User login
Are patients more satisfied with combination or monotherapy for hirsutism in PCOS?
EXPERT COMMENTARY
Ezeh and colleagues conducted a retrospective analysis to evaluate the effectiveness of long-term combination suppressive therapy on hirsutism, acne, and menstrual disturbances in patients with PCOS and to identify the elements that could predict therapeutic response.
Details of the study
This chart review examined data from 200 nondiabetic patients with PCOS who presented between October 1987 and June 2002. PCOS diagnosis was based on the National Institutes of Health (NIH) 1990 criteria. During the initial visit, patients underwent a detailed medical history and physical exam, including a modified Ferriman-Gallwey hirsutism score and hormonal evaluation.
Treatment regimens. Patients were treated with suppressive therapy that consisted of an oral contraceptive (OC) (35 µg ethinyl estradiol plus 1 mg ethynodiol diacetate), an antiandrogen (spironolactone 200 mg/day), or a combination of these drugs. They were followed every 4 to 12 months (mean follow-up time, 34.2 months; range, 6–155 months), and subjective therapy response was assessed from medical records and by improvements in hirsutism scores.
Study findings. The 138 patients treated with combination suppressive therapy reported higher rates of subjective improvement in hirsutism compared with patients treated with other regimens (89.9% vs 72.0%, P<.0001). They also had a significant objective reduction in their modified Ferriman-Gallwey hirsutism score (6.0 vs 3.2; P = .0001). The combination therapy was superior to either regimen alone; the response to therapy for symptom resolution took at least 6 months and continued for up to 60 months of combination suppressive therapy.
Adding electrolysis treatment to the combination regimen resulted in improved patient satisfaction, but the differences were not significant. Patients’ satisfaction with the therapeutic response could be predicted from their pretreatment hirsutism scores or circulating sex hormone–binding globulin levels.
Study strengths and weaknesses
The study’s major strengths are the large number of patients included, the uniformity of criteria for diagnosis, and the prolonged follow-up. This is one of the few studies to report the impact of therapy on health-related quality of life in patients with PCOS and to assess response to therapy with use of objective measures, such as changes in the modified Ferriman-Gallwey score.
However, the criteria used to diagnose PCOS—the NIH 1990 criteria—currently are used less commonly than the Rotterdam 2003 criteria, and they are less inclusive for the diagnosis of PCOS.
The OC pill formulation used in this study contained the progestogen ethynodiol diacetate, which is not used routinely in modern clinical practice. In addition, the majority of patients were non-Hispanic white, which limits extrapolating these findings to other races and ethnicities.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
This retrospective study offers Level II evidence confirming the superiority of a combined OC plus spironolactone (compared with either agent alone) in the treatment of hirsutism in women with PCOS. In addition, this study emphasizes the importance of using combination suppressive therapy for at least 6 months to see a clinical response. Electrolysis may be helpful to patients especially during the initial 6 months of suppressive treatment. Finally, spironolactone alone could be reserved for cases in which OCs are contraindicated in women not interested in becoming pregnant.
In our practice, we treat patients with hirsutism using OC pills containing the progestogen levonorgestrel plus spironolactone at a lower dose of 100 mg/day, since patients treated with higher spironolactone doses report irregular bleeding and fatigue.
--ELIE HOBEIKA, MD, AND BERT SCOCCIA, MD
EXPERT COMMENTARY
Ezeh and colleagues conducted a retrospective analysis to evaluate the effectiveness of long-term combination suppressive therapy on hirsutism, acne, and menstrual disturbances in patients with PCOS and to identify the elements that could predict therapeutic response.
Details of the study
This chart review examined data from 200 nondiabetic patients with PCOS who presented between October 1987 and June 2002. PCOS diagnosis was based on the National Institutes of Health (NIH) 1990 criteria. During the initial visit, patients underwent a detailed medical history and physical exam, including a modified Ferriman-Gallwey hirsutism score and hormonal evaluation.
Treatment regimens. Patients were treated with suppressive therapy that consisted of an oral contraceptive (OC) (35 µg ethinyl estradiol plus 1 mg ethynodiol diacetate), an antiandrogen (spironolactone 200 mg/day), or a combination of these drugs. They were followed every 4 to 12 months (mean follow-up time, 34.2 months; range, 6–155 months), and subjective therapy response was assessed from medical records and by improvements in hirsutism scores.
Study findings. The 138 patients treated with combination suppressive therapy reported higher rates of subjective improvement in hirsutism compared with patients treated with other regimens (89.9% vs 72.0%, P<.0001). They also had a significant objective reduction in their modified Ferriman-Gallwey hirsutism score (6.0 vs 3.2; P = .0001). The combination therapy was superior to either regimen alone; the response to therapy for symptom resolution took at least 6 months and continued for up to 60 months of combination suppressive therapy.
Adding electrolysis treatment to the combination regimen resulted in improved patient satisfaction, but the differences were not significant. Patients’ satisfaction with the therapeutic response could be predicted from their pretreatment hirsutism scores or circulating sex hormone–binding globulin levels.
Study strengths and weaknesses
The study’s major strengths are the large number of patients included, the uniformity of criteria for diagnosis, and the prolonged follow-up. This is one of the few studies to report the impact of therapy on health-related quality of life in patients with PCOS and to assess response to therapy with use of objective measures, such as changes in the modified Ferriman-Gallwey score.
However, the criteria used to diagnose PCOS—the NIH 1990 criteria—currently are used less commonly than the Rotterdam 2003 criteria, and they are less inclusive for the diagnosis of PCOS.
The OC pill formulation used in this study contained the progestogen ethynodiol diacetate, which is not used routinely in modern clinical practice. In addition, the majority of patients were non-Hispanic white, which limits extrapolating these findings to other races and ethnicities.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
This retrospective study offers Level II evidence confirming the superiority of a combined OC plus spironolactone (compared with either agent alone) in the treatment of hirsutism in women with PCOS. In addition, this study emphasizes the importance of using combination suppressive therapy for at least 6 months to see a clinical response. Electrolysis may be helpful to patients especially during the initial 6 months of suppressive treatment. Finally, spironolactone alone could be reserved for cases in which OCs are contraindicated in women not interested in becoming pregnant.
In our practice, we treat patients with hirsutism using OC pills containing the progestogen levonorgestrel plus spironolactone at a lower dose of 100 mg/day, since patients treated with higher spironolactone doses report irregular bleeding and fatigue.
--ELIE HOBEIKA, MD, AND BERT SCOCCIA, MD
EXPERT COMMENTARY
Ezeh and colleagues conducted a retrospective analysis to evaluate the effectiveness of long-term combination suppressive therapy on hirsutism, acne, and menstrual disturbances in patients with PCOS and to identify the elements that could predict therapeutic response.
Details of the study
This chart review examined data from 200 nondiabetic patients with PCOS who presented between October 1987 and June 2002. PCOS diagnosis was based on the National Institutes of Health (NIH) 1990 criteria. During the initial visit, patients underwent a detailed medical history and physical exam, including a modified Ferriman-Gallwey hirsutism score and hormonal evaluation.
Treatment regimens. Patients were treated with suppressive therapy that consisted of an oral contraceptive (OC) (35 µg ethinyl estradiol plus 1 mg ethynodiol diacetate), an antiandrogen (spironolactone 200 mg/day), or a combination of these drugs. They were followed every 4 to 12 months (mean follow-up time, 34.2 months; range, 6–155 months), and subjective therapy response was assessed from medical records and by improvements in hirsutism scores.
Study findings. The 138 patients treated with combination suppressive therapy reported higher rates of subjective improvement in hirsutism compared with patients treated with other regimens (89.9% vs 72.0%, P<.0001). They also had a significant objective reduction in their modified Ferriman-Gallwey hirsutism score (6.0 vs 3.2; P = .0001). The combination therapy was superior to either regimen alone; the response to therapy for symptom resolution took at least 6 months and continued for up to 60 months of combination suppressive therapy.
Adding electrolysis treatment to the combination regimen resulted in improved patient satisfaction, but the differences were not significant. Patients’ satisfaction with the therapeutic response could be predicted from their pretreatment hirsutism scores or circulating sex hormone–binding globulin levels.
Study strengths and weaknesses
The study’s major strengths are the large number of patients included, the uniformity of criteria for diagnosis, and the prolonged follow-up. This is one of the few studies to report the impact of therapy on health-related quality of life in patients with PCOS and to assess response to therapy with use of objective measures, such as changes in the modified Ferriman-Gallwey score.
However, the criteria used to diagnose PCOS—the NIH 1990 criteria—currently are used less commonly than the Rotterdam 2003 criteria, and they are less inclusive for the diagnosis of PCOS.
The OC pill formulation used in this study contained the progestogen ethynodiol diacetate, which is not used routinely in modern clinical practice. In addition, the majority of patients were non-Hispanic white, which limits extrapolating these findings to other races and ethnicities.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
This retrospective study offers Level II evidence confirming the superiority of a combined OC plus spironolactone (compared with either agent alone) in the treatment of hirsutism in women with PCOS. In addition, this study emphasizes the importance of using combination suppressive therapy for at least 6 months to see a clinical response. Electrolysis may be helpful to patients especially during the initial 6 months of suppressive treatment. Finally, spironolactone alone could be reserved for cases in which OCs are contraindicated in women not interested in becoming pregnant.
In our practice, we treat patients with hirsutism using OC pills containing the progestogen levonorgestrel plus spironolactone at a lower dose of 100 mg/day, since patients treated with higher spironolactone doses report irregular bleeding and fatigue.
--ELIE HOBEIKA, MD, AND BERT SCOCCIA, MD
With midterm elections near, PhRMA continues to spend
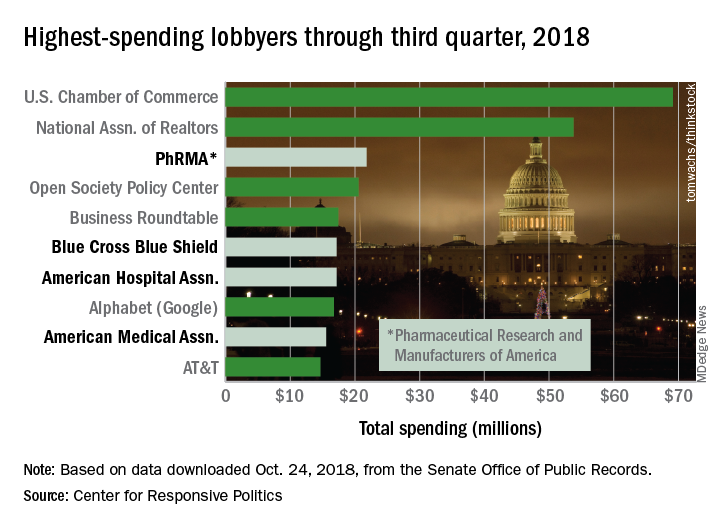
PhRMA’s $21.8 million worth of lobbying through September put it ahead of Blue Cross Blue Shield’s $17.2 million. PhRMA remains on pace to exceed its previous spending high of $27.2 million in 2009, and the health sector as a whole, with a lobbying bill of $421.5 million for three-quarters of 2018, is just slightly ahead of last year’s record of $561.3 million, the center reported on OpenSecrets.org.
PhRMA is third overall in lobbying spending so far this year, behind the U.S. Chamber of Commerce ($69.1 million) and the National Association of Realtors ($53.8 million). Blue Cross Blue Shield is sixth overall, with the American Hospital Association ($17.2 million, seventh) and the American Medical Association ($15.5 million, ninth) also in the top 10, according to the center’s analysis of data from the Senate Office of Public Records.
The pharmaceutical/health product industry leads health-sector lobbying with $216.1 million in spending so far in 2018, followed by hospitals/nursing homes at $73.6 million, health professionals at $68.6 million, and health services/HMOs at $57.5 million.

PhRMA’s $21.8 million worth of lobbying through September put it ahead of Blue Cross Blue Shield’s $17.2 million. PhRMA remains on pace to exceed its previous spending high of $27.2 million in 2009, and the health sector as a whole, with a lobbying bill of $421.5 million for three-quarters of 2018, is just slightly ahead of last year’s record of $561.3 million, the center reported on OpenSecrets.org.
PhRMA is third overall in lobbying spending so far this year, behind the U.S. Chamber of Commerce ($69.1 million) and the National Association of Realtors ($53.8 million). Blue Cross Blue Shield is sixth overall, with the American Hospital Association ($17.2 million, seventh) and the American Medical Association ($15.5 million, ninth) also in the top 10, according to the center’s analysis of data from the Senate Office of Public Records.
The pharmaceutical/health product industry leads health-sector lobbying with $216.1 million in spending so far in 2018, followed by hospitals/nursing homes at $73.6 million, health professionals at $68.6 million, and health services/HMOs at $57.5 million.

PhRMA’s $21.8 million worth of lobbying through September put it ahead of Blue Cross Blue Shield’s $17.2 million. PhRMA remains on pace to exceed its previous spending high of $27.2 million in 2009, and the health sector as a whole, with a lobbying bill of $421.5 million for three-quarters of 2018, is just slightly ahead of last year’s record of $561.3 million, the center reported on OpenSecrets.org.
PhRMA is third overall in lobbying spending so far this year, behind the U.S. Chamber of Commerce ($69.1 million) and the National Association of Realtors ($53.8 million). Blue Cross Blue Shield is sixth overall, with the American Hospital Association ($17.2 million, seventh) and the American Medical Association ($15.5 million, ninth) also in the top 10, according to the center’s analysis of data from the Senate Office of Public Records.
The pharmaceutical/health product industry leads health-sector lobbying with $216.1 million in spending so far in 2018, followed by hospitals/nursing homes at $73.6 million, health professionals at $68.6 million, and health services/HMOs at $57.5 million.
Genomic abnormalities shed light on racial disparity in myeloma
Researchers say they may have determined why African Americans have a two- to threefold increased risk of multiple myeloma (MM), compared with European Americans.
The team genotyped 881 MM samples from various racial groups and identified three gene subtypes – t(11;14), t(14;16), and t(14;20) – that explain the racial disparity.
They found that patients with African ancestry of 80% or more had a significantly higher occurrence of these subtypes, compared with individuals with African ancestry of less than 0.1%.
And these subtypes are driving the disparity in MM diagnoses between the populations.
Previous attempts to explain the disparity relied on self-reported race rather than quantitatively measured genetic ancestry, which could result in bias, Vincent Rajkumar, MD, of the Mayo Clinic in Rochester, Minn., and his colleagues reported in Blood Cancer Journal.
“A major new aspect of this study is that we identified the ancestry of each patient through DNA sequencing, which allowed us to determine ancestry more accurately,” Dr. Rajkumar said in a statement.
All 881 samples had abnormal plasma cell FISH, 851 had a normal chromosome study, and 30 had an abnormal study.
Median age for the entire group was 64 years. More samples were from men (54.3%) than women (45.7%). Researchers observed no significant difference between men and women in the proportion of primary cytogenetic abnormalities.
Of the 881 samples, the median African ancestry was 2.3%, the median European ancestry was 64.7%, and Northern European ancestry was 26.6%.
Thirty percent of the entire cohort had less than 0.1% African ancestry, and 13.6% had 80% or greater African ancestry.
Using a logistic regression model, the researchers determined that a 10% increase in the percentage of African ancestry was associated with a 6% increase in the odds of detecting t(11;14), t(14;16), or t(14;20) odds ratio, 1.06; 95% confidence interval, 1.02-1.11; P = .05).
The researchers plotted the probability of observing these cytogenetic abnormalities with the percentage of African ancestry and found the differences were most striking in the extreme populations – individuals with 80% or greater African ancestry and individuals with less than 0.1% African ancestry.
Upon further analysis, the team found a significantly higher prevalence of t(11;14), t(14;16), and t(14;20) in the group of patients with the greatest proportion of African ancestry (P = .008), compared with the European cohort.
The differences emerged in only the highest and lowest cohorts, they noted. Most patients (60%) were not included in these extreme populations because they had mixed ancestry.
The team observed no significant differences when the cutoff for African ancestry was greater than 50%.
The research was supported by the National Cancer Institute and the Mayo Clinic. One study author reported relationships with Celgene, Takeda, Prothena, Janssen, Pfizer, Alnylam, and GSK. Two authors reported relationships with the DNA Diagnostics Center.
SOURCE: Baughn LB et al. Blood Cancer J. 2018 Oct 10;8(10):96.
Researchers say they may have determined why African Americans have a two- to threefold increased risk of multiple myeloma (MM), compared with European Americans.
The team genotyped 881 MM samples from various racial groups and identified three gene subtypes – t(11;14), t(14;16), and t(14;20) – that explain the racial disparity.
They found that patients with African ancestry of 80% or more had a significantly higher occurrence of these subtypes, compared with individuals with African ancestry of less than 0.1%.
And these subtypes are driving the disparity in MM diagnoses between the populations.
Previous attempts to explain the disparity relied on self-reported race rather than quantitatively measured genetic ancestry, which could result in bias, Vincent Rajkumar, MD, of the Mayo Clinic in Rochester, Minn., and his colleagues reported in Blood Cancer Journal.
“A major new aspect of this study is that we identified the ancestry of each patient through DNA sequencing, which allowed us to determine ancestry more accurately,” Dr. Rajkumar said in a statement.
All 881 samples had abnormal plasma cell FISH, 851 had a normal chromosome study, and 30 had an abnormal study.
Median age for the entire group was 64 years. More samples were from men (54.3%) than women (45.7%). Researchers observed no significant difference between men and women in the proportion of primary cytogenetic abnormalities.
Of the 881 samples, the median African ancestry was 2.3%, the median European ancestry was 64.7%, and Northern European ancestry was 26.6%.
Thirty percent of the entire cohort had less than 0.1% African ancestry, and 13.6% had 80% or greater African ancestry.
Using a logistic regression model, the researchers determined that a 10% increase in the percentage of African ancestry was associated with a 6% increase in the odds of detecting t(11;14), t(14;16), or t(14;20) odds ratio, 1.06; 95% confidence interval, 1.02-1.11; P = .05).
The researchers plotted the probability of observing these cytogenetic abnormalities with the percentage of African ancestry and found the differences were most striking in the extreme populations – individuals with 80% or greater African ancestry and individuals with less than 0.1% African ancestry.
Upon further analysis, the team found a significantly higher prevalence of t(11;14), t(14;16), and t(14;20) in the group of patients with the greatest proportion of African ancestry (P = .008), compared with the European cohort.
The differences emerged in only the highest and lowest cohorts, they noted. Most patients (60%) were not included in these extreme populations because they had mixed ancestry.
The team observed no significant differences when the cutoff for African ancestry was greater than 50%.
The research was supported by the National Cancer Institute and the Mayo Clinic. One study author reported relationships with Celgene, Takeda, Prothena, Janssen, Pfizer, Alnylam, and GSK. Two authors reported relationships with the DNA Diagnostics Center.
SOURCE: Baughn LB et al. Blood Cancer J. 2018 Oct 10;8(10):96.
Researchers say they may have determined why African Americans have a two- to threefold increased risk of multiple myeloma (MM), compared with European Americans.
The team genotyped 881 MM samples from various racial groups and identified three gene subtypes – t(11;14), t(14;16), and t(14;20) – that explain the racial disparity.
They found that patients with African ancestry of 80% or more had a significantly higher occurrence of these subtypes, compared with individuals with African ancestry of less than 0.1%.
And these subtypes are driving the disparity in MM diagnoses between the populations.
Previous attempts to explain the disparity relied on self-reported race rather than quantitatively measured genetic ancestry, which could result in bias, Vincent Rajkumar, MD, of the Mayo Clinic in Rochester, Minn., and his colleagues reported in Blood Cancer Journal.
“A major new aspect of this study is that we identified the ancestry of each patient through DNA sequencing, which allowed us to determine ancestry more accurately,” Dr. Rajkumar said in a statement.
All 881 samples had abnormal plasma cell FISH, 851 had a normal chromosome study, and 30 had an abnormal study.
Median age for the entire group was 64 years. More samples were from men (54.3%) than women (45.7%). Researchers observed no significant difference between men and women in the proportion of primary cytogenetic abnormalities.
Of the 881 samples, the median African ancestry was 2.3%, the median European ancestry was 64.7%, and Northern European ancestry was 26.6%.
Thirty percent of the entire cohort had less than 0.1% African ancestry, and 13.6% had 80% or greater African ancestry.
Using a logistic regression model, the researchers determined that a 10% increase in the percentage of African ancestry was associated with a 6% increase in the odds of detecting t(11;14), t(14;16), or t(14;20) odds ratio, 1.06; 95% confidence interval, 1.02-1.11; P = .05).
The researchers plotted the probability of observing these cytogenetic abnormalities with the percentage of African ancestry and found the differences were most striking in the extreme populations – individuals with 80% or greater African ancestry and individuals with less than 0.1% African ancestry.
Upon further analysis, the team found a significantly higher prevalence of t(11;14), t(14;16), and t(14;20) in the group of patients with the greatest proportion of African ancestry (P = .008), compared with the European cohort.
The differences emerged in only the highest and lowest cohorts, they noted. Most patients (60%) were not included in these extreme populations because they had mixed ancestry.
The team observed no significant differences when the cutoff for African ancestry was greater than 50%.
The research was supported by the National Cancer Institute and the Mayo Clinic. One study author reported relationships with Celgene, Takeda, Prothena, Janssen, Pfizer, Alnylam, and GSK. Two authors reported relationships with the DNA Diagnostics Center.
SOURCE: Baughn LB et al. Blood Cancer J. 2018 Oct 10;8(10):96.
FROM BLOOD CANCER JOURNAL
Key clinical point:
Major finding: There was a significantly higher prevalence of t(11;14), t(14;16), and t(14:20) in patients with 80% or greater African ancestry, compared with the European cohort (P = .008).
Study details: The study included 881 samples from patients with an abnormal plasma cell proliferative disorder FISH result and concurrent conventional G-banded chromosome evaluation.
Disclosures: The research was supported by the National Cancer Institute and the Mayo Clinic. One study author reported relationships with Celgene, Takeda, Prothena, Janssen, Pfizer, Alnylam, and GSK. Two authors reported relationships with the DNA Diagnostics Center.
Source: Baughn LB et al. Blood Cancer J. 2018 Oct 10;8(10):96.
Leflunomide-hydroxychloroquine combo shows promise in primary Sjögren’s pilot study
CHICAGO – Combination therapy with leflunomide and hydroxychloroquine met all goals for efficacy, safety, and tolerability among patients with primary Sjögren’s syndrome in a randomized, placebo-controlled pilot study, lending support to evidence suggesting the two drugs have additive benefits.
The combined treatment was associated with a statistically significant decrease in the EULAR Sjögren’s syndrome disease activity index (ESSDAI) over 24 weeks – the primary endpoint of the study – in 21 patients in the treatment group. The ESSDAI score on combination treatment dropped from about 10 at baseline to about 6 at 24 weeks, compared with no change from a baseline of about 10 in eight patients in the placebo group. An ESSDAI decrease of 3 or more points occurred in 11 patients in the combination therapy group, compared with none in the placebo group, Joel A.G. van Roon, PhD, a researcher in the Laboratory of Translational Immunology at the University Medical Center Utrecht, the Netherlands, reported in a late-breaking poster at the annual meeting of the American College of Rheumatology.
Both leflunomide and hydroxychloroquine have been shown to inhibit B-cell hyperactivity, but the clinical benefits have been modest and not statistically significant. Since the two agents have complementary inhibitory properties on different immune cells – including B and T cells and plasmacytoid dendritic cells, and based on in vitro findings of additive benefits with respect to inhibition of T- and B-cell activation and CXCL13 production, Dr. van Roon and his colleagues conducted this double-blind, single-center, proof-of-concept pilot study (REPURpSS-1) to assess the efficacy, safety, and tolerability of combined treatment in primary Sjögren’s syndrome.
In all, 29 patients with clinically active disease, defined by ESSDAI of 5 or greater, were randomized 2:1 to receive either 20 mg of leflunomide daily plus 400 mg of hydroxychloroquine daily or placebo/placebo for 24 weeks.
Secondary endpoints such as oral dryness also improved significantly in the treatment group versus the placebo group. Stimulated whole saliva flow increased from about 800 mcL/5 min to about 1,400 mcL/5 min and decreased from about 1,250 to about 1,000 mcL/5 min in the groups, respectively. Median EULAR Sjögren’s syndrome patient reported index (ESSPRI), ESSPRI pain, and ESSPRI fatigue scores, as well as Physician’s and Patient’s Global Assessment scores each improved significantly in the treatment group (at least P less than .05 in all cases) but not in the placebo groups, said Dr. van Roon.
Additionally, serum IgG, IgM rheumatoid factor, and chemokine CXCL13 – a marker for lymphoid neogenesis – decreased significantly, and complement components 3 and 4 (C3 and C4) increased significantly by 24 weeks in the treatment group, but not in the placebo group. B-cell hyperactivity as measured by serum IgG decreased from about 20 g/L to about 14 g/L versus no change from about 15 g/L at baseline in the placebo group, he noted.
“Overall, combination leflunomide and hydroxychloroquine was safe and well tolerated, but larger randomized, controlled trials are needed to confirm the observed effects and to identify potential biomarkers for response,” he concluded.
This study was supported by ZonMw (the Netherlands Organization for Health Research and Development). Dr. van Roon reported having no relevant disclosures.
SOURCE: van Roon JAG et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L10.
CHICAGO – Combination therapy with leflunomide and hydroxychloroquine met all goals for efficacy, safety, and tolerability among patients with primary Sjögren’s syndrome in a randomized, placebo-controlled pilot study, lending support to evidence suggesting the two drugs have additive benefits.
The combined treatment was associated with a statistically significant decrease in the EULAR Sjögren’s syndrome disease activity index (ESSDAI) over 24 weeks – the primary endpoint of the study – in 21 patients in the treatment group. The ESSDAI score on combination treatment dropped from about 10 at baseline to about 6 at 24 weeks, compared with no change from a baseline of about 10 in eight patients in the placebo group. An ESSDAI decrease of 3 or more points occurred in 11 patients in the combination therapy group, compared with none in the placebo group, Joel A.G. van Roon, PhD, a researcher in the Laboratory of Translational Immunology at the University Medical Center Utrecht, the Netherlands, reported in a late-breaking poster at the annual meeting of the American College of Rheumatology.
Both leflunomide and hydroxychloroquine have been shown to inhibit B-cell hyperactivity, but the clinical benefits have been modest and not statistically significant. Since the two agents have complementary inhibitory properties on different immune cells – including B and T cells and plasmacytoid dendritic cells, and based on in vitro findings of additive benefits with respect to inhibition of T- and B-cell activation and CXCL13 production, Dr. van Roon and his colleagues conducted this double-blind, single-center, proof-of-concept pilot study (REPURpSS-1) to assess the efficacy, safety, and tolerability of combined treatment in primary Sjögren’s syndrome.
In all, 29 patients with clinically active disease, defined by ESSDAI of 5 or greater, were randomized 2:1 to receive either 20 mg of leflunomide daily plus 400 mg of hydroxychloroquine daily or placebo/placebo for 24 weeks.
Secondary endpoints such as oral dryness also improved significantly in the treatment group versus the placebo group. Stimulated whole saliva flow increased from about 800 mcL/5 min to about 1,400 mcL/5 min and decreased from about 1,250 to about 1,000 mcL/5 min in the groups, respectively. Median EULAR Sjögren’s syndrome patient reported index (ESSPRI), ESSPRI pain, and ESSPRI fatigue scores, as well as Physician’s and Patient’s Global Assessment scores each improved significantly in the treatment group (at least P less than .05 in all cases) but not in the placebo groups, said Dr. van Roon.
Additionally, serum IgG, IgM rheumatoid factor, and chemokine CXCL13 – a marker for lymphoid neogenesis – decreased significantly, and complement components 3 and 4 (C3 and C4) increased significantly by 24 weeks in the treatment group, but not in the placebo group. B-cell hyperactivity as measured by serum IgG decreased from about 20 g/L to about 14 g/L versus no change from about 15 g/L at baseline in the placebo group, he noted.
“Overall, combination leflunomide and hydroxychloroquine was safe and well tolerated, but larger randomized, controlled trials are needed to confirm the observed effects and to identify potential biomarkers for response,” he concluded.
This study was supported by ZonMw (the Netherlands Organization for Health Research and Development). Dr. van Roon reported having no relevant disclosures.
SOURCE: van Roon JAG et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L10.
CHICAGO – Combination therapy with leflunomide and hydroxychloroquine met all goals for efficacy, safety, and tolerability among patients with primary Sjögren’s syndrome in a randomized, placebo-controlled pilot study, lending support to evidence suggesting the two drugs have additive benefits.
The combined treatment was associated with a statistically significant decrease in the EULAR Sjögren’s syndrome disease activity index (ESSDAI) over 24 weeks – the primary endpoint of the study – in 21 patients in the treatment group. The ESSDAI score on combination treatment dropped from about 10 at baseline to about 6 at 24 weeks, compared with no change from a baseline of about 10 in eight patients in the placebo group. An ESSDAI decrease of 3 or more points occurred in 11 patients in the combination therapy group, compared with none in the placebo group, Joel A.G. van Roon, PhD, a researcher in the Laboratory of Translational Immunology at the University Medical Center Utrecht, the Netherlands, reported in a late-breaking poster at the annual meeting of the American College of Rheumatology.
Both leflunomide and hydroxychloroquine have been shown to inhibit B-cell hyperactivity, but the clinical benefits have been modest and not statistically significant. Since the two agents have complementary inhibitory properties on different immune cells – including B and T cells and plasmacytoid dendritic cells, and based on in vitro findings of additive benefits with respect to inhibition of T- and B-cell activation and CXCL13 production, Dr. van Roon and his colleagues conducted this double-blind, single-center, proof-of-concept pilot study (REPURpSS-1) to assess the efficacy, safety, and tolerability of combined treatment in primary Sjögren’s syndrome.
In all, 29 patients with clinically active disease, defined by ESSDAI of 5 or greater, were randomized 2:1 to receive either 20 mg of leflunomide daily plus 400 mg of hydroxychloroquine daily or placebo/placebo for 24 weeks.
Secondary endpoints such as oral dryness also improved significantly in the treatment group versus the placebo group. Stimulated whole saliva flow increased from about 800 mcL/5 min to about 1,400 mcL/5 min and decreased from about 1,250 to about 1,000 mcL/5 min in the groups, respectively. Median EULAR Sjögren’s syndrome patient reported index (ESSPRI), ESSPRI pain, and ESSPRI fatigue scores, as well as Physician’s and Patient’s Global Assessment scores each improved significantly in the treatment group (at least P less than .05 in all cases) but not in the placebo groups, said Dr. van Roon.
Additionally, serum IgG, IgM rheumatoid factor, and chemokine CXCL13 – a marker for lymphoid neogenesis – decreased significantly, and complement components 3 and 4 (C3 and C4) increased significantly by 24 weeks in the treatment group, but not in the placebo group. B-cell hyperactivity as measured by serum IgG decreased from about 20 g/L to about 14 g/L versus no change from about 15 g/L at baseline in the placebo group, he noted.
“Overall, combination leflunomide and hydroxychloroquine was safe and well tolerated, but larger randomized, controlled trials are needed to confirm the observed effects and to identify potential biomarkers for response,” he concluded.
This study was supported by ZonMw (the Netherlands Organization for Health Research and Development). Dr. van Roon reported having no relevant disclosures.
SOURCE: van Roon JAG et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L10.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: but larger randomized, controlled trials are needed to confirm the observed effects.
Major finding: Combined treatment was associated with a decline in EULAR Sjögren’s syndrome disease activity index score from about 10 at baseline to about 6 at 24 weeks.
Study details: A randomized, placebo-controlled pilot study of 29 patients.
Disclosures: This study was supported by ZonMw (the Netherlands Organization for Health Research and Development). Dr. van Roon reported having no relevant disclosures.
Source: van Roon JAG et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L10.
New and Noteworthy Information—November 2018
Thrombolysis Benefits Patients With Stroke
In patients with acute stroke with an unknown time of onset, IV alteplase guided by a mismatch between diffusion-weighted imaging and fluid-attenuated inversion recovery (FLAIR) in the region of ischemia results in a significantly better functional outcome and more intracranial hemorrhages at 90 days, compared with placebo, according to a study published August 16 in the New England Journal of Medicine. Researchers randomly assigned 254 participants to receive IV alteplase and 249 participants to receive placebo. Participants had an ischemic lesion on MRI diffusion-weighted imaging, but no parenchymal hyperintensity on FLAIR. A favorable outcome (ie, a score of 0 or 1 on the modified Rankin Scale at 90 days) occurred in 53.3% of the alteplase group versus 41.8% of the placebo group.
Thomalla G, Simonsen CZ, Boutitie F, et al. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379(7):611-622.
High Levels of Cortisol Linked to Impaired Memory
Middle-aged people with high levels of cortisol in their blood have impaired memory, compared with people with average levels of cortisol, according to a study published online ahead of print October 24 in Neurology. Researchers identified 2,231 people with an average age of 49 who did not have dementia. At the start of the study, each participant underwent a psychologic exam and assessments of memory and thinking skills. Participants’ memory and thinking skills were tested again at an average of eight years later. Participants also provided a blood sample. After adjusting for age, sex, smoking, and BMI, researchers found that people with high levels of cortisol had lower scores on tests of memory and thinking skills, compared with people with normal levels of cortisol.
Echouffo-Tcheugui JB, Conner SC, Himali JJ, et al. Circulating cortisol and cognitive and structural brain measures: The Framingham Heart Study. Neurology. 2018 Oct 24 [Epub ahead of print].
Is Nusinersen Effective If Initiated Later?
Patients with spinal muscular atrophy type 1 (SMA1) may benefit from nusinersen when the therapy is initiated after age 7 months, according to a study published online ahead of print August 29 in Neurology. In this study, 33 patients with SMA1 received intrathecal nusinersen injections. Researchers evaluated patients before treatment and at two months and six months after treatment. All patients were alive and continuing treatment at six months. Median progress on the modified Hammersmith Infant Neurologic Examination Part 2 score was 1.5 points after six months of treatment. The need for respiratory support significantly increased over time. The results are consistent with those of a phase III trial in which patients with SMA1 received nusinersen before age 7 months, the researchers said.
Aragon-Gawinska K, Seferian AM, Daron A, et al. Nusinersen in spinal muscular atrophy type 1 patients older than 7 months: a cohort study. Neurology. 2018 Aug 29 [Epub ahead of print].
Pre-Eclampsia Linked to Dementia in Late Life
Pre-eclampsia is associated with an increased risk of dementia, particularly vascular dementia, according to a study published October 17 in BMJ. The study cohort consisted of 1,178,005 Danish women with at least one live birth or stillbirth between 1978 and 2015. Women with a history of pre-eclampsia had more than three times the risk of vascular dementia later in life, compared with women with no history of pre-eclampsia. The association with vascular dementia seemed to be stronger for late-onset disease than for early-onset disease. Adjustment for diabetes, hypertension, and cardiovascular disease attenuated the hazard ratios moderately. Sensitivity analyses suggested that BMI was unlikely to explain the association with vascular dementia. In contrast, modest associations were observed for Alzheimer’s disease and other or unspecified dementia.
Basit S, Wohlfahrt J, Boyd HA. Pre-eclampsia and risk of dementia later in life: nationwide cohort study. BMJ. 2018;363:k4109.
Does Antiepileptic Drug Clearance Change During Pregnancy?
In pregnant women, antiepileptic drug (AED) clearance significantly changes by the first trimester for levetiracetam and by the second trimester for oxcarbazepine and topiramate, according to a study published September 25 in Neurology. This prospective, observational study included 40 women with epilepsy who were planning to conceive or were fewer than 16 weeks pregnant and who chose to continue their AEDs during pregnancy. Drug clearance values were obtained by blood draw at baseline and during pregnancy. Mean maximal clearances were 1.71 times the baseline clearance for levetiracetam, 1.63 times the baseline clearance for oxcarbazepine, and 1.39 the baseline clearance for topiramate. In 15 women on AED monotherapy, increased seizure frequency in the first, second, and all trimesters was associated with a lower ratio to target concentration.
Voinescu PE, Park S, Chen LQ, et al. Antiepileptic drug clearances during pregnancy and clinical implications for women with epilepsy. Neurology. 2018;91(13):e1228-e1236.
Do Dextroamphetamine and Physical Therapy Improve Function After Stroke?
Compared with placebo, dextroamphetamine combined with physical therapy does not improve recovery of motor function after stroke, according to a study published online ahead of print August 27 in JAMA Neurology. This pilot, double-blind, block-randomized clinical trial included patients with cortical or subcortical ischemic stroke and moderate or severe motor deficits. A total of 64 participants were randomized to receive 10 mg of dextroamphetamine or placebo one hour before a one-hour physical therapy session every four days for six sessions, in addition to standard rehabilitation. The primary outcome was the difference between groups in change in Fugl-Meyer motor scores from baseline to three months after stroke. Treatment was not associated with differences in the primary outcome, secondary measures, or subgroups based on stroke location or baseline severity.
Goldstein LB, Lennihan L, Rabadi MJ, et al. Effect of dextroamphetamine on poststroke motor recovery: a randomized clinical trial. JAMA Neurol. 2018 Aug 27 [Epub ahead of print].
FDA Approves Tegsedi for hATTR in Adults
The FDA has approved Tegsedi (inotersen) for the treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis (hATTR) in adults. The approval is based on data from the phase III randomized, double-blind, placebo-controlled, 15-month, international NEURO-TTR study in 172 patients with hATTR amyloidosis with symptoms of polyneuropathy. In NEURO-TTR, Tegsedi demonstrated significant benefit, compared with placebo, in neuropathy and quality of life, as measured by the modified Neuropathy Impairment Score +7 and the Norfolk Quality of Life Questionnaire-Diabetic Neuropathy total score. Patients treated with Tegsedi experienced similar benefit regardless of subgroup, such as age, sex, race, region, Neuropathy Impairment Score, Val30Met mutation status, and disease stage. Akcea Therapeutics, an affiliate of Ionis Pharmaceuticals that is headquartered in Boston, markets Tegsedi.
Data Review Evaluates Nusinersen’s Efficacy in SMA
A panel has reviewed the evidence for nusinersen treatment of spinal muscular atrophy (SMA). The results were published online ahead of print October 12 in Neurology. The authors systematically reviewed clinical trials of nusinersen in patients with SMA and assigned level of evidence statements. Among four published clinical trials identified, three were rated above Class IV. There is Class I evidence that in term infants with SMA and two copies of SMN2, treatment with nusinersen started in children younger than 7 months results in a better motor milestone response and higher rates of event-free survival than sham control. There is Class I evidence that in children aged 2 to 12 with SMA symptom onset after age 6 months, nusinersen yields greater improvement in motor function at 15 months than sham control.
Michelson D, Ciafaloni E, Ashwal S, et al. Evidence in focus: nusinersen use in spinal muscular atrophy: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018 Oct 12 [Epub ahead of print].
Aortic Stiffness Predicts Incident Dementia
Arterial stiffness can predict a person’s likelihood of developing dementia, according to a study published October 16 in the Journal of Alzheimer’s Disease. Researchers analyzed the association between arterial stiffness and dementia among 356 older adults with an average age of 78 who were part of the Cardiovascular Health Study Cognition Study. Eligible participants were dementia-free when the study began in 1998. Investigators tested participants’ aortic stiffness with carotid-femoral pulse wave velocity. Participants also underwent MRI of their brains to measure signs of subclinical brain disease. The researchers found that participants with high carotid-femoral pulse wave velocity readings were 60% more likely to develop dementia during the following 15 years, compared with people with lower carotid-femoral pulse wave velocity values.
Cui C, Sekikawa A, Kuller LH, et al. Aortic stiffness is associated with increased risk of incident dementia in older adults. J Alzheimers Dis. 2018;66(1):297-306.
Early MoCA Score Predicts Long-Term Outcome After Stroke
Early cognitive testing with the Montreal Cognitive Assessment (MoCA) predicts long-term cognitive outcome, functional outcome, and mortality after stroke, according to a study published online ahead of print October 17 in Neurology. In this international study, 274 people with stroke were administered MoCA within a week of stroke onset. Participants were divided into two groups: people with no problems with thinking and memory skills and people with cognitive impairment. People who had cognitive impairment within one week of stroke were seven times more likely to die during the three years of the study than people without cognitive impairment. Furthermore, the survival rate for people with cognitive impairment after three years was 83%, and the rate was 97% for people who did not have early cognitive impairment.
Zietemann V, Georgakis MK, Dondaine T, et al. Early MoCA predicts long-term cognitive and functional outcome and mortality after stroke. Neurology. 2018 Oct 17 [Epub ahead of print].
Antiepileptic Drug Use Related to Increased Stroke Risk
In Alzheimer’s disease, antiepileptic drug (AED) use is related to an increased risk of stroke, according to a study published September 18 in the Journal of the American Heart Association. Investigators examined the Medication Use and Alzheimer’s Disease cohort, which included all Finnish people who received a clinically verified diagnosis of Alzheimer’s disease from 2005 to 2011. People with previous stroke were excluded. For each incident AED user, the investigators matched one nonuser according to sex, age, and time since Alzheimer’s disease diagnosis. Analyses were conducted with Cox proportional hazards models and inverse probability of treatment weighting. Compared with nonuse, AED use was associated with an increased risk of stroke, and the risk of stroke was strongest during the first 90 days of AED use (adjusted hazard ratio, 2.36).
Sarycheva T, Lavikainen P, Taipale H, et al. Antiepileptic drug use and the risk of stroke among community-dwelling people with Alzheimer disease: A matched cohort study. J Am Heart Assoc. 2018;7(18):e009742.
Thrombolysis Benefits Patients With Stroke
In patients with acute stroke with an unknown time of onset, IV alteplase guided by a mismatch between diffusion-weighted imaging and fluid-attenuated inversion recovery (FLAIR) in the region of ischemia results in a significantly better functional outcome and more intracranial hemorrhages at 90 days, compared with placebo, according to a study published August 16 in the New England Journal of Medicine. Researchers randomly assigned 254 participants to receive IV alteplase and 249 participants to receive placebo. Participants had an ischemic lesion on MRI diffusion-weighted imaging, but no parenchymal hyperintensity on FLAIR. A favorable outcome (ie, a score of 0 or 1 on the modified Rankin Scale at 90 days) occurred in 53.3% of the alteplase group versus 41.8% of the placebo group.
Thomalla G, Simonsen CZ, Boutitie F, et al. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379(7):611-622.
High Levels of Cortisol Linked to Impaired Memory
Middle-aged people with high levels of cortisol in their blood have impaired memory, compared with people with average levels of cortisol, according to a study published online ahead of print October 24 in Neurology. Researchers identified 2,231 people with an average age of 49 who did not have dementia. At the start of the study, each participant underwent a psychologic exam and assessments of memory and thinking skills. Participants’ memory and thinking skills were tested again at an average of eight years later. Participants also provided a blood sample. After adjusting for age, sex, smoking, and BMI, researchers found that people with high levels of cortisol had lower scores on tests of memory and thinking skills, compared with people with normal levels of cortisol.
Echouffo-Tcheugui JB, Conner SC, Himali JJ, et al. Circulating cortisol and cognitive and structural brain measures: The Framingham Heart Study. Neurology. 2018 Oct 24 [Epub ahead of print].
Is Nusinersen Effective If Initiated Later?
Patients with spinal muscular atrophy type 1 (SMA1) may benefit from nusinersen when the therapy is initiated after age 7 months, according to a study published online ahead of print August 29 in Neurology. In this study, 33 patients with SMA1 received intrathecal nusinersen injections. Researchers evaluated patients before treatment and at two months and six months after treatment. All patients were alive and continuing treatment at six months. Median progress on the modified Hammersmith Infant Neurologic Examination Part 2 score was 1.5 points after six months of treatment. The need for respiratory support significantly increased over time. The results are consistent with those of a phase III trial in which patients with SMA1 received nusinersen before age 7 months, the researchers said.
Aragon-Gawinska K, Seferian AM, Daron A, et al. Nusinersen in spinal muscular atrophy type 1 patients older than 7 months: a cohort study. Neurology. 2018 Aug 29 [Epub ahead of print].
Pre-Eclampsia Linked to Dementia in Late Life
Pre-eclampsia is associated with an increased risk of dementia, particularly vascular dementia, according to a study published October 17 in BMJ. The study cohort consisted of 1,178,005 Danish women with at least one live birth or stillbirth between 1978 and 2015. Women with a history of pre-eclampsia had more than three times the risk of vascular dementia later in life, compared with women with no history of pre-eclampsia. The association with vascular dementia seemed to be stronger for late-onset disease than for early-onset disease. Adjustment for diabetes, hypertension, and cardiovascular disease attenuated the hazard ratios moderately. Sensitivity analyses suggested that BMI was unlikely to explain the association with vascular dementia. In contrast, modest associations were observed for Alzheimer’s disease and other or unspecified dementia.
Basit S, Wohlfahrt J, Boyd HA. Pre-eclampsia and risk of dementia later in life: nationwide cohort study. BMJ. 2018;363:k4109.
Does Antiepileptic Drug Clearance Change During Pregnancy?
In pregnant women, antiepileptic drug (AED) clearance significantly changes by the first trimester for levetiracetam and by the second trimester for oxcarbazepine and topiramate, according to a study published September 25 in Neurology. This prospective, observational study included 40 women with epilepsy who were planning to conceive or were fewer than 16 weeks pregnant and who chose to continue their AEDs during pregnancy. Drug clearance values were obtained by blood draw at baseline and during pregnancy. Mean maximal clearances were 1.71 times the baseline clearance for levetiracetam, 1.63 times the baseline clearance for oxcarbazepine, and 1.39 the baseline clearance for topiramate. In 15 women on AED monotherapy, increased seizure frequency in the first, second, and all trimesters was associated with a lower ratio to target concentration.
Voinescu PE, Park S, Chen LQ, et al. Antiepileptic drug clearances during pregnancy and clinical implications for women with epilepsy. Neurology. 2018;91(13):e1228-e1236.
Do Dextroamphetamine and Physical Therapy Improve Function After Stroke?
Compared with placebo, dextroamphetamine combined with physical therapy does not improve recovery of motor function after stroke, according to a study published online ahead of print August 27 in JAMA Neurology. This pilot, double-blind, block-randomized clinical trial included patients with cortical or subcortical ischemic stroke and moderate or severe motor deficits. A total of 64 participants were randomized to receive 10 mg of dextroamphetamine or placebo one hour before a one-hour physical therapy session every four days for six sessions, in addition to standard rehabilitation. The primary outcome was the difference between groups in change in Fugl-Meyer motor scores from baseline to three months after stroke. Treatment was not associated with differences in the primary outcome, secondary measures, or subgroups based on stroke location or baseline severity.
Goldstein LB, Lennihan L, Rabadi MJ, et al. Effect of dextroamphetamine on poststroke motor recovery: a randomized clinical trial. JAMA Neurol. 2018 Aug 27 [Epub ahead of print].
FDA Approves Tegsedi for hATTR in Adults
The FDA has approved Tegsedi (inotersen) for the treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis (hATTR) in adults. The approval is based on data from the phase III randomized, double-blind, placebo-controlled, 15-month, international NEURO-TTR study in 172 patients with hATTR amyloidosis with symptoms of polyneuropathy. In NEURO-TTR, Tegsedi demonstrated significant benefit, compared with placebo, in neuropathy and quality of life, as measured by the modified Neuropathy Impairment Score +7 and the Norfolk Quality of Life Questionnaire-Diabetic Neuropathy total score. Patients treated with Tegsedi experienced similar benefit regardless of subgroup, such as age, sex, race, region, Neuropathy Impairment Score, Val30Met mutation status, and disease stage. Akcea Therapeutics, an affiliate of Ionis Pharmaceuticals that is headquartered in Boston, markets Tegsedi.
Data Review Evaluates Nusinersen’s Efficacy in SMA
A panel has reviewed the evidence for nusinersen treatment of spinal muscular atrophy (SMA). The results were published online ahead of print October 12 in Neurology. The authors systematically reviewed clinical trials of nusinersen in patients with SMA and assigned level of evidence statements. Among four published clinical trials identified, three were rated above Class IV. There is Class I evidence that in term infants with SMA and two copies of SMN2, treatment with nusinersen started in children younger than 7 months results in a better motor milestone response and higher rates of event-free survival than sham control. There is Class I evidence that in children aged 2 to 12 with SMA symptom onset after age 6 months, nusinersen yields greater improvement in motor function at 15 months than sham control.
Michelson D, Ciafaloni E, Ashwal S, et al. Evidence in focus: nusinersen use in spinal muscular atrophy: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018 Oct 12 [Epub ahead of print].
Aortic Stiffness Predicts Incident Dementia
Arterial stiffness can predict a person’s likelihood of developing dementia, according to a study published October 16 in the Journal of Alzheimer’s Disease. Researchers analyzed the association between arterial stiffness and dementia among 356 older adults with an average age of 78 who were part of the Cardiovascular Health Study Cognition Study. Eligible participants were dementia-free when the study began in 1998. Investigators tested participants’ aortic stiffness with carotid-femoral pulse wave velocity. Participants also underwent MRI of their brains to measure signs of subclinical brain disease. The researchers found that participants with high carotid-femoral pulse wave velocity readings were 60% more likely to develop dementia during the following 15 years, compared with people with lower carotid-femoral pulse wave velocity values.
Cui C, Sekikawa A, Kuller LH, et al. Aortic stiffness is associated with increased risk of incident dementia in older adults. J Alzheimers Dis. 2018;66(1):297-306.
Early MoCA Score Predicts Long-Term Outcome After Stroke
Early cognitive testing with the Montreal Cognitive Assessment (MoCA) predicts long-term cognitive outcome, functional outcome, and mortality after stroke, according to a study published online ahead of print October 17 in Neurology. In this international study, 274 people with stroke were administered MoCA within a week of stroke onset. Participants were divided into two groups: people with no problems with thinking and memory skills and people with cognitive impairment. People who had cognitive impairment within one week of stroke were seven times more likely to die during the three years of the study than people without cognitive impairment. Furthermore, the survival rate for people with cognitive impairment after three years was 83%, and the rate was 97% for people who did not have early cognitive impairment.
Zietemann V, Georgakis MK, Dondaine T, et al. Early MoCA predicts long-term cognitive and functional outcome and mortality after stroke. Neurology. 2018 Oct 17 [Epub ahead of print].
Antiepileptic Drug Use Related to Increased Stroke Risk
In Alzheimer’s disease, antiepileptic drug (AED) use is related to an increased risk of stroke, according to a study published September 18 in the Journal of the American Heart Association. Investigators examined the Medication Use and Alzheimer’s Disease cohort, which included all Finnish people who received a clinically verified diagnosis of Alzheimer’s disease from 2005 to 2011. People with previous stroke were excluded. For each incident AED user, the investigators matched one nonuser according to sex, age, and time since Alzheimer’s disease diagnosis. Analyses were conducted with Cox proportional hazards models and inverse probability of treatment weighting. Compared with nonuse, AED use was associated with an increased risk of stroke, and the risk of stroke was strongest during the first 90 days of AED use (adjusted hazard ratio, 2.36).
Sarycheva T, Lavikainen P, Taipale H, et al. Antiepileptic drug use and the risk of stroke among community-dwelling people with Alzheimer disease: A matched cohort study. J Am Heart Assoc. 2018;7(18):e009742.
Thrombolysis Benefits Patients With Stroke
In patients with acute stroke with an unknown time of onset, IV alteplase guided by a mismatch between diffusion-weighted imaging and fluid-attenuated inversion recovery (FLAIR) in the region of ischemia results in a significantly better functional outcome and more intracranial hemorrhages at 90 days, compared with placebo, according to a study published August 16 in the New England Journal of Medicine. Researchers randomly assigned 254 participants to receive IV alteplase and 249 participants to receive placebo. Participants had an ischemic lesion on MRI diffusion-weighted imaging, but no parenchymal hyperintensity on FLAIR. A favorable outcome (ie, a score of 0 or 1 on the modified Rankin Scale at 90 days) occurred in 53.3% of the alteplase group versus 41.8% of the placebo group.
Thomalla G, Simonsen CZ, Boutitie F, et al. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379(7):611-622.
High Levels of Cortisol Linked to Impaired Memory
Middle-aged people with high levels of cortisol in their blood have impaired memory, compared with people with average levels of cortisol, according to a study published online ahead of print October 24 in Neurology. Researchers identified 2,231 people with an average age of 49 who did not have dementia. At the start of the study, each participant underwent a psychologic exam and assessments of memory and thinking skills. Participants’ memory and thinking skills were tested again at an average of eight years later. Participants also provided a blood sample. After adjusting for age, sex, smoking, and BMI, researchers found that people with high levels of cortisol had lower scores on tests of memory and thinking skills, compared with people with normal levels of cortisol.
Echouffo-Tcheugui JB, Conner SC, Himali JJ, et al. Circulating cortisol and cognitive and structural brain measures: The Framingham Heart Study. Neurology. 2018 Oct 24 [Epub ahead of print].
Is Nusinersen Effective If Initiated Later?
Patients with spinal muscular atrophy type 1 (SMA1) may benefit from nusinersen when the therapy is initiated after age 7 months, according to a study published online ahead of print August 29 in Neurology. In this study, 33 patients with SMA1 received intrathecal nusinersen injections. Researchers evaluated patients before treatment and at two months and six months after treatment. All patients were alive and continuing treatment at six months. Median progress on the modified Hammersmith Infant Neurologic Examination Part 2 score was 1.5 points after six months of treatment. The need for respiratory support significantly increased over time. The results are consistent with those of a phase III trial in which patients with SMA1 received nusinersen before age 7 months, the researchers said.
Aragon-Gawinska K, Seferian AM, Daron A, et al. Nusinersen in spinal muscular atrophy type 1 patients older than 7 months: a cohort study. Neurology. 2018 Aug 29 [Epub ahead of print].
Pre-Eclampsia Linked to Dementia in Late Life
Pre-eclampsia is associated with an increased risk of dementia, particularly vascular dementia, according to a study published October 17 in BMJ. The study cohort consisted of 1,178,005 Danish women with at least one live birth or stillbirth between 1978 and 2015. Women with a history of pre-eclampsia had more than three times the risk of vascular dementia later in life, compared with women with no history of pre-eclampsia. The association with vascular dementia seemed to be stronger for late-onset disease than for early-onset disease. Adjustment for diabetes, hypertension, and cardiovascular disease attenuated the hazard ratios moderately. Sensitivity analyses suggested that BMI was unlikely to explain the association with vascular dementia. In contrast, modest associations were observed for Alzheimer’s disease and other or unspecified dementia.
Basit S, Wohlfahrt J, Boyd HA. Pre-eclampsia and risk of dementia later in life: nationwide cohort study. BMJ. 2018;363:k4109.
Does Antiepileptic Drug Clearance Change During Pregnancy?
In pregnant women, antiepileptic drug (AED) clearance significantly changes by the first trimester for levetiracetam and by the second trimester for oxcarbazepine and topiramate, according to a study published September 25 in Neurology. This prospective, observational study included 40 women with epilepsy who were planning to conceive or were fewer than 16 weeks pregnant and who chose to continue their AEDs during pregnancy. Drug clearance values were obtained by blood draw at baseline and during pregnancy. Mean maximal clearances were 1.71 times the baseline clearance for levetiracetam, 1.63 times the baseline clearance for oxcarbazepine, and 1.39 the baseline clearance for topiramate. In 15 women on AED monotherapy, increased seizure frequency in the first, second, and all trimesters was associated with a lower ratio to target concentration.
Voinescu PE, Park S, Chen LQ, et al. Antiepileptic drug clearances during pregnancy and clinical implications for women with epilepsy. Neurology. 2018;91(13):e1228-e1236.
Do Dextroamphetamine and Physical Therapy Improve Function After Stroke?
Compared with placebo, dextroamphetamine combined with physical therapy does not improve recovery of motor function after stroke, according to a study published online ahead of print August 27 in JAMA Neurology. This pilot, double-blind, block-randomized clinical trial included patients with cortical or subcortical ischemic stroke and moderate or severe motor deficits. A total of 64 participants were randomized to receive 10 mg of dextroamphetamine or placebo one hour before a one-hour physical therapy session every four days for six sessions, in addition to standard rehabilitation. The primary outcome was the difference between groups in change in Fugl-Meyer motor scores from baseline to three months after stroke. Treatment was not associated with differences in the primary outcome, secondary measures, or subgroups based on stroke location or baseline severity.
Goldstein LB, Lennihan L, Rabadi MJ, et al. Effect of dextroamphetamine on poststroke motor recovery: a randomized clinical trial. JAMA Neurol. 2018 Aug 27 [Epub ahead of print].
FDA Approves Tegsedi for hATTR in Adults
The FDA has approved Tegsedi (inotersen) for the treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis (hATTR) in adults. The approval is based on data from the phase III randomized, double-blind, placebo-controlled, 15-month, international NEURO-TTR study in 172 patients with hATTR amyloidosis with symptoms of polyneuropathy. In NEURO-TTR, Tegsedi demonstrated significant benefit, compared with placebo, in neuropathy and quality of life, as measured by the modified Neuropathy Impairment Score +7 and the Norfolk Quality of Life Questionnaire-Diabetic Neuropathy total score. Patients treated with Tegsedi experienced similar benefit regardless of subgroup, such as age, sex, race, region, Neuropathy Impairment Score, Val30Met mutation status, and disease stage. Akcea Therapeutics, an affiliate of Ionis Pharmaceuticals that is headquartered in Boston, markets Tegsedi.
Data Review Evaluates Nusinersen’s Efficacy in SMA
A panel has reviewed the evidence for nusinersen treatment of spinal muscular atrophy (SMA). The results were published online ahead of print October 12 in Neurology. The authors systematically reviewed clinical trials of nusinersen in patients with SMA and assigned level of evidence statements. Among four published clinical trials identified, three were rated above Class IV. There is Class I evidence that in term infants with SMA and two copies of SMN2, treatment with nusinersen started in children younger than 7 months results in a better motor milestone response and higher rates of event-free survival than sham control. There is Class I evidence that in children aged 2 to 12 with SMA symptom onset after age 6 months, nusinersen yields greater improvement in motor function at 15 months than sham control.
Michelson D, Ciafaloni E, Ashwal S, et al. Evidence in focus: nusinersen use in spinal muscular atrophy: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018 Oct 12 [Epub ahead of print].
Aortic Stiffness Predicts Incident Dementia
Arterial stiffness can predict a person’s likelihood of developing dementia, according to a study published October 16 in the Journal of Alzheimer’s Disease. Researchers analyzed the association between arterial stiffness and dementia among 356 older adults with an average age of 78 who were part of the Cardiovascular Health Study Cognition Study. Eligible participants were dementia-free when the study began in 1998. Investigators tested participants’ aortic stiffness with carotid-femoral pulse wave velocity. Participants also underwent MRI of their brains to measure signs of subclinical brain disease. The researchers found that participants with high carotid-femoral pulse wave velocity readings were 60% more likely to develop dementia during the following 15 years, compared with people with lower carotid-femoral pulse wave velocity values.
Cui C, Sekikawa A, Kuller LH, et al. Aortic stiffness is associated with increased risk of incident dementia in older adults. J Alzheimers Dis. 2018;66(1):297-306.
Early MoCA Score Predicts Long-Term Outcome After Stroke
Early cognitive testing with the Montreal Cognitive Assessment (MoCA) predicts long-term cognitive outcome, functional outcome, and mortality after stroke, according to a study published online ahead of print October 17 in Neurology. In this international study, 274 people with stroke were administered MoCA within a week of stroke onset. Participants were divided into two groups: people with no problems with thinking and memory skills and people with cognitive impairment. People who had cognitive impairment within one week of stroke were seven times more likely to die during the three years of the study than people without cognitive impairment. Furthermore, the survival rate for people with cognitive impairment after three years was 83%, and the rate was 97% for people who did not have early cognitive impairment.
Zietemann V, Georgakis MK, Dondaine T, et al. Early MoCA predicts long-term cognitive and functional outcome and mortality after stroke. Neurology. 2018 Oct 17 [Epub ahead of print].
Antiepileptic Drug Use Related to Increased Stroke Risk
In Alzheimer’s disease, antiepileptic drug (AED) use is related to an increased risk of stroke, according to a study published September 18 in the Journal of the American Heart Association. Investigators examined the Medication Use and Alzheimer’s Disease cohort, which included all Finnish people who received a clinically verified diagnosis of Alzheimer’s disease from 2005 to 2011. People with previous stroke were excluded. For each incident AED user, the investigators matched one nonuser according to sex, age, and time since Alzheimer’s disease diagnosis. Analyses were conducted with Cox proportional hazards models and inverse probability of treatment weighting. Compared with nonuse, AED use was associated with an increased risk of stroke, and the risk of stroke was strongest during the first 90 days of AED use (adjusted hazard ratio, 2.36).
Sarycheva T, Lavikainen P, Taipale H, et al. Antiepileptic drug use and the risk of stroke among community-dwelling people with Alzheimer disease: A matched cohort study. J Am Heart Assoc. 2018;7(18):e009742.
Psychopharmacology 3.0
There is little doubt that the psychopharmacology revolution has been transformational for psychiatry and is also credited for sparking the momentous neuroscience advances of the past half century.
The field of psychiatry, dominated by Freudian psychology for decades, radically evolved from psychoanalysis to pharmacotherapy with the discovery that serious mental disorders are treatable with medications, thus dispensing with the couch.
Prior to 1952, the prevailing dogma was that “madness is irreversible.” That’s why millions of patients with various psychiatric disorders were locked up in institutions, which added to the stigma of mental illness. Then came the first antipsychotic drug, chlorpromazine, which “magically” eliminated the delusions and hallucinations of patients who had been hospitalized for years. That serendipitous and historic discovery was as transformational for psychiatry as penicillin was for infections (yet inexplicably, only the discovery of penicillin received a Nobel Prize). Most people today do not know that before chlorpromazine, 50% of all hospital beds in the U.S. were occupied by psychiatric patients. The massive shuttering of state hospitals in the 1970s and ’80s was a direct consequence of the widespread use of chlorpromazine and its cohort of first-generation antipsychotics (FGAs).
That was Psychopharmacology 1.0, spanning the period 1952 to 1987. It included dozens of FGAs belonging to 6 classes: phenothiazines, thioxanthenes, butyrophenones, dibenzazepines, dihydroindolones, and dibenzodiazepines. Psychopharmacology 1.0 also included monoamine oxidase inhibitors and tricyclic antidepressants for depression, and lithium for bipolar mania. Ironically, clozapine, the incognito seed template of the second-generation antipsychotic (SGA) class, was synthesized in 1959 with the early wave of FGAs, and launched in Europe in 1972, only to be withdrawn in 1974 due to agranulocytosis-induced deaths not recognized during the clinical trials.
The late 1980s ushered in Psychopharmacology 2.0, which was also transformative. It began in 1987 with the introduction of fluoxetine, the first selective serotonin receptor inhibitor. Then clozapine was resurrected in 1988 as the first FDA-approved drug for refractory schizophrenia. Being the first SGA (no acute extrapyramidal side effects at all, in contrast to all FGAs), it became the “mechanistic model” for all other SGA agents, which were introduced starting in 1993. All SGAs were designed by pharmaceutical companies’ medicinal chemists to mimic clozapine’s receptor profile: far stronger affinity to serotonin 5HT-2A receptors than to dopamine D2 receptors. Three partial agonists and several heterocyclic antidepressants were also introduced during this 2.0 era, which continued until approximately 2017. Of the 11 SGAs that were initially approved for schizophrenia, 7 also were approved for bipolar mania, and 2 received an FDA indication for bipolar depression, thus addressing a glaring unmet need.
Psychopharmacology 3.0 has already begun. Its seeds started sprouting over the past few years with the landmark studies of intravenous ketamine, which was demonstrated to reverse severe and refractory depression and suicidal urges within hours of injection. The first ketamine product, esketamine, an intranasal formulation, is expected to be approved by the FDA soon. In the same vein, other rapid-acting antidepressants, a welcome paradigm shift, are being developed, including IV scopolamine, IV rapastinel, and inhalable nitrous oxide.
Three novel and important pharmacologic agents have arrived in this 3.0 era:
- Pimavanserin, a serotonin 5HT-2A inverse agonist, the first and only non-dopamine–blocking antipsychotic approved by the FDA for the delusions and hallucinations of Parkinson’s disease psychosis. It is currently in clinical trials for schizophrenia and Alzheimer’s disease psychosis (for which nothing is yet approved).
- Valbenazine, the first drug approved for tardive dyskinesia (TD), the treatment of which had been elusive and remained a huge unmet need for 60 years. Its novel mechanism of action is inhibition of vesicular monoamine transporter 2 (VMAT2), which reduces the putative dopamine supersensitivity of TD.
- Deutetrabenazine, which was also approved for TD a few months after valbenazine, and has the same mechanism of action. It also was approved for Huntington’s chorea.
Continue to: Another important feature...
Another important feature of Psychopharmacology 3.0 is the repurposing of hallucinogens into novel therapies for posttraumatic stress disorder, anxiety, and depression.1 The opioid system is being recognized as another key player in depression, with many studies showing buprenorphine has antidepressant and anti-suicidal properties2 and the recent finding that pre-treatment with naloxone blocks the rapid antidepressive effects of ketamine.3 This finding casts doubt on the notion that the antidepressant mechanism of action of ketamine is solely mediated via its antagonism of the glutamate N-methyl-
These early developments in Psychopharmacology 3.0 augur well for the future. Companies in the pharmaceutical industry (which are hated by many, and even demonized and kept at arm’s length by major medical schools) are, in fact, the only entities in the world that develop new medications for psychiatric disorders, 82% of which still have no FDA-approved drug.4 Psychiatric researchers and clinicians should collaborate and advise the pharmaceutical companies about the urgent or unmet needs of psychiatric patients so they can target those unmet needs with their massive R&D resources.
In that spirit, here is my wish list of therapeutic targets that I hope will emerge during the Psychopharmacology 3.0 era and beyond:
1. New mechanisms of action for antipsychotics, based on emerging neurobiological research in schizophrenia and related psychoses, such as:
- Inhibit microglia activation
- Repair mitochondrial dysfunction
- Modulate the hypofunctional NMDA receptors
- Inhibit apoptosis
- Enhance neurogenesis
- Repair myelin pathology
- Inhibit neuroinflammation and oxidative stress
- Increase neurotropic growth factors
- Neurosteroid therapies (including estrogen)
- Exploit the microbiome influence on both the enteric and cephalic brains
2. Long-acting injectable antidepressants and mood stabilizers, because there is a malignant transformation into treatment-resistance in mood disorders after recurrent episodes due to nonadherence.5
3. Treatments for personality disorders, especially borderline and antisocial personality disorders.
4. An effective treatment for alcoholism.
5. Pharmacotherapy for aggression.
6. Vaccines for substance use.
7. Stage-specific pharmacotherapies (because the neurobiology of prodromal, first-episode, and multiple-episode patients have been shown to be quite different).
8. Drugs for epigenetic modulation to inhibit risk genes and to over-express protective genes.
It may take decades and hundreds of billions (even trillions) of R&D investment to accomplish the above, but I remain excited about the prospects of astounding psychopharmacologic advances to treat the disorders of the mind. Precision psychiatry advances will also expedite the selection of the right medication for each patient by employing predictive biomarkers. Breakthrough methodologies, such as pluripotent stem cells, opto-genetics, and clustered regularly interspaced short palindromic repeats (CRISPR), promise to revolutionize the biology, diagnosis, treatment, and prevention of various neuropsychiatric disorders.
The future of psychopharmacology is bright, if adequate resources are invested. The current direct and indirect costs of mental disorders and addictions are in the hundreds of billions of dollars annually. Only intensive research and disruptive discoveries will have the salutary dual effect of healing disease and reducing the economic burden of neuropsychiatric disorders. Psychopharmacology 3.0 advances, along with nonpharmacologic therapies such as neuromodulation (electroconvulsive therapy, transcranial magnetic stimulation, vagus nerve stimulation, and a dozen other techniques in development). Together with the indispensable evidence-based psychotherapies such as cognitive-behavioral therapy, dialectical behavior therapy, and interpersonal psychotherapy, psychopharmacology represents the leading edge of progress in psychiatric treatment. The psychiatrists of 1952 could only fantasize about what has since become a reality in healing ailing minds.
To comment on this editorial or other topics of interest: [email protected]
1. Nasrallah, HA. Maddening therapies: How hallucinogens morphed into novel treatments. Current Psychiatry. 2017;16(1):19-21.
2. Serafini G, Adavastro G, Canepa G, et al. The efficacy of buprenorphine in major depression, treatment-resistant depression and suicidal behavior: a systematic review. Int J Mol Sci. 2018;19(8). doi: 10.3390/ijms19082410.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018. doi: 10.1176/appi.ajp.2018.18020138. [Epub ahead of print].
4. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders. The majority of psychiatric diagnoses have no approved drug. Asian J Psychiatr. 2009;2(1):29-36.
5. Post RM. Preventing the malignant transformation of bipolar disorder. JAMA. 2018;319(12):1197-1198.
There is little doubt that the psychopharmacology revolution has been transformational for psychiatry and is also credited for sparking the momentous neuroscience advances of the past half century.
The field of psychiatry, dominated by Freudian psychology for decades, radically evolved from psychoanalysis to pharmacotherapy with the discovery that serious mental disorders are treatable with medications, thus dispensing with the couch.
Prior to 1952, the prevailing dogma was that “madness is irreversible.” That’s why millions of patients with various psychiatric disorders were locked up in institutions, which added to the stigma of mental illness. Then came the first antipsychotic drug, chlorpromazine, which “magically” eliminated the delusions and hallucinations of patients who had been hospitalized for years. That serendipitous and historic discovery was as transformational for psychiatry as penicillin was for infections (yet inexplicably, only the discovery of penicillin received a Nobel Prize). Most people today do not know that before chlorpromazine, 50% of all hospital beds in the U.S. were occupied by psychiatric patients. The massive shuttering of state hospitals in the 1970s and ’80s was a direct consequence of the widespread use of chlorpromazine and its cohort of first-generation antipsychotics (FGAs).
That was Psychopharmacology 1.0, spanning the period 1952 to 1987. It included dozens of FGAs belonging to 6 classes: phenothiazines, thioxanthenes, butyrophenones, dibenzazepines, dihydroindolones, and dibenzodiazepines. Psychopharmacology 1.0 also included monoamine oxidase inhibitors and tricyclic antidepressants for depression, and lithium for bipolar mania. Ironically, clozapine, the incognito seed template of the second-generation antipsychotic (SGA) class, was synthesized in 1959 with the early wave of FGAs, and launched in Europe in 1972, only to be withdrawn in 1974 due to agranulocytosis-induced deaths not recognized during the clinical trials.
The late 1980s ushered in Psychopharmacology 2.0, which was also transformative. It began in 1987 with the introduction of fluoxetine, the first selective serotonin receptor inhibitor. Then clozapine was resurrected in 1988 as the first FDA-approved drug for refractory schizophrenia. Being the first SGA (no acute extrapyramidal side effects at all, in contrast to all FGAs), it became the “mechanistic model” for all other SGA agents, which were introduced starting in 1993. All SGAs were designed by pharmaceutical companies’ medicinal chemists to mimic clozapine’s receptor profile: far stronger affinity to serotonin 5HT-2A receptors than to dopamine D2 receptors. Three partial agonists and several heterocyclic antidepressants were also introduced during this 2.0 era, which continued until approximately 2017. Of the 11 SGAs that were initially approved for schizophrenia, 7 also were approved for bipolar mania, and 2 received an FDA indication for bipolar depression, thus addressing a glaring unmet need.
Psychopharmacology 3.0 has already begun. Its seeds started sprouting over the past few years with the landmark studies of intravenous ketamine, which was demonstrated to reverse severe and refractory depression and suicidal urges within hours of injection. The first ketamine product, esketamine, an intranasal formulation, is expected to be approved by the FDA soon. In the same vein, other rapid-acting antidepressants, a welcome paradigm shift, are being developed, including IV scopolamine, IV rapastinel, and inhalable nitrous oxide.
Three novel and important pharmacologic agents have arrived in this 3.0 era:
- Pimavanserin, a serotonin 5HT-2A inverse agonist, the first and only non-dopamine–blocking antipsychotic approved by the FDA for the delusions and hallucinations of Parkinson’s disease psychosis. It is currently in clinical trials for schizophrenia and Alzheimer’s disease psychosis (for which nothing is yet approved).
- Valbenazine, the first drug approved for tardive dyskinesia (TD), the treatment of which had been elusive and remained a huge unmet need for 60 years. Its novel mechanism of action is inhibition of vesicular monoamine transporter 2 (VMAT2), which reduces the putative dopamine supersensitivity of TD.
- Deutetrabenazine, which was also approved for TD a few months after valbenazine, and has the same mechanism of action. It also was approved for Huntington’s chorea.
Continue to: Another important feature...
Another important feature of Psychopharmacology 3.0 is the repurposing of hallucinogens into novel therapies for posttraumatic stress disorder, anxiety, and depression.1 The opioid system is being recognized as another key player in depression, with many studies showing buprenorphine has antidepressant and anti-suicidal properties2 and the recent finding that pre-treatment with naloxone blocks the rapid antidepressive effects of ketamine.3 This finding casts doubt on the notion that the antidepressant mechanism of action of ketamine is solely mediated via its antagonism of the glutamate N-methyl-
These early developments in Psychopharmacology 3.0 augur well for the future. Companies in the pharmaceutical industry (which are hated by many, and even demonized and kept at arm’s length by major medical schools) are, in fact, the only entities in the world that develop new medications for psychiatric disorders, 82% of which still have no FDA-approved drug.4 Psychiatric researchers and clinicians should collaborate and advise the pharmaceutical companies about the urgent or unmet needs of psychiatric patients so they can target those unmet needs with their massive R&D resources.
In that spirit, here is my wish list of therapeutic targets that I hope will emerge during the Psychopharmacology 3.0 era and beyond:
1. New mechanisms of action for antipsychotics, based on emerging neurobiological research in schizophrenia and related psychoses, such as:
- Inhibit microglia activation
- Repair mitochondrial dysfunction
- Modulate the hypofunctional NMDA receptors
- Inhibit apoptosis
- Enhance neurogenesis
- Repair myelin pathology
- Inhibit neuroinflammation and oxidative stress
- Increase neurotropic growth factors
- Neurosteroid therapies (including estrogen)
- Exploit the microbiome influence on both the enteric and cephalic brains
2. Long-acting injectable antidepressants and mood stabilizers, because there is a malignant transformation into treatment-resistance in mood disorders after recurrent episodes due to nonadherence.5
3. Treatments for personality disorders, especially borderline and antisocial personality disorders.
4. An effective treatment for alcoholism.
5. Pharmacotherapy for aggression.
6. Vaccines for substance use.
7. Stage-specific pharmacotherapies (because the neurobiology of prodromal, first-episode, and multiple-episode patients have been shown to be quite different).
8. Drugs for epigenetic modulation to inhibit risk genes and to over-express protective genes.
It may take decades and hundreds of billions (even trillions) of R&D investment to accomplish the above, but I remain excited about the prospects of astounding psychopharmacologic advances to treat the disorders of the mind. Precision psychiatry advances will also expedite the selection of the right medication for each patient by employing predictive biomarkers. Breakthrough methodologies, such as pluripotent stem cells, opto-genetics, and clustered regularly interspaced short palindromic repeats (CRISPR), promise to revolutionize the biology, diagnosis, treatment, and prevention of various neuropsychiatric disorders.
The future of psychopharmacology is bright, if adequate resources are invested. The current direct and indirect costs of mental disorders and addictions are in the hundreds of billions of dollars annually. Only intensive research and disruptive discoveries will have the salutary dual effect of healing disease and reducing the economic burden of neuropsychiatric disorders. Psychopharmacology 3.0 advances, along with nonpharmacologic therapies such as neuromodulation (electroconvulsive therapy, transcranial magnetic stimulation, vagus nerve stimulation, and a dozen other techniques in development). Together with the indispensable evidence-based psychotherapies such as cognitive-behavioral therapy, dialectical behavior therapy, and interpersonal psychotherapy, psychopharmacology represents the leading edge of progress in psychiatric treatment. The psychiatrists of 1952 could only fantasize about what has since become a reality in healing ailing minds.
To comment on this editorial or other topics of interest: [email protected]
There is little doubt that the psychopharmacology revolution has been transformational for psychiatry and is also credited for sparking the momentous neuroscience advances of the past half century.
The field of psychiatry, dominated by Freudian psychology for decades, radically evolved from psychoanalysis to pharmacotherapy with the discovery that serious mental disorders are treatable with medications, thus dispensing with the couch.
Prior to 1952, the prevailing dogma was that “madness is irreversible.” That’s why millions of patients with various psychiatric disorders were locked up in institutions, which added to the stigma of mental illness. Then came the first antipsychotic drug, chlorpromazine, which “magically” eliminated the delusions and hallucinations of patients who had been hospitalized for years. That serendipitous and historic discovery was as transformational for psychiatry as penicillin was for infections (yet inexplicably, only the discovery of penicillin received a Nobel Prize). Most people today do not know that before chlorpromazine, 50% of all hospital beds in the U.S. were occupied by psychiatric patients. The massive shuttering of state hospitals in the 1970s and ’80s was a direct consequence of the widespread use of chlorpromazine and its cohort of first-generation antipsychotics (FGAs).
That was Psychopharmacology 1.0, spanning the period 1952 to 1987. It included dozens of FGAs belonging to 6 classes: phenothiazines, thioxanthenes, butyrophenones, dibenzazepines, dihydroindolones, and dibenzodiazepines. Psychopharmacology 1.0 also included monoamine oxidase inhibitors and tricyclic antidepressants for depression, and lithium for bipolar mania. Ironically, clozapine, the incognito seed template of the second-generation antipsychotic (SGA) class, was synthesized in 1959 with the early wave of FGAs, and launched in Europe in 1972, only to be withdrawn in 1974 due to agranulocytosis-induced deaths not recognized during the clinical trials.
The late 1980s ushered in Psychopharmacology 2.0, which was also transformative. It began in 1987 with the introduction of fluoxetine, the first selective serotonin receptor inhibitor. Then clozapine was resurrected in 1988 as the first FDA-approved drug for refractory schizophrenia. Being the first SGA (no acute extrapyramidal side effects at all, in contrast to all FGAs), it became the “mechanistic model” for all other SGA agents, which were introduced starting in 1993. All SGAs were designed by pharmaceutical companies’ medicinal chemists to mimic clozapine’s receptor profile: far stronger affinity to serotonin 5HT-2A receptors than to dopamine D2 receptors. Three partial agonists and several heterocyclic antidepressants were also introduced during this 2.0 era, which continued until approximately 2017. Of the 11 SGAs that were initially approved for schizophrenia, 7 also were approved for bipolar mania, and 2 received an FDA indication for bipolar depression, thus addressing a glaring unmet need.
Psychopharmacology 3.0 has already begun. Its seeds started sprouting over the past few years with the landmark studies of intravenous ketamine, which was demonstrated to reverse severe and refractory depression and suicidal urges within hours of injection. The first ketamine product, esketamine, an intranasal formulation, is expected to be approved by the FDA soon. In the same vein, other rapid-acting antidepressants, a welcome paradigm shift, are being developed, including IV scopolamine, IV rapastinel, and inhalable nitrous oxide.
Three novel and important pharmacologic agents have arrived in this 3.0 era:
- Pimavanserin, a serotonin 5HT-2A inverse agonist, the first and only non-dopamine–blocking antipsychotic approved by the FDA for the delusions and hallucinations of Parkinson’s disease psychosis. It is currently in clinical trials for schizophrenia and Alzheimer’s disease psychosis (for which nothing is yet approved).
- Valbenazine, the first drug approved for tardive dyskinesia (TD), the treatment of which had been elusive and remained a huge unmet need for 60 years. Its novel mechanism of action is inhibition of vesicular monoamine transporter 2 (VMAT2), which reduces the putative dopamine supersensitivity of TD.
- Deutetrabenazine, which was also approved for TD a few months after valbenazine, and has the same mechanism of action. It also was approved for Huntington’s chorea.
Continue to: Another important feature...
Another important feature of Psychopharmacology 3.0 is the repurposing of hallucinogens into novel therapies for posttraumatic stress disorder, anxiety, and depression.1 The opioid system is being recognized as another key player in depression, with many studies showing buprenorphine has antidepressant and anti-suicidal properties2 and the recent finding that pre-treatment with naloxone blocks the rapid antidepressive effects of ketamine.3 This finding casts doubt on the notion that the antidepressant mechanism of action of ketamine is solely mediated via its antagonism of the glutamate N-methyl-
These early developments in Psychopharmacology 3.0 augur well for the future. Companies in the pharmaceutical industry (which are hated by many, and even demonized and kept at arm’s length by major medical schools) are, in fact, the only entities in the world that develop new medications for psychiatric disorders, 82% of which still have no FDA-approved drug.4 Psychiatric researchers and clinicians should collaborate and advise the pharmaceutical companies about the urgent or unmet needs of psychiatric patients so they can target those unmet needs with their massive R&D resources.
In that spirit, here is my wish list of therapeutic targets that I hope will emerge during the Psychopharmacology 3.0 era and beyond:
1. New mechanisms of action for antipsychotics, based on emerging neurobiological research in schizophrenia and related psychoses, such as:
- Inhibit microglia activation
- Repair mitochondrial dysfunction
- Modulate the hypofunctional NMDA receptors
- Inhibit apoptosis
- Enhance neurogenesis
- Repair myelin pathology
- Inhibit neuroinflammation and oxidative stress
- Increase neurotropic growth factors
- Neurosteroid therapies (including estrogen)
- Exploit the microbiome influence on both the enteric and cephalic brains
2. Long-acting injectable antidepressants and mood stabilizers, because there is a malignant transformation into treatment-resistance in mood disorders after recurrent episodes due to nonadherence.5
3. Treatments for personality disorders, especially borderline and antisocial personality disorders.
4. An effective treatment for alcoholism.
5. Pharmacotherapy for aggression.
6. Vaccines for substance use.
7. Stage-specific pharmacotherapies (because the neurobiology of prodromal, first-episode, and multiple-episode patients have been shown to be quite different).
8. Drugs for epigenetic modulation to inhibit risk genes and to over-express protective genes.
It may take decades and hundreds of billions (even trillions) of R&D investment to accomplish the above, but I remain excited about the prospects of astounding psychopharmacologic advances to treat the disorders of the mind. Precision psychiatry advances will also expedite the selection of the right medication for each patient by employing predictive biomarkers. Breakthrough methodologies, such as pluripotent stem cells, opto-genetics, and clustered regularly interspaced short palindromic repeats (CRISPR), promise to revolutionize the biology, diagnosis, treatment, and prevention of various neuropsychiatric disorders.
The future of psychopharmacology is bright, if adequate resources are invested. The current direct and indirect costs of mental disorders and addictions are in the hundreds of billions of dollars annually. Only intensive research and disruptive discoveries will have the salutary dual effect of healing disease and reducing the economic burden of neuropsychiatric disorders. Psychopharmacology 3.0 advances, along with nonpharmacologic therapies such as neuromodulation (electroconvulsive therapy, transcranial magnetic stimulation, vagus nerve stimulation, and a dozen other techniques in development). Together with the indispensable evidence-based psychotherapies such as cognitive-behavioral therapy, dialectical behavior therapy, and interpersonal psychotherapy, psychopharmacology represents the leading edge of progress in psychiatric treatment. The psychiatrists of 1952 could only fantasize about what has since become a reality in healing ailing minds.
To comment on this editorial or other topics of interest: [email protected]
1. Nasrallah, HA. Maddening therapies: How hallucinogens morphed into novel treatments. Current Psychiatry. 2017;16(1):19-21.
2. Serafini G, Adavastro G, Canepa G, et al. The efficacy of buprenorphine in major depression, treatment-resistant depression and suicidal behavior: a systematic review. Int J Mol Sci. 2018;19(8). doi: 10.3390/ijms19082410.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018. doi: 10.1176/appi.ajp.2018.18020138. [Epub ahead of print].
4. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders. The majority of psychiatric diagnoses have no approved drug. Asian J Psychiatr. 2009;2(1):29-36.
5. Post RM. Preventing the malignant transformation of bipolar disorder. JAMA. 2018;319(12):1197-1198.
1. Nasrallah, HA. Maddening therapies: How hallucinogens morphed into novel treatments. Current Psychiatry. 2017;16(1):19-21.
2. Serafini G, Adavastro G, Canepa G, et al. The efficacy of buprenorphine in major depression, treatment-resistant depression and suicidal behavior: a systematic review. Int J Mol Sci. 2018;19(8). doi: 10.3390/ijms19082410.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018. doi: 10.1176/appi.ajp.2018.18020138. [Epub ahead of print].
4. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders. The majority of psychiatric diagnoses have no approved drug. Asian J Psychiatr. 2009;2(1):29-36.
5. Post RM. Preventing the malignant transformation of bipolar disorder. JAMA. 2018;319(12):1197-1198.
Can the 2017 McDonald Criteria Diagnose MS in Children?
Applying the new criteria to children may allow earlier diagnosis than the 2010 criteria do.
BERLIN—Applying the 2017 McDonald criteria to children is feasible and allows an earlier diagnosis of multiple sclerosis (MS), according to data described at ECTRIMS 2018. The presence of oligoclonal bands and negativity of myelin oligodendrocyte glycoprotein (MOG)-IgG are informative biomarkers when evaluating the risk of MS in children with a first demyelinating event, said the authors.
Few researchers have examined the application of the revised 2017 McDonald criteria to children. In addition, the role of biomarkers in confirming or ruling out a diagnosis of MS is uncertain. Georgina Arrambide, MD, PhD, a neurologist at Vall d’Hebron University Hospital in Barcelona, and colleagues conducted a prospective cohort study to compare the application of the 2017 and 2010 McDonald criteria in children with a first demyelinating event and to evaluate the contribution of biomarkers (ie, oligoclonal bands, aquaporin 4 [AQP4], and MOG-IgG) in predicting their clinical course.
The investigators followed a cohort of children from a first demyelinating episode. Serum and CSF samples were taken at fewer than three months from disease onset, and the researchers examined them for oligoclonal bands, AQP4, and MOG-IgG. Dr. Arrambide and colleagues also systematically analyzed 37 MRI items while blinded to clinical and immunologic data. They evaluated the proportion of patients fulfilling the 2010 and the 2017 McDonald criteria at baseline and the contribution of biomarkers to predicting the clinical course.
Clinical and baseline MRI data were available for 55 children (45% female) with a median age of 6.2. About 67% of children were younger than 12. At baseline, the diagnoses according to 2010 McDonald and 2013 International Pediatric MS Study Group criteria were acute disseminated encephalomyelitis (ADEM, 28 patients), MS (three patients), classical clinically isolated syndrome (CIS, 17 patients), radiologically isolated syndrome (RIS, one patient), and other (MRI suggestive of ADEM but without encephalopathy, six patients).
After a median follow-up of 16 months, the diagnosis changed in 10 patients because of clinical (five patients) or radiologic (five patients) activity. Seven patients evolved to MS (six patients with classical CIS and one with RIS), one to relapsing optic neuritis (ON), one to ADEM-ON (ie, ADEM followed by ON), and one to neuromyelitis optica spectrum disorder. None of the seven patients with available baseline samples who evolved to MS had MOG-IgG, compared with 22 of 38 (58%) patients who did not evolve. None had AQP4. In contrast, five of seven (71%) patients with MS had positive oligoclonal bands, compared with one of 26 (4%) who did not develop MS.
At baseline, three of 10 patients fulfilled the 2010 McDonald criteria. Four additional patients fulfilled the 2017 MS criteria thanks to the contribution of oligoclonal bands.
Applying the new criteria to children may allow earlier diagnosis than the 2010 criteria do.
Applying the new criteria to children may allow earlier diagnosis than the 2010 criteria do.
BERLIN—Applying the 2017 McDonald criteria to children is feasible and allows an earlier diagnosis of multiple sclerosis (MS), according to data described at ECTRIMS 2018. The presence of oligoclonal bands and negativity of myelin oligodendrocyte glycoprotein (MOG)-IgG are informative biomarkers when evaluating the risk of MS in children with a first demyelinating event, said the authors.
Few researchers have examined the application of the revised 2017 McDonald criteria to children. In addition, the role of biomarkers in confirming or ruling out a diagnosis of MS is uncertain. Georgina Arrambide, MD, PhD, a neurologist at Vall d’Hebron University Hospital in Barcelona, and colleagues conducted a prospective cohort study to compare the application of the 2017 and 2010 McDonald criteria in children with a first demyelinating event and to evaluate the contribution of biomarkers (ie, oligoclonal bands, aquaporin 4 [AQP4], and MOG-IgG) in predicting their clinical course.
The investigators followed a cohort of children from a first demyelinating episode. Serum and CSF samples were taken at fewer than three months from disease onset, and the researchers examined them for oligoclonal bands, AQP4, and MOG-IgG. Dr. Arrambide and colleagues also systematically analyzed 37 MRI items while blinded to clinical and immunologic data. They evaluated the proportion of patients fulfilling the 2010 and the 2017 McDonald criteria at baseline and the contribution of biomarkers to predicting the clinical course.
Clinical and baseline MRI data were available for 55 children (45% female) with a median age of 6.2. About 67% of children were younger than 12. At baseline, the diagnoses according to 2010 McDonald and 2013 International Pediatric MS Study Group criteria were acute disseminated encephalomyelitis (ADEM, 28 patients), MS (three patients), classical clinically isolated syndrome (CIS, 17 patients), radiologically isolated syndrome (RIS, one patient), and other (MRI suggestive of ADEM but without encephalopathy, six patients).
After a median follow-up of 16 months, the diagnosis changed in 10 patients because of clinical (five patients) or radiologic (five patients) activity. Seven patients evolved to MS (six patients with classical CIS and one with RIS), one to relapsing optic neuritis (ON), one to ADEM-ON (ie, ADEM followed by ON), and one to neuromyelitis optica spectrum disorder. None of the seven patients with available baseline samples who evolved to MS had MOG-IgG, compared with 22 of 38 (58%) patients who did not evolve. None had AQP4. In contrast, five of seven (71%) patients with MS had positive oligoclonal bands, compared with one of 26 (4%) who did not develop MS.
At baseline, three of 10 patients fulfilled the 2010 McDonald criteria. Four additional patients fulfilled the 2017 MS criteria thanks to the contribution of oligoclonal bands.
BERLIN—Applying the 2017 McDonald criteria to children is feasible and allows an earlier diagnosis of multiple sclerosis (MS), according to data described at ECTRIMS 2018. The presence of oligoclonal bands and negativity of myelin oligodendrocyte glycoprotein (MOG)-IgG are informative biomarkers when evaluating the risk of MS in children with a first demyelinating event, said the authors.
Few researchers have examined the application of the revised 2017 McDonald criteria to children. In addition, the role of biomarkers in confirming or ruling out a diagnosis of MS is uncertain. Georgina Arrambide, MD, PhD, a neurologist at Vall d’Hebron University Hospital in Barcelona, and colleagues conducted a prospective cohort study to compare the application of the 2017 and 2010 McDonald criteria in children with a first demyelinating event and to evaluate the contribution of biomarkers (ie, oligoclonal bands, aquaporin 4 [AQP4], and MOG-IgG) in predicting their clinical course.
The investigators followed a cohort of children from a first demyelinating episode. Serum and CSF samples were taken at fewer than three months from disease onset, and the researchers examined them for oligoclonal bands, AQP4, and MOG-IgG. Dr. Arrambide and colleagues also systematically analyzed 37 MRI items while blinded to clinical and immunologic data. They evaluated the proportion of patients fulfilling the 2010 and the 2017 McDonald criteria at baseline and the contribution of biomarkers to predicting the clinical course.
Clinical and baseline MRI data were available for 55 children (45% female) with a median age of 6.2. About 67% of children were younger than 12. At baseline, the diagnoses according to 2010 McDonald and 2013 International Pediatric MS Study Group criteria were acute disseminated encephalomyelitis (ADEM, 28 patients), MS (three patients), classical clinically isolated syndrome (CIS, 17 patients), radiologically isolated syndrome (RIS, one patient), and other (MRI suggestive of ADEM but without encephalopathy, six patients).
After a median follow-up of 16 months, the diagnosis changed in 10 patients because of clinical (five patients) or radiologic (five patients) activity. Seven patients evolved to MS (six patients with classical CIS and one with RIS), one to relapsing optic neuritis (ON), one to ADEM-ON (ie, ADEM followed by ON), and one to neuromyelitis optica spectrum disorder. None of the seven patients with available baseline samples who evolved to MS had MOG-IgG, compared with 22 of 38 (58%) patients who did not evolve. None had AQP4. In contrast, five of seven (71%) patients with MS had positive oligoclonal bands, compared with one of 26 (4%) who did not develop MS.
At baseline, three of 10 patients fulfilled the 2010 McDonald criteria. Four additional patients fulfilled the 2017 MS criteria thanks to the contribution of oligoclonal bands.
The art of manipulation: Simplifying hysterectomy by preparing the learner

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:
- Vaginal and bilateral thigh removal of a transobturator sling
- Morcellation at the time of vaginal hysterectomy
- Surgical management of non-tubal ectopic pregnancies
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:
- Vaginal and bilateral thigh removal of a transobturator sling
- Morcellation at the time of vaginal hysterectomy
- Surgical management of non-tubal ectopic pregnancies
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:
- Vaginal and bilateral thigh removal of a transobturator sling
- Morcellation at the time of vaginal hysterectomy
- Surgical management of non-tubal ectopic pregnancies
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
This video is brought to you by
Pembrolizumab extends survival of head and neck cancer
MUNICH – In patients with recurrent or metastatic head and neck squamous cell carcinoma expressing programmed death ligand-1 (PDL-1), the immune checkpoint inhibitor pembrolizumab alone or in combination with chemotherapy improved overall survival, compared with the EXTREME chemotherapy regimen, reported investigators in the Keynote 048 trial.
Overall survival (OS) among patients with a PD-L1 combined positive score (CPS) of 20 or greater treated with pembrolizumab (Keytruda) monotherapy was 14.9 months compared with 10.7 months for patients treated with the EXTREME regimen, a combination of cetuximab (Erbitux), carboplatin or cisplatin, and 5-fluorouracil.
A similar overall survival benefit was seen in patients with a CPS of 1 or greater, and in the total population of patients treated with pembrolizumab plus chemotherapy followed by pembrolizumab maintenance compared with EXTREME chemotherapy, Barbara Burtness, MD, of Yale Cancer Center, New Haven, Conn.
There were no differences in response rates between either pembrolizumab monotherapy or in combination compared with chemotherapy alone, but responses were more durable with the checkpoint inhibitor than with chemotherapy.
“Pembrolizumab alone and pembrolizumab given with platinum and 5-fluorouracil should represent new standards of care for the first-line treatment of metastatic head and neck carcinoma. Immune checkpoint monotherapy with pembrolizumab allows patients to live longer and has a better safety profile than the previous standard for those patients whose tumors express PD-L1,” she said at a briefing prior to her presentation of the data in a presidential symposium at the European Society for Medical Oncology Congress.
“This is the first time since 10 years that we show an improvement in survival for this group of patients,” said Jean-Pascal Machiels, MD, of University Clinic Saint-Luc, Brussels, the invited discussant for the briefing and the symposium.
The CPS is a ratio of PD-L1-positive tumor cells, lymphocytes, and macrophages to the total numbers of cells counted multiplied by 100. The investigators looked at progression-free survival (PFS) and OS in three cohorts of patients with squamous cell carcinomas of the oropharynx, oral cavity, hypopharynx, or larynx that were recurrent or metastatic and were incurable by local therapies. They compared pembrolizumab monotherapy with the EXTREME regimen, and pembrolizumab plus chemotherapy (as described in the following paragraph) with EXTREME.
A total of 882 patients were enrolled and stratified by PD-L1 expression (on 50% or greater of tumor cells, or less than 50%), p16 positive or negative status in the oropharynx, and Eastern Cooperative Oncology Group performance status of 0 or 1. The patients were then randomly assigned on a 1:1:1 basis to either pembrolizumab monotherapy at 200 mg every 3 weeks for up to 35 cycles, pembrolizumab plus a standard chemotherapy regimen (carboplatin to an area-under-the curve [AUC] of 5 or cisplatin 100 mg/m2 plus 5-FU 1000 mg/m2 per day for 4 days for six cycles, followed by pembrolizumab maintenance for up to 35 cycles or EXTREME (cetuximab at a loading dose of 400 mg/m2 followed by 250 mg/m2 once weekly plus the chemotherapy regimen described above, followed by maintenance cetuximab).
Pembrolizumab monotherapy vs. EXTREME
For the coprimary endpoint of OS in the CPS 20 or greater population, pembrolizumab was associated with significantly better survival than EXTREME at both the 12- and 24-month time points (56.9% vs. 44.9%, and 38.3% vs. 22,1%, respectively). After a minimum follow-up of 17 months, the median OS was 14.9 months with pembrolizumab, vs. 10.7 months for EXTREME. The hazard ratio (HR) for death with pembrolizumab was 0.61 (P = .0007).
The median OS in the CPS 1 or greater population was 12.3 months and 10.3 months, respectively (HR 0.78, P = .0086).
There were no differences between the arms in PFS, however, either in the CPS 20 or greater or CPS 1 or greater populations.
Although, as noted, response rates did not differ between the groups, the median duration of response was 20.9 months with pembrolizumab in both the CPS 20 and CPS 1 populations, compared with 4.2 and 4.5 months, respectively, for EXTREME.
Treatment-related adverse events of any grade occurred in 58% of patients in the monotherapy arm, vs. 96.9% in the EXTREME arm. Fatal adverse events occurred in 1% vs. 2.8%, and events leading to drug discontinuation occurred in 4.7% vs. 19.9%, respectively. There were more immune-mediated events in the pembrolizumab arm, including one death (from pneumonitis) vs. no deaths from immune-related causes in the EXTREME arm.
Pembrolizumab plus chemo vs. EXTREME
The combination of pembrolizumab was also superior to EXTREME in the total population, with 12- and 24-month OS rates of 53% vs. 43.9%, and 29% vs. 18.7%, respectively. The median OS was 13 months with the pembrolizumab/chemo combination, vs. 10.7 months for EXTREME, translating into an HR of 0.77 (P = .0034). In this analysis as well as in the pembrolizumab monotherapy combination, there was no difference in PFS or response rates, but responses in the pembrolizumab arm were more durable.
In this comparison, treatment-related adverse events were generally similar between the groups, although there were 10 treatment-related deaths with pembrolizumab, compared with eight in the EXTREME arm.
There was one immune-related death, from pneumonitis, in the pembrolizumab arm, vs. none in the EXTREME arm. Hypothyroidism, pneumonitis, hyperthyroidism and colitis were more frequent with pembrolizumab, whereas infusion reactions and severe skin reactions were more frequent with EXTREME.
“There are further analyses of biomarker and clinical predictors that will be forthcoming from this study, and these may eventually optimally guide the choice of whether to administer pembrolizumab alone or in the novel combination, Dr. Burtness said at the briefing.
“What’s extremely important also is that for a subgroup of patients with high expression of PD-L1, we can probably remove the cisplatin and have a good outcome with immunotherapy alone,” Dr. Machiels said.
He said that more work needs to be done to determine which patients are most likely to benefit from immunotherapy in the recurrent/metastatic setting, and “we have to see how we can now bring this active drug to the curative treatment of the patient, in combination with chemoradiation.”
The study was funded by Merck Sharp & Dohme. Dr. Burtness disclosed being and advisory board member and receiving travel expenses from MSD and others. Dr. Machiels disclosed speaker honoraria, travel expenses, and an uncompensated advisory role with MSD.
SOURCE: Burtness B et al. ESMO 2018. Abstract LBA8_PR.
MUNICH – In patients with recurrent or metastatic head and neck squamous cell carcinoma expressing programmed death ligand-1 (PDL-1), the immune checkpoint inhibitor pembrolizumab alone or in combination with chemotherapy improved overall survival, compared with the EXTREME chemotherapy regimen, reported investigators in the Keynote 048 trial.
Overall survival (OS) among patients with a PD-L1 combined positive score (CPS) of 20 or greater treated with pembrolizumab (Keytruda) monotherapy was 14.9 months compared with 10.7 months for patients treated with the EXTREME regimen, a combination of cetuximab (Erbitux), carboplatin or cisplatin, and 5-fluorouracil.
A similar overall survival benefit was seen in patients with a CPS of 1 or greater, and in the total population of patients treated with pembrolizumab plus chemotherapy followed by pembrolizumab maintenance compared with EXTREME chemotherapy, Barbara Burtness, MD, of Yale Cancer Center, New Haven, Conn.
There were no differences in response rates between either pembrolizumab monotherapy or in combination compared with chemotherapy alone, but responses were more durable with the checkpoint inhibitor than with chemotherapy.
“Pembrolizumab alone and pembrolizumab given with platinum and 5-fluorouracil should represent new standards of care for the first-line treatment of metastatic head and neck carcinoma. Immune checkpoint monotherapy with pembrolizumab allows patients to live longer and has a better safety profile than the previous standard for those patients whose tumors express PD-L1,” she said at a briefing prior to her presentation of the data in a presidential symposium at the European Society for Medical Oncology Congress.
“This is the first time since 10 years that we show an improvement in survival for this group of patients,” said Jean-Pascal Machiels, MD, of University Clinic Saint-Luc, Brussels, the invited discussant for the briefing and the symposium.
The CPS is a ratio of PD-L1-positive tumor cells, lymphocytes, and macrophages to the total numbers of cells counted multiplied by 100. The investigators looked at progression-free survival (PFS) and OS in three cohorts of patients with squamous cell carcinomas of the oropharynx, oral cavity, hypopharynx, or larynx that were recurrent or metastatic and were incurable by local therapies. They compared pembrolizumab monotherapy with the EXTREME regimen, and pembrolizumab plus chemotherapy (as described in the following paragraph) with EXTREME.
A total of 882 patients were enrolled and stratified by PD-L1 expression (on 50% or greater of tumor cells, or less than 50%), p16 positive or negative status in the oropharynx, and Eastern Cooperative Oncology Group performance status of 0 or 1. The patients were then randomly assigned on a 1:1:1 basis to either pembrolizumab monotherapy at 200 mg every 3 weeks for up to 35 cycles, pembrolizumab plus a standard chemotherapy regimen (carboplatin to an area-under-the curve [AUC] of 5 or cisplatin 100 mg/m2 plus 5-FU 1000 mg/m2 per day for 4 days for six cycles, followed by pembrolizumab maintenance for up to 35 cycles or EXTREME (cetuximab at a loading dose of 400 mg/m2 followed by 250 mg/m2 once weekly plus the chemotherapy regimen described above, followed by maintenance cetuximab).
Pembrolizumab monotherapy vs. EXTREME
For the coprimary endpoint of OS in the CPS 20 or greater population, pembrolizumab was associated with significantly better survival than EXTREME at both the 12- and 24-month time points (56.9% vs. 44.9%, and 38.3% vs. 22,1%, respectively). After a minimum follow-up of 17 months, the median OS was 14.9 months with pembrolizumab, vs. 10.7 months for EXTREME. The hazard ratio (HR) for death with pembrolizumab was 0.61 (P = .0007).
The median OS in the CPS 1 or greater population was 12.3 months and 10.3 months, respectively (HR 0.78, P = .0086).
There were no differences between the arms in PFS, however, either in the CPS 20 or greater or CPS 1 or greater populations.
Although, as noted, response rates did not differ between the groups, the median duration of response was 20.9 months with pembrolizumab in both the CPS 20 and CPS 1 populations, compared with 4.2 and 4.5 months, respectively, for EXTREME.
Treatment-related adverse events of any grade occurred in 58% of patients in the monotherapy arm, vs. 96.9% in the EXTREME arm. Fatal adverse events occurred in 1% vs. 2.8%, and events leading to drug discontinuation occurred in 4.7% vs. 19.9%, respectively. There were more immune-mediated events in the pembrolizumab arm, including one death (from pneumonitis) vs. no deaths from immune-related causes in the EXTREME arm.
Pembrolizumab plus chemo vs. EXTREME
The combination of pembrolizumab was also superior to EXTREME in the total population, with 12- and 24-month OS rates of 53% vs. 43.9%, and 29% vs. 18.7%, respectively. The median OS was 13 months with the pembrolizumab/chemo combination, vs. 10.7 months for EXTREME, translating into an HR of 0.77 (P = .0034). In this analysis as well as in the pembrolizumab monotherapy combination, there was no difference in PFS or response rates, but responses in the pembrolizumab arm were more durable.
In this comparison, treatment-related adverse events were generally similar between the groups, although there were 10 treatment-related deaths with pembrolizumab, compared with eight in the EXTREME arm.
There was one immune-related death, from pneumonitis, in the pembrolizumab arm, vs. none in the EXTREME arm. Hypothyroidism, pneumonitis, hyperthyroidism and colitis were more frequent with pembrolizumab, whereas infusion reactions and severe skin reactions were more frequent with EXTREME.
“There are further analyses of biomarker and clinical predictors that will be forthcoming from this study, and these may eventually optimally guide the choice of whether to administer pembrolizumab alone or in the novel combination, Dr. Burtness said at the briefing.
“What’s extremely important also is that for a subgroup of patients with high expression of PD-L1, we can probably remove the cisplatin and have a good outcome with immunotherapy alone,” Dr. Machiels said.
He said that more work needs to be done to determine which patients are most likely to benefit from immunotherapy in the recurrent/metastatic setting, and “we have to see how we can now bring this active drug to the curative treatment of the patient, in combination with chemoradiation.”
The study was funded by Merck Sharp & Dohme. Dr. Burtness disclosed being and advisory board member and receiving travel expenses from MSD and others. Dr. Machiels disclosed speaker honoraria, travel expenses, and an uncompensated advisory role with MSD.
SOURCE: Burtness B et al. ESMO 2018. Abstract LBA8_PR.
MUNICH – In patients with recurrent or metastatic head and neck squamous cell carcinoma expressing programmed death ligand-1 (PDL-1), the immune checkpoint inhibitor pembrolizumab alone or in combination with chemotherapy improved overall survival, compared with the EXTREME chemotherapy regimen, reported investigators in the Keynote 048 trial.
Overall survival (OS) among patients with a PD-L1 combined positive score (CPS) of 20 or greater treated with pembrolizumab (Keytruda) monotherapy was 14.9 months compared with 10.7 months for patients treated with the EXTREME regimen, a combination of cetuximab (Erbitux), carboplatin or cisplatin, and 5-fluorouracil.
A similar overall survival benefit was seen in patients with a CPS of 1 or greater, and in the total population of patients treated with pembrolizumab plus chemotherapy followed by pembrolizumab maintenance compared with EXTREME chemotherapy, Barbara Burtness, MD, of Yale Cancer Center, New Haven, Conn.
There were no differences in response rates between either pembrolizumab monotherapy or in combination compared with chemotherapy alone, but responses were more durable with the checkpoint inhibitor than with chemotherapy.
“Pembrolizumab alone and pembrolizumab given with platinum and 5-fluorouracil should represent new standards of care for the first-line treatment of metastatic head and neck carcinoma. Immune checkpoint monotherapy with pembrolizumab allows patients to live longer and has a better safety profile than the previous standard for those patients whose tumors express PD-L1,” she said at a briefing prior to her presentation of the data in a presidential symposium at the European Society for Medical Oncology Congress.
“This is the first time since 10 years that we show an improvement in survival for this group of patients,” said Jean-Pascal Machiels, MD, of University Clinic Saint-Luc, Brussels, the invited discussant for the briefing and the symposium.
The CPS is a ratio of PD-L1-positive tumor cells, lymphocytes, and macrophages to the total numbers of cells counted multiplied by 100. The investigators looked at progression-free survival (PFS) and OS in three cohorts of patients with squamous cell carcinomas of the oropharynx, oral cavity, hypopharynx, or larynx that were recurrent or metastatic and were incurable by local therapies. They compared pembrolizumab monotherapy with the EXTREME regimen, and pembrolizumab plus chemotherapy (as described in the following paragraph) with EXTREME.
A total of 882 patients were enrolled and stratified by PD-L1 expression (on 50% or greater of tumor cells, or less than 50%), p16 positive or negative status in the oropharynx, and Eastern Cooperative Oncology Group performance status of 0 or 1. The patients were then randomly assigned on a 1:1:1 basis to either pembrolizumab monotherapy at 200 mg every 3 weeks for up to 35 cycles, pembrolizumab plus a standard chemotherapy regimen (carboplatin to an area-under-the curve [AUC] of 5 or cisplatin 100 mg/m2 plus 5-FU 1000 mg/m2 per day for 4 days for six cycles, followed by pembrolizumab maintenance for up to 35 cycles or EXTREME (cetuximab at a loading dose of 400 mg/m2 followed by 250 mg/m2 once weekly plus the chemotherapy regimen described above, followed by maintenance cetuximab).
Pembrolizumab monotherapy vs. EXTREME
For the coprimary endpoint of OS in the CPS 20 or greater population, pembrolizumab was associated with significantly better survival than EXTREME at both the 12- and 24-month time points (56.9% vs. 44.9%, and 38.3% vs. 22,1%, respectively). After a minimum follow-up of 17 months, the median OS was 14.9 months with pembrolizumab, vs. 10.7 months for EXTREME. The hazard ratio (HR) for death with pembrolizumab was 0.61 (P = .0007).
The median OS in the CPS 1 or greater population was 12.3 months and 10.3 months, respectively (HR 0.78, P = .0086).
There were no differences between the arms in PFS, however, either in the CPS 20 or greater or CPS 1 or greater populations.
Although, as noted, response rates did not differ between the groups, the median duration of response was 20.9 months with pembrolizumab in both the CPS 20 and CPS 1 populations, compared with 4.2 and 4.5 months, respectively, for EXTREME.
Treatment-related adverse events of any grade occurred in 58% of patients in the monotherapy arm, vs. 96.9% in the EXTREME arm. Fatal adverse events occurred in 1% vs. 2.8%, and events leading to drug discontinuation occurred in 4.7% vs. 19.9%, respectively. There were more immune-mediated events in the pembrolizumab arm, including one death (from pneumonitis) vs. no deaths from immune-related causes in the EXTREME arm.
Pembrolizumab plus chemo vs. EXTREME
The combination of pembrolizumab was also superior to EXTREME in the total population, with 12- and 24-month OS rates of 53% vs. 43.9%, and 29% vs. 18.7%, respectively. The median OS was 13 months with the pembrolizumab/chemo combination, vs. 10.7 months for EXTREME, translating into an HR of 0.77 (P = .0034). In this analysis as well as in the pembrolizumab monotherapy combination, there was no difference in PFS or response rates, but responses in the pembrolizumab arm were more durable.
In this comparison, treatment-related adverse events were generally similar between the groups, although there were 10 treatment-related deaths with pembrolizumab, compared with eight in the EXTREME arm.
There was one immune-related death, from pneumonitis, in the pembrolizumab arm, vs. none in the EXTREME arm. Hypothyroidism, pneumonitis, hyperthyroidism and colitis were more frequent with pembrolizumab, whereas infusion reactions and severe skin reactions were more frequent with EXTREME.
“There are further analyses of biomarker and clinical predictors that will be forthcoming from this study, and these may eventually optimally guide the choice of whether to administer pembrolizumab alone or in the novel combination, Dr. Burtness said at the briefing.
“What’s extremely important also is that for a subgroup of patients with high expression of PD-L1, we can probably remove the cisplatin and have a good outcome with immunotherapy alone,” Dr. Machiels said.
He said that more work needs to be done to determine which patients are most likely to benefit from immunotherapy in the recurrent/metastatic setting, and “we have to see how we can now bring this active drug to the curative treatment of the patient, in combination with chemoradiation.”
The study was funded by Merck Sharp & Dohme. Dr. Burtness disclosed being and advisory board member and receiving travel expenses from MSD and others. Dr. Machiels disclosed speaker honoraria, travel expenses, and an uncompensated advisory role with MSD.
SOURCE: Burtness B et al. ESMO 2018. Abstract LBA8_PR.
REPORTING FROM ESMO 2018
Key clinical point: Pembrolizumab alone or in combination with chemotherapy was associated with better overall survival of squamous cell head and neck cancer, compared with the EXTREME chemotherapy regimen.
Major finding: Overall survival among patients with a PD-L1 combined positive score of 20 or greater treated with pembrolizumab (Keytruda) monotherapy was 14.9 compared with 10.7 months for patients treated with the EXTREME regimen.
Study details: Randomized phase 3 trial of 882 patients with recurrent or metastatic squamous cell carcinoma of the head and neck.
Disclosures: The study was funded by Merck Sharp & Dohme. Dr. Burtness disclosed being and advisory board member and receiving travel expenses from MSD and others. Dr. Machiels disclosed speaker honoraria, travel expenses, and an uncompensated advisory role with MSD.
Source: Burtness B et al. ESMO 2018. Abstract LBA8_PR.
Over one-third of psoriasis patients have PsA
Over one-third of psoriasis patients have PsA
About two-thirds of patients with psoriasis in a national registry also had psoriatic arthritis (PsA) and/or psoriasis in at least one challenging-to-treat (CTT) area, and one-quarter had both, according to Kristina Callis Duffin, MD, of the University of Utah, Salt Lake City, and her associates.
Their analysis included 2,042 psoriasis patients who were enrolled in the Corrona Psoriasis Registry between April 2015 and May 2018 and initiated biologic treatment during that time. The mean age was 49.6 years, 80% of the patients were white, and 51% were obese. Mean disease duration was 19.9 years and 89.2% of the patients had moderate to severe disease. CTT areas include the scalp, nails, and palmoplantar areas.
A total of 784 people in the cohort (38.4%) had PsA, 778 (38.1%) had scalp psoriasis, 326 (16.0%) had nail psoriasis, 223 (10.9%) had palmoplantar psoriasis, and 535 (26.2%) had both PsA and psoriasis in at least two CTT areas. The most common combinations were PsA plus scalp psoriasis and PsA plus nail and scalp psoriasis.
“These results indicate a need to further characterize patients with psoriasis who have PsA and CTT areas and evaluate the impact of these factors to better understand their treatment needs,” the investigators noted.
The Corrona registry has been supported by numerous pharmaceutical companies, and the study authors reported numerous financial relationships with industry; two authors are Novartis employees.
Secukinumab effective for slowing radiographic progression in active PsA
Treatment with secukinumab significantly reduced radiographic progression in patients with active PsA, according to Désirée van der Heijde, MD, PhD, professor of rheumatology at Leiden University Medical Center, and her associates.
The results come from an analysis of the FUTURE 5 trial, a study of 996 patients with active PsA despite previous NSAID treatment, disease-modifying antirheumatic drug treatment, or anti–tumor necrosis factor (TNF) therapy. Patients were randomized to receive 300 mg subcutaneous secukinumab with loading dose, 150 mg secukinumab with loading dose, 150 mg secukinumab without loading dose, or placebo, at baseline; weeks 1, 2, 3, and 4; then every 4 weeks.
After 24 weeks, the mean change in van der Heijde–modified Total Sharp Score for PsA was 0.08 for the 300-mg secukinumab group (P less than .01), 0.17 for the 150-mg secukinumab with loading dose group (P less than .05), a reduction of 0.09 for the 150-mg secukinumab without loading dose group (P less than .01), and 0.50 for the placebo group. Lower radiographic progression was seen regardless of prior anti-TNF or concomitant methotrexate treatment.
The study was funded by Novartis. The study authors reported financial disclosures with numerous companies; five authors are Novartis employees.
Tildrakizumab sustains efficacy in plaque psoriasis treatment after 1 year
Nearly all patients receiving the interleukin-23 inhibitor tildrakizumab for the treatment of moderate to severe plaque psoriasis maintained or improved their Psoriasis Area and Severity Index (PASI) response rate after 52 weeks of treatment, compared with their response after 28 weeks.
The analysis, conducted by Boni E. Elewski, MD, of the University of Alabama at Birmingham, and her associates, included 352 patients who received 100 mg tildrakizumab and 313 who received 200 mg tildrakizumab. Treatment was received at baseline, at 4 weeks, and then every 12 weeks afterward.
At week 28, the proportions of patients achieving PASI 100, PASI 90-99, PASI 75-89, and PASI 50-74 at week 28 were 25.9%, 38.4%, 25.3%, and 10.5%, respectively, among those treated with the 100-mg dose. The proportions were 24.6%, 24.3%, 19.5%, and 31.6%, respectively, among those treated with the 200-mg dose.
In patients who achieved at least PASI 90 on either dose at week 28, 88.9%-89.4% maintained that response at week 52. For patients with PASI 75-89, 39.3%-40.4% maintained that response and 33.7%-41.0% achieved a PASI 90 response. At week 52, in patients with PASI 50-74, 20.2%-29.7% achieved at least a PASI 90, 52.5%-64.9% achieved PASI 75, and only 2.6% of patients on either dose had fallen below PASI 50.
Four study authors reported being clinical investigators on studies sponsored by Merck and Sun Pharmaceuticals; five authors are employees of Sun Pharmaceuticals.
Halobetasol/tazarotene combination most effective for plaque psoriasis treatment
A fixed combination of halobetasol propionate 0.01% and tazarotene 0.045% lotion provided a synergistic effect over either component on its own for the treatment of plaque psoriasis, according to Leon H. Kircik, MD, of Indiana University, Indianapolis, and his associates.
The investigators performed a post hoc analysis of 212 patients with moderate to severe plaque psoriasis randomized to receive either the halobetasol/tazarotene combination, halobetasol only, tazarotene only, or vehicle only for 8 weeks, with follow-up at 12 weeks. Treatment success was based on the proportion of patients who achieved at least a 2-grade improvement in the Investigator Global Assessment (IGA) score, IGA scores of “clear” or “almost clear,” and percent change from baseline in IGA multiplied by Body Surface Area (BSA) composite score (IGAxBSA). “Synergy was calculated by summing up the contribution of the individual active ingredients (HP and TAZ) to overall efficacy and comparing to the efficacy achieved with HP/TAZ lotion relative to vehicle,” the authors explained.
Relative to vehicle, treatment success for halobetasol/tazarotene after 8 weeks was 42.8%, 23.6% for halobetasol alone, and 9.0% for tazarotene alone. After 12 weeks, the difference was 31.3%, 14.1%, and 5.9%, respectively. The percent change in IGAxBSA scores from baseline after 8 weeks, relative to vehicle, were 51.6%, 37.3%, and 3.3%, respectively. After 12 weeks, the change was 47.3%, 25.7%, and 8.6%, respectively.
After 8 weeks, the synergy ratio for treatment success and IGAxBSA scores for the halobetasol/tazarotene combination was 1.3. After 12 weeks, the synergy ratio for treatment success was 1.6 and the ratio for IGAxBSA scores was 1.4.
“By combining two agents into one once-daily formulation, this novel formulation reduces the number of product applications and may help patient adherence,” the study authors noted.
Dr. Kircik reported serving as a consultant and investigator for Valeant Pharmaceuticals. One study author is an employee of Bausch Health and Ortho Dermatologics, and another is an employee of Dow Pharmaceutical Sciences (a division of Valeant).
Brodalumab demonstrates low immunogenicity in moderate to severe psoriasis
The immunogenicity of brodalumab in patients with moderate to severe plaque psoriasis was low and did not compromise the efficacy or safety profile of the drug, according to Kristian Reich, MD, of Dermatologikum Berlin and SCIderm Research Institute in Hamburg, Germany, and his associates.
Data from a 12-week, phase 2 trial with a 352-week, open-label extension and three 52-week phase 3 trials were included in the analysis. Antidrug antibodies (ADAs) were tested, and positive samples were further analyzed for neutralizing ADAs by a cell-based assay.
Out of the 4,461 patients who received brodalumab, 122 (2.7%) were positive for ADAs after starting brodalumab. The incidence rate ranged from 1.9% to 3.4% between all dosing groups (140 mg, 210 mg, variable dosing, and 210 mg of brodalumab after ustekinumab). In 58 (1.4%) of patients, ADAs were transient. No patients had neutralizing ADAs, and no evidence of altered pharmacokinetics, loss of efficacy, or changes in the safety profile of brodalumab in subjects positive for ADAs was seen.
No significant difference was seen in the incidence rate of hypersensitivity or injection site reactions in brodalumab, compared with placebo or ustekinumab. The most common injection site reactions were injection site pain, erythema, and bruising.
The study was supported by Amgen. The study authors reported numerous disclosures. Two authors are employees of Leo Pharma, one author is a former employee of the company.
Secukinumab improves patient-reported outcomes in CTT psoriasis
Treatment with secukinumab significantly improved patient-reported outcomes such as fatigue, itch, pain, and quality of life measures in patients with CTT psoriasis after 6 months, according to Jerry Bagel, MD, of the Psoriasis Treatment Center of Central New Jersey, East Windsor, and his associates.
A total of 68 patients with psoriasis localized to at least one CTT area who were enrolled in the Corrona Psoriasis Registry from April 15, 2015, through May 10, 2018, and were receiving secukinumab for the entirety of the 6-month study period were included in the analysis. Patient-reported outcomes included in the analysis were fatigue, itch, pain, Dermatology Quality of Life Index (DLQI) score, and Work Productivity and Activity Impairment (WPAI) scale.
The mean age at enrollment was 51.2 years and almost 80% of patients were white. Mean psoriasis duration was 21.8 years and nearly half had PsA.
Visual analog scale scores improved over baseline for fatigue (mean, 23.2 vs. 33.2; P = .01), itch (20.9 vs. 49.6; P less than .0001), and pain (12.1 vs. 33.8; P less than .0001). DLQI scores also improved (2.9 vs. 8.1; P less than .0001), and the proportion of patients who reported that psoriasis had at least a moderate effect on their life was reduced after 6 months (22.1% vs. 59.7%; P less than .0001).
Based on WPAI results, patients experienced significant improvements in the percentage of daily activities impaired (mean, 9.5% vs 17.5%; P = .0075); of the 42 patients who were employed, both impairment percentage (3.7% vs. 11.2%; P = .0148) and percentage of work hours affected (4.9% vs. 11.9%; P = .0486) were reduced from baseline.
“These results are consistent with previous reports from secukinumab clinical trials; however, additional real-world studies are needed to evaluate the long-term effectiveness of secukinumab for improving [patient-reported outcomes] in patients with psoriasis in CTT areas,” the authors noted.
The Corrona registry has been supported by numerous pharmaceutical companies, and several study authors reported various disclosures with industry. Two authors are Novartis employees. The study was supported by Novartis; the company participated in the interpretation of data and review and approval of the abstract.
These posters were presented at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. SDEF and this news organization are owned by the same parent company.
Over one-third of psoriasis patients have PsA
About two-thirds of patients with psoriasis in a national registry also had psoriatic arthritis (PsA) and/or psoriasis in at least one challenging-to-treat (CTT) area, and one-quarter had both, according to Kristina Callis Duffin, MD, of the University of Utah, Salt Lake City, and her associates.
Their analysis included 2,042 psoriasis patients who were enrolled in the Corrona Psoriasis Registry between April 2015 and May 2018 and initiated biologic treatment during that time. The mean age was 49.6 years, 80% of the patients were white, and 51% were obese. Mean disease duration was 19.9 years and 89.2% of the patients had moderate to severe disease. CTT areas include the scalp, nails, and palmoplantar areas.
A total of 784 people in the cohort (38.4%) had PsA, 778 (38.1%) had scalp psoriasis, 326 (16.0%) had nail psoriasis, 223 (10.9%) had palmoplantar psoriasis, and 535 (26.2%) had both PsA and psoriasis in at least two CTT areas. The most common combinations were PsA plus scalp psoriasis and PsA plus nail and scalp psoriasis.
“These results indicate a need to further characterize patients with psoriasis who have PsA and CTT areas and evaluate the impact of these factors to better understand their treatment needs,” the investigators noted.
The Corrona registry has been supported by numerous pharmaceutical companies, and the study authors reported numerous financial relationships with industry; two authors are Novartis employees.
Secukinumab effective for slowing radiographic progression in active PsA
Treatment with secukinumab significantly reduced radiographic progression in patients with active PsA, according to Désirée van der Heijde, MD, PhD, professor of rheumatology at Leiden University Medical Center, and her associates.
The results come from an analysis of the FUTURE 5 trial, a study of 996 patients with active PsA despite previous NSAID treatment, disease-modifying antirheumatic drug treatment, or anti–tumor necrosis factor (TNF) therapy. Patients were randomized to receive 300 mg subcutaneous secukinumab with loading dose, 150 mg secukinumab with loading dose, 150 mg secukinumab without loading dose, or placebo, at baseline; weeks 1, 2, 3, and 4; then every 4 weeks.
After 24 weeks, the mean change in van der Heijde–modified Total Sharp Score for PsA was 0.08 for the 300-mg secukinumab group (P less than .01), 0.17 for the 150-mg secukinumab with loading dose group (P less than .05), a reduction of 0.09 for the 150-mg secukinumab without loading dose group (P less than .01), and 0.50 for the placebo group. Lower radiographic progression was seen regardless of prior anti-TNF or concomitant methotrexate treatment.
The study was funded by Novartis. The study authors reported financial disclosures with numerous companies; five authors are Novartis employees.
Tildrakizumab sustains efficacy in plaque psoriasis treatment after 1 year
Nearly all patients receiving the interleukin-23 inhibitor tildrakizumab for the treatment of moderate to severe plaque psoriasis maintained or improved their Psoriasis Area and Severity Index (PASI) response rate after 52 weeks of treatment, compared with their response after 28 weeks.
The analysis, conducted by Boni E. Elewski, MD, of the University of Alabama at Birmingham, and her associates, included 352 patients who received 100 mg tildrakizumab and 313 who received 200 mg tildrakizumab. Treatment was received at baseline, at 4 weeks, and then every 12 weeks afterward.
At week 28, the proportions of patients achieving PASI 100, PASI 90-99, PASI 75-89, and PASI 50-74 at week 28 were 25.9%, 38.4%, 25.3%, and 10.5%, respectively, among those treated with the 100-mg dose. The proportions were 24.6%, 24.3%, 19.5%, and 31.6%, respectively, among those treated with the 200-mg dose.
In patients who achieved at least PASI 90 on either dose at week 28, 88.9%-89.4% maintained that response at week 52. For patients with PASI 75-89, 39.3%-40.4% maintained that response and 33.7%-41.0% achieved a PASI 90 response. At week 52, in patients with PASI 50-74, 20.2%-29.7% achieved at least a PASI 90, 52.5%-64.9% achieved PASI 75, and only 2.6% of patients on either dose had fallen below PASI 50.
Four study authors reported being clinical investigators on studies sponsored by Merck and Sun Pharmaceuticals; five authors are employees of Sun Pharmaceuticals.
Halobetasol/tazarotene combination most effective for plaque psoriasis treatment
A fixed combination of halobetasol propionate 0.01% and tazarotene 0.045% lotion provided a synergistic effect over either component on its own for the treatment of plaque psoriasis, according to Leon H. Kircik, MD, of Indiana University, Indianapolis, and his associates.
The investigators performed a post hoc analysis of 212 patients with moderate to severe plaque psoriasis randomized to receive either the halobetasol/tazarotene combination, halobetasol only, tazarotene only, or vehicle only for 8 weeks, with follow-up at 12 weeks. Treatment success was based on the proportion of patients who achieved at least a 2-grade improvement in the Investigator Global Assessment (IGA) score, IGA scores of “clear” or “almost clear,” and percent change from baseline in IGA multiplied by Body Surface Area (BSA) composite score (IGAxBSA). “Synergy was calculated by summing up the contribution of the individual active ingredients (HP and TAZ) to overall efficacy and comparing to the efficacy achieved with HP/TAZ lotion relative to vehicle,” the authors explained.
Relative to vehicle, treatment success for halobetasol/tazarotene after 8 weeks was 42.8%, 23.6% for halobetasol alone, and 9.0% for tazarotene alone. After 12 weeks, the difference was 31.3%, 14.1%, and 5.9%, respectively. The percent change in IGAxBSA scores from baseline after 8 weeks, relative to vehicle, were 51.6%, 37.3%, and 3.3%, respectively. After 12 weeks, the change was 47.3%, 25.7%, and 8.6%, respectively.
After 8 weeks, the synergy ratio for treatment success and IGAxBSA scores for the halobetasol/tazarotene combination was 1.3. After 12 weeks, the synergy ratio for treatment success was 1.6 and the ratio for IGAxBSA scores was 1.4.
“By combining two agents into one once-daily formulation, this novel formulation reduces the number of product applications and may help patient adherence,” the study authors noted.
Dr. Kircik reported serving as a consultant and investigator for Valeant Pharmaceuticals. One study author is an employee of Bausch Health and Ortho Dermatologics, and another is an employee of Dow Pharmaceutical Sciences (a division of Valeant).
Brodalumab demonstrates low immunogenicity in moderate to severe psoriasis
The immunogenicity of brodalumab in patients with moderate to severe plaque psoriasis was low and did not compromise the efficacy or safety profile of the drug, according to Kristian Reich, MD, of Dermatologikum Berlin and SCIderm Research Institute in Hamburg, Germany, and his associates.
Data from a 12-week, phase 2 trial with a 352-week, open-label extension and three 52-week phase 3 trials were included in the analysis. Antidrug antibodies (ADAs) were tested, and positive samples were further analyzed for neutralizing ADAs by a cell-based assay.
Out of the 4,461 patients who received brodalumab, 122 (2.7%) were positive for ADAs after starting brodalumab. The incidence rate ranged from 1.9% to 3.4% between all dosing groups (140 mg, 210 mg, variable dosing, and 210 mg of brodalumab after ustekinumab). In 58 (1.4%) of patients, ADAs were transient. No patients had neutralizing ADAs, and no evidence of altered pharmacokinetics, loss of efficacy, or changes in the safety profile of brodalumab in subjects positive for ADAs was seen.
No significant difference was seen in the incidence rate of hypersensitivity or injection site reactions in brodalumab, compared with placebo or ustekinumab. The most common injection site reactions were injection site pain, erythema, and bruising.
The study was supported by Amgen. The study authors reported numerous disclosures. Two authors are employees of Leo Pharma, one author is a former employee of the company.
Secukinumab improves patient-reported outcomes in CTT psoriasis
Treatment with secukinumab significantly improved patient-reported outcomes such as fatigue, itch, pain, and quality of life measures in patients with CTT psoriasis after 6 months, according to Jerry Bagel, MD, of the Psoriasis Treatment Center of Central New Jersey, East Windsor, and his associates.
A total of 68 patients with psoriasis localized to at least one CTT area who were enrolled in the Corrona Psoriasis Registry from April 15, 2015, through May 10, 2018, and were receiving secukinumab for the entirety of the 6-month study period were included in the analysis. Patient-reported outcomes included in the analysis were fatigue, itch, pain, Dermatology Quality of Life Index (DLQI) score, and Work Productivity and Activity Impairment (WPAI) scale.
The mean age at enrollment was 51.2 years and almost 80% of patients were white. Mean psoriasis duration was 21.8 years and nearly half had PsA.
Visual analog scale scores improved over baseline for fatigue (mean, 23.2 vs. 33.2; P = .01), itch (20.9 vs. 49.6; P less than .0001), and pain (12.1 vs. 33.8; P less than .0001). DLQI scores also improved (2.9 vs. 8.1; P less than .0001), and the proportion of patients who reported that psoriasis had at least a moderate effect on their life was reduced after 6 months (22.1% vs. 59.7%; P less than .0001).
Based on WPAI results, patients experienced significant improvements in the percentage of daily activities impaired (mean, 9.5% vs 17.5%; P = .0075); of the 42 patients who were employed, both impairment percentage (3.7% vs. 11.2%; P = .0148) and percentage of work hours affected (4.9% vs. 11.9%; P = .0486) were reduced from baseline.
“These results are consistent with previous reports from secukinumab clinical trials; however, additional real-world studies are needed to evaluate the long-term effectiveness of secukinumab for improving [patient-reported outcomes] in patients with psoriasis in CTT areas,” the authors noted.
The Corrona registry has been supported by numerous pharmaceutical companies, and several study authors reported various disclosures with industry. Two authors are Novartis employees. The study was supported by Novartis; the company participated in the interpretation of data and review and approval of the abstract.
These posters were presented at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. SDEF and this news organization are owned by the same parent company.
Over one-third of psoriasis patients have PsA
About two-thirds of patients with psoriasis in a national registry also had psoriatic arthritis (PsA) and/or psoriasis in at least one challenging-to-treat (CTT) area, and one-quarter had both, according to Kristina Callis Duffin, MD, of the University of Utah, Salt Lake City, and her associates.
Their analysis included 2,042 psoriasis patients who were enrolled in the Corrona Psoriasis Registry between April 2015 and May 2018 and initiated biologic treatment during that time. The mean age was 49.6 years, 80% of the patients were white, and 51% were obese. Mean disease duration was 19.9 years and 89.2% of the patients had moderate to severe disease. CTT areas include the scalp, nails, and palmoplantar areas.
A total of 784 people in the cohort (38.4%) had PsA, 778 (38.1%) had scalp psoriasis, 326 (16.0%) had nail psoriasis, 223 (10.9%) had palmoplantar psoriasis, and 535 (26.2%) had both PsA and psoriasis in at least two CTT areas. The most common combinations were PsA plus scalp psoriasis and PsA plus nail and scalp psoriasis.
“These results indicate a need to further characterize patients with psoriasis who have PsA and CTT areas and evaluate the impact of these factors to better understand their treatment needs,” the investigators noted.
The Corrona registry has been supported by numerous pharmaceutical companies, and the study authors reported numerous financial relationships with industry; two authors are Novartis employees.
Secukinumab effective for slowing radiographic progression in active PsA
Treatment with secukinumab significantly reduced radiographic progression in patients with active PsA, according to Désirée van der Heijde, MD, PhD, professor of rheumatology at Leiden University Medical Center, and her associates.
The results come from an analysis of the FUTURE 5 trial, a study of 996 patients with active PsA despite previous NSAID treatment, disease-modifying antirheumatic drug treatment, or anti–tumor necrosis factor (TNF) therapy. Patients were randomized to receive 300 mg subcutaneous secukinumab with loading dose, 150 mg secukinumab with loading dose, 150 mg secukinumab without loading dose, or placebo, at baseline; weeks 1, 2, 3, and 4; then every 4 weeks.
After 24 weeks, the mean change in van der Heijde–modified Total Sharp Score for PsA was 0.08 for the 300-mg secukinumab group (P less than .01), 0.17 for the 150-mg secukinumab with loading dose group (P less than .05), a reduction of 0.09 for the 150-mg secukinumab without loading dose group (P less than .01), and 0.50 for the placebo group. Lower radiographic progression was seen regardless of prior anti-TNF or concomitant methotrexate treatment.
The study was funded by Novartis. The study authors reported financial disclosures with numerous companies; five authors are Novartis employees.
Tildrakizumab sustains efficacy in plaque psoriasis treatment after 1 year
Nearly all patients receiving the interleukin-23 inhibitor tildrakizumab for the treatment of moderate to severe plaque psoriasis maintained or improved their Psoriasis Area and Severity Index (PASI) response rate after 52 weeks of treatment, compared with their response after 28 weeks.
The analysis, conducted by Boni E. Elewski, MD, of the University of Alabama at Birmingham, and her associates, included 352 patients who received 100 mg tildrakizumab and 313 who received 200 mg tildrakizumab. Treatment was received at baseline, at 4 weeks, and then every 12 weeks afterward.
At week 28, the proportions of patients achieving PASI 100, PASI 90-99, PASI 75-89, and PASI 50-74 at week 28 were 25.9%, 38.4%, 25.3%, and 10.5%, respectively, among those treated with the 100-mg dose. The proportions were 24.6%, 24.3%, 19.5%, and 31.6%, respectively, among those treated with the 200-mg dose.
In patients who achieved at least PASI 90 on either dose at week 28, 88.9%-89.4% maintained that response at week 52. For patients with PASI 75-89, 39.3%-40.4% maintained that response and 33.7%-41.0% achieved a PASI 90 response. At week 52, in patients with PASI 50-74, 20.2%-29.7% achieved at least a PASI 90, 52.5%-64.9% achieved PASI 75, and only 2.6% of patients on either dose had fallen below PASI 50.
Four study authors reported being clinical investigators on studies sponsored by Merck and Sun Pharmaceuticals; five authors are employees of Sun Pharmaceuticals.
Halobetasol/tazarotene combination most effective for plaque psoriasis treatment
A fixed combination of halobetasol propionate 0.01% and tazarotene 0.045% lotion provided a synergistic effect over either component on its own for the treatment of plaque psoriasis, according to Leon H. Kircik, MD, of Indiana University, Indianapolis, and his associates.
The investigators performed a post hoc analysis of 212 patients with moderate to severe plaque psoriasis randomized to receive either the halobetasol/tazarotene combination, halobetasol only, tazarotene only, or vehicle only for 8 weeks, with follow-up at 12 weeks. Treatment success was based on the proportion of patients who achieved at least a 2-grade improvement in the Investigator Global Assessment (IGA) score, IGA scores of “clear” or “almost clear,” and percent change from baseline in IGA multiplied by Body Surface Area (BSA) composite score (IGAxBSA). “Synergy was calculated by summing up the contribution of the individual active ingredients (HP and TAZ) to overall efficacy and comparing to the efficacy achieved with HP/TAZ lotion relative to vehicle,” the authors explained.
Relative to vehicle, treatment success for halobetasol/tazarotene after 8 weeks was 42.8%, 23.6% for halobetasol alone, and 9.0% for tazarotene alone. After 12 weeks, the difference was 31.3%, 14.1%, and 5.9%, respectively. The percent change in IGAxBSA scores from baseline after 8 weeks, relative to vehicle, were 51.6%, 37.3%, and 3.3%, respectively. After 12 weeks, the change was 47.3%, 25.7%, and 8.6%, respectively.
After 8 weeks, the synergy ratio for treatment success and IGAxBSA scores for the halobetasol/tazarotene combination was 1.3. After 12 weeks, the synergy ratio for treatment success was 1.6 and the ratio for IGAxBSA scores was 1.4.
“By combining two agents into one once-daily formulation, this novel formulation reduces the number of product applications and may help patient adherence,” the study authors noted.
Dr. Kircik reported serving as a consultant and investigator for Valeant Pharmaceuticals. One study author is an employee of Bausch Health and Ortho Dermatologics, and another is an employee of Dow Pharmaceutical Sciences (a division of Valeant).
Brodalumab demonstrates low immunogenicity in moderate to severe psoriasis
The immunogenicity of brodalumab in patients with moderate to severe plaque psoriasis was low and did not compromise the efficacy or safety profile of the drug, according to Kristian Reich, MD, of Dermatologikum Berlin and SCIderm Research Institute in Hamburg, Germany, and his associates.
Data from a 12-week, phase 2 trial with a 352-week, open-label extension and three 52-week phase 3 trials were included in the analysis. Antidrug antibodies (ADAs) were tested, and positive samples were further analyzed for neutralizing ADAs by a cell-based assay.
Out of the 4,461 patients who received brodalumab, 122 (2.7%) were positive for ADAs after starting brodalumab. The incidence rate ranged from 1.9% to 3.4% between all dosing groups (140 mg, 210 mg, variable dosing, and 210 mg of brodalumab after ustekinumab). In 58 (1.4%) of patients, ADAs were transient. No patients had neutralizing ADAs, and no evidence of altered pharmacokinetics, loss of efficacy, or changes in the safety profile of brodalumab in subjects positive for ADAs was seen.
No significant difference was seen in the incidence rate of hypersensitivity or injection site reactions in brodalumab, compared with placebo or ustekinumab. The most common injection site reactions were injection site pain, erythema, and bruising.
The study was supported by Amgen. The study authors reported numerous disclosures. Two authors are employees of Leo Pharma, one author is a former employee of the company.
Secukinumab improves patient-reported outcomes in CTT psoriasis
Treatment with secukinumab significantly improved patient-reported outcomes such as fatigue, itch, pain, and quality of life measures in patients with CTT psoriasis after 6 months, according to Jerry Bagel, MD, of the Psoriasis Treatment Center of Central New Jersey, East Windsor, and his associates.
A total of 68 patients with psoriasis localized to at least one CTT area who were enrolled in the Corrona Psoriasis Registry from April 15, 2015, through May 10, 2018, and were receiving secukinumab for the entirety of the 6-month study period were included in the analysis. Patient-reported outcomes included in the analysis were fatigue, itch, pain, Dermatology Quality of Life Index (DLQI) score, and Work Productivity and Activity Impairment (WPAI) scale.
The mean age at enrollment was 51.2 years and almost 80% of patients were white. Mean psoriasis duration was 21.8 years and nearly half had PsA.
Visual analog scale scores improved over baseline for fatigue (mean, 23.2 vs. 33.2; P = .01), itch (20.9 vs. 49.6; P less than .0001), and pain (12.1 vs. 33.8; P less than .0001). DLQI scores also improved (2.9 vs. 8.1; P less than .0001), and the proportion of patients who reported that psoriasis had at least a moderate effect on their life was reduced after 6 months (22.1% vs. 59.7%; P less than .0001).
Based on WPAI results, patients experienced significant improvements in the percentage of daily activities impaired (mean, 9.5% vs 17.5%; P = .0075); of the 42 patients who were employed, both impairment percentage (3.7% vs. 11.2%; P = .0148) and percentage of work hours affected (4.9% vs. 11.9%; P = .0486) were reduced from baseline.
“These results are consistent with previous reports from secukinumab clinical trials; however, additional real-world studies are needed to evaluate the long-term effectiveness of secukinumab for improving [patient-reported outcomes] in patients with psoriasis in CTT areas,” the authors noted.
The Corrona registry has been supported by numerous pharmaceutical companies, and several study authors reported various disclosures with industry. Two authors are Novartis employees. The study was supported by Novartis; the company participated in the interpretation of data and review and approval of the abstract.
These posters were presented at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. SDEF and this news organization are owned by the same parent company.
FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR