User login
New feature debuts, how to address reviewer criticism, and more!
Dear Colleagues,
The November issue of The New Gastroenterologist is packed with some great articles! First, this issue’s In Focus article addresses the increasingly important topic of endoscopic management of obesity. In the article, the authors, Pichamol Jirapinyo and Christopher Thompson (Brigham and Women’s Hospital), provide an outstanding overview of the approved and up-and-coming endoscopic therapies that can be used to help treat the obesity epidemic. This is an area that we will inevitably see more of in our practices.
A new feature in this issue of The New Gastroenterologist is a column focused on early career gastroenterologists who are going into private practice, which was curated in conjunction with the Digestive Health Physicians Association. This month’s article by Fred Rosenberg (North Shore Endoscopy Center) provides an overview of private practice gastroenterology models. I look forward to making this column a recurring feature of future issues.
Additionally, using their wealth of experience, former CGH editor in chief Hashem El-Serag and current CGH editor in chief Fasiha Kanwal (Baylor) provide an enlightening piece on how to address reviewer criticism, which will no doubt be very helpful for those of us looking to publish. There is also a helpful article about grant writing tips authored by two successfully funded early career basic scientists, Arthur Beyder (Mayo) and Christina Twyman-Saint Victor (University of Pennsylvania).
For those considering pursuing extra training in IBD either during or after GI fellowship, Siddharth Singh (UCSD) goes through the different advanced training options that are now available in IBD. And finally, as many are laying down roots in new places, buying a house will almost inevitably be on the horizon. To help guide you through the mortgage preapproval process, Rob Wishnick (Guaranteed Rate) provides some useful insights from his many years of experience in the home loan industry.
Please check out “In Case You Missed It” to see other articles from the last quarter in AGA publications that may be of interest to you. And, if you have any ideas or want to contribute to The New Gastroenterologist, please contact me at [email protected] or the managing editor, Ryan Farrell, at [email protected].
Sincerely,
Bryson W. Katona, MD, PhD
Editor in Chief
Dr. Katona is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
Dear Colleagues,
The November issue of The New Gastroenterologist is packed with some great articles! First, this issue’s In Focus article addresses the increasingly important topic of endoscopic management of obesity. In the article, the authors, Pichamol Jirapinyo and Christopher Thompson (Brigham and Women’s Hospital), provide an outstanding overview of the approved and up-and-coming endoscopic therapies that can be used to help treat the obesity epidemic. This is an area that we will inevitably see more of in our practices.
A new feature in this issue of The New Gastroenterologist is a column focused on early career gastroenterologists who are going into private practice, which was curated in conjunction with the Digestive Health Physicians Association. This month’s article by Fred Rosenberg (North Shore Endoscopy Center) provides an overview of private practice gastroenterology models. I look forward to making this column a recurring feature of future issues.
Additionally, using their wealth of experience, former CGH editor in chief Hashem El-Serag and current CGH editor in chief Fasiha Kanwal (Baylor) provide an enlightening piece on how to address reviewer criticism, which will no doubt be very helpful for those of us looking to publish. There is also a helpful article about grant writing tips authored by two successfully funded early career basic scientists, Arthur Beyder (Mayo) and Christina Twyman-Saint Victor (University of Pennsylvania).
For those considering pursuing extra training in IBD either during or after GI fellowship, Siddharth Singh (UCSD) goes through the different advanced training options that are now available in IBD. And finally, as many are laying down roots in new places, buying a house will almost inevitably be on the horizon. To help guide you through the mortgage preapproval process, Rob Wishnick (Guaranteed Rate) provides some useful insights from his many years of experience in the home loan industry.
Please check out “In Case You Missed It” to see other articles from the last quarter in AGA publications that may be of interest to you. And, if you have any ideas or want to contribute to The New Gastroenterologist, please contact me at [email protected] or the managing editor, Ryan Farrell, at [email protected].
Sincerely,
Bryson W. Katona, MD, PhD
Editor in Chief
Dr. Katona is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
Dear Colleagues,
The November issue of The New Gastroenterologist is packed with some great articles! First, this issue’s In Focus article addresses the increasingly important topic of endoscopic management of obesity. In the article, the authors, Pichamol Jirapinyo and Christopher Thompson (Brigham and Women’s Hospital), provide an outstanding overview of the approved and up-and-coming endoscopic therapies that can be used to help treat the obesity epidemic. This is an area that we will inevitably see more of in our practices.
A new feature in this issue of The New Gastroenterologist is a column focused on early career gastroenterologists who are going into private practice, which was curated in conjunction with the Digestive Health Physicians Association. This month’s article by Fred Rosenberg (North Shore Endoscopy Center) provides an overview of private practice gastroenterology models. I look forward to making this column a recurring feature of future issues.
Additionally, using their wealth of experience, former CGH editor in chief Hashem El-Serag and current CGH editor in chief Fasiha Kanwal (Baylor) provide an enlightening piece on how to address reviewer criticism, which will no doubt be very helpful for those of us looking to publish. There is also a helpful article about grant writing tips authored by two successfully funded early career basic scientists, Arthur Beyder (Mayo) and Christina Twyman-Saint Victor (University of Pennsylvania).
For those considering pursuing extra training in IBD either during or after GI fellowship, Siddharth Singh (UCSD) goes through the different advanced training options that are now available in IBD. And finally, as many are laying down roots in new places, buying a house will almost inevitably be on the horizon. To help guide you through the mortgage preapproval process, Rob Wishnick (Guaranteed Rate) provides some useful insights from his many years of experience in the home loan industry.
Please check out “In Case You Missed It” to see other articles from the last quarter in AGA publications that may be of interest to you. And, if you have any ideas or want to contribute to The New Gastroenterologist, please contact me at [email protected] or the managing editor, Ryan Farrell, at [email protected].
Sincerely,
Bryson W. Katona, MD, PhD
Editor in Chief
Dr. Katona is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
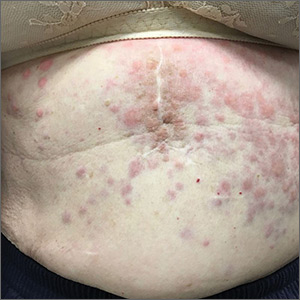
Persistent erythematous papulonodular rash
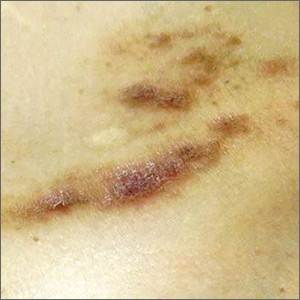
An 80-year-old white woman presented to our dermatology clinic with a rash across her abdomen that had been there for more than a year. While not itchy or painful, the rash was slowly expanding. The patient had tried treatments including topical antifungals and topical corticosteroids, but none had helped.
Her medical history was significant for dementia and stage III triple-negative breast cancer in the left breast (diagnosed 8 years prior), which was treated with a simple left mastectomy, chemotherapy, and radiation. She reported no history of skin cancer. She was not taking any medications and had no known drug allergies. A physical examination revealed an erythematous, papulonodular rash with diffuse induration in a band-like pattern across her entire upper abdomen and left flank (FIGURE).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Cutaneous metastasis of primary breast cancer
Based on our patient’s history, we gave a presumptive diagnosis of cutaneous breast cancer metastasis. A punch biopsy was performed. The pathology report showed nests of neoplastic cells within the dermis, which was consistent with this diagnosis. Immunohistochemical stains and fluorescence in-situ hybridization confirmed triple-negative breast markers for estrogen receptor, progesterone receptor, and human epidermal growth factor receptor-2.
An uncommon phenomenonseen mostly with breast cancer
Cutaneous metastatic carcinoma is relatively uncommon; one meta-analysis reported the overall incidence to be 5.3%.1 While it is unusual, any internal malignancy can metastasize to the skin. In women, the most common malignancy to do so is breast cancer. One study found breast cancer to be associated with 26.5% of cutaneous metastatic cases.2 These metastases often occur well after the patient has been treated for the primary malignancy.
Identifying features. Most cutaneous metastases occur near the site of the primary tumor, initially in the form of a firm, mobile, nonpainful nodule.3 This nodule is typically skin-colored or red, but in the case of cutaneous metastases of melanomas, it can appear blue or black. In the case of breast cancer, the lesions most often arise on the chest and abdomen.4 Occasionally, metastases can ulcerate through the skin.
Some forms of cutaneous metastasis, such as carcinoma erysipeloides, can appear in specific patterns. Carcinoma erysipeloides has a similar appearance to cellulitis; it manifests as a sharply demarcated, red, inflammatory patch in the skin adjacent to the primary tumor.
Consider the clinical picture
Cutaneous metastatic lesions have a wide range of differential diagnoses due to their varied appearances. It is important to view the overall clinical picture when distinguishing such lesions. Although cutaneous metastasis is uncommon, it should always be considered when asymptomatic skin lesions resist treatment—even in someone without a known history of malignancy.
Perform a biopsy. The diagnosis can be confirmed with a skin biopsy. A punch biopsy is preferable, as visualization of the dermis is crucial, and histology often reveals nests of pleomorphic cells. Further cellular cytology can elicit the primary malignancy of origin.
Making our diagnosis
We ruled out several possibilities before arriving at our diagnosis. An infectious etiology (eg, cutaneous candidiasis) was considered, as was a cutaneous change due to radiation therapy. We also considered shingles, the early stages of which would have been similar in appearance to our patient’s lesions, and urticaria, which can manifest as erythematous papules and wheals across various parts of the body. A lack of specific symptoms (eg, pruritis, pain, fever) made these alternative diagnoses less likely. The fact that our patient’s lesions persisted for more than a year without any response to treatment—and that they continued to grow—alerted us of a more sinister etiology.
Continue to: Treating the tumor is often not possible
Treating the tumor is often not possible
Treatment first involves treating the underlying tumor. For cases in which cutaneous lesions are the first manifestation of an internal malignancy, investigation as to the source should be performed. The lesions can then be treated with a combination of chemotherapy, radiation, and surgery.5,6
Unfortunately, in most cases of cutaneous metastases, the primary malignancy is already widespread and possibly untreatable. In such instances, palliative care is offered. Lesions are managed symptomatically, and prevention of skin irritation becomes the primary focus. Keeping the skin clean and dry helps to prevent ulceration and secondary infection.
In cases where the lesions ulcerate or crust, debridement can help. Excision of lesions, as well as pairing laser therapy with electrochemotherapy, may be helpful to improve the patient’s quality of life when lesions cause discomfort.
The prognosis for cutaneous metastasis due to breast cancer is often hard to predict because it is determined by other factors, such as the presence of internal metastases, which indicates a worse prognosis (on the scale of months). Some case reports have demonstrated that patients with metastases limited to the skin may have prolonged survival (on the scale of years).7
Our patient was offered an initial trial of radiation therapy, but she refused all treatment because the lesions did not cause discomfort, and she preferred to not go through further aggressive cancer treatment that could potentially cause complications and pain. We respected the patient’s wishes and counseled her on follow-up if the lesions became symptomatic or she decided she wanted to try treatment.
CORRESPONDENCE
Araya Zaesim, 1550 College St, Macon, GA, 31207; [email protected]
1. Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a meta-analysis of data. South Med J. 2003;96:164-167.
2. Brownstein MH, Helwig EB. Patterns of cutaneous metastasis. Arch Dermatol. 1972;105:862-868.
3. De Giorgi V, Grazzini M, Alfaioli B, et al. Cutaneous manifestations of breast carcinoma. Dermatol Ther. 2010;23:581-589.
4. Wong CYB, Helm MA, Kalb RE, et al. The presentation, pathology, and current management strategies of cutaneous metastasis. N Am J Med Sci. 2013;5:499-504.
5. Moore S. Cutaneous metastatic breast cancer. Clin J Oncol Nurs. 2002;6:255-260.
6. Ahmed M. Cutaneous metastases from breast carcinoma. BMJ Case Rep. 2011;2011: bcr0620114398.
7. Cho J, Park Y, Lee JC, et al. Case series of different onset of skin metastasis according to the breast cancer subtypes. Cancer Res Treat. 2014;46:194-199.
An 80-year-old white woman presented to our dermatology clinic with a rash across her abdomen that had been there for more than a year. While not itchy or painful, the rash was slowly expanding. The patient had tried treatments including topical antifungals and topical corticosteroids, but none had helped.
Her medical history was significant for dementia and stage III triple-negative breast cancer in the left breast (diagnosed 8 years prior), which was treated with a simple left mastectomy, chemotherapy, and radiation. She reported no history of skin cancer. She was not taking any medications and had no known drug allergies. A physical examination revealed an erythematous, papulonodular rash with diffuse induration in a band-like pattern across her entire upper abdomen and left flank (FIGURE).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Cutaneous metastasis of primary breast cancer
Based on our patient’s history, we gave a presumptive diagnosis of cutaneous breast cancer metastasis. A punch biopsy was performed. The pathology report showed nests of neoplastic cells within the dermis, which was consistent with this diagnosis. Immunohistochemical stains and fluorescence in-situ hybridization confirmed triple-negative breast markers for estrogen receptor, progesterone receptor, and human epidermal growth factor receptor-2.
An uncommon phenomenonseen mostly with breast cancer
Cutaneous metastatic carcinoma is relatively uncommon; one meta-analysis reported the overall incidence to be 5.3%.1 While it is unusual, any internal malignancy can metastasize to the skin. In women, the most common malignancy to do so is breast cancer. One study found breast cancer to be associated with 26.5% of cutaneous metastatic cases.2 These metastases often occur well after the patient has been treated for the primary malignancy.
Identifying features. Most cutaneous metastases occur near the site of the primary tumor, initially in the form of a firm, mobile, nonpainful nodule.3 This nodule is typically skin-colored or red, but in the case of cutaneous metastases of melanomas, it can appear blue or black. In the case of breast cancer, the lesions most often arise on the chest and abdomen.4 Occasionally, metastases can ulcerate through the skin.
Some forms of cutaneous metastasis, such as carcinoma erysipeloides, can appear in specific patterns. Carcinoma erysipeloides has a similar appearance to cellulitis; it manifests as a sharply demarcated, red, inflammatory patch in the skin adjacent to the primary tumor.
Consider the clinical picture
Cutaneous metastatic lesions have a wide range of differential diagnoses due to their varied appearances. It is important to view the overall clinical picture when distinguishing such lesions. Although cutaneous metastasis is uncommon, it should always be considered when asymptomatic skin lesions resist treatment—even in someone without a known history of malignancy.
Perform a biopsy. The diagnosis can be confirmed with a skin biopsy. A punch biopsy is preferable, as visualization of the dermis is crucial, and histology often reveals nests of pleomorphic cells. Further cellular cytology can elicit the primary malignancy of origin.
Making our diagnosis
We ruled out several possibilities before arriving at our diagnosis. An infectious etiology (eg, cutaneous candidiasis) was considered, as was a cutaneous change due to radiation therapy. We also considered shingles, the early stages of which would have been similar in appearance to our patient’s lesions, and urticaria, which can manifest as erythematous papules and wheals across various parts of the body. A lack of specific symptoms (eg, pruritis, pain, fever) made these alternative diagnoses less likely. The fact that our patient’s lesions persisted for more than a year without any response to treatment—and that they continued to grow—alerted us of a more sinister etiology.
Continue to: Treating the tumor is often not possible
Treating the tumor is often not possible
Treatment first involves treating the underlying tumor. For cases in which cutaneous lesions are the first manifestation of an internal malignancy, investigation as to the source should be performed. The lesions can then be treated with a combination of chemotherapy, radiation, and surgery.5,6
Unfortunately, in most cases of cutaneous metastases, the primary malignancy is already widespread and possibly untreatable. In such instances, palliative care is offered. Lesions are managed symptomatically, and prevention of skin irritation becomes the primary focus. Keeping the skin clean and dry helps to prevent ulceration and secondary infection.
In cases where the lesions ulcerate or crust, debridement can help. Excision of lesions, as well as pairing laser therapy with electrochemotherapy, may be helpful to improve the patient’s quality of life when lesions cause discomfort.
The prognosis for cutaneous metastasis due to breast cancer is often hard to predict because it is determined by other factors, such as the presence of internal metastases, which indicates a worse prognosis (on the scale of months). Some case reports have demonstrated that patients with metastases limited to the skin may have prolonged survival (on the scale of years).7
Our patient was offered an initial trial of radiation therapy, but she refused all treatment because the lesions did not cause discomfort, and she preferred to not go through further aggressive cancer treatment that could potentially cause complications and pain. We respected the patient’s wishes and counseled her on follow-up if the lesions became symptomatic or she decided she wanted to try treatment.
CORRESPONDENCE
Araya Zaesim, 1550 College St, Macon, GA, 31207; [email protected]
An 80-year-old white woman presented to our dermatology clinic with a rash across her abdomen that had been there for more than a year. While not itchy or painful, the rash was slowly expanding. The patient had tried treatments including topical antifungals and topical corticosteroids, but none had helped.
Her medical history was significant for dementia and stage III triple-negative breast cancer in the left breast (diagnosed 8 years prior), which was treated with a simple left mastectomy, chemotherapy, and radiation. She reported no history of skin cancer. She was not taking any medications and had no known drug allergies. A physical examination revealed an erythematous, papulonodular rash with diffuse induration in a band-like pattern across her entire upper abdomen and left flank (FIGURE).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Cutaneous metastasis of primary breast cancer
Based on our patient’s history, we gave a presumptive diagnosis of cutaneous breast cancer metastasis. A punch biopsy was performed. The pathology report showed nests of neoplastic cells within the dermis, which was consistent with this diagnosis. Immunohistochemical stains and fluorescence in-situ hybridization confirmed triple-negative breast markers for estrogen receptor, progesterone receptor, and human epidermal growth factor receptor-2.
An uncommon phenomenonseen mostly with breast cancer
Cutaneous metastatic carcinoma is relatively uncommon; one meta-analysis reported the overall incidence to be 5.3%.1 While it is unusual, any internal malignancy can metastasize to the skin. In women, the most common malignancy to do so is breast cancer. One study found breast cancer to be associated with 26.5% of cutaneous metastatic cases.2 These metastases often occur well after the patient has been treated for the primary malignancy.
Identifying features. Most cutaneous metastases occur near the site of the primary tumor, initially in the form of a firm, mobile, nonpainful nodule.3 This nodule is typically skin-colored or red, but in the case of cutaneous metastases of melanomas, it can appear blue or black. In the case of breast cancer, the lesions most often arise on the chest and abdomen.4 Occasionally, metastases can ulcerate through the skin.
Some forms of cutaneous metastasis, such as carcinoma erysipeloides, can appear in specific patterns. Carcinoma erysipeloides has a similar appearance to cellulitis; it manifests as a sharply demarcated, red, inflammatory patch in the skin adjacent to the primary tumor.
Consider the clinical picture
Cutaneous metastatic lesions have a wide range of differential diagnoses due to their varied appearances. It is important to view the overall clinical picture when distinguishing such lesions. Although cutaneous metastasis is uncommon, it should always be considered when asymptomatic skin lesions resist treatment—even in someone without a known history of malignancy.
Perform a biopsy. The diagnosis can be confirmed with a skin biopsy. A punch biopsy is preferable, as visualization of the dermis is crucial, and histology often reveals nests of pleomorphic cells. Further cellular cytology can elicit the primary malignancy of origin.
Making our diagnosis
We ruled out several possibilities before arriving at our diagnosis. An infectious etiology (eg, cutaneous candidiasis) was considered, as was a cutaneous change due to radiation therapy. We also considered shingles, the early stages of which would have been similar in appearance to our patient’s lesions, and urticaria, which can manifest as erythematous papules and wheals across various parts of the body. A lack of specific symptoms (eg, pruritis, pain, fever) made these alternative diagnoses less likely. The fact that our patient’s lesions persisted for more than a year without any response to treatment—and that they continued to grow—alerted us of a more sinister etiology.
Continue to: Treating the tumor is often not possible
Treating the tumor is often not possible
Treatment first involves treating the underlying tumor. For cases in which cutaneous lesions are the first manifestation of an internal malignancy, investigation as to the source should be performed. The lesions can then be treated with a combination of chemotherapy, radiation, and surgery.5,6
Unfortunately, in most cases of cutaneous metastases, the primary malignancy is already widespread and possibly untreatable. In such instances, palliative care is offered. Lesions are managed symptomatically, and prevention of skin irritation becomes the primary focus. Keeping the skin clean and dry helps to prevent ulceration and secondary infection.
In cases where the lesions ulcerate or crust, debridement can help. Excision of lesions, as well as pairing laser therapy with electrochemotherapy, may be helpful to improve the patient’s quality of life when lesions cause discomfort.
The prognosis for cutaneous metastasis due to breast cancer is often hard to predict because it is determined by other factors, such as the presence of internal metastases, which indicates a worse prognosis (on the scale of months). Some case reports have demonstrated that patients with metastases limited to the skin may have prolonged survival (on the scale of years).7
Our patient was offered an initial trial of radiation therapy, but she refused all treatment because the lesions did not cause discomfort, and she preferred to not go through further aggressive cancer treatment that could potentially cause complications and pain. We respected the patient’s wishes and counseled her on follow-up if the lesions became symptomatic or she decided she wanted to try treatment.
CORRESPONDENCE
Araya Zaesim, 1550 College St, Macon, GA, 31207; [email protected]
1. Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a meta-analysis of data. South Med J. 2003;96:164-167.
2. Brownstein MH, Helwig EB. Patterns of cutaneous metastasis. Arch Dermatol. 1972;105:862-868.
3. De Giorgi V, Grazzini M, Alfaioli B, et al. Cutaneous manifestations of breast carcinoma. Dermatol Ther. 2010;23:581-589.
4. Wong CYB, Helm MA, Kalb RE, et al. The presentation, pathology, and current management strategies of cutaneous metastasis. N Am J Med Sci. 2013;5:499-504.
5. Moore S. Cutaneous metastatic breast cancer. Clin J Oncol Nurs. 2002;6:255-260.
6. Ahmed M. Cutaneous metastases from breast carcinoma. BMJ Case Rep. 2011;2011: bcr0620114398.
7. Cho J, Park Y, Lee JC, et al. Case series of different onset of skin metastasis according to the breast cancer subtypes. Cancer Res Treat. 2014;46:194-199.
1. Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a meta-analysis of data. South Med J. 2003;96:164-167.
2. Brownstein MH, Helwig EB. Patterns of cutaneous metastasis. Arch Dermatol. 1972;105:862-868.
3. De Giorgi V, Grazzini M, Alfaioli B, et al. Cutaneous manifestations of breast carcinoma. Dermatol Ther. 2010;23:581-589.
4. Wong CYB, Helm MA, Kalb RE, et al. The presentation, pathology, and current management strategies of cutaneous metastasis. N Am J Med Sci. 2013;5:499-504.
5. Moore S. Cutaneous metastatic breast cancer. Clin J Oncol Nurs. 2002;6:255-260.
6. Ahmed M. Cutaneous metastases from breast carcinoma. BMJ Case Rep. 2011;2011: bcr0620114398.
7. Cho J, Park Y, Lee JC, et al. Case series of different onset of skin metastasis according to the breast cancer subtypes. Cancer Res Treat. 2014;46:194-199.
Primary hyperparathyroidism: Labs to order, Tx to consider
Since the advent of multichannel serum chemistry screening in the 1970s, large numbers of asymptomatic cases of primary hyperparathyroidism (PHPT) have been discovered. The clinical spectrum of the disease has changed from the classic “moans, groans, bones, and stones” to an asymptomatic and subtle presentation of hypercalcemia.1,2 PHPT and malignancy are the most common causes for hypercalcemia, accounting for 90% of cases.3 In the United States, the estimated incidence of PHPT between 1998 and 2010 was about 50 per 100,000 person-years. Most patients with PHPT are older women (ages >50 years) who are asymptomatic at the time of diagnosis.1
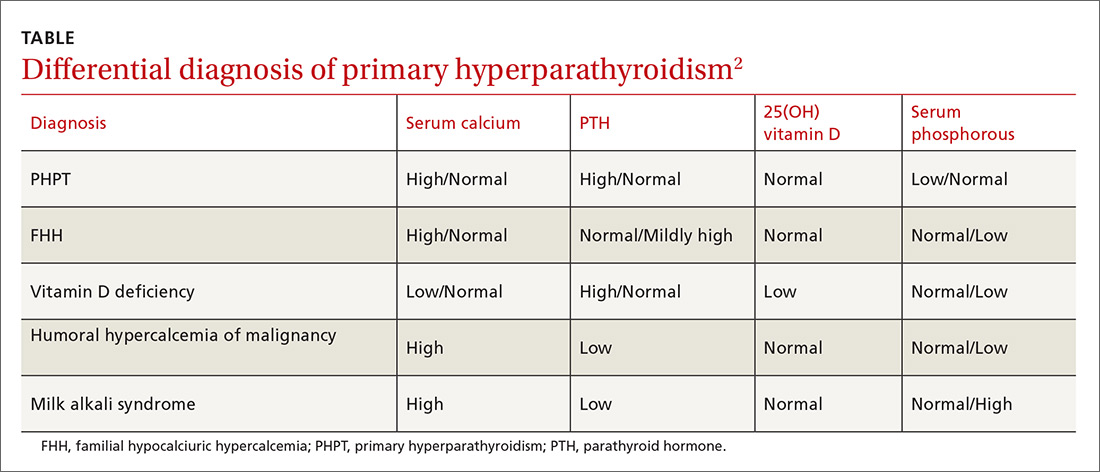
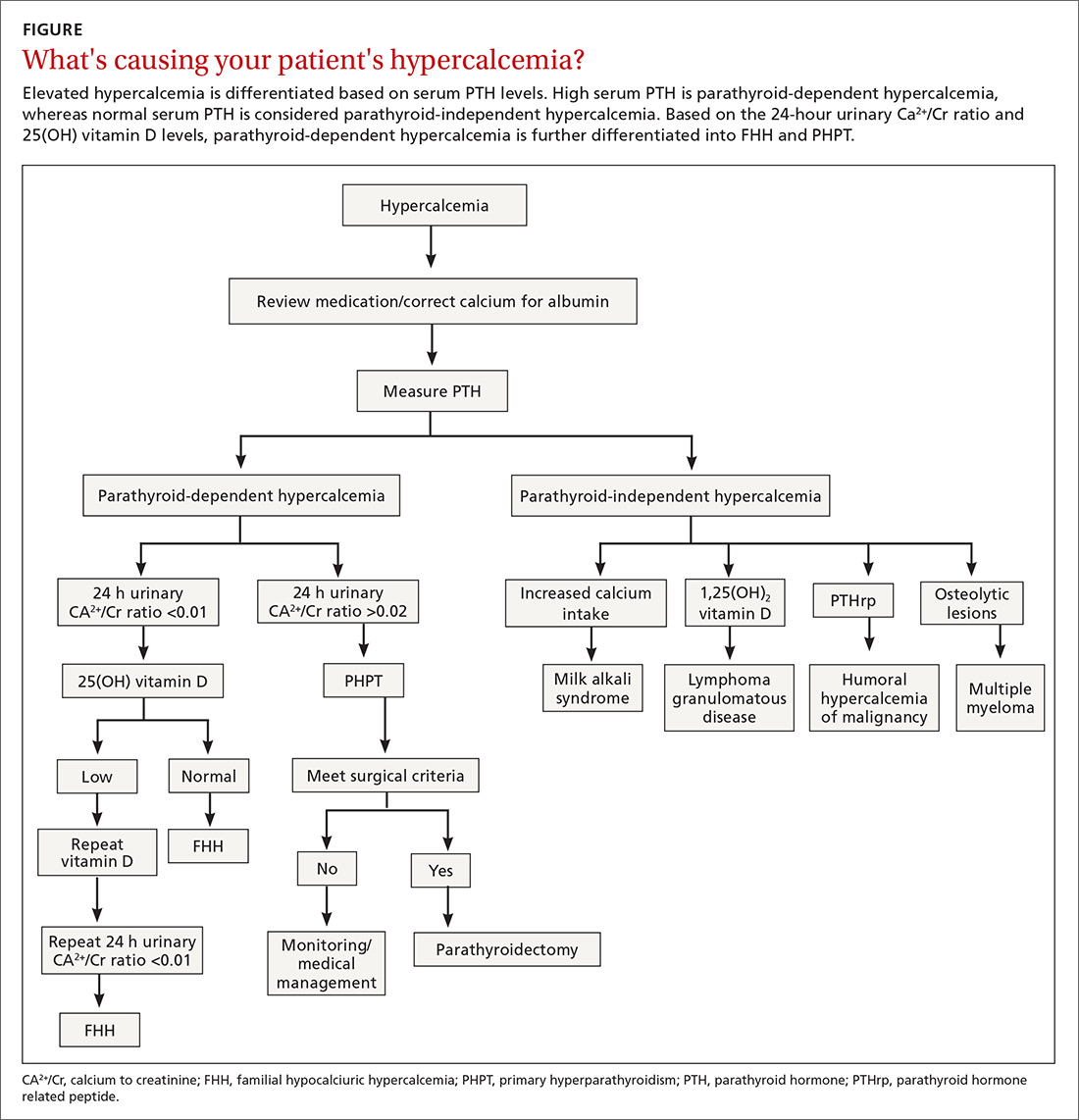
Vigilance needed in primary care. PHPT is slowly progressive, and the patient might accept symptoms as a process of aging. Therefore, it is essential that primary care physicians (PCPs) be aware of the diagnostic and management options. A systematic approach to the diagnosis of PHPT helps differentiate the causes of hypercalcemia (TABLE2; FIGURE2). But before we discuss PHPT diagnostic clues, it’s helpful to quickly review the workings of the parathyroid glands.
How the glands work, what can go wrong
Parathyroid hormone (PTH) is secreted by 4 pea-sized parathyroid glands located posterior to the thyroid. PTH regulates the levels of calcium (Ca2+) and phosphorous and controls the conversion of 25(OH) vitamin D to 1,25(OH)2 vitamin D by activating the enzyme 1 alpha-hydroxylase.
PHPT is regarded as an abnormal secretion of PTH that does not correlate with the levels of Ca2+ in the blood.1 Eighty percent of PHPT is due to a solitary adenoma in one of the parathyroid glands, 2% to 4% is secondary to multiple parathyroid adenomas, 15% is due to parathyroid hyperplasia, and 0.5% due to parathyroid carcinoma.4
Nonspecific symptomsare subtle clues of PHPT
Patients with PHPT can present with nonspecific symptoms, such as weakness, fatigue, anorexia, polyuria, polydipsia, bone and joint pain, mild depression, and mild cognitive or neuromuscular dysfunction.5 A careful history is essential to elicit these symptoms, as the patient may attribute these to aging or other causes. PHPT should also be considered when patients present with kidney stones, unexplained osteoporosis, or fragility fractures. A physical examination is seldom helpful, as parathyroid adenomas are hardly ever palpable. A slit-lamp examination may reveal corneal diseases in rare cases of hypercalcemia.6
Which lab tests, imaging should you order?
Serum Ca2+
Repeat measurements of serum Ca2+ to confirm hypercalcemia. Volume depletion, underlying malignancy, medications such as hydrochlorothiazide, lithium, and excess intake of Ca2+ carbonate can cause hypercalcemia.7 Therefore, a review of the patients’ home medications and dietary preferences in the evaluation of hypercalcemia is essential. The 2 most common causes of hypercalcemia are hyperparathyroidism and hypercalcemia of malignancy.
For hypercalcemia, establish a differential diagnosis by measuring intact PTH. An increased serum Ca2+ level along with an elevated PTH concentration should suggest PTH-dependent hypercalcemia, whereas hypercalcemia with suppressed or low-normal PTH values should suggest PTH-independent hypercalcemia (granulomatous disorders, hypercalcemia of malignancy).
Continue to: Hypercalcemia of malignancy is due to...
Hypercalcemia of malignancy is due to increased production of parathyroid hormone-related peptide from various tumor cells that initiate bone resorption and increased renal Ca2+ absorption. It can also be due to osteolysis from bone metastasis.7 It is generally severe and is a common cause of hypercalcemia in the inpatient setting.
Meticulous evaluation is vital to diagnose PHPT. Measurement of serum ionized Ca2+ reflects the biologically active Ca2+. Studies by Ong and colleagues suggest that about 24% of patients with the histologically proven parathyroid disease had isolated ionized hypercalcemia.8 It is also an important adjunct to diagnose the presumed normocalcemic PHPT in which both the ionized Ca2+ levels and serum total Ca2+ levels should be normal.9
In patients with hypoalbuminemia, a corrected serum Ca2+ is calculated using the equation: corrected Ca2+ = [0.8 × (normal albumin-patient’s albumin)] + serum Ca2+ level.
Serum PTH
Second-generation PTH assays (intact PTH) and third-generation PTH assays (bioactive PTH) are equally reliable in diagnosing PHPT.10 The results obtained with intact and bioactive PTH assays are highly correlated. Several studies have found no improvement in diagnostic accuracy when using the bioactive PTH assay.11,12
Serum PTH can be low, normal, or elevated in hypercalcemia. Hypercalcemia with a high PTH level is parathyroid-dependent hypercalcemia, whereas hypercalcemia with a suppressed PTH level is considered parathyroid-independent.
Continue to: Serum 25(OH) vitamin D
Serum 25(OH) vitamin D
Vitamin D levels are normal in PHPT and normocalcemic PHPT. However, measuring 25(OH) vitamin D in all patients with suspected PHPT is recommended to evaluate for secondary hyperparathyroidism that is due to hypocalcemia or renal failure, which can occur concomitantly with PHPT.
Normocalcemic PHPT can be differentiated from secondary hyperparathyroidism of chronic kidney disease by measuring the 1,25(OH)2 vitamin D level; it will be low in secondary hyperparathyroidism.4
Serum 1,25(OH)2 vitamin D
1,25(OH)2 vitaminD levels are elevated in about one-third of patients with PHPT, as PTH stimulates the conversion of 25(OH) vitamin D to 1,25(OH)2 vitamin D.13 Although this is not a routine test, it is useful in the evaluation of parathyroid-independent hypercalcemia caused by granulomatous disease, such as sarcoidosis where there is an autonomous production of 1,25(OH)2 vitamin D leading to hypercalcemia.14
Serum creatinine and estimated glomerular filtration rate
Serum creatinine (Cr) helps assess renal function. Reduction in serum Cr clearance to <60 mL/min with no other underlying cause is an indication for parathyroidectomy.10
Serum phosphorous
PTH increases the excretion of phosphorous by inhibiting reabsorption from the proximal tubule. Therefore, serum phosphorus tends to be in the lower range of normal in PHPT, but hypophosphatemia is present in less than a quarter of patients.4
Continue to: 24-hour urinary Ca2+
24-hour urinary Ca2+
A 24-hour urinary Ca2+ excretion is used to assess the risk of renal stones and to differentiate PHPT from familial hypocalciuric hypercalcemia (FHH). Patients with FHH have an abnormality in Ca2+ receptor gene expression in parathyroid cells and renal tubular cells that could lead to parathyroid-mediated hypercalcemia and hypocalciuria. FHH is differentiated from PHPT by calculating a 24-hour urinary Ca2+/Cr ratio. A value of <0.01 is diagnostic of FHH; whereas values >0.02 indicate PHPT. The test can be more accurate when the patient is on a normal Ca2+ and salt diet, when the estimated glomerular filtration rate is >60 mL/min/1.73 m2, and when the serum 25(OH) vitamin D level is >30 ng/dL.15 Adequate urine volume is necessary for the 24-hour Ca2+/Cr ratio to be valid.
Renal imaging
Kidney stones and high Ca2+ deposits in the kidneys are the common manifestations of PHPT. Renal X-ray, computed tomography (CT), or ultrasonography are recommended in the evaluation of patients with PHPT. An incidental finding of either kidney stones or high Ca2+ deposits in the kidneys is an indication for surgery.10
Bone density/DEXA (dual energy X-ray absorptiometry) scan with a vertebral fracture assessment (VFA)
Asymptomatic PHPT individuals with osteoporosis (T-score < 2.5) or vertebral compression fracture benefit from surgical management.10 It is essential to obtain densitometry at 3 sites: the lumbar spine, the hip, and the distal third of the radius. Due to differing amounts of cortical and cancellous bone at the 3 sites and the differential effects of PTH on the cortical and cancellous bone, measurement at all 3 sites allows a clear estimation of the severity of the hyperparathyroid process on the skeleton.16 Therefore, consider measuring serum PTH if the patient has severe osteoporosis or fragility fractures that cannot be explained or that are unresponsive to treatment.
Management
The primary modality of treatment in PHPT is parathyroidectomy. The benefits are many, including an increase in bone mineral density (BMD) and reduction in fractures and kidney stones.10 With modern imaging and intra-operative PTH measurement, the success of minimally invasive parathyroidectomy is high in experienced hands. Patients with PHPT should be referred to an endocrinologist before surgery.
Surgery
Consider surgery if the patient meets any one of the following criteria:
1) overt clinical manifestations (stones, fractures)
2) serum Ca2+ >1 mg/dL above the upper limit of normal
3) Cr clearance <60 mL/min
4) low BMD with a T score ≤2.5 at any site
5) age <50 years
6) uncertain prospect for follow-up.
Continue to: Perform imaging before surgery to identify...
Perform imaging before surgery to identify the overactive parathyroid glands. Ultrasound can detect enlargement of the parathyroid glands. A sestamibi scan, which measures the uptake of Tc99-sestamibi by the parathyroid glands, reflects the activity of the parathyroid glands. In cases of nonlocalization by these 2 modalities, other imaging techniques like 4D CT scan and contrast-enhanced ultrasound can be used. Of note: Imaging is used for localization, but not for diagnosis.
Intra-operative PTH measurement has added to the efficacy of minimally invasive parathyroidectomy. A drop in PTH of >50% after 10 to 15 minutes of excising the gland is considered to be positive.10
Medication management
Monitor patients who refuse surgery or those who do not meet the criteria after surgery. Serum Ca2+ and PTH are monitored annually. DEXA scan needs to be repeated every 1 to 2 years based on the clinical picture. Also assess patients for any fragility fractures and renal endpoints. Recommend taking vitamin D to keep the level above 20 ng/dL.10 Ca2+ intake should follow normally recommended guidelines.
Bisphosphonates are primarily used for the treatment of osteoporosis accompanying PHPT. They decrease bone resorption and, to a lesser extent, bone formation. Alendronate increases BMD at the lumbar spine, but does not have much effect on Ca2+ and PTH levels.
Calcimimetics act by mimicking the effects of Ca2+ on the Ca2+ receptors present on the surface of the parathyroid cells. Therefore, calcimimetics reduce the level of parathyroid hormone and Ca2+ levels. (Long-term benefits have not been established.) Bisphosphonates are prescribed for osteoporosis and calcimimetics for hypercalcemia.10
Continue to: Conclusion
Conclusion
Although largely asymptomatic, consider PHPT when patients present with unexplained kidney stones, osteoporosis, or any nonspecific symptoms described earlier. PHPT is diagnosed by detecting an inappropriately high or normal PTH in relation to the Ca2+ level. Medications need to be reviewed, and conditions such as FHH that produce similar symptoms need to be ruled out. Measurement of 25(OH) vitamin D levels is recommended in all patients with PHPT.
Parathyroidectomy is the definitive form of treatment and should be offered to patients who meet any one of the surgical criteria, as described earlier. It can also be offered to patients who do not meet the criteria if they prefer. It is known to decrease the risk of kidney stones and osteoporosis. Medical therapy is primarily for patients who do not meet the criteria as mentioned earlier and for those who cannot and/or are unwilling to undergo surgery.
CORRESPONDENCE
Padmaja Sanapureddy, MD, Department of Primary Care and Medicine, G.V. (Sonny) Montgomery VA Medical Center, 1500 E Woodrow Wilson Ave, Jackson, MS 39216; [email protected].
1. Griebeler ML, Kearns AE, Ryu E, et al. Secular trends in the incidence of primary hyperparathyroidism over five decades (1965-2010). Bone. 2015;73:1-7.
2. Melmed S, Polonsky, KS, Larsen PR, et al. Williams Textbook of Endocrinology: Hormones and Disorders of Mineral Metabolism, 12th ed. Philadelphia, PA: Elsevier Inc, 2011:1262-1263.
3. Assadi F. Hypercalcemia: an evidence-based approach to clinical cases. Iran J Kidney Dis. 2009;3(2):71-79.
4. Bilezikian JP, Cusano NE, Khan AA, et al. Primary hyperparathyroidism. Nat Rev Dis Primers. 2016;19;2:16033.
5. Roman S, Sosa JA. Psychiatric and cognitive aspects of primary hyperparathyroidism. Curr Opin Oncol. 2007;19:1-5.
6. Berkow JW, Fine BS, Zimmerman LE. Unusual ocular calcification in hyperparathyroidism. Am J Ophthalmol. 1968;66:812-824.
7. Carroll MF, Schade DS. A practical approach to hypercalcemia. Am Fam Physician. 2003;67:1959-1966.
8. Ong GSY, Walsh JP, Stuckey BGA, et al. The importance of measuring ionized calcium in characterizing calcium status and diagnosing primary hyperparathyroidism. J Clin Endocrinol Metab. 2012;97:3138-3145.
9. Silverberg SJ, Bilezikian JP. “Incipient” primary hyperparathyroidism: a “forme fruste” of an old disease. J Clin Endocrinol Metab. 2003;88:5348-5352.
10. Eastell R, Brandi ML, Costa AG, et al. Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99:3570-3579.
11. Boudou P, Ibrahim F, Cormier C, et al. Third- or second-generation parathyroid hormone assays: a remaining debate in the diagnosis of primary hyperparathyroidism. J Clin Endocrinol Metab. 2005;90:6370-6372.
12. Carnevale V, Dionisi S, Nofroni I, et al. Potential clinical utility of a new IRMA for parathyroid hormone in postmenopausal patients with primary hyperparathyroidism. Clin Chem. 2004;50:626-631.
13. Jameson JL, De Groot L. Endocrinology: Adult and Pediatric. 7thed. Philadelphia, PA: Elsevier Inc, 2016:1109.
14. Tebben PJ, Singh RJ, Kumar R. Vitamin d-mediated hypercalcemia: mechanisms, diagnosis and treatment. Endocr Rev. 2016;37:521-547.
15. Shinall MC Jr, Dahir KM, Broome JT. Differentiating familial hypocalciuric hypercalcemia from primary hyperparathyroidism. Endocr Pract. 2013;19:697-702.
16. Castellano E, Attanasio R, Gianotti L, et al. Forearm DXA increases the rate of patients with asymptomatic primary hyperparathyroidism meeting surgical criteria. J Clin Endocrinol Metab. 2016;101:2728-2732.
Since the advent of multichannel serum chemistry screening in the 1970s, large numbers of asymptomatic cases of primary hyperparathyroidism (PHPT) have been discovered. The clinical spectrum of the disease has changed from the classic “moans, groans, bones, and stones” to an asymptomatic and subtle presentation of hypercalcemia.1,2 PHPT and malignancy are the most common causes for hypercalcemia, accounting for 90% of cases.3 In the United States, the estimated incidence of PHPT between 1998 and 2010 was about 50 per 100,000 person-years. Most patients with PHPT are older women (ages >50 years) who are asymptomatic at the time of diagnosis.1

Vigilance needed in primary care. PHPT is slowly progressive, and the patient might accept symptoms as a process of aging. Therefore, it is essential that primary care physicians (PCPs) be aware of the diagnostic and management options. A systematic approach to the diagnosis of PHPT helps differentiate the causes of hypercalcemia (TABLE2; FIGURE2). But before we discuss PHPT diagnostic clues, it’s helpful to quickly review the workings of the parathyroid glands.

How the glands work, what can go wrong
Parathyroid hormone (PTH) is secreted by 4 pea-sized parathyroid glands located posterior to the thyroid. PTH regulates the levels of calcium (Ca2+) and phosphorous and controls the conversion of 25(OH) vitamin D to 1,25(OH)2 vitamin D by activating the enzyme 1 alpha-hydroxylase.
PHPT is regarded as an abnormal secretion of PTH that does not correlate with the levels of Ca2+ in the blood.1 Eighty percent of PHPT is due to a solitary adenoma in one of the parathyroid glands, 2% to 4% is secondary to multiple parathyroid adenomas, 15% is due to parathyroid hyperplasia, and 0.5% due to parathyroid carcinoma.4
Nonspecific symptomsare subtle clues of PHPT
Patients with PHPT can present with nonspecific symptoms, such as weakness, fatigue, anorexia, polyuria, polydipsia, bone and joint pain, mild depression, and mild cognitive or neuromuscular dysfunction.5 A careful history is essential to elicit these symptoms, as the patient may attribute these to aging or other causes. PHPT should also be considered when patients present with kidney stones, unexplained osteoporosis, or fragility fractures. A physical examination is seldom helpful, as parathyroid adenomas are hardly ever palpable. A slit-lamp examination may reveal corneal diseases in rare cases of hypercalcemia.6
Which lab tests, imaging should you order?
Serum Ca2+
Repeat measurements of serum Ca2+ to confirm hypercalcemia. Volume depletion, underlying malignancy, medications such as hydrochlorothiazide, lithium, and excess intake of Ca2+ carbonate can cause hypercalcemia.7 Therefore, a review of the patients’ home medications and dietary preferences in the evaluation of hypercalcemia is essential. The 2 most common causes of hypercalcemia are hyperparathyroidism and hypercalcemia of malignancy.
For hypercalcemia, establish a differential diagnosis by measuring intact PTH. An increased serum Ca2+ level along with an elevated PTH concentration should suggest PTH-dependent hypercalcemia, whereas hypercalcemia with suppressed or low-normal PTH values should suggest PTH-independent hypercalcemia (granulomatous disorders, hypercalcemia of malignancy).
Continue to: Hypercalcemia of malignancy is due to...
Hypercalcemia of malignancy is due to increased production of parathyroid hormone-related peptide from various tumor cells that initiate bone resorption and increased renal Ca2+ absorption. It can also be due to osteolysis from bone metastasis.7 It is generally severe and is a common cause of hypercalcemia in the inpatient setting.
Meticulous evaluation is vital to diagnose PHPT. Measurement of serum ionized Ca2+ reflects the biologically active Ca2+. Studies by Ong and colleagues suggest that about 24% of patients with the histologically proven parathyroid disease had isolated ionized hypercalcemia.8 It is also an important adjunct to diagnose the presumed normocalcemic PHPT in which both the ionized Ca2+ levels and serum total Ca2+ levels should be normal.9
In patients with hypoalbuminemia, a corrected serum Ca2+ is calculated using the equation: corrected Ca2+ = [0.8 × (normal albumin-patient’s albumin)] + serum Ca2+ level.
Serum PTH
Second-generation PTH assays (intact PTH) and third-generation PTH assays (bioactive PTH) are equally reliable in diagnosing PHPT.10 The results obtained with intact and bioactive PTH assays are highly correlated. Several studies have found no improvement in diagnostic accuracy when using the bioactive PTH assay.11,12
Serum PTH can be low, normal, or elevated in hypercalcemia. Hypercalcemia with a high PTH level is parathyroid-dependent hypercalcemia, whereas hypercalcemia with a suppressed PTH level is considered parathyroid-independent.
Continue to: Serum 25(OH) vitamin D
Serum 25(OH) vitamin D
Vitamin D levels are normal in PHPT and normocalcemic PHPT. However, measuring 25(OH) vitamin D in all patients with suspected PHPT is recommended to evaluate for secondary hyperparathyroidism that is due to hypocalcemia or renal failure, which can occur concomitantly with PHPT.
Normocalcemic PHPT can be differentiated from secondary hyperparathyroidism of chronic kidney disease by measuring the 1,25(OH)2 vitamin D level; it will be low in secondary hyperparathyroidism.4
Serum 1,25(OH)2 vitamin D
1,25(OH)2 vitaminD levels are elevated in about one-third of patients with PHPT, as PTH stimulates the conversion of 25(OH) vitamin D to 1,25(OH)2 vitamin D.13 Although this is not a routine test, it is useful in the evaluation of parathyroid-independent hypercalcemia caused by granulomatous disease, such as sarcoidosis where there is an autonomous production of 1,25(OH)2 vitamin D leading to hypercalcemia.14
Serum creatinine and estimated glomerular filtration rate
Serum creatinine (Cr) helps assess renal function. Reduction in serum Cr clearance to <60 mL/min with no other underlying cause is an indication for parathyroidectomy.10
Serum phosphorous
PTH increases the excretion of phosphorous by inhibiting reabsorption from the proximal tubule. Therefore, serum phosphorus tends to be in the lower range of normal in PHPT, but hypophosphatemia is present in less than a quarter of patients.4
Continue to: 24-hour urinary Ca2+
24-hour urinary Ca2+
A 24-hour urinary Ca2+ excretion is used to assess the risk of renal stones and to differentiate PHPT from familial hypocalciuric hypercalcemia (FHH). Patients with FHH have an abnormality in Ca2+ receptor gene expression in parathyroid cells and renal tubular cells that could lead to parathyroid-mediated hypercalcemia and hypocalciuria. FHH is differentiated from PHPT by calculating a 24-hour urinary Ca2+/Cr ratio. A value of <0.01 is diagnostic of FHH; whereas values >0.02 indicate PHPT. The test can be more accurate when the patient is on a normal Ca2+ and salt diet, when the estimated glomerular filtration rate is >60 mL/min/1.73 m2, and when the serum 25(OH) vitamin D level is >30 ng/dL.15 Adequate urine volume is necessary for the 24-hour Ca2+/Cr ratio to be valid.
Renal imaging
Kidney stones and high Ca2+ deposits in the kidneys are the common manifestations of PHPT. Renal X-ray, computed tomography (CT), or ultrasonography are recommended in the evaluation of patients with PHPT. An incidental finding of either kidney stones or high Ca2+ deposits in the kidneys is an indication for surgery.10
Bone density/DEXA (dual energy X-ray absorptiometry) scan with a vertebral fracture assessment (VFA)
Asymptomatic PHPT individuals with osteoporosis (T-score < 2.5) or vertebral compression fracture benefit from surgical management.10 It is essential to obtain densitometry at 3 sites: the lumbar spine, the hip, and the distal third of the radius. Due to differing amounts of cortical and cancellous bone at the 3 sites and the differential effects of PTH on the cortical and cancellous bone, measurement at all 3 sites allows a clear estimation of the severity of the hyperparathyroid process on the skeleton.16 Therefore, consider measuring serum PTH if the patient has severe osteoporosis or fragility fractures that cannot be explained or that are unresponsive to treatment.
Management
The primary modality of treatment in PHPT is parathyroidectomy. The benefits are many, including an increase in bone mineral density (BMD) and reduction in fractures and kidney stones.10 With modern imaging and intra-operative PTH measurement, the success of minimally invasive parathyroidectomy is high in experienced hands. Patients with PHPT should be referred to an endocrinologist before surgery.
Surgery
Consider surgery if the patient meets any one of the following criteria:
1) overt clinical manifestations (stones, fractures)
2) serum Ca2+ >1 mg/dL above the upper limit of normal
3) Cr clearance <60 mL/min
4) low BMD with a T score ≤2.5 at any site
5) age <50 years
6) uncertain prospect for follow-up.
Continue to: Perform imaging before surgery to identify...
Perform imaging before surgery to identify the overactive parathyroid glands. Ultrasound can detect enlargement of the parathyroid glands. A sestamibi scan, which measures the uptake of Tc99-sestamibi by the parathyroid glands, reflects the activity of the parathyroid glands. In cases of nonlocalization by these 2 modalities, other imaging techniques like 4D CT scan and contrast-enhanced ultrasound can be used. Of note: Imaging is used for localization, but not for diagnosis.
Intra-operative PTH measurement has added to the efficacy of minimally invasive parathyroidectomy. A drop in PTH of >50% after 10 to 15 minutes of excising the gland is considered to be positive.10
Medication management
Monitor patients who refuse surgery or those who do not meet the criteria after surgery. Serum Ca2+ and PTH are monitored annually. DEXA scan needs to be repeated every 1 to 2 years based on the clinical picture. Also assess patients for any fragility fractures and renal endpoints. Recommend taking vitamin D to keep the level above 20 ng/dL.10 Ca2+ intake should follow normally recommended guidelines.
Bisphosphonates are primarily used for the treatment of osteoporosis accompanying PHPT. They decrease bone resorption and, to a lesser extent, bone formation. Alendronate increases BMD at the lumbar spine, but does not have much effect on Ca2+ and PTH levels.
Calcimimetics act by mimicking the effects of Ca2+ on the Ca2+ receptors present on the surface of the parathyroid cells. Therefore, calcimimetics reduce the level of parathyroid hormone and Ca2+ levels. (Long-term benefits have not been established.) Bisphosphonates are prescribed for osteoporosis and calcimimetics for hypercalcemia.10
Continue to: Conclusion
Conclusion
Although largely asymptomatic, consider PHPT when patients present with unexplained kidney stones, osteoporosis, or any nonspecific symptoms described earlier. PHPT is diagnosed by detecting an inappropriately high or normal PTH in relation to the Ca2+ level. Medications need to be reviewed, and conditions such as FHH that produce similar symptoms need to be ruled out. Measurement of 25(OH) vitamin D levels is recommended in all patients with PHPT.
Parathyroidectomy is the definitive form of treatment and should be offered to patients who meet any one of the surgical criteria, as described earlier. It can also be offered to patients who do not meet the criteria if they prefer. It is known to decrease the risk of kidney stones and osteoporosis. Medical therapy is primarily for patients who do not meet the criteria as mentioned earlier and for those who cannot and/or are unwilling to undergo surgery.
CORRESPONDENCE
Padmaja Sanapureddy, MD, Department of Primary Care and Medicine, G.V. (Sonny) Montgomery VA Medical Center, 1500 E Woodrow Wilson Ave, Jackson, MS 39216; [email protected].
Since the advent of multichannel serum chemistry screening in the 1970s, large numbers of asymptomatic cases of primary hyperparathyroidism (PHPT) have been discovered. The clinical spectrum of the disease has changed from the classic “moans, groans, bones, and stones” to an asymptomatic and subtle presentation of hypercalcemia.1,2 PHPT and malignancy are the most common causes for hypercalcemia, accounting for 90% of cases.3 In the United States, the estimated incidence of PHPT between 1998 and 2010 was about 50 per 100,000 person-years. Most patients with PHPT are older women (ages >50 years) who are asymptomatic at the time of diagnosis.1

Vigilance needed in primary care. PHPT is slowly progressive, and the patient might accept symptoms as a process of aging. Therefore, it is essential that primary care physicians (PCPs) be aware of the diagnostic and management options. A systematic approach to the diagnosis of PHPT helps differentiate the causes of hypercalcemia (TABLE2; FIGURE2). But before we discuss PHPT diagnostic clues, it’s helpful to quickly review the workings of the parathyroid glands.

How the glands work, what can go wrong
Parathyroid hormone (PTH) is secreted by 4 pea-sized parathyroid glands located posterior to the thyroid. PTH regulates the levels of calcium (Ca2+) and phosphorous and controls the conversion of 25(OH) vitamin D to 1,25(OH)2 vitamin D by activating the enzyme 1 alpha-hydroxylase.
PHPT is regarded as an abnormal secretion of PTH that does not correlate with the levels of Ca2+ in the blood.1 Eighty percent of PHPT is due to a solitary adenoma in one of the parathyroid glands, 2% to 4% is secondary to multiple parathyroid adenomas, 15% is due to parathyroid hyperplasia, and 0.5% due to parathyroid carcinoma.4
Nonspecific symptomsare subtle clues of PHPT
Patients with PHPT can present with nonspecific symptoms, such as weakness, fatigue, anorexia, polyuria, polydipsia, bone and joint pain, mild depression, and mild cognitive or neuromuscular dysfunction.5 A careful history is essential to elicit these symptoms, as the patient may attribute these to aging or other causes. PHPT should also be considered when patients present with kidney stones, unexplained osteoporosis, or fragility fractures. A physical examination is seldom helpful, as parathyroid adenomas are hardly ever palpable. A slit-lamp examination may reveal corneal diseases in rare cases of hypercalcemia.6
Which lab tests, imaging should you order?
Serum Ca2+
Repeat measurements of serum Ca2+ to confirm hypercalcemia. Volume depletion, underlying malignancy, medications such as hydrochlorothiazide, lithium, and excess intake of Ca2+ carbonate can cause hypercalcemia.7 Therefore, a review of the patients’ home medications and dietary preferences in the evaluation of hypercalcemia is essential. The 2 most common causes of hypercalcemia are hyperparathyroidism and hypercalcemia of malignancy.
For hypercalcemia, establish a differential diagnosis by measuring intact PTH. An increased serum Ca2+ level along with an elevated PTH concentration should suggest PTH-dependent hypercalcemia, whereas hypercalcemia with suppressed or low-normal PTH values should suggest PTH-independent hypercalcemia (granulomatous disorders, hypercalcemia of malignancy).
Continue to: Hypercalcemia of malignancy is due to...
Hypercalcemia of malignancy is due to increased production of parathyroid hormone-related peptide from various tumor cells that initiate bone resorption and increased renal Ca2+ absorption. It can also be due to osteolysis from bone metastasis.7 It is generally severe and is a common cause of hypercalcemia in the inpatient setting.
Meticulous evaluation is vital to diagnose PHPT. Measurement of serum ionized Ca2+ reflects the biologically active Ca2+. Studies by Ong and colleagues suggest that about 24% of patients with the histologically proven parathyroid disease had isolated ionized hypercalcemia.8 It is also an important adjunct to diagnose the presumed normocalcemic PHPT in which both the ionized Ca2+ levels and serum total Ca2+ levels should be normal.9
In patients with hypoalbuminemia, a corrected serum Ca2+ is calculated using the equation: corrected Ca2+ = [0.8 × (normal albumin-patient’s albumin)] + serum Ca2+ level.
Serum PTH
Second-generation PTH assays (intact PTH) and third-generation PTH assays (bioactive PTH) are equally reliable in diagnosing PHPT.10 The results obtained with intact and bioactive PTH assays are highly correlated. Several studies have found no improvement in diagnostic accuracy when using the bioactive PTH assay.11,12
Serum PTH can be low, normal, or elevated in hypercalcemia. Hypercalcemia with a high PTH level is parathyroid-dependent hypercalcemia, whereas hypercalcemia with a suppressed PTH level is considered parathyroid-independent.
Continue to: Serum 25(OH) vitamin D
Serum 25(OH) vitamin D
Vitamin D levels are normal in PHPT and normocalcemic PHPT. However, measuring 25(OH) vitamin D in all patients with suspected PHPT is recommended to evaluate for secondary hyperparathyroidism that is due to hypocalcemia or renal failure, which can occur concomitantly with PHPT.
Normocalcemic PHPT can be differentiated from secondary hyperparathyroidism of chronic kidney disease by measuring the 1,25(OH)2 vitamin D level; it will be low in secondary hyperparathyroidism.4
Serum 1,25(OH)2 vitamin D
1,25(OH)2 vitaminD levels are elevated in about one-third of patients with PHPT, as PTH stimulates the conversion of 25(OH) vitamin D to 1,25(OH)2 vitamin D.13 Although this is not a routine test, it is useful in the evaluation of parathyroid-independent hypercalcemia caused by granulomatous disease, such as sarcoidosis where there is an autonomous production of 1,25(OH)2 vitamin D leading to hypercalcemia.14
Serum creatinine and estimated glomerular filtration rate
Serum creatinine (Cr) helps assess renal function. Reduction in serum Cr clearance to <60 mL/min with no other underlying cause is an indication for parathyroidectomy.10
Serum phosphorous
PTH increases the excretion of phosphorous by inhibiting reabsorption from the proximal tubule. Therefore, serum phosphorus tends to be in the lower range of normal in PHPT, but hypophosphatemia is present in less than a quarter of patients.4
Continue to: 24-hour urinary Ca2+
24-hour urinary Ca2+
A 24-hour urinary Ca2+ excretion is used to assess the risk of renal stones and to differentiate PHPT from familial hypocalciuric hypercalcemia (FHH). Patients with FHH have an abnormality in Ca2+ receptor gene expression in parathyroid cells and renal tubular cells that could lead to parathyroid-mediated hypercalcemia and hypocalciuria. FHH is differentiated from PHPT by calculating a 24-hour urinary Ca2+/Cr ratio. A value of <0.01 is diagnostic of FHH; whereas values >0.02 indicate PHPT. The test can be more accurate when the patient is on a normal Ca2+ and salt diet, when the estimated glomerular filtration rate is >60 mL/min/1.73 m2, and when the serum 25(OH) vitamin D level is >30 ng/dL.15 Adequate urine volume is necessary for the 24-hour Ca2+/Cr ratio to be valid.
Renal imaging
Kidney stones and high Ca2+ deposits in the kidneys are the common manifestations of PHPT. Renal X-ray, computed tomography (CT), or ultrasonography are recommended in the evaluation of patients with PHPT. An incidental finding of either kidney stones or high Ca2+ deposits in the kidneys is an indication for surgery.10
Bone density/DEXA (dual energy X-ray absorptiometry) scan with a vertebral fracture assessment (VFA)
Asymptomatic PHPT individuals with osteoporosis (T-score < 2.5) or vertebral compression fracture benefit from surgical management.10 It is essential to obtain densitometry at 3 sites: the lumbar spine, the hip, and the distal third of the radius. Due to differing amounts of cortical and cancellous bone at the 3 sites and the differential effects of PTH on the cortical and cancellous bone, measurement at all 3 sites allows a clear estimation of the severity of the hyperparathyroid process on the skeleton.16 Therefore, consider measuring serum PTH if the patient has severe osteoporosis or fragility fractures that cannot be explained or that are unresponsive to treatment.
Management
The primary modality of treatment in PHPT is parathyroidectomy. The benefits are many, including an increase in bone mineral density (BMD) and reduction in fractures and kidney stones.10 With modern imaging and intra-operative PTH measurement, the success of minimally invasive parathyroidectomy is high in experienced hands. Patients with PHPT should be referred to an endocrinologist before surgery.
Surgery
Consider surgery if the patient meets any one of the following criteria:
1) overt clinical manifestations (stones, fractures)
2) serum Ca2+ >1 mg/dL above the upper limit of normal
3) Cr clearance <60 mL/min
4) low BMD with a T score ≤2.5 at any site
5) age <50 years
6) uncertain prospect for follow-up.
Continue to: Perform imaging before surgery to identify...
Perform imaging before surgery to identify the overactive parathyroid glands. Ultrasound can detect enlargement of the parathyroid glands. A sestamibi scan, which measures the uptake of Tc99-sestamibi by the parathyroid glands, reflects the activity of the parathyroid glands. In cases of nonlocalization by these 2 modalities, other imaging techniques like 4D CT scan and contrast-enhanced ultrasound can be used. Of note: Imaging is used for localization, but not for diagnosis.
Intra-operative PTH measurement has added to the efficacy of minimally invasive parathyroidectomy. A drop in PTH of >50% after 10 to 15 minutes of excising the gland is considered to be positive.10
Medication management
Monitor patients who refuse surgery or those who do not meet the criteria after surgery. Serum Ca2+ and PTH are monitored annually. DEXA scan needs to be repeated every 1 to 2 years based on the clinical picture. Also assess patients for any fragility fractures and renal endpoints. Recommend taking vitamin D to keep the level above 20 ng/dL.10 Ca2+ intake should follow normally recommended guidelines.
Bisphosphonates are primarily used for the treatment of osteoporosis accompanying PHPT. They decrease bone resorption and, to a lesser extent, bone formation. Alendronate increases BMD at the lumbar spine, but does not have much effect on Ca2+ and PTH levels.
Calcimimetics act by mimicking the effects of Ca2+ on the Ca2+ receptors present on the surface of the parathyroid cells. Therefore, calcimimetics reduce the level of parathyroid hormone and Ca2+ levels. (Long-term benefits have not been established.) Bisphosphonates are prescribed for osteoporosis and calcimimetics for hypercalcemia.10
Continue to: Conclusion
Conclusion
Although largely asymptomatic, consider PHPT when patients present with unexplained kidney stones, osteoporosis, or any nonspecific symptoms described earlier. PHPT is diagnosed by detecting an inappropriately high or normal PTH in relation to the Ca2+ level. Medications need to be reviewed, and conditions such as FHH that produce similar symptoms need to be ruled out. Measurement of 25(OH) vitamin D levels is recommended in all patients with PHPT.
Parathyroidectomy is the definitive form of treatment and should be offered to patients who meet any one of the surgical criteria, as described earlier. It can also be offered to patients who do not meet the criteria if they prefer. It is known to decrease the risk of kidney stones and osteoporosis. Medical therapy is primarily for patients who do not meet the criteria as mentioned earlier and for those who cannot and/or are unwilling to undergo surgery.
CORRESPONDENCE
Padmaja Sanapureddy, MD, Department of Primary Care and Medicine, G.V. (Sonny) Montgomery VA Medical Center, 1500 E Woodrow Wilson Ave, Jackson, MS 39216; [email protected].
1. Griebeler ML, Kearns AE, Ryu E, et al. Secular trends in the incidence of primary hyperparathyroidism over five decades (1965-2010). Bone. 2015;73:1-7.
2. Melmed S, Polonsky, KS, Larsen PR, et al. Williams Textbook of Endocrinology: Hormones and Disorders of Mineral Metabolism, 12th ed. Philadelphia, PA: Elsevier Inc, 2011:1262-1263.
3. Assadi F. Hypercalcemia: an evidence-based approach to clinical cases. Iran J Kidney Dis. 2009;3(2):71-79.
4. Bilezikian JP, Cusano NE, Khan AA, et al. Primary hyperparathyroidism. Nat Rev Dis Primers. 2016;19;2:16033.
5. Roman S, Sosa JA. Psychiatric and cognitive aspects of primary hyperparathyroidism. Curr Opin Oncol. 2007;19:1-5.
6. Berkow JW, Fine BS, Zimmerman LE. Unusual ocular calcification in hyperparathyroidism. Am J Ophthalmol. 1968;66:812-824.
7. Carroll MF, Schade DS. A practical approach to hypercalcemia. Am Fam Physician. 2003;67:1959-1966.
8. Ong GSY, Walsh JP, Stuckey BGA, et al. The importance of measuring ionized calcium in characterizing calcium status and diagnosing primary hyperparathyroidism. J Clin Endocrinol Metab. 2012;97:3138-3145.
9. Silverberg SJ, Bilezikian JP. “Incipient” primary hyperparathyroidism: a “forme fruste” of an old disease. J Clin Endocrinol Metab. 2003;88:5348-5352.
10. Eastell R, Brandi ML, Costa AG, et al. Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99:3570-3579.
11. Boudou P, Ibrahim F, Cormier C, et al. Third- or second-generation parathyroid hormone assays: a remaining debate in the diagnosis of primary hyperparathyroidism. J Clin Endocrinol Metab. 2005;90:6370-6372.
12. Carnevale V, Dionisi S, Nofroni I, et al. Potential clinical utility of a new IRMA for parathyroid hormone in postmenopausal patients with primary hyperparathyroidism. Clin Chem. 2004;50:626-631.
13. Jameson JL, De Groot L. Endocrinology: Adult and Pediatric. 7thed. Philadelphia, PA: Elsevier Inc, 2016:1109.
14. Tebben PJ, Singh RJ, Kumar R. Vitamin d-mediated hypercalcemia: mechanisms, diagnosis and treatment. Endocr Rev. 2016;37:521-547.
15. Shinall MC Jr, Dahir KM, Broome JT. Differentiating familial hypocalciuric hypercalcemia from primary hyperparathyroidism. Endocr Pract. 2013;19:697-702.
16. Castellano E, Attanasio R, Gianotti L, et al. Forearm DXA increases the rate of patients with asymptomatic primary hyperparathyroidism meeting surgical criteria. J Clin Endocrinol Metab. 2016;101:2728-2732.
1. Griebeler ML, Kearns AE, Ryu E, et al. Secular trends in the incidence of primary hyperparathyroidism over five decades (1965-2010). Bone. 2015;73:1-7.
2. Melmed S, Polonsky, KS, Larsen PR, et al. Williams Textbook of Endocrinology: Hormones and Disorders of Mineral Metabolism, 12th ed. Philadelphia, PA: Elsevier Inc, 2011:1262-1263.
3. Assadi F. Hypercalcemia: an evidence-based approach to clinical cases. Iran J Kidney Dis. 2009;3(2):71-79.
4. Bilezikian JP, Cusano NE, Khan AA, et al. Primary hyperparathyroidism. Nat Rev Dis Primers. 2016;19;2:16033.
5. Roman S, Sosa JA. Psychiatric and cognitive aspects of primary hyperparathyroidism. Curr Opin Oncol. 2007;19:1-5.
6. Berkow JW, Fine BS, Zimmerman LE. Unusual ocular calcification in hyperparathyroidism. Am J Ophthalmol. 1968;66:812-824.
7. Carroll MF, Schade DS. A practical approach to hypercalcemia. Am Fam Physician. 2003;67:1959-1966.
8. Ong GSY, Walsh JP, Stuckey BGA, et al. The importance of measuring ionized calcium in characterizing calcium status and diagnosing primary hyperparathyroidism. J Clin Endocrinol Metab. 2012;97:3138-3145.
9. Silverberg SJ, Bilezikian JP. “Incipient” primary hyperparathyroidism: a “forme fruste” of an old disease. J Clin Endocrinol Metab. 2003;88:5348-5352.
10. Eastell R, Brandi ML, Costa AG, et al. Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99:3570-3579.
11. Boudou P, Ibrahim F, Cormier C, et al. Third- or second-generation parathyroid hormone assays: a remaining debate in the diagnosis of primary hyperparathyroidism. J Clin Endocrinol Metab. 2005;90:6370-6372.
12. Carnevale V, Dionisi S, Nofroni I, et al. Potential clinical utility of a new IRMA for parathyroid hormone in postmenopausal patients with primary hyperparathyroidism. Clin Chem. 2004;50:626-631.
13. Jameson JL, De Groot L. Endocrinology: Adult and Pediatric. 7thed. Philadelphia, PA: Elsevier Inc, 2016:1109.
14. Tebben PJ, Singh RJ, Kumar R. Vitamin d-mediated hypercalcemia: mechanisms, diagnosis and treatment. Endocr Rev. 2016;37:521-547.
15. Shinall MC Jr, Dahir KM, Broome JT. Differentiating familial hypocalciuric hypercalcemia from primary hyperparathyroidism. Endocr Pract. 2013;19:697-702.
16. Castellano E, Attanasio R, Gianotti L, et al. Forearm DXA increases the rate of patients with asymptomatic primary hyperparathyroidism meeting surgical criteria. J Clin Endocrinol Metab. 2016;101:2728-2732.
PRACTICE RECOMMENDATIONS
› Evaluate suspected cases of primary hyperparathyroidism (PHPT) with serum total calcium, parathyroid hormone (PTH), creatinine, and 25-hydroxy vitamin D levels. A
› Consider 24-hour urine measurement of calcium and creatinine in patients undergoing evaluation for possible PHPT. A
› Obtain bone densitometry at the spine, hip, and distal radius in patients with PHPT. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Adult foot fractures: A guide
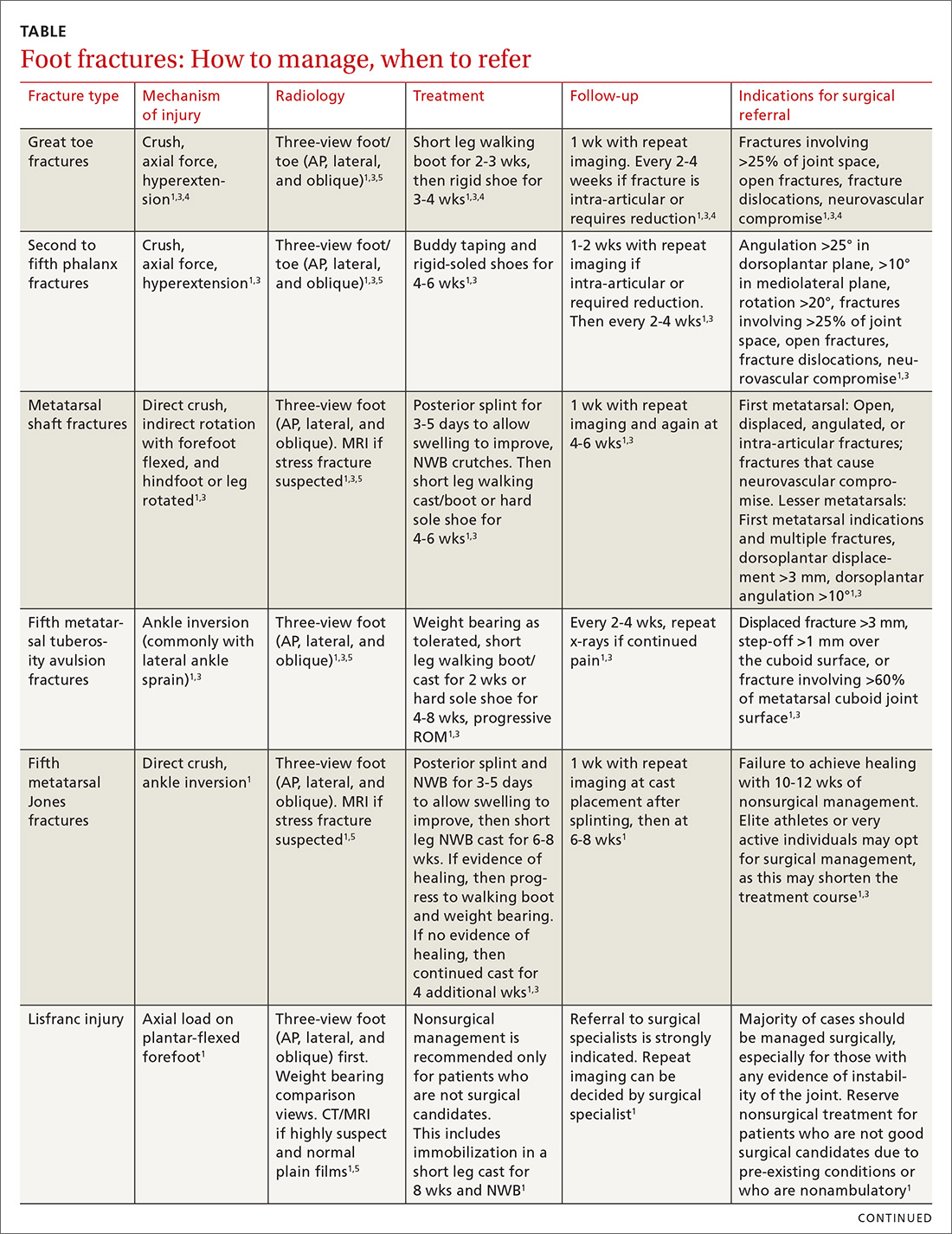
The evaluation and management of acute musculoskeletal conditions are frequently handled by primary care providers.1 It’s estimated that up to 14% of orthopedic complaints encountered by family physicians involve fractures,2 and approximately 15% of these are foot fractures.2 Diagnosis requires radiographic evaluation, but ultrasound is proving useful, too. This article reviews the diagnosis and management of adult foot fractures, with an emphasis on when advanced imaging and referral are indicated (TABLE1,3-10).
Phalanx fractures: The most common foot fractures
Phalanx fractures typically occur by crush injury, hyperextension, or direct axial force (eg, stubbing the toe).3 Patients with phalanx fractures typically present with pain at or near the site of injury, edema, ecchymosis, and erythema. Throbbing pain is characteristic, and dependent position may worsen the pain.1 Emergently evaluate any fracture causing tenting of the skin, protrusion from the skin, or neurovascular compromise, and attempt realignment to regain neurovascular function.

Most patients with phalanx fractures have point tenderness over the site of the fracture; however, this may also occur with contusions. Placing a gentle loading force along the long axis of the bone distal to the injury may help you differentiate between a contusion and a fracture.4 Pain observed with axial loading of the bone during examination points to a fracture rather than a contusion.
Differential diagnosis
Obtain imaging, including anterior-posterior (AP), lateral, and oblique views at a minimum, for all patients in whom you suspect fractures.5 Multiple fractures of the phalanges are common; therefore, always thoroughly examine the phalanges adjacent to the injured one.
Sesamoid bone fractures are uncommon but do occur and are usually due to direct injury from jumping or landing. The most common sesamoid to be injured is the medial sesamoid of the great toe, although the lateral sesamoid can also be injured. Bipartite sesamoids can occur and may confuse the examiner due to their similar appearance on x-rays to a sesamoid fracture.1 These normal variants often appear smooth and are commonly bilateral as opposed to the jagged or abrupt edges of a fracture. Stress fractures occur as well and are typically due to overuse-type injuries.
Other causes of pain similar to that experienced with phalanx fractures include soft tissue injuries to adjacent ligaments, tendons, and muscles. To help discern the cause of pain, evaluate nail beds for subungual hematomas, indicating injury to the nail bed causing bleeding and pressure under the nail. Obvious deformities of the toes or metatarsal-phalangeal joints signal the possibility of a fracture-dislocation. First metatarsophalangeal (MTP) sprain (“turf toe,” a condition common in athletes who hyperextend the toe, such as when pushing off from hard surfaces like turf) and gout can also present with acute pain in the first phalanx.
Treatment
Due to the role of the great toe in weight bearing and balance, great toe fractures are sometimes managed differently than fractures in Toes 2 through 5. Proper alignment and healing from a fracture in the first toe are critical to prevent future pain and other sequelae. Refer for orthopedic evaluation great toe fractures with displacement, angulation, rotational deformity, neurovascular compromise, >25% involvement of the joint space, or obvious dislocation.1 If referral is not indicated, treat great toe fractures with a short leg walking cast/boot for 2 to 3 weeks followed by buddy taping and use of a hard-soled shoe for 3 to 4 weeks.1
Continue to: With regard to the lesser toes...
With regard to the lesser toes, refer patients with fracture-dislocations, displaced intra-articular fractures, and fractures that do not reduce easily. Nondisplaced fractures of the lesser toes do not require surgical referral.1 These can be treated with splinting (buddy taping) and use of rigid-sole shoes for 4 to 6 weeks. Treatment duration depends largely on patient compliance; generally, continue treatment until point tenderness resolves.1
Treatment of sesamoid fractures consists of resting the affected foot with a walking boot, hard-soled shoe, or “donut” pad under the sesamoid bone to help distribute weight on the foot when standing. Length of treatment is approximately 6 to 8 weeks for most fractures.1 Consider surgical referral if nonoperative management is unsuccessful.
Metatarsal fractures: Look for malalignment
Metatarsal fractures account for 5% of all foot fractures encountered in primary care.2 These fractures typically occur as a result of falls, direct trauma, or rotational injuries (eg, ankle and foot sprains).1 In athletes, the most common cause of these fractures is high rotational force. Patients typically present with pain over the injury site, swelling, bruising, and pain with weight bearing.
As part of your exam, look for malalignment, rotational deformities, and evidence of open fracture. Palpation at the site of the fracture may increase the pain; however, as is true with phalanx fractures, contusions may also cause significant tenderness upon palpation. Also assess range of motion—with special attention to signs of malrotation—and evaluate the adjacent metatarsals, as multiple bones, ligaments, or both are often involved.1
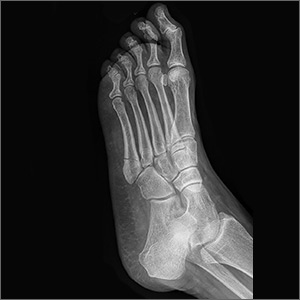
Fifth metatarsal fractures are the most common in adults, likely because of decreased cortical thickness as compared with the other metatarsals.3,11 In addition, multiple soft tissue attachments connect at the proximal aspect of the fifth metatarsal. Classification of these types of metatarsal fractures is based on anatomic location.3 Jones fractures are one type of fracture at the proximal aspect of the fifth metatarsal that occur at the metaphyseal-diaphyseal junction specifically (FIGURE 1). Because this area receives its blood supply from small terminal vessels, fractures here have a high risk of non-union and, thus, should be top of mind in any patient with tenderness at the base of the fifth metatarsal.
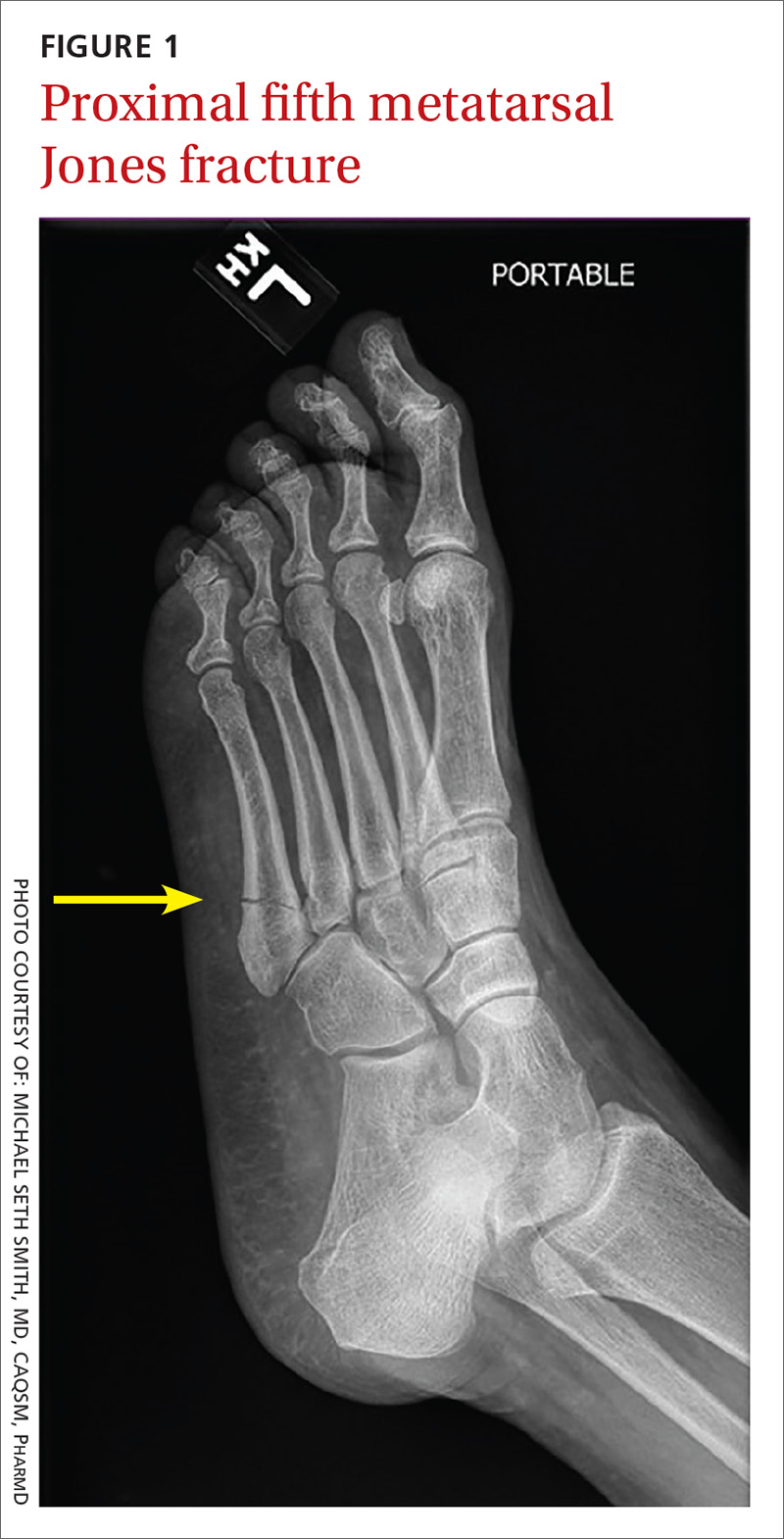
Continue to: MRI/ultrasound in addition to plain films?
MRI/ultrasound in addition to plain films?
Use the Ottawa Ankle and Foot Rules to determine the need for foot radiographs in the acute setting.12 If indicated, imaging should include AP, lateral, and oblique views of the foot.5 Consider magnetic resonance imaging (MRI) if you suspect a stress fracture, which typically presents as an overuse injury in athletes.
Ultrasound may be effective for identifying metatarsal fractures, as well.13 Ultrasound can be used to visualize fractures of the long metatarsal bones and identify displacement, angulation, and step-offs. Although its use in the United States has been limited for this purpose, studies in other countries are showing that it yields results comparable to plain films in the emergency department setting for diagnosis and initial management of these fractures.13
Differential diagnosis
Multiple other diagnoses present similarly to metatarsal fractures including: ligamentous/tendon/soft tissue injuries, interdigital neuroma, sesamoid fractures, and Lisfranc ligament injury (which we'll discuss in a bit). Stress fractures of the metatarsal, most commonly seen in the second metatarsal, are insidious in nature and are common with repetitive movement such as that made by gymnasts, dancers, and track athletes.14 Metatarsalgia, which causes pain at the ball of the foot, is a condition that can stem from a myriad of causes including a low or high arch, biomechanics, and previous injury.
Treatment
Nondisplaced, minimally displaced (<3 mm), and minimally angulated (<10° dorsal/plantar angulation) fractures of any metatarsal shaft may be managed conservatively with a hard-soled shoe or walking boot with weight bearing as tolerated for 4 to 6 weeks.1 Most stress fractures (excluding fifth metatarsal stress fractures) may be treated similarly. Surgical referral is indicated for patients with any open fracture, first metatarsal fractures with displacement, central metatarsal fractures with >10° deformity, >3 mm of translation, multiple fractures, or fifth metatarsal stress fracture.1
Base of fifth metatarsal tuberosity avulsion fractures can be managed nonoperatively with protected weight bearing in a boot or cast for 2 weeks or hard sole shoe for 4 to 8 weeks if the fracture is at the proximal tubercle (see Zone I, FIGURE 2). Jones fractures (Zone II, FIGURE 2) or fractures of the proximal diaphysis (Zone III, FIGURE 2) of the fifth metatarsal may also be managed nonoperatively in patients who are not competitive athletes. This can be done with a non-weight bearing (NWB) short leg cast for 6 to 8 weeks at a minimum.4 If the patient is an athlete who wishes to resume competition, surgical referral is indicated, as the risk of nonunion is high with these fractures.15
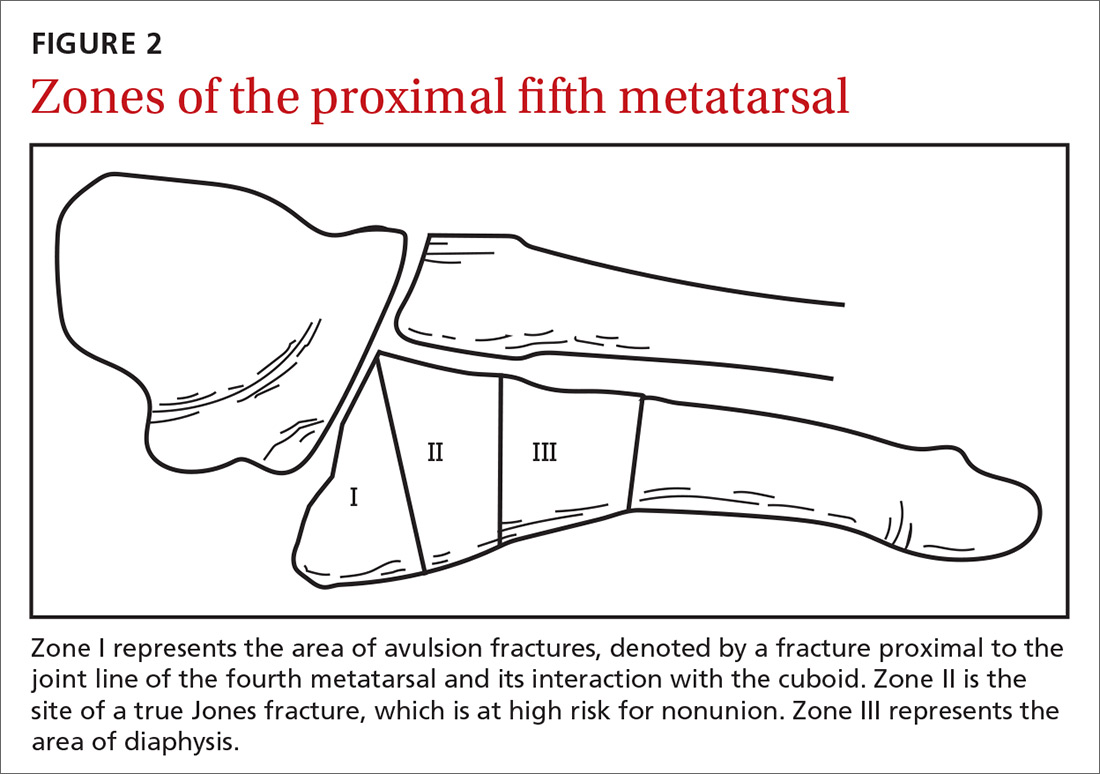
Continue to: Stress fractures and stress reactions...
Stress fractures and stress reactions (early evidence of bone edema seen on MRI, indicating progression to stress fracture) of the proximal fifth metatarsal should be managed with strict activity modifications and a NWB short leg cast for 6 to 8 weeks, given the high risk of nonunion.
Midfoot fractures: The cuboid, cuneiforms, and navicular bone
Although fractures are less common in the midfoot, the midfoot serves an important role in weight bearing and stabilization. In addition, along with the metatarsal bones, the midfoot is critical to proper alignment and articulation.
Cuboid and cuneiform fractures
Cuboid fractures may occur with high-velocity trauma, foot inversion with external rotation of the tibia, a direct blow, or axial load on a plantar-flexed heel. Pain with weight bearing or with walking on toes is usually present. Cuneiform fractures are less common and rarely occur in isolation.6 Mechanisms of injury for cuneiform fractures include direct impact, axial loading on a dorsiflexed or plantar-flexed foot with rotational force, and severe rotation of the midfoot section in a fixed foot. Pain is usually localized to the dorsal or dorsomedial aspect of the foot.
With these 2 midfoot injuries, the exam should include palpation of the cuboid, navicular, and cuneiform bones and careful inspection of the Lisfranc joint, as this can be injured in midfoot fractures.7 Obtain AP, lateral, and oblique views of the foot,5 and strongly consider bilateral weight-bearing films to evaluate for tarsometatarsal (TMT) joint complex injuries (typically Lisfranc joint complex injuries), given their association with midfoot fractures1 (FIGURE 3).
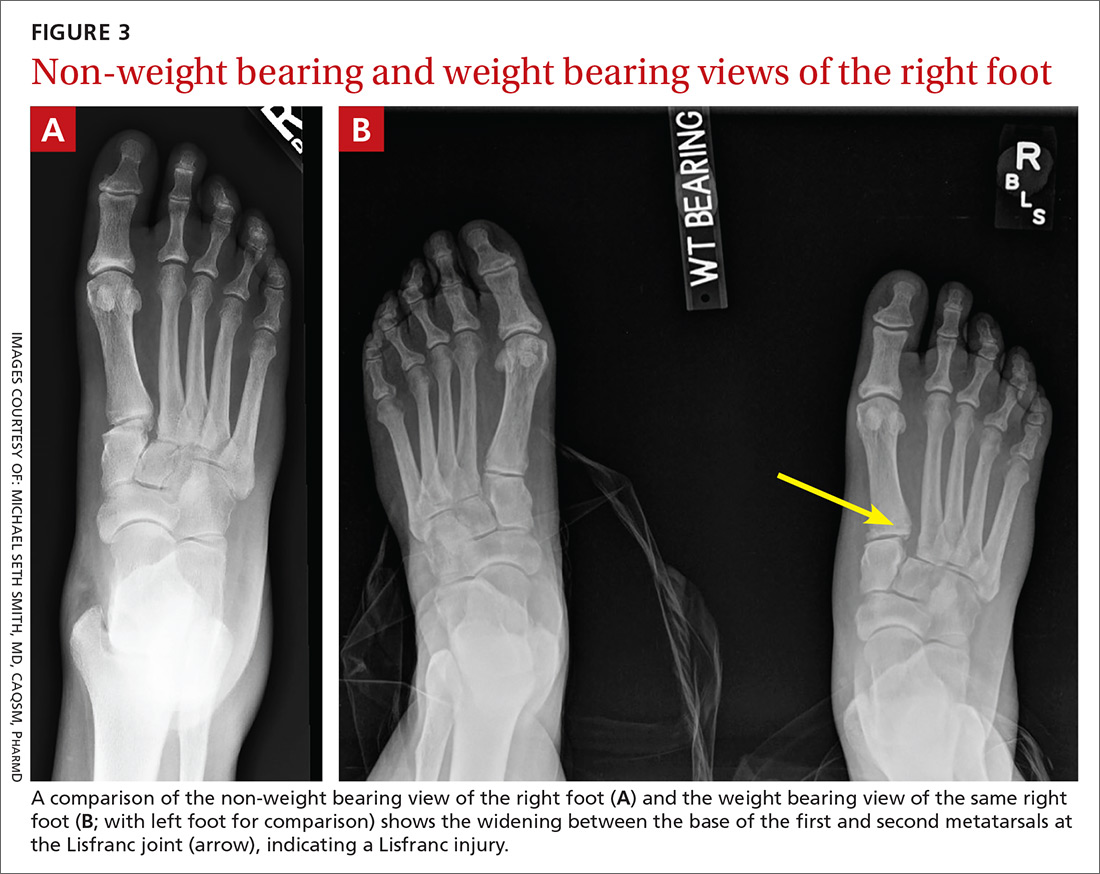
If the fracture is at or near the TMT joint complex, obtain a CT scan or MRI regardless of plain film findings to evaluate the Lisfranc joint complex. Because the Lisfranc joint is particularly important in midfoot stability, untreated injuries can lead to impaired gait or chronic foot pain and deformity. Early identification and surgical referral for these injuries is crucial.
Continue to: Navicular fractures
Navicular fractures
Navicular fractures are typically caused by a twisting mechanism with forced plantar flexion or forced dorsiflexion of the midfoot. They present with severe pain over the dorsal or dorsomedial foot, particularly while bearing weight. Tenderness to palpation over the navicular bone generally warrants imaging studies to rule out fracture, as undiagnosed fractures can lead to severe long-term disability. Use Ottawa Ankle and Foot Rules12 in the acute setting to determine the need for radiographs. If imaging is indicated, obtain AP, lateral, and oblique views.
Tuberosity fractures may be seen on the AP view, while dorsal avulsions, talonavicular joint disruptions, and naviculo-cuneiform joint injuries are better seen on lateral views.16 Patients, particularly cross-country and track athletes, presenting with insidious onset of pain over the navicular bone should be evaluated for stress fracture using MRI, even in the presence of normal radiographs.
Differential diagnosis. Suspect Lisfranc joint complex injuries in any patient with mid-foot pain or fracture. The transverse arch of the foot is reliant upon the articulation of the second metatarsal with all 5 neighboring bones. The Lisfranc ligament is the strongest of 3 supporting ligaments to anchor the TMT joint complex. Other causes of mid-foot pain include soft tissue injury, contusion, and tendinopathy. In addition, other conditions that may cause pain in this area include cuboid syndrome, peroneal tendinopathy, Jones fracture, stress fracture, anterior calcaneal fracture, and sinus tarsi syndrome.
Treatment. Nondisplaced fractures of the cuboid or cuneiform may be treated with a short leg walking cast/boot for 6 weeks followed by the use of a shoe with a thin, rigid, longitudinal arch support for an additional 6 weeks.1 Fractures requiring referral for surgical evaluation include fractures that are open and fractures with vascular or neurological compromise. Also refer comminuted fractures and those that present with >2 mm step-off. Lastly, midfoot fractures that involve the Lisfranc joint should be immobilized and referred for orthopedic evaluation with instructions to the patient to avoid weight bearing until orthopedic evaluation.8
Avulsion fractures of the navicular bone may be managed nonoperatively with a short leg walking cast/boot if there is <20% involvement of the talonavicular surface. Simple nondisplaced body fractures may also be managed conservatively with immobilization and protected weight bearing for 6 to 8 weeks.15 Refer for surgical evaluation avulsion fractures that are intra-articular or dorsal involving 20% or more of the talonavicular surface and tuberosity fractures, given their risk of nonunion.9 All navicular body fractures that are not longitudinal in nature should also be referred for surgical evaluation. Navicular stress fractures that do not extend into the plantar cortex may be managed conservatively with a minimum of 6 weeks of a short leg cast and strict NWB with close follow-up.17
Continue to: Calcaneal fractures
Calcaneal fractures
Calcaneal fractures typically occur from severe axial load or fall from a height. Weight bearing is usually limited and secondary to significant pain. Tenderness to palpation over the calcaneus or with squeezing of the heel will produce pain on exam. Initial x-rays should include a lateral and axial view of the calcaneus. Additional imaging, including a CT scan, may be indicated for further evaluation to determine the extent of the fracture or to determine if a fracture is present despite normal x-rays.10
Acute compartment syndrome occurs in 10% of calcaneal fractures and must be considered in patients with calcaneal fractures from severe trauma.18 Tendon injuries of the ankle, hindfoot, and midfoot may present similarly but can be ruled out with clinical exam and appropriate imaging.
Treatment
Avulsion fractures that do not involve more than 25% of the calcaneocuboid joint and nondisplaced calcaneal fractures may be managed conservatively by instructing patients to wear a NWB short leg cast/boot for 4 to 6 weeks.1 Refer for surgical evaluation patients with calcaneal fracture fragments >1 cm, displacement >3 mm, open fractures, joint involvement >25%, and those whose symptoms fail to resolve with conservative management. Stress fractures can be managed conservatively with cessation of aggravating activities and immobilization in a walking boot until symptoms resolve, which typically takes 4 to 6 weeks.1
CORRESPONDENCE
Michael Seth Smith, MD, CAQSM, PharmD, 3450 Hull Road, Gainesville, FL 32611; [email protected]
1. Eiff MP, Hatch RL, et al. Fracture Management for Primary Care. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012.
2. Hatch RL, Rosenbaum Cl. Fracture care by family physicians. A review of 295 cases. J Fam Pract. 1994;38:238-244.
3. Bica D, Sprouse RA, Armen J. Diagnosis and management of common foot fractures. Am Fam Physician. 2016;93:183-191.
4. Hatch RL, Hacking S. Evaluation and management of toe fractures. Am Fam Physician. 2003;68:2413-2418.
5. Snider RK. Essentials of Musculoskeletal Care. 2nd ed. Rosemont, IL: American Orthopedic Surgeons; 2001.
6. Guler F, Baz AB, Turan A, et al. Isolated medial cuneiform fractures: report of two cases and review of the literature. Foot Ankle Spec. 2011;4:306-309.
7. Borrelli J, De S, Van Pelt M. Fracture of the cuboid. J Am Acad Orthop Surg. 2012;20:472.
8. Pinney SJ, Sangeorzan BJ. Fractures of the tarsal bones. Orthop Clin North Am. 2001:32:21-33.
9. Rosenbaum AJ, Uhl R, DiPreta JA. Acute fractures of the tarsal navicular. Orthopedics. 2014;37:541-546.
10. Sanders RW, Clare MP. Calcaneous fractures. In: Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010:2064.
11. Singer G, Cichocki M, Schalamon J, et al. A study of metatarsal fractures in children. J Bone Joint Surg Am. 2008;90:772-776.
12. Stiell IG, Greenberg GH, McKnight RD, et al. A study to develop clinical decision rules for the use of radiography in acute ankle injuries. Ann Emerg Med. 1992;21:384-390.
13. Ekinci S, Polat O, Günalp M, et al. The accuracy of ultrasound evaluation in foot and ankle trauma. Am J Emerg Med. 2013;31:1551-1555.
14. Welck MJ, Hayes T, Pastides P, et al. Stress fractures of the foot and ankle. Injury. 2017;48:1722-1726.
15. Hatch RL, Alsobrook JA, Clugston JR. Diagnosis and management of metatarsal fractures. Am Fam Physician. 2007;76:817-826.
16. Schildhauer TA, Coulibaly MO, Hoffman MF. Fractures and dislocations of the midfoot and forefoot. In: Rockwood and Green’s Fractures in Adults. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2015:2690.
17. Mayer SW, Joyner PW, Almekinders LC, et al. Stress fractures of the foot and ankle in athletes. Sports Health. 2014;6:481-491.
18. Kalsi R, Dempsey A, Bunney EB. Compartment syndrome of the foot after calcaneal fracture. J Emerg Med. 2012;43:e101-106.
The evaluation and management of acute musculoskeletal conditions are frequently handled by primary care providers.1 It’s estimated that up to 14% of orthopedic complaints encountered by family physicians involve fractures,2 and approximately 15% of these are foot fractures.2 Diagnosis requires radiographic evaluation, but ultrasound is proving useful, too. This article reviews the diagnosis and management of adult foot fractures, with an emphasis on when advanced imaging and referral are indicated (TABLE1,3-10).

Phalanx fractures: The most common foot fractures
Phalanx fractures typically occur by crush injury, hyperextension, or direct axial force (eg, stubbing the toe).3 Patients with phalanx fractures typically present with pain at or near the site of injury, edema, ecchymosis, and erythema. Throbbing pain is characteristic, and dependent position may worsen the pain.1 Emergently evaluate any fracture causing tenting of the skin, protrusion from the skin, or neurovascular compromise, and attempt realignment to regain neurovascular function.

Most patients with phalanx fractures have point tenderness over the site of the fracture; however, this may also occur with contusions. Placing a gentle loading force along the long axis of the bone distal to the injury may help you differentiate between a contusion and a fracture.4 Pain observed with axial loading of the bone during examination points to a fracture rather than a contusion.
Differential diagnosis
Obtain imaging, including anterior-posterior (AP), lateral, and oblique views at a minimum, for all patients in whom you suspect fractures.5 Multiple fractures of the phalanges are common; therefore, always thoroughly examine the phalanges adjacent to the injured one.
Sesamoid bone fractures are uncommon but do occur and are usually due to direct injury from jumping or landing. The most common sesamoid to be injured is the medial sesamoid of the great toe, although the lateral sesamoid can also be injured. Bipartite sesamoids can occur and may confuse the examiner due to their similar appearance on x-rays to a sesamoid fracture.1 These normal variants often appear smooth and are commonly bilateral as opposed to the jagged or abrupt edges of a fracture. Stress fractures occur as well and are typically due to overuse-type injuries.
Other causes of pain similar to that experienced with phalanx fractures include soft tissue injuries to adjacent ligaments, tendons, and muscles. To help discern the cause of pain, evaluate nail beds for subungual hematomas, indicating injury to the nail bed causing bleeding and pressure under the nail. Obvious deformities of the toes or metatarsal-phalangeal joints signal the possibility of a fracture-dislocation. First metatarsophalangeal (MTP) sprain (“turf toe,” a condition common in athletes who hyperextend the toe, such as when pushing off from hard surfaces like turf) and gout can also present with acute pain in the first phalanx.
Treatment
Due to the role of the great toe in weight bearing and balance, great toe fractures are sometimes managed differently than fractures in Toes 2 through 5. Proper alignment and healing from a fracture in the first toe are critical to prevent future pain and other sequelae. Refer for orthopedic evaluation great toe fractures with displacement, angulation, rotational deformity, neurovascular compromise, >25% involvement of the joint space, or obvious dislocation.1 If referral is not indicated, treat great toe fractures with a short leg walking cast/boot for 2 to 3 weeks followed by buddy taping and use of a hard-soled shoe for 3 to 4 weeks.1
Continue to: With regard to the lesser toes...
With regard to the lesser toes, refer patients with fracture-dislocations, displaced intra-articular fractures, and fractures that do not reduce easily. Nondisplaced fractures of the lesser toes do not require surgical referral.1 These can be treated with splinting (buddy taping) and use of rigid-sole shoes for 4 to 6 weeks. Treatment duration depends largely on patient compliance; generally, continue treatment until point tenderness resolves.1
Treatment of sesamoid fractures consists of resting the affected foot with a walking boot, hard-soled shoe, or “donut” pad under the sesamoid bone to help distribute weight on the foot when standing. Length of treatment is approximately 6 to 8 weeks for most fractures.1 Consider surgical referral if nonoperative management is unsuccessful.
Metatarsal fractures: Look for malalignment
Metatarsal fractures account for 5% of all foot fractures encountered in primary care.2 These fractures typically occur as a result of falls, direct trauma, or rotational injuries (eg, ankle and foot sprains).1 In athletes, the most common cause of these fractures is high rotational force. Patients typically present with pain over the injury site, swelling, bruising, and pain with weight bearing.
As part of your exam, look for malalignment, rotational deformities, and evidence of open fracture. Palpation at the site of the fracture may increase the pain; however, as is true with phalanx fractures, contusions may also cause significant tenderness upon palpation. Also assess range of motion—with special attention to signs of malrotation—and evaluate the adjacent metatarsals, as multiple bones, ligaments, or both are often involved.1
Fifth metatarsal fractures are the most common in adults, likely because of decreased cortical thickness as compared with the other metatarsals.3,11 In addition, multiple soft tissue attachments connect at the proximal aspect of the fifth metatarsal. Classification of these types of metatarsal fractures is based on anatomic location.3 Jones fractures are one type of fracture at the proximal aspect of the fifth metatarsal that occur at the metaphyseal-diaphyseal junction specifically (FIGURE 1). Because this area receives its blood supply from small terminal vessels, fractures here have a high risk of non-union and, thus, should be top of mind in any patient with tenderness at the base of the fifth metatarsal.

Continue to: MRI/ultrasound in addition to plain films?
MRI/ultrasound in addition to plain films?
Use the Ottawa Ankle and Foot Rules to determine the need for foot radiographs in the acute setting.12 If indicated, imaging should include AP, lateral, and oblique views of the foot.5 Consider magnetic resonance imaging (MRI) if you suspect a stress fracture, which typically presents as an overuse injury in athletes.
Ultrasound may be effective for identifying metatarsal fractures, as well.13 Ultrasound can be used to visualize fractures of the long metatarsal bones and identify displacement, angulation, and step-offs. Although its use in the United States has been limited for this purpose, studies in other countries are showing that it yields results comparable to plain films in the emergency department setting for diagnosis and initial management of these fractures.13
Differential diagnosis
Multiple other diagnoses present similarly to metatarsal fractures including: ligamentous/tendon/soft tissue injuries, interdigital neuroma, sesamoid fractures, and Lisfranc ligament injury (which we'll discuss in a bit). Stress fractures of the metatarsal, most commonly seen in the second metatarsal, are insidious in nature and are common with repetitive movement such as that made by gymnasts, dancers, and track athletes.14 Metatarsalgia, which causes pain at the ball of the foot, is a condition that can stem from a myriad of causes including a low or high arch, biomechanics, and previous injury.
Treatment
Nondisplaced, minimally displaced (<3 mm), and minimally angulated (<10° dorsal/plantar angulation) fractures of any metatarsal shaft may be managed conservatively with a hard-soled shoe or walking boot with weight bearing as tolerated for 4 to 6 weeks.1 Most stress fractures (excluding fifth metatarsal stress fractures) may be treated similarly. Surgical referral is indicated for patients with any open fracture, first metatarsal fractures with displacement, central metatarsal fractures with >10° deformity, >3 mm of translation, multiple fractures, or fifth metatarsal stress fracture.1
Base of fifth metatarsal tuberosity avulsion fractures can be managed nonoperatively with protected weight bearing in a boot or cast for 2 weeks or hard sole shoe for 4 to 8 weeks if the fracture is at the proximal tubercle (see Zone I, FIGURE 2). Jones fractures (Zone II, FIGURE 2) or fractures of the proximal diaphysis (Zone III, FIGURE 2) of the fifth metatarsal may also be managed nonoperatively in patients who are not competitive athletes. This can be done with a non-weight bearing (NWB) short leg cast for 6 to 8 weeks at a minimum.4 If the patient is an athlete who wishes to resume competition, surgical referral is indicated, as the risk of nonunion is high with these fractures.15

Continue to: Stress fractures and stress reactions...
Stress fractures and stress reactions (early evidence of bone edema seen on MRI, indicating progression to stress fracture) of the proximal fifth metatarsal should be managed with strict activity modifications and a NWB short leg cast for 6 to 8 weeks, given the high risk of nonunion.
Midfoot fractures: The cuboid, cuneiforms, and navicular bone
Although fractures are less common in the midfoot, the midfoot serves an important role in weight bearing and stabilization. In addition, along with the metatarsal bones, the midfoot is critical to proper alignment and articulation.
Cuboid and cuneiform fractures
Cuboid fractures may occur with high-velocity trauma, foot inversion with external rotation of the tibia, a direct blow, or axial load on a plantar-flexed heel. Pain with weight bearing or with walking on toes is usually present. Cuneiform fractures are less common and rarely occur in isolation.6 Mechanisms of injury for cuneiform fractures include direct impact, axial loading on a dorsiflexed or plantar-flexed foot with rotational force, and severe rotation of the midfoot section in a fixed foot. Pain is usually localized to the dorsal or dorsomedial aspect of the foot.
With these 2 midfoot injuries, the exam should include palpation of the cuboid, navicular, and cuneiform bones and careful inspection of the Lisfranc joint, as this can be injured in midfoot fractures.7 Obtain AP, lateral, and oblique views of the foot,5 and strongly consider bilateral weight-bearing films to evaluate for tarsometatarsal (TMT) joint complex injuries (typically Lisfranc joint complex injuries), given their association with midfoot fractures1 (FIGURE 3).

If the fracture is at or near the TMT joint complex, obtain a CT scan or MRI regardless of plain film findings to evaluate the Lisfranc joint complex. Because the Lisfranc joint is particularly important in midfoot stability, untreated injuries can lead to impaired gait or chronic foot pain and deformity. Early identification and surgical referral for these injuries is crucial.
Continue to: Navicular fractures
Navicular fractures
Navicular fractures are typically caused by a twisting mechanism with forced plantar flexion or forced dorsiflexion of the midfoot. They present with severe pain over the dorsal or dorsomedial foot, particularly while bearing weight. Tenderness to palpation over the navicular bone generally warrants imaging studies to rule out fracture, as undiagnosed fractures can lead to severe long-term disability. Use Ottawa Ankle and Foot Rules12 in the acute setting to determine the need for radiographs. If imaging is indicated, obtain AP, lateral, and oblique views.
Tuberosity fractures may be seen on the AP view, while dorsal avulsions, talonavicular joint disruptions, and naviculo-cuneiform joint injuries are better seen on lateral views.16 Patients, particularly cross-country and track athletes, presenting with insidious onset of pain over the navicular bone should be evaluated for stress fracture using MRI, even in the presence of normal radiographs.
Differential diagnosis. Suspect Lisfranc joint complex injuries in any patient with mid-foot pain or fracture. The transverse arch of the foot is reliant upon the articulation of the second metatarsal with all 5 neighboring bones. The Lisfranc ligament is the strongest of 3 supporting ligaments to anchor the TMT joint complex. Other causes of mid-foot pain include soft tissue injury, contusion, and tendinopathy. In addition, other conditions that may cause pain in this area include cuboid syndrome, peroneal tendinopathy, Jones fracture, stress fracture, anterior calcaneal fracture, and sinus tarsi syndrome.
Treatment. Nondisplaced fractures of the cuboid or cuneiform may be treated with a short leg walking cast/boot for 6 weeks followed by the use of a shoe with a thin, rigid, longitudinal arch support for an additional 6 weeks.1 Fractures requiring referral for surgical evaluation include fractures that are open and fractures with vascular or neurological compromise. Also refer comminuted fractures and those that present with >2 mm step-off. Lastly, midfoot fractures that involve the Lisfranc joint should be immobilized and referred for orthopedic evaluation with instructions to the patient to avoid weight bearing until orthopedic evaluation.8
Avulsion fractures of the navicular bone may be managed nonoperatively with a short leg walking cast/boot if there is <20% involvement of the talonavicular surface. Simple nondisplaced body fractures may also be managed conservatively with immobilization and protected weight bearing for 6 to 8 weeks.15 Refer for surgical evaluation avulsion fractures that are intra-articular or dorsal involving 20% or more of the talonavicular surface and tuberosity fractures, given their risk of nonunion.9 All navicular body fractures that are not longitudinal in nature should also be referred for surgical evaluation. Navicular stress fractures that do not extend into the plantar cortex may be managed conservatively with a minimum of 6 weeks of a short leg cast and strict NWB with close follow-up.17
Continue to: Calcaneal fractures
Calcaneal fractures
Calcaneal fractures typically occur from severe axial load or fall from a height. Weight bearing is usually limited and secondary to significant pain. Tenderness to palpation over the calcaneus or with squeezing of the heel will produce pain on exam. Initial x-rays should include a lateral and axial view of the calcaneus. Additional imaging, including a CT scan, may be indicated for further evaluation to determine the extent of the fracture or to determine if a fracture is present despite normal x-rays.10
Acute compartment syndrome occurs in 10% of calcaneal fractures and must be considered in patients with calcaneal fractures from severe trauma.18 Tendon injuries of the ankle, hindfoot, and midfoot may present similarly but can be ruled out with clinical exam and appropriate imaging.
Treatment
Avulsion fractures that do not involve more than 25% of the calcaneocuboid joint and nondisplaced calcaneal fractures may be managed conservatively by instructing patients to wear a NWB short leg cast/boot for 4 to 6 weeks.1 Refer for surgical evaluation patients with calcaneal fracture fragments >1 cm, displacement >3 mm, open fractures, joint involvement >25%, and those whose symptoms fail to resolve with conservative management. Stress fractures can be managed conservatively with cessation of aggravating activities and immobilization in a walking boot until symptoms resolve, which typically takes 4 to 6 weeks.1
CORRESPONDENCE
Michael Seth Smith, MD, CAQSM, PharmD, 3450 Hull Road, Gainesville, FL 32611; [email protected]
The evaluation and management of acute musculoskeletal conditions are frequently handled by primary care providers.1 It’s estimated that up to 14% of orthopedic complaints encountered by family physicians involve fractures,2 and approximately 15% of these are foot fractures.2 Diagnosis requires radiographic evaluation, but ultrasound is proving useful, too. This article reviews the diagnosis and management of adult foot fractures, with an emphasis on when advanced imaging and referral are indicated (TABLE1,3-10).

Phalanx fractures: The most common foot fractures
Phalanx fractures typically occur by crush injury, hyperextension, or direct axial force (eg, stubbing the toe).3 Patients with phalanx fractures typically present with pain at or near the site of injury, edema, ecchymosis, and erythema. Throbbing pain is characteristic, and dependent position may worsen the pain.1 Emergently evaluate any fracture causing tenting of the skin, protrusion from the skin, or neurovascular compromise, and attempt realignment to regain neurovascular function.

Most patients with phalanx fractures have point tenderness over the site of the fracture; however, this may also occur with contusions. Placing a gentle loading force along the long axis of the bone distal to the injury may help you differentiate between a contusion and a fracture.4 Pain observed with axial loading of the bone during examination points to a fracture rather than a contusion.
Differential diagnosis
Obtain imaging, including anterior-posterior (AP), lateral, and oblique views at a minimum, for all patients in whom you suspect fractures.5 Multiple fractures of the phalanges are common; therefore, always thoroughly examine the phalanges adjacent to the injured one.
Sesamoid bone fractures are uncommon but do occur and are usually due to direct injury from jumping or landing. The most common sesamoid to be injured is the medial sesamoid of the great toe, although the lateral sesamoid can also be injured. Bipartite sesamoids can occur and may confuse the examiner due to their similar appearance on x-rays to a sesamoid fracture.1 These normal variants often appear smooth and are commonly bilateral as opposed to the jagged or abrupt edges of a fracture. Stress fractures occur as well and are typically due to overuse-type injuries.
Other causes of pain similar to that experienced with phalanx fractures include soft tissue injuries to adjacent ligaments, tendons, and muscles. To help discern the cause of pain, evaluate nail beds for subungual hematomas, indicating injury to the nail bed causing bleeding and pressure under the nail. Obvious deformities of the toes or metatarsal-phalangeal joints signal the possibility of a fracture-dislocation. First metatarsophalangeal (MTP) sprain (“turf toe,” a condition common in athletes who hyperextend the toe, such as when pushing off from hard surfaces like turf) and gout can also present with acute pain in the first phalanx.
Treatment
Due to the role of the great toe in weight bearing and balance, great toe fractures are sometimes managed differently than fractures in Toes 2 through 5. Proper alignment and healing from a fracture in the first toe are critical to prevent future pain and other sequelae. Refer for orthopedic evaluation great toe fractures with displacement, angulation, rotational deformity, neurovascular compromise, >25% involvement of the joint space, or obvious dislocation.1 If referral is not indicated, treat great toe fractures with a short leg walking cast/boot for 2 to 3 weeks followed by buddy taping and use of a hard-soled shoe for 3 to 4 weeks.1
Continue to: With regard to the lesser toes...
With regard to the lesser toes, refer patients with fracture-dislocations, displaced intra-articular fractures, and fractures that do not reduce easily. Nondisplaced fractures of the lesser toes do not require surgical referral.1 These can be treated with splinting (buddy taping) and use of rigid-sole shoes for 4 to 6 weeks. Treatment duration depends largely on patient compliance; generally, continue treatment until point tenderness resolves.1
Treatment of sesamoid fractures consists of resting the affected foot with a walking boot, hard-soled shoe, or “donut” pad under the sesamoid bone to help distribute weight on the foot when standing. Length of treatment is approximately 6 to 8 weeks for most fractures.1 Consider surgical referral if nonoperative management is unsuccessful.
Metatarsal fractures: Look for malalignment
Metatarsal fractures account for 5% of all foot fractures encountered in primary care.2 These fractures typically occur as a result of falls, direct trauma, or rotational injuries (eg, ankle and foot sprains).1 In athletes, the most common cause of these fractures is high rotational force. Patients typically present with pain over the injury site, swelling, bruising, and pain with weight bearing.
As part of your exam, look for malalignment, rotational deformities, and evidence of open fracture. Palpation at the site of the fracture may increase the pain; however, as is true with phalanx fractures, contusions may also cause significant tenderness upon palpation. Also assess range of motion—with special attention to signs of malrotation—and evaluate the adjacent metatarsals, as multiple bones, ligaments, or both are often involved.1
Fifth metatarsal fractures are the most common in adults, likely because of decreased cortical thickness as compared with the other metatarsals.3,11 In addition, multiple soft tissue attachments connect at the proximal aspect of the fifth metatarsal. Classification of these types of metatarsal fractures is based on anatomic location.3 Jones fractures are one type of fracture at the proximal aspect of the fifth metatarsal that occur at the metaphyseal-diaphyseal junction specifically (FIGURE 1). Because this area receives its blood supply from small terminal vessels, fractures here have a high risk of non-union and, thus, should be top of mind in any patient with tenderness at the base of the fifth metatarsal.

Continue to: MRI/ultrasound in addition to plain films?
MRI/ultrasound in addition to plain films?
Use the Ottawa Ankle and Foot Rules to determine the need for foot radiographs in the acute setting.12 If indicated, imaging should include AP, lateral, and oblique views of the foot.5 Consider magnetic resonance imaging (MRI) if you suspect a stress fracture, which typically presents as an overuse injury in athletes.
Ultrasound may be effective for identifying metatarsal fractures, as well.13 Ultrasound can be used to visualize fractures of the long metatarsal bones and identify displacement, angulation, and step-offs. Although its use in the United States has been limited for this purpose, studies in other countries are showing that it yields results comparable to plain films in the emergency department setting for diagnosis and initial management of these fractures.13
Differential diagnosis
Multiple other diagnoses present similarly to metatarsal fractures including: ligamentous/tendon/soft tissue injuries, interdigital neuroma, sesamoid fractures, and Lisfranc ligament injury (which we'll discuss in a bit). Stress fractures of the metatarsal, most commonly seen in the second metatarsal, are insidious in nature and are common with repetitive movement such as that made by gymnasts, dancers, and track athletes.14 Metatarsalgia, which causes pain at the ball of the foot, is a condition that can stem from a myriad of causes including a low or high arch, biomechanics, and previous injury.
Treatment
Nondisplaced, minimally displaced (<3 mm), and minimally angulated (<10° dorsal/plantar angulation) fractures of any metatarsal shaft may be managed conservatively with a hard-soled shoe or walking boot with weight bearing as tolerated for 4 to 6 weeks.1 Most stress fractures (excluding fifth metatarsal stress fractures) may be treated similarly. Surgical referral is indicated for patients with any open fracture, first metatarsal fractures with displacement, central metatarsal fractures with >10° deformity, >3 mm of translation, multiple fractures, or fifth metatarsal stress fracture.1
Base of fifth metatarsal tuberosity avulsion fractures can be managed nonoperatively with protected weight bearing in a boot or cast for 2 weeks or hard sole shoe for 4 to 8 weeks if the fracture is at the proximal tubercle (see Zone I, FIGURE 2). Jones fractures (Zone II, FIGURE 2) or fractures of the proximal diaphysis (Zone III, FIGURE 2) of the fifth metatarsal may also be managed nonoperatively in patients who are not competitive athletes. This can be done with a non-weight bearing (NWB) short leg cast for 6 to 8 weeks at a minimum.4 If the patient is an athlete who wishes to resume competition, surgical referral is indicated, as the risk of nonunion is high with these fractures.15

Continue to: Stress fractures and stress reactions...
Stress fractures and stress reactions (early evidence of bone edema seen on MRI, indicating progression to stress fracture) of the proximal fifth metatarsal should be managed with strict activity modifications and a NWB short leg cast for 6 to 8 weeks, given the high risk of nonunion.
Midfoot fractures: The cuboid, cuneiforms, and navicular bone
Although fractures are less common in the midfoot, the midfoot serves an important role in weight bearing and stabilization. In addition, along with the metatarsal bones, the midfoot is critical to proper alignment and articulation.
Cuboid and cuneiform fractures
Cuboid fractures may occur with high-velocity trauma, foot inversion with external rotation of the tibia, a direct blow, or axial load on a plantar-flexed heel. Pain with weight bearing or with walking on toes is usually present. Cuneiform fractures are less common and rarely occur in isolation.6 Mechanisms of injury for cuneiform fractures include direct impact, axial loading on a dorsiflexed or plantar-flexed foot with rotational force, and severe rotation of the midfoot section in a fixed foot. Pain is usually localized to the dorsal or dorsomedial aspect of the foot.
With these 2 midfoot injuries, the exam should include palpation of the cuboid, navicular, and cuneiform bones and careful inspection of the Lisfranc joint, as this can be injured in midfoot fractures.7 Obtain AP, lateral, and oblique views of the foot,5 and strongly consider bilateral weight-bearing films to evaluate for tarsometatarsal (TMT) joint complex injuries (typically Lisfranc joint complex injuries), given their association with midfoot fractures1 (FIGURE 3).

If the fracture is at or near the TMT joint complex, obtain a CT scan or MRI regardless of plain film findings to evaluate the Lisfranc joint complex. Because the Lisfranc joint is particularly important in midfoot stability, untreated injuries can lead to impaired gait or chronic foot pain and deformity. Early identification and surgical referral for these injuries is crucial.
Continue to: Navicular fractures
Navicular fractures
Navicular fractures are typically caused by a twisting mechanism with forced plantar flexion or forced dorsiflexion of the midfoot. They present with severe pain over the dorsal or dorsomedial foot, particularly while bearing weight. Tenderness to palpation over the navicular bone generally warrants imaging studies to rule out fracture, as undiagnosed fractures can lead to severe long-term disability. Use Ottawa Ankle and Foot Rules12 in the acute setting to determine the need for radiographs. If imaging is indicated, obtain AP, lateral, and oblique views.
Tuberosity fractures may be seen on the AP view, while dorsal avulsions, talonavicular joint disruptions, and naviculo-cuneiform joint injuries are better seen on lateral views.16 Patients, particularly cross-country and track athletes, presenting with insidious onset of pain over the navicular bone should be evaluated for stress fracture using MRI, even in the presence of normal radiographs.
Differential diagnosis. Suspect Lisfranc joint complex injuries in any patient with mid-foot pain or fracture. The transverse arch of the foot is reliant upon the articulation of the second metatarsal with all 5 neighboring bones. The Lisfranc ligament is the strongest of 3 supporting ligaments to anchor the TMT joint complex. Other causes of mid-foot pain include soft tissue injury, contusion, and tendinopathy. In addition, other conditions that may cause pain in this area include cuboid syndrome, peroneal tendinopathy, Jones fracture, stress fracture, anterior calcaneal fracture, and sinus tarsi syndrome.
Treatment. Nondisplaced fractures of the cuboid or cuneiform may be treated with a short leg walking cast/boot for 6 weeks followed by the use of a shoe with a thin, rigid, longitudinal arch support for an additional 6 weeks.1 Fractures requiring referral for surgical evaluation include fractures that are open and fractures with vascular or neurological compromise. Also refer comminuted fractures and those that present with >2 mm step-off. Lastly, midfoot fractures that involve the Lisfranc joint should be immobilized and referred for orthopedic evaluation with instructions to the patient to avoid weight bearing until orthopedic evaluation.8
Avulsion fractures of the navicular bone may be managed nonoperatively with a short leg walking cast/boot if there is <20% involvement of the talonavicular surface. Simple nondisplaced body fractures may also be managed conservatively with immobilization and protected weight bearing for 6 to 8 weeks.15 Refer for surgical evaluation avulsion fractures that are intra-articular or dorsal involving 20% or more of the talonavicular surface and tuberosity fractures, given their risk of nonunion.9 All navicular body fractures that are not longitudinal in nature should also be referred for surgical evaluation. Navicular stress fractures that do not extend into the plantar cortex may be managed conservatively with a minimum of 6 weeks of a short leg cast and strict NWB with close follow-up.17
Continue to: Calcaneal fractures
Calcaneal fractures
Calcaneal fractures typically occur from severe axial load or fall from a height. Weight bearing is usually limited and secondary to significant pain. Tenderness to palpation over the calcaneus or with squeezing of the heel will produce pain on exam. Initial x-rays should include a lateral and axial view of the calcaneus. Additional imaging, including a CT scan, may be indicated for further evaluation to determine the extent of the fracture or to determine if a fracture is present despite normal x-rays.10
Acute compartment syndrome occurs in 10% of calcaneal fractures and must be considered in patients with calcaneal fractures from severe trauma.18 Tendon injuries of the ankle, hindfoot, and midfoot may present similarly but can be ruled out with clinical exam and appropriate imaging.
Treatment
Avulsion fractures that do not involve more than 25% of the calcaneocuboid joint and nondisplaced calcaneal fractures may be managed conservatively by instructing patients to wear a NWB short leg cast/boot for 4 to 6 weeks.1 Refer for surgical evaluation patients with calcaneal fracture fragments >1 cm, displacement >3 mm, open fractures, joint involvement >25%, and those whose symptoms fail to resolve with conservative management. Stress fractures can be managed conservatively with cessation of aggravating activities and immobilization in a walking boot until symptoms resolve, which typically takes 4 to 6 weeks.1
CORRESPONDENCE
Michael Seth Smith, MD, CAQSM, PharmD, 3450 Hull Road, Gainesville, FL 32611; [email protected]
1. Eiff MP, Hatch RL, et al. Fracture Management for Primary Care. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012.
2. Hatch RL, Rosenbaum Cl. Fracture care by family physicians. A review of 295 cases. J Fam Pract. 1994;38:238-244.
3. Bica D, Sprouse RA, Armen J. Diagnosis and management of common foot fractures. Am Fam Physician. 2016;93:183-191.
4. Hatch RL, Hacking S. Evaluation and management of toe fractures. Am Fam Physician. 2003;68:2413-2418.
5. Snider RK. Essentials of Musculoskeletal Care. 2nd ed. Rosemont, IL: American Orthopedic Surgeons; 2001.
6. Guler F, Baz AB, Turan A, et al. Isolated medial cuneiform fractures: report of two cases and review of the literature. Foot Ankle Spec. 2011;4:306-309.
7. Borrelli J, De S, Van Pelt M. Fracture of the cuboid. J Am Acad Orthop Surg. 2012;20:472.
8. Pinney SJ, Sangeorzan BJ. Fractures of the tarsal bones. Orthop Clin North Am. 2001:32:21-33.
9. Rosenbaum AJ, Uhl R, DiPreta JA. Acute fractures of the tarsal navicular. Orthopedics. 2014;37:541-546.
10. Sanders RW, Clare MP. Calcaneous fractures. In: Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010:2064.
11. Singer G, Cichocki M, Schalamon J, et al. A study of metatarsal fractures in children. J Bone Joint Surg Am. 2008;90:772-776.
12. Stiell IG, Greenberg GH, McKnight RD, et al. A study to develop clinical decision rules for the use of radiography in acute ankle injuries. Ann Emerg Med. 1992;21:384-390.
13. Ekinci S, Polat O, Günalp M, et al. The accuracy of ultrasound evaluation in foot and ankle trauma. Am J Emerg Med. 2013;31:1551-1555.
14. Welck MJ, Hayes T, Pastides P, et al. Stress fractures of the foot and ankle. Injury. 2017;48:1722-1726.
15. Hatch RL, Alsobrook JA, Clugston JR. Diagnosis and management of metatarsal fractures. Am Fam Physician. 2007;76:817-826.
16. Schildhauer TA, Coulibaly MO, Hoffman MF. Fractures and dislocations of the midfoot and forefoot. In: Rockwood and Green’s Fractures in Adults. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2015:2690.
17. Mayer SW, Joyner PW, Almekinders LC, et al. Stress fractures of the foot and ankle in athletes. Sports Health. 2014;6:481-491.
18. Kalsi R, Dempsey A, Bunney EB. Compartment syndrome of the foot after calcaneal fracture. J Emerg Med. 2012;43:e101-106.
1. Eiff MP, Hatch RL, et al. Fracture Management for Primary Care. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012.
2. Hatch RL, Rosenbaum Cl. Fracture care by family physicians. A review of 295 cases. J Fam Pract. 1994;38:238-244.
3. Bica D, Sprouse RA, Armen J. Diagnosis and management of common foot fractures. Am Fam Physician. 2016;93:183-191.
4. Hatch RL, Hacking S. Evaluation and management of toe fractures. Am Fam Physician. 2003;68:2413-2418.
5. Snider RK. Essentials of Musculoskeletal Care. 2nd ed. Rosemont, IL: American Orthopedic Surgeons; 2001.
6. Guler F, Baz AB, Turan A, et al. Isolated medial cuneiform fractures: report of two cases and review of the literature. Foot Ankle Spec. 2011;4:306-309.
7. Borrelli J, De S, Van Pelt M. Fracture of the cuboid. J Am Acad Orthop Surg. 2012;20:472.
8. Pinney SJ, Sangeorzan BJ. Fractures of the tarsal bones. Orthop Clin North Am. 2001:32:21-33.
9. Rosenbaum AJ, Uhl R, DiPreta JA. Acute fractures of the tarsal navicular. Orthopedics. 2014;37:541-546.
10. Sanders RW, Clare MP. Calcaneous fractures. In: Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010:2064.
11. Singer G, Cichocki M, Schalamon J, et al. A study of metatarsal fractures in children. J Bone Joint Surg Am. 2008;90:772-776.
12. Stiell IG, Greenberg GH, McKnight RD, et al. A study to develop clinical decision rules for the use of radiography in acute ankle injuries. Ann Emerg Med. 1992;21:384-390.
13. Ekinci S, Polat O, Günalp M, et al. The accuracy of ultrasound evaluation in foot and ankle trauma. Am J Emerg Med. 2013;31:1551-1555.
14. Welck MJ, Hayes T, Pastides P, et al. Stress fractures of the foot and ankle. Injury. 2017;48:1722-1726.
15. Hatch RL, Alsobrook JA, Clugston JR. Diagnosis and management of metatarsal fractures. Am Fam Physician. 2007;76:817-826.
16. Schildhauer TA, Coulibaly MO, Hoffman MF. Fractures and dislocations of the midfoot and forefoot. In: Rockwood and Green’s Fractures in Adults. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2015:2690.
17. Mayer SW, Joyner PW, Almekinders LC, et al. Stress fractures of the foot and ankle in athletes. Sports Health. 2014;6:481-491.
18. Kalsi R, Dempsey A, Bunney EB. Compartment syndrome of the foot after calcaneal fracture. J Emerg Med. 2012;43:e101-106.
PRACTICE RECOMMENDATIONS
› Manage most fractures of the proximal fifth metatarsal metaphyseal-diaphyseal junction (Jones fracture) conservatively with appropriate treatment and close follow-up; refer for early surgical evaluation only those patients who are highly active and who are interested in a faster return to activity. B
› Use the Ottawa Ankle and Foot Rules to determine whether x-rays are needed in a patient with foot pain and a suspected fracture. A
› Start with weight-bearing x-rays and then consider computed tomography or magnetic resonance imaging for complete evaluation of suspected injury to the Lisfranc joint. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Obstructive sleep apnea: A better Dx model for primary care
ABSTRACT
Purpose To derive a predictive model for obstructive sleep apnea (OSA) in primary care practice, using home-based overnight oximetry results to refine posttest probability (PTP) of disease after initial risk stratification with the Sleep Apnea Clinical Score (SACS).
Methods We performed secondary analyses on data from a SACS validation cohort, to compare the diagnostic accuracy of 3 overnight oximetry measurements (oxygen desaturation index [ODI], mean saturation, and minimum saturation) in predicting OSA. Receiver operator characteristics (ROC) were computed for each measurement independently and sequentially after risk stratifying with SACS. We examined the implications of oximetry results for OSA PTP for participants categorized as intermediate risk (SACS 6-14; 66/191 participants [35%]; OSA probability 41%). We calculated positive likelihood ratios (LR) for multiple ODI results and determined which ones allowed recalibration to high- or low-risk PTP.
Results Among the 3 oximetry findings, ODI best predicted OSA (area under the curve [AUC], 0.88; 95% confidence interval [CI], 0.83-0.93). An ODI ≥8.4 (likelihood ratio [LR], 4.19; 95% CI, 2.87-6.10) created a PTP of 77%, while an ODI of 0 to <8.4 (LR, 0.19, 95% CI, 0.12-0.33) created a 14% PTP. Sequential application of SACS and ODI results yielded an AUC result of 0.90 (95% CI, 0.85-0.95).
Conclusions SACS risk stratification provides an advantage over clinical gestalt. In those at intermediate risk, ODI results provide a simple and clinically useful way to further refine diagnostic prediction. Sequential use of SACS and selectively employed overnight oximetry may limit unnecessary polysomnography. Oximetry testing should be avoided in patients deemed low or high risk by SACS, as positive results do not substantially recalibrate risk.
Obstructive sleep apnea (OSA) is a prevalent and underdiagnosed condition. The National Sleep Foundation estimates that 18 million Americans have OSA.1 Primary care practice may be the best setting in which to identify OSA, as many of our patients have conditions frequently associated with apnea (eg, hypertension, obesity, diabetes, arrhythmia, and neurologic illness). Up to a third of patients in primary care practice may be at increased risk.2,3
Clinical guidelines of the American Academy of Sleep Medicine (AASM) recommend obtaining a sleep history to evaluate for possible OSA in 3 instances: as part of a routine health maintenance examination, during evaluation of specific complaints associated with OSA (eg, snoring, apnea, daytime sleepiness), and during comprehensive evaluations for individuals with high-risk conditions (ie, obesity, congestive heart failure, refractory hypertension, diabetes, stroke history).4
The American College of Physicians (ACP) Clinical Practice Guideline suggests assessing individuals who have unexplained daytime sleepiness.5 The ACP considers this assessment “High-Value Care,” as “evidence shows that before diagnosis, patients with OSA have higher rates of health care use, more frequent and longer hospital stays, and higher health care costs than after diagnosis.”5
Continue to: We recently validated the diagnostic accuracy...
We recently validated the diagnostic accuracy of the Sleep Apnea Clinical Score (SACS) for use in a primary care patient population suspected of having OSA.6 SACS uses historical and clinical data to derive a score that identifies a patient’s risk level.7 However, as an alternative to the 2 levels described in Flemons’ SACS,7 we propose creating 3 risk strata (FIGURE 17,8). We believe that patients at high risk (SACS ≥15) should be encouraged to undergo sleep evaluations as their posttest probability (PTP) of OSA is 75% to 80%. Individuals at low risk (SACS ≤5; PTP <20%) could receive lifestyle advice and simple clinical interventions that decrease symptoms (eg, weight loss, increased physical activity, sleeping on one’s side). For low-risk patients, clinical observation and reevaluation could take place over time with their primary care provider, without additional testing or referral to specialists.
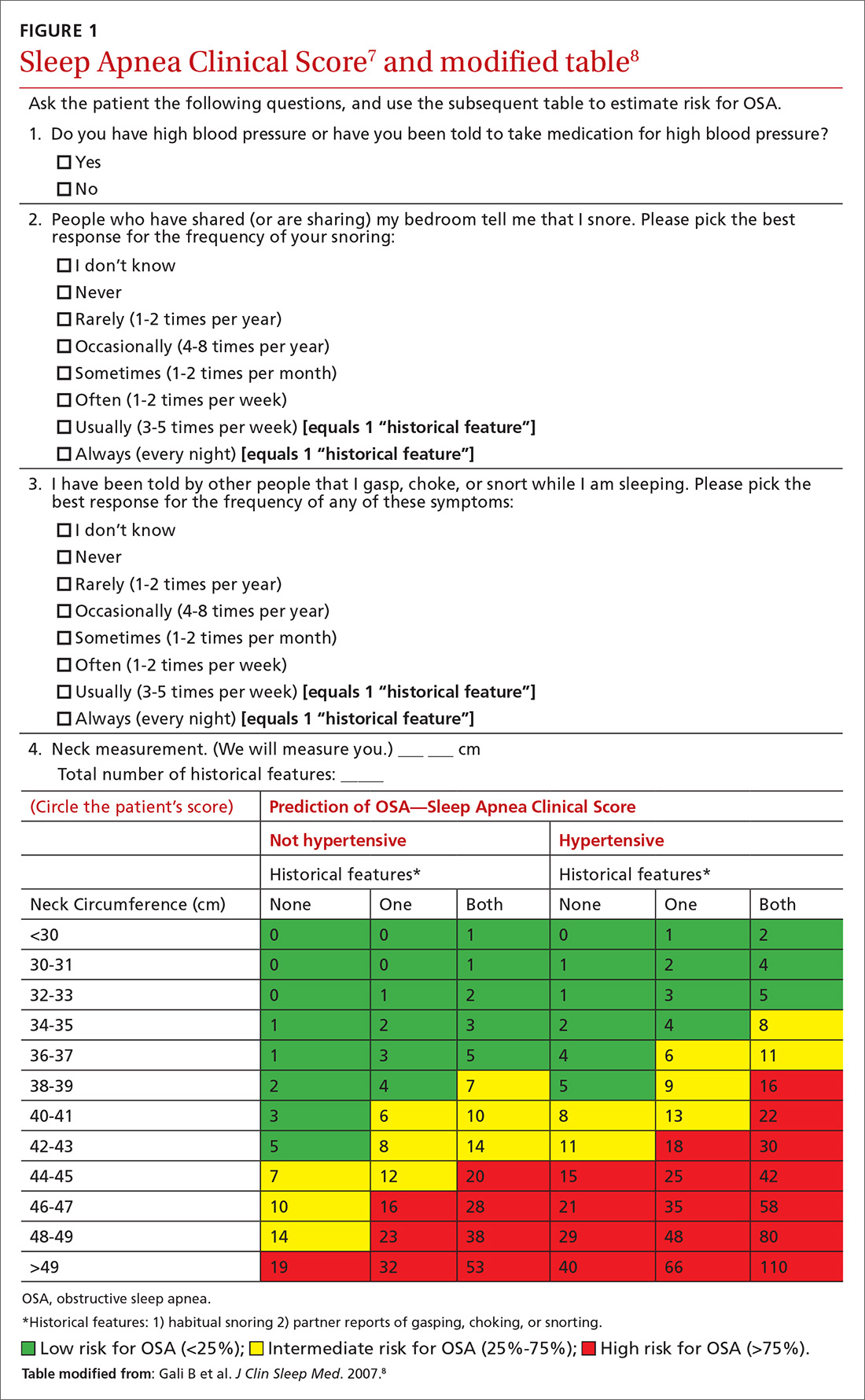
What about patients at intermediate risk? Many patients suspected of having OSA will be assigned to intermediate risk (SACS 6-14), and their PTP of OSA remains at 40% to 45%, the pre-test level most commonly encountered in suspected OSA. As polysomnography is a limited and expensive clinical resource, intermediate-risk patients would benefit from recalibration of their SACS-based risk assessment using an additional surrogate test such as home-based overnight oximetry. Our internal OSA practice guidelines recommend referral for sleep medicine consultation when oximetry results are abnormal—specifically, an oxygen desaturation index (ODI) of ≥5, a mean saturation less than 89%, and a minimum saturation of 75% or less.
Our objectives in this study were to compare the diagnostic implications of these 3 measurements from home-based overnight oximetry reports and use the most relevant result to derive a predictive model further refining PTP of OSA in a primary care patient population first stratified to intermediate risk by SACS.
METHODS
Subjects
We performed secondary analyses on data obtained from our SACS validation cohort.6 In brief, these were patients suspected of having OSA based on the presence of signs, symptoms, or associated risk factors. One hundred ninety-one patients completed all assessments. Sixty-six of 191 patients (35%) were categorized as intermediate risk (SACS 6-14; OSA probability 41% [27/66]).
Data collection and analyses
Participants completed home-based overnight oximetry using Nonin Model 2500 oximeters (Nonin Medical Inc., Plymouth, Minn). We transferred oximetry results from the sleep lab database to a statistical program for analyses of ODI, mean saturation, and minimal saturation. ODI was defined as the number of 4% drops in saturation from baseline divided by the number of hours of recording time. Although the AASM states that a diagnosis of OSA is confirmed if the number of obstructive events is more than 15 per hour or more than 5 per hour in a patient who reports related symptoms,4 we defined OSA as an apnea-hypopnea index (AHI) of >10 based on polysomnography (as this was the threshold used in the derivation cohort for SACS).7 We demonstrated the predictive ability of SACS at various AHI definitions of OSA in our validation cohort.6 The use of SACS in our validation cohort showed a statistically similar ability to predict OSA at both an AHI of 10 and 20, compared with the derivation cohort.
Continue to: We entered additional information...
We entered additional information reported directly by patients and obtained from their sleep studies into a REDCap database and transferred that to our statistical program. We used descriptive statistics to determine ranges and central tendencies of oximetry results. Receiver operator characteristic (ROC) analyses described the predictive abilities for each oximetry result individually and in serial application with prior SACS determinations. For comparison, we used the area under the ROC curve (AUC) from logistic regression to model the probability of OSA.
We calculated positive likelihood ratios (LR) and 95% confidence intervals (CI) to determine the degree of oximetry abnormality that would recalibrate risk either to a high PTP of OSA (>75%) or a low PTP (<25%). We sorted intermediate-risk SACS scores into quintiles based on ODI results to compare the resulting PTPs of OSA. We applied the PTP of OSA from our previous work (using the SACS score to compute the LR) as the new PTP, estimated the LR based on ODI, and computed an updated PTP of OSA. We also used ROC analysis to determine the optimal cutoff value of the ODI.
Finally, in accordance with our internal clinical practice recommendations, we examined the predictive ability of a “positive” ODI result of ≥5 to recalibrate risk prediction for OSA for patients in the low-risk group. We performed analyses using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
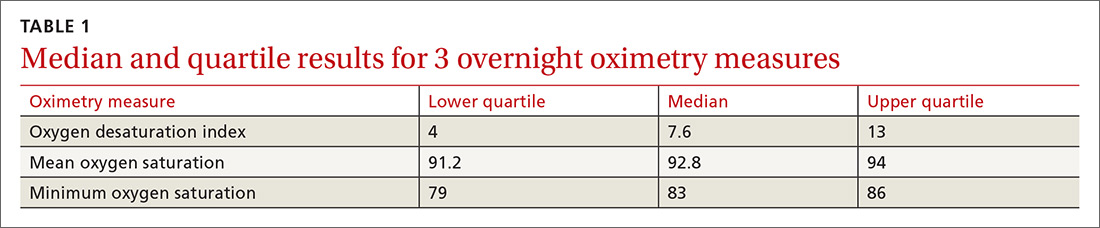
One hundred ninety-one subjects completed assessments. The median and quartile results for ODI, mean saturation, and minimum saturation are found in TABLE 1. TABLE 2 shows the distribution of patients with positive oximetry results. An ODI of 5 or greater was the most frequent abnormal result (135/191; 70.7%).
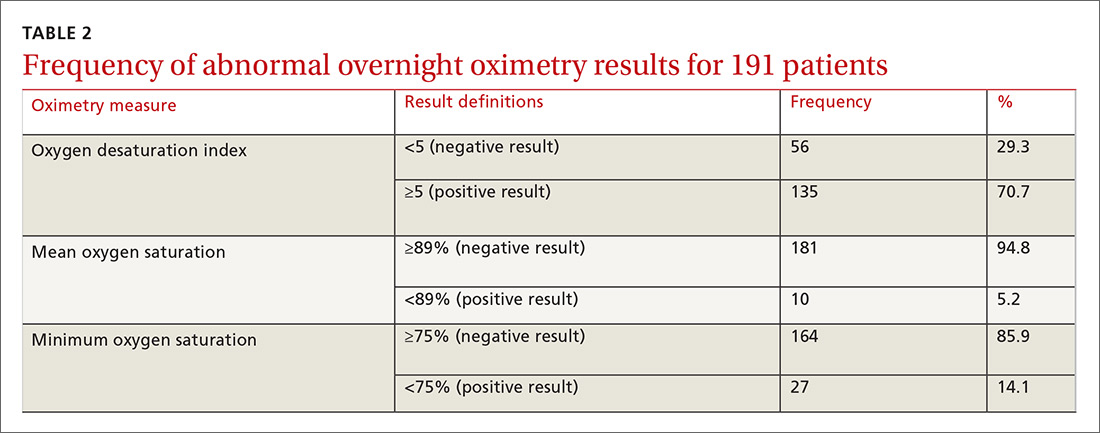
We used the AUC to measure the comparative abilities of SACS and the 3 overnight oximetry results in predicting OSA (TABLE 3). ODI results demonstrated the best ability to predict OSA, compared with polysomnography as the relative gold standard (AUC, 0.88; 95% confidence interval [CI], 0.83-0.93). Serial application of SACS and ODI yielded even better diagnostic results (AUC, 0.90; 95% CI, 0.85-0.95).
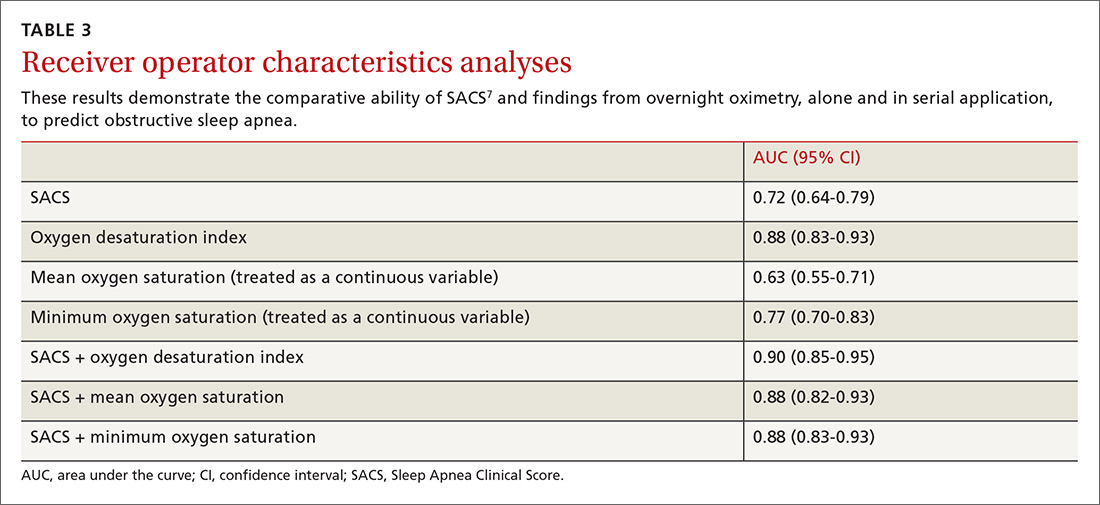
Continue to: As ODI was found to be the strongest predictor of OSA...
As ODI was found to be the strongest predictor of OSA, we grouped these results in quintiles and calculated positive LRs. TABLE 4 shows their effect on PTP of disease among patients with intermediate risk. An ODI result >10 effected an upward recalibration of disease probability (LR, 2.33; 95% CI, 1.27-4.26). The optimal cutoff of ODI to discriminate between those with and without OSA was determined by ROC analysis. An ODI greater than 8.4 created a PTP of disease of approximately 73% to 77%.
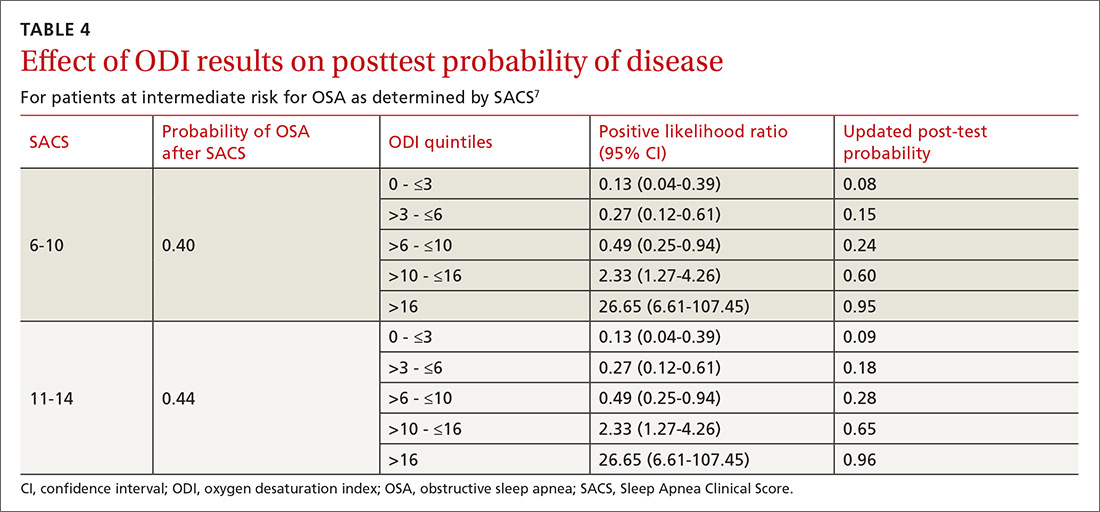
Our internal clinical guidelines recommend referring patients with an ODI of 5 or greater for sleep medicine consultation. We examined the ability of this ODI result to recalibrate disease suspicion for a patient at low risk (SACS ≤5). The LR for ODI of 5 or greater is 2.1, but this only results in a recalibration of risk from 24% pretest probability in our validation cohort to 41% PTP (95% CI, 33-49). This low cutoff for a positive test creates false-positive results more than 40% of the time due to low specificity (0.58). This is insufficient to change the suspicion of disease, resulting only in a shift to intermediate OSA risk.
DISCUSSION
Among 3 different oximetry measurements, an ODI ≥10 best predicts OSA, both independently and when used sequentially after the SACS. ODI was by far the most frequent abnormality on oximetry in our cohort, thereby increasing its utility in clinical decision making. For those subjects at intermediate risk, a cutoff of 10 for the ODI result may be a simple and clinically effective way to recalibrate risk and aid in making referral decisions. (This may also be simpler and more easily remembered by clinicians than the 8.4 ODI results from the ROC analyses.)
Assessment is inadequate without a clinical prediction rule. Unfortunately, providers cannot simply rely on clinical gestalt in diagnosing OSA. In their derivation cohort, Flemens et al examined the LRs created by SACS and by clinician prediction based on history and physical exam.7 The SACS LRs ranged from 5.17 to 0.25, a 20-fold range. This reflected superior diagnostic information compared with subjective physician impression, where LRs ranged from 3.7 to 0.52, a seven-fold range. Myers et al prepared a meta-analysis of 4 different trials that examined physicians’ ability to predict OSA.9 Despite the researchers’ use of experienced sleep medicine doctors, the overall diagnostic accuracy of clinical impression was modest (summary positive LR, 1.7; 95% CI, 1.5-2; I2 = 0%; summary negative LR, 0.67; 95% CI, 0.60-0.74; I2 = 10%; sensitivity, 58%; specificity, 67%). This is similar to reliance on a single clinical sign or symptom to predict OSA.
Wise use of oximetry augments SACS calculation. To limit unnecessary oximetry testing in low- and high-risk groups and to avoid polysomnography in cases of a low PTP of disease, we advocate limiting oximetry testing to individuals in the SACS intermediate-risk group (FIGURE 2) wherein ODI results can potentially recalibrate risk assessment up or down. (Those in the high- risk group should be referred to a sleep medicine specialist.) Our institutional recommendation of using an ODI result of ≥5 as a threshold to increase suspicion of disease requires a caveat for the low-risk group. “Positive” results at that low diagnostic threshold are frequently false.
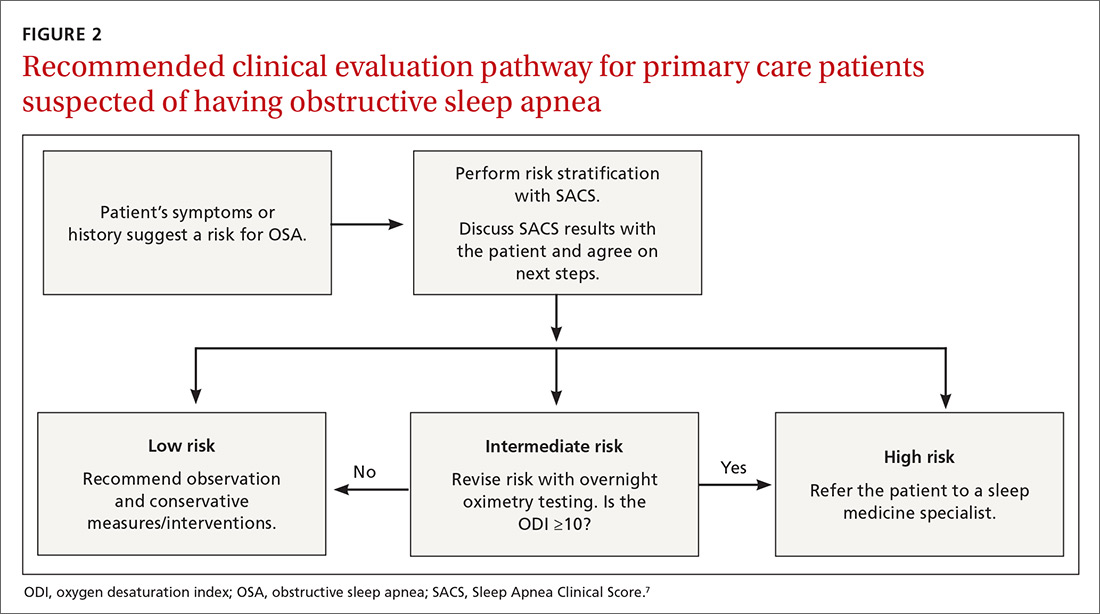
Continue to: Multiple benefits of SACS
Multiple benefits of SACS. We believe using the SACS calculation during clinical encounters with patients potentially at risk for OSA would increase diagnostic accuracy. Performing risk stratification with SACS should not be an undue burden on providers, and the increased time spent with patients has its own benefits, including helping them better understand their risk. Using this standardized process—augmented, as needed, with overnight ODI assessment—might also encourage more patients to follow through on subsequent recommendations, as their risk is further quantified objectively. Lastly, unnecessary testing with polysomnography could be avoided.
Limitations of our study. This study’s findings were derived from a patient population in a single institution. Replication of the findings from other settings would be helpful.
Looking forward. It is yet unclear if clinicians will embrace these strategies in real-world primary care practice. We have designed an implementation-and-dissemination trial to assess whether family physicians will use the SACS clinical predication rule in everyday practice and whether our evidence-based recommendations about overnight oximetry will be followed. Underlying our suggested clinical evaluation pathway (FIGURE 2) is the belief that there is value gained from sharing the decision-making process with patients. Although we provide new evidence that informs these conversations, the patient’s values and preferences are important when determining the best direction to proceed in the evaluation for suspected OSA. These recommendations are intended to aid, not replace, good clinical judgment.
Home-based sleep testing has become more widely available, is convenient for patients, and is less expensive than lab-based polysomnography. Our study did not directly address the appropriate circumstances for home studies in clinical evaluation. We rely on the expertise of our sleep medicine colleagues to determine which patients are appropriate candidates for home-based studies.
The AASM states that “portable monitors (PM) for the diagnosis of OSA should be [used] only in conjunction with a comprehensive sleep evaluation. Clinical sleep evaluations using PM must be supervised by a practitioner with board certification in sleep medicine or an individual who fulfills the eligibility criteria for the sleep medicine certification examination.”4 Additionally, the group recommends that PM “may be used in the unattended setting as an alternative to polysomnography for the diagnosis of OSA in patients with a high pretest probability of moderate to severe OSA and no comorbid sleep disorder or major comorbid medical disorders.”4
Continue to: GRANT SUPPORT
GRANT SUPPORT
The use of the REDCap database is supported by grant UL1 TR000135. This work was supported by a Mayo Foundation CR-20 grant awarded to Dr. Mookadam as Principal investigator and Dr. Grover as Coinvestigator.
Statistical analyses were supported, in part, by the Department of Family Medicine, Mayo Clinic, Scottsdale, Ariz.
CORRESPONDENCE
Michael Grover, DO, Mayo Clinic Thunderbird Primary Care Center-Family Medicine, 13737 N 92nd Street, Scottsdale, AZ 85260; [email protected]
1. National Sleep Foundation. Sleep apnea. https://sleepfoundation.org/sleep-disorders-problems/sleep-apnea. Accessed September 14, 2018.
2. Grover M, Mookadam M, Armas D, et al. Identifying patients at risk for obstructive sleep apnea in a primary care practice. J Am Board Fam Med. 2011;24:152-160.
3. Mold JW, Quattlebaum C, Schinnerer E, et al. Identification by primary care clinicians of patients with obstructive sleep apnea: a practice-based research network (PBRN) study. J Am Board Fam Med. 2011;24:138-145.
4. Epstein LJ, Kristo D, Strollo PJ, Jr., et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276.
5. Qaseem A, Dallas P, Owens DK, et al. Diagnosis of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2014;161:210-220.
6. Grover M, Mookadam M, Chang Y-H, et al. Validating the Sleep Apnea Clinical Score for use in primary care populations. Mayo Clin Proc. 2016;91:469-476.
7. Flemons WW, Whitelaw WA, Brant R, et al. Likelihood ratios for a sleep apnea clinical prediction rule. Am J Respir Crit Care Med. 1994;150:1279-1285.
8. Gali B, Whalen FX, Gay PC, et al. Management plan to reduce risks in perioperative care of patients with presumed obstructive sleep apnea syndrome. J Clin Sleep Med. 2007;3:582-588.
9. Myers KA, Mrkobrada M, Simel DL. Does this patient have obstructive sleep apnea?: The rational clinical examination systematic review. JAMA. 2013;310(7):731-741.
ABSTRACT
Purpose To derive a predictive model for obstructive sleep apnea (OSA) in primary care practice, using home-based overnight oximetry results to refine posttest probability (PTP) of disease after initial risk stratification with the Sleep Apnea Clinical Score (SACS).
Methods We performed secondary analyses on data from a SACS validation cohort, to compare the diagnostic accuracy of 3 overnight oximetry measurements (oxygen desaturation index [ODI], mean saturation, and minimum saturation) in predicting OSA. Receiver operator characteristics (ROC) were computed for each measurement independently and sequentially after risk stratifying with SACS. We examined the implications of oximetry results for OSA PTP for participants categorized as intermediate risk (SACS 6-14; 66/191 participants [35%]; OSA probability 41%). We calculated positive likelihood ratios (LR) for multiple ODI results and determined which ones allowed recalibration to high- or low-risk PTP.
Results Among the 3 oximetry findings, ODI best predicted OSA (area under the curve [AUC], 0.88; 95% confidence interval [CI], 0.83-0.93). An ODI ≥8.4 (likelihood ratio [LR], 4.19; 95% CI, 2.87-6.10) created a PTP of 77%, while an ODI of 0 to <8.4 (LR, 0.19, 95% CI, 0.12-0.33) created a 14% PTP. Sequential application of SACS and ODI results yielded an AUC result of 0.90 (95% CI, 0.85-0.95).
Conclusions SACS risk stratification provides an advantage over clinical gestalt. In those at intermediate risk, ODI results provide a simple and clinically useful way to further refine diagnostic prediction. Sequential use of SACS and selectively employed overnight oximetry may limit unnecessary polysomnography. Oximetry testing should be avoided in patients deemed low or high risk by SACS, as positive results do not substantially recalibrate risk.
Obstructive sleep apnea (OSA) is a prevalent and underdiagnosed condition. The National Sleep Foundation estimates that 18 million Americans have OSA.1 Primary care practice may be the best setting in which to identify OSA, as many of our patients have conditions frequently associated with apnea (eg, hypertension, obesity, diabetes, arrhythmia, and neurologic illness). Up to a third of patients in primary care practice may be at increased risk.2,3
Clinical guidelines of the American Academy of Sleep Medicine (AASM) recommend obtaining a sleep history to evaluate for possible OSA in 3 instances: as part of a routine health maintenance examination, during evaluation of specific complaints associated with OSA (eg, snoring, apnea, daytime sleepiness), and during comprehensive evaluations for individuals with high-risk conditions (ie, obesity, congestive heart failure, refractory hypertension, diabetes, stroke history).4
The American College of Physicians (ACP) Clinical Practice Guideline suggests assessing individuals who have unexplained daytime sleepiness.5 The ACP considers this assessment “High-Value Care,” as “evidence shows that before diagnosis, patients with OSA have higher rates of health care use, more frequent and longer hospital stays, and higher health care costs than after diagnosis.”5
Continue to: We recently validated the diagnostic accuracy...
We recently validated the diagnostic accuracy of the Sleep Apnea Clinical Score (SACS) for use in a primary care patient population suspected of having OSA.6 SACS uses historical and clinical data to derive a score that identifies a patient’s risk level.7 However, as an alternative to the 2 levels described in Flemons’ SACS,7 we propose creating 3 risk strata (FIGURE 17,8). We believe that patients at high risk (SACS ≥15) should be encouraged to undergo sleep evaluations as their posttest probability (PTP) of OSA is 75% to 80%. Individuals at low risk (SACS ≤5; PTP <20%) could receive lifestyle advice and simple clinical interventions that decrease symptoms (eg, weight loss, increased physical activity, sleeping on one’s side). For low-risk patients, clinical observation and reevaluation could take place over time with their primary care provider, without additional testing or referral to specialists.

What about patients at intermediate risk? Many patients suspected of having OSA will be assigned to intermediate risk (SACS 6-14), and their PTP of OSA remains at 40% to 45%, the pre-test level most commonly encountered in suspected OSA. As polysomnography is a limited and expensive clinical resource, intermediate-risk patients would benefit from recalibration of their SACS-based risk assessment using an additional surrogate test such as home-based overnight oximetry. Our internal OSA practice guidelines recommend referral for sleep medicine consultation when oximetry results are abnormal—specifically, an oxygen desaturation index (ODI) of ≥5, a mean saturation less than 89%, and a minimum saturation of 75% or less.
Our objectives in this study were to compare the diagnostic implications of these 3 measurements from home-based overnight oximetry reports and use the most relevant result to derive a predictive model further refining PTP of OSA in a primary care patient population first stratified to intermediate risk by SACS.
METHODS
Subjects
We performed secondary analyses on data obtained from our SACS validation cohort.6 In brief, these were patients suspected of having OSA based on the presence of signs, symptoms, or associated risk factors. One hundred ninety-one patients completed all assessments. Sixty-six of 191 patients (35%) were categorized as intermediate risk (SACS 6-14; OSA probability 41% [27/66]).
Data collection and analyses
Participants completed home-based overnight oximetry using Nonin Model 2500 oximeters (Nonin Medical Inc., Plymouth, Minn). We transferred oximetry results from the sleep lab database to a statistical program for analyses of ODI, mean saturation, and minimal saturation. ODI was defined as the number of 4% drops in saturation from baseline divided by the number of hours of recording time. Although the AASM states that a diagnosis of OSA is confirmed if the number of obstructive events is more than 15 per hour or more than 5 per hour in a patient who reports related symptoms,4 we defined OSA as an apnea-hypopnea index (AHI) of >10 based on polysomnography (as this was the threshold used in the derivation cohort for SACS).7 We demonstrated the predictive ability of SACS at various AHI definitions of OSA in our validation cohort.6 The use of SACS in our validation cohort showed a statistically similar ability to predict OSA at both an AHI of 10 and 20, compared with the derivation cohort.
Continue to: We entered additional information...
We entered additional information reported directly by patients and obtained from their sleep studies into a REDCap database and transferred that to our statistical program. We used descriptive statistics to determine ranges and central tendencies of oximetry results. Receiver operator characteristic (ROC) analyses described the predictive abilities for each oximetry result individually and in serial application with prior SACS determinations. For comparison, we used the area under the ROC curve (AUC) from logistic regression to model the probability of OSA.
We calculated positive likelihood ratios (LR) and 95% confidence intervals (CI) to determine the degree of oximetry abnormality that would recalibrate risk either to a high PTP of OSA (>75%) or a low PTP (<25%). We sorted intermediate-risk SACS scores into quintiles based on ODI results to compare the resulting PTPs of OSA. We applied the PTP of OSA from our previous work (using the SACS score to compute the LR) as the new PTP, estimated the LR based on ODI, and computed an updated PTP of OSA. We also used ROC analysis to determine the optimal cutoff value of the ODI.
Finally, in accordance with our internal clinical practice recommendations, we examined the predictive ability of a “positive” ODI result of ≥5 to recalibrate risk prediction for OSA for patients in the low-risk group. We performed analyses using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS

One hundred ninety-one subjects completed assessments. The median and quartile results for ODI, mean saturation, and minimum saturation are found in TABLE 1. TABLE 2 shows the distribution of patients with positive oximetry results. An ODI of 5 or greater was the most frequent abnormal result (135/191; 70.7%).

We used the AUC to measure the comparative abilities of SACS and the 3 overnight oximetry results in predicting OSA (TABLE 3). ODI results demonstrated the best ability to predict OSA, compared with polysomnography as the relative gold standard (AUC, 0.88; 95% confidence interval [CI], 0.83-0.93). Serial application of SACS and ODI yielded even better diagnostic results (AUC, 0.90; 95% CI, 0.85-0.95).

Continue to: As ODI was found to be the strongest predictor of OSA...
As ODI was found to be the strongest predictor of OSA, we grouped these results in quintiles and calculated positive LRs. TABLE 4 shows their effect on PTP of disease among patients with intermediate risk. An ODI result >10 effected an upward recalibration of disease probability (LR, 2.33; 95% CI, 1.27-4.26). The optimal cutoff of ODI to discriminate between those with and without OSA was determined by ROC analysis. An ODI greater than 8.4 created a PTP of disease of approximately 73% to 77%.

Our internal clinical guidelines recommend referring patients with an ODI of 5 or greater for sleep medicine consultation. We examined the ability of this ODI result to recalibrate disease suspicion for a patient at low risk (SACS ≤5). The LR for ODI of 5 or greater is 2.1, but this only results in a recalibration of risk from 24% pretest probability in our validation cohort to 41% PTP (95% CI, 33-49). This low cutoff for a positive test creates false-positive results more than 40% of the time due to low specificity (0.58). This is insufficient to change the suspicion of disease, resulting only in a shift to intermediate OSA risk.
DISCUSSION
Among 3 different oximetry measurements, an ODI ≥10 best predicts OSA, both independently and when used sequentially after the SACS. ODI was by far the most frequent abnormality on oximetry in our cohort, thereby increasing its utility in clinical decision making. For those subjects at intermediate risk, a cutoff of 10 for the ODI result may be a simple and clinically effective way to recalibrate risk and aid in making referral decisions. (This may also be simpler and more easily remembered by clinicians than the 8.4 ODI results from the ROC analyses.)
Assessment is inadequate without a clinical prediction rule. Unfortunately, providers cannot simply rely on clinical gestalt in diagnosing OSA. In their derivation cohort, Flemens et al examined the LRs created by SACS and by clinician prediction based on history and physical exam.7 The SACS LRs ranged from 5.17 to 0.25, a 20-fold range. This reflected superior diagnostic information compared with subjective physician impression, where LRs ranged from 3.7 to 0.52, a seven-fold range. Myers et al prepared a meta-analysis of 4 different trials that examined physicians’ ability to predict OSA.9 Despite the researchers’ use of experienced sleep medicine doctors, the overall diagnostic accuracy of clinical impression was modest (summary positive LR, 1.7; 95% CI, 1.5-2; I2 = 0%; summary negative LR, 0.67; 95% CI, 0.60-0.74; I2 = 10%; sensitivity, 58%; specificity, 67%). This is similar to reliance on a single clinical sign or symptom to predict OSA.
Wise use of oximetry augments SACS calculation. To limit unnecessary oximetry testing in low- and high-risk groups and to avoid polysomnography in cases of a low PTP of disease, we advocate limiting oximetry testing to individuals in the SACS intermediate-risk group (FIGURE 2) wherein ODI results can potentially recalibrate risk assessment up or down. (Those in the high- risk group should be referred to a sleep medicine specialist.) Our institutional recommendation of using an ODI result of ≥5 as a threshold to increase suspicion of disease requires a caveat for the low-risk group. “Positive” results at that low diagnostic threshold are frequently false.

Continue to: Multiple benefits of SACS
Multiple benefits of SACS. We believe using the SACS calculation during clinical encounters with patients potentially at risk for OSA would increase diagnostic accuracy. Performing risk stratification with SACS should not be an undue burden on providers, and the increased time spent with patients has its own benefits, including helping them better understand their risk. Using this standardized process—augmented, as needed, with overnight ODI assessment—might also encourage more patients to follow through on subsequent recommendations, as their risk is further quantified objectively. Lastly, unnecessary testing with polysomnography could be avoided.
Limitations of our study. This study’s findings were derived from a patient population in a single institution. Replication of the findings from other settings would be helpful.
Looking forward. It is yet unclear if clinicians will embrace these strategies in real-world primary care practice. We have designed an implementation-and-dissemination trial to assess whether family physicians will use the SACS clinical predication rule in everyday practice and whether our evidence-based recommendations about overnight oximetry will be followed. Underlying our suggested clinical evaluation pathway (FIGURE 2) is the belief that there is value gained from sharing the decision-making process with patients. Although we provide new evidence that informs these conversations, the patient’s values and preferences are important when determining the best direction to proceed in the evaluation for suspected OSA. These recommendations are intended to aid, not replace, good clinical judgment.
Home-based sleep testing has become more widely available, is convenient for patients, and is less expensive than lab-based polysomnography. Our study did not directly address the appropriate circumstances for home studies in clinical evaluation. We rely on the expertise of our sleep medicine colleagues to determine which patients are appropriate candidates for home-based studies.
The AASM states that “portable monitors (PM) for the diagnosis of OSA should be [used] only in conjunction with a comprehensive sleep evaluation. Clinical sleep evaluations using PM must be supervised by a practitioner with board certification in sleep medicine or an individual who fulfills the eligibility criteria for the sleep medicine certification examination.”4 Additionally, the group recommends that PM “may be used in the unattended setting as an alternative to polysomnography for the diagnosis of OSA in patients with a high pretest probability of moderate to severe OSA and no comorbid sleep disorder or major comorbid medical disorders.”4
Continue to: GRANT SUPPORT
GRANT SUPPORT
The use of the REDCap database is supported by grant UL1 TR000135. This work was supported by a Mayo Foundation CR-20 grant awarded to Dr. Mookadam as Principal investigator and Dr. Grover as Coinvestigator.
Statistical analyses were supported, in part, by the Department of Family Medicine, Mayo Clinic, Scottsdale, Ariz.
CORRESPONDENCE
Michael Grover, DO, Mayo Clinic Thunderbird Primary Care Center-Family Medicine, 13737 N 92nd Street, Scottsdale, AZ 85260; [email protected]
ABSTRACT
Purpose To derive a predictive model for obstructive sleep apnea (OSA) in primary care practice, using home-based overnight oximetry results to refine posttest probability (PTP) of disease after initial risk stratification with the Sleep Apnea Clinical Score (SACS).
Methods We performed secondary analyses on data from a SACS validation cohort, to compare the diagnostic accuracy of 3 overnight oximetry measurements (oxygen desaturation index [ODI], mean saturation, and minimum saturation) in predicting OSA. Receiver operator characteristics (ROC) were computed for each measurement independently and sequentially after risk stratifying with SACS. We examined the implications of oximetry results for OSA PTP for participants categorized as intermediate risk (SACS 6-14; 66/191 participants [35%]; OSA probability 41%). We calculated positive likelihood ratios (LR) for multiple ODI results and determined which ones allowed recalibration to high- or low-risk PTP.
Results Among the 3 oximetry findings, ODI best predicted OSA (area under the curve [AUC], 0.88; 95% confidence interval [CI], 0.83-0.93). An ODI ≥8.4 (likelihood ratio [LR], 4.19; 95% CI, 2.87-6.10) created a PTP of 77%, while an ODI of 0 to <8.4 (LR, 0.19, 95% CI, 0.12-0.33) created a 14% PTP. Sequential application of SACS and ODI results yielded an AUC result of 0.90 (95% CI, 0.85-0.95).
Conclusions SACS risk stratification provides an advantage over clinical gestalt. In those at intermediate risk, ODI results provide a simple and clinically useful way to further refine diagnostic prediction. Sequential use of SACS and selectively employed overnight oximetry may limit unnecessary polysomnography. Oximetry testing should be avoided in patients deemed low or high risk by SACS, as positive results do not substantially recalibrate risk.
Obstructive sleep apnea (OSA) is a prevalent and underdiagnosed condition. The National Sleep Foundation estimates that 18 million Americans have OSA.1 Primary care practice may be the best setting in which to identify OSA, as many of our patients have conditions frequently associated with apnea (eg, hypertension, obesity, diabetes, arrhythmia, and neurologic illness). Up to a third of patients in primary care practice may be at increased risk.2,3
Clinical guidelines of the American Academy of Sleep Medicine (AASM) recommend obtaining a sleep history to evaluate for possible OSA in 3 instances: as part of a routine health maintenance examination, during evaluation of specific complaints associated with OSA (eg, snoring, apnea, daytime sleepiness), and during comprehensive evaluations for individuals with high-risk conditions (ie, obesity, congestive heart failure, refractory hypertension, diabetes, stroke history).4
The American College of Physicians (ACP) Clinical Practice Guideline suggests assessing individuals who have unexplained daytime sleepiness.5 The ACP considers this assessment “High-Value Care,” as “evidence shows that before diagnosis, patients with OSA have higher rates of health care use, more frequent and longer hospital stays, and higher health care costs than after diagnosis.”5
Continue to: We recently validated the diagnostic accuracy...
We recently validated the diagnostic accuracy of the Sleep Apnea Clinical Score (SACS) for use in a primary care patient population suspected of having OSA.6 SACS uses historical and clinical data to derive a score that identifies a patient’s risk level.7 However, as an alternative to the 2 levels described in Flemons’ SACS,7 we propose creating 3 risk strata (FIGURE 17,8). We believe that patients at high risk (SACS ≥15) should be encouraged to undergo sleep evaluations as their posttest probability (PTP) of OSA is 75% to 80%. Individuals at low risk (SACS ≤5; PTP <20%) could receive lifestyle advice and simple clinical interventions that decrease symptoms (eg, weight loss, increased physical activity, sleeping on one’s side). For low-risk patients, clinical observation and reevaluation could take place over time with their primary care provider, without additional testing or referral to specialists.

What about patients at intermediate risk? Many patients suspected of having OSA will be assigned to intermediate risk (SACS 6-14), and their PTP of OSA remains at 40% to 45%, the pre-test level most commonly encountered in suspected OSA. As polysomnography is a limited and expensive clinical resource, intermediate-risk patients would benefit from recalibration of their SACS-based risk assessment using an additional surrogate test such as home-based overnight oximetry. Our internal OSA practice guidelines recommend referral for sleep medicine consultation when oximetry results are abnormal—specifically, an oxygen desaturation index (ODI) of ≥5, a mean saturation less than 89%, and a minimum saturation of 75% or less.
Our objectives in this study were to compare the diagnostic implications of these 3 measurements from home-based overnight oximetry reports and use the most relevant result to derive a predictive model further refining PTP of OSA in a primary care patient population first stratified to intermediate risk by SACS.
METHODS
Subjects
We performed secondary analyses on data obtained from our SACS validation cohort.6 In brief, these were patients suspected of having OSA based on the presence of signs, symptoms, or associated risk factors. One hundred ninety-one patients completed all assessments. Sixty-six of 191 patients (35%) were categorized as intermediate risk (SACS 6-14; OSA probability 41% [27/66]).
Data collection and analyses
Participants completed home-based overnight oximetry using Nonin Model 2500 oximeters (Nonin Medical Inc., Plymouth, Minn). We transferred oximetry results from the sleep lab database to a statistical program for analyses of ODI, mean saturation, and minimal saturation. ODI was defined as the number of 4% drops in saturation from baseline divided by the number of hours of recording time. Although the AASM states that a diagnosis of OSA is confirmed if the number of obstructive events is more than 15 per hour or more than 5 per hour in a patient who reports related symptoms,4 we defined OSA as an apnea-hypopnea index (AHI) of >10 based on polysomnography (as this was the threshold used in the derivation cohort for SACS).7 We demonstrated the predictive ability of SACS at various AHI definitions of OSA in our validation cohort.6 The use of SACS in our validation cohort showed a statistically similar ability to predict OSA at both an AHI of 10 and 20, compared with the derivation cohort.
Continue to: We entered additional information...
We entered additional information reported directly by patients and obtained from their sleep studies into a REDCap database and transferred that to our statistical program. We used descriptive statistics to determine ranges and central tendencies of oximetry results. Receiver operator characteristic (ROC) analyses described the predictive abilities for each oximetry result individually and in serial application with prior SACS determinations. For comparison, we used the area under the ROC curve (AUC) from logistic regression to model the probability of OSA.
We calculated positive likelihood ratios (LR) and 95% confidence intervals (CI) to determine the degree of oximetry abnormality that would recalibrate risk either to a high PTP of OSA (>75%) or a low PTP (<25%). We sorted intermediate-risk SACS scores into quintiles based on ODI results to compare the resulting PTPs of OSA. We applied the PTP of OSA from our previous work (using the SACS score to compute the LR) as the new PTP, estimated the LR based on ODI, and computed an updated PTP of OSA. We also used ROC analysis to determine the optimal cutoff value of the ODI.
Finally, in accordance with our internal clinical practice recommendations, we examined the predictive ability of a “positive” ODI result of ≥5 to recalibrate risk prediction for OSA for patients in the low-risk group. We performed analyses using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS

One hundred ninety-one subjects completed assessments. The median and quartile results for ODI, mean saturation, and minimum saturation are found in TABLE 1. TABLE 2 shows the distribution of patients with positive oximetry results. An ODI of 5 or greater was the most frequent abnormal result (135/191; 70.7%).

We used the AUC to measure the comparative abilities of SACS and the 3 overnight oximetry results in predicting OSA (TABLE 3). ODI results demonstrated the best ability to predict OSA, compared with polysomnography as the relative gold standard (AUC, 0.88; 95% confidence interval [CI], 0.83-0.93). Serial application of SACS and ODI yielded even better diagnostic results (AUC, 0.90; 95% CI, 0.85-0.95).

Continue to: As ODI was found to be the strongest predictor of OSA...
As ODI was found to be the strongest predictor of OSA, we grouped these results in quintiles and calculated positive LRs. TABLE 4 shows their effect on PTP of disease among patients with intermediate risk. An ODI result >10 effected an upward recalibration of disease probability (LR, 2.33; 95% CI, 1.27-4.26). The optimal cutoff of ODI to discriminate between those with and without OSA was determined by ROC analysis. An ODI greater than 8.4 created a PTP of disease of approximately 73% to 77%.

Our internal clinical guidelines recommend referring patients with an ODI of 5 or greater for sleep medicine consultation. We examined the ability of this ODI result to recalibrate disease suspicion for a patient at low risk (SACS ≤5). The LR for ODI of 5 or greater is 2.1, but this only results in a recalibration of risk from 24% pretest probability in our validation cohort to 41% PTP (95% CI, 33-49). This low cutoff for a positive test creates false-positive results more than 40% of the time due to low specificity (0.58). This is insufficient to change the suspicion of disease, resulting only in a shift to intermediate OSA risk.
DISCUSSION
Among 3 different oximetry measurements, an ODI ≥10 best predicts OSA, both independently and when used sequentially after the SACS. ODI was by far the most frequent abnormality on oximetry in our cohort, thereby increasing its utility in clinical decision making. For those subjects at intermediate risk, a cutoff of 10 for the ODI result may be a simple and clinically effective way to recalibrate risk and aid in making referral decisions. (This may also be simpler and more easily remembered by clinicians than the 8.4 ODI results from the ROC analyses.)
Assessment is inadequate without a clinical prediction rule. Unfortunately, providers cannot simply rely on clinical gestalt in diagnosing OSA. In their derivation cohort, Flemens et al examined the LRs created by SACS and by clinician prediction based on history and physical exam.7 The SACS LRs ranged from 5.17 to 0.25, a 20-fold range. This reflected superior diagnostic information compared with subjective physician impression, where LRs ranged from 3.7 to 0.52, a seven-fold range. Myers et al prepared a meta-analysis of 4 different trials that examined physicians’ ability to predict OSA.9 Despite the researchers’ use of experienced sleep medicine doctors, the overall diagnostic accuracy of clinical impression was modest (summary positive LR, 1.7; 95% CI, 1.5-2; I2 = 0%; summary negative LR, 0.67; 95% CI, 0.60-0.74; I2 = 10%; sensitivity, 58%; specificity, 67%). This is similar to reliance on a single clinical sign or symptom to predict OSA.
Wise use of oximetry augments SACS calculation. To limit unnecessary oximetry testing in low- and high-risk groups and to avoid polysomnography in cases of a low PTP of disease, we advocate limiting oximetry testing to individuals in the SACS intermediate-risk group (FIGURE 2) wherein ODI results can potentially recalibrate risk assessment up or down. (Those in the high- risk group should be referred to a sleep medicine specialist.) Our institutional recommendation of using an ODI result of ≥5 as a threshold to increase suspicion of disease requires a caveat for the low-risk group. “Positive” results at that low diagnostic threshold are frequently false.

Continue to: Multiple benefits of SACS
Multiple benefits of SACS. We believe using the SACS calculation during clinical encounters with patients potentially at risk for OSA would increase diagnostic accuracy. Performing risk stratification with SACS should not be an undue burden on providers, and the increased time spent with patients has its own benefits, including helping them better understand their risk. Using this standardized process—augmented, as needed, with overnight ODI assessment—might also encourage more patients to follow through on subsequent recommendations, as their risk is further quantified objectively. Lastly, unnecessary testing with polysomnography could be avoided.
Limitations of our study. This study’s findings were derived from a patient population in a single institution. Replication of the findings from other settings would be helpful.
Looking forward. It is yet unclear if clinicians will embrace these strategies in real-world primary care practice. We have designed an implementation-and-dissemination trial to assess whether family physicians will use the SACS clinical predication rule in everyday practice and whether our evidence-based recommendations about overnight oximetry will be followed. Underlying our suggested clinical evaluation pathway (FIGURE 2) is the belief that there is value gained from sharing the decision-making process with patients. Although we provide new evidence that informs these conversations, the patient’s values and preferences are important when determining the best direction to proceed in the evaluation for suspected OSA. These recommendations are intended to aid, not replace, good clinical judgment.
Home-based sleep testing has become more widely available, is convenient for patients, and is less expensive than lab-based polysomnography. Our study did not directly address the appropriate circumstances for home studies in clinical evaluation. We rely on the expertise of our sleep medicine colleagues to determine which patients are appropriate candidates for home-based studies.
The AASM states that “portable monitors (PM) for the diagnosis of OSA should be [used] only in conjunction with a comprehensive sleep evaluation. Clinical sleep evaluations using PM must be supervised by a practitioner with board certification in sleep medicine or an individual who fulfills the eligibility criteria for the sleep medicine certification examination.”4 Additionally, the group recommends that PM “may be used in the unattended setting as an alternative to polysomnography for the diagnosis of OSA in patients with a high pretest probability of moderate to severe OSA and no comorbid sleep disorder or major comorbid medical disorders.”4
Continue to: GRANT SUPPORT
GRANT SUPPORT
The use of the REDCap database is supported by grant UL1 TR000135. This work was supported by a Mayo Foundation CR-20 grant awarded to Dr. Mookadam as Principal investigator and Dr. Grover as Coinvestigator.
Statistical analyses were supported, in part, by the Department of Family Medicine, Mayo Clinic, Scottsdale, Ariz.
CORRESPONDENCE
Michael Grover, DO, Mayo Clinic Thunderbird Primary Care Center-Family Medicine, 13737 N 92nd Street, Scottsdale, AZ 85260; [email protected]
1. National Sleep Foundation. Sleep apnea. https://sleepfoundation.org/sleep-disorders-problems/sleep-apnea. Accessed September 14, 2018.
2. Grover M, Mookadam M, Armas D, et al. Identifying patients at risk for obstructive sleep apnea in a primary care practice. J Am Board Fam Med. 2011;24:152-160.
3. Mold JW, Quattlebaum C, Schinnerer E, et al. Identification by primary care clinicians of patients with obstructive sleep apnea: a practice-based research network (PBRN) study. J Am Board Fam Med. 2011;24:138-145.
4. Epstein LJ, Kristo D, Strollo PJ, Jr., et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276.
5. Qaseem A, Dallas P, Owens DK, et al. Diagnosis of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2014;161:210-220.
6. Grover M, Mookadam M, Chang Y-H, et al. Validating the Sleep Apnea Clinical Score for use in primary care populations. Mayo Clin Proc. 2016;91:469-476.
7. Flemons WW, Whitelaw WA, Brant R, et al. Likelihood ratios for a sleep apnea clinical prediction rule. Am J Respir Crit Care Med. 1994;150:1279-1285.
8. Gali B, Whalen FX, Gay PC, et al. Management plan to reduce risks in perioperative care of patients with presumed obstructive sleep apnea syndrome. J Clin Sleep Med. 2007;3:582-588.
9. Myers KA, Mrkobrada M, Simel DL. Does this patient have obstructive sleep apnea?: The rational clinical examination systematic review. JAMA. 2013;310(7):731-741.
1. National Sleep Foundation. Sleep apnea. https://sleepfoundation.org/sleep-disorders-problems/sleep-apnea. Accessed September 14, 2018.
2. Grover M, Mookadam M, Armas D, et al. Identifying patients at risk for obstructive sleep apnea in a primary care practice. J Am Board Fam Med. 2011;24:152-160.
3. Mold JW, Quattlebaum C, Schinnerer E, et al. Identification by primary care clinicians of patients with obstructive sleep apnea: a practice-based research network (PBRN) study. J Am Board Fam Med. 2011;24:138-145.
4. Epstein LJ, Kristo D, Strollo PJ, Jr., et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276.
5. Qaseem A, Dallas P, Owens DK, et al. Diagnosis of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2014;161:210-220.
6. Grover M, Mookadam M, Chang Y-H, et al. Validating the Sleep Apnea Clinical Score for use in primary care populations. Mayo Clin Proc. 2016;91:469-476.
7. Flemons WW, Whitelaw WA, Brant R, et al. Likelihood ratios for a sleep apnea clinical prediction rule. Am J Respir Crit Care Med. 1994;150:1279-1285.
8. Gali B, Whalen FX, Gay PC, et al. Management plan to reduce risks in perioperative care of patients with presumed obstructive sleep apnea syndrome. J Clin Sleep Med. 2007;3:582-588.
9. Myers KA, Mrkobrada M, Simel DL. Does this patient have obstructive sleep apnea?: The rational clinical examination systematic review. JAMA. 2013;310(7):731-741.
What medical therapies work for gastroparesis?
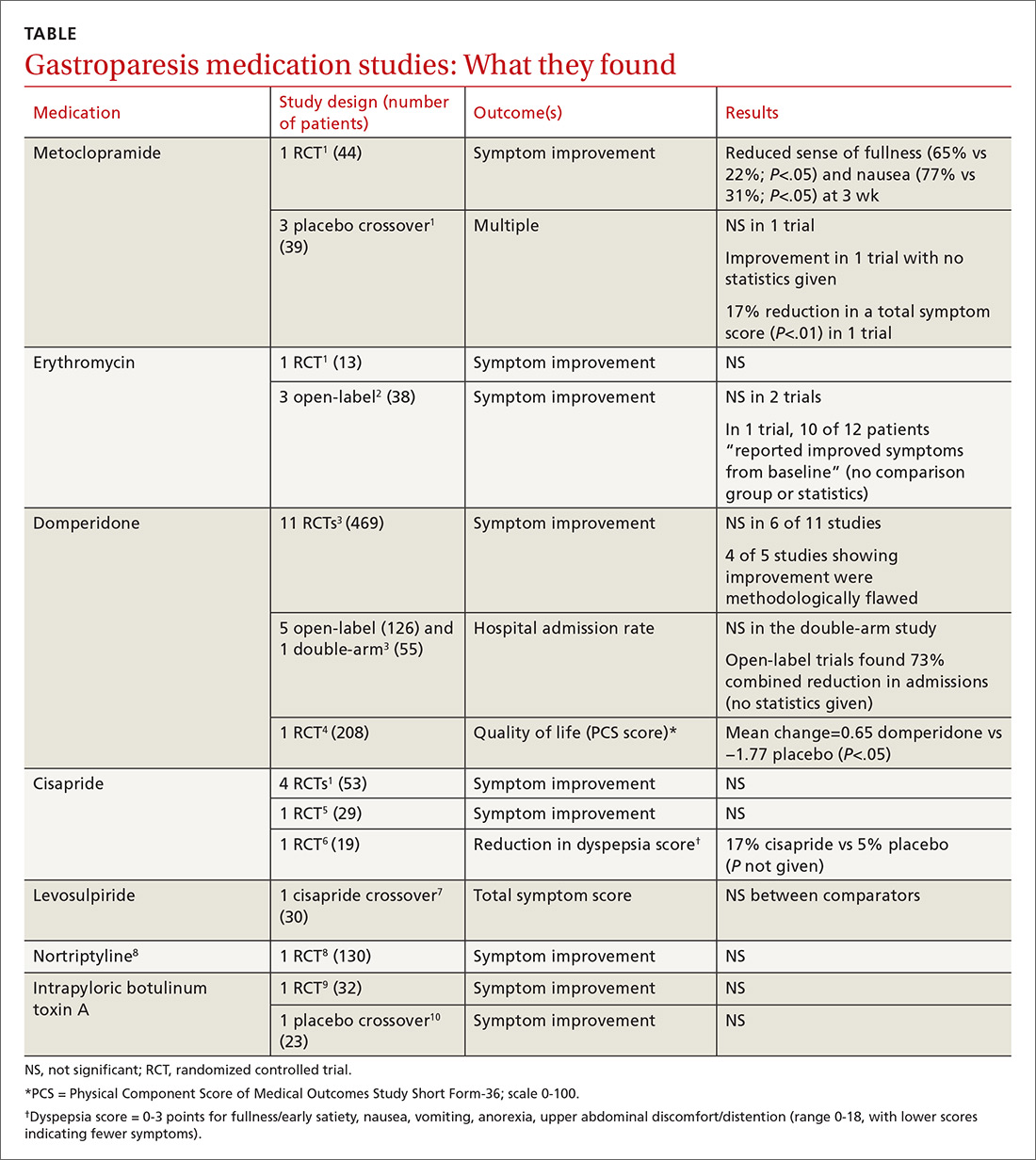
EVIDENCE SUMMARY
Metoclopramide. One systematic review that looked at the efficacy of metoclopramide for gastroparesis identified one small RCT and 3 smaller placebo-controlled crossover trials.1 The RCT (using 10 mg of metoclopramide after meals and at bedtime) found consistent improvement in the sense of fullness over 3 weeks of therapy, with reduction of nausea at one and 3 weeks, but not at 2 weeks. Vomiting, anorexia, and early satiety didn’t improve. The crossover trials had inconsistent results. The largest one, with only 16 patients, didn’t find an improvement in symptoms.
Erythromycin. Two systematic reviews looked at the efficacy of erythromycin, primarily identifying studies 20- to 30-years old. The first systematic review identified only one small (single-blind) RCT in which erythromycin treatment didn’t change symptoms.1 A second review identified 3 trials described as “open label,” all with fewer than 14 subjects and all lasting a month or less.2 Erythromycin improved patient symptoms in only 1 of the 3, and this trial (like the others) had significant methodologic flaws. The authors of the second review concluded that “the true efficacy of erythromycin in relieving symptoms … remains to be determined.”
Domperidone. A systematic review and one subsequent RCT evaluated domperidone. The systematic review identified 11 randomized, placebo-controlled trials (469 patients).3 Six studies found no impact on patient symptoms, while 5 reported a positive effect. The review also identified 6 trials that evaluated domperidone treatment on hospitalization rates. Open-label (single-arm, unblinded) trials tended to find a reduction in hospitalizations with domperidone, an effect not seen in the one double-arm study that evaluated this outcome.
The review authors noted that given the small size and low methodologic quality of most studies “it is not surprising … that there continues to be controversy about the efficacy of this drug” for symptoms of gastroparesis.
One subsequent RCT, using domperidone 20 mg 4 times daily for 4 weeks, found a 2% improvement over placebo in the physical component of a multifaceted quality-of-life measure.4 The improvement was statistically significant, but of unclear clinical importance.
Cisapride. One systematic review and 2 subsequent RCTs evaluated the clinical effects of cisapride. The systematic review included 4 small RCTs (53 patients) that didn’t individually find changes in patient symptoms.
In one subsequent RCT, comparing 10 mg cisapride 3 times daily to placebo for 2 weeks, cisapride yielded no significant change in symptoms.5 The other RCT compared oral cisapride 10 mg 3 times daily to placebo for one year. Cisapride treatment produced a 17% reduction in symptoms (P<.002 vs baseline), and placebo produced a 5% reduction (P=NS vs baseline). The study didn’t state if the difference between the 2 outcomes was statistically significant.6
Continue to: Levosulpiride
Levosulpiride. One crossover study compared 25 mg levosulpiride with 10 mg cisapride (both given orally 3 times a day) on gastroparesis symptoms and gastric emptying. Each medication was given for one month (washout duration not given). The study found similar efficacy between levosulpiride and cisapride in terms of improvement in gastric emptying rates and total symptom scores.7 No studies compare levosulpiride to placebo.
Nortriptyline. A multicenter, parallel-group, double-blind RCT comparing 75 mg/d nortriptyline for 15 weeks with placebo in adult patients with moderate to severe symptoms of idiopathic gastroparesis for at least 6 months found that nortriptyline didn’t improve symptoms.8
Botulinum toxin A. An RCT comparing a single injection of 200 units intrapyloric botulinum toxin A with placebo in adult patients with severe gastroparesis symptoms found that botulinum toxin A didn’t result in symptomatic improvement.9 A crossover trial comparing 100 units monthly intrapyloric botulinum toxin A for 3 months with placebo in patients with gastroparesis found that neither symptoms nor rate of gastric emptying changed with the toxin.10
RECOMMENDATIONS
The 2013 guidelines from the American College of Gastroenterology list metoclopramide as the first-line agent for gastroparesis requiring medical therapy, followed by domperidone and then erythromycin (all based on “moderate quality evidence”). Antiemetic agents are also recommended for symptom control.11
1. Sturm A, Holtmann G, Goebell H, et al. Prokinetics in patients with gastroparesis: a systematic analysis. Digestion. 1999;60:422-427.
2. Maganti K, Onyemere K, Jones MP. Oral erythromycin and symptomatic relief of gastroparesis: a systematic review. Am J Gastroenterol. 2003;98:259-263.
3. Sugumar A, Singh A, Pasricha PJ. A systematic review of the efficacy of domperidone for the treatment of diabetic gastroparesis. Clin Gastroenterol Hepatol. 2008;6:726-733.
4. Farup CE, Leidy NK, Murray M, et al. Effect of domperidone on the health-related quality of life of patients with symptoms of diabetic gastroparesis. Diabetes Care. 1998;21:1699-1706.
5. Dutta U, Padhy AK, Ahuja V, et al. Double blind controlled trial of effect of cisapride on gastric emptying in diabetics. Trop Gastroenterol. 1999;20:116-119.
6. Braden B, Enghofer M, Schaub M, et al. Long-term cisapride treatment improves diabetic gastroparesis but not glycaemic control. Aliment Pharmacol Ther. 2002;16:1341-1346.
7. Mansi C, Borro P, Giacomini M, et al. Comparative effects of levosulpiride and cisapride on gastric emptying and symptoms in patients with functional dyspepsia and gastroparesis. Aliment Pharmacol Ther. 2000;14:561-569.
8. Parkman HP, Van Natta ML, Abell TL, et al. Effect of nortriptyline on symptoms of idiopathic gastroparesis: the NORIG randomized clinical trial. JAMA. 2013;310:2640-2649.
9. Friedenberg FK, Palit A, Parkman HP, et al. Botulinum toxin A for the treatment of delayed gastric emptying. Am J Gastroenterol. 2008;103:416-423.
10. Arts J, Holvoet L, Caenepeel P, et al. Clinical trial: a randomized-controlled crossover study of intrapyloric injection of botulinum toxin in gastroparesis. Aliment Pharmacol Ther. 2007;26:1251-1258.
11. Camilleri M, Parkman HP, Shafi MA, et al. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18-38.

EVIDENCE SUMMARY
Metoclopramide. One systematic review that looked at the efficacy of metoclopramide for gastroparesis identified one small RCT and 3 smaller placebo-controlled crossover trials.1 The RCT (using 10 mg of metoclopramide after meals and at bedtime) found consistent improvement in the sense of fullness over 3 weeks of therapy, with reduction of nausea at one and 3 weeks, but not at 2 weeks. Vomiting, anorexia, and early satiety didn’t improve. The crossover trials had inconsistent results. The largest one, with only 16 patients, didn’t find an improvement in symptoms.
Erythromycin. Two systematic reviews looked at the efficacy of erythromycin, primarily identifying studies 20- to 30-years old. The first systematic review identified only one small (single-blind) RCT in which erythromycin treatment didn’t change symptoms.1 A second review identified 3 trials described as “open label,” all with fewer than 14 subjects and all lasting a month or less.2 Erythromycin improved patient symptoms in only 1 of the 3, and this trial (like the others) had significant methodologic flaws. The authors of the second review concluded that “the true efficacy of erythromycin in relieving symptoms … remains to be determined.”
Domperidone. A systematic review and one subsequent RCT evaluated domperidone. The systematic review identified 11 randomized, placebo-controlled trials (469 patients).3 Six studies found no impact on patient symptoms, while 5 reported a positive effect. The review also identified 6 trials that evaluated domperidone treatment on hospitalization rates. Open-label (single-arm, unblinded) trials tended to find a reduction in hospitalizations with domperidone, an effect not seen in the one double-arm study that evaluated this outcome.
The review authors noted that given the small size and low methodologic quality of most studies “it is not surprising … that there continues to be controversy about the efficacy of this drug” for symptoms of gastroparesis.
One subsequent RCT, using domperidone 20 mg 4 times daily for 4 weeks, found a 2% improvement over placebo in the physical component of a multifaceted quality-of-life measure.4 The improvement was statistically significant, but of unclear clinical importance.
Cisapride. One systematic review and 2 subsequent RCTs evaluated the clinical effects of cisapride. The systematic review included 4 small RCTs (53 patients) that didn’t individually find changes in patient symptoms.
In one subsequent RCT, comparing 10 mg cisapride 3 times daily to placebo for 2 weeks, cisapride yielded no significant change in symptoms.5 The other RCT compared oral cisapride 10 mg 3 times daily to placebo for one year. Cisapride treatment produced a 17% reduction in symptoms (P<.002 vs baseline), and placebo produced a 5% reduction (P=NS vs baseline). The study didn’t state if the difference between the 2 outcomes was statistically significant.6
Continue to: Levosulpiride
Levosulpiride. One crossover study compared 25 mg levosulpiride with 10 mg cisapride (both given orally 3 times a day) on gastroparesis symptoms and gastric emptying. Each medication was given for one month (washout duration not given). The study found similar efficacy between levosulpiride and cisapride in terms of improvement in gastric emptying rates and total symptom scores.7 No studies compare levosulpiride to placebo.
Nortriptyline. A multicenter, parallel-group, double-blind RCT comparing 75 mg/d nortriptyline for 15 weeks with placebo in adult patients with moderate to severe symptoms of idiopathic gastroparesis for at least 6 months found that nortriptyline didn’t improve symptoms.8
Botulinum toxin A. An RCT comparing a single injection of 200 units intrapyloric botulinum toxin A with placebo in adult patients with severe gastroparesis symptoms found that botulinum toxin A didn’t result in symptomatic improvement.9 A crossover trial comparing 100 units monthly intrapyloric botulinum toxin A for 3 months with placebo in patients with gastroparesis found that neither symptoms nor rate of gastric emptying changed with the toxin.10
RECOMMENDATIONS
The 2013 guidelines from the American College of Gastroenterology list metoclopramide as the first-line agent for gastroparesis requiring medical therapy, followed by domperidone and then erythromycin (all based on “moderate quality evidence”). Antiemetic agents are also recommended for symptom control.11

EVIDENCE SUMMARY
Metoclopramide. One systematic review that looked at the efficacy of metoclopramide for gastroparesis identified one small RCT and 3 smaller placebo-controlled crossover trials.1 The RCT (using 10 mg of metoclopramide after meals and at bedtime) found consistent improvement in the sense of fullness over 3 weeks of therapy, with reduction of nausea at one and 3 weeks, but not at 2 weeks. Vomiting, anorexia, and early satiety didn’t improve. The crossover trials had inconsistent results. The largest one, with only 16 patients, didn’t find an improvement in symptoms.
Erythromycin. Two systematic reviews looked at the efficacy of erythromycin, primarily identifying studies 20- to 30-years old. The first systematic review identified only one small (single-blind) RCT in which erythromycin treatment didn’t change symptoms.1 A second review identified 3 trials described as “open label,” all with fewer than 14 subjects and all lasting a month or less.2 Erythromycin improved patient symptoms in only 1 of the 3, and this trial (like the others) had significant methodologic flaws. The authors of the second review concluded that “the true efficacy of erythromycin in relieving symptoms … remains to be determined.”
Domperidone. A systematic review and one subsequent RCT evaluated domperidone. The systematic review identified 11 randomized, placebo-controlled trials (469 patients).3 Six studies found no impact on patient symptoms, while 5 reported a positive effect. The review also identified 6 trials that evaluated domperidone treatment on hospitalization rates. Open-label (single-arm, unblinded) trials tended to find a reduction in hospitalizations with domperidone, an effect not seen in the one double-arm study that evaluated this outcome.
The review authors noted that given the small size and low methodologic quality of most studies “it is not surprising … that there continues to be controversy about the efficacy of this drug” for symptoms of gastroparesis.
One subsequent RCT, using domperidone 20 mg 4 times daily for 4 weeks, found a 2% improvement over placebo in the physical component of a multifaceted quality-of-life measure.4 The improvement was statistically significant, but of unclear clinical importance.
Cisapride. One systematic review and 2 subsequent RCTs evaluated the clinical effects of cisapride. The systematic review included 4 small RCTs (53 patients) that didn’t individually find changes in patient symptoms.
In one subsequent RCT, comparing 10 mg cisapride 3 times daily to placebo for 2 weeks, cisapride yielded no significant change in symptoms.5 The other RCT compared oral cisapride 10 mg 3 times daily to placebo for one year. Cisapride treatment produced a 17% reduction in symptoms (P<.002 vs baseline), and placebo produced a 5% reduction (P=NS vs baseline). The study didn’t state if the difference between the 2 outcomes was statistically significant.6
Continue to: Levosulpiride
Levosulpiride. One crossover study compared 25 mg levosulpiride with 10 mg cisapride (both given orally 3 times a day) on gastroparesis symptoms and gastric emptying. Each medication was given for one month (washout duration not given). The study found similar efficacy between levosulpiride and cisapride in terms of improvement in gastric emptying rates and total symptom scores.7 No studies compare levosulpiride to placebo.
Nortriptyline. A multicenter, parallel-group, double-blind RCT comparing 75 mg/d nortriptyline for 15 weeks with placebo in adult patients with moderate to severe symptoms of idiopathic gastroparesis for at least 6 months found that nortriptyline didn’t improve symptoms.8
Botulinum toxin A. An RCT comparing a single injection of 200 units intrapyloric botulinum toxin A with placebo in adult patients with severe gastroparesis symptoms found that botulinum toxin A didn’t result in symptomatic improvement.9 A crossover trial comparing 100 units monthly intrapyloric botulinum toxin A for 3 months with placebo in patients with gastroparesis found that neither symptoms nor rate of gastric emptying changed with the toxin.10
RECOMMENDATIONS
The 2013 guidelines from the American College of Gastroenterology list metoclopramide as the first-line agent for gastroparesis requiring medical therapy, followed by domperidone and then erythromycin (all based on “moderate quality evidence”). Antiemetic agents are also recommended for symptom control.11
1. Sturm A, Holtmann G, Goebell H, et al. Prokinetics in patients with gastroparesis: a systematic analysis. Digestion. 1999;60:422-427.
2. Maganti K, Onyemere K, Jones MP. Oral erythromycin and symptomatic relief of gastroparesis: a systematic review. Am J Gastroenterol. 2003;98:259-263.
3. Sugumar A, Singh A, Pasricha PJ. A systematic review of the efficacy of domperidone for the treatment of diabetic gastroparesis. Clin Gastroenterol Hepatol. 2008;6:726-733.
4. Farup CE, Leidy NK, Murray M, et al. Effect of domperidone on the health-related quality of life of patients with symptoms of diabetic gastroparesis. Diabetes Care. 1998;21:1699-1706.
5. Dutta U, Padhy AK, Ahuja V, et al. Double blind controlled trial of effect of cisapride on gastric emptying in diabetics. Trop Gastroenterol. 1999;20:116-119.
6. Braden B, Enghofer M, Schaub M, et al. Long-term cisapride treatment improves diabetic gastroparesis but not glycaemic control. Aliment Pharmacol Ther. 2002;16:1341-1346.
7. Mansi C, Borro P, Giacomini M, et al. Comparative effects of levosulpiride and cisapride on gastric emptying and symptoms in patients with functional dyspepsia and gastroparesis. Aliment Pharmacol Ther. 2000;14:561-569.
8. Parkman HP, Van Natta ML, Abell TL, et al. Effect of nortriptyline on symptoms of idiopathic gastroparesis: the NORIG randomized clinical trial. JAMA. 2013;310:2640-2649.
9. Friedenberg FK, Palit A, Parkman HP, et al. Botulinum toxin A for the treatment of delayed gastric emptying. Am J Gastroenterol. 2008;103:416-423.
10. Arts J, Holvoet L, Caenepeel P, et al. Clinical trial: a randomized-controlled crossover study of intrapyloric injection of botulinum toxin in gastroparesis. Aliment Pharmacol Ther. 2007;26:1251-1258.
11. Camilleri M, Parkman HP, Shafi MA, et al. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18-38.
1. Sturm A, Holtmann G, Goebell H, et al. Prokinetics in patients with gastroparesis: a systematic analysis. Digestion. 1999;60:422-427.
2. Maganti K, Onyemere K, Jones MP. Oral erythromycin and symptomatic relief of gastroparesis: a systematic review. Am J Gastroenterol. 2003;98:259-263.
3. Sugumar A, Singh A, Pasricha PJ. A systematic review of the efficacy of domperidone for the treatment of diabetic gastroparesis. Clin Gastroenterol Hepatol. 2008;6:726-733.
4. Farup CE, Leidy NK, Murray M, et al. Effect of domperidone on the health-related quality of life of patients with symptoms of diabetic gastroparesis. Diabetes Care. 1998;21:1699-1706.
5. Dutta U, Padhy AK, Ahuja V, et al. Double blind controlled trial of effect of cisapride on gastric emptying in diabetics. Trop Gastroenterol. 1999;20:116-119.
6. Braden B, Enghofer M, Schaub M, et al. Long-term cisapride treatment improves diabetic gastroparesis but not glycaemic control. Aliment Pharmacol Ther. 2002;16:1341-1346.
7. Mansi C, Borro P, Giacomini M, et al. Comparative effects of levosulpiride and cisapride on gastric emptying and symptoms in patients with functional dyspepsia and gastroparesis. Aliment Pharmacol Ther. 2000;14:561-569.
8. Parkman HP, Van Natta ML, Abell TL, et al. Effect of nortriptyline on symptoms of idiopathic gastroparesis: the NORIG randomized clinical trial. JAMA. 2013;310:2640-2649.
9. Friedenberg FK, Palit A, Parkman HP, et al. Botulinum toxin A for the treatment of delayed gastric emptying. Am J Gastroenterol. 2008;103:416-423.
10. Arts J, Holvoet L, Caenepeel P, et al. Clinical trial: a randomized-controlled crossover study of intrapyloric injection of botulinum toxin in gastroparesis. Aliment Pharmacol Ther. 2007;26:1251-1258.
11. Camilleri M, Parkman HP, Shafi MA, et al. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18-38.
EVIDENCE-BASED ANSWER:
It’s unclear if there are any highly effective medications for gastroparesis (TABLE1-10). Metoclopramide improves the sense of fullness by about 40% for as long as 3 weeks, may improve nausea, and doesn’t affect vomiting or anorexia (strength of recommendation [SOR]: B, small randomized controlled trial [RCT]).
Whether or not erythromycin has an effect on symptoms is unclear (SOR: C, conflicting trials and expert opinion).
Domperidone may improve quality of life (by 2%) for as long as a year, but its effect on symptoms is also unclear (SOR: C, small RCTs).
Cisapride may not be effective for symptom relief (SOR: C, small conflicting RCTs), and levosulpiride is likely similar to cisapride (SOR: C, single small crossover trial).
Nortriptyline (SOR: B, single RCT) and intrapyloric botulinum toxin A (SOR: B, small RCT and crossover trial) aren’t effective for symptom relief.
Progressive discoloration over the right shoulder
A 15-year-old Caucasian boy presented for evaluation of an asymptomatic brown patch on his right shoulder. While the patient’s mother first noticed the patch when he was 5 years old, the discolored area had recently been expanding in size and had developed hypertrichosis. The patient was otherwise healthy; he took no medications and denied any symptoms or history of trauma to the area. None of his siblings were similarly affected.
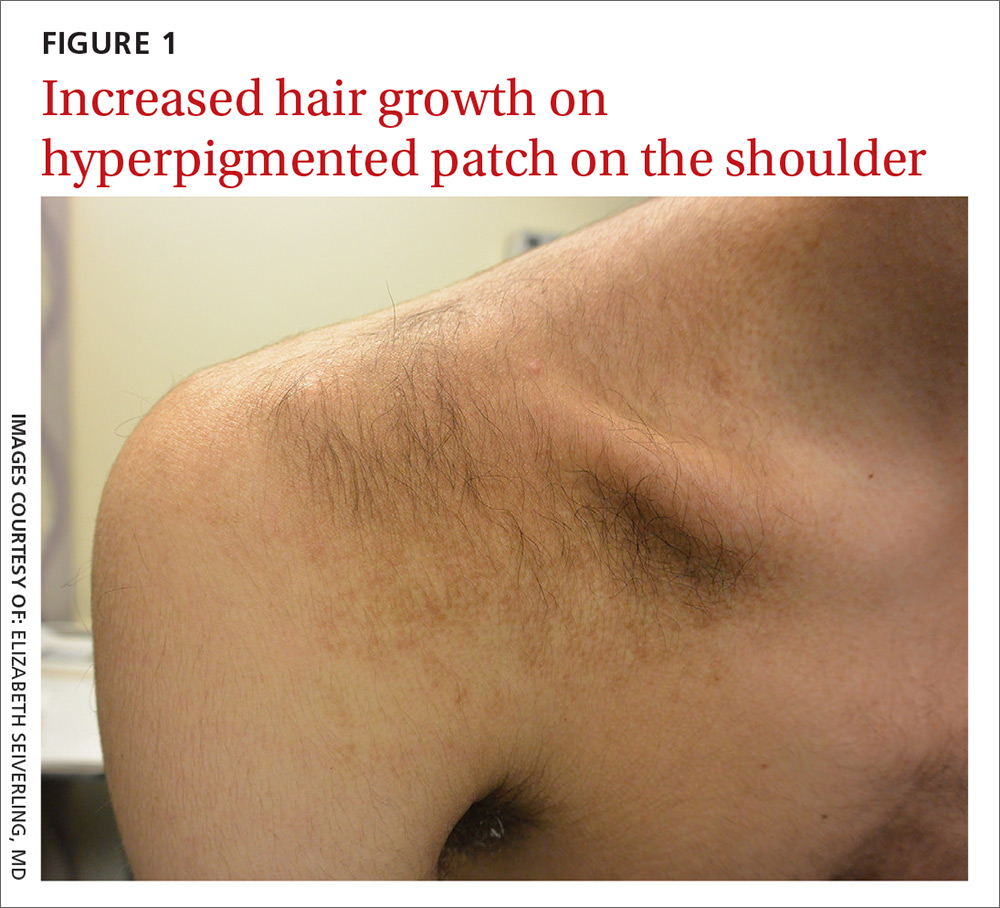
A physical examination revealed a well-demarcated hyperpigmented patch with an irregularly shaped border and an increased number of terminal hairs (FIGURE 1). The affected area was not indurated, and there were no muscular or skeletal abnormalities on inspection. Examination of the patch under a dermatoscope revealed islands of reticular (lattice-like) hyperpigmentation, focal hypopigmentation, and prominent follicles (FIGURE 2).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
DIAGNOSIS: Becker melanosis
Becker melanosis (also called Becker’s nevus or Becker’s pigmentary hamartoma) is an organoid hamartoma that is most common among males.1 This benign area of hyperpigmentation typically manifests as a circumscribed patch with an irregular border on the upper trunk, shoulders, or upper arms of young men. Becker melanosis is usually acquired and typically comes to medical attention around the time of puberty, although there may be a history of discoloration (as was true in this case).
A diagnosis that’s usually made clinically
Androgenic origin. Because of the male predominance and association with hypertrichosis (and for that matter, acne), androgens have been thought to play a role in the development of Becker melanosis.2 The condition affects about 1 in 200 young men.1 To date, no specific gene defect has been identified.
Underlying hypoplasia of the breast or musculoskeletal abnormalities are uncommonly associated with Becker melanosis. When these abnormalities are present, the condition is known as Becker’s nevus syndrome.3
Look for the pattern. Becker melanosis is associated with homogenous brown patches with perifollicular hypopigmentation, sometimes with a faint reticular pattern.4,5 The diagnosis can usually be made clinically, but a skin biopsy can be helpful to confirm questionable cases. Dermoscopy can also assist in diagnosis. In this case, our patient’s presentation was typical, and additional studies were not needed.
Other causes of hyperpigmentation
The differential diagnosis includes other localized disorders associated with hyperpigmentation (TABLE1,3,4).
Continue to: Morphea
Morphea represents a thickening of collagen bundles in the skin. Although morphea can affect the shoulder and trunk, as Becker melanosis does, lesions of morphea feel firm to the touch and are not associated with hypertrichosis.
Localized post-inflammatory hyperpigmentation occurs following a traumatic event, such as a burn, or a prior dermatosis, such as zoster. Careful history-taking can uncover an antecedent inflammatory condition. Post-inflammatory pigment changes do not typically result in hypertrichosis.
Café-au-lait macules can manifest as isolated areas of discoloration. These macules can be an important indicator of neurofibromatosis, a genetic disorder in which tumors grow in the nervous system. Melanocytic hamartomas of the iris (Lisch nodules), axillary freckling (Crowe’s sign), or multiple cutaneous neurofibromas serve as additional clues to neurofibromatosis. In ambiguous cases, a skin biopsy can help differentiate a café au lait macule from Becker melanosis.
To treat or not to treat?
No treatment other than reassurance is needed in most cases of Becker melanosis, as it is a benign condition. Protecting the area from sunlight can minimize darkening and contrast with the surrounding skin. Electrolysis and laser therapy can be used to treat the associated hypertrichosis; laser therapy can also reduce the hyperpigmentation. Nonablative fractional resurfacing accompanied by laser hair removal is also reported to be of value.6
Our patient was satisfied with reassurance of the benign nature of the condition and did not elect treatment.
CORRESPONDENCE
Matthew F. Helm, MD, 500 University Drive, Suite 4300, Department of Dermatology, HU14, UPC II, Hershey, PA 17033-2360; [email protected]
1. Rabinovitz HS, Barnhill RL. Benign melanocytic neoplasms. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. New York, NY: Elsevier Saunders; 2012;112:1853-1854.
2. Person JR, Longcope C. Becker’s nevus: an androgen-mediated hyperplasia with increased androgen receptors. J Am Acad Dermatol. 1984;10:235-238.
3. Cosendey FE, Martinez NS, Bernhard GA, et al. Becker nevus syndrome. An Bras Dermatol. 2010;85:379-384.
4. Ingordo V, Iannazzone SS, Cusano F, et al. Dermoscopic features of congenital melanocytic nevus and Becker nevus in an adult male population: an analysis with 10-fold magnification. Dermatology. 2006;212:354-360.
5. Luk DC, Lam SY, Cheung PC, et al. Dermoscopy for common skin problems in Chinese children using a novel Hong Kong-made dermoscope. Hong Kong Med J. 2014;20:495-503.
6. Balaraman B, Friedman PM. Hypertrichotic Becker’s nevi treated with combination 1,550nm non-ablative fractional photothermolysis and laser hair removal. Lasers Surg Med. 2016;48:350-353.
A 15-year-old Caucasian boy presented for evaluation of an asymptomatic brown patch on his right shoulder. While the patient’s mother first noticed the patch when he was 5 years old, the discolored area had recently been expanding in size and had developed hypertrichosis. The patient was otherwise healthy; he took no medications and denied any symptoms or history of trauma to the area. None of his siblings were similarly affected.

A physical examination revealed a well-demarcated hyperpigmented patch with an irregularly shaped border and an increased number of terminal hairs (FIGURE 1). The affected area was not indurated, and there were no muscular or skeletal abnormalities on inspection. Examination of the patch under a dermatoscope revealed islands of reticular (lattice-like) hyperpigmentation, focal hypopigmentation, and prominent follicles (FIGURE 2).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
DIAGNOSIS: Becker melanosis
Becker melanosis (also called Becker’s nevus or Becker’s pigmentary hamartoma) is an organoid hamartoma that is most common among males.1 This benign area of hyperpigmentation typically manifests as a circumscribed patch with an irregular border on the upper trunk, shoulders, or upper arms of young men. Becker melanosis is usually acquired and typically comes to medical attention around the time of puberty, although there may be a history of discoloration (as was true in this case).
A diagnosis that’s usually made clinically
Androgenic origin. Because of the male predominance and association with hypertrichosis (and for that matter, acne), androgens have been thought to play a role in the development of Becker melanosis.2 The condition affects about 1 in 200 young men.1 To date, no specific gene defect has been identified.
Underlying hypoplasia of the breast or musculoskeletal abnormalities are uncommonly associated with Becker melanosis. When these abnormalities are present, the condition is known as Becker’s nevus syndrome.3
Look for the pattern. Becker melanosis is associated with homogenous brown patches with perifollicular hypopigmentation, sometimes with a faint reticular pattern.4,5 The diagnosis can usually be made clinically, but a skin biopsy can be helpful to confirm questionable cases. Dermoscopy can also assist in diagnosis. In this case, our patient’s presentation was typical, and additional studies were not needed.
Other causes of hyperpigmentation
The differential diagnosis includes other localized disorders associated with hyperpigmentation (TABLE1,3,4).

Continue to: Morphea
Morphea represents a thickening of collagen bundles in the skin. Although morphea can affect the shoulder and trunk, as Becker melanosis does, lesions of morphea feel firm to the touch and are not associated with hypertrichosis.
Localized post-inflammatory hyperpigmentation occurs following a traumatic event, such as a burn, or a prior dermatosis, such as zoster. Careful history-taking can uncover an antecedent inflammatory condition. Post-inflammatory pigment changes do not typically result in hypertrichosis.
Café-au-lait macules can manifest as isolated areas of discoloration. These macules can be an important indicator of neurofibromatosis, a genetic disorder in which tumors grow in the nervous system. Melanocytic hamartomas of the iris (Lisch nodules), axillary freckling (Crowe’s sign), or multiple cutaneous neurofibromas serve as additional clues to neurofibromatosis. In ambiguous cases, a skin biopsy can help differentiate a café au lait macule from Becker melanosis.
To treat or not to treat?
No treatment other than reassurance is needed in most cases of Becker melanosis, as it is a benign condition. Protecting the area from sunlight can minimize darkening and contrast with the surrounding skin. Electrolysis and laser therapy can be used to treat the associated hypertrichosis; laser therapy can also reduce the hyperpigmentation. Nonablative fractional resurfacing accompanied by laser hair removal is also reported to be of value.6
Our patient was satisfied with reassurance of the benign nature of the condition and did not elect treatment.
CORRESPONDENCE
Matthew F. Helm, MD, 500 University Drive, Suite 4300, Department of Dermatology, HU14, UPC II, Hershey, PA 17033-2360; [email protected]
A 15-year-old Caucasian boy presented for evaluation of an asymptomatic brown patch on his right shoulder. While the patient’s mother first noticed the patch when he was 5 years old, the discolored area had recently been expanding in size and had developed hypertrichosis. The patient was otherwise healthy; he took no medications and denied any symptoms or history of trauma to the area. None of his siblings were similarly affected.

A physical examination revealed a well-demarcated hyperpigmented patch with an irregularly shaped border and an increased number of terminal hairs (FIGURE 1). The affected area was not indurated, and there were no muscular or skeletal abnormalities on inspection. Examination of the patch under a dermatoscope revealed islands of reticular (lattice-like) hyperpigmentation, focal hypopigmentation, and prominent follicles (FIGURE 2).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
DIAGNOSIS: Becker melanosis
Becker melanosis (also called Becker’s nevus or Becker’s pigmentary hamartoma) is an organoid hamartoma that is most common among males.1 This benign area of hyperpigmentation typically manifests as a circumscribed patch with an irregular border on the upper trunk, shoulders, or upper arms of young men. Becker melanosis is usually acquired and typically comes to medical attention around the time of puberty, although there may be a history of discoloration (as was true in this case).
A diagnosis that’s usually made clinically
Androgenic origin. Because of the male predominance and association with hypertrichosis (and for that matter, acne), androgens have been thought to play a role in the development of Becker melanosis.2 The condition affects about 1 in 200 young men.1 To date, no specific gene defect has been identified.
Underlying hypoplasia of the breast or musculoskeletal abnormalities are uncommonly associated with Becker melanosis. When these abnormalities are present, the condition is known as Becker’s nevus syndrome.3
Look for the pattern. Becker melanosis is associated with homogenous brown patches with perifollicular hypopigmentation, sometimes with a faint reticular pattern.4,5 The diagnosis can usually be made clinically, but a skin biopsy can be helpful to confirm questionable cases. Dermoscopy can also assist in diagnosis. In this case, our patient’s presentation was typical, and additional studies were not needed.
Other causes of hyperpigmentation
The differential diagnosis includes other localized disorders associated with hyperpigmentation (TABLE1,3,4).

Continue to: Morphea
Morphea represents a thickening of collagen bundles in the skin. Although morphea can affect the shoulder and trunk, as Becker melanosis does, lesions of morphea feel firm to the touch and are not associated with hypertrichosis.
Localized post-inflammatory hyperpigmentation occurs following a traumatic event, such as a burn, or a prior dermatosis, such as zoster. Careful history-taking can uncover an antecedent inflammatory condition. Post-inflammatory pigment changes do not typically result in hypertrichosis.
Café-au-lait macules can manifest as isolated areas of discoloration. These macules can be an important indicator of neurofibromatosis, a genetic disorder in which tumors grow in the nervous system. Melanocytic hamartomas of the iris (Lisch nodules), axillary freckling (Crowe’s sign), or multiple cutaneous neurofibromas serve as additional clues to neurofibromatosis. In ambiguous cases, a skin biopsy can help differentiate a café au lait macule from Becker melanosis.
To treat or not to treat?
No treatment other than reassurance is needed in most cases of Becker melanosis, as it is a benign condition. Protecting the area from sunlight can minimize darkening and contrast with the surrounding skin. Electrolysis and laser therapy can be used to treat the associated hypertrichosis; laser therapy can also reduce the hyperpigmentation. Nonablative fractional resurfacing accompanied by laser hair removal is also reported to be of value.6
Our patient was satisfied with reassurance of the benign nature of the condition and did not elect treatment.
CORRESPONDENCE
Matthew F. Helm, MD, 500 University Drive, Suite 4300, Department of Dermatology, HU14, UPC II, Hershey, PA 17033-2360; [email protected]
1. Rabinovitz HS, Barnhill RL. Benign melanocytic neoplasms. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. New York, NY: Elsevier Saunders; 2012;112:1853-1854.
2. Person JR, Longcope C. Becker’s nevus: an androgen-mediated hyperplasia with increased androgen receptors. J Am Acad Dermatol. 1984;10:235-238.
3. Cosendey FE, Martinez NS, Bernhard GA, et al. Becker nevus syndrome. An Bras Dermatol. 2010;85:379-384.
4. Ingordo V, Iannazzone SS, Cusano F, et al. Dermoscopic features of congenital melanocytic nevus and Becker nevus in an adult male population: an analysis with 10-fold magnification. Dermatology. 2006;212:354-360.
5. Luk DC, Lam SY, Cheung PC, et al. Dermoscopy for common skin problems in Chinese children using a novel Hong Kong-made dermoscope. Hong Kong Med J. 2014;20:495-503.
6. Balaraman B, Friedman PM. Hypertrichotic Becker’s nevi treated with combination 1,550nm non-ablative fractional photothermolysis and laser hair removal. Lasers Surg Med. 2016;48:350-353.
1. Rabinovitz HS, Barnhill RL. Benign melanocytic neoplasms. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. New York, NY: Elsevier Saunders; 2012;112:1853-1854.
2. Person JR, Longcope C. Becker’s nevus: an androgen-mediated hyperplasia with increased androgen receptors. J Am Acad Dermatol. 1984;10:235-238.
3. Cosendey FE, Martinez NS, Bernhard GA, et al. Becker nevus syndrome. An Bras Dermatol. 2010;85:379-384.
4. Ingordo V, Iannazzone SS, Cusano F, et al. Dermoscopic features of congenital melanocytic nevus and Becker nevus in an adult male population: an analysis with 10-fold magnification. Dermatology. 2006;212:354-360.
5. Luk DC, Lam SY, Cheung PC, et al. Dermoscopy for common skin problems in Chinese children using a novel Hong Kong-made dermoscope. Hong Kong Med J. 2014;20:495-503.
6. Balaraman B, Friedman PM. Hypertrichotic Becker’s nevi treated with combination 1,550nm non-ablative fractional photothermolysis and laser hair removal. Lasers Surg Med. 2016;48:350-353.
How could improved provider communication have improved the care this patient received?
THE CASE
A 40-year-old white woman presented to clinic with multiple pruritic skin lesions on her abdomen, arms, and legs that had developed over a 2-month period. The patient reported that she’d been feeling tired and had been experiencing psychological stressors in her personal life. Her medical history was significant for psoriasis (which was controlled), and her family history was significant for breast and bone cancer (mother) and asbestos-related lung cancer (maternal grandfather).
A physical examination, which included breast and pelvic exams, was unremarkable apart from the lesions located on her abdomen, arms, and legs. On skin examination, we noted multiple polygonal, planar papules and plaques of varying size with an overlying scale (FIGURE).
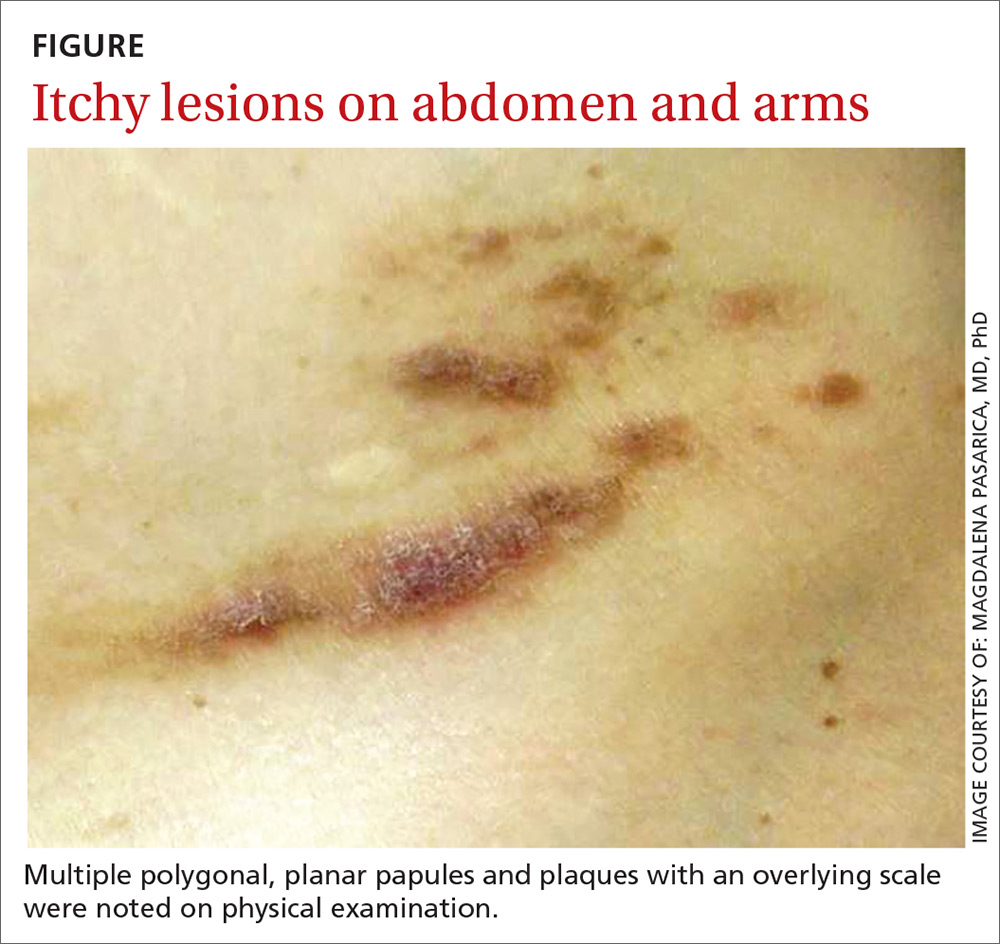
THE DIAGNOSIS
The physician obtained a biopsy of one of the skin lesions, and it was sent to a dermatopathologist to evaluate. Unfortunately, though, the patient’s history and a description of the lesion were not included with the initial biopsy requisition form. Based on the biopsy sample alone, the dermatopathologist’s report indicated a diagnosis of seborrheic keratosis.
A search for malignancy. Any case of sudden, extensive seborrheic keratosis is suspected to be a Leser-Trélat sign, which is known to be associated with human immunodeficiency virus or underlying malignancy—especially in the gastrointestinal system. The physician talked to the patient about the possibility of malignancy, and an extensive work-up was performed, including multiple laboratory tests, computed tomography (CT) imaging, an esophagogastroduodenoscopy, a colonoscopy, and mammography. None of the test results showed signs of an underlying malignancy.
In light of the negative findings, the physician reached out to the dermatopathologist to further discuss the case. It was determined that the dermatopathologist did not receive any clinical information (prior to this discussion) from the primary care office. This was surprising to the primary care physician, who was under the assumption that the clinical chart would be sent along with the biopsy sample. With this new information, the dermatopathologist reexamined the slides and diagnosed the lesion as lichen planus, a rather common skin disease not associated with cancer.
[polldaddy:10153197]
DISCUSSION
A root-cause analysis of this case identified multiple system failures, focused mainly on a lack of communication between providers:
- The description of the lesion and of the patient’s history were not included with the initial biopsy requisition form due to a lack of communication between the nurse and the physician performing the procedure.
- The dermatopathologist did not seek additional clinical information from the referring physician after receiving the sample.
- When the various providers did communicate, an accurate diagnosis was reached—but only after extensive investigation (and worry).
Communication is key to an accurate diagnosis
In 2000, it was estimated that health care costs due to preventable adverse events represent more than half of the $37.6 billion spent on health care.1 Since then, considerable effort has been made to address patient safety, misdiagnosis, and cost-effectiveness. Root cause analysis is one of the most popular methods used to evaluate and prevent future serious adverse events.2
Continue to: Diagnostic errors are often unreported...
Diagnostic errors are often unreported or unrecognized, especially in the outpatient setting.3 Studies focused on reducing diagnostic error show that a second review of pathology slides reduces error, controls costs, and improves quality of health care.4
Don’t rely (exclusively) on the health record. Gaps in effective communication between providers are a leading cause of preventable adverse events.5,6 The incorporation of electronic health records has allowed for more streamlined communication between providers. However, the mere presence of patient records in a common system does not guarantee the receipt or communication of information. The next step after entering the information into the record is to communicate it.
Our patient underwent a battery of costly and unnecessary tests and procedures, many of which were unwarranted at her age. In addition to being exposed to harmful radiation, she also experienced significant stress secondary to the tests and anticipation of the results. However, a root cause analysis of the case led to an improved protocol for communication between providers at the outpatient clinic. We now emphasize the necessity of including a clinical history and corresponding physical findings with all biopsies. We also encourage more direct communication between nursing staff, primary care physicians, and specialists.
THE TAKEAWAY
As medical professionals become increasingly reliant on the many emerging studies available to them, we sometimes forget that communication is key to optimal medical care, an accurate diagnosis, and patient safety.
Continue to: In addition, a second review...
In addition, a second review of dermatopathologic slides may be warranted if the pathologic diagnosis is inconsistent with the clinical picture or if the diagnosed condition is resistant to the usual therapies of choice. Incorrect diagnoses are more likely to occur when tests are interpreted in a vacuum without the corresponding clinical correlation. The weight of these mistakes is felt not only by the health care system, but by the patients themselves.
CORRESPONDENCE
Magdalena Pasarica, MD, PhD, University of Central Florida College of Medicine, 6850 Lake Nona Boulevard, Orlando, FL 32827; [email protected]
1. Kohn LT, Corrigan JM, Donaldson MS. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
2. U.S. Department of Health and Human Services. Patient safety primer: root cause analysis. https://psnet.ahrq.gov/primers/primer/10/root-cause-analysis. Updated August 2018. Accessed September 27, 2018.
3. Newman-Toker DE, Pronovost PJ. Diagnostic errors-the next frontier for patient safety. JAMA. 2009;301:1060-1062.
4. Kuijpers CC, Burger G, Al-Janabi S, et al. Improved quality of patient care through routine second review of histopathology specimens prior to multidisciplinary meetings. J Clin Pathol. 2016;69:866-871.
5. Leonard M, Graham S, Bonacum D. The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care. 2004;13:85-90.
6. Robinson NL. Promoting patient safety with perioperative hand-off communication. J Perianesth Nurs. 2016;31:245-253.
THE CASE
A 40-year-old white woman presented to clinic with multiple pruritic skin lesions on her abdomen, arms, and legs that had developed over a 2-month period. The patient reported that she’d been feeling tired and had been experiencing psychological stressors in her personal life. Her medical history was significant for psoriasis (which was controlled), and her family history was significant for breast and bone cancer (mother) and asbestos-related lung cancer (maternal grandfather).
A physical examination, which included breast and pelvic exams, was unremarkable apart from the lesions located on her abdomen, arms, and legs. On skin examination, we noted multiple polygonal, planar papules and plaques of varying size with an overlying scale (FIGURE).

THE DIAGNOSIS
The physician obtained a biopsy of one of the skin lesions, and it was sent to a dermatopathologist to evaluate. Unfortunately, though, the patient’s history and a description of the lesion were not included with the initial biopsy requisition form. Based on the biopsy sample alone, the dermatopathologist’s report indicated a diagnosis of seborrheic keratosis.
A search for malignancy. Any case of sudden, extensive seborrheic keratosis is suspected to be a Leser-Trélat sign, which is known to be associated with human immunodeficiency virus or underlying malignancy—especially in the gastrointestinal system. The physician talked to the patient about the possibility of malignancy, and an extensive work-up was performed, including multiple laboratory tests, computed tomography (CT) imaging, an esophagogastroduodenoscopy, a colonoscopy, and mammography. None of the test results showed signs of an underlying malignancy.
In light of the negative findings, the physician reached out to the dermatopathologist to further discuss the case. It was determined that the dermatopathologist did not receive any clinical information (prior to this discussion) from the primary care office. This was surprising to the primary care physician, who was under the assumption that the clinical chart would be sent along with the biopsy sample. With this new information, the dermatopathologist reexamined the slides and diagnosed the lesion as lichen planus, a rather common skin disease not associated with cancer.
[polldaddy:10153197]
DISCUSSION
A root-cause analysis of this case identified multiple system failures, focused mainly on a lack of communication between providers:
- The description of the lesion and of the patient’s history were not included with the initial biopsy requisition form due to a lack of communication between the nurse and the physician performing the procedure.
- The dermatopathologist did not seek additional clinical information from the referring physician after receiving the sample.
- When the various providers did communicate, an accurate diagnosis was reached—but only after extensive investigation (and worry).
Communication is key to an accurate diagnosis
In 2000, it was estimated that health care costs due to preventable adverse events represent more than half of the $37.6 billion spent on health care.1 Since then, considerable effort has been made to address patient safety, misdiagnosis, and cost-effectiveness. Root cause analysis is one of the most popular methods used to evaluate and prevent future serious adverse events.2
Continue to: Diagnostic errors are often unreported...
Diagnostic errors are often unreported or unrecognized, especially in the outpatient setting.3 Studies focused on reducing diagnostic error show that a second review of pathology slides reduces error, controls costs, and improves quality of health care.4
Don’t rely (exclusively) on the health record. Gaps in effective communication between providers are a leading cause of preventable adverse events.5,6 The incorporation of electronic health records has allowed for more streamlined communication between providers. However, the mere presence of patient records in a common system does not guarantee the receipt or communication of information. The next step after entering the information into the record is to communicate it.
Our patient underwent a battery of costly and unnecessary tests and procedures, many of which were unwarranted at her age. In addition to being exposed to harmful radiation, she also experienced significant stress secondary to the tests and anticipation of the results. However, a root cause analysis of the case led to an improved protocol for communication between providers at the outpatient clinic. We now emphasize the necessity of including a clinical history and corresponding physical findings with all biopsies. We also encourage more direct communication between nursing staff, primary care physicians, and specialists.
THE TAKEAWAY
As medical professionals become increasingly reliant on the many emerging studies available to them, we sometimes forget that communication is key to optimal medical care, an accurate diagnosis, and patient safety.
Continue to: In addition, a second review...
In addition, a second review of dermatopathologic slides may be warranted if the pathologic diagnosis is inconsistent with the clinical picture or if the diagnosed condition is resistant to the usual therapies of choice. Incorrect diagnoses are more likely to occur when tests are interpreted in a vacuum without the corresponding clinical correlation. The weight of these mistakes is felt not only by the health care system, but by the patients themselves.
CORRESPONDENCE
Magdalena Pasarica, MD, PhD, University of Central Florida College of Medicine, 6850 Lake Nona Boulevard, Orlando, FL 32827; [email protected]
THE CASE
A 40-year-old white woman presented to clinic with multiple pruritic skin lesions on her abdomen, arms, and legs that had developed over a 2-month period. The patient reported that she’d been feeling tired and had been experiencing psychological stressors in her personal life. Her medical history was significant for psoriasis (which was controlled), and her family history was significant for breast and bone cancer (mother) and asbestos-related lung cancer (maternal grandfather).
A physical examination, which included breast and pelvic exams, was unremarkable apart from the lesions located on her abdomen, arms, and legs. On skin examination, we noted multiple polygonal, planar papules and plaques of varying size with an overlying scale (FIGURE).

THE DIAGNOSIS
The physician obtained a biopsy of one of the skin lesions, and it was sent to a dermatopathologist to evaluate. Unfortunately, though, the patient’s history and a description of the lesion were not included with the initial biopsy requisition form. Based on the biopsy sample alone, the dermatopathologist’s report indicated a diagnosis of seborrheic keratosis.
A search for malignancy. Any case of sudden, extensive seborrheic keratosis is suspected to be a Leser-Trélat sign, which is known to be associated with human immunodeficiency virus or underlying malignancy—especially in the gastrointestinal system. The physician talked to the patient about the possibility of malignancy, and an extensive work-up was performed, including multiple laboratory tests, computed tomography (CT) imaging, an esophagogastroduodenoscopy, a colonoscopy, and mammography. None of the test results showed signs of an underlying malignancy.
In light of the negative findings, the physician reached out to the dermatopathologist to further discuss the case. It was determined that the dermatopathologist did not receive any clinical information (prior to this discussion) from the primary care office. This was surprising to the primary care physician, who was under the assumption that the clinical chart would be sent along with the biopsy sample. With this new information, the dermatopathologist reexamined the slides and diagnosed the lesion as lichen planus, a rather common skin disease not associated with cancer.
[polldaddy:10153197]
DISCUSSION
A root-cause analysis of this case identified multiple system failures, focused mainly on a lack of communication between providers:
- The description of the lesion and of the patient’s history were not included with the initial biopsy requisition form due to a lack of communication between the nurse and the physician performing the procedure.
- The dermatopathologist did not seek additional clinical information from the referring physician after receiving the sample.
- When the various providers did communicate, an accurate diagnosis was reached—but only after extensive investigation (and worry).
Communication is key to an accurate diagnosis
In 2000, it was estimated that health care costs due to preventable adverse events represent more than half of the $37.6 billion spent on health care.1 Since then, considerable effort has been made to address patient safety, misdiagnosis, and cost-effectiveness. Root cause analysis is one of the most popular methods used to evaluate and prevent future serious adverse events.2
Continue to: Diagnostic errors are often unreported...
Diagnostic errors are often unreported or unrecognized, especially in the outpatient setting.3 Studies focused on reducing diagnostic error show that a second review of pathology slides reduces error, controls costs, and improves quality of health care.4
Don’t rely (exclusively) on the health record. Gaps in effective communication between providers are a leading cause of preventable adverse events.5,6 The incorporation of electronic health records has allowed for more streamlined communication between providers. However, the mere presence of patient records in a common system does not guarantee the receipt or communication of information. The next step after entering the information into the record is to communicate it.
Our patient underwent a battery of costly and unnecessary tests and procedures, many of which were unwarranted at her age. In addition to being exposed to harmful radiation, she also experienced significant stress secondary to the tests and anticipation of the results. However, a root cause analysis of the case led to an improved protocol for communication between providers at the outpatient clinic. We now emphasize the necessity of including a clinical history and corresponding physical findings with all biopsies. We also encourage more direct communication between nursing staff, primary care physicians, and specialists.
THE TAKEAWAY
As medical professionals become increasingly reliant on the many emerging studies available to them, we sometimes forget that communication is key to optimal medical care, an accurate diagnosis, and patient safety.
Continue to: In addition, a second review...
In addition, a second review of dermatopathologic slides may be warranted if the pathologic diagnosis is inconsistent with the clinical picture or if the diagnosed condition is resistant to the usual therapies of choice. Incorrect diagnoses are more likely to occur when tests are interpreted in a vacuum without the corresponding clinical correlation. The weight of these mistakes is felt not only by the health care system, but by the patients themselves.
CORRESPONDENCE
Magdalena Pasarica, MD, PhD, University of Central Florida College of Medicine, 6850 Lake Nona Boulevard, Orlando, FL 32827; [email protected]
1. Kohn LT, Corrigan JM, Donaldson MS. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
2. U.S. Department of Health and Human Services. Patient safety primer: root cause analysis. https://psnet.ahrq.gov/primers/primer/10/root-cause-analysis. Updated August 2018. Accessed September 27, 2018.
3. Newman-Toker DE, Pronovost PJ. Diagnostic errors-the next frontier for patient safety. JAMA. 2009;301:1060-1062.
4. Kuijpers CC, Burger G, Al-Janabi S, et al. Improved quality of patient care through routine second review of histopathology specimens prior to multidisciplinary meetings. J Clin Pathol. 2016;69:866-871.
5. Leonard M, Graham S, Bonacum D. The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care. 2004;13:85-90.
6. Robinson NL. Promoting patient safety with perioperative hand-off communication. J Perianesth Nurs. 2016;31:245-253.
1. Kohn LT, Corrigan JM, Donaldson MS. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
2. U.S. Department of Health and Human Services. Patient safety primer: root cause analysis. https://psnet.ahrq.gov/primers/primer/10/root-cause-analysis. Updated August 2018. Accessed September 27, 2018.
3. Newman-Toker DE, Pronovost PJ. Diagnostic errors-the next frontier for patient safety. JAMA. 2009;301:1060-1062.
4. Kuijpers CC, Burger G, Al-Janabi S, et al. Improved quality of patient care through routine second review of histopathology specimens prior to multidisciplinary meetings. J Clin Pathol. 2016;69:866-871.
5. Leonard M, Graham S, Bonacum D. The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care. 2004;13:85-90.
6. Robinson NL. Promoting patient safety with perioperative hand-off communication. J Perianesth Nurs. 2016;31:245-253.
Should you reassess your patient’s asthma diagnosis?
ILLUSTRATIVE CASE
A 45-year-old woman presents to your office for a yearly visit. Two years ago she was started on an inhaled corticosteroid (ICS) and a bronchodilator rescue inhaler after being diagnosed with asthma based on her history and physical exam findings. She has had no exacerbations since then. Should you consider weaning her off the inhalers?
Asthma is a prevalent problem; 8% of adults ages 18 to 64 years have the chronic lung disease.2 Diagnosis can be challenging, partially because it requires measurement of transient airway resistance. And treatment entails significant costs and possible adverse effects. Without some sort of pulmonary function measurements or trials off medication, there is no clinical way to differentiate patients with well-controlled asthma from those who are being treated unnecessarily. Not surprisingly, studies have shown that ruling out active asthma and reducing medication usage is cost effective.3,4 This study followed a cohort of patients to see how many could be weaned off their asthma medications, and how they did in the subsequent year.
STUDY SUMMARY
About one-third of adults with asthma are “undiagnosed” within 5 years
The researchers recruited participants from the general population of the 10 largest cities and surrounding areas in Canada by randomly dialing cellular and landline phone numbers and asking about adult household members with asthma.1 The researchers focused on people with a recent (<5 years) asthma diagnosis, so as to represent contemporary diagnostic practice and to make it easier to collect medical records. Participants lived within 90 minutes of 10 medical centers in Canada. Patients were excluded if they were using long-term oral steroids, pregnant or breastfeeding, unable to tolerate spirometry or methacholine challenges, or had a history of more than 10 pack-years of smoking.
Of the 701 patients enrolled, 613 (87.4%) completed all study assessments. Patients progressed through a series of spirometry tests and were then tapered off their asthma-controlling medications.
The initial spirometry test confirmed asthma if bronchodilators caused a significant improvement in forced expiratory volume in the first second of expiration (FEV1). If there was no improvement, the patient took a methacholine challenge 1 week later; if they did well, their maintenance medications were reduced by half. If the patient did well with another methacholine challenge about 1 month later, maintenance medications were stopped, and the patient underwent a third methacholine challenge 3 weeks later.
Asthma was confirmed at any methacholine challenge if there was a 20% decrease in FEV1 from baseline at a methacholine concentration of ≤8 mg/mL; these patients were restarted on appropriate medications. If current asthma was ruled out, follow-up bronchial challenges were repeated at 6 and 12 months.
Results. Among the adults with physician-diagnosed asthma, 33.1% (95% confidence interval [CI], 29.4%-36.8%) no longer met criteria for an asthma diagnosis. Of those who no longer had asthma, 44% had previously undergone objective testing of airflow limitation. The investigators also found 12 patients (2%) had other serious cardiorespiratory conditions instead of asthma, including ischemic heart disease, subglottic stenosis, and bronchiectasis.
Continue to: During the 1-year follow-up period...
During the 1-year follow-up period, 22 (10.8%) of the 203 patients who were initially judged to no longer have asthma had a positive bronchial challenge test; 16 had no symptoms and continued to do well off all asthma medications. Six (3%) presented with respiratory symptoms and resumed treatment with asthma medications, but only 1 (0.5%) required oral corticosteroid therapy.
WHAT’S NEW?
Asthma meds are of no benefit for about one-third of patients taking them
This study found that one-third of patients with asthma diagnosed in the last 5 years no longer had symptoms or spirometry results consistent with asthma and did well in the subsequent year. For those patients, there appears to be no benefit to using asthma medications. The Global Institute for Asthma recommends stepping down treatment in adults with asthma that is well controlled for 3 months or more.5 While patients with objectively confirmed asthma diagnoses were more likely to still have asthma in this study, over 40% of patients who no longer had asthma were objectively proven to have had asthma at their original diagnosis.
CAVEATS
High level of rigor and the absence of a randomized trial
This study used a very structured protocol for tapering patients off their medications, including multiple spirometry tests, most including methacholine challenges, as well as oversight by pulmonologists. It is unclear whether this level of rigor is necessary for weaning in other clinical settings.
Also, this study was not a randomized trial, which is the gold standard for withdrawal of therapy. However, a cohort study is adequate to assess diagnostic testing, and this could be considered a trial of “undiagnosing” asthma in adults. The results here are consistent with those of a study that looked at asthma disappearance in groups of patients with and without obesity. In that study, approximately 30% of both groups of patients no longer had a diagnosis of asthma.6
Using random dialing is likely to have broadened the pool of patients this study drew upon. Also, there is a possibility that the patients who were lost to follow-up in this study represented those who had worsening symptoms. Some patients with mild asthma may have a waxing and waning course; it is possible that the study period was not long enough to capture this. In this study, only about 3% of patients who had their medications stopped reported worsening of symptoms.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
“Undiagnosis” is unusual
Using objective testing may provide some logistical or financial challenges for patients. Furthermore, “undiagnosing” a chronic disease like asthma is not a physician’s typical work, and it may take some time and effort to educate and monitor patients through the process.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
2. QuickStats: percentage of adults aged 18-64 years with current asthma,* by state - National Health Interview Survey,† 2014-2016. MMWR Morb Mortal Wkly Rep. 2018;67:590.
3. Pakhale S, Sumner A, Coyle D, et al. (Correcting) misdiagnoses of asthma: a cost effectiveness analysis. BMC Pulm Med. 2011;11:27.
4. Rank MA, Liesinger JT, Branda ME, et al. Comparative safety and costs of stepping down asthma medications in patients with controlled asthma. J Allergy Clin Immunol. 2016;137:1373-1379.
5. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2018. https://ginasthma.org. Accessed June 15, 2018.
6. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
ILLUSTRATIVE CASE
A 45-year-old woman presents to your office for a yearly visit. Two years ago she was started on an inhaled corticosteroid (ICS) and a bronchodilator rescue inhaler after being diagnosed with asthma based on her history and physical exam findings. She has had no exacerbations since then. Should you consider weaning her off the inhalers?
Asthma is a prevalent problem; 8% of adults ages 18 to 64 years have the chronic lung disease.2 Diagnosis can be challenging, partially because it requires measurement of transient airway resistance. And treatment entails significant costs and possible adverse effects. Without some sort of pulmonary function measurements or trials off medication, there is no clinical way to differentiate patients with well-controlled asthma from those who are being treated unnecessarily. Not surprisingly, studies have shown that ruling out active asthma and reducing medication usage is cost effective.3,4 This study followed a cohort of patients to see how many could be weaned off their asthma medications, and how they did in the subsequent year.
STUDY SUMMARY
About one-third of adults with asthma are “undiagnosed” within 5 years
The researchers recruited participants from the general population of the 10 largest cities and surrounding areas in Canada by randomly dialing cellular and landline phone numbers and asking about adult household members with asthma.1 The researchers focused on people with a recent (<5 years) asthma diagnosis, so as to represent contemporary diagnostic practice and to make it easier to collect medical records. Participants lived within 90 minutes of 10 medical centers in Canada. Patients were excluded if they were using long-term oral steroids, pregnant or breastfeeding, unable to tolerate spirometry or methacholine challenges, or had a history of more than 10 pack-years of smoking.
Of the 701 patients enrolled, 613 (87.4%) completed all study assessments. Patients progressed through a series of spirometry tests and were then tapered off their asthma-controlling medications.
The initial spirometry test confirmed asthma if bronchodilators caused a significant improvement in forced expiratory volume in the first second of expiration (FEV1). If there was no improvement, the patient took a methacholine challenge 1 week later; if they did well, their maintenance medications were reduced by half. If the patient did well with another methacholine challenge about 1 month later, maintenance medications were stopped, and the patient underwent a third methacholine challenge 3 weeks later.
Asthma was confirmed at any methacholine challenge if there was a 20% decrease in FEV1 from baseline at a methacholine concentration of ≤8 mg/mL; these patients were restarted on appropriate medications. If current asthma was ruled out, follow-up bronchial challenges were repeated at 6 and 12 months.
Results. Among the adults with physician-diagnosed asthma, 33.1% (95% confidence interval [CI], 29.4%-36.8%) no longer met criteria for an asthma diagnosis. Of those who no longer had asthma, 44% had previously undergone objective testing of airflow limitation. The investigators also found 12 patients (2%) had other serious cardiorespiratory conditions instead of asthma, including ischemic heart disease, subglottic stenosis, and bronchiectasis.
Continue to: During the 1-year follow-up period...
During the 1-year follow-up period, 22 (10.8%) of the 203 patients who were initially judged to no longer have asthma had a positive bronchial challenge test; 16 had no symptoms and continued to do well off all asthma medications. Six (3%) presented with respiratory symptoms and resumed treatment with asthma medications, but only 1 (0.5%) required oral corticosteroid therapy.
WHAT’S NEW?
Asthma meds are of no benefit for about one-third of patients taking them
This study found that one-third of patients with asthma diagnosed in the last 5 years no longer had symptoms or spirometry results consistent with asthma and did well in the subsequent year. For those patients, there appears to be no benefit to using asthma medications. The Global Institute for Asthma recommends stepping down treatment in adults with asthma that is well controlled for 3 months or more.5 While patients with objectively confirmed asthma diagnoses were more likely to still have asthma in this study, over 40% of patients who no longer had asthma were objectively proven to have had asthma at their original diagnosis.
CAVEATS
High level of rigor and the absence of a randomized trial
This study used a very structured protocol for tapering patients off their medications, including multiple spirometry tests, most including methacholine challenges, as well as oversight by pulmonologists. It is unclear whether this level of rigor is necessary for weaning in other clinical settings.
Also, this study was not a randomized trial, which is the gold standard for withdrawal of therapy. However, a cohort study is adequate to assess diagnostic testing, and this could be considered a trial of “undiagnosing” asthma in adults. The results here are consistent with those of a study that looked at asthma disappearance in groups of patients with and without obesity. In that study, approximately 30% of both groups of patients no longer had a diagnosis of asthma.6
Using random dialing is likely to have broadened the pool of patients this study drew upon. Also, there is a possibility that the patients who were lost to follow-up in this study represented those who had worsening symptoms. Some patients with mild asthma may have a waxing and waning course; it is possible that the study period was not long enough to capture this. In this study, only about 3% of patients who had their medications stopped reported worsening of symptoms.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
“Undiagnosis” is unusual
Using objective testing may provide some logistical or financial challenges for patients. Furthermore, “undiagnosing” a chronic disease like asthma is not a physician’s typical work, and it may take some time and effort to educate and monitor patients through the process.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 45-year-old woman presents to your office for a yearly visit. Two years ago she was started on an inhaled corticosteroid (ICS) and a bronchodilator rescue inhaler after being diagnosed with asthma based on her history and physical exam findings. She has had no exacerbations since then. Should you consider weaning her off the inhalers?
Asthma is a prevalent problem; 8% of adults ages 18 to 64 years have the chronic lung disease.2 Diagnosis can be challenging, partially because it requires measurement of transient airway resistance. And treatment entails significant costs and possible adverse effects. Without some sort of pulmonary function measurements or trials off medication, there is no clinical way to differentiate patients with well-controlled asthma from those who are being treated unnecessarily. Not surprisingly, studies have shown that ruling out active asthma and reducing medication usage is cost effective.3,4 This study followed a cohort of patients to see how many could be weaned off their asthma medications, and how they did in the subsequent year.
STUDY SUMMARY
About one-third of adults with asthma are “undiagnosed” within 5 years
The researchers recruited participants from the general population of the 10 largest cities and surrounding areas in Canada by randomly dialing cellular and landline phone numbers and asking about adult household members with asthma.1 The researchers focused on people with a recent (<5 years) asthma diagnosis, so as to represent contemporary diagnostic practice and to make it easier to collect medical records. Participants lived within 90 minutes of 10 medical centers in Canada. Patients were excluded if they were using long-term oral steroids, pregnant or breastfeeding, unable to tolerate spirometry or methacholine challenges, or had a history of more than 10 pack-years of smoking.
Of the 701 patients enrolled, 613 (87.4%) completed all study assessments. Patients progressed through a series of spirometry tests and were then tapered off their asthma-controlling medications.
The initial spirometry test confirmed asthma if bronchodilators caused a significant improvement in forced expiratory volume in the first second of expiration (FEV1). If there was no improvement, the patient took a methacholine challenge 1 week later; if they did well, their maintenance medications were reduced by half. If the patient did well with another methacholine challenge about 1 month later, maintenance medications were stopped, and the patient underwent a third methacholine challenge 3 weeks later.
Asthma was confirmed at any methacholine challenge if there was a 20% decrease in FEV1 from baseline at a methacholine concentration of ≤8 mg/mL; these patients were restarted on appropriate medications. If current asthma was ruled out, follow-up bronchial challenges were repeated at 6 and 12 months.
Results. Among the adults with physician-diagnosed asthma, 33.1% (95% confidence interval [CI], 29.4%-36.8%) no longer met criteria for an asthma diagnosis. Of those who no longer had asthma, 44% had previously undergone objective testing of airflow limitation. The investigators also found 12 patients (2%) had other serious cardiorespiratory conditions instead of asthma, including ischemic heart disease, subglottic stenosis, and bronchiectasis.
Continue to: During the 1-year follow-up period...
During the 1-year follow-up period, 22 (10.8%) of the 203 patients who were initially judged to no longer have asthma had a positive bronchial challenge test; 16 had no symptoms and continued to do well off all asthma medications. Six (3%) presented with respiratory symptoms and resumed treatment with asthma medications, but only 1 (0.5%) required oral corticosteroid therapy.
WHAT’S NEW?
Asthma meds are of no benefit for about one-third of patients taking them
This study found that one-third of patients with asthma diagnosed in the last 5 years no longer had symptoms or spirometry results consistent with asthma and did well in the subsequent year. For those patients, there appears to be no benefit to using asthma medications. The Global Institute for Asthma recommends stepping down treatment in adults with asthma that is well controlled for 3 months or more.5 While patients with objectively confirmed asthma diagnoses were more likely to still have asthma in this study, over 40% of patients who no longer had asthma were objectively proven to have had asthma at their original diagnosis.
CAVEATS
High level of rigor and the absence of a randomized trial
This study used a very structured protocol for tapering patients off their medications, including multiple spirometry tests, most including methacholine challenges, as well as oversight by pulmonologists. It is unclear whether this level of rigor is necessary for weaning in other clinical settings.
Also, this study was not a randomized trial, which is the gold standard for withdrawal of therapy. However, a cohort study is adequate to assess diagnostic testing, and this could be considered a trial of “undiagnosing” asthma in adults. The results here are consistent with those of a study that looked at asthma disappearance in groups of patients with and without obesity. In that study, approximately 30% of both groups of patients no longer had a diagnosis of asthma.6
Using random dialing is likely to have broadened the pool of patients this study drew upon. Also, there is a possibility that the patients who were lost to follow-up in this study represented those who had worsening symptoms. Some patients with mild asthma may have a waxing and waning course; it is possible that the study period was not long enough to capture this. In this study, only about 3% of patients who had their medications stopped reported worsening of symptoms.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
“Undiagnosis” is unusual
Using objective testing may provide some logistical or financial challenges for patients. Furthermore, “undiagnosing” a chronic disease like asthma is not a physician’s typical work, and it may take some time and effort to educate and monitor patients through the process.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
2. QuickStats: percentage of adults aged 18-64 years with current asthma,* by state - National Health Interview Survey,† 2014-2016. MMWR Morb Mortal Wkly Rep. 2018;67:590.
3. Pakhale S, Sumner A, Coyle D, et al. (Correcting) misdiagnoses of asthma: a cost effectiveness analysis. BMC Pulm Med. 2011;11:27.
4. Rank MA, Liesinger JT, Branda ME, et al. Comparative safety and costs of stepping down asthma medications in patients with controlled asthma. J Allergy Clin Immunol. 2016;137:1373-1379.
5. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2018. https://ginasthma.org. Accessed June 15, 2018.
6. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
1. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
2. QuickStats: percentage of adults aged 18-64 years with current asthma,* by state - National Health Interview Survey,† 2014-2016. MMWR Morb Mortal Wkly Rep. 2018;67:590.
3. Pakhale S, Sumner A, Coyle D, et al. (Correcting) misdiagnoses of asthma: a cost effectiveness analysis. BMC Pulm Med. 2011;11:27.
4. Rank MA, Liesinger JT, Branda ME, et al. Comparative safety and costs of stepping down asthma medications in patients with controlled asthma. J Allergy Clin Immunol. 2016;137:1373-1379.
5. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2018. https://ginasthma.org. Accessed June 15, 2018.
6. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
PRACTICE CHANGER
Consider tapering medications and retesting spirometry in adults with well-controlled asthma, as many may no longer have the disease.1
STRENGTH OF RECOMMENDATION
A: Based on a high-quality prospective cohort study and consistent findings in other studies.
Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
MSK injury? Make splinting choices based on the evidence
About 25% of all outpatient visits to family physicians include musculoskeletal (MSK) complaints.1 Splinting, bracing, or wrapping are used in 25% of these visits.2 The goals of splinting/bracing are multifold: accommodate a correct movement pattern, restrict poor movement patterns, and decrease the use of an injured area to allow for healing.
Splints and braces are generally noncircumferential and are easily put on and taken off. (The terms splints and braces can be used interchangeably.) The devices can be adjusted for swelling and are more comfortable than casts, but have the potential for poor patient adherence, may require frequent adjustment, and can allow for excessive motion.
Making the most of these devices requires an understanding of when the evidence supports (and doesn’t support) their use for particular injuries. In this article, we review the evidence for the use of splints/braces for common upper and lower extremity MSK conditions seen in family practice. We have confined our discussion to readily obtainable, off-the-shelf products. These products come in a variety of sizes and are easily kept on hand, or ordered through a durable medical equipment provider.
Carpal tunnel syndrome
Carpal tunnel syndrome (CTS) is a compression neuropathy of the median nerve at the level of the wrist. It is caused by several different conditions.
Goal of splinting: Minimize wrist movement to decrease any concomitant swelling in the carpal tunnel contributing to the compression. The two different types of orthoses commonly used are a neutral wrist splint (FIGURE 1) and a cock-up wrist splint (20° wrist extension).
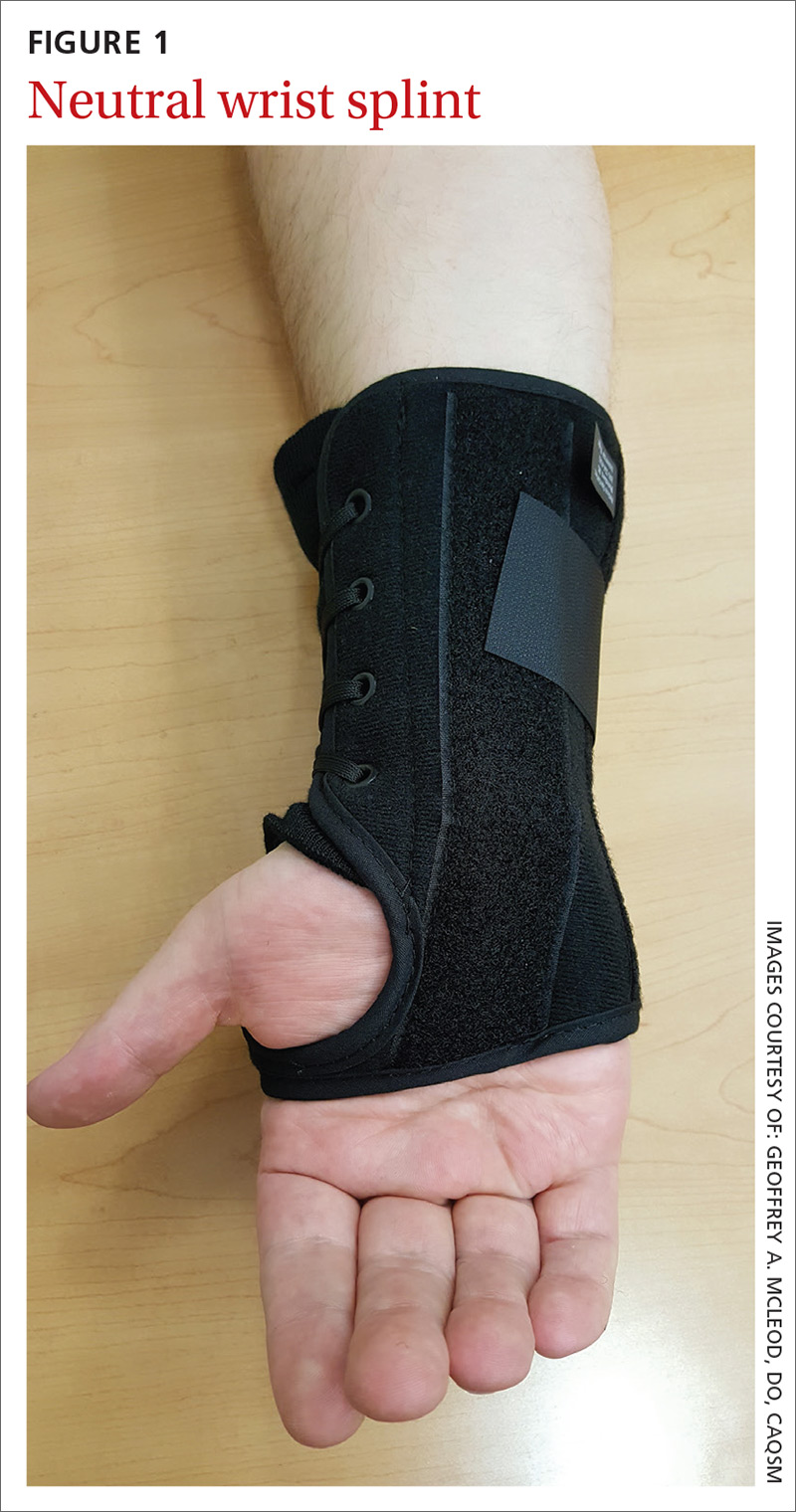
Evidence: A 2003 Cochrane review concluded that short-term symptom relief was achievable with bracing; however, better outcomes were seen with combination therapies (eg, medications, occupational therapy) vs splinting alone.3 A more recent Cochrane review in 2012 found poor or limited evidence that splint use at night was better than no treatment or any other nonsurgical treatment.4 There was also insufficient evidence to recommend one type of splint over another, although several poor-quality studies found neutral splinting to be more beneficial.5
A 2016 clinical practice guideline (CPG) from the American Academy of Orthopaedic Surgeons (AAOS) reported strong evidence supporting the use of immobilization.6 (Strong evidence is defined by the AAOS as 2 or more “high” strength studies with consistent findings for the intervention.6) Interestingly, of the 2 studies that AAOS used to make its conclusions,7,8 only the study by Manente et al8 was available at the time of the Cochrane 2012 review, and the Cochrane authors came to a different conclusion. The AAOS CPG does not comment on a specific type of brace.
Continue to: Harms
Harms: Both the 2012 Cochrane review and the AAOS statement indicate that there are no long-term harms other than some subjective discomfort in a minority of patients while wearing the splint.
Bottom line: A wrist splint should be considered in the treatment of CTS—especially if the condition is likely the result of repetitive wrist motion. If the patient can tolerate continuous use for 2 to 4 weeks, this should be employed. But at a minimum, nocturnal use for this duration would constitute a therapeutic trial. Combination therapy (ie, medication, occupational therapy, and splinting) is better than splinting alone.
de Quervain tendinopathy
This form of tendinopathy involves pain at the tendon sheaths of the abductor pollicis longus and the extensor pollicis brevis. Onset of symptoms has been attributed to overuse or repetitive movements of the wrist and thumb.
Goal of splinting: Immobilize the affected tendons to reduce irritation and/or inflammation. A thumb spica splint (FIGURE 2) is used to achieve this restriction.
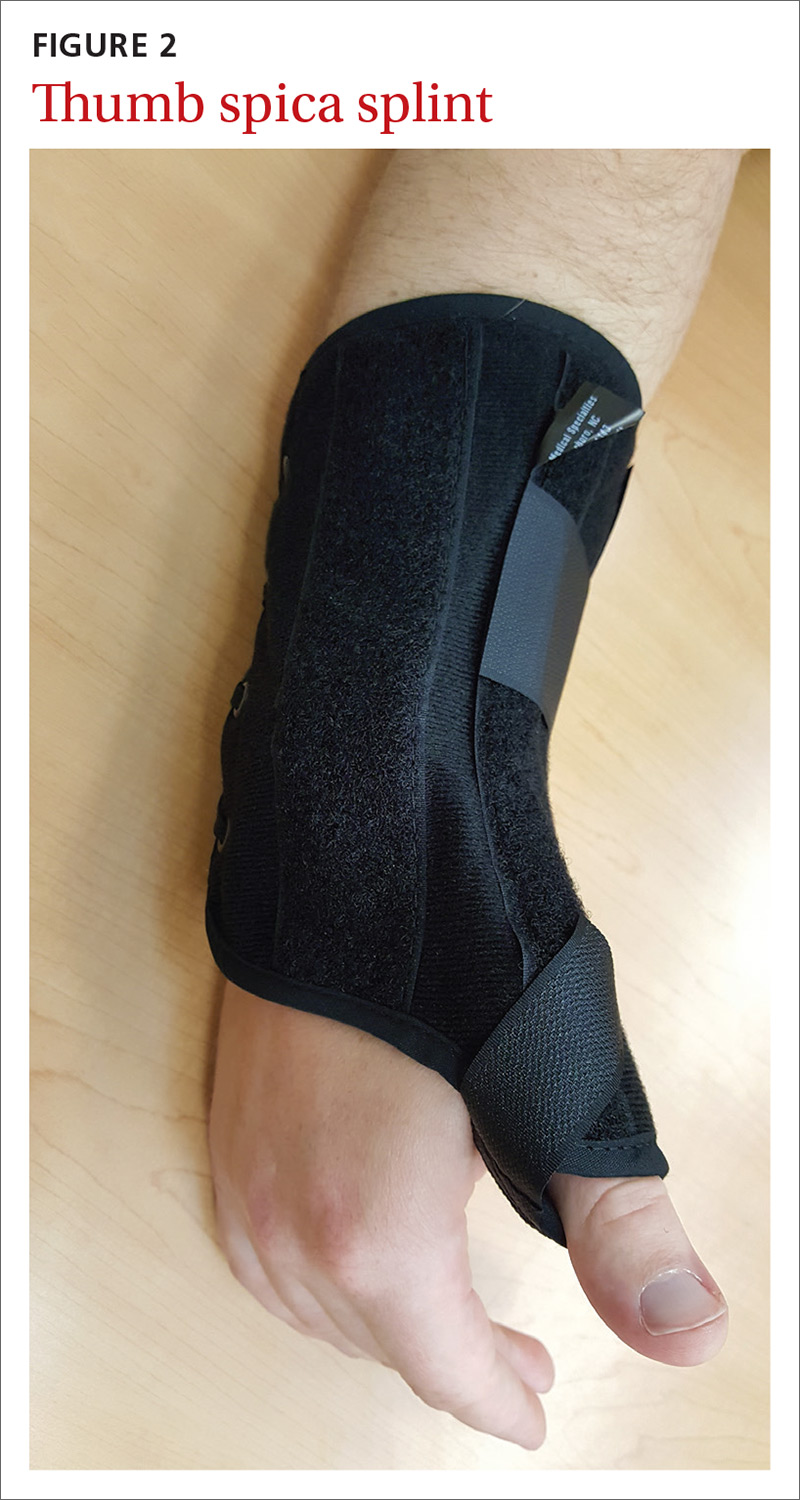
Evidence: Three randomized controlled trials (RCTs) suggested that the natural course is not affected by splint use for patients with prolonged symptoms (>3 months), and eventual resolution was noted in about 12 months—regardless of intervention with bracing. Symptoms improved more rapidly with the combination of medications and splint wear for those with a shorter duration of symptoms.9-11 Symptom severity driven wear compared with full-time use yielded equivalent outcomes.9 Those patients with longer duration and increased severity of symptoms fared worse regardless of treatment.10
Continue to: Harms
Harms: No documented harmful adverse effects (AEs) have been reported with splinting for this condition.
Bottom line: A thumb spica splint remains an option for de Quervain tendinopathy. It may provide symptomatic relief, especially if used early in the disease, but does not alter the natural disease course.
Lateral/medial epicondyle pain
Also known as tennis/golfer’s elbow, lateral/medial epicondyle pain is thought to result from overuse of the common wrist extensor/flexor muscle origins at the site of the myotendinous junctions.
Goal of splinting: To dampen or disperse the forces at the painful area via a counterforce brace (FIGURE 3). In addition, braces are used to decrease wrist use, specifically extension or flexion.
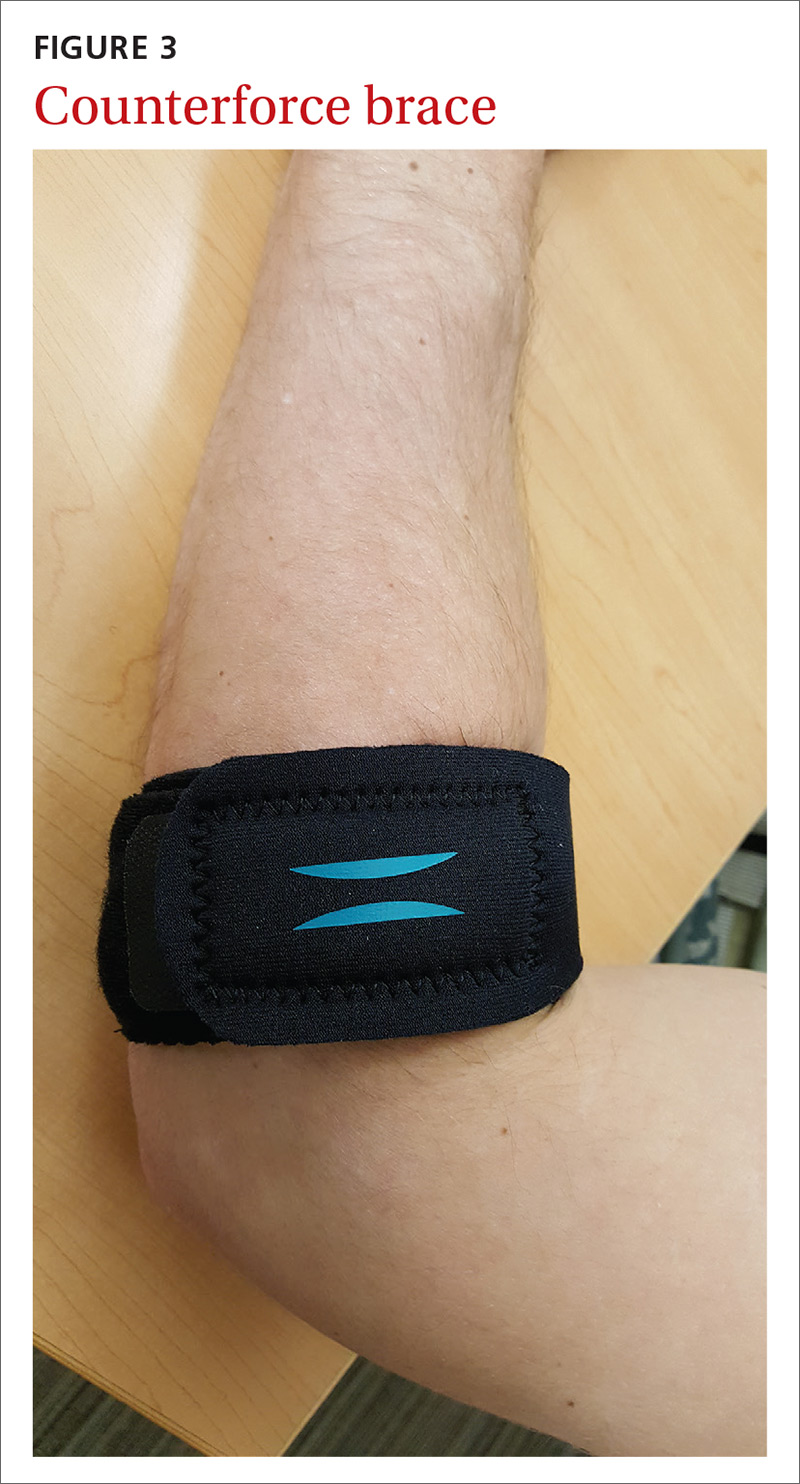
Evidence: A 2002 Cochrane Review found insufficient data to support the use of counterforce braces for relief of acute or chronic pain symptoms associated with epicondyle pain.Several studies supporting their use within this review were of varying quality with weak evidence.12
Continue to: Volar wrist braces have also been...
Volar wrist braces have also been studied for conservative management of epicondyle pain. Equivalent outcomes were noted comparing volar wrist bracing with a counterforce brace. Higher rates of recovery were seen in patients who participated in combination therapies (ie, bracing, physical therapy, and medication use).12
Harms: Use of counterforce braces for ≥30 days resulted in higher rates of braces restriction, more medical visits per patients, and higher medical costs. Derebery et al13 concluded that this was due to deconditioning on returning to normal activity. Use of a volar wrist brace should be discouraged as it reduces the active range of wrist motion, further contributing to deconditioning with long-term application.14
Bottom line: A trial of counterforce bracing should be used if pain precludes active rehabilitation or vocational pursuits, but should not be used as the sole therapy.
Knee osteoarthritis
Knee osteoarthritis (OA) can result from multiple (often commingled) etiologies, which ultimately result in loss of cartilage, ensuing bony abnormalities, and affected joint/soft tissue structures. Patients can present with severe symptoms with little loss of structural architecture or major structural changes with a paucity of symptoms.
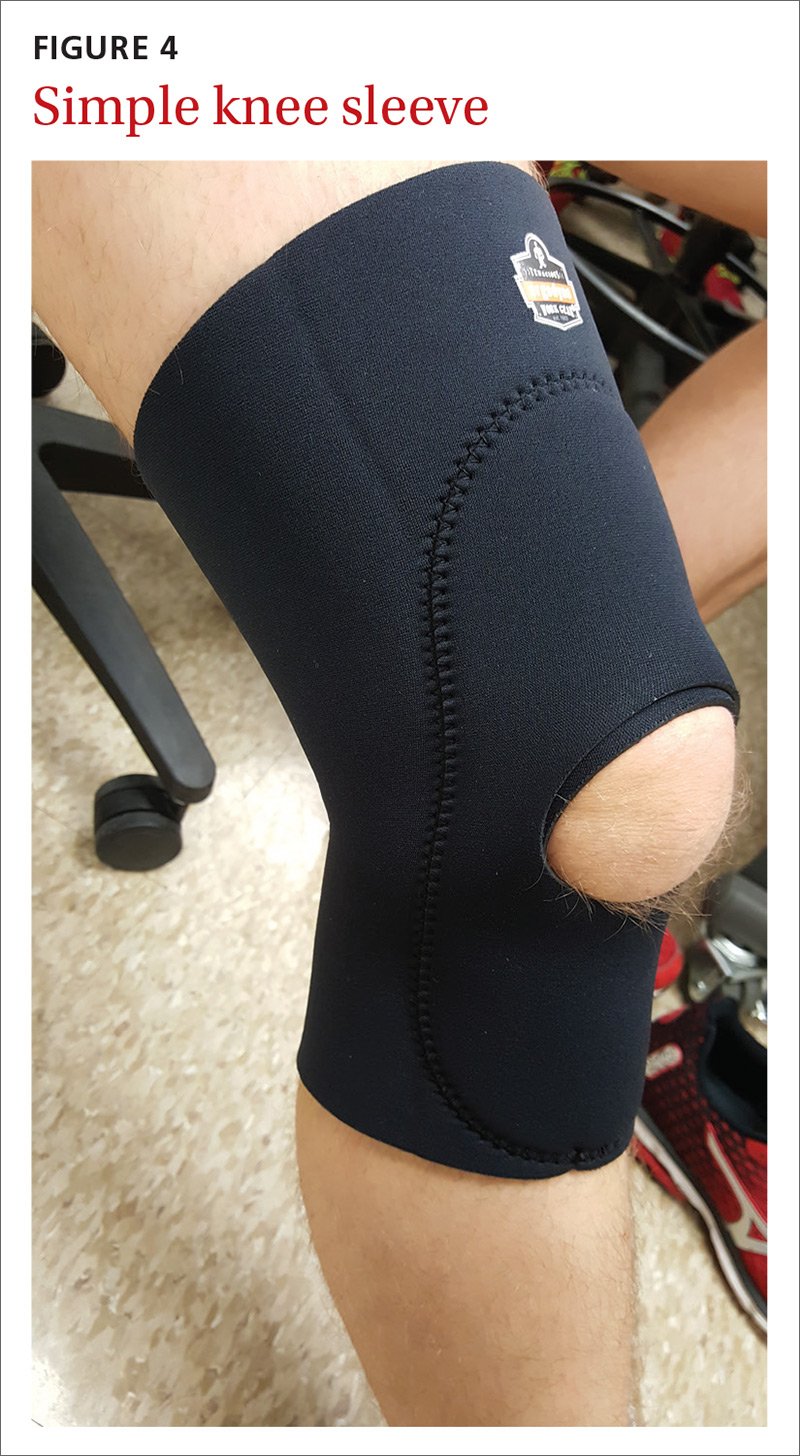
Goal of splinting: Depending on the orthoses used, the goals of splinting vary. A simple knee sleeve (FIGURE 4) provides warmth and proprioception, and a valgus unloader brace (FIGURE 5) provides valgus stress to open and unload the medial compartment.

Continue to: Evidence
Evidence: A single study evaluating a neutral knee sleeve vs control exhibited improved pain scores following several months of treatment. Mixed results were demonstrated with patient perceived quality of life improvement though.15 Currently, there is inconclusive evidence to support the use of valgus offloader braces per AAOS guidelines.16 This decision is based on 3 separate studies of moderate to high strength evidence. Improvements in the domains of pain, stiffness, self-reported functional capacity, and physical performance were unclear and no conclusions were able to be drawn.17,18
Harms: To date, no harmful AEs have been demonstrated with the use of knee sleeves. Valgus knee bracing can be uncomfortable, leading to poor adherence, but there are no long-term negative consequences.
Bottom line: Use of knee sleeves is worthwhile in patients with mild-to-moderate OA to improve functional scores. Inconclusive support for valgus knee bracing, along with the high cost of equipment, should reserve this option for patients with advanced OA who do not respond to typical conservative management and who are unwilling or ill-advised to undergo knee arthroplasty.16-18
Medial collateral ligament injury
An injury of the medial collateral ligament (MCL)—the medial stabilizer of the knee—can result from either a direct blow or a noncontact twisting injury. Grade 1 injuries have no actual ligament tear, grade 2 injuries have partial disruption, and grade 3 injuries denote a complete tear.
Goal of splinting: A hinged knee brace (FIGURE 6) allows for full extension but limited valgus and varus stresses.

Continue to: Evidence
Evidence: A conservative management strategy for an isolated injury is generally adequate to allow for sufficient healing, and “return to play” without prolonged disability. With conservative management, the affected joint is protected with a hinged knee brace for about 3 to 6 weeks.19,20 Data gathered on patients 9 years postinjury support the use of bracing of grades 1 to 2 injuries, but it is unclear what the optimal strategy is for grade 3 injuries.19
Harms: Generally well tolerated, and no harms have been reported.
Bottom line: Isolated grades 1 to 2 MCL injuries can be treated conservatively, and a hinged knee brace should be used as part of the rehabilitative process. It is unclear how to optimally manage grade 3 injuries.
Lateral ankle sprain
Lateral ankle sprains involve inversion injury to 1 or more of the 3 lateral ankle ligaments. Injuries are graded using the same grade schema as MCL injuries.
Goal of splinting: There are a variety of braces designed to provide lateral stability to patients with lateral ankle sprains. These stirrup braces differ in degree of support and additional fixation points—rigid (pneumatic) vs semirigid (Velcro, lace-up, etc) (FIGURE 7).
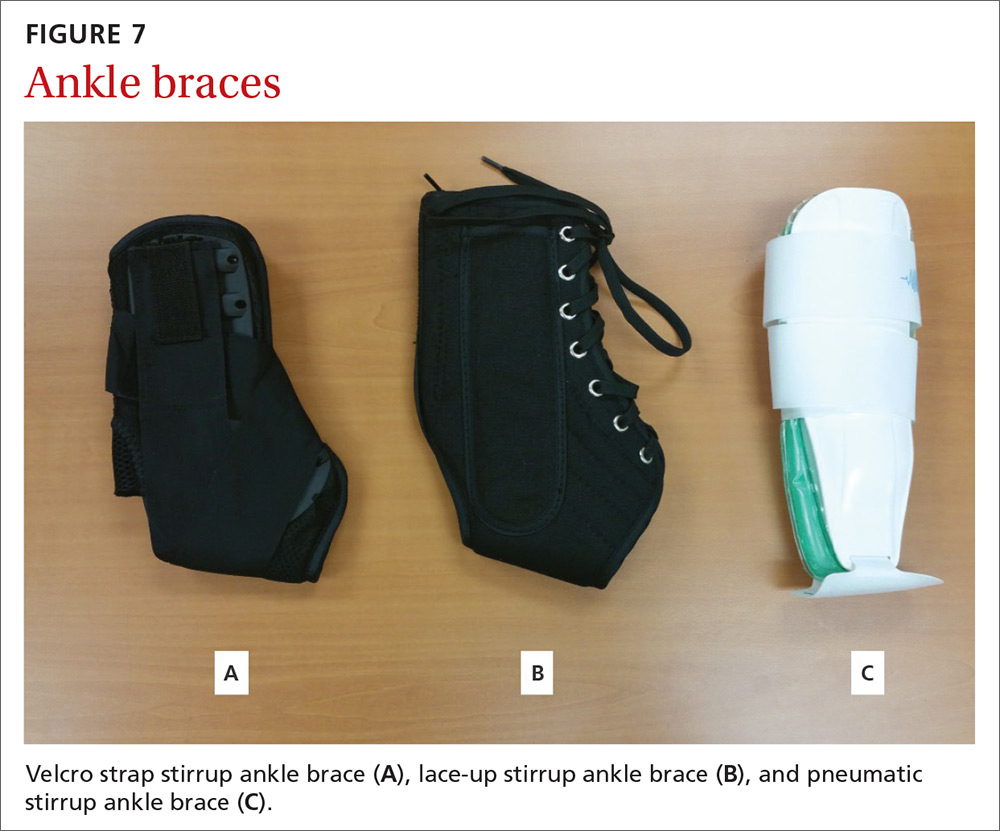
Continue to: Evidence
Evidence: A 2017 meta-analysis of systematic reviews found improved (self-reported) function when patients used external support devices such as tape, compression bandages, semirigid braces or boots, or walking casts.21 Secondary prevention utilizing brace wear during at-risk activities has been found to be the most important intervention to reduce recurrence.21,22
Harms: No direct injury from brace use has been reported, but consistent evidence exists that lack of early mobilization and rehabilitation can substantially affect the recovery from these injuries.
Bottom line: Consensus opinion recommends stirrup bracing for the treatment of grades 1 and 2 injuries.23,24 Controversy remains regarding brace use or complete immobilization for grade 3 injuries. Regardless of injury grade, early mobilization should be integrated into the treatment plan, coupled with active rehabilitation, including restoration of strength and proprioception. Prevention of second injuries is best accomplished with full rehabilitation and bracing during at-risk activities (eg, sports practices and competitions).21,22,25
A useful tool, but one not always covered by insurance
Bracing is a useful tool in the armamentarium of treating the common MSK complaints seen in everyday practice. Bracing must always be accompanied by a functional, active rehabilitation program.
Keep in mind, though, that many insurance plans may not cover the cost of bracing. Therefore, knowledge of its efficacy for a particular injury (or lack thereof) should guide treatment recommendations, along with shared decision making.
CORRESPONDENCE
Jeffrey C. Leggit, MD, CAQSM, 9706 Ethan Ridge Avenue, Frederick, MD 21704; [email protected].
1. MacKay C, Canizares M, Davis AM, et al. Health care utilization for musculoskeletal disorders. Arthritis Care Res (Hoboken). 2010;62:161-169.
2. CDC, National Center for Health Statistics. National hospital ambulatory medical care survey: 2010 outpatient department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_outpatient/2010_opd_web_tables.pdf. Accessed August 16, 2018.
3. O’Connor D, Marshall S, Massy-Westropp N. Non-surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database Syst Rev. 2003:CD003219.
4. Page MJ, Massy‐Westropp N, O’Connor D, et al. Splinting for carpal tunnel syndrome. Cochrane Database of Syst Rev. 2012:CD010003.
5. Burke DT, Burke MM, Stewart GW, et al. Splinting for carpal tunnel syndrome: in search of the optimal angle. Arch Phys Med Rehabil. 1994;75:1241-1244.
6. American Academy of Orthopaedic Surgeons. Management of Carpal Tunnel Syndrome Evidence-Based Clinical Practice Guideline. http://www.aaos.org/uploadedFiles/PreProduction/Quality/Guidelines_and_Reviews/guidelines/CTS%20CPG_2.29.16.pdf. Published February 29, 2016. Accessed August 16, 2018.
7. Hall B, Lee HC, Fitzgerald H, et al. Investigating the effectiveness of full-time wrist splinting and education in the treatment of carpal tunnel syndrome: a randomized controlled trial. Am J Occup Ther. 2013;7:448-459.
8. Manente G, Torrieri F, di Blasio F, et al. An innovative hand brace for carpal tunnel syndrome: a randomized controlled trial. Muscle Nerve. 2001;8:1020-1025.
9. Menendez ME, Thornton E, Kent S, et al. A prospective randomized clinical trial of prescription of full-time versus as-directed splint wear for de Quervain tendinopathy. Int Orthop. 2015;39:1563-1569.
10. Lane LB, Boretz RS, Stuchin SA. Treatment of de Quervain’s disease: role of conservative management. J Hand Surg Br. 2001;26:258-260.
11. Ring D, Schnellen A. Patient-centered care of de Quervain’s disease. J Hand Microsurg. 2009;1:68-71.
12. Struijs PA, Smidt N, Arola H, et al. Orthotic devices for the treatment of tennis elbow. Cochrane Database Syst Rev. 2002:CD001821.
13. Derebery VJ, Devenport JN, Giang GM, et al. The effects of splinting on outcomes of epicondylitis. Arch Phys Med Rehabil. 2005;86:1081-1088.
14. van de Streek MD, van der Schans CP, de Greef MH, et al. The effect of a forearm/hand splint compared with an elbow band as a treatment of lateral epicondylitis. Prosthet Orthot Int. 2004;28:183-189.
15. Brouwer RW, van Raaij TM, Verhaar JA, et al. Brace treatment for osteoarthritis of the knee: a prospective randomized multi-centre trial. Osteoarthritis Cartilage. 2006;14:777-783.
16. American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee, 2nd ed. Summary of recommendations. https://www.aaos.org/research/guidelines/OAKSummaryofRecommendations.pdf. Accessed August 16, 2018.
17. Kirkley A, Webster-Bogaert S, Litchfield R, et al. The effect of bracing on varus gonarthrosis. J Bone Joint Surg Am. 1999;81:539-548.
18. van Raaij TM, Reijman M, Brouwer RW, et al. Medial knee osteoarthritis treated by insoles or braces: a randomized trial. Clin Orthop Relat Res. 2010;468:1926-1932.
19. Kannus P. Long-term use of conservatively treated medial collateral ligament injuries of the knee joint. Clin Orthop Relat Res. 1988;226:103-112.
20. Stannard J. Medial and posteromedial instability of the knee: evaluation, treatment, and results. Sports Med Arthrosc Rev. 2010;18:263-268.
21. Doherty C, Bleakley C, Delahund E, et al. Treatment and prevention of acute and recurrent ankle sprain: an overview of systematic reviews with meta-analysis. Br J Sports Med. 2017;51:113-125.
22. Janssen KW, Hendriks MR, van Mechelen W, et al. The cost-effectiveness of measures to prevent recurrent ankle sprains: results of a 3-arm randomized controlled trial. Am J Sports Med. 2014;42:1534-1541.
23. Beynnon B, Renström P, Haugh L, et al. A prospective, randomized clinical investigation of the treatment of first-time ankle sprains. Am J Sports Med. 2006;34:1401-1412.
24. Seah R, Mani-Badu S. Managing ankle sprains in primary care: what is best practice? A systemic review of the last 10 years of evidence. Br Med Bull. 2011;97:105-135.
25. Kaminski TW, Hertel J, Amendola N, et al; National Athletic Trainers’ Association. National Athletic Trainers’ Association position statement: conservative management and prevention of ankle sprains in athletes. J Athl Train. 2013;48:528-545.
About 25% of all outpatient visits to family physicians include musculoskeletal (MSK) complaints.1 Splinting, bracing, or wrapping are used in 25% of these visits.2 The goals of splinting/bracing are multifold: accommodate a correct movement pattern, restrict poor movement patterns, and decrease the use of an injured area to allow for healing.
Splints and braces are generally noncircumferential and are easily put on and taken off. (The terms splints and braces can be used interchangeably.) The devices can be adjusted for swelling and are more comfortable than casts, but have the potential for poor patient adherence, may require frequent adjustment, and can allow for excessive motion.
Making the most of these devices requires an understanding of when the evidence supports (and doesn’t support) their use for particular injuries. In this article, we review the evidence for the use of splints/braces for common upper and lower extremity MSK conditions seen in family practice. We have confined our discussion to readily obtainable, off-the-shelf products. These products come in a variety of sizes and are easily kept on hand, or ordered through a durable medical equipment provider.
Carpal tunnel syndrome
Carpal tunnel syndrome (CTS) is a compression neuropathy of the median nerve at the level of the wrist. It is caused by several different conditions.
Goal of splinting: Minimize wrist movement to decrease any concomitant swelling in the carpal tunnel contributing to the compression. The two different types of orthoses commonly used are a neutral wrist splint (FIGURE 1) and a cock-up wrist splint (20° wrist extension).

Evidence: A 2003 Cochrane review concluded that short-term symptom relief was achievable with bracing; however, better outcomes were seen with combination therapies (eg, medications, occupational therapy) vs splinting alone.3 A more recent Cochrane review in 2012 found poor or limited evidence that splint use at night was better than no treatment or any other nonsurgical treatment.4 There was also insufficient evidence to recommend one type of splint over another, although several poor-quality studies found neutral splinting to be more beneficial.5
A 2016 clinical practice guideline (CPG) from the American Academy of Orthopaedic Surgeons (AAOS) reported strong evidence supporting the use of immobilization.6 (Strong evidence is defined by the AAOS as 2 or more “high” strength studies with consistent findings for the intervention.6) Interestingly, of the 2 studies that AAOS used to make its conclusions,7,8 only the study by Manente et al8 was available at the time of the Cochrane 2012 review, and the Cochrane authors came to a different conclusion. The AAOS CPG does not comment on a specific type of brace.
Continue to: Harms
Harms: Both the 2012 Cochrane review and the AAOS statement indicate that there are no long-term harms other than some subjective discomfort in a minority of patients while wearing the splint.
Bottom line: A wrist splint should be considered in the treatment of CTS—especially if the condition is likely the result of repetitive wrist motion. If the patient can tolerate continuous use for 2 to 4 weeks, this should be employed. But at a minimum, nocturnal use for this duration would constitute a therapeutic trial. Combination therapy (ie, medication, occupational therapy, and splinting) is better than splinting alone.
de Quervain tendinopathy
This form of tendinopathy involves pain at the tendon sheaths of the abductor pollicis longus and the extensor pollicis brevis. Onset of symptoms has been attributed to overuse or repetitive movements of the wrist and thumb.
Goal of splinting: Immobilize the affected tendons to reduce irritation and/or inflammation. A thumb spica splint (FIGURE 2) is used to achieve this restriction.

Evidence: Three randomized controlled trials (RCTs) suggested that the natural course is not affected by splint use for patients with prolonged symptoms (>3 months), and eventual resolution was noted in about 12 months—regardless of intervention with bracing. Symptoms improved more rapidly with the combination of medications and splint wear for those with a shorter duration of symptoms.9-11 Symptom severity driven wear compared with full-time use yielded equivalent outcomes.9 Those patients with longer duration and increased severity of symptoms fared worse regardless of treatment.10
Continue to: Harms
Harms: No documented harmful adverse effects (AEs) have been reported with splinting for this condition.
Bottom line: A thumb spica splint remains an option for de Quervain tendinopathy. It may provide symptomatic relief, especially if used early in the disease, but does not alter the natural disease course.
Lateral/medial epicondyle pain
Also known as tennis/golfer’s elbow, lateral/medial epicondyle pain is thought to result from overuse of the common wrist extensor/flexor muscle origins at the site of the myotendinous junctions.
Goal of splinting: To dampen or disperse the forces at the painful area via a counterforce brace (FIGURE 3). In addition, braces are used to decrease wrist use, specifically extension or flexion.

Evidence: A 2002 Cochrane Review found insufficient data to support the use of counterforce braces for relief of acute or chronic pain symptoms associated with epicondyle pain.Several studies supporting their use within this review were of varying quality with weak evidence.12
Continue to: Volar wrist braces have also been...
Volar wrist braces have also been studied for conservative management of epicondyle pain. Equivalent outcomes were noted comparing volar wrist bracing with a counterforce brace. Higher rates of recovery were seen in patients who participated in combination therapies (ie, bracing, physical therapy, and medication use).12
Harms: Use of counterforce braces for ≥30 days resulted in higher rates of braces restriction, more medical visits per patients, and higher medical costs. Derebery et al13 concluded that this was due to deconditioning on returning to normal activity. Use of a volar wrist brace should be discouraged as it reduces the active range of wrist motion, further contributing to deconditioning with long-term application.14
Bottom line: A trial of counterforce bracing should be used if pain precludes active rehabilitation or vocational pursuits, but should not be used as the sole therapy.
Knee osteoarthritis
Knee osteoarthritis (OA) can result from multiple (often commingled) etiologies, which ultimately result in loss of cartilage, ensuing bony abnormalities, and affected joint/soft tissue structures. Patients can present with severe symptoms with little loss of structural architecture or major structural changes with a paucity of symptoms.

Goal of splinting: Depending on the orthoses used, the goals of splinting vary. A simple knee sleeve (FIGURE 4) provides warmth and proprioception, and a valgus unloader brace (FIGURE 5) provides valgus stress to open and unload the medial compartment.

Continue to: Evidence
Evidence: A single study evaluating a neutral knee sleeve vs control exhibited improved pain scores following several months of treatment. Mixed results were demonstrated with patient perceived quality of life improvement though.15 Currently, there is inconclusive evidence to support the use of valgus offloader braces per AAOS guidelines.16 This decision is based on 3 separate studies of moderate to high strength evidence. Improvements in the domains of pain, stiffness, self-reported functional capacity, and physical performance were unclear and no conclusions were able to be drawn.17,18
Harms: To date, no harmful AEs have been demonstrated with the use of knee sleeves. Valgus knee bracing can be uncomfortable, leading to poor adherence, but there are no long-term negative consequences.
Bottom line: Use of knee sleeves is worthwhile in patients with mild-to-moderate OA to improve functional scores. Inconclusive support for valgus knee bracing, along with the high cost of equipment, should reserve this option for patients with advanced OA who do not respond to typical conservative management and who are unwilling or ill-advised to undergo knee arthroplasty.16-18
Medial collateral ligament injury
An injury of the medial collateral ligament (MCL)—the medial stabilizer of the knee—can result from either a direct blow or a noncontact twisting injury. Grade 1 injuries have no actual ligament tear, grade 2 injuries have partial disruption, and grade 3 injuries denote a complete tear.
Goal of splinting: A hinged knee brace (FIGURE 6) allows for full extension but limited valgus and varus stresses.

Continue to: Evidence
Evidence: A conservative management strategy for an isolated injury is generally adequate to allow for sufficient healing, and “return to play” without prolonged disability. With conservative management, the affected joint is protected with a hinged knee brace for about 3 to 6 weeks.19,20 Data gathered on patients 9 years postinjury support the use of bracing of grades 1 to 2 injuries, but it is unclear what the optimal strategy is for grade 3 injuries.19
Harms: Generally well tolerated, and no harms have been reported.
Bottom line: Isolated grades 1 to 2 MCL injuries can be treated conservatively, and a hinged knee brace should be used as part of the rehabilitative process. It is unclear how to optimally manage grade 3 injuries.
Lateral ankle sprain
Lateral ankle sprains involve inversion injury to 1 or more of the 3 lateral ankle ligaments. Injuries are graded using the same grade schema as MCL injuries.
Goal of splinting: There are a variety of braces designed to provide lateral stability to patients with lateral ankle sprains. These stirrup braces differ in degree of support and additional fixation points—rigid (pneumatic) vs semirigid (Velcro, lace-up, etc) (FIGURE 7).

Continue to: Evidence
Evidence: A 2017 meta-analysis of systematic reviews found improved (self-reported) function when patients used external support devices such as tape, compression bandages, semirigid braces or boots, or walking casts.21 Secondary prevention utilizing brace wear during at-risk activities has been found to be the most important intervention to reduce recurrence.21,22
Harms: No direct injury from brace use has been reported, but consistent evidence exists that lack of early mobilization and rehabilitation can substantially affect the recovery from these injuries.
Bottom line: Consensus opinion recommends stirrup bracing for the treatment of grades 1 and 2 injuries.23,24 Controversy remains regarding brace use or complete immobilization for grade 3 injuries. Regardless of injury grade, early mobilization should be integrated into the treatment plan, coupled with active rehabilitation, including restoration of strength and proprioception. Prevention of second injuries is best accomplished with full rehabilitation and bracing during at-risk activities (eg, sports practices and competitions).21,22,25
A useful tool, but one not always covered by insurance
Bracing is a useful tool in the armamentarium of treating the common MSK complaints seen in everyday practice. Bracing must always be accompanied by a functional, active rehabilitation program.
Keep in mind, though, that many insurance plans may not cover the cost of bracing. Therefore, knowledge of its efficacy for a particular injury (or lack thereof) should guide treatment recommendations, along with shared decision making.
CORRESPONDENCE
Jeffrey C. Leggit, MD, CAQSM, 9706 Ethan Ridge Avenue, Frederick, MD 21704; [email protected].
About 25% of all outpatient visits to family physicians include musculoskeletal (MSK) complaints.1 Splinting, bracing, or wrapping are used in 25% of these visits.2 The goals of splinting/bracing are multifold: accommodate a correct movement pattern, restrict poor movement patterns, and decrease the use of an injured area to allow for healing.
Splints and braces are generally noncircumferential and are easily put on and taken off. (The terms splints and braces can be used interchangeably.) The devices can be adjusted for swelling and are more comfortable than casts, but have the potential for poor patient adherence, may require frequent adjustment, and can allow for excessive motion.
Making the most of these devices requires an understanding of when the evidence supports (and doesn’t support) their use for particular injuries. In this article, we review the evidence for the use of splints/braces for common upper and lower extremity MSK conditions seen in family practice. We have confined our discussion to readily obtainable, off-the-shelf products. These products come in a variety of sizes and are easily kept on hand, or ordered through a durable medical equipment provider.
Carpal tunnel syndrome
Carpal tunnel syndrome (CTS) is a compression neuropathy of the median nerve at the level of the wrist. It is caused by several different conditions.
Goal of splinting: Minimize wrist movement to decrease any concomitant swelling in the carpal tunnel contributing to the compression. The two different types of orthoses commonly used are a neutral wrist splint (FIGURE 1) and a cock-up wrist splint (20° wrist extension).

Evidence: A 2003 Cochrane review concluded that short-term symptom relief was achievable with bracing; however, better outcomes were seen with combination therapies (eg, medications, occupational therapy) vs splinting alone.3 A more recent Cochrane review in 2012 found poor or limited evidence that splint use at night was better than no treatment or any other nonsurgical treatment.4 There was also insufficient evidence to recommend one type of splint over another, although several poor-quality studies found neutral splinting to be more beneficial.5
A 2016 clinical practice guideline (CPG) from the American Academy of Orthopaedic Surgeons (AAOS) reported strong evidence supporting the use of immobilization.6 (Strong evidence is defined by the AAOS as 2 or more “high” strength studies with consistent findings for the intervention.6) Interestingly, of the 2 studies that AAOS used to make its conclusions,7,8 only the study by Manente et al8 was available at the time of the Cochrane 2012 review, and the Cochrane authors came to a different conclusion. The AAOS CPG does not comment on a specific type of brace.
Continue to: Harms
Harms: Both the 2012 Cochrane review and the AAOS statement indicate that there are no long-term harms other than some subjective discomfort in a minority of patients while wearing the splint.
Bottom line: A wrist splint should be considered in the treatment of CTS—especially if the condition is likely the result of repetitive wrist motion. If the patient can tolerate continuous use for 2 to 4 weeks, this should be employed. But at a minimum, nocturnal use for this duration would constitute a therapeutic trial. Combination therapy (ie, medication, occupational therapy, and splinting) is better than splinting alone.
de Quervain tendinopathy
This form of tendinopathy involves pain at the tendon sheaths of the abductor pollicis longus and the extensor pollicis brevis. Onset of symptoms has been attributed to overuse or repetitive movements of the wrist and thumb.
Goal of splinting: Immobilize the affected tendons to reduce irritation and/or inflammation. A thumb spica splint (FIGURE 2) is used to achieve this restriction.

Evidence: Three randomized controlled trials (RCTs) suggested that the natural course is not affected by splint use for patients with prolonged symptoms (>3 months), and eventual resolution was noted in about 12 months—regardless of intervention with bracing. Symptoms improved more rapidly with the combination of medications and splint wear for those with a shorter duration of symptoms.9-11 Symptom severity driven wear compared with full-time use yielded equivalent outcomes.9 Those patients with longer duration and increased severity of symptoms fared worse regardless of treatment.10
Continue to: Harms
Harms: No documented harmful adverse effects (AEs) have been reported with splinting for this condition.
Bottom line: A thumb spica splint remains an option for de Quervain tendinopathy. It may provide symptomatic relief, especially if used early in the disease, but does not alter the natural disease course.
Lateral/medial epicondyle pain
Also known as tennis/golfer’s elbow, lateral/medial epicondyle pain is thought to result from overuse of the common wrist extensor/flexor muscle origins at the site of the myotendinous junctions.
Goal of splinting: To dampen or disperse the forces at the painful area via a counterforce brace (FIGURE 3). In addition, braces are used to decrease wrist use, specifically extension or flexion.

Evidence: A 2002 Cochrane Review found insufficient data to support the use of counterforce braces for relief of acute or chronic pain symptoms associated with epicondyle pain.Several studies supporting their use within this review were of varying quality with weak evidence.12
Continue to: Volar wrist braces have also been...
Volar wrist braces have also been studied for conservative management of epicondyle pain. Equivalent outcomes were noted comparing volar wrist bracing with a counterforce brace. Higher rates of recovery were seen in patients who participated in combination therapies (ie, bracing, physical therapy, and medication use).12
Harms: Use of counterforce braces for ≥30 days resulted in higher rates of braces restriction, more medical visits per patients, and higher medical costs. Derebery et al13 concluded that this was due to deconditioning on returning to normal activity. Use of a volar wrist brace should be discouraged as it reduces the active range of wrist motion, further contributing to deconditioning with long-term application.14
Bottom line: A trial of counterforce bracing should be used if pain precludes active rehabilitation or vocational pursuits, but should not be used as the sole therapy.
Knee osteoarthritis
Knee osteoarthritis (OA) can result from multiple (often commingled) etiologies, which ultimately result in loss of cartilage, ensuing bony abnormalities, and affected joint/soft tissue structures. Patients can present with severe symptoms with little loss of structural architecture or major structural changes with a paucity of symptoms.

Goal of splinting: Depending on the orthoses used, the goals of splinting vary. A simple knee sleeve (FIGURE 4) provides warmth and proprioception, and a valgus unloader brace (FIGURE 5) provides valgus stress to open and unload the medial compartment.

Continue to: Evidence
Evidence: A single study evaluating a neutral knee sleeve vs control exhibited improved pain scores following several months of treatment. Mixed results were demonstrated with patient perceived quality of life improvement though.15 Currently, there is inconclusive evidence to support the use of valgus offloader braces per AAOS guidelines.16 This decision is based on 3 separate studies of moderate to high strength evidence. Improvements in the domains of pain, stiffness, self-reported functional capacity, and physical performance were unclear and no conclusions were able to be drawn.17,18
Harms: To date, no harmful AEs have been demonstrated with the use of knee sleeves. Valgus knee bracing can be uncomfortable, leading to poor adherence, but there are no long-term negative consequences.
Bottom line: Use of knee sleeves is worthwhile in patients with mild-to-moderate OA to improve functional scores. Inconclusive support for valgus knee bracing, along with the high cost of equipment, should reserve this option for patients with advanced OA who do not respond to typical conservative management and who are unwilling or ill-advised to undergo knee arthroplasty.16-18
Medial collateral ligament injury
An injury of the medial collateral ligament (MCL)—the medial stabilizer of the knee—can result from either a direct blow or a noncontact twisting injury. Grade 1 injuries have no actual ligament tear, grade 2 injuries have partial disruption, and grade 3 injuries denote a complete tear.
Goal of splinting: A hinged knee brace (FIGURE 6) allows for full extension but limited valgus and varus stresses.

Continue to: Evidence
Evidence: A conservative management strategy for an isolated injury is generally adequate to allow for sufficient healing, and “return to play” without prolonged disability. With conservative management, the affected joint is protected with a hinged knee brace for about 3 to 6 weeks.19,20 Data gathered on patients 9 years postinjury support the use of bracing of grades 1 to 2 injuries, but it is unclear what the optimal strategy is for grade 3 injuries.19
Harms: Generally well tolerated, and no harms have been reported.
Bottom line: Isolated grades 1 to 2 MCL injuries can be treated conservatively, and a hinged knee brace should be used as part of the rehabilitative process. It is unclear how to optimally manage grade 3 injuries.
Lateral ankle sprain
Lateral ankle sprains involve inversion injury to 1 or more of the 3 lateral ankle ligaments. Injuries are graded using the same grade schema as MCL injuries.
Goal of splinting: There are a variety of braces designed to provide lateral stability to patients with lateral ankle sprains. These stirrup braces differ in degree of support and additional fixation points—rigid (pneumatic) vs semirigid (Velcro, lace-up, etc) (FIGURE 7).

Continue to: Evidence
Evidence: A 2017 meta-analysis of systematic reviews found improved (self-reported) function when patients used external support devices such as tape, compression bandages, semirigid braces or boots, or walking casts.21 Secondary prevention utilizing brace wear during at-risk activities has been found to be the most important intervention to reduce recurrence.21,22
Harms: No direct injury from brace use has been reported, but consistent evidence exists that lack of early mobilization and rehabilitation can substantially affect the recovery from these injuries.
Bottom line: Consensus opinion recommends stirrup bracing for the treatment of grades 1 and 2 injuries.23,24 Controversy remains regarding brace use or complete immobilization for grade 3 injuries. Regardless of injury grade, early mobilization should be integrated into the treatment plan, coupled with active rehabilitation, including restoration of strength and proprioception. Prevention of second injuries is best accomplished with full rehabilitation and bracing during at-risk activities (eg, sports practices and competitions).21,22,25
A useful tool, but one not always covered by insurance
Bracing is a useful tool in the armamentarium of treating the common MSK complaints seen in everyday practice. Bracing must always be accompanied by a functional, active rehabilitation program.
Keep in mind, though, that many insurance plans may not cover the cost of bracing. Therefore, knowledge of its efficacy for a particular injury (or lack thereof) should guide treatment recommendations, along with shared decision making.
CORRESPONDENCE
Jeffrey C. Leggit, MD, CAQSM, 9706 Ethan Ridge Avenue, Frederick, MD 21704; [email protected].
1. MacKay C, Canizares M, Davis AM, et al. Health care utilization for musculoskeletal disorders. Arthritis Care Res (Hoboken). 2010;62:161-169.
2. CDC, National Center for Health Statistics. National hospital ambulatory medical care survey: 2010 outpatient department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_outpatient/2010_opd_web_tables.pdf. Accessed August 16, 2018.
3. O’Connor D, Marshall S, Massy-Westropp N. Non-surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database Syst Rev. 2003:CD003219.
4. Page MJ, Massy‐Westropp N, O’Connor D, et al. Splinting for carpal tunnel syndrome. Cochrane Database of Syst Rev. 2012:CD010003.
5. Burke DT, Burke MM, Stewart GW, et al. Splinting for carpal tunnel syndrome: in search of the optimal angle. Arch Phys Med Rehabil. 1994;75:1241-1244.
6. American Academy of Orthopaedic Surgeons. Management of Carpal Tunnel Syndrome Evidence-Based Clinical Practice Guideline. http://www.aaos.org/uploadedFiles/PreProduction/Quality/Guidelines_and_Reviews/guidelines/CTS%20CPG_2.29.16.pdf. Published February 29, 2016. Accessed August 16, 2018.
7. Hall B, Lee HC, Fitzgerald H, et al. Investigating the effectiveness of full-time wrist splinting and education in the treatment of carpal tunnel syndrome: a randomized controlled trial. Am J Occup Ther. 2013;7:448-459.
8. Manente G, Torrieri F, di Blasio F, et al. An innovative hand brace for carpal tunnel syndrome: a randomized controlled trial. Muscle Nerve. 2001;8:1020-1025.
9. Menendez ME, Thornton E, Kent S, et al. A prospective randomized clinical trial of prescription of full-time versus as-directed splint wear for de Quervain tendinopathy. Int Orthop. 2015;39:1563-1569.
10. Lane LB, Boretz RS, Stuchin SA. Treatment of de Quervain’s disease: role of conservative management. J Hand Surg Br. 2001;26:258-260.
11. Ring D, Schnellen A. Patient-centered care of de Quervain’s disease. J Hand Microsurg. 2009;1:68-71.
12. Struijs PA, Smidt N, Arola H, et al. Orthotic devices for the treatment of tennis elbow. Cochrane Database Syst Rev. 2002:CD001821.
13. Derebery VJ, Devenport JN, Giang GM, et al. The effects of splinting on outcomes of epicondylitis. Arch Phys Med Rehabil. 2005;86:1081-1088.
14. van de Streek MD, van der Schans CP, de Greef MH, et al. The effect of a forearm/hand splint compared with an elbow band as a treatment of lateral epicondylitis. Prosthet Orthot Int. 2004;28:183-189.
15. Brouwer RW, van Raaij TM, Verhaar JA, et al. Brace treatment for osteoarthritis of the knee: a prospective randomized multi-centre trial. Osteoarthritis Cartilage. 2006;14:777-783.
16. American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee, 2nd ed. Summary of recommendations. https://www.aaos.org/research/guidelines/OAKSummaryofRecommendations.pdf. Accessed August 16, 2018.
17. Kirkley A, Webster-Bogaert S, Litchfield R, et al. The effect of bracing on varus gonarthrosis. J Bone Joint Surg Am. 1999;81:539-548.
18. van Raaij TM, Reijman M, Brouwer RW, et al. Medial knee osteoarthritis treated by insoles or braces: a randomized trial. Clin Orthop Relat Res. 2010;468:1926-1932.
19. Kannus P. Long-term use of conservatively treated medial collateral ligament injuries of the knee joint. Clin Orthop Relat Res. 1988;226:103-112.
20. Stannard J. Medial and posteromedial instability of the knee: evaluation, treatment, and results. Sports Med Arthrosc Rev. 2010;18:263-268.
21. Doherty C, Bleakley C, Delahund E, et al. Treatment and prevention of acute and recurrent ankle sprain: an overview of systematic reviews with meta-analysis. Br J Sports Med. 2017;51:113-125.
22. Janssen KW, Hendriks MR, van Mechelen W, et al. The cost-effectiveness of measures to prevent recurrent ankle sprains: results of a 3-arm randomized controlled trial. Am J Sports Med. 2014;42:1534-1541.
23. Beynnon B, Renström P, Haugh L, et al. A prospective, randomized clinical investigation of the treatment of first-time ankle sprains. Am J Sports Med. 2006;34:1401-1412.
24. Seah R, Mani-Badu S. Managing ankle sprains in primary care: what is best practice? A systemic review of the last 10 years of evidence. Br Med Bull. 2011;97:105-135.
25. Kaminski TW, Hertel J, Amendola N, et al; National Athletic Trainers’ Association. National Athletic Trainers’ Association position statement: conservative management and prevention of ankle sprains in athletes. J Athl Train. 2013;48:528-545.
1. MacKay C, Canizares M, Davis AM, et al. Health care utilization for musculoskeletal disorders. Arthritis Care Res (Hoboken). 2010;62:161-169.
2. CDC, National Center for Health Statistics. National hospital ambulatory medical care survey: 2010 outpatient department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_outpatient/2010_opd_web_tables.pdf. Accessed August 16, 2018.
3. O’Connor D, Marshall S, Massy-Westropp N. Non-surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database Syst Rev. 2003:CD003219.
4. Page MJ, Massy‐Westropp N, O’Connor D, et al. Splinting for carpal tunnel syndrome. Cochrane Database of Syst Rev. 2012:CD010003.
5. Burke DT, Burke MM, Stewart GW, et al. Splinting for carpal tunnel syndrome: in search of the optimal angle. Arch Phys Med Rehabil. 1994;75:1241-1244.
6. American Academy of Orthopaedic Surgeons. Management of Carpal Tunnel Syndrome Evidence-Based Clinical Practice Guideline. http://www.aaos.org/uploadedFiles/PreProduction/Quality/Guidelines_and_Reviews/guidelines/CTS%20CPG_2.29.16.pdf. Published February 29, 2016. Accessed August 16, 2018.
7. Hall B, Lee HC, Fitzgerald H, et al. Investigating the effectiveness of full-time wrist splinting and education in the treatment of carpal tunnel syndrome: a randomized controlled trial. Am J Occup Ther. 2013;7:448-459.
8. Manente G, Torrieri F, di Blasio F, et al. An innovative hand brace for carpal tunnel syndrome: a randomized controlled trial. Muscle Nerve. 2001;8:1020-1025.
9. Menendez ME, Thornton E, Kent S, et al. A prospective randomized clinical trial of prescription of full-time versus as-directed splint wear for de Quervain tendinopathy. Int Orthop. 2015;39:1563-1569.
10. Lane LB, Boretz RS, Stuchin SA. Treatment of de Quervain’s disease: role of conservative management. J Hand Surg Br. 2001;26:258-260.
11. Ring D, Schnellen A. Patient-centered care of de Quervain’s disease. J Hand Microsurg. 2009;1:68-71.
12. Struijs PA, Smidt N, Arola H, et al. Orthotic devices for the treatment of tennis elbow. Cochrane Database Syst Rev. 2002:CD001821.
13. Derebery VJ, Devenport JN, Giang GM, et al. The effects of splinting on outcomes of epicondylitis. Arch Phys Med Rehabil. 2005;86:1081-1088.
14. van de Streek MD, van der Schans CP, de Greef MH, et al. The effect of a forearm/hand splint compared with an elbow band as a treatment of lateral epicondylitis. Prosthet Orthot Int. 2004;28:183-189.
15. Brouwer RW, van Raaij TM, Verhaar JA, et al. Brace treatment for osteoarthritis of the knee: a prospective randomized multi-centre trial. Osteoarthritis Cartilage. 2006;14:777-783.
16. American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee, 2nd ed. Summary of recommendations. https://www.aaos.org/research/guidelines/OAKSummaryofRecommendations.pdf. Accessed August 16, 2018.
17. Kirkley A, Webster-Bogaert S, Litchfield R, et al. The effect of bracing on varus gonarthrosis. J Bone Joint Surg Am. 1999;81:539-548.
18. van Raaij TM, Reijman M, Brouwer RW, et al. Medial knee osteoarthritis treated by insoles or braces: a randomized trial. Clin Orthop Relat Res. 2010;468:1926-1932.
19. Kannus P. Long-term use of conservatively treated medial collateral ligament injuries of the knee joint. Clin Orthop Relat Res. 1988;226:103-112.
20. Stannard J. Medial and posteromedial instability of the knee: evaluation, treatment, and results. Sports Med Arthrosc Rev. 2010;18:263-268.
21. Doherty C, Bleakley C, Delahund E, et al. Treatment and prevention of acute and recurrent ankle sprain: an overview of systematic reviews with meta-analysis. Br J Sports Med. 2017;51:113-125.
22. Janssen KW, Hendriks MR, van Mechelen W, et al. The cost-effectiveness of measures to prevent recurrent ankle sprains: results of a 3-arm randomized controlled trial. Am J Sports Med. 2014;42:1534-1541.
23. Beynnon B, Renström P, Haugh L, et al. A prospective, randomized clinical investigation of the treatment of first-time ankle sprains. Am J Sports Med. 2006;34:1401-1412.
24. Seah R, Mani-Badu S. Managing ankle sprains in primary care: what is best practice? A systemic review of the last 10 years of evidence. Br Med Bull. 2011;97:105-135.
25. Kaminski TW, Hertel J, Amendola N, et al; National Athletic Trainers’ Association. National Athletic Trainers’ Association position statement: conservative management and prevention of ankle sprains in athletes. J Athl Train. 2013;48:528-545.
From The Journal of Family Practice | 2018;67(11):678-683.
PRACTICE RECOMMENDATIONS
› Consider a wrist splint for carpal tunnel syndrome secondary to repetitive motion. B
› Recommend a simple knee sleeve to help patients with osteoarthritis reduce their pain and improve daily function. B
› Use ankle bracing for secondary prevention of a recurrent ankle sprain. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series