User login
Autonomic Dysfunction Greater in PPTH than Migraine
Symptoms of autonomic dysfunction were greatest among those with persistent posttraumatic headaches (PPTH) compared to migraine and healthy controls (HCs), a recent study found. In addition, among individuals with PPTH, number of lifetime traumatic brain injuries (TBIs) was associated with greater symptoms of autonomic dysfunction, while greater headache burden was associated with higher vasomotor domain autonomic dysfunction subscores, potentially indicating that PPTH patients with higher disease burden have an increased risk for having autonomic dysfunction. Individuals with PPTH (n=56) (87.5% of whom had a migraine/probable migraine phenotype), migraine (n=30), and HCs (n=36) were prospectively assessed in this cross‐sectional cohort study using the COMPASS‐31 questionnaire. Total COMPASS‐31 scores and individual domain scores were compared between subject groups. Researchers found:
- COMPASS‐31 mean total weighted score was 37.22 ± 15.44 in the PPTH group, 27.15 ± 14.37 in the migraine group, and 11.67 ± 8.98 for HCs.
- COMPASS‐31 mean weighted total scores were significantly higher in those with PPTH vs migraine, for PPTH vs HCs, and for migraine vs HCs.
Howard L, Dumkrieger G, Chong CD, Ross K, Berisha V, Schwedt TJ. Symptoms of autonomic dysfunction among those with persistent posttraumatic headache attributed to mild traumatic brain injury: A comparison to migraine and healthy controls. [Published online ahead of print August 29, 2018]. Headache. doi:10.1111/head.13396.
Symptoms of autonomic dysfunction were greatest among those with persistent posttraumatic headaches (PPTH) compared to migraine and healthy controls (HCs), a recent study found. In addition, among individuals with PPTH, number of lifetime traumatic brain injuries (TBIs) was associated with greater symptoms of autonomic dysfunction, while greater headache burden was associated with higher vasomotor domain autonomic dysfunction subscores, potentially indicating that PPTH patients with higher disease burden have an increased risk for having autonomic dysfunction. Individuals with PPTH (n=56) (87.5% of whom had a migraine/probable migraine phenotype), migraine (n=30), and HCs (n=36) were prospectively assessed in this cross‐sectional cohort study using the COMPASS‐31 questionnaire. Total COMPASS‐31 scores and individual domain scores were compared between subject groups. Researchers found:
- COMPASS‐31 mean total weighted score was 37.22 ± 15.44 in the PPTH group, 27.15 ± 14.37 in the migraine group, and 11.67 ± 8.98 for HCs.
- COMPASS‐31 mean weighted total scores were significantly higher in those with PPTH vs migraine, for PPTH vs HCs, and for migraine vs HCs.
Howard L, Dumkrieger G, Chong CD, Ross K, Berisha V, Schwedt TJ. Symptoms of autonomic dysfunction among those with persistent posttraumatic headache attributed to mild traumatic brain injury: A comparison to migraine and healthy controls. [Published online ahead of print August 29, 2018]. Headache. doi:10.1111/head.13396.
Symptoms of autonomic dysfunction were greatest among those with persistent posttraumatic headaches (PPTH) compared to migraine and healthy controls (HCs), a recent study found. In addition, among individuals with PPTH, number of lifetime traumatic brain injuries (TBIs) was associated with greater symptoms of autonomic dysfunction, while greater headache burden was associated with higher vasomotor domain autonomic dysfunction subscores, potentially indicating that PPTH patients with higher disease burden have an increased risk for having autonomic dysfunction. Individuals with PPTH (n=56) (87.5% of whom had a migraine/probable migraine phenotype), migraine (n=30), and HCs (n=36) were prospectively assessed in this cross‐sectional cohort study using the COMPASS‐31 questionnaire. Total COMPASS‐31 scores and individual domain scores were compared between subject groups. Researchers found:
- COMPASS‐31 mean total weighted score was 37.22 ± 15.44 in the PPTH group, 27.15 ± 14.37 in the migraine group, and 11.67 ± 8.98 for HCs.
- COMPASS‐31 mean weighted total scores were significantly higher in those with PPTH vs migraine, for PPTH vs HCs, and for migraine vs HCs.
Howard L, Dumkrieger G, Chong CD, Ross K, Berisha V, Schwedt TJ. Symptoms of autonomic dysfunction among those with persistent posttraumatic headache attributed to mild traumatic brain injury: A comparison to migraine and healthy controls. [Published online ahead of print August 29, 2018]. Headache. doi:10.1111/head.13396.
What’s in that e-cigarette? It may be cannabis
Nearly 1 in 11 U.S. middle and high school students have used a cannabis product in an e-cigarette, according to a school-based survey of 20,675 students.
The survey found that 8.9% of students in grades 6-12 said they had used an e-cigarette with marijuana, tetrahydrocannabinol or hash oil, or tetrahydrocannabinol wax. Among the students who reported ever using e-cigarettes, 30.6% had used a cannabis product in the device. The findings were published in JAMA Pediatrics.
This translated to around 1.7 million high school students and 425,000 middle school students who had ever used cannabis in e-cigarettes; figures the authors said were consistent with or higher than previous reports among U.S. and Canadian students.
Katrina F. Trivers, PhD, and her colleagues from the Centers for Disease Control and Prevention, noted that the U.S. Surgeon General has found e-cigarette aerosol can contain potentially harmful ingredients. Additionally, the National Academies of Sciences has said youth cannabis use can harm learning and memory.
“Strategies to reduce cannabis use in e-cigarettes are critical for protecting young people from these potential health risks,” the researchers wrote.
Male students and high school students were significantly more likely to report using cannabis products in an e-cigarette (10.6% and 12.4%, respectively), compared with female or middle school students.
Among current users of e-cigarettes, 39.5% reported using cannabis in the e-cigarette, while among those who used other tobacco products, 38.5% used cannabis in e-cigarettes. Higher e-cigarette use was also associated with use of cannabis products in e-cigarettes.
Living with someone who used tobacco products was associated with a higher incidence of cannabis in e-cigarette use (13%). Researchers also saw a higher use of cannabis in e-cigarettes among students of Hispanic ethnicity, compared with other ethnicities.
In 2015, around one-third of U.S. middle and high school students said they had used nonnicotine substances in e-cigarettes, but the use of cannabis in e-cigarettes could increase as several states consider legalizing cannabis sales for adults. “Given the high concurrent use of tobacco and other substances, it is important to monitor the substances youth use in e-cigarettes,” they wrote.
The researchers reported having no financial disclosures.
SOURCE: Trivers KF et al. JAMA Pediatr. 2018 Sep 17. doi: 10.1001/jamapediatrics.2018.1920.
Nearly 1 in 11 U.S. middle and high school students have used a cannabis product in an e-cigarette, according to a school-based survey of 20,675 students.
The survey found that 8.9% of students in grades 6-12 said they had used an e-cigarette with marijuana, tetrahydrocannabinol or hash oil, or tetrahydrocannabinol wax. Among the students who reported ever using e-cigarettes, 30.6% had used a cannabis product in the device. The findings were published in JAMA Pediatrics.
This translated to around 1.7 million high school students and 425,000 middle school students who had ever used cannabis in e-cigarettes; figures the authors said were consistent with or higher than previous reports among U.S. and Canadian students.
Katrina F. Trivers, PhD, and her colleagues from the Centers for Disease Control and Prevention, noted that the U.S. Surgeon General has found e-cigarette aerosol can contain potentially harmful ingredients. Additionally, the National Academies of Sciences has said youth cannabis use can harm learning and memory.
“Strategies to reduce cannabis use in e-cigarettes are critical for protecting young people from these potential health risks,” the researchers wrote.
Male students and high school students were significantly more likely to report using cannabis products in an e-cigarette (10.6% and 12.4%, respectively), compared with female or middle school students.
Among current users of e-cigarettes, 39.5% reported using cannabis in the e-cigarette, while among those who used other tobacco products, 38.5% used cannabis in e-cigarettes. Higher e-cigarette use was also associated with use of cannabis products in e-cigarettes.
Living with someone who used tobacco products was associated with a higher incidence of cannabis in e-cigarette use (13%). Researchers also saw a higher use of cannabis in e-cigarettes among students of Hispanic ethnicity, compared with other ethnicities.
In 2015, around one-third of U.S. middle and high school students said they had used nonnicotine substances in e-cigarettes, but the use of cannabis in e-cigarettes could increase as several states consider legalizing cannabis sales for adults. “Given the high concurrent use of tobacco and other substances, it is important to monitor the substances youth use in e-cigarettes,” they wrote.
The researchers reported having no financial disclosures.
SOURCE: Trivers KF et al. JAMA Pediatr. 2018 Sep 17. doi: 10.1001/jamapediatrics.2018.1920.
Nearly 1 in 11 U.S. middle and high school students have used a cannabis product in an e-cigarette, according to a school-based survey of 20,675 students.
The survey found that 8.9% of students in grades 6-12 said they had used an e-cigarette with marijuana, tetrahydrocannabinol or hash oil, or tetrahydrocannabinol wax. Among the students who reported ever using e-cigarettes, 30.6% had used a cannabis product in the device. The findings were published in JAMA Pediatrics.
This translated to around 1.7 million high school students and 425,000 middle school students who had ever used cannabis in e-cigarettes; figures the authors said were consistent with or higher than previous reports among U.S. and Canadian students.
Katrina F. Trivers, PhD, and her colleagues from the Centers for Disease Control and Prevention, noted that the U.S. Surgeon General has found e-cigarette aerosol can contain potentially harmful ingredients. Additionally, the National Academies of Sciences has said youth cannabis use can harm learning and memory.
“Strategies to reduce cannabis use in e-cigarettes are critical for protecting young people from these potential health risks,” the researchers wrote.
Male students and high school students were significantly more likely to report using cannabis products in an e-cigarette (10.6% and 12.4%, respectively), compared with female or middle school students.
Among current users of e-cigarettes, 39.5% reported using cannabis in the e-cigarette, while among those who used other tobacco products, 38.5% used cannabis in e-cigarettes. Higher e-cigarette use was also associated with use of cannabis products in e-cigarettes.
Living with someone who used tobacco products was associated with a higher incidence of cannabis in e-cigarette use (13%). Researchers also saw a higher use of cannabis in e-cigarettes among students of Hispanic ethnicity, compared with other ethnicities.
In 2015, around one-third of U.S. middle and high school students said they had used nonnicotine substances in e-cigarettes, but the use of cannabis in e-cigarettes could increase as several states consider legalizing cannabis sales for adults. “Given the high concurrent use of tobacco and other substances, it is important to monitor the substances youth use in e-cigarettes,” they wrote.
The researchers reported having no financial disclosures.
SOURCE: Trivers KF et al. JAMA Pediatr. 2018 Sep 17. doi: 10.1001/jamapediatrics.2018.1920.
FROM JAMA PEDIATRICS
Key clinical point:
Major finding: A survey of U.S. students showed that 8.9% have used cannabis in an e-cigarette. Among e-cigarette users, cannabis use in e-cigarettes was reported by 30.6% of students.
Study details: A school-based survey of 20,675 students.
Disclosures: The researchers reported having no financial disclosures.
Source: Trivers KF et al. JAMA Pediatr. 2018 Sep 17. doi: 10.1001/jamapediatrics.2018.1920.
Metastatic breast cancer lesions immunologically depleted compared with primary
The immune microenvironment of metastatic breast cancer lesions is relatively inert and depleted versus primary tumors, results of a recent study suggest.
“These results predict that immune therapy may be more successful in early stage breast cancers rather than in metastatic disease,” Lajos Pusztai, MD, and study coinvestigators reported in Annals of Oncology.
However, metastatic breast cancers showed high expression levels of some targetable molecules that may provide a “foundation for rational immunotherapy combination strategies,” wrote Dr. Pusztai, director of breast cancer translational research at Yale Cancer Center, New Haven, Conn., and his coinvestigators.
The investigators looked at tumor PD-L1 protein expression, tumor infiltrating lymphocyte (TIL) count, and mRNA expression for 730 immune-related genes in both primary and metastatic cancer samples obtained from pathologists at Yale.
The study included one cohort with full sections of paired metastatic and primary tumors from 45 patients, and a second cohort of tissue microarrays from 55 other patients.
Compared with primary lesions, metastatic lesions had substantially lower levels of PD-L1 expression and TIL counts, the investigators found.
Staining of PD-L1 was primarily seen in stromal immune cells, rather than tumor cells, according to investigators. The median stromal PD-L1 positivity was 14% for metastases and 52% for primary tumors in the first cohort (P = .0004), and 7% for metastases and 22% for primary tumors in the second cohort (P = .03).
They also reported significant decreased TIL counts in metastatic lesions for both the first (P = .026) and second (P = .089) cohorts, the report shows.
Immune gene expression profiling results, similarly, showed that most immune cell types and functions were “depleted” in the metastatic lesions, including a decreased mRNA expression of CTLA4, Dr. Pusztai and his colleagues reported.
The “lesser immunogenicity” of metastatic breast cancer cells was shown by decreased expression of immune proteasome and MHC class I genes, along with increased expression of HLA-E, which has been shown to suppress immunity, and reduced presence of dendritic cells, they said.
However, they also found high expression of targetable molecules in metastatic lesions. Those included macrophage markers such as CD68 and CD163, cytokine ligand/receptor pairs that mediate pro-tumorigenic effects, such as CCL2/CCR2 and CXCR4/CXCL12, and signaling molecules such as STAT-3 and JAK2, among others.
“We suggest that targeting these molecules may lead to synergy with PD1/PD-L1 blockade in metastatic breast cancer,” they wrote.
The work by Dr. Pusztai and his colleagues was supported by the Breast Cancer Research Foundation, Susan G Komen for the Cure, Department of Defense Breast Cancer Research Program Awards, and the Rosztoczy Foundation. The authors declared no conflicts of interest.
SOURCE: Szekely B, et al. Ann Oncol. 2018 Sep 10. doi: 10.1093/annonc/mdy399.
The immune microenvironment of metastatic breast cancer lesions is relatively inert and depleted versus primary tumors, results of a recent study suggest.
“These results predict that immune therapy may be more successful in early stage breast cancers rather than in metastatic disease,” Lajos Pusztai, MD, and study coinvestigators reported in Annals of Oncology.
However, metastatic breast cancers showed high expression levels of some targetable molecules that may provide a “foundation for rational immunotherapy combination strategies,” wrote Dr. Pusztai, director of breast cancer translational research at Yale Cancer Center, New Haven, Conn., and his coinvestigators.
The investigators looked at tumor PD-L1 protein expression, tumor infiltrating lymphocyte (TIL) count, and mRNA expression for 730 immune-related genes in both primary and metastatic cancer samples obtained from pathologists at Yale.
The study included one cohort with full sections of paired metastatic and primary tumors from 45 patients, and a second cohort of tissue microarrays from 55 other patients.
Compared with primary lesions, metastatic lesions had substantially lower levels of PD-L1 expression and TIL counts, the investigators found.
Staining of PD-L1 was primarily seen in stromal immune cells, rather than tumor cells, according to investigators. The median stromal PD-L1 positivity was 14% for metastases and 52% for primary tumors in the first cohort (P = .0004), and 7% for metastases and 22% for primary tumors in the second cohort (P = .03).
They also reported significant decreased TIL counts in metastatic lesions for both the first (P = .026) and second (P = .089) cohorts, the report shows.
Immune gene expression profiling results, similarly, showed that most immune cell types and functions were “depleted” in the metastatic lesions, including a decreased mRNA expression of CTLA4, Dr. Pusztai and his colleagues reported.
The “lesser immunogenicity” of metastatic breast cancer cells was shown by decreased expression of immune proteasome and MHC class I genes, along with increased expression of HLA-E, which has been shown to suppress immunity, and reduced presence of dendritic cells, they said.
However, they also found high expression of targetable molecules in metastatic lesions. Those included macrophage markers such as CD68 and CD163, cytokine ligand/receptor pairs that mediate pro-tumorigenic effects, such as CCL2/CCR2 and CXCR4/CXCL12, and signaling molecules such as STAT-3 and JAK2, among others.
“We suggest that targeting these molecules may lead to synergy with PD1/PD-L1 blockade in metastatic breast cancer,” they wrote.
The work by Dr. Pusztai and his colleagues was supported by the Breast Cancer Research Foundation, Susan G Komen for the Cure, Department of Defense Breast Cancer Research Program Awards, and the Rosztoczy Foundation. The authors declared no conflicts of interest.
SOURCE: Szekely B, et al. Ann Oncol. 2018 Sep 10. doi: 10.1093/annonc/mdy399.
The immune microenvironment of metastatic breast cancer lesions is relatively inert and depleted versus primary tumors, results of a recent study suggest.
“These results predict that immune therapy may be more successful in early stage breast cancers rather than in metastatic disease,” Lajos Pusztai, MD, and study coinvestigators reported in Annals of Oncology.
However, metastatic breast cancers showed high expression levels of some targetable molecules that may provide a “foundation for rational immunotherapy combination strategies,” wrote Dr. Pusztai, director of breast cancer translational research at Yale Cancer Center, New Haven, Conn., and his coinvestigators.
The investigators looked at tumor PD-L1 protein expression, tumor infiltrating lymphocyte (TIL) count, and mRNA expression for 730 immune-related genes in both primary and metastatic cancer samples obtained from pathologists at Yale.
The study included one cohort with full sections of paired metastatic and primary tumors from 45 patients, and a second cohort of tissue microarrays from 55 other patients.
Compared with primary lesions, metastatic lesions had substantially lower levels of PD-L1 expression and TIL counts, the investigators found.
Staining of PD-L1 was primarily seen in stromal immune cells, rather than tumor cells, according to investigators. The median stromal PD-L1 positivity was 14% for metastases and 52% for primary tumors in the first cohort (P = .0004), and 7% for metastases and 22% for primary tumors in the second cohort (P = .03).
They also reported significant decreased TIL counts in metastatic lesions for both the first (P = .026) and second (P = .089) cohorts, the report shows.
Immune gene expression profiling results, similarly, showed that most immune cell types and functions were “depleted” in the metastatic lesions, including a decreased mRNA expression of CTLA4, Dr. Pusztai and his colleagues reported.
The “lesser immunogenicity” of metastatic breast cancer cells was shown by decreased expression of immune proteasome and MHC class I genes, along with increased expression of HLA-E, which has been shown to suppress immunity, and reduced presence of dendritic cells, they said.
However, they also found high expression of targetable molecules in metastatic lesions. Those included macrophage markers such as CD68 and CD163, cytokine ligand/receptor pairs that mediate pro-tumorigenic effects, such as CCL2/CCR2 and CXCR4/CXCL12, and signaling molecules such as STAT-3 and JAK2, among others.
“We suggest that targeting these molecules may lead to synergy with PD1/PD-L1 blockade in metastatic breast cancer,” they wrote.
The work by Dr. Pusztai and his colleagues was supported by the Breast Cancer Research Foundation, Susan G Komen for the Cure, Department of Defense Breast Cancer Research Program Awards, and the Rosztoczy Foundation. The authors declared no conflicts of interest.
SOURCE: Szekely B, et al. Ann Oncol. 2018 Sep 10. doi: 10.1093/annonc/mdy399.
FROM ANNALS OF ONCOLOGY
Key clinical point: The immune microenvironment of metastatic breast cancer lesions is relatively inert and depleted versus primary tumors.
Major finding: Median stromal PD-L1 positivity was 14% for metastases and 52% for primary tumors in one cohort (P = .0004), and 7% versus 22% in a second (P = .03).
Study details: Analysis of breast cancer tissue samples (primary tumor and metastatic lesions) from 90 patients
Disclosures: The work was supported by the Breast Cancer Research Foundation, the Susan Komen for the Cure, Department of Defense Breast Cancer Research Program Awards, and the Rosztoczy Foundation. The study authors declared no conflicts of interest.
Source: Szekely B et al. Ann Oncol. 2018 Sep 10. doi: 10.1093/annonc/mdy399.
Early CAR T data on P-BCMA-101 in refractory myeloma
Early results from a phase 1 trial suggest the chimeric antigen receptor (CAR) T-cell therapy P-BCMA-101 can produce responses in patients with relapsed/refractory multiple myeloma.
All 11 patients treated have experienced some clinical response, with 8 patients achieving a partial response (PR) or better.
The most common adverse events were neutropenia and thrombocytopenia. One patient was suspected to have cytokine release syndrome (CRS), but the condition resolved without use of tocilizumab or steroids.
These results were presented at the 2018 CAR-TCR Summit by Eric Ostertag, MD, PhD, chief executive officer of Poseida Therapeutics Inc., the company developing P-BCMA-101.
Dr. Ostertag presented data on 11 patients with heavily pretreated multiple myeloma. They had a median of six prior therapies. The median age was 60 years, and most of the patients were considered high risk.
Prior to receiving P-BCMA-101, patients received conditioning with fludarabine (30 mg/m2) and cyclophosphamide (300 mg/m2) for 3 days.
Patients were then treated across three dose groups with average CAR T-cell doses of 51 x 106 (n = 3), 152 x 106 (n = 7), and 430 x 106 (n = 1).
As of Aug. 10, 2018, all 11 patients were still on study.
There were no dose-limiting toxicities. Eight patients developed neutropenia, and five had thrombocytopenia.
Researchers suspected CRS in one patient, but the condition resolved without tocilizumab or steroid treatment. There was no neurotoxicity reported, and none of the patients required admission to an intensive care unit.
All patients showed improvement in biomarkers following treatment.
Ten patients were evaluable for response by International Myeloma Working Group criteria. Seven of these patients achieved at least a PR, including very good partial responses (VGPRs) and stringent complete response (CR).
The eleventh patient also responded to treatment, but this patient has oligosecretory disease and was evaluable only by PET. The patient had a near-CR by PET.
Poseida Therapeutics would not disclose additional details regarding how many patients achieved a PR, VGPR, or CR, but the company plans to release more information on response at an upcoming meeting.
“The latest data results show that P-BCMA-101 induces deep responses in a heavily pretreated population with relapsed/refractory multiple myeloma, with some patients reaching VGPR and even stringent CR at early efficacy assessments,” Dr. Ostertag said.
This study (NCT03288493) is funded by the California Institute for Regenerative Medicine and Poseida Therapeutics.
Early results from a phase 1 trial suggest the chimeric antigen receptor (CAR) T-cell therapy P-BCMA-101 can produce responses in patients with relapsed/refractory multiple myeloma.
All 11 patients treated have experienced some clinical response, with 8 patients achieving a partial response (PR) or better.
The most common adverse events were neutropenia and thrombocytopenia. One patient was suspected to have cytokine release syndrome (CRS), but the condition resolved without use of tocilizumab or steroids.
These results were presented at the 2018 CAR-TCR Summit by Eric Ostertag, MD, PhD, chief executive officer of Poseida Therapeutics Inc., the company developing P-BCMA-101.
Dr. Ostertag presented data on 11 patients with heavily pretreated multiple myeloma. They had a median of six prior therapies. The median age was 60 years, and most of the patients were considered high risk.
Prior to receiving P-BCMA-101, patients received conditioning with fludarabine (30 mg/m2) and cyclophosphamide (300 mg/m2) for 3 days.
Patients were then treated across three dose groups with average CAR T-cell doses of 51 x 106 (n = 3), 152 x 106 (n = 7), and 430 x 106 (n = 1).
As of Aug. 10, 2018, all 11 patients were still on study.
There were no dose-limiting toxicities. Eight patients developed neutropenia, and five had thrombocytopenia.
Researchers suspected CRS in one patient, but the condition resolved without tocilizumab or steroid treatment. There was no neurotoxicity reported, and none of the patients required admission to an intensive care unit.
All patients showed improvement in biomarkers following treatment.
Ten patients were evaluable for response by International Myeloma Working Group criteria. Seven of these patients achieved at least a PR, including very good partial responses (VGPRs) and stringent complete response (CR).
The eleventh patient also responded to treatment, but this patient has oligosecretory disease and was evaluable only by PET. The patient had a near-CR by PET.
Poseida Therapeutics would not disclose additional details regarding how many patients achieved a PR, VGPR, or CR, but the company plans to release more information on response at an upcoming meeting.
“The latest data results show that P-BCMA-101 induces deep responses in a heavily pretreated population with relapsed/refractory multiple myeloma, with some patients reaching VGPR and even stringent CR at early efficacy assessments,” Dr. Ostertag said.
This study (NCT03288493) is funded by the California Institute for Regenerative Medicine and Poseida Therapeutics.
Early results from a phase 1 trial suggest the chimeric antigen receptor (CAR) T-cell therapy P-BCMA-101 can produce responses in patients with relapsed/refractory multiple myeloma.
All 11 patients treated have experienced some clinical response, with 8 patients achieving a partial response (PR) or better.
The most common adverse events were neutropenia and thrombocytopenia. One patient was suspected to have cytokine release syndrome (CRS), but the condition resolved without use of tocilizumab or steroids.
These results were presented at the 2018 CAR-TCR Summit by Eric Ostertag, MD, PhD, chief executive officer of Poseida Therapeutics Inc., the company developing P-BCMA-101.
Dr. Ostertag presented data on 11 patients with heavily pretreated multiple myeloma. They had a median of six prior therapies. The median age was 60 years, and most of the patients were considered high risk.
Prior to receiving P-BCMA-101, patients received conditioning with fludarabine (30 mg/m2) and cyclophosphamide (300 mg/m2) for 3 days.
Patients were then treated across three dose groups with average CAR T-cell doses of 51 x 106 (n = 3), 152 x 106 (n = 7), and 430 x 106 (n = 1).
As of Aug. 10, 2018, all 11 patients were still on study.
There were no dose-limiting toxicities. Eight patients developed neutropenia, and five had thrombocytopenia.
Researchers suspected CRS in one patient, but the condition resolved without tocilizumab or steroid treatment. There was no neurotoxicity reported, and none of the patients required admission to an intensive care unit.
All patients showed improvement in biomarkers following treatment.
Ten patients were evaluable for response by International Myeloma Working Group criteria. Seven of these patients achieved at least a PR, including very good partial responses (VGPRs) and stringent complete response (CR).
The eleventh patient also responded to treatment, but this patient has oligosecretory disease and was evaluable only by PET. The patient had a near-CR by PET.
Poseida Therapeutics would not disclose additional details regarding how many patients achieved a PR, VGPR, or CR, but the company plans to release more information on response at an upcoming meeting.
“The latest data results show that P-BCMA-101 induces deep responses in a heavily pretreated population with relapsed/refractory multiple myeloma, with some patients reaching VGPR and even stringent CR at early efficacy assessments,” Dr. Ostertag said.
This study (NCT03288493) is funded by the California Institute for Regenerative Medicine and Poseida Therapeutics.
FROM THE 2018 CAR-TCR SUMMIT
Key clinical point: The chimeric antigen receptor .
Major finding: All 11 patients have shown signs of response, and 8 patients achieved a partial response or better.
Study details: Eleven patients have been treated thus far in this phase 1 trial.
Disclosures: This trial is funded by the California Institute for Regenerative Medicine and Poseida Therapeutics.
Nevus of Ota Associated With a Primary Uveal Melanoma and Intracranial Melanoma Metastasis
Nevus of Ota, originally referred to as nevus fusco-caeruleus ophthalmomaxillaris, initially was described in 1939 by Ota and Tanino.1 It is a dermal melanocytic hamartoma arising from incomplete migration of neural crest melanocytes to the epidermis during embryogenesis, resulting in nesting of subtle bands of dendritic melanocytes in the upper dermis. More common in Asians, Native Americans, and females, this hyperpigmented dermatosis most often is unilaterally distributed along the ophthalmic (V1) and maxillary (V2) branches of the trigeminal nerve.2 In some patients, nevus of Ota also is associated with ocular, orbital, and leptomeningeal melanocytosis. Approximately 15% of nevi of Ota have an activating guanine nucleotide-binding protein G(q) subunit alpha (GNAQ) or G protein subunit alpha 11 (GNAQ) mutation; 85% of uveal melanomas harbor one of these mutations.3 Although uncommon, neoplastic transformation with extension or metastasis to the brain has been reported in patients with nevus of Ota.4
We report the case of a 29-year-old woman with a long-standing history of nevus of Ota who presented acutely with an intracranial melanoma as an extension of a primary uveal melanoma.
Case Report
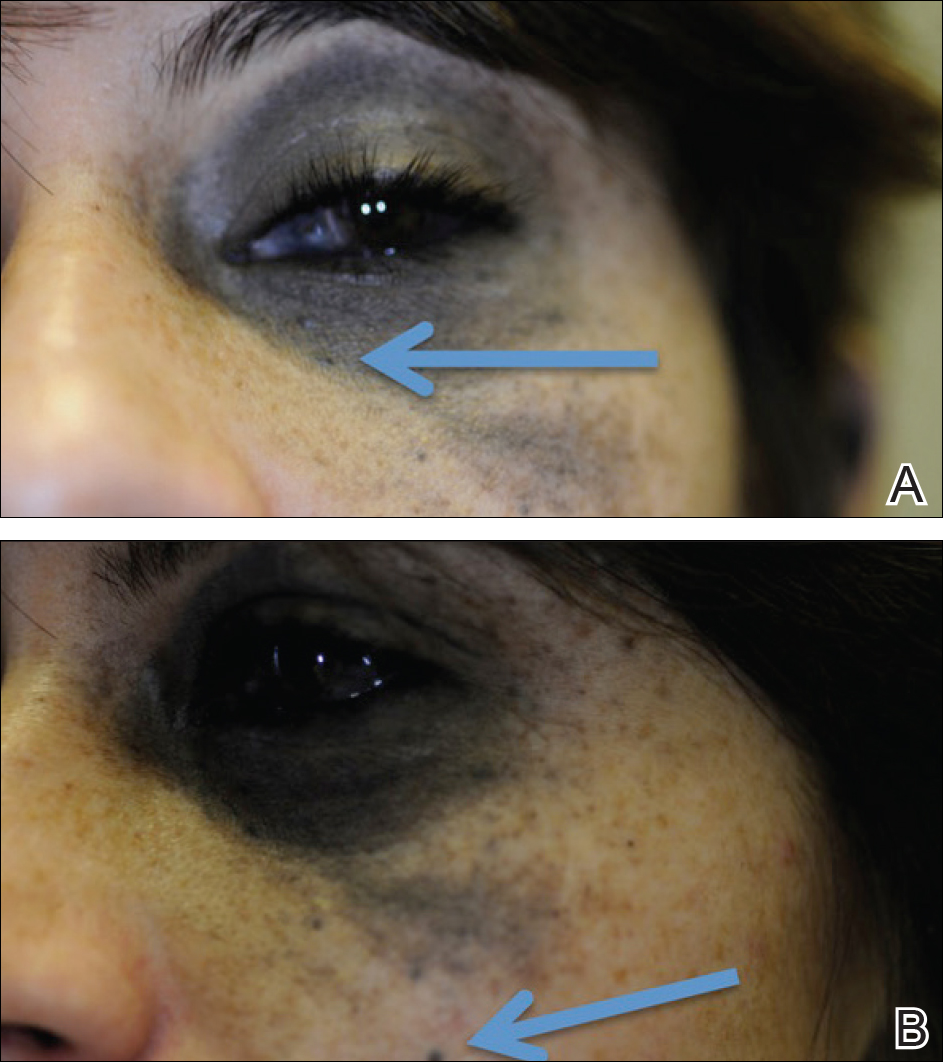
A 29-year-old woman with a history of a nevus of Ota involving the left inner canthus, eyelids, sclera, and superior malar cheek that had been present since birth presented to the emergency department with an acute onset of severe headache, blurred vision, and vomiting. Computed tomography (CT) and magnetic resonance imaging of the brain revealed a hemorrhagic mass in the left frontal lobe. Subsequent frontal craniotomy and resection revealed an intracranial melanoma.
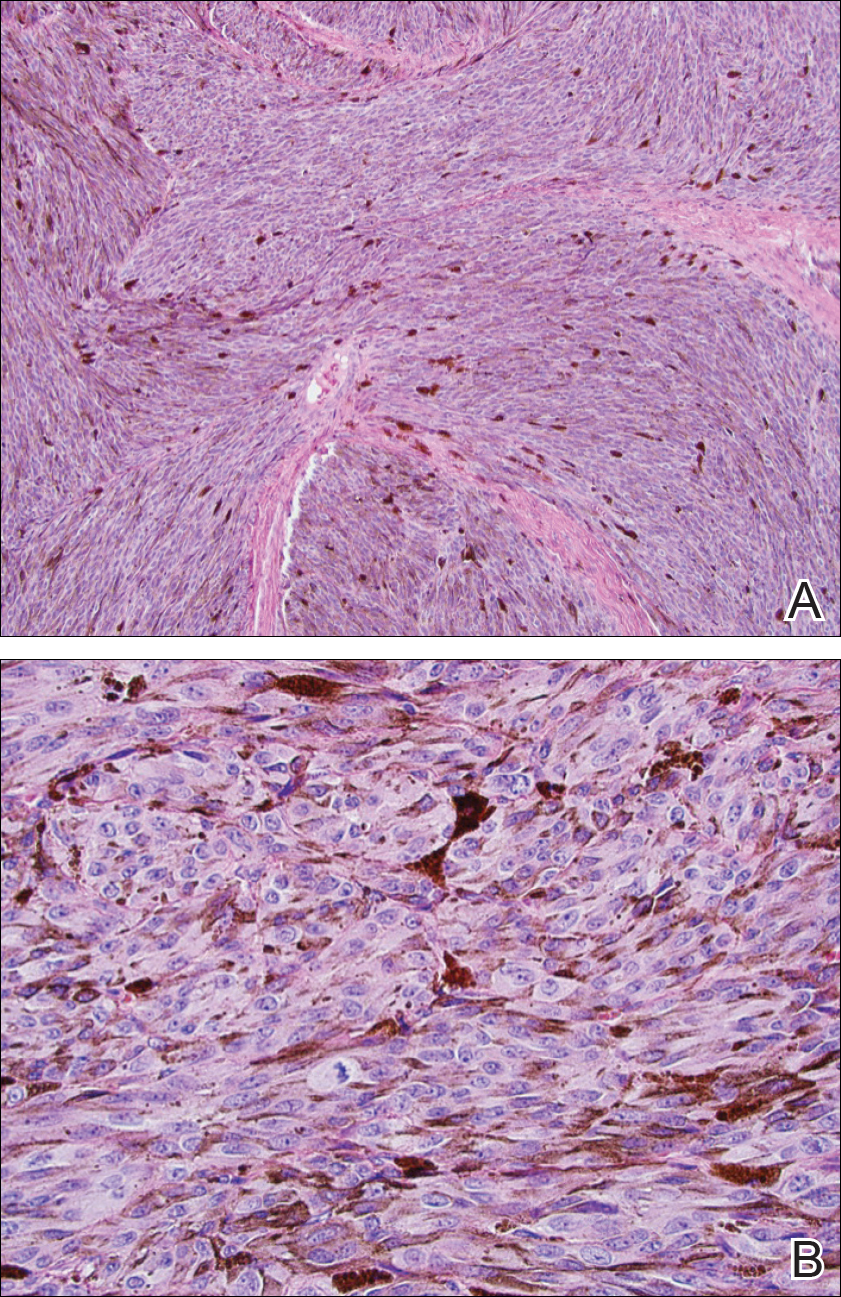
Two weeks following surgery, the patient underwent magnetic resonance imaging and combined positron emission tomography and CT scans that demonstrated a fluorodeoxyglucose-avid left retro-orbital mass. Histopathology of a biopsy from the left retro-orbital mass that had been obtained intraoperatively demonstrated a pigmented, spindled to epithelioid neoplasm with areas of marked atypia and a high mitotic rate that was compatible with malignant melanoma (Figure 1). Intracranial biopsies were sent for genetic study and were found to harbor GNAQ (Q209P) and BRCA1-associated protein 1 (BAP1)(p.P324fs*11) mutations.
The patient was referred to dermatology by neurosurgery for evaluation of a suspected primary cutaneous melanoma.

The patient entered a clinical trial at an outside institution several weeks after initial presentation to our institution for treatment with a mitogen-activated protein kinase MEK1 inhibitor as well as radiation therapy. The patient was lost to follow-up.
Comment
It has been demonstrated that homozygous loss of BAP1, located on the chromosome 3p21.1 locus, allows for progression to metastatic disease in uveal melanoma. The BAP1 gene codes for ubiquitin carboxyl-terminal hydrolase 7, which is involved in the removal of ubiquitin from proteins. This enzyme binds to BRCA1 (BRCA1, DNA repair associated) via the RING (Really Interesting New Gene) finger domain and acts as a tumor suppressor.5 Biallelic BAP1 mutations allow the transition to malignancy in concert with other mutations, such as GNAQ. Identification of a BAP1 mutation may serve as a valuable diagnostic and future therapeutic target in uveal melanoma.
Currently, there are no drugs that directly target mutated GNA11 and GNAQ proteins. Because aberrant GNA11 and GNAQ proteins activate MEK1, several MEK1 inhibitors are being tested with the hope of achieving indirect suppression of GNA11/GNAQ.6
We present a rare case of BAP1 and GNAQ mutations in intracranial melanoma associated with nevus of Ota. Although the uveal melanoma was not confirmed on histopathology, the clear mention of foci within the eye by ophthalmology, positron emission tomography–CT scan showing a fluorodeoxyglucose-avid left retro-orbital mass, and genetic studies of the intracranial biopsies were highly suggestive of a primary uveal melanoma.
Our case highlights the importance of ongoing ocular screening in patients with nevus of Ota, noting the possibility of malignant transformation. Furthermore, patients with nevus of Ota with ocular involvement may benefit from testing of BAP1 protein expression by immunohistochemistry.7 Identification of BAP1 and GNAQ mutations in patients with nevus of Ota place them at markedly higher risk for malignant melanoma. Therefore, dermatologic evaluation of patients with nevus of Ota should include a thorough review of the patient’s history and skin examination as well as referral for ophthalmologic evaluation.
- Ota M, Tanino H. A variety of nevus, frequently encountered in Japan, nevus fusco-caeruleus ophthalmomaxillaris and its relationship to pigmentary changes in the eye. Tokyo Med J. 1939;63:1243-1244.
- Swann PG, Kwong E. The naevus of Ota. Clin Exp Optom. 2010;93:264-267.
- Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma [published online November 17, 2010]. N Engl J Med. 2010;363:2191-2199.
- Nitta K, Kashima T, Mayuzumi H, et al. Animal-type malignancy melanoma associated with nevus of Ota in the orbit of a Japanese woman: a case report. Melanoma Res. 2014;24:286-289.
- Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas [published online November 4, 2010]. Science. 2010;330:1410-1413.
- Chen X, Wu Q, Tan L, et al. Combined PKC and MEK inhibition in uveal melanoma with GNAQ and GNA11 mutations. Oncogene. 2014;33:4724-4734.
- Kalirai H, Dodson A, Faqir S, et al. Lack of BAP1 protein expression in uveal melanoma is associated with increased metastatic risk and has utility in routine prognostic testing [published online July 24, 2010]. Br J Cancer. 2014;111:1373-1380.
Nevus of Ota, originally referred to as nevus fusco-caeruleus ophthalmomaxillaris, initially was described in 1939 by Ota and Tanino.1 It is a dermal melanocytic hamartoma arising from incomplete migration of neural crest melanocytes to the epidermis during embryogenesis, resulting in nesting of subtle bands of dendritic melanocytes in the upper dermis. More common in Asians, Native Americans, and females, this hyperpigmented dermatosis most often is unilaterally distributed along the ophthalmic (V1) and maxillary (V2) branches of the trigeminal nerve.2 In some patients, nevus of Ota also is associated with ocular, orbital, and leptomeningeal melanocytosis. Approximately 15% of nevi of Ota have an activating guanine nucleotide-binding protein G(q) subunit alpha (GNAQ) or G protein subunit alpha 11 (GNAQ) mutation; 85% of uveal melanomas harbor one of these mutations.3 Although uncommon, neoplastic transformation with extension or metastasis to the brain has been reported in patients with nevus of Ota.4
We report the case of a 29-year-old woman with a long-standing history of nevus of Ota who presented acutely with an intracranial melanoma as an extension of a primary uveal melanoma.
Case Report
A 29-year-old woman with a history of a nevus of Ota involving the left inner canthus, eyelids, sclera, and superior malar cheek that had been present since birth presented to the emergency department with an acute onset of severe headache, blurred vision, and vomiting. Computed tomography (CT) and magnetic resonance imaging of the brain revealed a hemorrhagic mass in the left frontal lobe. Subsequent frontal craniotomy and resection revealed an intracranial melanoma.
Two weeks following surgery, the patient underwent magnetic resonance imaging and combined positron emission tomography and CT scans that demonstrated a fluorodeoxyglucose-avid left retro-orbital mass. Histopathology of a biopsy from the left retro-orbital mass that had been obtained intraoperatively demonstrated a pigmented, spindled to epithelioid neoplasm with areas of marked atypia and a high mitotic rate that was compatible with malignant melanoma (Figure 1). Intracranial biopsies were sent for genetic study and were found to harbor GNAQ (Q209P) and BRCA1-associated protein 1 (BAP1)(p.P324fs*11) mutations.

The patient was referred to dermatology by neurosurgery for evaluation of a suspected primary cutaneous melanoma.


The patient entered a clinical trial at an outside institution several weeks after initial presentation to our institution for treatment with a mitogen-activated protein kinase MEK1 inhibitor as well as radiation therapy. The patient was lost to follow-up.
Comment
It has been demonstrated that homozygous loss of BAP1, located on the chromosome 3p21.1 locus, allows for progression to metastatic disease in uveal melanoma. The BAP1 gene codes for ubiquitin carboxyl-terminal hydrolase 7, which is involved in the removal of ubiquitin from proteins. This enzyme binds to BRCA1 (BRCA1, DNA repair associated) via the RING (Really Interesting New Gene) finger domain and acts as a tumor suppressor.5 Biallelic BAP1 mutations allow the transition to malignancy in concert with other mutations, such as GNAQ. Identification of a BAP1 mutation may serve as a valuable diagnostic and future therapeutic target in uveal melanoma.
Currently, there are no drugs that directly target mutated GNA11 and GNAQ proteins. Because aberrant GNA11 and GNAQ proteins activate MEK1, several MEK1 inhibitors are being tested with the hope of achieving indirect suppression of GNA11/GNAQ.6
We present a rare case of BAP1 and GNAQ mutations in intracranial melanoma associated with nevus of Ota. Although the uveal melanoma was not confirmed on histopathology, the clear mention of foci within the eye by ophthalmology, positron emission tomography–CT scan showing a fluorodeoxyglucose-avid left retro-orbital mass, and genetic studies of the intracranial biopsies were highly suggestive of a primary uveal melanoma.
Our case highlights the importance of ongoing ocular screening in patients with nevus of Ota, noting the possibility of malignant transformation. Furthermore, patients with nevus of Ota with ocular involvement may benefit from testing of BAP1 protein expression by immunohistochemistry.7 Identification of BAP1 and GNAQ mutations in patients with nevus of Ota place them at markedly higher risk for malignant melanoma. Therefore, dermatologic evaluation of patients with nevus of Ota should include a thorough review of the patient’s history and skin examination as well as referral for ophthalmologic evaluation.
Nevus of Ota, originally referred to as nevus fusco-caeruleus ophthalmomaxillaris, initially was described in 1939 by Ota and Tanino.1 It is a dermal melanocytic hamartoma arising from incomplete migration of neural crest melanocytes to the epidermis during embryogenesis, resulting in nesting of subtle bands of dendritic melanocytes in the upper dermis. More common in Asians, Native Americans, and females, this hyperpigmented dermatosis most often is unilaterally distributed along the ophthalmic (V1) and maxillary (V2) branches of the trigeminal nerve.2 In some patients, nevus of Ota also is associated with ocular, orbital, and leptomeningeal melanocytosis. Approximately 15% of nevi of Ota have an activating guanine nucleotide-binding protein G(q) subunit alpha (GNAQ) or G protein subunit alpha 11 (GNAQ) mutation; 85% of uveal melanomas harbor one of these mutations.3 Although uncommon, neoplastic transformation with extension or metastasis to the brain has been reported in patients with nevus of Ota.4
We report the case of a 29-year-old woman with a long-standing history of nevus of Ota who presented acutely with an intracranial melanoma as an extension of a primary uveal melanoma.
Case Report
A 29-year-old woman with a history of a nevus of Ota involving the left inner canthus, eyelids, sclera, and superior malar cheek that had been present since birth presented to the emergency department with an acute onset of severe headache, blurred vision, and vomiting. Computed tomography (CT) and magnetic resonance imaging of the brain revealed a hemorrhagic mass in the left frontal lobe. Subsequent frontal craniotomy and resection revealed an intracranial melanoma.
Two weeks following surgery, the patient underwent magnetic resonance imaging and combined positron emission tomography and CT scans that demonstrated a fluorodeoxyglucose-avid left retro-orbital mass. Histopathology of a biopsy from the left retro-orbital mass that had been obtained intraoperatively demonstrated a pigmented, spindled to epithelioid neoplasm with areas of marked atypia and a high mitotic rate that was compatible with malignant melanoma (Figure 1). Intracranial biopsies were sent for genetic study and were found to harbor GNAQ (Q209P) and BRCA1-associated protein 1 (BAP1)(p.P324fs*11) mutations.

The patient was referred to dermatology by neurosurgery for evaluation of a suspected primary cutaneous melanoma.


The patient entered a clinical trial at an outside institution several weeks after initial presentation to our institution for treatment with a mitogen-activated protein kinase MEK1 inhibitor as well as radiation therapy. The patient was lost to follow-up.
Comment
It has been demonstrated that homozygous loss of BAP1, located on the chromosome 3p21.1 locus, allows for progression to metastatic disease in uveal melanoma. The BAP1 gene codes for ubiquitin carboxyl-terminal hydrolase 7, which is involved in the removal of ubiquitin from proteins. This enzyme binds to BRCA1 (BRCA1, DNA repair associated) via the RING (Really Interesting New Gene) finger domain and acts as a tumor suppressor.5 Biallelic BAP1 mutations allow the transition to malignancy in concert with other mutations, such as GNAQ. Identification of a BAP1 mutation may serve as a valuable diagnostic and future therapeutic target in uveal melanoma.
Currently, there are no drugs that directly target mutated GNA11 and GNAQ proteins. Because aberrant GNA11 and GNAQ proteins activate MEK1, several MEK1 inhibitors are being tested with the hope of achieving indirect suppression of GNA11/GNAQ.6
We present a rare case of BAP1 and GNAQ mutations in intracranial melanoma associated with nevus of Ota. Although the uveal melanoma was not confirmed on histopathology, the clear mention of foci within the eye by ophthalmology, positron emission tomography–CT scan showing a fluorodeoxyglucose-avid left retro-orbital mass, and genetic studies of the intracranial biopsies were highly suggestive of a primary uveal melanoma.
Our case highlights the importance of ongoing ocular screening in patients with nevus of Ota, noting the possibility of malignant transformation. Furthermore, patients with nevus of Ota with ocular involvement may benefit from testing of BAP1 protein expression by immunohistochemistry.7 Identification of BAP1 and GNAQ mutations in patients with nevus of Ota place them at markedly higher risk for malignant melanoma. Therefore, dermatologic evaluation of patients with nevus of Ota should include a thorough review of the patient’s history and skin examination as well as referral for ophthalmologic evaluation.
- Ota M, Tanino H. A variety of nevus, frequently encountered in Japan, nevus fusco-caeruleus ophthalmomaxillaris and its relationship to pigmentary changes in the eye. Tokyo Med J. 1939;63:1243-1244.
- Swann PG, Kwong E. The naevus of Ota. Clin Exp Optom. 2010;93:264-267.
- Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma [published online November 17, 2010]. N Engl J Med. 2010;363:2191-2199.
- Nitta K, Kashima T, Mayuzumi H, et al. Animal-type malignancy melanoma associated with nevus of Ota in the orbit of a Japanese woman: a case report. Melanoma Res. 2014;24:286-289.
- Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas [published online November 4, 2010]. Science. 2010;330:1410-1413.
- Chen X, Wu Q, Tan L, et al. Combined PKC and MEK inhibition in uveal melanoma with GNAQ and GNA11 mutations. Oncogene. 2014;33:4724-4734.
- Kalirai H, Dodson A, Faqir S, et al. Lack of BAP1 protein expression in uveal melanoma is associated with increased metastatic risk and has utility in routine prognostic testing [published online July 24, 2010]. Br J Cancer. 2014;111:1373-1380.
- Ota M, Tanino H. A variety of nevus, frequently encountered in Japan, nevus fusco-caeruleus ophthalmomaxillaris and its relationship to pigmentary changes in the eye. Tokyo Med J. 1939;63:1243-1244.
- Swann PG, Kwong E. The naevus of Ota. Clin Exp Optom. 2010;93:264-267.
- Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma [published online November 17, 2010]. N Engl J Med. 2010;363:2191-2199.
- Nitta K, Kashima T, Mayuzumi H, et al. Animal-type malignancy melanoma associated with nevus of Ota in the orbit of a Japanese woman: a case report. Melanoma Res. 2014;24:286-289.
- Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas [published online November 4, 2010]. Science. 2010;330:1410-1413.
- Chen X, Wu Q, Tan L, et al. Combined PKC and MEK inhibition in uveal melanoma with GNAQ and GNA11 mutations. Oncogene. 2014;33:4724-4734.
- Kalirai H, Dodson A, Faqir S, et al. Lack of BAP1 protein expression in uveal melanoma is associated with increased metastatic risk and has utility in routine prognostic testing [published online July 24, 2010]. Br J Cancer. 2014;111:1373-1380.
Practice Points
- Nevus of Ota is a hyperpigmented dermatosis that typically is distributed along the ophthalmic (V1) and maxillary (V2) branches of the trigeminal nerve.
- GNAQ and BAP1 mutations in patients with nevus of Ota confer a greater risk for malignant melanoma and metastatic progression.
- Ongoing ophthalmologic screening is paramount in patients with nevus of Ota and may prevent devastating sequelae.
Value-based sleep: understanding and maximizing value in sleep medicine care
In addition to well-documented health consequences, obstructive sleep apnea (OSA) is associated with substantial economic costs borne by patients, payers, employers, and society at large. For example, in a recent white paper commissioned by the American Academy of Sleep Medicine, the total societal-level costs of OSA were estimated to exceed $150 billion per year in the United States alone. In addition to direct costs associated with OSA diagnosis and treatment, indirect costs were estimated at $86.9 billion for lost workplace productivity; $30 billion for increased health-care utilization (HCU); $26.2 billion for motor vehicle crashes (MVC); and $6.5 billion for workplace accidents and injuries.1
More important, evidence suggests that OSA treatments provide positive economic impact, for example reducing health-care utilization and reducing days missed from work. Our group at the University of Maryland is currently heavily involved in related research examining the health economic impact of sleep disorders and their treatments.
Value-based sleep is a concept that I created several years ago to guide a greater emphasis on health economic outcomes in order to advance our field. In addition to working with payers, industry partners, employers, and forward-thinking startups, we are investing much effort into provider education regarding the health economic aspects of sleep. This article examines what value-based sleep is, how to increase the value of sleep in your practice setting, and steps to prepare for payment models of the future.
Value is in the eye of the beholder
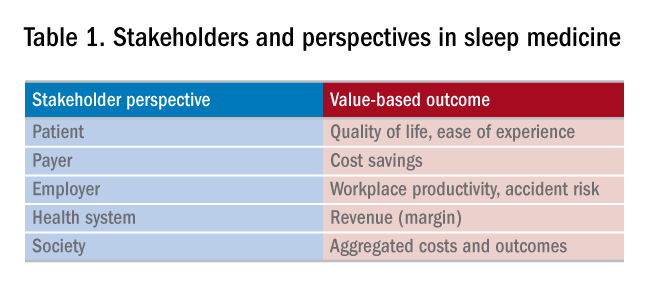
Unlike sleep medicine providers (and some patients), the majority of society views sleep as means to an end and not as an end-in-itself. That is, people only value sleep insofar as sleep will help them achieve their primary objectives, whatever they might be. In health economic terms, these distinct viewpoints are referred to as perspectives. For example, from the patient perspective, sleep is valued to the extent that it helps to increase quality of life. From the payer perspective, sleep is valued to the extent that reduces health-care utilization. From the employer perspective, sleep is valued to the extent that it increases workplace productivity and reduces health-care expenses. Table 1 summarizes common stakeholders and perspectives in sleep medicine.
Speaking the language of value
In order to define, demonstrate, and maximize the perceived value of sleep medicine services, sleep physicians must understand and clearly articulate the values of these multiple constituents. Most important, this means that sleep physicians must move beyond discussing the apnea-hypopnea index (AHI). To be clear, no one other than sleep medicine insiders care about the AHI! Of course, the AHI is an important (albeit imperfect) measure of OSA disease severity and treatment outcomes. However, when was the last time that a patient told you they woke up one morning dreaming about a lower AHI? It simply does not happen. Instead, stakeholders care about outcomes that matters to them, from their own unique perspectives. To speak directly to these interests and frame the value of sleep, sleep medicine providers must methodically develop value propositions with each unique target constituency in mind. Speak the language of your audience, and use terms that matter to them.
Adopting value-based payments
Much has been spoken about a transition from fee-for-service to value-based care in medicine. New health-care business models will soon impact patients, providers, payers, and health systems. To guide and ensure sustainable change, multi-stakeholder organizations, such as the Health Care Payment & Learning Action Network, are heavily engaged in the development and implementation of alternate payment models (APMs) to facilitate the transition from fee-for-service to population health. As depicted in Figure 1, sequential steps toward value-based care include increased fees corresponding to improved outcomes. A reimbursement model that is fully value-based centers on shared financial risks. Although private practitioners may be ill-equipped to provide population-level services or negotiate fully value-based models, sleep medicine providers should do well to increase familiarity with APMs and their impact on primary and specialty care services. 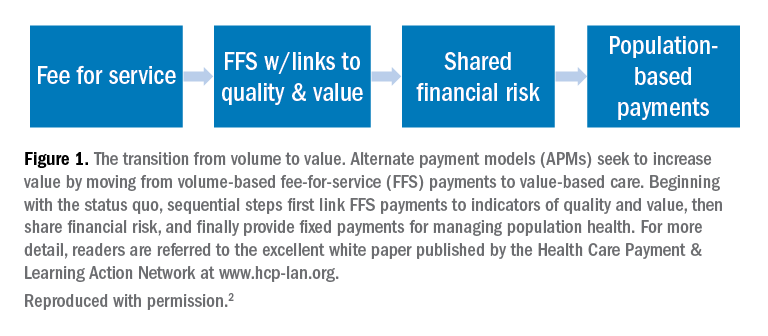
Five steps to a value-based approach
In the modern health-care climate of increasing costs on the one hand and limited resources on the other, sleep medicine providers must embrace a value-based perspective to survive, thrive, and grow in a new world of value-based care. This will require sleep medicine providers to learn, adapt, and adjust. The good news is that regardless of your practice or organizational setting, these strategies and tactics will help guide you:
1. Know thyself. What are your personal and organization objectives? Where are you, career-wise? Where do you want to be in 2, 3, and 5 years?
2. Know your customer. Whom do you serve? More broadly, whom does sleep serve? Listen carefully and identify the outcomes that matter to your constituents. Make these your endpoints.
3. Develop customer-centric language. Develop scripts. Rehearse them.
4. Understand trends in payments and technology. Is your region adopting bundled payments or paying more for improved outcomes? How might telemedicine or preauthorization for PAP impact your practice?
5. Know your numbers. To negotiate with confidence, you need to know your numbers. What are your costs per patient, per test, per outcome, and lifetime value of the patient?
Summary and next steps
To survive and thrive in a value-based future, you need to define, demonstrate, and maximize your perceived value. This will require greater attention to the language that you use, the results that you emphasize, and the data that you use to make decisions, all while attending to the perspectives of diverse stakeholders. The need for sleep medicine services has never been greater. Adopt a value-based sleep approach to ensure your bright future.
References
1. American Academy of Sleep M. Hidden health crisis costing America billions. Underdiagnosing and undertreating obstructive sleep apnea draining healthcare system. Mountain View, CA: Frost & Sullivan; 2016.
2. Wickwire EM, Verma T. Value and payment in sleep medicine. J Clin Sleep Med. 2018;14(5):881-884.
Dr. Wickwire is Associate Professor of Psychiatry and Medicine at the University of Maryland School of Medicine, where he directs the insomnia program. His current research interests include health and economic consequences of sleep disorders and their treatments and targeting sleep treatments for specific populations.
In addition to well-documented health consequences, obstructive sleep apnea (OSA) is associated with substantial economic costs borne by patients, payers, employers, and society at large. For example, in a recent white paper commissioned by the American Academy of Sleep Medicine, the total societal-level costs of OSA were estimated to exceed $150 billion per year in the United States alone. In addition to direct costs associated with OSA diagnosis and treatment, indirect costs were estimated at $86.9 billion for lost workplace productivity; $30 billion for increased health-care utilization (HCU); $26.2 billion for motor vehicle crashes (MVC); and $6.5 billion for workplace accidents and injuries.1
More important, evidence suggests that OSA treatments provide positive economic impact, for example reducing health-care utilization and reducing days missed from work. Our group at the University of Maryland is currently heavily involved in related research examining the health economic impact of sleep disorders and their treatments.
Value-based sleep is a concept that I created several years ago to guide a greater emphasis on health economic outcomes in order to advance our field. In addition to working with payers, industry partners, employers, and forward-thinking startups, we are investing much effort into provider education regarding the health economic aspects of sleep. This article examines what value-based sleep is, how to increase the value of sleep in your practice setting, and steps to prepare for payment models of the future.
Value is in the eye of the beholder
Unlike sleep medicine providers (and some patients), the majority of society views sleep as means to an end and not as an end-in-itself. That is, people only value sleep insofar as sleep will help them achieve their primary objectives, whatever they might be. In health economic terms, these distinct viewpoints are referred to as perspectives. For example, from the patient perspective, sleep is valued to the extent that it helps to increase quality of life. From the payer perspective, sleep is valued to the extent that reduces health-care utilization. From the employer perspective, sleep is valued to the extent that it increases workplace productivity and reduces health-care expenses. Table 1 summarizes common stakeholders and perspectives in sleep medicine.

Speaking the language of value
In order to define, demonstrate, and maximize the perceived value of sleep medicine services, sleep physicians must understand and clearly articulate the values of these multiple constituents. Most important, this means that sleep physicians must move beyond discussing the apnea-hypopnea index (AHI). To be clear, no one other than sleep medicine insiders care about the AHI! Of course, the AHI is an important (albeit imperfect) measure of OSA disease severity and treatment outcomes. However, when was the last time that a patient told you they woke up one morning dreaming about a lower AHI? It simply does not happen. Instead, stakeholders care about outcomes that matters to them, from their own unique perspectives. To speak directly to these interests and frame the value of sleep, sleep medicine providers must methodically develop value propositions with each unique target constituency in mind. Speak the language of your audience, and use terms that matter to them.
Adopting value-based payments
Much has been spoken about a transition from fee-for-service to value-based care in medicine. New health-care business models will soon impact patients, providers, payers, and health systems. To guide and ensure sustainable change, multi-stakeholder organizations, such as the Health Care Payment & Learning Action Network, are heavily engaged in the development and implementation of alternate payment models (APMs) to facilitate the transition from fee-for-service to population health. As depicted in Figure 1, sequential steps toward value-based care include increased fees corresponding to improved outcomes. A reimbursement model that is fully value-based centers on shared financial risks. Although private practitioners may be ill-equipped to provide population-level services or negotiate fully value-based models, sleep medicine providers should do well to increase familiarity with APMs and their impact on primary and specialty care services. 
Five steps to a value-based approach
In the modern health-care climate of increasing costs on the one hand and limited resources on the other, sleep medicine providers must embrace a value-based perspective to survive, thrive, and grow in a new world of value-based care. This will require sleep medicine providers to learn, adapt, and adjust. The good news is that regardless of your practice or organizational setting, these strategies and tactics will help guide you:
1. Know thyself. What are your personal and organization objectives? Where are you, career-wise? Where do you want to be in 2, 3, and 5 years?
2. Know your customer. Whom do you serve? More broadly, whom does sleep serve? Listen carefully and identify the outcomes that matter to your constituents. Make these your endpoints.
3. Develop customer-centric language. Develop scripts. Rehearse them.
4. Understand trends in payments and technology. Is your region adopting bundled payments or paying more for improved outcomes? How might telemedicine or preauthorization for PAP impact your practice?
5. Know your numbers. To negotiate with confidence, you need to know your numbers. What are your costs per patient, per test, per outcome, and lifetime value of the patient?
Summary and next steps
To survive and thrive in a value-based future, you need to define, demonstrate, and maximize your perceived value. This will require greater attention to the language that you use, the results that you emphasize, and the data that you use to make decisions, all while attending to the perspectives of diverse stakeholders. The need for sleep medicine services has never been greater. Adopt a value-based sleep approach to ensure your bright future.
References
1. American Academy of Sleep M. Hidden health crisis costing America billions. Underdiagnosing and undertreating obstructive sleep apnea draining healthcare system. Mountain View, CA: Frost & Sullivan; 2016.
2. Wickwire EM, Verma T. Value and payment in sleep medicine. J Clin Sleep Med. 2018;14(5):881-884.
Dr. Wickwire is Associate Professor of Psychiatry and Medicine at the University of Maryland School of Medicine, where he directs the insomnia program. His current research interests include health and economic consequences of sleep disorders and their treatments and targeting sleep treatments for specific populations.
In addition to well-documented health consequences, obstructive sleep apnea (OSA) is associated with substantial economic costs borne by patients, payers, employers, and society at large. For example, in a recent white paper commissioned by the American Academy of Sleep Medicine, the total societal-level costs of OSA were estimated to exceed $150 billion per year in the United States alone. In addition to direct costs associated with OSA diagnosis and treatment, indirect costs were estimated at $86.9 billion for lost workplace productivity; $30 billion for increased health-care utilization (HCU); $26.2 billion for motor vehicle crashes (MVC); and $6.5 billion for workplace accidents and injuries.1
More important, evidence suggests that OSA treatments provide positive economic impact, for example reducing health-care utilization and reducing days missed from work. Our group at the University of Maryland is currently heavily involved in related research examining the health economic impact of sleep disorders and their treatments.
Value-based sleep is a concept that I created several years ago to guide a greater emphasis on health economic outcomes in order to advance our field. In addition to working with payers, industry partners, employers, and forward-thinking startups, we are investing much effort into provider education regarding the health economic aspects of sleep. This article examines what value-based sleep is, how to increase the value of sleep in your practice setting, and steps to prepare for payment models of the future.
Value is in the eye of the beholder
Unlike sleep medicine providers (and some patients), the majority of society views sleep as means to an end and not as an end-in-itself. That is, people only value sleep insofar as sleep will help them achieve their primary objectives, whatever they might be. In health economic terms, these distinct viewpoints are referred to as perspectives. For example, from the patient perspective, sleep is valued to the extent that it helps to increase quality of life. From the payer perspective, sleep is valued to the extent that reduces health-care utilization. From the employer perspective, sleep is valued to the extent that it increases workplace productivity and reduces health-care expenses. Table 1 summarizes common stakeholders and perspectives in sleep medicine.

Speaking the language of value
In order to define, demonstrate, and maximize the perceived value of sleep medicine services, sleep physicians must understand and clearly articulate the values of these multiple constituents. Most important, this means that sleep physicians must move beyond discussing the apnea-hypopnea index (AHI). To be clear, no one other than sleep medicine insiders care about the AHI! Of course, the AHI is an important (albeit imperfect) measure of OSA disease severity and treatment outcomes. However, when was the last time that a patient told you they woke up one morning dreaming about a lower AHI? It simply does not happen. Instead, stakeholders care about outcomes that matters to them, from their own unique perspectives. To speak directly to these interests and frame the value of sleep, sleep medicine providers must methodically develop value propositions with each unique target constituency in mind. Speak the language of your audience, and use terms that matter to them.
Adopting value-based payments
Much has been spoken about a transition from fee-for-service to value-based care in medicine. New health-care business models will soon impact patients, providers, payers, and health systems. To guide and ensure sustainable change, multi-stakeholder organizations, such as the Health Care Payment & Learning Action Network, are heavily engaged in the development and implementation of alternate payment models (APMs) to facilitate the transition from fee-for-service to population health. As depicted in Figure 1, sequential steps toward value-based care include increased fees corresponding to improved outcomes. A reimbursement model that is fully value-based centers on shared financial risks. Although private practitioners may be ill-equipped to provide population-level services or negotiate fully value-based models, sleep medicine providers should do well to increase familiarity with APMs and their impact on primary and specialty care services. 
Five steps to a value-based approach
In the modern health-care climate of increasing costs on the one hand and limited resources on the other, sleep medicine providers must embrace a value-based perspective to survive, thrive, and grow in a new world of value-based care. This will require sleep medicine providers to learn, adapt, and adjust. The good news is that regardless of your practice or organizational setting, these strategies and tactics will help guide you:
1. Know thyself. What are your personal and organization objectives? Where are you, career-wise? Where do you want to be in 2, 3, and 5 years?
2. Know your customer. Whom do you serve? More broadly, whom does sleep serve? Listen carefully and identify the outcomes that matter to your constituents. Make these your endpoints.
3. Develop customer-centric language. Develop scripts. Rehearse them.
4. Understand trends in payments and technology. Is your region adopting bundled payments or paying more for improved outcomes? How might telemedicine or preauthorization for PAP impact your practice?
5. Know your numbers. To negotiate with confidence, you need to know your numbers. What are your costs per patient, per test, per outcome, and lifetime value of the patient?
Summary and next steps
To survive and thrive in a value-based future, you need to define, demonstrate, and maximize your perceived value. This will require greater attention to the language that you use, the results that you emphasize, and the data that you use to make decisions, all while attending to the perspectives of diverse stakeholders. The need for sleep medicine services has never been greater. Adopt a value-based sleep approach to ensure your bright future.
References
1. American Academy of Sleep M. Hidden health crisis costing America billions. Underdiagnosing and undertreating obstructive sleep apnea draining healthcare system. Mountain View, CA: Frost & Sullivan; 2016.
2. Wickwire EM, Verma T. Value and payment in sleep medicine. J Clin Sleep Med. 2018;14(5):881-884.
Dr. Wickwire is Associate Professor of Psychiatry and Medicine at the University of Maryland School of Medicine, where he directs the insomnia program. His current research interests include health and economic consequences of sleep disorders and their treatments and targeting sleep treatments for specific populations.
CHEST keynote to bridge the gap between generations
Scott Zimmer, a product of generation X, went through college with a passion for public speaking, as well as a deep interest in the generational divide. In 2013, he began working for a company called BridgeWorks and so began his career as one of three speakers at this firm of “generational junkies and trend spotters.”
Founded in 1998, Bridgeworks strives to bridge the generational gaps that are found in all workplaces through research, keynote speakers, workshops, blogs, training, trivia, and more. Bridgeworks is a team of 13 people coming from the baby boomer generation down to millennials on the cusp of being classified with generation Z (gen edgers, as Zimmer calls them). Each team member has their own interesting and diverse background with a passion for the topic of generations, and everyone engages this passion by conducting research with the BridgeWorks team.
There are generational clashes in every single industry, according to Zimmer. Just at BridgeWorks, he even notices when simply sending a text he perceives as “normal” to one of his millennial coworkers, that it is sometimes received as curt and leaves the recipient concerned that they have done something to offend him. This topic is not foreign to anyone— everyone has had a moment of saying “kids these days,” or “ugh, old people.” Because of this, Zimmer starts every session knowing that each person will leave with relevant insights and actionable takeaways.
Zimmer also loves to integrate nostalgia into his presentations, and working with generational theory at BridgeWorks allows him to do just that in a way that helps drive home points and makes ideas more relatable. “Some people like to say we are all just people and we grow out of certain things. But we develop specific traits and values at an impressionable age, and I love looking at what was happening in our lives during those formative years. What are these shared experiences that will form who we are?” This love of nostalgia set Zimmer up for a great opportunity to develop his own trivia gameshow at BridgeWorks. GenPOP! is an interactive trivia gameshow that pairs members of different generations up and quizzes them on all things pop culture from different decades, while also teaching audience members new things about the people they interact with every day.
“So much goes into who we are and who shows up to the workplace, what effects our behavior, and our motivation,” says Zimmer when asked where his passion for this topic stems. “It could be our gender, the region we grew up in, or birth order, and I personally like looking at it through the lens of these different generations.”
So, what will Zimmer bring to CHEST 2018? During his keynote presentation in San Antonio, Zimmer will examine the generational gaps that are existent in the medical community. “You don’t want your young medical professionals to feel like they are sitting at the ‘kids table’ or being talked down to when they have something to share because they do not have equal experience.”
Each generation and each member of a medical team communicates differently, and understanding those differences and feeling like an equal part of the team is very important. How information is conveyed to patients and medical team members of any age affects how they perceive given information and the level of comfort that is felt by each party. Finding ways to bridge the obvious gaps between the generations is a key component to making any team work efficiently.
Scott Zimmer, a product of generation X, went through college with a passion for public speaking, as well as a deep interest in the generational divide. In 2013, he began working for a company called BridgeWorks and so began his career as one of three speakers at this firm of “generational junkies and trend spotters.”
Founded in 1998, Bridgeworks strives to bridge the generational gaps that are found in all workplaces through research, keynote speakers, workshops, blogs, training, trivia, and more. Bridgeworks is a team of 13 people coming from the baby boomer generation down to millennials on the cusp of being classified with generation Z (gen edgers, as Zimmer calls them). Each team member has their own interesting and diverse background with a passion for the topic of generations, and everyone engages this passion by conducting research with the BridgeWorks team.
There are generational clashes in every single industry, according to Zimmer. Just at BridgeWorks, he even notices when simply sending a text he perceives as “normal” to one of his millennial coworkers, that it is sometimes received as curt and leaves the recipient concerned that they have done something to offend him. This topic is not foreign to anyone— everyone has had a moment of saying “kids these days,” or “ugh, old people.” Because of this, Zimmer starts every session knowing that each person will leave with relevant insights and actionable takeaways.
Zimmer also loves to integrate nostalgia into his presentations, and working with generational theory at BridgeWorks allows him to do just that in a way that helps drive home points and makes ideas more relatable. “Some people like to say we are all just people and we grow out of certain things. But we develop specific traits and values at an impressionable age, and I love looking at what was happening in our lives during those formative years. What are these shared experiences that will form who we are?” This love of nostalgia set Zimmer up for a great opportunity to develop his own trivia gameshow at BridgeWorks. GenPOP! is an interactive trivia gameshow that pairs members of different generations up and quizzes them on all things pop culture from different decades, while also teaching audience members new things about the people they interact with every day.
“So much goes into who we are and who shows up to the workplace, what effects our behavior, and our motivation,” says Zimmer when asked where his passion for this topic stems. “It could be our gender, the region we grew up in, or birth order, and I personally like looking at it through the lens of these different generations.”
So, what will Zimmer bring to CHEST 2018? During his keynote presentation in San Antonio, Zimmer will examine the generational gaps that are existent in the medical community. “You don’t want your young medical professionals to feel like they are sitting at the ‘kids table’ or being talked down to when they have something to share because they do not have equal experience.”
Each generation and each member of a medical team communicates differently, and understanding those differences and feeling like an equal part of the team is very important. How information is conveyed to patients and medical team members of any age affects how they perceive given information and the level of comfort that is felt by each party. Finding ways to bridge the obvious gaps between the generations is a key component to making any team work efficiently.
Scott Zimmer, a product of generation X, went through college with a passion for public speaking, as well as a deep interest in the generational divide. In 2013, he began working for a company called BridgeWorks and so began his career as one of three speakers at this firm of “generational junkies and trend spotters.”
Founded in 1998, Bridgeworks strives to bridge the generational gaps that are found in all workplaces through research, keynote speakers, workshops, blogs, training, trivia, and more. Bridgeworks is a team of 13 people coming from the baby boomer generation down to millennials on the cusp of being classified with generation Z (gen edgers, as Zimmer calls them). Each team member has their own interesting and diverse background with a passion for the topic of generations, and everyone engages this passion by conducting research with the BridgeWorks team.
There are generational clashes in every single industry, according to Zimmer. Just at BridgeWorks, he even notices when simply sending a text he perceives as “normal” to one of his millennial coworkers, that it is sometimes received as curt and leaves the recipient concerned that they have done something to offend him. This topic is not foreign to anyone— everyone has had a moment of saying “kids these days,” or “ugh, old people.” Because of this, Zimmer starts every session knowing that each person will leave with relevant insights and actionable takeaways.
Zimmer also loves to integrate nostalgia into his presentations, and working with generational theory at BridgeWorks allows him to do just that in a way that helps drive home points and makes ideas more relatable. “Some people like to say we are all just people and we grow out of certain things. But we develop specific traits and values at an impressionable age, and I love looking at what was happening in our lives during those formative years. What are these shared experiences that will form who we are?” This love of nostalgia set Zimmer up for a great opportunity to develop his own trivia gameshow at BridgeWorks. GenPOP! is an interactive trivia gameshow that pairs members of different generations up and quizzes them on all things pop culture from different decades, while also teaching audience members new things about the people they interact with every day.
“So much goes into who we are and who shows up to the workplace, what effects our behavior, and our motivation,” says Zimmer when asked where his passion for this topic stems. “It could be our gender, the region we grew up in, or birth order, and I personally like looking at it through the lens of these different generations.”
So, what will Zimmer bring to CHEST 2018? During his keynote presentation in San Antonio, Zimmer will examine the generational gaps that are existent in the medical community. “You don’t want your young medical professionals to feel like they are sitting at the ‘kids table’ or being talked down to when they have something to share because they do not have equal experience.”
Each generation and each member of a medical team communicates differently, and understanding those differences and feeling like an equal part of the team is very important. How information is conveyed to patients and medical team members of any age affects how they perceive given information and the level of comfort that is felt by each party. Finding ways to bridge the obvious gaps between the generations is a key component to making any team work efficiently.
Impact factor news for the journal CHEST®
The journal CHEST® was recently awarded a 2-year impact factor of 7.652, the highest in its history, which equates to a 24% increase over last year’s score. In addition, our 5-year impact factor is 7.854, a 7% increase over last year. With respect to the 2-year factor, CHEST® is ranked 4th out of 33 journals in the Critical Care category and 7th out of 59 journals in the Respiratory System category.
Our recent Eigenfactor places us as the second-highest ranked journal in both respiratory and critical care categories. The Eigenfactor metric adjusts the impact factor by eliminating self-citations and factoring in citations in the top-tier journals.
Congratulations to our journal CHEST® !
The journal CHEST® was recently awarded a 2-year impact factor of 7.652, the highest in its history, which equates to a 24% increase over last year’s score. In addition, our 5-year impact factor is 7.854, a 7% increase over last year. With respect to the 2-year factor, CHEST® is ranked 4th out of 33 journals in the Critical Care category and 7th out of 59 journals in the Respiratory System category.
Our recent Eigenfactor places us as the second-highest ranked journal in both respiratory and critical care categories. The Eigenfactor metric adjusts the impact factor by eliminating self-citations and factoring in citations in the top-tier journals.
Congratulations to our journal CHEST® !
The journal CHEST® was recently awarded a 2-year impact factor of 7.652, the highest in its history, which equates to a 24% increase over last year’s score. In addition, our 5-year impact factor is 7.854, a 7% increase over last year. With respect to the 2-year factor, CHEST® is ranked 4th out of 33 journals in the Critical Care category and 7th out of 59 journals in the Respiratory System category.
Our recent Eigenfactor places us as the second-highest ranked journal in both respiratory and critical care categories. The Eigenfactor metric adjusts the impact factor by eliminating self-citations and factoring in citations in the top-tier journals.
Congratulations to our journal CHEST® !
CHEST NetWorks
Palliative and End-of-Life Care
Patient-tailored goals-of-care discussions: Is this the new standard?
Goals-of-care discussions can be challenging conversations for even the most seasoned physicians. The challenge often is not just the timing but also knowing how to stitch together the content of the discussion. In most cases, physicians have minimal prior knowledge of patient and family preferences, and this adds to the complexity. In addition, the majority of these discussions happen in the inpatient setting (Mack et al. Ann Intern Med. 2012;156[3]:204) where the acuity of the illness adds to the barriers of effective communication (Fulmer et al. J Am Geriatr Soc. 2018;May 23. doi: 10.1111/jgs.15374. [Epub ahead of print]). Can these discussions be tailored to suit individual patient needs and can such attempts better goals-of-care communication? A recent publication by Curtis et al in JAMA Internal Medicine (2018;178[7]:930) attempts to shed light on these unanswered questions and provide physician guidance to better engage in these critical discussions. The cluster-randomized trial included both clinicians and patients. Patients were sent a survey assessing their individual preferences, and physicians were given a summary and communication tips based on these preferences (Jumpstart-Tips). This simple, cost-effective yet scalable intervention was able to improve the frequency, documentation, and patient-assessed quality of goals-of-care discussions in an outpatient setting. In addition, the delivery of goal-concordant care was increased at 3 months in the subgroup of patients with stable goals. A notable limitation of this study was the low participation among physicians. Further studies will be needed to further dissect the characteristics of participating and nonparticipating physicians. Research will also be needed to ascertain how to seamlessly integrate this into health-care delivery. But one irrefutable point is that interventions to improve communication hold the key to better end-of-life care delivery for our patients with serious illnesses. Drs. Paladino and Bernacki have aptly noted in their commentary (JAMA Intern Med. 2018;178[7]:940): “In the age of precision medicine, one can imagine a future of precision communication, where we….provide customized direction for clinicians to begin these discussions based on patient-specific needs.” One question remains. Will this be the new standard? The answer lies with us, the clinicians. My answer to this is a resounding “Yes,” and I hope early adopters will lead the way and prove me right.
Shine Raju, MD, MBBS
Steering Committee Member
Respiratory Care
Prevention of health-care professional errors in use of inhalers
Asthma affects approximately 300 million people worldwide. The 2018 Global Initiative for Asthma (GINA) guidelines recommend assessing the patient’s inhaler technique on a regular basis (www.ginasthma.org. Updated 2018. Accessed August 1, 2018).
The pressurized metered-dose inhaler (pMDI) and dry powder inhaler (DPI) are the most common aerosolized medication delivery devices.
Proper inhaler technique optimizes delivery of medication, and patients rely on a variety of their health-care providers (HCP) to teach them to use the devices. Unfortunately, evidence demonstrates patients are unable to use their inhalers properly (Sanchis et al. Chest. 2016;150[2]:394). Improper and inadequate inhaler technique is commonly associated with poor disease control, exacerbations, hospitalization stays, and need for systemic corticosteroids and antibiotic therapy (Capanoglu et al. J Asthma. 2015;52[8]:838; Levy et al. Prim Care Respir J. 2013;22:406; Westerik et al. J Asthma. 2015;53[3]:1).
Incorrect inhaler use is attributed to the design of the device, poor patient understanding, and HCPs having insufficient knowledge of the inhalers and performed the correct inhaled technique 15.5% of the time (Plaza et al. J Allergy Clin Immunol Prac. 2018;6[3]:987).
Health-care providers who are directly responsible for managing patients with pulmonary disease must have knowledge of correct inhaler techniques to effectively teach patients and properly assess their use of these devices. The quality of the HCP instruction to the patient is key to reducing poor inhaler technique (Klijn et al. NPJ Prim Care Respir Med. 2017;;27[1]:24. doi: 10.1038/s41533-017-0022-1). Targeted inhaler technique educational programs for HCPs have been shown to improve clinical outcomes of patients with asthma (Myers. Respir Care. 2015;60[8]:1190). The Respiratory Care NetWork is developing HCP and patient handouts for each aerosol delivery device which may be available in early 2019.
De De Gardner, DrPH, RRT-NPS, FCCP
Steering Committee Member
Sleep Medicine
Pediatric Sleep Disorders
The Sleep Medicine Network has worked hard to contribute to the CHEST 2018 exciting program of events by highlighting hot topics, discussing clinical controversies, and presenting challenging cases in sleep medicine. The goal of the Sleep Medicine Network has been to design content relevant to the diverse audience attending CHEST in San Antonio this year.
This goal includes topics relevant to pediatric sleep medicine. Why is this important to the larger audience at CHEST? Demand for pediatric sleep physicians significantly out paces access in many areas of this country (Phillips et al. Am J Respir Crit Care Med. 2015;192[8]:915). Adult sleep physicians may treat older children or adolescents in their practice, they may care for medically complex children when they transition to adulthood, and they may be asked for advice regarding the sleep concerns of children of their friends and colleagues. Sleep problems in children are common and may affect up to a quarter of children at some point during their lifetime (Owens. Prim Care. 2008;35[3]:533). The entire household is affected when children are not receiving adequate sleep; the sleep of their caregivers and family members is impacted. While many similarities exist between adult and pediatric sleep medicine, physicians who regularly care for children need to be aware of the important differences in the evaluation and treatment of pediatric sleep disorders.
How else can we connect with your practice? If you have an important topic you would like considered for CHEST 2019 please seek out the Sleep Medicine Network meeting in San Antonio, so we can continue to generate relevant content for your practice.
Julie Baughn, MD
Steering Committee Member
Palliative and End-of-Life Care
Patient-tailored goals-of-care discussions: Is this the new standard?
Goals-of-care discussions can be challenging conversations for even the most seasoned physicians. The challenge often is not just the timing but also knowing how to stitch together the content of the discussion. In most cases, physicians have minimal prior knowledge of patient and family preferences, and this adds to the complexity. In addition, the majority of these discussions happen in the inpatient setting (Mack et al. Ann Intern Med. 2012;156[3]:204) where the acuity of the illness adds to the barriers of effective communication (Fulmer et al. J Am Geriatr Soc. 2018;May 23. doi: 10.1111/jgs.15374. [Epub ahead of print]). Can these discussions be tailored to suit individual patient needs and can such attempts better goals-of-care communication? A recent publication by Curtis et al in JAMA Internal Medicine (2018;178[7]:930) attempts to shed light on these unanswered questions and provide physician guidance to better engage in these critical discussions. The cluster-randomized trial included both clinicians and patients. Patients were sent a survey assessing their individual preferences, and physicians were given a summary and communication tips based on these preferences (Jumpstart-Tips). This simple, cost-effective yet scalable intervention was able to improve the frequency, documentation, and patient-assessed quality of goals-of-care discussions in an outpatient setting. In addition, the delivery of goal-concordant care was increased at 3 months in the subgroup of patients with stable goals. A notable limitation of this study was the low participation among physicians. Further studies will be needed to further dissect the characteristics of participating and nonparticipating physicians. Research will also be needed to ascertain how to seamlessly integrate this into health-care delivery. But one irrefutable point is that interventions to improve communication hold the key to better end-of-life care delivery for our patients with serious illnesses. Drs. Paladino and Bernacki have aptly noted in their commentary (JAMA Intern Med. 2018;178[7]:940): “In the age of precision medicine, one can imagine a future of precision communication, where we….provide customized direction for clinicians to begin these discussions based on patient-specific needs.” One question remains. Will this be the new standard? The answer lies with us, the clinicians. My answer to this is a resounding “Yes,” and I hope early adopters will lead the way and prove me right.
Shine Raju, MD, MBBS
Steering Committee Member
Respiratory Care
Prevention of health-care professional errors in use of inhalers
Asthma affects approximately 300 million people worldwide. The 2018 Global Initiative for Asthma (GINA) guidelines recommend assessing the patient’s inhaler technique on a regular basis (www.ginasthma.org. Updated 2018. Accessed August 1, 2018).
The pressurized metered-dose inhaler (pMDI) and dry powder inhaler (DPI) are the most common aerosolized medication delivery devices.
Proper inhaler technique optimizes delivery of medication, and patients rely on a variety of their health-care providers (HCP) to teach them to use the devices. Unfortunately, evidence demonstrates patients are unable to use their inhalers properly (Sanchis et al. Chest. 2016;150[2]:394). Improper and inadequate inhaler technique is commonly associated with poor disease control, exacerbations, hospitalization stays, and need for systemic corticosteroids and antibiotic therapy (Capanoglu et al. J Asthma. 2015;52[8]:838; Levy et al. Prim Care Respir J. 2013;22:406; Westerik et al. J Asthma. 2015;53[3]:1).
Incorrect inhaler use is attributed to the design of the device, poor patient understanding, and HCPs having insufficient knowledge of the inhalers and performed the correct inhaled technique 15.5% of the time (Plaza et al. J Allergy Clin Immunol Prac. 2018;6[3]:987).
Health-care providers who are directly responsible for managing patients with pulmonary disease must have knowledge of correct inhaler techniques to effectively teach patients and properly assess their use of these devices. The quality of the HCP instruction to the patient is key to reducing poor inhaler technique (Klijn et al. NPJ Prim Care Respir Med. 2017;;27[1]:24. doi: 10.1038/s41533-017-0022-1). Targeted inhaler technique educational programs for HCPs have been shown to improve clinical outcomes of patients with asthma (Myers. Respir Care. 2015;60[8]:1190). The Respiratory Care NetWork is developing HCP and patient handouts for each aerosol delivery device which may be available in early 2019.
De De Gardner, DrPH, RRT-NPS, FCCP
Steering Committee Member
Sleep Medicine
Pediatric Sleep Disorders
The Sleep Medicine Network has worked hard to contribute to the CHEST 2018 exciting program of events by highlighting hot topics, discussing clinical controversies, and presenting challenging cases in sleep medicine. The goal of the Sleep Medicine Network has been to design content relevant to the diverse audience attending CHEST in San Antonio this year.
This goal includes topics relevant to pediatric sleep medicine. Why is this important to the larger audience at CHEST? Demand for pediatric sleep physicians significantly out paces access in many areas of this country (Phillips et al. Am J Respir Crit Care Med. 2015;192[8]:915). Adult sleep physicians may treat older children or adolescents in their practice, they may care for medically complex children when they transition to adulthood, and they may be asked for advice regarding the sleep concerns of children of their friends and colleagues. Sleep problems in children are common and may affect up to a quarter of children at some point during their lifetime (Owens. Prim Care. 2008;35[3]:533). The entire household is affected when children are not receiving adequate sleep; the sleep of their caregivers and family members is impacted. While many similarities exist between adult and pediatric sleep medicine, physicians who regularly care for children need to be aware of the important differences in the evaluation and treatment of pediatric sleep disorders.
How else can we connect with your practice? If you have an important topic you would like considered for CHEST 2019 please seek out the Sleep Medicine Network meeting in San Antonio, so we can continue to generate relevant content for your practice.
Julie Baughn, MD
Steering Committee Member
Palliative and End-of-Life Care
Patient-tailored goals-of-care discussions: Is this the new standard?
Goals-of-care discussions can be challenging conversations for even the most seasoned physicians. The challenge often is not just the timing but also knowing how to stitch together the content of the discussion. In most cases, physicians have minimal prior knowledge of patient and family preferences, and this adds to the complexity. In addition, the majority of these discussions happen in the inpatient setting (Mack et al. Ann Intern Med. 2012;156[3]:204) where the acuity of the illness adds to the barriers of effective communication (Fulmer et al. J Am Geriatr Soc. 2018;May 23. doi: 10.1111/jgs.15374. [Epub ahead of print]). Can these discussions be tailored to suit individual patient needs and can such attempts better goals-of-care communication? A recent publication by Curtis et al in JAMA Internal Medicine (2018;178[7]:930) attempts to shed light on these unanswered questions and provide physician guidance to better engage in these critical discussions. The cluster-randomized trial included both clinicians and patients. Patients were sent a survey assessing their individual preferences, and physicians were given a summary and communication tips based on these preferences (Jumpstart-Tips). This simple, cost-effective yet scalable intervention was able to improve the frequency, documentation, and patient-assessed quality of goals-of-care discussions in an outpatient setting. In addition, the delivery of goal-concordant care was increased at 3 months in the subgroup of patients with stable goals. A notable limitation of this study was the low participation among physicians. Further studies will be needed to further dissect the characteristics of participating and nonparticipating physicians. Research will also be needed to ascertain how to seamlessly integrate this into health-care delivery. But one irrefutable point is that interventions to improve communication hold the key to better end-of-life care delivery for our patients with serious illnesses. Drs. Paladino and Bernacki have aptly noted in their commentary (JAMA Intern Med. 2018;178[7]:940): “In the age of precision medicine, one can imagine a future of precision communication, where we….provide customized direction for clinicians to begin these discussions based on patient-specific needs.” One question remains. Will this be the new standard? The answer lies with us, the clinicians. My answer to this is a resounding “Yes,” and I hope early adopters will lead the way and prove me right.
Shine Raju, MD, MBBS
Steering Committee Member
Respiratory Care
Prevention of health-care professional errors in use of inhalers
Asthma affects approximately 300 million people worldwide. The 2018 Global Initiative for Asthma (GINA) guidelines recommend assessing the patient’s inhaler technique on a regular basis (www.ginasthma.org. Updated 2018. Accessed August 1, 2018).
The pressurized metered-dose inhaler (pMDI) and dry powder inhaler (DPI) are the most common aerosolized medication delivery devices.
Proper inhaler technique optimizes delivery of medication, and patients rely on a variety of their health-care providers (HCP) to teach them to use the devices. Unfortunately, evidence demonstrates patients are unable to use their inhalers properly (Sanchis et al. Chest. 2016;150[2]:394). Improper and inadequate inhaler technique is commonly associated with poor disease control, exacerbations, hospitalization stays, and need for systemic corticosteroids and antibiotic therapy (Capanoglu et al. J Asthma. 2015;52[8]:838; Levy et al. Prim Care Respir J. 2013;22:406; Westerik et al. J Asthma. 2015;53[3]:1).
Incorrect inhaler use is attributed to the design of the device, poor patient understanding, and HCPs having insufficient knowledge of the inhalers and performed the correct inhaled technique 15.5% of the time (Plaza et al. J Allergy Clin Immunol Prac. 2018;6[3]:987).
Health-care providers who are directly responsible for managing patients with pulmonary disease must have knowledge of correct inhaler techniques to effectively teach patients and properly assess their use of these devices. The quality of the HCP instruction to the patient is key to reducing poor inhaler technique (Klijn et al. NPJ Prim Care Respir Med. 2017;;27[1]:24. doi: 10.1038/s41533-017-0022-1). Targeted inhaler technique educational programs for HCPs have been shown to improve clinical outcomes of patients with asthma (Myers. Respir Care. 2015;60[8]:1190). The Respiratory Care NetWork is developing HCP and patient handouts for each aerosol delivery device which may be available in early 2019.
De De Gardner, DrPH, RRT-NPS, FCCP
Steering Committee Member
Sleep Medicine
Pediatric Sleep Disorders
The Sleep Medicine Network has worked hard to contribute to the CHEST 2018 exciting program of events by highlighting hot topics, discussing clinical controversies, and presenting challenging cases in sleep medicine. The goal of the Sleep Medicine Network has been to design content relevant to the diverse audience attending CHEST in San Antonio this year.
This goal includes topics relevant to pediatric sleep medicine. Why is this important to the larger audience at CHEST? Demand for pediatric sleep physicians significantly out paces access in many areas of this country (Phillips et al. Am J Respir Crit Care Med. 2015;192[8]:915). Adult sleep physicians may treat older children or adolescents in their practice, they may care for medically complex children when they transition to adulthood, and they may be asked for advice regarding the sleep concerns of children of their friends and colleagues. Sleep problems in children are common and may affect up to a quarter of children at some point during their lifetime (Owens. Prim Care. 2008;35[3]:533). The entire household is affected when children are not receiving adequate sleep; the sleep of their caregivers and family members is impacted. While many similarities exist between adult and pediatric sleep medicine, physicians who regularly care for children need to be aware of the important differences in the evaluation and treatment of pediatric sleep disorders.
How else can we connect with your practice? If you have an important topic you would like considered for CHEST 2019 please seek out the Sleep Medicine Network meeting in San Antonio, so we can continue to generate relevant content for your practice.
Julie Baughn, MD
Steering Committee Member
VA Releases Spanish Version of Health Benefits Application
Nearly 1.5 million veterans and more than 500,000 already enrolled in the VA health care system identify as being Hispanic or Latino. “Our veteran population is made up of an increasingly diverse group of people,” said VA Secretary Robert Wilkie. “It’s our duty to expand the ways we communicate with all veterans, so they’re properly informed about the benefits they’ve earned.”
To that end, the VA has released a Spanish version of the application for health benefits, “to simplify and improve the health care enrollment process.” The new language version follows on the VA Advisory Committee on Minority Veterans’ recommendation.
The form is available at VA medical facilities and online at https://www.va.gov/vaforms/medical/pdf/10-10EZ_Spanish.pdf.
Nearly 1.5 million veterans and more than 500,000 already enrolled in the VA health care system identify as being Hispanic or Latino. “Our veteran population is made up of an increasingly diverse group of people,” said VA Secretary Robert Wilkie. “It’s our duty to expand the ways we communicate with all veterans, so they’re properly informed about the benefits they’ve earned.”
To that end, the VA has released a Spanish version of the application for health benefits, “to simplify and improve the health care enrollment process.” The new language version follows on the VA Advisory Committee on Minority Veterans’ recommendation.
The form is available at VA medical facilities and online at https://www.va.gov/vaforms/medical/pdf/10-10EZ_Spanish.pdf.
Nearly 1.5 million veterans and more than 500,000 already enrolled in the VA health care system identify as being Hispanic or Latino. “Our veteran population is made up of an increasingly diverse group of people,” said VA Secretary Robert Wilkie. “It’s our duty to expand the ways we communicate with all veterans, so they’re properly informed about the benefits they’ve earned.”
To that end, the VA has released a Spanish version of the application for health benefits, “to simplify and improve the health care enrollment process.” The new language version follows on the VA Advisory Committee on Minority Veterans’ recommendation.
The form is available at VA medical facilities and online at https://www.va.gov/vaforms/medical/pdf/10-10EZ_Spanish.pdf.

