User login
Caring for patients with autism spectrum disorder
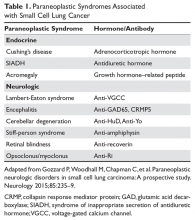
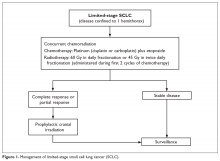
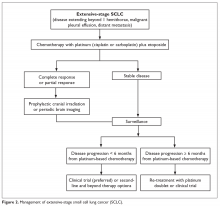
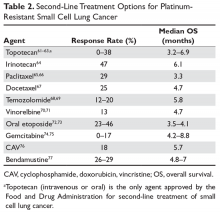
Autism spectrum disorder (ASD) is an umbrella term used to describe lifelong neurodevelopmental disorders characterized by impairment in social interactions and communication coupled with restricted, repetitive patterns of behaviors or interests that appear to share a common developmental course.1 In this article, we examine psychiatric care of patients with ASD and the most common symptom clusters treated with pharmacotherapy: irritability, anxiety, and hyperactivity/inattention.
First step: Keep the diagnosis in mind
Prior to 2013, ASD was comprised of 3 separate disorders distinguished by language delay and overall severity: autistic disorder, Asperger’s disorder, and pervasive developmental disorder, not otherwise specified.2 With the release of DSM-5 in 2013, these disorders were essentially collapsed into a single ASD.3 ASD prevalence is estimated to be 1 in 59 children,4 which represents a 20- to 30-fold increase since the 1960s.
In order to provide adequate psychiatric care for individuals with ASD, the first step is to remember the diagnosis; keep it in mind. This may be particularly important for clinicians who primarily care for adults, because such clinicians often receive limited training in disorders first manifesting in childhood and may not consider ASD in patients who have not been previously diagnosed. However, ASD diagnostic criteria have become broader, and public knowledge of the diagnosis has grown. DSM-5 acknowledges that although symptoms begin in early childhood, they may become more recognizable later in life with increasing social demand. The result is that many adults are likely undiagnosed. The estimated prevalence of ASD in adult psychiatric settings range from 1.5% to 4%.5-7 These patients have different treatment needs and unfortunately are often misdiagnosed with other psychiatric conditions.
A recent study in a state psychiatric facility found that 10% of patients in this setting met criteria for ASD.8 Almost all of those patients had been misdiagnosed with some form of schizophrenia, including one patient who had been previously diagnosed with autism by the father of autism himself, Leo Kanner, MD. Through the years, this patient’s autism diagnosis had fallen away, and at the time of the study, the patient carried a diagnosis of undifferentiated schizophrenia and was prescribed 8 psychotropic medications. The patient had repeatedly denied auditory or visual hallucinations; however, his stereotypies and odd behaviors were taken as evidence that he was responding to internal stimuli. This case highlights the importance of keeping the ASD diagnosis in mind when evaluating and treating patients.
Addressing 3 key symptom clusters
Even for patients with an established ASD diagnosis, comprehensive treatment is complex. It typically involves a multimodal approach that includes speech therapy, occupational therapy, applied behavioral analysis (ABA), and vocational training and support as well as management of associated medical conditions. Because medical comorbidities may play an important role in exacerbation of severe behaviors in ASD, often leading to acute behavioral regression and psychiatric admission, it is essential that they not be overlooked during evaluations.9,10
There are no effective pharmacologic treatments for the core social deficits seen in ASD. Novel pharmacotherapies to improve social impairment are in the early stages of research,11,12 but currently social impairment is best addressed through behavioral therapy and social skills training. Our role as psychiatrists is most often to treat co-occurring psychiatric symptoms so that individuals with ASD can fully participate in behavioral and school-based treatments that lead to improved social skills, activities of daily living, and quality of life. Three of the most common of these symptoms are irritability, anxiety, and hyperactivity/inattention.
Irritability
Irritability, marked by aggression, self-injury, and severe tantrums, causes serious distress for both patients and families, and this behavior cluster is the most frequently reported comorbid symptom in ASD.13-15 Nonpharmacologic treatment of irritability often involves ABA-based therapy and communication training.
Continued to: ABA includes an initial functional behavior assessment...
ABA includes an initial functional behavior assessment (FBA) of maladaptive behavior followed by the application of specific schedules of reinforcement for positive behavior. The FBA allows the therapist to determine what desirable consequences maintain a behavior. Without this knowledge, there is the risk of inadvertently rewarding a maladaptive behavior. For instance, if you are recommending a time-out for escape-motivated aggression, the result will likely be an increase rather than decrease in aggression.
Communication training teaches the patient to use communicative means to request a desired outcome to reduce inappropriate behaviors and improve independent functioning. Communication training can include speech therapy, teaching sign language, using picture exchange programs, or navigating communication devices. Consideration of nonpharmacologic management is vital in treatment planning. Continual inadvertent reward of behaviors will limit the effects of medications. Evidence suggests that pharmacotherapy is more effective when it occurs in the context of appropriate behavioral management techniques.16
Irritability has been the focus of significant pharmacotherapy research in ASD. Second-generation antipsychotics (SGAs) are first-line pharmacotherapy for severe irritability. Risperidone and aripiprazole are both FDA-approved for addressing irritability in youth with ASD. Their efficacy has been established in several large, placebo-controlled trials.17-23
Given issues with tolerability and cases refractory to the use of first-line agents,24 other SGAs are frequently used off-label for this indication with limited safety or efficacy data. Olanzapine demonstrated high response rates in early open-label studies,25,26 followed by efficacy over an 8-week double-blind placebo-controlled trial, although with significant weight gain.27 No other SGAs have been examined in double-blind placebo-controlled trials. Paliperidone demonstrated a particularly high response rate (84%) in a prospective open-label study of 25 adolescents and young adults with ASD.28 In a retrospective study of ziprasidone in 42 youth with ASD and irritability, we reported a response rate of 40%, which is lower than that seen for some other SGAs; however, ziprasidone can be an appealing option for patients for whom SGA-associated weight gain has been significant, because it is much more likely to be weight-neutral.29,30 Open-label studies with quetiapine in ASD have generally revealed only minimal efficacy for aggression,31,32 although sleep improvement may be more substantial.32 The safety and tolerability of lurasidone in treating irritability in youth with ASD has yet to be established.33 It is the only SGA with a published negative placebo-controlled trial in ASD.34 Use of SGAs may be limited by adverse effects, including weight gain, increased appetite, sedation, enuresis, and elevated prolactin. Monitoring of body mass index and metabolic profiles is indicated with all SGAs.
Haloperidol is the only first-generation antipsychotic with significant evidence (from multiple studies dating back to 1978) to support its use for ASD-associated irritability.35 However, due to the high incidence of dyskinesias and potential dystonias, use of haloperidol is reserved for severe treatment-refractory symptoms that have often not improved after multiple SGA trials.
Continued to: When severe self-inury and aggression fail to improve...
When severe self-injury and aggression fail to improve with multiple medication trials, the next steps include combination treatment with multiple antipsychotics,36 followed by clozapine, often as a last option.37 Research suggests that clozapine is effective and well-tolerated in ASD38-42; however, it has many potential severe adverse effects, including cardiomyopathy, lowered seizure threshold, severe constipation, weight gain, and agranulocytosis; due to risk of the latter, patients require regular blood draws for monitoring.
There is very little evidence to support the use of antiepileptic medications (AEDs) and mood stabilizers for irritability in ASD.43 Placebo-controlled trials have had mixed results. Some evidence suggests that AEDS may have more utility in individuals with ASD and abnormal EEGs without epilepsy44 or as an adjunct to SGA treatment.45 One study found that lithium may be beneficial for patients with ASD whose clinical presentation includes 2 or more mood symptoms.46
Anxiety
Anxiety is a significant issue for many individuals with ASD.47 Anxiety symptoms and disorders, including specific phobias, obsessive-compulsive disorder (OCD), social anxiety, and generalized anxiety disorder, are commonly seen in persons with ASD.48 Anxiety is often combined with restricted, repetitive behaviors (RBs) in ASD literature. Some evidence suggests that in individuals with ASD, sameness behaviors may limit sensory input and modulate anxiety.49 However, the core RBs symptom domain may not be related solely to anxiety, but rather represents deficits in executive processes that include cognitive flexibility and inhibitory control seen across multiple disorders with prominent RBs.50-54 Research indicates that anxiety is an independent and separable construct in ASD.55
Studies of treatments for both RBs and anxiety have focused primarily on selective serotonin reuptake inhibitors (SSRIs), hoping that the promising results for anxiety and OCD behaviors seen in neurotypical patients would translate to patients with ASD.56 Unfortunately, there is little evidence for effective pharmacologic management of ASD-associated anxiety.57 Large, randomized controlled trials (RCTs) are lacking. A Cochrane Database review of SSRIs for ASD58 examined 9 RCTs with a total of 320 patients. The authors concluded that there is no evidence to support the use of SSRIs for children with ASD, and limited evidence of utility in adults. Youth with ASD are particularly vulnerable to adverse effects from SSRIs, specifically impulsivity and agitation.57,59 However, SSRIs are among the most commonly prescribed medications for youth with ASD. Because there is limited evidence supporting SSRIs’ efficacy for this indication and issues with tolerability, there is significant concern for the overprescribing of SSRIs to patients with ASD. In comparison, there is some compelling evidence of efficacy for modified cognitive-behavioral therapy (CBT) for patients with high-functioning ASD. Seven RCTs have shown that CBT is superior to treatment as usual and waiting list control groups, with most effect sizes >0.8 and with no treatment-associated adverse effects.57
Risperidone has been shown to reduce RBs17,60 and anxiety17 in patients with ASD. In young children with co-occurring irritability, risperidone monotherapy is likely best to address both symptoms. When anxiety occurs in isolation and is severe, clinical experience suggests that SSRIs can be effective in a limited percentage of cases, though we recommend starting at low doses with frequent monitoring for activation and irritability. Treatment of anxiety is further complicated by the significant challenges presented by the diagnosis of true anxiety in the context of ASD.
Continued to: Hyperactivity and impulsivity
Hyperactivity and impulsivity
Hyperactivity and impulsivity are common among patients with ASD, with rates estimated from 41% to 78%.61 Hyperactivity and inattention are treated with a variety of medications. Research examining methylphenidate in ASD has demonstrated modest effects compared with placebo, though with frequent adverse effects, such as increased irritability and insomnia62,63 Other smaller studies have confirmed these results.64-66 One additional study found improvements not only in hyperactivity but also in joint attention and self-regulation of affective state following stimulant treatment.67 There is limited data on the efficacy and tolerability of amphetamine for treating hyperactivity and impulsivity in ASD. Stimulant medications often are avoided as the first-line treatment for hyperactivity because of concerns about increased irritability. Alpha-2 adrenergic receptor agonists often are used before stimulants because of their relatively benign adverse effect profile. Clonidine, guanfacine, and guanfacine ER all have demonstrated effectiveness in double-blind, placebo-controls trials in patients with ASD.68-70 In these trails, sedation was the most common adverse effect, although some studies have reported increased irritability with guanfacine.70,71
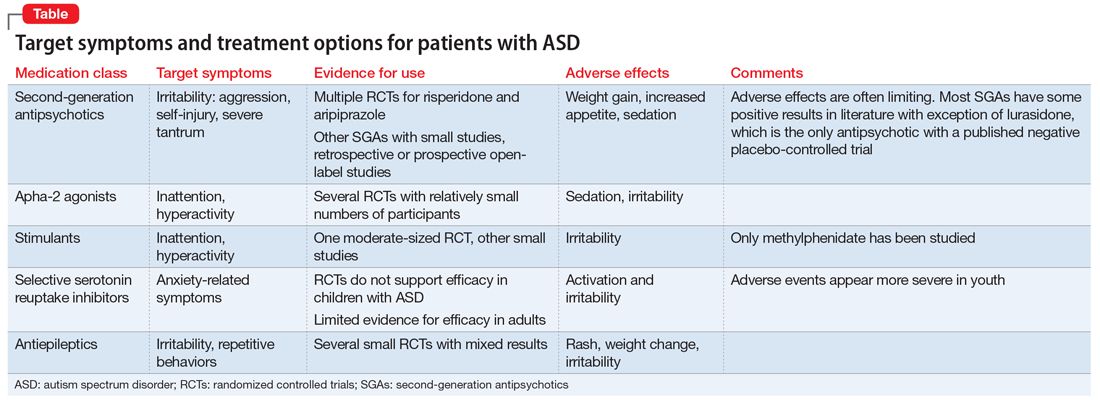
The Table provides a summary of the target symptoms and their treatment options for patients with ASD.
Improved diagnosis, but few evidence-based treatments
The rise in ASD cases observed over the past 20 years can be explained in part by a broader diagnostic algorithm and increased awareness. We are better at identifying ASD; however, there are still considerable gaps in identifying ASD in high-functioning patients and adults. One percent of the population has ASD,72,73 and this group is overrepresented in psychiatric clinic and hospital settings.74 Therefore, we must be aware of and understand the diagnosis.
Medication treatments are often less effective and less tolerable in patients with ASD than in patients without neurodevelopmental disability. There are differences in pharmacotherapy response and tolerability across development in ASD and limited evidence to guide prescribing in adults with ASD. SGAs appear to be effective across multiple symptom domains, but carry the risk of significant adverse effects. For anxiety and irritability, there is compelling evidence supporting the use of nonpharmacologic treatments.
Bottom Line
A subset of patients seen in psychiatry will have undiagnosed autism spectrum disorder (ASD). When evaluating worsening behaviors, first rule out organic causes. Second-generation antipsychotics have the most evidence for efficacy in ASD across multiple symptom domains. To sustain improvement in symptoms, it is vital to incorporate nonpharmacologic treatments.
Related Resources
- National Institute of Mental Health. Autism spectrum disorder. https://www.nimh.nih.gov/health/publications/autismspectrum-disorder/index.shtml.
- Centers for Disease Control and Prevention. Autism spectrum disorder (ASD). https://www.cdc.gov/ncbddd/ autism/index.html.
Drug Brand Names
Aripiprazole • Abilify
Clonidine • Catapres
Clozapine • Clozaril
Guanfacine • Tenex
Guanfacine Extended Release • Intuniv
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Methylphenidate • Ritalin
Olanzapine • Zyprexa
Paliperidone • Invega
Quetiapine • Seroquel
Risperidone • Risperdal
Ziprasidone • Geodon
1. Volkmar FR, Lord C, Bailey A, et al. Autism and pervasive developmental disorders. J Child Psychol Psychiatry. 2004;45(1):135-170.
2. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Baio J, Wiggins L, Christensen DL, et al. Prevalence of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2014. MMWR Surveill Summ 2018;67(6):1-23.
5. Scragg P, Shah A. Prevalence of Asperger’s syndrome in a secure hospital. Br J Psychiatry. 1994;165(5):679-682.
6. Hare DJ, Gould J, Mills R, et al. A preliminary study of individuals with autistic spectrum disorders in three special hospitals in England. London, UK: National Autistic Society; 1999.
7. Shah A, Holmes N, Wing L. Prevalence of autism and related conditions in adults in a mental handicap hospital. Appl Res Ment Retard. 1982;3(3):303-317.
8. Mandell DS, Lawer LJ, Branch K, et al. Prevalence and correlates of autism in a state psychiatric hospital. Autism. 2012;16(6):557-567.
9. Guinchat V, Cravero C, Diaz L, et al. Acute behavioral crises in psychiatric inpatients with autism spectrum disorder (ASD): recognition of concomitant medical or non-ASD psychiatric conditions predicts enhanced improvement. Res Devel Disabil. 2015;38:242-255.
10. Perisse D, Amiet C, Consoli A, et al. Risk factors of acute behavioral regression in psychiatrically hospitalized adolescents with autism. J Can Acad Child Adolesc Psychiatry. 2010;19(2):100-108.
11. Canitano R. New experimental treatments for core social domain in autism spectrum disorders. Front Pediatr. 2014;2:61.
12. Wink LK, Plawecki MH, Erickson CA, et al. Emerging drugs for the treatment of symptoms associated with autism spectrum disorders. Expert Opin Emerg Drugs. 2010;15(3):481-494.
13. Fitzpatrick SE, Srivorakiat L, Wink LK, et al. Aggression in autism spectrum disorder: presentation and treatment options. Neuropsychiatr Dis Treat. 2016;12:1525-1538.
14. Lecavalier L, Leone S, Wiltz J. The impact of behaviour problems on caregiver stress in young people with autism spectrum disorders. J Intellect Disabil Res. 2006;50(pt 3):172-183.
15. Mills R, Wing L. Researching interventions in ASD and priorities for research: surveying the membership of the NAS. London, UK: National Autistic Society; 2005.
16. Aman MG, McDougle CJ, Scahill L, et al. Medication and parent training in children with pervasive developmental disorders and serious behavior problems: results from a randomized clinical trial. J Am Acad Child Adolesc Psychiatry. 2009;48(12):1143-1154.
17. McDougle CJ, Holmes JP, Carlson DC, et al. A double-blind, placebo-controlled study of risperidone in adults with autistic disorder and other pervasive developmental disorders. Arch Gen Psychiatry. 1998;55(7):633-641.
18. Research Units on Pediatric Psychopharmacology Autism Network. Risperidone treatment of autistic disorder: longer-term benefits and blinded discontinuation after 6 months. Am J Psychiatry. 2005;162(7):1361-1369.
19. Shea S, Turgay A, Carroll A, et al. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics. 2004;114(5):e634-e641.
20. Zuddas A, Zanni R, Usala T. Second generation antipsychotics (SGAs) for non-psychotic disorders in children and adolescents: a review of the randomized controlled studies. Eur Neuropsychopharmacol. 2011;21(8):600-620.
21. Benton TD. Aripiprazole to treat irritability associated with autism: a placebo-controlled, fixed-dose trial. Curr Psychiatry Rep. 2011;13(2):77-79.
22. Marcus RN, Owen R, Kamen L, et al. A placebo-controlled, fixed-dose study of aripiprazole in children and adolescents with irritability associated with autistic disorder. J Am Acad Child Adolesc Psychiatry. 2009;48(11):1110-1119.
23. Owen R, Sikich L, Marcus RN, et al. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics. 2009;124(6):1533-1540.
24. Adler BA, Wink LK, Early M, et al. Drug-refractory aggression, self-injurious behavior, and severe tantrums in autism spectrum disorders: a chart review study. Autism. 2015;19(1):102-106.
25. Malone RP, Cater J, Sheikh RM, et al. Olanzapine versus haloperidol in children with autistic disorder: an open pilot study. J Am Acad Child Adolesc Psychiatry. 2001;40(8):887-894.
26. Potenza MN, Holmes JP, Kanes SJ, et al. Olanzapine treatment of children, adolescents, and adults with pervasive developmental disorders: an open-label pilot study. J Clin Psychopharmacol. 1999;19(1):37-44.
27. Hollander E, Wasserman S, Swanson EN, et al. A double-blind placebo-controlled pilot study of olanzapine in childhood/adolescent pervasive developmental disorder. J Child Adolesc Psychopharmacol. 2006;16(5):541-548.
28. Stigler KA, Erickson CA, Mullett JE, et al. Paliperidone for irritability in autistic disorder. J Child Adolesc Psychopharmacol. 2010;20(1):75-78.
29. Dominick K, Wink LK, McDougle CJ, et al. A retrospective naturalistic study of ziprasidone for irritability in youth with autism spectrum disorder. J Child Adolesc Psychopharmacol. 2015;25(5):397-401.
30. Malone RP, Delaney MA, Hyman SB, et al. Ziprasidone in adolescents with autism: an open-label pilot study. J Child Adolesc Psychopharmacol. 2007;17(6):779-790.
31. Findling RL, McNamara NK, Gracious BL, et al. Quetiapine in nine youths with autistic disorder. J Child Adolesc Psychopharmacol. 2004;14(2):287-294.
32. Golubchik P, Sever J, Weizman A. Low-dose quetiapine for adolescents with autistic spectrum disorder and aggressive behavior: open-label trial. Clin Neuropharmacol. 2011;34(6):216-219.
33. McClellan L, Dominick KC, Pedapati EV, et al. Lurasidone for the treatment of irritability and anger in autism spectrum disorders. Expert Opin Investig Drugs. 2017;26(8):985-989.
34. Loebel A, Brams M, Goldman RS, et al. Lurasidone for the treatment of irritability associated with autistic disorder. J Autism Dev Disord. 2016;46(4):1153-1163.
35. Campbell M, Anderson LT, Meier M, et al. A comparison of haloperidol and behavior therapy and their interaction in autistic children. J Am Acad Child Psychiatry. 1978;17(4):640-655.
36. Wink LK, Pedapati EV, Horn PS, et al. Multiple antipsychotic medication use in autism spectrum disorder. J Child Adolesc Psychopharmacol. 2017;27(1):91-94.
37. Wink LK, Badran I, Pedapati EV, et al. Clozapine for drug-refractory irritability in individuals with developmental disability. J Child Adolesc Psychopharmacol. 2016;26(9):843-846.
38. Chen NC, Bedair HS, McKay B, et al. Clozapine in the treatment of aggression in an adolescent with autistic disorder. J Clin Psychiatry. 2001;62(6):479-480.
39. Gobbi G, Pulvirenti L. Long-term treatment with clozapine in an adult with autistic disorder accompanied by aggressive behaviour. J Psychiatry Neurosci. 2001;26(4):340-341.
40. Lambrey S, Falissard B, Martin-Barrero M, et al. Effectiveness of clozapine for the treatment of aggression in an adolescent with autistic disorder. J Child Adolesc Psychopharmacol. 2010;20(1):79-80.
41. Yalcin O, Kaymak G, Erdogan A, et al. a retrospective investigation of clozapine treatment in autistic and nonautistic children and adolescents in an inpatient clinic in Turkey. J Child Adolesc Psychopharmacol. 2016;26(9):815-821.
42. Beherec L, Lambrey S, Quilici G, et al. Retrospective review of clozapine in the treatment of patients with autism spectrum disorder and severe disruptive behaviors. J Clin Psychopharmacol. 2011;31(3):341-344.
43. Hirota T, Veenstra-Vanderweele J, Hollander E, et al, Antiepileptic medications in autism spectrum disorder: a systematic review and meta-analysis. J Autism Dev Disord. 2014;44(4):948-957.
44. Hollander E, Chaplin W, Soorya L, et al. Divalproex sodium vs placebo for the treatment of irritability in children and adolescents with autism spectrum disorders. Neuropsychopharmacology. 2010;35(4):990-998.
45. Rezaei V, Mohammadi MR, Ghanizadeh A, et al. Double-blind, placebo-controlled trial of risperidone plus topiramate in children with autistic disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(7):1269-1272.
46. Siegel M, Beresford CA, Bunker M, et al. Preliminary investigation of lithium for mood disorder symptoms in children and adolescents with autism spectrum disorder. J Child Adolesc Psychopharmacol. 2014;24(7):399-402.
47. Costello EJ, Egger HL, Angold A. The developmental epidemiology of anxiety disorders: phenomenology, prevalence, and comorbidity. Child Adolesc Psychiatr Clin N Am. 2005;14(4):631-648,vii.
48. van Steensel FJ, Deutschman AA, Bogels SM. Examining the Screen for Child Anxiety-Related Emotional Disorder-71 as an assessment tool for anxiety in children with high-functioning autism spectrum disorders. Autism. 2013;17(6):681-692.
49. Lidstone J, Uljarevic M, Sullivan J, et al. Relations among restricted and repetitive behaviors, anxiety and sensory features in children with autism spectrum disorder. Research in Autism Spectrum Disorders. 2014;8(2):82-92.
50. Turner M. Annotation: Repetitive behaviour in autism: a review of psychological research. J Child Psychol Psychiatry. 1999;40(6):839-849.
51. Kuelz AK, Hohagen F, Voderholzer U. Neuropsychological performance in obsessive-compulsive disorder: a critical review. Biol Psychol. 2004;65(3):185-236.
52. Olley A, Malhi G, Sachdev P. Memory and executive functioning in obsessive-compulsive disorder: a selective review. J Affect Disord. 2007;104(1-3):15-23.
53. Channon S, Gunning A, Frankl J, et al. Tourette’s syndrome (TS): cognitive performance in adults with uncomplicated TS. Neuropsychology. 2006;20(1):58-65.
54. Crawford S, Channon S, Robertson MM. Tourette’s syndrome: performance on tests of behavioural inhibition, working memory and gambling. J Child Psychol Psychiatry. 2005;46(12):1327-1336.
55. Renno P, Wood JJ. Discriminant and convergent validity of the anxiety construct in children with autism spectrum disorders. J Autism Dev Disord. 2013;43(9):2135-2146.
56. Wink LK, Erickson CA, Stigler KA, et al. Riluzole in autistic disorder. J Child Adolesc Psychopharmacol. 2011;21(4):375-379.
57. Vasa RA, Carroll LM, Nozzolillo AA, et al. A systematic review of treatments for anxiety in youth with autism spectrum disorders. J Autism Dev Disord. 2014;44(12):3215-3229.
58. Williams K, Brignell A, Randall M, et al. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2013;(8):CD004677.
59. Wink LK, Erickson CA, McDougle CJ. Pharmacologic treatment of behavioral symptoms associated with autism and other pervasive developmental disorders. Curr Treat Options Neurol. 2010;12(6):529-538.
60. McDougle CJ, Scahill L, Aman MG, et al. Risperidone for the core symptom domains of autism: results from the study by the autism network of the research units on pediatric psychopharmacology. Am J Psychiatry. 2005;162(6):1142-1148.
61. Murray MJ, Attention-deficit/hyperactivity disorder in the context of autism spectrum disorders. Curr Psychiatry Rep. 2010;12(5):382-388.
62. Research Units on Pediatric Psychopharmacology Autism Network. Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch Gen Psychiatry. 2005;62(11):1266-1274.
63. Posey DJ, Aman MG, McCracken JT, et al. Positive effects of methylphenidate on inattention and hyperactivity in pervasive developmental disorders: an analysis of secondary measures. Biol Psychiatry. 2007;61(4):538-544.
64. Aman MG, Langworthy KS. Pharmacotherapy for hyperactivity in children with autism and other pervasive developmental disorders. J Autism Dev Disord. 2000;30(5):451-459.
65. Handen BL, Johnson CR, Lubetsky M. Efficacy of methylphenidate among children with autism and symptoms of attention-deficit hyperactivity disorder. J Autism Dev Disord. 2000;30(3):245-255.
66. Quintana H, Birmaher B, Stedge D, et al. Use of methylphenidate in the treatment of children with autistic disorder. J Autism Dev Disord. 1995;25(3):283-294.
67. Jahromi LB, Kasari CL, McCracken JT, et al. Positive effects of methylphenidate on social communication and self-regulation in children with pervasive developmental disorders and hyperactivity. J Autism Dev Disord. 2009;39(3):395-404.
68. Fankhauser MP, Karumanchi VC, German ML, et al. A double-blind, placebo-controlled study of the efficacy of transdermal clonidine in autism. J Clin Psychiatry. 1992;53(3):77-82.
69. Scahill L, McCracken JT, King BH, et al. Extended-release guanfacine for hyperactivity in children with autism spectrum disorder. Am J Psychiatry. 2015;172(12):1197-1206.
70. Handen BL, Sahl R, Hardan AY. Guanfacine in children with autism and/or intellectual disabilities. J Dev Behav Pediatr. 2008;29(4):303-308.
71. Scahill L, Aman MG, McDougle CJ, et al. A prospective open trial of guanfacine in children with pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2006;16(5):589-598.
72. Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators; Centers for Disease Control and Prevention (CDC). Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63(2):1-21.
73. Brugha TS, McManus S, Bankart J, et al. Epidemiology of autism spectrum disorders in adults in the community in England. Arch Gen Psychiatry. 2011;68(5):459-465.
74. Mandell DS, Psychiatric hospitalization among children with autism spectrum disorders. J Autism Dev Disord. 2008;38(6):1059-1065.
Autism spectrum disorder (ASD) is an umbrella term used to describe lifelong neurodevelopmental disorders characterized by impairment in social interactions and communication coupled with restricted, repetitive patterns of behaviors or interests that appear to share a common developmental course.1 In this article, we examine psychiatric care of patients with ASD and the most common symptom clusters treated with pharmacotherapy: irritability, anxiety, and hyperactivity/inattention.
First step: Keep the diagnosis in mind
Prior to 2013, ASD was comprised of 3 separate disorders distinguished by language delay and overall severity: autistic disorder, Asperger’s disorder, and pervasive developmental disorder, not otherwise specified.2 With the release of DSM-5 in 2013, these disorders were essentially collapsed into a single ASD.3 ASD prevalence is estimated to be 1 in 59 children,4 which represents a 20- to 30-fold increase since the 1960s.
In order to provide adequate psychiatric care for individuals with ASD, the first step is to remember the diagnosis; keep it in mind. This may be particularly important for clinicians who primarily care for adults, because such clinicians often receive limited training in disorders first manifesting in childhood and may not consider ASD in patients who have not been previously diagnosed. However, ASD diagnostic criteria have become broader, and public knowledge of the diagnosis has grown. DSM-5 acknowledges that although symptoms begin in early childhood, they may become more recognizable later in life with increasing social demand. The result is that many adults are likely undiagnosed. The estimated prevalence of ASD in adult psychiatric settings range from 1.5% to 4%.5-7 These patients have different treatment needs and unfortunately are often misdiagnosed with other psychiatric conditions.
A recent study in a state psychiatric facility found that 10% of patients in this setting met criteria for ASD.8 Almost all of those patients had been misdiagnosed with some form of schizophrenia, including one patient who had been previously diagnosed with autism by the father of autism himself, Leo Kanner, MD. Through the years, this patient’s autism diagnosis had fallen away, and at the time of the study, the patient carried a diagnosis of undifferentiated schizophrenia and was prescribed 8 psychotropic medications. The patient had repeatedly denied auditory or visual hallucinations; however, his stereotypies and odd behaviors were taken as evidence that he was responding to internal stimuli. This case highlights the importance of keeping the ASD diagnosis in mind when evaluating and treating patients.
Addressing 3 key symptom clusters
Even for patients with an established ASD diagnosis, comprehensive treatment is complex. It typically involves a multimodal approach that includes speech therapy, occupational therapy, applied behavioral analysis (ABA), and vocational training and support as well as management of associated medical conditions. Because medical comorbidities may play an important role in exacerbation of severe behaviors in ASD, often leading to acute behavioral regression and psychiatric admission, it is essential that they not be overlooked during evaluations.9,10
There are no effective pharmacologic treatments for the core social deficits seen in ASD. Novel pharmacotherapies to improve social impairment are in the early stages of research,11,12 but currently social impairment is best addressed through behavioral therapy and social skills training. Our role as psychiatrists is most often to treat co-occurring psychiatric symptoms so that individuals with ASD can fully participate in behavioral and school-based treatments that lead to improved social skills, activities of daily living, and quality of life. Three of the most common of these symptoms are irritability, anxiety, and hyperactivity/inattention.
Irritability
Irritability, marked by aggression, self-injury, and severe tantrums, causes serious distress for both patients and families, and this behavior cluster is the most frequently reported comorbid symptom in ASD.13-15 Nonpharmacologic treatment of irritability often involves ABA-based therapy and communication training.
Continued to: ABA includes an initial functional behavior assessment...
ABA includes an initial functional behavior assessment (FBA) of maladaptive behavior followed by the application of specific schedules of reinforcement for positive behavior. The FBA allows the therapist to determine what desirable consequences maintain a behavior. Without this knowledge, there is the risk of inadvertently rewarding a maladaptive behavior. For instance, if you are recommending a time-out for escape-motivated aggression, the result will likely be an increase rather than decrease in aggression.
Communication training teaches the patient to use communicative means to request a desired outcome to reduce inappropriate behaviors and improve independent functioning. Communication training can include speech therapy, teaching sign language, using picture exchange programs, or navigating communication devices. Consideration of nonpharmacologic management is vital in treatment planning. Continual inadvertent reward of behaviors will limit the effects of medications. Evidence suggests that pharmacotherapy is more effective when it occurs in the context of appropriate behavioral management techniques.16
Irritability has been the focus of significant pharmacotherapy research in ASD. Second-generation antipsychotics (SGAs) are first-line pharmacotherapy for severe irritability. Risperidone and aripiprazole are both FDA-approved for addressing irritability in youth with ASD. Their efficacy has been established in several large, placebo-controlled trials.17-23
Given issues with tolerability and cases refractory to the use of first-line agents,24 other SGAs are frequently used off-label for this indication with limited safety or efficacy data. Olanzapine demonstrated high response rates in early open-label studies,25,26 followed by efficacy over an 8-week double-blind placebo-controlled trial, although with significant weight gain.27 No other SGAs have been examined in double-blind placebo-controlled trials. Paliperidone demonstrated a particularly high response rate (84%) in a prospective open-label study of 25 adolescents and young adults with ASD.28 In a retrospective study of ziprasidone in 42 youth with ASD and irritability, we reported a response rate of 40%, which is lower than that seen for some other SGAs; however, ziprasidone can be an appealing option for patients for whom SGA-associated weight gain has been significant, because it is much more likely to be weight-neutral.29,30 Open-label studies with quetiapine in ASD have generally revealed only minimal efficacy for aggression,31,32 although sleep improvement may be more substantial.32 The safety and tolerability of lurasidone in treating irritability in youth with ASD has yet to be established.33 It is the only SGA with a published negative placebo-controlled trial in ASD.34 Use of SGAs may be limited by adverse effects, including weight gain, increased appetite, sedation, enuresis, and elevated prolactin. Monitoring of body mass index and metabolic profiles is indicated with all SGAs.
Haloperidol is the only first-generation antipsychotic with significant evidence (from multiple studies dating back to 1978) to support its use for ASD-associated irritability.35 However, due to the high incidence of dyskinesias and potential dystonias, use of haloperidol is reserved for severe treatment-refractory symptoms that have often not improved after multiple SGA trials.
Continued to: When severe self-inury and aggression fail to improve...
When severe self-injury and aggression fail to improve with multiple medication trials, the next steps include combination treatment with multiple antipsychotics,36 followed by clozapine, often as a last option.37 Research suggests that clozapine is effective and well-tolerated in ASD38-42; however, it has many potential severe adverse effects, including cardiomyopathy, lowered seizure threshold, severe constipation, weight gain, and agranulocytosis; due to risk of the latter, patients require regular blood draws for monitoring.
There is very little evidence to support the use of antiepileptic medications (AEDs) and mood stabilizers for irritability in ASD.43 Placebo-controlled trials have had mixed results. Some evidence suggests that AEDS may have more utility in individuals with ASD and abnormal EEGs without epilepsy44 or as an adjunct to SGA treatment.45 One study found that lithium may be beneficial for patients with ASD whose clinical presentation includes 2 or more mood symptoms.46
Anxiety
Anxiety is a significant issue for many individuals with ASD.47 Anxiety symptoms and disorders, including specific phobias, obsessive-compulsive disorder (OCD), social anxiety, and generalized anxiety disorder, are commonly seen in persons with ASD.48 Anxiety is often combined with restricted, repetitive behaviors (RBs) in ASD literature. Some evidence suggests that in individuals with ASD, sameness behaviors may limit sensory input and modulate anxiety.49 However, the core RBs symptom domain may not be related solely to anxiety, but rather represents deficits in executive processes that include cognitive flexibility and inhibitory control seen across multiple disorders with prominent RBs.50-54 Research indicates that anxiety is an independent and separable construct in ASD.55
Studies of treatments for both RBs and anxiety have focused primarily on selective serotonin reuptake inhibitors (SSRIs), hoping that the promising results for anxiety and OCD behaviors seen in neurotypical patients would translate to patients with ASD.56 Unfortunately, there is little evidence for effective pharmacologic management of ASD-associated anxiety.57 Large, randomized controlled trials (RCTs) are lacking. A Cochrane Database review of SSRIs for ASD58 examined 9 RCTs with a total of 320 patients. The authors concluded that there is no evidence to support the use of SSRIs for children with ASD, and limited evidence of utility in adults. Youth with ASD are particularly vulnerable to adverse effects from SSRIs, specifically impulsivity and agitation.57,59 However, SSRIs are among the most commonly prescribed medications for youth with ASD. Because there is limited evidence supporting SSRIs’ efficacy for this indication and issues with tolerability, there is significant concern for the overprescribing of SSRIs to patients with ASD. In comparison, there is some compelling evidence of efficacy for modified cognitive-behavioral therapy (CBT) for patients with high-functioning ASD. Seven RCTs have shown that CBT is superior to treatment as usual and waiting list control groups, with most effect sizes >0.8 and with no treatment-associated adverse effects.57
Risperidone has been shown to reduce RBs17,60 and anxiety17 in patients with ASD. In young children with co-occurring irritability, risperidone monotherapy is likely best to address both symptoms. When anxiety occurs in isolation and is severe, clinical experience suggests that SSRIs can be effective in a limited percentage of cases, though we recommend starting at low doses with frequent monitoring for activation and irritability. Treatment of anxiety is further complicated by the significant challenges presented by the diagnosis of true anxiety in the context of ASD.
Continued to: Hyperactivity and impulsivity
Hyperactivity and impulsivity
Hyperactivity and impulsivity are common among patients with ASD, with rates estimated from 41% to 78%.61 Hyperactivity and inattention are treated with a variety of medications. Research examining methylphenidate in ASD has demonstrated modest effects compared with placebo, though with frequent adverse effects, such as increased irritability and insomnia62,63 Other smaller studies have confirmed these results.64-66 One additional study found improvements not only in hyperactivity but also in joint attention and self-regulation of affective state following stimulant treatment.67 There is limited data on the efficacy and tolerability of amphetamine for treating hyperactivity and impulsivity in ASD. Stimulant medications often are avoided as the first-line treatment for hyperactivity because of concerns about increased irritability. Alpha-2 adrenergic receptor agonists often are used before stimulants because of their relatively benign adverse effect profile. Clonidine, guanfacine, and guanfacine ER all have demonstrated effectiveness in double-blind, placebo-controls trials in patients with ASD.68-70 In these trails, sedation was the most common adverse effect, although some studies have reported increased irritability with guanfacine.70,71
The Table provides a summary of the target symptoms and their treatment options for patients with ASD.

Improved diagnosis, but few evidence-based treatments
The rise in ASD cases observed over the past 20 years can be explained in part by a broader diagnostic algorithm and increased awareness. We are better at identifying ASD; however, there are still considerable gaps in identifying ASD in high-functioning patients and adults. One percent of the population has ASD,72,73 and this group is overrepresented in psychiatric clinic and hospital settings.74 Therefore, we must be aware of and understand the diagnosis.
Medication treatments are often less effective and less tolerable in patients with ASD than in patients without neurodevelopmental disability. There are differences in pharmacotherapy response and tolerability across development in ASD and limited evidence to guide prescribing in adults with ASD. SGAs appear to be effective across multiple symptom domains, but carry the risk of significant adverse effects. For anxiety and irritability, there is compelling evidence supporting the use of nonpharmacologic treatments.
Bottom Line
A subset of patients seen in psychiatry will have undiagnosed autism spectrum disorder (ASD). When evaluating worsening behaviors, first rule out organic causes. Second-generation antipsychotics have the most evidence for efficacy in ASD across multiple symptom domains. To sustain improvement in symptoms, it is vital to incorporate nonpharmacologic treatments.
Related Resources
- National Institute of Mental Health. Autism spectrum disorder. https://www.nimh.nih.gov/health/publications/autismspectrum-disorder/index.shtml.
- Centers for Disease Control and Prevention. Autism spectrum disorder (ASD). https://www.cdc.gov/ncbddd/ autism/index.html.
Drug Brand Names
Aripiprazole • Abilify
Clonidine • Catapres
Clozapine • Clozaril
Guanfacine • Tenex
Guanfacine Extended Release • Intuniv
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Methylphenidate • Ritalin
Olanzapine • Zyprexa
Paliperidone • Invega
Quetiapine • Seroquel
Risperidone • Risperdal
Ziprasidone • Geodon
Autism spectrum disorder (ASD) is an umbrella term used to describe lifelong neurodevelopmental disorders characterized by impairment in social interactions and communication coupled with restricted, repetitive patterns of behaviors or interests that appear to share a common developmental course.1 In this article, we examine psychiatric care of patients with ASD and the most common symptom clusters treated with pharmacotherapy: irritability, anxiety, and hyperactivity/inattention.
First step: Keep the diagnosis in mind
Prior to 2013, ASD was comprised of 3 separate disorders distinguished by language delay and overall severity: autistic disorder, Asperger’s disorder, and pervasive developmental disorder, not otherwise specified.2 With the release of DSM-5 in 2013, these disorders were essentially collapsed into a single ASD.3 ASD prevalence is estimated to be 1 in 59 children,4 which represents a 20- to 30-fold increase since the 1960s.
In order to provide adequate psychiatric care for individuals with ASD, the first step is to remember the diagnosis; keep it in mind. This may be particularly important for clinicians who primarily care for adults, because such clinicians often receive limited training in disorders first manifesting in childhood and may not consider ASD in patients who have not been previously diagnosed. However, ASD diagnostic criteria have become broader, and public knowledge of the diagnosis has grown. DSM-5 acknowledges that although symptoms begin in early childhood, they may become more recognizable later in life with increasing social demand. The result is that many adults are likely undiagnosed. The estimated prevalence of ASD in adult psychiatric settings range from 1.5% to 4%.5-7 These patients have different treatment needs and unfortunately are often misdiagnosed with other psychiatric conditions.
A recent study in a state psychiatric facility found that 10% of patients in this setting met criteria for ASD.8 Almost all of those patients had been misdiagnosed with some form of schizophrenia, including one patient who had been previously diagnosed with autism by the father of autism himself, Leo Kanner, MD. Through the years, this patient’s autism diagnosis had fallen away, and at the time of the study, the patient carried a diagnosis of undifferentiated schizophrenia and was prescribed 8 psychotropic medications. The patient had repeatedly denied auditory or visual hallucinations; however, his stereotypies and odd behaviors were taken as evidence that he was responding to internal stimuli. This case highlights the importance of keeping the ASD diagnosis in mind when evaluating and treating patients.
Addressing 3 key symptom clusters
Even for patients with an established ASD diagnosis, comprehensive treatment is complex. It typically involves a multimodal approach that includes speech therapy, occupational therapy, applied behavioral analysis (ABA), and vocational training and support as well as management of associated medical conditions. Because medical comorbidities may play an important role in exacerbation of severe behaviors in ASD, often leading to acute behavioral regression and psychiatric admission, it is essential that they not be overlooked during evaluations.9,10
There are no effective pharmacologic treatments for the core social deficits seen in ASD. Novel pharmacotherapies to improve social impairment are in the early stages of research,11,12 but currently social impairment is best addressed through behavioral therapy and social skills training. Our role as psychiatrists is most often to treat co-occurring psychiatric symptoms so that individuals with ASD can fully participate in behavioral and school-based treatments that lead to improved social skills, activities of daily living, and quality of life. Three of the most common of these symptoms are irritability, anxiety, and hyperactivity/inattention.
Irritability
Irritability, marked by aggression, self-injury, and severe tantrums, causes serious distress for both patients and families, and this behavior cluster is the most frequently reported comorbid symptom in ASD.13-15 Nonpharmacologic treatment of irritability often involves ABA-based therapy and communication training.
Continued to: ABA includes an initial functional behavior assessment...
ABA includes an initial functional behavior assessment (FBA) of maladaptive behavior followed by the application of specific schedules of reinforcement for positive behavior. The FBA allows the therapist to determine what desirable consequences maintain a behavior. Without this knowledge, there is the risk of inadvertently rewarding a maladaptive behavior. For instance, if you are recommending a time-out for escape-motivated aggression, the result will likely be an increase rather than decrease in aggression.
Communication training teaches the patient to use communicative means to request a desired outcome to reduce inappropriate behaviors and improve independent functioning. Communication training can include speech therapy, teaching sign language, using picture exchange programs, or navigating communication devices. Consideration of nonpharmacologic management is vital in treatment planning. Continual inadvertent reward of behaviors will limit the effects of medications. Evidence suggests that pharmacotherapy is more effective when it occurs in the context of appropriate behavioral management techniques.16
Irritability has been the focus of significant pharmacotherapy research in ASD. Second-generation antipsychotics (SGAs) are first-line pharmacotherapy for severe irritability. Risperidone and aripiprazole are both FDA-approved for addressing irritability in youth with ASD. Their efficacy has been established in several large, placebo-controlled trials.17-23
Given issues with tolerability and cases refractory to the use of first-line agents,24 other SGAs are frequently used off-label for this indication with limited safety or efficacy data. Olanzapine demonstrated high response rates in early open-label studies,25,26 followed by efficacy over an 8-week double-blind placebo-controlled trial, although with significant weight gain.27 No other SGAs have been examined in double-blind placebo-controlled trials. Paliperidone demonstrated a particularly high response rate (84%) in a prospective open-label study of 25 adolescents and young adults with ASD.28 In a retrospective study of ziprasidone in 42 youth with ASD and irritability, we reported a response rate of 40%, which is lower than that seen for some other SGAs; however, ziprasidone can be an appealing option for patients for whom SGA-associated weight gain has been significant, because it is much more likely to be weight-neutral.29,30 Open-label studies with quetiapine in ASD have generally revealed only minimal efficacy for aggression,31,32 although sleep improvement may be more substantial.32 The safety and tolerability of lurasidone in treating irritability in youth with ASD has yet to be established.33 It is the only SGA with a published negative placebo-controlled trial in ASD.34 Use of SGAs may be limited by adverse effects, including weight gain, increased appetite, sedation, enuresis, and elevated prolactin. Monitoring of body mass index and metabolic profiles is indicated with all SGAs.
Haloperidol is the only first-generation antipsychotic with significant evidence (from multiple studies dating back to 1978) to support its use for ASD-associated irritability.35 However, due to the high incidence of dyskinesias and potential dystonias, use of haloperidol is reserved for severe treatment-refractory symptoms that have often not improved after multiple SGA trials.
Continued to: When severe self-inury and aggression fail to improve...
When severe self-injury and aggression fail to improve with multiple medication trials, the next steps include combination treatment with multiple antipsychotics,36 followed by clozapine, often as a last option.37 Research suggests that clozapine is effective and well-tolerated in ASD38-42; however, it has many potential severe adverse effects, including cardiomyopathy, lowered seizure threshold, severe constipation, weight gain, and agranulocytosis; due to risk of the latter, patients require regular blood draws for monitoring.
There is very little evidence to support the use of antiepileptic medications (AEDs) and mood stabilizers for irritability in ASD.43 Placebo-controlled trials have had mixed results. Some evidence suggests that AEDS may have more utility in individuals with ASD and abnormal EEGs without epilepsy44 or as an adjunct to SGA treatment.45 One study found that lithium may be beneficial for patients with ASD whose clinical presentation includes 2 or more mood symptoms.46
Anxiety
Anxiety is a significant issue for many individuals with ASD.47 Anxiety symptoms and disorders, including specific phobias, obsessive-compulsive disorder (OCD), social anxiety, and generalized anxiety disorder, are commonly seen in persons with ASD.48 Anxiety is often combined with restricted, repetitive behaviors (RBs) in ASD literature. Some evidence suggests that in individuals with ASD, sameness behaviors may limit sensory input and modulate anxiety.49 However, the core RBs symptom domain may not be related solely to anxiety, but rather represents deficits in executive processes that include cognitive flexibility and inhibitory control seen across multiple disorders with prominent RBs.50-54 Research indicates that anxiety is an independent and separable construct in ASD.55
Studies of treatments for both RBs and anxiety have focused primarily on selective serotonin reuptake inhibitors (SSRIs), hoping that the promising results for anxiety and OCD behaviors seen in neurotypical patients would translate to patients with ASD.56 Unfortunately, there is little evidence for effective pharmacologic management of ASD-associated anxiety.57 Large, randomized controlled trials (RCTs) are lacking. A Cochrane Database review of SSRIs for ASD58 examined 9 RCTs with a total of 320 patients. The authors concluded that there is no evidence to support the use of SSRIs for children with ASD, and limited evidence of utility in adults. Youth with ASD are particularly vulnerable to adverse effects from SSRIs, specifically impulsivity and agitation.57,59 However, SSRIs are among the most commonly prescribed medications for youth with ASD. Because there is limited evidence supporting SSRIs’ efficacy for this indication and issues with tolerability, there is significant concern for the overprescribing of SSRIs to patients with ASD. In comparison, there is some compelling evidence of efficacy for modified cognitive-behavioral therapy (CBT) for patients with high-functioning ASD. Seven RCTs have shown that CBT is superior to treatment as usual and waiting list control groups, with most effect sizes >0.8 and with no treatment-associated adverse effects.57
Risperidone has been shown to reduce RBs17,60 and anxiety17 in patients with ASD. In young children with co-occurring irritability, risperidone monotherapy is likely best to address both symptoms. When anxiety occurs in isolation and is severe, clinical experience suggests that SSRIs can be effective in a limited percentage of cases, though we recommend starting at low doses with frequent monitoring for activation and irritability. Treatment of anxiety is further complicated by the significant challenges presented by the diagnosis of true anxiety in the context of ASD.
Continued to: Hyperactivity and impulsivity
Hyperactivity and impulsivity
Hyperactivity and impulsivity are common among patients with ASD, with rates estimated from 41% to 78%.61 Hyperactivity and inattention are treated with a variety of medications. Research examining methylphenidate in ASD has demonstrated modest effects compared with placebo, though with frequent adverse effects, such as increased irritability and insomnia62,63 Other smaller studies have confirmed these results.64-66 One additional study found improvements not only in hyperactivity but also in joint attention and self-regulation of affective state following stimulant treatment.67 There is limited data on the efficacy and tolerability of amphetamine for treating hyperactivity and impulsivity in ASD. Stimulant medications often are avoided as the first-line treatment for hyperactivity because of concerns about increased irritability. Alpha-2 adrenergic receptor agonists often are used before stimulants because of their relatively benign adverse effect profile. Clonidine, guanfacine, and guanfacine ER all have demonstrated effectiveness in double-blind, placebo-controls trials in patients with ASD.68-70 In these trails, sedation was the most common adverse effect, although some studies have reported increased irritability with guanfacine.70,71
The Table provides a summary of the target symptoms and their treatment options for patients with ASD.

Improved diagnosis, but few evidence-based treatments
The rise in ASD cases observed over the past 20 years can be explained in part by a broader diagnostic algorithm and increased awareness. We are better at identifying ASD; however, there are still considerable gaps in identifying ASD in high-functioning patients and adults. One percent of the population has ASD,72,73 and this group is overrepresented in psychiatric clinic and hospital settings.74 Therefore, we must be aware of and understand the diagnosis.
Medication treatments are often less effective and less tolerable in patients with ASD than in patients without neurodevelopmental disability. There are differences in pharmacotherapy response and tolerability across development in ASD and limited evidence to guide prescribing in adults with ASD. SGAs appear to be effective across multiple symptom domains, but carry the risk of significant adverse effects. For anxiety and irritability, there is compelling evidence supporting the use of nonpharmacologic treatments.
Bottom Line
A subset of patients seen in psychiatry will have undiagnosed autism spectrum disorder (ASD). When evaluating worsening behaviors, first rule out organic causes. Second-generation antipsychotics have the most evidence for efficacy in ASD across multiple symptom domains. To sustain improvement in symptoms, it is vital to incorporate nonpharmacologic treatments.
Related Resources
- National Institute of Mental Health. Autism spectrum disorder. https://www.nimh.nih.gov/health/publications/autismspectrum-disorder/index.shtml.
- Centers for Disease Control and Prevention. Autism spectrum disorder (ASD). https://www.cdc.gov/ncbddd/ autism/index.html.
Drug Brand Names
Aripiprazole • Abilify
Clonidine • Catapres
Clozapine • Clozaril
Guanfacine • Tenex
Guanfacine Extended Release • Intuniv
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Methylphenidate • Ritalin
Olanzapine • Zyprexa
Paliperidone • Invega
Quetiapine • Seroquel
Risperidone • Risperdal
Ziprasidone • Geodon
1. Volkmar FR, Lord C, Bailey A, et al. Autism and pervasive developmental disorders. J Child Psychol Psychiatry. 2004;45(1):135-170.
2. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Baio J, Wiggins L, Christensen DL, et al. Prevalence of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2014. MMWR Surveill Summ 2018;67(6):1-23.
5. Scragg P, Shah A. Prevalence of Asperger’s syndrome in a secure hospital. Br J Psychiatry. 1994;165(5):679-682.
6. Hare DJ, Gould J, Mills R, et al. A preliminary study of individuals with autistic spectrum disorders in three special hospitals in England. London, UK: National Autistic Society; 1999.
7. Shah A, Holmes N, Wing L. Prevalence of autism and related conditions in adults in a mental handicap hospital. Appl Res Ment Retard. 1982;3(3):303-317.
8. Mandell DS, Lawer LJ, Branch K, et al. Prevalence and correlates of autism in a state psychiatric hospital. Autism. 2012;16(6):557-567.
9. Guinchat V, Cravero C, Diaz L, et al. Acute behavioral crises in psychiatric inpatients with autism spectrum disorder (ASD): recognition of concomitant medical or non-ASD psychiatric conditions predicts enhanced improvement. Res Devel Disabil. 2015;38:242-255.
10. Perisse D, Amiet C, Consoli A, et al. Risk factors of acute behavioral regression in psychiatrically hospitalized adolescents with autism. J Can Acad Child Adolesc Psychiatry. 2010;19(2):100-108.
11. Canitano R. New experimental treatments for core social domain in autism spectrum disorders. Front Pediatr. 2014;2:61.
12. Wink LK, Plawecki MH, Erickson CA, et al. Emerging drugs for the treatment of symptoms associated with autism spectrum disorders. Expert Opin Emerg Drugs. 2010;15(3):481-494.
13. Fitzpatrick SE, Srivorakiat L, Wink LK, et al. Aggression in autism spectrum disorder: presentation and treatment options. Neuropsychiatr Dis Treat. 2016;12:1525-1538.
14. Lecavalier L, Leone S, Wiltz J. The impact of behaviour problems on caregiver stress in young people with autism spectrum disorders. J Intellect Disabil Res. 2006;50(pt 3):172-183.
15. Mills R, Wing L. Researching interventions in ASD and priorities for research: surveying the membership of the NAS. London, UK: National Autistic Society; 2005.
16. Aman MG, McDougle CJ, Scahill L, et al. Medication and parent training in children with pervasive developmental disorders and serious behavior problems: results from a randomized clinical trial. J Am Acad Child Adolesc Psychiatry. 2009;48(12):1143-1154.
17. McDougle CJ, Holmes JP, Carlson DC, et al. A double-blind, placebo-controlled study of risperidone in adults with autistic disorder and other pervasive developmental disorders. Arch Gen Psychiatry. 1998;55(7):633-641.
18. Research Units on Pediatric Psychopharmacology Autism Network. Risperidone treatment of autistic disorder: longer-term benefits and blinded discontinuation after 6 months. Am J Psychiatry. 2005;162(7):1361-1369.
19. Shea S, Turgay A, Carroll A, et al. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics. 2004;114(5):e634-e641.
20. Zuddas A, Zanni R, Usala T. Second generation antipsychotics (SGAs) for non-psychotic disorders in children and adolescents: a review of the randomized controlled studies. Eur Neuropsychopharmacol. 2011;21(8):600-620.
21. Benton TD. Aripiprazole to treat irritability associated with autism: a placebo-controlled, fixed-dose trial. Curr Psychiatry Rep. 2011;13(2):77-79.
22. Marcus RN, Owen R, Kamen L, et al. A placebo-controlled, fixed-dose study of aripiprazole in children and adolescents with irritability associated with autistic disorder. J Am Acad Child Adolesc Psychiatry. 2009;48(11):1110-1119.
23. Owen R, Sikich L, Marcus RN, et al. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics. 2009;124(6):1533-1540.
24. Adler BA, Wink LK, Early M, et al. Drug-refractory aggression, self-injurious behavior, and severe tantrums in autism spectrum disorders: a chart review study. Autism. 2015;19(1):102-106.
25. Malone RP, Cater J, Sheikh RM, et al. Olanzapine versus haloperidol in children with autistic disorder: an open pilot study. J Am Acad Child Adolesc Psychiatry. 2001;40(8):887-894.
26. Potenza MN, Holmes JP, Kanes SJ, et al. Olanzapine treatment of children, adolescents, and adults with pervasive developmental disorders: an open-label pilot study. J Clin Psychopharmacol. 1999;19(1):37-44.
27. Hollander E, Wasserman S, Swanson EN, et al. A double-blind placebo-controlled pilot study of olanzapine in childhood/adolescent pervasive developmental disorder. J Child Adolesc Psychopharmacol. 2006;16(5):541-548.
28. Stigler KA, Erickson CA, Mullett JE, et al. Paliperidone for irritability in autistic disorder. J Child Adolesc Psychopharmacol. 2010;20(1):75-78.
29. Dominick K, Wink LK, McDougle CJ, et al. A retrospective naturalistic study of ziprasidone for irritability in youth with autism spectrum disorder. J Child Adolesc Psychopharmacol. 2015;25(5):397-401.
30. Malone RP, Delaney MA, Hyman SB, et al. Ziprasidone in adolescents with autism: an open-label pilot study. J Child Adolesc Psychopharmacol. 2007;17(6):779-790.
31. Findling RL, McNamara NK, Gracious BL, et al. Quetiapine in nine youths with autistic disorder. J Child Adolesc Psychopharmacol. 2004;14(2):287-294.
32. Golubchik P, Sever J, Weizman A. Low-dose quetiapine for adolescents with autistic spectrum disorder and aggressive behavior: open-label trial. Clin Neuropharmacol. 2011;34(6):216-219.
33. McClellan L, Dominick KC, Pedapati EV, et al. Lurasidone for the treatment of irritability and anger in autism spectrum disorders. Expert Opin Investig Drugs. 2017;26(8):985-989.
34. Loebel A, Brams M, Goldman RS, et al. Lurasidone for the treatment of irritability associated with autistic disorder. J Autism Dev Disord. 2016;46(4):1153-1163.
35. Campbell M, Anderson LT, Meier M, et al. A comparison of haloperidol and behavior therapy and their interaction in autistic children. J Am Acad Child Psychiatry. 1978;17(4):640-655.
36. Wink LK, Pedapati EV, Horn PS, et al. Multiple antipsychotic medication use in autism spectrum disorder. J Child Adolesc Psychopharmacol. 2017;27(1):91-94.
37. Wink LK, Badran I, Pedapati EV, et al. Clozapine for drug-refractory irritability in individuals with developmental disability. J Child Adolesc Psychopharmacol. 2016;26(9):843-846.
38. Chen NC, Bedair HS, McKay B, et al. Clozapine in the treatment of aggression in an adolescent with autistic disorder. J Clin Psychiatry. 2001;62(6):479-480.
39. Gobbi G, Pulvirenti L. Long-term treatment with clozapine in an adult with autistic disorder accompanied by aggressive behaviour. J Psychiatry Neurosci. 2001;26(4):340-341.
40. Lambrey S, Falissard B, Martin-Barrero M, et al. Effectiveness of clozapine for the treatment of aggression in an adolescent with autistic disorder. J Child Adolesc Psychopharmacol. 2010;20(1):79-80.
41. Yalcin O, Kaymak G, Erdogan A, et al. a retrospective investigation of clozapine treatment in autistic and nonautistic children and adolescents in an inpatient clinic in Turkey. J Child Adolesc Psychopharmacol. 2016;26(9):815-821.
42. Beherec L, Lambrey S, Quilici G, et al. Retrospective review of clozapine in the treatment of patients with autism spectrum disorder and severe disruptive behaviors. J Clin Psychopharmacol. 2011;31(3):341-344.
43. Hirota T, Veenstra-Vanderweele J, Hollander E, et al, Antiepileptic medications in autism spectrum disorder: a systematic review and meta-analysis. J Autism Dev Disord. 2014;44(4):948-957.
44. Hollander E, Chaplin W, Soorya L, et al. Divalproex sodium vs placebo for the treatment of irritability in children and adolescents with autism spectrum disorders. Neuropsychopharmacology. 2010;35(4):990-998.
45. Rezaei V, Mohammadi MR, Ghanizadeh A, et al. Double-blind, placebo-controlled trial of risperidone plus topiramate in children with autistic disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(7):1269-1272.
46. Siegel M, Beresford CA, Bunker M, et al. Preliminary investigation of lithium for mood disorder symptoms in children and adolescents with autism spectrum disorder. J Child Adolesc Psychopharmacol. 2014;24(7):399-402.
47. Costello EJ, Egger HL, Angold A. The developmental epidemiology of anxiety disorders: phenomenology, prevalence, and comorbidity. Child Adolesc Psychiatr Clin N Am. 2005;14(4):631-648,vii.
48. van Steensel FJ, Deutschman AA, Bogels SM. Examining the Screen for Child Anxiety-Related Emotional Disorder-71 as an assessment tool for anxiety in children with high-functioning autism spectrum disorders. Autism. 2013;17(6):681-692.
49. Lidstone J, Uljarevic M, Sullivan J, et al. Relations among restricted and repetitive behaviors, anxiety and sensory features in children with autism spectrum disorder. Research in Autism Spectrum Disorders. 2014;8(2):82-92.
50. Turner M. Annotation: Repetitive behaviour in autism: a review of psychological research. J Child Psychol Psychiatry. 1999;40(6):839-849.
51. Kuelz AK, Hohagen F, Voderholzer U. Neuropsychological performance in obsessive-compulsive disorder: a critical review. Biol Psychol. 2004;65(3):185-236.
52. Olley A, Malhi G, Sachdev P. Memory and executive functioning in obsessive-compulsive disorder: a selective review. J Affect Disord. 2007;104(1-3):15-23.
53. Channon S, Gunning A, Frankl J, et al. Tourette’s syndrome (TS): cognitive performance in adults with uncomplicated TS. Neuropsychology. 2006;20(1):58-65.
54. Crawford S, Channon S, Robertson MM. Tourette’s syndrome: performance on tests of behavioural inhibition, working memory and gambling. J Child Psychol Psychiatry. 2005;46(12):1327-1336.
55. Renno P, Wood JJ. Discriminant and convergent validity of the anxiety construct in children with autism spectrum disorders. J Autism Dev Disord. 2013;43(9):2135-2146.
56. Wink LK, Erickson CA, Stigler KA, et al. Riluzole in autistic disorder. J Child Adolesc Psychopharmacol. 2011;21(4):375-379.
57. Vasa RA, Carroll LM, Nozzolillo AA, et al. A systematic review of treatments for anxiety in youth with autism spectrum disorders. J Autism Dev Disord. 2014;44(12):3215-3229.
58. Williams K, Brignell A, Randall M, et al. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2013;(8):CD004677.
59. Wink LK, Erickson CA, McDougle CJ. Pharmacologic treatment of behavioral symptoms associated with autism and other pervasive developmental disorders. Curr Treat Options Neurol. 2010;12(6):529-538.
60. McDougle CJ, Scahill L, Aman MG, et al. Risperidone for the core symptom domains of autism: results from the study by the autism network of the research units on pediatric psychopharmacology. Am J Psychiatry. 2005;162(6):1142-1148.
61. Murray MJ, Attention-deficit/hyperactivity disorder in the context of autism spectrum disorders. Curr Psychiatry Rep. 2010;12(5):382-388.
62. Research Units on Pediatric Psychopharmacology Autism Network. Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch Gen Psychiatry. 2005;62(11):1266-1274.
63. Posey DJ, Aman MG, McCracken JT, et al. Positive effects of methylphenidate on inattention and hyperactivity in pervasive developmental disorders: an analysis of secondary measures. Biol Psychiatry. 2007;61(4):538-544.
64. Aman MG, Langworthy KS. Pharmacotherapy for hyperactivity in children with autism and other pervasive developmental disorders. J Autism Dev Disord. 2000;30(5):451-459.
65. Handen BL, Johnson CR, Lubetsky M. Efficacy of methylphenidate among children with autism and symptoms of attention-deficit hyperactivity disorder. J Autism Dev Disord. 2000;30(3):245-255.
66. Quintana H, Birmaher B, Stedge D, et al. Use of methylphenidate in the treatment of children with autistic disorder. J Autism Dev Disord. 1995;25(3):283-294.
67. Jahromi LB, Kasari CL, McCracken JT, et al. Positive effects of methylphenidate on social communication and self-regulation in children with pervasive developmental disorders and hyperactivity. J Autism Dev Disord. 2009;39(3):395-404.
68. Fankhauser MP, Karumanchi VC, German ML, et al. A double-blind, placebo-controlled study of the efficacy of transdermal clonidine in autism. J Clin Psychiatry. 1992;53(3):77-82.
69. Scahill L, McCracken JT, King BH, et al. Extended-release guanfacine for hyperactivity in children with autism spectrum disorder. Am J Psychiatry. 2015;172(12):1197-1206.
70. Handen BL, Sahl R, Hardan AY. Guanfacine in children with autism and/or intellectual disabilities. J Dev Behav Pediatr. 2008;29(4):303-308.
71. Scahill L, Aman MG, McDougle CJ, et al. A prospective open trial of guanfacine in children with pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2006;16(5):589-598.
72. Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators; Centers for Disease Control and Prevention (CDC). Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63(2):1-21.
73. Brugha TS, McManus S, Bankart J, et al. Epidemiology of autism spectrum disorders in adults in the community in England. Arch Gen Psychiatry. 2011;68(5):459-465.
74. Mandell DS, Psychiatric hospitalization among children with autism spectrum disorders. J Autism Dev Disord. 2008;38(6):1059-1065.
1. Volkmar FR, Lord C, Bailey A, et al. Autism and pervasive developmental disorders. J Child Psychol Psychiatry. 2004;45(1):135-170.
2. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Baio J, Wiggins L, Christensen DL, et al. Prevalence of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2014. MMWR Surveill Summ 2018;67(6):1-23.
5. Scragg P, Shah A. Prevalence of Asperger’s syndrome in a secure hospital. Br J Psychiatry. 1994;165(5):679-682.
6. Hare DJ, Gould J, Mills R, et al. A preliminary study of individuals with autistic spectrum disorders in three special hospitals in England. London, UK: National Autistic Society; 1999.
7. Shah A, Holmes N, Wing L. Prevalence of autism and related conditions in adults in a mental handicap hospital. Appl Res Ment Retard. 1982;3(3):303-317.
8. Mandell DS, Lawer LJ, Branch K, et al. Prevalence and correlates of autism in a state psychiatric hospital. Autism. 2012;16(6):557-567.
9. Guinchat V, Cravero C, Diaz L, et al. Acute behavioral crises in psychiatric inpatients with autism spectrum disorder (ASD): recognition of concomitant medical or non-ASD psychiatric conditions predicts enhanced improvement. Res Devel Disabil. 2015;38:242-255.
10. Perisse D, Amiet C, Consoli A, et al. Risk factors of acute behavioral regression in psychiatrically hospitalized adolescents with autism. J Can Acad Child Adolesc Psychiatry. 2010;19(2):100-108.
11. Canitano R. New experimental treatments for core social domain in autism spectrum disorders. Front Pediatr. 2014;2:61.
12. Wink LK, Plawecki MH, Erickson CA, et al. Emerging drugs for the treatment of symptoms associated with autism spectrum disorders. Expert Opin Emerg Drugs. 2010;15(3):481-494.
13. Fitzpatrick SE, Srivorakiat L, Wink LK, et al. Aggression in autism spectrum disorder: presentation and treatment options. Neuropsychiatr Dis Treat. 2016;12:1525-1538.
14. Lecavalier L, Leone S, Wiltz J. The impact of behaviour problems on caregiver stress in young people with autism spectrum disorders. J Intellect Disabil Res. 2006;50(pt 3):172-183.
15. Mills R, Wing L. Researching interventions in ASD and priorities for research: surveying the membership of the NAS. London, UK: National Autistic Society; 2005.
16. Aman MG, McDougle CJ, Scahill L, et al. Medication and parent training in children with pervasive developmental disorders and serious behavior problems: results from a randomized clinical trial. J Am Acad Child Adolesc Psychiatry. 2009;48(12):1143-1154.
17. McDougle CJ, Holmes JP, Carlson DC, et al. A double-blind, placebo-controlled study of risperidone in adults with autistic disorder and other pervasive developmental disorders. Arch Gen Psychiatry. 1998;55(7):633-641.
18. Research Units on Pediatric Psychopharmacology Autism Network. Risperidone treatment of autistic disorder: longer-term benefits and blinded discontinuation after 6 months. Am J Psychiatry. 2005;162(7):1361-1369.
19. Shea S, Turgay A, Carroll A, et al. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics. 2004;114(5):e634-e641.
20. Zuddas A, Zanni R, Usala T. Second generation antipsychotics (SGAs) for non-psychotic disorders in children and adolescents: a review of the randomized controlled studies. Eur Neuropsychopharmacol. 2011;21(8):600-620.
21. Benton TD. Aripiprazole to treat irritability associated with autism: a placebo-controlled, fixed-dose trial. Curr Psychiatry Rep. 2011;13(2):77-79.
22. Marcus RN, Owen R, Kamen L, et al. A placebo-controlled, fixed-dose study of aripiprazole in children and adolescents with irritability associated with autistic disorder. J Am Acad Child Adolesc Psychiatry. 2009;48(11):1110-1119.
23. Owen R, Sikich L, Marcus RN, et al. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics. 2009;124(6):1533-1540.
24. Adler BA, Wink LK, Early M, et al. Drug-refractory aggression, self-injurious behavior, and severe tantrums in autism spectrum disorders: a chart review study. Autism. 2015;19(1):102-106.
25. Malone RP, Cater J, Sheikh RM, et al. Olanzapine versus haloperidol in children with autistic disorder: an open pilot study. J Am Acad Child Adolesc Psychiatry. 2001;40(8):887-894.
26. Potenza MN, Holmes JP, Kanes SJ, et al. Olanzapine treatment of children, adolescents, and adults with pervasive developmental disorders: an open-label pilot study. J Clin Psychopharmacol. 1999;19(1):37-44.
27. Hollander E, Wasserman S, Swanson EN, et al. A double-blind placebo-controlled pilot study of olanzapine in childhood/adolescent pervasive developmental disorder. J Child Adolesc Psychopharmacol. 2006;16(5):541-548.
28. Stigler KA, Erickson CA, Mullett JE, et al. Paliperidone for irritability in autistic disorder. J Child Adolesc Psychopharmacol. 2010;20(1):75-78.
29. Dominick K, Wink LK, McDougle CJ, et al. A retrospective naturalistic study of ziprasidone for irritability in youth with autism spectrum disorder. J Child Adolesc Psychopharmacol. 2015;25(5):397-401.
30. Malone RP, Delaney MA, Hyman SB, et al. Ziprasidone in adolescents with autism: an open-label pilot study. J Child Adolesc Psychopharmacol. 2007;17(6):779-790.
31. Findling RL, McNamara NK, Gracious BL, et al. Quetiapine in nine youths with autistic disorder. J Child Adolesc Psychopharmacol. 2004;14(2):287-294.
32. Golubchik P, Sever J, Weizman A. Low-dose quetiapine for adolescents with autistic spectrum disorder and aggressive behavior: open-label trial. Clin Neuropharmacol. 2011;34(6):216-219.
33. McClellan L, Dominick KC, Pedapati EV, et al. Lurasidone for the treatment of irritability and anger in autism spectrum disorders. Expert Opin Investig Drugs. 2017;26(8):985-989.
34. Loebel A, Brams M, Goldman RS, et al. Lurasidone for the treatment of irritability associated with autistic disorder. J Autism Dev Disord. 2016;46(4):1153-1163.
35. Campbell M, Anderson LT, Meier M, et al. A comparison of haloperidol and behavior therapy and their interaction in autistic children. J Am Acad Child Psychiatry. 1978;17(4):640-655.
36. Wink LK, Pedapati EV, Horn PS, et al. Multiple antipsychotic medication use in autism spectrum disorder. J Child Adolesc Psychopharmacol. 2017;27(1):91-94.
37. Wink LK, Badran I, Pedapati EV, et al. Clozapine for drug-refractory irritability in individuals with developmental disability. J Child Adolesc Psychopharmacol. 2016;26(9):843-846.
38. Chen NC, Bedair HS, McKay B, et al. Clozapine in the treatment of aggression in an adolescent with autistic disorder. J Clin Psychiatry. 2001;62(6):479-480.
39. Gobbi G, Pulvirenti L. Long-term treatment with clozapine in an adult with autistic disorder accompanied by aggressive behaviour. J Psychiatry Neurosci. 2001;26(4):340-341.
40. Lambrey S, Falissard B, Martin-Barrero M, et al. Effectiveness of clozapine for the treatment of aggression in an adolescent with autistic disorder. J Child Adolesc Psychopharmacol. 2010;20(1):79-80.
41. Yalcin O, Kaymak G, Erdogan A, et al. a retrospective investigation of clozapine treatment in autistic and nonautistic children and adolescents in an inpatient clinic in Turkey. J Child Adolesc Psychopharmacol. 2016;26(9):815-821.
42. Beherec L, Lambrey S, Quilici G, et al. Retrospective review of clozapine in the treatment of patients with autism spectrum disorder and severe disruptive behaviors. J Clin Psychopharmacol. 2011;31(3):341-344.
43. Hirota T, Veenstra-Vanderweele J, Hollander E, et al, Antiepileptic medications in autism spectrum disorder: a systematic review and meta-analysis. J Autism Dev Disord. 2014;44(4):948-957.
44. Hollander E, Chaplin W, Soorya L, et al. Divalproex sodium vs placebo for the treatment of irritability in children and adolescents with autism spectrum disorders. Neuropsychopharmacology. 2010;35(4):990-998.
45. Rezaei V, Mohammadi MR, Ghanizadeh A, et al. Double-blind, placebo-controlled trial of risperidone plus topiramate in children with autistic disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(7):1269-1272.
46. Siegel M, Beresford CA, Bunker M, et al. Preliminary investigation of lithium for mood disorder symptoms in children and adolescents with autism spectrum disorder. J Child Adolesc Psychopharmacol. 2014;24(7):399-402.
47. Costello EJ, Egger HL, Angold A. The developmental epidemiology of anxiety disorders: phenomenology, prevalence, and comorbidity. Child Adolesc Psychiatr Clin N Am. 2005;14(4):631-648,vii.
48. van Steensel FJ, Deutschman AA, Bogels SM. Examining the Screen for Child Anxiety-Related Emotional Disorder-71 as an assessment tool for anxiety in children with high-functioning autism spectrum disorders. Autism. 2013;17(6):681-692.
49. Lidstone J, Uljarevic M, Sullivan J, et al. Relations among restricted and repetitive behaviors, anxiety and sensory features in children with autism spectrum disorder. Research in Autism Spectrum Disorders. 2014;8(2):82-92.
50. Turner M. Annotation: Repetitive behaviour in autism: a review of psychological research. J Child Psychol Psychiatry. 1999;40(6):839-849.
51. Kuelz AK, Hohagen F, Voderholzer U. Neuropsychological performance in obsessive-compulsive disorder: a critical review. Biol Psychol. 2004;65(3):185-236.
52. Olley A, Malhi G, Sachdev P. Memory and executive functioning in obsessive-compulsive disorder: a selective review. J Affect Disord. 2007;104(1-3):15-23.
53. Channon S, Gunning A, Frankl J, et al. Tourette’s syndrome (TS): cognitive performance in adults with uncomplicated TS. Neuropsychology. 2006;20(1):58-65.
54. Crawford S, Channon S, Robertson MM. Tourette’s syndrome: performance on tests of behavioural inhibition, working memory and gambling. J Child Psychol Psychiatry. 2005;46(12):1327-1336.
55. Renno P, Wood JJ. Discriminant and convergent validity of the anxiety construct in children with autism spectrum disorders. J Autism Dev Disord. 2013;43(9):2135-2146.
56. Wink LK, Erickson CA, Stigler KA, et al. Riluzole in autistic disorder. J Child Adolesc Psychopharmacol. 2011;21(4):375-379.
57. Vasa RA, Carroll LM, Nozzolillo AA, et al. A systematic review of treatments for anxiety in youth with autism spectrum disorders. J Autism Dev Disord. 2014;44(12):3215-3229.
58. Williams K, Brignell A, Randall M, et al. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2013;(8):CD004677.
59. Wink LK, Erickson CA, McDougle CJ. Pharmacologic treatment of behavioral symptoms associated with autism and other pervasive developmental disorders. Curr Treat Options Neurol. 2010;12(6):529-538.
60. McDougle CJ, Scahill L, Aman MG, et al. Risperidone for the core symptom domains of autism: results from the study by the autism network of the research units on pediatric psychopharmacology. Am J Psychiatry. 2005;162(6):1142-1148.
61. Murray MJ, Attention-deficit/hyperactivity disorder in the context of autism spectrum disorders. Curr Psychiatry Rep. 2010;12(5):382-388.
62. Research Units on Pediatric Psychopharmacology Autism Network. Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch Gen Psychiatry. 2005;62(11):1266-1274.
63. Posey DJ, Aman MG, McCracken JT, et al. Positive effects of methylphenidate on inattention and hyperactivity in pervasive developmental disorders: an analysis of secondary measures. Biol Psychiatry. 2007;61(4):538-544.
64. Aman MG, Langworthy KS. Pharmacotherapy for hyperactivity in children with autism and other pervasive developmental disorders. J Autism Dev Disord. 2000;30(5):451-459.
65. Handen BL, Johnson CR, Lubetsky M. Efficacy of methylphenidate among children with autism and symptoms of attention-deficit hyperactivity disorder. J Autism Dev Disord. 2000;30(3):245-255.
66. Quintana H, Birmaher B, Stedge D, et al. Use of methylphenidate in the treatment of children with autistic disorder. J Autism Dev Disord. 1995;25(3):283-294.
67. Jahromi LB, Kasari CL, McCracken JT, et al. Positive effects of methylphenidate on social communication and self-regulation in children with pervasive developmental disorders and hyperactivity. J Autism Dev Disord. 2009;39(3):395-404.
68. Fankhauser MP, Karumanchi VC, German ML, et al. A double-blind, placebo-controlled study of the efficacy of transdermal clonidine in autism. J Clin Psychiatry. 1992;53(3):77-82.
69. Scahill L, McCracken JT, King BH, et al. Extended-release guanfacine for hyperactivity in children with autism spectrum disorder. Am J Psychiatry. 2015;172(12):1197-1206.
70. Handen BL, Sahl R, Hardan AY. Guanfacine in children with autism and/or intellectual disabilities. J Dev Behav Pediatr. 2008;29(4):303-308.
71. Scahill L, Aman MG, McDougle CJ, et al. A prospective open trial of guanfacine in children with pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2006;16(5):589-598.
72. Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators; Centers for Disease Control and Prevention (CDC). Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63(2):1-21.
73. Brugha TS, McManus S, Bankart J, et al. Epidemiology of autism spectrum disorders in adults in the community in England. Arch Gen Psychiatry. 2011;68(5):459-465.
74. Mandell DS, Psychiatric hospitalization among children with autism spectrum disorders. J Autism Dev Disord. 2008;38(6):1059-1065.
Neuropolitics in the age of extremism: Brain regions involved in hatred
We psychiatrists encounter a wide variety of intense negative emotions in our patients on a daily basis, whether in the clinic or on an inpatient unit. These include rage, irritability, hostility, paranoia, loathing, and unadulterated hatred.
We evaluate, diagnose, and treat the underlying psychiatric brain disorders that generate such maladaptive emotions, and have our patients regain their baseline functioning by resolving the psychopathology that ignited their amygdala and their limbic circuitry.
But while we can manage the microcosm of one patient’s mental state, we are unable to intervene in the macrocosm of an entire society ravaged by extreme hyper-partisanship and naked bidirectional hatred. It is literally impossible for even the most skillful psychiatrists to repair a nation caught up in poisonous emotional turmoil, irreconcilable political differences, and a veritable war of belief systems that mimic religious fanaticism, which history tells us led to so many tragic wars over the centuries and millennia.
Ideally, politics is supposed to be an elegant cerebral process, a debate of ideas across disparate ideologies, the product of which is expected to be the advancement of the welfare of the nation and its citizens. But what we are currently witnessing is a distressing degeneration of politics into personal hatred and ad hominem attacks, with partisans frothing at the mouth as they describe the utter stupidity and dangerousness of their despised political opponents-cum-bitter enemies. They even declare each other “mentally ill,” which is an absurd explanation of why other people do not agree with their belief system. Neither side can find an iota of redeeming value in the political views of the “other side” and hurl insults and epithets verbally and in writing via dueling books that become instant best sellers among the partisan aficionados on both sides.
This disastrous political “climate change” may have ominous repercussions for the brains of the political combatants themselves, and even for those on the sidelines who are subjected to the relentless stress of witnessing a social train wreck in the making. As a neuropsychiatrist, I wonder if the collective national amygdala of the country is on fire, and the national prefrontal cortex is being corroded by the pervasive and ugly negativity that engulfs us all, with social media that incites its users night and day, adding gasoline to the fire. Chronic stress and its associated hypercortisolemia are known to be neurotoxic to the hippocampus and eventuate in clinical depression and its grave consequences.
Continued to: I think I sensed this odious scenario coming...
I think I sensed this odious scenario coming 2 years ago during the bizarre presidential election, when I wrote an editorial describing the “fear and loathing” that permeated the political process and the unusual behavior of the candidates.1 A year after the election, I commented about the toxic zeitgeist of political extremism from a psychiatric perspective.2 The situation appears to be getting worse, and the folie en masse is intensifying and its hateful cacophony is deafening to our sensibilities.
Aaron Beck, MD, the father of cognitive-behavioral therapy (CBT), wrote a book about hate.3 It may be a fantasy, but I wish the leaders on both sides would agree to a course of CBT to recognize the destructive path of intransigent hyper-partisanship. They might then transcend their egocentric attitudes and inspire millions of their followers to communicate rationally, instead of stoking the fires of resentment and enmity toward the “other side.”
Let’s get back to science: Where are the pathways of hate located in the brain? An interesting study was conducted to detect the neural circuits that mediate hate.4 The researchers obtained functional magnetic resonance imaging scans of participants while they were viewing the face of a person they hate compared with the face of an acquaintance toward whom they have neutral feelings. They also calculated a “hate score” for each participant for the analysis. They found that viewing a hated person increased the activity in several brain regions, including the medial frontal gyrus, right putamen, premotor cortex, frontal pole, and medial insula bilaterally. The activation in 3 areas correlated with the intensity of the hatred: right insula, right premotor cortex, and right frontal-medial gyrus. At the same time, the right superior frontal gyrus showed deactivation. Interestingly, hate and romantic love shared activation in 2 areas: the putamen and insula. This suggests that passionate love and passionate hate are 2 sides of the same neural coin! It prompts me to wonder what happens to the capacity to love among political extremists when their putamen and insula are filled up with hate. It also makes me wonder if unbridled hatred can be “enjoyable” and even addictive, as passionate romantic love is.
The bottom line: Consider the brain changes that are occurring on a large scale in at least a hundred million political partisans, and whether those neural circuits get even more intensely activated following the elections, regardless of the outcome.
Finally, we must remain cognizant of the epigenetic consequences of emotions and stress.5 There is solid scientific evidence that extremes of human experiences can modify gene expression in sperm and fetuses, resulting in a transgenerational effect upon the children of the extreme partisans, and also the children of nonpartisan observers, who experience unmitigated anxiety due to the inescapable cloud of negative affect shrouding their daily lives.6 So politicians should be cognizant that perpetuating a bitter war against each other may be detrimental to their progeny and future generations. I am frankly worried about the epigenetically disrupted emotional stability of voters circa 2035, born in these days of unprecedented and tumultuous hatred by their hyper-partisan parents.
Henry A. Nasrallah, MD
Editor-in-Chief
1. Nasrallah HA. Fear and loathing abound in the ‘off-label’ presidential election of 2016. Current Psychiatry. 2016;15(7):21,26.
2. Nasrallah HA. The toxic zeitgeist of hyper-partisanship: a psychiatric perspective. Current Psychiatry. 2018;17(2):17-18.
3. Beck AT. Prisoners of hate: the cognitive basis of anger, hostility, and violence. New York, NY: Harper-Collins; 1999.
4. Zeki S, Romaya JP. Neural correlates of hate. PloS One. 2008;3(10):e3556. doi: 10.1371/journal.pone.0003556.
5. Griffiths BB, Hunter RG. Neuroepigenetics of stress. Neuroscience. 2014;275:420-435.
6. Bartlett AA, Singh R, Hunter RG. Anxiety and epigenetics. Adv Exp Med Biol.2017;978:145-166.
We psychiatrists encounter a wide variety of intense negative emotions in our patients on a daily basis, whether in the clinic or on an inpatient unit. These include rage, irritability, hostility, paranoia, loathing, and unadulterated hatred.
We evaluate, diagnose, and treat the underlying psychiatric brain disorders that generate such maladaptive emotions, and have our patients regain their baseline functioning by resolving the psychopathology that ignited their amygdala and their limbic circuitry.
But while we can manage the microcosm of one patient’s mental state, we are unable to intervene in the macrocosm of an entire society ravaged by extreme hyper-partisanship and naked bidirectional hatred. It is literally impossible for even the most skillful psychiatrists to repair a nation caught up in poisonous emotional turmoil, irreconcilable political differences, and a veritable war of belief systems that mimic religious fanaticism, which history tells us led to so many tragic wars over the centuries and millennia.
Ideally, politics is supposed to be an elegant cerebral process, a debate of ideas across disparate ideologies, the product of which is expected to be the advancement of the welfare of the nation and its citizens. But what we are currently witnessing is a distressing degeneration of politics into personal hatred and ad hominem attacks, with partisans frothing at the mouth as they describe the utter stupidity and dangerousness of their despised political opponents-cum-bitter enemies. They even declare each other “mentally ill,” which is an absurd explanation of why other people do not agree with their belief system. Neither side can find an iota of redeeming value in the political views of the “other side” and hurl insults and epithets verbally and in writing via dueling books that become instant best sellers among the partisan aficionados on both sides.
This disastrous political “climate change” may have ominous repercussions for the brains of the political combatants themselves, and even for those on the sidelines who are subjected to the relentless stress of witnessing a social train wreck in the making. As a neuropsychiatrist, I wonder if the collective national amygdala of the country is on fire, and the national prefrontal cortex is being corroded by the pervasive and ugly negativity that engulfs us all, with social media that incites its users night and day, adding gasoline to the fire. Chronic stress and its associated hypercortisolemia are known to be neurotoxic to the hippocampus and eventuate in clinical depression and its grave consequences.
Continued to: I think I sensed this odious scenario coming...
I think I sensed this odious scenario coming 2 years ago during the bizarre presidential election, when I wrote an editorial describing the “fear and loathing” that permeated the political process and the unusual behavior of the candidates.1 A year after the election, I commented about the toxic zeitgeist of political extremism from a psychiatric perspective.2 The situation appears to be getting worse, and the folie en masse is intensifying and its hateful cacophony is deafening to our sensibilities.
Aaron Beck, MD, the father of cognitive-behavioral therapy (CBT), wrote a book about hate.3 It may be a fantasy, but I wish the leaders on both sides would agree to a course of CBT to recognize the destructive path of intransigent hyper-partisanship. They might then transcend their egocentric attitudes and inspire millions of their followers to communicate rationally, instead of stoking the fires of resentment and enmity toward the “other side.”
Let’s get back to science: Where are the pathways of hate located in the brain? An interesting study was conducted to detect the neural circuits that mediate hate.4 The researchers obtained functional magnetic resonance imaging scans of participants while they were viewing the face of a person they hate compared with the face of an acquaintance toward whom they have neutral feelings. They also calculated a “hate score” for each participant for the analysis. They found that viewing a hated person increased the activity in several brain regions, including the medial frontal gyrus, right putamen, premotor cortex, frontal pole, and medial insula bilaterally. The activation in 3 areas correlated with the intensity of the hatred: right insula, right premotor cortex, and right frontal-medial gyrus. At the same time, the right superior frontal gyrus showed deactivation. Interestingly, hate and romantic love shared activation in 2 areas: the putamen and insula. This suggests that passionate love and passionate hate are 2 sides of the same neural coin! It prompts me to wonder what happens to the capacity to love among political extremists when their putamen and insula are filled up with hate. It also makes me wonder if unbridled hatred can be “enjoyable” and even addictive, as passionate romantic love is.
The bottom line: Consider the brain changes that are occurring on a large scale in at least a hundred million political partisans, and whether those neural circuits get even more intensely activated following the elections, regardless of the outcome.
Finally, we must remain cognizant of the epigenetic consequences of emotions and stress.5 There is solid scientific evidence that extremes of human experiences can modify gene expression in sperm and fetuses, resulting in a transgenerational effect upon the children of the extreme partisans, and also the children of nonpartisan observers, who experience unmitigated anxiety due to the inescapable cloud of negative affect shrouding their daily lives.6 So politicians should be cognizant that perpetuating a bitter war against each other may be detrimental to their progeny and future generations. I am frankly worried about the epigenetically disrupted emotional stability of voters circa 2035, born in these days of unprecedented and tumultuous hatred by their hyper-partisan parents.
Henry A. Nasrallah, MD
Editor-in-Chief
We psychiatrists encounter a wide variety of intense negative emotions in our patients on a daily basis, whether in the clinic or on an inpatient unit. These include rage, irritability, hostility, paranoia, loathing, and unadulterated hatred.
We evaluate, diagnose, and treat the underlying psychiatric brain disorders that generate such maladaptive emotions, and have our patients regain their baseline functioning by resolving the psychopathology that ignited their amygdala and their limbic circuitry.
But while we can manage the microcosm of one patient’s mental state, we are unable to intervene in the macrocosm of an entire society ravaged by extreme hyper-partisanship and naked bidirectional hatred. It is literally impossible for even the most skillful psychiatrists to repair a nation caught up in poisonous emotional turmoil, irreconcilable political differences, and a veritable war of belief systems that mimic religious fanaticism, which history tells us led to so many tragic wars over the centuries and millennia.
Ideally, politics is supposed to be an elegant cerebral process, a debate of ideas across disparate ideologies, the product of which is expected to be the advancement of the welfare of the nation and its citizens. But what we are currently witnessing is a distressing degeneration of politics into personal hatred and ad hominem attacks, with partisans frothing at the mouth as they describe the utter stupidity and dangerousness of their despised political opponents-cum-bitter enemies. They even declare each other “mentally ill,” which is an absurd explanation of why other people do not agree with their belief system. Neither side can find an iota of redeeming value in the political views of the “other side” and hurl insults and epithets verbally and in writing via dueling books that become instant best sellers among the partisan aficionados on both sides.
This disastrous political “climate change” may have ominous repercussions for the brains of the political combatants themselves, and even for those on the sidelines who are subjected to the relentless stress of witnessing a social train wreck in the making. As a neuropsychiatrist, I wonder if the collective national amygdala of the country is on fire, and the national prefrontal cortex is being corroded by the pervasive and ugly negativity that engulfs us all, with social media that incites its users night and day, adding gasoline to the fire. Chronic stress and its associated hypercortisolemia are known to be neurotoxic to the hippocampus and eventuate in clinical depression and its grave consequences.
Continued to: I think I sensed this odious scenario coming...
I think I sensed this odious scenario coming 2 years ago during the bizarre presidential election, when I wrote an editorial describing the “fear and loathing” that permeated the political process and the unusual behavior of the candidates.1 A year after the election, I commented about the toxic zeitgeist of political extremism from a psychiatric perspective.2 The situation appears to be getting worse, and the folie en masse is intensifying and its hateful cacophony is deafening to our sensibilities.
Aaron Beck, MD, the father of cognitive-behavioral therapy (CBT), wrote a book about hate.3 It may be a fantasy, but I wish the leaders on both sides would agree to a course of CBT to recognize the destructive path of intransigent hyper-partisanship. They might then transcend their egocentric attitudes and inspire millions of their followers to communicate rationally, instead of stoking the fires of resentment and enmity toward the “other side.”
Let’s get back to science: Where are the pathways of hate located in the brain? An interesting study was conducted to detect the neural circuits that mediate hate.4 The researchers obtained functional magnetic resonance imaging scans of participants while they were viewing the face of a person they hate compared with the face of an acquaintance toward whom they have neutral feelings. They also calculated a “hate score” for each participant for the analysis. They found that viewing a hated person increased the activity in several brain regions, including the medial frontal gyrus, right putamen, premotor cortex, frontal pole, and medial insula bilaterally. The activation in 3 areas correlated with the intensity of the hatred: right insula, right premotor cortex, and right frontal-medial gyrus. At the same time, the right superior frontal gyrus showed deactivation. Interestingly, hate and romantic love shared activation in 2 areas: the putamen and insula. This suggests that passionate love and passionate hate are 2 sides of the same neural coin! It prompts me to wonder what happens to the capacity to love among political extremists when their putamen and insula are filled up with hate. It also makes me wonder if unbridled hatred can be “enjoyable” and even addictive, as passionate romantic love is.
The bottom line: Consider the brain changes that are occurring on a large scale in at least a hundred million political partisans, and whether those neural circuits get even more intensely activated following the elections, regardless of the outcome.
Finally, we must remain cognizant of the epigenetic consequences of emotions and stress.5 There is solid scientific evidence that extremes of human experiences can modify gene expression in sperm and fetuses, resulting in a transgenerational effect upon the children of the extreme partisans, and also the children of nonpartisan observers, who experience unmitigated anxiety due to the inescapable cloud of negative affect shrouding their daily lives.6 So politicians should be cognizant that perpetuating a bitter war against each other may be detrimental to their progeny and future generations. I am frankly worried about the epigenetically disrupted emotional stability of voters circa 2035, born in these days of unprecedented and tumultuous hatred by their hyper-partisan parents.
Henry A. Nasrallah, MD
Editor-in-Chief
1. Nasrallah HA. Fear and loathing abound in the ‘off-label’ presidential election of 2016. Current Psychiatry. 2016;15(7):21,26.
2. Nasrallah HA. The toxic zeitgeist of hyper-partisanship: a psychiatric perspective. Current Psychiatry. 2018;17(2):17-18.
3. Beck AT. Prisoners of hate: the cognitive basis of anger, hostility, and violence. New York, NY: Harper-Collins; 1999.
4. Zeki S, Romaya JP. Neural correlates of hate. PloS One. 2008;3(10):e3556. doi: 10.1371/journal.pone.0003556.
5. Griffiths BB, Hunter RG. Neuroepigenetics of stress. Neuroscience. 2014;275:420-435.
6. Bartlett AA, Singh R, Hunter RG. Anxiety and epigenetics. Adv Exp Med Biol.2017;978:145-166.
1. Nasrallah HA. Fear and loathing abound in the ‘off-label’ presidential election of 2016. Current Psychiatry. 2016;15(7):21,26.
2. Nasrallah HA. The toxic zeitgeist of hyper-partisanship: a psychiatric perspective. Current Psychiatry. 2018;17(2):17-18.
3. Beck AT. Prisoners of hate: the cognitive basis of anger, hostility, and violence. New York, NY: Harper-Collins; 1999.
4. Zeki S, Romaya JP. Neural correlates of hate. PloS One. 2008;3(10):e3556. doi: 10.1371/journal.pone.0003556.
5. Griffiths BB, Hunter RG. Neuroepigenetics of stress. Neuroscience. 2014;275:420-435.
6. Bartlett AA, Singh R, Hunter RG. Anxiety and epigenetics. Adv Exp Med Biol.2017;978:145-166.
Preventing brain damage in psychosis
I read with great interest Dr. Nasrallah’s editorial, “FAST and RAPID: Acronyms to prevent brain damage in stroke and psychosis” (From the Editor,
Mitchell L. Glaser, MD
Board-Certified Child/Adolescent and General Psychiatrist
Assistant Professor of Psychiatry
Rush University Medical Center
Chairman
Department of Psychiatry
Medical Director of Child/Adolescent Psychiatry
St. Mary/Elizabeth Medical Center
Clinical Assistant Professor of Psychiatry
Rosalind Franklin University
Chicago, Illinois
Thank you, Dr. Nasrallah, for your incisive thinking and for bringing our attention as psychiatrists to the crucial issues of our clinical practice. I’d like to offer some nuance on the RAPID acronym. First, I’d like to counterpropose DASH: Delusions, Auditory hallucinations, Strange behavior, Hospital now. This is more in line with getting physicians to tune in to the symptoms that should alarm them and bring them to action. I agree that neurodegeneration and illness recurrence are the problems to address. One unsettled issue remains: With early intervention, can we eventually taper patients off antipsychotics to spare them the metabolic and immune morbidity associated with these medications? There is some evidence that this is possible, but it is difficult to collect data. One of the factors delaying treatment, other than lack of recognition, is the general public’s belief that the treatment is sometimes worse than the disease. If we can address this issue in a nuanced fashion, we may get more “early adopters” of these neuron-sparing treatments.
Michael S. Diamond, MD
Private psychiatric practice
Chevy Chase, Maryland
Dr. Nasrallah is right to focus on brain injury patterns, including inflammation and de-myelination, during psychotic episodes. He and Dr. Roque note that starting a patient on a long-acting injectable antipsychotic as soon as possible may prevent subsequent relapse and further brain damage. However, their editorial omits 2 treatments—minocycline and clemastine—that can help stop CNS inflammation, reduce brain damage, and promote remyelination.
Minocycline has been shown to reduce stroke infarct penumbra size and improve outcomes in functional recovery from stroke.1,2 Minocycline’s effects as a potent CNS anti-inflammatory and antiapoptotic agent are well established.
Clemastine has been shown to improve function in multiple sclerosis by activating oligodendrocyte precursor cells into active agents of myelination and fiber bundle stabilization.3 Clemastine reverses acute leukoencephalopathy.4
If we are to treat acute psychosis as a neurologic emergency, we cannot rely on long-acting injectable antipsychotics as the sole treatment. Psychiatric medication alone is not sufficient across every neuropsychiatric condition in which inflammation and white matter damage are part of the etiology, destruction, and pattern of relapse.
The adverse effects risk of adjunctive minocycline and clemastine is low compared with the potential benefits of stopping inflammation, reducing apoptosis, and jump-starting white matter repair. Doses of oral minocycline in the 50- to 100-mg/d range and oral clemastine in the 1.34- to 2.68-mg/d range together can lead to reduced cranial heat, improved cranial suture mobility, and improved elasticity of white matter bundle tracts palpable on physical examination. Both medications show clinical results in improved emotional self-regulation, according to family reports and clinical observations in the outpatient setting. There is no reason to delay neurologic-based adjunctive treatment when our goal is to prevent and reverse brain damage.
Daniel Kerlinsky, MD
Child Psychiatrist
Clinical Assistant Professor
Burrell College of Osteopathic Medicine
Albuquerque, New Mexico
References
1. Hess DH, Fagan SC. Repurposing an old drug to improve the use and safety of tissue plasminogen activator for acute ischemic stroke: minocycline. Rev Neurol Dis. 2010;30(7 pt 2):55S-61S.
2. Vedantam S, Moller AR. Minocycline: a novel stroke therapy. J Neurol Stroke.
3. Green AJ, Gelfand JM, Cree BA, et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. Lancet. 2017;390(10111):2481-2489.
4. Cree BAC, Niu J, Hoi KK, et al. Clemastine rescues myelination defects and promotes functional recovery in hypoxic brain injury. Brain. 2018;141(1):85-98.
Continue to: Dr. Nasrallah responds
Dr. Nasrallah responds
Thanks to my colleagues, Drs. Diamond, Glaser, and Kerlinsky, for their cogent letters about my editorial.
To Dr. Glaser: The “ethics” of conducting placebo-controlled studies when developing a new antipsychotic has been raging for some time. For decades, the FDA has insisted on using a placebo group because around 25% to 30% of research participants respond to placebo, and because participants receiving placebo also complain of many adverse effects. So a new drug has to demonstrate a statistically higher efficacy than a placebo, and the adverse effect profile of the placebo group will put the safety and tolerability profile of a new drug in proper perspective. However, in Europe, they do not conduct placebo-controlled studies; instead, they conduct what is called a “non-inferiority” trial of a new antipsychotic compared with a well-established antipsychotic.
Interestingly, even though the discovery of the neurodegenerative effects of untreated psychosis was only 20 years ago (in 1997 after serial MRI scans revealed progressive atrophy), in the 1960s, the first antipsychotic, chlorpromazine, was compared with placebo in a large national study for 6 months. This study showed without a doubt that chlorpromazine has a higher efficacy than placebo. After the study was done, Dr. Philip May at University of California, Los Angeles looked at what happened to the psychotic patients who received placebo for 6 months and found that they became less responsive to treatment, were re-hospitalized more often, and had more negative symptoms and a poorer overall outcome. That was a clue that untreated psychosis can be harmful, and it supports your point about the ethics of using placebo. In contemporary studies, a trial of oral antipsychotics is 6 weeks, not 6 months. In the year-long, placebo-controlled studies of injectable antipsychotics in stable patients, those who show the slightest increase in delusions, hallucinations, or suicidal/homicidal behavior were promptly taken out of the study and treated. This reduced the “harm,” although not completely. Perhaps the FDA will change its policies and adopt the non-inferiority model. That’s what is done with nonpsychiatric disorders such as pneumonia, stroke, or diabetes. But one last fact has to be stated: The placebo response in anxiety, depression, or psychosis is much higher (25% to 35%) than the 1% placebo response in pneumonia.
To Dr. Diamond: I really like DASH, and it is an acronym for quick symptomatic diagnosis. Speedy treatment then follows with the acronym RAPID to prevent brain damage that gets worse with delay.
As for the second issue of tapering off the antipsychotic medication, the evidence is overwhelming in favor of continuous pharmacotherapy. Just as hypertension and diabetes will return if medications are tapered or stopped, so will psychosis, and vengefully so because treatment resistance increases with each relapse.1 This is also true for bipolar disorder recurrences.2 A recent 20-year follow-up study showed that stopping antipsychotic treatment is associated with a much higher mortality rate than continuation therapy.3 Another 7-year study showed the same thing.4 It is literally deadly, and not just neurodegenerative, for persons with schizophrenia to stop their medications.
To Dr. Kerlinsky: I agree with you about using certain adjunctive pharmacotherapies for acute psychosis, which is associated with neuroinflammation, oxidative stress, and neuropil and myelin damage. I support using agents with anti-inflammatory effects (such as minocycline and omega-3 fatty acid), antioxidant effects (such as N-acetylcysteine), and neuroprotective effects (such as minocycline, clemastine, lithium, vitamin D, erythropoietin, etc.). I refer you to my past editorial, “Are you neuroprotecting your patients? 10 Adjunctive therapies to consider,”5 in which I mentioned all the above. I also pointed out the many neuroprotective effects of atypical antipsychotics in another editorial.6 Although off-label, those supplements can be useful interventions that can ameliorate the gray and white matter damage associated with acute psychotic relapses in patients with schizophrenia.
Henry A. Nasrallah, MD
Editor-in-Chief
The Sydney W. Souers Endowed Chair
Professor and Chairman
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
References
1. Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
2. Post RM. Preventing the maligna
3. Tiihonen J, Tanskanen A, Taipale H. 20-year nationwide follow-up study on discontinuation of antipsychotic treatment in first-episode schizophrenia. Am J Psychiatry. 2018;175(8):765-773.
4. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
5. Nasrallah HA. Are you neuroprotecting your patients? 10 Adjunctive therapies to consider. Current Psychiatry. 2016;15(12):12-14.
6. Nasrallah HA. A decade after the CATIE study, the focus has shifted from effectiveness to neuroprotection. Current Psychiatry. 2015;14(2):19-21.
I read with great interest Dr. Nasrallah’s editorial, “FAST and RAPID: Acronyms to prevent brain damage in stroke and psychosis” (From the Editor,
Mitchell L. Glaser, MD
Board-Certified Child/Adolescent and General Psychiatrist
Assistant Professor of Psychiatry
Rush University Medical Center
Chairman
Department of Psychiatry
Medical Director of Child/Adolescent Psychiatry
St. Mary/Elizabeth Medical Center
Clinical Assistant Professor of Psychiatry
Rosalind Franklin University
Chicago, Illinois
Thank you, Dr. Nasrallah, for your incisive thinking and for bringing our attention as psychiatrists to the crucial issues of our clinical practice. I’d like to offer some nuance on the RAPID acronym. First, I’d like to counterpropose DASH: Delusions, Auditory hallucinations, Strange behavior, Hospital now. This is more in line with getting physicians to tune in to the symptoms that should alarm them and bring them to action. I agree that neurodegeneration and illness recurrence are the problems to address. One unsettled issue remains: With early intervention, can we eventually taper patients off antipsychotics to spare them the metabolic and immune morbidity associated with these medications? There is some evidence that this is possible, but it is difficult to collect data. One of the factors delaying treatment, other than lack of recognition, is the general public’s belief that the treatment is sometimes worse than the disease. If we can address this issue in a nuanced fashion, we may get more “early adopters” of these neuron-sparing treatments.
Michael S. Diamond, MD
Private psychiatric practice
Chevy Chase, Maryland
Dr. Nasrallah is right to focus on brain injury patterns, including inflammation and de-myelination, during psychotic episodes. He and Dr. Roque note that starting a patient on a long-acting injectable antipsychotic as soon as possible may prevent subsequent relapse and further brain damage. However, their editorial omits 2 treatments—minocycline and clemastine—that can help stop CNS inflammation, reduce brain damage, and promote remyelination.
Minocycline has been shown to reduce stroke infarct penumbra size and improve outcomes in functional recovery from stroke.1,2 Minocycline’s effects as a potent CNS anti-inflammatory and antiapoptotic agent are well established.
Clemastine has been shown to improve function in multiple sclerosis by activating oligodendrocyte precursor cells into active agents of myelination and fiber bundle stabilization.3 Clemastine reverses acute leukoencephalopathy.4
If we are to treat acute psychosis as a neurologic emergency, we cannot rely on long-acting injectable antipsychotics as the sole treatment. Psychiatric medication alone is not sufficient across every neuropsychiatric condition in which inflammation and white matter damage are part of the etiology, destruction, and pattern of relapse.
The adverse effects risk of adjunctive minocycline and clemastine is low compared with the potential benefits of stopping inflammation, reducing apoptosis, and jump-starting white matter repair. Doses of oral minocycline in the 50- to 100-mg/d range and oral clemastine in the 1.34- to 2.68-mg/d range together can lead to reduced cranial heat, improved cranial suture mobility, and improved elasticity of white matter bundle tracts palpable on physical examination. Both medications show clinical results in improved emotional self-regulation, according to family reports and clinical observations in the outpatient setting. There is no reason to delay neurologic-based adjunctive treatment when our goal is to prevent and reverse brain damage.
Daniel Kerlinsky, MD
Child Psychiatrist
Clinical Assistant Professor
Burrell College of Osteopathic Medicine
Albuquerque, New Mexico
References
1. Hess DH, Fagan SC. Repurposing an old drug to improve the use and safety of tissue plasminogen activator for acute ischemic stroke: minocycline. Rev Neurol Dis. 2010;30(7 pt 2):55S-61S.
2. Vedantam S, Moller AR. Minocycline: a novel stroke therapy. J Neurol Stroke.
3. Green AJ, Gelfand JM, Cree BA, et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. Lancet. 2017;390(10111):2481-2489.
4. Cree BAC, Niu J, Hoi KK, et al. Clemastine rescues myelination defects and promotes functional recovery in hypoxic brain injury. Brain. 2018;141(1):85-98.
Continue to: Dr. Nasrallah responds
Dr. Nasrallah responds
Thanks to my colleagues, Drs. Diamond, Glaser, and Kerlinsky, for their cogent letters about my editorial.
To Dr. Glaser: The “ethics” of conducting placebo-controlled studies when developing a new antipsychotic has been raging for some time. For decades, the FDA has insisted on using a placebo group because around 25% to 30% of research participants respond to placebo, and because participants receiving placebo also complain of many adverse effects. So a new drug has to demonstrate a statistically higher efficacy than a placebo, and the adverse effect profile of the placebo group will put the safety and tolerability profile of a new drug in proper perspective. However, in Europe, they do not conduct placebo-controlled studies; instead, they conduct what is called a “non-inferiority” trial of a new antipsychotic compared with a well-established antipsychotic.
Interestingly, even though the discovery of the neurodegenerative effects of untreated psychosis was only 20 years ago (in 1997 after serial MRI scans revealed progressive atrophy), in the 1960s, the first antipsychotic, chlorpromazine, was compared with placebo in a large national study for 6 months. This study showed without a doubt that chlorpromazine has a higher efficacy than placebo. After the study was done, Dr. Philip May at University of California, Los Angeles looked at what happened to the psychotic patients who received placebo for 6 months and found that they became less responsive to treatment, were re-hospitalized more often, and had more negative symptoms and a poorer overall outcome. That was a clue that untreated psychosis can be harmful, and it supports your point about the ethics of using placebo. In contemporary studies, a trial of oral antipsychotics is 6 weeks, not 6 months. In the year-long, placebo-controlled studies of injectable antipsychotics in stable patients, those who show the slightest increase in delusions, hallucinations, or suicidal/homicidal behavior were promptly taken out of the study and treated. This reduced the “harm,” although not completely. Perhaps the FDA will change its policies and adopt the non-inferiority model. That’s what is done with nonpsychiatric disorders such as pneumonia, stroke, or diabetes. But one last fact has to be stated: The placebo response in anxiety, depression, or psychosis is much higher (25% to 35%) than the 1% placebo response in pneumonia.
To Dr. Diamond: I really like DASH, and it is an acronym for quick symptomatic diagnosis. Speedy treatment then follows with the acronym RAPID to prevent brain damage that gets worse with delay.
As for the second issue of tapering off the antipsychotic medication, the evidence is overwhelming in favor of continuous pharmacotherapy. Just as hypertension and diabetes will return if medications are tapered or stopped, so will psychosis, and vengefully so because treatment resistance increases with each relapse.1 This is also true for bipolar disorder recurrences.2 A recent 20-year follow-up study showed that stopping antipsychotic treatment is associated with a much higher mortality rate than continuation therapy.3 Another 7-year study showed the same thing.4 It is literally deadly, and not just neurodegenerative, for persons with schizophrenia to stop their medications.
To Dr. Kerlinsky: I agree with you about using certain adjunctive pharmacotherapies for acute psychosis, which is associated with neuroinflammation, oxidative stress, and neuropil and myelin damage. I support using agents with anti-inflammatory effects (such as minocycline and omega-3 fatty acid), antioxidant effects (such as N-acetylcysteine), and neuroprotective effects (such as minocycline, clemastine, lithium, vitamin D, erythropoietin, etc.). I refer you to my past editorial, “Are you neuroprotecting your patients? 10 Adjunctive therapies to consider,”5 in which I mentioned all the above. I also pointed out the many neuroprotective effects of atypical antipsychotics in another editorial.6 Although off-label, those supplements can be useful interventions that can ameliorate the gray and white matter damage associated with acute psychotic relapses in patients with schizophrenia.
Henry A. Nasrallah, MD
Editor-in-Chief
The Sydney W. Souers Endowed Chair
Professor and Chairman
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
References
1. Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
2. Post RM. Preventing the maligna
3. Tiihonen J, Tanskanen A, Taipale H. 20-year nationwide follow-up study on discontinuation of antipsychotic treatment in first-episode schizophrenia. Am J Psychiatry. 2018;175(8):765-773.
4. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
5. Nasrallah HA. Are you neuroprotecting your patients? 10 Adjunctive therapies to consider. Current Psychiatry. 2016;15(12):12-14.
6. Nasrallah HA. A decade after the CATIE study, the focus has shifted from effectiveness to neuroprotection. Current Psychiatry. 2015;14(2):19-21.
I read with great interest Dr. Nasrallah’s editorial, “FAST and RAPID: Acronyms to prevent brain damage in stroke and psychosis” (From the Editor,
Mitchell L. Glaser, MD
Board-Certified Child/Adolescent and General Psychiatrist
Assistant Professor of Psychiatry
Rush University Medical Center
Chairman
Department of Psychiatry
Medical Director of Child/Adolescent Psychiatry
St. Mary/Elizabeth Medical Center
Clinical Assistant Professor of Psychiatry
Rosalind Franklin University
Chicago, Illinois
Thank you, Dr. Nasrallah, for your incisive thinking and for bringing our attention as psychiatrists to the crucial issues of our clinical practice. I’d like to offer some nuance on the RAPID acronym. First, I’d like to counterpropose DASH: Delusions, Auditory hallucinations, Strange behavior, Hospital now. This is more in line with getting physicians to tune in to the symptoms that should alarm them and bring them to action. I agree that neurodegeneration and illness recurrence are the problems to address. One unsettled issue remains: With early intervention, can we eventually taper patients off antipsychotics to spare them the metabolic and immune morbidity associated with these medications? There is some evidence that this is possible, but it is difficult to collect data. One of the factors delaying treatment, other than lack of recognition, is the general public’s belief that the treatment is sometimes worse than the disease. If we can address this issue in a nuanced fashion, we may get more “early adopters” of these neuron-sparing treatments.
Michael S. Diamond, MD
Private psychiatric practice
Chevy Chase, Maryland
Dr. Nasrallah is right to focus on brain injury patterns, including inflammation and de-myelination, during psychotic episodes. He and Dr. Roque note that starting a patient on a long-acting injectable antipsychotic as soon as possible may prevent subsequent relapse and further brain damage. However, their editorial omits 2 treatments—minocycline and clemastine—that can help stop CNS inflammation, reduce brain damage, and promote remyelination.
Minocycline has been shown to reduce stroke infarct penumbra size and improve outcomes in functional recovery from stroke.1,2 Minocycline’s effects as a potent CNS anti-inflammatory and antiapoptotic agent are well established.
Clemastine has been shown to improve function in multiple sclerosis by activating oligodendrocyte precursor cells into active agents of myelination and fiber bundle stabilization.3 Clemastine reverses acute leukoencephalopathy.4
If we are to treat acute psychosis as a neurologic emergency, we cannot rely on long-acting injectable antipsychotics as the sole treatment. Psychiatric medication alone is not sufficient across every neuropsychiatric condition in which inflammation and white matter damage are part of the etiology, destruction, and pattern of relapse.
The adverse effects risk of adjunctive minocycline and clemastine is low compared with the potential benefits of stopping inflammation, reducing apoptosis, and jump-starting white matter repair. Doses of oral minocycline in the 50- to 100-mg/d range and oral clemastine in the 1.34- to 2.68-mg/d range together can lead to reduced cranial heat, improved cranial suture mobility, and improved elasticity of white matter bundle tracts palpable on physical examination. Both medications show clinical results in improved emotional self-regulation, according to family reports and clinical observations in the outpatient setting. There is no reason to delay neurologic-based adjunctive treatment when our goal is to prevent and reverse brain damage.
Daniel Kerlinsky, MD
Child Psychiatrist
Clinical Assistant Professor
Burrell College of Osteopathic Medicine
Albuquerque, New Mexico
References
1. Hess DH, Fagan SC. Repurposing an old drug to improve the use and safety of tissue plasminogen activator for acute ischemic stroke: minocycline. Rev Neurol Dis. 2010;30(7 pt 2):55S-61S.
2. Vedantam S, Moller AR. Minocycline: a novel stroke therapy. J Neurol Stroke.
3. Green AJ, Gelfand JM, Cree BA, et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. Lancet. 2017;390(10111):2481-2489.
4. Cree BAC, Niu J, Hoi KK, et al. Clemastine rescues myelination defects and promotes functional recovery in hypoxic brain injury. Brain. 2018;141(1):85-98.
Continue to: Dr. Nasrallah responds
Dr. Nasrallah responds
Thanks to my colleagues, Drs. Diamond, Glaser, and Kerlinsky, for their cogent letters about my editorial.
To Dr. Glaser: The “ethics” of conducting placebo-controlled studies when developing a new antipsychotic has been raging for some time. For decades, the FDA has insisted on using a placebo group because around 25% to 30% of research participants respond to placebo, and because participants receiving placebo also complain of many adverse effects. So a new drug has to demonstrate a statistically higher efficacy than a placebo, and the adverse effect profile of the placebo group will put the safety and tolerability profile of a new drug in proper perspective. However, in Europe, they do not conduct placebo-controlled studies; instead, they conduct what is called a “non-inferiority” trial of a new antipsychotic compared with a well-established antipsychotic.
Interestingly, even though the discovery of the neurodegenerative effects of untreated psychosis was only 20 years ago (in 1997 after serial MRI scans revealed progressive atrophy), in the 1960s, the first antipsychotic, chlorpromazine, was compared with placebo in a large national study for 6 months. This study showed without a doubt that chlorpromazine has a higher efficacy than placebo. After the study was done, Dr. Philip May at University of California, Los Angeles looked at what happened to the psychotic patients who received placebo for 6 months and found that they became less responsive to treatment, were re-hospitalized more often, and had more negative symptoms and a poorer overall outcome. That was a clue that untreated psychosis can be harmful, and it supports your point about the ethics of using placebo. In contemporary studies, a trial of oral antipsychotics is 6 weeks, not 6 months. In the year-long, placebo-controlled studies of injectable antipsychotics in stable patients, those who show the slightest increase in delusions, hallucinations, or suicidal/homicidal behavior were promptly taken out of the study and treated. This reduced the “harm,” although not completely. Perhaps the FDA will change its policies and adopt the non-inferiority model. That’s what is done with nonpsychiatric disorders such as pneumonia, stroke, or diabetes. But one last fact has to be stated: The placebo response in anxiety, depression, or psychosis is much higher (25% to 35%) than the 1% placebo response in pneumonia.
To Dr. Diamond: I really like DASH, and it is an acronym for quick symptomatic diagnosis. Speedy treatment then follows with the acronym RAPID to prevent brain damage that gets worse with delay.
As for the second issue of tapering off the antipsychotic medication, the evidence is overwhelming in favor of continuous pharmacotherapy. Just as hypertension and diabetes will return if medications are tapered or stopped, so will psychosis, and vengefully so because treatment resistance increases with each relapse.1 This is also true for bipolar disorder recurrences.2 A recent 20-year follow-up study showed that stopping antipsychotic treatment is associated with a much higher mortality rate than continuation therapy.3 Another 7-year study showed the same thing.4 It is literally deadly, and not just neurodegenerative, for persons with schizophrenia to stop their medications.
To Dr. Kerlinsky: I agree with you about using certain adjunctive pharmacotherapies for acute psychosis, which is associated with neuroinflammation, oxidative stress, and neuropil and myelin damage. I support using agents with anti-inflammatory effects (such as minocycline and omega-3 fatty acid), antioxidant effects (such as N-acetylcysteine), and neuroprotective effects (such as minocycline, clemastine, lithium, vitamin D, erythropoietin, etc.). I refer you to my past editorial, “Are you neuroprotecting your patients? 10 Adjunctive therapies to consider,”5 in which I mentioned all the above. I also pointed out the many neuroprotective effects of atypical antipsychotics in another editorial.6 Although off-label, those supplements can be useful interventions that can ameliorate the gray and white matter damage associated with acute psychotic relapses in patients with schizophrenia.
Henry A. Nasrallah, MD
Editor-in-Chief
The Sydney W. Souers Endowed Chair
Professor and Chairman
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
References
1. Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
2. Post RM. Preventing the maligna
3. Tiihonen J, Tanskanen A, Taipale H. 20-year nationwide follow-up study on discontinuation of antipsychotic treatment in first-episode schizophrenia. Am J Psychiatry. 2018;175(8):765-773.
4. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
5. Nasrallah HA. Are you neuroprotecting your patients? 10 Adjunctive therapies to consider. Current Psychiatry. 2016;15(12):12-14.
6. Nasrallah HA. A decade after the CATIE study, the focus has shifted from effectiveness to neuroprotection. Current Psychiatry. 2015;14(2):19-21.
Protein binding changes and drug interactions: What do we know?
Mr. S, age 47, weighs 209 lb and has a history of seizure disorder, bipolar disorder not otherwise specified, hypertension, and type 2 diabetes mellitus. He presents to the emergency department after not taking his medications for 2 days while on vacation. He has increased energy, decreased sleep, and pressured speech, and insists on walking for up to 10 hours per day “in preparation for a marathon,” even though he has a 4-cm foot ulcer. His family reports that he had been compliant with his medications until the present incident.
Mr. S has no known drug allergies. His medications include oral divalproex sodium delayed release (valproic acid [VPA]), 1,000 mg twice a day, oral lisinopril, 20 mg every morning, and insulin glargine, 22 units subcutaneously every evening.
A complete blood count, basic metabolic panel, creatine kinase level, VPA level, and urine drug screen are ordered. Relevant results include a serum creatinine level of 1.4 mg/dL (normal range: 0.6 to 1.2 mg/dL), a glucose serum level of 188 mg/dL (normal range: 70 to 100 mg/dL), and a VPA level of 23 mcg/mL (therapeutic range: 50 to 125 mcg/mL). A liver function panel is within normal limits: albumin level of 3.9 g/dL, aspartate aminotransferase level of 18 IU/L, and alanine aminotransferase level of 14 IU/L. In light of Mr. S’s seizure history, neurology is consulted and the decision is made to continue treating him with VPA because he has been seizure-free for 4.5 years and this medication has also helped with his bipolar disorder.
Mr. S is admitted to the hospital and his home medications are resumed at the current doses. On hospital Day 3, Mr. S’s VPA level is 62 mcg/mL, his obsession with a marathon has remitted, and his sleep pattern has normalized. Infectious disease and podiatry services are consulted for his diabetic foot infection, which has ulcerated down to the bone. IV ertapenem, 1,000 mg/d, is initiated with plans for debridement the following week. Two days later, Mr. S has a witnessed seizure; his VPA level is 9 mcg/mL.
A common question asked of pharmacists is, “Will protein binding changes affect drug dosages?” In this article, I describe how protein binding changes may occur, and the complexity of the dynamic. Being highly bound to a protein typically does not mean all medications will interact, but some interactions can be important. This article does not cover medications that bind to hormones.
Why is protein binding important? When a medication is bound to plasma protein, it is not free to act. There can be a delay in therapeutic effect (because no drug is available to react), delayed elimination, or possibly displacement of another protein-bound medication. Additionally, medications tend not to cross the blood-brain barrier or be eliminated when bound. For example, if a drug is 99% bound (leaving 1% free) and displacement now leaves 2% of the drug free, this event has doubled the amount of free drug. As the unbound medication is eliminated, the drug that is bound to the protein can act as a reservoir. A dynamic relationship exists between bound drug, unbound drug, and rate of elimination.
Which proteins do drugs commonly bind to? The proteins often associated with binding include albumin, alpha-1-acid glycoprotein (AAG), and lipoproteins. Albumin comprises 60% of total plasma protein in the plasma. Lipoproteins include very high-density lipoprotein (VHDL), high-density lipoprotein (HDL), very low-density lipoprotein (VLDL), and low-density lipoprotein (LDL).1 Medications that bind to lipoproteins include cyclosporine, tacrolimus, and propofol.2
Continued to: What common disease states can cause hypoalbuminemia?
What common disease states can cause hypoalbuminemia? Many disease states can result in low albumin levels. The most common ones are malnutrition, malignancies, stress, injury, burns, pregnancy, and diabetes.3 When there is less albumin to bind to, free drug levels may be increased.
Can AAG levels change with disease states as well? Because AAG accounts for a lower percentage of total plasma protein than albumin, there may be less clinical concern regarding AAG. AAG levels usually do not drop, but instead can become elevated during times of trauma, inflammation, and acute myocardial infarction. This could result in increased binding of the free drug.4Which medications bind to red blood cells (RBCs)? There are several locations for drugs to bind to RBCs, including to hemoglobin and the plasma membrane. Medications that commonly bind to RBCs include barbiturates, chlorpromazine, imipramine, and phenytoin.5
What are common highly-bound medications? The Table1 provides examples of medications that are >90% protein-bound. However, this information may be misleading because many medications are highly bound. Zhang et al1 compiled binding data for 222 drugs, half of which bind 90% to 100%. However, the literature does not indicate that they all have clinically significant interactions. Benet and Hoener6 discuss how factors other than protein binding affect potential drug interactions, and the complexity of the body’s ability to compensate for increased free drug. Medication characteristics that may contribute to producing a significant interaction include, but are not limited to:
- free vs protein-bound drug in the plasma or tissue
- volume of distribution
- organs affected
- hepatic bioavailability
- drug clearance.
For example, VPA is 93% protein-bound and phenytoin is 91% protein-bound.1 However, this interaction is affected by more than just protein binding. VPA not only displaces the protein-bound phenytoin, but also inhibits its metabolism, which together result in increased free phenytoin levels.
Continued to: Another area of concern is a critically ill patient...
Another area of concern is a critically ill patient who has a change in his or her pH. Medications that are highly bound and have high clearance rates may be affected. This is of particular concern when prescribing antibiotics that are time-dependent, such as beta-lactams.3
What happened to Mr. S? Mr. S likely experienced a drug–drug interaction that resulted in a subtherapeutic VPA level and subsequent seizure. Case reports have shown evidence that the carbapenem class of antibiotics, which includes ertapenem, interacts with VPA.7 Proposed mechanisms include a lowering of VPA serum levels due to a redistribution of the VPA onto the RBCs due to carbapenem. Other theories include the possibility that carbapenems may limit oral VPA absorption, decrease VPA enterohepatic recirculation, and increase VPA metabolism.7 Using VPA and ertapenem together is discouraged because seizures have been reported among patients receiving this combination. If it is medically necessary to administer VPA and ertapenem, closely monitor VPA levels. In Mr. S’s case, another broad-spectrum antibiotic, such as piperacillin-tazobactam, could have been used, for his diabetic foot infection.
While many medications may have high protein binding, there are few clinically important known interactions. However, our understanding of the relationship between protein binding and drug interactions may improve with additional research.
CASE CONTINUED
Under neurology’s care, lacosamide is added for treatment of Mr. S’s seizures. No more seizures are noted during the remainder of his hospitalization. Infectious disease services change his antibiotic to piperacillin-tazobactam. Mr. S continues to progress well and is discharged to a rehabilitation center 2 days later.
Related Resource
- DrugBank. www.drugbank.ca. Canadian Institutes of Health Research.
Drug Brand Names
Amiodarone • Cordarone, Pacerone
Bumetanide • Bumex
Bupivacaine • Marcaine, Sensorcaine
Buprenorphine • Belbuca, Subutex
Ceftriaxone • Rocephin
Chlordiazepoxide • Librium
Chlorpromazine • Thorazine
Clozapine • Clozaril
Cyclosporine • Gengraf, Neoral
Diazepam • Valium
Doxycycline • Acticlate, Doryx
Duloxetine • Cymbalta
Ertapenem • Invanz
Fluoxetine • Prozac, Sarafem
Furosemide • Lasix
Glargine (Insulin) • Lantus, Toujeo
Glipizide • Glucotrol
Haloperidol • Haldol
Ibuprofen • Advil, Motrin
Imipramine • Tofranil
Lacosamide • Vimpat
Lisinopril • Prinivil, Zestril
Lorazepam • Ativan
Nicardipine • Cardene
Nortriptyline • Pamelor
Paclitaxel • Abraxane, Taxol
Phenytoin • Dilantin, Phenytek
Piperacillin-tazobactam • Zosyn
Propofol • Diprivan
Sertraline • Zoloft
Tacrolimus • Prograf
Tamoxifen • Soltamox
Valproic acid • Depakene, Depakote
Verapamil • Calan, Verelan
Warfarin • Coumadin, Jantoven
1. Zhang F, Xue J, Shao J, et al. Compilation of 222 drugs’ plasma protein binding data and guidance for study designs. Drug Discov Today. 2012;17(9-10):475-485.
2. Mehvar R. Role of protein binding in pharmacokinetics. Am J Pharm Edu. 2005;69(5): Article 103;1-8.
3. Roberts JA, Pea F, Lipman J. The clinical relevance of plasma protein binding changes. Clin Pharmacokinet. 2013;52(1):1-8.
4. Schmidt S, Gonzalez D, Derendork H. Significance of protein binding in pharmacokinetics and pharmacodynamics. J Pharm Sci. 2010;99(3):1107-1122.
5. Hinderling P. Red blood cells: a neglected compartment in pharmacokinetics and pharmacodynamics. Pharmacol Rev. 1997;49(3):279-295.
6. Benet LZ, Hoener B. Changes in plasma protein binding have little clinical relevance. Clin Pharmacol Ther. 2002;71(3):115-121.
7. Park MK, Lim KS, Kim T, et al. Reduced valproic acid serum concentrations due to drug interactions with carbapenem antibiotics: overview of 6 cases. Ther Drug Monit. 2012;34(5):599-603.
Mr. S, age 47, weighs 209 lb and has a history of seizure disorder, bipolar disorder not otherwise specified, hypertension, and type 2 diabetes mellitus. He presents to the emergency department after not taking his medications for 2 days while on vacation. He has increased energy, decreased sleep, and pressured speech, and insists on walking for up to 10 hours per day “in preparation for a marathon,” even though he has a 4-cm foot ulcer. His family reports that he had been compliant with his medications until the present incident.
Mr. S has no known drug allergies. His medications include oral divalproex sodium delayed release (valproic acid [VPA]), 1,000 mg twice a day, oral lisinopril, 20 mg every morning, and insulin glargine, 22 units subcutaneously every evening.
A complete blood count, basic metabolic panel, creatine kinase level, VPA level, and urine drug screen are ordered. Relevant results include a serum creatinine level of 1.4 mg/dL (normal range: 0.6 to 1.2 mg/dL), a glucose serum level of 188 mg/dL (normal range: 70 to 100 mg/dL), and a VPA level of 23 mcg/mL (therapeutic range: 50 to 125 mcg/mL). A liver function panel is within normal limits: albumin level of 3.9 g/dL, aspartate aminotransferase level of 18 IU/L, and alanine aminotransferase level of 14 IU/L. In light of Mr. S’s seizure history, neurology is consulted and the decision is made to continue treating him with VPA because he has been seizure-free for 4.5 years and this medication has also helped with his bipolar disorder.
Mr. S is admitted to the hospital and his home medications are resumed at the current doses. On hospital Day 3, Mr. S’s VPA level is 62 mcg/mL, his obsession with a marathon has remitted, and his sleep pattern has normalized. Infectious disease and podiatry services are consulted for his diabetic foot infection, which has ulcerated down to the bone. IV ertapenem, 1,000 mg/d, is initiated with plans for debridement the following week. Two days later, Mr. S has a witnessed seizure; his VPA level is 9 mcg/mL.

A common question asked of pharmacists is, “Will protein binding changes affect drug dosages?” In this article, I describe how protein binding changes may occur, and the complexity of the dynamic. Being highly bound to a protein typically does not mean all medications will interact, but some interactions can be important. This article does not cover medications that bind to hormones.
Why is protein binding important? When a medication is bound to plasma protein, it is not free to act. There can be a delay in therapeutic effect (because no drug is available to react), delayed elimination, or possibly displacement of another protein-bound medication. Additionally, medications tend not to cross the blood-brain barrier or be eliminated when bound. For example, if a drug is 99% bound (leaving 1% free) and displacement now leaves 2% of the drug free, this event has doubled the amount of free drug. As the unbound medication is eliminated, the drug that is bound to the protein can act as a reservoir. A dynamic relationship exists between bound drug, unbound drug, and rate of elimination.
Which proteins do drugs commonly bind to? The proteins often associated with binding include albumin, alpha-1-acid glycoprotein (AAG), and lipoproteins. Albumin comprises 60% of total plasma protein in the plasma. Lipoproteins include very high-density lipoprotein (VHDL), high-density lipoprotein (HDL), very low-density lipoprotein (VLDL), and low-density lipoprotein (LDL).1 Medications that bind to lipoproteins include cyclosporine, tacrolimus, and propofol.2
Continued to: What common disease states can cause hypoalbuminemia?
What common disease states can cause hypoalbuminemia? Many disease states can result in low albumin levels. The most common ones are malnutrition, malignancies, stress, injury, burns, pregnancy, and diabetes.3 When there is less albumin to bind to, free drug levels may be increased.
Can AAG levels change with disease states as well? Because AAG accounts for a lower percentage of total plasma protein than albumin, there may be less clinical concern regarding AAG. AAG levels usually do not drop, but instead can become elevated during times of trauma, inflammation, and acute myocardial infarction. This could result in increased binding of the free drug.4Which medications bind to red blood cells (RBCs)? There are several locations for drugs to bind to RBCs, including to hemoglobin and the plasma membrane. Medications that commonly bind to RBCs include barbiturates, chlorpromazine, imipramine, and phenytoin.5
What are common highly-bound medications? The Table1 provides examples of medications that are >90% protein-bound. However, this information may be misleading because many medications are highly bound. Zhang et al1 compiled binding data for 222 drugs, half of which bind 90% to 100%. However, the literature does not indicate that they all have clinically significant interactions. Benet and Hoener6 discuss how factors other than protein binding affect potential drug interactions, and the complexity of the body’s ability to compensate for increased free drug. Medication characteristics that may contribute to producing a significant interaction include, but are not limited to:
- free vs protein-bound drug in the plasma or tissue
- volume of distribution
- organs affected
- hepatic bioavailability
- drug clearance.

For example, VPA is 93% protein-bound and phenytoin is 91% protein-bound.1 However, this interaction is affected by more than just protein binding. VPA not only displaces the protein-bound phenytoin, but also inhibits its metabolism, which together result in increased free phenytoin levels.
Continued to: Another area of concern is a critically ill patient...
Another area of concern is a critically ill patient who has a change in his or her pH. Medications that are highly bound and have high clearance rates may be affected. This is of particular concern when prescribing antibiotics that are time-dependent, such as beta-lactams.3
What happened to Mr. S? Mr. S likely experienced a drug–drug interaction that resulted in a subtherapeutic VPA level and subsequent seizure. Case reports have shown evidence that the carbapenem class of antibiotics, which includes ertapenem, interacts with VPA.7 Proposed mechanisms include a lowering of VPA serum levels due to a redistribution of the VPA onto the RBCs due to carbapenem. Other theories include the possibility that carbapenems may limit oral VPA absorption, decrease VPA enterohepatic recirculation, and increase VPA metabolism.7 Using VPA and ertapenem together is discouraged because seizures have been reported among patients receiving this combination. If it is medically necessary to administer VPA and ertapenem, closely monitor VPA levels. In Mr. S’s case, another broad-spectrum antibiotic, such as piperacillin-tazobactam, could have been used, for his diabetic foot infection.
While many medications may have high protein binding, there are few clinically important known interactions. However, our understanding of the relationship between protein binding and drug interactions may improve with additional research.
CASE CONTINUED
Under neurology’s care, lacosamide is added for treatment of Mr. S’s seizures. No more seizures are noted during the remainder of his hospitalization. Infectious disease services change his antibiotic to piperacillin-tazobactam. Mr. S continues to progress well and is discharged to a rehabilitation center 2 days later.
Related Resource
- DrugBank. www.drugbank.ca. Canadian Institutes of Health Research.
Drug Brand Names
Amiodarone • Cordarone, Pacerone
Bumetanide • Bumex
Bupivacaine • Marcaine, Sensorcaine
Buprenorphine • Belbuca, Subutex
Ceftriaxone • Rocephin
Chlordiazepoxide • Librium
Chlorpromazine • Thorazine
Clozapine • Clozaril
Cyclosporine • Gengraf, Neoral
Diazepam • Valium
Doxycycline • Acticlate, Doryx
Duloxetine • Cymbalta
Ertapenem • Invanz
Fluoxetine • Prozac, Sarafem
Furosemide • Lasix
Glargine (Insulin) • Lantus, Toujeo
Glipizide • Glucotrol
Haloperidol • Haldol
Ibuprofen • Advil, Motrin
Imipramine • Tofranil
Lacosamide • Vimpat
Lisinopril • Prinivil, Zestril
Lorazepam • Ativan
Nicardipine • Cardene
Nortriptyline • Pamelor
Paclitaxel • Abraxane, Taxol
Phenytoin • Dilantin, Phenytek
Piperacillin-tazobactam • Zosyn
Propofol • Diprivan
Sertraline • Zoloft
Tacrolimus • Prograf
Tamoxifen • Soltamox
Valproic acid • Depakene, Depakote
Verapamil • Calan, Verelan
Warfarin • Coumadin, Jantoven
Mr. S, age 47, weighs 209 lb and has a history of seizure disorder, bipolar disorder not otherwise specified, hypertension, and type 2 diabetes mellitus. He presents to the emergency department after not taking his medications for 2 days while on vacation. He has increased energy, decreased sleep, and pressured speech, and insists on walking for up to 10 hours per day “in preparation for a marathon,” even though he has a 4-cm foot ulcer. His family reports that he had been compliant with his medications until the present incident.
Mr. S has no known drug allergies. His medications include oral divalproex sodium delayed release (valproic acid [VPA]), 1,000 mg twice a day, oral lisinopril, 20 mg every morning, and insulin glargine, 22 units subcutaneously every evening.
A complete blood count, basic metabolic panel, creatine kinase level, VPA level, and urine drug screen are ordered. Relevant results include a serum creatinine level of 1.4 mg/dL (normal range: 0.6 to 1.2 mg/dL), a glucose serum level of 188 mg/dL (normal range: 70 to 100 mg/dL), and a VPA level of 23 mcg/mL (therapeutic range: 50 to 125 mcg/mL). A liver function panel is within normal limits: albumin level of 3.9 g/dL, aspartate aminotransferase level of 18 IU/L, and alanine aminotransferase level of 14 IU/L. In light of Mr. S’s seizure history, neurology is consulted and the decision is made to continue treating him with VPA because he has been seizure-free for 4.5 years and this medication has also helped with his bipolar disorder.
Mr. S is admitted to the hospital and his home medications are resumed at the current doses. On hospital Day 3, Mr. S’s VPA level is 62 mcg/mL, his obsession with a marathon has remitted, and his sleep pattern has normalized. Infectious disease and podiatry services are consulted for his diabetic foot infection, which has ulcerated down to the bone. IV ertapenem, 1,000 mg/d, is initiated with plans for debridement the following week. Two days later, Mr. S has a witnessed seizure; his VPA level is 9 mcg/mL.

A common question asked of pharmacists is, “Will protein binding changes affect drug dosages?” In this article, I describe how protein binding changes may occur, and the complexity of the dynamic. Being highly bound to a protein typically does not mean all medications will interact, but some interactions can be important. This article does not cover medications that bind to hormones.
Why is protein binding important? When a medication is bound to plasma protein, it is not free to act. There can be a delay in therapeutic effect (because no drug is available to react), delayed elimination, or possibly displacement of another protein-bound medication. Additionally, medications tend not to cross the blood-brain barrier or be eliminated when bound. For example, if a drug is 99% bound (leaving 1% free) and displacement now leaves 2% of the drug free, this event has doubled the amount of free drug. As the unbound medication is eliminated, the drug that is bound to the protein can act as a reservoir. A dynamic relationship exists between bound drug, unbound drug, and rate of elimination.
Which proteins do drugs commonly bind to? The proteins often associated with binding include albumin, alpha-1-acid glycoprotein (AAG), and lipoproteins. Albumin comprises 60% of total plasma protein in the plasma. Lipoproteins include very high-density lipoprotein (VHDL), high-density lipoprotein (HDL), very low-density lipoprotein (VLDL), and low-density lipoprotein (LDL).1 Medications that bind to lipoproteins include cyclosporine, tacrolimus, and propofol.2
Continued to: What common disease states can cause hypoalbuminemia?
What common disease states can cause hypoalbuminemia? Many disease states can result in low albumin levels. The most common ones are malnutrition, malignancies, stress, injury, burns, pregnancy, and diabetes.3 When there is less albumin to bind to, free drug levels may be increased.
Can AAG levels change with disease states as well? Because AAG accounts for a lower percentage of total plasma protein than albumin, there may be less clinical concern regarding AAG. AAG levels usually do not drop, but instead can become elevated during times of trauma, inflammation, and acute myocardial infarction. This could result in increased binding of the free drug.4Which medications bind to red blood cells (RBCs)? There are several locations for drugs to bind to RBCs, including to hemoglobin and the plasma membrane. Medications that commonly bind to RBCs include barbiturates, chlorpromazine, imipramine, and phenytoin.5
What are common highly-bound medications? The Table1 provides examples of medications that are >90% protein-bound. However, this information may be misleading because many medications are highly bound. Zhang et al1 compiled binding data for 222 drugs, half of which bind 90% to 100%. However, the literature does not indicate that they all have clinically significant interactions. Benet and Hoener6 discuss how factors other than protein binding affect potential drug interactions, and the complexity of the body’s ability to compensate for increased free drug. Medication characteristics that may contribute to producing a significant interaction include, but are not limited to:
- free vs protein-bound drug in the plasma or tissue
- volume of distribution
- organs affected
- hepatic bioavailability
- drug clearance.

For example, VPA is 93% protein-bound and phenytoin is 91% protein-bound.1 However, this interaction is affected by more than just protein binding. VPA not only displaces the protein-bound phenytoin, but also inhibits its metabolism, which together result in increased free phenytoin levels.
Continued to: Another area of concern is a critically ill patient...
Another area of concern is a critically ill patient who has a change in his or her pH. Medications that are highly bound and have high clearance rates may be affected. This is of particular concern when prescribing antibiotics that are time-dependent, such as beta-lactams.3
What happened to Mr. S? Mr. S likely experienced a drug–drug interaction that resulted in a subtherapeutic VPA level and subsequent seizure. Case reports have shown evidence that the carbapenem class of antibiotics, which includes ertapenem, interacts with VPA.7 Proposed mechanisms include a lowering of VPA serum levels due to a redistribution of the VPA onto the RBCs due to carbapenem. Other theories include the possibility that carbapenems may limit oral VPA absorption, decrease VPA enterohepatic recirculation, and increase VPA metabolism.7 Using VPA and ertapenem together is discouraged because seizures have been reported among patients receiving this combination. If it is medically necessary to administer VPA and ertapenem, closely monitor VPA levels. In Mr. S’s case, another broad-spectrum antibiotic, such as piperacillin-tazobactam, could have been used, for his diabetic foot infection.
While many medications may have high protein binding, there are few clinically important known interactions. However, our understanding of the relationship between protein binding and drug interactions may improve with additional research.
CASE CONTINUED
Under neurology’s care, lacosamide is added for treatment of Mr. S’s seizures. No more seizures are noted during the remainder of his hospitalization. Infectious disease services change his antibiotic to piperacillin-tazobactam. Mr. S continues to progress well and is discharged to a rehabilitation center 2 days later.
Related Resource
- DrugBank. www.drugbank.ca. Canadian Institutes of Health Research.
Drug Brand Names
Amiodarone • Cordarone, Pacerone
Bumetanide • Bumex
Bupivacaine • Marcaine, Sensorcaine
Buprenorphine • Belbuca, Subutex
Ceftriaxone • Rocephin
Chlordiazepoxide • Librium
Chlorpromazine • Thorazine
Clozapine • Clozaril
Cyclosporine • Gengraf, Neoral
Diazepam • Valium
Doxycycline • Acticlate, Doryx
Duloxetine • Cymbalta
Ertapenem • Invanz
Fluoxetine • Prozac, Sarafem
Furosemide • Lasix
Glargine (Insulin) • Lantus, Toujeo
Glipizide • Glucotrol
Haloperidol • Haldol
Ibuprofen • Advil, Motrin
Imipramine • Tofranil
Lacosamide • Vimpat
Lisinopril • Prinivil, Zestril
Lorazepam • Ativan
Nicardipine • Cardene
Nortriptyline • Pamelor
Paclitaxel • Abraxane, Taxol
Phenytoin • Dilantin, Phenytek
Piperacillin-tazobactam • Zosyn
Propofol • Diprivan
Sertraline • Zoloft
Tacrolimus • Prograf
Tamoxifen • Soltamox
Valproic acid • Depakene, Depakote
Verapamil • Calan, Verelan
Warfarin • Coumadin, Jantoven
1. Zhang F, Xue J, Shao J, et al. Compilation of 222 drugs’ plasma protein binding data and guidance for study designs. Drug Discov Today. 2012;17(9-10):475-485.
2. Mehvar R. Role of protein binding in pharmacokinetics. Am J Pharm Edu. 2005;69(5): Article 103;1-8.
3. Roberts JA, Pea F, Lipman J. The clinical relevance of plasma protein binding changes. Clin Pharmacokinet. 2013;52(1):1-8.
4. Schmidt S, Gonzalez D, Derendork H. Significance of protein binding in pharmacokinetics and pharmacodynamics. J Pharm Sci. 2010;99(3):1107-1122.
5. Hinderling P. Red blood cells: a neglected compartment in pharmacokinetics and pharmacodynamics. Pharmacol Rev. 1997;49(3):279-295.
6. Benet LZ, Hoener B. Changes in plasma protein binding have little clinical relevance. Clin Pharmacol Ther. 2002;71(3):115-121.
7. Park MK, Lim KS, Kim T, et al. Reduced valproic acid serum concentrations due to drug interactions with carbapenem antibiotics: overview of 6 cases. Ther Drug Monit. 2012;34(5):599-603.
1. Zhang F, Xue J, Shao J, et al. Compilation of 222 drugs’ plasma protein binding data and guidance for study designs. Drug Discov Today. 2012;17(9-10):475-485.
2. Mehvar R. Role of protein binding in pharmacokinetics. Am J Pharm Edu. 2005;69(5): Article 103;1-8.
3. Roberts JA, Pea F, Lipman J. The clinical relevance of plasma protein binding changes. Clin Pharmacokinet. 2013;52(1):1-8.
4. Schmidt S, Gonzalez D, Derendork H. Significance of protein binding in pharmacokinetics and pharmacodynamics. J Pharm Sci. 2010;99(3):1107-1122.
5. Hinderling P. Red blood cells: a neglected compartment in pharmacokinetics and pharmacodynamics. Pharmacol Rev. 1997;49(3):279-295.
6. Benet LZ, Hoener B. Changes in plasma protein binding have little clinical relevance. Clin Pharmacol Ther. 2002;71(3):115-121.
7. Park MK, Lim KS, Kim T, et al. Reduced valproic acid serum concentrations due to drug interactions with carbapenem antibiotics: overview of 6 cases. Ther Drug Monit. 2012;34(5):599-603.
Unrelenting depression: ‘I would rather be dead than feel this way’
CASE Suicidal ideation, flare-up of ulcerative colitis
Mr. J, age 56, who has a history of major depressive disorder (MDD), generalized anxiety disorder (GAD), and ulcerative colitis (UC), presents to the emergency department (ED) with suicidal ideation and a plan to overdose on his medications. He reports no current emotional or financial stressors in his personal life. Home medications documented at the time of his arrival to the ED include sertraline, 100 mg/d, bupropion, 150 mg/d, buspirone, 10 mg 3 times daily, diazepam 10 mg 3 times daily, as needed, adalimumab, 40 mg IM every 2 weeks, and diphenhydramine, 50 mg every night.
A recent flare-up of UC resulted in Mr. J being placed on a 15-week prednisone taper, beginning at 80 mg/d and decreasing by 5 mg weekly, which was completed 2 weeks before he presented to the ED. After completing the prednisone taper, Mr. J went to his primary care physician (PCP) on 3 separate occasions due to episodes of severe depression. Although the PCP prescribed multiple medications to target Mr. J’s depressive symptoms, he continued to decline.
Subsequently, Mr. J came to the ED and is admitted to the psychiatric unit for safety and stabilization. Upon admission, Mr. J becomes bedridden, and reports that his current depressive episode is the most severe that he has ever experienced in his more than 30 years of having MDD. He says that neither bupropion nor buspirone are helping with his depression, anxiety, or any related symptom.
[polldaddy:10120537]
The authors’ observations
At admission, all of Mr. J’s home medications, except sertraline and adalimumab, which had been prescribed to treat UC (Box1,2), were discontinued. His diazepam was discontinued because the clinician felt it may have been contributing to Mr. J’s inability to walk or get out of bed. Diazepam was not tapered because it was initiated 7 days prior to admission and was thought to be exacerbating his depression and suicidal ideation. Bupropion and buspirone, which were initiated 2 weeks prior, were discontinued because Mr. J reported that neither medication was helping with his depression, anxiety, or any related symptom.
Box
Ulcerative colitis and depressive episodes
Ulcerative colitis (UC) is a chronic condition associated with inflammation in the colon causing extreme abdominal discomfort during acute flare-ups. Moderate to severe UC flare-ups are commonly treated with corticosteroids due to these medications’ anti-inflammatory properties. Although rare, corticosteroid withdrawal has been documented to induce episodes of depression. The pathophysiology of corticosteroid withdrawal inducing neuropsychiatric sequelae remains unclear; however, it is thought to be due to hypothalamic-pituitary-adrenocortical suppression.1 Fardet et al2 concluded that incident rates per 100 person-years at risk during the withdrawal period were 11.1 (95% confidence interval, 10.0, 12.3) for depression.
EVALUATION Poor appetite, anxiety, and continued suicidality
During evaluation, vital signs, laboratory findings, and diagnostic testing are found to be unremarkable. Mr. J’s presentation and complaints are entirely subjective, and include poor appetite, fatigue, difficulty sleeping, sorrow, anxiety, and continued suicidality. Mr. J reports that he feels miserable, which is reflected by his poor eye contact, soft speech, and body language.
Continued to: The authors' observations
The authors’ observations
MDD is a mood disorder characterized by depressed mood and/or loss of interest or pleasure for more than 2 weeks.3 First-line pharmacotherapy for MDD includes monotherapy with a selective serotonin reuptake inhibitor (SSRI), serotonin-norepinephrine reuptake inhibitor (SNRI), mirtazapine, or bupropion.4 Medication selection is typically based on patient-specific factors, adverse effect profile, drug–drug interactions, and cost. Other treatments include electroconvulsive therapy (ECT) or cognitive-behavioral therapy (CBT).4,5 Augmentation agents, such as second-generation antipsychotics, lithium, thyroid hormone supplementation, buspirone, anticonvulsants, and combinations of antidepressants, may also be considered.4
TREATMENT Condition worsens
On Day 2 of hospitalization, Mr. J is started on aripiprazole, 5 mg/d, clonazepam, 1 mg twice daily, and melatonin, 5 mg, each night for sleep. Aripiprazole, 5 mg/d, is initiated as an adjunct to sertraline for MDD because Mr. J reports feeling much worse and continues to report that he would “rather die than feel this way.” Mr. J begins to believe that his current state is his new baseline, and that feeling better is no longer possible.
On Day 3 of hospitalization, records are obtained from a clinician at an outside facility who previously treated Mr
By Day 8 of hospitalization, there is no notable change in Mr. J’s depressive symptoms. On Day 9, sertraline is increased to 200 mg/d, with little improvement from Mr. J’s perspective. The multidisciplinary team evaluates him, and when directly asked, Mr. J cites his 4 greatest complaints to be poor sleep, fatigue, no appetite, and depressed mood. Once again, he states, “I would rather be dead than go on feeling this way.”
[polldaddy:10120587]
The authors’ observations
Due to Mr. J’s severe, unrelenting depressive episode, the treatment team obtained his informed consent to undergo ECT. On Day 9, before initiating ECT, the pharmacist recommended mirtazapine, even though the patient weighed almost 89 kg (196.21 lb) and had a body mass index of 27.8 kg/m2. The treatment team thought that mirtazapine augmentation could potentially help the sertraline work more quickly while targeting Mr. J’s 4 greatest complaints.
Mirtazapine is a central alpha-2 antagonist or noradrenergic and specific serotonergic antidepressant (NaSSA) that works through antagonism of the presynaptic alpha-2 adrenergic receptors to indirectly regulate release of monoamines and increase the release of serotonin and norepinephrine.6 Additionally, mirtazapine has antagonist actions at 5HT2A, 5HT2C, 5HT3, and histamine-1 receptors.6 Potential adverse effects include drowsiness and increased appetite leading to weight gain.7 Mirtazapine’s therapeutic efficacy is similar to SSRIs for treating depression.4 Mirtazapine in combination with an SNRI has been referred to as “California rocket fuel” due to the theoretical pharmacologic synergy and resulting strong antidepressant action.6 It was hypothesized that similar effects could be seen by augmenting the SSRI sertraline with mirtazapine.
Continued to: The time to efficacy with mirtazapine...
The time to efficacy with mirtazapine is approximately 2 to 4 weeks, but anxiety symptoms and poor sleep or insomnia may improve in the first week.8 Studies have suggested the possibility of a more rapid onset of efficacy with mirtazapine than with SSRIs, as well as potential response acceleration in MDD and other psychiatric illnesses such as anxiety disorders or obsessive-compulsive disorder (OCD).9,10 A review that included several double-blind studies and compared mirtazapine with SSRIs found the amount of responders with persistent improvement with onset in Week 1 was more pronounced with mirtazapine.9
Augmenting an SSRI with mirtazapine is a potential therapeutic option because it can help boost the efficacy of the prescribed SSRI while enhancing appetite and blunting the activating or anxiety-like effects of some SSRIs, which may help with relaxation and sleep.4 The combination of an SSRI plus mirtazapine has been studied in patients with MDD, posttraumatic stress disorder, and OCD; it was found to improve symptoms of those conditions due to the medications’ complementary mechanisms of action.4,11-13 Also, mirtazapine has been shown to decrease the rates of relapse after an acute phase of depression.4,14
OUTCOME Rapid improvement
On Day 9, Mr. J receives the first dose of mirtazapine, 7.5 mg at bedtime. On Day 10, when Mr. J wakes, his mood is notably improved. He is more interactive (sitting up in bed reading and making eye contact with the staff during an interview), and he reports improved sleep and eats most of his breakfast.
After receiving 3 doses of mirtazapine, Mr. J reports that he feels back to his normal self; he is interactive, alert, and eating well. Due to the rapid improvement in mood, ECT is discontinued, and he does not receive any ECT treatment during the remainder of his hospitalization.
On Day 11, divalproex is discontinued. Because Mr. J receives only 5 days of therapy with this agent, his divalproex level is not checked. At this point, the treatment team feels confident in ruling out bipolar disorder.
On Day 15, Mr. J is discharged with sertraline, 200 mg/d, mirtazapine, 7.5 mg/d at 7
Ten months after his depressive episode, Mr. J has had no further admissions at the hospital where he received the treatment described here.
Bottom Line
Evidence for the treatment of major depressive disorder induced by corticosteroid withdrawal is limited. Despite trials of agents from multiple medication classes, the depressive episode may not improve. Adding mirtazapine to a selective serotonin reuptake inhibitor or serotonin-norepinephrine reuptake inhibitor may prove successful.
Related Resources
- Watanabe N, Omori IM, Nakagawa A, et al. Mirtazapine versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2011;(12):CD006528.
- Kenna HA, Poon AW, de los Angeles CP, et al. Psychiatric complications of treatment with corticosteroids: review with case report. Psychiatry Clin Neurosci. 2011;65(6):549-560.
Drug Brand Names
Adalimumab • Humira
Aripiprazole • Abilify
Bupropion • Wellbutrin, Zyban
Buspirone • Buspar
Clonazepam • Klonopin
Diazepam • Valium
Diphenhydramine • Benadryl
Divalproex • Depakote, Depakote ER
Lithium • Eskalith, Lithobid
Mirtazapine • Remeron
Prednisone • Deltasone
Sertraline • Zoloft
1. Dixon R, Christy N. On the various forms of corticosteroid withdrawal syndrome. Am J Med. 1980;68(2):224-30.
2. Fardet L, Petersen I, Nazareth I. Suicidal behavior and severe neuropsychiatric disorders following glucocorticoid therapy in primary care. Am J Psychiatry. 2012;169(5):491-497.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder, 3rd ed. Arlington Virginia: American Psychiatric Association. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published October 2010. Accessed March 15, 2017.
5. National Institute for Health and Clinical Excellence (NICE) Clinical Guideline 90. Depression in adults: recognition and management. https://www.nice.org.uk/guidance/cg90. Accessed March 15, 2017.
6. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications, 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013;317-322; 363-364.
7. Remeron [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2018.
8. Gorman JM. Mirtazapine: clinical overview. J Clin Psychiatry. 1999;60(suppl 17):9-13; discussion 46-48.
9. Quitkin FM, Taylor BP, Kremer C. Does mirtazapine have a more rapid onset than SSRIs? J Clin Psychiatry. 2001;62(5):358-361.
10. Pallanti S, Quercioli L, Bruscoli M. Response acceleration with mirtazapine augmentation of citalopram in obsessive-compulsive disorder patients without comorbid depression: a pilot study. J Clin Psychiatry. 2004;65(10):1394-1399.
11. Blier P, Gobbi G, Turcotte JE, et al. Mirtazapine and paroxetine in major depression: a comparison of monotherapy versus their combination from treatment initiation. Eur Neuropsychopharmacol. 2009;19(7):457-465.
12. Blier P, Ward HE, Tremblay P, et al. Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. Am J Psychiatry. 2010;167(3):281-288.
13. Carpenter LL, Yasmin S, Price LH. A double-blind, placebo-controlled study of antidepressant augmentation with mirtazapine. Biol Psychiatry. 2002;51(2):183-188.
14. Schneier FR, Campeas R, Carcamo J, et al. Combined mirtazapine and SSRI treatment of PTSD: a placebo-controlled trial. Depress Anxiety. 2015;32(8):570-579.
CASE Suicidal ideation, flare-up of ulcerative colitis
Mr. J, age 56, who has a history of major depressive disorder (MDD), generalized anxiety disorder (GAD), and ulcerative colitis (UC), presents to the emergency department (ED) with suicidal ideation and a plan to overdose on his medications. He reports no current emotional or financial stressors in his personal life. Home medications documented at the time of his arrival to the ED include sertraline, 100 mg/d, bupropion, 150 mg/d, buspirone, 10 mg 3 times daily, diazepam 10 mg 3 times daily, as needed, adalimumab, 40 mg IM every 2 weeks, and diphenhydramine, 50 mg every night.
A recent flare-up of UC resulted in Mr. J being placed on a 15-week prednisone taper, beginning at 80 mg/d and decreasing by 5 mg weekly, which was completed 2 weeks before he presented to the ED. After completing the prednisone taper, Mr. J went to his primary care physician (PCP) on 3 separate occasions due to episodes of severe depression. Although the PCP prescribed multiple medications to target Mr. J’s depressive symptoms, he continued to decline.
Subsequently, Mr. J came to the ED and is admitted to the psychiatric unit for safety and stabilization. Upon admission, Mr. J becomes bedridden, and reports that his current depressive episode is the most severe that he has ever experienced in his more than 30 years of having MDD. He says that neither bupropion nor buspirone are helping with his depression, anxiety, or any related symptom.
[polldaddy:10120537]
The authors’ observations
At admission, all of Mr. J’s home medications, except sertraline and adalimumab, which had been prescribed to treat UC (Box1,2), were discontinued. His diazepam was discontinued because the clinician felt it may have been contributing to Mr. J’s inability to walk or get out of bed. Diazepam was not tapered because it was initiated 7 days prior to admission and was thought to be exacerbating his depression and suicidal ideation. Bupropion and buspirone, which were initiated 2 weeks prior, were discontinued because Mr. J reported that neither medication was helping with his depression, anxiety, or any related symptom.
Box
Ulcerative colitis and depressive episodes
Ulcerative colitis (UC) is a chronic condition associated with inflammation in the colon causing extreme abdominal discomfort during acute flare-ups. Moderate to severe UC flare-ups are commonly treated with corticosteroids due to these medications’ anti-inflammatory properties. Although rare, corticosteroid withdrawal has been documented to induce episodes of depression. The pathophysiology of corticosteroid withdrawal inducing neuropsychiatric sequelae remains unclear; however, it is thought to be due to hypothalamic-pituitary-adrenocortical suppression.1 Fardet et al2 concluded that incident rates per 100 person-years at risk during the withdrawal period were 11.1 (95% confidence interval, 10.0, 12.3) for depression.
EVALUATION Poor appetite, anxiety, and continued suicidality
During evaluation, vital signs, laboratory findings, and diagnostic testing are found to be unremarkable. Mr. J’s presentation and complaints are entirely subjective, and include poor appetite, fatigue, difficulty sleeping, sorrow, anxiety, and continued suicidality. Mr. J reports that he feels miserable, which is reflected by his poor eye contact, soft speech, and body language.
Continued to: The authors' observations
The authors’ observations
MDD is a mood disorder characterized by depressed mood and/or loss of interest or pleasure for more than 2 weeks.3 First-line pharmacotherapy for MDD includes monotherapy with a selective serotonin reuptake inhibitor (SSRI), serotonin-norepinephrine reuptake inhibitor (SNRI), mirtazapine, or bupropion.4 Medication selection is typically based on patient-specific factors, adverse effect profile, drug–drug interactions, and cost. Other treatments include electroconvulsive therapy (ECT) or cognitive-behavioral therapy (CBT).4,5 Augmentation agents, such as second-generation antipsychotics, lithium, thyroid hormone supplementation, buspirone, anticonvulsants, and combinations of antidepressants, may also be considered.4
TREATMENT Condition worsens
On Day 2 of hospitalization, Mr. J is started on aripiprazole, 5 mg/d, clonazepam, 1 mg twice daily, and melatonin, 5 mg, each night for sleep. Aripiprazole, 5 mg/d, is initiated as an adjunct to sertraline for MDD because Mr. J reports feeling much worse and continues to report that he would “rather die than feel this way.” Mr. J begins to believe that his current state is his new baseline, and that feeling better is no longer possible.
On Day 3 of hospitalization, records are obtained from a clinician at an outside facility who previously treated Mr
By Day 8 of hospitalization, there is no notable change in Mr. J’s depressive symptoms. On Day 9, sertraline is increased to 200 mg/d, with little improvement from Mr. J’s perspective. The multidisciplinary team evaluates him, and when directly asked, Mr. J cites his 4 greatest complaints to be poor sleep, fatigue, no appetite, and depressed mood. Once again, he states, “I would rather be dead than go on feeling this way.”
[polldaddy:10120587]
The authors’ observations
Due to Mr. J’s severe, unrelenting depressive episode, the treatment team obtained his informed consent to undergo ECT. On Day 9, before initiating ECT, the pharmacist recommended mirtazapine, even though the patient weighed almost 89 kg (196.21 lb) and had a body mass index of 27.8 kg/m2. The treatment team thought that mirtazapine augmentation could potentially help the sertraline work more quickly while targeting Mr. J’s 4 greatest complaints.
Mirtazapine is a central alpha-2 antagonist or noradrenergic and specific serotonergic antidepressant (NaSSA) that works through antagonism of the presynaptic alpha-2 adrenergic receptors to indirectly regulate release of monoamines and increase the release of serotonin and norepinephrine.6 Additionally, mirtazapine has antagonist actions at 5HT2A, 5HT2C, 5HT3, and histamine-1 receptors.6 Potential adverse effects include drowsiness and increased appetite leading to weight gain.7 Mirtazapine’s therapeutic efficacy is similar to SSRIs for treating depression.4 Mirtazapine in combination with an SNRI has been referred to as “California rocket fuel” due to the theoretical pharmacologic synergy and resulting strong antidepressant action.6 It was hypothesized that similar effects could be seen by augmenting the SSRI sertraline with mirtazapine.
Continued to: The time to efficacy with mirtazapine...
The time to efficacy with mirtazapine is approximately 2 to 4 weeks, but anxiety symptoms and poor sleep or insomnia may improve in the first week.8 Studies have suggested the possibility of a more rapid onset of efficacy with mirtazapine than with SSRIs, as well as potential response acceleration in MDD and other psychiatric illnesses such as anxiety disorders or obsessive-compulsive disorder (OCD).9,10 A review that included several double-blind studies and compared mirtazapine with SSRIs found the amount of responders with persistent improvement with onset in Week 1 was more pronounced with mirtazapine.9
Augmenting an SSRI with mirtazapine is a potential therapeutic option because it can help boost the efficacy of the prescribed SSRI while enhancing appetite and blunting the activating or anxiety-like effects of some SSRIs, which may help with relaxation and sleep.4 The combination of an SSRI plus mirtazapine has been studied in patients with MDD, posttraumatic stress disorder, and OCD; it was found to improve symptoms of those conditions due to the medications’ complementary mechanisms of action.4,11-13 Also, mirtazapine has been shown to decrease the rates of relapse after an acute phase of depression.4,14
OUTCOME Rapid improvement
On Day 9, Mr. J receives the first dose of mirtazapine, 7.5 mg at bedtime. On Day 10, when Mr. J wakes, his mood is notably improved. He is more interactive (sitting up in bed reading and making eye contact with the staff during an interview), and he reports improved sleep and eats most of his breakfast.
After receiving 3 doses of mirtazapine, Mr. J reports that he feels back to his normal self; he is interactive, alert, and eating well. Due to the rapid improvement in mood, ECT is discontinued, and he does not receive any ECT treatment during the remainder of his hospitalization.
On Day 11, divalproex is discontinued. Because Mr. J receives only 5 days of therapy with this agent, his divalproex level is not checked. At this point, the treatment team feels confident in ruling out bipolar disorder.
On Day 15, Mr. J is discharged with sertraline, 200 mg/d, mirtazapine, 7.5 mg/d at 7
Ten months after his depressive episode, Mr. J has had no further admissions at the hospital where he received the treatment described here.
Bottom Line
Evidence for the treatment of major depressive disorder induced by corticosteroid withdrawal is limited. Despite trials of agents from multiple medication classes, the depressive episode may not improve. Adding mirtazapine to a selective serotonin reuptake inhibitor or serotonin-norepinephrine reuptake inhibitor may prove successful.
Related Resources
- Watanabe N, Omori IM, Nakagawa A, et al. Mirtazapine versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2011;(12):CD006528.
- Kenna HA, Poon AW, de los Angeles CP, et al. Psychiatric complications of treatment with corticosteroids: review with case report. Psychiatry Clin Neurosci. 2011;65(6):549-560.
Drug Brand Names
Adalimumab • Humira
Aripiprazole • Abilify
Bupropion • Wellbutrin, Zyban
Buspirone • Buspar
Clonazepam • Klonopin
Diazepam • Valium
Diphenhydramine • Benadryl
Divalproex • Depakote, Depakote ER
Lithium • Eskalith, Lithobid
Mirtazapine • Remeron
Prednisone • Deltasone
Sertraline • Zoloft
CASE Suicidal ideation, flare-up of ulcerative colitis
Mr. J, age 56, who has a history of major depressive disorder (MDD), generalized anxiety disorder (GAD), and ulcerative colitis (UC), presents to the emergency department (ED) with suicidal ideation and a plan to overdose on his medications. He reports no current emotional or financial stressors in his personal life. Home medications documented at the time of his arrival to the ED include sertraline, 100 mg/d, bupropion, 150 mg/d, buspirone, 10 mg 3 times daily, diazepam 10 mg 3 times daily, as needed, adalimumab, 40 mg IM every 2 weeks, and diphenhydramine, 50 mg every night.
A recent flare-up of UC resulted in Mr. J being placed on a 15-week prednisone taper, beginning at 80 mg/d and decreasing by 5 mg weekly, which was completed 2 weeks before he presented to the ED. After completing the prednisone taper, Mr. J went to his primary care physician (PCP) on 3 separate occasions due to episodes of severe depression. Although the PCP prescribed multiple medications to target Mr. J’s depressive symptoms, he continued to decline.
Subsequently, Mr. J came to the ED and is admitted to the psychiatric unit for safety and stabilization. Upon admission, Mr. J becomes bedridden, and reports that his current depressive episode is the most severe that he has ever experienced in his more than 30 years of having MDD. He says that neither bupropion nor buspirone are helping with his depression, anxiety, or any related symptom.
[polldaddy:10120537]
The authors’ observations
At admission, all of Mr. J’s home medications, except sertraline and adalimumab, which had been prescribed to treat UC (Box1,2), were discontinued. His diazepam was discontinued because the clinician felt it may have been contributing to Mr. J’s inability to walk or get out of bed. Diazepam was not tapered because it was initiated 7 days prior to admission and was thought to be exacerbating his depression and suicidal ideation. Bupropion and buspirone, which were initiated 2 weeks prior, were discontinued because Mr. J reported that neither medication was helping with his depression, anxiety, or any related symptom.
Box
Ulcerative colitis and depressive episodes
Ulcerative colitis (UC) is a chronic condition associated with inflammation in the colon causing extreme abdominal discomfort during acute flare-ups. Moderate to severe UC flare-ups are commonly treated with corticosteroids due to these medications’ anti-inflammatory properties. Although rare, corticosteroid withdrawal has been documented to induce episodes of depression. The pathophysiology of corticosteroid withdrawal inducing neuropsychiatric sequelae remains unclear; however, it is thought to be due to hypothalamic-pituitary-adrenocortical suppression.1 Fardet et al2 concluded that incident rates per 100 person-years at risk during the withdrawal period were 11.1 (95% confidence interval, 10.0, 12.3) for depression.
EVALUATION Poor appetite, anxiety, and continued suicidality
During evaluation, vital signs, laboratory findings, and diagnostic testing are found to be unremarkable. Mr. J’s presentation and complaints are entirely subjective, and include poor appetite, fatigue, difficulty sleeping, sorrow, anxiety, and continued suicidality. Mr. J reports that he feels miserable, which is reflected by his poor eye contact, soft speech, and body language.
Continued to: The authors' observations
The authors’ observations
MDD is a mood disorder characterized by depressed mood and/or loss of interest or pleasure for more than 2 weeks.3 First-line pharmacotherapy for MDD includes monotherapy with a selective serotonin reuptake inhibitor (SSRI), serotonin-norepinephrine reuptake inhibitor (SNRI), mirtazapine, or bupropion.4 Medication selection is typically based on patient-specific factors, adverse effect profile, drug–drug interactions, and cost. Other treatments include electroconvulsive therapy (ECT) or cognitive-behavioral therapy (CBT).4,5 Augmentation agents, such as second-generation antipsychotics, lithium, thyroid hormone supplementation, buspirone, anticonvulsants, and combinations of antidepressants, may also be considered.4
TREATMENT Condition worsens
On Day 2 of hospitalization, Mr. J is started on aripiprazole, 5 mg/d, clonazepam, 1 mg twice daily, and melatonin, 5 mg, each night for sleep. Aripiprazole, 5 mg/d, is initiated as an adjunct to sertraline for MDD because Mr. J reports feeling much worse and continues to report that he would “rather die than feel this way.” Mr. J begins to believe that his current state is his new baseline, and that feeling better is no longer possible.
On Day 3 of hospitalization, records are obtained from a clinician at an outside facility who previously treated Mr
By Day 8 of hospitalization, there is no notable change in Mr. J’s depressive symptoms. On Day 9, sertraline is increased to 200 mg/d, with little improvement from Mr. J’s perspective. The multidisciplinary team evaluates him, and when directly asked, Mr. J cites his 4 greatest complaints to be poor sleep, fatigue, no appetite, and depressed mood. Once again, he states, “I would rather be dead than go on feeling this way.”
[polldaddy:10120587]
The authors’ observations
Due to Mr. J’s severe, unrelenting depressive episode, the treatment team obtained his informed consent to undergo ECT. On Day 9, before initiating ECT, the pharmacist recommended mirtazapine, even though the patient weighed almost 89 kg (196.21 lb) and had a body mass index of 27.8 kg/m2. The treatment team thought that mirtazapine augmentation could potentially help the sertraline work more quickly while targeting Mr. J’s 4 greatest complaints.
Mirtazapine is a central alpha-2 antagonist or noradrenergic and specific serotonergic antidepressant (NaSSA) that works through antagonism of the presynaptic alpha-2 adrenergic receptors to indirectly regulate release of monoamines and increase the release of serotonin and norepinephrine.6 Additionally, mirtazapine has antagonist actions at 5HT2A, 5HT2C, 5HT3, and histamine-1 receptors.6 Potential adverse effects include drowsiness and increased appetite leading to weight gain.7 Mirtazapine’s therapeutic efficacy is similar to SSRIs for treating depression.4 Mirtazapine in combination with an SNRI has been referred to as “California rocket fuel” due to the theoretical pharmacologic synergy and resulting strong antidepressant action.6 It was hypothesized that similar effects could be seen by augmenting the SSRI sertraline with mirtazapine.
Continued to: The time to efficacy with mirtazapine...
The time to efficacy with mirtazapine is approximately 2 to 4 weeks, but anxiety symptoms and poor sleep or insomnia may improve in the first week.8 Studies have suggested the possibility of a more rapid onset of efficacy with mirtazapine than with SSRIs, as well as potential response acceleration in MDD and other psychiatric illnesses such as anxiety disorders or obsessive-compulsive disorder (OCD).9,10 A review that included several double-blind studies and compared mirtazapine with SSRIs found the amount of responders with persistent improvement with onset in Week 1 was more pronounced with mirtazapine.9
Augmenting an SSRI with mirtazapine is a potential therapeutic option because it can help boost the efficacy of the prescribed SSRI while enhancing appetite and blunting the activating or anxiety-like effects of some SSRIs, which may help with relaxation and sleep.4 The combination of an SSRI plus mirtazapine has been studied in patients with MDD, posttraumatic stress disorder, and OCD; it was found to improve symptoms of those conditions due to the medications’ complementary mechanisms of action.4,11-13 Also, mirtazapine has been shown to decrease the rates of relapse after an acute phase of depression.4,14
OUTCOME Rapid improvement
On Day 9, Mr. J receives the first dose of mirtazapine, 7.5 mg at bedtime. On Day 10, when Mr. J wakes, his mood is notably improved. He is more interactive (sitting up in bed reading and making eye contact with the staff during an interview), and he reports improved sleep and eats most of his breakfast.
After receiving 3 doses of mirtazapine, Mr. J reports that he feels back to his normal self; he is interactive, alert, and eating well. Due to the rapid improvement in mood, ECT is discontinued, and he does not receive any ECT treatment during the remainder of his hospitalization.
On Day 11, divalproex is discontinued. Because Mr. J receives only 5 days of therapy with this agent, his divalproex level is not checked. At this point, the treatment team feels confident in ruling out bipolar disorder.
On Day 15, Mr. J is discharged with sertraline, 200 mg/d, mirtazapine, 7.5 mg/d at 7
Ten months after his depressive episode, Mr. J has had no further admissions at the hospital where he received the treatment described here.
Bottom Line
Evidence for the treatment of major depressive disorder induced by corticosteroid withdrawal is limited. Despite trials of agents from multiple medication classes, the depressive episode may not improve. Adding mirtazapine to a selective serotonin reuptake inhibitor or serotonin-norepinephrine reuptake inhibitor may prove successful.
Related Resources
- Watanabe N, Omori IM, Nakagawa A, et al. Mirtazapine versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2011;(12):CD006528.
- Kenna HA, Poon AW, de los Angeles CP, et al. Psychiatric complications of treatment with corticosteroids: review with case report. Psychiatry Clin Neurosci. 2011;65(6):549-560.
Drug Brand Names
Adalimumab • Humira
Aripiprazole • Abilify
Bupropion • Wellbutrin, Zyban
Buspirone • Buspar
Clonazepam • Klonopin
Diazepam • Valium
Diphenhydramine • Benadryl
Divalproex • Depakote, Depakote ER
Lithium • Eskalith, Lithobid
Mirtazapine • Remeron
Prednisone • Deltasone
Sertraline • Zoloft
1. Dixon R, Christy N. On the various forms of corticosteroid withdrawal syndrome. Am J Med. 1980;68(2):224-30.
2. Fardet L, Petersen I, Nazareth I. Suicidal behavior and severe neuropsychiatric disorders following glucocorticoid therapy in primary care. Am J Psychiatry. 2012;169(5):491-497.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder, 3rd ed. Arlington Virginia: American Psychiatric Association. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published October 2010. Accessed March 15, 2017.
5. National Institute for Health and Clinical Excellence (NICE) Clinical Guideline 90. Depression in adults: recognition and management. https://www.nice.org.uk/guidance/cg90. Accessed March 15, 2017.
6. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications, 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013;317-322; 363-364.
7. Remeron [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2018.
8. Gorman JM. Mirtazapine: clinical overview. J Clin Psychiatry. 1999;60(suppl 17):9-13; discussion 46-48.
9. Quitkin FM, Taylor BP, Kremer C. Does mirtazapine have a more rapid onset than SSRIs? J Clin Psychiatry. 2001;62(5):358-361.
10. Pallanti S, Quercioli L, Bruscoli M. Response acceleration with mirtazapine augmentation of citalopram in obsessive-compulsive disorder patients without comorbid depression: a pilot study. J Clin Psychiatry. 2004;65(10):1394-1399.
11. Blier P, Gobbi G, Turcotte JE, et al. Mirtazapine and paroxetine in major depression: a comparison of monotherapy versus their combination from treatment initiation. Eur Neuropsychopharmacol. 2009;19(7):457-465.
12. Blier P, Ward HE, Tremblay P, et al. Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. Am J Psychiatry. 2010;167(3):281-288.
13. Carpenter LL, Yasmin S, Price LH. A double-blind, placebo-controlled study of antidepressant augmentation with mirtazapine. Biol Psychiatry. 2002;51(2):183-188.
14. Schneier FR, Campeas R, Carcamo J, et al. Combined mirtazapine and SSRI treatment of PTSD: a placebo-controlled trial. Depress Anxiety. 2015;32(8):570-579.
1. Dixon R, Christy N. On the various forms of corticosteroid withdrawal syndrome. Am J Med. 1980;68(2):224-30.
2. Fardet L, Petersen I, Nazareth I. Suicidal behavior and severe neuropsychiatric disorders following glucocorticoid therapy in primary care. Am J Psychiatry. 2012;169(5):491-497.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder, 3rd ed. Arlington Virginia: American Psychiatric Association. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published October 2010. Accessed March 15, 2017.
5. National Institute for Health and Clinical Excellence (NICE) Clinical Guideline 90. Depression in adults: recognition and management. https://www.nice.org.uk/guidance/cg90. Accessed March 15, 2017.
6. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications, 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013;317-322; 363-364.
7. Remeron [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2018.
8. Gorman JM. Mirtazapine: clinical overview. J Clin Psychiatry. 1999;60(suppl 17):9-13; discussion 46-48.
9. Quitkin FM, Taylor BP, Kremer C. Does mirtazapine have a more rapid onset than SSRIs? J Clin Psychiatry. 2001;62(5):358-361.
10. Pallanti S, Quercioli L, Bruscoli M. Response acceleration with mirtazapine augmentation of citalopram in obsessive-compulsive disorder patients without comorbid depression: a pilot study. J Clin Psychiatry. 2004;65(10):1394-1399.
11. Blier P, Gobbi G, Turcotte JE, et al. Mirtazapine and paroxetine in major depression: a comparison of monotherapy versus their combination from treatment initiation. Eur Neuropsychopharmacol. 2009;19(7):457-465.
12. Blier P, Ward HE, Tremblay P, et al. Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. Am J Psychiatry. 2010;167(3):281-288.
13. Carpenter LL, Yasmin S, Price LH. A double-blind, placebo-controlled study of antidepressant augmentation with mirtazapine. Biol Psychiatry. 2002;51(2):183-188.
14. Schneier FR, Campeas R, Carcamo J, et al. Combined mirtazapine and SSRI treatment of PTSD: a placebo-controlled trial. Depress Anxiety. 2015;32(8):570-579.
Vitamin B6 for tardive dyskinesia?
Although antipsychotics have revolutionized the treatment of severe mental illnesses, adverse effects often present a substantial obstacle to adherence. One of the most tenacious and difficult-to-treat adverse effects is tardive dyskinesia (TD), a neuromotor syndrome with characteristic involuntary repetitive movements, typically of the muscles of the jaw, lips, and tongue. In addition to spasms and grimacing, patients can have choreoathetoid movements of the neck. In more extreme presentations, some patients can have difficulty breathing. TD is a largely irreversible condition. It is often a disfiguring lifelong disability that can further stigmatize patients who already suffer scorn and derision. TD usually has a delayed onset after a patient is started on an antipsychotic.1 The syndrome is more commonly associated with first-generation antipsychotics, but affects up to 20% of patients who are treated with second-generation antipsychotics.1 In the United States, TD affects as many as 500,000 patients.1
There are several palliative interventions for TD, but the evidence for a consistently reliable treatment is weak. Branched-chain amino acids, ginkgo biloba, melatonin, and vitamin E have been investigated as interventions. Other approaches include switching to an alternate antipsychotic such as clozapine, adjusting the antipsychotic dose, using anticholinergic medications, adjunctive amantadine, gamma aminobutyric acid agonists, or adding tetrabenazine.
The FDA recently approved two vesicular monoamine transporter 2 (VMAT2) inhibitors, deutetrabenazine and valbenazine, for addressing symptoms of TD. However, these medications can cost tens of thousands of dollars per year, and also carry the risk of adverse effects such as sedation, akathisia, urinary retention, constipation, and muscle pain.2 When treating a patient who develops TD, one might consider other potentially effective therapies with low adverse effect profiles that may be more cost-effective than existing treatments. The bioactive form of vitamin B6 (pyridoxine), pyridoxal-5-phosphate, has been used to treat various antipsychotic-induced movement disorders. Preliminary evidence suggests that vitamin B6 may help reduce the symptoms of TD.
A recent Cochrane Database Review (2015)3 of pyridoxal-5-phosphate treatment for TD found a significant improvement in symptoms compared with placebo. Although the studies included in this review were limited by modest sample sizes and short follow-up periods, 2 of the investigations revealed improvements of >40% in extrapyramidal symptoms with vitamin B6 compared with placebo. Lerner et al (2001)4 conducted a randomized, double-blind, placebo-controlled crossover trial in which 15 inpatients with schizophrenia who met the criteria for TD were assigned to vitamin B6, 400 mg/d, or placebo for 4 weeks. After a 2-week washout period, the placebo group was given vitamin B6 and vice versa. Compared with placebo, mean scores on the parkinsonism and dyskinetic movement subscales of the Extrapyramidal Symptom Rating Scale were significantly better in the third week of treatment with vitamin B6.
Lerner et al (2007)5 later conducted a separate crossover study using the same design with a washout period. This trial included a larger sample size (50 inpatients with DSM-IV diagnoses of schizophrenia or schizoaffective disorder and TD) and the dosage of vitamin B6 was increased to 1,200 mg/d over 26 weeks. Patients who received vitamin B6 experienced a significantly greater decrease in Extrapyramidal Symptom Rating Scale scores compared with those in the placebo group.
Continued to: A 29-year-old woman with treatment-resistant schizophrenia...
Umar et al (2016)6 published a case review of a 29-year-old woman with treatment-resistant schizophrenia with TD who was treated with clozapine, 400 mg/d. She was started on vitamin B6, 450 mg/d, for 4 weeks, and then her dose was increased to 600 mg/d. At 6 months, she experienced a 78% reduction in the severity of her TD symptoms, as measured by the Abnormal Involuntary Movement Scale. The authors reported that this improvement was maintained for 1 year after vitamin B6 was stopped.
Miodownik et al (2008)7 reported in a study of 89 patients with schizophrenia that those with TD (n = 40) had diminished amounts of vitamin B6 in their plasma compared with patients without symptoms of motor disturbances (n = 49).
Vitamin B6 has been known to improve other psychotropic-induced movement disorders. In a study of lithium-induced tremors, treatment with pyridoxine, 900 to 1,200 mg/d, resulted in “impressive improvement until total disappearance of tremor.”8 Lerner et al (2004)9 also reported significant improvement for patients with neuroleptic-induced akathisia who were treated with vitamin B6.
Some proposed mechanisms of action
Pyridoxal-5-phosphate is a coenzyme in the synthesis of dopamine and other neurotransmitters. This might explain in part the biochemical mechanism of vitamin B6 in attenuating motor symptoms following long-term dopamine blockade. Chronic neurotransmitter antagonism may result in an upregulation of dopamine receptors in response. This compensatory reaction might create a dopamine receptor super-sensitivity in the nigrostriatal pathways.10
Another potential mechanism of action might be vitamin B6’s potent antioxidant properties and its scavenging of free radicals. The neurotoxicity of oxidative stress has been implicated in various movement disorders and psychiatric conditions.
In all of the studies described here, patients continued to receive daily antipsychotic treatment. In these trials, the adverse effects of vitamin B6 were minimal or negligible. In one study, vitamin B6 was reported to have had a better adverse effect profile than placebo.4
1. Carbon M, Hsieh CH, Kane JM, et al. Tardive dyskinesia prevalence in the period of second-generation antipsychotic use: a meta-analysis. J Clin Psychiatry. 2017;78(3):e264-e278.
2. Smith Mosley LL, Mosely II JF, Fleischfresser JR, et al. Vesicular monoamine transporter type 2 (VMAT2) inhibitors in the management of tardive dyskinesia. Clin Med Rev Case Rep. 2017;4(12):1-5.
3. Adelufosi AO, Abayomi O, Ojo M. Pyridoxal 5 phosphate for neuroleptic-induced tardive dyskinesia. Cochrane Database Syst Rev. 2015;(4):CD010501.
4. Lerner V, Miodownik C, Kapstan A, et al. Vitamin B(6) in the treatment of tardive dyskinesia: a double-blind, placebo-controlled, crossover study. Am J Psychiatry. 2001;158(9):1511-1514.
5. Lerner V, Miodownik C, Kapstan A, et al. Vitamin B6 treatment for tardive dyskinesia: a randomized, double-blind, placebo-controlled, crossover study. J Clin Psychiatry. 2007;68(11):1648-1654.
6. Umar MU, Isa AA, Abba AH. High dose pyridoxine for the treatment of tardive dyskinesia: clinical case and review of literature. Ther Adv Psychopharmacol. 2016;6(2):152-156.
7. Miodownik C, Meoded A, Libov I, et al. Pyridoxal plasma level in schizophrenic and schizoaffective patients with and without tardive dyskinesia. Clin Neuropharmacol. 2008;31(4):197-203.
8. Miodownik C, Witztum E, Lerner V. Lithium-induced tremor treated with vitamin B6: a preliminary case series. Int J Psychiatry Med. 2002;32(1):103-108.
9. Lerner V, Bergman J, Statsenko N, et al. Vitamin B6 treatment in acute neuroleptic-induced akathisia: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2004;65(11):1550-1554.
10. Miller, BJ. Tardive dyskinesia: a review of the literature. Psychiatric Times. http://www.psychiatrictimes.com/articles/tardive-dyskinesia-review-literature. Published June 27, 2017. Accessed July 31, 2018.
Although antipsychotics have revolutionized the treatment of severe mental illnesses, adverse effects often present a substantial obstacle to adherence. One of the most tenacious and difficult-to-treat adverse effects is tardive dyskinesia (TD), a neuromotor syndrome with characteristic involuntary repetitive movements, typically of the muscles of the jaw, lips, and tongue. In addition to spasms and grimacing, patients can have choreoathetoid movements of the neck. In more extreme presentations, some patients can have difficulty breathing. TD is a largely irreversible condition. It is often a disfiguring lifelong disability that can further stigmatize patients who already suffer scorn and derision. TD usually has a delayed onset after a patient is started on an antipsychotic.1 The syndrome is more commonly associated with first-generation antipsychotics, but affects up to 20% of patients who are treated with second-generation antipsychotics.1 In the United States, TD affects as many as 500,000 patients.1
There are several palliative interventions for TD, but the evidence for a consistently reliable treatment is weak. Branched-chain amino acids, ginkgo biloba, melatonin, and vitamin E have been investigated as interventions. Other approaches include switching to an alternate antipsychotic such as clozapine, adjusting the antipsychotic dose, using anticholinergic medications, adjunctive amantadine, gamma aminobutyric acid agonists, or adding tetrabenazine.
The FDA recently approved two vesicular monoamine transporter 2 (VMAT2) inhibitors, deutetrabenazine and valbenazine, for addressing symptoms of TD. However, these medications can cost tens of thousands of dollars per year, and also carry the risk of adverse effects such as sedation, akathisia, urinary retention, constipation, and muscle pain.2 When treating a patient who develops TD, one might consider other potentially effective therapies with low adverse effect profiles that may be more cost-effective than existing treatments. The bioactive form of vitamin B6 (pyridoxine), pyridoxal-5-phosphate, has been used to treat various antipsychotic-induced movement disorders. Preliminary evidence suggests that vitamin B6 may help reduce the symptoms of TD.
A recent Cochrane Database Review (2015)3 of pyridoxal-5-phosphate treatment for TD found a significant improvement in symptoms compared with placebo. Although the studies included in this review were limited by modest sample sizes and short follow-up periods, 2 of the investigations revealed improvements of >40% in extrapyramidal symptoms with vitamin B6 compared with placebo. Lerner et al (2001)4 conducted a randomized, double-blind, placebo-controlled crossover trial in which 15 inpatients with schizophrenia who met the criteria for TD were assigned to vitamin B6, 400 mg/d, or placebo for 4 weeks. After a 2-week washout period, the placebo group was given vitamin B6 and vice versa. Compared with placebo, mean scores on the parkinsonism and dyskinetic movement subscales of the Extrapyramidal Symptom Rating Scale were significantly better in the third week of treatment with vitamin B6.
Lerner et al (2007)5 later conducted a separate crossover study using the same design with a washout period. This trial included a larger sample size (50 inpatients with DSM-IV diagnoses of schizophrenia or schizoaffective disorder and TD) and the dosage of vitamin B6 was increased to 1,200 mg/d over 26 weeks. Patients who received vitamin B6 experienced a significantly greater decrease in Extrapyramidal Symptom Rating Scale scores compared with those in the placebo group.
Continued to: A 29-year-old woman with treatment-resistant schizophrenia...
Umar et al (2016)6 published a case review of a 29-year-old woman with treatment-resistant schizophrenia with TD who was treated with clozapine, 400 mg/d. She was started on vitamin B6, 450 mg/d, for 4 weeks, and then her dose was increased to 600 mg/d. At 6 months, she experienced a 78% reduction in the severity of her TD symptoms, as measured by the Abnormal Involuntary Movement Scale. The authors reported that this improvement was maintained for 1 year after vitamin B6 was stopped.
Miodownik et al (2008)7 reported in a study of 89 patients with schizophrenia that those with TD (n = 40) had diminished amounts of vitamin B6 in their plasma compared with patients without symptoms of motor disturbances (n = 49).
Vitamin B6 has been known to improve other psychotropic-induced movement disorders. In a study of lithium-induced tremors, treatment with pyridoxine, 900 to 1,200 mg/d, resulted in “impressive improvement until total disappearance of tremor.”8 Lerner et al (2004)9 also reported significant improvement for patients with neuroleptic-induced akathisia who were treated with vitamin B6.
Some proposed mechanisms of action
Pyridoxal-5-phosphate is a coenzyme in the synthesis of dopamine and other neurotransmitters. This might explain in part the biochemical mechanism of vitamin B6 in attenuating motor symptoms following long-term dopamine blockade. Chronic neurotransmitter antagonism may result in an upregulation of dopamine receptors in response. This compensatory reaction might create a dopamine receptor super-sensitivity in the nigrostriatal pathways.10
Another potential mechanism of action might be vitamin B6’s potent antioxidant properties and its scavenging of free radicals. The neurotoxicity of oxidative stress has been implicated in various movement disorders and psychiatric conditions.
In all of the studies described here, patients continued to receive daily antipsychotic treatment. In these trials, the adverse effects of vitamin B6 were minimal or negligible. In one study, vitamin B6 was reported to have had a better adverse effect profile than placebo.4
Although antipsychotics have revolutionized the treatment of severe mental illnesses, adverse effects often present a substantial obstacle to adherence. One of the most tenacious and difficult-to-treat adverse effects is tardive dyskinesia (TD), a neuromotor syndrome with characteristic involuntary repetitive movements, typically of the muscles of the jaw, lips, and tongue. In addition to spasms and grimacing, patients can have choreoathetoid movements of the neck. In more extreme presentations, some patients can have difficulty breathing. TD is a largely irreversible condition. It is often a disfiguring lifelong disability that can further stigmatize patients who already suffer scorn and derision. TD usually has a delayed onset after a patient is started on an antipsychotic.1 The syndrome is more commonly associated with first-generation antipsychotics, but affects up to 20% of patients who are treated with second-generation antipsychotics.1 In the United States, TD affects as many as 500,000 patients.1
There are several palliative interventions for TD, but the evidence for a consistently reliable treatment is weak. Branched-chain amino acids, ginkgo biloba, melatonin, and vitamin E have been investigated as interventions. Other approaches include switching to an alternate antipsychotic such as clozapine, adjusting the antipsychotic dose, using anticholinergic medications, adjunctive amantadine, gamma aminobutyric acid agonists, or adding tetrabenazine.
The FDA recently approved two vesicular monoamine transporter 2 (VMAT2) inhibitors, deutetrabenazine and valbenazine, for addressing symptoms of TD. However, these medications can cost tens of thousands of dollars per year, and also carry the risk of adverse effects such as sedation, akathisia, urinary retention, constipation, and muscle pain.2 When treating a patient who develops TD, one might consider other potentially effective therapies with low adverse effect profiles that may be more cost-effective than existing treatments. The bioactive form of vitamin B6 (pyridoxine), pyridoxal-5-phosphate, has been used to treat various antipsychotic-induced movement disorders. Preliminary evidence suggests that vitamin B6 may help reduce the symptoms of TD.
A recent Cochrane Database Review (2015)3 of pyridoxal-5-phosphate treatment for TD found a significant improvement in symptoms compared with placebo. Although the studies included in this review were limited by modest sample sizes and short follow-up periods, 2 of the investigations revealed improvements of >40% in extrapyramidal symptoms with vitamin B6 compared with placebo. Lerner et al (2001)4 conducted a randomized, double-blind, placebo-controlled crossover trial in which 15 inpatients with schizophrenia who met the criteria for TD were assigned to vitamin B6, 400 mg/d, or placebo for 4 weeks. After a 2-week washout period, the placebo group was given vitamin B6 and vice versa. Compared with placebo, mean scores on the parkinsonism and dyskinetic movement subscales of the Extrapyramidal Symptom Rating Scale were significantly better in the third week of treatment with vitamin B6.
Lerner et al (2007)5 later conducted a separate crossover study using the same design with a washout period. This trial included a larger sample size (50 inpatients with DSM-IV diagnoses of schizophrenia or schizoaffective disorder and TD) and the dosage of vitamin B6 was increased to 1,200 mg/d over 26 weeks. Patients who received vitamin B6 experienced a significantly greater decrease in Extrapyramidal Symptom Rating Scale scores compared with those in the placebo group.
Continued to: A 29-year-old woman with treatment-resistant schizophrenia...
Umar et al (2016)6 published a case review of a 29-year-old woman with treatment-resistant schizophrenia with TD who was treated with clozapine, 400 mg/d. She was started on vitamin B6, 450 mg/d, for 4 weeks, and then her dose was increased to 600 mg/d. At 6 months, she experienced a 78% reduction in the severity of her TD symptoms, as measured by the Abnormal Involuntary Movement Scale. The authors reported that this improvement was maintained for 1 year after vitamin B6 was stopped.
Miodownik et al (2008)7 reported in a study of 89 patients with schizophrenia that those with TD (n = 40) had diminished amounts of vitamin B6 in their plasma compared with patients without symptoms of motor disturbances (n = 49).
Vitamin B6 has been known to improve other psychotropic-induced movement disorders. In a study of lithium-induced tremors, treatment with pyridoxine, 900 to 1,200 mg/d, resulted in “impressive improvement until total disappearance of tremor.”8 Lerner et al (2004)9 also reported significant improvement for patients with neuroleptic-induced akathisia who were treated with vitamin B6.
Some proposed mechanisms of action
Pyridoxal-5-phosphate is a coenzyme in the synthesis of dopamine and other neurotransmitters. This might explain in part the biochemical mechanism of vitamin B6 in attenuating motor symptoms following long-term dopamine blockade. Chronic neurotransmitter antagonism may result in an upregulation of dopamine receptors in response. This compensatory reaction might create a dopamine receptor super-sensitivity in the nigrostriatal pathways.10
Another potential mechanism of action might be vitamin B6’s potent antioxidant properties and its scavenging of free radicals. The neurotoxicity of oxidative stress has been implicated in various movement disorders and psychiatric conditions.
In all of the studies described here, patients continued to receive daily antipsychotic treatment. In these trials, the adverse effects of vitamin B6 were minimal or negligible. In one study, vitamin B6 was reported to have had a better adverse effect profile than placebo.4
1. Carbon M, Hsieh CH, Kane JM, et al. Tardive dyskinesia prevalence in the period of second-generation antipsychotic use: a meta-analysis. J Clin Psychiatry. 2017;78(3):e264-e278.
2. Smith Mosley LL, Mosely II JF, Fleischfresser JR, et al. Vesicular monoamine transporter type 2 (VMAT2) inhibitors in the management of tardive dyskinesia. Clin Med Rev Case Rep. 2017;4(12):1-5.
3. Adelufosi AO, Abayomi O, Ojo M. Pyridoxal 5 phosphate for neuroleptic-induced tardive dyskinesia. Cochrane Database Syst Rev. 2015;(4):CD010501.
4. Lerner V, Miodownik C, Kapstan A, et al. Vitamin B(6) in the treatment of tardive dyskinesia: a double-blind, placebo-controlled, crossover study. Am J Psychiatry. 2001;158(9):1511-1514.
5. Lerner V, Miodownik C, Kapstan A, et al. Vitamin B6 treatment for tardive dyskinesia: a randomized, double-blind, placebo-controlled, crossover study. J Clin Psychiatry. 2007;68(11):1648-1654.
6. Umar MU, Isa AA, Abba AH. High dose pyridoxine for the treatment of tardive dyskinesia: clinical case and review of literature. Ther Adv Psychopharmacol. 2016;6(2):152-156.
7. Miodownik C, Meoded A, Libov I, et al. Pyridoxal plasma level in schizophrenic and schizoaffective patients with and without tardive dyskinesia. Clin Neuropharmacol. 2008;31(4):197-203.
8. Miodownik C, Witztum E, Lerner V. Lithium-induced tremor treated with vitamin B6: a preliminary case series. Int J Psychiatry Med. 2002;32(1):103-108.
9. Lerner V, Bergman J, Statsenko N, et al. Vitamin B6 treatment in acute neuroleptic-induced akathisia: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2004;65(11):1550-1554.
10. Miller, BJ. Tardive dyskinesia: a review of the literature. Psychiatric Times. http://www.psychiatrictimes.com/articles/tardive-dyskinesia-review-literature. Published June 27, 2017. Accessed July 31, 2018.
1. Carbon M, Hsieh CH, Kane JM, et al. Tardive dyskinesia prevalence in the period of second-generation antipsychotic use: a meta-analysis. J Clin Psychiatry. 2017;78(3):e264-e278.
2. Smith Mosley LL, Mosely II JF, Fleischfresser JR, et al. Vesicular monoamine transporter type 2 (VMAT2) inhibitors in the management of tardive dyskinesia. Clin Med Rev Case Rep. 2017;4(12):1-5.
3. Adelufosi AO, Abayomi O, Ojo M. Pyridoxal 5 phosphate for neuroleptic-induced tardive dyskinesia. Cochrane Database Syst Rev. 2015;(4):CD010501.
4. Lerner V, Miodownik C, Kapstan A, et al. Vitamin B(6) in the treatment of tardive dyskinesia: a double-blind, placebo-controlled, crossover study. Am J Psychiatry. 2001;158(9):1511-1514.
5. Lerner V, Miodownik C, Kapstan A, et al. Vitamin B6 treatment for tardive dyskinesia: a randomized, double-blind, placebo-controlled, crossover study. J Clin Psychiatry. 2007;68(11):1648-1654.
6. Umar MU, Isa AA, Abba AH. High dose pyridoxine for the treatment of tardive dyskinesia: clinical case and review of literature. Ther Adv Psychopharmacol. 2016;6(2):152-156.
7. Miodownik C, Meoded A, Libov I, et al. Pyridoxal plasma level in schizophrenic and schizoaffective patients with and without tardive dyskinesia. Clin Neuropharmacol. 2008;31(4):197-203.
8. Miodownik C, Witztum E, Lerner V. Lithium-induced tremor treated with vitamin B6: a preliminary case series. Int J Psychiatry Med. 2002;32(1):103-108.
9. Lerner V, Bergman J, Statsenko N, et al. Vitamin B6 treatment in acute neuroleptic-induced akathisia: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2004;65(11):1550-1554.
10. Miller, BJ. Tardive dyskinesia: a review of the literature. Psychiatric Times. http://www.psychiatrictimes.com/articles/tardive-dyskinesia-review-literature. Published June 27, 2017. Accessed July 31, 2018.
Proactive consultation: A new model of care in consultation-liaison psychiatry
During my residency training, I was trained in the standard “reactive” psychiatric consultation model. In this system, I would see consults placed by the primary team after they identified a behavioral issue in a patient. As a trainee, I experienced frequent frustrations working in this model: Consults that are discharge-dependent (“Can you see the patient before he is discharged this morning?”), consults for acute behavioral dysregulation (“The patient is near the elevator, can you come see him ASAP?”), or consults for consequences of poor management of alcohol/benzodiazepine withdrawal (“The patient is confused and trying to leave”).
As a fellow in consultation-liaison (C-L) psychiatry, I was introduced to the “proactive” consultation model, which avoids some of these issues. In this article, which is intended for residents who have not been exposed to this new approach, I explain how the proactive model changes our experience as C-L clinicians.
The Behavioral Intervention Team
At Yale New Haven Hospital, the Behavioral Intervention Team (BIT) is a proactive, multidisciplinary psychiatric consultation service that serves the internal medicine units at the hospital. The team consists of nurse practitioners, nurse liaison specialists, social workers, and psychiatrists. The team identifies and removes behavioral barriers in the care of hospitalized mentally ill patients.
The BIT collaborates closely with the medical team through formal and informal consultation; co-management of behavioral issues; education of medical, nursing, and social work staff; and direct care of complex patients with behavioral disorders. The BIT assists the medical team with transitions to appropriate outpatient and inpatient psychiatric care. The team also manages the relationship with the insurer when a patient requires a stay in a psychiatric unit.
This model has a critical financial benefit in reducing the length of stay, but it also has many other benefits. It focuses on early recognition and treatment, and helps mitigate the effects of mental or substance use disorders on patients’ recovery. BIT members educate their peers regarding management of a multitude of behavioral issues. This fosters extensive informal collaboration (“curbside consultation”), which helps patients who did not receive a formal consult. The model distributes work more rationally among different professional specialists. It yields a relationship with medical teams that is not only more effective, but also more enjoyable. In the BIT model, psychiatrists pick the cases where they feel they can have the most impact, and avoid the cases they feel they cannot have any.1-3
CASE A better approach to alcohol withdrawal
Mr. X, age 56, has a history of alcohol use disorder, hypertension, and coronary artery disease. He’s had multiple past admissions for complicated alcohol withdrawal. He is transferred from a local community hospital, where he had presented with chest pain. His last drink was 2 days prior to admission, and his blood alcohol level is <10 mg/dL.
During Mr. X’s previous hospitalizations, psychiatric consults were performed in the standard reactive model. The primary team initially prescribed an ineffective dosage of benzodiazepines for his alcohol withdrawal. This escalated his withdrawal into delirium tremens, after which psychiatry was involved. Due to this early ineffective management, the patient had a prolonged medical ICU stay and overall stay, experienced increased medical complications, and required increased staff resources because he was extremely agitated.
Continued to: During this hospitalization...
During this hospitalization, Mr. X arrives with similar medical complaints. The nurse practitioner on the BIT service, who screened all admissions each day, examines the prior notes (she finds the team sign-outs to be particularly useful). She suggests a psychiatric consult on Day 1 of the admission, which the primary medical team orders. The BIT nurse practitioner gives apt recommendations of evidence-based management, including a benzodiazepine taper, high-dose thiamine, and psychopharmacologic approaches to severe agitation. The nurse liaison specialist on the service makes behavioral plans for managing agitation, which she communicates to the nurses caring for Mr. X.
Because his withdrawal is managed more promptly, Mr. X’s length of stay is shorter and he does not experience any medical complications. The BIT social worker helps find appropriate aftercare options, including residential treatment and Alcoholics Anonymous meetings, to which the patient agrees.
Participating in this case was highly educational for me as a trainee. This case is but one example among many where proactive consultation provided prompt care, lowered the rate of complications, reduced length of stay, and resulted in greater provider satisfaction. The Table4 contrasts the proactive and reactive consultation models. The following 5 factors are critical in the proactive consultation model4,5:
1. Standardized and reliable procedure screening of all admissions, involving a mental health professional, through record review and staff contact. This screening should identify patients with issues who will benefit specifically from in-hospital services, rather than just patients with any psychiatric issue. An electronic medical record is essential to efficient screening, team communication, and progress monitoring. Truly integrated consultation would be impossible with a paper chart.
Continued to: 2. Rapid intervention...
2. Rapid intervention that anticipates impending problems before a cascade of complications starts.
3. Collaborative engagement with the primary medical team, sharing the burden of caring for the complex inpatient, and transmitting critical behavioral management skills to all caregivers, including the skill of recognizing patients who can benefit from a psychiatric consultation.
4. Daily and close contact between behavioral and medical teams, ensuring that treatment recommendations are understood, enacted, and reinforced, ineffective treatments are discontinued, and new problems are addressed before complicating consequences arise. Dedicating specific personnel to specific hospital units and placing them in rounds simplifies communication and speeds intervention implementation.
5. A multidisciplinary consultation team, offering a range of responses, including informal curbside consultation, consultation with an advanced practice registered nurse, social work interventions, advice to discharge planning teams, psychological services, and access to specialized providers, such as addiction teams, as well as traditional consultation with an experienced psychiatrist.
Research has shown the effectiveness of proactive, embedded, multidisciplinary approaches.1-3,5 It was a gratifying experience to work in this model. I worked intimately with medical clinicians, and shared the burden of responsibilities leading to optimal patient outcomes. The proactive consultation model truly re-emphasizes the “liaison” component of C-L psychiatry, as it was originally envisioned.
1. Sledge WH, Gueorguieva R, Desan P, et al. Multidisciplinary proactive psychiatric consultation service: impact on length of stay for medical inpatients. Psychother Psychosom. 2015;84(4):208-216.
2. Desan PH, Zimbrean PC, Weinstein AJ, et al. Proactive psychiatric consultation services reduce length of stay for admissions to an inpatient medical team. Psychosomatics. 2011;52(6):513-520.
3. Sledge WH, Bozzo J, White-McCullum BA, et al. The cost-benefit from the perspective of the hospital of a proactive psychiatric consultation service on inpatient general medicine services. Health Econ Outcome Res Open Access. 2016;2(4):122.
4. Sledge WH, Lee HB. Proactive psychiatric consultation for hospitalized patients, a plan for the future. Health Affairs. www.healthaffairs.org/do/10.1377/hblog20150528.048026/full/. Published May 28, 2015. Accessed September 12, 2018.
5. Desan P, Lee H, Zimbrean P, et al. New models of psychiatric consultation in the general medical hospital: liaison psychiatry is back. Psychiatr Ann. 2017;47:355-361.
During my residency training, I was trained in the standard “reactive” psychiatric consultation model. In this system, I would see consults placed by the primary team after they identified a behavioral issue in a patient. As a trainee, I experienced frequent frustrations working in this model: Consults that are discharge-dependent (“Can you see the patient before he is discharged this morning?”), consults for acute behavioral dysregulation (“The patient is near the elevator, can you come see him ASAP?”), or consults for consequences of poor management of alcohol/benzodiazepine withdrawal (“The patient is confused and trying to leave”).
As a fellow in consultation-liaison (C-L) psychiatry, I was introduced to the “proactive” consultation model, which avoids some of these issues. In this article, which is intended for residents who have not been exposed to this new approach, I explain how the proactive model changes our experience as C-L clinicians.
The Behavioral Intervention Team
At Yale New Haven Hospital, the Behavioral Intervention Team (BIT) is a proactive, multidisciplinary psychiatric consultation service that serves the internal medicine units at the hospital. The team consists of nurse practitioners, nurse liaison specialists, social workers, and psychiatrists. The team identifies and removes behavioral barriers in the care of hospitalized mentally ill patients.
The BIT collaborates closely with the medical team through formal and informal consultation; co-management of behavioral issues; education of medical, nursing, and social work staff; and direct care of complex patients with behavioral disorders. The BIT assists the medical team with transitions to appropriate outpatient and inpatient psychiatric care. The team also manages the relationship with the insurer when a patient requires a stay in a psychiatric unit.
This model has a critical financial benefit in reducing the length of stay, but it also has many other benefits. It focuses on early recognition and treatment, and helps mitigate the effects of mental or substance use disorders on patients’ recovery. BIT members educate their peers regarding management of a multitude of behavioral issues. This fosters extensive informal collaboration (“curbside consultation”), which helps patients who did not receive a formal consult. The model distributes work more rationally among different professional specialists. It yields a relationship with medical teams that is not only more effective, but also more enjoyable. In the BIT model, psychiatrists pick the cases where they feel they can have the most impact, and avoid the cases they feel they cannot have any.1-3
CASE A better approach to alcohol withdrawal
Mr. X, age 56, has a history of alcohol use disorder, hypertension, and coronary artery disease. He’s had multiple past admissions for complicated alcohol withdrawal. He is transferred from a local community hospital, where he had presented with chest pain. His last drink was 2 days prior to admission, and his blood alcohol level is <10 mg/dL.
During Mr. X’s previous hospitalizations, psychiatric consults were performed in the standard reactive model. The primary team initially prescribed an ineffective dosage of benzodiazepines for his alcohol withdrawal. This escalated his withdrawal into delirium tremens, after which psychiatry was involved. Due to this early ineffective management, the patient had a prolonged medical ICU stay and overall stay, experienced increased medical complications, and required increased staff resources because he was extremely agitated.
Continued to: During this hospitalization...
During this hospitalization, Mr. X arrives with similar medical complaints. The nurse practitioner on the BIT service, who screened all admissions each day, examines the prior notes (she finds the team sign-outs to be particularly useful). She suggests a psychiatric consult on Day 1 of the admission, which the primary medical team orders. The BIT nurse practitioner gives apt recommendations of evidence-based management, including a benzodiazepine taper, high-dose thiamine, and psychopharmacologic approaches to severe agitation. The nurse liaison specialist on the service makes behavioral plans for managing agitation, which she communicates to the nurses caring for Mr. X.
Because his withdrawal is managed more promptly, Mr. X’s length of stay is shorter and he does not experience any medical complications. The BIT social worker helps find appropriate aftercare options, including residential treatment and Alcoholics Anonymous meetings, to which the patient agrees.
Participating in this case was highly educational for me as a trainee. This case is but one example among many where proactive consultation provided prompt care, lowered the rate of complications, reduced length of stay, and resulted in greater provider satisfaction. The Table4 contrasts the proactive and reactive consultation models. The following 5 factors are critical in the proactive consultation model4,5:
1. Standardized and reliable procedure screening of all admissions, involving a mental health professional, through record review and staff contact. This screening should identify patients with issues who will benefit specifically from in-hospital services, rather than just patients with any psychiatric issue. An electronic medical record is essential to efficient screening, team communication, and progress monitoring. Truly integrated consultation would be impossible with a paper chart.

Continued to: 2. Rapid intervention...
2. Rapid intervention that anticipates impending problems before a cascade of complications starts.
3. Collaborative engagement with the primary medical team, sharing the burden of caring for the complex inpatient, and transmitting critical behavioral management skills to all caregivers, including the skill of recognizing patients who can benefit from a psychiatric consultation.
4. Daily and close contact between behavioral and medical teams, ensuring that treatment recommendations are understood, enacted, and reinforced, ineffective treatments are discontinued, and new problems are addressed before complicating consequences arise. Dedicating specific personnel to specific hospital units and placing them in rounds simplifies communication and speeds intervention implementation.
5. A multidisciplinary consultation team, offering a range of responses, including informal curbside consultation, consultation with an advanced practice registered nurse, social work interventions, advice to discharge planning teams, psychological services, and access to specialized providers, such as addiction teams, as well as traditional consultation with an experienced psychiatrist.
Research has shown the effectiveness of proactive, embedded, multidisciplinary approaches.1-3,5 It was a gratifying experience to work in this model. I worked intimately with medical clinicians, and shared the burden of responsibilities leading to optimal patient outcomes. The proactive consultation model truly re-emphasizes the “liaison” component of C-L psychiatry, as it was originally envisioned.
During my residency training, I was trained in the standard “reactive” psychiatric consultation model. In this system, I would see consults placed by the primary team after they identified a behavioral issue in a patient. As a trainee, I experienced frequent frustrations working in this model: Consults that are discharge-dependent (“Can you see the patient before he is discharged this morning?”), consults for acute behavioral dysregulation (“The patient is near the elevator, can you come see him ASAP?”), or consults for consequences of poor management of alcohol/benzodiazepine withdrawal (“The patient is confused and trying to leave”).
As a fellow in consultation-liaison (C-L) psychiatry, I was introduced to the “proactive” consultation model, which avoids some of these issues. In this article, which is intended for residents who have not been exposed to this new approach, I explain how the proactive model changes our experience as C-L clinicians.
The Behavioral Intervention Team
At Yale New Haven Hospital, the Behavioral Intervention Team (BIT) is a proactive, multidisciplinary psychiatric consultation service that serves the internal medicine units at the hospital. The team consists of nurse practitioners, nurse liaison specialists, social workers, and psychiatrists. The team identifies and removes behavioral barriers in the care of hospitalized mentally ill patients.
The BIT collaborates closely with the medical team through formal and informal consultation; co-management of behavioral issues; education of medical, nursing, and social work staff; and direct care of complex patients with behavioral disorders. The BIT assists the medical team with transitions to appropriate outpatient and inpatient psychiatric care. The team also manages the relationship with the insurer when a patient requires a stay in a psychiatric unit.
This model has a critical financial benefit in reducing the length of stay, but it also has many other benefits. It focuses on early recognition and treatment, and helps mitigate the effects of mental or substance use disorders on patients’ recovery. BIT members educate their peers regarding management of a multitude of behavioral issues. This fosters extensive informal collaboration (“curbside consultation”), which helps patients who did not receive a formal consult. The model distributes work more rationally among different professional specialists. It yields a relationship with medical teams that is not only more effective, but also more enjoyable. In the BIT model, psychiatrists pick the cases where they feel they can have the most impact, and avoid the cases they feel they cannot have any.1-3
CASE A better approach to alcohol withdrawal
Mr. X, age 56, has a history of alcohol use disorder, hypertension, and coronary artery disease. He’s had multiple past admissions for complicated alcohol withdrawal. He is transferred from a local community hospital, where he had presented with chest pain. His last drink was 2 days prior to admission, and his blood alcohol level is <10 mg/dL.
During Mr. X’s previous hospitalizations, psychiatric consults were performed in the standard reactive model. The primary team initially prescribed an ineffective dosage of benzodiazepines for his alcohol withdrawal. This escalated his withdrawal into delirium tremens, after which psychiatry was involved. Due to this early ineffective management, the patient had a prolonged medical ICU stay and overall stay, experienced increased medical complications, and required increased staff resources because he was extremely agitated.
Continued to: During this hospitalization...
During this hospitalization, Mr. X arrives with similar medical complaints. The nurse practitioner on the BIT service, who screened all admissions each day, examines the prior notes (she finds the team sign-outs to be particularly useful). She suggests a psychiatric consult on Day 1 of the admission, which the primary medical team orders. The BIT nurse practitioner gives apt recommendations of evidence-based management, including a benzodiazepine taper, high-dose thiamine, and psychopharmacologic approaches to severe agitation. The nurse liaison specialist on the service makes behavioral plans for managing agitation, which she communicates to the nurses caring for Mr. X.
Because his withdrawal is managed more promptly, Mr. X’s length of stay is shorter and he does not experience any medical complications. The BIT social worker helps find appropriate aftercare options, including residential treatment and Alcoholics Anonymous meetings, to which the patient agrees.
Participating in this case was highly educational for me as a trainee. This case is but one example among many where proactive consultation provided prompt care, lowered the rate of complications, reduced length of stay, and resulted in greater provider satisfaction. The Table4 contrasts the proactive and reactive consultation models. The following 5 factors are critical in the proactive consultation model4,5:
1. Standardized and reliable procedure screening of all admissions, involving a mental health professional, through record review and staff contact. This screening should identify patients with issues who will benefit specifically from in-hospital services, rather than just patients with any psychiatric issue. An electronic medical record is essential to efficient screening, team communication, and progress monitoring. Truly integrated consultation would be impossible with a paper chart.

Continued to: 2. Rapid intervention...
2. Rapid intervention that anticipates impending problems before a cascade of complications starts.
3. Collaborative engagement with the primary medical team, sharing the burden of caring for the complex inpatient, and transmitting critical behavioral management skills to all caregivers, including the skill of recognizing patients who can benefit from a psychiatric consultation.
4. Daily and close contact between behavioral and medical teams, ensuring that treatment recommendations are understood, enacted, and reinforced, ineffective treatments are discontinued, and new problems are addressed before complicating consequences arise. Dedicating specific personnel to specific hospital units and placing them in rounds simplifies communication and speeds intervention implementation.
5. A multidisciplinary consultation team, offering a range of responses, including informal curbside consultation, consultation with an advanced practice registered nurse, social work interventions, advice to discharge planning teams, psychological services, and access to specialized providers, such as addiction teams, as well as traditional consultation with an experienced psychiatrist.
Research has shown the effectiveness of proactive, embedded, multidisciplinary approaches.1-3,5 It was a gratifying experience to work in this model. I worked intimately with medical clinicians, and shared the burden of responsibilities leading to optimal patient outcomes. The proactive consultation model truly re-emphasizes the “liaison” component of C-L psychiatry, as it was originally envisioned.
1. Sledge WH, Gueorguieva R, Desan P, et al. Multidisciplinary proactive psychiatric consultation service: impact on length of stay for medical inpatients. Psychother Psychosom. 2015;84(4):208-216.
2. Desan PH, Zimbrean PC, Weinstein AJ, et al. Proactive psychiatric consultation services reduce length of stay for admissions to an inpatient medical team. Psychosomatics. 2011;52(6):513-520.
3. Sledge WH, Bozzo J, White-McCullum BA, et al. The cost-benefit from the perspective of the hospital of a proactive psychiatric consultation service on inpatient general medicine services. Health Econ Outcome Res Open Access. 2016;2(4):122.
4. Sledge WH, Lee HB. Proactive psychiatric consultation for hospitalized patients, a plan for the future. Health Affairs. www.healthaffairs.org/do/10.1377/hblog20150528.048026/full/. Published May 28, 2015. Accessed September 12, 2018.
5. Desan P, Lee H, Zimbrean P, et al. New models of psychiatric consultation in the general medical hospital: liaison psychiatry is back. Psychiatr Ann. 2017;47:355-361.
1. Sledge WH, Gueorguieva R, Desan P, et al. Multidisciplinary proactive psychiatric consultation service: impact on length of stay for medical inpatients. Psychother Psychosom. 2015;84(4):208-216.
2. Desan PH, Zimbrean PC, Weinstein AJ, et al. Proactive psychiatric consultation services reduce length of stay for admissions to an inpatient medical team. Psychosomatics. 2011;52(6):513-520.
3. Sledge WH, Bozzo J, White-McCullum BA, et al. The cost-benefit from the perspective of the hospital of a proactive psychiatric consultation service on inpatient general medicine services. Health Econ Outcome Res Open Access. 2016;2(4):122.
4. Sledge WH, Lee HB. Proactive psychiatric consultation for hospitalized patients, a plan for the future. Health Affairs. www.healthaffairs.org/do/10.1377/hblog20150528.048026/full/. Published May 28, 2015. Accessed September 12, 2018.
5. Desan P, Lee H, Zimbrean P, et al. New models of psychiatric consultation in the general medical hospital: liaison psychiatry is back. Psychiatr Ann. 2017;47:355-361.
BCMA-targeted platforms could alter MM therapy
New York—Three novel treatment strategies that target B-cell maturation antigen (BCMA) are showing promise in recent multiple myeloma (MM) clinical trials, according to Shaji K. Kumar, MD, of Mayo Clinic Cancer Center in Rochester, Minnesota.
The strategies include B-cell maturation antigen (BCMA) antibody-drug conjugate, BCMA-specific chimeric antigen receptor (CAR) T-cell therapies, and bispecific T-cell engagers (BiTEs).
“Clearly, there are a lot of exciting drugs that are currently in clinical trials, but these 3 platforms appear to be much more advanced than the others, and hopefully we will see that in the clinic in the near future,” Dr. Kumar told attendees at the NCCN 13th Annual Congress: Hematologic Malignancies.
BCMA is required for plasma cell survival and is broadly expressed on malignant plasma cells.
BCMA antibody-drug conjugate
The antibody-drug conjugate, GSK2857916, is a humanized IgG1 anti-BCMA antibody conjugated to a microtubule-disrupting agent. It produced an overall response rate of 67% at the 2 highest dose levels in 9 MM patients who had previously received multiple standard-of-care agents.
“Some of the responses were quite durable, lasting several months,” he said.
Now, GSK2857916 is being evaluated in a variety of different combinations, he said, including in an upcoming phase 2 study of the antibody-drug conjugate in combination with lenalidomide plus dexamethasone or bortezomib plus dexamethasone in patients with relapsed or refractory disease.
BCMA-specific CAR T-cell therapy
Some of the most “exciting” data with anti-BCMA CAR T-cell therapy in myeloma, according to Dr. Kumar, involves bb2121. bb2121 showed durable clinical responses in heavily pretreated patients, according to an ASH 2017 presentation.
“The overall response rate is quite significant,” Dr. Kumar said. He related a 94% rate of overall response that was even higher in patients treated with doses of 150 x 106 CAR+ T cells or more. Many of the responses were lasting, he said, with 5 patients in ongoing response for more than a year.
“The results are exciting enough that this is actually moving forward with registration trials,” Dr. Kumar added.
Another novel CAR T-cell product, LCAR-B38M, has demonstrated promising results. LCAR-B38M principally targets BCMA and has led to a significant number of patients achieving stringent complete response that lasted beyond 1 year.
Multiple BCMA-targeting CAR T-cell products that use different vectors and different costimulatory molecules are currently in clinical trials, Dr. Kumar said.
BiTEs
In contrast to CAR T-cell products that must be customized to each patient in a process that takes weeks, BiTEs are a ready-made approach to allow T cells to engage with tumor cells.
“In patients with advanced disease, a lot can change in that short timeframe, so having an approach that is off-the-shelf, which is not patient specific, is quite attractive,” Dr. Kumar said.
BCMA-directed BiTE therapies to watch that are under investigation include AMG 420 and PF-06863135, he said.
New York—Three novel treatment strategies that target B-cell maturation antigen (BCMA) are showing promise in recent multiple myeloma (MM) clinical trials, according to Shaji K. Kumar, MD, of Mayo Clinic Cancer Center in Rochester, Minnesota.
The strategies include B-cell maturation antigen (BCMA) antibody-drug conjugate, BCMA-specific chimeric antigen receptor (CAR) T-cell therapies, and bispecific T-cell engagers (BiTEs).
“Clearly, there are a lot of exciting drugs that are currently in clinical trials, but these 3 platforms appear to be much more advanced than the others, and hopefully we will see that in the clinic in the near future,” Dr. Kumar told attendees at the NCCN 13th Annual Congress: Hematologic Malignancies.
BCMA is required for plasma cell survival and is broadly expressed on malignant plasma cells.
BCMA antibody-drug conjugate
The antibody-drug conjugate, GSK2857916, is a humanized IgG1 anti-BCMA antibody conjugated to a microtubule-disrupting agent. It produced an overall response rate of 67% at the 2 highest dose levels in 9 MM patients who had previously received multiple standard-of-care agents.
“Some of the responses were quite durable, lasting several months,” he said.
Now, GSK2857916 is being evaluated in a variety of different combinations, he said, including in an upcoming phase 2 study of the antibody-drug conjugate in combination with lenalidomide plus dexamethasone or bortezomib plus dexamethasone in patients with relapsed or refractory disease.
BCMA-specific CAR T-cell therapy
Some of the most “exciting” data with anti-BCMA CAR T-cell therapy in myeloma, according to Dr. Kumar, involves bb2121. bb2121 showed durable clinical responses in heavily pretreated patients, according to an ASH 2017 presentation.
“The overall response rate is quite significant,” Dr. Kumar said. He related a 94% rate of overall response that was even higher in patients treated with doses of 150 x 106 CAR+ T cells or more. Many of the responses were lasting, he said, with 5 patients in ongoing response for more than a year.
“The results are exciting enough that this is actually moving forward with registration trials,” Dr. Kumar added.
Another novel CAR T-cell product, LCAR-B38M, has demonstrated promising results. LCAR-B38M principally targets BCMA and has led to a significant number of patients achieving stringent complete response that lasted beyond 1 year.
Multiple BCMA-targeting CAR T-cell products that use different vectors and different costimulatory molecules are currently in clinical trials, Dr. Kumar said.
BiTEs
In contrast to CAR T-cell products that must be customized to each patient in a process that takes weeks, BiTEs are a ready-made approach to allow T cells to engage with tumor cells.
“In patients with advanced disease, a lot can change in that short timeframe, so having an approach that is off-the-shelf, which is not patient specific, is quite attractive,” Dr. Kumar said.
BCMA-directed BiTE therapies to watch that are under investigation include AMG 420 and PF-06863135, he said.
New York—Three novel treatment strategies that target B-cell maturation antigen (BCMA) are showing promise in recent multiple myeloma (MM) clinical trials, according to Shaji K. Kumar, MD, of Mayo Clinic Cancer Center in Rochester, Minnesota.
The strategies include B-cell maturation antigen (BCMA) antibody-drug conjugate, BCMA-specific chimeric antigen receptor (CAR) T-cell therapies, and bispecific T-cell engagers (BiTEs).
“Clearly, there are a lot of exciting drugs that are currently in clinical trials, but these 3 platforms appear to be much more advanced than the others, and hopefully we will see that in the clinic in the near future,” Dr. Kumar told attendees at the NCCN 13th Annual Congress: Hematologic Malignancies.
BCMA is required for plasma cell survival and is broadly expressed on malignant plasma cells.
BCMA antibody-drug conjugate
The antibody-drug conjugate, GSK2857916, is a humanized IgG1 anti-BCMA antibody conjugated to a microtubule-disrupting agent. It produced an overall response rate of 67% at the 2 highest dose levels in 9 MM patients who had previously received multiple standard-of-care agents.
“Some of the responses were quite durable, lasting several months,” he said.
Now, GSK2857916 is being evaluated in a variety of different combinations, he said, including in an upcoming phase 2 study of the antibody-drug conjugate in combination with lenalidomide plus dexamethasone or bortezomib plus dexamethasone in patients with relapsed or refractory disease.
BCMA-specific CAR T-cell therapy
Some of the most “exciting” data with anti-BCMA CAR T-cell therapy in myeloma, according to Dr. Kumar, involves bb2121. bb2121 showed durable clinical responses in heavily pretreated patients, according to an ASH 2017 presentation.
“The overall response rate is quite significant,” Dr. Kumar said. He related a 94% rate of overall response that was even higher in patients treated with doses of 150 x 106 CAR+ T cells or more. Many of the responses were lasting, he said, with 5 patients in ongoing response for more than a year.
“The results are exciting enough that this is actually moving forward with registration trials,” Dr. Kumar added.
Another novel CAR T-cell product, LCAR-B38M, has demonstrated promising results. LCAR-B38M principally targets BCMA and has led to a significant number of patients achieving stringent complete response that lasted beyond 1 year.
Multiple BCMA-targeting CAR T-cell products that use different vectors and different costimulatory molecules are currently in clinical trials, Dr. Kumar said.
BiTEs
In contrast to CAR T-cell products that must be customized to each patient in a process that takes weeks, BiTEs are a ready-made approach to allow T cells to engage with tumor cells.
“In patients with advanced disease, a lot can change in that short timeframe, so having an approach that is off-the-shelf, which is not patient specific, is quite attractive,” Dr. Kumar said.
BCMA-directed BiTE therapies to watch that are under investigation include AMG 420 and PF-06863135, he said.
Brentuximab improves survival in older HL patients
Older patients with untreated Hodgkin lymphoma (HL) can achieve significantly improved survival by adding brentuximab vedotin to their treatment before and after standard chemotherapy, a recent study found.
In patients with low comorbidity scores, responses were even more robust, reported lead author Andrew M. Evens, DO, of the Rutgers Cancer Institute of New Jersey, and colleagues.
“Causes of poor outcomes for older patients with HL are not fully understood but have been attributed to a combination of factors, including presence of comorbidities, poorer performance status, disease and biological differences, inability to tolerate chemotherapy at the full dose, and increased treatment-related toxicities,” the authors wrote in the Journal of Clinical Oncology.
The primary goal of the study was to improve outcomes for untreated, older patients, a group that’s historically been a difficult-to-treat patient population.
The phase 2 trial included 48 HL patients with a median age of 69 (range, 60 – 88).
All patients underwent geriatric assessment for comorbidities and loss of activities of daily living.
Treatment consisted of two doses of brentuximab followed by six cycles of doxorubicin, vinblastine, and dacarbazine (AVD), then four more doses of brentuximab (consolidation doses).
The primary endpoint was complete remission at completion of AVD.
Secondary outcomes included overall response rate, 2-year progression-free survival, 2-year overall survival, and safety.
Just over half the patients (52%) completed all cycles of therapy, and almost three quarters (73%) received at least one consolidation dose of brentuximab.
Among the first 23 evaluable patients, both the complete remission rate and overall response rate were 96%. Intention-to-treat survival rates for all 48 patients were 84% for 2-year progression-free survival and 93% for 2-year overall survival.
Historical 2-year progression-free survival rates in similar older patients is poor, at 50%, so the progression-free survival rate of 84% in this study represents a significant improvement.
Of note, patients with fewer comorbidities and without loss of instrumental activities of daily living showed more robust responses.
Patients with Cumulative Illness Rating Scale for Geriatrics (CIRS-G) comorbidity scores of less than 10 had a 2-year progression-free survival rate of 100% versus 45% for those with higher scores.
Similarly, patients without loss of instrumental activities achieved a progression-free survival rate of 94% versus 25% for those who had lost some instrumental activities.
Grade 3 or 4 adverse events occurred in 42% of patients, with neutropenia being the most common (44%).
“This study represents among the best-reported outcomes to date for untreated older patients with HL,” the investigators concluded.
Seattle Genetics supported the investigator-initiated trial.
Older patients with untreated Hodgkin lymphoma (HL) can achieve significantly improved survival by adding brentuximab vedotin to their treatment before and after standard chemotherapy, a recent study found.
In patients with low comorbidity scores, responses were even more robust, reported lead author Andrew M. Evens, DO, of the Rutgers Cancer Institute of New Jersey, and colleagues.
“Causes of poor outcomes for older patients with HL are not fully understood but have been attributed to a combination of factors, including presence of comorbidities, poorer performance status, disease and biological differences, inability to tolerate chemotherapy at the full dose, and increased treatment-related toxicities,” the authors wrote in the Journal of Clinical Oncology.
The primary goal of the study was to improve outcomes for untreated, older patients, a group that’s historically been a difficult-to-treat patient population.
The phase 2 trial included 48 HL patients with a median age of 69 (range, 60 – 88).
All patients underwent geriatric assessment for comorbidities and loss of activities of daily living.
Treatment consisted of two doses of brentuximab followed by six cycles of doxorubicin, vinblastine, and dacarbazine (AVD), then four more doses of brentuximab (consolidation doses).
The primary endpoint was complete remission at completion of AVD.
Secondary outcomes included overall response rate, 2-year progression-free survival, 2-year overall survival, and safety.
Just over half the patients (52%) completed all cycles of therapy, and almost three quarters (73%) received at least one consolidation dose of brentuximab.
Among the first 23 evaluable patients, both the complete remission rate and overall response rate were 96%. Intention-to-treat survival rates for all 48 patients were 84% for 2-year progression-free survival and 93% for 2-year overall survival.
Historical 2-year progression-free survival rates in similar older patients is poor, at 50%, so the progression-free survival rate of 84% in this study represents a significant improvement.
Of note, patients with fewer comorbidities and without loss of instrumental activities of daily living showed more robust responses.
Patients with Cumulative Illness Rating Scale for Geriatrics (CIRS-G) comorbidity scores of less than 10 had a 2-year progression-free survival rate of 100% versus 45% for those with higher scores.
Similarly, patients without loss of instrumental activities achieved a progression-free survival rate of 94% versus 25% for those who had lost some instrumental activities.
Grade 3 or 4 adverse events occurred in 42% of patients, with neutropenia being the most common (44%).
“This study represents among the best-reported outcomes to date for untreated older patients with HL,” the investigators concluded.
Seattle Genetics supported the investigator-initiated trial.
Older patients with untreated Hodgkin lymphoma (HL) can achieve significantly improved survival by adding brentuximab vedotin to their treatment before and after standard chemotherapy, a recent study found.
In patients with low comorbidity scores, responses were even more robust, reported lead author Andrew M. Evens, DO, of the Rutgers Cancer Institute of New Jersey, and colleagues.
“Causes of poor outcomes for older patients with HL are not fully understood but have been attributed to a combination of factors, including presence of comorbidities, poorer performance status, disease and biological differences, inability to tolerate chemotherapy at the full dose, and increased treatment-related toxicities,” the authors wrote in the Journal of Clinical Oncology.
The primary goal of the study was to improve outcomes for untreated, older patients, a group that’s historically been a difficult-to-treat patient population.
The phase 2 trial included 48 HL patients with a median age of 69 (range, 60 – 88).
All patients underwent geriatric assessment for comorbidities and loss of activities of daily living.
Treatment consisted of two doses of brentuximab followed by six cycles of doxorubicin, vinblastine, and dacarbazine (AVD), then four more doses of brentuximab (consolidation doses).
The primary endpoint was complete remission at completion of AVD.
Secondary outcomes included overall response rate, 2-year progression-free survival, 2-year overall survival, and safety.
Just over half the patients (52%) completed all cycles of therapy, and almost three quarters (73%) received at least one consolidation dose of brentuximab.
Among the first 23 evaluable patients, both the complete remission rate and overall response rate were 96%. Intention-to-treat survival rates for all 48 patients were 84% for 2-year progression-free survival and 93% for 2-year overall survival.
Historical 2-year progression-free survival rates in similar older patients is poor, at 50%, so the progression-free survival rate of 84% in this study represents a significant improvement.
Of note, patients with fewer comorbidities and without loss of instrumental activities of daily living showed more robust responses.
Patients with Cumulative Illness Rating Scale for Geriatrics (CIRS-G) comorbidity scores of less than 10 had a 2-year progression-free survival rate of 100% versus 45% for those with higher scores.
Similarly, patients without loss of instrumental activities achieved a progression-free survival rate of 94% versus 25% for those who had lost some instrumental activities.
Grade 3 or 4 adverse events occurred in 42% of patients, with neutropenia being the most common (44%).
“This study represents among the best-reported outcomes to date for untreated older patients with HL,” the investigators concluded.
Seattle Genetics supported the investigator-initiated trial.
Small Cell Lung Cancer
INTRODUCTION
Small cell lung cancer (SCLC) is an aggressive cancer of neuroendocrine origin that accounts for approximately 15% of all lung cancer cases, with approximately 33,000 patients diagnosed annually.1 The incidence of SCLC in the United States has steadily declined over the past 30 years, presumably because of a decrease in the number of smokers and a change to low-tar filter cigarettes.2 Although the overall incidence of SCLC has been decreasing, the incidence in women is increasing and the male-to-female incidence ratio is now 1:1.3 Nearly all cases of SCLC are associated with heavy tobacco exposure, making it a heterogeneous disease with a complex genomic landscape consisting of thousands of mutations.4,5 Despite recent advances in the treatment of non-small cell lung cancer, the therapeutic options for SCLC remain limited, with a median overall survival (OS) of 9 months in patients with advanced disease.
DIAGNOSIS AND STAGING
CASE PRESENTATION
A 61-year-old man presents to the emergency department with progressive shortness of breath and cough over the past 6 weeks. He also reports a 20-lb weight loss over the same period. He is a current smoker and has been smoking 1 pack of cigarettes per day since the age of 18 years. A chest radiograph obtained in the emergency department shows a right hilar mass. Computed tomography (CT) scan confirms the presence of a 4.5-cm right hilar mass and enlarged mediastinal lymph nodes bilaterally.
• What are the next steps in diagnosis?
SCLC is characterized by rapid growth and early hematogenous metastasis. Consequently, only 25% of patients have limited-stage disease at the time of diagnosis. According to the Veterans Administration Lung Study Group (VALSG) staging system, limited-stage disease is defined as tumor that is confined to 1 hemithorax and can be encompassed within 1 radiation field. This typically includes mediastinal lymph nodes and ipsilateral supraclavicular lymph nodes. Approximately 75% of patients present with extensive-stage disease, which is defined as disease that cannot be classified as limited, including disease that extends beyond 1 hemithorax. Extensive-stage disease includes the presence of malignant pleural effusion and/or distant metastasis.6 The VALSG classification and staging system is more commonly used in clinical practice than the American Joint Committee on Cancer TNM staging system because it is less complex and directs treatment decisions, as most of the literature on SCLC classifies patients based on the VALSG system.7
Given SCLC’s propensity to metastasize quickly, none of the currently available screening methods have proven successful in early detection of SCLC. In the National Lung Cancer Screening Trial, 86% of the 125 patients who were diagnosed with SCLC while undergoing annual low-dose chest CT scans had advanced disease at diagnosis.8,9 These results highlight the fact that most cases of SCLC develop in the interval between annual screening imaging.
SCLC frequently presents with a large hilar mass that is symptomatic. Common symptoms include shortness of breath and cough. In addition, patients with SCLC usually have bulky mediastinal adenopathy at presentation. SCLC is commonly located submucosally in the bronchus, and therefore hemoptysis is not a very common symptom at the time of presentation. Patients may present with superior vena cava syndrome from local compression by the tumor. Not infrequently, SCLC is associated with paraneoplastic syndromes that arise due to ectopic secretion of hormones or antibodies by the tumor cells. The paraneoplastic syndromes can be broadly categorized as endocrine or neurologic (Table 1). The presence of a paraneoplastic syndrome is often a clue to the potential diagnosis of SCLC in the presence of a hilar mass. Additionally, some paraneoplastic syndromes, more specifically endocrine paraneoplastic syndromes, follow the pattern of disease response and relapse, and therefore can sometimes serve as an early marker of disease relapse or progression.
The common sites of metastases include brain, liver, and bone. Therefore, the staging workup should include fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT scan. Contrast-enhanced CT scan of the chest and abdomen and bone scan can be obtained for staging in lieu of PET scan. Due to the physiologic FDG uptake, cerebral metastases cannot be assessed with sufficient certainty using PET-CT.10 Therefore, brain imaging with contrast-enhanced CT or magnetic resonance imaging (MRI) is also necessary. Although the incidence of metastasis to bone marrow is less than 10%, bone marrow aspiration and biopsy are warranted in patients with unexplained cytopenias, especially when the cytopenia is associated with teardrop-shaped red cells or nucleated red cells on peripheral blood smear, findings indicative of a marrow infiltrative process.7 The tissue diagnosis is established by obtaining a biopsy of the primary tumor or 1 of the metastatic sites. In localized disease, bronchoscopy (with endobronchial ultrasound, if necessary) with biopsy of the centrally located tumor and/or lymph node is required. Histologically, SCLC consists of monomorphic cells, a high nuclear-cytoplasmic ratio, and confluent necrosis. The tumor cells are positive for chromogranin, synaptophysin, and CD56 by immunohistochemistry, and very frequently are also positive for thyroid transcription factor 1.11 Although serum tumor markers, including neuron-specific enolase and progastrin-releasing peptide, are frequently elevated in patients with SCLC, these markers are of limited value in clinical practice because they lack sensitivity and specificity.12
MANAGEMENT OF LIMITED-STAGE DISEASE
CASE CONTINUED
The patient undergoes FDG PET scan, which shows the presence of a hypermetabolic right hilar mass in addition to enlarged and hypermetabolic bilateral mediastinal lymph nodes. There are no other areas of FDG avidity. Brain MRI does not show any evidence of brain metastasis. Thus, the patient is confirmed to have limited-stage SCLC.
• What is the standard of care treatment for limited-stage SCLC?
SCLC is exquisitely sensitive to both chemotherapy and radiation, especially at the time of initial presentation. The standard of care treatment of limited-stage SCLC is 4 cycles of platinum-based chemotherapy in combination with thoracic radiation started within the first 2 cycles of chemotherapy (Figure 1).
CHOICE OF CHEMOTHERAPY
Etoposide and cisplatin is the most commonly used initial combination chemotherapy regimen in limited-stage SCLC.14 This combination has largely replaced anthracycline-based regimens given its favorable efficacy and toxicity profile.15–17 Several small randomized trials have shown comparable efficacy of carboplatin and etoposide in extensive-stage SCLC.18–20 A meta-analysis of 4 randomized trials comparing cisplatin-based versus carboplatin-based regimens in 663 patients with SCLC (32% had limited-stage disease and 68% had extensive-stage disease) showed no statistically significant difference in response rate, progression-free survival (PFS), or OS between the 2 regimens.21 Therefore, in clinical practice carboplatin is frequently used instead of cisplatin in patients with extensive-stage disease. In patients with limited-stage disease, cisplatin is still the drug of choice. However, the toxicity profile of the 2 regimens is different. Cisplatin-based regimens are more commonly associated with neuropathy, nephrotoxicity, and chemotherapy-induced nausea/vomiting,18 while carboplatin-based regimens are more myelosuppressive.22 In addition, the combination of thoracic radiation with either of these regimens is associated with a higher risk of esophagitis, pneumonitis, and myelosuppression.23 The use of myeloid growth factors is not recommended in patients undergoing concurrent chemoradiation.24 Of note, intravenous etoposide is always preferred over oral etoposide, especially in the curative setting given the unreliable absorption and bioavailability of oral formulations.
THORACIC RADIOTHERAPY
Adding thoracic radiotherapy to platinum-etoposide chemotherapy improves local control and OS. Two meta-analyses of 13 trials including more than 2000 patients have shown a 25% to 30% decrease in local failure and a 5% to 7% increase in 2-year OS with chemoradiation compared to chemotherapy alone in limited-stage SCLC.25,26 Early (within the first 2 cycles) concurrent thoracic radiation is superior to delayed and/or sequential radiation in terms of local control and OS.23,27,28 The dose and fractionation of thoracic radiation in limited-stage SCLC has remained a controversial issue. The Eastern Cooperative Oncology Group/Radiation Therapy Oncology Group randomized trial compared 45 Gy of radiotherapy delivered twice daily over a period of 3 weeks to 45 Gy once daily over 5 weeks concurrently with chemotherapy. The twice daily regimen led to a 10% improvement in 5-year OS (26% versus 16%), but a higher incidence of grade 3 and 4 adverse events.13 Despite the survival advantage demonstrated by hyperfractionated radiotherapy, the results need to be interpreted with caution because the radiation doses are not biologically equivalent. In addition, the difficult logistics of patients receiving radiation twice a day has limited the routine implementation of this strategy. Subsequently, another randomized phase 3 trial (CONVERT) compared 45 Gy radiotherapy twice daily with 66 Gy radiotherapy once daily in limited-stage SCLC.29 This trial did not show any difference in OS. The patients in the twice daily arm had a higher incidence of grade 4 neutropenia. Considering the results of these trials, both strategies—45 Gy fractionated twice daily or 60 Gy fractionated once daily, delivered concurrently with chemotherapy—are acceptable in the setting of limited-stage SCLC. However, quite often a hyperfractionated regimen is not feasible for patients and many radiation oncology centers. Hopefully, the ongoing CALGB 30610 study will clarify the optimal radiation schedule for limited-stage disease.
PROPHYLACTIC CRANIAL IRRADIATION
Approximately 75% of patients with limited-stage disease experience disease recurrence, and brain is the site of recurrence in approximately half of these patients.30 Prophylactic cranial irradiation (PCI) consisting of 25 Gy radiotherapy delivered in 10 fractions has been shown to be effective in decreasing the incidence of cerebral metastases.30–32 Although individual small studies have not shown a survival benefit of PCI because of small sample size and limited power, a meta-analysis of these studies has shown a 25% decrease in the 3-year incidence of brain metastasis and 5.4% increase in 3-year OS.30 Most patients included in these studies had limited-stage disease. Therefore, PCI is the standard of care for patients with limited-stage disease who attain a partial or complete response to chemoradiation.
ROLE OF SURGERY
Surgical resection may be an acceptable choice in a very limited subset of patients with peripherally located small (< 5 cm) tumors where mediastinal lymph nodes have been confirmed to be uninvolved with complete mediastinal staging.33,34 Most of the data in this setting are derived from retrospective studies.35,36 A 5-year OS between 40% and 60% has been reported with this strategy in patients with clinical stage I disease. In general, when surgery is considered, lobectomy with mediastinal lymph node dissection followed by chemotherapy (if there is no nodal involvement) or chemoradiation (if nodal involvement) is recommended.37,38 Wedge or segmental resections are not considered to be optimal surgical options.
MANAGEMENT OF EXTENSIVE-STAGE DISEASE
CASE CONTINUED
The patient receives 4 cycles of cisplatin and etoposide along with 70 Gy radiotherapy concurrently with the first 2 cycles of chemotherapy. His post-treatment CT scans show a partial response. He undergoes PCI 6 weeks after completion of treatment. At routine follow-up 18 months later, he is doing generally well except for mildly decreased appetite and an unintentional weight loss of 5 lb. CT scans demonstrate multiple hypodense liver lesions ranging from 7 mm to 2 cm in size and a 2-cm left adrenal gland lesion highly concerning for metastasis. FDG PET scan confirms that the adrenal and liver lesions are hypermetabolic. In addition, the PET scan shows multiple FDG-avid bone lesions throughout the spine. Brain MRI is negative for brain metastasis.
• What is the standard of care for treatment of extensive-stage disease?
Chemotherapy is the mainstay of treatment for extensive-stage SCLC; the goals of treatment are prolongation of survival, prevention or alleviation of cancer-related symptoms, and improvement in quality of life. The combination of etoposide with a platinum agent (carboplatin or cisplatin) is the preferred first-line treatment option. Carboplatin is more commonly used in clinical practice in this setting because of its comparable efficacy and better tolerability compared to cisplatin (Figure 2).21 A Japanese phase 3 trial comparing cisplatin plus irinotecan with cisplatin plus etoposide in the first-line setting in extensive-stage SCLC showed improvement in median and 2-year OS with the cisplatin/irinotecan regimen; however, 2 subsequent phase 3 trials conducted in the United States comparing these 2 regimens did not show any difference in OS. In addition, the cisplatin/irinotecan regimen was more toxic than the etoposide-based regimen.39,40 Therefore, 4 to 6 cycles of platinum/etoposide remains the standard of care first-line treatment for extensive-stage SCLC in the United States. The combination yields a 60% to 70% response rate, but the majority of patients invariably experience disease progression, with a median OS of 9 to 11 months.41 Maintenance chemotherapy beyond the initial 4 to 6 cycles does not improve survival and is associated with higher cumulative toxicity.42
Multiple attempts at improving first-line chemotherapy in extensive-stage disease have failed to show any meaningful difference in OS. For example, the addition of ifosfamide, palifosfamide, cyclophosphamide, taxane, or anthracycline to platinum doublet failed to show improvement in OS and led to more toxicity.43–46 Additionally, the use of alternating or cyclic chemotherapies in an attempt to curb drug resistance has also failed to show survival benefit.47–49 The addition of the antiangiogenic agent bevacizumab to standard platinum-based doublet has not prolonged OS in SCLC and has led to an unacceptably higher rate of tracheoesophageal fistula when used in conjunction with chemoradiation in limited-stage disease.50–55 Finally, the immune checkpoint inhibitor ipilimumab in combination with platinum plus etoposide failed to improve PFS or OS compared to platinum plus etoposide alone in a recent phase 3 trial, and maintenance pembrolizumab after completion of platinum-based chemotherapy did not improve PFS.56,57
More recently, a phase 2 study of pembrolizumab in extensive-stage SCLC (KEYNOTE 158) reported an overall response rate of 35.7%, median PFS of 2.1 months, and median OS of 14.6 months in patients who tested positive for programmed death ligand-1 (PD-L1) expression (which was defined as a PD-L1 Combined Positive Score ≥ 1).58 The median duration of response has not been reached in this study, indicating that pembrolizumab may be a promising approach in patients with extensive-stage SCLC, especially for those with PD-L1–positive tumors.
Patients with extensive-stage disease who have brain metastasis at the time of diagnosis can be treated with systemic chemotherapy first if the brain metastases are asymptomatic and there is significant extracranial disease burden. In that case, whole brain radiotherapy should be given after completion of systemic therapy.
SECOND-LINE CHEMOTHERAPY
Despite being exquisitely chemosensitive, SCLC is associated with a very poor prognosis largely because of invariable disease progression following first-line therapy and lack of effective second-line treatment options that can lead to appreciable disease control. The choice of second-line treatment is predominantly determined by the time of disease relapse after first-line platinum-based therapy. If this interval is 6 months or longer, re-treatment utilizing the same platinum doublet is appropriate. However, if the interval is 6 months or less, second-line systemic therapy options should be explored. Unfortunately, the response rate tends to be less than 10% with most of the second-line therapies in platinum-resistant disease (defined as disease progression within 3 months of receiving platinum-based therapy). If disease progression occurs between 3 and 6 months after completion of platinum-based therapy, the response rate with second-line chemotherapy is in the range of 25%.59,60
A number of second-line chemotherapy options have been explored in small studies, including topotecan, irinotecan, paclitaxel, docetaxel, temozolomide, vinorelbine, oral etoposide, gemcitabine, bendamustine, and CAV (
IMMUNOTHERAPY
The role of immune checkpoint inhibitors in the treatment of SCLC is evolving, and currently there are no FDA-approved immunotherapy agents for treating SCLC. A recently conducted phase 1/2 trial (CheckMate 032) studied the anti-programmed death(PD)-1 antibody nivolumab with or without the anti-cytotoxic T-lymphocyte–associated antigen (CTLA) -4 antibody ipilimumab in patients with relapsed SCLC. The authors reported response rates of 10% with nivolumab 3 mg/kg and 21% with nivolumab 1 mg/kg plus ipilimumab 3 mg/kg.78,79 The 2-year OS was 26% with the combination and 14% with single-agent nivolumab. Only 18% of patients had PD-L1 expression of ≥ 1%, and the response rate did not correlate with PD-L1 status. The rate of grade 3 or 4 adverse events was approximately 20%, and only 10% of patients discontinued treatment because of toxicity. Based on these data, nivolumab plus ipilimumab is now included in the National Comprehensive Cancer Network guidelines as an option for patients with SCLC who experience disease relapse within 6 months of receiving platinum-based therapy;7 however, it is questionable whether routine use of this combination is justified based on currently available data. The evidence for the combination of nivolumab and ipilimumab remains limited. The efficacy and toxicity data from both randomized and nonrandomized cohorts were presented together, making it hard to interpret the results.
Another phase 1b study (KEYNOTE-028) evaluated the anti-PD-1 antibody pembrolizumab (10 mg/kg intravenously every 2 weeks) in patients with relapsed SCLC who had received 1 or more prior lines of therapy and had PD-L1 expression of ≥ 1%. This study showed a response rate of 33%, with a median duration of response of 19 months and 1-year OS of 38%.80 Although only 28% of screened patients had PD-L1 expression of ≥ 1%, these results indicated that at least a subset of SCLC patients are able to achieve durable responses with immune checkpoint inhibition. A number of clinical trials utilizing immune checkpoint inhibitors in various combinations and settings are currently underway.
ROLE OF PROPHYLACTIC CRANIAL IRRADIATION
The role of PCI in extensive-stage SCLC is not clearly defined. A randomized phase 3 trial conducted by the European Organization for Research and Treatment of Cancer (EORTC) comparing PCI with no PCI in patients with extensive-stage SCLC who had a partial or complete response to initial platinum-based chemotherapy showed a decrease in the incidence of symptomatic brain metastasis and improvement in 1-year OS with PCI.81 However, this trial did not require mandatory brain imaging prior to PCI, and thus it is unclear if some patients in the PCI group had asymptomatic brain metastasis prior to enrollment and therefore received therapeutic benefit from brain radiation. Additionally, the dose and fractionation of PCI was not standardized across patient groups.
A more recent phase 3 study conducted in Japan that compared PCI (25 Gy in 10 fractions) with no PCI reported no difference in survival between the 2 groups.82 As opposed to the EORTC study, the Japanese study did require baseline brain imaging to confirm the absence of brain metastasis prior to enrollment. In addition, the control patients underwent periodic brain MRI to allow early detection of brain metastasis. Given the emergence of the new data, the impact of PCI on survival in patients with extensive-stage SCLC is unproven, and PCI likely has a role in a highly selected small group of patients with extensive-stage SCLC. PCI is not recommended for patients with poor performance status (ECOG performance score of 3 or 4) or underlying neurocognitive disorders.34,83
The NMDA-receptor antagonist memantine can be used in patients undergoing PCI to delay the occurrence of cognitive dysfunction.61 Memantine 20 mg daily delayed time to cognitive decline and reduced the rate of decline in memory, executive function, and processing speed compared to placebo in patients receiving whole brain radiotherapy.84
ROLE OF RADIOTHERAPY
A subset of patients with extensive-stage SCLC may benefit from consolidative thoracic radiotherapy after completion of platinum-based chemotherapy. A randomized trial that enrolled patients who achieved complete or near complete response after 3 cycles of cisplatin plus etoposide compared thoracic radiotherapy in combination with continued chemotherapy versus chemotherapy alone.85 The median OS was longer with the addition of thoracic radiotherapy compared to chemotherapy alone. Another phase 3 trial did not show improvement in 1-year OS with consolidative thoracic radiotherapy, but 2-year OS and 6-month PFS were longer.86 In general, consolidative thoracic radiotherapy benefits patients who have residual thoracic disease and low-bulk extrathoracic disease that has responded to systemic therapy.87 In addition, patients who initially presented with bulky symptomatic thoracic disease should also be considered for consolidative radiation.
Similar to other solid tumors, radiotherapy should be utilized for palliative purposes in patients with painful bone metastasis, spinal cord compression, or brain metastasis. Surgery is generally not recommended for spinal cord compression given the short life expectancy of patients with extensive-stage disease. Whole brain radiotherapy is preferred over stereotactic radiosurgery because micrometastasis is frequently present even in the setting of 1 or 2 radiographically evident brain metastasis.
NOVEL THERAPIES
The very complex genetic landscape of SCLC accounts for its resistance to conventional therapy and high recurrence rate; however, at the same time this complexity can form the basis for effective targeted therapy for the disease. One of the major factors hindering the development of targeted therapies in SCLC is limited availability of tissue due to small tissue samples and the frequent presence of significant necrosis in the samples. In recent years, several different therapeutic strategies and targeted agents have been investigated for their potential role in SCLC. Several of them, including EGFR tyrosine kinase inhibitors (TKIs), BCR-ABL TKIs, mTOR inhibitors, and VEGF inhibitors, have not been shown to provide a survival advantage in this disease. Several others, including PARP inhibitors, cellular developmental pathway inhibitors, and antibody-drug conjugates, are being tested. A phase 1 study of veliparib combined with cisplatin and etoposide in patients with previously untreated extensive-stage SCLC demonstrated a complete response in 14.3%, a partial response in 57.1%, and stable disease in 28.6% of patients with an acceptable safety profile.88 So far, none of these agents are approved for use in SCLC, and the majority are in early- phase clinical trials.89
One of the emerging targets in the treatment of SCLC is delta-like protein 3 (DLL3). DLL3 is expressed on more than 80% of SCLC tumor cells and cancer stem cells. Rovalpituzumab tesirine is an antibody-drug conjugate consisting of humanized anti-DLL3 monoclonal antibody linked to SC-DR002, a DNA-crosslinking agent. A phase 1 trial of rovalpituzumab in patients with relapsed SCLC after 1 or 2 prior lines of therapy reported a response rate of 31% in patients with DLL3 expression of ≥ 50%. The median duration of response and median PFS were both 4.6 months.90 Rovalpituzumab is currently in later phases of clinical trials and has a potential to serve as an option for patients with extensive-stage disease after disease progression on platinum-based therapy.
SUMMARY
Four to 6 cycles of carboplatin and etoposide remain the standard of care first-line treatment for patients with extensive stage SCLC. The only FDA-approved second-line treatment option is topotecan. Re-treatment with the original platinum doublet is a reasonable option for patients who have disease progression 6 months or longer after completion of platinum-based therapy. The immune checkpoint inhibitors pembrolizumab and combination nivolumab and ipilimumab have shown promising results in the second-line setting and beyond. The role of PCI has become more controversial in recent years, and periodic brain MRI in lieu of PCI is now an acceptable approach.
RESPONSE ASSESSMENT/SURVEILLANCE
For patients undergoing treatment for limited-stage SCLC, response assessment with contrast-enhanced CT of the chest/abdomen should be performed after completion of 4 cycles of chemotherapy and thoracic radiation.7 The surveillance guidelines consist of history, physical exam, and imaging every 3 months during the first 2 years, every 6 months during the third year, and annually thereafter. If PCI is not performed, brain MRI or contrast-enhanced CT scan should be performed every 3 or 4 months during the first 2 years of follow up. For extensive-stage disease, response assessment should be performed after every 2 cycles of therapy. After completion of therapy, history, physical exam, and imaging should be done every 2 months during the first year, every 3 or 4 months during years 2 and 3, every 6 months during years 4 and 5, and annually thereafter. Routine use of PET scan for surveillance is not recommended. Any new pulmonary nodule should prompt evaluation for a second primary lung malignancy. Finally, smoking cessation counseling is an integral part of management of any patient with SCLC and should be included with every clinic visit.
CONCLUSION
SCLC is a heterogeneous and genetically complex disease with a very high mortality rate. The current standard of care includes concurrent chemoradiation with cisplatin and etoposide for limited-stage SCLC and the combination of platinum and etoposide for extensive SCLC. A number of novel treatment approaches, including immune checkpoint inhibitors and antibody-drug conjugates, have had promising results in early clinical trials. Given the limited treatment options and large unmet need for new treatment options, enrollment in clinical trials is strongly recommended for patients with SCLC.
1. American Cancer Society. Cancer Facts & Figures 2017. American Cancer Society website. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. Published 2017. Accessed July 11, 2018.
2. Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24:4539–44.
3. Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2014. National Cancer Institute website. https://seer.cancer.gov/csr/1975_2014/. Updated April 2, 2018. Accessed July 11, 2018.
4. Varghese AM, Zakowski MF, Yu HA, et al. Small-cell lung cancers in patients who never smoked cigarettes. J Thorac Oncol 2014;9:892–6.
5. Pleasance ED, Stephens PJ, O’Meara S, et al. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature 2010;463:184–90.
6. Green RA, Humphrey E, Close H, Patno ME. Alkylating agents in bronchogenic carcinoma. Am J Med 1969;46:516–25.
7. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology for small cell lung cancer (Version 2.2018). www.nccn.org/professionals/physician_gls/pdf/sclc.pdf. Accessed August 12, 2018.
8. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409.
9. Aberle DR, DeMello S, Berg CD, et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med 2013;369:920–31.
10. Kitajima K, Nakamoto Y, Okizuka H, et al. Accuracy of whole-body FDG-PET/CT for detecting brain metastases from non-central nervous system tumors. Ann Nucl Med 2008;22:595–602.
11. Ordonez NG. Value of thyroid transcription factor-1 immunostaining in distinguishing small cell lung carcinomas from other small cell carcinomas. Am J Surg Pathol 2000;24:1217–23.
12. Karnak D, Beder S, Kayacan O, et al. Neuron-specific enolase and lung cancer. Am J Clin Oncol 2005;28:586–90.
13. Turrisi AT 3rd, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 1999;340:265–71.
14. Evans WK, Shepherd FA, Feld R, et al. VP-16 and cisplatin as first-line therapy for small-cell lung cancer. J Clin Oncol 1985;3:1471–7.
15. Pujol JL, Carestia L, Daurés JP. Is there a case for cisplatin in the treatment of small-cell lung cancer? A meta-analysis of randomized trials of a cisplatin-containing regimen versus a regimen without this alkylating agent. Br J Cancer 2000;83:8–15.
16. Mascaux C, Paesmans M, Berghmans T, et al; European Lung Cancer Working Party (ELCWP). A systematic review of the role of etoposide and cisplatin in the chemotherapy of small cell lung cancer with methodology assessment and meta-analysis. Lung Cancer 2000;30:23–36.
17. Sundstrøm S, Bremnes RM, Kaasa S, et al; Norwegian Lung Cancer Study Group. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years’ follow-up. J Clin Oncol 2002;20:4665–72.
18. Hatfield LA, Huskamp HA, Lamont EB. Survival and toxicity after cisplatin plus etoposide versus carboplatin plus etoposide for extensive-stage small-cell lung cancer in elderly patients. J Oncol Pract 2016;12:666–73.
19. Okamoto H, Watanabe K, Kunikane H, et al. Randomised phase III trial of carboplatin plus etoposide vs split doses of cisplatin plus etoposide in elderly or poor-risk patients with extensive disease small-cell lung cancer: JCOG 9702. Br J Cancer 2007;97:162–9.
20. Skarlos DV, Samantas E, Kosmidis P, et al. Randomized comparison of etoposide-cisplatin vs. etoposide-carboplatin and irradiation in small-cell lung cancer. A Hellenic Co-operative Oncology Group study. Ann Oncol 1994;5:601–7.
21. Rossi A, Di Maio M, Chiodini P, et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data J Clin Oncol 2012;30:1692–8.
22. Bishop JF, Raghavan D, Stuart-Harris R, et al. Carboplatin (CBDCA, JM-8) and VP-16-213 in previously untreated patients with small-cell lung cancer. J Clin Oncol 1987;5:1574–8.
23. Takada M, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol 2002;20:3054–60.
24. Bunn PA Jr, Crowley J, Kelly K, et al. Chemoradiotherapy with or without granulocyte-macrophage colony-stimulating factor in the treatment of limited-stage small-cell lung cancer: a prospective phase III randomized study of the Southwest Oncology Group. J Clin Oncol 1995;13:1632–41.
25. Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 1992;327:1618–24.
26. Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol 1992;10:890–5.
27. Murray N, Coy P, Pater JL, et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 1993;11:336–44.
28. De Ruysscher D, Lueza B, Le Péchoux C, et al. Impact of thoracic radiotherapy timing in limited-stage small-cell lung cancer: usefulness of the individual patient data meta-analysis. Ann Oncol 2016;27:1818–28.
29. Faivre-Finn C, Snee M, Ashcroft L, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol 2017;18:1116–25.
30. Aupérin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341:476–84.
31. Arriagada R, Le Chevalier T, Borie F, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst 1995;87:183–90.
32. Le Péchoux C, Dunant A, Senan S, et al; Prophylactic Cranial Irradiation (PCI) Collaborative Group. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): a randomised clinical trial. Lancet Oncol 2009;10:467–74.
33. Schneider BJ, Saxena A, Downey RJ. Surgery for early-stage small cell lung cancer. J Natl Compr Canc Netw 2011;9:1132–9.
34. Inoue M, Nakagawa K, Fujiwara K, et al. Results of preoperative mediastinoscopy for small cell lung cancer. Ann Thorac Surg 2000;70:1620–3.
35. Lim E, Belcher E, Yap YK, et al. The role of surgery in the treatment of limited disease small cell lung cancer: time to reevaluate. J Thorac Oncol 2008;3:1267–71.
36. Inoue M, Miyoshi S, Yasumitsu T, et al. Surgical results for small cell lung cancer based on the new TNM staging system. Thoracic Surgery Study Group of Osaka University, Osaka, Japan. Ann Thorac Surg 2000;70:1615–9.
37. Yang CF, Chan DY, Speicher PJ, et al. Role of adjuvant therapy in a population-based cohort of patients with early-stage small-cell lung cancer. J Clin Oncol 2016;34:1057–64.
38. Shepherd FA, Evans WK, Feld R, et al. Adjuvant chemotherapy following surgical resection for small-cell carcinoma of the lung. J Clin Oncol 1988;6:832–8.
39. Noda K, Nishiwaki Y, Kawahara M, et al; Japan Clinical Oncology Group. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 2002;346:85–91.
40. Lara PN Jr, Natale R, Crowley J, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol 2009;27:2530–5.
41. Chute JP, Chen T, Feigal E, et al. Twenty years of phase III trials for patients with extensive-stage small-cell lung cancer: perceptible progress. J Clin Oncol 1999;17:1794–801.
42. Zhou H, Zeng C, Wei Y, et al. Duration of chemotherapy for small cell lung cancer: a meta-analysis. PloS One 2013;8:e73805.
43. Loehrer PJ Sr, Ansari R, Gonin R, et al. Cisplatin plus etoposide with and without ifosfamide in extensive small-cell lung cancer: a Hoosier Oncology Group study. J Clin Oncol;13:2594–9.
44. Pujol JL, Daurés JP, Riviére A, et al. Etoposide plus cisplatin with or without the combination of 4’-epidoxorubicin plus cyclophosphamide in treatment of extensive small-cell lung cancer: a French Federation of Cancer Institutes multicenter phase III randomized study. J Natl Cancer Inst 2001;93:300–8.
45. Berghmans T, Scherpereel A, Meert AP, et al; European Lung Cancer Working Party (ELCWP). A phase III randomized study comparing a chemotherapy with cisplatin and etoposide to a etoposide regimen without cisplatin for patients with extensive small-cell lung cancer. Front Oncol 2017;7:217.
46. Jalal SI, Lavin P, Lo G, et al. Carboplatin and etoposide with or without palifosfamide in untreated extensive-stage small-cell lung cancer: a Multicenter, Adaptive, Randomized Phase III Study (MATISSE). J Clin Oncol 2017;35:2619–23.
47. Fukuoka M, Furuse K, Saijo N, et al. Randomized trial of cyclophosphamide, doxorubicin, and vincristine versus cisplatin and etoposide versus alternation of these regimens in small-cell lung cancer. J Natl Cancer Inst 1991;83:855–61.
48. Roth BJ, Johnson DH, Einhorn LH, et al. Randomized study of cyclophosphamide, doxorubicin, and vincristine versus etoposide and cisplatin versus alternation of these two regimens in extensive small-cell lung cancer: a phase III trial of the Southeastern Cancer Study Group. J Clin Oncol 1992;10:282–91.
49. Miles DW, Earl HM, Souhami RL, et al. Intensive weekly chemotherapy for good-prognosis patients with small-cell lung cancer. J Clin Oncol 1991;9:280–5.
50. Petrioli R, Roviello G, Laera L, et al. Cisplatin, etoposide, and bevacizumab regimen followed by oral etoposide and bevacizumab maintenance treatment in patients with extensive-stage small cell lung cancer: a single-institution experience. Clin Lung Cancer 2015;16:e229–34.
51. Spigel DR, Greco FA, Zubkus JD, et al. Phase II trial of irinotecan, carboplatin, and bevacizumab in the treatment of patients with extensive-stage small-cell lung cancer. J Thorac Oncol 2009;4:1555–60.
52. Spigel DR, Townley PM, Waterhouse DM, et al. Randomized phase II study of bevacizumab in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer: results from the SALUTE trial. J Clin Oncol 2011;29:2215–22.
53. Horn L, Dahlberg SE, Sandler AB, et al. Phase II study of cisplatin plus etoposide and bevacizumab for previously untreated, extensive-stage small-cell lung cancer: Eastern Cooperative Oncology Group Study E3501. J Clin Oncol 2009;27:6006–11.
54. Tiseo M, Boni L, Ambrosio F, et al. Italian, multicenter, phase III, randomized study of cisplatin plus etoposide with or without bevacizumab as first-line treatment in extensive-disease small-cell lung cancer: the GOIRC-AIFA FARM6PMFJM trial. J Clin Oncol 2017;35:1281–7.
55. Pujol JL, Lavole A, Quoix E, et al. Randomized phase II-III study of bevacizumab in combination with chemotherapy in previously untreated extensive small-cell lung cancer: results from the IFCT-0802 trial. Ann Oncol 2015;26:908–14.
56. Gadgeel SM, Ventimiglia J, Kalemkerian GP, et al. Phase II study of maintenance pembrolizumab (pembro) in extensive stage small cell lung cancer (ES-SCLC) patients (pts) [abstract]. J Clin Oncol 2017;35(15_suppl):8504.
57. Reck M, Luft A, Szczesna A, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol 2016;34:3740–8.
58. Chung HC, Lopez-Martin JA, Kao SC, et al. Phase 2 study of pembrolizumab in advanced small-cell lung cancer (SCLC): KEYNOTE-158 [abstract]. J Clin Oncol 2018;36(suppl):8506.
59. Owonikoko TK, Behera M, Chen Z, et al. A systematic analysis of efficacy of second-line chemotherapy in sensitive and refractory small-cell lung cancer. J Thorac Oncol 2012;7:866–72.
60. Postmus PE, Berendsen HH, van Zandwijk N, et al. Retreatment with the induction regimen in small cell lung cancer relapsing after an initial response to short term chemotherapy. Eur J Cancer Clin Oncol 1987;23:1409–11.
61. von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol 1999;17:658–667.
62. O’Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol 2006;24:5441–7.
63. Eckardt JR, von Pawel J, Pujol JL, et al. Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. J Clin Oncol 2007;25:2086–92.
64. Masuda N, Fukuoka M, Kusunoki Y, et al. CPT-11: a new derivative of camptothecin for the treatment of refractory or relapsed small-cell lung cancer. J Clin Oncol 1992;10:1225–9.
65. Smit EF, Fokkema E, Biesma B, et al. A phase II study of paclitaxel in heavily pretreated patients with small-cell lung cancer. Br J Cancer 1998;77:347–51.
66. Yamamoto N, Tsurutani J, Yoshimura N, et al. Phase II study of weekly paclitaxel for relapsed and refractory small cell lung cancer. Anticancer Res 2006;26:777–81.
67. Smyth JF, Smith IE, Sessa C, et al. Activity of docetaxel (Taxotere) in small cell lung cancer. Eur J Cancer 1994;30A:1058–60.
68. Pietanza MC, Kadota K, Huberman K, et al. Phase II trial of temozolomide with relapsed sensitive or refractory small cell lung cancer, with assessment of methylguanine-DNA methyltransferase as a potential biomarker. Clin Cancer Res 2012;18:1138–45.
69. Zauderer MG, Drilon A, Kadota K, et al. Trial of a 5-day dosing regimen of temozolomide in patients with relapsed small cell lung cancers with assessment of methylguanine-DNA methyltransferase. Lung Cancer 2014;86:237–40.
70. Jassem J, Karnicka-Mlodkowska H, van Pottelsberghe C, et al. Phase II study of vinorelbine (Navelbine) in previously treated small cell lung cancer patients. Eur J Cancer 1993;29A:1720–2.
71. Furuse K, Kuboa K, Kawahara M, et al. Phase II study of vinorelbine in heavily previously treated small cell lung cancer. Oncology 1996;53:169–72.
72. Einhorn LH, Pennington K, McClean J. Phase II trial of daily oral VP-16 in refractory small cell lung cancer. Semin Oncol 1990;17:32–5.
73. Johnson DH, Greco FA, Strupp J, et al. Prolonged administration of oral etoposide in patients with relapsed or refractory small-cell lung cancer: a phase II trial. J Clin Oncol 1990;8:1613–7.
74. Van der Lee I, Smit EF, van Putten JW, et al. Single-agent gemcitabine in patients with resistant small-cell lung cancer. Ann Oncol 2001;12:557–61.
75. Masters GA, Declerck L, Blanke C, et al. Phase II trial of gemcitabine in refractory or relapsed small-cell lung cancer. J Clin Oncol 2003;21:1550–5.
76. von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol 1999;17:658–67.
77. Lammers PE, Shyr Y, Li CI, et al. Phase II study of bendamustine in relapsed chemotherapy sensitive or resistant small-cell lung cancer. J Thorac Oncol 2014;9:559–62.
78. Hellmann MD, Ott PA, Zugazagoitia J, et al. Nivolumab (nivo) ± ipilimumab (ipi) in advanced small-cell lung cancer (SCLC): First report of a randomized expansion cohort from CheckMate 032 [abstract]. J Clin Oncol 2017;35(15_suppl):8503.
79. Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883–95.
80. Ott PA, Elez E, Hiret S, et al. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the Phase Ib KEYNOTE-028 study. J Clin Oncol 2017;35:3823–9.
81. Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med 2007;357:664–72.
82. Takahashi T, Yamanaka T, Seto T, et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:663–71.
83. Slotman BJ, Mauer ME, Bottomley A, et al. Prophylactic cranial irradiation in extensive disease small-cell lung cancer: short-term health-related quality of life and patient reported symptoms: results of an international Phase III randomized controlled trial by the EORTC Radiation Oncology and Lung Cancer Groups. J Clin Oncol 2009;27:78–84.
84. Brown PD, Pugh S, Laack NN, et al; Radiation Therapy Oncology Group (RTOG). Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol 2013;15:1429–37.
85. Jeremic B, Shibamoto Y, Nikolic N, et al. Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: a randomized study. J Clin Oncol 1999;17:2092–9.
86. Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet 2015;385:36–42.
87. Slotman BJ, van Tinteren H, Praag JO, et al. Radiotherapy for extensive stage small-cell lung cancer - authors’ reply. Lancet 2015;385:1292–3.
88. Owonikoko TK, Dahlberg SE, Khan SA, et al. A phase 1 safety study of veliparib combined with cisplatin and etoposide in extensive stage small cell lung cancer: A trial of the ECOG-ACRIN Cancer Research Group (E2511). Lung Cancer 2015;89:66–70.
89. Mamdani H, Induru R, Jalal SI. Novel therapies in small cell lung cancer. Transl Lung Cancer Res 2015;4:533–44.
90. Rudin CM, Pietanza MC, Bauer TM, et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol 2017;18:42–51.
INTRODUCTION
Small cell lung cancer (SCLC) is an aggressive cancer of neuroendocrine origin that accounts for approximately 15% of all lung cancer cases, with approximately 33,000 patients diagnosed annually.1 The incidence of SCLC in the United States has steadily declined over the past 30 years, presumably because of a decrease in the number of smokers and a change to low-tar filter cigarettes.2 Although the overall incidence of SCLC has been decreasing, the incidence in women is increasing and the male-to-female incidence ratio is now 1:1.3 Nearly all cases of SCLC are associated with heavy tobacco exposure, making it a heterogeneous disease with a complex genomic landscape consisting of thousands of mutations.4,5 Despite recent advances in the treatment of non-small cell lung cancer, the therapeutic options for SCLC remain limited, with a median overall survival (OS) of 9 months in patients with advanced disease.
DIAGNOSIS AND STAGING
CASE PRESENTATION
A 61-year-old man presents to the emergency department with progressive shortness of breath and cough over the past 6 weeks. He also reports a 20-lb weight loss over the same period. He is a current smoker and has been smoking 1 pack of cigarettes per day since the age of 18 years. A chest radiograph obtained in the emergency department shows a right hilar mass. Computed tomography (CT) scan confirms the presence of a 4.5-cm right hilar mass and enlarged mediastinal lymph nodes bilaterally.
• What are the next steps in diagnosis?
SCLC is characterized by rapid growth and early hematogenous metastasis. Consequently, only 25% of patients have limited-stage disease at the time of diagnosis. According to the Veterans Administration Lung Study Group (VALSG) staging system, limited-stage disease is defined as tumor that is confined to 1 hemithorax and can be encompassed within 1 radiation field. This typically includes mediastinal lymph nodes and ipsilateral supraclavicular lymph nodes. Approximately 75% of patients present with extensive-stage disease, which is defined as disease that cannot be classified as limited, including disease that extends beyond 1 hemithorax. Extensive-stage disease includes the presence of malignant pleural effusion and/or distant metastasis.6 The VALSG classification and staging system is more commonly used in clinical practice than the American Joint Committee on Cancer TNM staging system because it is less complex and directs treatment decisions, as most of the literature on SCLC classifies patients based on the VALSG system.7
Given SCLC’s propensity to metastasize quickly, none of the currently available screening methods have proven successful in early detection of SCLC. In the National Lung Cancer Screening Trial, 86% of the 125 patients who were diagnosed with SCLC while undergoing annual low-dose chest CT scans had advanced disease at diagnosis.8,9 These results highlight the fact that most cases of SCLC develop in the interval between annual screening imaging.
SCLC frequently presents with a large hilar mass that is symptomatic. Common symptoms include shortness of breath and cough. In addition, patients with SCLC usually have bulky mediastinal adenopathy at presentation. SCLC is commonly located submucosally in the bronchus, and therefore hemoptysis is not a very common symptom at the time of presentation. Patients may present with superior vena cava syndrome from local compression by the tumor. Not infrequently, SCLC is associated with paraneoplastic syndromes that arise due to ectopic secretion of hormones or antibodies by the tumor cells. The paraneoplastic syndromes can be broadly categorized as endocrine or neurologic (Table 1). The presence of a paraneoplastic syndrome is often a clue to the potential diagnosis of SCLC in the presence of a hilar mass. Additionally, some paraneoplastic syndromes, more specifically endocrine paraneoplastic syndromes, follow the pattern of disease response and relapse, and therefore can sometimes serve as an early marker of disease relapse or progression.
The common sites of metastases include brain, liver, and bone. Therefore, the staging workup should include fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT scan. Contrast-enhanced CT scan of the chest and abdomen and bone scan can be obtained for staging in lieu of PET scan. Due to the physiologic FDG uptake, cerebral metastases cannot be assessed with sufficient certainty using PET-CT.10 Therefore, brain imaging with contrast-enhanced CT or magnetic resonance imaging (MRI) is also necessary. Although the incidence of metastasis to bone marrow is less than 10%, bone marrow aspiration and biopsy are warranted in patients with unexplained cytopenias, especially when the cytopenia is associated with teardrop-shaped red cells or nucleated red cells on peripheral blood smear, findings indicative of a marrow infiltrative process.7 The tissue diagnosis is established by obtaining a biopsy of the primary tumor or 1 of the metastatic sites. In localized disease, bronchoscopy (with endobronchial ultrasound, if necessary) with biopsy of the centrally located tumor and/or lymph node is required. Histologically, SCLC consists of monomorphic cells, a high nuclear-cytoplasmic ratio, and confluent necrosis. The tumor cells are positive for chromogranin, synaptophysin, and CD56 by immunohistochemistry, and very frequently are also positive for thyroid transcription factor 1.11 Although serum tumor markers, including neuron-specific enolase and progastrin-releasing peptide, are frequently elevated in patients with SCLC, these markers are of limited value in clinical practice because they lack sensitivity and specificity.12
MANAGEMENT OF LIMITED-STAGE DISEASE
CASE CONTINUED
The patient undergoes FDG PET scan, which shows the presence of a hypermetabolic right hilar mass in addition to enlarged and hypermetabolic bilateral mediastinal lymph nodes. There are no other areas of FDG avidity. Brain MRI does not show any evidence of brain metastasis. Thus, the patient is confirmed to have limited-stage SCLC.
• What is the standard of care treatment for limited-stage SCLC?
SCLC is exquisitely sensitive to both chemotherapy and radiation, especially at the time of initial presentation. The standard of care treatment of limited-stage SCLC is 4 cycles of platinum-based chemotherapy in combination with thoracic radiation started within the first 2 cycles of chemotherapy (Figure 1).
CHOICE OF CHEMOTHERAPY
Etoposide and cisplatin is the most commonly used initial combination chemotherapy regimen in limited-stage SCLC.14 This combination has largely replaced anthracycline-based regimens given its favorable efficacy and toxicity profile.15–17 Several small randomized trials have shown comparable efficacy of carboplatin and etoposide in extensive-stage SCLC.18–20 A meta-analysis of 4 randomized trials comparing cisplatin-based versus carboplatin-based regimens in 663 patients with SCLC (32% had limited-stage disease and 68% had extensive-stage disease) showed no statistically significant difference in response rate, progression-free survival (PFS), or OS between the 2 regimens.21 Therefore, in clinical practice carboplatin is frequently used instead of cisplatin in patients with extensive-stage disease. In patients with limited-stage disease, cisplatin is still the drug of choice. However, the toxicity profile of the 2 regimens is different. Cisplatin-based regimens are more commonly associated with neuropathy, nephrotoxicity, and chemotherapy-induced nausea/vomiting,18 while carboplatin-based regimens are more myelosuppressive.22 In addition, the combination of thoracic radiation with either of these regimens is associated with a higher risk of esophagitis, pneumonitis, and myelosuppression.23 The use of myeloid growth factors is not recommended in patients undergoing concurrent chemoradiation.24 Of note, intravenous etoposide is always preferred over oral etoposide, especially in the curative setting given the unreliable absorption and bioavailability of oral formulations.
THORACIC RADIOTHERAPY
Adding thoracic radiotherapy to platinum-etoposide chemotherapy improves local control and OS. Two meta-analyses of 13 trials including more than 2000 patients have shown a 25% to 30% decrease in local failure and a 5% to 7% increase in 2-year OS with chemoradiation compared to chemotherapy alone in limited-stage SCLC.25,26 Early (within the first 2 cycles) concurrent thoracic radiation is superior to delayed and/or sequential radiation in terms of local control and OS.23,27,28 The dose and fractionation of thoracic radiation in limited-stage SCLC has remained a controversial issue. The Eastern Cooperative Oncology Group/Radiation Therapy Oncology Group randomized trial compared 45 Gy of radiotherapy delivered twice daily over a period of 3 weeks to 45 Gy once daily over 5 weeks concurrently with chemotherapy. The twice daily regimen led to a 10% improvement in 5-year OS (26% versus 16%), but a higher incidence of grade 3 and 4 adverse events.13 Despite the survival advantage demonstrated by hyperfractionated radiotherapy, the results need to be interpreted with caution because the radiation doses are not biologically equivalent. In addition, the difficult logistics of patients receiving radiation twice a day has limited the routine implementation of this strategy. Subsequently, another randomized phase 3 trial (CONVERT) compared 45 Gy radiotherapy twice daily with 66 Gy radiotherapy once daily in limited-stage SCLC.29 This trial did not show any difference in OS. The patients in the twice daily arm had a higher incidence of grade 4 neutropenia. Considering the results of these trials, both strategies—45 Gy fractionated twice daily or 60 Gy fractionated once daily, delivered concurrently with chemotherapy—are acceptable in the setting of limited-stage SCLC. However, quite often a hyperfractionated regimen is not feasible for patients and many radiation oncology centers. Hopefully, the ongoing CALGB 30610 study will clarify the optimal radiation schedule for limited-stage disease.
PROPHYLACTIC CRANIAL IRRADIATION
Approximately 75% of patients with limited-stage disease experience disease recurrence, and brain is the site of recurrence in approximately half of these patients.30 Prophylactic cranial irradiation (PCI) consisting of 25 Gy radiotherapy delivered in 10 fractions has been shown to be effective in decreasing the incidence of cerebral metastases.30–32 Although individual small studies have not shown a survival benefit of PCI because of small sample size and limited power, a meta-analysis of these studies has shown a 25% decrease in the 3-year incidence of brain metastasis and 5.4% increase in 3-year OS.30 Most patients included in these studies had limited-stage disease. Therefore, PCI is the standard of care for patients with limited-stage disease who attain a partial or complete response to chemoradiation.
ROLE OF SURGERY
Surgical resection may be an acceptable choice in a very limited subset of patients with peripherally located small (< 5 cm) tumors where mediastinal lymph nodes have been confirmed to be uninvolved with complete mediastinal staging.33,34 Most of the data in this setting are derived from retrospective studies.35,36 A 5-year OS between 40% and 60% has been reported with this strategy in patients with clinical stage I disease. In general, when surgery is considered, lobectomy with mediastinal lymph node dissection followed by chemotherapy (if there is no nodal involvement) or chemoradiation (if nodal involvement) is recommended.37,38 Wedge or segmental resections are not considered to be optimal surgical options.
MANAGEMENT OF EXTENSIVE-STAGE DISEASE
CASE CONTINUED
The patient receives 4 cycles of cisplatin and etoposide along with 70 Gy radiotherapy concurrently with the first 2 cycles of chemotherapy. His post-treatment CT scans show a partial response. He undergoes PCI 6 weeks after completion of treatment. At routine follow-up 18 months later, he is doing generally well except for mildly decreased appetite and an unintentional weight loss of 5 lb. CT scans demonstrate multiple hypodense liver lesions ranging from 7 mm to 2 cm in size and a 2-cm left adrenal gland lesion highly concerning for metastasis. FDG PET scan confirms that the adrenal and liver lesions are hypermetabolic. In addition, the PET scan shows multiple FDG-avid bone lesions throughout the spine. Brain MRI is negative for brain metastasis.
• What is the standard of care for treatment of extensive-stage disease?
Chemotherapy is the mainstay of treatment for extensive-stage SCLC; the goals of treatment are prolongation of survival, prevention or alleviation of cancer-related symptoms, and improvement in quality of life. The combination of etoposide with a platinum agent (carboplatin or cisplatin) is the preferred first-line treatment option. Carboplatin is more commonly used in clinical practice in this setting because of its comparable efficacy and better tolerability compared to cisplatin (Figure 2).21 A Japanese phase 3 trial comparing cisplatin plus irinotecan with cisplatin plus etoposide in the first-line setting in extensive-stage SCLC showed improvement in median and 2-year OS with the cisplatin/irinotecan regimen; however, 2 subsequent phase 3 trials conducted in the United States comparing these 2 regimens did not show any difference in OS. In addition, the cisplatin/irinotecan regimen was more toxic than the etoposide-based regimen.39,40 Therefore, 4 to 6 cycles of platinum/etoposide remains the standard of care first-line treatment for extensive-stage SCLC in the United States. The combination yields a 60% to 70% response rate, but the majority of patients invariably experience disease progression, with a median OS of 9 to 11 months.41 Maintenance chemotherapy beyond the initial 4 to 6 cycles does not improve survival and is associated with higher cumulative toxicity.42
Multiple attempts at improving first-line chemotherapy in extensive-stage disease have failed to show any meaningful difference in OS. For example, the addition of ifosfamide, palifosfamide, cyclophosphamide, taxane, or anthracycline to platinum doublet failed to show improvement in OS and led to more toxicity.43–46 Additionally, the use of alternating or cyclic chemotherapies in an attempt to curb drug resistance has also failed to show survival benefit.47–49 The addition of the antiangiogenic agent bevacizumab to standard platinum-based doublet has not prolonged OS in SCLC and has led to an unacceptably higher rate of tracheoesophageal fistula when used in conjunction with chemoradiation in limited-stage disease.50–55 Finally, the immune checkpoint inhibitor ipilimumab in combination with platinum plus etoposide failed to improve PFS or OS compared to platinum plus etoposide alone in a recent phase 3 trial, and maintenance pembrolizumab after completion of platinum-based chemotherapy did not improve PFS.56,57
More recently, a phase 2 study of pembrolizumab in extensive-stage SCLC (KEYNOTE 158) reported an overall response rate of 35.7%, median PFS of 2.1 months, and median OS of 14.6 months in patients who tested positive for programmed death ligand-1 (PD-L1) expression (which was defined as a PD-L1 Combined Positive Score ≥ 1).58 The median duration of response has not been reached in this study, indicating that pembrolizumab may be a promising approach in patients with extensive-stage SCLC, especially for those with PD-L1–positive tumors.
Patients with extensive-stage disease who have brain metastasis at the time of diagnosis can be treated with systemic chemotherapy first if the brain metastases are asymptomatic and there is significant extracranial disease burden. In that case, whole brain radiotherapy should be given after completion of systemic therapy.
SECOND-LINE CHEMOTHERAPY
Despite being exquisitely chemosensitive, SCLC is associated with a very poor prognosis largely because of invariable disease progression following first-line therapy and lack of effective second-line treatment options that can lead to appreciable disease control. The choice of second-line treatment is predominantly determined by the time of disease relapse after first-line platinum-based therapy. If this interval is 6 months or longer, re-treatment utilizing the same platinum doublet is appropriate. However, if the interval is 6 months or less, second-line systemic therapy options should be explored. Unfortunately, the response rate tends to be less than 10% with most of the second-line therapies in platinum-resistant disease (defined as disease progression within 3 months of receiving platinum-based therapy). If disease progression occurs between 3 and 6 months after completion of platinum-based therapy, the response rate with second-line chemotherapy is in the range of 25%.59,60
A number of second-line chemotherapy options have been explored in small studies, including topotecan, irinotecan, paclitaxel, docetaxel, temozolomide, vinorelbine, oral etoposide, gemcitabine, bendamustine, and CAV (
IMMUNOTHERAPY
The role of immune checkpoint inhibitors in the treatment of SCLC is evolving, and currently there are no FDA-approved immunotherapy agents for treating SCLC. A recently conducted phase 1/2 trial (CheckMate 032) studied the anti-programmed death(PD)-1 antibody nivolumab with or without the anti-cytotoxic T-lymphocyte–associated antigen (CTLA) -4 antibody ipilimumab in patients with relapsed SCLC. The authors reported response rates of 10% with nivolumab 3 mg/kg and 21% with nivolumab 1 mg/kg plus ipilimumab 3 mg/kg.78,79 The 2-year OS was 26% with the combination and 14% with single-agent nivolumab. Only 18% of patients had PD-L1 expression of ≥ 1%, and the response rate did not correlate with PD-L1 status. The rate of grade 3 or 4 adverse events was approximately 20%, and only 10% of patients discontinued treatment because of toxicity. Based on these data, nivolumab plus ipilimumab is now included in the National Comprehensive Cancer Network guidelines as an option for patients with SCLC who experience disease relapse within 6 months of receiving platinum-based therapy;7 however, it is questionable whether routine use of this combination is justified based on currently available data. The evidence for the combination of nivolumab and ipilimumab remains limited. The efficacy and toxicity data from both randomized and nonrandomized cohorts were presented together, making it hard to interpret the results.
Another phase 1b study (KEYNOTE-028) evaluated the anti-PD-1 antibody pembrolizumab (10 mg/kg intravenously every 2 weeks) in patients with relapsed SCLC who had received 1 or more prior lines of therapy and had PD-L1 expression of ≥ 1%. This study showed a response rate of 33%, with a median duration of response of 19 months and 1-year OS of 38%.80 Although only 28% of screened patients had PD-L1 expression of ≥ 1%, these results indicated that at least a subset of SCLC patients are able to achieve durable responses with immune checkpoint inhibition. A number of clinical trials utilizing immune checkpoint inhibitors in various combinations and settings are currently underway.
ROLE OF PROPHYLACTIC CRANIAL IRRADIATION
The role of PCI in extensive-stage SCLC is not clearly defined. A randomized phase 3 trial conducted by the European Organization for Research and Treatment of Cancer (EORTC) comparing PCI with no PCI in patients with extensive-stage SCLC who had a partial or complete response to initial platinum-based chemotherapy showed a decrease in the incidence of symptomatic brain metastasis and improvement in 1-year OS with PCI.81 However, this trial did not require mandatory brain imaging prior to PCI, and thus it is unclear if some patients in the PCI group had asymptomatic brain metastasis prior to enrollment and therefore received therapeutic benefit from brain radiation. Additionally, the dose and fractionation of PCI was not standardized across patient groups.
A more recent phase 3 study conducted in Japan that compared PCI (25 Gy in 10 fractions) with no PCI reported no difference in survival between the 2 groups.82 As opposed to the EORTC study, the Japanese study did require baseline brain imaging to confirm the absence of brain metastasis prior to enrollment. In addition, the control patients underwent periodic brain MRI to allow early detection of brain metastasis. Given the emergence of the new data, the impact of PCI on survival in patients with extensive-stage SCLC is unproven, and PCI likely has a role in a highly selected small group of patients with extensive-stage SCLC. PCI is not recommended for patients with poor performance status (ECOG performance score of 3 or 4) or underlying neurocognitive disorders.34,83
The NMDA-receptor antagonist memantine can be used in patients undergoing PCI to delay the occurrence of cognitive dysfunction.61 Memantine 20 mg daily delayed time to cognitive decline and reduced the rate of decline in memory, executive function, and processing speed compared to placebo in patients receiving whole brain radiotherapy.84
ROLE OF RADIOTHERAPY
A subset of patients with extensive-stage SCLC may benefit from consolidative thoracic radiotherapy after completion of platinum-based chemotherapy. A randomized trial that enrolled patients who achieved complete or near complete response after 3 cycles of cisplatin plus etoposide compared thoracic radiotherapy in combination with continued chemotherapy versus chemotherapy alone.85 The median OS was longer with the addition of thoracic radiotherapy compared to chemotherapy alone. Another phase 3 trial did not show improvement in 1-year OS with consolidative thoracic radiotherapy, but 2-year OS and 6-month PFS were longer.86 In general, consolidative thoracic radiotherapy benefits patients who have residual thoracic disease and low-bulk extrathoracic disease that has responded to systemic therapy.87 In addition, patients who initially presented with bulky symptomatic thoracic disease should also be considered for consolidative radiation.
Similar to other solid tumors, radiotherapy should be utilized for palliative purposes in patients with painful bone metastasis, spinal cord compression, or brain metastasis. Surgery is generally not recommended for spinal cord compression given the short life expectancy of patients with extensive-stage disease. Whole brain radiotherapy is preferred over stereotactic radiosurgery because micrometastasis is frequently present even in the setting of 1 or 2 radiographically evident brain metastasis.
NOVEL THERAPIES
The very complex genetic landscape of SCLC accounts for its resistance to conventional therapy and high recurrence rate; however, at the same time this complexity can form the basis for effective targeted therapy for the disease. One of the major factors hindering the development of targeted therapies in SCLC is limited availability of tissue due to small tissue samples and the frequent presence of significant necrosis in the samples. In recent years, several different therapeutic strategies and targeted agents have been investigated for their potential role in SCLC. Several of them, including EGFR tyrosine kinase inhibitors (TKIs), BCR-ABL TKIs, mTOR inhibitors, and VEGF inhibitors, have not been shown to provide a survival advantage in this disease. Several others, including PARP inhibitors, cellular developmental pathway inhibitors, and antibody-drug conjugates, are being tested. A phase 1 study of veliparib combined with cisplatin and etoposide in patients with previously untreated extensive-stage SCLC demonstrated a complete response in 14.3%, a partial response in 57.1%, and stable disease in 28.6% of patients with an acceptable safety profile.88 So far, none of these agents are approved for use in SCLC, and the majority are in early- phase clinical trials.89
One of the emerging targets in the treatment of SCLC is delta-like protein 3 (DLL3). DLL3 is expressed on more than 80% of SCLC tumor cells and cancer stem cells. Rovalpituzumab tesirine is an antibody-drug conjugate consisting of humanized anti-DLL3 monoclonal antibody linked to SC-DR002, a DNA-crosslinking agent. A phase 1 trial of rovalpituzumab in patients with relapsed SCLC after 1 or 2 prior lines of therapy reported a response rate of 31% in patients with DLL3 expression of ≥ 50%. The median duration of response and median PFS were both 4.6 months.90 Rovalpituzumab is currently in later phases of clinical trials and has a potential to serve as an option for patients with extensive-stage disease after disease progression on platinum-based therapy.
SUMMARY
Four to 6 cycles of carboplatin and etoposide remain the standard of care first-line treatment for patients with extensive stage SCLC. The only FDA-approved second-line treatment option is topotecan. Re-treatment with the original platinum doublet is a reasonable option for patients who have disease progression 6 months or longer after completion of platinum-based therapy. The immune checkpoint inhibitors pembrolizumab and combination nivolumab and ipilimumab have shown promising results in the second-line setting and beyond. The role of PCI has become more controversial in recent years, and periodic brain MRI in lieu of PCI is now an acceptable approach.
RESPONSE ASSESSMENT/SURVEILLANCE
For patients undergoing treatment for limited-stage SCLC, response assessment with contrast-enhanced CT of the chest/abdomen should be performed after completion of 4 cycles of chemotherapy and thoracic radiation.7 The surveillance guidelines consist of history, physical exam, and imaging every 3 months during the first 2 years, every 6 months during the third year, and annually thereafter. If PCI is not performed, brain MRI or contrast-enhanced CT scan should be performed every 3 or 4 months during the first 2 years of follow up. For extensive-stage disease, response assessment should be performed after every 2 cycles of therapy. After completion of therapy, history, physical exam, and imaging should be done every 2 months during the first year, every 3 or 4 months during years 2 and 3, every 6 months during years 4 and 5, and annually thereafter. Routine use of PET scan for surveillance is not recommended. Any new pulmonary nodule should prompt evaluation for a second primary lung malignancy. Finally, smoking cessation counseling is an integral part of management of any patient with SCLC and should be included with every clinic visit.
CONCLUSION
SCLC is a heterogeneous and genetically complex disease with a very high mortality rate. The current standard of care includes concurrent chemoradiation with cisplatin and etoposide for limited-stage SCLC and the combination of platinum and etoposide for extensive SCLC. A number of novel treatment approaches, including immune checkpoint inhibitors and antibody-drug conjugates, have had promising results in early clinical trials. Given the limited treatment options and large unmet need for new treatment options, enrollment in clinical trials is strongly recommended for patients with SCLC.
INTRODUCTION
Small cell lung cancer (SCLC) is an aggressive cancer of neuroendocrine origin that accounts for approximately 15% of all lung cancer cases, with approximately 33,000 patients diagnosed annually.1 The incidence of SCLC in the United States has steadily declined over the past 30 years, presumably because of a decrease in the number of smokers and a change to low-tar filter cigarettes.2 Although the overall incidence of SCLC has been decreasing, the incidence in women is increasing and the male-to-female incidence ratio is now 1:1.3 Nearly all cases of SCLC are associated with heavy tobacco exposure, making it a heterogeneous disease with a complex genomic landscape consisting of thousands of mutations.4,5 Despite recent advances in the treatment of non-small cell lung cancer, the therapeutic options for SCLC remain limited, with a median overall survival (OS) of 9 months in patients with advanced disease.
DIAGNOSIS AND STAGING
CASE PRESENTATION
A 61-year-old man presents to the emergency department with progressive shortness of breath and cough over the past 6 weeks. He also reports a 20-lb weight loss over the same period. He is a current smoker and has been smoking 1 pack of cigarettes per day since the age of 18 years. A chest radiograph obtained in the emergency department shows a right hilar mass. Computed tomography (CT) scan confirms the presence of a 4.5-cm right hilar mass and enlarged mediastinal lymph nodes bilaterally.
• What are the next steps in diagnosis?
SCLC is characterized by rapid growth and early hematogenous metastasis. Consequently, only 25% of patients have limited-stage disease at the time of diagnosis. According to the Veterans Administration Lung Study Group (VALSG) staging system, limited-stage disease is defined as tumor that is confined to 1 hemithorax and can be encompassed within 1 radiation field. This typically includes mediastinal lymph nodes and ipsilateral supraclavicular lymph nodes. Approximately 75% of patients present with extensive-stage disease, which is defined as disease that cannot be classified as limited, including disease that extends beyond 1 hemithorax. Extensive-stage disease includes the presence of malignant pleural effusion and/or distant metastasis.6 The VALSG classification and staging system is more commonly used in clinical practice than the American Joint Committee on Cancer TNM staging system because it is less complex and directs treatment decisions, as most of the literature on SCLC classifies patients based on the VALSG system.7
Given SCLC’s propensity to metastasize quickly, none of the currently available screening methods have proven successful in early detection of SCLC. In the National Lung Cancer Screening Trial, 86% of the 125 patients who were diagnosed with SCLC while undergoing annual low-dose chest CT scans had advanced disease at diagnosis.8,9 These results highlight the fact that most cases of SCLC develop in the interval between annual screening imaging.
SCLC frequently presents with a large hilar mass that is symptomatic. Common symptoms include shortness of breath and cough. In addition, patients with SCLC usually have bulky mediastinal adenopathy at presentation. SCLC is commonly located submucosally in the bronchus, and therefore hemoptysis is not a very common symptom at the time of presentation. Patients may present with superior vena cava syndrome from local compression by the tumor. Not infrequently, SCLC is associated with paraneoplastic syndromes that arise due to ectopic secretion of hormones or antibodies by the tumor cells. The paraneoplastic syndromes can be broadly categorized as endocrine or neurologic (Table 1). The presence of a paraneoplastic syndrome is often a clue to the potential diagnosis of SCLC in the presence of a hilar mass. Additionally, some paraneoplastic syndromes, more specifically endocrine paraneoplastic syndromes, follow the pattern of disease response and relapse, and therefore can sometimes serve as an early marker of disease relapse or progression.
The common sites of metastases include brain, liver, and bone. Therefore, the staging workup should include fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT scan. Contrast-enhanced CT scan of the chest and abdomen and bone scan can be obtained for staging in lieu of PET scan. Due to the physiologic FDG uptake, cerebral metastases cannot be assessed with sufficient certainty using PET-CT.10 Therefore, brain imaging with contrast-enhanced CT or magnetic resonance imaging (MRI) is also necessary. Although the incidence of metastasis to bone marrow is less than 10%, bone marrow aspiration and biopsy are warranted in patients with unexplained cytopenias, especially when the cytopenia is associated with teardrop-shaped red cells or nucleated red cells on peripheral blood smear, findings indicative of a marrow infiltrative process.7 The tissue diagnosis is established by obtaining a biopsy of the primary tumor or 1 of the metastatic sites. In localized disease, bronchoscopy (with endobronchial ultrasound, if necessary) with biopsy of the centrally located tumor and/or lymph node is required. Histologically, SCLC consists of monomorphic cells, a high nuclear-cytoplasmic ratio, and confluent necrosis. The tumor cells are positive for chromogranin, synaptophysin, and CD56 by immunohistochemistry, and very frequently are also positive for thyroid transcription factor 1.11 Although serum tumor markers, including neuron-specific enolase and progastrin-releasing peptide, are frequently elevated in patients with SCLC, these markers are of limited value in clinical practice because they lack sensitivity and specificity.12
MANAGEMENT OF LIMITED-STAGE DISEASE
CASE CONTINUED
The patient undergoes FDG PET scan, which shows the presence of a hypermetabolic right hilar mass in addition to enlarged and hypermetabolic bilateral mediastinal lymph nodes. There are no other areas of FDG avidity. Brain MRI does not show any evidence of brain metastasis. Thus, the patient is confirmed to have limited-stage SCLC.
• What is the standard of care treatment for limited-stage SCLC?
SCLC is exquisitely sensitive to both chemotherapy and radiation, especially at the time of initial presentation. The standard of care treatment of limited-stage SCLC is 4 cycles of platinum-based chemotherapy in combination with thoracic radiation started within the first 2 cycles of chemotherapy (Figure 1).
CHOICE OF CHEMOTHERAPY
Etoposide and cisplatin is the most commonly used initial combination chemotherapy regimen in limited-stage SCLC.14 This combination has largely replaced anthracycline-based regimens given its favorable efficacy and toxicity profile.15–17 Several small randomized trials have shown comparable efficacy of carboplatin and etoposide in extensive-stage SCLC.18–20 A meta-analysis of 4 randomized trials comparing cisplatin-based versus carboplatin-based regimens in 663 patients with SCLC (32% had limited-stage disease and 68% had extensive-stage disease) showed no statistically significant difference in response rate, progression-free survival (PFS), or OS between the 2 regimens.21 Therefore, in clinical practice carboplatin is frequently used instead of cisplatin in patients with extensive-stage disease. In patients with limited-stage disease, cisplatin is still the drug of choice. However, the toxicity profile of the 2 regimens is different. Cisplatin-based regimens are more commonly associated with neuropathy, nephrotoxicity, and chemotherapy-induced nausea/vomiting,18 while carboplatin-based regimens are more myelosuppressive.22 In addition, the combination of thoracic radiation with either of these regimens is associated with a higher risk of esophagitis, pneumonitis, and myelosuppression.23 The use of myeloid growth factors is not recommended in patients undergoing concurrent chemoradiation.24 Of note, intravenous etoposide is always preferred over oral etoposide, especially in the curative setting given the unreliable absorption and bioavailability of oral formulations.
THORACIC RADIOTHERAPY
Adding thoracic radiotherapy to platinum-etoposide chemotherapy improves local control and OS. Two meta-analyses of 13 trials including more than 2000 patients have shown a 25% to 30% decrease in local failure and a 5% to 7% increase in 2-year OS with chemoradiation compared to chemotherapy alone in limited-stage SCLC.25,26 Early (within the first 2 cycles) concurrent thoracic radiation is superior to delayed and/or sequential radiation in terms of local control and OS.23,27,28 The dose and fractionation of thoracic radiation in limited-stage SCLC has remained a controversial issue. The Eastern Cooperative Oncology Group/Radiation Therapy Oncology Group randomized trial compared 45 Gy of radiotherapy delivered twice daily over a period of 3 weeks to 45 Gy once daily over 5 weeks concurrently with chemotherapy. The twice daily regimen led to a 10% improvement in 5-year OS (26% versus 16%), but a higher incidence of grade 3 and 4 adverse events.13 Despite the survival advantage demonstrated by hyperfractionated radiotherapy, the results need to be interpreted with caution because the radiation doses are not biologically equivalent. In addition, the difficult logistics of patients receiving radiation twice a day has limited the routine implementation of this strategy. Subsequently, another randomized phase 3 trial (CONVERT) compared 45 Gy radiotherapy twice daily with 66 Gy radiotherapy once daily in limited-stage SCLC.29 This trial did not show any difference in OS. The patients in the twice daily arm had a higher incidence of grade 4 neutropenia. Considering the results of these trials, both strategies—45 Gy fractionated twice daily or 60 Gy fractionated once daily, delivered concurrently with chemotherapy—are acceptable in the setting of limited-stage SCLC. However, quite often a hyperfractionated regimen is not feasible for patients and many radiation oncology centers. Hopefully, the ongoing CALGB 30610 study will clarify the optimal radiation schedule for limited-stage disease.
PROPHYLACTIC CRANIAL IRRADIATION
Approximately 75% of patients with limited-stage disease experience disease recurrence, and brain is the site of recurrence in approximately half of these patients.30 Prophylactic cranial irradiation (PCI) consisting of 25 Gy radiotherapy delivered in 10 fractions has been shown to be effective in decreasing the incidence of cerebral metastases.30–32 Although individual small studies have not shown a survival benefit of PCI because of small sample size and limited power, a meta-analysis of these studies has shown a 25% decrease in the 3-year incidence of brain metastasis and 5.4% increase in 3-year OS.30 Most patients included in these studies had limited-stage disease. Therefore, PCI is the standard of care for patients with limited-stage disease who attain a partial or complete response to chemoradiation.
ROLE OF SURGERY
Surgical resection may be an acceptable choice in a very limited subset of patients with peripherally located small (< 5 cm) tumors where mediastinal lymph nodes have been confirmed to be uninvolved with complete mediastinal staging.33,34 Most of the data in this setting are derived from retrospective studies.35,36 A 5-year OS between 40% and 60% has been reported with this strategy in patients with clinical stage I disease. In general, when surgery is considered, lobectomy with mediastinal lymph node dissection followed by chemotherapy (if there is no nodal involvement) or chemoradiation (if nodal involvement) is recommended.37,38 Wedge or segmental resections are not considered to be optimal surgical options.
MANAGEMENT OF EXTENSIVE-STAGE DISEASE
CASE CONTINUED
The patient receives 4 cycles of cisplatin and etoposide along with 70 Gy radiotherapy concurrently with the first 2 cycles of chemotherapy. His post-treatment CT scans show a partial response. He undergoes PCI 6 weeks after completion of treatment. At routine follow-up 18 months later, he is doing generally well except for mildly decreased appetite and an unintentional weight loss of 5 lb. CT scans demonstrate multiple hypodense liver lesions ranging from 7 mm to 2 cm in size and a 2-cm left adrenal gland lesion highly concerning for metastasis. FDG PET scan confirms that the adrenal and liver lesions are hypermetabolic. In addition, the PET scan shows multiple FDG-avid bone lesions throughout the spine. Brain MRI is negative for brain metastasis.
• What is the standard of care for treatment of extensive-stage disease?
Chemotherapy is the mainstay of treatment for extensive-stage SCLC; the goals of treatment are prolongation of survival, prevention or alleviation of cancer-related symptoms, and improvement in quality of life. The combination of etoposide with a platinum agent (carboplatin or cisplatin) is the preferred first-line treatment option. Carboplatin is more commonly used in clinical practice in this setting because of its comparable efficacy and better tolerability compared to cisplatin (Figure 2).21 A Japanese phase 3 trial comparing cisplatin plus irinotecan with cisplatin plus etoposide in the first-line setting in extensive-stage SCLC showed improvement in median and 2-year OS with the cisplatin/irinotecan regimen; however, 2 subsequent phase 3 trials conducted in the United States comparing these 2 regimens did not show any difference in OS. In addition, the cisplatin/irinotecan regimen was more toxic than the etoposide-based regimen.39,40 Therefore, 4 to 6 cycles of platinum/etoposide remains the standard of care first-line treatment for extensive-stage SCLC in the United States. The combination yields a 60% to 70% response rate, but the majority of patients invariably experience disease progression, with a median OS of 9 to 11 months.41 Maintenance chemotherapy beyond the initial 4 to 6 cycles does not improve survival and is associated with higher cumulative toxicity.42
Multiple attempts at improving first-line chemotherapy in extensive-stage disease have failed to show any meaningful difference in OS. For example, the addition of ifosfamide, palifosfamide, cyclophosphamide, taxane, or anthracycline to platinum doublet failed to show improvement in OS and led to more toxicity.43–46 Additionally, the use of alternating or cyclic chemotherapies in an attempt to curb drug resistance has also failed to show survival benefit.47–49 The addition of the antiangiogenic agent bevacizumab to standard platinum-based doublet has not prolonged OS in SCLC and has led to an unacceptably higher rate of tracheoesophageal fistula when used in conjunction with chemoradiation in limited-stage disease.50–55 Finally, the immune checkpoint inhibitor ipilimumab in combination with platinum plus etoposide failed to improve PFS or OS compared to platinum plus etoposide alone in a recent phase 3 trial, and maintenance pembrolizumab after completion of platinum-based chemotherapy did not improve PFS.56,57
More recently, a phase 2 study of pembrolizumab in extensive-stage SCLC (KEYNOTE 158) reported an overall response rate of 35.7%, median PFS of 2.1 months, and median OS of 14.6 months in patients who tested positive for programmed death ligand-1 (PD-L1) expression (which was defined as a PD-L1 Combined Positive Score ≥ 1).58 The median duration of response has not been reached in this study, indicating that pembrolizumab may be a promising approach in patients with extensive-stage SCLC, especially for those with PD-L1–positive tumors.
Patients with extensive-stage disease who have brain metastasis at the time of diagnosis can be treated with systemic chemotherapy first if the brain metastases are asymptomatic and there is significant extracranial disease burden. In that case, whole brain radiotherapy should be given after completion of systemic therapy.
SECOND-LINE CHEMOTHERAPY
Despite being exquisitely chemosensitive, SCLC is associated with a very poor prognosis largely because of invariable disease progression following first-line therapy and lack of effective second-line treatment options that can lead to appreciable disease control. The choice of second-line treatment is predominantly determined by the time of disease relapse after first-line platinum-based therapy. If this interval is 6 months or longer, re-treatment utilizing the same platinum doublet is appropriate. However, if the interval is 6 months or less, second-line systemic therapy options should be explored. Unfortunately, the response rate tends to be less than 10% with most of the second-line therapies in platinum-resistant disease (defined as disease progression within 3 months of receiving platinum-based therapy). If disease progression occurs between 3 and 6 months after completion of platinum-based therapy, the response rate with second-line chemotherapy is in the range of 25%.59,60
A number of second-line chemotherapy options have been explored in small studies, including topotecan, irinotecan, paclitaxel, docetaxel, temozolomide, vinorelbine, oral etoposide, gemcitabine, bendamustine, and CAV (
IMMUNOTHERAPY
The role of immune checkpoint inhibitors in the treatment of SCLC is evolving, and currently there are no FDA-approved immunotherapy agents for treating SCLC. A recently conducted phase 1/2 trial (CheckMate 032) studied the anti-programmed death(PD)-1 antibody nivolumab with or without the anti-cytotoxic T-lymphocyte–associated antigen (CTLA) -4 antibody ipilimumab in patients with relapsed SCLC. The authors reported response rates of 10% with nivolumab 3 mg/kg and 21% with nivolumab 1 mg/kg plus ipilimumab 3 mg/kg.78,79 The 2-year OS was 26% with the combination and 14% with single-agent nivolumab. Only 18% of patients had PD-L1 expression of ≥ 1%, and the response rate did not correlate with PD-L1 status. The rate of grade 3 or 4 adverse events was approximately 20%, and only 10% of patients discontinued treatment because of toxicity. Based on these data, nivolumab plus ipilimumab is now included in the National Comprehensive Cancer Network guidelines as an option for patients with SCLC who experience disease relapse within 6 months of receiving platinum-based therapy;7 however, it is questionable whether routine use of this combination is justified based on currently available data. The evidence for the combination of nivolumab and ipilimumab remains limited. The efficacy and toxicity data from both randomized and nonrandomized cohorts were presented together, making it hard to interpret the results.
Another phase 1b study (KEYNOTE-028) evaluated the anti-PD-1 antibody pembrolizumab (10 mg/kg intravenously every 2 weeks) in patients with relapsed SCLC who had received 1 or more prior lines of therapy and had PD-L1 expression of ≥ 1%. This study showed a response rate of 33%, with a median duration of response of 19 months and 1-year OS of 38%.80 Although only 28% of screened patients had PD-L1 expression of ≥ 1%, these results indicated that at least a subset of SCLC patients are able to achieve durable responses with immune checkpoint inhibition. A number of clinical trials utilizing immune checkpoint inhibitors in various combinations and settings are currently underway.
ROLE OF PROPHYLACTIC CRANIAL IRRADIATION
The role of PCI in extensive-stage SCLC is not clearly defined. A randomized phase 3 trial conducted by the European Organization for Research and Treatment of Cancer (EORTC) comparing PCI with no PCI in patients with extensive-stage SCLC who had a partial or complete response to initial platinum-based chemotherapy showed a decrease in the incidence of symptomatic brain metastasis and improvement in 1-year OS with PCI.81 However, this trial did not require mandatory brain imaging prior to PCI, and thus it is unclear if some patients in the PCI group had asymptomatic brain metastasis prior to enrollment and therefore received therapeutic benefit from brain radiation. Additionally, the dose and fractionation of PCI was not standardized across patient groups.
A more recent phase 3 study conducted in Japan that compared PCI (25 Gy in 10 fractions) with no PCI reported no difference in survival between the 2 groups.82 As opposed to the EORTC study, the Japanese study did require baseline brain imaging to confirm the absence of brain metastasis prior to enrollment. In addition, the control patients underwent periodic brain MRI to allow early detection of brain metastasis. Given the emergence of the new data, the impact of PCI on survival in patients with extensive-stage SCLC is unproven, and PCI likely has a role in a highly selected small group of patients with extensive-stage SCLC. PCI is not recommended for patients with poor performance status (ECOG performance score of 3 or 4) or underlying neurocognitive disorders.34,83
The NMDA-receptor antagonist memantine can be used in patients undergoing PCI to delay the occurrence of cognitive dysfunction.61 Memantine 20 mg daily delayed time to cognitive decline and reduced the rate of decline in memory, executive function, and processing speed compared to placebo in patients receiving whole brain radiotherapy.84
ROLE OF RADIOTHERAPY
A subset of patients with extensive-stage SCLC may benefit from consolidative thoracic radiotherapy after completion of platinum-based chemotherapy. A randomized trial that enrolled patients who achieved complete or near complete response after 3 cycles of cisplatin plus etoposide compared thoracic radiotherapy in combination with continued chemotherapy versus chemotherapy alone.85 The median OS was longer with the addition of thoracic radiotherapy compared to chemotherapy alone. Another phase 3 trial did not show improvement in 1-year OS with consolidative thoracic radiotherapy, but 2-year OS and 6-month PFS were longer.86 In general, consolidative thoracic radiotherapy benefits patients who have residual thoracic disease and low-bulk extrathoracic disease that has responded to systemic therapy.87 In addition, patients who initially presented with bulky symptomatic thoracic disease should also be considered for consolidative radiation.
Similar to other solid tumors, radiotherapy should be utilized for palliative purposes in patients with painful bone metastasis, spinal cord compression, or brain metastasis. Surgery is generally not recommended for spinal cord compression given the short life expectancy of patients with extensive-stage disease. Whole brain radiotherapy is preferred over stereotactic radiosurgery because micrometastasis is frequently present even in the setting of 1 or 2 radiographically evident brain metastasis.
NOVEL THERAPIES
The very complex genetic landscape of SCLC accounts for its resistance to conventional therapy and high recurrence rate; however, at the same time this complexity can form the basis for effective targeted therapy for the disease. One of the major factors hindering the development of targeted therapies in SCLC is limited availability of tissue due to small tissue samples and the frequent presence of significant necrosis in the samples. In recent years, several different therapeutic strategies and targeted agents have been investigated for their potential role in SCLC. Several of them, including EGFR tyrosine kinase inhibitors (TKIs), BCR-ABL TKIs, mTOR inhibitors, and VEGF inhibitors, have not been shown to provide a survival advantage in this disease. Several others, including PARP inhibitors, cellular developmental pathway inhibitors, and antibody-drug conjugates, are being tested. A phase 1 study of veliparib combined with cisplatin and etoposide in patients with previously untreated extensive-stage SCLC demonstrated a complete response in 14.3%, a partial response in 57.1%, and stable disease in 28.6% of patients with an acceptable safety profile.88 So far, none of these agents are approved for use in SCLC, and the majority are in early- phase clinical trials.89
One of the emerging targets in the treatment of SCLC is delta-like protein 3 (DLL3). DLL3 is expressed on more than 80% of SCLC tumor cells and cancer stem cells. Rovalpituzumab tesirine is an antibody-drug conjugate consisting of humanized anti-DLL3 monoclonal antibody linked to SC-DR002, a DNA-crosslinking agent. A phase 1 trial of rovalpituzumab in patients with relapsed SCLC after 1 or 2 prior lines of therapy reported a response rate of 31% in patients with DLL3 expression of ≥ 50%. The median duration of response and median PFS were both 4.6 months.90 Rovalpituzumab is currently in later phases of clinical trials and has a potential to serve as an option for patients with extensive-stage disease after disease progression on platinum-based therapy.
SUMMARY
Four to 6 cycles of carboplatin and etoposide remain the standard of care first-line treatment for patients with extensive stage SCLC. The only FDA-approved second-line treatment option is topotecan. Re-treatment with the original platinum doublet is a reasonable option for patients who have disease progression 6 months or longer after completion of platinum-based therapy. The immune checkpoint inhibitors pembrolizumab and combination nivolumab and ipilimumab have shown promising results in the second-line setting and beyond. The role of PCI has become more controversial in recent years, and periodic brain MRI in lieu of PCI is now an acceptable approach.
RESPONSE ASSESSMENT/SURVEILLANCE
For patients undergoing treatment for limited-stage SCLC, response assessment with contrast-enhanced CT of the chest/abdomen should be performed after completion of 4 cycles of chemotherapy and thoracic radiation.7 The surveillance guidelines consist of history, physical exam, and imaging every 3 months during the first 2 years, every 6 months during the third year, and annually thereafter. If PCI is not performed, brain MRI or contrast-enhanced CT scan should be performed every 3 or 4 months during the first 2 years of follow up. For extensive-stage disease, response assessment should be performed after every 2 cycles of therapy. After completion of therapy, history, physical exam, and imaging should be done every 2 months during the first year, every 3 or 4 months during years 2 and 3, every 6 months during years 4 and 5, and annually thereafter. Routine use of PET scan for surveillance is not recommended. Any new pulmonary nodule should prompt evaluation for a second primary lung malignancy. Finally, smoking cessation counseling is an integral part of management of any patient with SCLC and should be included with every clinic visit.
CONCLUSION
SCLC is a heterogeneous and genetically complex disease with a very high mortality rate. The current standard of care includes concurrent chemoradiation with cisplatin and etoposide for limited-stage SCLC and the combination of platinum and etoposide for extensive SCLC. A number of novel treatment approaches, including immune checkpoint inhibitors and antibody-drug conjugates, have had promising results in early clinical trials. Given the limited treatment options and large unmet need for new treatment options, enrollment in clinical trials is strongly recommended for patients with SCLC.
1. American Cancer Society. Cancer Facts & Figures 2017. American Cancer Society website. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. Published 2017. Accessed July 11, 2018.
2. Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24:4539–44.
3. Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2014. National Cancer Institute website. https://seer.cancer.gov/csr/1975_2014/. Updated April 2, 2018. Accessed July 11, 2018.
4. Varghese AM, Zakowski MF, Yu HA, et al. Small-cell lung cancers in patients who never smoked cigarettes. J Thorac Oncol 2014;9:892–6.
5. Pleasance ED, Stephens PJ, O’Meara S, et al. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature 2010;463:184–90.
6. Green RA, Humphrey E, Close H, Patno ME. Alkylating agents in bronchogenic carcinoma. Am J Med 1969;46:516–25.
7. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology for small cell lung cancer (Version 2.2018). www.nccn.org/professionals/physician_gls/pdf/sclc.pdf. Accessed August 12, 2018.
8. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409.
9. Aberle DR, DeMello S, Berg CD, et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med 2013;369:920–31.
10. Kitajima K, Nakamoto Y, Okizuka H, et al. Accuracy of whole-body FDG-PET/CT for detecting brain metastases from non-central nervous system tumors. Ann Nucl Med 2008;22:595–602.
11. Ordonez NG. Value of thyroid transcription factor-1 immunostaining in distinguishing small cell lung carcinomas from other small cell carcinomas. Am J Surg Pathol 2000;24:1217–23.
12. Karnak D, Beder S, Kayacan O, et al. Neuron-specific enolase and lung cancer. Am J Clin Oncol 2005;28:586–90.
13. Turrisi AT 3rd, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 1999;340:265–71.
14. Evans WK, Shepherd FA, Feld R, et al. VP-16 and cisplatin as first-line therapy for small-cell lung cancer. J Clin Oncol 1985;3:1471–7.
15. Pujol JL, Carestia L, Daurés JP. Is there a case for cisplatin in the treatment of small-cell lung cancer? A meta-analysis of randomized trials of a cisplatin-containing regimen versus a regimen without this alkylating agent. Br J Cancer 2000;83:8–15.
16. Mascaux C, Paesmans M, Berghmans T, et al; European Lung Cancer Working Party (ELCWP). A systematic review of the role of etoposide and cisplatin in the chemotherapy of small cell lung cancer with methodology assessment and meta-analysis. Lung Cancer 2000;30:23–36.
17. Sundstrøm S, Bremnes RM, Kaasa S, et al; Norwegian Lung Cancer Study Group. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years’ follow-up. J Clin Oncol 2002;20:4665–72.
18. Hatfield LA, Huskamp HA, Lamont EB. Survival and toxicity after cisplatin plus etoposide versus carboplatin plus etoposide for extensive-stage small-cell lung cancer in elderly patients. J Oncol Pract 2016;12:666–73.
19. Okamoto H, Watanabe K, Kunikane H, et al. Randomised phase III trial of carboplatin plus etoposide vs split doses of cisplatin plus etoposide in elderly or poor-risk patients with extensive disease small-cell lung cancer: JCOG 9702. Br J Cancer 2007;97:162–9.
20. Skarlos DV, Samantas E, Kosmidis P, et al. Randomized comparison of etoposide-cisplatin vs. etoposide-carboplatin and irradiation in small-cell lung cancer. A Hellenic Co-operative Oncology Group study. Ann Oncol 1994;5:601–7.
21. Rossi A, Di Maio M, Chiodini P, et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data J Clin Oncol 2012;30:1692–8.
22. Bishop JF, Raghavan D, Stuart-Harris R, et al. Carboplatin (CBDCA, JM-8) and VP-16-213 in previously untreated patients with small-cell lung cancer. J Clin Oncol 1987;5:1574–8.
23. Takada M, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol 2002;20:3054–60.
24. Bunn PA Jr, Crowley J, Kelly K, et al. Chemoradiotherapy with or without granulocyte-macrophage colony-stimulating factor in the treatment of limited-stage small-cell lung cancer: a prospective phase III randomized study of the Southwest Oncology Group. J Clin Oncol 1995;13:1632–41.
25. Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 1992;327:1618–24.
26. Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol 1992;10:890–5.
27. Murray N, Coy P, Pater JL, et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 1993;11:336–44.
28. De Ruysscher D, Lueza B, Le Péchoux C, et al. Impact of thoracic radiotherapy timing in limited-stage small-cell lung cancer: usefulness of the individual patient data meta-analysis. Ann Oncol 2016;27:1818–28.
29. Faivre-Finn C, Snee M, Ashcroft L, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol 2017;18:1116–25.
30. Aupérin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341:476–84.
31. Arriagada R, Le Chevalier T, Borie F, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst 1995;87:183–90.
32. Le Péchoux C, Dunant A, Senan S, et al; Prophylactic Cranial Irradiation (PCI) Collaborative Group. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): a randomised clinical trial. Lancet Oncol 2009;10:467–74.
33. Schneider BJ, Saxena A, Downey RJ. Surgery for early-stage small cell lung cancer. J Natl Compr Canc Netw 2011;9:1132–9.
34. Inoue M, Nakagawa K, Fujiwara K, et al. Results of preoperative mediastinoscopy for small cell lung cancer. Ann Thorac Surg 2000;70:1620–3.
35. Lim E, Belcher E, Yap YK, et al. The role of surgery in the treatment of limited disease small cell lung cancer: time to reevaluate. J Thorac Oncol 2008;3:1267–71.
36. Inoue M, Miyoshi S, Yasumitsu T, et al. Surgical results for small cell lung cancer based on the new TNM staging system. Thoracic Surgery Study Group of Osaka University, Osaka, Japan. Ann Thorac Surg 2000;70:1615–9.
37. Yang CF, Chan DY, Speicher PJ, et al. Role of adjuvant therapy in a population-based cohort of patients with early-stage small-cell lung cancer. J Clin Oncol 2016;34:1057–64.
38. Shepherd FA, Evans WK, Feld R, et al. Adjuvant chemotherapy following surgical resection for small-cell carcinoma of the lung. J Clin Oncol 1988;6:832–8.
39. Noda K, Nishiwaki Y, Kawahara M, et al; Japan Clinical Oncology Group. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 2002;346:85–91.
40. Lara PN Jr, Natale R, Crowley J, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol 2009;27:2530–5.
41. Chute JP, Chen T, Feigal E, et al. Twenty years of phase III trials for patients with extensive-stage small-cell lung cancer: perceptible progress. J Clin Oncol 1999;17:1794–801.
42. Zhou H, Zeng C, Wei Y, et al. Duration of chemotherapy for small cell lung cancer: a meta-analysis. PloS One 2013;8:e73805.
43. Loehrer PJ Sr, Ansari R, Gonin R, et al. Cisplatin plus etoposide with and without ifosfamide in extensive small-cell lung cancer: a Hoosier Oncology Group study. J Clin Oncol;13:2594–9.
44. Pujol JL, Daurés JP, Riviére A, et al. Etoposide plus cisplatin with or without the combination of 4’-epidoxorubicin plus cyclophosphamide in treatment of extensive small-cell lung cancer: a French Federation of Cancer Institutes multicenter phase III randomized study. J Natl Cancer Inst 2001;93:300–8.
45. Berghmans T, Scherpereel A, Meert AP, et al; European Lung Cancer Working Party (ELCWP). A phase III randomized study comparing a chemotherapy with cisplatin and etoposide to a etoposide regimen without cisplatin for patients with extensive small-cell lung cancer. Front Oncol 2017;7:217.
46. Jalal SI, Lavin P, Lo G, et al. Carboplatin and etoposide with or without palifosfamide in untreated extensive-stage small-cell lung cancer: a Multicenter, Adaptive, Randomized Phase III Study (MATISSE). J Clin Oncol 2017;35:2619–23.
47. Fukuoka M, Furuse K, Saijo N, et al. Randomized trial of cyclophosphamide, doxorubicin, and vincristine versus cisplatin and etoposide versus alternation of these regimens in small-cell lung cancer. J Natl Cancer Inst 1991;83:855–61.
48. Roth BJ, Johnson DH, Einhorn LH, et al. Randomized study of cyclophosphamide, doxorubicin, and vincristine versus etoposide and cisplatin versus alternation of these two regimens in extensive small-cell lung cancer: a phase III trial of the Southeastern Cancer Study Group. J Clin Oncol 1992;10:282–91.
49. Miles DW, Earl HM, Souhami RL, et al. Intensive weekly chemotherapy for good-prognosis patients with small-cell lung cancer. J Clin Oncol 1991;9:280–5.
50. Petrioli R, Roviello G, Laera L, et al. Cisplatin, etoposide, and bevacizumab regimen followed by oral etoposide and bevacizumab maintenance treatment in patients with extensive-stage small cell lung cancer: a single-institution experience. Clin Lung Cancer 2015;16:e229–34.
51. Spigel DR, Greco FA, Zubkus JD, et al. Phase II trial of irinotecan, carboplatin, and bevacizumab in the treatment of patients with extensive-stage small-cell lung cancer. J Thorac Oncol 2009;4:1555–60.
52. Spigel DR, Townley PM, Waterhouse DM, et al. Randomized phase II study of bevacizumab in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer: results from the SALUTE trial. J Clin Oncol 2011;29:2215–22.
53. Horn L, Dahlberg SE, Sandler AB, et al. Phase II study of cisplatin plus etoposide and bevacizumab for previously untreated, extensive-stage small-cell lung cancer: Eastern Cooperative Oncology Group Study E3501. J Clin Oncol 2009;27:6006–11.
54. Tiseo M, Boni L, Ambrosio F, et al. Italian, multicenter, phase III, randomized study of cisplatin plus etoposide with or without bevacizumab as first-line treatment in extensive-disease small-cell lung cancer: the GOIRC-AIFA FARM6PMFJM trial. J Clin Oncol 2017;35:1281–7.
55. Pujol JL, Lavole A, Quoix E, et al. Randomized phase II-III study of bevacizumab in combination with chemotherapy in previously untreated extensive small-cell lung cancer: results from the IFCT-0802 trial. Ann Oncol 2015;26:908–14.
56. Gadgeel SM, Ventimiglia J, Kalemkerian GP, et al. Phase II study of maintenance pembrolizumab (pembro) in extensive stage small cell lung cancer (ES-SCLC) patients (pts) [abstract]. J Clin Oncol 2017;35(15_suppl):8504.
57. Reck M, Luft A, Szczesna A, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol 2016;34:3740–8.
58. Chung HC, Lopez-Martin JA, Kao SC, et al. Phase 2 study of pembrolizumab in advanced small-cell lung cancer (SCLC): KEYNOTE-158 [abstract]. J Clin Oncol 2018;36(suppl):8506.
59. Owonikoko TK, Behera M, Chen Z, et al. A systematic analysis of efficacy of second-line chemotherapy in sensitive and refractory small-cell lung cancer. J Thorac Oncol 2012;7:866–72.
60. Postmus PE, Berendsen HH, van Zandwijk N, et al. Retreatment with the induction regimen in small cell lung cancer relapsing after an initial response to short term chemotherapy. Eur J Cancer Clin Oncol 1987;23:1409–11.
61. von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol 1999;17:658–667.
62. O’Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol 2006;24:5441–7.
63. Eckardt JR, von Pawel J, Pujol JL, et al. Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. J Clin Oncol 2007;25:2086–92.
64. Masuda N, Fukuoka M, Kusunoki Y, et al. CPT-11: a new derivative of camptothecin for the treatment of refractory or relapsed small-cell lung cancer. J Clin Oncol 1992;10:1225–9.
65. Smit EF, Fokkema E, Biesma B, et al. A phase II study of paclitaxel in heavily pretreated patients with small-cell lung cancer. Br J Cancer 1998;77:347–51.
66. Yamamoto N, Tsurutani J, Yoshimura N, et al. Phase II study of weekly paclitaxel for relapsed and refractory small cell lung cancer. Anticancer Res 2006;26:777–81.
67. Smyth JF, Smith IE, Sessa C, et al. Activity of docetaxel (Taxotere) in small cell lung cancer. Eur J Cancer 1994;30A:1058–60.
68. Pietanza MC, Kadota K, Huberman K, et al. Phase II trial of temozolomide with relapsed sensitive or refractory small cell lung cancer, with assessment of methylguanine-DNA methyltransferase as a potential biomarker. Clin Cancer Res 2012;18:1138–45.
69. Zauderer MG, Drilon A, Kadota K, et al. Trial of a 5-day dosing regimen of temozolomide in patients with relapsed small cell lung cancers with assessment of methylguanine-DNA methyltransferase. Lung Cancer 2014;86:237–40.
70. Jassem J, Karnicka-Mlodkowska H, van Pottelsberghe C, et al. Phase II study of vinorelbine (Navelbine) in previously treated small cell lung cancer patients. Eur J Cancer 1993;29A:1720–2.
71. Furuse K, Kuboa K, Kawahara M, et al. Phase II study of vinorelbine in heavily previously treated small cell lung cancer. Oncology 1996;53:169–72.
72. Einhorn LH, Pennington K, McClean J. Phase II trial of daily oral VP-16 in refractory small cell lung cancer. Semin Oncol 1990;17:32–5.
73. Johnson DH, Greco FA, Strupp J, et al. Prolonged administration of oral etoposide in patients with relapsed or refractory small-cell lung cancer: a phase II trial. J Clin Oncol 1990;8:1613–7.
74. Van der Lee I, Smit EF, van Putten JW, et al. Single-agent gemcitabine in patients with resistant small-cell lung cancer. Ann Oncol 2001;12:557–61.
75. Masters GA, Declerck L, Blanke C, et al. Phase II trial of gemcitabine in refractory or relapsed small-cell lung cancer. J Clin Oncol 2003;21:1550–5.
76. von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol 1999;17:658–67.
77. Lammers PE, Shyr Y, Li CI, et al. Phase II study of bendamustine in relapsed chemotherapy sensitive or resistant small-cell lung cancer. J Thorac Oncol 2014;9:559–62.
78. Hellmann MD, Ott PA, Zugazagoitia J, et al. Nivolumab (nivo) ± ipilimumab (ipi) in advanced small-cell lung cancer (SCLC): First report of a randomized expansion cohort from CheckMate 032 [abstract]. J Clin Oncol 2017;35(15_suppl):8503.
79. Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883–95.
80. Ott PA, Elez E, Hiret S, et al. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the Phase Ib KEYNOTE-028 study. J Clin Oncol 2017;35:3823–9.
81. Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med 2007;357:664–72.
82. Takahashi T, Yamanaka T, Seto T, et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:663–71.
83. Slotman BJ, Mauer ME, Bottomley A, et al. Prophylactic cranial irradiation in extensive disease small-cell lung cancer: short-term health-related quality of life and patient reported symptoms: results of an international Phase III randomized controlled trial by the EORTC Radiation Oncology and Lung Cancer Groups. J Clin Oncol 2009;27:78–84.
84. Brown PD, Pugh S, Laack NN, et al; Radiation Therapy Oncology Group (RTOG). Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol 2013;15:1429–37.
85. Jeremic B, Shibamoto Y, Nikolic N, et al. Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: a randomized study. J Clin Oncol 1999;17:2092–9.
86. Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet 2015;385:36–42.
87. Slotman BJ, van Tinteren H, Praag JO, et al. Radiotherapy for extensive stage small-cell lung cancer - authors’ reply. Lancet 2015;385:1292–3.
88. Owonikoko TK, Dahlberg SE, Khan SA, et al. A phase 1 safety study of veliparib combined with cisplatin and etoposide in extensive stage small cell lung cancer: A trial of the ECOG-ACRIN Cancer Research Group (E2511). Lung Cancer 2015;89:66–70.
89. Mamdani H, Induru R, Jalal SI. Novel therapies in small cell lung cancer. Transl Lung Cancer Res 2015;4:533–44.
90. Rudin CM, Pietanza MC, Bauer TM, et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol 2017;18:42–51.
1. American Cancer Society. Cancer Facts & Figures 2017. American Cancer Society website. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. Published 2017. Accessed July 11, 2018.
2. Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24:4539–44.
3. Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2014. National Cancer Institute website. https://seer.cancer.gov/csr/1975_2014/. Updated April 2, 2018. Accessed July 11, 2018.
4. Varghese AM, Zakowski MF, Yu HA, et al. Small-cell lung cancers in patients who never smoked cigarettes. J Thorac Oncol 2014;9:892–6.
5. Pleasance ED, Stephens PJ, O’Meara S, et al. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature 2010;463:184–90.
6. Green RA, Humphrey E, Close H, Patno ME. Alkylating agents in bronchogenic carcinoma. Am J Med 1969;46:516–25.
7. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology for small cell lung cancer (Version 2.2018). www.nccn.org/professionals/physician_gls/pdf/sclc.pdf. Accessed August 12, 2018.
8. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409.
9. Aberle DR, DeMello S, Berg CD, et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med 2013;369:920–31.
10. Kitajima K, Nakamoto Y, Okizuka H, et al. Accuracy of whole-body FDG-PET/CT for detecting brain metastases from non-central nervous system tumors. Ann Nucl Med 2008;22:595–602.
11. Ordonez NG. Value of thyroid transcription factor-1 immunostaining in distinguishing small cell lung carcinomas from other small cell carcinomas. Am J Surg Pathol 2000;24:1217–23.
12. Karnak D, Beder S, Kayacan O, et al. Neuron-specific enolase and lung cancer. Am J Clin Oncol 2005;28:586–90.
13. Turrisi AT 3rd, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 1999;340:265–71.
14. Evans WK, Shepherd FA, Feld R, et al. VP-16 and cisplatin as first-line therapy for small-cell lung cancer. J Clin Oncol 1985;3:1471–7.
15. Pujol JL, Carestia L, Daurés JP. Is there a case for cisplatin in the treatment of small-cell lung cancer? A meta-analysis of randomized trials of a cisplatin-containing regimen versus a regimen without this alkylating agent. Br J Cancer 2000;83:8–15.
16. Mascaux C, Paesmans M, Berghmans T, et al; European Lung Cancer Working Party (ELCWP). A systematic review of the role of etoposide and cisplatin in the chemotherapy of small cell lung cancer with methodology assessment and meta-analysis. Lung Cancer 2000;30:23–36.
17. Sundstrøm S, Bremnes RM, Kaasa S, et al; Norwegian Lung Cancer Study Group. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years’ follow-up. J Clin Oncol 2002;20:4665–72.
18. Hatfield LA, Huskamp HA, Lamont EB. Survival and toxicity after cisplatin plus etoposide versus carboplatin plus etoposide for extensive-stage small-cell lung cancer in elderly patients. J Oncol Pract 2016;12:666–73.
19. Okamoto H, Watanabe K, Kunikane H, et al. Randomised phase III trial of carboplatin plus etoposide vs split doses of cisplatin plus etoposide in elderly or poor-risk patients with extensive disease small-cell lung cancer: JCOG 9702. Br J Cancer 2007;97:162–9.
20. Skarlos DV, Samantas E, Kosmidis P, et al. Randomized comparison of etoposide-cisplatin vs. etoposide-carboplatin and irradiation in small-cell lung cancer. A Hellenic Co-operative Oncology Group study. Ann Oncol 1994;5:601–7.
21. Rossi A, Di Maio M, Chiodini P, et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data J Clin Oncol 2012;30:1692–8.
22. Bishop JF, Raghavan D, Stuart-Harris R, et al. Carboplatin (CBDCA, JM-8) and VP-16-213 in previously untreated patients with small-cell lung cancer. J Clin Oncol 1987;5:1574–8.
23. Takada M, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol 2002;20:3054–60.
24. Bunn PA Jr, Crowley J, Kelly K, et al. Chemoradiotherapy with or without granulocyte-macrophage colony-stimulating factor in the treatment of limited-stage small-cell lung cancer: a prospective phase III randomized study of the Southwest Oncology Group. J Clin Oncol 1995;13:1632–41.
25. Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 1992;327:1618–24.
26. Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol 1992;10:890–5.
27. Murray N, Coy P, Pater JL, et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 1993;11:336–44.
28. De Ruysscher D, Lueza B, Le Péchoux C, et al. Impact of thoracic radiotherapy timing in limited-stage small-cell lung cancer: usefulness of the individual patient data meta-analysis. Ann Oncol 2016;27:1818–28.
29. Faivre-Finn C, Snee M, Ashcroft L, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol 2017;18:1116–25.
30. Aupérin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341:476–84.
31. Arriagada R, Le Chevalier T, Borie F, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst 1995;87:183–90.
32. Le Péchoux C, Dunant A, Senan S, et al; Prophylactic Cranial Irradiation (PCI) Collaborative Group. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): a randomised clinical trial. Lancet Oncol 2009;10:467–74.
33. Schneider BJ, Saxena A, Downey RJ. Surgery for early-stage small cell lung cancer. J Natl Compr Canc Netw 2011;9:1132–9.
34. Inoue M, Nakagawa K, Fujiwara K, et al. Results of preoperative mediastinoscopy for small cell lung cancer. Ann Thorac Surg 2000;70:1620–3.
35. Lim E, Belcher E, Yap YK, et al. The role of surgery in the treatment of limited disease small cell lung cancer: time to reevaluate. J Thorac Oncol 2008;3:1267–71.
36. Inoue M, Miyoshi S, Yasumitsu T, et al. Surgical results for small cell lung cancer based on the new TNM staging system. Thoracic Surgery Study Group of Osaka University, Osaka, Japan. Ann Thorac Surg 2000;70:1615–9.
37. Yang CF, Chan DY, Speicher PJ, et al. Role of adjuvant therapy in a population-based cohort of patients with early-stage small-cell lung cancer. J Clin Oncol 2016;34:1057–64.
38. Shepherd FA, Evans WK, Feld R, et al. Adjuvant chemotherapy following surgical resection for small-cell carcinoma of the lung. J Clin Oncol 1988;6:832–8.
39. Noda K, Nishiwaki Y, Kawahara M, et al; Japan Clinical Oncology Group. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 2002;346:85–91.
40. Lara PN Jr, Natale R, Crowley J, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol 2009;27:2530–5.
41. Chute JP, Chen T, Feigal E, et al. Twenty years of phase III trials for patients with extensive-stage small-cell lung cancer: perceptible progress. J Clin Oncol 1999;17:1794–801.
42. Zhou H, Zeng C, Wei Y, et al. Duration of chemotherapy for small cell lung cancer: a meta-analysis. PloS One 2013;8:e73805.
43. Loehrer PJ Sr, Ansari R, Gonin R, et al. Cisplatin plus etoposide with and without ifosfamide in extensive small-cell lung cancer: a Hoosier Oncology Group study. J Clin Oncol;13:2594–9.
44. Pujol JL, Daurés JP, Riviére A, et al. Etoposide plus cisplatin with or without the combination of 4’-epidoxorubicin plus cyclophosphamide in treatment of extensive small-cell lung cancer: a French Federation of Cancer Institutes multicenter phase III randomized study. J Natl Cancer Inst 2001;93:300–8.
45. Berghmans T, Scherpereel A, Meert AP, et al; European Lung Cancer Working Party (ELCWP). A phase III randomized study comparing a chemotherapy with cisplatin and etoposide to a etoposide regimen without cisplatin for patients with extensive small-cell lung cancer. Front Oncol 2017;7:217.
46. Jalal SI, Lavin P, Lo G, et al. Carboplatin and etoposide with or without palifosfamide in untreated extensive-stage small-cell lung cancer: a Multicenter, Adaptive, Randomized Phase III Study (MATISSE). J Clin Oncol 2017;35:2619–23.
47. Fukuoka M, Furuse K, Saijo N, et al. Randomized trial of cyclophosphamide, doxorubicin, and vincristine versus cisplatin and etoposide versus alternation of these regimens in small-cell lung cancer. J Natl Cancer Inst 1991;83:855–61.
48. Roth BJ, Johnson DH, Einhorn LH, et al. Randomized study of cyclophosphamide, doxorubicin, and vincristine versus etoposide and cisplatin versus alternation of these two regimens in extensive small-cell lung cancer: a phase III trial of the Southeastern Cancer Study Group. J Clin Oncol 1992;10:282–91.
49. Miles DW, Earl HM, Souhami RL, et al. Intensive weekly chemotherapy for good-prognosis patients with small-cell lung cancer. J Clin Oncol 1991;9:280–5.
50. Petrioli R, Roviello G, Laera L, et al. Cisplatin, etoposide, and bevacizumab regimen followed by oral etoposide and bevacizumab maintenance treatment in patients with extensive-stage small cell lung cancer: a single-institution experience. Clin Lung Cancer 2015;16:e229–34.
51. Spigel DR, Greco FA, Zubkus JD, et al. Phase II trial of irinotecan, carboplatin, and bevacizumab in the treatment of patients with extensive-stage small-cell lung cancer. J Thorac Oncol 2009;4:1555–60.
52. Spigel DR, Townley PM, Waterhouse DM, et al. Randomized phase II study of bevacizumab in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer: results from the SALUTE trial. J Clin Oncol 2011;29:2215–22.
53. Horn L, Dahlberg SE, Sandler AB, et al. Phase II study of cisplatin plus etoposide and bevacizumab for previously untreated, extensive-stage small-cell lung cancer: Eastern Cooperative Oncology Group Study E3501. J Clin Oncol 2009;27:6006–11.
54. Tiseo M, Boni L, Ambrosio F, et al. Italian, multicenter, phase III, randomized study of cisplatin plus etoposide with or without bevacizumab as first-line treatment in extensive-disease small-cell lung cancer: the GOIRC-AIFA FARM6PMFJM trial. J Clin Oncol 2017;35:1281–7.
55. Pujol JL, Lavole A, Quoix E, et al. Randomized phase II-III study of bevacizumab in combination with chemotherapy in previously untreated extensive small-cell lung cancer: results from the IFCT-0802 trial. Ann Oncol 2015;26:908–14.
56. Gadgeel SM, Ventimiglia J, Kalemkerian GP, et al. Phase II study of maintenance pembrolizumab (pembro) in extensive stage small cell lung cancer (ES-SCLC) patients (pts) [abstract]. J Clin Oncol 2017;35(15_suppl):8504.
57. Reck M, Luft A, Szczesna A, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol 2016;34:3740–8.
58. Chung HC, Lopez-Martin JA, Kao SC, et al. Phase 2 study of pembrolizumab in advanced small-cell lung cancer (SCLC): KEYNOTE-158 [abstract]. J Clin Oncol 2018;36(suppl):8506.
59. Owonikoko TK, Behera M, Chen Z, et al. A systematic analysis of efficacy of second-line chemotherapy in sensitive and refractory small-cell lung cancer. J Thorac Oncol 2012;7:866–72.
60. Postmus PE, Berendsen HH, van Zandwijk N, et al. Retreatment with the induction regimen in small cell lung cancer relapsing after an initial response to short term chemotherapy. Eur J Cancer Clin Oncol 1987;23:1409–11.
61. von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol 1999;17:658–667.
62. O’Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol 2006;24:5441–7.
63. Eckardt JR, von Pawel J, Pujol JL, et al. Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. J Clin Oncol 2007;25:2086–92.
64. Masuda N, Fukuoka M, Kusunoki Y, et al. CPT-11: a new derivative of camptothecin for the treatment of refractory or relapsed small-cell lung cancer. J Clin Oncol 1992;10:1225–9.
65. Smit EF, Fokkema E, Biesma B, et al. A phase II study of paclitaxel in heavily pretreated patients with small-cell lung cancer. Br J Cancer 1998;77:347–51.
66. Yamamoto N, Tsurutani J, Yoshimura N, et al. Phase II study of weekly paclitaxel for relapsed and refractory small cell lung cancer. Anticancer Res 2006;26:777–81.
67. Smyth JF, Smith IE, Sessa C, et al. Activity of docetaxel (Taxotere) in small cell lung cancer. Eur J Cancer 1994;30A:1058–60.
68. Pietanza MC, Kadota K, Huberman K, et al. Phase II trial of temozolomide with relapsed sensitive or refractory small cell lung cancer, with assessment of methylguanine-DNA methyltransferase as a potential biomarker. Clin Cancer Res 2012;18:1138–45.
69. Zauderer MG, Drilon A, Kadota K, et al. Trial of a 5-day dosing regimen of temozolomide in patients with relapsed small cell lung cancers with assessment of methylguanine-DNA methyltransferase. Lung Cancer 2014;86:237–40.
70. Jassem J, Karnicka-Mlodkowska H, van Pottelsberghe C, et al. Phase II study of vinorelbine (Navelbine) in previously treated small cell lung cancer patients. Eur J Cancer 1993;29A:1720–2.
71. Furuse K, Kuboa K, Kawahara M, et al. Phase II study of vinorelbine in heavily previously treated small cell lung cancer. Oncology 1996;53:169–72.
72. Einhorn LH, Pennington K, McClean J. Phase II trial of daily oral VP-16 in refractory small cell lung cancer. Semin Oncol 1990;17:32–5.
73. Johnson DH, Greco FA, Strupp J, et al. Prolonged administration of oral etoposide in patients with relapsed or refractory small-cell lung cancer: a phase II trial. J Clin Oncol 1990;8:1613–7.
74. Van der Lee I, Smit EF, van Putten JW, et al. Single-agent gemcitabine in patients with resistant small-cell lung cancer. Ann Oncol 2001;12:557–61.
75. Masters GA, Declerck L, Blanke C, et al. Phase II trial of gemcitabine in refractory or relapsed small-cell lung cancer. J Clin Oncol 2003;21:1550–5.
76. von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol 1999;17:658–67.
77. Lammers PE, Shyr Y, Li CI, et al. Phase II study of bendamustine in relapsed chemotherapy sensitive or resistant small-cell lung cancer. J Thorac Oncol 2014;9:559–62.
78. Hellmann MD, Ott PA, Zugazagoitia J, et al. Nivolumab (nivo) ± ipilimumab (ipi) in advanced small-cell lung cancer (SCLC): First report of a randomized expansion cohort from CheckMate 032 [abstract]. J Clin Oncol 2017;35(15_suppl):8503.
79. Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883–95.
80. Ott PA, Elez E, Hiret S, et al. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the Phase Ib KEYNOTE-028 study. J Clin Oncol 2017;35:3823–9.
81. Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med 2007;357:664–72.
82. Takahashi T, Yamanaka T, Seto T, et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:663–71.
83. Slotman BJ, Mauer ME, Bottomley A, et al. Prophylactic cranial irradiation in extensive disease small-cell lung cancer: short-term health-related quality of life and patient reported symptoms: results of an international Phase III randomized controlled trial by the EORTC Radiation Oncology and Lung Cancer Groups. J Clin Oncol 2009;27:78–84.
84. Brown PD, Pugh S, Laack NN, et al; Radiation Therapy Oncology Group (RTOG). Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol 2013;15:1429–37.
85. Jeremic B, Shibamoto Y, Nikolic N, et al. Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: a randomized study. J Clin Oncol 1999;17:2092–9.
86. Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet 2015;385:36–42.
87. Slotman BJ, van Tinteren H, Praag JO, et al. Radiotherapy for extensive stage small-cell lung cancer - authors’ reply. Lancet 2015;385:1292–3.
88. Owonikoko TK, Dahlberg SE, Khan SA, et al. A phase 1 safety study of veliparib combined with cisplatin and etoposide in extensive stage small cell lung cancer: A trial of the ECOG-ACRIN Cancer Research Group (E2511). Lung Cancer 2015;89:66–70.
89. Mamdani H, Induru R, Jalal SI. Novel therapies in small cell lung cancer. Transl Lung Cancer Res 2015;4:533–44.
90. Rudin CM, Pietanza MC, Bauer TM, et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol 2017;18:42–51.