancer predisposition genes have not been established. Thus, a better understanding of gene-specific risks for TNBC is needed to identify the genes that should be tested in the setting of TNBC,” they wrote.
The investigators looked for associations between deleterious mutations in cancer predisposition genes and TNBC among 8,753 patients with TNBC testing with a 21-gene assay, and among 2,148 women tested for 17 genes in studies conducted by the Triple Negative Breast Cancer Consortium.
They found that among white women, germline pathogenic variants were associated with the following odds ratios (OR) for TNBC (all P values less than .0001):
- BRCA2 = 5.42
- BARD1 = 5.92
- RAD51D = 6.97
- PALB2 = 14.41
- BRCA1 = 16.27
Although there were insufficient data on the risks for African American women, an exploratory analysis showed that risks for TNBC associated with specific pathogenic variants were similar to those for white women, the authors said.
The pathogenic variants were detected in 12% of all patients in the study.
“Continued study of gene-specific risks for breast cancer subtypes may lead to tailored medical management recommendations for PV [pathogenic variant] carriers. Consistent with this hypothesis, initial studies evaluating intensified screening in high-risk women have suggested that a decrease in mortality from TNBC can be achieved,” they wrote.
The study was supported in part by the National Institutes of Health and the Breast Cancer Research Foundation, and was sponsored by Ambry Genetics Inc. The authors reported having no conflicts of interest.
SOURCE: Shimelis H et al. J Natl Cancer Inst. 2018 Aug 7. doi: 10.1093/jnci/djy106.
Women with germline pathogenic variants in five genes are at high risk for developing triple-negative breast cancer, and have a greater than 20% lifetime risk for breast cancer in general, results of a large study suggest.
Multigene testing of nearly 11,000 women with triple-negative breast cancer (TNBC; lacking estrogen, progesterone, and human epidermal growth factor receptors) showed that germline pathogenic variants in BARD1, BRCA1, BRCA2, PALB2, and RAD51D were associated with risk for clinical TNBC ranging from nearly sixfold to more than 16-fold higher than that of women without the genetic variants, reported Fergus J. Couch, PhD, of the Mayo Clinic, Rochester, Minn., and his colleagues.
“The results suggest that all TNBC patients should undergo multigene panel testing, regardless of age at diagnosis or family history of cancer, for improved cancer risk assessment and because of the ongoing development of targeted therapeutic approaches for TNBC patients with mutations in predisposition genes,” they wrote. Their report is in the Journal of the National Cancer Institute.
Although National Comprehensive Cancer Network guidelines recommend testing for the cancer predisposition genes BRCA1 and BRCA2 in women with a TNBC diagnosis at age 60 or younger or those with a family history of breast and/or ovarian cancer, the picture is less clear regarding genetic predisposition to TNBC, the authors noted.
“[R]ecommendations for testing of other genes are not fully established because the risks of TNBC associated with mutations in cancer predisposition genes have not been established. Thus, a better understanding of gene-specific risks for TNBC is needed to identify the genes that should be tested in the setting of TNBC,” they wrote.
The investigators looked for associations between deleterious mutations in cancer predisposition genes and TNBC among 8,753 patients with TNBC testing with a 21-gene assay, and among 2,148 women tested for 17 genes in studies conducted by the Triple Negative Breast Cancer Consortium.
They found that among white women, germline pathogenic variants were associated with the following odds ratios (OR) for TNBC (all P values less than .0001):
- BRCA2 = 5.42
- BARD1 = 5.92
- RAD51D = 6.97
- PALB2 = 14.41
- BRCA1 = 16.27
Although there were insufficient data on the risks for African American women, an exploratory analysis showed that risks for TNBC associated with specific pathogenic variants were similar to those for white women, the authors said.
The pathogenic variants were detected in 12% of all patients in the study.
“Continued study of gene-specific risks for breast cancer subtypes may lead to tailored medical management recommendations for PV [pathogenic variant] carriers. Consistent with this hypothesis, initial studies evaluating intensified screening in high-risk women have suggested that a decrease in mortality from TNBC can be achieved,” they wrote.
The study was supported in part by the National Institutes of Health and the Breast Cancer Research Foundation, and was sponsored by Ambry Genetics Inc. The authors reported having no conflicts of interest.
SOURCE: Shimelis H et al. J Natl Cancer Inst. 2018 Aug 7. doi: 10.1093/jnci/djy106.
Women with germline pathogenic variants in five genes are at high risk for developing triple-negative breast cancer, and have a greater than 20% lifetime risk for breast cancer in general, results of a large study suggest.
Multigene testing of nearly 11,000 women with triple-negative breast cancer (TNBC; lacking estrogen, progesterone, and human epidermal growth factor receptors) showed that germline pathogenic variants in BARD1, BRCA1, BRCA2, PALB2, and RAD51D were associated with risk for clinical TNBC ranging from nearly sixfold to more than 16-fold higher than that of women without the genetic variants, reported Fergus J. Couch, PhD, of the Mayo Clinic, Rochester, Minn., and his colleagues.
“The results suggest that all TNBC patients should undergo multigene panel testing, regardless of age at diagnosis or family history of cancer, for improved cancer risk assessment and because of the ongoing development of targeted therapeutic approaches for TNBC patients with mutations in predisposition genes,” they wrote. Their report is in the Journal of the National Cancer Institute.
Although National Comprehensive Cancer Network guidelines recommend testing for the cancer predisposition genes BRCA1 and BRCA2 in women with a TNBC diagnosis at age 60 or younger or those with a family history of breast and/or ovarian cancer, the picture is less clear regarding genetic predisposition to TNBC, the authors noted.
“[R]ecommendations for testing of other genes are not fully established because the risks of TNBC associated with mutations in cancer predisposition genes have not been established. Thus, a better understanding of gene-specific risks for TNBC is needed to identify the genes that should be tested in the setting of TNBC,” they wrote.
The investigators looked for associations between deleterious mutations in cancer predisposition genes and TNBC among 8,753 patients with TNBC testing with a 21-gene assay, and among 2,148 women tested for 17 genes in studies conducted by the Triple Negative Breast Cancer Consortium.
They found that among white women, germline pathogenic variants were associated with the following odds ratios (OR) for TNBC (all P values less than .0001):
- BRCA2 = 5.42
- BARD1 = 5.92
- RAD51D = 6.97
- PALB2 = 14.41
- BRCA1 = 16.27
Although there were insufficient data on the risks for African American women, an exploratory analysis showed that risks for TNBC associated with specific pathogenic variants were similar to those for white women, the authors said.
The pathogenic variants were detected in 12% of all patients in the study.
“Continued study of gene-specific risks for breast cancer subtypes may lead to tailored medical management recommendations for PV [pathogenic variant] carriers. Consistent with this hypothesis, initial studies evaluating intensified screening in high-risk women have suggested that a decrease in mortality from TNBC can be achieved,” they wrote.
The study was supported in part by the National Institutes of Health and the Breast Cancer Research Foundation, and was sponsored by Ambry Genetics Inc. The authors reported having no conflicts of interest.
SOURCE: Shimelis H et al. J Natl Cancer Inst. 2018 Aug 7. doi: 10.1093/jnci/djy106.
FROM JOURNAL OF THE NATIONAL CANCER INSTITUTE
Key clinical point: Pathogenic variants in five cancer predisposition genes are associated with significantly increased risk for triple negative breast cancer (TNBC).
Major finding: Pathogenic variants in BRCA1 were associated with a more than 16-fold risk for TNBC.
Study details: Retrospective review of multigene assay testing in 10,901 women with TNBC.
Disclosures: The study was supported in part by the National Institutes of Health and the Breast Cancer Research Foundation, and was sponsored by Ambry Genetics Inc. The authors reported having no conflicts of interest.
Source: Shimelis H et al. J Natl Cancer Inst. 2018 Aug 7. doi: 10.1093/jnci/djy106.
Docs push back on step therapy in Medicare Advantage
A new policy that allows Medicare Advantage plans to use step therapy to control spending on prescription drug administered in the office is not going over well with doctors.
The Centers for Medicare & Medicaid Services announced the policy change Aug. 7, which will give Medicare Advantage plan sponsors the “choice of implementing step therapy to manage Part B drugs, beginning Jan. 1, 2019,” the agency said in a statement. Step therapy, as described by the announcement “is a type of prior authorization for drugs that begins medication for a medical condition with the most preferred drug therapy and progresses to other therapies only if necessary, promoting better clinical decisions.”
Doctors aren’t having it.
“Put simply, this policy change is a gross affront to America’s sickest Medicare patients – individuals living with diseases like inflammatory arthritis and cancer – who depend on timely access to safe, affordable, and high-quality treatments,” American College of Rheumatology President David Daikh, MD, PhD, said in a statement.
“Utilization management techniques like step therapy prevent and delay important treatments for rheumatic disease patients, which can result in irreversible joint or organ damage,” Dr. Daikh continued. “At the same time that medical research is showing that early institution of effective treatment prevents such damage, CMS is instituting a policy that will makes it much more difficult for patients to get this treatment in time.”
The action is part of the broader Trump administration initiative to lower the prices and out-of-pocket costs of prescription drugs as outlined in the American Patients First blueprint.
By “implementing step therapy along with care coordination and drug adherence programs in [Medicare Advantage], it will lower costs and improve the quality of care for Medicare beneficiaries,” CMS officials said in a statement. The move to allow step therapy will give Medicare Advantage plan sponsors the ability to negotiate the designation of a preferred drug, something the agency believes could result in lower prices for these drugs, which in turn will lower the copays for Medicare beneficiaries.
Plan sponsors will be required to pass savings onto beneficiaries through some sort of rewards program, according to a memo detailing the policy change, which also notes that plan rewards “cannot be offered in the form of cash or monetary rebate, but may be offered as gift cards or other items value to all eligible enrollees.”
The value of the rewards must be more than half of the savings generated from implementing the step therapy program, according to the memo.
CMS officials noted that there will be a process that beneficiaries can follow if they believe they need direct access to a drug that would otherwise be available only after failing on another drug.
The American Society of Clinical Oncology also voiced its objection.
“ASCO strongly opposes the Centers for Medicare & Medicaid Services decision to allow Medicare Advantage plans to employ step therapy,” ASCO President Monica Bertagnolli, MD, said in a statement. “Step therapy requires patients to try and fail to have a desired clinical outcome on a lower-cost medications before they can access the medication prescribed by their health care provider. This not only delays patient access to proper treatments, [but it also] potentially leads to irreversible disease progression and other significant patient health risks.”
Further, the American Gastroenterology Association “is concerned that the proposal could limit access for current and future beneficiaries and could add to the growing regulatory burden that physicians already face,” according to a statement.
Barbara L. McAneny, MD, president of the American Medical Association, said that physicians “are concerned with patients getting the most effective treatment, and step therapy requirements frequently get in the way. ... Physicians have no easy access to patient benefit and formulary information at the point of prescribing, so they will not be able to readily determine which drugs are preferred by their patients’ [Medicare Advantage] plans. This results in treatment delays and unnecessary red tape for physicians and patients.”
The new policy applies to only new prescriptions or administrations of Part B drugs. Patients will not have current treatments disrupted if that drug is not the first drug on the step therapy ladder. Additionally, patients will have the opportunity to make a one-time change in plans during the first quarter annually if they are finding the plan is not working for them. Plan sponsors must disclose that Part B drugs may be subject to step therapy.
A new policy that allows Medicare Advantage plans to use step therapy to control spending on prescription drug administered in the office is not going over well with doctors.
The Centers for Medicare & Medicaid Services announced the policy change Aug. 7, which will give Medicare Advantage plan sponsors the “choice of implementing step therapy to manage Part B drugs, beginning Jan. 1, 2019,” the agency said in a statement. Step therapy, as described by the announcement “is a type of prior authorization for drugs that begins medication for a medical condition with the most preferred drug therapy and progresses to other therapies only if necessary, promoting better clinical decisions.”
Doctors aren’t having it.
“Put simply, this policy change is a gross affront to America’s sickest Medicare patients – individuals living with diseases like inflammatory arthritis and cancer – who depend on timely access to safe, affordable, and high-quality treatments,” American College of Rheumatology President David Daikh, MD, PhD, said in a statement.
“Utilization management techniques like step therapy prevent and delay important treatments for rheumatic disease patients, which can result in irreversible joint or organ damage,” Dr. Daikh continued. “At the same time that medical research is showing that early institution of effective treatment prevents such damage, CMS is instituting a policy that will makes it much more difficult for patients to get this treatment in time.”
The action is part of the broader Trump administration initiative to lower the prices and out-of-pocket costs of prescription drugs as outlined in the American Patients First blueprint.
By “implementing step therapy along with care coordination and drug adherence programs in [Medicare Advantage], it will lower costs and improve the quality of care for Medicare beneficiaries,” CMS officials said in a statement. The move to allow step therapy will give Medicare Advantage plan sponsors the ability to negotiate the designation of a preferred drug, something the agency believes could result in lower prices for these drugs, which in turn will lower the copays for Medicare beneficiaries.
Plan sponsors will be required to pass savings onto beneficiaries through some sort of rewards program, according to a memo detailing the policy change, which also notes that plan rewards “cannot be offered in the form of cash or monetary rebate, but may be offered as gift cards or other items value to all eligible enrollees.”
The value of the rewards must be more than half of the savings generated from implementing the step therapy program, according to the memo.
CMS officials noted that there will be a process that beneficiaries can follow if they believe they need direct access to a drug that would otherwise be available only after failing on another drug.
The American Society of Clinical Oncology also voiced its objection.
“ASCO strongly opposes the Centers for Medicare & Medicaid Services decision to allow Medicare Advantage plans to employ step therapy,” ASCO President Monica Bertagnolli, MD, said in a statement. “Step therapy requires patients to try and fail to have a desired clinical outcome on a lower-cost medications before they can access the medication prescribed by their health care provider. This not only delays patient access to proper treatments, [but it also] potentially leads to irreversible disease progression and other significant patient health risks.”
Further, the American Gastroenterology Association “is concerned that the proposal could limit access for current and future beneficiaries and could add to the growing regulatory burden that physicians already face,” according to a statement.
Barbara L. McAneny, MD, president of the American Medical Association, said that physicians “are concerned with patients getting the most effective treatment, and step therapy requirements frequently get in the way. ... Physicians have no easy access to patient benefit and formulary information at the point of prescribing, so they will not be able to readily determine which drugs are preferred by their patients’ [Medicare Advantage] plans. This results in treatment delays and unnecessary red tape for physicians and patients.”
The new policy applies to only new prescriptions or administrations of Part B drugs. Patients will not have current treatments disrupted if that drug is not the first drug on the step therapy ladder. Additionally, patients will have the opportunity to make a one-time change in plans during the first quarter annually if they are finding the plan is not working for them. Plan sponsors must disclose that Part B drugs may be subject to step therapy.
A new policy that allows Medicare Advantage plans to use step therapy to control spending on prescription drug administered in the office is not going over well with doctors.
The Centers for Medicare & Medicaid Services announced the policy change Aug. 7, which will give Medicare Advantage plan sponsors the “choice of implementing step therapy to manage Part B drugs, beginning Jan. 1, 2019,” the agency said in a statement. Step therapy, as described by the announcement “is a type of prior authorization for drugs that begins medication for a medical condition with the most preferred drug therapy and progresses to other therapies only if necessary, promoting better clinical decisions.”
Doctors aren’t having it.
“Put simply, this policy change is a gross affront to America’s sickest Medicare patients – individuals living with diseases like inflammatory arthritis and cancer – who depend on timely access to safe, affordable, and high-quality treatments,” American College of Rheumatology President David Daikh, MD, PhD, said in a statement.
“Utilization management techniques like step therapy prevent and delay important treatments for rheumatic disease patients, which can result in irreversible joint or organ damage,” Dr. Daikh continued. “At the same time that medical research is showing that early institution of effective treatment prevents such damage, CMS is instituting a policy that will makes it much more difficult for patients to get this treatment in time.”
The action is part of the broader Trump administration initiative to lower the prices and out-of-pocket costs of prescription drugs as outlined in the American Patients First blueprint.
By “implementing step therapy along with care coordination and drug adherence programs in [Medicare Advantage], it will lower costs and improve the quality of care for Medicare beneficiaries,” CMS officials said in a statement. The move to allow step therapy will give Medicare Advantage plan sponsors the ability to negotiate the designation of a preferred drug, something the agency believes could result in lower prices for these drugs, which in turn will lower the copays for Medicare beneficiaries.
Plan sponsors will be required to pass savings onto beneficiaries through some sort of rewards program, according to a memo detailing the policy change, which also notes that plan rewards “cannot be offered in the form of cash or monetary rebate, but may be offered as gift cards or other items value to all eligible enrollees.”
The value of the rewards must be more than half of the savings generated from implementing the step therapy program, according to the memo.
CMS officials noted that there will be a process that beneficiaries can follow if they believe they need direct access to a drug that would otherwise be available only after failing on another drug.
The American Society of Clinical Oncology also voiced its objection.
“ASCO strongly opposes the Centers for Medicare & Medicaid Services decision to allow Medicare Advantage plans to employ step therapy,” ASCO President Monica Bertagnolli, MD, said in a statement. “Step therapy requires patients to try and fail to have a desired clinical outcome on a lower-cost medications before they can access the medication prescribed by their health care provider. This not only delays patient access to proper treatments, [but it also] potentially leads to irreversible disease progression and other significant patient health risks.”
Further, the American Gastroenterology Association “is concerned that the proposal could limit access for current and future beneficiaries and could add to the growing regulatory burden that physicians already face,” according to a statement.
Barbara L. McAneny, MD, president of the American Medical Association, said that physicians “are concerned with patients getting the most effective treatment, and step therapy requirements frequently get in the way. ... Physicians have no easy access to patient benefit and formulary information at the point of prescribing, so they will not be able to readily determine which drugs are preferred by their patients’ [Medicare Advantage] plans. This results in treatment delays and unnecessary red tape for physicians and patients.”
The new policy applies to only new prescriptions or administrations of Part B drugs. Patients will not have current treatments disrupted if that drug is not the first drug on the step therapy ladder. Additionally, patients will have the opportunity to make a one-time change in plans during the first quarter annually if they are finding the plan is not working for them. Plan sponsors must disclose that Part B drugs may be subject to step therapy.
Key clinical point: Medicare Advantage plans will now be able to implement step therapy on Part B drugs.
Major finding: Only new prescriptions will be eligible for step therapy; existing treatments will not be disrupted.
Study details: CMS made the decision to reverse previously existing policy on step therapy as part of an ongoing effort to help lower the cost of prescription drugs.
Disclosures: There were no relevant disclosures.
Source: Prior Authorization and Step Therapy for Part B Drugs in Medicare Advantage;