User login
Perioperative cardiovascular medicine: 5 questions for 2018
A plethora of studies are under way in the field of perioperative medicine. As a result, evidence-based care of surgical patients is evolving at an exponential rate.
We performed a literature search and, using consensus, identified recent articles we believe will have a great impact on perioperative cardiovascular medicine. These articles report studies that were presented at national meetings in 2018, including the Perioperative Medicine Summit, Society of General Internal Medicine, and Society of Hospital Medicine. These articles are grouped under 5 questions that will help guide clinical practice in perioperative cardiovascular medicine.
SHOULD ASPIRIN BE CONTINUED PERIOPERATIVELY IN PATIENTS WITH A CORONARY STENT?
The Perioperative Ischemic Evaluation 2 (POISE-2) trial1 found that giving aspirin before surgery and throughout the early postoperative period had no significant effect on the rate of a composite of death or nonfatal myocardial infarction; moreover, aspirin increased the risk of major bleeding. However, many experts felt uncomfortable stopping aspirin preoperatively in patients taking it for secondary prophylaxis, particularly patients with a coronary stent.
[Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244.]
This post hoc subgroup analysis2 of POISE-2 evaluated the benefit and harm of perioperative aspirin in patients who had previously undergone percutaneous coronary intervention, more than 90% of whom had received a stent. Patients were age 45 or older with atherosclerotic heart disease or risk factors for it who had previously undergone percutaneous coronary intervention and were now undergoing noncardiac surgery.
Patients who had received a bare-metal stent within the previous 6 weeks or a drug-eluting stent within 12 months before surgery were excluded because guidelines at that time said to continue dual antiplatelet therapy for that long. Recommendations have since changed; the optimal duration for dual antiplatelet therapy with drug-eluting stents is now 6 months. Second-generation drug-eluting stents pose a lower risk of stent thrombosis and require a shorter duration of dual antiplatelet therapy than first-generation drug-eluting stents. Approximately 25% of the percutaneous coronary intervention subgroup had a drug-eluting stent, but the authors did not specify the type of drug-eluting stent.
The post hoc analysis2 included a subgroup of 234 of 4,998 patients receiving aspirin and 236 of 5,012 patients receiving placebo initiated within 4 hours before surgery and continued postoperatively. The primary outcome measured was the rate of death or nonfatal myocardial infarction within 30 days after surgery, and bleeding was a secondary outcome.
Findings. Although the overall POISE-2 study found no benefit from aspirin, in the subgroup who had previously undergone percutaneous coronary intervention, aspirin significantly reduced the risk of the primary outcome, which occurred in 6% vs 11.5% of the patients:
- Absolute risk reduction 5.5% (95% confidence interval 0.4%–10.5%)
- Hazard ratio 0.50 (0.26–0.95).
The reduction was primarily due to fewer myocardial infarctions:
- Absolute risk reduction 5.9% (1.0%–10.8%)
- Hazard ratio 0.44 (0.22–0.87).
The type of stent had no effect on the primary outcome, although this subgroup analysis had limited power. In the nonpercutaneous coronary intervention subgroup, there was no significant difference in outcomes between the aspirin and placebo groups. This subgroup analysis was underpowered to evaluate the effect of aspirin on the composite of major and life-threatening bleeding in patients with prior percutaneous coronary intervention, which was reported as “uncertain” due to wide confidence intervals (absolute risk increase 1.3%, 95% confidence interval –2.6% to 5.2%), but the increased risk of major or life-threatening bleeding with aspirin demonstrated in the overall POISE-2 study population likely applies:
- Absolute risk increase 0.8% (0.1%–1.6%)
- Hazard ratio 1.22 (1.01–1.48).
Limitations. This was a nonspecified subgroup analysis that was underpowered and had a relatively small sample size with few events.
Conclusion. In the absence of a very high bleeding risk, continuing aspirin perioperatively in patients with prior percutaneous coronary intervention undergoing noncardiac surgery is more likely to result in benefit than harm. This finding is in agreement with current recommendations from the American College Cardiology and American Heart Association (class I; level of evidence C).3
WHAT IS THE INCIDENCE OF MINS? IS MEASURING TROPONIN USEFUL?
Despite advances in anesthesia and surgical techniques, about 1% of patients over age 45 die within 30 days of noncardiac surgery.4 Studies have demonstrated a high mortality rate in patients who experience myocardial injury after noncardiac surgery (MINS), defined as elevations of troponin T with or without ischemic symptoms or electrocardiographic changes.5 Most of these studies used earlier, “non-high-sensitivity” troponin T assays. Fifth-generation, highly sensitive troponin T assays are now available that can detect troponin T at lower concentrations, but their utility in predicting postoperative outcomes remains uncertain. Two recent studies provide further insight into these issues.
[Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, Sigamani A, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017; 317(16):1642–1651.]
The Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study5 was an international, prospective cohort study that initially evaluated the association between MINS and the 30-day mortality rate using a non-high-sensitivity troponin T assay (Roche fourth-generation Elecsys TnT assay) in patients age 45 or older undergoing noncardiac surgery and requiring hospital admission for at least 1 night. After the first 15,000 patients, the study switched to the Roche fifth-generation assay, with measurements at 6 to 12 hours after surgery and on postoperative days 1, 2, and 3.
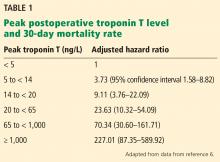
A 2017 analysis by Devereaux et al6 included only these later-enrolled patients and correlated their high-sensitivity troponin T levels with 30-day mortality rates. Patients with a level 14 ng/L or higher, the upper limit of normal in this study, were also assessed for ischemic symptoms and electrocardiographic changes. Although not required by the study, more than 7,800 patients had their troponin T levels measured before surgery, and the absolute change was also analyzed for an association with the 30-day mortality rate.
Findings. Of the 21,842 patients, about two-thirds underwent some form of major surgery; some of them had more than 1 type. A total of 1.2% of the patients died within 30 days of surgery.
Based on their analysis, the authors proposed that MINS be defined as:
- A postoperative troponin T level of 65 ng/L or higher, or
- A level in the range of 20 ng/L to less than 65 ng/L with an absolute increase from the preoperative level at least 5 ng/L, not attributable to a nonischemic cause.
Seventeen percent of the study patients met these criteria, and of these, 21.7% met the universal definition of myocardial infarction, although only 6.9% had symptoms of it.
Limitations. Only 40.4% of the patients had a preoperative high-sensitivity troponin T measurement for comparison, and in 13.8% of patients who had an elevated perioperative measurement, their preoperative value was the same or higher than their postoperative one. Thus, the incidence of MINS may have been overestimated if patients were otherwise not known to have troponin T elevations before surgery.
[Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation 2018; 137(12):1221–1232.]
Puelacher et al7 investigated the prevalence of MINS in 2,018 patients at increased cardiovascular risk (age ≥ 65, or age ≥ 45 with a history of coronary artery disease, peripheral vascular disease, or stroke) who underwent major noncardiac surgery (planned overnight stay ≥ 24 hours) at a university hospital in Switzerland. Patients had their troponin T measured with a high-sensitivity assay within 30 days before surgery and on postoperative days 1 and 2.
Instead of MINS, the investigators used the term “perioperative myocardial injury” (PMI), defined as an absolute increase in troponin T of at least 14 ng/L from before surgery to the peak postoperative reading. Similar to MINS, PMI did not require ischemic features, but in this study, noncardiac triggers (sepsis, stroke, or pulmonary embolus) were not excluded.
Findings. PMI occurred in 16% of surgeries, and of the patients with PMI, 6% had typical chest pain and 18% had any ischemic symptoms. Unlike in the POISE-2 study discussed above, PMI triggered an automatic referral to a cardiologist.
The unadjusted 30-day mortality rate was 8.9% among patients with PMI and 1.5% in those without. Multivariable logistic regression analysis showed an adjusted hazard ratio for 30-day mortality of 2.7 (95% CI 1.5–4.8) for those with PMI vs without, and this difference persisted for at least 1 year.
In patients with PMI, the authors compared the 30-day mortality rate of those with no ischemic signs or symptoms (71% of the patients) with those who met the criteria for myocardial infarction and found no difference. Patients with PMI triggered by a noncardiac event had a worse prognosis than those with a presumed cardiac etiology.
Limitations. Despite the multivariate analysis that included adjustment for age, nonelective surgery, and Revised Cardiac Risk Index (RCRI), the increased risk associated with PMI could simply reflect higher risk at baseline. Although PMI resulted in automatic referral to a cardiologist, only 10% of patients eventually underwent coronary angiography; a similar percentage were discharged with additional medical therapy such as aspirin, a statin, or a beta-blocker. The effect of these interventions is not known.
Conclusions. MINS is common and has a strong association with mortality risk proportional to the degree of troponin T elevation using high-sensitivity assays, consistent with data from previous studies of earlier assays. Because the mechanism of MINS may differ from that of myocardial infarction, its prevention and treatment may differ, and it remains unclear how serial measurement in postoperative patients should change clinical practice.
The recently published Dabigatran in Patients With Myocardial Injury After Non-cardiac Surgery (MANAGE) trial8 suggests that dabigatran may reduce arterial and venous complications in patients with MINS, but the study had a number of limitations that may restrict the clinical applicability of this finding.
While awaiting further clinical outcomes data, pre- and postoperative troponin T measurement may be beneficial in higher-risk patients (such as those with cardiovascular disease or multiple RCRI risk factors) if the information will change perioperative management.
WHAT IS THE ROLE OF HYPOTENSION OR BLOOD PRESSURE CONTROL?
Intraoperative hypotension is associated with organ ischemia, which may cause postoperative myocardial infarction, myocardial injury, and acute kidney injury.9 Traditional anesthesia practice is to maintain intraoperative blood pressure within 20% of the preoperative baseline, based on the notion that hypertensive patients require higher perfusion pressures.
[Futier E, Lefrant J-Y, Guinot P-G, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA 2017; 318(14):1346–1357.]
Futier et al10 sought to address uncertainty in intraoperative and immediate postoperative management of systolic blood pressure. In this multicenter, randomized, parallel-group trial, 298 patients at increased risk of postoperative renal complications were randomized to blood pressure management that was either “individualized” (within 10% of resting systolic pressure) or “standard” (≥ 80 mm Hg or ≥ 40% of resting systolic pressure) from induction to 4 hours postoperatively.
Blood pressure was monitored using radial arterial lines and maintained using a combination of intravenous fluids, norepinephrine (the first-line agent for the individualized group), and ephedrine (in the standard treatment group only). The primary outcome was a composite of systemic inflammatory response syndrome (SIRS) and organ dysfunction affecting at least 1 organ system (cardiovascular, respiratory, renal, hematologic, or neurologic).
Findings. Data on the primary outcome were available for 292 of 298 patients enrolled. The mean age was 70 years, 15% were women, and 82% had previously diagnosed hypertension. Despite the requirement for an elevated risk of acute kidney injury, only 13% of the patients had a baseline estimated glomerular filtration rate of less than 60 mL/min/1.73 m2, and the median was 88 mL/min/1.73 m2. Ninety-five percent of patients underwent abdominal surgery, and 50% of the surgeries were elective.
The mean systolic blood pressure was 123 mm Hg in the individualized treatment group compared with 116 mm Hg in the standard treatment group. Despite this small difference, 96% of individualized treatment patients received norepinephrine, compared with 26% in the standard treatment group.
The primary outcome of SIRS with organ dysfunction occurred in 38.1% of patients in the individualized treatment group and 51.7% of those in the standard treatment group. After adjusting for center, surgical urgency, surgical site, and acute kidney injury risk index, the relative risk of developing SIRS in those receiving individualized management was 0.73 (P = .02). Renal dysfunction (based on Acute Dialysis Quality Initiative criteria11) occurred in 32.7% of individualized treatment patients and 49% of standardized treatment patients.
Limitations of this study included differences in pharmacologic approach to maintain blood pressure in the 2 protocols (ephedrine and fluids vs norepinephrine) and a modest sample size.
Conclusions. Despite this, the difference in organ dysfunction was striking, with a number needed to treat of only 7 patients. This intervention extended 4 hours postoperatively, a time when many of these patients have left the postanesthesia care unit and have returned to hospitalist care on inpatient wards.
While optimal management of intraoperative and immediate postoperative blood pressure may not be settled, this study suggests that even mild relative hypotension may justify immediate action. Further studies may be useful to delineate high- and low-risk populations, the timing of greatest risk, and indications for intraarterial blood pressure monitoring.
[Salmasi V, Maheswari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology 2017; 126(1):47–65.]
This retrospective cohort study12 assessed the association between myocardial or kidney injury and absolute or relative thresholds of intraoperative mean arterial pressure. It included 57,315 adults who underwent inpatient noncardiac surgery, had a preoperative and at least 1 postoperative serum creatinine measurement within 7 days, and had blood pressure recorded in preoperative appointments within 6 months. Patients with chronic kidney disease (glomerular filtration rate < 60 mL/min/1.73 m2) and those on dialysis were excluded. The outcomes were MINS5 and acute kidney injury as defined by the Acute Kidney Injury Network.9
Findings. A mean arterial pressure below an absolute threshold of 65 mm Hg or a relative threshold of 20% lower than baseline value was associated with myocardial and kidney injury. At each threshold, prolonged periods of hypotension were associated with progressively increased risk.
An important conclusion of the study was that relative thresholds of mean arterial pressure were not any more predictive than absolute thresholds. Absolute thresholds are easier to use intraoperatively, especially when baseline values are not available. The authors did not find a clinically significant interaction between baseline blood pressure and the association of hypotension and myocardial and kidney injury.
Limitations included use of cardiac enzymes postoperatively to define MINS. Since these were not routinely collected, clinically silent myocardial injury may have been missed. Baseline blood pressure may have important implications in other forms of organ injury (ie, cerebral ischemia) that were not studied.
Summary. The lowest absolute mean arterial pressure is as predictive of postoperative myocardial and kidney injury as the relative pressure reduction, at least in patients with normal renal function. Limiting exposure to intraoperative hypotension is important. Baseline blood pressure values may have limited utility for intraoperative management.
In combination, these studies confirm that intraoperative hypotension is a predictor of postoperative organ dysfunction, but the definition and management remain unclear. While aggressive intraoperative management is likely beneficial, how to manage the antihypertensive therapy the patient has been taking as an outpatient when he or she comes into the hospital for surgery remains uncertain.
DOES PATENT FORAMEN OVALE INCREASE THE RISK OF STROKE?
Perioperative stroke is an uncommon, severe complication of noncardiac surgery. The pathophysiology has been better defined in cardiac than in noncardiac surgeries. In nonsurgical patients, patent foramen ovale (PFO) is associated with stroke, even in patients considered to be at low risk.13 Perioperative patients have additional risk for venous thromboembolism and may have periprocedural antithrombotic medications altered, increasing their risk of paradoxical embolism through the PFO.
[Ng PY, Ng AK, Subramaniam B, et al. Association of preoperatively diagnosed patent foramen ovale with perioperative ischemic stroke. JAMA 2018; 319(5):452–462.]
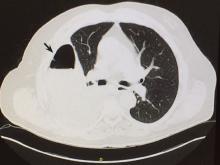
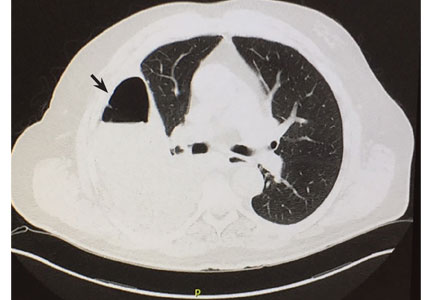
This retrospective cohort study of noncardiac surgery patients at 3 hospitals14 sought to determine the association of preoperatively diagnosed PFO with the risk of perioperative ischemic stroke identified by International Classification of Diseases diagnoses.
Of 150,198 patients, 1.0% had a preoperative diagnosis of PFO, and at baseline, those with PFO had significantly more comorbidities than those without PFO. Stroke occurred in 3.2% of patients with PFO vs 0.5% of those without. Patients known to have a PFO were much more likely to have cardiovascular and thromboembolic risk factors for stroke. In the adjusted analysis, the absolute risk difference between groups was 0.4% (95% CI 0.2–0.6%), with an estimated perioperative stroke risk of 5.9 per 1,000 in patients with known patent foramen ovale and 2.2 per 1,000 in those without. A diagnosis of PFO was also associated with increased risk of large-vessel-territory stroke and more severe neurologic deficit.
Further attempts to adjust for baseline risk factors and other potential bias, including a propensity score-matched cohort analysis and an analysis limited to patients who had echocardiography performed in the same healthcare system, still showed a higher risk of perioperative stroke among patients with preoperatively detected patent foramen ovale.
Limitations. The study was retrospective and observational, used administrative data, and had a low rate of PFO diagnosis (1%), compared with about 25% in population-based studies.15 Indications for preoperative echocardiography are unknown. In addition, the study specifically examined preoperatively diagnosed PFO, rather than including those diagnosed in the postoperative period.
Discussion. How does this study affect clinical practice? The absolute stroke risk was increased by 0.4% in patients with PFO compared with those without. Although this is a relatively small increase, millions of patients undergo noncardiac surgery annually. The risks of therapeutic anticoagulation or PFO closure are likely too high in this context; however, clinicians may approach the perioperative management of antiplatelet agents and venous thromboembolism prophylaxis in patients with known PFO with additional caution.
HOW DOES TIMING OF EMERGENCY SURGERY AFTER PRIOR STROKE AFFECT OUTCOMES?
A history of stroke or transient ischemic attack is a known risk factor for perioperative vascular complications. A recent large cohort study demonstrated that a history of stroke within 9 months of elective surgery was associated with increased adverse outcomes.16 Little is known, however, of the perioperative risk in patients with a history of stroke who undergo emergency surgery.
[Christiansen MN, Andersson C, Gislason GH, et al. Risks of cardiovascular adverse events and death in patients with previous stroke undergoing emergency noncardiac, nonintracranial surgery: the importance of operative timing. Anesthesiology 2017; 127(1):9–19.]
In this study,17 all emergency noncardiac and nonintracranial surgeries from 2005 to 2011 were analyzed using multiple national patient registries in Denmark according to time elapsed between previous stroke and surgery. Primary outcomes were 30-day all-cause mortality and 30-day major adverse cardiac events (MACE), defined as nonfatal ischemic stroke, nonfatal myocardial infarction, and cardiovascular death. Statistical analysis to assess the risk of adverse outcomes included logistic regression models, spline analyses, and propensity-score matching.
Findings. The authors identified 146,694 emergency surgeries, with 7,861 patients (5.4%) having had a previous stroke (transient ischemic attacks and hemorrhagic strokes were not included). Rates of postoperative stroke were as follows:
- 9.9% in patents with a history of ischemic stroke within 3 months of surgery
- 2.8% in patients with a history of stroke 3 to 9 months before surgery
- 0.3% in patients with no previous stroke.
The risk plateaued when the time between stroke and surgery exceeded 4 to 5 months.15
Interestingly, in patients who underwent emergency surgery within 14 days of stroke, the risk of MACE was significantly lower immediately after surgery (1–3 days after stroke) compared with surgery that took place 4 to 14 days after stroke. The authors hypothesized that because cerebral autoregulation does not become compromised until approximately 5 days after a stroke, the risk was lower 1 to 3 days after surgery and increased thereafter.
Limitations of this study included the possibility of residual confounding, given its retrospective design using administrative data, not accounting for preoperative antithrombotic and anticoagulation therapy, and lack of information regarding the etiology of recurrent stroke (eg, thromboembolic, atherothrombotic, hypoperfusion).
Conclusions. Although it would be impractical to postpone emergency surgery in a patient who recently had a stroke, this study shows that the incidence rates of postoperative recurrent stroke and MACE are high. Therefore, it is important that the patient and perioperative team be aware of the risk. Further research is needed to confirm these estimates of postoperative adverse events in more diverse patient populations.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244. doi:10.7326/M17-2341
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 130(24):2215–2245. doi:10.1161/CIR.0000000000000105
- Smilowitz NR, Gupta N, Ramakrishna H, Guo Y, Berger JS, Bangalore S. Perioperative major adverse cardiovascular and cerebrovascular events associated with noncardiac surgery. JAMA Cardiol 2017; 2(2):181–187. doi:10.1001/jamacardio.2016.4792
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120(3):564–578. doi:10.1097/ALN.0000000000000113
- Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, Sigamani A, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017; 317(16):1642–1651. doi:10.1001/jama.2017.4360
- Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation 2018; 137(12):1221–1232. doi:10.1161/CIRCULATIONAHA.117.030114
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8
- Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology 2013; 119(3):507–515. doi:10.1097/ALN.0b013e3182a10e26
- Futier E, Lefrant JY, Guinot PG, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA 2017; 318(14):1346–1357. doi:10.1001/jama.2017.14172
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) group. Crit Care 2004; 8:R204. doi:10.1186/cc2872
- Salmasi V, Maheswari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology 2017; 126(1):47–65. doi:10.1097/ALN.0000000000001432
- Lechat P, Mas JL, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med 1988; 318(18):1148–1152. doi:10.1056/NEJM198805053181802
- Ng PY, Ng AK, Subramaniam B, et al. Association of preoperatively diagnosed patent foramen ovale with perioperative ischemic stroke. JAMA 2018; 319(5):452–462. doi:10.1001/jama.2017.21899
- Meissner I, Whisnant JP, Khandheria BK, et al. Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC study. Stroke Prevention: Assessment of Risk in a Community. Mayo Clin Proc 1999; 74(9):862–869. pmid:10488786
- Jørgensen ME, Torp-Pedersen C, Gislason GH, et al. Time elapsed after ischemic stroke and risk of adverse cardiovascular events and mortality following elective noncardiac surgery. JAMA 2014; 312:269–277. doi:10.1001/jama.2014.8165
- Christiansen MN, Andersson C, Gislason GH, et al. Risks of cardiovascular adverse events and death in patients with previous stroke undergoing emergency noncardiac, nonintracranial surgery: the importance of operative timing. Anesthesiology 2017; 127(1):9–19. doi:10.1097/ALN.0000000000001685
A plethora of studies are under way in the field of perioperative medicine. As a result, evidence-based care of surgical patients is evolving at an exponential rate.
We performed a literature search and, using consensus, identified recent articles we believe will have a great impact on perioperative cardiovascular medicine. These articles report studies that were presented at national meetings in 2018, including the Perioperative Medicine Summit, Society of General Internal Medicine, and Society of Hospital Medicine. These articles are grouped under 5 questions that will help guide clinical practice in perioperative cardiovascular medicine.
SHOULD ASPIRIN BE CONTINUED PERIOPERATIVELY IN PATIENTS WITH A CORONARY STENT?
The Perioperative Ischemic Evaluation 2 (POISE-2) trial1 found that giving aspirin before surgery and throughout the early postoperative period had no significant effect on the rate of a composite of death or nonfatal myocardial infarction; moreover, aspirin increased the risk of major bleeding. However, many experts felt uncomfortable stopping aspirin preoperatively in patients taking it for secondary prophylaxis, particularly patients with a coronary stent.
[Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244.]
This post hoc subgroup analysis2 of POISE-2 evaluated the benefit and harm of perioperative aspirin in patients who had previously undergone percutaneous coronary intervention, more than 90% of whom had received a stent. Patients were age 45 or older with atherosclerotic heart disease or risk factors for it who had previously undergone percutaneous coronary intervention and were now undergoing noncardiac surgery.
Patients who had received a bare-metal stent within the previous 6 weeks or a drug-eluting stent within 12 months before surgery were excluded because guidelines at that time said to continue dual antiplatelet therapy for that long. Recommendations have since changed; the optimal duration for dual antiplatelet therapy with drug-eluting stents is now 6 months. Second-generation drug-eluting stents pose a lower risk of stent thrombosis and require a shorter duration of dual antiplatelet therapy than first-generation drug-eluting stents. Approximately 25% of the percutaneous coronary intervention subgroup had a drug-eluting stent, but the authors did not specify the type of drug-eluting stent.
The post hoc analysis2 included a subgroup of 234 of 4,998 patients receiving aspirin and 236 of 5,012 patients receiving placebo initiated within 4 hours before surgery and continued postoperatively. The primary outcome measured was the rate of death or nonfatal myocardial infarction within 30 days after surgery, and bleeding was a secondary outcome.
Findings. Although the overall POISE-2 study found no benefit from aspirin, in the subgroup who had previously undergone percutaneous coronary intervention, aspirin significantly reduced the risk of the primary outcome, which occurred in 6% vs 11.5% of the patients:
- Absolute risk reduction 5.5% (95% confidence interval 0.4%–10.5%)
- Hazard ratio 0.50 (0.26–0.95).
The reduction was primarily due to fewer myocardial infarctions:
- Absolute risk reduction 5.9% (1.0%–10.8%)
- Hazard ratio 0.44 (0.22–0.87).
The type of stent had no effect on the primary outcome, although this subgroup analysis had limited power. In the nonpercutaneous coronary intervention subgroup, there was no significant difference in outcomes between the aspirin and placebo groups. This subgroup analysis was underpowered to evaluate the effect of aspirin on the composite of major and life-threatening bleeding in patients with prior percutaneous coronary intervention, which was reported as “uncertain” due to wide confidence intervals (absolute risk increase 1.3%, 95% confidence interval –2.6% to 5.2%), but the increased risk of major or life-threatening bleeding with aspirin demonstrated in the overall POISE-2 study population likely applies:
- Absolute risk increase 0.8% (0.1%–1.6%)
- Hazard ratio 1.22 (1.01–1.48).
Limitations. This was a nonspecified subgroup analysis that was underpowered and had a relatively small sample size with few events.
Conclusion. In the absence of a very high bleeding risk, continuing aspirin perioperatively in patients with prior percutaneous coronary intervention undergoing noncardiac surgery is more likely to result in benefit than harm. This finding is in agreement with current recommendations from the American College Cardiology and American Heart Association (class I; level of evidence C).3
WHAT IS THE INCIDENCE OF MINS? IS MEASURING TROPONIN USEFUL?
Despite advances in anesthesia and surgical techniques, about 1% of patients over age 45 die within 30 days of noncardiac surgery.4 Studies have demonstrated a high mortality rate in patients who experience myocardial injury after noncardiac surgery (MINS), defined as elevations of troponin T with or without ischemic symptoms or electrocardiographic changes.5 Most of these studies used earlier, “non-high-sensitivity” troponin T assays. Fifth-generation, highly sensitive troponin T assays are now available that can detect troponin T at lower concentrations, but their utility in predicting postoperative outcomes remains uncertain. Two recent studies provide further insight into these issues.
[Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, Sigamani A, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017; 317(16):1642–1651.]
The Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study5 was an international, prospective cohort study that initially evaluated the association between MINS and the 30-day mortality rate using a non-high-sensitivity troponin T assay (Roche fourth-generation Elecsys TnT assay) in patients age 45 or older undergoing noncardiac surgery and requiring hospital admission for at least 1 night. After the first 15,000 patients, the study switched to the Roche fifth-generation assay, with measurements at 6 to 12 hours after surgery and on postoperative days 1, 2, and 3.
A 2017 analysis by Devereaux et al6 included only these later-enrolled patients and correlated their high-sensitivity troponin T levels with 30-day mortality rates. Patients with a level 14 ng/L or higher, the upper limit of normal in this study, were also assessed for ischemic symptoms and electrocardiographic changes. Although not required by the study, more than 7,800 patients had their troponin T levels measured before surgery, and the absolute change was also analyzed for an association with the 30-day mortality rate.
Findings. Of the 21,842 patients, about two-thirds underwent some form of major surgery; some of them had more than 1 type. A total of 1.2% of the patients died within 30 days of surgery.
Based on their analysis, the authors proposed that MINS be defined as:
- A postoperative troponin T level of 65 ng/L or higher, or
- A level in the range of 20 ng/L to less than 65 ng/L with an absolute increase from the preoperative level at least 5 ng/L, not attributable to a nonischemic cause.
Seventeen percent of the study patients met these criteria, and of these, 21.7% met the universal definition of myocardial infarction, although only 6.9% had symptoms of it.
Limitations. Only 40.4% of the patients had a preoperative high-sensitivity troponin T measurement for comparison, and in 13.8% of patients who had an elevated perioperative measurement, their preoperative value was the same or higher than their postoperative one. Thus, the incidence of MINS may have been overestimated if patients were otherwise not known to have troponin T elevations before surgery.
[Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation 2018; 137(12):1221–1232.]
Puelacher et al7 investigated the prevalence of MINS in 2,018 patients at increased cardiovascular risk (age ≥ 65, or age ≥ 45 with a history of coronary artery disease, peripheral vascular disease, or stroke) who underwent major noncardiac surgery (planned overnight stay ≥ 24 hours) at a university hospital in Switzerland. Patients had their troponin T measured with a high-sensitivity assay within 30 days before surgery and on postoperative days 1 and 2.
Instead of MINS, the investigators used the term “perioperative myocardial injury” (PMI), defined as an absolute increase in troponin T of at least 14 ng/L from before surgery to the peak postoperative reading. Similar to MINS, PMI did not require ischemic features, but in this study, noncardiac triggers (sepsis, stroke, or pulmonary embolus) were not excluded.
Findings. PMI occurred in 16% of surgeries, and of the patients with PMI, 6% had typical chest pain and 18% had any ischemic symptoms. Unlike in the POISE-2 study discussed above, PMI triggered an automatic referral to a cardiologist.
The unadjusted 30-day mortality rate was 8.9% among patients with PMI and 1.5% in those without. Multivariable logistic regression analysis showed an adjusted hazard ratio for 30-day mortality of 2.7 (95% CI 1.5–4.8) for those with PMI vs without, and this difference persisted for at least 1 year.
In patients with PMI, the authors compared the 30-day mortality rate of those with no ischemic signs or symptoms (71% of the patients) with those who met the criteria for myocardial infarction and found no difference. Patients with PMI triggered by a noncardiac event had a worse prognosis than those with a presumed cardiac etiology.
Limitations. Despite the multivariate analysis that included adjustment for age, nonelective surgery, and Revised Cardiac Risk Index (RCRI), the increased risk associated with PMI could simply reflect higher risk at baseline. Although PMI resulted in automatic referral to a cardiologist, only 10% of patients eventually underwent coronary angiography; a similar percentage were discharged with additional medical therapy such as aspirin, a statin, or a beta-blocker. The effect of these interventions is not known.
Conclusions. MINS is common and has a strong association with mortality risk proportional to the degree of troponin T elevation using high-sensitivity assays, consistent with data from previous studies of earlier assays. Because the mechanism of MINS may differ from that of myocardial infarction, its prevention and treatment may differ, and it remains unclear how serial measurement in postoperative patients should change clinical practice.
The recently published Dabigatran in Patients With Myocardial Injury After Non-cardiac Surgery (MANAGE) trial8 suggests that dabigatran may reduce arterial and venous complications in patients with MINS, but the study had a number of limitations that may restrict the clinical applicability of this finding.
While awaiting further clinical outcomes data, pre- and postoperative troponin T measurement may be beneficial in higher-risk patients (such as those with cardiovascular disease or multiple RCRI risk factors) if the information will change perioperative management.
WHAT IS THE ROLE OF HYPOTENSION OR BLOOD PRESSURE CONTROL?
Intraoperative hypotension is associated with organ ischemia, which may cause postoperative myocardial infarction, myocardial injury, and acute kidney injury.9 Traditional anesthesia practice is to maintain intraoperative blood pressure within 20% of the preoperative baseline, based on the notion that hypertensive patients require higher perfusion pressures.
[Futier E, Lefrant J-Y, Guinot P-G, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA 2017; 318(14):1346–1357.]
Futier et al10 sought to address uncertainty in intraoperative and immediate postoperative management of systolic blood pressure. In this multicenter, randomized, parallel-group trial, 298 patients at increased risk of postoperative renal complications were randomized to blood pressure management that was either “individualized” (within 10% of resting systolic pressure) or “standard” (≥ 80 mm Hg or ≥ 40% of resting systolic pressure) from induction to 4 hours postoperatively.
Blood pressure was monitored using radial arterial lines and maintained using a combination of intravenous fluids, norepinephrine (the first-line agent for the individualized group), and ephedrine (in the standard treatment group only). The primary outcome was a composite of systemic inflammatory response syndrome (SIRS) and organ dysfunction affecting at least 1 organ system (cardiovascular, respiratory, renal, hematologic, or neurologic).
Findings. Data on the primary outcome were available for 292 of 298 patients enrolled. The mean age was 70 years, 15% were women, and 82% had previously diagnosed hypertension. Despite the requirement for an elevated risk of acute kidney injury, only 13% of the patients had a baseline estimated glomerular filtration rate of less than 60 mL/min/1.73 m2, and the median was 88 mL/min/1.73 m2. Ninety-five percent of patients underwent abdominal surgery, and 50% of the surgeries were elective.
The mean systolic blood pressure was 123 mm Hg in the individualized treatment group compared with 116 mm Hg in the standard treatment group. Despite this small difference, 96% of individualized treatment patients received norepinephrine, compared with 26% in the standard treatment group.
The primary outcome of SIRS with organ dysfunction occurred in 38.1% of patients in the individualized treatment group and 51.7% of those in the standard treatment group. After adjusting for center, surgical urgency, surgical site, and acute kidney injury risk index, the relative risk of developing SIRS in those receiving individualized management was 0.73 (P = .02). Renal dysfunction (based on Acute Dialysis Quality Initiative criteria11) occurred in 32.7% of individualized treatment patients and 49% of standardized treatment patients.
Limitations of this study included differences in pharmacologic approach to maintain blood pressure in the 2 protocols (ephedrine and fluids vs norepinephrine) and a modest sample size.
Conclusions. Despite this, the difference in organ dysfunction was striking, with a number needed to treat of only 7 patients. This intervention extended 4 hours postoperatively, a time when many of these patients have left the postanesthesia care unit and have returned to hospitalist care on inpatient wards.
While optimal management of intraoperative and immediate postoperative blood pressure may not be settled, this study suggests that even mild relative hypotension may justify immediate action. Further studies may be useful to delineate high- and low-risk populations, the timing of greatest risk, and indications for intraarterial blood pressure monitoring.
[Salmasi V, Maheswari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology 2017; 126(1):47–65.]
This retrospective cohort study12 assessed the association between myocardial or kidney injury and absolute or relative thresholds of intraoperative mean arterial pressure. It included 57,315 adults who underwent inpatient noncardiac surgery, had a preoperative and at least 1 postoperative serum creatinine measurement within 7 days, and had blood pressure recorded in preoperative appointments within 6 months. Patients with chronic kidney disease (glomerular filtration rate < 60 mL/min/1.73 m2) and those on dialysis were excluded. The outcomes were MINS5 and acute kidney injury as defined by the Acute Kidney Injury Network.9
Findings. A mean arterial pressure below an absolute threshold of 65 mm Hg or a relative threshold of 20% lower than baseline value was associated with myocardial and kidney injury. At each threshold, prolonged periods of hypotension were associated with progressively increased risk.
An important conclusion of the study was that relative thresholds of mean arterial pressure were not any more predictive than absolute thresholds. Absolute thresholds are easier to use intraoperatively, especially when baseline values are not available. The authors did not find a clinically significant interaction between baseline blood pressure and the association of hypotension and myocardial and kidney injury.
Limitations included use of cardiac enzymes postoperatively to define MINS. Since these were not routinely collected, clinically silent myocardial injury may have been missed. Baseline blood pressure may have important implications in other forms of organ injury (ie, cerebral ischemia) that were not studied.
Summary. The lowest absolute mean arterial pressure is as predictive of postoperative myocardial and kidney injury as the relative pressure reduction, at least in patients with normal renal function. Limiting exposure to intraoperative hypotension is important. Baseline blood pressure values may have limited utility for intraoperative management.
In combination, these studies confirm that intraoperative hypotension is a predictor of postoperative organ dysfunction, but the definition and management remain unclear. While aggressive intraoperative management is likely beneficial, how to manage the antihypertensive therapy the patient has been taking as an outpatient when he or she comes into the hospital for surgery remains uncertain.
DOES PATENT FORAMEN OVALE INCREASE THE RISK OF STROKE?
Perioperative stroke is an uncommon, severe complication of noncardiac surgery. The pathophysiology has been better defined in cardiac than in noncardiac surgeries. In nonsurgical patients, patent foramen ovale (PFO) is associated with stroke, even in patients considered to be at low risk.13 Perioperative patients have additional risk for venous thromboembolism and may have periprocedural antithrombotic medications altered, increasing their risk of paradoxical embolism through the PFO.
[Ng PY, Ng AK, Subramaniam B, et al. Association of preoperatively diagnosed patent foramen ovale with perioperative ischemic stroke. JAMA 2018; 319(5):452–462.]
This retrospective cohort study of noncardiac surgery patients at 3 hospitals14 sought to determine the association of preoperatively diagnosed PFO with the risk of perioperative ischemic stroke identified by International Classification of Diseases diagnoses.
Of 150,198 patients, 1.0% had a preoperative diagnosis of PFO, and at baseline, those with PFO had significantly more comorbidities than those without PFO. Stroke occurred in 3.2% of patients with PFO vs 0.5% of those without. Patients known to have a PFO were much more likely to have cardiovascular and thromboembolic risk factors for stroke. In the adjusted analysis, the absolute risk difference between groups was 0.4% (95% CI 0.2–0.6%), with an estimated perioperative stroke risk of 5.9 per 1,000 in patients with known patent foramen ovale and 2.2 per 1,000 in those without. A diagnosis of PFO was also associated with increased risk of large-vessel-territory stroke and more severe neurologic deficit.
Further attempts to adjust for baseline risk factors and other potential bias, including a propensity score-matched cohort analysis and an analysis limited to patients who had echocardiography performed in the same healthcare system, still showed a higher risk of perioperative stroke among patients with preoperatively detected patent foramen ovale.
Limitations. The study was retrospective and observational, used administrative data, and had a low rate of PFO diagnosis (1%), compared with about 25% in population-based studies.15 Indications for preoperative echocardiography are unknown. In addition, the study specifically examined preoperatively diagnosed PFO, rather than including those diagnosed in the postoperative period.
Discussion. How does this study affect clinical practice? The absolute stroke risk was increased by 0.4% in patients with PFO compared with those without. Although this is a relatively small increase, millions of patients undergo noncardiac surgery annually. The risks of therapeutic anticoagulation or PFO closure are likely too high in this context; however, clinicians may approach the perioperative management of antiplatelet agents and venous thromboembolism prophylaxis in patients with known PFO with additional caution.
HOW DOES TIMING OF EMERGENCY SURGERY AFTER PRIOR STROKE AFFECT OUTCOMES?
A history of stroke or transient ischemic attack is a known risk factor for perioperative vascular complications. A recent large cohort study demonstrated that a history of stroke within 9 months of elective surgery was associated with increased adverse outcomes.16 Little is known, however, of the perioperative risk in patients with a history of stroke who undergo emergency surgery.
[Christiansen MN, Andersson C, Gislason GH, et al. Risks of cardiovascular adverse events and death in patients with previous stroke undergoing emergency noncardiac, nonintracranial surgery: the importance of operative timing. Anesthesiology 2017; 127(1):9–19.]
In this study,17 all emergency noncardiac and nonintracranial surgeries from 2005 to 2011 were analyzed using multiple national patient registries in Denmark according to time elapsed between previous stroke and surgery. Primary outcomes were 30-day all-cause mortality and 30-day major adverse cardiac events (MACE), defined as nonfatal ischemic stroke, nonfatal myocardial infarction, and cardiovascular death. Statistical analysis to assess the risk of adverse outcomes included logistic regression models, spline analyses, and propensity-score matching.
Findings. The authors identified 146,694 emergency surgeries, with 7,861 patients (5.4%) having had a previous stroke (transient ischemic attacks and hemorrhagic strokes were not included). Rates of postoperative stroke were as follows:
- 9.9% in patents with a history of ischemic stroke within 3 months of surgery
- 2.8% in patients with a history of stroke 3 to 9 months before surgery
- 0.3% in patients with no previous stroke.
The risk plateaued when the time between stroke and surgery exceeded 4 to 5 months.15
Interestingly, in patients who underwent emergency surgery within 14 days of stroke, the risk of MACE was significantly lower immediately after surgery (1–3 days after stroke) compared with surgery that took place 4 to 14 days after stroke. The authors hypothesized that because cerebral autoregulation does not become compromised until approximately 5 days after a stroke, the risk was lower 1 to 3 days after surgery and increased thereafter.
Limitations of this study included the possibility of residual confounding, given its retrospective design using administrative data, not accounting for preoperative antithrombotic and anticoagulation therapy, and lack of information regarding the etiology of recurrent stroke (eg, thromboembolic, atherothrombotic, hypoperfusion).
Conclusions. Although it would be impractical to postpone emergency surgery in a patient who recently had a stroke, this study shows that the incidence rates of postoperative recurrent stroke and MACE are high. Therefore, it is important that the patient and perioperative team be aware of the risk. Further research is needed to confirm these estimates of postoperative adverse events in more diverse patient populations.
A plethora of studies are under way in the field of perioperative medicine. As a result, evidence-based care of surgical patients is evolving at an exponential rate.
We performed a literature search and, using consensus, identified recent articles we believe will have a great impact on perioperative cardiovascular medicine. These articles report studies that were presented at national meetings in 2018, including the Perioperative Medicine Summit, Society of General Internal Medicine, and Society of Hospital Medicine. These articles are grouped under 5 questions that will help guide clinical practice in perioperative cardiovascular medicine.
SHOULD ASPIRIN BE CONTINUED PERIOPERATIVELY IN PATIENTS WITH A CORONARY STENT?
The Perioperative Ischemic Evaluation 2 (POISE-2) trial1 found that giving aspirin before surgery and throughout the early postoperative period had no significant effect on the rate of a composite of death or nonfatal myocardial infarction; moreover, aspirin increased the risk of major bleeding. However, many experts felt uncomfortable stopping aspirin preoperatively in patients taking it for secondary prophylaxis, particularly patients with a coronary stent.
[Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244.]
This post hoc subgroup analysis2 of POISE-2 evaluated the benefit and harm of perioperative aspirin in patients who had previously undergone percutaneous coronary intervention, more than 90% of whom had received a stent. Patients were age 45 or older with atherosclerotic heart disease or risk factors for it who had previously undergone percutaneous coronary intervention and were now undergoing noncardiac surgery.
Patients who had received a bare-metal stent within the previous 6 weeks or a drug-eluting stent within 12 months before surgery were excluded because guidelines at that time said to continue dual antiplatelet therapy for that long. Recommendations have since changed; the optimal duration for dual antiplatelet therapy with drug-eluting stents is now 6 months. Second-generation drug-eluting stents pose a lower risk of stent thrombosis and require a shorter duration of dual antiplatelet therapy than first-generation drug-eluting stents. Approximately 25% of the percutaneous coronary intervention subgroup had a drug-eluting stent, but the authors did not specify the type of drug-eluting stent.
The post hoc analysis2 included a subgroup of 234 of 4,998 patients receiving aspirin and 236 of 5,012 patients receiving placebo initiated within 4 hours before surgery and continued postoperatively. The primary outcome measured was the rate of death or nonfatal myocardial infarction within 30 days after surgery, and bleeding was a secondary outcome.
Findings. Although the overall POISE-2 study found no benefit from aspirin, in the subgroup who had previously undergone percutaneous coronary intervention, aspirin significantly reduced the risk of the primary outcome, which occurred in 6% vs 11.5% of the patients:
- Absolute risk reduction 5.5% (95% confidence interval 0.4%–10.5%)
- Hazard ratio 0.50 (0.26–0.95).
The reduction was primarily due to fewer myocardial infarctions:
- Absolute risk reduction 5.9% (1.0%–10.8%)
- Hazard ratio 0.44 (0.22–0.87).
The type of stent had no effect on the primary outcome, although this subgroup analysis had limited power. In the nonpercutaneous coronary intervention subgroup, there was no significant difference in outcomes between the aspirin and placebo groups. This subgroup analysis was underpowered to evaluate the effect of aspirin on the composite of major and life-threatening bleeding in patients with prior percutaneous coronary intervention, which was reported as “uncertain” due to wide confidence intervals (absolute risk increase 1.3%, 95% confidence interval –2.6% to 5.2%), but the increased risk of major or life-threatening bleeding with aspirin demonstrated in the overall POISE-2 study population likely applies:
- Absolute risk increase 0.8% (0.1%–1.6%)
- Hazard ratio 1.22 (1.01–1.48).
Limitations. This was a nonspecified subgroup analysis that was underpowered and had a relatively small sample size with few events.
Conclusion. In the absence of a very high bleeding risk, continuing aspirin perioperatively in patients with prior percutaneous coronary intervention undergoing noncardiac surgery is more likely to result in benefit than harm. This finding is in agreement with current recommendations from the American College Cardiology and American Heart Association (class I; level of evidence C).3
WHAT IS THE INCIDENCE OF MINS? IS MEASURING TROPONIN USEFUL?
Despite advances in anesthesia and surgical techniques, about 1% of patients over age 45 die within 30 days of noncardiac surgery.4 Studies have demonstrated a high mortality rate in patients who experience myocardial injury after noncardiac surgery (MINS), defined as elevations of troponin T with or without ischemic symptoms or electrocardiographic changes.5 Most of these studies used earlier, “non-high-sensitivity” troponin T assays. Fifth-generation, highly sensitive troponin T assays are now available that can detect troponin T at lower concentrations, but their utility in predicting postoperative outcomes remains uncertain. Two recent studies provide further insight into these issues.
[Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, Sigamani A, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017; 317(16):1642–1651.]
The Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study5 was an international, prospective cohort study that initially evaluated the association between MINS and the 30-day mortality rate using a non-high-sensitivity troponin T assay (Roche fourth-generation Elecsys TnT assay) in patients age 45 or older undergoing noncardiac surgery and requiring hospital admission for at least 1 night. After the first 15,000 patients, the study switched to the Roche fifth-generation assay, with measurements at 6 to 12 hours after surgery and on postoperative days 1, 2, and 3.
A 2017 analysis by Devereaux et al6 included only these later-enrolled patients and correlated their high-sensitivity troponin T levels with 30-day mortality rates. Patients with a level 14 ng/L or higher, the upper limit of normal in this study, were also assessed for ischemic symptoms and electrocardiographic changes. Although not required by the study, more than 7,800 patients had their troponin T levels measured before surgery, and the absolute change was also analyzed for an association with the 30-day mortality rate.
Findings. Of the 21,842 patients, about two-thirds underwent some form of major surgery; some of them had more than 1 type. A total of 1.2% of the patients died within 30 days of surgery.
Based on their analysis, the authors proposed that MINS be defined as:
- A postoperative troponin T level of 65 ng/L or higher, or
- A level in the range of 20 ng/L to less than 65 ng/L with an absolute increase from the preoperative level at least 5 ng/L, not attributable to a nonischemic cause.
Seventeen percent of the study patients met these criteria, and of these, 21.7% met the universal definition of myocardial infarction, although only 6.9% had symptoms of it.
Limitations. Only 40.4% of the patients had a preoperative high-sensitivity troponin T measurement for comparison, and in 13.8% of patients who had an elevated perioperative measurement, their preoperative value was the same or higher than their postoperative one. Thus, the incidence of MINS may have been overestimated if patients were otherwise not known to have troponin T elevations before surgery.
[Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation 2018; 137(12):1221–1232.]
Puelacher et al7 investigated the prevalence of MINS in 2,018 patients at increased cardiovascular risk (age ≥ 65, or age ≥ 45 with a history of coronary artery disease, peripheral vascular disease, or stroke) who underwent major noncardiac surgery (planned overnight stay ≥ 24 hours) at a university hospital in Switzerland. Patients had their troponin T measured with a high-sensitivity assay within 30 days before surgery and on postoperative days 1 and 2.
Instead of MINS, the investigators used the term “perioperative myocardial injury” (PMI), defined as an absolute increase in troponin T of at least 14 ng/L from before surgery to the peak postoperative reading. Similar to MINS, PMI did not require ischemic features, but in this study, noncardiac triggers (sepsis, stroke, or pulmonary embolus) were not excluded.
Findings. PMI occurred in 16% of surgeries, and of the patients with PMI, 6% had typical chest pain and 18% had any ischemic symptoms. Unlike in the POISE-2 study discussed above, PMI triggered an automatic referral to a cardiologist.
The unadjusted 30-day mortality rate was 8.9% among patients with PMI and 1.5% in those without. Multivariable logistic regression analysis showed an adjusted hazard ratio for 30-day mortality of 2.7 (95% CI 1.5–4.8) for those with PMI vs without, and this difference persisted for at least 1 year.
In patients with PMI, the authors compared the 30-day mortality rate of those with no ischemic signs or symptoms (71% of the patients) with those who met the criteria for myocardial infarction and found no difference. Patients with PMI triggered by a noncardiac event had a worse prognosis than those with a presumed cardiac etiology.
Limitations. Despite the multivariate analysis that included adjustment for age, nonelective surgery, and Revised Cardiac Risk Index (RCRI), the increased risk associated with PMI could simply reflect higher risk at baseline. Although PMI resulted in automatic referral to a cardiologist, only 10% of patients eventually underwent coronary angiography; a similar percentage were discharged with additional medical therapy such as aspirin, a statin, or a beta-blocker. The effect of these interventions is not known.
Conclusions. MINS is common and has a strong association with mortality risk proportional to the degree of troponin T elevation using high-sensitivity assays, consistent with data from previous studies of earlier assays. Because the mechanism of MINS may differ from that of myocardial infarction, its prevention and treatment may differ, and it remains unclear how serial measurement in postoperative patients should change clinical practice.
The recently published Dabigatran in Patients With Myocardial Injury After Non-cardiac Surgery (MANAGE) trial8 suggests that dabigatran may reduce arterial and venous complications in patients with MINS, but the study had a number of limitations that may restrict the clinical applicability of this finding.
While awaiting further clinical outcomes data, pre- and postoperative troponin T measurement may be beneficial in higher-risk patients (such as those with cardiovascular disease or multiple RCRI risk factors) if the information will change perioperative management.
WHAT IS THE ROLE OF HYPOTENSION OR BLOOD PRESSURE CONTROL?
Intraoperative hypotension is associated with organ ischemia, which may cause postoperative myocardial infarction, myocardial injury, and acute kidney injury.9 Traditional anesthesia practice is to maintain intraoperative blood pressure within 20% of the preoperative baseline, based on the notion that hypertensive patients require higher perfusion pressures.
[Futier E, Lefrant J-Y, Guinot P-G, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA 2017; 318(14):1346–1357.]
Futier et al10 sought to address uncertainty in intraoperative and immediate postoperative management of systolic blood pressure. In this multicenter, randomized, parallel-group trial, 298 patients at increased risk of postoperative renal complications were randomized to blood pressure management that was either “individualized” (within 10% of resting systolic pressure) or “standard” (≥ 80 mm Hg or ≥ 40% of resting systolic pressure) from induction to 4 hours postoperatively.
Blood pressure was monitored using radial arterial lines and maintained using a combination of intravenous fluids, norepinephrine (the first-line agent for the individualized group), and ephedrine (in the standard treatment group only). The primary outcome was a composite of systemic inflammatory response syndrome (SIRS) and organ dysfunction affecting at least 1 organ system (cardiovascular, respiratory, renal, hematologic, or neurologic).
Findings. Data on the primary outcome were available for 292 of 298 patients enrolled. The mean age was 70 years, 15% were women, and 82% had previously diagnosed hypertension. Despite the requirement for an elevated risk of acute kidney injury, only 13% of the patients had a baseline estimated glomerular filtration rate of less than 60 mL/min/1.73 m2, and the median was 88 mL/min/1.73 m2. Ninety-five percent of patients underwent abdominal surgery, and 50% of the surgeries were elective.
The mean systolic blood pressure was 123 mm Hg in the individualized treatment group compared with 116 mm Hg in the standard treatment group. Despite this small difference, 96% of individualized treatment patients received norepinephrine, compared with 26% in the standard treatment group.
The primary outcome of SIRS with organ dysfunction occurred in 38.1% of patients in the individualized treatment group and 51.7% of those in the standard treatment group. After adjusting for center, surgical urgency, surgical site, and acute kidney injury risk index, the relative risk of developing SIRS in those receiving individualized management was 0.73 (P = .02). Renal dysfunction (based on Acute Dialysis Quality Initiative criteria11) occurred in 32.7% of individualized treatment patients and 49% of standardized treatment patients.
Limitations of this study included differences in pharmacologic approach to maintain blood pressure in the 2 protocols (ephedrine and fluids vs norepinephrine) and a modest sample size.
Conclusions. Despite this, the difference in organ dysfunction was striking, with a number needed to treat of only 7 patients. This intervention extended 4 hours postoperatively, a time when many of these patients have left the postanesthesia care unit and have returned to hospitalist care on inpatient wards.
While optimal management of intraoperative and immediate postoperative blood pressure may not be settled, this study suggests that even mild relative hypotension may justify immediate action. Further studies may be useful to delineate high- and low-risk populations, the timing of greatest risk, and indications for intraarterial blood pressure monitoring.
[Salmasi V, Maheswari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology 2017; 126(1):47–65.]
This retrospective cohort study12 assessed the association between myocardial or kidney injury and absolute or relative thresholds of intraoperative mean arterial pressure. It included 57,315 adults who underwent inpatient noncardiac surgery, had a preoperative and at least 1 postoperative serum creatinine measurement within 7 days, and had blood pressure recorded in preoperative appointments within 6 months. Patients with chronic kidney disease (glomerular filtration rate < 60 mL/min/1.73 m2) and those on dialysis were excluded. The outcomes were MINS5 and acute kidney injury as defined by the Acute Kidney Injury Network.9
Findings. A mean arterial pressure below an absolute threshold of 65 mm Hg or a relative threshold of 20% lower than baseline value was associated with myocardial and kidney injury. At each threshold, prolonged periods of hypotension were associated with progressively increased risk.
An important conclusion of the study was that relative thresholds of mean arterial pressure were not any more predictive than absolute thresholds. Absolute thresholds are easier to use intraoperatively, especially when baseline values are not available. The authors did not find a clinically significant interaction between baseline blood pressure and the association of hypotension and myocardial and kidney injury.
Limitations included use of cardiac enzymes postoperatively to define MINS. Since these were not routinely collected, clinically silent myocardial injury may have been missed. Baseline blood pressure may have important implications in other forms of organ injury (ie, cerebral ischemia) that were not studied.
Summary. The lowest absolute mean arterial pressure is as predictive of postoperative myocardial and kidney injury as the relative pressure reduction, at least in patients with normal renal function. Limiting exposure to intraoperative hypotension is important. Baseline blood pressure values may have limited utility for intraoperative management.
In combination, these studies confirm that intraoperative hypotension is a predictor of postoperative organ dysfunction, but the definition and management remain unclear. While aggressive intraoperative management is likely beneficial, how to manage the antihypertensive therapy the patient has been taking as an outpatient when he or she comes into the hospital for surgery remains uncertain.
DOES PATENT FORAMEN OVALE INCREASE THE RISK OF STROKE?
Perioperative stroke is an uncommon, severe complication of noncardiac surgery. The pathophysiology has been better defined in cardiac than in noncardiac surgeries. In nonsurgical patients, patent foramen ovale (PFO) is associated with stroke, even in patients considered to be at low risk.13 Perioperative patients have additional risk for venous thromboembolism and may have periprocedural antithrombotic medications altered, increasing their risk of paradoxical embolism through the PFO.
[Ng PY, Ng AK, Subramaniam B, et al. Association of preoperatively diagnosed patent foramen ovale with perioperative ischemic stroke. JAMA 2018; 319(5):452–462.]
This retrospective cohort study of noncardiac surgery patients at 3 hospitals14 sought to determine the association of preoperatively diagnosed PFO with the risk of perioperative ischemic stroke identified by International Classification of Diseases diagnoses.
Of 150,198 patients, 1.0% had a preoperative diagnosis of PFO, and at baseline, those with PFO had significantly more comorbidities than those without PFO. Stroke occurred in 3.2% of patients with PFO vs 0.5% of those without. Patients known to have a PFO were much more likely to have cardiovascular and thromboembolic risk factors for stroke. In the adjusted analysis, the absolute risk difference between groups was 0.4% (95% CI 0.2–0.6%), with an estimated perioperative stroke risk of 5.9 per 1,000 in patients with known patent foramen ovale and 2.2 per 1,000 in those without. A diagnosis of PFO was also associated with increased risk of large-vessel-territory stroke and more severe neurologic deficit.
Further attempts to adjust for baseline risk factors and other potential bias, including a propensity score-matched cohort analysis and an analysis limited to patients who had echocardiography performed in the same healthcare system, still showed a higher risk of perioperative stroke among patients with preoperatively detected patent foramen ovale.
Limitations. The study was retrospective and observational, used administrative data, and had a low rate of PFO diagnosis (1%), compared with about 25% in population-based studies.15 Indications for preoperative echocardiography are unknown. In addition, the study specifically examined preoperatively diagnosed PFO, rather than including those diagnosed in the postoperative period.
Discussion. How does this study affect clinical practice? The absolute stroke risk was increased by 0.4% in patients with PFO compared with those without. Although this is a relatively small increase, millions of patients undergo noncardiac surgery annually. The risks of therapeutic anticoagulation or PFO closure are likely too high in this context; however, clinicians may approach the perioperative management of antiplatelet agents and venous thromboembolism prophylaxis in patients with known PFO with additional caution.
HOW DOES TIMING OF EMERGENCY SURGERY AFTER PRIOR STROKE AFFECT OUTCOMES?
A history of stroke or transient ischemic attack is a known risk factor for perioperative vascular complications. A recent large cohort study demonstrated that a history of stroke within 9 months of elective surgery was associated with increased adverse outcomes.16 Little is known, however, of the perioperative risk in patients with a history of stroke who undergo emergency surgery.
[Christiansen MN, Andersson C, Gislason GH, et al. Risks of cardiovascular adverse events and death in patients with previous stroke undergoing emergency noncardiac, nonintracranial surgery: the importance of operative timing. Anesthesiology 2017; 127(1):9–19.]
In this study,17 all emergency noncardiac and nonintracranial surgeries from 2005 to 2011 were analyzed using multiple national patient registries in Denmark according to time elapsed between previous stroke and surgery. Primary outcomes were 30-day all-cause mortality and 30-day major adverse cardiac events (MACE), defined as nonfatal ischemic stroke, nonfatal myocardial infarction, and cardiovascular death. Statistical analysis to assess the risk of adverse outcomes included logistic regression models, spline analyses, and propensity-score matching.
Findings. The authors identified 146,694 emergency surgeries, with 7,861 patients (5.4%) having had a previous stroke (transient ischemic attacks and hemorrhagic strokes were not included). Rates of postoperative stroke were as follows:
- 9.9% in patents with a history of ischemic stroke within 3 months of surgery
- 2.8% in patients with a history of stroke 3 to 9 months before surgery
- 0.3% in patients with no previous stroke.
The risk plateaued when the time between stroke and surgery exceeded 4 to 5 months.15
Interestingly, in patients who underwent emergency surgery within 14 days of stroke, the risk of MACE was significantly lower immediately after surgery (1–3 days after stroke) compared with surgery that took place 4 to 14 days after stroke. The authors hypothesized that because cerebral autoregulation does not become compromised until approximately 5 days after a stroke, the risk was lower 1 to 3 days after surgery and increased thereafter.
Limitations of this study included the possibility of residual confounding, given its retrospective design using administrative data, not accounting for preoperative antithrombotic and anticoagulation therapy, and lack of information regarding the etiology of recurrent stroke (eg, thromboembolic, atherothrombotic, hypoperfusion).
Conclusions. Although it would be impractical to postpone emergency surgery in a patient who recently had a stroke, this study shows that the incidence rates of postoperative recurrent stroke and MACE are high. Therefore, it is important that the patient and perioperative team be aware of the risk. Further research is needed to confirm these estimates of postoperative adverse events in more diverse patient populations.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244. doi:10.7326/M17-2341
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 130(24):2215–2245. doi:10.1161/CIR.0000000000000105
- Smilowitz NR, Gupta N, Ramakrishna H, Guo Y, Berger JS, Bangalore S. Perioperative major adverse cardiovascular and cerebrovascular events associated with noncardiac surgery. JAMA Cardiol 2017; 2(2):181–187. doi:10.1001/jamacardio.2016.4792
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120(3):564–578. doi:10.1097/ALN.0000000000000113
- Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, Sigamani A, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017; 317(16):1642–1651. doi:10.1001/jama.2017.4360
- Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation 2018; 137(12):1221–1232. doi:10.1161/CIRCULATIONAHA.117.030114
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8
- Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology 2013; 119(3):507–515. doi:10.1097/ALN.0b013e3182a10e26
- Futier E, Lefrant JY, Guinot PG, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA 2017; 318(14):1346–1357. doi:10.1001/jama.2017.14172
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) group. Crit Care 2004; 8:R204. doi:10.1186/cc2872
- Salmasi V, Maheswari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology 2017; 126(1):47–65. doi:10.1097/ALN.0000000000001432
- Lechat P, Mas JL, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med 1988; 318(18):1148–1152. doi:10.1056/NEJM198805053181802
- Ng PY, Ng AK, Subramaniam B, et al. Association of preoperatively diagnosed patent foramen ovale with perioperative ischemic stroke. JAMA 2018; 319(5):452–462. doi:10.1001/jama.2017.21899
- Meissner I, Whisnant JP, Khandheria BK, et al. Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC study. Stroke Prevention: Assessment of Risk in a Community. Mayo Clin Proc 1999; 74(9):862–869. pmid:10488786
- Jørgensen ME, Torp-Pedersen C, Gislason GH, et al. Time elapsed after ischemic stroke and risk of adverse cardiovascular events and mortality following elective noncardiac surgery. JAMA 2014; 312:269–277. doi:10.1001/jama.2014.8165
- Christiansen MN, Andersson C, Gislason GH, et al. Risks of cardiovascular adverse events and death in patients with previous stroke undergoing emergency noncardiac, nonintracranial surgery: the importance of operative timing. Anesthesiology 2017; 127(1):9–19. doi:10.1097/ALN.0000000000001685
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244. doi:10.7326/M17-2341
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 130(24):2215–2245. doi:10.1161/CIR.0000000000000105
- Smilowitz NR, Gupta N, Ramakrishna H, Guo Y, Berger JS, Bangalore S. Perioperative major adverse cardiovascular and cerebrovascular events associated with noncardiac surgery. JAMA Cardiol 2017; 2(2):181–187. doi:10.1001/jamacardio.2016.4792
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120(3):564–578. doi:10.1097/ALN.0000000000000113
- Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, Sigamani A, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017; 317(16):1642–1651. doi:10.1001/jama.2017.4360
- Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation 2018; 137(12):1221–1232. doi:10.1161/CIRCULATIONAHA.117.030114
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8
- Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology 2013; 119(3):507–515. doi:10.1097/ALN.0b013e3182a10e26
- Futier E, Lefrant JY, Guinot PG, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA 2017; 318(14):1346–1357. doi:10.1001/jama.2017.14172
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) group. Crit Care 2004; 8:R204. doi:10.1186/cc2872
- Salmasi V, Maheswari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology 2017; 126(1):47–65. doi:10.1097/ALN.0000000000001432
- Lechat P, Mas JL, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med 1988; 318(18):1148–1152. doi:10.1056/NEJM198805053181802
- Ng PY, Ng AK, Subramaniam B, et al. Association of preoperatively diagnosed patent foramen ovale with perioperative ischemic stroke. JAMA 2018; 319(5):452–462. doi:10.1001/jama.2017.21899
- Meissner I, Whisnant JP, Khandheria BK, et al. Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC study. Stroke Prevention: Assessment of Risk in a Community. Mayo Clin Proc 1999; 74(9):862–869. pmid:10488786
- Jørgensen ME, Torp-Pedersen C, Gislason GH, et al. Time elapsed after ischemic stroke and risk of adverse cardiovascular events and mortality following elective noncardiac surgery. JAMA 2014; 312:269–277. doi:10.1001/jama.2014.8165
- Christiansen MN, Andersson C, Gislason GH, et al. Risks of cardiovascular adverse events and death in patients with previous stroke undergoing emergency noncardiac, nonintracranial surgery: the importance of operative timing. Anesthesiology 2017; 127(1):9–19. doi:10.1097/ALN.0000000000001685
KEY POINTS
- Patients undergoing noncardiac surgery who have a history of percutaneous coronary intervention will benefit from continuing aspirin perioperatively if they are not at very high risk of bleeding.
- Myocardial injury after noncardiac surgery is strongly associated with a risk of death, and the higher the troponin level, the higher the risk. Measuring troponin T before and after surgery may be beneficial in patients at high risk if the information leads to a change in management.
- Perioperative hypotension can lead to end-organ dysfunction postoperatively. There is conflicting evidence whether the absolute or relative reduction in blood pressure is more predictive.
- Perioperative risk of stroke is higher in patients with patent foramen ovale than in those without.
- Many patients who recently had a stroke suffer recurrent stroke and major adverse cardiac events if they undergo emergency surgery.
Men’s health 2018: BPH, prostate cancer, erectile dysfunction, supplements
Primary care physicians are tasked with a wide variety of issues affecting men. This article reviews the latest research in 4 areas of men’s health commonly addressed in primary care:
- Medical management of benign prostatic hyperplasia (BPH)
- Prostate cancer screening and treatment
- Medical management of erectile dysfunction
- Use of supplements.
MEDICAL MANAGEMENT OF BPH
An 84-year-old man with a history of hypertension, type 2 diabetes, hyperlipidemia, BPH, mild cognitive impairment, and osteoarthritis presents for a 6-month follow-up, accompanied by his son.
Two years ago he was started on a 5-alpha reductase inhibitor and an alpha-blocker for worsening BPH symptoms. His BPH symptoms are currently under control, with an American Urological Association (AUA) symptom index score of 7 of a possible 35 (higher scores being worse).
However, both the patient and son are concerned about the number of medications he is on and wonder if some could be eliminated.
Assessment tools
BPH is a common cause of lower urinary tract symptoms in older men. Evidence-based tools to help the clinician and patient decide on when to consider treatment for symptoms are:
- The AUA symptom index1
- The International Prostate Symptom Score (IPSS).2
An AUA symptom index score or IPSS score of 8 through 19 of a possible 35 is consistent with moderate symptoms, while a score of 20 or higher indicates severe symptoms.
Combination therapy or monotherapy?
Monotherapy with an alpha-blocker or a 5-alpha reductase inhibitor is often the first-line treatment for BPH-related lower urinary tract symptoms.3 However, combination therapy with both an alpha-blocker and a 5-alpha reductase inhibitor is another evidence-based option.
The Medical Therapy of Prostatic Symptoms study,4 a randomized controlled trial, reported that long-term combination therapy reduced the risk of BPH clinical progression better than monotherapy. The same trial also found that either combination therapy or finasteride alone (a 5-alpha reductase inhibitor) reduced the risk of acute urinary retention and the future need for invasive therapy.
Monotherapy after a period of combination therapy?
There is also evidence to support switching from combination to monotherapy after an initial treatment period.
Matsukawa et al5 examined the effects of withdrawing the alpha-blocker from BPH combination therapy in a study in 140 patients. For 12 months, all patients received the alpha-blocker silodosin and the 5-alpha reductase inhibitor dutasteride. At 12 months, the remaining 132 patients (8 patients had been lost to follow-up) were randomized to continue combination therapy or to take dutasteride alone for another 12 months. They were evaluated at 0, 12, and 24 months by questionnaires (the IPSS and Overactive Bladder Symptom Score) and urodynamic testing (uroflowmetry, cystometrography, and pressure-flow studies).
There were no significant differences in subjective symptoms and bladder outlet obstruction between patients who continued combination therapy and those who switched to dutasteride monotherapy. In the monotherapy group, those whose symptoms worsened weighed more (68.8 kg vs 62.6 kg, P =.002) and had a higher body mass index (BMI) (26.2 kg/m2 vs 22.8 kg/m2, P < .001) than those whose symptoms stayed the same or got better.
These findings of successful alpha-blocker withdrawal were consistent with those of other studies.
The Symptom Management After Reducing Therapy study6 showed that 80% of men with an IPSS score less than 20 who changed to dutasteride monotherapy did not have a noticeable worsening of their symptoms.
Baldwin et al7 noted similar success after withdrawing the alpha-blocker doxazosin in patients on finasteride.
Review all medications
The National Health and Nutrition Examination Survey noted that the estimated prevalence of polypharmacy increased from 8% in 1999 to 15% in 2011.8 Many commonly used medications, such as decongestants, antihistamines, and anticholinergic agents, can worsen BPH symptoms,9 so it is reasonable to consistently review the patient’s medications to weigh the risks and benefits and determine which ones align with the patient’s personal care goals.
BPH: Take-home points
- Combination therapy with an alpha-blocker and a 5-alpha reductase inhibitor is an effective regimen for BPH.
- Polypharmacy is a significant problem in the elderly.
- Withdrawing the alpha-blocker component from BPH combination therapy can be considered after 1 year of combination therapy in patients whose symptoms have been well controlled.
PROSTATE CANCER SCREENING AND TREATMENT
A 60-year-old patient calls you after receiving his laboratory testing report from his insurance physical. His prostate-specific antigen (PSA) level is 5.1 ng/mL, and he has several questions:
- Should he have agreed to the screening?
- How effective is the screening?
- What are the next steps?
Is PSA screening useful?
Over the last few years, there has been great debate as to the utility of screening for prostate cancer.
The US Centers for Disease Control and Prevention10 reported that in 2014, an estimated 172,258 men in the United States were diagnosed with prostate cancer, but only 28,343 men died of it. These statistics support the notion that screening programs may be detecting what might otherwise be a silent disease.
The US Preventive Services Task Force (USPSTF)11 recommends against blanket PSA screening, in view of the low probability that it reduces the risk of death from prostate cancer. For men ages 55 through 69, current guidelines give a grade C recommendation to PSA screening, meaning there is moderate agreement that the benefit is likely small, and screening should be selectively offered based on professional judgment and patient preference. In men ages 70 and older who are not at high risk, the guideline gives screening a grade D recommendation, meaning there is moderate evidence that there is no benefit from the practice. This is a change from the 2012 USPSTF guidelines,12 which gave a grade D recommendation to PSA screening for all ages.
The American Urological Association13 recommends against PSA screening in men under age 40 or ages 70 and older. It does not recommend routine screening in those ages 40 to 54 at average risk, but it says the decision should be individualized in this age group in those at higher risk (eg, with a positive family history, African American). At ages 55 through 69, it recommends shared decision-making, taking into account cancer risk and life expectancy. In those who opt for screening, an interval of 2 years or more may be preferred over annual screening to reduce the risk of overdiagnosis.
The USPSTF recommendations rely heavily on data from 2 trials: the European Randomized Study of Screening for Prostate Cancer (ERSPC)14 and the Prostate, Lung, Colorectal, and Ovarian Screening (PLCO) trial.15
The ERSPC14 demonstrated that screening for prostate cancer reduced deaths from prostate cancer by 20%, with an absolute risk difference of 0.71 deaths per 1,000 men; 1,410 men would need to be screened and 48 additional cases of prostate cancer would need to be treated to prevent 1 death from prostate cancer. Screening also decreased the risk of developing metastatic disease by 30%.16 On the negative side, screening increased the risk of overdiagnosis and other harms such as bleeding, sepsis, and incontinence.
The PLCO trial,15 in contrast, found no difference in death rates between men randomly assigned to annual screening and those assigned to usual care. Differences between the trial results were thought to be due to different practice settings as well as study implementation and compliance.
Tsodikov et al17 reanalyzed data from the ERSPC and the PLCO trial using 3 different mathematical models to estimate the effects of screening in both trials compared with no screening. The analysis found no evidence that the effects of screening vs not screening differed between the 2 trials, ultimately concluding that PSA screening reduced prostate cancer deaths by 25% to 32%, which the authors inferred was primarily a result of earlier detection of cancer.
The Cluster Randomized Trial of PSA Testing for Prostate Cancer,18 published in March 2018, explored the effect of single PSA screening vs no screening on prostate cancer mortality rates in 419,582 men ages 50 through 69. Although screening detected more cases of low-risk prostate cancer, there was no significant difference in prostate cancer mortality rates after a median follow-up of 10 years. However, 10% to 15% of the control group was estimated to have also been screened, and these results do not directly speak to the efficacy of serial PSA screening.
Extended follow-up of this trial is planned to report on long-term survival benefits and whether screening lowers the risk of metastasis.
Imaging-guided prostate biopsy
Once a patient is found to have an elevated PSA level, standard practice has been to perform transrectal ultrasonography to obtain 12 core biopsy samples. The results indicate whether the prostate contains cancer, how aggressive the cancer is (Gleason score), and whether there is extracapsular extension.
In the past, magnetic resonance imaging (MRI) of the prostate before biopsy was thought to be too costly, and many insurance plans do not currently cover it.
Pahwa et al,19 however, in a cost-effectiveness study using a decision-analysis model, found that using MRI to detect lesions and then guide biopsy by triaging patients into proper treatment pathways added health benefits in a cost-effective manner in 94.05% of simulations. These benefits were found across all age groups.
This study demonstrated that doctors could use MRI to better evaluate patients for potentially harmful lesions. If a focus of cancer is found, it can be biopsied; if no cancer is seen on MRI, the patient can avoid biopsy completely. Additionally, though MRI tended to miss low-risk cancers, these cancers are thought to disproportionately lead to higher healthcare costs through unnecessary treatment. Therefore, a negative MRI study was believed to be an excellent sign that the patient does not have aggressive prostate cancer. This approach led to a net gain of 0.251 additional quality-adjusted life years compared with the standard biopsy strategy.
The Prostate MRI Imaging Study20 also found MRI to be effective in the prostate cancer workup. In this trial, 576 men who had never undergone biopsy underwent multiparametric MRI, transrectal ultrasonography-guided biopsy, and the reference standard, ie, transperineal template prostate mapping biopsy. Of those who underwent biopsy, 71% received a diagnosis of prostate cancer, and 40% had clinically significant disease. In patients with clinically significant disease, MRI was more sensitive than ultrasonography-guided biopsy (93% vs 48%, P < .0001) but less specific (41% vs 96%, P < .0001).
Based on these findings, if biopsy were performed only in those who had suspicious lesions on MRI, 27% of men with elevated PSA could avoid biopsy and its potential complications such as bleeding and sepsis, which occurred in 5.9% of the biopsy group.
The Prostate Evaluation for Clinically Important Disease: Sampling Using Image Guidance or Not? trial21 more recently studied MRI with or without targeted biopsy vs standard transrectal ultrasonography-guided biopsy in 500 men who had not undergone biopsy before, and reported similar results. MRI with or without biopsy led to fewer biopsies and less overdetection of clinically insignificant prostate cancers compared with the standard approach. Furthermore, those in the MRI-targeted biopsy group were 13% less likely to receive a diagnosis of clinically insignificant cancer than those who received the standard biopsy (adjusted difference −13 percentage points, 95% confidence interval [CI] −19 to −7, P < .001).
Together, these data provide another argument for adding multiparametric MRI to the workup of men with an elevated PSA level.
Surveillance vs treatment for prostate cancer
Once prostate cancer is diagnosed, surveillance is becoming an increasingly common management strategy.
The Prostate Cancer Intervention Versus Observation Trial (PIVOT),22 one of the largest and longest trials involving cancer patients, offered further evidence that active surveillance and less intervention for men with prostate cancer is a better approach in many cases. This trial compared prostatectomy and observation alone in a randomized fashion. Inclusion for the study required men to be medically fit for radical prostatectomy, along with having histologically confirmed localized prostate cancer (stage T1-T2NxM0 in the tumor-node-metastasis classification system) of any grade diagnosed within the last 12 months.
During 19.5 years of follow-up, 223 (61.3%) of the 364 men randomly assigned to radical prostatectomy died, compared with 245 (66.8%) of 367 men in the observation group; the difference was not statistically different (P = .06). Only 9.4% of the deaths were due to prostate cancer, 7.4% in the surgery group and 11.4% in the observation group (P = .06).
Surgery was associated with a lower all-cause mortality rate than observation in the subgroup of patients with intermediate-risk prostate cancer (defined as PSA 10–20 ng/mL and a Gleason score of 7). Surgery was also associated with less disease progression.22
This finding is in line with previous data from the Scandinavian Prostate Cancer Group Study Number 4,23 as well as the much larger Prostate Testing for Cancer and Treatment (ProtecT) trial,24 both of which reported that metastasis was 1.5 and 2.6 times as common, respectively, in participants in the active surveillance groups. However, in the PIVOT trial, those in the surgery group were significantly more likely than those in the observation group to have erectile dysfunction and urinary incontinence at 10 years.
Therefore, in men with localized disease and in those with low-risk PSA levels, both the PIVOT and ProtecT trials suggest that death from prostate cancer is uncommon and that observation may be more appropriate.
Prostate cancer: Take-home points
- A new look at 2 large trials of PSA screening strengthened evidence that testing in the right patient population can reduce deaths from prostate cancer, but a third recently published trial that found no benefit from 1-time screening may reopen debate on the topic.
- MRI offers a better method than ultrasonography-guided biopsy to triage patients thought to be at high risk of prostate cancer and tends to limit costly overtreatment of disease that likely would not cause death.
- Surgery for prostate cancer may not prolong life but could reduce disease progression, at the risk of more adverse effects.
- Shared decision-making should be practiced when deciding whether to use active surveillance or active treatment of diagnosed prostate cancer.
MANAGEMENT OF ERECTILE DYSFUNCTION
A 62-year-old man with hypertension, hyperlipidemia, peripheral artery disease, and type 2 diabetes presents for a 6-month follow-up. His medications include aspirin, metformin, lisinopril, and atorvastatin, all of which he takes without problems. Over the past several months, he has noticed that his erections are not adequate for sexual intercourse. He recently heard that a generic version of sildenafil has just become available, and he wonders if it might benefit him.
Erectile dysfunction is common, associated with chronic diseases
Erectile dysfunction, ie, persistent inability to obtain and maintain an erection sufficient to permit satisfactory sexual intercourse,25,26 is estimated to affect nearly 20% of men over the age of 20 and 75% of men over the age of 75.27
In age-adjusted models, erectile dysfunction has been shown28 to be associated with:
- History of cardiovascular disease (odds ratio [OR] 1.63, 95% CI 1.02–2.63)
- Diabetes (OR 3.90, 95% CI 2.16–7.04)
- Treated hypertension vs no hypertension (OR 2.22, 95% CI 1.30–3.80)
- Current smoking vs never smoking (OR 1.63, 95% CI 1.01–2.62)
- BMI greater than 30 kg/m2 vs less than 25 kg/m2 (OR 1.80, 95% CI 1.03–3.14).
Because of the strong association between cardiovascular disease and erectile dysfunction, the presence of one often suggests the need to screen for the other.29 While tools such as the International Index of Erectile Function (IIEF-5) have been developed to evaluate erectile dysfunction, it is most often diagnosed on the basis of clinical impression, while validated assessment methods are reserved for clinical trials.28
Multiple causes of erectile dysfunction
Erectile dysfunction arises from inadequate penile tissue response to a sexual signal. The response can be disrupted at several points. For example, damage to vascular smooth muscle cells (eg, from age or obesity) and endothelial cells (from smoking or diabetes) and narrowing of the vascular lumen (from atherosclerosis or hypertension) have all been shown to impair engorgement of the corpus cavernosum.30 In addition, denervation from prostate surgery or spinal trauma and psychogenic causes should be recognized in discussions with patients.
Drugs for erectile dysfunction
Pharmacologic management of erectile dysfunction includes oral, sublingual, intracavernosal, and intraurethral therapies.31 Treatment in primary care settings usually includes addressing underlying chronic diseases32 and prescribing phosphodiesterase-5 inhibitors (sildenafil, tadalafil, vardenafil, and avanafil). These drugs work by increasing local concentrations of cyclic guanosine monophosphate in the corpus cavernosum to induce vasodilation.33
While these 4 drugs are still patent-protected, a manufacturer has been allowed to introduce a generic version of sildenafil into US markets, and a generic version of tadalafil is expected to be available soon.
Sildenafil, tadalafil, and vardenafil have been studied and found to have some degree of effectiveness in erectile dysfunction caused by damage to the penile vasculature, denervation, and spinal cord injury.34 All drugs of this class have adverse effects including headache, facial flushing, and nasal congestion, but the drugs are generally well tolerated.35
Sildenafil and tadalafil improve IIEF-5 scores by a similar margin, raising scores on the erectile domain subsection from approximately 14 of a possible 30 to approximately 24 of 30 in a trial of both drugs.36 However, multiple crossover studies comparing the 2 drugs have shown that nearly 75% of patients prefer tadalafil to sildenafil,36,37 perhaps because of tadalafil’s longer duration of action.34
There is little evidence to suggest that vardenafil is more effective or more often preferred by patients than tadalafil or sidenafil.34,38 And though data on the newest drug on the market, avanafil, are limited, a meta-analysis concluded that it may be less effective than tadalafil and without significant differences in terms of safety.39
Other treatments
Lifestyle modifications, especially smoking cessation and exercise, have been shown to reduce the risk of erectile dysfunction with varying effect sizes across studies.40–42 Moreover, factors such as obesity, alcohol use, and smoking may cause irreversible harm, and thus a healthy lifestyle should be encouraged.41
While there is only weak evidence for the use of psychological interventions alone for treating most types of erectile dysfunction, one meta-analysis found that the combination of psychological intervention and a phosphodiesterase-5 inhibitor improved sexual satisfaction more than drug therapy alone.43
Erectile dysfunction: Take-home points
- Erectile dysfunction is common, affecting nearly 20% of men over the age of 20 and over 75% of men over the age of 75.
- Erectile dysfunction is often associated with chronic disease and may suggest the need to screen for cardiovascular disease.
- Treating underlying chronic diseases may help, and phosphodiesterase-5 inhibitors are effective; tadalafil may be most often preferred.
SUPPLEMENT USE AND MEN’S HEALTH
A 68-year-old man with a history of hypertension, BPH, and erectile dysfunction presents for a 6-month follow-up. His medication use includes lisinopril, which he takes without problems. He denies any new physical symptoms. His physical examination is unremarkable. He says he has heard about supplements that might help with his sexual performance and hopes to discuss recommendations during the visit.
A burgeoning, unregulated industry
Since the passage of the Dietary Supplement and Health Education Act in 1994, a law that decreased oversight of the supplement industry, spending on supplements has skyrocketed to over $41.1 billion each year.44 Advertisements for these products typically claim that they improve general mental and physical health, sexual and romantic performance, leanness, and muscularity.45 A national survey of men ages 57 and older reported that the most popular products were aimed at nutrition (such as multivitamins), cardiovascular health (such as omega-3 fatty acids), and chronic conditions (such as saw palmetto for BPH).46
Little evidence of efficacy
There is little evidence to support the use of most supplements to improve men’s health. For example, a study in 82,405 men found no association between mortality rates and multivitamin use (hazard ratio [HR] 1.07, 95% CI 0.96–1.19).47 Even for specific uses, such as cognitive performance, randomized trials exploring the effects of multivitamins in men have been largely negative.48
The positive trials that have been reported are often of low quality and are funded by supplement manufacturers. For example, one of the few trials that reported a positive association between multivitamin supplementation and cognition in men was underpowered (N = 51) and found improvement in only 1 of 19 cognitive domains.49 Despite the poor design and results to the contrary, this industry-funded study nevertheless concluded that multivitamins may play a role in improving elements of memory.
Evidence of possible harm from antioxidants
While not always specific to men, many meta-analyses have explored the effects of antioxidant supplements on cardiovascular and mortality risk. Most of them concluded that antioxidant supplements have no benefit and that some may actually be harmful.
For example, multiple meta-analyses of vitamin E supplementation found no cardiovascular benefit but possible increases in all-cause mortality rates in those taking high doses (risk ratio 1.04, 95% CI 1.01–1.07).50,51
Another meta-analysis of 180,938 participants in high-quality studies found an increased risk of all-cause mortality associated with independent intake of several antioxidant vitamins, including beta-carotene (risk ratio 1.07, 95% CI 1.02–1.11) and vitamin A (risk ratio 1.16, 95% CI 1.10–1.24), while intake of vitamin C and selenium had no impact on mortality.52
Similarly, although nearly 10% of US adults report taking omega-3 fatty acid supplements, a review of 24 randomized controlled trials and meta-analyses published between 2005 and 2012 concluded that only 2 supported the use of these supplements for any health benefit.53
Can supplements improve sexual function, prostate health?
To improve sexual function. A 2015 narrative review of the ingredients in General Nutrition Center’s top 30 best-selling products targeted at improving men’s sexual performance (including improving libido and erectile dysfunction) found only poor evidence for any efficacy.54 The few studies that did support the use of select supplements, including B vitamins in people with diabetes, L-arginine, and yohimbine, were deemed to be of poor quality or showed a smaller effect size compared with standard medical therapy.
To prevent prostate cancer. Studies of supplement use to improve prostate health have had mixed results. For example, multiple large case-control studies have suggested that taking vitamin D55,56 or vitamin C57 is not associated with prostate cancer risk, while increased vitamin A58,59 and E60,61 intake is associated with inconsistent increases in prostate cancer risk.
In the Selenium and Vitamin E Cancer Prevention Trial,62 a randomized controlled trial in 35,533 men, those assigned to receive vitamin E supplementation were 17% more likely to get prostate cancer than were those assigned to placebo (HR 1.17, 99% CI 1.004–1.36, P = .008).
However, there are plausible biologic links between nutraceuticals and prostate cancer. For example, studies have linked genetic polymorphisms in vitamin D receptors63 as well as intake of natural androgen receptor modulators, such as the most active polyphenol in green tea,64 to prostate cancer risk and aggressiveness in certain populations. This led a recent review to conclude that there is some biologic plausibility, but at present little epidemiologic evidence, to support any dietary supplement’s ability to broadly affect prostate cancer risk.65
Interest continues in exploring the targeted use of nutraceuticals as adjuvant therapy in specific populations at risk of prostate cancer.66,67
To treat BPH. There is a similar dearth of clinical or population-based evidence that supplements can broadly affect BPH symptoms. For example, in a 2012 Cochrane review of Serenoa repens (saw palmetto) utilizing only high-quality evidence, there was no evidence that supplement use significantly reduced lower urinary tract symptoms, nocturia, or peak urine flow in BPH patients, and this was true even when the supplement was taken at triple-strength doses.68
For other diseases. There is also limited evidence that supplements can affect other chronic diseases. For example, a meta-analysis of 3,803 patients found that glucosamine, chondroitin, and their combination had no impact on joint pain or joint space narrowing in patients with osteoarthritis of the knee or hip.69
Even when there is some evidence to suggest benefit from supplementation, study heterogeneity and varying evidence quality limit confidence in the conclusions. For example, meta-analyses suggest garlic may improve blood pressure control in those with hypertension70 and improve lipid and blood glucose control in type 2 diabetes.71 However, most of the trials included in those systematic reviews were underpowered, with samples as low as 10 patients, and many suffered from improper design, such as inadequate blinding of researchers. In addition, these meta-analyses often do not report adverse events, suggesting that higher quality studies would be needed to adequately measure event rates. As such, there is need for caution and a case-by-case review before recommending even a seemingly benign supplement like garlic to patients.
In total, there is only limited evidence to support the efficacy of supplements across many diseases and concerns common to men in primary care. This includes improving general health, cardiovascular health, sexual functioning, or other chronic diseases. While a supplement’s placebo effect may at times provide some benefit, supplements are much less strictly regulated since the passing of the 1994 act, and even vitamin supplementation has been shown to be associated with negative health outcomes. As such, a patient’s use of supplements requires careful consideration and shared decision-making.
Supplements: Take-home points
- Supplements are only loosely regulated by the federal government.
- There is some biologic but limited epidemiologic evidence for the use of multivitamins to improve cognition or mortality rates; for the use of antioxidant vitamins or omega-3 fatty acids to improve cardiovascular health; for the use of any of the top-selling sexual enhancement supplements to improve libido or erectile function; and for the use of vitamins or other supplements for improving BPH or reducing prostate cancer risk. Using supplements may in some cases be harmful.
- Given the heterogeneity of studies of supplements to manage chronic diseases and a lack of reporting of adverse events, careful consideration is needed when recommending supplements to patients.
- Barry MJ, Fowler FJ Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. J Urol 2017; 197(2S):S189–S197. doi:10.1016/j.juro.2016.10.071
- Urological Sciences Research Foundation. International Prostate Symptom Score (IPSS). http://www.usrf.org/questionnaires/AUA_SymptomScore.html. Accessed October 16, 2018.
- McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 2011; 185(5):1793–1803. doi:10.1016/j.juro.2011.01.074
- McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 2003; 349(25):2387–2398. doi:10.1056/NEJMoa030656
- Matsukawa Y, Takai S, Funahashi Y, et al. Effects of withdrawing alpha-1 blocker from the combination therapy with alpha-1 blocker and 5-alpha-reductase inhibitor in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a prospective and comparative trial using urodynamics. J Urol 2017; 198(4):905–912. doi:10.1016/j.juro.2017.05.031
- Barkin J, Guimaraes M, Jacobi G, Pushkar D, Taylor S, van Vierssen Trip OB. Alpha-blocker therapy can be withdrawn in the majority of men following initial combination therapy with the dual 5a-reductase inhibitor dutasteride. Eur Urol 2003; 44(4):461–466. pmid:14499682
- Baldwin KC, Ginsberg PC, Roehrborn CG, Harkaway RC. Discontinuation of alpha-blockade after initial treatment with finasteride and doxazosin in men with lower urinary tract symptoms and clinical evidence of benign prostatic hyperplasia. Urology 2001; 58(2):203–209. pmid:11489700
- Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA 2015; 314(17):1818–1831. doi:10.1001/jama.2015.13766
- DuBeau CE, Yalla SV, Resnick NM. Improving the utility of urine flow rate to exclude outlet obstruction in men with voiding symptoms. J Am Geriatr Soc 1998; 46(9):1118–1124. pmid:9736105
- US Department of Health and Human Services Health Resources and Services Administration. United States Cancer Statistics: 1999-2014 Incidence and Mortality Web-Based Report. Atlanta; 2017. https://nccd.cdc.gov/uscs/. Accessed October 17, 2018.
- US Preventive Services Task Force. Final recommendation statement. Prostate cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1. Accessed October 16, 2018.
- US Preventive Services Task Force. Archived: prostate cancer: screening. Original release date: May 2012. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/prostate-cancer-screening. Accessed October 16, 2018.
- Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA guideline. J Urol 2013; 190(2):419–426. doi:10.1016/j.juro.2013.04.119
- Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009; 360(13):1320–1328. doi:10.1056/NEJMoa0810084
- Andriole GL, Crawford ED, Grubb RL, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 2009; 360(13):1310–1319. doi:10.1056/NEJMoa0810696
- Schröder FH, Hugosson J, Carlsson S, et al. Screening for prostate cancer decreases the risk of developing metastatic disease: findings from the European Randomized Study of Screening for Prostate Cancer (ERSPC). Eur Urol 2012; 62(5):745–752. doi:10.1016/j.eururo.2012.05.068
- Tsodikov A, Gulati R, Heijnsdijk EAM, et al. Reconciling the effects of screening on prostate cancer mortality in the ERSPC and PLCO trials. Ann Intern Med 2017; 167(7):449–455. doi:10.7326/M16-2586
- Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA 2018; 319(9):883–895. doi:10.1001/jama.2018.0154
- Pahwa S, Schiltz NK, Ponsky LE, Lu Z, Griswold MA, Gulani V. Cost-effectiveness of MR imaging–guided strategies for detection of prostate cancer in biopsy-naive men. Radiology 2017; 285(1):157–166. doi:10.1148/radiol.2017162181
- Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017; 389(10071):815–822. doi:10.1016/S0140-6736(16)32401-1
- Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018; 378(19):1767–1777. doi:10.1056/NEJMoa1801993
- Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med 2017; 377(2):132–142. doi:10.1056/NEJMoa1615869
- Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med 2014; 370(10):932–942. doi:10.1056/NEJMoa1311593
- Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016; 375(15):1415–1424. doi:10.1056/NEJMoa1606220
- Morley JE. Impotence. Am J Med 1986; 80(5):897–905. pmid:3518438
- NIH Consensus Development Panel on Impotence. NIH Consensus Conference. Impotence. JAMA 1993; 270(1):83–90. pmid:8510302
- Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med 2007; 120(2):151–157. doi:10.1016/j.amjmed.2006.06.010
- Rosen RC, Cappelleri JC, Gendrano N 3rd. The International Index of Erectile Function (IIEF): a state-of-the-science review. Int J Impot Res 2002; 14(4):226–244. doi:10.1038/sj.ijir.3900857
- Gandaglia G, Briganti A, Jackson G, et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur Urol 2014; 65(5):968–978. doi:10.1016/j.eururo.2013.08.023
- Heaton JPW, Adams MA. Causes of erectile dysfunction. Endocrine 2004; 23(2-3):119–123. doi:10.1385/ENDO:23:2-3:119
- Montorsi F, Salonia A, Deho F, et al. Pharmacological management of erectile dysfunction. BJU Int 2003; 91(5):446–454. pmid:12603396
- Cai X, Tian Y, Wu T, Cao CX, Bu SY, Wang KJ. The role of statins in erectile dysfunction: a systematic review and meta-analysis. Asian J Androl 2014; 16(3):461–466. doi:10.4103/1008-682X.123678
- Webb DJ, Freestone S, Allen MJ, Muirhead GJ. Sildenafil citrate and blood-pressure–lowering drugs: results of drug interaction studies with an organic nitrate and a calcium antagonist. Am J Cardiol 1999; 83(5):21C–28C. pmid:10078539
- Doggrell SA. Comparison of clinical trials with sildenafil, vardenafil and tadalafil in erectile dysfunction. Expert Opin Pharmacother 2005; 6(1):75–84. doi:10.1517/14656566.6.1.75
- Gresser U, Gleiter CH. Erectile dysfunction: comparison of efficacy and side effects of the PDE-5 inhibitors sildenafil, vardenafil and tadalafil—review of the literature. Eur J Med Res 2002; 7(10):435–446. pmid:12435622
- Eardley I, Mirone V, Montorsi F, et al. An open-label, multicentre, randomized, crossover study comparing sildenafil citrate and tadalafil for treating erectile dysfunction in men naive to phosphodiesterase 5 inhibitor therapy. BJU Int 2005; 96(9):1323–1332. doi:10.1111/j.1464-410X.2005.05892.x
- von Keitz A, Rajfer J, Segal S, et al. A multicenter, randomized, double-blind, crossover study to evaluate patient preference between tadalafil and sildenafil. Eur Urol 2004; 45(4):499–509. doi:10.1016/j.eururo.2003.11.030
- Martin-Morales A, Haro JM, Beardsworth A, Bertsch J, Kontodimas S; EDOS Group. Therapeutic effectiveness and patient satisfaction after 6 months of treatment with tadalafil, sildenafil, and vardenafil: results from the erectile dysfunction observational study (EDOS). Eur Urol 2007; 51(2):541–550. doi:10.1016/j.eururo.2006.09.027
- Yuan J, Zhang R, Yang Z, et al. Comparative effectiveness and safety of oral phosphodiesterase type 5 inhibitors for erectile dysfunction: a systematic review and network meta-analysis. Eur Urol 2013; 63(5):902–912. doi:10.1016/j.eururo.2013.01.012
- Cao S, Yin X, Wang Y, Zhou H, Song F, Lu Z. Smoking and risk of erectile dysfunction: systematic review of observational studies with meta-analysis. PLoS One 2013; 8(4):e60443. doi:10.1371/journal.pone.0060443
- Derby CA, Mohr BA, Goldstein I, Feldman HA, Johannes CB, McKinlay JB. Modifiable risk factors and erectile dysfunction: can lifestyle changes modify risk? Urology 2000; 56(2):302–306. pmid:10925098
- Esposito K, Giugliano F, Di Palo C, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA 2004; 291(24):2978–2984. doi:10.1001/jama.291.24.2978
- Schmidt HM, Munder T, Gerger H, Frühauf S, Barth J. Combination of psychological intervention and phosphodiesterase-5 inhibitors for erectile dysfunction: a narrative review and meta-analysis. J Sex Med 2014; 11(6):1376–1391. doi:10.1111/jsm.12520
- New Hope Network. Supplement Business Report 2017. Boulder; 2017. http://images.info.newhope.com/Web/NewHopeNaturalMedia/%7B3a3f3b03-6130-41d4-9e66-84f29eeebe44%7D_2017_Supplement_Business_Report_-_Extended_TOC.pdf. Accessed October 16, 2018.
- Labre MP. Burn fat, build muscle: a content analysis of men’s health and men’s fitness. Int J Mens Health 2005; 4(2):187–200.
- Qato DM, Alexander GC, Conti RM, Johnson M, Schumm P, Lindau ST. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA 2008; 300(24):2867–2878. doi:10.1001/jama.2008.892
- Park SY, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Multivitamin use and the risk of mortality and cancer incidence: the multiethnic cohort study. Am J Epidemiol 2011; 173(8):906–914. doi:10.1093/aje/kwq447
- McNeill G, Avenell A, Campbell MK, et al. Effect of multivitamin and multimineral supplementation on cognitive function in men and women aged 65 years and over: a randomised controlled trial. Nutr J 2007; 6(1):10. doi:10.1186/1475-2891-6-10
- Harris E, Macpherson H, Vitetta L, Kirk J, Sali A, Pipingas A. Effects of a multivitamin, mineral and herbal supplement on cognition and blood biomarkers in older men: a randomised, placebo-controlled trial. Hum Psychopharmacol Clin Exp 2012; 27(4):370–377. doi:10.1002/hup.2236
- Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet 2003; 361(9374):2017–2023. doi:10.1016/S0140-6736(03)13637-9
- Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 2005; 142(1):37–46. pmid:15537682
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 2007; 297(8):842–857. doi:10.1001/jama.297.8.842
- Grey A, Bolland M. Clinical trial evidence and use of fish oil supplements. JAMA Intern Med 2014; 174(3):460–462. doi:10.1001/jamainternmed.2013.12765
- Cui T, Kovell RC, Brooks DC, Terlecki RP. A urologist’s guide to ingredients found in top-selling nutraceuticals for men’s sexual health. J Sex Med 2015; 12(11):2105–2117. doi:10.1111/jsm.13013
- Schenk JM, Till CA, Tangen CM, et al. Serum 25-hydroxyvitamin D concentrations and risk of prostate cancer: results from the Prostate Cancer Prevention Trial. Cancer Epidemiol Prev Biomarkers 2014; 23(8):1484–1493. doi:10.1158/1055-9965.EPI-13-1340
- Albanes D, Mondul AM, Yu K, et al. Serum 25-hydroxy vitamin D and prostate cancer risk in a large nested case-control study. Cancer Epidemiol Prev Biomarkers 2011; 20(9):1850–1860. doi:10.1158/1055-9965.EPI-11-0403
- Roswall N, Larsen SB, Friis S, et al. Micronutrient intake and risk of prostate cancer in a cohort of middle-aged, Danish men. Cancer Causes Control 2013; 24(6):1129–1135. doi:10.1007/s10552-013-0190-4
- Mondul AM, Watters JL, Männistö S, et al. Serum retinol and risk of prostate cancer. Am J Epidemiol 2011; 173(7):813-821. doi:10.1093/aje/kwq429
- Schenk JM, Riboli E, Chatterjee N, et al. Serum retinol and prostate cancer risk: a nested case-control study in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Prev Biomarkers 2009; 18(4):1227–1231. doi:10.1158/1055-9965.EPI-08-0984
- Bidoli E, Talamini R, Zucchetto A, et al. Dietary vitamins E and C and prostate cancer risk. Acta Oncol 2009; 48(6):890–894. doi:10.1080/02841860902946546
- Wright ME, Weinstein SJ, Lawson KA, et al. Supplemental and dietary vitamin E intakes and risk of prostate cancer in a large prospective study. Cancer Epidemiol Prev Biomarkers 2007; 16(6):1128–1135. doi:10.1158/1055-9965.EPI-06-1071
- Klein EA, Thompson IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011; 306(14):1549–1556. doi:10.1001/jama.2011.1437
- Jingwi EY, Abbas M, Ricks-Santi L, et al. Vitamin D receptor genetic polymorphisms are associated with PSA level, Gleason score and prostate cancer risk in African-American men. Anticancer Res 2015; 35(3):1549–1558. pmid:25750310
- Siddiqui IA, Asim M, Hafeez BB, Adhami VM, Tarapore RS, Mukhtar H. Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB J 2011; 25(4):1198–1207. doi:10.1096/fj.10-167924
- Yacoubian A, Dargham RA, Khauli RB, Bachir BG. Overview of dietary supplements in prostate cancer. Curr Urol Rep 2016; 17(11):78. doi:10.1007/s11934-016-0637-8
- Kallifatidis G, Hoy JJ, Lokeshwar BL. Bioactive natural products for chemoprevention and treatment of castration-resistant prostate cancer. Semin Cancer Biol 2016; 40:160–169. doi:10.1016/j.semcancer.2016.06.003
- Shui IM, Mondul AM, Lindström S, et al. Circulating vitamin D, vitamin D–related genetic variation, and risk of fatal prostate cancer in the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. Cancer 2015; 121(12):1949–1956. doi:10.1002/cncr.29320
- Tacklind J, MacDonald R, Rutks I, Stanke JU, Wilt TJ. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev 2012; 12:CD001423. doi:10.1002/14651858.CD001423.pub3
- Wandel S, Jüni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ 2010; 341:c4675. doi:10.1136/bmj.c4675
- Reinhart KM, Coleman CI, Teevan C, Vachhani P, White CM. Effects of garlic on blood pressure in patients with and without systolic hypertension: a meta-analysis. Ann Pharmacother 2008; 42(12):1766–1771. doi:10.1345/aph.1L319
- Wang J, Zhang X, Lan H, Wang W. Effect of garlic supplement in the management of type 2 diabetes mellitus (T2DM): a meta-analysis of randomized controlled trials. Food Nutr Res 2017; 61(1):1377571. doi:10.1080/16546628.2017.1377571
Primary care physicians are tasked with a wide variety of issues affecting men. This article reviews the latest research in 4 areas of men’s health commonly addressed in primary care:
- Medical management of benign prostatic hyperplasia (BPH)
- Prostate cancer screening and treatment
- Medical management of erectile dysfunction
- Use of supplements.
MEDICAL MANAGEMENT OF BPH
An 84-year-old man with a history of hypertension, type 2 diabetes, hyperlipidemia, BPH, mild cognitive impairment, and osteoarthritis presents for a 6-month follow-up, accompanied by his son.
Two years ago he was started on a 5-alpha reductase inhibitor and an alpha-blocker for worsening BPH symptoms. His BPH symptoms are currently under control, with an American Urological Association (AUA) symptom index score of 7 of a possible 35 (higher scores being worse).
However, both the patient and son are concerned about the number of medications he is on and wonder if some could be eliminated.
Assessment tools
BPH is a common cause of lower urinary tract symptoms in older men. Evidence-based tools to help the clinician and patient decide on when to consider treatment for symptoms are:
- The AUA symptom index1
- The International Prostate Symptom Score (IPSS).2
An AUA symptom index score or IPSS score of 8 through 19 of a possible 35 is consistent with moderate symptoms, while a score of 20 or higher indicates severe symptoms.
Combination therapy or monotherapy?
Monotherapy with an alpha-blocker or a 5-alpha reductase inhibitor is often the first-line treatment for BPH-related lower urinary tract symptoms.3 However, combination therapy with both an alpha-blocker and a 5-alpha reductase inhibitor is another evidence-based option.
The Medical Therapy of Prostatic Symptoms study,4 a randomized controlled trial, reported that long-term combination therapy reduced the risk of BPH clinical progression better than monotherapy. The same trial also found that either combination therapy or finasteride alone (a 5-alpha reductase inhibitor) reduced the risk of acute urinary retention and the future need for invasive therapy.
Monotherapy after a period of combination therapy?
There is also evidence to support switching from combination to monotherapy after an initial treatment period.
Matsukawa et al5 examined the effects of withdrawing the alpha-blocker from BPH combination therapy in a study in 140 patients. For 12 months, all patients received the alpha-blocker silodosin and the 5-alpha reductase inhibitor dutasteride. At 12 months, the remaining 132 patients (8 patients had been lost to follow-up) were randomized to continue combination therapy or to take dutasteride alone for another 12 months. They were evaluated at 0, 12, and 24 months by questionnaires (the IPSS and Overactive Bladder Symptom Score) and urodynamic testing (uroflowmetry, cystometrography, and pressure-flow studies).
There were no significant differences in subjective symptoms and bladder outlet obstruction between patients who continued combination therapy and those who switched to dutasteride monotherapy. In the monotherapy group, those whose symptoms worsened weighed more (68.8 kg vs 62.6 kg, P =.002) and had a higher body mass index (BMI) (26.2 kg/m2 vs 22.8 kg/m2, P < .001) than those whose symptoms stayed the same or got better.
These findings of successful alpha-blocker withdrawal were consistent with those of other studies.
The Symptom Management After Reducing Therapy study6 showed that 80% of men with an IPSS score less than 20 who changed to dutasteride monotherapy did not have a noticeable worsening of their symptoms.
Baldwin et al7 noted similar success after withdrawing the alpha-blocker doxazosin in patients on finasteride.
Review all medications
The National Health and Nutrition Examination Survey noted that the estimated prevalence of polypharmacy increased from 8% in 1999 to 15% in 2011.8 Many commonly used medications, such as decongestants, antihistamines, and anticholinergic agents, can worsen BPH symptoms,9 so it is reasonable to consistently review the patient’s medications to weigh the risks and benefits and determine which ones align with the patient’s personal care goals.
BPH: Take-home points
- Combination therapy with an alpha-blocker and a 5-alpha reductase inhibitor is an effective regimen for BPH.
- Polypharmacy is a significant problem in the elderly.
- Withdrawing the alpha-blocker component from BPH combination therapy can be considered after 1 year of combination therapy in patients whose symptoms have been well controlled.
PROSTATE CANCER SCREENING AND TREATMENT
A 60-year-old patient calls you after receiving his laboratory testing report from his insurance physical. His prostate-specific antigen (PSA) level is 5.1 ng/mL, and he has several questions:
- Should he have agreed to the screening?
- How effective is the screening?
- What are the next steps?
Is PSA screening useful?
Over the last few years, there has been great debate as to the utility of screening for prostate cancer.
The US Centers for Disease Control and Prevention10 reported that in 2014, an estimated 172,258 men in the United States were diagnosed with prostate cancer, but only 28,343 men died of it. These statistics support the notion that screening programs may be detecting what might otherwise be a silent disease.
The US Preventive Services Task Force (USPSTF)11 recommends against blanket PSA screening, in view of the low probability that it reduces the risk of death from prostate cancer. For men ages 55 through 69, current guidelines give a grade C recommendation to PSA screening, meaning there is moderate agreement that the benefit is likely small, and screening should be selectively offered based on professional judgment and patient preference. In men ages 70 and older who are not at high risk, the guideline gives screening a grade D recommendation, meaning there is moderate evidence that there is no benefit from the practice. This is a change from the 2012 USPSTF guidelines,12 which gave a grade D recommendation to PSA screening for all ages.
The American Urological Association13 recommends against PSA screening in men under age 40 or ages 70 and older. It does not recommend routine screening in those ages 40 to 54 at average risk, but it says the decision should be individualized in this age group in those at higher risk (eg, with a positive family history, African American). At ages 55 through 69, it recommends shared decision-making, taking into account cancer risk and life expectancy. In those who opt for screening, an interval of 2 years or more may be preferred over annual screening to reduce the risk of overdiagnosis.
The USPSTF recommendations rely heavily on data from 2 trials: the European Randomized Study of Screening for Prostate Cancer (ERSPC)14 and the Prostate, Lung, Colorectal, and Ovarian Screening (PLCO) trial.15
The ERSPC14 demonstrated that screening for prostate cancer reduced deaths from prostate cancer by 20%, with an absolute risk difference of 0.71 deaths per 1,000 men; 1,410 men would need to be screened and 48 additional cases of prostate cancer would need to be treated to prevent 1 death from prostate cancer. Screening also decreased the risk of developing metastatic disease by 30%.16 On the negative side, screening increased the risk of overdiagnosis and other harms such as bleeding, sepsis, and incontinence.
The PLCO trial,15 in contrast, found no difference in death rates between men randomly assigned to annual screening and those assigned to usual care. Differences between the trial results were thought to be due to different practice settings as well as study implementation and compliance.
Tsodikov et al17 reanalyzed data from the ERSPC and the PLCO trial using 3 different mathematical models to estimate the effects of screening in both trials compared with no screening. The analysis found no evidence that the effects of screening vs not screening differed between the 2 trials, ultimately concluding that PSA screening reduced prostate cancer deaths by 25% to 32%, which the authors inferred was primarily a result of earlier detection of cancer.
The Cluster Randomized Trial of PSA Testing for Prostate Cancer,18 published in March 2018, explored the effect of single PSA screening vs no screening on prostate cancer mortality rates in 419,582 men ages 50 through 69. Although screening detected more cases of low-risk prostate cancer, there was no significant difference in prostate cancer mortality rates after a median follow-up of 10 years. However, 10% to 15% of the control group was estimated to have also been screened, and these results do not directly speak to the efficacy of serial PSA screening.
Extended follow-up of this trial is planned to report on long-term survival benefits and whether screening lowers the risk of metastasis.
Imaging-guided prostate biopsy
Once a patient is found to have an elevated PSA level, standard practice has been to perform transrectal ultrasonography to obtain 12 core biopsy samples. The results indicate whether the prostate contains cancer, how aggressive the cancer is (Gleason score), and whether there is extracapsular extension.
In the past, magnetic resonance imaging (MRI) of the prostate before biopsy was thought to be too costly, and many insurance plans do not currently cover it.
Pahwa et al,19 however, in a cost-effectiveness study using a decision-analysis model, found that using MRI to detect lesions and then guide biopsy by triaging patients into proper treatment pathways added health benefits in a cost-effective manner in 94.05% of simulations. These benefits were found across all age groups.
This study demonstrated that doctors could use MRI to better evaluate patients for potentially harmful lesions. If a focus of cancer is found, it can be biopsied; if no cancer is seen on MRI, the patient can avoid biopsy completely. Additionally, though MRI tended to miss low-risk cancers, these cancers are thought to disproportionately lead to higher healthcare costs through unnecessary treatment. Therefore, a negative MRI study was believed to be an excellent sign that the patient does not have aggressive prostate cancer. This approach led to a net gain of 0.251 additional quality-adjusted life years compared with the standard biopsy strategy.
The Prostate MRI Imaging Study20 also found MRI to be effective in the prostate cancer workup. In this trial, 576 men who had never undergone biopsy underwent multiparametric MRI, transrectal ultrasonography-guided biopsy, and the reference standard, ie, transperineal template prostate mapping biopsy. Of those who underwent biopsy, 71% received a diagnosis of prostate cancer, and 40% had clinically significant disease. In patients with clinically significant disease, MRI was more sensitive than ultrasonography-guided biopsy (93% vs 48%, P < .0001) but less specific (41% vs 96%, P < .0001).
Based on these findings, if biopsy were performed only in those who had suspicious lesions on MRI, 27% of men with elevated PSA could avoid biopsy and its potential complications such as bleeding and sepsis, which occurred in 5.9% of the biopsy group.
The Prostate Evaluation for Clinically Important Disease: Sampling Using Image Guidance or Not? trial21 more recently studied MRI with or without targeted biopsy vs standard transrectal ultrasonography-guided biopsy in 500 men who had not undergone biopsy before, and reported similar results. MRI with or without biopsy led to fewer biopsies and less overdetection of clinically insignificant prostate cancers compared with the standard approach. Furthermore, those in the MRI-targeted biopsy group were 13% less likely to receive a diagnosis of clinically insignificant cancer than those who received the standard biopsy (adjusted difference −13 percentage points, 95% confidence interval [CI] −19 to −7, P < .001).
Together, these data provide another argument for adding multiparametric MRI to the workup of men with an elevated PSA level.
Surveillance vs treatment for prostate cancer
Once prostate cancer is diagnosed, surveillance is becoming an increasingly common management strategy.
The Prostate Cancer Intervention Versus Observation Trial (PIVOT),22 one of the largest and longest trials involving cancer patients, offered further evidence that active surveillance and less intervention for men with prostate cancer is a better approach in many cases. This trial compared prostatectomy and observation alone in a randomized fashion. Inclusion for the study required men to be medically fit for radical prostatectomy, along with having histologically confirmed localized prostate cancer (stage T1-T2NxM0 in the tumor-node-metastasis classification system) of any grade diagnosed within the last 12 months.
During 19.5 years of follow-up, 223 (61.3%) of the 364 men randomly assigned to radical prostatectomy died, compared with 245 (66.8%) of 367 men in the observation group; the difference was not statistically different (P = .06). Only 9.4% of the deaths were due to prostate cancer, 7.4% in the surgery group and 11.4% in the observation group (P = .06).
Surgery was associated with a lower all-cause mortality rate than observation in the subgroup of patients with intermediate-risk prostate cancer (defined as PSA 10–20 ng/mL and a Gleason score of 7). Surgery was also associated with less disease progression.22
This finding is in line with previous data from the Scandinavian Prostate Cancer Group Study Number 4,23 as well as the much larger Prostate Testing for Cancer and Treatment (ProtecT) trial,24 both of which reported that metastasis was 1.5 and 2.6 times as common, respectively, in participants in the active surveillance groups. However, in the PIVOT trial, those in the surgery group were significantly more likely than those in the observation group to have erectile dysfunction and urinary incontinence at 10 years.
Therefore, in men with localized disease and in those with low-risk PSA levels, both the PIVOT and ProtecT trials suggest that death from prostate cancer is uncommon and that observation may be more appropriate.
Prostate cancer: Take-home points
- A new look at 2 large trials of PSA screening strengthened evidence that testing in the right patient population can reduce deaths from prostate cancer, but a third recently published trial that found no benefit from 1-time screening may reopen debate on the topic.
- MRI offers a better method than ultrasonography-guided biopsy to triage patients thought to be at high risk of prostate cancer and tends to limit costly overtreatment of disease that likely would not cause death.
- Surgery for prostate cancer may not prolong life but could reduce disease progression, at the risk of more adverse effects.
- Shared decision-making should be practiced when deciding whether to use active surveillance or active treatment of diagnosed prostate cancer.
MANAGEMENT OF ERECTILE DYSFUNCTION
A 62-year-old man with hypertension, hyperlipidemia, peripheral artery disease, and type 2 diabetes presents for a 6-month follow-up. His medications include aspirin, metformin, lisinopril, and atorvastatin, all of which he takes without problems. Over the past several months, he has noticed that his erections are not adequate for sexual intercourse. He recently heard that a generic version of sildenafil has just become available, and he wonders if it might benefit him.
Erectile dysfunction is common, associated with chronic diseases
Erectile dysfunction, ie, persistent inability to obtain and maintain an erection sufficient to permit satisfactory sexual intercourse,25,26 is estimated to affect nearly 20% of men over the age of 20 and 75% of men over the age of 75.27
In age-adjusted models, erectile dysfunction has been shown28 to be associated with:
- History of cardiovascular disease (odds ratio [OR] 1.63, 95% CI 1.02–2.63)
- Diabetes (OR 3.90, 95% CI 2.16–7.04)
- Treated hypertension vs no hypertension (OR 2.22, 95% CI 1.30–3.80)
- Current smoking vs never smoking (OR 1.63, 95% CI 1.01–2.62)
- BMI greater than 30 kg/m2 vs less than 25 kg/m2 (OR 1.80, 95% CI 1.03–3.14).
Because of the strong association between cardiovascular disease and erectile dysfunction, the presence of one often suggests the need to screen for the other.29 While tools such as the International Index of Erectile Function (IIEF-5) have been developed to evaluate erectile dysfunction, it is most often diagnosed on the basis of clinical impression, while validated assessment methods are reserved for clinical trials.28
Multiple causes of erectile dysfunction
Erectile dysfunction arises from inadequate penile tissue response to a sexual signal. The response can be disrupted at several points. For example, damage to vascular smooth muscle cells (eg, from age or obesity) and endothelial cells (from smoking or diabetes) and narrowing of the vascular lumen (from atherosclerosis or hypertension) have all been shown to impair engorgement of the corpus cavernosum.30 In addition, denervation from prostate surgery or spinal trauma and psychogenic causes should be recognized in discussions with patients.
Drugs for erectile dysfunction
Pharmacologic management of erectile dysfunction includes oral, sublingual, intracavernosal, and intraurethral therapies.31 Treatment in primary care settings usually includes addressing underlying chronic diseases32 and prescribing phosphodiesterase-5 inhibitors (sildenafil, tadalafil, vardenafil, and avanafil). These drugs work by increasing local concentrations of cyclic guanosine monophosphate in the corpus cavernosum to induce vasodilation.33
While these 4 drugs are still patent-protected, a manufacturer has been allowed to introduce a generic version of sildenafil into US markets, and a generic version of tadalafil is expected to be available soon.
Sildenafil, tadalafil, and vardenafil have been studied and found to have some degree of effectiveness in erectile dysfunction caused by damage to the penile vasculature, denervation, and spinal cord injury.34 All drugs of this class have adverse effects including headache, facial flushing, and nasal congestion, but the drugs are generally well tolerated.35
Sildenafil and tadalafil improve IIEF-5 scores by a similar margin, raising scores on the erectile domain subsection from approximately 14 of a possible 30 to approximately 24 of 30 in a trial of both drugs.36 However, multiple crossover studies comparing the 2 drugs have shown that nearly 75% of patients prefer tadalafil to sildenafil,36,37 perhaps because of tadalafil’s longer duration of action.34
There is little evidence to suggest that vardenafil is more effective or more often preferred by patients than tadalafil or sidenafil.34,38 And though data on the newest drug on the market, avanafil, are limited, a meta-analysis concluded that it may be less effective than tadalafil and without significant differences in terms of safety.39
Other treatments
Lifestyle modifications, especially smoking cessation and exercise, have been shown to reduce the risk of erectile dysfunction with varying effect sizes across studies.40–42 Moreover, factors such as obesity, alcohol use, and smoking may cause irreversible harm, and thus a healthy lifestyle should be encouraged.41
While there is only weak evidence for the use of psychological interventions alone for treating most types of erectile dysfunction, one meta-analysis found that the combination of psychological intervention and a phosphodiesterase-5 inhibitor improved sexual satisfaction more than drug therapy alone.43
Erectile dysfunction: Take-home points
- Erectile dysfunction is common, affecting nearly 20% of men over the age of 20 and over 75% of men over the age of 75.
- Erectile dysfunction is often associated with chronic disease and may suggest the need to screen for cardiovascular disease.
- Treating underlying chronic diseases may help, and phosphodiesterase-5 inhibitors are effective; tadalafil may be most often preferred.
SUPPLEMENT USE AND MEN’S HEALTH
A 68-year-old man with a history of hypertension, BPH, and erectile dysfunction presents for a 6-month follow-up. His medication use includes lisinopril, which he takes without problems. He denies any new physical symptoms. His physical examination is unremarkable. He says he has heard about supplements that might help with his sexual performance and hopes to discuss recommendations during the visit.
A burgeoning, unregulated industry
Since the passage of the Dietary Supplement and Health Education Act in 1994, a law that decreased oversight of the supplement industry, spending on supplements has skyrocketed to over $41.1 billion each year.44 Advertisements for these products typically claim that they improve general mental and physical health, sexual and romantic performance, leanness, and muscularity.45 A national survey of men ages 57 and older reported that the most popular products were aimed at nutrition (such as multivitamins), cardiovascular health (such as omega-3 fatty acids), and chronic conditions (such as saw palmetto for BPH).46
Little evidence of efficacy
There is little evidence to support the use of most supplements to improve men’s health. For example, a study in 82,405 men found no association between mortality rates and multivitamin use (hazard ratio [HR] 1.07, 95% CI 0.96–1.19).47 Even for specific uses, such as cognitive performance, randomized trials exploring the effects of multivitamins in men have been largely negative.48
The positive trials that have been reported are often of low quality and are funded by supplement manufacturers. For example, one of the few trials that reported a positive association between multivitamin supplementation and cognition in men was underpowered (N = 51) and found improvement in only 1 of 19 cognitive domains.49 Despite the poor design and results to the contrary, this industry-funded study nevertheless concluded that multivitamins may play a role in improving elements of memory.
Evidence of possible harm from antioxidants
While not always specific to men, many meta-analyses have explored the effects of antioxidant supplements on cardiovascular and mortality risk. Most of them concluded that antioxidant supplements have no benefit and that some may actually be harmful.
For example, multiple meta-analyses of vitamin E supplementation found no cardiovascular benefit but possible increases in all-cause mortality rates in those taking high doses (risk ratio 1.04, 95% CI 1.01–1.07).50,51
Another meta-analysis of 180,938 participants in high-quality studies found an increased risk of all-cause mortality associated with independent intake of several antioxidant vitamins, including beta-carotene (risk ratio 1.07, 95% CI 1.02–1.11) and vitamin A (risk ratio 1.16, 95% CI 1.10–1.24), while intake of vitamin C and selenium had no impact on mortality.52
Similarly, although nearly 10% of US adults report taking omega-3 fatty acid supplements, a review of 24 randomized controlled trials and meta-analyses published between 2005 and 2012 concluded that only 2 supported the use of these supplements for any health benefit.53
Can supplements improve sexual function, prostate health?
To improve sexual function. A 2015 narrative review of the ingredients in General Nutrition Center’s top 30 best-selling products targeted at improving men’s sexual performance (including improving libido and erectile dysfunction) found only poor evidence for any efficacy.54 The few studies that did support the use of select supplements, including B vitamins in people with diabetes, L-arginine, and yohimbine, were deemed to be of poor quality or showed a smaller effect size compared with standard medical therapy.
To prevent prostate cancer. Studies of supplement use to improve prostate health have had mixed results. For example, multiple large case-control studies have suggested that taking vitamin D55,56 or vitamin C57 is not associated with prostate cancer risk, while increased vitamin A58,59 and E60,61 intake is associated with inconsistent increases in prostate cancer risk.
In the Selenium and Vitamin E Cancer Prevention Trial,62 a randomized controlled trial in 35,533 men, those assigned to receive vitamin E supplementation were 17% more likely to get prostate cancer than were those assigned to placebo (HR 1.17, 99% CI 1.004–1.36, P = .008).
However, there are plausible biologic links between nutraceuticals and prostate cancer. For example, studies have linked genetic polymorphisms in vitamin D receptors63 as well as intake of natural androgen receptor modulators, such as the most active polyphenol in green tea,64 to prostate cancer risk and aggressiveness in certain populations. This led a recent review to conclude that there is some biologic plausibility, but at present little epidemiologic evidence, to support any dietary supplement’s ability to broadly affect prostate cancer risk.65
Interest continues in exploring the targeted use of nutraceuticals as adjuvant therapy in specific populations at risk of prostate cancer.66,67
To treat BPH. There is a similar dearth of clinical or population-based evidence that supplements can broadly affect BPH symptoms. For example, in a 2012 Cochrane review of Serenoa repens (saw palmetto) utilizing only high-quality evidence, there was no evidence that supplement use significantly reduced lower urinary tract symptoms, nocturia, or peak urine flow in BPH patients, and this was true even when the supplement was taken at triple-strength doses.68
For other diseases. There is also limited evidence that supplements can affect other chronic diseases. For example, a meta-analysis of 3,803 patients found that glucosamine, chondroitin, and their combination had no impact on joint pain or joint space narrowing in patients with osteoarthritis of the knee or hip.69
Even when there is some evidence to suggest benefit from supplementation, study heterogeneity and varying evidence quality limit confidence in the conclusions. For example, meta-analyses suggest garlic may improve blood pressure control in those with hypertension70 and improve lipid and blood glucose control in type 2 diabetes.71 However, most of the trials included in those systematic reviews were underpowered, with samples as low as 10 patients, and many suffered from improper design, such as inadequate blinding of researchers. In addition, these meta-analyses often do not report adverse events, suggesting that higher quality studies would be needed to adequately measure event rates. As such, there is need for caution and a case-by-case review before recommending even a seemingly benign supplement like garlic to patients.
In total, there is only limited evidence to support the efficacy of supplements across many diseases and concerns common to men in primary care. This includes improving general health, cardiovascular health, sexual functioning, or other chronic diseases. While a supplement’s placebo effect may at times provide some benefit, supplements are much less strictly regulated since the passing of the 1994 act, and even vitamin supplementation has been shown to be associated with negative health outcomes. As such, a patient’s use of supplements requires careful consideration and shared decision-making.
Supplements: Take-home points
- Supplements are only loosely regulated by the federal government.
- There is some biologic but limited epidemiologic evidence for the use of multivitamins to improve cognition or mortality rates; for the use of antioxidant vitamins or omega-3 fatty acids to improve cardiovascular health; for the use of any of the top-selling sexual enhancement supplements to improve libido or erectile function; and for the use of vitamins or other supplements for improving BPH or reducing prostate cancer risk. Using supplements may in some cases be harmful.
- Given the heterogeneity of studies of supplements to manage chronic diseases and a lack of reporting of adverse events, careful consideration is needed when recommending supplements to patients.
Primary care physicians are tasked with a wide variety of issues affecting men. This article reviews the latest research in 4 areas of men’s health commonly addressed in primary care:
- Medical management of benign prostatic hyperplasia (BPH)
- Prostate cancer screening and treatment
- Medical management of erectile dysfunction
- Use of supplements.
MEDICAL MANAGEMENT OF BPH
An 84-year-old man with a history of hypertension, type 2 diabetes, hyperlipidemia, BPH, mild cognitive impairment, and osteoarthritis presents for a 6-month follow-up, accompanied by his son.
Two years ago he was started on a 5-alpha reductase inhibitor and an alpha-blocker for worsening BPH symptoms. His BPH symptoms are currently under control, with an American Urological Association (AUA) symptom index score of 7 of a possible 35 (higher scores being worse).
However, both the patient and son are concerned about the number of medications he is on and wonder if some could be eliminated.
Assessment tools
BPH is a common cause of lower urinary tract symptoms in older men. Evidence-based tools to help the clinician and patient decide on when to consider treatment for symptoms are:
- The AUA symptom index1
- The International Prostate Symptom Score (IPSS).2
An AUA symptom index score or IPSS score of 8 through 19 of a possible 35 is consistent with moderate symptoms, while a score of 20 or higher indicates severe symptoms.
Combination therapy or monotherapy?
Monotherapy with an alpha-blocker or a 5-alpha reductase inhibitor is often the first-line treatment for BPH-related lower urinary tract symptoms.3 However, combination therapy with both an alpha-blocker and a 5-alpha reductase inhibitor is another evidence-based option.
The Medical Therapy of Prostatic Symptoms study,4 a randomized controlled trial, reported that long-term combination therapy reduced the risk of BPH clinical progression better than monotherapy. The same trial also found that either combination therapy or finasteride alone (a 5-alpha reductase inhibitor) reduced the risk of acute urinary retention and the future need for invasive therapy.
Monotherapy after a period of combination therapy?
There is also evidence to support switching from combination to monotherapy after an initial treatment period.
Matsukawa et al5 examined the effects of withdrawing the alpha-blocker from BPH combination therapy in a study in 140 patients. For 12 months, all patients received the alpha-blocker silodosin and the 5-alpha reductase inhibitor dutasteride. At 12 months, the remaining 132 patients (8 patients had been lost to follow-up) were randomized to continue combination therapy or to take dutasteride alone for another 12 months. They were evaluated at 0, 12, and 24 months by questionnaires (the IPSS and Overactive Bladder Symptom Score) and urodynamic testing (uroflowmetry, cystometrography, and pressure-flow studies).
There were no significant differences in subjective symptoms and bladder outlet obstruction between patients who continued combination therapy and those who switched to dutasteride monotherapy. In the monotherapy group, those whose symptoms worsened weighed more (68.8 kg vs 62.6 kg, P =.002) and had a higher body mass index (BMI) (26.2 kg/m2 vs 22.8 kg/m2, P < .001) than those whose symptoms stayed the same or got better.
These findings of successful alpha-blocker withdrawal were consistent with those of other studies.
The Symptom Management After Reducing Therapy study6 showed that 80% of men with an IPSS score less than 20 who changed to dutasteride monotherapy did not have a noticeable worsening of their symptoms.
Baldwin et al7 noted similar success after withdrawing the alpha-blocker doxazosin in patients on finasteride.
Review all medications
The National Health and Nutrition Examination Survey noted that the estimated prevalence of polypharmacy increased from 8% in 1999 to 15% in 2011.8 Many commonly used medications, such as decongestants, antihistamines, and anticholinergic agents, can worsen BPH symptoms,9 so it is reasonable to consistently review the patient’s medications to weigh the risks and benefits and determine which ones align with the patient’s personal care goals.
BPH: Take-home points
- Combination therapy with an alpha-blocker and a 5-alpha reductase inhibitor is an effective regimen for BPH.
- Polypharmacy is a significant problem in the elderly.
- Withdrawing the alpha-blocker component from BPH combination therapy can be considered after 1 year of combination therapy in patients whose symptoms have been well controlled.
PROSTATE CANCER SCREENING AND TREATMENT
A 60-year-old patient calls you after receiving his laboratory testing report from his insurance physical. His prostate-specific antigen (PSA) level is 5.1 ng/mL, and he has several questions:
- Should he have agreed to the screening?
- How effective is the screening?
- What are the next steps?
Is PSA screening useful?
Over the last few years, there has been great debate as to the utility of screening for prostate cancer.
The US Centers for Disease Control and Prevention10 reported that in 2014, an estimated 172,258 men in the United States were diagnosed with prostate cancer, but only 28,343 men died of it. These statistics support the notion that screening programs may be detecting what might otherwise be a silent disease.
The US Preventive Services Task Force (USPSTF)11 recommends against blanket PSA screening, in view of the low probability that it reduces the risk of death from prostate cancer. For men ages 55 through 69, current guidelines give a grade C recommendation to PSA screening, meaning there is moderate agreement that the benefit is likely small, and screening should be selectively offered based on professional judgment and patient preference. In men ages 70 and older who are not at high risk, the guideline gives screening a grade D recommendation, meaning there is moderate evidence that there is no benefit from the practice. This is a change from the 2012 USPSTF guidelines,12 which gave a grade D recommendation to PSA screening for all ages.
The American Urological Association13 recommends against PSA screening in men under age 40 or ages 70 and older. It does not recommend routine screening in those ages 40 to 54 at average risk, but it says the decision should be individualized in this age group in those at higher risk (eg, with a positive family history, African American). At ages 55 through 69, it recommends shared decision-making, taking into account cancer risk and life expectancy. In those who opt for screening, an interval of 2 years or more may be preferred over annual screening to reduce the risk of overdiagnosis.
The USPSTF recommendations rely heavily on data from 2 trials: the European Randomized Study of Screening for Prostate Cancer (ERSPC)14 and the Prostate, Lung, Colorectal, and Ovarian Screening (PLCO) trial.15
The ERSPC14 demonstrated that screening for prostate cancer reduced deaths from prostate cancer by 20%, with an absolute risk difference of 0.71 deaths per 1,000 men; 1,410 men would need to be screened and 48 additional cases of prostate cancer would need to be treated to prevent 1 death from prostate cancer. Screening also decreased the risk of developing metastatic disease by 30%.16 On the negative side, screening increased the risk of overdiagnosis and other harms such as bleeding, sepsis, and incontinence.
The PLCO trial,15 in contrast, found no difference in death rates between men randomly assigned to annual screening and those assigned to usual care. Differences between the trial results were thought to be due to different practice settings as well as study implementation and compliance.
Tsodikov et al17 reanalyzed data from the ERSPC and the PLCO trial using 3 different mathematical models to estimate the effects of screening in both trials compared with no screening. The analysis found no evidence that the effects of screening vs not screening differed between the 2 trials, ultimately concluding that PSA screening reduced prostate cancer deaths by 25% to 32%, which the authors inferred was primarily a result of earlier detection of cancer.
The Cluster Randomized Trial of PSA Testing for Prostate Cancer,18 published in March 2018, explored the effect of single PSA screening vs no screening on prostate cancer mortality rates in 419,582 men ages 50 through 69. Although screening detected more cases of low-risk prostate cancer, there was no significant difference in prostate cancer mortality rates after a median follow-up of 10 years. However, 10% to 15% of the control group was estimated to have also been screened, and these results do not directly speak to the efficacy of serial PSA screening.
Extended follow-up of this trial is planned to report on long-term survival benefits and whether screening lowers the risk of metastasis.
Imaging-guided prostate biopsy
Once a patient is found to have an elevated PSA level, standard practice has been to perform transrectal ultrasonography to obtain 12 core biopsy samples. The results indicate whether the prostate contains cancer, how aggressive the cancer is (Gleason score), and whether there is extracapsular extension.
In the past, magnetic resonance imaging (MRI) of the prostate before biopsy was thought to be too costly, and many insurance plans do not currently cover it.
Pahwa et al,19 however, in a cost-effectiveness study using a decision-analysis model, found that using MRI to detect lesions and then guide biopsy by triaging patients into proper treatment pathways added health benefits in a cost-effective manner in 94.05% of simulations. These benefits were found across all age groups.
This study demonstrated that doctors could use MRI to better evaluate patients for potentially harmful lesions. If a focus of cancer is found, it can be biopsied; if no cancer is seen on MRI, the patient can avoid biopsy completely. Additionally, though MRI tended to miss low-risk cancers, these cancers are thought to disproportionately lead to higher healthcare costs through unnecessary treatment. Therefore, a negative MRI study was believed to be an excellent sign that the patient does not have aggressive prostate cancer. This approach led to a net gain of 0.251 additional quality-adjusted life years compared with the standard biopsy strategy.
The Prostate MRI Imaging Study20 also found MRI to be effective in the prostate cancer workup. In this trial, 576 men who had never undergone biopsy underwent multiparametric MRI, transrectal ultrasonography-guided biopsy, and the reference standard, ie, transperineal template prostate mapping biopsy. Of those who underwent biopsy, 71% received a diagnosis of prostate cancer, and 40% had clinically significant disease. In patients with clinically significant disease, MRI was more sensitive than ultrasonography-guided biopsy (93% vs 48%, P < .0001) but less specific (41% vs 96%, P < .0001).
Based on these findings, if biopsy were performed only in those who had suspicious lesions on MRI, 27% of men with elevated PSA could avoid biopsy and its potential complications such as bleeding and sepsis, which occurred in 5.9% of the biopsy group.
The Prostate Evaluation for Clinically Important Disease: Sampling Using Image Guidance or Not? trial21 more recently studied MRI with or without targeted biopsy vs standard transrectal ultrasonography-guided biopsy in 500 men who had not undergone biopsy before, and reported similar results. MRI with or without biopsy led to fewer biopsies and less overdetection of clinically insignificant prostate cancers compared with the standard approach. Furthermore, those in the MRI-targeted biopsy group were 13% less likely to receive a diagnosis of clinically insignificant cancer than those who received the standard biopsy (adjusted difference −13 percentage points, 95% confidence interval [CI] −19 to −7, P < .001).
Together, these data provide another argument for adding multiparametric MRI to the workup of men with an elevated PSA level.
Surveillance vs treatment for prostate cancer
Once prostate cancer is diagnosed, surveillance is becoming an increasingly common management strategy.
The Prostate Cancer Intervention Versus Observation Trial (PIVOT),22 one of the largest and longest trials involving cancer patients, offered further evidence that active surveillance and less intervention for men with prostate cancer is a better approach in many cases. This trial compared prostatectomy and observation alone in a randomized fashion. Inclusion for the study required men to be medically fit for radical prostatectomy, along with having histologically confirmed localized prostate cancer (stage T1-T2NxM0 in the tumor-node-metastasis classification system) of any grade diagnosed within the last 12 months.
During 19.5 years of follow-up, 223 (61.3%) of the 364 men randomly assigned to radical prostatectomy died, compared with 245 (66.8%) of 367 men in the observation group; the difference was not statistically different (P = .06). Only 9.4% of the deaths were due to prostate cancer, 7.4% in the surgery group and 11.4% in the observation group (P = .06).
Surgery was associated with a lower all-cause mortality rate than observation in the subgroup of patients with intermediate-risk prostate cancer (defined as PSA 10–20 ng/mL and a Gleason score of 7). Surgery was also associated with less disease progression.22
This finding is in line with previous data from the Scandinavian Prostate Cancer Group Study Number 4,23 as well as the much larger Prostate Testing for Cancer and Treatment (ProtecT) trial,24 both of which reported that metastasis was 1.5 and 2.6 times as common, respectively, in participants in the active surveillance groups. However, in the PIVOT trial, those in the surgery group were significantly more likely than those in the observation group to have erectile dysfunction and urinary incontinence at 10 years.
Therefore, in men with localized disease and in those with low-risk PSA levels, both the PIVOT and ProtecT trials suggest that death from prostate cancer is uncommon and that observation may be more appropriate.
Prostate cancer: Take-home points
- A new look at 2 large trials of PSA screening strengthened evidence that testing in the right patient population can reduce deaths from prostate cancer, but a third recently published trial that found no benefit from 1-time screening may reopen debate on the topic.
- MRI offers a better method than ultrasonography-guided biopsy to triage patients thought to be at high risk of prostate cancer and tends to limit costly overtreatment of disease that likely would not cause death.
- Surgery for prostate cancer may not prolong life but could reduce disease progression, at the risk of more adverse effects.
- Shared decision-making should be practiced when deciding whether to use active surveillance or active treatment of diagnosed prostate cancer.
MANAGEMENT OF ERECTILE DYSFUNCTION
A 62-year-old man with hypertension, hyperlipidemia, peripheral artery disease, and type 2 diabetes presents for a 6-month follow-up. His medications include aspirin, metformin, lisinopril, and atorvastatin, all of which he takes without problems. Over the past several months, he has noticed that his erections are not adequate for sexual intercourse. He recently heard that a generic version of sildenafil has just become available, and he wonders if it might benefit him.
Erectile dysfunction is common, associated with chronic diseases
Erectile dysfunction, ie, persistent inability to obtain and maintain an erection sufficient to permit satisfactory sexual intercourse,25,26 is estimated to affect nearly 20% of men over the age of 20 and 75% of men over the age of 75.27
In age-adjusted models, erectile dysfunction has been shown28 to be associated with:
- History of cardiovascular disease (odds ratio [OR] 1.63, 95% CI 1.02–2.63)
- Diabetes (OR 3.90, 95% CI 2.16–7.04)
- Treated hypertension vs no hypertension (OR 2.22, 95% CI 1.30–3.80)
- Current smoking vs never smoking (OR 1.63, 95% CI 1.01–2.62)
- BMI greater than 30 kg/m2 vs less than 25 kg/m2 (OR 1.80, 95% CI 1.03–3.14).
Because of the strong association between cardiovascular disease and erectile dysfunction, the presence of one often suggests the need to screen for the other.29 While tools such as the International Index of Erectile Function (IIEF-5) have been developed to evaluate erectile dysfunction, it is most often diagnosed on the basis of clinical impression, while validated assessment methods are reserved for clinical trials.28
Multiple causes of erectile dysfunction
Erectile dysfunction arises from inadequate penile tissue response to a sexual signal. The response can be disrupted at several points. For example, damage to vascular smooth muscle cells (eg, from age or obesity) and endothelial cells (from smoking or diabetes) and narrowing of the vascular lumen (from atherosclerosis or hypertension) have all been shown to impair engorgement of the corpus cavernosum.30 In addition, denervation from prostate surgery or spinal trauma and psychogenic causes should be recognized in discussions with patients.
Drugs for erectile dysfunction
Pharmacologic management of erectile dysfunction includes oral, sublingual, intracavernosal, and intraurethral therapies.31 Treatment in primary care settings usually includes addressing underlying chronic diseases32 and prescribing phosphodiesterase-5 inhibitors (sildenafil, tadalafil, vardenafil, and avanafil). These drugs work by increasing local concentrations of cyclic guanosine monophosphate in the corpus cavernosum to induce vasodilation.33
While these 4 drugs are still patent-protected, a manufacturer has been allowed to introduce a generic version of sildenafil into US markets, and a generic version of tadalafil is expected to be available soon.
Sildenafil, tadalafil, and vardenafil have been studied and found to have some degree of effectiveness in erectile dysfunction caused by damage to the penile vasculature, denervation, and spinal cord injury.34 All drugs of this class have adverse effects including headache, facial flushing, and nasal congestion, but the drugs are generally well tolerated.35
Sildenafil and tadalafil improve IIEF-5 scores by a similar margin, raising scores on the erectile domain subsection from approximately 14 of a possible 30 to approximately 24 of 30 in a trial of both drugs.36 However, multiple crossover studies comparing the 2 drugs have shown that nearly 75% of patients prefer tadalafil to sildenafil,36,37 perhaps because of tadalafil’s longer duration of action.34
There is little evidence to suggest that vardenafil is more effective or more often preferred by patients than tadalafil or sidenafil.34,38 And though data on the newest drug on the market, avanafil, are limited, a meta-analysis concluded that it may be less effective than tadalafil and without significant differences in terms of safety.39
Other treatments
Lifestyle modifications, especially smoking cessation and exercise, have been shown to reduce the risk of erectile dysfunction with varying effect sizes across studies.40–42 Moreover, factors such as obesity, alcohol use, and smoking may cause irreversible harm, and thus a healthy lifestyle should be encouraged.41
While there is only weak evidence for the use of psychological interventions alone for treating most types of erectile dysfunction, one meta-analysis found that the combination of psychological intervention and a phosphodiesterase-5 inhibitor improved sexual satisfaction more than drug therapy alone.43
Erectile dysfunction: Take-home points
- Erectile dysfunction is common, affecting nearly 20% of men over the age of 20 and over 75% of men over the age of 75.
- Erectile dysfunction is often associated with chronic disease and may suggest the need to screen for cardiovascular disease.
- Treating underlying chronic diseases may help, and phosphodiesterase-5 inhibitors are effective; tadalafil may be most often preferred.
SUPPLEMENT USE AND MEN’S HEALTH
A 68-year-old man with a history of hypertension, BPH, and erectile dysfunction presents for a 6-month follow-up. His medication use includes lisinopril, which he takes without problems. He denies any new physical symptoms. His physical examination is unremarkable. He says he has heard about supplements that might help with his sexual performance and hopes to discuss recommendations during the visit.
A burgeoning, unregulated industry
Since the passage of the Dietary Supplement and Health Education Act in 1994, a law that decreased oversight of the supplement industry, spending on supplements has skyrocketed to over $41.1 billion each year.44 Advertisements for these products typically claim that they improve general mental and physical health, sexual and romantic performance, leanness, and muscularity.45 A national survey of men ages 57 and older reported that the most popular products were aimed at nutrition (such as multivitamins), cardiovascular health (such as omega-3 fatty acids), and chronic conditions (such as saw palmetto for BPH).46
Little evidence of efficacy
There is little evidence to support the use of most supplements to improve men’s health. For example, a study in 82,405 men found no association between mortality rates and multivitamin use (hazard ratio [HR] 1.07, 95% CI 0.96–1.19).47 Even for specific uses, such as cognitive performance, randomized trials exploring the effects of multivitamins in men have been largely negative.48
The positive trials that have been reported are often of low quality and are funded by supplement manufacturers. For example, one of the few trials that reported a positive association between multivitamin supplementation and cognition in men was underpowered (N = 51) and found improvement in only 1 of 19 cognitive domains.49 Despite the poor design and results to the contrary, this industry-funded study nevertheless concluded that multivitamins may play a role in improving elements of memory.
Evidence of possible harm from antioxidants
While not always specific to men, many meta-analyses have explored the effects of antioxidant supplements on cardiovascular and mortality risk. Most of them concluded that antioxidant supplements have no benefit and that some may actually be harmful.
For example, multiple meta-analyses of vitamin E supplementation found no cardiovascular benefit but possible increases in all-cause mortality rates in those taking high doses (risk ratio 1.04, 95% CI 1.01–1.07).50,51
Another meta-analysis of 180,938 participants in high-quality studies found an increased risk of all-cause mortality associated with independent intake of several antioxidant vitamins, including beta-carotene (risk ratio 1.07, 95% CI 1.02–1.11) and vitamin A (risk ratio 1.16, 95% CI 1.10–1.24), while intake of vitamin C and selenium had no impact on mortality.52
Similarly, although nearly 10% of US adults report taking omega-3 fatty acid supplements, a review of 24 randomized controlled trials and meta-analyses published between 2005 and 2012 concluded that only 2 supported the use of these supplements for any health benefit.53
Can supplements improve sexual function, prostate health?
To improve sexual function. A 2015 narrative review of the ingredients in General Nutrition Center’s top 30 best-selling products targeted at improving men’s sexual performance (including improving libido and erectile dysfunction) found only poor evidence for any efficacy.54 The few studies that did support the use of select supplements, including B vitamins in people with diabetes, L-arginine, and yohimbine, were deemed to be of poor quality or showed a smaller effect size compared with standard medical therapy.
To prevent prostate cancer. Studies of supplement use to improve prostate health have had mixed results. For example, multiple large case-control studies have suggested that taking vitamin D55,56 or vitamin C57 is not associated with prostate cancer risk, while increased vitamin A58,59 and E60,61 intake is associated with inconsistent increases in prostate cancer risk.
In the Selenium and Vitamin E Cancer Prevention Trial,62 a randomized controlled trial in 35,533 men, those assigned to receive vitamin E supplementation were 17% more likely to get prostate cancer than were those assigned to placebo (HR 1.17, 99% CI 1.004–1.36, P = .008).
However, there are plausible biologic links between nutraceuticals and prostate cancer. For example, studies have linked genetic polymorphisms in vitamin D receptors63 as well as intake of natural androgen receptor modulators, such as the most active polyphenol in green tea,64 to prostate cancer risk and aggressiveness in certain populations. This led a recent review to conclude that there is some biologic plausibility, but at present little epidemiologic evidence, to support any dietary supplement’s ability to broadly affect prostate cancer risk.65
Interest continues in exploring the targeted use of nutraceuticals as adjuvant therapy in specific populations at risk of prostate cancer.66,67
To treat BPH. There is a similar dearth of clinical or population-based evidence that supplements can broadly affect BPH symptoms. For example, in a 2012 Cochrane review of Serenoa repens (saw palmetto) utilizing only high-quality evidence, there was no evidence that supplement use significantly reduced lower urinary tract symptoms, nocturia, or peak urine flow in BPH patients, and this was true even when the supplement was taken at triple-strength doses.68
For other diseases. There is also limited evidence that supplements can affect other chronic diseases. For example, a meta-analysis of 3,803 patients found that glucosamine, chondroitin, and their combination had no impact on joint pain or joint space narrowing in patients with osteoarthritis of the knee or hip.69
Even when there is some evidence to suggest benefit from supplementation, study heterogeneity and varying evidence quality limit confidence in the conclusions. For example, meta-analyses suggest garlic may improve blood pressure control in those with hypertension70 and improve lipid and blood glucose control in type 2 diabetes.71 However, most of the trials included in those systematic reviews were underpowered, with samples as low as 10 patients, and many suffered from improper design, such as inadequate blinding of researchers. In addition, these meta-analyses often do not report adverse events, suggesting that higher quality studies would be needed to adequately measure event rates. As such, there is need for caution and a case-by-case review before recommending even a seemingly benign supplement like garlic to patients.
In total, there is only limited evidence to support the efficacy of supplements across many diseases and concerns common to men in primary care. This includes improving general health, cardiovascular health, sexual functioning, or other chronic diseases. While a supplement’s placebo effect may at times provide some benefit, supplements are much less strictly regulated since the passing of the 1994 act, and even vitamin supplementation has been shown to be associated with negative health outcomes. As such, a patient’s use of supplements requires careful consideration and shared decision-making.
Supplements: Take-home points
- Supplements are only loosely regulated by the federal government.
- There is some biologic but limited epidemiologic evidence for the use of multivitamins to improve cognition or mortality rates; for the use of antioxidant vitamins or omega-3 fatty acids to improve cardiovascular health; for the use of any of the top-selling sexual enhancement supplements to improve libido or erectile function; and for the use of vitamins or other supplements for improving BPH or reducing prostate cancer risk. Using supplements may in some cases be harmful.
- Given the heterogeneity of studies of supplements to manage chronic diseases and a lack of reporting of adverse events, careful consideration is needed when recommending supplements to patients.
- Barry MJ, Fowler FJ Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. J Urol 2017; 197(2S):S189–S197. doi:10.1016/j.juro.2016.10.071
- Urological Sciences Research Foundation. International Prostate Symptom Score (IPSS). http://www.usrf.org/questionnaires/AUA_SymptomScore.html. Accessed October 16, 2018.
- McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 2011; 185(5):1793–1803. doi:10.1016/j.juro.2011.01.074
- McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 2003; 349(25):2387–2398. doi:10.1056/NEJMoa030656
- Matsukawa Y, Takai S, Funahashi Y, et al. Effects of withdrawing alpha-1 blocker from the combination therapy with alpha-1 blocker and 5-alpha-reductase inhibitor in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a prospective and comparative trial using urodynamics. J Urol 2017; 198(4):905–912. doi:10.1016/j.juro.2017.05.031
- Barkin J, Guimaraes M, Jacobi G, Pushkar D, Taylor S, van Vierssen Trip OB. Alpha-blocker therapy can be withdrawn in the majority of men following initial combination therapy with the dual 5a-reductase inhibitor dutasteride. Eur Urol 2003; 44(4):461–466. pmid:14499682
- Baldwin KC, Ginsberg PC, Roehrborn CG, Harkaway RC. Discontinuation of alpha-blockade after initial treatment with finasteride and doxazosin in men with lower urinary tract symptoms and clinical evidence of benign prostatic hyperplasia. Urology 2001; 58(2):203–209. pmid:11489700
- Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA 2015; 314(17):1818–1831. doi:10.1001/jama.2015.13766
- DuBeau CE, Yalla SV, Resnick NM. Improving the utility of urine flow rate to exclude outlet obstruction in men with voiding symptoms. J Am Geriatr Soc 1998; 46(9):1118–1124. pmid:9736105
- US Department of Health and Human Services Health Resources and Services Administration. United States Cancer Statistics: 1999-2014 Incidence and Mortality Web-Based Report. Atlanta; 2017. https://nccd.cdc.gov/uscs/. Accessed October 17, 2018.
- US Preventive Services Task Force. Final recommendation statement. Prostate cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1. Accessed October 16, 2018.
- US Preventive Services Task Force. Archived: prostate cancer: screening. Original release date: May 2012. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/prostate-cancer-screening. Accessed October 16, 2018.
- Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA guideline. J Urol 2013; 190(2):419–426. doi:10.1016/j.juro.2013.04.119
- Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009; 360(13):1320–1328. doi:10.1056/NEJMoa0810084
- Andriole GL, Crawford ED, Grubb RL, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 2009; 360(13):1310–1319. doi:10.1056/NEJMoa0810696
- Schröder FH, Hugosson J, Carlsson S, et al. Screening for prostate cancer decreases the risk of developing metastatic disease: findings from the European Randomized Study of Screening for Prostate Cancer (ERSPC). Eur Urol 2012; 62(5):745–752. doi:10.1016/j.eururo.2012.05.068
- Tsodikov A, Gulati R, Heijnsdijk EAM, et al. Reconciling the effects of screening on prostate cancer mortality in the ERSPC and PLCO trials. Ann Intern Med 2017; 167(7):449–455. doi:10.7326/M16-2586
- Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA 2018; 319(9):883–895. doi:10.1001/jama.2018.0154
- Pahwa S, Schiltz NK, Ponsky LE, Lu Z, Griswold MA, Gulani V. Cost-effectiveness of MR imaging–guided strategies for detection of prostate cancer in biopsy-naive men. Radiology 2017; 285(1):157–166. doi:10.1148/radiol.2017162181
- Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017; 389(10071):815–822. doi:10.1016/S0140-6736(16)32401-1
- Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018; 378(19):1767–1777. doi:10.1056/NEJMoa1801993
- Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med 2017; 377(2):132–142. doi:10.1056/NEJMoa1615869
- Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med 2014; 370(10):932–942. doi:10.1056/NEJMoa1311593
- Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016; 375(15):1415–1424. doi:10.1056/NEJMoa1606220
- Morley JE. Impotence. Am J Med 1986; 80(5):897–905. pmid:3518438
- NIH Consensus Development Panel on Impotence. NIH Consensus Conference. Impotence. JAMA 1993; 270(1):83–90. pmid:8510302
- Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med 2007; 120(2):151–157. doi:10.1016/j.amjmed.2006.06.010
- Rosen RC, Cappelleri JC, Gendrano N 3rd. The International Index of Erectile Function (IIEF): a state-of-the-science review. Int J Impot Res 2002; 14(4):226–244. doi:10.1038/sj.ijir.3900857
- Gandaglia G, Briganti A, Jackson G, et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur Urol 2014; 65(5):968–978. doi:10.1016/j.eururo.2013.08.023
- Heaton JPW, Adams MA. Causes of erectile dysfunction. Endocrine 2004; 23(2-3):119–123. doi:10.1385/ENDO:23:2-3:119
- Montorsi F, Salonia A, Deho F, et al. Pharmacological management of erectile dysfunction. BJU Int 2003; 91(5):446–454. pmid:12603396
- Cai X, Tian Y, Wu T, Cao CX, Bu SY, Wang KJ. The role of statins in erectile dysfunction: a systematic review and meta-analysis. Asian J Androl 2014; 16(3):461–466. doi:10.4103/1008-682X.123678
- Webb DJ, Freestone S, Allen MJ, Muirhead GJ. Sildenafil citrate and blood-pressure–lowering drugs: results of drug interaction studies with an organic nitrate and a calcium antagonist. Am J Cardiol 1999; 83(5):21C–28C. pmid:10078539
- Doggrell SA. Comparison of clinical trials with sildenafil, vardenafil and tadalafil in erectile dysfunction. Expert Opin Pharmacother 2005; 6(1):75–84. doi:10.1517/14656566.6.1.75
- Gresser U, Gleiter CH. Erectile dysfunction: comparison of efficacy and side effects of the PDE-5 inhibitors sildenafil, vardenafil and tadalafil—review of the literature. Eur J Med Res 2002; 7(10):435–446. pmid:12435622
- Eardley I, Mirone V, Montorsi F, et al. An open-label, multicentre, randomized, crossover study comparing sildenafil citrate and tadalafil for treating erectile dysfunction in men naive to phosphodiesterase 5 inhibitor therapy. BJU Int 2005; 96(9):1323–1332. doi:10.1111/j.1464-410X.2005.05892.x
- von Keitz A, Rajfer J, Segal S, et al. A multicenter, randomized, double-blind, crossover study to evaluate patient preference between tadalafil and sildenafil. Eur Urol 2004; 45(4):499–509. doi:10.1016/j.eururo.2003.11.030
- Martin-Morales A, Haro JM, Beardsworth A, Bertsch J, Kontodimas S; EDOS Group. Therapeutic effectiveness and patient satisfaction after 6 months of treatment with tadalafil, sildenafil, and vardenafil: results from the erectile dysfunction observational study (EDOS). Eur Urol 2007; 51(2):541–550. doi:10.1016/j.eururo.2006.09.027
- Yuan J, Zhang R, Yang Z, et al. Comparative effectiveness and safety of oral phosphodiesterase type 5 inhibitors for erectile dysfunction: a systematic review and network meta-analysis. Eur Urol 2013; 63(5):902–912. doi:10.1016/j.eururo.2013.01.012
- Cao S, Yin X, Wang Y, Zhou H, Song F, Lu Z. Smoking and risk of erectile dysfunction: systematic review of observational studies with meta-analysis. PLoS One 2013; 8(4):e60443. doi:10.1371/journal.pone.0060443
- Derby CA, Mohr BA, Goldstein I, Feldman HA, Johannes CB, McKinlay JB. Modifiable risk factors and erectile dysfunction: can lifestyle changes modify risk? Urology 2000; 56(2):302–306. pmid:10925098
- Esposito K, Giugliano F, Di Palo C, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA 2004; 291(24):2978–2984. doi:10.1001/jama.291.24.2978
- Schmidt HM, Munder T, Gerger H, Frühauf S, Barth J. Combination of psychological intervention and phosphodiesterase-5 inhibitors for erectile dysfunction: a narrative review and meta-analysis. J Sex Med 2014; 11(6):1376–1391. doi:10.1111/jsm.12520
- New Hope Network. Supplement Business Report 2017. Boulder; 2017. http://images.info.newhope.com/Web/NewHopeNaturalMedia/%7B3a3f3b03-6130-41d4-9e66-84f29eeebe44%7D_2017_Supplement_Business_Report_-_Extended_TOC.pdf. Accessed October 16, 2018.
- Labre MP. Burn fat, build muscle: a content analysis of men’s health and men’s fitness. Int J Mens Health 2005; 4(2):187–200.
- Qato DM, Alexander GC, Conti RM, Johnson M, Schumm P, Lindau ST. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA 2008; 300(24):2867–2878. doi:10.1001/jama.2008.892
- Park SY, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Multivitamin use and the risk of mortality and cancer incidence: the multiethnic cohort study. Am J Epidemiol 2011; 173(8):906–914. doi:10.1093/aje/kwq447
- McNeill G, Avenell A, Campbell MK, et al. Effect of multivitamin and multimineral supplementation on cognitive function in men and women aged 65 years and over: a randomised controlled trial. Nutr J 2007; 6(1):10. doi:10.1186/1475-2891-6-10
- Harris E, Macpherson H, Vitetta L, Kirk J, Sali A, Pipingas A. Effects of a multivitamin, mineral and herbal supplement on cognition and blood biomarkers in older men: a randomised, placebo-controlled trial. Hum Psychopharmacol Clin Exp 2012; 27(4):370–377. doi:10.1002/hup.2236
- Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet 2003; 361(9374):2017–2023. doi:10.1016/S0140-6736(03)13637-9
- Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 2005; 142(1):37–46. pmid:15537682
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 2007; 297(8):842–857. doi:10.1001/jama.297.8.842
- Grey A, Bolland M. Clinical trial evidence and use of fish oil supplements. JAMA Intern Med 2014; 174(3):460–462. doi:10.1001/jamainternmed.2013.12765
- Cui T, Kovell RC, Brooks DC, Terlecki RP. A urologist’s guide to ingredients found in top-selling nutraceuticals for men’s sexual health. J Sex Med 2015; 12(11):2105–2117. doi:10.1111/jsm.13013
- Schenk JM, Till CA, Tangen CM, et al. Serum 25-hydroxyvitamin D concentrations and risk of prostate cancer: results from the Prostate Cancer Prevention Trial. Cancer Epidemiol Prev Biomarkers 2014; 23(8):1484–1493. doi:10.1158/1055-9965.EPI-13-1340
- Albanes D, Mondul AM, Yu K, et al. Serum 25-hydroxy vitamin D and prostate cancer risk in a large nested case-control study. Cancer Epidemiol Prev Biomarkers 2011; 20(9):1850–1860. doi:10.1158/1055-9965.EPI-11-0403
- Roswall N, Larsen SB, Friis S, et al. Micronutrient intake and risk of prostate cancer in a cohort of middle-aged, Danish men. Cancer Causes Control 2013; 24(6):1129–1135. doi:10.1007/s10552-013-0190-4
- Mondul AM, Watters JL, Männistö S, et al. Serum retinol and risk of prostate cancer. Am J Epidemiol 2011; 173(7):813-821. doi:10.1093/aje/kwq429
- Schenk JM, Riboli E, Chatterjee N, et al. Serum retinol and prostate cancer risk: a nested case-control study in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Prev Biomarkers 2009; 18(4):1227–1231. doi:10.1158/1055-9965.EPI-08-0984
- Bidoli E, Talamini R, Zucchetto A, et al. Dietary vitamins E and C and prostate cancer risk. Acta Oncol 2009; 48(6):890–894. doi:10.1080/02841860902946546
- Wright ME, Weinstein SJ, Lawson KA, et al. Supplemental and dietary vitamin E intakes and risk of prostate cancer in a large prospective study. Cancer Epidemiol Prev Biomarkers 2007; 16(6):1128–1135. doi:10.1158/1055-9965.EPI-06-1071
- Klein EA, Thompson IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011; 306(14):1549–1556. doi:10.1001/jama.2011.1437
- Jingwi EY, Abbas M, Ricks-Santi L, et al. Vitamin D receptor genetic polymorphisms are associated with PSA level, Gleason score and prostate cancer risk in African-American men. Anticancer Res 2015; 35(3):1549–1558. pmid:25750310
- Siddiqui IA, Asim M, Hafeez BB, Adhami VM, Tarapore RS, Mukhtar H. Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB J 2011; 25(4):1198–1207. doi:10.1096/fj.10-167924
- Yacoubian A, Dargham RA, Khauli RB, Bachir BG. Overview of dietary supplements in prostate cancer. Curr Urol Rep 2016; 17(11):78. doi:10.1007/s11934-016-0637-8
- Kallifatidis G, Hoy JJ, Lokeshwar BL. Bioactive natural products for chemoprevention and treatment of castration-resistant prostate cancer. Semin Cancer Biol 2016; 40:160–169. doi:10.1016/j.semcancer.2016.06.003
- Shui IM, Mondul AM, Lindström S, et al. Circulating vitamin D, vitamin D–related genetic variation, and risk of fatal prostate cancer in the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. Cancer 2015; 121(12):1949–1956. doi:10.1002/cncr.29320
- Tacklind J, MacDonald R, Rutks I, Stanke JU, Wilt TJ. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev 2012; 12:CD001423. doi:10.1002/14651858.CD001423.pub3
- Wandel S, Jüni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ 2010; 341:c4675. doi:10.1136/bmj.c4675
- Reinhart KM, Coleman CI, Teevan C, Vachhani P, White CM. Effects of garlic on blood pressure in patients with and without systolic hypertension: a meta-analysis. Ann Pharmacother 2008; 42(12):1766–1771. doi:10.1345/aph.1L319
- Wang J, Zhang X, Lan H, Wang W. Effect of garlic supplement in the management of type 2 diabetes mellitus (T2DM): a meta-analysis of randomized controlled trials. Food Nutr Res 2017; 61(1):1377571. doi:10.1080/16546628.2017.1377571
- Barry MJ, Fowler FJ Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. J Urol 2017; 197(2S):S189–S197. doi:10.1016/j.juro.2016.10.071
- Urological Sciences Research Foundation. International Prostate Symptom Score (IPSS). http://www.usrf.org/questionnaires/AUA_SymptomScore.html. Accessed October 16, 2018.
- McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 2011; 185(5):1793–1803. doi:10.1016/j.juro.2011.01.074
- McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 2003; 349(25):2387–2398. doi:10.1056/NEJMoa030656
- Matsukawa Y, Takai S, Funahashi Y, et al. Effects of withdrawing alpha-1 blocker from the combination therapy with alpha-1 blocker and 5-alpha-reductase inhibitor in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a prospective and comparative trial using urodynamics. J Urol 2017; 198(4):905–912. doi:10.1016/j.juro.2017.05.031
- Barkin J, Guimaraes M, Jacobi G, Pushkar D, Taylor S, van Vierssen Trip OB. Alpha-blocker therapy can be withdrawn in the majority of men following initial combination therapy with the dual 5a-reductase inhibitor dutasteride. Eur Urol 2003; 44(4):461–466. pmid:14499682
- Baldwin KC, Ginsberg PC, Roehrborn CG, Harkaway RC. Discontinuation of alpha-blockade after initial treatment with finasteride and doxazosin in men with lower urinary tract symptoms and clinical evidence of benign prostatic hyperplasia. Urology 2001; 58(2):203–209. pmid:11489700
- Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA 2015; 314(17):1818–1831. doi:10.1001/jama.2015.13766
- DuBeau CE, Yalla SV, Resnick NM. Improving the utility of urine flow rate to exclude outlet obstruction in men with voiding symptoms. J Am Geriatr Soc 1998; 46(9):1118–1124. pmid:9736105
- US Department of Health and Human Services Health Resources and Services Administration. United States Cancer Statistics: 1999-2014 Incidence and Mortality Web-Based Report. Atlanta; 2017. https://nccd.cdc.gov/uscs/. Accessed October 17, 2018.
- US Preventive Services Task Force. Final recommendation statement. Prostate cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1. Accessed October 16, 2018.
- US Preventive Services Task Force. Archived: prostate cancer: screening. Original release date: May 2012. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/prostate-cancer-screening. Accessed October 16, 2018.
- Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA guideline. J Urol 2013; 190(2):419–426. doi:10.1016/j.juro.2013.04.119
- Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009; 360(13):1320–1328. doi:10.1056/NEJMoa0810084
- Andriole GL, Crawford ED, Grubb RL, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 2009; 360(13):1310–1319. doi:10.1056/NEJMoa0810696
- Schröder FH, Hugosson J, Carlsson S, et al. Screening for prostate cancer decreases the risk of developing metastatic disease: findings from the European Randomized Study of Screening for Prostate Cancer (ERSPC). Eur Urol 2012; 62(5):745–752. doi:10.1016/j.eururo.2012.05.068
- Tsodikov A, Gulati R, Heijnsdijk EAM, et al. Reconciling the effects of screening on prostate cancer mortality in the ERSPC and PLCO trials. Ann Intern Med 2017; 167(7):449–455. doi:10.7326/M16-2586
- Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA 2018; 319(9):883–895. doi:10.1001/jama.2018.0154
- Pahwa S, Schiltz NK, Ponsky LE, Lu Z, Griswold MA, Gulani V. Cost-effectiveness of MR imaging–guided strategies for detection of prostate cancer in biopsy-naive men. Radiology 2017; 285(1):157–166. doi:10.1148/radiol.2017162181
- Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017; 389(10071):815–822. doi:10.1016/S0140-6736(16)32401-1
- Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018; 378(19):1767–1777. doi:10.1056/NEJMoa1801993
- Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med 2017; 377(2):132–142. doi:10.1056/NEJMoa1615869
- Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med 2014; 370(10):932–942. doi:10.1056/NEJMoa1311593
- Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016; 375(15):1415–1424. doi:10.1056/NEJMoa1606220
- Morley JE. Impotence. Am J Med 1986; 80(5):897–905. pmid:3518438
- NIH Consensus Development Panel on Impotence. NIH Consensus Conference. Impotence. JAMA 1993; 270(1):83–90. pmid:8510302
- Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med 2007; 120(2):151–157. doi:10.1016/j.amjmed.2006.06.010
- Rosen RC, Cappelleri JC, Gendrano N 3rd. The International Index of Erectile Function (IIEF): a state-of-the-science review. Int J Impot Res 2002; 14(4):226–244. doi:10.1038/sj.ijir.3900857
- Gandaglia G, Briganti A, Jackson G, et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur Urol 2014; 65(5):968–978. doi:10.1016/j.eururo.2013.08.023
- Heaton JPW, Adams MA. Causes of erectile dysfunction. Endocrine 2004; 23(2-3):119–123. doi:10.1385/ENDO:23:2-3:119
- Montorsi F, Salonia A, Deho F, et al. Pharmacological management of erectile dysfunction. BJU Int 2003; 91(5):446–454. pmid:12603396
- Cai X, Tian Y, Wu T, Cao CX, Bu SY, Wang KJ. The role of statins in erectile dysfunction: a systematic review and meta-analysis. Asian J Androl 2014; 16(3):461–466. doi:10.4103/1008-682X.123678
- Webb DJ, Freestone S, Allen MJ, Muirhead GJ. Sildenafil citrate and blood-pressure–lowering drugs: results of drug interaction studies with an organic nitrate and a calcium antagonist. Am J Cardiol 1999; 83(5):21C–28C. pmid:10078539
- Doggrell SA. Comparison of clinical trials with sildenafil, vardenafil and tadalafil in erectile dysfunction. Expert Opin Pharmacother 2005; 6(1):75–84. doi:10.1517/14656566.6.1.75
- Gresser U, Gleiter CH. Erectile dysfunction: comparison of efficacy and side effects of the PDE-5 inhibitors sildenafil, vardenafil and tadalafil—review of the literature. Eur J Med Res 2002; 7(10):435–446. pmid:12435622
- Eardley I, Mirone V, Montorsi F, et al. An open-label, multicentre, randomized, crossover study comparing sildenafil citrate and tadalafil for treating erectile dysfunction in men naive to phosphodiesterase 5 inhibitor therapy. BJU Int 2005; 96(9):1323–1332. doi:10.1111/j.1464-410X.2005.05892.x
- von Keitz A, Rajfer J, Segal S, et al. A multicenter, randomized, double-blind, crossover study to evaluate patient preference between tadalafil and sildenafil. Eur Urol 2004; 45(4):499–509. doi:10.1016/j.eururo.2003.11.030
- Martin-Morales A, Haro JM, Beardsworth A, Bertsch J, Kontodimas S; EDOS Group. Therapeutic effectiveness and patient satisfaction after 6 months of treatment with tadalafil, sildenafil, and vardenafil: results from the erectile dysfunction observational study (EDOS). Eur Urol 2007; 51(2):541–550. doi:10.1016/j.eururo.2006.09.027
- Yuan J, Zhang R, Yang Z, et al. Comparative effectiveness and safety of oral phosphodiesterase type 5 inhibitors for erectile dysfunction: a systematic review and network meta-analysis. Eur Urol 2013; 63(5):902–912. doi:10.1016/j.eururo.2013.01.012
- Cao S, Yin X, Wang Y, Zhou H, Song F, Lu Z. Smoking and risk of erectile dysfunction: systematic review of observational studies with meta-analysis. PLoS One 2013; 8(4):e60443. doi:10.1371/journal.pone.0060443
- Derby CA, Mohr BA, Goldstein I, Feldman HA, Johannes CB, McKinlay JB. Modifiable risk factors and erectile dysfunction: can lifestyle changes modify risk? Urology 2000; 56(2):302–306. pmid:10925098
- Esposito K, Giugliano F, Di Palo C, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA 2004; 291(24):2978–2984. doi:10.1001/jama.291.24.2978
- Schmidt HM, Munder T, Gerger H, Frühauf S, Barth J. Combination of psychological intervention and phosphodiesterase-5 inhibitors for erectile dysfunction: a narrative review and meta-analysis. J Sex Med 2014; 11(6):1376–1391. doi:10.1111/jsm.12520
- New Hope Network. Supplement Business Report 2017. Boulder; 2017. http://images.info.newhope.com/Web/NewHopeNaturalMedia/%7B3a3f3b03-6130-41d4-9e66-84f29eeebe44%7D_2017_Supplement_Business_Report_-_Extended_TOC.pdf. Accessed October 16, 2018.
- Labre MP. Burn fat, build muscle: a content analysis of men’s health and men’s fitness. Int J Mens Health 2005; 4(2):187–200.
- Qato DM, Alexander GC, Conti RM, Johnson M, Schumm P, Lindau ST. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA 2008; 300(24):2867–2878. doi:10.1001/jama.2008.892
- Park SY, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Multivitamin use and the risk of mortality and cancer incidence: the multiethnic cohort study. Am J Epidemiol 2011; 173(8):906–914. doi:10.1093/aje/kwq447
- McNeill G, Avenell A, Campbell MK, et al. Effect of multivitamin and multimineral supplementation on cognitive function in men and women aged 65 years and over: a randomised controlled trial. Nutr J 2007; 6(1):10. doi:10.1186/1475-2891-6-10
- Harris E, Macpherson H, Vitetta L, Kirk J, Sali A, Pipingas A. Effects of a multivitamin, mineral and herbal supplement on cognition and blood biomarkers in older men: a randomised, placebo-controlled trial. Hum Psychopharmacol Clin Exp 2012; 27(4):370–377. doi:10.1002/hup.2236
- Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet 2003; 361(9374):2017–2023. doi:10.1016/S0140-6736(03)13637-9
- Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 2005; 142(1):37–46. pmid:15537682
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 2007; 297(8):842–857. doi:10.1001/jama.297.8.842
- Grey A, Bolland M. Clinical trial evidence and use of fish oil supplements. JAMA Intern Med 2014; 174(3):460–462. doi:10.1001/jamainternmed.2013.12765
- Cui T, Kovell RC, Brooks DC, Terlecki RP. A urologist’s guide to ingredients found in top-selling nutraceuticals for men’s sexual health. J Sex Med 2015; 12(11):2105–2117. doi:10.1111/jsm.13013
- Schenk JM, Till CA, Tangen CM, et al. Serum 25-hydroxyvitamin D concentrations and risk of prostate cancer: results from the Prostate Cancer Prevention Trial. Cancer Epidemiol Prev Biomarkers 2014; 23(8):1484–1493. doi:10.1158/1055-9965.EPI-13-1340
- Albanes D, Mondul AM, Yu K, et al. Serum 25-hydroxy vitamin D and prostate cancer risk in a large nested case-control study. Cancer Epidemiol Prev Biomarkers 2011; 20(9):1850–1860. doi:10.1158/1055-9965.EPI-11-0403
- Roswall N, Larsen SB, Friis S, et al. Micronutrient intake and risk of prostate cancer in a cohort of middle-aged, Danish men. Cancer Causes Control 2013; 24(6):1129–1135. doi:10.1007/s10552-013-0190-4
- Mondul AM, Watters JL, Männistö S, et al. Serum retinol and risk of prostate cancer. Am J Epidemiol 2011; 173(7):813-821. doi:10.1093/aje/kwq429
- Schenk JM, Riboli E, Chatterjee N, et al. Serum retinol and prostate cancer risk: a nested case-control study in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Prev Biomarkers 2009; 18(4):1227–1231. doi:10.1158/1055-9965.EPI-08-0984
- Bidoli E, Talamini R, Zucchetto A, et al. Dietary vitamins E and C and prostate cancer risk. Acta Oncol 2009; 48(6):890–894. doi:10.1080/02841860902946546
- Wright ME, Weinstein SJ, Lawson KA, et al. Supplemental and dietary vitamin E intakes and risk of prostate cancer in a large prospective study. Cancer Epidemiol Prev Biomarkers 2007; 16(6):1128–1135. doi:10.1158/1055-9965.EPI-06-1071
- Klein EA, Thompson IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011; 306(14):1549–1556. doi:10.1001/jama.2011.1437
- Jingwi EY, Abbas M, Ricks-Santi L, et al. Vitamin D receptor genetic polymorphisms are associated with PSA level, Gleason score and prostate cancer risk in African-American men. Anticancer Res 2015; 35(3):1549–1558. pmid:25750310
- Siddiqui IA, Asim M, Hafeez BB, Adhami VM, Tarapore RS, Mukhtar H. Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB J 2011; 25(4):1198–1207. doi:10.1096/fj.10-167924
- Yacoubian A, Dargham RA, Khauli RB, Bachir BG. Overview of dietary supplements in prostate cancer. Curr Urol Rep 2016; 17(11):78. doi:10.1007/s11934-016-0637-8
- Kallifatidis G, Hoy JJ, Lokeshwar BL. Bioactive natural products for chemoprevention and treatment of castration-resistant prostate cancer. Semin Cancer Biol 2016; 40:160–169. doi:10.1016/j.semcancer.2016.06.003
- Shui IM, Mondul AM, Lindström S, et al. Circulating vitamin D, vitamin D–related genetic variation, and risk of fatal prostate cancer in the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. Cancer 2015; 121(12):1949–1956. doi:10.1002/cncr.29320
- Tacklind J, MacDonald R, Rutks I, Stanke JU, Wilt TJ. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev 2012; 12:CD001423. doi:10.1002/14651858.CD001423.pub3
- Wandel S, Jüni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ 2010; 341:c4675. doi:10.1136/bmj.c4675
- Reinhart KM, Coleman CI, Teevan C, Vachhani P, White CM. Effects of garlic on blood pressure in patients with and without systolic hypertension: a meta-analysis. Ann Pharmacother 2008; 42(12):1766–1771. doi:10.1345/aph.1L319
- Wang J, Zhang X, Lan H, Wang W. Effect of garlic supplement in the management of type 2 diabetes mellitus (T2DM): a meta-analysis of randomized controlled trials. Food Nutr Res 2017; 61(1):1377571. doi:10.1080/16546628.2017.1377571
KEY POINTS
- The combination of an alpha-blocker and a 5-alpha reductase inhibitor is an effective regimen for BPH. Withdrawing the alpha-blocker from the combination can be considered if symptoms have been well controlled after 1 year of combination therapy.
- A new look at 2 large trials of prostate-specific antigen screening strengthened evidence that testing in the right patient population can reduce deaths from prostate cancer, but a third recently published trial found no benefit to 1-time screening.
- Magnetic resonance imaging offers a better method than ultrasonography-guided biopsy to triage patients thought to be at high risk of prostate cancer and tends to limit costly overtreatment of disease that likely would not cause death.
- Erectile dysfunction is often associated with chronic disease and may suggest the need to screen for cardiovascular disease.
Bisphosphonate-related atypical femoral fracture: Managing a rare but serious complication
Bisphosphonate therapy minimizes bone loss and reduces fracture risk by up to 50% in patients with osteoporosis,1 but it is also associated with increased risks of osteonecrosis of the jaw and atypical femoral fracture. Although atypical femoral fractures are rare, they can have a devastating effect. Patient concern about this complication has contributed to a decrease in bisphosphonate use by about half in the last decade or so,2,3 and we fear this could result in an increase in hip fracture rates.
In this article, we examine the evidence on bisphosphonate-associated atypical femoral fractures, including risks, pathogenesis, treatment, and prevention.
ATYPICAL FRACTURES INVOLVE THE FEMORAL SHAFT, NOT THE HEAD
An atypical femoral fracture is a transverse fracture of the femoral shaft (diaphysis), defined by both clinical criteria and radiographic appearance.
To be defined as atypical, a femoral fracture must meet 4 of the following 5 criteria4:
- Occurs with minimal or no trauma
- Has a predominantly transverse fracture line, originating at the lateral cortex and sometimes becoming oblique as it progresses medially across the femur
- Extends through both cortices and may be associated with a medial spike (complete fractures); or involves only the lateral cortex (incomplete fractures)
- Is noncomminuted or minimally comminuted
- Shows localized periosteal or endosteal thickening (termed “beaking” or “flaring”) of the lateral cortex at the fracture site.
Several minor features are also important but are not required, eg:
- Cortical thickening of the femoral shaft
- Unilateral or bilateral prodromal pain preceding the fracture
- Bilateral incomplete or complete femoral diaphysis fractures
- Delayed fracture healing.
Atypical femoral fracture can occur anywhere along the shaft, from just distal to the lesser trochanter to just proximal to the supracondylar flare. However, most occur in 2 areas, with 1 cluster centered at about 41 mm from the lesser trochanter (more common in relatively younger patients) and the other at 187 mm.5
ABSOLUTE RISK IS LOW BUT INCREASES WITH LONGER USE
Atypical femoral fractures are rare. Schilcher et al6 reviewed radiographs of 1,234 women who had a subtrochanteric or shaft fracture and found 59 (4.6%) of fractures were atypical. In a systematic review of 14 studies,7 the incidence ranged from 3.0 to 9.8 cases per 100,000 patient-years.
Furthermore, not all atypical femoral fractures are in bisphosphonate users: 7.4% were in nonusers in 1 series8 and 22% in another.9
Nevertheless, most studies show that bisphosphonate use increases the incidence of atypical femoral fracture, and the incidence increases with duration of use, especially after 3 years.7
An international task force of the American Society for Bone and Mineral Research listed the absolute risk as between 3.2 and 50 cases per 100,000 patient-years, with longer use (> 5 years) increasing the risk to about 100 per 100,000 patient-years.4 After stopping bisphosphonate therapy, the risk diminished by 70% per year.9
In another study, for 0.1 to 1.9 years of therapy, the age-adjusted atypical fracture rates were 1.78 per 100,000 per year (95% confidence interval [CI] 1.5–2.0), increasing to 113.1 per 100,000 per year (95% CI 69.3–156.8) with exposure from 8 to 9.9 years.10
A case-control study found that more than 5 years of bisphosphonate use increased the fracture risk by an odds ratio of 2.74 (95% CI 1.25–6.02).11
The incidence of typical femoral fracture was higher in those who adhered better to their oral bisphosphonate regimen in some studies,12 but the opposite was true in others.13
The benefits of bisphosphonate therapy in reducing fracture risk, however, outweigh the risk of atypical fracture.4
We do not know whether the rate of atypical femoral fracture is increasing. A review of Kaiser Permanente Northwest records found that the rates of atypical femoral shaft fracture had remained stable from 1996 to 2009. However, 61.9% of patients who met the strict radiographic criteria had taken oral bisphosphonates.14 These data suggest that bisphosphonate use has not increased the overall population-based risk for subtrochanteric and femoral shaft fractures, but that bisphosphonates and other risk factors may have increased the likelihood that such fractures will exhibit atypical radiographic features.
A population-based study in Denmark13 found that alendronate use longer than 10 years was associated with an adjusted 30% lower risk of hip fracture and no increase in the risk of subtrochanteric and femoral shaft fracture. In addition, the risk of subtrochanteric and femoral shaft fracture was lower with high adherence to alendronate treatment (based on medication possession ratio > 80%) compared with low adherence (ratio < 50%) (odds ratio 0.88, 95% CI 0.77–0.99). The risk was not increased in current vs past users.
The Danish study13 used the coding of the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) to identify subtrochanteric and femoral shaft fractures without radiologic review for atypical radiographic features. The lack of specific ICD-10 coding for subtrochanteric and femoral shaft fractures with atypical radiographic features has limited our knowledge of their incidence.
Contralateral fracture in more than one-fourth of cases
After an atypical femoral fracture, patients have a significant risk of fracture on the contralateral side. In a case-control study, 28% of patients with atypical femoral fracture suffered a contralateral fracture, compared with 0.9% of patients presenting with a typical fracture pattern (odds ratio 42.6, 95% CI 12.8–142.4).15
Contralateral fracture occurs from 1 month to 4 years after the index atypical femoral fracture.16
There are reports of bisphosphonate-related low-impact fractures in other sites such as the tibia17 and forearm.18 However, they may be too rare to warrant screening.
Mortality rates
A Swedish database study found that patients with atypical femoral fractures, whether bisphosphonate users or nonusers, do not have higher mortality rates than patients with ordinary subtrochanteric or femoral shaft fractures.19 Furthermore, the mortality rates for those with atypical femoral fracture were similar to rates in the general population. In contrast, patients with an ordinary femoral fracture had a higher mortality risk than the general population.19
Other studies suggest that atypical femoral fracture may be associated with a less favorable prognosis in older patients,20 but this could be due to differences in demographics, treatment adherence, or postfracture care.21
In addition, functional outcomes as measured by independent mobility at discharge and at 3 months were comparable between patients with atypical fracture and those with typical fracture.22
IMAGING STUDIES
If a long-term bisphosphonate user presents with hip, thigh, or groin pain, imaging studies are recommended.
Plain radiography
Radiography is usually the first step and should include a frontal view of the pelvis (Figure 1) and 2 views of the full length of each femur. If radiography is not conclusive, bone scan or magnetic resonance imaging (MRI) should be considered.
A linear cortex transverse fracture pattern and focal lateral cortical thickening are the most sensitive and specific radiographic features.23,24 Because of the risk of fracture on the contralateral side, radiographic study of that side is recommended as well.
Computed tomography
Computed tomography (CT) is not sensitive for early stress fractures and, given the radiation burden, is not recommended in the workup of atypical fracture.
Bone scanning
Bone scanning using technetium 99m-labeled methylene diphosphonate with a gamma camera shows active bone turnover. Stress fractures and atypical femoral fractures are most easily identified in the third (delayed) phase of the bone scan. Although bone scanning is highly sensitive, the specificity is limited by lack of spatial resolution. Atypical femoral fracture appears as increased activity in the subtrochanteric region with a predilection for the lateral cortex.
Dual-energy x-ray absorptiometry
Conventional dual-energy x-ray absorptiometry (DXA) extends only to 1 to 2 cm below the lesser trochanter and can therefore miss atypical fractures, which usually occur farther down. The overall detection rate for DXA was 61% in a sample of 33 patients.25
Newer scanners can look at the entire femoral shaft.26 In addition, newer software can quantify focal thickening (beaking) of the lateral cortex and screen patients who have no symptoms. The results of serial measurements can be graphed so that the practitioner can view trends to help assess or rule out potential asymptomatic atypical femoral fracture.
A localized reaction (periosteal thickening of the lateral cortex or beaking) often precedes atypical femoral fracture. A 2017 study reported that patients with high localized reaction (mean height 3.3 mm) that was of the pointed type and was accompanied by prodromal pain had an increased risk of complete or incomplete atypical femoral fracture at that site.27 This finding is used by the newer DXA software. The predictive value of beaking on extended femoral DXA may be as high as 83%.26
Magnetic resonance imaging
The MRI characteristics of atypical femoral fracture are similar to those of other stress fractures except that there is a lateral-to-medial pattern rather than a medial pattern. The earliest findings include periosteal reaction about the lateral cortex with a normal marrow signal.
MRI may be of particular benefit in patients with known atypical femoral fracture to screen the contralateral leg. It should image the entire length of both femurs. Contrast enhancement is not needed.
Regardless of whether initial findings were discovered on conventional radiographs or DXA, MRI confirmation is needed. Radionuclide bone scanning is currently not recommended because it lacks specificity. Combination imaging is recommended, with either radiography plus MRI or DXA plus MRI.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of atypical femoral fracture includes stress fracture, pathologic fracture, hypophosphatasia, and osteogenesis imperfecta.28 Hypophosphatemic osteomalacia can cause Looser zones, which can be confused with atypical femoral fractures but usually occur on the medial side.4 Stress fracture of the femur can occur below the lesser trochanter but usually begins in the medial, not the lateral, cortex.
Pathologic fractures from underlying osseous lesions can mimic the cortical beaking of bisphosphonate-related fracture, but they usually show the associated underlying lucent lesion and poorly defined margins. A sinus tract along the region of a chronic osteomyelitis may also appear similar.
Hypophosphatasia is an inborn error of metabolism caused by a loss-of-function mutation in the gene encoding alkaline phosphatase, resulting in pyrophosphate accumulation and causing osteomalacia from impaired mineralization. This can result in femoral pseudofracture that is often bilateral and occurs in the subtrochanteric region.29
ADDITIONAL RISK FACTORS
Patients with atypical femoral fracture are generally a heterogeneous group, but there are risk factors to note other than bisphosphonate exposure.
Asian women had a risk 8 times higher than white women in 1 study.30
Bone geometry. Mahjoub et al8 reported that compared with controls, patients with atypical femoral fracture had greater offset of the femoral shaft from the center of rotation of the femoral head, a more acute angle between the femoral neck and shaft, and greater proximal cortical thickness.
Medications. In addition to bisphosphonates, other drugs associated with atypical femoral fracture include RANK-ligand inhibitors such as denosumab (another drug for osteoporosis),31 glucocorticoids,32,33 and proton pump inhibitors.32,33
Genetics. Three sisters with atypical femoral fracture were found to have 37 rare mutations in 34 genes, including one in the GGPS1 gene, which codes for geranylgeranyl pyrophosphate synthase—an enzyme that bisphosphonates inhibit.34
Medical conditions other than osteoporosis include collagen diseases, chronic pulmonary disease, asthma, rheumatoid arthritis, and diabetes.35
Clinical recommendations
Current recommendations are to reevaluate bisphosphonate use in patients with osteoporosis after 5 or more years of therapy.36
Given that patients with osteoporosis are at increased risk of typical fracture, those at higher risk should be considered for continued bisphosphonate therapy. Factors for high risk include the following:
- History of fracture on therapy
- Hip T score –2.5 or lower
- Older age (≥ 70)
- Other strong risk factors for fracture such as smoking, alcohol use, corticosteroid use, rheumatoid arthritis, and family history
- World Health Organization FRAX fracture risk score above the country-specific threshold.
Those at lower risk should be considered for a 2- to 3-year bisphosphonate holiday with periodic reevaluation of bone density and, possibly, bone markers.36
WHAT IS THE UNDERLYING PATHOPHYSIOLOGY?
The mechanism by which bisphosphonates increase the risk of atypical femoral fracture is not clear. These drugs work by suppressing bone turnover; however, in theory, prolonged use could suppress it too much and increase bone fragility.
One hypothesis is that bisphosphonates impair the toughening of cortical bone, an important barrier to clinical fracture. This is supported by a study that found bisphosphonate users with atypical femoral fracture had deficits in intrinsic and extrinsic bone toughness, perhaps due to treatment-related increases in matrix mineralization.37 Although this study and others showed an increase in matrix mineralization and reduced mineralization heterogeneity with bisphosphonate use,38,39 it is unclear whether such changes contributed to reduced toughness or to atypical femoral fracture.
Changes in the skeletal geometry of the lower limb such as femoral neck-shaft angle and femoral curvature alter the stresses and strains experienced by the femoral diaphysis with loading. Because the incidence of incomplete atypical femoral fracture is much greater than that of complete fracture, most incomplete atypical femoral fractures heal before the fracture progresses.
Ultimately, all fractures, including atypical femoral fractures, occur when mechanical stress and strain exceed bone strength.
Antiresorptive drugs such as bisphosphonates, estrogen, calcitonin, and RANK ligand inhibitors prevent hip fracture by increasing the strength of the proximal femur—perhaps at the expense of the strength (or toughness) of the subtrochanteric shaft. It is also possible that treatment-related increases in hip strength (and reduced hip fracture rates) promote or sustain the transfer of stress and strain to femoral regions that experience lesser or no increases in strength from treatment, which likely includes the shaft.40,41
CT studies in Japanese women with osteoporosis have shown that 2 years of zoledronate therapy had greater effects in the hip than in the femoral shaft, with significant increases in cortical thickness and volumetric bone mineral density at the femoral neck and intertrochanteric region compared with baseline.42 But zoledronate did not increase femoral shaft cortical thickness and caused only a minor increase in femoral shaft volumetric bone mineral density. Fracture patterns may have depended on damage and effects of bone turnover on mass and structure.
This hypothetical scenario portrays a possible “hip survival bias” mechanism for atypical femoral fracture, with the association with antiresorptive drugs arising from greater stress and strain in cortical regions where these fractures occur rather than from treatment-related reductions in cortical bone strength or toughness.
PRODROMAL PAIN IS COMMON
From 32% to 76% of patients who have incomplete or developing atypical femoral fracture present with a prodrome of groin or hip pain.4,43 Prodromal pain occurs any time from 2 weeks to several years before the fracture, presenting as pain in the anterior or lateral thigh or in the groin.
Prodromal pain in a patient on antiresorptive therapy should be a signal for the clinician to obtain a radiograph of the hip and to look for contralateral symptoms and fractures. The most common mechanism of injury appears to be a ground-level fall or even a nontraumatic activity such as walking or stepping off a curb.
MEDICAL MANAGEMENT
In bisphosphonate users with radiographic evidence of atypical femoral fracture, the bisphosphonate should be discontinued and the patient assessed for calcium and vitamin D deficiency, with supplements prescribed if needed.4
For patients with incomplete fracture and persistent pain after 3 months of medical management, prophylactic surgical nail fixation is recommended to prevent complete fracture.
Teriparatide, which has been associated with enhanced bone fracture healing, is a possible treatment to promote healing of atypical femoral fracture, either alone or as an adjunct to surgical fixation. A systematic review published in 2015 supported the use of teriparatide for enhancing fracture healing in atypical femoral fracture.44 In addition, a 10-patient series45 showed that incomplete fractures without radiolucent lines responded to teriparatide alone, whereas those with radiolucent lines needed intramedullary nailing.
These results suggest that teriparatide works best when the fracture site is stable, either inherently or with surgical fixation.
ORTHOPEDIC CARE
Orthopedic care for atypical femoral fracture differs depending on whether the patient experiences pain and whether the fracture is incomplete or complete. Figure 2 shows a treatment algorithm for atypical femoral fracture.
These are difficult fractures to manage, complicated by delayed healing in the elderly, complex displacement patterns, altered bone geometry, and risk of fracture in the opposite limb, all of which raise questions about recommending protected weight-bearing exercise.
Furthermore, atypical femoral fracture is often associated with increased anterolateral bowing of the femur, making it difficult to insert an intramedullary nail: the radius of curvature of the bone is shorter than that of a standard femoral nail. This mismatch can lead to intraoperative complications such as iatrogenic fracture during prophylactic nailing, malunion from excess straightening of the femur (which can itself lead to leg length discrepancy), and gapping of the fracture site, particularly on the medial side.
Intramedullary nailing for complete fracture
Intramedullary nailing is the first-line treatment for complete atypical femoral fracture, although the risk of delayed healing and revision surgery may be somewhat higher than with typical femoral fracture.46 Prophylactic intramedullary nailing should be considered for a patient with intractable pain.2
A radiograph of the opposite leg should be obtained routinely, looking for an asymptomatic fracture. Bisphosphonates should be discontinued and calcium and vitamin D continued. Teriparatide therapy can be considered as an alternative treatment.
Conservative management for incomplete fracture without pain
Incomplete atypical femoral fracture unaccompanied by pain can be followed conservatively.47 In addition to stopping antiresorptive therapy, patients need to avoid high-impact and repetitive-impact activities such as jumping or running. If pain occurs, patients should begin protected weight-bearing exercise.
Treatment is uncertain for incomplete fracture with pain
For patients with incomplete atypical femoral fracture and pain, treatment is controversial. Regimens that include 2 to 3 months of protected weight-bearing exercise, a full metabolic bone workup, calcium and vitamin D supplementation, and anabolic bone agents have produced some success. Some authors have reported poor results from conservative care, with few patients achieving pain relief or signs of complete healing.48,49 Additionally, if an incomplete fracture is found in the opposite femur, protected weight-bearing of both legs may not be possible.
Patients with incomplete fracture should be monitored regularly with radiography and physical examination. If there is progression of the fracture, escalation of pain, or failure to heal within 2 to 3 months, then surgical treatment is necessary.
Prophylactic placement of an intramedullary nail to prevent completion of the fracture and allow a return to full weight-bearing is generally advised.50 A long locking plate can be used if bone deformities make it difficult to place an intramedullary nail; however, nails are preferred because they allow formation of endochondral callus, which can be helpful in these difficult-to-heal fractures.
Results from retrospective reviews have shown that surgically treated patients with bisphosphonate-associated incomplete atypical femoral fracture were more likely than those treated nonsurgically to be pain-free (81% vs 64%) and have radiographic healing (100% vs 18% at final follow-up).46 Results have also been positive for those with complete atypical femoral fracture. At 6 months, 64% of surgically treated patients were pain-free and 98% were radiographically healed.51
The unusual geometry of the femur in patients with atypical femoral fracture and the presence of intramedullary cortical callus makes the placement of an intramedullary femoral rod more complex than in typical femoral fracture.8
Intramedullary nailing of atypical femoral fracture is a challenge for even the most experienced surgeon, and vigilance is imperative to avoid iatrogenic fracture and malunion.
MANY QUESTIONS REMAIN
We need more studies on the pathophysiology of bisphosphonate-associated atypical femoral fracture, the value of periodic screening with DXA, and which factors predict high risk (eg, Asian ethnicity, use of certain medications, femoral geometry). In addition, we need more data on the success of conservative management of incomplete fracture, including use of teriparatide.
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348(9041):1535–1541. pmid:8950879
- Jha S, Wang Z, Laucis N, Bhattacharyya T. Trends in media reports, oral bisphosphonate prescriptions, and hip fractures 1996–2012: an ecological analysis. J Bone Miner Res 2015; 30(12):2179–2187. doi:10.1002/jbmr.2565
- Solomon DH, Johnston SS, Boytsov NN, McMorrow D, Lane JM, Krohn KD. Osteoporosis medication use after hip fracture in US patients between 2002 and 2011. J Bone Miner Res 2014; 29(9):1929–1937. doi:10.1002/jbmr.2202
- Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2014; 29(1):1–23. doi:10.1002/jbmr.1998
- Koeppen VA, Schilcher J, Aspenberg P. Dichotomous location of 160 atypical femoral fractures. Acta Orthop 2013; 84(6):561–564. doi:10.3109/17453674.2013.866193
- Schilcher J, Koeppen V, Aspenberg P, Michäelsson K. Risk of atypical femoral fracture during and after bisphosphonate use. Acta Orthop 2015; 86(1):100–107. doi:10.3109/17453674.2015.1004149
- Khow KS, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Epidemiology and postoperative outcomes of atypical femoral fractures in older adults: a systematic review. J Nutr Health Aging 2017; 21(1):83–91. doi:10.1007/s12603-015-0652-3
- Mahjoub Z, Jean S, Leclerc JT, et al. Incidence and characteristics of atypical femoral fractures: clinical and geometrical data. J Bone Miner Res 2016; 31(4):767–776. doi:10.1002/jbmr.2748
- Schilcher J, Michaelsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med 2011; 364(18):1728–1737. doi:10.1056/NEJMoa1010650
- Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res 2012; 27(12):2544–2550. doi:10.1002/jbmr.1719
- Park-Wyllie LY, Mamdani MM, Juurlink DN, et al. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA 2011; 305(8):783–789. doi:10.1001/jama.2011.190
- Wang Z, Ward MM, Chan L, Bhattacharyya T. Adherence to oral bisphosphonates and the risk of subtrochanteric and femoral shaft fractures among female Medicare beneficiaries. Osteoporos Int 2014; 25(8):2109–2116. doi:10.1007/s00198-014-2738-x
- Abrahamsen B, Eiken P, Prieto-Alhambra D, Eastell R. Risk of hip, subtrochanteric, and femoral shaft fractures among mid and long term users of alendronate: nationwide cohort and nested case-control study. BMJ 2016; 353:i3365. doi:10.1136/bmj.i3365
- Feldstein AC, Black D, Perrin N, et al. Incidence and demography of femur fractures with and without atypical features. J Bone Miner Res 2012; 27(5):977–986. doi:10.1002/jbmr.1550
- Meier RP, Perneger TV, Stern R, Rizzoli R, Peter RE. Increasing occurrence of atypical femoral fractures associated with bisphosphonate use. Arch Intern Med 2012; 172(12):930–936. doi:10.1001/archinternmed.2012.1796
- La Rocca Vieira R, Rosenberg ZS, Allison MB, Im SA, Babb J, Peck V. Frequency of incomplete atypical femoral fractures in asymptomatic patients on long term bisphosphonate therapy. AJR Am J Roentgenol 2012; 198(5):1144–1151. doi:10.2214/AJR.11.7442
- Bissonnette L, April PM, Dumais R, Boire G, Roux S. Atypical fracture of the tibial diaphysis associated with bisphosphonate therapy: a case report. Bone 2013; 56(2):406–409. doi:10.1016/j.bone.2013.07.012
- Moon J, Bither N, Lee T. Atypical forearm fractures associated with long-term use of bisphosphonate. Arch Orthop Trauma Surg 2013; 133(7):889–892. doi:10.1007/s00402-013-1760-3
- Kharazmi M, Hallberg P, Schilcher J, Aspenberg P, Michaëlsson K. Mortality after atypical femoral fractures: a cohort study. J Bone Miner Res 2016; 31(3):491–497. doi:10.1002/jbmr.2767
- Medin E, Goude F, Melberg HO, Tediosi F, Belicza E, Peltola M; EuroHOPE Study Group. European regional differences in all-cause mortality and length of stay for patients with hip fracture. Health Econ 2015; 24(suppl 2):53–64. doi:10.1002/hec.3278
- Abrahamsen B, Prieto-Alhambra D. Patients with atypical femur fractures have the same mortality as the background population-drug channeling bias, bisphosphonate effects and public health implications. J Bone Miner Res 2016; 31(3):488–490. doi:10.1002/jbmr.2801
- Khow KS, Paterson F, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Outcomes between older adults with atypical and typical femoral fractures are comparable. Injury 2017; 48(2):394–398. doi:10.1016/j.injury.2016.10.035
- Adams AL, Xue F, Chantra JQ, et al. Sensitivity and specificity of radiographic characteristics in atypical femoral fractures. Osteoporos Int 2017; 28(1):413–417. doi:10.1007/s00198-016-3809-y
- Rosenberg ZS, La Rocca Vieira R, Chan SS, et al. Bisphosphonate-related complete atypical subtrochanteric femoral fractures: diagnostic utility of radiography. AJR Am J Roentgenol 2011; 197(4):954–960. doi:10.2214/AJR.10.6262
- Kim S, Yang KH, Lim H, et al. Detection of prefracture hip lesions in atypical subtrochanteric fracture with dual-energy x-ray absorptiometry images. Radiology 2014; 270(2):487–495. doi:10.1148/radiol.13122691
- van de Laarschot DM, Smits AA, Buitendijk SK, Stegenga MT, Zillikens MC. Screening for atypical femur fractures using extended femur scans by DXA. J Bone Miner Res 2017; 32(8):1632–1639. doi:10.1002/jbmr.3164
- Sato H, Kondo N, Nakatsue T, et al. High and pointed type of femoral localized reaction frequently extends to complete an incomplete atypical femoral fracture in patients with autoimmune diseases on long-term glucocorticoids and bisphosphonates. Osteoporos Int 2017; 28(8):2367–2376. doi:10.1007/s00198-017-4038-8
- Giaconi JC, Watterson CT. Bisphosphonate-related atypical femur fractures and the radiographic features. In: Silverman SL, Abrahamsen B, eds. The Duration and Safety of Osteoporosis Treatment. Switzerland: Springer International Publishing; 2016:107–124. doi:10.1007/978-3-319-23639-1
- Whyte MP. Atypical femoral fractures, bisphosphonates, and adult hypophosphatasia. J Bone Miner Res 2009; 24(6):1132–1134. doi:10.1359/jbmr.081253
- Lo JC, Hui RL, Grimsrud CD, et al. The association of race/ethnicity and risk of atypical femoral fracture among older women receiving oral bisphosphonate therapy. Bone 2016; 85:142–147. doi:10.1016/j.bone.2016.01.002
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 2017; 5(7):513–523. doi:10.1016/S2213-8587(17)30138-9
- Koh JH, Myong JP, Yoo J, et al. Predisposing factors associated with atypical femur fracture among postmenopausal Korean women receiving bisphosphonate therapy: 8 years' experience in a single center. Osteoporos Int 2017; 28(11):3251–3259. doi:10.1007/s00198-017-4169-y
- Kim D, Sung YK, Cho SK, Han M, Kim YS. Factors associated with atypical femoral fracture. Rheumatol Int 2016; 36(1):65–71. doi:10.1007/s00296-015-3323-0
- Roca-Ayats N, Balcells S, Garcia-Giralt N, et al. GGPS1 mutation and atypical femoral fractures with bisphosphonates. N Engl J Med 2017; 376(18):1794–1795. doi:10.1056/NEJMc1612804
- Giusti A, Hamdy NA, Dekkers OM, Ramautar SR, Dijkstra S, Papapoulos SE. Atypical fractures and bisphosphonate therapy: a cohort study of patients with femoral fracture with radiographic adjudication of fracture site and features. Bone 2011; 48(5):966–971. doi:10.1016/j.bone.2010.12.033
- Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2016; 31(1):16–35. doi:10.1002/jbmr.2708
- Lloyd AA, Gludovatz B, Riedel C, et al. Atypical fracture with long-term bisphosphonate therapy is associated with altered cortical composition and reduced fracture resistance. Proc Natl Acad Sci USA 2017; 114(33):8722–8727. doi:10.1073/pnas.1704460114
- Ettinger B, Burr DB, Ritchie RO. Proposed pathogenesis for atypical femoral fractures; lessons from materials research. Bone 2013; 55(2):495–500. doi:10.1016/j.bone.2013.02.004
- Burr DB, Liu Z, Allen MR. Duration-dependent effects of clinically relevant oral alendronate doses on cortical bone toughness in beagle dogs. Bone 2015; 71:58–62. doi:10.1016/j.bone.2014.10.010
- Sasaki S, Miyakoshi N, Hongo M, Kasukawa Y, Shimada Y. Low-energy diaphyseal femoral fractures associated with bisphosphonate use and severe curved femur: a case series. J Bone Miner Metab 2012; 30(5):561–567. doi:10.1007/s00774-012-0358-0
- Pulkkinen P, Gluer C, Jamsa T. Investigation of differences between hip fracture types: a worthy strategy of improved risk assessment and fracture prevention. Bone 2011; 49(4):600–604. doi:10.1016/j.bone.2011.07.022
- Ito M, Sone T, Shiraki M, et al. The effect of once-yearly zoledronic acid on hip structural and biomechanical properties derived using computed tomography (CT) in Japanese women with osteoporosis. Bone 2018; 106:179–186. doi:10.1016/j.bone.2017.10.013
- Bogdan Y, Einhorn TA. Clinical presentation of atypical femur fractures. In: Silverman SL, Abrahamsen B, eds. The Duration and Safety of Osteoporosis Treatment. Switzerland: Springer International Publishing; 2016:137–140. doi:10.1007/978-3-319-23639-1
- Im GI, Lee SH. Effect of teriparatide on healing of atypical femoral fractures: a systemic review. J Bone Metab 2015; 22(4):183–189. doi:10.11005/jbm.2015.22.4.183
- Saleh A, Hegde VV, Potty AG, Schneider R, Cornell CN, Lane JM. Management strategy for symptomatic bisphosphonate-associated incomplete atypical femoral fractures. HSS J 2012; 8(2):103–110. doi:10.1007/s11420-012-9275-y
- Egol KA, Park JH, Prensky C, Rosenberg ZS, Peck V, Tejwani NC. Surgical treatment improves clinical and functional outcomes for patients who sustain incomplete bisphosphonate-related femur fractures. J Orthop Trauma 2013; 27(6):331–335. doi:10.1097/BOT.0b013e31827240ae
- Koh A, Guerado E, Giannoudis PV. Atypical femoral fractures related to bisphosphonate treatment: issues and controversies related to their surgical management. Bone Joint J 2017; 99-B(3):295–302. doi:10.1302/0301-620X.99B3.BJJ-2016-0276.R2
- Oh CW, Oh JK, Park KC, Kim JW, Yoon YC. Prophylactic nailing of incomplete atypical femoral fractures. ScientificWorldJournal 2013; 2013:450148. doi:10.1155/2013/450148
- Ha YC, Cho MR, Park KH, Kim SY, Koo KH. Is surgery necessary for femoral insufficiency fractures after long-term bisphosphonate therapy? Clin Orthop Relat Res 2010; 468(12):3393–3398. doi:10.1007/s11999-010-1583-2
- Tosounidis TH, Lampropoulou-Adamidou, Kanakaris NK. Intramedullary nailing of sequential bilateral atypical subtrochanteric fractures and the management of distal femoral intraoperative fracture. J Orthop Trauma 2015 Jun 11. Epub ahead of print. doi:10.1097/BOT.0000000000000370
- Egol KA, Park JH, Rosenberg ZS, Peck V, Tejwani NC. Healing delayed but generally reliable after bisphosphonate-associated complete femur fractures treated with IM nails. Clin Orthop Relat Res 2014; 472(9):2728–2734. doi:10.1007/s11999-013-2963-1
Bisphosphonate therapy minimizes bone loss and reduces fracture risk by up to 50% in patients with osteoporosis,1 but it is also associated with increased risks of osteonecrosis of the jaw and atypical femoral fracture. Although atypical femoral fractures are rare, they can have a devastating effect. Patient concern about this complication has contributed to a decrease in bisphosphonate use by about half in the last decade or so,2,3 and we fear this could result in an increase in hip fracture rates.
In this article, we examine the evidence on bisphosphonate-associated atypical femoral fractures, including risks, pathogenesis, treatment, and prevention.
ATYPICAL FRACTURES INVOLVE THE FEMORAL SHAFT, NOT THE HEAD
An atypical femoral fracture is a transverse fracture of the femoral shaft (diaphysis), defined by both clinical criteria and radiographic appearance.
To be defined as atypical, a femoral fracture must meet 4 of the following 5 criteria4:
- Occurs with minimal or no trauma
- Has a predominantly transverse fracture line, originating at the lateral cortex and sometimes becoming oblique as it progresses medially across the femur
- Extends through both cortices and may be associated with a medial spike (complete fractures); or involves only the lateral cortex (incomplete fractures)
- Is noncomminuted or minimally comminuted
- Shows localized periosteal or endosteal thickening (termed “beaking” or “flaring”) of the lateral cortex at the fracture site.
Several minor features are also important but are not required, eg:
- Cortical thickening of the femoral shaft
- Unilateral or bilateral prodromal pain preceding the fracture
- Bilateral incomplete or complete femoral diaphysis fractures
- Delayed fracture healing.
Atypical femoral fracture can occur anywhere along the shaft, from just distal to the lesser trochanter to just proximal to the supracondylar flare. However, most occur in 2 areas, with 1 cluster centered at about 41 mm from the lesser trochanter (more common in relatively younger patients) and the other at 187 mm.5
ABSOLUTE RISK IS LOW BUT INCREASES WITH LONGER USE
Atypical femoral fractures are rare. Schilcher et al6 reviewed radiographs of 1,234 women who had a subtrochanteric or shaft fracture and found 59 (4.6%) of fractures were atypical. In a systematic review of 14 studies,7 the incidence ranged from 3.0 to 9.8 cases per 100,000 patient-years.
Furthermore, not all atypical femoral fractures are in bisphosphonate users: 7.4% were in nonusers in 1 series8 and 22% in another.9
Nevertheless, most studies show that bisphosphonate use increases the incidence of atypical femoral fracture, and the incidence increases with duration of use, especially after 3 years.7
An international task force of the American Society for Bone and Mineral Research listed the absolute risk as between 3.2 and 50 cases per 100,000 patient-years, with longer use (> 5 years) increasing the risk to about 100 per 100,000 patient-years.4 After stopping bisphosphonate therapy, the risk diminished by 70% per year.9
In another study, for 0.1 to 1.9 years of therapy, the age-adjusted atypical fracture rates were 1.78 per 100,000 per year (95% confidence interval [CI] 1.5–2.0), increasing to 113.1 per 100,000 per year (95% CI 69.3–156.8) with exposure from 8 to 9.9 years.10
A case-control study found that more than 5 years of bisphosphonate use increased the fracture risk by an odds ratio of 2.74 (95% CI 1.25–6.02).11
The incidence of typical femoral fracture was higher in those who adhered better to their oral bisphosphonate regimen in some studies,12 but the opposite was true in others.13
The benefits of bisphosphonate therapy in reducing fracture risk, however, outweigh the risk of atypical fracture.4
We do not know whether the rate of atypical femoral fracture is increasing. A review of Kaiser Permanente Northwest records found that the rates of atypical femoral shaft fracture had remained stable from 1996 to 2009. However, 61.9% of patients who met the strict radiographic criteria had taken oral bisphosphonates.14 These data suggest that bisphosphonate use has not increased the overall population-based risk for subtrochanteric and femoral shaft fractures, but that bisphosphonates and other risk factors may have increased the likelihood that such fractures will exhibit atypical radiographic features.
A population-based study in Denmark13 found that alendronate use longer than 10 years was associated with an adjusted 30% lower risk of hip fracture and no increase in the risk of subtrochanteric and femoral shaft fracture. In addition, the risk of subtrochanteric and femoral shaft fracture was lower with high adherence to alendronate treatment (based on medication possession ratio > 80%) compared with low adherence (ratio < 50%) (odds ratio 0.88, 95% CI 0.77–0.99). The risk was not increased in current vs past users.
The Danish study13 used the coding of the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) to identify subtrochanteric and femoral shaft fractures without radiologic review for atypical radiographic features. The lack of specific ICD-10 coding for subtrochanteric and femoral shaft fractures with atypical radiographic features has limited our knowledge of their incidence.
Contralateral fracture in more than one-fourth of cases
After an atypical femoral fracture, patients have a significant risk of fracture on the contralateral side. In a case-control study, 28% of patients with atypical femoral fracture suffered a contralateral fracture, compared with 0.9% of patients presenting with a typical fracture pattern (odds ratio 42.6, 95% CI 12.8–142.4).15
Contralateral fracture occurs from 1 month to 4 years after the index atypical femoral fracture.16
There are reports of bisphosphonate-related low-impact fractures in other sites such as the tibia17 and forearm.18 However, they may be too rare to warrant screening.
Mortality rates
A Swedish database study found that patients with atypical femoral fractures, whether bisphosphonate users or nonusers, do not have higher mortality rates than patients with ordinary subtrochanteric or femoral shaft fractures.19 Furthermore, the mortality rates for those with atypical femoral fracture were similar to rates in the general population. In contrast, patients with an ordinary femoral fracture had a higher mortality risk than the general population.19
Other studies suggest that atypical femoral fracture may be associated with a less favorable prognosis in older patients,20 but this could be due to differences in demographics, treatment adherence, or postfracture care.21
In addition, functional outcomes as measured by independent mobility at discharge and at 3 months were comparable between patients with atypical fracture and those with typical fracture.22
IMAGING STUDIES
If a long-term bisphosphonate user presents with hip, thigh, or groin pain, imaging studies are recommended.
Plain radiography
Radiography is usually the first step and should include a frontal view of the pelvis (Figure 1) and 2 views of the full length of each femur. If radiography is not conclusive, bone scan or magnetic resonance imaging (MRI) should be considered.
A linear cortex transverse fracture pattern and focal lateral cortical thickening are the most sensitive and specific radiographic features.23,24 Because of the risk of fracture on the contralateral side, radiographic study of that side is recommended as well.
Computed tomography
Computed tomography (CT) is not sensitive for early stress fractures and, given the radiation burden, is not recommended in the workup of atypical fracture.
Bone scanning
Bone scanning using technetium 99m-labeled methylene diphosphonate with a gamma camera shows active bone turnover. Stress fractures and atypical femoral fractures are most easily identified in the third (delayed) phase of the bone scan. Although bone scanning is highly sensitive, the specificity is limited by lack of spatial resolution. Atypical femoral fracture appears as increased activity in the subtrochanteric region with a predilection for the lateral cortex.
Dual-energy x-ray absorptiometry
Conventional dual-energy x-ray absorptiometry (DXA) extends only to 1 to 2 cm below the lesser trochanter and can therefore miss atypical fractures, which usually occur farther down. The overall detection rate for DXA was 61% in a sample of 33 patients.25
Newer scanners can look at the entire femoral shaft.26 In addition, newer software can quantify focal thickening (beaking) of the lateral cortex and screen patients who have no symptoms. The results of serial measurements can be graphed so that the practitioner can view trends to help assess or rule out potential asymptomatic atypical femoral fracture.
A localized reaction (periosteal thickening of the lateral cortex or beaking) often precedes atypical femoral fracture. A 2017 study reported that patients with high localized reaction (mean height 3.3 mm) that was of the pointed type and was accompanied by prodromal pain had an increased risk of complete or incomplete atypical femoral fracture at that site.27 This finding is used by the newer DXA software. The predictive value of beaking on extended femoral DXA may be as high as 83%.26
Magnetic resonance imaging
The MRI characteristics of atypical femoral fracture are similar to those of other stress fractures except that there is a lateral-to-medial pattern rather than a medial pattern. The earliest findings include periosteal reaction about the lateral cortex with a normal marrow signal.
MRI may be of particular benefit in patients with known atypical femoral fracture to screen the contralateral leg. It should image the entire length of both femurs. Contrast enhancement is not needed.
Regardless of whether initial findings were discovered on conventional radiographs or DXA, MRI confirmation is needed. Radionuclide bone scanning is currently not recommended because it lacks specificity. Combination imaging is recommended, with either radiography plus MRI or DXA plus MRI.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of atypical femoral fracture includes stress fracture, pathologic fracture, hypophosphatasia, and osteogenesis imperfecta.28 Hypophosphatemic osteomalacia can cause Looser zones, which can be confused with atypical femoral fractures but usually occur on the medial side.4 Stress fracture of the femur can occur below the lesser trochanter but usually begins in the medial, not the lateral, cortex.
Pathologic fractures from underlying osseous lesions can mimic the cortical beaking of bisphosphonate-related fracture, but they usually show the associated underlying lucent lesion and poorly defined margins. A sinus tract along the region of a chronic osteomyelitis may also appear similar.
Hypophosphatasia is an inborn error of metabolism caused by a loss-of-function mutation in the gene encoding alkaline phosphatase, resulting in pyrophosphate accumulation and causing osteomalacia from impaired mineralization. This can result in femoral pseudofracture that is often bilateral and occurs in the subtrochanteric region.29
ADDITIONAL RISK FACTORS
Patients with atypical femoral fracture are generally a heterogeneous group, but there are risk factors to note other than bisphosphonate exposure.
Asian women had a risk 8 times higher than white women in 1 study.30
Bone geometry. Mahjoub et al8 reported that compared with controls, patients with atypical femoral fracture had greater offset of the femoral shaft from the center of rotation of the femoral head, a more acute angle between the femoral neck and shaft, and greater proximal cortical thickness.
Medications. In addition to bisphosphonates, other drugs associated with atypical femoral fracture include RANK-ligand inhibitors such as denosumab (another drug for osteoporosis),31 glucocorticoids,32,33 and proton pump inhibitors.32,33
Genetics. Three sisters with atypical femoral fracture were found to have 37 rare mutations in 34 genes, including one in the GGPS1 gene, which codes for geranylgeranyl pyrophosphate synthase—an enzyme that bisphosphonates inhibit.34
Medical conditions other than osteoporosis include collagen diseases, chronic pulmonary disease, asthma, rheumatoid arthritis, and diabetes.35
Clinical recommendations
Current recommendations are to reevaluate bisphosphonate use in patients with osteoporosis after 5 or more years of therapy.36
Given that patients with osteoporosis are at increased risk of typical fracture, those at higher risk should be considered for continued bisphosphonate therapy. Factors for high risk include the following:
- History of fracture on therapy
- Hip T score –2.5 or lower
- Older age (≥ 70)
- Other strong risk factors for fracture such as smoking, alcohol use, corticosteroid use, rheumatoid arthritis, and family history
- World Health Organization FRAX fracture risk score above the country-specific threshold.
Those at lower risk should be considered for a 2- to 3-year bisphosphonate holiday with periodic reevaluation of bone density and, possibly, bone markers.36
WHAT IS THE UNDERLYING PATHOPHYSIOLOGY?
The mechanism by which bisphosphonates increase the risk of atypical femoral fracture is not clear. These drugs work by suppressing bone turnover; however, in theory, prolonged use could suppress it too much and increase bone fragility.
One hypothesis is that bisphosphonates impair the toughening of cortical bone, an important barrier to clinical fracture. This is supported by a study that found bisphosphonate users with atypical femoral fracture had deficits in intrinsic and extrinsic bone toughness, perhaps due to treatment-related increases in matrix mineralization.37 Although this study and others showed an increase in matrix mineralization and reduced mineralization heterogeneity with bisphosphonate use,38,39 it is unclear whether such changes contributed to reduced toughness or to atypical femoral fracture.
Changes in the skeletal geometry of the lower limb such as femoral neck-shaft angle and femoral curvature alter the stresses and strains experienced by the femoral diaphysis with loading. Because the incidence of incomplete atypical femoral fracture is much greater than that of complete fracture, most incomplete atypical femoral fractures heal before the fracture progresses.
Ultimately, all fractures, including atypical femoral fractures, occur when mechanical stress and strain exceed bone strength.
Antiresorptive drugs such as bisphosphonates, estrogen, calcitonin, and RANK ligand inhibitors prevent hip fracture by increasing the strength of the proximal femur—perhaps at the expense of the strength (or toughness) of the subtrochanteric shaft. It is also possible that treatment-related increases in hip strength (and reduced hip fracture rates) promote or sustain the transfer of stress and strain to femoral regions that experience lesser or no increases in strength from treatment, which likely includes the shaft.40,41
CT studies in Japanese women with osteoporosis have shown that 2 years of zoledronate therapy had greater effects in the hip than in the femoral shaft, with significant increases in cortical thickness and volumetric bone mineral density at the femoral neck and intertrochanteric region compared with baseline.42 But zoledronate did not increase femoral shaft cortical thickness and caused only a minor increase in femoral shaft volumetric bone mineral density. Fracture patterns may have depended on damage and effects of bone turnover on mass and structure.
This hypothetical scenario portrays a possible “hip survival bias” mechanism for atypical femoral fracture, with the association with antiresorptive drugs arising from greater stress and strain in cortical regions where these fractures occur rather than from treatment-related reductions in cortical bone strength or toughness.
PRODROMAL PAIN IS COMMON
From 32% to 76% of patients who have incomplete or developing atypical femoral fracture present with a prodrome of groin or hip pain.4,43 Prodromal pain occurs any time from 2 weeks to several years before the fracture, presenting as pain in the anterior or lateral thigh or in the groin.
Prodromal pain in a patient on antiresorptive therapy should be a signal for the clinician to obtain a radiograph of the hip and to look for contralateral symptoms and fractures. The most common mechanism of injury appears to be a ground-level fall or even a nontraumatic activity such as walking or stepping off a curb.
MEDICAL MANAGEMENT
In bisphosphonate users with radiographic evidence of atypical femoral fracture, the bisphosphonate should be discontinued and the patient assessed for calcium and vitamin D deficiency, with supplements prescribed if needed.4
For patients with incomplete fracture and persistent pain after 3 months of medical management, prophylactic surgical nail fixation is recommended to prevent complete fracture.
Teriparatide, which has been associated with enhanced bone fracture healing, is a possible treatment to promote healing of atypical femoral fracture, either alone or as an adjunct to surgical fixation. A systematic review published in 2015 supported the use of teriparatide for enhancing fracture healing in atypical femoral fracture.44 In addition, a 10-patient series45 showed that incomplete fractures without radiolucent lines responded to teriparatide alone, whereas those with radiolucent lines needed intramedullary nailing.
These results suggest that teriparatide works best when the fracture site is stable, either inherently or with surgical fixation.
ORTHOPEDIC CARE
Orthopedic care for atypical femoral fracture differs depending on whether the patient experiences pain and whether the fracture is incomplete or complete. Figure 2 shows a treatment algorithm for atypical femoral fracture.
These are difficult fractures to manage, complicated by delayed healing in the elderly, complex displacement patterns, altered bone geometry, and risk of fracture in the opposite limb, all of which raise questions about recommending protected weight-bearing exercise.
Furthermore, atypical femoral fracture is often associated with increased anterolateral bowing of the femur, making it difficult to insert an intramedullary nail: the radius of curvature of the bone is shorter than that of a standard femoral nail. This mismatch can lead to intraoperative complications such as iatrogenic fracture during prophylactic nailing, malunion from excess straightening of the femur (which can itself lead to leg length discrepancy), and gapping of the fracture site, particularly on the medial side.
Intramedullary nailing for complete fracture
Intramedullary nailing is the first-line treatment for complete atypical femoral fracture, although the risk of delayed healing and revision surgery may be somewhat higher than with typical femoral fracture.46 Prophylactic intramedullary nailing should be considered for a patient with intractable pain.2
A radiograph of the opposite leg should be obtained routinely, looking for an asymptomatic fracture. Bisphosphonates should be discontinued and calcium and vitamin D continued. Teriparatide therapy can be considered as an alternative treatment.
Conservative management for incomplete fracture without pain
Incomplete atypical femoral fracture unaccompanied by pain can be followed conservatively.47 In addition to stopping antiresorptive therapy, patients need to avoid high-impact and repetitive-impact activities such as jumping or running. If pain occurs, patients should begin protected weight-bearing exercise.
Treatment is uncertain for incomplete fracture with pain
For patients with incomplete atypical femoral fracture and pain, treatment is controversial. Regimens that include 2 to 3 months of protected weight-bearing exercise, a full metabolic bone workup, calcium and vitamin D supplementation, and anabolic bone agents have produced some success. Some authors have reported poor results from conservative care, with few patients achieving pain relief or signs of complete healing.48,49 Additionally, if an incomplete fracture is found in the opposite femur, protected weight-bearing of both legs may not be possible.
Patients with incomplete fracture should be monitored regularly with radiography and physical examination. If there is progression of the fracture, escalation of pain, or failure to heal within 2 to 3 months, then surgical treatment is necessary.
Prophylactic placement of an intramedullary nail to prevent completion of the fracture and allow a return to full weight-bearing is generally advised.50 A long locking plate can be used if bone deformities make it difficult to place an intramedullary nail; however, nails are preferred because they allow formation of endochondral callus, which can be helpful in these difficult-to-heal fractures.
Results from retrospective reviews have shown that surgically treated patients with bisphosphonate-associated incomplete atypical femoral fracture were more likely than those treated nonsurgically to be pain-free (81% vs 64%) and have radiographic healing (100% vs 18% at final follow-up).46 Results have also been positive for those with complete atypical femoral fracture. At 6 months, 64% of surgically treated patients were pain-free and 98% were radiographically healed.51
The unusual geometry of the femur in patients with atypical femoral fracture and the presence of intramedullary cortical callus makes the placement of an intramedullary femoral rod more complex than in typical femoral fracture.8
Intramedullary nailing of atypical femoral fracture is a challenge for even the most experienced surgeon, and vigilance is imperative to avoid iatrogenic fracture and malunion.
MANY QUESTIONS REMAIN
We need more studies on the pathophysiology of bisphosphonate-associated atypical femoral fracture, the value of periodic screening with DXA, and which factors predict high risk (eg, Asian ethnicity, use of certain medications, femoral geometry). In addition, we need more data on the success of conservative management of incomplete fracture, including use of teriparatide.
Bisphosphonate therapy minimizes bone loss and reduces fracture risk by up to 50% in patients with osteoporosis,1 but it is also associated with increased risks of osteonecrosis of the jaw and atypical femoral fracture. Although atypical femoral fractures are rare, they can have a devastating effect. Patient concern about this complication has contributed to a decrease in bisphosphonate use by about half in the last decade or so,2,3 and we fear this could result in an increase in hip fracture rates.
In this article, we examine the evidence on bisphosphonate-associated atypical femoral fractures, including risks, pathogenesis, treatment, and prevention.
ATYPICAL FRACTURES INVOLVE THE FEMORAL SHAFT, NOT THE HEAD
An atypical femoral fracture is a transverse fracture of the femoral shaft (diaphysis), defined by both clinical criteria and radiographic appearance.
To be defined as atypical, a femoral fracture must meet 4 of the following 5 criteria4:
- Occurs with minimal or no trauma
- Has a predominantly transverse fracture line, originating at the lateral cortex and sometimes becoming oblique as it progresses medially across the femur
- Extends through both cortices and may be associated with a medial spike (complete fractures); or involves only the lateral cortex (incomplete fractures)
- Is noncomminuted or minimally comminuted
- Shows localized periosteal or endosteal thickening (termed “beaking” or “flaring”) of the lateral cortex at the fracture site.
Several minor features are also important but are not required, eg:
- Cortical thickening of the femoral shaft
- Unilateral or bilateral prodromal pain preceding the fracture
- Bilateral incomplete or complete femoral diaphysis fractures
- Delayed fracture healing.
Atypical femoral fracture can occur anywhere along the shaft, from just distal to the lesser trochanter to just proximal to the supracondylar flare. However, most occur in 2 areas, with 1 cluster centered at about 41 mm from the lesser trochanter (more common in relatively younger patients) and the other at 187 mm.5
ABSOLUTE RISK IS LOW BUT INCREASES WITH LONGER USE
Atypical femoral fractures are rare. Schilcher et al6 reviewed radiographs of 1,234 women who had a subtrochanteric or shaft fracture and found 59 (4.6%) of fractures were atypical. In a systematic review of 14 studies,7 the incidence ranged from 3.0 to 9.8 cases per 100,000 patient-years.
Furthermore, not all atypical femoral fractures are in bisphosphonate users: 7.4% were in nonusers in 1 series8 and 22% in another.9
Nevertheless, most studies show that bisphosphonate use increases the incidence of atypical femoral fracture, and the incidence increases with duration of use, especially after 3 years.7
An international task force of the American Society for Bone and Mineral Research listed the absolute risk as between 3.2 and 50 cases per 100,000 patient-years, with longer use (> 5 years) increasing the risk to about 100 per 100,000 patient-years.4 After stopping bisphosphonate therapy, the risk diminished by 70% per year.9
In another study, for 0.1 to 1.9 years of therapy, the age-adjusted atypical fracture rates were 1.78 per 100,000 per year (95% confidence interval [CI] 1.5–2.0), increasing to 113.1 per 100,000 per year (95% CI 69.3–156.8) with exposure from 8 to 9.9 years.10
A case-control study found that more than 5 years of bisphosphonate use increased the fracture risk by an odds ratio of 2.74 (95% CI 1.25–6.02).11
The incidence of typical femoral fracture was higher in those who adhered better to their oral bisphosphonate regimen in some studies,12 but the opposite was true in others.13
The benefits of bisphosphonate therapy in reducing fracture risk, however, outweigh the risk of atypical fracture.4
We do not know whether the rate of atypical femoral fracture is increasing. A review of Kaiser Permanente Northwest records found that the rates of atypical femoral shaft fracture had remained stable from 1996 to 2009. However, 61.9% of patients who met the strict radiographic criteria had taken oral bisphosphonates.14 These data suggest that bisphosphonate use has not increased the overall population-based risk for subtrochanteric and femoral shaft fractures, but that bisphosphonates and other risk factors may have increased the likelihood that such fractures will exhibit atypical radiographic features.
A population-based study in Denmark13 found that alendronate use longer than 10 years was associated with an adjusted 30% lower risk of hip fracture and no increase in the risk of subtrochanteric and femoral shaft fracture. In addition, the risk of subtrochanteric and femoral shaft fracture was lower with high adherence to alendronate treatment (based on medication possession ratio > 80%) compared with low adherence (ratio < 50%) (odds ratio 0.88, 95% CI 0.77–0.99). The risk was not increased in current vs past users.
The Danish study13 used the coding of the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) to identify subtrochanteric and femoral shaft fractures without radiologic review for atypical radiographic features. The lack of specific ICD-10 coding for subtrochanteric and femoral shaft fractures with atypical radiographic features has limited our knowledge of their incidence.
Contralateral fracture in more than one-fourth of cases
After an atypical femoral fracture, patients have a significant risk of fracture on the contralateral side. In a case-control study, 28% of patients with atypical femoral fracture suffered a contralateral fracture, compared with 0.9% of patients presenting with a typical fracture pattern (odds ratio 42.6, 95% CI 12.8–142.4).15
Contralateral fracture occurs from 1 month to 4 years after the index atypical femoral fracture.16
There are reports of bisphosphonate-related low-impact fractures in other sites such as the tibia17 and forearm.18 However, they may be too rare to warrant screening.
Mortality rates
A Swedish database study found that patients with atypical femoral fractures, whether bisphosphonate users or nonusers, do not have higher mortality rates than patients with ordinary subtrochanteric or femoral shaft fractures.19 Furthermore, the mortality rates for those with atypical femoral fracture were similar to rates in the general population. In contrast, patients with an ordinary femoral fracture had a higher mortality risk than the general population.19
Other studies suggest that atypical femoral fracture may be associated with a less favorable prognosis in older patients,20 but this could be due to differences in demographics, treatment adherence, or postfracture care.21
In addition, functional outcomes as measured by independent mobility at discharge and at 3 months were comparable between patients with atypical fracture and those with typical fracture.22
IMAGING STUDIES
If a long-term bisphosphonate user presents with hip, thigh, or groin pain, imaging studies are recommended.
Plain radiography
Radiography is usually the first step and should include a frontal view of the pelvis (Figure 1) and 2 views of the full length of each femur. If radiography is not conclusive, bone scan or magnetic resonance imaging (MRI) should be considered.
A linear cortex transverse fracture pattern and focal lateral cortical thickening are the most sensitive and specific radiographic features.23,24 Because of the risk of fracture on the contralateral side, radiographic study of that side is recommended as well.
Computed tomography
Computed tomography (CT) is not sensitive for early stress fractures and, given the radiation burden, is not recommended in the workup of atypical fracture.
Bone scanning
Bone scanning using technetium 99m-labeled methylene diphosphonate with a gamma camera shows active bone turnover. Stress fractures and atypical femoral fractures are most easily identified in the third (delayed) phase of the bone scan. Although bone scanning is highly sensitive, the specificity is limited by lack of spatial resolution. Atypical femoral fracture appears as increased activity in the subtrochanteric region with a predilection for the lateral cortex.
Dual-energy x-ray absorptiometry
Conventional dual-energy x-ray absorptiometry (DXA) extends only to 1 to 2 cm below the lesser trochanter and can therefore miss atypical fractures, which usually occur farther down. The overall detection rate for DXA was 61% in a sample of 33 patients.25
Newer scanners can look at the entire femoral shaft.26 In addition, newer software can quantify focal thickening (beaking) of the lateral cortex and screen patients who have no symptoms. The results of serial measurements can be graphed so that the practitioner can view trends to help assess or rule out potential asymptomatic atypical femoral fracture.
A localized reaction (periosteal thickening of the lateral cortex or beaking) often precedes atypical femoral fracture. A 2017 study reported that patients with high localized reaction (mean height 3.3 mm) that was of the pointed type and was accompanied by prodromal pain had an increased risk of complete or incomplete atypical femoral fracture at that site.27 This finding is used by the newer DXA software. The predictive value of beaking on extended femoral DXA may be as high as 83%.26
Magnetic resonance imaging
The MRI characteristics of atypical femoral fracture are similar to those of other stress fractures except that there is a lateral-to-medial pattern rather than a medial pattern. The earliest findings include periosteal reaction about the lateral cortex with a normal marrow signal.
MRI may be of particular benefit in patients with known atypical femoral fracture to screen the contralateral leg. It should image the entire length of both femurs. Contrast enhancement is not needed.
Regardless of whether initial findings were discovered on conventional radiographs or DXA, MRI confirmation is needed. Radionuclide bone scanning is currently not recommended because it lacks specificity. Combination imaging is recommended, with either radiography plus MRI or DXA plus MRI.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of atypical femoral fracture includes stress fracture, pathologic fracture, hypophosphatasia, and osteogenesis imperfecta.28 Hypophosphatemic osteomalacia can cause Looser zones, which can be confused with atypical femoral fractures but usually occur on the medial side.4 Stress fracture of the femur can occur below the lesser trochanter but usually begins in the medial, not the lateral, cortex.
Pathologic fractures from underlying osseous lesions can mimic the cortical beaking of bisphosphonate-related fracture, but they usually show the associated underlying lucent lesion and poorly defined margins. A sinus tract along the region of a chronic osteomyelitis may also appear similar.
Hypophosphatasia is an inborn error of metabolism caused by a loss-of-function mutation in the gene encoding alkaline phosphatase, resulting in pyrophosphate accumulation and causing osteomalacia from impaired mineralization. This can result in femoral pseudofracture that is often bilateral and occurs in the subtrochanteric region.29
ADDITIONAL RISK FACTORS
Patients with atypical femoral fracture are generally a heterogeneous group, but there are risk factors to note other than bisphosphonate exposure.
Asian women had a risk 8 times higher than white women in 1 study.30
Bone geometry. Mahjoub et al8 reported that compared with controls, patients with atypical femoral fracture had greater offset of the femoral shaft from the center of rotation of the femoral head, a more acute angle between the femoral neck and shaft, and greater proximal cortical thickness.
Medications. In addition to bisphosphonates, other drugs associated with atypical femoral fracture include RANK-ligand inhibitors such as denosumab (another drug for osteoporosis),31 glucocorticoids,32,33 and proton pump inhibitors.32,33
Genetics. Three sisters with atypical femoral fracture were found to have 37 rare mutations in 34 genes, including one in the GGPS1 gene, which codes for geranylgeranyl pyrophosphate synthase—an enzyme that bisphosphonates inhibit.34
Medical conditions other than osteoporosis include collagen diseases, chronic pulmonary disease, asthma, rheumatoid arthritis, and diabetes.35
Clinical recommendations
Current recommendations are to reevaluate bisphosphonate use in patients with osteoporosis after 5 or more years of therapy.36
Given that patients with osteoporosis are at increased risk of typical fracture, those at higher risk should be considered for continued bisphosphonate therapy. Factors for high risk include the following:
- History of fracture on therapy
- Hip T score –2.5 or lower
- Older age (≥ 70)
- Other strong risk factors for fracture such as smoking, alcohol use, corticosteroid use, rheumatoid arthritis, and family history
- World Health Organization FRAX fracture risk score above the country-specific threshold.
Those at lower risk should be considered for a 2- to 3-year bisphosphonate holiday with periodic reevaluation of bone density and, possibly, bone markers.36
WHAT IS THE UNDERLYING PATHOPHYSIOLOGY?
The mechanism by which bisphosphonates increase the risk of atypical femoral fracture is not clear. These drugs work by suppressing bone turnover; however, in theory, prolonged use could suppress it too much and increase bone fragility.
One hypothesis is that bisphosphonates impair the toughening of cortical bone, an important barrier to clinical fracture. This is supported by a study that found bisphosphonate users with atypical femoral fracture had deficits in intrinsic and extrinsic bone toughness, perhaps due to treatment-related increases in matrix mineralization.37 Although this study and others showed an increase in matrix mineralization and reduced mineralization heterogeneity with bisphosphonate use,38,39 it is unclear whether such changes contributed to reduced toughness or to atypical femoral fracture.
Changes in the skeletal geometry of the lower limb such as femoral neck-shaft angle and femoral curvature alter the stresses and strains experienced by the femoral diaphysis with loading. Because the incidence of incomplete atypical femoral fracture is much greater than that of complete fracture, most incomplete atypical femoral fractures heal before the fracture progresses.
Ultimately, all fractures, including atypical femoral fractures, occur when mechanical stress and strain exceed bone strength.
Antiresorptive drugs such as bisphosphonates, estrogen, calcitonin, and RANK ligand inhibitors prevent hip fracture by increasing the strength of the proximal femur—perhaps at the expense of the strength (or toughness) of the subtrochanteric shaft. It is also possible that treatment-related increases in hip strength (and reduced hip fracture rates) promote or sustain the transfer of stress and strain to femoral regions that experience lesser or no increases in strength from treatment, which likely includes the shaft.40,41
CT studies in Japanese women with osteoporosis have shown that 2 years of zoledronate therapy had greater effects in the hip than in the femoral shaft, with significant increases in cortical thickness and volumetric bone mineral density at the femoral neck and intertrochanteric region compared with baseline.42 But zoledronate did not increase femoral shaft cortical thickness and caused only a minor increase in femoral shaft volumetric bone mineral density. Fracture patterns may have depended on damage and effects of bone turnover on mass and structure.
This hypothetical scenario portrays a possible “hip survival bias” mechanism for atypical femoral fracture, with the association with antiresorptive drugs arising from greater stress and strain in cortical regions where these fractures occur rather than from treatment-related reductions in cortical bone strength or toughness.
PRODROMAL PAIN IS COMMON
From 32% to 76% of patients who have incomplete or developing atypical femoral fracture present with a prodrome of groin or hip pain.4,43 Prodromal pain occurs any time from 2 weeks to several years before the fracture, presenting as pain in the anterior or lateral thigh or in the groin.
Prodromal pain in a patient on antiresorptive therapy should be a signal for the clinician to obtain a radiograph of the hip and to look for contralateral symptoms and fractures. The most common mechanism of injury appears to be a ground-level fall or even a nontraumatic activity such as walking or stepping off a curb.
MEDICAL MANAGEMENT
In bisphosphonate users with radiographic evidence of atypical femoral fracture, the bisphosphonate should be discontinued and the patient assessed for calcium and vitamin D deficiency, with supplements prescribed if needed.4
For patients with incomplete fracture and persistent pain after 3 months of medical management, prophylactic surgical nail fixation is recommended to prevent complete fracture.
Teriparatide, which has been associated with enhanced bone fracture healing, is a possible treatment to promote healing of atypical femoral fracture, either alone or as an adjunct to surgical fixation. A systematic review published in 2015 supported the use of teriparatide for enhancing fracture healing in atypical femoral fracture.44 In addition, a 10-patient series45 showed that incomplete fractures without radiolucent lines responded to teriparatide alone, whereas those with radiolucent lines needed intramedullary nailing.
These results suggest that teriparatide works best when the fracture site is stable, either inherently or with surgical fixation.
ORTHOPEDIC CARE
Orthopedic care for atypical femoral fracture differs depending on whether the patient experiences pain and whether the fracture is incomplete or complete. Figure 2 shows a treatment algorithm for atypical femoral fracture.
These are difficult fractures to manage, complicated by delayed healing in the elderly, complex displacement patterns, altered bone geometry, and risk of fracture in the opposite limb, all of which raise questions about recommending protected weight-bearing exercise.
Furthermore, atypical femoral fracture is often associated with increased anterolateral bowing of the femur, making it difficult to insert an intramedullary nail: the radius of curvature of the bone is shorter than that of a standard femoral nail. This mismatch can lead to intraoperative complications such as iatrogenic fracture during prophylactic nailing, malunion from excess straightening of the femur (which can itself lead to leg length discrepancy), and gapping of the fracture site, particularly on the medial side.
Intramedullary nailing for complete fracture
Intramedullary nailing is the first-line treatment for complete atypical femoral fracture, although the risk of delayed healing and revision surgery may be somewhat higher than with typical femoral fracture.46 Prophylactic intramedullary nailing should be considered for a patient with intractable pain.2
A radiograph of the opposite leg should be obtained routinely, looking for an asymptomatic fracture. Bisphosphonates should be discontinued and calcium and vitamin D continued. Teriparatide therapy can be considered as an alternative treatment.
Conservative management for incomplete fracture without pain
Incomplete atypical femoral fracture unaccompanied by pain can be followed conservatively.47 In addition to stopping antiresorptive therapy, patients need to avoid high-impact and repetitive-impact activities such as jumping or running. If pain occurs, patients should begin protected weight-bearing exercise.
Treatment is uncertain for incomplete fracture with pain
For patients with incomplete atypical femoral fracture and pain, treatment is controversial. Regimens that include 2 to 3 months of protected weight-bearing exercise, a full metabolic bone workup, calcium and vitamin D supplementation, and anabolic bone agents have produced some success. Some authors have reported poor results from conservative care, with few patients achieving pain relief or signs of complete healing.48,49 Additionally, if an incomplete fracture is found in the opposite femur, protected weight-bearing of both legs may not be possible.
Patients with incomplete fracture should be monitored regularly with radiography and physical examination. If there is progression of the fracture, escalation of pain, or failure to heal within 2 to 3 months, then surgical treatment is necessary.
Prophylactic placement of an intramedullary nail to prevent completion of the fracture and allow a return to full weight-bearing is generally advised.50 A long locking plate can be used if bone deformities make it difficult to place an intramedullary nail; however, nails are preferred because they allow formation of endochondral callus, which can be helpful in these difficult-to-heal fractures.
Results from retrospective reviews have shown that surgically treated patients with bisphosphonate-associated incomplete atypical femoral fracture were more likely than those treated nonsurgically to be pain-free (81% vs 64%) and have radiographic healing (100% vs 18% at final follow-up).46 Results have also been positive for those with complete atypical femoral fracture. At 6 months, 64% of surgically treated patients were pain-free and 98% were radiographically healed.51
The unusual geometry of the femur in patients with atypical femoral fracture and the presence of intramedullary cortical callus makes the placement of an intramedullary femoral rod more complex than in typical femoral fracture.8
Intramedullary nailing of atypical femoral fracture is a challenge for even the most experienced surgeon, and vigilance is imperative to avoid iatrogenic fracture and malunion.
MANY QUESTIONS REMAIN
We need more studies on the pathophysiology of bisphosphonate-associated atypical femoral fracture, the value of periodic screening with DXA, and which factors predict high risk (eg, Asian ethnicity, use of certain medications, femoral geometry). In addition, we need more data on the success of conservative management of incomplete fracture, including use of teriparatide.
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348(9041):1535–1541. pmid:8950879
- Jha S, Wang Z, Laucis N, Bhattacharyya T. Trends in media reports, oral bisphosphonate prescriptions, and hip fractures 1996–2012: an ecological analysis. J Bone Miner Res 2015; 30(12):2179–2187. doi:10.1002/jbmr.2565
- Solomon DH, Johnston SS, Boytsov NN, McMorrow D, Lane JM, Krohn KD. Osteoporosis medication use after hip fracture in US patients between 2002 and 2011. J Bone Miner Res 2014; 29(9):1929–1937. doi:10.1002/jbmr.2202
- Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2014; 29(1):1–23. doi:10.1002/jbmr.1998
- Koeppen VA, Schilcher J, Aspenberg P. Dichotomous location of 160 atypical femoral fractures. Acta Orthop 2013; 84(6):561–564. doi:10.3109/17453674.2013.866193
- Schilcher J, Koeppen V, Aspenberg P, Michäelsson K. Risk of atypical femoral fracture during and after bisphosphonate use. Acta Orthop 2015; 86(1):100–107. doi:10.3109/17453674.2015.1004149
- Khow KS, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Epidemiology and postoperative outcomes of atypical femoral fractures in older adults: a systematic review. J Nutr Health Aging 2017; 21(1):83–91. doi:10.1007/s12603-015-0652-3
- Mahjoub Z, Jean S, Leclerc JT, et al. Incidence and characteristics of atypical femoral fractures: clinical and geometrical data. J Bone Miner Res 2016; 31(4):767–776. doi:10.1002/jbmr.2748
- Schilcher J, Michaelsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med 2011; 364(18):1728–1737. doi:10.1056/NEJMoa1010650
- Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res 2012; 27(12):2544–2550. doi:10.1002/jbmr.1719
- Park-Wyllie LY, Mamdani MM, Juurlink DN, et al. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA 2011; 305(8):783–789. doi:10.1001/jama.2011.190
- Wang Z, Ward MM, Chan L, Bhattacharyya T. Adherence to oral bisphosphonates and the risk of subtrochanteric and femoral shaft fractures among female Medicare beneficiaries. Osteoporos Int 2014; 25(8):2109–2116. doi:10.1007/s00198-014-2738-x
- Abrahamsen B, Eiken P, Prieto-Alhambra D, Eastell R. Risk of hip, subtrochanteric, and femoral shaft fractures among mid and long term users of alendronate: nationwide cohort and nested case-control study. BMJ 2016; 353:i3365. doi:10.1136/bmj.i3365
- Feldstein AC, Black D, Perrin N, et al. Incidence and demography of femur fractures with and without atypical features. J Bone Miner Res 2012; 27(5):977–986. doi:10.1002/jbmr.1550
- Meier RP, Perneger TV, Stern R, Rizzoli R, Peter RE. Increasing occurrence of atypical femoral fractures associated with bisphosphonate use. Arch Intern Med 2012; 172(12):930–936. doi:10.1001/archinternmed.2012.1796
- La Rocca Vieira R, Rosenberg ZS, Allison MB, Im SA, Babb J, Peck V. Frequency of incomplete atypical femoral fractures in asymptomatic patients on long term bisphosphonate therapy. AJR Am J Roentgenol 2012; 198(5):1144–1151. doi:10.2214/AJR.11.7442
- Bissonnette L, April PM, Dumais R, Boire G, Roux S. Atypical fracture of the tibial diaphysis associated with bisphosphonate therapy: a case report. Bone 2013; 56(2):406–409. doi:10.1016/j.bone.2013.07.012
- Moon J, Bither N, Lee T. Atypical forearm fractures associated with long-term use of bisphosphonate. Arch Orthop Trauma Surg 2013; 133(7):889–892. doi:10.1007/s00402-013-1760-3
- Kharazmi M, Hallberg P, Schilcher J, Aspenberg P, Michaëlsson K. Mortality after atypical femoral fractures: a cohort study. J Bone Miner Res 2016; 31(3):491–497. doi:10.1002/jbmr.2767
- Medin E, Goude F, Melberg HO, Tediosi F, Belicza E, Peltola M; EuroHOPE Study Group. European regional differences in all-cause mortality and length of stay for patients with hip fracture. Health Econ 2015; 24(suppl 2):53–64. doi:10.1002/hec.3278
- Abrahamsen B, Prieto-Alhambra D. Patients with atypical femur fractures have the same mortality as the background population-drug channeling bias, bisphosphonate effects and public health implications. J Bone Miner Res 2016; 31(3):488–490. doi:10.1002/jbmr.2801
- Khow KS, Paterson F, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Outcomes between older adults with atypical and typical femoral fractures are comparable. Injury 2017; 48(2):394–398. doi:10.1016/j.injury.2016.10.035
- Adams AL, Xue F, Chantra JQ, et al. Sensitivity and specificity of radiographic characteristics in atypical femoral fractures. Osteoporos Int 2017; 28(1):413–417. doi:10.1007/s00198-016-3809-y
- Rosenberg ZS, La Rocca Vieira R, Chan SS, et al. Bisphosphonate-related complete atypical subtrochanteric femoral fractures: diagnostic utility of radiography. AJR Am J Roentgenol 2011; 197(4):954–960. doi:10.2214/AJR.10.6262
- Kim S, Yang KH, Lim H, et al. Detection of prefracture hip lesions in atypical subtrochanteric fracture with dual-energy x-ray absorptiometry images. Radiology 2014; 270(2):487–495. doi:10.1148/radiol.13122691
- van de Laarschot DM, Smits AA, Buitendijk SK, Stegenga MT, Zillikens MC. Screening for atypical femur fractures using extended femur scans by DXA. J Bone Miner Res 2017; 32(8):1632–1639. doi:10.1002/jbmr.3164
- Sato H, Kondo N, Nakatsue T, et al. High and pointed type of femoral localized reaction frequently extends to complete an incomplete atypical femoral fracture in patients with autoimmune diseases on long-term glucocorticoids and bisphosphonates. Osteoporos Int 2017; 28(8):2367–2376. doi:10.1007/s00198-017-4038-8
- Giaconi JC, Watterson CT. Bisphosphonate-related atypical femur fractures and the radiographic features. In: Silverman SL, Abrahamsen B, eds. The Duration and Safety of Osteoporosis Treatment. Switzerland: Springer International Publishing; 2016:107–124. doi:10.1007/978-3-319-23639-1
- Whyte MP. Atypical femoral fractures, bisphosphonates, and adult hypophosphatasia. J Bone Miner Res 2009; 24(6):1132–1134. doi:10.1359/jbmr.081253
- Lo JC, Hui RL, Grimsrud CD, et al. The association of race/ethnicity and risk of atypical femoral fracture among older women receiving oral bisphosphonate therapy. Bone 2016; 85:142–147. doi:10.1016/j.bone.2016.01.002
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 2017; 5(7):513–523. doi:10.1016/S2213-8587(17)30138-9
- Koh JH, Myong JP, Yoo J, et al. Predisposing factors associated with atypical femur fracture among postmenopausal Korean women receiving bisphosphonate therapy: 8 years' experience in a single center. Osteoporos Int 2017; 28(11):3251–3259. doi:10.1007/s00198-017-4169-y
- Kim D, Sung YK, Cho SK, Han M, Kim YS. Factors associated with atypical femoral fracture. Rheumatol Int 2016; 36(1):65–71. doi:10.1007/s00296-015-3323-0
- Roca-Ayats N, Balcells S, Garcia-Giralt N, et al. GGPS1 mutation and atypical femoral fractures with bisphosphonates. N Engl J Med 2017; 376(18):1794–1795. doi:10.1056/NEJMc1612804
- Giusti A, Hamdy NA, Dekkers OM, Ramautar SR, Dijkstra S, Papapoulos SE. Atypical fractures and bisphosphonate therapy: a cohort study of patients with femoral fracture with radiographic adjudication of fracture site and features. Bone 2011; 48(5):966–971. doi:10.1016/j.bone.2010.12.033
- Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2016; 31(1):16–35. doi:10.1002/jbmr.2708
- Lloyd AA, Gludovatz B, Riedel C, et al. Atypical fracture with long-term bisphosphonate therapy is associated with altered cortical composition and reduced fracture resistance. Proc Natl Acad Sci USA 2017; 114(33):8722–8727. doi:10.1073/pnas.1704460114
- Ettinger B, Burr DB, Ritchie RO. Proposed pathogenesis for atypical femoral fractures; lessons from materials research. Bone 2013; 55(2):495–500. doi:10.1016/j.bone.2013.02.004
- Burr DB, Liu Z, Allen MR. Duration-dependent effects of clinically relevant oral alendronate doses on cortical bone toughness in beagle dogs. Bone 2015; 71:58–62. doi:10.1016/j.bone.2014.10.010
- Sasaki S, Miyakoshi N, Hongo M, Kasukawa Y, Shimada Y. Low-energy diaphyseal femoral fractures associated with bisphosphonate use and severe curved femur: a case series. J Bone Miner Metab 2012; 30(5):561–567. doi:10.1007/s00774-012-0358-0
- Pulkkinen P, Gluer C, Jamsa T. Investigation of differences between hip fracture types: a worthy strategy of improved risk assessment and fracture prevention. Bone 2011; 49(4):600–604. doi:10.1016/j.bone.2011.07.022
- Ito M, Sone T, Shiraki M, et al. The effect of once-yearly zoledronic acid on hip structural and biomechanical properties derived using computed tomography (CT) in Japanese women with osteoporosis. Bone 2018; 106:179–186. doi:10.1016/j.bone.2017.10.013
- Bogdan Y, Einhorn TA. Clinical presentation of atypical femur fractures. In: Silverman SL, Abrahamsen B, eds. The Duration and Safety of Osteoporosis Treatment. Switzerland: Springer International Publishing; 2016:137–140. doi:10.1007/978-3-319-23639-1
- Im GI, Lee SH. Effect of teriparatide on healing of atypical femoral fractures: a systemic review. J Bone Metab 2015; 22(4):183–189. doi:10.11005/jbm.2015.22.4.183
- Saleh A, Hegde VV, Potty AG, Schneider R, Cornell CN, Lane JM. Management strategy for symptomatic bisphosphonate-associated incomplete atypical femoral fractures. HSS J 2012; 8(2):103–110. doi:10.1007/s11420-012-9275-y
- Egol KA, Park JH, Prensky C, Rosenberg ZS, Peck V, Tejwani NC. Surgical treatment improves clinical and functional outcomes for patients who sustain incomplete bisphosphonate-related femur fractures. J Orthop Trauma 2013; 27(6):331–335. doi:10.1097/BOT.0b013e31827240ae
- Koh A, Guerado E, Giannoudis PV. Atypical femoral fractures related to bisphosphonate treatment: issues and controversies related to their surgical management. Bone Joint J 2017; 99-B(3):295–302. doi:10.1302/0301-620X.99B3.BJJ-2016-0276.R2
- Oh CW, Oh JK, Park KC, Kim JW, Yoon YC. Prophylactic nailing of incomplete atypical femoral fractures. ScientificWorldJournal 2013; 2013:450148. doi:10.1155/2013/450148
- Ha YC, Cho MR, Park KH, Kim SY, Koo KH. Is surgery necessary for femoral insufficiency fractures after long-term bisphosphonate therapy? Clin Orthop Relat Res 2010; 468(12):3393–3398. doi:10.1007/s11999-010-1583-2
- Tosounidis TH, Lampropoulou-Adamidou, Kanakaris NK. Intramedullary nailing of sequential bilateral atypical subtrochanteric fractures and the management of distal femoral intraoperative fracture. J Orthop Trauma 2015 Jun 11. Epub ahead of print. doi:10.1097/BOT.0000000000000370
- Egol KA, Park JH, Rosenberg ZS, Peck V, Tejwani NC. Healing delayed but generally reliable after bisphosphonate-associated complete femur fractures treated with IM nails. Clin Orthop Relat Res 2014; 472(9):2728–2734. doi:10.1007/s11999-013-2963-1
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348(9041):1535–1541. pmid:8950879
- Jha S, Wang Z, Laucis N, Bhattacharyya T. Trends in media reports, oral bisphosphonate prescriptions, and hip fractures 1996–2012: an ecological analysis. J Bone Miner Res 2015; 30(12):2179–2187. doi:10.1002/jbmr.2565
- Solomon DH, Johnston SS, Boytsov NN, McMorrow D, Lane JM, Krohn KD. Osteoporosis medication use after hip fracture in US patients between 2002 and 2011. J Bone Miner Res 2014; 29(9):1929–1937. doi:10.1002/jbmr.2202
- Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2014; 29(1):1–23. doi:10.1002/jbmr.1998
- Koeppen VA, Schilcher J, Aspenberg P. Dichotomous location of 160 atypical femoral fractures. Acta Orthop 2013; 84(6):561–564. doi:10.3109/17453674.2013.866193
- Schilcher J, Koeppen V, Aspenberg P, Michäelsson K. Risk of atypical femoral fracture during and after bisphosphonate use. Acta Orthop 2015; 86(1):100–107. doi:10.3109/17453674.2015.1004149
- Khow KS, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Epidemiology and postoperative outcomes of atypical femoral fractures in older adults: a systematic review. J Nutr Health Aging 2017; 21(1):83–91. doi:10.1007/s12603-015-0652-3
- Mahjoub Z, Jean S, Leclerc JT, et al. Incidence and characteristics of atypical femoral fractures: clinical and geometrical data. J Bone Miner Res 2016; 31(4):767–776. doi:10.1002/jbmr.2748
- Schilcher J, Michaelsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med 2011; 364(18):1728–1737. doi:10.1056/NEJMoa1010650
- Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res 2012; 27(12):2544–2550. doi:10.1002/jbmr.1719
- Park-Wyllie LY, Mamdani MM, Juurlink DN, et al. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA 2011; 305(8):783–789. doi:10.1001/jama.2011.190
- Wang Z, Ward MM, Chan L, Bhattacharyya T. Adherence to oral bisphosphonates and the risk of subtrochanteric and femoral shaft fractures among female Medicare beneficiaries. Osteoporos Int 2014; 25(8):2109–2116. doi:10.1007/s00198-014-2738-x
- Abrahamsen B, Eiken P, Prieto-Alhambra D, Eastell R. Risk of hip, subtrochanteric, and femoral shaft fractures among mid and long term users of alendronate: nationwide cohort and nested case-control study. BMJ 2016; 353:i3365. doi:10.1136/bmj.i3365
- Feldstein AC, Black D, Perrin N, et al. Incidence and demography of femur fractures with and without atypical features. J Bone Miner Res 2012; 27(5):977–986. doi:10.1002/jbmr.1550
- Meier RP, Perneger TV, Stern R, Rizzoli R, Peter RE. Increasing occurrence of atypical femoral fractures associated with bisphosphonate use. Arch Intern Med 2012; 172(12):930–936. doi:10.1001/archinternmed.2012.1796
- La Rocca Vieira R, Rosenberg ZS, Allison MB, Im SA, Babb J, Peck V. Frequency of incomplete atypical femoral fractures in asymptomatic patients on long term bisphosphonate therapy. AJR Am J Roentgenol 2012; 198(5):1144–1151. doi:10.2214/AJR.11.7442
- Bissonnette L, April PM, Dumais R, Boire G, Roux S. Atypical fracture of the tibial diaphysis associated with bisphosphonate therapy: a case report. Bone 2013; 56(2):406–409. doi:10.1016/j.bone.2013.07.012
- Moon J, Bither N, Lee T. Atypical forearm fractures associated with long-term use of bisphosphonate. Arch Orthop Trauma Surg 2013; 133(7):889–892. doi:10.1007/s00402-013-1760-3
- Kharazmi M, Hallberg P, Schilcher J, Aspenberg P, Michaëlsson K. Mortality after atypical femoral fractures: a cohort study. J Bone Miner Res 2016; 31(3):491–497. doi:10.1002/jbmr.2767
- Medin E, Goude F, Melberg HO, Tediosi F, Belicza E, Peltola M; EuroHOPE Study Group. European regional differences in all-cause mortality and length of stay for patients with hip fracture. Health Econ 2015; 24(suppl 2):53–64. doi:10.1002/hec.3278
- Abrahamsen B, Prieto-Alhambra D. Patients with atypical femur fractures have the same mortality as the background population-drug channeling bias, bisphosphonate effects and public health implications. J Bone Miner Res 2016; 31(3):488–490. doi:10.1002/jbmr.2801
- Khow KS, Paterson F, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Outcomes between older adults with atypical and typical femoral fractures are comparable. Injury 2017; 48(2):394–398. doi:10.1016/j.injury.2016.10.035
- Adams AL, Xue F, Chantra JQ, et al. Sensitivity and specificity of radiographic characteristics in atypical femoral fractures. Osteoporos Int 2017; 28(1):413–417. doi:10.1007/s00198-016-3809-y
- Rosenberg ZS, La Rocca Vieira R, Chan SS, et al. Bisphosphonate-related complete atypical subtrochanteric femoral fractures: diagnostic utility of radiography. AJR Am J Roentgenol 2011; 197(4):954–960. doi:10.2214/AJR.10.6262
- Kim S, Yang KH, Lim H, et al. Detection of prefracture hip lesions in atypical subtrochanteric fracture with dual-energy x-ray absorptiometry images. Radiology 2014; 270(2):487–495. doi:10.1148/radiol.13122691
- van de Laarschot DM, Smits AA, Buitendijk SK, Stegenga MT, Zillikens MC. Screening for atypical femur fractures using extended femur scans by DXA. J Bone Miner Res 2017; 32(8):1632–1639. doi:10.1002/jbmr.3164
- Sato H, Kondo N, Nakatsue T, et al. High and pointed type of femoral localized reaction frequently extends to complete an incomplete atypical femoral fracture in patients with autoimmune diseases on long-term glucocorticoids and bisphosphonates. Osteoporos Int 2017; 28(8):2367–2376. doi:10.1007/s00198-017-4038-8
- Giaconi JC, Watterson CT. Bisphosphonate-related atypical femur fractures and the radiographic features. In: Silverman SL, Abrahamsen B, eds. The Duration and Safety of Osteoporosis Treatment. Switzerland: Springer International Publishing; 2016:107–124. doi:10.1007/978-3-319-23639-1
- Whyte MP. Atypical femoral fractures, bisphosphonates, and adult hypophosphatasia. J Bone Miner Res 2009; 24(6):1132–1134. doi:10.1359/jbmr.081253
- Lo JC, Hui RL, Grimsrud CD, et al. The association of race/ethnicity and risk of atypical femoral fracture among older women receiving oral bisphosphonate therapy. Bone 2016; 85:142–147. doi:10.1016/j.bone.2016.01.002
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 2017; 5(7):513–523. doi:10.1016/S2213-8587(17)30138-9
- Koh JH, Myong JP, Yoo J, et al. Predisposing factors associated with atypical femur fracture among postmenopausal Korean women receiving bisphosphonate therapy: 8 years' experience in a single center. Osteoporos Int 2017; 28(11):3251–3259. doi:10.1007/s00198-017-4169-y
- Kim D, Sung YK, Cho SK, Han M, Kim YS. Factors associated with atypical femoral fracture. Rheumatol Int 2016; 36(1):65–71. doi:10.1007/s00296-015-3323-0
- Roca-Ayats N, Balcells S, Garcia-Giralt N, et al. GGPS1 mutation and atypical femoral fractures with bisphosphonates. N Engl J Med 2017; 376(18):1794–1795. doi:10.1056/NEJMc1612804
- Giusti A, Hamdy NA, Dekkers OM, Ramautar SR, Dijkstra S, Papapoulos SE. Atypical fractures and bisphosphonate therapy: a cohort study of patients with femoral fracture with radiographic adjudication of fracture site and features. Bone 2011; 48(5):966–971. doi:10.1016/j.bone.2010.12.033
- Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2016; 31(1):16–35. doi:10.1002/jbmr.2708
- Lloyd AA, Gludovatz B, Riedel C, et al. Atypical fracture with long-term bisphosphonate therapy is associated with altered cortical composition and reduced fracture resistance. Proc Natl Acad Sci USA 2017; 114(33):8722–8727. doi:10.1073/pnas.1704460114
- Ettinger B, Burr DB, Ritchie RO. Proposed pathogenesis for atypical femoral fractures; lessons from materials research. Bone 2013; 55(2):495–500. doi:10.1016/j.bone.2013.02.004
- Burr DB, Liu Z, Allen MR. Duration-dependent effects of clinically relevant oral alendronate doses on cortical bone toughness in beagle dogs. Bone 2015; 71:58–62. doi:10.1016/j.bone.2014.10.010
- Sasaki S, Miyakoshi N, Hongo M, Kasukawa Y, Shimada Y. Low-energy diaphyseal femoral fractures associated with bisphosphonate use and severe curved femur: a case series. J Bone Miner Metab 2012; 30(5):561–567. doi:10.1007/s00774-012-0358-0
- Pulkkinen P, Gluer C, Jamsa T. Investigation of differences between hip fracture types: a worthy strategy of improved risk assessment and fracture prevention. Bone 2011; 49(4):600–604. doi:10.1016/j.bone.2011.07.022
- Ito M, Sone T, Shiraki M, et al. The effect of once-yearly zoledronic acid on hip structural and biomechanical properties derived using computed tomography (CT) in Japanese women with osteoporosis. Bone 2018; 106:179–186. doi:10.1016/j.bone.2017.10.013
- Bogdan Y, Einhorn TA. Clinical presentation of atypical femur fractures. In: Silverman SL, Abrahamsen B, eds. The Duration and Safety of Osteoporosis Treatment. Switzerland: Springer International Publishing; 2016:137–140. doi:10.1007/978-3-319-23639-1
- Im GI, Lee SH. Effect of teriparatide on healing of atypical femoral fractures: a systemic review. J Bone Metab 2015; 22(4):183–189. doi:10.11005/jbm.2015.22.4.183
- Saleh A, Hegde VV, Potty AG, Schneider R, Cornell CN, Lane JM. Management strategy for symptomatic bisphosphonate-associated incomplete atypical femoral fractures. HSS J 2012; 8(2):103–110. doi:10.1007/s11420-012-9275-y
- Egol KA, Park JH, Prensky C, Rosenberg ZS, Peck V, Tejwani NC. Surgical treatment improves clinical and functional outcomes for patients who sustain incomplete bisphosphonate-related femur fractures. J Orthop Trauma 2013; 27(6):331–335. doi:10.1097/BOT.0b013e31827240ae
- Koh A, Guerado E, Giannoudis PV. Atypical femoral fractures related to bisphosphonate treatment: issues and controversies related to their surgical management. Bone Joint J 2017; 99-B(3):295–302. doi:10.1302/0301-620X.99B3.BJJ-2016-0276.R2
- Oh CW, Oh JK, Park KC, Kim JW, Yoon YC. Prophylactic nailing of incomplete atypical femoral fractures. ScientificWorldJournal 2013; 2013:450148. doi:10.1155/2013/450148
- Ha YC, Cho MR, Park KH, Kim SY, Koo KH. Is surgery necessary for femoral insufficiency fractures after long-term bisphosphonate therapy? Clin Orthop Relat Res 2010; 468(12):3393–3398. doi:10.1007/s11999-010-1583-2
- Tosounidis TH, Lampropoulou-Adamidou, Kanakaris NK. Intramedullary nailing of sequential bilateral atypical subtrochanteric fractures and the management of distal femoral intraoperative fracture. J Orthop Trauma 2015 Jun 11. Epub ahead of print. doi:10.1097/BOT.0000000000000370
- Egol KA, Park JH, Rosenberg ZS, Peck V, Tejwani NC. Healing delayed but generally reliable after bisphosphonate-associated complete femur fractures treated with IM nails. Clin Orthop Relat Res 2014; 472(9):2728–2734. doi:10.1007/s11999-013-2963-1
KEY POINTS
- The benefits of bisphosphonate therapy in reducing fracture risk outweigh the risk of atypical fracture.
- Bisphosphonate use for longer than 5 years greatly increases the risk of atypical femoral fracture.
- Treatment of atypical femoral fracture varies depending on whether the patient has pain and whether the fracture is complete or incomplete.
A physician’s response to observational studies of opioid prescribing
Several months ago, we invited readers to submit short personalized commentaries on articles that changed the way they approach a specific clinical problem and the way they take care of patients. In this issue of the Journal, addiction specialist Charles Reznikoff, MD, discusses 3 observational studies that focused on how prescribing opioids for acute pain can lead to chronic opioid use and addiction, and how these studies have influenced his practice.
Although observational studies rank lower on the level-of-evidence scale than randomized controlled trials, they can intellectually stimulate and inform us in ways that lead us to modify how we deliver clinical care.
The initial prescribing of pain medications and the management of patients with chronic pain are currently under intense scrutiny, and are the topic of much discussion in the United States. The opioid epidemic has spilled over into all aspects of daily life, far beyond the medical community. But since we physicians are the only legal and regulated source of narcotics and other pain medications, we are under the microscope—and rightly so.
We, our patients, the pharmaceutical industry, legislators, and the law enforcement community struggle to navigate a complex maze, one with moving walls. Not long ago, physicians were told that we were not attentive enough to our patients’ suffering and needed to do better at relieving it. “Pain” became a vital sign and a recorded metric of quality care. Some excellent changes evolved from this focus, such as increased emphasis on postoperative regional and local pain control. But pain measurements continue to be recorded at every outpatient visit, an almost mindless requirement.
Recently, a patient with lupus nephritis whom I was seeing for blood pressure management reported a pain level of 8 on a scale of 10. I confess that I usually don’t even look at these metrics, but for whatever reason I saw her answer. I asked her about it. She had burned her finger while cooking and said, “I had no idea what number to pick. I picked 8. It’s no big deal.”
But the ongoing emphasis on this metric may lead some patients to expect total pain relief, a problematic expectation in those with chronic pain syndromes such as fibromyalgia. As Dr. Reznikoff points out, a large proportion of patients report they have chronic pain, and many (but clearly not all) suffer from recognized or masked chronic anxiety and depression disorders1 that may well influence how they use pain medications.
Thus, while physicians indeed are on the front lines of offering initial prescriptions for pain medications, we remain betwixt and between in the challenges of responding to the immediate needs of our patients while trying to predict the long-term effects of our prescription on the individual patient and of our prescribing patterns on society in general.
I again welcome your submissions describing how individual publications have affected your personal approach to managing patients and specific diseases. We will publish selected contributions in print and online.
- Tsang A, Von Korff M, Lee S, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain 2008; 9(10):883–891. doi:10.1016/j.jpain.2008.05.005
Several months ago, we invited readers to submit short personalized commentaries on articles that changed the way they approach a specific clinical problem and the way they take care of patients. In this issue of the Journal, addiction specialist Charles Reznikoff, MD, discusses 3 observational studies that focused on how prescribing opioids for acute pain can lead to chronic opioid use and addiction, and how these studies have influenced his practice.
Although observational studies rank lower on the level-of-evidence scale than randomized controlled trials, they can intellectually stimulate and inform us in ways that lead us to modify how we deliver clinical care.
The initial prescribing of pain medications and the management of patients with chronic pain are currently under intense scrutiny, and are the topic of much discussion in the United States. The opioid epidemic has spilled over into all aspects of daily life, far beyond the medical community. But since we physicians are the only legal and regulated source of narcotics and other pain medications, we are under the microscope—and rightly so.
We, our patients, the pharmaceutical industry, legislators, and the law enforcement community struggle to navigate a complex maze, one with moving walls. Not long ago, physicians were told that we were not attentive enough to our patients’ suffering and needed to do better at relieving it. “Pain” became a vital sign and a recorded metric of quality care. Some excellent changes evolved from this focus, such as increased emphasis on postoperative regional and local pain control. But pain measurements continue to be recorded at every outpatient visit, an almost mindless requirement.
Recently, a patient with lupus nephritis whom I was seeing for blood pressure management reported a pain level of 8 on a scale of 10. I confess that I usually don’t even look at these metrics, but for whatever reason I saw her answer. I asked her about it. She had burned her finger while cooking and said, “I had no idea what number to pick. I picked 8. It’s no big deal.”
But the ongoing emphasis on this metric may lead some patients to expect total pain relief, a problematic expectation in those with chronic pain syndromes such as fibromyalgia. As Dr. Reznikoff points out, a large proportion of patients report they have chronic pain, and many (but clearly not all) suffer from recognized or masked chronic anxiety and depression disorders1 that may well influence how they use pain medications.
Thus, while physicians indeed are on the front lines of offering initial prescriptions for pain medications, we remain betwixt and between in the challenges of responding to the immediate needs of our patients while trying to predict the long-term effects of our prescription on the individual patient and of our prescribing patterns on society in general.
I again welcome your submissions describing how individual publications have affected your personal approach to managing patients and specific diseases. We will publish selected contributions in print and online.
Several months ago, we invited readers to submit short personalized commentaries on articles that changed the way they approach a specific clinical problem and the way they take care of patients. In this issue of the Journal, addiction specialist Charles Reznikoff, MD, discusses 3 observational studies that focused on how prescribing opioids for acute pain can lead to chronic opioid use and addiction, and how these studies have influenced his practice.
Although observational studies rank lower on the level-of-evidence scale than randomized controlled trials, they can intellectually stimulate and inform us in ways that lead us to modify how we deliver clinical care.
The initial prescribing of pain medications and the management of patients with chronic pain are currently under intense scrutiny, and are the topic of much discussion in the United States. The opioid epidemic has spilled over into all aspects of daily life, far beyond the medical community. But since we physicians are the only legal and regulated source of narcotics and other pain medications, we are under the microscope—and rightly so.
We, our patients, the pharmaceutical industry, legislators, and the law enforcement community struggle to navigate a complex maze, one with moving walls. Not long ago, physicians were told that we were not attentive enough to our patients’ suffering and needed to do better at relieving it. “Pain” became a vital sign and a recorded metric of quality care. Some excellent changes evolved from this focus, such as increased emphasis on postoperative regional and local pain control. But pain measurements continue to be recorded at every outpatient visit, an almost mindless requirement.
Recently, a patient with lupus nephritis whom I was seeing for blood pressure management reported a pain level of 8 on a scale of 10. I confess that I usually don’t even look at these metrics, but for whatever reason I saw her answer. I asked her about it. She had burned her finger while cooking and said, “I had no idea what number to pick. I picked 8. It’s no big deal.”
But the ongoing emphasis on this metric may lead some patients to expect total pain relief, a problematic expectation in those with chronic pain syndromes such as fibromyalgia. As Dr. Reznikoff points out, a large proportion of patients report they have chronic pain, and many (but clearly not all) suffer from recognized or masked chronic anxiety and depression disorders1 that may well influence how they use pain medications.
Thus, while physicians indeed are on the front lines of offering initial prescriptions for pain medications, we remain betwixt and between in the challenges of responding to the immediate needs of our patients while trying to predict the long-term effects of our prescription on the individual patient and of our prescribing patterns on society in general.
I again welcome your submissions describing how individual publications have affected your personal approach to managing patients and specific diseases. We will publish selected contributions in print and online.
- Tsang A, Von Korff M, Lee S, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain 2008; 9(10):883–891. doi:10.1016/j.jpain.2008.05.005
- Tsang A, Von Korff M, Lee S, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain 2008; 9(10):883–891. doi:10.1016/j.jpain.2008.05.005
Renal vein thrombosis and pulmonary embolism
A 49-year-old man developed nephrotic-range proteinuria (urine protein–creatinine ratio 4.1 g/g), and primary membranous nephropathy was diagnosed by kidney biopsy. He declined therapy apart from angiotensin receptor blockade.
Five months after undergoing the biopsy, he presented to the emergency room with marked dyspnea, cough, and epigastric discomfort. His blood pressure was 160/100 mm Hg, heart rate 95 beats/minute, and oxygen saturation by pulse oximetry 97% at rest on ambient air, decreasing to 92% with ambulation.
Initial laboratory testing results were as follows:
- Sodium 135 mmol/L (reference range 136–144)
- Potassium 3.9 mmol/L (3.7–5.1)
- Chloride 104 mmol/L (97–105)
- Bicarbonate 21 mmol/L (22–30)
- Blood urea nitrogen 14 mg/dL (9–24)
- Serum creatinine 1.1 mg/dL (0.73–1.22)
- Albumin 2.1 g/dL (3.4–4.9).
Urinalysis revealed the following:
- 5 red blood cells per high-power field, compared with 1 to 2 previously
- 3+ proteinuria
- Urine protein–creatinine ratio 11 g/g
- No glucosuria.
Electrocardiography revealed normal sinus rhythm without ischemic changes. Chest radiography did not show consolidation.
At 7 months after the thrombotic event, there was no evidence of residual renal vein thrombosis on magnetic resonance venography, and at 14 months his serum creatinine level was 0.9 mg/dL, albumin 4.0 g/dL, and urine protein–creatinine ratio 0.8 g/g.
RENAL VEIN THROMBOSIS: RISK FACTORS AND CLINICAL FEATURES
Severe hypoalbuminemia in the setting of nephrotic syndrome due to membranous nephropathy is associated with the highest risk of venous thromboembolic events, with renal vein thrombus being the classic complication.1 Venous thromboembolic events also occur in other nephrotic syndromes, albeit at a lower frequency.2
Venous thromboembolic events are estimated to occur in 7% to 33% of patients with membranous glomerulopathy, with albumin levels less than 2.8 g/dL considered a notable risk factor.1,2
While often a chronic complication, acute renal vein thrombosis may present with flank pain and hematuria.3 In our patient, the dramatic increase in proteinuria and possibly the increase in hematuria suggested renal vein thrombosis. Proximal tubular dysfunction, such as glucosuria, can be seen on occasion.
DIAGNOSIS AND TREATMENT
Screening asymptomatic patients for renal vein thrombosis is not recommended, and the decision to start prophylactic anticoagulation must be individualized.4
Although renal venography historically was the gold standard test to diagnose renal vein thrombosis, it has been replaced by noninvasive imaging such as computed tomography and magnetic resonance venography.
While anticoagulation remains the treatment of choice, catheter-directed thrombectomy or surgical thrombectomy can be considered for some patients with acute renal vein thrombosis.5
- Couser WG. Primary membranous nephropathy. Clin J Am Soc Nephrol 2017; 12(6):983–997. doi:10.2215/CJN.11761116
- Barbour SJ, Greenwald A, Djurdjev O, et al. Disease-specific risk of venous thromboembolic events is increased in idiopathic glomerulonephritis. Kidney Int 2012; 81(2):190–195. doi:10.1038/ki.2011.312
- Lionaki S, Derebail VK, Hogan SL, et al. Venous thromboembolism in patients with membranous nephropathy. Clin J Am Soc Nephrol 2012; 7(1):43–51. doi:10.2215/CJN.04250511
- Lee T, Biddle AK, Lionaki S, et al. Personalized prophylactic anticoagulation decision analysis in patients with membranous nephropathy. Kidney Int 2014; 85(6):1412–1420. doi:10.1038/ki.2013.476
- Jaar BG, Kim HS, Samaniego MD, Lund GB, Atta MG. Percutaneous mechanical thrombectomy: a new approach in the treatment of acute renal-vein thrombosis. Nephrol Dial Transplant 2002; 17(6):1122–1125. pmid:12032209
A 49-year-old man developed nephrotic-range proteinuria (urine protein–creatinine ratio 4.1 g/g), and primary membranous nephropathy was diagnosed by kidney biopsy. He declined therapy apart from angiotensin receptor blockade.
Five months after undergoing the biopsy, he presented to the emergency room with marked dyspnea, cough, and epigastric discomfort. His blood pressure was 160/100 mm Hg, heart rate 95 beats/minute, and oxygen saturation by pulse oximetry 97% at rest on ambient air, decreasing to 92% with ambulation.
Initial laboratory testing results were as follows:
- Sodium 135 mmol/L (reference range 136–144)
- Potassium 3.9 mmol/L (3.7–5.1)
- Chloride 104 mmol/L (97–105)
- Bicarbonate 21 mmol/L (22–30)
- Blood urea nitrogen 14 mg/dL (9–24)
- Serum creatinine 1.1 mg/dL (0.73–1.22)
- Albumin 2.1 g/dL (3.4–4.9).
Urinalysis revealed the following:
- 5 red blood cells per high-power field, compared with 1 to 2 previously
- 3+ proteinuria
- Urine protein–creatinine ratio 11 g/g
- No glucosuria.
Electrocardiography revealed normal sinus rhythm without ischemic changes. Chest radiography did not show consolidation.
At 7 months after the thrombotic event, there was no evidence of residual renal vein thrombosis on magnetic resonance venography, and at 14 months his serum creatinine level was 0.9 mg/dL, albumin 4.0 g/dL, and urine protein–creatinine ratio 0.8 g/g.
RENAL VEIN THROMBOSIS: RISK FACTORS AND CLINICAL FEATURES
Severe hypoalbuminemia in the setting of nephrotic syndrome due to membranous nephropathy is associated with the highest risk of venous thromboembolic events, with renal vein thrombus being the classic complication.1 Venous thromboembolic events also occur in other nephrotic syndromes, albeit at a lower frequency.2
Venous thromboembolic events are estimated to occur in 7% to 33% of patients with membranous glomerulopathy, with albumin levels less than 2.8 g/dL considered a notable risk factor.1,2
While often a chronic complication, acute renal vein thrombosis may present with flank pain and hematuria.3 In our patient, the dramatic increase in proteinuria and possibly the increase in hematuria suggested renal vein thrombosis. Proximal tubular dysfunction, such as glucosuria, can be seen on occasion.
DIAGNOSIS AND TREATMENT
Screening asymptomatic patients for renal vein thrombosis is not recommended, and the decision to start prophylactic anticoagulation must be individualized.4
Although renal venography historically was the gold standard test to diagnose renal vein thrombosis, it has been replaced by noninvasive imaging such as computed tomography and magnetic resonance venography.
While anticoagulation remains the treatment of choice, catheter-directed thrombectomy or surgical thrombectomy can be considered for some patients with acute renal vein thrombosis.5
A 49-year-old man developed nephrotic-range proteinuria (urine protein–creatinine ratio 4.1 g/g), and primary membranous nephropathy was diagnosed by kidney biopsy. He declined therapy apart from angiotensin receptor blockade.
Five months after undergoing the biopsy, he presented to the emergency room with marked dyspnea, cough, and epigastric discomfort. His blood pressure was 160/100 mm Hg, heart rate 95 beats/minute, and oxygen saturation by pulse oximetry 97% at rest on ambient air, decreasing to 92% with ambulation.
Initial laboratory testing results were as follows:
- Sodium 135 mmol/L (reference range 136–144)
- Potassium 3.9 mmol/L (3.7–5.1)
- Chloride 104 mmol/L (97–105)
- Bicarbonate 21 mmol/L (22–30)
- Blood urea nitrogen 14 mg/dL (9–24)
- Serum creatinine 1.1 mg/dL (0.73–1.22)
- Albumin 2.1 g/dL (3.4–4.9).
Urinalysis revealed the following:
- 5 red blood cells per high-power field, compared with 1 to 2 previously
- 3+ proteinuria
- Urine protein–creatinine ratio 11 g/g
- No glucosuria.
Electrocardiography revealed normal sinus rhythm without ischemic changes. Chest radiography did not show consolidation.
At 7 months after the thrombotic event, there was no evidence of residual renal vein thrombosis on magnetic resonance venography, and at 14 months his serum creatinine level was 0.9 mg/dL, albumin 4.0 g/dL, and urine protein–creatinine ratio 0.8 g/g.
RENAL VEIN THROMBOSIS: RISK FACTORS AND CLINICAL FEATURES
Severe hypoalbuminemia in the setting of nephrotic syndrome due to membranous nephropathy is associated with the highest risk of venous thromboembolic events, with renal vein thrombus being the classic complication.1 Venous thromboembolic events also occur in other nephrotic syndromes, albeit at a lower frequency.2
Venous thromboembolic events are estimated to occur in 7% to 33% of patients with membranous glomerulopathy, with albumin levels less than 2.8 g/dL considered a notable risk factor.1,2
While often a chronic complication, acute renal vein thrombosis may present with flank pain and hematuria.3 In our patient, the dramatic increase in proteinuria and possibly the increase in hematuria suggested renal vein thrombosis. Proximal tubular dysfunction, such as glucosuria, can be seen on occasion.
DIAGNOSIS AND TREATMENT
Screening asymptomatic patients for renal vein thrombosis is not recommended, and the decision to start prophylactic anticoagulation must be individualized.4
Although renal venography historically was the gold standard test to diagnose renal vein thrombosis, it has been replaced by noninvasive imaging such as computed tomography and magnetic resonance venography.
While anticoagulation remains the treatment of choice, catheter-directed thrombectomy or surgical thrombectomy can be considered for some patients with acute renal vein thrombosis.5
- Couser WG. Primary membranous nephropathy. Clin J Am Soc Nephrol 2017; 12(6):983–997. doi:10.2215/CJN.11761116
- Barbour SJ, Greenwald A, Djurdjev O, et al. Disease-specific risk of venous thromboembolic events is increased in idiopathic glomerulonephritis. Kidney Int 2012; 81(2):190–195. doi:10.1038/ki.2011.312
- Lionaki S, Derebail VK, Hogan SL, et al. Venous thromboembolism in patients with membranous nephropathy. Clin J Am Soc Nephrol 2012; 7(1):43–51. doi:10.2215/CJN.04250511
- Lee T, Biddle AK, Lionaki S, et al. Personalized prophylactic anticoagulation decision analysis in patients with membranous nephropathy. Kidney Int 2014; 85(6):1412–1420. doi:10.1038/ki.2013.476
- Jaar BG, Kim HS, Samaniego MD, Lund GB, Atta MG. Percutaneous mechanical thrombectomy: a new approach in the treatment of acute renal-vein thrombosis. Nephrol Dial Transplant 2002; 17(6):1122–1125. pmid:12032209
- Couser WG. Primary membranous nephropathy. Clin J Am Soc Nephrol 2017; 12(6):983–997. doi:10.2215/CJN.11761116
- Barbour SJ, Greenwald A, Djurdjev O, et al. Disease-specific risk of venous thromboembolic events is increased in idiopathic glomerulonephritis. Kidney Int 2012; 81(2):190–195. doi:10.1038/ki.2011.312
- Lionaki S, Derebail VK, Hogan SL, et al. Venous thromboembolism in patients with membranous nephropathy. Clin J Am Soc Nephrol 2012; 7(1):43–51. doi:10.2215/CJN.04250511
- Lee T, Biddle AK, Lionaki S, et al. Personalized prophylactic anticoagulation decision analysis in patients with membranous nephropathy. Kidney Int 2014; 85(6):1412–1420. doi:10.1038/ki.2013.476
- Jaar BG, Kim HS, Samaniego MD, Lund GB, Atta MG. Percutaneous mechanical thrombectomy: a new approach in the treatment of acute renal-vein thrombosis. Nephrol Dial Transplant 2002; 17(6):1122–1125. pmid:12032209
Back pain as a sign of inferior vena cava filter complications
A 63-year-old woman presented with an acute exacerbation of chronic back pain after a fall. She was taking warfarin because of a history of factor V Leiden, deep vein thrombosis, and pulmonary embolism, for which a temporary inferior vena cava (IVC) filter had been placed 8 years ago. Her physicians had subsequently tried to remove the filter, without success. Some time after that, 1 of the filter struts had been removed after it migrated through her abdominal wall.
Laboratory testing revealed a supratherapeutic international normalized ratio of 8.5.
The patient underwent endovascular aneurysm repair with adequate placement of a vascular graft. She was discharged on therapeutic anticoagulation, and her back pain had notably improved.
COMPLICATIONS OF IVC FILTERS
In the United States, the use of IVC filters has increased significantly over the last decade, with placement rates ranging from 12% to 17% in patients with venous thromboembolism.1
The American Heart Association recommends filter placement for patients with venous thromboembolism for whom anticoagulation has failed or is contraindicated, patients unable to withstand pulmonary embolism, and patients who are hemodynamically unstable.2 While indications vary in the guidelines released by different societies, filters are most often placed in patients who have an acute bleed, significant surgery after admission for venous thromboembolism, metastatic cancer, and severe illness.3
Complications can occur during and after insertion and during removal. They are more frequent with temporary than with permanent filters, and include filter movement and fracture as well as occlusion and penetration.4,5
In our patient, we believe that the 3 remaining filter struts likely penetrated the wall of the IVC to the extent that they encountered adjacent structures (aorta, duodenum, kidney).
Of cases of IVC filter penetration reported to a US Food and Drug Administration database, 13.1% involved small bowel perforation, 6.5% involved aortic perforation, and 4.2% involved retroperitoneal bleeding. Symptoms such as abdominal and back pain were present in 38.3% of cases involving IVC penetration.5
Therefore, the differential diagnosis for patients with a history of IVC filter placement presenting with these symptoms should address filter complications, including occlusion, incorrect placement, fracture, migration, and penetration of the filter.4 If complications occur, treatment options include anticoagulation, endovascular repair, and surgical intervention.
- Alkhouli M, Bashir R. Inferior vena cava filters in the United States: less is more. Int J Cardiol 2014; 177(3):742–743. doi:10.1016/j.ijcard.2014.08.010
- Jaff MR, McMurtry MS, Archer SL, et al; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123(16):1788–1830. doi:10.1161/CIR.0b013e318214914f
- White RH, Geraghty EM, Brunson A, et al. High variation between hospitals in vena cava filter use for venous thromboembolism. JAMA Intern Med 2013; 173(7):506–512. doi:10.1001/jamainternmed.2013.2352
- Sella DM, Oldenburg WA. Complications of inferior vena cava filters. Semin Vasc Surg 2013; 26(1):23–28. doi:10.1053/j.semvascsurg.2013.04.005
- Andreoli JM, Lewandowski RJ, Vogelzang RL, Ryu RK. Comparison of complication rates associated with permanent and retrievable inferior vena cava filters: a review of the MAUDE database. J Vasc Interv Radiol 2014; 25(8):1181–1185. doi:10.1016/j.jvir.2014.04.016
A 63-year-old woman presented with an acute exacerbation of chronic back pain after a fall. She was taking warfarin because of a history of factor V Leiden, deep vein thrombosis, and pulmonary embolism, for which a temporary inferior vena cava (IVC) filter had been placed 8 years ago. Her physicians had subsequently tried to remove the filter, without success. Some time after that, 1 of the filter struts had been removed after it migrated through her abdominal wall.
Laboratory testing revealed a supratherapeutic international normalized ratio of 8.5.
The patient underwent endovascular aneurysm repair with adequate placement of a vascular graft. She was discharged on therapeutic anticoagulation, and her back pain had notably improved.
COMPLICATIONS OF IVC FILTERS
In the United States, the use of IVC filters has increased significantly over the last decade, with placement rates ranging from 12% to 17% in patients with venous thromboembolism.1
The American Heart Association recommends filter placement for patients with venous thromboembolism for whom anticoagulation has failed or is contraindicated, patients unable to withstand pulmonary embolism, and patients who are hemodynamically unstable.2 While indications vary in the guidelines released by different societies, filters are most often placed in patients who have an acute bleed, significant surgery after admission for venous thromboembolism, metastatic cancer, and severe illness.3
Complications can occur during and after insertion and during removal. They are more frequent with temporary than with permanent filters, and include filter movement and fracture as well as occlusion and penetration.4,5
In our patient, we believe that the 3 remaining filter struts likely penetrated the wall of the IVC to the extent that they encountered adjacent structures (aorta, duodenum, kidney).
Of cases of IVC filter penetration reported to a US Food and Drug Administration database, 13.1% involved small bowel perforation, 6.5% involved aortic perforation, and 4.2% involved retroperitoneal bleeding. Symptoms such as abdominal and back pain were present in 38.3% of cases involving IVC penetration.5
Therefore, the differential diagnosis for patients with a history of IVC filter placement presenting with these symptoms should address filter complications, including occlusion, incorrect placement, fracture, migration, and penetration of the filter.4 If complications occur, treatment options include anticoagulation, endovascular repair, and surgical intervention.
A 63-year-old woman presented with an acute exacerbation of chronic back pain after a fall. She was taking warfarin because of a history of factor V Leiden, deep vein thrombosis, and pulmonary embolism, for which a temporary inferior vena cava (IVC) filter had been placed 8 years ago. Her physicians had subsequently tried to remove the filter, without success. Some time after that, 1 of the filter struts had been removed after it migrated through her abdominal wall.
Laboratory testing revealed a supratherapeutic international normalized ratio of 8.5.
The patient underwent endovascular aneurysm repair with adequate placement of a vascular graft. She was discharged on therapeutic anticoagulation, and her back pain had notably improved.
COMPLICATIONS OF IVC FILTERS
In the United States, the use of IVC filters has increased significantly over the last decade, with placement rates ranging from 12% to 17% in patients with venous thromboembolism.1
The American Heart Association recommends filter placement for patients with venous thromboembolism for whom anticoagulation has failed or is contraindicated, patients unable to withstand pulmonary embolism, and patients who are hemodynamically unstable.2 While indications vary in the guidelines released by different societies, filters are most often placed in patients who have an acute bleed, significant surgery after admission for venous thromboembolism, metastatic cancer, and severe illness.3
Complications can occur during and after insertion and during removal. They are more frequent with temporary than with permanent filters, and include filter movement and fracture as well as occlusion and penetration.4,5
In our patient, we believe that the 3 remaining filter struts likely penetrated the wall of the IVC to the extent that they encountered adjacent structures (aorta, duodenum, kidney).
Of cases of IVC filter penetration reported to a US Food and Drug Administration database, 13.1% involved small bowel perforation, 6.5% involved aortic perforation, and 4.2% involved retroperitoneal bleeding. Symptoms such as abdominal and back pain were present in 38.3% of cases involving IVC penetration.5
Therefore, the differential diagnosis for patients with a history of IVC filter placement presenting with these symptoms should address filter complications, including occlusion, incorrect placement, fracture, migration, and penetration of the filter.4 If complications occur, treatment options include anticoagulation, endovascular repair, and surgical intervention.
- Alkhouli M, Bashir R. Inferior vena cava filters in the United States: less is more. Int J Cardiol 2014; 177(3):742–743. doi:10.1016/j.ijcard.2014.08.010
- Jaff MR, McMurtry MS, Archer SL, et al; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123(16):1788–1830. doi:10.1161/CIR.0b013e318214914f
- White RH, Geraghty EM, Brunson A, et al. High variation between hospitals in vena cava filter use for venous thromboembolism. JAMA Intern Med 2013; 173(7):506–512. doi:10.1001/jamainternmed.2013.2352
- Sella DM, Oldenburg WA. Complications of inferior vena cava filters. Semin Vasc Surg 2013; 26(1):23–28. doi:10.1053/j.semvascsurg.2013.04.005
- Andreoli JM, Lewandowski RJ, Vogelzang RL, Ryu RK. Comparison of complication rates associated with permanent and retrievable inferior vena cava filters: a review of the MAUDE database. J Vasc Interv Radiol 2014; 25(8):1181–1185. doi:10.1016/j.jvir.2014.04.016
- Alkhouli M, Bashir R. Inferior vena cava filters in the United States: less is more. Int J Cardiol 2014; 177(3):742–743. doi:10.1016/j.ijcard.2014.08.010
- Jaff MR, McMurtry MS, Archer SL, et al; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123(16):1788–1830. doi:10.1161/CIR.0b013e318214914f
- White RH, Geraghty EM, Brunson A, et al. High variation between hospitals in vena cava filter use for venous thromboembolism. JAMA Intern Med 2013; 173(7):506–512. doi:10.1001/jamainternmed.2013.2352
- Sella DM, Oldenburg WA. Complications of inferior vena cava filters. Semin Vasc Surg 2013; 26(1):23–28. doi:10.1053/j.semvascsurg.2013.04.005
- Andreoli JM, Lewandowski RJ, Vogelzang RL, Ryu RK. Comparison of complication rates associated with permanent and retrievable inferior vena cava filters: a review of the MAUDE database. J Vasc Interv Radiol 2014; 25(8):1181–1185. doi:10.1016/j.jvir.2014.04.016
Which patients with pulmonary embolism need echocardiography?
Most patients admitted with pulmonary embolism (PE) do not need transthoracic echocardiography (TTE); it should be performed in hemodynamically unstable patients, as well as in hemodynamically stable patients with specific elevated cardiac biomarkers and imaging features.
The decision to perform TTE should be based on clinical presentation, PE burden, and imaging findings (eg, computed tomographic angiography). TTE helps to stratify risk, guide management, monitor response to therapy, and give prognostic information for a subset of patients at increased risk for PE-related adverse events.
RISK STRATIFICATION IN PULMONARY EMBOLISM
PE has a spectrum of presentations ranging from no symptoms to shock. Based on the clinical presentation, PE can be categorized as high, intermediate, or low risk.
High-risk PE, often referred to as “massive” PE, is defined in current American Heart Association guidelines as acute PE with sustained hypotension (systolic blood pressure < 90 mm Hg for at least 15 minutes or requiring inotropic support), persistent profound bradycardia (heart rate < 40 beats per minute with signs or symptoms of shock), syncope, or cardiac arrest.1
Intermediate-risk or “submassive” PE is more challenging to identify because patients are more hemodynamically stable, yet have evidence on electrocardiography, TTE, computed tomography, or cardiac biomarker testing—ie, N-terminal pro-B-type natriuretic peptide (NT-proBNP) or troponin—that indicates myocardial injury or volume overload.1
Low-risk PE is acute PE in the absence of clinical markers of adverse prognosis that define massive or submassive PE.1
ECHOCARDIOGRAPHIC FEATURES OF HIGH-RISK PULMONARY EMBOLISM
Certain TTE findings suggest increased risk of a poor outcome and may warrant therapy that is more invasive and aggressive. High-risk features include the following:
- Impaired right ventricular function
- Interventricular septum bulging into the left ventricle (“D-shaped” septum)
- Dilated proximal pulmonary arteries
- Increased severity of tricuspid regurgitation
- Elevated right atrial pressure
- Elevated pulmonary artery pressure
- Free-floating right ventricular thrombi, which are associated with a mortality rate of up to 45% and can be detected in 7% to 18% of patients6
- Tricuspid annular plane systolic excursion, an echocardiographic measure of right ventricular function1; a value less than 17 mm suggests impaired right ventricular systolic function7
- The McConnell sign, a feature of acute massive PE: akinesia of the mid-free wall of the right ventricle and hypercontractility of the apex.
These TTE findings often lead to treatment with thrombolysis, transfer to the intensive care unit, and activation of the interventional team for catheter-based therapies.1,8 Free-floating right heart thrombi or thrombus straddling the interatrial septum (“thrombus in transit”) through a patent foramen ovale may require surgical embolectomy.8
PATIENT SELECTION AND INDICATIONS FOR ECHOCARDIOGRAPHY
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension. Circulation 2011; 123:1788–1830. doi:10.1161/CIR.0b013e318214914f
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389. doi:10.1001/archinternmed.2010.199
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005; 172:1041–1046. doi:10.1164/rccm.200506-862OC
- Bova C, Pesavento R, Marchiori A, et al; TELESIO Study Group. Risk stratification and outcomes in hemodynamically stable patients with acute pulmonary embolism. J Thromb Haemost 2009; 7:938–944. doi:10.1111/j.1538-7836.2009.03345.x
- Fernandez C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest 2015; 148:211–218. doi:10.1378/chest.14-2551
- Chartier L, Bera J, Delomez M, et al. Free-floating thrombi in the right heart: diagnosis, management, and prognostic indexes in 38 consecutive patients. Circulation 1999; 99:2779–2783. pmid:10351972
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults. J Am Soc Echocardiogr 2010; 23:685–713. doi:10.1016/j.echo.2010.05.010
- Konstantinides S, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35:3033–3069a–k. doi:10.1093/eurheartj/ehu283
- Saric M, Armour AC, Arnaout MS, et al. Guidelines for the use of echocardiography in the evaluation of a cardiac source of embolism. J Am Soc Echocardiogr 2016; 29:1–42. doi:10.1016/j.echo.2015.09.011
Most patients admitted with pulmonary embolism (PE) do not need transthoracic echocardiography (TTE); it should be performed in hemodynamically unstable patients, as well as in hemodynamically stable patients with specific elevated cardiac biomarkers and imaging features.
The decision to perform TTE should be based on clinical presentation, PE burden, and imaging findings (eg, computed tomographic angiography). TTE helps to stratify risk, guide management, monitor response to therapy, and give prognostic information for a subset of patients at increased risk for PE-related adverse events.
RISK STRATIFICATION IN PULMONARY EMBOLISM
PE has a spectrum of presentations ranging from no symptoms to shock. Based on the clinical presentation, PE can be categorized as high, intermediate, or low risk.
High-risk PE, often referred to as “massive” PE, is defined in current American Heart Association guidelines as acute PE with sustained hypotension (systolic blood pressure < 90 mm Hg for at least 15 minutes or requiring inotropic support), persistent profound bradycardia (heart rate < 40 beats per minute with signs or symptoms of shock), syncope, or cardiac arrest.1
Intermediate-risk or “submassive” PE is more challenging to identify because patients are more hemodynamically stable, yet have evidence on electrocardiography, TTE, computed tomography, or cardiac biomarker testing—ie, N-terminal pro-B-type natriuretic peptide (NT-proBNP) or troponin—that indicates myocardial injury or volume overload.1
Low-risk PE is acute PE in the absence of clinical markers of adverse prognosis that define massive or submassive PE.1
ECHOCARDIOGRAPHIC FEATURES OF HIGH-RISK PULMONARY EMBOLISM
Certain TTE findings suggest increased risk of a poor outcome and may warrant therapy that is more invasive and aggressive. High-risk features include the following:
- Impaired right ventricular function
- Interventricular septum bulging into the left ventricle (“D-shaped” septum)
- Dilated proximal pulmonary arteries
- Increased severity of tricuspid regurgitation
- Elevated right atrial pressure
- Elevated pulmonary artery pressure
- Free-floating right ventricular thrombi, which are associated with a mortality rate of up to 45% and can be detected in 7% to 18% of patients6
- Tricuspid annular plane systolic excursion, an echocardiographic measure of right ventricular function1; a value less than 17 mm suggests impaired right ventricular systolic function7
- The McConnell sign, a feature of acute massive PE: akinesia of the mid-free wall of the right ventricle and hypercontractility of the apex.
These TTE findings often lead to treatment with thrombolysis, transfer to the intensive care unit, and activation of the interventional team for catheter-based therapies.1,8 Free-floating right heart thrombi or thrombus straddling the interatrial septum (“thrombus in transit”) through a patent foramen ovale may require surgical embolectomy.8
PATIENT SELECTION AND INDICATIONS FOR ECHOCARDIOGRAPHY
Most patients admitted with pulmonary embolism (PE) do not need transthoracic echocardiography (TTE); it should be performed in hemodynamically unstable patients, as well as in hemodynamically stable patients with specific elevated cardiac biomarkers and imaging features.
The decision to perform TTE should be based on clinical presentation, PE burden, and imaging findings (eg, computed tomographic angiography). TTE helps to stratify risk, guide management, monitor response to therapy, and give prognostic information for a subset of patients at increased risk for PE-related adverse events.
RISK STRATIFICATION IN PULMONARY EMBOLISM
PE has a spectrum of presentations ranging from no symptoms to shock. Based on the clinical presentation, PE can be categorized as high, intermediate, or low risk.
High-risk PE, often referred to as “massive” PE, is defined in current American Heart Association guidelines as acute PE with sustained hypotension (systolic blood pressure < 90 mm Hg for at least 15 minutes or requiring inotropic support), persistent profound bradycardia (heart rate < 40 beats per minute with signs or symptoms of shock), syncope, or cardiac arrest.1
Intermediate-risk or “submassive” PE is more challenging to identify because patients are more hemodynamically stable, yet have evidence on electrocardiography, TTE, computed tomography, or cardiac biomarker testing—ie, N-terminal pro-B-type natriuretic peptide (NT-proBNP) or troponin—that indicates myocardial injury or volume overload.1
Low-risk PE is acute PE in the absence of clinical markers of adverse prognosis that define massive or submassive PE.1
ECHOCARDIOGRAPHIC FEATURES OF HIGH-RISK PULMONARY EMBOLISM
Certain TTE findings suggest increased risk of a poor outcome and may warrant therapy that is more invasive and aggressive. High-risk features include the following:
- Impaired right ventricular function
- Interventricular septum bulging into the left ventricle (“D-shaped” septum)
- Dilated proximal pulmonary arteries
- Increased severity of tricuspid regurgitation
- Elevated right atrial pressure
- Elevated pulmonary artery pressure
- Free-floating right ventricular thrombi, which are associated with a mortality rate of up to 45% and can be detected in 7% to 18% of patients6
- Tricuspid annular plane systolic excursion, an echocardiographic measure of right ventricular function1; a value less than 17 mm suggests impaired right ventricular systolic function7
- The McConnell sign, a feature of acute massive PE: akinesia of the mid-free wall of the right ventricle and hypercontractility of the apex.
These TTE findings often lead to treatment with thrombolysis, transfer to the intensive care unit, and activation of the interventional team for catheter-based therapies.1,8 Free-floating right heart thrombi or thrombus straddling the interatrial septum (“thrombus in transit”) through a patent foramen ovale may require surgical embolectomy.8
PATIENT SELECTION AND INDICATIONS FOR ECHOCARDIOGRAPHY
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension. Circulation 2011; 123:1788–1830. doi:10.1161/CIR.0b013e318214914f
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389. doi:10.1001/archinternmed.2010.199
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005; 172:1041–1046. doi:10.1164/rccm.200506-862OC
- Bova C, Pesavento R, Marchiori A, et al; TELESIO Study Group. Risk stratification and outcomes in hemodynamically stable patients with acute pulmonary embolism. J Thromb Haemost 2009; 7:938–944. doi:10.1111/j.1538-7836.2009.03345.x
- Fernandez C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest 2015; 148:211–218. doi:10.1378/chest.14-2551
- Chartier L, Bera J, Delomez M, et al. Free-floating thrombi in the right heart: diagnosis, management, and prognostic indexes in 38 consecutive patients. Circulation 1999; 99:2779–2783. pmid:10351972
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults. J Am Soc Echocardiogr 2010; 23:685–713. doi:10.1016/j.echo.2010.05.010
- Konstantinides S, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35:3033–3069a–k. doi:10.1093/eurheartj/ehu283
- Saric M, Armour AC, Arnaout MS, et al. Guidelines for the use of echocardiography in the evaluation of a cardiac source of embolism. J Am Soc Echocardiogr 2016; 29:1–42. doi:10.1016/j.echo.2015.09.011
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension. Circulation 2011; 123:1788–1830. doi:10.1161/CIR.0b013e318214914f
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389. doi:10.1001/archinternmed.2010.199
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005; 172:1041–1046. doi:10.1164/rccm.200506-862OC
- Bova C, Pesavento R, Marchiori A, et al; TELESIO Study Group. Risk stratification and outcomes in hemodynamically stable patients with acute pulmonary embolism. J Thromb Haemost 2009; 7:938–944. doi:10.1111/j.1538-7836.2009.03345.x
- Fernandez C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest 2015; 148:211–218. doi:10.1378/chest.14-2551
- Chartier L, Bera J, Delomez M, et al. Free-floating thrombi in the right heart: diagnosis, management, and prognostic indexes in 38 consecutive patients. Circulation 1999; 99:2779–2783. pmid:10351972
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults. J Am Soc Echocardiogr 2010; 23:685–713. doi:10.1016/j.echo.2010.05.010
- Konstantinides S, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35:3033–3069a–k. doi:10.1093/eurheartj/ehu283
- Saric M, Armour AC, Arnaout MS, et al. Guidelines for the use of echocardiography in the evaluation of a cardiac source of embolism. J Am Soc Echocardiogr 2016; 29:1–42. doi:10.1016/j.echo.2015.09.011
Pulmonary infarction due to pulmonary embolism
A 76-year-old man whose history included abdominal aortic aneurysm repair, bilateral femoral artery bypass for popliteal artery aneurysm, hypertension, and peptic ulcer disease was admitted to a community hospital with pleuritic chest pain and shortness of breath. Two days earlier, he had undergone repair of a ventral hernia.
At the time of that admission, he reported no fever, chills, night sweats, cough, or history of heart or lung disease. His vital signs were normal, and physical examination had revealed no apparent respiratory distress, no jugular venous distention, normal heart sounds, and no pedal edema; however, decreased air entry was noted in the right lung base. Initial serum levels of troponin and N-terminal pro-B-type natriuretic peptide were normal.
At that time, computed tomographic angiography of the chest showed segmental pulmonary emboli in the left upper and right lower lobes of the lungs and right pleural effusion. Transthoracic echocardiography showed normal atrial and ventricular sizes with no right or left ventricular systolic dysfunction and a left ventricular ejection fraction of 59%.
Treatment with intravenous heparin was started, and the patient was transferred to our hospital.
PLEURAL EFFUSION AND PULMONARY EMBOLISM
1. Which of the following is true about pleural effusion?
- It is rarely, if ever, associated with pulmonary embolism
- Most patients with pleural effusion due to pulmonary embolism do not have pleuritic chest pain
- Pulmonary embolism should be excluded in all cases of pleural effusion without a clear cause
Pulmonary embolism should be excluded in all cases of pleural effusion that do not have a clear cause. As for the other answer choices:
- Pulmonary embolism is the fourth leading cause of pleural effusion in the United States, after heart failure, pneumonia, and malignancy.1
- About 75% of patients who develop pleural effusion in the setting of pulmonary embolism complain of pleuritic chest pain on the side of the effusion.2 Most effusions are unilateral, small, and usually exudative.3
EVALUATION BEGINS: RESULTS OF THORACENTESIS
Our patient continued to receive intravenous heparin.
He underwent thoracentesis on hospital day 3, and 1,000 mL of turbid sanguineous pleural fluid was removed. Analysis of the fluid showed pH 7.27, white blood cell count 3.797 × 109/L with 80% neutrophils, and lactate dehydrogenase (LDH) concentration 736 U/L (a ratio of pleural fluid LDH to a concurrent serum LDH > 0.6 is suggestive of an exudate); the fluid was also sent for culture and cytology. Thoracentesis was terminated early due to cough, and follow-up chest radiography showed a moderate-sized pneumothorax.
Computed tomography (CT) of the chest at this time showed a small wedge-shaped area of lung consolidation in the right lower lobe (also seen on CT done 1 day before admission to our hospital), with an intrinsic air-fluid level suggesting a focal infarct or lung abscess, now obscured by adjacent consolidation and atelectasis. In the interval since the previous CT, the multiloculated right pleural effusion had increased in size (Figure 1).
THE NEXT STEP
2. What is the most appropriate next step for this patient?
- Consult an interventional radiologist for chest tube placement
- Start empiric antibiotic therapy and ask an interventional radiologist to place a chest tube
- Start empiric antibiotic therapy, withhold anticoagulation, and consult a thoracic surgeon
- Start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation
The most appropriate next step is to start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation.
In this patient, it is appropriate to initiate antibiotics empirically on the basis of his significant pleural loculations, a wedge-shaped consolidation, and 80% neutrophils in the pleural fluid, all of which suggest infection. The unmasking of a wedge-shaped consolidation after thoracentesis, with a previously noted air-fluid level and an interval increase in multiloculated pleural fluid, raises suspicion of a necrotic infection that may have ruptured into the pleural space, a possible lung infarct, or a malignancy. Hence, simply placing a chest tube may not be enough.
Blood in the pleural fluid does not necessitate withholding anticoagulation unless the bleeding is heavy. A pleural fluid hematocrit greater than 50% of the peripheral blood hematocrit suggests hemothorax and is an indication to withhold anticoagulation.1 Our patient’s pleural fluid was qualitatively sanguineous but not frankly bloody, and therefore we judged that it was not necessary to stop his heparin.
HOW DOES PULMONARY INFARCTION PRESENT CLINICALLY?
3. Which of the following statements about pulmonary infarction is incorrect?
- Cavitation and infarction are more common with larger emboli
- Cavitation occurs in fewer than 10% of pulmonary infarctions
- Lung abscess develops in more than 50% of pulmonary infarctions
- Pulmonary thromboembolism is the most common cause of pulmonary infarction
Lung abscess develops in far fewer than 50% of cases of pulmonary infarction. The rest of the statements are correct.
Cavitation complicates about 4% to 7% of infarctions and is more common when the infarction is 4 cm or greater in diameter.4 These cavities are usually single and predominantly on the right side in the apical or posterior segment of the upper lobe or the apical segment of the right lower lobe, as in our patient.5–8 CT demonstrating scalloped inner margins and cross-cavity band shadows suggests a cavitary pulmonary infarction.9,10
Infection and abscess in pulmonary infarction are poorly understood but have been linked to larger infarctions, coexistent congestion or atelectasis, and dental or oropharyngeal infection. In an early series of 550 cases of pulmonary infarction, 23 patients (4.2%) developed lung abscess and 6 (1.1%) developed empyema.11 The mean time to cavitation for an infected pulmonary infarction has been reported to be 18 days.12
A reversed halo sign, generally described as a focal, rounded area of ground-glass opacity surrounded by a nearly complete ring of consolidation, has been reported to be more frequent with pulmonary infarction than with other diseases, especially when in the lower lobes.13
CASE CONTINUED: THORACOSCOPY
A cardiothoracic surgeon was consulted, intravenous heparin was discontinued, an inferior vena cava filter was placed, and the patient underwent video-assisted thoracoscopy.
Purulent fluid was noted on the lateral aspect of right lower lobe; this appeared to be the ruptured cavitary lesion functioning like an uncontrolled bronchopleural fistula. Two chest tubes, sizes 32F and 28F, were placed after decortication, resection of the lung abscess, and closure of the bronchopleural fistula. No significant air leak was noted after resection of this segment of lung.
Pathologic study showed acute organizing pneumonia with abscess formation; no malignant cells or granulomas were seen (Figure 2). Pleural fluid cultures grew Streptococcus intermedius, while the tissue culture was negative for any growth, including acid-fast bacilli and fungi.
On 3 different occasions, both chest tubes were shortened, backed out 2 cm, and resecured with sutures and pins, and Heimlich valves were applied before the patient was discharged.
Intravenous piperacillin-tazobactam was started on the fifth hospital day. On discharge, the patient was advised to continue this treatment for 3 weeks at home.
The patient was receiving enoxaparin subcutaneously in prophylactic doses; 72 hours after the thorascopic procedure this was increased to therapeutic doses, continuing after discharge. Bridging to warfarin was not advised in view of his chest tubes.
Our patient appeared to have developed a right lower lobe infarction that cavitated and ruptured into the pleural space, causing a bronchopleural fistula with empyema after a recent pulmonary embolism. Other reported causes of pulmonary infarction in pulmonary embolism are malignancy and heavy clot burden,6 but these have not been confirmed in subsequent studies.5 Malignancy was ruled out by biopsy of the resected portion of the lung, and our patient did not have a history of heart failure. A clear cavity was not noted (because it ruptured into the pleura), but an air-fluid level was described in a wedge-shaped consolidation, suggesting infarction.
How common is pulmonary infarction after pulmonary embolism?
Pulmonary infarction occurs in few patients with pulmonary embolism.13 Since the lungs receive oxygen from the airways and have a dual blood supply from the pulmonary and bronchial arteries, they are not particularly vulnerable to ischemia. However, the reported incidence of pulmonary infarction in patients with pulmonary embolism has ranged from 10% to higher than 30%.5,14,15
The reasons behind pulmonary infarction with complications after pulmonary embolism have varied in different case series in different eras. CT, biopsy, or autopsy studies reveal pulmonary infarction after pulmonary embolism to be more common than suspected by clinical symptoms.
In a Mayo Clinic series of 43 cases of pulmonary infarction diagnosed over a 6-year period by surgical lung biopsy, 18 (42%) of the patients had underlying pulmonary thromboembolism, which was the most common cause.16
RISK FACTORS FOR PULMONARY INFARCTION
4. Which statement about risk factors for pulmonary infarction in pulmonary embolism is incorrect?
- Heart failure may be a risk factor for pulmonary infarction
- Pulmonary hemorrhage is a risk factor for pulmonary infarction
- Pulmonary infarction is more common with more proximal sites of pulmonary embolism
- Collateral circulation may protect against pulmonary infarction
Infarction is more common with emboli that are distal rather than proximal.
Dalen et al15 suggested that after pulmonary embolism, pulmonary hemorrhage is an important contributor to the development of pulmonary infarction independent of the presence or absence of associated cardiac or pulmonary disease, but that the effect depends on the site of obstruction.
This idea was first proposed in 1913, when Karsner and Ghoreyeb17 showed that when pulmonary arteries are completely obstructed, the bronchial arteries take over, except when the embolism is present in a small branch of the pulmonary artery. This is because the physiologic anastomosis between the pulmonary artery and the bronchial arteries is located at the precapillary level of the pulmonary artery, and the bronchial circulation does not take over until the pulmonary arterial pressure in the area of the embolism drops to zero.
Using CT data, Kirchner et al5 confirmed that the risk of pulmonary infarction is higher if the obstruction is peripheral, ie, distal.
Using autopsy data, Tsao et al18 reported a higher risk of pulmonary infarction in embolic occlusion of pulmonary vessels less than 3 mm in diameter.
Collateral circulation has been shown to protect against pulmonary infarction. For example, Miniati et al14 showed that healthy young patients with pulmonary embolism were more prone to develop pulmonary infarction, probably because they had less efficient collateral systems in the peripheral lung fields. In lung transplant recipients, it has been shown that the risk of infarction decreased with development of collateral circulation.19
Dalen et al,15 however, attributed delayed resolution of pulmonary hemorrhage (as measured by resolution of infiltrate on chest radiography) to higher underlying pulmonary venous pressure in patients with heart failure and consequent pulmonary infarction. In comparison, healthy patients without cardiac or pulmonary disease have faster resolution of pulmonary hemorrhage when present, and less likelihood of pulmonary infarction (and death in submassive pulmonary embolism).
Data on the management of infected pulmonary infarction are limited. Mortality rates have been as high as 41% with noninfected and 73% with infected cavitary infarctions.4 Some authors have advocated early surgical resection in view of high rates of failure of medical treatment due to lack of blood supply within the cavity and continued risk of infection.
KEY POINTS
In patients with a recently diagnosed pulmonary embolism and concurrent symptoms of bacterial pneumonia, a diagnosis of cavitary pulmonary infarction should be considered.
Consolidations that are pleural-based with sharp, rounded margins and with focal areas of central hyperlucencies representing hemorrhage on the mediastinal windows on CT are more likely to represent a pulmonary infarct.20
- Light RW. Pleural Diseases. 4th ed. Baltimore, MD: Lippincott, Williams & Wilkins; 2001.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100(3):598–603. pmid:1909617
- Light RW. Pleural effusion due to pulmonary emboli. Curr Opin Pulm Med 2001; 7(4):198–201. pmid:11470974
- Libby LS, King TE, LaForce FM, Schwarz MI. Pulmonary cavitation following pulmonary infarction. Medicine (Baltimore) 1985; 64(5):342–348. pmid:4033411
- Kirchner J, Obermann A, Stuckradt S, et al. Lung infarction following pulmonary embolism: a comparative study on clinical conditions and CT findings to identify predisposing factors. Rofo 2015; 187(6):440–444. doi:10.1055/s-0034-1399006
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging 2006; 21(1):1–7. doi:10.1097/01.rti.0000187433.06762.fb
- Scharf J, Nahir AM, Munk J, Lichtig C. Aseptic cavitation in pulmonary infarction. Chest 1971; 59(4):456–458. pmid:5551596
- Wilson AG, Joseph AE, Butland RJ. The radiology of aseptic cavitation in pulmonary infarction. Clin Radiol 1986; 37(4):327–333. pmid:3731699
- Butler MD, Biscardi FH, Schain DC, Humphries JE, Blow O, Spotnitz WD. Pulmonary resection for treatment of cavitary pulmonary infarction. Ann Thorac Surg 1997; 63(3):849–850. pmid:9066420
- Koroscil MT, Hauser TR. Acute pulmonary embolism leading to cavitation and large pulmonary abscess: a rare complication of pulmonary infarction. Respir Med Case Rep 2016; 20:72–74. doi:10.1016/j.rmcr.2016.12.001
- Levin L, Kernohan JW, Moersch HJ. Pulmonary abscess secondary to bland pulmonary infarction. Dis Chest 1948; 14(2):218–232. pmid:18904835
- Marchiori E, Menna Barreto M, Pereira Freitas HM, et al. Morphological characteristics of the reversed halo sign that may strongly suggest pulmonary infarction. Clin Radiol 2018; 73(5):503.e7–503.e13. doi:10.1016/j.crad.2017.11.022
- Smith GT, Dexter L, Dammin GJ. Postmortem quantitative studies in pulmonary embolism. In: Sasahara AA, Stein M, eds. Pulmonary Embolic Disease. New York, NY: Grune & Stratton, Inc; 1965:120–126.
- Miniati M, Bottai M, Ciccotosto C, Roberto L, Monti S. Predictors of pulmonary infarction. Medicine (Baltimore) 2015; 94(41):e1488. doi:10.1097/MD.0000000000001488
- Dalen JE, Haffajee CI, Alpert JS, Howe JP, Ockene IS, Paraskos JA. Pulmonary embolism, pulmonary hemorrhage and pulmonary infarction. N Engl J Med 1977; 296(25):1431–1435. doi:10.1056/NEJM197706232962503
- Parambil JG, Savci CD, Tazelaar HD, Ryu JH. Causes and presenting features of pulmonary infarctions in 43 cases identified by surgical lung biopsy. Chest 2005; 127(4):1178–1183. doi:10.1378/chest.127.4.1178
- Karsner HT, Ghoreyeb AA. Studies in infarction: III. The circulation in experimental pulmonary embolism. J Exp Med 1913; 18(5):507–511. pmid:19867725
- Tsao MS, Schraufnagel D, Wang NS. Pathogenesis of pulmonary infarction. Am J Med 1982; 72(4):599–606. pmid:6462058
- Burns KE, Iacono AT. Incidence of clinically unsuspected pulmonary embolism in mechanically ventilated lung transplant recipients. Transplantation 2003; 76(6):964–968. doi:10.1097/01.TP.0000084523.58610.BA
- Yousem SA. The surgical pathology of pulmonary infarcts: diagnostic confusion with granulomatous disease, vasculitis, and neoplasia. Mod Pathol 2009; 22(5):679–685. doi:10.1038/modpathol.2009.20
A 76-year-old man whose history included abdominal aortic aneurysm repair, bilateral femoral artery bypass for popliteal artery aneurysm, hypertension, and peptic ulcer disease was admitted to a community hospital with pleuritic chest pain and shortness of breath. Two days earlier, he had undergone repair of a ventral hernia.
At the time of that admission, he reported no fever, chills, night sweats, cough, or history of heart or lung disease. His vital signs were normal, and physical examination had revealed no apparent respiratory distress, no jugular venous distention, normal heart sounds, and no pedal edema; however, decreased air entry was noted in the right lung base. Initial serum levels of troponin and N-terminal pro-B-type natriuretic peptide were normal.
At that time, computed tomographic angiography of the chest showed segmental pulmonary emboli in the left upper and right lower lobes of the lungs and right pleural effusion. Transthoracic echocardiography showed normal atrial and ventricular sizes with no right or left ventricular systolic dysfunction and a left ventricular ejection fraction of 59%.
Treatment with intravenous heparin was started, and the patient was transferred to our hospital.
PLEURAL EFFUSION AND PULMONARY EMBOLISM
1. Which of the following is true about pleural effusion?
- It is rarely, if ever, associated with pulmonary embolism
- Most patients with pleural effusion due to pulmonary embolism do not have pleuritic chest pain
- Pulmonary embolism should be excluded in all cases of pleural effusion without a clear cause
Pulmonary embolism should be excluded in all cases of pleural effusion that do not have a clear cause. As for the other answer choices:
- Pulmonary embolism is the fourth leading cause of pleural effusion in the United States, after heart failure, pneumonia, and malignancy.1
- About 75% of patients who develop pleural effusion in the setting of pulmonary embolism complain of pleuritic chest pain on the side of the effusion.2 Most effusions are unilateral, small, and usually exudative.3
EVALUATION BEGINS: RESULTS OF THORACENTESIS
Our patient continued to receive intravenous heparin.
He underwent thoracentesis on hospital day 3, and 1,000 mL of turbid sanguineous pleural fluid was removed. Analysis of the fluid showed pH 7.27, white blood cell count 3.797 × 109/L with 80% neutrophils, and lactate dehydrogenase (LDH) concentration 736 U/L (a ratio of pleural fluid LDH to a concurrent serum LDH > 0.6 is suggestive of an exudate); the fluid was also sent for culture and cytology. Thoracentesis was terminated early due to cough, and follow-up chest radiography showed a moderate-sized pneumothorax.
Computed tomography (CT) of the chest at this time showed a small wedge-shaped area of lung consolidation in the right lower lobe (also seen on CT done 1 day before admission to our hospital), with an intrinsic air-fluid level suggesting a focal infarct or lung abscess, now obscured by adjacent consolidation and atelectasis. In the interval since the previous CT, the multiloculated right pleural effusion had increased in size (Figure 1).
THE NEXT STEP
2. What is the most appropriate next step for this patient?
- Consult an interventional radiologist for chest tube placement
- Start empiric antibiotic therapy and ask an interventional radiologist to place a chest tube
- Start empiric antibiotic therapy, withhold anticoagulation, and consult a thoracic surgeon
- Start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation
The most appropriate next step is to start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation.
In this patient, it is appropriate to initiate antibiotics empirically on the basis of his significant pleural loculations, a wedge-shaped consolidation, and 80% neutrophils in the pleural fluid, all of which suggest infection. The unmasking of a wedge-shaped consolidation after thoracentesis, with a previously noted air-fluid level and an interval increase in multiloculated pleural fluid, raises suspicion of a necrotic infection that may have ruptured into the pleural space, a possible lung infarct, or a malignancy. Hence, simply placing a chest tube may not be enough.
Blood in the pleural fluid does not necessitate withholding anticoagulation unless the bleeding is heavy. A pleural fluid hematocrit greater than 50% of the peripheral blood hematocrit suggests hemothorax and is an indication to withhold anticoagulation.1 Our patient’s pleural fluid was qualitatively sanguineous but not frankly bloody, and therefore we judged that it was not necessary to stop his heparin.
HOW DOES PULMONARY INFARCTION PRESENT CLINICALLY?
3. Which of the following statements about pulmonary infarction is incorrect?
- Cavitation and infarction are more common with larger emboli
- Cavitation occurs in fewer than 10% of pulmonary infarctions
- Lung abscess develops in more than 50% of pulmonary infarctions
- Pulmonary thromboembolism is the most common cause of pulmonary infarction
Lung abscess develops in far fewer than 50% of cases of pulmonary infarction. The rest of the statements are correct.
Cavitation complicates about 4% to 7% of infarctions and is more common when the infarction is 4 cm or greater in diameter.4 These cavities are usually single and predominantly on the right side in the apical or posterior segment of the upper lobe or the apical segment of the right lower lobe, as in our patient.5–8 CT demonstrating scalloped inner margins and cross-cavity band shadows suggests a cavitary pulmonary infarction.9,10
Infection and abscess in pulmonary infarction are poorly understood but have been linked to larger infarctions, coexistent congestion or atelectasis, and dental or oropharyngeal infection. In an early series of 550 cases of pulmonary infarction, 23 patients (4.2%) developed lung abscess and 6 (1.1%) developed empyema.11 The mean time to cavitation for an infected pulmonary infarction has been reported to be 18 days.12
A reversed halo sign, generally described as a focal, rounded area of ground-glass opacity surrounded by a nearly complete ring of consolidation, has been reported to be more frequent with pulmonary infarction than with other diseases, especially when in the lower lobes.13
CASE CONTINUED: THORACOSCOPY
A cardiothoracic surgeon was consulted, intravenous heparin was discontinued, an inferior vena cava filter was placed, and the patient underwent video-assisted thoracoscopy.
Purulent fluid was noted on the lateral aspect of right lower lobe; this appeared to be the ruptured cavitary lesion functioning like an uncontrolled bronchopleural fistula. Two chest tubes, sizes 32F and 28F, were placed after decortication, resection of the lung abscess, and closure of the bronchopleural fistula. No significant air leak was noted after resection of this segment of lung.
Pathologic study showed acute organizing pneumonia with abscess formation; no malignant cells or granulomas were seen (Figure 2). Pleural fluid cultures grew Streptococcus intermedius, while the tissue culture was negative for any growth, including acid-fast bacilli and fungi.
On 3 different occasions, both chest tubes were shortened, backed out 2 cm, and resecured with sutures and pins, and Heimlich valves were applied before the patient was discharged.
Intravenous piperacillin-tazobactam was started on the fifth hospital day. On discharge, the patient was advised to continue this treatment for 3 weeks at home.
The patient was receiving enoxaparin subcutaneously in prophylactic doses; 72 hours after the thorascopic procedure this was increased to therapeutic doses, continuing after discharge. Bridging to warfarin was not advised in view of his chest tubes.
Our patient appeared to have developed a right lower lobe infarction that cavitated and ruptured into the pleural space, causing a bronchopleural fistula with empyema after a recent pulmonary embolism. Other reported causes of pulmonary infarction in pulmonary embolism are malignancy and heavy clot burden,6 but these have not been confirmed in subsequent studies.5 Malignancy was ruled out by biopsy of the resected portion of the lung, and our patient did not have a history of heart failure. A clear cavity was not noted (because it ruptured into the pleura), but an air-fluid level was described in a wedge-shaped consolidation, suggesting infarction.
How common is pulmonary infarction after pulmonary embolism?
Pulmonary infarction occurs in few patients with pulmonary embolism.13 Since the lungs receive oxygen from the airways and have a dual blood supply from the pulmonary and bronchial arteries, they are not particularly vulnerable to ischemia. However, the reported incidence of pulmonary infarction in patients with pulmonary embolism has ranged from 10% to higher than 30%.5,14,15
The reasons behind pulmonary infarction with complications after pulmonary embolism have varied in different case series in different eras. CT, biopsy, or autopsy studies reveal pulmonary infarction after pulmonary embolism to be more common than suspected by clinical symptoms.
In a Mayo Clinic series of 43 cases of pulmonary infarction diagnosed over a 6-year period by surgical lung biopsy, 18 (42%) of the patients had underlying pulmonary thromboembolism, which was the most common cause.16
RISK FACTORS FOR PULMONARY INFARCTION
4. Which statement about risk factors for pulmonary infarction in pulmonary embolism is incorrect?
- Heart failure may be a risk factor for pulmonary infarction
- Pulmonary hemorrhage is a risk factor for pulmonary infarction
- Pulmonary infarction is more common with more proximal sites of pulmonary embolism
- Collateral circulation may protect against pulmonary infarction
Infarction is more common with emboli that are distal rather than proximal.
Dalen et al15 suggested that after pulmonary embolism, pulmonary hemorrhage is an important contributor to the development of pulmonary infarction independent of the presence or absence of associated cardiac or pulmonary disease, but that the effect depends on the site of obstruction.
This idea was first proposed in 1913, when Karsner and Ghoreyeb17 showed that when pulmonary arteries are completely obstructed, the bronchial arteries take over, except when the embolism is present in a small branch of the pulmonary artery. This is because the physiologic anastomosis between the pulmonary artery and the bronchial arteries is located at the precapillary level of the pulmonary artery, and the bronchial circulation does not take over until the pulmonary arterial pressure in the area of the embolism drops to zero.
Using CT data, Kirchner et al5 confirmed that the risk of pulmonary infarction is higher if the obstruction is peripheral, ie, distal.
Using autopsy data, Tsao et al18 reported a higher risk of pulmonary infarction in embolic occlusion of pulmonary vessels less than 3 mm in diameter.
Collateral circulation has been shown to protect against pulmonary infarction. For example, Miniati et al14 showed that healthy young patients with pulmonary embolism were more prone to develop pulmonary infarction, probably because they had less efficient collateral systems in the peripheral lung fields. In lung transplant recipients, it has been shown that the risk of infarction decreased with development of collateral circulation.19
Dalen et al,15 however, attributed delayed resolution of pulmonary hemorrhage (as measured by resolution of infiltrate on chest radiography) to higher underlying pulmonary venous pressure in patients with heart failure and consequent pulmonary infarction. In comparison, healthy patients without cardiac or pulmonary disease have faster resolution of pulmonary hemorrhage when present, and less likelihood of pulmonary infarction (and death in submassive pulmonary embolism).
Data on the management of infected pulmonary infarction are limited. Mortality rates have been as high as 41% with noninfected and 73% with infected cavitary infarctions.4 Some authors have advocated early surgical resection in view of high rates of failure of medical treatment due to lack of blood supply within the cavity and continued risk of infection.
KEY POINTS
In patients with a recently diagnosed pulmonary embolism and concurrent symptoms of bacterial pneumonia, a diagnosis of cavitary pulmonary infarction should be considered.
Consolidations that are pleural-based with sharp, rounded margins and with focal areas of central hyperlucencies representing hemorrhage on the mediastinal windows on CT are more likely to represent a pulmonary infarct.20
A 76-year-old man whose history included abdominal aortic aneurysm repair, bilateral femoral artery bypass for popliteal artery aneurysm, hypertension, and peptic ulcer disease was admitted to a community hospital with pleuritic chest pain and shortness of breath. Two days earlier, he had undergone repair of a ventral hernia.
At the time of that admission, he reported no fever, chills, night sweats, cough, or history of heart or lung disease. His vital signs were normal, and physical examination had revealed no apparent respiratory distress, no jugular venous distention, normal heart sounds, and no pedal edema; however, decreased air entry was noted in the right lung base. Initial serum levels of troponin and N-terminal pro-B-type natriuretic peptide were normal.
At that time, computed tomographic angiography of the chest showed segmental pulmonary emboli in the left upper and right lower lobes of the lungs and right pleural effusion. Transthoracic echocardiography showed normal atrial and ventricular sizes with no right or left ventricular systolic dysfunction and a left ventricular ejection fraction of 59%.
Treatment with intravenous heparin was started, and the patient was transferred to our hospital.
PLEURAL EFFUSION AND PULMONARY EMBOLISM
1. Which of the following is true about pleural effusion?
- It is rarely, if ever, associated with pulmonary embolism
- Most patients with pleural effusion due to pulmonary embolism do not have pleuritic chest pain
- Pulmonary embolism should be excluded in all cases of pleural effusion without a clear cause
Pulmonary embolism should be excluded in all cases of pleural effusion that do not have a clear cause. As for the other answer choices:
- Pulmonary embolism is the fourth leading cause of pleural effusion in the United States, after heart failure, pneumonia, and malignancy.1
- About 75% of patients who develop pleural effusion in the setting of pulmonary embolism complain of pleuritic chest pain on the side of the effusion.2 Most effusions are unilateral, small, and usually exudative.3
EVALUATION BEGINS: RESULTS OF THORACENTESIS
Our patient continued to receive intravenous heparin.
He underwent thoracentesis on hospital day 3, and 1,000 mL of turbid sanguineous pleural fluid was removed. Analysis of the fluid showed pH 7.27, white blood cell count 3.797 × 109/L with 80% neutrophils, and lactate dehydrogenase (LDH) concentration 736 U/L (a ratio of pleural fluid LDH to a concurrent serum LDH > 0.6 is suggestive of an exudate); the fluid was also sent for culture and cytology. Thoracentesis was terminated early due to cough, and follow-up chest radiography showed a moderate-sized pneumothorax.
Computed tomography (CT) of the chest at this time showed a small wedge-shaped area of lung consolidation in the right lower lobe (also seen on CT done 1 day before admission to our hospital), with an intrinsic air-fluid level suggesting a focal infarct or lung abscess, now obscured by adjacent consolidation and atelectasis. In the interval since the previous CT, the multiloculated right pleural effusion had increased in size (Figure 1).
THE NEXT STEP
2. What is the most appropriate next step for this patient?
- Consult an interventional radiologist for chest tube placement
- Start empiric antibiotic therapy and ask an interventional radiologist to place a chest tube
- Start empiric antibiotic therapy, withhold anticoagulation, and consult a thoracic surgeon
- Start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation
The most appropriate next step is to start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation.
In this patient, it is appropriate to initiate antibiotics empirically on the basis of his significant pleural loculations, a wedge-shaped consolidation, and 80% neutrophils in the pleural fluid, all of which suggest infection. The unmasking of a wedge-shaped consolidation after thoracentesis, with a previously noted air-fluid level and an interval increase in multiloculated pleural fluid, raises suspicion of a necrotic infection that may have ruptured into the pleural space, a possible lung infarct, or a malignancy. Hence, simply placing a chest tube may not be enough.
Blood in the pleural fluid does not necessitate withholding anticoagulation unless the bleeding is heavy. A pleural fluid hematocrit greater than 50% of the peripheral blood hematocrit suggests hemothorax and is an indication to withhold anticoagulation.1 Our patient’s pleural fluid was qualitatively sanguineous but not frankly bloody, and therefore we judged that it was not necessary to stop his heparin.
HOW DOES PULMONARY INFARCTION PRESENT CLINICALLY?
3. Which of the following statements about pulmonary infarction is incorrect?
- Cavitation and infarction are more common with larger emboli
- Cavitation occurs in fewer than 10% of pulmonary infarctions
- Lung abscess develops in more than 50% of pulmonary infarctions
- Pulmonary thromboembolism is the most common cause of pulmonary infarction
Lung abscess develops in far fewer than 50% of cases of pulmonary infarction. The rest of the statements are correct.
Cavitation complicates about 4% to 7% of infarctions and is more common when the infarction is 4 cm or greater in diameter.4 These cavities are usually single and predominantly on the right side in the apical or posterior segment of the upper lobe or the apical segment of the right lower lobe, as in our patient.5–8 CT demonstrating scalloped inner margins and cross-cavity band shadows suggests a cavitary pulmonary infarction.9,10
Infection and abscess in pulmonary infarction are poorly understood but have been linked to larger infarctions, coexistent congestion or atelectasis, and dental or oropharyngeal infection. In an early series of 550 cases of pulmonary infarction, 23 patients (4.2%) developed lung abscess and 6 (1.1%) developed empyema.11 The mean time to cavitation for an infected pulmonary infarction has been reported to be 18 days.12
A reversed halo sign, generally described as a focal, rounded area of ground-glass opacity surrounded by a nearly complete ring of consolidation, has been reported to be more frequent with pulmonary infarction than with other diseases, especially when in the lower lobes.13
CASE CONTINUED: THORACOSCOPY
A cardiothoracic surgeon was consulted, intravenous heparin was discontinued, an inferior vena cava filter was placed, and the patient underwent video-assisted thoracoscopy.
Purulent fluid was noted on the lateral aspect of right lower lobe; this appeared to be the ruptured cavitary lesion functioning like an uncontrolled bronchopleural fistula. Two chest tubes, sizes 32F and 28F, were placed after decortication, resection of the lung abscess, and closure of the bronchopleural fistula. No significant air leak was noted after resection of this segment of lung.
Pathologic study showed acute organizing pneumonia with abscess formation; no malignant cells or granulomas were seen (Figure 2). Pleural fluid cultures grew Streptococcus intermedius, while the tissue culture was negative for any growth, including acid-fast bacilli and fungi.
On 3 different occasions, both chest tubes were shortened, backed out 2 cm, and resecured with sutures and pins, and Heimlich valves were applied before the patient was discharged.
Intravenous piperacillin-tazobactam was started on the fifth hospital day. On discharge, the patient was advised to continue this treatment for 3 weeks at home.
The patient was receiving enoxaparin subcutaneously in prophylactic doses; 72 hours after the thorascopic procedure this was increased to therapeutic doses, continuing after discharge. Bridging to warfarin was not advised in view of his chest tubes.
Our patient appeared to have developed a right lower lobe infarction that cavitated and ruptured into the pleural space, causing a bronchopleural fistula with empyema after a recent pulmonary embolism. Other reported causes of pulmonary infarction in pulmonary embolism are malignancy and heavy clot burden,6 but these have not been confirmed in subsequent studies.5 Malignancy was ruled out by biopsy of the resected portion of the lung, and our patient did not have a history of heart failure. A clear cavity was not noted (because it ruptured into the pleura), but an air-fluid level was described in a wedge-shaped consolidation, suggesting infarction.
How common is pulmonary infarction after pulmonary embolism?
Pulmonary infarction occurs in few patients with pulmonary embolism.13 Since the lungs receive oxygen from the airways and have a dual blood supply from the pulmonary and bronchial arteries, they are not particularly vulnerable to ischemia. However, the reported incidence of pulmonary infarction in patients with pulmonary embolism has ranged from 10% to higher than 30%.5,14,15
The reasons behind pulmonary infarction with complications after pulmonary embolism have varied in different case series in different eras. CT, biopsy, or autopsy studies reveal pulmonary infarction after pulmonary embolism to be more common than suspected by clinical symptoms.
In a Mayo Clinic series of 43 cases of pulmonary infarction diagnosed over a 6-year period by surgical lung biopsy, 18 (42%) of the patients had underlying pulmonary thromboembolism, which was the most common cause.16
RISK FACTORS FOR PULMONARY INFARCTION
4. Which statement about risk factors for pulmonary infarction in pulmonary embolism is incorrect?
- Heart failure may be a risk factor for pulmonary infarction
- Pulmonary hemorrhage is a risk factor for pulmonary infarction
- Pulmonary infarction is more common with more proximal sites of pulmonary embolism
- Collateral circulation may protect against pulmonary infarction
Infarction is more common with emboli that are distal rather than proximal.
Dalen et al15 suggested that after pulmonary embolism, pulmonary hemorrhage is an important contributor to the development of pulmonary infarction independent of the presence or absence of associated cardiac or pulmonary disease, but that the effect depends on the site of obstruction.
This idea was first proposed in 1913, when Karsner and Ghoreyeb17 showed that when pulmonary arteries are completely obstructed, the bronchial arteries take over, except when the embolism is present in a small branch of the pulmonary artery. This is because the physiologic anastomosis between the pulmonary artery and the bronchial arteries is located at the precapillary level of the pulmonary artery, and the bronchial circulation does not take over until the pulmonary arterial pressure in the area of the embolism drops to zero.
Using CT data, Kirchner et al5 confirmed that the risk of pulmonary infarction is higher if the obstruction is peripheral, ie, distal.
Using autopsy data, Tsao et al18 reported a higher risk of pulmonary infarction in embolic occlusion of pulmonary vessels less than 3 mm in diameter.
Collateral circulation has been shown to protect against pulmonary infarction. For example, Miniati et al14 showed that healthy young patients with pulmonary embolism were more prone to develop pulmonary infarction, probably because they had less efficient collateral systems in the peripheral lung fields. In lung transplant recipients, it has been shown that the risk of infarction decreased with development of collateral circulation.19
Dalen et al,15 however, attributed delayed resolution of pulmonary hemorrhage (as measured by resolution of infiltrate on chest radiography) to higher underlying pulmonary venous pressure in patients with heart failure and consequent pulmonary infarction. In comparison, healthy patients without cardiac or pulmonary disease have faster resolution of pulmonary hemorrhage when present, and less likelihood of pulmonary infarction (and death in submassive pulmonary embolism).
Data on the management of infected pulmonary infarction are limited. Mortality rates have been as high as 41% with noninfected and 73% with infected cavitary infarctions.4 Some authors have advocated early surgical resection in view of high rates of failure of medical treatment due to lack of blood supply within the cavity and continued risk of infection.
KEY POINTS
In patients with a recently diagnosed pulmonary embolism and concurrent symptoms of bacterial pneumonia, a diagnosis of cavitary pulmonary infarction should be considered.
Consolidations that are pleural-based with sharp, rounded margins and with focal areas of central hyperlucencies representing hemorrhage on the mediastinal windows on CT are more likely to represent a pulmonary infarct.20
- Light RW. Pleural Diseases. 4th ed. Baltimore, MD: Lippincott, Williams & Wilkins; 2001.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100(3):598–603. pmid:1909617
- Light RW. Pleural effusion due to pulmonary emboli. Curr Opin Pulm Med 2001; 7(4):198–201. pmid:11470974
- Libby LS, King TE, LaForce FM, Schwarz MI. Pulmonary cavitation following pulmonary infarction. Medicine (Baltimore) 1985; 64(5):342–348. pmid:4033411
- Kirchner J, Obermann A, Stuckradt S, et al. Lung infarction following pulmonary embolism: a comparative study on clinical conditions and CT findings to identify predisposing factors. Rofo 2015; 187(6):440–444. doi:10.1055/s-0034-1399006
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging 2006; 21(1):1–7. doi:10.1097/01.rti.0000187433.06762.fb
- Scharf J, Nahir AM, Munk J, Lichtig C. Aseptic cavitation in pulmonary infarction. Chest 1971; 59(4):456–458. pmid:5551596
- Wilson AG, Joseph AE, Butland RJ. The radiology of aseptic cavitation in pulmonary infarction. Clin Radiol 1986; 37(4):327–333. pmid:3731699
- Butler MD, Biscardi FH, Schain DC, Humphries JE, Blow O, Spotnitz WD. Pulmonary resection for treatment of cavitary pulmonary infarction. Ann Thorac Surg 1997; 63(3):849–850. pmid:9066420
- Koroscil MT, Hauser TR. Acute pulmonary embolism leading to cavitation and large pulmonary abscess: a rare complication of pulmonary infarction. Respir Med Case Rep 2016; 20:72–74. doi:10.1016/j.rmcr.2016.12.001
- Levin L, Kernohan JW, Moersch HJ. Pulmonary abscess secondary to bland pulmonary infarction. Dis Chest 1948; 14(2):218–232. pmid:18904835
- Marchiori E, Menna Barreto M, Pereira Freitas HM, et al. Morphological characteristics of the reversed halo sign that may strongly suggest pulmonary infarction. Clin Radiol 2018; 73(5):503.e7–503.e13. doi:10.1016/j.crad.2017.11.022
- Smith GT, Dexter L, Dammin GJ. Postmortem quantitative studies in pulmonary embolism. In: Sasahara AA, Stein M, eds. Pulmonary Embolic Disease. New York, NY: Grune & Stratton, Inc; 1965:120–126.
- Miniati M, Bottai M, Ciccotosto C, Roberto L, Monti S. Predictors of pulmonary infarction. Medicine (Baltimore) 2015; 94(41):e1488. doi:10.1097/MD.0000000000001488
- Dalen JE, Haffajee CI, Alpert JS, Howe JP, Ockene IS, Paraskos JA. Pulmonary embolism, pulmonary hemorrhage and pulmonary infarction. N Engl J Med 1977; 296(25):1431–1435. doi:10.1056/NEJM197706232962503
- Parambil JG, Savci CD, Tazelaar HD, Ryu JH. Causes and presenting features of pulmonary infarctions in 43 cases identified by surgical lung biopsy. Chest 2005; 127(4):1178–1183. doi:10.1378/chest.127.4.1178
- Karsner HT, Ghoreyeb AA. Studies in infarction: III. The circulation in experimental pulmonary embolism. J Exp Med 1913; 18(5):507–511. pmid:19867725
- Tsao MS, Schraufnagel D, Wang NS. Pathogenesis of pulmonary infarction. Am J Med 1982; 72(4):599–606. pmid:6462058
- Burns KE, Iacono AT. Incidence of clinically unsuspected pulmonary embolism in mechanically ventilated lung transplant recipients. Transplantation 2003; 76(6):964–968. doi:10.1097/01.TP.0000084523.58610.BA
- Yousem SA. The surgical pathology of pulmonary infarcts: diagnostic confusion with granulomatous disease, vasculitis, and neoplasia. Mod Pathol 2009; 22(5):679–685. doi:10.1038/modpathol.2009.20
- Light RW. Pleural Diseases. 4th ed. Baltimore, MD: Lippincott, Williams & Wilkins; 2001.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100(3):598–603. pmid:1909617
- Light RW. Pleural effusion due to pulmonary emboli. Curr Opin Pulm Med 2001; 7(4):198–201. pmid:11470974
- Libby LS, King TE, LaForce FM, Schwarz MI. Pulmonary cavitation following pulmonary infarction. Medicine (Baltimore) 1985; 64(5):342–348. pmid:4033411
- Kirchner J, Obermann A, Stuckradt S, et al. Lung infarction following pulmonary embolism: a comparative study on clinical conditions and CT findings to identify predisposing factors. Rofo 2015; 187(6):440–444. doi:10.1055/s-0034-1399006
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging 2006; 21(1):1–7. doi:10.1097/01.rti.0000187433.06762.fb
- Scharf J, Nahir AM, Munk J, Lichtig C. Aseptic cavitation in pulmonary infarction. Chest 1971; 59(4):456–458. pmid:5551596
- Wilson AG, Joseph AE, Butland RJ. The radiology of aseptic cavitation in pulmonary infarction. Clin Radiol 1986; 37(4):327–333. pmid:3731699
- Butler MD, Biscardi FH, Schain DC, Humphries JE, Blow O, Spotnitz WD. Pulmonary resection for treatment of cavitary pulmonary infarction. Ann Thorac Surg 1997; 63(3):849–850. pmid:9066420
- Koroscil MT, Hauser TR. Acute pulmonary embolism leading to cavitation and large pulmonary abscess: a rare complication of pulmonary infarction. Respir Med Case Rep 2016; 20:72–74. doi:10.1016/j.rmcr.2016.12.001
- Levin L, Kernohan JW, Moersch HJ. Pulmonary abscess secondary to bland pulmonary infarction. Dis Chest 1948; 14(2):218–232. pmid:18904835
- Marchiori E, Menna Barreto M, Pereira Freitas HM, et al. Morphological characteristics of the reversed halo sign that may strongly suggest pulmonary infarction. Clin Radiol 2018; 73(5):503.e7–503.e13. doi:10.1016/j.crad.2017.11.022
- Smith GT, Dexter L, Dammin GJ. Postmortem quantitative studies in pulmonary embolism. In: Sasahara AA, Stein M, eds. Pulmonary Embolic Disease. New York, NY: Grune & Stratton, Inc; 1965:120–126.
- Miniati M, Bottai M, Ciccotosto C, Roberto L, Monti S. Predictors of pulmonary infarction. Medicine (Baltimore) 2015; 94(41):e1488. doi:10.1097/MD.0000000000001488
- Dalen JE, Haffajee CI, Alpert JS, Howe JP, Ockene IS, Paraskos JA. Pulmonary embolism, pulmonary hemorrhage and pulmonary infarction. N Engl J Med 1977; 296(25):1431–1435. doi:10.1056/NEJM197706232962503
- Parambil JG, Savci CD, Tazelaar HD, Ryu JH. Causes and presenting features of pulmonary infarctions in 43 cases identified by surgical lung biopsy. Chest 2005; 127(4):1178–1183. doi:10.1378/chest.127.4.1178
- Karsner HT, Ghoreyeb AA. Studies in infarction: III. The circulation in experimental pulmonary embolism. J Exp Med 1913; 18(5):507–511. pmid:19867725
- Tsao MS, Schraufnagel D, Wang NS. Pathogenesis of pulmonary infarction. Am J Med 1982; 72(4):599–606. pmid:6462058
- Burns KE, Iacono AT. Incidence of clinically unsuspected pulmonary embolism in mechanically ventilated lung transplant recipients. Transplantation 2003; 76(6):964–968. doi:10.1097/01.TP.0000084523.58610.BA
- Yousem SA. The surgical pathology of pulmonary infarcts: diagnostic confusion with granulomatous disease, vasculitis, and neoplasia. Mod Pathol 2009; 22(5):679–685. doi:10.1038/modpathol.2009.20
How acute pain leads to chronic opioid use
Mary, age 38, was hospitalized for acute cholecystitis requiring laparoscopic surgery. Her hospital course was uneventful. At the time of discharge, I, her inpatient doctor, prescribed 15 hydrocodone tablets for postoperative pain. I never saw her again. Did she struggle to stop taking the hydrocodone I prescribed?
Heather is a 50-year-old patient in my addiction medicine clinic who developed opioid use disorder while being treated for chronic pain. After much hardship and to her credit, she is now in long-term remission. Did her opioid use disorder start with an opioid prescription for an accepted indication?
The issues Mary and Heather face seem unrelated, but these 2 patients may be at different time points in the progression of the same disease. As a hospitalist, I want to optimize the chances that patients taking opioids for acute pain will be able to stop taking them.
CHRONIC USE VS OPIOID USE DISORDER
There is a distinction between chronic use of opioids and opioid use disorder. The latter is also known as addiction.
Patients who take opioids daily do not necessarily have opioid use disorder, even if they have physiologic dependence on them. Physiologic opioid dependence is commonly confused with opioid use disorder, but it is the expected result of regularly taking these drugs.
Opioid use disorder is a chronic disease of the brain characterized by loss of control over opioid use, resulting in harm. The Diagnostic and Statistical Manual, fifth edition, excludes physiologic dependence on opioids (tolerance and withdrawal) from its criteria for opioid use disorder if the patient is taking opioids solely under medical supervision.1 To be diagnosed with opioid use disorder, patients need to do only 2 of the following within 12 months:
- Take more of the drug than intended
- Want or try to cut down without success
- Spend a lot of time in getting, using, or recovering from the drug
- Crave the drug
- Fail to meet commitments due to the drug
- Continue to use the drug, even though it causes social or relationship problems
- Give up or reduce other activities because of the drug
- Use the drug even when it isn’t safe
- Continue to use even when it causes physical or psychological problems
- Develop tolerance (but, as noted, not if taking the drug as directed under a doctor’s supervision)
- Experience withdrawal (again, but not if taking the drug under medical supervision).
WHY DO SOME PATIENTS STRUGGLE TO STOP TAKING OPIOIDS?
Studying opioid use disorder as an outcome in large groups of patients is complicated by imperfect medical documentation. However, using pharmacy claims data, researchers can accurately describe opioid prescription patterns in large groups of patients over time. This means we can count how many patients keep taking prescribed opioids but not how many become addicted.
In a country where nearly 40% of adults are prescribed an opioid annually, the question is not why people start taking opioids, but why some have to struggle to stop.2 Several recent studies used pharmacy claims data to identify factors that may predict chronic opioid use in patients prescribed opioids for acute pain. The findings suggest that we can better treat acute pain to prevent chronic opioid use.
We don’t yet know how to protect patients like Mary from opioid use disorder, but the following 3 studies have already changed my practice.
HIGHER TOTAL DOSE MEANS HIGHER RISK
[Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use—United States, 2006–2015. MMWR Morb Mortal Wkly Rep 2017; 66(10):265–269.]
Shah et al3 reported a study of nearly 1.3 million opioid-naive patients who received opioid prescriptions. Of those prescribed at least 1 day of opioids, 6% were still taking them 1 year later, and 2.9% were still taking them 3 years later.
Opioid exposure in acute pain was measured in total “morphine milligram equivalents” (MME), ie, the cumulative amount of opioids prescribed in the treatment episode, standardized across different types of opioids. We usually think of exposure in terms of how many milligrams a patient takes per day, which correlates with mortality in chronic opioid use.4 But this study showed a linear relationship between total MME prescribed for acute pain and ongoing opioid use in opioid-naive patients. By itself, the difference between daily and total MME made the article revelatory.
But the study went further, asking how much is too much: ie, What is the cutoff MME above which the patient is at risk of chronic opioid use? The relationship between acute opioid dose and chronic use is linear and starts early. Shah et al suggested that a total threshold of 700 MME predicts chronic opioid use—140 hydrocodone tablets, or 1 month of regular use.3
Many doctors worry that specific opioids such as oxycodone, hydromorphone, and fentanyl may be more habit-forming. Surprisingly, this study showed that these drugs were associated with rates of chronic use similar to those of other opioids when they controlled for potency.
Bottom line. Total opioid use in acute pain was the best predictor of chronic opioid use, and it showed that chronicity begins earlier than thought.
DON’T BE A ‘HIGH-INTENSITY’ PRESCRIBER
[Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med 2017; 376(7):663–673.]
Barnett et al5 analyzed opioid prescribing for acute pain in the emergency department, using Medicare pharmacy data from 377,629 previously opioid-naive patients. They categorized the emergency providers into quartiles based on the frequency of opioid prescribing.
The relative risk of ongoing opioid use 1 year after being treated by a “high-intensity” prescriber (ie, one in the top quartile) was 30% greater than in similar patients seen by a low-intensity prescriber (ie, one in the bottom quartile). In addition, those who were treated by high-intensity prescribers were more likely to have a serious fall.
In designing the study, the authors assumed that patients visiting an emergency department had their doctor assigned randomly. They controlled for many patient variables that might have confounded the results, such as age, sex, race, depression, medical comorbidities, and geographic region. Were the higher rates of ongoing opioid use in the high-intensity-prescriber group due to the higher prescribing rates of their emergency providers, or did the providers counsel patients differently? This is not known.
Bottom line. Different doctors manage similar patients differently when it comes to pain, and those who prescribe more opioids for acute pain put their patients at risk of chronic opioid use and falls. I don’t want to be a high-intensity opioid prescriber.
SURGERY AND CHRONIC OPIOID USE
[Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg 2017; 152(6):e170504.]
Brummett et al6 examined ongoing opioid use after surgery in 36,177 opioid-naive patients and in a nonsurgical control group. After 3 months, 6% of the patients who underwent surgery remained on opioids, compared with only 0.4% of the nonsurgical controls. Whether the surgery was major or minor did not affect the rate of postoperative opioid use.
Risk factors for ongoing opioid use were preexisting addiction to anything (including tobacco), mood disorders, and preoperative pain disorders. These risk factors have previously been reported in nonsurgical patients.7
Brummett et al speculated that patients are counseled about postoperative opioids in a way that leads them to overestimate the safety and efficacy of these drugs for treating other common pain conditions.6
Bottom line. Patients with mental health comorbidities have a hard time stopping opioids. The remarkable finding in this study was the similarity between major and minor surgery in terms of chronic opioid use. If postoperative opioids treat only the pain caused by the surgery, major surgery should be associated with greater opioid use. The similarity suggests that a mechanism other than postoperative pain confers risk of chronic opioid use.
THINKING ABOUT OPIOIDS
Collectively, these articles describe elements of acute pain treatment that correlate with chronic ongoing opioid use: a higher cumulative dose,3 being seen by a physician who prescribes a lot of opioids,5 undergoing surgery,6 and psychiatric comorbidity.6 They made me wonder if opioid use for acute pain acts as an inoculation, analogous to inoculating a Petri dish with bacteria. The likelihood of chronic opioid use arises from the inoculum dose, the host response, and the context of inoculation.
These articles do not show how patients taking opioids chronically for pain become addicted. Stumbo et al8 interviewed 283 opioid-dependent patients and identified 5 pathways to opioid use disorder, 3 of which were related to pain control: inadequately controlled chronic pain, exposure to opioids during acute pain episodes, and chronic pain in patients who already had substance use disorders. Brat et al9 recently estimated the risk of opioid use disorder after receiving opioids postoperatively to be less than 1%, but it increased dramatically with duration of opioid treatment.
A patient who fills an opioid prescription does not necessarily have chronic pain. Nor do all patients with chronic pain require an opioid prescription. These studies did not establish whether the patients had a pain syndrome. In practice, we call our patients who chronically take opioids our “chronic pain patients.” But 40% of Americans have chronic pain, while only 5% take opioids daily for pain.11,12
We assume that those taking opioids have the most severe pain. But Brummett et al suggested that continued opioid use is predicted less by pain and more by psychiatric comorbidity.6 More than half of the opioid prescriptions in the United States are written for patients with serious mental illness, who represent one-sixth of that population.11 Maybe chronic opioid use for pain has more to do with vulnerability to opioids and less to do with a pain syndrome.
I now think about daily opioid use in much the same way as I think about daily prednisone use. Patients on daily prednisone have a characteristic set of medical risks from the prednisone itself, regardless of its indication. Yet we do not consider these patients addicted to prednisone. Opioid use may be similar.
Like most doctors, I am troubled by the continued rise in the opioid overdose rate.13 Yet addiction and death from overdose are not the only risks that patients on chronic opioids face; they also have higher rates of falls, cardiovascular death, pneumonia, death from chronic obstructive pulmonary disease, and motor vehicle crashes.14–17 Patients on chronic opioids for pain have greater mental health comorbidity and worse function.18
Most concerning, chronic opioid treatment for pain lacks proof of benefit. In fact, a recent study disproved the benefit of opioids for chronic pain compared with nonopioid options.19 When I meet with patients who are taking chronic opioids for pain, I often can’t identify why the drugs were started or ought to be continued, and I anticipate a bad outcome. Yet the patient is afraid to stop the drug. For these reasons, chronic opioid use for pain strikes me as worth considering separately from opioid use disorder.
HOW THIS CHANGED MY PRACTICE
The studies described above have had a powerful effect on my clinical care as a hospitalist.
I now talk to all patients starting opioids about how hard it can be to stop. Some patients are defensive at first, believing this does not apply to them. But I politely continue.
People with depression and anxiety can have a harder time stopping opioids. Addiction is both a risk with ongoing opioid use and a possible outcome of acute opioid use.8 But one can struggle to stop opioids without being addicted or depressed. Even the healthiest person may wish to continue opioids past the point of benefit.
I am careful not to invalidate the patient’s experience of pain. It is challenging for patients to find the balance between current discomfort and a possible future adverse effect. In these conversations, I imagine how I would want a loved one counseled on their pain control. This centers me as I choose my words and my tone.
I now monitor the total amount of opioid I prescribe for acute pain in addition to the daily dose. I give my patients as few opioids as reasonable, and advise them to take the minimum dose required for tolerable comfort. I offer nonopioid options as the preferred choice, presenting them as effective and safe. I do this irrespective of the indication for opioids.
I limit opioids in all patients, not just those with comorbidities. I include in my shared decision-making process the risk of chronic opioid use when I prescribe opioids for acute pain, carefully distinguishing it from opioid use disorder. Instead of excess opioids, I give patients my office phone number to call in case they struggle. I rarely get calls. But I find patients would rather have access to a doctor than extra pills. And offering them my contact information lets me limit opioids while letting them know that I am committed to their comfort and health.
As an addiction medicine doctor, I consult on patients not taking their opioids as prescribed. Caring for these patients is intellectually and emotionally draining; they suffer daily, and the opioids they take provide a modicum of relief at a high cost. The publications I have discussed here provide insight into how a troubled relationship with opioids begins. I remind myself that these patients have an iatrogenic condition. Their behaviors that we label “aberrant” may reflect an adverse reaction to medications prescribed to them for acute pain.
Mary, my patient with postoperative pain after cholecystectomy, may over time develop opioid use disorder as Heather did. That progression may have begun with the hydrocodone I prescribed and the counseling I gave her, and it may proceed to chronic opioid use and then opioid use disorder.
I am looking closely at the care I give for acute pain in light of these innovative studies. But even more so, they have increased the compassion with which I care for patients like Heather, those harmed by prescribed opioids.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association Publishing; 2013:541–546.
- Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription opioid use, misuse, and use disorders in US adults: 2015 national survey on drug use and health. Ann Intern Med 2017; 167(5):293–301. doi:10.7326/M17-0865
- Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use—United States, 2006–2015. MMWR Morb Mortal Wkly Rep 2017; 66(10):265–269. doi:10.15585/mmwr.mm6610a1
- Dasgupta N, Funk MJ, Proescholdbell S, Hirsch A, Ribisl KM, Marshall S. Cohort study of the impact of high-dose opioid analgesics on overdose mortality. Pain Med 2016; 17(1):85–98. doi:10.1111/pme.12907
- Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med 2017; 376(7):663–673. doi:10.1056/NEJMsa1610524
- Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg 2017; 152(6):e170504. doi:10.1001/jamasurg.2017.0504
- Volkow ND, McLellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med 2016; 374(13):1253–1263. doi:10.1056/NEJMra1507771
- Stumbo SP, Yarborough BJ, McCarty D, Weisner C, Green CA. Patient-reported pathways to opioid use disorders and pain-related barriers to treatment engagement. J Subst Abuse Treat 2017; 73:47–54. doi:10.1016/j.jsat.2016.11.003
- Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ 2018; 360:j5790. doi:10.1136/bmj.j5790
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain 2015; 156(4):569–576. doi:10.1097/01.j.pain.0000460357.01998.f1
- Davis MA, Lin LA, Liu H, Sites BD. Prescription opioid use among adults with mental health disorders in the United States. J Am Board Fam Med 2017; 30(4):407–417. doi:10.3122/jabfm.2017.04.170112
- Tsang A, Von Korff M, Lee S, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain 2008; 9(10):883–891. doi:10.1016/j.jpain.2008.05.005
- QuickStats: age-adjusted death rates for drug overdose, by race/ethnicity—national vital statistics system, United States, 2015–2016. MMWR Morb Mortal Wkly Rep 2018; 67(12):374. doi:10.15585/mmwr.mm6712a9
- Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med 2010; 170(22):1968–1976. doi:10.1001/archinternmed.2010.391
- Vozoris NT, Wang X, Fischer HD, et al. Incident opioid drug use and adverse respiratory outcomes among older adults with COPD. Eur Respir J 2016; 48(3):683–693. doi:10.1183/13993003.01967-2015
- Wiese AD, Griffin MR, Schaffner W, et al. Opioid analgesic use and risk for invasive pneumococcal diseases: a nested case-control study. Ann Intern Med 2018; 168(6):396–404. doi:10.7326/M17-1907
- Chihuri S, Li G. Use of prescription opioids and motor vehicle crashes: a meta analysis. Accid Anal Prev 2017; 109:123–131. doi:10.1016/j.aap.2017.10.004
- Morasco BJ, Yarborough BJ, Smith NX, et al. Higher prescription opioid dose is associated with worse patient-reported pain outcomes and more health care utilization. J Pain 2017; 18(4):437–445. doi:10.1016/j.jpain.2016.12.004
- Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA 2018; 319(9):872–882. doi:10.1001/jama.2018.0899
Mary, age 38, was hospitalized for acute cholecystitis requiring laparoscopic surgery. Her hospital course was uneventful. At the time of discharge, I, her inpatient doctor, prescribed 15 hydrocodone tablets for postoperative pain. I never saw her again. Did she struggle to stop taking the hydrocodone I prescribed?
Heather is a 50-year-old patient in my addiction medicine clinic who developed opioid use disorder while being treated for chronic pain. After much hardship and to her credit, she is now in long-term remission. Did her opioid use disorder start with an opioid prescription for an accepted indication?
The issues Mary and Heather face seem unrelated, but these 2 patients may be at different time points in the progression of the same disease. As a hospitalist, I want to optimize the chances that patients taking opioids for acute pain will be able to stop taking them.
CHRONIC USE VS OPIOID USE DISORDER
There is a distinction between chronic use of opioids and opioid use disorder. The latter is also known as addiction.
Patients who take opioids daily do not necessarily have opioid use disorder, even if they have physiologic dependence on them. Physiologic opioid dependence is commonly confused with opioid use disorder, but it is the expected result of regularly taking these drugs.
Opioid use disorder is a chronic disease of the brain characterized by loss of control over opioid use, resulting in harm. The Diagnostic and Statistical Manual, fifth edition, excludes physiologic dependence on opioids (tolerance and withdrawal) from its criteria for opioid use disorder if the patient is taking opioids solely under medical supervision.1 To be diagnosed with opioid use disorder, patients need to do only 2 of the following within 12 months:
- Take more of the drug than intended
- Want or try to cut down without success
- Spend a lot of time in getting, using, or recovering from the drug
- Crave the drug
- Fail to meet commitments due to the drug
- Continue to use the drug, even though it causes social or relationship problems
- Give up or reduce other activities because of the drug
- Use the drug even when it isn’t safe
- Continue to use even when it causes physical or psychological problems
- Develop tolerance (but, as noted, not if taking the drug as directed under a doctor’s supervision)
- Experience withdrawal (again, but not if taking the drug under medical supervision).
WHY DO SOME PATIENTS STRUGGLE TO STOP TAKING OPIOIDS?
Studying opioid use disorder as an outcome in large groups of patients is complicated by imperfect medical documentation. However, using pharmacy claims data, researchers can accurately describe opioid prescription patterns in large groups of patients over time. This means we can count how many patients keep taking prescribed opioids but not how many become addicted.
In a country where nearly 40% of adults are prescribed an opioid annually, the question is not why people start taking opioids, but why some have to struggle to stop.2 Several recent studies used pharmacy claims data to identify factors that may predict chronic opioid use in patients prescribed opioids for acute pain. The findings suggest that we can better treat acute pain to prevent chronic opioid use.
We don’t yet know how to protect patients like Mary from opioid use disorder, but the following 3 studies have already changed my practice.
HIGHER TOTAL DOSE MEANS HIGHER RISK
[Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use—United States, 2006–2015. MMWR Morb Mortal Wkly Rep 2017; 66(10):265–269.]
Shah et al3 reported a study of nearly 1.3 million opioid-naive patients who received opioid prescriptions. Of those prescribed at least 1 day of opioids, 6% were still taking them 1 year later, and 2.9% were still taking them 3 years later.
Opioid exposure in acute pain was measured in total “morphine milligram equivalents” (MME), ie, the cumulative amount of opioids prescribed in the treatment episode, standardized across different types of opioids. We usually think of exposure in terms of how many milligrams a patient takes per day, which correlates with mortality in chronic opioid use.4 But this study showed a linear relationship between total MME prescribed for acute pain and ongoing opioid use in opioid-naive patients. By itself, the difference between daily and total MME made the article revelatory.
But the study went further, asking how much is too much: ie, What is the cutoff MME above which the patient is at risk of chronic opioid use? The relationship between acute opioid dose and chronic use is linear and starts early. Shah et al suggested that a total threshold of 700 MME predicts chronic opioid use—140 hydrocodone tablets, or 1 month of regular use.3
Many doctors worry that specific opioids such as oxycodone, hydromorphone, and fentanyl may be more habit-forming. Surprisingly, this study showed that these drugs were associated with rates of chronic use similar to those of other opioids when they controlled for potency.
Bottom line. Total opioid use in acute pain was the best predictor of chronic opioid use, and it showed that chronicity begins earlier than thought.
DON’T BE A ‘HIGH-INTENSITY’ PRESCRIBER
[Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med 2017; 376(7):663–673.]
Barnett et al5 analyzed opioid prescribing for acute pain in the emergency department, using Medicare pharmacy data from 377,629 previously opioid-naive patients. They categorized the emergency providers into quartiles based on the frequency of opioid prescribing.
The relative risk of ongoing opioid use 1 year after being treated by a “high-intensity” prescriber (ie, one in the top quartile) was 30% greater than in similar patients seen by a low-intensity prescriber (ie, one in the bottom quartile). In addition, those who were treated by high-intensity prescribers were more likely to have a serious fall.
In designing the study, the authors assumed that patients visiting an emergency department had their doctor assigned randomly. They controlled for many patient variables that might have confounded the results, such as age, sex, race, depression, medical comorbidities, and geographic region. Were the higher rates of ongoing opioid use in the high-intensity-prescriber group due to the higher prescribing rates of their emergency providers, or did the providers counsel patients differently? This is not known.
Bottom line. Different doctors manage similar patients differently when it comes to pain, and those who prescribe more opioids for acute pain put their patients at risk of chronic opioid use and falls. I don’t want to be a high-intensity opioid prescriber.
SURGERY AND CHRONIC OPIOID USE
[Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg 2017; 152(6):e170504.]
Brummett et al6 examined ongoing opioid use after surgery in 36,177 opioid-naive patients and in a nonsurgical control group. After 3 months, 6% of the patients who underwent surgery remained on opioids, compared with only 0.4% of the nonsurgical controls. Whether the surgery was major or minor did not affect the rate of postoperative opioid use.
Risk factors for ongoing opioid use were preexisting addiction to anything (including tobacco), mood disorders, and preoperative pain disorders. These risk factors have previously been reported in nonsurgical patients.7
Brummett et al speculated that patients are counseled about postoperative opioids in a way that leads them to overestimate the safety and efficacy of these drugs for treating other common pain conditions.6
Bottom line. Patients with mental health comorbidities have a hard time stopping opioids. The remarkable finding in this study was the similarity between major and minor surgery in terms of chronic opioid use. If postoperative opioids treat only the pain caused by the surgery, major surgery should be associated with greater opioid use. The similarity suggests that a mechanism other than postoperative pain confers risk of chronic opioid use.
THINKING ABOUT OPIOIDS
Collectively, these articles describe elements of acute pain treatment that correlate with chronic ongoing opioid use: a higher cumulative dose,3 being seen by a physician who prescribes a lot of opioids,5 undergoing surgery,6 and psychiatric comorbidity.6 They made me wonder if opioid use for acute pain acts as an inoculation, analogous to inoculating a Petri dish with bacteria. The likelihood of chronic opioid use arises from the inoculum dose, the host response, and the context of inoculation.
These articles do not show how patients taking opioids chronically for pain become addicted. Stumbo et al8 interviewed 283 opioid-dependent patients and identified 5 pathways to opioid use disorder, 3 of which were related to pain control: inadequately controlled chronic pain, exposure to opioids during acute pain episodes, and chronic pain in patients who already had substance use disorders. Brat et al9 recently estimated the risk of opioid use disorder after receiving opioids postoperatively to be less than 1%, but it increased dramatically with duration of opioid treatment.
A patient who fills an opioid prescription does not necessarily have chronic pain. Nor do all patients with chronic pain require an opioid prescription. These studies did not establish whether the patients had a pain syndrome. In practice, we call our patients who chronically take opioids our “chronic pain patients.” But 40% of Americans have chronic pain, while only 5% take opioids daily for pain.11,12
We assume that those taking opioids have the most severe pain. But Brummett et al suggested that continued opioid use is predicted less by pain and more by psychiatric comorbidity.6 More than half of the opioid prescriptions in the United States are written for patients with serious mental illness, who represent one-sixth of that population.11 Maybe chronic opioid use for pain has more to do with vulnerability to opioids and less to do with a pain syndrome.
I now think about daily opioid use in much the same way as I think about daily prednisone use. Patients on daily prednisone have a characteristic set of medical risks from the prednisone itself, regardless of its indication. Yet we do not consider these patients addicted to prednisone. Opioid use may be similar.
Like most doctors, I am troubled by the continued rise in the opioid overdose rate.13 Yet addiction and death from overdose are not the only risks that patients on chronic opioids face; they also have higher rates of falls, cardiovascular death, pneumonia, death from chronic obstructive pulmonary disease, and motor vehicle crashes.14–17 Patients on chronic opioids for pain have greater mental health comorbidity and worse function.18
Most concerning, chronic opioid treatment for pain lacks proof of benefit. In fact, a recent study disproved the benefit of opioids for chronic pain compared with nonopioid options.19 When I meet with patients who are taking chronic opioids for pain, I often can’t identify why the drugs were started or ought to be continued, and I anticipate a bad outcome. Yet the patient is afraid to stop the drug. For these reasons, chronic opioid use for pain strikes me as worth considering separately from opioid use disorder.
HOW THIS CHANGED MY PRACTICE
The studies described above have had a powerful effect on my clinical care as a hospitalist.
I now talk to all patients starting opioids about how hard it can be to stop. Some patients are defensive at first, believing this does not apply to them. But I politely continue.
People with depression and anxiety can have a harder time stopping opioids. Addiction is both a risk with ongoing opioid use and a possible outcome of acute opioid use.8 But one can struggle to stop opioids without being addicted or depressed. Even the healthiest person may wish to continue opioids past the point of benefit.
I am careful not to invalidate the patient’s experience of pain. It is challenging for patients to find the balance between current discomfort and a possible future adverse effect. In these conversations, I imagine how I would want a loved one counseled on their pain control. This centers me as I choose my words and my tone.
I now monitor the total amount of opioid I prescribe for acute pain in addition to the daily dose. I give my patients as few opioids as reasonable, and advise them to take the minimum dose required for tolerable comfort. I offer nonopioid options as the preferred choice, presenting them as effective and safe. I do this irrespective of the indication for opioids.
I limit opioids in all patients, not just those with comorbidities. I include in my shared decision-making process the risk of chronic opioid use when I prescribe opioids for acute pain, carefully distinguishing it from opioid use disorder. Instead of excess opioids, I give patients my office phone number to call in case they struggle. I rarely get calls. But I find patients would rather have access to a doctor than extra pills. And offering them my contact information lets me limit opioids while letting them know that I am committed to their comfort and health.
As an addiction medicine doctor, I consult on patients not taking their opioids as prescribed. Caring for these patients is intellectually and emotionally draining; they suffer daily, and the opioids they take provide a modicum of relief at a high cost. The publications I have discussed here provide insight into how a troubled relationship with opioids begins. I remind myself that these patients have an iatrogenic condition. Their behaviors that we label “aberrant” may reflect an adverse reaction to medications prescribed to them for acute pain.
Mary, my patient with postoperative pain after cholecystectomy, may over time develop opioid use disorder as Heather did. That progression may have begun with the hydrocodone I prescribed and the counseling I gave her, and it may proceed to chronic opioid use and then opioid use disorder.
I am looking closely at the care I give for acute pain in light of these innovative studies. But even more so, they have increased the compassion with which I care for patients like Heather, those harmed by prescribed opioids.
Mary, age 38, was hospitalized for acute cholecystitis requiring laparoscopic surgery. Her hospital course was uneventful. At the time of discharge, I, her inpatient doctor, prescribed 15 hydrocodone tablets for postoperative pain. I never saw her again. Did she struggle to stop taking the hydrocodone I prescribed?
Heather is a 50-year-old patient in my addiction medicine clinic who developed opioid use disorder while being treated for chronic pain. After much hardship and to her credit, she is now in long-term remission. Did her opioid use disorder start with an opioid prescription for an accepted indication?
The issues Mary and Heather face seem unrelated, but these 2 patients may be at different time points in the progression of the same disease. As a hospitalist, I want to optimize the chances that patients taking opioids for acute pain will be able to stop taking them.
CHRONIC USE VS OPIOID USE DISORDER
There is a distinction between chronic use of opioids and opioid use disorder. The latter is also known as addiction.
Patients who take opioids daily do not necessarily have opioid use disorder, even if they have physiologic dependence on them. Physiologic opioid dependence is commonly confused with opioid use disorder, but it is the expected result of regularly taking these drugs.
Opioid use disorder is a chronic disease of the brain characterized by loss of control over opioid use, resulting in harm. The Diagnostic and Statistical Manual, fifth edition, excludes physiologic dependence on opioids (tolerance and withdrawal) from its criteria for opioid use disorder if the patient is taking opioids solely under medical supervision.1 To be diagnosed with opioid use disorder, patients need to do only 2 of the following within 12 months:
- Take more of the drug than intended
- Want or try to cut down without success
- Spend a lot of time in getting, using, or recovering from the drug
- Crave the drug
- Fail to meet commitments due to the drug
- Continue to use the drug, even though it causes social or relationship problems
- Give up or reduce other activities because of the drug
- Use the drug even when it isn’t safe
- Continue to use even when it causes physical or psychological problems
- Develop tolerance (but, as noted, not if taking the drug as directed under a doctor’s supervision)
- Experience withdrawal (again, but not if taking the drug under medical supervision).
WHY DO SOME PATIENTS STRUGGLE TO STOP TAKING OPIOIDS?
Studying opioid use disorder as an outcome in large groups of patients is complicated by imperfect medical documentation. However, using pharmacy claims data, researchers can accurately describe opioid prescription patterns in large groups of patients over time. This means we can count how many patients keep taking prescribed opioids but not how many become addicted.
In a country where nearly 40% of adults are prescribed an opioid annually, the question is not why people start taking opioids, but why some have to struggle to stop.2 Several recent studies used pharmacy claims data to identify factors that may predict chronic opioid use in patients prescribed opioids for acute pain. The findings suggest that we can better treat acute pain to prevent chronic opioid use.
We don’t yet know how to protect patients like Mary from opioid use disorder, but the following 3 studies have already changed my practice.
HIGHER TOTAL DOSE MEANS HIGHER RISK
[Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use—United States, 2006–2015. MMWR Morb Mortal Wkly Rep 2017; 66(10):265–269.]
Shah et al3 reported a study of nearly 1.3 million opioid-naive patients who received opioid prescriptions. Of those prescribed at least 1 day of opioids, 6% were still taking them 1 year later, and 2.9% were still taking them 3 years later.
Opioid exposure in acute pain was measured in total “morphine milligram equivalents” (MME), ie, the cumulative amount of opioids prescribed in the treatment episode, standardized across different types of opioids. We usually think of exposure in terms of how many milligrams a patient takes per day, which correlates with mortality in chronic opioid use.4 But this study showed a linear relationship between total MME prescribed for acute pain and ongoing opioid use in opioid-naive patients. By itself, the difference between daily and total MME made the article revelatory.
But the study went further, asking how much is too much: ie, What is the cutoff MME above which the patient is at risk of chronic opioid use? The relationship between acute opioid dose and chronic use is linear and starts early. Shah et al suggested that a total threshold of 700 MME predicts chronic opioid use—140 hydrocodone tablets, or 1 month of regular use.3
Many doctors worry that specific opioids such as oxycodone, hydromorphone, and fentanyl may be more habit-forming. Surprisingly, this study showed that these drugs were associated with rates of chronic use similar to those of other opioids when they controlled for potency.
Bottom line. Total opioid use in acute pain was the best predictor of chronic opioid use, and it showed that chronicity begins earlier than thought.
DON’T BE A ‘HIGH-INTENSITY’ PRESCRIBER
[Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med 2017; 376(7):663–673.]
Barnett et al5 analyzed opioid prescribing for acute pain in the emergency department, using Medicare pharmacy data from 377,629 previously opioid-naive patients. They categorized the emergency providers into quartiles based on the frequency of opioid prescribing.
The relative risk of ongoing opioid use 1 year after being treated by a “high-intensity” prescriber (ie, one in the top quartile) was 30% greater than in similar patients seen by a low-intensity prescriber (ie, one in the bottom quartile). In addition, those who were treated by high-intensity prescribers were more likely to have a serious fall.
In designing the study, the authors assumed that patients visiting an emergency department had their doctor assigned randomly. They controlled for many patient variables that might have confounded the results, such as age, sex, race, depression, medical comorbidities, and geographic region. Were the higher rates of ongoing opioid use in the high-intensity-prescriber group due to the higher prescribing rates of their emergency providers, or did the providers counsel patients differently? This is not known.
Bottom line. Different doctors manage similar patients differently when it comes to pain, and those who prescribe more opioids for acute pain put their patients at risk of chronic opioid use and falls. I don’t want to be a high-intensity opioid prescriber.
SURGERY AND CHRONIC OPIOID USE
[Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg 2017; 152(6):e170504.]
Brummett et al6 examined ongoing opioid use after surgery in 36,177 opioid-naive patients and in a nonsurgical control group. After 3 months, 6% of the patients who underwent surgery remained on opioids, compared with only 0.4% of the nonsurgical controls. Whether the surgery was major or minor did not affect the rate of postoperative opioid use.
Risk factors for ongoing opioid use were preexisting addiction to anything (including tobacco), mood disorders, and preoperative pain disorders. These risk factors have previously been reported in nonsurgical patients.7
Brummett et al speculated that patients are counseled about postoperative opioids in a way that leads them to overestimate the safety and efficacy of these drugs for treating other common pain conditions.6
Bottom line. Patients with mental health comorbidities have a hard time stopping opioids. The remarkable finding in this study was the similarity between major and minor surgery in terms of chronic opioid use. If postoperative opioids treat only the pain caused by the surgery, major surgery should be associated with greater opioid use. The similarity suggests that a mechanism other than postoperative pain confers risk of chronic opioid use.
THINKING ABOUT OPIOIDS
Collectively, these articles describe elements of acute pain treatment that correlate with chronic ongoing opioid use: a higher cumulative dose,3 being seen by a physician who prescribes a lot of opioids,5 undergoing surgery,6 and psychiatric comorbidity.6 They made me wonder if opioid use for acute pain acts as an inoculation, analogous to inoculating a Petri dish with bacteria. The likelihood of chronic opioid use arises from the inoculum dose, the host response, and the context of inoculation.
These articles do not show how patients taking opioids chronically for pain become addicted. Stumbo et al8 interviewed 283 opioid-dependent patients and identified 5 pathways to opioid use disorder, 3 of which were related to pain control: inadequately controlled chronic pain, exposure to opioids during acute pain episodes, and chronic pain in patients who already had substance use disorders. Brat et al9 recently estimated the risk of opioid use disorder after receiving opioids postoperatively to be less than 1%, but it increased dramatically with duration of opioid treatment.
A patient who fills an opioid prescription does not necessarily have chronic pain. Nor do all patients with chronic pain require an opioid prescription. These studies did not establish whether the patients had a pain syndrome. In practice, we call our patients who chronically take opioids our “chronic pain patients.” But 40% of Americans have chronic pain, while only 5% take opioids daily for pain.11,12
We assume that those taking opioids have the most severe pain. But Brummett et al suggested that continued opioid use is predicted less by pain and more by psychiatric comorbidity.6 More than half of the opioid prescriptions in the United States are written for patients with serious mental illness, who represent one-sixth of that population.11 Maybe chronic opioid use for pain has more to do with vulnerability to opioids and less to do with a pain syndrome.
I now think about daily opioid use in much the same way as I think about daily prednisone use. Patients on daily prednisone have a characteristic set of medical risks from the prednisone itself, regardless of its indication. Yet we do not consider these patients addicted to prednisone. Opioid use may be similar.
Like most doctors, I am troubled by the continued rise in the opioid overdose rate.13 Yet addiction and death from overdose are not the only risks that patients on chronic opioids face; they also have higher rates of falls, cardiovascular death, pneumonia, death from chronic obstructive pulmonary disease, and motor vehicle crashes.14–17 Patients on chronic opioids for pain have greater mental health comorbidity and worse function.18
Most concerning, chronic opioid treatment for pain lacks proof of benefit. In fact, a recent study disproved the benefit of opioids for chronic pain compared with nonopioid options.19 When I meet with patients who are taking chronic opioids for pain, I often can’t identify why the drugs were started or ought to be continued, and I anticipate a bad outcome. Yet the patient is afraid to stop the drug. For these reasons, chronic opioid use for pain strikes me as worth considering separately from opioid use disorder.
HOW THIS CHANGED MY PRACTICE
The studies described above have had a powerful effect on my clinical care as a hospitalist.
I now talk to all patients starting opioids about how hard it can be to stop. Some patients are defensive at first, believing this does not apply to them. But I politely continue.
People with depression and anxiety can have a harder time stopping opioids. Addiction is both a risk with ongoing opioid use and a possible outcome of acute opioid use.8 But one can struggle to stop opioids without being addicted or depressed. Even the healthiest person may wish to continue opioids past the point of benefit.
I am careful not to invalidate the patient’s experience of pain. It is challenging for patients to find the balance between current discomfort and a possible future adverse effect. In these conversations, I imagine how I would want a loved one counseled on their pain control. This centers me as I choose my words and my tone.
I now monitor the total amount of opioid I prescribe for acute pain in addition to the daily dose. I give my patients as few opioids as reasonable, and advise them to take the minimum dose required for tolerable comfort. I offer nonopioid options as the preferred choice, presenting them as effective and safe. I do this irrespective of the indication for opioids.
I limit opioids in all patients, not just those with comorbidities. I include in my shared decision-making process the risk of chronic opioid use when I prescribe opioids for acute pain, carefully distinguishing it from opioid use disorder. Instead of excess opioids, I give patients my office phone number to call in case they struggle. I rarely get calls. But I find patients would rather have access to a doctor than extra pills. And offering them my contact information lets me limit opioids while letting them know that I am committed to their comfort and health.
As an addiction medicine doctor, I consult on patients not taking their opioids as prescribed. Caring for these patients is intellectually and emotionally draining; they suffer daily, and the opioids they take provide a modicum of relief at a high cost. The publications I have discussed here provide insight into how a troubled relationship with opioids begins. I remind myself that these patients have an iatrogenic condition. Their behaviors that we label “aberrant” may reflect an adverse reaction to medications prescribed to them for acute pain.
Mary, my patient with postoperative pain after cholecystectomy, may over time develop opioid use disorder as Heather did. That progression may have begun with the hydrocodone I prescribed and the counseling I gave her, and it may proceed to chronic opioid use and then opioid use disorder.
I am looking closely at the care I give for acute pain in light of these innovative studies. But even more so, they have increased the compassion with which I care for patients like Heather, those harmed by prescribed opioids.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association Publishing; 2013:541–546.
- Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription opioid use, misuse, and use disorders in US adults: 2015 national survey on drug use and health. Ann Intern Med 2017; 167(5):293–301. doi:10.7326/M17-0865
- Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use—United States, 2006–2015. MMWR Morb Mortal Wkly Rep 2017; 66(10):265–269. doi:10.15585/mmwr.mm6610a1
- Dasgupta N, Funk MJ, Proescholdbell S, Hirsch A, Ribisl KM, Marshall S. Cohort study of the impact of high-dose opioid analgesics on overdose mortality. Pain Med 2016; 17(1):85–98. doi:10.1111/pme.12907
- Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med 2017; 376(7):663–673. doi:10.1056/NEJMsa1610524
- Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg 2017; 152(6):e170504. doi:10.1001/jamasurg.2017.0504
- Volkow ND, McLellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med 2016; 374(13):1253–1263. doi:10.1056/NEJMra1507771
- Stumbo SP, Yarborough BJ, McCarty D, Weisner C, Green CA. Patient-reported pathways to opioid use disorders and pain-related barriers to treatment engagement. J Subst Abuse Treat 2017; 73:47–54. doi:10.1016/j.jsat.2016.11.003
- Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ 2018; 360:j5790. doi:10.1136/bmj.j5790
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain 2015; 156(4):569–576. doi:10.1097/01.j.pain.0000460357.01998.f1
- Davis MA, Lin LA, Liu H, Sites BD. Prescription opioid use among adults with mental health disorders in the United States. J Am Board Fam Med 2017; 30(4):407–417. doi:10.3122/jabfm.2017.04.170112
- Tsang A, Von Korff M, Lee S, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain 2008; 9(10):883–891. doi:10.1016/j.jpain.2008.05.005
- QuickStats: age-adjusted death rates for drug overdose, by race/ethnicity—national vital statistics system, United States, 2015–2016. MMWR Morb Mortal Wkly Rep 2018; 67(12):374. doi:10.15585/mmwr.mm6712a9
- Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med 2010; 170(22):1968–1976. doi:10.1001/archinternmed.2010.391
- Vozoris NT, Wang X, Fischer HD, et al. Incident opioid drug use and adverse respiratory outcomes among older adults with COPD. Eur Respir J 2016; 48(3):683–693. doi:10.1183/13993003.01967-2015
- Wiese AD, Griffin MR, Schaffner W, et al. Opioid analgesic use and risk for invasive pneumococcal diseases: a nested case-control study. Ann Intern Med 2018; 168(6):396–404. doi:10.7326/M17-1907
- Chihuri S, Li G. Use of prescription opioids and motor vehicle crashes: a meta analysis. Accid Anal Prev 2017; 109:123–131. doi:10.1016/j.aap.2017.10.004
- Morasco BJ, Yarborough BJ, Smith NX, et al. Higher prescription opioid dose is associated with worse patient-reported pain outcomes and more health care utilization. J Pain 2017; 18(4):437–445. doi:10.1016/j.jpain.2016.12.004
- Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA 2018; 319(9):872–882. doi:10.1001/jama.2018.0899
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association Publishing; 2013:541–546.
- Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription opioid use, misuse, and use disorders in US adults: 2015 national survey on drug use and health. Ann Intern Med 2017; 167(5):293–301. doi:10.7326/M17-0865
- Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use—United States, 2006–2015. MMWR Morb Mortal Wkly Rep 2017; 66(10):265–269. doi:10.15585/mmwr.mm6610a1
- Dasgupta N, Funk MJ, Proescholdbell S, Hirsch A, Ribisl KM, Marshall S. Cohort study of the impact of high-dose opioid analgesics on overdose mortality. Pain Med 2016; 17(1):85–98. doi:10.1111/pme.12907
- Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med 2017; 376(7):663–673. doi:10.1056/NEJMsa1610524
- Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg 2017; 152(6):e170504. doi:10.1001/jamasurg.2017.0504
- Volkow ND, McLellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med 2016; 374(13):1253–1263. doi:10.1056/NEJMra1507771
- Stumbo SP, Yarborough BJ, McCarty D, Weisner C, Green CA. Patient-reported pathways to opioid use disorders and pain-related barriers to treatment engagement. J Subst Abuse Treat 2017; 73:47–54. doi:10.1016/j.jsat.2016.11.003
- Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ 2018; 360:j5790. doi:10.1136/bmj.j5790
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain 2015; 156(4):569–576. doi:10.1097/01.j.pain.0000460357.01998.f1
- Davis MA, Lin LA, Liu H, Sites BD. Prescription opioid use among adults with mental health disorders in the United States. J Am Board Fam Med 2017; 30(4):407–417. doi:10.3122/jabfm.2017.04.170112
- Tsang A, Von Korff M, Lee S, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain 2008; 9(10):883–891. doi:10.1016/j.jpain.2008.05.005
- QuickStats: age-adjusted death rates for drug overdose, by race/ethnicity—national vital statistics system, United States, 2015–2016. MMWR Morb Mortal Wkly Rep 2018; 67(12):374. doi:10.15585/mmwr.mm6712a9
- Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med 2010; 170(22):1968–1976. doi:10.1001/archinternmed.2010.391
- Vozoris NT, Wang X, Fischer HD, et al. Incident opioid drug use and adverse respiratory outcomes among older adults with COPD. Eur Respir J 2016; 48(3):683–693. doi:10.1183/13993003.01967-2015
- Wiese AD, Griffin MR, Schaffner W, et al. Opioid analgesic use and risk for invasive pneumococcal diseases: a nested case-control study. Ann Intern Med 2018; 168(6):396–404. doi:10.7326/M17-1907
- Chihuri S, Li G. Use of prescription opioids and motor vehicle crashes: a meta analysis. Accid Anal Prev 2017; 109:123–131. doi:10.1016/j.aap.2017.10.004
- Morasco BJ, Yarborough BJ, Smith NX, et al. Higher prescription opioid dose is associated with worse patient-reported pain outcomes and more health care utilization. J Pain 2017; 18(4):437–445. doi:10.1016/j.jpain.2016.12.004
- Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA 2018; 319(9):872–882. doi:10.1001/jama.2018.0899
PSA screening: Back to the future
My urologic career began in the late 1980s, just before prostate-specific antigen (PSA) testing was introduced. Ever since, a busy prostate cancer practice has given me a frontline view of the benefits and possible harms of PSA screening.
In the pre-PSA era, about half of men with newly diagnosed prostate cancer presented with incurable disease, either locally advanced or metastatic. The most common treatment was bilateral orchiectomy, which was the only safe form of androgen deprivation available.
Fast-forward a few years to the mid-1990s. Within 5 years after the introduction of PSA testing, the rate of incurable disease at diagnosis fell to just 5%, and treatment for localized disease skyrocketed, including radical prostatectomy, external beam radiation, and brachytherapy. As a result of earlier diagnosis and improved treatments, the death rate from prostate cancer in US men has fallen more than 30% since 1990.
The first-hand experience of seeing this massive stage migration to curable disease has forever convinced me that PSA screening is beneficial. Robust statistical models lend credence to this belief, with estimates that screening is responsible for 45% to 70% of this decline in mortality.1
Fast-forward again to 2012, when the US Preventive Services Task Force (USPSTF) published a strong recommendation against screening. The recommendation had so much force that as recently as 2014, only 11% of men at highest risk of prostate cancer in the Cleveland Clinic system were screened for it,2 mirroring national trends.
What happened? Colored by the experience in the era before PSA, when men presented frequently with painful metastatic disease and had an average life expectancy of 18 to 24 months, it was widely believed that all detected prostate cancer required treatment. What was not appreciated was that while PSA detects lots of prostate cancer, the most common reason for PSA levels to reach a range worrisome enough to trigger biopsy was actually benign prostatic hypertrophy.
The resulting increase in the number of biopsies resulted in the detection of a substantial number of low-grade cancers that were never destined to cause clinical harm but that got treated anyway, based on the fear that all cancers had metastatic potential. The USPSTF based its recommendation against screening on the harms caused by this overdetection and overtreatment of nonlethal disease, focusing on risks of biopsy such as sepsis, and on treatment-related adverse effects such as changes in urinary, bowel, and sexual function.
RANDOMIZED TRIALS SHOW A BENEFIT FROM SCREENING
As a result of this controversy, several large randomized trials designed to test whether PSA screening was beneficial were organized and begun in the 1990s, with one in the United States and another in Europe.3,4 Mature data from both trials have now established that there is indeed benefit to population-level screening.
The US Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO), was initially reported to show no difference in prostate cancer-specific mortality rates in those screened vs not screened, but because more than 90% of the men in the no-screening arm were screened anyway, that conclusion is erroneous.3
With 13-year follow-up and far less PSA contamination in the unscreened arm, the European Randomized Study of Screening for Prostate Cancer (ERSPC) in men ages 55 to 69 demonstrated a 27% reduction in the rate of death and a 35% reduction in the need for palliative treatments (androgen deprivation or radiation, or both) for metastatic disease in those screened vs not screened, clearly establishing substantial clinical benefit to PSA screening.4
A recent analysis of both trials that controlled for PSA drop-ins (comparing those actually screened with those actually not screened) concluded that the benefit of screening in terms of mortality reduction (estimated at about 30%) are equal in both trials.5 A large cohort study from Kaiser Permanente with 16-year follow-up has suggested that PSA screening has both a prostate cancer-specific benefit and an overall mortality benefit.6
ACTIVE SURVEILLANCE CAN REDUCE OVERTREATMENT
In parallel with the design and completion of these trials, there was a significant effort to better identify and manage patients initially overdiagnosed with nonlethal cancers by developing active surveillance regimens.
This management strategy recognizes that most low-grade cancers pose no short-term risk to the patient’s health or longevity, that definitive therapy can be deferred, and that with regular monitoring by digital rectal examination, PSA measurement, and repeat biopsy, cancers that progress can still be cured. The result of this strategy is a marked reduction in the harms caused by overtreatment (ie, the aforementioned adverse effects), as well as the avoidance of unnecessary treatment in many patients.
A randomized trial and 2 large prospective cohort studies have confirmed the long-term safety of this approach,7–9 and the development of commercially available, biopsy-based gene expression profiling tools promises to further improve risk stratification at diagnosis and during follow-up for individual patients.10
NEW USPSTF RECOMMENDATIONS: AN INDIVIDUAL, INFORMED DECISION
Based on the results of the ERSPC and the widespread adoption and safety of active surveillance, which together show benefit to screening and fewer harms in overdetection and overtreatment, in 2018 the USPSTF recast its recommendations. In upgrading the recommendation from “D” to “C,” the recommendation now states that for men ages 55 to 69, PSA screening should be an individual decision after a discussion with an informed provider, although men over 70 are still advised not to undergo screening at all.11
Some may think that this recommendation has arrived just in time, or that it should be made even stronger to actually recommend screening, as recent data from 2 national registries—the Surveillance, Epidemiology, and End Results program and the National Cancer Database—show that the fall in screening after the 2012 USPSTF guidelines has resulted in an increase in men presenting with advanced stage disease.12,13 (All of you Back to the Future fans, please return to the mid to late 1980s to see how that plays out.)
So the pendulum has now swung back in favor of screening, largely supported by solid data showing meaningful clinical benefit, better understanding of PSA and prostate cancer biology, and adoption of active surveillance.
AN IDEAL SCREENING PROGRAM
An ideal screening program would detect only biologically significant cancers, thus eliminating overdetection and overtreatment. There is reason for optimism on this front.
Second-generation PSA tests have better diagnostic accuracy for high-grade disease than earlier tests. Two such tests, the Prostate Health Index (Beckman Coulter) and the 4K-score (Opko Health), are commercially available though not usually covered by commercial insurers.14 A third test, IsoPSA (Cleveland Diagnostics), is under development. Most hospital laboratories will be able to be run this test with no need for a central laboratory.15 All 3 tests have been shown to reduce unnecessary biopsies (because of a low probability of finding a biologically significant cancer) by 30% to 45% and will help reduce overdetection.
Moreover, multiparametric magnetic resonance imaging of the prostate has been shown to improve detection of high-grade cancers,16 and a randomized trial has suggested that its incorporation into a screening strategy is cost-effective and could be better than PSA testing plus transrectal ultrasonography alone (the current standard of care).17
Several risk scores based on germline genomics also hold promise for better identifying those at risk and for helping to de-intensify screening for those unlikely to have high-grade cancer.18
Screening for prostate cancer reduces mortality rates and the burden of metastatic disease, and the paradigm continues to evolve. Men at risk by virtue of age (55 to 69, and healthy men > 70), family history, race, and newly identified factors (germline genetics) all deserve an informed discussion on the benefits and risks of screening
- Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control 2008; 19(2):175–181. doi:10.1007/s10552-007-9083-8
- Misra-Hebert AD, Hu B, Klein EA, et al. Prostate cancer screening practices in a large, integrated health system: 2007-2014. BJU Int 2017; 120(2):257–264. doi:10.1111/bju.13793
- Shoag JE, Mittal S, Hu JC. Reevaluating PSA testing rates in the PLCO trial. N Engl J Med 2016; 374(18):1795–1796. doi:10.1056/NEJMc1515131
- Schröder FH, Hugosson J, Roobol MJ, et al; ERSPC Investigators. Screening and prostate cancer mortality: results of the European randomised study of screening for prostate cancer (ERSPC) at 13 years of follow-up. Lancet 2014; 384(9959):2027–2035. doi:10.1016/S0140-6736(14)60525-0
- Tsodikov A, Gulati R, Heijnsdijk EAM, et al. Reconciling the effects of screening on prostate cancer mortality in the ERSPC and PLCO trials. Ann Intern Med 2017; 167(7):449–455. doi:10.7326/M16-2586
- Alpert PF. New evidence for the benefit of prostate-specific antigen screening: data from 400,887 Kaiser Permanente patients. Urology 2018; 118:119–126. doi:10.1016/j.urology.2018.02.049
- Lane JA, Donovan JL, Davis M, et al; ProtecT Study Group. Active monitoring, radical prostatectomy, or radiotherapy for localised prostate cancer: study design and diagnostic and baseline results of the ProtecT randomised phase 3 trial. Lancet Oncol 2014; 15(10):1109–1118. doi:10.1016/S1470-2045(14)70361-4
- Tosoian JJ, Mamawala M, Epstein JI, et al. Intermediate and longer-term outcomes from a prospective active-surveillance program for favorable-risk prostate cancer. J Clin Oncol 2015; 33(30):3379–3385. doi:10.1200/JCO.2015.62.5764
- Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 2015; 33(3):272–277. doi:10.1200/JCO.2014.55.1192
- Nyame YA, Grimberg DC, Greene DJ, et al. Genomic scores are independent of disease volume in men with favorable risk prostate cancer: implications for choosing men for active surveillance. J Urol 2018; 199(2):438–444. doi:10.1016/j.juro.2017.09.077
- US Preventive Services Task Force. Final recommendation statement. Prostate cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1. Accessed October 2, 2018.
- Negoita S, Feuer EJ, Mariotto A, et al. Annual report to the nation on the status of cancer, part II: recent changes in prostate cancer trends and disease characteristics. Cancer 2018; 124(13):2801–2814. doi:10.1002/cncr.31549
- Weiner AB, Matulewicz RS, Eggener SE, Schaeffer EM. Increasing incidence of metastatic prostate cancer in the United States (2004–2013). Prostate Cancer Prostatic Dis 2016; 19(4):395–397. doi:10.1038/pcan.2016.30
- Loeb S. Biomarkers for prostate biopsy and risk stratification of newly diagnosed prostate cancer patients. Urol Pract 2017; 4(4):315–321. doi:10.1016/j.urpr.2016.08.001
- Klein EA, Chait A, Hafron JM, et al. The single-parameter, structure-based IsoPSA assay demonstrates improved diagnostic accuracy for detection of any prostate cancer and high-grade prostate cancer compared to a concentration-based assay of total prostate-specific antigen: a preliminary report. Eur Urol 2017; 72(6):942–949. doi:10.1016/j.eururo.2017.03.025
- Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015; 313(4):390–397. doi:10.1001/jama.2014.17942
- Kasivisvanathan V, Rannikko AS, Borghi M, et al; PRECISION Study Group Collaborators. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018; 378(19):1767–1777. doi:10.1056/NEJMoa1801993
- Seibert TM, Fan CC, Wang Y, et al. PRACTICAL Consortium. Polygenic hazard score to guide screening for aggressive prostate cancer: development and validation in large scale cohorts. BMJ 2018; 360:j5757. doi:10.1136/bmj.j5757
My urologic career began in the late 1980s, just before prostate-specific antigen (PSA) testing was introduced. Ever since, a busy prostate cancer practice has given me a frontline view of the benefits and possible harms of PSA screening.
In the pre-PSA era, about half of men with newly diagnosed prostate cancer presented with incurable disease, either locally advanced or metastatic. The most common treatment was bilateral orchiectomy, which was the only safe form of androgen deprivation available.
Fast-forward a few years to the mid-1990s. Within 5 years after the introduction of PSA testing, the rate of incurable disease at diagnosis fell to just 5%, and treatment for localized disease skyrocketed, including radical prostatectomy, external beam radiation, and brachytherapy. As a result of earlier diagnosis and improved treatments, the death rate from prostate cancer in US men has fallen more than 30% since 1990.
The first-hand experience of seeing this massive stage migration to curable disease has forever convinced me that PSA screening is beneficial. Robust statistical models lend credence to this belief, with estimates that screening is responsible for 45% to 70% of this decline in mortality.1
Fast-forward again to 2012, when the US Preventive Services Task Force (USPSTF) published a strong recommendation against screening. The recommendation had so much force that as recently as 2014, only 11% of men at highest risk of prostate cancer in the Cleveland Clinic system were screened for it,2 mirroring national trends.
What happened? Colored by the experience in the era before PSA, when men presented frequently with painful metastatic disease and had an average life expectancy of 18 to 24 months, it was widely believed that all detected prostate cancer required treatment. What was not appreciated was that while PSA detects lots of prostate cancer, the most common reason for PSA levels to reach a range worrisome enough to trigger biopsy was actually benign prostatic hypertrophy.
The resulting increase in the number of biopsies resulted in the detection of a substantial number of low-grade cancers that were never destined to cause clinical harm but that got treated anyway, based on the fear that all cancers had metastatic potential. The USPSTF based its recommendation against screening on the harms caused by this overdetection and overtreatment of nonlethal disease, focusing on risks of biopsy such as sepsis, and on treatment-related adverse effects such as changes in urinary, bowel, and sexual function.
RANDOMIZED TRIALS SHOW A BENEFIT FROM SCREENING
As a result of this controversy, several large randomized trials designed to test whether PSA screening was beneficial were organized and begun in the 1990s, with one in the United States and another in Europe.3,4 Mature data from both trials have now established that there is indeed benefit to population-level screening.
The US Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO), was initially reported to show no difference in prostate cancer-specific mortality rates in those screened vs not screened, but because more than 90% of the men in the no-screening arm were screened anyway, that conclusion is erroneous.3
With 13-year follow-up and far less PSA contamination in the unscreened arm, the European Randomized Study of Screening for Prostate Cancer (ERSPC) in men ages 55 to 69 demonstrated a 27% reduction in the rate of death and a 35% reduction in the need for palliative treatments (androgen deprivation or radiation, or both) for metastatic disease in those screened vs not screened, clearly establishing substantial clinical benefit to PSA screening.4
A recent analysis of both trials that controlled for PSA drop-ins (comparing those actually screened with those actually not screened) concluded that the benefit of screening in terms of mortality reduction (estimated at about 30%) are equal in both trials.5 A large cohort study from Kaiser Permanente with 16-year follow-up has suggested that PSA screening has both a prostate cancer-specific benefit and an overall mortality benefit.6
ACTIVE SURVEILLANCE CAN REDUCE OVERTREATMENT
In parallel with the design and completion of these trials, there was a significant effort to better identify and manage patients initially overdiagnosed with nonlethal cancers by developing active surveillance regimens.
This management strategy recognizes that most low-grade cancers pose no short-term risk to the patient’s health or longevity, that definitive therapy can be deferred, and that with regular monitoring by digital rectal examination, PSA measurement, and repeat biopsy, cancers that progress can still be cured. The result of this strategy is a marked reduction in the harms caused by overtreatment (ie, the aforementioned adverse effects), as well as the avoidance of unnecessary treatment in many patients.
A randomized trial and 2 large prospective cohort studies have confirmed the long-term safety of this approach,7–9 and the development of commercially available, biopsy-based gene expression profiling tools promises to further improve risk stratification at diagnosis and during follow-up for individual patients.10
NEW USPSTF RECOMMENDATIONS: AN INDIVIDUAL, INFORMED DECISION
Based on the results of the ERSPC and the widespread adoption and safety of active surveillance, which together show benefit to screening and fewer harms in overdetection and overtreatment, in 2018 the USPSTF recast its recommendations. In upgrading the recommendation from “D” to “C,” the recommendation now states that for men ages 55 to 69, PSA screening should be an individual decision after a discussion with an informed provider, although men over 70 are still advised not to undergo screening at all.11
Some may think that this recommendation has arrived just in time, or that it should be made even stronger to actually recommend screening, as recent data from 2 national registries—the Surveillance, Epidemiology, and End Results program and the National Cancer Database—show that the fall in screening after the 2012 USPSTF guidelines has resulted in an increase in men presenting with advanced stage disease.12,13 (All of you Back to the Future fans, please return to the mid to late 1980s to see how that plays out.)
So the pendulum has now swung back in favor of screening, largely supported by solid data showing meaningful clinical benefit, better understanding of PSA and prostate cancer biology, and adoption of active surveillance.
AN IDEAL SCREENING PROGRAM
An ideal screening program would detect only biologically significant cancers, thus eliminating overdetection and overtreatment. There is reason for optimism on this front.
Second-generation PSA tests have better diagnostic accuracy for high-grade disease than earlier tests. Two such tests, the Prostate Health Index (Beckman Coulter) and the 4K-score (Opko Health), are commercially available though not usually covered by commercial insurers.14 A third test, IsoPSA (Cleveland Diagnostics), is under development. Most hospital laboratories will be able to be run this test with no need for a central laboratory.15 All 3 tests have been shown to reduce unnecessary biopsies (because of a low probability of finding a biologically significant cancer) by 30% to 45% and will help reduce overdetection.
Moreover, multiparametric magnetic resonance imaging of the prostate has been shown to improve detection of high-grade cancers,16 and a randomized trial has suggested that its incorporation into a screening strategy is cost-effective and could be better than PSA testing plus transrectal ultrasonography alone (the current standard of care).17
Several risk scores based on germline genomics also hold promise for better identifying those at risk and for helping to de-intensify screening for those unlikely to have high-grade cancer.18
Screening for prostate cancer reduces mortality rates and the burden of metastatic disease, and the paradigm continues to evolve. Men at risk by virtue of age (55 to 69, and healthy men > 70), family history, race, and newly identified factors (germline genetics) all deserve an informed discussion on the benefits and risks of screening
My urologic career began in the late 1980s, just before prostate-specific antigen (PSA) testing was introduced. Ever since, a busy prostate cancer practice has given me a frontline view of the benefits and possible harms of PSA screening.
In the pre-PSA era, about half of men with newly diagnosed prostate cancer presented with incurable disease, either locally advanced or metastatic. The most common treatment was bilateral orchiectomy, which was the only safe form of androgen deprivation available.
Fast-forward a few years to the mid-1990s. Within 5 years after the introduction of PSA testing, the rate of incurable disease at diagnosis fell to just 5%, and treatment for localized disease skyrocketed, including radical prostatectomy, external beam radiation, and brachytherapy. As a result of earlier diagnosis and improved treatments, the death rate from prostate cancer in US men has fallen more than 30% since 1990.
The first-hand experience of seeing this massive stage migration to curable disease has forever convinced me that PSA screening is beneficial. Robust statistical models lend credence to this belief, with estimates that screening is responsible for 45% to 70% of this decline in mortality.1
Fast-forward again to 2012, when the US Preventive Services Task Force (USPSTF) published a strong recommendation against screening. The recommendation had so much force that as recently as 2014, only 11% of men at highest risk of prostate cancer in the Cleveland Clinic system were screened for it,2 mirroring national trends.
What happened? Colored by the experience in the era before PSA, when men presented frequently with painful metastatic disease and had an average life expectancy of 18 to 24 months, it was widely believed that all detected prostate cancer required treatment. What was not appreciated was that while PSA detects lots of prostate cancer, the most common reason for PSA levels to reach a range worrisome enough to trigger biopsy was actually benign prostatic hypertrophy.
The resulting increase in the number of biopsies resulted in the detection of a substantial number of low-grade cancers that were never destined to cause clinical harm but that got treated anyway, based on the fear that all cancers had metastatic potential. The USPSTF based its recommendation against screening on the harms caused by this overdetection and overtreatment of nonlethal disease, focusing on risks of biopsy such as sepsis, and on treatment-related adverse effects such as changes in urinary, bowel, and sexual function.
RANDOMIZED TRIALS SHOW A BENEFIT FROM SCREENING
As a result of this controversy, several large randomized trials designed to test whether PSA screening was beneficial were organized and begun in the 1990s, with one in the United States and another in Europe.3,4 Mature data from both trials have now established that there is indeed benefit to population-level screening.
The US Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO), was initially reported to show no difference in prostate cancer-specific mortality rates in those screened vs not screened, but because more than 90% of the men in the no-screening arm were screened anyway, that conclusion is erroneous.3
With 13-year follow-up and far less PSA contamination in the unscreened arm, the European Randomized Study of Screening for Prostate Cancer (ERSPC) in men ages 55 to 69 demonstrated a 27% reduction in the rate of death and a 35% reduction in the need for palliative treatments (androgen deprivation or radiation, or both) for metastatic disease in those screened vs not screened, clearly establishing substantial clinical benefit to PSA screening.4
A recent analysis of both trials that controlled for PSA drop-ins (comparing those actually screened with those actually not screened) concluded that the benefit of screening in terms of mortality reduction (estimated at about 30%) are equal in both trials.5 A large cohort study from Kaiser Permanente with 16-year follow-up has suggested that PSA screening has both a prostate cancer-specific benefit and an overall mortality benefit.6
ACTIVE SURVEILLANCE CAN REDUCE OVERTREATMENT
In parallel with the design and completion of these trials, there was a significant effort to better identify and manage patients initially overdiagnosed with nonlethal cancers by developing active surveillance regimens.
This management strategy recognizes that most low-grade cancers pose no short-term risk to the patient’s health or longevity, that definitive therapy can be deferred, and that with regular monitoring by digital rectal examination, PSA measurement, and repeat biopsy, cancers that progress can still be cured. The result of this strategy is a marked reduction in the harms caused by overtreatment (ie, the aforementioned adverse effects), as well as the avoidance of unnecessary treatment in many patients.
A randomized trial and 2 large prospective cohort studies have confirmed the long-term safety of this approach,7–9 and the development of commercially available, biopsy-based gene expression profiling tools promises to further improve risk stratification at diagnosis and during follow-up for individual patients.10
NEW USPSTF RECOMMENDATIONS: AN INDIVIDUAL, INFORMED DECISION
Based on the results of the ERSPC and the widespread adoption and safety of active surveillance, which together show benefit to screening and fewer harms in overdetection and overtreatment, in 2018 the USPSTF recast its recommendations. In upgrading the recommendation from “D” to “C,” the recommendation now states that for men ages 55 to 69, PSA screening should be an individual decision after a discussion with an informed provider, although men over 70 are still advised not to undergo screening at all.11
Some may think that this recommendation has arrived just in time, or that it should be made even stronger to actually recommend screening, as recent data from 2 national registries—the Surveillance, Epidemiology, and End Results program and the National Cancer Database—show that the fall in screening after the 2012 USPSTF guidelines has resulted in an increase in men presenting with advanced stage disease.12,13 (All of you Back to the Future fans, please return to the mid to late 1980s to see how that plays out.)
So the pendulum has now swung back in favor of screening, largely supported by solid data showing meaningful clinical benefit, better understanding of PSA and prostate cancer biology, and adoption of active surveillance.
AN IDEAL SCREENING PROGRAM
An ideal screening program would detect only biologically significant cancers, thus eliminating overdetection and overtreatment. There is reason for optimism on this front.
Second-generation PSA tests have better diagnostic accuracy for high-grade disease than earlier tests. Two such tests, the Prostate Health Index (Beckman Coulter) and the 4K-score (Opko Health), are commercially available though not usually covered by commercial insurers.14 A third test, IsoPSA (Cleveland Diagnostics), is under development. Most hospital laboratories will be able to be run this test with no need for a central laboratory.15 All 3 tests have been shown to reduce unnecessary biopsies (because of a low probability of finding a biologically significant cancer) by 30% to 45% and will help reduce overdetection.
Moreover, multiparametric magnetic resonance imaging of the prostate has been shown to improve detection of high-grade cancers,16 and a randomized trial has suggested that its incorporation into a screening strategy is cost-effective and could be better than PSA testing plus transrectal ultrasonography alone (the current standard of care).17
Several risk scores based on germline genomics also hold promise for better identifying those at risk and for helping to de-intensify screening for those unlikely to have high-grade cancer.18
Screening for prostate cancer reduces mortality rates and the burden of metastatic disease, and the paradigm continues to evolve. Men at risk by virtue of age (55 to 69, and healthy men > 70), family history, race, and newly identified factors (germline genetics) all deserve an informed discussion on the benefits and risks of screening
- Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control 2008; 19(2):175–181. doi:10.1007/s10552-007-9083-8
- Misra-Hebert AD, Hu B, Klein EA, et al. Prostate cancer screening practices in a large, integrated health system: 2007-2014. BJU Int 2017; 120(2):257–264. doi:10.1111/bju.13793
- Shoag JE, Mittal S, Hu JC. Reevaluating PSA testing rates in the PLCO trial. N Engl J Med 2016; 374(18):1795–1796. doi:10.1056/NEJMc1515131
- Schröder FH, Hugosson J, Roobol MJ, et al; ERSPC Investigators. Screening and prostate cancer mortality: results of the European randomised study of screening for prostate cancer (ERSPC) at 13 years of follow-up. Lancet 2014; 384(9959):2027–2035. doi:10.1016/S0140-6736(14)60525-0
- Tsodikov A, Gulati R, Heijnsdijk EAM, et al. Reconciling the effects of screening on prostate cancer mortality in the ERSPC and PLCO trials. Ann Intern Med 2017; 167(7):449–455. doi:10.7326/M16-2586
- Alpert PF. New evidence for the benefit of prostate-specific antigen screening: data from 400,887 Kaiser Permanente patients. Urology 2018; 118:119–126. doi:10.1016/j.urology.2018.02.049
- Lane JA, Donovan JL, Davis M, et al; ProtecT Study Group. Active monitoring, radical prostatectomy, or radiotherapy for localised prostate cancer: study design and diagnostic and baseline results of the ProtecT randomised phase 3 trial. Lancet Oncol 2014; 15(10):1109–1118. doi:10.1016/S1470-2045(14)70361-4
- Tosoian JJ, Mamawala M, Epstein JI, et al. Intermediate and longer-term outcomes from a prospective active-surveillance program for favorable-risk prostate cancer. J Clin Oncol 2015; 33(30):3379–3385. doi:10.1200/JCO.2015.62.5764
- Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 2015; 33(3):272–277. doi:10.1200/JCO.2014.55.1192
- Nyame YA, Grimberg DC, Greene DJ, et al. Genomic scores are independent of disease volume in men with favorable risk prostate cancer: implications for choosing men for active surveillance. J Urol 2018; 199(2):438–444. doi:10.1016/j.juro.2017.09.077
- US Preventive Services Task Force. Final recommendation statement. Prostate cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1. Accessed October 2, 2018.
- Negoita S, Feuer EJ, Mariotto A, et al. Annual report to the nation on the status of cancer, part II: recent changes in prostate cancer trends and disease characteristics. Cancer 2018; 124(13):2801–2814. doi:10.1002/cncr.31549
- Weiner AB, Matulewicz RS, Eggener SE, Schaeffer EM. Increasing incidence of metastatic prostate cancer in the United States (2004–2013). Prostate Cancer Prostatic Dis 2016; 19(4):395–397. doi:10.1038/pcan.2016.30
- Loeb S. Biomarkers for prostate biopsy and risk stratification of newly diagnosed prostate cancer patients. Urol Pract 2017; 4(4):315–321. doi:10.1016/j.urpr.2016.08.001
- Klein EA, Chait A, Hafron JM, et al. The single-parameter, structure-based IsoPSA assay demonstrates improved diagnostic accuracy for detection of any prostate cancer and high-grade prostate cancer compared to a concentration-based assay of total prostate-specific antigen: a preliminary report. Eur Urol 2017; 72(6):942–949. doi:10.1016/j.eururo.2017.03.025
- Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015; 313(4):390–397. doi:10.1001/jama.2014.17942
- Kasivisvanathan V, Rannikko AS, Borghi M, et al; PRECISION Study Group Collaborators. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018; 378(19):1767–1777. doi:10.1056/NEJMoa1801993
- Seibert TM, Fan CC, Wang Y, et al. PRACTICAL Consortium. Polygenic hazard score to guide screening for aggressive prostate cancer: development and validation in large scale cohorts. BMJ 2018; 360:j5757. doi:10.1136/bmj.j5757
- Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control 2008; 19(2):175–181. doi:10.1007/s10552-007-9083-8
- Misra-Hebert AD, Hu B, Klein EA, et al. Prostate cancer screening practices in a large, integrated health system: 2007-2014. BJU Int 2017; 120(2):257–264. doi:10.1111/bju.13793
- Shoag JE, Mittal S, Hu JC. Reevaluating PSA testing rates in the PLCO trial. N Engl J Med 2016; 374(18):1795–1796. doi:10.1056/NEJMc1515131
- Schröder FH, Hugosson J, Roobol MJ, et al; ERSPC Investigators. Screening and prostate cancer mortality: results of the European randomised study of screening for prostate cancer (ERSPC) at 13 years of follow-up. Lancet 2014; 384(9959):2027–2035. doi:10.1016/S0140-6736(14)60525-0
- Tsodikov A, Gulati R, Heijnsdijk EAM, et al. Reconciling the effects of screening on prostate cancer mortality in the ERSPC and PLCO trials. Ann Intern Med 2017; 167(7):449–455. doi:10.7326/M16-2586
- Alpert PF. New evidence for the benefit of prostate-specific antigen screening: data from 400,887 Kaiser Permanente patients. Urology 2018; 118:119–126. doi:10.1016/j.urology.2018.02.049
- Lane JA, Donovan JL, Davis M, et al; ProtecT Study Group. Active monitoring, radical prostatectomy, or radiotherapy for localised prostate cancer: study design and diagnostic and baseline results of the ProtecT randomised phase 3 trial. Lancet Oncol 2014; 15(10):1109–1118. doi:10.1016/S1470-2045(14)70361-4
- Tosoian JJ, Mamawala M, Epstein JI, et al. Intermediate and longer-term outcomes from a prospective active-surveillance program for favorable-risk prostate cancer. J Clin Oncol 2015; 33(30):3379–3385. doi:10.1200/JCO.2015.62.5764
- Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 2015; 33(3):272–277. doi:10.1200/JCO.2014.55.1192
- Nyame YA, Grimberg DC, Greene DJ, et al. Genomic scores are independent of disease volume in men with favorable risk prostate cancer: implications for choosing men for active surveillance. J Urol 2018; 199(2):438–444. doi:10.1016/j.juro.2017.09.077
- US Preventive Services Task Force. Final recommendation statement. Prostate cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1. Accessed October 2, 2018.
- Negoita S, Feuer EJ, Mariotto A, et al. Annual report to the nation on the status of cancer, part II: recent changes in prostate cancer trends and disease characteristics. Cancer 2018; 124(13):2801–2814. doi:10.1002/cncr.31549
- Weiner AB, Matulewicz RS, Eggener SE, Schaeffer EM. Increasing incidence of metastatic prostate cancer in the United States (2004–2013). Prostate Cancer Prostatic Dis 2016; 19(4):395–397. doi:10.1038/pcan.2016.30
- Loeb S. Biomarkers for prostate biopsy and risk stratification of newly diagnosed prostate cancer patients. Urol Pract 2017; 4(4):315–321. doi:10.1016/j.urpr.2016.08.001
- Klein EA, Chait A, Hafron JM, et al. The single-parameter, structure-based IsoPSA assay demonstrates improved diagnostic accuracy for detection of any prostate cancer and high-grade prostate cancer compared to a concentration-based assay of total prostate-specific antigen: a preliminary report. Eur Urol 2017; 72(6):942–949. doi:10.1016/j.eururo.2017.03.025
- Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015; 313(4):390–397. doi:10.1001/jama.2014.17942
- Kasivisvanathan V, Rannikko AS, Borghi M, et al; PRECISION Study Group Collaborators. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018; 378(19):1767–1777. doi:10.1056/NEJMoa1801993
- Seibert TM, Fan CC, Wang Y, et al. PRACTICAL Consortium. Polygenic hazard score to guide screening for aggressive prostate cancer: development and validation in large scale cohorts. BMJ 2018; 360:j5757. doi:10.1136/bmj.j5757