User login
IV Dihydroergotamine Is Associated With Chest Pain in Pediatric Patients With Headache
Patients who continue DHE despite chest pain are more likely than patients who stop DHE to experience acute headache resolution.
CHICAGO—Among pediatric patients who receive IV dihydroergotamine (DHE) for headache, chest pain is a common side effect and reason for early cessation of DHE, according to a study presented at the 47th Annual Meeting of the Child Neurology Society. Chest pain may not represent a serious cardiovascular problem, and patients who continue DHE despite chest pain have better chances of acute headache resolution, compared with patients who stop DHE, said Sara Fridinger, MD, a fellow with the Division of Neurology at Children’s Hospital of Philadelphia.
IV DHE is an effective headache treatment for children, but it has many side effects, including chest pain. Chest pain in pediatric patients who receive IV DHE may result from esophageal spasms, but it raises concerns about myocardial ischemia because of the drug’s vasospastic qualities, the researchers said.
To determine the incidence and significance of chest pain among pediatric patients who received IV DHE for headache, Dr. Fridinger and Christina Szperka, MD, Director of the Pediatric Headache Program at Children’s Hospital of Philadelphia, conducted a retrospective chart review. They examined data from pediatric patients at their hospital who received IV DHE between January 2014 and July 2016. They excluded patients who received DHE for secondary headache. Data from 183 patients (median age, 15.7; 81% female) were included in their analysis, including reports of chest pain and other side effects, EKG data, and cardiac enzymes.
Chest pain occurred in 27% (n = 49) of patients who received DHE. Chest pain occurred after the first dose in 33% of patients and after the second dose in 61%. All patients received premedication before the dose that caused chest pain, and metoclopramide was used as premedication in 80% of cases. No patients with chest pain had elevated troponin. Of the 31% of patients with chest pain who had EKG abnormalities, the abnormalities were either unchanged from baseline or deemed not clinically significant. Of patients with chest pain, 39% stopped DHE due to chest pain, whereas 61% continued with the DHE protocol.
Thirty-seven percent of patients who stopped DHE due to chest pain and 50% of those who continued DHE despite chest pain achieved resolution of the acute headache.
“It is reassuring that no patients were found to have elevated cardiac enzymes and no patients had frankly abnormal EKGs,” said Drs. Fridinger and Szperka.
Patients who continue DHE despite chest pain are more likely than patients who stop DHE to experience acute headache resolution.
Patients who continue DHE despite chest pain are more likely than patients who stop DHE to experience acute headache resolution.
CHICAGO—Among pediatric patients who receive IV dihydroergotamine (DHE) for headache, chest pain is a common side effect and reason for early cessation of DHE, according to a study presented at the 47th Annual Meeting of the Child Neurology Society. Chest pain may not represent a serious cardiovascular problem, and patients who continue DHE despite chest pain have better chances of acute headache resolution, compared with patients who stop DHE, said Sara Fridinger, MD, a fellow with the Division of Neurology at Children’s Hospital of Philadelphia.
IV DHE is an effective headache treatment for children, but it has many side effects, including chest pain. Chest pain in pediatric patients who receive IV DHE may result from esophageal spasms, but it raises concerns about myocardial ischemia because of the drug’s vasospastic qualities, the researchers said.
To determine the incidence and significance of chest pain among pediatric patients who received IV DHE for headache, Dr. Fridinger and Christina Szperka, MD, Director of the Pediatric Headache Program at Children’s Hospital of Philadelphia, conducted a retrospective chart review. They examined data from pediatric patients at their hospital who received IV DHE between January 2014 and July 2016. They excluded patients who received DHE for secondary headache. Data from 183 patients (median age, 15.7; 81% female) were included in their analysis, including reports of chest pain and other side effects, EKG data, and cardiac enzymes.
Chest pain occurred in 27% (n = 49) of patients who received DHE. Chest pain occurred after the first dose in 33% of patients and after the second dose in 61%. All patients received premedication before the dose that caused chest pain, and metoclopramide was used as premedication in 80% of cases. No patients with chest pain had elevated troponin. Of the 31% of patients with chest pain who had EKG abnormalities, the abnormalities were either unchanged from baseline or deemed not clinically significant. Of patients with chest pain, 39% stopped DHE due to chest pain, whereas 61% continued with the DHE protocol.
Thirty-seven percent of patients who stopped DHE due to chest pain and 50% of those who continued DHE despite chest pain achieved resolution of the acute headache.
“It is reassuring that no patients were found to have elevated cardiac enzymes and no patients had frankly abnormal EKGs,” said Drs. Fridinger and Szperka.
CHICAGO—Among pediatric patients who receive IV dihydroergotamine (DHE) for headache, chest pain is a common side effect and reason for early cessation of DHE, according to a study presented at the 47th Annual Meeting of the Child Neurology Society. Chest pain may not represent a serious cardiovascular problem, and patients who continue DHE despite chest pain have better chances of acute headache resolution, compared with patients who stop DHE, said Sara Fridinger, MD, a fellow with the Division of Neurology at Children’s Hospital of Philadelphia.
IV DHE is an effective headache treatment for children, but it has many side effects, including chest pain. Chest pain in pediatric patients who receive IV DHE may result from esophageal spasms, but it raises concerns about myocardial ischemia because of the drug’s vasospastic qualities, the researchers said.
To determine the incidence and significance of chest pain among pediatric patients who received IV DHE for headache, Dr. Fridinger and Christina Szperka, MD, Director of the Pediatric Headache Program at Children’s Hospital of Philadelphia, conducted a retrospective chart review. They examined data from pediatric patients at their hospital who received IV DHE between January 2014 and July 2016. They excluded patients who received DHE for secondary headache. Data from 183 patients (median age, 15.7; 81% female) were included in their analysis, including reports of chest pain and other side effects, EKG data, and cardiac enzymes.
Chest pain occurred in 27% (n = 49) of patients who received DHE. Chest pain occurred after the first dose in 33% of patients and after the second dose in 61%. All patients received premedication before the dose that caused chest pain, and metoclopramide was used as premedication in 80% of cases. No patients with chest pain had elevated troponin. Of the 31% of patients with chest pain who had EKG abnormalities, the abnormalities were either unchanged from baseline or deemed not clinically significant. Of patients with chest pain, 39% stopped DHE due to chest pain, whereas 61% continued with the DHE protocol.
Thirty-seven percent of patients who stopped DHE due to chest pain and 50% of those who continued DHE despite chest pain achieved resolution of the acute headache.
“It is reassuring that no patients were found to have elevated cardiac enzymes and no patients had frankly abnormal EKGs,” said Drs. Fridinger and Szperka.
Military Grooming Standards and Their Impact on Skin Diseases of the Head and Neck
The US military enforces grooming standards to ensure the professional appearance and serviceability of soldiers in all operational settings. Although most individuals are able to uphold these regulations without incident, there is a growing cohort of servicemembers with skin diseases that were exacerbated or even initiated by haircuts, hairstyling, and shaving required to conform to these grooming standards. These skin diseases, which can affect both sexes and may not be appreciated until years into a soldier's service commitment, can have consequences related to individual morbidity and medical readiness for deployment, making it an important issue for medical practitioners to recognize and manage in servicemembers.
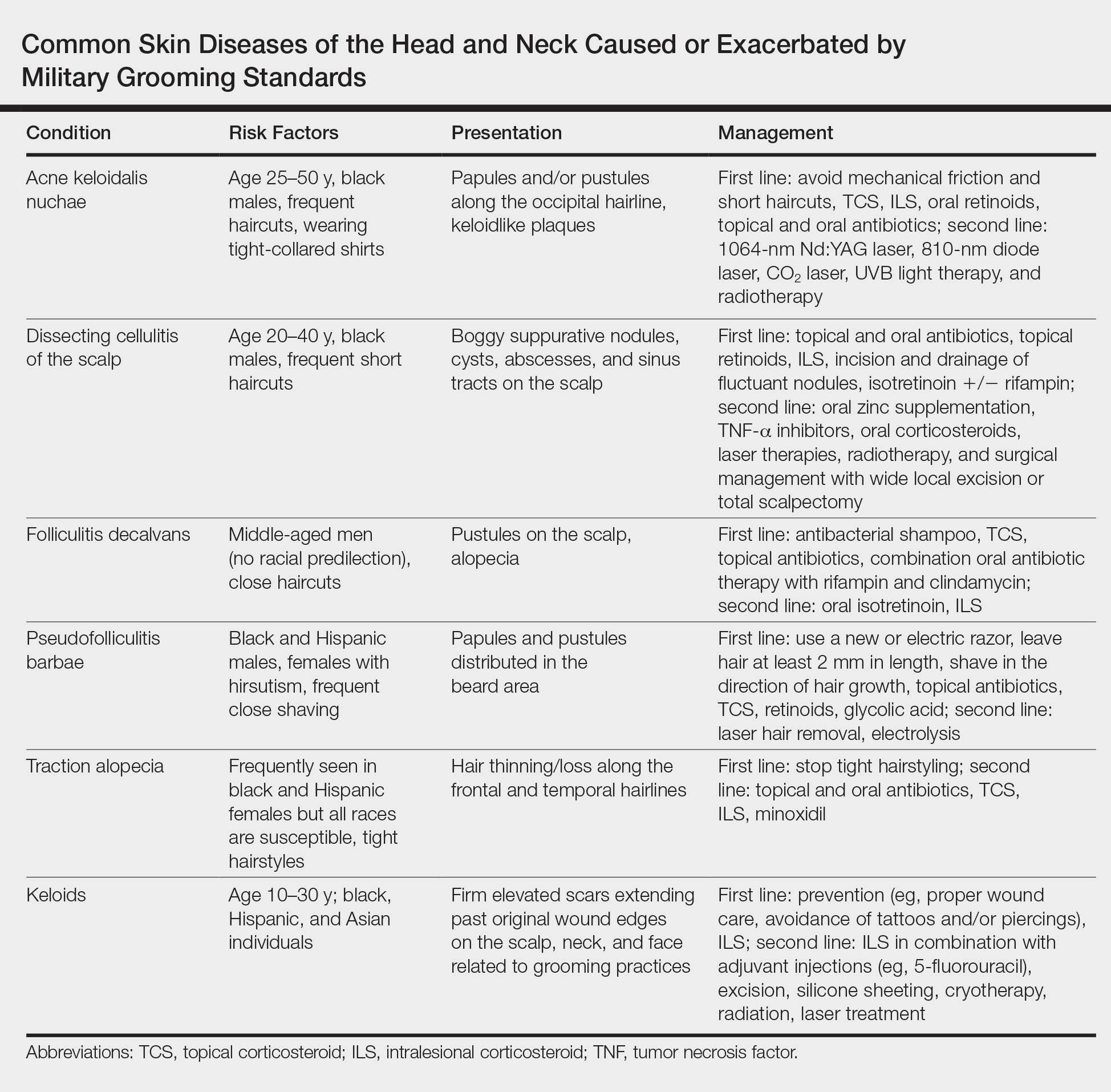
This review highlights several disorders of the pilosebaceous unit of the head and neck that can be caused or exacerbated by military grooming standards, including inflammatory hair disorders, traction alopecia, and pseudofolliculitis barbae. Discussion of each entity will include a review of susceptibility and causality as well as initial treatment options to consider (Table).
Inflammatory Hair Disorders
The proper appearance of servicemembers in uniform represents self-discipline and conformity to the high standards of the military. This transition occurs as a rite of passage for many new male recruits who receive shaved haircuts during their first days of basic training. Thereafter, male servicemembers are required to maintain a tapered appearance of the hair per military regulations.1 Clipping hair closely to the scalp or shaving the head entirely are authorized and often encouraged; therefore, high and tight haircuts and buzz cuts are popular among male soldiers due to the general ease of care and ability to maintain the haircut themselves. Conversely, these styles require servicemembers to get weekly or biweekly haircuts that in turn can lead to chronic trauma and irritation. In more susceptible populations, inflammatory hair disorders such as acne keloidalis nuchae (AKN), dissecting cellulitis of the scalp, and folliculitis decalvans may be incited.
Acne Keloidalis Nuchae
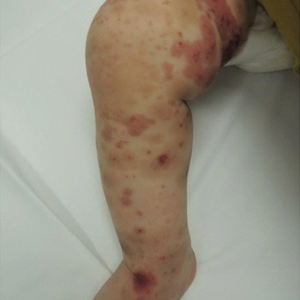
Acne keloidalis nuchae, also called folliculitis keloidalis, is a chronic scarring folliculitis presenting with papules and plaques on the occiput and nape of the neck that may merge to form hypertrophic scars or keloids. This disorder most commonly develops in young black men but also can be seen in black females and white patients of both sexes.2 Acne keloidalis nuchae shares many histologic features with central centrifugal cicatricial alopecia, which may suggest a similar pathogenesis. Apart from frequent haircuts, tight-collared shirts, such as those on military service uniforms, also have been associated with AKN. Because of these suspected etiologies, first-line treatment focuses on preventing further trauma by avoiding mechanical irritation and short haircuts, which may be difficult in the military setting. For earlier disease stages, topical and intralesional corticosteroids, oral retinoids, and topical and oral antibiotics are used for their anti-inflammatory properties.3 In refractory cases, surgical excision with healing by secondary intention may be attempted.4 Additional treatment options include the 1064-nm Nd:YAG and 810-nm diode lasers,3 UVB light therapy, CO2 laser, and radiotherapy.
Dissecting Cellulitis of the Scalp
Similar to AKN, dissecting cellulitis of the scalp is another inflammatory hair disorder that is worsened by frequent short haircuts.5 Dissecting cellulitis of the scalp is a primary cicatricial alopecia proposed to be secondary to follicular occlusion. It often is seen in black males aged 20 to 40 years and is characterized by boggy suppurative nodules and cysts with draining sinus tracts, abscesses, and resultant scarring alopecia. Dissecting cellulitis of the scalp is part of the follicular occlusion tetrad, which also includes hidradenitis suppurativa, acne conglobata, and pilonidal cysts. First-line therapies include topical and oral antibiotics, topical retinoids, intralesional corticosteroids, incision and drainage of fluctuant nodules, and oral isotretinoin with or without rifampin. Alternative treatments include oral zinc supplementation, oral corticosteroids, tumor necrosis factor α inhibitors, laser therapies, radiotherapy, and surgical management with wide local excision or total scalpectomy.6,7
Folliculitis Decalvans
Folliculitis decalvans is a primary cicatricial alopecia of the scalp that most commonly presents in middle-aged men without racial predilection.8 Folliculitis decalvans presents with multiple pustules, crusts, tufted hairs, and perifollicular hyperkeratosis, leading to scarring of the scalp, which often is most severe on the posterior vertex. Staphylococcus aureus is a presumed player in the pathogenesis of folliculitis decalvans with superantigens causing release of cytokines stimulating follicular destruction. Close haircuts in conformation with military grooming standards can contribute to this condition due to mechanical trauma and subsequent inflammation. It typically is diagnosed clinically, but if histologic confirmation is desired, a sample from the periphery of early lesions is preferred.9 Initial treatment consists of antibacterial shampoos, topical corticosteroids, topical antibiotics, and combination oral antibiotic therapy with rifampin and clindamycin. Studies using oral isotretinoin have shown variable results,10,11 and the most effective treatment of recalcitrant lesions appears to be intralesional corticosteroids.12
Follicular and Scarring Disorders
In addition to inflammatory hair disorders, military grooming standards have been linked to the pathogenesis of diseases such as pseudofolliculitis barbae, traction alopecia, and keloids, specifically through irritation of the face, neck, and scalp, as well as damage to the follicular unit.5 These conditions develop because grooming regulations necessitate certain hair practices such as close shaving of facial and neck hair and keeping long hair secured relatively tightly to the scalp.
Pseudofolliculitis Barbae
Males in the military are obligated to keep their faces clean-shaven.1 They may acquire a medical waiver for a specified beard length if deemed appropriate by the treating physician,1 which often leads to the need for continual waiver renewal and also may warrant possible negative perception from peers, subordinates, and leadership. One of the most prevalent conditions that is closely associated with shaving is pseudofolliculitis barbae. The combination of close shaving and tightly coiled hairs causes the hairs to grow toward and penetrate the skin, particularly on the neck.13 In some cases, the hairs never actually exit the skin and simply curl within the superficial epidermis. A foreign body reaction often arises, leading to inflamed follicular papules and pustules. Affected individuals may experience pain, pruritus, and secondary infections. Postinflammatory hyperpigmentation, hypertrophic scarring, and keloid formation are common sequelae in cases of untreated disease. Pseudofolliculitis barbae also is exacerbated by pulling the skin taut and shaving against the grain, making behavioral interventions a key component in management of this condition. Preliminary recommendations include using a new or electric razor, leaving hair at least 2 mm in length, and shaving in the direction of hair growth. Other treatment options with varying effectiveness include daily alternation of a mild topical corticosteroid and one of the following: a topical retinoid, topical antibiotics, or glycolic acid. The only treatments that approach definitive cure are laser hair removal and electrolysis for which patient skin type plays an important role in laser selection.5
Traction Alopecia
Similar to their male counterparts, female military members must also present a conservative professional appearance, including hair that is neatly groomed.1 If the length of the hair extends beyond the uniform collar, it must be inconspicuously fastened or pinned above the collar. As a result, loosely tied hair is unauthorized, and females with long hair must secure their hair tightly on a daily basis. Traction alopecia results from tight hairstyling over a prolonged period and commonly affects female soldiers. The etiology is presumed to be mechanical loosening of hair within the follicles, leading to inflammation. Although traditionally seen in black women along the frontal and temporal hairlines, traction alopecia has been identified in individuals of all races and can occur anywhere on the scalp.5 Perifollicular erythema may be the first sign, and papules and pustules may be visible. Although the hair loss in traction alopecia usually is reversible if the traction is ceased, end-stage disease may be permanent.6 Halting traction-inducing practices is paramount, and other treatment options that may slow progression include topical or oral antibiotics and topical or intralesional corticosteroids. Recovery of hair loss also may be aided by topical minoxidil.5
Keloids
Keloid formation is an important pathology to address, as it may result from several of the aforementioned conditions. Keloids are most commonly seen in black individuals but also can occur in Hispanic and Asian patients. The cause has not been fully elucidated but is thought to be a combination of dysfunctional fibroblasts with a genetic component based on racial predilection and twin concordance studies.5 The chest, shoulders, upper back, neck, and earlobes are particularly susceptible to keloid formation, which can appear from 1 to 24 years following dermal trauma.5 Unlike hypertrophic scars, keloids generally do not regress and frequently cause discomfort, pruritus, and emotional distress. They also can hinder wearing a military uniform. Sustained remission is problematic, making prevention a first-line approach, including proper care of wounds when they occur and avoiding elective procedures such as piercings and tattoos. Intralesional corticosteroids, adjuvant injections (eg, 5-fluorouracil), silicone sheeting, cryotherapy, radiation, laser therapy, and excision are some of the treatment options when keloids have formed.5
Final Comment
It is important to recognize military grooming standards as a cause or contributor to several diseases of the head and neck in military servicemembers. Specifically, frequent haircuts in male soldiers are associated with several inflammatory hair disorders, including AKN, dissecting cellulitis of the scalp, and folliculitis decalvans, while daily shaving predisposes individuals to pseudofolliculitis barbae with possible keloid formation. Females may develop traction alopecia from chronically tight, pulled back hairstyles. All of these conditions have health implications for the affected individuals and can compromise the military mission. Awareness, prevention, and recognition are key along with the knowledge base to provide anticipatory avoidance and initiate appropriate treatments, thereby mitigating these potential consequences.
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Washington, DC: Department of the Army; 2017. https://history.army.mil/html/forcestruc/docs/AR670-1.pdf. Accessed October 11, 2018.
- East-Innis AD, Stylianou K, Paolino A, et al. Acne keloidalis nuchae: risk factors and associated disorders--a retrospective study. Int J Dermatol. 2017;56:828-832.
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systematic review of the literature. Dermatol Ther (Heidelb). 2016;6:363-378.
- Glenn MJ, Bennett RG, Kelly AP. Acne keloidalis nuchae: treatment with excision and second-intention healing. J Am Acad Dermatol. 1995;33:243-246.
- Madu P, Kundu RV. Follicular and scarring disorders in skin of color: presentation and management. Am J Clin Dermatol. 2014;15:307-321.
- Rodney IJ, Onwudiwe OC. Hair and scalp disorders in ethnic populations. J Drugs Dermatol. 2013;12:420-427.
- Lindsey SF, Tosti A. Ethnic hair disorders. Curr Probl Dermatol. 2015;47:139-148.
- Whiting DA. Cicatricial alopecia: clinico-pathological findings and treatment. Clin Dermatol. 2001;19:211-225.
- Sperling LC, Cowper SE, Knopp EA. An Atlas of Hair Pathology with Clinical Correlations. 2nd ed. Boca Raton, FL: CRC Press; 2012.
- Gemmeke A, Wollina U. Folliculitis decalvans of the scalp: response to triple therapy with isotretinoin, clindamycin, and prednisolone. Acta Dermatovenerol Alp Pannonica Adriat. 2006;15:184-186.
- Hallai N, Thompson I, Williams P, et al. Folliculitis spinulosa decalvans: failure to respond to oral isotretinoin. J Eur Acad Dermatol Venereol. 2006;20:223-224.
- Bolduc C, Sperling LC, Shapiro J. Primary cicatricial alopecia. J Am Acad Dermatol. 2016;75:101-117.
- Perry PK, Cook-Bolden FE, Rahman Z, et al. Defining pseudofolliculitis barbae in 2001: a review of the literature and current trends. J Am Acad Dermatol. 2002;46(2 suppl):S113-S119.
The US military enforces grooming standards to ensure the professional appearance and serviceability of soldiers in all operational settings. Although most individuals are able to uphold these regulations without incident, there is a growing cohort of servicemembers with skin diseases that were exacerbated or even initiated by haircuts, hairstyling, and shaving required to conform to these grooming standards. These skin diseases, which can affect both sexes and may not be appreciated until years into a soldier's service commitment, can have consequences related to individual morbidity and medical readiness for deployment, making it an important issue for medical practitioners to recognize and manage in servicemembers.
This review highlights several disorders of the pilosebaceous unit of the head and neck that can be caused or exacerbated by military grooming standards, including inflammatory hair disorders, traction alopecia, and pseudofolliculitis barbae. Discussion of each entity will include a review of susceptibility and causality as well as initial treatment options to consider (Table).

Inflammatory Hair Disorders
The proper appearance of servicemembers in uniform represents self-discipline and conformity to the high standards of the military. This transition occurs as a rite of passage for many new male recruits who receive shaved haircuts during their first days of basic training. Thereafter, male servicemembers are required to maintain a tapered appearance of the hair per military regulations.1 Clipping hair closely to the scalp or shaving the head entirely are authorized and often encouraged; therefore, high and tight haircuts and buzz cuts are popular among male soldiers due to the general ease of care and ability to maintain the haircut themselves. Conversely, these styles require servicemembers to get weekly or biweekly haircuts that in turn can lead to chronic trauma and irritation. In more susceptible populations, inflammatory hair disorders such as acne keloidalis nuchae (AKN), dissecting cellulitis of the scalp, and folliculitis decalvans may be incited.
Acne Keloidalis Nuchae
Acne keloidalis nuchae, also called folliculitis keloidalis, is a chronic scarring folliculitis presenting with papules and plaques on the occiput and nape of the neck that may merge to form hypertrophic scars or keloids. This disorder most commonly develops in young black men but also can be seen in black females and white patients of both sexes.2 Acne keloidalis nuchae shares many histologic features with central centrifugal cicatricial alopecia, which may suggest a similar pathogenesis. Apart from frequent haircuts, tight-collared shirts, such as those on military service uniforms, also have been associated with AKN. Because of these suspected etiologies, first-line treatment focuses on preventing further trauma by avoiding mechanical irritation and short haircuts, which may be difficult in the military setting. For earlier disease stages, topical and intralesional corticosteroids, oral retinoids, and topical and oral antibiotics are used for their anti-inflammatory properties.3 In refractory cases, surgical excision with healing by secondary intention may be attempted.4 Additional treatment options include the 1064-nm Nd:YAG and 810-nm diode lasers,3 UVB light therapy, CO2 laser, and radiotherapy.
Dissecting Cellulitis of the Scalp
Similar to AKN, dissecting cellulitis of the scalp is another inflammatory hair disorder that is worsened by frequent short haircuts.5 Dissecting cellulitis of the scalp is a primary cicatricial alopecia proposed to be secondary to follicular occlusion. It often is seen in black males aged 20 to 40 years and is characterized by boggy suppurative nodules and cysts with draining sinus tracts, abscesses, and resultant scarring alopecia. Dissecting cellulitis of the scalp is part of the follicular occlusion tetrad, which also includes hidradenitis suppurativa, acne conglobata, and pilonidal cysts. First-line therapies include topical and oral antibiotics, topical retinoids, intralesional corticosteroids, incision and drainage of fluctuant nodules, and oral isotretinoin with or without rifampin. Alternative treatments include oral zinc supplementation, oral corticosteroids, tumor necrosis factor α inhibitors, laser therapies, radiotherapy, and surgical management with wide local excision or total scalpectomy.6,7
Folliculitis Decalvans
Folliculitis decalvans is a primary cicatricial alopecia of the scalp that most commonly presents in middle-aged men without racial predilection.8 Folliculitis decalvans presents with multiple pustules, crusts, tufted hairs, and perifollicular hyperkeratosis, leading to scarring of the scalp, which often is most severe on the posterior vertex. Staphylococcus aureus is a presumed player in the pathogenesis of folliculitis decalvans with superantigens causing release of cytokines stimulating follicular destruction. Close haircuts in conformation with military grooming standards can contribute to this condition due to mechanical trauma and subsequent inflammation. It typically is diagnosed clinically, but if histologic confirmation is desired, a sample from the periphery of early lesions is preferred.9 Initial treatment consists of antibacterial shampoos, topical corticosteroids, topical antibiotics, and combination oral antibiotic therapy with rifampin and clindamycin. Studies using oral isotretinoin have shown variable results,10,11 and the most effective treatment of recalcitrant lesions appears to be intralesional corticosteroids.12
Follicular and Scarring Disorders
In addition to inflammatory hair disorders, military grooming standards have been linked to the pathogenesis of diseases such as pseudofolliculitis barbae, traction alopecia, and keloids, specifically through irritation of the face, neck, and scalp, as well as damage to the follicular unit.5 These conditions develop because grooming regulations necessitate certain hair practices such as close shaving of facial and neck hair and keeping long hair secured relatively tightly to the scalp.
Pseudofolliculitis Barbae
Males in the military are obligated to keep their faces clean-shaven.1 They may acquire a medical waiver for a specified beard length if deemed appropriate by the treating physician,1 which often leads to the need for continual waiver renewal and also may warrant possible negative perception from peers, subordinates, and leadership. One of the most prevalent conditions that is closely associated with shaving is pseudofolliculitis barbae. The combination of close shaving and tightly coiled hairs causes the hairs to grow toward and penetrate the skin, particularly on the neck.13 In some cases, the hairs never actually exit the skin and simply curl within the superficial epidermis. A foreign body reaction often arises, leading to inflamed follicular papules and pustules. Affected individuals may experience pain, pruritus, and secondary infections. Postinflammatory hyperpigmentation, hypertrophic scarring, and keloid formation are common sequelae in cases of untreated disease. Pseudofolliculitis barbae also is exacerbated by pulling the skin taut and shaving against the grain, making behavioral interventions a key component in management of this condition. Preliminary recommendations include using a new or electric razor, leaving hair at least 2 mm in length, and shaving in the direction of hair growth. Other treatment options with varying effectiveness include daily alternation of a mild topical corticosteroid and one of the following: a topical retinoid, topical antibiotics, or glycolic acid. The only treatments that approach definitive cure are laser hair removal and electrolysis for which patient skin type plays an important role in laser selection.5
Traction Alopecia
Similar to their male counterparts, female military members must also present a conservative professional appearance, including hair that is neatly groomed.1 If the length of the hair extends beyond the uniform collar, it must be inconspicuously fastened or pinned above the collar. As a result, loosely tied hair is unauthorized, and females with long hair must secure their hair tightly on a daily basis. Traction alopecia results from tight hairstyling over a prolonged period and commonly affects female soldiers. The etiology is presumed to be mechanical loosening of hair within the follicles, leading to inflammation. Although traditionally seen in black women along the frontal and temporal hairlines, traction alopecia has been identified in individuals of all races and can occur anywhere on the scalp.5 Perifollicular erythema may be the first sign, and papules and pustules may be visible. Although the hair loss in traction alopecia usually is reversible if the traction is ceased, end-stage disease may be permanent.6 Halting traction-inducing practices is paramount, and other treatment options that may slow progression include topical or oral antibiotics and topical or intralesional corticosteroids. Recovery of hair loss also may be aided by topical minoxidil.5
Keloids
Keloid formation is an important pathology to address, as it may result from several of the aforementioned conditions. Keloids are most commonly seen in black individuals but also can occur in Hispanic and Asian patients. The cause has not been fully elucidated but is thought to be a combination of dysfunctional fibroblasts with a genetic component based on racial predilection and twin concordance studies.5 The chest, shoulders, upper back, neck, and earlobes are particularly susceptible to keloid formation, which can appear from 1 to 24 years following dermal trauma.5 Unlike hypertrophic scars, keloids generally do not regress and frequently cause discomfort, pruritus, and emotional distress. They also can hinder wearing a military uniform. Sustained remission is problematic, making prevention a first-line approach, including proper care of wounds when they occur and avoiding elective procedures such as piercings and tattoos. Intralesional corticosteroids, adjuvant injections (eg, 5-fluorouracil), silicone sheeting, cryotherapy, radiation, laser therapy, and excision are some of the treatment options when keloids have formed.5
Final Comment
It is important to recognize military grooming standards as a cause or contributor to several diseases of the head and neck in military servicemembers. Specifically, frequent haircuts in male soldiers are associated with several inflammatory hair disorders, including AKN, dissecting cellulitis of the scalp, and folliculitis decalvans, while daily shaving predisposes individuals to pseudofolliculitis barbae with possible keloid formation. Females may develop traction alopecia from chronically tight, pulled back hairstyles. All of these conditions have health implications for the affected individuals and can compromise the military mission. Awareness, prevention, and recognition are key along with the knowledge base to provide anticipatory avoidance and initiate appropriate treatments, thereby mitigating these potential consequences.
The US military enforces grooming standards to ensure the professional appearance and serviceability of soldiers in all operational settings. Although most individuals are able to uphold these regulations without incident, there is a growing cohort of servicemembers with skin diseases that were exacerbated or even initiated by haircuts, hairstyling, and shaving required to conform to these grooming standards. These skin diseases, which can affect both sexes and may not be appreciated until years into a soldier's service commitment, can have consequences related to individual morbidity and medical readiness for deployment, making it an important issue for medical practitioners to recognize and manage in servicemembers.
This review highlights several disorders of the pilosebaceous unit of the head and neck that can be caused or exacerbated by military grooming standards, including inflammatory hair disorders, traction alopecia, and pseudofolliculitis barbae. Discussion of each entity will include a review of susceptibility and causality as well as initial treatment options to consider (Table).

Inflammatory Hair Disorders
The proper appearance of servicemembers in uniform represents self-discipline and conformity to the high standards of the military. This transition occurs as a rite of passage for many new male recruits who receive shaved haircuts during their first days of basic training. Thereafter, male servicemembers are required to maintain a tapered appearance of the hair per military regulations.1 Clipping hair closely to the scalp or shaving the head entirely are authorized and often encouraged; therefore, high and tight haircuts and buzz cuts are popular among male soldiers due to the general ease of care and ability to maintain the haircut themselves. Conversely, these styles require servicemembers to get weekly or biweekly haircuts that in turn can lead to chronic trauma and irritation. In more susceptible populations, inflammatory hair disorders such as acne keloidalis nuchae (AKN), dissecting cellulitis of the scalp, and folliculitis decalvans may be incited.
Acne Keloidalis Nuchae
Acne keloidalis nuchae, also called folliculitis keloidalis, is a chronic scarring folliculitis presenting with papules and plaques on the occiput and nape of the neck that may merge to form hypertrophic scars or keloids. This disorder most commonly develops in young black men but also can be seen in black females and white patients of both sexes.2 Acne keloidalis nuchae shares many histologic features with central centrifugal cicatricial alopecia, which may suggest a similar pathogenesis. Apart from frequent haircuts, tight-collared shirts, such as those on military service uniforms, also have been associated with AKN. Because of these suspected etiologies, first-line treatment focuses on preventing further trauma by avoiding mechanical irritation and short haircuts, which may be difficult in the military setting. For earlier disease stages, topical and intralesional corticosteroids, oral retinoids, and topical and oral antibiotics are used for their anti-inflammatory properties.3 In refractory cases, surgical excision with healing by secondary intention may be attempted.4 Additional treatment options include the 1064-nm Nd:YAG and 810-nm diode lasers,3 UVB light therapy, CO2 laser, and radiotherapy.
Dissecting Cellulitis of the Scalp
Similar to AKN, dissecting cellulitis of the scalp is another inflammatory hair disorder that is worsened by frequent short haircuts.5 Dissecting cellulitis of the scalp is a primary cicatricial alopecia proposed to be secondary to follicular occlusion. It often is seen in black males aged 20 to 40 years and is characterized by boggy suppurative nodules and cysts with draining sinus tracts, abscesses, and resultant scarring alopecia. Dissecting cellulitis of the scalp is part of the follicular occlusion tetrad, which also includes hidradenitis suppurativa, acne conglobata, and pilonidal cysts. First-line therapies include topical and oral antibiotics, topical retinoids, intralesional corticosteroids, incision and drainage of fluctuant nodules, and oral isotretinoin with or without rifampin. Alternative treatments include oral zinc supplementation, oral corticosteroids, tumor necrosis factor α inhibitors, laser therapies, radiotherapy, and surgical management with wide local excision or total scalpectomy.6,7
Folliculitis Decalvans
Folliculitis decalvans is a primary cicatricial alopecia of the scalp that most commonly presents in middle-aged men without racial predilection.8 Folliculitis decalvans presents with multiple pustules, crusts, tufted hairs, and perifollicular hyperkeratosis, leading to scarring of the scalp, which often is most severe on the posterior vertex. Staphylococcus aureus is a presumed player in the pathogenesis of folliculitis decalvans with superantigens causing release of cytokines stimulating follicular destruction. Close haircuts in conformation with military grooming standards can contribute to this condition due to mechanical trauma and subsequent inflammation. It typically is diagnosed clinically, but if histologic confirmation is desired, a sample from the periphery of early lesions is preferred.9 Initial treatment consists of antibacterial shampoos, topical corticosteroids, topical antibiotics, and combination oral antibiotic therapy with rifampin and clindamycin. Studies using oral isotretinoin have shown variable results,10,11 and the most effective treatment of recalcitrant lesions appears to be intralesional corticosteroids.12
Follicular and Scarring Disorders
In addition to inflammatory hair disorders, military grooming standards have been linked to the pathogenesis of diseases such as pseudofolliculitis barbae, traction alopecia, and keloids, specifically through irritation of the face, neck, and scalp, as well as damage to the follicular unit.5 These conditions develop because grooming regulations necessitate certain hair practices such as close shaving of facial and neck hair and keeping long hair secured relatively tightly to the scalp.
Pseudofolliculitis Barbae
Males in the military are obligated to keep their faces clean-shaven.1 They may acquire a medical waiver for a specified beard length if deemed appropriate by the treating physician,1 which often leads to the need for continual waiver renewal and also may warrant possible negative perception from peers, subordinates, and leadership. One of the most prevalent conditions that is closely associated with shaving is pseudofolliculitis barbae. The combination of close shaving and tightly coiled hairs causes the hairs to grow toward and penetrate the skin, particularly on the neck.13 In some cases, the hairs never actually exit the skin and simply curl within the superficial epidermis. A foreign body reaction often arises, leading to inflamed follicular papules and pustules. Affected individuals may experience pain, pruritus, and secondary infections. Postinflammatory hyperpigmentation, hypertrophic scarring, and keloid formation are common sequelae in cases of untreated disease. Pseudofolliculitis barbae also is exacerbated by pulling the skin taut and shaving against the grain, making behavioral interventions a key component in management of this condition. Preliminary recommendations include using a new or electric razor, leaving hair at least 2 mm in length, and shaving in the direction of hair growth. Other treatment options with varying effectiveness include daily alternation of a mild topical corticosteroid and one of the following: a topical retinoid, topical antibiotics, or glycolic acid. The only treatments that approach definitive cure are laser hair removal and electrolysis for which patient skin type plays an important role in laser selection.5
Traction Alopecia
Similar to their male counterparts, female military members must also present a conservative professional appearance, including hair that is neatly groomed.1 If the length of the hair extends beyond the uniform collar, it must be inconspicuously fastened or pinned above the collar. As a result, loosely tied hair is unauthorized, and females with long hair must secure their hair tightly on a daily basis. Traction alopecia results from tight hairstyling over a prolonged period and commonly affects female soldiers. The etiology is presumed to be mechanical loosening of hair within the follicles, leading to inflammation. Although traditionally seen in black women along the frontal and temporal hairlines, traction alopecia has been identified in individuals of all races and can occur anywhere on the scalp.5 Perifollicular erythema may be the first sign, and papules and pustules may be visible. Although the hair loss in traction alopecia usually is reversible if the traction is ceased, end-stage disease may be permanent.6 Halting traction-inducing practices is paramount, and other treatment options that may slow progression include topical or oral antibiotics and topical or intralesional corticosteroids. Recovery of hair loss also may be aided by topical minoxidil.5
Keloids
Keloid formation is an important pathology to address, as it may result from several of the aforementioned conditions. Keloids are most commonly seen in black individuals but also can occur in Hispanic and Asian patients. The cause has not been fully elucidated but is thought to be a combination of dysfunctional fibroblasts with a genetic component based on racial predilection and twin concordance studies.5 The chest, shoulders, upper back, neck, and earlobes are particularly susceptible to keloid formation, which can appear from 1 to 24 years following dermal trauma.5 Unlike hypertrophic scars, keloids generally do not regress and frequently cause discomfort, pruritus, and emotional distress. They also can hinder wearing a military uniform. Sustained remission is problematic, making prevention a first-line approach, including proper care of wounds when they occur and avoiding elective procedures such as piercings and tattoos. Intralesional corticosteroids, adjuvant injections (eg, 5-fluorouracil), silicone sheeting, cryotherapy, radiation, laser therapy, and excision are some of the treatment options when keloids have formed.5
Final Comment
It is important to recognize military grooming standards as a cause or contributor to several diseases of the head and neck in military servicemembers. Specifically, frequent haircuts in male soldiers are associated with several inflammatory hair disorders, including AKN, dissecting cellulitis of the scalp, and folliculitis decalvans, while daily shaving predisposes individuals to pseudofolliculitis barbae with possible keloid formation. Females may develop traction alopecia from chronically tight, pulled back hairstyles. All of these conditions have health implications for the affected individuals and can compromise the military mission. Awareness, prevention, and recognition are key along with the knowledge base to provide anticipatory avoidance and initiate appropriate treatments, thereby mitigating these potential consequences.
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Washington, DC: Department of the Army; 2017. https://history.army.mil/html/forcestruc/docs/AR670-1.pdf. Accessed October 11, 2018.
- East-Innis AD, Stylianou K, Paolino A, et al. Acne keloidalis nuchae: risk factors and associated disorders--a retrospective study. Int J Dermatol. 2017;56:828-832.
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systematic review of the literature. Dermatol Ther (Heidelb). 2016;6:363-378.
- Glenn MJ, Bennett RG, Kelly AP. Acne keloidalis nuchae: treatment with excision and second-intention healing. J Am Acad Dermatol. 1995;33:243-246.
- Madu P, Kundu RV. Follicular and scarring disorders in skin of color: presentation and management. Am J Clin Dermatol. 2014;15:307-321.
- Rodney IJ, Onwudiwe OC. Hair and scalp disorders in ethnic populations. J Drugs Dermatol. 2013;12:420-427.
- Lindsey SF, Tosti A. Ethnic hair disorders. Curr Probl Dermatol. 2015;47:139-148.
- Whiting DA. Cicatricial alopecia: clinico-pathological findings and treatment. Clin Dermatol. 2001;19:211-225.
- Sperling LC, Cowper SE, Knopp EA. An Atlas of Hair Pathology with Clinical Correlations. 2nd ed. Boca Raton, FL: CRC Press; 2012.
- Gemmeke A, Wollina U. Folliculitis decalvans of the scalp: response to triple therapy with isotretinoin, clindamycin, and prednisolone. Acta Dermatovenerol Alp Pannonica Adriat. 2006;15:184-186.
- Hallai N, Thompson I, Williams P, et al. Folliculitis spinulosa decalvans: failure to respond to oral isotretinoin. J Eur Acad Dermatol Venereol. 2006;20:223-224.
- Bolduc C, Sperling LC, Shapiro J. Primary cicatricial alopecia. J Am Acad Dermatol. 2016;75:101-117.
- Perry PK, Cook-Bolden FE, Rahman Z, et al. Defining pseudofolliculitis barbae in 2001: a review of the literature and current trends. J Am Acad Dermatol. 2002;46(2 suppl):S113-S119.
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Washington, DC: Department of the Army; 2017. https://history.army.mil/html/forcestruc/docs/AR670-1.pdf. Accessed October 11, 2018.
- East-Innis AD, Stylianou K, Paolino A, et al. Acne keloidalis nuchae: risk factors and associated disorders--a retrospective study. Int J Dermatol. 2017;56:828-832.
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systematic review of the literature. Dermatol Ther (Heidelb). 2016;6:363-378.
- Glenn MJ, Bennett RG, Kelly AP. Acne keloidalis nuchae: treatment with excision and second-intention healing. J Am Acad Dermatol. 1995;33:243-246.
- Madu P, Kundu RV. Follicular and scarring disorders in skin of color: presentation and management. Am J Clin Dermatol. 2014;15:307-321.
- Rodney IJ, Onwudiwe OC. Hair and scalp disorders in ethnic populations. J Drugs Dermatol. 2013;12:420-427.
- Lindsey SF, Tosti A. Ethnic hair disorders. Curr Probl Dermatol. 2015;47:139-148.
- Whiting DA. Cicatricial alopecia: clinico-pathological findings and treatment. Clin Dermatol. 2001;19:211-225.
- Sperling LC, Cowper SE, Knopp EA. An Atlas of Hair Pathology with Clinical Correlations. 2nd ed. Boca Raton, FL: CRC Press; 2012.
- Gemmeke A, Wollina U. Folliculitis decalvans of the scalp: response to triple therapy with isotretinoin, clindamycin, and prednisolone. Acta Dermatovenerol Alp Pannonica Adriat. 2006;15:184-186.
- Hallai N, Thompson I, Williams P, et al. Folliculitis spinulosa decalvans: failure to respond to oral isotretinoin. J Eur Acad Dermatol Venereol. 2006;20:223-224.
- Bolduc C, Sperling LC, Shapiro J. Primary cicatricial alopecia. J Am Acad Dermatol. 2016;75:101-117.
- Perry PK, Cook-Bolden FE, Rahman Z, et al. Defining pseudofolliculitis barbae in 2001: a review of the literature and current trends. J Am Acad Dermatol. 2002;46(2 suppl):S113-S119.
Practice Points
- The short frequent haircuts required to maintain a tapered appearance of the hair per US military regulations may lead to inflammatory hair disorders such as acne keloidalis nuchae, dissecting cellulitis of the scalp, and folliculitis decalvans.
- The mainstay of prevention for these conditions is avoidance of inciting factors such as short haircuts, tight-collared shirts, frequent shaving, or tight hairstyles.
- Early identification and treatment of inflammatory follicular and scarring disorders can prevent further scarring, pigmentation changes, and/or disfigurement.
Smartphone Versus Holter Monitoring for Poststroke Atrial Fibrillation Detection
Although guidelines recommend Holter monitoring for all patients with ischemic stroke or TIA, comparatively few receive it.
MONTREAL—In patients hospitalized for a recent acute ischemic stroke or transient ischemic attack (TIA), a smartphone-based method identified three times more patients with atrial fibrillation than did 24-hour Holter monitoring after discharge, according to a study presented at the 11th World Stroke Congress.
This high level of atrial fibrillation detection suggests that this relatively cheap and noninvasive device is a good complement to conventional monitoring by a 24-hour Holter recording or an implanted loop recorder in patients with recent stroke. The device may thus satisfy the requirements of current guidelines from the world’s cardiology societies.
In-Hospital and Postdischarge Monitoring
In the study, 294 of 1,079 patients with acute ischemic stroke or TIA underwent serial, 30-second monitoring with the AliveCor device while hospitalized. The device was designed for smartphone-enabled ECG measurement. After discharge, the same patients underwent Holter monitoring. The latter technique identified eight patients (3%) with atrial fibrillation, compared with 25 patients (9%) who were identified using the AliveCor device, said Bernard Yan, MD, a consultant neurologist and endovascular neurointerventionist in the Comprehensive Stroke Center of the Royal Melbourne Hospital. Seven of the eight patients identified with atrial fibrillation by Holter monitoring were also found to have atrial fibrillation by the AliveCor device.
Dr. Yan attributed the higher in-hospital detection rate for atrial fibrillation to the timing of screening, which occurred within days of the stroke or TIA, rather than after the patient had left the hospital. “The difference may be because we monitored patients [with the AliveCor device] much earlier, during their hot period right after their stroke.”
Practice Does Not Match Recommendations
The trial, which was called SPOT-AF, was conducted at several centers in Australia, China, and Hong Kong. All patients underwent AliveCor monitoring during their stay in the hospital, which lasted for a median of four days. Patients performed a 30-second heart rhythm check every time a nurse saw them for a routine vital-sign examination, which usually was three or four times per day. The current analysis focused on the 294 patients (27% of the 1,079 patients) who also underwent 24-hour Holter monitoring following hospital discharge when ordered by their personal physician.
This 27% rate of postdischarge Holter monitoring was consistent with that of a 2016 review of more than 17,000 patients with stroke or TIA in Canada. That study found that 31% of participants underwent 24-hour Holter monitoring for atrial fibrillation during the 30 days following their index event. Guidelines, however, call for atrial fibrillation screening in all patients with recent ischemic stroke and TIA.
Although screening for atrial fibrillation with a smartphone-based device is inexpensive and easy, Dr. Yan did not suggest that this approach could replace a Holter monitor or an implanted loop recorder, which is what current guidelines recommend. “To change the guidelines, we need a different study that compares these approaches head to head.”
SPOT-AF received partial funding from Boehringer Ingelheim. Dr. Yan has spoken on behalf of Bayer, Boehringer Ingelheim, Pfizer, and Stryker.
—Mitchel L. Zoler
Suggested Reading
Edwards JD, Kapral MK, Fang J, et al. Underutilization of ambulatory ECG monitoring after stroke and transient ischemic attack: missed opportunities for atrial fibrillation detection. Stroke. 2016;47(8):1982-1989.
Tu HT, Chen Z, Swift C, et al. Smartphone electrographic monitoring for atrial fibrillation in acute ischemic stroke and transient ischemic attack. Int J Stroke. 2017;12(7):786-789.
Although guidelines recommend Holter monitoring for all patients with ischemic stroke or TIA, comparatively few receive it.
Although guidelines recommend Holter monitoring for all patients with ischemic stroke or TIA, comparatively few receive it.
MONTREAL—In patients hospitalized for a recent acute ischemic stroke or transient ischemic attack (TIA), a smartphone-based method identified three times more patients with atrial fibrillation than did 24-hour Holter monitoring after discharge, according to a study presented at the 11th World Stroke Congress.
This high level of atrial fibrillation detection suggests that this relatively cheap and noninvasive device is a good complement to conventional monitoring by a 24-hour Holter recording or an implanted loop recorder in patients with recent stroke. The device may thus satisfy the requirements of current guidelines from the world’s cardiology societies.
In-Hospital and Postdischarge Monitoring
In the study, 294 of 1,079 patients with acute ischemic stroke or TIA underwent serial, 30-second monitoring with the AliveCor device while hospitalized. The device was designed for smartphone-enabled ECG measurement. After discharge, the same patients underwent Holter monitoring. The latter technique identified eight patients (3%) with atrial fibrillation, compared with 25 patients (9%) who were identified using the AliveCor device, said Bernard Yan, MD, a consultant neurologist and endovascular neurointerventionist in the Comprehensive Stroke Center of the Royal Melbourne Hospital. Seven of the eight patients identified with atrial fibrillation by Holter monitoring were also found to have atrial fibrillation by the AliveCor device.
Dr. Yan attributed the higher in-hospital detection rate for atrial fibrillation to the timing of screening, which occurred within days of the stroke or TIA, rather than after the patient had left the hospital. “The difference may be because we monitored patients [with the AliveCor device] much earlier, during their hot period right after their stroke.”
Practice Does Not Match Recommendations
The trial, which was called SPOT-AF, was conducted at several centers in Australia, China, and Hong Kong. All patients underwent AliveCor monitoring during their stay in the hospital, which lasted for a median of four days. Patients performed a 30-second heart rhythm check every time a nurse saw them for a routine vital-sign examination, which usually was three or four times per day. The current analysis focused on the 294 patients (27% of the 1,079 patients) who also underwent 24-hour Holter monitoring following hospital discharge when ordered by their personal physician.
This 27% rate of postdischarge Holter monitoring was consistent with that of a 2016 review of more than 17,000 patients with stroke or TIA in Canada. That study found that 31% of participants underwent 24-hour Holter monitoring for atrial fibrillation during the 30 days following their index event. Guidelines, however, call for atrial fibrillation screening in all patients with recent ischemic stroke and TIA.
Although screening for atrial fibrillation with a smartphone-based device is inexpensive and easy, Dr. Yan did not suggest that this approach could replace a Holter monitor or an implanted loop recorder, which is what current guidelines recommend. “To change the guidelines, we need a different study that compares these approaches head to head.”
SPOT-AF received partial funding from Boehringer Ingelheim. Dr. Yan has spoken on behalf of Bayer, Boehringer Ingelheim, Pfizer, and Stryker.
—Mitchel L. Zoler
Suggested Reading
Edwards JD, Kapral MK, Fang J, et al. Underutilization of ambulatory ECG monitoring after stroke and transient ischemic attack: missed opportunities for atrial fibrillation detection. Stroke. 2016;47(8):1982-1989.
Tu HT, Chen Z, Swift C, et al. Smartphone electrographic monitoring for atrial fibrillation in acute ischemic stroke and transient ischemic attack. Int J Stroke. 2017;12(7):786-789.
MONTREAL—In patients hospitalized for a recent acute ischemic stroke or transient ischemic attack (TIA), a smartphone-based method identified three times more patients with atrial fibrillation than did 24-hour Holter monitoring after discharge, according to a study presented at the 11th World Stroke Congress.
This high level of atrial fibrillation detection suggests that this relatively cheap and noninvasive device is a good complement to conventional monitoring by a 24-hour Holter recording or an implanted loop recorder in patients with recent stroke. The device may thus satisfy the requirements of current guidelines from the world’s cardiology societies.
In-Hospital and Postdischarge Monitoring
In the study, 294 of 1,079 patients with acute ischemic stroke or TIA underwent serial, 30-second monitoring with the AliveCor device while hospitalized. The device was designed for smartphone-enabled ECG measurement. After discharge, the same patients underwent Holter monitoring. The latter technique identified eight patients (3%) with atrial fibrillation, compared with 25 patients (9%) who were identified using the AliveCor device, said Bernard Yan, MD, a consultant neurologist and endovascular neurointerventionist in the Comprehensive Stroke Center of the Royal Melbourne Hospital. Seven of the eight patients identified with atrial fibrillation by Holter monitoring were also found to have atrial fibrillation by the AliveCor device.
Dr. Yan attributed the higher in-hospital detection rate for atrial fibrillation to the timing of screening, which occurred within days of the stroke or TIA, rather than after the patient had left the hospital. “The difference may be because we monitored patients [with the AliveCor device] much earlier, during their hot period right after their stroke.”
Practice Does Not Match Recommendations
The trial, which was called SPOT-AF, was conducted at several centers in Australia, China, and Hong Kong. All patients underwent AliveCor monitoring during their stay in the hospital, which lasted for a median of four days. Patients performed a 30-second heart rhythm check every time a nurse saw them for a routine vital-sign examination, which usually was three or four times per day. The current analysis focused on the 294 patients (27% of the 1,079 patients) who also underwent 24-hour Holter monitoring following hospital discharge when ordered by their personal physician.
This 27% rate of postdischarge Holter monitoring was consistent with that of a 2016 review of more than 17,000 patients with stroke or TIA in Canada. That study found that 31% of participants underwent 24-hour Holter monitoring for atrial fibrillation during the 30 days following their index event. Guidelines, however, call for atrial fibrillation screening in all patients with recent ischemic stroke and TIA.
Although screening for atrial fibrillation with a smartphone-based device is inexpensive and easy, Dr. Yan did not suggest that this approach could replace a Holter monitor or an implanted loop recorder, which is what current guidelines recommend. “To change the guidelines, we need a different study that compares these approaches head to head.”
SPOT-AF received partial funding from Boehringer Ingelheim. Dr. Yan has spoken on behalf of Bayer, Boehringer Ingelheim, Pfizer, and Stryker.
—Mitchel L. Zoler
Suggested Reading
Edwards JD, Kapral MK, Fang J, et al. Underutilization of ambulatory ECG monitoring after stroke and transient ischemic attack: missed opportunities for atrial fibrillation detection. Stroke. 2016;47(8):1982-1989.
Tu HT, Chen Z, Swift C, et al. Smartphone electrographic monitoring for atrial fibrillation in acute ischemic stroke and transient ischemic attack. Int J Stroke. 2017;12(7):786-789.
'Liver first' for select stage IV colon cancer gaining traction
BOSTON –
It’s an alternative to usual care, meaning simultaneous bowel and liver resection or bowel resection with liver surgery later on.
Systemic chemotherapy comes first, followed by liver resection. If margins are microscopically negative, the patient gets another round of chemotherapy. If no additional lesions emerge, the primary tumor is taken out. The entire process can take up to a year.
The approach was developed in the Netherlands for rectal cancer with advanced liver metastases. The idea was to get the liver lesions out before they became unresectable, then remove the primary tumor later on. It’s gaining traction now for colon cancer, and beginning to trickle into the United States at a few academic medical centers.
It comes down to what’s more dangerous, the metastases or the primary tumor? Tumor science hasn’t answered that question yet. There’s general agreement that metastases are what kill people with cancer, but it’s not known if they come mostly from previous metastases or from the primary tumor. The liver-first approach assumes the former.
Liver-first is “extremely controversial. For older surgeons who are not in tertiary care centers, liver-first doesn’t make sense, and it doesn’t seem to make sense to patients. They wonder why you would go after the liver when they were diagnosed with a colon tumor,” said Janice Rafferty, MD, FACS, professor of surgery at the University of Cincinnati, at the annual clinical congress of the American College of Surgeons.
“Well, it’s because the primary tumor doesn’t limit your life,” she continued. “The life-limiting disease is in the liver, not the colon. If you explain it to them that way, it makes sense. If we cannot get an R0 resection on the liver, it doesn’t make sense to go after the primary, unless it’s symptomatic with obstruction, bleeding, or fistula.”
There have been about 10 attempts at a randomized trial of this approach versus usual care, but they were not successful because of the difficulty of recruiting patients. Patients – and no doubt, some surgeons – may have some resistance to the logic of going after metastases first.
Dr. Rafferty moderated a review of research from Yale University, New Haven, Conn., that attempted to plug the evidence gap. The Yale investigators “presented really interesting data that shows that liver-first has improved survival,” she said.
The Yale team used the National Cancer Database to compare 2010-2015 outcomes from liver-first patients with patients who had simultaneous or bowel-first resections, followed by later liver resections. The database didn’t allow them to tease out simultaneous from bowel-first cases, so they lumped them together as usual care. To avoid confounding, rectal carcinomas and metastases to the lung, brain, and other organs were excluded.
Median survival was 34 months among 358 liver-first patients versus 24 months among 18,042 usual care patients in an intention-to-treat analysis. Among patients who completed their resections, median survival was 57 months among 140 liver-first patients versus 36 months with usual care in 3,988.
The benefit held after adjustment for patient and tumor characteristics (hazard ratio for death 0.77 in favor of liver first). When further adjusted for chemotherapy timing, there was a strong trend for liver-first but it was not statistically significant, suggesting that up-front chemotherapy contributed to the results (HR, 0.88; 95% confidence interval, 0.75-1.01; P = .09).
There were many caveats. The liver-first patients were younger, with over half under the age of 60 years versus just over 40% in usual care. They were also healthier based on Charlson comorbidity scores and more likely to have upfront chemotherapy and be treated at an academic center.
So, what should surgeons make of these findings? Lead investigator Vadim Kurbatov, MD, a Yale surgery resident, argued that, at the very least, they suggest that liver-first is a viable option for stage IV colon cancer with isolated liver metastases. Going further, they suggest that liver first may be the right way to go for younger, healthier patients at academic centers.
For sicker stage IV patients, however, the role of liver-first is unclear. “We really do need a randomized trial,” he said.
Dr. Kurbatov and Dr. Rafferty had no relevant disclosures to report. The work was funded in part by the National Institutes of Health.
BOSTON –
It’s an alternative to usual care, meaning simultaneous bowel and liver resection or bowel resection with liver surgery later on.
Systemic chemotherapy comes first, followed by liver resection. If margins are microscopically negative, the patient gets another round of chemotherapy. If no additional lesions emerge, the primary tumor is taken out. The entire process can take up to a year.
The approach was developed in the Netherlands for rectal cancer with advanced liver metastases. The idea was to get the liver lesions out before they became unresectable, then remove the primary tumor later on. It’s gaining traction now for colon cancer, and beginning to trickle into the United States at a few academic medical centers.
It comes down to what’s more dangerous, the metastases or the primary tumor? Tumor science hasn’t answered that question yet. There’s general agreement that metastases are what kill people with cancer, but it’s not known if they come mostly from previous metastases or from the primary tumor. The liver-first approach assumes the former.
Liver-first is “extremely controversial. For older surgeons who are not in tertiary care centers, liver-first doesn’t make sense, and it doesn’t seem to make sense to patients. They wonder why you would go after the liver when they were diagnosed with a colon tumor,” said Janice Rafferty, MD, FACS, professor of surgery at the University of Cincinnati, at the annual clinical congress of the American College of Surgeons.
“Well, it’s because the primary tumor doesn’t limit your life,” she continued. “The life-limiting disease is in the liver, not the colon. If you explain it to them that way, it makes sense. If we cannot get an R0 resection on the liver, it doesn’t make sense to go after the primary, unless it’s symptomatic with obstruction, bleeding, or fistula.”
There have been about 10 attempts at a randomized trial of this approach versus usual care, but they were not successful because of the difficulty of recruiting patients. Patients – and no doubt, some surgeons – may have some resistance to the logic of going after metastases first.
Dr. Rafferty moderated a review of research from Yale University, New Haven, Conn., that attempted to plug the evidence gap. The Yale investigators “presented really interesting data that shows that liver-first has improved survival,” she said.
The Yale team used the National Cancer Database to compare 2010-2015 outcomes from liver-first patients with patients who had simultaneous or bowel-first resections, followed by later liver resections. The database didn’t allow them to tease out simultaneous from bowel-first cases, so they lumped them together as usual care. To avoid confounding, rectal carcinomas and metastases to the lung, brain, and other organs were excluded.
Median survival was 34 months among 358 liver-first patients versus 24 months among 18,042 usual care patients in an intention-to-treat analysis. Among patients who completed their resections, median survival was 57 months among 140 liver-first patients versus 36 months with usual care in 3,988.
The benefit held after adjustment for patient and tumor characteristics (hazard ratio for death 0.77 in favor of liver first). When further adjusted for chemotherapy timing, there was a strong trend for liver-first but it was not statistically significant, suggesting that up-front chemotherapy contributed to the results (HR, 0.88; 95% confidence interval, 0.75-1.01; P = .09).
There were many caveats. The liver-first patients were younger, with over half under the age of 60 years versus just over 40% in usual care. They were also healthier based on Charlson comorbidity scores and more likely to have upfront chemotherapy and be treated at an academic center.
So, what should surgeons make of these findings? Lead investigator Vadim Kurbatov, MD, a Yale surgery resident, argued that, at the very least, they suggest that liver-first is a viable option for stage IV colon cancer with isolated liver metastases. Going further, they suggest that liver first may be the right way to go for younger, healthier patients at academic centers.
For sicker stage IV patients, however, the role of liver-first is unclear. “We really do need a randomized trial,” he said.
Dr. Kurbatov and Dr. Rafferty had no relevant disclosures to report. The work was funded in part by the National Institutes of Health.
BOSTON –
It’s an alternative to usual care, meaning simultaneous bowel and liver resection or bowel resection with liver surgery later on.
Systemic chemotherapy comes first, followed by liver resection. If margins are microscopically negative, the patient gets another round of chemotherapy. If no additional lesions emerge, the primary tumor is taken out. The entire process can take up to a year.
The approach was developed in the Netherlands for rectal cancer with advanced liver metastases. The idea was to get the liver lesions out before they became unresectable, then remove the primary tumor later on. It’s gaining traction now for colon cancer, and beginning to trickle into the United States at a few academic medical centers.
It comes down to what’s more dangerous, the metastases or the primary tumor? Tumor science hasn’t answered that question yet. There’s general agreement that metastases are what kill people with cancer, but it’s not known if they come mostly from previous metastases or from the primary tumor. The liver-first approach assumes the former.
Liver-first is “extremely controversial. For older surgeons who are not in tertiary care centers, liver-first doesn’t make sense, and it doesn’t seem to make sense to patients. They wonder why you would go after the liver when they were diagnosed with a colon tumor,” said Janice Rafferty, MD, FACS, professor of surgery at the University of Cincinnati, at the annual clinical congress of the American College of Surgeons.
“Well, it’s because the primary tumor doesn’t limit your life,” she continued. “The life-limiting disease is in the liver, not the colon. If you explain it to them that way, it makes sense. If we cannot get an R0 resection on the liver, it doesn’t make sense to go after the primary, unless it’s symptomatic with obstruction, bleeding, or fistula.”
There have been about 10 attempts at a randomized trial of this approach versus usual care, but they were not successful because of the difficulty of recruiting patients. Patients – and no doubt, some surgeons – may have some resistance to the logic of going after metastases first.
Dr. Rafferty moderated a review of research from Yale University, New Haven, Conn., that attempted to plug the evidence gap. The Yale investigators “presented really interesting data that shows that liver-first has improved survival,” she said.
The Yale team used the National Cancer Database to compare 2010-2015 outcomes from liver-first patients with patients who had simultaneous or bowel-first resections, followed by later liver resections. The database didn’t allow them to tease out simultaneous from bowel-first cases, so they lumped them together as usual care. To avoid confounding, rectal carcinomas and metastases to the lung, brain, and other organs were excluded.
Median survival was 34 months among 358 liver-first patients versus 24 months among 18,042 usual care patients in an intention-to-treat analysis. Among patients who completed their resections, median survival was 57 months among 140 liver-first patients versus 36 months with usual care in 3,988.
The benefit held after adjustment for patient and tumor characteristics (hazard ratio for death 0.77 in favor of liver first). When further adjusted for chemotherapy timing, there was a strong trend for liver-first but it was not statistically significant, suggesting that up-front chemotherapy contributed to the results (HR, 0.88; 95% confidence interval, 0.75-1.01; P = .09).
There were many caveats. The liver-first patients were younger, with over half under the age of 60 years versus just over 40% in usual care. They were also healthier based on Charlson comorbidity scores and more likely to have upfront chemotherapy and be treated at an academic center.
So, what should surgeons make of these findings? Lead investigator Vadim Kurbatov, MD, a Yale surgery resident, argued that, at the very least, they suggest that liver-first is a viable option for stage IV colon cancer with isolated liver metastases. Going further, they suggest that liver first may be the right way to go for younger, healthier patients at academic centers.
For sicker stage IV patients, however, the role of liver-first is unclear. “We really do need a randomized trial,” he said.
Dr. Kurbatov and Dr. Rafferty had no relevant disclosures to report. The work was funded in part by the National Institutes of Health.
REPORTING FROM THE ACS CLINICAL CONGRESS
Key clinical point: The liver-first approach may be appropriate for younger, healthier patients at academic centers.
Major finding: Median survival was 34 months among 358 liver-first patients versus 24 months among 18,042 usual care patients in an intention-to-treat analysis.
Study details: A review of over 18,000 patients in the National Cancer Database
Disclosures: The lead investigator had no disclosures to report. The work was funded in part by the National Institutes of Health.
Tinea Incognito in an Urban Pediatric Population
Tinea incognito (TI) describes a dermatophytosis with often atypical clinical features attributed to prior use of topical corticosteroids or other immunomodulating agents. Tinea incognito may lack the scale and elevated margin typical of cutaneous dermatophytoses and can be mistaken for other pediatric cutaneous diseases, particularly atopic dermatitis. 1 Given the prevalence of TI and its susceptibility to misdiagnosis, we conducted a retrospective medical record review of cases of pediatric dermatophytosis presenting from 2005 to 2016.
Methods
We reviewed medical records for patients younger than 18 years who had been seen at the Faculty Group Practice of the Ronald O. Perelman Department of Dermatology, New York University School of Medicine (New York, New York), between January 1, 2005, and October 21, 2016, using International Classification of Diseases, Ninth Revision (ICD-9) codes 110.0 (tinea capitis), 110.1 (onychomycosis/tinea unguium), 110.3 (tinea cruris), 110.4 (tinea pedis), 110.5 (tinea corporis), and 110.9 (tinea, unspecified site). Cases were included in this study if there was documentation of dermatophytosis previously treated with topical corticosteroids or calcineurin inhibitors as well as positive potassium hydroxide (KOH) preparation or fungal culture with dermatophyte growth obtained from lesions satisfying the first criterion. This study was approved by the New York University School of Medicine institutional review board (study no. S15-01388).
Statistical analyses were conducted in SPSS 19.0 for Windows. Categorical variables were assessed using the χ2 test for independence and the Fisher exact test.
Results
A total of 464 cases were reviewed. A positive KOH preparation or dermatophyte fungal culture was documented in 83 cases. Of them, 29 (34.9%) were treated with topical steroids and/or calcineurin inhibitors prior to presentation to dermatology (Table). The mean age at presentation was 8 years. Duration of symptoms prior to presentation was recorded for 23 of 29 patients (79.3%). Of them, 6 (26.1%) experienced symptoms for 1 month or less, 12 (52.2%) for 1 to 6 months, and 5 (21.7%) for 6 months to 1 year.
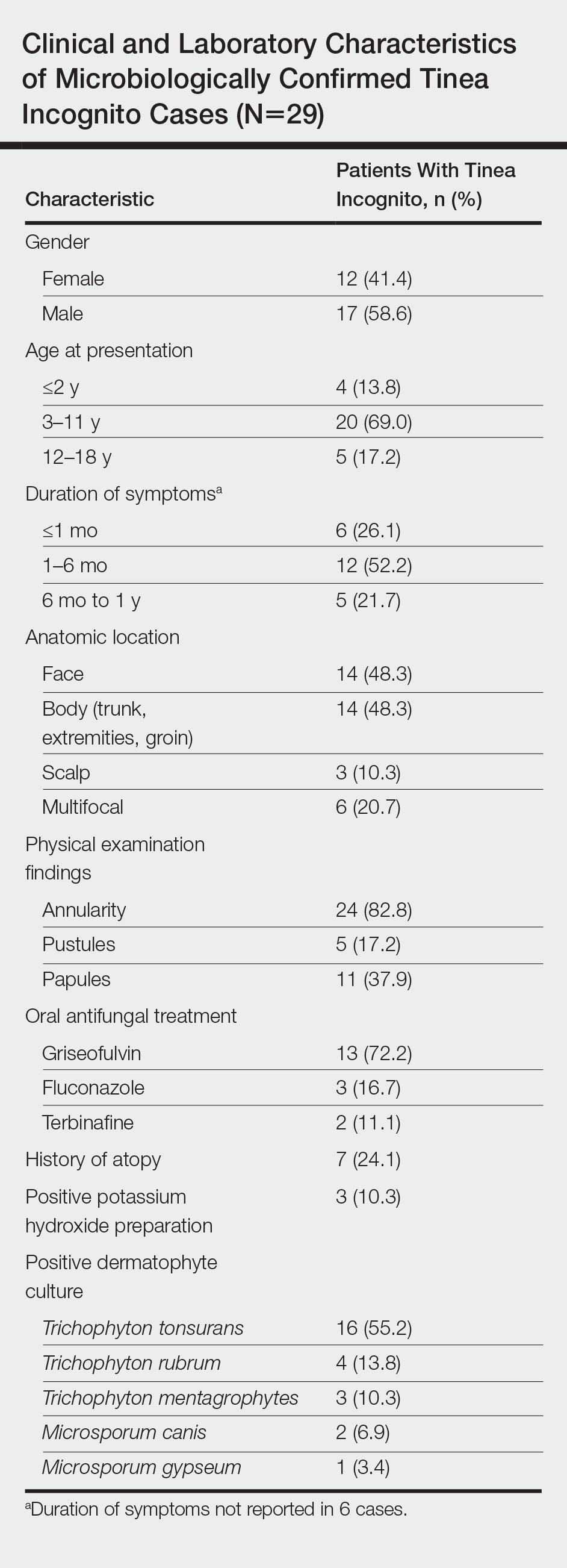
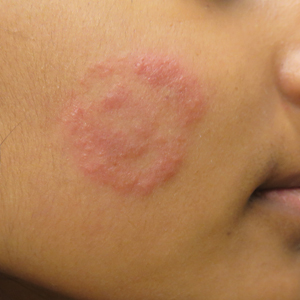
Physical examination findings (Figure) were documented in all 29 cases. Annular lesions were noted in 24 patients (82.8%). Pustules were present in 5 patients (17.2%) and papules in 11 patients (37.9%). Fourteen patients (48.3%) had involvement of the face, 14 (48.3%) of the body (ie, trunk, extremities, or groin), and 3 (10.3%) of the scalp. Six patients (20.7%) demonstrated findings at more than one body site.
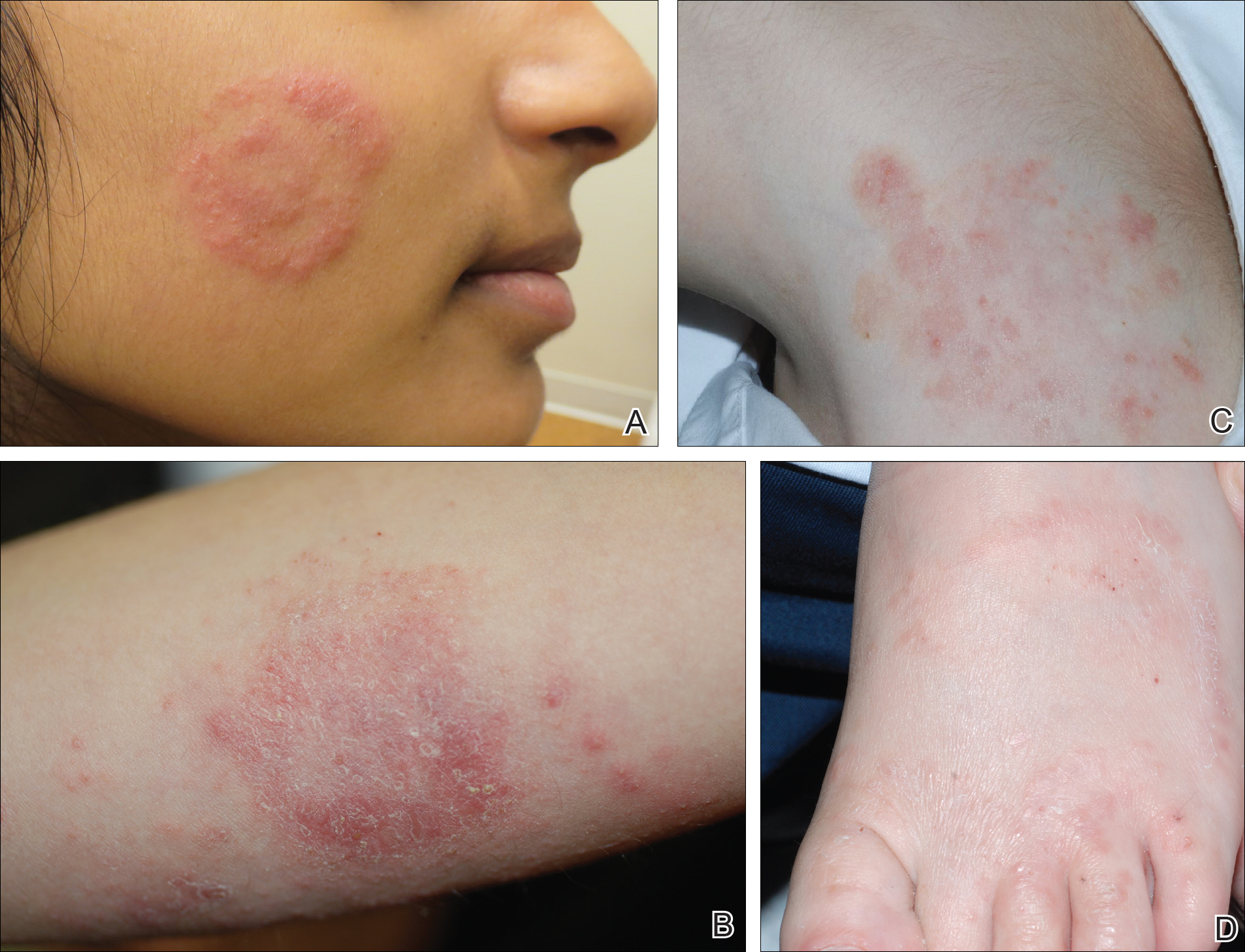
Females were more likely to demonstrate facial lesions (P=.02), while males were more likely to present with body lesions (P=.04). Of 26 patients diagnosed via fungal culture, 16 (55.2%) grew Trichophyton tonsurans, 4 (13.8%) grew Trichophyton rubrum, 3 (10.3%) grew Trichophyton mentagrophytes, 2 (6.9%) grew Microsporum canis, and 1 (3.4%) grew Microsporum gypseum. Treatment entailed oral medication in 18 cases (62.1%). Of them, 13 (72.2%) were treated with griseofulvin, 3 (16.7%) with fluconazole, and 2 (11.1%) with terbinafine. Topical antifungals were prescribed in the remaining 11 cases (37.9%); no further treatment was documented.
Comment
Since the initial description of TI, approximately 60 case reports and small series as well as several larger observational studies describing TI have been published. In our series of pediatric patients, 29 of 83 culture- or KOH-confirmed dermatophytosis cases (34.9%) were considered to be TI due to treatment with topical corticosteroids and/or calcineurin inhibitors prior to presentation. This high prevalence contrasts with the 5.6% prevalence reported in the only prior large case series examining TI in childhood.2 These authors further reported that in their pediatric population, TI was significantly (odds ratio, 8.7; 95% CI, 4.7-16.1) more likely to occur on the face relative to other dermatophytoses and significantly (odds ratio, 0.014; 95% CI, 0.002-0.099) less likely to occur on the scalp.2 We noted a significant association between female gender and facial symptoms as well as between male gender and truncal symptoms. Taken together, these findings suggest an increased likelihood of pediatric tinea faciei to be inappropriately treated, particularly in females.
Although TI treated with topical corticosteroids or calcineurin inhibitors can mimic other skin diseases, a majority of patients in our series demonstrated findings associated with classic tinea, such as annularity and scale. Further, we found that T tonsurans was the causative organism in most cases with T rubrum uncommonly seen, though it is the most prevalent dermatophyte observed worldwide and in 2 large TI case series.3,4 Regional variation in dermatophytes may account for these differences. In our study, griseofulvin was used most frequently in TI treatment, though a systematic review of oral antifungals in tinea capitis supported terbinafine’s greater efficacy in patients infected with T tonsurans.5
Conclusion
Our case series demonstrated a 35% prevalence of TI cases in a population of children with confirmed dermatophytosis presenting to dermatologists at an American academic medical center. We hope that noting the high prevalence and manifold presentations of this disease will aid practitioners in maintaining clinical suspicion for dermatophytosis and thereby facilitate appropriate identification and treatment of TI.
- Paloni G, Valerio E, Berti I, et al. Tinea incognito [published online September 28, 2015]. J Pediatr. 2015;167:1450-e2.
- del Boz J, Crespo V, Rivas‐Ruiz F, et al. Tinea incognito in children: 54 cases. Mycoses. 2011;54:254-258.
- Romano C, Maritati E, Gianni C. Tinea incognito in Italy: a 15-year survey. Mycoses. 2006;49:383-387.
- Kim WJ, Kim TW, Mun JH, et al. Tinea incognito in Korea and itsrisk factors: nine-year multicenter survey. J Korean Med Sci. 2013;28:145-151.
- Chen X, Jiang X, Yang M, et al. Systemic antifungal therapy for tinea capitis in children: an abridged Cochrane review. J Am Acad Dermatol. 2017;76:368-374.
Tinea incognito (TI) describes a dermatophytosis with often atypical clinical features attributed to prior use of topical corticosteroids or other immunomodulating agents. Tinea incognito may lack the scale and elevated margin typical of cutaneous dermatophytoses and can be mistaken for other pediatric cutaneous diseases, particularly atopic dermatitis. 1 Given the prevalence of TI and its susceptibility to misdiagnosis, we conducted a retrospective medical record review of cases of pediatric dermatophytosis presenting from 2005 to 2016.
Methods
We reviewed medical records for patients younger than 18 years who had been seen at the Faculty Group Practice of the Ronald O. Perelman Department of Dermatology, New York University School of Medicine (New York, New York), between January 1, 2005, and October 21, 2016, using International Classification of Diseases, Ninth Revision (ICD-9) codes 110.0 (tinea capitis), 110.1 (onychomycosis/tinea unguium), 110.3 (tinea cruris), 110.4 (tinea pedis), 110.5 (tinea corporis), and 110.9 (tinea, unspecified site). Cases were included in this study if there was documentation of dermatophytosis previously treated with topical corticosteroids or calcineurin inhibitors as well as positive potassium hydroxide (KOH) preparation or fungal culture with dermatophyte growth obtained from lesions satisfying the first criterion. This study was approved by the New York University School of Medicine institutional review board (study no. S15-01388).
Statistical analyses were conducted in SPSS 19.0 for Windows. Categorical variables were assessed using the χ2 test for independence and the Fisher exact test.
Results
A total of 464 cases were reviewed. A positive KOH preparation or dermatophyte fungal culture was documented in 83 cases. Of them, 29 (34.9%) were treated with topical steroids and/or calcineurin inhibitors prior to presentation to dermatology (Table). The mean age at presentation was 8 years. Duration of symptoms prior to presentation was recorded for 23 of 29 patients (79.3%). Of them, 6 (26.1%) experienced symptoms for 1 month or less, 12 (52.2%) for 1 to 6 months, and 5 (21.7%) for 6 months to 1 year.

Physical examination findings (Figure) were documented in all 29 cases. Annular lesions were noted in 24 patients (82.8%). Pustules were present in 5 patients (17.2%) and papules in 11 patients (37.9%). Fourteen patients (48.3%) had involvement of the face, 14 (48.3%) of the body (ie, trunk, extremities, or groin), and 3 (10.3%) of the scalp. Six patients (20.7%) demonstrated findings at more than one body site.

Females were more likely to demonstrate facial lesions (P=.02), while males were more likely to present with body lesions (P=.04). Of 26 patients diagnosed via fungal culture, 16 (55.2%) grew Trichophyton tonsurans, 4 (13.8%) grew Trichophyton rubrum, 3 (10.3%) grew Trichophyton mentagrophytes, 2 (6.9%) grew Microsporum canis, and 1 (3.4%) grew Microsporum gypseum. Treatment entailed oral medication in 18 cases (62.1%). Of them, 13 (72.2%) were treated with griseofulvin, 3 (16.7%) with fluconazole, and 2 (11.1%) with terbinafine. Topical antifungals were prescribed in the remaining 11 cases (37.9%); no further treatment was documented.
Comment
Since the initial description of TI, approximately 60 case reports and small series as well as several larger observational studies describing TI have been published. In our series of pediatric patients, 29 of 83 culture- or KOH-confirmed dermatophytosis cases (34.9%) were considered to be TI due to treatment with topical corticosteroids and/or calcineurin inhibitors prior to presentation. This high prevalence contrasts with the 5.6% prevalence reported in the only prior large case series examining TI in childhood.2 These authors further reported that in their pediatric population, TI was significantly (odds ratio, 8.7; 95% CI, 4.7-16.1) more likely to occur on the face relative to other dermatophytoses and significantly (odds ratio, 0.014; 95% CI, 0.002-0.099) less likely to occur on the scalp.2 We noted a significant association between female gender and facial symptoms as well as between male gender and truncal symptoms. Taken together, these findings suggest an increased likelihood of pediatric tinea faciei to be inappropriately treated, particularly in females.
Although TI treated with topical corticosteroids or calcineurin inhibitors can mimic other skin diseases, a majority of patients in our series demonstrated findings associated with classic tinea, such as annularity and scale. Further, we found that T tonsurans was the causative organism in most cases with T rubrum uncommonly seen, though it is the most prevalent dermatophyte observed worldwide and in 2 large TI case series.3,4 Regional variation in dermatophytes may account for these differences. In our study, griseofulvin was used most frequently in TI treatment, though a systematic review of oral antifungals in tinea capitis supported terbinafine’s greater efficacy in patients infected with T tonsurans.5
Conclusion
Our case series demonstrated a 35% prevalence of TI cases in a population of children with confirmed dermatophytosis presenting to dermatologists at an American academic medical center. We hope that noting the high prevalence and manifold presentations of this disease will aid practitioners in maintaining clinical suspicion for dermatophytosis and thereby facilitate appropriate identification and treatment of TI.
Tinea incognito (TI) describes a dermatophytosis with often atypical clinical features attributed to prior use of topical corticosteroids or other immunomodulating agents. Tinea incognito may lack the scale and elevated margin typical of cutaneous dermatophytoses and can be mistaken for other pediatric cutaneous diseases, particularly atopic dermatitis. 1 Given the prevalence of TI and its susceptibility to misdiagnosis, we conducted a retrospective medical record review of cases of pediatric dermatophytosis presenting from 2005 to 2016.
Methods
We reviewed medical records for patients younger than 18 years who had been seen at the Faculty Group Practice of the Ronald O. Perelman Department of Dermatology, New York University School of Medicine (New York, New York), between January 1, 2005, and October 21, 2016, using International Classification of Diseases, Ninth Revision (ICD-9) codes 110.0 (tinea capitis), 110.1 (onychomycosis/tinea unguium), 110.3 (tinea cruris), 110.4 (tinea pedis), 110.5 (tinea corporis), and 110.9 (tinea, unspecified site). Cases were included in this study if there was documentation of dermatophytosis previously treated with topical corticosteroids or calcineurin inhibitors as well as positive potassium hydroxide (KOH) preparation or fungal culture with dermatophyte growth obtained from lesions satisfying the first criterion. This study was approved by the New York University School of Medicine institutional review board (study no. S15-01388).
Statistical analyses were conducted in SPSS 19.0 for Windows. Categorical variables were assessed using the χ2 test for independence and the Fisher exact test.
Results
A total of 464 cases were reviewed. A positive KOH preparation or dermatophyte fungal culture was documented in 83 cases. Of them, 29 (34.9%) were treated with topical steroids and/or calcineurin inhibitors prior to presentation to dermatology (Table). The mean age at presentation was 8 years. Duration of symptoms prior to presentation was recorded for 23 of 29 patients (79.3%). Of them, 6 (26.1%) experienced symptoms for 1 month or less, 12 (52.2%) for 1 to 6 months, and 5 (21.7%) for 6 months to 1 year.

Physical examination findings (Figure) were documented in all 29 cases. Annular lesions were noted in 24 patients (82.8%). Pustules were present in 5 patients (17.2%) and papules in 11 patients (37.9%). Fourteen patients (48.3%) had involvement of the face, 14 (48.3%) of the body (ie, trunk, extremities, or groin), and 3 (10.3%) of the scalp. Six patients (20.7%) demonstrated findings at more than one body site.

Females were more likely to demonstrate facial lesions (P=.02), while males were more likely to present with body lesions (P=.04). Of 26 patients diagnosed via fungal culture, 16 (55.2%) grew Trichophyton tonsurans, 4 (13.8%) grew Trichophyton rubrum, 3 (10.3%) grew Trichophyton mentagrophytes, 2 (6.9%) grew Microsporum canis, and 1 (3.4%) grew Microsporum gypseum. Treatment entailed oral medication in 18 cases (62.1%). Of them, 13 (72.2%) were treated with griseofulvin, 3 (16.7%) with fluconazole, and 2 (11.1%) with terbinafine. Topical antifungals were prescribed in the remaining 11 cases (37.9%); no further treatment was documented.
Comment
Since the initial description of TI, approximately 60 case reports and small series as well as several larger observational studies describing TI have been published. In our series of pediatric patients, 29 of 83 culture- or KOH-confirmed dermatophytosis cases (34.9%) were considered to be TI due to treatment with topical corticosteroids and/or calcineurin inhibitors prior to presentation. This high prevalence contrasts with the 5.6% prevalence reported in the only prior large case series examining TI in childhood.2 These authors further reported that in their pediatric population, TI was significantly (odds ratio, 8.7; 95% CI, 4.7-16.1) more likely to occur on the face relative to other dermatophytoses and significantly (odds ratio, 0.014; 95% CI, 0.002-0.099) less likely to occur on the scalp.2 We noted a significant association between female gender and facial symptoms as well as between male gender and truncal symptoms. Taken together, these findings suggest an increased likelihood of pediatric tinea faciei to be inappropriately treated, particularly in females.
Although TI treated with topical corticosteroids or calcineurin inhibitors can mimic other skin diseases, a majority of patients in our series demonstrated findings associated with classic tinea, such as annularity and scale. Further, we found that T tonsurans was the causative organism in most cases with T rubrum uncommonly seen, though it is the most prevalent dermatophyte observed worldwide and in 2 large TI case series.3,4 Regional variation in dermatophytes may account for these differences. In our study, griseofulvin was used most frequently in TI treatment, though a systematic review of oral antifungals in tinea capitis supported terbinafine’s greater efficacy in patients infected with T tonsurans.5
Conclusion
Our case series demonstrated a 35% prevalence of TI cases in a population of children with confirmed dermatophytosis presenting to dermatologists at an American academic medical center. We hope that noting the high prevalence and manifold presentations of this disease will aid practitioners in maintaining clinical suspicion for dermatophytosis and thereby facilitate appropriate identification and treatment of TI.
- Paloni G, Valerio E, Berti I, et al. Tinea incognito [published online September 28, 2015]. J Pediatr. 2015;167:1450-e2.
- del Boz J, Crespo V, Rivas‐Ruiz F, et al. Tinea incognito in children: 54 cases. Mycoses. 2011;54:254-258.
- Romano C, Maritati E, Gianni C. Tinea incognito in Italy: a 15-year survey. Mycoses. 2006;49:383-387.
- Kim WJ, Kim TW, Mun JH, et al. Tinea incognito in Korea and itsrisk factors: nine-year multicenter survey. J Korean Med Sci. 2013;28:145-151.
- Chen X, Jiang X, Yang M, et al. Systemic antifungal therapy for tinea capitis in children: an abridged Cochrane review. J Am Acad Dermatol. 2017;76:368-374.
- Paloni G, Valerio E, Berti I, et al. Tinea incognito [published online September 28, 2015]. J Pediatr. 2015;167:1450-e2.
- del Boz J, Crespo V, Rivas‐Ruiz F, et al. Tinea incognito in children: 54 cases. Mycoses. 2011;54:254-258.
- Romano C, Maritati E, Gianni C. Tinea incognito in Italy: a 15-year survey. Mycoses. 2006;49:383-387.
- Kim WJ, Kim TW, Mun JH, et al. Tinea incognito in Korea and itsrisk factors: nine-year multicenter survey. J Korean Med Sci. 2013;28:145-151.
- Chen X, Jiang X, Yang M, et al. Systemic antifungal therapy for tinea capitis in children: an abridged Cochrane review. J Am Acad Dermatol. 2017;76:368-374.
Practice Points
- Within our pediatric study population of microbiologically confirmed tinea cases at an American academic center, we found a 35% prevalence of tinea incognito (TI).
- Unlike investigations of TI in other countries, Trichophyton tonsurans was found to be the most common causative dermatophyte.
- Our data suggest that facial tinea may be more likely to be improperly treated in females and likewise tinea of the trunk or extremities in males.
Researchers Develop Guidelines for Evaluating Cognitive and Behavioral Syndromes in Adults
A care partner almost always should be involved in the evaluation, the guidelines advise.
CHICAGO—An Alzheimer’s Association workgroup has developed 20 recommendations for the clinical evaluation of patients with cognitive or behavioral complaints. All middle-aged or older individuals who report or whose care partner or clinician reports cognitive, behavioral, or functional changes should undergo a timely evaluation, the guidelines advise. A care partner almost always should be involved the evaluation, according to the guidelines.
The recommendations cover the recognition and evaluation of symptoms, selection of brain imaging and other tests, and communication with and support of affected individuals and their caregivers.
Alireza Atri, MD, PhD, cochair of the workgroup, presented the recommendations at AAIC 2018. The authors plan to finalize and publish the guidelines in 2018.
“Until now, we have not had highly specific and multispecialty US national guidelines that can inform the diagnostic process across all care settings and that provide standards meant to improve patient autonomy, care, and outcomes,” said Dr. Atri, Director of the Banner Sun Health Research Institute in Sun City, Arizona, and Lecturer in Neurology at the Center for Brain/Mind Medicine at Brigham and Women’s Hospital and Harvard Medical School in Boston.
Cognitive Behavioral Syndromes
The clinical practice guidelines recognize a broad category of cognitive behavioral syndromes marked by memory and thinking symptoms as well as changes in sleep, anxiety, personality, and relationships.
The Alzheimer’s Association in 2017 convened a Diagnostic Evaluation Clinical Practice Guideline workgroup to develop evidence-based guidelines. The group includes experts in medical, neuropsychologic, and nursing specialties. The members conducted a systematic review of the literature and made recommendations using a modified Delphi consensus process. They graded the recommendations as “A” (must be done; will improve outcomes in almost all cases), “B” (should be done), and “C” (may be done).
The recommendations emphasize obtaining a history from not only the patient, but also from someone who knows the patient well to establish the presence and characteristics of any substantial changes and to categorize the cognitive behavioral syndrome.
Other recommendations for evaluating patients with cognitive behavioral syndromes include the following:
- For patients with atypical or rapidly progressive cognitive behavioral symptoms, the clinician should expedite an evaluation and strongly consider referral to a specialist. (Level A)
- The evaluation process should use tiers of assessments and tests based on a patient’s presentation, risk factors, and profile. (Level A)
- The clinician should involve an informant to obtain reliable information about changes in cognition, activities of daily living, mood and other neuropsychiatric symptoms, and sensory and motor function. Use of structured instruments for assessing these domains is helpful. (Level A)
- Clinicians should use validated tools to assess cognition. (Level A)
- When office-based cognitive assessment is not sufficiently informative (eg, when interpretation of results is uncertain due to a complex clinical profile or confounding demographic characteristics), neuropsychologic evaluation is recommended. (Level A)
- The clinician should obtain MRI as a first-tier approach to aid in establishing etiology. If MRI is not available or is contraindicated, CT should be obtained. (Level B)
- If etiology remains uncertain after interpretation of structural imaging, a dementia specialist can obtain molecular imaging with FDG-PET to improve diagnostic accuracy. (Level B)
- In cases with continued diagnostic uncertainty, a dementia specialist can obtain CSF according to appropriate use criteria for analysis of aβ42 amyloid and tau/p-tau profiles to evaluate for Alzheimer’s disease pathology. (Level C)
- If diagnostic uncertainty remains after obtaining structural imaging and FDG-PET, and CSF aβ and tau/p-tau profiles are unavailable or uninterpretable, the dementia specialist can obtain an amyloid PET scan according to the appropriate use criteria. (Level C)
- In a patient with an established cognitive behavioral syndrome and a likely autosomal dominant family history, the dementia specialist should consider whether genetic testing is warranted. A genetic counselor should be involved throughout the process. (Level A)
A Tool for Medical Professionals
According to the workgroup, a timely and accurate diagnosis of Alzheimer’s disease and related dementias increases patient autonomy when he or she is most able to participate in goals of treatment and life and care decisions. It also allows for early intervention to maximize support opportunities and treatment outcomes.
“These new guidelines will provide an important new tool for medical professionals to more accurately diagnose Alzheimer’s [disease] and other dementias. As a result, people will get the right care and appropriate treatments; families will get the right support and be able to plan for the future,” said James Hendrix, PhD, Alzheimer’s Association Director of Global Science Initiatives and a member of the workgroup. “Too often, cognitive and behavioral symptoms due to Alzheimer’s disease and other dementias are unrecognized or attributed to something else.”
“The guidelines can empower patients, families, and clinicians to expect that symptoms will be evaluated in a patient-centered, structured, and collaborative manner,” Dr. Atri said. “In addition, they help to ensure that, regardless of the specific diagnosis, the results are communicated in a timely and compassionate way to help patients and families live the best lives possible.”
A care partner almost always should be involved in the evaluation, the guidelines advise.
A care partner almost always should be involved in the evaluation, the guidelines advise.
CHICAGO—An Alzheimer’s Association workgroup has developed 20 recommendations for the clinical evaluation of patients with cognitive or behavioral complaints. All middle-aged or older individuals who report or whose care partner or clinician reports cognitive, behavioral, or functional changes should undergo a timely evaluation, the guidelines advise. A care partner almost always should be involved the evaluation, according to the guidelines.
The recommendations cover the recognition and evaluation of symptoms, selection of brain imaging and other tests, and communication with and support of affected individuals and their caregivers.
Alireza Atri, MD, PhD, cochair of the workgroup, presented the recommendations at AAIC 2018. The authors plan to finalize and publish the guidelines in 2018.
“Until now, we have not had highly specific and multispecialty US national guidelines that can inform the diagnostic process across all care settings and that provide standards meant to improve patient autonomy, care, and outcomes,” said Dr. Atri, Director of the Banner Sun Health Research Institute in Sun City, Arizona, and Lecturer in Neurology at the Center for Brain/Mind Medicine at Brigham and Women’s Hospital and Harvard Medical School in Boston.
Cognitive Behavioral Syndromes
The clinical practice guidelines recognize a broad category of cognitive behavioral syndromes marked by memory and thinking symptoms as well as changes in sleep, anxiety, personality, and relationships.
The Alzheimer’s Association in 2017 convened a Diagnostic Evaluation Clinical Practice Guideline workgroup to develop evidence-based guidelines. The group includes experts in medical, neuropsychologic, and nursing specialties. The members conducted a systematic review of the literature and made recommendations using a modified Delphi consensus process. They graded the recommendations as “A” (must be done; will improve outcomes in almost all cases), “B” (should be done), and “C” (may be done).
The recommendations emphasize obtaining a history from not only the patient, but also from someone who knows the patient well to establish the presence and characteristics of any substantial changes and to categorize the cognitive behavioral syndrome.
Other recommendations for evaluating patients with cognitive behavioral syndromes include the following:
- For patients with atypical or rapidly progressive cognitive behavioral symptoms, the clinician should expedite an evaluation and strongly consider referral to a specialist. (Level A)
- The evaluation process should use tiers of assessments and tests based on a patient’s presentation, risk factors, and profile. (Level A)
- The clinician should involve an informant to obtain reliable information about changes in cognition, activities of daily living, mood and other neuropsychiatric symptoms, and sensory and motor function. Use of structured instruments for assessing these domains is helpful. (Level A)
- Clinicians should use validated tools to assess cognition. (Level A)
- When office-based cognitive assessment is not sufficiently informative (eg, when interpretation of results is uncertain due to a complex clinical profile or confounding demographic characteristics), neuropsychologic evaluation is recommended. (Level A)
- The clinician should obtain MRI as a first-tier approach to aid in establishing etiology. If MRI is not available or is contraindicated, CT should be obtained. (Level B)
- If etiology remains uncertain after interpretation of structural imaging, a dementia specialist can obtain molecular imaging with FDG-PET to improve diagnostic accuracy. (Level B)
- In cases with continued diagnostic uncertainty, a dementia specialist can obtain CSF according to appropriate use criteria for analysis of aβ42 amyloid and tau/p-tau profiles to evaluate for Alzheimer’s disease pathology. (Level C)
- If diagnostic uncertainty remains after obtaining structural imaging and FDG-PET, and CSF aβ and tau/p-tau profiles are unavailable or uninterpretable, the dementia specialist can obtain an amyloid PET scan according to the appropriate use criteria. (Level C)
- In a patient with an established cognitive behavioral syndrome and a likely autosomal dominant family history, the dementia specialist should consider whether genetic testing is warranted. A genetic counselor should be involved throughout the process. (Level A)
A Tool for Medical Professionals
According to the workgroup, a timely and accurate diagnosis of Alzheimer’s disease and related dementias increases patient autonomy when he or she is most able to participate in goals of treatment and life and care decisions. It also allows for early intervention to maximize support opportunities and treatment outcomes.
“These new guidelines will provide an important new tool for medical professionals to more accurately diagnose Alzheimer’s [disease] and other dementias. As a result, people will get the right care and appropriate treatments; families will get the right support and be able to plan for the future,” said James Hendrix, PhD, Alzheimer’s Association Director of Global Science Initiatives and a member of the workgroup. “Too often, cognitive and behavioral symptoms due to Alzheimer’s disease and other dementias are unrecognized or attributed to something else.”
“The guidelines can empower patients, families, and clinicians to expect that symptoms will be evaluated in a patient-centered, structured, and collaborative manner,” Dr. Atri said. “In addition, they help to ensure that, regardless of the specific diagnosis, the results are communicated in a timely and compassionate way to help patients and families live the best lives possible.”
CHICAGO—An Alzheimer’s Association workgroup has developed 20 recommendations for the clinical evaluation of patients with cognitive or behavioral complaints. All middle-aged or older individuals who report or whose care partner or clinician reports cognitive, behavioral, or functional changes should undergo a timely evaluation, the guidelines advise. A care partner almost always should be involved the evaluation, according to the guidelines.
The recommendations cover the recognition and evaluation of symptoms, selection of brain imaging and other tests, and communication with and support of affected individuals and their caregivers.
Alireza Atri, MD, PhD, cochair of the workgroup, presented the recommendations at AAIC 2018. The authors plan to finalize and publish the guidelines in 2018.
“Until now, we have not had highly specific and multispecialty US national guidelines that can inform the diagnostic process across all care settings and that provide standards meant to improve patient autonomy, care, and outcomes,” said Dr. Atri, Director of the Banner Sun Health Research Institute in Sun City, Arizona, and Lecturer in Neurology at the Center for Brain/Mind Medicine at Brigham and Women’s Hospital and Harvard Medical School in Boston.
Cognitive Behavioral Syndromes
The clinical practice guidelines recognize a broad category of cognitive behavioral syndromes marked by memory and thinking symptoms as well as changes in sleep, anxiety, personality, and relationships.
The Alzheimer’s Association in 2017 convened a Diagnostic Evaluation Clinical Practice Guideline workgroup to develop evidence-based guidelines. The group includes experts in medical, neuropsychologic, and nursing specialties. The members conducted a systematic review of the literature and made recommendations using a modified Delphi consensus process. They graded the recommendations as “A” (must be done; will improve outcomes in almost all cases), “B” (should be done), and “C” (may be done).
The recommendations emphasize obtaining a history from not only the patient, but also from someone who knows the patient well to establish the presence and characteristics of any substantial changes and to categorize the cognitive behavioral syndrome.
Other recommendations for evaluating patients with cognitive behavioral syndromes include the following:
- For patients with atypical or rapidly progressive cognitive behavioral symptoms, the clinician should expedite an evaluation and strongly consider referral to a specialist. (Level A)
- The evaluation process should use tiers of assessments and tests based on a patient’s presentation, risk factors, and profile. (Level A)
- The clinician should involve an informant to obtain reliable information about changes in cognition, activities of daily living, mood and other neuropsychiatric symptoms, and sensory and motor function. Use of structured instruments for assessing these domains is helpful. (Level A)
- Clinicians should use validated tools to assess cognition. (Level A)
- When office-based cognitive assessment is not sufficiently informative (eg, when interpretation of results is uncertain due to a complex clinical profile or confounding demographic characteristics), neuropsychologic evaluation is recommended. (Level A)
- The clinician should obtain MRI as a first-tier approach to aid in establishing etiology. If MRI is not available or is contraindicated, CT should be obtained. (Level B)
- If etiology remains uncertain after interpretation of structural imaging, a dementia specialist can obtain molecular imaging with FDG-PET to improve diagnostic accuracy. (Level B)
- In cases with continued diagnostic uncertainty, a dementia specialist can obtain CSF according to appropriate use criteria for analysis of aβ42 amyloid and tau/p-tau profiles to evaluate for Alzheimer’s disease pathology. (Level C)
- If diagnostic uncertainty remains after obtaining structural imaging and FDG-PET, and CSF aβ and tau/p-tau profiles are unavailable or uninterpretable, the dementia specialist can obtain an amyloid PET scan according to the appropriate use criteria. (Level C)
- In a patient with an established cognitive behavioral syndrome and a likely autosomal dominant family history, the dementia specialist should consider whether genetic testing is warranted. A genetic counselor should be involved throughout the process. (Level A)
A Tool for Medical Professionals
According to the workgroup, a timely and accurate diagnosis of Alzheimer’s disease and related dementias increases patient autonomy when he or she is most able to participate in goals of treatment and life and care decisions. It also allows for early intervention to maximize support opportunities and treatment outcomes.
“These new guidelines will provide an important new tool for medical professionals to more accurately diagnose Alzheimer’s [disease] and other dementias. As a result, people will get the right care and appropriate treatments; families will get the right support and be able to plan for the future,” said James Hendrix, PhD, Alzheimer’s Association Director of Global Science Initiatives and a member of the workgroup. “Too often, cognitive and behavioral symptoms due to Alzheimer’s disease and other dementias are unrecognized or attributed to something else.”
“The guidelines can empower patients, families, and clinicians to expect that symptoms will be evaluated in a patient-centered, structured, and collaborative manner,” Dr. Atri said. “In addition, they help to ensure that, regardless of the specific diagnosis, the results are communicated in a timely and compassionate way to help patients and families live the best lives possible.”
Eruptive Vellus Hair Cysts in Identical Triplets With Dermoscopic Findings
Case Report
Four-year-old identical triplet girls with numerous asymptomatic scattered papules on the chest of 4 months’ duration were referred to a dermatologist by their pediatrician for molluscum contagiosum. The patients’ father reported that there was no history of trauma, irritation, or manipulation to the affected area. Their medical history was notable for prematurity at 32 weeks’ gestation and congenital dermal melanocytosis. Family history was notable for their father having acne and similar papules on the chest during adolescence that resolved with isotretinoin therapy.
On physical examination there were multiple smooth, hyperpigmented to erythematous, comedonal, 1- to 2-mm papules dispersed on the anterior central chest of all 3 patients (Figure 1). Clinically, these lesions were fairly indistinguishable from other common dermatologic conditions such as acne or milia. Dermoscopic examination revealed homogenous yellow-white areas surrounded by light brown to erythematous halos (Figure 2). Histopathologic examination was not performed given the benign clinical diagnosis and avoidance of biopsy in pediatric populations. Based on dermoscopic features and history, a diagnosis of eruptive vellus hair cysts (EVHCs) in identical triplets was made.
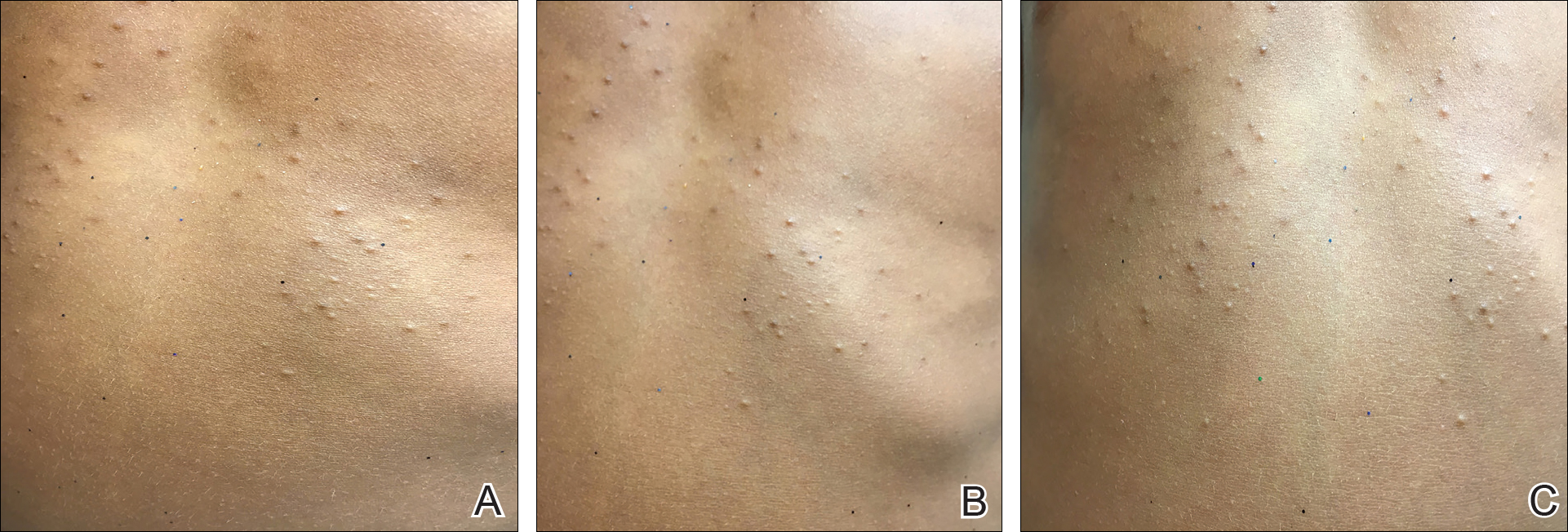
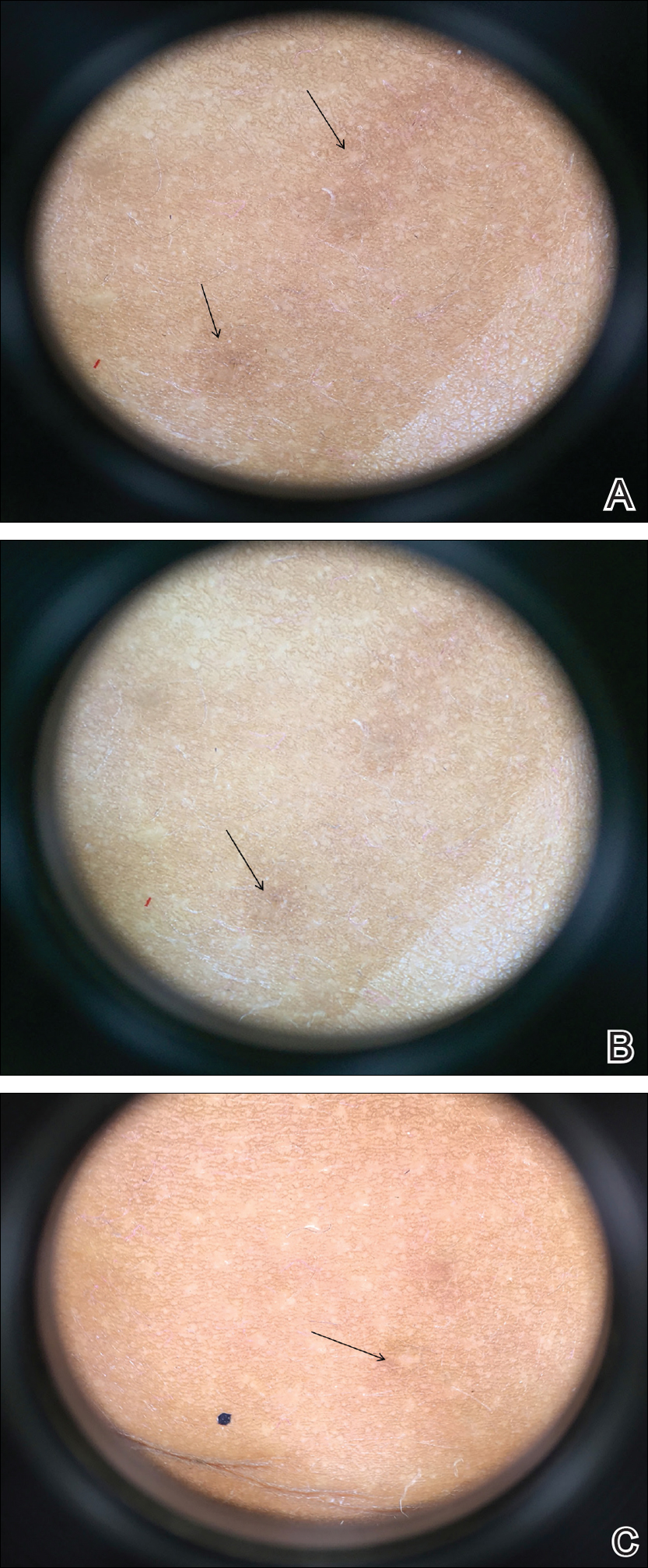
Comment
Pathogenesis
Eruptive vellus hair cysts, first introduced by Esterly et al1 in 1977, are uncommon benign lesions presumed to be caused by an abnormal development of the infundibular portion of the hair follicle.2 They are usually 1- to 3-mm, reddish brown, monomorphous papules overlapping with pilosebaceous and apocrine units.3 Although the lesions typically are located on the chest and extremities, they may occur on the face, abdomen, axillae, buttocks, or genital area.1,3 The inheritance of EVHCs is unclear. The majority of reported cases are sporadic; however, the literature mentions 19 families affected by autosomal-dominant EVHCs based on phylogeny.3 In 2015, EVHCs were reported in identical twins, further supporting the case for a genetic mutation.4 We augment this autosomal-dominant inheritance pattern by presenting a case of identical triplets with EVHCs. The patients’ father reported similar lesions in childhood, further underscoring a genetic basis.
The pathogenesis of EVHC is uncertain, with 2 main theories. Some propose retention of vellus hair and keratin in a cavity formed by an abnormal vellus hair follicle causing infundibular occlusion. Others consider the growth of benign follicular hamartomas that differentiate to become vellus hairs.1
Clinical Presentation
The sporadic form of EVHCs is noted to be more common and clinically presents later, with an average age at onset of 16 years and an average age at diagnosis of 24 years.3 The sporadic form occurs without trauma or manipulation as a precursor. Less commonly, lesions present at birth or in early infancy and may show an autosomal-dominant inheritance pattern with a similar distribution across relatives.3
Other variants of EVHCs have been described. Late-onset EVHC usually occurs at 35 years or older (average age, 57 years), with a female to male predominance of 2.5 to 1.3 This late onset may be attributed to proliferation of ductal follicular keratinocytes or loss of perifollicular elastic fibers exacerbated by exogenous factors such as manipulation, UV rays, or trauma.5
For unilesional EVHC, the average age at diagnosis is 27 years.3 Some of these lesions may be pedunculated and greater than 8 mm. There is a female to male predominance of 2 to 1. Eruptive vellus hair cysts with steatocystoma multiplex can be seen with an average age at onset of 19 years and a female to male predominance of 0.2 to 1. There may be a family history of this subset, as reported in 3 patients with this pattern.3
Diagnosis
The recommended workup for EVHCs varies by patient and age. Eruptive vellus hair cysts present an opportunity to utilize noninvasive diagnostic procedures, especially for the pediatric population, to avoid scarring and pain from manipulation or biopsies. Although many practitioners may comfortably diagnose EVHCs clinically, 6 cases were misdiagnosed as steatocystoma multiplex, keratosis pilaris, or milia prior to histopathology revealing vellus hair cysts.6
Dermoscopy presents as a useful diagnostic aid. Eruptive vellus hair cysts exhibit light yellow homogenous circular structures with a maroon or erythematous halo.2,7 A central gray-blue color point may be seen due to melanin in the pigmented hair shaft.7 A dermoscopy review of EVHCs reported radiating capillaries.2 Occasionally, nonfollicular homogenous blue pigmentation may be seen due to a connection to atrophic hair follicles in the mid dermis and no normal hair follicle around the cysts.8 In comparison, dermoscopic characteristics of molluscum contagiosum demonstrated a polylobular, white-yellow, amorphous structure at the center with a hardened central umbilicated core and a crown of hairpin vessels at the periphery. Additionally, comedonal acne, commonly mistaken for EVHCs, reveals a brown-yellow hard central plug with sparse inflammation under dermoscopy.2 Thus, differentiation of these entities with dermoscopy should be highly prioritized to better aid in the diagnosis of pediatric dermatologic conditions using painless noninvasive techniques.
Treatment
The main indication for treatment of EVHCs is cosmetic concern. Twenty-five percent of EVHCs spontaneously resolve with transepidermal hair elimination or a granulomatous reaction.4,5 A case report of 4 siblings with congenital EVHCs also described a mother with similar lesions that resolved spontaneously in early adulthood,3 as our patients’ father also noted. Treatment modalities including topical keratolytic agents such as urea 10%, retinoic acid 0.05%, tazarotene cream 0.1%, and lactic acid 12%; incision and drainage; CO2 laser; or erbium-doped YAG laser ablation have been tried with minimal improvement.9 Of note, tazarotene cream 0.1% has demonstrated better results than both erbium-doped YAG laser and drainage and incision of EVHCs.4 Additionally, another report evidenced partial improvement with calcipotriene within 2 months with some lesions completely resolved and others flattened, which may be attributed to the antiproliferative and prodifferentiating effects on the ductal follicular keratinocytes by calcipotriene.5 Lastly, an additional study indicated that isotretinoin and vitamin A derivatives were ineffective for clearing EVHCs.10
Conclusion
We presented 3 identical triplets with the classic pediatric onset and dermoscopic findings of EVHCs on the trunk. Although the definitive diagnosis of EVHCs relies on histopathology, we argue that their unique dermoscopic findings combined with a thorough clinical examination is sufficient to recognize this benign condition and avoid painful procedures in the pediatric population.
- Esterly NB, Fretzin DF, Pinkus H. Eruptive vellus hair cysts. Arch Dermatol. 1977;113:500-503.
- Alfaro-Castellón P, Mejía-Rodríguez SA, Valencia-Herrera A, et al. Dermoscopy distinction of eruptive vellus hair cysts with molluscum contagiosum and acne lesions. Pediatr Dermatol. 2012;29:772-773.
- Torchia D, Vega J, Schachner LA. Eruptive vellus hair cysts: a systematic review. Am J Clin Dermatol. 2012;13:19-28.
- Pauline G, Alain H, Jean-Jaques R, et al. Eruptive vellus hair cysts: an original case occurring in twins [published online July 11, 2014]. Int J Dermatol. 2015;54:E209-E212.
- Erkek E, Kurtipek GS, Duman D, et al. Eruptive vellus hair cysts: report of a pediatric case with partial response to calcipotriene therapy. Cutis. 2009;84:295-298.
- Shi G, Zhou Y, Cai YX, et al. Clinicopathological features and expression of four keratins (K10, K14, K17 and K19) in six cases of eruptive vellus hair cysts. Clin Exp Dermatol. 2014;39:496-499.
- Panchaprateep R, Tanus A, Tosti A. Clinical, dermoscopic, and histopathologic features of body hair disorders. J Am Acad Dermatol. 2015;72:890-900.
- Takada S, Togawa Y, Wakabayashii S, et al. Dermoscopic findings in eruptive vellus hair cysts: a case report. Austin J Dermatol. 2014;1:1004.
- Khatu S, Vasani R, Amin S. Eruptive vellus hair cyst presenting as asymptomatic follicular papules on extremities. Indian Dermatol Online J. 2013;4:213-215.
- Urbina-Gonzalez F, Aguilar-Martinez A, Cristobal-Gil M, et al. The treatment of eruptive vellus hair cysts with isotretinoin. Br J Dermatol. 1987;116:465-466.
Case Report
Four-year-old identical triplet girls with numerous asymptomatic scattered papules on the chest of 4 months’ duration were referred to a dermatologist by their pediatrician for molluscum contagiosum. The patients’ father reported that there was no history of trauma, irritation, or manipulation to the affected area. Their medical history was notable for prematurity at 32 weeks’ gestation and congenital dermal melanocytosis. Family history was notable for their father having acne and similar papules on the chest during adolescence that resolved with isotretinoin therapy.
On physical examination there were multiple smooth, hyperpigmented to erythematous, comedonal, 1- to 2-mm papules dispersed on the anterior central chest of all 3 patients (Figure 1). Clinically, these lesions were fairly indistinguishable from other common dermatologic conditions such as acne or milia. Dermoscopic examination revealed homogenous yellow-white areas surrounded by light brown to erythematous halos (Figure 2). Histopathologic examination was not performed given the benign clinical diagnosis and avoidance of biopsy in pediatric populations. Based on dermoscopic features and history, a diagnosis of eruptive vellus hair cysts (EVHCs) in identical triplets was made.


Comment
Pathogenesis
Eruptive vellus hair cysts, first introduced by Esterly et al1 in 1977, are uncommon benign lesions presumed to be caused by an abnormal development of the infundibular portion of the hair follicle.2 They are usually 1- to 3-mm, reddish brown, monomorphous papules overlapping with pilosebaceous and apocrine units.3 Although the lesions typically are located on the chest and extremities, they may occur on the face, abdomen, axillae, buttocks, or genital area.1,3 The inheritance of EVHCs is unclear. The majority of reported cases are sporadic; however, the literature mentions 19 families affected by autosomal-dominant EVHCs based on phylogeny.3 In 2015, EVHCs were reported in identical twins, further supporting the case for a genetic mutation.4 We augment this autosomal-dominant inheritance pattern by presenting a case of identical triplets with EVHCs. The patients’ father reported similar lesions in childhood, further underscoring a genetic basis.
The pathogenesis of EVHC is uncertain, with 2 main theories. Some propose retention of vellus hair and keratin in a cavity formed by an abnormal vellus hair follicle causing infundibular occlusion. Others consider the growth of benign follicular hamartomas that differentiate to become vellus hairs.1
Clinical Presentation
The sporadic form of EVHCs is noted to be more common and clinically presents later, with an average age at onset of 16 years and an average age at diagnosis of 24 years.3 The sporadic form occurs without trauma or manipulation as a precursor. Less commonly, lesions present at birth or in early infancy and may show an autosomal-dominant inheritance pattern with a similar distribution across relatives.3
Other variants of EVHCs have been described. Late-onset EVHC usually occurs at 35 years or older (average age, 57 years), with a female to male predominance of 2.5 to 1.3 This late onset may be attributed to proliferation of ductal follicular keratinocytes or loss of perifollicular elastic fibers exacerbated by exogenous factors such as manipulation, UV rays, or trauma.5
For unilesional EVHC, the average age at diagnosis is 27 years.3 Some of these lesions may be pedunculated and greater than 8 mm. There is a female to male predominance of 2 to 1. Eruptive vellus hair cysts with steatocystoma multiplex can be seen with an average age at onset of 19 years and a female to male predominance of 0.2 to 1. There may be a family history of this subset, as reported in 3 patients with this pattern.3
Diagnosis
The recommended workup for EVHCs varies by patient and age. Eruptive vellus hair cysts present an opportunity to utilize noninvasive diagnostic procedures, especially for the pediatric population, to avoid scarring and pain from manipulation or biopsies. Although many practitioners may comfortably diagnose EVHCs clinically, 6 cases were misdiagnosed as steatocystoma multiplex, keratosis pilaris, or milia prior to histopathology revealing vellus hair cysts.6
Dermoscopy presents as a useful diagnostic aid. Eruptive vellus hair cysts exhibit light yellow homogenous circular structures with a maroon or erythematous halo.2,7 A central gray-blue color point may be seen due to melanin in the pigmented hair shaft.7 A dermoscopy review of EVHCs reported radiating capillaries.2 Occasionally, nonfollicular homogenous blue pigmentation may be seen due to a connection to atrophic hair follicles in the mid dermis and no normal hair follicle around the cysts.8 In comparison, dermoscopic characteristics of molluscum contagiosum demonstrated a polylobular, white-yellow, amorphous structure at the center with a hardened central umbilicated core and a crown of hairpin vessels at the periphery. Additionally, comedonal acne, commonly mistaken for EVHCs, reveals a brown-yellow hard central plug with sparse inflammation under dermoscopy.2 Thus, differentiation of these entities with dermoscopy should be highly prioritized to better aid in the diagnosis of pediatric dermatologic conditions using painless noninvasive techniques.
Treatment
The main indication for treatment of EVHCs is cosmetic concern. Twenty-five percent of EVHCs spontaneously resolve with transepidermal hair elimination or a granulomatous reaction.4,5 A case report of 4 siblings with congenital EVHCs also described a mother with similar lesions that resolved spontaneously in early adulthood,3 as our patients’ father also noted. Treatment modalities including topical keratolytic agents such as urea 10%, retinoic acid 0.05%, tazarotene cream 0.1%, and lactic acid 12%; incision and drainage; CO2 laser; or erbium-doped YAG laser ablation have been tried with minimal improvement.9 Of note, tazarotene cream 0.1% has demonstrated better results than both erbium-doped YAG laser and drainage and incision of EVHCs.4 Additionally, another report evidenced partial improvement with calcipotriene within 2 months with some lesions completely resolved and others flattened, which may be attributed to the antiproliferative and prodifferentiating effects on the ductal follicular keratinocytes by calcipotriene.5 Lastly, an additional study indicated that isotretinoin and vitamin A derivatives were ineffective for clearing EVHCs.10
Conclusion
We presented 3 identical triplets with the classic pediatric onset and dermoscopic findings of EVHCs on the trunk. Although the definitive diagnosis of EVHCs relies on histopathology, we argue that their unique dermoscopic findings combined with a thorough clinical examination is sufficient to recognize this benign condition and avoid painful procedures in the pediatric population.
Case Report
Four-year-old identical triplet girls with numerous asymptomatic scattered papules on the chest of 4 months’ duration were referred to a dermatologist by their pediatrician for molluscum contagiosum. The patients’ father reported that there was no history of trauma, irritation, or manipulation to the affected area. Their medical history was notable for prematurity at 32 weeks’ gestation and congenital dermal melanocytosis. Family history was notable for their father having acne and similar papules on the chest during adolescence that resolved with isotretinoin therapy.
On physical examination there were multiple smooth, hyperpigmented to erythematous, comedonal, 1- to 2-mm papules dispersed on the anterior central chest of all 3 patients (Figure 1). Clinically, these lesions were fairly indistinguishable from other common dermatologic conditions such as acne or milia. Dermoscopic examination revealed homogenous yellow-white areas surrounded by light brown to erythematous halos (Figure 2). Histopathologic examination was not performed given the benign clinical diagnosis and avoidance of biopsy in pediatric populations. Based on dermoscopic features and history, a diagnosis of eruptive vellus hair cysts (EVHCs) in identical triplets was made.


Comment
Pathogenesis
Eruptive vellus hair cysts, first introduced by Esterly et al1 in 1977, are uncommon benign lesions presumed to be caused by an abnormal development of the infundibular portion of the hair follicle.2 They are usually 1- to 3-mm, reddish brown, monomorphous papules overlapping with pilosebaceous and apocrine units.3 Although the lesions typically are located on the chest and extremities, they may occur on the face, abdomen, axillae, buttocks, or genital area.1,3 The inheritance of EVHCs is unclear. The majority of reported cases are sporadic; however, the literature mentions 19 families affected by autosomal-dominant EVHCs based on phylogeny.3 In 2015, EVHCs were reported in identical twins, further supporting the case for a genetic mutation.4 We augment this autosomal-dominant inheritance pattern by presenting a case of identical triplets with EVHCs. The patients’ father reported similar lesions in childhood, further underscoring a genetic basis.
The pathogenesis of EVHC is uncertain, with 2 main theories. Some propose retention of vellus hair and keratin in a cavity formed by an abnormal vellus hair follicle causing infundibular occlusion. Others consider the growth of benign follicular hamartomas that differentiate to become vellus hairs.1
Clinical Presentation
The sporadic form of EVHCs is noted to be more common and clinically presents later, with an average age at onset of 16 years and an average age at diagnosis of 24 years.3 The sporadic form occurs without trauma or manipulation as a precursor. Less commonly, lesions present at birth or in early infancy and may show an autosomal-dominant inheritance pattern with a similar distribution across relatives.3
Other variants of EVHCs have been described. Late-onset EVHC usually occurs at 35 years or older (average age, 57 years), with a female to male predominance of 2.5 to 1.3 This late onset may be attributed to proliferation of ductal follicular keratinocytes or loss of perifollicular elastic fibers exacerbated by exogenous factors such as manipulation, UV rays, or trauma.5
For unilesional EVHC, the average age at diagnosis is 27 years.3 Some of these lesions may be pedunculated and greater than 8 mm. There is a female to male predominance of 2 to 1. Eruptive vellus hair cysts with steatocystoma multiplex can be seen with an average age at onset of 19 years and a female to male predominance of 0.2 to 1. There may be a family history of this subset, as reported in 3 patients with this pattern.3
Diagnosis
The recommended workup for EVHCs varies by patient and age. Eruptive vellus hair cysts present an opportunity to utilize noninvasive diagnostic procedures, especially for the pediatric population, to avoid scarring and pain from manipulation or biopsies. Although many practitioners may comfortably diagnose EVHCs clinically, 6 cases were misdiagnosed as steatocystoma multiplex, keratosis pilaris, or milia prior to histopathology revealing vellus hair cysts.6
Dermoscopy presents as a useful diagnostic aid. Eruptive vellus hair cysts exhibit light yellow homogenous circular structures with a maroon or erythematous halo.2,7 A central gray-blue color point may be seen due to melanin in the pigmented hair shaft.7 A dermoscopy review of EVHCs reported radiating capillaries.2 Occasionally, nonfollicular homogenous blue pigmentation may be seen due to a connection to atrophic hair follicles in the mid dermis and no normal hair follicle around the cysts.8 In comparison, dermoscopic characteristics of molluscum contagiosum demonstrated a polylobular, white-yellow, amorphous structure at the center with a hardened central umbilicated core and a crown of hairpin vessels at the periphery. Additionally, comedonal acne, commonly mistaken for EVHCs, reveals a brown-yellow hard central plug with sparse inflammation under dermoscopy.2 Thus, differentiation of these entities with dermoscopy should be highly prioritized to better aid in the diagnosis of pediatric dermatologic conditions using painless noninvasive techniques.
Treatment
The main indication for treatment of EVHCs is cosmetic concern. Twenty-five percent of EVHCs spontaneously resolve with transepidermal hair elimination or a granulomatous reaction.4,5 A case report of 4 siblings with congenital EVHCs also described a mother with similar lesions that resolved spontaneously in early adulthood,3 as our patients’ father also noted. Treatment modalities including topical keratolytic agents such as urea 10%, retinoic acid 0.05%, tazarotene cream 0.1%, and lactic acid 12%; incision and drainage; CO2 laser; or erbium-doped YAG laser ablation have been tried with minimal improvement.9 Of note, tazarotene cream 0.1% has demonstrated better results than both erbium-doped YAG laser and drainage and incision of EVHCs.4 Additionally, another report evidenced partial improvement with calcipotriene within 2 months with some lesions completely resolved and others flattened, which may be attributed to the antiproliferative and prodifferentiating effects on the ductal follicular keratinocytes by calcipotriene.5 Lastly, an additional study indicated that isotretinoin and vitamin A derivatives were ineffective for clearing EVHCs.10
Conclusion
We presented 3 identical triplets with the classic pediatric onset and dermoscopic findings of EVHCs on the trunk. Although the definitive diagnosis of EVHCs relies on histopathology, we argue that their unique dermoscopic findings combined with a thorough clinical examination is sufficient to recognize this benign condition and avoid painful procedures in the pediatric population.
- Esterly NB, Fretzin DF, Pinkus H. Eruptive vellus hair cysts. Arch Dermatol. 1977;113:500-503.
- Alfaro-Castellón P, Mejía-Rodríguez SA, Valencia-Herrera A, et al. Dermoscopy distinction of eruptive vellus hair cysts with molluscum contagiosum and acne lesions. Pediatr Dermatol. 2012;29:772-773.
- Torchia D, Vega J, Schachner LA. Eruptive vellus hair cysts: a systematic review. Am J Clin Dermatol. 2012;13:19-28.
- Pauline G, Alain H, Jean-Jaques R, et al. Eruptive vellus hair cysts: an original case occurring in twins [published online July 11, 2014]. Int J Dermatol. 2015;54:E209-E212.
- Erkek E, Kurtipek GS, Duman D, et al. Eruptive vellus hair cysts: report of a pediatric case with partial response to calcipotriene therapy. Cutis. 2009;84:295-298.
- Shi G, Zhou Y, Cai YX, et al. Clinicopathological features and expression of four keratins (K10, K14, K17 and K19) in six cases of eruptive vellus hair cysts. Clin Exp Dermatol. 2014;39:496-499.
- Panchaprateep R, Tanus A, Tosti A. Clinical, dermoscopic, and histopathologic features of body hair disorders. J Am Acad Dermatol. 2015;72:890-900.
- Takada S, Togawa Y, Wakabayashii S, et al. Dermoscopic findings in eruptive vellus hair cysts: a case report. Austin J Dermatol. 2014;1:1004.
- Khatu S, Vasani R, Amin S. Eruptive vellus hair cyst presenting as asymptomatic follicular papules on extremities. Indian Dermatol Online J. 2013;4:213-215.
- Urbina-Gonzalez F, Aguilar-Martinez A, Cristobal-Gil M, et al. The treatment of eruptive vellus hair cysts with isotretinoin. Br J Dermatol. 1987;116:465-466.
- Esterly NB, Fretzin DF, Pinkus H. Eruptive vellus hair cysts. Arch Dermatol. 1977;113:500-503.
- Alfaro-Castellón P, Mejía-Rodríguez SA, Valencia-Herrera A, et al. Dermoscopy distinction of eruptive vellus hair cysts with molluscum contagiosum and acne lesions. Pediatr Dermatol. 2012;29:772-773.
- Torchia D, Vega J, Schachner LA. Eruptive vellus hair cysts: a systematic review. Am J Clin Dermatol. 2012;13:19-28.
- Pauline G, Alain H, Jean-Jaques R, et al. Eruptive vellus hair cysts: an original case occurring in twins [published online July 11, 2014]. Int J Dermatol. 2015;54:E209-E212.
- Erkek E, Kurtipek GS, Duman D, et al. Eruptive vellus hair cysts: report of a pediatric case with partial response to calcipotriene therapy. Cutis. 2009;84:295-298.
- Shi G, Zhou Y, Cai YX, et al. Clinicopathological features and expression of four keratins (K10, K14, K17 and K19) in six cases of eruptive vellus hair cysts. Clin Exp Dermatol. 2014;39:496-499.
- Panchaprateep R, Tanus A, Tosti A. Clinical, dermoscopic, and histopathologic features of body hair disorders. J Am Acad Dermatol. 2015;72:890-900.
- Takada S, Togawa Y, Wakabayashii S, et al. Dermoscopic findings in eruptive vellus hair cysts: a case report. Austin J Dermatol. 2014;1:1004.
- Khatu S, Vasani R, Amin S. Eruptive vellus hair cyst presenting as asymptomatic follicular papules on extremities. Indian Dermatol Online J. 2013;4:213-215.
- Urbina-Gonzalez F, Aguilar-Martinez A, Cristobal-Gil M, et al. The treatment of eruptive vellus hair cysts with isotretinoin. Br J Dermatol. 1987;116:465-466.
Practice Points
- Eruptive vellus hair cysts (EVHCs) are 1- to 3-mm round, dome-shaped, flesh-colored, asymptomatic, benign papules typically occurring on the chest and extremities.
- Pathogenesis and inheritance are unclear. Although the majority of EVHC cases are sporadic, the strong influence of genes is indicated by numerous reports of families in whom 2 or more members were affected.
- Dermoscopy is a noninvasive diagnostic procedure that should be utilized to diagnose EVHCs in the pediatric population; specifically, EVHCs exhibit light yellow, homogenous, circular structures with a maroon or erythematous halo.
- The main indication for treatment of EVHCs is cosmetic concern; however, one-quarter of cases may resolve spontaneously.
Growth on right cheek
Figure 1
The FP suspected that this was a nodular basal cell carcinoma (BCC) with pigmentation. The physical exam was suspicious because of the pearly appearance, superficial ulcerations, and presence of telangiectasias with a loss of the normal pore pattern. Dermoscopy gave further evidence for a nodular BCC by revealing arborizing “tree-like” telangiectasias, ulcerations, shiny white areas, and gray-blue globules. Skin cancers often produce their own vascular supply and also ulcerate. The shiny white areas (which are the result of collagen deposition and occur in many skin cancers) are best seen with polarized dermoscopy.
The FP recommended a shave biopsy and performed one immediately after obtaining patient consent. (See the Watch & Learn video on “Shave biopsy.”) Knowing that the BCC would be vascular, the FP injected 1% lidocaine with epinephrine and waited 15 minutes for the epinephrine to work.
After seeing another patient, he performed the shave biopsy with a Dermablade, and used a cotton-tipped applicator to vigorously apply the aluminum chloride to the site. He used a twisting motion and pressure to stop most of the bleeding and then used his electrosurgical instrument—with a sharp tipped electrode—to stop recalcitrant bleeders.
The patient was given a diagnosis of BCC on the follow-up visit. The FP referred the patient for Mohs surgery because of the large size and location of the tumor.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Basal cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:989-998.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Figure 1

The FP suspected that this was a nodular basal cell carcinoma (BCC) with pigmentation. The physical exam was suspicious because of the pearly appearance, superficial ulcerations, and presence of telangiectasias with a loss of the normal pore pattern. Dermoscopy gave further evidence for a nodular BCC by revealing arborizing “tree-like” telangiectasias, ulcerations, shiny white areas, and gray-blue globules. Skin cancers often produce their own vascular supply and also ulcerate. The shiny white areas (which are the result of collagen deposition and occur in many skin cancers) are best seen with polarized dermoscopy.
The FP recommended a shave biopsy and performed one immediately after obtaining patient consent. (See the Watch & Learn video on “Shave biopsy.”) Knowing that the BCC would be vascular, the FP injected 1% lidocaine with epinephrine and waited 15 minutes for the epinephrine to work.
After seeing another patient, he performed the shave biopsy with a Dermablade, and used a cotton-tipped applicator to vigorously apply the aluminum chloride to the site. He used a twisting motion and pressure to stop most of the bleeding and then used his electrosurgical instrument—with a sharp tipped electrode—to stop recalcitrant bleeders.
The patient was given a diagnosis of BCC on the follow-up visit. The FP referred the patient for Mohs surgery because of the large size and location of the tumor.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Basal cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:989-998.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Figure 1

The FP suspected that this was a nodular basal cell carcinoma (BCC) with pigmentation. The physical exam was suspicious because of the pearly appearance, superficial ulcerations, and presence of telangiectasias with a loss of the normal pore pattern. Dermoscopy gave further evidence for a nodular BCC by revealing arborizing “tree-like” telangiectasias, ulcerations, shiny white areas, and gray-blue globules. Skin cancers often produce their own vascular supply and also ulcerate. The shiny white areas (which are the result of collagen deposition and occur in many skin cancers) are best seen with polarized dermoscopy.
The FP recommended a shave biopsy and performed one immediately after obtaining patient consent. (See the Watch & Learn video on “Shave biopsy.”) Knowing that the BCC would be vascular, the FP injected 1% lidocaine with epinephrine and waited 15 minutes for the epinephrine to work.
After seeing another patient, he performed the shave biopsy with a Dermablade, and used a cotton-tipped applicator to vigorously apply the aluminum chloride to the site. He used a twisting motion and pressure to stop most of the bleeding and then used his electrosurgical instrument—with a sharp tipped electrode—to stop recalcitrant bleeders.
The patient was given a diagnosis of BCC on the follow-up visit. The FP referred the patient for Mohs surgery because of the large size and location of the tumor.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Basal cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:989-998.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Pediatric Primary Cutaneous Blastomycosis Clinically Responsive to Itraconazole
Blastomycosis is a polymorphic disease caused by the thermally dimorphic fungus Blastomyces dermatitidis, which is naturally occurring worldwide but particularly prominent in the Great Lakes, Mississippi, and Ohio River areas of the United States. The disease was first described by Thomas Caspar Gilchrist in 1894 and historically has been referred to as Gilchrist disease, North American blastomycosis, or Chicago disease.1,2 Cutaneous blastomycosis can occur by dissemination of yeast to the skin from systemic and pulmonary disease or rarely via direct inoculation of the skin resulting in primary cutaneous disease. Clinically, the lesions are polymorphic and may appear as well-demarcated verrucous plaques containing foci of pustules or ulcerations. Lesions typically heal centrifugally with a cribriform scar.3
We describe an adolescent with a unique history of inoculation 2 weeks prior to the development of a biopsy-confirmed lesion of cutaneous blastomycosis on the left chest wall that clinically resolved following 6 months of itraconazole.
Case Report
A 16-year-old adolescent boy with a history of morbid obesity, asthma, and seasonal allergies presented for evaluation of a painful, slowly enlarging skin lesion on the left chest wall of 2 months’ duration. According to the patient, a “small pimple” appeared at the site of impact 2 weeks following a fall into a muddy flowerbed in Madison, Wisconsin. The patient recalled that although he had soiled his clothing, there was no identifiable puncture of the skin. Despite daily application of hydrogen peroxide and a 1-week course of trimethoprim-sulfamethoxazole, the lesion gradually enlarged. Complete review of systems as well as exposure and travel history were otherwise negative.
Physical examination revealed a 5.0×2.5-cm exophytic, firm, well-circumscribed plaque with a papillated crusted surface on the left side of the chest near the posterior axillary line (Figure 1). There was no palpable regional lymphadenopathy. Pulmonary examination was unremarkable. Diagnostic workup, including complete blood cell count with differential, hemoglobin A1c, human immunodeficiency virus antibody/antigen testing, interferon-gamma release assay, and chest radiograph were all within normal limits.
Histologic examination of a biopsy specimen showed pseudoepitheliomatous hyperplasia of the epidermis with a brisk mixed inflammatory infiltrate (Figure 2). Displayed in Figure 3 is the Grocott-Gomori methenamine-silver stain that highlighted the thick double-contoured wall-budding yeasts.
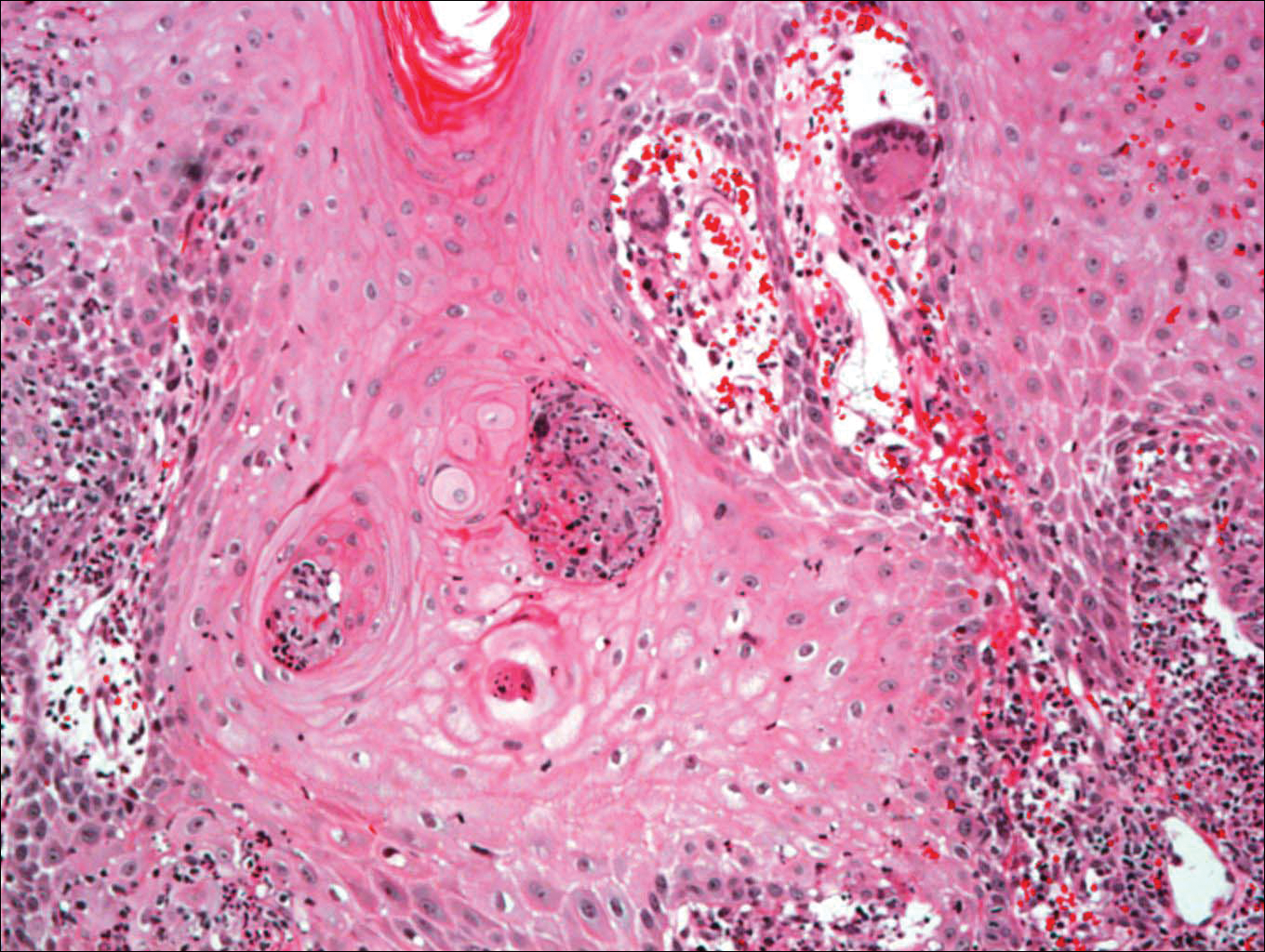
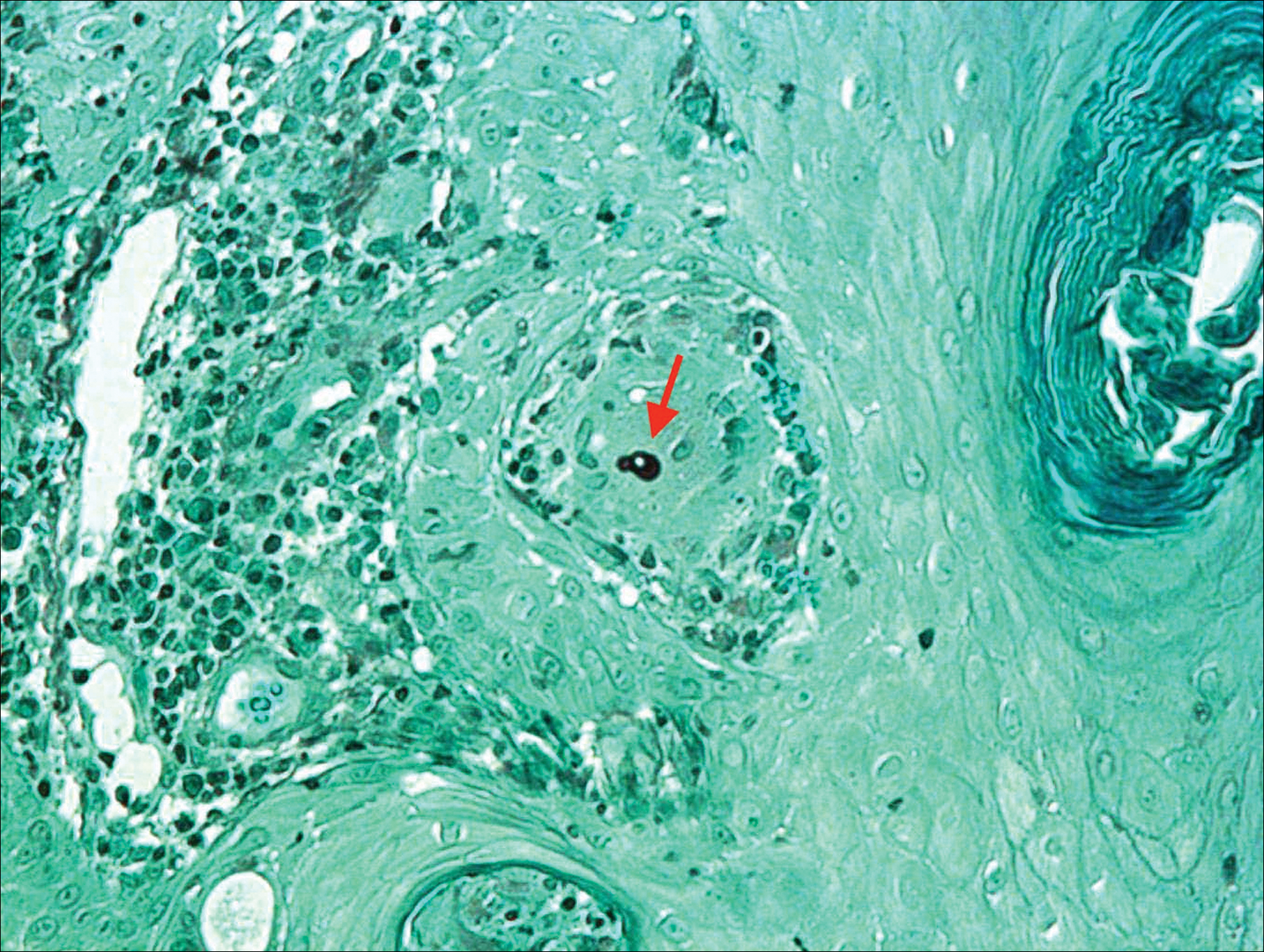
The patient was diagnosed with primary cutaneous blastomycosis. Treatment was initiated with itraconazole 200 mg 3 times daily for 3 days, followed by 200 mg 2 times daily for 6 months. Following 3 months of therapy, the lesion had markedly improved with violaceous dyschromia and no residual surface changes. After 5 months of itraconazole, the patient stopped taking the medication for 2 months due to pharmacy issues and then resumed. After 6 total months of therapy, the lesion healed with only residual dyschromia and itraconazole was discontinued.
Comment
Epidemiology
Blastomycosis is a polymorphic pyogranulomatous disease caused by the dimorphic fungus B dermatitidis, naturally occurring in the soil with a worldwide distribution.4 Individuals affected by the disease often reside in locations where the fungus is endemic, specifically in areas that border the Mississippi and Ohio rivers, the Great Lakes, and Canadian provinces near the Saint Lawrence Seaway. More recently there has been an increased incidence of blastomycosis, with the highest proportion found in Wisconsin and Michigan.1,2 Exposures often are associated with recreational and occupational activities near streams or rivers where there may be decaying vegetation.1 Despite the ubiquitous presence of B dermatitidis in regions where the species is endemic, it is likely that many individuals who are exposed to the organism do not develop infection.
Pathogenesis
The exact pathogenesis for the development of disease in a particular individual remains unclear. Immunosuppression is not a prerequisite for susceptibility, as evidenced by a review of 123 cases of blastomycosis in which a preceding immunodepressive disorder was present in only 25% of patients. The same study found that it was almost equally common as diabetes mellitus and present in 22% of patients.5 The organism is considered a true pathogen given its ability to affect healthy individuals and the presence of a newly identified novel 120-kD glycoprotein antigen (WI-1) on the cell wall that may confer virulence via extracellular matrix and macrophage binding. Intact cell-mediated immunity that prevents the conversion of conidia (the infectious agent) to yeast (the form that exists at body temperature) plays a key role in conferring natural resistance.6,7
Cutaneous infection may occur by either dissemination of yeast to the skin from systemic disease or less commonly via direct inoculation of the skin, resulting in primary cutaneous disease. With respect to systemic disease, infection occurs through inhalation of conidia from moist soil containing organic debris, with an incubation period of 4 to 6 weeks. In the lungs, in a process largely dependent on host cell-mediated immunity, the mold quickly converts to yeast and may then either multiply or be phagocytized.2,6,7 Transmission does not occur from person to person.7 Asymptomatic infection may occur in at least 50% of patients, often leading to a delay in diagnosis. Symptomatic pulmonary disease may range from mild flulike symptoms to overt pneumonia, clinically indistinguishable from community-acquired bacterial pneumonia, tuberculosis, other fungal infections, and cancer. Of patients with primary pulmonary disease, 25% to 80% have been reported to develop secondary organ involvement via lymphohematogenous spread most commonly to the skin, followed respectively by the skeletal, genitourinary, and central nervous systems. Currently, there are 54 documented cases of secondary disseminated cutaneous blastomycosis in children reported in the literature.3,8-14
Presentation
Primary cutaneous disease resulting from direct cutaneous inoculation is rare, especially among children.14 Of 28 cases of isolated cutaneous blastomycosis reported in the literature, 12 (42%) were pediatric.3,8-21 Inoculation blastomycosis typically presents as a papule that expands to a well-demarcated verrucous plaque, often up to several centimeters in diameter, and is located on the skin at the site of contact. The lesion may exhibit a myriad of features ranging from pustules or nodules to focal ulcerations, either present centrally or within raised borders that ultimately may communicate via sinus tracking.7 Lesions that are purely pustular in morphology also have been reported. Healing typically begins centrally and expands centrifugally, often with cribriform scarring.2,4,22 Histologic features of primary and secondary blastomycosis include pseudoepitheliomatous hyperplasia, intraepidermal microabscesses, and dermal suppurative granulomatous inflammation.4 Classically, broad-based budding yeast are identified with a doubly refractile cell wall that is best visualized on periodic acid–Schiff staining.2
Diagnosis
In approximately 50% of patients with cutaneous blastomycosis resulting from secondary spread, there may be an absence of clinically active pulmonary disease, posing a diagnostic dilemma when differentiating from primary cutaneous disease.1,2,4 Furthermore, the skin findings exhibited in primary and secondary cutaneous blastomycosis cannot be distinguished by clinical inspection.19 To fulfill the criteria for diagnosis of primary cutaneous blastomycosis, there must be an identifiable source of infection from the environment, a lesion at the site of contact, a proven absence of systemic infection, and visualization and/or isolation of fungus from the lesion.4,12 The incubation period of lesions is shorter in primary cutaneous disease (2 weeks) and may aid in its differentiation from secondary disease, which typically is longer with lesions presenting 4 to 6 weeks following initial exposure.4
Treatment
Under the current 2015 guidelines from the American Academy of Pediatrics Committee on Infectious Diseases, 6 to 12 months of itraconazole is the treatment recommendation for mild to moderate pulmonary systemic disease without central nervous system involvement.7 Central nervous system disease and moderate to severe pulmonary and systemic disease are treated with intravenous amphotericin B followed by 12 months of oral itraconazole.1,7 Primary cutaneous disease, unlike secondary disease, may self-resolve; however, primary cutaneous disease usually is treated with 6 months of itraconazole, though successful therapy with surgical excision, radiation therapy, and incision and drainage have been reported.19
Unlike secondary cutaneous blastomycosis, primary inoculation disease may be self-limited; however, as treatment with antifungal therapy has become the standard of care, the disease’s propensity to self-resolve has not been well studied.4 Oral itraconazole for 6 to 12 months is the treatment of choice for mild to moderate cutaneous disease.1,22 Effective treatment duration may be difficult to definitively assess because of the self-limited nature of the disease. Our patient showed marked improvement after 3 months and resolution of the skin lesion following 6 months of itraconazole therapy. Our findings support the previously documented observation that systemic therapy might potentially be needed only for the time required to eliminate the clinical evidence of cutaneous disease.19 Our patient received the full 6 months of treatment according to current guidelines. Among a review of 22 cases of primary inoculation blastomycosis, the 5 patients who were treated with an azole agent alone showed disease clearance with an average treatment course of 3.2 months, ranging from 1 to 6 months.19 Further studies that assess the time to clearance with antifungal therapy and subsequent recurrence rates may be warranted.
Conclusion
Pediatric primary cutaneous blastomycosis is a rare cutaneous disease. Identifying sources of probable inoculation from the environment for this patient was unique in that the patient fell into a muddy puddle within a flowerbed. Given the patient’s atopic history, a predominance of humoral over cell-mediated immunity may have placed him at risk. He responded well to 6 months of oral itraconazole and there was no ulceration or scar formation. An increased awareness of this infection, particularly in geographic areas where its reported incidence is on the rise, could be helpful in reducing delays in diagnosis and treatment.
Acknowledgments
We thank Wenhua Liu, MD (Libertyville, Illinois), for reviewing the pathology and Pravin Muniyappa, MD (Chicago, Illinois), for referring the case.
- Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:1801-1812.
- Smith JA, Riddell Jt, Kauffman CA. Cutaneous manifestations of endemic mycoses. Curr Infect Dis Rep. 2013;15:440-449.
- Fisher KR, Baselski V, Beard G, et al. Pustular blastomycosis. J Am Acad Dermatol. 2009;6:355-358.
- Mason AR, Cortes GY, Cook J, et al. Cutaneous blastomycosis: a diagnostic challenge. Int J Dermatol. 2008;47:824-830.
- Lemos LB, Baliga M, Guo M. Blastomycosis: the great pretender can also be an opportunist. initial clinical diagnosis and underlying diseases in 123 patients. Ann Diagn Pathol. 2002;6:194-203.
- Bradsher RW, Chapman SW, Pappas PG. Blastomycosis. Infect Dis Clin North Am. 2003;17:21-40, vii.
- Blastomycosis. In: Kimberlin DW, ed. Red Book: 2015 Report of the Committee on Infectious Diseases. 30th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2015:263-264.
- Brick KE, Drolet BA, Lyon VB, et al. Cutaneous and disseminated blastomycosis: a pediatric case series. Pediatr Dermatol. 2013;30:23-28.
- Fanella S, Skinner S, Trepman E, et al. Blastomycosis in children and adolescents: a 30-year experience from Manitoba. Med Mycol. 2011;49:627-632.
- Frost HM, Anderson J, Ivacic L, et al. Blastomycosis in children: an analysis of clinical, epidemiologic, and genetic features. J Pediatr Infect Dis Soc. 2017;6:49-56.
- Shukla S, Singh S, Jain M, et al. Paediatric cutaneous blastomycosis: a rare case diagnosed on FNAC. Diagn Cytopathol. 2009;37:119-121.
- Smith RJ, Boos MD, Burnham JM, et al. Atypical cutaneous blastomycosis in a child with juvenile idiopathic arthritis on infliximab. Pediatrics. 2015;136:E1386-E1389.
- Wilson JW, Cawley EP, Weidman FD, et al. Primary cutaneous North American blastomycosis. AMA Arch Derm. 1955;71:39-45.
- Zampogna JC, Hoy MJ, Ramos-Caro FA. Primary cutaneous north american blastomycosis in an immunosuppressed child. Pediatr Dermatol. 2003;20:128-130.
- Balasaraswathy P, Theerthanath. Cutaneous blastomycosis presenting as non-healing ulcer and responding to oral ketoconazole. Dermatol Online J. 2003;9:19.
- Bonifaz A, Morales D, Morales N, et al. Cutaneous blastomycosis. an imported case with good response to itraconazole. Rev Iberoam Micol. 2016;33:51-54.
- Clinton TS, Timko AL. Cutaneous blastomycosis without evidence of pulmonary involvement. Mil Med. 2003;168:651-653.
- Dhamija A, D’Souza P, Salgia P, et al. Blastomycosis presenting as solitary nodule: a rare presentation. Indian J Dermatol. 2012;57:133-135.
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Motswaledi HM, Monyemangene FM, Maloba BR, et al. Blastomycosis: a case report and review of the literature. Int J Dermatol. 2012;51:1090-1093.
- Rodríguez-Mena A, Mayorga J, Solís-Ledesma G, et al. Blastomycosis: report of an imported case in Mexico, with only cutaneous lesions [in Spanish]. Rev Iberoam Micol. 2010;27:210-212.
- Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367-381.
Blastomycosis is a polymorphic disease caused by the thermally dimorphic fungus Blastomyces dermatitidis, which is naturally occurring worldwide but particularly prominent in the Great Lakes, Mississippi, and Ohio River areas of the United States. The disease was first described by Thomas Caspar Gilchrist in 1894 and historically has been referred to as Gilchrist disease, North American blastomycosis, or Chicago disease.1,2 Cutaneous blastomycosis can occur by dissemination of yeast to the skin from systemic and pulmonary disease or rarely via direct inoculation of the skin resulting in primary cutaneous disease. Clinically, the lesions are polymorphic and may appear as well-demarcated verrucous plaques containing foci of pustules or ulcerations. Lesions typically heal centrifugally with a cribriform scar.3
We describe an adolescent with a unique history of inoculation 2 weeks prior to the development of a biopsy-confirmed lesion of cutaneous blastomycosis on the left chest wall that clinically resolved following 6 months of itraconazole.
Case Report
A 16-year-old adolescent boy with a history of morbid obesity, asthma, and seasonal allergies presented for evaluation of a painful, slowly enlarging skin lesion on the left chest wall of 2 months’ duration. According to the patient, a “small pimple” appeared at the site of impact 2 weeks following a fall into a muddy flowerbed in Madison, Wisconsin. The patient recalled that although he had soiled his clothing, there was no identifiable puncture of the skin. Despite daily application of hydrogen peroxide and a 1-week course of trimethoprim-sulfamethoxazole, the lesion gradually enlarged. Complete review of systems as well as exposure and travel history were otherwise negative.
Physical examination revealed a 5.0×2.5-cm exophytic, firm, well-circumscribed plaque with a papillated crusted surface on the left side of the chest near the posterior axillary line (Figure 1). There was no palpable regional lymphadenopathy. Pulmonary examination was unremarkable. Diagnostic workup, including complete blood cell count with differential, hemoglobin A1c, human immunodeficiency virus antibody/antigen testing, interferon-gamma release assay, and chest radiograph were all within normal limits.

Histologic examination of a biopsy specimen showed pseudoepitheliomatous hyperplasia of the epidermis with a brisk mixed inflammatory infiltrate (Figure 2). Displayed in Figure 3 is the Grocott-Gomori methenamine-silver stain that highlighted the thick double-contoured wall-budding yeasts.


The patient was diagnosed with primary cutaneous blastomycosis. Treatment was initiated with itraconazole 200 mg 3 times daily for 3 days, followed by 200 mg 2 times daily for 6 months. Following 3 months of therapy, the lesion had markedly improved with violaceous dyschromia and no residual surface changes. After 5 months of itraconazole, the patient stopped taking the medication for 2 months due to pharmacy issues and then resumed. After 6 total months of therapy, the lesion healed with only residual dyschromia and itraconazole was discontinued.
Comment
Epidemiology
Blastomycosis is a polymorphic pyogranulomatous disease caused by the dimorphic fungus B dermatitidis, naturally occurring in the soil with a worldwide distribution.4 Individuals affected by the disease often reside in locations where the fungus is endemic, specifically in areas that border the Mississippi and Ohio rivers, the Great Lakes, and Canadian provinces near the Saint Lawrence Seaway. More recently there has been an increased incidence of blastomycosis, with the highest proportion found in Wisconsin and Michigan.1,2 Exposures often are associated with recreational and occupational activities near streams or rivers where there may be decaying vegetation.1 Despite the ubiquitous presence of B dermatitidis in regions where the species is endemic, it is likely that many individuals who are exposed to the organism do not develop infection.
Pathogenesis
The exact pathogenesis for the development of disease in a particular individual remains unclear. Immunosuppression is not a prerequisite for susceptibility, as evidenced by a review of 123 cases of blastomycosis in which a preceding immunodepressive disorder was present in only 25% of patients. The same study found that it was almost equally common as diabetes mellitus and present in 22% of patients.5 The organism is considered a true pathogen given its ability to affect healthy individuals and the presence of a newly identified novel 120-kD glycoprotein antigen (WI-1) on the cell wall that may confer virulence via extracellular matrix and macrophage binding. Intact cell-mediated immunity that prevents the conversion of conidia (the infectious agent) to yeast (the form that exists at body temperature) plays a key role in conferring natural resistance.6,7
Cutaneous infection may occur by either dissemination of yeast to the skin from systemic disease or less commonly via direct inoculation of the skin, resulting in primary cutaneous disease. With respect to systemic disease, infection occurs through inhalation of conidia from moist soil containing organic debris, with an incubation period of 4 to 6 weeks. In the lungs, in a process largely dependent on host cell-mediated immunity, the mold quickly converts to yeast and may then either multiply or be phagocytized.2,6,7 Transmission does not occur from person to person.7 Asymptomatic infection may occur in at least 50% of patients, often leading to a delay in diagnosis. Symptomatic pulmonary disease may range from mild flulike symptoms to overt pneumonia, clinically indistinguishable from community-acquired bacterial pneumonia, tuberculosis, other fungal infections, and cancer. Of patients with primary pulmonary disease, 25% to 80% have been reported to develop secondary organ involvement via lymphohematogenous spread most commonly to the skin, followed respectively by the skeletal, genitourinary, and central nervous systems. Currently, there are 54 documented cases of secondary disseminated cutaneous blastomycosis in children reported in the literature.3,8-14
Presentation
Primary cutaneous disease resulting from direct cutaneous inoculation is rare, especially among children.14 Of 28 cases of isolated cutaneous blastomycosis reported in the literature, 12 (42%) were pediatric.3,8-21 Inoculation blastomycosis typically presents as a papule that expands to a well-demarcated verrucous plaque, often up to several centimeters in diameter, and is located on the skin at the site of contact. The lesion may exhibit a myriad of features ranging from pustules or nodules to focal ulcerations, either present centrally or within raised borders that ultimately may communicate via sinus tracking.7 Lesions that are purely pustular in morphology also have been reported. Healing typically begins centrally and expands centrifugally, often with cribriform scarring.2,4,22 Histologic features of primary and secondary blastomycosis include pseudoepitheliomatous hyperplasia, intraepidermal microabscesses, and dermal suppurative granulomatous inflammation.4 Classically, broad-based budding yeast are identified with a doubly refractile cell wall that is best visualized on periodic acid–Schiff staining.2
Diagnosis
In approximately 50% of patients with cutaneous blastomycosis resulting from secondary spread, there may be an absence of clinically active pulmonary disease, posing a diagnostic dilemma when differentiating from primary cutaneous disease.1,2,4 Furthermore, the skin findings exhibited in primary and secondary cutaneous blastomycosis cannot be distinguished by clinical inspection.19 To fulfill the criteria for diagnosis of primary cutaneous blastomycosis, there must be an identifiable source of infection from the environment, a lesion at the site of contact, a proven absence of systemic infection, and visualization and/or isolation of fungus from the lesion.4,12 The incubation period of lesions is shorter in primary cutaneous disease (2 weeks) and may aid in its differentiation from secondary disease, which typically is longer with lesions presenting 4 to 6 weeks following initial exposure.4
Treatment
Under the current 2015 guidelines from the American Academy of Pediatrics Committee on Infectious Diseases, 6 to 12 months of itraconazole is the treatment recommendation for mild to moderate pulmonary systemic disease without central nervous system involvement.7 Central nervous system disease and moderate to severe pulmonary and systemic disease are treated with intravenous amphotericin B followed by 12 months of oral itraconazole.1,7 Primary cutaneous disease, unlike secondary disease, may self-resolve; however, primary cutaneous disease usually is treated with 6 months of itraconazole, though successful therapy with surgical excision, radiation therapy, and incision and drainage have been reported.19
Unlike secondary cutaneous blastomycosis, primary inoculation disease may be self-limited; however, as treatment with antifungal therapy has become the standard of care, the disease’s propensity to self-resolve has not been well studied.4 Oral itraconazole for 6 to 12 months is the treatment of choice for mild to moderate cutaneous disease.1,22 Effective treatment duration may be difficult to definitively assess because of the self-limited nature of the disease. Our patient showed marked improvement after 3 months and resolution of the skin lesion following 6 months of itraconazole therapy. Our findings support the previously documented observation that systemic therapy might potentially be needed only for the time required to eliminate the clinical evidence of cutaneous disease.19 Our patient received the full 6 months of treatment according to current guidelines. Among a review of 22 cases of primary inoculation blastomycosis, the 5 patients who were treated with an azole agent alone showed disease clearance with an average treatment course of 3.2 months, ranging from 1 to 6 months.19 Further studies that assess the time to clearance with antifungal therapy and subsequent recurrence rates may be warranted.
Conclusion
Pediatric primary cutaneous blastomycosis is a rare cutaneous disease. Identifying sources of probable inoculation from the environment for this patient was unique in that the patient fell into a muddy puddle within a flowerbed. Given the patient’s atopic history, a predominance of humoral over cell-mediated immunity may have placed him at risk. He responded well to 6 months of oral itraconazole and there was no ulceration or scar formation. An increased awareness of this infection, particularly in geographic areas where its reported incidence is on the rise, could be helpful in reducing delays in diagnosis and treatment.
Acknowledgments
We thank Wenhua Liu, MD (Libertyville, Illinois), for reviewing the pathology and Pravin Muniyappa, MD (Chicago, Illinois), for referring the case.
Blastomycosis is a polymorphic disease caused by the thermally dimorphic fungus Blastomyces dermatitidis, which is naturally occurring worldwide but particularly prominent in the Great Lakes, Mississippi, and Ohio River areas of the United States. The disease was first described by Thomas Caspar Gilchrist in 1894 and historically has been referred to as Gilchrist disease, North American blastomycosis, or Chicago disease.1,2 Cutaneous blastomycosis can occur by dissemination of yeast to the skin from systemic and pulmonary disease or rarely via direct inoculation of the skin resulting in primary cutaneous disease. Clinically, the lesions are polymorphic and may appear as well-demarcated verrucous plaques containing foci of pustules or ulcerations. Lesions typically heal centrifugally with a cribriform scar.3
We describe an adolescent with a unique history of inoculation 2 weeks prior to the development of a biopsy-confirmed lesion of cutaneous blastomycosis on the left chest wall that clinically resolved following 6 months of itraconazole.
Case Report
A 16-year-old adolescent boy with a history of morbid obesity, asthma, and seasonal allergies presented for evaluation of a painful, slowly enlarging skin lesion on the left chest wall of 2 months’ duration. According to the patient, a “small pimple” appeared at the site of impact 2 weeks following a fall into a muddy flowerbed in Madison, Wisconsin. The patient recalled that although he had soiled his clothing, there was no identifiable puncture of the skin. Despite daily application of hydrogen peroxide and a 1-week course of trimethoprim-sulfamethoxazole, the lesion gradually enlarged. Complete review of systems as well as exposure and travel history were otherwise negative.
Physical examination revealed a 5.0×2.5-cm exophytic, firm, well-circumscribed plaque with a papillated crusted surface on the left side of the chest near the posterior axillary line (Figure 1). There was no palpable regional lymphadenopathy. Pulmonary examination was unremarkable. Diagnostic workup, including complete blood cell count with differential, hemoglobin A1c, human immunodeficiency virus antibody/antigen testing, interferon-gamma release assay, and chest radiograph were all within normal limits.

Histologic examination of a biopsy specimen showed pseudoepitheliomatous hyperplasia of the epidermis with a brisk mixed inflammatory infiltrate (Figure 2). Displayed in Figure 3 is the Grocott-Gomori methenamine-silver stain that highlighted the thick double-contoured wall-budding yeasts.


The patient was diagnosed with primary cutaneous blastomycosis. Treatment was initiated with itraconazole 200 mg 3 times daily for 3 days, followed by 200 mg 2 times daily for 6 months. Following 3 months of therapy, the lesion had markedly improved with violaceous dyschromia and no residual surface changes. After 5 months of itraconazole, the patient stopped taking the medication for 2 months due to pharmacy issues and then resumed. After 6 total months of therapy, the lesion healed with only residual dyschromia and itraconazole was discontinued.
Comment
Epidemiology
Blastomycosis is a polymorphic pyogranulomatous disease caused by the dimorphic fungus B dermatitidis, naturally occurring in the soil with a worldwide distribution.4 Individuals affected by the disease often reside in locations where the fungus is endemic, specifically in areas that border the Mississippi and Ohio rivers, the Great Lakes, and Canadian provinces near the Saint Lawrence Seaway. More recently there has been an increased incidence of blastomycosis, with the highest proportion found in Wisconsin and Michigan.1,2 Exposures often are associated with recreational and occupational activities near streams or rivers where there may be decaying vegetation.1 Despite the ubiquitous presence of B dermatitidis in regions where the species is endemic, it is likely that many individuals who are exposed to the organism do not develop infection.
Pathogenesis
The exact pathogenesis for the development of disease in a particular individual remains unclear. Immunosuppression is not a prerequisite for susceptibility, as evidenced by a review of 123 cases of blastomycosis in which a preceding immunodepressive disorder was present in only 25% of patients. The same study found that it was almost equally common as diabetes mellitus and present in 22% of patients.5 The organism is considered a true pathogen given its ability to affect healthy individuals and the presence of a newly identified novel 120-kD glycoprotein antigen (WI-1) on the cell wall that may confer virulence via extracellular matrix and macrophage binding. Intact cell-mediated immunity that prevents the conversion of conidia (the infectious agent) to yeast (the form that exists at body temperature) plays a key role in conferring natural resistance.6,7
Cutaneous infection may occur by either dissemination of yeast to the skin from systemic disease or less commonly via direct inoculation of the skin, resulting in primary cutaneous disease. With respect to systemic disease, infection occurs through inhalation of conidia from moist soil containing organic debris, with an incubation period of 4 to 6 weeks. In the lungs, in a process largely dependent on host cell-mediated immunity, the mold quickly converts to yeast and may then either multiply or be phagocytized.2,6,7 Transmission does not occur from person to person.7 Asymptomatic infection may occur in at least 50% of patients, often leading to a delay in diagnosis. Symptomatic pulmonary disease may range from mild flulike symptoms to overt pneumonia, clinically indistinguishable from community-acquired bacterial pneumonia, tuberculosis, other fungal infections, and cancer. Of patients with primary pulmonary disease, 25% to 80% have been reported to develop secondary organ involvement via lymphohematogenous spread most commonly to the skin, followed respectively by the skeletal, genitourinary, and central nervous systems. Currently, there are 54 documented cases of secondary disseminated cutaneous blastomycosis in children reported in the literature.3,8-14
Presentation
Primary cutaneous disease resulting from direct cutaneous inoculation is rare, especially among children.14 Of 28 cases of isolated cutaneous blastomycosis reported in the literature, 12 (42%) were pediatric.3,8-21 Inoculation blastomycosis typically presents as a papule that expands to a well-demarcated verrucous plaque, often up to several centimeters in diameter, and is located on the skin at the site of contact. The lesion may exhibit a myriad of features ranging from pustules or nodules to focal ulcerations, either present centrally or within raised borders that ultimately may communicate via sinus tracking.7 Lesions that are purely pustular in morphology also have been reported. Healing typically begins centrally and expands centrifugally, often with cribriform scarring.2,4,22 Histologic features of primary and secondary blastomycosis include pseudoepitheliomatous hyperplasia, intraepidermal microabscesses, and dermal suppurative granulomatous inflammation.4 Classically, broad-based budding yeast are identified with a doubly refractile cell wall that is best visualized on periodic acid–Schiff staining.2
Diagnosis
In approximately 50% of patients with cutaneous blastomycosis resulting from secondary spread, there may be an absence of clinically active pulmonary disease, posing a diagnostic dilemma when differentiating from primary cutaneous disease.1,2,4 Furthermore, the skin findings exhibited in primary and secondary cutaneous blastomycosis cannot be distinguished by clinical inspection.19 To fulfill the criteria for diagnosis of primary cutaneous blastomycosis, there must be an identifiable source of infection from the environment, a lesion at the site of contact, a proven absence of systemic infection, and visualization and/or isolation of fungus from the lesion.4,12 The incubation period of lesions is shorter in primary cutaneous disease (2 weeks) and may aid in its differentiation from secondary disease, which typically is longer with lesions presenting 4 to 6 weeks following initial exposure.4
Treatment
Under the current 2015 guidelines from the American Academy of Pediatrics Committee on Infectious Diseases, 6 to 12 months of itraconazole is the treatment recommendation for mild to moderate pulmonary systemic disease without central nervous system involvement.7 Central nervous system disease and moderate to severe pulmonary and systemic disease are treated with intravenous amphotericin B followed by 12 months of oral itraconazole.1,7 Primary cutaneous disease, unlike secondary disease, may self-resolve; however, primary cutaneous disease usually is treated with 6 months of itraconazole, though successful therapy with surgical excision, radiation therapy, and incision and drainage have been reported.19
Unlike secondary cutaneous blastomycosis, primary inoculation disease may be self-limited; however, as treatment with antifungal therapy has become the standard of care, the disease’s propensity to self-resolve has not been well studied.4 Oral itraconazole for 6 to 12 months is the treatment of choice for mild to moderate cutaneous disease.1,22 Effective treatment duration may be difficult to definitively assess because of the self-limited nature of the disease. Our patient showed marked improvement after 3 months and resolution of the skin lesion following 6 months of itraconazole therapy. Our findings support the previously documented observation that systemic therapy might potentially be needed only for the time required to eliminate the clinical evidence of cutaneous disease.19 Our patient received the full 6 months of treatment according to current guidelines. Among a review of 22 cases of primary inoculation blastomycosis, the 5 patients who were treated with an azole agent alone showed disease clearance with an average treatment course of 3.2 months, ranging from 1 to 6 months.19 Further studies that assess the time to clearance with antifungal therapy and subsequent recurrence rates may be warranted.
Conclusion
Pediatric primary cutaneous blastomycosis is a rare cutaneous disease. Identifying sources of probable inoculation from the environment for this patient was unique in that the patient fell into a muddy puddle within a flowerbed. Given the patient’s atopic history, a predominance of humoral over cell-mediated immunity may have placed him at risk. He responded well to 6 months of oral itraconazole and there was no ulceration or scar formation. An increased awareness of this infection, particularly in geographic areas where its reported incidence is on the rise, could be helpful in reducing delays in diagnosis and treatment.
Acknowledgments
We thank Wenhua Liu, MD (Libertyville, Illinois), for reviewing the pathology and Pravin Muniyappa, MD (Chicago, Illinois), for referring the case.
- Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:1801-1812.
- Smith JA, Riddell Jt, Kauffman CA. Cutaneous manifestations of endemic mycoses. Curr Infect Dis Rep. 2013;15:440-449.
- Fisher KR, Baselski V, Beard G, et al. Pustular blastomycosis. J Am Acad Dermatol. 2009;6:355-358.
- Mason AR, Cortes GY, Cook J, et al. Cutaneous blastomycosis: a diagnostic challenge. Int J Dermatol. 2008;47:824-830.
- Lemos LB, Baliga M, Guo M. Blastomycosis: the great pretender can also be an opportunist. initial clinical diagnosis and underlying diseases in 123 patients. Ann Diagn Pathol. 2002;6:194-203.
- Bradsher RW, Chapman SW, Pappas PG. Blastomycosis. Infect Dis Clin North Am. 2003;17:21-40, vii.
- Blastomycosis. In: Kimberlin DW, ed. Red Book: 2015 Report of the Committee on Infectious Diseases. 30th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2015:263-264.
- Brick KE, Drolet BA, Lyon VB, et al. Cutaneous and disseminated blastomycosis: a pediatric case series. Pediatr Dermatol. 2013;30:23-28.
- Fanella S, Skinner S, Trepman E, et al. Blastomycosis in children and adolescents: a 30-year experience from Manitoba. Med Mycol. 2011;49:627-632.
- Frost HM, Anderson J, Ivacic L, et al. Blastomycosis in children: an analysis of clinical, epidemiologic, and genetic features. J Pediatr Infect Dis Soc. 2017;6:49-56.
- Shukla S, Singh S, Jain M, et al. Paediatric cutaneous blastomycosis: a rare case diagnosed on FNAC. Diagn Cytopathol. 2009;37:119-121.
- Smith RJ, Boos MD, Burnham JM, et al. Atypical cutaneous blastomycosis in a child with juvenile idiopathic arthritis on infliximab. Pediatrics. 2015;136:E1386-E1389.
- Wilson JW, Cawley EP, Weidman FD, et al. Primary cutaneous North American blastomycosis. AMA Arch Derm. 1955;71:39-45.
- Zampogna JC, Hoy MJ, Ramos-Caro FA. Primary cutaneous north american blastomycosis in an immunosuppressed child. Pediatr Dermatol. 2003;20:128-130.
- Balasaraswathy P, Theerthanath. Cutaneous blastomycosis presenting as non-healing ulcer and responding to oral ketoconazole. Dermatol Online J. 2003;9:19.
- Bonifaz A, Morales D, Morales N, et al. Cutaneous blastomycosis. an imported case with good response to itraconazole. Rev Iberoam Micol. 2016;33:51-54.
- Clinton TS, Timko AL. Cutaneous blastomycosis without evidence of pulmonary involvement. Mil Med. 2003;168:651-653.
- Dhamija A, D’Souza P, Salgia P, et al. Blastomycosis presenting as solitary nodule: a rare presentation. Indian J Dermatol. 2012;57:133-135.
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Motswaledi HM, Monyemangene FM, Maloba BR, et al. Blastomycosis: a case report and review of the literature. Int J Dermatol. 2012;51:1090-1093.
- Rodríguez-Mena A, Mayorga J, Solís-Ledesma G, et al. Blastomycosis: report of an imported case in Mexico, with only cutaneous lesions [in Spanish]. Rev Iberoam Micol. 2010;27:210-212.
- Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367-381.
- Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:1801-1812.
- Smith JA, Riddell Jt, Kauffman CA. Cutaneous manifestations of endemic mycoses. Curr Infect Dis Rep. 2013;15:440-449.
- Fisher KR, Baselski V, Beard G, et al. Pustular blastomycosis. J Am Acad Dermatol. 2009;6:355-358.
- Mason AR, Cortes GY, Cook J, et al. Cutaneous blastomycosis: a diagnostic challenge. Int J Dermatol. 2008;47:824-830.
- Lemos LB, Baliga M, Guo M. Blastomycosis: the great pretender can also be an opportunist. initial clinical diagnosis and underlying diseases in 123 patients. Ann Diagn Pathol. 2002;6:194-203.
- Bradsher RW, Chapman SW, Pappas PG. Blastomycosis. Infect Dis Clin North Am. 2003;17:21-40, vii.
- Blastomycosis. In: Kimberlin DW, ed. Red Book: 2015 Report of the Committee on Infectious Diseases. 30th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2015:263-264.
- Brick KE, Drolet BA, Lyon VB, et al. Cutaneous and disseminated blastomycosis: a pediatric case series. Pediatr Dermatol. 2013;30:23-28.
- Fanella S, Skinner S, Trepman E, et al. Blastomycosis in children and adolescents: a 30-year experience from Manitoba. Med Mycol. 2011;49:627-632.
- Frost HM, Anderson J, Ivacic L, et al. Blastomycosis in children: an analysis of clinical, epidemiologic, and genetic features. J Pediatr Infect Dis Soc. 2017;6:49-56.
- Shukla S, Singh S, Jain M, et al. Paediatric cutaneous blastomycosis: a rare case diagnosed on FNAC. Diagn Cytopathol. 2009;37:119-121.
- Smith RJ, Boos MD, Burnham JM, et al. Atypical cutaneous blastomycosis in a child with juvenile idiopathic arthritis on infliximab. Pediatrics. 2015;136:E1386-E1389.
- Wilson JW, Cawley EP, Weidman FD, et al. Primary cutaneous North American blastomycosis. AMA Arch Derm. 1955;71:39-45.
- Zampogna JC, Hoy MJ, Ramos-Caro FA. Primary cutaneous north american blastomycosis in an immunosuppressed child. Pediatr Dermatol. 2003;20:128-130.
- Balasaraswathy P, Theerthanath. Cutaneous blastomycosis presenting as non-healing ulcer and responding to oral ketoconazole. Dermatol Online J. 2003;9:19.
- Bonifaz A, Morales D, Morales N, et al. Cutaneous blastomycosis. an imported case with good response to itraconazole. Rev Iberoam Micol. 2016;33:51-54.
- Clinton TS, Timko AL. Cutaneous blastomycosis without evidence of pulmonary involvement. Mil Med. 2003;168:651-653.
- Dhamija A, D’Souza P, Salgia P, et al. Blastomycosis presenting as solitary nodule: a rare presentation. Indian J Dermatol. 2012;57:133-135.
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Motswaledi HM, Monyemangene FM, Maloba BR, et al. Blastomycosis: a case report and review of the literature. Int J Dermatol. 2012;51:1090-1093.
- Rodríguez-Mena A, Mayorga J, Solís-Ledesma G, et al. Blastomycosis: report of an imported case in Mexico, with only cutaneous lesions [in Spanish]. Rev Iberoam Micol. 2010;27:210-212.
- Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367-381.
Practice Points
- Cutaneous blastomycosis can occur by dissemination of yeast to the skin from systemic and pulmonary disease or rarely via direct inoculation of the skin, resulting in primary cutaneous disease.
- Exposures often are associated with recreational and occupational activities near streams or rivers where there may be decaying vegetation.
- Oral itraconazole for 6 to 12 months is the treatment of choice for mild to moderate cutaneous disease.
- Increased awareness of this rare infection, particularly in geographic areas where its reported incidence is on the rise, could be helpful in reducing delays in diagnosis and treatment.
Acute Hemorrhagic Edema of Infancy: Guide to Prevent Misdiagnosis
Acute hemorrhagic edema of infancy (AHEI) is an uncommon leukocytoclastic vasculitis affecting children aged 6 to 24 months; Henoch-Schönlein purpura (HSP) is the most common misdiagnosis. The 2 entities should be differentiated, as HSP may have renal and gastrointestinal (GI) comorbidities that need serial follow-up, whereas AHEI follows a benign course without systemic sequelae. Patient history and physical examination are the most important factors in differentiating the 2 diseases; histopathologic and direct immunofluorescence (DIF) analyses may lend further diagnostic confidence.
We report the case of a 10-month-old previously healthy boy who presented with acute rash, edema, and low-grade fever in the setting of recent diarrhea. We differentiate between AHEI and HSP to help prevent misdiagnosis by health care providers.
Case Report
A 10-month-old previously healthy boy presented to the emergency department (ED) for evaluation of a rash and swelling of 4 days’ duration. He had nonbloody diarrhea 1 week prior; soon after, he developed bilateral lower leg edema and rash. On evaluation in a different ED, he had a low-grade fever (rectal temperature, 38.0°C) but normal blood work, including complete blood cell count, basic metabolic panel, and coagulation studies. The patient was discharged to outpatient follow-up with his pediatrician who reported normal urinalysis.
Due to progression of the rash, the patient presented to our ED 3 days after his initial ED assessment. Dermatology was consulted. At the time of presentation, he was afebrile but with GI upset and fussiness. His parents denied additional symptoms or blood in urine or stool. Physical examination revealed a nontoxic-appearing infant with scattered palpable, annular, purpuric papules coalescing into plaques on both legs and feet (Figure 1), with sparse petechiae noted on the lower abdomen. The cheeks had scattered purpuric papules and plaques bilaterally, a few with a small central crust (Figure 2), and the right superior helix had a faint purpuric macule. The hands had a few pink edematous coalescing papules.
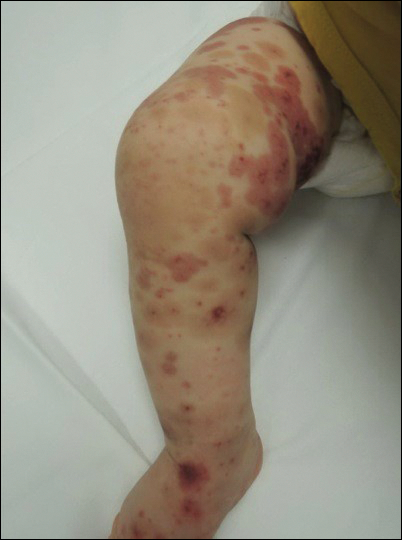
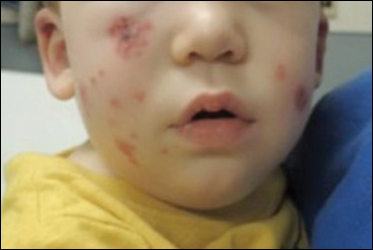
Histopathologic analyses with hematoxylin and eosin staining (Figure 3) and DIF (Figure 4) were performed from within a representative purpuric plaque on the right hip. Direct immunofluorescence was performed to evaluate for an IgA vasculitis versus an alternative type of vasculitis. The hematoxylin and eosin–stained specimen demonstrated a dermal perivascular infiltrate involving superficial and deep vessels with neutrophils, karyorrhexis, and erythrocyte extravasation. The endothelium was intact, with a mild suggestion of fibrinoid change of the blood vessel walls. Direct immunofluorescence revealed granular deposition of IgA, C3, and fibrinogen in multiple dermal blood vessels. Combined, the specimens were interpreted as evolving IgA-associated leukocytoclastic vasculitis.
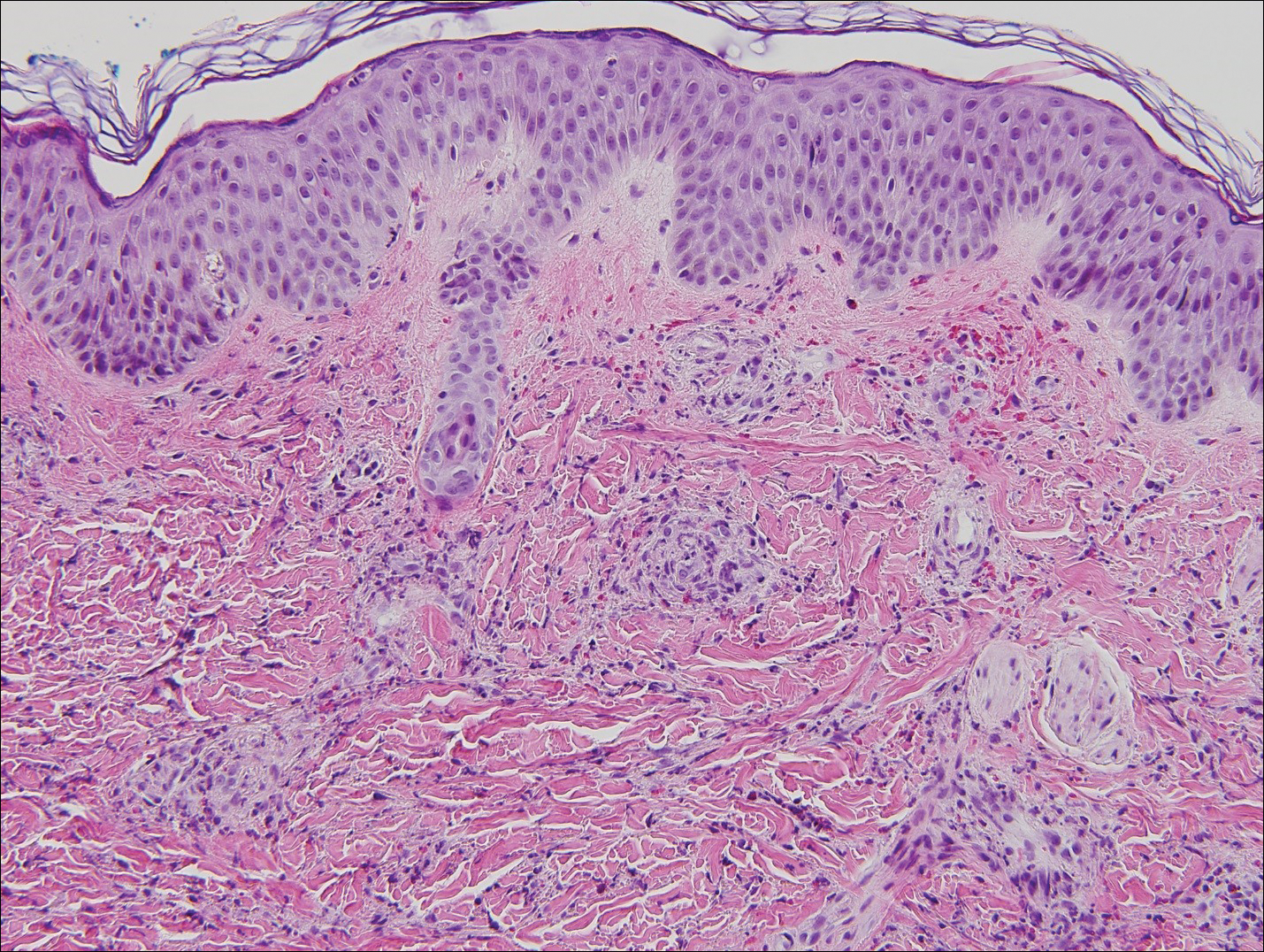
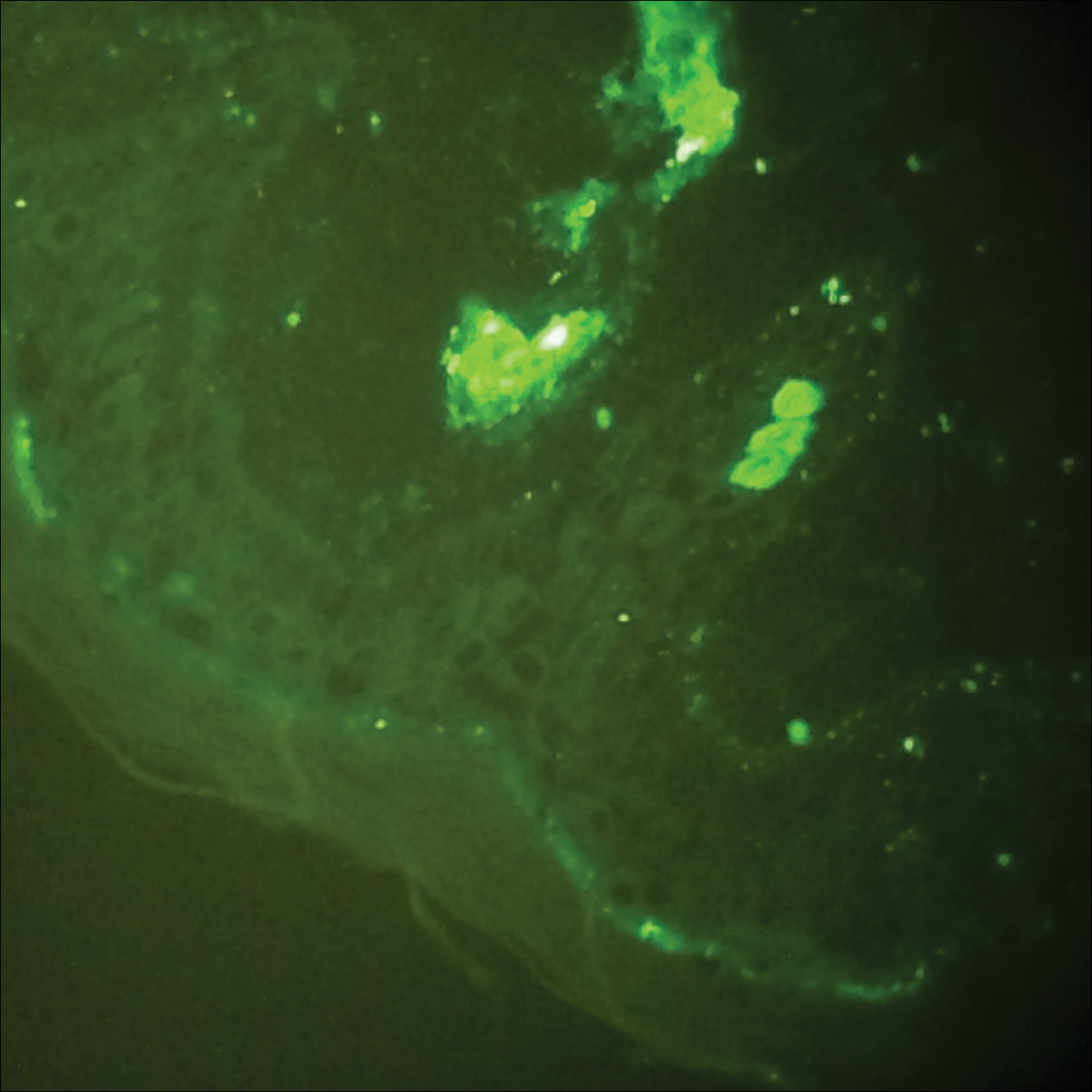
The case was reviewed with our 2 department pediatric dermatologists; a diagnosis of AHEI was made based on the clinical and supportive histopathological presentations. The patient’s parents chose active treatment with a 2-week taper of oral prednisone because of the patient’s discomfort with edema. No GI or adverse renal sequelae, including findings on urinalysis, were reported at 1-month hospital follow-up with dermatology and pediatrics.
Comment
Incidence and Clinical Characteristics
Acute hemorrhagic edema of infancy is an uncommon leukocytoclastic vasculitis first described in the United States by Snow1 in 1913. Other names for the disorder include acute hemorrhagic edema of young children, cockade purpura and edema, Finkelstein disease, and Seidlmayer disease.2 Boys are affected more often than girls, with most children presenting at 6 to 24 months of age. Most affected children experience a prodrome of simple respiratory tract illness (most common), diarrhea (as in our case), or urinary tract infection.2 The exact pathophysiology behind AHEI is unknown, but it is thought to be an immune complex–mediated disease evidenced by the fact that infection, use of medication, or immunization precedes most cases.3,4
Diagnosis
Acute hemorrhagic edema of infancy is diagnosed clinically, with or without the support of skin biopsy. It should be differentiated from HSP because of renal and GI sequelae that HSP portends compared to the benign course of AHEI.2 Notably, some health care providers consider AHEI a benign variant of HSP.2,3
Characteristically, AHEI patients are nontoxic-appearing infants with a low-grade fever who develop relatively large (1–5 cm) targetoid purpuric lesions and indurated nonpitting edema of the extremities.2,5 Purpura in AHEI frequently occurs on the face, ears, and upper and lower extremities, whereas purpura in HSP most commonly presents on the buttocks and extensor legs with sparing of the face. Henoch-Schönlein purpura most often affects children aged 3 to 6 years compared to AHEI’s younger demographic (age <2 years).4,5 Clinically, HSP presents with palpable purpura and 1 or more of the following features: diffuse abdominal pain, arthritis/arthralgia, renal involvement, and skin or renal biopsy showing predominant IgA deposition.2,6
Both AHEI and HSP show leukocytoclastic vasculitis on histopathology.2-4,6,7 Positive perivascular IgA staining on DIF is strongly associated with HSP, but nearly one-quarter of AHEI cases also show this deposition pattern2,4,7; therefore, DIF alone cannot exclude a diagnosis of AHEI.
Differential Diagnosis
Alternative diagnoses to consider with AHEI include drug-induced vasculitis, erythema multiforme, HSP, Kawasaki disease, meningococcemia, nonaccidental skin bruising, Rocky Mountain spotted fever, septic vasculitis, and urticarial vasculitis (Table).2-4,6-8

Treatment
Acute hemorrhagic edema of infancy is self-limited, with only rare reports of extracutaneous involvement. Supportive treatment is indicated because spontaneous recovery without sequelae is expected within 21 days.2,3,6 If edema is symptomatic, as was the case with our patient, corticosteroids may shorten the disease course.3
Conclusion
Our case highlights the need to combine clinical history, physical examination, and histopathologic analysis to differentiate between AHEI and HSP, which is important for 2 reasons: (1) it helps with the decision to undertake active or observational treatment, and (2) it helps the clinician counsel the patient and guardians regarding potential associated renal and GI risks.
- Snow IM. Purpura, urticaria and angioneurotic edema of the hands and feet in a nursing baby. JAMA. 1913;61:18-19.
- Fiore E, Rizzi M, Ragazzi M, et al. Acute hemorrhagic edema of young children (cockade purpura and edema): a case series and systematic review. J Am Acad Dermatol. 2008;59:684-695.
- Freitas P, Bygum A. Visual impairment caused by periorbital edema in an infant with acute hemorrhagic edema of infancy. Pediatr Dermatol. 2013;30:e132-e135.
- Legrain V, Lejean S, Taïeb A, et al. Infantile acute hemorrhagic edema of the skin: study of ten cases. J Am Acad Dermatol. 1991;24:17-22.
- Breda L, Franchini S, Marzetti V, et al. Escherichia coli urinary infection as a cause of acute hemorrhagic edema in infancy. Pediatr Dermatol. 2015;32:e309-e311.
- Ozen S, Ruperto N, Dillon MJ, et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis. 2006;65:936-941.
- Saraclar Y, Tinaztepe K, Adalioğlu G, et al. Acute hemorrhagic edema of infancy (AHEI)—a variant of Henoch-Schönlein purpura or a distinct clinical entity? J Allergy Clin Immunol. 1990;86:473-483.
- Shinkai K, Fox L. Cutaneous vasculitis. In: Bolognia J, Jorizzo J, Schaffer J, eds. Dermatology. 3rd ed. China: Elsevier Limited; 2012:385-410.
Acute hemorrhagic edema of infancy (AHEI) is an uncommon leukocytoclastic vasculitis affecting children aged 6 to 24 months; Henoch-Schönlein purpura (HSP) is the most common misdiagnosis. The 2 entities should be differentiated, as HSP may have renal and gastrointestinal (GI) comorbidities that need serial follow-up, whereas AHEI follows a benign course without systemic sequelae. Patient history and physical examination are the most important factors in differentiating the 2 diseases; histopathologic and direct immunofluorescence (DIF) analyses may lend further diagnostic confidence.
We report the case of a 10-month-old previously healthy boy who presented with acute rash, edema, and low-grade fever in the setting of recent diarrhea. We differentiate between AHEI and HSP to help prevent misdiagnosis by health care providers.
Case Report
A 10-month-old previously healthy boy presented to the emergency department (ED) for evaluation of a rash and swelling of 4 days’ duration. He had nonbloody diarrhea 1 week prior; soon after, he developed bilateral lower leg edema and rash. On evaluation in a different ED, he had a low-grade fever (rectal temperature, 38.0°C) but normal blood work, including complete blood cell count, basic metabolic panel, and coagulation studies. The patient was discharged to outpatient follow-up with his pediatrician who reported normal urinalysis.
Due to progression of the rash, the patient presented to our ED 3 days after his initial ED assessment. Dermatology was consulted. At the time of presentation, he was afebrile but with GI upset and fussiness. His parents denied additional symptoms or blood in urine or stool. Physical examination revealed a nontoxic-appearing infant with scattered palpable, annular, purpuric papules coalescing into plaques on both legs and feet (Figure 1), with sparse petechiae noted on the lower abdomen. The cheeks had scattered purpuric papules and plaques bilaterally, a few with a small central crust (Figure 2), and the right superior helix had a faint purpuric macule. The hands had a few pink edematous coalescing papules.


Histopathologic analyses with hematoxylin and eosin staining (Figure 3) and DIF (Figure 4) were performed from within a representative purpuric plaque on the right hip. Direct immunofluorescence was performed to evaluate for an IgA vasculitis versus an alternative type of vasculitis. The hematoxylin and eosin–stained specimen demonstrated a dermal perivascular infiltrate involving superficial and deep vessels with neutrophils, karyorrhexis, and erythrocyte extravasation. The endothelium was intact, with a mild suggestion of fibrinoid change of the blood vessel walls. Direct immunofluorescence revealed granular deposition of IgA, C3, and fibrinogen in multiple dermal blood vessels. Combined, the specimens were interpreted as evolving IgA-associated leukocytoclastic vasculitis.


The case was reviewed with our 2 department pediatric dermatologists; a diagnosis of AHEI was made based on the clinical and supportive histopathological presentations. The patient’s parents chose active treatment with a 2-week taper of oral prednisone because of the patient’s discomfort with edema. No GI or adverse renal sequelae, including findings on urinalysis, were reported at 1-month hospital follow-up with dermatology and pediatrics.
Comment
Incidence and Clinical Characteristics
Acute hemorrhagic edema of infancy is an uncommon leukocytoclastic vasculitis first described in the United States by Snow1 in 1913. Other names for the disorder include acute hemorrhagic edema of young children, cockade purpura and edema, Finkelstein disease, and Seidlmayer disease.2 Boys are affected more often than girls, with most children presenting at 6 to 24 months of age. Most affected children experience a prodrome of simple respiratory tract illness (most common), diarrhea (as in our case), or urinary tract infection.2 The exact pathophysiology behind AHEI is unknown, but it is thought to be an immune complex–mediated disease evidenced by the fact that infection, use of medication, or immunization precedes most cases.3,4
Diagnosis
Acute hemorrhagic edema of infancy is diagnosed clinically, with or without the support of skin biopsy. It should be differentiated from HSP because of renal and GI sequelae that HSP portends compared to the benign course of AHEI.2 Notably, some health care providers consider AHEI a benign variant of HSP.2,3
Characteristically, AHEI patients are nontoxic-appearing infants with a low-grade fever who develop relatively large (1–5 cm) targetoid purpuric lesions and indurated nonpitting edema of the extremities.2,5 Purpura in AHEI frequently occurs on the face, ears, and upper and lower extremities, whereas purpura in HSP most commonly presents on the buttocks and extensor legs with sparing of the face. Henoch-Schönlein purpura most often affects children aged 3 to 6 years compared to AHEI’s younger demographic (age <2 years).4,5 Clinically, HSP presents with palpable purpura and 1 or more of the following features: diffuse abdominal pain, arthritis/arthralgia, renal involvement, and skin or renal biopsy showing predominant IgA deposition.2,6
Both AHEI and HSP show leukocytoclastic vasculitis on histopathology.2-4,6,7 Positive perivascular IgA staining on DIF is strongly associated with HSP, but nearly one-quarter of AHEI cases also show this deposition pattern2,4,7; therefore, DIF alone cannot exclude a diagnosis of AHEI.
Differential Diagnosis
Alternative diagnoses to consider with AHEI include drug-induced vasculitis, erythema multiforme, HSP, Kawasaki disease, meningococcemia, nonaccidental skin bruising, Rocky Mountain spotted fever, septic vasculitis, and urticarial vasculitis (Table).2-4,6-8

Treatment
Acute hemorrhagic edema of infancy is self-limited, with only rare reports of extracutaneous involvement. Supportive treatment is indicated because spontaneous recovery without sequelae is expected within 21 days.2,3,6 If edema is symptomatic, as was the case with our patient, corticosteroids may shorten the disease course.3
Conclusion
Our case highlights the need to combine clinical history, physical examination, and histopathologic analysis to differentiate between AHEI and HSP, which is important for 2 reasons: (1) it helps with the decision to undertake active or observational treatment, and (2) it helps the clinician counsel the patient and guardians regarding potential associated renal and GI risks.
Acute hemorrhagic edema of infancy (AHEI) is an uncommon leukocytoclastic vasculitis affecting children aged 6 to 24 months; Henoch-Schönlein purpura (HSP) is the most common misdiagnosis. The 2 entities should be differentiated, as HSP may have renal and gastrointestinal (GI) comorbidities that need serial follow-up, whereas AHEI follows a benign course without systemic sequelae. Patient history and physical examination are the most important factors in differentiating the 2 diseases; histopathologic and direct immunofluorescence (DIF) analyses may lend further diagnostic confidence.
We report the case of a 10-month-old previously healthy boy who presented with acute rash, edema, and low-grade fever in the setting of recent diarrhea. We differentiate between AHEI and HSP to help prevent misdiagnosis by health care providers.
Case Report
A 10-month-old previously healthy boy presented to the emergency department (ED) for evaluation of a rash and swelling of 4 days’ duration. He had nonbloody diarrhea 1 week prior; soon after, he developed bilateral lower leg edema and rash. On evaluation in a different ED, he had a low-grade fever (rectal temperature, 38.0°C) but normal blood work, including complete blood cell count, basic metabolic panel, and coagulation studies. The patient was discharged to outpatient follow-up with his pediatrician who reported normal urinalysis.
Due to progression of the rash, the patient presented to our ED 3 days after his initial ED assessment. Dermatology was consulted. At the time of presentation, he was afebrile but with GI upset and fussiness. His parents denied additional symptoms or blood in urine or stool. Physical examination revealed a nontoxic-appearing infant with scattered palpable, annular, purpuric papules coalescing into plaques on both legs and feet (Figure 1), with sparse petechiae noted on the lower abdomen. The cheeks had scattered purpuric papules and plaques bilaterally, a few with a small central crust (Figure 2), and the right superior helix had a faint purpuric macule. The hands had a few pink edematous coalescing papules.


Histopathologic analyses with hematoxylin and eosin staining (Figure 3) and DIF (Figure 4) were performed from within a representative purpuric plaque on the right hip. Direct immunofluorescence was performed to evaluate for an IgA vasculitis versus an alternative type of vasculitis. The hematoxylin and eosin–stained specimen demonstrated a dermal perivascular infiltrate involving superficial and deep vessels with neutrophils, karyorrhexis, and erythrocyte extravasation. The endothelium was intact, with a mild suggestion of fibrinoid change of the blood vessel walls. Direct immunofluorescence revealed granular deposition of IgA, C3, and fibrinogen in multiple dermal blood vessels. Combined, the specimens were interpreted as evolving IgA-associated leukocytoclastic vasculitis.


The case was reviewed with our 2 department pediatric dermatologists; a diagnosis of AHEI was made based on the clinical and supportive histopathological presentations. The patient’s parents chose active treatment with a 2-week taper of oral prednisone because of the patient’s discomfort with edema. No GI or adverse renal sequelae, including findings on urinalysis, were reported at 1-month hospital follow-up with dermatology and pediatrics.
Comment
Incidence and Clinical Characteristics
Acute hemorrhagic edema of infancy is an uncommon leukocytoclastic vasculitis first described in the United States by Snow1 in 1913. Other names for the disorder include acute hemorrhagic edema of young children, cockade purpura and edema, Finkelstein disease, and Seidlmayer disease.2 Boys are affected more often than girls, with most children presenting at 6 to 24 months of age. Most affected children experience a prodrome of simple respiratory tract illness (most common), diarrhea (as in our case), or urinary tract infection.2 The exact pathophysiology behind AHEI is unknown, but it is thought to be an immune complex–mediated disease evidenced by the fact that infection, use of medication, or immunization precedes most cases.3,4
Diagnosis
Acute hemorrhagic edema of infancy is diagnosed clinically, with or without the support of skin biopsy. It should be differentiated from HSP because of renal and GI sequelae that HSP portends compared to the benign course of AHEI.2 Notably, some health care providers consider AHEI a benign variant of HSP.2,3
Characteristically, AHEI patients are nontoxic-appearing infants with a low-grade fever who develop relatively large (1–5 cm) targetoid purpuric lesions and indurated nonpitting edema of the extremities.2,5 Purpura in AHEI frequently occurs on the face, ears, and upper and lower extremities, whereas purpura in HSP most commonly presents on the buttocks and extensor legs with sparing of the face. Henoch-Schönlein purpura most often affects children aged 3 to 6 years compared to AHEI’s younger demographic (age <2 years).4,5 Clinically, HSP presents with palpable purpura and 1 or more of the following features: diffuse abdominal pain, arthritis/arthralgia, renal involvement, and skin or renal biopsy showing predominant IgA deposition.2,6
Both AHEI and HSP show leukocytoclastic vasculitis on histopathology.2-4,6,7 Positive perivascular IgA staining on DIF is strongly associated with HSP, but nearly one-quarter of AHEI cases also show this deposition pattern2,4,7; therefore, DIF alone cannot exclude a diagnosis of AHEI.
Differential Diagnosis
Alternative diagnoses to consider with AHEI include drug-induced vasculitis, erythema multiforme, HSP, Kawasaki disease, meningococcemia, nonaccidental skin bruising, Rocky Mountain spotted fever, septic vasculitis, and urticarial vasculitis (Table).2-4,6-8

Treatment
Acute hemorrhagic edema of infancy is self-limited, with only rare reports of extracutaneous involvement. Supportive treatment is indicated because spontaneous recovery without sequelae is expected within 21 days.2,3,6 If edema is symptomatic, as was the case with our patient, corticosteroids may shorten the disease course.3
Conclusion
Our case highlights the need to combine clinical history, physical examination, and histopathologic analysis to differentiate between AHEI and HSP, which is important for 2 reasons: (1) it helps with the decision to undertake active or observational treatment, and (2) it helps the clinician counsel the patient and guardians regarding potential associated renal and GI risks.
- Snow IM. Purpura, urticaria and angioneurotic edema of the hands and feet in a nursing baby. JAMA. 1913;61:18-19.
- Fiore E, Rizzi M, Ragazzi M, et al. Acute hemorrhagic edema of young children (cockade purpura and edema): a case series and systematic review. J Am Acad Dermatol. 2008;59:684-695.
- Freitas P, Bygum A. Visual impairment caused by periorbital edema in an infant with acute hemorrhagic edema of infancy. Pediatr Dermatol. 2013;30:e132-e135.
- Legrain V, Lejean S, Taïeb A, et al. Infantile acute hemorrhagic edema of the skin: study of ten cases. J Am Acad Dermatol. 1991;24:17-22.
- Breda L, Franchini S, Marzetti V, et al. Escherichia coli urinary infection as a cause of acute hemorrhagic edema in infancy. Pediatr Dermatol. 2015;32:e309-e311.
- Ozen S, Ruperto N, Dillon MJ, et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis. 2006;65:936-941.
- Saraclar Y, Tinaztepe K, Adalioğlu G, et al. Acute hemorrhagic edema of infancy (AHEI)—a variant of Henoch-Schönlein purpura or a distinct clinical entity? J Allergy Clin Immunol. 1990;86:473-483.
- Shinkai K, Fox L. Cutaneous vasculitis. In: Bolognia J, Jorizzo J, Schaffer J, eds. Dermatology. 3rd ed. China: Elsevier Limited; 2012:385-410.
- Snow IM. Purpura, urticaria and angioneurotic edema of the hands and feet in a nursing baby. JAMA. 1913;61:18-19.
- Fiore E, Rizzi M, Ragazzi M, et al. Acute hemorrhagic edema of young children (cockade purpura and edema): a case series and systematic review. J Am Acad Dermatol. 2008;59:684-695.
- Freitas P, Bygum A. Visual impairment caused by periorbital edema in an infant with acute hemorrhagic edema of infancy. Pediatr Dermatol. 2013;30:e132-e135.
- Legrain V, Lejean S, Taïeb A, et al. Infantile acute hemorrhagic edema of the skin: study of ten cases. J Am Acad Dermatol. 1991;24:17-22.
- Breda L, Franchini S, Marzetti V, et al. Escherichia coli urinary infection as a cause of acute hemorrhagic edema in infancy. Pediatr Dermatol. 2015;32:e309-e311.
- Ozen S, Ruperto N, Dillon MJ, et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis. 2006;65:936-941.
- Saraclar Y, Tinaztepe K, Adalioğlu G, et al. Acute hemorrhagic edema of infancy (AHEI)—a variant of Henoch-Schönlein purpura or a distinct clinical entity? J Allergy Clin Immunol. 1990;86:473-483.
- Shinkai K, Fox L. Cutaneous vasculitis. In: Bolognia J, Jorizzo J, Schaffer J, eds. Dermatology. 3rd ed. China: Elsevier Limited; 2012:385-410.
Practice Points
- Acute hemorrhagic edema of infancy (AHEI) is an uncommon benign leukocytoclastic vasculitis of unknown precise pathophysiology that is thought be immune complex mediated.
- Clinical history, physical examination, and histopathologic analysis combine to allow the important differentiation between AHEI and Henoch-Schönlein purpura (HSP).
- Differentiation between AHEI and HSP determines treatment decisions and indicates the need for counseling on potential associated renal and gastrointestinal risks of HSP.