User login
What medical therapies work for gastroparesis?
EVIDENCE SUMMARY
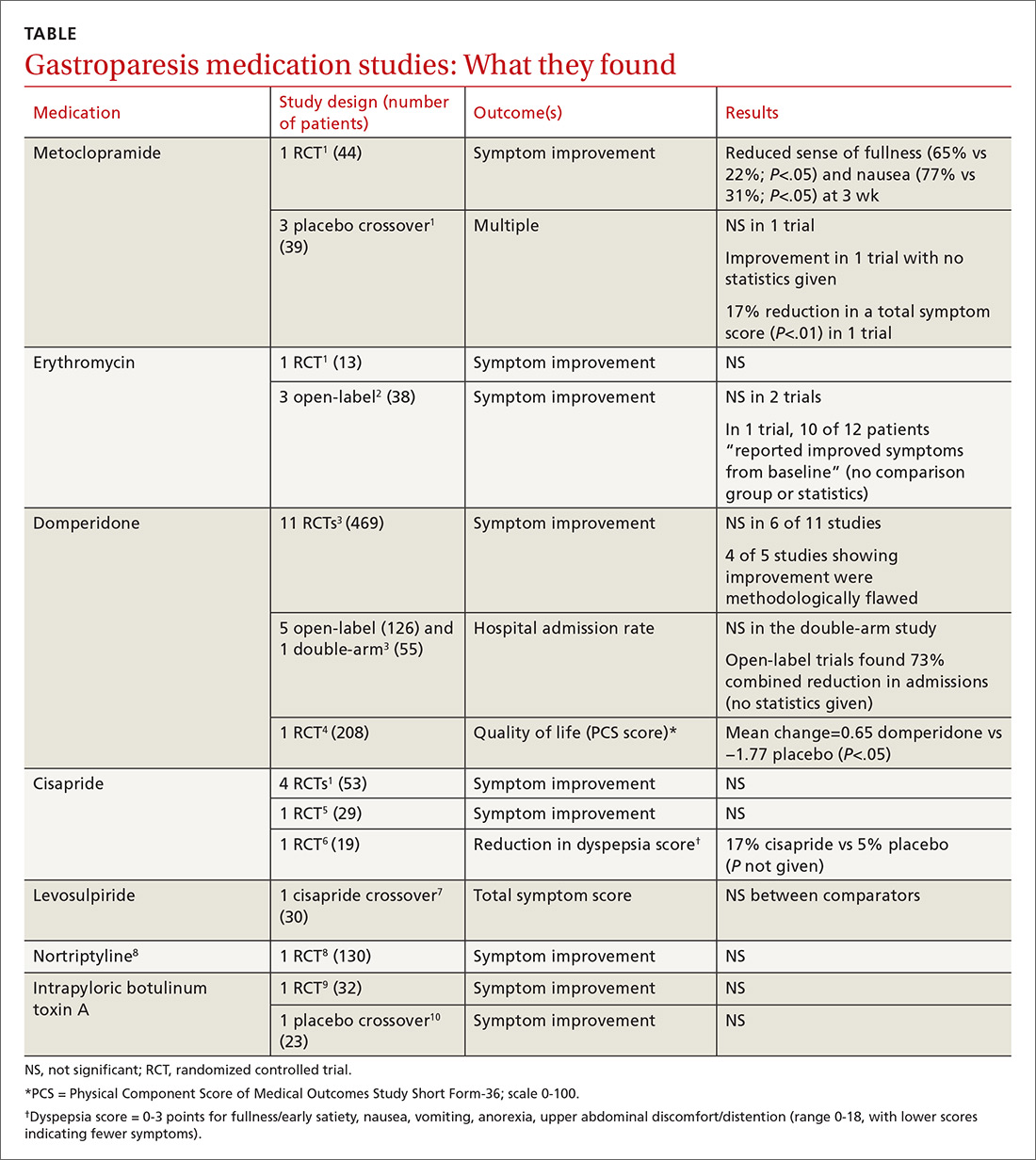
Metoclopramide. One systematic review that looked at the efficacy of metoclopramide for gastroparesis identified one small RCT and 3 smaller placebo-controlled crossover trials.1 The RCT (using 10 mg of metoclopramide after meals and at bedtime) found consistent improvement in the sense of fullness over 3 weeks of therapy, with reduction of nausea at one and 3 weeks, but not at 2 weeks. Vomiting, anorexia, and early satiety didn’t improve. The crossover trials had inconsistent results. The largest one, with only 16 patients, didn’t find an improvement in symptoms.
Erythromycin. Two systematic reviews looked at the efficacy of erythromycin, primarily identifying studies 20- to 30-years old. The first systematic review identified only one small (single-blind) RCT in which erythromycin treatment didn’t change symptoms.1 A second review identified 3 trials described as “open label,” all with fewer than 14 subjects and all lasting a month or less.2 Erythromycin improved patient symptoms in only 1 of the 3, and this trial (like the others) had significant methodologic flaws. The authors of the second review concluded that “the true efficacy of erythromycin in relieving symptoms … remains to be determined.”
Domperidone. A systematic review and one subsequent RCT evaluated domperidone. The systematic review identified 11 randomized, placebo-controlled trials (469 patients).3 Six studies found no impact on patient symptoms, while 5 reported a positive effect. The review also identified 6 trials that evaluated domperidone treatment on hospitalization rates. Open-label (single-arm, unblinded) trials tended to find a reduction in hospitalizations with domperidone, an effect not seen in the one double-arm study that evaluated this outcome.
The review authors noted that given the small size and low methodologic quality of most studies “it is not surprising … that there continues to be controversy about the efficacy of this drug” for symptoms of gastroparesis.
One subsequent RCT, using domperidone 20 mg 4 times daily for 4 weeks, found a 2% improvement over placebo in the physical component of a multifaceted quality-of-life measure.4 The improvement was statistically significant, but of unclear clinical importance.
Cisapride. One systematic review and 2 subsequent RCTs evaluated the clinical effects of cisapride. The systematic review included 4 small RCTs (53 patients) that didn’t individually find changes in patient symptoms.
In one subsequent RCT, comparing 10 mg cisapride 3 times daily to placebo for 2 weeks, cisapride yielded no significant change in symptoms.5 The other RCT compared oral cisapride 10 mg 3 times daily to placebo for one year. Cisapride treatment produced a 17% reduction in symptoms (P<.002 vs baseline), and placebo produced a 5% reduction (P=NS vs baseline). The study didn’t state if the difference between the 2 outcomes was statistically significant.6
Continue to: Levosulpiride
Levosulpiride. One crossover study compared 25 mg levosulpiride with 10 mg cisapride (both given orally 3 times a day) on gastroparesis symptoms and gastric emptying. Each medication was given for one month (washout duration not given). The study found similar efficacy between levosulpiride and cisapride in terms of improvement in gastric emptying rates and total symptom scores.7 No studies compare levosulpiride to placebo.
Nortriptyline. A multicenter, parallel-group, double-blind RCT comparing 75 mg/d nortriptyline for 15 weeks with placebo in adult patients with moderate to severe symptoms of idiopathic gastroparesis for at least 6 months found that nortriptyline didn’t improve symptoms.8
Botulinum toxin A. An RCT comparing a single injection of 200 units intrapyloric botulinum toxin A with placebo in adult patients with severe gastroparesis symptoms found that botulinum toxin A didn’t result in symptomatic improvement.9 A crossover trial comparing 100 units monthly intrapyloric botulinum toxin A for 3 months with placebo in patients with gastroparesis found that neither symptoms nor rate of gastric emptying changed with the toxin.10
RECOMMENDATIONS
The 2013 guidelines from the American College of Gastroenterology list metoclopramide as the first-line agent for gastroparesis requiring medical therapy, followed by domperidone and then erythromycin (all based on “moderate quality evidence”). Antiemetic agents are also recommended for symptom control.11
1. Sturm A, Holtmann G, Goebell H, et al. Prokinetics in patients with gastroparesis: a systematic analysis. Digestion. 1999;60:422-427.
2. Maganti K, Onyemere K, Jones MP. Oral erythromycin and symptomatic relief of gastroparesis: a systematic review. Am J Gastroenterol. 2003;98:259-263.
3. Sugumar A, Singh A, Pasricha PJ. A systematic review of the efficacy of domperidone for the treatment of diabetic gastroparesis. Clin Gastroenterol Hepatol. 2008;6:726-733.
4. Farup CE, Leidy NK, Murray M, et al. Effect of domperidone on the health-related quality of life of patients with symptoms of diabetic gastroparesis. Diabetes Care. 1998;21:1699-1706.
5. Dutta U, Padhy AK, Ahuja V, et al. Double blind controlled trial of effect of cisapride on gastric emptying in diabetics. Trop Gastroenterol. 1999;20:116-119.
6. Braden B, Enghofer M, Schaub M, et al. Long-term cisapride treatment improves diabetic gastroparesis but not glycaemic control. Aliment Pharmacol Ther. 2002;16:1341-1346.
7. Mansi C, Borro P, Giacomini M, et al. Comparative effects of levosulpiride and cisapride on gastric emptying and symptoms in patients with functional dyspepsia and gastroparesis. Aliment Pharmacol Ther. 2000;14:561-569.
8. Parkman HP, Van Natta ML, Abell TL, et al. Effect of nortriptyline on symptoms of idiopathic gastroparesis: the NORIG randomized clinical trial. JAMA. 2013;310:2640-2649.
9. Friedenberg FK, Palit A, Parkman HP, et al. Botulinum toxin A for the treatment of delayed gastric emptying. Am J Gastroenterol. 2008;103:416-423.
10. Arts J, Holvoet L, Caenepeel P, et al. Clinical trial: a randomized-controlled crossover study of intrapyloric injection of botulinum toxin in gastroparesis. Aliment Pharmacol Ther. 2007;26:1251-1258.
11. Camilleri M, Parkman HP, Shafi MA, et al. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18-38.

EVIDENCE SUMMARY
Metoclopramide. One systematic review that looked at the efficacy of metoclopramide for gastroparesis identified one small RCT and 3 smaller placebo-controlled crossover trials.1 The RCT (using 10 mg of metoclopramide after meals and at bedtime) found consistent improvement in the sense of fullness over 3 weeks of therapy, with reduction of nausea at one and 3 weeks, but not at 2 weeks. Vomiting, anorexia, and early satiety didn’t improve. The crossover trials had inconsistent results. The largest one, with only 16 patients, didn’t find an improvement in symptoms.
Erythromycin. Two systematic reviews looked at the efficacy of erythromycin, primarily identifying studies 20- to 30-years old. The first systematic review identified only one small (single-blind) RCT in which erythromycin treatment didn’t change symptoms.1 A second review identified 3 trials described as “open label,” all with fewer than 14 subjects and all lasting a month or less.2 Erythromycin improved patient symptoms in only 1 of the 3, and this trial (like the others) had significant methodologic flaws. The authors of the second review concluded that “the true efficacy of erythromycin in relieving symptoms … remains to be determined.”
Domperidone. A systematic review and one subsequent RCT evaluated domperidone. The systematic review identified 11 randomized, placebo-controlled trials (469 patients).3 Six studies found no impact on patient symptoms, while 5 reported a positive effect. The review also identified 6 trials that evaluated domperidone treatment on hospitalization rates. Open-label (single-arm, unblinded) trials tended to find a reduction in hospitalizations with domperidone, an effect not seen in the one double-arm study that evaluated this outcome.
The review authors noted that given the small size and low methodologic quality of most studies “it is not surprising … that there continues to be controversy about the efficacy of this drug” for symptoms of gastroparesis.
One subsequent RCT, using domperidone 20 mg 4 times daily for 4 weeks, found a 2% improvement over placebo in the physical component of a multifaceted quality-of-life measure.4 The improvement was statistically significant, but of unclear clinical importance.
Cisapride. One systematic review and 2 subsequent RCTs evaluated the clinical effects of cisapride. The systematic review included 4 small RCTs (53 patients) that didn’t individually find changes in patient symptoms.
In one subsequent RCT, comparing 10 mg cisapride 3 times daily to placebo for 2 weeks, cisapride yielded no significant change in symptoms.5 The other RCT compared oral cisapride 10 mg 3 times daily to placebo for one year. Cisapride treatment produced a 17% reduction in symptoms (P<.002 vs baseline), and placebo produced a 5% reduction (P=NS vs baseline). The study didn’t state if the difference between the 2 outcomes was statistically significant.6
Continue to: Levosulpiride
Levosulpiride. One crossover study compared 25 mg levosulpiride with 10 mg cisapride (both given orally 3 times a day) on gastroparesis symptoms and gastric emptying. Each medication was given for one month (washout duration not given). The study found similar efficacy between levosulpiride and cisapride in terms of improvement in gastric emptying rates and total symptom scores.7 No studies compare levosulpiride to placebo.
Nortriptyline. A multicenter, parallel-group, double-blind RCT comparing 75 mg/d nortriptyline for 15 weeks with placebo in adult patients with moderate to severe symptoms of idiopathic gastroparesis for at least 6 months found that nortriptyline didn’t improve symptoms.8
Botulinum toxin A. An RCT comparing a single injection of 200 units intrapyloric botulinum toxin A with placebo in adult patients with severe gastroparesis symptoms found that botulinum toxin A didn’t result in symptomatic improvement.9 A crossover trial comparing 100 units monthly intrapyloric botulinum toxin A for 3 months with placebo in patients with gastroparesis found that neither symptoms nor rate of gastric emptying changed with the toxin.10
RECOMMENDATIONS
The 2013 guidelines from the American College of Gastroenterology list metoclopramide as the first-line agent for gastroparesis requiring medical therapy, followed by domperidone and then erythromycin (all based on “moderate quality evidence”). Antiemetic agents are also recommended for symptom control.11

EVIDENCE SUMMARY
Metoclopramide. One systematic review that looked at the efficacy of metoclopramide for gastroparesis identified one small RCT and 3 smaller placebo-controlled crossover trials.1 The RCT (using 10 mg of metoclopramide after meals and at bedtime) found consistent improvement in the sense of fullness over 3 weeks of therapy, with reduction of nausea at one and 3 weeks, but not at 2 weeks. Vomiting, anorexia, and early satiety didn’t improve. The crossover trials had inconsistent results. The largest one, with only 16 patients, didn’t find an improvement in symptoms.
Erythromycin. Two systematic reviews looked at the efficacy of erythromycin, primarily identifying studies 20- to 30-years old. The first systematic review identified only one small (single-blind) RCT in which erythromycin treatment didn’t change symptoms.1 A second review identified 3 trials described as “open label,” all with fewer than 14 subjects and all lasting a month or less.2 Erythromycin improved patient symptoms in only 1 of the 3, and this trial (like the others) had significant methodologic flaws. The authors of the second review concluded that “the true efficacy of erythromycin in relieving symptoms … remains to be determined.”
Domperidone. A systematic review and one subsequent RCT evaluated domperidone. The systematic review identified 11 randomized, placebo-controlled trials (469 patients).3 Six studies found no impact on patient symptoms, while 5 reported a positive effect. The review also identified 6 trials that evaluated domperidone treatment on hospitalization rates. Open-label (single-arm, unblinded) trials tended to find a reduction in hospitalizations with domperidone, an effect not seen in the one double-arm study that evaluated this outcome.
The review authors noted that given the small size and low methodologic quality of most studies “it is not surprising … that there continues to be controversy about the efficacy of this drug” for symptoms of gastroparesis.
One subsequent RCT, using domperidone 20 mg 4 times daily for 4 weeks, found a 2% improvement over placebo in the physical component of a multifaceted quality-of-life measure.4 The improvement was statistically significant, but of unclear clinical importance.
Cisapride. One systematic review and 2 subsequent RCTs evaluated the clinical effects of cisapride. The systematic review included 4 small RCTs (53 patients) that didn’t individually find changes in patient symptoms.
In one subsequent RCT, comparing 10 mg cisapride 3 times daily to placebo for 2 weeks, cisapride yielded no significant change in symptoms.5 The other RCT compared oral cisapride 10 mg 3 times daily to placebo for one year. Cisapride treatment produced a 17% reduction in symptoms (P<.002 vs baseline), and placebo produced a 5% reduction (P=NS vs baseline). The study didn’t state if the difference between the 2 outcomes was statistically significant.6
Continue to: Levosulpiride
Levosulpiride. One crossover study compared 25 mg levosulpiride with 10 mg cisapride (both given orally 3 times a day) on gastroparesis symptoms and gastric emptying. Each medication was given for one month (washout duration not given). The study found similar efficacy between levosulpiride and cisapride in terms of improvement in gastric emptying rates and total symptom scores.7 No studies compare levosulpiride to placebo.
Nortriptyline. A multicenter, parallel-group, double-blind RCT comparing 75 mg/d nortriptyline for 15 weeks with placebo in adult patients with moderate to severe symptoms of idiopathic gastroparesis for at least 6 months found that nortriptyline didn’t improve symptoms.8
Botulinum toxin A. An RCT comparing a single injection of 200 units intrapyloric botulinum toxin A with placebo in adult patients with severe gastroparesis symptoms found that botulinum toxin A didn’t result in symptomatic improvement.9 A crossover trial comparing 100 units monthly intrapyloric botulinum toxin A for 3 months with placebo in patients with gastroparesis found that neither symptoms nor rate of gastric emptying changed with the toxin.10
RECOMMENDATIONS
The 2013 guidelines from the American College of Gastroenterology list metoclopramide as the first-line agent for gastroparesis requiring medical therapy, followed by domperidone and then erythromycin (all based on “moderate quality evidence”). Antiemetic agents are also recommended for symptom control.11
1. Sturm A, Holtmann G, Goebell H, et al. Prokinetics in patients with gastroparesis: a systematic analysis. Digestion. 1999;60:422-427.
2. Maganti K, Onyemere K, Jones MP. Oral erythromycin and symptomatic relief of gastroparesis: a systematic review. Am J Gastroenterol. 2003;98:259-263.
3. Sugumar A, Singh A, Pasricha PJ. A systematic review of the efficacy of domperidone for the treatment of diabetic gastroparesis. Clin Gastroenterol Hepatol. 2008;6:726-733.
4. Farup CE, Leidy NK, Murray M, et al. Effect of domperidone on the health-related quality of life of patients with symptoms of diabetic gastroparesis. Diabetes Care. 1998;21:1699-1706.
5. Dutta U, Padhy AK, Ahuja V, et al. Double blind controlled trial of effect of cisapride on gastric emptying in diabetics. Trop Gastroenterol. 1999;20:116-119.
6. Braden B, Enghofer M, Schaub M, et al. Long-term cisapride treatment improves diabetic gastroparesis but not glycaemic control. Aliment Pharmacol Ther. 2002;16:1341-1346.
7. Mansi C, Borro P, Giacomini M, et al. Comparative effects of levosulpiride and cisapride on gastric emptying and symptoms in patients with functional dyspepsia and gastroparesis. Aliment Pharmacol Ther. 2000;14:561-569.
8. Parkman HP, Van Natta ML, Abell TL, et al. Effect of nortriptyline on symptoms of idiopathic gastroparesis: the NORIG randomized clinical trial. JAMA. 2013;310:2640-2649.
9. Friedenberg FK, Palit A, Parkman HP, et al. Botulinum toxin A for the treatment of delayed gastric emptying. Am J Gastroenterol. 2008;103:416-423.
10. Arts J, Holvoet L, Caenepeel P, et al. Clinical trial: a randomized-controlled crossover study of intrapyloric injection of botulinum toxin in gastroparesis. Aliment Pharmacol Ther. 2007;26:1251-1258.
11. Camilleri M, Parkman HP, Shafi MA, et al. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18-38.
1. Sturm A, Holtmann G, Goebell H, et al. Prokinetics in patients with gastroparesis: a systematic analysis. Digestion. 1999;60:422-427.
2. Maganti K, Onyemere K, Jones MP. Oral erythromycin and symptomatic relief of gastroparesis: a systematic review. Am J Gastroenterol. 2003;98:259-263.
3. Sugumar A, Singh A, Pasricha PJ. A systematic review of the efficacy of domperidone for the treatment of diabetic gastroparesis. Clin Gastroenterol Hepatol. 2008;6:726-733.
4. Farup CE, Leidy NK, Murray M, et al. Effect of domperidone on the health-related quality of life of patients with symptoms of diabetic gastroparesis. Diabetes Care. 1998;21:1699-1706.
5. Dutta U, Padhy AK, Ahuja V, et al. Double blind controlled trial of effect of cisapride on gastric emptying in diabetics. Trop Gastroenterol. 1999;20:116-119.
6. Braden B, Enghofer M, Schaub M, et al. Long-term cisapride treatment improves diabetic gastroparesis but not glycaemic control. Aliment Pharmacol Ther. 2002;16:1341-1346.
7. Mansi C, Borro P, Giacomini M, et al. Comparative effects of levosulpiride and cisapride on gastric emptying and symptoms in patients with functional dyspepsia and gastroparesis. Aliment Pharmacol Ther. 2000;14:561-569.
8. Parkman HP, Van Natta ML, Abell TL, et al. Effect of nortriptyline on symptoms of idiopathic gastroparesis: the NORIG randomized clinical trial. JAMA. 2013;310:2640-2649.
9. Friedenberg FK, Palit A, Parkman HP, et al. Botulinum toxin A for the treatment of delayed gastric emptying. Am J Gastroenterol. 2008;103:416-423.
10. Arts J, Holvoet L, Caenepeel P, et al. Clinical trial: a randomized-controlled crossover study of intrapyloric injection of botulinum toxin in gastroparesis. Aliment Pharmacol Ther. 2007;26:1251-1258.
11. Camilleri M, Parkman HP, Shafi MA, et al. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18-38.
EVIDENCE-BASED ANSWER:
It’s unclear if there are any highly effective medications for gastroparesis (TABLE1-10). Metoclopramide improves the sense of fullness by about 40% for as long as 3 weeks, may improve nausea, and doesn’t affect vomiting or anorexia (strength of recommendation [SOR]: B, small randomized controlled trial [RCT]).
Whether or not erythromycin has an effect on symptoms is unclear (SOR: C, conflicting trials and expert opinion).
Domperidone may improve quality of life (by 2%) for as long as a year, but its effect on symptoms is also unclear (SOR: C, small RCTs).
Cisapride may not be effective for symptom relief (SOR: C, small conflicting RCTs), and levosulpiride is likely similar to cisapride (SOR: C, single small crossover trial).
Nortriptyline (SOR: B, single RCT) and intrapyloric botulinum toxin A (SOR: B, small RCT and crossover trial) aren’t effective for symptom relief.
Progressive discoloration over the right shoulder
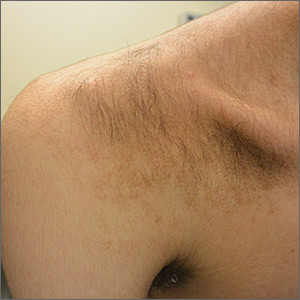
A 15-year-old Caucasian boy presented for evaluation of an asymptomatic brown patch on his right shoulder. While the patient’s mother first noticed the patch when he was 5 years old, the discolored area had recently been expanding in size and had developed hypertrichosis. The patient was otherwise healthy; he took no medications and denied any symptoms or history of trauma to the area. None of his siblings were similarly affected.
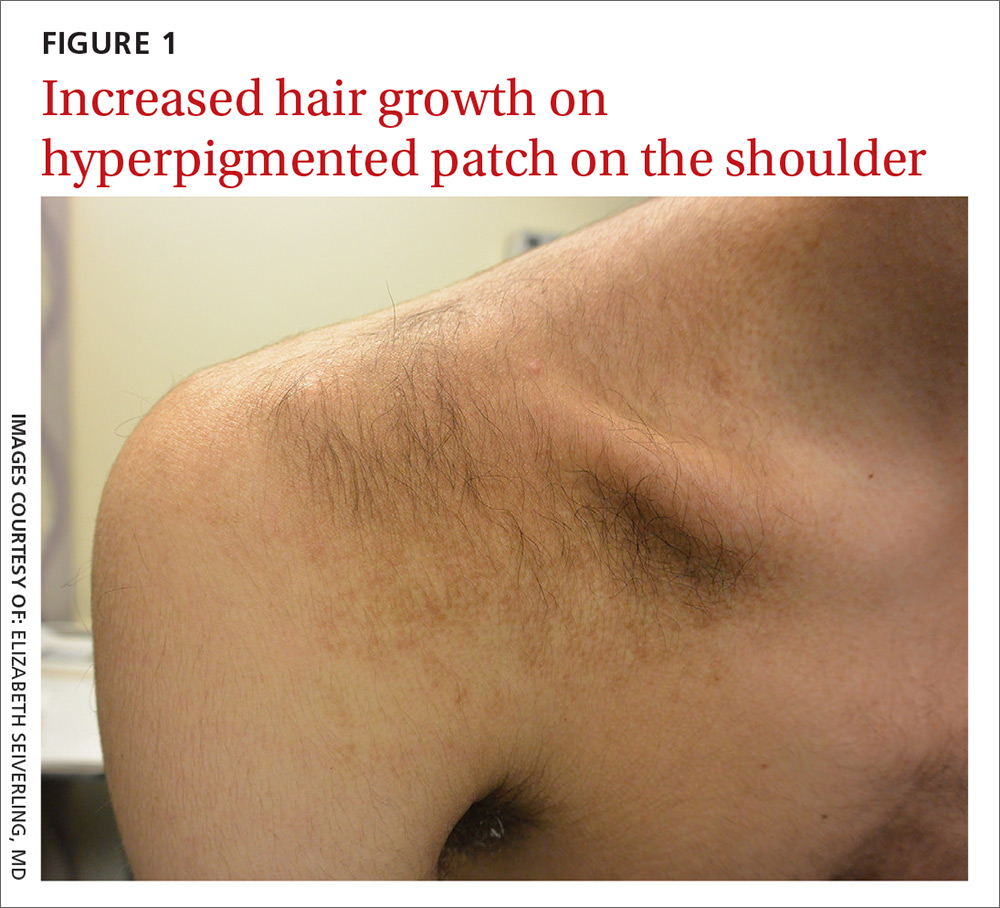
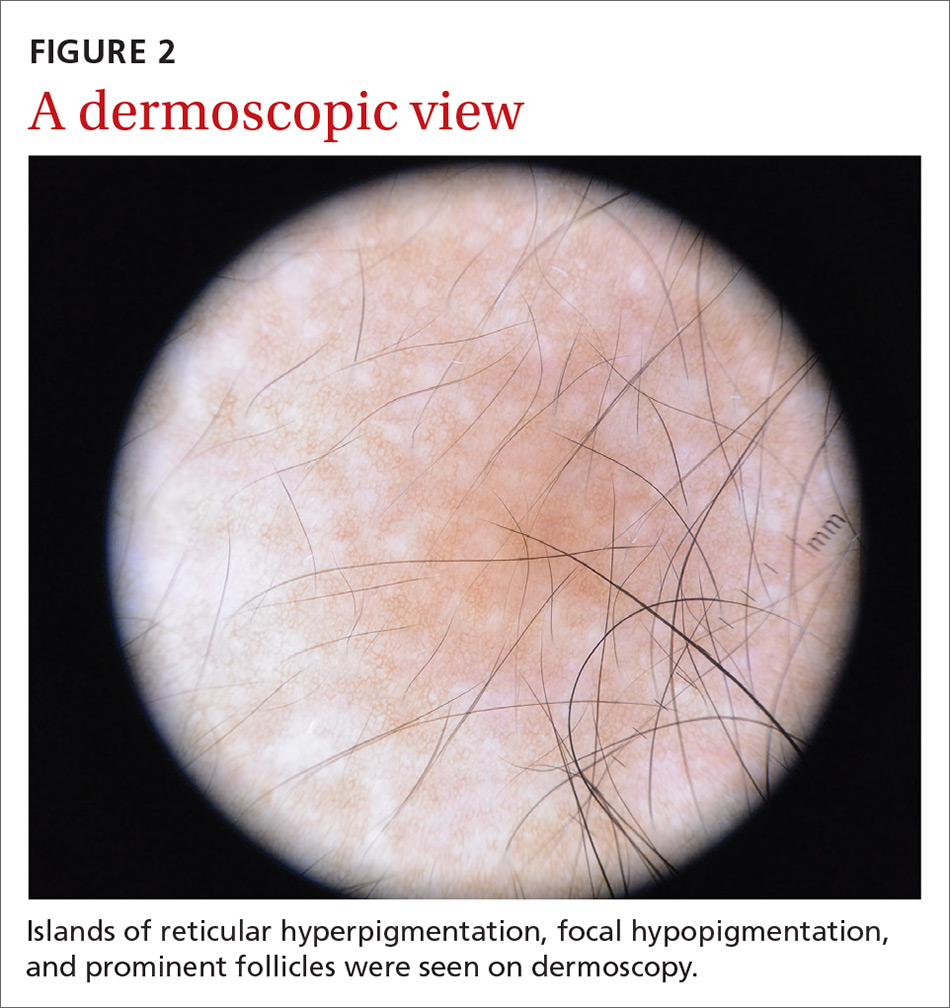
A physical examination revealed a well-demarcated hyperpigmented patch with an irregularly shaped border and an increased number of terminal hairs (FIGURE 1). The affected area was not indurated, and there were no muscular or skeletal abnormalities on inspection. Examination of the patch under a dermatoscope revealed islands of reticular (lattice-like) hyperpigmentation, focal hypopigmentation, and prominent follicles (FIGURE 2).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
DIAGNOSIS: Becker melanosis
Becker melanosis (also called Becker’s nevus or Becker’s pigmentary hamartoma) is an organoid hamartoma that is most common among males.1 This benign area of hyperpigmentation typically manifests as a circumscribed patch with an irregular border on the upper trunk, shoulders, or upper arms of young men. Becker melanosis is usually acquired and typically comes to medical attention around the time of puberty, although there may be a history of discoloration (as was true in this case).
A diagnosis that’s usually made clinically
Androgenic origin. Because of the male predominance and association with hypertrichosis (and for that matter, acne), androgens have been thought to play a role in the development of Becker melanosis.2 The condition affects about 1 in 200 young men.1 To date, no specific gene defect has been identified.
Underlying hypoplasia of the breast or musculoskeletal abnormalities are uncommonly associated with Becker melanosis. When these abnormalities are present, the condition is known as Becker’s nevus syndrome.3
Look for the pattern. Becker melanosis is associated with homogenous brown patches with perifollicular hypopigmentation, sometimes with a faint reticular pattern.4,5 The diagnosis can usually be made clinically, but a skin biopsy can be helpful to confirm questionable cases. Dermoscopy can also assist in diagnosis. In this case, our patient’s presentation was typical, and additional studies were not needed.
Other causes of hyperpigmentation
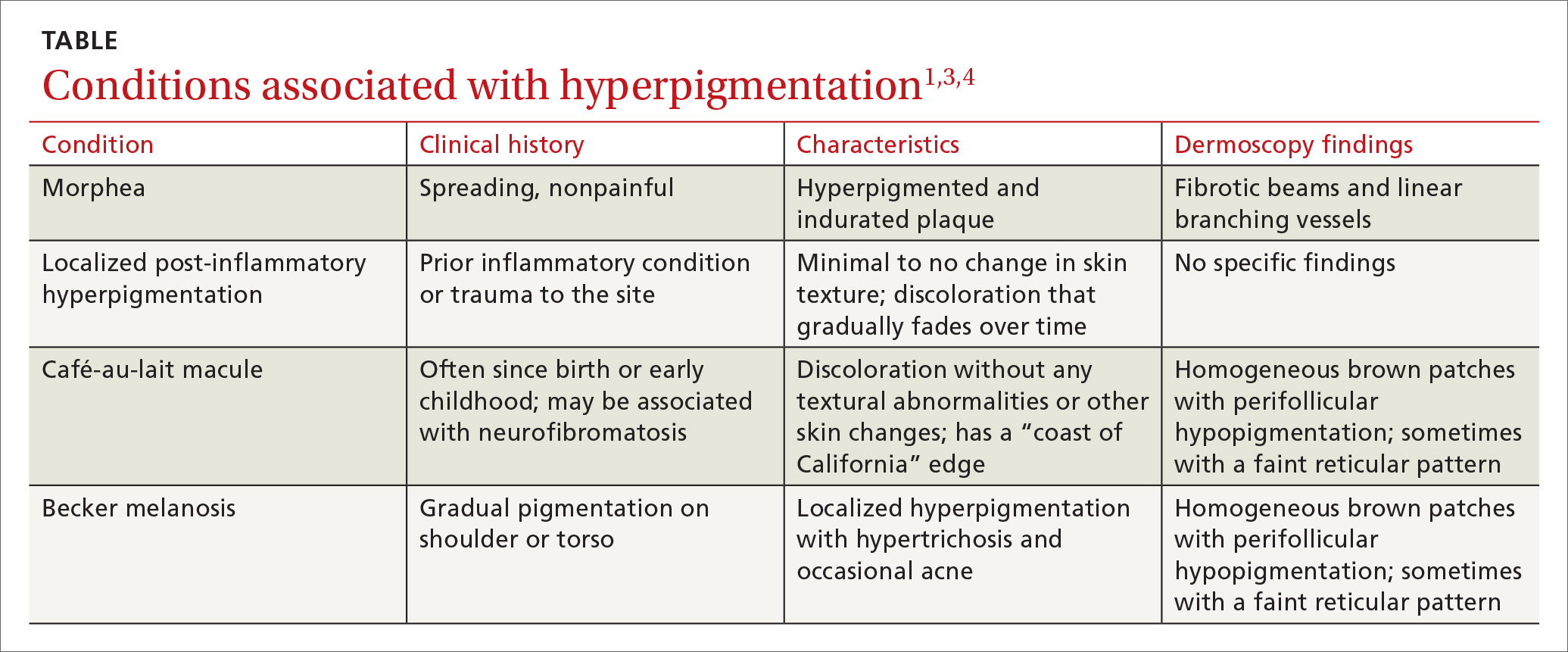
The differential diagnosis includes other localized disorders associated with hyperpigmentation (TABLE1,3,4).
Continue to: Morphea
Morphea represents a thickening of collagen bundles in the skin. Although morphea can affect the shoulder and trunk, as Becker melanosis does, lesions of morphea feel firm to the touch and are not associated with hypertrichosis.
Localized post-inflammatory hyperpigmentation occurs following a traumatic event, such as a burn, or a prior dermatosis, such as zoster. Careful history-taking can uncover an antecedent inflammatory condition. Post-inflammatory pigment changes do not typically result in hypertrichosis.
Café-au-lait macules can manifest as isolated areas of discoloration. These macules can be an important indicator of neurofibromatosis, a genetic disorder in which tumors grow in the nervous system. Melanocytic hamartomas of the iris (Lisch nodules), axillary freckling (Crowe’s sign), or multiple cutaneous neurofibromas serve as additional clues to neurofibromatosis. In ambiguous cases, a skin biopsy can help differentiate a café au lait macule from Becker melanosis.
To treat or not to treat?
No treatment other than reassurance is needed in most cases of Becker melanosis, as it is a benign condition. Protecting the area from sunlight can minimize darkening and contrast with the surrounding skin. Electrolysis and laser therapy can be used to treat the associated hypertrichosis; laser therapy can also reduce the hyperpigmentation. Nonablative fractional resurfacing accompanied by laser hair removal is also reported to be of value.6
Our patient was satisfied with reassurance of the benign nature of the condition and did not elect treatment.
CORRESPONDENCE
Matthew F. Helm, MD, 500 University Drive, Suite 4300, Department of Dermatology, HU14, UPC II, Hershey, PA 17033-2360; [email protected]
1. Rabinovitz HS, Barnhill RL. Benign melanocytic neoplasms. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. New York, NY: Elsevier Saunders; 2012;112:1853-1854.
2. Person JR, Longcope C. Becker’s nevus: an androgen-mediated hyperplasia with increased androgen receptors. J Am Acad Dermatol. 1984;10:235-238.
3. Cosendey FE, Martinez NS, Bernhard GA, et al. Becker nevus syndrome. An Bras Dermatol. 2010;85:379-384.
4. Ingordo V, Iannazzone SS, Cusano F, et al. Dermoscopic features of congenital melanocytic nevus and Becker nevus in an adult male population: an analysis with 10-fold magnification. Dermatology. 2006;212:354-360.
5. Luk DC, Lam SY, Cheung PC, et al. Dermoscopy for common skin problems in Chinese children using a novel Hong Kong-made dermoscope. Hong Kong Med J. 2014;20:495-503.
6. Balaraman B, Friedman PM. Hypertrichotic Becker’s nevi treated with combination 1,550nm non-ablative fractional photothermolysis and laser hair removal. Lasers Surg Med. 2016;48:350-353.
A 15-year-old Caucasian boy presented for evaluation of an asymptomatic brown patch on his right shoulder. While the patient’s mother first noticed the patch when he was 5 years old, the discolored area had recently been expanding in size and had developed hypertrichosis. The patient was otherwise healthy; he took no medications and denied any symptoms or history of trauma to the area. None of his siblings were similarly affected.

A physical examination revealed a well-demarcated hyperpigmented patch with an irregularly shaped border and an increased number of terminal hairs (FIGURE 1). The affected area was not indurated, and there were no muscular or skeletal abnormalities on inspection. Examination of the patch under a dermatoscope revealed islands of reticular (lattice-like) hyperpigmentation, focal hypopigmentation, and prominent follicles (FIGURE 2).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
DIAGNOSIS: Becker melanosis
Becker melanosis (also called Becker’s nevus or Becker’s pigmentary hamartoma) is an organoid hamartoma that is most common among males.1 This benign area of hyperpigmentation typically manifests as a circumscribed patch with an irregular border on the upper trunk, shoulders, or upper arms of young men. Becker melanosis is usually acquired and typically comes to medical attention around the time of puberty, although there may be a history of discoloration (as was true in this case).
A diagnosis that’s usually made clinically
Androgenic origin. Because of the male predominance and association with hypertrichosis (and for that matter, acne), androgens have been thought to play a role in the development of Becker melanosis.2 The condition affects about 1 in 200 young men.1 To date, no specific gene defect has been identified.
Underlying hypoplasia of the breast or musculoskeletal abnormalities are uncommonly associated with Becker melanosis. When these abnormalities are present, the condition is known as Becker’s nevus syndrome.3
Look for the pattern. Becker melanosis is associated with homogenous brown patches with perifollicular hypopigmentation, sometimes with a faint reticular pattern.4,5 The diagnosis can usually be made clinically, but a skin biopsy can be helpful to confirm questionable cases. Dermoscopy can also assist in diagnosis. In this case, our patient’s presentation was typical, and additional studies were not needed.
Other causes of hyperpigmentation
The differential diagnosis includes other localized disorders associated with hyperpigmentation (TABLE1,3,4).

Continue to: Morphea
Morphea represents a thickening of collagen bundles in the skin. Although morphea can affect the shoulder and trunk, as Becker melanosis does, lesions of morphea feel firm to the touch and are not associated with hypertrichosis.
Localized post-inflammatory hyperpigmentation occurs following a traumatic event, such as a burn, or a prior dermatosis, such as zoster. Careful history-taking can uncover an antecedent inflammatory condition. Post-inflammatory pigment changes do not typically result in hypertrichosis.
Café-au-lait macules can manifest as isolated areas of discoloration. These macules can be an important indicator of neurofibromatosis, a genetic disorder in which tumors grow in the nervous system. Melanocytic hamartomas of the iris (Lisch nodules), axillary freckling (Crowe’s sign), or multiple cutaneous neurofibromas serve as additional clues to neurofibromatosis. In ambiguous cases, a skin biopsy can help differentiate a café au lait macule from Becker melanosis.
To treat or not to treat?
No treatment other than reassurance is needed in most cases of Becker melanosis, as it is a benign condition. Protecting the area from sunlight can minimize darkening and contrast with the surrounding skin. Electrolysis and laser therapy can be used to treat the associated hypertrichosis; laser therapy can also reduce the hyperpigmentation. Nonablative fractional resurfacing accompanied by laser hair removal is also reported to be of value.6
Our patient was satisfied with reassurance of the benign nature of the condition and did not elect treatment.
CORRESPONDENCE
Matthew F. Helm, MD, 500 University Drive, Suite 4300, Department of Dermatology, HU14, UPC II, Hershey, PA 17033-2360; [email protected]
A 15-year-old Caucasian boy presented for evaluation of an asymptomatic brown patch on his right shoulder. While the patient’s mother first noticed the patch when he was 5 years old, the discolored area had recently been expanding in size and had developed hypertrichosis. The patient was otherwise healthy; he took no medications and denied any symptoms or history of trauma to the area. None of his siblings were similarly affected.

A physical examination revealed a well-demarcated hyperpigmented patch with an irregularly shaped border and an increased number of terminal hairs (FIGURE 1). The affected area was not indurated, and there were no muscular or skeletal abnormalities on inspection. Examination of the patch under a dermatoscope revealed islands of reticular (lattice-like) hyperpigmentation, focal hypopigmentation, and prominent follicles (FIGURE 2).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
DIAGNOSIS: Becker melanosis
Becker melanosis (also called Becker’s nevus or Becker’s pigmentary hamartoma) is an organoid hamartoma that is most common among males.1 This benign area of hyperpigmentation typically manifests as a circumscribed patch with an irregular border on the upper trunk, shoulders, or upper arms of young men. Becker melanosis is usually acquired and typically comes to medical attention around the time of puberty, although there may be a history of discoloration (as was true in this case).
A diagnosis that’s usually made clinically
Androgenic origin. Because of the male predominance and association with hypertrichosis (and for that matter, acne), androgens have been thought to play a role in the development of Becker melanosis.2 The condition affects about 1 in 200 young men.1 To date, no specific gene defect has been identified.
Underlying hypoplasia of the breast or musculoskeletal abnormalities are uncommonly associated with Becker melanosis. When these abnormalities are present, the condition is known as Becker’s nevus syndrome.3
Look for the pattern. Becker melanosis is associated with homogenous brown patches with perifollicular hypopigmentation, sometimes with a faint reticular pattern.4,5 The diagnosis can usually be made clinically, but a skin biopsy can be helpful to confirm questionable cases. Dermoscopy can also assist in diagnosis. In this case, our patient’s presentation was typical, and additional studies were not needed.
Other causes of hyperpigmentation
The differential diagnosis includes other localized disorders associated with hyperpigmentation (TABLE1,3,4).

Continue to: Morphea
Morphea represents a thickening of collagen bundles in the skin. Although morphea can affect the shoulder and trunk, as Becker melanosis does, lesions of morphea feel firm to the touch and are not associated with hypertrichosis.
Localized post-inflammatory hyperpigmentation occurs following a traumatic event, such as a burn, or a prior dermatosis, such as zoster. Careful history-taking can uncover an antecedent inflammatory condition. Post-inflammatory pigment changes do not typically result in hypertrichosis.
Café-au-lait macules can manifest as isolated areas of discoloration. These macules can be an important indicator of neurofibromatosis, a genetic disorder in which tumors grow in the nervous system. Melanocytic hamartomas of the iris (Lisch nodules), axillary freckling (Crowe’s sign), or multiple cutaneous neurofibromas serve as additional clues to neurofibromatosis. In ambiguous cases, a skin biopsy can help differentiate a café au lait macule from Becker melanosis.
To treat or not to treat?
No treatment other than reassurance is needed in most cases of Becker melanosis, as it is a benign condition. Protecting the area from sunlight can minimize darkening and contrast with the surrounding skin. Electrolysis and laser therapy can be used to treat the associated hypertrichosis; laser therapy can also reduce the hyperpigmentation. Nonablative fractional resurfacing accompanied by laser hair removal is also reported to be of value.6
Our patient was satisfied with reassurance of the benign nature of the condition and did not elect treatment.
CORRESPONDENCE
Matthew F. Helm, MD, 500 University Drive, Suite 4300, Department of Dermatology, HU14, UPC II, Hershey, PA 17033-2360; [email protected]
1. Rabinovitz HS, Barnhill RL. Benign melanocytic neoplasms. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. New York, NY: Elsevier Saunders; 2012;112:1853-1854.
2. Person JR, Longcope C. Becker’s nevus: an androgen-mediated hyperplasia with increased androgen receptors. J Am Acad Dermatol. 1984;10:235-238.
3. Cosendey FE, Martinez NS, Bernhard GA, et al. Becker nevus syndrome. An Bras Dermatol. 2010;85:379-384.
4. Ingordo V, Iannazzone SS, Cusano F, et al. Dermoscopic features of congenital melanocytic nevus and Becker nevus in an adult male population: an analysis with 10-fold magnification. Dermatology. 2006;212:354-360.
5. Luk DC, Lam SY, Cheung PC, et al. Dermoscopy for common skin problems in Chinese children using a novel Hong Kong-made dermoscope. Hong Kong Med J. 2014;20:495-503.
6. Balaraman B, Friedman PM. Hypertrichotic Becker’s nevi treated with combination 1,550nm non-ablative fractional photothermolysis and laser hair removal. Lasers Surg Med. 2016;48:350-353.
1. Rabinovitz HS, Barnhill RL. Benign melanocytic neoplasms. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. New York, NY: Elsevier Saunders; 2012;112:1853-1854.
2. Person JR, Longcope C. Becker’s nevus: an androgen-mediated hyperplasia with increased androgen receptors. J Am Acad Dermatol. 1984;10:235-238.
3. Cosendey FE, Martinez NS, Bernhard GA, et al. Becker nevus syndrome. An Bras Dermatol. 2010;85:379-384.
4. Ingordo V, Iannazzone SS, Cusano F, et al. Dermoscopic features of congenital melanocytic nevus and Becker nevus in an adult male population: an analysis with 10-fold magnification. Dermatology. 2006;212:354-360.
5. Luk DC, Lam SY, Cheung PC, et al. Dermoscopy for common skin problems in Chinese children using a novel Hong Kong-made dermoscope. Hong Kong Med J. 2014;20:495-503.
6. Balaraman B, Friedman PM. Hypertrichotic Becker’s nevi treated with combination 1,550nm non-ablative fractional photothermolysis and laser hair removal. Lasers Surg Med. 2016;48:350-353.
How could improved provider communication have improved the care this patient received?
THE CASE
A 40-year-old white woman presented to clinic with multiple pruritic skin lesions on her abdomen, arms, and legs that had developed over a 2-month period. The patient reported that she’d been feeling tired and had been experiencing psychological stressors in her personal life. Her medical history was significant for psoriasis (which was controlled), and her family history was significant for breast and bone cancer (mother) and asbestos-related lung cancer (maternal grandfather).
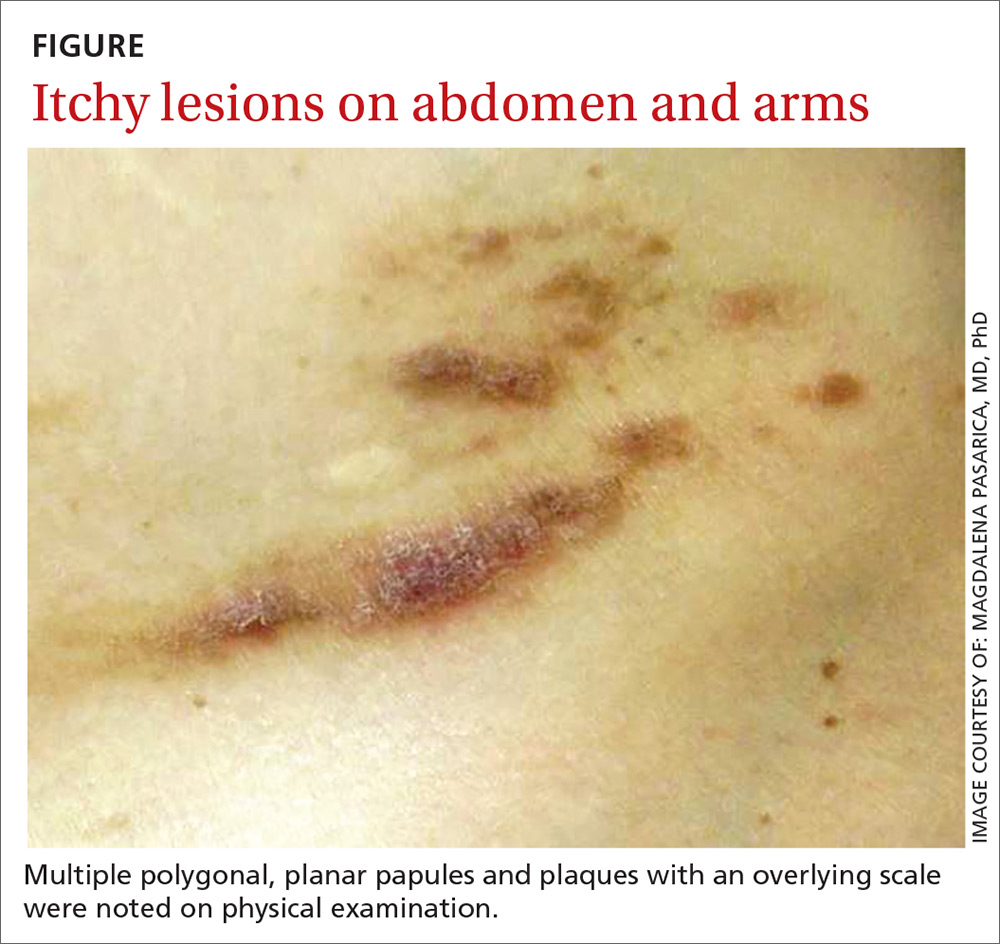
A physical examination, which included breast and pelvic exams, was unremarkable apart from the lesions located on her abdomen, arms, and legs. On skin examination, we noted multiple polygonal, planar papules and plaques of varying size with an overlying scale (FIGURE).
THE DIAGNOSIS
The physician obtained a biopsy of one of the skin lesions, and it was sent to a dermatopathologist to evaluate. Unfortunately, though, the patient’s history and a description of the lesion were not included with the initial biopsy requisition form. Based on the biopsy sample alone, the dermatopathologist’s report indicated a diagnosis of seborrheic keratosis.
A search for malignancy. Any case of sudden, extensive seborrheic keratosis is suspected to be a Leser-Trélat sign, which is known to be associated with human immunodeficiency virus or underlying malignancy—especially in the gastrointestinal system. The physician talked to the patient about the possibility of malignancy, and an extensive work-up was performed, including multiple laboratory tests, computed tomography (CT) imaging, an esophagogastroduodenoscopy, a colonoscopy, and mammography. None of the test results showed signs of an underlying malignancy.
In light of the negative findings, the physician reached out to the dermatopathologist to further discuss the case. It was determined that the dermatopathologist did not receive any clinical information (prior to this discussion) from the primary care office. This was surprising to the primary care physician, who was under the assumption that the clinical chart would be sent along with the biopsy sample. With this new information, the dermatopathologist reexamined the slides and diagnosed the lesion as lichen planus, a rather common skin disease not associated with cancer.
[polldaddy:10153197]
DISCUSSION
A root-cause analysis of this case identified multiple system failures, focused mainly on a lack of communication between providers:
- The description of the lesion and of the patient’s history were not included with the initial biopsy requisition form due to a lack of communication between the nurse and the physician performing the procedure.
- The dermatopathologist did not seek additional clinical information from the referring physician after receiving the sample.
- When the various providers did communicate, an accurate diagnosis was reached—but only after extensive investigation (and worry).
Communication is key to an accurate diagnosis
In 2000, it was estimated that health care costs due to preventable adverse events represent more than half of the $37.6 billion spent on health care.1 Since then, considerable effort has been made to address patient safety, misdiagnosis, and cost-effectiveness. Root cause analysis is one of the most popular methods used to evaluate and prevent future serious adverse events.2
Continue to: Diagnostic errors are often unreported...
Diagnostic errors are often unreported or unrecognized, especially in the outpatient setting.3 Studies focused on reducing diagnostic error show that a second review of pathology slides reduces error, controls costs, and improves quality of health care.4
Don’t rely (exclusively) on the health record. Gaps in effective communication between providers are a leading cause of preventable adverse events.5,6 The incorporation of electronic health records has allowed for more streamlined communication between providers. However, the mere presence of patient records in a common system does not guarantee the receipt or communication of information. The next step after entering the information into the record is to communicate it.
Our patient underwent a battery of costly and unnecessary tests and procedures, many of which were unwarranted at her age. In addition to being exposed to harmful radiation, she also experienced significant stress secondary to the tests and anticipation of the results. However, a root cause analysis of the case led to an improved protocol for communication between providers at the outpatient clinic. We now emphasize the necessity of including a clinical history and corresponding physical findings with all biopsies. We also encourage more direct communication between nursing staff, primary care physicians, and specialists.
THE TAKEAWAY
As medical professionals become increasingly reliant on the many emerging studies available to them, we sometimes forget that communication is key to optimal medical care, an accurate diagnosis, and patient safety.
Continue to: In addition, a second review...
In addition, a second review of dermatopathologic slides may be warranted if the pathologic diagnosis is inconsistent with the clinical picture or if the diagnosed condition is resistant to the usual therapies of choice. Incorrect diagnoses are more likely to occur when tests are interpreted in a vacuum without the corresponding clinical correlation. The weight of these mistakes is felt not only by the health care system, but by the patients themselves.
CORRESPONDENCE
Magdalena Pasarica, MD, PhD, University of Central Florida College of Medicine, 6850 Lake Nona Boulevard, Orlando, FL 32827; [email protected]
1. Kohn LT, Corrigan JM, Donaldson MS. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
2. U.S. Department of Health and Human Services. Patient safety primer: root cause analysis. https://psnet.ahrq.gov/primers/primer/10/root-cause-analysis. Updated August 2018. Accessed September 27, 2018.
3. Newman-Toker DE, Pronovost PJ. Diagnostic errors-the next frontier for patient safety. JAMA. 2009;301:1060-1062.
4. Kuijpers CC, Burger G, Al-Janabi S, et al. Improved quality of patient care through routine second review of histopathology specimens prior to multidisciplinary meetings. J Clin Pathol. 2016;69:866-871.
5. Leonard M, Graham S, Bonacum D. The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care. 2004;13:85-90.
6. Robinson NL. Promoting patient safety with perioperative hand-off communication. J Perianesth Nurs. 2016;31:245-253.
THE CASE
A 40-year-old white woman presented to clinic with multiple pruritic skin lesions on her abdomen, arms, and legs that had developed over a 2-month period. The patient reported that she’d been feeling tired and had been experiencing psychological stressors in her personal life. Her medical history was significant for psoriasis (which was controlled), and her family history was significant for breast and bone cancer (mother) and asbestos-related lung cancer (maternal grandfather).
A physical examination, which included breast and pelvic exams, was unremarkable apart from the lesions located on her abdomen, arms, and legs. On skin examination, we noted multiple polygonal, planar papules and plaques of varying size with an overlying scale (FIGURE).

THE DIAGNOSIS
The physician obtained a biopsy of one of the skin lesions, and it was sent to a dermatopathologist to evaluate. Unfortunately, though, the patient’s history and a description of the lesion were not included with the initial biopsy requisition form. Based on the biopsy sample alone, the dermatopathologist’s report indicated a diagnosis of seborrheic keratosis.
A search for malignancy. Any case of sudden, extensive seborrheic keratosis is suspected to be a Leser-Trélat sign, which is known to be associated with human immunodeficiency virus or underlying malignancy—especially in the gastrointestinal system. The physician talked to the patient about the possibility of malignancy, and an extensive work-up was performed, including multiple laboratory tests, computed tomography (CT) imaging, an esophagogastroduodenoscopy, a colonoscopy, and mammography. None of the test results showed signs of an underlying malignancy.
In light of the negative findings, the physician reached out to the dermatopathologist to further discuss the case. It was determined that the dermatopathologist did not receive any clinical information (prior to this discussion) from the primary care office. This was surprising to the primary care physician, who was under the assumption that the clinical chart would be sent along with the biopsy sample. With this new information, the dermatopathologist reexamined the slides and diagnosed the lesion as lichen planus, a rather common skin disease not associated with cancer.
[polldaddy:10153197]
DISCUSSION
A root-cause analysis of this case identified multiple system failures, focused mainly on a lack of communication between providers:
- The description of the lesion and of the patient’s history were not included with the initial biopsy requisition form due to a lack of communication between the nurse and the physician performing the procedure.
- The dermatopathologist did not seek additional clinical information from the referring physician after receiving the sample.
- When the various providers did communicate, an accurate diagnosis was reached—but only after extensive investigation (and worry).
Communication is key to an accurate diagnosis
In 2000, it was estimated that health care costs due to preventable adverse events represent more than half of the $37.6 billion spent on health care.1 Since then, considerable effort has been made to address patient safety, misdiagnosis, and cost-effectiveness. Root cause analysis is one of the most popular methods used to evaluate and prevent future serious adverse events.2
Continue to: Diagnostic errors are often unreported...
Diagnostic errors are often unreported or unrecognized, especially in the outpatient setting.3 Studies focused on reducing diagnostic error show that a second review of pathology slides reduces error, controls costs, and improves quality of health care.4
Don’t rely (exclusively) on the health record. Gaps in effective communication between providers are a leading cause of preventable adverse events.5,6 The incorporation of electronic health records has allowed for more streamlined communication between providers. However, the mere presence of patient records in a common system does not guarantee the receipt or communication of information. The next step after entering the information into the record is to communicate it.
Our patient underwent a battery of costly and unnecessary tests and procedures, many of which were unwarranted at her age. In addition to being exposed to harmful radiation, she also experienced significant stress secondary to the tests and anticipation of the results. However, a root cause analysis of the case led to an improved protocol for communication between providers at the outpatient clinic. We now emphasize the necessity of including a clinical history and corresponding physical findings with all biopsies. We also encourage more direct communication between nursing staff, primary care physicians, and specialists.
THE TAKEAWAY
As medical professionals become increasingly reliant on the many emerging studies available to them, we sometimes forget that communication is key to optimal medical care, an accurate diagnosis, and patient safety.
Continue to: In addition, a second review...
In addition, a second review of dermatopathologic slides may be warranted if the pathologic diagnosis is inconsistent with the clinical picture or if the diagnosed condition is resistant to the usual therapies of choice. Incorrect diagnoses are more likely to occur when tests are interpreted in a vacuum without the corresponding clinical correlation. The weight of these mistakes is felt not only by the health care system, but by the patients themselves.
CORRESPONDENCE
Magdalena Pasarica, MD, PhD, University of Central Florida College of Medicine, 6850 Lake Nona Boulevard, Orlando, FL 32827; [email protected]
THE CASE
A 40-year-old white woman presented to clinic with multiple pruritic skin lesions on her abdomen, arms, and legs that had developed over a 2-month period. The patient reported that she’d been feeling tired and had been experiencing psychological stressors in her personal life. Her medical history was significant for psoriasis (which was controlled), and her family history was significant for breast and bone cancer (mother) and asbestos-related lung cancer (maternal grandfather).
A physical examination, which included breast and pelvic exams, was unremarkable apart from the lesions located on her abdomen, arms, and legs. On skin examination, we noted multiple polygonal, planar papules and plaques of varying size with an overlying scale (FIGURE).

THE DIAGNOSIS
The physician obtained a biopsy of one of the skin lesions, and it was sent to a dermatopathologist to evaluate. Unfortunately, though, the patient’s history and a description of the lesion were not included with the initial biopsy requisition form. Based on the biopsy sample alone, the dermatopathologist’s report indicated a diagnosis of seborrheic keratosis.
A search for malignancy. Any case of sudden, extensive seborrheic keratosis is suspected to be a Leser-Trélat sign, which is known to be associated with human immunodeficiency virus or underlying malignancy—especially in the gastrointestinal system. The physician talked to the patient about the possibility of malignancy, and an extensive work-up was performed, including multiple laboratory tests, computed tomography (CT) imaging, an esophagogastroduodenoscopy, a colonoscopy, and mammography. None of the test results showed signs of an underlying malignancy.
In light of the negative findings, the physician reached out to the dermatopathologist to further discuss the case. It was determined that the dermatopathologist did not receive any clinical information (prior to this discussion) from the primary care office. This was surprising to the primary care physician, who was under the assumption that the clinical chart would be sent along with the biopsy sample. With this new information, the dermatopathologist reexamined the slides and diagnosed the lesion as lichen planus, a rather common skin disease not associated with cancer.
[polldaddy:10153197]
DISCUSSION
A root-cause analysis of this case identified multiple system failures, focused mainly on a lack of communication between providers:
- The description of the lesion and of the patient’s history were not included with the initial biopsy requisition form due to a lack of communication between the nurse and the physician performing the procedure.
- The dermatopathologist did not seek additional clinical information from the referring physician after receiving the sample.
- When the various providers did communicate, an accurate diagnosis was reached—but only after extensive investigation (and worry).
Communication is key to an accurate diagnosis
In 2000, it was estimated that health care costs due to preventable adverse events represent more than half of the $37.6 billion spent on health care.1 Since then, considerable effort has been made to address patient safety, misdiagnosis, and cost-effectiveness. Root cause analysis is one of the most popular methods used to evaluate and prevent future serious adverse events.2
Continue to: Diagnostic errors are often unreported...
Diagnostic errors are often unreported or unrecognized, especially in the outpatient setting.3 Studies focused on reducing diagnostic error show that a second review of pathology slides reduces error, controls costs, and improves quality of health care.4
Don’t rely (exclusively) on the health record. Gaps in effective communication between providers are a leading cause of preventable adverse events.5,6 The incorporation of electronic health records has allowed for more streamlined communication between providers. However, the mere presence of patient records in a common system does not guarantee the receipt or communication of information. The next step after entering the information into the record is to communicate it.
Our patient underwent a battery of costly and unnecessary tests and procedures, many of which were unwarranted at her age. In addition to being exposed to harmful radiation, she also experienced significant stress secondary to the tests and anticipation of the results. However, a root cause analysis of the case led to an improved protocol for communication between providers at the outpatient clinic. We now emphasize the necessity of including a clinical history and corresponding physical findings with all biopsies. We also encourage more direct communication between nursing staff, primary care physicians, and specialists.
THE TAKEAWAY
As medical professionals become increasingly reliant on the many emerging studies available to them, we sometimes forget that communication is key to optimal medical care, an accurate diagnosis, and patient safety.
Continue to: In addition, a second review...
In addition, a second review of dermatopathologic slides may be warranted if the pathologic diagnosis is inconsistent with the clinical picture or if the diagnosed condition is resistant to the usual therapies of choice. Incorrect diagnoses are more likely to occur when tests are interpreted in a vacuum without the corresponding clinical correlation. The weight of these mistakes is felt not only by the health care system, but by the patients themselves.
CORRESPONDENCE
Magdalena Pasarica, MD, PhD, University of Central Florida College of Medicine, 6850 Lake Nona Boulevard, Orlando, FL 32827; [email protected]
1. Kohn LT, Corrigan JM, Donaldson MS. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
2. U.S. Department of Health and Human Services. Patient safety primer: root cause analysis. https://psnet.ahrq.gov/primers/primer/10/root-cause-analysis. Updated August 2018. Accessed September 27, 2018.
3. Newman-Toker DE, Pronovost PJ. Diagnostic errors-the next frontier for patient safety. JAMA. 2009;301:1060-1062.
4. Kuijpers CC, Burger G, Al-Janabi S, et al. Improved quality of patient care through routine second review of histopathology specimens prior to multidisciplinary meetings. J Clin Pathol. 2016;69:866-871.
5. Leonard M, Graham S, Bonacum D. The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care. 2004;13:85-90.
6. Robinson NL. Promoting patient safety with perioperative hand-off communication. J Perianesth Nurs. 2016;31:245-253.
1. Kohn LT, Corrigan JM, Donaldson MS. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
2. U.S. Department of Health and Human Services. Patient safety primer: root cause analysis. https://psnet.ahrq.gov/primers/primer/10/root-cause-analysis. Updated August 2018. Accessed September 27, 2018.
3. Newman-Toker DE, Pronovost PJ. Diagnostic errors-the next frontier for patient safety. JAMA. 2009;301:1060-1062.
4. Kuijpers CC, Burger G, Al-Janabi S, et al. Improved quality of patient care through routine second review of histopathology specimens prior to multidisciplinary meetings. J Clin Pathol. 2016;69:866-871.
5. Leonard M, Graham S, Bonacum D. The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care. 2004;13:85-90.
6. Robinson NL. Promoting patient safety with perioperative hand-off communication. J Perianesth Nurs. 2016;31:245-253.
Should you reassess your patient’s asthma diagnosis?
ILLUSTRATIVE CASE
A 45-year-old woman presents to your office for a yearly visit. Two years ago she was started on an inhaled corticosteroid (ICS) and a bronchodilator rescue inhaler after being diagnosed with asthma based on her history and physical exam findings. She has had no exacerbations since then. Should you consider weaning her off the inhalers?
Asthma is a prevalent problem; 8% of adults ages 18 to 64 years have the chronic lung disease.2 Diagnosis can be challenging, partially because it requires measurement of transient airway resistance. And treatment entails significant costs and possible adverse effects. Without some sort of pulmonary function measurements or trials off medication, there is no clinical way to differentiate patients with well-controlled asthma from those who are being treated unnecessarily. Not surprisingly, studies have shown that ruling out active asthma and reducing medication usage is cost effective.3,4 This study followed a cohort of patients to see how many could be weaned off their asthma medications, and how they did in the subsequent year.
STUDY SUMMARY
About one-third of adults with asthma are “undiagnosed” within 5 years
The researchers recruited participants from the general population of the 10 largest cities and surrounding areas in Canada by randomly dialing cellular and landline phone numbers and asking about adult household members with asthma.1 The researchers focused on people with a recent (<5 years) asthma diagnosis, so as to represent contemporary diagnostic practice and to make it easier to collect medical records. Participants lived within 90 minutes of 10 medical centers in Canada. Patients were excluded if they were using long-term oral steroids, pregnant or breastfeeding, unable to tolerate spirometry or methacholine challenges, or had a history of more than 10 pack-years of smoking.
Of the 701 patients enrolled, 613 (87.4%) completed all study assessments. Patients progressed through a series of spirometry tests and were then tapered off their asthma-controlling medications.
The initial spirometry test confirmed asthma if bronchodilators caused a significant improvement in forced expiratory volume in the first second of expiration (FEV1). If there was no improvement, the patient took a methacholine challenge 1 week later; if they did well, their maintenance medications were reduced by half. If the patient did well with another methacholine challenge about 1 month later, maintenance medications were stopped, and the patient underwent a third methacholine challenge 3 weeks later.
Asthma was confirmed at any methacholine challenge if there was a 20% decrease in FEV1 from baseline at a methacholine concentration of ≤8 mg/mL; these patients were restarted on appropriate medications. If current asthma was ruled out, follow-up bronchial challenges were repeated at 6 and 12 months.
Results. Among the adults with physician-diagnosed asthma, 33.1% (95% confidence interval [CI], 29.4%-36.8%) no longer met criteria for an asthma diagnosis. Of those who no longer had asthma, 44% had previously undergone objective testing of airflow limitation. The investigators also found 12 patients (2%) had other serious cardiorespiratory conditions instead of asthma, including ischemic heart disease, subglottic stenosis, and bronchiectasis.
Continue to: During the 1-year follow-up period...
During the 1-year follow-up period, 22 (10.8%) of the 203 patients who were initially judged to no longer have asthma had a positive bronchial challenge test; 16 had no symptoms and continued to do well off all asthma medications. Six (3%) presented with respiratory symptoms and resumed treatment with asthma medications, but only 1 (0.5%) required oral corticosteroid therapy.
WHAT’S NEW?
Asthma meds are of no benefit for about one-third of patients taking them
This study found that one-third of patients with asthma diagnosed in the last 5 years no longer had symptoms or spirometry results consistent with asthma and did well in the subsequent year. For those patients, there appears to be no benefit to using asthma medications. The Global Institute for Asthma recommends stepping down treatment in adults with asthma that is well controlled for 3 months or more.5 While patients with objectively confirmed asthma diagnoses were more likely to still have asthma in this study, over 40% of patients who no longer had asthma were objectively proven to have had asthma at their original diagnosis.
CAVEATS
High level of rigor and the absence of a randomized trial
This study used a very structured protocol for tapering patients off their medications, including multiple spirometry tests, most including methacholine challenges, as well as oversight by pulmonologists. It is unclear whether this level of rigor is necessary for weaning in other clinical settings.
Also, this study was not a randomized trial, which is the gold standard for withdrawal of therapy. However, a cohort study is adequate to assess diagnostic testing, and this could be considered a trial of “undiagnosing” asthma in adults. The results here are consistent with those of a study that looked at asthma disappearance in groups of patients with and without obesity. In that study, approximately 30% of both groups of patients no longer had a diagnosis of asthma.6
Using random dialing is likely to have broadened the pool of patients this study drew upon. Also, there is a possibility that the patients who were lost to follow-up in this study represented those who had worsening symptoms. Some patients with mild asthma may have a waxing and waning course; it is possible that the study period was not long enough to capture this. In this study, only about 3% of patients who had their medications stopped reported worsening of symptoms.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
“Undiagnosis” is unusual
Using objective testing may provide some logistical or financial challenges for patients. Furthermore, “undiagnosing” a chronic disease like asthma is not a physician’s typical work, and it may take some time and effort to educate and monitor patients through the process.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
2. QuickStats: percentage of adults aged 18-64 years with current asthma,* by state - National Health Interview Survey,† 2014-2016. MMWR Morb Mortal Wkly Rep. 2018;67:590.
3. Pakhale S, Sumner A, Coyle D, et al. (Correcting) misdiagnoses of asthma: a cost effectiveness analysis. BMC Pulm Med. 2011;11:27.
4. Rank MA, Liesinger JT, Branda ME, et al. Comparative safety and costs of stepping down asthma medications in patients with controlled asthma. J Allergy Clin Immunol. 2016;137:1373-1379.
5. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2018. https://ginasthma.org. Accessed June 15, 2018.
6. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
ILLUSTRATIVE CASE
A 45-year-old woman presents to your office for a yearly visit. Two years ago she was started on an inhaled corticosteroid (ICS) and a bronchodilator rescue inhaler after being diagnosed with asthma based on her history and physical exam findings. She has had no exacerbations since then. Should you consider weaning her off the inhalers?
Asthma is a prevalent problem; 8% of adults ages 18 to 64 years have the chronic lung disease.2 Diagnosis can be challenging, partially because it requires measurement of transient airway resistance. And treatment entails significant costs and possible adverse effects. Without some sort of pulmonary function measurements or trials off medication, there is no clinical way to differentiate patients with well-controlled asthma from those who are being treated unnecessarily. Not surprisingly, studies have shown that ruling out active asthma and reducing medication usage is cost effective.3,4 This study followed a cohort of patients to see how many could be weaned off their asthma medications, and how they did in the subsequent year.
STUDY SUMMARY
About one-third of adults with asthma are “undiagnosed” within 5 years
The researchers recruited participants from the general population of the 10 largest cities and surrounding areas in Canada by randomly dialing cellular and landline phone numbers and asking about adult household members with asthma.1 The researchers focused on people with a recent (<5 years) asthma diagnosis, so as to represent contemporary diagnostic practice and to make it easier to collect medical records. Participants lived within 90 minutes of 10 medical centers in Canada. Patients were excluded if they were using long-term oral steroids, pregnant or breastfeeding, unable to tolerate spirometry or methacholine challenges, or had a history of more than 10 pack-years of smoking.
Of the 701 patients enrolled, 613 (87.4%) completed all study assessments. Patients progressed through a series of spirometry tests and were then tapered off their asthma-controlling medications.
The initial spirometry test confirmed asthma if bronchodilators caused a significant improvement in forced expiratory volume in the first second of expiration (FEV1). If there was no improvement, the patient took a methacholine challenge 1 week later; if they did well, their maintenance medications were reduced by half. If the patient did well with another methacholine challenge about 1 month later, maintenance medications were stopped, and the patient underwent a third methacholine challenge 3 weeks later.
Asthma was confirmed at any methacholine challenge if there was a 20% decrease in FEV1 from baseline at a methacholine concentration of ≤8 mg/mL; these patients were restarted on appropriate medications. If current asthma was ruled out, follow-up bronchial challenges were repeated at 6 and 12 months.
Results. Among the adults with physician-diagnosed asthma, 33.1% (95% confidence interval [CI], 29.4%-36.8%) no longer met criteria for an asthma diagnosis. Of those who no longer had asthma, 44% had previously undergone objective testing of airflow limitation. The investigators also found 12 patients (2%) had other serious cardiorespiratory conditions instead of asthma, including ischemic heart disease, subglottic stenosis, and bronchiectasis.
Continue to: During the 1-year follow-up period...
During the 1-year follow-up period, 22 (10.8%) of the 203 patients who were initially judged to no longer have asthma had a positive bronchial challenge test; 16 had no symptoms and continued to do well off all asthma medications. Six (3%) presented with respiratory symptoms and resumed treatment with asthma medications, but only 1 (0.5%) required oral corticosteroid therapy.
WHAT’S NEW?
Asthma meds are of no benefit for about one-third of patients taking them
This study found that one-third of patients with asthma diagnosed in the last 5 years no longer had symptoms or spirometry results consistent with asthma and did well in the subsequent year. For those patients, there appears to be no benefit to using asthma medications. The Global Institute for Asthma recommends stepping down treatment in adults with asthma that is well controlled for 3 months or more.5 While patients with objectively confirmed asthma diagnoses were more likely to still have asthma in this study, over 40% of patients who no longer had asthma were objectively proven to have had asthma at their original diagnosis.
CAVEATS
High level of rigor and the absence of a randomized trial
This study used a very structured protocol for tapering patients off their medications, including multiple spirometry tests, most including methacholine challenges, as well as oversight by pulmonologists. It is unclear whether this level of rigor is necessary for weaning in other clinical settings.
Also, this study was not a randomized trial, which is the gold standard for withdrawal of therapy. However, a cohort study is adequate to assess diagnostic testing, and this could be considered a trial of “undiagnosing” asthma in adults. The results here are consistent with those of a study that looked at asthma disappearance in groups of patients with and without obesity. In that study, approximately 30% of both groups of patients no longer had a diagnosis of asthma.6
Using random dialing is likely to have broadened the pool of patients this study drew upon. Also, there is a possibility that the patients who were lost to follow-up in this study represented those who had worsening symptoms. Some patients with mild asthma may have a waxing and waning course; it is possible that the study period was not long enough to capture this. In this study, only about 3% of patients who had their medications stopped reported worsening of symptoms.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
“Undiagnosis” is unusual
Using objective testing may provide some logistical or financial challenges for patients. Furthermore, “undiagnosing” a chronic disease like asthma is not a physician’s typical work, and it may take some time and effort to educate and monitor patients through the process.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 45-year-old woman presents to your office for a yearly visit. Two years ago she was started on an inhaled corticosteroid (ICS) and a bronchodilator rescue inhaler after being diagnosed with asthma based on her history and physical exam findings. She has had no exacerbations since then. Should you consider weaning her off the inhalers?
Asthma is a prevalent problem; 8% of adults ages 18 to 64 years have the chronic lung disease.2 Diagnosis can be challenging, partially because it requires measurement of transient airway resistance. And treatment entails significant costs and possible adverse effects. Without some sort of pulmonary function measurements or trials off medication, there is no clinical way to differentiate patients with well-controlled asthma from those who are being treated unnecessarily. Not surprisingly, studies have shown that ruling out active asthma and reducing medication usage is cost effective.3,4 This study followed a cohort of patients to see how many could be weaned off their asthma medications, and how they did in the subsequent year.
STUDY SUMMARY
About one-third of adults with asthma are “undiagnosed” within 5 years
The researchers recruited participants from the general population of the 10 largest cities and surrounding areas in Canada by randomly dialing cellular and landline phone numbers and asking about adult household members with asthma.1 The researchers focused on people with a recent (<5 years) asthma diagnosis, so as to represent contemporary diagnostic practice and to make it easier to collect medical records. Participants lived within 90 minutes of 10 medical centers in Canada. Patients were excluded if they were using long-term oral steroids, pregnant or breastfeeding, unable to tolerate spirometry or methacholine challenges, or had a history of more than 10 pack-years of smoking.
Of the 701 patients enrolled, 613 (87.4%) completed all study assessments. Patients progressed through a series of spirometry tests and were then tapered off their asthma-controlling medications.
The initial spirometry test confirmed asthma if bronchodilators caused a significant improvement in forced expiratory volume in the first second of expiration (FEV1). If there was no improvement, the patient took a methacholine challenge 1 week later; if they did well, their maintenance medications were reduced by half. If the patient did well with another methacholine challenge about 1 month later, maintenance medications were stopped, and the patient underwent a third methacholine challenge 3 weeks later.
Asthma was confirmed at any methacholine challenge if there was a 20% decrease in FEV1 from baseline at a methacholine concentration of ≤8 mg/mL; these patients were restarted on appropriate medications. If current asthma was ruled out, follow-up bronchial challenges were repeated at 6 and 12 months.
Results. Among the adults with physician-diagnosed asthma, 33.1% (95% confidence interval [CI], 29.4%-36.8%) no longer met criteria for an asthma diagnosis. Of those who no longer had asthma, 44% had previously undergone objective testing of airflow limitation. The investigators also found 12 patients (2%) had other serious cardiorespiratory conditions instead of asthma, including ischemic heart disease, subglottic stenosis, and bronchiectasis.
Continue to: During the 1-year follow-up period...
During the 1-year follow-up period, 22 (10.8%) of the 203 patients who were initially judged to no longer have asthma had a positive bronchial challenge test; 16 had no symptoms and continued to do well off all asthma medications. Six (3%) presented with respiratory symptoms and resumed treatment with asthma medications, but only 1 (0.5%) required oral corticosteroid therapy.
WHAT’S NEW?
Asthma meds are of no benefit for about one-third of patients taking them
This study found that one-third of patients with asthma diagnosed in the last 5 years no longer had symptoms or spirometry results consistent with asthma and did well in the subsequent year. For those patients, there appears to be no benefit to using asthma medications. The Global Institute for Asthma recommends stepping down treatment in adults with asthma that is well controlled for 3 months or more.5 While patients with objectively confirmed asthma diagnoses were more likely to still have asthma in this study, over 40% of patients who no longer had asthma were objectively proven to have had asthma at their original diagnosis.
CAVEATS
High level of rigor and the absence of a randomized trial
This study used a very structured protocol for tapering patients off their medications, including multiple spirometry tests, most including methacholine challenges, as well as oversight by pulmonologists. It is unclear whether this level of rigor is necessary for weaning in other clinical settings.
Also, this study was not a randomized trial, which is the gold standard for withdrawal of therapy. However, a cohort study is adequate to assess diagnostic testing, and this could be considered a trial of “undiagnosing” asthma in adults. The results here are consistent with those of a study that looked at asthma disappearance in groups of patients with and without obesity. In that study, approximately 30% of both groups of patients no longer had a diagnosis of asthma.6
Using random dialing is likely to have broadened the pool of patients this study drew upon. Also, there is a possibility that the patients who were lost to follow-up in this study represented those who had worsening symptoms. Some patients with mild asthma may have a waxing and waning course; it is possible that the study period was not long enough to capture this. In this study, only about 3% of patients who had their medications stopped reported worsening of symptoms.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
“Undiagnosis” is unusual
Using objective testing may provide some logistical or financial challenges for patients. Furthermore, “undiagnosing” a chronic disease like asthma is not a physician’s typical work, and it may take some time and effort to educate and monitor patients through the process.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
2. QuickStats: percentage of adults aged 18-64 years with current asthma,* by state - National Health Interview Survey,† 2014-2016. MMWR Morb Mortal Wkly Rep. 2018;67:590.
3. Pakhale S, Sumner A, Coyle D, et al. (Correcting) misdiagnoses of asthma: a cost effectiveness analysis. BMC Pulm Med. 2011;11:27.
4. Rank MA, Liesinger JT, Branda ME, et al. Comparative safety and costs of stepping down asthma medications in patients with controlled asthma. J Allergy Clin Immunol. 2016;137:1373-1379.
5. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2018. https://ginasthma.org. Accessed June 15, 2018.
6. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
1. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
2. QuickStats: percentage of adults aged 18-64 years with current asthma,* by state - National Health Interview Survey,† 2014-2016. MMWR Morb Mortal Wkly Rep. 2018;67:590.
3. Pakhale S, Sumner A, Coyle D, et al. (Correcting) misdiagnoses of asthma: a cost effectiveness analysis. BMC Pulm Med. 2011;11:27.
4. Rank MA, Liesinger JT, Branda ME, et al. Comparative safety and costs of stepping down asthma medications in patients with controlled asthma. J Allergy Clin Immunol. 2016;137:1373-1379.
5. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2018. https://ginasthma.org. Accessed June 15, 2018.
6. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
PRACTICE CHANGER
Consider tapering medications and retesting spirometry in adults with well-controlled asthma, as many may no longer have the disease.1
STRENGTH OF RECOMMENDATION
A: Based on a high-quality prospective cohort study and consistent findings in other studies.
Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
MSK injury? Make splinting choices based on the evidence
About 25% of all outpatient visits to family physicians include musculoskeletal (MSK) complaints.1 Splinting, bracing, or wrapping are used in 25% of these visits.2 The goals of splinting/bracing are multifold: accommodate a correct movement pattern, restrict poor movement patterns, and decrease the use of an injured area to allow for healing.
Splints and braces are generally noncircumferential and are easily put on and taken off. (The terms splints and braces can be used interchangeably.) The devices can be adjusted for swelling and are more comfortable than casts, but have the potential for poor patient adherence, may require frequent adjustment, and can allow for excessive motion.
Making the most of these devices requires an understanding of when the evidence supports (and doesn’t support) their use for particular injuries. In this article, we review the evidence for the use of splints/braces for common upper and lower extremity MSK conditions seen in family practice. We have confined our discussion to readily obtainable, off-the-shelf products. These products come in a variety of sizes and are easily kept on hand, or ordered through a durable medical equipment provider.
Carpal tunnel syndrome
Carpal tunnel syndrome (CTS) is a compression neuropathy of the median nerve at the level of the wrist. It is caused by several different conditions.
Goal of splinting: Minimize wrist movement to decrease any concomitant swelling in the carpal tunnel contributing to the compression. The two different types of orthoses commonly used are a neutral wrist splint (FIGURE 1) and a cock-up wrist splint (20° wrist extension).
Evidence: A 2003 Cochrane review concluded that short-term symptom relief was achievable with bracing; however, better outcomes were seen with combination therapies (eg, medications, occupational therapy) vs splinting alone.3 A more recent Cochrane review in 2012 found poor or limited evidence that splint use at night was better than no treatment or any other nonsurgical treatment.4 There was also insufficient evidence to recommend one type of splint over another, although several poor-quality studies found neutral splinting to be more beneficial.5
A 2016 clinical practice guideline (CPG) from the American Academy of Orthopaedic Surgeons (AAOS) reported strong evidence supporting the use of immobilization.6 (Strong evidence is defined by the AAOS as 2 or more “high” strength studies with consistent findings for the intervention.6) Interestingly, of the 2 studies that AAOS used to make its conclusions,7,8 only the study by Manente et al8 was available at the time of the Cochrane 2012 review, and the Cochrane authors came to a different conclusion. The AAOS CPG does not comment on a specific type of brace.
Continue to: Harms
Harms: Both the 2012 Cochrane review and the AAOS statement indicate that there are no long-term harms other than some subjective discomfort in a minority of patients while wearing the splint.
Bottom line: A wrist splint should be considered in the treatment of CTS—especially if the condition is likely the result of repetitive wrist motion. If the patient can tolerate continuous use for 2 to 4 weeks, this should be employed. But at a minimum, nocturnal use for this duration would constitute a therapeutic trial. Combination therapy (ie, medication, occupational therapy, and splinting) is better than splinting alone.
de Quervain tendinopathy
This form of tendinopathy involves pain at the tendon sheaths of the abductor pollicis longus and the extensor pollicis brevis. Onset of symptoms has been attributed to overuse or repetitive movements of the wrist and thumb.
Goal of splinting: Immobilize the affected tendons to reduce irritation and/or inflammation. A thumb spica splint (FIGURE 2) is used to achieve this restriction.
Evidence: Three randomized controlled trials (RCTs) suggested that the natural course is not affected by splint use for patients with prolonged symptoms (>3 months), and eventual resolution was noted in about 12 months—regardless of intervention with bracing. Symptoms improved more rapidly with the combination of medications and splint wear for those with a shorter duration of symptoms.9-11 Symptom severity driven wear compared with full-time use yielded equivalent outcomes.9 Those patients with longer duration and increased severity of symptoms fared worse regardless of treatment.10
Continue to: Harms
Harms: No documented harmful adverse effects (AEs) have been reported with splinting for this condition.
Bottom line: A thumb spica splint remains an option for de Quervain tendinopathy. It may provide symptomatic relief, especially if used early in the disease, but does not alter the natural disease course.
Lateral/medial epicondyle pain
Also known as tennis/golfer’s elbow, lateral/medial epicondyle pain is thought to result from overuse of the common wrist extensor/flexor muscle origins at the site of the myotendinous junctions.
Goal of splinting: To dampen or disperse the forces at the painful area via a counterforce brace (FIGURE 3). In addition, braces are used to decrease wrist use, specifically extension or flexion.
Evidence: A 2002 Cochrane Review found insufficient data to support the use of counterforce braces for relief of acute or chronic pain symptoms associated with epicondyle pain.Several studies supporting their use within this review were of varying quality with weak evidence.12
Continue to: Volar wrist braces have also been...
Volar wrist braces have also been studied for conservative management of epicondyle pain. Equivalent outcomes were noted comparing volar wrist bracing with a counterforce brace. Higher rates of recovery were seen in patients who participated in combination therapies (ie, bracing, physical therapy, and medication use).12
Harms: Use of counterforce braces for ≥30 days resulted in higher rates of braces restriction, more medical visits per patients, and higher medical costs. Derebery et al13 concluded that this was due to deconditioning on returning to normal activity. Use of a volar wrist brace should be discouraged as it reduces the active range of wrist motion, further contributing to deconditioning with long-term application.14
Bottom line: A trial of counterforce bracing should be used if pain precludes active rehabilitation or vocational pursuits, but should not be used as the sole therapy.
Knee osteoarthritis
Knee osteoarthritis (OA) can result from multiple (often commingled) etiologies, which ultimately result in loss of cartilage, ensuing bony abnormalities, and affected joint/soft tissue structures. Patients can present with severe symptoms with little loss of structural architecture or major structural changes with a paucity of symptoms.
Goal of splinting: Depending on the orthoses used, the goals of splinting vary. A simple knee sleeve (FIGURE 4) provides warmth and proprioception, and a valgus unloader brace (FIGURE 5) provides valgus stress to open and unload the medial compartment.

Continue to: Evidence
Evidence: A single study evaluating a neutral knee sleeve vs control exhibited improved pain scores following several months of treatment. Mixed results were demonstrated with patient perceived quality of life improvement though.15 Currently, there is inconclusive evidence to support the use of valgus offloader braces per AAOS guidelines.16 This decision is based on 3 separate studies of moderate to high strength evidence. Improvements in the domains of pain, stiffness, self-reported functional capacity, and physical performance were unclear and no conclusions were able to be drawn.17,18
Harms: To date, no harmful AEs have been demonstrated with the use of knee sleeves. Valgus knee bracing can be uncomfortable, leading to poor adherence, but there are no long-term negative consequences.
Bottom line: Use of knee sleeves is worthwhile in patients with mild-to-moderate OA to improve functional scores. Inconclusive support for valgus knee bracing, along with the high cost of equipment, should reserve this option for patients with advanced OA who do not respond to typical conservative management and who are unwilling or ill-advised to undergo knee arthroplasty.16-18
Medial collateral ligament injury
An injury of the medial collateral ligament (MCL)—the medial stabilizer of the knee—can result from either a direct blow or a noncontact twisting injury. Grade 1 injuries have no actual ligament tear, grade 2 injuries have partial disruption, and grade 3 injuries denote a complete tear.
Goal of splinting: A hinged knee brace (FIGURE 6) allows for full extension but limited valgus and varus stresses.

Continue to: Evidence
Evidence: A conservative management strategy for an isolated injury is generally adequate to allow for sufficient healing, and “return to play” without prolonged disability. With conservative management, the affected joint is protected with a hinged knee brace for about 3 to 6 weeks.19,20 Data gathered on patients 9 years postinjury support the use of bracing of grades 1 to 2 injuries, but it is unclear what the optimal strategy is for grade 3 injuries.19
Harms: Generally well tolerated, and no harms have been reported.
Bottom line: Isolated grades 1 to 2 MCL injuries can be treated conservatively, and a hinged knee brace should be used as part of the rehabilitative process. It is unclear how to optimally manage grade 3 injuries.
Lateral ankle sprain
Lateral ankle sprains involve inversion injury to 1 or more of the 3 lateral ankle ligaments. Injuries are graded using the same grade schema as MCL injuries.
Goal of splinting: There are a variety of braces designed to provide lateral stability to patients with lateral ankle sprains. These stirrup braces differ in degree of support and additional fixation points—rigid (pneumatic) vs semirigid (Velcro, lace-up, etc) (FIGURE 7).
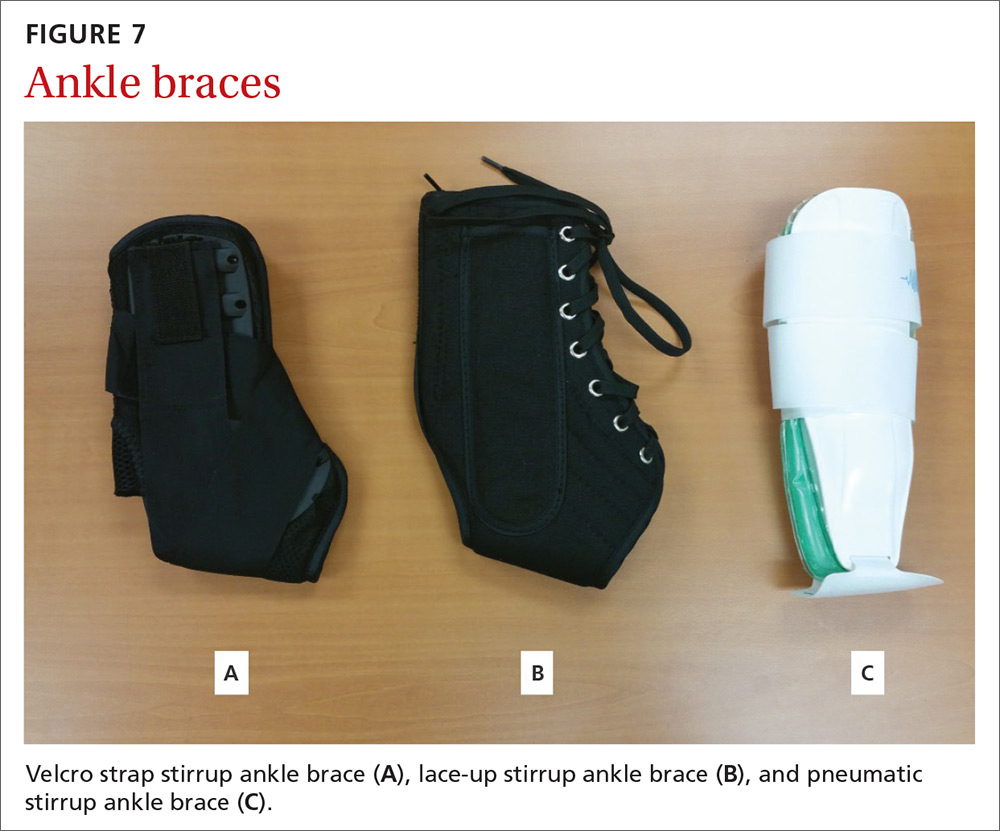
Continue to: Evidence
Evidence: A 2017 meta-analysis of systematic reviews found improved (self-reported) function when patients used external support devices such as tape, compression bandages, semirigid braces or boots, or walking casts.21 Secondary prevention utilizing brace wear during at-risk activities has been found to be the most important intervention to reduce recurrence.21,22
Harms: No direct injury from brace use has been reported, but consistent evidence exists that lack of early mobilization and rehabilitation can substantially affect the recovery from these injuries.
Bottom line: Consensus opinion recommends stirrup bracing for the treatment of grades 1 and 2 injuries.23,24 Controversy remains regarding brace use or complete immobilization for grade 3 injuries. Regardless of injury grade, early mobilization should be integrated into the treatment plan, coupled with active rehabilitation, including restoration of strength and proprioception. Prevention of second injuries is best accomplished with full rehabilitation and bracing during at-risk activities (eg, sports practices and competitions).21,22,25
A useful tool, but one not always covered by insurance
Bracing is a useful tool in the armamentarium of treating the common MSK complaints seen in everyday practice. Bracing must always be accompanied by a functional, active rehabilitation program.
Keep in mind, though, that many insurance plans may not cover the cost of bracing. Therefore, knowledge of its efficacy for a particular injury (or lack thereof) should guide treatment recommendations, along with shared decision making.
CORRESPONDENCE
Jeffrey C. Leggit, MD, CAQSM, 9706 Ethan Ridge Avenue, Frederick, MD 21704; [email protected].
1. MacKay C, Canizares M, Davis AM, et al. Health care utilization for musculoskeletal disorders. Arthritis Care Res (Hoboken). 2010;62:161-169.
2. CDC, National Center for Health Statistics. National hospital ambulatory medical care survey: 2010 outpatient department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_outpatient/2010_opd_web_tables.pdf. Accessed August 16, 2018.
3. O’Connor D, Marshall S, Massy-Westropp N. Non-surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database Syst Rev. 2003:CD003219.
4. Page MJ, Massy‐Westropp N, O’Connor D, et al. Splinting for carpal tunnel syndrome. Cochrane Database of Syst Rev. 2012:CD010003.
5. Burke DT, Burke MM, Stewart GW, et al. Splinting for carpal tunnel syndrome: in search of the optimal angle. Arch Phys Med Rehabil. 1994;75:1241-1244.
6. American Academy of Orthopaedic Surgeons. Management of Carpal Tunnel Syndrome Evidence-Based Clinical Practice Guideline. http://www.aaos.org/uploadedFiles/PreProduction/Quality/Guidelines_and_Reviews/guidelines/CTS%20CPG_2.29.16.pdf. Published February 29, 2016. Accessed August 16, 2018.
7. Hall B, Lee HC, Fitzgerald H, et al. Investigating the effectiveness of full-time wrist splinting and education in the treatment of carpal tunnel syndrome: a randomized controlled trial. Am J Occup Ther. 2013;7:448-459.
8. Manente G, Torrieri F, di Blasio F, et al. An innovative hand brace for carpal tunnel syndrome: a randomized controlled trial. Muscle Nerve. 2001;8:1020-1025.
9. Menendez ME, Thornton E, Kent S, et al. A prospective randomized clinical trial of prescription of full-time versus as-directed splint wear for de Quervain tendinopathy. Int Orthop. 2015;39:1563-1569.
10. Lane LB, Boretz RS, Stuchin SA. Treatment of de Quervain’s disease: role of conservative management. J Hand Surg Br. 2001;26:258-260.
11. Ring D, Schnellen A. Patient-centered care of de Quervain’s disease. J Hand Microsurg. 2009;1:68-71.
12. Struijs PA, Smidt N, Arola H, et al. Orthotic devices for the treatment of tennis elbow. Cochrane Database Syst Rev. 2002:CD001821.
13. Derebery VJ, Devenport JN, Giang GM, et al. The effects of splinting on outcomes of epicondylitis. Arch Phys Med Rehabil. 2005;86:1081-1088.
14. van de Streek MD, van der Schans CP, de Greef MH, et al. The effect of a forearm/hand splint compared with an elbow band as a treatment of lateral epicondylitis. Prosthet Orthot Int. 2004;28:183-189.
15. Brouwer RW, van Raaij TM, Verhaar JA, et al. Brace treatment for osteoarthritis of the knee: a prospective randomized multi-centre trial. Osteoarthritis Cartilage. 2006;14:777-783.
16. American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee, 2nd ed. Summary of recommendations. https://www.aaos.org/research/guidelines/OAKSummaryofRecommendations.pdf. Accessed August 16, 2018.
17. Kirkley A, Webster-Bogaert S, Litchfield R, et al. The effect of bracing on varus gonarthrosis. J Bone Joint Surg Am. 1999;81:539-548.
18. van Raaij TM, Reijman M, Brouwer RW, et al. Medial knee osteoarthritis treated by insoles or braces: a randomized trial. Clin Orthop Relat Res. 2010;468:1926-1932.
19. Kannus P. Long-term use of conservatively treated medial collateral ligament injuries of the knee joint. Clin Orthop Relat Res. 1988;226:103-112.
20. Stannard J. Medial and posteromedial instability of the knee: evaluation, treatment, and results. Sports Med Arthrosc Rev. 2010;18:263-268.
21. Doherty C, Bleakley C, Delahund E, et al. Treatment and prevention of acute and recurrent ankle sprain: an overview of systematic reviews with meta-analysis. Br J Sports Med. 2017;51:113-125.
22. Janssen KW, Hendriks MR, van Mechelen W, et al. The cost-effectiveness of measures to prevent recurrent ankle sprains: results of a 3-arm randomized controlled trial. Am J Sports Med. 2014;42:1534-1541.
23. Beynnon B, Renström P, Haugh L, et al. A prospective, randomized clinical investigation of the treatment of first-time ankle sprains. Am J Sports Med. 2006;34:1401-1412.
24. Seah R, Mani-Badu S. Managing ankle sprains in primary care: what is best practice? A systemic review of the last 10 years of evidence. Br Med Bull. 2011;97:105-135.
25. Kaminski TW, Hertel J, Amendola N, et al; National Athletic Trainers’ Association. National Athletic Trainers’ Association position statement: conservative management and prevention of ankle sprains in athletes. J Athl Train. 2013;48:528-545.
About 25% of all outpatient visits to family physicians include musculoskeletal (MSK) complaints.1 Splinting, bracing, or wrapping are used in 25% of these visits.2 The goals of splinting/bracing are multifold: accommodate a correct movement pattern, restrict poor movement patterns, and decrease the use of an injured area to allow for healing.
Splints and braces are generally noncircumferential and are easily put on and taken off. (The terms splints and braces can be used interchangeably.) The devices can be adjusted for swelling and are more comfortable than casts, but have the potential for poor patient adherence, may require frequent adjustment, and can allow for excessive motion.
Making the most of these devices requires an understanding of when the evidence supports (and doesn’t support) their use for particular injuries. In this article, we review the evidence for the use of splints/braces for common upper and lower extremity MSK conditions seen in family practice. We have confined our discussion to readily obtainable, off-the-shelf products. These products come in a variety of sizes and are easily kept on hand, or ordered through a durable medical equipment provider.
Carpal tunnel syndrome
Carpal tunnel syndrome (CTS) is a compression neuropathy of the median nerve at the level of the wrist. It is caused by several different conditions.
Goal of splinting: Minimize wrist movement to decrease any concomitant swelling in the carpal tunnel contributing to the compression. The two different types of orthoses commonly used are a neutral wrist splint (FIGURE 1) and a cock-up wrist splint (20° wrist extension).

Evidence: A 2003 Cochrane review concluded that short-term symptom relief was achievable with bracing; however, better outcomes were seen with combination therapies (eg, medications, occupational therapy) vs splinting alone.3 A more recent Cochrane review in 2012 found poor or limited evidence that splint use at night was better than no treatment or any other nonsurgical treatment.4 There was also insufficient evidence to recommend one type of splint over another, although several poor-quality studies found neutral splinting to be more beneficial.5
A 2016 clinical practice guideline (CPG) from the American Academy of Orthopaedic Surgeons (AAOS) reported strong evidence supporting the use of immobilization.6 (Strong evidence is defined by the AAOS as 2 or more “high” strength studies with consistent findings for the intervention.6) Interestingly, of the 2 studies that AAOS used to make its conclusions,7,8 only the study by Manente et al8 was available at the time of the Cochrane 2012 review, and the Cochrane authors came to a different conclusion. The AAOS CPG does not comment on a specific type of brace.
Continue to: Harms
Harms: Both the 2012 Cochrane review and the AAOS statement indicate that there are no long-term harms other than some subjective discomfort in a minority of patients while wearing the splint.
Bottom line: A wrist splint should be considered in the treatment of CTS—especially if the condition is likely the result of repetitive wrist motion. If the patient can tolerate continuous use for 2 to 4 weeks, this should be employed. But at a minimum, nocturnal use for this duration would constitute a therapeutic trial. Combination therapy (ie, medication, occupational therapy, and splinting) is better than splinting alone.
de Quervain tendinopathy
This form of tendinopathy involves pain at the tendon sheaths of the abductor pollicis longus and the extensor pollicis brevis. Onset of symptoms has been attributed to overuse or repetitive movements of the wrist and thumb.
Goal of splinting: Immobilize the affected tendons to reduce irritation and/or inflammation. A thumb spica splint (FIGURE 2) is used to achieve this restriction.

Evidence: Three randomized controlled trials (RCTs) suggested that the natural course is not affected by splint use for patients with prolonged symptoms (>3 months), and eventual resolution was noted in about 12 months—regardless of intervention with bracing. Symptoms improved more rapidly with the combination of medications and splint wear for those with a shorter duration of symptoms.9-11 Symptom severity driven wear compared with full-time use yielded equivalent outcomes.9 Those patients with longer duration and increased severity of symptoms fared worse regardless of treatment.10
Continue to: Harms
Harms: No documented harmful adverse effects (AEs) have been reported with splinting for this condition.
Bottom line: A thumb spica splint remains an option for de Quervain tendinopathy. It may provide symptomatic relief, especially if used early in the disease, but does not alter the natural disease course.
Lateral/medial epicondyle pain
Also known as tennis/golfer’s elbow, lateral/medial epicondyle pain is thought to result from overuse of the common wrist extensor/flexor muscle origins at the site of the myotendinous junctions.
Goal of splinting: To dampen or disperse the forces at the painful area via a counterforce brace (FIGURE 3). In addition, braces are used to decrease wrist use, specifically extension or flexion.

Evidence: A 2002 Cochrane Review found insufficient data to support the use of counterforce braces for relief of acute or chronic pain symptoms associated with epicondyle pain.Several studies supporting their use within this review were of varying quality with weak evidence.12
Continue to: Volar wrist braces have also been...
Volar wrist braces have also been studied for conservative management of epicondyle pain. Equivalent outcomes were noted comparing volar wrist bracing with a counterforce brace. Higher rates of recovery were seen in patients who participated in combination therapies (ie, bracing, physical therapy, and medication use).12
Harms: Use of counterforce braces for ≥30 days resulted in higher rates of braces restriction, more medical visits per patients, and higher medical costs. Derebery et al13 concluded that this was due to deconditioning on returning to normal activity. Use of a volar wrist brace should be discouraged as it reduces the active range of wrist motion, further contributing to deconditioning with long-term application.14
Bottom line: A trial of counterforce bracing should be used if pain precludes active rehabilitation or vocational pursuits, but should not be used as the sole therapy.
Knee osteoarthritis
Knee osteoarthritis (OA) can result from multiple (often commingled) etiologies, which ultimately result in loss of cartilage, ensuing bony abnormalities, and affected joint/soft tissue structures. Patients can present with severe symptoms with little loss of structural architecture or major structural changes with a paucity of symptoms.

Goal of splinting: Depending on the orthoses used, the goals of splinting vary. A simple knee sleeve (FIGURE 4) provides warmth and proprioception, and a valgus unloader brace (FIGURE 5) provides valgus stress to open and unload the medial compartment.

Continue to: Evidence
Evidence: A single study evaluating a neutral knee sleeve vs control exhibited improved pain scores following several months of treatment. Mixed results were demonstrated with patient perceived quality of life improvement though.15 Currently, there is inconclusive evidence to support the use of valgus offloader braces per AAOS guidelines.16 This decision is based on 3 separate studies of moderate to high strength evidence. Improvements in the domains of pain, stiffness, self-reported functional capacity, and physical performance were unclear and no conclusions were able to be drawn.17,18
Harms: To date, no harmful AEs have been demonstrated with the use of knee sleeves. Valgus knee bracing can be uncomfortable, leading to poor adherence, but there are no long-term negative consequences.
Bottom line: Use of knee sleeves is worthwhile in patients with mild-to-moderate OA to improve functional scores. Inconclusive support for valgus knee bracing, along with the high cost of equipment, should reserve this option for patients with advanced OA who do not respond to typical conservative management and who are unwilling or ill-advised to undergo knee arthroplasty.16-18
Medial collateral ligament injury
An injury of the medial collateral ligament (MCL)—the medial stabilizer of the knee—can result from either a direct blow or a noncontact twisting injury. Grade 1 injuries have no actual ligament tear, grade 2 injuries have partial disruption, and grade 3 injuries denote a complete tear.
Goal of splinting: A hinged knee brace (FIGURE 6) allows for full extension but limited valgus and varus stresses.

Continue to: Evidence
Evidence: A conservative management strategy for an isolated injury is generally adequate to allow for sufficient healing, and “return to play” without prolonged disability. With conservative management, the affected joint is protected with a hinged knee brace for about 3 to 6 weeks.19,20 Data gathered on patients 9 years postinjury support the use of bracing of grades 1 to 2 injuries, but it is unclear what the optimal strategy is for grade 3 injuries.19
Harms: Generally well tolerated, and no harms have been reported.
Bottom line: Isolated grades 1 to 2 MCL injuries can be treated conservatively, and a hinged knee brace should be used as part of the rehabilitative process. It is unclear how to optimally manage grade 3 injuries.
Lateral ankle sprain
Lateral ankle sprains involve inversion injury to 1 or more of the 3 lateral ankle ligaments. Injuries are graded using the same grade schema as MCL injuries.
Goal of splinting: There are a variety of braces designed to provide lateral stability to patients with lateral ankle sprains. These stirrup braces differ in degree of support and additional fixation points—rigid (pneumatic) vs semirigid (Velcro, lace-up, etc) (FIGURE 7).

Continue to: Evidence
Evidence: A 2017 meta-analysis of systematic reviews found improved (self-reported) function when patients used external support devices such as tape, compression bandages, semirigid braces or boots, or walking casts.21 Secondary prevention utilizing brace wear during at-risk activities has been found to be the most important intervention to reduce recurrence.21,22
Harms: No direct injury from brace use has been reported, but consistent evidence exists that lack of early mobilization and rehabilitation can substantially affect the recovery from these injuries.
Bottom line: Consensus opinion recommends stirrup bracing for the treatment of grades 1 and 2 injuries.23,24 Controversy remains regarding brace use or complete immobilization for grade 3 injuries. Regardless of injury grade, early mobilization should be integrated into the treatment plan, coupled with active rehabilitation, including restoration of strength and proprioception. Prevention of second injuries is best accomplished with full rehabilitation and bracing during at-risk activities (eg, sports practices and competitions).21,22,25
A useful tool, but one not always covered by insurance
Bracing is a useful tool in the armamentarium of treating the common MSK complaints seen in everyday practice. Bracing must always be accompanied by a functional, active rehabilitation program.
Keep in mind, though, that many insurance plans may not cover the cost of bracing. Therefore, knowledge of its efficacy for a particular injury (or lack thereof) should guide treatment recommendations, along with shared decision making.
CORRESPONDENCE
Jeffrey C. Leggit, MD, CAQSM, 9706 Ethan Ridge Avenue, Frederick, MD 21704; [email protected].
About 25% of all outpatient visits to family physicians include musculoskeletal (MSK) complaints.1 Splinting, bracing, or wrapping are used in 25% of these visits.2 The goals of splinting/bracing are multifold: accommodate a correct movement pattern, restrict poor movement patterns, and decrease the use of an injured area to allow for healing.
Splints and braces are generally noncircumferential and are easily put on and taken off. (The terms splints and braces can be used interchangeably.) The devices can be adjusted for swelling and are more comfortable than casts, but have the potential for poor patient adherence, may require frequent adjustment, and can allow for excessive motion.
Making the most of these devices requires an understanding of when the evidence supports (and doesn’t support) their use for particular injuries. In this article, we review the evidence for the use of splints/braces for common upper and lower extremity MSK conditions seen in family practice. We have confined our discussion to readily obtainable, off-the-shelf products. These products come in a variety of sizes and are easily kept on hand, or ordered through a durable medical equipment provider.
Carpal tunnel syndrome
Carpal tunnel syndrome (CTS) is a compression neuropathy of the median nerve at the level of the wrist. It is caused by several different conditions.
Goal of splinting: Minimize wrist movement to decrease any concomitant swelling in the carpal tunnel contributing to the compression. The two different types of orthoses commonly used are a neutral wrist splint (FIGURE 1) and a cock-up wrist splint (20° wrist extension).

Evidence: A 2003 Cochrane review concluded that short-term symptom relief was achievable with bracing; however, better outcomes were seen with combination therapies (eg, medications, occupational therapy) vs splinting alone.3 A more recent Cochrane review in 2012 found poor or limited evidence that splint use at night was better than no treatment or any other nonsurgical treatment.4 There was also insufficient evidence to recommend one type of splint over another, although several poor-quality studies found neutral splinting to be more beneficial.5
A 2016 clinical practice guideline (CPG) from the American Academy of Orthopaedic Surgeons (AAOS) reported strong evidence supporting the use of immobilization.6 (Strong evidence is defined by the AAOS as 2 or more “high” strength studies with consistent findings for the intervention.6) Interestingly, of the 2 studies that AAOS used to make its conclusions,7,8 only the study by Manente et al8 was available at the time of the Cochrane 2012 review, and the Cochrane authors came to a different conclusion. The AAOS CPG does not comment on a specific type of brace.
Continue to: Harms
Harms: Both the 2012 Cochrane review and the AAOS statement indicate that there are no long-term harms other than some subjective discomfort in a minority of patients while wearing the splint.
Bottom line: A wrist splint should be considered in the treatment of CTS—especially if the condition is likely the result of repetitive wrist motion. If the patient can tolerate continuous use for 2 to 4 weeks, this should be employed. But at a minimum, nocturnal use for this duration would constitute a therapeutic trial. Combination therapy (ie, medication, occupational therapy, and splinting) is better than splinting alone.
de Quervain tendinopathy
This form of tendinopathy involves pain at the tendon sheaths of the abductor pollicis longus and the extensor pollicis brevis. Onset of symptoms has been attributed to overuse or repetitive movements of the wrist and thumb.
Goal of splinting: Immobilize the affected tendons to reduce irritation and/or inflammation. A thumb spica splint (FIGURE 2) is used to achieve this restriction.

Evidence: Three randomized controlled trials (RCTs) suggested that the natural course is not affected by splint use for patients with prolonged symptoms (>3 months), and eventual resolution was noted in about 12 months—regardless of intervention with bracing. Symptoms improved more rapidly with the combination of medications and splint wear for those with a shorter duration of symptoms.9-11 Symptom severity driven wear compared with full-time use yielded equivalent outcomes.9 Those patients with longer duration and increased severity of symptoms fared worse regardless of treatment.10
Continue to: Harms
Harms: No documented harmful adverse effects (AEs) have been reported with splinting for this condition.
Bottom line: A thumb spica splint remains an option for de Quervain tendinopathy. It may provide symptomatic relief, especially if used early in the disease, but does not alter the natural disease course.
Lateral/medial epicondyle pain
Also known as tennis/golfer’s elbow, lateral/medial epicondyle pain is thought to result from overuse of the common wrist extensor/flexor muscle origins at the site of the myotendinous junctions.
Goal of splinting: To dampen or disperse the forces at the painful area via a counterforce brace (FIGURE 3). In addition, braces are used to decrease wrist use, specifically extension or flexion.

Evidence: A 2002 Cochrane Review found insufficient data to support the use of counterforce braces for relief of acute or chronic pain symptoms associated with epicondyle pain.Several studies supporting their use within this review were of varying quality with weak evidence.12
Continue to: Volar wrist braces have also been...
Volar wrist braces have also been studied for conservative management of epicondyle pain. Equivalent outcomes were noted comparing volar wrist bracing with a counterforce brace. Higher rates of recovery were seen in patients who participated in combination therapies (ie, bracing, physical therapy, and medication use).12
Harms: Use of counterforce braces for ≥30 days resulted in higher rates of braces restriction, more medical visits per patients, and higher medical costs. Derebery et al13 concluded that this was due to deconditioning on returning to normal activity. Use of a volar wrist brace should be discouraged as it reduces the active range of wrist motion, further contributing to deconditioning with long-term application.14
Bottom line: A trial of counterforce bracing should be used if pain precludes active rehabilitation or vocational pursuits, but should not be used as the sole therapy.
Knee osteoarthritis
Knee osteoarthritis (OA) can result from multiple (often commingled) etiologies, which ultimately result in loss of cartilage, ensuing bony abnormalities, and affected joint/soft tissue structures. Patients can present with severe symptoms with little loss of structural architecture or major structural changes with a paucity of symptoms.

Goal of splinting: Depending on the orthoses used, the goals of splinting vary. A simple knee sleeve (FIGURE 4) provides warmth and proprioception, and a valgus unloader brace (FIGURE 5) provides valgus stress to open and unload the medial compartment.

Continue to: Evidence
Evidence: A single study evaluating a neutral knee sleeve vs control exhibited improved pain scores following several months of treatment. Mixed results were demonstrated with patient perceived quality of life improvement though.15 Currently, there is inconclusive evidence to support the use of valgus offloader braces per AAOS guidelines.16 This decision is based on 3 separate studies of moderate to high strength evidence. Improvements in the domains of pain, stiffness, self-reported functional capacity, and physical performance were unclear and no conclusions were able to be drawn.17,18
Harms: To date, no harmful AEs have been demonstrated with the use of knee sleeves. Valgus knee bracing can be uncomfortable, leading to poor adherence, but there are no long-term negative consequences.
Bottom line: Use of knee sleeves is worthwhile in patients with mild-to-moderate OA to improve functional scores. Inconclusive support for valgus knee bracing, along with the high cost of equipment, should reserve this option for patients with advanced OA who do not respond to typical conservative management and who are unwilling or ill-advised to undergo knee arthroplasty.16-18
Medial collateral ligament injury
An injury of the medial collateral ligament (MCL)—the medial stabilizer of the knee—can result from either a direct blow or a noncontact twisting injury. Grade 1 injuries have no actual ligament tear, grade 2 injuries have partial disruption, and grade 3 injuries denote a complete tear.
Goal of splinting: A hinged knee brace (FIGURE 6) allows for full extension but limited valgus and varus stresses.

Continue to: Evidence
Evidence: A conservative management strategy for an isolated injury is generally adequate to allow for sufficient healing, and “return to play” without prolonged disability. With conservative management, the affected joint is protected with a hinged knee brace for about 3 to 6 weeks.19,20 Data gathered on patients 9 years postinjury support the use of bracing of grades 1 to 2 injuries, but it is unclear what the optimal strategy is for grade 3 injuries.19
Harms: Generally well tolerated, and no harms have been reported.
Bottom line: Isolated grades 1 to 2 MCL injuries can be treated conservatively, and a hinged knee brace should be used as part of the rehabilitative process. It is unclear how to optimally manage grade 3 injuries.
Lateral ankle sprain
Lateral ankle sprains involve inversion injury to 1 or more of the 3 lateral ankle ligaments. Injuries are graded using the same grade schema as MCL injuries.
Goal of splinting: There are a variety of braces designed to provide lateral stability to patients with lateral ankle sprains. These stirrup braces differ in degree of support and additional fixation points—rigid (pneumatic) vs semirigid (Velcro, lace-up, etc) (FIGURE 7).

Continue to: Evidence
Evidence: A 2017 meta-analysis of systematic reviews found improved (self-reported) function when patients used external support devices such as tape, compression bandages, semirigid braces or boots, or walking casts.21 Secondary prevention utilizing brace wear during at-risk activities has been found to be the most important intervention to reduce recurrence.21,22
Harms: No direct injury from brace use has been reported, but consistent evidence exists that lack of early mobilization and rehabilitation can substantially affect the recovery from these injuries.
Bottom line: Consensus opinion recommends stirrup bracing for the treatment of grades 1 and 2 injuries.23,24 Controversy remains regarding brace use or complete immobilization for grade 3 injuries. Regardless of injury grade, early mobilization should be integrated into the treatment plan, coupled with active rehabilitation, including restoration of strength and proprioception. Prevention of second injuries is best accomplished with full rehabilitation and bracing during at-risk activities (eg, sports practices and competitions).21,22,25
A useful tool, but one not always covered by insurance
Bracing is a useful tool in the armamentarium of treating the common MSK complaints seen in everyday practice. Bracing must always be accompanied by a functional, active rehabilitation program.
Keep in mind, though, that many insurance plans may not cover the cost of bracing. Therefore, knowledge of its efficacy for a particular injury (or lack thereof) should guide treatment recommendations, along with shared decision making.
CORRESPONDENCE
Jeffrey C. Leggit, MD, CAQSM, 9706 Ethan Ridge Avenue, Frederick, MD 21704; [email protected].
1. MacKay C, Canizares M, Davis AM, et al. Health care utilization for musculoskeletal disorders. Arthritis Care Res (Hoboken). 2010;62:161-169.
2. CDC, National Center for Health Statistics. National hospital ambulatory medical care survey: 2010 outpatient department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_outpatient/2010_opd_web_tables.pdf. Accessed August 16, 2018.
3. O’Connor D, Marshall S, Massy-Westropp N. Non-surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database Syst Rev. 2003:CD003219.
4. Page MJ, Massy‐Westropp N, O’Connor D, et al. Splinting for carpal tunnel syndrome. Cochrane Database of Syst Rev. 2012:CD010003.
5. Burke DT, Burke MM, Stewart GW, et al. Splinting for carpal tunnel syndrome: in search of the optimal angle. Arch Phys Med Rehabil. 1994;75:1241-1244.
6. American Academy of Orthopaedic Surgeons. Management of Carpal Tunnel Syndrome Evidence-Based Clinical Practice Guideline. http://www.aaos.org/uploadedFiles/PreProduction/Quality/Guidelines_and_Reviews/guidelines/CTS%20CPG_2.29.16.pdf. Published February 29, 2016. Accessed August 16, 2018.
7. Hall B, Lee HC, Fitzgerald H, et al. Investigating the effectiveness of full-time wrist splinting and education in the treatment of carpal tunnel syndrome: a randomized controlled trial. Am J Occup Ther. 2013;7:448-459.
8. Manente G, Torrieri F, di Blasio F, et al. An innovative hand brace for carpal tunnel syndrome: a randomized controlled trial. Muscle Nerve. 2001;8:1020-1025.
9. Menendez ME, Thornton E, Kent S, et al. A prospective randomized clinical trial of prescription of full-time versus as-directed splint wear for de Quervain tendinopathy. Int Orthop. 2015;39:1563-1569.
10. Lane LB, Boretz RS, Stuchin SA. Treatment of de Quervain’s disease: role of conservative management. J Hand Surg Br. 2001;26:258-260.
11. Ring D, Schnellen A. Patient-centered care of de Quervain’s disease. J Hand Microsurg. 2009;1:68-71.
12. Struijs PA, Smidt N, Arola H, et al. Orthotic devices for the treatment of tennis elbow. Cochrane Database Syst Rev. 2002:CD001821.
13. Derebery VJ, Devenport JN, Giang GM, et al. The effects of splinting on outcomes of epicondylitis. Arch Phys Med Rehabil. 2005;86:1081-1088.
14. van de Streek MD, van der Schans CP, de Greef MH, et al. The effect of a forearm/hand splint compared with an elbow band as a treatment of lateral epicondylitis. Prosthet Orthot Int. 2004;28:183-189.
15. Brouwer RW, van Raaij TM, Verhaar JA, et al. Brace treatment for osteoarthritis of the knee: a prospective randomized multi-centre trial. Osteoarthritis Cartilage. 2006;14:777-783.
16. American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee, 2nd ed. Summary of recommendations. https://www.aaos.org/research/guidelines/OAKSummaryofRecommendations.pdf. Accessed August 16, 2018.
17. Kirkley A, Webster-Bogaert S, Litchfield R, et al. The effect of bracing on varus gonarthrosis. J Bone Joint Surg Am. 1999;81:539-548.
18. van Raaij TM, Reijman M, Brouwer RW, et al. Medial knee osteoarthritis treated by insoles or braces: a randomized trial. Clin Orthop Relat Res. 2010;468:1926-1932.
19. Kannus P. Long-term use of conservatively treated medial collateral ligament injuries of the knee joint. Clin Orthop Relat Res. 1988;226:103-112.
20. Stannard J. Medial and posteromedial instability of the knee: evaluation, treatment, and results. Sports Med Arthrosc Rev. 2010;18:263-268.
21. Doherty C, Bleakley C, Delahund E, et al. Treatment and prevention of acute and recurrent ankle sprain: an overview of systematic reviews with meta-analysis. Br J Sports Med. 2017;51:113-125.
22. Janssen KW, Hendriks MR, van Mechelen W, et al. The cost-effectiveness of measures to prevent recurrent ankle sprains: results of a 3-arm randomized controlled trial. Am J Sports Med. 2014;42:1534-1541.
23. Beynnon B, Renström P, Haugh L, et al. A prospective, randomized clinical investigation of the treatment of first-time ankle sprains. Am J Sports Med. 2006;34:1401-1412.
24. Seah R, Mani-Badu S. Managing ankle sprains in primary care: what is best practice? A systemic review of the last 10 years of evidence. Br Med Bull. 2011;97:105-135.
25. Kaminski TW, Hertel J, Amendola N, et al; National Athletic Trainers’ Association. National Athletic Trainers’ Association position statement: conservative management and prevention of ankle sprains in athletes. J Athl Train. 2013;48:528-545.
1. MacKay C, Canizares M, Davis AM, et al. Health care utilization for musculoskeletal disorders. Arthritis Care Res (Hoboken). 2010;62:161-169.
2. CDC, National Center for Health Statistics. National hospital ambulatory medical care survey: 2010 outpatient department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_outpatient/2010_opd_web_tables.pdf. Accessed August 16, 2018.
3. O’Connor D, Marshall S, Massy-Westropp N. Non-surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database Syst Rev. 2003:CD003219.
4. Page MJ, Massy‐Westropp N, O’Connor D, et al. Splinting for carpal tunnel syndrome. Cochrane Database of Syst Rev. 2012:CD010003.
5. Burke DT, Burke MM, Stewart GW, et al. Splinting for carpal tunnel syndrome: in search of the optimal angle. Arch Phys Med Rehabil. 1994;75:1241-1244.
6. American Academy of Orthopaedic Surgeons. Management of Carpal Tunnel Syndrome Evidence-Based Clinical Practice Guideline. http://www.aaos.org/uploadedFiles/PreProduction/Quality/Guidelines_and_Reviews/guidelines/CTS%20CPG_2.29.16.pdf. Published February 29, 2016. Accessed August 16, 2018.
7. Hall B, Lee HC, Fitzgerald H, et al. Investigating the effectiveness of full-time wrist splinting and education in the treatment of carpal tunnel syndrome: a randomized controlled trial. Am J Occup Ther. 2013;7:448-459.
8. Manente G, Torrieri F, di Blasio F, et al. An innovative hand brace for carpal tunnel syndrome: a randomized controlled trial. Muscle Nerve. 2001;8:1020-1025.
9. Menendez ME, Thornton E, Kent S, et al. A prospective randomized clinical trial of prescription of full-time versus as-directed splint wear for de Quervain tendinopathy. Int Orthop. 2015;39:1563-1569.
10. Lane LB, Boretz RS, Stuchin SA. Treatment of de Quervain’s disease: role of conservative management. J Hand Surg Br. 2001;26:258-260.
11. Ring D, Schnellen A. Patient-centered care of de Quervain’s disease. J Hand Microsurg. 2009;1:68-71.
12. Struijs PA, Smidt N, Arola H, et al. Orthotic devices for the treatment of tennis elbow. Cochrane Database Syst Rev. 2002:CD001821.
13. Derebery VJ, Devenport JN, Giang GM, et al. The effects of splinting on outcomes of epicondylitis. Arch Phys Med Rehabil. 2005;86:1081-1088.
14. van de Streek MD, van der Schans CP, de Greef MH, et al. The effect of a forearm/hand splint compared with an elbow band as a treatment of lateral epicondylitis. Prosthet Orthot Int. 2004;28:183-189.
15. Brouwer RW, van Raaij TM, Verhaar JA, et al. Brace treatment for osteoarthritis of the knee: a prospective randomized multi-centre trial. Osteoarthritis Cartilage. 2006;14:777-783.
16. American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee, 2nd ed. Summary of recommendations. https://www.aaos.org/research/guidelines/OAKSummaryofRecommendations.pdf. Accessed August 16, 2018.
17. Kirkley A, Webster-Bogaert S, Litchfield R, et al. The effect of bracing on varus gonarthrosis. J Bone Joint Surg Am. 1999;81:539-548.
18. van Raaij TM, Reijman M, Brouwer RW, et al. Medial knee osteoarthritis treated by insoles or braces: a randomized trial. Clin Orthop Relat Res. 2010;468:1926-1932.
19. Kannus P. Long-term use of conservatively treated medial collateral ligament injuries of the knee joint. Clin Orthop Relat Res. 1988;226:103-112.
20. Stannard J. Medial and posteromedial instability of the knee: evaluation, treatment, and results. Sports Med Arthrosc Rev. 2010;18:263-268.
21. Doherty C, Bleakley C, Delahund E, et al. Treatment and prevention of acute and recurrent ankle sprain: an overview of systematic reviews with meta-analysis. Br J Sports Med. 2017;51:113-125.
22. Janssen KW, Hendriks MR, van Mechelen W, et al. The cost-effectiveness of measures to prevent recurrent ankle sprains: results of a 3-arm randomized controlled trial. Am J Sports Med. 2014;42:1534-1541.
23. Beynnon B, Renström P, Haugh L, et al. A prospective, randomized clinical investigation of the treatment of first-time ankle sprains. Am J Sports Med. 2006;34:1401-1412.
24. Seah R, Mani-Badu S. Managing ankle sprains in primary care: what is best practice? A systemic review of the last 10 years of evidence. Br Med Bull. 2011;97:105-135.
25. Kaminski TW, Hertel J, Amendola N, et al; National Athletic Trainers’ Association. National Athletic Trainers’ Association position statement: conservative management and prevention of ankle sprains in athletes. J Athl Train. 2013;48:528-545.
From The Journal of Family Practice | 2018;67(11):678-683.
PRACTICE RECOMMENDATIONS
› Consider a wrist splint for carpal tunnel syndrome secondary to repetitive motion. B
› Recommend a simple knee sleeve to help patients with osteoarthritis reduce their pain and improve daily function. B
› Use ankle bracing for secondary prevention of a recurrent ankle sprain. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
3 steps we can take to address childhood adversity
Twenty years ago, the American Journal of Preventive Medicine published Felitti and colleagues’ seminal publication on the relationship between adverse childhood experiences (ACEs) and poor mental and physical health.1 It is astonishing that mainstream medicine is only now taking this finding seriously under the current banner of “trauma informed care.” Better late than never.
In this issue of JFP, Stillerman provides a cogent summary of the research on diagnosis and treatment of ACEs performed over the past 20 years. There are good data supporting the effectiveness of identifying and treating ACEs to lessen the adverse health outcomes that can result. More important, however, is taking a public health approach to preventing the adverse health effects of ACEs by staging community interventions and providing support to new mothers and families.
Research strongly supports a causal relationship between ACEs and a host of mental and physical ailments. Felitti found that adults with 4 or more ACEs compared with none had a 4- to 12-fold increased health risk for alcoholism, drug abuse, depression, and suicide attempt. ACEs also increased the risk of ischemic heart disease, cancer, chronic lung disease, skeletal fractures, and liver disease.1
There is need for further research on screening for, and treating, ACEs. A large randomized trial using one of the practical brief screeners would help us learn more about the impact that screening can have on the mental and physical health of those affected. Does the identification and empathetic acknowledgement of the traumatic events lead to improved health? If it does not, what type of treatment is most effective?
Continue to: Pending further research...
Pending further research, here are 3 steps that family physicians can take today:
- Be aware of the strength of the relationship between ACEs and health problems.
- Begin screening adults and children for ACEs using one of the simple, validated screening tools described by Stillerman. In a large follow-up study, screening along with discussion of the results with the patient’s physician led to remarkable decreases in health care utilization in the year following screening, which suggests that there are therapeutic benefits to bringing ACEs to light and fostering discussion.2
- Remain ever compassionate in your interactions with all patients, knowing that many have significant childhood scars.
1. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245-258.
2. Felitti VJ, Anda RF. The relationship of adverse childhood experiences to adult medical disease, psychiatric disorders and sexual behavior: implications for healthcare. In: Lanius RA, Vermetten E, Pain C, eds. The Impact of Early Life Trauma on Health and Disease: The Hidden Epidemic. Cambridge, UK: Cambridge University Press; 2011:77-87.
Twenty years ago, the American Journal of Preventive Medicine published Felitti and colleagues’ seminal publication on the relationship between adverse childhood experiences (ACEs) and poor mental and physical health.1 It is astonishing that mainstream medicine is only now taking this finding seriously under the current banner of “trauma informed care.” Better late than never.
In this issue of JFP, Stillerman provides a cogent summary of the research on diagnosis and treatment of ACEs performed over the past 20 years. There are good data supporting the effectiveness of identifying and treating ACEs to lessen the adverse health outcomes that can result. More important, however, is taking a public health approach to preventing the adverse health effects of ACEs by staging community interventions and providing support to new mothers and families.
Research strongly supports a causal relationship between ACEs and a host of mental and physical ailments. Felitti found that adults with 4 or more ACEs compared with none had a 4- to 12-fold increased health risk for alcoholism, drug abuse, depression, and suicide attempt. ACEs also increased the risk of ischemic heart disease, cancer, chronic lung disease, skeletal fractures, and liver disease.1
There is need for further research on screening for, and treating, ACEs. A large randomized trial using one of the practical brief screeners would help us learn more about the impact that screening can have on the mental and physical health of those affected. Does the identification and empathetic acknowledgement of the traumatic events lead to improved health? If it does not, what type of treatment is most effective?
Continue to: Pending further research...
Pending further research, here are 3 steps that family physicians can take today:
- Be aware of the strength of the relationship between ACEs and health problems.
- Begin screening adults and children for ACEs using one of the simple, validated screening tools described by Stillerman. In a large follow-up study, screening along with discussion of the results with the patient’s physician led to remarkable decreases in health care utilization in the year following screening, which suggests that there are therapeutic benefits to bringing ACEs to light and fostering discussion.2
- Remain ever compassionate in your interactions with all patients, knowing that many have significant childhood scars.
Twenty years ago, the American Journal of Preventive Medicine published Felitti and colleagues’ seminal publication on the relationship between adverse childhood experiences (ACEs) and poor mental and physical health.1 It is astonishing that mainstream medicine is only now taking this finding seriously under the current banner of “trauma informed care.” Better late than never.
In this issue of JFP, Stillerman provides a cogent summary of the research on diagnosis and treatment of ACEs performed over the past 20 years. There are good data supporting the effectiveness of identifying and treating ACEs to lessen the adverse health outcomes that can result. More important, however, is taking a public health approach to preventing the adverse health effects of ACEs by staging community interventions and providing support to new mothers and families.
Research strongly supports a causal relationship between ACEs and a host of mental and physical ailments. Felitti found that adults with 4 or more ACEs compared with none had a 4- to 12-fold increased health risk for alcoholism, drug abuse, depression, and suicide attempt. ACEs also increased the risk of ischemic heart disease, cancer, chronic lung disease, skeletal fractures, and liver disease.1
There is need for further research on screening for, and treating, ACEs. A large randomized trial using one of the practical brief screeners would help us learn more about the impact that screening can have on the mental and physical health of those affected. Does the identification and empathetic acknowledgement of the traumatic events lead to improved health? If it does not, what type of treatment is most effective?
Continue to: Pending further research...
Pending further research, here are 3 steps that family physicians can take today:
- Be aware of the strength of the relationship between ACEs and health problems.
- Begin screening adults and children for ACEs using one of the simple, validated screening tools described by Stillerman. In a large follow-up study, screening along with discussion of the results with the patient’s physician led to remarkable decreases in health care utilization in the year following screening, which suggests that there are therapeutic benefits to bringing ACEs to light and fostering discussion.2
- Remain ever compassionate in your interactions with all patients, knowing that many have significant childhood scars.
1. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245-258.
2. Felitti VJ, Anda RF. The relationship of adverse childhood experiences to adult medical disease, psychiatric disorders and sexual behavior: implications for healthcare. In: Lanius RA, Vermetten E, Pain C, eds. The Impact of Early Life Trauma on Health and Disease: The Hidden Epidemic. Cambridge, UK: Cambridge University Press; 2011:77-87.
1. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245-258.
2. Felitti VJ, Anda RF. The relationship of adverse childhood experiences to adult medical disease, psychiatric disorders and sexual behavior: implications for healthcare. In: Lanius RA, Vermetten E, Pain C, eds. The Impact of Early Life Trauma on Health and Disease: The Hidden Epidemic. Cambridge, UK: Cambridge University Press; 2011:77-87.
Childhood adversity & lifelong health: From research to action
The rising prevalence of obesity, widespread community violence, and the opioid epidemic are urgent health crises that we have, so far, failed to solve. Physicians must therefore ask: Are we employing the right framework to effectively understand and address these complex problems?
Careful review of the literature reveals that these problems and many others begin with, and are profoundly affected by, childhood adversity. Compounding this, studies over the past 20 years that have focused on abuse and neglect without including community, structural, and historical adversity demonstrate that our definitions of adversity and trauma have been too narrow. The prevalence and diversity of factors affecting development and health is much greater than our medical model anticipates.1,2
CASE
Eileen W, a 55-year-old married, self-employed woman with a 20-year history of autoimmune thyroiditis, longstanding insomnia, and anxiety presents with intense episodes of terror related to public speaking, which are compromising her work performance. Her history is significant for tobacco and alcohol use beginning in early adolescence and continuing into young adulthood, as well as 2 unplanned pregnancies in her 20s. Additional adversities included the murder of her maternal aunt while Ms. W was in utero, resulting in her parents having fostered 2 young cousins; bullying; and the premature death of a special-needs sibling.
What treatment strategies might have been undertaken to manage consequences of the adversities of Ms. W’s childhood—both on her own initiative and as interventions by her health care providers?

Our medical model must be updated to be effective
Because at least 60% of Americans have had 1 or more experiences of childhood adversity, family physicians care for affected patients every day—a reality incompletely addressed by our conventional theories and practices.1,3 Consequently, updating our medical model to incorporate research that confirms the critical and widespread impact of childhood experience on health and illness is an essential task for family medicine.
Core values of family medicine integrate biological, clinical, and behavioral sciences. They include comprehensive and compassionate care that is provided within the context of family and community across the lifespan.4,5 Family medicine is therefore the ideal specialty to lead a movement that will translate scientific evidence of the effects of childhood adversity on health into training, delivery of care, and research—transforming clinical practice and patient health across the lifespan.
This article describes the dramatic impact of childhood adversity on health and well-being and calls on family physicians to play a crucial role in preventing, mitigating, and treating the consequences of childhood adversity, an important root cause of disease.
Continue to: Childhood adversity makes us sick
Childhood adversity makes us sick
The first paper about the landmark Adverse Childhood Experiences (ACE) Study, published 20 years ago, is 1 of more than 90 on this topic.3 This study explored the relationship of physical, emotional, and social health in adulthood and self-reported childhood adversity, and comprised 10 categories of abuse, neglect, and household distress between birth and 18 years of age. One of the largest epidemiological studies of its kind, the ACE Study surveyed more than 17,000 mostly white, middle-aged, educated, and insured participants. Study researchers developed an “ACE Score”—the total number of ACEs faced by a person before her (his) 18th birthday—and found that 64% of respondents endorsed 1 or more ACEs; 27% reported 3 or more ACEs; and 5% experienced 6 or more.
The ACE Study revealed a dose–response relationship between ACEs and more than 40 health-compromising behaviors, negative health conditions, and poor social outcomes. Examples include cardiac, autoimmune disease, obesity, intravenous drug abuse, depression and anxiety, adolescent pregnancy, and worker absenteeism. Tragically, an ACE score of ≥6 conferred a significant risk for premature death.1
ACE data have been collected in diverse populations in 32 states and many countries through the Behavioral Risk Factor Surveillance Survey conducted by the Centers for Disease Control and Prevention3; the Child & Adolescent Health Measurement Initiative’s National Survey of Children’s Health6; and The World Health Organization’s ACE International Questionnaire7—underscoring the pervasiveness of childhood adversity. Evaluation of ACEs in special populations, such as people experiencing homelessness,8 incarcerated youth,9 people struggling with addiction,10 and even health care workers,11 uncovers notably higher rates of ACEs in these populations than in the general population.
Is childhood adversity a true cause of bad outcomes?
Or is the relationship between the 2 entities merely an association? To help answer this question, researchers evaluated the ACE Study using Bradford Hill criteria—9 epidemiological principles employed to infer causation. Their findings strongly support the hypothesis that not only are ACEs associated with myriad negative outcomes, they are their root cause12 and therefore a powerful determinant of our most pressing and expensive health and social problems.Nevertheless, strategies to prevent and address childhood adversity, which are critical to meeting national health goals of successful prevention and treatment of myriad conditions, are absent from the paradigm and practice of most physicians.
The body of research about the health impact of additional adverse experiences is growing to include community violence, poverty, longstanding discrimination,2 and other experiences that we describe as social determinants of health. Furthermore, social determinants of health, or adverse community experiences, appear to maintain a dose–response relationship with health and social outcomes.2 ,13 Along with adverse collective historical experiences (historical trauma),14 these community experiences are forcing further re-examination of existing paradigms of health.
Continue to: The biological pathway from experience to illness
The biological pathway from experience to illness
Neuroscience supports the epidemiology of ACEs.12 The brain develops from the bottom up, in a use-dependent fashion, contingent on genetic potential and, most importantly, on our experiences, which also influence genetic expression. Although present across the lifespan, the brain’s capacity to change—neuroplasticity—is most robust from the prenatal period until about 3 years of age.15 The autonomic nervous system receives information from the body about our internal world and from sensory organs about our external environment and sends it to the brain for processing and interpretation, resulting in micro- and macro-adaptations in structure and function, both within the brain and in the rest of the body.16
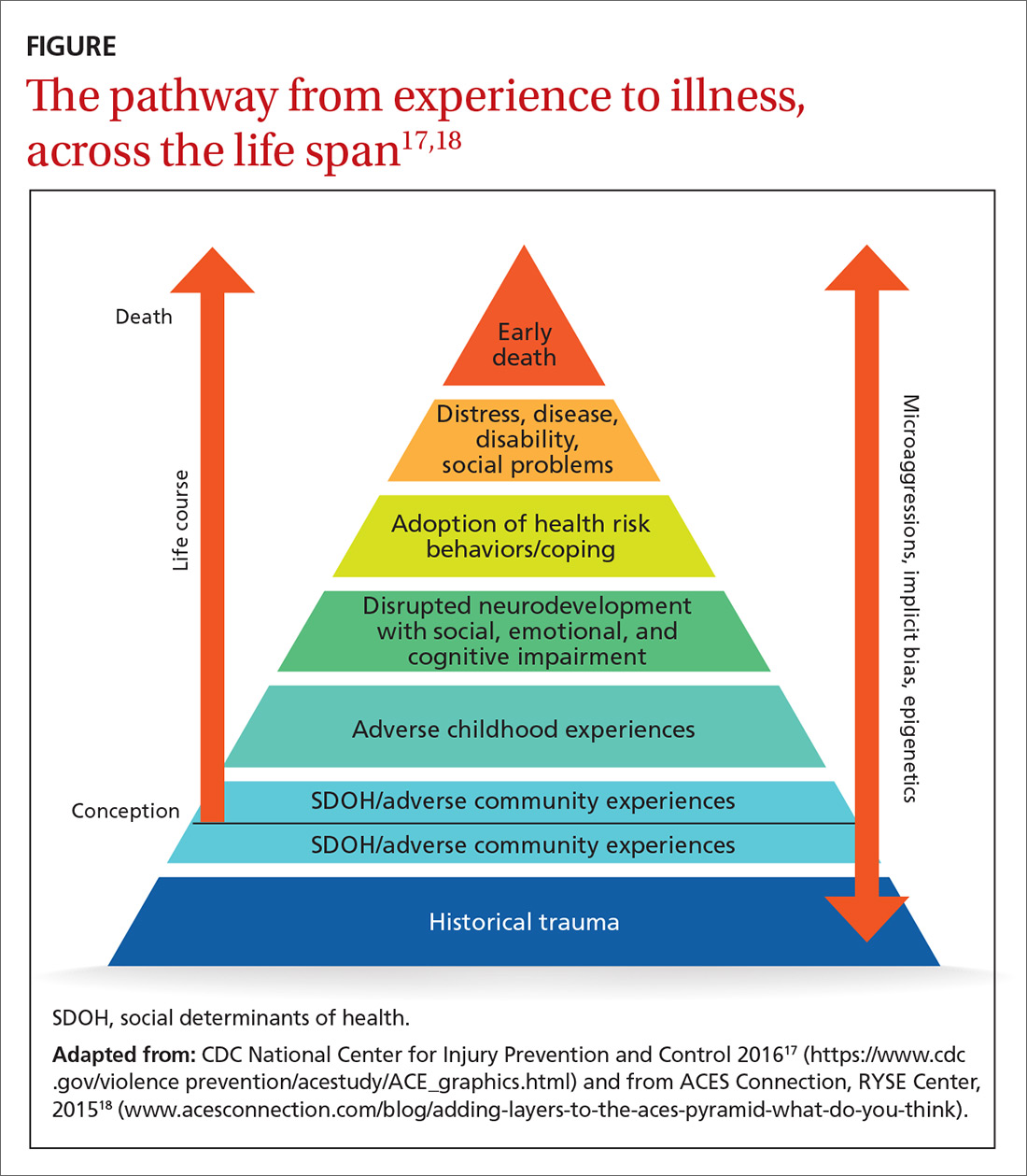
Neuroscience demonstrates that adverse experiences, in the context of insufficient protective factors and depending on their timing, severity, and frequency, cause overactivation or prolonged activation, or both, of the stress response system, thus derailing optimal growth and development of the brain and disrupting healthy signaling in all body systems. The dysregulated stress response drives inflammation and subsequent chronic disease (FIGURE17,18), and may influence genetic expression in this, and future, generations.12,14,19 Using neuroimaging and assessment of biomarkers, researchers can see the harm caused by inadequately buffered adversity on overall anatomy and physiology. Protective factors such as a safe environment and positive relationships provide hope that normal biological responses to adverse circumstances can be prevented or reversed, leading to clinical, cognitive, and functional improvement11 (TABLE 120-22).
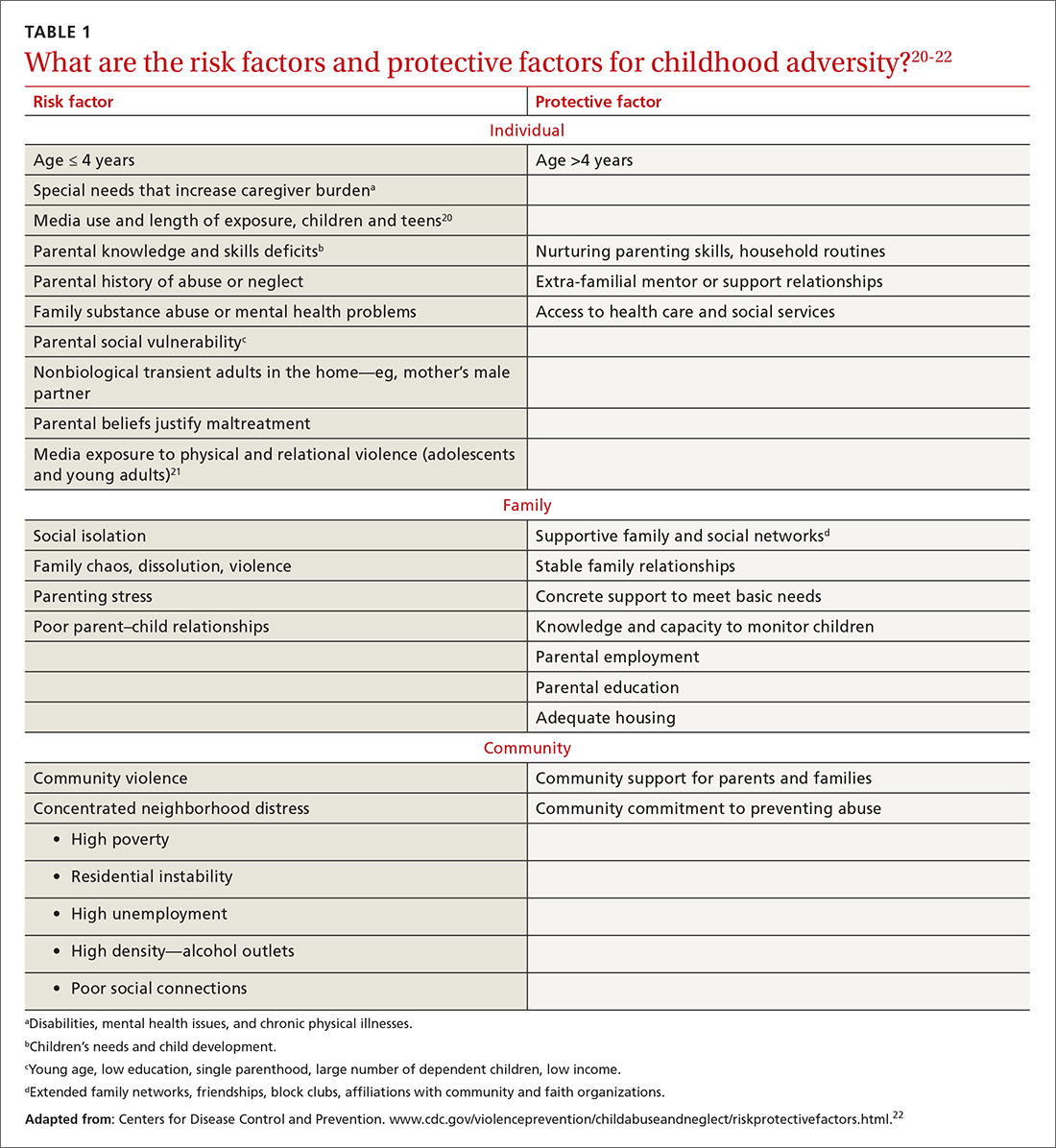
Evidence-based primary prevention of childhood adversity succeeds
Primary prevention of childhood adversity offers significant benefits across the lifespan and, likely, into the next generation. It ensures that every infant has at least 1 nurturing, attuned caregiver with whom to develop a secure attachment relationship that is essential for optimal growth and development of brain and body.
Primary prevention is most effective when it focuses on supporting caregivers during the perinatal and early childhood periods of their families, before children’s brains are fully organized. Primary prevention involves evidence-based program implementation; collaboration among multiple sectors, including early childhood education, child welfare, criminal justice, business, faith, and health care; and, ultimately, policy change. It incorporates individual, family, and community-based strategies to meet basic needs, ensure safety, fortify a sense of love and belonging in families, and support parents in developing optimal parenting skills. This allows caregivers to devote attention to their children, thus strengthening attunement and attachment, reducing toxic stress, and building protective factors and resilience. Evidence-based and -informed prevention programs include the Nurse–Family Partnership (NFP), Positive Parenting Program (Triple P), and the Family-Centered Medical Home.
NFP. Randomized controlled trials of the NFP, a perinatal home visiting program for low-income, first-time pregnant women and their offspring, showed a reduction in the incidence of domestic violence, child maltreatment, and maternal smoking, with improvement in maternal financial stability, cognitive and socioemotional outcomes, and rates of substance abuse and incarceration in children and/or youth.23
Continue to: Triple P
Triple P. A randomized controlled trial of Triple P, an evidence-based, multilevel, population-based preventive intervention system that was designed to support parents and enhance parenting practices for families with at least 1 child (birth to 12 years old), demonstrated a statistically significant reduction in substantiated child maltreatment cases, out-of-home placements, and emergency room visits and hospitalizations for childhood injuries that were the result of child maltreatment.24
The Family-Centered Medical Home, a primary care strategy to reduce premature and low-birth-weight deliveries, used Medicaid dollars for services not traditionally considered “medical” to address all physical and emotional needs of mothers and families as part of the medical relationship. This program eliminated premature delivery and low birth weight,25 both considered evidence of in utero toxic stress.26
Screening can be brief: In some cases, a single question
The prevalence and impact of childhood adversity, along with the opportunity for significant health improvements and savings, inspires providers to explore screening. Existing screening programs have consistent goals27,28:
- identify unique experiences shaping our patients’ health
- reframe “What’s wrong with you?” as “What happened to you?” “What’s right with you?” and “What matters to you?”
- facilitate health education and neuro-education, particularly meaning-making and self-regulation
- prevent and mitigate the sequelae of exposure to ACEs
- promote health in this and subsequent generations.
The ACE Study screened patients in the context of a comprehensive periodic health assessment. Study participants completed an at-home questionnaire and reviewed it with their physician.1 The Urban ACE Survey added important community stressors such as neighborhood violence, bullying, and food insecurity to the original ACE questionnaire.2
Primary care tool. Wade developed a short, 2-question ACE pre-screener for primary care29 and is exploring screening for childhood adversity in pediatric practice, as are primary care clinicians around the country.
Continue to: Single-question screener
Single-question screener. A Chicago internist interviewed more than 500 patients using a single-question screener that asked whether growing up was “mostly okay or pretty difficult.” This tool accurately confirmed childhood adversity in patients with complex chronic illness, prevented re-traumatization by allowing patients control over disclosure, and opened the door to collaborative healing work over time.30
The Hague Protocol, now mandated in the Netherlands for health and justice professionals, focuses its efforts upstream by offering early detection of children at risk for adverse experiences. The protocol requires asking adults who present with intimate partner violence, suicidality, psychiatric disturbance, or severe substance abuse whether they care for children in any capacity. Those who are so identified are referred to a center at which support services are offered.31
Uncertainty about the utility of existing tools. Many screening tools appear to be promising in terms of identification of the risk for, or actual, childhood adversity, patient and provider satisfaction, and their “fit” in the clinical workflow. Even so, no best practice guidelines exist in primary care to steer screening efforts. Questions remain about27-29:
- broad implementation of a specific tool
- how, when, and where screening should take place
- whether to screen adults, parents, or children—or all 3
- how best to use the content and pacing of screening questions to promote self-regulation and prevent re-traumatization
- best strategies for training and supporting health care workers around screening activities
- how to optimally manage a positive screen.
How best to approach treatment
Treatment includes trauma-informed care, an organizational transformation process (described in TABLE 232; in “The lexicon of childhood adversity: Concepts and tools for care”33-45; and in the subsection, “Lessons from neuroscience”), and individual treatment strategies. The Substance Abuse and Mental Health Services Administration (SAMHSA) of the US Department of Health and Human Services is advocating for implementation of trauma-informed approaches in health systems.

Continue to: The lexicon of childhood adversity...
SIDEBAR
The lexicon of childhood adversity: Concepts and tools for care33-45
Adversity A state or instance of serious or continued difficulty or misfortune. 33
Attachment A special, enduring form of emotional relationship with a specific person involving soothing, pleasure, and comfort.34
Attunement The ability to read and respond to the cues of another.35
Eye-movement desensitization and reprocessing (EMDR) An evidence-based psychotherapy for posttraumatic stress disorder and other psychiatric disorders, mental health problems, and somatic symptoms. EMDR facilitates resumption of normal information processing and integration; the patient attends to emotionally disturbing material in brief sequential doses while simultaneously focusing on an external stimulus. EMDR targets past experience, current triggers, and future potential challenges, and results in alleviation of presenting symptoms; a decrease or elimination of distress from the disturbing memory; improved view of the self; relief from bodily disturbance; and resolution of present and future anticipated triggers.36
Historical trauma Cumulative emotional and psychological wounding, resulting from group traumatic experiences, transmitted across generations within a community.37
Neurofeedback Electroencephalographic biofeedback is a method for retraining brainwave patterns through operant conditioning; it is used to treat posttraumatic stress disorder, various mental health conditions, addiction, chronic pain, epilepsy, and other disorders.38
Neuromodulatory Having the capacity to alter nerve activity through targeted delivery of a stimulus, such as electrical stimulation or chemical agents, to specific neurological sites in the body to help restore function or relieve symptoms.39
Social determinants of health/adverse community experiences Conditions in which people are born, grow, live, work, and age and that are shaped by distribution of money, power, and resources at all levels.40,41
Trauma An event or circumstance experienced or observed by a person as physically or emotionally harmful or threatening and having lasting adverse effects on that person's functioning and well-being.42
Trauma-focused cognitive behavioral therapy An evidence-based trauma treatment for children 3 to 18 years and their parents comprising the elements of the acronym PRACTICE: Psychoeducation and parenting; Relaxation methods; Affective expression and regulation skills; Cognitive coping skills and processing; Trauma narrative and processing; In vivo exposure; Conjoint parent-child therapy sessions; and Enhancing personal safety and growth.43
Trauma-informed approach This "4-R" approach can be implemented in any type of service setting, organization, or program that: Realizes the widespread impact of trauma and understands potential paths for recovery; recognizes signs and symptoms of trauma in clients, families, staff, and others involved with the system; responds by fully integrating knowledge about trauma into policies, procedures, and practices; and seeks to actively resist re-traumatization.44
Use-dependent The organization and function of neurons, the neural system, and the brain depends on repetitive, patterned stimulation.45
Continue to: Trauma-informed care is a model...
Trauma-informed care is a model intended to promote healing and reduce the risk for re-traumatization of patients by staff—significant concerns in clinical settings, where the dynamics of loss of power, control, and safety that are inherent in traumatic experience can be replicated.46 To operationalize trauma-informed care more formally, the Center for Health Care Strategies, Inc., and the National Council for Behavioral Health are developing recommendations for 1) standardized screening and assessment tools, evidence-based clinical interventions, implementation processes, and relevant and replicable outcome measures, and 2) policy changes to improve patient and staff engagement, enhance health outcomes, and reduce avoidable care and excess costs.47,48
Lessons from neuroscience guide effective treatment.16 Treatment begins with bottom-up strategies that are focused on decreasing suboptimal excitatory input from the survival brainstem to create safety, connect patients to resources to meet basic needs, teach self-regulation skills, and improve relational health in and outside of the office. Later-stage top-down methods, such as education and other cognitive activities, focus on strengthening the regulatory capacity of the thinking cortex.16 In many ways, treatment mirrors prevention: It emphasizes first helping patients feel safe and loved.
In a follow-up to the ACE Study, 100,000 patients had a primary care visit in which their practitioner reviewed the ACE questionnaire with them; said “I see that you have________. Tell me how that has affected you later in your life” for every “Yes” response; and listened to the answers without passing judgment. This simple intervention profoundly decreased health resource utilization by these patients during the following year: a reduction of 35% in office visits, 11% in emergency room visits, and 3% in hospitalizations.1
The neurosequential model of therapeutics assesses neurodevelopment in the context of childhood adversity and relational health to evaluate consequences of childhood adversity and direct treatment. Adopted domestically and internationally, this model has had statistically significant success facilitating improvement in patients’ physical, emotional, and social health status.16,49
Trauma-specific treatment modalities such as trauma-focused cognitive behavioral therapy and eye-movement desensitization and reprocessing (EMDR),50 a trauma-specific treatment effective in resolving painful childhood memories, are evidence-based treatments that reduce trauma-related symptoms; evidence is also emerging about the efficacy of yoga51 and neurofeedback.52 These therapies have been best studied as treatment for posttraumatic stress disorder and other mental health disorders and also hold promise for addressing physical and social consequences of adversity. They present a low risk for harm, appear to be cost-effective, and improve outcomes.
Continue to: Best regimens involve a multifaceted approach that combines...
Best regimens involve a multifaceted approach that combines health-system resources with referral to other community practitioners and agencies. An excellent example is a current collaboration between health systems and affordable housing programs to reduce and, ultimately, eliminate chronic homelessness. Positive outcomes of this collaboration include both improved health and life satisfaction for participants and cost savings to the health system.53
CASE
Beginning in adulthood, Ms. W began long-term psychotherapy and had a therapeutic trial of antidepressants, without significant improvement. None of her medical or mental-health providers educated her about the connection between childhood adversity and illness to help her make sense of her health history and autoimmune disease, or to guide treatment. She learned from a friend about the relationship between childhood adversity and poor health and self-administered the ACE questionnaire, scoring 5 points out of a possible 10.
Ms. W enjoyed loving relationships with her mother, sisters, and friends. She had long-standing personal practices of individual and group physical activity, journaling, and spending time in nature.
About 10 years ago, Ms. W committed to regular yoga practice and later saw a functional medicine provider, who focused on nutrition and restorative sleep. She noticed improvement in all signs and symptoms; however, the terror of public speaking remained. Through friends, she found a practitioner who offered EMDR. Over the past 2 years, her terror has resolved and general anxiety and insomnia have continued to improve; she is now able to speak with fluency and comfort in any arena.
Addressing childhood adversity: Our “natural domain”
Experiences, positive and negative, shape our psychology and biology; they are powerful determinants of health—or illness. Prevention of, and response to, childhood adversity demand a systems approach to the whole person in context—the natural domain of family medicine.
Continue to: Although clinical translation is still unfolding...
Although clinical translation is still unfolding, the risks of implementing promising prevention and treatment strategies are low, the stakes are high, and the potential benefits are vast. Therefore, we as family physicians can—must—learn and incorporate the science of childhood adversity, neurobiology, and life course into our training, research, and clinical paradigm and practice; we can do that by embedding this framework throughout our training and continuing education in formal didactics, case discussions, hands-on skill-building, scientific investigation, and patient care.
We must make our offices and hospitals trauma-informed; connect patients with resources to meet basic needs and with home-visiting and parent education programs; educate patients about the impact of protective and adverse factors on health; provide and practice self-regulation training in our offices or by referral; and advocate for equity.
Using these strategies, family physicians will play a crucial role in the prevention, mitigation, and treatment of the root cause of disease and society’s deepest individual and collective suffering.
CORRESPONDENCE
Audrey Stillerman, MD, ABFM, ABIHM, ABOIM, Office of Community Engagement and Neighborhood Health Partnerships, 808 South Wolcott Street, Room 809, Chicago, IL 60612; [email protected].
ACKNOWLEDGMENT
Patricia Rush, MD, MBA, and Adrienne Williams, PhD, reviewed the manuscript of this article.
1. Felitti V, Anda R. The relationship of adverse childhood experiences to adult medical disease, psychiatric disorders and sexual behavior: implications for healthcare. In: Lanius RA, Vermetten E, Pain C, eds. The Impact of Early Life Trauma on Health and Disease: The Hidden Epidemic. Cambridge, UK: Cambridge University Press; 2011:77-87.
2. Wade R Jr, Shea JA, Rubin D, et al. Adverse childhood experiences of low-income urban youth. Pediatrics. 2014;134:e13-e20.
3. Centers for Disease Control and Prevention. Child abuse and neglect prevention. April 10, 2018. www.cdc.gov/violenceprevention/childabuseandneglect/index.html. Accessed September 20, 2018.
4. American Academy of Family Physicians. Definition of family medicine. www.aafp.org/about/policies/all/family-medicine-definition.html. Accessed March 5, 2018.
5. Martin JC, Avant RF, Bowman MA, et al; The Future of Family Medicine Project Leadership Committee. The Future of Family Medicine: a collaborative project of the family medicine community. Ann Fam Med. 2004;2 Suppl 1:S3-S32.
6. Child & Adolescent Health Measurement Initiative (CAHMI). A national and across-state profile on Adverse Childhood Experiences among U.S. children and possibilities to heal and thrive. Issue Brief. October 2017. www.cahmi.org/wp-content/uploads/2018/05/aces_brief_final.pdf. Accessed September 20, 2018.
7. World Health Organization. Adverse Childhood Experiences International Questionnaire (ACE-IQ). www.who.int/violence_injury_prevention/violence/activities/adverse_childhood_experiences/en/. Accessed September 20, 2018.
8. Roos LE, Mota N, Afifi TO, et al. Relationship between adverse childhood experiences and homelessness and the impact of axis I and II disorders. Am J Public Health. 2013;103(Suppl 2):S275-S281.
9. Baglivio MT. Wolff KT. Piquero AR, et al. The relationship between adverse childhood experiences (ACE) and juvenile offending trajectories in a juvenile offender sample. J Crim Justice. 2015;43:229-241.
10. Dube SR. Felitti VF. Dong M, et al. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics. 2003;111:564-572.
11. Maunder RG, Peladeau N, Savage D, et al. The prevalence of childhood adversity among healthcare workers and its relationship to adult life events, distress and impairment. Child Abuse Negl. 2010;34:114-123.
12. Anda RF, Felitti VJ, Bremner JD, et al. The enduring effects of abuse and related adverse experiences in childhood: a convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256:174-186.
13. Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic disparities in health in the United States: what the patterns tell us. Am J Public Health. 2010;100(Suppl 1):S186-S196.
14. Bowers ME, Yehuda R. Intergenerational transmission of stress in humans. Neuropsychopharmacology. 2016;41:232-244.
15. Perry BD. Memories of fears: How the brain stores and retrieves traumatic experiences. In: Goodwin J, Attias R, eds. Splintered Reflections: Images of the Body in Trauma. New York, NY: Basic Books; 1999:9-38.
16. Perry BD. Examining child maltreatment through a neurodevelopmental lens: clinical application of the Neurosequential Model of Therapeutics. J Loss Trauma. 2009;14:240-255.
17.
18. Adding layers to the ACEs pyramid—What do you think? Trauma and social location. ACES Connection, RYSE Center. 2015. www.acesconnection.com/blog/adding-layers-to-the-aces-pyramid-what-do-you-think. Accessed October 10, 2018.
19. Berens AE, Jensen SKG, Nelson CA 3rd. Biological embedding of childhood adversity: from physiological mechanisms to clinical implications. BMC Med. 2017;15:135.
20. Rostad WL, Basile KC, Clayton HB. Association among television and computer/video game use, victimization, and suicide risk among U.S. high school students. J Interpers Violence. 2018 Mar 1:886260518760020.
21. Coyne SM, Nelson DA, Graham-Kevan N, et al. Media depictions of physical and relational aggression: connections with aggression in young adults’ romantic relationships. Aggress Behav. 2011;37:56-62.
22. Centers for Disease Control and Prevention. Violence prevention: Child abuse and neglect: risk and protective factors. April 10, 2018. www.cdc.gov/violenceprevention/childabuseandneglect/riskprotectivefactors.html. Accessed October 10, 2018.
23. Miller TR. Projected outcomes of nurse-family partnership home visitation during 1996-2013, United States. Prev Sci. 2015;16:765-777.
24. Prinz RJ, Sanders MR, Shapiro CJ, et al. Population-based prevention of child maltreatment: the U.S. Triple P system population trial. Prev Sci. 2009;10:1-12.
25. Kraft C. Building capacity & support for two generation primary care. 2015 Midwest Regional Summit on Adverse Childhood Experiences. March 13, 2015. www.hmprg.org/assets/root/PDFs/2015/Summit%20Notes%20for%20Day%20Two.pdf. Accessed September 20, 2018.
26. Smith MV, Gotman N, Yonkers KA. Early childhood adversity and pregnancy outcomes. Matern Child Health J. 2016;20:790-798.
27. Leitch L. Action steps using ACEs and trauma-informed care: a resilience model. Health & Justice. 2017;5:1-10.
28. Bethell CD, Carle A, Hudziak J, et al. Methods to assess adverse childhood experiences of children and families: toward approaches to promote child well-being in policy and practice. Acad Pediatr. 2017;17:S51-S69.
29. Wade R Jr, Becker BD, Bevans KB, et al. Development and evaluation of a short adverse childhood experiences measure. Am J Prev Med. 2017;52:163-172.
30. Rush P. How learning about emotional trauma led me to a new understanding of chronic illness and health disparity. Becoming trauma-informed: Perspectives from public health, faith communities, education and medicine. Presented at 2016 Advocate Symposium, “Becoming a Trauma-Informed Children’s Hospital and Community: Building Foundations of Care, Collaboration and Practice.” Oaklawn, IL: Advocate Children’s Hospital; November 16, 2016.
31. Diderich HM, Fekkes M, Verkerk PH, et al. A new protocol for screening adults presenting with their own medical problems at the Emergency Department to identify children at high risk for maltreatment. Child Abuse Negl. 2013;37:1122-1131.
32. Fact Sheet: Key ingredients for trauma-informed care. Center for Health Care Strategies, Inc. August 2017. www.chcs.org/media/ATC-Key-Ingredients-Fact-Sheet_081417.pdf. Accessed September 22, 2018.
33. Adversity. In: Merriam-Webster Online Dictionary. Springfield, MA: Merriam-Webster, Inc. www.merriam-webster.com/dictionary/adversity. Accessed September 21, 2018.
34. Perry BD. Understanding traumatized and maltreated children: the core concepts. Child Trauma Academy Video Training Series, Video 4;2004:12. Child Trauma Academy (http://childtrauma.org/).
35. Perry BD. Understanding traumatized and maltreated children: the core concepts. Child Trauma Academy Video Training Series, Video 4;2004:19. Child Trauma Academy (http://childtrauma.org/).
36. EMDRIA’s definition of EMDR (eye movement desensitization and reprocessing). Austin, TX: EMDRIA: EMDR International Association. http://c.ymcdn.com/sites/www.emdria.org/resource/resmgr/imported/EMDRIA%20Definition%20of%20EMDR.pdf. Revised February 25 2012. Accessed September 21, 2018.
37. Types of trauma and violence: Historical trauma. Washington, DC: Substance Abuse and Mental Health Services Administration. www.samhsa.gov/trauma-violence/types. Accessed September 21, 2018.
38. Hammond DC. What is neurofeedback? An update. J Neurotherapy. 2011;15:305-336.
39. International Neuromodulation Society. Neuromodulation, or neuromodulatory effect. www.neuromodulation.com/neuromodulation-defined. November 9, 2017. Accessed September 21, 2018.
40. World Health Organization. Social determinants of health. www.who.int/social_determinants/sdh_definition/en/. Accessed September 21, 2018.
41. Davis R, Pinderhughes H, Williams M. Adverse community experiences and resilience: a framework for addressing and preventing community trauma. Oakland, CA: Prevention Institute; 2015:4-5. www.preventioninstitute.org/publications/adverse-community-experiences-and-resilience-framework-addressing-and-preventing. Accessed September 30, 2018.
42. SAMHSA-HRSA Center for Integrated Health Solutions. Trauma. Rockville, MD: Substance Abuse and Mental Health Services Administration and Health Resources and Services Administration, US Department of Health and Human Services. www.integration.samhsa.gov/clinical-practice/trauma. Accessed September 21, 2018.
43. Cohen JA, Mandarino AP. Trauma-focused cognitive behavioural therapy for children and parents. Child Adolesc Ment Health. 2008;13:158-162.
44. Trauma-informed approach and trauma-specific interventions: Trauma-informed approach. Washington, DC: National Center for Trauma Informed Care and Alternatives to Seclusion and Restraints; Substance Abuse and Mental Health Services Administration. www.samhsa.gov/nctic/trauma-interventions. Accessed September 21, 2018.
45. Perry BD. How the brain develops: the importance of early childhood. Child Trauma Academy Video Training Series, Video 1;2004:21. Child Trauma Academy (http://childtrauma.org/).
46. Huang LN, Sharp CS, Gunther T. It’s just good medicine: trauma-informed primary care. (SAMHSA-HRSA Center for Integrated Health Solutions webinar); August 6, 2013. www.integration.samhsa.gov/about-us/CIHS_TIC_Webinar_PDF.pdf. Accessed September 20, 2018.
47. CHCS: Center for Health Care Strategies, Inc. Fact sheet: Key ingredients for trauma-informed care. August 2017. www.chcs.org/media/ATC-Key-Ingredients-Fact-Sheet_081417.pdf. Accessed September 20, 2018.
48. National Council for Behavioral Health. Trauma-informed primary care: fostering resilience and recovery. www.thenationalcouncil.org/consulting-areas-of-expertise/trauma-informed-primary-care/. Accessed September 20, 2018.
49. Child Trauma Academy. The Neurosequential Model of Therapeutics as evidence-based practice. https://childtrauma.org/wp-content/uploads/2015/05/NMT_EvidenceBasedPract_5_2_15.pdf. Accessed September 30, 2018.
50. Bisson JI, Ehlers A, Matthews R, et al. Psychological treatments for chronic post-traumatic stress disorder. Systematic review and meta-analysis. Br J Psychiatry. 2007;190:97-104.
51. Metcalf O, Varker T, Forbes D, et al. Efficacy of fifteen emerging interventions for the treatment of posttraumatic stress disorder: a systematic review. J Trauma Stress. 2016;29:88-92.
52. van der Kolk BA, Hodgdon H, Gapen M, et al. A randomized controlled study of neurofeedback for chronic PTSD. 2016; PLoS One. 2016;11:e0166752.
53. Bryan M. A hospital offers frequent ER patients an out—free housing. “All Things Considered.” National Public Radio. June 29, 2016. www.npr.org/sections/health-shots/2016/06/29/482994000/a-hospital-offers-frequent-er-patients-an-out-free-housing. Acces-sed September 20, 2018.
The rising prevalence of obesity, widespread community violence, and the opioid epidemic are urgent health crises that we have, so far, failed to solve. Physicians must therefore ask: Are we employing the right framework to effectively understand and address these complex problems?
Careful review of the literature reveals that these problems and many others begin with, and are profoundly affected by, childhood adversity. Compounding this, studies over the past 20 years that have focused on abuse and neglect without including community, structural, and historical adversity demonstrate that our definitions of adversity and trauma have been too narrow. The prevalence and diversity of factors affecting development and health is much greater than our medical model anticipates.1,2
CASE
Eileen W, a 55-year-old married, self-employed woman with a 20-year history of autoimmune thyroiditis, longstanding insomnia, and anxiety presents with intense episodes of terror related to public speaking, which are compromising her work performance. Her history is significant for tobacco and alcohol use beginning in early adolescence and continuing into young adulthood, as well as 2 unplanned pregnancies in her 20s. Additional adversities included the murder of her maternal aunt while Ms. W was in utero, resulting in her parents having fostered 2 young cousins; bullying; and the premature death of a special-needs sibling.
What treatment strategies might have been undertaken to manage consequences of the adversities of Ms. W’s childhood—both on her own initiative and as interventions by her health care providers?

Our medical model must be updated to be effective
Because at least 60% of Americans have had 1 or more experiences of childhood adversity, family physicians care for affected patients every day—a reality incompletely addressed by our conventional theories and practices.1,3 Consequently, updating our medical model to incorporate research that confirms the critical and widespread impact of childhood experience on health and illness is an essential task for family medicine.
Core values of family medicine integrate biological, clinical, and behavioral sciences. They include comprehensive and compassionate care that is provided within the context of family and community across the lifespan.4,5 Family medicine is therefore the ideal specialty to lead a movement that will translate scientific evidence of the effects of childhood adversity on health into training, delivery of care, and research—transforming clinical practice and patient health across the lifespan.
This article describes the dramatic impact of childhood adversity on health and well-being and calls on family physicians to play a crucial role in preventing, mitigating, and treating the consequences of childhood adversity, an important root cause of disease.
Continue to: Childhood adversity makes us sick
Childhood adversity makes us sick
The first paper about the landmark Adverse Childhood Experiences (ACE) Study, published 20 years ago, is 1 of more than 90 on this topic.3 This study explored the relationship of physical, emotional, and social health in adulthood and self-reported childhood adversity, and comprised 10 categories of abuse, neglect, and household distress between birth and 18 years of age. One of the largest epidemiological studies of its kind, the ACE Study surveyed more than 17,000 mostly white, middle-aged, educated, and insured participants. Study researchers developed an “ACE Score”—the total number of ACEs faced by a person before her (his) 18th birthday—and found that 64% of respondents endorsed 1 or more ACEs; 27% reported 3 or more ACEs; and 5% experienced 6 or more.
The ACE Study revealed a dose–response relationship between ACEs and more than 40 health-compromising behaviors, negative health conditions, and poor social outcomes. Examples include cardiac, autoimmune disease, obesity, intravenous drug abuse, depression and anxiety, adolescent pregnancy, and worker absenteeism. Tragically, an ACE score of ≥6 conferred a significant risk for premature death.1
ACE data have been collected in diverse populations in 32 states and many countries through the Behavioral Risk Factor Surveillance Survey conducted by the Centers for Disease Control and Prevention3; the Child & Adolescent Health Measurement Initiative’s National Survey of Children’s Health6; and The World Health Organization’s ACE International Questionnaire7—underscoring the pervasiveness of childhood adversity. Evaluation of ACEs in special populations, such as people experiencing homelessness,8 incarcerated youth,9 people struggling with addiction,10 and even health care workers,11 uncovers notably higher rates of ACEs in these populations than in the general population.
Is childhood adversity a true cause of bad outcomes?
Or is the relationship between the 2 entities merely an association? To help answer this question, researchers evaluated the ACE Study using Bradford Hill criteria—9 epidemiological principles employed to infer causation. Their findings strongly support the hypothesis that not only are ACEs associated with myriad negative outcomes, they are their root cause12 and therefore a powerful determinant of our most pressing and expensive health and social problems.Nevertheless, strategies to prevent and address childhood adversity, which are critical to meeting national health goals of successful prevention and treatment of myriad conditions, are absent from the paradigm and practice of most physicians.
The body of research about the health impact of additional adverse experiences is growing to include community violence, poverty, longstanding discrimination,2 and other experiences that we describe as social determinants of health. Furthermore, social determinants of health, or adverse community experiences, appear to maintain a dose–response relationship with health and social outcomes.2 ,13 Along with adverse collective historical experiences (historical trauma),14 these community experiences are forcing further re-examination of existing paradigms of health.
Continue to: The biological pathway from experience to illness
The biological pathway from experience to illness
Neuroscience supports the epidemiology of ACEs.12 The brain develops from the bottom up, in a use-dependent fashion, contingent on genetic potential and, most importantly, on our experiences, which also influence genetic expression. Although present across the lifespan, the brain’s capacity to change—neuroplasticity—is most robust from the prenatal period until about 3 years of age.15 The autonomic nervous system receives information from the body about our internal world and from sensory organs about our external environment and sends it to the brain for processing and interpretation, resulting in micro- and macro-adaptations in structure and function, both within the brain and in the rest of the body.16

Neuroscience demonstrates that adverse experiences, in the context of insufficient protective factors and depending on their timing, severity, and frequency, cause overactivation or prolonged activation, or both, of the stress response system, thus derailing optimal growth and development of the brain and disrupting healthy signaling in all body systems. The dysregulated stress response drives inflammation and subsequent chronic disease (FIGURE17,18), and may influence genetic expression in this, and future, generations.12,14,19 Using neuroimaging and assessment of biomarkers, researchers can see the harm caused by inadequately buffered adversity on overall anatomy and physiology. Protective factors such as a safe environment and positive relationships provide hope that normal biological responses to adverse circumstances can be prevented or reversed, leading to clinical, cognitive, and functional improvement11 (TABLE 120-22).

Evidence-based primary prevention of childhood adversity succeeds
Primary prevention of childhood adversity offers significant benefits across the lifespan and, likely, into the next generation. It ensures that every infant has at least 1 nurturing, attuned caregiver with whom to develop a secure attachment relationship that is essential for optimal growth and development of brain and body.
Primary prevention is most effective when it focuses on supporting caregivers during the perinatal and early childhood periods of their families, before children’s brains are fully organized. Primary prevention involves evidence-based program implementation; collaboration among multiple sectors, including early childhood education, child welfare, criminal justice, business, faith, and health care; and, ultimately, policy change. It incorporates individual, family, and community-based strategies to meet basic needs, ensure safety, fortify a sense of love and belonging in families, and support parents in developing optimal parenting skills. This allows caregivers to devote attention to their children, thus strengthening attunement and attachment, reducing toxic stress, and building protective factors and resilience. Evidence-based and -informed prevention programs include the Nurse–Family Partnership (NFP), Positive Parenting Program (Triple P), and the Family-Centered Medical Home.
NFP. Randomized controlled trials of the NFP, a perinatal home visiting program for low-income, first-time pregnant women and their offspring, showed a reduction in the incidence of domestic violence, child maltreatment, and maternal smoking, with improvement in maternal financial stability, cognitive and socioemotional outcomes, and rates of substance abuse and incarceration in children and/or youth.23
Continue to: Triple P
Triple P. A randomized controlled trial of Triple P, an evidence-based, multilevel, population-based preventive intervention system that was designed to support parents and enhance parenting practices for families with at least 1 child (birth to 12 years old), demonstrated a statistically significant reduction in substantiated child maltreatment cases, out-of-home placements, and emergency room visits and hospitalizations for childhood injuries that were the result of child maltreatment.24
The Family-Centered Medical Home, a primary care strategy to reduce premature and low-birth-weight deliveries, used Medicaid dollars for services not traditionally considered “medical” to address all physical and emotional needs of mothers and families as part of the medical relationship. This program eliminated premature delivery and low birth weight,25 both considered evidence of in utero toxic stress.26
Screening can be brief: In some cases, a single question
The prevalence and impact of childhood adversity, along with the opportunity for significant health improvements and savings, inspires providers to explore screening. Existing screening programs have consistent goals27,28:
- identify unique experiences shaping our patients’ health
- reframe “What’s wrong with you?” as “What happened to you?” “What’s right with you?” and “What matters to you?”
- facilitate health education and neuro-education, particularly meaning-making and self-regulation
- prevent and mitigate the sequelae of exposure to ACEs
- promote health in this and subsequent generations.
The ACE Study screened patients in the context of a comprehensive periodic health assessment. Study participants completed an at-home questionnaire and reviewed it with their physician.1 The Urban ACE Survey added important community stressors such as neighborhood violence, bullying, and food insecurity to the original ACE questionnaire.2
Primary care tool. Wade developed a short, 2-question ACE pre-screener for primary care29 and is exploring screening for childhood adversity in pediatric practice, as are primary care clinicians around the country.
Continue to: Single-question screener
Single-question screener. A Chicago internist interviewed more than 500 patients using a single-question screener that asked whether growing up was “mostly okay or pretty difficult.” This tool accurately confirmed childhood adversity in patients with complex chronic illness, prevented re-traumatization by allowing patients control over disclosure, and opened the door to collaborative healing work over time.30
The Hague Protocol, now mandated in the Netherlands for health and justice professionals, focuses its efforts upstream by offering early detection of children at risk for adverse experiences. The protocol requires asking adults who present with intimate partner violence, suicidality, psychiatric disturbance, or severe substance abuse whether they care for children in any capacity. Those who are so identified are referred to a center at which support services are offered.31
Uncertainty about the utility of existing tools. Many screening tools appear to be promising in terms of identification of the risk for, or actual, childhood adversity, patient and provider satisfaction, and their “fit” in the clinical workflow. Even so, no best practice guidelines exist in primary care to steer screening efforts. Questions remain about27-29:
- broad implementation of a specific tool
- how, when, and where screening should take place
- whether to screen adults, parents, or children—or all 3
- how best to use the content and pacing of screening questions to promote self-regulation and prevent re-traumatization
- best strategies for training and supporting health care workers around screening activities
- how to optimally manage a positive screen.
How best to approach treatment
Treatment includes trauma-informed care, an organizational transformation process (described in TABLE 232; in “The lexicon of childhood adversity: Concepts and tools for care”33-45; and in the subsection, “Lessons from neuroscience”), and individual treatment strategies. The Substance Abuse and Mental Health Services Administration (SAMHSA) of the US Department of Health and Human Services is advocating for implementation of trauma-informed approaches in health systems.

Continue to: The lexicon of childhood adversity...
SIDEBAR
The lexicon of childhood adversity: Concepts and tools for care33-45
Adversity A state or instance of serious or continued difficulty or misfortune. 33
Attachment A special, enduring form of emotional relationship with a specific person involving soothing, pleasure, and comfort.34
Attunement The ability to read and respond to the cues of another.35
Eye-movement desensitization and reprocessing (EMDR) An evidence-based psychotherapy for posttraumatic stress disorder and other psychiatric disorders, mental health problems, and somatic symptoms. EMDR facilitates resumption of normal information processing and integration; the patient attends to emotionally disturbing material in brief sequential doses while simultaneously focusing on an external stimulus. EMDR targets past experience, current triggers, and future potential challenges, and results in alleviation of presenting symptoms; a decrease or elimination of distress from the disturbing memory; improved view of the self; relief from bodily disturbance; and resolution of present and future anticipated triggers.36
Historical trauma Cumulative emotional and psychological wounding, resulting from group traumatic experiences, transmitted across generations within a community.37
Neurofeedback Electroencephalographic biofeedback is a method for retraining brainwave patterns through operant conditioning; it is used to treat posttraumatic stress disorder, various mental health conditions, addiction, chronic pain, epilepsy, and other disorders.38
Neuromodulatory Having the capacity to alter nerve activity through targeted delivery of a stimulus, such as electrical stimulation or chemical agents, to specific neurological sites in the body to help restore function or relieve symptoms.39
Social determinants of health/adverse community experiences Conditions in which people are born, grow, live, work, and age and that are shaped by distribution of money, power, and resources at all levels.40,41
Trauma An event or circumstance experienced or observed by a person as physically or emotionally harmful or threatening and having lasting adverse effects on that person's functioning and well-being.42
Trauma-focused cognitive behavioral therapy An evidence-based trauma treatment for children 3 to 18 years and their parents comprising the elements of the acronym PRACTICE: Psychoeducation and parenting; Relaxation methods; Affective expression and regulation skills; Cognitive coping skills and processing; Trauma narrative and processing; In vivo exposure; Conjoint parent-child therapy sessions; and Enhancing personal safety and growth.43
Trauma-informed approach This "4-R" approach can be implemented in any type of service setting, organization, or program that: Realizes the widespread impact of trauma and understands potential paths for recovery; recognizes signs and symptoms of trauma in clients, families, staff, and others involved with the system; responds by fully integrating knowledge about trauma into policies, procedures, and practices; and seeks to actively resist re-traumatization.44
Use-dependent The organization and function of neurons, the neural system, and the brain depends on repetitive, patterned stimulation.45
Continue to: Trauma-informed care is a model...
Trauma-informed care is a model intended to promote healing and reduce the risk for re-traumatization of patients by staff—significant concerns in clinical settings, where the dynamics of loss of power, control, and safety that are inherent in traumatic experience can be replicated.46 To operationalize trauma-informed care more formally, the Center for Health Care Strategies, Inc., and the National Council for Behavioral Health are developing recommendations for 1) standardized screening and assessment tools, evidence-based clinical interventions, implementation processes, and relevant and replicable outcome measures, and 2) policy changes to improve patient and staff engagement, enhance health outcomes, and reduce avoidable care and excess costs.47,48
Lessons from neuroscience guide effective treatment.16 Treatment begins with bottom-up strategies that are focused on decreasing suboptimal excitatory input from the survival brainstem to create safety, connect patients to resources to meet basic needs, teach self-regulation skills, and improve relational health in and outside of the office. Later-stage top-down methods, such as education and other cognitive activities, focus on strengthening the regulatory capacity of the thinking cortex.16 In many ways, treatment mirrors prevention: It emphasizes first helping patients feel safe and loved.
In a follow-up to the ACE Study, 100,000 patients had a primary care visit in which their practitioner reviewed the ACE questionnaire with them; said “I see that you have________. Tell me how that has affected you later in your life” for every “Yes” response; and listened to the answers without passing judgment. This simple intervention profoundly decreased health resource utilization by these patients during the following year: a reduction of 35% in office visits, 11% in emergency room visits, and 3% in hospitalizations.1
The neurosequential model of therapeutics assesses neurodevelopment in the context of childhood adversity and relational health to evaluate consequences of childhood adversity and direct treatment. Adopted domestically and internationally, this model has had statistically significant success facilitating improvement in patients’ physical, emotional, and social health status.16,49
Trauma-specific treatment modalities such as trauma-focused cognitive behavioral therapy and eye-movement desensitization and reprocessing (EMDR),50 a trauma-specific treatment effective in resolving painful childhood memories, are evidence-based treatments that reduce trauma-related symptoms; evidence is also emerging about the efficacy of yoga51 and neurofeedback.52 These therapies have been best studied as treatment for posttraumatic stress disorder and other mental health disorders and also hold promise for addressing physical and social consequences of adversity. They present a low risk for harm, appear to be cost-effective, and improve outcomes.
Continue to: Best regimens involve a multifaceted approach that combines...
Best regimens involve a multifaceted approach that combines health-system resources with referral to other community practitioners and agencies. An excellent example is a current collaboration between health systems and affordable housing programs to reduce and, ultimately, eliminate chronic homelessness. Positive outcomes of this collaboration include both improved health and life satisfaction for participants and cost savings to the health system.53
CASE
Beginning in adulthood, Ms. W began long-term psychotherapy and had a therapeutic trial of antidepressants, without significant improvement. None of her medical or mental-health providers educated her about the connection between childhood adversity and illness to help her make sense of her health history and autoimmune disease, or to guide treatment. She learned from a friend about the relationship between childhood adversity and poor health and self-administered the ACE questionnaire, scoring 5 points out of a possible 10.
Ms. W enjoyed loving relationships with her mother, sisters, and friends. She had long-standing personal practices of individual and group physical activity, journaling, and spending time in nature.
About 10 years ago, Ms. W committed to regular yoga practice and later saw a functional medicine provider, who focused on nutrition and restorative sleep. She noticed improvement in all signs and symptoms; however, the terror of public speaking remained. Through friends, she found a practitioner who offered EMDR. Over the past 2 years, her terror has resolved and general anxiety and insomnia have continued to improve; she is now able to speak with fluency and comfort in any arena.
Addressing childhood adversity: Our “natural domain”
Experiences, positive and negative, shape our psychology and biology; they are powerful determinants of health—or illness. Prevention of, and response to, childhood adversity demand a systems approach to the whole person in context—the natural domain of family medicine.
Continue to: Although clinical translation is still unfolding...
Although clinical translation is still unfolding, the risks of implementing promising prevention and treatment strategies are low, the stakes are high, and the potential benefits are vast. Therefore, we as family physicians can—must—learn and incorporate the science of childhood adversity, neurobiology, and life course into our training, research, and clinical paradigm and practice; we can do that by embedding this framework throughout our training and continuing education in formal didactics, case discussions, hands-on skill-building, scientific investigation, and patient care.
We must make our offices and hospitals trauma-informed; connect patients with resources to meet basic needs and with home-visiting and parent education programs; educate patients about the impact of protective and adverse factors on health; provide and practice self-regulation training in our offices or by referral; and advocate for equity.
Using these strategies, family physicians will play a crucial role in the prevention, mitigation, and treatment of the root cause of disease and society’s deepest individual and collective suffering.
CORRESPONDENCE
Audrey Stillerman, MD, ABFM, ABIHM, ABOIM, Office of Community Engagement and Neighborhood Health Partnerships, 808 South Wolcott Street, Room 809, Chicago, IL 60612; [email protected].
ACKNOWLEDGMENT
Patricia Rush, MD, MBA, and Adrienne Williams, PhD, reviewed the manuscript of this article.
The rising prevalence of obesity, widespread community violence, and the opioid epidemic are urgent health crises that we have, so far, failed to solve. Physicians must therefore ask: Are we employing the right framework to effectively understand and address these complex problems?
Careful review of the literature reveals that these problems and many others begin with, and are profoundly affected by, childhood adversity. Compounding this, studies over the past 20 years that have focused on abuse and neglect without including community, structural, and historical adversity demonstrate that our definitions of adversity and trauma have been too narrow. The prevalence and diversity of factors affecting development and health is much greater than our medical model anticipates.1,2
CASE
Eileen W, a 55-year-old married, self-employed woman with a 20-year history of autoimmune thyroiditis, longstanding insomnia, and anxiety presents with intense episodes of terror related to public speaking, which are compromising her work performance. Her history is significant for tobacco and alcohol use beginning in early adolescence and continuing into young adulthood, as well as 2 unplanned pregnancies in her 20s. Additional adversities included the murder of her maternal aunt while Ms. W was in utero, resulting in her parents having fostered 2 young cousins; bullying; and the premature death of a special-needs sibling.
What treatment strategies might have been undertaken to manage consequences of the adversities of Ms. W’s childhood—both on her own initiative and as interventions by her health care providers?

Our medical model must be updated to be effective
Because at least 60% of Americans have had 1 or more experiences of childhood adversity, family physicians care for affected patients every day—a reality incompletely addressed by our conventional theories and practices.1,3 Consequently, updating our medical model to incorporate research that confirms the critical and widespread impact of childhood experience on health and illness is an essential task for family medicine.
Core values of family medicine integrate biological, clinical, and behavioral sciences. They include comprehensive and compassionate care that is provided within the context of family and community across the lifespan.4,5 Family medicine is therefore the ideal specialty to lead a movement that will translate scientific evidence of the effects of childhood adversity on health into training, delivery of care, and research—transforming clinical practice and patient health across the lifespan.
This article describes the dramatic impact of childhood adversity on health and well-being and calls on family physicians to play a crucial role in preventing, mitigating, and treating the consequences of childhood adversity, an important root cause of disease.
Continue to: Childhood adversity makes us sick
Childhood adversity makes us sick
The first paper about the landmark Adverse Childhood Experiences (ACE) Study, published 20 years ago, is 1 of more than 90 on this topic.3 This study explored the relationship of physical, emotional, and social health in adulthood and self-reported childhood adversity, and comprised 10 categories of abuse, neglect, and household distress between birth and 18 years of age. One of the largest epidemiological studies of its kind, the ACE Study surveyed more than 17,000 mostly white, middle-aged, educated, and insured participants. Study researchers developed an “ACE Score”—the total number of ACEs faced by a person before her (his) 18th birthday—and found that 64% of respondents endorsed 1 or more ACEs; 27% reported 3 or more ACEs; and 5% experienced 6 or more.
The ACE Study revealed a dose–response relationship between ACEs and more than 40 health-compromising behaviors, negative health conditions, and poor social outcomes. Examples include cardiac, autoimmune disease, obesity, intravenous drug abuse, depression and anxiety, adolescent pregnancy, and worker absenteeism. Tragically, an ACE score of ≥6 conferred a significant risk for premature death.1
ACE data have been collected in diverse populations in 32 states and many countries through the Behavioral Risk Factor Surveillance Survey conducted by the Centers for Disease Control and Prevention3; the Child & Adolescent Health Measurement Initiative’s National Survey of Children’s Health6; and The World Health Organization’s ACE International Questionnaire7—underscoring the pervasiveness of childhood adversity. Evaluation of ACEs in special populations, such as people experiencing homelessness,8 incarcerated youth,9 people struggling with addiction,10 and even health care workers,11 uncovers notably higher rates of ACEs in these populations than in the general population.
Is childhood adversity a true cause of bad outcomes?
Or is the relationship between the 2 entities merely an association? To help answer this question, researchers evaluated the ACE Study using Bradford Hill criteria—9 epidemiological principles employed to infer causation. Their findings strongly support the hypothesis that not only are ACEs associated with myriad negative outcomes, they are their root cause12 and therefore a powerful determinant of our most pressing and expensive health and social problems.Nevertheless, strategies to prevent and address childhood adversity, which are critical to meeting national health goals of successful prevention and treatment of myriad conditions, are absent from the paradigm and practice of most physicians.
The body of research about the health impact of additional adverse experiences is growing to include community violence, poverty, longstanding discrimination,2 and other experiences that we describe as social determinants of health. Furthermore, social determinants of health, or adverse community experiences, appear to maintain a dose–response relationship with health and social outcomes.2 ,13 Along with adverse collective historical experiences (historical trauma),14 these community experiences are forcing further re-examination of existing paradigms of health.
Continue to: The biological pathway from experience to illness
The biological pathway from experience to illness
Neuroscience supports the epidemiology of ACEs.12 The brain develops from the bottom up, in a use-dependent fashion, contingent on genetic potential and, most importantly, on our experiences, which also influence genetic expression. Although present across the lifespan, the brain’s capacity to change—neuroplasticity—is most robust from the prenatal period until about 3 years of age.15 The autonomic nervous system receives information from the body about our internal world and from sensory organs about our external environment and sends it to the brain for processing and interpretation, resulting in micro- and macro-adaptations in structure and function, both within the brain and in the rest of the body.16

Neuroscience demonstrates that adverse experiences, in the context of insufficient protective factors and depending on their timing, severity, and frequency, cause overactivation or prolonged activation, or both, of the stress response system, thus derailing optimal growth and development of the brain and disrupting healthy signaling in all body systems. The dysregulated stress response drives inflammation and subsequent chronic disease (FIGURE17,18), and may influence genetic expression in this, and future, generations.12,14,19 Using neuroimaging and assessment of biomarkers, researchers can see the harm caused by inadequately buffered adversity on overall anatomy and physiology. Protective factors such as a safe environment and positive relationships provide hope that normal biological responses to adverse circumstances can be prevented or reversed, leading to clinical, cognitive, and functional improvement11 (TABLE 120-22).

Evidence-based primary prevention of childhood adversity succeeds
Primary prevention of childhood adversity offers significant benefits across the lifespan and, likely, into the next generation. It ensures that every infant has at least 1 nurturing, attuned caregiver with whom to develop a secure attachment relationship that is essential for optimal growth and development of brain and body.
Primary prevention is most effective when it focuses on supporting caregivers during the perinatal and early childhood periods of their families, before children’s brains are fully organized. Primary prevention involves evidence-based program implementation; collaboration among multiple sectors, including early childhood education, child welfare, criminal justice, business, faith, and health care; and, ultimately, policy change. It incorporates individual, family, and community-based strategies to meet basic needs, ensure safety, fortify a sense of love and belonging in families, and support parents in developing optimal parenting skills. This allows caregivers to devote attention to their children, thus strengthening attunement and attachment, reducing toxic stress, and building protective factors and resilience. Evidence-based and -informed prevention programs include the Nurse–Family Partnership (NFP), Positive Parenting Program (Triple P), and the Family-Centered Medical Home.
NFP. Randomized controlled trials of the NFP, a perinatal home visiting program for low-income, first-time pregnant women and their offspring, showed a reduction in the incidence of domestic violence, child maltreatment, and maternal smoking, with improvement in maternal financial stability, cognitive and socioemotional outcomes, and rates of substance abuse and incarceration in children and/or youth.23
Continue to: Triple P
Triple P. A randomized controlled trial of Triple P, an evidence-based, multilevel, population-based preventive intervention system that was designed to support parents and enhance parenting practices for families with at least 1 child (birth to 12 years old), demonstrated a statistically significant reduction in substantiated child maltreatment cases, out-of-home placements, and emergency room visits and hospitalizations for childhood injuries that were the result of child maltreatment.24
The Family-Centered Medical Home, a primary care strategy to reduce premature and low-birth-weight deliveries, used Medicaid dollars for services not traditionally considered “medical” to address all physical and emotional needs of mothers and families as part of the medical relationship. This program eliminated premature delivery and low birth weight,25 both considered evidence of in utero toxic stress.26
Screening can be brief: In some cases, a single question
The prevalence and impact of childhood adversity, along with the opportunity for significant health improvements and savings, inspires providers to explore screening. Existing screening programs have consistent goals27,28:
- identify unique experiences shaping our patients’ health
- reframe “What’s wrong with you?” as “What happened to you?” “What’s right with you?” and “What matters to you?”
- facilitate health education and neuro-education, particularly meaning-making and self-regulation
- prevent and mitigate the sequelae of exposure to ACEs
- promote health in this and subsequent generations.
The ACE Study screened patients in the context of a comprehensive periodic health assessment. Study participants completed an at-home questionnaire and reviewed it with their physician.1 The Urban ACE Survey added important community stressors such as neighborhood violence, bullying, and food insecurity to the original ACE questionnaire.2
Primary care tool. Wade developed a short, 2-question ACE pre-screener for primary care29 and is exploring screening for childhood adversity in pediatric practice, as are primary care clinicians around the country.
Continue to: Single-question screener
Single-question screener. A Chicago internist interviewed more than 500 patients using a single-question screener that asked whether growing up was “mostly okay or pretty difficult.” This tool accurately confirmed childhood adversity in patients with complex chronic illness, prevented re-traumatization by allowing patients control over disclosure, and opened the door to collaborative healing work over time.30
The Hague Protocol, now mandated in the Netherlands for health and justice professionals, focuses its efforts upstream by offering early detection of children at risk for adverse experiences. The protocol requires asking adults who present with intimate partner violence, suicidality, psychiatric disturbance, or severe substance abuse whether they care for children in any capacity. Those who are so identified are referred to a center at which support services are offered.31
Uncertainty about the utility of existing tools. Many screening tools appear to be promising in terms of identification of the risk for, or actual, childhood adversity, patient and provider satisfaction, and their “fit” in the clinical workflow. Even so, no best practice guidelines exist in primary care to steer screening efforts. Questions remain about27-29:
- broad implementation of a specific tool
- how, when, and where screening should take place
- whether to screen adults, parents, or children—or all 3
- how best to use the content and pacing of screening questions to promote self-regulation and prevent re-traumatization
- best strategies for training and supporting health care workers around screening activities
- how to optimally manage a positive screen.
How best to approach treatment
Treatment includes trauma-informed care, an organizational transformation process (described in TABLE 232; in “The lexicon of childhood adversity: Concepts and tools for care”33-45; and in the subsection, “Lessons from neuroscience”), and individual treatment strategies. The Substance Abuse and Mental Health Services Administration (SAMHSA) of the US Department of Health and Human Services is advocating for implementation of trauma-informed approaches in health systems.

Continue to: The lexicon of childhood adversity...
SIDEBAR
The lexicon of childhood adversity: Concepts and tools for care33-45
Adversity A state or instance of serious or continued difficulty or misfortune. 33
Attachment A special, enduring form of emotional relationship with a specific person involving soothing, pleasure, and comfort.34
Attunement The ability to read and respond to the cues of another.35
Eye-movement desensitization and reprocessing (EMDR) An evidence-based psychotherapy for posttraumatic stress disorder and other psychiatric disorders, mental health problems, and somatic symptoms. EMDR facilitates resumption of normal information processing and integration; the patient attends to emotionally disturbing material in brief sequential doses while simultaneously focusing on an external stimulus. EMDR targets past experience, current triggers, and future potential challenges, and results in alleviation of presenting symptoms; a decrease or elimination of distress from the disturbing memory; improved view of the self; relief from bodily disturbance; and resolution of present and future anticipated triggers.36
Historical trauma Cumulative emotional and psychological wounding, resulting from group traumatic experiences, transmitted across generations within a community.37
Neurofeedback Electroencephalographic biofeedback is a method for retraining brainwave patterns through operant conditioning; it is used to treat posttraumatic stress disorder, various mental health conditions, addiction, chronic pain, epilepsy, and other disorders.38
Neuromodulatory Having the capacity to alter nerve activity through targeted delivery of a stimulus, such as electrical stimulation or chemical agents, to specific neurological sites in the body to help restore function or relieve symptoms.39
Social determinants of health/adverse community experiences Conditions in which people are born, grow, live, work, and age and that are shaped by distribution of money, power, and resources at all levels.40,41
Trauma An event or circumstance experienced or observed by a person as physically or emotionally harmful or threatening and having lasting adverse effects on that person's functioning and well-being.42
Trauma-focused cognitive behavioral therapy An evidence-based trauma treatment for children 3 to 18 years and their parents comprising the elements of the acronym PRACTICE: Psychoeducation and parenting; Relaxation methods; Affective expression and regulation skills; Cognitive coping skills and processing; Trauma narrative and processing; In vivo exposure; Conjoint parent-child therapy sessions; and Enhancing personal safety and growth.43
Trauma-informed approach This "4-R" approach can be implemented in any type of service setting, organization, or program that: Realizes the widespread impact of trauma and understands potential paths for recovery; recognizes signs and symptoms of trauma in clients, families, staff, and others involved with the system; responds by fully integrating knowledge about trauma into policies, procedures, and practices; and seeks to actively resist re-traumatization.44
Use-dependent The organization and function of neurons, the neural system, and the brain depends on repetitive, patterned stimulation.45
Continue to: Trauma-informed care is a model...
Trauma-informed care is a model intended to promote healing and reduce the risk for re-traumatization of patients by staff—significant concerns in clinical settings, where the dynamics of loss of power, control, and safety that are inherent in traumatic experience can be replicated.46 To operationalize trauma-informed care more formally, the Center for Health Care Strategies, Inc., and the National Council for Behavioral Health are developing recommendations for 1) standardized screening and assessment tools, evidence-based clinical interventions, implementation processes, and relevant and replicable outcome measures, and 2) policy changes to improve patient and staff engagement, enhance health outcomes, and reduce avoidable care and excess costs.47,48
Lessons from neuroscience guide effective treatment.16 Treatment begins with bottom-up strategies that are focused on decreasing suboptimal excitatory input from the survival brainstem to create safety, connect patients to resources to meet basic needs, teach self-regulation skills, and improve relational health in and outside of the office. Later-stage top-down methods, such as education and other cognitive activities, focus on strengthening the regulatory capacity of the thinking cortex.16 In many ways, treatment mirrors prevention: It emphasizes first helping patients feel safe and loved.
In a follow-up to the ACE Study, 100,000 patients had a primary care visit in which their practitioner reviewed the ACE questionnaire with them; said “I see that you have________. Tell me how that has affected you later in your life” for every “Yes” response; and listened to the answers without passing judgment. This simple intervention profoundly decreased health resource utilization by these patients during the following year: a reduction of 35% in office visits, 11% in emergency room visits, and 3% in hospitalizations.1
The neurosequential model of therapeutics assesses neurodevelopment in the context of childhood adversity and relational health to evaluate consequences of childhood adversity and direct treatment. Adopted domestically and internationally, this model has had statistically significant success facilitating improvement in patients’ physical, emotional, and social health status.16,49
Trauma-specific treatment modalities such as trauma-focused cognitive behavioral therapy and eye-movement desensitization and reprocessing (EMDR),50 a trauma-specific treatment effective in resolving painful childhood memories, are evidence-based treatments that reduce trauma-related symptoms; evidence is also emerging about the efficacy of yoga51 and neurofeedback.52 These therapies have been best studied as treatment for posttraumatic stress disorder and other mental health disorders and also hold promise for addressing physical and social consequences of adversity. They present a low risk for harm, appear to be cost-effective, and improve outcomes.
Continue to: Best regimens involve a multifaceted approach that combines...
Best regimens involve a multifaceted approach that combines health-system resources with referral to other community practitioners and agencies. An excellent example is a current collaboration between health systems and affordable housing programs to reduce and, ultimately, eliminate chronic homelessness. Positive outcomes of this collaboration include both improved health and life satisfaction for participants and cost savings to the health system.53
CASE
Beginning in adulthood, Ms. W began long-term psychotherapy and had a therapeutic trial of antidepressants, without significant improvement. None of her medical or mental-health providers educated her about the connection between childhood adversity and illness to help her make sense of her health history and autoimmune disease, or to guide treatment. She learned from a friend about the relationship between childhood adversity and poor health and self-administered the ACE questionnaire, scoring 5 points out of a possible 10.
Ms. W enjoyed loving relationships with her mother, sisters, and friends. She had long-standing personal practices of individual and group physical activity, journaling, and spending time in nature.
About 10 years ago, Ms. W committed to regular yoga practice and later saw a functional medicine provider, who focused on nutrition and restorative sleep. She noticed improvement in all signs and symptoms; however, the terror of public speaking remained. Through friends, she found a practitioner who offered EMDR. Over the past 2 years, her terror has resolved and general anxiety and insomnia have continued to improve; she is now able to speak with fluency and comfort in any arena.
Addressing childhood adversity: Our “natural domain”
Experiences, positive and negative, shape our psychology and biology; they are powerful determinants of health—or illness. Prevention of, and response to, childhood adversity demand a systems approach to the whole person in context—the natural domain of family medicine.
Continue to: Although clinical translation is still unfolding...
Although clinical translation is still unfolding, the risks of implementing promising prevention and treatment strategies are low, the stakes are high, and the potential benefits are vast. Therefore, we as family physicians can—must—learn and incorporate the science of childhood adversity, neurobiology, and life course into our training, research, and clinical paradigm and practice; we can do that by embedding this framework throughout our training and continuing education in formal didactics, case discussions, hands-on skill-building, scientific investigation, and patient care.
We must make our offices and hospitals trauma-informed; connect patients with resources to meet basic needs and with home-visiting and parent education programs; educate patients about the impact of protective and adverse factors on health; provide and practice self-regulation training in our offices or by referral; and advocate for equity.
Using these strategies, family physicians will play a crucial role in the prevention, mitigation, and treatment of the root cause of disease and society’s deepest individual and collective suffering.
CORRESPONDENCE
Audrey Stillerman, MD, ABFM, ABIHM, ABOIM, Office of Community Engagement and Neighborhood Health Partnerships, 808 South Wolcott Street, Room 809, Chicago, IL 60612; [email protected].
ACKNOWLEDGMENT
Patricia Rush, MD, MBA, and Adrienne Williams, PhD, reviewed the manuscript of this article.
1. Felitti V, Anda R. The relationship of adverse childhood experiences to adult medical disease, psychiatric disorders and sexual behavior: implications for healthcare. In: Lanius RA, Vermetten E, Pain C, eds. The Impact of Early Life Trauma on Health and Disease: The Hidden Epidemic. Cambridge, UK: Cambridge University Press; 2011:77-87.
2. Wade R Jr, Shea JA, Rubin D, et al. Adverse childhood experiences of low-income urban youth. Pediatrics. 2014;134:e13-e20.
3. Centers for Disease Control and Prevention. Child abuse and neglect prevention. April 10, 2018. www.cdc.gov/violenceprevention/childabuseandneglect/index.html. Accessed September 20, 2018.
4. American Academy of Family Physicians. Definition of family medicine. www.aafp.org/about/policies/all/family-medicine-definition.html. Accessed March 5, 2018.
5. Martin JC, Avant RF, Bowman MA, et al; The Future of Family Medicine Project Leadership Committee. The Future of Family Medicine: a collaborative project of the family medicine community. Ann Fam Med. 2004;2 Suppl 1:S3-S32.
6. Child & Adolescent Health Measurement Initiative (CAHMI). A national and across-state profile on Adverse Childhood Experiences among U.S. children and possibilities to heal and thrive. Issue Brief. October 2017. www.cahmi.org/wp-content/uploads/2018/05/aces_brief_final.pdf. Accessed September 20, 2018.
7. World Health Organization. Adverse Childhood Experiences International Questionnaire (ACE-IQ). www.who.int/violence_injury_prevention/violence/activities/adverse_childhood_experiences/en/. Accessed September 20, 2018.
8. Roos LE, Mota N, Afifi TO, et al. Relationship between adverse childhood experiences and homelessness and the impact of axis I and II disorders. Am J Public Health. 2013;103(Suppl 2):S275-S281.
9. Baglivio MT. Wolff KT. Piquero AR, et al. The relationship between adverse childhood experiences (ACE) and juvenile offending trajectories in a juvenile offender sample. J Crim Justice. 2015;43:229-241.
10. Dube SR. Felitti VF. Dong M, et al. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics. 2003;111:564-572.
11. Maunder RG, Peladeau N, Savage D, et al. The prevalence of childhood adversity among healthcare workers and its relationship to adult life events, distress and impairment. Child Abuse Negl. 2010;34:114-123.
12. Anda RF, Felitti VJ, Bremner JD, et al. The enduring effects of abuse and related adverse experiences in childhood: a convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256:174-186.
13. Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic disparities in health in the United States: what the patterns tell us. Am J Public Health. 2010;100(Suppl 1):S186-S196.
14. Bowers ME, Yehuda R. Intergenerational transmission of stress in humans. Neuropsychopharmacology. 2016;41:232-244.
15. Perry BD. Memories of fears: How the brain stores and retrieves traumatic experiences. In: Goodwin J, Attias R, eds. Splintered Reflections: Images of the Body in Trauma. New York, NY: Basic Books; 1999:9-38.
16. Perry BD. Examining child maltreatment through a neurodevelopmental lens: clinical application of the Neurosequential Model of Therapeutics. J Loss Trauma. 2009;14:240-255.
17.
18. Adding layers to the ACEs pyramid—What do you think? Trauma and social location. ACES Connection, RYSE Center. 2015. www.acesconnection.com/blog/adding-layers-to-the-aces-pyramid-what-do-you-think. Accessed October 10, 2018.
19. Berens AE, Jensen SKG, Nelson CA 3rd. Biological embedding of childhood adversity: from physiological mechanisms to clinical implications. BMC Med. 2017;15:135.
20. Rostad WL, Basile KC, Clayton HB. Association among television and computer/video game use, victimization, and suicide risk among U.S. high school students. J Interpers Violence. 2018 Mar 1:886260518760020.
21. Coyne SM, Nelson DA, Graham-Kevan N, et al. Media depictions of physical and relational aggression: connections with aggression in young adults’ romantic relationships. Aggress Behav. 2011;37:56-62.
22. Centers for Disease Control and Prevention. Violence prevention: Child abuse and neglect: risk and protective factors. April 10, 2018. www.cdc.gov/violenceprevention/childabuseandneglect/riskprotectivefactors.html. Accessed October 10, 2018.
23. Miller TR. Projected outcomes of nurse-family partnership home visitation during 1996-2013, United States. Prev Sci. 2015;16:765-777.
24. Prinz RJ, Sanders MR, Shapiro CJ, et al. Population-based prevention of child maltreatment: the U.S. Triple P system population trial. Prev Sci. 2009;10:1-12.
25. Kraft C. Building capacity & support for two generation primary care. 2015 Midwest Regional Summit on Adverse Childhood Experiences. March 13, 2015. www.hmprg.org/assets/root/PDFs/2015/Summit%20Notes%20for%20Day%20Two.pdf. Accessed September 20, 2018.
26. Smith MV, Gotman N, Yonkers KA. Early childhood adversity and pregnancy outcomes. Matern Child Health J. 2016;20:790-798.
27. Leitch L. Action steps using ACEs and trauma-informed care: a resilience model. Health & Justice. 2017;5:1-10.
28. Bethell CD, Carle A, Hudziak J, et al. Methods to assess adverse childhood experiences of children and families: toward approaches to promote child well-being in policy and practice. Acad Pediatr. 2017;17:S51-S69.
29. Wade R Jr, Becker BD, Bevans KB, et al. Development and evaluation of a short adverse childhood experiences measure. Am J Prev Med. 2017;52:163-172.
30. Rush P. How learning about emotional trauma led me to a new understanding of chronic illness and health disparity. Becoming trauma-informed: Perspectives from public health, faith communities, education and medicine. Presented at 2016 Advocate Symposium, “Becoming a Trauma-Informed Children’s Hospital and Community: Building Foundations of Care, Collaboration and Practice.” Oaklawn, IL: Advocate Children’s Hospital; November 16, 2016.
31. Diderich HM, Fekkes M, Verkerk PH, et al. A new protocol for screening adults presenting with their own medical problems at the Emergency Department to identify children at high risk for maltreatment. Child Abuse Negl. 2013;37:1122-1131.
32. Fact Sheet: Key ingredients for trauma-informed care. Center for Health Care Strategies, Inc. August 2017. www.chcs.org/media/ATC-Key-Ingredients-Fact-Sheet_081417.pdf. Accessed September 22, 2018.
33. Adversity. In: Merriam-Webster Online Dictionary. Springfield, MA: Merriam-Webster, Inc. www.merriam-webster.com/dictionary/adversity. Accessed September 21, 2018.
34. Perry BD. Understanding traumatized and maltreated children: the core concepts. Child Trauma Academy Video Training Series, Video 4;2004:12. Child Trauma Academy (http://childtrauma.org/).
35. Perry BD. Understanding traumatized and maltreated children: the core concepts. Child Trauma Academy Video Training Series, Video 4;2004:19. Child Trauma Academy (http://childtrauma.org/).
36. EMDRIA’s definition of EMDR (eye movement desensitization and reprocessing). Austin, TX: EMDRIA: EMDR International Association. http://c.ymcdn.com/sites/www.emdria.org/resource/resmgr/imported/EMDRIA%20Definition%20of%20EMDR.pdf. Revised February 25 2012. Accessed September 21, 2018.
37. Types of trauma and violence: Historical trauma. Washington, DC: Substance Abuse and Mental Health Services Administration. www.samhsa.gov/trauma-violence/types. Accessed September 21, 2018.
38. Hammond DC. What is neurofeedback? An update. J Neurotherapy. 2011;15:305-336.
39. International Neuromodulation Society. Neuromodulation, or neuromodulatory effect. www.neuromodulation.com/neuromodulation-defined. November 9, 2017. Accessed September 21, 2018.
40. World Health Organization. Social determinants of health. www.who.int/social_determinants/sdh_definition/en/. Accessed September 21, 2018.
41. Davis R, Pinderhughes H, Williams M. Adverse community experiences and resilience: a framework for addressing and preventing community trauma. Oakland, CA: Prevention Institute; 2015:4-5. www.preventioninstitute.org/publications/adverse-community-experiences-and-resilience-framework-addressing-and-preventing. Accessed September 30, 2018.
42. SAMHSA-HRSA Center for Integrated Health Solutions. Trauma. Rockville, MD: Substance Abuse and Mental Health Services Administration and Health Resources and Services Administration, US Department of Health and Human Services. www.integration.samhsa.gov/clinical-practice/trauma. Accessed September 21, 2018.
43. Cohen JA, Mandarino AP. Trauma-focused cognitive behavioural therapy for children and parents. Child Adolesc Ment Health. 2008;13:158-162.
44. Trauma-informed approach and trauma-specific interventions: Trauma-informed approach. Washington, DC: National Center for Trauma Informed Care and Alternatives to Seclusion and Restraints; Substance Abuse and Mental Health Services Administration. www.samhsa.gov/nctic/trauma-interventions. Accessed September 21, 2018.
45. Perry BD. How the brain develops: the importance of early childhood. Child Trauma Academy Video Training Series, Video 1;2004:21. Child Trauma Academy (http://childtrauma.org/).
46. Huang LN, Sharp CS, Gunther T. It’s just good medicine: trauma-informed primary care. (SAMHSA-HRSA Center for Integrated Health Solutions webinar); August 6, 2013. www.integration.samhsa.gov/about-us/CIHS_TIC_Webinar_PDF.pdf. Accessed September 20, 2018.
47. CHCS: Center for Health Care Strategies, Inc. Fact sheet: Key ingredients for trauma-informed care. August 2017. www.chcs.org/media/ATC-Key-Ingredients-Fact-Sheet_081417.pdf. Accessed September 20, 2018.
48. National Council for Behavioral Health. Trauma-informed primary care: fostering resilience and recovery. www.thenationalcouncil.org/consulting-areas-of-expertise/trauma-informed-primary-care/. Accessed September 20, 2018.
49. Child Trauma Academy. The Neurosequential Model of Therapeutics as evidence-based practice. https://childtrauma.org/wp-content/uploads/2015/05/NMT_EvidenceBasedPract_5_2_15.pdf. Accessed September 30, 2018.
50. Bisson JI, Ehlers A, Matthews R, et al. Psychological treatments for chronic post-traumatic stress disorder. Systematic review and meta-analysis. Br J Psychiatry. 2007;190:97-104.
51. Metcalf O, Varker T, Forbes D, et al. Efficacy of fifteen emerging interventions for the treatment of posttraumatic stress disorder: a systematic review. J Trauma Stress. 2016;29:88-92.
52. van der Kolk BA, Hodgdon H, Gapen M, et al. A randomized controlled study of neurofeedback for chronic PTSD. 2016; PLoS One. 2016;11:e0166752.
53. Bryan M. A hospital offers frequent ER patients an out—free housing. “All Things Considered.” National Public Radio. June 29, 2016. www.npr.org/sections/health-shots/2016/06/29/482994000/a-hospital-offers-frequent-er-patients-an-out-free-housing. Acces-sed September 20, 2018.
1. Felitti V, Anda R. The relationship of adverse childhood experiences to adult medical disease, psychiatric disorders and sexual behavior: implications for healthcare. In: Lanius RA, Vermetten E, Pain C, eds. The Impact of Early Life Trauma on Health and Disease: The Hidden Epidemic. Cambridge, UK: Cambridge University Press; 2011:77-87.
2. Wade R Jr, Shea JA, Rubin D, et al. Adverse childhood experiences of low-income urban youth. Pediatrics. 2014;134:e13-e20.
3. Centers for Disease Control and Prevention. Child abuse and neglect prevention. April 10, 2018. www.cdc.gov/violenceprevention/childabuseandneglect/index.html. Accessed September 20, 2018.
4. American Academy of Family Physicians. Definition of family medicine. www.aafp.org/about/policies/all/family-medicine-definition.html. Accessed March 5, 2018.
5. Martin JC, Avant RF, Bowman MA, et al; The Future of Family Medicine Project Leadership Committee. The Future of Family Medicine: a collaborative project of the family medicine community. Ann Fam Med. 2004;2 Suppl 1:S3-S32.
6. Child & Adolescent Health Measurement Initiative (CAHMI). A national and across-state profile on Adverse Childhood Experiences among U.S. children and possibilities to heal and thrive. Issue Brief. October 2017. www.cahmi.org/wp-content/uploads/2018/05/aces_brief_final.pdf. Accessed September 20, 2018.
7. World Health Organization. Adverse Childhood Experiences International Questionnaire (ACE-IQ). www.who.int/violence_injury_prevention/violence/activities/adverse_childhood_experiences/en/. Accessed September 20, 2018.
8. Roos LE, Mota N, Afifi TO, et al. Relationship between adverse childhood experiences and homelessness and the impact of axis I and II disorders. Am J Public Health. 2013;103(Suppl 2):S275-S281.
9. Baglivio MT. Wolff KT. Piquero AR, et al. The relationship between adverse childhood experiences (ACE) and juvenile offending trajectories in a juvenile offender sample. J Crim Justice. 2015;43:229-241.
10. Dube SR. Felitti VF. Dong M, et al. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics. 2003;111:564-572.
11. Maunder RG, Peladeau N, Savage D, et al. The prevalence of childhood adversity among healthcare workers and its relationship to adult life events, distress and impairment. Child Abuse Negl. 2010;34:114-123.
12. Anda RF, Felitti VJ, Bremner JD, et al. The enduring effects of abuse and related adverse experiences in childhood: a convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256:174-186.
13. Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic disparities in health in the United States: what the patterns tell us. Am J Public Health. 2010;100(Suppl 1):S186-S196.
14. Bowers ME, Yehuda R. Intergenerational transmission of stress in humans. Neuropsychopharmacology. 2016;41:232-244.
15. Perry BD. Memories of fears: How the brain stores and retrieves traumatic experiences. In: Goodwin J, Attias R, eds. Splintered Reflections: Images of the Body in Trauma. New York, NY: Basic Books; 1999:9-38.
16. Perry BD. Examining child maltreatment through a neurodevelopmental lens: clinical application of the Neurosequential Model of Therapeutics. J Loss Trauma. 2009;14:240-255.
17.
18. Adding layers to the ACEs pyramid—What do you think? Trauma and social location. ACES Connection, RYSE Center. 2015. www.acesconnection.com/blog/adding-layers-to-the-aces-pyramid-what-do-you-think. Accessed October 10, 2018.
19. Berens AE, Jensen SKG, Nelson CA 3rd. Biological embedding of childhood adversity: from physiological mechanisms to clinical implications. BMC Med. 2017;15:135.
20. Rostad WL, Basile KC, Clayton HB. Association among television and computer/video game use, victimization, and suicide risk among U.S. high school students. J Interpers Violence. 2018 Mar 1:886260518760020.
21. Coyne SM, Nelson DA, Graham-Kevan N, et al. Media depictions of physical and relational aggression: connections with aggression in young adults’ romantic relationships. Aggress Behav. 2011;37:56-62.
22. Centers for Disease Control and Prevention. Violence prevention: Child abuse and neglect: risk and protective factors. April 10, 2018. www.cdc.gov/violenceprevention/childabuseandneglect/riskprotectivefactors.html. Accessed October 10, 2018.
23. Miller TR. Projected outcomes of nurse-family partnership home visitation during 1996-2013, United States. Prev Sci. 2015;16:765-777.
24. Prinz RJ, Sanders MR, Shapiro CJ, et al. Population-based prevention of child maltreatment: the U.S. Triple P system population trial. Prev Sci. 2009;10:1-12.
25. Kraft C. Building capacity & support for two generation primary care. 2015 Midwest Regional Summit on Adverse Childhood Experiences. March 13, 2015. www.hmprg.org/assets/root/PDFs/2015/Summit%20Notes%20for%20Day%20Two.pdf. Accessed September 20, 2018.
26. Smith MV, Gotman N, Yonkers KA. Early childhood adversity and pregnancy outcomes. Matern Child Health J. 2016;20:790-798.
27. Leitch L. Action steps using ACEs and trauma-informed care: a resilience model. Health & Justice. 2017;5:1-10.
28. Bethell CD, Carle A, Hudziak J, et al. Methods to assess adverse childhood experiences of children and families: toward approaches to promote child well-being in policy and practice. Acad Pediatr. 2017;17:S51-S69.
29. Wade R Jr, Becker BD, Bevans KB, et al. Development and evaluation of a short adverse childhood experiences measure. Am J Prev Med. 2017;52:163-172.
30. Rush P. How learning about emotional trauma led me to a new understanding of chronic illness and health disparity. Becoming trauma-informed: Perspectives from public health, faith communities, education and medicine. Presented at 2016 Advocate Symposium, “Becoming a Trauma-Informed Children’s Hospital and Community: Building Foundations of Care, Collaboration and Practice.” Oaklawn, IL: Advocate Children’s Hospital; November 16, 2016.
31. Diderich HM, Fekkes M, Verkerk PH, et al. A new protocol for screening adults presenting with their own medical problems at the Emergency Department to identify children at high risk for maltreatment. Child Abuse Negl. 2013;37:1122-1131.
32. Fact Sheet: Key ingredients for trauma-informed care. Center for Health Care Strategies, Inc. August 2017. www.chcs.org/media/ATC-Key-Ingredients-Fact-Sheet_081417.pdf. Accessed September 22, 2018.
33. Adversity. In: Merriam-Webster Online Dictionary. Springfield, MA: Merriam-Webster, Inc. www.merriam-webster.com/dictionary/adversity. Accessed September 21, 2018.
34. Perry BD. Understanding traumatized and maltreated children: the core concepts. Child Trauma Academy Video Training Series, Video 4;2004:12. Child Trauma Academy (http://childtrauma.org/).
35. Perry BD. Understanding traumatized and maltreated children: the core concepts. Child Trauma Academy Video Training Series, Video 4;2004:19. Child Trauma Academy (http://childtrauma.org/).
36. EMDRIA’s definition of EMDR (eye movement desensitization and reprocessing). Austin, TX: EMDRIA: EMDR International Association. http://c.ymcdn.com/sites/www.emdria.org/resource/resmgr/imported/EMDRIA%20Definition%20of%20EMDR.pdf. Revised February 25 2012. Accessed September 21, 2018.
37. Types of trauma and violence: Historical trauma. Washington, DC: Substance Abuse and Mental Health Services Administration. www.samhsa.gov/trauma-violence/types. Accessed September 21, 2018.
38. Hammond DC. What is neurofeedback? An update. J Neurotherapy. 2011;15:305-336.
39. International Neuromodulation Society. Neuromodulation, or neuromodulatory effect. www.neuromodulation.com/neuromodulation-defined. November 9, 2017. Accessed September 21, 2018.
40. World Health Organization. Social determinants of health. www.who.int/social_determinants/sdh_definition/en/. Accessed September 21, 2018.
41. Davis R, Pinderhughes H, Williams M. Adverse community experiences and resilience: a framework for addressing and preventing community trauma. Oakland, CA: Prevention Institute; 2015:4-5. www.preventioninstitute.org/publications/adverse-community-experiences-and-resilience-framework-addressing-and-preventing. Accessed September 30, 2018.
42. SAMHSA-HRSA Center for Integrated Health Solutions. Trauma. Rockville, MD: Substance Abuse and Mental Health Services Administration and Health Resources and Services Administration, US Department of Health and Human Services. www.integration.samhsa.gov/clinical-practice/trauma. Accessed September 21, 2018.
43. Cohen JA, Mandarino AP. Trauma-focused cognitive behavioural therapy for children and parents. Child Adolesc Ment Health. 2008;13:158-162.
44. Trauma-informed approach and trauma-specific interventions: Trauma-informed approach. Washington, DC: National Center for Trauma Informed Care and Alternatives to Seclusion and Restraints; Substance Abuse and Mental Health Services Administration. www.samhsa.gov/nctic/trauma-interventions. Accessed September 21, 2018.
45. Perry BD. How the brain develops: the importance of early childhood. Child Trauma Academy Video Training Series, Video 1;2004:21. Child Trauma Academy (http://childtrauma.org/).
46. Huang LN, Sharp CS, Gunther T. It’s just good medicine: trauma-informed primary care. (SAMHSA-HRSA Center for Integrated Health Solutions webinar); August 6, 2013. www.integration.samhsa.gov/about-us/CIHS_TIC_Webinar_PDF.pdf. Accessed September 20, 2018.
47. CHCS: Center for Health Care Strategies, Inc. Fact sheet: Key ingredients for trauma-informed care. August 2017. www.chcs.org/media/ATC-Key-Ingredients-Fact-Sheet_081417.pdf. Accessed September 20, 2018.
48. National Council for Behavioral Health. Trauma-informed primary care: fostering resilience and recovery. www.thenationalcouncil.org/consulting-areas-of-expertise/trauma-informed-primary-care/. Accessed September 20, 2018.
49. Child Trauma Academy. The Neurosequential Model of Therapeutics as evidence-based practice. https://childtrauma.org/wp-content/uploads/2015/05/NMT_EvidenceBasedPract_5_2_15.pdf. Accessed September 30, 2018.
50. Bisson JI, Ehlers A, Matthews R, et al. Psychological treatments for chronic post-traumatic stress disorder. Systematic review and meta-analysis. Br J Psychiatry. 2007;190:97-104.
51. Metcalf O, Varker T, Forbes D, et al. Efficacy of fifteen emerging interventions for the treatment of posttraumatic stress disorder: a systematic review. J Trauma Stress. 2016;29:88-92.
52. van der Kolk BA, Hodgdon H, Gapen M, et al. A randomized controlled study of neurofeedback for chronic PTSD. 2016; PLoS One. 2016;11:e0166752.
53. Bryan M. A hospital offers frequent ER patients an out—free housing. “All Things Considered.” National Public Radio. June 29, 2016. www.npr.org/sections/health-shots/2016/06/29/482994000/a-hospital-offers-frequent-er-patients-an-out-free-housing. Acces-sed September 20, 2018.
PRACTICE RECOMMENDATIONS
› Refer eligible patients to an evidence-based perinatal home-visiting program and all parents to an evidence-based parenting program to prevent childhood adversity. A
› Consider screening adult patients and parents for their own history (and their children’s history) of childhood adversity. B
› Recommend trauma-focused cognitive behavioral therapy and eye-movement desensitization and reprocessing as first-line treatments for adversity and trauma. A
› Consider prescribing yoga, neurofeedback, and other neuromodulatory modalities to treat the consequences of childhood adversity and trauma. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Click for Credit: Short-term NSAIDs; endometriosis; more
Here are 5 articles from the November issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Short-term NSAIDs appear safe for high-risk patients
To take the posttest, go to: https://bit.ly/2PgXKGx
Expires October 8, 2019
2. Chronic liver disease raises death risk in pneumonia patients
To take the posttest, go to: https://bit.ly/2NPSXXA
Expires October 8, 2019
3. Half of outpatient antibiotics prescribed with no infectious disease code
To take the posttest, go to: https://bit.ly/2pWEWxU
Expires October 6, 2019
4. Secondary fractures in older men spike soon after first, but exercise may help
To take the posttest, go to: https://bit.ly/2OCNl8A
Expires October 3, 2019
5. Consider ART for younger endometriosis patients
To take the posttest, go to: https://bit.ly/2NO1Sc4
Expires October 5, 2019
Here are 5 articles from the November issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Short-term NSAIDs appear safe for high-risk patients
To take the posttest, go to: https://bit.ly/2PgXKGx
Expires October 8, 2019
2. Chronic liver disease raises death risk in pneumonia patients
To take the posttest, go to: https://bit.ly/2NPSXXA
Expires October 8, 2019
3. Half of outpatient antibiotics prescribed with no infectious disease code
To take the posttest, go to: https://bit.ly/2pWEWxU
Expires October 6, 2019
4. Secondary fractures in older men spike soon after first, but exercise may help
To take the posttest, go to: https://bit.ly/2OCNl8A
Expires October 3, 2019
5. Consider ART for younger endometriosis patients
To take the posttest, go to: https://bit.ly/2NO1Sc4
Expires October 5, 2019
Here are 5 articles from the November issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Short-term NSAIDs appear safe for high-risk patients
To take the posttest, go to: https://bit.ly/2PgXKGx
Expires October 8, 2019
2. Chronic liver disease raises death risk in pneumonia patients
To take the posttest, go to: https://bit.ly/2NPSXXA
Expires October 8, 2019
3. Half of outpatient antibiotics prescribed with no infectious disease code
To take the posttest, go to: https://bit.ly/2pWEWxU
Expires October 6, 2019
4. Secondary fractures in older men spike soon after first, but exercise may help
To take the posttest, go to: https://bit.ly/2OCNl8A
Expires October 3, 2019
5. Consider ART for younger endometriosis patients
To take the posttest, go to: https://bit.ly/2NO1Sc4
Expires October 5, 2019
ECG to screen asymptomatic adults? Not so fast, says USPSTF
Resources
US Preventive Services Task Force. Final recommendation statement: Cardiovascular disease risk: screening with electrocardiography.
https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cardiovascular-disease-risk-screening-with-electrocardiography. Published June 2018. Accessed October 23, 2018.
US Preventive Services Task Force. Final recommendation statement: Atrial fibrillation: screening with electrocardiography.
https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/atrial-fibrillation-screening-with-electrocardiography. Published August 2018. Accessed October 23, 2018.
Resources
US Preventive Services Task Force. Final recommendation statement: Cardiovascular disease risk: screening with electrocardiography.
https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cardiovascular-disease-risk-screening-with-electrocardiography. Published June 2018. Accessed October 23, 2018.
US Preventive Services Task Force. Final recommendation statement: Atrial fibrillation: screening with electrocardiography.
https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/atrial-fibrillation-screening-with-electrocardiography. Published August 2018. Accessed October 23, 2018.
Resources
US Preventive Services Task Force. Final recommendation statement: Cardiovascular disease risk: screening with electrocardiography.
https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cardiovascular-disease-risk-screening-with-electrocardiography. Published June 2018. Accessed October 23, 2018.
US Preventive Services Task Force. Final recommendation statement: Atrial fibrillation: screening with electrocardiography.
https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/atrial-fibrillation-screening-with-electrocardiography. Published August 2018. Accessed October 23, 2018.
Barriers to Self-Management in African American Adolescents with Asthma
From Wayne State University, Detroit, MI (Dr. Gibson-Scipio), and the University of Texas Rio Grande Valley, Edinburg, TX (Dr. Krouse).
Abstract
- Objective: To review the literature on barriers to asthma self-management amo
ng African American adolescents. - Methods: Review of the literature.
- Results: Asthma self-management barriers experienced by African American adolescents are often related to developmental needs, lack of knowledge, and personal perspectives and experiences. Adolescents find managing their symptoms and adhering to prescriptive therapies a burden and desire to be more like healthy peers. As they struggle to identify with peers, they may engage in risky behaviors such as ignoring symptoms and delaying treatment, thus leading to poorer asthma control and health outcomes. African American adolescents struggle with perceptions of racial biases from health care providers and teachers that interfere with self-management behaviors. They also describe the influence of culturally based practices learned from caregivers that contribute to their misconceptions and inadequate skills in effectively managing their asthma.
- Conclusion: Researchers should seek to develop interventions to address the unique contextual and culturally based needs of African American adolescents that support the development of effective asthma self-management behaviors. This may include making use of family members (especially mothers) and extended support for self-management during this period of rapid growth and transition. Health care providers should consider a team-based approach to the adolescent patient. Such an approach should be grounded in recommendations from national guidelines that suggest a patient-centered approach to care that includes a partnership between the patient and the provider to address unique barriers to effective self-management.
Keywords: youth; caregiver; drug-therapy; self-efficacy; disease-management; patient-centered care.
Effective asthma self-management by urban African American adolescents is a critical aspect of care that should be addressed with vigilance due to the persistent disparities in disease prevalence, morbidity, and mortality compared to Caucasians.1-3 The overarching goal of asthma self-management is to achieve symptom control, maintain normal activity levels, and minimize future risk of exacerbations and medication side effects.4,5 Best practices for asthma self-management begin with a partnership between health care providers and clients (including parent/caregiver). This relationship should help affected individuals gain asthma control based on knowledge of their disease and treatment options, confidence and skills in trigger avoidance, medication administration, and management of acute exacerbations.4,5
Among youth aged 18 years and younger, African Americans have the highest asthma prevalence rates of all racial and ethnic groups, and between 2001 and 2009 asthma prevalence rates rose by 50% among African American youth.6 As of 2015, prevalence rates for asthma among African American youth were 13.4%, as compared to 7.4% for white youth.7 African American youth have been found to have more frequent asthma exacerbations and related school absences than white youth.8 Furthermore, African American youth younger than 18 years are more likely to be admitted to the hospital for asthma and are 10 times more likely to die from asthma compared to non-Hispanic white children.6
Urban African American adolescents with asthma are particularly vulnerable to poor asthma self-management due to the complexity of the disease in this population.3 African American youth must deal with multiple adverse environmental conditions, lack of knowledge or disbelief concerning effective disease self-management strategies, variable access and quality of care, and the psychosocial dynamics of being young while having a chronic disease.2,3,9-11 It is important to understand and address barriers to successful asthma self-management during adolescence, as behaviors developed during this stage of life often persist into adulthood.9 In this article, we review the literature on barriers to asthma self-management among African American adolescents and offer suggestions on clinical strategies for improving self-management in this vulnerable population.
Methods
The initial search strategy was developed in collaboration with an experienced librarian. Keywords, MeSH terms, and potential databases were identified. Keywords included urban, African American, adolescent, asthma, self-management, and barriers. These terms were expanded based on search results and a review of abstracts that fit the intent of our review. The search was limited to U.S. studies published between 2005 and 2017. Excluded from the search were conference abstracts, doctoral dissertations, master’s theses, meta-analyses, systematic reviews, and studies conducted outside of the United States. Additional articles for the review were identified during the review process from the reference lists of the publications.
Abstracts were reviewed for articles that reported a study population inclusive of African American adolescents with asthma and that were related to self-management. Studies that used qualitative and other descriptive methods and cohort and randomized control trials were reviewed. Due to the limited number of articles found that exclusively focused on African Americans, the authors set a threshold for African American participants at 40% or greater for inclusion in this review.
Full papers were retrieved that met the inclusion criteria for a full review. Each author initially independently reviewed a selected number of papers and abstracted the study purpose, sample, study design, results, conclusions, and limitations. Subsequently, both authors reviewed in tandem and then discussed each selected manuscript to assure the appropriateness for inclusion. The subject matter was considered the priority for inclusion in the review. Study methods, sample size, and noted limitations were categorized but were not considered as a basis for exclusion. Thematic analysis was used to identify common themes across studies.
Results
We identified 23 papers that met our criteria (Table). Five common themes were found that related to barriers in disease self-management for African American adolescents: (a) knowledge and skills, (b) beliefs and attitudes, (c) personal/emotional factors, (d) caregivers, and (e) schools.
Knowledge and Skills
Adequate knowledge of the elements of asthma self-management is critical for achieving control of this condition. Asthma knowledge includes a basic understanding of the disease process and treatment strategies, an awareness of early signs and symptoms of worsening asthma, and an understanding of how to manage environmental triggers.4,5 Sin and colleagues conducted one of the earlier studies to examine the influence of asthma knowledge on asthma self-management in African American adolescents and found a significant positive association between knowledge and asthma self-management behaviors.12
Adherence to an asthma medication, especially inhaled corticosteroids (ICS), is one of the cornerstones to successful self-management of asthma.13,14 Consistent use of ICS therapy to control asthma symptoms and disease progression is often suboptimal in African American adolescents and tends to worsen as they age;15 studies have found lower adherence levels were more prominent in older African American adolescents and males.13,16 In a recent study of adolescents with persistent asthma who were prescribed daily ICS, youth with greater ICS knowledge as assessed using a standardized instrument demonstrated significantly higher adherence rates.13 Proper technique in the use of an inhaler is also important in medication administration. Asthma ICS medication delivery devices vary significantly and require different techniques for medication administration. However, inhaler device skills have been found to be very inadequate in high-risk African American adolescents.17 Thus, knowledge related to ICS therapy and proper skills in the use of inhaler devices is an important aspect of asthma self-management that have been found to be inadequate in African American Adolescents.
Interventions and programs geared to improving education may lead to improved self-management. Multisystemic Therapy-Health Care (MST-HC) is a tailored home-based intervention that includes knowledge and skill-building components. In a study of African American youth with poorly controlled asthma, the program was found to improve illness management.18 In addition, adolescents who complete formal asthma education programs demonstrate significantly higher scores in self-management than those youth who do not participate in these programs.13,19 Unfortunately, few African American teens report participation in an asthma education program.19 In a study of a motivational interviewing intervention to improve controller medication adherence for African American adolescents,14 youth reported gaining more knowledge about their asthma medications and were significantly more motivated to take their controller medications after participating in the intervention; however, while adherence to controller medications was greater than baseline, it was not significantly different.14 This study demonstrated the value of asthma education and the feasibility of a motivational intervention to support controller medication adherence. However, this study also demonstrated the complexity of medication adherence in that neither knowledge or motivation led to significant changes in medication adherence among African American adolescents.
Low health literacy can also act as a barrier to asthma self-management. Health literacy requires skills and knowledge that enable an individual to communicate, process, and understand basic health information that informs health decisions.20 Health literacy was found to be associated with indicators of poor disease self-management among urban African American adolescents in grades 9 through 12.21 In this study, health literacy was established using questions about confidence in filling out medical forms, self-reported problems with learning about the youth’s medical condition, and the need for assistance in reading hospital materials. Adolescents with poor health literacy scores were more likely to reside in a household with the following characteristics: mother with less than a high school education, Medicaid health insurance, family members with a body mass index exceeding the 85th percentile, and lack of rescue medication. Poor health literacy was most common among younger adolescents (ie, ninth graders). Some youth with poor health literacy also reported more emergency department visits, hospitalizations, and lower overall quality of life.21
Beliefs and Attitudes
Beliefs and attitudes towards taking asthma medications can act as barriers to adherence in the adolescent. African American adolescents often report the belief that ICS are not helpful or necessary.16,22-25 These beliefs have been correlated with a lack of understanding of the inflammatory mechanisms of asthma, reports of asthma attacks despite use of controller medications, fear of addiction to medications, and a belief that nontraditional interventions (eg, exercise) will work better to get rid of asthma or abate symptoms.16-19,22-24 African American adolescents also report beliefs that asthma will go away or get better as they age, and they are willing to forgo the use of controller medications based on these beliefs.24
African American adolescents often engage in asthma self-management independent of caregivers. These youth describe asthma self-management activities an annoyance and of low priority in part due to competing tasks and negative interactions with caregivers.25 During early adolescence asthma self-management is often suboptimal, and as youth age they become less observant regarding their asthma and are less likely to seek help.26 Adolescents’ beliefs and low prioritization of asthma self-management may contribute to forgetfulness and loss of inhalers, which are common reasons reported for poor adherence to ICS.16,23-26 Further, the role of caregivers during this period has often been overlooked. Caregivers of African American adolescents have been found to be stressed and overwhelmed with personal responsibilities and neighborhood conditions, leaving them little time to attend to the asthma self-management behavior of youth. Due to these contextual factors, interactions with chronically ill youth may be strained, resulting in negative interactions with youth related to asthma self-management. However, in an intervention study that used multisystemic therapy (an approach that targets the affected individual, family, and community), improvement in positive parenting behaviors related to asthma self-management contributed to improved ICS adherence by adolescents.27
Adolescents can perceive traditional asthma self-management as conflicting with their own personal and/or cultural beliefs. They may seek options beyond the use of medicine and have voiced preferences for behaviors that they believe will “strengthen their lungs” more naturally.24 An appreciation of how youth might use complementary/alternative medicine (CAM) as an adjunctive therapy or in place of evidence-based asthma care is important to understanding the potential effect on morbidity and mortality. Behaviors and beliefs about the use of CAM have not been well studied among urban African American adolescents with asthma. Only one study was found that assessed the use of CAM among a primarily urban African American adolescent population. In that study, 71% of the population reported using some form of CAM during the past 30 days.28 Prayer and relaxation were the most frequently used strategies in the management of asthma symptoms. Perceived efficacy of relaxation and prayer among teens who engaged in this form of CAM was 87% and 85%, respectively. Other CAM strategies included yoga, meditation, guided imagery, and biofeedback. When adolescents were asked if they shared their use of CAM in asthma management with a health care provider, most reported sharing the use of yoga and dietary changes but were least likely to share their use of prayer and guided imagery.28
Personal/Emotional Factors
African American adolescents have reported asthma as a limiting factor in terms of both physical and social activities. They perceive asthma as a burden to themselves and others (eg, peers, family, coaches).9,25 The burden of asthma is further exemplified in the emotional response to the symptoms of the disease and the self-management responsibilities. The need to prevent and respond to asthma symptoms is associated with being embarrassed, frustrated, angry, annoyed, worried, lonely, and isolated.9,11,25 Negative coping strategies by youth in response to psychosocial experiences include decisions to disregard or give minimal attention to asthma symptoms and to delay or not take prescribed medications. Students report ignoring asthma symptom management while engaging in physical activities to maintain a sense of normalcy among peers and as a way of dealing with perceptions by coaches or teachers that they are weak or in need of being protected.24,25
Negative thoughts and experiences can result in depressive disorders and poor quality of life. Depression is a common finding among urban youth with asthma.29,30 Youth diagnosed with asthma who have comorbid depression may benefit from interventions to improve self-management. In a secondary analysis from a Web-based asthma management intervention targeting African American adolescents, depression was found to have a modifying effect on the emotional domain of quality of life for youth in the intervention arm of the study. This finding indicates that participants who were depressed and who reported low levels of emotional quality of life benefited from the Web-based interventions that targeted self-management.31
Caregivers
Caregivers (especially moms) are a common source of support for the development and implementation of asthma self-management behaviors in adolescents.32 Caregivers sometimes hold beliefs similar to those of youth and believe the urban environment can act as a barrier to asthma management.9,25,32 They describe the complexity of asthma treatment plans, a lack of understanding of the disease process, and insensitivity of health care providers to their expressed needs along with the providers’ limited cultural awareness in the development of self-management plans.9,22,33 Caregivers describe how family finances, insurance gaps, access to care, and their own familial/cultural beliefs influence their decisions and ability to support their child’s asthma management.33 When faced with the cost of care they report instances of having to decide between necessities such as food and housing or co-pays for medications and office visits.22,33 They also report concerns about visits with multiple providers due to an inability to access their primary care provider, which can lead to delays in their child being diagnosed with asthma.22
Caregivers report a need to include culturally based practices, past experiences, and personal beliefs into the adolescents’ asthma management plan.22,32,33 In a small interview-based study of caregivers residing in 3 New Jersey public housing communities, caregivers reported preferring “familial” methods of controlling asthma (eg, restriction of activities; use of showers, steam, vaporizers, and nebulizers) over evidence-based recommendations. Many caregivers were confused or lacked knowledge about asthma action plans.33 Caregivers have also been found to lack adequate or accurate knowledge related to asthma medications and factors that improved or worsened asthma. While caregivers report a desire to help educate their teens by passing on what they know, their lack of adequate asthma knowledge may hamper their efforts and potentially worsen the teens’ asthma self-management.32
While African American caregivers often describe themselves as hypervigilant concerning their child’s asthma, they may report different information than their adolescent when both are questioned about asthma symptom experiences and functional status.34 Factors increasing the level of congruence between caregiver and teen asthma symptom reports were found to be related to the adolescents’ age and asthma disease classification. Symptom questionnaire responses of older teens and those with mild intermittent asthma were more likely to be similar to caregiver reports. The researchers concluded that clinicians and researchers may obtain reliable asthma symptom and functional status reports by asking the adolescent directly.34
Schools
Caregivers and adolescents describe schools as a threat to self-management and the overall health of youth with asthma.9,32 They perceive that a lack of knowledge by staff, teachers, and coaches contributes to inattentiveness or disbelief in the credibility of reported asthma symptoms by youth.11,23 These misperceptions and the lack of attentiveness by adults in the school may pose safety and health issues for African American youth.9,25,33,34 For example, adolescents report pressure from teacher, coaches, and peers in school settings to partake in sports and/or gym classes. Youth want to identify with healthy peers and thus often choose not to take asthma medications during such activities or opt to continue participating while being compromised by airway obstruction. Of great concern were reports by caregivers and teens of not being allowed to call a parent for support or retrieve their medications when needed for asthma symptoms.32
Future Research and Practice Implications
In this review, we identified 5 common themes around barriers to asthma self-management for African American adolescents (knowledge and skills, beliefs and attitudes, personal/emotional factors, caregivers, and schools). Caregivers, especially mothers, play a pivotal role in the development of effective asthma self-management behaviors. Depsite good intentions, there is evidence of caregivers passing on ineffective experiential and culturally based beliefs and practices to their adolescents that can negatively influence self-care behaviors.13,28,38 Studies are needed to further investigate these findings among caregivers as their beliefs and practices for asthma self-management have been found to coexist among adolescents. Studies that investigate how to incorporate caregiver asthma knowledge, cultural beliefs and behaviors in developing self-management interventions have the potential to positively influence asthma outcomes among African American adolescents.27 The unique cultural beliefs, contextual environmental, and social disparities faced by African American caregivers should not be neglected.
African American adolescents, like adolescents in other racial or ethnic groups, desire to be autonomous in their asthma self-management. However, as adolescents age their adherence behaviors often decline. This may suggest a need for a longer transition period to self-management that extends into emerging adulthood (18-25 years). While youth want to feel supported, there appears to be a fine line between receiving needed support and what youth describe as “nagging” behaviors by adults. Additional investigations into how asthma responsibilities are transitioned from the parent to youth and how best to support the development and maintenance of related behaviors and skills are warranted. In addition, teens described problems related to communicating with health care providers, noting a lack of clarity in explanations received about how to manage their asthma. Some teens believed the communication challenges were based on beliefs and biases held by providers that African American youth had limited capacities for self-management.9 There is a need to better understand interactions among African American adolescents, parents, and clinicians so that communication and transitioning asthma care to the youth will produce optimal health outcomes.
According to asthma guidelines, the patient-provider relationship is essential to effective asthma self-management.4,5 However, there is little mention in the literature of team-based care. Clinicians such as physicians, physician assistants, and nurse practitioners provide direct care to adolescents in terms of disease management and the overall effectiveness of treatment plans. African American youth demonstrate a need for asthma education that is comprehensive and that is contextualized to their daily lives. A team-based approach to care that includes social workers and community health workers may help to extend the reach of clinicians. Follow-up times with families and youth between office visits can be used to support adolescents to develop asthma self-management and allow them a safe space to describe frustrations and other emotions that contribute to their desire to be disease-free.
Summary
Asthma is a chronic disease that is often more severe and difficult to manage in African American adolescents. While African American adolescents describe developmental needs like those of other youth, cultural beliefs and contextual experiences influence their self-care management in unique ways. Opportunities exist to better understand the needs of African American adolescents and to help them successfully gain the knowledge, skills, and behaviors needed to effectively engage in self-management of their asthma.
Corresponding author: Wanda Gibson-Scipio, PhD, FNP-BC, FAANP, 5557 Cass Ave., 346 Cohn Building, Detroit, MI 48324; [email protected].
Financial disclosures: None.
1. Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001-2010. NCHS Data Brief. 2012:1-8.
2. Bruzzese JM, Bonner S, Vincent EJ, et al. Asthma education: the adolescent experience. Patient Educ Couns. 2004;55:396-406.
3. Bryant-Stephens T. Asthma disparities in urban environments. J Allergy Clin Immunol. 2009;123:1199-1206.
4. National Heart, Lung, and Blood Institute. National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007.
5. GINA. Global strategy for asthma management and prevention. 2017. www.ginaasthma.org. Accessed Dec 15, 2017.
6. Centers for Disease Control and Prevention. Vital signs. 2011. https://www.cdc.gov/vitalsigns/asthma/index.html. Accessed December 15, 2017.
7. Centers for Disease Control and Prevention. 2015 National Health Interview Survey (NHIS) Data. National Center for Environmental Health, 2017. https://www.cdc.gov/asthma/nhis/2015/table4-1.htm. Accessed December 15, 2017.
8. Gupta RS, Carrión-Carire V, Weiss KB. The widening black/white gap in asthma hospitalizations and mortality. J Allergy Clin Immunol. 2006;117:351-358.
9. Evans-Agnew R. Asthma management disparities: a photovoice investigation with African American youth. J Sch Nurs. 2016;32:99-111.
10. Naar-King S, Ellis, D, Kolmodin, K. Feasibility of adapting multisystemic therapy to improve illness management behaviors and reduce asthma morbidity in high risk African American youth: a case series. J Child Fam Stud. 2009;18:564-573.
11. Rhee H, Wenzel J, Steeves RH. Adolescents’ psychosocial experiences living with asthma: a focus group study. J Pediatr Health Care. 2007;21:99-107.
12. Sin MK, Kang DH, Weaver M. Relationships of asthma knowledge, self-management, and social support in African American adolescents with asthma. Int J Nurs Stud. 2005;42:307-313.
13. Mosnaim G, Li H, Martin M, et al. Factors associated with levels of adherence to inhaled corticosteroids in minority adolescents with asthma. Ann Allergy Asthma Immunol. 2014;112:116-120.
14. Riekert KA, Borrelli B, Bilderback A, Rand CS. The development of a motivational interviewing intervention to promote medication adherence among inner-city, African American adolescents with asthma. Patient Educ Couns. 2011;82:117-122.
15. Bruzzese JM, Stepney C, Fiorino EK, et al. Asthma self-management is sub-optimal in urban Hispanic and African American/black early adolescents with uncontrolled persistent asthma. J Asthma. 2012;49:90-97.
16. Naimi DR, Freedman TG, Ginsburg KR, et al. Adolescents and asthma: why bother with our meds? J Allergy Clin Immunol. 2009;123:1335-1341.
17. Naar-King S, Lam P, Ellis D, et al. Asthma medication device skills in high-risk African American adolescents. J Asthma. 2013;50:579-582.
18. Ellis DA, King P, Naar-King S. Mediators of treatment effects in a randomized clinical trial of multisystemic therapy-health care in adolescents with poorly controlled asthma: disease knowledge and device use skills. J Pediatr Psychol. 2016;41:522-530.
19. Crowder SJ, Hanna KM, Carpenter JS, Broome ME. Factors associated with asthma self-management in African American adolescents. J Pediatric Nurs. 2015;30:e35-e43.
20. U.S. Department of Health and Human Services. Healthy people 2010: understanding and improving health. 2nd ed. Washington (DC): U.S. Government Printing Office; November 2000.
21. Valerio MA, Peterson EL, Wittich AR, Joseph CLM. Examining health literacy among urban African-American adolescents with asthma. J Asthma. 2016;53:1041-1047.
22. Laster N, Holsey CN, Shendell DG, et al. Barriers to asthma management among urban families: caregiver and child perspectives. J Asthma. 2009;46:731-739.
23. Ayala GX, Miller D, Zagami E, et al. Asthma in middle schools: what students have to say about their asthma. J Sch Health. 2006;76:208-214.
24. Gibson-Scipio W, Gourdin D, Krouse, HJ. Asthma self-management goals, beliefs and behaviors of urban African American adolescents prior to transitioning to adult health care. J Pediatric Nurs. 2015;30:e53-e61.
25. Blaakman SW, Cohen A, Fagnano M, Halterman JS. Asthma medication adherence among urban teens: a qualitative analysis of barriers, facilitators and experiences with school-based care. J Asthma. 2014;51:522-529.
26. Bruzzese JM, Idalski Carcone A, Lam P, et al. Adherence to asthma medication regimens in urban African American adolescents: application of self-determination theory. Health Psychol. 2014;33:461-464.
27. Ellis DA, King P, Naar-King S, et al. Effects of family treatment on parenting beliefs among caregivers of youth with poorly controlled asthma. J Dev Behav Pediatr. 2014;35:486-493.
28. Cotton S, Luberto CM, Yi MS, Tsevat J. Complementary and alternative medicine behaviors and beliefs in urban adolescents with asthma. J Asthma. 2011;48:531-538.
29. Bahreinian S, Ball GDC, Colman I, et al. Depression is more common in girls with nonatopic asthma. Chest. 2011;140:1138-1145.
30. Bender BG. Risk taking, depression, adherence, and symptom control in adolescents and young adults with asthma. Am J Respir Crit Care Med. 2006;173:953-957.
31. Guglani L, Havstad SL, Johnson CC, et al. Effect of depressive symptoms on asthma intervention in urban teens. Ann Allergy Asthma Immunol. 2012;109:237-242.
32. Gibson-Scipio W, Krouse HJ. Goals, beliefs, and concerns of urban caregivers of middle and older adolescents with asthma. J Asthma. 2013;50:242-249.
33. Wagner F, Steefel L. Beliefs regarding asthma management relating to asthma action plans (AAPs) of African American caregivers residing in Newark, New Jersey public housing communities. J Pediatr Nurs. 2017;36:92-97.
34. Houle CR, Joseph CL, Caldwell CH, et al. Congruence between urban adolescent and caregiver responses to questions about the adolescent’s asthma. J Urban Health. 2011;88:30-40.
From Wayne State University, Detroit, MI (Dr. Gibson-Scipio), and the University of Texas Rio Grande Valley, Edinburg, TX (Dr. Krouse).
Abstract
- Objective: To review the literature on barriers to asthma self-management amo
ng African American adolescents. - Methods: Review of the literature.
- Results: Asthma self-management barriers experienced by African American adolescents are often related to developmental needs, lack of knowledge, and personal perspectives and experiences. Adolescents find managing their symptoms and adhering to prescriptive therapies a burden and desire to be more like healthy peers. As they struggle to identify with peers, they may engage in risky behaviors such as ignoring symptoms and delaying treatment, thus leading to poorer asthma control and health outcomes. African American adolescents struggle with perceptions of racial biases from health care providers and teachers that interfere with self-management behaviors. They also describe the influence of culturally based practices learned from caregivers that contribute to their misconceptions and inadequate skills in effectively managing their asthma.
- Conclusion: Researchers should seek to develop interventions to address the unique contextual and culturally based needs of African American adolescents that support the development of effective asthma self-management behaviors. This may include making use of family members (especially mothers) and extended support for self-management during this period of rapid growth and transition. Health care providers should consider a team-based approach to the adolescent patient. Such an approach should be grounded in recommendations from national guidelines that suggest a patient-centered approach to care that includes a partnership between the patient and the provider to address unique barriers to effective self-management.
Keywords: youth; caregiver; drug-therapy; self-efficacy; disease-management; patient-centered care.
Effective asthma self-management by urban African American adolescents is a critical aspect of care that should be addressed with vigilance due to the persistent disparities in disease prevalence, morbidity, and mortality compared to Caucasians.1-3 The overarching goal of asthma self-management is to achieve symptom control, maintain normal activity levels, and minimize future risk of exacerbations and medication side effects.4,5 Best practices for asthma self-management begin with a partnership between health care providers and clients (including parent/caregiver). This relationship should help affected individuals gain asthma control based on knowledge of their disease and treatment options, confidence and skills in trigger avoidance, medication administration, and management of acute exacerbations.4,5
Among youth aged 18 years and younger, African Americans have the highest asthma prevalence rates of all racial and ethnic groups, and between 2001 and 2009 asthma prevalence rates rose by 50% among African American youth.6 As of 2015, prevalence rates for asthma among African American youth were 13.4%, as compared to 7.4% for white youth.7 African American youth have been found to have more frequent asthma exacerbations and related school absences than white youth.8 Furthermore, African American youth younger than 18 years are more likely to be admitted to the hospital for asthma and are 10 times more likely to die from asthma compared to non-Hispanic white children.6
Urban African American adolescents with asthma are particularly vulnerable to poor asthma self-management due to the complexity of the disease in this population.3 African American youth must deal with multiple adverse environmental conditions, lack of knowledge or disbelief concerning effective disease self-management strategies, variable access and quality of care, and the psychosocial dynamics of being young while having a chronic disease.2,3,9-11 It is important to understand and address barriers to successful asthma self-management during adolescence, as behaviors developed during this stage of life often persist into adulthood.9 In this article, we review the literature on barriers to asthma self-management among African American adolescents and offer suggestions on clinical strategies for improving self-management in this vulnerable population.
Methods
The initial search strategy was developed in collaboration with an experienced librarian. Keywords, MeSH terms, and potential databases were identified. Keywords included urban, African American, adolescent, asthma, self-management, and barriers. These terms were expanded based on search results and a review of abstracts that fit the intent of our review. The search was limited to U.S. studies published between 2005 and 2017. Excluded from the search were conference abstracts, doctoral dissertations, master’s theses, meta-analyses, systematic reviews, and studies conducted outside of the United States. Additional articles for the review were identified during the review process from the reference lists of the publications.
Abstracts were reviewed for articles that reported a study population inclusive of African American adolescents with asthma and that were related to self-management. Studies that used qualitative and other descriptive methods and cohort and randomized control trials were reviewed. Due to the limited number of articles found that exclusively focused on African Americans, the authors set a threshold for African American participants at 40% or greater for inclusion in this review.
Full papers were retrieved that met the inclusion criteria for a full review. Each author initially independently reviewed a selected number of papers and abstracted the study purpose, sample, study design, results, conclusions, and limitations. Subsequently, both authors reviewed in tandem and then discussed each selected manuscript to assure the appropriateness for inclusion. The subject matter was considered the priority for inclusion in the review. Study methods, sample size, and noted limitations were categorized but were not considered as a basis for exclusion. Thematic analysis was used to identify common themes across studies.
Results
We identified 23 papers that met our criteria (Table). Five common themes were found that related to barriers in disease self-management for African American adolescents: (a) knowledge and skills, (b) beliefs and attitudes, (c) personal/emotional factors, (d) caregivers, and (e) schools.
Knowledge and Skills
Adequate knowledge of the elements of asthma self-management is critical for achieving control of this condition. Asthma knowledge includes a basic understanding of the disease process and treatment strategies, an awareness of early signs and symptoms of worsening asthma, and an understanding of how to manage environmental triggers.4,5 Sin and colleagues conducted one of the earlier studies to examine the influence of asthma knowledge on asthma self-management in African American adolescents and found a significant positive association between knowledge and asthma self-management behaviors.12
Adherence to an asthma medication, especially inhaled corticosteroids (ICS), is one of the cornerstones to successful self-management of asthma.13,14 Consistent use of ICS therapy to control asthma symptoms and disease progression is often suboptimal in African American adolescents and tends to worsen as they age;15 studies have found lower adherence levels were more prominent in older African American adolescents and males.13,16 In a recent study of adolescents with persistent asthma who were prescribed daily ICS, youth with greater ICS knowledge as assessed using a standardized instrument demonstrated significantly higher adherence rates.13 Proper technique in the use of an inhaler is also important in medication administration. Asthma ICS medication delivery devices vary significantly and require different techniques for medication administration. However, inhaler device skills have been found to be very inadequate in high-risk African American adolescents.17 Thus, knowledge related to ICS therapy and proper skills in the use of inhaler devices is an important aspect of asthma self-management that have been found to be inadequate in African American Adolescents.
Interventions and programs geared to improving education may lead to improved self-management. Multisystemic Therapy-Health Care (MST-HC) is a tailored home-based intervention that includes knowledge and skill-building components. In a study of African American youth with poorly controlled asthma, the program was found to improve illness management.18 In addition, adolescents who complete formal asthma education programs demonstrate significantly higher scores in self-management than those youth who do not participate in these programs.13,19 Unfortunately, few African American teens report participation in an asthma education program.19 In a study of a motivational interviewing intervention to improve controller medication adherence for African American adolescents,14 youth reported gaining more knowledge about their asthma medications and were significantly more motivated to take their controller medications after participating in the intervention; however, while adherence to controller medications was greater than baseline, it was not significantly different.14 This study demonstrated the value of asthma education and the feasibility of a motivational intervention to support controller medication adherence. However, this study also demonstrated the complexity of medication adherence in that neither knowledge or motivation led to significant changes in medication adherence among African American adolescents.
Low health literacy can also act as a barrier to asthma self-management. Health literacy requires skills and knowledge that enable an individual to communicate, process, and understand basic health information that informs health decisions.20 Health literacy was found to be associated with indicators of poor disease self-management among urban African American adolescents in grades 9 through 12.21 In this study, health literacy was established using questions about confidence in filling out medical forms, self-reported problems with learning about the youth’s medical condition, and the need for assistance in reading hospital materials. Adolescents with poor health literacy scores were more likely to reside in a household with the following characteristics: mother with less than a high school education, Medicaid health insurance, family members with a body mass index exceeding the 85th percentile, and lack of rescue medication. Poor health literacy was most common among younger adolescents (ie, ninth graders). Some youth with poor health literacy also reported more emergency department visits, hospitalizations, and lower overall quality of life.21
Beliefs and Attitudes
Beliefs and attitudes towards taking asthma medications can act as barriers to adherence in the adolescent. African American adolescents often report the belief that ICS are not helpful or necessary.16,22-25 These beliefs have been correlated with a lack of understanding of the inflammatory mechanisms of asthma, reports of asthma attacks despite use of controller medications, fear of addiction to medications, and a belief that nontraditional interventions (eg, exercise) will work better to get rid of asthma or abate symptoms.16-19,22-24 African American adolescents also report beliefs that asthma will go away or get better as they age, and they are willing to forgo the use of controller medications based on these beliefs.24
African American adolescents often engage in asthma self-management independent of caregivers. These youth describe asthma self-management activities an annoyance and of low priority in part due to competing tasks and negative interactions with caregivers.25 During early adolescence asthma self-management is often suboptimal, and as youth age they become less observant regarding their asthma and are less likely to seek help.26 Adolescents’ beliefs and low prioritization of asthma self-management may contribute to forgetfulness and loss of inhalers, which are common reasons reported for poor adherence to ICS.16,23-26 Further, the role of caregivers during this period has often been overlooked. Caregivers of African American adolescents have been found to be stressed and overwhelmed with personal responsibilities and neighborhood conditions, leaving them little time to attend to the asthma self-management behavior of youth. Due to these contextual factors, interactions with chronically ill youth may be strained, resulting in negative interactions with youth related to asthma self-management. However, in an intervention study that used multisystemic therapy (an approach that targets the affected individual, family, and community), improvement in positive parenting behaviors related to asthma self-management contributed to improved ICS adherence by adolescents.27
Adolescents can perceive traditional asthma self-management as conflicting with their own personal and/or cultural beliefs. They may seek options beyond the use of medicine and have voiced preferences for behaviors that they believe will “strengthen their lungs” more naturally.24 An appreciation of how youth might use complementary/alternative medicine (CAM) as an adjunctive therapy or in place of evidence-based asthma care is important to understanding the potential effect on morbidity and mortality. Behaviors and beliefs about the use of CAM have not been well studied among urban African American adolescents with asthma. Only one study was found that assessed the use of CAM among a primarily urban African American adolescent population. In that study, 71% of the population reported using some form of CAM during the past 30 days.28 Prayer and relaxation were the most frequently used strategies in the management of asthma symptoms. Perceived efficacy of relaxation and prayer among teens who engaged in this form of CAM was 87% and 85%, respectively. Other CAM strategies included yoga, meditation, guided imagery, and biofeedback. When adolescents were asked if they shared their use of CAM in asthma management with a health care provider, most reported sharing the use of yoga and dietary changes but were least likely to share their use of prayer and guided imagery.28
Personal/Emotional Factors
African American adolescents have reported asthma as a limiting factor in terms of both physical and social activities. They perceive asthma as a burden to themselves and others (eg, peers, family, coaches).9,25 The burden of asthma is further exemplified in the emotional response to the symptoms of the disease and the self-management responsibilities. The need to prevent and respond to asthma symptoms is associated with being embarrassed, frustrated, angry, annoyed, worried, lonely, and isolated.9,11,25 Negative coping strategies by youth in response to psychosocial experiences include decisions to disregard or give minimal attention to asthma symptoms and to delay or not take prescribed medications. Students report ignoring asthma symptom management while engaging in physical activities to maintain a sense of normalcy among peers and as a way of dealing with perceptions by coaches or teachers that they are weak or in need of being protected.24,25
Negative thoughts and experiences can result in depressive disorders and poor quality of life. Depression is a common finding among urban youth with asthma.29,30 Youth diagnosed with asthma who have comorbid depression may benefit from interventions to improve self-management. In a secondary analysis from a Web-based asthma management intervention targeting African American adolescents, depression was found to have a modifying effect on the emotional domain of quality of life for youth in the intervention arm of the study. This finding indicates that participants who were depressed and who reported low levels of emotional quality of life benefited from the Web-based interventions that targeted self-management.31
Caregivers
Caregivers (especially moms) are a common source of support for the development and implementation of asthma self-management behaviors in adolescents.32 Caregivers sometimes hold beliefs similar to those of youth and believe the urban environment can act as a barrier to asthma management.9,25,32 They describe the complexity of asthma treatment plans, a lack of understanding of the disease process, and insensitivity of health care providers to their expressed needs along with the providers’ limited cultural awareness in the development of self-management plans.9,22,33 Caregivers describe how family finances, insurance gaps, access to care, and their own familial/cultural beliefs influence their decisions and ability to support their child’s asthma management.33 When faced with the cost of care they report instances of having to decide between necessities such as food and housing or co-pays for medications and office visits.22,33 They also report concerns about visits with multiple providers due to an inability to access their primary care provider, which can lead to delays in their child being diagnosed with asthma.22
Caregivers report a need to include culturally based practices, past experiences, and personal beliefs into the adolescents’ asthma management plan.22,32,33 In a small interview-based study of caregivers residing in 3 New Jersey public housing communities, caregivers reported preferring “familial” methods of controlling asthma (eg, restriction of activities; use of showers, steam, vaporizers, and nebulizers) over evidence-based recommendations. Many caregivers were confused or lacked knowledge about asthma action plans.33 Caregivers have also been found to lack adequate or accurate knowledge related to asthma medications and factors that improved or worsened asthma. While caregivers report a desire to help educate their teens by passing on what they know, their lack of adequate asthma knowledge may hamper their efforts and potentially worsen the teens’ asthma self-management.32
While African American caregivers often describe themselves as hypervigilant concerning their child’s asthma, they may report different information than their adolescent when both are questioned about asthma symptom experiences and functional status.34 Factors increasing the level of congruence between caregiver and teen asthma symptom reports were found to be related to the adolescents’ age and asthma disease classification. Symptom questionnaire responses of older teens and those with mild intermittent asthma were more likely to be similar to caregiver reports. The researchers concluded that clinicians and researchers may obtain reliable asthma symptom and functional status reports by asking the adolescent directly.34
Schools
Caregivers and adolescents describe schools as a threat to self-management and the overall health of youth with asthma.9,32 They perceive that a lack of knowledge by staff, teachers, and coaches contributes to inattentiveness or disbelief in the credibility of reported asthma symptoms by youth.11,23 These misperceptions and the lack of attentiveness by adults in the school may pose safety and health issues for African American youth.9,25,33,34 For example, adolescents report pressure from teacher, coaches, and peers in school settings to partake in sports and/or gym classes. Youth want to identify with healthy peers and thus often choose not to take asthma medications during such activities or opt to continue participating while being compromised by airway obstruction. Of great concern were reports by caregivers and teens of not being allowed to call a parent for support or retrieve their medications when needed for asthma symptoms.32
Future Research and Practice Implications
In this review, we identified 5 common themes around barriers to asthma self-management for African American adolescents (knowledge and skills, beliefs and attitudes, personal/emotional factors, caregivers, and schools). Caregivers, especially mothers, play a pivotal role in the development of effective asthma self-management behaviors. Depsite good intentions, there is evidence of caregivers passing on ineffective experiential and culturally based beliefs and practices to their adolescents that can negatively influence self-care behaviors.13,28,38 Studies are needed to further investigate these findings among caregivers as their beliefs and practices for asthma self-management have been found to coexist among adolescents. Studies that investigate how to incorporate caregiver asthma knowledge, cultural beliefs and behaviors in developing self-management interventions have the potential to positively influence asthma outcomes among African American adolescents.27 The unique cultural beliefs, contextual environmental, and social disparities faced by African American caregivers should not be neglected.
African American adolescents, like adolescents in other racial or ethnic groups, desire to be autonomous in their asthma self-management. However, as adolescents age their adherence behaviors often decline. This may suggest a need for a longer transition period to self-management that extends into emerging adulthood (18-25 years). While youth want to feel supported, there appears to be a fine line between receiving needed support and what youth describe as “nagging” behaviors by adults. Additional investigations into how asthma responsibilities are transitioned from the parent to youth and how best to support the development and maintenance of related behaviors and skills are warranted. In addition, teens described problems related to communicating with health care providers, noting a lack of clarity in explanations received about how to manage their asthma. Some teens believed the communication challenges were based on beliefs and biases held by providers that African American youth had limited capacities for self-management.9 There is a need to better understand interactions among African American adolescents, parents, and clinicians so that communication and transitioning asthma care to the youth will produce optimal health outcomes.
According to asthma guidelines, the patient-provider relationship is essential to effective asthma self-management.4,5 However, there is little mention in the literature of team-based care. Clinicians such as physicians, physician assistants, and nurse practitioners provide direct care to adolescents in terms of disease management and the overall effectiveness of treatment plans. African American youth demonstrate a need for asthma education that is comprehensive and that is contextualized to their daily lives. A team-based approach to care that includes social workers and community health workers may help to extend the reach of clinicians. Follow-up times with families and youth between office visits can be used to support adolescents to develop asthma self-management and allow them a safe space to describe frustrations and other emotions that contribute to their desire to be disease-free.
Summary
Asthma is a chronic disease that is often more severe and difficult to manage in African American adolescents. While African American adolescents describe developmental needs like those of other youth, cultural beliefs and contextual experiences influence their self-care management in unique ways. Opportunities exist to better understand the needs of African American adolescents and to help them successfully gain the knowledge, skills, and behaviors needed to effectively engage in self-management of their asthma.
Corresponding author: Wanda Gibson-Scipio, PhD, FNP-BC, FAANP, 5557 Cass Ave., 346 Cohn Building, Detroit, MI 48324; [email protected].
Financial disclosures: None.
From Wayne State University, Detroit, MI (Dr. Gibson-Scipio), and the University of Texas Rio Grande Valley, Edinburg, TX (Dr. Krouse).
Abstract
- Objective: To review the literature on barriers to asthma self-management amo
ng African American adolescents. - Methods: Review of the literature.
- Results: Asthma self-management barriers experienced by African American adolescents are often related to developmental needs, lack of knowledge, and personal perspectives and experiences. Adolescents find managing their symptoms and adhering to prescriptive therapies a burden and desire to be more like healthy peers. As they struggle to identify with peers, they may engage in risky behaviors such as ignoring symptoms and delaying treatment, thus leading to poorer asthma control and health outcomes. African American adolescents struggle with perceptions of racial biases from health care providers and teachers that interfere with self-management behaviors. They also describe the influence of culturally based practices learned from caregivers that contribute to their misconceptions and inadequate skills in effectively managing their asthma.
- Conclusion: Researchers should seek to develop interventions to address the unique contextual and culturally based needs of African American adolescents that support the development of effective asthma self-management behaviors. This may include making use of family members (especially mothers) and extended support for self-management during this period of rapid growth and transition. Health care providers should consider a team-based approach to the adolescent patient. Such an approach should be grounded in recommendations from national guidelines that suggest a patient-centered approach to care that includes a partnership between the patient and the provider to address unique barriers to effective self-management.
Keywords: youth; caregiver; drug-therapy; self-efficacy; disease-management; patient-centered care.
Effective asthma self-management by urban African American adolescents is a critical aspect of care that should be addressed with vigilance due to the persistent disparities in disease prevalence, morbidity, and mortality compared to Caucasians.1-3 The overarching goal of asthma self-management is to achieve symptom control, maintain normal activity levels, and minimize future risk of exacerbations and medication side effects.4,5 Best practices for asthma self-management begin with a partnership between health care providers and clients (including parent/caregiver). This relationship should help affected individuals gain asthma control based on knowledge of their disease and treatment options, confidence and skills in trigger avoidance, medication administration, and management of acute exacerbations.4,5
Among youth aged 18 years and younger, African Americans have the highest asthma prevalence rates of all racial and ethnic groups, and between 2001 and 2009 asthma prevalence rates rose by 50% among African American youth.6 As of 2015, prevalence rates for asthma among African American youth were 13.4%, as compared to 7.4% for white youth.7 African American youth have been found to have more frequent asthma exacerbations and related school absences than white youth.8 Furthermore, African American youth younger than 18 years are more likely to be admitted to the hospital for asthma and are 10 times more likely to die from asthma compared to non-Hispanic white children.6
Urban African American adolescents with asthma are particularly vulnerable to poor asthma self-management due to the complexity of the disease in this population.3 African American youth must deal with multiple adverse environmental conditions, lack of knowledge or disbelief concerning effective disease self-management strategies, variable access and quality of care, and the psychosocial dynamics of being young while having a chronic disease.2,3,9-11 It is important to understand and address barriers to successful asthma self-management during adolescence, as behaviors developed during this stage of life often persist into adulthood.9 In this article, we review the literature on barriers to asthma self-management among African American adolescents and offer suggestions on clinical strategies for improving self-management in this vulnerable population.
Methods
The initial search strategy was developed in collaboration with an experienced librarian. Keywords, MeSH terms, and potential databases were identified. Keywords included urban, African American, adolescent, asthma, self-management, and barriers. These terms were expanded based on search results and a review of abstracts that fit the intent of our review. The search was limited to U.S. studies published between 2005 and 2017. Excluded from the search were conference abstracts, doctoral dissertations, master’s theses, meta-analyses, systematic reviews, and studies conducted outside of the United States. Additional articles for the review were identified during the review process from the reference lists of the publications.
Abstracts were reviewed for articles that reported a study population inclusive of African American adolescents with asthma and that were related to self-management. Studies that used qualitative and other descriptive methods and cohort and randomized control trials were reviewed. Due to the limited number of articles found that exclusively focused on African Americans, the authors set a threshold for African American participants at 40% or greater for inclusion in this review.
Full papers were retrieved that met the inclusion criteria for a full review. Each author initially independently reviewed a selected number of papers and abstracted the study purpose, sample, study design, results, conclusions, and limitations. Subsequently, both authors reviewed in tandem and then discussed each selected manuscript to assure the appropriateness for inclusion. The subject matter was considered the priority for inclusion in the review. Study methods, sample size, and noted limitations were categorized but were not considered as a basis for exclusion. Thematic analysis was used to identify common themes across studies.
Results
We identified 23 papers that met our criteria (Table). Five common themes were found that related to barriers in disease self-management for African American adolescents: (a) knowledge and skills, (b) beliefs and attitudes, (c) personal/emotional factors, (d) caregivers, and (e) schools.
Knowledge and Skills
Adequate knowledge of the elements of asthma self-management is critical for achieving control of this condition. Asthma knowledge includes a basic understanding of the disease process and treatment strategies, an awareness of early signs and symptoms of worsening asthma, and an understanding of how to manage environmental triggers.4,5 Sin and colleagues conducted one of the earlier studies to examine the influence of asthma knowledge on asthma self-management in African American adolescents and found a significant positive association between knowledge and asthma self-management behaviors.12
Adherence to an asthma medication, especially inhaled corticosteroids (ICS), is one of the cornerstones to successful self-management of asthma.13,14 Consistent use of ICS therapy to control asthma symptoms and disease progression is often suboptimal in African American adolescents and tends to worsen as they age;15 studies have found lower adherence levels were more prominent in older African American adolescents and males.13,16 In a recent study of adolescents with persistent asthma who were prescribed daily ICS, youth with greater ICS knowledge as assessed using a standardized instrument demonstrated significantly higher adherence rates.13 Proper technique in the use of an inhaler is also important in medication administration. Asthma ICS medication delivery devices vary significantly and require different techniques for medication administration. However, inhaler device skills have been found to be very inadequate in high-risk African American adolescents.17 Thus, knowledge related to ICS therapy and proper skills in the use of inhaler devices is an important aspect of asthma self-management that have been found to be inadequate in African American Adolescents.
Interventions and programs geared to improving education may lead to improved self-management. Multisystemic Therapy-Health Care (MST-HC) is a tailored home-based intervention that includes knowledge and skill-building components. In a study of African American youth with poorly controlled asthma, the program was found to improve illness management.18 In addition, adolescents who complete formal asthma education programs demonstrate significantly higher scores in self-management than those youth who do not participate in these programs.13,19 Unfortunately, few African American teens report participation in an asthma education program.19 In a study of a motivational interviewing intervention to improve controller medication adherence for African American adolescents,14 youth reported gaining more knowledge about their asthma medications and were significantly more motivated to take their controller medications after participating in the intervention; however, while adherence to controller medications was greater than baseline, it was not significantly different.14 This study demonstrated the value of asthma education and the feasibility of a motivational intervention to support controller medication adherence. However, this study also demonstrated the complexity of medication adherence in that neither knowledge or motivation led to significant changes in medication adherence among African American adolescents.
Low health literacy can also act as a barrier to asthma self-management. Health literacy requires skills and knowledge that enable an individual to communicate, process, and understand basic health information that informs health decisions.20 Health literacy was found to be associated with indicators of poor disease self-management among urban African American adolescents in grades 9 through 12.21 In this study, health literacy was established using questions about confidence in filling out medical forms, self-reported problems with learning about the youth’s medical condition, and the need for assistance in reading hospital materials. Adolescents with poor health literacy scores were more likely to reside in a household with the following characteristics: mother with less than a high school education, Medicaid health insurance, family members with a body mass index exceeding the 85th percentile, and lack of rescue medication. Poor health literacy was most common among younger adolescents (ie, ninth graders). Some youth with poor health literacy also reported more emergency department visits, hospitalizations, and lower overall quality of life.21
Beliefs and Attitudes
Beliefs and attitudes towards taking asthma medications can act as barriers to adherence in the adolescent. African American adolescents often report the belief that ICS are not helpful or necessary.16,22-25 These beliefs have been correlated with a lack of understanding of the inflammatory mechanisms of asthma, reports of asthma attacks despite use of controller medications, fear of addiction to medications, and a belief that nontraditional interventions (eg, exercise) will work better to get rid of asthma or abate symptoms.16-19,22-24 African American adolescents also report beliefs that asthma will go away or get better as they age, and they are willing to forgo the use of controller medications based on these beliefs.24
African American adolescents often engage in asthma self-management independent of caregivers. These youth describe asthma self-management activities an annoyance and of low priority in part due to competing tasks and negative interactions with caregivers.25 During early adolescence asthma self-management is often suboptimal, and as youth age they become less observant regarding their asthma and are less likely to seek help.26 Adolescents’ beliefs and low prioritization of asthma self-management may contribute to forgetfulness and loss of inhalers, which are common reasons reported for poor adherence to ICS.16,23-26 Further, the role of caregivers during this period has often been overlooked. Caregivers of African American adolescents have been found to be stressed and overwhelmed with personal responsibilities and neighborhood conditions, leaving them little time to attend to the asthma self-management behavior of youth. Due to these contextual factors, interactions with chronically ill youth may be strained, resulting in negative interactions with youth related to asthma self-management. However, in an intervention study that used multisystemic therapy (an approach that targets the affected individual, family, and community), improvement in positive parenting behaviors related to asthma self-management contributed to improved ICS adherence by adolescents.27
Adolescents can perceive traditional asthma self-management as conflicting with their own personal and/or cultural beliefs. They may seek options beyond the use of medicine and have voiced preferences for behaviors that they believe will “strengthen their lungs” more naturally.24 An appreciation of how youth might use complementary/alternative medicine (CAM) as an adjunctive therapy or in place of evidence-based asthma care is important to understanding the potential effect on morbidity and mortality. Behaviors and beliefs about the use of CAM have not been well studied among urban African American adolescents with asthma. Only one study was found that assessed the use of CAM among a primarily urban African American adolescent population. In that study, 71% of the population reported using some form of CAM during the past 30 days.28 Prayer and relaxation were the most frequently used strategies in the management of asthma symptoms. Perceived efficacy of relaxation and prayer among teens who engaged in this form of CAM was 87% and 85%, respectively. Other CAM strategies included yoga, meditation, guided imagery, and biofeedback. When adolescents were asked if they shared their use of CAM in asthma management with a health care provider, most reported sharing the use of yoga and dietary changes but were least likely to share their use of prayer and guided imagery.28
Personal/Emotional Factors
African American adolescents have reported asthma as a limiting factor in terms of both physical and social activities. They perceive asthma as a burden to themselves and others (eg, peers, family, coaches).9,25 The burden of asthma is further exemplified in the emotional response to the symptoms of the disease and the self-management responsibilities. The need to prevent and respond to asthma symptoms is associated with being embarrassed, frustrated, angry, annoyed, worried, lonely, and isolated.9,11,25 Negative coping strategies by youth in response to psychosocial experiences include decisions to disregard or give minimal attention to asthma symptoms and to delay or not take prescribed medications. Students report ignoring asthma symptom management while engaging in physical activities to maintain a sense of normalcy among peers and as a way of dealing with perceptions by coaches or teachers that they are weak or in need of being protected.24,25
Negative thoughts and experiences can result in depressive disorders and poor quality of life. Depression is a common finding among urban youth with asthma.29,30 Youth diagnosed with asthma who have comorbid depression may benefit from interventions to improve self-management. In a secondary analysis from a Web-based asthma management intervention targeting African American adolescents, depression was found to have a modifying effect on the emotional domain of quality of life for youth in the intervention arm of the study. This finding indicates that participants who were depressed and who reported low levels of emotional quality of life benefited from the Web-based interventions that targeted self-management.31
Caregivers
Caregivers (especially moms) are a common source of support for the development and implementation of asthma self-management behaviors in adolescents.32 Caregivers sometimes hold beliefs similar to those of youth and believe the urban environment can act as a barrier to asthma management.9,25,32 They describe the complexity of asthma treatment plans, a lack of understanding of the disease process, and insensitivity of health care providers to their expressed needs along with the providers’ limited cultural awareness in the development of self-management plans.9,22,33 Caregivers describe how family finances, insurance gaps, access to care, and their own familial/cultural beliefs influence their decisions and ability to support their child’s asthma management.33 When faced with the cost of care they report instances of having to decide between necessities such as food and housing or co-pays for medications and office visits.22,33 They also report concerns about visits with multiple providers due to an inability to access their primary care provider, which can lead to delays in their child being diagnosed with asthma.22
Caregivers report a need to include culturally based practices, past experiences, and personal beliefs into the adolescents’ asthma management plan.22,32,33 In a small interview-based study of caregivers residing in 3 New Jersey public housing communities, caregivers reported preferring “familial” methods of controlling asthma (eg, restriction of activities; use of showers, steam, vaporizers, and nebulizers) over evidence-based recommendations. Many caregivers were confused or lacked knowledge about asthma action plans.33 Caregivers have also been found to lack adequate or accurate knowledge related to asthma medications and factors that improved or worsened asthma. While caregivers report a desire to help educate their teens by passing on what they know, their lack of adequate asthma knowledge may hamper their efforts and potentially worsen the teens’ asthma self-management.32
While African American caregivers often describe themselves as hypervigilant concerning their child’s asthma, they may report different information than their adolescent when both are questioned about asthma symptom experiences and functional status.34 Factors increasing the level of congruence between caregiver and teen asthma symptom reports were found to be related to the adolescents’ age and asthma disease classification. Symptom questionnaire responses of older teens and those with mild intermittent asthma were more likely to be similar to caregiver reports. The researchers concluded that clinicians and researchers may obtain reliable asthma symptom and functional status reports by asking the adolescent directly.34
Schools
Caregivers and adolescents describe schools as a threat to self-management and the overall health of youth with asthma.9,32 They perceive that a lack of knowledge by staff, teachers, and coaches contributes to inattentiveness or disbelief in the credibility of reported asthma symptoms by youth.11,23 These misperceptions and the lack of attentiveness by adults in the school may pose safety and health issues for African American youth.9,25,33,34 For example, adolescents report pressure from teacher, coaches, and peers in school settings to partake in sports and/or gym classes. Youth want to identify with healthy peers and thus often choose not to take asthma medications during such activities or opt to continue participating while being compromised by airway obstruction. Of great concern were reports by caregivers and teens of not being allowed to call a parent for support or retrieve their medications when needed for asthma symptoms.32
Future Research and Practice Implications
In this review, we identified 5 common themes around barriers to asthma self-management for African American adolescents (knowledge and skills, beliefs and attitudes, personal/emotional factors, caregivers, and schools). Caregivers, especially mothers, play a pivotal role in the development of effective asthma self-management behaviors. Depsite good intentions, there is evidence of caregivers passing on ineffective experiential and culturally based beliefs and practices to their adolescents that can negatively influence self-care behaviors.13,28,38 Studies are needed to further investigate these findings among caregivers as their beliefs and practices for asthma self-management have been found to coexist among adolescents. Studies that investigate how to incorporate caregiver asthma knowledge, cultural beliefs and behaviors in developing self-management interventions have the potential to positively influence asthma outcomes among African American adolescents.27 The unique cultural beliefs, contextual environmental, and social disparities faced by African American caregivers should not be neglected.
African American adolescents, like adolescents in other racial or ethnic groups, desire to be autonomous in their asthma self-management. However, as adolescents age their adherence behaviors often decline. This may suggest a need for a longer transition period to self-management that extends into emerging adulthood (18-25 years). While youth want to feel supported, there appears to be a fine line between receiving needed support and what youth describe as “nagging” behaviors by adults. Additional investigations into how asthma responsibilities are transitioned from the parent to youth and how best to support the development and maintenance of related behaviors and skills are warranted. In addition, teens described problems related to communicating with health care providers, noting a lack of clarity in explanations received about how to manage their asthma. Some teens believed the communication challenges were based on beliefs and biases held by providers that African American youth had limited capacities for self-management.9 There is a need to better understand interactions among African American adolescents, parents, and clinicians so that communication and transitioning asthma care to the youth will produce optimal health outcomes.
According to asthma guidelines, the patient-provider relationship is essential to effective asthma self-management.4,5 However, there is little mention in the literature of team-based care. Clinicians such as physicians, physician assistants, and nurse practitioners provide direct care to adolescents in terms of disease management and the overall effectiveness of treatment plans. African American youth demonstrate a need for asthma education that is comprehensive and that is contextualized to their daily lives. A team-based approach to care that includes social workers and community health workers may help to extend the reach of clinicians. Follow-up times with families and youth between office visits can be used to support adolescents to develop asthma self-management and allow them a safe space to describe frustrations and other emotions that contribute to their desire to be disease-free.
Summary
Asthma is a chronic disease that is often more severe and difficult to manage in African American adolescents. While African American adolescents describe developmental needs like those of other youth, cultural beliefs and contextual experiences influence their self-care management in unique ways. Opportunities exist to better understand the needs of African American adolescents and to help them successfully gain the knowledge, skills, and behaviors needed to effectively engage in self-management of their asthma.
Corresponding author: Wanda Gibson-Scipio, PhD, FNP-BC, FAANP, 5557 Cass Ave., 346 Cohn Building, Detroit, MI 48324; [email protected].
Financial disclosures: None.
1. Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001-2010. NCHS Data Brief. 2012:1-8.
2. Bruzzese JM, Bonner S, Vincent EJ, et al. Asthma education: the adolescent experience. Patient Educ Couns. 2004;55:396-406.
3. Bryant-Stephens T. Asthma disparities in urban environments. J Allergy Clin Immunol. 2009;123:1199-1206.
4. National Heart, Lung, and Blood Institute. National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007.
5. GINA. Global strategy for asthma management and prevention. 2017. www.ginaasthma.org. Accessed Dec 15, 2017.
6. Centers for Disease Control and Prevention. Vital signs. 2011. https://www.cdc.gov/vitalsigns/asthma/index.html. Accessed December 15, 2017.
7. Centers for Disease Control and Prevention. 2015 National Health Interview Survey (NHIS) Data. National Center for Environmental Health, 2017. https://www.cdc.gov/asthma/nhis/2015/table4-1.htm. Accessed December 15, 2017.
8. Gupta RS, Carrión-Carire V, Weiss KB. The widening black/white gap in asthma hospitalizations and mortality. J Allergy Clin Immunol. 2006;117:351-358.
9. Evans-Agnew R. Asthma management disparities: a photovoice investigation with African American youth. J Sch Nurs. 2016;32:99-111.
10. Naar-King S, Ellis, D, Kolmodin, K. Feasibility of adapting multisystemic therapy to improve illness management behaviors and reduce asthma morbidity in high risk African American youth: a case series. J Child Fam Stud. 2009;18:564-573.
11. Rhee H, Wenzel J, Steeves RH. Adolescents’ psychosocial experiences living with asthma: a focus group study. J Pediatr Health Care. 2007;21:99-107.
12. Sin MK, Kang DH, Weaver M. Relationships of asthma knowledge, self-management, and social support in African American adolescents with asthma. Int J Nurs Stud. 2005;42:307-313.
13. Mosnaim G, Li H, Martin M, et al. Factors associated with levels of adherence to inhaled corticosteroids in minority adolescents with asthma. Ann Allergy Asthma Immunol. 2014;112:116-120.
14. Riekert KA, Borrelli B, Bilderback A, Rand CS. The development of a motivational interviewing intervention to promote medication adherence among inner-city, African American adolescents with asthma. Patient Educ Couns. 2011;82:117-122.
15. Bruzzese JM, Stepney C, Fiorino EK, et al. Asthma self-management is sub-optimal in urban Hispanic and African American/black early adolescents with uncontrolled persistent asthma. J Asthma. 2012;49:90-97.
16. Naimi DR, Freedman TG, Ginsburg KR, et al. Adolescents and asthma: why bother with our meds? J Allergy Clin Immunol. 2009;123:1335-1341.
17. Naar-King S, Lam P, Ellis D, et al. Asthma medication device skills in high-risk African American adolescents. J Asthma. 2013;50:579-582.
18. Ellis DA, King P, Naar-King S. Mediators of treatment effects in a randomized clinical trial of multisystemic therapy-health care in adolescents with poorly controlled asthma: disease knowledge and device use skills. J Pediatr Psychol. 2016;41:522-530.
19. Crowder SJ, Hanna KM, Carpenter JS, Broome ME. Factors associated with asthma self-management in African American adolescents. J Pediatric Nurs. 2015;30:e35-e43.
20. U.S. Department of Health and Human Services. Healthy people 2010: understanding and improving health. 2nd ed. Washington (DC): U.S. Government Printing Office; November 2000.
21. Valerio MA, Peterson EL, Wittich AR, Joseph CLM. Examining health literacy among urban African-American adolescents with asthma. J Asthma. 2016;53:1041-1047.
22. Laster N, Holsey CN, Shendell DG, et al. Barriers to asthma management among urban families: caregiver and child perspectives. J Asthma. 2009;46:731-739.
23. Ayala GX, Miller D, Zagami E, et al. Asthma in middle schools: what students have to say about their asthma. J Sch Health. 2006;76:208-214.
24. Gibson-Scipio W, Gourdin D, Krouse, HJ. Asthma self-management goals, beliefs and behaviors of urban African American adolescents prior to transitioning to adult health care. J Pediatric Nurs. 2015;30:e53-e61.
25. Blaakman SW, Cohen A, Fagnano M, Halterman JS. Asthma medication adherence among urban teens: a qualitative analysis of barriers, facilitators and experiences with school-based care. J Asthma. 2014;51:522-529.
26. Bruzzese JM, Idalski Carcone A, Lam P, et al. Adherence to asthma medication regimens in urban African American adolescents: application of self-determination theory. Health Psychol. 2014;33:461-464.
27. Ellis DA, King P, Naar-King S, et al. Effects of family treatment on parenting beliefs among caregivers of youth with poorly controlled asthma. J Dev Behav Pediatr. 2014;35:486-493.
28. Cotton S, Luberto CM, Yi MS, Tsevat J. Complementary and alternative medicine behaviors and beliefs in urban adolescents with asthma. J Asthma. 2011;48:531-538.
29. Bahreinian S, Ball GDC, Colman I, et al. Depression is more common in girls with nonatopic asthma. Chest. 2011;140:1138-1145.
30. Bender BG. Risk taking, depression, adherence, and symptom control in adolescents and young adults with asthma. Am J Respir Crit Care Med. 2006;173:953-957.
31. Guglani L, Havstad SL, Johnson CC, et al. Effect of depressive symptoms on asthma intervention in urban teens. Ann Allergy Asthma Immunol. 2012;109:237-242.
32. Gibson-Scipio W, Krouse HJ. Goals, beliefs, and concerns of urban caregivers of middle and older adolescents with asthma. J Asthma. 2013;50:242-249.
33. Wagner F, Steefel L. Beliefs regarding asthma management relating to asthma action plans (AAPs) of African American caregivers residing in Newark, New Jersey public housing communities. J Pediatr Nurs. 2017;36:92-97.
34. Houle CR, Joseph CL, Caldwell CH, et al. Congruence between urban adolescent and caregiver responses to questions about the adolescent’s asthma. J Urban Health. 2011;88:30-40.
1. Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001-2010. NCHS Data Brief. 2012:1-8.
2. Bruzzese JM, Bonner S, Vincent EJ, et al. Asthma education: the adolescent experience. Patient Educ Couns. 2004;55:396-406.
3. Bryant-Stephens T. Asthma disparities in urban environments. J Allergy Clin Immunol. 2009;123:1199-1206.
4. National Heart, Lung, and Blood Institute. National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007.
5. GINA. Global strategy for asthma management and prevention. 2017. www.ginaasthma.org. Accessed Dec 15, 2017.
6. Centers for Disease Control and Prevention. Vital signs. 2011. https://www.cdc.gov/vitalsigns/asthma/index.html. Accessed December 15, 2017.
7. Centers for Disease Control and Prevention. 2015 National Health Interview Survey (NHIS) Data. National Center for Environmental Health, 2017. https://www.cdc.gov/asthma/nhis/2015/table4-1.htm. Accessed December 15, 2017.
8. Gupta RS, Carrión-Carire V, Weiss KB. The widening black/white gap in asthma hospitalizations and mortality. J Allergy Clin Immunol. 2006;117:351-358.
9. Evans-Agnew R. Asthma management disparities: a photovoice investigation with African American youth. J Sch Nurs. 2016;32:99-111.
10. Naar-King S, Ellis, D, Kolmodin, K. Feasibility of adapting multisystemic therapy to improve illness management behaviors and reduce asthma morbidity in high risk African American youth: a case series. J Child Fam Stud. 2009;18:564-573.
11. Rhee H, Wenzel J, Steeves RH. Adolescents’ psychosocial experiences living with asthma: a focus group study. J Pediatr Health Care. 2007;21:99-107.
12. Sin MK, Kang DH, Weaver M. Relationships of asthma knowledge, self-management, and social support in African American adolescents with asthma. Int J Nurs Stud. 2005;42:307-313.
13. Mosnaim G, Li H, Martin M, et al. Factors associated with levels of adherence to inhaled corticosteroids in minority adolescents with asthma. Ann Allergy Asthma Immunol. 2014;112:116-120.
14. Riekert KA, Borrelli B, Bilderback A, Rand CS. The development of a motivational interviewing intervention to promote medication adherence among inner-city, African American adolescents with asthma. Patient Educ Couns. 2011;82:117-122.
15. Bruzzese JM, Stepney C, Fiorino EK, et al. Asthma self-management is sub-optimal in urban Hispanic and African American/black early adolescents with uncontrolled persistent asthma. J Asthma. 2012;49:90-97.
16. Naimi DR, Freedman TG, Ginsburg KR, et al. Adolescents and asthma: why bother with our meds? J Allergy Clin Immunol. 2009;123:1335-1341.
17. Naar-King S, Lam P, Ellis D, et al. Asthma medication device skills in high-risk African American adolescents. J Asthma. 2013;50:579-582.
18. Ellis DA, King P, Naar-King S. Mediators of treatment effects in a randomized clinical trial of multisystemic therapy-health care in adolescents with poorly controlled asthma: disease knowledge and device use skills. J Pediatr Psychol. 2016;41:522-530.
19. Crowder SJ, Hanna KM, Carpenter JS, Broome ME. Factors associated with asthma self-management in African American adolescents. J Pediatric Nurs. 2015;30:e35-e43.
20. U.S. Department of Health and Human Services. Healthy people 2010: understanding and improving health. 2nd ed. Washington (DC): U.S. Government Printing Office; November 2000.
21. Valerio MA, Peterson EL, Wittich AR, Joseph CLM. Examining health literacy among urban African-American adolescents with asthma. J Asthma. 2016;53:1041-1047.
22. Laster N, Holsey CN, Shendell DG, et al. Barriers to asthma management among urban families: caregiver and child perspectives. J Asthma. 2009;46:731-739.
23. Ayala GX, Miller D, Zagami E, et al. Asthma in middle schools: what students have to say about their asthma. J Sch Health. 2006;76:208-214.
24. Gibson-Scipio W, Gourdin D, Krouse, HJ. Asthma self-management goals, beliefs and behaviors of urban African American adolescents prior to transitioning to adult health care. J Pediatric Nurs. 2015;30:e53-e61.
25. Blaakman SW, Cohen A, Fagnano M, Halterman JS. Asthma medication adherence among urban teens: a qualitative analysis of barriers, facilitators and experiences with school-based care. J Asthma. 2014;51:522-529.
26. Bruzzese JM, Idalski Carcone A, Lam P, et al. Adherence to asthma medication regimens in urban African American adolescents: application of self-determination theory. Health Psychol. 2014;33:461-464.
27. Ellis DA, King P, Naar-King S, et al. Effects of family treatment on parenting beliefs among caregivers of youth with poorly controlled asthma. J Dev Behav Pediatr. 2014;35:486-493.
28. Cotton S, Luberto CM, Yi MS, Tsevat J. Complementary and alternative medicine behaviors and beliefs in urban adolescents with asthma. J Asthma. 2011;48:531-538.
29. Bahreinian S, Ball GDC, Colman I, et al. Depression is more common in girls with nonatopic asthma. Chest. 2011;140:1138-1145.
30. Bender BG. Risk taking, depression, adherence, and symptom control in adolescents and young adults with asthma. Am J Respir Crit Care Med. 2006;173:953-957.
31. Guglani L, Havstad SL, Johnson CC, et al. Effect of depressive symptoms on asthma intervention in urban teens. Ann Allergy Asthma Immunol. 2012;109:237-242.
32. Gibson-Scipio W, Krouse HJ. Goals, beliefs, and concerns of urban caregivers of middle and older adolescents with asthma. J Asthma. 2013;50:242-249.
33. Wagner F, Steefel L. Beliefs regarding asthma management relating to asthma action plans (AAPs) of African American caregivers residing in Newark, New Jersey public housing communities. J Pediatr Nurs. 2017;36:92-97.
34. Houle CR, Joseph CL, Caldwell CH, et al. Congruence between urban adolescent and caregiver responses to questions about the adolescent’s asthma. J Urban Health. 2011;88:30-40.