User login
New guidelines frame treatment options for Castleman disease
The (MCD), although adjuvant chemotherapy can be critical in severe cases, according to the first-ever consensus treatment guidelines based on symptom severity.
Early intervention with combination chemotherapy may prevent a fatal outcome in patients with severe idiopathic MCD, according to the guidelines, which are based on input from 42 experts from 10 countries.
The guidelines should help clinicians select therapy, evaluate response, and thereby improve outcomes for this difficult-to-treat disease, according to guideline co-author David C. Fajgenbaum, MD, who is himself an idiopathic MCD patient. Frits van Rhee, MD, PhD, is the first author of the guidelines.*
“Right now we recommend siltuximab first line for everyone,” Dr. Fajgenbaum said in an interview, “but if we continue to dig deeper, it may be that there are clinical cases within idiopathic MCD that we think are even better candidates than others, and there may be alternative therapies for other patients.”
Experts reviewed published literature and a series of 344 clinical cases to develop the guidelines, which are published in the journal Blood.
Treating idiopathic MCD is challenging because of the rarity and heterogeneity of the disease, among other factors, said Dr. Fajgenbaum, of the division of translational medicine and human genetics at the University of Pennsylvania, Philadelphia.
Some 6,000-7,000 cases of Castleman disease are diagnosed yearly, and of those, only about 1,000 cases are idiopathic MCD, according to Dr. Fajgenbaum.
“Even within idiopathic MCD, there is some heterogeneity,” he said. “Some patients present in the intensive care unit with life-threatening multiple organ failure and will die within weeks of presentation, whereas others will have a slower presentation and certainly not nearly as aggressive presentation.”
Although the exact etiology of idiopathic MCD is unknown, human interleukin (IL)-6 is the most common pathological driver, experts said in the guidelines.
Siltuximab and tocilizumab are two IL-6–directed therapies used to treat multicentric Castleman disease, with siltuximab targeting IL-6 itself and tocilizumab targeting the IL-6 receptor. Siltuximab is recommended as the first choice because of rigorous data supporting its use, including randomized clinical trial data, while tocilizumab is recommended if siltuximab is not available.
However, clinicians need to carefully monitor laboratory results and clinical features for patients on these drugs because about 50% of idiopathic MCD patients don’t have a satisfactory response to first-line anti–IL-6 treatments, Dr. Fajgenbaum said.
“Once you get to second-line therapies, that’s really where the level of evidence is lower,” he said.
Second-line therapy should include rituximab, and immunomodulatory/immunosuppressive agents or steroids may be added, according to the guidelines.
Third line therapy is “less well defined,” according to the guidelines, and experts generally recommended immunomodulatory/immunosuppressive agents such as cyclosporine A, sirolimus, thalidomide, and lenalidomide.
Cytotoxic chemotherapy has a high response rate but also a high rate of relapse and significant toxicities, according to the data analysis conducted as part of the guideline development process. Based on that, the experts said to avoid it unless the patient progresses to severe idiopathic MCD.
“Patients who are literally dying in the intensive care unit, given the right combination chemotherapy, can improve within days to weeks and can even leave the hospital,” Dr. Fajgenbaum said. “It’s not necessarily going to be the answer long term, but it can be life saving in the short term. So we recommended a really quite aggressive approach for these patients.”
To bolster the evidence base, investigators in the Castleman Disease Collaborative Network (CDCN) set up an international registry to collect treatment and outcome data for 500 patients. After the first year and a half, 150 patients were enrolled, and the investigators have identified more than 30 drugs that have been used off label to treat idiopathic MCD, according to Dr. Fajgenbaum.
“Some of the drugs are demonstrating efficacy in small numbers,” he said. “With the goal of 500 patients total, we can certainly hope to see some trends.”
Dr. Fajgenbaum was diagnosed with idiopathic MCD as a medical student.
“That certainly served as a very strong personal motivator for me to get involved in the disease,” he said. “But as I’ve gotten more and more involved, I’ve obviously met a lot of other patients, and that really is a huge motivator for all members of the CDCN. We want more options for more patients more quickly so we can help as many people as possible.”
Dr. Fajgenbaum reported research funding from Janssen. Coauthors reported disclosures related to Janssen, Bristol-Myers Squibb, Genentech, Merck, Celgene, Incyte, Pfizer, Sequenom, and Foundation Medicine, among others.
SOURCE: van Rhee F et al. Blood. 2018 Sep 4. doi: 10.1182/blood-2018-07-862334.
This story was updated 10/01/2018.
The (MCD), although adjuvant chemotherapy can be critical in severe cases, according to the first-ever consensus treatment guidelines based on symptom severity.
Early intervention with combination chemotherapy may prevent a fatal outcome in patients with severe idiopathic MCD, according to the guidelines, which are based on input from 42 experts from 10 countries.
The guidelines should help clinicians select therapy, evaluate response, and thereby improve outcomes for this difficult-to-treat disease, according to guideline co-author David C. Fajgenbaum, MD, who is himself an idiopathic MCD patient. Frits van Rhee, MD, PhD, is the first author of the guidelines.*
“Right now we recommend siltuximab first line for everyone,” Dr. Fajgenbaum said in an interview, “but if we continue to dig deeper, it may be that there are clinical cases within idiopathic MCD that we think are even better candidates than others, and there may be alternative therapies for other patients.”
Experts reviewed published literature and a series of 344 clinical cases to develop the guidelines, which are published in the journal Blood.
Treating idiopathic MCD is challenging because of the rarity and heterogeneity of the disease, among other factors, said Dr. Fajgenbaum, of the division of translational medicine and human genetics at the University of Pennsylvania, Philadelphia.
Some 6,000-7,000 cases of Castleman disease are diagnosed yearly, and of those, only about 1,000 cases are idiopathic MCD, according to Dr. Fajgenbaum.
“Even within idiopathic MCD, there is some heterogeneity,” he said. “Some patients present in the intensive care unit with life-threatening multiple organ failure and will die within weeks of presentation, whereas others will have a slower presentation and certainly not nearly as aggressive presentation.”
Although the exact etiology of idiopathic MCD is unknown, human interleukin (IL)-6 is the most common pathological driver, experts said in the guidelines.
Siltuximab and tocilizumab are two IL-6–directed therapies used to treat multicentric Castleman disease, with siltuximab targeting IL-6 itself and tocilizumab targeting the IL-6 receptor. Siltuximab is recommended as the first choice because of rigorous data supporting its use, including randomized clinical trial data, while tocilizumab is recommended if siltuximab is not available.
However, clinicians need to carefully monitor laboratory results and clinical features for patients on these drugs because about 50% of idiopathic MCD patients don’t have a satisfactory response to first-line anti–IL-6 treatments, Dr. Fajgenbaum said.
“Once you get to second-line therapies, that’s really where the level of evidence is lower,” he said.
Second-line therapy should include rituximab, and immunomodulatory/immunosuppressive agents or steroids may be added, according to the guidelines.
Third line therapy is “less well defined,” according to the guidelines, and experts generally recommended immunomodulatory/immunosuppressive agents such as cyclosporine A, sirolimus, thalidomide, and lenalidomide.
Cytotoxic chemotherapy has a high response rate but also a high rate of relapse and significant toxicities, according to the data analysis conducted as part of the guideline development process. Based on that, the experts said to avoid it unless the patient progresses to severe idiopathic MCD.
“Patients who are literally dying in the intensive care unit, given the right combination chemotherapy, can improve within days to weeks and can even leave the hospital,” Dr. Fajgenbaum said. “It’s not necessarily going to be the answer long term, but it can be life saving in the short term. So we recommended a really quite aggressive approach for these patients.”
To bolster the evidence base, investigators in the Castleman Disease Collaborative Network (CDCN) set up an international registry to collect treatment and outcome data for 500 patients. After the first year and a half, 150 patients were enrolled, and the investigators have identified more than 30 drugs that have been used off label to treat idiopathic MCD, according to Dr. Fajgenbaum.
“Some of the drugs are demonstrating efficacy in small numbers,” he said. “With the goal of 500 patients total, we can certainly hope to see some trends.”
Dr. Fajgenbaum was diagnosed with idiopathic MCD as a medical student.
“That certainly served as a very strong personal motivator for me to get involved in the disease,” he said. “But as I’ve gotten more and more involved, I’ve obviously met a lot of other patients, and that really is a huge motivator for all members of the CDCN. We want more options for more patients more quickly so we can help as many people as possible.”
Dr. Fajgenbaum reported research funding from Janssen. Coauthors reported disclosures related to Janssen, Bristol-Myers Squibb, Genentech, Merck, Celgene, Incyte, Pfizer, Sequenom, and Foundation Medicine, among others.
SOURCE: van Rhee F et al. Blood. 2018 Sep 4. doi: 10.1182/blood-2018-07-862334.
This story was updated 10/01/2018.
The (MCD), although adjuvant chemotherapy can be critical in severe cases, according to the first-ever consensus treatment guidelines based on symptom severity.
Early intervention with combination chemotherapy may prevent a fatal outcome in patients with severe idiopathic MCD, according to the guidelines, which are based on input from 42 experts from 10 countries.
The guidelines should help clinicians select therapy, evaluate response, and thereby improve outcomes for this difficult-to-treat disease, according to guideline co-author David C. Fajgenbaum, MD, who is himself an idiopathic MCD patient. Frits van Rhee, MD, PhD, is the first author of the guidelines.*
“Right now we recommend siltuximab first line for everyone,” Dr. Fajgenbaum said in an interview, “but if we continue to dig deeper, it may be that there are clinical cases within idiopathic MCD that we think are even better candidates than others, and there may be alternative therapies for other patients.”
Experts reviewed published literature and a series of 344 clinical cases to develop the guidelines, which are published in the journal Blood.
Treating idiopathic MCD is challenging because of the rarity and heterogeneity of the disease, among other factors, said Dr. Fajgenbaum, of the division of translational medicine and human genetics at the University of Pennsylvania, Philadelphia.
Some 6,000-7,000 cases of Castleman disease are diagnosed yearly, and of those, only about 1,000 cases are idiopathic MCD, according to Dr. Fajgenbaum.
“Even within idiopathic MCD, there is some heterogeneity,” he said. “Some patients present in the intensive care unit with life-threatening multiple organ failure and will die within weeks of presentation, whereas others will have a slower presentation and certainly not nearly as aggressive presentation.”
Although the exact etiology of idiopathic MCD is unknown, human interleukin (IL)-6 is the most common pathological driver, experts said in the guidelines.
Siltuximab and tocilizumab are two IL-6–directed therapies used to treat multicentric Castleman disease, with siltuximab targeting IL-6 itself and tocilizumab targeting the IL-6 receptor. Siltuximab is recommended as the first choice because of rigorous data supporting its use, including randomized clinical trial data, while tocilizumab is recommended if siltuximab is not available.
However, clinicians need to carefully monitor laboratory results and clinical features for patients on these drugs because about 50% of idiopathic MCD patients don’t have a satisfactory response to first-line anti–IL-6 treatments, Dr. Fajgenbaum said.
“Once you get to second-line therapies, that’s really where the level of evidence is lower,” he said.
Second-line therapy should include rituximab, and immunomodulatory/immunosuppressive agents or steroids may be added, according to the guidelines.
Third line therapy is “less well defined,” according to the guidelines, and experts generally recommended immunomodulatory/immunosuppressive agents such as cyclosporine A, sirolimus, thalidomide, and lenalidomide.
Cytotoxic chemotherapy has a high response rate but also a high rate of relapse and significant toxicities, according to the data analysis conducted as part of the guideline development process. Based on that, the experts said to avoid it unless the patient progresses to severe idiopathic MCD.
“Patients who are literally dying in the intensive care unit, given the right combination chemotherapy, can improve within days to weeks and can even leave the hospital,” Dr. Fajgenbaum said. “It’s not necessarily going to be the answer long term, but it can be life saving in the short term. So we recommended a really quite aggressive approach for these patients.”
To bolster the evidence base, investigators in the Castleman Disease Collaborative Network (CDCN) set up an international registry to collect treatment and outcome data for 500 patients. After the first year and a half, 150 patients were enrolled, and the investigators have identified more than 30 drugs that have been used off label to treat idiopathic MCD, according to Dr. Fajgenbaum.
“Some of the drugs are demonstrating efficacy in small numbers,” he said. “With the goal of 500 patients total, we can certainly hope to see some trends.”
Dr. Fajgenbaum was diagnosed with idiopathic MCD as a medical student.
“That certainly served as a very strong personal motivator for me to get involved in the disease,” he said. “But as I’ve gotten more and more involved, I’ve obviously met a lot of other patients, and that really is a huge motivator for all members of the CDCN. We want more options for more patients more quickly so we can help as many people as possible.”
Dr. Fajgenbaum reported research funding from Janssen. Coauthors reported disclosures related to Janssen, Bristol-Myers Squibb, Genentech, Merck, Celgene, Incyte, Pfizer, Sequenom, and Foundation Medicine, among others.
SOURCE: van Rhee F et al. Blood. 2018 Sep 4. doi: 10.1182/blood-2018-07-862334.
This story was updated 10/01/2018.
FROM BLOOD
Employing irritable bowel syndrome patient-reported outcomes in the clinical trenches
Patients often seek care because they experience symptoms that negatively affect their health-related quality of life (HRQOL). Health care providers must then elicit, measure, and interpret patient symptoms as part of their clinical evaluation. To assist with this goal and to help bridge the gap between patients and providers, investigators have developed and validated a wide range of patient-reported outcomes (PROs) across the breadth and depth of the human health and illness experience. These PROs, which measure any aspect of a patient’s biopsychosocial health and come directly from the patient, may help direct care and improve outcomes. When PROs are collected systematically, efficiently, and in the right place at the right time, they may enhance the patient–provider relationship by improving communication and facilitating shared decision making.1-3
Within gastroenterology and hepatology, PROs have been developed for a number of conditions, including irritable bowel syndrome (IBS), chronic idiopathic constipation, cirrhosis, eosinophilic esophagitis, inflammatory bowel disease, and gastroesophageal reflux disease, among many other chronic diseases. IBS in particular is well suited for PRO measurement because it is symptom based and significantly impacts patients’ HRQOL and emotional health. Moreover, it is the most commonly diagnosed gastrointestinal condition and imparts a significant economic burden. In this article, we review the rationale for measuring IBS PROs in routine clinical practice and detail available measurement instruments.
Importance of IBS PROs in clinical practice
IBS is a functional GI disorder that is characterized by recurrent abdominal pain and altered bowel habits (i.e., diarrhea, constipation, or a mix of both). It has an estimated worldwide prevalence of 11%, and total costs are estimated at $30 billion annually in the United States alone.4 Because of the chronic relapsing nature of IBS, along with its impact on physical, mental, and social distress, it becomes important to accurately capture a patient’s illness experience with PROs. This is especially relevant to patients with IBS because we currently lack objective measurable biomarkers to assess their GI symptom burden. Instead, clinicians often are relegated to informal assessments of the severity of a patient’s symptoms, which ultimately guide their treatment recommendations: How many bowel movements have you had in the past week? Were your bowel movements hard or soft? How bad is your abdominal pain on a scale of 1 to 10? However, these traditional outcomes measured by health care providers often fail to capture other aspects of their health. For example, simply asking patients about the frequency and character of their stools will not provide any insight into how their symptoms impact their HRQOL and psychosocial health. An individual may report only two loose stools per day, but this may lead to substantial anxiety and negatively affect his or her performance at work. Similarly, the significance of the symptoms will vary from person to person; a patient with IBS who has five loose daily bowel movements may not be bothered by it, whereas another individual with three loose stools per day may feel that it severely hampers his or her daily activities. This is where PROs provide value because they provide a key component to understanding the true burden of IBS, and accounts for the HRQOL and psychosocial decrement engendered by the disease.
Although past literature has reported inconsistent benefits of using PROs in clinical practice,5 including that from our own work,6 there is a growing number of studies that have noted improved clinical outcomes through employing PROs.7-9 A compelling example is provided by Basch et al,7 who randomized patients receiving chemotherapy for metastatic cancer to either weekly symptom reporting using electronically delivered PRO instruments vs. usual care, which consisted of symptom reporting at the discretion of clinicians. Here, they found that the intervention group had higher HRQOL, were less likely to visit the emergency department, and remained on chemotherapy for a longer period of time. Interestingly, the benefits from PROs were greater among participants who lacked prior computer experience vs. those who were computer savvy. Basch et al9 also noted that the use of PROs extended survival by 5 months when compared with the control group (31.2 vs. 26.0 mo; P = .03). Longitudinal symptom reporting among IBS patients using PROs, when implemented well, may similarly lead to improved patient satisfaction, HRQOL, and clinical outcomes.
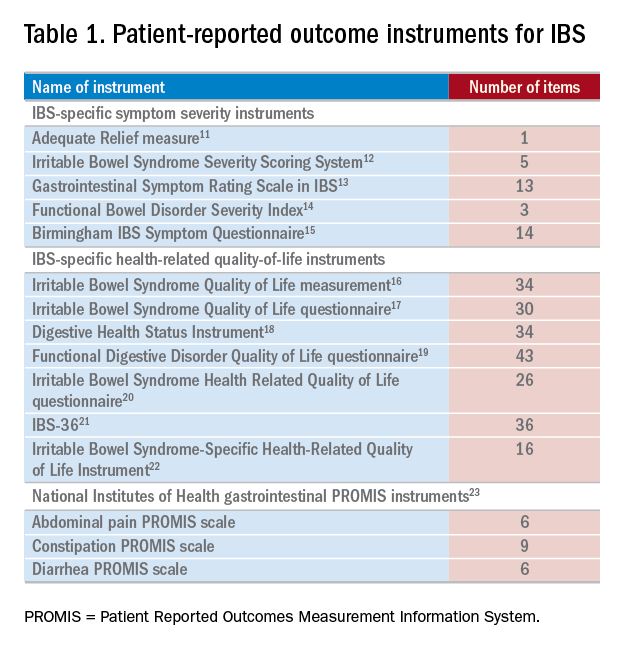
Measuring PROs in the clinical trenches
PROs generally are measured with patient questionnaires that collect data across several areas, including physical, social, and psychological functioning. Although PROs may enhance the patient–provider relationship and improve communication and shared decision making, we acknowledge that there are important barriers to its use in routine clinical practice.10 First, many providers (and their patients) may find use of PRO instruments burdensome, and it can be time consuming to collect PROs from patients and securely transmit the data into the electronic health record (EHR). This can make it untenable for use in busy practices. Second, many gastroenterologists have not received formal training in performing complete biopsychosocial evaluations with PROs, and it can be difficult to understand and act upon PRO scores. Third, there are many PROs to choose from and there is a lack of measurement standards across questionnaires. These challenges limit widespread use of PROs in clinical practice, and it is understandable why most providers instead opt for informal measurement of symptoms and function. Later, we detail strategies for overcoming the earlier-described challenges in employing PROs in everyday practice along with relevant IBS PRO instruments.
IBS–specific PRO instruments
There have been several IBS-specific PRO instruments described in the literature, all of which vary in length, content, and amount of data supporting their validity (Table 1). Examples of IBS symptoms scales include the Adequate Relief measure, Irritable Bowel Syndrome Severity Scoring System, Gastrointestinal Symptom Rating Scale in IBS, Functional Bowel Disorder Severity Index, IBS Symptom Questionnaire, and Birmingham IBS Symptom Questionnaire.15,24 There are also IBS-specific HRQOL instruments, such as the Irritable Bowel Syndrome Quality of Life measurement, Digestive Health Status Instrument, Functional Digestive Disorder Quality of Life questionnaire, Irritable Bowel Syndrome Health Related Quality of Life questionnaire, and IBS-36, among others.21,24
Bijkerk et al24 evaluated and compared the validity and appropriateness of both the symptom and QOL scales. Among the examined IBS symptom instruments, they found that the Adequate Relief question (Did you have adequate relief of IBS-related abdominal pain or discomfort?) is the best choice for assessing global symptomatology, and the Irritable Bowel Syndrome Severity Scoring System is optimal for obtaining information on more specific symptoms.24 As for the QOL scales, the Irritable Bowel Syndrome Quality of Life measurement, which comprises 34 items, is the preferred instrument for assessing changes in HRQOL because it is the most extensively validated.24 Bijkerk et al24 also concluded that although the studied instruments showed reasonable psychometric and methodologic qualities, the use of these instruments in daily clinical practice is debatable because the measures (save for the Adequate Relief question) are lengthy and/or cumbersome to use. Because these instruments may not be practical for use during everyday care, this leads to a discussion of the National Institutes of Health (NIH) Patient Reported Outcomes Measurement Information System (PROMIS), a novel approach to measuring PROs in the clinical trenches.
NIH PROMIS
Although there have been many efforts to implement PROs in routine clinical care, a recent confluence of scientific, regulatory, and political factors, coupled with technological advancements in PRO measurement techniques, have justified re-evaluation of the use of PROs in everyday practice. In response to the practical and technical challenges to employing PROs in the clinical trenches as described earlier, the NIH PROMIS (www.healthmeasures.net) was created in 2004 with the goal of developing and validating a toolbox of PROs that cover the breadth and depth of the human health and illness experience. The PROMIS initiative also was borne from the realization that patients are the ultimate consumers of health care and are the final judge on whether their health care needs are being addressed adequately.
By using modern psychometric techniques, such as item response theory and computerized adaptive testing, PROMIS offers state-of-the-art psychometrics, establishes common-language benchmarks for symptoms across conditions, and identifies clinical thresholds for action and meaningful clinical improvement or decline. PROMIS questionnaires, in light of accelerated EHR adoption in recent years, also are designed to be administered electronically and efficiently, allowing implementation in busy clinical settings. As of December 2017, these instruments can be administered and scored through EHRs such as Epic (Epic Systems, Verona, WI) and Cerner (Cerner Corporation, North Kansas City, MO), the PROMIS iPad (Apple Inc, Cupertino, CA) App, and online data collection tools such as the Assessment Center (www.assessmentcenter.net) and REDCap (Research Electronic Data Capture; Vanderbilt University, Nashville, TN).25 An increasing number of health systems are making PROMIS measures available through their EHRs. For example, the University of Rochester Medical Center collects PROMIS scores for physical function, pain interference, and depression from more than 80% of their patients with in-clinic testing, and individual departments are able to further tailor their administered questionnaires.26
Gastrointestinal PROs measurement information system scales
Because of the extraordinary burden of illness from digestive diseases, the PROMIS consortium added a GI item bank, which our research group developed.23 By using the NIH PROMIS framework, we constructed and validated eight GI PROMIS symptom scales: abdominal pain, bloating/gas, constipation, diarrhea, bowel incontinence, dysphagia, heartburn/reflux, and nausea/vomiting.23 GI PROMIS was designed from the outset to not be a disease-targeted item bank (e.g., IBS-, cirrhosis-, or inflammatory bowel disease specific), but rather symptom targeted, measuring the physical symptoms of the GI tract, because it is more useful across the population as a whole. In Supplementary Figure 1 (at https://doi.org/10.1016/j.cgh.2017.12.026), we include the abdominal pain, constipation, and diarrhea PROMIS scales because they form the cardinal symptoms of IBS.
GI PROMIS scales are readily accessible via the Assessment Center,25 and we also have made them freely available via MyGiHealth — an iOS (Apple Inc) and online app (go.mygihealth.io) endorsed by the American Gastroenterological Association. The patient’s responses to the questionnaires are converted to percentile scores and compared with the general U.S. population, and then displayed in a symptom heat map. The app also allows users to track GI PROMIS scores longitudinally, empowering IBS patients (and any patient with GI symptoms for that matter) and their providers to see if they are objectively responding to prescribed therapies and potentially improving satisfaction and patient–provider communication.
Conclusions
IBS is a common, chronic, relapsing disease that often leads to physical, mental, and social distress. Without objective measurable biomarkers to assess IBS patients’ GI symptom burden, along with health care’s increased emphasis on patient-centered care, it becomes important to accurately capture a patient’s illness experience with PROs. A number of IBS symptom and QOL PRO instruments have been described in the literature, but most are beset by lengthy completion times and are impractical for use in everyday care. GI PROMIS, on the other hand, is a versatile and efficient instrument for collecting PRO data from not only IBS patients, but also all those who seek care in our GI clinics. Improvements in PRO and implementation science combined with technological advances have lessened the barriers to employing PROs in routine clinical care, and an increasing number of institutions are beginning to take up this challenge. In doing so and by seamlessly incorporating PROs in clinical practice, it facilitates placement of our patients’ voices at the forefront of their health care, changes how we monitor and manage patients, and, ultimately, may improve patient satisfaction and clinical outcomes.
Content from this column was originally published in the “Practice Management: The Road Ahead” section of Clinical Gastroenterology and Hepatology (2018;16:462-6).
References
1. Detmar S.B., Muller M.J., Schornagel J.H., et al. Role of health-related quality of life in palliative chemotherapy treatment decisions. J Clin Oncol. 2002;20:1056-62.
2. Detmar S.B., Muller M.J., Schornagel J.H., et al. Health-related quality-of-life assessments and patient-physician communication: a randomized controlled trial. JAMA. 2002;288:3027-34.
3. Neumann M., Edelhauser F., Kreps G.L., et al. Can patient-provider interaction increase the effectiveness of medical treatment or even substitute it? An exploration on why and how to study the specific effect of the provider. Patient Educ Couns. 2010;80:307-14.
4. Lembo A.J. The clinical and economic burden of irritable bowel syndrome. Pract Gastroenterol. 2007;31:3-9.
5. Valderas J.M., Kotzeva A., Espallargues M., et al. The impact of measuring patient-reported outcomes in clinical practice: a systematic review of the literature. Qual Life Res. 2008;17:179-93.
6. Almario C.V., Chey W.D., Khanna D., et al. Impact of National Institutes of Health gastrointestinal PROMIS measures in clinical practice: results of a multicenter controlled trial. Am J Gastroenterol. 2016;111:1546-56.
7. Basch E., Deal A.M., Kris M.G., et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34:557-65.
8. Kotronoulas G., Kearney N., Maguire R., et al. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol. 2014;32:1480-501.
9. Basch E., Deal A.M., Dueck A.C., et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318:197-8.
10. Spiegel B.M. Patient-reported outcomes in gastroenterology: clinical and research applications. J Neurogastroenterol Motil. 2013;19:137-48.
11. Mangel A.W., Hahn B.A., Heath A.T., et al. Adequate relief as an endpoint in clinical trials in irritable bowel syndrome. J Int Med Res. 1998;26:76-81.
12. Francis C.Y., Morris J., Whorwell P.J. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395-402.
13. Wiklund I.K., Fullerton S., Hawkey C.J., et al. An irritable bowel syndrome-specific symptom questionnaire: development and validation. Scand J Gastroenterol. 2003;38:947-54.
14. Drossman D.A., Li Z., Toner B.B., et al. Functional bowel disorders. A multicenter comparison of health status and development of illness severity index. Dig Dis Sci. 1995;40:986-95.
15. Roalfe A.K., Roberts L.M., Wilson S. Evaluation of the Birmingham IBS symptom questionnaire. BMC Gastroenterol. 2008;8:30.
16. Patrick D.L., Drossman D.A., Frederick I.O., et al. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43:400-11.
17. Hahn B.A., Kirchdoerfer L.J., Fullerton S., et al. Evaluation of a new quality of life questionnaire for patients with irritable bowel syndrome. Aliment Pharmacol Ther. 1997;11:547-52.
18. Shaw M., Talley N.J., Adlis S., et al. Development of a digestive health status instrument: tests of scaling assumptions, structure and reliability in a primary care population. Aliment Pharmacol Ther. 1998;12:1067-78.
19. Chassany O., Marquis P., Scherrer B., et al. Validation of a specific quality of life questionnaire for functional digestive disorders. Gut. 1999;44:527-33.
20. Wong E., Guyatt G.H., Cook D.J., et al. Development of a questionnaire to measure quality of life in patients with irritable bowel syndrome. Eur J Surg Suppl. 1998;583:50-6.
21. Groll D., Vanner S.J., Depew W.T., et al. The IBS-36: a new quality of life measure for irritable bowel syndrome. Am J Gastroenterol. 2002;97:962-71.
22. Lee E.H., Kwon O., Hahm K.B., et al. Irritable bowel syndrome-specific health-related quality of life instrument: development and psychometric evaluation. Health Qual Life Outcomes. 2016;14:22.
23. Spiegel B.M., Hays R.D., Bolus R., et al. Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) gastrointestinal symptom scales. Am J Gastroenterol. 2014;109:1804-14.
24. Bijkerk C.J., de Wit N.J., Muris J.W., et al. Outcome measures in irritable bowel syndrome: comparison of psychometric and methodological characteristics. Am J Gastroenterol. 2003;98:122-7.
25. HealthMeasures. Data collection tools. 2017. Available from http://www.healthmeasures.net/resource-center/data-collection-tools. Accessed August 20, 2017.
26. Baumhauer J.F. Patient-reported outcomes – are they living up to their potential?. N Engl J Med. 2017;377:6-9.
Dr. Almario is assistant professor-in-residence of medicine and public health, division of digestive and liver diseases, Dr. Spiegel professor-in-residence of medicine and public health, division of digestive and liver diseases, Cedars-Sinai Medical Center; Cedars-Sinai Center for Outcomes Research and Education, Los Angeles. Dr. Spiegel is a principal at My Total Health, which operates and maintains MyGiHealth; Dr. Almario has a stock option grant in My Total Health.
Patients often seek care because they experience symptoms that negatively affect their health-related quality of life (HRQOL). Health care providers must then elicit, measure, and interpret patient symptoms as part of their clinical evaluation. To assist with this goal and to help bridge the gap between patients and providers, investigators have developed and validated a wide range of patient-reported outcomes (PROs) across the breadth and depth of the human health and illness experience. These PROs, which measure any aspect of a patient’s biopsychosocial health and come directly from the patient, may help direct care and improve outcomes. When PROs are collected systematically, efficiently, and in the right place at the right time, they may enhance the patient–provider relationship by improving communication and facilitating shared decision making.1-3
Within gastroenterology and hepatology, PROs have been developed for a number of conditions, including irritable bowel syndrome (IBS), chronic idiopathic constipation, cirrhosis, eosinophilic esophagitis, inflammatory bowel disease, and gastroesophageal reflux disease, among many other chronic diseases. IBS in particular is well suited for PRO measurement because it is symptom based and significantly impacts patients’ HRQOL and emotional health. Moreover, it is the most commonly diagnosed gastrointestinal condition and imparts a significant economic burden. In this article, we review the rationale for measuring IBS PROs in routine clinical practice and detail available measurement instruments.
Importance of IBS PROs in clinical practice
IBS is a functional GI disorder that is characterized by recurrent abdominal pain and altered bowel habits (i.e., diarrhea, constipation, or a mix of both). It has an estimated worldwide prevalence of 11%, and total costs are estimated at $30 billion annually in the United States alone.4 Because of the chronic relapsing nature of IBS, along with its impact on physical, mental, and social distress, it becomes important to accurately capture a patient’s illness experience with PROs. This is especially relevant to patients with IBS because we currently lack objective measurable biomarkers to assess their GI symptom burden. Instead, clinicians often are relegated to informal assessments of the severity of a patient’s symptoms, which ultimately guide their treatment recommendations: How many bowel movements have you had in the past week? Were your bowel movements hard or soft? How bad is your abdominal pain on a scale of 1 to 10? However, these traditional outcomes measured by health care providers often fail to capture other aspects of their health. For example, simply asking patients about the frequency and character of their stools will not provide any insight into how their symptoms impact their HRQOL and psychosocial health. An individual may report only two loose stools per day, but this may lead to substantial anxiety and negatively affect his or her performance at work. Similarly, the significance of the symptoms will vary from person to person; a patient with IBS who has five loose daily bowel movements may not be bothered by it, whereas another individual with three loose stools per day may feel that it severely hampers his or her daily activities. This is where PROs provide value because they provide a key component to understanding the true burden of IBS, and accounts for the HRQOL and psychosocial decrement engendered by the disease.
Although past literature has reported inconsistent benefits of using PROs in clinical practice,5 including that from our own work,6 there is a growing number of studies that have noted improved clinical outcomes through employing PROs.7-9 A compelling example is provided by Basch et al,7 who randomized patients receiving chemotherapy for metastatic cancer to either weekly symptom reporting using electronically delivered PRO instruments vs. usual care, which consisted of symptom reporting at the discretion of clinicians. Here, they found that the intervention group had higher HRQOL, were less likely to visit the emergency department, and remained on chemotherapy for a longer period of time. Interestingly, the benefits from PROs were greater among participants who lacked prior computer experience vs. those who were computer savvy. Basch et al9 also noted that the use of PROs extended survival by 5 months when compared with the control group (31.2 vs. 26.0 mo; P = .03). Longitudinal symptom reporting among IBS patients using PROs, when implemented well, may similarly lead to improved patient satisfaction, HRQOL, and clinical outcomes.

Measuring PROs in the clinical trenches
PROs generally are measured with patient questionnaires that collect data across several areas, including physical, social, and psychological functioning. Although PROs may enhance the patient–provider relationship and improve communication and shared decision making, we acknowledge that there are important barriers to its use in routine clinical practice.10 First, many providers (and their patients) may find use of PRO instruments burdensome, and it can be time consuming to collect PROs from patients and securely transmit the data into the electronic health record (EHR). This can make it untenable for use in busy practices. Second, many gastroenterologists have not received formal training in performing complete biopsychosocial evaluations with PROs, and it can be difficult to understand and act upon PRO scores. Third, there are many PROs to choose from and there is a lack of measurement standards across questionnaires. These challenges limit widespread use of PROs in clinical practice, and it is understandable why most providers instead opt for informal measurement of symptoms and function. Later, we detail strategies for overcoming the earlier-described challenges in employing PROs in everyday practice along with relevant IBS PRO instruments.
IBS–specific PRO instruments
There have been several IBS-specific PRO instruments described in the literature, all of which vary in length, content, and amount of data supporting their validity (Table 1). Examples of IBS symptoms scales include the Adequate Relief measure, Irritable Bowel Syndrome Severity Scoring System, Gastrointestinal Symptom Rating Scale in IBS, Functional Bowel Disorder Severity Index, IBS Symptom Questionnaire, and Birmingham IBS Symptom Questionnaire.15,24 There are also IBS-specific HRQOL instruments, such as the Irritable Bowel Syndrome Quality of Life measurement, Digestive Health Status Instrument, Functional Digestive Disorder Quality of Life questionnaire, Irritable Bowel Syndrome Health Related Quality of Life questionnaire, and IBS-36, among others.21,24
Bijkerk et al24 evaluated and compared the validity and appropriateness of both the symptom and QOL scales. Among the examined IBS symptom instruments, they found that the Adequate Relief question (Did you have adequate relief of IBS-related abdominal pain or discomfort?) is the best choice for assessing global symptomatology, and the Irritable Bowel Syndrome Severity Scoring System is optimal for obtaining information on more specific symptoms.24 As for the QOL scales, the Irritable Bowel Syndrome Quality of Life measurement, which comprises 34 items, is the preferred instrument for assessing changes in HRQOL because it is the most extensively validated.24 Bijkerk et al24 also concluded that although the studied instruments showed reasonable psychometric and methodologic qualities, the use of these instruments in daily clinical practice is debatable because the measures (save for the Adequate Relief question) are lengthy and/or cumbersome to use. Because these instruments may not be practical for use during everyday care, this leads to a discussion of the National Institutes of Health (NIH) Patient Reported Outcomes Measurement Information System (PROMIS), a novel approach to measuring PROs in the clinical trenches.
NIH PROMIS
Although there have been many efforts to implement PROs in routine clinical care, a recent confluence of scientific, regulatory, and political factors, coupled with technological advancements in PRO measurement techniques, have justified re-evaluation of the use of PROs in everyday practice. In response to the practical and technical challenges to employing PROs in the clinical trenches as described earlier, the NIH PROMIS (www.healthmeasures.net) was created in 2004 with the goal of developing and validating a toolbox of PROs that cover the breadth and depth of the human health and illness experience. The PROMIS initiative also was borne from the realization that patients are the ultimate consumers of health care and are the final judge on whether their health care needs are being addressed adequately.
By using modern psychometric techniques, such as item response theory and computerized adaptive testing, PROMIS offers state-of-the-art psychometrics, establishes common-language benchmarks for symptoms across conditions, and identifies clinical thresholds for action and meaningful clinical improvement or decline. PROMIS questionnaires, in light of accelerated EHR adoption in recent years, also are designed to be administered electronically and efficiently, allowing implementation in busy clinical settings. As of December 2017, these instruments can be administered and scored through EHRs such as Epic (Epic Systems, Verona, WI) and Cerner (Cerner Corporation, North Kansas City, MO), the PROMIS iPad (Apple Inc, Cupertino, CA) App, and online data collection tools such as the Assessment Center (www.assessmentcenter.net) and REDCap (Research Electronic Data Capture; Vanderbilt University, Nashville, TN).25 An increasing number of health systems are making PROMIS measures available through their EHRs. For example, the University of Rochester Medical Center collects PROMIS scores for physical function, pain interference, and depression from more than 80% of their patients with in-clinic testing, and individual departments are able to further tailor their administered questionnaires.26
Gastrointestinal PROs measurement information system scales
Because of the extraordinary burden of illness from digestive diseases, the PROMIS consortium added a GI item bank, which our research group developed.23 By using the NIH PROMIS framework, we constructed and validated eight GI PROMIS symptom scales: abdominal pain, bloating/gas, constipation, diarrhea, bowel incontinence, dysphagia, heartburn/reflux, and nausea/vomiting.23 GI PROMIS was designed from the outset to not be a disease-targeted item bank (e.g., IBS-, cirrhosis-, or inflammatory bowel disease specific), but rather symptom targeted, measuring the physical symptoms of the GI tract, because it is more useful across the population as a whole. In Supplementary Figure 1 (at https://doi.org/10.1016/j.cgh.2017.12.026), we include the abdominal pain, constipation, and diarrhea PROMIS scales because they form the cardinal symptoms of IBS.
GI PROMIS scales are readily accessible via the Assessment Center,25 and we also have made them freely available via MyGiHealth — an iOS (Apple Inc) and online app (go.mygihealth.io) endorsed by the American Gastroenterological Association. The patient’s responses to the questionnaires are converted to percentile scores and compared with the general U.S. population, and then displayed in a symptom heat map. The app also allows users to track GI PROMIS scores longitudinally, empowering IBS patients (and any patient with GI symptoms for that matter) and their providers to see if they are objectively responding to prescribed therapies and potentially improving satisfaction and patient–provider communication.
Conclusions
IBS is a common, chronic, relapsing disease that often leads to physical, mental, and social distress. Without objective measurable biomarkers to assess IBS patients’ GI symptom burden, along with health care’s increased emphasis on patient-centered care, it becomes important to accurately capture a patient’s illness experience with PROs. A number of IBS symptom and QOL PRO instruments have been described in the literature, but most are beset by lengthy completion times and are impractical for use in everyday care. GI PROMIS, on the other hand, is a versatile and efficient instrument for collecting PRO data from not only IBS patients, but also all those who seek care in our GI clinics. Improvements in PRO and implementation science combined with technological advances have lessened the barriers to employing PROs in routine clinical care, and an increasing number of institutions are beginning to take up this challenge. In doing so and by seamlessly incorporating PROs in clinical practice, it facilitates placement of our patients’ voices at the forefront of their health care, changes how we monitor and manage patients, and, ultimately, may improve patient satisfaction and clinical outcomes.
Content from this column was originally published in the “Practice Management: The Road Ahead” section of Clinical Gastroenterology and Hepatology (2018;16:462-6).
References
1. Detmar S.B., Muller M.J., Schornagel J.H., et al. Role of health-related quality of life in palliative chemotherapy treatment decisions. J Clin Oncol. 2002;20:1056-62.
2. Detmar S.B., Muller M.J., Schornagel J.H., et al. Health-related quality-of-life assessments and patient-physician communication: a randomized controlled trial. JAMA. 2002;288:3027-34.
3. Neumann M., Edelhauser F., Kreps G.L., et al. Can patient-provider interaction increase the effectiveness of medical treatment or even substitute it? An exploration on why and how to study the specific effect of the provider. Patient Educ Couns. 2010;80:307-14.
4. Lembo A.J. The clinical and economic burden of irritable bowel syndrome. Pract Gastroenterol. 2007;31:3-9.
5. Valderas J.M., Kotzeva A., Espallargues M., et al. The impact of measuring patient-reported outcomes in clinical practice: a systematic review of the literature. Qual Life Res. 2008;17:179-93.
6. Almario C.V., Chey W.D., Khanna D., et al. Impact of National Institutes of Health gastrointestinal PROMIS measures in clinical practice: results of a multicenter controlled trial. Am J Gastroenterol. 2016;111:1546-56.
7. Basch E., Deal A.M., Kris M.G., et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34:557-65.
8. Kotronoulas G., Kearney N., Maguire R., et al. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol. 2014;32:1480-501.
9. Basch E., Deal A.M., Dueck A.C., et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318:197-8.
10. Spiegel B.M. Patient-reported outcomes in gastroenterology: clinical and research applications. J Neurogastroenterol Motil. 2013;19:137-48.
11. Mangel A.W., Hahn B.A., Heath A.T., et al. Adequate relief as an endpoint in clinical trials in irritable bowel syndrome. J Int Med Res. 1998;26:76-81.
12. Francis C.Y., Morris J., Whorwell P.J. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395-402.
13. Wiklund I.K., Fullerton S., Hawkey C.J., et al. An irritable bowel syndrome-specific symptom questionnaire: development and validation. Scand J Gastroenterol. 2003;38:947-54.
14. Drossman D.A., Li Z., Toner B.B., et al. Functional bowel disorders. A multicenter comparison of health status and development of illness severity index. Dig Dis Sci. 1995;40:986-95.
15. Roalfe A.K., Roberts L.M., Wilson S. Evaluation of the Birmingham IBS symptom questionnaire. BMC Gastroenterol. 2008;8:30.
16. Patrick D.L., Drossman D.A., Frederick I.O., et al. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43:400-11.
17. Hahn B.A., Kirchdoerfer L.J., Fullerton S., et al. Evaluation of a new quality of life questionnaire for patients with irritable bowel syndrome. Aliment Pharmacol Ther. 1997;11:547-52.
18. Shaw M., Talley N.J., Adlis S., et al. Development of a digestive health status instrument: tests of scaling assumptions, structure and reliability in a primary care population. Aliment Pharmacol Ther. 1998;12:1067-78.
19. Chassany O., Marquis P., Scherrer B., et al. Validation of a specific quality of life questionnaire for functional digestive disorders. Gut. 1999;44:527-33.
20. Wong E., Guyatt G.H., Cook D.J., et al. Development of a questionnaire to measure quality of life in patients with irritable bowel syndrome. Eur J Surg Suppl. 1998;583:50-6.
21. Groll D., Vanner S.J., Depew W.T., et al. The IBS-36: a new quality of life measure for irritable bowel syndrome. Am J Gastroenterol. 2002;97:962-71.
22. Lee E.H., Kwon O., Hahm K.B., et al. Irritable bowel syndrome-specific health-related quality of life instrument: development and psychometric evaluation. Health Qual Life Outcomes. 2016;14:22.
23. Spiegel B.M., Hays R.D., Bolus R., et al. Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) gastrointestinal symptom scales. Am J Gastroenterol. 2014;109:1804-14.
24. Bijkerk C.J., de Wit N.J., Muris J.W., et al. Outcome measures in irritable bowel syndrome: comparison of psychometric and methodological characteristics. Am J Gastroenterol. 2003;98:122-7.
25. HealthMeasures. Data collection tools. 2017. Available from http://www.healthmeasures.net/resource-center/data-collection-tools. Accessed August 20, 2017.
26. Baumhauer J.F. Patient-reported outcomes – are they living up to their potential?. N Engl J Med. 2017;377:6-9.
Dr. Almario is assistant professor-in-residence of medicine and public health, division of digestive and liver diseases, Dr. Spiegel professor-in-residence of medicine and public health, division of digestive and liver diseases, Cedars-Sinai Medical Center; Cedars-Sinai Center for Outcomes Research and Education, Los Angeles. Dr. Spiegel is a principal at My Total Health, which operates and maintains MyGiHealth; Dr. Almario has a stock option grant in My Total Health.
Patients often seek care because they experience symptoms that negatively affect their health-related quality of life (HRQOL). Health care providers must then elicit, measure, and interpret patient symptoms as part of their clinical evaluation. To assist with this goal and to help bridge the gap between patients and providers, investigators have developed and validated a wide range of patient-reported outcomes (PROs) across the breadth and depth of the human health and illness experience. These PROs, which measure any aspect of a patient’s biopsychosocial health and come directly from the patient, may help direct care and improve outcomes. When PROs are collected systematically, efficiently, and in the right place at the right time, they may enhance the patient–provider relationship by improving communication and facilitating shared decision making.1-3
Within gastroenterology and hepatology, PROs have been developed for a number of conditions, including irritable bowel syndrome (IBS), chronic idiopathic constipation, cirrhosis, eosinophilic esophagitis, inflammatory bowel disease, and gastroesophageal reflux disease, among many other chronic diseases. IBS in particular is well suited for PRO measurement because it is symptom based and significantly impacts patients’ HRQOL and emotional health. Moreover, it is the most commonly diagnosed gastrointestinal condition and imparts a significant economic burden. In this article, we review the rationale for measuring IBS PROs in routine clinical practice and detail available measurement instruments.
Importance of IBS PROs in clinical practice
IBS is a functional GI disorder that is characterized by recurrent abdominal pain and altered bowel habits (i.e., diarrhea, constipation, or a mix of both). It has an estimated worldwide prevalence of 11%, and total costs are estimated at $30 billion annually in the United States alone.4 Because of the chronic relapsing nature of IBS, along with its impact on physical, mental, and social distress, it becomes important to accurately capture a patient’s illness experience with PROs. This is especially relevant to patients with IBS because we currently lack objective measurable biomarkers to assess their GI symptom burden. Instead, clinicians often are relegated to informal assessments of the severity of a patient’s symptoms, which ultimately guide their treatment recommendations: How many bowel movements have you had in the past week? Were your bowel movements hard or soft? How bad is your abdominal pain on a scale of 1 to 10? However, these traditional outcomes measured by health care providers often fail to capture other aspects of their health. For example, simply asking patients about the frequency and character of their stools will not provide any insight into how their symptoms impact their HRQOL and psychosocial health. An individual may report only two loose stools per day, but this may lead to substantial anxiety and negatively affect his or her performance at work. Similarly, the significance of the symptoms will vary from person to person; a patient with IBS who has five loose daily bowel movements may not be bothered by it, whereas another individual with three loose stools per day may feel that it severely hampers his or her daily activities. This is where PROs provide value because they provide a key component to understanding the true burden of IBS, and accounts for the HRQOL and psychosocial decrement engendered by the disease.
Although past literature has reported inconsistent benefits of using PROs in clinical practice,5 including that from our own work,6 there is a growing number of studies that have noted improved clinical outcomes through employing PROs.7-9 A compelling example is provided by Basch et al,7 who randomized patients receiving chemotherapy for metastatic cancer to either weekly symptom reporting using electronically delivered PRO instruments vs. usual care, which consisted of symptom reporting at the discretion of clinicians. Here, they found that the intervention group had higher HRQOL, were less likely to visit the emergency department, and remained on chemotherapy for a longer period of time. Interestingly, the benefits from PROs were greater among participants who lacked prior computer experience vs. those who were computer savvy. Basch et al9 also noted that the use of PROs extended survival by 5 months when compared with the control group (31.2 vs. 26.0 mo; P = .03). Longitudinal symptom reporting among IBS patients using PROs, when implemented well, may similarly lead to improved patient satisfaction, HRQOL, and clinical outcomes.

Measuring PROs in the clinical trenches
PROs generally are measured with patient questionnaires that collect data across several areas, including physical, social, and psychological functioning. Although PROs may enhance the patient–provider relationship and improve communication and shared decision making, we acknowledge that there are important barriers to its use in routine clinical practice.10 First, many providers (and their patients) may find use of PRO instruments burdensome, and it can be time consuming to collect PROs from patients and securely transmit the data into the electronic health record (EHR). This can make it untenable for use in busy practices. Second, many gastroenterologists have not received formal training in performing complete biopsychosocial evaluations with PROs, and it can be difficult to understand and act upon PRO scores. Third, there are many PROs to choose from and there is a lack of measurement standards across questionnaires. These challenges limit widespread use of PROs in clinical practice, and it is understandable why most providers instead opt for informal measurement of symptoms and function. Later, we detail strategies for overcoming the earlier-described challenges in employing PROs in everyday practice along with relevant IBS PRO instruments.
IBS–specific PRO instruments
There have been several IBS-specific PRO instruments described in the literature, all of which vary in length, content, and amount of data supporting their validity (Table 1). Examples of IBS symptoms scales include the Adequate Relief measure, Irritable Bowel Syndrome Severity Scoring System, Gastrointestinal Symptom Rating Scale in IBS, Functional Bowel Disorder Severity Index, IBS Symptom Questionnaire, and Birmingham IBS Symptom Questionnaire.15,24 There are also IBS-specific HRQOL instruments, such as the Irritable Bowel Syndrome Quality of Life measurement, Digestive Health Status Instrument, Functional Digestive Disorder Quality of Life questionnaire, Irritable Bowel Syndrome Health Related Quality of Life questionnaire, and IBS-36, among others.21,24
Bijkerk et al24 evaluated and compared the validity and appropriateness of both the symptom and QOL scales. Among the examined IBS symptom instruments, they found that the Adequate Relief question (Did you have adequate relief of IBS-related abdominal pain or discomfort?) is the best choice for assessing global symptomatology, and the Irritable Bowel Syndrome Severity Scoring System is optimal for obtaining information on more specific symptoms.24 As for the QOL scales, the Irritable Bowel Syndrome Quality of Life measurement, which comprises 34 items, is the preferred instrument for assessing changes in HRQOL because it is the most extensively validated.24 Bijkerk et al24 also concluded that although the studied instruments showed reasonable psychometric and methodologic qualities, the use of these instruments in daily clinical practice is debatable because the measures (save for the Adequate Relief question) are lengthy and/or cumbersome to use. Because these instruments may not be practical for use during everyday care, this leads to a discussion of the National Institutes of Health (NIH) Patient Reported Outcomes Measurement Information System (PROMIS), a novel approach to measuring PROs in the clinical trenches.
NIH PROMIS
Although there have been many efforts to implement PROs in routine clinical care, a recent confluence of scientific, regulatory, and political factors, coupled with technological advancements in PRO measurement techniques, have justified re-evaluation of the use of PROs in everyday practice. In response to the practical and technical challenges to employing PROs in the clinical trenches as described earlier, the NIH PROMIS (www.healthmeasures.net) was created in 2004 with the goal of developing and validating a toolbox of PROs that cover the breadth and depth of the human health and illness experience. The PROMIS initiative also was borne from the realization that patients are the ultimate consumers of health care and are the final judge on whether their health care needs are being addressed adequately.
By using modern psychometric techniques, such as item response theory and computerized adaptive testing, PROMIS offers state-of-the-art psychometrics, establishes common-language benchmarks for symptoms across conditions, and identifies clinical thresholds for action and meaningful clinical improvement or decline. PROMIS questionnaires, in light of accelerated EHR adoption in recent years, also are designed to be administered electronically and efficiently, allowing implementation in busy clinical settings. As of December 2017, these instruments can be administered and scored through EHRs such as Epic (Epic Systems, Verona, WI) and Cerner (Cerner Corporation, North Kansas City, MO), the PROMIS iPad (Apple Inc, Cupertino, CA) App, and online data collection tools such as the Assessment Center (www.assessmentcenter.net) and REDCap (Research Electronic Data Capture; Vanderbilt University, Nashville, TN).25 An increasing number of health systems are making PROMIS measures available through their EHRs. For example, the University of Rochester Medical Center collects PROMIS scores for physical function, pain interference, and depression from more than 80% of their patients with in-clinic testing, and individual departments are able to further tailor their administered questionnaires.26
Gastrointestinal PROs measurement information system scales
Because of the extraordinary burden of illness from digestive diseases, the PROMIS consortium added a GI item bank, which our research group developed.23 By using the NIH PROMIS framework, we constructed and validated eight GI PROMIS symptom scales: abdominal pain, bloating/gas, constipation, diarrhea, bowel incontinence, dysphagia, heartburn/reflux, and nausea/vomiting.23 GI PROMIS was designed from the outset to not be a disease-targeted item bank (e.g., IBS-, cirrhosis-, or inflammatory bowel disease specific), but rather symptom targeted, measuring the physical symptoms of the GI tract, because it is more useful across the population as a whole. In Supplementary Figure 1 (at https://doi.org/10.1016/j.cgh.2017.12.026), we include the abdominal pain, constipation, and diarrhea PROMIS scales because they form the cardinal symptoms of IBS.
GI PROMIS scales are readily accessible via the Assessment Center,25 and we also have made them freely available via MyGiHealth — an iOS (Apple Inc) and online app (go.mygihealth.io) endorsed by the American Gastroenterological Association. The patient’s responses to the questionnaires are converted to percentile scores and compared with the general U.S. population, and then displayed in a symptom heat map. The app also allows users to track GI PROMIS scores longitudinally, empowering IBS patients (and any patient with GI symptoms for that matter) and their providers to see if they are objectively responding to prescribed therapies and potentially improving satisfaction and patient–provider communication.
Conclusions
IBS is a common, chronic, relapsing disease that often leads to physical, mental, and social distress. Without objective measurable biomarkers to assess IBS patients’ GI symptom burden, along with health care’s increased emphasis on patient-centered care, it becomes important to accurately capture a patient’s illness experience with PROs. A number of IBS symptom and QOL PRO instruments have been described in the literature, but most are beset by lengthy completion times and are impractical for use in everyday care. GI PROMIS, on the other hand, is a versatile and efficient instrument for collecting PRO data from not only IBS patients, but also all those who seek care in our GI clinics. Improvements in PRO and implementation science combined with technological advances have lessened the barriers to employing PROs in routine clinical care, and an increasing number of institutions are beginning to take up this challenge. In doing so and by seamlessly incorporating PROs in clinical practice, it facilitates placement of our patients’ voices at the forefront of their health care, changes how we monitor and manage patients, and, ultimately, may improve patient satisfaction and clinical outcomes.
Content from this column was originally published in the “Practice Management: The Road Ahead” section of Clinical Gastroenterology and Hepatology (2018;16:462-6).
References
1. Detmar S.B., Muller M.J., Schornagel J.H., et al. Role of health-related quality of life in palliative chemotherapy treatment decisions. J Clin Oncol. 2002;20:1056-62.
2. Detmar S.B., Muller M.J., Schornagel J.H., et al. Health-related quality-of-life assessments and patient-physician communication: a randomized controlled trial. JAMA. 2002;288:3027-34.
3. Neumann M., Edelhauser F., Kreps G.L., et al. Can patient-provider interaction increase the effectiveness of medical treatment or even substitute it? An exploration on why and how to study the specific effect of the provider. Patient Educ Couns. 2010;80:307-14.
4. Lembo A.J. The clinical and economic burden of irritable bowel syndrome. Pract Gastroenterol. 2007;31:3-9.
5. Valderas J.M., Kotzeva A., Espallargues M., et al. The impact of measuring patient-reported outcomes in clinical practice: a systematic review of the literature. Qual Life Res. 2008;17:179-93.
6. Almario C.V., Chey W.D., Khanna D., et al. Impact of National Institutes of Health gastrointestinal PROMIS measures in clinical practice: results of a multicenter controlled trial. Am J Gastroenterol. 2016;111:1546-56.
7. Basch E., Deal A.M., Kris M.G., et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34:557-65.
8. Kotronoulas G., Kearney N., Maguire R., et al. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol. 2014;32:1480-501.
9. Basch E., Deal A.M., Dueck A.C., et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318:197-8.
10. Spiegel B.M. Patient-reported outcomes in gastroenterology: clinical and research applications. J Neurogastroenterol Motil. 2013;19:137-48.
11. Mangel A.W., Hahn B.A., Heath A.T., et al. Adequate relief as an endpoint in clinical trials in irritable bowel syndrome. J Int Med Res. 1998;26:76-81.
12. Francis C.Y., Morris J., Whorwell P.J. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395-402.
13. Wiklund I.K., Fullerton S., Hawkey C.J., et al. An irritable bowel syndrome-specific symptom questionnaire: development and validation. Scand J Gastroenterol. 2003;38:947-54.
14. Drossman D.A., Li Z., Toner B.B., et al. Functional bowel disorders. A multicenter comparison of health status and development of illness severity index. Dig Dis Sci. 1995;40:986-95.
15. Roalfe A.K., Roberts L.M., Wilson S. Evaluation of the Birmingham IBS symptom questionnaire. BMC Gastroenterol. 2008;8:30.
16. Patrick D.L., Drossman D.A., Frederick I.O., et al. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43:400-11.
17. Hahn B.A., Kirchdoerfer L.J., Fullerton S., et al. Evaluation of a new quality of life questionnaire for patients with irritable bowel syndrome. Aliment Pharmacol Ther. 1997;11:547-52.
18. Shaw M., Talley N.J., Adlis S., et al. Development of a digestive health status instrument: tests of scaling assumptions, structure and reliability in a primary care population. Aliment Pharmacol Ther. 1998;12:1067-78.
19. Chassany O., Marquis P., Scherrer B., et al. Validation of a specific quality of life questionnaire for functional digestive disorders. Gut. 1999;44:527-33.
20. Wong E., Guyatt G.H., Cook D.J., et al. Development of a questionnaire to measure quality of life in patients with irritable bowel syndrome. Eur J Surg Suppl. 1998;583:50-6.
21. Groll D., Vanner S.J., Depew W.T., et al. The IBS-36: a new quality of life measure for irritable bowel syndrome. Am J Gastroenterol. 2002;97:962-71.
22. Lee E.H., Kwon O., Hahm K.B., et al. Irritable bowel syndrome-specific health-related quality of life instrument: development and psychometric evaluation. Health Qual Life Outcomes. 2016;14:22.
23. Spiegel B.M., Hays R.D., Bolus R., et al. Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) gastrointestinal symptom scales. Am J Gastroenterol. 2014;109:1804-14.
24. Bijkerk C.J., de Wit N.J., Muris J.W., et al. Outcome measures in irritable bowel syndrome: comparison of psychometric and methodological characteristics. Am J Gastroenterol. 2003;98:122-7.
25. HealthMeasures. Data collection tools. 2017. Available from http://www.healthmeasures.net/resource-center/data-collection-tools. Accessed August 20, 2017.
26. Baumhauer J.F. Patient-reported outcomes – are they living up to their potential?. N Engl J Med. 2017;377:6-9.
Dr. Almario is assistant professor-in-residence of medicine and public health, division of digestive and liver diseases, Dr. Spiegel professor-in-residence of medicine and public health, division of digestive and liver diseases, Cedars-Sinai Medical Center; Cedars-Sinai Center for Outcomes Research and Education, Los Angeles. Dr. Spiegel is a principal at My Total Health, which operates and maintains MyGiHealth; Dr. Almario has a stock option grant in My Total Health.
Mayo Clinic announces new president and CEO: Gianrico Farrugia, MD
The Mayo Clinic Board of Trustees has announced that Gianrico Farrugia, MD, vice president and CEO of Mayo Clinic Florida, will take over as president and CEO of Mayo Clinic at the end of the year. AGA congratulates Dr. Farrugia on this accomplishment.
Here’s three reasons why AGA is excited by this news:
1. Dr. Farrugia is an accomplished GI investigator. Dr. Farrugia runs an NIH-funded translational laboratory focused on disorders of GI motility. The aim of Dr. Farrugia’s work is to understand at a cellular, subcellular and molecular level how the normal functions of the GI tract determine the defects that result in diseases such as diabetic gastroparesis, slow transit constipation, and irritable bowel syndrome (IBS), which will ultimately lead to new strategies to treat these diseases by developing targeted disease-modifying agents.
2. Dr. Farrugia is an alumnus of the AGA Research Foundation Research Scholar Award program. Dr. Farrugia received his Research Scholar Award in 1994 for his project titled “Jejunal Smooth Muscle Ion Channel Regulation in Health and Disease.”
3. Dr. Farrugia has given back to AGA both with his time – serving on the AGA Nominating Committee, AGA Institute Council, and Cellular and Molecular Gastroenterology and Hepatology editorial board – and by contributing, with his wife Geraldine Farrugia, to the AGA Research Foundation at the highest level as an AGA Legacy Society member.
Join AGA members in congratulating Dr. Farrugia in the AGA Community, community.gastro.org.
The Mayo Clinic Board of Trustees has announced that Gianrico Farrugia, MD, vice president and CEO of Mayo Clinic Florida, will take over as president and CEO of Mayo Clinic at the end of the year. AGA congratulates Dr. Farrugia on this accomplishment.
Here’s three reasons why AGA is excited by this news:
1. Dr. Farrugia is an accomplished GI investigator. Dr. Farrugia runs an NIH-funded translational laboratory focused on disorders of GI motility. The aim of Dr. Farrugia’s work is to understand at a cellular, subcellular and molecular level how the normal functions of the GI tract determine the defects that result in diseases such as diabetic gastroparesis, slow transit constipation, and irritable bowel syndrome (IBS), which will ultimately lead to new strategies to treat these diseases by developing targeted disease-modifying agents.
2. Dr. Farrugia is an alumnus of the AGA Research Foundation Research Scholar Award program. Dr. Farrugia received his Research Scholar Award in 1994 for his project titled “Jejunal Smooth Muscle Ion Channel Regulation in Health and Disease.”
3. Dr. Farrugia has given back to AGA both with his time – serving on the AGA Nominating Committee, AGA Institute Council, and Cellular and Molecular Gastroenterology and Hepatology editorial board – and by contributing, with his wife Geraldine Farrugia, to the AGA Research Foundation at the highest level as an AGA Legacy Society member.
Join AGA members in congratulating Dr. Farrugia in the AGA Community, community.gastro.org.
The Mayo Clinic Board of Trustees has announced that Gianrico Farrugia, MD, vice president and CEO of Mayo Clinic Florida, will take over as president and CEO of Mayo Clinic at the end of the year. AGA congratulates Dr. Farrugia on this accomplishment.
Here’s three reasons why AGA is excited by this news:
1. Dr. Farrugia is an accomplished GI investigator. Dr. Farrugia runs an NIH-funded translational laboratory focused on disorders of GI motility. The aim of Dr. Farrugia’s work is to understand at a cellular, subcellular and molecular level how the normal functions of the GI tract determine the defects that result in diseases such as diabetic gastroparesis, slow transit constipation, and irritable bowel syndrome (IBS), which will ultimately lead to new strategies to treat these diseases by developing targeted disease-modifying agents.
2. Dr. Farrugia is an alumnus of the AGA Research Foundation Research Scholar Award program. Dr. Farrugia received his Research Scholar Award in 1994 for his project titled “Jejunal Smooth Muscle Ion Channel Regulation in Health and Disease.”
3. Dr. Farrugia has given back to AGA both with his time – serving on the AGA Nominating Committee, AGA Institute Council, and Cellular and Molecular Gastroenterology and Hepatology editorial board – and by contributing, with his wife Geraldine Farrugia, to the AGA Research Foundation at the highest level as an AGA Legacy Society member.
Join AGA members in congratulating Dr. Farrugia in the AGA Community, community.gastro.org.
Rising microbiome investigator: Ting-Chin David Shen, MD, PhD
We spoke with Dr. Shen, instructor of medicine at the University of Pennsylvania and the recipient of the AGA Research Foundation’s 2016 Microbiome Junior Investigator Award, to learn about his passion for gut microbiome research.
How would you sum up your research in one sentence?
My research examines the metabolic interactions between the gut microbiota and the mammalian host, with a particular emphasis on amino acid metabolism and nitrogen flux via the bacterial enzyme urease.
What impact do you hope your research will have on patients?
My hope is that by better understanding the biological mechanisms by which the gut microbiota impacts host metabolism, we can modulate its effects to treat a variety of conditions and diseases including hepatic encephalopathy, inborn errors of metabolism, obesity, malnutrition, etc.
What inspired you to focus your research career on the gut microbiome?
My clinical experience as a gastroenterologist inspired my interest in metabolic and nutritional research. When I learned of the impact that the gut microbiota has on host metabolism, it created an entirely different perspective for me in terms of thinking about how to treat metabolic and nutritional disorders. There are tremendous opportunities in modifying our gut microbiota in concert with dietary interventions in order to modulate our metabolism.
What recent publication from your lab best represents your work, if anyone wants to learn more?
The following work examined how the use of a defined bacterial consortium without urease activity can reduce colonic ammonia level upon inoculation into the gut and ameliorate morbidity and mortality in a murine model of liver disease: Shen T.D., Albenberg L.A., Bittinger K., et al, Engineering the gut microbiota to treat hyperammonemia. J Clin Invest. 2015 Jul 1;125(7):2841-50. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4563680/.
We spoke with Dr. Shen, instructor of medicine at the University of Pennsylvania and the recipient of the AGA Research Foundation’s 2016 Microbiome Junior Investigator Award, to learn about his passion for gut microbiome research.
How would you sum up your research in one sentence?
My research examines the metabolic interactions between the gut microbiota and the mammalian host, with a particular emphasis on amino acid metabolism and nitrogen flux via the bacterial enzyme urease.
What impact do you hope your research will have on patients?
My hope is that by better understanding the biological mechanisms by which the gut microbiota impacts host metabolism, we can modulate its effects to treat a variety of conditions and diseases including hepatic encephalopathy, inborn errors of metabolism, obesity, malnutrition, etc.
What inspired you to focus your research career on the gut microbiome?
My clinical experience as a gastroenterologist inspired my interest in metabolic and nutritional research. When I learned of the impact that the gut microbiota has on host metabolism, it created an entirely different perspective for me in terms of thinking about how to treat metabolic and nutritional disorders. There are tremendous opportunities in modifying our gut microbiota in concert with dietary interventions in order to modulate our metabolism.
What recent publication from your lab best represents your work, if anyone wants to learn more?
The following work examined how the use of a defined bacterial consortium without urease activity can reduce colonic ammonia level upon inoculation into the gut and ameliorate morbidity and mortality in a murine model of liver disease: Shen T.D., Albenberg L.A., Bittinger K., et al, Engineering the gut microbiota to treat hyperammonemia. J Clin Invest. 2015 Jul 1;125(7):2841-50. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4563680/.
We spoke with Dr. Shen, instructor of medicine at the University of Pennsylvania and the recipient of the AGA Research Foundation’s 2016 Microbiome Junior Investigator Award, to learn about his passion for gut microbiome research.
How would you sum up your research in one sentence?
My research examines the metabolic interactions between the gut microbiota and the mammalian host, with a particular emphasis on amino acid metabolism and nitrogen flux via the bacterial enzyme urease.
What impact do you hope your research will have on patients?
My hope is that by better understanding the biological mechanisms by which the gut microbiota impacts host metabolism, we can modulate its effects to treat a variety of conditions and diseases including hepatic encephalopathy, inborn errors of metabolism, obesity, malnutrition, etc.
What inspired you to focus your research career on the gut microbiome?
My clinical experience as a gastroenterologist inspired my interest in metabolic and nutritional research. When I learned of the impact that the gut microbiota has on host metabolism, it created an entirely different perspective for me in terms of thinking about how to treat metabolic and nutritional disorders. There are tremendous opportunities in modifying our gut microbiota in concert with dietary interventions in order to modulate our metabolism.
What recent publication from your lab best represents your work, if anyone wants to learn more?
The following work examined how the use of a defined bacterial consortium without urease activity can reduce colonic ammonia level upon inoculation into the gut and ameliorate morbidity and mortality in a murine model of liver disease: Shen T.D., Albenberg L.A., Bittinger K., et al, Engineering the gut microbiota to treat hyperammonemia. J Clin Invest. 2015 Jul 1;125(7):2841-50. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4563680/.
Red flag raised on CMS indication-based formulary policy
Physician groups are expressing concerns regarding a new policy that will allow indication-based formulary design in the Medicare Part D prescription drug benefit.
The Centers for Medicare & Medicaid Services announced the new policy in an Aug. 29 memo to Part D plan sponsors.
According to a fact sheet issued by CMS on the same day, indication-based formulary design “is a formulary management tool that allows health plans to tailor on-formulary coverage of drugs predicated on specific indications.”
Current Part D policy requires plan sponsors to cover all Food and Drug Administration–approved indications for each drug that is on a plan formulary. Sponsors can begin to implement the new indication-based formulary design policy for plans issued in 2020.
The memo notes that, if a Part D plan sponsor chooses to opt into this policy, “it must ensure that there is another therapeutically similar drug on formulary for the nonformulary indication. For example, if a tumor necrosis factor (TNF) blocker is FDA-approved for both Crohn’s disease and plaque psoriasis, but the Part D plan will include it on formulary for plaque psoriasis only, the plan must ensure that there is another TNF blocker on formulary that will be covered for Crohn’s disease.”
Beneficiaries can use the exceptions process to get coverage for a drug that has an indication not on the formulary.
“By allowing Medicare’s prescription drug plans to cover the best drug for each patient condition, plans will have more negotiating power with drug companies, which will result in lower prices for Medicare beneficiaries,” CMS Administrator Seema Verma said in a statement.
However, physician groups should be concerned about the definition of “best drug.” Is this definition based upon efficacy, results of clinical trials, clinical effectiveness research, or just cost? Will there be transparenecy surrounding rebates?
The “proposed changes will exacerbate many of the access issues patients currently face with plan usage of existing utilization management practices, such as step therapy,” the American College of Rheumatology said in a statement. “Unlike step therapy, which often delays effective treatments, this proposal would go even further and allow plans to remove therapies from the formulary altogether, leaving patients completely unable to access treatments that doctors and patients choose together. ... We also have concerns on what this would mean for work being done on compendia inclusion to secure off-label drug coverage if plans don’t have to cover all FDA-approved indications.”
A similar situation exists in patients with inflammatory bowel disease in which step therapy has largely been replaced by risk assessments. The AGA Crohn’s and UC Care Pathways are based on this principle.
Under the plan, Medicare patients will face increased challenges as they navigate health plans to make sure that their needed drug is on their selected formulary, which can change based on what health conditions they have,” AMA President Barbara McAneny, MD, said in a statement. Dr. McAneny added that it will be even more difficult for physicians who are working with patients to get them on the best medicines covered by the patient’s formulary.
“Physicians already lack ready access to accurate formulary information – preferred/tier status, on/off formulary, PA [prior authorization] and step therapy requirements – at the point of care in their EHRs,” she said. “These transparency problems will expand by an order of magnitude by the complications this change introduces.”
Physician groups are expressing concerns regarding a new policy that will allow indication-based formulary design in the Medicare Part D prescription drug benefit.
The Centers for Medicare & Medicaid Services announced the new policy in an Aug. 29 memo to Part D plan sponsors.
According to a fact sheet issued by CMS on the same day, indication-based formulary design “is a formulary management tool that allows health plans to tailor on-formulary coverage of drugs predicated on specific indications.”
Current Part D policy requires plan sponsors to cover all Food and Drug Administration–approved indications for each drug that is on a plan formulary. Sponsors can begin to implement the new indication-based formulary design policy for plans issued in 2020.
The memo notes that, if a Part D plan sponsor chooses to opt into this policy, “it must ensure that there is another therapeutically similar drug on formulary for the nonformulary indication. For example, if a tumor necrosis factor (TNF) blocker is FDA-approved for both Crohn’s disease and plaque psoriasis, but the Part D plan will include it on formulary for plaque psoriasis only, the plan must ensure that there is another TNF blocker on formulary that will be covered for Crohn’s disease.”
Beneficiaries can use the exceptions process to get coverage for a drug that has an indication not on the formulary.
“By allowing Medicare’s prescription drug plans to cover the best drug for each patient condition, plans will have more negotiating power with drug companies, which will result in lower prices for Medicare beneficiaries,” CMS Administrator Seema Verma said in a statement.
However, physician groups should be concerned about the definition of “best drug.” Is this definition based upon efficacy, results of clinical trials, clinical effectiveness research, or just cost? Will there be transparenecy surrounding rebates?
The “proposed changes will exacerbate many of the access issues patients currently face with plan usage of existing utilization management practices, such as step therapy,” the American College of Rheumatology said in a statement. “Unlike step therapy, which often delays effective treatments, this proposal would go even further and allow plans to remove therapies from the formulary altogether, leaving patients completely unable to access treatments that doctors and patients choose together. ... We also have concerns on what this would mean for work being done on compendia inclusion to secure off-label drug coverage if plans don’t have to cover all FDA-approved indications.”
A similar situation exists in patients with inflammatory bowel disease in which step therapy has largely been replaced by risk assessments. The AGA Crohn’s and UC Care Pathways are based on this principle.
Under the plan, Medicare patients will face increased challenges as they navigate health plans to make sure that their needed drug is on their selected formulary, which can change based on what health conditions they have,” AMA President Barbara McAneny, MD, said in a statement. Dr. McAneny added that it will be even more difficult for physicians who are working with patients to get them on the best medicines covered by the patient’s formulary.
“Physicians already lack ready access to accurate formulary information – preferred/tier status, on/off formulary, PA [prior authorization] and step therapy requirements – at the point of care in their EHRs,” she said. “These transparency problems will expand by an order of magnitude by the complications this change introduces.”
Physician groups are expressing concerns regarding a new policy that will allow indication-based formulary design in the Medicare Part D prescription drug benefit.
The Centers for Medicare & Medicaid Services announced the new policy in an Aug. 29 memo to Part D plan sponsors.
According to a fact sheet issued by CMS on the same day, indication-based formulary design “is a formulary management tool that allows health plans to tailor on-formulary coverage of drugs predicated on specific indications.”
Current Part D policy requires plan sponsors to cover all Food and Drug Administration–approved indications for each drug that is on a plan formulary. Sponsors can begin to implement the new indication-based formulary design policy for plans issued in 2020.
The memo notes that, if a Part D plan sponsor chooses to opt into this policy, “it must ensure that there is another therapeutically similar drug on formulary for the nonformulary indication. For example, if a tumor necrosis factor (TNF) blocker is FDA-approved for both Crohn’s disease and plaque psoriasis, but the Part D plan will include it on formulary for plaque psoriasis only, the plan must ensure that there is another TNF blocker on formulary that will be covered for Crohn’s disease.”
Beneficiaries can use the exceptions process to get coverage for a drug that has an indication not on the formulary.
“By allowing Medicare’s prescription drug plans to cover the best drug for each patient condition, plans will have more negotiating power with drug companies, which will result in lower prices for Medicare beneficiaries,” CMS Administrator Seema Verma said in a statement.
However, physician groups should be concerned about the definition of “best drug.” Is this definition based upon efficacy, results of clinical trials, clinical effectiveness research, or just cost? Will there be transparenecy surrounding rebates?
The “proposed changes will exacerbate many of the access issues patients currently face with plan usage of existing utilization management practices, such as step therapy,” the American College of Rheumatology said in a statement. “Unlike step therapy, which often delays effective treatments, this proposal would go even further and allow plans to remove therapies from the formulary altogether, leaving patients completely unable to access treatments that doctors and patients choose together. ... We also have concerns on what this would mean for work being done on compendia inclusion to secure off-label drug coverage if plans don’t have to cover all FDA-approved indications.”
A similar situation exists in patients with inflammatory bowel disease in which step therapy has largely been replaced by risk assessments. The AGA Crohn’s and UC Care Pathways are based on this principle.
Under the plan, Medicare patients will face increased challenges as they navigate health plans to make sure that their needed drug is on their selected formulary, which can change based on what health conditions they have,” AMA President Barbara McAneny, MD, said in a statement. Dr. McAneny added that it will be even more difficult for physicians who are working with patients to get them on the best medicines covered by the patient’s formulary.
“Physicians already lack ready access to accurate formulary information – preferred/tier status, on/off formulary, PA [prior authorization] and step therapy requirements – at the point of care in their EHRs,” she said. “These transparency problems will expand by an order of magnitude by the complications this change introduces.”
Treating cannabinoid hyperemesis syndrome
Incidence may increase as marijuana use rises
Case
WS is a 54-year-old African American male with a medical history of diabetes mellitus type 2, hypertension, obstructive sleep apnea, and gastroparesis. He has multiple admissions for intractable nausea, vomiting, and abdominal pain believed to be from diabetic gastroparesis despite a normal gastric-emptying study. Endoscopy done in prior admission showed duodenitis, gastritis, and esophagitis, and colonoscopy revealed diverticulosis. He had a negative gastric-emptying study of 6% retention at 4 hrs. His last hemoglobin A1c was 5 and his glucose has been well controlled. He is hospitalized again for intractable abdominal pain, nausea, and vomiting. His examination was unremarkable except for dry mucosa and epigastric tenderness. His labs were also insignificant except for prerenal azotemia. Upon further questioning he admitted to significant marijuana use, and his symptoms transiently improved with a hot shower in the hospital. He was diagnosed with cannabinoid hyperemesis syndrome (CHS) and admitted for further management.
Background
In the United States, 9 states and the District of Columbia have legalized recreational marijuana use, and 29 states and DC have legalized medical marijuana. Marijuana use is likely to rise, and with it may arise an increasing incidence of CHS.
The exact prevalence of CHS is not known. Diagnosis is often delayed as there is no reliable diagnostic test. A high index of suspicion is needed for prompt diagnosis.
CHS was first described in 2004 in South Australia and since then many case reports have been published. Marijuana has both proemetic and antiemetic effects. Unlike its antiemetic effect, the pathophysiology of the proemetic effect of marijuana is not well understood.
Key clinical features
CHS typically has three phases. Initially patients present with prodromal symptoms of abdominal discomfort and nausea. There is no emesis at this early phase. Patients are still able to tolerate a liquid diet in this prodromal phase.
This is followed by a more active phase of intractable vomiting, which is relieved by hot showers or baths. Most patients take compulsively long hot showers or baths many times a day. Also, they develop diaphoresis, restlessness, agitation, and weight loss.
The active phase is followed by a recovery phase when symptoms resolve and patients return to baseline, only to have it recur if marijuana use continues.
Diagnostic approach and management
CHS should be suspected in patients coming in with recurrent symptoms of abdominal pain, nausea and vomiting, and who have normal CBC, basic metabolic panel, lipase, and liver function tests. Patients should be directly questioned about marijuana use and whether symptoms are relieved with hot showers. A toxicology screen should be done. For patients with marijuana use and compulsive hot showers, further work up of their symptoms (e.g., upper endoscopy, abdominal ultrasound, and/or nuclear medicine emptying study) should be avoided. Figure 1 shows the suggested work-up.
The differential diagnosis for recurrent abdominal pain, nausea, and vomiting is chronic pancreatitis, gastroparesis, severe gastritis, medication adverse effects (especially GLP1 receptor agonists), cyclic vomiting syndrome, psychogenic vomiting, and (with the rise of narcotic abuse) narcotic bowel syndrome.
Our patient had a history of diabetes with an HbA1c at goal and a normal nuclear medicine gastric-emptying study (6% retention at 4 hours). He was also on liraglutide, but his symptoms predated this medicine use.
The mainstay of treatment for CHS is supportive therapy with intravenous fluids and antiemetics like 5-HT3-receptor antagonists (ondansetron); D2-receptor antagonists (metoclopramide); and H1-receptor antagonists (diphenhydramine). The effectiveness of these agents is limited, which is also a clue for the diagnosis of CHS. If traditional agents fail in controlling the symptoms, haloperidol can be tried, but it has been used with limited success. Our patient did not respond to traditional antiemetics, but responded well to a small dose of lorazepam. Even though a benzodiazepine is not the mainstay of treatment, it may be tried if other agents fail. Acid-suppression therapy with a proton pump inhibitor should be used as esophagogastroduodenoscopy (EGD) usually reveals mild gastritis and esophagitis, as in our patient. Narcotic use should be avoided for management of abdominal pain.
Patients should be counseled against marijuana use. This may be difficult if marijuana is being used as an appetite stimulant or for treatment of chemotherapy-induced nausea and vomiting. If willing, patients should be referred to a substance abuse rehabilitation center.
Back to the case
In this case, after a diagnosis of CHS was made, the patient was counseled against marijuana use. His abdominal pain and intractable vomiting did not improve with conservative management of n.p.o status, prochlorperazine, metoclopramide, and ondansetron. He was given a trial of low-dose lorazepam with significant improvement in his symptoms. He was counseled extensively against marijuana use and discharged. A follow-up phone call at 3 months showed continued abstinence and no recurrence of symptoms.
Dr. Gupta is a hospitalist at Yale New Haven Health and Bridgeport (Conn.) Hospital.
References
1. Bajgoric S et al. Cannabinoid hyperemesis syndrome: A guide for the practising clinician. BMJ Case Rep. 2015. doi: 10.1136/bcr-2015-210246.
2. Batke M et al. The cannabis hyperemesis syndrome characterized by persistent nausea and vomiting, abdominal pain, and compulsive bathing associated with chronic marijuana use: A report of eight cases in the united states. Dig Dis Sci. 2010 Nov;55(11):3113-9.
3. Iacopetti CL et al. Cannabinoid hyperemesis syndrome: a case report and review of pathophysiology. Clin Med Res. 2014 Sep;12(1-2):65-7.
4. Hickey JL et al. Haloperidol for treatment of cannabinoid hyperemesis syndrome. Am J Emerg Med. 2013 Jun. 31(6):1003.e5-6. Epub 2013 Apr 10.
Key points
Suspect CHS for patients with recurrent abdominal pain, nausea, and vomiting with negative initial work-up.
- Ask directly about marijuana use.
- Ask whether symptoms are relieved with hot shower/ bath.
- Send a toxicology screen.
- Make a diagnosis of CHS if:
1. Positive marijuana use.
2. Symptom improvement with hot baths or
3. Toxicology positive for marijuana.
- Manage conservatively with hydration and antiemetics.
- Suspect CHS if traditional antiemetics are not providing relief .
- If traditional antiemetics fail, trial of haloperidol or low-dose benzodiazepines.
- Avoid narcotics.
- Avoid unnecessary investigations.
- Counsel patients against marijuana use and refer to substance abuse center if patient agrees.
Incidence may increase as marijuana use rises
Incidence may increase as marijuana use rises
Case
WS is a 54-year-old African American male with a medical history of diabetes mellitus type 2, hypertension, obstructive sleep apnea, and gastroparesis. He has multiple admissions for intractable nausea, vomiting, and abdominal pain believed to be from diabetic gastroparesis despite a normal gastric-emptying study. Endoscopy done in prior admission showed duodenitis, gastritis, and esophagitis, and colonoscopy revealed diverticulosis. He had a negative gastric-emptying study of 6% retention at 4 hrs. His last hemoglobin A1c was 5 and his glucose has been well controlled. He is hospitalized again for intractable abdominal pain, nausea, and vomiting. His examination was unremarkable except for dry mucosa and epigastric tenderness. His labs were also insignificant except for prerenal azotemia. Upon further questioning he admitted to significant marijuana use, and his symptoms transiently improved with a hot shower in the hospital. He was diagnosed with cannabinoid hyperemesis syndrome (CHS) and admitted for further management.
Background
In the United States, 9 states and the District of Columbia have legalized recreational marijuana use, and 29 states and DC have legalized medical marijuana. Marijuana use is likely to rise, and with it may arise an increasing incidence of CHS.
The exact prevalence of CHS is not known. Diagnosis is often delayed as there is no reliable diagnostic test. A high index of suspicion is needed for prompt diagnosis.
CHS was first described in 2004 in South Australia and since then many case reports have been published. Marijuana has both proemetic and antiemetic effects. Unlike its antiemetic effect, the pathophysiology of the proemetic effect of marijuana is not well understood.
Key clinical features
CHS typically has three phases. Initially patients present with prodromal symptoms of abdominal discomfort and nausea. There is no emesis at this early phase. Patients are still able to tolerate a liquid diet in this prodromal phase.
This is followed by a more active phase of intractable vomiting, which is relieved by hot showers or baths. Most patients take compulsively long hot showers or baths many times a day. Also, they develop diaphoresis, restlessness, agitation, and weight loss.
The active phase is followed by a recovery phase when symptoms resolve and patients return to baseline, only to have it recur if marijuana use continues.
Diagnostic approach and management
CHS should be suspected in patients coming in with recurrent symptoms of abdominal pain, nausea and vomiting, and who have normal CBC, basic metabolic panel, lipase, and liver function tests. Patients should be directly questioned about marijuana use and whether symptoms are relieved with hot showers. A toxicology screen should be done. For patients with marijuana use and compulsive hot showers, further work up of their symptoms (e.g., upper endoscopy, abdominal ultrasound, and/or nuclear medicine emptying study) should be avoided. Figure 1 shows the suggested work-up.

The differential diagnosis for recurrent abdominal pain, nausea, and vomiting is chronic pancreatitis, gastroparesis, severe gastritis, medication adverse effects (especially GLP1 receptor agonists), cyclic vomiting syndrome, psychogenic vomiting, and (with the rise of narcotic abuse) narcotic bowel syndrome.
Our patient had a history of diabetes with an HbA1c at goal and a normal nuclear medicine gastric-emptying study (6% retention at 4 hours). He was also on liraglutide, but his symptoms predated this medicine use.
The mainstay of treatment for CHS is supportive therapy with intravenous fluids and antiemetics like 5-HT3-receptor antagonists (ondansetron); D2-receptor antagonists (metoclopramide); and H1-receptor antagonists (diphenhydramine). The effectiveness of these agents is limited, which is also a clue for the diagnosis of CHS. If traditional agents fail in controlling the symptoms, haloperidol can be tried, but it has been used with limited success. Our patient did not respond to traditional antiemetics, but responded well to a small dose of lorazepam. Even though a benzodiazepine is not the mainstay of treatment, it may be tried if other agents fail. Acid-suppression therapy with a proton pump inhibitor should be used as esophagogastroduodenoscopy (EGD) usually reveals mild gastritis and esophagitis, as in our patient. Narcotic use should be avoided for management of abdominal pain.
Patients should be counseled against marijuana use. This may be difficult if marijuana is being used as an appetite stimulant or for treatment of chemotherapy-induced nausea and vomiting. If willing, patients should be referred to a substance abuse rehabilitation center.
Back to the case
In this case, after a diagnosis of CHS was made, the patient was counseled against marijuana use. His abdominal pain and intractable vomiting did not improve with conservative management of n.p.o status, prochlorperazine, metoclopramide, and ondansetron. He was given a trial of low-dose lorazepam with significant improvement in his symptoms. He was counseled extensively against marijuana use and discharged. A follow-up phone call at 3 months showed continued abstinence and no recurrence of symptoms.
Dr. Gupta is a hospitalist at Yale New Haven Health and Bridgeport (Conn.) Hospital.
References
1. Bajgoric S et al. Cannabinoid hyperemesis syndrome: A guide for the practising clinician. BMJ Case Rep. 2015. doi: 10.1136/bcr-2015-210246.
2. Batke M et al. The cannabis hyperemesis syndrome characterized by persistent nausea and vomiting, abdominal pain, and compulsive bathing associated with chronic marijuana use: A report of eight cases in the united states. Dig Dis Sci. 2010 Nov;55(11):3113-9.
3. Iacopetti CL et al. Cannabinoid hyperemesis syndrome: a case report and review of pathophysiology. Clin Med Res. 2014 Sep;12(1-2):65-7.
4. Hickey JL et al. Haloperidol for treatment of cannabinoid hyperemesis syndrome. Am J Emerg Med. 2013 Jun. 31(6):1003.e5-6. Epub 2013 Apr 10.
Key points
Suspect CHS for patients with recurrent abdominal pain, nausea, and vomiting with negative initial work-up.
- Ask directly about marijuana use.
- Ask whether symptoms are relieved with hot shower/ bath.
- Send a toxicology screen.
- Make a diagnosis of CHS if:
1. Positive marijuana use.
2. Symptom improvement with hot baths or
3. Toxicology positive for marijuana.
- Manage conservatively with hydration and antiemetics.
- Suspect CHS if traditional antiemetics are not providing relief .
- If traditional antiemetics fail, trial of haloperidol or low-dose benzodiazepines.
- Avoid narcotics.
- Avoid unnecessary investigations.
- Counsel patients against marijuana use and refer to substance abuse center if patient agrees.
Case
WS is a 54-year-old African American male with a medical history of diabetes mellitus type 2, hypertension, obstructive sleep apnea, and gastroparesis. He has multiple admissions for intractable nausea, vomiting, and abdominal pain believed to be from diabetic gastroparesis despite a normal gastric-emptying study. Endoscopy done in prior admission showed duodenitis, gastritis, and esophagitis, and colonoscopy revealed diverticulosis. He had a negative gastric-emptying study of 6% retention at 4 hrs. His last hemoglobin A1c was 5 and his glucose has been well controlled. He is hospitalized again for intractable abdominal pain, nausea, and vomiting. His examination was unremarkable except for dry mucosa and epigastric tenderness. His labs were also insignificant except for prerenal azotemia. Upon further questioning he admitted to significant marijuana use, and his symptoms transiently improved with a hot shower in the hospital. He was diagnosed with cannabinoid hyperemesis syndrome (CHS) and admitted for further management.
Background
In the United States, 9 states and the District of Columbia have legalized recreational marijuana use, and 29 states and DC have legalized medical marijuana. Marijuana use is likely to rise, and with it may arise an increasing incidence of CHS.
The exact prevalence of CHS is not known. Diagnosis is often delayed as there is no reliable diagnostic test. A high index of suspicion is needed for prompt diagnosis.
CHS was first described in 2004 in South Australia and since then many case reports have been published. Marijuana has both proemetic and antiemetic effects. Unlike its antiemetic effect, the pathophysiology of the proemetic effect of marijuana is not well understood.
Key clinical features
CHS typically has three phases. Initially patients present with prodromal symptoms of abdominal discomfort and nausea. There is no emesis at this early phase. Patients are still able to tolerate a liquid diet in this prodromal phase.
This is followed by a more active phase of intractable vomiting, which is relieved by hot showers or baths. Most patients take compulsively long hot showers or baths many times a day. Also, they develop diaphoresis, restlessness, agitation, and weight loss.
The active phase is followed by a recovery phase when symptoms resolve and patients return to baseline, only to have it recur if marijuana use continues.
Diagnostic approach and management
CHS should be suspected in patients coming in with recurrent symptoms of abdominal pain, nausea and vomiting, and who have normal CBC, basic metabolic panel, lipase, and liver function tests. Patients should be directly questioned about marijuana use and whether symptoms are relieved with hot showers. A toxicology screen should be done. For patients with marijuana use and compulsive hot showers, further work up of their symptoms (e.g., upper endoscopy, abdominal ultrasound, and/or nuclear medicine emptying study) should be avoided. Figure 1 shows the suggested work-up.

The differential diagnosis for recurrent abdominal pain, nausea, and vomiting is chronic pancreatitis, gastroparesis, severe gastritis, medication adverse effects (especially GLP1 receptor agonists), cyclic vomiting syndrome, psychogenic vomiting, and (with the rise of narcotic abuse) narcotic bowel syndrome.
Our patient had a history of diabetes with an HbA1c at goal and a normal nuclear medicine gastric-emptying study (6% retention at 4 hours). He was also on liraglutide, but his symptoms predated this medicine use.
The mainstay of treatment for CHS is supportive therapy with intravenous fluids and antiemetics like 5-HT3-receptor antagonists (ondansetron); D2-receptor antagonists (metoclopramide); and H1-receptor antagonists (diphenhydramine). The effectiveness of these agents is limited, which is also a clue for the diagnosis of CHS. If traditional agents fail in controlling the symptoms, haloperidol can be tried, but it has been used with limited success. Our patient did not respond to traditional antiemetics, but responded well to a small dose of lorazepam. Even though a benzodiazepine is not the mainstay of treatment, it may be tried if other agents fail. Acid-suppression therapy with a proton pump inhibitor should be used as esophagogastroduodenoscopy (EGD) usually reveals mild gastritis and esophagitis, as in our patient. Narcotic use should be avoided for management of abdominal pain.
Patients should be counseled against marijuana use. This may be difficult if marijuana is being used as an appetite stimulant or for treatment of chemotherapy-induced nausea and vomiting. If willing, patients should be referred to a substance abuse rehabilitation center.
Back to the case
In this case, after a diagnosis of CHS was made, the patient was counseled against marijuana use. His abdominal pain and intractable vomiting did not improve with conservative management of n.p.o status, prochlorperazine, metoclopramide, and ondansetron. He was given a trial of low-dose lorazepam with significant improvement in his symptoms. He was counseled extensively against marijuana use and discharged. A follow-up phone call at 3 months showed continued abstinence and no recurrence of symptoms.
Dr. Gupta is a hospitalist at Yale New Haven Health and Bridgeport (Conn.) Hospital.
References
1. Bajgoric S et al. Cannabinoid hyperemesis syndrome: A guide for the practising clinician. BMJ Case Rep. 2015. doi: 10.1136/bcr-2015-210246.
2. Batke M et al. The cannabis hyperemesis syndrome characterized by persistent nausea and vomiting, abdominal pain, and compulsive bathing associated with chronic marijuana use: A report of eight cases in the united states. Dig Dis Sci. 2010 Nov;55(11):3113-9.
3. Iacopetti CL et al. Cannabinoid hyperemesis syndrome: a case report and review of pathophysiology. Clin Med Res. 2014 Sep;12(1-2):65-7.
4. Hickey JL et al. Haloperidol for treatment of cannabinoid hyperemesis syndrome. Am J Emerg Med. 2013 Jun. 31(6):1003.e5-6. Epub 2013 Apr 10.
Key points
Suspect CHS for patients with recurrent abdominal pain, nausea, and vomiting with negative initial work-up.
- Ask directly about marijuana use.
- Ask whether symptoms are relieved with hot shower/ bath.
- Send a toxicology screen.
- Make a diagnosis of CHS if:
1. Positive marijuana use.
2. Symptom improvement with hot baths or
3. Toxicology positive for marijuana.
- Manage conservatively with hydration and antiemetics.
- Suspect CHS if traditional antiemetics are not providing relief .
- If traditional antiemetics fail, trial of haloperidol or low-dose benzodiazepines.
- Avoid narcotics.
- Avoid unnecessary investigations.
- Counsel patients against marijuana use and refer to substance abuse center if patient agrees.
FDA Approves Galcanezumab for Migraine Prevention
The treatment is the third anti-CGRP antibody to receive regulatory approval.
ROCKVILLE, MD—The FDA has approved galcanezumab-gnlm (Emgality) for the preventive treatment of migraine in adults. Eli Lilly and Company manufactures the therapy. Emgality is the third calcitonin gene-related peptide (CGRP) antagonist to receive regulatory approval.
The approval is based on the results of three phase III clinical trials: EVOLVE-1, EVOLVE-2, and REGAIN. EVOLVE-1 and EVOLVE-2 were six-month, double-blind, placebo-controlled studies that included adults with episodic migraine. REGAIN was a three-month, double-blind, placebo-controlled study of adults with chronic migraine. The primary end point of all three trials was mean change from baseline in the number of monthly headache days.
In all trials, patients received either placebo, 120 mg of galcanezumab-gnlm after an initial loading dose of 240 mg, or 240 mg of galcanezumab-gnlm. In EVOLVE-1 and EVOLVE-2, people who received galcanezumab-gnlm had significantly fewer headache days per month than people who received placebo, and those who received galcanezumab-gnlm were also more likely to achieve a 50%, 75%, and 100% reduction in headache days.
In REGAIN, patients who received galcanezumab-gnlm experienced fewer monthly headache days than those who received placebo and were more likely to achieve a 50% reduction in headache days. There was no difference between groups in the likelihood of achieving a 75% or 100% reduction.
The recommended dosage, according to the label, is a monthly, 120-mg subcutaneous injection with an initial loading dose of 240 mg. The most common adverse event associated with galcanezumab-gnlm is injection-site reaction.
Galcanezumab-gnlm is under final review by the European Commission for approval in Europe.
—Lucas Franki
The treatment is the third anti-CGRP antibody to receive regulatory approval.
The treatment is the third anti-CGRP antibody to receive regulatory approval.
ROCKVILLE, MD—The FDA has approved galcanezumab-gnlm (Emgality) for the preventive treatment of migraine in adults. Eli Lilly and Company manufactures the therapy. Emgality is the third calcitonin gene-related peptide (CGRP) antagonist to receive regulatory approval.
The approval is based on the results of three phase III clinical trials: EVOLVE-1, EVOLVE-2, and REGAIN. EVOLVE-1 and EVOLVE-2 were six-month, double-blind, placebo-controlled studies that included adults with episodic migraine. REGAIN was a three-month, double-blind, placebo-controlled study of adults with chronic migraine. The primary end point of all three trials was mean change from baseline in the number of monthly headache days.
In all trials, patients received either placebo, 120 mg of galcanezumab-gnlm after an initial loading dose of 240 mg, or 240 mg of galcanezumab-gnlm. In EVOLVE-1 and EVOLVE-2, people who received galcanezumab-gnlm had significantly fewer headache days per month than people who received placebo, and those who received galcanezumab-gnlm were also more likely to achieve a 50%, 75%, and 100% reduction in headache days.
In REGAIN, patients who received galcanezumab-gnlm experienced fewer monthly headache days than those who received placebo and were more likely to achieve a 50% reduction in headache days. There was no difference between groups in the likelihood of achieving a 75% or 100% reduction.
The recommended dosage, according to the label, is a monthly, 120-mg subcutaneous injection with an initial loading dose of 240 mg. The most common adverse event associated with galcanezumab-gnlm is injection-site reaction.
Galcanezumab-gnlm is under final review by the European Commission for approval in Europe.
—Lucas Franki
ROCKVILLE, MD—The FDA has approved galcanezumab-gnlm (Emgality) for the preventive treatment of migraine in adults. Eli Lilly and Company manufactures the therapy. Emgality is the third calcitonin gene-related peptide (CGRP) antagonist to receive regulatory approval.
The approval is based on the results of three phase III clinical trials: EVOLVE-1, EVOLVE-2, and REGAIN. EVOLVE-1 and EVOLVE-2 were six-month, double-blind, placebo-controlled studies that included adults with episodic migraine. REGAIN was a three-month, double-blind, placebo-controlled study of adults with chronic migraine. The primary end point of all three trials was mean change from baseline in the number of monthly headache days.
In all trials, patients received either placebo, 120 mg of galcanezumab-gnlm after an initial loading dose of 240 mg, or 240 mg of galcanezumab-gnlm. In EVOLVE-1 and EVOLVE-2, people who received galcanezumab-gnlm had significantly fewer headache days per month than people who received placebo, and those who received galcanezumab-gnlm were also more likely to achieve a 50%, 75%, and 100% reduction in headache days.
In REGAIN, patients who received galcanezumab-gnlm experienced fewer monthly headache days than those who received placebo and were more likely to achieve a 50% reduction in headache days. There was no difference between groups in the likelihood of achieving a 75% or 100% reduction.
The recommended dosage, according to the label, is a monthly, 120-mg subcutaneous injection with an initial loading dose of 240 mg. The most common adverse event associated with galcanezumab-gnlm is injection-site reaction.
Galcanezumab-gnlm is under final review by the European Commission for approval in Europe.
—Lucas Franki
New MS Subtype Shows Absence of Cerebral White Matter Demyelination
A new subtype of multiple sclerosis (MS) called myelocortical MS is characterized by demyelination only in the spinal cord and cerebral cortex and not in the cerebral white matter, according to a study published online ahead of print August 21 in Lancet Neurology. The findings are based on an examination of the brains and spinal cords of 100 patients who died of MS.
Bruce D. Trapp, PhD, the Morris R. and Ruth V. Graham Endowed Chair in Biomedical Research at the Lerner Research Institute at the Cleveland Clinic in Ohio, and his coauthors said that while the demyelination of cerebral white matter is a pathologic hallmark of MS, previous research has found that only around half of cerebral T2-weighted hyperintense white matter lesions are demyelinated, and these lesions account for less than a third of variance in the rate of brain atrophy.
“In the absence of specific MRI metrics for demyelination, the relationship between cerebral white-matter demyelination and neurodegeneration remains speculative,” they said.
In this study, researchers scanned the brains with MRI before autopsy, then took centimeter-thick hemispheric slices to study the white-matter lesions. They identified 12 individuals as having what they describe as myelocortical MS, characterized by the absence of areas of cerebral white-matter discoloration indicative of demyelinated lesions.
The authors then compared these individuals with 12 individuals with typical MS matched by age, sex, MRI protocol, MS disease subtype, disease duration, and Expanded Disability Status Scale score.
Not Typical MS
They found that while individuals with myelocortical MS did not have demyelinated lesions in the cerebral white matter, they had areas of demyelinated lesions in the cerebral cortex similar to those of individuals with typical MS (median 4.45% vs 9.74%, respectively). However, the individuals with myelocortical MS had a significantly smaller area of spinal cord demyelination (median 3.81% vs 13.81%).
Individuals with myelocortical MS also had significantly lower mean cortical neuronal densities, compared with healthy control brains, in layer III, layer V, and layer VI. But individuals with typical MS only had a lower cortical neuronal density in layer V when compared with controls.
Dr. Trapp and colleagues also saw that in typical MS, neuronal density decreased as the area of brain white-matter demyelination increased. However, this negative linear correlation was not seen in myelocortical MS.
On MRI, researchers were still able to see abnormalities in the cerebral white matter in individuals with myelocortical MS, in T2-weighted, T1-weighted, and magnetization transfer ratios (MTR) images. They also found similar total T2-weighted and T1-weighted lesion volumes in individuals with myelocortical MS and those with typical MS, although individuals with typical MS had significantly greater MTR lesion volumes.
The Hallmarks of Myelocortical MS
“We propose that myelocortical MS is characterized by spinal cord demyelination, subpial cortical demyelination, and an absence of cerebral white-matter demyelination,” Dr. Trapp and colleagues wrote. “Our findings indicate that abnormal cerebral white-matter T2-T1-MTR regions of interest are not always demyelinated, and this pathologic evidence suggests that cerebral white-matter demyelination and cortical neuronal degeneration can be independent events in myelocortical MS.”
The authors acknowledged that one limitation of their study may have been selection bias, as all the patients in the study died from complications of advanced MS. They suggested that it was therefore not appropriate to conclude that the prevalence of myelocortical MS seen in their sample would be similar across the entire MS population, nor were the findings likely to apply to pateints with earlier stage disease.
The study received funding from the NIH and the National MS Society. One author is an employee of Renovo Neural, and three authors are employees of Biogen. One author declared a pending patent related to automated lesion segmentation from MRI images, and four authors declared funding, fees, and nonfinancial support from pharmaceutical companies.
—Bianca Nogrady
Suggested Reading
Trapp BD, Vignos M, Dudman J, et al. Cortical neuronal densities and cerebral white matter demyelination in multiple sclerosis: a retrospective study. Lancet Neurol. 2018 Aug 21 [Epub ahead of print].
A new subtype of multiple sclerosis (MS) called myelocortical MS is characterized by demyelination only in the spinal cord and cerebral cortex and not in the cerebral white matter, according to a study published online ahead of print August 21 in Lancet Neurology. The findings are based on an examination of the brains and spinal cords of 100 patients who died of MS.
Bruce D. Trapp, PhD, the Morris R. and Ruth V. Graham Endowed Chair in Biomedical Research at the Lerner Research Institute at the Cleveland Clinic in Ohio, and his coauthors said that while the demyelination of cerebral white matter is a pathologic hallmark of MS, previous research has found that only around half of cerebral T2-weighted hyperintense white matter lesions are demyelinated, and these lesions account for less than a third of variance in the rate of brain atrophy.
“In the absence of specific MRI metrics for demyelination, the relationship between cerebral white-matter demyelination and neurodegeneration remains speculative,” they said.
In this study, researchers scanned the brains with MRI before autopsy, then took centimeter-thick hemispheric slices to study the white-matter lesions. They identified 12 individuals as having what they describe as myelocortical MS, characterized by the absence of areas of cerebral white-matter discoloration indicative of demyelinated lesions.
The authors then compared these individuals with 12 individuals with typical MS matched by age, sex, MRI protocol, MS disease subtype, disease duration, and Expanded Disability Status Scale score.
Not Typical MS
They found that while individuals with myelocortical MS did not have demyelinated lesions in the cerebral white matter, they had areas of demyelinated lesions in the cerebral cortex similar to those of individuals with typical MS (median 4.45% vs 9.74%, respectively). However, the individuals with myelocortical MS had a significantly smaller area of spinal cord demyelination (median 3.81% vs 13.81%).
Individuals with myelocortical MS also had significantly lower mean cortical neuronal densities, compared with healthy control brains, in layer III, layer V, and layer VI. But individuals with typical MS only had a lower cortical neuronal density in layer V when compared with controls.
Dr. Trapp and colleagues also saw that in typical MS, neuronal density decreased as the area of brain white-matter demyelination increased. However, this negative linear correlation was not seen in myelocortical MS.
On MRI, researchers were still able to see abnormalities in the cerebral white matter in individuals with myelocortical MS, in T2-weighted, T1-weighted, and magnetization transfer ratios (MTR) images. They also found similar total T2-weighted and T1-weighted lesion volumes in individuals with myelocortical MS and those with typical MS, although individuals with typical MS had significantly greater MTR lesion volumes.
The Hallmarks of Myelocortical MS
“We propose that myelocortical MS is characterized by spinal cord demyelination, subpial cortical demyelination, and an absence of cerebral white-matter demyelination,” Dr. Trapp and colleagues wrote. “Our findings indicate that abnormal cerebral white-matter T2-T1-MTR regions of interest are not always demyelinated, and this pathologic evidence suggests that cerebral white-matter demyelination and cortical neuronal degeneration can be independent events in myelocortical MS.”
The authors acknowledged that one limitation of their study may have been selection bias, as all the patients in the study died from complications of advanced MS. They suggested that it was therefore not appropriate to conclude that the prevalence of myelocortical MS seen in their sample would be similar across the entire MS population, nor were the findings likely to apply to pateints with earlier stage disease.
The study received funding from the NIH and the National MS Society. One author is an employee of Renovo Neural, and three authors are employees of Biogen. One author declared a pending patent related to automated lesion segmentation from MRI images, and four authors declared funding, fees, and nonfinancial support from pharmaceutical companies.
—Bianca Nogrady
Suggested Reading
Trapp BD, Vignos M, Dudman J, et al. Cortical neuronal densities and cerebral white matter demyelination in multiple sclerosis: a retrospective study. Lancet Neurol. 2018 Aug 21 [Epub ahead of print].
A new subtype of multiple sclerosis (MS) called myelocortical MS is characterized by demyelination only in the spinal cord and cerebral cortex and not in the cerebral white matter, according to a study published online ahead of print August 21 in Lancet Neurology. The findings are based on an examination of the brains and spinal cords of 100 patients who died of MS.
Bruce D. Trapp, PhD, the Morris R. and Ruth V. Graham Endowed Chair in Biomedical Research at the Lerner Research Institute at the Cleveland Clinic in Ohio, and his coauthors said that while the demyelination of cerebral white matter is a pathologic hallmark of MS, previous research has found that only around half of cerebral T2-weighted hyperintense white matter lesions are demyelinated, and these lesions account for less than a third of variance in the rate of brain atrophy.
“In the absence of specific MRI metrics for demyelination, the relationship between cerebral white-matter demyelination and neurodegeneration remains speculative,” they said.
In this study, researchers scanned the brains with MRI before autopsy, then took centimeter-thick hemispheric slices to study the white-matter lesions. They identified 12 individuals as having what they describe as myelocortical MS, characterized by the absence of areas of cerebral white-matter discoloration indicative of demyelinated lesions.
The authors then compared these individuals with 12 individuals with typical MS matched by age, sex, MRI protocol, MS disease subtype, disease duration, and Expanded Disability Status Scale score.
Not Typical MS
They found that while individuals with myelocortical MS did not have demyelinated lesions in the cerebral white matter, they had areas of demyelinated lesions in the cerebral cortex similar to those of individuals with typical MS (median 4.45% vs 9.74%, respectively). However, the individuals with myelocortical MS had a significantly smaller area of spinal cord demyelination (median 3.81% vs 13.81%).
Individuals with myelocortical MS also had significantly lower mean cortical neuronal densities, compared with healthy control brains, in layer III, layer V, and layer VI. But individuals with typical MS only had a lower cortical neuronal density in layer V when compared with controls.
Dr. Trapp and colleagues also saw that in typical MS, neuronal density decreased as the area of brain white-matter demyelination increased. However, this negative linear correlation was not seen in myelocortical MS.
On MRI, researchers were still able to see abnormalities in the cerebral white matter in individuals with myelocortical MS, in T2-weighted, T1-weighted, and magnetization transfer ratios (MTR) images. They also found similar total T2-weighted and T1-weighted lesion volumes in individuals with myelocortical MS and those with typical MS, although individuals with typical MS had significantly greater MTR lesion volumes.
The Hallmarks of Myelocortical MS
“We propose that myelocortical MS is characterized by spinal cord demyelination, subpial cortical demyelination, and an absence of cerebral white-matter demyelination,” Dr. Trapp and colleagues wrote. “Our findings indicate that abnormal cerebral white-matter T2-T1-MTR regions of interest are not always demyelinated, and this pathologic evidence suggests that cerebral white-matter demyelination and cortical neuronal degeneration can be independent events in myelocortical MS.”
The authors acknowledged that one limitation of their study may have been selection bias, as all the patients in the study died from complications of advanced MS. They suggested that it was therefore not appropriate to conclude that the prevalence of myelocortical MS seen in their sample would be similar across the entire MS population, nor were the findings likely to apply to pateints with earlier stage disease.
The study received funding from the NIH and the National MS Society. One author is an employee of Renovo Neural, and three authors are employees of Biogen. One author declared a pending patent related to automated lesion segmentation from MRI images, and four authors declared funding, fees, and nonfinancial support from pharmaceutical companies.
—Bianca Nogrady
Suggested Reading
Trapp BD, Vignos M, Dudman J, et al. Cortical neuronal densities and cerebral white matter demyelination in multiple sclerosis: a retrospective study. Lancet Neurol. 2018 Aug 21 [Epub ahead of print].
U.S. obesity continues to advance
The prevalence of adult obesity was at or above 35% for seven states in 2017, which is up from five states in 2016 and no states in 2012, according to the Centers for Disease Control and Prevention.
Iowa and Oklahoma, the two newest states with prevalences at or exceeding 35%, joined Alabama, Arkansas, Louisiana, Mississippi, and West Virginia, which has the country’s highest rate of adult obesity at 38.1%. Colorado’s 22.6% rate is the lowest prevalence among all states. The District of Columbia and Hawaii also have prevalences under 25%; previously, Massachusetts also was in this group, but its prevalence went up to 25.9% last year, the CDC reported.
Regional disparities in self-reported adult obesity put the South (32.4%) and the Midwest (32.3%) well ahead of the Northeast (27.7%) and the West (26.1%) in 2017. Racial and ethnic disparities also were seen, with large gaps between blacks, who had a prevalence of 39%, and Hispanics (32.4%) and whites (29.3%). Obesity prevalence was 35% or higher among black adults in 31 states and D.C., while this was true among Hispanics in eight states and among whites in one (West Virginia), although the prevalence was at or above 35% for multiple racial groups in some of these states, the CDC reported based on data from the Behavioral Risk Factor Surveillance System.
“Obesity costs the United States health care system over $147 billion a year [and] research has shown that obesity affects work productivity and military readiness,” the CDC said in a written statement. “To protect the health of the next generation, support for healthy behaviors such as healthy eating, better sleep, stress management, and physical activity should start early and expand to reach Americans across the lifespan in the communities where they live, learn, work, and play.”
The AGA Obesity Practice Guide provides physicians with a comprehensive, multi-disciplinary process to guide and personalize innovative obesity care for safe and effective weight management. Learn more at http://ow.ly/p1Fh30lOXYD
The prevalence of adult obesity was at or above 35% for seven states in 2017, which is up from five states in 2016 and no states in 2012, according to the Centers for Disease Control and Prevention.
Iowa and Oklahoma, the two newest states with prevalences at or exceeding 35%, joined Alabama, Arkansas, Louisiana, Mississippi, and West Virginia, which has the country’s highest rate of adult obesity at 38.1%. Colorado’s 22.6% rate is the lowest prevalence among all states. The District of Columbia and Hawaii also have prevalences under 25%; previously, Massachusetts also was in this group, but its prevalence went up to 25.9% last year, the CDC reported.
Regional disparities in self-reported adult obesity put the South (32.4%) and the Midwest (32.3%) well ahead of the Northeast (27.7%) and the West (26.1%) in 2017. Racial and ethnic disparities also were seen, with large gaps between blacks, who had a prevalence of 39%, and Hispanics (32.4%) and whites (29.3%). Obesity prevalence was 35% or higher among black adults in 31 states and D.C., while this was true among Hispanics in eight states and among whites in one (West Virginia), although the prevalence was at or above 35% for multiple racial groups in some of these states, the CDC reported based on data from the Behavioral Risk Factor Surveillance System.
“Obesity costs the United States health care system over $147 billion a year [and] research has shown that obesity affects work productivity and military readiness,” the CDC said in a written statement. “To protect the health of the next generation, support for healthy behaviors such as healthy eating, better sleep, stress management, and physical activity should start early and expand to reach Americans across the lifespan in the communities where they live, learn, work, and play.”
The AGA Obesity Practice Guide provides physicians with a comprehensive, multi-disciplinary process to guide and personalize innovative obesity care for safe and effective weight management. Learn more at http://ow.ly/p1Fh30lOXYD
The prevalence of adult obesity was at or above 35% for seven states in 2017, which is up from five states in 2016 and no states in 2012, according to the Centers for Disease Control and Prevention.
Iowa and Oklahoma, the two newest states with prevalences at or exceeding 35%, joined Alabama, Arkansas, Louisiana, Mississippi, and West Virginia, which has the country’s highest rate of adult obesity at 38.1%. Colorado’s 22.6% rate is the lowest prevalence among all states. The District of Columbia and Hawaii also have prevalences under 25%; previously, Massachusetts also was in this group, but its prevalence went up to 25.9% last year, the CDC reported.
Regional disparities in self-reported adult obesity put the South (32.4%) and the Midwest (32.3%) well ahead of the Northeast (27.7%) and the West (26.1%) in 2017. Racial and ethnic disparities also were seen, with large gaps between blacks, who had a prevalence of 39%, and Hispanics (32.4%) and whites (29.3%). Obesity prevalence was 35% or higher among black adults in 31 states and D.C., while this was true among Hispanics in eight states and among whites in one (West Virginia), although the prevalence was at or above 35% for multiple racial groups in some of these states, the CDC reported based on data from the Behavioral Risk Factor Surveillance System.
“Obesity costs the United States health care system over $147 billion a year [and] research has shown that obesity affects work productivity and military readiness,” the CDC said in a written statement. “To protect the health of the next generation, support for healthy behaviors such as healthy eating, better sleep, stress management, and physical activity should start early and expand to reach Americans across the lifespan in the communities where they live, learn, work, and play.”
The AGA Obesity Practice Guide provides physicians with a comprehensive, multi-disciplinary process to guide and personalize innovative obesity care for safe and effective weight management. Learn more at http://ow.ly/p1Fh30lOXYD
Autologous fecal transplant restores microbiota after allo-HSCT
For patients who undergo allogeneic hematopoietic stem cell transplant (allo-HSCT) with intensive antibiotics, a subsequent autologous fecal transplant (auto-FMT) can restore intestinal microbiota, a recent study found.
Loss of normal gut bacteria after allo-HSCT and antibiotics is a common occurrence and known risk factor for graft-versus-host disease (GVHD) and intestinal infection.
“Overall, patients who lose gut microbiota diversity at the time of hematopoietic stem cell engraftment have higher rates of transplant-related death,” reported Ying Taur, MD, of the Memorial Sloan Kettering Cancer Center in New York, and his colleagues. “We explored whether the microbiota could be restored in allo-HSCT patients through the use of auto-FMT.”
Allo-HSCT patients are immune suppressed for months after engraftment, so safety concerns led the investigators to use auto-FMT rather than a fecal transplant from another individual. The complex population of viruses, fungi, archaea, bacteria, and protozoa that inhabit the human gut remains poorly understood, as does the infectious potential of a heterologous fecal donor.
“Despite remarkable advances in recent years, current technologies are incapable of comprehensively determining fecal composition,” the authors wrote in Science Translational Medicine.
The study involved 25 patients undergoing allo-HSCT with intensive antibiotic therapy. Prior to engraftment, fecal samples were collected from all patients and analyzed for composition and diversity, measured by inverse Simpson index.
Samples were then frozen and stored. Fecal analysis also was performed after engraftment, and again after the auto-FMT time point. Auto-FMT was performed in 14 patients; 11 patients served as controls and did not receive treatment. Patients were followed for 1 year.
The investigators found that all of the patients who underwent auto-FMT recovered their pre–allo-HSCT microbiota composition and diversity, compared with none of the controls (P less than .0001). Further analysis showed that auto-FMT increased diversity (inverse Simpson index) by 64%, compared with 38% in controls.
“We have demonstrated the potential of auto-FMT as a clinical intervention to restore intestinal microbiota diversity to levels deemed safe in patients, thereby reversing the disruptive effects of broad-spectrum antibiotic treatment for patients undergoing allo-HSCT transplant,” the investigators concluded.
Study funding was provided by the Leonard Tow Foundation and the Memorial Sloan Kettering’s Center for Microbes, Inflammation, and Cancer. The authors reported financial relationships with Merck, AbbVie, Nektar Therapeutics, Novartis, and others.
SOURCE: Taur Y et al. Sci Transl Med. 2018 Sep 26. doi: 10.1126/scitranslmed.aap9489.
For patients who undergo allogeneic hematopoietic stem cell transplant (allo-HSCT) with intensive antibiotics, a subsequent autologous fecal transplant (auto-FMT) can restore intestinal microbiota, a recent study found.
Loss of normal gut bacteria after allo-HSCT and antibiotics is a common occurrence and known risk factor for graft-versus-host disease (GVHD) and intestinal infection.
“Overall, patients who lose gut microbiota diversity at the time of hematopoietic stem cell engraftment have higher rates of transplant-related death,” reported Ying Taur, MD, of the Memorial Sloan Kettering Cancer Center in New York, and his colleagues. “We explored whether the microbiota could be restored in allo-HSCT patients through the use of auto-FMT.”
Allo-HSCT patients are immune suppressed for months after engraftment, so safety concerns led the investigators to use auto-FMT rather than a fecal transplant from another individual. The complex population of viruses, fungi, archaea, bacteria, and protozoa that inhabit the human gut remains poorly understood, as does the infectious potential of a heterologous fecal donor.
“Despite remarkable advances in recent years, current technologies are incapable of comprehensively determining fecal composition,” the authors wrote in Science Translational Medicine.
The study involved 25 patients undergoing allo-HSCT with intensive antibiotic therapy. Prior to engraftment, fecal samples were collected from all patients and analyzed for composition and diversity, measured by inverse Simpson index.
Samples were then frozen and stored. Fecal analysis also was performed after engraftment, and again after the auto-FMT time point. Auto-FMT was performed in 14 patients; 11 patients served as controls and did not receive treatment. Patients were followed for 1 year.
The investigators found that all of the patients who underwent auto-FMT recovered their pre–allo-HSCT microbiota composition and diversity, compared with none of the controls (P less than .0001). Further analysis showed that auto-FMT increased diversity (inverse Simpson index) by 64%, compared with 38% in controls.
“We have demonstrated the potential of auto-FMT as a clinical intervention to restore intestinal microbiota diversity to levels deemed safe in patients, thereby reversing the disruptive effects of broad-spectrum antibiotic treatment for patients undergoing allo-HSCT transplant,” the investigators concluded.
Study funding was provided by the Leonard Tow Foundation and the Memorial Sloan Kettering’s Center for Microbes, Inflammation, and Cancer. The authors reported financial relationships with Merck, AbbVie, Nektar Therapeutics, Novartis, and others.
SOURCE: Taur Y et al. Sci Transl Med. 2018 Sep 26. doi: 10.1126/scitranslmed.aap9489.
For patients who undergo allogeneic hematopoietic stem cell transplant (allo-HSCT) with intensive antibiotics, a subsequent autologous fecal transplant (auto-FMT) can restore intestinal microbiota, a recent study found.
Loss of normal gut bacteria after allo-HSCT and antibiotics is a common occurrence and known risk factor for graft-versus-host disease (GVHD) and intestinal infection.
“Overall, patients who lose gut microbiota diversity at the time of hematopoietic stem cell engraftment have higher rates of transplant-related death,” reported Ying Taur, MD, of the Memorial Sloan Kettering Cancer Center in New York, and his colleagues. “We explored whether the microbiota could be restored in allo-HSCT patients through the use of auto-FMT.”
Allo-HSCT patients are immune suppressed for months after engraftment, so safety concerns led the investigators to use auto-FMT rather than a fecal transplant from another individual. The complex population of viruses, fungi, archaea, bacteria, and protozoa that inhabit the human gut remains poorly understood, as does the infectious potential of a heterologous fecal donor.
“Despite remarkable advances in recent years, current technologies are incapable of comprehensively determining fecal composition,” the authors wrote in Science Translational Medicine.
The study involved 25 patients undergoing allo-HSCT with intensive antibiotic therapy. Prior to engraftment, fecal samples were collected from all patients and analyzed for composition and diversity, measured by inverse Simpson index.
Samples were then frozen and stored. Fecal analysis also was performed after engraftment, and again after the auto-FMT time point. Auto-FMT was performed in 14 patients; 11 patients served as controls and did not receive treatment. Patients were followed for 1 year.
The investigators found that all of the patients who underwent auto-FMT recovered their pre–allo-HSCT microbiota composition and diversity, compared with none of the controls (P less than .0001). Further analysis showed that auto-FMT increased diversity (inverse Simpson index) by 64%, compared with 38% in controls.
“We have demonstrated the potential of auto-FMT as a clinical intervention to restore intestinal microbiota diversity to levels deemed safe in patients, thereby reversing the disruptive effects of broad-spectrum antibiotic treatment for patients undergoing allo-HSCT transplant,” the investigators concluded.
Study funding was provided by the Leonard Tow Foundation and the Memorial Sloan Kettering’s Center for Microbes, Inflammation, and Cancer. The authors reported financial relationships with Merck, AbbVie, Nektar Therapeutics, Novartis, and others.
SOURCE: Taur Y et al. Sci Transl Med. 2018 Sep 26. doi: 10.1126/scitranslmed.aap9489.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point:
Major finding: All patients who received auto-FMT regained pre–allo-HSCT microbiota composition and diversity (P less than .0001).
Study details: An open-label study involving 25 allo-HSCT patients that compared auto-FMT with no treatment.
Disclosures: Study funding was provided by the Leonard Tow Foundation and the Memorial Sloan Kettering’s Center for Microbes, Inflammation, and Cancer. The authors reported disclosures related Merck, AbbVie, Nektar Therapeutics, Novartis, and others.
Source: Taur Y et al. Sci Transl Med. 2018 Sep 26. doi: 10.1126/scitranslmed.aap9489.

















