User login
FDA approves assay to screen blood for Zika
The US Food and Drug Administration (FDA) has approved the Procleix® Zika Virus Assay for blood screening.
The assay is approved to detect Zika virus in individual or pooled plasma specimens from human donors, including volunteer donors of whole blood and blood components for transfusion.
The assay is also approved for testing plasma or serum specimens to screen other living or cadaveric organ donors and human cells, tissues, and cellular and tissue-based products.
The Procleix Zika Virus Assay, which was developed by Hologic, Inc. and Grifols, is designed to run on the Procleix Panther System. The Procleix Panther System automates all aspects of nucleic acid technology-based blood screening on a single, integrated platform.
The Procleix Zika Virus Assay has been in use in the US since June 2016, when it was authorized under an investigational new drug protocol to screen donated blood collected in the US.
Also in 2016, the assay was CE-marked for use in European countries conforming to CE Mark regulations.
The US Food and Drug Administration (FDA) has approved the Procleix® Zika Virus Assay for blood screening.
The assay is approved to detect Zika virus in individual or pooled plasma specimens from human donors, including volunteer donors of whole blood and blood components for transfusion.
The assay is also approved for testing plasma or serum specimens to screen other living or cadaveric organ donors and human cells, tissues, and cellular and tissue-based products.
The Procleix Zika Virus Assay, which was developed by Hologic, Inc. and Grifols, is designed to run on the Procleix Panther System. The Procleix Panther System automates all aspects of nucleic acid technology-based blood screening on a single, integrated platform.
The Procleix Zika Virus Assay has been in use in the US since June 2016, when it was authorized under an investigational new drug protocol to screen donated blood collected in the US.
Also in 2016, the assay was CE-marked for use in European countries conforming to CE Mark regulations.
The US Food and Drug Administration (FDA) has approved the Procleix® Zika Virus Assay for blood screening.
The assay is approved to detect Zika virus in individual or pooled plasma specimens from human donors, including volunteer donors of whole blood and blood components for transfusion.
The assay is also approved for testing plasma or serum specimens to screen other living or cadaveric organ donors and human cells, tissues, and cellular and tissue-based products.
The Procleix Zika Virus Assay, which was developed by Hologic, Inc. and Grifols, is designed to run on the Procleix Panther System. The Procleix Panther System automates all aspects of nucleic acid technology-based blood screening on a single, integrated platform.
The Procleix Zika Virus Assay has been in use in the US since June 2016, when it was authorized under an investigational new drug protocol to screen donated blood collected in the US.
Also in 2016, the assay was CE-marked for use in European countries conforming to CE Mark regulations.
Lenalidomide may be best maintenance for MM
Lenalidomide may be the best maintenance treatment option for patients with newly diagnosed multiple myeloma (MM), according to the authors of a systematic review and meta-analysis.
Francesca M. Gay, MD, PhD, of the University of Torino in Italy, and her coauthors wrote that, despite the well-recognized importance of novel agent–based maintenance therapy for MM, there is a lack of direct or indirect comparisons between the available regimens.
In a paper published in JAMA Oncology, the researchers reported the results of a systematic review and meta-analysis of 11 prospective, phase 3, randomized, controlled trials of 8 varieties of maintenance therapy in 5073 participants with newly diagnosed MM.
The researchers found that lenalidomide-based regimens showed the best progression-free survival rates, compared with placebo. The hazard ratio (HR) was 0.39 for lenalidomide plus prednisone, and the HR was 0.47 for lenalidomide alone.
In 74% of the network meta-analysis simulations, lenalidomide-based regimens were the most effective options.
Four other maintenance treatment options—thalidomide-interferon (HR, 0.50), thalidomide-bortezomib (HR, 0.58), bortezomib-prednisone (HR, 0.72), and thalidomide alone (HR, 0.73)—also showed progression-free survival gains, but interferon therapy (HR, 0.91) failed to show any benefit.
For overall survival, lenalidomide alone (HR, 0.76) was the best option, followed by thalidomide-bortezomib (HR, 0.82) and bortezomib-prednisone (HR, 0.84). None of the other regimens considered showed benefits for overall survival.
“Long-term use of lenalidomide undoubtedly has advantages, owing to the lack of neuropathy, which is the main factor limiting the long-term use of both thalidomide and bortezomib,” the authors wrote.
When the authors restricted their analysis to trials conducted in the setting of autologous stem cell transplant, they found similar results, with lenalidomide-based regimens having the best progression-free and overall survival.
Patients with a good prognosis and standard-risk chromosomal abnormalities also did best with lenalidomide-based maintenance, while those with a poor prognosis—for example, with ISS stage III disease—benefited more from bortezomib-based maintenance.
However, patients with high-risk chromosomal abnormalities gained no advantage from any regimen, which the authors suggested may relate to small sample size, different cut-off points, or the patients’ extremely poor prognosis.
The authors noted that their analysis did not take into account adverse events, drug discontinuations, or quality of life but focused solely on progression-free survival and overall survival.
“An increase in second primary malignant disease with prolonged lenalidomide therapy has been reported, but the survival benefit overcame the risk in all the trials,” they wrote.
The authors also commented that better treatment options are needed for patients with aggressive disease, and there are ongoing trials looking at second-generation proteasome inhibitors, immunomodulatory agents, and monoclonal antibodies for maintenance therapy.
Most authors declared research funding, advisory board positions, fees, and honoraria from the pharmaceutical industry, including lenalidomide manufacturer Celgene.
Lenalidomide may be the best maintenance treatment option for patients with newly diagnosed multiple myeloma (MM), according to the authors of a systematic review and meta-analysis.
Francesca M. Gay, MD, PhD, of the University of Torino in Italy, and her coauthors wrote that, despite the well-recognized importance of novel agent–based maintenance therapy for MM, there is a lack of direct or indirect comparisons between the available regimens.
In a paper published in JAMA Oncology, the researchers reported the results of a systematic review and meta-analysis of 11 prospective, phase 3, randomized, controlled trials of 8 varieties of maintenance therapy in 5073 participants with newly diagnosed MM.
The researchers found that lenalidomide-based regimens showed the best progression-free survival rates, compared with placebo. The hazard ratio (HR) was 0.39 for lenalidomide plus prednisone, and the HR was 0.47 for lenalidomide alone.
In 74% of the network meta-analysis simulations, lenalidomide-based regimens were the most effective options.
Four other maintenance treatment options—thalidomide-interferon (HR, 0.50), thalidomide-bortezomib (HR, 0.58), bortezomib-prednisone (HR, 0.72), and thalidomide alone (HR, 0.73)—also showed progression-free survival gains, but interferon therapy (HR, 0.91) failed to show any benefit.
For overall survival, lenalidomide alone (HR, 0.76) was the best option, followed by thalidomide-bortezomib (HR, 0.82) and bortezomib-prednisone (HR, 0.84). None of the other regimens considered showed benefits for overall survival.
“Long-term use of lenalidomide undoubtedly has advantages, owing to the lack of neuropathy, which is the main factor limiting the long-term use of both thalidomide and bortezomib,” the authors wrote.
When the authors restricted their analysis to trials conducted in the setting of autologous stem cell transplant, they found similar results, with lenalidomide-based regimens having the best progression-free and overall survival.
Patients with a good prognosis and standard-risk chromosomal abnormalities also did best with lenalidomide-based maintenance, while those with a poor prognosis—for example, with ISS stage III disease—benefited more from bortezomib-based maintenance.
However, patients with high-risk chromosomal abnormalities gained no advantage from any regimen, which the authors suggested may relate to small sample size, different cut-off points, or the patients’ extremely poor prognosis.
The authors noted that their analysis did not take into account adverse events, drug discontinuations, or quality of life but focused solely on progression-free survival and overall survival.
“An increase in second primary malignant disease with prolonged lenalidomide therapy has been reported, but the survival benefit overcame the risk in all the trials,” they wrote.
The authors also commented that better treatment options are needed for patients with aggressive disease, and there are ongoing trials looking at second-generation proteasome inhibitors, immunomodulatory agents, and monoclonal antibodies for maintenance therapy.
Most authors declared research funding, advisory board positions, fees, and honoraria from the pharmaceutical industry, including lenalidomide manufacturer Celgene.
Lenalidomide may be the best maintenance treatment option for patients with newly diagnosed multiple myeloma (MM), according to the authors of a systematic review and meta-analysis.
Francesca M. Gay, MD, PhD, of the University of Torino in Italy, and her coauthors wrote that, despite the well-recognized importance of novel agent–based maintenance therapy for MM, there is a lack of direct or indirect comparisons between the available regimens.
In a paper published in JAMA Oncology, the researchers reported the results of a systematic review and meta-analysis of 11 prospective, phase 3, randomized, controlled trials of 8 varieties of maintenance therapy in 5073 participants with newly diagnosed MM.
The researchers found that lenalidomide-based regimens showed the best progression-free survival rates, compared with placebo. The hazard ratio (HR) was 0.39 for lenalidomide plus prednisone, and the HR was 0.47 for lenalidomide alone.
In 74% of the network meta-analysis simulations, lenalidomide-based regimens were the most effective options.
Four other maintenance treatment options—thalidomide-interferon (HR, 0.50), thalidomide-bortezomib (HR, 0.58), bortezomib-prednisone (HR, 0.72), and thalidomide alone (HR, 0.73)—also showed progression-free survival gains, but interferon therapy (HR, 0.91) failed to show any benefit.
For overall survival, lenalidomide alone (HR, 0.76) was the best option, followed by thalidomide-bortezomib (HR, 0.82) and bortezomib-prednisone (HR, 0.84). None of the other regimens considered showed benefits for overall survival.
“Long-term use of lenalidomide undoubtedly has advantages, owing to the lack of neuropathy, which is the main factor limiting the long-term use of both thalidomide and bortezomib,” the authors wrote.
When the authors restricted their analysis to trials conducted in the setting of autologous stem cell transplant, they found similar results, with lenalidomide-based regimens having the best progression-free and overall survival.
Patients with a good prognosis and standard-risk chromosomal abnormalities also did best with lenalidomide-based maintenance, while those with a poor prognosis—for example, with ISS stage III disease—benefited more from bortezomib-based maintenance.
However, patients with high-risk chromosomal abnormalities gained no advantage from any regimen, which the authors suggested may relate to small sample size, different cut-off points, or the patients’ extremely poor prognosis.
The authors noted that their analysis did not take into account adverse events, drug discontinuations, or quality of life but focused solely on progression-free survival and overall survival.
“An increase in second primary malignant disease with prolonged lenalidomide therapy has been reported, but the survival benefit overcame the risk in all the trials,” they wrote.
The authors also commented that better treatment options are needed for patients with aggressive disease, and there are ongoing trials looking at second-generation proteasome inhibitors, immunomodulatory agents, and monoclonal antibodies for maintenance therapy.
Most authors declared research funding, advisory board positions, fees, and honoraria from the pharmaceutical industry, including lenalidomide manufacturer Celgene.
Real-world bleeding risk with ibrutinib
The Bruton tyrosine kinase inhibitor ibrutinib has been linked to a 20-fold increased risk of major bleeding in blood cancer patients taking concomitant antiplatelet and anticoagulation therapy in a clinical setting.
Caution should be used when weighing the risks and benefits of ibrutinib for patients already taking antiplatelet or anticoagulation therapy, or both, wrote Joseph Mock, MD, of the University of Virginia Health System in Charlottesville, and his colleagues.
Their report was published in Clinical Lymphoma, Myeloma & Leukemia.
Ibrutinib had been associated with an increased risk of bleeding, albeit low, in the clinical trial setting, but the authors suggested this rate could be higher in everyday clinical practice.
“Much of the information [from clinical trials] on the bleeding risk with ibrutinib, included pooled analyses, was from patients exclusively treated in clinical trials with specific exclusion criteria,” the researchers wrote. “These criteria have generally excluded patients with significant comorbidities. However, these patients are seen in clinical practice.”
The researchers conducted a review of patients treated within the University of Virginia Health System between January 2012 and May 2016.
The team identified 70 patients, with an average age of 72, who were taking ibrutinib for chronic lymphocytic leukemia (64%), mantle cell lymphoma (27%), diffuse large B-cell lymphoma (4%), lymphoblastic lymphoma (3%), and Waldenström’s macroglobulinemia (1%).
Bleeding of any grade occurred in 56% of patients, mostly grade 1-2 bruising and epistaxis.
However, major bleeding, defined as grade 3 or higher, occurred in 19% of patients (n=13). Seven of these patients were taking combined antiplatelet and anticoagulant therapy, 4 were taking antiplatelet agents alone, 1 was taking an anticoagulant agent alone, and 1 was taking only ibrutinib.
Univariate analysis showed that the factors associated with an increased risk of major bleeding were antiplatelet or anticoagulant medication, the combination of the 2 medications, interacting medications, anemia (hemoglobin less than 12 g/dL), and an elevated international normalized ratio (INR, > 1.5).
In a multivariate analysis, only the following factors were associated with an increased risk of major bleeding:
- Concomitant antiplatelet and anticoagulant use—hazard ratio=20.0 (95% CI, 2.1-200.0; P=0.0005) vs no antiplatelet/anticoagulant therapy
- Elevated INR—hazard ratio=4.6 (95% CI, 1.1-19.6; P=0.0409).
The researchers said the risk of major bleeding in patients taking both antiplatelet and anticoagulant therapy was “unacceptably high” and “medications other than ibrutinib should be considered” in this patient population.
Overall, the team said their findings confirm “the increasingly recognized risk of major bleeding complications with ibrutinib compared with what was originally reported in the clinical trial setting.”
They noted that this study was limited by the relatively small population size. Their finding that platelet count was not associated with bleeding risk was also “counterintuitive.”
The Bruton tyrosine kinase inhibitor ibrutinib has been linked to a 20-fold increased risk of major bleeding in blood cancer patients taking concomitant antiplatelet and anticoagulation therapy in a clinical setting.
Caution should be used when weighing the risks and benefits of ibrutinib for patients already taking antiplatelet or anticoagulation therapy, or both, wrote Joseph Mock, MD, of the University of Virginia Health System in Charlottesville, and his colleagues.
Their report was published in Clinical Lymphoma, Myeloma & Leukemia.
Ibrutinib had been associated with an increased risk of bleeding, albeit low, in the clinical trial setting, but the authors suggested this rate could be higher in everyday clinical practice.
“Much of the information [from clinical trials] on the bleeding risk with ibrutinib, included pooled analyses, was from patients exclusively treated in clinical trials with specific exclusion criteria,” the researchers wrote. “These criteria have generally excluded patients with significant comorbidities. However, these patients are seen in clinical practice.”
The researchers conducted a review of patients treated within the University of Virginia Health System between January 2012 and May 2016.
The team identified 70 patients, with an average age of 72, who were taking ibrutinib for chronic lymphocytic leukemia (64%), mantle cell lymphoma (27%), diffuse large B-cell lymphoma (4%), lymphoblastic lymphoma (3%), and Waldenström’s macroglobulinemia (1%).
Bleeding of any grade occurred in 56% of patients, mostly grade 1-2 bruising and epistaxis.
However, major bleeding, defined as grade 3 or higher, occurred in 19% of patients (n=13). Seven of these patients were taking combined antiplatelet and anticoagulant therapy, 4 were taking antiplatelet agents alone, 1 was taking an anticoagulant agent alone, and 1 was taking only ibrutinib.
Univariate analysis showed that the factors associated with an increased risk of major bleeding were antiplatelet or anticoagulant medication, the combination of the 2 medications, interacting medications, anemia (hemoglobin less than 12 g/dL), and an elevated international normalized ratio (INR, > 1.5).
In a multivariate analysis, only the following factors were associated with an increased risk of major bleeding:
- Concomitant antiplatelet and anticoagulant use—hazard ratio=20.0 (95% CI, 2.1-200.0; P=0.0005) vs no antiplatelet/anticoagulant therapy
- Elevated INR—hazard ratio=4.6 (95% CI, 1.1-19.6; P=0.0409).
The researchers said the risk of major bleeding in patients taking both antiplatelet and anticoagulant therapy was “unacceptably high” and “medications other than ibrutinib should be considered” in this patient population.
Overall, the team said their findings confirm “the increasingly recognized risk of major bleeding complications with ibrutinib compared with what was originally reported in the clinical trial setting.”
They noted that this study was limited by the relatively small population size. Their finding that platelet count was not associated with bleeding risk was also “counterintuitive.”
The Bruton tyrosine kinase inhibitor ibrutinib has been linked to a 20-fold increased risk of major bleeding in blood cancer patients taking concomitant antiplatelet and anticoagulation therapy in a clinical setting.
Caution should be used when weighing the risks and benefits of ibrutinib for patients already taking antiplatelet or anticoagulation therapy, or both, wrote Joseph Mock, MD, of the University of Virginia Health System in Charlottesville, and his colleagues.
Their report was published in Clinical Lymphoma, Myeloma & Leukemia.
Ibrutinib had been associated with an increased risk of bleeding, albeit low, in the clinical trial setting, but the authors suggested this rate could be higher in everyday clinical practice.
“Much of the information [from clinical trials] on the bleeding risk with ibrutinib, included pooled analyses, was from patients exclusively treated in clinical trials with specific exclusion criteria,” the researchers wrote. “These criteria have generally excluded patients with significant comorbidities. However, these patients are seen in clinical practice.”
The researchers conducted a review of patients treated within the University of Virginia Health System between January 2012 and May 2016.
The team identified 70 patients, with an average age of 72, who were taking ibrutinib for chronic lymphocytic leukemia (64%), mantle cell lymphoma (27%), diffuse large B-cell lymphoma (4%), lymphoblastic lymphoma (3%), and Waldenström’s macroglobulinemia (1%).
Bleeding of any grade occurred in 56% of patients, mostly grade 1-2 bruising and epistaxis.
However, major bleeding, defined as grade 3 or higher, occurred in 19% of patients (n=13). Seven of these patients were taking combined antiplatelet and anticoagulant therapy, 4 were taking antiplatelet agents alone, 1 was taking an anticoagulant agent alone, and 1 was taking only ibrutinib.
Univariate analysis showed that the factors associated with an increased risk of major bleeding were antiplatelet or anticoagulant medication, the combination of the 2 medications, interacting medications, anemia (hemoglobin less than 12 g/dL), and an elevated international normalized ratio (INR, > 1.5).
In a multivariate analysis, only the following factors were associated with an increased risk of major bleeding:
- Concomitant antiplatelet and anticoagulant use—hazard ratio=20.0 (95% CI, 2.1-200.0; P=0.0005) vs no antiplatelet/anticoagulant therapy
- Elevated INR—hazard ratio=4.6 (95% CI, 1.1-19.6; P=0.0409).
The researchers said the risk of major bleeding in patients taking both antiplatelet and anticoagulant therapy was “unacceptably high” and “medications other than ibrutinib should be considered” in this patient population.
Overall, the team said their findings confirm “the increasingly recognized risk of major bleeding complications with ibrutinib compared with what was originally reported in the clinical trial setting.”
They noted that this study was limited by the relatively small population size. Their finding that platelet count was not associated with bleeding risk was also “counterintuitive.”
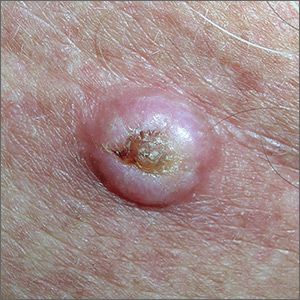
Growth on chest
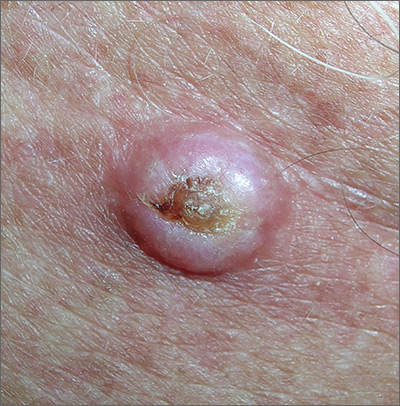
The FP noted that the lesion had a central keratin core (resembling a volcano) surrounded by a somewhat pearly raised border. He suspected that this was a squamous cell carcinoma (SCC) of the keratoacanthoma type and recommended a biopsy.
A shave biopsy was performed, and the result confirmed the diagnosis of SCC of the keratoacanthoma type. (See the Watch & Learn video on “Shave biopsy.”) The pathologist added that the lesion was well-differentiated (which was to be expected with a keratoacanthoma). While some keratoacanthomas actually resolve spontaneously, the standard of care is to treat them with either excision, electrodesiccation and curettage, or cryotherapy. On the follow-up visit, these options were presented to the patient and he decided to have cryotherapy. He accepted the risk of hypopigmentation in the treated area, but said that he preferred to avoid surgery.
The area was anesthetized with an injection of 1% lidocaine and epinephrine, and cryotherapy was performed with a 5 mm margin. The area was frozen with liquid nitrogen spray for 30 seconds and allowed to thaw. A second freeze for 30 seconds was then applied. Sun protection and sun avoidance were encouraged. At a 1-year follow-up, there was no recurrence and there was hypopigmentation at the treatment site.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Guzman A, Usatine R. Keratoacanthoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:977-980.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP noted that the lesion had a central keratin core (resembling a volcano) surrounded by a somewhat pearly raised border. He suspected that this was a squamous cell carcinoma (SCC) of the keratoacanthoma type and recommended a biopsy.
A shave biopsy was performed, and the result confirmed the diagnosis of SCC of the keratoacanthoma type. (See the Watch & Learn video on “Shave biopsy.”) The pathologist added that the lesion was well-differentiated (which was to be expected with a keratoacanthoma). While some keratoacanthomas actually resolve spontaneously, the standard of care is to treat them with either excision, electrodesiccation and curettage, or cryotherapy. On the follow-up visit, these options were presented to the patient and he decided to have cryotherapy. He accepted the risk of hypopigmentation in the treated area, but said that he preferred to avoid surgery.
The area was anesthetized with an injection of 1% lidocaine and epinephrine, and cryotherapy was performed with a 5 mm margin. The area was frozen with liquid nitrogen spray for 30 seconds and allowed to thaw. A second freeze for 30 seconds was then applied. Sun protection and sun avoidance were encouraged. At a 1-year follow-up, there was no recurrence and there was hypopigmentation at the treatment site.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Guzman A, Usatine R. Keratoacanthoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:977-980.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP noted that the lesion had a central keratin core (resembling a volcano) surrounded by a somewhat pearly raised border. He suspected that this was a squamous cell carcinoma (SCC) of the keratoacanthoma type and recommended a biopsy.
A shave biopsy was performed, and the result confirmed the diagnosis of SCC of the keratoacanthoma type. (See the Watch & Learn video on “Shave biopsy.”) The pathologist added that the lesion was well-differentiated (which was to be expected with a keratoacanthoma). While some keratoacanthomas actually resolve spontaneously, the standard of care is to treat them with either excision, electrodesiccation and curettage, or cryotherapy. On the follow-up visit, these options were presented to the patient and he decided to have cryotherapy. He accepted the risk of hypopigmentation in the treated area, but said that he preferred to avoid surgery.
The area was anesthetized with an injection of 1% lidocaine and epinephrine, and cryotherapy was performed with a 5 mm margin. The area was frozen with liquid nitrogen spray for 30 seconds and allowed to thaw. A second freeze for 30 seconds was then applied. Sun protection and sun avoidance were encouraged. At a 1-year follow-up, there was no recurrence and there was hypopigmentation at the treatment site.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Guzman A, Usatine R. Keratoacanthoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:977-980.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Opioids, other causes linked to shorter lifespans, rising midlife mortality
Two reports in the BMJ regarding new research offer grim tidings about mortality in the United States: One study has suggested the opioid epidemic is driving down life expectancy, and another has found that midlife death rates from multiple causes are on the rise across ethnic groups.
“Death rates are increasing across the U.S. population for dozens of conditions,” according to Steven H. Woolf, MD, MPH, a professor at Virginia Commonwealth University, Richmond, and his associates, who together wrote the second study. “Of particular urgency is recognizing that the unfavorable mortality pattern that began for some groups in the 1990s is now unfolding among Hispanics and non-Hispanic blacks, a development made more consequential by their high baseline mortality rates.”
Dr. Woolf and his coinvestigators tracked midlife mortality (ages 25-64 years) from 1999-2016 across the U.S. population.
They found that all-cause mortality jumped among whites, American Indians, and Alaska Natives over that time. A trend toward decreasing all-cause mortality ended in 2009-2011 among African Americans, Hispanic American, Asian Americans, and Americans of Pacific Islander descent.
“Drug overdoses were the leading cause of increased mortality in midlife in each population, but mortality also increased for alcohol related conditions, suicides, and organ diseases involving multiple body systems,” according to the researchers.
For the life expectancy study, which was published in the BMJ, researchers led by Jessica Y. Ho, PhD, of the University of Southern California, Los Angeles, tracked all-cause and cause-specific mortality in 18 high-income nations. They focused on the years 2014-2016.
Most of the nations saw declines in life expectancy from 2014-2015, mainly caused by older people dying from physical diseases and mental disorders. From 2015-2016, most of these nations saw their life expectancy levels rebound and experienced “robust gains.”
In the United States, however, life expectancy at birth for women fell from 81.47 years in 2014 to 81.35 years in 2015, and rose to 81.40 years in 2016.
For men, life expectancy fell continuously from 76.67 years in 2014 to 76.50 years in 2015 to 76.40 years in 2016.
In 2016, these life expectancies were the lowest of 17 nations examined in the study. (Statistics from the 18th nation in the study, Canada, were not included for 2016).
The United States was an outlier in other ways. For one, deaths among people younger than 65 years were the major contributor to the life expectancy decline.
And causes of death were also different than other countries: “For American women, drug overdose and external causes, and respiratory and cardiovascular diseases, contributed roughly equally to the decline in life expectancy, but for American men, nearly all of the decline was attributable to drug overdose and external causes.”
No funding is reported for the death rates study. The study authors report no relevant disclosures.
The life expectancy study was supported by the Robert Wood Johnson Foundation. Authors variously report support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute on Aging, and the National Institute of Child Health and Human Development.
SOURCE: Ho JY et al. BMJ. 2018;362:k2562; Woolf SH et al. BMJ 2018;362:k3096.
Life expectancy in some high-income nations has declined, at least temporarily, in recent years for apparent causes such as flu epidemics and “deaths of despair” because of drug abuse and suicide. While these newer trends may be short lived, they’re worrisome because life expectancy reflects human progress. Meanwhile, some nations and subpopulations continue to lag behind high-income nations despite the benefits of the modern era. Moving forward, we must back up policies with better scientific evidence. Improvements are needed in areas such as timely national mortality data, comparable statistics, and reliable numbers.
Domantas Jasilionis, PhD, is with the Max Planck Institute for Demographic Research, Rostock, Germany
Life expectancy in some high-income nations has declined, at least temporarily, in recent years for apparent causes such as flu epidemics and “deaths of despair” because of drug abuse and suicide. While these newer trends may be short lived, they’re worrisome because life expectancy reflects human progress. Meanwhile, some nations and subpopulations continue to lag behind high-income nations despite the benefits of the modern era. Moving forward, we must back up policies with better scientific evidence. Improvements are needed in areas such as timely national mortality data, comparable statistics, and reliable numbers.
Domantas Jasilionis, PhD, is with the Max Planck Institute for Demographic Research, Rostock, Germany
Life expectancy in some high-income nations has declined, at least temporarily, in recent years for apparent causes such as flu epidemics and “deaths of despair” because of drug abuse and suicide. While these newer trends may be short lived, they’re worrisome because life expectancy reflects human progress. Meanwhile, some nations and subpopulations continue to lag behind high-income nations despite the benefits of the modern era. Moving forward, we must back up policies with better scientific evidence. Improvements are needed in areas such as timely national mortality data, comparable statistics, and reliable numbers.
Domantas Jasilionis, PhD, is with the Max Planck Institute for Demographic Research, Rostock, Germany
Two reports in the BMJ regarding new research offer grim tidings about mortality in the United States: One study has suggested the opioid epidemic is driving down life expectancy, and another has found that midlife death rates from multiple causes are on the rise across ethnic groups.
“Death rates are increasing across the U.S. population for dozens of conditions,” according to Steven H. Woolf, MD, MPH, a professor at Virginia Commonwealth University, Richmond, and his associates, who together wrote the second study. “Of particular urgency is recognizing that the unfavorable mortality pattern that began for some groups in the 1990s is now unfolding among Hispanics and non-Hispanic blacks, a development made more consequential by their high baseline mortality rates.”
Dr. Woolf and his coinvestigators tracked midlife mortality (ages 25-64 years) from 1999-2016 across the U.S. population.
They found that all-cause mortality jumped among whites, American Indians, and Alaska Natives over that time. A trend toward decreasing all-cause mortality ended in 2009-2011 among African Americans, Hispanic American, Asian Americans, and Americans of Pacific Islander descent.
“Drug overdoses were the leading cause of increased mortality in midlife in each population, but mortality also increased for alcohol related conditions, suicides, and organ diseases involving multiple body systems,” according to the researchers.
For the life expectancy study, which was published in the BMJ, researchers led by Jessica Y. Ho, PhD, of the University of Southern California, Los Angeles, tracked all-cause and cause-specific mortality in 18 high-income nations. They focused on the years 2014-2016.
Most of the nations saw declines in life expectancy from 2014-2015, mainly caused by older people dying from physical diseases and mental disorders. From 2015-2016, most of these nations saw their life expectancy levels rebound and experienced “robust gains.”
In the United States, however, life expectancy at birth for women fell from 81.47 years in 2014 to 81.35 years in 2015, and rose to 81.40 years in 2016.
For men, life expectancy fell continuously from 76.67 years in 2014 to 76.50 years in 2015 to 76.40 years in 2016.
In 2016, these life expectancies were the lowest of 17 nations examined in the study. (Statistics from the 18th nation in the study, Canada, were not included for 2016).
The United States was an outlier in other ways. For one, deaths among people younger than 65 years were the major contributor to the life expectancy decline.
And causes of death were also different than other countries: “For American women, drug overdose and external causes, and respiratory and cardiovascular diseases, contributed roughly equally to the decline in life expectancy, but for American men, nearly all of the decline was attributable to drug overdose and external causes.”
No funding is reported for the death rates study. The study authors report no relevant disclosures.
The life expectancy study was supported by the Robert Wood Johnson Foundation. Authors variously report support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute on Aging, and the National Institute of Child Health and Human Development.
SOURCE: Ho JY et al. BMJ. 2018;362:k2562; Woolf SH et al. BMJ 2018;362:k3096.
Two reports in the BMJ regarding new research offer grim tidings about mortality in the United States: One study has suggested the opioid epidemic is driving down life expectancy, and another has found that midlife death rates from multiple causes are on the rise across ethnic groups.
“Death rates are increasing across the U.S. population for dozens of conditions,” according to Steven H. Woolf, MD, MPH, a professor at Virginia Commonwealth University, Richmond, and his associates, who together wrote the second study. “Of particular urgency is recognizing that the unfavorable mortality pattern that began for some groups in the 1990s is now unfolding among Hispanics and non-Hispanic blacks, a development made more consequential by their high baseline mortality rates.”
Dr. Woolf and his coinvestigators tracked midlife mortality (ages 25-64 years) from 1999-2016 across the U.S. population.
They found that all-cause mortality jumped among whites, American Indians, and Alaska Natives over that time. A trend toward decreasing all-cause mortality ended in 2009-2011 among African Americans, Hispanic American, Asian Americans, and Americans of Pacific Islander descent.
“Drug overdoses were the leading cause of increased mortality in midlife in each population, but mortality also increased for alcohol related conditions, suicides, and organ diseases involving multiple body systems,” according to the researchers.
For the life expectancy study, which was published in the BMJ, researchers led by Jessica Y. Ho, PhD, of the University of Southern California, Los Angeles, tracked all-cause and cause-specific mortality in 18 high-income nations. They focused on the years 2014-2016.
Most of the nations saw declines in life expectancy from 2014-2015, mainly caused by older people dying from physical diseases and mental disorders. From 2015-2016, most of these nations saw their life expectancy levels rebound and experienced “robust gains.”
In the United States, however, life expectancy at birth for women fell from 81.47 years in 2014 to 81.35 years in 2015, and rose to 81.40 years in 2016.
For men, life expectancy fell continuously from 76.67 years in 2014 to 76.50 years in 2015 to 76.40 years in 2016.
In 2016, these life expectancies were the lowest of 17 nations examined in the study. (Statistics from the 18th nation in the study, Canada, were not included for 2016).
The United States was an outlier in other ways. For one, deaths among people younger than 65 years were the major contributor to the life expectancy decline.
And causes of death were also different than other countries: “For American women, drug overdose and external causes, and respiratory and cardiovascular diseases, contributed roughly equally to the decline in life expectancy, but for American men, nearly all of the decline was attributable to drug overdose and external causes.”
No funding is reported for the death rates study. The study authors report no relevant disclosures.
The life expectancy study was supported by the Robert Wood Johnson Foundation. Authors variously report support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute on Aging, and the National Institute of Child Health and Human Development.
SOURCE: Ho JY et al. BMJ. 2018;362:k2562; Woolf SH et al. BMJ 2018;362:k3096.
FROM THE BMJ
Ibalizumab shows promise against MDR HIV-1
In combination with an optimized background regimen, the humanized IgG4 monoclonal antibody ibalizumab can reduce viral load and increase CD4 counts in patients with multidrug-resistant HIV type 1 (MDR HIV-1), according to a study published in the New England Journal of Medicine. It does so by binding noncompetitively to CD4 receptors, which can thereby block HIV-1 entry.
TMB-301 (NCT02475629) was a 24-week, open-label, single-group, phase 3 study that included patients with MDR HIV-1. During July 2015 to October 2016, patients first continued on their previous treatment for 7 days, then were given a 2,000-mg loading dose of ibalizumab as monotherapy, and then continued on 800 mg ibalizumab every 14 days plus an individualized regimen that was optimized based on patients’ past treatments for the remainder of the study period.
The primary endpoint was how many patients experienced a decrease in viral load of at least 0.5 log10 copies/mL from baseline to day 14 – that is, after the 7-day control period plus the 7-days ibalizumab monotherapy period. This occurred in 33 of the 40 patients (83%; 95% confidence interval, 67%-93%; P less than .001); in fact, 60% of patients (n = 24) experienced a decrease of at least 1.0 log10 copies/mL during that period, and the mean and median reductions were 1.1 log10 copies/mL. One secondary end point was increase in CD4 counts: The mean increase was 62 cells/mcL, although smaller increases were seen among patients with lower starting counts. Of the 10 patients who exhibited virologic failure or rebound, 9 showed less susceptibility to ibalizumab at week 25 than they had shown at baseline.
At least one adverse event was seen in 32 patients (80%), although most (87%) of those events were mild to moderate in severity, such as diarrhea (20%) and nausea, fatigue, pyrexia, rash, and dizziness (13% each). More serious events (grade 3 or 4) were seen in 28% of patients (n = 11); serious events were seen in nine patients – including immune reconstitution inflammatory syndrome in one. Four patients died during the study, although none of the deaths were believed to have been related to treatment with ibalizumab.
The investigators noted that the study’s limitations – its small size, uncontrolled design, and limited time for analyzing secondary and safety endpoints – were largely related to small number of patients with MDR HIV-1. The investigators noted that, in accordance with HIV treatment guidelines, patients who experience resistance-related treatment failure should be started on a regimen that includes at least two fully active agents; the investigators suggest that the addition of a drug like ibalizumab provides new opportunities for effective treatments in these situations.
This study was originally presented at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
The study was supported by the Orphan Products Clinical Trials Grants Program of the Food and Drug Administration and TaiMed, which developed ibalizumab. The authors disclosed ties to various industry entities, including grants, personal fees, nonfinancial support, and other forms of support from TaiMed.
SOURCE: Emu B et al. New Engl J Med. 2018 Aug 16;379(9):645-54.
In combination with an optimized background regimen, the humanized IgG4 monoclonal antibody ibalizumab can reduce viral load and increase CD4 counts in patients with multidrug-resistant HIV type 1 (MDR HIV-1), according to a study published in the New England Journal of Medicine. It does so by binding noncompetitively to CD4 receptors, which can thereby block HIV-1 entry.
TMB-301 (NCT02475629) was a 24-week, open-label, single-group, phase 3 study that included patients with MDR HIV-1. During July 2015 to October 2016, patients first continued on their previous treatment for 7 days, then were given a 2,000-mg loading dose of ibalizumab as monotherapy, and then continued on 800 mg ibalizumab every 14 days plus an individualized regimen that was optimized based on patients’ past treatments for the remainder of the study period.
The primary endpoint was how many patients experienced a decrease in viral load of at least 0.5 log10 copies/mL from baseline to day 14 – that is, after the 7-day control period plus the 7-days ibalizumab monotherapy period. This occurred in 33 of the 40 patients (83%; 95% confidence interval, 67%-93%; P less than .001); in fact, 60% of patients (n = 24) experienced a decrease of at least 1.0 log10 copies/mL during that period, and the mean and median reductions were 1.1 log10 copies/mL. One secondary end point was increase in CD4 counts: The mean increase was 62 cells/mcL, although smaller increases were seen among patients with lower starting counts. Of the 10 patients who exhibited virologic failure or rebound, 9 showed less susceptibility to ibalizumab at week 25 than they had shown at baseline.
At least one adverse event was seen in 32 patients (80%), although most (87%) of those events were mild to moderate in severity, such as diarrhea (20%) and nausea, fatigue, pyrexia, rash, and dizziness (13% each). More serious events (grade 3 or 4) were seen in 28% of patients (n = 11); serious events were seen in nine patients – including immune reconstitution inflammatory syndrome in one. Four patients died during the study, although none of the deaths were believed to have been related to treatment with ibalizumab.
The investigators noted that the study’s limitations – its small size, uncontrolled design, and limited time for analyzing secondary and safety endpoints – were largely related to small number of patients with MDR HIV-1. The investigators noted that, in accordance with HIV treatment guidelines, patients who experience resistance-related treatment failure should be started on a regimen that includes at least two fully active agents; the investigators suggest that the addition of a drug like ibalizumab provides new opportunities for effective treatments in these situations.
This study was originally presented at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
The study was supported by the Orphan Products Clinical Trials Grants Program of the Food and Drug Administration and TaiMed, which developed ibalizumab. The authors disclosed ties to various industry entities, including grants, personal fees, nonfinancial support, and other forms of support from TaiMed.
SOURCE: Emu B et al. New Engl J Med. 2018 Aug 16;379(9):645-54.
In combination with an optimized background regimen, the humanized IgG4 monoclonal antibody ibalizumab can reduce viral load and increase CD4 counts in patients with multidrug-resistant HIV type 1 (MDR HIV-1), according to a study published in the New England Journal of Medicine. It does so by binding noncompetitively to CD4 receptors, which can thereby block HIV-1 entry.
TMB-301 (NCT02475629) was a 24-week, open-label, single-group, phase 3 study that included patients with MDR HIV-1. During July 2015 to October 2016, patients first continued on their previous treatment for 7 days, then were given a 2,000-mg loading dose of ibalizumab as monotherapy, and then continued on 800 mg ibalizumab every 14 days plus an individualized regimen that was optimized based on patients’ past treatments for the remainder of the study period.
The primary endpoint was how many patients experienced a decrease in viral load of at least 0.5 log10 copies/mL from baseline to day 14 – that is, after the 7-day control period plus the 7-days ibalizumab monotherapy period. This occurred in 33 of the 40 patients (83%; 95% confidence interval, 67%-93%; P less than .001); in fact, 60% of patients (n = 24) experienced a decrease of at least 1.0 log10 copies/mL during that period, and the mean and median reductions were 1.1 log10 copies/mL. One secondary end point was increase in CD4 counts: The mean increase was 62 cells/mcL, although smaller increases were seen among patients with lower starting counts. Of the 10 patients who exhibited virologic failure or rebound, 9 showed less susceptibility to ibalizumab at week 25 than they had shown at baseline.
At least one adverse event was seen in 32 patients (80%), although most (87%) of those events were mild to moderate in severity, such as diarrhea (20%) and nausea, fatigue, pyrexia, rash, and dizziness (13% each). More serious events (grade 3 or 4) were seen in 28% of patients (n = 11); serious events were seen in nine patients – including immune reconstitution inflammatory syndrome in one. Four patients died during the study, although none of the deaths were believed to have been related to treatment with ibalizumab.
The investigators noted that the study’s limitations – its small size, uncontrolled design, and limited time for analyzing secondary and safety endpoints – were largely related to small number of patients with MDR HIV-1. The investigators noted that, in accordance with HIV treatment guidelines, patients who experience resistance-related treatment failure should be started on a regimen that includes at least two fully active agents; the investigators suggest that the addition of a drug like ibalizumab provides new opportunities for effective treatments in these situations.
This study was originally presented at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
The study was supported by the Orphan Products Clinical Trials Grants Program of the Food and Drug Administration and TaiMed, which developed ibalizumab. The authors disclosed ties to various industry entities, including grants, personal fees, nonfinancial support, and other forms of support from TaiMed.
SOURCE: Emu B et al. New Engl J Med. 2018 Aug 16;379(9):645-54.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Studies support cardiovascular risk management in T2DM
Taking steps to manage cardiovascular risk can help improve long-term outcomes for type 2 diabetes mellitus, according to two new studies published Aug. 16 in the New England Journal of Medicine.
The first study, which examined weight gain after smoking cessation, found that despite a temporary increase in T2DM risk, post-cessation weight gain did not diminish the long-term benefits of reduced cardiovascular and all-cause mortality.
The analysis included three cohort studies: the Nurses’ Health Study (NHS), Nurses’ Health Study II (NHS II), and the Health Professionals Follow-Up Study (HPFS), with follow-up questionnaires every 2 years. After exclusions, a total of 162,807 patients were included in the diabetes analysis and 170,723 in the mortality analysis, reported Yang Hu of the department of nutrition at Harvard T.H. Chan School of Public Health, Boston, and his coauthors (N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMoa1803626).
In each follow-up cycle, participants who reported being smokers in the previous cycle but “past” smokers in the current cycle were identified. Quitters were defined as either transient quitters (past smokers in the current cycle but current smokers in previous and next cycles), recent quitters (2-6 consecutive years since smoking cessation), and long-term quitters (6 or more consecutive years since cessation). Weight change was observed for the first 6 years after quitting.
Overall, 12,384 cases of T2DM were confirmed. Diabetes risk was higher for recent quitters than for current smokers (hazard ratio, 1.22; 95% confidence interval, 1.12-1.32); this risk peaked 5-7 years after quitting and then gradually decreased. In analysis of patients with the longest follow-up time, diabetes risk dropped after 30 years of cessation to that of participants who had never smoked, the authors reported.
Compared with current smokers, hazard ratios for T2DM in recent quitters were 1.08 (95% CI, 0.93-1.26) for those without weight gain, 1.15 (95% CI, 0.99-1.33) for those with weight gain of 0.1-5.0 kg, 1.36 (95% CI, 1.16-1.58) for those with weight gain of 5.1-10 kg, and 1.59 (95% CI, 1.36-1.85) in those with weight gain of more than 10 kg.
In the mortality analysis, 23,867 deaths occurred, of which 5,492 were due to cardiovascular disease. Compared with current smokers, hazard ratios for death from cardiovascular disease in recent quitters were 0.69 (95% CI, 0.54-0.88) in those without weight gain; 0.47 (95% CI, 0.35-0.63) in those with weight gain of 0.1-5 kg; 0.25 (95% CI, 0.15-0.42) in those with weight gain of 5.1-10 kg; 0.33 (95% CI, 0.18-0.60) in those with weight gain of more than 10 kg; and 0.50 (95% CI, 0.46-0.55) for longer term quitters. The corresponding hazard ratios for all-cause deaths in the same weight gain groups were 0.81 (95% CI, 0.73-0.90); 0.52 (95% CI, 0.46-0.59); 0.46 (95% CI, 0.38-0.55); 0.50 (95% CI, 0.40-0.63); and 0.57 (95% CI, 0.54-0.59).
The findings suggest that weight gain after quitting smoking “did not attenuate the apparent benefits of smoking cessation on reducing cardiovascular mortality or extending longevity,” the authors said. “However, preventing excessive weight gain may maximize the health benefits of smoking cessation through reducing the short-term risk of diabetes and further lowering the long-term risk of death.”
The second study, which included 271,174 patients with T2DM from the Swedish National Diabetes Register and 1,355,870 controls, examined five risk factors: elevated glycated hemoglobin level, elevated low-density lipoprotein cholesterol level, albuminuria, smoking, and elevated blood pressure.
All-cause mortality, myocardial infarction, stroke, and hospitalization for heart failure were evaluated. The risk of each outcome among patients with T2DM was estimated according to the number of risk-factor variables within guideline-recommended target ranges, compared with matched controls, wrote Aidin Rawshani, MD, of the department of molecular and clinical medicine at the University of Gothenburg (Sweden), and his coauthors (N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMoa1800256).
Among the T2DM patients, the excess risk of outcomes was reduced with each risk factor variable within the recommended target range. A total of 37,825 patients with T2DM (13.9%) and 137,520 controls (10.1%) died during the study period.
Among T2DM patients with all variables within target range, the hazard ratio was 1.06 for all-cause death (95% CI, 1.00-1.12); 0.84 for acute myocardial infarction (95% CI, 0.75-0.93); and 0.95 for stroke (95% CI, 0.84-1.07). Smoking was the strongest predictor of death, followed by physical activity, marital status, glycated hemoglobin level, and use of statins.
The study results “indicate that having all five risk-factor variables within the target ranges could theoretically eliminate the excess risk of acute myocardial infarction,” Dr. Rawshani and his colleagues wrote. “Patients with type 2 diabetes who had five risk-factor variables within target ranges appeared to have little or no excess risks of death, myocardial infarction, and stroke as compared with the general population.”
Dr. Hu and his coauthors did not report any disclosures. Dr. Rawshani’s coauthors disclosed relationships with numerous companies including Amgen, Astra Zeneca, and Boehringer Ingelheim.
SOURCE: Hu Y et al. N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMoa1803626. Rawshani A et al. N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMoa1800256.
“Given the high prevalence of obesity and the related increase in type 2 diabetes, prevention of cardiovascular complications is essential,” Steven A. Schroeder, MD, wrote in an editorial published along with the studies (N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMe1809004).
The findings reported by Hu and colleagues demonstrate that “the cardiovascular and overall mortality benefits of stopping smoking far outweigh the risks of acquiring type 2 diabetes,” he wrote.
The results reported by Rawshani and coauthors “provide clear support for active management of risk factors” because of the fact that patients with risk factor variables within recommended range had little or no excess risk of death or cardiovascular events.
The results of these two studies “provide support for control of cardiovascular risk factors in patients with diabetes, as well as reassurance that the benefits of smoking cessation outweigh the risks of obesity-associated diabetes,” he concluded.
Dr. Schroeder is on the faculty of the department of medicine, University of California, San Francisco. He had no financial conflicts of interest to disclose.
“Given the high prevalence of obesity and the related increase in type 2 diabetes, prevention of cardiovascular complications is essential,” Steven A. Schroeder, MD, wrote in an editorial published along with the studies (N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMe1809004).
The findings reported by Hu and colleagues demonstrate that “the cardiovascular and overall mortality benefits of stopping smoking far outweigh the risks of acquiring type 2 diabetes,” he wrote.
The results reported by Rawshani and coauthors “provide clear support for active management of risk factors” because of the fact that patients with risk factor variables within recommended range had little or no excess risk of death or cardiovascular events.
The results of these two studies “provide support for control of cardiovascular risk factors in patients with diabetes, as well as reassurance that the benefits of smoking cessation outweigh the risks of obesity-associated diabetes,” he concluded.
Dr. Schroeder is on the faculty of the department of medicine, University of California, San Francisco. He had no financial conflicts of interest to disclose.
“Given the high prevalence of obesity and the related increase in type 2 diabetes, prevention of cardiovascular complications is essential,” Steven A. Schroeder, MD, wrote in an editorial published along with the studies (N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMe1809004).
The findings reported by Hu and colleagues demonstrate that “the cardiovascular and overall mortality benefits of stopping smoking far outweigh the risks of acquiring type 2 diabetes,” he wrote.
The results reported by Rawshani and coauthors “provide clear support for active management of risk factors” because of the fact that patients with risk factor variables within recommended range had little or no excess risk of death or cardiovascular events.
The results of these two studies “provide support for control of cardiovascular risk factors in patients with diabetes, as well as reassurance that the benefits of smoking cessation outweigh the risks of obesity-associated diabetes,” he concluded.
Dr. Schroeder is on the faculty of the department of medicine, University of California, San Francisco. He had no financial conflicts of interest to disclose.
Taking steps to manage cardiovascular risk can help improve long-term outcomes for type 2 diabetes mellitus, according to two new studies published Aug. 16 in the New England Journal of Medicine.
The first study, which examined weight gain after smoking cessation, found that despite a temporary increase in T2DM risk, post-cessation weight gain did not diminish the long-term benefits of reduced cardiovascular and all-cause mortality.
The analysis included three cohort studies: the Nurses’ Health Study (NHS), Nurses’ Health Study II (NHS II), and the Health Professionals Follow-Up Study (HPFS), with follow-up questionnaires every 2 years. After exclusions, a total of 162,807 patients were included in the diabetes analysis and 170,723 in the mortality analysis, reported Yang Hu of the department of nutrition at Harvard T.H. Chan School of Public Health, Boston, and his coauthors (N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMoa1803626).
In each follow-up cycle, participants who reported being smokers in the previous cycle but “past” smokers in the current cycle were identified. Quitters were defined as either transient quitters (past smokers in the current cycle but current smokers in previous and next cycles), recent quitters (2-6 consecutive years since smoking cessation), and long-term quitters (6 or more consecutive years since cessation). Weight change was observed for the first 6 years after quitting.
Overall, 12,384 cases of T2DM were confirmed. Diabetes risk was higher for recent quitters than for current smokers (hazard ratio, 1.22; 95% confidence interval, 1.12-1.32); this risk peaked 5-7 years after quitting and then gradually decreased. In analysis of patients with the longest follow-up time, diabetes risk dropped after 30 years of cessation to that of participants who had never smoked, the authors reported.
Compared with current smokers, hazard ratios for T2DM in recent quitters were 1.08 (95% CI, 0.93-1.26) for those without weight gain, 1.15 (95% CI, 0.99-1.33) for those with weight gain of 0.1-5.0 kg, 1.36 (95% CI, 1.16-1.58) for those with weight gain of 5.1-10 kg, and 1.59 (95% CI, 1.36-1.85) in those with weight gain of more than 10 kg.
In the mortality analysis, 23,867 deaths occurred, of which 5,492 were due to cardiovascular disease. Compared with current smokers, hazard ratios for death from cardiovascular disease in recent quitters were 0.69 (95% CI, 0.54-0.88) in those without weight gain; 0.47 (95% CI, 0.35-0.63) in those with weight gain of 0.1-5 kg; 0.25 (95% CI, 0.15-0.42) in those with weight gain of 5.1-10 kg; 0.33 (95% CI, 0.18-0.60) in those with weight gain of more than 10 kg; and 0.50 (95% CI, 0.46-0.55) for longer term quitters. The corresponding hazard ratios for all-cause deaths in the same weight gain groups were 0.81 (95% CI, 0.73-0.90); 0.52 (95% CI, 0.46-0.59); 0.46 (95% CI, 0.38-0.55); 0.50 (95% CI, 0.40-0.63); and 0.57 (95% CI, 0.54-0.59).
The findings suggest that weight gain after quitting smoking “did not attenuate the apparent benefits of smoking cessation on reducing cardiovascular mortality or extending longevity,” the authors said. “However, preventing excessive weight gain may maximize the health benefits of smoking cessation through reducing the short-term risk of diabetes and further lowering the long-term risk of death.”
The second study, which included 271,174 patients with T2DM from the Swedish National Diabetes Register and 1,355,870 controls, examined five risk factors: elevated glycated hemoglobin level, elevated low-density lipoprotein cholesterol level, albuminuria, smoking, and elevated blood pressure.
All-cause mortality, myocardial infarction, stroke, and hospitalization for heart failure were evaluated. The risk of each outcome among patients with T2DM was estimated according to the number of risk-factor variables within guideline-recommended target ranges, compared with matched controls, wrote Aidin Rawshani, MD, of the department of molecular and clinical medicine at the University of Gothenburg (Sweden), and his coauthors (N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMoa1800256).
Among the T2DM patients, the excess risk of outcomes was reduced with each risk factor variable within the recommended target range. A total of 37,825 patients with T2DM (13.9%) and 137,520 controls (10.1%) died during the study period.
Among T2DM patients with all variables within target range, the hazard ratio was 1.06 for all-cause death (95% CI, 1.00-1.12); 0.84 for acute myocardial infarction (95% CI, 0.75-0.93); and 0.95 for stroke (95% CI, 0.84-1.07). Smoking was the strongest predictor of death, followed by physical activity, marital status, glycated hemoglobin level, and use of statins.
The study results “indicate that having all five risk-factor variables within the target ranges could theoretically eliminate the excess risk of acute myocardial infarction,” Dr. Rawshani and his colleagues wrote. “Patients with type 2 diabetes who had five risk-factor variables within target ranges appeared to have little or no excess risks of death, myocardial infarction, and stroke as compared with the general population.”
Dr. Hu and his coauthors did not report any disclosures. Dr. Rawshani’s coauthors disclosed relationships with numerous companies including Amgen, Astra Zeneca, and Boehringer Ingelheim.
SOURCE: Hu Y et al. N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMoa1803626. Rawshani A et al. N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMoa1800256.
Taking steps to manage cardiovascular risk can help improve long-term outcomes for type 2 diabetes mellitus, according to two new studies published Aug. 16 in the New England Journal of Medicine.
The first study, which examined weight gain after smoking cessation, found that despite a temporary increase in T2DM risk, post-cessation weight gain did not diminish the long-term benefits of reduced cardiovascular and all-cause mortality.
The analysis included three cohort studies: the Nurses’ Health Study (NHS), Nurses’ Health Study II (NHS II), and the Health Professionals Follow-Up Study (HPFS), with follow-up questionnaires every 2 years. After exclusions, a total of 162,807 patients were included in the diabetes analysis and 170,723 in the mortality analysis, reported Yang Hu of the department of nutrition at Harvard T.H. Chan School of Public Health, Boston, and his coauthors (N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMoa1803626).
In each follow-up cycle, participants who reported being smokers in the previous cycle but “past” smokers in the current cycle were identified. Quitters were defined as either transient quitters (past smokers in the current cycle but current smokers in previous and next cycles), recent quitters (2-6 consecutive years since smoking cessation), and long-term quitters (6 or more consecutive years since cessation). Weight change was observed for the first 6 years after quitting.
Overall, 12,384 cases of T2DM were confirmed. Diabetes risk was higher for recent quitters than for current smokers (hazard ratio, 1.22; 95% confidence interval, 1.12-1.32); this risk peaked 5-7 years after quitting and then gradually decreased. In analysis of patients with the longest follow-up time, diabetes risk dropped after 30 years of cessation to that of participants who had never smoked, the authors reported.
Compared with current smokers, hazard ratios for T2DM in recent quitters were 1.08 (95% CI, 0.93-1.26) for those without weight gain, 1.15 (95% CI, 0.99-1.33) for those with weight gain of 0.1-5.0 kg, 1.36 (95% CI, 1.16-1.58) for those with weight gain of 5.1-10 kg, and 1.59 (95% CI, 1.36-1.85) in those with weight gain of more than 10 kg.
In the mortality analysis, 23,867 deaths occurred, of which 5,492 were due to cardiovascular disease. Compared with current smokers, hazard ratios for death from cardiovascular disease in recent quitters were 0.69 (95% CI, 0.54-0.88) in those without weight gain; 0.47 (95% CI, 0.35-0.63) in those with weight gain of 0.1-5 kg; 0.25 (95% CI, 0.15-0.42) in those with weight gain of 5.1-10 kg; 0.33 (95% CI, 0.18-0.60) in those with weight gain of more than 10 kg; and 0.50 (95% CI, 0.46-0.55) for longer term quitters. The corresponding hazard ratios for all-cause deaths in the same weight gain groups were 0.81 (95% CI, 0.73-0.90); 0.52 (95% CI, 0.46-0.59); 0.46 (95% CI, 0.38-0.55); 0.50 (95% CI, 0.40-0.63); and 0.57 (95% CI, 0.54-0.59).
The findings suggest that weight gain after quitting smoking “did not attenuate the apparent benefits of smoking cessation on reducing cardiovascular mortality or extending longevity,” the authors said. “However, preventing excessive weight gain may maximize the health benefits of smoking cessation through reducing the short-term risk of diabetes and further lowering the long-term risk of death.”
The second study, which included 271,174 patients with T2DM from the Swedish National Diabetes Register and 1,355,870 controls, examined five risk factors: elevated glycated hemoglobin level, elevated low-density lipoprotein cholesterol level, albuminuria, smoking, and elevated blood pressure.
All-cause mortality, myocardial infarction, stroke, and hospitalization for heart failure were evaluated. The risk of each outcome among patients with T2DM was estimated according to the number of risk-factor variables within guideline-recommended target ranges, compared with matched controls, wrote Aidin Rawshani, MD, of the department of molecular and clinical medicine at the University of Gothenburg (Sweden), and his coauthors (N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMoa1800256).
Among the T2DM patients, the excess risk of outcomes was reduced with each risk factor variable within the recommended target range. A total of 37,825 patients with T2DM (13.9%) and 137,520 controls (10.1%) died during the study period.
Among T2DM patients with all variables within target range, the hazard ratio was 1.06 for all-cause death (95% CI, 1.00-1.12); 0.84 for acute myocardial infarction (95% CI, 0.75-0.93); and 0.95 for stroke (95% CI, 0.84-1.07). Smoking was the strongest predictor of death, followed by physical activity, marital status, glycated hemoglobin level, and use of statins.
The study results “indicate that having all five risk-factor variables within the target ranges could theoretically eliminate the excess risk of acute myocardial infarction,” Dr. Rawshani and his colleagues wrote. “Patients with type 2 diabetes who had five risk-factor variables within target ranges appeared to have little or no excess risks of death, myocardial infarction, and stroke as compared with the general population.”
Dr. Hu and his coauthors did not report any disclosures. Dr. Rawshani’s coauthors disclosed relationships with numerous companies including Amgen, Astra Zeneca, and Boehringer Ingelheim.
SOURCE: Hu Y et al. N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMoa1803626. Rawshani A et al. N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMoa1800256.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Taking steps to manage cardiovascular risk can help improve the long-term outlook for type 2 diabetes mellitus.
Major finding: Despite a temporary increase in type 2 diabetes mellitus risk, post–smoking cessation weight gain did not diminish the long-term benefits of reduced cardiovascular and all-cause mortality. In patients with type 2 diabetes, the excess risk of adverse outcomes was reduced with each risk factor variable within the recommended target range.
Study details: An analysis of three cohort studies and a separate analysis of 271,174 patients with T2DM from the Swedish National Diabetes Register and 1,355,870 controls.
Disclosures: Dr. Hu and coauthors did not report any disclosures. Dr. Rawshani’s coauthors disclosed relationships with numerous companies including Amgen, Astra Zeneca, and Boehringer Ingelheim.
Source: Hu Y et al. N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMoa1803626. Rawshani A et al. N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMoa1800256.
Spontaneous intracranial hypotension: a triple misnomer
SAN FRANCISCO – Spontaneous intracranial hypotension couldn’t have a more misleading moniker.
First of all, spontaneous intracranial hypotension (SIH) is not always spontaneous; often there is an antecedent causal event. Second, the main problem isn’t intracranial, it’s a leak in the spinal column, most often in the low cervical or thoracic zone. And cerebrospinal fluid (CSF) pressure is usually normal in these patients, Deborah I. Friedman, MD, said in the annual Seymour Solomon Award Lecture at the annual meeting of the American Headache Society.
Moreover, although SIH is usually labeled a rare disorder, with a published annual incidence of 5 cases per 100,000, that’s doubtless a gross underestimate stemming from the absence of an ICD-9 or -10 code for the condition, according to Dr. Friedman, chief of the division of headache medicine, professor of neurology, and professor of ophthalmology at the University of Texas Southwestern Medical Center, Dallas.
“[SIH is] much more common than we think,” she asserted. “These people are out there. They’re in your offices. I can guarantee you, there are patients you’ve been seeing for years in your practice that have [SIH]. I’ve missed it. I bet you have, too.”
She focused her award lecture on the diagnosis of SIH from the perspective of a headache specialist.
“Most of the literature that’s out there – and it is good literature – wasn’t written by headache medicine specialists, it was written by very famous and prominent neurosurgeons and neuroradiologists. But the people we see are not necessarily the people they see,” Dr. Friedman explained.
SIH can be a challenging diagnosis because of its myriad presentations.
“You need to be a detective,” she emphasized.
Headache is the most common symptom of SIH and the reason patients with the disorder seek out a headache specialist. It’s time to consider the diagnosis in a patient with a new daily persistent headache, or in the patient running around with a diagnosis of chronic migraine for which nothing works.
“The people who come in with a huge list of medications they’ve tried and nothing works? That’s unusual for migraine. Usually something works for migraine,” she observed.
The headache of SIH can be thunderclap in onset, although not necessarily so. The most common location is posterior, but the pain can be centered anywhere in the head or face. Bilateral pain is more common than unilateral.
The most typical headache pattern is one that’s orthostatic or gets worse at the end of the day. The longer a patient has SIH, though, the less likely that they’ll have a postural component. The majority of patients are awakened by their headache in the middle of the night. The headache is often exertional and usually worsens with Valsalva maneuvers, including coughing, sneezing, lifting, bending forward, straining, singing, and/or sexual activity. People with SIH often find that caffeine works very well for them. Ask nonleading questions about these issues, she suggested.
Besides headache, other common symptoms of SIH include tinnitus, abnormal hearing as if underwater, neck pain, imbalance, pain between the shoulder blades, and blurred or double vision.
Risk factors that are a tipoff include joint hypermobility, previous lumbar puncture, epidural or spinal anesthesia, known disc disease, or a personal or family history of retinal detachment at a young age, aneurysm, dissection, or valvular heart disease.
Joint hypermobility is really common in patients with SIH. These are often headache patients who enjoy yoga and were superflexible as children, participating in gymnastics, ballet, or cheerleading.
“We’re looking for Cirque du Soleil here, so you have to ask the Cirque du Soleil questions,” Dr. Friedman said.
On physical examination, she looks for joint hypermobility, makes sure that spontaneous retinal venous pulsations indicative of normal CSF pressure are present when she looks in the eyes, and puts the patient in 5 degrees of Trendelenburg position for 5-10 minutes to see if that improves the headache and other symptoms.
One of the first things Dr. Friedman does when she suspects the diagnosis is to send a patient to the recommended website of the Spinal CSF Leak Foundation. She asks them to take a look around the site and tell her if the descriptions sound like them.
There is broad agreement that the first-line diagnostic test is brain imaging via MRI with gadolinium enhancement. The diagnostic challenge posed by SIH is that the test is normal in 30% of affected patients.
At present there is no consensus as to the next move when the brain MRI is negative. Some favor CT with or without MR myelography, others a T2-weighted spine MRI. But despite a thorough search, no leak is found in about half of individuals with SIH.
Although Dr. Friedman focused on diagnosis, she turned briefly to treatment. She has found that conservative measures don’t work very well. A reasonable strategy, even if a leak site hasn’t been identified, is to treat with a high-volume epidural CT-guided targeted blood patch with fibrin sealant.
“It gives relief about a third of the time each time you do it,” according to Dr. Friedman.
SAN FRANCISCO – Spontaneous intracranial hypotension couldn’t have a more misleading moniker.
First of all, spontaneous intracranial hypotension (SIH) is not always spontaneous; often there is an antecedent causal event. Second, the main problem isn’t intracranial, it’s a leak in the spinal column, most often in the low cervical or thoracic zone. And cerebrospinal fluid (CSF) pressure is usually normal in these patients, Deborah I. Friedman, MD, said in the annual Seymour Solomon Award Lecture at the annual meeting of the American Headache Society.
Moreover, although SIH is usually labeled a rare disorder, with a published annual incidence of 5 cases per 100,000, that’s doubtless a gross underestimate stemming from the absence of an ICD-9 or -10 code for the condition, according to Dr. Friedman, chief of the division of headache medicine, professor of neurology, and professor of ophthalmology at the University of Texas Southwestern Medical Center, Dallas.
“[SIH is] much more common than we think,” she asserted. “These people are out there. They’re in your offices. I can guarantee you, there are patients you’ve been seeing for years in your practice that have [SIH]. I’ve missed it. I bet you have, too.”
She focused her award lecture on the diagnosis of SIH from the perspective of a headache specialist.
“Most of the literature that’s out there – and it is good literature – wasn’t written by headache medicine specialists, it was written by very famous and prominent neurosurgeons and neuroradiologists. But the people we see are not necessarily the people they see,” Dr. Friedman explained.
SIH can be a challenging diagnosis because of its myriad presentations.
“You need to be a detective,” she emphasized.
Headache is the most common symptom of SIH and the reason patients with the disorder seek out a headache specialist. It’s time to consider the diagnosis in a patient with a new daily persistent headache, or in the patient running around with a diagnosis of chronic migraine for which nothing works.
“The people who come in with a huge list of medications they’ve tried and nothing works? That’s unusual for migraine. Usually something works for migraine,” she observed.
The headache of SIH can be thunderclap in onset, although not necessarily so. The most common location is posterior, but the pain can be centered anywhere in the head or face. Bilateral pain is more common than unilateral.
The most typical headache pattern is one that’s orthostatic or gets worse at the end of the day. The longer a patient has SIH, though, the less likely that they’ll have a postural component. The majority of patients are awakened by their headache in the middle of the night. The headache is often exertional and usually worsens with Valsalva maneuvers, including coughing, sneezing, lifting, bending forward, straining, singing, and/or sexual activity. People with SIH often find that caffeine works very well for them. Ask nonleading questions about these issues, she suggested.
Besides headache, other common symptoms of SIH include tinnitus, abnormal hearing as if underwater, neck pain, imbalance, pain between the shoulder blades, and blurred or double vision.
Risk factors that are a tipoff include joint hypermobility, previous lumbar puncture, epidural or spinal anesthesia, known disc disease, or a personal or family history of retinal detachment at a young age, aneurysm, dissection, or valvular heart disease.
Joint hypermobility is really common in patients with SIH. These are often headache patients who enjoy yoga and were superflexible as children, participating in gymnastics, ballet, or cheerleading.
“We’re looking for Cirque du Soleil here, so you have to ask the Cirque du Soleil questions,” Dr. Friedman said.
On physical examination, she looks for joint hypermobility, makes sure that spontaneous retinal venous pulsations indicative of normal CSF pressure are present when she looks in the eyes, and puts the patient in 5 degrees of Trendelenburg position for 5-10 minutes to see if that improves the headache and other symptoms.
One of the first things Dr. Friedman does when she suspects the diagnosis is to send a patient to the recommended website of the Spinal CSF Leak Foundation. She asks them to take a look around the site and tell her if the descriptions sound like them.
There is broad agreement that the first-line diagnostic test is brain imaging via MRI with gadolinium enhancement. The diagnostic challenge posed by SIH is that the test is normal in 30% of affected patients.
At present there is no consensus as to the next move when the brain MRI is negative. Some favor CT with or without MR myelography, others a T2-weighted spine MRI. But despite a thorough search, no leak is found in about half of individuals with SIH.
Although Dr. Friedman focused on diagnosis, she turned briefly to treatment. She has found that conservative measures don’t work very well. A reasonable strategy, even if a leak site hasn’t been identified, is to treat with a high-volume epidural CT-guided targeted blood patch with fibrin sealant.
“It gives relief about a third of the time each time you do it,” according to Dr. Friedman.
SAN FRANCISCO – Spontaneous intracranial hypotension couldn’t have a more misleading moniker.
First of all, spontaneous intracranial hypotension (SIH) is not always spontaneous; often there is an antecedent causal event. Second, the main problem isn’t intracranial, it’s a leak in the spinal column, most often in the low cervical or thoracic zone. And cerebrospinal fluid (CSF) pressure is usually normal in these patients, Deborah I. Friedman, MD, said in the annual Seymour Solomon Award Lecture at the annual meeting of the American Headache Society.
Moreover, although SIH is usually labeled a rare disorder, with a published annual incidence of 5 cases per 100,000, that’s doubtless a gross underestimate stemming from the absence of an ICD-9 or -10 code for the condition, according to Dr. Friedman, chief of the division of headache medicine, professor of neurology, and professor of ophthalmology at the University of Texas Southwestern Medical Center, Dallas.
“[SIH is] much more common than we think,” she asserted. “These people are out there. They’re in your offices. I can guarantee you, there are patients you’ve been seeing for years in your practice that have [SIH]. I’ve missed it. I bet you have, too.”
She focused her award lecture on the diagnosis of SIH from the perspective of a headache specialist.
“Most of the literature that’s out there – and it is good literature – wasn’t written by headache medicine specialists, it was written by very famous and prominent neurosurgeons and neuroradiologists. But the people we see are not necessarily the people they see,” Dr. Friedman explained.
SIH can be a challenging diagnosis because of its myriad presentations.
“You need to be a detective,” she emphasized.
Headache is the most common symptom of SIH and the reason patients with the disorder seek out a headache specialist. It’s time to consider the diagnosis in a patient with a new daily persistent headache, or in the patient running around with a diagnosis of chronic migraine for which nothing works.
“The people who come in with a huge list of medications they’ve tried and nothing works? That’s unusual for migraine. Usually something works for migraine,” she observed.
The headache of SIH can be thunderclap in onset, although not necessarily so. The most common location is posterior, but the pain can be centered anywhere in the head or face. Bilateral pain is more common than unilateral.
The most typical headache pattern is one that’s orthostatic or gets worse at the end of the day. The longer a patient has SIH, though, the less likely that they’ll have a postural component. The majority of patients are awakened by their headache in the middle of the night. The headache is often exertional and usually worsens with Valsalva maneuvers, including coughing, sneezing, lifting, bending forward, straining, singing, and/or sexual activity. People with SIH often find that caffeine works very well for them. Ask nonleading questions about these issues, she suggested.
Besides headache, other common symptoms of SIH include tinnitus, abnormal hearing as if underwater, neck pain, imbalance, pain between the shoulder blades, and blurred or double vision.
Risk factors that are a tipoff include joint hypermobility, previous lumbar puncture, epidural or spinal anesthesia, known disc disease, or a personal or family history of retinal detachment at a young age, aneurysm, dissection, or valvular heart disease.
Joint hypermobility is really common in patients with SIH. These are often headache patients who enjoy yoga and were superflexible as children, participating in gymnastics, ballet, or cheerleading.
“We’re looking for Cirque du Soleil here, so you have to ask the Cirque du Soleil questions,” Dr. Friedman said.
On physical examination, she looks for joint hypermobility, makes sure that spontaneous retinal venous pulsations indicative of normal CSF pressure are present when she looks in the eyes, and puts the patient in 5 degrees of Trendelenburg position for 5-10 minutes to see if that improves the headache and other symptoms.
One of the first things Dr. Friedman does when she suspects the diagnosis is to send a patient to the recommended website of the Spinal CSF Leak Foundation. She asks them to take a look around the site and tell her if the descriptions sound like them.
There is broad agreement that the first-line diagnostic test is brain imaging via MRI with gadolinium enhancement. The diagnostic challenge posed by SIH is that the test is normal in 30% of affected patients.
At present there is no consensus as to the next move when the brain MRI is negative. Some favor CT with or without MR myelography, others a T2-weighted spine MRI. But despite a thorough search, no leak is found in about half of individuals with SIH.
Although Dr. Friedman focused on diagnosis, she turned briefly to treatment. She has found that conservative measures don’t work very well. A reasonable strategy, even if a leak site hasn’t been identified, is to treat with a high-volume epidural CT-guided targeted blood patch with fibrin sealant.
“It gives relief about a third of the time each time you do it,” according to Dr. Friedman.
REPORTING FROM THE AHS ANNUAL MEETING
Workouts tailored to personality can be more satisfying
Exercise is good for the body and can be beneficial for the mind. But when it comes to the type of activity, the quality of the sweat might vary from mundane to wonderful depending on your personality.
“People tend to think that there’s one best way to go about an exercise routine, but one-size-fits-all doesn’t apply here. Instead, it’s important to know who you are and select a type of exercise that fits,” said John Hackston, a psychologist with the British firm OPP, which markets personality assessment programs and business psychology solutions. He also led a study presented at the last meeting of the British Psychological Society.
“We were keen to investigate how organizations could help their staffs’ development through exercise, finding that matching an individual’s personality type to a particular type of exercise can increase both the effectiveness and the person’s enjoyment of it,” he explained in an interview with Science Daily.
The survey of more than 800 people from diverse businesses in several countries showed that certain exercise activities and settings attract certain personalities.
Extroverts might prefer the hustle bustle of a health club, where group exercise classes and rows of equipment provide the opportunity for the shedding of sweat in a social setting and can offer the group motivation to keep the exercise going. By contrast, introverts might prefer the rejuvenating peace and solitude of a long-distance run or walk.
The findings went further. Regimented exercise was appealing to those whose outlook on the world was through the lens of objective logic, but not those who place more importance on feelings and values.
The changing nature of the outdoors attracted people with a more creative mindset, particularly those open to new ideas. For these folks, outdoor activities like running and cycling fit the bill better than the local gym.
“The most important piece of advice to come out of this research is that ” Mr. Hackston said. “There can be pressure to follow the crowd to the gym or sign up to the latest exercise fad, but it would be much more effective for them to match their personality type to an exercise plan that is more likely to last the test of time.”
Click here to read about the study.
Exercise is good for the body and can be beneficial for the mind. But when it comes to the type of activity, the quality of the sweat might vary from mundane to wonderful depending on your personality.
“People tend to think that there’s one best way to go about an exercise routine, but one-size-fits-all doesn’t apply here. Instead, it’s important to know who you are and select a type of exercise that fits,” said John Hackston, a psychologist with the British firm OPP, which markets personality assessment programs and business psychology solutions. He also led a study presented at the last meeting of the British Psychological Society.
“We were keen to investigate how organizations could help their staffs’ development through exercise, finding that matching an individual’s personality type to a particular type of exercise can increase both the effectiveness and the person’s enjoyment of it,” he explained in an interview with Science Daily.
The survey of more than 800 people from diverse businesses in several countries showed that certain exercise activities and settings attract certain personalities.
Extroverts might prefer the hustle bustle of a health club, where group exercise classes and rows of equipment provide the opportunity for the shedding of sweat in a social setting and can offer the group motivation to keep the exercise going. By contrast, introverts might prefer the rejuvenating peace and solitude of a long-distance run or walk.
The findings went further. Regimented exercise was appealing to those whose outlook on the world was through the lens of objective logic, but not those who place more importance on feelings and values.
The changing nature of the outdoors attracted people with a more creative mindset, particularly those open to new ideas. For these folks, outdoor activities like running and cycling fit the bill better than the local gym.
“The most important piece of advice to come out of this research is that ” Mr. Hackston said. “There can be pressure to follow the crowd to the gym or sign up to the latest exercise fad, but it would be much more effective for them to match their personality type to an exercise plan that is more likely to last the test of time.”
Click here to read about the study.
Exercise is good for the body and can be beneficial for the mind. But when it comes to the type of activity, the quality of the sweat might vary from mundane to wonderful depending on your personality.
“People tend to think that there’s one best way to go about an exercise routine, but one-size-fits-all doesn’t apply here. Instead, it’s important to know who you are and select a type of exercise that fits,” said John Hackston, a psychologist with the British firm OPP, which markets personality assessment programs and business psychology solutions. He also led a study presented at the last meeting of the British Psychological Society.
“We were keen to investigate how organizations could help their staffs’ development through exercise, finding that matching an individual’s personality type to a particular type of exercise can increase both the effectiveness and the person’s enjoyment of it,” he explained in an interview with Science Daily.
The survey of more than 800 people from diverse businesses in several countries showed that certain exercise activities and settings attract certain personalities.
Extroverts might prefer the hustle bustle of a health club, where group exercise classes and rows of equipment provide the opportunity for the shedding of sweat in a social setting and can offer the group motivation to keep the exercise going. By contrast, introverts might prefer the rejuvenating peace and solitude of a long-distance run or walk.
The findings went further. Regimented exercise was appealing to those whose outlook on the world was through the lens of objective logic, but not those who place more importance on feelings and values.
The changing nature of the outdoors attracted people with a more creative mindset, particularly those open to new ideas. For these folks, outdoor activities like running and cycling fit the bill better than the local gym.
“The most important piece of advice to come out of this research is that ” Mr. Hackston said. “There can be pressure to follow the crowd to the gym or sign up to the latest exercise fad, but it would be much more effective for them to match their personality type to an exercise plan that is more likely to last the test of time.”
Click here to read about the study.
Latex Allergy From Biologic Injectable Devices