User login
Correction: Genitourinary syndrome of menopause
Taurine
Taurine, also known as 2-aminoethanesulfonic acid, is a naturally occurring beta-amino acid (which has a sulphonic acid group instead of carboxylic acid, differentiating it from other amino acids) yielded by methionine and cysteine metabolism in the liver.1,2 An important free beta-amino acid in mammals, it is often the free amino acid present in the greatest concentrations in several cell types in humans.1,2 Dietary intake of taurine also plays an important role in maintaining the body’s taurine levels because of mammals’ limited ability to synthesize it.1
Notably in terms of dermatologic treatment options, the combination product taurine bromamine is known to impart antioxidant, anti-inflammatory, and antibacterial activities.3 And taurine itself is associated with antioxidant, anti-inflammatory, antifibrotic, and immunomodulatory characteristics,1,4 and is noted for conferring antiaging benefits.5
Acne and other inflammatory conditions
The use of .6,7
In response to the problem of evolving antibiotic resistance, Marcinkiewicz reported in 2009 on the then-new therapeutic option of topical taurine bromamine for the treatment of inflammatory skin disorders such as acne. The author pointed out that Propionibacterium acnes is particularly sensitive to taurine bromamine, with the substance now known to suppress H2O2 production by activated neutrophils, likely contributing to moderating the severity and lowering the number of inflammatory acne lesions. In a 6-week double-blind pilot clinical study, Marcinkiewicz and his team compared the efficacy of 0.5% taurine bromamine cream with 1% clindamycin gel in 40 patients with mild to moderate acne. Treatments, which were randomly assigned, occurred twice daily through the study. Amelioration of acne symptoms was comparable in the two groups, with more than 90% of patients improving clinically and experiencing similar decreases in acne lesions (65% in the taurine bromamine group and 68% in the clindamycin group). Marcinkiewicz concluded that these results indicate the viability of taurine bromamine as an option for inflammatory acne therapy, particularly for patients who have shown antibiotic resistance.3
Wide-ranging protection potential
In 2003, Janeke et al. conducted analyses that showed that taurine accumulation defended cultured human keratinocytes from osmotically- and UV-induced apoptosis, suggesting the importance of taurine as an epidermal osmolyte necessary for maintaining keratinocyte hydration in a dry environment.2
Three years later, Collin et al. demonstrated the dynamic protective effects of taurine on the human hair follicle in an in vitro study in which taurine promoted hair survival and protected against TGF-beta1-induced damage.1
Taurine has also been found to stabilize and protect the catalytic activity of the hemoprotein cytochrome P450 3A4, which is a key enzyme responsible for metabolizing various endogenous as well as foreign substances, including drugs.8
Penetration enhancement
In 2016, Mueller et al. studied the effects of urea and taurine as hydrophilic penetration enhancers on stratum corneum lipid models as both substances are known to exert such effects. With inconclusive results as to the roots of such activity, they speculated that both entities enhance penetration through the introduction of copious water into the corneocytes, resulting from the robust water-binding capacity of urea and the consequent osmotic pressure related to taurine.9
Possible skin whitening and anti-aging roles and other promising lab results
Based on their previous work demonstrating that azelaic acid, a saturated dicarboxylic acid found naturally in wheat, rye, and barley, suppressed melanogenesis, Yu and Kim investigated the antimelanogenic activity of azelaic acid and taurine in B16F10 mouse melanoma cells in 2010. They found that the combination of the two substances exhibited a greater inhibitory effect in melanocytes than azelaic acid alone, with melanin production and tyrosinase activity suppressed without inducing cytotoxicity. The investigators concluded the combination of azelaic acid and taurine may be an effective approach for treating hyperpigmentation.10
In 2015, Ito et al. investigated the possible anti-aging role of taurine using a taurine transporter knockout mouse model. They noted that aging-related disorders affecting the skin, heart, skeletal muscle, and liver and resulting in a shorter lifespan have been correlated with tissue taurine depletion. The researchers proposed that proper protein folding allows endogenous taurine to perform as an antiaging molecule.5
Also in 2015, Kim et al. investigated potential mechanisms of the antiproliferative activity of taurine on murine B16F10 melanoma cells via the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and neutral red assays and microscopic analysis. They found that taurine prevented cell proliferation and engendered apoptosis in B16F10 cells, concluding that taurine may have a role to play as a chemotherapeutic agent for skin cancer.11
In 2014, Ashkani-Esfahani et al. studied the impact of taurine on cutaneous leishmaniasis wounds in a mouse model. Investigators induced 18 mice with wounds using L. major promastigotes, and divided them into a taurine injection group, taurine gel group, and no treatment group, performing treatments every 24 hours over 21 days. The taurine treatment groups exhibited significantly greater numerical fibroblast density, collagen bundle volume density, and vessel length densities compared with the nontreatment group. The taurine injection group displayed higher fibroblast numerical density than did the taurine gel group. The researchers concluded that taurine has the capacity to enhance wound healing and tissue regeneration but showed no direct anti-leishmaniasis effect.4
Conclusion
Taurine has been found over the last few decades to impart salutary effects for human health. This beta-amino acid that occurs naturally in humans and other mammals also appears to hold promising potential in the dermatologic realm, particularly for its anti-inflammatory and antioxidant effects. More research is needed to ascertain just how pivotal this compound can be for skin health.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Int J Cosmet Sci. 2006 Aug;28(4):289-98.
2. J Invest Dermatol. 2003 Aug;121(2):354-61.
3. Pol Arch Med Wewn. 2009 Oct;119(10):673-6.
4. Adv Biomed Res. 2014 Oct 7;3:204.
5. Adv Exp Med Biol. 2015;803:481-7.
6. Am J Clin Dermatol. 2012 Dec 1;13(6):357-64.
7. Eur J Dermatol. 2008 Jul-Aug;18(4):433-9.
8. Biochemistry (Mosc). 2015 Mar;80(3):366-73.
9. Biochim Biophys Acta. 2016 Sep;1858(9):2006-18.
10. J Biomed Sci. 2010 Aug 24;17 Suppl 1:S45.
11. Adv Exp Med Biol. 2015;803:167-77.
Taurine, also known as 2-aminoethanesulfonic acid, is a naturally occurring beta-amino acid (which has a sulphonic acid group instead of carboxylic acid, differentiating it from other amino acids) yielded by methionine and cysteine metabolism in the liver.1,2 An important free beta-amino acid in mammals, it is often the free amino acid present in the greatest concentrations in several cell types in humans.1,2 Dietary intake of taurine also plays an important role in maintaining the body’s taurine levels because of mammals’ limited ability to synthesize it.1
Notably in terms of dermatologic treatment options, the combination product taurine bromamine is known to impart antioxidant, anti-inflammatory, and antibacterial activities.3 And taurine itself is associated with antioxidant, anti-inflammatory, antifibrotic, and immunomodulatory characteristics,1,4 and is noted for conferring antiaging benefits.5
Acne and other inflammatory conditions
The use of .6,7
In response to the problem of evolving antibiotic resistance, Marcinkiewicz reported in 2009 on the then-new therapeutic option of topical taurine bromamine for the treatment of inflammatory skin disorders such as acne. The author pointed out that Propionibacterium acnes is particularly sensitive to taurine bromamine, with the substance now known to suppress H2O2 production by activated neutrophils, likely contributing to moderating the severity and lowering the number of inflammatory acne lesions. In a 6-week double-blind pilot clinical study, Marcinkiewicz and his team compared the efficacy of 0.5% taurine bromamine cream with 1% clindamycin gel in 40 patients with mild to moderate acne. Treatments, which were randomly assigned, occurred twice daily through the study. Amelioration of acne symptoms was comparable in the two groups, with more than 90% of patients improving clinically and experiencing similar decreases in acne lesions (65% in the taurine bromamine group and 68% in the clindamycin group). Marcinkiewicz concluded that these results indicate the viability of taurine bromamine as an option for inflammatory acne therapy, particularly for patients who have shown antibiotic resistance.3
Wide-ranging protection potential
In 2003, Janeke et al. conducted analyses that showed that taurine accumulation defended cultured human keratinocytes from osmotically- and UV-induced apoptosis, suggesting the importance of taurine as an epidermal osmolyte necessary for maintaining keratinocyte hydration in a dry environment.2
Three years later, Collin et al. demonstrated the dynamic protective effects of taurine on the human hair follicle in an in vitro study in which taurine promoted hair survival and protected against TGF-beta1-induced damage.1
Taurine has also been found to stabilize and protect the catalytic activity of the hemoprotein cytochrome P450 3A4, which is a key enzyme responsible for metabolizing various endogenous as well as foreign substances, including drugs.8
Penetration enhancement
In 2016, Mueller et al. studied the effects of urea and taurine as hydrophilic penetration enhancers on stratum corneum lipid models as both substances are known to exert such effects. With inconclusive results as to the roots of such activity, they speculated that both entities enhance penetration through the introduction of copious water into the corneocytes, resulting from the robust water-binding capacity of urea and the consequent osmotic pressure related to taurine.9
Possible skin whitening and anti-aging roles and other promising lab results
Based on their previous work demonstrating that azelaic acid, a saturated dicarboxylic acid found naturally in wheat, rye, and barley, suppressed melanogenesis, Yu and Kim investigated the antimelanogenic activity of azelaic acid and taurine in B16F10 mouse melanoma cells in 2010. They found that the combination of the two substances exhibited a greater inhibitory effect in melanocytes than azelaic acid alone, with melanin production and tyrosinase activity suppressed without inducing cytotoxicity. The investigators concluded the combination of azelaic acid and taurine may be an effective approach for treating hyperpigmentation.10
In 2015, Ito et al. investigated the possible anti-aging role of taurine using a taurine transporter knockout mouse model. They noted that aging-related disorders affecting the skin, heart, skeletal muscle, and liver and resulting in a shorter lifespan have been correlated with tissue taurine depletion. The researchers proposed that proper protein folding allows endogenous taurine to perform as an antiaging molecule.5
Also in 2015, Kim et al. investigated potential mechanisms of the antiproliferative activity of taurine on murine B16F10 melanoma cells via the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and neutral red assays and microscopic analysis. They found that taurine prevented cell proliferation and engendered apoptosis in B16F10 cells, concluding that taurine may have a role to play as a chemotherapeutic agent for skin cancer.11
In 2014, Ashkani-Esfahani et al. studied the impact of taurine on cutaneous leishmaniasis wounds in a mouse model. Investigators induced 18 mice with wounds using L. major promastigotes, and divided them into a taurine injection group, taurine gel group, and no treatment group, performing treatments every 24 hours over 21 days. The taurine treatment groups exhibited significantly greater numerical fibroblast density, collagen bundle volume density, and vessel length densities compared with the nontreatment group. The taurine injection group displayed higher fibroblast numerical density than did the taurine gel group. The researchers concluded that taurine has the capacity to enhance wound healing and tissue regeneration but showed no direct anti-leishmaniasis effect.4
Conclusion
Taurine has been found over the last few decades to impart salutary effects for human health. This beta-amino acid that occurs naturally in humans and other mammals also appears to hold promising potential in the dermatologic realm, particularly for its anti-inflammatory and antioxidant effects. More research is needed to ascertain just how pivotal this compound can be for skin health.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Int J Cosmet Sci. 2006 Aug;28(4):289-98.
2. J Invest Dermatol. 2003 Aug;121(2):354-61.
3. Pol Arch Med Wewn. 2009 Oct;119(10):673-6.
4. Adv Biomed Res. 2014 Oct 7;3:204.
5. Adv Exp Med Biol. 2015;803:481-7.
6. Am J Clin Dermatol. 2012 Dec 1;13(6):357-64.
7. Eur J Dermatol. 2008 Jul-Aug;18(4):433-9.
8. Biochemistry (Mosc). 2015 Mar;80(3):366-73.
9. Biochim Biophys Acta. 2016 Sep;1858(9):2006-18.
10. J Biomed Sci. 2010 Aug 24;17 Suppl 1:S45.
11. Adv Exp Med Biol. 2015;803:167-77.
Taurine, also known as 2-aminoethanesulfonic acid, is a naturally occurring beta-amino acid (which has a sulphonic acid group instead of carboxylic acid, differentiating it from other amino acids) yielded by methionine and cysteine metabolism in the liver.1,2 An important free beta-amino acid in mammals, it is often the free amino acid present in the greatest concentrations in several cell types in humans.1,2 Dietary intake of taurine also plays an important role in maintaining the body’s taurine levels because of mammals’ limited ability to synthesize it.1
Notably in terms of dermatologic treatment options, the combination product taurine bromamine is known to impart antioxidant, anti-inflammatory, and antibacterial activities.3 And taurine itself is associated with antioxidant, anti-inflammatory, antifibrotic, and immunomodulatory characteristics,1,4 and is noted for conferring antiaging benefits.5
Acne and other inflammatory conditions
The use of .6,7
In response to the problem of evolving antibiotic resistance, Marcinkiewicz reported in 2009 on the then-new therapeutic option of topical taurine bromamine for the treatment of inflammatory skin disorders such as acne. The author pointed out that Propionibacterium acnes is particularly sensitive to taurine bromamine, with the substance now known to suppress H2O2 production by activated neutrophils, likely contributing to moderating the severity and lowering the number of inflammatory acne lesions. In a 6-week double-blind pilot clinical study, Marcinkiewicz and his team compared the efficacy of 0.5% taurine bromamine cream with 1% clindamycin gel in 40 patients with mild to moderate acne. Treatments, which were randomly assigned, occurred twice daily through the study. Amelioration of acne symptoms was comparable in the two groups, with more than 90% of patients improving clinically and experiencing similar decreases in acne lesions (65% in the taurine bromamine group and 68% in the clindamycin group). Marcinkiewicz concluded that these results indicate the viability of taurine bromamine as an option for inflammatory acne therapy, particularly for patients who have shown antibiotic resistance.3
Wide-ranging protection potential
In 2003, Janeke et al. conducted analyses that showed that taurine accumulation defended cultured human keratinocytes from osmotically- and UV-induced apoptosis, suggesting the importance of taurine as an epidermal osmolyte necessary for maintaining keratinocyte hydration in a dry environment.2
Three years later, Collin et al. demonstrated the dynamic protective effects of taurine on the human hair follicle in an in vitro study in which taurine promoted hair survival and protected against TGF-beta1-induced damage.1
Taurine has also been found to stabilize and protect the catalytic activity of the hemoprotein cytochrome P450 3A4, which is a key enzyme responsible for metabolizing various endogenous as well as foreign substances, including drugs.8
Penetration enhancement
In 2016, Mueller et al. studied the effects of urea and taurine as hydrophilic penetration enhancers on stratum corneum lipid models as both substances are known to exert such effects. With inconclusive results as to the roots of such activity, they speculated that both entities enhance penetration through the introduction of copious water into the corneocytes, resulting from the robust water-binding capacity of urea and the consequent osmotic pressure related to taurine.9
Possible skin whitening and anti-aging roles and other promising lab results
Based on their previous work demonstrating that azelaic acid, a saturated dicarboxylic acid found naturally in wheat, rye, and barley, suppressed melanogenesis, Yu and Kim investigated the antimelanogenic activity of azelaic acid and taurine in B16F10 mouse melanoma cells in 2010. They found that the combination of the two substances exhibited a greater inhibitory effect in melanocytes than azelaic acid alone, with melanin production and tyrosinase activity suppressed without inducing cytotoxicity. The investigators concluded the combination of azelaic acid and taurine may be an effective approach for treating hyperpigmentation.10
In 2015, Ito et al. investigated the possible anti-aging role of taurine using a taurine transporter knockout mouse model. They noted that aging-related disorders affecting the skin, heart, skeletal muscle, and liver and resulting in a shorter lifespan have been correlated with tissue taurine depletion. The researchers proposed that proper protein folding allows endogenous taurine to perform as an antiaging molecule.5
Also in 2015, Kim et al. investigated potential mechanisms of the antiproliferative activity of taurine on murine B16F10 melanoma cells via the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and neutral red assays and microscopic analysis. They found that taurine prevented cell proliferation and engendered apoptosis in B16F10 cells, concluding that taurine may have a role to play as a chemotherapeutic agent for skin cancer.11
In 2014, Ashkani-Esfahani et al. studied the impact of taurine on cutaneous leishmaniasis wounds in a mouse model. Investigators induced 18 mice with wounds using L. major promastigotes, and divided them into a taurine injection group, taurine gel group, and no treatment group, performing treatments every 24 hours over 21 days. The taurine treatment groups exhibited significantly greater numerical fibroblast density, collagen bundle volume density, and vessel length densities compared with the nontreatment group. The taurine injection group displayed higher fibroblast numerical density than did the taurine gel group. The researchers concluded that taurine has the capacity to enhance wound healing and tissue regeneration but showed no direct anti-leishmaniasis effect.4
Conclusion
Taurine has been found over the last few decades to impart salutary effects for human health. This beta-amino acid that occurs naturally in humans and other mammals also appears to hold promising potential in the dermatologic realm, particularly for its anti-inflammatory and antioxidant effects. More research is needed to ascertain just how pivotal this compound can be for skin health.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Int J Cosmet Sci. 2006 Aug;28(4):289-98.
2. J Invest Dermatol. 2003 Aug;121(2):354-61.
3. Pol Arch Med Wewn. 2009 Oct;119(10):673-6.
4. Adv Biomed Res. 2014 Oct 7;3:204.
5. Adv Exp Med Biol. 2015;803:481-7.
6. Am J Clin Dermatol. 2012 Dec 1;13(6):357-64.
7. Eur J Dermatol. 2008 Jul-Aug;18(4):433-9.
8. Biochemistry (Mosc). 2015 Mar;80(3):366-73.
9. Biochim Biophys Acta. 2016 Sep;1858(9):2006-18.
10. J Biomed Sci. 2010 Aug 24;17 Suppl 1:S45.
11. Adv Exp Med Biol. 2015;803:167-77.
November 2018 Digital Edition
Click here to access the November 2018 Digital Edition.
Table of Contents
Providing Rural Veterans With Access to Exercise Through Gerofit
Reducing COPD Readmission Rates: Using a COPD Care Service During Care Transitions
Initiative to Minimize Pharmaceutical Risk in Older Veterans (IMPROVE) Polypharmacy Clinic

Click here to access the November 2018 Digital Edition.
Table of Contents
Providing Rural Veterans With Access to Exercise Through Gerofit
Reducing COPD Readmission Rates: Using a COPD Care Service During Care Transitions
Initiative to Minimize Pharmaceutical Risk in Older Veterans (IMPROVE) Polypharmacy Clinic

Click here to access the November 2018 Digital Edition.
Table of Contents
Providing Rural Veterans With Access to Exercise Through Gerofit
Reducing COPD Readmission Rates: Using a COPD Care Service During Care Transitions
Initiative to Minimize Pharmaceutical Risk in Older Veterans (IMPROVE) Polypharmacy Clinic

Reply to “Increasing Inpatient Consultation: Hospitalist Perceptions and Objective Findings. In Reference to: ‘Hospitalist Perspective of Interactions with Medicine Subspecialty Consult Services’”
The finding by Kachman et al. that consultations have decreased at their institution is an interesting and important observation.1 In contrast, our study found that more than a third of hospitalists reported an increase in consultation requests.2 There may be several explanations for this discrepancy. First, as Kachman et al. suggest, there may be differences between hospitalist perception and actual consultation use. Second, a significant variability in consultation may exist between hospitals. Although our study examined four institutions, we were unable to examine the variability between them, which requires further study. Third, there may be considerable variability between individual hospitalist practices, which is consistent with the findings reported by Kachman et al. Finally, the fact that our study examined only nonteaching services may be another explanation as Kachman et al. found that hospitalists on nonteaching services ordered more consultations than those on teaching services. These findings are consistent with a recent study conducted by Perez et al., who found that hospitalists on teaching services utilized fewer consultations and had lower direct care costs and shorter lengths of stay compared with those on nonteaching services.3 This finding raises the question of whether consultations impact care costs and lengths of stay, a topic that should be explored in future studies.
Disclosures
The authors report no conflicts of interest.
1. Kachman M, Carter K, Martin S. Increasing inpatient consultation: hospitalist perceptions and objective findings. In Reference to: “Hospitalist perspective of interactions with medicine subspecialty consult services”. J Hosp Med. 2018;13(11):802. doi: 10.12788/jhm.2992.
2. Adams TN, Bonsall J, Hunt D, et al. Hospitalist perspective of interactions with medicine subspecialty consult services. J Hosp Med. 2018;13(5):318-323. doi: 10.12788/jhm.2882. PubMed
3. Perez JA Jr, Awar M, Nezamabadi A, et al. Comparison of direct patient care costs and quality outcomes of the teaching and nonteaching hospitalist services at a large academic medical center. Acad Med. 2018;93(3):491-497. doi: 10.1097/ACM.0000000000002026. PubMed
The finding by Kachman et al. that consultations have decreased at their institution is an interesting and important observation.1 In contrast, our study found that more than a third of hospitalists reported an increase in consultation requests.2 There may be several explanations for this discrepancy. First, as Kachman et al. suggest, there may be differences between hospitalist perception and actual consultation use. Second, a significant variability in consultation may exist between hospitals. Although our study examined four institutions, we were unable to examine the variability between them, which requires further study. Third, there may be considerable variability between individual hospitalist practices, which is consistent with the findings reported by Kachman et al. Finally, the fact that our study examined only nonteaching services may be another explanation as Kachman et al. found that hospitalists on nonteaching services ordered more consultations than those on teaching services. These findings are consistent with a recent study conducted by Perez et al., who found that hospitalists on teaching services utilized fewer consultations and had lower direct care costs and shorter lengths of stay compared with those on nonteaching services.3 This finding raises the question of whether consultations impact care costs and lengths of stay, a topic that should be explored in future studies.
Disclosures
The authors report no conflicts of interest.
The finding by Kachman et al. that consultations have decreased at their institution is an interesting and important observation.1 In contrast, our study found that more than a third of hospitalists reported an increase in consultation requests.2 There may be several explanations for this discrepancy. First, as Kachman et al. suggest, there may be differences between hospitalist perception and actual consultation use. Second, a significant variability in consultation may exist between hospitals. Although our study examined four institutions, we were unable to examine the variability between them, which requires further study. Third, there may be considerable variability between individual hospitalist practices, which is consistent with the findings reported by Kachman et al. Finally, the fact that our study examined only nonteaching services may be another explanation as Kachman et al. found that hospitalists on nonteaching services ordered more consultations than those on teaching services. These findings are consistent with a recent study conducted by Perez et al., who found that hospitalists on teaching services utilized fewer consultations and had lower direct care costs and shorter lengths of stay compared with those on nonteaching services.3 This finding raises the question of whether consultations impact care costs and lengths of stay, a topic that should be explored in future studies.
Disclosures
The authors report no conflicts of interest.
1. Kachman M, Carter K, Martin S. Increasing inpatient consultation: hospitalist perceptions and objective findings. In Reference to: “Hospitalist perspective of interactions with medicine subspecialty consult services”. J Hosp Med. 2018;13(11):802. doi: 10.12788/jhm.2992.
2. Adams TN, Bonsall J, Hunt D, et al. Hospitalist perspective of interactions with medicine subspecialty consult services. J Hosp Med. 2018;13(5):318-323. doi: 10.12788/jhm.2882. PubMed
3. Perez JA Jr, Awar M, Nezamabadi A, et al. Comparison of direct patient care costs and quality outcomes of the teaching and nonteaching hospitalist services at a large academic medical center. Acad Med. 2018;93(3):491-497. doi: 10.1097/ACM.0000000000002026. PubMed
1. Kachman M, Carter K, Martin S. Increasing inpatient consultation: hospitalist perceptions and objective findings. In Reference to: “Hospitalist perspective of interactions with medicine subspecialty consult services”. J Hosp Med. 2018;13(11):802. doi: 10.12788/jhm.2992.
2. Adams TN, Bonsall J, Hunt D, et al. Hospitalist perspective of interactions with medicine subspecialty consult services. J Hosp Med. 2018;13(5):318-323. doi: 10.12788/jhm.2882. PubMed
3. Perez JA Jr, Awar M, Nezamabadi A, et al. Comparison of direct patient care costs and quality outcomes of the teaching and nonteaching hospitalist services at a large academic medical center. Acad Med. 2018;93(3):491-497. doi: 10.1097/ACM.0000000000002026. PubMed
© 2018 Society of Hospital Medicine
In Reply to “Diving Into Diagnostic Uncertainty: Strategies to Mitigate Cognitive Load. In Reference to: ‘Focused Ethnography of Diagnosis in Academic Medical Centers’”
We thank Dr. Santhosh and colleagues for their letter concerning our article.1 We agree that the diagnostic journey includes interactions both between and across teams, not just those within the patient’s team. In an article currently in press in Diagnosis, we examine how systems and cognitive factors interact during the process of diagnosis. Specifically, we reported on how communication between consultants can be both a barrier and facilitator to the diagnostic process.2 We found that the frequency, quality, and pace of communication between and across inpatient teams and specialists are essential to timely diagnoses. As diagnostic errors remain a costly and morbid issue in the hospital setting, efforts to improve communication are clearly needed.3
Santhosh et al. raise an interesting point regarding cognitive load in evaluating diagnosis. Cognitive load is a multidimensional construct that represents the load that performing a specific task poses on a learner’s cognitive system.4 Components often used for measuring load include (a) task characteristics such as format, complexity, and time pressure; (b) subject characteristics such as expertise level, age, and spatial abilities; and (c) mental load and effort that originate from the interaction between task and subject characteristics.5 While there is little doubt that measuring these constructs has face value in diagnosis, we know of no instruments that are nimble
Disclosures
The authors have nothing to disclose.
Funding
This project was supported by grant number P30HS024385 from the Agency for Healthcare Research and Quality. The funding source played no role in study design, data acquisition, analysis or decision to report these data.
1. Chopra V, Harrod M, Winter S, et al. Focused ethnography of diagnosis in academic medical centers. J Hosp Med. 2018;13(10):668-672. doi: 10.12788/jhm.2966 PubMed
2. Gupta A, Harrod M, Quinn M, et al. Mind the overlap: how system problems contribute to cognitive failure and diagnostic errors. Diagnosis. 2018; In Press PubMed
3. Gupta A, Snyder A, Kachalia A, et al. Malpractice claims related to diagnostic errors in the hospital [published online ahead of print August 11, 2017]. BMJ Qual Saf. 2017. doi: 10.1136/bmjqs-2017-006774 PubMed
4. Paas FG, Van Merrienboer JJ, Adam JJ. Measurement of cognitive load in instructional research. Percept Mot Skills. 1994;79(1 Pt 2):419-30. doi: 10.2466/pms.1994.79.1.419 PubMed
5. Paas FG, Tuovinen JE, Tabbers H, et al. Cognitive load measurement as a means to advance cognitive load theory. Educational Psychologist. 2003;38(1):63-71. doi: 10.1207/S15326985EP3801_8
We thank Dr. Santhosh and colleagues for their letter concerning our article.1 We agree that the diagnostic journey includes interactions both between and across teams, not just those within the patient’s team. In an article currently in press in Diagnosis, we examine how systems and cognitive factors interact during the process of diagnosis. Specifically, we reported on how communication between consultants can be both a barrier and facilitator to the diagnostic process.2 We found that the frequency, quality, and pace of communication between and across inpatient teams and specialists are essential to timely diagnoses. As diagnostic errors remain a costly and morbid issue in the hospital setting, efforts to improve communication are clearly needed.3
Santhosh et al. raise an interesting point regarding cognitive load in evaluating diagnosis. Cognitive load is a multidimensional construct that represents the load that performing a specific task poses on a learner’s cognitive system.4 Components often used for measuring load include (a) task characteristics such as format, complexity, and time pressure; (b) subject characteristics such as expertise level, age, and spatial abilities; and (c) mental load and effort that originate from the interaction between task and subject characteristics.5 While there is little doubt that measuring these constructs has face value in diagnosis, we know of no instruments that are nimble
Disclosures
The authors have nothing to disclose.
Funding
This project was supported by grant number P30HS024385 from the Agency for Healthcare Research and Quality. The funding source played no role in study design, data acquisition, analysis or decision to report these data.
We thank Dr. Santhosh and colleagues for their letter concerning our article.1 We agree that the diagnostic journey includes interactions both between and across teams, not just those within the patient’s team. In an article currently in press in Diagnosis, we examine how systems and cognitive factors interact during the process of diagnosis. Specifically, we reported on how communication between consultants can be both a barrier and facilitator to the diagnostic process.2 We found that the frequency, quality, and pace of communication between and across inpatient teams and specialists are essential to timely diagnoses. As diagnostic errors remain a costly and morbid issue in the hospital setting, efforts to improve communication are clearly needed.3
Santhosh et al. raise an interesting point regarding cognitive load in evaluating diagnosis. Cognitive load is a multidimensional construct that represents the load that performing a specific task poses on a learner’s cognitive system.4 Components often used for measuring load include (a) task characteristics such as format, complexity, and time pressure; (b) subject characteristics such as expertise level, age, and spatial abilities; and (c) mental load and effort that originate from the interaction between task and subject characteristics.5 While there is little doubt that measuring these constructs has face value in diagnosis, we know of no instruments that are nimble
Disclosures
The authors have nothing to disclose.
Funding
This project was supported by grant number P30HS024385 from the Agency for Healthcare Research and Quality. The funding source played no role in study design, data acquisition, analysis or decision to report these data.
1. Chopra V, Harrod M, Winter S, et al. Focused ethnography of diagnosis in academic medical centers. J Hosp Med. 2018;13(10):668-672. doi: 10.12788/jhm.2966 PubMed
2. Gupta A, Harrod M, Quinn M, et al. Mind the overlap: how system problems contribute to cognitive failure and diagnostic errors. Diagnosis. 2018; In Press PubMed
3. Gupta A, Snyder A, Kachalia A, et al. Malpractice claims related to diagnostic errors in the hospital [published online ahead of print August 11, 2017]. BMJ Qual Saf. 2017. doi: 10.1136/bmjqs-2017-006774 PubMed
4. Paas FG, Van Merrienboer JJ, Adam JJ. Measurement of cognitive load in instructional research. Percept Mot Skills. 1994;79(1 Pt 2):419-30. doi: 10.2466/pms.1994.79.1.419 PubMed
5. Paas FG, Tuovinen JE, Tabbers H, et al. Cognitive load measurement as a means to advance cognitive load theory. Educational Psychologist. 2003;38(1):63-71. doi: 10.1207/S15326985EP3801_8
1. Chopra V, Harrod M, Winter S, et al. Focused ethnography of diagnosis in academic medical centers. J Hosp Med. 2018;13(10):668-672. doi: 10.12788/jhm.2966 PubMed
2. Gupta A, Harrod M, Quinn M, et al. Mind the overlap: how system problems contribute to cognitive failure and diagnostic errors. Diagnosis. 2018; In Press PubMed
3. Gupta A, Snyder A, Kachalia A, et al. Malpractice claims related to diagnostic errors in the hospital [published online ahead of print August 11, 2017]. BMJ Qual Saf. 2017. doi: 10.1136/bmjqs-2017-006774 PubMed
4. Paas FG, Van Merrienboer JJ, Adam JJ. Measurement of cognitive load in instructional research. Percept Mot Skills. 1994;79(1 Pt 2):419-30. doi: 10.2466/pms.1994.79.1.419 PubMed
5. Paas FG, Tuovinen JE, Tabbers H, et al. Cognitive load measurement as a means to advance cognitive load theory. Educational Psychologist. 2003;38(1):63-71. doi: 10.1207/S15326985EP3801_8
© 2018 Society of Hospital Medicine
Diving Into Diagnostic Uncertainty: Strategies to Mitigate Cognitive Load: In Reference to: “Focused Ethnography of Diagnosis in Academic Medical Centers”
We read the article by Chopra et al. “Focused Ethnography of Diagnosis in Academic Medical Centers” with great interest.1 This ethnographic study provided valuable insights into possible interventions to encourage diagnostic thinking.
Duty hour regulations and the resulting increase in handoffs have shifted the social experience of diagnosis from one that occurs within teams to one that often occurs between teams during handoffs between providers.2 While the article highlighted barriers to diagnosis, including distractions and time pressure, it did not explicitly discuss cognitive load theory. Cognitive load theory is an educational framework that has been described by Young et al.3 to improve instructions in the handoff process. These investigators showed how progressively experienced learners retain more information when using a structured scaffold or framework for information, such as the IPASS mnemonic,4 for example.
To mitigate the effects of distraction on the transfer of information, especially in cases with high diagnostic uncertainty, cognitive load must be explicitly considered. A structured framework for communication about diagnostic uncertainty informed by cognitive load theory would be a novel innovation that would help not only graduate medical education but could also improve diagnostic accuracy.
Disclosures
The authors have no conflicts of interest to disclose
1. Chopra V, Harrod M, Winter S, et al. Focused Ethnography of Diagnosis in Academic Medical Centers. J Hosp Med. 2018;13(10):668-672. doi: 10.12788/jhm.2966. PubMed
2. Duong JA, Jensen TP, Morduchowicz, S, Mourad M, Harrison JD, Ranji SR. Exploring physician perspectives of residency holdover handoffs: a qualitative study to understand an increasingly important type of handoff. J Gen Intern Med. 2017;32(6):654-659. doi: 10.1007/s11606-017-4009-y PubMed
3. Young JQ, ten Cate O, O’Sullivan PS, Irby DM. Unpacking the complexity of patient handoffs through the lens of cognitive load theory. Teach Learn Med. 2016;28(1):88-96. doi: 10.1080/10401334.2015.1107491. PubMed
4. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. doi: 10.1056/NEJMc1414788. PubMed
We read the article by Chopra et al. “Focused Ethnography of Diagnosis in Academic Medical Centers” with great interest.1 This ethnographic study provided valuable insights into possible interventions to encourage diagnostic thinking.
Duty hour regulations and the resulting increase in handoffs have shifted the social experience of diagnosis from one that occurs within teams to one that often occurs between teams during handoffs between providers.2 While the article highlighted barriers to diagnosis, including distractions and time pressure, it did not explicitly discuss cognitive load theory. Cognitive load theory is an educational framework that has been described by Young et al.3 to improve instructions in the handoff process. These investigators showed how progressively experienced learners retain more information when using a structured scaffold or framework for information, such as the IPASS mnemonic,4 for example.
To mitigate the effects of distraction on the transfer of information, especially in cases with high diagnostic uncertainty, cognitive load must be explicitly considered. A structured framework for communication about diagnostic uncertainty informed by cognitive load theory would be a novel innovation that would help not only graduate medical education but could also improve diagnostic accuracy.
Disclosures
The authors have no conflicts of interest to disclose
We read the article by Chopra et al. “Focused Ethnography of Diagnosis in Academic Medical Centers” with great interest.1 This ethnographic study provided valuable insights into possible interventions to encourage diagnostic thinking.
Duty hour regulations and the resulting increase in handoffs have shifted the social experience of diagnosis from one that occurs within teams to one that often occurs between teams during handoffs between providers.2 While the article highlighted barriers to diagnosis, including distractions and time pressure, it did not explicitly discuss cognitive load theory. Cognitive load theory is an educational framework that has been described by Young et al.3 to improve instructions in the handoff process. These investigators showed how progressively experienced learners retain more information when using a structured scaffold or framework for information, such as the IPASS mnemonic,4 for example.
To mitigate the effects of distraction on the transfer of information, especially in cases with high diagnostic uncertainty, cognitive load must be explicitly considered. A structured framework for communication about diagnostic uncertainty informed by cognitive load theory would be a novel innovation that would help not only graduate medical education but could also improve diagnostic accuracy.
Disclosures
The authors have no conflicts of interest to disclose
1. Chopra V, Harrod M, Winter S, et al. Focused Ethnography of Diagnosis in Academic Medical Centers. J Hosp Med. 2018;13(10):668-672. doi: 10.12788/jhm.2966. PubMed
2. Duong JA, Jensen TP, Morduchowicz, S, Mourad M, Harrison JD, Ranji SR. Exploring physician perspectives of residency holdover handoffs: a qualitative study to understand an increasingly important type of handoff. J Gen Intern Med. 2017;32(6):654-659. doi: 10.1007/s11606-017-4009-y PubMed
3. Young JQ, ten Cate O, O’Sullivan PS, Irby DM. Unpacking the complexity of patient handoffs through the lens of cognitive load theory. Teach Learn Med. 2016;28(1):88-96. doi: 10.1080/10401334.2015.1107491. PubMed
4. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. doi: 10.1056/NEJMc1414788. PubMed
1. Chopra V, Harrod M, Winter S, et al. Focused Ethnography of Diagnosis in Academic Medical Centers. J Hosp Med. 2018;13(10):668-672. doi: 10.12788/jhm.2966. PubMed
2. Duong JA, Jensen TP, Morduchowicz, S, Mourad M, Harrison JD, Ranji SR. Exploring physician perspectives of residency holdover handoffs: a qualitative study to understand an increasingly important type of handoff. J Gen Intern Med. 2017;32(6):654-659. doi: 10.1007/s11606-017-4009-y PubMed
3. Young JQ, ten Cate O, O’Sullivan PS, Irby DM. Unpacking the complexity of patient handoffs through the lens of cognitive load theory. Teach Learn Med. 2016;28(1):88-96. doi: 10.1080/10401334.2015.1107491. PubMed
4. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. doi: 10.1056/NEJMc1414788. PubMed
© 2018 Society of Hospital Medicine
Increasing Inpatient Consultation: Hospitalist Perceptions and Objective Findings. In Reference to: “Hospitalist Perspective of Interactions with Medicine Subspecialty Consult Services”
We read with interest the article, “Hospitalist Perspective of Interactions with Medicine Subspecialty Consult Services.”1 We applaud the authors for their work, but were surprised by the authors’ findings of hospitalist perceptions of consultation utilization. The authors reported that more hospitalists felt that their personal use of consultation was increasing (38.5%) versus those who reported that use was decreasing (30.3%).1 The lack of true consensus on this issue may hint at significant variability in hospitalist use of inpatient consultation. We examined consultation use in 4,023 general medicine admissions to the University of Chicago from 2011 to 2015. Consultation use varied widely, with a 3.5-fold difference between the lowest and the highest quartiles of use (P < .01).2 Contrary to the survey findings, we found that the number of consultations per admission actually decreased with each year in our sample.2 In addition, a particularly interesting effect was observed in hospitalists; in multivariate regression, hospitalists on nonteaching services ordered more consultations than those on teaching services.2 These findings suggest a gap between hospitalist self-reported perceptions of consultation use and actual use, which is important to understand, and highlight the need for further characterization of factors driving the use of this valuable resource.
Disclosures
The authors have no conflicts of interest to disclose.
1. Adams TN, Bonsall J, Hunt D, et al. Hospitalist perspective of interactions with medicine subspecialty consult services. J Hosp Med. 2018:13(5):318-323. doi: 10.12788/jhm.2882. PubMed
2. Kachman M, Carter K, Martin S, et al. Describing variability of inpatient consultation practices on general medicine services: patient, admission and physician-level factors. Abstract from: Hospital Medicine 2018; April 8-11, 2018; Orlando, Florida.
We read with interest the article, “Hospitalist Perspective of Interactions with Medicine Subspecialty Consult Services.”1 We applaud the authors for their work, but were surprised by the authors’ findings of hospitalist perceptions of consultation utilization. The authors reported that more hospitalists felt that their personal use of consultation was increasing (38.5%) versus those who reported that use was decreasing (30.3%).1 The lack of true consensus on this issue may hint at significant variability in hospitalist use of inpatient consultation. We examined consultation use in 4,023 general medicine admissions to the University of Chicago from 2011 to 2015. Consultation use varied widely, with a 3.5-fold difference between the lowest and the highest quartiles of use (P < .01).2 Contrary to the survey findings, we found that the number of consultations per admission actually decreased with each year in our sample.2 In addition, a particularly interesting effect was observed in hospitalists; in multivariate regression, hospitalists on nonteaching services ordered more consultations than those on teaching services.2 These findings suggest a gap between hospitalist self-reported perceptions of consultation use and actual use, which is important to understand, and highlight the need for further characterization of factors driving the use of this valuable resource.
Disclosures
The authors have no conflicts of interest to disclose.
We read with interest the article, “Hospitalist Perspective of Interactions with Medicine Subspecialty Consult Services.”1 We applaud the authors for their work, but were surprised by the authors’ findings of hospitalist perceptions of consultation utilization. The authors reported that more hospitalists felt that their personal use of consultation was increasing (38.5%) versus those who reported that use was decreasing (30.3%).1 The lack of true consensus on this issue may hint at significant variability in hospitalist use of inpatient consultation. We examined consultation use in 4,023 general medicine admissions to the University of Chicago from 2011 to 2015. Consultation use varied widely, with a 3.5-fold difference between the lowest and the highest quartiles of use (P < .01).2 Contrary to the survey findings, we found that the number of consultations per admission actually decreased with each year in our sample.2 In addition, a particularly interesting effect was observed in hospitalists; in multivariate regression, hospitalists on nonteaching services ordered more consultations than those on teaching services.2 These findings suggest a gap between hospitalist self-reported perceptions of consultation use and actual use, which is important to understand, and highlight the need for further characterization of factors driving the use of this valuable resource.
Disclosures
The authors have no conflicts of interest to disclose.
1. Adams TN, Bonsall J, Hunt D, et al. Hospitalist perspective of interactions with medicine subspecialty consult services. J Hosp Med. 2018:13(5):318-323. doi: 10.12788/jhm.2882. PubMed
2. Kachman M, Carter K, Martin S, et al. Describing variability of inpatient consultation practices on general medicine services: patient, admission and physician-level factors. Abstract from: Hospital Medicine 2018; April 8-11, 2018; Orlando, Florida.
1. Adams TN, Bonsall J, Hunt D, et al. Hospitalist perspective of interactions with medicine subspecialty consult services. J Hosp Med. 2018:13(5):318-323. doi: 10.12788/jhm.2882. PubMed
2. Kachman M, Carter K, Martin S, et al. Describing variability of inpatient consultation practices on general medicine services: patient, admission and physician-level factors. Abstract from: Hospital Medicine 2018; April 8-11, 2018; Orlando, Florida.
© 2018 Society of Hospital Medicine
Healthcare Quality for Children and Adolescents with Suicidality Admitted to Acute Care Hospitals in the United States
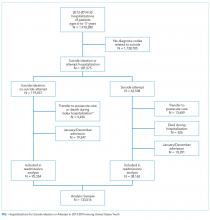
Suicide is the second most common cause of death among children, adolescents, and young adults in the United States. In 2016, over 6,000 children and youth 5 to 24 years of age succumbed to suicide, thus reflecting a mortality rate nearly three times higher than deaths from malignancies and 28 times higher than deaths from sepsis in this age group.1 Suicidal ideation and suicide attempts are even more common, with 17% of high school students reporting seriously considering suicide and 8% reporting suicide attempts in the previous 12 months.2 These tragic statistics are reflected in our health system use, emergency department (ED) utilization for suicide attempts and suicidal ideation is growing at a tremendous rate, and over 50% of the children seen in EDs are subsequently admitted to the hospital for ongoing care.3,4
In this issue of Journal of Hospital Medicine, Doupnik and colleagues present an analysis of pediatric hospitalizations for suicide attempts and suicidal ideation at acute care hospitals contained within the 2013 and 2014 National Readmissions Dataset.5 This dataset reflects a nationally representative sample of pediatric hospitalizations, weighted to allow for national estimates. Although their focus was on hospital readmission, their analysis yielded additional valuable data about suicide attempts and suicidal ideation in American youth. The investigators identified 181,575 pediatric acute care hospitalizations for suicide attempts and suicidal ideation over the two-year study period, accounting for 9.5% of all acute care hospitalizations among children and adolescents 6 to 17 years of age nationally. This number exceeds the biennial number of pediatric hospitalizations for cellulitis, dehydration, and urinary tract infections, all of which are generally considered the “bread and butter” of pediatric hospital medicine.6
Doupnik and colleagues rightly pointed out that hospital readmission is not a nationally endorsed measure to evaluate the quality of pediatric mental health hospitalizations. At the same time, their work highlights that acute care hospitals need strategies to measure the quality of pediatric hospitalizations for suicide attempts and suicidal ideation. Beyond readmissions, how should the quality of these hospital stays be evaluated? A recent review of 15 national quality measure sets identified 257 unique measures to evaluate pediatric quality of care.7 Of these, only one focused on mental health hospitalization. This measure, which was endorsed by the National Quality Forum, determines the percentage of discharges for patients six years of age and older who were hospitalized for mental health diagnoses and who had a follow-up visit with a mental health practitioner within 7 and 30 days of hospital discharge.8 Given Doupnik et al.’s finding that one-third of all 30-day hospital readmissions occurred within seven days of hospital discharge, early follow-up visits with mental health practitioners is arguably essential.
Although evidence-based quality measures to evaluate hospital-based mental healthcare are limited, quality measure development is ongoing, facilitated by recent federal health policy and associated research efforts. Four newly developed measures focus on the quality of inpatient care for suicidality, including two evaluated using data from health records and two derived from caregiver surveys. The first medical records-based measure identifies whether caregivers of patients admitted to hospital for dangerous self-harm or suicidality have documentation that they were counseled on how to restrict their child’s or adolescent’s access to potentially lethal means of suicide before discharge. The second record-based measure evaluates documentation in the medical record of discussion between the hospital provider and the patient’s outpatient provider regarding the plan for follow-up.9 The two survey-based measures ask caregivers whether they were counseled on how to restrict access to potentially lethal means of suicide, and, for children and adolescents started on a new antidepressant medication or dose, whether they were counseled regarding the potential benefits and risks of the medication.10 All measures were field-tested at children’s hospitals to ensure feasibility in data collection. However, as shown by Doupnik et al., only 7.4% of acute care hospitalizations for suicide attempts and suicidal ideation occurred at freestanding children’s hospitals; most occurred at urban nonteaching centers. Evaluation of these new quality measures across structurally diverse hospitals is an important next step.
Beyond the healthcare constructs evaluated by these quality measures, many foundational questions about what constitutes high quality inpatient healthcare for suicide attempts and suicidal ideation remain. An American Academy of Child and Adolescent Psychiatry (AACAP) practice parameter, which was published in 2001, established minimal standards for the assessment and treatment of children and adolescents with suicidal behavior.11 This guideline recommends inpatient treatment until the mental state or level of suicidality has stabilized, with discharge considered only when the clinician is satisfied that adequate supervision and support will be available and when a responsible adult has agreed to secure or dispose of potentially lethal medications and firearms. It further recommends that the clinician treating the child or adolescent during the days following a suicide attempt be available to the patient and family – for example, to receive and make telephone calls outside of regular clinic hours. Recognizing the growing prevalence of suicidality in American children and youth, coupled with critical shortages in pediatric psychiatrists and fragmentation of inpatient and outpatient care, these minimal standards may be difficult to implement across the many settings where children receive their mental healthcare.4,12,13
The large number of children and adolescents being hospitalized for suicide attempts and suicidal ideation at acute care hospitals demands that we take stock of how we manage this vulnerable population. Although Doupnik and colleagues suggest that exclusion of specialty psychiatric hospitals from their dataset is a limitation, their presentation of suicide attempts and suicidal ideation epidemiology at acute care hospitals provides valuable data for pediatric hospitalists. Given the presence of pediatric hospitalists at many acute care hospitals, comanagement by hospital medicine and psychiatry services may prove both efficient and effective while breaking down the silos that traditionally separate these specialties. Alternatively, extending the role of collaborative care teams, which are increasingly embedded in pediatric primary care, into inpatient settings may enable continuity of care and improve healthcare quality.14 Finally, nearly 20 years have passed since the AACAP published its practice parameter for the assessment and treatment of children and adolescents with suicidal behavior. An update to reflect contemporary suicide attempts and suicidal ideation statistics and evidence-based practices is needed, and collaboration between professional pediatric and psychiatric organizations in the creation of this update would recognize the growing role of pediatricians, including hospitalists, in the provision of mental healthcare for children.
Updated guidelines must take into account the transitions of care experienced by children and adolescents throughout their hospital stay: at admission, at discharge, and during their hospitalization if they move from medical to psychiatric care. Research is needed to determine what proportion of children and adolescents receive evidence-based mental health therapies while in hospital and how many are connected with wraparound mental health services before hospital discharge.15 Doupnik et al. excluded children and adolescents who were transferred to other hospitals, which included over 18,000 youth. How long did these patients spend “boarding,” and did they receive any mental health assessment or treatment during this period? Although the Joint Commission recommends that holding times for patients awaiting bed placement should not exceed four4 hours, hospitals have described average pediatric inpatient boarding times of 2-3 days while awaiting inpatient psychiatric care.16,17 In one study of children and adolescents awaiting transfer for inpatient psychiatric care, mental health counseling was received by only 6%, which reflects lost time that could have been spent treating this highly vulnerable population.16 Multidisciplinary collaboration is needed to address these issues and inform best practices.
Although mortality is a rare outcome for most conditions we treat in pediatric hospital medicine, mortality following suicide attempts is all too common. The data presented by Doupnik and colleagues provide a powerful call to improve healthcare quality across the diverse settings where children with suicidality receive their care.
Disclosures
The authors have no financial relationships relevant to this article to disclose.
Funding
Dr. Leyenaar was supported by grant number K08HS024133 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of AHRQ.
1. Centers for Disease Control and Prevention, National Center for Health Statistics. Underlying Cause of Death 1999-2016 on CDC WONDER Online Database, released December, 2017.
2. Kann L, Kinchen S, Shanklin S, et al. Youth risk behavior surveillance-United States, 2013. MMWR. 2014;63(4):1-168. PubMed
3. Olfson M, Gameroff MJ, Marcus SC, Greenberg T, Shaffer D. Emergency treatment of young people following deliberate self-harm. Arch Gen Psychiatry. 2005;62(10):1122-1128. doi: 10.1001/archpsyc.62.10.1122 PubMed
4. Mercado MC, Holland K, Leemis RW, Stone DM, Wang J. Trends in emergency department visits for nonfatal self-inflicted injuries among youth aged 10 to 24 years in the United States, 2001-2015. JAMA. 2017;318(19):1931-1932. doi: 10.1001/jama.2017.13317 PubMed
5. Doupnik S, Rodean J, Zima B, et al. Readmissions after pediatric hospitalization for suicide ideation and suicide attempt [published online ahead of print October 31, 2018]. J Hosp Med. doi: 10.12788/jhm.3070
6. Leyenaar JK, Ralston SL, Shieh M, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. doi: 10.1002/jhm.2624 PubMed
7. House SA, Coon ER, Schroeder AR, Ralston SL. Categorization of national pediatric quality measures. Pediatrics. 2017;139(4):e20163269. PubMed
8. National Quality Forum. Follow-up after hospitalization for mental illness. Available at www.qualityforum.org. Accessed July 21, 2018.
9. Bardach N, Burkhart Q, Richardson L, et al. Hospital-based quality measures for pediatric mental health care. Pediatrics. 2018;141(6):e20173554. PubMed
10. Parast L, Bardach N, Burkhart Q, et al. Development of new quality measures for hospital-based care of suicidal youth. Acad Pediatr. 2018;18(3):248-255. doi: 10.1016/j.acap.2017.09.017 PubMed
11. Shaffer D, Pfeffer C. Practice parameters for the assessment and treatment of children and adolescents with suicidal behavior. J Am Acad Child Adolesc Psychiatry. 2001;40(7 Suppl):24-51. doi: 10.1097/00004583-200107001-00003
12. Thomas C, Holtzer C. The continuing shortage of child and adolescent psychiatrists. J Am Acad Child Adolesc Psychiatry. 2006;45(9):1023-1031. doi: 10.1097/01.chi.0000225353.16831.5d PubMed
13. Plemmons G, Hall M, Doupnik S, et al. Hospitalization for suicide ideation or attempt: 2008–2015. Pediatrics. 2018;141(6):e20172426. PubMed
14. Beach SR, Walker J, Celano CM, Mastromauro CA, Sharpe M, Huffman JC. Implementing collaborative care programs for psychiatric disorders in medical settings: a practical guide. Gen Hosp Psychiatry. 2015;37(6):522-527. doi: 10.1016/j.genhosppsych.2015.06.015 PubMed
15. Winters N, Pumariega A. Practice parameter on child and adolescent mental health care in community systems of care. J Am Acad Child Adolsc Psychiatry. 2007;46(2):284-299. DOI: 10.1097/01.chi.0000246061.70330.b8 PubMed
16. Claudius I, Donofrio J, Lam CN, Santillanes G. Impact of boarding pediatric psychiatric patients on a medical ward. Hosp Pediatr. 2014;4(3):125-131. doi: 10.1542/hpeds.2013-0079 PubMed
17. Gallagher KAS, Bujoreanu IS, Cheung P, Choi C, Golden S, Brodziak K. Psychiatric boarding in the pediatric inpatient medical setting: a retrospective analysis. Hosp Pediatr. 2013;7(8):444-450. doi: 10.1542/hpeds.2017-0005 PubMed
Suicide is the second most common cause of death among children, adolescents, and young adults in the United States. In 2016, over 6,000 children and youth 5 to 24 years of age succumbed to suicide, thus reflecting a mortality rate nearly three times higher than deaths from malignancies and 28 times higher than deaths from sepsis in this age group.1 Suicidal ideation and suicide attempts are even more common, with 17% of high school students reporting seriously considering suicide and 8% reporting suicide attempts in the previous 12 months.2 These tragic statistics are reflected in our health system use, emergency department (ED) utilization for suicide attempts and suicidal ideation is growing at a tremendous rate, and over 50% of the children seen in EDs are subsequently admitted to the hospital for ongoing care.3,4
In this issue of Journal of Hospital Medicine, Doupnik and colleagues present an analysis of pediatric hospitalizations for suicide attempts and suicidal ideation at acute care hospitals contained within the 2013 and 2014 National Readmissions Dataset.5 This dataset reflects a nationally representative sample of pediatric hospitalizations, weighted to allow for national estimates. Although their focus was on hospital readmission, their analysis yielded additional valuable data about suicide attempts and suicidal ideation in American youth. The investigators identified 181,575 pediatric acute care hospitalizations for suicide attempts and suicidal ideation over the two-year study period, accounting for 9.5% of all acute care hospitalizations among children and adolescents 6 to 17 years of age nationally. This number exceeds the biennial number of pediatric hospitalizations for cellulitis, dehydration, and urinary tract infections, all of which are generally considered the “bread and butter” of pediatric hospital medicine.6
Doupnik and colleagues rightly pointed out that hospital readmission is not a nationally endorsed measure to evaluate the quality of pediatric mental health hospitalizations. At the same time, their work highlights that acute care hospitals need strategies to measure the quality of pediatric hospitalizations for suicide attempts and suicidal ideation. Beyond readmissions, how should the quality of these hospital stays be evaluated? A recent review of 15 national quality measure sets identified 257 unique measures to evaluate pediatric quality of care.7 Of these, only one focused on mental health hospitalization. This measure, which was endorsed by the National Quality Forum, determines the percentage of discharges for patients six years of age and older who were hospitalized for mental health diagnoses and who had a follow-up visit with a mental health practitioner within 7 and 30 days of hospital discharge.8 Given Doupnik et al.’s finding that one-third of all 30-day hospital readmissions occurred within seven days of hospital discharge, early follow-up visits with mental health practitioners is arguably essential.
Although evidence-based quality measures to evaluate hospital-based mental healthcare are limited, quality measure development is ongoing, facilitated by recent federal health policy and associated research efforts. Four newly developed measures focus on the quality of inpatient care for suicidality, including two evaluated using data from health records and two derived from caregiver surveys. The first medical records-based measure identifies whether caregivers of patients admitted to hospital for dangerous self-harm or suicidality have documentation that they were counseled on how to restrict their child’s or adolescent’s access to potentially lethal means of suicide before discharge. The second record-based measure evaluates documentation in the medical record of discussion between the hospital provider and the patient’s outpatient provider regarding the plan for follow-up.9 The two survey-based measures ask caregivers whether they were counseled on how to restrict access to potentially lethal means of suicide, and, for children and adolescents started on a new antidepressant medication or dose, whether they were counseled regarding the potential benefits and risks of the medication.10 All measures were field-tested at children’s hospitals to ensure feasibility in data collection. However, as shown by Doupnik et al., only 7.4% of acute care hospitalizations for suicide attempts and suicidal ideation occurred at freestanding children’s hospitals; most occurred at urban nonteaching centers. Evaluation of these new quality measures across structurally diverse hospitals is an important next step.
Beyond the healthcare constructs evaluated by these quality measures, many foundational questions about what constitutes high quality inpatient healthcare for suicide attempts and suicidal ideation remain. An American Academy of Child and Adolescent Psychiatry (AACAP) practice parameter, which was published in 2001, established minimal standards for the assessment and treatment of children and adolescents with suicidal behavior.11 This guideline recommends inpatient treatment until the mental state or level of suicidality has stabilized, with discharge considered only when the clinician is satisfied that adequate supervision and support will be available and when a responsible adult has agreed to secure or dispose of potentially lethal medications and firearms. It further recommends that the clinician treating the child or adolescent during the days following a suicide attempt be available to the patient and family – for example, to receive and make telephone calls outside of regular clinic hours. Recognizing the growing prevalence of suicidality in American children and youth, coupled with critical shortages in pediatric psychiatrists and fragmentation of inpatient and outpatient care, these minimal standards may be difficult to implement across the many settings where children receive their mental healthcare.4,12,13
The large number of children and adolescents being hospitalized for suicide attempts and suicidal ideation at acute care hospitals demands that we take stock of how we manage this vulnerable population. Although Doupnik and colleagues suggest that exclusion of specialty psychiatric hospitals from their dataset is a limitation, their presentation of suicide attempts and suicidal ideation epidemiology at acute care hospitals provides valuable data for pediatric hospitalists. Given the presence of pediatric hospitalists at many acute care hospitals, comanagement by hospital medicine and psychiatry services may prove both efficient and effective while breaking down the silos that traditionally separate these specialties. Alternatively, extending the role of collaborative care teams, which are increasingly embedded in pediatric primary care, into inpatient settings may enable continuity of care and improve healthcare quality.14 Finally, nearly 20 years have passed since the AACAP published its practice parameter for the assessment and treatment of children and adolescents with suicidal behavior. An update to reflect contemporary suicide attempts and suicidal ideation statistics and evidence-based practices is needed, and collaboration between professional pediatric and psychiatric organizations in the creation of this update would recognize the growing role of pediatricians, including hospitalists, in the provision of mental healthcare for children.
Updated guidelines must take into account the transitions of care experienced by children and adolescents throughout their hospital stay: at admission, at discharge, and during their hospitalization if they move from medical to psychiatric care. Research is needed to determine what proportion of children and adolescents receive evidence-based mental health therapies while in hospital and how many are connected with wraparound mental health services before hospital discharge.15 Doupnik et al. excluded children and adolescents who were transferred to other hospitals, which included over 18,000 youth. How long did these patients spend “boarding,” and did they receive any mental health assessment or treatment during this period? Although the Joint Commission recommends that holding times for patients awaiting bed placement should not exceed four4 hours, hospitals have described average pediatric inpatient boarding times of 2-3 days while awaiting inpatient psychiatric care.16,17 In one study of children and adolescents awaiting transfer for inpatient psychiatric care, mental health counseling was received by only 6%, which reflects lost time that could have been spent treating this highly vulnerable population.16 Multidisciplinary collaboration is needed to address these issues and inform best practices.
Although mortality is a rare outcome for most conditions we treat in pediatric hospital medicine, mortality following suicide attempts is all too common. The data presented by Doupnik and colleagues provide a powerful call to improve healthcare quality across the diverse settings where children with suicidality receive their care.
Disclosures
The authors have no financial relationships relevant to this article to disclose.
Funding
Dr. Leyenaar was supported by grant number K08HS024133 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of AHRQ.
Suicide is the second most common cause of death among children, adolescents, and young adults in the United States. In 2016, over 6,000 children and youth 5 to 24 years of age succumbed to suicide, thus reflecting a mortality rate nearly three times higher than deaths from malignancies and 28 times higher than deaths from sepsis in this age group.1 Suicidal ideation and suicide attempts are even more common, with 17% of high school students reporting seriously considering suicide and 8% reporting suicide attempts in the previous 12 months.2 These tragic statistics are reflected in our health system use, emergency department (ED) utilization for suicide attempts and suicidal ideation is growing at a tremendous rate, and over 50% of the children seen in EDs are subsequently admitted to the hospital for ongoing care.3,4
In this issue of Journal of Hospital Medicine, Doupnik and colleagues present an analysis of pediatric hospitalizations for suicide attempts and suicidal ideation at acute care hospitals contained within the 2013 and 2014 National Readmissions Dataset.5 This dataset reflects a nationally representative sample of pediatric hospitalizations, weighted to allow for national estimates. Although their focus was on hospital readmission, their analysis yielded additional valuable data about suicide attempts and suicidal ideation in American youth. The investigators identified 181,575 pediatric acute care hospitalizations for suicide attempts and suicidal ideation over the two-year study period, accounting for 9.5% of all acute care hospitalizations among children and adolescents 6 to 17 years of age nationally. This number exceeds the biennial number of pediatric hospitalizations for cellulitis, dehydration, and urinary tract infections, all of which are generally considered the “bread and butter” of pediatric hospital medicine.6
Doupnik and colleagues rightly pointed out that hospital readmission is not a nationally endorsed measure to evaluate the quality of pediatric mental health hospitalizations. At the same time, their work highlights that acute care hospitals need strategies to measure the quality of pediatric hospitalizations for suicide attempts and suicidal ideation. Beyond readmissions, how should the quality of these hospital stays be evaluated? A recent review of 15 national quality measure sets identified 257 unique measures to evaluate pediatric quality of care.7 Of these, only one focused on mental health hospitalization. This measure, which was endorsed by the National Quality Forum, determines the percentage of discharges for patients six years of age and older who were hospitalized for mental health diagnoses and who had a follow-up visit with a mental health practitioner within 7 and 30 days of hospital discharge.8 Given Doupnik et al.’s finding that one-third of all 30-day hospital readmissions occurred within seven days of hospital discharge, early follow-up visits with mental health practitioners is arguably essential.
Although evidence-based quality measures to evaluate hospital-based mental healthcare are limited, quality measure development is ongoing, facilitated by recent federal health policy and associated research efforts. Four newly developed measures focus on the quality of inpatient care for suicidality, including two evaluated using data from health records and two derived from caregiver surveys. The first medical records-based measure identifies whether caregivers of patients admitted to hospital for dangerous self-harm or suicidality have documentation that they were counseled on how to restrict their child’s or adolescent’s access to potentially lethal means of suicide before discharge. The second record-based measure evaluates documentation in the medical record of discussion between the hospital provider and the patient’s outpatient provider regarding the plan for follow-up.9 The two survey-based measures ask caregivers whether they were counseled on how to restrict access to potentially lethal means of suicide, and, for children and adolescents started on a new antidepressant medication or dose, whether they were counseled regarding the potential benefits and risks of the medication.10 All measures were field-tested at children’s hospitals to ensure feasibility in data collection. However, as shown by Doupnik et al., only 7.4% of acute care hospitalizations for suicide attempts and suicidal ideation occurred at freestanding children’s hospitals; most occurred at urban nonteaching centers. Evaluation of these new quality measures across structurally diverse hospitals is an important next step.
Beyond the healthcare constructs evaluated by these quality measures, many foundational questions about what constitutes high quality inpatient healthcare for suicide attempts and suicidal ideation remain. An American Academy of Child and Adolescent Psychiatry (AACAP) practice parameter, which was published in 2001, established minimal standards for the assessment and treatment of children and adolescents with suicidal behavior.11 This guideline recommends inpatient treatment until the mental state or level of suicidality has stabilized, with discharge considered only when the clinician is satisfied that adequate supervision and support will be available and when a responsible adult has agreed to secure or dispose of potentially lethal medications and firearms. It further recommends that the clinician treating the child or adolescent during the days following a suicide attempt be available to the patient and family – for example, to receive and make telephone calls outside of regular clinic hours. Recognizing the growing prevalence of suicidality in American children and youth, coupled with critical shortages in pediatric psychiatrists and fragmentation of inpatient and outpatient care, these minimal standards may be difficult to implement across the many settings where children receive their mental healthcare.4,12,13
The large number of children and adolescents being hospitalized for suicide attempts and suicidal ideation at acute care hospitals demands that we take stock of how we manage this vulnerable population. Although Doupnik and colleagues suggest that exclusion of specialty psychiatric hospitals from their dataset is a limitation, their presentation of suicide attempts and suicidal ideation epidemiology at acute care hospitals provides valuable data for pediatric hospitalists. Given the presence of pediatric hospitalists at many acute care hospitals, comanagement by hospital medicine and psychiatry services may prove both efficient and effective while breaking down the silos that traditionally separate these specialties. Alternatively, extending the role of collaborative care teams, which are increasingly embedded in pediatric primary care, into inpatient settings may enable continuity of care and improve healthcare quality.14 Finally, nearly 20 years have passed since the AACAP published its practice parameter for the assessment and treatment of children and adolescents with suicidal behavior. An update to reflect contemporary suicide attempts and suicidal ideation statistics and evidence-based practices is needed, and collaboration between professional pediatric and psychiatric organizations in the creation of this update would recognize the growing role of pediatricians, including hospitalists, in the provision of mental healthcare for children.
Updated guidelines must take into account the transitions of care experienced by children and adolescents throughout their hospital stay: at admission, at discharge, and during their hospitalization if they move from medical to psychiatric care. Research is needed to determine what proportion of children and adolescents receive evidence-based mental health therapies while in hospital and how many are connected with wraparound mental health services before hospital discharge.15 Doupnik et al. excluded children and adolescents who were transferred to other hospitals, which included over 18,000 youth. How long did these patients spend “boarding,” and did they receive any mental health assessment or treatment during this period? Although the Joint Commission recommends that holding times for patients awaiting bed placement should not exceed four4 hours, hospitals have described average pediatric inpatient boarding times of 2-3 days while awaiting inpatient psychiatric care.16,17 In one study of children and adolescents awaiting transfer for inpatient psychiatric care, mental health counseling was received by only 6%, which reflects lost time that could have been spent treating this highly vulnerable population.16 Multidisciplinary collaboration is needed to address these issues and inform best practices.
Although mortality is a rare outcome for most conditions we treat in pediatric hospital medicine, mortality following suicide attempts is all too common. The data presented by Doupnik and colleagues provide a powerful call to improve healthcare quality across the diverse settings where children with suicidality receive their care.
Disclosures
The authors have no financial relationships relevant to this article to disclose.
Funding
Dr. Leyenaar was supported by grant number K08HS024133 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of AHRQ.
1. Centers for Disease Control and Prevention, National Center for Health Statistics. Underlying Cause of Death 1999-2016 on CDC WONDER Online Database, released December, 2017.
2. Kann L, Kinchen S, Shanklin S, et al. Youth risk behavior surveillance-United States, 2013. MMWR. 2014;63(4):1-168. PubMed
3. Olfson M, Gameroff MJ, Marcus SC, Greenberg T, Shaffer D. Emergency treatment of young people following deliberate self-harm. Arch Gen Psychiatry. 2005;62(10):1122-1128. doi: 10.1001/archpsyc.62.10.1122 PubMed
4. Mercado MC, Holland K, Leemis RW, Stone DM, Wang J. Trends in emergency department visits for nonfatal self-inflicted injuries among youth aged 10 to 24 years in the United States, 2001-2015. JAMA. 2017;318(19):1931-1932. doi: 10.1001/jama.2017.13317 PubMed
5. Doupnik S, Rodean J, Zima B, et al. Readmissions after pediatric hospitalization for suicide ideation and suicide attempt [published online ahead of print October 31, 2018]. J Hosp Med. doi: 10.12788/jhm.3070
6. Leyenaar JK, Ralston SL, Shieh M, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. doi: 10.1002/jhm.2624 PubMed
7. House SA, Coon ER, Schroeder AR, Ralston SL. Categorization of national pediatric quality measures. Pediatrics. 2017;139(4):e20163269. PubMed
8. National Quality Forum. Follow-up after hospitalization for mental illness. Available at www.qualityforum.org. Accessed July 21, 2018.
9. Bardach N, Burkhart Q, Richardson L, et al. Hospital-based quality measures for pediatric mental health care. Pediatrics. 2018;141(6):e20173554. PubMed
10. Parast L, Bardach N, Burkhart Q, et al. Development of new quality measures for hospital-based care of suicidal youth. Acad Pediatr. 2018;18(3):248-255. doi: 10.1016/j.acap.2017.09.017 PubMed
11. Shaffer D, Pfeffer C. Practice parameters for the assessment and treatment of children and adolescents with suicidal behavior. J Am Acad Child Adolesc Psychiatry. 2001;40(7 Suppl):24-51. doi: 10.1097/00004583-200107001-00003
12. Thomas C, Holtzer C. The continuing shortage of child and adolescent psychiatrists. J Am Acad Child Adolesc Psychiatry. 2006;45(9):1023-1031. doi: 10.1097/01.chi.0000225353.16831.5d PubMed
13. Plemmons G, Hall M, Doupnik S, et al. Hospitalization for suicide ideation or attempt: 2008–2015. Pediatrics. 2018;141(6):e20172426. PubMed
14. Beach SR, Walker J, Celano CM, Mastromauro CA, Sharpe M, Huffman JC. Implementing collaborative care programs for psychiatric disorders in medical settings: a practical guide. Gen Hosp Psychiatry. 2015;37(6):522-527. doi: 10.1016/j.genhosppsych.2015.06.015 PubMed
15. Winters N, Pumariega A. Practice parameter on child and adolescent mental health care in community systems of care. J Am Acad Child Adolsc Psychiatry. 2007;46(2):284-299. DOI: 10.1097/01.chi.0000246061.70330.b8 PubMed
16. Claudius I, Donofrio J, Lam CN, Santillanes G. Impact of boarding pediatric psychiatric patients on a medical ward. Hosp Pediatr. 2014;4(3):125-131. doi: 10.1542/hpeds.2013-0079 PubMed
17. Gallagher KAS, Bujoreanu IS, Cheung P, Choi C, Golden S, Brodziak K. Psychiatric boarding in the pediatric inpatient medical setting: a retrospective analysis. Hosp Pediatr. 2013;7(8):444-450. doi: 10.1542/hpeds.2017-0005 PubMed
1. Centers for Disease Control and Prevention, National Center for Health Statistics. Underlying Cause of Death 1999-2016 on CDC WONDER Online Database, released December, 2017.
2. Kann L, Kinchen S, Shanklin S, et al. Youth risk behavior surveillance-United States, 2013. MMWR. 2014;63(4):1-168. PubMed
3. Olfson M, Gameroff MJ, Marcus SC, Greenberg T, Shaffer D. Emergency treatment of young people following deliberate self-harm. Arch Gen Psychiatry. 2005;62(10):1122-1128. doi: 10.1001/archpsyc.62.10.1122 PubMed
4. Mercado MC, Holland K, Leemis RW, Stone DM, Wang J. Trends in emergency department visits for nonfatal self-inflicted injuries among youth aged 10 to 24 years in the United States, 2001-2015. JAMA. 2017;318(19):1931-1932. doi: 10.1001/jama.2017.13317 PubMed
5. Doupnik S, Rodean J, Zima B, et al. Readmissions after pediatric hospitalization for suicide ideation and suicide attempt [published online ahead of print October 31, 2018]. J Hosp Med. doi: 10.12788/jhm.3070
6. Leyenaar JK, Ralston SL, Shieh M, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. doi: 10.1002/jhm.2624 PubMed
7. House SA, Coon ER, Schroeder AR, Ralston SL. Categorization of national pediatric quality measures. Pediatrics. 2017;139(4):e20163269. PubMed
8. National Quality Forum. Follow-up after hospitalization for mental illness. Available at www.qualityforum.org. Accessed July 21, 2018.
9. Bardach N, Burkhart Q, Richardson L, et al. Hospital-based quality measures for pediatric mental health care. Pediatrics. 2018;141(6):e20173554. PubMed
10. Parast L, Bardach N, Burkhart Q, et al. Development of new quality measures for hospital-based care of suicidal youth. Acad Pediatr. 2018;18(3):248-255. doi: 10.1016/j.acap.2017.09.017 PubMed
11. Shaffer D, Pfeffer C. Practice parameters for the assessment and treatment of children and adolescents with suicidal behavior. J Am Acad Child Adolesc Psychiatry. 2001;40(7 Suppl):24-51. doi: 10.1097/00004583-200107001-00003
12. Thomas C, Holtzer C. The continuing shortage of child and adolescent psychiatrists. J Am Acad Child Adolesc Psychiatry. 2006;45(9):1023-1031. doi: 10.1097/01.chi.0000225353.16831.5d PubMed
13. Plemmons G, Hall M, Doupnik S, et al. Hospitalization for suicide ideation or attempt: 2008–2015. Pediatrics. 2018;141(6):e20172426. PubMed
14. Beach SR, Walker J, Celano CM, Mastromauro CA, Sharpe M, Huffman JC. Implementing collaborative care programs for psychiatric disorders in medical settings: a practical guide. Gen Hosp Psychiatry. 2015;37(6):522-527. doi: 10.1016/j.genhosppsych.2015.06.015 PubMed
15. Winters N, Pumariega A. Practice parameter on child and adolescent mental health care in community systems of care. J Am Acad Child Adolsc Psychiatry. 2007;46(2):284-299. DOI: 10.1097/01.chi.0000246061.70330.b8 PubMed
16. Claudius I, Donofrio J, Lam CN, Santillanes G. Impact of boarding pediatric psychiatric patients on a medical ward. Hosp Pediatr. 2014;4(3):125-131. doi: 10.1542/hpeds.2013-0079 PubMed
17. Gallagher KAS, Bujoreanu IS, Cheung P, Choi C, Golden S, Brodziak K. Psychiatric boarding in the pediatric inpatient medical setting: a retrospective analysis. Hosp Pediatr. 2013;7(8):444-450. doi: 10.1542/hpeds.2017-0005 PubMed
© 2018 Society of Hospital Medicine
Readmissions after Pediatric Hospitalization for Suicide Ideation and Suicide Attempt
Suicide is a leading cause of death among 10- to 34-year-olds in the United States.1,2 During the past two decades, the youth suicide death rate has risen by 24%, and more than 5,000 young people die from suicide each year.3 Suicide ideation (SI) and suicide attempts (SAs) are well-established risk factors for suicide death and a source of morbidity for patients and families. One-third of youth with SI attempt suicide at some point in their lifetime.4 Approximately 11% of youth SAs result in suicide death, and 2% of youth who attempt suicide subsequently go on to die from suicide after recovering from a prior SA.5 More than 60,000 youth are hospitalized for SI or SA each year,6 and young people hospitalized for SA are at high short-term risk of repeat SA and suicide death.7 Hospitals need strategies for measuring the quality of SI and SA hospitalizations, monitoring postdischarge outcomes, and identifying the patients at the highest risk of poor outcomes. Readmissions are a useful hospital quality measure that can indicate re-emergence of SI, repeat SA, or inadequate community-based mental health treatment, and interventions designed for patients with readmissions can potentially avert morbidity or mortality.
The National Committee on Quality Assurance recommends measurement of quality metrics for 30-day mental health follow-up after psychiatric hospitalizations, 30-day readmissions after adult (but not pediatric) psychiatric hospitalizations, and 30-day readmissions in pediatric medical and surgical hospitalizations. Readmission measures are not consistently used to evaluate pediatric psychiatric hospitalizations, and psychiatric quality measures are not used to evaluate medical or surgical hospitalizations for SA. Recent research has investigated transfers to postacute care,8 readmission prevalence, variation in hospital readmission performance, and risk factors for readmissions after pediatric psychiatric hospitalizations.9–11 However, no national study has investigated 30-day readmissions in youth hospitalized specifically for SI or SA.
To inform hospital quality measurement and improve hospital and postdischarge care for youth at risk of suicide, more information is needed about the characteristics of and the risk factors for readmissions after index SI/SA hospitalization. To address this knowledge gap, among SI/SA hospitalizations in 6- to 17-year-olds, we examined (1) unplanned 30-day readmissions and characteristics of hospitalizations by 30-day readmission status; (2) patient, hospital, and regional characteristics associated with 30-day readmissions; and (3) characteristics of 30-day readmissions.
METHODS
Study Design and Data Source
We conducted a national, retrospective cohort study of hospitalizations for patients aged 6-17 years using the Agency for Healthcare Research and Quality (AHRQ) 2013 and 2014 Nationwide Readmissions Database (NRD). The combined 2013-2014 NRD includes administrative data from a nationally representative sample of 29 million hospitalizations in 22 states, accounting for 49.3% of all US hospitalizations, and is weighted for national projections. The NRD includes hospital information, patient demographic information, and International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis, procedure, and external cause of injury codes (E-codes). The database includes one primary diagnosis, up to 24 additional diagnoses, and up to 4 E-codes for each hospitalization. The NRD includes information about hospitalizations in acute-care general hospitals (including their psychiatric units) but not from specialty psychiatric hospitals. The database also includes de-identified, verified patient linkage numbers so that patients can be tracked across multiple hospitalizations at the same institution or different institutions within the same state. This study was considered to be exempt from review by the Children’s Hospital of Philadelphia Institutional Review Board.
Sample
We identified a sample of 181,575 hospitalizations for SI (n = 119,037) or SA (n = 62,538) among 6- to 17-year-olds between January 1, 2013, and December 31, 2014 (Figure). We included children as young as 6 years because validated methods exist to identify SI and SA in this age group,12 and because suicide deaths have recently increased among younger children.3 We excluded patients aged 18 years and older from this study since delivery of mental health services differs for adults.13 To create the sample, we first identified all hospitalizations of patients aged 6-17 years. We then used a validated algorithm relying on ICD-9-CM diagnosis codes for poisonings and E-codes for self-injury (E950-959) to identify hospitalizations related to SA.12 Because E-code completeness varies among states,14 we also used the combination of having both a diagnosis code for injury (800-999) and an ICD-9-CM code for SI (V62.84) as a proxy for SA. Among hospitalizations without SA, we identified hospitalizations with SI using the ICD-9-CM code for SI (V62.84) in any position.
We identified 133,516 index hospitalizations with complete data at risk for an unplanned readmission. Because NRD data cannot be linked between calendar years, we limited the study time period for each calendar year to 10 months. We excluded hospitalizations in January because the full 30-day time frame to determine whether a hospitalization had occurred in the preceding 30 days, a known risk factor for readmissions in other samples,15 was not available. We excluded index hospitalizations occurring in December, because the full 30-day time frame to ascertain readmissions was not available. We excluded hospitalizations resulting in death, since these are not at risk for readmission, and hospitalizations resulting in transfer, since the timing of discharge to the community was not known. Readmission hospitalizations were eligible to be included as index hospitalizations if they met sample inclusion criteria. The final sample for readmission analyses included 95,354 SI hospitalizations and 38,162 SA hospitalizations (Figure).
Primary Outcome
The primary outcome was any unplanned, all-cause readmission within 30 days of index hospitalization for SI or SA. Among 30-day readmissions, we examined readmission timing, whether the readmission was to the same hospital or a different hospital, length of stay, and indication for readmission (medical/surgical or psychiatric, and presence of SI or SA diagnoses). Planned readmissions were identified using measure specifications endorsed by the AHRQ and the National Quality Forum16 and excluded from measurement.
Among index hospitalizations for SI, we specifically examined 30-day readmissions for subsequent SA, since one objective of hospitalization for SI is to prevent progression to SA or death. We could not identify hospitalizations for repeat SA after index hospitalization for SA, because diagnosis codes did not differentiate between readmission for complications of index SA and readmission for repeat SA.
Independent Variables
We analyzed demographic, clinical, and hospital factors associated with readmissions in other samples.17–20 Demographic characteristics included patient gender and age, urban or rural residence, payer, and median national income quartile for a patient’s ZIP code. Race and ethnicity data are not available in the NRD.
Clinical characteristics included hospitalization in the 30-days preceding the index hospitalization, index hospitalization length of stay, and admission via the emergency department (ED) versus direct admission. A patient’s chronic condition profile was determined using index hospitalization diagnosis codes. Complex chronic conditions (eg, cancer, cystic fibrosis) were identified using a classification system used in several prior studies of hospital administrative datasets,21 and other noncomplex chronic medical conditions (eg, asthma, obesity) were identified using the Healthcare Cost and Utilization Project (HCUP) chronic condition indicator system.22 Psychiatric conditions (eg, anxiety disorders, substance abuse, autism) were identified and categorized using a classification system used in studies of hospital administrative datasets.23 The number of psychiatric conditions was determined by counting the number of psychiatric condition categories in which a patient had a diagnosis. SA was categorized as having lower risk of death (eg, medication overdose, injury from cutting or piercing) or higher risk of death (eg, hanging, suffocation, or firearm injury).24
Because of known temporal trends in SI and SA,25,26 the month and year of admission were included as covariates. Hospital characteristics included teaching hospital and children’s hospital designations.
Statistical Analysis
We compared descriptive, summary statistics for characteristics of index hospitalizations with and without a 30-day readmission using Rao-Scott chi-square tests. In multivariable analyses, we derived logistic regression models to measure the associations of patient, hospital, and temporal factors with 30-day hospital readmissions. Analyses were conducted in SAS PROC SURVEYLOGISTIC and were weighted to achieve national estimates, clustered by sample stratum and hospital to account for the complex survey design,27 clustered by patients to account for multiple index visits per patient, and adjusted for clinical, demographic, and hospital characteristics. SAS version 9.4 (SAS Institute, Cary, North Carolina) was used for all analyses. All tests were two-sided, and a P value <.05 was considered as statistically significant.
RESULTS
Sample Characteristics
In the weighted analyses, we identified 133,516 hospitalizations in acute-care hospitals for SI or SA, and 8.5% (n = 11,375) of hospitalizations had at least one unplanned 30-day readmission to an acute-care hospital. Unweighted, the sample included 37,683 patients and 42,198 hospitalizations. Among all patients represented in the sample, 90.5% had only a single SI or SA hospitalization, 7.7% had two hospitalizations, and 1.8% had >2 hospitalizations in one year.
Table 1 summarizes the sample characteristics and displays the demographic, clinical, and hospital characteristics of index hospitalizations by the 30-day readmission status. Patients represented in the index hospitalizations were 64.9% female, 3.6% were aged 6-9 years, 40.1% were aged 10-14 years, and 56.3% were aged 15-17 years. Nearly half of the patients (49.1%) used public insurance. Nearly half (44.9%) lived in metropolitan areas with >1 million residents, 36.1% lived in metropolitan areas with 50,000 to 1 million residents, and 14.7% lived in rural areas.
Median length of stay for the index hospitalization was 5 days (interquartile range [IQR] 3-7). Nearly one-third (32.3%) of patients had a noncomplex chronic medical condition, 7.8% had a complex chronic medical condition, and 98.1% had a psychiatric condition. The most common psychiatric conditions were depressive disorders (60.0%) and anxiety disorders (42.2%). More than half (55.0%) of the patients had >2 psychiatric conditions. Most hospitalizations in the sample had SI only (71.4%). Among patients with SA, 81.0% had a lower lethality mechanism of injury and 19.0% had a higher lethality mechanism.
Patients experiencing a readmission were more likely to be 10-14 years old and use public insurance than patients without a readmission (P < .001 for both). For clinical characteristics, patients with a readmission were more likely to have longer index hospital stays (6 vs. 5 days), >2 psychiatric conditions (SI vs. SA), a prior admission in the 30 days preceding the index hospitalization, and admission via the ED (vs. direct admission) (P < .001 for all).
Association of Patient and Hospital Characteristics with Readmissions
Table 2 displays the patient and hospital characteristics associated with readmissions. Among demographic characteristics, 10- to 14-year-old patients had higher odds of readmission (odds ratio [OR]: 1.18, 95% confidence interval [CI]: 1.07-1.29) than 15- to 17-year-old patients. Having public insurance was associated with higher odds of readmission (OR: 1.14, 95% CI: 1.04-1.25). We found no differences in readmission rates based on sex, urban or rural location, or patient’s ZIP code income quartile.
Among clinical characteristics, hospitalizations with an admission for SI or SA in the preceding 30 days, meaning that the index hospitalization itself was a readmission, had the strongest association with readmissions (OR: 3.14, 95% CI: 2.73-3.61). In addition, patients admitted via the ED for the index hospitalization had higher odds of readmission (OR: 1.25, 95% CI: 1.15-1.36). Chronic psychiatric conditions associated with higher odds of readmission included psychotic disorders (OR: 1.39, 95% CI: 1.16-1.67) and bipolar disorder (OR: 1.27, 95% CI: 1.13-1.44).
Characteristics of 30-day Readmissions
Table 3 displays the characteristics of readmissions after SI compared to that after SA. Among the combined sample of 11,375 30-day readmissions, 34.1% occurred within 7 days, and 65.9% in 8-30 days. Eleven percent of patients with any readmission had more than one readmission within 30 days. Among readmissions, 94.5% were for a psychiatric problem and 5.5% were for a medical or surgical problem. A total of 43.9% had a diagnosis of SI and 19.5% a diagnosis of SA. Readmissions were more likely to occur at a different hospital after SI than after SA (48.1% vs. 31.3%, P < .001). Medical and surgical indications for readmission were less common after SI than after SA (4.4% vs. 8.7%, P < .001). Only 1.2% of SI hospitalizations had a readmission for SA within 30 days. Of these cases, 55.6% were aged 15-17 years, 43.3% were aged 10-14 years, and 1.1% were aged 6-9 years; 73.1% of the patients were female, and 49.1% used public insurance.
DISCUSSION
SI and SA in children and adolescents are substantial public health problems associated with significant hospital resource utilization. In 2013 and 2014, there were 181,575 pediatric acute-care hospitalizations for SI or SA, accounting for 9.5% of all hospitalizations in 6- to 17-year-old patients nationally. Among acute-care SI and SA hospitalizations, 8.5% had a readmission to an acute-care hospital within 30 days. The study data source did not include psychiatric specialty hospitals, and the number of index hospitalizations is likely substantially higher when psychiatric specialty hospitalizations are included. Readmissions may also be higher if patients were readmitted to psychiatric specialty hospitals after discharge from acute-care hospitals. The strongest risk factor for unplanned 30-day readmissions was a previous hospitalization in the 30 days before the index admission, likely a marker for severity or complexity of psychiatric illness. Other characteristics associated with higher odds of readmission were bipolar disorder, psychotic disorders, and age 10-14 years. More than one-third of readmissions occurred within the first 7 days after hospital discharge. The prevalence of SI and SA hospitalizations and readmissions was similar to findings in previous analyses of mental health hospitalizations.10,28
A patient’s psychiatric illness type and severity, as evidenced by the need for frequent repeat hospitalizations, was highly associated with the risk of 30-day readmission. Any hospitalization in the 30 days preceding the index hospitalization, whether for SI/SA or for another problem, was a strong risk factor for readmissions. We suspect that prior SI/SA hospitalizations reflect a patient’s chronic elevated risk for suicide. Prior hospitalizations not for SI or SA could be hospitalizations for mental illness exacerbations that increase the risk of SI or SA, eg, bipolar disorder with acute mania, or they could represent physical health problems. Chronic physical health problems are a known risk factor for SI and SA.29
A knowledge of those characteristics that increase the readmission risk can inform future resource allocation, research, and policy in several ways. First, longer hospital stays could mitigate readmission risk in some patients with severe psychiatric illness. European studies in older adolescents and adults show that for severe psychiatric illness, a longer hospital stay is associated with a lower risk of hospital readmission.15,30 Second, better access to intensive community-based mental health (MH) services, including evidence-based psychotherapy and medication management, improves symptoms in young people.31 Access to these services likely affects the risk of hospital readmission. We found that readmission risk was highest in 10- to 14-year-olds. Taken in the context of existing evidence that suicide rates are rising in younger patients,1,3 our findings suggest that particular attention to community services for younger patients is needed. Third, care coordination could help patients access beneficial services to reduce readmissions and improve other outcomes. Enhanced discharge care coordination reduced suicide deaths in high-risk populations in Europe32 and Japan33 and improved attendance at mental health follow-up after pediatric ED discharge in a small United States sample.34 Given that one-third of readmissions occurred within seven days, care coordination designed to ensure access to ambulatory services in the immediate postdischarge period may be particularly beneficial.
We found that ZIP code income quartile was not associated with readmissions. We suspect that poverty is not as closely correlated with MH hospitalization outcomes as it is with physical health hospitalization outcomes for several reasons. Medicaid insurance historically has more robust coverage of mental health services than some private insurance plans, which might offset some of the risk of poor mental health outcomes associated with poverty. Low-income families are eligible to use social services, and families accessing social services might have more opportunities to become familiar with community mental health programs. Further, the expectation of high achievement found in some higher income families is associated with MH problems in children and adolescents.35 Therefore, being in a higher income quartile might not be as protective against poor mental health outcomes as it is against poor physical health outcomes.
Although the NRD provides a rich source of readmission data across hospitals nationally, several limitations are inherent to this administrative dataset. First, data from specialty psychiatric hospitals were not included in the NRD. The study underestimates the total number of index hospitalizations and readmissions, since index SI/SA hospitalizations at psychiatric hospitals are not included, and readmissions are not included if they occurred at specialty psychiatric hospitals. Second, because data cannot be linked between calendar years, we excluded January and December hospitalizations, and findings might not generalize to hospitalizations in January and December. Seasonal trends in SI/SA hospitalizations are known to occur.36 Third, race, ethnicity, primary language, gender identity, and sexual orientation are not available in the NRD, and we could not examine the association of these characteristics with the likelihood of readmissions. Fourth, we did not have information about pre- or posthospitalization insurance enrollment or outpatient services that could affect the risk of readmission. Nevertheless, this study offers information on the characteristics of readmissions after hospitalizations for SI and SA in a large nationally representative sample of youth, and the findings can inform resource planning to prevent suicides.
CONCLUSION
Hospital readmissions are common in patients with SI and SA, and patients with a recent previous hospitalization have the highest risk of readmission. More than one-third of readmissions after SI or SA occurred within the first seven days. Due to the dearth of mental health services in the community, hospitals offer an important safety net for youth experiencing acute suicidal crises. Strategies to improve the continuum of care for patients at risk of suicide that solely focus on reducing readmissions are not likely to benefit patients. However, readmissions can identify opportunities for improving hospital discharge processes and outpatient services. Future research and clinical innovation to investigate and improve hospital discharge planning and access to community mental health services is likely to benefit patients and could reduce 30-day hospital readmissions.
Acknowledgments
The authors thank John Lawlor for his assistance with the analysis.
Disclosures
The authors have no potential conflicts of interest to disclose.
Funding
Dr. Zima received funding from the Behavioral Health Centers of Excellence for California (SB852).
1. Sheftall AH, Asti L, Horowitz LM, et al. Suicide in elementary school-aged children and early adolescents. Pediatrics. 2016;138(4):e20160436. doi: 10.1542/peds.2016-0436. PubMed
2. Prevention CNC for I. Suicide facts at a Glance 2015 nonfatal suicidal thoughts and behavior. In: 2015:3-4. https://stacks.cdc.gov/view/cdc/34181/cdc_34181_DS1.pdf. Accessed September 30, 2016.
3. Curtin S, Warner M, Hedegaard H. Increase in Suicide in the United States, 1999-2014. Hyattsville, MD; 2016. http://www.cdc.gov/nchs/data/databriefs/db241.pdf. Accessed November 7, 2016. PubMed
4. Nock MK, Green JG, Hwang I, et al. Prevalence, correlates, and treatment of lifetime suicidal behavior among adolescents: results from the national comorbidity survey replication adolescent supplement. JAMA Psychiatry. 2013;70(3):300. doi: 10.1001/2013.jamapsychiatry.55. PubMed
5. Bostwick JM, Pabbati C, Geske JR, Mckean AJ. Suicide attempt as a risk factor for completed suicide: even more lethal than we knew. Am J Psychiatry. 2016;173(11):1094-1100. doi: 10.1176/appi.ajp.2016.15070854. PubMed
6. Torio CM, Encinosa W, Berdahl T, McCormick MC, Simpson LA. Annual report on health care for children and youth in the united states: national estimates of cost, utilization and expenditures for children with mental health conditions. Acad Pediatr. 2015;15(1):19-35. doi: 10.1016/j.acap.2014.07.007. PubMed
7. Olfson M, Wall M, Wang S, et al. Suicide after deliberate self-harm in adolescents and young adults. Pediatrics. 2018;141(4):e20173517. doi: 10.1542/peds.2017-3517. PubMed
8. Gay JC, Zima BT, Coker TR, et al. Postacute care after pediatric hospitalizations for a primary mental health condition. J Pediatr. 2018;193:222-228.e1. doi: 10.1016/j.jpeds.2017.09.058. PubMed
9. Heslin KC, Weiss AJ. Hospital Readmissions Involving Psychiatric Disorders, 2012. 2015. https://www.ncbi.nlm.nih.gov/books/NBK305353/pdf/Bookshelf_NBK305353.pdf. Accessed September 8, 2017. PubMed
10. Feng JY, Toomey SL, Zaslavsky AM, Nakamura MM, Schuster MA. Readmission after pediatric mental health admissions. Pediatrics. 2017;140(6):e20171571. doi: 10.1542/peds.2017-1571. PubMed
11. Bardach NS, Vittinghoff E, Asteria-Peñaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. doi: 10.1542/peds.2012-3527. PubMed
12. Callahan ST, Fuchs DC, Shelton RC, et al. Identifying suicidal behavior among adolescents using administrative claims data. Pharmacoepidemiol Drug Saf. 2013;22(7):769-775. doi: 10.1002/pds.3421. PubMed
13. SAMHSA, HHS, Synectics for Management Decisions, Mathematica Policy Research. National Mental Health Services Survey: 2010: Data on Mental Health Treatment Facilities. http://media.samhsa.gov/data/DASIS/NMHSS2010D/NMHSS2010_Web.pdf. Accessed November 13, 2015.
14. Patrick AR, Miller M, Barber CW, Wang PS, Canning CF, Schneeweiss S. Identification of hospitalizations for intentional self-harm when E-codes are incompletely recorded. Pharmacoepidemiol Drug Saf. 2010;19(12):1263-1275. doi: 10.1002/pds.2037. PubMed
15. Mellesdal L, Mehlum L, Wentzel-Larsen T, Kroken R, Jørgensen HA. Suicide risk and acute psychiatric readmissions: a prospective cohort study. Psychiatr Serv. 2010;61(1):25-31. doi: 10.1176/appi.ps.61.1.25. PubMed
16. Agency for Healthcare Research and Quality, Centers for Medicare and Medicaid. Measure: Pediatric All-Condition Readmission Measure Measure Developer: Center of Excellence for Pediatric Quality Measurement (CEPQM). https://www.ahrq.gov/sites/default/files/wysiwyg/policymakers/chipra/factsheets/chipra_14-p008-1-ef.pdf. Accessed November 15, 2017.
17. Cancino RS, Culpepper L, Sadikova E, Martin J, Jack BW, Mitchell SE. Dose-response relationship between depressive symptoms and hospital readmission. J Hosp Med. 2014;9(6):358-364. doi: 10.1002/jhm.2180. PubMed
18. Carlisle CE, Mamdani M, Schachar R, To T. Aftercare, emergency department visits, and readmission in adolescents. J Am Acad Child Adolesc Psychiatry. 2012;51(3):283-293. http://www.sciencedirect.com/science/article/pii/S0890856711011002. Accessed November 2, 2015. PubMed
19. Fadum EA, Stanley B, Qin P, Diep LM, Mehlum L. Self-poisoning with medications in adolescents: a national register study of hospital admissions and readmissions. Gen Hosp Psychiatry. 2014;36(6):709-715. doi: 10.1016/j.genhosppsych.2014.09.004. PubMed
20. Bernet AC. Predictors of psychiatric readmission among veterans at high risk of suicide: the impact of post-discharge aftercare. Arch Psychiatr Nurs. 2013;27(5):260-261. doi: 10.1016/j.apnu.2013.07.001. PubMed
21. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14(1):199. doi: 10.1186/1471-2431-14-199. PubMed
22. HCUP. HCUP-US Tools & Software Page. http://www.hcup-us.ahrq.gov/toolssoftware/chronic/chronic.jsp. Published 2015. Accessed October 30, 2015.
23. Zima BT, Rodean J, Hall M, Bardach NS, Coker TR, Berry JG. Psychiatric disorders and trends in resource use in pediatric hospitals. Pediatrics. 2016;138(5):e20160909-e20160909. doi: 10.1542/peds.2016-0909. PubMed
24. Spicer RS, Miller TR. Suicide acts in 8 states: incidence and case fatality rates by demographics and method. Am J Public Health. 2000;90(12):1885-1891. doi: 10.2105/AJPH.90.12.1885. PubMed
25. Hansen B, Lang M. Back to school blues: Seasonality of youth suicide and the academic calendar. Econ Educ Rev. 2011;30(5):850-861. doi: 10.1016/j.econedurev.2011.04.012.
26. Lueck C, Kearl L, Lam CN, Claudius I. Do emergency pediatric psychiatric visits for danger to self or others correspond to times of school attendance? Am J Emerg Med. 2015;33(5):682-684. doi: 10.1016/J.AJEM.2015.02.055. PubMed
27. Healthcare Cost And Utilization Project. Introduction to the HCUP Nationwide Readmissions Database. Rockville, MD; 2017. https://www.hcup-us.ahrq.gov/db/nation/nrd/Introduction_NRD_2010-2014.pdf. Accessed November 14, 2017.
28. Bardach NS, Coker TR, Zima BT, et al. Common and costly hospitalizations for pediatric mental health disorders. Pediatrics. 2014;133(4):602-609. doi: 10.1542/peds.2013-3165. PubMed
29. Ahmedani BK, Peterson EL, Hu Y, et al. Major physical health conditions and risk of suicide. Am J Prev Med. 2017;53(3):308-315. doi: 10.1016/J.AMEPRE.2017.04.001. PubMed
30. Gunnell D, Hawton K, Ho D, et al. Hospital admissions for self harm after discharge from psychiatric inpatient care: cohort study. BMJ. 2008;337:a2278. doi: 10.1136/bmj.a2278. PubMed
31. The TADS Team. The treatment for adolescents with depression study (TADS). Arch Gen Psychiatry. 2007;64(10):1132. doi: 10.1001/archpsyc.64.10.1132. PubMed
32. While D, Bickley H, Roscoe A, et al. Implementation of mental health service recommendations in England and Wales and suicide rates, 1997-2006: A cross-sectional and before-and-after observational study. Lancet. 2012;379(9820):1005-1012. doi: 10.1016/S0140-6736(11)61712-1. PubMed
33. Kawanishi C, Aruga T, Ishizuka N, et al. Assertive case management versus enhanced usual care for people with mental health problems who had attempted suicide and were admitted to hospital emergency departments in Japan (ACTION-J): a multicentre, randomised controlled trial. Lancet Psychiatry. 2014;1(3):193-201. doi: 10.1016/S2215-0366(14)70259-7. PubMed
34. Grupp-Phelan J, McGuire L, Husky MM, Olfson M. A randomized controlled trial to engage in care of adolescent emergency department patients with mental health problems that increase suicide risk. Pediatr Emerg Care. 2012;28(12):1263-1268. doi: 10.1097/PEC.0b013e3182767ac8. PubMed
35. Ciciolla L, Curlee AS, Karageorge J, Luthar SS. When mothers and fathers are seen as disproportionately valuing achievements: implications for adjustment among upper middle class youth. J Youth Adolesc. 2017;46(5):1057-1075. doi: 10.1007/s10964-016-0596-x. PubMed
36. Plemmons G, Hall M, Doupnik S, et al. Hospitalization for suicide ideation or attempt: 2008–2015. Pediatrics. May 2018:e20172426. doi: 10.1542/peds.2017-2426. PubMed
Suicide is a leading cause of death among 10- to 34-year-olds in the United States.1,2 During the past two decades, the youth suicide death rate has risen by 24%, and more than 5,000 young people die from suicide each year.3 Suicide ideation (SI) and suicide attempts (SAs) are well-established risk factors for suicide death and a source of morbidity for patients and families. One-third of youth with SI attempt suicide at some point in their lifetime.4 Approximately 11% of youth SAs result in suicide death, and 2% of youth who attempt suicide subsequently go on to die from suicide after recovering from a prior SA.5 More than 60,000 youth are hospitalized for SI or SA each year,6 and young people hospitalized for SA are at high short-term risk of repeat SA and suicide death.7 Hospitals need strategies for measuring the quality of SI and SA hospitalizations, monitoring postdischarge outcomes, and identifying the patients at the highest risk of poor outcomes. Readmissions are a useful hospital quality measure that can indicate re-emergence of SI, repeat SA, or inadequate community-based mental health treatment, and interventions designed for patients with readmissions can potentially avert morbidity or mortality.
The National Committee on Quality Assurance recommends measurement of quality metrics for 30-day mental health follow-up after psychiatric hospitalizations, 30-day readmissions after adult (but not pediatric) psychiatric hospitalizations, and 30-day readmissions in pediatric medical and surgical hospitalizations. Readmission measures are not consistently used to evaluate pediatric psychiatric hospitalizations, and psychiatric quality measures are not used to evaluate medical or surgical hospitalizations for SA. Recent research has investigated transfers to postacute care,8 readmission prevalence, variation in hospital readmission performance, and risk factors for readmissions after pediatric psychiatric hospitalizations.9–11 However, no national study has investigated 30-day readmissions in youth hospitalized specifically for SI or SA.
To inform hospital quality measurement and improve hospital and postdischarge care for youth at risk of suicide, more information is needed about the characteristics of and the risk factors for readmissions after index SI/SA hospitalization. To address this knowledge gap, among SI/SA hospitalizations in 6- to 17-year-olds, we examined (1) unplanned 30-day readmissions and characteristics of hospitalizations by 30-day readmission status; (2) patient, hospital, and regional characteristics associated with 30-day readmissions; and (3) characteristics of 30-day readmissions.
METHODS
Study Design and Data Source
We conducted a national, retrospective cohort study of hospitalizations for patients aged 6-17 years using the Agency for Healthcare Research and Quality (AHRQ) 2013 and 2014 Nationwide Readmissions Database (NRD). The combined 2013-2014 NRD includes administrative data from a nationally representative sample of 29 million hospitalizations in 22 states, accounting for 49.3% of all US hospitalizations, and is weighted for national projections. The NRD includes hospital information, patient demographic information, and International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis, procedure, and external cause of injury codes (E-codes). The database includes one primary diagnosis, up to 24 additional diagnoses, and up to 4 E-codes for each hospitalization. The NRD includes information about hospitalizations in acute-care general hospitals (including their psychiatric units) but not from specialty psychiatric hospitals. The database also includes de-identified, verified patient linkage numbers so that patients can be tracked across multiple hospitalizations at the same institution or different institutions within the same state. This study was considered to be exempt from review by the Children’s Hospital of Philadelphia Institutional Review Board.
Sample
We identified a sample of 181,575 hospitalizations for SI (n = 119,037) or SA (n = 62,538) among 6- to 17-year-olds between January 1, 2013, and December 31, 2014 (Figure). We included children as young as 6 years because validated methods exist to identify SI and SA in this age group,12 and because suicide deaths have recently increased among younger children.3 We excluded patients aged 18 years and older from this study since delivery of mental health services differs for adults.13 To create the sample, we first identified all hospitalizations of patients aged 6-17 years. We then used a validated algorithm relying on ICD-9-CM diagnosis codes for poisonings and E-codes for self-injury (E950-959) to identify hospitalizations related to SA.12 Because E-code completeness varies among states,14 we also used the combination of having both a diagnosis code for injury (800-999) and an ICD-9-CM code for SI (V62.84) as a proxy for SA. Among hospitalizations without SA, we identified hospitalizations with SI using the ICD-9-CM code for SI (V62.84) in any position.
We identified 133,516 index hospitalizations with complete data at risk for an unplanned readmission. Because NRD data cannot be linked between calendar years, we limited the study time period for each calendar year to 10 months. We excluded hospitalizations in January because the full 30-day time frame to determine whether a hospitalization had occurred in the preceding 30 days, a known risk factor for readmissions in other samples,15 was not available. We excluded index hospitalizations occurring in December, because the full 30-day time frame to ascertain readmissions was not available. We excluded hospitalizations resulting in death, since these are not at risk for readmission, and hospitalizations resulting in transfer, since the timing of discharge to the community was not known. Readmission hospitalizations were eligible to be included as index hospitalizations if they met sample inclusion criteria. The final sample for readmission analyses included 95,354 SI hospitalizations and 38,162 SA hospitalizations (Figure).
Primary Outcome
The primary outcome was any unplanned, all-cause readmission within 30 days of index hospitalization for SI or SA. Among 30-day readmissions, we examined readmission timing, whether the readmission was to the same hospital or a different hospital, length of stay, and indication for readmission (medical/surgical or psychiatric, and presence of SI or SA diagnoses). Planned readmissions were identified using measure specifications endorsed by the AHRQ and the National Quality Forum16 and excluded from measurement.
Among index hospitalizations for SI, we specifically examined 30-day readmissions for subsequent SA, since one objective of hospitalization for SI is to prevent progression to SA or death. We could not identify hospitalizations for repeat SA after index hospitalization for SA, because diagnosis codes did not differentiate between readmission for complications of index SA and readmission for repeat SA.
Independent Variables
We analyzed demographic, clinical, and hospital factors associated with readmissions in other samples.17–20 Demographic characteristics included patient gender and age, urban or rural residence, payer, and median national income quartile for a patient’s ZIP code. Race and ethnicity data are not available in the NRD.
Clinical characteristics included hospitalization in the 30-days preceding the index hospitalization, index hospitalization length of stay, and admission via the emergency department (ED) versus direct admission. A patient’s chronic condition profile was determined using index hospitalization diagnosis codes. Complex chronic conditions (eg, cancer, cystic fibrosis) were identified using a classification system used in several prior studies of hospital administrative datasets,21 and other noncomplex chronic medical conditions (eg, asthma, obesity) were identified using the Healthcare Cost and Utilization Project (HCUP) chronic condition indicator system.22 Psychiatric conditions (eg, anxiety disorders, substance abuse, autism) were identified and categorized using a classification system used in studies of hospital administrative datasets.23 The number of psychiatric conditions was determined by counting the number of psychiatric condition categories in which a patient had a diagnosis. SA was categorized as having lower risk of death (eg, medication overdose, injury from cutting or piercing) or higher risk of death (eg, hanging, suffocation, or firearm injury).24
Because of known temporal trends in SI and SA,25,26 the month and year of admission were included as covariates. Hospital characteristics included teaching hospital and children’s hospital designations.
Statistical Analysis
We compared descriptive, summary statistics for characteristics of index hospitalizations with and without a 30-day readmission using Rao-Scott chi-square tests. In multivariable analyses, we derived logistic regression models to measure the associations of patient, hospital, and temporal factors with 30-day hospital readmissions. Analyses were conducted in SAS PROC SURVEYLOGISTIC and were weighted to achieve national estimates, clustered by sample stratum and hospital to account for the complex survey design,27 clustered by patients to account for multiple index visits per patient, and adjusted for clinical, demographic, and hospital characteristics. SAS version 9.4 (SAS Institute, Cary, North Carolina) was used for all analyses. All tests were two-sided, and a P value <.05 was considered as statistically significant.
RESULTS
Sample Characteristics
In the weighted analyses, we identified 133,516 hospitalizations in acute-care hospitals for SI or SA, and 8.5% (n = 11,375) of hospitalizations had at least one unplanned 30-day readmission to an acute-care hospital. Unweighted, the sample included 37,683 patients and 42,198 hospitalizations. Among all patients represented in the sample, 90.5% had only a single SI or SA hospitalization, 7.7% had two hospitalizations, and 1.8% had >2 hospitalizations in one year.
Table 1 summarizes the sample characteristics and displays the demographic, clinical, and hospital characteristics of index hospitalizations by the 30-day readmission status. Patients represented in the index hospitalizations were 64.9% female, 3.6% were aged 6-9 years, 40.1% were aged 10-14 years, and 56.3% were aged 15-17 years. Nearly half of the patients (49.1%) used public insurance. Nearly half (44.9%) lived in metropolitan areas with >1 million residents, 36.1% lived in metropolitan areas with 50,000 to 1 million residents, and 14.7% lived in rural areas.
Median length of stay for the index hospitalization was 5 days (interquartile range [IQR] 3-7). Nearly one-third (32.3%) of patients had a noncomplex chronic medical condition, 7.8% had a complex chronic medical condition, and 98.1% had a psychiatric condition. The most common psychiatric conditions were depressive disorders (60.0%) and anxiety disorders (42.2%). More than half (55.0%) of the patients had >2 psychiatric conditions. Most hospitalizations in the sample had SI only (71.4%). Among patients with SA, 81.0% had a lower lethality mechanism of injury and 19.0% had a higher lethality mechanism.
Patients experiencing a readmission were more likely to be 10-14 years old and use public insurance than patients without a readmission (P < .001 for both). For clinical characteristics, patients with a readmission were more likely to have longer index hospital stays (6 vs. 5 days), >2 psychiatric conditions (SI vs. SA), a prior admission in the 30 days preceding the index hospitalization, and admission via the ED (vs. direct admission) (P < .001 for all).
Association of Patient and Hospital Characteristics with Readmissions
Table 2 displays the patient and hospital characteristics associated with readmissions. Among demographic characteristics, 10- to 14-year-old patients had higher odds of readmission (odds ratio [OR]: 1.18, 95% confidence interval [CI]: 1.07-1.29) than 15- to 17-year-old patients. Having public insurance was associated with higher odds of readmission (OR: 1.14, 95% CI: 1.04-1.25). We found no differences in readmission rates based on sex, urban or rural location, or patient’s ZIP code income quartile.
Among clinical characteristics, hospitalizations with an admission for SI or SA in the preceding 30 days, meaning that the index hospitalization itself was a readmission, had the strongest association with readmissions (OR: 3.14, 95% CI: 2.73-3.61). In addition, patients admitted via the ED for the index hospitalization had higher odds of readmission (OR: 1.25, 95% CI: 1.15-1.36). Chronic psychiatric conditions associated with higher odds of readmission included psychotic disorders (OR: 1.39, 95% CI: 1.16-1.67) and bipolar disorder (OR: 1.27, 95% CI: 1.13-1.44).
Characteristics of 30-day Readmissions
Table 3 displays the characteristics of readmissions after SI compared to that after SA. Among the combined sample of 11,375 30-day readmissions, 34.1% occurred within 7 days, and 65.9% in 8-30 days. Eleven percent of patients with any readmission had more than one readmission within 30 days. Among readmissions, 94.5% were for a psychiatric problem and 5.5% were for a medical or surgical problem. A total of 43.9% had a diagnosis of SI and 19.5% a diagnosis of SA. Readmissions were more likely to occur at a different hospital after SI than after SA (48.1% vs. 31.3%, P < .001). Medical and surgical indications for readmission were less common after SI than after SA (4.4% vs. 8.7%, P < .001). Only 1.2% of SI hospitalizations had a readmission for SA within 30 days. Of these cases, 55.6% were aged 15-17 years, 43.3% were aged 10-14 years, and 1.1% were aged 6-9 years; 73.1% of the patients were female, and 49.1% used public insurance.
DISCUSSION
SI and SA in children and adolescents are substantial public health problems associated with significant hospital resource utilization. In 2013 and 2014, there were 181,575 pediatric acute-care hospitalizations for SI or SA, accounting for 9.5% of all hospitalizations in 6- to 17-year-old patients nationally. Among acute-care SI and SA hospitalizations, 8.5% had a readmission to an acute-care hospital within 30 days. The study data source did not include psychiatric specialty hospitals, and the number of index hospitalizations is likely substantially higher when psychiatric specialty hospitalizations are included. Readmissions may also be higher if patients were readmitted to psychiatric specialty hospitals after discharge from acute-care hospitals. The strongest risk factor for unplanned 30-day readmissions was a previous hospitalization in the 30 days before the index admission, likely a marker for severity or complexity of psychiatric illness. Other characteristics associated with higher odds of readmission were bipolar disorder, psychotic disorders, and age 10-14 years. More than one-third of readmissions occurred within the first 7 days after hospital discharge. The prevalence of SI and SA hospitalizations and readmissions was similar to findings in previous analyses of mental health hospitalizations.10,28
A patient’s psychiatric illness type and severity, as evidenced by the need for frequent repeat hospitalizations, was highly associated with the risk of 30-day readmission. Any hospitalization in the 30 days preceding the index hospitalization, whether for SI/SA or for another problem, was a strong risk factor for readmissions. We suspect that prior SI/SA hospitalizations reflect a patient’s chronic elevated risk for suicide. Prior hospitalizations not for SI or SA could be hospitalizations for mental illness exacerbations that increase the risk of SI or SA, eg, bipolar disorder with acute mania, or they could represent physical health problems. Chronic physical health problems are a known risk factor for SI and SA.29
A knowledge of those characteristics that increase the readmission risk can inform future resource allocation, research, and policy in several ways. First, longer hospital stays could mitigate readmission risk in some patients with severe psychiatric illness. European studies in older adolescents and adults show that for severe psychiatric illness, a longer hospital stay is associated with a lower risk of hospital readmission.15,30 Second, better access to intensive community-based mental health (MH) services, including evidence-based psychotherapy and medication management, improves symptoms in young people.31 Access to these services likely affects the risk of hospital readmission. We found that readmission risk was highest in 10- to 14-year-olds. Taken in the context of existing evidence that suicide rates are rising in younger patients,1,3 our findings suggest that particular attention to community services for younger patients is needed. Third, care coordination could help patients access beneficial services to reduce readmissions and improve other outcomes. Enhanced discharge care coordination reduced suicide deaths in high-risk populations in Europe32 and Japan33 and improved attendance at mental health follow-up after pediatric ED discharge in a small United States sample.34 Given that one-third of readmissions occurred within seven days, care coordination designed to ensure access to ambulatory services in the immediate postdischarge period may be particularly beneficial.
We found that ZIP code income quartile was not associated with readmissions. We suspect that poverty is not as closely correlated with MH hospitalization outcomes as it is with physical health hospitalization outcomes for several reasons. Medicaid insurance historically has more robust coverage of mental health services than some private insurance plans, which might offset some of the risk of poor mental health outcomes associated with poverty. Low-income families are eligible to use social services, and families accessing social services might have more opportunities to become familiar with community mental health programs. Further, the expectation of high achievement found in some higher income families is associated with MH problems in children and adolescents.35 Therefore, being in a higher income quartile might not be as protective against poor mental health outcomes as it is against poor physical health outcomes.
Although the NRD provides a rich source of readmission data across hospitals nationally, several limitations are inherent to this administrative dataset. First, data from specialty psychiatric hospitals were not included in the NRD. The study underestimates the total number of index hospitalizations and readmissions, since index SI/SA hospitalizations at psychiatric hospitals are not included, and readmissions are not included if they occurred at specialty psychiatric hospitals. Second, because data cannot be linked between calendar years, we excluded January and December hospitalizations, and findings might not generalize to hospitalizations in January and December. Seasonal trends in SI/SA hospitalizations are known to occur.36 Third, race, ethnicity, primary language, gender identity, and sexual orientation are not available in the NRD, and we could not examine the association of these characteristics with the likelihood of readmissions. Fourth, we did not have information about pre- or posthospitalization insurance enrollment or outpatient services that could affect the risk of readmission. Nevertheless, this study offers information on the characteristics of readmissions after hospitalizations for SI and SA in a large nationally representative sample of youth, and the findings can inform resource planning to prevent suicides.
CONCLUSION
Hospital readmissions are common in patients with SI and SA, and patients with a recent previous hospitalization have the highest risk of readmission. More than one-third of readmissions after SI or SA occurred within the first seven days. Due to the dearth of mental health services in the community, hospitals offer an important safety net for youth experiencing acute suicidal crises. Strategies to improve the continuum of care for patients at risk of suicide that solely focus on reducing readmissions are not likely to benefit patients. However, readmissions can identify opportunities for improving hospital discharge processes and outpatient services. Future research and clinical innovation to investigate and improve hospital discharge planning and access to community mental health services is likely to benefit patients and could reduce 30-day hospital readmissions.
Acknowledgments
The authors thank John Lawlor for his assistance with the analysis.
Disclosures
The authors have no potential conflicts of interest to disclose.
Funding
Dr. Zima received funding from the Behavioral Health Centers of Excellence for California (SB852).
Suicide is a leading cause of death among 10- to 34-year-olds in the United States.1,2 During the past two decades, the youth suicide death rate has risen by 24%, and more than 5,000 young people die from suicide each year.3 Suicide ideation (SI) and suicide attempts (SAs) are well-established risk factors for suicide death and a source of morbidity for patients and families. One-third of youth with SI attempt suicide at some point in their lifetime.4 Approximately 11% of youth SAs result in suicide death, and 2% of youth who attempt suicide subsequently go on to die from suicide after recovering from a prior SA.5 More than 60,000 youth are hospitalized for SI or SA each year,6 and young people hospitalized for SA are at high short-term risk of repeat SA and suicide death.7 Hospitals need strategies for measuring the quality of SI and SA hospitalizations, monitoring postdischarge outcomes, and identifying the patients at the highest risk of poor outcomes. Readmissions are a useful hospital quality measure that can indicate re-emergence of SI, repeat SA, or inadequate community-based mental health treatment, and interventions designed for patients with readmissions can potentially avert morbidity or mortality.
The National Committee on Quality Assurance recommends measurement of quality metrics for 30-day mental health follow-up after psychiatric hospitalizations, 30-day readmissions after adult (but not pediatric) psychiatric hospitalizations, and 30-day readmissions in pediatric medical and surgical hospitalizations. Readmission measures are not consistently used to evaluate pediatric psychiatric hospitalizations, and psychiatric quality measures are not used to evaluate medical or surgical hospitalizations for SA. Recent research has investigated transfers to postacute care,8 readmission prevalence, variation in hospital readmission performance, and risk factors for readmissions after pediatric psychiatric hospitalizations.9–11 However, no national study has investigated 30-day readmissions in youth hospitalized specifically for SI or SA.
To inform hospital quality measurement and improve hospital and postdischarge care for youth at risk of suicide, more information is needed about the characteristics of and the risk factors for readmissions after index SI/SA hospitalization. To address this knowledge gap, among SI/SA hospitalizations in 6- to 17-year-olds, we examined (1) unplanned 30-day readmissions and characteristics of hospitalizations by 30-day readmission status; (2) patient, hospital, and regional characteristics associated with 30-day readmissions; and (3) characteristics of 30-day readmissions.
METHODS
Study Design and Data Source
We conducted a national, retrospective cohort study of hospitalizations for patients aged 6-17 years using the Agency for Healthcare Research and Quality (AHRQ) 2013 and 2014 Nationwide Readmissions Database (NRD). The combined 2013-2014 NRD includes administrative data from a nationally representative sample of 29 million hospitalizations in 22 states, accounting for 49.3% of all US hospitalizations, and is weighted for national projections. The NRD includes hospital information, patient demographic information, and International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis, procedure, and external cause of injury codes (E-codes). The database includes one primary diagnosis, up to 24 additional diagnoses, and up to 4 E-codes for each hospitalization. The NRD includes information about hospitalizations in acute-care general hospitals (including their psychiatric units) but not from specialty psychiatric hospitals. The database also includes de-identified, verified patient linkage numbers so that patients can be tracked across multiple hospitalizations at the same institution or different institutions within the same state. This study was considered to be exempt from review by the Children’s Hospital of Philadelphia Institutional Review Board.
Sample
We identified a sample of 181,575 hospitalizations for SI (n = 119,037) or SA (n = 62,538) among 6- to 17-year-olds between January 1, 2013, and December 31, 2014 (Figure). We included children as young as 6 years because validated methods exist to identify SI and SA in this age group,12 and because suicide deaths have recently increased among younger children.3 We excluded patients aged 18 years and older from this study since delivery of mental health services differs for adults.13 To create the sample, we first identified all hospitalizations of patients aged 6-17 years. We then used a validated algorithm relying on ICD-9-CM diagnosis codes for poisonings and E-codes for self-injury (E950-959) to identify hospitalizations related to SA.12 Because E-code completeness varies among states,14 we also used the combination of having both a diagnosis code for injury (800-999) and an ICD-9-CM code for SI (V62.84) as a proxy for SA. Among hospitalizations without SA, we identified hospitalizations with SI using the ICD-9-CM code for SI (V62.84) in any position.
We identified 133,516 index hospitalizations with complete data at risk for an unplanned readmission. Because NRD data cannot be linked between calendar years, we limited the study time period for each calendar year to 10 months. We excluded hospitalizations in January because the full 30-day time frame to determine whether a hospitalization had occurred in the preceding 30 days, a known risk factor for readmissions in other samples,15 was not available. We excluded index hospitalizations occurring in December, because the full 30-day time frame to ascertain readmissions was not available. We excluded hospitalizations resulting in death, since these are not at risk for readmission, and hospitalizations resulting in transfer, since the timing of discharge to the community was not known. Readmission hospitalizations were eligible to be included as index hospitalizations if they met sample inclusion criteria. The final sample for readmission analyses included 95,354 SI hospitalizations and 38,162 SA hospitalizations (Figure).
Primary Outcome
The primary outcome was any unplanned, all-cause readmission within 30 days of index hospitalization for SI or SA. Among 30-day readmissions, we examined readmission timing, whether the readmission was to the same hospital or a different hospital, length of stay, and indication for readmission (medical/surgical or psychiatric, and presence of SI or SA diagnoses). Planned readmissions were identified using measure specifications endorsed by the AHRQ and the National Quality Forum16 and excluded from measurement.
Among index hospitalizations for SI, we specifically examined 30-day readmissions for subsequent SA, since one objective of hospitalization for SI is to prevent progression to SA or death. We could not identify hospitalizations for repeat SA after index hospitalization for SA, because diagnosis codes did not differentiate between readmission for complications of index SA and readmission for repeat SA.
Independent Variables
We analyzed demographic, clinical, and hospital factors associated with readmissions in other samples.17–20 Demographic characteristics included patient gender and age, urban or rural residence, payer, and median national income quartile for a patient’s ZIP code. Race and ethnicity data are not available in the NRD.
Clinical characteristics included hospitalization in the 30-days preceding the index hospitalization, index hospitalization length of stay, and admission via the emergency department (ED) versus direct admission. A patient’s chronic condition profile was determined using index hospitalization diagnosis codes. Complex chronic conditions (eg, cancer, cystic fibrosis) were identified using a classification system used in several prior studies of hospital administrative datasets,21 and other noncomplex chronic medical conditions (eg, asthma, obesity) were identified using the Healthcare Cost and Utilization Project (HCUP) chronic condition indicator system.22 Psychiatric conditions (eg, anxiety disorders, substance abuse, autism) were identified and categorized using a classification system used in studies of hospital administrative datasets.23 The number of psychiatric conditions was determined by counting the number of psychiatric condition categories in which a patient had a diagnosis. SA was categorized as having lower risk of death (eg, medication overdose, injury from cutting or piercing) or higher risk of death (eg, hanging, suffocation, or firearm injury).24
Because of known temporal trends in SI and SA,25,26 the month and year of admission were included as covariates. Hospital characteristics included teaching hospital and children’s hospital designations.
Statistical Analysis
We compared descriptive, summary statistics for characteristics of index hospitalizations with and without a 30-day readmission using Rao-Scott chi-square tests. In multivariable analyses, we derived logistic regression models to measure the associations of patient, hospital, and temporal factors with 30-day hospital readmissions. Analyses were conducted in SAS PROC SURVEYLOGISTIC and were weighted to achieve national estimates, clustered by sample stratum and hospital to account for the complex survey design,27 clustered by patients to account for multiple index visits per patient, and adjusted for clinical, demographic, and hospital characteristics. SAS version 9.4 (SAS Institute, Cary, North Carolina) was used for all analyses. All tests were two-sided, and a P value <.05 was considered as statistically significant.
RESULTS
Sample Characteristics
In the weighted analyses, we identified 133,516 hospitalizations in acute-care hospitals for SI or SA, and 8.5% (n = 11,375) of hospitalizations had at least one unplanned 30-day readmission to an acute-care hospital. Unweighted, the sample included 37,683 patients and 42,198 hospitalizations. Among all patients represented in the sample, 90.5% had only a single SI or SA hospitalization, 7.7% had two hospitalizations, and 1.8% had >2 hospitalizations in one year.
Table 1 summarizes the sample characteristics and displays the demographic, clinical, and hospital characteristics of index hospitalizations by the 30-day readmission status. Patients represented in the index hospitalizations were 64.9% female, 3.6% were aged 6-9 years, 40.1% were aged 10-14 years, and 56.3% were aged 15-17 years. Nearly half of the patients (49.1%) used public insurance. Nearly half (44.9%) lived in metropolitan areas with >1 million residents, 36.1% lived in metropolitan areas with 50,000 to 1 million residents, and 14.7% lived in rural areas.
Median length of stay for the index hospitalization was 5 days (interquartile range [IQR] 3-7). Nearly one-third (32.3%) of patients had a noncomplex chronic medical condition, 7.8% had a complex chronic medical condition, and 98.1% had a psychiatric condition. The most common psychiatric conditions were depressive disorders (60.0%) and anxiety disorders (42.2%). More than half (55.0%) of the patients had >2 psychiatric conditions. Most hospitalizations in the sample had SI only (71.4%). Among patients with SA, 81.0% had a lower lethality mechanism of injury and 19.0% had a higher lethality mechanism.
Patients experiencing a readmission were more likely to be 10-14 years old and use public insurance than patients without a readmission (P < .001 for both). For clinical characteristics, patients with a readmission were more likely to have longer index hospital stays (6 vs. 5 days), >2 psychiatric conditions (SI vs. SA), a prior admission in the 30 days preceding the index hospitalization, and admission via the ED (vs. direct admission) (P < .001 for all).
Association of Patient and Hospital Characteristics with Readmissions
Table 2 displays the patient and hospital characteristics associated with readmissions. Among demographic characteristics, 10- to 14-year-old patients had higher odds of readmission (odds ratio [OR]: 1.18, 95% confidence interval [CI]: 1.07-1.29) than 15- to 17-year-old patients. Having public insurance was associated with higher odds of readmission (OR: 1.14, 95% CI: 1.04-1.25). We found no differences in readmission rates based on sex, urban or rural location, or patient’s ZIP code income quartile.
Among clinical characteristics, hospitalizations with an admission for SI or SA in the preceding 30 days, meaning that the index hospitalization itself was a readmission, had the strongest association with readmissions (OR: 3.14, 95% CI: 2.73-3.61). In addition, patients admitted via the ED for the index hospitalization had higher odds of readmission (OR: 1.25, 95% CI: 1.15-1.36). Chronic psychiatric conditions associated with higher odds of readmission included psychotic disorders (OR: 1.39, 95% CI: 1.16-1.67) and bipolar disorder (OR: 1.27, 95% CI: 1.13-1.44).
Characteristics of 30-day Readmissions
Table 3 displays the characteristics of readmissions after SI compared to that after SA. Among the combined sample of 11,375 30-day readmissions, 34.1% occurred within 7 days, and 65.9% in 8-30 days. Eleven percent of patients with any readmission had more than one readmission within 30 days. Among readmissions, 94.5% were for a psychiatric problem and 5.5% were for a medical or surgical problem. A total of 43.9% had a diagnosis of SI and 19.5% a diagnosis of SA. Readmissions were more likely to occur at a different hospital after SI than after SA (48.1% vs. 31.3%, P < .001). Medical and surgical indications for readmission were less common after SI than after SA (4.4% vs. 8.7%, P < .001). Only 1.2% of SI hospitalizations had a readmission for SA within 30 days. Of these cases, 55.6% were aged 15-17 years, 43.3% were aged 10-14 years, and 1.1% were aged 6-9 years; 73.1% of the patients were female, and 49.1% used public insurance.
DISCUSSION
SI and SA in children and adolescents are substantial public health problems associated with significant hospital resource utilization. In 2013 and 2014, there were 181,575 pediatric acute-care hospitalizations for SI or SA, accounting for 9.5% of all hospitalizations in 6- to 17-year-old patients nationally. Among acute-care SI and SA hospitalizations, 8.5% had a readmission to an acute-care hospital within 30 days. The study data source did not include psychiatric specialty hospitals, and the number of index hospitalizations is likely substantially higher when psychiatric specialty hospitalizations are included. Readmissions may also be higher if patients were readmitted to psychiatric specialty hospitals after discharge from acute-care hospitals. The strongest risk factor for unplanned 30-day readmissions was a previous hospitalization in the 30 days before the index admission, likely a marker for severity or complexity of psychiatric illness. Other characteristics associated with higher odds of readmission were bipolar disorder, psychotic disorders, and age 10-14 years. More than one-third of readmissions occurred within the first 7 days after hospital discharge. The prevalence of SI and SA hospitalizations and readmissions was similar to findings in previous analyses of mental health hospitalizations.10,28
A patient’s psychiatric illness type and severity, as evidenced by the need for frequent repeat hospitalizations, was highly associated with the risk of 30-day readmission. Any hospitalization in the 30 days preceding the index hospitalization, whether for SI/SA or for another problem, was a strong risk factor for readmissions. We suspect that prior SI/SA hospitalizations reflect a patient’s chronic elevated risk for suicide. Prior hospitalizations not for SI or SA could be hospitalizations for mental illness exacerbations that increase the risk of SI or SA, eg, bipolar disorder with acute mania, or they could represent physical health problems. Chronic physical health problems are a known risk factor for SI and SA.29
A knowledge of those characteristics that increase the readmission risk can inform future resource allocation, research, and policy in several ways. First, longer hospital stays could mitigate readmission risk in some patients with severe psychiatric illness. European studies in older adolescents and adults show that for severe psychiatric illness, a longer hospital stay is associated with a lower risk of hospital readmission.15,30 Second, better access to intensive community-based mental health (MH) services, including evidence-based psychotherapy and medication management, improves symptoms in young people.31 Access to these services likely affects the risk of hospital readmission. We found that readmission risk was highest in 10- to 14-year-olds. Taken in the context of existing evidence that suicide rates are rising in younger patients,1,3 our findings suggest that particular attention to community services for younger patients is needed. Third, care coordination could help patients access beneficial services to reduce readmissions and improve other outcomes. Enhanced discharge care coordination reduced suicide deaths in high-risk populations in Europe32 and Japan33 and improved attendance at mental health follow-up after pediatric ED discharge in a small United States sample.34 Given that one-third of readmissions occurred within seven days, care coordination designed to ensure access to ambulatory services in the immediate postdischarge period may be particularly beneficial.
We found that ZIP code income quartile was not associated with readmissions. We suspect that poverty is not as closely correlated with MH hospitalization outcomes as it is with physical health hospitalization outcomes for several reasons. Medicaid insurance historically has more robust coverage of mental health services than some private insurance plans, which might offset some of the risk of poor mental health outcomes associated with poverty. Low-income families are eligible to use social services, and families accessing social services might have more opportunities to become familiar with community mental health programs. Further, the expectation of high achievement found in some higher income families is associated with MH problems in children and adolescents.35 Therefore, being in a higher income quartile might not be as protective against poor mental health outcomes as it is against poor physical health outcomes.
Although the NRD provides a rich source of readmission data across hospitals nationally, several limitations are inherent to this administrative dataset. First, data from specialty psychiatric hospitals were not included in the NRD. The study underestimates the total number of index hospitalizations and readmissions, since index SI/SA hospitalizations at psychiatric hospitals are not included, and readmissions are not included if they occurred at specialty psychiatric hospitals. Second, because data cannot be linked between calendar years, we excluded January and December hospitalizations, and findings might not generalize to hospitalizations in January and December. Seasonal trends in SI/SA hospitalizations are known to occur.36 Third, race, ethnicity, primary language, gender identity, and sexual orientation are not available in the NRD, and we could not examine the association of these characteristics with the likelihood of readmissions. Fourth, we did not have information about pre- or posthospitalization insurance enrollment or outpatient services that could affect the risk of readmission. Nevertheless, this study offers information on the characteristics of readmissions after hospitalizations for SI and SA in a large nationally representative sample of youth, and the findings can inform resource planning to prevent suicides.
CONCLUSION
Hospital readmissions are common in patients with SI and SA, and patients with a recent previous hospitalization have the highest risk of readmission. More than one-third of readmissions after SI or SA occurred within the first seven days. Due to the dearth of mental health services in the community, hospitals offer an important safety net for youth experiencing acute suicidal crises. Strategies to improve the continuum of care for patients at risk of suicide that solely focus on reducing readmissions are not likely to benefit patients. However, readmissions can identify opportunities for improving hospital discharge processes and outpatient services. Future research and clinical innovation to investigate and improve hospital discharge planning and access to community mental health services is likely to benefit patients and could reduce 30-day hospital readmissions.
Acknowledgments
The authors thank John Lawlor for his assistance with the analysis.
Disclosures
The authors have no potential conflicts of interest to disclose.
Funding
Dr. Zima received funding from the Behavioral Health Centers of Excellence for California (SB852).
1. Sheftall AH, Asti L, Horowitz LM, et al. Suicide in elementary school-aged children and early adolescents. Pediatrics. 2016;138(4):e20160436. doi: 10.1542/peds.2016-0436. PubMed
2. Prevention CNC for I. Suicide facts at a Glance 2015 nonfatal suicidal thoughts and behavior. In: 2015:3-4. https://stacks.cdc.gov/view/cdc/34181/cdc_34181_DS1.pdf. Accessed September 30, 2016.
3. Curtin S, Warner M, Hedegaard H. Increase in Suicide in the United States, 1999-2014. Hyattsville, MD; 2016. http://www.cdc.gov/nchs/data/databriefs/db241.pdf. Accessed November 7, 2016. PubMed
4. Nock MK, Green JG, Hwang I, et al. Prevalence, correlates, and treatment of lifetime suicidal behavior among adolescents: results from the national comorbidity survey replication adolescent supplement. JAMA Psychiatry. 2013;70(3):300. doi: 10.1001/2013.jamapsychiatry.55. PubMed
5. Bostwick JM, Pabbati C, Geske JR, Mckean AJ. Suicide attempt as a risk factor for completed suicide: even more lethal than we knew. Am J Psychiatry. 2016;173(11):1094-1100. doi: 10.1176/appi.ajp.2016.15070854. PubMed
6. Torio CM, Encinosa W, Berdahl T, McCormick MC, Simpson LA. Annual report on health care for children and youth in the united states: national estimates of cost, utilization and expenditures for children with mental health conditions. Acad Pediatr. 2015;15(1):19-35. doi: 10.1016/j.acap.2014.07.007. PubMed
7. Olfson M, Wall M, Wang S, et al. Suicide after deliberate self-harm in adolescents and young adults. Pediatrics. 2018;141(4):e20173517. doi: 10.1542/peds.2017-3517. PubMed
8. Gay JC, Zima BT, Coker TR, et al. Postacute care after pediatric hospitalizations for a primary mental health condition. J Pediatr. 2018;193:222-228.e1. doi: 10.1016/j.jpeds.2017.09.058. PubMed
9. Heslin KC, Weiss AJ. Hospital Readmissions Involving Psychiatric Disorders, 2012. 2015. https://www.ncbi.nlm.nih.gov/books/NBK305353/pdf/Bookshelf_NBK305353.pdf. Accessed September 8, 2017. PubMed
10. Feng JY, Toomey SL, Zaslavsky AM, Nakamura MM, Schuster MA. Readmission after pediatric mental health admissions. Pediatrics. 2017;140(6):e20171571. doi: 10.1542/peds.2017-1571. PubMed
11. Bardach NS, Vittinghoff E, Asteria-Peñaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. doi: 10.1542/peds.2012-3527. PubMed
12. Callahan ST, Fuchs DC, Shelton RC, et al. Identifying suicidal behavior among adolescents using administrative claims data. Pharmacoepidemiol Drug Saf. 2013;22(7):769-775. doi: 10.1002/pds.3421. PubMed
13. SAMHSA, HHS, Synectics for Management Decisions, Mathematica Policy Research. National Mental Health Services Survey: 2010: Data on Mental Health Treatment Facilities. http://media.samhsa.gov/data/DASIS/NMHSS2010D/NMHSS2010_Web.pdf. Accessed November 13, 2015.
14. Patrick AR, Miller M, Barber CW, Wang PS, Canning CF, Schneeweiss S. Identification of hospitalizations for intentional self-harm when E-codes are incompletely recorded. Pharmacoepidemiol Drug Saf. 2010;19(12):1263-1275. doi: 10.1002/pds.2037. PubMed
15. Mellesdal L, Mehlum L, Wentzel-Larsen T, Kroken R, Jørgensen HA. Suicide risk and acute psychiatric readmissions: a prospective cohort study. Psychiatr Serv. 2010;61(1):25-31. doi: 10.1176/appi.ps.61.1.25. PubMed
16. Agency for Healthcare Research and Quality, Centers for Medicare and Medicaid. Measure: Pediatric All-Condition Readmission Measure Measure Developer: Center of Excellence for Pediatric Quality Measurement (CEPQM). https://www.ahrq.gov/sites/default/files/wysiwyg/policymakers/chipra/factsheets/chipra_14-p008-1-ef.pdf. Accessed November 15, 2017.
17. Cancino RS, Culpepper L, Sadikova E, Martin J, Jack BW, Mitchell SE. Dose-response relationship between depressive symptoms and hospital readmission. J Hosp Med. 2014;9(6):358-364. doi: 10.1002/jhm.2180. PubMed
18. Carlisle CE, Mamdani M, Schachar R, To T. Aftercare, emergency department visits, and readmission in adolescents. J Am Acad Child Adolesc Psychiatry. 2012;51(3):283-293. http://www.sciencedirect.com/science/article/pii/S0890856711011002. Accessed November 2, 2015. PubMed
19. Fadum EA, Stanley B, Qin P, Diep LM, Mehlum L. Self-poisoning with medications in adolescents: a national register study of hospital admissions and readmissions. Gen Hosp Psychiatry. 2014;36(6):709-715. doi: 10.1016/j.genhosppsych.2014.09.004. PubMed
20. Bernet AC. Predictors of psychiatric readmission among veterans at high risk of suicide: the impact of post-discharge aftercare. Arch Psychiatr Nurs. 2013;27(5):260-261. doi: 10.1016/j.apnu.2013.07.001. PubMed
21. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14(1):199. doi: 10.1186/1471-2431-14-199. PubMed
22. HCUP. HCUP-US Tools & Software Page. http://www.hcup-us.ahrq.gov/toolssoftware/chronic/chronic.jsp. Published 2015. Accessed October 30, 2015.
23. Zima BT, Rodean J, Hall M, Bardach NS, Coker TR, Berry JG. Psychiatric disorders and trends in resource use in pediatric hospitals. Pediatrics. 2016;138(5):e20160909-e20160909. doi: 10.1542/peds.2016-0909. PubMed
24. Spicer RS, Miller TR. Suicide acts in 8 states: incidence and case fatality rates by demographics and method. Am J Public Health. 2000;90(12):1885-1891. doi: 10.2105/AJPH.90.12.1885. PubMed
25. Hansen B, Lang M. Back to school blues: Seasonality of youth suicide and the academic calendar. Econ Educ Rev. 2011;30(5):850-861. doi: 10.1016/j.econedurev.2011.04.012.
26. Lueck C, Kearl L, Lam CN, Claudius I. Do emergency pediatric psychiatric visits for danger to self or others correspond to times of school attendance? Am J Emerg Med. 2015;33(5):682-684. doi: 10.1016/J.AJEM.2015.02.055. PubMed
27. Healthcare Cost And Utilization Project. Introduction to the HCUP Nationwide Readmissions Database. Rockville, MD; 2017. https://www.hcup-us.ahrq.gov/db/nation/nrd/Introduction_NRD_2010-2014.pdf. Accessed November 14, 2017.
28. Bardach NS, Coker TR, Zima BT, et al. Common and costly hospitalizations for pediatric mental health disorders. Pediatrics. 2014;133(4):602-609. doi: 10.1542/peds.2013-3165. PubMed
29. Ahmedani BK, Peterson EL, Hu Y, et al. Major physical health conditions and risk of suicide. Am J Prev Med. 2017;53(3):308-315. doi: 10.1016/J.AMEPRE.2017.04.001. PubMed
30. Gunnell D, Hawton K, Ho D, et al. Hospital admissions for self harm after discharge from psychiatric inpatient care: cohort study. BMJ. 2008;337:a2278. doi: 10.1136/bmj.a2278. PubMed
31. The TADS Team. The treatment for adolescents with depression study (TADS). Arch Gen Psychiatry. 2007;64(10):1132. doi: 10.1001/archpsyc.64.10.1132. PubMed
32. While D, Bickley H, Roscoe A, et al. Implementation of mental health service recommendations in England and Wales and suicide rates, 1997-2006: A cross-sectional and before-and-after observational study. Lancet. 2012;379(9820):1005-1012. doi: 10.1016/S0140-6736(11)61712-1. PubMed
33. Kawanishi C, Aruga T, Ishizuka N, et al. Assertive case management versus enhanced usual care for people with mental health problems who had attempted suicide and were admitted to hospital emergency departments in Japan (ACTION-J): a multicentre, randomised controlled trial. Lancet Psychiatry. 2014;1(3):193-201. doi: 10.1016/S2215-0366(14)70259-7. PubMed
34. Grupp-Phelan J, McGuire L, Husky MM, Olfson M. A randomized controlled trial to engage in care of adolescent emergency department patients with mental health problems that increase suicide risk. Pediatr Emerg Care. 2012;28(12):1263-1268. doi: 10.1097/PEC.0b013e3182767ac8. PubMed
35. Ciciolla L, Curlee AS, Karageorge J, Luthar SS. When mothers and fathers are seen as disproportionately valuing achievements: implications for adjustment among upper middle class youth. J Youth Adolesc. 2017;46(5):1057-1075. doi: 10.1007/s10964-016-0596-x. PubMed
36. Plemmons G, Hall M, Doupnik S, et al. Hospitalization for suicide ideation or attempt: 2008–2015. Pediatrics. May 2018:e20172426. doi: 10.1542/peds.2017-2426. PubMed
1. Sheftall AH, Asti L, Horowitz LM, et al. Suicide in elementary school-aged children and early adolescents. Pediatrics. 2016;138(4):e20160436. doi: 10.1542/peds.2016-0436. PubMed
2. Prevention CNC for I. Suicide facts at a Glance 2015 nonfatal suicidal thoughts and behavior. In: 2015:3-4. https://stacks.cdc.gov/view/cdc/34181/cdc_34181_DS1.pdf. Accessed September 30, 2016.
3. Curtin S, Warner M, Hedegaard H. Increase in Suicide in the United States, 1999-2014. Hyattsville, MD; 2016. http://www.cdc.gov/nchs/data/databriefs/db241.pdf. Accessed November 7, 2016. PubMed
4. Nock MK, Green JG, Hwang I, et al. Prevalence, correlates, and treatment of lifetime suicidal behavior among adolescents: results from the national comorbidity survey replication adolescent supplement. JAMA Psychiatry. 2013;70(3):300. doi: 10.1001/2013.jamapsychiatry.55. PubMed
5. Bostwick JM, Pabbati C, Geske JR, Mckean AJ. Suicide attempt as a risk factor for completed suicide: even more lethal than we knew. Am J Psychiatry. 2016;173(11):1094-1100. doi: 10.1176/appi.ajp.2016.15070854. PubMed
6. Torio CM, Encinosa W, Berdahl T, McCormick MC, Simpson LA. Annual report on health care for children and youth in the united states: national estimates of cost, utilization and expenditures for children with mental health conditions. Acad Pediatr. 2015;15(1):19-35. doi: 10.1016/j.acap.2014.07.007. PubMed
7. Olfson M, Wall M, Wang S, et al. Suicide after deliberate self-harm in adolescents and young adults. Pediatrics. 2018;141(4):e20173517. doi: 10.1542/peds.2017-3517. PubMed
8. Gay JC, Zima BT, Coker TR, et al. Postacute care after pediatric hospitalizations for a primary mental health condition. J Pediatr. 2018;193:222-228.e1. doi: 10.1016/j.jpeds.2017.09.058. PubMed
9. Heslin KC, Weiss AJ. Hospital Readmissions Involving Psychiatric Disorders, 2012. 2015. https://www.ncbi.nlm.nih.gov/books/NBK305353/pdf/Bookshelf_NBK305353.pdf. Accessed September 8, 2017. PubMed
10. Feng JY, Toomey SL, Zaslavsky AM, Nakamura MM, Schuster MA. Readmission after pediatric mental health admissions. Pediatrics. 2017;140(6):e20171571. doi: 10.1542/peds.2017-1571. PubMed
11. Bardach NS, Vittinghoff E, Asteria-Peñaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. doi: 10.1542/peds.2012-3527. PubMed
12. Callahan ST, Fuchs DC, Shelton RC, et al. Identifying suicidal behavior among adolescents using administrative claims data. Pharmacoepidemiol Drug Saf. 2013;22(7):769-775. doi: 10.1002/pds.3421. PubMed
13. SAMHSA, HHS, Synectics for Management Decisions, Mathematica Policy Research. National Mental Health Services Survey: 2010: Data on Mental Health Treatment Facilities. http://media.samhsa.gov/data/DASIS/NMHSS2010D/NMHSS2010_Web.pdf. Accessed November 13, 2015.
14. Patrick AR, Miller M, Barber CW, Wang PS, Canning CF, Schneeweiss S. Identification of hospitalizations for intentional self-harm when E-codes are incompletely recorded. Pharmacoepidemiol Drug Saf. 2010;19(12):1263-1275. doi: 10.1002/pds.2037. PubMed
15. Mellesdal L, Mehlum L, Wentzel-Larsen T, Kroken R, Jørgensen HA. Suicide risk and acute psychiatric readmissions: a prospective cohort study. Psychiatr Serv. 2010;61(1):25-31. doi: 10.1176/appi.ps.61.1.25. PubMed
16. Agency for Healthcare Research and Quality, Centers for Medicare and Medicaid. Measure: Pediatric All-Condition Readmission Measure Measure Developer: Center of Excellence for Pediatric Quality Measurement (CEPQM). https://www.ahrq.gov/sites/default/files/wysiwyg/policymakers/chipra/factsheets/chipra_14-p008-1-ef.pdf. Accessed November 15, 2017.
17. Cancino RS, Culpepper L, Sadikova E, Martin J, Jack BW, Mitchell SE. Dose-response relationship between depressive symptoms and hospital readmission. J Hosp Med. 2014;9(6):358-364. doi: 10.1002/jhm.2180. PubMed
18. Carlisle CE, Mamdani M, Schachar R, To T. Aftercare, emergency department visits, and readmission in adolescents. J Am Acad Child Adolesc Psychiatry. 2012;51(3):283-293. http://www.sciencedirect.com/science/article/pii/S0890856711011002. Accessed November 2, 2015. PubMed
19. Fadum EA, Stanley B, Qin P, Diep LM, Mehlum L. Self-poisoning with medications in adolescents: a national register study of hospital admissions and readmissions. Gen Hosp Psychiatry. 2014;36(6):709-715. doi: 10.1016/j.genhosppsych.2014.09.004. PubMed
20. Bernet AC. Predictors of psychiatric readmission among veterans at high risk of suicide: the impact of post-discharge aftercare. Arch Psychiatr Nurs. 2013;27(5):260-261. doi: 10.1016/j.apnu.2013.07.001. PubMed
21. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14(1):199. doi: 10.1186/1471-2431-14-199. PubMed
22. HCUP. HCUP-US Tools & Software Page. http://www.hcup-us.ahrq.gov/toolssoftware/chronic/chronic.jsp. Published 2015. Accessed October 30, 2015.
23. Zima BT, Rodean J, Hall M, Bardach NS, Coker TR, Berry JG. Psychiatric disorders and trends in resource use in pediatric hospitals. Pediatrics. 2016;138(5):e20160909-e20160909. doi: 10.1542/peds.2016-0909. PubMed
24. Spicer RS, Miller TR. Suicide acts in 8 states: incidence and case fatality rates by demographics and method. Am J Public Health. 2000;90(12):1885-1891. doi: 10.2105/AJPH.90.12.1885. PubMed
25. Hansen B, Lang M. Back to school blues: Seasonality of youth suicide and the academic calendar. Econ Educ Rev. 2011;30(5):850-861. doi: 10.1016/j.econedurev.2011.04.012.
26. Lueck C, Kearl L, Lam CN, Claudius I. Do emergency pediatric psychiatric visits for danger to self or others correspond to times of school attendance? Am J Emerg Med. 2015;33(5):682-684. doi: 10.1016/J.AJEM.2015.02.055. PubMed
27. Healthcare Cost And Utilization Project. Introduction to the HCUP Nationwide Readmissions Database. Rockville, MD; 2017. https://www.hcup-us.ahrq.gov/db/nation/nrd/Introduction_NRD_2010-2014.pdf. Accessed November 14, 2017.
28. Bardach NS, Coker TR, Zima BT, et al. Common and costly hospitalizations for pediatric mental health disorders. Pediatrics. 2014;133(4):602-609. doi: 10.1542/peds.2013-3165. PubMed
29. Ahmedani BK, Peterson EL, Hu Y, et al. Major physical health conditions and risk of suicide. Am J Prev Med. 2017;53(3):308-315. doi: 10.1016/J.AMEPRE.2017.04.001. PubMed
30. Gunnell D, Hawton K, Ho D, et al. Hospital admissions for self harm after discharge from psychiatric inpatient care: cohort study. BMJ. 2008;337:a2278. doi: 10.1136/bmj.a2278. PubMed
31. The TADS Team. The treatment for adolescents with depression study (TADS). Arch Gen Psychiatry. 2007;64(10):1132. doi: 10.1001/archpsyc.64.10.1132. PubMed
32. While D, Bickley H, Roscoe A, et al. Implementation of mental health service recommendations in England and Wales and suicide rates, 1997-2006: A cross-sectional and before-and-after observational study. Lancet. 2012;379(9820):1005-1012. doi: 10.1016/S0140-6736(11)61712-1. PubMed
33. Kawanishi C, Aruga T, Ishizuka N, et al. Assertive case management versus enhanced usual care for people with mental health problems who had attempted suicide and were admitted to hospital emergency departments in Japan (ACTION-J): a multicentre, randomised controlled trial. Lancet Psychiatry. 2014;1(3):193-201. doi: 10.1016/S2215-0366(14)70259-7. PubMed
34. Grupp-Phelan J, McGuire L, Husky MM, Olfson M. A randomized controlled trial to engage in care of adolescent emergency department patients with mental health problems that increase suicide risk. Pediatr Emerg Care. 2012;28(12):1263-1268. doi: 10.1097/PEC.0b013e3182767ac8. PubMed
35. Ciciolla L, Curlee AS, Karageorge J, Luthar SS. When mothers and fathers are seen as disproportionately valuing achievements: implications for adjustment among upper middle class youth. J Youth Adolesc. 2017;46(5):1057-1075. doi: 10.1007/s10964-016-0596-x. PubMed
36. Plemmons G, Hall M, Doupnik S, et al. Hospitalization for suicide ideation or attempt: 2008–2015. Pediatrics. May 2018:e20172426. doi: 10.1542/peds.2017-2426. PubMed
© 2018 Society of Hospital Medicine
The Virtual Hospitalist: The Future is Now
Compared with other industries, medicine has been slow to embrace the digital age. Electronic health records have only recently become ubiquitous, and that was only realized after governmental prodding through Meaningful Use legislation. Other digital tools, such as video or remote sensor technologies, have been available for decades but had not been introduced into routine medical care until recently for various reasons, ranging from costs to security to reimbursement rules. However, we are currently in the midst of a paradigm shift in medicine toward virtual care, as exemplified by the Kaiser Permanente CEO’s proclamation in 2017 that this capitated care system had moved over half of its 100 million annual patient encounters to the virtual environment.1
Regulation – both at the state and federal levels – has been the largest barrier to the adoption of virtual care. State licensure regulations for practicing medicine hamper virtual visits, which can otherwise be easily achieved without regard to geography. Although the Centers for Medicare & Medicaid Services (CMS) has had provisions for telehealth billing, these have been largely limited to rural areas. However, regulations are constantly evolving as the Interstate Medical Licensure Compact list is not CMS. The Interstate Medical Licensure Compact (www.imlcc.org) is an agreement involving 24 states that permits licensed physicians to practice medicine across state lines. CMS has recently proposed to add payments for virtual check-in visits, which will not be subject to the prior limitations on Medicare telehealth services.2 These and future changes in regulation will likely spur the rapid adoption and evolution of virtual services.
In this context, the article by Kuperman et al.3 provides a welcoming view of the future of hospital medicine. The authors demonstrated the feasibility of using a “virtual hospitalist” to manage patients admitted to a small rural hospital that lacked the patient volumes and resources to justify on-site hospitalist staffing. The patients benefited from the clinical expertise of an experienced inpatient provider while staying near their homes. This article adds to the growing literature on the use of these technologies in the hospital settings, which range from the management of patients in the intensive care unit4 to stroke patients in the ED5 and to inpatient psychiatric consultation.6
What are the implications for hospitalists? We need to prepare the current and future generations of hospitalists for practice in an evolving digital environment. “Choosing Wisely®: Things We Do For No Reason” is one of the most popular segments of JHM for a good reason: there are many things in the field of medicine because “that’s the way we always did it.” The capabilities unleashed by digital technologies will require hospitalists to rethink how we manage patients in acute and subacute settings and after discharge. Although these tools show a substantial promise to help us achieve the Triple Aim, we will need considerably more research to understand the costs and effectiveness of these new digital technologies and approaches.7,8 We also need new payment models that recognize their value. Finally, we also need to be aware that doctoring elements, such as human touch, physical presence, and emotional connection, can be encumbered and not enhanced by digital technologies.9
Disclosures
Dr. Ong and Dr. Brotman have nothing to disclose.
1. Why Digital Transformations Are Hard. Wall Street Journal. March 7, 2017, 2017.
2. Medicare Program: Revisions to Payment Policies under the Physician Fee Schedule and Other Revisions to Part B for CY 2019; Medicare Shared Savings Program Requirements; etc. In: Centers for Medicare & Medicaid Services, ed: Federal Register; 2018:1472.
3. Kuperman EF, Linson EL, Klefstad K, Perry E, Glenn K. The virtual hospitalist: a single-site implementation bringing hospitalist coverage to critical access hospitals. J Hosp Med. 2018. In Press. PubMed
4. Lilly CM, Cody S, Zhao H, et al. Hospital mortality, length of stay, and preventable complications among critically ill patients before and after tele-ICU reengineering of critical care processes. JAMA. 2011;305(21):2175-2183. doi: 10.1001/jama.2011.697. PubMed
5. Meyer BC, Raman R, Hemmen T, et al. Efficacy of site-independent telemedicine in the STRokE DOC trial: a randomised, blinded, prospective study. Lancet Neurol. 2008;7(9):787-795. doi: 10.1016/S1474-4422(08)70171-6. PubMed
6. Arevian AC, Jeffrey J, Young AS, Ong MK. Opportunities for flexible, on-demand care delivery through telemedicine. Psychiatr Serv. 2018;69(1):5-8. doi: 10.1176/appi.ps.201600589. PubMed
7. Ashwood JS, Mehrotra A, Cowling D, Uscher-Pines L. Direct-to-consumer telehealth may increase access to care but does not decrease spending. Health Aff (Millwood). 2017;36(3):485-491. doi: 10.1377/hlthaff.2016.1130. PubMed
8. Ong MK, Romano PS, Edgington S, et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the better effectiveness after transition -- Heart Failure (BEAT-HF) Randomized Clinical Trial. JAMA Intern Med. 2016;176(3):310-318. doi: 10.1001/jamainternmed.2015.7712. PubMed
9. Verghese A. Culture shock--patient as icon, icon as patient. N Engl J Med. 2008;359(26):2748-2751. doi: 10.1056/NEJMp0807461. PubMed
Compared with other industries, medicine has been slow to embrace the digital age. Electronic health records have only recently become ubiquitous, and that was only realized after governmental prodding through Meaningful Use legislation. Other digital tools, such as video or remote sensor technologies, have been available for decades but had not been introduced into routine medical care until recently for various reasons, ranging from costs to security to reimbursement rules. However, we are currently in the midst of a paradigm shift in medicine toward virtual care, as exemplified by the Kaiser Permanente CEO’s proclamation in 2017 that this capitated care system had moved over half of its 100 million annual patient encounters to the virtual environment.1
Regulation – both at the state and federal levels – has been the largest barrier to the adoption of virtual care. State licensure regulations for practicing medicine hamper virtual visits, which can otherwise be easily achieved without regard to geography. Although the Centers for Medicare & Medicaid Services (CMS) has had provisions for telehealth billing, these have been largely limited to rural areas. However, regulations are constantly evolving as the Interstate Medical Licensure Compact list is not CMS. The Interstate Medical Licensure Compact (www.imlcc.org) is an agreement involving 24 states that permits licensed physicians to practice medicine across state lines. CMS has recently proposed to add payments for virtual check-in visits, which will not be subject to the prior limitations on Medicare telehealth services.2 These and future changes in regulation will likely spur the rapid adoption and evolution of virtual services.
In this context, the article by Kuperman et al.3 provides a welcoming view of the future of hospital medicine. The authors demonstrated the feasibility of using a “virtual hospitalist” to manage patients admitted to a small rural hospital that lacked the patient volumes and resources to justify on-site hospitalist staffing. The patients benefited from the clinical expertise of an experienced inpatient provider while staying near their homes. This article adds to the growing literature on the use of these technologies in the hospital settings, which range from the management of patients in the intensive care unit4 to stroke patients in the ED5 and to inpatient psychiatric consultation.6
What are the implications for hospitalists? We need to prepare the current and future generations of hospitalists for practice in an evolving digital environment. “Choosing Wisely®: Things We Do For No Reason” is one of the most popular segments of JHM for a good reason: there are many things in the field of medicine because “that’s the way we always did it.” The capabilities unleashed by digital technologies will require hospitalists to rethink how we manage patients in acute and subacute settings and after discharge. Although these tools show a substantial promise to help us achieve the Triple Aim, we will need considerably more research to understand the costs and effectiveness of these new digital technologies and approaches.7,8 We also need new payment models that recognize their value. Finally, we also need to be aware that doctoring elements, such as human touch, physical presence, and emotional connection, can be encumbered and not enhanced by digital technologies.9
Disclosures
Dr. Ong and Dr. Brotman have nothing to disclose.
Compared with other industries, medicine has been slow to embrace the digital age. Electronic health records have only recently become ubiquitous, and that was only realized after governmental prodding through Meaningful Use legislation. Other digital tools, such as video or remote sensor technologies, have been available for decades but had not been introduced into routine medical care until recently for various reasons, ranging from costs to security to reimbursement rules. However, we are currently in the midst of a paradigm shift in medicine toward virtual care, as exemplified by the Kaiser Permanente CEO’s proclamation in 2017 that this capitated care system had moved over half of its 100 million annual patient encounters to the virtual environment.1
Regulation – both at the state and federal levels – has been the largest barrier to the adoption of virtual care. State licensure regulations for practicing medicine hamper virtual visits, which can otherwise be easily achieved without regard to geography. Although the Centers for Medicare & Medicaid Services (CMS) has had provisions for telehealth billing, these have been largely limited to rural areas. However, regulations are constantly evolving as the Interstate Medical Licensure Compact list is not CMS. The Interstate Medical Licensure Compact (www.imlcc.org) is an agreement involving 24 states that permits licensed physicians to practice medicine across state lines. CMS has recently proposed to add payments for virtual check-in visits, which will not be subject to the prior limitations on Medicare telehealth services.2 These and future changes in regulation will likely spur the rapid adoption and evolution of virtual services.
In this context, the article by Kuperman et al.3 provides a welcoming view of the future of hospital medicine. The authors demonstrated the feasibility of using a “virtual hospitalist” to manage patients admitted to a small rural hospital that lacked the patient volumes and resources to justify on-site hospitalist staffing. The patients benefited from the clinical expertise of an experienced inpatient provider while staying near their homes. This article adds to the growing literature on the use of these technologies in the hospital settings, which range from the management of patients in the intensive care unit4 to stroke patients in the ED5 and to inpatient psychiatric consultation.6
What are the implications for hospitalists? We need to prepare the current and future generations of hospitalists for practice in an evolving digital environment. “Choosing Wisely®: Things We Do For No Reason” is one of the most popular segments of JHM for a good reason: there are many things in the field of medicine because “that’s the way we always did it.” The capabilities unleashed by digital technologies will require hospitalists to rethink how we manage patients in acute and subacute settings and after discharge. Although these tools show a substantial promise to help us achieve the Triple Aim, we will need considerably more research to understand the costs and effectiveness of these new digital technologies and approaches.7,8 We also need new payment models that recognize their value. Finally, we also need to be aware that doctoring elements, such as human touch, physical presence, and emotional connection, can be encumbered and not enhanced by digital technologies.9
Disclosures
Dr. Ong and Dr. Brotman have nothing to disclose.
1. Why Digital Transformations Are Hard. Wall Street Journal. March 7, 2017, 2017.
2. Medicare Program: Revisions to Payment Policies under the Physician Fee Schedule and Other Revisions to Part B for CY 2019; Medicare Shared Savings Program Requirements; etc. In: Centers for Medicare & Medicaid Services, ed: Federal Register; 2018:1472.
3. Kuperman EF, Linson EL, Klefstad K, Perry E, Glenn K. The virtual hospitalist: a single-site implementation bringing hospitalist coverage to critical access hospitals. J Hosp Med. 2018. In Press. PubMed
4. Lilly CM, Cody S, Zhao H, et al. Hospital mortality, length of stay, and preventable complications among critically ill patients before and after tele-ICU reengineering of critical care processes. JAMA. 2011;305(21):2175-2183. doi: 10.1001/jama.2011.697. PubMed
5. Meyer BC, Raman R, Hemmen T, et al. Efficacy of site-independent telemedicine in the STRokE DOC trial: a randomised, blinded, prospective study. Lancet Neurol. 2008;7(9):787-795. doi: 10.1016/S1474-4422(08)70171-6. PubMed
6. Arevian AC, Jeffrey J, Young AS, Ong MK. Opportunities for flexible, on-demand care delivery through telemedicine. Psychiatr Serv. 2018;69(1):5-8. doi: 10.1176/appi.ps.201600589. PubMed
7. Ashwood JS, Mehrotra A, Cowling D, Uscher-Pines L. Direct-to-consumer telehealth may increase access to care but does not decrease spending. Health Aff (Millwood). 2017;36(3):485-491. doi: 10.1377/hlthaff.2016.1130. PubMed
8. Ong MK, Romano PS, Edgington S, et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the better effectiveness after transition -- Heart Failure (BEAT-HF) Randomized Clinical Trial. JAMA Intern Med. 2016;176(3):310-318. doi: 10.1001/jamainternmed.2015.7712. PubMed
9. Verghese A. Culture shock--patient as icon, icon as patient. N Engl J Med. 2008;359(26):2748-2751. doi: 10.1056/NEJMp0807461. PubMed
1. Why Digital Transformations Are Hard. Wall Street Journal. March 7, 2017, 2017.
2. Medicare Program: Revisions to Payment Policies under the Physician Fee Schedule and Other Revisions to Part B for CY 2019; Medicare Shared Savings Program Requirements; etc. In: Centers for Medicare & Medicaid Services, ed: Federal Register; 2018:1472.
3. Kuperman EF, Linson EL, Klefstad K, Perry E, Glenn K. The virtual hospitalist: a single-site implementation bringing hospitalist coverage to critical access hospitals. J Hosp Med. 2018. In Press. PubMed
4. Lilly CM, Cody S, Zhao H, et al. Hospital mortality, length of stay, and preventable complications among critically ill patients before and after tele-ICU reengineering of critical care processes. JAMA. 2011;305(21):2175-2183. doi: 10.1001/jama.2011.697. PubMed
5. Meyer BC, Raman R, Hemmen T, et al. Efficacy of site-independent telemedicine in the STRokE DOC trial: a randomised, blinded, prospective study. Lancet Neurol. 2008;7(9):787-795. doi: 10.1016/S1474-4422(08)70171-6. PubMed
6. Arevian AC, Jeffrey J, Young AS, Ong MK. Opportunities for flexible, on-demand care delivery through telemedicine. Psychiatr Serv. 2018;69(1):5-8. doi: 10.1176/appi.ps.201600589. PubMed
7. Ashwood JS, Mehrotra A, Cowling D, Uscher-Pines L. Direct-to-consumer telehealth may increase access to care but does not decrease spending. Health Aff (Millwood). 2017;36(3):485-491. doi: 10.1377/hlthaff.2016.1130. PubMed
8. Ong MK, Romano PS, Edgington S, et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the better effectiveness after transition -- Heart Failure (BEAT-HF) Randomized Clinical Trial. JAMA Intern Med. 2016;176(3):310-318. doi: 10.1001/jamainternmed.2015.7712. PubMed
9. Verghese A. Culture shock--patient as icon, icon as patient. N Engl J Med. 2008;359(26):2748-2751. doi: 10.1056/NEJMp0807461. PubMed
© 2018 Society of Hospital Medicine