User login
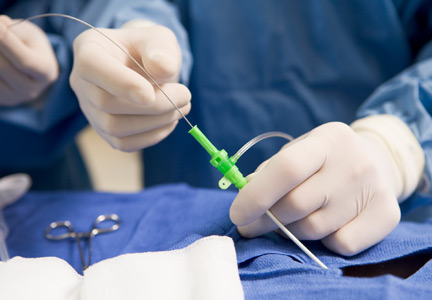
Transcatheter aortic valve replacement for bicuspid aortic valve stenosis
Bicuspid aortic valve is the most common congenital cardiac abnormality in humans and is a significant risk factor for premature aortic valve dysfunction due to accelerated leaflet deterioration and calcification from altered hemodynamics. From 20% to 50% of patients with bicuspid aortic valve need aortic valve replacement during their lifetime, mostly for aortic stenosis.1,2
While 0.5% to 2% of the general population are born with a bicuspid aortic valve, more than 40% of patients (mainly younger patients) who undergo surgical or transcatheter intervention for aortic valve disease in some cohorts have this abnormality, suggesting that its true prevalence may be underreported.3
In the past decade, transcatheter aortic valve replacement (TAVR) has cemented its place as an option for patients with severe tricuspid aortic stenosis who cannot undergo surgery because their surgical risk is intermediate or high.4 However, most of the studies of balloon-expandable and self-expanding TAVR devices have excluded patients with bicuspid aortic valve.
BICUSPID AORTIC VALVE POSES CHALLENGES FOR TAVR
As TAVR is explored in younger and lower-risk populations, in which the prevalence of bicuspid aortic valve is presumably higher, the discussion of feasibility, safety, and efficacy of TAVR in patients with bicuspid aortic valve is both important and timely.
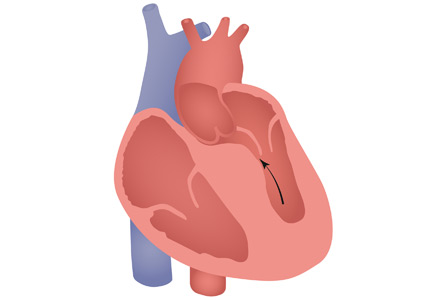
Bicuspid aortic valve is commonly categorized according to the Sievers classification,5 which describes 3 main morphologic types (designated types 0, 1, and 2) according to the number of raphes connecting the leaflets. Unique anatomic features of bicuspid aortic valve render the TAVR procedure challenging in these patients and merit consideration. These include, but are not limited to:
- Asymmetric calcification of the valve leaflets and calcified raphes. This results in asymmetric and incomplete expansion of the prosthesis, leading to incomplete sealing and paravalvular leak.
- A larger and more elliptically shaped aortic annulus, leading to challenges with proper sizing and apposition of the prosthesis
- Concomitant aortopathy, posing a higher risk of aortic rupture, dissection, paravalvular leak, and other complications during implantation.
Thus, compared with patients with tricuspid degenerative aortic stenosis, patients undergoing TAVR who have bicuspid aortic valve have a higher short-term risk of death and a higher risk of residual aortic regurgitation, and are more likely to need implantation of a second valve.
PARAVALVULAR LEAK
Paravalvular leak, arguably an independent marker of higher morbidity and mortality risk after TAVR, is more common in patients with bicuspid aortic valve undergoing TAVR than in those with tricuspid aortic valve. Earlier studies reported rates of moderate or severe paravalvular leak between 16% and 32%.6,7
The newer-generation balloon-expandable Sapien 3 valve (Edwards Lifesciences, Irvine, CA) is associated with a lower incidence of moderate or severe paravalvular leak than earlier devices, mainly attributable to its outer skirt, which allows more complete sealing.8 There are also reports of successful treatment of bicuspid aortic valve stenosis using the Lotus device (Boston Scientific, Marlborough, MA).9 This device features adaptive sealing along with retrievability and repositioning ability, which may facilitate optimal positioning and prevent paravalvular leak.
SIZING OF THE PROSTHESIS
Sizing of the prosthesis in patients with bicuspid aortic valve stenosis remains a challenge: some experts advocate the usual practice of measuring the perimeter and area at the level of the annulus, while others advocate measuring at the level of the commissures, 4 to 8 mm above the annulus. Balloon valvuloplasty may be a useful sizing tool, though it carries the hazards of severe aortic regurgitation and periprocedural stroke.
Angiography of the ascending aorta during balloon valvuloplasty can help verify whether an adequate seal is achievable and aid in selecting an appropriately sized prosthesis. Liu et al10 performed sequential balloon aortic valvuloplasty before TAVR with a self-expanding valve in 12 patients. Of these, 11 (91.7%) received a smaller device than they would have with multidetector computed tomography-guided annulus measurement.
Given that a larger valve may increase the risk of annular rupture, implantation of a smaller valve is always reasonable in bicuspid aortic valve as long as it achieves appropriate sealing with no paravalvular leak.
THE NEED FOR A PACEMAKER
After undergoing TAVR, more patients who have a bicuspid aortic valve need a permanent pacemaker than those who have a tricuspid aortic valve. This group appears to be more vulnerable to conduction abnormalities after TAVR, and rates of new pacemaker implantation as high as 25% have been reported with the newer-generation devices. Perlman et al8 observed that even when the Sapien 3 valve was implanted high in the left ventricular outflow tract, nearly 10% of patients needed a new pacemaker.
This is an important issue, as most patients with bicuspid aortic valve with severe aortic stenosis are relatively young and may endure deleterious effects from long-term pacing.
LONG-TERM OUTCOMES
The data on long-term outcomes of patients with bicuspid aortic valve who undergo TAVR are limited, and the available studies were small, with relatively short-term follow-up. However, Yoon et al compared TAVR outcomes between bicuspid and tricuspid aortic stenosis patients using propensity-score matching and demonstrated comparable all-cause mortality rates at 2 years (17.2% vs 19.4%, P = .28).6
Given the relatively long life expectancy of patients with bicuspid aortic valve undergoing TAVR, who tend to be younger than those with tricuspid aortic valve stenosis, longer-term data are needed to draw meaningful conclusions about the durability of transcatheter valves in this population. The bicuspid aortic valve is asymmetric, so that during TAVR the stent may not expand completely, and this in theory may result in more strain on the prosthesis and accelerate its structural deterioration.
In a recent meta-analysis, Reddy et al11 analyzed 13 observational studies in 758 patients with severe bicuspid aortic valve stenosis undergoing TAVR with older and newer devices. The mean Society of Thoracic Surgeons Predicted Risk of Mortality score, which predicts the risk of death within 30 days, was 5.0%, but the actual rate was 3.7% (95% confidence interval [CI] 2.1%–5.6%). A high procedural success rate of 95% (95% CI 90.2%–98.5%] was also noted, but the rates of new permanent pacemaker implantation (17.9%, 95% CI 14.2%–22%) and severe perivalvular leak (12.2%, 95% CI 3.1%–24.8%) were somewhat elevated.11
NOT FOR ALL, BUT AN EMERGING, VIABLE OPTION
As implanted prostheses and TAVR techniques undergo continuous improvement and as the experience of operators and institutions advances, procedural outcomes will likely improve.
The available data suggest that TAVR with the newer devices, when performed by experienced hands, is a viable option across most of the risk spectrum in patients with severe tricuspid aortic stenosis, including low-risk patients,12 and selectively in patients with bicuspid aortic valve stenosis. However, for patients with bicuspid aortic valve with severe aortic stenosis and associated aortopathy, surgery remains the standard of care.
More study is needed to identify patients with bicuspid aortic valve who can be safely and effectively treated with TAVR.
- Ward C. Clinical significance of the bicuspid aortic valve. Heart 2000; 83(1):81–85. pmid:10618341
- Michelena HI, Desjardins VA, Avierinos JF, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008; 117(21):2776–2784. doi:10.1161/CIRCULATIONAHA.107.740878
- Jilaihawi H, Wu Y, Yang Y, et al. Morphological characteristics of severe aortic stenosis in China: imaging corelab observations from the first Chinese transcatheter aortic valve trial. Catheter Cardiovasc Interv 2015; 85(suppl 1):752–761. doi:10.1002/ccd.25863
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice. J Am Coll Cardiol 2017; 70(2):252–289. doi:10.1016/j.jacc.2017.03.011
- Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 2007; 133(5):1226–1233. doi:10.1016/j.jtcvs.2007.01.039
- Yoon SH, Bleiziffer S, De Backer O, et al. Outcomes in transcatheter aortic valve replacement for bicuspid versus tricuspid aortic valve stenosis. J Am Coll Cardiol 2017; 69(21):2579–2589. doi:10.1016/j.jacc.2017.03.017
- Mylotte D, Lefevre T, Sondergaard L, et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J Am Coll Cardiol 2014; 64(22):2330–2339. doi:10.1016/j.jacc.2014.09.039
- Perlman GY, Blanke P, Dvir D, et al. Bicuspid aortic valve stenosis: favorable early outcomes with a next-generation transcatheter heart valve in a multicenter study. JACC Cardiovasc Interv 2016; 9(8):817–824. doi:10.1016/j.jcin.2016.01.002
- Chan AW, Wong D, Charania J. Transcatheter aortic valve replacement in bicuspid aortic stenosis using Lotus valve system. Catheter Cardiovasc Interv 2017; 90(1):157–163. doi:10.1002/ccd.26506
- Liu X, He Y, Zhu Q, et al. Supra-annular structure assessment for self-expanding transcatheter heart valve size selection in patients with bicuspid aortic valve. Catheter Cardiovasc Interv 2018; 91(5):986–994. doi:10.1002/ccd.27467
- Reddy G, Wang Z, Nishimura RA, et al. Transcatheter aortic valve replacement for stenotic bicuspid aortic valves: systematic review and meta analyses of observational studies. Catheter Cardiovasc Interv 2018; 91(5):975–983. doi:10.1002/ccd.27340
- Waksman R, Rogers T, Torguson R, et al. Transcatheter aortic valve replacement in low-risk patients with symptomatic severe aortic stenosis. J Am Coll Cardiol 2018; pii:S0735-1097(18)36852–36859. Epub ahead of print. doi:10.1016/j.jacc.2018.08.1033
Bicuspid aortic valve is the most common congenital cardiac abnormality in humans and is a significant risk factor for premature aortic valve dysfunction due to accelerated leaflet deterioration and calcification from altered hemodynamics. From 20% to 50% of patients with bicuspid aortic valve need aortic valve replacement during their lifetime, mostly for aortic stenosis.1,2
While 0.5% to 2% of the general population are born with a bicuspid aortic valve, more than 40% of patients (mainly younger patients) who undergo surgical or transcatheter intervention for aortic valve disease in some cohorts have this abnormality, suggesting that its true prevalence may be underreported.3
In the past decade, transcatheter aortic valve replacement (TAVR) has cemented its place as an option for patients with severe tricuspid aortic stenosis who cannot undergo surgery because their surgical risk is intermediate or high.4 However, most of the studies of balloon-expandable and self-expanding TAVR devices have excluded patients with bicuspid aortic valve.
BICUSPID AORTIC VALVE POSES CHALLENGES FOR TAVR
As TAVR is explored in younger and lower-risk populations, in which the prevalence of bicuspid aortic valve is presumably higher, the discussion of feasibility, safety, and efficacy of TAVR in patients with bicuspid aortic valve is both important and timely.
Bicuspid aortic valve is commonly categorized according to the Sievers classification,5 which describes 3 main morphologic types (designated types 0, 1, and 2) according to the number of raphes connecting the leaflets. Unique anatomic features of bicuspid aortic valve render the TAVR procedure challenging in these patients and merit consideration. These include, but are not limited to:
- Asymmetric calcification of the valve leaflets and calcified raphes. This results in asymmetric and incomplete expansion of the prosthesis, leading to incomplete sealing and paravalvular leak.
- A larger and more elliptically shaped aortic annulus, leading to challenges with proper sizing and apposition of the prosthesis
- Concomitant aortopathy, posing a higher risk of aortic rupture, dissection, paravalvular leak, and other complications during implantation.
Thus, compared with patients with tricuspid degenerative aortic stenosis, patients undergoing TAVR who have bicuspid aortic valve have a higher short-term risk of death and a higher risk of residual aortic regurgitation, and are more likely to need implantation of a second valve.
PARAVALVULAR LEAK
Paravalvular leak, arguably an independent marker of higher morbidity and mortality risk after TAVR, is more common in patients with bicuspid aortic valve undergoing TAVR than in those with tricuspid aortic valve. Earlier studies reported rates of moderate or severe paravalvular leak between 16% and 32%.6,7
The newer-generation balloon-expandable Sapien 3 valve (Edwards Lifesciences, Irvine, CA) is associated with a lower incidence of moderate or severe paravalvular leak than earlier devices, mainly attributable to its outer skirt, which allows more complete sealing.8 There are also reports of successful treatment of bicuspid aortic valve stenosis using the Lotus device (Boston Scientific, Marlborough, MA).9 This device features adaptive sealing along with retrievability and repositioning ability, which may facilitate optimal positioning and prevent paravalvular leak.
SIZING OF THE PROSTHESIS
Sizing of the prosthesis in patients with bicuspid aortic valve stenosis remains a challenge: some experts advocate the usual practice of measuring the perimeter and area at the level of the annulus, while others advocate measuring at the level of the commissures, 4 to 8 mm above the annulus. Balloon valvuloplasty may be a useful sizing tool, though it carries the hazards of severe aortic regurgitation and periprocedural stroke.
Angiography of the ascending aorta during balloon valvuloplasty can help verify whether an adequate seal is achievable and aid in selecting an appropriately sized prosthesis. Liu et al10 performed sequential balloon aortic valvuloplasty before TAVR with a self-expanding valve in 12 patients. Of these, 11 (91.7%) received a smaller device than they would have with multidetector computed tomography-guided annulus measurement.
Given that a larger valve may increase the risk of annular rupture, implantation of a smaller valve is always reasonable in bicuspid aortic valve as long as it achieves appropriate sealing with no paravalvular leak.
THE NEED FOR A PACEMAKER
After undergoing TAVR, more patients who have a bicuspid aortic valve need a permanent pacemaker than those who have a tricuspid aortic valve. This group appears to be more vulnerable to conduction abnormalities after TAVR, and rates of new pacemaker implantation as high as 25% have been reported with the newer-generation devices. Perlman et al8 observed that even when the Sapien 3 valve was implanted high in the left ventricular outflow tract, nearly 10% of patients needed a new pacemaker.
This is an important issue, as most patients with bicuspid aortic valve with severe aortic stenosis are relatively young and may endure deleterious effects from long-term pacing.
LONG-TERM OUTCOMES
The data on long-term outcomes of patients with bicuspid aortic valve who undergo TAVR are limited, and the available studies were small, with relatively short-term follow-up. However, Yoon et al compared TAVR outcomes between bicuspid and tricuspid aortic stenosis patients using propensity-score matching and demonstrated comparable all-cause mortality rates at 2 years (17.2% vs 19.4%, P = .28).6
Given the relatively long life expectancy of patients with bicuspid aortic valve undergoing TAVR, who tend to be younger than those with tricuspid aortic valve stenosis, longer-term data are needed to draw meaningful conclusions about the durability of transcatheter valves in this population. The bicuspid aortic valve is asymmetric, so that during TAVR the stent may not expand completely, and this in theory may result in more strain on the prosthesis and accelerate its structural deterioration.
In a recent meta-analysis, Reddy et al11 analyzed 13 observational studies in 758 patients with severe bicuspid aortic valve stenosis undergoing TAVR with older and newer devices. The mean Society of Thoracic Surgeons Predicted Risk of Mortality score, which predicts the risk of death within 30 days, was 5.0%, but the actual rate was 3.7% (95% confidence interval [CI] 2.1%–5.6%). A high procedural success rate of 95% (95% CI 90.2%–98.5%] was also noted, but the rates of new permanent pacemaker implantation (17.9%, 95% CI 14.2%–22%) and severe perivalvular leak (12.2%, 95% CI 3.1%–24.8%) were somewhat elevated.11
NOT FOR ALL, BUT AN EMERGING, VIABLE OPTION
As implanted prostheses and TAVR techniques undergo continuous improvement and as the experience of operators and institutions advances, procedural outcomes will likely improve.
The available data suggest that TAVR with the newer devices, when performed by experienced hands, is a viable option across most of the risk spectrum in patients with severe tricuspid aortic stenosis, including low-risk patients,12 and selectively in patients with bicuspid aortic valve stenosis. However, for patients with bicuspid aortic valve with severe aortic stenosis and associated aortopathy, surgery remains the standard of care.
More study is needed to identify patients with bicuspid aortic valve who can be safely and effectively treated with TAVR.
Bicuspid aortic valve is the most common congenital cardiac abnormality in humans and is a significant risk factor for premature aortic valve dysfunction due to accelerated leaflet deterioration and calcification from altered hemodynamics. From 20% to 50% of patients with bicuspid aortic valve need aortic valve replacement during their lifetime, mostly for aortic stenosis.1,2
While 0.5% to 2% of the general population are born with a bicuspid aortic valve, more than 40% of patients (mainly younger patients) who undergo surgical or transcatheter intervention for aortic valve disease in some cohorts have this abnormality, suggesting that its true prevalence may be underreported.3
In the past decade, transcatheter aortic valve replacement (TAVR) has cemented its place as an option for patients with severe tricuspid aortic stenosis who cannot undergo surgery because their surgical risk is intermediate or high.4 However, most of the studies of balloon-expandable and self-expanding TAVR devices have excluded patients with bicuspid aortic valve.
BICUSPID AORTIC VALVE POSES CHALLENGES FOR TAVR
As TAVR is explored in younger and lower-risk populations, in which the prevalence of bicuspid aortic valve is presumably higher, the discussion of feasibility, safety, and efficacy of TAVR in patients with bicuspid aortic valve is both important and timely.
Bicuspid aortic valve is commonly categorized according to the Sievers classification,5 which describes 3 main morphologic types (designated types 0, 1, and 2) according to the number of raphes connecting the leaflets. Unique anatomic features of bicuspid aortic valve render the TAVR procedure challenging in these patients and merit consideration. These include, but are not limited to:
- Asymmetric calcification of the valve leaflets and calcified raphes. This results in asymmetric and incomplete expansion of the prosthesis, leading to incomplete sealing and paravalvular leak.
- A larger and more elliptically shaped aortic annulus, leading to challenges with proper sizing and apposition of the prosthesis
- Concomitant aortopathy, posing a higher risk of aortic rupture, dissection, paravalvular leak, and other complications during implantation.
Thus, compared with patients with tricuspid degenerative aortic stenosis, patients undergoing TAVR who have bicuspid aortic valve have a higher short-term risk of death and a higher risk of residual aortic regurgitation, and are more likely to need implantation of a second valve.
PARAVALVULAR LEAK
Paravalvular leak, arguably an independent marker of higher morbidity and mortality risk after TAVR, is more common in patients with bicuspid aortic valve undergoing TAVR than in those with tricuspid aortic valve. Earlier studies reported rates of moderate or severe paravalvular leak between 16% and 32%.6,7
The newer-generation balloon-expandable Sapien 3 valve (Edwards Lifesciences, Irvine, CA) is associated with a lower incidence of moderate or severe paravalvular leak than earlier devices, mainly attributable to its outer skirt, which allows more complete sealing.8 There are also reports of successful treatment of bicuspid aortic valve stenosis using the Lotus device (Boston Scientific, Marlborough, MA).9 This device features adaptive sealing along with retrievability and repositioning ability, which may facilitate optimal positioning and prevent paravalvular leak.
SIZING OF THE PROSTHESIS
Sizing of the prosthesis in patients with bicuspid aortic valve stenosis remains a challenge: some experts advocate the usual practice of measuring the perimeter and area at the level of the annulus, while others advocate measuring at the level of the commissures, 4 to 8 mm above the annulus. Balloon valvuloplasty may be a useful sizing tool, though it carries the hazards of severe aortic regurgitation and periprocedural stroke.
Angiography of the ascending aorta during balloon valvuloplasty can help verify whether an adequate seal is achievable and aid in selecting an appropriately sized prosthesis. Liu et al10 performed sequential balloon aortic valvuloplasty before TAVR with a self-expanding valve in 12 patients. Of these, 11 (91.7%) received a smaller device than they would have with multidetector computed tomography-guided annulus measurement.
Given that a larger valve may increase the risk of annular rupture, implantation of a smaller valve is always reasonable in bicuspid aortic valve as long as it achieves appropriate sealing with no paravalvular leak.
THE NEED FOR A PACEMAKER
After undergoing TAVR, more patients who have a bicuspid aortic valve need a permanent pacemaker than those who have a tricuspid aortic valve. This group appears to be more vulnerable to conduction abnormalities after TAVR, and rates of new pacemaker implantation as high as 25% have been reported with the newer-generation devices. Perlman et al8 observed that even when the Sapien 3 valve was implanted high in the left ventricular outflow tract, nearly 10% of patients needed a new pacemaker.
This is an important issue, as most patients with bicuspid aortic valve with severe aortic stenosis are relatively young and may endure deleterious effects from long-term pacing.
LONG-TERM OUTCOMES
The data on long-term outcomes of patients with bicuspid aortic valve who undergo TAVR are limited, and the available studies were small, with relatively short-term follow-up. However, Yoon et al compared TAVR outcomes between bicuspid and tricuspid aortic stenosis patients using propensity-score matching and demonstrated comparable all-cause mortality rates at 2 years (17.2% vs 19.4%, P = .28).6
Given the relatively long life expectancy of patients with bicuspid aortic valve undergoing TAVR, who tend to be younger than those with tricuspid aortic valve stenosis, longer-term data are needed to draw meaningful conclusions about the durability of transcatheter valves in this population. The bicuspid aortic valve is asymmetric, so that during TAVR the stent may not expand completely, and this in theory may result in more strain on the prosthesis and accelerate its structural deterioration.
In a recent meta-analysis, Reddy et al11 analyzed 13 observational studies in 758 patients with severe bicuspid aortic valve stenosis undergoing TAVR with older and newer devices. The mean Society of Thoracic Surgeons Predicted Risk of Mortality score, which predicts the risk of death within 30 days, was 5.0%, but the actual rate was 3.7% (95% confidence interval [CI] 2.1%–5.6%). A high procedural success rate of 95% (95% CI 90.2%–98.5%] was also noted, but the rates of new permanent pacemaker implantation (17.9%, 95% CI 14.2%–22%) and severe perivalvular leak (12.2%, 95% CI 3.1%–24.8%) were somewhat elevated.11
NOT FOR ALL, BUT AN EMERGING, VIABLE OPTION
As implanted prostheses and TAVR techniques undergo continuous improvement and as the experience of operators and institutions advances, procedural outcomes will likely improve.
The available data suggest that TAVR with the newer devices, when performed by experienced hands, is a viable option across most of the risk spectrum in patients with severe tricuspid aortic stenosis, including low-risk patients,12 and selectively in patients with bicuspid aortic valve stenosis. However, for patients with bicuspid aortic valve with severe aortic stenosis and associated aortopathy, surgery remains the standard of care.
More study is needed to identify patients with bicuspid aortic valve who can be safely and effectively treated with TAVR.
- Ward C. Clinical significance of the bicuspid aortic valve. Heart 2000; 83(1):81–85. pmid:10618341
- Michelena HI, Desjardins VA, Avierinos JF, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008; 117(21):2776–2784. doi:10.1161/CIRCULATIONAHA.107.740878
- Jilaihawi H, Wu Y, Yang Y, et al. Morphological characteristics of severe aortic stenosis in China: imaging corelab observations from the first Chinese transcatheter aortic valve trial. Catheter Cardiovasc Interv 2015; 85(suppl 1):752–761. doi:10.1002/ccd.25863
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice. J Am Coll Cardiol 2017; 70(2):252–289. doi:10.1016/j.jacc.2017.03.011
- Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 2007; 133(5):1226–1233. doi:10.1016/j.jtcvs.2007.01.039
- Yoon SH, Bleiziffer S, De Backer O, et al. Outcomes in transcatheter aortic valve replacement for bicuspid versus tricuspid aortic valve stenosis. J Am Coll Cardiol 2017; 69(21):2579–2589. doi:10.1016/j.jacc.2017.03.017
- Mylotte D, Lefevre T, Sondergaard L, et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J Am Coll Cardiol 2014; 64(22):2330–2339. doi:10.1016/j.jacc.2014.09.039
- Perlman GY, Blanke P, Dvir D, et al. Bicuspid aortic valve stenosis: favorable early outcomes with a next-generation transcatheter heart valve in a multicenter study. JACC Cardiovasc Interv 2016; 9(8):817–824. doi:10.1016/j.jcin.2016.01.002
- Chan AW, Wong D, Charania J. Transcatheter aortic valve replacement in bicuspid aortic stenosis using Lotus valve system. Catheter Cardiovasc Interv 2017; 90(1):157–163. doi:10.1002/ccd.26506
- Liu X, He Y, Zhu Q, et al. Supra-annular structure assessment for self-expanding transcatheter heart valve size selection in patients with bicuspid aortic valve. Catheter Cardiovasc Interv 2018; 91(5):986–994. doi:10.1002/ccd.27467
- Reddy G, Wang Z, Nishimura RA, et al. Transcatheter aortic valve replacement for stenotic bicuspid aortic valves: systematic review and meta analyses of observational studies. Catheter Cardiovasc Interv 2018; 91(5):975–983. doi:10.1002/ccd.27340
- Waksman R, Rogers T, Torguson R, et al. Transcatheter aortic valve replacement in low-risk patients with symptomatic severe aortic stenosis. J Am Coll Cardiol 2018; pii:S0735-1097(18)36852–36859. Epub ahead of print. doi:10.1016/j.jacc.2018.08.1033
- Ward C. Clinical significance of the bicuspid aortic valve. Heart 2000; 83(1):81–85. pmid:10618341
- Michelena HI, Desjardins VA, Avierinos JF, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008; 117(21):2776–2784. doi:10.1161/CIRCULATIONAHA.107.740878
- Jilaihawi H, Wu Y, Yang Y, et al. Morphological characteristics of severe aortic stenosis in China: imaging corelab observations from the first Chinese transcatheter aortic valve trial. Catheter Cardiovasc Interv 2015; 85(suppl 1):752–761. doi:10.1002/ccd.25863
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice. J Am Coll Cardiol 2017; 70(2):252–289. doi:10.1016/j.jacc.2017.03.011
- Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 2007; 133(5):1226–1233. doi:10.1016/j.jtcvs.2007.01.039
- Yoon SH, Bleiziffer S, De Backer O, et al. Outcomes in transcatheter aortic valve replacement for bicuspid versus tricuspid aortic valve stenosis. J Am Coll Cardiol 2017; 69(21):2579–2589. doi:10.1016/j.jacc.2017.03.017
- Mylotte D, Lefevre T, Sondergaard L, et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J Am Coll Cardiol 2014; 64(22):2330–2339. doi:10.1016/j.jacc.2014.09.039
- Perlman GY, Blanke P, Dvir D, et al. Bicuspid aortic valve stenosis: favorable early outcomes with a next-generation transcatheter heart valve in a multicenter study. JACC Cardiovasc Interv 2016; 9(8):817–824. doi:10.1016/j.jcin.2016.01.002
- Chan AW, Wong D, Charania J. Transcatheter aortic valve replacement in bicuspid aortic stenosis using Lotus valve system. Catheter Cardiovasc Interv 2017; 90(1):157–163. doi:10.1002/ccd.26506
- Liu X, He Y, Zhu Q, et al. Supra-annular structure assessment for self-expanding transcatheter heart valve size selection in patients with bicuspid aortic valve. Catheter Cardiovasc Interv 2018; 91(5):986–994. doi:10.1002/ccd.27467
- Reddy G, Wang Z, Nishimura RA, et al. Transcatheter aortic valve replacement for stenotic bicuspid aortic valves: systematic review and meta analyses of observational studies. Catheter Cardiovasc Interv 2018; 91(5):975–983. doi:10.1002/ccd.27340
- Waksman R, Rogers T, Torguson R, et al. Transcatheter aortic valve replacement in low-risk patients with symptomatic severe aortic stenosis. J Am Coll Cardiol 2018; pii:S0735-1097(18)36852–36859. Epub ahead of print. doi:10.1016/j.jacc.2018.08.1033
Hypertrophic cardiomyopathy
To the Editor: We read with interest the article by Young et al on hypertrophic cardiomyopathy (HCM)1 and would like to raise a few important points.
HCM has a complex phenotypic expression and doesn’t necessarily involve left ventricular outflow obstruction. Midventricular obstruction is a unique subtype of HCM, with increased risk of left ventricular apical aneurysm (LVAA) formation. We reported that 25% of HCM patients with midventricular obstruction progress to LVAA compared with 0.3% of patients with other HCM subtypes.2 Magnetic resonance imaging plays a pivotal role in assessing midventricular obstruction, owing to asymmetric geometry of the left ventricle and the shortcomings of echocardiography in assessing the apical aneurysm.2
Anticoagulation remains one of the cornerstones in treating midventricular obstruction with LVAA. We performed a systematic review and found a high prevalence of atrial arrhythmia, apical thrombus, and stroke, which necessitated anticoagulation in one-fifth of patients.2
Ventricular arrhythmias are prevalent in midventricular obstruction with LVAA, mainly from increased fibrosis formation at the apical rim.3 In our review, 25.7% of patients with midventricular obstruction with LVAA and an implantable cardioverter-defibrillator (ICD) experienced appropriate shocks.2 Our finding was in line with those of Rowin et al,3 who showed appropriate ICD shocks in one-third of HCM patients with apical aneurysm. Apical aneurysm is currently considered an independent risk factor for sudden cardiac death in HCM, with an increased rate of sudden death of up to 5% every year.3,4
It is imperative to distinguish midventricular obstruction with LVAA as a unique disease imposing a higher risk of thromboembolism, ventricular arrhythmia, and progression to end-stage heart failure.3 We suggest that those patients be evaluated early in the course of disease for anticoagulation, ICD implantation, and early surgical intervention.2
- Young L, Smedira NG, Tower-Rader A, Lever H, Desai MY. Hypertrophic cardiomyopathy: a complex disease. Cleve Clin J Med 2018; 85(5):399–411. doi:10.3949/ccjm.85a.17076
- Elsheshtawy MO, Mahmoud AN, Abdelghany M, Suen IH, Sadiq A, Shani J. Left ventricular aneurysms in hypertrophic cardiomyopathy with midventricular obstruction: a systematic review of literature. Pacing Clin Electrophysiol 2018 May 22. doi:10.1111/pace.13380. [Epub ahead of print].
- Rowin EJ, Maron BJ, Haas TS, et al. Hypertrophic cardiomyopathy with left ventricular apical aneurysm: implications for risk stratification and management. J Am Coll Cardiol 2017; 69(7):761–773. doi:10.1016/j.jacc.2016.11.063
- Spirito P. Saving more lives. J Am Coll Cardiol 2017; 69(7): 774–776. doi:10.1016/j.jacc.2016.12.010
To the Editor: We read with interest the article by Young et al on hypertrophic cardiomyopathy (HCM)1 and would like to raise a few important points.
HCM has a complex phenotypic expression and doesn’t necessarily involve left ventricular outflow obstruction. Midventricular obstruction is a unique subtype of HCM, with increased risk of left ventricular apical aneurysm (LVAA) formation. We reported that 25% of HCM patients with midventricular obstruction progress to LVAA compared with 0.3% of patients with other HCM subtypes.2 Magnetic resonance imaging plays a pivotal role in assessing midventricular obstruction, owing to asymmetric geometry of the left ventricle and the shortcomings of echocardiography in assessing the apical aneurysm.2
Anticoagulation remains one of the cornerstones in treating midventricular obstruction with LVAA. We performed a systematic review and found a high prevalence of atrial arrhythmia, apical thrombus, and stroke, which necessitated anticoagulation in one-fifth of patients.2
Ventricular arrhythmias are prevalent in midventricular obstruction with LVAA, mainly from increased fibrosis formation at the apical rim.3 In our review, 25.7% of patients with midventricular obstruction with LVAA and an implantable cardioverter-defibrillator (ICD) experienced appropriate shocks.2 Our finding was in line with those of Rowin et al,3 who showed appropriate ICD shocks in one-third of HCM patients with apical aneurysm. Apical aneurysm is currently considered an independent risk factor for sudden cardiac death in HCM, with an increased rate of sudden death of up to 5% every year.3,4
It is imperative to distinguish midventricular obstruction with LVAA as a unique disease imposing a higher risk of thromboembolism, ventricular arrhythmia, and progression to end-stage heart failure.3 We suggest that those patients be evaluated early in the course of disease for anticoagulation, ICD implantation, and early surgical intervention.2
To the Editor: We read with interest the article by Young et al on hypertrophic cardiomyopathy (HCM)1 and would like to raise a few important points.
HCM has a complex phenotypic expression and doesn’t necessarily involve left ventricular outflow obstruction. Midventricular obstruction is a unique subtype of HCM, with increased risk of left ventricular apical aneurysm (LVAA) formation. We reported that 25% of HCM patients with midventricular obstruction progress to LVAA compared with 0.3% of patients with other HCM subtypes.2 Magnetic resonance imaging plays a pivotal role in assessing midventricular obstruction, owing to asymmetric geometry of the left ventricle and the shortcomings of echocardiography in assessing the apical aneurysm.2
Anticoagulation remains one of the cornerstones in treating midventricular obstruction with LVAA. We performed a systematic review and found a high prevalence of atrial arrhythmia, apical thrombus, and stroke, which necessitated anticoagulation in one-fifth of patients.2
Ventricular arrhythmias are prevalent in midventricular obstruction with LVAA, mainly from increased fibrosis formation at the apical rim.3 In our review, 25.7% of patients with midventricular obstruction with LVAA and an implantable cardioverter-defibrillator (ICD) experienced appropriate shocks.2 Our finding was in line with those of Rowin et al,3 who showed appropriate ICD shocks in one-third of HCM patients with apical aneurysm. Apical aneurysm is currently considered an independent risk factor for sudden cardiac death in HCM, with an increased rate of sudden death of up to 5% every year.3,4
It is imperative to distinguish midventricular obstruction with LVAA as a unique disease imposing a higher risk of thromboembolism, ventricular arrhythmia, and progression to end-stage heart failure.3 We suggest that those patients be evaluated early in the course of disease for anticoagulation, ICD implantation, and early surgical intervention.2
- Young L, Smedira NG, Tower-Rader A, Lever H, Desai MY. Hypertrophic cardiomyopathy: a complex disease. Cleve Clin J Med 2018; 85(5):399–411. doi:10.3949/ccjm.85a.17076
- Elsheshtawy MO, Mahmoud AN, Abdelghany M, Suen IH, Sadiq A, Shani J. Left ventricular aneurysms in hypertrophic cardiomyopathy with midventricular obstruction: a systematic review of literature. Pacing Clin Electrophysiol 2018 May 22. doi:10.1111/pace.13380. [Epub ahead of print].
- Rowin EJ, Maron BJ, Haas TS, et al. Hypertrophic cardiomyopathy with left ventricular apical aneurysm: implications for risk stratification and management. J Am Coll Cardiol 2017; 69(7):761–773. doi:10.1016/j.jacc.2016.11.063
- Spirito P. Saving more lives. J Am Coll Cardiol 2017; 69(7): 774–776. doi:10.1016/j.jacc.2016.12.010
- Young L, Smedira NG, Tower-Rader A, Lever H, Desai MY. Hypertrophic cardiomyopathy: a complex disease. Cleve Clin J Med 2018; 85(5):399–411. doi:10.3949/ccjm.85a.17076
- Elsheshtawy MO, Mahmoud AN, Abdelghany M, Suen IH, Sadiq A, Shani J. Left ventricular aneurysms in hypertrophic cardiomyopathy with midventricular obstruction: a systematic review of literature. Pacing Clin Electrophysiol 2018 May 22. doi:10.1111/pace.13380. [Epub ahead of print].
- Rowin EJ, Maron BJ, Haas TS, et al. Hypertrophic cardiomyopathy with left ventricular apical aneurysm: implications for risk stratification and management. J Am Coll Cardiol 2017; 69(7):761–773. doi:10.1016/j.jacc.2016.11.063
- Spirito P. Saving more lives. J Am Coll Cardiol 2017; 69(7): 774–776. doi:10.1016/j.jacc.2016.12.010
Postsurgical hypoparathyroidism is not primary hypoparathyroidism
To the Editor: I read with interest the case of a 67-year-old woman with bilateral hand numbness, published in the March 2018 issue of the Journal, and I would like to suggest 2 important corrections to this article.1
The authors present a case of hypocalcemia secondary to postsurgical hypoparathyroidism but describe it as due to primary hypoparathyroidism. The patient had undergone thyroidectomy 10 years earlier and since then had hypocalcemia, secondary to postsurgical hypoparathyroidism, that was treated with calcium and vitamin D, until she stopped taking these agents. Postsurgical hypothyroidism is the most common cause of acquired or secondary hypoparathyroidism and is not primary hypoparathyroidism. I strongly feel that this requires an update or correction to the article. This patient may have associated malabsorption, as the authors alluded to, as the cause of her “normal” serum parathyroid hormone level.
The patient also had hypomagnesemia, which the authors state could have been due to furosemide use and “uncontrolled” diabetes mellitus. Diabetes doesn’t need to be uncontrolled to cause hypomagnesemia. Hypomagnesemia is common in patients with type 2 diabetes mellitus, with a prevalence of 14% to 48% in patients with diabetes compared with 2.5% to 15% in the general population.2 It is often multifactorial and may be secondary to one or more of the following factors: poor dietary intake, autonomic dysfunction, altered insulin resistance, glomerular hyperfiltration, osmotic diuresis (uncontrolled diabetes), recurrent metabolic acidosis, hypophosphatemia, hypokalemia, and therapy with drugs such as metformin and sulfonylureas.
Patients with type 2 diabetes and hypomagnesemia often enter a vicious cycle in which hypomagnesemia worsens insulin resistance and insulin resistance, by reducing the activity of renal magnesium channel transient receptor potential melastatin (TRPM) type 6, perpetuates hypomagnesemia.3
- Radwan SS, Hamo KN, Zayed AA. A 67-year-old woman with bilateral hand numbness. Cleve Clin J Med 2018; 85(3):200–208. doi:10.3949/ccjm.85a.17026
- Pham PC, Pham PM, Pham SV, Miller JM, Pham PT. Hypomagnesemia in patients with type 2 diabetes. Clin J Am Soc Nephrol 2007; 2(2):366–373. doi:10.2215/CJN.02960906
- Gommers LM, Hoenderop JG, Bindels RJ, de Baaij JH. Hypomagnesemia in type 2 diabetes: a vicious circle? Diabetes 2016; 65(1):3–13. doi:10.2337/db15-1028
To the Editor: I read with interest the case of a 67-year-old woman with bilateral hand numbness, published in the March 2018 issue of the Journal, and I would like to suggest 2 important corrections to this article.1
The authors present a case of hypocalcemia secondary to postsurgical hypoparathyroidism but describe it as due to primary hypoparathyroidism. The patient had undergone thyroidectomy 10 years earlier and since then had hypocalcemia, secondary to postsurgical hypoparathyroidism, that was treated with calcium and vitamin D, until she stopped taking these agents. Postsurgical hypothyroidism is the most common cause of acquired or secondary hypoparathyroidism and is not primary hypoparathyroidism. I strongly feel that this requires an update or correction to the article. This patient may have associated malabsorption, as the authors alluded to, as the cause of her “normal” serum parathyroid hormone level.
The patient also had hypomagnesemia, which the authors state could have been due to furosemide use and “uncontrolled” diabetes mellitus. Diabetes doesn’t need to be uncontrolled to cause hypomagnesemia. Hypomagnesemia is common in patients with type 2 diabetes mellitus, with a prevalence of 14% to 48% in patients with diabetes compared with 2.5% to 15% in the general population.2 It is often multifactorial and may be secondary to one or more of the following factors: poor dietary intake, autonomic dysfunction, altered insulin resistance, glomerular hyperfiltration, osmotic diuresis (uncontrolled diabetes), recurrent metabolic acidosis, hypophosphatemia, hypokalemia, and therapy with drugs such as metformin and sulfonylureas.
Patients with type 2 diabetes and hypomagnesemia often enter a vicious cycle in which hypomagnesemia worsens insulin resistance and insulin resistance, by reducing the activity of renal magnesium channel transient receptor potential melastatin (TRPM) type 6, perpetuates hypomagnesemia.3
To the Editor: I read with interest the case of a 67-year-old woman with bilateral hand numbness, published in the March 2018 issue of the Journal, and I would like to suggest 2 important corrections to this article.1
The authors present a case of hypocalcemia secondary to postsurgical hypoparathyroidism but describe it as due to primary hypoparathyroidism. The patient had undergone thyroidectomy 10 years earlier and since then had hypocalcemia, secondary to postsurgical hypoparathyroidism, that was treated with calcium and vitamin D, until she stopped taking these agents. Postsurgical hypothyroidism is the most common cause of acquired or secondary hypoparathyroidism and is not primary hypoparathyroidism. I strongly feel that this requires an update or correction to the article. This patient may have associated malabsorption, as the authors alluded to, as the cause of her “normal” serum parathyroid hormone level.
The patient also had hypomagnesemia, which the authors state could have been due to furosemide use and “uncontrolled” diabetes mellitus. Diabetes doesn’t need to be uncontrolled to cause hypomagnesemia. Hypomagnesemia is common in patients with type 2 diabetes mellitus, with a prevalence of 14% to 48% in patients with diabetes compared with 2.5% to 15% in the general population.2 It is often multifactorial and may be secondary to one or more of the following factors: poor dietary intake, autonomic dysfunction, altered insulin resistance, glomerular hyperfiltration, osmotic diuresis (uncontrolled diabetes), recurrent metabolic acidosis, hypophosphatemia, hypokalemia, and therapy with drugs such as metformin and sulfonylureas.
Patients with type 2 diabetes and hypomagnesemia often enter a vicious cycle in which hypomagnesemia worsens insulin resistance and insulin resistance, by reducing the activity of renal magnesium channel transient receptor potential melastatin (TRPM) type 6, perpetuates hypomagnesemia.3
- Radwan SS, Hamo KN, Zayed AA. A 67-year-old woman with bilateral hand numbness. Cleve Clin J Med 2018; 85(3):200–208. doi:10.3949/ccjm.85a.17026
- Pham PC, Pham PM, Pham SV, Miller JM, Pham PT. Hypomagnesemia in patients with type 2 diabetes. Clin J Am Soc Nephrol 2007; 2(2):366–373. doi:10.2215/CJN.02960906
- Gommers LM, Hoenderop JG, Bindels RJ, de Baaij JH. Hypomagnesemia in type 2 diabetes: a vicious circle? Diabetes 2016; 65(1):3–13. doi:10.2337/db15-1028
- Radwan SS, Hamo KN, Zayed AA. A 67-year-old woman with bilateral hand numbness. Cleve Clin J Med 2018; 85(3):200–208. doi:10.3949/ccjm.85a.17026
- Pham PC, Pham PM, Pham SV, Miller JM, Pham PT. Hypomagnesemia in patients with type 2 diabetes. Clin J Am Soc Nephrol 2007; 2(2):366–373. doi:10.2215/CJN.02960906
- Gommers LM, Hoenderop JG, Bindels RJ, de Baaij JH. Hypomagnesemia in type 2 diabetes: a vicious circle? Diabetes 2016; 65(1):3–13. doi:10.2337/db15-1028
In reply: Postsurgical hypoparathyroidism is not primary hypoparathyroidism
In Reply: We thank Dr. Parmar and appreciate his important comments.
Regarding the difference between primary and secondary hypoparathyroidism, the definition varies among investigators. Some define primary hypoparathyroidism as a condition characterized by primary absence or deficiency of parathyroid hormone (PTH), which results in hypocalcemia and which can be congenital or acquired, including postsurgical hypoparathyroidism.1–4 In principle, this is similar to the classification of disorders affecting other endocrine glands as primary and secondary. For example, primary hypothyroidism refers to a state of low thyroid hormones resulting from impairment or loss of function of the thyroid gland itself, such as in Hashimoto thyroiditis, radioactive iodine therapy, or thyroidectomy, among others.5 We adopted this definition in our article. In contrast, secondary hypoparathyroidism is characterized by low PTH secretion in response to certain conditions that cause hypercalcemia. Non-PTH-mediated hypercalcemia is a more common term used to describe this state of secondary hypoparathyroidism.
Other investigators restrict the term “primary hypoparathyroidism” to nonacquired (congenital or hereditary) etiologies, while applying the term “secondary hypoparathyroidism” to acquired etiologies.6
Concerning the association between diabetes mellitus and hypomagnesemia, we agree that diabetes does not need to be uncontrolled to cause hypomagnesemia. However, the patient described in our article presented with severe hypomagnesemia (serum level 0.6 mg/dL), which is not commonly associated with diabetes. Most cases of hypomagnesemia in patients with type 2 diabetes mellitus are mild and asymptomatic, whereas severe manifestations including seizures, cardiac arrhythmias, and acute tetany are rarely encountered in clinical practice.7 Furthermore, numerous studies have shown a negative correlation between serum magnesium level and glycemic control.7–11 A recent study reported that plasma triglyceride and glucose levels are the main determinants of the plasma magnesium concentration in patients with type 2 diabetes.12
Our patient’s diabetes was uncontrolled, as evidenced by her hemoglobin A1c level of 9.7% and her random serum glucose level of 224 mg/dL. Therefore, it is more likely that “uncontrolled diabetes mellitus” (in addition to diuretic use) was the cause of her symptomatic severe hypomagnesemia rather than controlled diabetes mellitus.
- Mendes EM, Meireles-Brandão L, Meira C, Morais N, Ribeiro C, Guerra D. Primary hypoparathyroidism presenting as basal ganglia calcification secondary to extreme hypocalcemia. Clin Pract 2018; 8(1):1007. doi:10.4081/cp.2018.1007
- Vadiveloo T, Donnan PT, Leese GP. A population-based study of the epidemiology of chronic hypoparathyroidism. J Bone Miner Res 2018; 33(3):478-485. doi:10.1002/jbmr.3329
- Hendy GN, Cole DEC, Bastepe M. Hypoparathyroidism and pseudohypoparathyroidism. In: De Groot LJ, Chrousos G, Dungan K, et al, eds. Endotext [Internet], South Dartmouth (MA): MDText.com, Inc.; 2017. www.ncbi.nlm.nih.gov/books/NBK279165. Accessed August 20, 2018.
- Rosa RG, Barros AJ, de Lima AR, et al. Mood disorder as a manifestation of primary hypoparathyroidism: a case report. J Med Case Rep 2014; 8:326. doi:10.1186/1752-1947-8-326
- Almandoz JP, Gharib H. Hypothyroidism: etiology, diagnosis, and management. Med Clin North Am 2012; 96(2):203–221. doi:10.1016/j.mcna.2012.01.005
- Fouda UM, Fouda RM, Ammar HM, Salem M, Darouti ME. Impetigo herpetiformis during the puerperium triggered by secondary hypoparathyroidism: a case report. Cases J 2009; 2:9338. doi:10.1186/1757-1626-2-9338
- Tosiello L. Hypomagnesemia and diabetes mellitus. A review of clinical implications. Arch Intern Med 1996; 156(11):1143–1148. pmid: 8639008
- Pham PC, Pham PM, Pham PA, et al. Lower serum magnesium levels are associated with more rapid decline of renal function in patients with diabetes mellitus type 2. Clin Nephrol 2005; 63(6):429–436. pmid:15960144
- Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med 2005; 20(1):3–17. doi:10.1177/0885066604271539
- Resnick LM, Altura BT, Gupta RK, Laragh JH, Alderman MH, Altura BM. Intracellular and extracellular magnesium depletion in type 2 (non-insulin-independent) diabetes mellitus. Diabetologia 1993; 36(8):767–770. pmid:8405745
- Pun KK, Ho PW. Subclinical hyponatremia, hyperkalemia and hypomagnesemia in patients with poorly controlled diabetes mellitus. Diabetes Res Clin Pract 1989; 7(3)163–167. pmid: 2605984
- Kurstjens S, de Baaij JH, Bouras H, Bindels RJ, Tack CJ, Hoenderop JG. Determinants of hypomagnesemia in patients with type 2 diabetes mellitus. Eur J Endocrinol 2017; 176(1):11–19. doi:10.1530/EJE-16-0517
In Reply: We thank Dr. Parmar and appreciate his important comments.
Regarding the difference between primary and secondary hypoparathyroidism, the definition varies among investigators. Some define primary hypoparathyroidism as a condition characterized by primary absence or deficiency of parathyroid hormone (PTH), which results in hypocalcemia and which can be congenital or acquired, including postsurgical hypoparathyroidism.1–4 In principle, this is similar to the classification of disorders affecting other endocrine glands as primary and secondary. For example, primary hypothyroidism refers to a state of low thyroid hormones resulting from impairment or loss of function of the thyroid gland itself, such as in Hashimoto thyroiditis, radioactive iodine therapy, or thyroidectomy, among others.5 We adopted this definition in our article. In contrast, secondary hypoparathyroidism is characterized by low PTH secretion in response to certain conditions that cause hypercalcemia. Non-PTH-mediated hypercalcemia is a more common term used to describe this state of secondary hypoparathyroidism.
Other investigators restrict the term “primary hypoparathyroidism” to nonacquired (congenital or hereditary) etiologies, while applying the term “secondary hypoparathyroidism” to acquired etiologies.6
Concerning the association between diabetes mellitus and hypomagnesemia, we agree that diabetes does not need to be uncontrolled to cause hypomagnesemia. However, the patient described in our article presented with severe hypomagnesemia (serum level 0.6 mg/dL), which is not commonly associated with diabetes. Most cases of hypomagnesemia in patients with type 2 diabetes mellitus are mild and asymptomatic, whereas severe manifestations including seizures, cardiac arrhythmias, and acute tetany are rarely encountered in clinical practice.7 Furthermore, numerous studies have shown a negative correlation between serum magnesium level and glycemic control.7–11 A recent study reported that plasma triglyceride and glucose levels are the main determinants of the plasma magnesium concentration in patients with type 2 diabetes.12
Our patient’s diabetes was uncontrolled, as evidenced by her hemoglobin A1c level of 9.7% and her random serum glucose level of 224 mg/dL. Therefore, it is more likely that “uncontrolled diabetes mellitus” (in addition to diuretic use) was the cause of her symptomatic severe hypomagnesemia rather than controlled diabetes mellitus.
In Reply: We thank Dr. Parmar and appreciate his important comments.
Regarding the difference between primary and secondary hypoparathyroidism, the definition varies among investigators. Some define primary hypoparathyroidism as a condition characterized by primary absence or deficiency of parathyroid hormone (PTH), which results in hypocalcemia and which can be congenital or acquired, including postsurgical hypoparathyroidism.1–4 In principle, this is similar to the classification of disorders affecting other endocrine glands as primary and secondary. For example, primary hypothyroidism refers to a state of low thyroid hormones resulting from impairment or loss of function of the thyroid gland itself, such as in Hashimoto thyroiditis, radioactive iodine therapy, or thyroidectomy, among others.5 We adopted this definition in our article. In contrast, secondary hypoparathyroidism is characterized by low PTH secretion in response to certain conditions that cause hypercalcemia. Non-PTH-mediated hypercalcemia is a more common term used to describe this state of secondary hypoparathyroidism.
Other investigators restrict the term “primary hypoparathyroidism” to nonacquired (congenital or hereditary) etiologies, while applying the term “secondary hypoparathyroidism” to acquired etiologies.6
Concerning the association between diabetes mellitus and hypomagnesemia, we agree that diabetes does not need to be uncontrolled to cause hypomagnesemia. However, the patient described in our article presented with severe hypomagnesemia (serum level 0.6 mg/dL), which is not commonly associated with diabetes. Most cases of hypomagnesemia in patients with type 2 diabetes mellitus are mild and asymptomatic, whereas severe manifestations including seizures, cardiac arrhythmias, and acute tetany are rarely encountered in clinical practice.7 Furthermore, numerous studies have shown a negative correlation between serum magnesium level and glycemic control.7–11 A recent study reported that plasma triglyceride and glucose levels are the main determinants of the plasma magnesium concentration in patients with type 2 diabetes.12
Our patient’s diabetes was uncontrolled, as evidenced by her hemoglobin A1c level of 9.7% and her random serum glucose level of 224 mg/dL. Therefore, it is more likely that “uncontrolled diabetes mellitus” (in addition to diuretic use) was the cause of her symptomatic severe hypomagnesemia rather than controlled diabetes mellitus.
- Mendes EM, Meireles-Brandão L, Meira C, Morais N, Ribeiro C, Guerra D. Primary hypoparathyroidism presenting as basal ganglia calcification secondary to extreme hypocalcemia. Clin Pract 2018; 8(1):1007. doi:10.4081/cp.2018.1007
- Vadiveloo T, Donnan PT, Leese GP. A population-based study of the epidemiology of chronic hypoparathyroidism. J Bone Miner Res 2018; 33(3):478-485. doi:10.1002/jbmr.3329
- Hendy GN, Cole DEC, Bastepe M. Hypoparathyroidism and pseudohypoparathyroidism. In: De Groot LJ, Chrousos G, Dungan K, et al, eds. Endotext [Internet], South Dartmouth (MA): MDText.com, Inc.; 2017. www.ncbi.nlm.nih.gov/books/NBK279165. Accessed August 20, 2018.
- Rosa RG, Barros AJ, de Lima AR, et al. Mood disorder as a manifestation of primary hypoparathyroidism: a case report. J Med Case Rep 2014; 8:326. doi:10.1186/1752-1947-8-326
- Almandoz JP, Gharib H. Hypothyroidism: etiology, diagnosis, and management. Med Clin North Am 2012; 96(2):203–221. doi:10.1016/j.mcna.2012.01.005
- Fouda UM, Fouda RM, Ammar HM, Salem M, Darouti ME. Impetigo herpetiformis during the puerperium triggered by secondary hypoparathyroidism: a case report. Cases J 2009; 2:9338. doi:10.1186/1757-1626-2-9338
- Tosiello L. Hypomagnesemia and diabetes mellitus. A review of clinical implications. Arch Intern Med 1996; 156(11):1143–1148. pmid: 8639008
- Pham PC, Pham PM, Pham PA, et al. Lower serum magnesium levels are associated with more rapid decline of renal function in patients with diabetes mellitus type 2. Clin Nephrol 2005; 63(6):429–436. pmid:15960144
- Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med 2005; 20(1):3–17. doi:10.1177/0885066604271539
- Resnick LM, Altura BT, Gupta RK, Laragh JH, Alderman MH, Altura BM. Intracellular and extracellular magnesium depletion in type 2 (non-insulin-independent) diabetes mellitus. Diabetologia 1993; 36(8):767–770. pmid:8405745
- Pun KK, Ho PW. Subclinical hyponatremia, hyperkalemia and hypomagnesemia in patients with poorly controlled diabetes mellitus. Diabetes Res Clin Pract 1989; 7(3)163–167. pmid: 2605984
- Kurstjens S, de Baaij JH, Bouras H, Bindels RJ, Tack CJ, Hoenderop JG. Determinants of hypomagnesemia in patients with type 2 diabetes mellitus. Eur J Endocrinol 2017; 176(1):11–19. doi:10.1530/EJE-16-0517
- Mendes EM, Meireles-Brandão L, Meira C, Morais N, Ribeiro C, Guerra D. Primary hypoparathyroidism presenting as basal ganglia calcification secondary to extreme hypocalcemia. Clin Pract 2018; 8(1):1007. doi:10.4081/cp.2018.1007
- Vadiveloo T, Donnan PT, Leese GP. A population-based study of the epidemiology of chronic hypoparathyroidism. J Bone Miner Res 2018; 33(3):478-485. doi:10.1002/jbmr.3329
- Hendy GN, Cole DEC, Bastepe M. Hypoparathyroidism and pseudohypoparathyroidism. In: De Groot LJ, Chrousos G, Dungan K, et al, eds. Endotext [Internet], South Dartmouth (MA): MDText.com, Inc.; 2017. www.ncbi.nlm.nih.gov/books/NBK279165. Accessed August 20, 2018.
- Rosa RG, Barros AJ, de Lima AR, et al. Mood disorder as a manifestation of primary hypoparathyroidism: a case report. J Med Case Rep 2014; 8:326. doi:10.1186/1752-1947-8-326
- Almandoz JP, Gharib H. Hypothyroidism: etiology, diagnosis, and management. Med Clin North Am 2012; 96(2):203–221. doi:10.1016/j.mcna.2012.01.005
- Fouda UM, Fouda RM, Ammar HM, Salem M, Darouti ME. Impetigo herpetiformis during the puerperium triggered by secondary hypoparathyroidism: a case report. Cases J 2009; 2:9338. doi:10.1186/1757-1626-2-9338
- Tosiello L. Hypomagnesemia and diabetes mellitus. A review of clinical implications. Arch Intern Med 1996; 156(11):1143–1148. pmid: 8639008
- Pham PC, Pham PM, Pham PA, et al. Lower serum magnesium levels are associated with more rapid decline of renal function in patients with diabetes mellitus type 2. Clin Nephrol 2005; 63(6):429–436. pmid:15960144
- Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med 2005; 20(1):3–17. doi:10.1177/0885066604271539
- Resnick LM, Altura BT, Gupta RK, Laragh JH, Alderman MH, Altura BM. Intracellular and extracellular magnesium depletion in type 2 (non-insulin-independent) diabetes mellitus. Diabetologia 1993; 36(8):767–770. pmid:8405745
- Pun KK, Ho PW. Subclinical hyponatremia, hyperkalemia and hypomagnesemia in patients with poorly controlled diabetes mellitus. Diabetes Res Clin Pract 1989; 7(3)163–167. pmid: 2605984
- Kurstjens S, de Baaij JH, Bouras H, Bindels RJ, Tack CJ, Hoenderop JG. Determinants of hypomagnesemia in patients with type 2 diabetes mellitus. Eur J Endocrinol 2017; 176(1):11–19. doi:10.1530/EJE-16-0517
Correction: Liver enzymes
In the article by Agganis B, Lee D, Sepe T (Liver enzymes: No trivial elevations, even if asymptomatic. Cleve Clin J Med 2018; 85(8):612–617, doi:10.3949/ccjm.85a.17103), an error occurred on page 613, in the second paragraph in the section about alcohol intake. The words ALT and AST were reversed. The paragraph should read as follows:
The exact pathogenesis of alcoholic hepatitis is incompletely understood, but alcohol is primarily metabolized by the liver, and damage likely occurs during metabolism of the ingested alcohol. AST elevations tend to be higher than ALT elevations; the reason is ascribed to hepatic deficiency of pyridoxal 5´-phosphate, a cofactor of the enzymatic activity of ALT, which leads to a lesser increase in ALT than in AST.
We thank Avinash Alexander, MD, Texas Tech University Health Sciences Center, for calling this to our attention. The correction has been made online.
In the article by Agganis B, Lee D, Sepe T (Liver enzymes: No trivial elevations, even if asymptomatic. Cleve Clin J Med 2018; 85(8):612–617, doi:10.3949/ccjm.85a.17103), an error occurred on page 613, in the second paragraph in the section about alcohol intake. The words ALT and AST were reversed. The paragraph should read as follows:
The exact pathogenesis of alcoholic hepatitis is incompletely understood, but alcohol is primarily metabolized by the liver, and damage likely occurs during metabolism of the ingested alcohol. AST elevations tend to be higher than ALT elevations; the reason is ascribed to hepatic deficiency of pyridoxal 5´-phosphate, a cofactor of the enzymatic activity of ALT, which leads to a lesser increase in ALT than in AST.
We thank Avinash Alexander, MD, Texas Tech University Health Sciences Center, for calling this to our attention. The correction has been made online.
In the article by Agganis B, Lee D, Sepe T (Liver enzymes: No trivial elevations, even if asymptomatic. Cleve Clin J Med 2018; 85(8):612–617, doi:10.3949/ccjm.85a.17103), an error occurred on page 613, in the second paragraph in the section about alcohol intake. The words ALT and AST were reversed. The paragraph should read as follows:
The exact pathogenesis of alcoholic hepatitis is incompletely understood, but alcohol is primarily metabolized by the liver, and damage likely occurs during metabolism of the ingested alcohol. AST elevations tend to be higher than ALT elevations; the reason is ascribed to hepatic deficiency of pyridoxal 5´-phosphate, a cofactor of the enzymatic activity of ALT, which leads to a lesser increase in ALT than in AST.
We thank Avinash Alexander, MD, Texas Tech University Health Sciences Center, for calling this to our attention. The correction has been made online.
Clove
Cloves (Syzygium aromaticum, also known as Eugenia caryophyllata) are the aromatic flower buds of a tree in the Myrtaceae family native to Indonesia. The essential oil of clove is known to exhibit antioxidant, anti-inflammatory, antimicrobial, antifungal, antiviral, anticancer, cytotoxic, insect repellent, and anesthetic activities.1,2 It is used topically in herbal medicine to alleviate pain and facilitate healing,3 and has been used in traditional medicine to confer analgesic, anti-inflammatory, antimicrobial, antiviral, and antiseptic activity.4 Cloves also are used in fragrances and for food flavoring.2
The two main constituents of clove oil are eugenol (78%) and beta-caryophyllene (13%). Although clove oil and its primary components are generally recognized as safe, a 2006 in vitro study by Prashar et al. found that clove oil and eugenol displayed cytotoxicity toward human fibroblasts and endothelial cells. Clove oil, in concentrations as low as 0.03%, was noted for being exceedingly cytotoxic, with up to 73% of this effect ascribed to eugenol, with beta-caryophyllene displaying no toxicity.3 In addition to beta-caryophyllene and the phenylpropanoid eugenol, other important constituents of clove essential oil are the phenylpropanoids carvacrol, thymol, and cinnamaldehyde.2
Topical applications and human studies
constituent, eugenol.5 It also has been used as a penetration enhancer in various forms of topical products, including creams, ointments, gels, and patches.6
Palmar hyperhidrosis
In 2017, Ibrahim et al. treated 45 patients with palmar hyperhidrosis with clove oil 45% in liposome, with 20 patients in a control group treated with 0.9% saline solution. Subjects were assessed by gravimetry testing and hyperhidrosis disease severity scale to determine the impact of clove oil on decreasing the sweating rate in patients with idiopathic palmar hyperhidrosis. Gravimetry testing revealed that the sweating rate decreased significantly in the clove oil group but that there was no significant improvement in the placebo group. The investigators concluded that twice-daily topical application of 45% clove oil in liposome for 2 weeks showed promise in significantly reducing palmar sweating.5
Pruritus
That same year Ibrahim et al. evaluated the effects of topically applied clove oil in treating 50 patients with chronic pruritus due to hepatic, renal, or diabetic origin. The investigators divided the subjects into two groups of 25, with the first directed to hydrate their skin before applying topical clove oil twice daily for 2 weeks. The second group was instructed to apply topical petrolatum by hand on the same schedule. Using the 5-D itch scale, researchers noted a significant improvement in all parameters in the patients using clove oil and no such improvements in the petrolatum group. They concluded that particularly for patients whose topical or systemic treatments are not well tolerated or are contraindicated.7
Anal fissure
In 2007, Elwakeel et al. evaluated the use of a clove oil 1% cream for the treatment of chronic anal fissure as opposed to the traditional treatment of stool softeners and lignocaine cream 5% in a single-blind randomized comparative trial over 6 weeks. Healing was observed in 60% of the 30 patients in the clove oil group and in 12% of the 25 patients in the control group at the 3-month follow-up visit. The researchers concluded that topically applied clove oil cream yielded significant benefits in the treatment of chronic anal fissures.8
More recently, Nelson et al. conducted a literature survey to evaluate the efficacy and morbidity of nonsurgical treatments for anal fissures from 1966 to August 2010. Clove oil was among 17 agents used in the 77 cited studies. While no medical therapies were found to display the efficacy of surgical sphincterotomy (or, fortunately, linked to the risk of incontinence), clove oil was identified as one of the “newer” agents demonstrating promise.9
Musculoskeletal pain
Clove oil is included among several herbal ingredients (i.e., eucalyptus oil, gaultheria oil, turpentine oil, menthol, and camphor) associated with analgesic and anti-inflammatory properties that are used in the topical spray Eezpain. Nawaz et al. showed in a prospective pilot study with 20 male and female subjects that the polyherbal formulation was efficacious in relieving mild to moderate knee and wrist joint pain.10
Laboratory studies
Just over a decade ago, Chaieb et al. assessed the antioxidant characteristics of the essential oil of clove, finding that it displayed a robust radical scavenging capacity against 2,2-diphenyl-1-picrylhydrazyl in comparison to the synthetic antioxidant tert-butylated hydroxytoluene. It also showed potent antifungal activity against 53 test strains of human pathogenic yeasts. The authors noted that clove oil is a readily available source of natural antioxidants and is a worthy ingredient in pharmaceutical products.11
Anti-inflammatory activity
In 2017, Han and Parker studied the biological activity of four concentrations of a commercially available clove essential oil product on 17 protein biomarkers important in inflammation in a model of human skin disease. They found that the 0.011% concentration of the oil enacted strong antiproliferative effects on human dermal skin fibroblasts, and significantly suppressed multiple proinflammatory biomarkers as well as tissue remodeling protein molecules. The investigators also observed that essential clove oil significantly influenced global gene expression and signaling pathways involved in inflammation, tissue remodeling, and cancer processes. They concluded that their results indicate anti-inflammatory, anticancer, and tissue-remodeling properties of clove essential oil, and its main active ingredient eugenol, in human dermal fibroblasts.1
UVB protection
Recently, Patwardhan and Bhatt assessed the capacity of flavonoids from clove buds to protect human dermal fibroblasts from UVB exposure. They found that the flavonoid-enriched fraction of clove demonstrated significant potential, as it mitigated the effects of UVB radiation, and delivered protection via the nuclear factor E2-related factor 2-antioxidant response pathway. The flavonoid-enriched clove fraction, they concluded, warrants consideration as a topically applied cutaneous protectant against the effects of UVB exposure.4
Antiviral and immunomodulatory activity
Based on their earlier work showing the antiviral activity of clove bud oil against Pseudomonas aeruginosa PAO1, Haripriyan et al. reported this year that clove bud oil affects pseudomonal proteases (elastase A, elastase B, protease IV, and alkaline protease), attenuating significant viral mechanisms of this noted human disease agent while bolstering host immunomodulatory functions. They concluded that their results suggest the viability of clove bud oil as a topical treatment for infections resistant to antibiotics.12
Acne
In 2017, Owen et al. developed a topical preparation incorporating clove bud, rosewood, and litsea essential oils that compared favorably with the topical antibiotics Dalacin T and Stiemycin in controlling acne vulgaris-linked bacteria. Specifically, the herbal formulation exhibited synergistic activity against Propionibacterium acnes, although not to Staphylococcus epidermidis, and its antimicrobial activity exceeded or equated to that of the tested antibiotics. The investigators suggested that the polyherbal preparation may serve as an option for treating acne-linked bacteria.13
Scabies
In a study 2 years ago to ascertain the efficacy of 10 essential oils against Sarcoptes scabiei, Fang et al. conducted contact bioassays and fumigation bioassays using clove, palmarosa, geranium, tea tree, lavender, Manuka, bitter orange, eucalyptus, Japanese cedar, and cade oil. In the contact bioassays, clove oil 1%, the most effective of the oils, eliminated the mites within 20 minutes. In the fumigation bioassay, clove was second to tea tree oil in efficacy. The investigators concluded that clove, tea tree, palmarosa, and eucalyptus oils demonstrate potential in pest control and for treating scabies infections in humans or animals.14
Conclusion
Clove oil is an active ingredient in various topical treatments. While not typically a first-line therapy, it shows promise for a wider range of applications. Research continues to determine the extent to which this botanical agent can reach into the dermatologic armamentarium and, more importantly, how effective it can be in treating cutaneous disorders.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC.
References
1. Pharm Biol. 2017 Dec;55(1):1619-22.
2. Phytother Res. 2007 Jun;21(6):501-6.
3. Cell Prolif. 2006 Aug;39(4):241-8.
4. Pharmacogn Mag. 2015 Oct;11(Suppl 3):S397-406.
5. J Cosmet Dermatol. 2017 Dec 28. doi: 10.1111/jocd.12471.
6. Curr Drug Deliv. 2012 Mar;9(2):219-30.
7. J Cosmet Dermatol. 2017 Dec;16(4):508-11.
8. Colorectal Dis. 2007 Jul;9(6):549-52
9. Cochrane Database Syst Rev. 2012 Feb 15;(2):CD003431.
10. Pak J Pharm Sci. 2015 Jan;28(1):43-7.
11. Mycoses. 2007 Sep;50(5):403-6.
12. Sci Rep. 2018 Feb 21;8(1):3437.
13. Phytother Res. 2017 Mar;31(3):410-7.
14. Parasit Vectors. 2016 Nov 22;9(1):594.
Cloves (Syzygium aromaticum, also known as Eugenia caryophyllata) are the aromatic flower buds of a tree in the Myrtaceae family native to Indonesia. The essential oil of clove is known to exhibit antioxidant, anti-inflammatory, antimicrobial, antifungal, antiviral, anticancer, cytotoxic, insect repellent, and anesthetic activities.1,2 It is used topically in herbal medicine to alleviate pain and facilitate healing,3 and has been used in traditional medicine to confer analgesic, anti-inflammatory, antimicrobial, antiviral, and antiseptic activity.4 Cloves also are used in fragrances and for food flavoring.2
The two main constituents of clove oil are eugenol (78%) and beta-caryophyllene (13%). Although clove oil and its primary components are generally recognized as safe, a 2006 in vitro study by Prashar et al. found that clove oil and eugenol displayed cytotoxicity toward human fibroblasts and endothelial cells. Clove oil, in concentrations as low as 0.03%, was noted for being exceedingly cytotoxic, with up to 73% of this effect ascribed to eugenol, with beta-caryophyllene displaying no toxicity.3 In addition to beta-caryophyllene and the phenylpropanoid eugenol, other important constituents of clove essential oil are the phenylpropanoids carvacrol, thymol, and cinnamaldehyde.2
Topical applications and human studies
constituent, eugenol.5 It also has been used as a penetration enhancer in various forms of topical products, including creams, ointments, gels, and patches.6
Palmar hyperhidrosis
In 2017, Ibrahim et al. treated 45 patients with palmar hyperhidrosis with clove oil 45% in liposome, with 20 patients in a control group treated with 0.9% saline solution. Subjects were assessed by gravimetry testing and hyperhidrosis disease severity scale to determine the impact of clove oil on decreasing the sweating rate in patients with idiopathic palmar hyperhidrosis. Gravimetry testing revealed that the sweating rate decreased significantly in the clove oil group but that there was no significant improvement in the placebo group. The investigators concluded that twice-daily topical application of 45% clove oil in liposome for 2 weeks showed promise in significantly reducing palmar sweating.5
Pruritus
That same year Ibrahim et al. evaluated the effects of topically applied clove oil in treating 50 patients with chronic pruritus due to hepatic, renal, or diabetic origin. The investigators divided the subjects into two groups of 25, with the first directed to hydrate their skin before applying topical clove oil twice daily for 2 weeks. The second group was instructed to apply topical petrolatum by hand on the same schedule. Using the 5-D itch scale, researchers noted a significant improvement in all parameters in the patients using clove oil and no such improvements in the petrolatum group. They concluded that particularly for patients whose topical or systemic treatments are not well tolerated or are contraindicated.7
Anal fissure
In 2007, Elwakeel et al. evaluated the use of a clove oil 1% cream for the treatment of chronic anal fissure as opposed to the traditional treatment of stool softeners and lignocaine cream 5% in a single-blind randomized comparative trial over 6 weeks. Healing was observed in 60% of the 30 patients in the clove oil group and in 12% of the 25 patients in the control group at the 3-month follow-up visit. The researchers concluded that topically applied clove oil cream yielded significant benefits in the treatment of chronic anal fissures.8
More recently, Nelson et al. conducted a literature survey to evaluate the efficacy and morbidity of nonsurgical treatments for anal fissures from 1966 to August 2010. Clove oil was among 17 agents used in the 77 cited studies. While no medical therapies were found to display the efficacy of surgical sphincterotomy (or, fortunately, linked to the risk of incontinence), clove oil was identified as one of the “newer” agents demonstrating promise.9
Musculoskeletal pain
Clove oil is included among several herbal ingredients (i.e., eucalyptus oil, gaultheria oil, turpentine oil, menthol, and camphor) associated with analgesic and anti-inflammatory properties that are used in the topical spray Eezpain. Nawaz et al. showed in a prospective pilot study with 20 male and female subjects that the polyherbal formulation was efficacious in relieving mild to moderate knee and wrist joint pain.10
Laboratory studies
Just over a decade ago, Chaieb et al. assessed the antioxidant characteristics of the essential oil of clove, finding that it displayed a robust radical scavenging capacity against 2,2-diphenyl-1-picrylhydrazyl in comparison to the synthetic antioxidant tert-butylated hydroxytoluene. It also showed potent antifungal activity against 53 test strains of human pathogenic yeasts. The authors noted that clove oil is a readily available source of natural antioxidants and is a worthy ingredient in pharmaceutical products.11
Anti-inflammatory activity
In 2017, Han and Parker studied the biological activity of four concentrations of a commercially available clove essential oil product on 17 protein biomarkers important in inflammation in a model of human skin disease. They found that the 0.011% concentration of the oil enacted strong antiproliferative effects on human dermal skin fibroblasts, and significantly suppressed multiple proinflammatory biomarkers as well as tissue remodeling protein molecules. The investigators also observed that essential clove oil significantly influenced global gene expression and signaling pathways involved in inflammation, tissue remodeling, and cancer processes. They concluded that their results indicate anti-inflammatory, anticancer, and tissue-remodeling properties of clove essential oil, and its main active ingredient eugenol, in human dermal fibroblasts.1
UVB protection
Recently, Patwardhan and Bhatt assessed the capacity of flavonoids from clove buds to protect human dermal fibroblasts from UVB exposure. They found that the flavonoid-enriched fraction of clove demonstrated significant potential, as it mitigated the effects of UVB radiation, and delivered protection via the nuclear factor E2-related factor 2-antioxidant response pathway. The flavonoid-enriched clove fraction, they concluded, warrants consideration as a topically applied cutaneous protectant against the effects of UVB exposure.4
Antiviral and immunomodulatory activity
Based on their earlier work showing the antiviral activity of clove bud oil against Pseudomonas aeruginosa PAO1, Haripriyan et al. reported this year that clove bud oil affects pseudomonal proteases (elastase A, elastase B, protease IV, and alkaline protease), attenuating significant viral mechanisms of this noted human disease agent while bolstering host immunomodulatory functions. They concluded that their results suggest the viability of clove bud oil as a topical treatment for infections resistant to antibiotics.12
Acne
In 2017, Owen et al. developed a topical preparation incorporating clove bud, rosewood, and litsea essential oils that compared favorably with the topical antibiotics Dalacin T and Stiemycin in controlling acne vulgaris-linked bacteria. Specifically, the herbal formulation exhibited synergistic activity against Propionibacterium acnes, although not to Staphylococcus epidermidis, and its antimicrobial activity exceeded or equated to that of the tested antibiotics. The investigators suggested that the polyherbal preparation may serve as an option for treating acne-linked bacteria.13
Scabies
In a study 2 years ago to ascertain the efficacy of 10 essential oils against Sarcoptes scabiei, Fang et al. conducted contact bioassays and fumigation bioassays using clove, palmarosa, geranium, tea tree, lavender, Manuka, bitter orange, eucalyptus, Japanese cedar, and cade oil. In the contact bioassays, clove oil 1%, the most effective of the oils, eliminated the mites within 20 minutes. In the fumigation bioassay, clove was second to tea tree oil in efficacy. The investigators concluded that clove, tea tree, palmarosa, and eucalyptus oils demonstrate potential in pest control and for treating scabies infections in humans or animals.14
Conclusion
Clove oil is an active ingredient in various topical treatments. While not typically a first-line therapy, it shows promise for a wider range of applications. Research continues to determine the extent to which this botanical agent can reach into the dermatologic armamentarium and, more importantly, how effective it can be in treating cutaneous disorders.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC.
References
1. Pharm Biol. 2017 Dec;55(1):1619-22.
2. Phytother Res. 2007 Jun;21(6):501-6.
3. Cell Prolif. 2006 Aug;39(4):241-8.
4. Pharmacogn Mag. 2015 Oct;11(Suppl 3):S397-406.
5. J Cosmet Dermatol. 2017 Dec 28. doi: 10.1111/jocd.12471.
6. Curr Drug Deliv. 2012 Mar;9(2):219-30.
7. J Cosmet Dermatol. 2017 Dec;16(4):508-11.
8. Colorectal Dis. 2007 Jul;9(6):549-52
9. Cochrane Database Syst Rev. 2012 Feb 15;(2):CD003431.
10. Pak J Pharm Sci. 2015 Jan;28(1):43-7.
11. Mycoses. 2007 Sep;50(5):403-6.
12. Sci Rep. 2018 Feb 21;8(1):3437.
13. Phytother Res. 2017 Mar;31(3):410-7.
14. Parasit Vectors. 2016 Nov 22;9(1):594.
Cloves (Syzygium aromaticum, also known as Eugenia caryophyllata) are the aromatic flower buds of a tree in the Myrtaceae family native to Indonesia. The essential oil of clove is known to exhibit antioxidant, anti-inflammatory, antimicrobial, antifungal, antiviral, anticancer, cytotoxic, insect repellent, and anesthetic activities.1,2 It is used topically in herbal medicine to alleviate pain and facilitate healing,3 and has been used in traditional medicine to confer analgesic, anti-inflammatory, antimicrobial, antiviral, and antiseptic activity.4 Cloves also are used in fragrances and for food flavoring.2
The two main constituents of clove oil are eugenol (78%) and beta-caryophyllene (13%). Although clove oil and its primary components are generally recognized as safe, a 2006 in vitro study by Prashar et al. found that clove oil and eugenol displayed cytotoxicity toward human fibroblasts and endothelial cells. Clove oil, in concentrations as low as 0.03%, was noted for being exceedingly cytotoxic, with up to 73% of this effect ascribed to eugenol, with beta-caryophyllene displaying no toxicity.3 In addition to beta-caryophyllene and the phenylpropanoid eugenol, other important constituents of clove essential oil are the phenylpropanoids carvacrol, thymol, and cinnamaldehyde.2
Topical applications and human studies
constituent, eugenol.5 It also has been used as a penetration enhancer in various forms of topical products, including creams, ointments, gels, and patches.6
Palmar hyperhidrosis
In 2017, Ibrahim et al. treated 45 patients with palmar hyperhidrosis with clove oil 45% in liposome, with 20 patients in a control group treated with 0.9% saline solution. Subjects were assessed by gravimetry testing and hyperhidrosis disease severity scale to determine the impact of clove oil on decreasing the sweating rate in patients with idiopathic palmar hyperhidrosis. Gravimetry testing revealed that the sweating rate decreased significantly in the clove oil group but that there was no significant improvement in the placebo group. The investigators concluded that twice-daily topical application of 45% clove oil in liposome for 2 weeks showed promise in significantly reducing palmar sweating.5
Pruritus
That same year Ibrahim et al. evaluated the effects of topically applied clove oil in treating 50 patients with chronic pruritus due to hepatic, renal, or diabetic origin. The investigators divided the subjects into two groups of 25, with the first directed to hydrate their skin before applying topical clove oil twice daily for 2 weeks. The second group was instructed to apply topical petrolatum by hand on the same schedule. Using the 5-D itch scale, researchers noted a significant improvement in all parameters in the patients using clove oil and no such improvements in the petrolatum group. They concluded that particularly for patients whose topical or systemic treatments are not well tolerated or are contraindicated.7
Anal fissure
In 2007, Elwakeel et al. evaluated the use of a clove oil 1% cream for the treatment of chronic anal fissure as opposed to the traditional treatment of stool softeners and lignocaine cream 5% in a single-blind randomized comparative trial over 6 weeks. Healing was observed in 60% of the 30 patients in the clove oil group and in 12% of the 25 patients in the control group at the 3-month follow-up visit. The researchers concluded that topically applied clove oil cream yielded significant benefits in the treatment of chronic anal fissures.8
More recently, Nelson et al. conducted a literature survey to evaluate the efficacy and morbidity of nonsurgical treatments for anal fissures from 1966 to August 2010. Clove oil was among 17 agents used in the 77 cited studies. While no medical therapies were found to display the efficacy of surgical sphincterotomy (or, fortunately, linked to the risk of incontinence), clove oil was identified as one of the “newer” agents demonstrating promise.9
Musculoskeletal pain
Clove oil is included among several herbal ingredients (i.e., eucalyptus oil, gaultheria oil, turpentine oil, menthol, and camphor) associated with analgesic and anti-inflammatory properties that are used in the topical spray Eezpain. Nawaz et al. showed in a prospective pilot study with 20 male and female subjects that the polyherbal formulation was efficacious in relieving mild to moderate knee and wrist joint pain.10
Laboratory studies
Just over a decade ago, Chaieb et al. assessed the antioxidant characteristics of the essential oil of clove, finding that it displayed a robust radical scavenging capacity against 2,2-diphenyl-1-picrylhydrazyl in comparison to the synthetic antioxidant tert-butylated hydroxytoluene. It also showed potent antifungal activity against 53 test strains of human pathogenic yeasts. The authors noted that clove oil is a readily available source of natural antioxidants and is a worthy ingredient in pharmaceutical products.11
Anti-inflammatory activity
In 2017, Han and Parker studied the biological activity of four concentrations of a commercially available clove essential oil product on 17 protein biomarkers important in inflammation in a model of human skin disease. They found that the 0.011% concentration of the oil enacted strong antiproliferative effects on human dermal skin fibroblasts, and significantly suppressed multiple proinflammatory biomarkers as well as tissue remodeling protein molecules. The investigators also observed that essential clove oil significantly influenced global gene expression and signaling pathways involved in inflammation, tissue remodeling, and cancer processes. They concluded that their results indicate anti-inflammatory, anticancer, and tissue-remodeling properties of clove essential oil, and its main active ingredient eugenol, in human dermal fibroblasts.1
UVB protection
Recently, Patwardhan and Bhatt assessed the capacity of flavonoids from clove buds to protect human dermal fibroblasts from UVB exposure. They found that the flavonoid-enriched fraction of clove demonstrated significant potential, as it mitigated the effects of UVB radiation, and delivered protection via the nuclear factor E2-related factor 2-antioxidant response pathway. The flavonoid-enriched clove fraction, they concluded, warrants consideration as a topically applied cutaneous protectant against the effects of UVB exposure.4
Antiviral and immunomodulatory activity
Based on their earlier work showing the antiviral activity of clove bud oil against Pseudomonas aeruginosa PAO1, Haripriyan et al. reported this year that clove bud oil affects pseudomonal proteases (elastase A, elastase B, protease IV, and alkaline protease), attenuating significant viral mechanisms of this noted human disease agent while bolstering host immunomodulatory functions. They concluded that their results suggest the viability of clove bud oil as a topical treatment for infections resistant to antibiotics.12
Acne
In 2017, Owen et al. developed a topical preparation incorporating clove bud, rosewood, and litsea essential oils that compared favorably with the topical antibiotics Dalacin T and Stiemycin in controlling acne vulgaris-linked bacteria. Specifically, the herbal formulation exhibited synergistic activity against Propionibacterium acnes, although not to Staphylococcus epidermidis, and its antimicrobial activity exceeded or equated to that of the tested antibiotics. The investigators suggested that the polyherbal preparation may serve as an option for treating acne-linked bacteria.13
Scabies
In a study 2 years ago to ascertain the efficacy of 10 essential oils against Sarcoptes scabiei, Fang et al. conducted contact bioassays and fumigation bioassays using clove, palmarosa, geranium, tea tree, lavender, Manuka, bitter orange, eucalyptus, Japanese cedar, and cade oil. In the contact bioassays, clove oil 1%, the most effective of the oils, eliminated the mites within 20 minutes. In the fumigation bioassay, clove was second to tea tree oil in efficacy. The investigators concluded that clove, tea tree, palmarosa, and eucalyptus oils demonstrate potential in pest control and for treating scabies infections in humans or animals.14
Conclusion
Clove oil is an active ingredient in various topical treatments. While not typically a first-line therapy, it shows promise for a wider range of applications. Research continues to determine the extent to which this botanical agent can reach into the dermatologic armamentarium and, more importantly, how effective it can be in treating cutaneous disorders.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC.
References
1. Pharm Biol. 2017 Dec;55(1):1619-22.
2. Phytother Res. 2007 Jun;21(6):501-6.
3. Cell Prolif. 2006 Aug;39(4):241-8.
4. Pharmacogn Mag. 2015 Oct;11(Suppl 3):S397-406.
5. J Cosmet Dermatol. 2017 Dec 28. doi: 10.1111/jocd.12471.
6. Curr Drug Deliv. 2012 Mar;9(2):219-30.
7. J Cosmet Dermatol. 2017 Dec;16(4):508-11.
8. Colorectal Dis. 2007 Jul;9(6):549-52
9. Cochrane Database Syst Rev. 2012 Feb 15;(2):CD003431.
10. Pak J Pharm Sci. 2015 Jan;28(1):43-7.
11. Mycoses. 2007 Sep;50(5):403-6.
12. Sci Rep. 2018 Feb 21;8(1):3437.
13. Phytother Res. 2017 Mar;31(3):410-7.
14. Parasit Vectors. 2016 Nov 22;9(1):594.
Swings in four metabolic measures predicted death in healthy people
, based on data from a 5.5-year population-based study in Korea.
In a model adjusted for age, sex, smoking, alcohol consumption, regular exercise, and income status the group with high variability for all four parameters had a significantly higher risk for all-cause mortality (hazard ratio, 2.27; 95% confidence interval, 2.13-2.42), for MI (HR, 1.43; 95% CI, 1.25-1.64), and for stroke (HR, 1.41; 95% CI, 1.25-1.60), compared with the group with low variability for all four parameters. The association with risk was graded and persisted after multivariable adjustment.
“Variability in metabolic parameters may be prognostic surrogate markers for predicting mortality and cardiovascular outcomes,” wrote senior author Seung-Hwan Lee, MD, PhD, and professor of endocrinology at the College of Medicine of the Catholic University of Korea in Seoul, South Korea, and colleagues. “High variability in metabolic parameters (may be) associated with adverse health outcomes not only in a diseased population, but also in the relatively healthy population although the mechanism could be somewhat different.”
Korea has a single-payer system, the Korean National Health Insurance system, that includes health information on its entire population. The researchers selected data from 6,748,773 people who were free of diabetes mellitus, hypertension, and dyslipidemia, and who underwent three or more health examinations during 2005-2012 that documented body mass index (BMI), fasting blood glucose, systolic blood pressure, and total cholesterol. Participants were followed to the end of 2015, for a median follow-up of 5.5 years. There were 54,785 deaths (0.8%), 22,498 cases of stroke (0.3%), and 21,452 MIs (0.3%).
The research team defined high variability as the highest quartile, classifying participants according to the number of high-variability parameters. A score of 4 indicated high variability in all four metabolic parameters – body weight, systolic blood pressure, total cholesterol, and fasting blood glucose.
In the highest quartile in fasting blood glucose variability, compared with the lowest quartile, the risk of all-cause mortality increased by 20% (HR, 1.20; 95% CI, 1.18-1.23), MI by 16% (HR, 1.16; 95% CI, 1.12-1.21), and stroke by 13% (HR, 1.13; 95% CI, 1.09-1.17).
For the highest quartile in total cholesterol variability, compared with the lowest quartile, the risk of all-cause mortality increased by 31% (HR, 1.31; 95% CI, 1.28-1.34), MI by 10% (HR, 1.10; 95% CI, 1.06-1.14), and stroke by 6% (HR, 1.06; 95% CI, 1.03-1.10).
For the highest quartile in systolic BP variability, compared with the lowest quartile, the risk of all-cause mortality increased by 19% (HR, 1.19; 95% CI, 1.16-1.22), MI by 7% (HR, 1.07; 95% CI, 1.03-1.11), and stroke by 14% (HR, 1.14; 95% CI, 1.10-1.18).
For the highest quartile in BMI variability, compared with the lowest quartile, the risk of all-cause mortality increased by 53% (HR, 1.53; 95% CI, 1.50-1.57), MI by 14% (HR, 1.14; 95% CI, 1.09-1.18), and stroke by 14% (HR, 1.14; 95% CI, 1.10-1.18).
“It is not certain whether these results from Korea would apply to the United States. However, several previous studies on variability were performed in other populations, suggesting that it is likely to be a common phenomenon,” the authors wrote.
The study was supported in part by the National Research Foundation of Korea Grant funded by the Korean Government. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
SOURCE: Lee S-H et al. Circulation. 2018 Oct.
, based on data from a 5.5-year population-based study in Korea.
In a model adjusted for age, sex, smoking, alcohol consumption, regular exercise, and income status the group with high variability for all four parameters had a significantly higher risk for all-cause mortality (hazard ratio, 2.27; 95% confidence interval, 2.13-2.42), for MI (HR, 1.43; 95% CI, 1.25-1.64), and for stroke (HR, 1.41; 95% CI, 1.25-1.60), compared with the group with low variability for all four parameters. The association with risk was graded and persisted after multivariable adjustment.
“Variability in metabolic parameters may be prognostic surrogate markers for predicting mortality and cardiovascular outcomes,” wrote senior author Seung-Hwan Lee, MD, PhD, and professor of endocrinology at the College of Medicine of the Catholic University of Korea in Seoul, South Korea, and colleagues. “High variability in metabolic parameters (may be) associated with adverse health outcomes not only in a diseased population, but also in the relatively healthy population although the mechanism could be somewhat different.”
Korea has a single-payer system, the Korean National Health Insurance system, that includes health information on its entire population. The researchers selected data from 6,748,773 people who were free of diabetes mellitus, hypertension, and dyslipidemia, and who underwent three or more health examinations during 2005-2012 that documented body mass index (BMI), fasting blood glucose, systolic blood pressure, and total cholesterol. Participants were followed to the end of 2015, for a median follow-up of 5.5 years. There were 54,785 deaths (0.8%), 22,498 cases of stroke (0.3%), and 21,452 MIs (0.3%).
The research team defined high variability as the highest quartile, classifying participants according to the number of high-variability parameters. A score of 4 indicated high variability in all four metabolic parameters – body weight, systolic blood pressure, total cholesterol, and fasting blood glucose.
In the highest quartile in fasting blood glucose variability, compared with the lowest quartile, the risk of all-cause mortality increased by 20% (HR, 1.20; 95% CI, 1.18-1.23), MI by 16% (HR, 1.16; 95% CI, 1.12-1.21), and stroke by 13% (HR, 1.13; 95% CI, 1.09-1.17).
For the highest quartile in total cholesterol variability, compared with the lowest quartile, the risk of all-cause mortality increased by 31% (HR, 1.31; 95% CI, 1.28-1.34), MI by 10% (HR, 1.10; 95% CI, 1.06-1.14), and stroke by 6% (HR, 1.06; 95% CI, 1.03-1.10).
For the highest quartile in systolic BP variability, compared with the lowest quartile, the risk of all-cause mortality increased by 19% (HR, 1.19; 95% CI, 1.16-1.22), MI by 7% (HR, 1.07; 95% CI, 1.03-1.11), and stroke by 14% (HR, 1.14; 95% CI, 1.10-1.18).
For the highest quartile in BMI variability, compared with the lowest quartile, the risk of all-cause mortality increased by 53% (HR, 1.53; 95% CI, 1.50-1.57), MI by 14% (HR, 1.14; 95% CI, 1.09-1.18), and stroke by 14% (HR, 1.14; 95% CI, 1.10-1.18).
“It is not certain whether these results from Korea would apply to the United States. However, several previous studies on variability were performed in other populations, suggesting that it is likely to be a common phenomenon,” the authors wrote.
The study was supported in part by the National Research Foundation of Korea Grant funded by the Korean Government. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
SOURCE: Lee S-H et al. Circulation. 2018 Oct.
, based on data from a 5.5-year population-based study in Korea.
In a model adjusted for age, sex, smoking, alcohol consumption, regular exercise, and income status the group with high variability for all four parameters had a significantly higher risk for all-cause mortality (hazard ratio, 2.27; 95% confidence interval, 2.13-2.42), for MI (HR, 1.43; 95% CI, 1.25-1.64), and for stroke (HR, 1.41; 95% CI, 1.25-1.60), compared with the group with low variability for all four parameters. The association with risk was graded and persisted after multivariable adjustment.
“Variability in metabolic parameters may be prognostic surrogate markers for predicting mortality and cardiovascular outcomes,” wrote senior author Seung-Hwan Lee, MD, PhD, and professor of endocrinology at the College of Medicine of the Catholic University of Korea in Seoul, South Korea, and colleagues. “High variability in metabolic parameters (may be) associated with adverse health outcomes not only in a diseased population, but also in the relatively healthy population although the mechanism could be somewhat different.”
Korea has a single-payer system, the Korean National Health Insurance system, that includes health information on its entire population. The researchers selected data from 6,748,773 people who were free of diabetes mellitus, hypertension, and dyslipidemia, and who underwent three or more health examinations during 2005-2012 that documented body mass index (BMI), fasting blood glucose, systolic blood pressure, and total cholesterol. Participants were followed to the end of 2015, for a median follow-up of 5.5 years. There were 54,785 deaths (0.8%), 22,498 cases of stroke (0.3%), and 21,452 MIs (0.3%).
The research team defined high variability as the highest quartile, classifying participants according to the number of high-variability parameters. A score of 4 indicated high variability in all four metabolic parameters – body weight, systolic blood pressure, total cholesterol, and fasting blood glucose.
In the highest quartile in fasting blood glucose variability, compared with the lowest quartile, the risk of all-cause mortality increased by 20% (HR, 1.20; 95% CI, 1.18-1.23), MI by 16% (HR, 1.16; 95% CI, 1.12-1.21), and stroke by 13% (HR, 1.13; 95% CI, 1.09-1.17).
For the highest quartile in total cholesterol variability, compared with the lowest quartile, the risk of all-cause mortality increased by 31% (HR, 1.31; 95% CI, 1.28-1.34), MI by 10% (HR, 1.10; 95% CI, 1.06-1.14), and stroke by 6% (HR, 1.06; 95% CI, 1.03-1.10).
For the highest quartile in systolic BP variability, compared with the lowest quartile, the risk of all-cause mortality increased by 19% (HR, 1.19; 95% CI, 1.16-1.22), MI by 7% (HR, 1.07; 95% CI, 1.03-1.11), and stroke by 14% (HR, 1.14; 95% CI, 1.10-1.18).
For the highest quartile in BMI variability, compared with the lowest quartile, the risk of all-cause mortality increased by 53% (HR, 1.53; 95% CI, 1.50-1.57), MI by 14% (HR, 1.14; 95% CI, 1.09-1.18), and stroke by 14% (HR, 1.14; 95% CI, 1.10-1.18).
“It is not certain whether these results from Korea would apply to the United States. However, several previous studies on variability were performed in other populations, suggesting that it is likely to be a common phenomenon,” the authors wrote.
The study was supported in part by the National Research Foundation of Korea Grant funded by the Korean Government. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
SOURCE: Lee S-H et al. Circulation. 2018 Oct.
FROM CIRCULATION
Key clinical point: Fluctuations in fasting glucose and cholesterol levels, systolic blood pressure, and body mass index are associated with a higher risk for all-cause mortality, myocardial infarction, and stroke in otherwise healthy people.
Major finding: The hazard ratios were 2.27 (95% CI, 2.13-2.42) for all-cause mortality, 1.43 (95% CI, 1.25-1.64) for MI, and 1.41 (95% CI, 1.25-1.60) for stroke.
Study details: An observational population-based study involving more than 6.7 million Koreans age 20 and older.
Disclosures: The study was funded by the National Research Foundation of Korea. The authors had no relevant conflicts of interest to declare.
Source: Lee S-H et al. Circulation. 2018 Oct.
Reply to “In Reference to 'Improving the Safety of Opioid Use for Acute Noncancer Pain in Hospitalized Adults: A Consensus Statement from the Society of Hospital Medicine'”
Hall et al. draw attention to the important question of whether some patients may benefit from a naloxone prescription when discharged from the hospital with a short-term opioid prescription for acute pain. Although all members of the working group agreed that naloxone is appropriate in some cases, we were hesitant to recommend this as a standard practice for several reasons.
First, the intent of our Consensus Statement1 was to synthesize and summarize the areas of consensus in existing guidelines; none of the existing guidelines included in our systematic review make a recommendation for naloxone prescription in the setting of short-term opioid use for acute pain.2 We believe that this may relate to the fact that the risk factors for overdose and the threshold of risk above which naloxone would be beneficial have yet to be defined for this population and are likely to differ from those defined in patients using opioids chronically.
Additionally, if practitioners follow the recommendations to limit prescribing for acute pain to the minimum dose and duration of an opioid that was presumably administered in the hospital with an observed response, then the risk of overdose and the potential benefit of naloxone will decrease. Furthermore, emerging data from randomized controlled trials demonstrating noninferiority of nonopioid analgesics in the management of acute pain suggest that we should not so readily presume opioids to be the necessary or the best option.3-5 Data questioning the benefits of opioids over other safer therapies have particularly important implications for patients in whom the risks are felt to be high enough to warrant consideration of naloxone.
Disclosures
Dr. Herzig reports receiving compensation from the Society of Hospital Medicine for her editorial role in the Journal of Hospital Medicine (unrelated to the present work). None of the other authors have any conflicts of interest to disclose.
Funding
Dr. Herzig is funded by a grant number K23AG042459 from the National Institute on Aging. Dr. Mosher is supported in part by the Department of Veterans Affairs Office of Academic Affiliations and the Office of Research and Development and Health Services Research and Development Service (HSR&D) through the Comprehensive Access and Delivery Research and Evaluation Center (CIN 13-412). The views expressed in this manuscript do not necessarily represent the views of the funding agencies.
1. Herzig SJ, Mosher HJ, Calcaterra SL, Jena AB, Nuckols TK. Improving the safety of opioid use for acute noncancer pain in hospitalized adults: a consensus statement from the Society of Hospital Medicine. J Hosp Med. 2018;13(4):263-271. doi: 10.12788/jhm.2980. PubMed
2. Herzig SJ, Calcaterra SL, Mosher HJ, et al. Safe opioid prescribing for acute noncancer pain in hospitalized adults: a systematic review of existing guidelines.. J Hosp Med. 2018;13(4):256-262. doi: 10.12788/jhm.2979. PubMed
3. Chang AK, Bijur PE, Esses D, Barnaby DP, Baer J. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA. 2017;318(17):1661-1667. doi: 10.1001/jama.2017.16190. PubMed
4. Graudins A, Meek R, Parkinson J, Egerton-Warburton D, Meyer A. A randomised controlled trial of paracetamol and ibuprofen with or without codeine or oxycodone as initial analgesia for adults with moderate pain from limb injury. Emerg Med Australas. 2016;28(6):666-672. doi: 10.1111/1742-6723.12672 PubMed
5. Holdgate A, Pollock T. Nonsteroidal anti-inflammatory drugs (NSAIDs) versus opioids for acute renal colic. Cochrane Database Syst Rev. 2005:CD004137. doi: 10.1002/14651858.CD004137.pub3 PubMed
Hall et al. draw attention to the important question of whether some patients may benefit from a naloxone prescription when discharged from the hospital with a short-term opioid prescription for acute pain. Although all members of the working group agreed that naloxone is appropriate in some cases, we were hesitant to recommend this as a standard practice for several reasons.
First, the intent of our Consensus Statement1 was to synthesize and summarize the areas of consensus in existing guidelines; none of the existing guidelines included in our systematic review make a recommendation for naloxone prescription in the setting of short-term opioid use for acute pain.2 We believe that this may relate to the fact that the risk factors for overdose and the threshold of risk above which naloxone would be beneficial have yet to be defined for this population and are likely to differ from those defined in patients using opioids chronically.
Additionally, if practitioners follow the recommendations to limit prescribing for acute pain to the minimum dose and duration of an opioid that was presumably administered in the hospital with an observed response, then the risk of overdose and the potential benefit of naloxone will decrease. Furthermore, emerging data from randomized controlled trials demonstrating noninferiority of nonopioid analgesics in the management of acute pain suggest that we should not so readily presume opioids to be the necessary or the best option.3-5 Data questioning the benefits of opioids over other safer therapies have particularly important implications for patients in whom the risks are felt to be high enough to warrant consideration of naloxone.
Disclosures
Dr. Herzig reports receiving compensation from the Society of Hospital Medicine for her editorial role in the Journal of Hospital Medicine (unrelated to the present work). None of the other authors have any conflicts of interest to disclose.
Funding
Dr. Herzig is funded by a grant number K23AG042459 from the National Institute on Aging. Dr. Mosher is supported in part by the Department of Veterans Affairs Office of Academic Affiliations and the Office of Research and Development and Health Services Research and Development Service (HSR&D) through the Comprehensive Access and Delivery Research and Evaluation Center (CIN 13-412). The views expressed in this manuscript do not necessarily represent the views of the funding agencies.
Hall et al. draw attention to the important question of whether some patients may benefit from a naloxone prescription when discharged from the hospital with a short-term opioid prescription for acute pain. Although all members of the working group agreed that naloxone is appropriate in some cases, we were hesitant to recommend this as a standard practice for several reasons.
First, the intent of our Consensus Statement1 was to synthesize and summarize the areas of consensus in existing guidelines; none of the existing guidelines included in our systematic review make a recommendation for naloxone prescription in the setting of short-term opioid use for acute pain.2 We believe that this may relate to the fact that the risk factors for overdose and the threshold of risk above which naloxone would be beneficial have yet to be defined for this population and are likely to differ from those defined in patients using opioids chronically.
Additionally, if practitioners follow the recommendations to limit prescribing for acute pain to the minimum dose and duration of an opioid that was presumably administered in the hospital with an observed response, then the risk of overdose and the potential benefit of naloxone will decrease. Furthermore, emerging data from randomized controlled trials demonstrating noninferiority of nonopioid analgesics in the management of acute pain suggest that we should not so readily presume opioids to be the necessary or the best option.3-5 Data questioning the benefits of opioids over other safer therapies have particularly important implications for patients in whom the risks are felt to be high enough to warrant consideration of naloxone.
Disclosures
Dr. Herzig reports receiving compensation from the Society of Hospital Medicine for her editorial role in the Journal of Hospital Medicine (unrelated to the present work). None of the other authors have any conflicts of interest to disclose.
Funding
Dr. Herzig is funded by a grant number K23AG042459 from the National Institute on Aging. Dr. Mosher is supported in part by the Department of Veterans Affairs Office of Academic Affiliations and the Office of Research and Development and Health Services Research and Development Service (HSR&D) through the Comprehensive Access and Delivery Research and Evaluation Center (CIN 13-412). The views expressed in this manuscript do not necessarily represent the views of the funding agencies.
1. Herzig SJ, Mosher HJ, Calcaterra SL, Jena AB, Nuckols TK. Improving the safety of opioid use for acute noncancer pain in hospitalized adults: a consensus statement from the Society of Hospital Medicine. J Hosp Med. 2018;13(4):263-271. doi: 10.12788/jhm.2980. PubMed
2. Herzig SJ, Calcaterra SL, Mosher HJ, et al. Safe opioid prescribing for acute noncancer pain in hospitalized adults: a systematic review of existing guidelines.. J Hosp Med. 2018;13(4):256-262. doi: 10.12788/jhm.2979. PubMed
3. Chang AK, Bijur PE, Esses D, Barnaby DP, Baer J. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA. 2017;318(17):1661-1667. doi: 10.1001/jama.2017.16190. PubMed
4. Graudins A, Meek R, Parkinson J, Egerton-Warburton D, Meyer A. A randomised controlled trial of paracetamol and ibuprofen with or without codeine or oxycodone as initial analgesia for adults with moderate pain from limb injury. Emerg Med Australas. 2016;28(6):666-672. doi: 10.1111/1742-6723.12672 PubMed
5. Holdgate A, Pollock T. Nonsteroidal anti-inflammatory drugs (NSAIDs) versus opioids for acute renal colic. Cochrane Database Syst Rev. 2005:CD004137. doi: 10.1002/14651858.CD004137.pub3 PubMed
1. Herzig SJ, Mosher HJ, Calcaterra SL, Jena AB, Nuckols TK. Improving the safety of opioid use for acute noncancer pain in hospitalized adults: a consensus statement from the Society of Hospital Medicine. J Hosp Med. 2018;13(4):263-271. doi: 10.12788/jhm.2980. PubMed
2. Herzig SJ, Calcaterra SL, Mosher HJ, et al. Safe opioid prescribing for acute noncancer pain in hospitalized adults: a systematic review of existing guidelines.. J Hosp Med. 2018;13(4):256-262. doi: 10.12788/jhm.2979. PubMed
3. Chang AK, Bijur PE, Esses D, Barnaby DP, Baer J. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA. 2017;318(17):1661-1667. doi: 10.1001/jama.2017.16190. PubMed
4. Graudins A, Meek R, Parkinson J, Egerton-Warburton D, Meyer A. A randomised controlled trial of paracetamol and ibuprofen with or without codeine or oxycodone as initial analgesia for adults with moderate pain from limb injury. Emerg Med Australas. 2016;28(6):666-672. doi: 10.1111/1742-6723.12672 PubMed
5. Holdgate A, Pollock T. Nonsteroidal anti-inflammatory drugs (NSAIDs) versus opioids for acute renal colic. Cochrane Database Syst Rev. 2005:CD004137. doi: 10.1002/14651858.CD004137.pub3 PubMed
In Reference to “Improving the Safety of Opioid Use for Acute Noncancer Pain in Hospitalized Adults: A Consensus Statement from the Society of Hospital Medicine”
We read with great interest the consensus statement on improving the safety of opioid use for acute noncancer pain by Herzig et al.1 We strongly support the recommendations outlined in the document.
However, we would like to advocate for an additional recommendation that was considered but not included by the authors. Given the proven benefit—with minimal risk—in providing naloxone to patients and family members, we encourage naloxone prescriptions at discharge for all patients at risk for opioid overdose independent of therapy duration.2 Even opioid-naive patients who are prescribed opioids at hospital discharge have a significantly higher risk for chronic opioid use.3
We support extrapolating recommendations from the Centers for Disease Control and Prevention and Substance Abuse and Mental Health Services Administration to prescribe naloxone to all patients at discharge who are at risk for an opioid overdose, including those with a history of overdose or substance use disorder as well as those receiving a prescription of ≥50 mg morphine equivalents per day or who use opioids and benzodiazepines.4,5
Given the current barriers to healthcare access, prescribing naloxone at discharge may be a rare opportunity to provide a potential life-saving intervention to prevent a fatal opioid overdose.
Disclosures
We have no relevant conflicts of interest to report. No payment or services from a third party were received for any aspect of this submitted work. We have no financial relationships with entities in the biomedical arena that could be perceived to influence, or that give the appearance of potentially influencing, what was written in this submitted work.
1. Herzig SJ, Mosher HJ, Calcaterra SL, Jena AB, Nuckols TK. Improving the safety of opioid use for acute noncancer pain in hospitalized adults: a consensus statement from the society of hospital medicine. J Hosp Med. 2018;13(4);263-271. doi: 10.12788/jhm.2980. PubMed
2. McDonald R, Strang J. Are take-home naloxone programmes effective? Systematic review utilizing application of the Bradford Hill criteria. Addiction 2016;111(7):1177-1187. doi: 10.1111/add.13326. PubMed
3. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswanger IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2016;31(5):478-485. doi: 10.1007/s11606-015-3539-4. PubMed
4. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain--United States, 2016. JAMA. 2016;315(15):1624-1645. doi: 10.1001/jama.2016.1464. PubMed
5. Substance Abuse and Mental Health Services Administration. Medications for Opioid Use Disorder. Treatment Improvement Protocol (TIP) Series 63, Full Document. HHS Publication No. (SMA) 18- 5063FULLDOC. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2018. Available at: https://store.samhsa.gov/shin/content//SMA18-5063FULLDOC/SMA18-5063FULLDOC.pdf. Accessed April 12, 2018.
We read with great interest the consensus statement on improving the safety of opioid use for acute noncancer pain by Herzig et al.1 We strongly support the recommendations outlined in the document.
However, we would like to advocate for an additional recommendation that was considered but not included by the authors. Given the proven benefit—with minimal risk—in providing naloxone to patients and family members, we encourage naloxone prescriptions at discharge for all patients at risk for opioid overdose independent of therapy duration.2 Even opioid-naive patients who are prescribed opioids at hospital discharge have a significantly higher risk for chronic opioid use.3
We support extrapolating recommendations from the Centers for Disease Control and Prevention and Substance Abuse and Mental Health Services Administration to prescribe naloxone to all patients at discharge who are at risk for an opioid overdose, including those with a history of overdose or substance use disorder as well as those receiving a prescription of ≥50 mg morphine equivalents per day or who use opioids and benzodiazepines.4,5
Given the current barriers to healthcare access, prescribing naloxone at discharge may be a rare opportunity to provide a potential life-saving intervention to prevent a fatal opioid overdose.
Disclosures
We have no relevant conflicts of interest to report. No payment or services from a third party were received for any aspect of this submitted work. We have no financial relationships with entities in the biomedical arena that could be perceived to influence, or that give the appearance of potentially influencing, what was written in this submitted work.
We read with great interest the consensus statement on improving the safety of opioid use for acute noncancer pain by Herzig et al.1 We strongly support the recommendations outlined in the document.
However, we would like to advocate for an additional recommendation that was considered but not included by the authors. Given the proven benefit—with minimal risk—in providing naloxone to patients and family members, we encourage naloxone prescriptions at discharge for all patients at risk for opioid overdose independent of therapy duration.2 Even opioid-naive patients who are prescribed opioids at hospital discharge have a significantly higher risk for chronic opioid use.3
We support extrapolating recommendations from the Centers for Disease Control and Prevention and Substance Abuse and Mental Health Services Administration to prescribe naloxone to all patients at discharge who are at risk for an opioid overdose, including those with a history of overdose or substance use disorder as well as those receiving a prescription of ≥50 mg morphine equivalents per day or who use opioids and benzodiazepines.4,5
Given the current barriers to healthcare access, prescribing naloxone at discharge may be a rare opportunity to provide a potential life-saving intervention to prevent a fatal opioid overdose.
Disclosures
We have no relevant conflicts of interest to report. No payment or services from a third party were received for any aspect of this submitted work. We have no financial relationships with entities in the biomedical arena that could be perceived to influence, or that give the appearance of potentially influencing, what was written in this submitted work.
1. Herzig SJ, Mosher HJ, Calcaterra SL, Jena AB, Nuckols TK. Improving the safety of opioid use for acute noncancer pain in hospitalized adults: a consensus statement from the society of hospital medicine. J Hosp Med. 2018;13(4);263-271. doi: 10.12788/jhm.2980. PubMed
2. McDonald R, Strang J. Are take-home naloxone programmes effective? Systematic review utilizing application of the Bradford Hill criteria. Addiction 2016;111(7):1177-1187. doi: 10.1111/add.13326. PubMed
3. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswanger IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2016;31(5):478-485. doi: 10.1007/s11606-015-3539-4. PubMed
4. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain--United States, 2016. JAMA. 2016;315(15):1624-1645. doi: 10.1001/jama.2016.1464. PubMed
5. Substance Abuse and Mental Health Services Administration. Medications for Opioid Use Disorder. Treatment Improvement Protocol (TIP) Series 63, Full Document. HHS Publication No. (SMA) 18- 5063FULLDOC. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2018. Available at: https://store.samhsa.gov/shin/content//SMA18-5063FULLDOC/SMA18-5063FULLDOC.pdf. Accessed April 12, 2018.
1. Herzig SJ, Mosher HJ, Calcaterra SL, Jena AB, Nuckols TK. Improving the safety of opioid use for acute noncancer pain in hospitalized adults: a consensus statement from the society of hospital medicine. J Hosp Med. 2018;13(4);263-271. doi: 10.12788/jhm.2980. PubMed
2. McDonald R, Strang J. Are take-home naloxone programmes effective? Systematic review utilizing application of the Bradford Hill criteria. Addiction 2016;111(7):1177-1187. doi: 10.1111/add.13326. PubMed
3. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswanger IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2016;31(5):478-485. doi: 10.1007/s11606-015-3539-4. PubMed
4. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain--United States, 2016. JAMA. 2016;315(15):1624-1645. doi: 10.1001/jama.2016.1464. PubMed
5. Substance Abuse and Mental Health Services Administration. Medications for Opioid Use Disorder. Treatment Improvement Protocol (TIP) Series 63, Full Document. HHS Publication No. (SMA) 18- 5063FULLDOC. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2018. Available at: https://store.samhsa.gov/shin/content//SMA18-5063FULLDOC/SMA18-5063FULLDOC.pdf. Accessed April 12, 2018.
© 2018 Society of Hospital Medicine
A Shooting in the Hospital: When Domestic Violence Occurs in the Hospital, Reflection, and Response
On September 12, 2017, a son walked into his mother’s room in the surgical intensive care unit (ICU) of Dartmouth-Hitchcock Medical Center (DHMC) in Lebanon, New Hampshire, and shot her with a handgun. As an actively practicing hospitalist and the Chief Clinical Officer for DHMC, I immediately became involved with our hospitals’ response to domestic violence, a homicide, and an issue that to this point we felt lived outside our walls.
Several hospital systems are struggling with violence entering their institutions, particularly in their psychiatry and emergency service areas, fueled in part by untreated mental health and the rising opioid epidemic. Although gun violence in hospitals is indeed rare, inside the hospital, it occurs often in the emergency department.1 In New Hampshire, we suffer from a woefully underfunded state mental health infrastructure and one of the highest opioid death rates in the United States.2
DHMC is a 400-bed academic medical center, level 1 trauma center, and a National Cancer Institute (NCI)-designated cancer center that serves New Hampshire and eastern Vermont with its community and critical access hospitals and community group practices across the two states. With a wide geographic catchment area, our academic hospital at DHMC has one of the highest case-mix indices in the northeastern United States and is in the top 30 among hospitals of >300 beds in the United States.
After the shooting, the patient’s son left the ICU without targeting anyone else, and despite video surveillance systems, he was not seen leaving the hospital. At the same time, a Code Blue was called to address the victim and her needs. The Critical Care staff struggled to attend to and resuscitate the victim, and my Medicine team, on call that day, was paged and rushed to the ICU to assist. In a unit trained to manage the sequelae of trauma, this event was painfully surreal. Ultimately, the surgical critical-care physician, attending to the patient, ended the resuscitation efforts when it was clear that the patient, now a homicide victim, could not be saved.
With the shooter’s whereabouts unknown, a Code Silver (Active Shooter alert) was called. Then, following our “Run-Hide-Fight” training protocol, staff, patients, and visitors exited the building in large numbers and those that could not, sheltered in place. The operating room and the emergency department were secured and continued to function.
More than 160 law enforcement officers, including trained tactical and SWAT teams, from 13 different agencies arrived on scene. Ninety minutes after the shooting, the son was apprehended at a police traffic checkpoint, attempting to leave the hospital campus.
Our involvement in this event did not end at this point. Concerned about the possibility of other suspects or devices left in the hospital, the law enforcement officers swept our hospital. With a 1.2 million square foot campus, this would take another two hours, during which we still provided care to our patients and asked the staff and families to continue to seek safe shelter.
The shock of this terrible day was immediate and profound, leading to a thorough debrief and systematic analysis of how we might improve our processes and in turn help other organizations that might unfortunately face similar situations.
We reflected on how to better secure our hospital and to strengthen our coordination and collaboration with law enforcement. We increased our security presence not only in the ICU but also in our emergency department and developed individual unit-based security measures. We fast-tracked a unit-based shutdown plan that was already in process and increased our commitments to plan and drill for larger scenarios in conjunction with law enforcement agencies.
The physical location of our hospital was important in how our response unfolded. DHMC’s unique rural location in northern New England added challenges specific to our location, which may provide an opportunity for other hospitals to consider. Although we were able to provide care, water, and transport during this tragedy on a warm day in September, caring for thousands of people outside a hospital during a typical subzero February would be a different story.
Communication during the event and how specifically to ask people to act were identified as a key area of improvement. We realized that our language and training around the various codes lacked clarity and specificity. As is familiar to many, in our hospital with Red, Blue, Black, Purple, and White codes, some staff (and certainly families and visitors) were not sure what to do in a “Code Silver.” We worked to better define our language so that in a future event or in a drill, we would state in plain language that we have “an active shooter” or a “violence with weapons” event in progress with clear instructions on next steps. Our term “Run-Hide-Fight” was changed to “Avoid-Hide-Fight” to better reflect updated training and best practice for a future event. We revised our teaching and training materials and protocols, so that in the event of a similar situation, we could provide information in plain language, across numerous formats, and with some frequency to keep people apprised, even if the situation is not changing.
Our methods of ongoing communications were also reassessed. In our reviews, it became clear that the notification systems and the computer-based alerts seen on the computers of hospital staff were different from those at the medical school. Communication protocols on pagers and mobile phones and across social media such as Facebook and Twitter were redesigned. Though our institution has long had the ability to provide cell phone notifications during emergencies, not all employees and staff had elected to activate this feature. We also improved our speaker systems so that overhead paging and alerts could be heard outside the building.
Having improved personal reference materials on hand is important. We updated the cards attached to our ID badges with clear instructions about “active shooter” or “violence with weapon” situations. We also developed different response scenarios dependent on the campus location. An event in the ICU, for example, might require leaving the scene, although sheltering-in-place might be more appropriate for an offsite administrative building.
A significant challenge to our active-shooter situation was making sure that our staff, patients, visitors, and their families were adequately supported following the event. Learning from the experiences of other hospitals and communities, we undertook a deliberate process of preparedness and healing.3 From our surgical ICU to our distant community group practices, we provided communication and avenues for personal support. Our Employee Assistance Program provided 24/7 support in a conference room in the surgical ICU and in other areas, on and off site, for all staff at Dartmouth-Hitchcock. The shooting affected those in the vicinity, as well as far away. Staff who had experienced domestic and other violence in their past were impacted in ways that required special care and attention. Some who were in adjacent rooms during the event were able to return to work immediately, whereas other staff, in separate units and more distant clinics, struggled and required leaves of absence. Through this event, we witnessed the personal and deep psychological impact of such violence. We held town halls, updated daily communications from our Incident Command Team, and maintained an open dialog across the organization.
In reflection, it is challenging to face this experience without the greater context of what we unfortunately experience all too often in America today. We have seen the spectrum from the shootings at Marjory Stoneman Douglas High School in Parkland, Florida, to the isolated events that rarely reach our national news and collective consciousness. It seems that we have already experienced a shooting at a school every week in the US.
There is even an overlap seen in domestic and mass shootings as we saw in the Sandy Hook Elementary School shootings in 2012, in which the tragic event was preceded by the shooter murdering his mother in her home.4 Today, in the US, women are disproportionally the subject of domestic violence, and more than half of all killed are done so by a male family member. The presence of a gun in domestic violence situations increases the risk for homicide for women by 500%.5- 7 Our experience indeed mirrored this reality.
Many readers of this piece will recognize how similar their situation is to that of our hospital, that this happens elsewhere, not here. Although my institution has faced this as a tragedy that has tested our organization, one cannot also be deeply troubled by the greater impact of domestic and gun violence on healthcare and the American society today. Our staff and physicians have been witness and at times subject to such violence, and this experience has now made it even more poignant. Ultimately, and sadly, we feel that we are more prepared.
Disclosures
The author has nothing to disclose.
1. Kelen GD, Catlett CL, Kunitz JG, Hsieh YH. Hospital-based shootings in the United States: 2000 to 2011. Ann Emerg Med. 2012;60(6):790-798. doi: 10.1016/j.annemergmed.2012.08.012. PubMed
2. Center for Disease Control and Preventions (CDC) Drug Overdose Death Data. https://www.cdc.gov/drugoverdose/data/statedeaths.html. Accessed April 10, 2018
3. Van Den Bos J, Creten N, Davenport S, Roberts, M. Cost of community violence to hospitals and health systems. Report for the American Hospital Association. July 26, 2017
4. Krouse WJ, Richardson DJ. Mass murder with firearms: incidents and victims, 1999-2013. Congressional Research Service. https://fas.org/sgp/crs/misc/R44126.pdf. Accessed April 10, 2018
5. Campbell JC, Webster D, Koziol-McLain J, et al. Risk factors for femicide within physically abusive intimate relationships. Am J Public Health. 2003;93(7):1089-1097. https:/doi.org/10.2105/AJPH.93.7.1089.
6. Fox JA, Zawitz MW. Homicide trends in the United States: Bureau of Justice Statistics; 2009.
7. Federal Bureau of Investigation. 2014 Crime in their United States. https://ucr.fbi.gov/crime-in-the-u.s/2014/crime-in-the-u.s.-2014/cius-home. Accessed April 10, 2018
On September 12, 2017, a son walked into his mother’s room in the surgical intensive care unit (ICU) of Dartmouth-Hitchcock Medical Center (DHMC) in Lebanon, New Hampshire, and shot her with a handgun. As an actively practicing hospitalist and the Chief Clinical Officer for DHMC, I immediately became involved with our hospitals’ response to domestic violence, a homicide, and an issue that to this point we felt lived outside our walls.
Several hospital systems are struggling with violence entering their institutions, particularly in their psychiatry and emergency service areas, fueled in part by untreated mental health and the rising opioid epidemic. Although gun violence in hospitals is indeed rare, inside the hospital, it occurs often in the emergency department.1 In New Hampshire, we suffer from a woefully underfunded state mental health infrastructure and one of the highest opioid death rates in the United States.2
DHMC is a 400-bed academic medical center, level 1 trauma center, and a National Cancer Institute (NCI)-designated cancer center that serves New Hampshire and eastern Vermont with its community and critical access hospitals and community group practices across the two states. With a wide geographic catchment area, our academic hospital at DHMC has one of the highest case-mix indices in the northeastern United States and is in the top 30 among hospitals of >300 beds in the United States.
After the shooting, the patient’s son left the ICU without targeting anyone else, and despite video surveillance systems, he was not seen leaving the hospital. At the same time, a Code Blue was called to address the victim and her needs. The Critical Care staff struggled to attend to and resuscitate the victim, and my Medicine team, on call that day, was paged and rushed to the ICU to assist. In a unit trained to manage the sequelae of trauma, this event was painfully surreal. Ultimately, the surgical critical-care physician, attending to the patient, ended the resuscitation efforts when it was clear that the patient, now a homicide victim, could not be saved.
With the shooter’s whereabouts unknown, a Code Silver (Active Shooter alert) was called. Then, following our “Run-Hide-Fight” training protocol, staff, patients, and visitors exited the building in large numbers and those that could not, sheltered in place. The operating room and the emergency department were secured and continued to function.
More than 160 law enforcement officers, including trained tactical and SWAT teams, from 13 different agencies arrived on scene. Ninety minutes after the shooting, the son was apprehended at a police traffic checkpoint, attempting to leave the hospital campus.
Our involvement in this event did not end at this point. Concerned about the possibility of other suspects or devices left in the hospital, the law enforcement officers swept our hospital. With a 1.2 million square foot campus, this would take another two hours, during which we still provided care to our patients and asked the staff and families to continue to seek safe shelter.
The shock of this terrible day was immediate and profound, leading to a thorough debrief and systematic analysis of how we might improve our processes and in turn help other organizations that might unfortunately face similar situations.
We reflected on how to better secure our hospital and to strengthen our coordination and collaboration with law enforcement. We increased our security presence not only in the ICU but also in our emergency department and developed individual unit-based security measures. We fast-tracked a unit-based shutdown plan that was already in process and increased our commitments to plan and drill for larger scenarios in conjunction with law enforcement agencies.
The physical location of our hospital was important in how our response unfolded. DHMC’s unique rural location in northern New England added challenges specific to our location, which may provide an opportunity for other hospitals to consider. Although we were able to provide care, water, and transport during this tragedy on a warm day in September, caring for thousands of people outside a hospital during a typical subzero February would be a different story.
Communication during the event and how specifically to ask people to act were identified as a key area of improvement. We realized that our language and training around the various codes lacked clarity and specificity. As is familiar to many, in our hospital with Red, Blue, Black, Purple, and White codes, some staff (and certainly families and visitors) were not sure what to do in a “Code Silver.” We worked to better define our language so that in a future event or in a drill, we would state in plain language that we have “an active shooter” or a “violence with weapons” event in progress with clear instructions on next steps. Our term “Run-Hide-Fight” was changed to “Avoid-Hide-Fight” to better reflect updated training and best practice for a future event. We revised our teaching and training materials and protocols, so that in the event of a similar situation, we could provide information in plain language, across numerous formats, and with some frequency to keep people apprised, even if the situation is not changing.
Our methods of ongoing communications were also reassessed. In our reviews, it became clear that the notification systems and the computer-based alerts seen on the computers of hospital staff were different from those at the medical school. Communication protocols on pagers and mobile phones and across social media such as Facebook and Twitter were redesigned. Though our institution has long had the ability to provide cell phone notifications during emergencies, not all employees and staff had elected to activate this feature. We also improved our speaker systems so that overhead paging and alerts could be heard outside the building.
Having improved personal reference materials on hand is important. We updated the cards attached to our ID badges with clear instructions about “active shooter” or “violence with weapon” situations. We also developed different response scenarios dependent on the campus location. An event in the ICU, for example, might require leaving the scene, although sheltering-in-place might be more appropriate for an offsite administrative building.
A significant challenge to our active-shooter situation was making sure that our staff, patients, visitors, and their families were adequately supported following the event. Learning from the experiences of other hospitals and communities, we undertook a deliberate process of preparedness and healing.3 From our surgical ICU to our distant community group practices, we provided communication and avenues for personal support. Our Employee Assistance Program provided 24/7 support in a conference room in the surgical ICU and in other areas, on and off site, for all staff at Dartmouth-Hitchcock. The shooting affected those in the vicinity, as well as far away. Staff who had experienced domestic and other violence in their past were impacted in ways that required special care and attention. Some who were in adjacent rooms during the event were able to return to work immediately, whereas other staff, in separate units and more distant clinics, struggled and required leaves of absence. Through this event, we witnessed the personal and deep psychological impact of such violence. We held town halls, updated daily communications from our Incident Command Team, and maintained an open dialog across the organization.
In reflection, it is challenging to face this experience without the greater context of what we unfortunately experience all too often in America today. We have seen the spectrum from the shootings at Marjory Stoneman Douglas High School in Parkland, Florida, to the isolated events that rarely reach our national news and collective consciousness. It seems that we have already experienced a shooting at a school every week in the US.
There is even an overlap seen in domestic and mass shootings as we saw in the Sandy Hook Elementary School shootings in 2012, in which the tragic event was preceded by the shooter murdering his mother in her home.4 Today, in the US, women are disproportionally the subject of domestic violence, and more than half of all killed are done so by a male family member. The presence of a gun in domestic violence situations increases the risk for homicide for women by 500%.5- 7 Our experience indeed mirrored this reality.
Many readers of this piece will recognize how similar their situation is to that of our hospital, that this happens elsewhere, not here. Although my institution has faced this as a tragedy that has tested our organization, one cannot also be deeply troubled by the greater impact of domestic and gun violence on healthcare and the American society today. Our staff and physicians have been witness and at times subject to such violence, and this experience has now made it even more poignant. Ultimately, and sadly, we feel that we are more prepared.
Disclosures
The author has nothing to disclose.
On September 12, 2017, a son walked into his mother’s room in the surgical intensive care unit (ICU) of Dartmouth-Hitchcock Medical Center (DHMC) in Lebanon, New Hampshire, and shot her with a handgun. As an actively practicing hospitalist and the Chief Clinical Officer for DHMC, I immediately became involved with our hospitals’ response to domestic violence, a homicide, and an issue that to this point we felt lived outside our walls.
Several hospital systems are struggling with violence entering their institutions, particularly in their psychiatry and emergency service areas, fueled in part by untreated mental health and the rising opioid epidemic. Although gun violence in hospitals is indeed rare, inside the hospital, it occurs often in the emergency department.1 In New Hampshire, we suffer from a woefully underfunded state mental health infrastructure and one of the highest opioid death rates in the United States.2
DHMC is a 400-bed academic medical center, level 1 trauma center, and a National Cancer Institute (NCI)-designated cancer center that serves New Hampshire and eastern Vermont with its community and critical access hospitals and community group practices across the two states. With a wide geographic catchment area, our academic hospital at DHMC has one of the highest case-mix indices in the northeastern United States and is in the top 30 among hospitals of >300 beds in the United States.
After the shooting, the patient’s son left the ICU without targeting anyone else, and despite video surveillance systems, he was not seen leaving the hospital. At the same time, a Code Blue was called to address the victim and her needs. The Critical Care staff struggled to attend to and resuscitate the victim, and my Medicine team, on call that day, was paged and rushed to the ICU to assist. In a unit trained to manage the sequelae of trauma, this event was painfully surreal. Ultimately, the surgical critical-care physician, attending to the patient, ended the resuscitation efforts when it was clear that the patient, now a homicide victim, could not be saved.
With the shooter’s whereabouts unknown, a Code Silver (Active Shooter alert) was called. Then, following our “Run-Hide-Fight” training protocol, staff, patients, and visitors exited the building in large numbers and those that could not, sheltered in place. The operating room and the emergency department were secured and continued to function.
More than 160 law enforcement officers, including trained tactical and SWAT teams, from 13 different agencies arrived on scene. Ninety minutes after the shooting, the son was apprehended at a police traffic checkpoint, attempting to leave the hospital campus.
Our involvement in this event did not end at this point. Concerned about the possibility of other suspects or devices left in the hospital, the law enforcement officers swept our hospital. With a 1.2 million square foot campus, this would take another two hours, during which we still provided care to our patients and asked the staff and families to continue to seek safe shelter.
The shock of this terrible day was immediate and profound, leading to a thorough debrief and systematic analysis of how we might improve our processes and in turn help other organizations that might unfortunately face similar situations.
We reflected on how to better secure our hospital and to strengthen our coordination and collaboration with law enforcement. We increased our security presence not only in the ICU but also in our emergency department and developed individual unit-based security measures. We fast-tracked a unit-based shutdown plan that was already in process and increased our commitments to plan and drill for larger scenarios in conjunction with law enforcement agencies.
The physical location of our hospital was important in how our response unfolded. DHMC’s unique rural location in northern New England added challenges specific to our location, which may provide an opportunity for other hospitals to consider. Although we were able to provide care, water, and transport during this tragedy on a warm day in September, caring for thousands of people outside a hospital during a typical subzero February would be a different story.
Communication during the event and how specifically to ask people to act were identified as a key area of improvement. We realized that our language and training around the various codes lacked clarity and specificity. As is familiar to many, in our hospital with Red, Blue, Black, Purple, and White codes, some staff (and certainly families and visitors) were not sure what to do in a “Code Silver.” We worked to better define our language so that in a future event or in a drill, we would state in plain language that we have “an active shooter” or a “violence with weapons” event in progress with clear instructions on next steps. Our term “Run-Hide-Fight” was changed to “Avoid-Hide-Fight” to better reflect updated training and best practice for a future event. We revised our teaching and training materials and protocols, so that in the event of a similar situation, we could provide information in plain language, across numerous formats, and with some frequency to keep people apprised, even if the situation is not changing.
Our methods of ongoing communications were also reassessed. In our reviews, it became clear that the notification systems and the computer-based alerts seen on the computers of hospital staff were different from those at the medical school. Communication protocols on pagers and mobile phones and across social media such as Facebook and Twitter were redesigned. Though our institution has long had the ability to provide cell phone notifications during emergencies, not all employees and staff had elected to activate this feature. We also improved our speaker systems so that overhead paging and alerts could be heard outside the building.
Having improved personal reference materials on hand is important. We updated the cards attached to our ID badges with clear instructions about “active shooter” or “violence with weapon” situations. We also developed different response scenarios dependent on the campus location. An event in the ICU, for example, might require leaving the scene, although sheltering-in-place might be more appropriate for an offsite administrative building.
A significant challenge to our active-shooter situation was making sure that our staff, patients, visitors, and their families were adequately supported following the event. Learning from the experiences of other hospitals and communities, we undertook a deliberate process of preparedness and healing.3 From our surgical ICU to our distant community group practices, we provided communication and avenues for personal support. Our Employee Assistance Program provided 24/7 support in a conference room in the surgical ICU and in other areas, on and off site, for all staff at Dartmouth-Hitchcock. The shooting affected those in the vicinity, as well as far away. Staff who had experienced domestic and other violence in their past were impacted in ways that required special care and attention. Some who were in adjacent rooms during the event were able to return to work immediately, whereas other staff, in separate units and more distant clinics, struggled and required leaves of absence. Through this event, we witnessed the personal and deep psychological impact of such violence. We held town halls, updated daily communications from our Incident Command Team, and maintained an open dialog across the organization.
In reflection, it is challenging to face this experience without the greater context of what we unfortunately experience all too often in America today. We have seen the spectrum from the shootings at Marjory Stoneman Douglas High School in Parkland, Florida, to the isolated events that rarely reach our national news and collective consciousness. It seems that we have already experienced a shooting at a school every week in the US.
There is even an overlap seen in domestic and mass shootings as we saw in the Sandy Hook Elementary School shootings in 2012, in which the tragic event was preceded by the shooter murdering his mother in her home.4 Today, in the US, women are disproportionally the subject of domestic violence, and more than half of all killed are done so by a male family member. The presence of a gun in domestic violence situations increases the risk for homicide for women by 500%.5- 7 Our experience indeed mirrored this reality.
Many readers of this piece will recognize how similar their situation is to that of our hospital, that this happens elsewhere, not here. Although my institution has faced this as a tragedy that has tested our organization, one cannot also be deeply troubled by the greater impact of domestic and gun violence on healthcare and the American society today. Our staff and physicians have been witness and at times subject to such violence, and this experience has now made it even more poignant. Ultimately, and sadly, we feel that we are more prepared.
Disclosures
The author has nothing to disclose.
1. Kelen GD, Catlett CL, Kunitz JG, Hsieh YH. Hospital-based shootings in the United States: 2000 to 2011. Ann Emerg Med. 2012;60(6):790-798. doi: 10.1016/j.annemergmed.2012.08.012. PubMed
2. Center for Disease Control and Preventions (CDC) Drug Overdose Death Data. https://www.cdc.gov/drugoverdose/data/statedeaths.html. Accessed April 10, 2018
3. Van Den Bos J, Creten N, Davenport S, Roberts, M. Cost of community violence to hospitals and health systems. Report for the American Hospital Association. July 26, 2017
4. Krouse WJ, Richardson DJ. Mass murder with firearms: incidents and victims, 1999-2013. Congressional Research Service. https://fas.org/sgp/crs/misc/R44126.pdf. Accessed April 10, 2018
5. Campbell JC, Webster D, Koziol-McLain J, et al. Risk factors for femicide within physically abusive intimate relationships. Am J Public Health. 2003;93(7):1089-1097. https:/doi.org/10.2105/AJPH.93.7.1089.
6. Fox JA, Zawitz MW. Homicide trends in the United States: Bureau of Justice Statistics; 2009.
7. Federal Bureau of Investigation. 2014 Crime in their United States. https://ucr.fbi.gov/crime-in-the-u.s/2014/crime-in-the-u.s.-2014/cius-home. Accessed April 10, 2018
1. Kelen GD, Catlett CL, Kunitz JG, Hsieh YH. Hospital-based shootings in the United States: 2000 to 2011. Ann Emerg Med. 2012;60(6):790-798. doi: 10.1016/j.annemergmed.2012.08.012. PubMed
2. Center for Disease Control and Preventions (CDC) Drug Overdose Death Data. https://www.cdc.gov/drugoverdose/data/statedeaths.html. Accessed April 10, 2018
3. Van Den Bos J, Creten N, Davenport S, Roberts, M. Cost of community violence to hospitals and health systems. Report for the American Hospital Association. July 26, 2017
4. Krouse WJ, Richardson DJ. Mass murder with firearms: incidents and victims, 1999-2013. Congressional Research Service. https://fas.org/sgp/crs/misc/R44126.pdf. Accessed April 10, 2018
5. Campbell JC, Webster D, Koziol-McLain J, et al. Risk factors for femicide within physically abusive intimate relationships. Am J Public Health. 2003;93(7):1089-1097. https:/doi.org/10.2105/AJPH.93.7.1089.
6. Fox JA, Zawitz MW. Homicide trends in the United States: Bureau of Justice Statistics; 2009.
7. Federal Bureau of Investigation. 2014 Crime in their United States. https://ucr.fbi.gov/crime-in-the-u.s/2014/crime-in-the-u.s.-2014/cius-home. Accessed April 10, 2018
© 2018 Society of Hospital Medicine