User login
Management of Metastatic Gastric Cancer
INTRODUCTION
According to the Surveillance, Epidemiology and End Results database, in 2017 there were 28,000 new cases of gastric cancer, accounting for 1.8% of all malignancies in the United States, and an estimated 10,960 gastric cancer–related deaths.1 Worldwide, gastric cancer is the fifth most common malignancy and the third most common cause of death from any cancer.2 The incidence of gastric cancer varies significantly by geographic region, with countries in Eastern Asia (China, Japan), Eastern Europe, and Central and South America accounting for 50% of all new cases.3 Although the incidence of gastric cancer has declined in recent years, this decrease has not been observed consistently across all nations.2 In particular, the incidence of gastric cancers arising from the cardia has been increasing, which is perhaps due to a higher prevalence of obesity in Western societies.4
In this article, we review key aspects of management of metastatic gastric cancer, including selection of first- and second-line therapy, and discuss targeted agents and upcoming clinical trials.
EPIDEMIOLOGY AND RISK FACTORS
Chronic infection with Helicobacter pylori, a gram-negative bacterium, is a strong etiological factor for the development of gastric cancer, contributing to up to 70% of cases.2 The pathogen can colonize the gastric mucosa, leading to chronic inflammation. Although most patients remain asymptomatic, 1% to 3% develop gastric cancer and another 0.1% develop mucosa-associated lymphoid tissue lymphoma.5 H. pylori infection is more commonly associated with cancer of the gastric body than with cancer of the gastroesophageal junction (GEJ). The increased burden of gastric cancer in countries in Eastern Asia, Latin America, and Eastern Europe has been correlated to the prevalence of chronic H. pylori infection in these areas.
Carcinogenesis secondary to H. pylori infection may occur via several mechanisms. First, H. pylori can release virulence factors, such as cytotoxin-associated gene A, vacuolating cytotoxin, and outer membrane proteins, into the cytosol of host cells, leading to changes in patterns of cell proliferation and apoptosis.6 These virulence factors can modulate the host immune system, attenuating it to promote dysplasia. In addition, continued recognition of these factors by the immune system leads to a persistent inflammatory response, with the release of cytokines such as interleukin (IL) -1β, IL-6, and IL-8. This leads to chronic mucosal damage, further promoting dysplasia with eventual transformation into adenocarcinoma.7 In Japan and Korea, where screening for H. pylori infection is routinely performed, there have been improvements in overall survival (OS) rates for gastric cancer, with 5-year OS rates of 70%.8 The International Agency for Research on Cancer recommends further research into population-based screening and treatment programs for patients with chronic H. pylori infection. However, despite this recommendation, optimal screening strategies are not clearly defined.9
Other risk factors for the development of gastric cancer include chronic gastroesophageal reflux disease; smoking; alcohol use; exposure to radiation; diets high in fats, salt, and smoked items and low in fruits and vegetables; obesity; and exposure to chemotherapeutic agents such as procarbazine.10 Another pathogen suspected, but not proven, to be associated with increased risk for gastric cancer is the Epstein-Barr virus, a human herpesvirus found in 80% of all gastric carcinomas with lymphoid features.11 In addition, whether the use of medications such as statins and nonsteroidal anti-inflammatory drugs confers a decreased risk of gastric cancers remains unclear.10
EVALUATION
CASE PRESENTATION
A 55-year-old Caucasian man with a history of type 2 diabetes mellitus presents to the gastrointestinal (GI) clinic with a 6-month history of dysphagia. The dysphagia is worsened with ingestion of solids, particularly towards the end of the day. He states that the food often gets “stuck in the middle of the chest.” The patient denies any nausea or emesis but notes that he has a poor appetite. He reports having worsening mid-epigastric abdominal pain that is non-radiating, dull in character, and 6/10 in intensity. He also reports a 10-lb weight loss over the past 2 months. He has no previous history of reflux, chest pain, dyspnea, or cough. Review of systems is otherwise benign. Physical exam is within normal limits.
• Which tests should be conducted when gastric cancer is suspected?
Persistent epigastric abdominal pain and weight loss are the most common early symptoms of gastric cancer. Nausea, early satiety, dysphagia, and occult GI bleeding can be other presenting signs. Patients presenting with alarm symptoms of nausea, emesis, early satiety, abdominal pain, or weight loss should be fully evaluated with upper GI endoscopy. Early diagnosis of gastric cancer is essential in obtaining a curative resection. However, at least 40% of patients present with de novo metastatic disease at the time of initial diagnosis.12 Gastric cancer spreads by direct extension through the gastric wall, with the liver, peritoneum, and regional lymph nodes being the most common sites of metastatic deposits.13 Classically, Virchow’s node, the left supraclavicular lymph node, is involved with metastatic gastric cancer. Involvement of the left axillary lymph node (Irish node) or a periumbilical nodule (Sister Mary Joseph node) may also be observed. Other, less commonly noted sites of metastatic disease include the ovaries, central nervous system, bone, lung, and soft tissues.13
Upper GI endoscopy is the best method for determining tumor location and extent and obtaining a specimen for a definitive tissue diagnosis.14 It is essential to accurately identify the location of the tumor in the stomach and relative to the GEJ. The American Joint Committee on Cancer classification defines tumors involving the GEJ with an epicenter no more than 2 cm into the proximal stomach as esophageal cancers.15 Tumors of the GEJ with their epicenter more than 2 cm into the proximal stomach are defined as gastric cancers. If metastatic disease is suspected, computed tomography (CT) scan of the chest, abdomen, and pelvis with oral and intravenous contrast can be obtained to determine the extent of disease spread. In the absence of any metastatic disease, endoscopic ultrasound (EUS) should be conducted to determine the depth of tumor invasion (T staging) and lymph node status. In the era of targeted therapy, patients with metastatic disease should undergo testing for human epidermal growth factor-2 (HER-2) expression, microsatellite instability (MSI), and programmed death ligand 1 (PD-L1) expression. Patients should be staged according to the TNM staging system.
FIRST-LINE TREATMENT OPTIONS
CASE CONTINUED
The patient undergoes esophagoduodenoscopy (EGD) and is found to have a gastric cardia mass extending into the distal esophagus. EUS also demonstrates multiple abdominal and mediastinal lymph nodes. No gastric outlet obstruction is found. Biopsy shows poorly differentiated invasive adenocarcinoma. Warthin–Starry stain is negative for H. pylori organism. The tumor cells are positive for cytokeratin (CK7), CK19, and mucin-1 gene (MUC1); focally positive for CK20; and negative for MUC2. HER2 testing results are reported as immunohistochemistry (IHC) 3+, consistent with strongly positive HER2 protein expression. Further IHC testing for mismatch repair (MMR) proteins shows intact nuclear expression of MLH1, MSH2, MSH6, and PMS2 protein, consistent with a low probability of MSI-high tumor. The tumor is found to be PD-L1 positive. Imaging reveals abnormal mass-like nodular thickening of the gastric wall, with an infiltrative opacity within the pancreatico-duodenal groove, suspicious for tumor infiltration. Multiple metastatic deposits are noted in the liver, peritoneum, and bilateral lungs. There is extensive gastrohepatic ligament and periportal lymphadenopathy and mild enlargement of the pulmonary hilar lymph nodes. These findings are consistent with stage 4 (T4bN3aM1) gastric cancer. Given these findings, staging laparoscopy is deferred.
• What are the first-line treatment options for patients with metastatic gastric cancer?
Patients with metastatic gastric cancer have a poor prognosis, and management is stratified based on performance status (Figure). In patients with good performance status, systemic chemotherapy is the mainstay of treatment. The goal of therapy is not curative, but rather treatment focuses on palliation of symptoms arising from tumor spread. Given this treatment goal, there has been considerable interest in clarifying the utility of chemotherapy as opposed to best supportive care. In a recent Cochrane review of 64 randomized control trials involving 11,698 patients, chemotherapy was found to improve OS by 6.7 months as compared to best supportive care (hazard ratio [HR] 0.3 [95% confidence interval {CI} 0.24 to 0.55]).16 Five classes of cytotoxic chemotherapeutic agents have demonstrated activity in gastric cancer. These include fluoropyrimidine (either infusional fluorouracil or capecitabine), platinum agents (cisplatin or oxaliplatin), taxanes (docetaxel or paclitaxel), anthracyclines (epirubicin), and irinotecan.13 Treatment options are further divided based on whether the patient has HER2-overexpressing or non-expressing malignancy.
HER2-NEGATIVE DISEASE
For patients with HER2-negative disease, National Comprehensive Cancer Network (NCCN) guidelines recommend using 2-drug combination regimens rather than 3 drugs, given concern for increased toxicity with 3-drug regimens.17 For patients with a performance status of 0 to 1, utilization of a 3-drug regimen is a reasonable alternative. The combination of a fluoropyrimidine with a platinum agent is considered the standard of care, with regimens such as fluorouracil, leucovorin, and oxaliplatin (FOLFOX) being commonly used.
Epirubicin-containing regimens have also been extensively studied in advanced gastric cancer. In a study of 274 previously untreated patients with GEJ cancers, the combination of epirubicin, cisplatin, and fluorouracil (ECF) was compared to fluorouracil, doxorubicin, and methotrexate (FAMTX). There was an OS benefit favoring ECF (8.9 months versus 5.7 months) at 1 year (95% CI 27% to 45%, P = 0.0009). The ECF regimen was associated with an increased risk of nausea, emesis, and alopecia, while more hematologic toxicity and infections were noted with the FAMTX regimen.18 In addition, in a phase 3 trial, Van Cutsem and colleagues examined the role of docetaxel in combination with cisplatin and fluorouracil (DCF) compared to cisplatin and fluorouracil alone. Addition of docetaxel led to improved OS and time to progression (9.2 months versus 8.6 months for cisplatin and fluorouracil alone, P = 0.02) but with an increased risk of grade 3 and 4 toxicities (69% versus 59%). These adverse events included neutropenia (82% versus 57% of cisplatin and fluorouracil patients), diarrhea (19% versus 8%), stomatitis (21% versus 27%), and fatigue (19% versus 14%).19
The landmark phase 3 REAL-2 study compared 4 chemotherapy regimens in patients with untreated advanced esophagogastric cancer. This study was conducted to determine if the efficacy of cisplatin and oxaliplatin, a third-generation platinum agent, is equivalent to that of fluorouracil and capecitabine, an oral fluoropyrimidine. In this trial, a 2 × 2 design was used to compare 4 regimens: ECF versus epirubicin, cisplatin, and capecitabine (ECX) versus epirubicin, oxaliplatin, and fluorouracil (EOF) versus epirubicin, oxaliplatin, and capecitabine (EOX). The study found EOX to be noninferior to ECF, with a trend towards improved OS compared to other combination regimens (11.2 months versus 9.9 months, HR 0.80 [95% CI 0.66 to 0.97], P = 0.02).20 Thus, the study demonstrated that an oxaliplatin and capecitabine-based regimen could replace cisplatin and fluorouracil. Given that fluorouracil administration requires long continuous infusions, the oral-based capecitabine regimen is an attractive option for patients.
Several trials have demonstrated the equivalency of oxaliplatin with cisplatin in combination regimens for the treatment of advanced gastric cancer. Oxaliplatin has the benefit of an improved toxicity profile as compared to cisplatin, with the major dose-limiting toxicity being peripheral neuropathy
Given previous evidence that DCF (docetaxel, cisplatin, fluorouracil) is superior to cisplatin and fluorouracil alone, there was interest in determining if the addition of docetaxel to a backbone of fluorouracil, oxaliplatin, and leucovorin (FLO) could elicit a higher response rate. This concept was investigated in a phase 2 trial that assigned 54 patients with metastatic gastric or GEJ adenocarcinoma to receive biweekly infusions of oxaliplatin, leucovorin, fluorouracil, and docetaxel.21 Median time to response was 1.54 months, and the overall response rate was 57.7%. Median progression-free survival (PFS) was 5.2 months, and OS was 11.1 months. The most common grade 3 or 4 toxicities included neutropenia (48%), leukopenia (27.8%), diarrhea (14.8%), and fatigue (11.1%).
Irinotecan-based regimens have also been extensively studied in the first-line treatment of metastatic gastric cancer, particularly as an alternative to platinum-based therapy, but superiority has not been established. The combination of fluorouracil, leucovorin, and irinotecan (FOLFIRI) was compared to ECX in a phase 3 trial.22 The study enrolled 416 patients with locally advanced or metastatic gastric or GEJ cancer. At a median follow up of 31 months, the time to progression was longer in the FOLFIRI arm as compared to the ECX arm (5.1 months versus 4.2 months, P = 0.008), but there was no difference in OS (9.5 months versus 9.7 months, P = 0.95), median PFS (5.3 months versus 5.8 months, P = 0.96), or response rate (39.2% versus 37.8%). However, the FOLFIRI regimen had an improved toxicity profile, with a lower overall rate of grade 3 or 4 toxicity (69% versus 84%, P < 0.001). Given these findings, the FOLFIRI regimen is an acceptable alternative to platinum-based therapy in suitable patients.22
HER2-POSITIVE DISEASE
The HER2 proto-oncogene, initially described in breast cancer, has been implicated in several malignancies, including gastric and esophageal cancer. Overexpression or amplification of HER2 can be found in up to 30% of gastric cancers.23 For these patients, adding trastuzumab to a standard regimen of platinum and fluoropyrimidine is the standard of care. The prospective phase 3 Trastuzumab for Gastric Cancer (ToGA) trial randomly assigned 594 patients with HER2-positive gastric cancer to receive either cisplatin and fluorouracil or capecitabine and cisplatin with trastuzumab (n = 294) or without (n = 290) trastuzumab every 3 weeks for a total of 6 cycles, followed by maintenance trastuzumab until disease progression was noted.24 HER2 positivity was defined as HER2 protein overexpression by IHC (cutoff of 3+) or gene amplification by fluorescence in situ hybridization (FISH); tumors with IHC 2+ patterns were followed with FISH studies to confirm positivity. The study found a higher incidence of HER2-positive tumors in patients with GEJ tumors compared to patients with distal gastric cancers (33% versus 20%).24 In this trial, the addition of trastuzumab was associated with an improvement in OS: 13.5 months in the trastuzumab cohort versus 11.1 months in those receiving chemotherapy alone (HR 0.74, P = 0.0048). There was not a significant difference in toxicities between the 2 cohorts, with nausea, emesis, and neutropenia being the most common adverse events. Rates of overall grade 3 or 4 events were similar as well (68% in each cohort). Further exploratory analysis was also conducted according to HER2 status by dividing patients into a “high-expressor” group (n = 446), defined as patients with IHC 3+ tumors or IHC 2+ and FISH positivity, and a “low-expressor” group (n = 131), which included patients with IHC 0 or 1+ tumors. Analysis of patients in the 2 subgroups demonstrated an improved OS with the addition of trastuzumab for the high-expressor cohort, with a median OS of 16 months (HR 0.65 [95% CI 0.51 to 0.83]) compared to 11.8 months in those receiving only chemotherapy.
Dual HER2 blockade has been investigated in metastatic gastric cancer. The phase 3 randomized JACOB trial assigned 780 patients to receive either trastuzumab with a cisplatin/fluoropyrimidine regimen with or without the addition of pertuzumab; the primary end point was OS.25 A non-statistically significant trend towards improvement in OS was found in the pertuzumab arm (17.5 months) as compared with the standard of care arm (14.2 months, HR 0.84, P = 0.0565). The pertuzumab/trastuzumab/chemotherapy cohort experienced a higher incidence of diarrhea (61.6% versus 35.1% in control arm). Cardiac toxicity was comparable in the 2 cohorts.
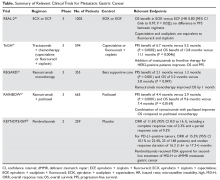
The Table provides a summary of relevant clinical trials in metastatic gastric cancer.
SECOND-LINE THERAPY
CASE CONTINUED
The patient receives capecitabine, oxaliplatin, and trastuzumab therapy for 6 cycles, followed by trastuzumab for another 3 cycles. While on therapy, he develops a painful right clavicular lesion. He undergoes magnetic resonance imaging of the right clavicle, which shows a lesion in the distal two-thirds of the right clavicle measuring 9.7 × 3.7 × 3.8 cm. The patient is started on palliative radiation to the clavicle. However, repeat CT imaging shows progressive liver metastases.
• What is the approach to second-line therapy for metastatic gastric cancer?
Improvements in our understanding of the molecular pathways that lead to tumorigenesis have contributed to the development of several targeted agents whose efficacy in gastric cancer is being investigated. The NCCN guidelines recommend that for all patients who progress on frontline therapy, second-line therapy consists of a combination of ramucirumab and paclitaxel. Other options include single-agent docetaxel, paclitaxel, irinotecan, or ramucirumab. Combination therapy using irinotecan with either docetaxel, fluorouracil, or cisplatin may also be used.
Ramucirumab, a human IgG1 monoclonal antibody that targets the vascular endothelial growth factor receptor 2 (VEGFR2), was initially approved in 2014 as monotherapy for patients who had previously progressed on first-line chemotherapy. Its approval was based on the results of the phase 3 randomized, double-blind placebo-controlled REGARD study.26 The trial randomly assigned 355 patients with advanced gastric or GEJ adenocarcinoma and disease progression after first-line platinum-containing or fluoropyrimidine-containing chemotherapy to receive best supportive care plus either ramucirumab (n = 238) or placebo (n = 117). Monotherapy with ramucirumab significantly improved median OS compared with placebo (5.2 months versus. 3.8 months; HR 0.776 [95% CI 0.6 to 0.99], P = 0.047). There was also an improvement in PFS of 2.1 months in the ramucirumab cohort, as compared to 1.3 months in the placebo cohort (P < 0.0001). Patients in the ramucirumab arm experienced a higher incidence of hypertension (16% versus 8%), but all other adverse events occurred at comparable rates. Five deaths in the ramucirumab group were thought to be secondary to the study drug, as compared to 2 deaths in the placebo group.
In the subsequent phase 3 RAINBOW trial, the addition of ramucirumab to paclitaxel was investigated, with 330 patients assigned to the combination group and 335 to the paclitaxel-only group.27 The trial again showed that combination therapy afforded patients a significant survival advantage compared to paclitaxel alone, with a median OS of 9.6 months versus 7.4 months for the monotherapy group (HR 0.807 [95% CI 0.678 to 0.962], P = 0.017). A PFS benefit of 4.4 months was observed in the combination therapy groups, as compared with 2.9 months in the monotherapy group (HR 0.635, P < 0.0001). The ramucirumab/paclitaxel group also had a higher overall response rate of 28% versus 16%. The combination cohort had an increased incidence of grade 3 or higher adverse hypertensive events (14% versus 2%) and neutropenia (41% versus 19%), while the incidence of grade 3 febrile neutropenic events was similar between the groups (3% versus 2%).
The addition of bevacizumab, another monoclonal antibody against VEGF, to standard chemotherapy regimens has been explored, but studies have failed to show a survival benefit with this agent in the first-line treatment of advanced gastric cancer. The phase 3 Avastin in Gastric Cancer (AVAGAST) trial was a multinational, randomized study where patients received either bevacizumab (n = 387) or placebo (n = 387) in addition to cisplatin and capecitabine.28 The substitution of fluorouracil for capecitabine was permitted for patients who were unable to tolerate oral medications. Cisplatin was administered for a maximum of 6 cycles, while capecitabine and bevacizumab were administered until disease progression. The study failed to show an improvement in OS, with a median OS of 12.1 months noted in the bevacizumab cohort, as compared to 10.1 months in the placebo arm (HR 0.87 [95% CI 0.73 to 1.03], P = 0.1002). However, there was a modest improvement in median PFS (6.7 months versus 5.3 months; HR 0.80 [95% CI 0.68 to 0.93], P = 0.0037) and overall response rate (46% versus 37.4%, P = 0.0315). The most commonly reported grade 3 to 5 adverse events included neutropenia (35%), anemia (10%), and loss of appetite (8%). Interestingly, in a follow-up report, higher serum levels of VEGF-A were thought to correlate with an enhanced response to bevacizumab.29 However, the routine use of biomarker analysis in selecting patients for treatment with bevacizumab in metastatic gastric cancer remains to be further clarified.
Use of other agents with anti-HER2 activity in the second-line treatment of patients who have experienced progression while on trastuzumab remains unclear. In the recent T-ACT trial, patients with disease refractory to frontline therapy with combination trastuzumab and fluoropyrimidine/platinum agents were randomly assigned to receive either weekly paclitaxel (n = 45) or weekly paclitaxel plus trastuzumab (n = 44).30 Patients in the combination cohort received an initial dose of trastuzumab 8 mg/kg followed by 6 mg/kg every 3 weeks until progression. The study did not find a difference in either PFS (3.19 months versus 3.68 months; HR 0.91 [95% CI 0.67 to 1.22], P = 0.33) or OS (9.95 months versus 10.2 months; HR 1.23 [95% CI 0.75 to 1.99], P = 0.20). The study thus failed to show a benefit to continuing trastuzumab after progression in the first-line setting.
Lapatinib in combination with paclitaxel has been compared to paclitaxel alone for the treatment of advanced HER2-positive gastric cancer in an Asian population in the phase 3 TyTAN trial.31 With a primary end point of OS, the study randomly assigned 129 patients to receive paclitaxel alone and 132 patients to receive paclitaxel with lapatinib. There was a nonsignificant trend towards improvement in OS in the combination group (11 months) as compared to the paclitaxel-only group(8.9 months, P = 0.1044), with no significant difference in median PFS (5.4 months versus 4.4 months). However, it is important to note that only 15 patients in this trial had previously been exposed to trastuzumab. Another trial, the phase 3 GATSBY study, examined the efficacy of trastuzumab emtansine in the second-line setting compared to taxanes alone and failed to show any improvement in PFS or OS.32 Given these results, no alternative anti-HER2 therapy has been proven to be efficacious for patients who are trastuzumab refractory. Therefore, including anti-HER2 therapy in the second-line treatment of HER2-positive gastric cancer is not recommended.
IMMUNOTHERAPY AND OTHER TARGETED THERAPIES
Several other targeted therapies have been studied in advanced gastric cancer, without any demonstrable survival benefit. The PI3K/AKT/mTOR pathway is known to be involved in regulation of cell growth and angiogenesis, and the mTOR inhibitor everolimus is widely used to treat other malignancies, including breast cancer. The use of everolimus in the second-line setting was studied in the phase 3 GRANITE-1 trial, where it was compared to best supportive care and failed to provide any survival benefit.33 Cetuximab, a recombinant human and mouse chimeric monoclonal antibody, and panitumumab, a recombinant human antibody against the epidermal growth factor receptor (EGFR), have also been examined in gastric and GEJ cancer patients. However, the large phase 3 EXPAND and REAL-3 trials did not show a survival benefit when these agents were added to standard chemotherapy.34,35
Overexpression of MET, a proto-oncogene and tyrosine kinase receptor, has also been implicated in gastric cancer progression. The ligand for MET is the hepatocyte growth factor (HGF), and aberrant signaling of this pathway has been shown to correlate with an aggressive gastric cancer phenotype and poorer OS by promoting tumor growth and angiogenesis. However, no MET inhibitors thus far have been found to be clinically effective. RILOMET-1 and RILOMET-2 were phase 3 trials examining the efficacy of rilotumumab, a humanized anti-HGF antibody, in combination with chemotherapy (ECX and cisplatin with capecitabine, respectively) for the frontline treatment of MET-positive GEJ and gastric cancers. Both studies were discontinued due to a higher treatment-related mortality in patients receiving rilotumumab, with a higher incidence of adverse events due to disease progression being noted.36 Similarly, onartuzumab, a monovalent monoclonal antibody against the MET receptor, was investigated in the phase 3 METGastric trial in combination with modified FOLFOX6 as first-line therapy for HER2-negative, MET-positive metastatic GEJ and gastric cancers. The study did not demonstrate any significant improvements in OS or PFS.37
There has been significant interest in incorporating immunotherapy in the treatment of early and metastatic gastric cancer. Pembrolizumab is the first programmed death receptor (PD-1) inhibitor to be approved for treatment of patients with PD-L1−positive advanced gastric cancer who had previously received 2 or more lines of chemotherapy. Although earlier studies of pembrolizumab in lung cancer utilized the tumor proportion score (TPS) to determine PD-L1 positivity, this was not found to be applicable to gastric cancer. Instead, the combined positive score (CPS) is used in gastric cancer. The CPS evaluates the number of tumor cells and immune cells (macrophages and lymphocytes) that stain positive for PD-L1 relative to all viable tumor cells. Comparatively, the TPS only examines the percentage of viable tumor cells that show complete or partial positive staining for PD-L1. A CPS score of 1 or greater identifies patients who would be suitable candidates for pembrolizumab.
The approval of pembrolizumab was based on the positive findings from the recent KEYNOTE-059 trial.38 The study included 259 patients who had previously received either fluoropyrimidine, cisplatin, or anti-HER2 therapy, with 148 patients (55%) of these patients having PD-L1−positive tumors. The PD-L1 status was determined using a pharmDx Kit, which is now approved by the US Food and Drug Administration to select patients who could benefit from pembrolizumab treatment. CPS was calculated as the number of PD-L1−staining cells divided by the total number of evaluated cells. The study included patients with microsatellite stable (MSI-S), undetermined, or deficient MMR status. The overall response rate to pembrolizumab across all patients was 11.6%, median PFS was 2 months, and the 12-month OS rate was 23.4%. In the subset of patients with MSI-H tumors, the overall response rate was 57.1%, with a complete response rate of 14.3%; in those with MSI-S tumors, the overall response rate was 9% and the complete response rate was 2.4%. Among patients with PD-L1–positive tumors, the overall response rate was 15.5% (95% CI 10.1% to 22.4). Common adverse events included fatigue, hypothyroidism, nausea, diarrhea, and arthralgia.38
CASE CONCLUSION
This patient with metastatic gastric cancer receives second-line chemotherapy with ramucirumab and paclitaxel. Follow-up imaging shows persistent liver metastases and new lung metastasis. Because the tumor is PD-L1–positive, the patient receives 4 cycles of pembrolizumab, with no significant change noted in disease burden. He notes a significant decline in functional status with increased weight loss, nausea, emesis, and fatigue. The patient opts to forego any further therapy and instead chooses to pursue supportive care only.
SUMMARY
Gastric cancer is the third most common cause of cancer death worldwide. Common risk factors for developing gastric cancer include H. pylori infection, smoking, alcohol abuse, radiation exposure, high-fat diet, and obesity. Patients presenting with alarm symptoms of nausea, emesis, early satiety, abdominal pain, or weight loss should be fully evaluated with upper GI endoscopy. If there is suspicion for metastatic disease, CT evaluation of the chest, abdomen, and pelvis with oral and intravenous contrast should be obtained. Treatment of patients with metastatic gastric cancer is guided by their performance status at presentation. For patients with good performance status, a combination of platinum and fluoropyrimidine therapy, such as FOLFOX, can be considered. Doublet chemotherapy regimens are preferred over triplet chemotherapy regimens given their better tolerability. For patients with HER2-positive disease, the addition of trastuzumab to the platinum and fluoropyrimidine backbone is the standard of care in the first line.
Several targeted agents have been studied in patients progressing on initial therapy, with ramucirumab and paclitaxel being considered the regimen of choice in the second line. No anti-HER2 therapy has been approved for patients who are refractory to trastuzumab. Pembrolizumab is approved for use in patients who are PD-L1–positive and have previously progressed on at least 2 lines of chemotherapy. Pembrolizumab is also approved for the treatment of patients with unresectable or metastatic, MSI-H or MMR-deficient gastric cancers that have progressed after prior treatment and who have no satisfactory alternative treatment options.
1. Noone AM, Cronin KA, Altekruse SF, et al. Cancer incidence and survival trends by subtype using data from the Surveillance Epidemiology and End Results Program, 1992-2013. Cancer Epidemiol Biomarkers Prev 2017;26:632–41
2. Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol 2017;3:524–8.
3. Sitarz R, Skierucha M, Mielko J, et al. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res 2018;10:239–48.
4. Olefson S, Moss SF. Obesity and related risk factors in gastric cardia adenocarcinoma. Gastric Cancer 2015;18:23–32.
5. Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett 2014;345:196–202.
6. Espinoza JL, Matsumoto A, Tanaka H, Matsumura I. Gastric microbiota: An emerging player in Helicobacter pylori-induced gastric malignancies. Cancer Lett 2018;414:147–52.
7. Chmiela M, Gonciarz W. Molecular mimicry in Helicobacter pylori infections. World J Gastroenterol 2017;23:3964–77.
8. Isobe Y, Nashimoto A, Akazawa K, et al. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer 2011;14:301–16.
9. Ford A, Gurusamy KS, Delaney B, et al. Eradication therapy for peptic ulcer disease in Helicobacter pylori-positive patients. Cochrane Database Syst Rev 2004(4):CD003840.
10. Karimi P, Islami F, Anandasabapathy S, et al. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 2014;23:700–13.
11. Van Cutsem E, Sagaert X, Topal B, et al. Gastric cancer. Lancet 2016;388:2654–64.
12. Chan BA , Sim HW, Natori A, et al. Survival outcomes for de novo versus relapsed stage IV gastric and gastroesophageal junction (GEJ) adenocarcinoma [abstract]. J Clin Oncol 2018;36(no. 4 suppl):148.
13. DeVita VT, Lawrence TS, Rosenberg SA. DeVita, Hellman, and Rosenberg’s cancer: principles & practice of oncology. 9th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2011.
14. Siewert JR, Hölscher AH, Becker K, Gössner W. [Cardia cancer: attempt at a therapeutically relevant classification]. [Article in German.] Chirurg 1987;58:25–32.
15. Amin MB, Edge SB, Greene FL, et al, eds. AJCC cancer staging manual. 8th ed. New York: Springer; 2017.
16. Wagner AD, Syn NL, Moehler M, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 2017;8:CD004064.
17. Qiu H, Zhou Z. [Updates and interpretation on NCCN clinical practice guidelines for gastric cancer 2017 version 5]. [Article in Chinese.] Zhonghua Wei Chang Wai Ke Za Zhi 2018;21:160–4.
18. Webb A, Cunningham D, Scarffe JH, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol 1997;15:261–7.
19. Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006;24:4991–7.
20. Cunningham D, Okines AF, Ashley S. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2010;362:858–9.
21. Al-Batran SE, Hartmann JT, Hofheinz R, et al. Biweekly fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) for patients with metastatic adenocarcinoma of the stomach or esophagogastric junction: a phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol 2008;19:1882–7.
22. Guimbaud R, Louvet C, Ries P, et al. Prospective, randomized, multicenter, phase III study of fluorouracil, leucovorin, and irinotecan versus epirubicin, cisplatin, and capecitabine in advanced gastric adenocarcinoma: a French intergroup (Federation Francophone de Cancerologie Digestive, Federation Nationale des Centres de Lutte Contre le Cancer, and Groupe Cooperateur Multidisciplinaire en Oncologie) study. J Clin Oncol 2014;32:3520–6.
23. Boku N. HER2-positive gastric cancer. Gastric Cancer 2014;17:1–12.
24. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687–97.
25. Tabernero J, Hoff PM, Shen L, et al. Pertuzumab + trastuzumab + chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer: Final analysis of a Phase III study (JACOB) [abstract]. Ann Oncol 2017;28(suppl 5):6160.
26. Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31–9.
27. Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224–35.
28. Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968–76.
29. Van Cutsem E, de Haas S, Kang YK, et al, Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol 2012;30:2119–27.
30. Makiyama A, Sagara K, Kawada J, et al. A randomized phase II study of weekly paclitaxel ± trastuzumab in patients with HER2-positive advanced gastric or gastro-esophageal junction cancer refractory to trastuzumab combined with fluoropyrimidine and platinum: WJOG7112G (T-ACT) [abstract]. J Clin Oncol 2018;36(no. 15 suppl):4011.
31. Satoh T, Xu RH, Chung HC, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol 2014;32:2039–49.
32. Thuss-Patience PC, Shah MA, Ohtsu A, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol 2017;18:640–53.
33. Ohtsu A, Ajani JA, Bai YX, et al. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol 2013;31:3935–43.
34. Lordick F, Kang YK, Chung HC, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol 2013;14:490–9.
35. Waddell T, Chau I, Cunningham D, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol 2013;14:481–9.
36. Catenacci DVT, Tebbutt NC, Davidenko I, et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:1467–82.
37. Shah MA, Bang YJ, Lordick F, et al. Effect of fluorouracil, leucovorin, and oxaliplatin with or without onartuzumab in HER2-negative, MET-positive gastroesophageal adenocarcinoma: the METGastric randomized clinical trial. JAMA Oncol 2017;3:620–7.
38. Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol 2018;4(5):e180013.
INTRODUCTION
According to the Surveillance, Epidemiology and End Results database, in 2017 there were 28,000 new cases of gastric cancer, accounting for 1.8% of all malignancies in the United States, and an estimated 10,960 gastric cancer–related deaths.1 Worldwide, gastric cancer is the fifth most common malignancy and the third most common cause of death from any cancer.2 The incidence of gastric cancer varies significantly by geographic region, with countries in Eastern Asia (China, Japan), Eastern Europe, and Central and South America accounting for 50% of all new cases.3 Although the incidence of gastric cancer has declined in recent years, this decrease has not been observed consistently across all nations.2 In particular, the incidence of gastric cancers arising from the cardia has been increasing, which is perhaps due to a higher prevalence of obesity in Western societies.4
In this article, we review key aspects of management of metastatic gastric cancer, including selection of first- and second-line therapy, and discuss targeted agents and upcoming clinical trials.
EPIDEMIOLOGY AND RISK FACTORS
Chronic infection with Helicobacter pylori, a gram-negative bacterium, is a strong etiological factor for the development of gastric cancer, contributing to up to 70% of cases.2 The pathogen can colonize the gastric mucosa, leading to chronic inflammation. Although most patients remain asymptomatic, 1% to 3% develop gastric cancer and another 0.1% develop mucosa-associated lymphoid tissue lymphoma.5 H. pylori infection is more commonly associated with cancer of the gastric body than with cancer of the gastroesophageal junction (GEJ). The increased burden of gastric cancer in countries in Eastern Asia, Latin America, and Eastern Europe has been correlated to the prevalence of chronic H. pylori infection in these areas.
Carcinogenesis secondary to H. pylori infection may occur via several mechanisms. First, H. pylori can release virulence factors, such as cytotoxin-associated gene A, vacuolating cytotoxin, and outer membrane proteins, into the cytosol of host cells, leading to changes in patterns of cell proliferation and apoptosis.6 These virulence factors can modulate the host immune system, attenuating it to promote dysplasia. In addition, continued recognition of these factors by the immune system leads to a persistent inflammatory response, with the release of cytokines such as interleukin (IL) -1β, IL-6, and IL-8. This leads to chronic mucosal damage, further promoting dysplasia with eventual transformation into adenocarcinoma.7 In Japan and Korea, where screening for H. pylori infection is routinely performed, there have been improvements in overall survival (OS) rates for gastric cancer, with 5-year OS rates of 70%.8 The International Agency for Research on Cancer recommends further research into population-based screening and treatment programs for patients with chronic H. pylori infection. However, despite this recommendation, optimal screening strategies are not clearly defined.9
Other risk factors for the development of gastric cancer include chronic gastroesophageal reflux disease; smoking; alcohol use; exposure to radiation; diets high in fats, salt, and smoked items and low in fruits and vegetables; obesity; and exposure to chemotherapeutic agents such as procarbazine.10 Another pathogen suspected, but not proven, to be associated with increased risk for gastric cancer is the Epstein-Barr virus, a human herpesvirus found in 80% of all gastric carcinomas with lymphoid features.11 In addition, whether the use of medications such as statins and nonsteroidal anti-inflammatory drugs confers a decreased risk of gastric cancers remains unclear.10
EVALUATION
CASE PRESENTATION
A 55-year-old Caucasian man with a history of type 2 diabetes mellitus presents to the gastrointestinal (GI) clinic with a 6-month history of dysphagia. The dysphagia is worsened with ingestion of solids, particularly towards the end of the day. He states that the food often gets “stuck in the middle of the chest.” The patient denies any nausea or emesis but notes that he has a poor appetite. He reports having worsening mid-epigastric abdominal pain that is non-radiating, dull in character, and 6/10 in intensity. He also reports a 10-lb weight loss over the past 2 months. He has no previous history of reflux, chest pain, dyspnea, or cough. Review of systems is otherwise benign. Physical exam is within normal limits.
• Which tests should be conducted when gastric cancer is suspected?
Persistent epigastric abdominal pain and weight loss are the most common early symptoms of gastric cancer. Nausea, early satiety, dysphagia, and occult GI bleeding can be other presenting signs. Patients presenting with alarm symptoms of nausea, emesis, early satiety, abdominal pain, or weight loss should be fully evaluated with upper GI endoscopy. Early diagnosis of gastric cancer is essential in obtaining a curative resection. However, at least 40% of patients present with de novo metastatic disease at the time of initial diagnosis.12 Gastric cancer spreads by direct extension through the gastric wall, with the liver, peritoneum, and regional lymph nodes being the most common sites of metastatic deposits.13 Classically, Virchow’s node, the left supraclavicular lymph node, is involved with metastatic gastric cancer. Involvement of the left axillary lymph node (Irish node) or a periumbilical nodule (Sister Mary Joseph node) may also be observed. Other, less commonly noted sites of metastatic disease include the ovaries, central nervous system, bone, lung, and soft tissues.13
Upper GI endoscopy is the best method for determining tumor location and extent and obtaining a specimen for a definitive tissue diagnosis.14 It is essential to accurately identify the location of the tumor in the stomach and relative to the GEJ. The American Joint Committee on Cancer classification defines tumors involving the GEJ with an epicenter no more than 2 cm into the proximal stomach as esophageal cancers.15 Tumors of the GEJ with their epicenter more than 2 cm into the proximal stomach are defined as gastric cancers. If metastatic disease is suspected, computed tomography (CT) scan of the chest, abdomen, and pelvis with oral and intravenous contrast can be obtained to determine the extent of disease spread. In the absence of any metastatic disease, endoscopic ultrasound (EUS) should be conducted to determine the depth of tumor invasion (T staging) and lymph node status. In the era of targeted therapy, patients with metastatic disease should undergo testing for human epidermal growth factor-2 (HER-2) expression, microsatellite instability (MSI), and programmed death ligand 1 (PD-L1) expression. Patients should be staged according to the TNM staging system.
FIRST-LINE TREATMENT OPTIONS
CASE CONTINUED
The patient undergoes esophagoduodenoscopy (EGD) and is found to have a gastric cardia mass extending into the distal esophagus. EUS also demonstrates multiple abdominal and mediastinal lymph nodes. No gastric outlet obstruction is found. Biopsy shows poorly differentiated invasive adenocarcinoma. Warthin–Starry stain is negative for H. pylori organism. The tumor cells are positive for cytokeratin (CK7), CK19, and mucin-1 gene (MUC1); focally positive for CK20; and negative for MUC2. HER2 testing results are reported as immunohistochemistry (IHC) 3+, consistent with strongly positive HER2 protein expression. Further IHC testing for mismatch repair (MMR) proteins shows intact nuclear expression of MLH1, MSH2, MSH6, and PMS2 protein, consistent with a low probability of MSI-high tumor. The tumor is found to be PD-L1 positive. Imaging reveals abnormal mass-like nodular thickening of the gastric wall, with an infiltrative opacity within the pancreatico-duodenal groove, suspicious for tumor infiltration. Multiple metastatic deposits are noted in the liver, peritoneum, and bilateral lungs. There is extensive gastrohepatic ligament and periportal lymphadenopathy and mild enlargement of the pulmonary hilar lymph nodes. These findings are consistent with stage 4 (T4bN3aM1) gastric cancer. Given these findings, staging laparoscopy is deferred.
• What are the first-line treatment options for patients with metastatic gastric cancer?
Patients with metastatic gastric cancer have a poor prognosis, and management is stratified based on performance status (Figure). In patients with good performance status, systemic chemotherapy is the mainstay of treatment. The goal of therapy is not curative, but rather treatment focuses on palliation of symptoms arising from tumor spread. Given this treatment goal, there has been considerable interest in clarifying the utility of chemotherapy as opposed to best supportive care. In a recent Cochrane review of 64 randomized control trials involving 11,698 patients, chemotherapy was found to improve OS by 6.7 months as compared to best supportive care (hazard ratio [HR] 0.3 [95% confidence interval {CI} 0.24 to 0.55]).16 Five classes of cytotoxic chemotherapeutic agents have demonstrated activity in gastric cancer. These include fluoropyrimidine (either infusional fluorouracil or capecitabine), platinum agents (cisplatin or oxaliplatin), taxanes (docetaxel or paclitaxel), anthracyclines (epirubicin), and irinotecan.13 Treatment options are further divided based on whether the patient has HER2-overexpressing or non-expressing malignancy.
HER2-NEGATIVE DISEASE
For patients with HER2-negative disease, National Comprehensive Cancer Network (NCCN) guidelines recommend using 2-drug combination regimens rather than 3 drugs, given concern for increased toxicity with 3-drug regimens.17 For patients with a performance status of 0 to 1, utilization of a 3-drug regimen is a reasonable alternative. The combination of a fluoropyrimidine with a platinum agent is considered the standard of care, with regimens such as fluorouracil, leucovorin, and oxaliplatin (FOLFOX) being commonly used.
Epirubicin-containing regimens have also been extensively studied in advanced gastric cancer. In a study of 274 previously untreated patients with GEJ cancers, the combination of epirubicin, cisplatin, and fluorouracil (ECF) was compared to fluorouracil, doxorubicin, and methotrexate (FAMTX). There was an OS benefit favoring ECF (8.9 months versus 5.7 months) at 1 year (95% CI 27% to 45%, P = 0.0009). The ECF regimen was associated with an increased risk of nausea, emesis, and alopecia, while more hematologic toxicity and infections were noted with the FAMTX regimen.18 In addition, in a phase 3 trial, Van Cutsem and colleagues examined the role of docetaxel in combination with cisplatin and fluorouracil (DCF) compared to cisplatin and fluorouracil alone. Addition of docetaxel led to improved OS and time to progression (9.2 months versus 8.6 months for cisplatin and fluorouracil alone, P = 0.02) but with an increased risk of grade 3 and 4 toxicities (69% versus 59%). These adverse events included neutropenia (82% versus 57% of cisplatin and fluorouracil patients), diarrhea (19% versus 8%), stomatitis (21% versus 27%), and fatigue (19% versus 14%).19
The landmark phase 3 REAL-2 study compared 4 chemotherapy regimens in patients with untreated advanced esophagogastric cancer. This study was conducted to determine if the efficacy of cisplatin and oxaliplatin, a third-generation platinum agent, is equivalent to that of fluorouracil and capecitabine, an oral fluoropyrimidine. In this trial, a 2 × 2 design was used to compare 4 regimens: ECF versus epirubicin, cisplatin, and capecitabine (ECX) versus epirubicin, oxaliplatin, and fluorouracil (EOF) versus epirubicin, oxaliplatin, and capecitabine (EOX). The study found EOX to be noninferior to ECF, with a trend towards improved OS compared to other combination regimens (11.2 months versus 9.9 months, HR 0.80 [95% CI 0.66 to 0.97], P = 0.02).20 Thus, the study demonstrated that an oxaliplatin and capecitabine-based regimen could replace cisplatin and fluorouracil. Given that fluorouracil administration requires long continuous infusions, the oral-based capecitabine regimen is an attractive option for patients.
Several trials have demonstrated the equivalency of oxaliplatin with cisplatin in combination regimens for the treatment of advanced gastric cancer. Oxaliplatin has the benefit of an improved toxicity profile as compared to cisplatin, with the major dose-limiting toxicity being peripheral neuropathy
Given previous evidence that DCF (docetaxel, cisplatin, fluorouracil) is superior to cisplatin and fluorouracil alone, there was interest in determining if the addition of docetaxel to a backbone of fluorouracil, oxaliplatin, and leucovorin (FLO) could elicit a higher response rate. This concept was investigated in a phase 2 trial that assigned 54 patients with metastatic gastric or GEJ adenocarcinoma to receive biweekly infusions of oxaliplatin, leucovorin, fluorouracil, and docetaxel.21 Median time to response was 1.54 months, and the overall response rate was 57.7%. Median progression-free survival (PFS) was 5.2 months, and OS was 11.1 months. The most common grade 3 or 4 toxicities included neutropenia (48%), leukopenia (27.8%), diarrhea (14.8%), and fatigue (11.1%).
Irinotecan-based regimens have also been extensively studied in the first-line treatment of metastatic gastric cancer, particularly as an alternative to platinum-based therapy, but superiority has not been established. The combination of fluorouracil, leucovorin, and irinotecan (FOLFIRI) was compared to ECX in a phase 3 trial.22 The study enrolled 416 patients with locally advanced or metastatic gastric or GEJ cancer. At a median follow up of 31 months, the time to progression was longer in the FOLFIRI arm as compared to the ECX arm (5.1 months versus 4.2 months, P = 0.008), but there was no difference in OS (9.5 months versus 9.7 months, P = 0.95), median PFS (5.3 months versus 5.8 months, P = 0.96), or response rate (39.2% versus 37.8%). However, the FOLFIRI regimen had an improved toxicity profile, with a lower overall rate of grade 3 or 4 toxicity (69% versus 84%, P < 0.001). Given these findings, the FOLFIRI regimen is an acceptable alternative to platinum-based therapy in suitable patients.22
HER2-POSITIVE DISEASE
The HER2 proto-oncogene, initially described in breast cancer, has been implicated in several malignancies, including gastric and esophageal cancer. Overexpression or amplification of HER2 can be found in up to 30% of gastric cancers.23 For these patients, adding trastuzumab to a standard regimen of platinum and fluoropyrimidine is the standard of care. The prospective phase 3 Trastuzumab for Gastric Cancer (ToGA) trial randomly assigned 594 patients with HER2-positive gastric cancer to receive either cisplatin and fluorouracil or capecitabine and cisplatin with trastuzumab (n = 294) or without (n = 290) trastuzumab every 3 weeks for a total of 6 cycles, followed by maintenance trastuzumab until disease progression was noted.24 HER2 positivity was defined as HER2 protein overexpression by IHC (cutoff of 3+) or gene amplification by fluorescence in situ hybridization (FISH); tumors with IHC 2+ patterns were followed with FISH studies to confirm positivity. The study found a higher incidence of HER2-positive tumors in patients with GEJ tumors compared to patients with distal gastric cancers (33% versus 20%).24 In this trial, the addition of trastuzumab was associated with an improvement in OS: 13.5 months in the trastuzumab cohort versus 11.1 months in those receiving chemotherapy alone (HR 0.74, P = 0.0048). There was not a significant difference in toxicities between the 2 cohorts, with nausea, emesis, and neutropenia being the most common adverse events. Rates of overall grade 3 or 4 events were similar as well (68% in each cohort). Further exploratory analysis was also conducted according to HER2 status by dividing patients into a “high-expressor” group (n = 446), defined as patients with IHC 3+ tumors or IHC 2+ and FISH positivity, and a “low-expressor” group (n = 131), which included patients with IHC 0 or 1+ tumors. Analysis of patients in the 2 subgroups demonstrated an improved OS with the addition of trastuzumab for the high-expressor cohort, with a median OS of 16 months (HR 0.65 [95% CI 0.51 to 0.83]) compared to 11.8 months in those receiving only chemotherapy.
Dual HER2 blockade has been investigated in metastatic gastric cancer. The phase 3 randomized JACOB trial assigned 780 patients to receive either trastuzumab with a cisplatin/fluoropyrimidine regimen with or without the addition of pertuzumab; the primary end point was OS.25 A non-statistically significant trend towards improvement in OS was found in the pertuzumab arm (17.5 months) as compared with the standard of care arm (14.2 months, HR 0.84, P = 0.0565). The pertuzumab/trastuzumab/chemotherapy cohort experienced a higher incidence of diarrhea (61.6% versus 35.1% in control arm). Cardiac toxicity was comparable in the 2 cohorts.
The Table provides a summary of relevant clinical trials in metastatic gastric cancer.
SECOND-LINE THERAPY
CASE CONTINUED
The patient receives capecitabine, oxaliplatin, and trastuzumab therapy for 6 cycles, followed by trastuzumab for another 3 cycles. While on therapy, he develops a painful right clavicular lesion. He undergoes magnetic resonance imaging of the right clavicle, which shows a lesion in the distal two-thirds of the right clavicle measuring 9.7 × 3.7 × 3.8 cm. The patient is started on palliative radiation to the clavicle. However, repeat CT imaging shows progressive liver metastases.
• What is the approach to second-line therapy for metastatic gastric cancer?
Improvements in our understanding of the molecular pathways that lead to tumorigenesis have contributed to the development of several targeted agents whose efficacy in gastric cancer is being investigated. The NCCN guidelines recommend that for all patients who progress on frontline therapy, second-line therapy consists of a combination of ramucirumab and paclitaxel. Other options include single-agent docetaxel, paclitaxel, irinotecan, or ramucirumab. Combination therapy using irinotecan with either docetaxel, fluorouracil, or cisplatin may also be used.
Ramucirumab, a human IgG1 monoclonal antibody that targets the vascular endothelial growth factor receptor 2 (VEGFR2), was initially approved in 2014 as monotherapy for patients who had previously progressed on first-line chemotherapy. Its approval was based on the results of the phase 3 randomized, double-blind placebo-controlled REGARD study.26 The trial randomly assigned 355 patients with advanced gastric or GEJ adenocarcinoma and disease progression after first-line platinum-containing or fluoropyrimidine-containing chemotherapy to receive best supportive care plus either ramucirumab (n = 238) or placebo (n = 117). Monotherapy with ramucirumab significantly improved median OS compared with placebo (5.2 months versus. 3.8 months; HR 0.776 [95% CI 0.6 to 0.99], P = 0.047). There was also an improvement in PFS of 2.1 months in the ramucirumab cohort, as compared to 1.3 months in the placebo cohort (P < 0.0001). Patients in the ramucirumab arm experienced a higher incidence of hypertension (16% versus 8%), but all other adverse events occurred at comparable rates. Five deaths in the ramucirumab group were thought to be secondary to the study drug, as compared to 2 deaths in the placebo group.
In the subsequent phase 3 RAINBOW trial, the addition of ramucirumab to paclitaxel was investigated, with 330 patients assigned to the combination group and 335 to the paclitaxel-only group.27 The trial again showed that combination therapy afforded patients a significant survival advantage compared to paclitaxel alone, with a median OS of 9.6 months versus 7.4 months for the monotherapy group (HR 0.807 [95% CI 0.678 to 0.962], P = 0.017). A PFS benefit of 4.4 months was observed in the combination therapy groups, as compared with 2.9 months in the monotherapy group (HR 0.635, P < 0.0001). The ramucirumab/paclitaxel group also had a higher overall response rate of 28% versus 16%. The combination cohort had an increased incidence of grade 3 or higher adverse hypertensive events (14% versus 2%) and neutropenia (41% versus 19%), while the incidence of grade 3 febrile neutropenic events was similar between the groups (3% versus 2%).
The addition of bevacizumab, another monoclonal antibody against VEGF, to standard chemotherapy regimens has been explored, but studies have failed to show a survival benefit with this agent in the first-line treatment of advanced gastric cancer. The phase 3 Avastin in Gastric Cancer (AVAGAST) trial was a multinational, randomized study where patients received either bevacizumab (n = 387) or placebo (n = 387) in addition to cisplatin and capecitabine.28 The substitution of fluorouracil for capecitabine was permitted for patients who were unable to tolerate oral medications. Cisplatin was administered for a maximum of 6 cycles, while capecitabine and bevacizumab were administered until disease progression. The study failed to show an improvement in OS, with a median OS of 12.1 months noted in the bevacizumab cohort, as compared to 10.1 months in the placebo arm (HR 0.87 [95% CI 0.73 to 1.03], P = 0.1002). However, there was a modest improvement in median PFS (6.7 months versus 5.3 months; HR 0.80 [95% CI 0.68 to 0.93], P = 0.0037) and overall response rate (46% versus 37.4%, P = 0.0315). The most commonly reported grade 3 to 5 adverse events included neutropenia (35%), anemia (10%), and loss of appetite (8%). Interestingly, in a follow-up report, higher serum levels of VEGF-A were thought to correlate with an enhanced response to bevacizumab.29 However, the routine use of biomarker analysis in selecting patients for treatment with bevacizumab in metastatic gastric cancer remains to be further clarified.
Use of other agents with anti-HER2 activity in the second-line treatment of patients who have experienced progression while on trastuzumab remains unclear. In the recent T-ACT trial, patients with disease refractory to frontline therapy with combination trastuzumab and fluoropyrimidine/platinum agents were randomly assigned to receive either weekly paclitaxel (n = 45) or weekly paclitaxel plus trastuzumab (n = 44).30 Patients in the combination cohort received an initial dose of trastuzumab 8 mg/kg followed by 6 mg/kg every 3 weeks until progression. The study did not find a difference in either PFS (3.19 months versus 3.68 months; HR 0.91 [95% CI 0.67 to 1.22], P = 0.33) or OS (9.95 months versus 10.2 months; HR 1.23 [95% CI 0.75 to 1.99], P = 0.20). The study thus failed to show a benefit to continuing trastuzumab after progression in the first-line setting.
Lapatinib in combination with paclitaxel has been compared to paclitaxel alone for the treatment of advanced HER2-positive gastric cancer in an Asian population in the phase 3 TyTAN trial.31 With a primary end point of OS, the study randomly assigned 129 patients to receive paclitaxel alone and 132 patients to receive paclitaxel with lapatinib. There was a nonsignificant trend towards improvement in OS in the combination group (11 months) as compared to the paclitaxel-only group(8.9 months, P = 0.1044), with no significant difference in median PFS (5.4 months versus 4.4 months). However, it is important to note that only 15 patients in this trial had previously been exposed to trastuzumab. Another trial, the phase 3 GATSBY study, examined the efficacy of trastuzumab emtansine in the second-line setting compared to taxanes alone and failed to show any improvement in PFS or OS.32 Given these results, no alternative anti-HER2 therapy has been proven to be efficacious for patients who are trastuzumab refractory. Therefore, including anti-HER2 therapy in the second-line treatment of HER2-positive gastric cancer is not recommended.
IMMUNOTHERAPY AND OTHER TARGETED THERAPIES
Several other targeted therapies have been studied in advanced gastric cancer, without any demonstrable survival benefit. The PI3K/AKT/mTOR pathway is known to be involved in regulation of cell growth and angiogenesis, and the mTOR inhibitor everolimus is widely used to treat other malignancies, including breast cancer. The use of everolimus in the second-line setting was studied in the phase 3 GRANITE-1 trial, where it was compared to best supportive care and failed to provide any survival benefit.33 Cetuximab, a recombinant human and mouse chimeric monoclonal antibody, and panitumumab, a recombinant human antibody against the epidermal growth factor receptor (EGFR), have also been examined in gastric and GEJ cancer patients. However, the large phase 3 EXPAND and REAL-3 trials did not show a survival benefit when these agents were added to standard chemotherapy.34,35
Overexpression of MET, a proto-oncogene and tyrosine kinase receptor, has also been implicated in gastric cancer progression. The ligand for MET is the hepatocyte growth factor (HGF), and aberrant signaling of this pathway has been shown to correlate with an aggressive gastric cancer phenotype and poorer OS by promoting tumor growth and angiogenesis. However, no MET inhibitors thus far have been found to be clinically effective. RILOMET-1 and RILOMET-2 were phase 3 trials examining the efficacy of rilotumumab, a humanized anti-HGF antibody, in combination with chemotherapy (ECX and cisplatin with capecitabine, respectively) for the frontline treatment of MET-positive GEJ and gastric cancers. Both studies were discontinued due to a higher treatment-related mortality in patients receiving rilotumumab, with a higher incidence of adverse events due to disease progression being noted.36 Similarly, onartuzumab, a monovalent monoclonal antibody against the MET receptor, was investigated in the phase 3 METGastric trial in combination with modified FOLFOX6 as first-line therapy for HER2-negative, MET-positive metastatic GEJ and gastric cancers. The study did not demonstrate any significant improvements in OS or PFS.37
There has been significant interest in incorporating immunotherapy in the treatment of early and metastatic gastric cancer. Pembrolizumab is the first programmed death receptor (PD-1) inhibitor to be approved for treatment of patients with PD-L1−positive advanced gastric cancer who had previously received 2 or more lines of chemotherapy. Although earlier studies of pembrolizumab in lung cancer utilized the tumor proportion score (TPS) to determine PD-L1 positivity, this was not found to be applicable to gastric cancer. Instead, the combined positive score (CPS) is used in gastric cancer. The CPS evaluates the number of tumor cells and immune cells (macrophages and lymphocytes) that stain positive for PD-L1 relative to all viable tumor cells. Comparatively, the TPS only examines the percentage of viable tumor cells that show complete or partial positive staining for PD-L1. A CPS score of 1 or greater identifies patients who would be suitable candidates for pembrolizumab.
The approval of pembrolizumab was based on the positive findings from the recent KEYNOTE-059 trial.38 The study included 259 patients who had previously received either fluoropyrimidine, cisplatin, or anti-HER2 therapy, with 148 patients (55%) of these patients having PD-L1−positive tumors. The PD-L1 status was determined using a pharmDx Kit, which is now approved by the US Food and Drug Administration to select patients who could benefit from pembrolizumab treatment. CPS was calculated as the number of PD-L1−staining cells divided by the total number of evaluated cells. The study included patients with microsatellite stable (MSI-S), undetermined, or deficient MMR status. The overall response rate to pembrolizumab across all patients was 11.6%, median PFS was 2 months, and the 12-month OS rate was 23.4%. In the subset of patients with MSI-H tumors, the overall response rate was 57.1%, with a complete response rate of 14.3%; in those with MSI-S tumors, the overall response rate was 9% and the complete response rate was 2.4%. Among patients with PD-L1–positive tumors, the overall response rate was 15.5% (95% CI 10.1% to 22.4). Common adverse events included fatigue, hypothyroidism, nausea, diarrhea, and arthralgia.38
CASE CONCLUSION
This patient with metastatic gastric cancer receives second-line chemotherapy with ramucirumab and paclitaxel. Follow-up imaging shows persistent liver metastases and new lung metastasis. Because the tumor is PD-L1–positive, the patient receives 4 cycles of pembrolizumab, with no significant change noted in disease burden. He notes a significant decline in functional status with increased weight loss, nausea, emesis, and fatigue. The patient opts to forego any further therapy and instead chooses to pursue supportive care only.
SUMMARY
Gastric cancer is the third most common cause of cancer death worldwide. Common risk factors for developing gastric cancer include H. pylori infection, smoking, alcohol abuse, radiation exposure, high-fat diet, and obesity. Patients presenting with alarm symptoms of nausea, emesis, early satiety, abdominal pain, or weight loss should be fully evaluated with upper GI endoscopy. If there is suspicion for metastatic disease, CT evaluation of the chest, abdomen, and pelvis with oral and intravenous contrast should be obtained. Treatment of patients with metastatic gastric cancer is guided by their performance status at presentation. For patients with good performance status, a combination of platinum and fluoropyrimidine therapy, such as FOLFOX, can be considered. Doublet chemotherapy regimens are preferred over triplet chemotherapy regimens given their better tolerability. For patients with HER2-positive disease, the addition of trastuzumab to the platinum and fluoropyrimidine backbone is the standard of care in the first line.
Several targeted agents have been studied in patients progressing on initial therapy, with ramucirumab and paclitaxel being considered the regimen of choice in the second line. No anti-HER2 therapy has been approved for patients who are refractory to trastuzumab. Pembrolizumab is approved for use in patients who are PD-L1–positive and have previously progressed on at least 2 lines of chemotherapy. Pembrolizumab is also approved for the treatment of patients with unresectable or metastatic, MSI-H or MMR-deficient gastric cancers that have progressed after prior treatment and who have no satisfactory alternative treatment options.
INTRODUCTION
According to the Surveillance, Epidemiology and End Results database, in 2017 there were 28,000 new cases of gastric cancer, accounting for 1.8% of all malignancies in the United States, and an estimated 10,960 gastric cancer–related deaths.1 Worldwide, gastric cancer is the fifth most common malignancy and the third most common cause of death from any cancer.2 The incidence of gastric cancer varies significantly by geographic region, with countries in Eastern Asia (China, Japan), Eastern Europe, and Central and South America accounting for 50% of all new cases.3 Although the incidence of gastric cancer has declined in recent years, this decrease has not been observed consistently across all nations.2 In particular, the incidence of gastric cancers arising from the cardia has been increasing, which is perhaps due to a higher prevalence of obesity in Western societies.4
In this article, we review key aspects of management of metastatic gastric cancer, including selection of first- and second-line therapy, and discuss targeted agents and upcoming clinical trials.
EPIDEMIOLOGY AND RISK FACTORS
Chronic infection with Helicobacter pylori, a gram-negative bacterium, is a strong etiological factor for the development of gastric cancer, contributing to up to 70% of cases.2 The pathogen can colonize the gastric mucosa, leading to chronic inflammation. Although most patients remain asymptomatic, 1% to 3% develop gastric cancer and another 0.1% develop mucosa-associated lymphoid tissue lymphoma.5 H. pylori infection is more commonly associated with cancer of the gastric body than with cancer of the gastroesophageal junction (GEJ). The increased burden of gastric cancer in countries in Eastern Asia, Latin America, and Eastern Europe has been correlated to the prevalence of chronic H. pylori infection in these areas.
Carcinogenesis secondary to H. pylori infection may occur via several mechanisms. First, H. pylori can release virulence factors, such as cytotoxin-associated gene A, vacuolating cytotoxin, and outer membrane proteins, into the cytosol of host cells, leading to changes in patterns of cell proliferation and apoptosis.6 These virulence factors can modulate the host immune system, attenuating it to promote dysplasia. In addition, continued recognition of these factors by the immune system leads to a persistent inflammatory response, with the release of cytokines such as interleukin (IL) -1β, IL-6, and IL-8. This leads to chronic mucosal damage, further promoting dysplasia with eventual transformation into adenocarcinoma.7 In Japan and Korea, where screening for H. pylori infection is routinely performed, there have been improvements in overall survival (OS) rates for gastric cancer, with 5-year OS rates of 70%.8 The International Agency for Research on Cancer recommends further research into population-based screening and treatment programs for patients with chronic H. pylori infection. However, despite this recommendation, optimal screening strategies are not clearly defined.9
Other risk factors for the development of gastric cancer include chronic gastroesophageal reflux disease; smoking; alcohol use; exposure to radiation; diets high in fats, salt, and smoked items and low in fruits and vegetables; obesity; and exposure to chemotherapeutic agents such as procarbazine.10 Another pathogen suspected, but not proven, to be associated with increased risk for gastric cancer is the Epstein-Barr virus, a human herpesvirus found in 80% of all gastric carcinomas with lymphoid features.11 In addition, whether the use of medications such as statins and nonsteroidal anti-inflammatory drugs confers a decreased risk of gastric cancers remains unclear.10
EVALUATION
CASE PRESENTATION
A 55-year-old Caucasian man with a history of type 2 diabetes mellitus presents to the gastrointestinal (GI) clinic with a 6-month history of dysphagia. The dysphagia is worsened with ingestion of solids, particularly towards the end of the day. He states that the food often gets “stuck in the middle of the chest.” The patient denies any nausea or emesis but notes that he has a poor appetite. He reports having worsening mid-epigastric abdominal pain that is non-radiating, dull in character, and 6/10 in intensity. He also reports a 10-lb weight loss over the past 2 months. He has no previous history of reflux, chest pain, dyspnea, or cough. Review of systems is otherwise benign. Physical exam is within normal limits.
• Which tests should be conducted when gastric cancer is suspected?
Persistent epigastric abdominal pain and weight loss are the most common early symptoms of gastric cancer. Nausea, early satiety, dysphagia, and occult GI bleeding can be other presenting signs. Patients presenting with alarm symptoms of nausea, emesis, early satiety, abdominal pain, or weight loss should be fully evaluated with upper GI endoscopy. Early diagnosis of gastric cancer is essential in obtaining a curative resection. However, at least 40% of patients present with de novo metastatic disease at the time of initial diagnosis.12 Gastric cancer spreads by direct extension through the gastric wall, with the liver, peritoneum, and regional lymph nodes being the most common sites of metastatic deposits.13 Classically, Virchow’s node, the left supraclavicular lymph node, is involved with metastatic gastric cancer. Involvement of the left axillary lymph node (Irish node) or a periumbilical nodule (Sister Mary Joseph node) may also be observed. Other, less commonly noted sites of metastatic disease include the ovaries, central nervous system, bone, lung, and soft tissues.13
Upper GI endoscopy is the best method for determining tumor location and extent and obtaining a specimen for a definitive tissue diagnosis.14 It is essential to accurately identify the location of the tumor in the stomach and relative to the GEJ. The American Joint Committee on Cancer classification defines tumors involving the GEJ with an epicenter no more than 2 cm into the proximal stomach as esophageal cancers.15 Tumors of the GEJ with their epicenter more than 2 cm into the proximal stomach are defined as gastric cancers. If metastatic disease is suspected, computed tomography (CT) scan of the chest, abdomen, and pelvis with oral and intravenous contrast can be obtained to determine the extent of disease spread. In the absence of any metastatic disease, endoscopic ultrasound (EUS) should be conducted to determine the depth of tumor invasion (T staging) and lymph node status. In the era of targeted therapy, patients with metastatic disease should undergo testing for human epidermal growth factor-2 (HER-2) expression, microsatellite instability (MSI), and programmed death ligand 1 (PD-L1) expression. Patients should be staged according to the TNM staging system.
FIRST-LINE TREATMENT OPTIONS
CASE CONTINUED
The patient undergoes esophagoduodenoscopy (EGD) and is found to have a gastric cardia mass extending into the distal esophagus. EUS also demonstrates multiple abdominal and mediastinal lymph nodes. No gastric outlet obstruction is found. Biopsy shows poorly differentiated invasive adenocarcinoma. Warthin–Starry stain is negative for H. pylori organism. The tumor cells are positive for cytokeratin (CK7), CK19, and mucin-1 gene (MUC1); focally positive for CK20; and negative for MUC2. HER2 testing results are reported as immunohistochemistry (IHC) 3+, consistent with strongly positive HER2 protein expression. Further IHC testing for mismatch repair (MMR) proteins shows intact nuclear expression of MLH1, MSH2, MSH6, and PMS2 protein, consistent with a low probability of MSI-high tumor. The tumor is found to be PD-L1 positive. Imaging reveals abnormal mass-like nodular thickening of the gastric wall, with an infiltrative opacity within the pancreatico-duodenal groove, suspicious for tumor infiltration. Multiple metastatic deposits are noted in the liver, peritoneum, and bilateral lungs. There is extensive gastrohepatic ligament and periportal lymphadenopathy and mild enlargement of the pulmonary hilar lymph nodes. These findings are consistent with stage 4 (T4bN3aM1) gastric cancer. Given these findings, staging laparoscopy is deferred.
• What are the first-line treatment options for patients with metastatic gastric cancer?
Patients with metastatic gastric cancer have a poor prognosis, and management is stratified based on performance status (Figure). In patients with good performance status, systemic chemotherapy is the mainstay of treatment. The goal of therapy is not curative, but rather treatment focuses on palliation of symptoms arising from tumor spread. Given this treatment goal, there has been considerable interest in clarifying the utility of chemotherapy as opposed to best supportive care. In a recent Cochrane review of 64 randomized control trials involving 11,698 patients, chemotherapy was found to improve OS by 6.7 months as compared to best supportive care (hazard ratio [HR] 0.3 [95% confidence interval {CI} 0.24 to 0.55]).16 Five classes of cytotoxic chemotherapeutic agents have demonstrated activity in gastric cancer. These include fluoropyrimidine (either infusional fluorouracil or capecitabine), platinum agents (cisplatin or oxaliplatin), taxanes (docetaxel or paclitaxel), anthracyclines (epirubicin), and irinotecan.13 Treatment options are further divided based on whether the patient has HER2-overexpressing or non-expressing malignancy.
HER2-NEGATIVE DISEASE
For patients with HER2-negative disease, National Comprehensive Cancer Network (NCCN) guidelines recommend using 2-drug combination regimens rather than 3 drugs, given concern for increased toxicity with 3-drug regimens.17 For patients with a performance status of 0 to 1, utilization of a 3-drug regimen is a reasonable alternative. The combination of a fluoropyrimidine with a platinum agent is considered the standard of care, with regimens such as fluorouracil, leucovorin, and oxaliplatin (FOLFOX) being commonly used.
Epirubicin-containing regimens have also been extensively studied in advanced gastric cancer. In a study of 274 previously untreated patients with GEJ cancers, the combination of epirubicin, cisplatin, and fluorouracil (ECF) was compared to fluorouracil, doxorubicin, and methotrexate (FAMTX). There was an OS benefit favoring ECF (8.9 months versus 5.7 months) at 1 year (95% CI 27% to 45%, P = 0.0009). The ECF regimen was associated with an increased risk of nausea, emesis, and alopecia, while more hematologic toxicity and infections were noted with the FAMTX regimen.18 In addition, in a phase 3 trial, Van Cutsem and colleagues examined the role of docetaxel in combination with cisplatin and fluorouracil (DCF) compared to cisplatin and fluorouracil alone. Addition of docetaxel led to improved OS and time to progression (9.2 months versus 8.6 months for cisplatin and fluorouracil alone, P = 0.02) but with an increased risk of grade 3 and 4 toxicities (69% versus 59%). These adverse events included neutropenia (82% versus 57% of cisplatin and fluorouracil patients), diarrhea (19% versus 8%), stomatitis (21% versus 27%), and fatigue (19% versus 14%).19
The landmark phase 3 REAL-2 study compared 4 chemotherapy regimens in patients with untreated advanced esophagogastric cancer. This study was conducted to determine if the efficacy of cisplatin and oxaliplatin, a third-generation platinum agent, is equivalent to that of fluorouracil and capecitabine, an oral fluoropyrimidine. In this trial, a 2 × 2 design was used to compare 4 regimens: ECF versus epirubicin, cisplatin, and capecitabine (ECX) versus epirubicin, oxaliplatin, and fluorouracil (EOF) versus epirubicin, oxaliplatin, and capecitabine (EOX). The study found EOX to be noninferior to ECF, with a trend towards improved OS compared to other combination regimens (11.2 months versus 9.9 months, HR 0.80 [95% CI 0.66 to 0.97], P = 0.02).20 Thus, the study demonstrated that an oxaliplatin and capecitabine-based regimen could replace cisplatin and fluorouracil. Given that fluorouracil administration requires long continuous infusions, the oral-based capecitabine regimen is an attractive option for patients.
Several trials have demonstrated the equivalency of oxaliplatin with cisplatin in combination regimens for the treatment of advanced gastric cancer. Oxaliplatin has the benefit of an improved toxicity profile as compared to cisplatin, with the major dose-limiting toxicity being peripheral neuropathy
Given previous evidence that DCF (docetaxel, cisplatin, fluorouracil) is superior to cisplatin and fluorouracil alone, there was interest in determining if the addition of docetaxel to a backbone of fluorouracil, oxaliplatin, and leucovorin (FLO) could elicit a higher response rate. This concept was investigated in a phase 2 trial that assigned 54 patients with metastatic gastric or GEJ adenocarcinoma to receive biweekly infusions of oxaliplatin, leucovorin, fluorouracil, and docetaxel.21 Median time to response was 1.54 months, and the overall response rate was 57.7%. Median progression-free survival (PFS) was 5.2 months, and OS was 11.1 months. The most common grade 3 or 4 toxicities included neutropenia (48%), leukopenia (27.8%), diarrhea (14.8%), and fatigue (11.1%).
Irinotecan-based regimens have also been extensively studied in the first-line treatment of metastatic gastric cancer, particularly as an alternative to platinum-based therapy, but superiority has not been established. The combination of fluorouracil, leucovorin, and irinotecan (FOLFIRI) was compared to ECX in a phase 3 trial.22 The study enrolled 416 patients with locally advanced or metastatic gastric or GEJ cancer. At a median follow up of 31 months, the time to progression was longer in the FOLFIRI arm as compared to the ECX arm (5.1 months versus 4.2 months, P = 0.008), but there was no difference in OS (9.5 months versus 9.7 months, P = 0.95), median PFS (5.3 months versus 5.8 months, P = 0.96), or response rate (39.2% versus 37.8%). However, the FOLFIRI regimen had an improved toxicity profile, with a lower overall rate of grade 3 or 4 toxicity (69% versus 84%, P < 0.001). Given these findings, the FOLFIRI regimen is an acceptable alternative to platinum-based therapy in suitable patients.22
HER2-POSITIVE DISEASE
The HER2 proto-oncogene, initially described in breast cancer, has been implicated in several malignancies, including gastric and esophageal cancer. Overexpression or amplification of HER2 can be found in up to 30% of gastric cancers.23 For these patients, adding trastuzumab to a standard regimen of platinum and fluoropyrimidine is the standard of care. The prospective phase 3 Trastuzumab for Gastric Cancer (ToGA) trial randomly assigned 594 patients with HER2-positive gastric cancer to receive either cisplatin and fluorouracil or capecitabine and cisplatin with trastuzumab (n = 294) or without (n = 290) trastuzumab every 3 weeks for a total of 6 cycles, followed by maintenance trastuzumab until disease progression was noted.24 HER2 positivity was defined as HER2 protein overexpression by IHC (cutoff of 3+) or gene amplification by fluorescence in situ hybridization (FISH); tumors with IHC 2+ patterns were followed with FISH studies to confirm positivity. The study found a higher incidence of HER2-positive tumors in patients with GEJ tumors compared to patients with distal gastric cancers (33% versus 20%).24 In this trial, the addition of trastuzumab was associated with an improvement in OS: 13.5 months in the trastuzumab cohort versus 11.1 months in those receiving chemotherapy alone (HR 0.74, P = 0.0048). There was not a significant difference in toxicities between the 2 cohorts, with nausea, emesis, and neutropenia being the most common adverse events. Rates of overall grade 3 or 4 events were similar as well (68% in each cohort). Further exploratory analysis was also conducted according to HER2 status by dividing patients into a “high-expressor” group (n = 446), defined as patients with IHC 3+ tumors or IHC 2+ and FISH positivity, and a “low-expressor” group (n = 131), which included patients with IHC 0 or 1+ tumors. Analysis of patients in the 2 subgroups demonstrated an improved OS with the addition of trastuzumab for the high-expressor cohort, with a median OS of 16 months (HR 0.65 [95% CI 0.51 to 0.83]) compared to 11.8 months in those receiving only chemotherapy.
Dual HER2 blockade has been investigated in metastatic gastric cancer. The phase 3 randomized JACOB trial assigned 780 patients to receive either trastuzumab with a cisplatin/fluoropyrimidine regimen with or without the addition of pertuzumab; the primary end point was OS.25 A non-statistically significant trend towards improvement in OS was found in the pertuzumab arm (17.5 months) as compared with the standard of care arm (14.2 months, HR 0.84, P = 0.0565). The pertuzumab/trastuzumab/chemotherapy cohort experienced a higher incidence of diarrhea (61.6% versus 35.1% in control arm). Cardiac toxicity was comparable in the 2 cohorts.
The Table provides a summary of relevant clinical trials in metastatic gastric cancer.
SECOND-LINE THERAPY
CASE CONTINUED
The patient receives capecitabine, oxaliplatin, and trastuzumab therapy for 6 cycles, followed by trastuzumab for another 3 cycles. While on therapy, he develops a painful right clavicular lesion. He undergoes magnetic resonance imaging of the right clavicle, which shows a lesion in the distal two-thirds of the right clavicle measuring 9.7 × 3.7 × 3.8 cm. The patient is started on palliative radiation to the clavicle. However, repeat CT imaging shows progressive liver metastases.
• What is the approach to second-line therapy for metastatic gastric cancer?
Improvements in our understanding of the molecular pathways that lead to tumorigenesis have contributed to the development of several targeted agents whose efficacy in gastric cancer is being investigated. The NCCN guidelines recommend that for all patients who progress on frontline therapy, second-line therapy consists of a combination of ramucirumab and paclitaxel. Other options include single-agent docetaxel, paclitaxel, irinotecan, or ramucirumab. Combination therapy using irinotecan with either docetaxel, fluorouracil, or cisplatin may also be used.
Ramucirumab, a human IgG1 monoclonal antibody that targets the vascular endothelial growth factor receptor 2 (VEGFR2), was initially approved in 2014 as monotherapy for patients who had previously progressed on first-line chemotherapy. Its approval was based on the results of the phase 3 randomized, double-blind placebo-controlled REGARD study.26 The trial randomly assigned 355 patients with advanced gastric or GEJ adenocarcinoma and disease progression after first-line platinum-containing or fluoropyrimidine-containing chemotherapy to receive best supportive care plus either ramucirumab (n = 238) or placebo (n = 117). Monotherapy with ramucirumab significantly improved median OS compared with placebo (5.2 months versus. 3.8 months; HR 0.776 [95% CI 0.6 to 0.99], P = 0.047). There was also an improvement in PFS of 2.1 months in the ramucirumab cohort, as compared to 1.3 months in the placebo cohort (P < 0.0001). Patients in the ramucirumab arm experienced a higher incidence of hypertension (16% versus 8%), but all other adverse events occurred at comparable rates. Five deaths in the ramucirumab group were thought to be secondary to the study drug, as compared to 2 deaths in the placebo group.
In the subsequent phase 3 RAINBOW trial, the addition of ramucirumab to paclitaxel was investigated, with 330 patients assigned to the combination group and 335 to the paclitaxel-only group.27 The trial again showed that combination therapy afforded patients a significant survival advantage compared to paclitaxel alone, with a median OS of 9.6 months versus 7.4 months for the monotherapy group (HR 0.807 [95% CI 0.678 to 0.962], P = 0.017). A PFS benefit of 4.4 months was observed in the combination therapy groups, as compared with 2.9 months in the monotherapy group (HR 0.635, P < 0.0001). The ramucirumab/paclitaxel group also had a higher overall response rate of 28% versus 16%. The combination cohort had an increased incidence of grade 3 or higher adverse hypertensive events (14% versus 2%) and neutropenia (41% versus 19%), while the incidence of grade 3 febrile neutropenic events was similar between the groups (3% versus 2%).
The addition of bevacizumab, another monoclonal antibody against VEGF, to standard chemotherapy regimens has been explored, but studies have failed to show a survival benefit with this agent in the first-line treatment of advanced gastric cancer. The phase 3 Avastin in Gastric Cancer (AVAGAST) trial was a multinational, randomized study where patients received either bevacizumab (n = 387) or placebo (n = 387) in addition to cisplatin and capecitabine.28 The substitution of fluorouracil for capecitabine was permitted for patients who were unable to tolerate oral medications. Cisplatin was administered for a maximum of 6 cycles, while capecitabine and bevacizumab were administered until disease progression. The study failed to show an improvement in OS, with a median OS of 12.1 months noted in the bevacizumab cohort, as compared to 10.1 months in the placebo arm (HR 0.87 [95% CI 0.73 to 1.03], P = 0.1002). However, there was a modest improvement in median PFS (6.7 months versus 5.3 months; HR 0.80 [95% CI 0.68 to 0.93], P = 0.0037) and overall response rate (46% versus 37.4%, P = 0.0315). The most commonly reported grade 3 to 5 adverse events included neutropenia (35%), anemia (10%), and loss of appetite (8%). Interestingly, in a follow-up report, higher serum levels of VEGF-A were thought to correlate with an enhanced response to bevacizumab.29 However, the routine use of biomarker analysis in selecting patients for treatment with bevacizumab in metastatic gastric cancer remains to be further clarified.
Use of other agents with anti-HER2 activity in the second-line treatment of patients who have experienced progression while on trastuzumab remains unclear. In the recent T-ACT trial, patients with disease refractory to frontline therapy with combination trastuzumab and fluoropyrimidine/platinum agents were randomly assigned to receive either weekly paclitaxel (n = 45) or weekly paclitaxel plus trastuzumab (n = 44).30 Patients in the combination cohort received an initial dose of trastuzumab 8 mg/kg followed by 6 mg/kg every 3 weeks until progression. The study did not find a difference in either PFS (3.19 months versus 3.68 months; HR 0.91 [95% CI 0.67 to 1.22], P = 0.33) or OS (9.95 months versus 10.2 months; HR 1.23 [95% CI 0.75 to 1.99], P = 0.20). The study thus failed to show a benefit to continuing trastuzumab after progression in the first-line setting.
Lapatinib in combination with paclitaxel has been compared to paclitaxel alone for the treatment of advanced HER2-positive gastric cancer in an Asian population in the phase 3 TyTAN trial.31 With a primary end point of OS, the study randomly assigned 129 patients to receive paclitaxel alone and 132 patients to receive paclitaxel with lapatinib. There was a nonsignificant trend towards improvement in OS in the combination group (11 months) as compared to the paclitaxel-only group(8.9 months, P = 0.1044), with no significant difference in median PFS (5.4 months versus 4.4 months). However, it is important to note that only 15 patients in this trial had previously been exposed to trastuzumab. Another trial, the phase 3 GATSBY study, examined the efficacy of trastuzumab emtansine in the second-line setting compared to taxanes alone and failed to show any improvement in PFS or OS.32 Given these results, no alternative anti-HER2 therapy has been proven to be efficacious for patients who are trastuzumab refractory. Therefore, including anti-HER2 therapy in the second-line treatment of HER2-positive gastric cancer is not recommended.
IMMUNOTHERAPY AND OTHER TARGETED THERAPIES
Several other targeted therapies have been studied in advanced gastric cancer, without any demonstrable survival benefit. The PI3K/AKT/mTOR pathway is known to be involved in regulation of cell growth and angiogenesis, and the mTOR inhibitor everolimus is widely used to treat other malignancies, including breast cancer. The use of everolimus in the second-line setting was studied in the phase 3 GRANITE-1 trial, where it was compared to best supportive care and failed to provide any survival benefit.33 Cetuximab, a recombinant human and mouse chimeric monoclonal antibody, and panitumumab, a recombinant human antibody against the epidermal growth factor receptor (EGFR), have also been examined in gastric and GEJ cancer patients. However, the large phase 3 EXPAND and REAL-3 trials did not show a survival benefit when these agents were added to standard chemotherapy.34,35
Overexpression of MET, a proto-oncogene and tyrosine kinase receptor, has also been implicated in gastric cancer progression. The ligand for MET is the hepatocyte growth factor (HGF), and aberrant signaling of this pathway has been shown to correlate with an aggressive gastric cancer phenotype and poorer OS by promoting tumor growth and angiogenesis. However, no MET inhibitors thus far have been found to be clinically effective. RILOMET-1 and RILOMET-2 were phase 3 trials examining the efficacy of rilotumumab, a humanized anti-HGF antibody, in combination with chemotherapy (ECX and cisplatin with capecitabine, respectively) for the frontline treatment of MET-positive GEJ and gastric cancers. Both studies were discontinued due to a higher treatment-related mortality in patients receiving rilotumumab, with a higher incidence of adverse events due to disease progression being noted.36 Similarly, onartuzumab, a monovalent monoclonal antibody against the MET receptor, was investigated in the phase 3 METGastric trial in combination with modified FOLFOX6 as first-line therapy for HER2-negative, MET-positive metastatic GEJ and gastric cancers. The study did not demonstrate any significant improvements in OS or PFS.37
There has been significant interest in incorporating immunotherapy in the treatment of early and metastatic gastric cancer. Pembrolizumab is the first programmed death receptor (PD-1) inhibitor to be approved for treatment of patients with PD-L1−positive advanced gastric cancer who had previously received 2 or more lines of chemotherapy. Although earlier studies of pembrolizumab in lung cancer utilized the tumor proportion score (TPS) to determine PD-L1 positivity, this was not found to be applicable to gastric cancer. Instead, the combined positive score (CPS) is used in gastric cancer. The CPS evaluates the number of tumor cells and immune cells (macrophages and lymphocytes) that stain positive for PD-L1 relative to all viable tumor cells. Comparatively, the TPS only examines the percentage of viable tumor cells that show complete or partial positive staining for PD-L1. A CPS score of 1 or greater identifies patients who would be suitable candidates for pembrolizumab.
The approval of pembrolizumab was based on the positive findings from the recent KEYNOTE-059 trial.38 The study included 259 patients who had previously received either fluoropyrimidine, cisplatin, or anti-HER2 therapy, with 148 patients (55%) of these patients having PD-L1−positive tumors. The PD-L1 status was determined using a pharmDx Kit, which is now approved by the US Food and Drug Administration to select patients who could benefit from pembrolizumab treatment. CPS was calculated as the number of PD-L1−staining cells divided by the total number of evaluated cells. The study included patients with microsatellite stable (MSI-S), undetermined, or deficient MMR status. The overall response rate to pembrolizumab across all patients was 11.6%, median PFS was 2 months, and the 12-month OS rate was 23.4%. In the subset of patients with MSI-H tumors, the overall response rate was 57.1%, with a complete response rate of 14.3%; in those with MSI-S tumors, the overall response rate was 9% and the complete response rate was 2.4%. Among patients with PD-L1–positive tumors, the overall response rate was 15.5% (95% CI 10.1% to 22.4). Common adverse events included fatigue, hypothyroidism, nausea, diarrhea, and arthralgia.38
CASE CONCLUSION
This patient with metastatic gastric cancer receives second-line chemotherapy with ramucirumab and paclitaxel. Follow-up imaging shows persistent liver metastases and new lung metastasis. Because the tumor is PD-L1–positive, the patient receives 4 cycles of pembrolizumab, with no significant change noted in disease burden. He notes a significant decline in functional status with increased weight loss, nausea, emesis, and fatigue. The patient opts to forego any further therapy and instead chooses to pursue supportive care only.
SUMMARY
Gastric cancer is the third most common cause of cancer death worldwide. Common risk factors for developing gastric cancer include H. pylori infection, smoking, alcohol abuse, radiation exposure, high-fat diet, and obesity. Patients presenting with alarm symptoms of nausea, emesis, early satiety, abdominal pain, or weight loss should be fully evaluated with upper GI endoscopy. If there is suspicion for metastatic disease, CT evaluation of the chest, abdomen, and pelvis with oral and intravenous contrast should be obtained. Treatment of patients with metastatic gastric cancer is guided by their performance status at presentation. For patients with good performance status, a combination of platinum and fluoropyrimidine therapy, such as FOLFOX, can be considered. Doublet chemotherapy regimens are preferred over triplet chemotherapy regimens given their better tolerability. For patients with HER2-positive disease, the addition of trastuzumab to the platinum and fluoropyrimidine backbone is the standard of care in the first line.
Several targeted agents have been studied in patients progressing on initial therapy, with ramucirumab and paclitaxel being considered the regimen of choice in the second line. No anti-HER2 therapy has been approved for patients who are refractory to trastuzumab. Pembrolizumab is approved for use in patients who are PD-L1–positive and have previously progressed on at least 2 lines of chemotherapy. Pembrolizumab is also approved for the treatment of patients with unresectable or metastatic, MSI-H or MMR-deficient gastric cancers that have progressed after prior treatment and who have no satisfactory alternative treatment options.
1. Noone AM, Cronin KA, Altekruse SF, et al. Cancer incidence and survival trends by subtype using data from the Surveillance Epidemiology and End Results Program, 1992-2013. Cancer Epidemiol Biomarkers Prev 2017;26:632–41
2. Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol 2017;3:524–8.
3. Sitarz R, Skierucha M, Mielko J, et al. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res 2018;10:239–48.
4. Olefson S, Moss SF. Obesity and related risk factors in gastric cardia adenocarcinoma. Gastric Cancer 2015;18:23–32.
5. Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett 2014;345:196–202.
6. Espinoza JL, Matsumoto A, Tanaka H, Matsumura I. Gastric microbiota: An emerging player in Helicobacter pylori-induced gastric malignancies. Cancer Lett 2018;414:147–52.
7. Chmiela M, Gonciarz W. Molecular mimicry in Helicobacter pylori infections. World J Gastroenterol 2017;23:3964–77.
8. Isobe Y, Nashimoto A, Akazawa K, et al. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer 2011;14:301–16.
9. Ford A, Gurusamy KS, Delaney B, et al. Eradication therapy for peptic ulcer disease in Helicobacter pylori-positive patients. Cochrane Database Syst Rev 2004(4):CD003840.
10. Karimi P, Islami F, Anandasabapathy S, et al. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 2014;23:700–13.
11. Van Cutsem E, Sagaert X, Topal B, et al. Gastric cancer. Lancet 2016;388:2654–64.
12. Chan BA , Sim HW, Natori A, et al. Survival outcomes for de novo versus relapsed stage IV gastric and gastroesophageal junction (GEJ) adenocarcinoma [abstract]. J Clin Oncol 2018;36(no. 4 suppl):148.
13. DeVita VT, Lawrence TS, Rosenberg SA. DeVita, Hellman, and Rosenberg’s cancer: principles & practice of oncology. 9th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2011.
14. Siewert JR, Hölscher AH, Becker K, Gössner W. [Cardia cancer: attempt at a therapeutically relevant classification]. [Article in German.] Chirurg 1987;58:25–32.
15. Amin MB, Edge SB, Greene FL, et al, eds. AJCC cancer staging manual. 8th ed. New York: Springer; 2017.
16. Wagner AD, Syn NL, Moehler M, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 2017;8:CD004064.
17. Qiu H, Zhou Z. [Updates and interpretation on NCCN clinical practice guidelines for gastric cancer 2017 version 5]. [Article in Chinese.] Zhonghua Wei Chang Wai Ke Za Zhi 2018;21:160–4.
18. Webb A, Cunningham D, Scarffe JH, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol 1997;15:261–7.
19. Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006;24:4991–7.
20. Cunningham D, Okines AF, Ashley S. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2010;362:858–9.
21. Al-Batran SE, Hartmann JT, Hofheinz R, et al. Biweekly fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) for patients with metastatic adenocarcinoma of the stomach or esophagogastric junction: a phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol 2008;19:1882–7.
22. Guimbaud R, Louvet C, Ries P, et al. Prospective, randomized, multicenter, phase III study of fluorouracil, leucovorin, and irinotecan versus epirubicin, cisplatin, and capecitabine in advanced gastric adenocarcinoma: a French intergroup (Federation Francophone de Cancerologie Digestive, Federation Nationale des Centres de Lutte Contre le Cancer, and Groupe Cooperateur Multidisciplinaire en Oncologie) study. J Clin Oncol 2014;32:3520–6.
23. Boku N. HER2-positive gastric cancer. Gastric Cancer 2014;17:1–12.
24. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687–97.
25. Tabernero J, Hoff PM, Shen L, et al. Pertuzumab + trastuzumab + chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer: Final analysis of a Phase III study (JACOB) [abstract]. Ann Oncol 2017;28(suppl 5):6160.
26. Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31–9.
27. Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224–35.
28. Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968–76.
29. Van Cutsem E, de Haas S, Kang YK, et al, Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol 2012;30:2119–27.
30. Makiyama A, Sagara K, Kawada J, et al. A randomized phase II study of weekly paclitaxel ± trastuzumab in patients with HER2-positive advanced gastric or gastro-esophageal junction cancer refractory to trastuzumab combined with fluoropyrimidine and platinum: WJOG7112G (T-ACT) [abstract]. J Clin Oncol 2018;36(no. 15 suppl):4011.
31. Satoh T, Xu RH, Chung HC, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol 2014;32:2039–49.
32. Thuss-Patience PC, Shah MA, Ohtsu A, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol 2017;18:640–53.
33. Ohtsu A, Ajani JA, Bai YX, et al. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol 2013;31:3935–43.
34. Lordick F, Kang YK, Chung HC, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol 2013;14:490–9.
35. Waddell T, Chau I, Cunningham D, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol 2013;14:481–9.
36. Catenacci DVT, Tebbutt NC, Davidenko I, et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:1467–82.
37. Shah MA, Bang YJ, Lordick F, et al. Effect of fluorouracil, leucovorin, and oxaliplatin with or without onartuzumab in HER2-negative, MET-positive gastroesophageal adenocarcinoma: the METGastric randomized clinical trial. JAMA Oncol 2017;3:620–7.
38. Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol 2018;4(5):e180013.
1. Noone AM, Cronin KA, Altekruse SF, et al. Cancer incidence and survival trends by subtype using data from the Surveillance Epidemiology and End Results Program, 1992-2013. Cancer Epidemiol Biomarkers Prev 2017;26:632–41
2. Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol 2017;3:524–8.
3. Sitarz R, Skierucha M, Mielko J, et al. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res 2018;10:239–48.
4. Olefson S, Moss SF. Obesity and related risk factors in gastric cardia adenocarcinoma. Gastric Cancer 2015;18:23–32.
5. Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett 2014;345:196–202.
6. Espinoza JL, Matsumoto A, Tanaka H, Matsumura I. Gastric microbiota: An emerging player in Helicobacter pylori-induced gastric malignancies. Cancer Lett 2018;414:147–52.
7. Chmiela M, Gonciarz W. Molecular mimicry in Helicobacter pylori infections. World J Gastroenterol 2017;23:3964–77.
8. Isobe Y, Nashimoto A, Akazawa K, et al. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer 2011;14:301–16.
9. Ford A, Gurusamy KS, Delaney B, et al. Eradication therapy for peptic ulcer disease in Helicobacter pylori-positive patients. Cochrane Database Syst Rev 2004(4):CD003840.
10. Karimi P, Islami F, Anandasabapathy S, et al. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 2014;23:700–13.
11. Van Cutsem E, Sagaert X, Topal B, et al. Gastric cancer. Lancet 2016;388:2654–64.
12. Chan BA , Sim HW, Natori A, et al. Survival outcomes for de novo versus relapsed stage IV gastric and gastroesophageal junction (GEJ) adenocarcinoma [abstract]. J Clin Oncol 2018;36(no. 4 suppl):148.
13. DeVita VT, Lawrence TS, Rosenberg SA. DeVita, Hellman, and Rosenberg’s cancer: principles & practice of oncology. 9th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2011.
14. Siewert JR, Hölscher AH, Becker K, Gössner W. [Cardia cancer: attempt at a therapeutically relevant classification]. [Article in German.] Chirurg 1987;58:25–32.
15. Amin MB, Edge SB, Greene FL, et al, eds. AJCC cancer staging manual. 8th ed. New York: Springer; 2017.
16. Wagner AD, Syn NL, Moehler M, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 2017;8:CD004064.
17. Qiu H, Zhou Z. [Updates and interpretation on NCCN clinical practice guidelines for gastric cancer 2017 version 5]. [Article in Chinese.] Zhonghua Wei Chang Wai Ke Za Zhi 2018;21:160–4.
18. Webb A, Cunningham D, Scarffe JH, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol 1997;15:261–7.
19. Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006;24:4991–7.
20. Cunningham D, Okines AF, Ashley S. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2010;362:858–9.
21. Al-Batran SE, Hartmann JT, Hofheinz R, et al. Biweekly fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) for patients with metastatic adenocarcinoma of the stomach or esophagogastric junction: a phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol 2008;19:1882–7.
22. Guimbaud R, Louvet C, Ries P, et al. Prospective, randomized, multicenter, phase III study of fluorouracil, leucovorin, and irinotecan versus epirubicin, cisplatin, and capecitabine in advanced gastric adenocarcinoma: a French intergroup (Federation Francophone de Cancerologie Digestive, Federation Nationale des Centres de Lutte Contre le Cancer, and Groupe Cooperateur Multidisciplinaire en Oncologie) study. J Clin Oncol 2014;32:3520–6.
23. Boku N. HER2-positive gastric cancer. Gastric Cancer 2014;17:1–12.
24. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687–97.
25. Tabernero J, Hoff PM, Shen L, et al. Pertuzumab + trastuzumab + chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer: Final analysis of a Phase III study (JACOB) [abstract]. Ann Oncol 2017;28(suppl 5):6160.
26. Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31–9.
27. Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224–35.
28. Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968–76.
29. Van Cutsem E, de Haas S, Kang YK, et al, Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol 2012;30:2119–27.
30. Makiyama A, Sagara K, Kawada J, et al. A randomized phase II study of weekly paclitaxel ± trastuzumab in patients with HER2-positive advanced gastric or gastro-esophageal junction cancer refractory to trastuzumab combined with fluoropyrimidine and platinum: WJOG7112G (T-ACT) [abstract]. J Clin Oncol 2018;36(no. 15 suppl):4011.
31. Satoh T, Xu RH, Chung HC, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol 2014;32:2039–49.
32. Thuss-Patience PC, Shah MA, Ohtsu A, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol 2017;18:640–53.
33. Ohtsu A, Ajani JA, Bai YX, et al. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol 2013;31:3935–43.
34. Lordick F, Kang YK, Chung HC, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol 2013;14:490–9.
35. Waddell T, Chau I, Cunningham D, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol 2013;14:481–9.
36. Catenacci DVT, Tebbutt NC, Davidenko I, et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:1467–82.
37. Shah MA, Bang YJ, Lordick F, et al. Effect of fluorouracil, leucovorin, and oxaliplatin with or without onartuzumab in HER2-negative, MET-positive gastroesophageal adenocarcinoma: the METGastric randomized clinical trial. JAMA Oncol 2017;3:620–7.
38. Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol 2018;4(5):e180013.
Transfusion Medicine
INTRODUCTION
Transfusion therapy is an essential part of hematology practice, allowing for curative therapy of diseases such as leukemia, aplastic anemia, and aggressive lymphomas. Nonetheless, transfusions are associated with significant risks, including transmission of infections and transfusion-related reactions. Controversy remains about key issues in transfusion therapy, such as triggers for red cell transfusions. This article reviews the available blood products (Table 1) and indications for transfusion along with the associated risks, and also discusses specific clinical situations, such as massive transfusion.
BLOOD PRODUCTS
WHOLE BLOOD
Whole blood is the product of 1 unit of donated blood plus anticoagulant/preservative, and by definition contains 1 unit of plasma and red cells. Whole blood can be stored for 5 weeks. Although it was the standard product in the past, whole blood is rarely used since 1 unit of donated blood can now be fractionated into 1 unit of red blood cells (RBC), 1 unit of platelets, and 1 unit of fresh frozen plasma (FFP). Thus, the use of whole blood for just a single transfusion represents a waste of resources. There are 2 exceptions. One is autologous blood donations, which are whole blood units. Second, whole blood is increasingly being used in massive transfusions for trauma patients, with the rationale being that all essential blood components are being transfused at once.1
PACKED RED CELLS
The remaining red cell mass after most of the plasma is removed is called the “packed” red cell unit (hematocrit = 70%–80%), and so red cells are often called “packed” red cells, or PRBC. A preservative is added to improve the flow of blood and to provide “nutrients” for the red cells, and this reduces the hematocrit to approximately 60%. The volume of a red cell unit is approximately 340 mL. In the average adult, 1 unit of RBC raises the hematocrit by 3%. The indications for transfusion of red cells are to increase red cell mass, and thus oxygen delivery, in patients who are compromised by their anemia.
Several randomized trials have helped define the indications for red cell transfusions and justify lower hematocrit thresholds for initiating transfusion. The TRICC (Transfusion Requirements in Critical Care Investigators for the Canadian Critical Care Trials Group) trial showed that in critical care patients (30-day mortality, 18.7%–23.3%), a conservative transfusion strategy of waiting until the hematocrit was below 21% had the same outcomes as transfusing at a threshold of 24%.2 The TRACS (Transfusion Requirements After Cardiac Surgery) trial showed that a hematocrit target of 24% had the same benefit as a target of 30% in patients who had undergone cardiac bypass surgery.3 For patients with acute myocardial infarction, the outcomes were worse with aggressive transfusion at a hematocrit of 30% compared to 24%.4 In patients with upper gastrointestinal bleeding, a hemoglobin transfusion trigger of 7 g/dL was associated with a lower mortality than a trigger of 9 g/dL (5% versus 9%).5 Finally, the FOCUS (Transfusion Trigger Trial for Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair) trial showed that in older patients (average age 80 years) who had undergone hip fracture surgery, transfusions based on symptoms and not a fixed trigger of 30% had the same outcomes but considerable savings in blood products.6 Based on these trials, decisions regarding when to transfuse patients should be based on symptoms and not “numbers.” Young patients, especially those with reversible anemias, can tolerate low blood counts and should not be transfused based on an arbitrary number.
PLATELETS
Several types of platelet products exist. One unit of platelet concentrate is derived from 1 unit of donor blood. Plateletpheresis from volunteer donors is also used to harvest platelets, with the resulting product referred to as plateletpheresis platelets. One unit of single-donor (pheresis) platelets is equivalent to 6 platelet concentrates. Finally, HLA-matched platelets are single-donor pheresis units that are obtained from an HLA-matched donor. This product should be ordered only if there is evidence of HLA antibodies (see Platelet Alloimmunization section).
The dose of platelets for the average patient is 6 units of platelet concentrate or 1 pheresis unit. In theory, 1 unit of platelet concentrate can raise the count by 5 to 7 × 103/µL, but often this response is blunted by concurrent illness or bleeding. In patients who appear to have a poor response, the platelet count can be checked 15 minutes after platelet infusion. No rise or a minimal rise (< 2 × 103/µL) in the platelet count is suggestive of platelet refractoriness, while a good 15-minute response but poor 24-hour count is more suggestive of consumption—fever, sepsis, drug, or splenomegaly—and not refractoriness.
The indication for platelet transfusion depends on the clinical situation. For patients with immune thrombocytopenia, platelets should not be transfused unless there is life-threatening bleeding. For stable patients with marrow aplasia from chemotherapy, a cut-off of a morning platelet count of less than 10 × 103/µL has been shown to be as safe as higher levels for prophylactic transfusions.7 For patients with active bleeding, the platelet count should be kept above 50 × 103/µL. Patients with acquired or inherited platelet dysfunction may benefit from transfusion no matter the platelet count.
Platelet Alloimmunization
Patients exposed to transfused white cells with different HLA antigens can develop antibodies to these antigens.8 Anti-HLA antibodies are common in patients who previously have received transfused blood that is not leukodepleted and in patients who have been pregnant. Since platelets carry class I HLA antigens, they will be rapidly destroyed by anti-HLA antibodies when transfused into these patients. In patients transfused for aplastic anemia or myelodysplasia, as many as 90% will become HLA-immunized. The incidence is lower in patients receiving chemotherapy but still can be as high as 60% to 90%.9,10 Patients who have developed anti-HLA antibodies can respond to transfused platelets matched for HLA antigens. Unfortunately, some patients will either be a rare HLA type or be so heavily immunized that they will not respond to any platelet transfusion.
The significance of alloimmunization centers on 2 concepts: recognition and avoidance. Patients with HLA antibodies will fail to have an increment of their platelet counts with transfusions. Accordingly, patients who do not experience an increase in their count 15 minutes after the transfusion may have HLA antibodies. One can test for the presence of anti-HLA antibodies, although some patients instead have specific antiplatelet antibodies that will not respond to HLA-matched platelets. In patients who have been pregnant or previously transfused and are scheduled to undergo transplant or aggressive chemotherapy, it is wise to test for anti-HLA antibodies in order to plan their transfusion needs. The evidence suggests that transfused white cells are responsible for initiating the anti-HLA response. Trials have shown that giving leukodepleted blood products may reduce the incidence of alloimmunization, so patients who are not HLA-alloimmunized should receive only leukodepleted products.11A difficult problem is bleeding in patients who are refractory to platelet transfusion.12 Patients who test positive for the presence of anti-HLA antibodies can receive transfusions of HLA-matched platelets.13 Unfortunately, matched platelet transfusions are not effective in 20% to 70% of these patients. Also, since some loci are difficult to match, effective products may be unavailable. Finally, as many as 25% of patients have antiplatelet antibodies in which HLA-matched products will be ineffective. Platelet cross-matching can be performed to find compatible units for these patients, but this may not always be successful. In the patient who is totally refractory to platelet transfusion, consider drugs as an etiology of antiplatelet antibodies (especially vancomycin).14 Use of antifibrinolytic agents such as epsilon-aminocaproic acid or tranexamic acid may decrease the incidence of minor bleeding, but these are ineffective for major bleeding. “Platelet drips”—infusing either a platelet concentrate per hour or 1 plateletpheresis unit every 6 hours—may be given as a continuous infusion, but there is no evidence that this is helpful.15
FRESH FROZEN PLASMA
FFP is made from 1 unit of donated whole blood, with an average volume of 225 mL per unit. One unit of FFP can increase coagulation factor levels by 5% and fibrinogen by 10 mg/dL in the average stable patient. FFP can take 20 to 30 minutes to thaw before use, so in situations where FFP is needed quickly, the blood bank must be informed to “keep ahead” some units. Units of FFP that have been thawed but not used can be stored refrigerated for 5 days to prevent wasting blood products.
The indications for FFP are limited to several situations. These include a documented coagulation defect that can be corrected by a reasonable amount of FFP, such as factor V deficiency and factor XI deficiency, disseminated intravascular coagulation (DIC), reversal of warfarin, and massive transfusions. FFP is also used for the therapy of thrombotic thrombocytopenic purpura.
There is little justification for FFP transfusion in many of the clinical settings in which it is commonly used. For example, FFP is given for minor elevations of the INR in patients with liver disease, despite literature showing not only that the INR rise is not reflective of coagulation defects, but also that patients with liver disease may even be thrombophilic.16,17 Reviews of FFP use found limited evidence-based indications for its use.18,19 Also, several studies have shown that transfusion of FFP is not effective at reversing minor elevations of the INR (1.3–1.8).20 In a meta-analysis, FFP was associated with increased risk for lung injury and a trend toward increased mortality.18
CRYOPRECIPITATE
Cryoprecipitate is produced from 1 unit of FFP that is thawed at 4°C. The precipitate is resuspended with 10 mL of saline or FFP and refrozen for storage. One unit contains at least 150 mg of fibrinogen and 80 units of factor VIII, along with von Willebrand factor. Thawing time for cryoprecipitate is approximately 20 minutes.
Cryoprecipitate is used to raise the fibrinogen level in patients with DIC or massive transfusion with hemodilution. It is third-line therapy in the treatment of type 1 von Willebrand disease and is second-line therapy in the treatment of patients with other types of von Willebrand disease. Currently, von Willebrand factor concentrates are the preferred replacement product for von Willebrand disease. Cryoprecipitate can be used as a source of factor VIII for hemophiliacs, but the preferred product for these patients is the super pure factor VIII concentrates or recombinant products. Cryoprecipitate can also be used to shorten the bleeding time of uremic patients, but its effectiveness for this is controversial.
GRANULOCYTES
Granulocytes are harvested by leukopheresis of normal donors, with a target yield of 1010 granulocytes from each donor. To reach this target, the donors are often “stimulated” with neutrophil growth factors. The harvesting procedure can take 3 hours and is associated with some risks to the donor (eg, citrate toxicity). The current indications for granulocytes are very limited since the advent of neutrophil growth factors and improved antimicrobials.21 They can be useful in the neutropenic patient with a documented bacterial infection in whom the white blood cell count is not expected to recover in the near future. Given the difficulty of keeping the count up, these transfusions have been mainly used in treating small children.
SPECIAL BLOOD PRODUCTS
IRRADIATED BLOOD PRODUCTS
Irradiation of blood is performed for only one reason: to prevent transfusion-related graft-versus-host disease (TGVHD) (Table 2).22 The irradiation can be performed at the blood center or in the transfusion service of larger hospitals. The units are not radioactive and can be transfused safely to other patients. There is increased leakage of potassium in irradiated units of blood, so the units need to be transfused within 14 days; in patients potentially sensitive to potassium (eg, neonates), the units must be transfused within 24 hours. Patients undergoing stem cell transplant, those receiving either interuterine transfusions or products from relatives, any patient with Hodgkin disease or receiving purine analogs or alemtuzumab, and patients with severe congenital immune deficiencies should receive irradiated blood. Most would also advocate that patients with hematologic malignances receiving chemotherapy receive irradiated products, but this is more controversial.
LEUKPDEPLETED BLOOD
Contamination of blood products by white blood cells is increasingly being recognized as a possible cause of adverse effects in transfused patients, including febrile transfusion reactions, inducing HLA alloimmunization, immunosuppression, disease transmission, and TGVHD. Reducing white cells can reduce the incidence of all of these complications except TGVHD. Currently, white cells are removed by infusion through filters that trap the cells. This can be done either at the bedside, in the blood bank, or at the donor center. The majority of red cells provided by blood centers in many areas of the country are already leukoreduced, eliminating the need for labor-intensive filtration at the transfusion center or bedside. Platelets collected by plateletpheresis methods can also be made leukocyte-poor. The current indications for leukodepleted productions are:
- Prevention of febrile transfusion reactions in patients with previous documented reactions
- Prevention of HLA alloimmunization (ineffective if patient has received 1 or more blood products not leukodepleted or is already HLA immunized)
- Prevention of cytomegalovirus (CMV) infection
CMV-NEGATIVE BLOOD
CMV can be transmitted through any cellular blood product—red cells and platelets. For patients who are CMV-negative and receiving transplants, especially stem cell transplants, a new CMV infection can be devastating.21 For years only blood from CMV-negative donors was used to transfuse CMV-negative patients. This policy is effective in preventing CMV infection, but because 50% of the population is positive for CMV antibodies, it may potentially lead to shortages of products that could be transfused to the patient. Currently, leukoreduced blood products are used since leukofiltration of the blood is just as effective as transfusion of CMV-negative blood in preventing infections and allows greater use of all blood products.23
COMPLICATIONS OF TRANSFUSIONS
HEMOLYTIC TRANSFUSION REACTION
There are 2 forms of hemolytic reactions—immediate and delayed.24 The immediate reaction is associated with fevers, hypotension, back pain, and oliguria. In severe cases, DIC and renal failure may occur. The immediate reaction is due to transfusion of blood that reacts with the recipient’s preformed high-titer blood antigen antibodies, most often to ABO. This is fatal 2% of the time and occurs almost always as a result of errors in correct identification of the patient. Reactions are due to recipient antibodies attacking donated RBCs, resulting in release of hemoglobin and red cell membrane–antigen complexes. These complexes are believed to lead to the hypotension, fevers, chills, and renal damage associated with the hemolytic reaction. Treatment consists of immediately stopping the transfusion, notifying the blood bank, vigorous intravenous hydration to keep the urine output over 100 mL/hr, and supportive therapy.
The delayed reaction can range in severity from an abrupt drop in the hematocrit to normal response to transfusion but the patient developing a positive Coombs’ test. The delayed response is due to an anamnestic response to blood-group antigens. When the patient is exposed to the same antigen, there is a rise in antibody titer leading to the reaction. Some alloantibodies can lead to a brisk reaction, most often anti-Kidd. The frequency with which delayed transfusion reactions occur is underestimated because mild reactions often do not get worked up or even discovered.
ALLERGIC REACTIONS
Allergic reactions are common (1%–3% of transfusions) and occur in patients having antibodies to proteins in donor blood, which can lead to hives and itching with transfusions. Most of the time these allergic reactions are mild and can be treated with antihistamines. Prophylaxis with antihistamines is not indicated for future transfusions unless the reactions are frequent. Rarely these reactions can be associated with shock and hypotension. Patients who are immunoglobulin (Ig) A–deficient can develop anaphylactic reactions to IgA-containing blood products. Patients with severe allergic reactions need to have their IgA measured and, if deficient, receive only washed units or plasma from IgA-deficient donors to prevent future severe reactions.
FEBRILE REACTIONS
The most common transfusion reaction is a febrile reaction that occurs after the transfusion starts and that sometimes can be complicated by chills. This reaction often occurs due to the presence of leukocyte debris and cytokines in the donated blood. Therapy is supportive and involves stopping the transfusion and administering acetaminophen, but since hemolytic transfusion reactions can present with fever all patients need to be thoroughly evaluated. The incidence of reactions can be decreased by using leukodepleted blood and plateletpheresis platelets. Most patients do not benefit from receiving prophylactic acetaminophen for future transfusion unless they have multiple reactions.
TRANSFUSION-RELATED ACUTE LUNG INJURY
Once thought a rare complication, transfusion-related acute lung injury (TRALI) is increasingly being recognized, with an incidence of approximately 1:5000 patients; it is now the most frequent cause of transfusion-related death.24,25 TRALI is noncardiac pulmonary edema and typically manifests clinically with hypoxemia, fever, bilateral infiltrates, and hypotension 2 to 6 hours after blood is given. Ventilatory support is often required. Recovery is usually rapid (24–48 hours) and complete. The etiology is complex. In many cases, transfused anti-HLA antibodies react with the recipient’s white cells leading to pulmonary damage. Another theory is that transfusion of preformed cytokines leads to pulmonary damage. Because plasma products from multiparous women are most often associated with anti-HLA antibodies, the restricted use of blood products from women has decreased the incidence of TRALI over the past few years.26
TRANSFUSION-ASSOCIATED CIRCULATORY OVERLOAD
Increasingly it being recognized that volume overload resulting from transfusions can lead to significant morbidity.27 Patients with heart or renal disease or patients who already have compromised fluid status are at risk for transfusion-associated circulatory overload (TACO). Another risk factor is transfusion of multiple blood products. Patients with TACO develop dyspnea within 6 hours of transfusion, but do not have fever or rash with the dyspnea. The diagnosis is made by demonstrating circulatory overload (eg, high venous pressure, B-type natriuretic peptide). Treatment is aggressive diuresis. Strategies to prevent TACO include judicious use of blood products, especially in patients at risk for TACO, and the use of prophylactic diuretics, especially with red cell or plasma transfusions.28
TRANSFUSION-RELATED GRAFT-VERSUS-HOST DISEASE
TGVHD is a rare reaction, but one that is most often fatal.29 TGVHD occurs when donor lymphocytes attack the blood recipient’s organs—skin, liver, intestines, and marrow. This is very rare in the normal blood recipient unless the donor and recipient share some HLA haplotypes.30 In immunosuppressed patients, TGVHD can occur with lesser degrees of HLA similarity, with cases reported in blood recipients who are mainly patients with Hodgkin disease or acute leukemia undergoing chemotherapy, and in patients receiving purine analogs. TGVHD had not been reported in AIDS patients despite profound immunosuppression, perhaps because the milieu of the patient does not allow lymphocyte expansion. Symptoms of TGVHD are an erythematous rash that may progress to epidermal toxic necrolysis, liver dysfunction, diarrhea, and pancytopenia. TGVHD is prevented by irradiating blood products given to at-risk patients with 2500 to 3500 rads. Directed blood donation from all blood relatives should also be irradiated. TGVHD cannot be prevented by leukopoor blood because the minute amount of lymphocytes that are not filtered still can lead to these complications.
POST-TRANSFUSION PURPURA
Patients with post-transfusion purpura (PTP) develop severe thrombocytopenia (< 10 × 103/µL) with often severe bleeding 1 to 2 weeks after receiving any type of blood product.31 Patients who develop PTP most often lack platelet antigen PLA1 or other platelet antigens. For unknown reasons, exposure to the antigens from the transfusion leads to rapid destruction of the patient’s own platelets. The diagnostic clue is thrombocytopenia in a patient, typically female, who has received a red cell or platelet blood product in the past 7 to 10 days. Treatment consists of intravenous immunoglobulin32 and plasmapheresis to remove the offending antibody. If patients with a history of PTP require further transfusions, only PLA1-negative platelets should be given.
IRON OVERLOAD
Every transfusion of red cells delivers approximately 250 mg of iron to the recipient. Since there is no natural way of ridding the body of iron, heavily transfused patients are at risk of iron overload. This is most often seen in children heavily transfused for thalassemia. Starting in the second decade of life, these individuals will develop endocrinopathies due to iron overload, liver problems, and often fatal cardiomyopathies. Studies have shown that chelation of iron with deferoxamine can be effective in preventing this fatal complication.33 Oral iron chelators such as deferasirox and deferiprone are also effective. The risk of iron overload in heavily transfused patients with myelodysplasia or other transfusion-dependent anemias is unclear, and uncertainty exists about the need for chelation.34
Young patients who face years of transfusions should be started on iron chelation to avoid iron overload. For older patients with transfusion-dependent anemia, iron chelation therapy should be considered if their life expectancy is long (years to decades) or special studies such as T2-weighted cardiac magnetic resonance imaging showing iron overloading.35
INFECTIOUS COMPLICATIONS
Concern over transmission of HIV infection via blood products in the late 1980s led to both a reduction in blood product use and a greater awareness of infectious complications of transfusion and their prevention. However, no blood product can ever be assumed to be safe for 2 reasons. One is that blood products can transmit infections during a “window period”—the time before a contaminated product can be detected by testing. The second is that blood is not screened for all potential infections (eg, babesiosis or new infections such as West Nile virus at the start of the outbreak). Risk of infection is reduced in 2 ways: deferral of potential infectious donors and blood product testing.
As part of the donation process, potential blood donors are asked a series of questions to see if they have risk factors for infections (eg, recent travel to malarious areas, recent tattoos), and if they answer positive are deferred from donating blood. Blood products are then tested for infectious agents by a combination of methods including detection of viral antigen, antibody response to infections, and more recently polymerase chain reaction (PCR).36 Current screening includes syphilis testing; testing for antibodies to HIV, HTLV (human T-lymphotropic virus), hepatitis C virus, hepatitis B core antigen (HBcAg), hepatitis B surface antigen, and PCR for HIV, hepatitis B virus, HCV, and West Nile virus. Some centers also test for Trypanosoma cruzi, the cause of Chagas disease.
In the past, the numerically most common transfusion-related disease was hepatitis, first B and then C.37,38 The first step in eliminating these infections was to stop paying donors for blood products. With the introduction of effective testing for hepatitis B and then C, the incidence of transfusion-related hepatitis has plummeted.36 For example, with the introduction of a diagnostic test for hepatitis C, the estimated risk has fallen from 5% to less than 1 per million. Currently, the risk of transmission of hepatitis B and C, HIV, and HTLV is less than 1 in a million.38
Despite this testing, blood transfusions can transmit a variety of infections, including malaria and babesiosis.39 Any new blood-borne infection introduced into the population can get into the blood supply as well. For example, at the start of the West Nile virus epidemic, there was a cluster of transfusion-transmitted cases that resulted in severe and sometimes fatal illness in immunosuppressed patients, but this issue has been addressed with the development of a PCR assay for screening blood.40 The rate of transfusion-related babesiosis has been increasing and screening for the causative parasite is being considered.
MASSIVE TRANSFUSIONS
Acutely bleeding patients can require large amounts of transfusion products. Early data showed high mortality rates with transfusion of more than 20 units of blood,41 but with modern blood banking techniques and improved laboratory testing, this rate has decreased dramatically, with survival rates of 43% to 70% in patients transfused with more than 50 units of blood.42
The basic approach to massive transfusions is to first transfuse the patient to maintain hemodynamic stability while specific blood tests are being obtained, and then to use the results of these early tests to guide the rest of the resuscitation. An important component is the ability to rapidly deliver standard packages of red cells, usually 6 to 10 units at a time, to the bleeding patient. To avoid delay while the patient’s blood is being typed, the first products delivered are blood group O Rh-positive units. Given the shortage of Rh-negative blood, this should be reserved for only empiric therapy of women of child-bearing age. Once the blood type is known, the patient can be switched over to type-specific blood.
In the past decade, there has been a shift toward increasing the amount of plasma given to patients receiving massive transfusions. This shift has occurred for 2 reasons. One is that modeling of coagulation changes in massive bleeding suggests the need for larger amounts of plasma to correct defects than have previously been recommended.43 The other reason is based on analysis of resuscitation protocols used in military and civilian trauma centers showing that giving red cells and plasma units in a 1:1 ratio appears to be associated with improved outcomes in massive transfusion. Several studies have extended this concept to platelets, again suggesting improved survival with 1 unit of random donor platelets given 1:1 with red cells and plasma units. The PROPPR (Prospective Observational Multicenter Major Trauma Transfusion) study compared a 1:1:1 to 1:1:2 ratio in patients with severe trauma and major bleeding and found less exsanguination and faster achievement of hemostasis in the first 24 hours.44 This has led to the widespread adoption of the 1:1 ratio by most trauma centers, and by default to other massive transfusion situations despite the lack of clinical trial data.45
One barrier to increased use is that plasma is kept frozen and requires 20 minutes to thaw. Many institutions are now keeping inventories of thawed plasma available for immediate use, ranging from 2 to 4 units of group AB plasma to keeping their entire inventory as liquid plasma.46 Plasma that is thawed but not used can be relabeled as “thawed plasma” and kept for up to 5 days. Also, many centers now use group A plasma for massive transfusions as this rarely leads to transfusion reactions and is much more available.47 Research is currently under way on lyophilized plasma, which can be stored at room temperature and can be rapidly reconstituted for emergency use.
The standard approach for laboratory testing is obtaining 5 tests: hematocrit, platelet count, INR/prothrombin time, activated partial thromboplastin time (aPTT), and fibrinogen.48 Product selection is guided by these tests, and they are repeated at regular intervals during the massive transfusion. A typical protocol is shown in Table 3. It is important as part of any protocol to have a flow chart that records laboratory results and products given that any member of the team can easily view.
The transfusion threshold for a low hematocrit depends on the stability of the patient. If the hematocrit is below 30% and the patient is bleeding or hemodynamically unstable, one should transfuse packed red cells. Stable patients can tolerate lower hematocrits, and an aggressive transfusion policy may even be detrimental.2,49 If the patient is bleeding, has florid DIC, or has received platelet aggregation inhibitors, then keeping the platelet count above 50 ×
While in the past fibrinogen targets of 50 to 100 mg/dL were recommended, recent data indicate that a target of 150 mg/dL or higher may be more appropriate.51–53 Severe fibrinolysis may occur in certain clinical situations such as brain injuries, hepatic trauma, or ischemic limb reperfusion, and the use of large amounts of cryoprecipitate can be anticipated. In patients with an INR greater than 2 and an abnormal aPTT, one can give 2 to 4 units of FFP. For an aPTT greater than 1.5 times normal, 2 to 4 units of plasma should be given. Elevation of the aPTT above 1.8 times normal control is associated with microvascular bleeding in trauma patients.54 Patients with marked abnormalities (eg, anaPTT more than 2 times normal) may require aggressive therapy with at least 15 to 30 mL/kg (4–8 units for an average adult) of plasma.55
Recently there has been increasing interest in the use of thromboelastography (TEG) in massive transfusion.56 This is a point-of-care assay performed on fresh whole blood that can assess multiple facets of hemostasis, including coagulation, platelet function, and fibrinolysis.57,58 TEG is performed by placing a 0.35-mL sample of whole blood into an oscillating container with a sensor pin that measures the force of thrombus formation. TEG measures 5 parameters:
- r time: time from starting TEG until clot formation
- K time: time needed for tracing to go from 2 mm to 20 mm
- alpha angle: slope of tracing between r and K time
- MA: greatest amplitude of TEG tracing
- Whole blood lysis index: amplitude of tracing 60 minutes after MA.
Several centers have incorporated TEG into resuscitation protocols that include standardized strategies for responding to abnormalities. Data suggest that use of TEG may decrease the use of blood products, especially in cardiac surgery, but this has not been prospectively studied in massive transfusions.56,59
COMPLICATIONS OF MASSIVE TRANSFUSIONS
Electrolyte abnormalities are unusual even in patients who receive massive transfusions.60 Platelet concentrates and plasma contain citrate that can chelate calcium. However, the citrate is rapidly metabolized, and it is rare to see clinically significant hypocalcemia. Although empiric calcium replacement is often recommended, one study suggests that this is associated with a worse outcome and should not be done.61 If hypocalcemia is a clinical concern, then levels should be drawn to guide therapy. Stored blood is acidic, with a pH of 6.5 to 6.9. However, acidosis attributed solely to transfused blood is rare and most often is a reflection of the patient’s stability. Empirical bicarbonate replacement has been associated with severe alkalosis and is not recom mended.62,63 Although potassium leaks out of stored red cells, even older units of blood contain only 8 mEq/L of potassium, so hyperkalemia is usually not a concern.
PATIENTS WITH AUTOIMMUNE HEMOLYTIC ANEMIA
Patients with autoimmune hemolytic anemia can be difficult to transfuse,64 because the autoantibody can interfere with several aspects of the transfusion services evaluation. In some patients the autoantibody can be so strong that the patient’s blood type cannot be determined. In most patients, the final step of the cross-match—mixing the donor blood with recipient plasma—will show noncompatibility due to the autoantibodies reacting with any red cells.
The first step when transfusing a patient with autoimmune hemolytic anemia is to draw several tubes of blood for the transfusion service before any potential transfusions. This allows the transfusion service to remove the autoantibodies so they can screen for underlying alloantibodies. Second, if the patient requires immediate transfusion, then type-specific or O-negative blood should be given. If the patient has not been recently (months) transfused, the incidence of a severe transfusion reaction is low. The first unit should be infused slowly with close observation of the patient. For patients who have been multiply transfused, the use of an “in-vivo” cross-match may be helpful. This is where the patient is slowly transfused 10 to 15 mL of blood over 15 minutes. The the plasma and urine are then assessed for signs of hemolysis and, if negative, the remaining product is given.
REFUSAL OF BLOOD PRODUCTS
The initial step in managing patients who refuse blood products is to find out why they are refusing them. Many patients have an exaggerated fear of HIV and other infectious agents, so discussing the very low risk for infection transmission can often resolve the situation. The most common reason for refusal of blood products is religious belief. Jehovah’s Witness patients will refuse blood products due to their interpretation of the Bible.65 All members will refuse red cells, plasma, and platelets, while decisions about “derived” blood products—products made by manipulation of the original donated units—are a matter of conscience. These include cryoprecipitate, intravenous gammaglobulin, and albumin.
In an elective situation, the first step is to discuss with the patient those products that are a matter of conscience and clearly document this. The patient’s blood count and iron stores should be assessed to identify any correctible causes of anemia or low iron stores before surgery. The use of erythropoietin to correct blood counts before surgery is controversial, as this may increase thrombosis risk and is contraindicated in patients with curable tumors.
For patients with acute blood loss, use of intravenous iron combined with high-dose erythropoietin is the most common approach to raise the blood count.65 A recommended erythropoietin dose is 300 units/kg 3 times a week, dropping to 100 units/kg 3 times weekly until the goal hematocrit is reached. Another often overlooked step is to consolidate and minimize laboratory testing. The most important step is to be respectful of the patient and their beliefs. Many larger cities have liaisons that can help with interactions between Jehovah’s Witness patients and the health care system.
NON-TRANSFUSION THERAPIES FOR ACUTE BLEEDING
DESMOPRESSIN
Desmopressin (DDAVP) is a synthetic analog of antidiuretic hormone that raises the levels of both factor VIII and von Willebrand protein severalfold.66 Desmopressin is effective in supporting hemostasis in patients with a wide variety of congenital and acquired bleeding disorders. However, desmopressin does not reduce blood loss before routine surgery in a healthy patient and should not be used for this purpose.67
TRANEXAMIC ACID
Tranexamic acid is an antifibrinolytic agent that blocks the binding of plasmin to fibrin.68 This agent was first shown to be useful in disorders that involve excessive fibrinolysis69–73 or as adjunctive therapy for oral or dental procedures in patients with a bleeding diathesis. In patients with severe thrombocytopenia, the use of antifibrinolytic agents may reduce bleeding. Increasing data shows that tranexamic acid can prevent blood loss in a variety of surgeries including heart bypass, liver transplantation, and orthopedic surgery.74 Patients across these settings have decreased blood loss and need for transfusion with no increased risk of thrombosis. The CRASH-2 study showed that the use of tranexamic acid significantly reduced mortality in trauma patients.75 The WOMEN trial demonstrated that 1 g of tranexamic acid given to women with blood loss of more than 500 mL after vaginal delivery or 1000 mL after cesarean section has a risk reduction of death of 0.81 with no increased risk of thrombosis.76 Given this abundant data, it is clear tranexamic acid needs to be part of any massive transfusion protocol.77
RECOMBINANT FACTOR VIIa
Recombinant factor VIIa (rVIIa) was originally developed as a “bypass” agent to support hemostasis in hemophiliacs.78 However, the use of rVIIa for a wide array of bleeding disorders, including patients with factor VII and XI deficiency and Glanzmann thrombasthenia, has been reported.79 Increasingly, rVIIa is being used as a “universal hemostatic agent” for patients with uncontrolled bleeding from any mechanism.80 Multiple case reports have described the use of rVIIa for bleeding in cardiac surgery patients, obstetrical bleeding, reversal of anticoagulation, and trauma.81 Unfortunately, little formal trial data exists to put these anecdotes into perspective, and formal review of clinical trial results has shown no benefit.82,83 However, when used in older patients, especially those with vascular risk factors, the risk of arterial thrombosis appears to increase.84 In the trials for intracranial hemorrhage, the thrombosis rate was 5% to 9%, and rates up to 10% for arterial events were seen in older patients in a review of all trials.85–87 Given the lack of data but the evidence of risk, rVIIa use should be restricted to patients with documented bleeding disorders that have been shown to benefit by its use.
1. Yazer MH, Cap AP, Spinella PC, et al. How do I implement a whole blood program for massively bleeding patients? Transfusion 2018;58:622–8.
2. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999;340:409–17.
3. Hajjar LA, Vincent JL, Galas FR, et al. Tranfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA 2010;304:1559–67.
4. Cooper HA, Rao SV, Greenberg MD, et al. Conservative versus liberal red cell transfusion in acute myocardial infarction (the CRIT Randomized Pilot Study). Am J Cardiol 2011;108:1108–11.
5. Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013;368:11–21.
6. Carson JL, Terrin ML, Noveck H, et al; the FOCUS Investigators. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011 Dec 4. [Epub ahead of print]
7. Schiffer CA, Anderson KC, Bennett CL, et al. Platelet transfusion for patients with cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol 2001;19:1519–38.
8. Schiffer CA. Prevention of alloimmunization against platelets. Blood 1991;77:1–4.
9. Hod E, Schwartz J. Platelet transfusion refractoriness. Br J Haematol 2008;142:348–60.
10. Novotny VMJ, Van Doorn R, Witvliet MD, et al. Occurrence of allogeneic HLA and non-HLA antibodies after transfusion of prestorage filtered platelets and red blood cells: A prospective study. Blood 1995;85:1736–41.
11. McFarland J, Menitove J, Kagen L et al. Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. N Engl J Med 1997;337:1861–9.
12. Juskewitch JE, Norgan AP, De Goey SR, et al. How do I … manage the platelet transfusion-refractory patient? Transfusion 2017;57:2828–35.
13. Schiffer CA. Diagnosis and management of refractoriness to platelet transfusion. Blood Rev 2001;15:175–80.
14. Christie DJ, van Buren N, Lennon SS, Putnam JL. Vancomycin-dependent antibodies associated with thrombocytopenia and refractoriness to platelet transfusion in patients with leukemia. Blood 1990;75:518–23.
15. Dzik S. How I do it: platelet support for refractory patients. Transfusion 2007;47:374–8.
16. Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med 2011;365:147–56.
17. Lisman T, Porte RJ. Pathogenesis, prevention, and management of bleeding and thrombosis in patients with liver diseases. Res Pract Thromb Haemost 2017;1:150–61.
18. Murad MH, Stubbs JR, Gandhi MJ, et al. The effect of plasma transfusion on morbidity and mortality: a systematic review and meta-analysis. Transfusion 2010;50:1370–83.
19. Green L, Bolton-Maggs P, Beattie C, et al. British Society of Haematology Guidelines on the spectrum of fresh frozen plasma and cryoprecipitate products: their handling and use in various patient groups in the absence of major bleeding. Br J Haematol 2018;181:54–67.
20. Abdel-Wahab OI, Healy B, Dzik WH. Effect of fresh-frozen plasma transfusion on prothrombin time and bleeding in patients with mild coagulation abnormalities. Transfusion 2006;46:1279–85.
21. Price TH. Granulocyte transfusion: current status. Semin Hematol 2007;44:15–23.
22. Treleaven J, Gennery A, Marsh J, et al. Guidelines on the use of irradiated blood components prepared by the British Committee for Standards in Haematology blood transfusion task force. Br J Haematol 2011;152:35–51.
23. Thiele T, Kruger W, Zimmermann K, et al. Transmission of cytomegalovirus (CMV) infection by leukoreduced blood products not tested for CMV antibodies: a single-center prospective study in high-risk patients undergoing allogeneic hematopoietic stem cell transplantation (CME). Transfusion 2011;51:2620–6.
24. Delaney M, Wendel S, Bercovitz RS, et al; Biomedical Excellence for Safer Transfusion (BEST) Collaborative. Transfusion reactions: prevention, diagnosis, and treatment. Lancet 2016;388:2825–36.
25. Toy P, Gajic O, Bacchetti P, et al. Transfusion related acute lung injury: incidence and risk factors. Blood 2011 Nov 23. [Epub ahead of print]
26. Wiersum-Osselton JC, Middelburg RA, Beckers EA, et al. Male-only fresh-frozen plasma for transfusion-related acute lung injury prevention: before-and-after comparative cohort study. Transfusion 2011;51:1278–83.
27. Friedman T, Javidroozi M, Lobel G, Shander A. Complications of allogeneic blood product administration, with emphasis on transfusion-related acute lung injury and transfusion-associated circulatory overload. Adv Anesth 2017;35:159–73.
28. Lin CR, Armali C, Callum J, et al. Transfusion-associated circulatory overload prevention: a retrospective observational study of diuretic use. Vox Sang 2018;113:386–92.
29. Sun X, Yu H, Xu Z, et al. Transfusion-associated graft-versus-host-disease: case report and review of literature. Transfus Apher Sci 2010;43:331–4.
30. Petz LD, Calhoun L, Yam P, et al. Transfusion-associated graft-versus-host disease in immunocompetent patients: report of a fatal case associated with transfusion of blood from a second-degree relative, and a survey of predisposing factors. Transfusion 1993;33:742–50.
31. Mueller-Eckhardt C. Post-transfusion purpura. Br J Hematol 1986;64:419–24.
32. Mueller-Eckhardt C, Kiefel V. High-dose IgG for posttransfusion purpura-revisited. Blut 1988;57:163–7.
33. Modell B, Khan M, Darlison M. Survival in beta-thalassaemia major in the UK: data from the UK Thalassaemia Register. Lancet 2000;355(9220):2051–2.
34. Zeidan AM, Griffiths EA. To chelate or not to chelate in MDS: That is the question! Blood Rev 2018. pii: S0268-960X(17)30128-5.
35. Konen E, Ghoti H, Goitein O, et al. No evidence for myocardial iron overload in multitransfused patients with myelodysplastic syndrome using cardiac magnetic resonance T2 technique. Am J Hematol 2007;82:1013–16.
36. Squires JE. Risks of transfusion. South Med J 2011;104:762–9.
37. Sharma S, Sharma P, Tyler LN. Transfusion of blood and blood products: indications and complications. Am Fam Physician 2011;83:719–24.
38. Jacquot C, Delaney M. Efforts toward elimination of infectious agents in blood products. J Intensive Care Med 2018 Jan 1:885066618756589 [Epub ahead of print].
39. Herwaldt BL, Linden JV, Bosserman E, et al. Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med 2011;155:509–19.
40. Biggerstaff BJ, Petersen LR. Estimated risk of West Nile virus transmission through blood transfusion during an epidemic in Queens, New York City. Transfusion 2002;42:1019–26.
41. Wilson RF, Mammen E, Walt AJ. Eight years of experience with massive blood transfusions. J Trauma 1971;11:275–85.
42. Wade CE, del Junco DJ, Holcomb JB, et al. Variations between level I trauma centers in 24-hour mortality in severely injured patients requiring a massive transfusion. J Trauma 2011;71(2 Suppl 3):S389–S393.
43. Hirshberg A, Dugas M, Banez EI, et al. Minimizing dilutional coagulopathy in exsanguinating hemorrhage: a computer simulation. J Trauma 2003;54:454–63.
44. Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 2015;313:471–82.
45. Pasquier P, Gayat E, Rackelboom T, et al. An observational study of the fresh frozen plasma: red blood cell ratio in postpartum hemorrhage. Anesth Analg 2013;116:155–61.
46. Yuan S, Ziman A, Anthony MA, et al. How do we provide blood products to trauma patients? Transfusion 2009;49:1045–9.
47. Stevens WT, Morse BC, Bernard A, et al. Incompatible type A plasma transfusion in patients requiring massive transfusion protocol: Outcomes of an Eastern Association for the Surgery of Trauma multicenter study. J Trauma Acute Care Surg 2017;83:25–9.
48. DeLoughery TG. Coagulation defects in trauma patients: etiology, recognition, and therapy. Crit Care Clin 2004;20:13–24.
49. Blair SD, Janvrin SB, McCollum CN, Greenhalgh RM. Effect of early blood transfusion on gastrointestinal haemorrhage. Br J Surg 1986;73:783–5.
50. Counts RB, Haisch C, Simon TL, et al. Hemostasis in massively transfused trauma patients. Ann Surg 1979;190:91–9.
51. Gerlach R, Tolle F, Raabe A, et al. Increased risk for postoperative hemorrhage after intracranial surgery in patients with decreased factor XIII activity: implications of a prospective study. Stroke 2002;33:1618–23.
52. Fenger-Eriksen C, Lindberg-Larsen M, Christensen AQ, et al. Fibrinogen concentrate substitution therapy in patients with massive haemorrhage and low plasma fibrinogen concentrations. Br J Anaesth 2008;101:769–73.
53. Charbit B, Mandelbrot L, Samain E, et al. The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. J Thromb Haemost 2007;5:266–73.
54. Ciavarella D, Reed RL, Counts RB, et al. Clotting factor levels and the risk of diffuse microvascular bleeding in the massively transfused patient. Br J Haematol 1987;67:365–8.
55. Chowdhury P, Saayman AG, Paulus U, et al. Efficacy of standard dose and 30 ml/kg fresh frozen plasma in correcting laboratory parameters of haemostasis in critically ill patients. Br J Haematol 2004;125:69–73.
56. Curry NS, Davenport R, Pavord S, et al.The use of viscoelastic haemostatic assays in the management of major bleeding: A British Society for Haematology Guideline. Br J Haematol 2018. doi: 10.1111/bjh.15524. [Epub ahead of print].
57. Kashuk JL, Moore EE, Sawyer M, et al. Postinjury coagulopathy management: goal directed resuscitation via POC thrombelastography. Ann Surg 2010;251:604–14.
58. Whitten CW, Greilich PE. Thromboelastography: past, present, and future. Anesthesiology 2000;92:1223–5.
59. Girdauskas E, Kempfert J, Kuntze T, et al. Thromboelastometrically guided transfusion protocol during aortic surgery with circulatory arrest: a prospective, randomized trial. J Thorac Cardiovasc Surg 2010;140:1117–24.
60. Goskowicz R. The complications of massive tranfusion. Anesthesiology Clin North Am 1999;17:959–78.
61. Howland WS, Schwiezer O, Boyan CP. Massive blood replacement without calcuim administration. Surg Gynecol Obstet 1964;159:171–7.
62. Miller RD, Tong MJ, Robbins TO. Effects of massive transfusion of blood on acid-base balance. JAMA 1971;216:1762–5.
63. Collins JA. Problems associated with the massive transfusion of stored blood. Surgery 1974;75:274–95.
64. Petz LD. A physician’s guide to transfusion in autoimmune haemolytic anaemia. Br J Haematol 2004;124:712–6.
65. Scharman CD, Burger D, Shatzel JJ, et al. Treatment of individuals who cannot receive blood products for religious or other reasons. Am J Hematol 2017;92:1370–81
66. Leissinger C, Carcao M, Gill JC, et al. Desmopressin (DDAVP) in the management of patients with congenital bleeding disorders. Haemophilia 2014;20:158–67.
67. Desborough MJ, Oakland KA, Landoni G, et al. Desmopressin for treatment of platelet dysfunction and reversal of antiplatelet agents: a systematic review and meta-analysis of randomized controlled trials. J Thromb Haemost 2017;15:263–72.
68. Ng W, Jerath A, Wa˛sowicz M. Tranexamic acid: a clinical review. Anaesthesiol Intensive Ther 2015;47:339–50.
69. Amitrano L, Guardascione MA, Brancaccio V, Balzano A. Coagulation disorders in liver disease. Semin Liver Dis 2002;22:83–96.
70. Chang JC, Kane KK. Pathologic hyperfibrinolysis associated with amyloidosis: clinical response to epsilon amino caproic acid. Am J Clin Pathol 1984;81:382–7.
71. Anonymous. Tranexamic acid. Med Letter Drugs Therapeutics 1987;29:89–90.
72. Schwartz BS, Williams EC, Conlan MG, Mosher DF. Epsilon-aminocaproic acid in the treatment of patients with acute promyelocytic leukemia and acquired alpha-2-plasmin inhibitor defiency. Ann Intern Med 1986;105:873–7.
73. Takahashi H, Tatewaki W, Wada K, et al. Fibrinolysis and fibrinogenolysis in liver disease. Am J Hematol 1990;34:241-–5.
74. Ker K, Edwards P, Perel P, et al. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ 2012;344:e3054.
75. Shakur H, Roberts I, Bautista R, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376(9734):23–32.
76. WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet 2017;389:2105–16.
77. Godbey EA, Schwartz J. ‘Massive transfusion protocols and the use of tranexamic acid’. Curr Opin Hematol 2018 Aug 16. doi: 10.1097/MOH.0000000000000457. [Epub ahead of print]
78. Hay CR, Negrier C, Ludlam CA. The treatment of bleeding in acquired haemophilia with recombinant factor VIIa: a multicentre study. Thromb Haemost 1997;78:1463–7.
79. DeLoughery TG. Management of bleeding emergencies: when to use recombinant activated factor VII. Expert Opin Pharmacother 2006;7:25–34.
80. Aledort L. Recombinant factor VIIa Is a pan-hemostatic agent? Thromb Haemost 2000;83:637–8.
81. Logan AC, Yank V, Stafford RS. Off-label use of recombinant factor VIIa in U.S. hospitals: analysis of hospital records. Ann Intern Med 2011;154:516–22.
82. Lin Y, Stanworth S, Birchall J, Doree C, Hyde C. Use of recombinant factor VIIa for the prevention and treatment of bleeding in patients without hemophilia: a systematic review and meta-analysis. CMAJ 2011;183:E9–19.
83. Yank V, Tuohy CV, Logan AC et al. Systematic review: benefits and harms of in-hospital use of recombinant factor VIIa for off-label indications. Ann Intern Med 2011;154:529–40.
84. Pavese P, Bonadona A, Beaubien J, et al. FVIIa corrects the coagulopathy of fulminant hepatic failure but may be associated with thrombosis: a report of four cases. Can J Anaesth 2005;52:26–29.
85. Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2005;352:777–85.
86. Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2008;358:2127–37.
87. Levi M, Levy JH, Andersen HF, Truloff D. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med 2010;363:1791–1800.
INTRODUCTION
Transfusion therapy is an essential part of hematology practice, allowing for curative therapy of diseases such as leukemia, aplastic anemia, and aggressive lymphomas. Nonetheless, transfusions are associated with significant risks, including transmission of infections and transfusion-related reactions. Controversy remains about key issues in transfusion therapy, such as triggers for red cell transfusions. This article reviews the available blood products (Table 1) and indications for transfusion along with the associated risks, and also discusses specific clinical situations, such as massive transfusion.
BLOOD PRODUCTS
WHOLE BLOOD
Whole blood is the product of 1 unit of donated blood plus anticoagulant/preservative, and by definition contains 1 unit of plasma and red cells. Whole blood can be stored for 5 weeks. Although it was the standard product in the past, whole blood is rarely used since 1 unit of donated blood can now be fractionated into 1 unit of red blood cells (RBC), 1 unit of platelets, and 1 unit of fresh frozen plasma (FFP). Thus, the use of whole blood for just a single transfusion represents a waste of resources. There are 2 exceptions. One is autologous blood donations, which are whole blood units. Second, whole blood is increasingly being used in massive transfusions for trauma patients, with the rationale being that all essential blood components are being transfused at once.1
PACKED RED CELLS
The remaining red cell mass after most of the plasma is removed is called the “packed” red cell unit (hematocrit = 70%–80%), and so red cells are often called “packed” red cells, or PRBC. A preservative is added to improve the flow of blood and to provide “nutrients” for the red cells, and this reduces the hematocrit to approximately 60%. The volume of a red cell unit is approximately 340 mL. In the average adult, 1 unit of RBC raises the hematocrit by 3%. The indications for transfusion of red cells are to increase red cell mass, and thus oxygen delivery, in patients who are compromised by their anemia.
Several randomized trials have helped define the indications for red cell transfusions and justify lower hematocrit thresholds for initiating transfusion. The TRICC (Transfusion Requirements in Critical Care Investigators for the Canadian Critical Care Trials Group) trial showed that in critical care patients (30-day mortality, 18.7%–23.3%), a conservative transfusion strategy of waiting until the hematocrit was below 21% had the same outcomes as transfusing at a threshold of 24%.2 The TRACS (Transfusion Requirements After Cardiac Surgery) trial showed that a hematocrit target of 24% had the same benefit as a target of 30% in patients who had undergone cardiac bypass surgery.3 For patients with acute myocardial infarction, the outcomes were worse with aggressive transfusion at a hematocrit of 30% compared to 24%.4 In patients with upper gastrointestinal bleeding, a hemoglobin transfusion trigger of 7 g/dL was associated with a lower mortality than a trigger of 9 g/dL (5% versus 9%).5 Finally, the FOCUS (Transfusion Trigger Trial for Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair) trial showed that in older patients (average age 80 years) who had undergone hip fracture surgery, transfusions based on symptoms and not a fixed trigger of 30% had the same outcomes but considerable savings in blood products.6 Based on these trials, decisions regarding when to transfuse patients should be based on symptoms and not “numbers.” Young patients, especially those with reversible anemias, can tolerate low blood counts and should not be transfused based on an arbitrary number.
PLATELETS
Several types of platelet products exist. One unit of platelet concentrate is derived from 1 unit of donor blood. Plateletpheresis from volunteer donors is also used to harvest platelets, with the resulting product referred to as plateletpheresis platelets. One unit of single-donor (pheresis) platelets is equivalent to 6 platelet concentrates. Finally, HLA-matched platelets are single-donor pheresis units that are obtained from an HLA-matched donor. This product should be ordered only if there is evidence of HLA antibodies (see Platelet Alloimmunization section).
The dose of platelets for the average patient is 6 units of platelet concentrate or 1 pheresis unit. In theory, 1 unit of platelet concentrate can raise the count by 5 to 7 × 103/µL, but often this response is blunted by concurrent illness or bleeding. In patients who appear to have a poor response, the platelet count can be checked 15 minutes after platelet infusion. No rise or a minimal rise (< 2 × 103/µL) in the platelet count is suggestive of platelet refractoriness, while a good 15-minute response but poor 24-hour count is more suggestive of consumption—fever, sepsis, drug, or splenomegaly—and not refractoriness.
The indication for platelet transfusion depends on the clinical situation. For patients with immune thrombocytopenia, platelets should not be transfused unless there is life-threatening bleeding. For stable patients with marrow aplasia from chemotherapy, a cut-off of a morning platelet count of less than 10 × 103/µL has been shown to be as safe as higher levels for prophylactic transfusions.7 For patients with active bleeding, the platelet count should be kept above 50 × 103/µL. Patients with acquired or inherited platelet dysfunction may benefit from transfusion no matter the platelet count.
Platelet Alloimmunization
Patients exposed to transfused white cells with different HLA antigens can develop antibodies to these antigens.8 Anti-HLA antibodies are common in patients who previously have received transfused blood that is not leukodepleted and in patients who have been pregnant. Since platelets carry class I HLA antigens, they will be rapidly destroyed by anti-HLA antibodies when transfused into these patients. In patients transfused for aplastic anemia or myelodysplasia, as many as 90% will become HLA-immunized. The incidence is lower in patients receiving chemotherapy but still can be as high as 60% to 90%.9,10 Patients who have developed anti-HLA antibodies can respond to transfused platelets matched for HLA antigens. Unfortunately, some patients will either be a rare HLA type or be so heavily immunized that they will not respond to any platelet transfusion.
The significance of alloimmunization centers on 2 concepts: recognition and avoidance. Patients with HLA antibodies will fail to have an increment of their platelet counts with transfusions. Accordingly, patients who do not experience an increase in their count 15 minutes after the transfusion may have HLA antibodies. One can test for the presence of anti-HLA antibodies, although some patients instead have specific antiplatelet antibodies that will not respond to HLA-matched platelets. In patients who have been pregnant or previously transfused and are scheduled to undergo transplant or aggressive chemotherapy, it is wise to test for anti-HLA antibodies in order to plan their transfusion needs. The evidence suggests that transfused white cells are responsible for initiating the anti-HLA response. Trials have shown that giving leukodepleted blood products may reduce the incidence of alloimmunization, so patients who are not HLA-alloimmunized should receive only leukodepleted products.11A difficult problem is bleeding in patients who are refractory to platelet transfusion.12 Patients who test positive for the presence of anti-HLA antibodies can receive transfusions of HLA-matched platelets.13 Unfortunately, matched platelet transfusions are not effective in 20% to 70% of these patients. Also, since some loci are difficult to match, effective products may be unavailable. Finally, as many as 25% of patients have antiplatelet antibodies in which HLA-matched products will be ineffective. Platelet cross-matching can be performed to find compatible units for these patients, but this may not always be successful. In the patient who is totally refractory to platelet transfusion, consider drugs as an etiology of antiplatelet antibodies (especially vancomycin).14 Use of antifibrinolytic agents such as epsilon-aminocaproic acid or tranexamic acid may decrease the incidence of minor bleeding, but these are ineffective for major bleeding. “Platelet drips”—infusing either a platelet concentrate per hour or 1 plateletpheresis unit every 6 hours—may be given as a continuous infusion, but there is no evidence that this is helpful.15
FRESH FROZEN PLASMA
FFP is made from 1 unit of donated whole blood, with an average volume of 225 mL per unit. One unit of FFP can increase coagulation factor levels by 5% and fibrinogen by 10 mg/dL in the average stable patient. FFP can take 20 to 30 minutes to thaw before use, so in situations where FFP is needed quickly, the blood bank must be informed to “keep ahead” some units. Units of FFP that have been thawed but not used can be stored refrigerated for 5 days to prevent wasting blood products.
The indications for FFP are limited to several situations. These include a documented coagulation defect that can be corrected by a reasonable amount of FFP, such as factor V deficiency and factor XI deficiency, disseminated intravascular coagulation (DIC), reversal of warfarin, and massive transfusions. FFP is also used for the therapy of thrombotic thrombocytopenic purpura.
There is little justification for FFP transfusion in many of the clinical settings in which it is commonly used. For example, FFP is given for minor elevations of the INR in patients with liver disease, despite literature showing not only that the INR rise is not reflective of coagulation defects, but also that patients with liver disease may even be thrombophilic.16,17 Reviews of FFP use found limited evidence-based indications for its use.18,19 Also, several studies have shown that transfusion of FFP is not effective at reversing minor elevations of the INR (1.3–1.8).20 In a meta-analysis, FFP was associated with increased risk for lung injury and a trend toward increased mortality.18
CRYOPRECIPITATE
Cryoprecipitate is produced from 1 unit of FFP that is thawed at 4°C. The precipitate is resuspended with 10 mL of saline or FFP and refrozen for storage. One unit contains at least 150 mg of fibrinogen and 80 units of factor VIII, along with von Willebrand factor. Thawing time for cryoprecipitate is approximately 20 minutes.
Cryoprecipitate is used to raise the fibrinogen level in patients with DIC or massive transfusion with hemodilution. It is third-line therapy in the treatment of type 1 von Willebrand disease and is second-line therapy in the treatment of patients with other types of von Willebrand disease. Currently, von Willebrand factor concentrates are the preferred replacement product for von Willebrand disease. Cryoprecipitate can be used as a source of factor VIII for hemophiliacs, but the preferred product for these patients is the super pure factor VIII concentrates or recombinant products. Cryoprecipitate can also be used to shorten the bleeding time of uremic patients, but its effectiveness for this is controversial.
GRANULOCYTES
Granulocytes are harvested by leukopheresis of normal donors, with a target yield of 1010 granulocytes from each donor. To reach this target, the donors are often “stimulated” with neutrophil growth factors. The harvesting procedure can take 3 hours and is associated with some risks to the donor (eg, citrate toxicity). The current indications for granulocytes are very limited since the advent of neutrophil growth factors and improved antimicrobials.21 They can be useful in the neutropenic patient with a documented bacterial infection in whom the white blood cell count is not expected to recover in the near future. Given the difficulty of keeping the count up, these transfusions have been mainly used in treating small children.
SPECIAL BLOOD PRODUCTS
IRRADIATED BLOOD PRODUCTS
Irradiation of blood is performed for only one reason: to prevent transfusion-related graft-versus-host disease (TGVHD) (Table 2).22 The irradiation can be performed at the blood center or in the transfusion service of larger hospitals. The units are not radioactive and can be transfused safely to other patients. There is increased leakage of potassium in irradiated units of blood, so the units need to be transfused within 14 days; in patients potentially sensitive to potassium (eg, neonates), the units must be transfused within 24 hours. Patients undergoing stem cell transplant, those receiving either interuterine transfusions or products from relatives, any patient with Hodgkin disease or receiving purine analogs or alemtuzumab, and patients with severe congenital immune deficiencies should receive irradiated blood. Most would also advocate that patients with hematologic malignances receiving chemotherapy receive irradiated products, but this is more controversial.
LEUKPDEPLETED BLOOD
Contamination of blood products by white blood cells is increasingly being recognized as a possible cause of adverse effects in transfused patients, including febrile transfusion reactions, inducing HLA alloimmunization, immunosuppression, disease transmission, and TGVHD. Reducing white cells can reduce the incidence of all of these complications except TGVHD. Currently, white cells are removed by infusion through filters that trap the cells. This can be done either at the bedside, in the blood bank, or at the donor center. The majority of red cells provided by blood centers in many areas of the country are already leukoreduced, eliminating the need for labor-intensive filtration at the transfusion center or bedside. Platelets collected by plateletpheresis methods can also be made leukocyte-poor. The current indications for leukodepleted productions are:
- Prevention of febrile transfusion reactions in patients with previous documented reactions
- Prevention of HLA alloimmunization (ineffective if patient has received 1 or more blood products not leukodepleted or is already HLA immunized)
- Prevention of cytomegalovirus (CMV) infection
CMV-NEGATIVE BLOOD
CMV can be transmitted through any cellular blood product—red cells and platelets. For patients who are CMV-negative and receiving transplants, especially stem cell transplants, a new CMV infection can be devastating.21 For years only blood from CMV-negative donors was used to transfuse CMV-negative patients. This policy is effective in preventing CMV infection, but because 50% of the population is positive for CMV antibodies, it may potentially lead to shortages of products that could be transfused to the patient. Currently, leukoreduced blood products are used since leukofiltration of the blood is just as effective as transfusion of CMV-negative blood in preventing infections and allows greater use of all blood products.23
COMPLICATIONS OF TRANSFUSIONS
HEMOLYTIC TRANSFUSION REACTION
There are 2 forms of hemolytic reactions—immediate and delayed.24 The immediate reaction is associated with fevers, hypotension, back pain, and oliguria. In severe cases, DIC and renal failure may occur. The immediate reaction is due to transfusion of blood that reacts with the recipient’s preformed high-titer blood antigen antibodies, most often to ABO. This is fatal 2% of the time and occurs almost always as a result of errors in correct identification of the patient. Reactions are due to recipient antibodies attacking donated RBCs, resulting in release of hemoglobin and red cell membrane–antigen complexes. These complexes are believed to lead to the hypotension, fevers, chills, and renal damage associated with the hemolytic reaction. Treatment consists of immediately stopping the transfusion, notifying the blood bank, vigorous intravenous hydration to keep the urine output over 100 mL/hr, and supportive therapy.
The delayed reaction can range in severity from an abrupt drop in the hematocrit to normal response to transfusion but the patient developing a positive Coombs’ test. The delayed response is due to an anamnestic response to blood-group antigens. When the patient is exposed to the same antigen, there is a rise in antibody titer leading to the reaction. Some alloantibodies can lead to a brisk reaction, most often anti-Kidd. The frequency with which delayed transfusion reactions occur is underestimated because mild reactions often do not get worked up or even discovered.
ALLERGIC REACTIONS
Allergic reactions are common (1%–3% of transfusions) and occur in patients having antibodies to proteins in donor blood, which can lead to hives and itching with transfusions. Most of the time these allergic reactions are mild and can be treated with antihistamines. Prophylaxis with antihistamines is not indicated for future transfusions unless the reactions are frequent. Rarely these reactions can be associated with shock and hypotension. Patients who are immunoglobulin (Ig) A–deficient can develop anaphylactic reactions to IgA-containing blood products. Patients with severe allergic reactions need to have their IgA measured and, if deficient, receive only washed units or plasma from IgA-deficient donors to prevent future severe reactions.
FEBRILE REACTIONS
The most common transfusion reaction is a febrile reaction that occurs after the transfusion starts and that sometimes can be complicated by chills. This reaction often occurs due to the presence of leukocyte debris and cytokines in the donated blood. Therapy is supportive and involves stopping the transfusion and administering acetaminophen, but since hemolytic transfusion reactions can present with fever all patients need to be thoroughly evaluated. The incidence of reactions can be decreased by using leukodepleted blood and plateletpheresis platelets. Most patients do not benefit from receiving prophylactic acetaminophen for future transfusion unless they have multiple reactions.
TRANSFUSION-RELATED ACUTE LUNG INJURY
Once thought a rare complication, transfusion-related acute lung injury (TRALI) is increasingly being recognized, with an incidence of approximately 1:5000 patients; it is now the most frequent cause of transfusion-related death.24,25 TRALI is noncardiac pulmonary edema and typically manifests clinically with hypoxemia, fever, bilateral infiltrates, and hypotension 2 to 6 hours after blood is given. Ventilatory support is often required. Recovery is usually rapid (24–48 hours) and complete. The etiology is complex. In many cases, transfused anti-HLA antibodies react with the recipient’s white cells leading to pulmonary damage. Another theory is that transfusion of preformed cytokines leads to pulmonary damage. Because plasma products from multiparous women are most often associated with anti-HLA antibodies, the restricted use of blood products from women has decreased the incidence of TRALI over the past few years.26
TRANSFUSION-ASSOCIATED CIRCULATORY OVERLOAD
Increasingly it being recognized that volume overload resulting from transfusions can lead to significant morbidity.27 Patients with heart or renal disease or patients who already have compromised fluid status are at risk for transfusion-associated circulatory overload (TACO). Another risk factor is transfusion of multiple blood products. Patients with TACO develop dyspnea within 6 hours of transfusion, but do not have fever or rash with the dyspnea. The diagnosis is made by demonstrating circulatory overload (eg, high venous pressure, B-type natriuretic peptide). Treatment is aggressive diuresis. Strategies to prevent TACO include judicious use of blood products, especially in patients at risk for TACO, and the use of prophylactic diuretics, especially with red cell or plasma transfusions.28
TRANSFUSION-RELATED GRAFT-VERSUS-HOST DISEASE
TGVHD is a rare reaction, but one that is most often fatal.29 TGVHD occurs when donor lymphocytes attack the blood recipient’s organs—skin, liver, intestines, and marrow. This is very rare in the normal blood recipient unless the donor and recipient share some HLA haplotypes.30 In immunosuppressed patients, TGVHD can occur with lesser degrees of HLA similarity, with cases reported in blood recipients who are mainly patients with Hodgkin disease or acute leukemia undergoing chemotherapy, and in patients receiving purine analogs. TGVHD had not been reported in AIDS patients despite profound immunosuppression, perhaps because the milieu of the patient does not allow lymphocyte expansion. Symptoms of TGVHD are an erythematous rash that may progress to epidermal toxic necrolysis, liver dysfunction, diarrhea, and pancytopenia. TGVHD is prevented by irradiating blood products given to at-risk patients with 2500 to 3500 rads. Directed blood donation from all blood relatives should also be irradiated. TGVHD cannot be prevented by leukopoor blood because the minute amount of lymphocytes that are not filtered still can lead to these complications.
POST-TRANSFUSION PURPURA
Patients with post-transfusion purpura (PTP) develop severe thrombocytopenia (< 10 × 103/µL) with often severe bleeding 1 to 2 weeks after receiving any type of blood product.31 Patients who develop PTP most often lack platelet antigen PLA1 or other platelet antigens. For unknown reasons, exposure to the antigens from the transfusion leads to rapid destruction of the patient’s own platelets. The diagnostic clue is thrombocytopenia in a patient, typically female, who has received a red cell or platelet blood product in the past 7 to 10 days. Treatment consists of intravenous immunoglobulin32 and plasmapheresis to remove the offending antibody. If patients with a history of PTP require further transfusions, only PLA1-negative platelets should be given.
IRON OVERLOAD
Every transfusion of red cells delivers approximately 250 mg of iron to the recipient. Since there is no natural way of ridding the body of iron, heavily transfused patients are at risk of iron overload. This is most often seen in children heavily transfused for thalassemia. Starting in the second decade of life, these individuals will develop endocrinopathies due to iron overload, liver problems, and often fatal cardiomyopathies. Studies have shown that chelation of iron with deferoxamine can be effective in preventing this fatal complication.33 Oral iron chelators such as deferasirox and deferiprone are also effective. The risk of iron overload in heavily transfused patients with myelodysplasia or other transfusion-dependent anemias is unclear, and uncertainty exists about the need for chelation.34
Young patients who face years of transfusions should be started on iron chelation to avoid iron overload. For older patients with transfusion-dependent anemia, iron chelation therapy should be considered if their life expectancy is long (years to decades) or special studies such as T2-weighted cardiac magnetic resonance imaging showing iron overloading.35
INFECTIOUS COMPLICATIONS
Concern over transmission of HIV infection via blood products in the late 1980s led to both a reduction in blood product use and a greater awareness of infectious complications of transfusion and their prevention. However, no blood product can ever be assumed to be safe for 2 reasons. One is that blood products can transmit infections during a “window period”—the time before a contaminated product can be detected by testing. The second is that blood is not screened for all potential infections (eg, babesiosis or new infections such as West Nile virus at the start of the outbreak). Risk of infection is reduced in 2 ways: deferral of potential infectious donors and blood product testing.
As part of the donation process, potential blood donors are asked a series of questions to see if they have risk factors for infections (eg, recent travel to malarious areas, recent tattoos), and if they answer positive are deferred from donating blood. Blood products are then tested for infectious agents by a combination of methods including detection of viral antigen, antibody response to infections, and more recently polymerase chain reaction (PCR).36 Current screening includes syphilis testing; testing for antibodies to HIV, HTLV (human T-lymphotropic virus), hepatitis C virus, hepatitis B core antigen (HBcAg), hepatitis B surface antigen, and PCR for HIV, hepatitis B virus, HCV, and West Nile virus. Some centers also test for Trypanosoma cruzi, the cause of Chagas disease.
In the past, the numerically most common transfusion-related disease was hepatitis, first B and then C.37,38 The first step in eliminating these infections was to stop paying donors for blood products. With the introduction of effective testing for hepatitis B and then C, the incidence of transfusion-related hepatitis has plummeted.36 For example, with the introduction of a diagnostic test for hepatitis C, the estimated risk has fallen from 5% to less than 1 per million. Currently, the risk of transmission of hepatitis B and C, HIV, and HTLV is less than 1 in a million.38
Despite this testing, blood transfusions can transmit a variety of infections, including malaria and babesiosis.39 Any new blood-borne infection introduced into the population can get into the blood supply as well. For example, at the start of the West Nile virus epidemic, there was a cluster of transfusion-transmitted cases that resulted in severe and sometimes fatal illness in immunosuppressed patients, but this issue has been addressed with the development of a PCR assay for screening blood.40 The rate of transfusion-related babesiosis has been increasing and screening for the causative parasite is being considered.
MASSIVE TRANSFUSIONS
Acutely bleeding patients can require large amounts of transfusion products. Early data showed high mortality rates with transfusion of more than 20 units of blood,41 but with modern blood banking techniques and improved laboratory testing, this rate has decreased dramatically, with survival rates of 43% to 70% in patients transfused with more than 50 units of blood.42
The basic approach to massive transfusions is to first transfuse the patient to maintain hemodynamic stability while specific blood tests are being obtained, and then to use the results of these early tests to guide the rest of the resuscitation. An important component is the ability to rapidly deliver standard packages of red cells, usually 6 to 10 units at a time, to the bleeding patient. To avoid delay while the patient’s blood is being typed, the first products delivered are blood group O Rh-positive units. Given the shortage of Rh-negative blood, this should be reserved for only empiric therapy of women of child-bearing age. Once the blood type is known, the patient can be switched over to type-specific blood.
In the past decade, there has been a shift toward increasing the amount of plasma given to patients receiving massive transfusions. This shift has occurred for 2 reasons. One is that modeling of coagulation changes in massive bleeding suggests the need for larger amounts of plasma to correct defects than have previously been recommended.43 The other reason is based on analysis of resuscitation protocols used in military and civilian trauma centers showing that giving red cells and plasma units in a 1:1 ratio appears to be associated with improved outcomes in massive transfusion. Several studies have extended this concept to platelets, again suggesting improved survival with 1 unit of random donor platelets given 1:1 with red cells and plasma units. The PROPPR (Prospective Observational Multicenter Major Trauma Transfusion) study compared a 1:1:1 to 1:1:2 ratio in patients with severe trauma and major bleeding and found less exsanguination and faster achievement of hemostasis in the first 24 hours.44 This has led to the widespread adoption of the 1:1 ratio by most trauma centers, and by default to other massive transfusion situations despite the lack of clinical trial data.45
One barrier to increased use is that plasma is kept frozen and requires 20 minutes to thaw. Many institutions are now keeping inventories of thawed plasma available for immediate use, ranging from 2 to 4 units of group AB plasma to keeping their entire inventory as liquid plasma.46 Plasma that is thawed but not used can be relabeled as “thawed plasma” and kept for up to 5 days. Also, many centers now use group A plasma for massive transfusions as this rarely leads to transfusion reactions and is much more available.47 Research is currently under way on lyophilized plasma, which can be stored at room temperature and can be rapidly reconstituted for emergency use.
The standard approach for laboratory testing is obtaining 5 tests: hematocrit, platelet count, INR/prothrombin time, activated partial thromboplastin time (aPTT), and fibrinogen.48 Product selection is guided by these tests, and they are repeated at regular intervals during the massive transfusion. A typical protocol is shown in Table 3. It is important as part of any protocol to have a flow chart that records laboratory results and products given that any member of the team can easily view.
The transfusion threshold for a low hematocrit depends on the stability of the patient. If the hematocrit is below 30% and the patient is bleeding or hemodynamically unstable, one should transfuse packed red cells. Stable patients can tolerate lower hematocrits, and an aggressive transfusion policy may even be detrimental.2,49 If the patient is bleeding, has florid DIC, or has received platelet aggregation inhibitors, then keeping the platelet count above 50 ×
While in the past fibrinogen targets of 50 to 100 mg/dL were recommended, recent data indicate that a target of 150 mg/dL or higher may be more appropriate.51–53 Severe fibrinolysis may occur in certain clinical situations such as brain injuries, hepatic trauma, or ischemic limb reperfusion, and the use of large amounts of cryoprecipitate can be anticipated. In patients with an INR greater than 2 and an abnormal aPTT, one can give 2 to 4 units of FFP. For an aPTT greater than 1.5 times normal, 2 to 4 units of plasma should be given. Elevation of the aPTT above 1.8 times normal control is associated with microvascular bleeding in trauma patients.54 Patients with marked abnormalities (eg, anaPTT more than 2 times normal) may require aggressive therapy with at least 15 to 30 mL/kg (4–8 units for an average adult) of plasma.55
Recently there has been increasing interest in the use of thromboelastography (TEG) in massive transfusion.56 This is a point-of-care assay performed on fresh whole blood that can assess multiple facets of hemostasis, including coagulation, platelet function, and fibrinolysis.57,58 TEG is performed by placing a 0.35-mL sample of whole blood into an oscillating container with a sensor pin that measures the force of thrombus formation. TEG measures 5 parameters:
- r time: time from starting TEG until clot formation
- K time: time needed for tracing to go from 2 mm to 20 mm
- alpha angle: slope of tracing between r and K time
- MA: greatest amplitude of TEG tracing
- Whole blood lysis index: amplitude of tracing 60 minutes after MA.
Several centers have incorporated TEG into resuscitation protocols that include standardized strategies for responding to abnormalities. Data suggest that use of TEG may decrease the use of blood products, especially in cardiac surgery, but this has not been prospectively studied in massive transfusions.56,59
COMPLICATIONS OF MASSIVE TRANSFUSIONS
Electrolyte abnormalities are unusual even in patients who receive massive transfusions.60 Platelet concentrates and plasma contain citrate that can chelate calcium. However, the citrate is rapidly metabolized, and it is rare to see clinically significant hypocalcemia. Although empiric calcium replacement is often recommended, one study suggests that this is associated with a worse outcome and should not be done.61 If hypocalcemia is a clinical concern, then levels should be drawn to guide therapy. Stored blood is acidic, with a pH of 6.5 to 6.9. However, acidosis attributed solely to transfused blood is rare and most often is a reflection of the patient’s stability. Empirical bicarbonate replacement has been associated with severe alkalosis and is not recom mended.62,63 Although potassium leaks out of stored red cells, even older units of blood contain only 8 mEq/L of potassium, so hyperkalemia is usually not a concern.
PATIENTS WITH AUTOIMMUNE HEMOLYTIC ANEMIA
Patients with autoimmune hemolytic anemia can be difficult to transfuse,64 because the autoantibody can interfere with several aspects of the transfusion services evaluation. In some patients the autoantibody can be so strong that the patient’s blood type cannot be determined. In most patients, the final step of the cross-match—mixing the donor blood with recipient plasma—will show noncompatibility due to the autoantibodies reacting with any red cells.
The first step when transfusing a patient with autoimmune hemolytic anemia is to draw several tubes of blood for the transfusion service before any potential transfusions. This allows the transfusion service to remove the autoantibodies so they can screen for underlying alloantibodies. Second, if the patient requires immediate transfusion, then type-specific or O-negative blood should be given. If the patient has not been recently (months) transfused, the incidence of a severe transfusion reaction is low. The first unit should be infused slowly with close observation of the patient. For patients who have been multiply transfused, the use of an “in-vivo” cross-match may be helpful. This is where the patient is slowly transfused 10 to 15 mL of blood over 15 minutes. The the plasma and urine are then assessed for signs of hemolysis and, if negative, the remaining product is given.
REFUSAL OF BLOOD PRODUCTS
The initial step in managing patients who refuse blood products is to find out why they are refusing them. Many patients have an exaggerated fear of HIV and other infectious agents, so discussing the very low risk for infection transmission can often resolve the situation. The most common reason for refusal of blood products is religious belief. Jehovah’s Witness patients will refuse blood products due to their interpretation of the Bible.65 All members will refuse red cells, plasma, and platelets, while decisions about “derived” blood products—products made by manipulation of the original donated units—are a matter of conscience. These include cryoprecipitate, intravenous gammaglobulin, and albumin.
In an elective situation, the first step is to discuss with the patient those products that are a matter of conscience and clearly document this. The patient’s blood count and iron stores should be assessed to identify any correctible causes of anemia or low iron stores before surgery. The use of erythropoietin to correct blood counts before surgery is controversial, as this may increase thrombosis risk and is contraindicated in patients with curable tumors.
For patients with acute blood loss, use of intravenous iron combined with high-dose erythropoietin is the most common approach to raise the blood count.65 A recommended erythropoietin dose is 300 units/kg 3 times a week, dropping to 100 units/kg 3 times weekly until the goal hematocrit is reached. Another often overlooked step is to consolidate and minimize laboratory testing. The most important step is to be respectful of the patient and their beliefs. Many larger cities have liaisons that can help with interactions between Jehovah’s Witness patients and the health care system.
NON-TRANSFUSION THERAPIES FOR ACUTE BLEEDING
DESMOPRESSIN
Desmopressin (DDAVP) is a synthetic analog of antidiuretic hormone that raises the levels of both factor VIII and von Willebrand protein severalfold.66 Desmopressin is effective in supporting hemostasis in patients with a wide variety of congenital and acquired bleeding disorders. However, desmopressin does not reduce blood loss before routine surgery in a healthy patient and should not be used for this purpose.67
TRANEXAMIC ACID
Tranexamic acid is an antifibrinolytic agent that blocks the binding of plasmin to fibrin.68 This agent was first shown to be useful in disorders that involve excessive fibrinolysis69–73 or as adjunctive therapy for oral or dental procedures in patients with a bleeding diathesis. In patients with severe thrombocytopenia, the use of antifibrinolytic agents may reduce bleeding. Increasing data shows that tranexamic acid can prevent blood loss in a variety of surgeries including heart bypass, liver transplantation, and orthopedic surgery.74 Patients across these settings have decreased blood loss and need for transfusion with no increased risk of thrombosis. The CRASH-2 study showed that the use of tranexamic acid significantly reduced mortality in trauma patients.75 The WOMEN trial demonstrated that 1 g of tranexamic acid given to women with blood loss of more than 500 mL after vaginal delivery or 1000 mL after cesarean section has a risk reduction of death of 0.81 with no increased risk of thrombosis.76 Given this abundant data, it is clear tranexamic acid needs to be part of any massive transfusion protocol.77
RECOMBINANT FACTOR VIIa
Recombinant factor VIIa (rVIIa) was originally developed as a “bypass” agent to support hemostasis in hemophiliacs.78 However, the use of rVIIa for a wide array of bleeding disorders, including patients with factor VII and XI deficiency and Glanzmann thrombasthenia, has been reported.79 Increasingly, rVIIa is being used as a “universal hemostatic agent” for patients with uncontrolled bleeding from any mechanism.80 Multiple case reports have described the use of rVIIa for bleeding in cardiac surgery patients, obstetrical bleeding, reversal of anticoagulation, and trauma.81 Unfortunately, little formal trial data exists to put these anecdotes into perspective, and formal review of clinical trial results has shown no benefit.82,83 However, when used in older patients, especially those with vascular risk factors, the risk of arterial thrombosis appears to increase.84 In the trials for intracranial hemorrhage, the thrombosis rate was 5% to 9%, and rates up to 10% for arterial events were seen in older patients in a review of all trials.85–87 Given the lack of data but the evidence of risk, rVIIa use should be restricted to patients with documented bleeding disorders that have been shown to benefit by its use.
INTRODUCTION
Transfusion therapy is an essential part of hematology practice, allowing for curative therapy of diseases such as leukemia, aplastic anemia, and aggressive lymphomas. Nonetheless, transfusions are associated with significant risks, including transmission of infections and transfusion-related reactions. Controversy remains about key issues in transfusion therapy, such as triggers for red cell transfusions. This article reviews the available blood products (Table 1) and indications for transfusion along with the associated risks, and also discusses specific clinical situations, such as massive transfusion.
BLOOD PRODUCTS
WHOLE BLOOD
Whole blood is the product of 1 unit of donated blood plus anticoagulant/preservative, and by definition contains 1 unit of plasma and red cells. Whole blood can be stored for 5 weeks. Although it was the standard product in the past, whole blood is rarely used since 1 unit of donated blood can now be fractionated into 1 unit of red blood cells (RBC), 1 unit of platelets, and 1 unit of fresh frozen plasma (FFP). Thus, the use of whole blood for just a single transfusion represents a waste of resources. There are 2 exceptions. One is autologous blood donations, which are whole blood units. Second, whole blood is increasingly being used in massive transfusions for trauma patients, with the rationale being that all essential blood components are being transfused at once.1
PACKED RED CELLS
The remaining red cell mass after most of the plasma is removed is called the “packed” red cell unit (hematocrit = 70%–80%), and so red cells are often called “packed” red cells, or PRBC. A preservative is added to improve the flow of blood and to provide “nutrients” for the red cells, and this reduces the hematocrit to approximately 60%. The volume of a red cell unit is approximately 340 mL. In the average adult, 1 unit of RBC raises the hematocrit by 3%. The indications for transfusion of red cells are to increase red cell mass, and thus oxygen delivery, in patients who are compromised by their anemia.
Several randomized trials have helped define the indications for red cell transfusions and justify lower hematocrit thresholds for initiating transfusion. The TRICC (Transfusion Requirements in Critical Care Investigators for the Canadian Critical Care Trials Group) trial showed that in critical care patients (30-day mortality, 18.7%–23.3%), a conservative transfusion strategy of waiting until the hematocrit was below 21% had the same outcomes as transfusing at a threshold of 24%.2 The TRACS (Transfusion Requirements After Cardiac Surgery) trial showed that a hematocrit target of 24% had the same benefit as a target of 30% in patients who had undergone cardiac bypass surgery.3 For patients with acute myocardial infarction, the outcomes were worse with aggressive transfusion at a hematocrit of 30% compared to 24%.4 In patients with upper gastrointestinal bleeding, a hemoglobin transfusion trigger of 7 g/dL was associated with a lower mortality than a trigger of 9 g/dL (5% versus 9%).5 Finally, the FOCUS (Transfusion Trigger Trial for Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair) trial showed that in older patients (average age 80 years) who had undergone hip fracture surgery, transfusions based on symptoms and not a fixed trigger of 30% had the same outcomes but considerable savings in blood products.6 Based on these trials, decisions regarding when to transfuse patients should be based on symptoms and not “numbers.” Young patients, especially those with reversible anemias, can tolerate low blood counts and should not be transfused based on an arbitrary number.
PLATELETS
Several types of platelet products exist. One unit of platelet concentrate is derived from 1 unit of donor blood. Plateletpheresis from volunteer donors is also used to harvest platelets, with the resulting product referred to as plateletpheresis platelets. One unit of single-donor (pheresis) platelets is equivalent to 6 platelet concentrates. Finally, HLA-matched platelets are single-donor pheresis units that are obtained from an HLA-matched donor. This product should be ordered only if there is evidence of HLA antibodies (see Platelet Alloimmunization section).
The dose of platelets for the average patient is 6 units of platelet concentrate or 1 pheresis unit. In theory, 1 unit of platelet concentrate can raise the count by 5 to 7 × 103/µL, but often this response is blunted by concurrent illness or bleeding. In patients who appear to have a poor response, the platelet count can be checked 15 minutes after platelet infusion. No rise or a minimal rise (< 2 × 103/µL) in the platelet count is suggestive of platelet refractoriness, while a good 15-minute response but poor 24-hour count is more suggestive of consumption—fever, sepsis, drug, or splenomegaly—and not refractoriness.
The indication for platelet transfusion depends on the clinical situation. For patients with immune thrombocytopenia, platelets should not be transfused unless there is life-threatening bleeding. For stable patients with marrow aplasia from chemotherapy, a cut-off of a morning platelet count of less than 10 × 103/µL has been shown to be as safe as higher levels for prophylactic transfusions.7 For patients with active bleeding, the platelet count should be kept above 50 × 103/µL. Patients with acquired or inherited platelet dysfunction may benefit from transfusion no matter the platelet count.
Platelet Alloimmunization
Patients exposed to transfused white cells with different HLA antigens can develop antibodies to these antigens.8 Anti-HLA antibodies are common in patients who previously have received transfused blood that is not leukodepleted and in patients who have been pregnant. Since platelets carry class I HLA antigens, they will be rapidly destroyed by anti-HLA antibodies when transfused into these patients. In patients transfused for aplastic anemia or myelodysplasia, as many as 90% will become HLA-immunized. The incidence is lower in patients receiving chemotherapy but still can be as high as 60% to 90%.9,10 Patients who have developed anti-HLA antibodies can respond to transfused platelets matched for HLA antigens. Unfortunately, some patients will either be a rare HLA type or be so heavily immunized that they will not respond to any platelet transfusion.
The significance of alloimmunization centers on 2 concepts: recognition and avoidance. Patients with HLA antibodies will fail to have an increment of their platelet counts with transfusions. Accordingly, patients who do not experience an increase in their count 15 minutes after the transfusion may have HLA antibodies. One can test for the presence of anti-HLA antibodies, although some patients instead have specific antiplatelet antibodies that will not respond to HLA-matched platelets. In patients who have been pregnant or previously transfused and are scheduled to undergo transplant or aggressive chemotherapy, it is wise to test for anti-HLA antibodies in order to plan their transfusion needs. The evidence suggests that transfused white cells are responsible for initiating the anti-HLA response. Trials have shown that giving leukodepleted blood products may reduce the incidence of alloimmunization, so patients who are not HLA-alloimmunized should receive only leukodepleted products.11A difficult problem is bleeding in patients who are refractory to platelet transfusion.12 Patients who test positive for the presence of anti-HLA antibodies can receive transfusions of HLA-matched platelets.13 Unfortunately, matched platelet transfusions are not effective in 20% to 70% of these patients. Also, since some loci are difficult to match, effective products may be unavailable. Finally, as many as 25% of patients have antiplatelet antibodies in which HLA-matched products will be ineffective. Platelet cross-matching can be performed to find compatible units for these patients, but this may not always be successful. In the patient who is totally refractory to platelet transfusion, consider drugs as an etiology of antiplatelet antibodies (especially vancomycin).14 Use of antifibrinolytic agents such as epsilon-aminocaproic acid or tranexamic acid may decrease the incidence of minor bleeding, but these are ineffective for major bleeding. “Platelet drips”—infusing either a platelet concentrate per hour or 1 plateletpheresis unit every 6 hours—may be given as a continuous infusion, but there is no evidence that this is helpful.15
FRESH FROZEN PLASMA
FFP is made from 1 unit of donated whole blood, with an average volume of 225 mL per unit. One unit of FFP can increase coagulation factor levels by 5% and fibrinogen by 10 mg/dL in the average stable patient. FFP can take 20 to 30 minutes to thaw before use, so in situations where FFP is needed quickly, the blood bank must be informed to “keep ahead” some units. Units of FFP that have been thawed but not used can be stored refrigerated for 5 days to prevent wasting blood products.
The indications for FFP are limited to several situations. These include a documented coagulation defect that can be corrected by a reasonable amount of FFP, such as factor V deficiency and factor XI deficiency, disseminated intravascular coagulation (DIC), reversal of warfarin, and massive transfusions. FFP is also used for the therapy of thrombotic thrombocytopenic purpura.
There is little justification for FFP transfusion in many of the clinical settings in which it is commonly used. For example, FFP is given for minor elevations of the INR in patients with liver disease, despite literature showing not only that the INR rise is not reflective of coagulation defects, but also that patients with liver disease may even be thrombophilic.16,17 Reviews of FFP use found limited evidence-based indications for its use.18,19 Also, several studies have shown that transfusion of FFP is not effective at reversing minor elevations of the INR (1.3–1.8).20 In a meta-analysis, FFP was associated with increased risk for lung injury and a trend toward increased mortality.18
CRYOPRECIPITATE
Cryoprecipitate is produced from 1 unit of FFP that is thawed at 4°C. The precipitate is resuspended with 10 mL of saline or FFP and refrozen for storage. One unit contains at least 150 mg of fibrinogen and 80 units of factor VIII, along with von Willebrand factor. Thawing time for cryoprecipitate is approximately 20 minutes.
Cryoprecipitate is used to raise the fibrinogen level in patients with DIC or massive transfusion with hemodilution. It is third-line therapy in the treatment of type 1 von Willebrand disease and is second-line therapy in the treatment of patients with other types of von Willebrand disease. Currently, von Willebrand factor concentrates are the preferred replacement product for von Willebrand disease. Cryoprecipitate can be used as a source of factor VIII for hemophiliacs, but the preferred product for these patients is the super pure factor VIII concentrates or recombinant products. Cryoprecipitate can also be used to shorten the bleeding time of uremic patients, but its effectiveness for this is controversial.
GRANULOCYTES
Granulocytes are harvested by leukopheresis of normal donors, with a target yield of 1010 granulocytes from each donor. To reach this target, the donors are often “stimulated” with neutrophil growth factors. The harvesting procedure can take 3 hours and is associated with some risks to the donor (eg, citrate toxicity). The current indications for granulocytes are very limited since the advent of neutrophil growth factors and improved antimicrobials.21 They can be useful in the neutropenic patient with a documented bacterial infection in whom the white blood cell count is not expected to recover in the near future. Given the difficulty of keeping the count up, these transfusions have been mainly used in treating small children.
SPECIAL BLOOD PRODUCTS
IRRADIATED BLOOD PRODUCTS
Irradiation of blood is performed for only one reason: to prevent transfusion-related graft-versus-host disease (TGVHD) (Table 2).22 The irradiation can be performed at the blood center or in the transfusion service of larger hospitals. The units are not radioactive and can be transfused safely to other patients. There is increased leakage of potassium in irradiated units of blood, so the units need to be transfused within 14 days; in patients potentially sensitive to potassium (eg, neonates), the units must be transfused within 24 hours. Patients undergoing stem cell transplant, those receiving either interuterine transfusions or products from relatives, any patient with Hodgkin disease or receiving purine analogs or alemtuzumab, and patients with severe congenital immune deficiencies should receive irradiated blood. Most would also advocate that patients with hematologic malignances receiving chemotherapy receive irradiated products, but this is more controversial.
LEUKPDEPLETED BLOOD
Contamination of blood products by white blood cells is increasingly being recognized as a possible cause of adverse effects in transfused patients, including febrile transfusion reactions, inducing HLA alloimmunization, immunosuppression, disease transmission, and TGVHD. Reducing white cells can reduce the incidence of all of these complications except TGVHD. Currently, white cells are removed by infusion through filters that trap the cells. This can be done either at the bedside, in the blood bank, or at the donor center. The majority of red cells provided by blood centers in many areas of the country are already leukoreduced, eliminating the need for labor-intensive filtration at the transfusion center or bedside. Platelets collected by plateletpheresis methods can also be made leukocyte-poor. The current indications for leukodepleted productions are:
- Prevention of febrile transfusion reactions in patients with previous documented reactions
- Prevention of HLA alloimmunization (ineffective if patient has received 1 or more blood products not leukodepleted or is already HLA immunized)
- Prevention of cytomegalovirus (CMV) infection
CMV-NEGATIVE BLOOD
CMV can be transmitted through any cellular blood product—red cells and platelets. For patients who are CMV-negative and receiving transplants, especially stem cell transplants, a new CMV infection can be devastating.21 For years only blood from CMV-negative donors was used to transfuse CMV-negative patients. This policy is effective in preventing CMV infection, but because 50% of the population is positive for CMV antibodies, it may potentially lead to shortages of products that could be transfused to the patient. Currently, leukoreduced blood products are used since leukofiltration of the blood is just as effective as transfusion of CMV-negative blood in preventing infections and allows greater use of all blood products.23
COMPLICATIONS OF TRANSFUSIONS
HEMOLYTIC TRANSFUSION REACTION
There are 2 forms of hemolytic reactions—immediate and delayed.24 The immediate reaction is associated with fevers, hypotension, back pain, and oliguria. In severe cases, DIC and renal failure may occur. The immediate reaction is due to transfusion of blood that reacts with the recipient’s preformed high-titer blood antigen antibodies, most often to ABO. This is fatal 2% of the time and occurs almost always as a result of errors in correct identification of the patient. Reactions are due to recipient antibodies attacking donated RBCs, resulting in release of hemoglobin and red cell membrane–antigen complexes. These complexes are believed to lead to the hypotension, fevers, chills, and renal damage associated with the hemolytic reaction. Treatment consists of immediately stopping the transfusion, notifying the blood bank, vigorous intravenous hydration to keep the urine output over 100 mL/hr, and supportive therapy.
The delayed reaction can range in severity from an abrupt drop in the hematocrit to normal response to transfusion but the patient developing a positive Coombs’ test. The delayed response is due to an anamnestic response to blood-group antigens. When the patient is exposed to the same antigen, there is a rise in antibody titer leading to the reaction. Some alloantibodies can lead to a brisk reaction, most often anti-Kidd. The frequency with which delayed transfusion reactions occur is underestimated because mild reactions often do not get worked up or even discovered.
ALLERGIC REACTIONS
Allergic reactions are common (1%–3% of transfusions) and occur in patients having antibodies to proteins in donor blood, which can lead to hives and itching with transfusions. Most of the time these allergic reactions are mild and can be treated with antihistamines. Prophylaxis with antihistamines is not indicated for future transfusions unless the reactions are frequent. Rarely these reactions can be associated with shock and hypotension. Patients who are immunoglobulin (Ig) A–deficient can develop anaphylactic reactions to IgA-containing blood products. Patients with severe allergic reactions need to have their IgA measured and, if deficient, receive only washed units or plasma from IgA-deficient donors to prevent future severe reactions.
FEBRILE REACTIONS
The most common transfusion reaction is a febrile reaction that occurs after the transfusion starts and that sometimes can be complicated by chills. This reaction often occurs due to the presence of leukocyte debris and cytokines in the donated blood. Therapy is supportive and involves stopping the transfusion and administering acetaminophen, but since hemolytic transfusion reactions can present with fever all patients need to be thoroughly evaluated. The incidence of reactions can be decreased by using leukodepleted blood and plateletpheresis platelets. Most patients do not benefit from receiving prophylactic acetaminophen for future transfusion unless they have multiple reactions.
TRANSFUSION-RELATED ACUTE LUNG INJURY
Once thought a rare complication, transfusion-related acute lung injury (TRALI) is increasingly being recognized, with an incidence of approximately 1:5000 patients; it is now the most frequent cause of transfusion-related death.24,25 TRALI is noncardiac pulmonary edema and typically manifests clinically with hypoxemia, fever, bilateral infiltrates, and hypotension 2 to 6 hours after blood is given. Ventilatory support is often required. Recovery is usually rapid (24–48 hours) and complete. The etiology is complex. In many cases, transfused anti-HLA antibodies react with the recipient’s white cells leading to pulmonary damage. Another theory is that transfusion of preformed cytokines leads to pulmonary damage. Because plasma products from multiparous women are most often associated with anti-HLA antibodies, the restricted use of blood products from women has decreased the incidence of TRALI over the past few years.26
TRANSFUSION-ASSOCIATED CIRCULATORY OVERLOAD
Increasingly it being recognized that volume overload resulting from transfusions can lead to significant morbidity.27 Patients with heart or renal disease or patients who already have compromised fluid status are at risk for transfusion-associated circulatory overload (TACO). Another risk factor is transfusion of multiple blood products. Patients with TACO develop dyspnea within 6 hours of transfusion, but do not have fever or rash with the dyspnea. The diagnosis is made by demonstrating circulatory overload (eg, high venous pressure, B-type natriuretic peptide). Treatment is aggressive diuresis. Strategies to prevent TACO include judicious use of blood products, especially in patients at risk for TACO, and the use of prophylactic diuretics, especially with red cell or plasma transfusions.28
TRANSFUSION-RELATED GRAFT-VERSUS-HOST DISEASE
TGVHD is a rare reaction, but one that is most often fatal.29 TGVHD occurs when donor lymphocytes attack the blood recipient’s organs—skin, liver, intestines, and marrow. This is very rare in the normal blood recipient unless the donor and recipient share some HLA haplotypes.30 In immunosuppressed patients, TGVHD can occur with lesser degrees of HLA similarity, with cases reported in blood recipients who are mainly patients with Hodgkin disease or acute leukemia undergoing chemotherapy, and in patients receiving purine analogs. TGVHD had not been reported in AIDS patients despite profound immunosuppression, perhaps because the milieu of the patient does not allow lymphocyte expansion. Symptoms of TGVHD are an erythematous rash that may progress to epidermal toxic necrolysis, liver dysfunction, diarrhea, and pancytopenia. TGVHD is prevented by irradiating blood products given to at-risk patients with 2500 to 3500 rads. Directed blood donation from all blood relatives should also be irradiated. TGVHD cannot be prevented by leukopoor blood because the minute amount of lymphocytes that are not filtered still can lead to these complications.
POST-TRANSFUSION PURPURA
Patients with post-transfusion purpura (PTP) develop severe thrombocytopenia (< 10 × 103/µL) with often severe bleeding 1 to 2 weeks after receiving any type of blood product.31 Patients who develop PTP most often lack platelet antigen PLA1 or other platelet antigens. For unknown reasons, exposure to the antigens from the transfusion leads to rapid destruction of the patient’s own platelets. The diagnostic clue is thrombocytopenia in a patient, typically female, who has received a red cell or platelet blood product in the past 7 to 10 days. Treatment consists of intravenous immunoglobulin32 and plasmapheresis to remove the offending antibody. If patients with a history of PTP require further transfusions, only PLA1-negative platelets should be given.
IRON OVERLOAD
Every transfusion of red cells delivers approximately 250 mg of iron to the recipient. Since there is no natural way of ridding the body of iron, heavily transfused patients are at risk of iron overload. This is most often seen in children heavily transfused for thalassemia. Starting in the second decade of life, these individuals will develop endocrinopathies due to iron overload, liver problems, and often fatal cardiomyopathies. Studies have shown that chelation of iron with deferoxamine can be effective in preventing this fatal complication.33 Oral iron chelators such as deferasirox and deferiprone are also effective. The risk of iron overload in heavily transfused patients with myelodysplasia or other transfusion-dependent anemias is unclear, and uncertainty exists about the need for chelation.34
Young patients who face years of transfusions should be started on iron chelation to avoid iron overload. For older patients with transfusion-dependent anemia, iron chelation therapy should be considered if their life expectancy is long (years to decades) or special studies such as T2-weighted cardiac magnetic resonance imaging showing iron overloading.35
INFECTIOUS COMPLICATIONS
Concern over transmission of HIV infection via blood products in the late 1980s led to both a reduction in blood product use and a greater awareness of infectious complications of transfusion and their prevention. However, no blood product can ever be assumed to be safe for 2 reasons. One is that blood products can transmit infections during a “window period”—the time before a contaminated product can be detected by testing. The second is that blood is not screened for all potential infections (eg, babesiosis or new infections such as West Nile virus at the start of the outbreak). Risk of infection is reduced in 2 ways: deferral of potential infectious donors and blood product testing.
As part of the donation process, potential blood donors are asked a series of questions to see if they have risk factors for infections (eg, recent travel to malarious areas, recent tattoos), and if they answer positive are deferred from donating blood. Blood products are then tested for infectious agents by a combination of methods including detection of viral antigen, antibody response to infections, and more recently polymerase chain reaction (PCR).36 Current screening includes syphilis testing; testing for antibodies to HIV, HTLV (human T-lymphotropic virus), hepatitis C virus, hepatitis B core antigen (HBcAg), hepatitis B surface antigen, and PCR for HIV, hepatitis B virus, HCV, and West Nile virus. Some centers also test for Trypanosoma cruzi, the cause of Chagas disease.
In the past, the numerically most common transfusion-related disease was hepatitis, first B and then C.37,38 The first step in eliminating these infections was to stop paying donors for blood products. With the introduction of effective testing for hepatitis B and then C, the incidence of transfusion-related hepatitis has plummeted.36 For example, with the introduction of a diagnostic test for hepatitis C, the estimated risk has fallen from 5% to less than 1 per million. Currently, the risk of transmission of hepatitis B and C, HIV, and HTLV is less than 1 in a million.38
Despite this testing, blood transfusions can transmit a variety of infections, including malaria and babesiosis.39 Any new blood-borne infection introduced into the population can get into the blood supply as well. For example, at the start of the West Nile virus epidemic, there was a cluster of transfusion-transmitted cases that resulted in severe and sometimes fatal illness in immunosuppressed patients, but this issue has been addressed with the development of a PCR assay for screening blood.40 The rate of transfusion-related babesiosis has been increasing and screening for the causative parasite is being considered.
MASSIVE TRANSFUSIONS
Acutely bleeding patients can require large amounts of transfusion products. Early data showed high mortality rates with transfusion of more than 20 units of blood,41 but with modern blood banking techniques and improved laboratory testing, this rate has decreased dramatically, with survival rates of 43% to 70% in patients transfused with more than 50 units of blood.42
The basic approach to massive transfusions is to first transfuse the patient to maintain hemodynamic stability while specific blood tests are being obtained, and then to use the results of these early tests to guide the rest of the resuscitation. An important component is the ability to rapidly deliver standard packages of red cells, usually 6 to 10 units at a time, to the bleeding patient. To avoid delay while the patient’s blood is being typed, the first products delivered are blood group O Rh-positive units. Given the shortage of Rh-negative blood, this should be reserved for only empiric therapy of women of child-bearing age. Once the blood type is known, the patient can be switched over to type-specific blood.
In the past decade, there has been a shift toward increasing the amount of plasma given to patients receiving massive transfusions. This shift has occurred for 2 reasons. One is that modeling of coagulation changes in massive bleeding suggests the need for larger amounts of plasma to correct defects than have previously been recommended.43 The other reason is based on analysis of resuscitation protocols used in military and civilian trauma centers showing that giving red cells and plasma units in a 1:1 ratio appears to be associated with improved outcomes in massive transfusion. Several studies have extended this concept to platelets, again suggesting improved survival with 1 unit of random donor platelets given 1:1 with red cells and plasma units. The PROPPR (Prospective Observational Multicenter Major Trauma Transfusion) study compared a 1:1:1 to 1:1:2 ratio in patients with severe trauma and major bleeding and found less exsanguination and faster achievement of hemostasis in the first 24 hours.44 This has led to the widespread adoption of the 1:1 ratio by most trauma centers, and by default to other massive transfusion situations despite the lack of clinical trial data.45
One barrier to increased use is that plasma is kept frozen and requires 20 minutes to thaw. Many institutions are now keeping inventories of thawed plasma available for immediate use, ranging from 2 to 4 units of group AB plasma to keeping their entire inventory as liquid plasma.46 Plasma that is thawed but not used can be relabeled as “thawed plasma” and kept for up to 5 days. Also, many centers now use group A plasma for massive transfusions as this rarely leads to transfusion reactions and is much more available.47 Research is currently under way on lyophilized plasma, which can be stored at room temperature and can be rapidly reconstituted for emergency use.
The standard approach for laboratory testing is obtaining 5 tests: hematocrit, platelet count, INR/prothrombin time, activated partial thromboplastin time (aPTT), and fibrinogen.48 Product selection is guided by these tests, and they are repeated at regular intervals during the massive transfusion. A typical protocol is shown in Table 3. It is important as part of any protocol to have a flow chart that records laboratory results and products given that any member of the team can easily view.
The transfusion threshold for a low hematocrit depends on the stability of the patient. If the hematocrit is below 30% and the patient is bleeding or hemodynamically unstable, one should transfuse packed red cells. Stable patients can tolerate lower hematocrits, and an aggressive transfusion policy may even be detrimental.2,49 If the patient is bleeding, has florid DIC, or has received platelet aggregation inhibitors, then keeping the platelet count above 50 ×
While in the past fibrinogen targets of 50 to 100 mg/dL were recommended, recent data indicate that a target of 150 mg/dL or higher may be more appropriate.51–53 Severe fibrinolysis may occur in certain clinical situations such as brain injuries, hepatic trauma, or ischemic limb reperfusion, and the use of large amounts of cryoprecipitate can be anticipated. In patients with an INR greater than 2 and an abnormal aPTT, one can give 2 to 4 units of FFP. For an aPTT greater than 1.5 times normal, 2 to 4 units of plasma should be given. Elevation of the aPTT above 1.8 times normal control is associated with microvascular bleeding in trauma patients.54 Patients with marked abnormalities (eg, anaPTT more than 2 times normal) may require aggressive therapy with at least 15 to 30 mL/kg (4–8 units for an average adult) of plasma.55
Recently there has been increasing interest in the use of thromboelastography (TEG) in massive transfusion.56 This is a point-of-care assay performed on fresh whole blood that can assess multiple facets of hemostasis, including coagulation, platelet function, and fibrinolysis.57,58 TEG is performed by placing a 0.35-mL sample of whole blood into an oscillating container with a sensor pin that measures the force of thrombus formation. TEG measures 5 parameters:
- r time: time from starting TEG until clot formation
- K time: time needed for tracing to go from 2 mm to 20 mm
- alpha angle: slope of tracing between r and K time
- MA: greatest amplitude of TEG tracing
- Whole blood lysis index: amplitude of tracing 60 minutes after MA.
Several centers have incorporated TEG into resuscitation protocols that include standardized strategies for responding to abnormalities. Data suggest that use of TEG may decrease the use of blood products, especially in cardiac surgery, but this has not been prospectively studied in massive transfusions.56,59
COMPLICATIONS OF MASSIVE TRANSFUSIONS
Electrolyte abnormalities are unusual even in patients who receive massive transfusions.60 Platelet concentrates and plasma contain citrate that can chelate calcium. However, the citrate is rapidly metabolized, and it is rare to see clinically significant hypocalcemia. Although empiric calcium replacement is often recommended, one study suggests that this is associated with a worse outcome and should not be done.61 If hypocalcemia is a clinical concern, then levels should be drawn to guide therapy. Stored blood is acidic, with a pH of 6.5 to 6.9. However, acidosis attributed solely to transfused blood is rare and most often is a reflection of the patient’s stability. Empirical bicarbonate replacement has been associated with severe alkalosis and is not recom mended.62,63 Although potassium leaks out of stored red cells, even older units of blood contain only 8 mEq/L of potassium, so hyperkalemia is usually not a concern.
PATIENTS WITH AUTOIMMUNE HEMOLYTIC ANEMIA
Patients with autoimmune hemolytic anemia can be difficult to transfuse,64 because the autoantibody can interfere with several aspects of the transfusion services evaluation. In some patients the autoantibody can be so strong that the patient’s blood type cannot be determined. In most patients, the final step of the cross-match—mixing the donor blood with recipient plasma—will show noncompatibility due to the autoantibodies reacting with any red cells.
The first step when transfusing a patient with autoimmune hemolytic anemia is to draw several tubes of blood for the transfusion service before any potential transfusions. This allows the transfusion service to remove the autoantibodies so they can screen for underlying alloantibodies. Second, if the patient requires immediate transfusion, then type-specific or O-negative blood should be given. If the patient has not been recently (months) transfused, the incidence of a severe transfusion reaction is low. The first unit should be infused slowly with close observation of the patient. For patients who have been multiply transfused, the use of an “in-vivo” cross-match may be helpful. This is where the patient is slowly transfused 10 to 15 mL of blood over 15 minutes. The the plasma and urine are then assessed for signs of hemolysis and, if negative, the remaining product is given.
REFUSAL OF BLOOD PRODUCTS
The initial step in managing patients who refuse blood products is to find out why they are refusing them. Many patients have an exaggerated fear of HIV and other infectious agents, so discussing the very low risk for infection transmission can often resolve the situation. The most common reason for refusal of blood products is religious belief. Jehovah’s Witness patients will refuse blood products due to their interpretation of the Bible.65 All members will refuse red cells, plasma, and platelets, while decisions about “derived” blood products—products made by manipulation of the original donated units—are a matter of conscience. These include cryoprecipitate, intravenous gammaglobulin, and albumin.
In an elective situation, the first step is to discuss with the patient those products that are a matter of conscience and clearly document this. The patient’s blood count and iron stores should be assessed to identify any correctible causes of anemia or low iron stores before surgery. The use of erythropoietin to correct blood counts before surgery is controversial, as this may increase thrombosis risk and is contraindicated in patients with curable tumors.
For patients with acute blood loss, use of intravenous iron combined with high-dose erythropoietin is the most common approach to raise the blood count.65 A recommended erythropoietin dose is 300 units/kg 3 times a week, dropping to 100 units/kg 3 times weekly until the goal hematocrit is reached. Another often overlooked step is to consolidate and minimize laboratory testing. The most important step is to be respectful of the patient and their beliefs. Many larger cities have liaisons that can help with interactions between Jehovah’s Witness patients and the health care system.
NON-TRANSFUSION THERAPIES FOR ACUTE BLEEDING
DESMOPRESSIN
Desmopressin (DDAVP) is a synthetic analog of antidiuretic hormone that raises the levels of both factor VIII and von Willebrand protein severalfold.66 Desmopressin is effective in supporting hemostasis in patients with a wide variety of congenital and acquired bleeding disorders. However, desmopressin does not reduce blood loss before routine surgery in a healthy patient and should not be used for this purpose.67
TRANEXAMIC ACID
Tranexamic acid is an antifibrinolytic agent that blocks the binding of plasmin to fibrin.68 This agent was first shown to be useful in disorders that involve excessive fibrinolysis69–73 or as adjunctive therapy for oral or dental procedures in patients with a bleeding diathesis. In patients with severe thrombocytopenia, the use of antifibrinolytic agents may reduce bleeding. Increasing data shows that tranexamic acid can prevent blood loss in a variety of surgeries including heart bypass, liver transplantation, and orthopedic surgery.74 Patients across these settings have decreased blood loss and need for transfusion with no increased risk of thrombosis. The CRASH-2 study showed that the use of tranexamic acid significantly reduced mortality in trauma patients.75 The WOMEN trial demonstrated that 1 g of tranexamic acid given to women with blood loss of more than 500 mL after vaginal delivery or 1000 mL after cesarean section has a risk reduction of death of 0.81 with no increased risk of thrombosis.76 Given this abundant data, it is clear tranexamic acid needs to be part of any massive transfusion protocol.77
RECOMBINANT FACTOR VIIa
Recombinant factor VIIa (rVIIa) was originally developed as a “bypass” agent to support hemostasis in hemophiliacs.78 However, the use of rVIIa for a wide array of bleeding disorders, including patients with factor VII and XI deficiency and Glanzmann thrombasthenia, has been reported.79 Increasingly, rVIIa is being used as a “universal hemostatic agent” for patients with uncontrolled bleeding from any mechanism.80 Multiple case reports have described the use of rVIIa for bleeding in cardiac surgery patients, obstetrical bleeding, reversal of anticoagulation, and trauma.81 Unfortunately, little formal trial data exists to put these anecdotes into perspective, and formal review of clinical trial results has shown no benefit.82,83 However, when used in older patients, especially those with vascular risk factors, the risk of arterial thrombosis appears to increase.84 In the trials for intracranial hemorrhage, the thrombosis rate was 5% to 9%, and rates up to 10% for arterial events were seen in older patients in a review of all trials.85–87 Given the lack of data but the evidence of risk, rVIIa use should be restricted to patients with documented bleeding disorders that have been shown to benefit by its use.
1. Yazer MH, Cap AP, Spinella PC, et al. How do I implement a whole blood program for massively bleeding patients? Transfusion 2018;58:622–8.
2. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999;340:409–17.
3. Hajjar LA, Vincent JL, Galas FR, et al. Tranfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA 2010;304:1559–67.
4. Cooper HA, Rao SV, Greenberg MD, et al. Conservative versus liberal red cell transfusion in acute myocardial infarction (the CRIT Randomized Pilot Study). Am J Cardiol 2011;108:1108–11.
5. Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013;368:11–21.
6. Carson JL, Terrin ML, Noveck H, et al; the FOCUS Investigators. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011 Dec 4. [Epub ahead of print]
7. Schiffer CA, Anderson KC, Bennett CL, et al. Platelet transfusion for patients with cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol 2001;19:1519–38.
8. Schiffer CA. Prevention of alloimmunization against platelets. Blood 1991;77:1–4.
9. Hod E, Schwartz J. Platelet transfusion refractoriness. Br J Haematol 2008;142:348–60.
10. Novotny VMJ, Van Doorn R, Witvliet MD, et al. Occurrence of allogeneic HLA and non-HLA antibodies after transfusion of prestorage filtered platelets and red blood cells: A prospective study. Blood 1995;85:1736–41.
11. McFarland J, Menitove J, Kagen L et al. Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. N Engl J Med 1997;337:1861–9.
12. Juskewitch JE, Norgan AP, De Goey SR, et al. How do I … manage the platelet transfusion-refractory patient? Transfusion 2017;57:2828–35.
13. Schiffer CA. Diagnosis and management of refractoriness to platelet transfusion. Blood Rev 2001;15:175–80.
14. Christie DJ, van Buren N, Lennon SS, Putnam JL. Vancomycin-dependent antibodies associated with thrombocytopenia and refractoriness to platelet transfusion in patients with leukemia. Blood 1990;75:518–23.
15. Dzik S. How I do it: platelet support for refractory patients. Transfusion 2007;47:374–8.
16. Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med 2011;365:147–56.
17. Lisman T, Porte RJ. Pathogenesis, prevention, and management of bleeding and thrombosis in patients with liver diseases. Res Pract Thromb Haemost 2017;1:150–61.
18. Murad MH, Stubbs JR, Gandhi MJ, et al. The effect of plasma transfusion on morbidity and mortality: a systematic review and meta-analysis. Transfusion 2010;50:1370–83.
19. Green L, Bolton-Maggs P, Beattie C, et al. British Society of Haematology Guidelines on the spectrum of fresh frozen plasma and cryoprecipitate products: their handling and use in various patient groups in the absence of major bleeding. Br J Haematol 2018;181:54–67.
20. Abdel-Wahab OI, Healy B, Dzik WH. Effect of fresh-frozen plasma transfusion on prothrombin time and bleeding in patients with mild coagulation abnormalities. Transfusion 2006;46:1279–85.
21. Price TH. Granulocyte transfusion: current status. Semin Hematol 2007;44:15–23.
22. Treleaven J, Gennery A, Marsh J, et al. Guidelines on the use of irradiated blood components prepared by the British Committee for Standards in Haematology blood transfusion task force. Br J Haematol 2011;152:35–51.
23. Thiele T, Kruger W, Zimmermann K, et al. Transmission of cytomegalovirus (CMV) infection by leukoreduced blood products not tested for CMV antibodies: a single-center prospective study in high-risk patients undergoing allogeneic hematopoietic stem cell transplantation (CME). Transfusion 2011;51:2620–6.
24. Delaney M, Wendel S, Bercovitz RS, et al; Biomedical Excellence for Safer Transfusion (BEST) Collaborative. Transfusion reactions: prevention, diagnosis, and treatment. Lancet 2016;388:2825–36.
25. Toy P, Gajic O, Bacchetti P, et al. Transfusion related acute lung injury: incidence and risk factors. Blood 2011 Nov 23. [Epub ahead of print]
26. Wiersum-Osselton JC, Middelburg RA, Beckers EA, et al. Male-only fresh-frozen plasma for transfusion-related acute lung injury prevention: before-and-after comparative cohort study. Transfusion 2011;51:1278–83.
27. Friedman T, Javidroozi M, Lobel G, Shander A. Complications of allogeneic blood product administration, with emphasis on transfusion-related acute lung injury and transfusion-associated circulatory overload. Adv Anesth 2017;35:159–73.
28. Lin CR, Armali C, Callum J, et al. Transfusion-associated circulatory overload prevention: a retrospective observational study of diuretic use. Vox Sang 2018;113:386–92.
29. Sun X, Yu H, Xu Z, et al. Transfusion-associated graft-versus-host-disease: case report and review of literature. Transfus Apher Sci 2010;43:331–4.
30. Petz LD, Calhoun L, Yam P, et al. Transfusion-associated graft-versus-host disease in immunocompetent patients: report of a fatal case associated with transfusion of blood from a second-degree relative, and a survey of predisposing factors. Transfusion 1993;33:742–50.
31. Mueller-Eckhardt C. Post-transfusion purpura. Br J Hematol 1986;64:419–24.
32. Mueller-Eckhardt C, Kiefel V. High-dose IgG for posttransfusion purpura-revisited. Blut 1988;57:163–7.
33. Modell B, Khan M, Darlison M. Survival in beta-thalassaemia major in the UK: data from the UK Thalassaemia Register. Lancet 2000;355(9220):2051–2.
34. Zeidan AM, Griffiths EA. To chelate or not to chelate in MDS: That is the question! Blood Rev 2018. pii: S0268-960X(17)30128-5.
35. Konen E, Ghoti H, Goitein O, et al. No evidence for myocardial iron overload in multitransfused patients with myelodysplastic syndrome using cardiac magnetic resonance T2 technique. Am J Hematol 2007;82:1013–16.
36. Squires JE. Risks of transfusion. South Med J 2011;104:762–9.
37. Sharma S, Sharma P, Tyler LN. Transfusion of blood and blood products: indications and complications. Am Fam Physician 2011;83:719–24.
38. Jacquot C, Delaney M. Efforts toward elimination of infectious agents in blood products. J Intensive Care Med 2018 Jan 1:885066618756589 [Epub ahead of print].
39. Herwaldt BL, Linden JV, Bosserman E, et al. Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med 2011;155:509–19.
40. Biggerstaff BJ, Petersen LR. Estimated risk of West Nile virus transmission through blood transfusion during an epidemic in Queens, New York City. Transfusion 2002;42:1019–26.
41. Wilson RF, Mammen E, Walt AJ. Eight years of experience with massive blood transfusions. J Trauma 1971;11:275–85.
42. Wade CE, del Junco DJ, Holcomb JB, et al. Variations between level I trauma centers in 24-hour mortality in severely injured patients requiring a massive transfusion. J Trauma 2011;71(2 Suppl 3):S389–S393.
43. Hirshberg A, Dugas M, Banez EI, et al. Minimizing dilutional coagulopathy in exsanguinating hemorrhage: a computer simulation. J Trauma 2003;54:454–63.
44. Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 2015;313:471–82.
45. Pasquier P, Gayat E, Rackelboom T, et al. An observational study of the fresh frozen plasma: red blood cell ratio in postpartum hemorrhage. Anesth Analg 2013;116:155–61.
46. Yuan S, Ziman A, Anthony MA, et al. How do we provide blood products to trauma patients? Transfusion 2009;49:1045–9.
47. Stevens WT, Morse BC, Bernard A, et al. Incompatible type A plasma transfusion in patients requiring massive transfusion protocol: Outcomes of an Eastern Association for the Surgery of Trauma multicenter study. J Trauma Acute Care Surg 2017;83:25–9.
48. DeLoughery TG. Coagulation defects in trauma patients: etiology, recognition, and therapy. Crit Care Clin 2004;20:13–24.
49. Blair SD, Janvrin SB, McCollum CN, Greenhalgh RM. Effect of early blood transfusion on gastrointestinal haemorrhage. Br J Surg 1986;73:783–5.
50. Counts RB, Haisch C, Simon TL, et al. Hemostasis in massively transfused trauma patients. Ann Surg 1979;190:91–9.
51. Gerlach R, Tolle F, Raabe A, et al. Increased risk for postoperative hemorrhage after intracranial surgery in patients with decreased factor XIII activity: implications of a prospective study. Stroke 2002;33:1618–23.
52. Fenger-Eriksen C, Lindberg-Larsen M, Christensen AQ, et al. Fibrinogen concentrate substitution therapy in patients with massive haemorrhage and low plasma fibrinogen concentrations. Br J Anaesth 2008;101:769–73.
53. Charbit B, Mandelbrot L, Samain E, et al. The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. J Thromb Haemost 2007;5:266–73.
54. Ciavarella D, Reed RL, Counts RB, et al. Clotting factor levels and the risk of diffuse microvascular bleeding in the massively transfused patient. Br J Haematol 1987;67:365–8.
55. Chowdhury P, Saayman AG, Paulus U, et al. Efficacy of standard dose and 30 ml/kg fresh frozen plasma in correcting laboratory parameters of haemostasis in critically ill patients. Br J Haematol 2004;125:69–73.
56. Curry NS, Davenport R, Pavord S, et al.The use of viscoelastic haemostatic assays in the management of major bleeding: A British Society for Haematology Guideline. Br J Haematol 2018. doi: 10.1111/bjh.15524. [Epub ahead of print].
57. Kashuk JL, Moore EE, Sawyer M, et al. Postinjury coagulopathy management: goal directed resuscitation via POC thrombelastography. Ann Surg 2010;251:604–14.
58. Whitten CW, Greilich PE. Thromboelastography: past, present, and future. Anesthesiology 2000;92:1223–5.
59. Girdauskas E, Kempfert J, Kuntze T, et al. Thromboelastometrically guided transfusion protocol during aortic surgery with circulatory arrest: a prospective, randomized trial. J Thorac Cardiovasc Surg 2010;140:1117–24.
60. Goskowicz R. The complications of massive tranfusion. Anesthesiology Clin North Am 1999;17:959–78.
61. Howland WS, Schwiezer O, Boyan CP. Massive blood replacement without calcuim administration. Surg Gynecol Obstet 1964;159:171–7.
62. Miller RD, Tong MJ, Robbins TO. Effects of massive transfusion of blood on acid-base balance. JAMA 1971;216:1762–5.
63. Collins JA. Problems associated with the massive transfusion of stored blood. Surgery 1974;75:274–95.
64. Petz LD. A physician’s guide to transfusion in autoimmune haemolytic anaemia. Br J Haematol 2004;124:712–6.
65. Scharman CD, Burger D, Shatzel JJ, et al. Treatment of individuals who cannot receive blood products for religious or other reasons. Am J Hematol 2017;92:1370–81
66. Leissinger C, Carcao M, Gill JC, et al. Desmopressin (DDAVP) in the management of patients with congenital bleeding disorders. Haemophilia 2014;20:158–67.
67. Desborough MJ, Oakland KA, Landoni G, et al. Desmopressin for treatment of platelet dysfunction and reversal of antiplatelet agents: a systematic review and meta-analysis of randomized controlled trials. J Thromb Haemost 2017;15:263–72.
68. Ng W, Jerath A, Wa˛sowicz M. Tranexamic acid: a clinical review. Anaesthesiol Intensive Ther 2015;47:339–50.
69. Amitrano L, Guardascione MA, Brancaccio V, Balzano A. Coagulation disorders in liver disease. Semin Liver Dis 2002;22:83–96.
70. Chang JC, Kane KK. Pathologic hyperfibrinolysis associated with amyloidosis: clinical response to epsilon amino caproic acid. Am J Clin Pathol 1984;81:382–7.
71. Anonymous. Tranexamic acid. Med Letter Drugs Therapeutics 1987;29:89–90.
72. Schwartz BS, Williams EC, Conlan MG, Mosher DF. Epsilon-aminocaproic acid in the treatment of patients with acute promyelocytic leukemia and acquired alpha-2-plasmin inhibitor defiency. Ann Intern Med 1986;105:873–7.
73. Takahashi H, Tatewaki W, Wada K, et al. Fibrinolysis and fibrinogenolysis in liver disease. Am J Hematol 1990;34:241-–5.
74. Ker K, Edwards P, Perel P, et al. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ 2012;344:e3054.
75. Shakur H, Roberts I, Bautista R, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376(9734):23–32.
76. WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet 2017;389:2105–16.
77. Godbey EA, Schwartz J. ‘Massive transfusion protocols and the use of tranexamic acid’. Curr Opin Hematol 2018 Aug 16. doi: 10.1097/MOH.0000000000000457. [Epub ahead of print]
78. Hay CR, Negrier C, Ludlam CA. The treatment of bleeding in acquired haemophilia with recombinant factor VIIa: a multicentre study. Thromb Haemost 1997;78:1463–7.
79. DeLoughery TG. Management of bleeding emergencies: when to use recombinant activated factor VII. Expert Opin Pharmacother 2006;7:25–34.
80. Aledort L. Recombinant factor VIIa Is a pan-hemostatic agent? Thromb Haemost 2000;83:637–8.
81. Logan AC, Yank V, Stafford RS. Off-label use of recombinant factor VIIa in U.S. hospitals: analysis of hospital records. Ann Intern Med 2011;154:516–22.
82. Lin Y, Stanworth S, Birchall J, Doree C, Hyde C. Use of recombinant factor VIIa for the prevention and treatment of bleeding in patients without hemophilia: a systematic review and meta-analysis. CMAJ 2011;183:E9–19.
83. Yank V, Tuohy CV, Logan AC et al. Systematic review: benefits and harms of in-hospital use of recombinant factor VIIa for off-label indications. Ann Intern Med 2011;154:529–40.
84. Pavese P, Bonadona A, Beaubien J, et al. FVIIa corrects the coagulopathy of fulminant hepatic failure but may be associated with thrombosis: a report of four cases. Can J Anaesth 2005;52:26–29.
85. Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2005;352:777–85.
86. Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2008;358:2127–37.
87. Levi M, Levy JH, Andersen HF, Truloff D. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med 2010;363:1791–1800.
1. Yazer MH, Cap AP, Spinella PC, et al. How do I implement a whole blood program for massively bleeding patients? Transfusion 2018;58:622–8.
2. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999;340:409–17.
3. Hajjar LA, Vincent JL, Galas FR, et al. Tranfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA 2010;304:1559–67.
4. Cooper HA, Rao SV, Greenberg MD, et al. Conservative versus liberal red cell transfusion in acute myocardial infarction (the CRIT Randomized Pilot Study). Am J Cardiol 2011;108:1108–11.
5. Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013;368:11–21.
6. Carson JL, Terrin ML, Noveck H, et al; the FOCUS Investigators. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011 Dec 4. [Epub ahead of print]
7. Schiffer CA, Anderson KC, Bennett CL, et al. Platelet transfusion for patients with cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol 2001;19:1519–38.
8. Schiffer CA. Prevention of alloimmunization against platelets. Blood 1991;77:1–4.
9. Hod E, Schwartz J. Platelet transfusion refractoriness. Br J Haematol 2008;142:348–60.
10. Novotny VMJ, Van Doorn R, Witvliet MD, et al. Occurrence of allogeneic HLA and non-HLA antibodies after transfusion of prestorage filtered platelets and red blood cells: A prospective study. Blood 1995;85:1736–41.
11. McFarland J, Menitove J, Kagen L et al. Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. N Engl J Med 1997;337:1861–9.
12. Juskewitch JE, Norgan AP, De Goey SR, et al. How do I … manage the platelet transfusion-refractory patient? Transfusion 2017;57:2828–35.
13. Schiffer CA. Diagnosis and management of refractoriness to platelet transfusion. Blood Rev 2001;15:175–80.
14. Christie DJ, van Buren N, Lennon SS, Putnam JL. Vancomycin-dependent antibodies associated with thrombocytopenia and refractoriness to platelet transfusion in patients with leukemia. Blood 1990;75:518–23.
15. Dzik S. How I do it: platelet support for refractory patients. Transfusion 2007;47:374–8.
16. Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med 2011;365:147–56.
17. Lisman T, Porte RJ. Pathogenesis, prevention, and management of bleeding and thrombosis in patients with liver diseases. Res Pract Thromb Haemost 2017;1:150–61.
18. Murad MH, Stubbs JR, Gandhi MJ, et al. The effect of plasma transfusion on morbidity and mortality: a systematic review and meta-analysis. Transfusion 2010;50:1370–83.
19. Green L, Bolton-Maggs P, Beattie C, et al. British Society of Haematology Guidelines on the spectrum of fresh frozen plasma and cryoprecipitate products: their handling and use in various patient groups in the absence of major bleeding. Br J Haematol 2018;181:54–67.
20. Abdel-Wahab OI, Healy B, Dzik WH. Effect of fresh-frozen plasma transfusion on prothrombin time and bleeding in patients with mild coagulation abnormalities. Transfusion 2006;46:1279–85.
21. Price TH. Granulocyte transfusion: current status. Semin Hematol 2007;44:15–23.
22. Treleaven J, Gennery A, Marsh J, et al. Guidelines on the use of irradiated blood components prepared by the British Committee for Standards in Haematology blood transfusion task force. Br J Haematol 2011;152:35–51.
23. Thiele T, Kruger W, Zimmermann K, et al. Transmission of cytomegalovirus (CMV) infection by leukoreduced blood products not tested for CMV antibodies: a single-center prospective study in high-risk patients undergoing allogeneic hematopoietic stem cell transplantation (CME). Transfusion 2011;51:2620–6.
24. Delaney M, Wendel S, Bercovitz RS, et al; Biomedical Excellence for Safer Transfusion (BEST) Collaborative. Transfusion reactions: prevention, diagnosis, and treatment. Lancet 2016;388:2825–36.
25. Toy P, Gajic O, Bacchetti P, et al. Transfusion related acute lung injury: incidence and risk factors. Blood 2011 Nov 23. [Epub ahead of print]
26. Wiersum-Osselton JC, Middelburg RA, Beckers EA, et al. Male-only fresh-frozen plasma for transfusion-related acute lung injury prevention: before-and-after comparative cohort study. Transfusion 2011;51:1278–83.
27. Friedman T, Javidroozi M, Lobel G, Shander A. Complications of allogeneic blood product administration, with emphasis on transfusion-related acute lung injury and transfusion-associated circulatory overload. Adv Anesth 2017;35:159–73.
28. Lin CR, Armali C, Callum J, et al. Transfusion-associated circulatory overload prevention: a retrospective observational study of diuretic use. Vox Sang 2018;113:386–92.
29. Sun X, Yu H, Xu Z, et al. Transfusion-associated graft-versus-host-disease: case report and review of literature. Transfus Apher Sci 2010;43:331–4.
30. Petz LD, Calhoun L, Yam P, et al. Transfusion-associated graft-versus-host disease in immunocompetent patients: report of a fatal case associated with transfusion of blood from a second-degree relative, and a survey of predisposing factors. Transfusion 1993;33:742–50.
31. Mueller-Eckhardt C. Post-transfusion purpura. Br J Hematol 1986;64:419–24.
32. Mueller-Eckhardt C, Kiefel V. High-dose IgG for posttransfusion purpura-revisited. Blut 1988;57:163–7.
33. Modell B, Khan M, Darlison M. Survival in beta-thalassaemia major in the UK: data from the UK Thalassaemia Register. Lancet 2000;355(9220):2051–2.
34. Zeidan AM, Griffiths EA. To chelate or not to chelate in MDS: That is the question! Blood Rev 2018. pii: S0268-960X(17)30128-5.
35. Konen E, Ghoti H, Goitein O, et al. No evidence for myocardial iron overload in multitransfused patients with myelodysplastic syndrome using cardiac magnetic resonance T2 technique. Am J Hematol 2007;82:1013–16.
36. Squires JE. Risks of transfusion. South Med J 2011;104:762–9.
37. Sharma S, Sharma P, Tyler LN. Transfusion of blood and blood products: indications and complications. Am Fam Physician 2011;83:719–24.
38. Jacquot C, Delaney M. Efforts toward elimination of infectious agents in blood products. J Intensive Care Med 2018 Jan 1:885066618756589 [Epub ahead of print].
39. Herwaldt BL, Linden JV, Bosserman E, et al. Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med 2011;155:509–19.
40. Biggerstaff BJ, Petersen LR. Estimated risk of West Nile virus transmission through blood transfusion during an epidemic in Queens, New York City. Transfusion 2002;42:1019–26.
41. Wilson RF, Mammen E, Walt AJ. Eight years of experience with massive blood transfusions. J Trauma 1971;11:275–85.
42. Wade CE, del Junco DJ, Holcomb JB, et al. Variations between level I trauma centers in 24-hour mortality in severely injured patients requiring a massive transfusion. J Trauma 2011;71(2 Suppl 3):S389–S393.
43. Hirshberg A, Dugas M, Banez EI, et al. Minimizing dilutional coagulopathy in exsanguinating hemorrhage: a computer simulation. J Trauma 2003;54:454–63.
44. Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 2015;313:471–82.
45. Pasquier P, Gayat E, Rackelboom T, et al. An observational study of the fresh frozen plasma: red blood cell ratio in postpartum hemorrhage. Anesth Analg 2013;116:155–61.
46. Yuan S, Ziman A, Anthony MA, et al. How do we provide blood products to trauma patients? Transfusion 2009;49:1045–9.
47. Stevens WT, Morse BC, Bernard A, et al. Incompatible type A plasma transfusion in patients requiring massive transfusion protocol: Outcomes of an Eastern Association for the Surgery of Trauma multicenter study. J Trauma Acute Care Surg 2017;83:25–9.
48. DeLoughery TG. Coagulation defects in trauma patients: etiology, recognition, and therapy. Crit Care Clin 2004;20:13–24.
49. Blair SD, Janvrin SB, McCollum CN, Greenhalgh RM. Effect of early blood transfusion on gastrointestinal haemorrhage. Br J Surg 1986;73:783–5.
50. Counts RB, Haisch C, Simon TL, et al. Hemostasis in massively transfused trauma patients. Ann Surg 1979;190:91–9.
51. Gerlach R, Tolle F, Raabe A, et al. Increased risk for postoperative hemorrhage after intracranial surgery in patients with decreased factor XIII activity: implications of a prospective study. Stroke 2002;33:1618–23.
52. Fenger-Eriksen C, Lindberg-Larsen M, Christensen AQ, et al. Fibrinogen concentrate substitution therapy in patients with massive haemorrhage and low plasma fibrinogen concentrations. Br J Anaesth 2008;101:769–73.
53. Charbit B, Mandelbrot L, Samain E, et al. The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. J Thromb Haemost 2007;5:266–73.
54. Ciavarella D, Reed RL, Counts RB, et al. Clotting factor levels and the risk of diffuse microvascular bleeding in the massively transfused patient. Br J Haematol 1987;67:365–8.
55. Chowdhury P, Saayman AG, Paulus U, et al. Efficacy of standard dose and 30 ml/kg fresh frozen plasma in correcting laboratory parameters of haemostasis in critically ill patients. Br J Haematol 2004;125:69–73.
56. Curry NS, Davenport R, Pavord S, et al.The use of viscoelastic haemostatic assays in the management of major bleeding: A British Society for Haematology Guideline. Br J Haematol 2018. doi: 10.1111/bjh.15524. [Epub ahead of print].
57. Kashuk JL, Moore EE, Sawyer M, et al. Postinjury coagulopathy management: goal directed resuscitation via POC thrombelastography. Ann Surg 2010;251:604–14.
58. Whitten CW, Greilich PE. Thromboelastography: past, present, and future. Anesthesiology 2000;92:1223–5.
59. Girdauskas E, Kempfert J, Kuntze T, et al. Thromboelastometrically guided transfusion protocol during aortic surgery with circulatory arrest: a prospective, randomized trial. J Thorac Cardiovasc Surg 2010;140:1117–24.
60. Goskowicz R. The complications of massive tranfusion. Anesthesiology Clin North Am 1999;17:959–78.
61. Howland WS, Schwiezer O, Boyan CP. Massive blood replacement without calcuim administration. Surg Gynecol Obstet 1964;159:171–7.
62. Miller RD, Tong MJ, Robbins TO. Effects of massive transfusion of blood on acid-base balance. JAMA 1971;216:1762–5.
63. Collins JA. Problems associated with the massive transfusion of stored blood. Surgery 1974;75:274–95.
64. Petz LD. A physician’s guide to transfusion in autoimmune haemolytic anaemia. Br J Haematol 2004;124:712–6.
65. Scharman CD, Burger D, Shatzel JJ, et al. Treatment of individuals who cannot receive blood products for religious or other reasons. Am J Hematol 2017;92:1370–81
66. Leissinger C, Carcao M, Gill JC, et al. Desmopressin (DDAVP) in the management of patients with congenital bleeding disorders. Haemophilia 2014;20:158–67.
67. Desborough MJ, Oakland KA, Landoni G, et al. Desmopressin for treatment of platelet dysfunction and reversal of antiplatelet agents: a systematic review and meta-analysis of randomized controlled trials. J Thromb Haemost 2017;15:263–72.
68. Ng W, Jerath A, Wa˛sowicz M. Tranexamic acid: a clinical review. Anaesthesiol Intensive Ther 2015;47:339–50.
69. Amitrano L, Guardascione MA, Brancaccio V, Balzano A. Coagulation disorders in liver disease. Semin Liver Dis 2002;22:83–96.
70. Chang JC, Kane KK. Pathologic hyperfibrinolysis associated with amyloidosis: clinical response to epsilon amino caproic acid. Am J Clin Pathol 1984;81:382–7.
71. Anonymous. Tranexamic acid. Med Letter Drugs Therapeutics 1987;29:89–90.
72. Schwartz BS, Williams EC, Conlan MG, Mosher DF. Epsilon-aminocaproic acid in the treatment of patients with acute promyelocytic leukemia and acquired alpha-2-plasmin inhibitor defiency. Ann Intern Med 1986;105:873–7.
73. Takahashi H, Tatewaki W, Wada K, et al. Fibrinolysis and fibrinogenolysis in liver disease. Am J Hematol 1990;34:241-–5.
74. Ker K, Edwards P, Perel P, et al. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ 2012;344:e3054.
75. Shakur H, Roberts I, Bautista R, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376(9734):23–32.
76. WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet 2017;389:2105–16.
77. Godbey EA, Schwartz J. ‘Massive transfusion protocols and the use of tranexamic acid’. Curr Opin Hematol 2018 Aug 16. doi: 10.1097/MOH.0000000000000457. [Epub ahead of print]
78. Hay CR, Negrier C, Ludlam CA. The treatment of bleeding in acquired haemophilia with recombinant factor VIIa: a multicentre study. Thromb Haemost 1997;78:1463–7.
79. DeLoughery TG. Management of bleeding emergencies: when to use recombinant activated factor VII. Expert Opin Pharmacother 2006;7:25–34.
80. Aledort L. Recombinant factor VIIa Is a pan-hemostatic agent? Thromb Haemost 2000;83:637–8.
81. Logan AC, Yank V, Stafford RS. Off-label use of recombinant factor VIIa in U.S. hospitals: analysis of hospital records. Ann Intern Med 2011;154:516–22.
82. Lin Y, Stanworth S, Birchall J, Doree C, Hyde C. Use of recombinant factor VIIa for the prevention and treatment of bleeding in patients without hemophilia: a systematic review and meta-analysis. CMAJ 2011;183:E9–19.
83. Yank V, Tuohy CV, Logan AC et al. Systematic review: benefits and harms of in-hospital use of recombinant factor VIIa for off-label indications. Ann Intern Med 2011;154:529–40.
84. Pavese P, Bonadona A, Beaubien J, et al. FVIIa corrects the coagulopathy of fulminant hepatic failure but may be associated with thrombosis: a report of four cases. Can J Anaesth 2005;52:26–29.
85. Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2005;352:777–85.
86. Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2008;358:2127–37.
87. Levi M, Levy JH, Andersen HF, Truloff D. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med 2010;363:1791–1800.
Does this help the patient?
Twenty minutes after Ronald Reagan finished his inaugural address, the Islamic Republic of Iran announced the release of 52 American hostages that Iran had held for the last year of Jimmy Carter’s presidency. The timing led to the “October Surprise conspiracy theory,” in which some inferred a deliberate plan to influence an American election. We now refer to any political event timed to an election as an “October Surprise.” We are awaiting some type of surprise prior to this November’s elections. Events unfolding this fall will have generational effects on American politics, our health care delivery models, our financial security, individual rights and the democratic infrastructure of our country. This is not an election to sit out.
The proposed rule the Centers for Medicare & Medicaid published in the summer has generated a massive public response. The major issues (as discussed in last month’s GI & Hepatology News issue, including the editorial) include a dramatic change in documentation requirements and payment for evaluation and management (E/M) codes for both new and returning patients. While the reduction in documentation is laudable, the reduction in reimbursement for complex visits is not. At Michigan Medicine (2.2 million outpatient visits per year), reimbursements would go down by $3.5 million annually for our E/M visits. Most responses to the proposed rule requested a year’s delay and intensive analysis of work involved prior to reducing payments (see further comments at gastro.org, the AGA website).
This month we cover a new anti-obesity drug that shows cardiovascular safety. This is a welcome potential addition to our therapies since another story updates us on the relentless rise in obesity in this country. We cover the world’s alcohol use this month. On a financial note, the anticipated savings from biosimilars may be less than we hoped if data from Medicare Part D can be generalized. We also cover a diagnostic update about eosinophilic esophagitis.
I hope you enjoy this issue of GI & Hepatology News and all the AGA publications that provide you with up-to-date science, clinical information, and news about gastroenterology in general. Remember to vote. On the wall across from my desk as I sit as a leader in ambulatory care at Michigan Medicine, there is a large sign that grounds me. It reads, “Does this help the patient?”
John I. Allen, MD, MBA, AGAF
Editor in Chief
Twenty minutes after Ronald Reagan finished his inaugural address, the Islamic Republic of Iran announced the release of 52 American hostages that Iran had held for the last year of Jimmy Carter’s presidency. The timing led to the “October Surprise conspiracy theory,” in which some inferred a deliberate plan to influence an American election. We now refer to any political event timed to an election as an “October Surprise.” We are awaiting some type of surprise prior to this November’s elections. Events unfolding this fall will have generational effects on American politics, our health care delivery models, our financial security, individual rights and the democratic infrastructure of our country. This is not an election to sit out.
The proposed rule the Centers for Medicare & Medicaid published in the summer has generated a massive public response. The major issues (as discussed in last month’s GI & Hepatology News issue, including the editorial) include a dramatic change in documentation requirements and payment for evaluation and management (E/M) codes for both new and returning patients. While the reduction in documentation is laudable, the reduction in reimbursement for complex visits is not. At Michigan Medicine (2.2 million outpatient visits per year), reimbursements would go down by $3.5 million annually for our E/M visits. Most responses to the proposed rule requested a year’s delay and intensive analysis of work involved prior to reducing payments (see further comments at gastro.org, the AGA website).
This month we cover a new anti-obesity drug that shows cardiovascular safety. This is a welcome potential addition to our therapies since another story updates us on the relentless rise in obesity in this country. We cover the world’s alcohol use this month. On a financial note, the anticipated savings from biosimilars may be less than we hoped if data from Medicare Part D can be generalized. We also cover a diagnostic update about eosinophilic esophagitis.
I hope you enjoy this issue of GI & Hepatology News and all the AGA publications that provide you with up-to-date science, clinical information, and news about gastroenterology in general. Remember to vote. On the wall across from my desk as I sit as a leader in ambulatory care at Michigan Medicine, there is a large sign that grounds me. It reads, “Does this help the patient?”
John I. Allen, MD, MBA, AGAF
Editor in Chief
Twenty minutes after Ronald Reagan finished his inaugural address, the Islamic Republic of Iran announced the release of 52 American hostages that Iran had held for the last year of Jimmy Carter’s presidency. The timing led to the “October Surprise conspiracy theory,” in which some inferred a deliberate plan to influence an American election. We now refer to any political event timed to an election as an “October Surprise.” We are awaiting some type of surprise prior to this November’s elections. Events unfolding this fall will have generational effects on American politics, our health care delivery models, our financial security, individual rights and the democratic infrastructure of our country. This is not an election to sit out.
The proposed rule the Centers for Medicare & Medicaid published in the summer has generated a massive public response. The major issues (as discussed in last month’s GI & Hepatology News issue, including the editorial) include a dramatic change in documentation requirements and payment for evaluation and management (E/M) codes for both new and returning patients. While the reduction in documentation is laudable, the reduction in reimbursement for complex visits is not. At Michigan Medicine (2.2 million outpatient visits per year), reimbursements would go down by $3.5 million annually for our E/M visits. Most responses to the proposed rule requested a year’s delay and intensive analysis of work involved prior to reducing payments (see further comments at gastro.org, the AGA website).
This month we cover a new anti-obesity drug that shows cardiovascular safety. This is a welcome potential addition to our therapies since another story updates us on the relentless rise in obesity in this country. We cover the world’s alcohol use this month. On a financial note, the anticipated savings from biosimilars may be less than we hoped if data from Medicare Part D can be generalized. We also cover a diagnostic update about eosinophilic esophagitis.
I hope you enjoy this issue of GI & Hepatology News and all the AGA publications that provide you with up-to-date science, clinical information, and news about gastroenterology in general. Remember to vote. On the wall across from my desk as I sit as a leader in ambulatory care at Michigan Medicine, there is a large sign that grounds me. It reads, “Does this help the patient?”
John I. Allen, MD, MBA, AGAF
Editor in Chief
What’s the Buzz? Treatment Strategies in Chronic Subjective Tinnitus
CE/CME No: CR-1810
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Distinguish primary tinnitus from secondary tinnitus.
• Understand and implement a full clinical evaluation of tinnitus, including imaging studies when appropriate.
• Discuss expectations regarding treatment options and realistic outcomes of currently recommended therapy.
• Direct patients to specialist care for cognitive behavioral therapy or tinnitus retraining therapy.
• Know when pharmacotherapeutic intervention is indicated.
FACULTY
Wendy Gillian Ross practices urgent care medicine in Lake Grove, New York, and primary care in Patchogue, New York. Randy Danielsen is Professor and Dean, Arizona School of Health Sciences, and Director, Center for the Future of the Health Professions, both at A.T. Still University, in Mesa, Arizona. He is Physician Assistant Editor-in-Chief of Clinician Reviews.
The authors have no financial relationships to disclose.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through September 30, 2019.
Article begins on next page >>
Tinnitus can be a debilitating condition that affects quality of life and is often not treated according to guidelines. Cognitive behavioral therapy and tinnitus retraining therapy have been successful in reducing tinnitus bother; pharmacotherapy is not widely accepted as successful, and can, in fact, be deleterious. This article describes pathophysiologic disturbances of hearing and how they relate to chronic subjective tinnitus, discusses the clinical evaluation of tinnitus as a presenting symptom, and reviews current treatments.
Primary chronic subjective tinnitus, often thought of more as a symptom than a diagnosis, affects millions of people worldwide. This troublesome condition has been chronicled as far back as the first century
It is estimated that only 20% of people who experience tinnitus actively seek treatment.2 In the United States, 2 to 3 million of the 12 million patients who do request treatment report lasting symptoms that they describe as debilitating.3 For patients who seek help, the treatment recommended by physicians is typically pharmacotherapeutic—which does not follow guidelines.4
The aim of this article is to reinforce a greater understanding of the mechanisms of tinnitus and integrate that knowledge into treatment guidelines. The article does not discuss surgical treatment of tinnitus.
DEFINITION AND CLASSIFICATION
A universal standard definition of chronic tinnitus does not exist; Trevis et al define it as a phantom sound that persists for more than three months.5 The quality and loudness of tinnitus is variable but is often described as a buzz, hiss, or ringing. Prevalence increases with age, smoking, male gender, and ethnicity, with the non-Latino white population statistically at greater risk.3 Comorbid conditions (eg, diabetes and other autoimmune diseases) are risk factors for tinnitus. A history of exposure to loud sound—occupational, environmental, or recreational—also can predispose a person to tinnitus.3
The American Academy of Otolaryngology–Head and Neck Surgery (AAO–HNS) classifies tinnitus as primary (subjective) or secondary (objective). Primary tinnitus—representing the majority of cases—has no identifiable cause; there may be accompanying sensorineural hearing loss or hyperacusis. Secondary tinnitus can also be associated with sensorineural hearing loss but has an identifiable underlying cause.6 The differential diagnosis of tinnitus is listed in the Table.7
Tinnitus is further defined by its persistence. Persistent tinnitus is defined as tinnitus lasting more than six months, slightly longer than the duration offered by Trevis et al, who also define tinnitus as bothersome or non-bothersome, depending on its impact on quality of life.5,6 Causes of reduced quality of life include depression, anxiety, insomnia, and neurocognitive decline—all of which have been associated with chronic subjective tinnitus.8
Continue to: Researchers have discovered that...
Researchers have discovered that tinnitus is not simply a cochlear phenomenon. The pathology extends well beyond the auditory complex, having a deleterious effect on both the somatosensory and central nervous systems, providing some explanation for the prevalence of anxiety and depression associated with the disorder (see "Pathophysiology of tinnitus").9-17

Because of the insidious nature of tinnitus and lack of standard measures of severity, true prevalence is difficult to calculate.18
CLINICAL EVALUATION
Tinnitus can be a presenting complaint or elicited during history-taking. Symptomatic patients should receive full evaluation, including a complete physical exam, medication history, and laboratory workup.
Adverse effect of drugs
Medications that commonly cause tinnitus symptoms are NSAIDs, chemotherapeutic agents, and antibiotics (eg, macrolides and fluoroquinolones). Amiodarone, ACE inhibitors, proton-pump inhibitors, and calcium-channel blockers have also been implicated. Paradoxically, anxiolytics and tricyclic antidepressants, which are sometimes used to treat tinnitus, have been linked to causing the condition.7
Laboratory tests and imaging
Testing should include investigation for infectious disease, autoimmune disorders, and vitamin deficiency.7 According to the American College of Radiology, imaging is unnecessary in the workup of primary tinnitus. Any suspicion of a vascular cause noted on the physical exam (eg, an associated bruit or venous hum), however, should be explored with imaging. Furthermore, any case of tinnitus that lateralizes also requires additional investigation. Modalities of choice are MRI, CT, and CT angiography.19
Continue to: Referral for audiology evaluation
Referral for audiology evaluation
When no underlying pathology can be identified for tinnitus, the patient should be sent for a full audiology evaluation to screen for associated hearing loss. Discussion of audiology screening tests is beyond the scope of this article; however, testing includes otoscopy, audiography, tympanography, otoacoustic emission testing, auditory brainstem-response testing, and vestibular evoked myogenic potential testing.7
Probing nonphysical impacts
Quality of life and overall emotional wellness, including cognitive function, should be investigated in patients with tinnitus. Two questionnaires commonly used in the assessment of tinnitus bother are the Tinnitus Handicap Inventory and the Tinnitus Reaction Questionnaire.7 In a large, systematic review, Trevis et al report that “64% of studies investigating depression found an increase in depressive symptoms in people with chronic tinnitus compared to hearing control groups, and 62% of studies investigating anxiety reported significantly increased anxiety symptoms.”5
MANAGEMENT
Tinnitus management should be viewed two ways: treatment of perceived loudness and treatment of comorbid symptoms relating to tinnitus bother.6 In the same meta-analysis, Trevis and colleagues found that patients with tinnitus had higher rates of anxiety, depression, and overall decline in cognitive function, including processing speed, concentration, and sleep disorders.5 It is useful to keep this observation in mind when reviewing treatment options for tinnitus.
Five classic pharmacotherapeutic approaches to tinnitus management are
- Anticonvulsants
- Antidepressants
- Anesthetics
- Anxiolytics
- Lidocaine.
Newer medications that show some promise are N-methyl-D-aspartate (NMDA) receptor antagonists, notably neramexane. Alternative pharmaceuticals include vitamin-based treatments, cannabinoids, and herbal compounds.
Continue to: The AAOS-HNS supports...
The AAO–HNS supports nonpharmacotherapeutic treatment of tinnitus; its guidelines include a recommendation for cognitive behavioral therapy (CBT) as primary therapy.6 In addition, tinnitus-retraining therapy, tinnitus-masking therapy/sound therapy, meditation/mindfulness, and yoga all have been studied for their ability to alleviate tinnitus bother.
Pharmacotherapeutic management
Anticonvulsants have failed to provide strong evidence of usefulness in the treatment of tinnitus and are not supported by the AAO–HNS as such.6 This conclusion notwithstanding, the anticonvulsants carbamazepine and gabapentin have historically been two of the more common medications used to treat tinnitus.
Carbamazepine is a glutamate receptor antagonist that suppresses seizure activity. Based on prior research suggesting that spontaneous firing within the auditory complex is similar to seizure activity, Iranian researchers explored the hypothesis that carbamazepine might lessen tinnitus severity. Their study revealed, however, that carbamazepine did not statistically significantly reduce the severity of tinnitus, compared to placebo.20 While carbamazepine may be of limited use in the treatment of subjective tinnitus, recent literature confirms that it is not only useful, but also diagnostic, in typewriter tinnitus (ie, having a staccato quality, like the sound of typewriter keys being depressed). Typewriter tinnitus is a secondary cause of tinnitus related to disruption of the stapes in the middle ear.21
Gabapentin works by promoting gamma-aminobutyric acid (GABA) production in the brain. GABA is an inhibitory neurotransmitter, thus slowing down signals between neurons. Following on preliminary research that detected low levels of GABA in the inferior colliculus of rodents with salicylate-induced tinnitus, Aazh and colleagues conducted a double-blind study of gabapentin—and concluded that it yielded no improvement in symptoms, compared to placebo.22
Valproic acid has not been formally investigated but is commonly incorporated in the treatment of tinnitus.23 Lamotrigine has provided similarly disappointing results in the treatment of tinnitus.24
Continue to: Antidepressants and anxiolytics
Antidepressants and anxiolytics. Based on the results of their early clinical trials, Sullivan and colleagues concluded that tricyclic antidepressants produced significant improvement in tinnitus symptoms, due to the analgesic effects of these drugs. The researchers studied nortriptyline specifically; in severely depressed patients, the drug reduced the loudness of tinnitus and depressive symptoms. In non-depressed subjects, however, nortriptyline was not as efficacious.25
Selective serotonin reuptake inhibitors have not had the same success as nortriptyline. In a study of paroxetine conducted by Oishi and colleagues, there was little evidence that the drug reduced the loudness of tinnitus, although overall, it did reduce tinnitus bother and anxiety.26
Included in the category of anxiolytics, benzodiazepines have long been used to treat severe tinnitus-induced anxiety, with some success. However, as Elgoyhen and Langguth point out, studies of benzodiazepines for tinnitus have been limited in size.23
The AAO–HNS does not support routine use of antidepressants and anxiolytics for tinnitus bother.7
NMDA receptor antagonists. In a recent clinical trial, neramexane was studied for its efficacy in tinnitus. Neramexane acts at the cholinergic nicotinic and NMDA receptors in the efferent auditory system. Its complex reaction is thought to prevent transmission of unwanted sound not only to structures within the auditory system but beyond, to the medial geniculate body and lateral nucleus of the amygdala. The trial has proved some benefit concerning overall perception of tinnitus loudness; a phase 2 trial is being conducted.27
Continue to: Intra-tympanic anesthetics
Intra-tympanic anesthetics. Anesthetics, such as lidocaine, have had limited success and results have not been found to be sustained.
Alternative medical managements
Traditional Chinese herbal medications have been used for centuries and are increasingly popular in Western culture. Hilton and colleagues studied Ginkgo biloba, or maidenhair tree, a traditional Chinese herbal supplement available as an extract and as dried leaves. The main action of the extract is vasoregulatory; antiplatelet effects are also seen. Adverse effects include gastrointestinal upset and headache. In a systematic review, Hilton and colleagues concluded that Ginkgo did not reduce overall tinnitus loudness or severity; the review was limited, however, by the fact that only two studies met criteria for inclusion.28
Vitamins, lipoflavinoids, zinc, manganese, and melatonin are all supplements marketed to improve tinnitus symptoms. However, a cross-sectional study confirmed prior research that did not show any benefit from the use of these supplements.29
Cannabinoids are being studied for their proposed antiepileptic effects. There is a popular misconception of Cannabis as a singular chemical when in fact, it is a plant that contains hundreds of chemicals that each act differently on the brain. In a review, Smith and Zheng30 explain that two cannabinoid receptors, CB1 and CB2, are represented, and exert their effects, in different areas of the brain. CB1 receptors block calcium influx in presynaptic terminals, resulting in an inhibitory effect on neurotransmitter release.
CB1 receptors have been found in the dorsal cochlear nuclei, prompting research interest in how cannabinoids affect neurotransmission of unwanted sounds of tinnitus. To date, however, there are conflicting data concerning the benefit of cannabinoids and tinnitus. In fact, Smith and Zheng state that some data suggest that cannabinoids might make tinnitus worse.30
Continue to: Nonpharmacotherapeutic management
Nonpharmacotherapeutic management
Cognitive behavioral therapy. Conceptualized by Aaron T. Beck in the 1960s, cognitive behavioral therapy (CBT) is the leading recommendation made by the AAO–HNS in its tinnitus treatment guidelines.6 Beck’s work centered on the idea that behaviors are modifiable thoughts, through analysis of past experiences and assumptions based on those experiences. By understanding the core belief that a patient attaches to a feeling, Beck hypothesized that behaviors or responses to those feelings could be changed; this is accomplished through discussion to dispel unwarranted fears and by teaching coping mechanisms, such as relaxation. The idea behind CBT in the management of tinnitus is clear: The sound cannot be eliminated, but the patient’s response to the sound can be modified. Ultimately, through this modified response or habituation, the patient can relax and live with the sound.31
Since anxiety, depression, and insomnia are common comorbidities of tinnitus, a psychologic approach remains in the forefront of treatment recommendations. Hoare and colleagues reported that in “a meta-analysis of 10 randomized trials evaluating different forms of CBT (by the therapist and over the Internet), CBT improved tinnitus symptoms compared to non-CBT controls.”7
Tinnitus retraining therapy (TRT) is another form of habituation therapy, introduced by Jastreboff in the 1990s. His work furthered the idea that tinnitus could be reframed, as it is in CBT. Simply, he proposed that systems outside the auditory complex—namely the autonomic nervous system and the limbic system—respond to the signal produced by damaged hair cells in the cochlear nuclei. TRT retrains connections to block or ignore these signals.13 Unlike CBT, the aim of TRT is to eliminate the perception of sound.
By educating patients about the physiologic mechanisms of tinnitus, TRT reduces patient anxiety related to the sound. The process of habituation follows counseling. To accomplish this, the patient wears a sound generator, similar in appearance to hearing aids, using broadband noise. The sound does not mask the tinnitus but closes the gap between silence and the perception of tinnitus. The sound generator is worn for six hours daily for approximately 12 months.
Multiple studies have employed Jastreboff’s original technique, including a clinical trial by Bauer and colleagues. The published outcome of this study confirmed that patients experienced a positive and lasting effect with TRT.32 In addition, a small study of TRT conducted by Barozzi and colleagues, using different colors of sound (ie, how the frequency of a given sound corresponds to the light-wave frequency of a particular color), found statistically significant improvement. Allowing patients to pick a sound that they found more pleasant increased the effectiveness of the treatment.33 (Patients can learn more about TRT by visiting www.tinnitus-pjj.com, hosted by tinnitus researcher Pawel J. Jastreboff.)
Continue to: Alternative nonmedical therapies...
Alternative nonmedical therapies have become popular; they include meditation, yoga, physical therapy, mindfulness, and tinnitus-masking treatment with sound.
Results of a study of yoga and meditation showed that patients felt more relaxed, but that these interventions had no effect on the severity of tinnitus. The principle behind yoga practice, according to Köksoy and colleagues, is that the discipline is thought to affect the limbic system by deactivating the sympathetic response to stimulation from surrounding sounds. In addition, Köksoy states, other researchers have provided evidence that yoga increases circulating levels of antioxidants, which in turn reduce oxidative stress.34
Particularly among members of the millennial generation, mindfulness has become a buzzword. The practice refers to a “method for facing, exploring, and alleviating suffering by relating to present experiences.”35 Roland and colleagues conducted a clinical trial of mindfulness practiced by a cohort of patients with bothersome tinnitus; results were based on scores gleaned from standard rating scales (eg, Global Bothersome Scale, Cognitive and Affective Mindfulness Scale-Revised, Cognitive Failures Questionnaire, Tinnitus Handicap Inventory, and Tinnitus Functional Index). Evaluated before and four weeks after cessation of therapy, subjects reported that tinnitus bother was reduced, but none showed statistically significant improvement in depression, anxiety, or cognitive ability.35
Used for more than 40 years, sound-based therapy has been discussed in conjunction with TRT.36 It is recognized as an approved but optional treatment by the AAO–HNS. In response to a 2010 study by Hobson that used sound-based therapy alone for tinnitus, Tunkel and colleagues cautioned that the modality showed little benefit. The major downside to acoustic therapy, according to the AAO–HNS clinical guidelines, is cost and patients’ excessive expectation of effectiveness.6
According to the AAO–HNS, repetitive-transcranial magnetic stimulation is not supported as a valid treatment for tinnitus because it can lead to seizures in patients who are taking medication that lowers the seizure threshold or who have a secondary cause of tinnitus, such as a tumor—therefore creating risk that outweighs any benefit.6
Continue to: CONCLUSION
CONCLUSION
For a large percentage of the population, chronic subjective tinnitus is a significant variable in the evaluation of quality of life. The condition is not completely understood and often displays features unique to the individual. Much of the initial response to research linking tinnitus with shared pathways typical for chronic pain, anxiety, and depression has resulted in pharmacotherapeutic management that is not always warranted—or successful.
Clinical research into the pathophysiology of tinnitus is providing a better understanding of the neurophysiologic mechanisms that underpin the science of chronic tinnitus. With this information, researchers can one day design medical management that targets specific receptors, resulting in greater management success.
The psychologic impact of tinnitus cannot be underestimated. When almost one-third of patients complain of debilitating symptoms that can also result in neurocognitive decline, tinnitus becomes a condition that cannot be ignored. Guidelines set forth by the AAO–HNS state that CBT and TRT offer some reprieve from symptoms and teach patients habituation without further damage to hearing. The use of broad-based sound generators has been well established as a useful management tool, although it is not curative.
The limitations of some studies that reviewed alternative medicines include small sample size and difficulty comparing research analysis because of disparities in tinnitus rating scales. Also, age bias, comorbid conditions, and study drop-out rates affected overall statistical significance of some studies. Additional, high-quality research is warranted in this area.
Continue to: Prevention of tinnitus...
Prevention of tinnitus through education on hearing loss and its causes should be regarded as implicit; occupational noise and recreational use of music devices put people at heightened risk for hearing loss and tinnitus. Information and open discussion that include the discovery of tinnitus symptoms during routine physical examination are recommended.
Last, providers who adhere to recognized guidelines will aid patients in coping with the challenges that tinnitus presents. As research continues to unravel the complex interaction between neurons, medical science is hopeful that curative treatments will become available.
1. Maltby MT. Ancient voices on tinnitus: the pathology and treatment of tinnitus in Celsus and the Hippocratic Corpus compared and contrasted. Int Tinnitus J. 2012;17(2):140-145.
2. Wolever RQ, Price R, Hazelton GA, et al. Complementary therapies for significant dysfunction from tinnitus: treatment review and potential for integrative medicine. Evid Based Complement Alternat Med. 2015;15:931418.
3. Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med. 2010;123(8):711-718.
4. Bhatt JM, Lin HW, Bhattacharyya N. Prevalence, severity, exposures, and treatment patterns of tinnitus in the United States. JAMA Otolaryngol Head Neck Surg. 2016;142(10):959-965.
5. Trevis KJ, McLachlan NM, Wilson SJ. A systematic review and meta-analysis of psychological functioning in chronic tinnitus. Clin Psychol Rev. 2018;60:62-86.
6. Tunkel DE, Bauer CA, Sun GH, et al. Clinical practice guideline: tinnitus. Otolaryngol Head Neck Surg. 2014;151(suppl 2):S1-S40.
7. Dinces EA. Treatment of tinnitus. UpToDate. April 12, 2018. www.uptodate.com/contents/treatment-of-tinnitus. Accessed September 17, 2018.
8. Gudwani S, Munjal SK, Panda NK, Kohli A. Association of chronic subjective tinnitus with neuro-cognitive performance. Int Tinnitus J. 2017;21:90-97.
9. Jastreboff PJ. 25 years of tinnitus retraining therapy. HNO. 2015;63:307-311.
10. Pujol R. Journey into the world of hearing. 2016. www.cochlea.eu/en. Accessed September 17, 2018.
11. Adjamian P, Hall DA, Palmer AR, et al. Neuroanatomical abnormalities in chronic tinnitus in the human brain.Neurosci Biobehav Rev. 2014;45:119-133.
12. Shore SE, Roberts LE, Langguth B. Maladaptive plasticity in tinnitus—triggers, mechanisms and treatment. Nat Rev Neurol. 2016;12(3):150-160.
13. Jastreboff PJ, Gray WC, Gold SL. Neurophysiological approach to tinnitus patients. Am J Otol. 1996;17(2):236-240.
14. Kaltenbach JA. Tinnitus: models and mechanisms. Hear Res. 2011;276:52-60.
15. Rauschecker JP, Leaver AM, Mühlau M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron. 2010;66(6):819-826.
16. Møller AR. Sensorineural tinnitus: its pathology and probable therapies. Int J Otolaryngol. 2016;2016:2830157.
17. Chen YC, Xia W, Chen H, et al. Tinnitus distress is linked to enhanced resting‐state functional connectivity from the limbic system to the auditory cortex. Hum Brain Mapp. 2017;38(5):2384-2397.
18. McCormack A, Edmonson-Jones M, Somerset S, Hall D. A systematic review of the reporting of tinnitus prevalence and severity. Hear Res. 2016;337:70-79.
19. Kessler MM, Moussa M, Bykowski J, et al; Expert Panel on Neurologic Imaging. ACR Appropriateness Criteria® Tinnitus. J Am Coll Radiol. 2017;14:S584-S591.
20. Gerami H, Saberi A, Nemati, S, et al. Effects of oxcarbazepine versus carbamazepine on tinnitus: a randomized double-blind placebo-controlled clinical trial. Iran J Neurol. 2012;11(3):106-110.
21. Sunwoo W, Jeon YJ, Bae YJ, et al. Typewriter tinnitus revisited: the typical symptoms and the initial response to carbamazepine are the most reliable diagnostic clues. Sci Rep. 2017;7:10615.
22. Aazh H, El Refaie A, Humphriss R. Gabapentin for tinnitus: a systematic review. Am J Audiol. 2011;20:151-158.
23. Elgoyhen AB, Langguth B. Pharmacological approaches to the treatment of tinnitus. Drug Discov Today. 2010;15:300-305.
24. Langguth B, Kreuzer PM, Kleinjung T, De Ridder D. Tinnitus: causes and clinical management. Lancet Neurol. 2013;12(9):920-930.
25. Sullivan M, Katon W, Russo J, et al. A randomized trial of nortriptyline for severe chronic tinnitus. Effects on depression, disability, and tinnitus symptoms. Arch Intern Med. 1993;153(19):2251-2259.
26. Oishi N, Kanzaki S, Shinden S, et al. Effects of selective serotonin reuptake inhibitor on treating tinnitus in patients stratified for presence of depression or anxiety. Audiol Neurootol. 2010;15(3):187-193.
27. Suckfüll M, Althaus M, Ellers-Lenz B, et al. A randomized, double-blind, placebo-controlled clinical trial to evaluate the efficacy and safety of neramexane in patients with moderate to severe subjective tinnitus. BMC Ear Nose Throat Disord. 2011;11:1.
28. Hilton MP, Zimmermann EF, Hunt WT. Ginkgo biloba for tinnitus. Cochrane Database Syst Rev. 2013;CD003852. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD003852.pub3/full. Accessed September 17, 2018.
29. Coelho C, Tyler R, Ji H, et al. Survey on the effectiveness of dietary supplements to treat tinnitus. Am J Audiol. 2016;25:184-205.
30. Smith PF, Zheng Y. Cannabinoids, cannabinoid receptors and tinnitus. Hear Res. 2015;332:210-216.
31. Martinez-Devesa P, Perera R, Theodoulou M, Waddell A. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev. 2010:CD005233. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD005233.pub3/full. Accessed September 17, 2018.
32. Bauer CA, Berry JL, Brozoski TJ. The effect of tinnitus retraining therapy on chronic tinnitus: a controlled trial. Laryngoscope Investig Otolaryngol. 2017;2(4):166-177.
33. Barozzi S, Ambrosetti U, Callaway SL, et al. Effects of tinnitus retraining therapy with different colours of sound. Int Tinnitus J. 2017;21:139-143.
34. Köksoy S, Eti CM, Karatas¸ M, Vayisoglu Y. The effects of yoga in patients suffering from subjective tinnitus. Int Arch Otorhinolaryngol. 2018;22(1):9-13.
35. Roland LT, Lenze EJ, Hardin FM, et al. Effects of mindfulness based stress reduction therapy on subjective bother and neural connectivity in chronic tinnitus. Otolaryngol Head Neck Surg. 2015;152(5):919-926.
36. Ibarra D, Tavira-Sanchez F, Recuero-Lopez M, Anthony BW. In-ear medical devices for acoustic therapies in tinnitus treatments, state of the art. Auris Nasus Larynx. 2018;45:6-12.
CE/CME No: CR-1810
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Distinguish primary tinnitus from secondary tinnitus.
• Understand and implement a full clinical evaluation of tinnitus, including imaging studies when appropriate.
• Discuss expectations regarding treatment options and realistic outcomes of currently recommended therapy.
• Direct patients to specialist care for cognitive behavioral therapy or tinnitus retraining therapy.
• Know when pharmacotherapeutic intervention is indicated.
FACULTY
Wendy Gillian Ross practices urgent care medicine in Lake Grove, New York, and primary care in Patchogue, New York. Randy Danielsen is Professor and Dean, Arizona School of Health Sciences, and Director, Center for the Future of the Health Professions, both at A.T. Still University, in Mesa, Arizona. He is Physician Assistant Editor-in-Chief of Clinician Reviews.
The authors have no financial relationships to disclose.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through September 30, 2019.
Article begins on next page >>
Tinnitus can be a debilitating condition that affects quality of life and is often not treated according to guidelines. Cognitive behavioral therapy and tinnitus retraining therapy have been successful in reducing tinnitus bother; pharmacotherapy is not widely accepted as successful, and can, in fact, be deleterious. This article describes pathophysiologic disturbances of hearing and how they relate to chronic subjective tinnitus, discusses the clinical evaluation of tinnitus as a presenting symptom, and reviews current treatments.
Primary chronic subjective tinnitus, often thought of more as a symptom than a diagnosis, affects millions of people worldwide. This troublesome condition has been chronicled as far back as the first century
It is estimated that only 20% of people who experience tinnitus actively seek treatment.2 In the United States, 2 to 3 million of the 12 million patients who do request treatment report lasting symptoms that they describe as debilitating.3 For patients who seek help, the treatment recommended by physicians is typically pharmacotherapeutic—which does not follow guidelines.4
The aim of this article is to reinforce a greater understanding of the mechanisms of tinnitus and integrate that knowledge into treatment guidelines. The article does not discuss surgical treatment of tinnitus.
DEFINITION AND CLASSIFICATION
A universal standard definition of chronic tinnitus does not exist; Trevis et al define it as a phantom sound that persists for more than three months.5 The quality and loudness of tinnitus is variable but is often described as a buzz, hiss, or ringing. Prevalence increases with age, smoking, male gender, and ethnicity, with the non-Latino white population statistically at greater risk.3 Comorbid conditions (eg, diabetes and other autoimmune diseases) are risk factors for tinnitus. A history of exposure to loud sound—occupational, environmental, or recreational—also can predispose a person to tinnitus.3
The American Academy of Otolaryngology–Head and Neck Surgery (AAO–HNS) classifies tinnitus as primary (subjective) or secondary (objective). Primary tinnitus—representing the majority of cases—has no identifiable cause; there may be accompanying sensorineural hearing loss or hyperacusis. Secondary tinnitus can also be associated with sensorineural hearing loss but has an identifiable underlying cause.6 The differential diagnosis of tinnitus is listed in the Table.7

Tinnitus is further defined by its persistence. Persistent tinnitus is defined as tinnitus lasting more than six months, slightly longer than the duration offered by Trevis et al, who also define tinnitus as bothersome or non-bothersome, depending on its impact on quality of life.5,6 Causes of reduced quality of life include depression, anxiety, insomnia, and neurocognitive decline—all of which have been associated with chronic subjective tinnitus.8
Continue to: Researchers have discovered that...
Researchers have discovered that tinnitus is not simply a cochlear phenomenon. The pathology extends well beyond the auditory complex, having a deleterious effect on both the somatosensory and central nervous systems, providing some explanation for the prevalence of anxiety and depression associated with the disorder (see "Pathophysiology of tinnitus").9-17

Because of the insidious nature of tinnitus and lack of standard measures of severity, true prevalence is difficult to calculate.18
CLINICAL EVALUATION
Tinnitus can be a presenting complaint or elicited during history-taking. Symptomatic patients should receive full evaluation, including a complete physical exam, medication history, and laboratory workup.
Adverse effect of drugs
Medications that commonly cause tinnitus symptoms are NSAIDs, chemotherapeutic agents, and antibiotics (eg, macrolides and fluoroquinolones). Amiodarone, ACE inhibitors, proton-pump inhibitors, and calcium-channel blockers have also been implicated. Paradoxically, anxiolytics and tricyclic antidepressants, which are sometimes used to treat tinnitus, have been linked to causing the condition.7
Laboratory tests and imaging
Testing should include investigation for infectious disease, autoimmune disorders, and vitamin deficiency.7 According to the American College of Radiology, imaging is unnecessary in the workup of primary tinnitus. Any suspicion of a vascular cause noted on the physical exam (eg, an associated bruit or venous hum), however, should be explored with imaging. Furthermore, any case of tinnitus that lateralizes also requires additional investigation. Modalities of choice are MRI, CT, and CT angiography.19
Continue to: Referral for audiology evaluation
Referral for audiology evaluation
When no underlying pathology can be identified for tinnitus, the patient should be sent for a full audiology evaluation to screen for associated hearing loss. Discussion of audiology screening tests is beyond the scope of this article; however, testing includes otoscopy, audiography, tympanography, otoacoustic emission testing, auditory brainstem-response testing, and vestibular evoked myogenic potential testing.7
Probing nonphysical impacts
Quality of life and overall emotional wellness, including cognitive function, should be investigated in patients with tinnitus. Two questionnaires commonly used in the assessment of tinnitus bother are the Tinnitus Handicap Inventory and the Tinnitus Reaction Questionnaire.7 In a large, systematic review, Trevis et al report that “64% of studies investigating depression found an increase in depressive symptoms in people with chronic tinnitus compared to hearing control groups, and 62% of studies investigating anxiety reported significantly increased anxiety symptoms.”5
MANAGEMENT
Tinnitus management should be viewed two ways: treatment of perceived loudness and treatment of comorbid symptoms relating to tinnitus bother.6 In the same meta-analysis, Trevis and colleagues found that patients with tinnitus had higher rates of anxiety, depression, and overall decline in cognitive function, including processing speed, concentration, and sleep disorders.5 It is useful to keep this observation in mind when reviewing treatment options for tinnitus.
Five classic pharmacotherapeutic approaches to tinnitus management are
- Anticonvulsants
- Antidepressants
- Anesthetics
- Anxiolytics
- Lidocaine.
Newer medications that show some promise are N-methyl-D-aspartate (NMDA) receptor antagonists, notably neramexane. Alternative pharmaceuticals include vitamin-based treatments, cannabinoids, and herbal compounds.
Continue to: The AAOS-HNS supports...
The AAO–HNS supports nonpharmacotherapeutic treatment of tinnitus; its guidelines include a recommendation for cognitive behavioral therapy (CBT) as primary therapy.6 In addition, tinnitus-retraining therapy, tinnitus-masking therapy/sound therapy, meditation/mindfulness, and yoga all have been studied for their ability to alleviate tinnitus bother.
Pharmacotherapeutic management
Anticonvulsants have failed to provide strong evidence of usefulness in the treatment of tinnitus and are not supported by the AAO–HNS as such.6 This conclusion notwithstanding, the anticonvulsants carbamazepine and gabapentin have historically been two of the more common medications used to treat tinnitus.
Carbamazepine is a glutamate receptor antagonist that suppresses seizure activity. Based on prior research suggesting that spontaneous firing within the auditory complex is similar to seizure activity, Iranian researchers explored the hypothesis that carbamazepine might lessen tinnitus severity. Their study revealed, however, that carbamazepine did not statistically significantly reduce the severity of tinnitus, compared to placebo.20 While carbamazepine may be of limited use in the treatment of subjective tinnitus, recent literature confirms that it is not only useful, but also diagnostic, in typewriter tinnitus (ie, having a staccato quality, like the sound of typewriter keys being depressed). Typewriter tinnitus is a secondary cause of tinnitus related to disruption of the stapes in the middle ear.21
Gabapentin works by promoting gamma-aminobutyric acid (GABA) production in the brain. GABA is an inhibitory neurotransmitter, thus slowing down signals between neurons. Following on preliminary research that detected low levels of GABA in the inferior colliculus of rodents with salicylate-induced tinnitus, Aazh and colleagues conducted a double-blind study of gabapentin—and concluded that it yielded no improvement in symptoms, compared to placebo.22
Valproic acid has not been formally investigated but is commonly incorporated in the treatment of tinnitus.23 Lamotrigine has provided similarly disappointing results in the treatment of tinnitus.24
Continue to: Antidepressants and anxiolytics
Antidepressants and anxiolytics. Based on the results of their early clinical trials, Sullivan and colleagues concluded that tricyclic antidepressants produced significant improvement in tinnitus symptoms, due to the analgesic effects of these drugs. The researchers studied nortriptyline specifically; in severely depressed patients, the drug reduced the loudness of tinnitus and depressive symptoms. In non-depressed subjects, however, nortriptyline was not as efficacious.25
Selective serotonin reuptake inhibitors have not had the same success as nortriptyline. In a study of paroxetine conducted by Oishi and colleagues, there was little evidence that the drug reduced the loudness of tinnitus, although overall, it did reduce tinnitus bother and anxiety.26
Included in the category of anxiolytics, benzodiazepines have long been used to treat severe tinnitus-induced anxiety, with some success. However, as Elgoyhen and Langguth point out, studies of benzodiazepines for tinnitus have been limited in size.23
The AAO–HNS does not support routine use of antidepressants and anxiolytics for tinnitus bother.7
NMDA receptor antagonists. In a recent clinical trial, neramexane was studied for its efficacy in tinnitus. Neramexane acts at the cholinergic nicotinic and NMDA receptors in the efferent auditory system. Its complex reaction is thought to prevent transmission of unwanted sound not only to structures within the auditory system but beyond, to the medial geniculate body and lateral nucleus of the amygdala. The trial has proved some benefit concerning overall perception of tinnitus loudness; a phase 2 trial is being conducted.27
Continue to: Intra-tympanic anesthetics
Intra-tympanic anesthetics. Anesthetics, such as lidocaine, have had limited success and results have not been found to be sustained.
Alternative medical managements
Traditional Chinese herbal medications have been used for centuries and are increasingly popular in Western culture. Hilton and colleagues studied Ginkgo biloba, or maidenhair tree, a traditional Chinese herbal supplement available as an extract and as dried leaves. The main action of the extract is vasoregulatory; antiplatelet effects are also seen. Adverse effects include gastrointestinal upset and headache. In a systematic review, Hilton and colleagues concluded that Ginkgo did not reduce overall tinnitus loudness or severity; the review was limited, however, by the fact that only two studies met criteria for inclusion.28
Vitamins, lipoflavinoids, zinc, manganese, and melatonin are all supplements marketed to improve tinnitus symptoms. However, a cross-sectional study confirmed prior research that did not show any benefit from the use of these supplements.29
Cannabinoids are being studied for their proposed antiepileptic effects. There is a popular misconception of Cannabis as a singular chemical when in fact, it is a plant that contains hundreds of chemicals that each act differently on the brain. In a review, Smith and Zheng30 explain that two cannabinoid receptors, CB1 and CB2, are represented, and exert their effects, in different areas of the brain. CB1 receptors block calcium influx in presynaptic terminals, resulting in an inhibitory effect on neurotransmitter release.
CB1 receptors have been found in the dorsal cochlear nuclei, prompting research interest in how cannabinoids affect neurotransmission of unwanted sounds of tinnitus. To date, however, there are conflicting data concerning the benefit of cannabinoids and tinnitus. In fact, Smith and Zheng state that some data suggest that cannabinoids might make tinnitus worse.30
Continue to: Nonpharmacotherapeutic management
Nonpharmacotherapeutic management
Cognitive behavioral therapy. Conceptualized by Aaron T. Beck in the 1960s, cognitive behavioral therapy (CBT) is the leading recommendation made by the AAO–HNS in its tinnitus treatment guidelines.6 Beck’s work centered on the idea that behaviors are modifiable thoughts, through analysis of past experiences and assumptions based on those experiences. By understanding the core belief that a patient attaches to a feeling, Beck hypothesized that behaviors or responses to those feelings could be changed; this is accomplished through discussion to dispel unwarranted fears and by teaching coping mechanisms, such as relaxation. The idea behind CBT in the management of tinnitus is clear: The sound cannot be eliminated, but the patient’s response to the sound can be modified. Ultimately, through this modified response or habituation, the patient can relax and live with the sound.31
Since anxiety, depression, and insomnia are common comorbidities of tinnitus, a psychologic approach remains in the forefront of treatment recommendations. Hoare and colleagues reported that in “a meta-analysis of 10 randomized trials evaluating different forms of CBT (by the therapist and over the Internet), CBT improved tinnitus symptoms compared to non-CBT controls.”7
Tinnitus retraining therapy (TRT) is another form of habituation therapy, introduced by Jastreboff in the 1990s. His work furthered the idea that tinnitus could be reframed, as it is in CBT. Simply, he proposed that systems outside the auditory complex—namely the autonomic nervous system and the limbic system—respond to the signal produced by damaged hair cells in the cochlear nuclei. TRT retrains connections to block or ignore these signals.13 Unlike CBT, the aim of TRT is to eliminate the perception of sound.
By educating patients about the physiologic mechanisms of tinnitus, TRT reduces patient anxiety related to the sound. The process of habituation follows counseling. To accomplish this, the patient wears a sound generator, similar in appearance to hearing aids, using broadband noise. The sound does not mask the tinnitus but closes the gap between silence and the perception of tinnitus. The sound generator is worn for six hours daily for approximately 12 months.
Multiple studies have employed Jastreboff’s original technique, including a clinical trial by Bauer and colleagues. The published outcome of this study confirmed that patients experienced a positive and lasting effect with TRT.32 In addition, a small study of TRT conducted by Barozzi and colleagues, using different colors of sound (ie, how the frequency of a given sound corresponds to the light-wave frequency of a particular color), found statistically significant improvement. Allowing patients to pick a sound that they found more pleasant increased the effectiveness of the treatment.33 (Patients can learn more about TRT by visiting www.tinnitus-pjj.com, hosted by tinnitus researcher Pawel J. Jastreboff.)
Continue to: Alternative nonmedical therapies...
Alternative nonmedical therapies have become popular; they include meditation, yoga, physical therapy, mindfulness, and tinnitus-masking treatment with sound.
Results of a study of yoga and meditation showed that patients felt more relaxed, but that these interventions had no effect on the severity of tinnitus. The principle behind yoga practice, according to Köksoy and colleagues, is that the discipline is thought to affect the limbic system by deactivating the sympathetic response to stimulation from surrounding sounds. In addition, Köksoy states, other researchers have provided evidence that yoga increases circulating levels of antioxidants, which in turn reduce oxidative stress.34
Particularly among members of the millennial generation, mindfulness has become a buzzword. The practice refers to a “method for facing, exploring, and alleviating suffering by relating to present experiences.”35 Roland and colleagues conducted a clinical trial of mindfulness practiced by a cohort of patients with bothersome tinnitus; results were based on scores gleaned from standard rating scales (eg, Global Bothersome Scale, Cognitive and Affective Mindfulness Scale-Revised, Cognitive Failures Questionnaire, Tinnitus Handicap Inventory, and Tinnitus Functional Index). Evaluated before and four weeks after cessation of therapy, subjects reported that tinnitus bother was reduced, but none showed statistically significant improvement in depression, anxiety, or cognitive ability.35
Used for more than 40 years, sound-based therapy has been discussed in conjunction with TRT.36 It is recognized as an approved but optional treatment by the AAO–HNS. In response to a 2010 study by Hobson that used sound-based therapy alone for tinnitus, Tunkel and colleagues cautioned that the modality showed little benefit. The major downside to acoustic therapy, according to the AAO–HNS clinical guidelines, is cost and patients’ excessive expectation of effectiveness.6
According to the AAO–HNS, repetitive-transcranial magnetic stimulation is not supported as a valid treatment for tinnitus because it can lead to seizures in patients who are taking medication that lowers the seizure threshold or who have a secondary cause of tinnitus, such as a tumor—therefore creating risk that outweighs any benefit.6
Continue to: CONCLUSION
CONCLUSION
For a large percentage of the population, chronic subjective tinnitus is a significant variable in the evaluation of quality of life. The condition is not completely understood and often displays features unique to the individual. Much of the initial response to research linking tinnitus with shared pathways typical for chronic pain, anxiety, and depression has resulted in pharmacotherapeutic management that is not always warranted—or successful.
Clinical research into the pathophysiology of tinnitus is providing a better understanding of the neurophysiologic mechanisms that underpin the science of chronic tinnitus. With this information, researchers can one day design medical management that targets specific receptors, resulting in greater management success.
The psychologic impact of tinnitus cannot be underestimated. When almost one-third of patients complain of debilitating symptoms that can also result in neurocognitive decline, tinnitus becomes a condition that cannot be ignored. Guidelines set forth by the AAO–HNS state that CBT and TRT offer some reprieve from symptoms and teach patients habituation without further damage to hearing. The use of broad-based sound generators has been well established as a useful management tool, although it is not curative.
The limitations of some studies that reviewed alternative medicines include small sample size and difficulty comparing research analysis because of disparities in tinnitus rating scales. Also, age bias, comorbid conditions, and study drop-out rates affected overall statistical significance of some studies. Additional, high-quality research is warranted in this area.
Continue to: Prevention of tinnitus...
Prevention of tinnitus through education on hearing loss and its causes should be regarded as implicit; occupational noise and recreational use of music devices put people at heightened risk for hearing loss and tinnitus. Information and open discussion that include the discovery of tinnitus symptoms during routine physical examination are recommended.
Last, providers who adhere to recognized guidelines will aid patients in coping with the challenges that tinnitus presents. As research continues to unravel the complex interaction between neurons, medical science is hopeful that curative treatments will become available.
CE/CME No: CR-1810
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Distinguish primary tinnitus from secondary tinnitus.
• Understand and implement a full clinical evaluation of tinnitus, including imaging studies when appropriate.
• Discuss expectations regarding treatment options and realistic outcomes of currently recommended therapy.
• Direct patients to specialist care for cognitive behavioral therapy or tinnitus retraining therapy.
• Know when pharmacotherapeutic intervention is indicated.
FACULTY
Wendy Gillian Ross practices urgent care medicine in Lake Grove, New York, and primary care in Patchogue, New York. Randy Danielsen is Professor and Dean, Arizona School of Health Sciences, and Director, Center for the Future of the Health Professions, both at A.T. Still University, in Mesa, Arizona. He is Physician Assistant Editor-in-Chief of Clinician Reviews.
The authors have no financial relationships to disclose.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through September 30, 2019.
Article begins on next page >>
Tinnitus can be a debilitating condition that affects quality of life and is often not treated according to guidelines. Cognitive behavioral therapy and tinnitus retraining therapy have been successful in reducing tinnitus bother; pharmacotherapy is not widely accepted as successful, and can, in fact, be deleterious. This article describes pathophysiologic disturbances of hearing and how they relate to chronic subjective tinnitus, discusses the clinical evaluation of tinnitus as a presenting symptom, and reviews current treatments.
Primary chronic subjective tinnitus, often thought of more as a symptom than a diagnosis, affects millions of people worldwide. This troublesome condition has been chronicled as far back as the first century
It is estimated that only 20% of people who experience tinnitus actively seek treatment.2 In the United States, 2 to 3 million of the 12 million patients who do request treatment report lasting symptoms that they describe as debilitating.3 For patients who seek help, the treatment recommended by physicians is typically pharmacotherapeutic—which does not follow guidelines.4
The aim of this article is to reinforce a greater understanding of the mechanisms of tinnitus and integrate that knowledge into treatment guidelines. The article does not discuss surgical treatment of tinnitus.
DEFINITION AND CLASSIFICATION
A universal standard definition of chronic tinnitus does not exist; Trevis et al define it as a phantom sound that persists for more than three months.5 The quality and loudness of tinnitus is variable but is often described as a buzz, hiss, or ringing. Prevalence increases with age, smoking, male gender, and ethnicity, with the non-Latino white population statistically at greater risk.3 Comorbid conditions (eg, diabetes and other autoimmune diseases) are risk factors for tinnitus. A history of exposure to loud sound—occupational, environmental, or recreational—also can predispose a person to tinnitus.3
The American Academy of Otolaryngology–Head and Neck Surgery (AAO–HNS) classifies tinnitus as primary (subjective) or secondary (objective). Primary tinnitus—representing the majority of cases—has no identifiable cause; there may be accompanying sensorineural hearing loss or hyperacusis. Secondary tinnitus can also be associated with sensorineural hearing loss but has an identifiable underlying cause.6 The differential diagnosis of tinnitus is listed in the Table.7

Tinnitus is further defined by its persistence. Persistent tinnitus is defined as tinnitus lasting more than six months, slightly longer than the duration offered by Trevis et al, who also define tinnitus as bothersome or non-bothersome, depending on its impact on quality of life.5,6 Causes of reduced quality of life include depression, anxiety, insomnia, and neurocognitive decline—all of which have been associated with chronic subjective tinnitus.8
Continue to: Researchers have discovered that...
Researchers have discovered that tinnitus is not simply a cochlear phenomenon. The pathology extends well beyond the auditory complex, having a deleterious effect on both the somatosensory and central nervous systems, providing some explanation for the prevalence of anxiety and depression associated with the disorder (see "Pathophysiology of tinnitus").9-17

Because of the insidious nature of tinnitus and lack of standard measures of severity, true prevalence is difficult to calculate.18
CLINICAL EVALUATION
Tinnitus can be a presenting complaint or elicited during history-taking. Symptomatic patients should receive full evaluation, including a complete physical exam, medication history, and laboratory workup.
Adverse effect of drugs
Medications that commonly cause tinnitus symptoms are NSAIDs, chemotherapeutic agents, and antibiotics (eg, macrolides and fluoroquinolones). Amiodarone, ACE inhibitors, proton-pump inhibitors, and calcium-channel blockers have also been implicated. Paradoxically, anxiolytics and tricyclic antidepressants, which are sometimes used to treat tinnitus, have been linked to causing the condition.7
Laboratory tests and imaging
Testing should include investigation for infectious disease, autoimmune disorders, and vitamin deficiency.7 According to the American College of Radiology, imaging is unnecessary in the workup of primary tinnitus. Any suspicion of a vascular cause noted on the physical exam (eg, an associated bruit or venous hum), however, should be explored with imaging. Furthermore, any case of tinnitus that lateralizes also requires additional investigation. Modalities of choice are MRI, CT, and CT angiography.19
Continue to: Referral for audiology evaluation
Referral for audiology evaluation
When no underlying pathology can be identified for tinnitus, the patient should be sent for a full audiology evaluation to screen for associated hearing loss. Discussion of audiology screening tests is beyond the scope of this article; however, testing includes otoscopy, audiography, tympanography, otoacoustic emission testing, auditory brainstem-response testing, and vestibular evoked myogenic potential testing.7
Probing nonphysical impacts
Quality of life and overall emotional wellness, including cognitive function, should be investigated in patients with tinnitus. Two questionnaires commonly used in the assessment of tinnitus bother are the Tinnitus Handicap Inventory and the Tinnitus Reaction Questionnaire.7 In a large, systematic review, Trevis et al report that “64% of studies investigating depression found an increase in depressive symptoms in people with chronic tinnitus compared to hearing control groups, and 62% of studies investigating anxiety reported significantly increased anxiety symptoms.”5
MANAGEMENT
Tinnitus management should be viewed two ways: treatment of perceived loudness and treatment of comorbid symptoms relating to tinnitus bother.6 In the same meta-analysis, Trevis and colleagues found that patients with tinnitus had higher rates of anxiety, depression, and overall decline in cognitive function, including processing speed, concentration, and sleep disorders.5 It is useful to keep this observation in mind when reviewing treatment options for tinnitus.
Five classic pharmacotherapeutic approaches to tinnitus management are
- Anticonvulsants
- Antidepressants
- Anesthetics
- Anxiolytics
- Lidocaine.
Newer medications that show some promise are N-methyl-D-aspartate (NMDA) receptor antagonists, notably neramexane. Alternative pharmaceuticals include vitamin-based treatments, cannabinoids, and herbal compounds.
Continue to: The AAOS-HNS supports...
The AAO–HNS supports nonpharmacotherapeutic treatment of tinnitus; its guidelines include a recommendation for cognitive behavioral therapy (CBT) as primary therapy.6 In addition, tinnitus-retraining therapy, tinnitus-masking therapy/sound therapy, meditation/mindfulness, and yoga all have been studied for their ability to alleviate tinnitus bother.
Pharmacotherapeutic management
Anticonvulsants have failed to provide strong evidence of usefulness in the treatment of tinnitus and are not supported by the AAO–HNS as such.6 This conclusion notwithstanding, the anticonvulsants carbamazepine and gabapentin have historically been two of the more common medications used to treat tinnitus.
Carbamazepine is a glutamate receptor antagonist that suppresses seizure activity. Based on prior research suggesting that spontaneous firing within the auditory complex is similar to seizure activity, Iranian researchers explored the hypothesis that carbamazepine might lessen tinnitus severity. Their study revealed, however, that carbamazepine did not statistically significantly reduce the severity of tinnitus, compared to placebo.20 While carbamazepine may be of limited use in the treatment of subjective tinnitus, recent literature confirms that it is not only useful, but also diagnostic, in typewriter tinnitus (ie, having a staccato quality, like the sound of typewriter keys being depressed). Typewriter tinnitus is a secondary cause of tinnitus related to disruption of the stapes in the middle ear.21
Gabapentin works by promoting gamma-aminobutyric acid (GABA) production in the brain. GABA is an inhibitory neurotransmitter, thus slowing down signals between neurons. Following on preliminary research that detected low levels of GABA in the inferior colliculus of rodents with salicylate-induced tinnitus, Aazh and colleagues conducted a double-blind study of gabapentin—and concluded that it yielded no improvement in symptoms, compared to placebo.22
Valproic acid has not been formally investigated but is commonly incorporated in the treatment of tinnitus.23 Lamotrigine has provided similarly disappointing results in the treatment of tinnitus.24
Continue to: Antidepressants and anxiolytics
Antidepressants and anxiolytics. Based on the results of their early clinical trials, Sullivan and colleagues concluded that tricyclic antidepressants produced significant improvement in tinnitus symptoms, due to the analgesic effects of these drugs. The researchers studied nortriptyline specifically; in severely depressed patients, the drug reduced the loudness of tinnitus and depressive symptoms. In non-depressed subjects, however, nortriptyline was not as efficacious.25
Selective serotonin reuptake inhibitors have not had the same success as nortriptyline. In a study of paroxetine conducted by Oishi and colleagues, there was little evidence that the drug reduced the loudness of tinnitus, although overall, it did reduce tinnitus bother and anxiety.26
Included in the category of anxiolytics, benzodiazepines have long been used to treat severe tinnitus-induced anxiety, with some success. However, as Elgoyhen and Langguth point out, studies of benzodiazepines for tinnitus have been limited in size.23
The AAO–HNS does not support routine use of antidepressants and anxiolytics for tinnitus bother.7
NMDA receptor antagonists. In a recent clinical trial, neramexane was studied for its efficacy in tinnitus. Neramexane acts at the cholinergic nicotinic and NMDA receptors in the efferent auditory system. Its complex reaction is thought to prevent transmission of unwanted sound not only to structures within the auditory system but beyond, to the medial geniculate body and lateral nucleus of the amygdala. The trial has proved some benefit concerning overall perception of tinnitus loudness; a phase 2 trial is being conducted.27
Continue to: Intra-tympanic anesthetics
Intra-tympanic anesthetics. Anesthetics, such as lidocaine, have had limited success and results have not been found to be sustained.
Alternative medical managements
Traditional Chinese herbal medications have been used for centuries and are increasingly popular in Western culture. Hilton and colleagues studied Ginkgo biloba, or maidenhair tree, a traditional Chinese herbal supplement available as an extract and as dried leaves. The main action of the extract is vasoregulatory; antiplatelet effects are also seen. Adverse effects include gastrointestinal upset and headache. In a systematic review, Hilton and colleagues concluded that Ginkgo did not reduce overall tinnitus loudness or severity; the review was limited, however, by the fact that only two studies met criteria for inclusion.28
Vitamins, lipoflavinoids, zinc, manganese, and melatonin are all supplements marketed to improve tinnitus symptoms. However, a cross-sectional study confirmed prior research that did not show any benefit from the use of these supplements.29
Cannabinoids are being studied for their proposed antiepileptic effects. There is a popular misconception of Cannabis as a singular chemical when in fact, it is a plant that contains hundreds of chemicals that each act differently on the brain. In a review, Smith and Zheng30 explain that two cannabinoid receptors, CB1 and CB2, are represented, and exert their effects, in different areas of the brain. CB1 receptors block calcium influx in presynaptic terminals, resulting in an inhibitory effect on neurotransmitter release.
CB1 receptors have been found in the dorsal cochlear nuclei, prompting research interest in how cannabinoids affect neurotransmission of unwanted sounds of tinnitus. To date, however, there are conflicting data concerning the benefit of cannabinoids and tinnitus. In fact, Smith and Zheng state that some data suggest that cannabinoids might make tinnitus worse.30
Continue to: Nonpharmacotherapeutic management
Nonpharmacotherapeutic management
Cognitive behavioral therapy. Conceptualized by Aaron T. Beck in the 1960s, cognitive behavioral therapy (CBT) is the leading recommendation made by the AAO–HNS in its tinnitus treatment guidelines.6 Beck’s work centered on the idea that behaviors are modifiable thoughts, through analysis of past experiences and assumptions based on those experiences. By understanding the core belief that a patient attaches to a feeling, Beck hypothesized that behaviors or responses to those feelings could be changed; this is accomplished through discussion to dispel unwarranted fears and by teaching coping mechanisms, such as relaxation. The idea behind CBT in the management of tinnitus is clear: The sound cannot be eliminated, but the patient’s response to the sound can be modified. Ultimately, through this modified response or habituation, the patient can relax and live with the sound.31
Since anxiety, depression, and insomnia are common comorbidities of tinnitus, a psychologic approach remains in the forefront of treatment recommendations. Hoare and colleagues reported that in “a meta-analysis of 10 randomized trials evaluating different forms of CBT (by the therapist and over the Internet), CBT improved tinnitus symptoms compared to non-CBT controls.”7
Tinnitus retraining therapy (TRT) is another form of habituation therapy, introduced by Jastreboff in the 1990s. His work furthered the idea that tinnitus could be reframed, as it is in CBT. Simply, he proposed that systems outside the auditory complex—namely the autonomic nervous system and the limbic system—respond to the signal produced by damaged hair cells in the cochlear nuclei. TRT retrains connections to block or ignore these signals.13 Unlike CBT, the aim of TRT is to eliminate the perception of sound.
By educating patients about the physiologic mechanisms of tinnitus, TRT reduces patient anxiety related to the sound. The process of habituation follows counseling. To accomplish this, the patient wears a sound generator, similar in appearance to hearing aids, using broadband noise. The sound does not mask the tinnitus but closes the gap between silence and the perception of tinnitus. The sound generator is worn for six hours daily for approximately 12 months.
Multiple studies have employed Jastreboff’s original technique, including a clinical trial by Bauer and colleagues. The published outcome of this study confirmed that patients experienced a positive and lasting effect with TRT.32 In addition, a small study of TRT conducted by Barozzi and colleagues, using different colors of sound (ie, how the frequency of a given sound corresponds to the light-wave frequency of a particular color), found statistically significant improvement. Allowing patients to pick a sound that they found more pleasant increased the effectiveness of the treatment.33 (Patients can learn more about TRT by visiting www.tinnitus-pjj.com, hosted by tinnitus researcher Pawel J. Jastreboff.)
Continue to: Alternative nonmedical therapies...
Alternative nonmedical therapies have become popular; they include meditation, yoga, physical therapy, mindfulness, and tinnitus-masking treatment with sound.
Results of a study of yoga and meditation showed that patients felt more relaxed, but that these interventions had no effect on the severity of tinnitus. The principle behind yoga practice, according to Köksoy and colleagues, is that the discipline is thought to affect the limbic system by deactivating the sympathetic response to stimulation from surrounding sounds. In addition, Köksoy states, other researchers have provided evidence that yoga increases circulating levels of antioxidants, which in turn reduce oxidative stress.34
Particularly among members of the millennial generation, mindfulness has become a buzzword. The practice refers to a “method for facing, exploring, and alleviating suffering by relating to present experiences.”35 Roland and colleagues conducted a clinical trial of mindfulness practiced by a cohort of patients with bothersome tinnitus; results were based on scores gleaned from standard rating scales (eg, Global Bothersome Scale, Cognitive and Affective Mindfulness Scale-Revised, Cognitive Failures Questionnaire, Tinnitus Handicap Inventory, and Tinnitus Functional Index). Evaluated before and four weeks after cessation of therapy, subjects reported that tinnitus bother was reduced, but none showed statistically significant improvement in depression, anxiety, or cognitive ability.35
Used for more than 40 years, sound-based therapy has been discussed in conjunction with TRT.36 It is recognized as an approved but optional treatment by the AAO–HNS. In response to a 2010 study by Hobson that used sound-based therapy alone for tinnitus, Tunkel and colleagues cautioned that the modality showed little benefit. The major downside to acoustic therapy, according to the AAO–HNS clinical guidelines, is cost and patients’ excessive expectation of effectiveness.6
According to the AAO–HNS, repetitive-transcranial magnetic stimulation is not supported as a valid treatment for tinnitus because it can lead to seizures in patients who are taking medication that lowers the seizure threshold or who have a secondary cause of tinnitus, such as a tumor—therefore creating risk that outweighs any benefit.6
Continue to: CONCLUSION
CONCLUSION
For a large percentage of the population, chronic subjective tinnitus is a significant variable in the evaluation of quality of life. The condition is not completely understood and often displays features unique to the individual. Much of the initial response to research linking tinnitus with shared pathways typical for chronic pain, anxiety, and depression has resulted in pharmacotherapeutic management that is not always warranted—or successful.
Clinical research into the pathophysiology of tinnitus is providing a better understanding of the neurophysiologic mechanisms that underpin the science of chronic tinnitus. With this information, researchers can one day design medical management that targets specific receptors, resulting in greater management success.
The psychologic impact of tinnitus cannot be underestimated. When almost one-third of patients complain of debilitating symptoms that can also result in neurocognitive decline, tinnitus becomes a condition that cannot be ignored. Guidelines set forth by the AAO–HNS state that CBT and TRT offer some reprieve from symptoms and teach patients habituation without further damage to hearing. The use of broad-based sound generators has been well established as a useful management tool, although it is not curative.
The limitations of some studies that reviewed alternative medicines include small sample size and difficulty comparing research analysis because of disparities in tinnitus rating scales. Also, age bias, comorbid conditions, and study drop-out rates affected overall statistical significance of some studies. Additional, high-quality research is warranted in this area.
Continue to: Prevention of tinnitus...
Prevention of tinnitus through education on hearing loss and its causes should be regarded as implicit; occupational noise and recreational use of music devices put people at heightened risk for hearing loss and tinnitus. Information and open discussion that include the discovery of tinnitus symptoms during routine physical examination are recommended.
Last, providers who adhere to recognized guidelines will aid patients in coping with the challenges that tinnitus presents. As research continues to unravel the complex interaction between neurons, medical science is hopeful that curative treatments will become available.
1. Maltby MT. Ancient voices on tinnitus: the pathology and treatment of tinnitus in Celsus and the Hippocratic Corpus compared and contrasted. Int Tinnitus J. 2012;17(2):140-145.
2. Wolever RQ, Price R, Hazelton GA, et al. Complementary therapies for significant dysfunction from tinnitus: treatment review and potential for integrative medicine. Evid Based Complement Alternat Med. 2015;15:931418.
3. Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med. 2010;123(8):711-718.
4. Bhatt JM, Lin HW, Bhattacharyya N. Prevalence, severity, exposures, and treatment patterns of tinnitus in the United States. JAMA Otolaryngol Head Neck Surg. 2016;142(10):959-965.
5. Trevis KJ, McLachlan NM, Wilson SJ. A systematic review and meta-analysis of psychological functioning in chronic tinnitus. Clin Psychol Rev. 2018;60:62-86.
6. Tunkel DE, Bauer CA, Sun GH, et al. Clinical practice guideline: tinnitus. Otolaryngol Head Neck Surg. 2014;151(suppl 2):S1-S40.
7. Dinces EA. Treatment of tinnitus. UpToDate. April 12, 2018. www.uptodate.com/contents/treatment-of-tinnitus. Accessed September 17, 2018.
8. Gudwani S, Munjal SK, Panda NK, Kohli A. Association of chronic subjective tinnitus with neuro-cognitive performance. Int Tinnitus J. 2017;21:90-97.
9. Jastreboff PJ. 25 years of tinnitus retraining therapy. HNO. 2015;63:307-311.
10. Pujol R. Journey into the world of hearing. 2016. www.cochlea.eu/en. Accessed September 17, 2018.
11. Adjamian P, Hall DA, Palmer AR, et al. Neuroanatomical abnormalities in chronic tinnitus in the human brain.Neurosci Biobehav Rev. 2014;45:119-133.
12. Shore SE, Roberts LE, Langguth B. Maladaptive plasticity in tinnitus—triggers, mechanisms and treatment. Nat Rev Neurol. 2016;12(3):150-160.
13. Jastreboff PJ, Gray WC, Gold SL. Neurophysiological approach to tinnitus patients. Am J Otol. 1996;17(2):236-240.
14. Kaltenbach JA. Tinnitus: models and mechanisms. Hear Res. 2011;276:52-60.
15. Rauschecker JP, Leaver AM, Mühlau M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron. 2010;66(6):819-826.
16. Møller AR. Sensorineural tinnitus: its pathology and probable therapies. Int J Otolaryngol. 2016;2016:2830157.
17. Chen YC, Xia W, Chen H, et al. Tinnitus distress is linked to enhanced resting‐state functional connectivity from the limbic system to the auditory cortex. Hum Brain Mapp. 2017;38(5):2384-2397.
18. McCormack A, Edmonson-Jones M, Somerset S, Hall D. A systematic review of the reporting of tinnitus prevalence and severity. Hear Res. 2016;337:70-79.
19. Kessler MM, Moussa M, Bykowski J, et al; Expert Panel on Neurologic Imaging. ACR Appropriateness Criteria® Tinnitus. J Am Coll Radiol. 2017;14:S584-S591.
20. Gerami H, Saberi A, Nemati, S, et al. Effects of oxcarbazepine versus carbamazepine on tinnitus: a randomized double-blind placebo-controlled clinical trial. Iran J Neurol. 2012;11(3):106-110.
21. Sunwoo W, Jeon YJ, Bae YJ, et al. Typewriter tinnitus revisited: the typical symptoms and the initial response to carbamazepine are the most reliable diagnostic clues. Sci Rep. 2017;7:10615.
22. Aazh H, El Refaie A, Humphriss R. Gabapentin for tinnitus: a systematic review. Am J Audiol. 2011;20:151-158.
23. Elgoyhen AB, Langguth B. Pharmacological approaches to the treatment of tinnitus. Drug Discov Today. 2010;15:300-305.
24. Langguth B, Kreuzer PM, Kleinjung T, De Ridder D. Tinnitus: causes and clinical management. Lancet Neurol. 2013;12(9):920-930.
25. Sullivan M, Katon W, Russo J, et al. A randomized trial of nortriptyline for severe chronic tinnitus. Effects on depression, disability, and tinnitus symptoms. Arch Intern Med. 1993;153(19):2251-2259.
26. Oishi N, Kanzaki S, Shinden S, et al. Effects of selective serotonin reuptake inhibitor on treating tinnitus in patients stratified for presence of depression or anxiety. Audiol Neurootol. 2010;15(3):187-193.
27. Suckfüll M, Althaus M, Ellers-Lenz B, et al. A randomized, double-blind, placebo-controlled clinical trial to evaluate the efficacy and safety of neramexane in patients with moderate to severe subjective tinnitus. BMC Ear Nose Throat Disord. 2011;11:1.
28. Hilton MP, Zimmermann EF, Hunt WT. Ginkgo biloba for tinnitus. Cochrane Database Syst Rev. 2013;CD003852. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD003852.pub3/full. Accessed September 17, 2018.
29. Coelho C, Tyler R, Ji H, et al. Survey on the effectiveness of dietary supplements to treat tinnitus. Am J Audiol. 2016;25:184-205.
30. Smith PF, Zheng Y. Cannabinoids, cannabinoid receptors and tinnitus. Hear Res. 2015;332:210-216.
31. Martinez-Devesa P, Perera R, Theodoulou M, Waddell A. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev. 2010:CD005233. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD005233.pub3/full. Accessed September 17, 2018.
32. Bauer CA, Berry JL, Brozoski TJ. The effect of tinnitus retraining therapy on chronic tinnitus: a controlled trial. Laryngoscope Investig Otolaryngol. 2017;2(4):166-177.
33. Barozzi S, Ambrosetti U, Callaway SL, et al. Effects of tinnitus retraining therapy with different colours of sound. Int Tinnitus J. 2017;21:139-143.
34. Köksoy S, Eti CM, Karatas¸ M, Vayisoglu Y. The effects of yoga in patients suffering from subjective tinnitus. Int Arch Otorhinolaryngol. 2018;22(1):9-13.
35. Roland LT, Lenze EJ, Hardin FM, et al. Effects of mindfulness based stress reduction therapy on subjective bother and neural connectivity in chronic tinnitus. Otolaryngol Head Neck Surg. 2015;152(5):919-926.
36. Ibarra D, Tavira-Sanchez F, Recuero-Lopez M, Anthony BW. In-ear medical devices for acoustic therapies in tinnitus treatments, state of the art. Auris Nasus Larynx. 2018;45:6-12.
1. Maltby MT. Ancient voices on tinnitus: the pathology and treatment of tinnitus in Celsus and the Hippocratic Corpus compared and contrasted. Int Tinnitus J. 2012;17(2):140-145.
2. Wolever RQ, Price R, Hazelton GA, et al. Complementary therapies for significant dysfunction from tinnitus: treatment review and potential for integrative medicine. Evid Based Complement Alternat Med. 2015;15:931418.
3. Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med. 2010;123(8):711-718.
4. Bhatt JM, Lin HW, Bhattacharyya N. Prevalence, severity, exposures, and treatment patterns of tinnitus in the United States. JAMA Otolaryngol Head Neck Surg. 2016;142(10):959-965.
5. Trevis KJ, McLachlan NM, Wilson SJ. A systematic review and meta-analysis of psychological functioning in chronic tinnitus. Clin Psychol Rev. 2018;60:62-86.
6. Tunkel DE, Bauer CA, Sun GH, et al. Clinical practice guideline: tinnitus. Otolaryngol Head Neck Surg. 2014;151(suppl 2):S1-S40.
7. Dinces EA. Treatment of tinnitus. UpToDate. April 12, 2018. www.uptodate.com/contents/treatment-of-tinnitus. Accessed September 17, 2018.
8. Gudwani S, Munjal SK, Panda NK, Kohli A. Association of chronic subjective tinnitus with neuro-cognitive performance. Int Tinnitus J. 2017;21:90-97.
9. Jastreboff PJ. 25 years of tinnitus retraining therapy. HNO. 2015;63:307-311.
10. Pujol R. Journey into the world of hearing. 2016. www.cochlea.eu/en. Accessed September 17, 2018.
11. Adjamian P, Hall DA, Palmer AR, et al. Neuroanatomical abnormalities in chronic tinnitus in the human brain.Neurosci Biobehav Rev. 2014;45:119-133.
12. Shore SE, Roberts LE, Langguth B. Maladaptive plasticity in tinnitus—triggers, mechanisms and treatment. Nat Rev Neurol. 2016;12(3):150-160.
13. Jastreboff PJ, Gray WC, Gold SL. Neurophysiological approach to tinnitus patients. Am J Otol. 1996;17(2):236-240.
14. Kaltenbach JA. Tinnitus: models and mechanisms. Hear Res. 2011;276:52-60.
15. Rauschecker JP, Leaver AM, Mühlau M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron. 2010;66(6):819-826.
16. Møller AR. Sensorineural tinnitus: its pathology and probable therapies. Int J Otolaryngol. 2016;2016:2830157.
17. Chen YC, Xia W, Chen H, et al. Tinnitus distress is linked to enhanced resting‐state functional connectivity from the limbic system to the auditory cortex. Hum Brain Mapp. 2017;38(5):2384-2397.
18. McCormack A, Edmonson-Jones M, Somerset S, Hall D. A systematic review of the reporting of tinnitus prevalence and severity. Hear Res. 2016;337:70-79.
19. Kessler MM, Moussa M, Bykowski J, et al; Expert Panel on Neurologic Imaging. ACR Appropriateness Criteria® Tinnitus. J Am Coll Radiol. 2017;14:S584-S591.
20. Gerami H, Saberi A, Nemati, S, et al. Effects of oxcarbazepine versus carbamazepine on tinnitus: a randomized double-blind placebo-controlled clinical trial. Iran J Neurol. 2012;11(3):106-110.
21. Sunwoo W, Jeon YJ, Bae YJ, et al. Typewriter tinnitus revisited: the typical symptoms and the initial response to carbamazepine are the most reliable diagnostic clues. Sci Rep. 2017;7:10615.
22. Aazh H, El Refaie A, Humphriss R. Gabapentin for tinnitus: a systematic review. Am J Audiol. 2011;20:151-158.
23. Elgoyhen AB, Langguth B. Pharmacological approaches to the treatment of tinnitus. Drug Discov Today. 2010;15:300-305.
24. Langguth B, Kreuzer PM, Kleinjung T, De Ridder D. Tinnitus: causes and clinical management. Lancet Neurol. 2013;12(9):920-930.
25. Sullivan M, Katon W, Russo J, et al. A randomized trial of nortriptyline for severe chronic tinnitus. Effects on depression, disability, and tinnitus symptoms. Arch Intern Med. 1993;153(19):2251-2259.
26. Oishi N, Kanzaki S, Shinden S, et al. Effects of selective serotonin reuptake inhibitor on treating tinnitus in patients stratified for presence of depression or anxiety. Audiol Neurootol. 2010;15(3):187-193.
27. Suckfüll M, Althaus M, Ellers-Lenz B, et al. A randomized, double-blind, placebo-controlled clinical trial to evaluate the efficacy and safety of neramexane in patients with moderate to severe subjective tinnitus. BMC Ear Nose Throat Disord. 2011;11:1.
28. Hilton MP, Zimmermann EF, Hunt WT. Ginkgo biloba for tinnitus. Cochrane Database Syst Rev. 2013;CD003852. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD003852.pub3/full. Accessed September 17, 2018.
29. Coelho C, Tyler R, Ji H, et al. Survey on the effectiveness of dietary supplements to treat tinnitus. Am J Audiol. 2016;25:184-205.
30. Smith PF, Zheng Y. Cannabinoids, cannabinoid receptors and tinnitus. Hear Res. 2015;332:210-216.
31. Martinez-Devesa P, Perera R, Theodoulou M, Waddell A. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev. 2010:CD005233. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD005233.pub3/full. Accessed September 17, 2018.
32. Bauer CA, Berry JL, Brozoski TJ. The effect of tinnitus retraining therapy on chronic tinnitus: a controlled trial. Laryngoscope Investig Otolaryngol. 2017;2(4):166-177.
33. Barozzi S, Ambrosetti U, Callaway SL, et al. Effects of tinnitus retraining therapy with different colours of sound. Int Tinnitus J. 2017;21:139-143.
34. Köksoy S, Eti CM, Karatas¸ M, Vayisoglu Y. The effects of yoga in patients suffering from subjective tinnitus. Int Arch Otorhinolaryngol. 2018;22(1):9-13.
35. Roland LT, Lenze EJ, Hardin FM, et al. Effects of mindfulness based stress reduction therapy on subjective bother and neural connectivity in chronic tinnitus. Otolaryngol Head Neck Surg. 2015;152(5):919-926.
36. Ibarra D, Tavira-Sanchez F, Recuero-Lopez M, Anthony BW. In-ear medical devices for acoustic therapies in tinnitus treatments, state of the art. Auris Nasus Larynx. 2018;45:6-12.
FDA lifts partial hold on tazemetostat trials
The U.S. Food and Drug Administration has lifted the partial clinical hold on trials of tazemetostat, an EZH2 inhibitor being developed to treat solid tumors and lymphomas, according to a press release from the drug’s developer Epizyme.
The patient had been on study for approximately 15 months and had achieved a confirmed partial response. The patient has since discontinued tazemetostat and responded to treatment for T-LBL.
“This remains the only case of T-LBL we’ve seen in more than 750 patients treated with tazemetostat,” Robert Bazemore, president and chief executive officer of Epizyme, said in a webcast on Sept. 24.
Epizyme assessed the risk of secondary malignancies, including T-LBL, as well as the overall risks and benefits of tazemetostat treatment, conducting a review of the published literature and an examination of efficacy and safety data across all of its tazemetostat trials. A panel of external scientific and medical experts who reviewed the findings concluded that T-LBL risks appear to be confined to pediatric patients who received higher doses of the drug. The phase 1 pediatric study in which the patient developed T-LBL included higher doses of tazemetostat than those used in the phase 2 adult studies.
“The team at Epizyme has worked diligently in collaboration with external experts and the FDA over the past several months,” Mr. Bazemore said.
The company is not making any substantial changes to trial designs or the patient populations involved in tazemetostat trials. However, Epizyme is modifying dosing in the pediatric studies, improving patient monitoring, and making changes to exclusion criteria to reduce the potential risk of T-LBL and other secondary malignancies. Mr. Bazemore said Epizyme hopes to submit a New Drug Application for tazemetostat in the treatment of epithelioid sarcoma.
Tazemetostat is under investigation as monotherapy in phase 2 trials of follicular lymphoma and solid-tumor malignancies. The drug is also being studied as part of combination therapy for non–small cell lung cancer and diffuse large B-cell lymphoma (DLBCL).
In August, Epizyme announced its decision to stop developing tazemetostat for use as monotherapy or in combination with prednisolone for patients with DLBCL. However, tazemetostat is still under investigation as a potential treatment for DLBCL as part of other combination regimens.
Epizyme is now working to resolve partial clinical holds placed on tazemetostat in France and Germany in order to resume trial enrollment in those countries.
The U.S. Food and Drug Administration has lifted the partial clinical hold on trials of tazemetostat, an EZH2 inhibitor being developed to treat solid tumors and lymphomas, according to a press release from the drug’s developer Epizyme.
The patient had been on study for approximately 15 months and had achieved a confirmed partial response. The patient has since discontinued tazemetostat and responded to treatment for T-LBL.
“This remains the only case of T-LBL we’ve seen in more than 750 patients treated with tazemetostat,” Robert Bazemore, president and chief executive officer of Epizyme, said in a webcast on Sept. 24.
Epizyme assessed the risk of secondary malignancies, including T-LBL, as well as the overall risks and benefits of tazemetostat treatment, conducting a review of the published literature and an examination of efficacy and safety data across all of its tazemetostat trials. A panel of external scientific and medical experts who reviewed the findings concluded that T-LBL risks appear to be confined to pediatric patients who received higher doses of the drug. The phase 1 pediatric study in which the patient developed T-LBL included higher doses of tazemetostat than those used in the phase 2 adult studies.
“The team at Epizyme has worked diligently in collaboration with external experts and the FDA over the past several months,” Mr. Bazemore said.
The company is not making any substantial changes to trial designs or the patient populations involved in tazemetostat trials. However, Epizyme is modifying dosing in the pediatric studies, improving patient monitoring, and making changes to exclusion criteria to reduce the potential risk of T-LBL and other secondary malignancies. Mr. Bazemore said Epizyme hopes to submit a New Drug Application for tazemetostat in the treatment of epithelioid sarcoma.
Tazemetostat is under investigation as monotherapy in phase 2 trials of follicular lymphoma and solid-tumor malignancies. The drug is also being studied as part of combination therapy for non–small cell lung cancer and diffuse large B-cell lymphoma (DLBCL).
In August, Epizyme announced its decision to stop developing tazemetostat for use as monotherapy or in combination with prednisolone for patients with DLBCL. However, tazemetostat is still under investigation as a potential treatment for DLBCL as part of other combination regimens.
Epizyme is now working to resolve partial clinical holds placed on tazemetostat in France and Germany in order to resume trial enrollment in those countries.
The U.S. Food and Drug Administration has lifted the partial clinical hold on trials of tazemetostat, an EZH2 inhibitor being developed to treat solid tumors and lymphomas, according to a press release from the drug’s developer Epizyme.
The patient had been on study for approximately 15 months and had achieved a confirmed partial response. The patient has since discontinued tazemetostat and responded to treatment for T-LBL.
“This remains the only case of T-LBL we’ve seen in more than 750 patients treated with tazemetostat,” Robert Bazemore, president and chief executive officer of Epizyme, said in a webcast on Sept. 24.
Epizyme assessed the risk of secondary malignancies, including T-LBL, as well as the overall risks and benefits of tazemetostat treatment, conducting a review of the published literature and an examination of efficacy and safety data across all of its tazemetostat trials. A panel of external scientific and medical experts who reviewed the findings concluded that T-LBL risks appear to be confined to pediatric patients who received higher doses of the drug. The phase 1 pediatric study in which the patient developed T-LBL included higher doses of tazemetostat than those used in the phase 2 adult studies.
“The team at Epizyme has worked diligently in collaboration with external experts and the FDA over the past several months,” Mr. Bazemore said.
The company is not making any substantial changes to trial designs or the patient populations involved in tazemetostat trials. However, Epizyme is modifying dosing in the pediatric studies, improving patient monitoring, and making changes to exclusion criteria to reduce the potential risk of T-LBL and other secondary malignancies. Mr. Bazemore said Epizyme hopes to submit a New Drug Application for tazemetostat in the treatment of epithelioid sarcoma.
Tazemetostat is under investigation as monotherapy in phase 2 trials of follicular lymphoma and solid-tumor malignancies. The drug is also being studied as part of combination therapy for non–small cell lung cancer and diffuse large B-cell lymphoma (DLBCL).
In August, Epizyme announced its decision to stop developing tazemetostat for use as monotherapy or in combination with prednisolone for patients with DLBCL. However, tazemetostat is still under investigation as a potential treatment for DLBCL as part of other combination regimens.
Epizyme is now working to resolve partial clinical holds placed on tazemetostat in France and Germany in order to resume trial enrollment in those countries.
TV and mental health story lines; when doctors don’t listen – to women
Turn on the television, and chances are good that popular dramas (especially hospital-oriented shows) will be showing an episode revolving around a mental health issue.
, in time for a commercial. Real life is messier.
“Unfortunately mental health story lines are much more likely to be fear-mongering and wildly wrong. As a psychiatrist, this both piques my interest and upends my work-life balance,” Dr. Gold writes. “Whether I’m watching everyone’s favorite medical drama or ‘reality TV,’ it’s impossible not to switch into physician mode, angry on behalf of all of my patients and the many viewers who are being misled.”
Do physicians hear women?
“Rebecca continues to be paranoid.”
That was a note written by someone involved in the medical care of a 30-something woman diagnosed with stage IIB cervical cancer, according to an article in New York magazine.
“There’s a whiff of old ‘female hysteria’ to [the note], with more than a hint of dismissal,” writes the patient’s sister, Kate Beaton. “Becky was scared, and perhaps that was the main takeaway that day. But she was also right.”
The article tells the story of a vibrant woman who, according to her sister, asked her doctors lots of questions, wrote everything down, and faced years of being dismissed when she explained her symptoms. Becky’s sister says she is telling her sister’s story in an effort to make a difference in the lives of other patients.
“[Becky] did not want anyone to go through what she went through, ever again,” Ms. Beaton writes.
Letting children roam free
Children of the 1950s and 1960s can remember tearing out the door after dinner with the parental order to be home before dark. Where we went and what we did was known only to us. Our parents trusted we knew how to look out after ourselves.
In that tradition, as explained by National Public Radio, some parents are actively turning away from the to-the-second scheduling of their children’s lives and Teflon coating them against the perceived danger of everyday life. Instead, they are letting their children be independent. It can be a powerful life benefit for a child. But it can come at a cost to parents. Parents in several states have been arrested for actions that include letting their children walk to school unattended.
“This very pessimistic, fearful way of looking at childhood isn’t based in reality,” says Leonore Skenazy in a story on NPR. “It is something that we have been taught.” Ms. Skenazy is founder of Free Range Kids, a group that promotes childhood independence.
Boundaries and remote work
More and more Americans are working from home, according to a 2017 Gallup survey. The survey says that 43% of people worked remotely for part of the time in 2016, compared with 39% in 2012.
But for parents who work remotely, separating their business and family lives is especially challenging, Marie Elizabeth Oliver writes in an article published in The Washington Post.
“Being a parent is isolating, but being a parent and working from home is really isolating,” author Karen Alpert says. “Especially as a mom, there’s so much pressure to do your job as fast and efficiently as possible.”
Experts advise setting boundaries by taking steps such as setting timers and checking in every hour, for example. Or using clothes to make the mental shifts between being on the clock, so to speak, and being in leisure mode.
“It’s not that you have to dress up,” author David Heinemeier Hansson says in the article, describing one of his employees who came up with a system that enabled him to set these boundaries using clothes. “It’s just that he knew, ‘I have my home slippers on right now, so I’m not responding to this email.’ “
Neuroscience as a remedy to heartbreak
The end of a romance can be both frustrating and embarrassing. A person may wallow in the emotional muck for a long time.
Such was the case for Dessa, a well-known rapper, singer, and writer from Minneapolis, who carried the emotional baggage of an ex-boyfriend.
“You’re not only suffering,” she comments in an interview on NPR. “You’re just sort of ridiculous. Discipline and dedication are my strong suits – it really bothered me that, no matter how much effort I tried to expend in trying to solve this problem, I was stuck.”
The stalemate ended when she viewed a TED Talk by Helen Fisher, PhD, a biological anthropologist and visiting research associate at Rutgers University, New Brunswick, N.J. Dr. Fisher used functional MRI to examine some people in the throes of lost love. The examinations revealed revved-up activity of certain parts of their brains.
This prompted the idea that techniques of neurofeedback could be used to wipe the pangs of love from the brain circuitry. It seems to have worked for Dessa, although a placebo effect cannot be ruled out.
“Before [the feedback], I felt that I was really under the thumb of a fixation and a compulsion,” she says. “And now it feels like those feelings have been scaled down.”
Turn on the television, and chances are good that popular dramas (especially hospital-oriented shows) will be showing an episode revolving around a mental health issue.
, in time for a commercial. Real life is messier.
“Unfortunately mental health story lines are much more likely to be fear-mongering and wildly wrong. As a psychiatrist, this both piques my interest and upends my work-life balance,” Dr. Gold writes. “Whether I’m watching everyone’s favorite medical drama or ‘reality TV,’ it’s impossible not to switch into physician mode, angry on behalf of all of my patients and the many viewers who are being misled.”
Do physicians hear women?
“Rebecca continues to be paranoid.”
That was a note written by someone involved in the medical care of a 30-something woman diagnosed with stage IIB cervical cancer, according to an article in New York magazine.
“There’s a whiff of old ‘female hysteria’ to [the note], with more than a hint of dismissal,” writes the patient’s sister, Kate Beaton. “Becky was scared, and perhaps that was the main takeaway that day. But she was also right.”
The article tells the story of a vibrant woman who, according to her sister, asked her doctors lots of questions, wrote everything down, and faced years of being dismissed when she explained her symptoms. Becky’s sister says she is telling her sister’s story in an effort to make a difference in the lives of other patients.
“[Becky] did not want anyone to go through what she went through, ever again,” Ms. Beaton writes.
Letting children roam free
Children of the 1950s and 1960s can remember tearing out the door after dinner with the parental order to be home before dark. Where we went and what we did was known only to us. Our parents trusted we knew how to look out after ourselves.
In that tradition, as explained by National Public Radio, some parents are actively turning away from the to-the-second scheduling of their children’s lives and Teflon coating them against the perceived danger of everyday life. Instead, they are letting their children be independent. It can be a powerful life benefit for a child. But it can come at a cost to parents. Parents in several states have been arrested for actions that include letting their children walk to school unattended.
“This very pessimistic, fearful way of looking at childhood isn’t based in reality,” says Leonore Skenazy in a story on NPR. “It is something that we have been taught.” Ms. Skenazy is founder of Free Range Kids, a group that promotes childhood independence.
Boundaries and remote work
More and more Americans are working from home, according to a 2017 Gallup survey. The survey says that 43% of people worked remotely for part of the time in 2016, compared with 39% in 2012.
But for parents who work remotely, separating their business and family lives is especially challenging, Marie Elizabeth Oliver writes in an article published in The Washington Post.
“Being a parent is isolating, but being a parent and working from home is really isolating,” author Karen Alpert says. “Especially as a mom, there’s so much pressure to do your job as fast and efficiently as possible.”
Experts advise setting boundaries by taking steps such as setting timers and checking in every hour, for example. Or using clothes to make the mental shifts between being on the clock, so to speak, and being in leisure mode.
“It’s not that you have to dress up,” author David Heinemeier Hansson says in the article, describing one of his employees who came up with a system that enabled him to set these boundaries using clothes. “It’s just that he knew, ‘I have my home slippers on right now, so I’m not responding to this email.’ “
Neuroscience as a remedy to heartbreak
The end of a romance can be both frustrating and embarrassing. A person may wallow in the emotional muck for a long time.
Such was the case for Dessa, a well-known rapper, singer, and writer from Minneapolis, who carried the emotional baggage of an ex-boyfriend.
“You’re not only suffering,” she comments in an interview on NPR. “You’re just sort of ridiculous. Discipline and dedication are my strong suits – it really bothered me that, no matter how much effort I tried to expend in trying to solve this problem, I was stuck.”
The stalemate ended when she viewed a TED Talk by Helen Fisher, PhD, a biological anthropologist and visiting research associate at Rutgers University, New Brunswick, N.J. Dr. Fisher used functional MRI to examine some people in the throes of lost love. The examinations revealed revved-up activity of certain parts of their brains.
This prompted the idea that techniques of neurofeedback could be used to wipe the pangs of love from the brain circuitry. It seems to have worked for Dessa, although a placebo effect cannot be ruled out.
“Before [the feedback], I felt that I was really under the thumb of a fixation and a compulsion,” she says. “And now it feels like those feelings have been scaled down.”
Turn on the television, and chances are good that popular dramas (especially hospital-oriented shows) will be showing an episode revolving around a mental health issue.
, in time for a commercial. Real life is messier.
“Unfortunately mental health story lines are much more likely to be fear-mongering and wildly wrong. As a psychiatrist, this both piques my interest and upends my work-life balance,” Dr. Gold writes. “Whether I’m watching everyone’s favorite medical drama or ‘reality TV,’ it’s impossible not to switch into physician mode, angry on behalf of all of my patients and the many viewers who are being misled.”
Do physicians hear women?
“Rebecca continues to be paranoid.”
That was a note written by someone involved in the medical care of a 30-something woman diagnosed with stage IIB cervical cancer, according to an article in New York magazine.
“There’s a whiff of old ‘female hysteria’ to [the note], with more than a hint of dismissal,” writes the patient’s sister, Kate Beaton. “Becky was scared, and perhaps that was the main takeaway that day. But she was also right.”
The article tells the story of a vibrant woman who, according to her sister, asked her doctors lots of questions, wrote everything down, and faced years of being dismissed when she explained her symptoms. Becky’s sister says she is telling her sister’s story in an effort to make a difference in the lives of other patients.
“[Becky] did not want anyone to go through what she went through, ever again,” Ms. Beaton writes.
Letting children roam free
Children of the 1950s and 1960s can remember tearing out the door after dinner with the parental order to be home before dark. Where we went and what we did was known only to us. Our parents trusted we knew how to look out after ourselves.
In that tradition, as explained by National Public Radio, some parents are actively turning away from the to-the-second scheduling of their children’s lives and Teflon coating them against the perceived danger of everyday life. Instead, they are letting their children be independent. It can be a powerful life benefit for a child. But it can come at a cost to parents. Parents in several states have been arrested for actions that include letting their children walk to school unattended.
“This very pessimistic, fearful way of looking at childhood isn’t based in reality,” says Leonore Skenazy in a story on NPR. “It is something that we have been taught.” Ms. Skenazy is founder of Free Range Kids, a group that promotes childhood independence.
Boundaries and remote work
More and more Americans are working from home, according to a 2017 Gallup survey. The survey says that 43% of people worked remotely for part of the time in 2016, compared with 39% in 2012.
But for parents who work remotely, separating their business and family lives is especially challenging, Marie Elizabeth Oliver writes in an article published in The Washington Post.
“Being a parent is isolating, but being a parent and working from home is really isolating,” author Karen Alpert says. “Especially as a mom, there’s so much pressure to do your job as fast and efficiently as possible.”
Experts advise setting boundaries by taking steps such as setting timers and checking in every hour, for example. Or using clothes to make the mental shifts between being on the clock, so to speak, and being in leisure mode.
“It’s not that you have to dress up,” author David Heinemeier Hansson says in the article, describing one of his employees who came up with a system that enabled him to set these boundaries using clothes. “It’s just that he knew, ‘I have my home slippers on right now, so I’m not responding to this email.’ “
Neuroscience as a remedy to heartbreak
The end of a romance can be both frustrating and embarrassing. A person may wallow in the emotional muck for a long time.
Such was the case for Dessa, a well-known rapper, singer, and writer from Minneapolis, who carried the emotional baggage of an ex-boyfriend.
“You’re not only suffering,” she comments in an interview on NPR. “You’re just sort of ridiculous. Discipline and dedication are my strong suits – it really bothered me that, no matter how much effort I tried to expend in trying to solve this problem, I was stuck.”
The stalemate ended when she viewed a TED Talk by Helen Fisher, PhD, a biological anthropologist and visiting research associate at Rutgers University, New Brunswick, N.J. Dr. Fisher used functional MRI to examine some people in the throes of lost love. The examinations revealed revved-up activity of certain parts of their brains.
This prompted the idea that techniques of neurofeedback could be used to wipe the pangs of love from the brain circuitry. It seems to have worked for Dessa, although a placebo effect cannot be ruled out.
“Before [the feedback], I felt that I was really under the thumb of a fixation and a compulsion,” she says. “And now it feels like those feelings have been scaled down.”
Coronary CT FFR sharpens patient assessment in two studies
MUNICH – Noninvasive assessment of fractional flow reserve (FFR) within coronary arteries using data collected by CT angiography again has been shown to provide important additional diagnostic information that better guides patient management.
“The value of FFRCT is to reduce the number of patients who go to the cath lab. For patients with a stenosis of 60% that is not likely to have functional significance we can avoid catheterization and treat the patient medically. FFRCT is a valuable technology, but my concern is that currently it costs about $1,400 for this test,” commented Todd C. Villines, MD, a cardiologist at Georgetown University in Washington who was a discussant for the study. “Given the cost, we need to better define the patients on whom we use FFRCT and integrate it into clinical decision making,” Dr. Villines said in an interview.
Perhaps the best demonstration of the potential role for FFRCT came from a single-center study at Aarhus (Denmark) University with 3,674 patients with stable chest pain who underwent CCTA as their initial assessment for suspected coronary artery disease between May 2014 and December 2016. More than two-thirds of these patients had coronary stenoses of less than 30% and had no further assessment or treatment, and 11% had at least one coronary stenosis of at least 70% on CCTA and then had follow-up testing by either conventional angiography or myocardial perfusion imaging. The report at the congress focused on the 697 patients with an inconclusive result based on CCTA alone and at least one stenosis of 30%-69% who underwent FFRCT analysis, and focused specifically on 677 patients with a useful FFRCT result.
Of these patients, 410 (61% of this subgroup) had no coronary lesion that created a FFRCT of 0.8 or less. All received treatment with optimal medical therapy only, and after a median follow-up had a 3.9% incidence of the primary endpoint, the combined rate of all-cause death, nonfatal MI, hospitalization for unstable angina, or unplanned revascularization. This 3.9% rate was not significantly different from the 2.8% rate seen during follow-up of the patients with no coronary stenosis of 30% or greater.
The remaining 267 patients (39% of the subgroup) with a FFRCT that showed 80% or less flow reserve either received optimal medical therapy (112 patients, 42% of this group) or angiography by coronary catheterization (155 patients, 58% of this group).
The second report used data collected from 5,083 patients entered into a multinational registry, ADVANCE, with symptoms suggestive of coronary artery disease and results from CCTA that suggested coronary stenosis. The collaborating researchers then used the CCTA results to generate a FFR analysis for 4,893 (96%) of the patients, and the analysis was usable for 4,737 of them. The FFRCT results led to reclassification of the management strategy for 67% of the patients, the primary endpoint for this analysis, reported Timothy A. Fairbairn, MD, a cardiologist at the Liverpool (England) Heart and Chest Hospital.
One limitation of this study was the relatively brief, 90-day follow-up, but it is the first real-world, multicenter assessment of the utility and safety of FFRCT.
These findings highlight what a “disruptive technology” FFRCT represents, commented Dr. Villines. He also noted that the reclassifications triggered by the FFRCT analysis led to fewer patients undergoing invasive angiography, a good outcome from a cost-effectiveness perspective.
Concurrently with Dr. Fairbairn’s report the results from ADVANCE also appeared in an article published online (Euro Heart J. 2018 Aug 25. doi: 10.1093/eurheartj/ehy530).
A third FFRCT study reported at the session, the Computed Tomographic Evaluation of Atherosclerotic Determinants of Myocardial Ischemia (CREDENCE) study, enrolled 612 patients with suspected coronary artery disease who had been referred for and underwent invasive coronary angiography with FFR evaluation at 13 international centers, including several in the United States. All 612 patients also had assessment by CCTA and FFRCT, and also some type of functional myocardial perfusion assessment using positron emission tomography, single-photon emission CT, or coronary MR.
The Aarhus University study received no commercial funding. Dr. Nørgaard has received research funding from Edwards; Siemens; and HeartFlow, the company that markets FFR analysis for coronary CT angiography data. The ADVANCE registry was sponsored by HeartFlow. Dr. Fairbairn has been a speaker for Heartflow. Dr. Stuijfzand and Dr. Villines had no relevant disclosures.
MUNICH – Noninvasive assessment of fractional flow reserve (FFR) within coronary arteries using data collected by CT angiography again has been shown to provide important additional diagnostic information that better guides patient management.
“The value of FFRCT is to reduce the number of patients who go to the cath lab. For patients with a stenosis of 60% that is not likely to have functional significance we can avoid catheterization and treat the patient medically. FFRCT is a valuable technology, but my concern is that currently it costs about $1,400 for this test,” commented Todd C. Villines, MD, a cardiologist at Georgetown University in Washington who was a discussant for the study. “Given the cost, we need to better define the patients on whom we use FFRCT and integrate it into clinical decision making,” Dr. Villines said in an interview.
Perhaps the best demonstration of the potential role for FFRCT came from a single-center study at Aarhus (Denmark) University with 3,674 patients with stable chest pain who underwent CCTA as their initial assessment for suspected coronary artery disease between May 2014 and December 2016. More than two-thirds of these patients had coronary stenoses of less than 30% and had no further assessment or treatment, and 11% had at least one coronary stenosis of at least 70% on CCTA and then had follow-up testing by either conventional angiography or myocardial perfusion imaging. The report at the congress focused on the 697 patients with an inconclusive result based on CCTA alone and at least one stenosis of 30%-69% who underwent FFRCT analysis, and focused specifically on 677 patients with a useful FFRCT result.
Of these patients, 410 (61% of this subgroup) had no coronary lesion that created a FFRCT of 0.8 or less. All received treatment with optimal medical therapy only, and after a median follow-up had a 3.9% incidence of the primary endpoint, the combined rate of all-cause death, nonfatal MI, hospitalization for unstable angina, or unplanned revascularization. This 3.9% rate was not significantly different from the 2.8% rate seen during follow-up of the patients with no coronary stenosis of 30% or greater.
The remaining 267 patients (39% of the subgroup) with a FFRCT that showed 80% or less flow reserve either received optimal medical therapy (112 patients, 42% of this group) or angiography by coronary catheterization (155 patients, 58% of this group).
The second report used data collected from 5,083 patients entered into a multinational registry, ADVANCE, with symptoms suggestive of coronary artery disease and results from CCTA that suggested coronary stenosis. The collaborating researchers then used the CCTA results to generate a FFR analysis for 4,893 (96%) of the patients, and the analysis was usable for 4,737 of them. The FFRCT results led to reclassification of the management strategy for 67% of the patients, the primary endpoint for this analysis, reported Timothy A. Fairbairn, MD, a cardiologist at the Liverpool (England) Heart and Chest Hospital.
One limitation of this study was the relatively brief, 90-day follow-up, but it is the first real-world, multicenter assessment of the utility and safety of FFRCT.
These findings highlight what a “disruptive technology” FFRCT represents, commented Dr. Villines. He also noted that the reclassifications triggered by the FFRCT analysis led to fewer patients undergoing invasive angiography, a good outcome from a cost-effectiveness perspective.
Concurrently with Dr. Fairbairn’s report the results from ADVANCE also appeared in an article published online (Euro Heart J. 2018 Aug 25. doi: 10.1093/eurheartj/ehy530).
A third FFRCT study reported at the session, the Computed Tomographic Evaluation of Atherosclerotic Determinants of Myocardial Ischemia (CREDENCE) study, enrolled 612 patients with suspected coronary artery disease who had been referred for and underwent invasive coronary angiography with FFR evaluation at 13 international centers, including several in the United States. All 612 patients also had assessment by CCTA and FFRCT, and also some type of functional myocardial perfusion assessment using positron emission tomography, single-photon emission CT, or coronary MR.
The Aarhus University study received no commercial funding. Dr. Nørgaard has received research funding from Edwards; Siemens; and HeartFlow, the company that markets FFR analysis for coronary CT angiography data. The ADVANCE registry was sponsored by HeartFlow. Dr. Fairbairn has been a speaker for Heartflow. Dr. Stuijfzand and Dr. Villines had no relevant disclosures.
MUNICH – Noninvasive assessment of fractional flow reserve (FFR) within coronary arteries using data collected by CT angiography again has been shown to provide important additional diagnostic information that better guides patient management.
“The value of FFRCT is to reduce the number of patients who go to the cath lab. For patients with a stenosis of 60% that is not likely to have functional significance we can avoid catheterization and treat the patient medically. FFRCT is a valuable technology, but my concern is that currently it costs about $1,400 for this test,” commented Todd C. Villines, MD, a cardiologist at Georgetown University in Washington who was a discussant for the study. “Given the cost, we need to better define the patients on whom we use FFRCT and integrate it into clinical decision making,” Dr. Villines said in an interview.
Perhaps the best demonstration of the potential role for FFRCT came from a single-center study at Aarhus (Denmark) University with 3,674 patients with stable chest pain who underwent CCTA as their initial assessment for suspected coronary artery disease between May 2014 and December 2016. More than two-thirds of these patients had coronary stenoses of less than 30% and had no further assessment or treatment, and 11% had at least one coronary stenosis of at least 70% on CCTA and then had follow-up testing by either conventional angiography or myocardial perfusion imaging. The report at the congress focused on the 697 patients with an inconclusive result based on CCTA alone and at least one stenosis of 30%-69% who underwent FFRCT analysis, and focused specifically on 677 patients with a useful FFRCT result.
Of these patients, 410 (61% of this subgroup) had no coronary lesion that created a FFRCT of 0.8 or less. All received treatment with optimal medical therapy only, and after a median follow-up had a 3.9% incidence of the primary endpoint, the combined rate of all-cause death, nonfatal MI, hospitalization for unstable angina, or unplanned revascularization. This 3.9% rate was not significantly different from the 2.8% rate seen during follow-up of the patients with no coronary stenosis of 30% or greater.
The remaining 267 patients (39% of the subgroup) with a FFRCT that showed 80% or less flow reserve either received optimal medical therapy (112 patients, 42% of this group) or angiography by coronary catheterization (155 patients, 58% of this group).
The second report used data collected from 5,083 patients entered into a multinational registry, ADVANCE, with symptoms suggestive of coronary artery disease and results from CCTA that suggested coronary stenosis. The collaborating researchers then used the CCTA results to generate a FFR analysis for 4,893 (96%) of the patients, and the analysis was usable for 4,737 of them. The FFRCT results led to reclassification of the management strategy for 67% of the patients, the primary endpoint for this analysis, reported Timothy A. Fairbairn, MD, a cardiologist at the Liverpool (England) Heart and Chest Hospital.
One limitation of this study was the relatively brief, 90-day follow-up, but it is the first real-world, multicenter assessment of the utility and safety of FFRCT.
These findings highlight what a “disruptive technology” FFRCT represents, commented Dr. Villines. He also noted that the reclassifications triggered by the FFRCT analysis led to fewer patients undergoing invasive angiography, a good outcome from a cost-effectiveness perspective.
Concurrently with Dr. Fairbairn’s report the results from ADVANCE also appeared in an article published online (Euro Heart J. 2018 Aug 25. doi: 10.1093/eurheartj/ehy530).
A third FFRCT study reported at the session, the Computed Tomographic Evaluation of Atherosclerotic Determinants of Myocardial Ischemia (CREDENCE) study, enrolled 612 patients with suspected coronary artery disease who had been referred for and underwent invasive coronary angiography with FFR evaluation at 13 international centers, including several in the United States. All 612 patients also had assessment by CCTA and FFRCT, and also some type of functional myocardial perfusion assessment using positron emission tomography, single-photon emission CT, or coronary MR.
The Aarhus University study received no commercial funding. Dr. Nørgaard has received research funding from Edwards; Siemens; and HeartFlow, the company that markets FFR analysis for coronary CT angiography data. The ADVANCE registry was sponsored by HeartFlow. Dr. Fairbairn has been a speaker for Heartflow. Dr. Stuijfzand and Dr. Villines had no relevant disclosures.
REPORTING FROM THE ESC CONGRESS 2018
Trials need standardized reporting of pediatric fever after flu vaccine
Researchers found a lower rate of pediatric fever after applying a standard definition of fever across three different clinical trials of pediatric patients receiving influenza vaccinations, according to research published in the Pediatric Infectious Disease Journal.
Investigators in future studies must adopt a standardized definition of pediatric fever after an influenza vaccination. “Our study demonstrates the variability in results which occur due to minor differences in the definition of fever, methods of analysis and reporting of results,” Jean Li-Kim-Moy, MBBS, of the University of Sydney, and colleagues wrote.
Dr. Li-Kim-Moy and colleagues analyzed pediatric datasets from three different clinical trials using trivalent influenza vaccine (TIV); the primary trial included 3,317 children aged 6-35 months who were randomized to receive Fluarix at 0.25 mL or 0.5 mL, or receive 0.25 mL of Fluzone. The other two trials studied children receiving TIV between 6 months–17 years and 3-17 years. The researchers also performed a multivariable regression analysis to determine the relationship between immunogenicity, antipyretic use, and postvaccination fever.
The primary study initially reported the fever rate 0 days–3 days after vaccination was between 6% and 7%. After reporting the rate of fever separately for each dose and changing the criteria to “defining fever as greater than or equal to 38.0°C by any route of measurement” for the primary study, the researchers found a rate of any-cause fever was 3%-4% for the first dose and 4%-5% for the second dose. The rate of vaccine-related fever in the primary study was 3% for the first dose and 3%-4% for the second dose, with researchers noting vaccine-related fever occurred significantly earlier compared with any-cause fever (mean 1 days vs. 2 days after vaccination; P equals .04).
Impact of fever, antipyretics
The researchers also performed a pooled immunogenicity analysis of 5,902 children from all three trials and found a strong association between fever after vaccination and increased geometric mean titer (GMT) ratios (1.21-1.39; P less than or equal to .01) and an association between antipyretic use and reduced GMT ratios (0.80-0.87; P less than .0006).
“Our pooled analysis of the three trials demonstrated highly significant associations, for all strains, between postvaccination fever and up to 39% higher GMT; Similarly, strong evidence of associations in the opposite direction was found between postvaccination antipyretic use (days 0-3), adjusting for all other factors including fever, and decreased immunogenicity against all vaccine strains in children,” Dr. Li-Kim-Moy and colleagues said.
Antipyretic use was common in the primary study, occurring in one in six of the children, they said. These findings of “significant associations between fever and increased vaccine immunogenicity, and between antipyretic use and reduced immunogenicity in children after influenza vaccination” suggest the need for further study, especially because parents often give antipyretics if their children are febrile after vaccinations.
“There is uncertainty whether our findings, and those of others, on immunogenicity translate into clinically significant effects,” they wrote. “However, the fact that influenza vaccine, unlike many routine childhood vaccines, is only moderately protective may mean that modest reductions in antibody response are more likely to correlate to less protection.”
Dr. Wood reported receiving a fellowship from the National Health and Medical Research Council (NHMRC) and being an investigator for GlaxoSmithKline trials. Dr. Booy reported being an advisor for influenza vaccine manufacturing, an advisory board member, on the speaker’s bureau, and a researcher of vaccines for several manufacturers. The other authors reported no relevant conflicts of interest.
SOURCE: Li-Kim-Moy J et al. Pediatr Infect Dis J. 2018 Oct;37(10):971-5.
Researchers found a lower rate of pediatric fever after applying a standard definition of fever across three different clinical trials of pediatric patients receiving influenza vaccinations, according to research published in the Pediatric Infectious Disease Journal.
Investigators in future studies must adopt a standardized definition of pediatric fever after an influenza vaccination. “Our study demonstrates the variability in results which occur due to minor differences in the definition of fever, methods of analysis and reporting of results,” Jean Li-Kim-Moy, MBBS, of the University of Sydney, and colleagues wrote.
Dr. Li-Kim-Moy and colleagues analyzed pediatric datasets from three different clinical trials using trivalent influenza vaccine (TIV); the primary trial included 3,317 children aged 6-35 months who were randomized to receive Fluarix at 0.25 mL or 0.5 mL, or receive 0.25 mL of Fluzone. The other two trials studied children receiving TIV between 6 months–17 years and 3-17 years. The researchers also performed a multivariable regression analysis to determine the relationship between immunogenicity, antipyretic use, and postvaccination fever.
The primary study initially reported the fever rate 0 days–3 days after vaccination was between 6% and 7%. After reporting the rate of fever separately for each dose and changing the criteria to “defining fever as greater than or equal to 38.0°C by any route of measurement” for the primary study, the researchers found a rate of any-cause fever was 3%-4% for the first dose and 4%-5% for the second dose. The rate of vaccine-related fever in the primary study was 3% for the first dose and 3%-4% for the second dose, with researchers noting vaccine-related fever occurred significantly earlier compared with any-cause fever (mean 1 days vs. 2 days after vaccination; P equals .04).
Impact of fever, antipyretics
The researchers also performed a pooled immunogenicity analysis of 5,902 children from all three trials and found a strong association between fever after vaccination and increased geometric mean titer (GMT) ratios (1.21-1.39; P less than or equal to .01) and an association between antipyretic use and reduced GMT ratios (0.80-0.87; P less than .0006).
“Our pooled analysis of the three trials demonstrated highly significant associations, for all strains, between postvaccination fever and up to 39% higher GMT; Similarly, strong evidence of associations in the opposite direction was found between postvaccination antipyretic use (days 0-3), adjusting for all other factors including fever, and decreased immunogenicity against all vaccine strains in children,” Dr. Li-Kim-Moy and colleagues said.
Antipyretic use was common in the primary study, occurring in one in six of the children, they said. These findings of “significant associations between fever and increased vaccine immunogenicity, and between antipyretic use and reduced immunogenicity in children after influenza vaccination” suggest the need for further study, especially because parents often give antipyretics if their children are febrile after vaccinations.
“There is uncertainty whether our findings, and those of others, on immunogenicity translate into clinically significant effects,” they wrote. “However, the fact that influenza vaccine, unlike many routine childhood vaccines, is only moderately protective may mean that modest reductions in antibody response are more likely to correlate to less protection.”
Dr. Wood reported receiving a fellowship from the National Health and Medical Research Council (NHMRC) and being an investigator for GlaxoSmithKline trials. Dr. Booy reported being an advisor for influenza vaccine manufacturing, an advisory board member, on the speaker’s bureau, and a researcher of vaccines for several manufacturers. The other authors reported no relevant conflicts of interest.
SOURCE: Li-Kim-Moy J et al. Pediatr Infect Dis J. 2018 Oct;37(10):971-5.
Researchers found a lower rate of pediatric fever after applying a standard definition of fever across three different clinical trials of pediatric patients receiving influenza vaccinations, according to research published in the Pediatric Infectious Disease Journal.
Investigators in future studies must adopt a standardized definition of pediatric fever after an influenza vaccination. “Our study demonstrates the variability in results which occur due to minor differences in the definition of fever, methods of analysis and reporting of results,” Jean Li-Kim-Moy, MBBS, of the University of Sydney, and colleagues wrote.
Dr. Li-Kim-Moy and colleagues analyzed pediatric datasets from three different clinical trials using trivalent influenza vaccine (TIV); the primary trial included 3,317 children aged 6-35 months who were randomized to receive Fluarix at 0.25 mL or 0.5 mL, or receive 0.25 mL of Fluzone. The other two trials studied children receiving TIV between 6 months–17 years and 3-17 years. The researchers also performed a multivariable regression analysis to determine the relationship between immunogenicity, antipyretic use, and postvaccination fever.
The primary study initially reported the fever rate 0 days–3 days after vaccination was between 6% and 7%. After reporting the rate of fever separately for each dose and changing the criteria to “defining fever as greater than or equal to 38.0°C by any route of measurement” for the primary study, the researchers found a rate of any-cause fever was 3%-4% for the first dose and 4%-5% for the second dose. The rate of vaccine-related fever in the primary study was 3% for the first dose and 3%-4% for the second dose, with researchers noting vaccine-related fever occurred significantly earlier compared with any-cause fever (mean 1 days vs. 2 days after vaccination; P equals .04).
Impact of fever, antipyretics
The researchers also performed a pooled immunogenicity analysis of 5,902 children from all three trials and found a strong association between fever after vaccination and increased geometric mean titer (GMT) ratios (1.21-1.39; P less than or equal to .01) and an association between antipyretic use and reduced GMT ratios (0.80-0.87; P less than .0006).
“Our pooled analysis of the three trials demonstrated highly significant associations, for all strains, between postvaccination fever and up to 39% higher GMT; Similarly, strong evidence of associations in the opposite direction was found between postvaccination antipyretic use (days 0-3), adjusting for all other factors including fever, and decreased immunogenicity against all vaccine strains in children,” Dr. Li-Kim-Moy and colleagues said.
Antipyretic use was common in the primary study, occurring in one in six of the children, they said. These findings of “significant associations between fever and increased vaccine immunogenicity, and between antipyretic use and reduced immunogenicity in children after influenza vaccination” suggest the need for further study, especially because parents often give antipyretics if their children are febrile after vaccinations.
“There is uncertainty whether our findings, and those of others, on immunogenicity translate into clinically significant effects,” they wrote. “However, the fact that influenza vaccine, unlike many routine childhood vaccines, is only moderately protective may mean that modest reductions in antibody response are more likely to correlate to less protection.”
Dr. Wood reported receiving a fellowship from the National Health and Medical Research Council (NHMRC) and being an investigator for GlaxoSmithKline trials. Dr. Booy reported being an advisor for influenza vaccine manufacturing, an advisory board member, on the speaker’s bureau, and a researcher of vaccines for several manufacturers. The other authors reported no relevant conflicts of interest.
SOURCE: Li-Kim-Moy J et al. Pediatr Infect Dis J. 2018 Oct;37(10):971-5.
FROM THE PEDIATRIC INFECTIOUS DISEASE JOURNAL
Key clinical point: There is variability in reporting and analysis of pediatric fever rates after administration of the influenza vaccine.
Major finding: Applying the Brighton Collaboration standardized definition for vaccine-related fever to three clinical trials yielded significantly lower rates of fever (3%-4%), compared with the rates reported in the trials (6%-7%).
Study details: An analysis of pediatric fever data from three different clinical trials using Brighton Collaboration criteria.
Disclosures: Dr. Wood reported receiving a fellowship from the National Health and Medical Research Council and being an investigator for GlaxoSmithKline trials. Dr. Booy reported being an advisor for influenza vaccine manufacturing, an advisory board member, on the speaker’s bureau, and a researcher of vaccines for several manufacturers. The other authors reported no relevant conflicts of interest.
Source: Li-Kim-Moy J et al. Pediatr Infect Dis J. 2018 Oct;37(10):971-5.
It may be time to rethink damage-control laparotomy
SAN ANTONIO – A , but it can cost fewer resources: fewer days in intensive care, fewer days on a ventilator, and a shorter overall length of stay.
Among damage-control abdominal trauma cases that could have been closed, definitive laparotomy (DEF) was associated with a 56% probability of major abdominal complications – very close to the probability associated with damage-control closure, John Harvin, MD, said at the annual meeting of the American Association for the Surgery of Trauma.
Definitive closure was also associated with about a 72% chance of more ventilator- and hospital-free days and a 77% chance of more ICU-free days than damage-control closure, said Dr. Harvin of the University of Texas, Austin.
“Our analysis tells us that there’s a minimal chance of reducing complications with a definitive laparotomy, compared to leaving them open, but this also comes with more than a 70% chance of having a shorter hospital staying and getting off the ventilator faster.”
The data from a 2-year quality review process reaffirms what trauma surgeons have been seeing, and reporting, since damage-control closure became more popular in the past decade, said Peter Rhee, MD, commenting on the study.
“There are three congregations in this faith of damage-control laparotomy,” said Dr. Rhee, a surgeon in Atlanta, Ga. “The first believes it should be the default for all these types of operations and that being preemptive is better than anything. The second believes that it doesn’t hurt the patient too much, and that it can be done when absolutely needed to save a life. The third belief is that there’s minimal data to support it, that it should rarely be used, and that it’s always costly. We all know that the pendulum of damage-control laparotomy is finally swinging back to the center.”
The study arose from an effort at the Red Duke Trauma Institute, Austin, Tex., to reduce its rate of damage-control laparotomy (DCL). Surgeons examined all emergent trauma laparotomies conducted from 2013 to 2015. They discussed each case and compared morbidity and mortality rates to a published control group. This work was published last year in the Journal of the American College of Surgeons.
By adopting this review procedure, the hospital was able to decrease its 39% DCL rate to 23% over 2 years (an absolute reduction of 68 cases) without any change in infection rates, fascial dehiscence, unplanned reoperation, or mortality. The improvements continued, Dr. Harvin noted, with a farther 17% reduction in DCL after the project concluded.
Dr. Harvin’s analysis looked at 44 of these procedures which, according to the adjudication panel, could have been managed by DEF. Each was matched to a DEF case, and the outcomes were calculated with a Bayesian statistical model.
The primary outcome was major abdominal complication, a composite measure of fascial dehiscence, organ or space surgical-site infection, reopening of fascia, and enteric suture line failure. Secondary outcomes were days off the ventilator and out of the ICU and hospital.
Of the 872 patients in the study, most (639; 73%) were managed by DEF; the rest (209; 24%) were managed by DCL. Of these, the panel agreed that 44 (22%) could have been safely closed at the time of surgery and survived at least 5 days. The propensity-matching scheme comprised 39 of these cases matched with 39 DEF cases.
Most were male (74%); the mean age was 38 years. Penetrating injuries were most common (54%). The abdominal Abbreviated Injury Score was 3. The mean Injury Severity Score was 22 in the DEF cases and 25 in the DCL cases, but this was not a significant difference. There were no differences in blood pressure at presentation or at the end of the surgery, no differences in blood transfusion needs, and no differences in body temperature.
A major abdominal complication occurred in 31% of the DEF cases and 21% of the DCL cases, a relative risk of 0.99. This amounted to a 56% posterior probability of a complication associated with DEF.
Comparing DCL cases with DEF cases, the mean number of hospital-free days was 15 vs.13; ICU-free days, 26 vs. 21; and ventilator-free days, 29 vs. 26. These differences amount to a 72% chance that DEL would result in more hospital-free days and more ventilator-free days, and a 77% chance that DEL would result in more ICU-free days.
The numbers underscore the need to rethink DCL for abdominal trauma, Dr. Rhee said.
“I too once believed in this procedure and used to do it all the time. After 2 decades, we now know that it contributed to the frozen bellies, abdominal wound hernias, fascial dehiscence, missed complications, and to the never-ending enteroatmospheric fistulas. If we want to reduce fistulas, we must first reduce damage-control laparotomy. Nurses will love you for not creating the narcotic-addicted, total parenteral nutrition–dependent patient with a fragrant open belly and a fistula.”
Neither Dr. Harvin nor Dr Rhee had any relevant financial disclosures.
SAN ANTONIO – A , but it can cost fewer resources: fewer days in intensive care, fewer days on a ventilator, and a shorter overall length of stay.
Among damage-control abdominal trauma cases that could have been closed, definitive laparotomy (DEF) was associated with a 56% probability of major abdominal complications – very close to the probability associated with damage-control closure, John Harvin, MD, said at the annual meeting of the American Association for the Surgery of Trauma.
Definitive closure was also associated with about a 72% chance of more ventilator- and hospital-free days and a 77% chance of more ICU-free days than damage-control closure, said Dr. Harvin of the University of Texas, Austin.
“Our analysis tells us that there’s a minimal chance of reducing complications with a definitive laparotomy, compared to leaving them open, but this also comes with more than a 70% chance of having a shorter hospital staying and getting off the ventilator faster.”
The data from a 2-year quality review process reaffirms what trauma surgeons have been seeing, and reporting, since damage-control closure became more popular in the past decade, said Peter Rhee, MD, commenting on the study.
“There are three congregations in this faith of damage-control laparotomy,” said Dr. Rhee, a surgeon in Atlanta, Ga. “The first believes it should be the default for all these types of operations and that being preemptive is better than anything. The second believes that it doesn’t hurt the patient too much, and that it can be done when absolutely needed to save a life. The third belief is that there’s minimal data to support it, that it should rarely be used, and that it’s always costly. We all know that the pendulum of damage-control laparotomy is finally swinging back to the center.”
The study arose from an effort at the Red Duke Trauma Institute, Austin, Tex., to reduce its rate of damage-control laparotomy (DCL). Surgeons examined all emergent trauma laparotomies conducted from 2013 to 2015. They discussed each case and compared morbidity and mortality rates to a published control group. This work was published last year in the Journal of the American College of Surgeons.
By adopting this review procedure, the hospital was able to decrease its 39% DCL rate to 23% over 2 years (an absolute reduction of 68 cases) without any change in infection rates, fascial dehiscence, unplanned reoperation, or mortality. The improvements continued, Dr. Harvin noted, with a farther 17% reduction in DCL after the project concluded.
Dr. Harvin’s analysis looked at 44 of these procedures which, according to the adjudication panel, could have been managed by DEF. Each was matched to a DEF case, and the outcomes were calculated with a Bayesian statistical model.
The primary outcome was major abdominal complication, a composite measure of fascial dehiscence, organ or space surgical-site infection, reopening of fascia, and enteric suture line failure. Secondary outcomes were days off the ventilator and out of the ICU and hospital.
Of the 872 patients in the study, most (639; 73%) were managed by DEF; the rest (209; 24%) were managed by DCL. Of these, the panel agreed that 44 (22%) could have been safely closed at the time of surgery and survived at least 5 days. The propensity-matching scheme comprised 39 of these cases matched with 39 DEF cases.
Most were male (74%); the mean age was 38 years. Penetrating injuries were most common (54%). The abdominal Abbreviated Injury Score was 3. The mean Injury Severity Score was 22 in the DEF cases and 25 in the DCL cases, but this was not a significant difference. There were no differences in blood pressure at presentation or at the end of the surgery, no differences in blood transfusion needs, and no differences in body temperature.
A major abdominal complication occurred in 31% of the DEF cases and 21% of the DCL cases, a relative risk of 0.99. This amounted to a 56% posterior probability of a complication associated with DEF.
Comparing DCL cases with DEF cases, the mean number of hospital-free days was 15 vs.13; ICU-free days, 26 vs. 21; and ventilator-free days, 29 vs. 26. These differences amount to a 72% chance that DEL would result in more hospital-free days and more ventilator-free days, and a 77% chance that DEL would result in more ICU-free days.
The numbers underscore the need to rethink DCL for abdominal trauma, Dr. Rhee said.
“I too once believed in this procedure and used to do it all the time. After 2 decades, we now know that it contributed to the frozen bellies, abdominal wound hernias, fascial dehiscence, missed complications, and to the never-ending enteroatmospheric fistulas. If we want to reduce fistulas, we must first reduce damage-control laparotomy. Nurses will love you for not creating the narcotic-addicted, total parenteral nutrition–dependent patient with a fragrant open belly and a fistula.”
Neither Dr. Harvin nor Dr Rhee had any relevant financial disclosures.
SAN ANTONIO – A , but it can cost fewer resources: fewer days in intensive care, fewer days on a ventilator, and a shorter overall length of stay.
Among damage-control abdominal trauma cases that could have been closed, definitive laparotomy (DEF) was associated with a 56% probability of major abdominal complications – very close to the probability associated with damage-control closure, John Harvin, MD, said at the annual meeting of the American Association for the Surgery of Trauma.
Definitive closure was also associated with about a 72% chance of more ventilator- and hospital-free days and a 77% chance of more ICU-free days than damage-control closure, said Dr. Harvin of the University of Texas, Austin.
“Our analysis tells us that there’s a minimal chance of reducing complications with a definitive laparotomy, compared to leaving them open, but this also comes with more than a 70% chance of having a shorter hospital staying and getting off the ventilator faster.”
The data from a 2-year quality review process reaffirms what trauma surgeons have been seeing, and reporting, since damage-control closure became more popular in the past decade, said Peter Rhee, MD, commenting on the study.
“There are three congregations in this faith of damage-control laparotomy,” said Dr. Rhee, a surgeon in Atlanta, Ga. “The first believes it should be the default for all these types of operations and that being preemptive is better than anything. The second believes that it doesn’t hurt the patient too much, and that it can be done when absolutely needed to save a life. The third belief is that there’s minimal data to support it, that it should rarely be used, and that it’s always costly. We all know that the pendulum of damage-control laparotomy is finally swinging back to the center.”
The study arose from an effort at the Red Duke Trauma Institute, Austin, Tex., to reduce its rate of damage-control laparotomy (DCL). Surgeons examined all emergent trauma laparotomies conducted from 2013 to 2015. They discussed each case and compared morbidity and mortality rates to a published control group. This work was published last year in the Journal of the American College of Surgeons.
By adopting this review procedure, the hospital was able to decrease its 39% DCL rate to 23% over 2 years (an absolute reduction of 68 cases) without any change in infection rates, fascial dehiscence, unplanned reoperation, or mortality. The improvements continued, Dr. Harvin noted, with a farther 17% reduction in DCL after the project concluded.
Dr. Harvin’s analysis looked at 44 of these procedures which, according to the adjudication panel, could have been managed by DEF. Each was matched to a DEF case, and the outcomes were calculated with a Bayesian statistical model.
The primary outcome was major abdominal complication, a composite measure of fascial dehiscence, organ or space surgical-site infection, reopening of fascia, and enteric suture line failure. Secondary outcomes were days off the ventilator and out of the ICU and hospital.
Of the 872 patients in the study, most (639; 73%) were managed by DEF; the rest (209; 24%) were managed by DCL. Of these, the panel agreed that 44 (22%) could have been safely closed at the time of surgery and survived at least 5 days. The propensity-matching scheme comprised 39 of these cases matched with 39 DEF cases.
Most were male (74%); the mean age was 38 years. Penetrating injuries were most common (54%). The abdominal Abbreviated Injury Score was 3. The mean Injury Severity Score was 22 in the DEF cases and 25 in the DCL cases, but this was not a significant difference. There were no differences in blood pressure at presentation or at the end of the surgery, no differences in blood transfusion needs, and no differences in body temperature.
A major abdominal complication occurred in 31% of the DEF cases and 21% of the DCL cases, a relative risk of 0.99. This amounted to a 56% posterior probability of a complication associated with DEF.
Comparing DCL cases with DEF cases, the mean number of hospital-free days was 15 vs.13; ICU-free days, 26 vs. 21; and ventilator-free days, 29 vs. 26. These differences amount to a 72% chance that DEL would result in more hospital-free days and more ventilator-free days, and a 77% chance that DEL would result in more ICU-free days.
The numbers underscore the need to rethink DCL for abdominal trauma, Dr. Rhee said.
“I too once believed in this procedure and used to do it all the time. After 2 decades, we now know that it contributed to the frozen bellies, abdominal wound hernias, fascial dehiscence, missed complications, and to the never-ending enteroatmospheric fistulas. If we want to reduce fistulas, we must first reduce damage-control laparotomy. Nurses will love you for not creating the narcotic-addicted, total parenteral nutrition–dependent patient with a fragrant open belly and a fistula.”
Neither Dr. Harvin nor Dr Rhee had any relevant financial disclosures.
AT THE AAST ANNUAL MEETING
Key clinical point: Definitive laparotomy for abdominal trauma added little risk of complications but shortened ventilator and ICU days.
Major finding: Definitive closure was associated with a 72% chance of more ventilator- and hospital-free days and a 77% chance of more ICU-free days than damage-control closure.
Study details: The Bayseian analysis comprised 39 definitive and 39 damage-control laparotomy patients.
Disclosures: Neither Dr. Harvin nor Dr. Rhee had any financial disclosures.
Source: Harvin J et al. AAST 2018, Oral paper 12.
Improved survival in liposarcoma
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel