User login
FDA expands approval of pembrolizumab in NSCLC
The Food and Drug Administration .
The drug is now approved for use in combination with carboplatin and either paclitaxel or nanoparticle albumin–bound (nab) paclitaxel for the first-line treatment of NSCLC, regardless of PD-L1 expression status.
This makes pembrolizumab the first anti-PD-1 therapy approved in the first-line setting both as monotherapy and in combination treatment for certain patients with metastatic NSCLC. All appropriate patients with metastatic squamous NSCLC or metastatic nonsquamous NSCLC and no EGFR or ALK mutations are now eligible to receive pembrolizumab-based treatment first-line.
The FDA’s approval is based on results from the phase 3 KEYNOTE-407 trial. This randomized, double-blind study enrolled patients with metastatic squamous NSCLC, regardless of tumor PD-L1 expression status, who had received no prior systemic treatment for metastatic disease.
Patients in the pembrolizumab arm (n = 278) received pembrolizumab and carboplatin every 3 weeks for four cycles, plus paclitaxel every 3 weeks for four cycles or nab-paclitaxel on days 1, 8, and 15 of every 3-week cycle for four cycles, followed by pembrolizumab every 3 weeks.
Patients in the control arm (n = 281) received the same regimen of carboplatin and paclitaxel/nab-paclitaxel, but placebo instead of pembrolizumab.
There was a significant improvement in overall response rate, progression-free survival, and overall survival in patients who received pembrolizumab.
The overall response rate was 58% in the pembrolizumab arm and 35% in the placebo arm (P = .0008). The median duration of response was 7.2 months and 4.9 months, respectively.
The median progression-free survival was 6.4 months in the pembrolizumab arm and 4.8 months in the placebo arm (P less than .0001). The median overall survival was 15.9 months and 11.3 months, respectively (P = .0017).
Safety data are available for the first 203 patients treated on the trial, 101 of them in the pembrolizumab arm.
Fifteen percent of patients discontinued pembrolizumab because of adverse events (AEs), and 43% of patients on pembrolizumab experienced AEs leading to dose interruption.
The most common AEs leading to dose interruption in the pembrolizumab arm were thrombocytopenia, neutropenia, anemia, asthenia, and diarrhea. The most frequent serious AEs in the pembrolizumab arm were febrile neutropenia, pneumonia, and urinary tract infection.
Additional details on this trial are available in the prescribing information, which can be found on the Keytruda website.
The Food and Drug Administration .
The drug is now approved for use in combination with carboplatin and either paclitaxel or nanoparticle albumin–bound (nab) paclitaxel for the first-line treatment of NSCLC, regardless of PD-L1 expression status.
This makes pembrolizumab the first anti-PD-1 therapy approved in the first-line setting both as monotherapy and in combination treatment for certain patients with metastatic NSCLC. All appropriate patients with metastatic squamous NSCLC or metastatic nonsquamous NSCLC and no EGFR or ALK mutations are now eligible to receive pembrolizumab-based treatment first-line.
The FDA’s approval is based on results from the phase 3 KEYNOTE-407 trial. This randomized, double-blind study enrolled patients with metastatic squamous NSCLC, regardless of tumor PD-L1 expression status, who had received no prior systemic treatment for metastatic disease.
Patients in the pembrolizumab arm (n = 278) received pembrolizumab and carboplatin every 3 weeks for four cycles, plus paclitaxel every 3 weeks for four cycles or nab-paclitaxel on days 1, 8, and 15 of every 3-week cycle for four cycles, followed by pembrolizumab every 3 weeks.
Patients in the control arm (n = 281) received the same regimen of carboplatin and paclitaxel/nab-paclitaxel, but placebo instead of pembrolizumab.
There was a significant improvement in overall response rate, progression-free survival, and overall survival in patients who received pembrolizumab.
The overall response rate was 58% in the pembrolizumab arm and 35% in the placebo arm (P = .0008). The median duration of response was 7.2 months and 4.9 months, respectively.
The median progression-free survival was 6.4 months in the pembrolizumab arm and 4.8 months in the placebo arm (P less than .0001). The median overall survival was 15.9 months and 11.3 months, respectively (P = .0017).
Safety data are available for the first 203 patients treated on the trial, 101 of them in the pembrolizumab arm.
Fifteen percent of patients discontinued pembrolizumab because of adverse events (AEs), and 43% of patients on pembrolizumab experienced AEs leading to dose interruption.
The most common AEs leading to dose interruption in the pembrolizumab arm were thrombocytopenia, neutropenia, anemia, asthenia, and diarrhea. The most frequent serious AEs in the pembrolizumab arm were febrile neutropenia, pneumonia, and urinary tract infection.
Additional details on this trial are available in the prescribing information, which can be found on the Keytruda website.
The Food and Drug Administration .
The drug is now approved for use in combination with carboplatin and either paclitaxel or nanoparticle albumin–bound (nab) paclitaxel for the first-line treatment of NSCLC, regardless of PD-L1 expression status.
This makes pembrolizumab the first anti-PD-1 therapy approved in the first-line setting both as monotherapy and in combination treatment for certain patients with metastatic NSCLC. All appropriate patients with metastatic squamous NSCLC or metastatic nonsquamous NSCLC and no EGFR or ALK mutations are now eligible to receive pembrolizumab-based treatment first-line.
The FDA’s approval is based on results from the phase 3 KEYNOTE-407 trial. This randomized, double-blind study enrolled patients with metastatic squamous NSCLC, regardless of tumor PD-L1 expression status, who had received no prior systemic treatment for metastatic disease.
Patients in the pembrolizumab arm (n = 278) received pembrolizumab and carboplatin every 3 weeks for four cycles, plus paclitaxel every 3 weeks for four cycles or nab-paclitaxel on days 1, 8, and 15 of every 3-week cycle for four cycles, followed by pembrolizumab every 3 weeks.
Patients in the control arm (n = 281) received the same regimen of carboplatin and paclitaxel/nab-paclitaxel, but placebo instead of pembrolizumab.
There was a significant improvement in overall response rate, progression-free survival, and overall survival in patients who received pembrolizumab.
The overall response rate was 58% in the pembrolizumab arm and 35% in the placebo arm (P = .0008). The median duration of response was 7.2 months and 4.9 months, respectively.
The median progression-free survival was 6.4 months in the pembrolizumab arm and 4.8 months in the placebo arm (P less than .0001). The median overall survival was 15.9 months and 11.3 months, respectively (P = .0017).
Safety data are available for the first 203 patients treated on the trial, 101 of them in the pembrolizumab arm.
Fifteen percent of patients discontinued pembrolizumab because of adverse events (AEs), and 43% of patients on pembrolizumab experienced AEs leading to dose interruption.
The most common AEs leading to dose interruption in the pembrolizumab arm were thrombocytopenia, neutropenia, anemia, asthenia, and diarrhea. The most frequent serious AEs in the pembrolizumab arm were febrile neutropenia, pneumonia, and urinary tract infection.
Additional details on this trial are available in the prescribing information, which can be found on the Keytruda website.
VA Reaches Milestone in Digitizing Claims Process
In a “significant modernization effort,” the VA has removed nearly 8 million paper files from 60 locations in under 22 months. The files are scanned into the electronic claims process system, which means faster claims decisions, the VA says.
According to the VA, it is a milestone in a years-long project to improve the veteran experience and streamline claims processes. The project began in 2013 when the VA began removing paper records from its regional offices to save space and money. It then expanded in 2016 when the VA launched the File Bank Extraction initiative, which removed more than 1.7 million paper claims files. In 2017, the agency began extracting 6.1 million paper records held in the Records Control Division (RCD) in St. Louis.
The records are temporarily stored in a secure facility certified by the National Archives and Records Administration, where they are inventoried, prioritized, and sent to VA vendors for scanning into the VA’s Veterans Benefits Management System.
The VA is negotiating to return the RCD’s leased warehouse space to the General Services Administration, estimating the move will save roughly $1.8 million per year.
Source:
VA achieves major milestone in effort to modernize claims processing [news release]. Washington, DC: U.S. Department of Veteran Affairs Office of Public Affairs and Media Relations; October 23,2018. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5131. Accessed October 31, 2018.
In a “significant modernization effort,” the VA has removed nearly 8 million paper files from 60 locations in under 22 months. The files are scanned into the electronic claims process system, which means faster claims decisions, the VA says.
According to the VA, it is a milestone in a years-long project to improve the veteran experience and streamline claims processes. The project began in 2013 when the VA began removing paper records from its regional offices to save space and money. It then expanded in 2016 when the VA launched the File Bank Extraction initiative, which removed more than 1.7 million paper claims files. In 2017, the agency began extracting 6.1 million paper records held in the Records Control Division (RCD) in St. Louis.
The records are temporarily stored in a secure facility certified by the National Archives and Records Administration, where they are inventoried, prioritized, and sent to VA vendors for scanning into the VA’s Veterans Benefits Management System.
The VA is negotiating to return the RCD’s leased warehouse space to the General Services Administration, estimating the move will save roughly $1.8 million per year.
Source:
VA achieves major milestone in effort to modernize claims processing [news release]. Washington, DC: U.S. Department of Veteran Affairs Office of Public Affairs and Media Relations; October 23,2018. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5131. Accessed October 31, 2018.
In a “significant modernization effort,” the VA has removed nearly 8 million paper files from 60 locations in under 22 months. The files are scanned into the electronic claims process system, which means faster claims decisions, the VA says.
According to the VA, it is a milestone in a years-long project to improve the veteran experience and streamline claims processes. The project began in 2013 when the VA began removing paper records from its regional offices to save space and money. It then expanded in 2016 when the VA launched the File Bank Extraction initiative, which removed more than 1.7 million paper claims files. In 2017, the agency began extracting 6.1 million paper records held in the Records Control Division (RCD) in St. Louis.
The records are temporarily stored in a secure facility certified by the National Archives and Records Administration, where they are inventoried, prioritized, and sent to VA vendors for scanning into the VA’s Veterans Benefits Management System.
The VA is negotiating to return the RCD’s leased warehouse space to the General Services Administration, estimating the move will save roughly $1.8 million per year.
Source:
VA achieves major milestone in effort to modernize claims processing [news release]. Washington, DC: U.S. Department of Veteran Affairs Office of Public Affairs and Media Relations; October 23,2018. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5131. Accessed October 31, 2018.
Progressive and Translucent Plaques on the Soles
The Diagnosis: Cutaneous Macroglobulinosis
Waldenström macroglobulinemia is a lymphoplasmacytic lymphoma that produces a circulating monoclonal IgM. Incidence in the United States is 1500 patients annually, most commonly men in their 70s.1 The disease process is largely indolent, with early symptoms consisting of generalized weakness, weight loss, and fatigue. Signs of lymphadenopathy, hepatosplenomegaly, and cytopenia may emerge as the disease progresses. Diagnostic criteria include bone marrow biopsy with plasmacytoid/plasmacellular infiltrate; IgM monoclonal gammopathy; and end-organ damage, which may include cutaneous manifestations.2
Cutaneous findings in Waldenström macroglobulinemia are nonspecific and secondary to the disease's hematologic manifestations, presenting as livedo reticularis, purpura, and mucosal bleeding.3 True cutaneous involvement of the disease is rare and was first described in 1978 by Tichenor.4 Specific cutaneous lesions have 2 separate clinical presentations: (1) a primary cutaneous infiltrate of lymphoplasmacytic cells, and (2) deposition of IgM in the dermis.5 Although the primary infiltrate of neoplastic cells appears as erythematous firm papules or plaques on the face and trunk, similar to other manifestations of leukemia cutis, deposition of IgM presents as translucent papules and plaques and is located more distally, particularly on the extensor extremities.6 These depositional plaques are not pruritic but may be tender if located over sites of pressure, as seen with the plantar presentation in our patient.
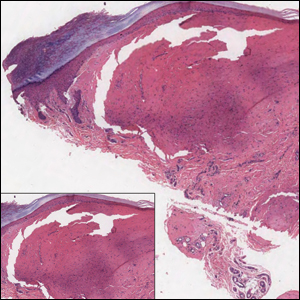
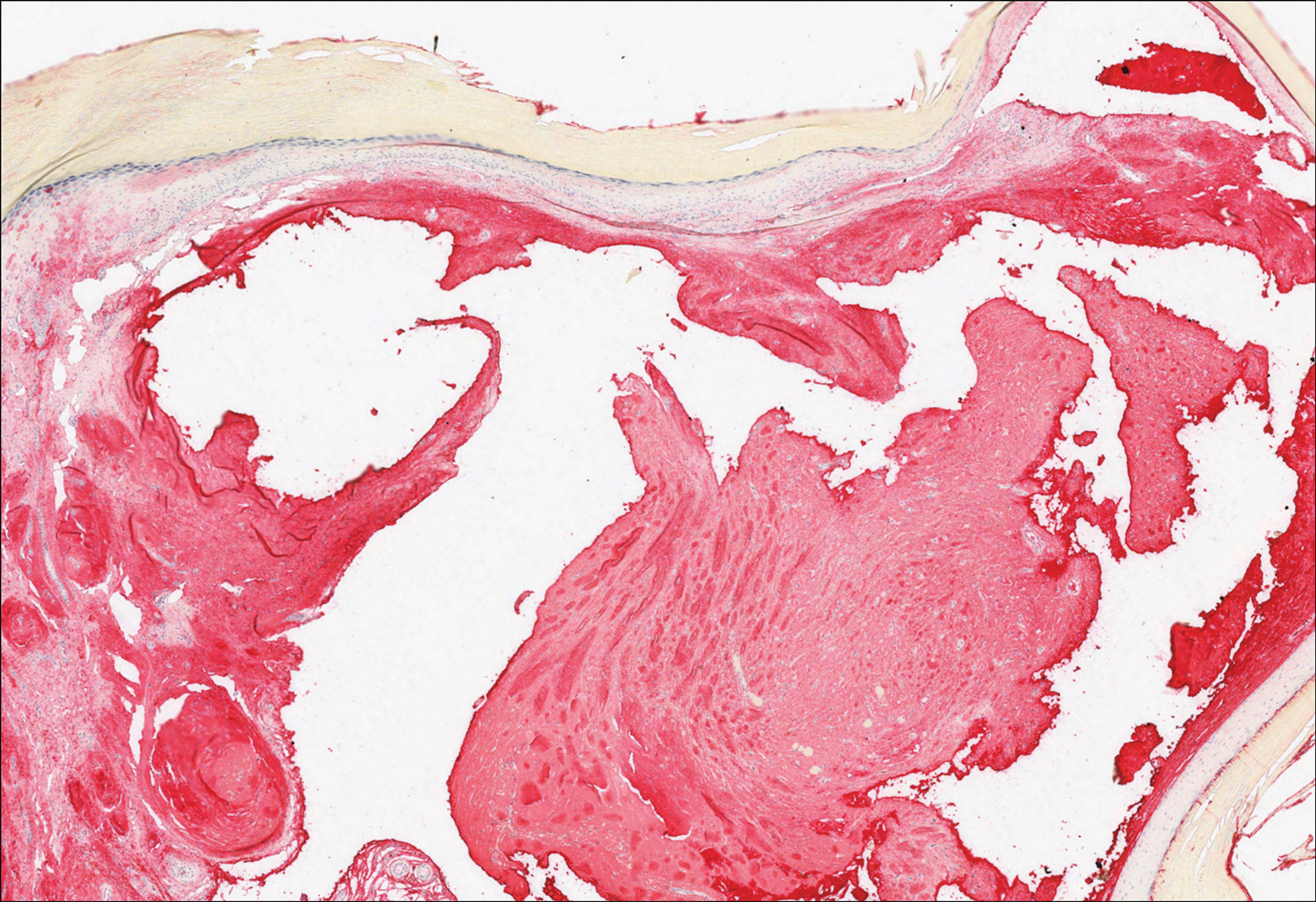
Histologically, cutaneous macroglobulinosis demonstrates IgM deposition in perieccrine, perivascular, or intravascular tissue that is periodic acid-Schiff (PAS) positive.7 Staining with Congo red and Alcian blue is negative. In our case, biopsy showed a nodular deposition of hypocellular globular material that stained brightly with PAS and PAS diastase. With Masson trichome stain, intensity of staining diminished, suggesting that the deposition was not composed of collagen; rather, this deposition appeared to consist of IgM storage papules on immunohistochemistry (Figure 1). Further workup revealed borderline pancytopenia and elevated globulins with a monoclonal peak on serum protein electrophoresis, confirming the diagnosis of cutaneous macroglobulinosis secondary to Waldenström macroglobulinemia.
A PubMed search of articles indexed for MEDLINE using the terms cutaneous, macroglobulinosis, macroglobulinemia, Waldenström's macroglobulinemia, Waldenström's macroglobulinaemia, and macroglobulinemia cutis revealed a total of 19 cases of cutaneous macroglobulinosis (including this case). The average age of presentation in these cases is 60 years (range, 29-83 years) with a predisposition for men (68% [13/19]). The development of cutaneous macroglobulinosis primarily has been noted following diagnosis of Waldenström macroglobulinemia (53% [10/19]), with some cases prior to diagnosis (37% [7/19]) or at the time of diagnosis (11% [2/19]). The presence of cutaneous lesions does not correlate with prognosis of the underlying malignancy.5,8,9
Systemic treatment of the underlying macroglobulinemia has been suggested for symptomatic cases of cutaneous macroglobulinosis.3 Prior therapy has consisted primarily of chlorambucil; however, treatment with rituximab, occasionally in conjunction with the proteasome inhibitor bortezomib, recently has been reported.10 Because of the symptomatic nature of our patient's lesions, she was referred to the oncology department and started on rituximab therapy. The lesions improved with therapy and have remained stable following treatment.
The differential diagnosis for tender pink papules and plaques on the arms and legs includes tophaceous gout, plantar fibromatosis, erythropoietic protoporphyria, and acral fibrokeratoma.
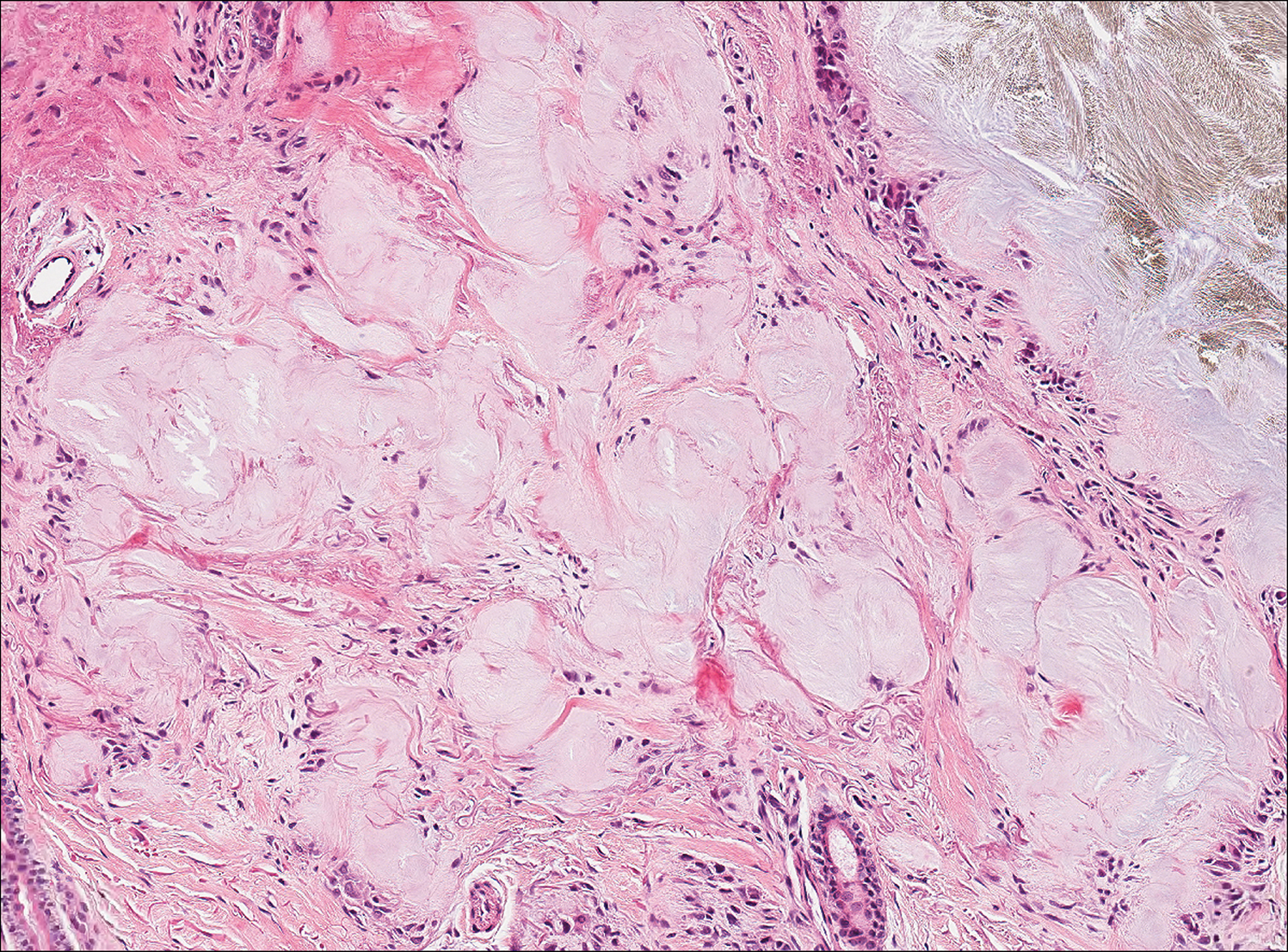
Gouty tophi commonly accumulate as painful, edematous, yellow to whitish nodules and tumors with erythema, often overlying joints or extensor surfaces. Histopathologic examination after formalin fixation shows needle-shaped clefts within feathery amorphous pink areas surrounded by granuloma (Figure 2).11 Yellow, needle-shaped, negatively birefringent crystals can be viewed under polarized microscopy in alcohol-fixed samples.
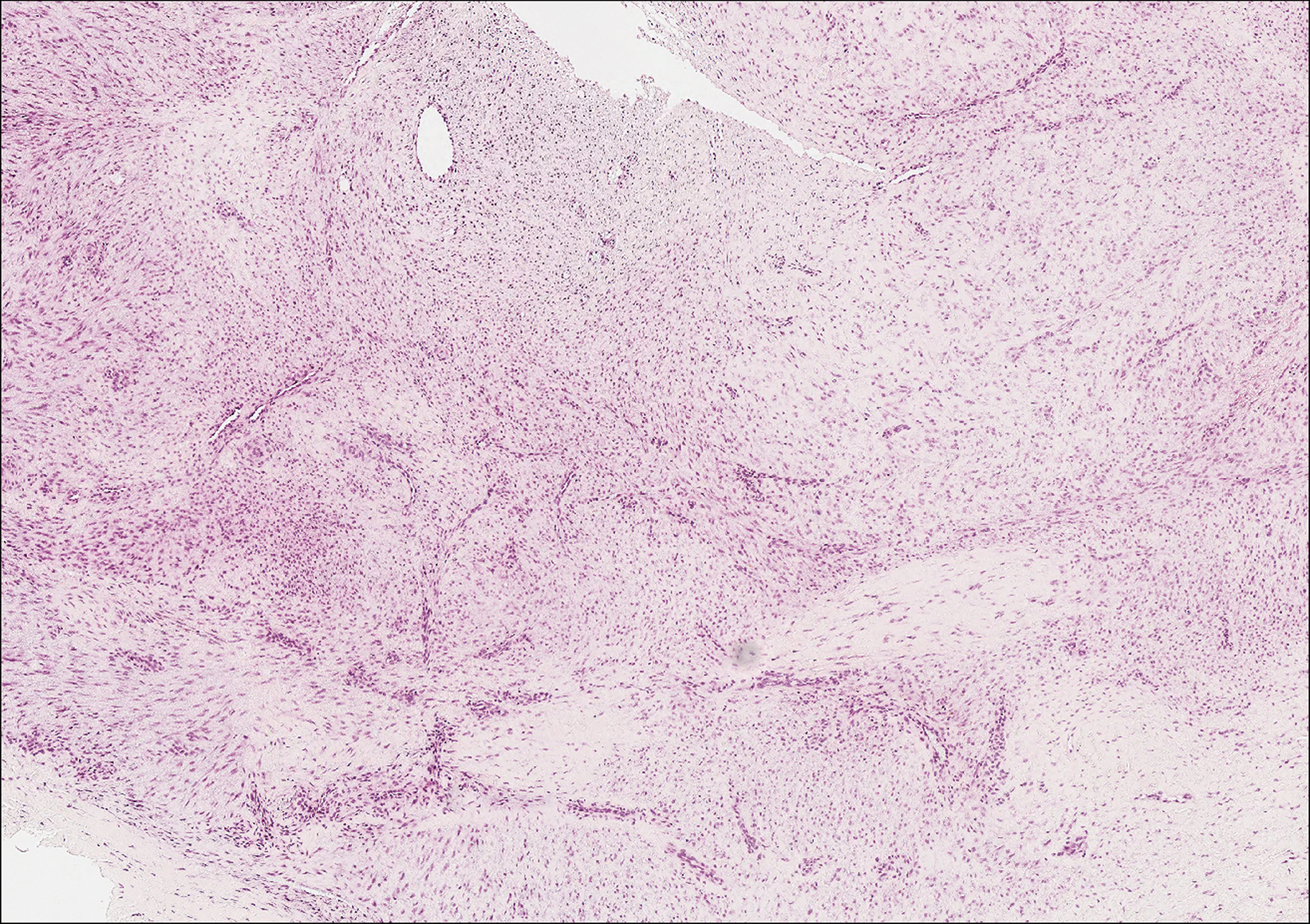
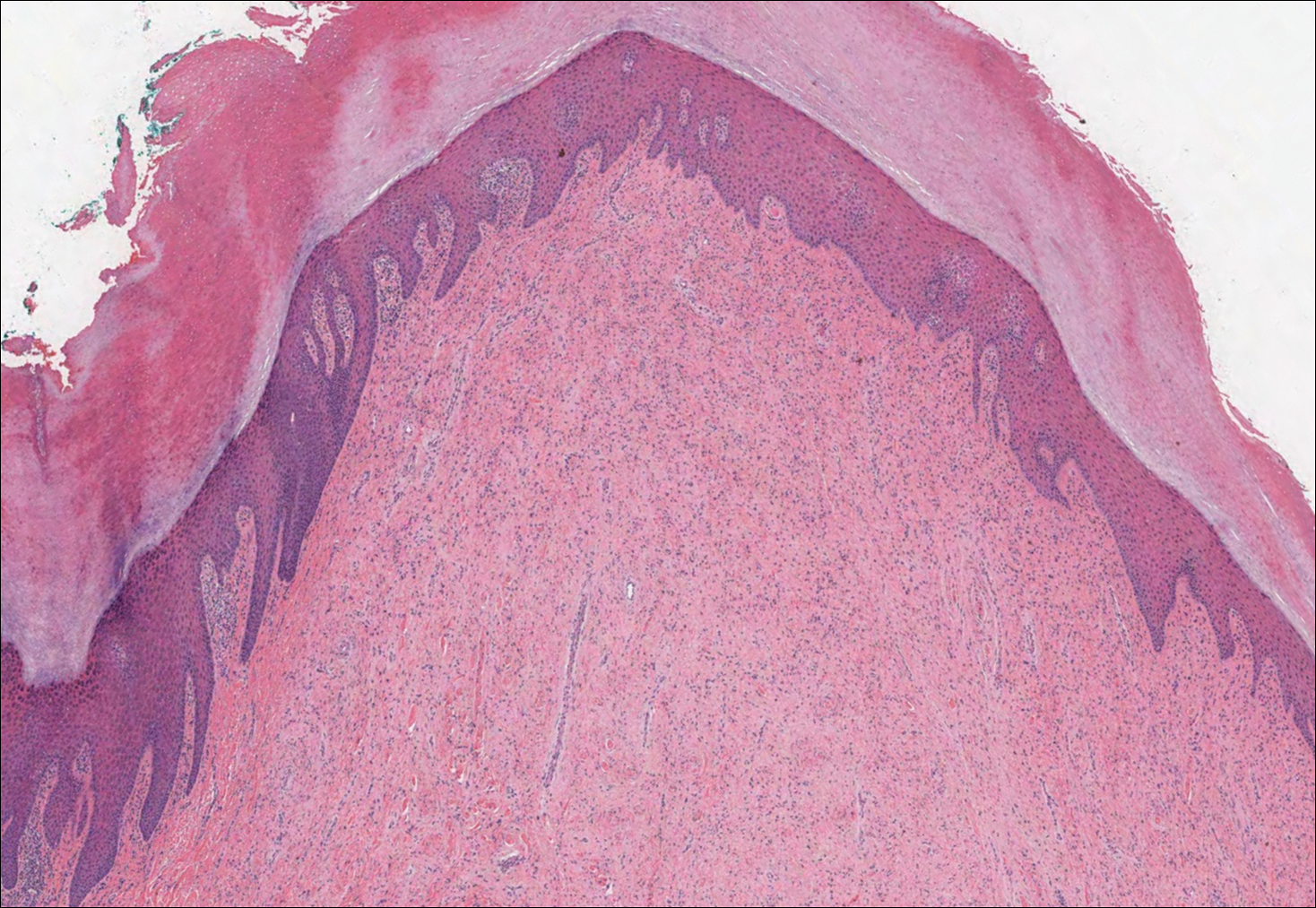
Plantar fibromatosis (Ledderhose disease) is a benign proliferation of the plantar aponeurosis linked to alcohol use; liver disease; and notably epilepsy,12 a component of our patient's medical history. Large nodules appear grossly on the plantar feet and may progress to contractures in more advanced lesions. Biopsy reveals bland hyperproliferation of fibroblasts in a background of fascial fibrous tissue (Figure 3).12 Clinically, this diagnosis is part of the differential diagnosis of plantar nodules but appears histologically different than cutaneous macroglobulinosis because there are no hyaline deposits in plantar fibromatosis.
Erythropoietic protoporphyria is a rare disorder that primarily arises due to a congenital deficiency in the ferrochelatase enzyme involved in heme biosynthesis. Erythropoietic protoporphyria is the most common porphyria among children and typically presents in infancy or early childhood as a painful photosensitivity with ensuing cutaneous manifestations and possible hepatobiliary disease. Edema and severe burning pain can be noted within minutes of sun exposure in a dose-response relationship.13 Histologic findings of erythropoietic protoporphyria differ based on acute or chronic skin changes. Acute lesions exhibit a predominantly neutrophilic interstitial dermal infiltrate with vacuoles and intercellular edema. Chronic changes include the accumulation of a PAS-positive, amorphous, hyalinelike substance, similar to the microscopic findings of cutaneous macroglobulinosis (Figure 4).13
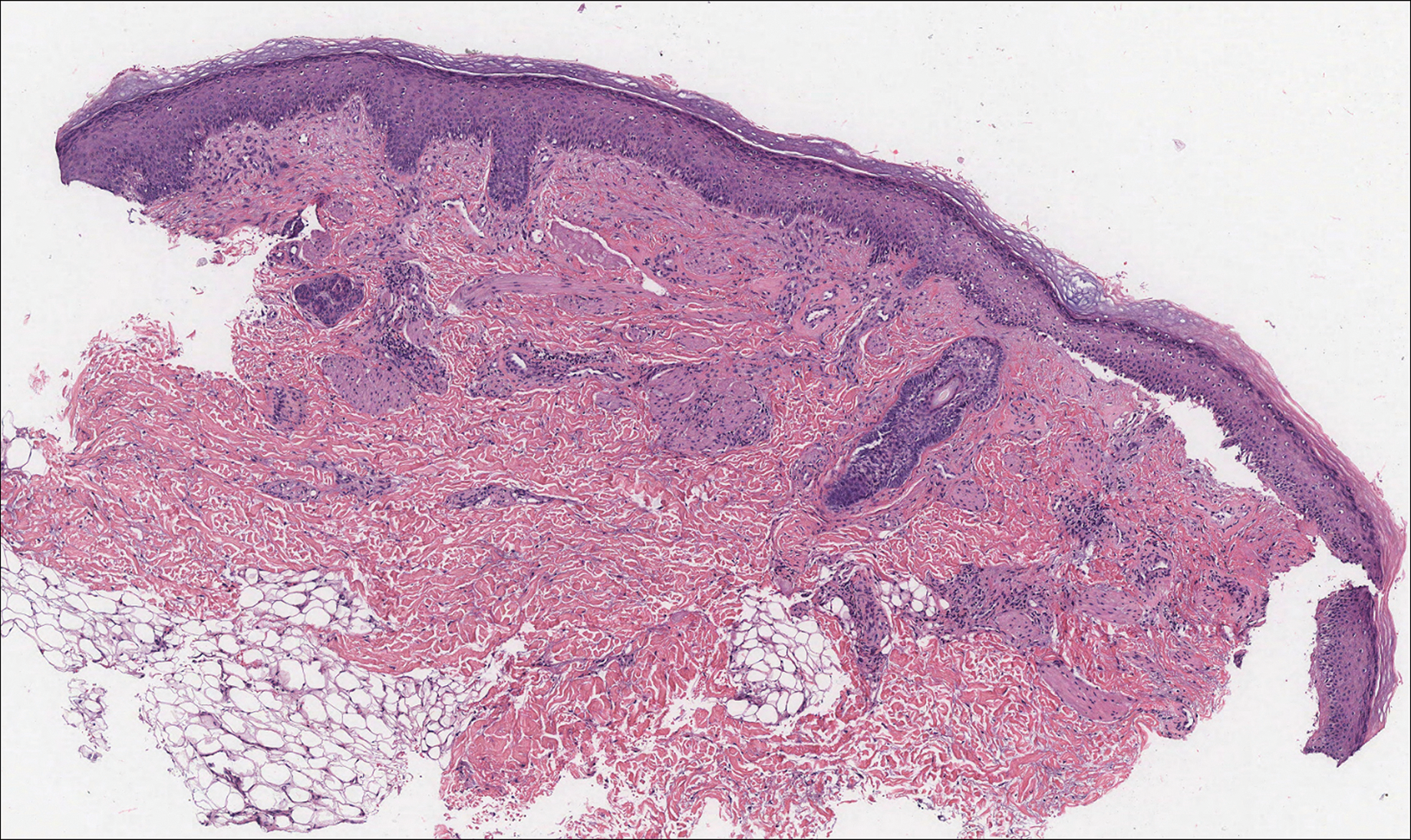
An acral fibrokeratoma is a benign fibroepithelial tumor that clinically appears as a flesh-colored or slightly erythematous exophytic nodule that most commonly is found on the fingers or toes. Thought to arise from trauma to the affected area, it is histologically characterized by interwoven collagenous bundles with overlying epidermal hyperkeratosis, acanthosis, and deep thickened rete ridges14 (Figure 5). Although multiple acral fibrokeratomas have been reported (similar to presentations of prurigo nodularis),15 they more commonly appear as solitary lesions as opposed to the numerous translucent papules seen in our patient.
- Camp BJ, Magro CM. Cutaneous macroglobulinosis: a case series. J Cutan Pathol. 2012;39:962-970.
- Dimopoulos MA, Alexanian R. Waldenstrom's macroglobulinemia. Blood. 1994;83:1452-1459.
- D'Acunto C, Nigrisoli E, Liardo EV, et al. Painful plantar nodules: a specific manifestation of cutaneous macroglobulinosis. J Am Acad Dermatol. 2014;71:E251-E252.
- Tichenor RE. Macroglobulinemia cutis. Arch Dermatol. 1978;114:280-281.
- Gressier L, Hotz C, Lelièvre JD, et al. Cutaneous macroglobulinosis: a report of 2 cases. Arch Dermatol. 2010;146:165-169.
- Spicknall KE, Dubas LE, Mutasim DF. Cutaneous macroglobulinosis with monotypic plasma cells: a specific manifestation of Waldenström macroglobulinemia. J Cutan Pathol. 2013;40:442-444.
- Lüftl M, Sauter-Jenne B, Gramatzki M, et al. Cutaneous macroglobulinosis deposits in a patient with IgM paraproteinemia/incipient Waldenström macroglobulinemia. J Dtsch Dermatol Ges. 2010;8:1000-1003.
- Mascaro JM, Montserrat E, Estrach T, et al. Specific cutaneous manifestations of Waldenstrom macroglobulinaemia: a report of two cases. Br J Dermatol. 1982;106:217-222.
- Hanke CW, Steck WD, Bergfeld WF, et al. Cutaneous macroglobulinosis. Arch Dermatol. 1980;116:575-577.
- Oshio-Yoshii A, Fujimoto N, Shiba Y, et al. Cutaneous macroglobulinosis: successful treatment with rituximab. J Eur Acad Dermatol Venereol. 2017;31:E30-E31.
- Gupta A, Rai S, Sinha R, et al. Tophi as an initial manifestation of gout. J Cytol. 2009;26:165-166.
- Carroll P, Henshaw RM, Garwood C, et al. Plantar fibromatosis: pathophysiology, surgical and nonsurgical therapies: an evidence-based review. Foot Ankle Spec. 2018;11:168-176.
- Michaels BD, Del Rosso JQ, Mobini N, et al. Erythropoietic protoporphyria: a case report and literature review. J Clin Aesthet Dermatol. 2010;3:44-48.
- Boffeli TJ, Abben KW. Acral fibrokeratoma of the foot treated with excision and trap door flap closure: a case report. J Foot Ankle Surg. 2014;53:449-452.
- Reed RJ. Multiple acral fibrokeratomas (a variant of prurigo nodularis). discussion of classification of acral fibrous nodules and of histogenesis of acral fibrokeratomas. Arch Dermatol. 1971;103:287-297.
The Diagnosis: Cutaneous Macroglobulinosis
Waldenström macroglobulinemia is a lymphoplasmacytic lymphoma that produces a circulating monoclonal IgM. Incidence in the United States is 1500 patients annually, most commonly men in their 70s.1 The disease process is largely indolent, with early symptoms consisting of generalized weakness, weight loss, and fatigue. Signs of lymphadenopathy, hepatosplenomegaly, and cytopenia may emerge as the disease progresses. Diagnostic criteria include bone marrow biopsy with plasmacytoid/plasmacellular infiltrate; IgM monoclonal gammopathy; and end-organ damage, which may include cutaneous manifestations.2
Cutaneous findings in Waldenström macroglobulinemia are nonspecific and secondary to the disease's hematologic manifestations, presenting as livedo reticularis, purpura, and mucosal bleeding.3 True cutaneous involvement of the disease is rare and was first described in 1978 by Tichenor.4 Specific cutaneous lesions have 2 separate clinical presentations: (1) a primary cutaneous infiltrate of lymphoplasmacytic cells, and (2) deposition of IgM in the dermis.5 Although the primary infiltrate of neoplastic cells appears as erythematous firm papules or plaques on the face and trunk, similar to other manifestations of leukemia cutis, deposition of IgM presents as translucent papules and plaques and is located more distally, particularly on the extensor extremities.6 These depositional plaques are not pruritic but may be tender if located over sites of pressure, as seen with the plantar presentation in our patient.
Histologically, cutaneous macroglobulinosis demonstrates IgM deposition in perieccrine, perivascular, or intravascular tissue that is periodic acid-Schiff (PAS) positive.7 Staining with Congo red and Alcian blue is negative. In our case, biopsy showed a nodular deposition of hypocellular globular material that stained brightly with PAS and PAS diastase. With Masson trichome stain, intensity of staining diminished, suggesting that the deposition was not composed of collagen; rather, this deposition appeared to consist of IgM storage papules on immunohistochemistry (Figure 1). Further workup revealed borderline pancytopenia and elevated globulins with a monoclonal peak on serum protein electrophoresis, confirming the diagnosis of cutaneous macroglobulinosis secondary to Waldenström macroglobulinemia.

A PubMed search of articles indexed for MEDLINE using the terms cutaneous, macroglobulinosis, macroglobulinemia, Waldenström's macroglobulinemia, Waldenström's macroglobulinaemia, and macroglobulinemia cutis revealed a total of 19 cases of cutaneous macroglobulinosis (including this case). The average age of presentation in these cases is 60 years (range, 29-83 years) with a predisposition for men (68% [13/19]). The development of cutaneous macroglobulinosis primarily has been noted following diagnosis of Waldenström macroglobulinemia (53% [10/19]), with some cases prior to diagnosis (37% [7/19]) or at the time of diagnosis (11% [2/19]). The presence of cutaneous lesions does not correlate with prognosis of the underlying malignancy.5,8,9
Systemic treatment of the underlying macroglobulinemia has been suggested for symptomatic cases of cutaneous macroglobulinosis.3 Prior therapy has consisted primarily of chlorambucil; however, treatment with rituximab, occasionally in conjunction with the proteasome inhibitor bortezomib, recently has been reported.10 Because of the symptomatic nature of our patient's lesions, she was referred to the oncology department and started on rituximab therapy. The lesions improved with therapy and have remained stable following treatment.
The differential diagnosis for tender pink papules and plaques on the arms and legs includes tophaceous gout, plantar fibromatosis, erythropoietic protoporphyria, and acral fibrokeratoma.
Gouty tophi commonly accumulate as painful, edematous, yellow to whitish nodules and tumors with erythema, often overlying joints or extensor surfaces. Histopathologic examination after formalin fixation shows needle-shaped clefts within feathery amorphous pink areas surrounded by granuloma (Figure 2).11 Yellow, needle-shaped, negatively birefringent crystals can be viewed under polarized microscopy in alcohol-fixed samples.

Plantar fibromatosis (Ledderhose disease) is a benign proliferation of the plantar aponeurosis linked to alcohol use; liver disease; and notably epilepsy,12 a component of our patient's medical history. Large nodules appear grossly on the plantar feet and may progress to contractures in more advanced lesions. Biopsy reveals bland hyperproliferation of fibroblasts in a background of fascial fibrous tissue (Figure 3).12 Clinically, this diagnosis is part of the differential diagnosis of plantar nodules but appears histologically different than cutaneous macroglobulinosis because there are no hyaline deposits in plantar fibromatosis.

Erythropoietic protoporphyria is a rare disorder that primarily arises due to a congenital deficiency in the ferrochelatase enzyme involved in heme biosynthesis. Erythropoietic protoporphyria is the most common porphyria among children and typically presents in infancy or early childhood as a painful photosensitivity with ensuing cutaneous manifestations and possible hepatobiliary disease. Edema and severe burning pain can be noted within minutes of sun exposure in a dose-response relationship.13 Histologic findings of erythropoietic protoporphyria differ based on acute or chronic skin changes. Acute lesions exhibit a predominantly neutrophilic interstitial dermal infiltrate with vacuoles and intercellular edema. Chronic changes include the accumulation of a PAS-positive, amorphous, hyalinelike substance, similar to the microscopic findings of cutaneous macroglobulinosis (Figure 4).13

An acral fibrokeratoma is a benign fibroepithelial tumor that clinically appears as a flesh-colored or slightly erythematous exophytic nodule that most commonly is found on the fingers or toes. Thought to arise from trauma to the affected area, it is histologically characterized by interwoven collagenous bundles with overlying epidermal hyperkeratosis, acanthosis, and deep thickened rete ridges14 (Figure 5). Although multiple acral fibrokeratomas have been reported (similar to presentations of prurigo nodularis),15 they more commonly appear as solitary lesions as opposed to the numerous translucent papules seen in our patient.

The Diagnosis: Cutaneous Macroglobulinosis
Waldenström macroglobulinemia is a lymphoplasmacytic lymphoma that produces a circulating monoclonal IgM. Incidence in the United States is 1500 patients annually, most commonly men in their 70s.1 The disease process is largely indolent, with early symptoms consisting of generalized weakness, weight loss, and fatigue. Signs of lymphadenopathy, hepatosplenomegaly, and cytopenia may emerge as the disease progresses. Diagnostic criteria include bone marrow biopsy with plasmacytoid/plasmacellular infiltrate; IgM monoclonal gammopathy; and end-organ damage, which may include cutaneous manifestations.2
Cutaneous findings in Waldenström macroglobulinemia are nonspecific and secondary to the disease's hematologic manifestations, presenting as livedo reticularis, purpura, and mucosal bleeding.3 True cutaneous involvement of the disease is rare and was first described in 1978 by Tichenor.4 Specific cutaneous lesions have 2 separate clinical presentations: (1) a primary cutaneous infiltrate of lymphoplasmacytic cells, and (2) deposition of IgM in the dermis.5 Although the primary infiltrate of neoplastic cells appears as erythematous firm papules or plaques on the face and trunk, similar to other manifestations of leukemia cutis, deposition of IgM presents as translucent papules and plaques and is located more distally, particularly on the extensor extremities.6 These depositional plaques are not pruritic but may be tender if located over sites of pressure, as seen with the plantar presentation in our patient.
Histologically, cutaneous macroglobulinosis demonstrates IgM deposition in perieccrine, perivascular, or intravascular tissue that is periodic acid-Schiff (PAS) positive.7 Staining with Congo red and Alcian blue is negative. In our case, biopsy showed a nodular deposition of hypocellular globular material that stained brightly with PAS and PAS diastase. With Masson trichome stain, intensity of staining diminished, suggesting that the deposition was not composed of collagen; rather, this deposition appeared to consist of IgM storage papules on immunohistochemistry (Figure 1). Further workup revealed borderline pancytopenia and elevated globulins with a monoclonal peak on serum protein electrophoresis, confirming the diagnosis of cutaneous macroglobulinosis secondary to Waldenström macroglobulinemia.

A PubMed search of articles indexed for MEDLINE using the terms cutaneous, macroglobulinosis, macroglobulinemia, Waldenström's macroglobulinemia, Waldenström's macroglobulinaemia, and macroglobulinemia cutis revealed a total of 19 cases of cutaneous macroglobulinosis (including this case). The average age of presentation in these cases is 60 years (range, 29-83 years) with a predisposition for men (68% [13/19]). The development of cutaneous macroglobulinosis primarily has been noted following diagnosis of Waldenström macroglobulinemia (53% [10/19]), with some cases prior to diagnosis (37% [7/19]) or at the time of diagnosis (11% [2/19]). The presence of cutaneous lesions does not correlate with prognosis of the underlying malignancy.5,8,9
Systemic treatment of the underlying macroglobulinemia has been suggested for symptomatic cases of cutaneous macroglobulinosis.3 Prior therapy has consisted primarily of chlorambucil; however, treatment with rituximab, occasionally in conjunction with the proteasome inhibitor bortezomib, recently has been reported.10 Because of the symptomatic nature of our patient's lesions, she was referred to the oncology department and started on rituximab therapy. The lesions improved with therapy and have remained stable following treatment.
The differential diagnosis for tender pink papules and plaques on the arms and legs includes tophaceous gout, plantar fibromatosis, erythropoietic protoporphyria, and acral fibrokeratoma.
Gouty tophi commonly accumulate as painful, edematous, yellow to whitish nodules and tumors with erythema, often overlying joints or extensor surfaces. Histopathologic examination after formalin fixation shows needle-shaped clefts within feathery amorphous pink areas surrounded by granuloma (Figure 2).11 Yellow, needle-shaped, negatively birefringent crystals can be viewed under polarized microscopy in alcohol-fixed samples.

Plantar fibromatosis (Ledderhose disease) is a benign proliferation of the plantar aponeurosis linked to alcohol use; liver disease; and notably epilepsy,12 a component of our patient's medical history. Large nodules appear grossly on the plantar feet and may progress to contractures in more advanced lesions. Biopsy reveals bland hyperproliferation of fibroblasts in a background of fascial fibrous tissue (Figure 3).12 Clinically, this diagnosis is part of the differential diagnosis of plantar nodules but appears histologically different than cutaneous macroglobulinosis because there are no hyaline deposits in plantar fibromatosis.

Erythropoietic protoporphyria is a rare disorder that primarily arises due to a congenital deficiency in the ferrochelatase enzyme involved in heme biosynthesis. Erythropoietic protoporphyria is the most common porphyria among children and typically presents in infancy or early childhood as a painful photosensitivity with ensuing cutaneous manifestations and possible hepatobiliary disease. Edema and severe burning pain can be noted within minutes of sun exposure in a dose-response relationship.13 Histologic findings of erythropoietic protoporphyria differ based on acute or chronic skin changes. Acute lesions exhibit a predominantly neutrophilic interstitial dermal infiltrate with vacuoles and intercellular edema. Chronic changes include the accumulation of a PAS-positive, amorphous, hyalinelike substance, similar to the microscopic findings of cutaneous macroglobulinosis (Figure 4).13

An acral fibrokeratoma is a benign fibroepithelial tumor that clinically appears as a flesh-colored or slightly erythematous exophytic nodule that most commonly is found on the fingers or toes. Thought to arise from trauma to the affected area, it is histologically characterized by interwoven collagenous bundles with overlying epidermal hyperkeratosis, acanthosis, and deep thickened rete ridges14 (Figure 5). Although multiple acral fibrokeratomas have been reported (similar to presentations of prurigo nodularis),15 they more commonly appear as solitary lesions as opposed to the numerous translucent papules seen in our patient.

- Camp BJ, Magro CM. Cutaneous macroglobulinosis: a case series. J Cutan Pathol. 2012;39:962-970.
- Dimopoulos MA, Alexanian R. Waldenstrom's macroglobulinemia. Blood. 1994;83:1452-1459.
- D'Acunto C, Nigrisoli E, Liardo EV, et al. Painful plantar nodules: a specific manifestation of cutaneous macroglobulinosis. J Am Acad Dermatol. 2014;71:E251-E252.
- Tichenor RE. Macroglobulinemia cutis. Arch Dermatol. 1978;114:280-281.
- Gressier L, Hotz C, Lelièvre JD, et al. Cutaneous macroglobulinosis: a report of 2 cases. Arch Dermatol. 2010;146:165-169.
- Spicknall KE, Dubas LE, Mutasim DF. Cutaneous macroglobulinosis with monotypic plasma cells: a specific manifestation of Waldenström macroglobulinemia. J Cutan Pathol. 2013;40:442-444.
- Lüftl M, Sauter-Jenne B, Gramatzki M, et al. Cutaneous macroglobulinosis deposits in a patient with IgM paraproteinemia/incipient Waldenström macroglobulinemia. J Dtsch Dermatol Ges. 2010;8:1000-1003.
- Mascaro JM, Montserrat E, Estrach T, et al. Specific cutaneous manifestations of Waldenstrom macroglobulinaemia: a report of two cases. Br J Dermatol. 1982;106:217-222.
- Hanke CW, Steck WD, Bergfeld WF, et al. Cutaneous macroglobulinosis. Arch Dermatol. 1980;116:575-577.
- Oshio-Yoshii A, Fujimoto N, Shiba Y, et al. Cutaneous macroglobulinosis: successful treatment with rituximab. J Eur Acad Dermatol Venereol. 2017;31:E30-E31.
- Gupta A, Rai S, Sinha R, et al. Tophi as an initial manifestation of gout. J Cytol. 2009;26:165-166.
- Carroll P, Henshaw RM, Garwood C, et al. Plantar fibromatosis: pathophysiology, surgical and nonsurgical therapies: an evidence-based review. Foot Ankle Spec. 2018;11:168-176.
- Michaels BD, Del Rosso JQ, Mobini N, et al. Erythropoietic protoporphyria: a case report and literature review. J Clin Aesthet Dermatol. 2010;3:44-48.
- Boffeli TJ, Abben KW. Acral fibrokeratoma of the foot treated with excision and trap door flap closure: a case report. J Foot Ankle Surg. 2014;53:449-452.
- Reed RJ. Multiple acral fibrokeratomas (a variant of prurigo nodularis). discussion of classification of acral fibrous nodules and of histogenesis of acral fibrokeratomas. Arch Dermatol. 1971;103:287-297.
- Camp BJ, Magro CM. Cutaneous macroglobulinosis: a case series. J Cutan Pathol. 2012;39:962-970.
- Dimopoulos MA, Alexanian R. Waldenstrom's macroglobulinemia. Blood. 1994;83:1452-1459.
- D'Acunto C, Nigrisoli E, Liardo EV, et al. Painful plantar nodules: a specific manifestation of cutaneous macroglobulinosis. J Am Acad Dermatol. 2014;71:E251-E252.
- Tichenor RE. Macroglobulinemia cutis. Arch Dermatol. 1978;114:280-281.
- Gressier L, Hotz C, Lelièvre JD, et al. Cutaneous macroglobulinosis: a report of 2 cases. Arch Dermatol. 2010;146:165-169.
- Spicknall KE, Dubas LE, Mutasim DF. Cutaneous macroglobulinosis with monotypic plasma cells: a specific manifestation of Waldenström macroglobulinemia. J Cutan Pathol. 2013;40:442-444.
- Lüftl M, Sauter-Jenne B, Gramatzki M, et al. Cutaneous macroglobulinosis deposits in a patient with IgM paraproteinemia/incipient Waldenström macroglobulinemia. J Dtsch Dermatol Ges. 2010;8:1000-1003.
- Mascaro JM, Montserrat E, Estrach T, et al. Specific cutaneous manifestations of Waldenstrom macroglobulinaemia: a report of two cases. Br J Dermatol. 1982;106:217-222.
- Hanke CW, Steck WD, Bergfeld WF, et al. Cutaneous macroglobulinosis. Arch Dermatol. 1980;116:575-577.
- Oshio-Yoshii A, Fujimoto N, Shiba Y, et al. Cutaneous macroglobulinosis: successful treatment with rituximab. J Eur Acad Dermatol Venereol. 2017;31:E30-E31.
- Gupta A, Rai S, Sinha R, et al. Tophi as an initial manifestation of gout. J Cytol. 2009;26:165-166.
- Carroll P, Henshaw RM, Garwood C, et al. Plantar fibromatosis: pathophysiology, surgical and nonsurgical therapies: an evidence-based review. Foot Ankle Spec. 2018;11:168-176.
- Michaels BD, Del Rosso JQ, Mobini N, et al. Erythropoietic protoporphyria: a case report and literature review. J Clin Aesthet Dermatol. 2010;3:44-48.
- Boffeli TJ, Abben KW. Acral fibrokeratoma of the foot treated with excision and trap door flap closure: a case report. J Foot Ankle Surg. 2014;53:449-452.
- Reed RJ. Multiple acral fibrokeratomas (a variant of prurigo nodularis). discussion of classification of acral fibrous nodules and of histogenesis of acral fibrokeratomas. Arch Dermatol. 1971;103:287-297.
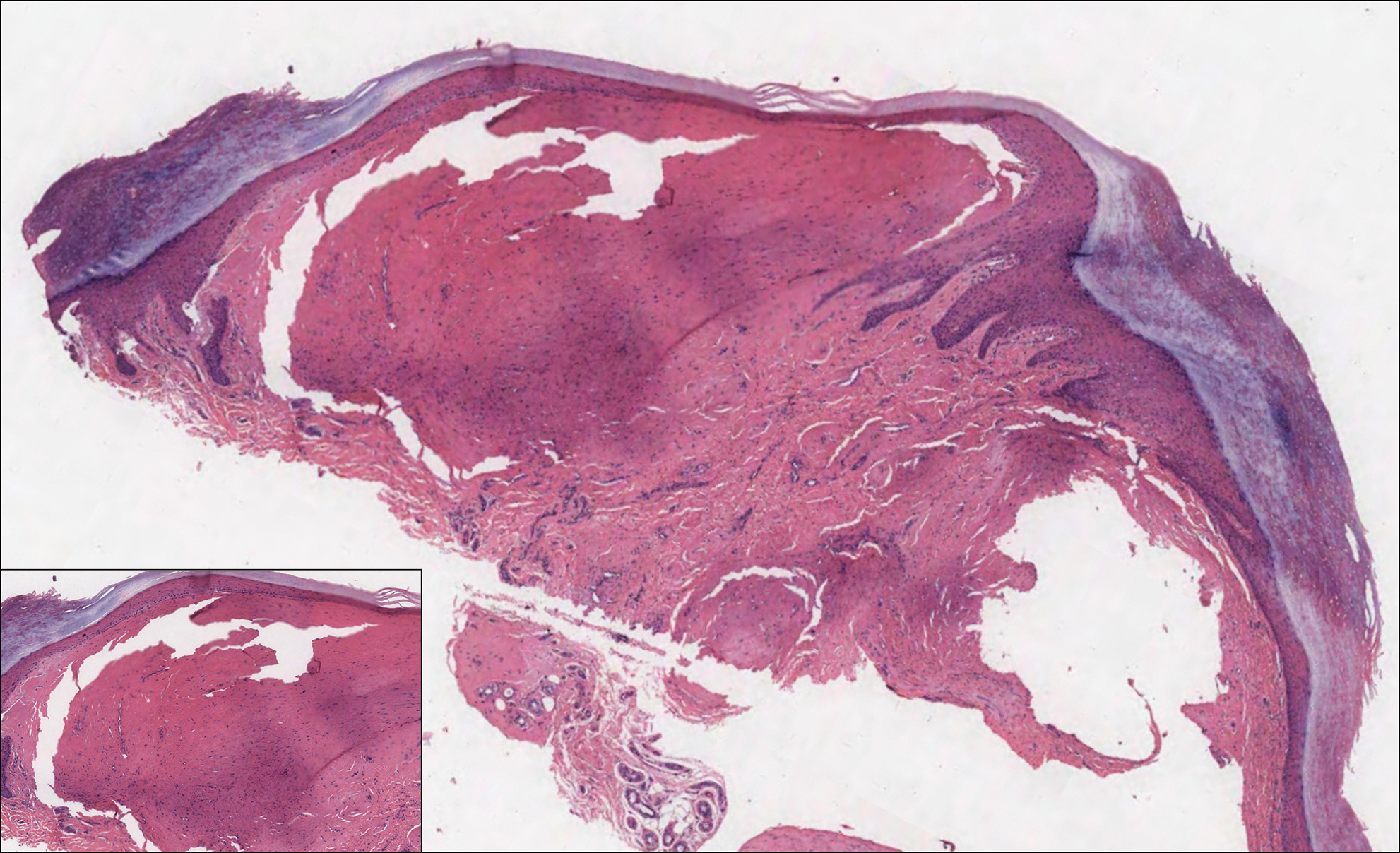
A 64-year-old woman with a medical history of Waldenström macroglobulinemia, multiple sclerosis, and epilepsy presented with slowly growing papules on the plantar feet of 21 months' duration. She was diagnosed with Waldenström macroglobulinemia incidentally on routine blood work 3 years prior and declined treatment because she was asymptomatic. Physical examination revealed a total of 20 firm, variably sized, light pink to purple, partially translucent and telangiectatic papules and plaques bilaterally on the plantar feet. A plaque from the right sole was biopsied.
Elagolix: A new treatment for pelvic pain caused by endometriosis
Endometriosis is the presence of tissue resembling endometrial glands and stroma outside of the uterine cavity. Women with endometriosis often present for medical care with at least one of 3 problems: pelvic pain, infertility, and/or an adnexal mass due to endometriosis.1 Many clinical observations demonstrate that endometriosis lesions require estrogen to grow and maintain their viability, including that: (1) endometriosis is uncommon before puberty or after menopause, (2) surgical removal of both ovaries results in regression of endometriosis lesions, and (3) gonadotropin-releasing hormone (GnRH) analogues cause a hypo‑estrogenic hormonal environment, resulting in regression of endometriosis lesions and improvement in pelvic pain. Since endometriosis lesions require estrogen to maintain their viability, suppressing estradiol is a logical approach to hormonal treatment of the disease.
The estrogen threshold hypothesis
The estradiol concentration that causes endometriosis lesions to grow or regress varies among women, but a concentration less than 20 pg/mL usually causes lesions to regress, and a concentration greater than 60 pg/mL usually supports lesion growth and maintains lesion viability.2 Although an estradiol concentration below 20 pg/mL may cause lesions to regress, it also is associated with moderate to severe hot flashes and accelerated bone loss. These adverse effects limit the use of strong suppression of estrogen as a long-term treatment strategy. The estrogen threshold hypothesis posits that gently suppressing estradiol to a concentration between 20 and 45 pg/mL may simultaneously cause endometriosis lesions to regress, resulting in reduced pelvic pain, minimal bone loss, and few hot flashes.2
Building on the estrogen threshold hypothesis, clinicians have two options for treatment of pelvic pain caused by endometriosis:
- strong suppression of estradiol to a concentration below 20 pg/mL
- gentle suppression of estradiol to a concentration in the range of 20 to 45 pg/mL.
Strong suppression of estradiol to levels below 20 pg/mL will reliably induce amenorrhea and cause regression of endometriosis lesions, thereby reducing pelvic pain. Strong suppression of estradiol also will cause moderate to severe hot flashes and accelerated bone loss in many women. By contrast, gentle suppression of circulating estradiol to a concentration in the range of 20 to 45 pg/mL may result in amenorrhea or oligomenorrhea, suppression of the growth of endometriosis lesions, a modest reduction in pelvic pain, mild hot flashes, and minimal bone loss.
Recently, the US Food and Drug Administration (FDA) approved elagolix, an oral GnRH antagonist, for treatment of endometriosis.3 Elagolix blocks GnRH receptors in the pituitary gland, resulting in reduced production of luteinizing hormone and follicle stimulating hormone and a decrease in sex steroid secretion in the ovarian follicles, which leads to a reduction in the production and circulating concentration of estradiol. The FDA approved two doses of elagolix: 150 mg once daily for up to 24 months and 200 mg twice daily for up to 6 months. Importantly, elagolix at a dose of 150 mg once daily results in a mean circulating estradiol concentration of 41 pg/mL, indicating gentle suppression of ovarian estradiol production, and 200 mg twice daily results in a mean circulating ovarian estradiol concentration of 12 pg/mL, indicating strong suppression of ovarian estradiol production.3 For clinicians treating women with pelvic pain caused by endometriosis, these two elagolix regimens permit the individualization of hormonal therapy to the unique needs of each woman.
Continue to: Safety information for elagolix
- Contraindications: Elagolix should not be prescribed to women who are currently pregnant or have known osteoporosis or severe hepatic impairment. Elagolix should not be used in women taking cyclosporine or gemfibrozil (organic anion transporting polypeptide inhibitors).
- Elagolix may cause dose-dependent bone loss.
- Elagolix reduces menstrual bleeding, which may make it difficult to recognize the occurrence of pregnancy. Nonhormonal contraceptives should be utilized during elagolix treatment.
- Elagolix may be associated with an increase in reported depressive symptoms and mood changes.
- Elagolix may be associated with an increase in alanine aminotransferase more than 3 times the upper limit of the reference range. If elevated liver function tests are detected, the benefits and risks of continuing elagolix treatment should be evaluated.
Elagolix benefits and adverse effects
In one large clinical trial (Elaris Endometriosis I), 872 women were randomly assigned to treatment with one of two doses of elagolix (200 mg twice daily [high-dose group] or 150 mgonce daily [low-dose group]) or placebo.4 After 3 months of treatment, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of women in the high-dose, low-dose, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). In addition, at 3 months, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of women in the high-dose, low-dose, and placebo groups, respectively (low-dose vs placebo, P<.01; high-dose vs placebo, P<.001). Hot flashes that were severe enough to be classified as adverse events by study participants were reported by 42%, 24%, and 7% of the women in the high-dose, low-dose, and placebo groups, respectively. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47%, and hip/femoral/neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose, low-dose, and placebo groups, respectively.
Another large clinical trial of elagolix for treatment of pelvic pain caused by endometriosis (Elaris II) involving 817 women produced results that were similar to those reported in Elaris I.4 The elagolix continuation studies, Elaris III and IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.5
Depot leuprolide acetate and nafarelin acetate
Depot leuprolide acetate and nafarelin acetate are GnRH analogues approved by the FDA more than 25 years ago for treatment of pelvic pain caused by endometriosis. Over the past two decades, depot leuprolide acetate has been one of the most commonly used hormonal treatments for endometriosis in the United States. A 3-month formulation of depot leuprolide acetate with an 11.25-mg injection has resulted in mean circulating estradiol concentrations of 8 pg/mL, indicating very strong suppression of estradiol production.6 A twice-daily 200-µg dose of nafarelin acetate nasal spray has resulted in a circulating estradiol concentration of approximately 28 pg/mL, indicating gentle suppression of estradiol production.7
At current prices, elagolix treatment is substantially less expensive than treatment with leuprolide or nafarelin. In addition, many women in my practice prefer to use an oral medication over an intramuscular injection or a nasal spray medication. It is likely that clinicians and patients will evolve to prioritize and favor elagolix therapy over depot leuprolide or nafarelin treatment.
Continue to: 5 options for using elagolix
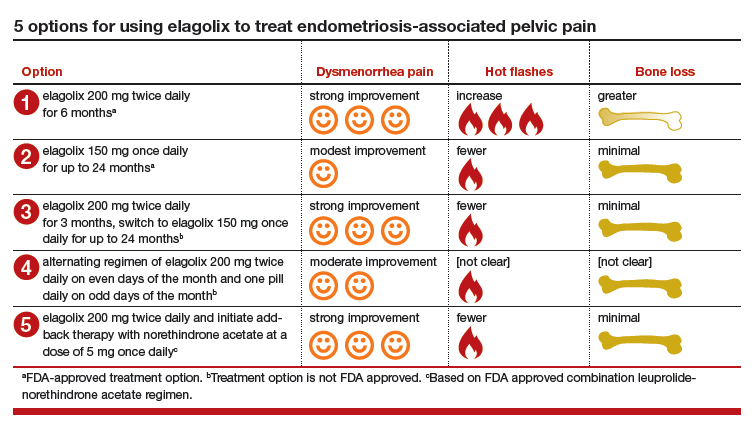
5 options for using elagolix
There are many potential options for using elagolix in the treatment of pelvic pain caused by endometriosis.
Option 1. Prescribe elagolix 200 mg twice daily for 6 months to achieve strong suppression of estradiol and marked improvement in dysmenorrhea, although at the cost of more hot flashes and greater bone loss.
Option 2. Prescribe elagolix 150 mg once daily for up to 24 months to achieve gentle suppression of estradiol and modest improvement in dysmenorrhea with fewer hot flashes and minimal bone loss.
Options 1 and 2 have been studied in high quality clinical trials involving more than 1,500 women and are approved by the FDA.
Option 3. Initiate treatment with elagolix 200 mg twice daily for 3 months, immediately accruing the benefits of strong suppression of estradiol, and then switch to elagolix 150 mg once daily for up to 24 months to achieve continuing pain control with fewer adverse effects. This regimen combines strong initial suppression of estradiol, which will result in marked improvement in dysmenorrhea, along with long-term gentle suppression of estradiol, which is likely to maintain decreased pain symptoms with minimal long-term bone loss and fewer hot flashes.
Option 4. Prescribe an alternating regimen of elagolix 200 mg twice daily on even days of the month (two pills daily is an even number of pills) and one pill daily on odd days of the month (1 pill daily is an odd number of pills). This regimen should produce a mean estradiol concentration between 12 and 41 pg/mL, resulting in moderate rather than strong or gentle suppression of estradiol.
Options 3 and 4 are based on extrapolation using our knowledge about the hormonal treatment of endometriosis and are not regimens approved by the FDA.
Option 5. Prescribe elagolix 200 mg twice daily and initiate add-back therapy with norethindrone acetate 5 mg once daily. Substantial evidence supports the combination of a GnRH analogue that strongly suppresses estradiol production with norethindrone acetate add-back, which helps mitigate the bone loss that occurs with strong suppression of estradiol and reduces the frequency of moderate to severe hot flashes.
Option 5 is based on extrapolation from high-quality studies of leuprolide acetate depot plus norethindrone acetate add-back.8 The combination regimen is approved by the FDA.3
Elagolix availability increases treatment choices for women
Pelvic pain caused by endometriosis is common, affecting approximately 8% of women of reproductive age.9 Endometriosis is a vexing disease because diagnosis is often delayed many years after the onset of symptoms, causing great frustration among patients.10 Some effective hormonal treatment options, including danazol and depot leuprolide, are poorly tolerated by patients because of adverse effects, including weight gain (danazol), hot flashes, and bone loss (depot leuprolide). Combination oral contraceptives used in a continuous or cyclic fashion often result in inadequate improvement in pelvic pain.11 The synthesis of an orally active, small-molecule GnRH antagonist is an innovative advance in endocrine pharmacology. The Elaris Endometriosis clinical trials have demonstrated that elagolix is effective in the treatment of pelvic pain caused by endometriosis.4,5 A great advantage of elagolix is that dosing can be tailored for each patient to achieve reduction in pain while minimizing unwanted adverse effects such as hot flashes and bone loss. In Elaris Endometriosis I, fewer than 10% of women discontinued elagolix due to adverse effects.4 Elagolix is also less expensive than depot leuprolide and nafarelin.
Millions of women in the United States have pelvic pain caused by endometriosis. Obstetrician-gynecologists are the clinicians best trained to care for these women, and patients trust that we will effectively treat their problem.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol. 2018;131:557-571.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Orlissa [package insert]. North Chicago, IL: AbbVie Inc; 2018.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017; 377: 28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Lupron Depot [package insert]. North Chicago, IL: Abbott Laboratories: 2012.
- Henzl MR, Corson SL, Moghissi K, et al. Administration of nasal nafarelin as compared with oral danazol for endometriosis. a multicenter double-blind comparative clinical trial. N Engl J Med. 1988;318:485-489.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Missmer SA, Hankinson SE, Spiegelman D, et al. The incidence of laparoscopically-confirmed endometriosis by demographic, anthropomorphic and lifestyle factors. Am J Epidemiol. 2004;160:784-796.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8,10-11,16.
- Jensen JT, Schlaff W, Gordon K. Use of combined hormonal contraceptives for the treatment of endometriosis-related pain: a systematic review of the evidence. Fertil Steril. 2018;110:137-152.
Endometriosis is the presence of tissue resembling endometrial glands and stroma outside of the uterine cavity. Women with endometriosis often present for medical care with at least one of 3 problems: pelvic pain, infertility, and/or an adnexal mass due to endometriosis.1 Many clinical observations demonstrate that endometriosis lesions require estrogen to grow and maintain their viability, including that: (1) endometriosis is uncommon before puberty or after menopause, (2) surgical removal of both ovaries results in regression of endometriosis lesions, and (3) gonadotropin-releasing hormone (GnRH) analogues cause a hypo‑estrogenic hormonal environment, resulting in regression of endometriosis lesions and improvement in pelvic pain. Since endometriosis lesions require estrogen to maintain their viability, suppressing estradiol is a logical approach to hormonal treatment of the disease.
The estrogen threshold hypothesis
The estradiol concentration that causes endometriosis lesions to grow or regress varies among women, but a concentration less than 20 pg/mL usually causes lesions to regress, and a concentration greater than 60 pg/mL usually supports lesion growth and maintains lesion viability.2 Although an estradiol concentration below 20 pg/mL may cause lesions to regress, it also is associated with moderate to severe hot flashes and accelerated bone loss. These adverse effects limit the use of strong suppression of estrogen as a long-term treatment strategy. The estrogen threshold hypothesis posits that gently suppressing estradiol to a concentration between 20 and 45 pg/mL may simultaneously cause endometriosis lesions to regress, resulting in reduced pelvic pain, minimal bone loss, and few hot flashes.2
Building on the estrogen threshold hypothesis, clinicians have two options for treatment of pelvic pain caused by endometriosis:
- strong suppression of estradiol to a concentration below 20 pg/mL
- gentle suppression of estradiol to a concentration in the range of 20 to 45 pg/mL.
Strong suppression of estradiol to levels below 20 pg/mL will reliably induce amenorrhea and cause regression of endometriosis lesions, thereby reducing pelvic pain. Strong suppression of estradiol also will cause moderate to severe hot flashes and accelerated bone loss in many women. By contrast, gentle suppression of circulating estradiol to a concentration in the range of 20 to 45 pg/mL may result in amenorrhea or oligomenorrhea, suppression of the growth of endometriosis lesions, a modest reduction in pelvic pain, mild hot flashes, and minimal bone loss.
Recently, the US Food and Drug Administration (FDA) approved elagolix, an oral GnRH antagonist, for treatment of endometriosis.3 Elagolix blocks GnRH receptors in the pituitary gland, resulting in reduced production of luteinizing hormone and follicle stimulating hormone and a decrease in sex steroid secretion in the ovarian follicles, which leads to a reduction in the production and circulating concentration of estradiol. The FDA approved two doses of elagolix: 150 mg once daily for up to 24 months and 200 mg twice daily for up to 6 months. Importantly, elagolix at a dose of 150 mg once daily results in a mean circulating estradiol concentration of 41 pg/mL, indicating gentle suppression of ovarian estradiol production, and 200 mg twice daily results in a mean circulating ovarian estradiol concentration of 12 pg/mL, indicating strong suppression of ovarian estradiol production.3 For clinicians treating women with pelvic pain caused by endometriosis, these two elagolix regimens permit the individualization of hormonal therapy to the unique needs of each woman.
Continue to: Safety information for elagolix
- Contraindications: Elagolix should not be prescribed to women who are currently pregnant or have known osteoporosis or severe hepatic impairment. Elagolix should not be used in women taking cyclosporine or gemfibrozil (organic anion transporting polypeptide inhibitors).
- Elagolix may cause dose-dependent bone loss.
- Elagolix reduces menstrual bleeding, which may make it difficult to recognize the occurrence of pregnancy. Nonhormonal contraceptives should be utilized during elagolix treatment.
- Elagolix may be associated with an increase in reported depressive symptoms and mood changes.
- Elagolix may be associated with an increase in alanine aminotransferase more than 3 times the upper limit of the reference range. If elevated liver function tests are detected, the benefits and risks of continuing elagolix treatment should be evaluated.
Elagolix benefits and adverse effects
In one large clinical trial (Elaris Endometriosis I), 872 women were randomly assigned to treatment with one of two doses of elagolix (200 mg twice daily [high-dose group] or 150 mgonce daily [low-dose group]) or placebo.4 After 3 months of treatment, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of women in the high-dose, low-dose, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). In addition, at 3 months, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of women in the high-dose, low-dose, and placebo groups, respectively (low-dose vs placebo, P<.01; high-dose vs placebo, P<.001). Hot flashes that were severe enough to be classified as adverse events by study participants were reported by 42%, 24%, and 7% of the women in the high-dose, low-dose, and placebo groups, respectively. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47%, and hip/femoral/neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose, low-dose, and placebo groups, respectively.
Another large clinical trial of elagolix for treatment of pelvic pain caused by endometriosis (Elaris II) involving 817 women produced results that were similar to those reported in Elaris I.4 The elagolix continuation studies, Elaris III and IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.5
Depot leuprolide acetate and nafarelin acetate
Depot leuprolide acetate and nafarelin acetate are GnRH analogues approved by the FDA more than 25 years ago for treatment of pelvic pain caused by endometriosis. Over the past two decades, depot leuprolide acetate has been one of the most commonly used hormonal treatments for endometriosis in the United States. A 3-month formulation of depot leuprolide acetate with an 11.25-mg injection has resulted in mean circulating estradiol concentrations of 8 pg/mL, indicating very strong suppression of estradiol production.6 A twice-daily 200-µg dose of nafarelin acetate nasal spray has resulted in a circulating estradiol concentration of approximately 28 pg/mL, indicating gentle suppression of estradiol production.7
At current prices, elagolix treatment is substantially less expensive than treatment with leuprolide or nafarelin. In addition, many women in my practice prefer to use an oral medication over an intramuscular injection or a nasal spray medication. It is likely that clinicians and patients will evolve to prioritize and favor elagolix therapy over depot leuprolide or nafarelin treatment.
Continue to: 5 options for using elagolix
5 options for using elagolix
There are many potential options for using elagolix in the treatment of pelvic pain caused by endometriosis.
Option 1. Prescribe elagolix 200 mg twice daily for 6 months to achieve strong suppression of estradiol and marked improvement in dysmenorrhea, although at the cost of more hot flashes and greater bone loss.
Option 2. Prescribe elagolix 150 mg once daily for up to 24 months to achieve gentle suppression of estradiol and modest improvement in dysmenorrhea with fewer hot flashes and minimal bone loss.
Options 1 and 2 have been studied in high quality clinical trials involving more than 1,500 women and are approved by the FDA.
Option 3. Initiate treatment with elagolix 200 mg twice daily for 3 months, immediately accruing the benefits of strong suppression of estradiol, and then switch to elagolix 150 mg once daily for up to 24 months to achieve continuing pain control with fewer adverse effects. This regimen combines strong initial suppression of estradiol, which will result in marked improvement in dysmenorrhea, along with long-term gentle suppression of estradiol, which is likely to maintain decreased pain symptoms with minimal long-term bone loss and fewer hot flashes.
Option 4. Prescribe an alternating regimen of elagolix 200 mg twice daily on even days of the month (two pills daily is an even number of pills) and one pill daily on odd days of the month (1 pill daily is an odd number of pills). This regimen should produce a mean estradiol concentration between 12 and 41 pg/mL, resulting in moderate rather than strong or gentle suppression of estradiol.
Options 3 and 4 are based on extrapolation using our knowledge about the hormonal treatment of endometriosis and are not regimens approved by the FDA.
Option 5. Prescribe elagolix 200 mg twice daily and initiate add-back therapy with norethindrone acetate 5 mg once daily. Substantial evidence supports the combination of a GnRH analogue that strongly suppresses estradiol production with norethindrone acetate add-back, which helps mitigate the bone loss that occurs with strong suppression of estradiol and reduces the frequency of moderate to severe hot flashes.
Option 5 is based on extrapolation from high-quality studies of leuprolide acetate depot plus norethindrone acetate add-back.8 The combination regimen is approved by the FDA.3

Elagolix availability increases treatment choices for women
Pelvic pain caused by endometriosis is common, affecting approximately 8% of women of reproductive age.9 Endometriosis is a vexing disease because diagnosis is often delayed many years after the onset of symptoms, causing great frustration among patients.10 Some effective hormonal treatment options, including danazol and depot leuprolide, are poorly tolerated by patients because of adverse effects, including weight gain (danazol), hot flashes, and bone loss (depot leuprolide). Combination oral contraceptives used in a continuous or cyclic fashion often result in inadequate improvement in pelvic pain.11 The synthesis of an orally active, small-molecule GnRH antagonist is an innovative advance in endocrine pharmacology. The Elaris Endometriosis clinical trials have demonstrated that elagolix is effective in the treatment of pelvic pain caused by endometriosis.4,5 A great advantage of elagolix is that dosing can be tailored for each patient to achieve reduction in pain while minimizing unwanted adverse effects such as hot flashes and bone loss. In Elaris Endometriosis I, fewer than 10% of women discontinued elagolix due to adverse effects.4 Elagolix is also less expensive than depot leuprolide and nafarelin.
Millions of women in the United States have pelvic pain caused by endometriosis. Obstetrician-gynecologists are the clinicians best trained to care for these women, and patients trust that we will effectively treat their problem.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Endometriosis is the presence of tissue resembling endometrial glands and stroma outside of the uterine cavity. Women with endometriosis often present for medical care with at least one of 3 problems: pelvic pain, infertility, and/or an adnexal mass due to endometriosis.1 Many clinical observations demonstrate that endometriosis lesions require estrogen to grow and maintain their viability, including that: (1) endometriosis is uncommon before puberty or after menopause, (2) surgical removal of both ovaries results in regression of endometriosis lesions, and (3) gonadotropin-releasing hormone (GnRH) analogues cause a hypo‑estrogenic hormonal environment, resulting in regression of endometriosis lesions and improvement in pelvic pain. Since endometriosis lesions require estrogen to maintain their viability, suppressing estradiol is a logical approach to hormonal treatment of the disease.
The estrogen threshold hypothesis
The estradiol concentration that causes endometriosis lesions to grow or regress varies among women, but a concentration less than 20 pg/mL usually causes lesions to regress, and a concentration greater than 60 pg/mL usually supports lesion growth and maintains lesion viability.2 Although an estradiol concentration below 20 pg/mL may cause lesions to regress, it also is associated with moderate to severe hot flashes and accelerated bone loss. These adverse effects limit the use of strong suppression of estrogen as a long-term treatment strategy. The estrogen threshold hypothesis posits that gently suppressing estradiol to a concentration between 20 and 45 pg/mL may simultaneously cause endometriosis lesions to regress, resulting in reduced pelvic pain, minimal bone loss, and few hot flashes.2
Building on the estrogen threshold hypothesis, clinicians have two options for treatment of pelvic pain caused by endometriosis:
- strong suppression of estradiol to a concentration below 20 pg/mL
- gentle suppression of estradiol to a concentration in the range of 20 to 45 pg/mL.
Strong suppression of estradiol to levels below 20 pg/mL will reliably induce amenorrhea and cause regression of endometriosis lesions, thereby reducing pelvic pain. Strong suppression of estradiol also will cause moderate to severe hot flashes and accelerated bone loss in many women. By contrast, gentle suppression of circulating estradiol to a concentration in the range of 20 to 45 pg/mL may result in amenorrhea or oligomenorrhea, suppression of the growth of endometriosis lesions, a modest reduction in pelvic pain, mild hot flashes, and minimal bone loss.
Recently, the US Food and Drug Administration (FDA) approved elagolix, an oral GnRH antagonist, for treatment of endometriosis.3 Elagolix blocks GnRH receptors in the pituitary gland, resulting in reduced production of luteinizing hormone and follicle stimulating hormone and a decrease in sex steroid secretion in the ovarian follicles, which leads to a reduction in the production and circulating concentration of estradiol. The FDA approved two doses of elagolix: 150 mg once daily for up to 24 months and 200 mg twice daily for up to 6 months. Importantly, elagolix at a dose of 150 mg once daily results in a mean circulating estradiol concentration of 41 pg/mL, indicating gentle suppression of ovarian estradiol production, and 200 mg twice daily results in a mean circulating ovarian estradiol concentration of 12 pg/mL, indicating strong suppression of ovarian estradiol production.3 For clinicians treating women with pelvic pain caused by endometriosis, these two elagolix regimens permit the individualization of hormonal therapy to the unique needs of each woman.
Continue to: Safety information for elagolix
- Contraindications: Elagolix should not be prescribed to women who are currently pregnant or have known osteoporosis or severe hepatic impairment. Elagolix should not be used in women taking cyclosporine or gemfibrozil (organic anion transporting polypeptide inhibitors).
- Elagolix may cause dose-dependent bone loss.
- Elagolix reduces menstrual bleeding, which may make it difficult to recognize the occurrence of pregnancy. Nonhormonal contraceptives should be utilized during elagolix treatment.
- Elagolix may be associated with an increase in reported depressive symptoms and mood changes.
- Elagolix may be associated with an increase in alanine aminotransferase more than 3 times the upper limit of the reference range. If elevated liver function tests are detected, the benefits and risks of continuing elagolix treatment should be evaluated.
Elagolix benefits and adverse effects
In one large clinical trial (Elaris Endometriosis I), 872 women were randomly assigned to treatment with one of two doses of elagolix (200 mg twice daily [high-dose group] or 150 mgonce daily [low-dose group]) or placebo.4 After 3 months of treatment, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of women in the high-dose, low-dose, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). In addition, at 3 months, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of women in the high-dose, low-dose, and placebo groups, respectively (low-dose vs placebo, P<.01; high-dose vs placebo, P<.001). Hot flashes that were severe enough to be classified as adverse events by study participants were reported by 42%, 24%, and 7% of the women in the high-dose, low-dose, and placebo groups, respectively. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47%, and hip/femoral/neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose, low-dose, and placebo groups, respectively.
Another large clinical trial of elagolix for treatment of pelvic pain caused by endometriosis (Elaris II) involving 817 women produced results that were similar to those reported in Elaris I.4 The elagolix continuation studies, Elaris III and IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.5
Depot leuprolide acetate and nafarelin acetate
Depot leuprolide acetate and nafarelin acetate are GnRH analogues approved by the FDA more than 25 years ago for treatment of pelvic pain caused by endometriosis. Over the past two decades, depot leuprolide acetate has been one of the most commonly used hormonal treatments for endometriosis in the United States. A 3-month formulation of depot leuprolide acetate with an 11.25-mg injection has resulted in mean circulating estradiol concentrations of 8 pg/mL, indicating very strong suppression of estradiol production.6 A twice-daily 200-µg dose of nafarelin acetate nasal spray has resulted in a circulating estradiol concentration of approximately 28 pg/mL, indicating gentle suppression of estradiol production.7
At current prices, elagolix treatment is substantially less expensive than treatment with leuprolide or nafarelin. In addition, many women in my practice prefer to use an oral medication over an intramuscular injection or a nasal spray medication. It is likely that clinicians and patients will evolve to prioritize and favor elagolix therapy over depot leuprolide or nafarelin treatment.
Continue to: 5 options for using elagolix
5 options for using elagolix
There are many potential options for using elagolix in the treatment of pelvic pain caused by endometriosis.
Option 1. Prescribe elagolix 200 mg twice daily for 6 months to achieve strong suppression of estradiol and marked improvement in dysmenorrhea, although at the cost of more hot flashes and greater bone loss.
Option 2. Prescribe elagolix 150 mg once daily for up to 24 months to achieve gentle suppression of estradiol and modest improvement in dysmenorrhea with fewer hot flashes and minimal bone loss.
Options 1 and 2 have been studied in high quality clinical trials involving more than 1,500 women and are approved by the FDA.
Option 3. Initiate treatment with elagolix 200 mg twice daily for 3 months, immediately accruing the benefits of strong suppression of estradiol, and then switch to elagolix 150 mg once daily for up to 24 months to achieve continuing pain control with fewer adverse effects. This regimen combines strong initial suppression of estradiol, which will result in marked improvement in dysmenorrhea, along with long-term gentle suppression of estradiol, which is likely to maintain decreased pain symptoms with minimal long-term bone loss and fewer hot flashes.
Option 4. Prescribe an alternating regimen of elagolix 200 mg twice daily on even days of the month (two pills daily is an even number of pills) and one pill daily on odd days of the month (1 pill daily is an odd number of pills). This regimen should produce a mean estradiol concentration between 12 and 41 pg/mL, resulting in moderate rather than strong or gentle suppression of estradiol.
Options 3 and 4 are based on extrapolation using our knowledge about the hormonal treatment of endometriosis and are not regimens approved by the FDA.
Option 5. Prescribe elagolix 200 mg twice daily and initiate add-back therapy with norethindrone acetate 5 mg once daily. Substantial evidence supports the combination of a GnRH analogue that strongly suppresses estradiol production with norethindrone acetate add-back, which helps mitigate the bone loss that occurs with strong suppression of estradiol and reduces the frequency of moderate to severe hot flashes.
Option 5 is based on extrapolation from high-quality studies of leuprolide acetate depot plus norethindrone acetate add-back.8 The combination regimen is approved by the FDA.3

Elagolix availability increases treatment choices for women
Pelvic pain caused by endometriosis is common, affecting approximately 8% of women of reproductive age.9 Endometriosis is a vexing disease because diagnosis is often delayed many years after the onset of symptoms, causing great frustration among patients.10 Some effective hormonal treatment options, including danazol and depot leuprolide, are poorly tolerated by patients because of adverse effects, including weight gain (danazol), hot flashes, and bone loss (depot leuprolide). Combination oral contraceptives used in a continuous or cyclic fashion often result in inadequate improvement in pelvic pain.11 The synthesis of an orally active, small-molecule GnRH antagonist is an innovative advance in endocrine pharmacology. The Elaris Endometriosis clinical trials have demonstrated that elagolix is effective in the treatment of pelvic pain caused by endometriosis.4,5 A great advantage of elagolix is that dosing can be tailored for each patient to achieve reduction in pain while minimizing unwanted adverse effects such as hot flashes and bone loss. In Elaris Endometriosis I, fewer than 10% of women discontinued elagolix due to adverse effects.4 Elagolix is also less expensive than depot leuprolide and nafarelin.
Millions of women in the United States have pelvic pain caused by endometriosis. Obstetrician-gynecologists are the clinicians best trained to care for these women, and patients trust that we will effectively treat their problem.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol. 2018;131:557-571.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Orlissa [package insert]. North Chicago, IL: AbbVie Inc; 2018.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017; 377: 28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Lupron Depot [package insert]. North Chicago, IL: Abbott Laboratories: 2012.
- Henzl MR, Corson SL, Moghissi K, et al. Administration of nasal nafarelin as compared with oral danazol for endometriosis. a multicenter double-blind comparative clinical trial. N Engl J Med. 1988;318:485-489.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Missmer SA, Hankinson SE, Spiegelman D, et al. The incidence of laparoscopically-confirmed endometriosis by demographic, anthropomorphic and lifestyle factors. Am J Epidemiol. 2004;160:784-796.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8,10-11,16.
- Jensen JT, Schlaff W, Gordon K. Use of combined hormonal contraceptives for the treatment of endometriosis-related pain: a systematic review of the evidence. Fertil Steril. 2018;110:137-152.
- Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol. 2018;131:557-571.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Orlissa [package insert]. North Chicago, IL: AbbVie Inc; 2018.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017; 377: 28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Lupron Depot [package insert]. North Chicago, IL: Abbott Laboratories: 2012.
- Henzl MR, Corson SL, Moghissi K, et al. Administration of nasal nafarelin as compared with oral danazol for endometriosis. a multicenter double-blind comparative clinical trial. N Engl J Med. 1988;318:485-489.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Missmer SA, Hankinson SE, Spiegelman D, et al. The incidence of laparoscopically-confirmed endometriosis by demographic, anthropomorphic and lifestyle factors. Am J Epidemiol. 2004;160:784-796.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8,10-11,16.
- Jensen JT, Schlaff W, Gordon K. Use of combined hormonal contraceptives for the treatment of endometriosis-related pain: a systematic review of the evidence. Fertil Steril. 2018;110:137-152.
Pediatric Dermatology Workforce Shortage Explained
The Society for Pediatric Dermatology (SPD) was established in 1975, and the pediatric dermatology workforce shortage began shortly after. In 1986, Honig and Burke1 reported that opportunities in pediatric dermatology were limited and that pediatric dermatologists were predominantly located in larger teaching hospitals and selected private practice settings; furthermore, only approximately 20% had patient populations comprising more than 75% children.1 Positive changes have occurred since that time, with more practitioners dedicated to pediatric dermatology and increased opportunities within the specialty. The SPD has expanded to a thriving group of collegial pediatric dermatologists now topping 1200 members worldwide.
Although the SPD has strongly influenced practice development in pediatric dermatology, there are fewer than 300 board-certified pediatric dermatologists in the United States and approximately double that number of pediatric dermatology practitioners. The deficiency is glaring based on the national population alone. The US Census Bureau reported 325,719,178 individuals living in the United States (as of July 1, 2017).2 With approximately 75 million children in the United States and estimates that 22.8% of the population is younger than 18 years,3 there currently is 1 pediatric dermatologist for every 120,000 children or more.
As if the numbers alone were not adequate, a number of publications have addressed the benefits of pediatric dermatologists in both dermatology and pediatrics training and furthermore in pediatric care. A 2004 survey of dermatology program directors and chairpersons regarding the issue of the pediatric dermatology workforce shortage revealed that 45 of 94 (47.9%) programs employed a pediatric dermatologist and 24 (25.5%) had been looking to hire one for more than a year.4 Although more pediatric dermatologists have joined the workforce, it is not surprising that programs with no pediatric dermatologists want them. First, pediatric dermatologists dramatically improve the quality of training with regard to pediatric dermatology education and can increase dermatology residents’ comfort level with children. In a survey of a group of graduating third-year dermatology residents, dermatology residency program directors, and pediatric dermatology fellowship program directors by Nijhawan et al,5 residents who were trained in a program with one or more full-time pediatric dermatologists were more likely to feel competent treating children and to feel satisfied with their training program’s pediatric dermatology curriculum than residents without contact with a full-time pediatric dermatologist (50.0% vs 5.9% [P=.002] and 85.3% vs 52.9% [P<.001], respectively). The availability of a pediatric dermatology fellowship further enhanced satisfaction. Residents in programs with no full-time pediatric dermatologist on staff were more likely to be somewhat or extremely dissatisfied with their pediatric dermatology training. Residency program directors were more satisfied with their curriculums when there was one or more pediatric dermatologist on staff (P<.01).5
Programs with pediatric dermatologists also offer easy access to a mentor in the field. In a 2010 survey of pediatric dermatologists (published in 2014), Admani et al6 reported that 84% (91/109) of respondents (board-certified pediatric dermatologists) cited mentorship as the most important factor influencing their career choice. Exposure to the specialty was noted as a key motivating factor. In my opinion, the actual inclusion of a pediatric dermatology fellowship, whether the position is filled or not, appears to increase the chances of expansion and retention in the field.
Furthermore, due to the outpatient burden of skin disease in a pediatrics practice, providing pediatric trainees with contact with a pediatric dermatologist is needed.
As if there was not enough evidence that pediatric dermatologists are in high demand, SPD pediatric dermatology workforce surveys from the last 5 years, which will soon be updated, show similar indications.7,10 Fogel and Teng11 showed that 60% of surveyed pediatric dermatologists (N=226) were academic and 81% were salaried. Unlike previous data,1 the investigators showed that children constituted 79.5% of respondents’ patient populations.
For the medical student or resident seeking a career in pediatric dermatology, it appears that finding and working on projects with mentors likely is the key to stepping in the field. From my own experience, pediatric dermatologists are extremely friendly and open to supporting career development in earnest students. Reach out to potential mentors months before starting desired electives, as you are competing with other students and pediatrics, dermatology, and emergency medicine residents. Joining and attending meetings of the SPD is a great way to find direction in this friendly and collegial field. Additionally, pediatric dermatology sessions at the annual meetings of the American Academy of Dermatology are a wonderful way to experience the excitement of the field. As a pediatric dermatologist in practice for almost 2 decades, I can honestly say that the field is always intellectually stimulating and evolving rapidly through enhanced understanding of disease pathogenesis, genetics, and therapeutics. Helping children and their parents/guardians never gets boring.
The solution to improving the size and accessibility of the pediatric dermatology workforce is not simple and likely starts from the bottom up. More than 75% of pediatric dermatologists favor implementing systems to encourage medical students to pursue a career in pediatric dermatology.7 Increasing resident exposure to dedicated pediatric dermatology training time enhances satisfaction.5 Increased funding of fellowships can help these students and residents meet their goals. Current fellowship training programs now total 36, but not all approved institutions have been able to support a postgraduate year 5 (PGY-5) or higher fellow, and in my experience some institutions have avoided adding a fellow due to lack of funding internally. The average pediatric dermatologist earns $100,000 less than colleagues who treat adults, which is an impediment to the expansion of the field.10 This disparity may chase away practitioners, especially those with medical school debt. Debt forgiveness programs, enhanced practice development, and better base pay for pediatric dermatologists could positively impact growth in this specialty. Dermatology and pediatrics training programs need to dedicate more money and developmental support for pediatric dermatologists as a way to invest in the quality of pediatric dermatology education for their trainees. By recognizing the true value of the academic contributions of pediatric dermatologists, dermatology residency programs can invest in producing trainees with greater aplomb and acumen in pediatric dermatology.
- Honig PJ, Burke L. The subspecialty of pediatric dermatology. J Am Acad Dermatol. 1986;15:123-126.
- United States Census Bureau. QuickFacts. https://www.census.gov/quickfacts/fact/table/US/PST045217#PST045217. Accessed October 19, 2018.
- An aging nation: projected number of children and older adults. United States Census Bureau website. https://www.census.gov/library/visualizations/2018/comm/historic-first.html. Published March 13, 2018. Accessed October 9, 2018.
- Hester EJ, McNealy KM, Kelloff JN, et al. Demand outstrips supply of US pediatric dermatologists: results from a national survey. J Am Acad Dermatol. 2004;50:431-434.
- Nijhawan RI, Mazza JM, Silverberg NB. Pediatric dermatology training survey of United States dermatology residency programs. Pediatr Dermatol. 2014;31:131-137.
- Admani S, Caufield M, Kim SS, et al. Understanding the pediatric dermatology workforce shortage: mentoring matters. J Pediatr. 2014;164:372-375.
- 2014 Society for Pediatric Dermatology Peds Derm Training Survey. Society for Pediatric Dermatology website. https://pedsderm.net/site/assets/files/8639/06b-peds_training_survey_responses_final.pdf. Accessed October 9, 2018.
- ABD approved pediatric dermatology fellowship programs. Society for Pediatric Dermatology website. https://pedsderm.net/training/fellowships/abd-approved-pediatric-dermatology-fellowship-programs/. Accessed October 9, 2018.
- Prindaville B, Simon SD, Horii KA. Dermatology-related outpatient visits by children: implications for workforce and pediatric education. J Am Acad Dermatol. 2016;75:228-229.
- Prindaville B, Antaya RJ, Siegfried EC. Pediatric dermatology: past, present and future [published online July 21, 2014]. Pediatr Dermatol. 2015;32:1-12.
- Fogel AL, Teng JM. The US pediatric dermatology workforce: an assessment of productivity and practice patterns. Pediatr Dermatol. 2015;32:825-829.
The Society for Pediatric Dermatology (SPD) was established in 1975, and the pediatric dermatology workforce shortage began shortly after. In 1986, Honig and Burke1 reported that opportunities in pediatric dermatology were limited and that pediatric dermatologists were predominantly located in larger teaching hospitals and selected private practice settings; furthermore, only approximately 20% had patient populations comprising more than 75% children.1 Positive changes have occurred since that time, with more practitioners dedicated to pediatric dermatology and increased opportunities within the specialty. The SPD has expanded to a thriving group of collegial pediatric dermatologists now topping 1200 members worldwide.
Although the SPD has strongly influenced practice development in pediatric dermatology, there are fewer than 300 board-certified pediatric dermatologists in the United States and approximately double that number of pediatric dermatology practitioners. The deficiency is glaring based on the national population alone. The US Census Bureau reported 325,719,178 individuals living in the United States (as of July 1, 2017).2 With approximately 75 million children in the United States and estimates that 22.8% of the population is younger than 18 years,3 there currently is 1 pediatric dermatologist for every 120,000 children or more.
As if the numbers alone were not adequate, a number of publications have addressed the benefits of pediatric dermatologists in both dermatology and pediatrics training and furthermore in pediatric care. A 2004 survey of dermatology program directors and chairpersons regarding the issue of the pediatric dermatology workforce shortage revealed that 45 of 94 (47.9%) programs employed a pediatric dermatologist and 24 (25.5%) had been looking to hire one for more than a year.4 Although more pediatric dermatologists have joined the workforce, it is not surprising that programs with no pediatric dermatologists want them. First, pediatric dermatologists dramatically improve the quality of training with regard to pediatric dermatology education and can increase dermatology residents’ comfort level with children. In a survey of a group of graduating third-year dermatology residents, dermatology residency program directors, and pediatric dermatology fellowship program directors by Nijhawan et al,5 residents who were trained in a program with one or more full-time pediatric dermatologists were more likely to feel competent treating children and to feel satisfied with their training program’s pediatric dermatology curriculum than residents without contact with a full-time pediatric dermatologist (50.0% vs 5.9% [P=.002] and 85.3% vs 52.9% [P<.001], respectively). The availability of a pediatric dermatology fellowship further enhanced satisfaction. Residents in programs with no full-time pediatric dermatologist on staff were more likely to be somewhat or extremely dissatisfied with their pediatric dermatology training. Residency program directors were more satisfied with their curriculums when there was one or more pediatric dermatologist on staff (P<.01).5
Programs with pediatric dermatologists also offer easy access to a mentor in the field. In a 2010 survey of pediatric dermatologists (published in 2014), Admani et al6 reported that 84% (91/109) of respondents (board-certified pediatric dermatologists) cited mentorship as the most important factor influencing their career choice. Exposure to the specialty was noted as a key motivating factor. In my opinion, the actual inclusion of a pediatric dermatology fellowship, whether the position is filled or not, appears to increase the chances of expansion and retention in the field.
Furthermore, due to the outpatient burden of skin disease in a pediatrics practice, providing pediatric trainees with contact with a pediatric dermatologist is needed.
As if there was not enough evidence that pediatric dermatologists are in high demand, SPD pediatric dermatology workforce surveys from the last 5 years, which will soon be updated, show similar indications.7,10 Fogel and Teng11 showed that 60% of surveyed pediatric dermatologists (N=226) were academic and 81% were salaried. Unlike previous data,1 the investigators showed that children constituted 79.5% of respondents’ patient populations.
For the medical student or resident seeking a career in pediatric dermatology, it appears that finding and working on projects with mentors likely is the key to stepping in the field. From my own experience, pediatric dermatologists are extremely friendly and open to supporting career development in earnest students. Reach out to potential mentors months before starting desired electives, as you are competing with other students and pediatrics, dermatology, and emergency medicine residents. Joining and attending meetings of the SPD is a great way to find direction in this friendly and collegial field. Additionally, pediatric dermatology sessions at the annual meetings of the American Academy of Dermatology are a wonderful way to experience the excitement of the field. As a pediatric dermatologist in practice for almost 2 decades, I can honestly say that the field is always intellectually stimulating and evolving rapidly through enhanced understanding of disease pathogenesis, genetics, and therapeutics. Helping children and their parents/guardians never gets boring.
The solution to improving the size and accessibility of the pediatric dermatology workforce is not simple and likely starts from the bottom up. More than 75% of pediatric dermatologists favor implementing systems to encourage medical students to pursue a career in pediatric dermatology.7 Increasing resident exposure to dedicated pediatric dermatology training time enhances satisfaction.5 Increased funding of fellowships can help these students and residents meet their goals. Current fellowship training programs now total 36, but not all approved institutions have been able to support a postgraduate year 5 (PGY-5) or higher fellow, and in my experience some institutions have avoided adding a fellow due to lack of funding internally. The average pediatric dermatologist earns $100,000 less than colleagues who treat adults, which is an impediment to the expansion of the field.10 This disparity may chase away practitioners, especially those with medical school debt. Debt forgiveness programs, enhanced practice development, and better base pay for pediatric dermatologists could positively impact growth in this specialty. Dermatology and pediatrics training programs need to dedicate more money and developmental support for pediatric dermatologists as a way to invest in the quality of pediatric dermatology education for their trainees. By recognizing the true value of the academic contributions of pediatric dermatologists, dermatology residency programs can invest in producing trainees with greater aplomb and acumen in pediatric dermatology.
The Society for Pediatric Dermatology (SPD) was established in 1975, and the pediatric dermatology workforce shortage began shortly after. In 1986, Honig and Burke1 reported that opportunities in pediatric dermatology were limited and that pediatric dermatologists were predominantly located in larger teaching hospitals and selected private practice settings; furthermore, only approximately 20% had patient populations comprising more than 75% children.1 Positive changes have occurred since that time, with more practitioners dedicated to pediatric dermatology and increased opportunities within the specialty. The SPD has expanded to a thriving group of collegial pediatric dermatologists now topping 1200 members worldwide.
Although the SPD has strongly influenced practice development in pediatric dermatology, there are fewer than 300 board-certified pediatric dermatologists in the United States and approximately double that number of pediatric dermatology practitioners. The deficiency is glaring based on the national population alone. The US Census Bureau reported 325,719,178 individuals living in the United States (as of July 1, 2017).2 With approximately 75 million children in the United States and estimates that 22.8% of the population is younger than 18 years,3 there currently is 1 pediatric dermatologist for every 120,000 children or more.
As if the numbers alone were not adequate, a number of publications have addressed the benefits of pediatric dermatologists in both dermatology and pediatrics training and furthermore in pediatric care. A 2004 survey of dermatology program directors and chairpersons regarding the issue of the pediatric dermatology workforce shortage revealed that 45 of 94 (47.9%) programs employed a pediatric dermatologist and 24 (25.5%) had been looking to hire one for more than a year.4 Although more pediatric dermatologists have joined the workforce, it is not surprising that programs with no pediatric dermatologists want them. First, pediatric dermatologists dramatically improve the quality of training with regard to pediatric dermatology education and can increase dermatology residents’ comfort level with children. In a survey of a group of graduating third-year dermatology residents, dermatology residency program directors, and pediatric dermatology fellowship program directors by Nijhawan et al,5 residents who were trained in a program with one or more full-time pediatric dermatologists were more likely to feel competent treating children and to feel satisfied with their training program’s pediatric dermatology curriculum than residents without contact with a full-time pediatric dermatologist (50.0% vs 5.9% [P=.002] and 85.3% vs 52.9% [P<.001], respectively). The availability of a pediatric dermatology fellowship further enhanced satisfaction. Residents in programs with no full-time pediatric dermatologist on staff were more likely to be somewhat or extremely dissatisfied with their pediatric dermatology training. Residency program directors were more satisfied with their curriculums when there was one or more pediatric dermatologist on staff (P<.01).5
Programs with pediatric dermatologists also offer easy access to a mentor in the field. In a 2010 survey of pediatric dermatologists (published in 2014), Admani et al6 reported that 84% (91/109) of respondents (board-certified pediatric dermatologists) cited mentorship as the most important factor influencing their career choice. Exposure to the specialty was noted as a key motivating factor. In my opinion, the actual inclusion of a pediatric dermatology fellowship, whether the position is filled or not, appears to increase the chances of expansion and retention in the field.
Furthermore, due to the outpatient burden of skin disease in a pediatrics practice, providing pediatric trainees with contact with a pediatric dermatologist is needed.
As if there was not enough evidence that pediatric dermatologists are in high demand, SPD pediatric dermatology workforce surveys from the last 5 years, which will soon be updated, show similar indications.7,10 Fogel and Teng11 showed that 60% of surveyed pediatric dermatologists (N=226) were academic and 81% were salaried. Unlike previous data,1 the investigators showed that children constituted 79.5% of respondents’ patient populations.
For the medical student or resident seeking a career in pediatric dermatology, it appears that finding and working on projects with mentors likely is the key to stepping in the field. From my own experience, pediatric dermatologists are extremely friendly and open to supporting career development in earnest students. Reach out to potential mentors months before starting desired electives, as you are competing with other students and pediatrics, dermatology, and emergency medicine residents. Joining and attending meetings of the SPD is a great way to find direction in this friendly and collegial field. Additionally, pediatric dermatology sessions at the annual meetings of the American Academy of Dermatology are a wonderful way to experience the excitement of the field. As a pediatric dermatologist in practice for almost 2 decades, I can honestly say that the field is always intellectually stimulating and evolving rapidly through enhanced understanding of disease pathogenesis, genetics, and therapeutics. Helping children and their parents/guardians never gets boring.
The solution to improving the size and accessibility of the pediatric dermatology workforce is not simple and likely starts from the bottom up. More than 75% of pediatric dermatologists favor implementing systems to encourage medical students to pursue a career in pediatric dermatology.7 Increasing resident exposure to dedicated pediatric dermatology training time enhances satisfaction.5 Increased funding of fellowships can help these students and residents meet their goals. Current fellowship training programs now total 36, but not all approved institutions have been able to support a postgraduate year 5 (PGY-5) or higher fellow, and in my experience some institutions have avoided adding a fellow due to lack of funding internally. The average pediatric dermatologist earns $100,000 less than colleagues who treat adults, which is an impediment to the expansion of the field.10 This disparity may chase away practitioners, especially those with medical school debt. Debt forgiveness programs, enhanced practice development, and better base pay for pediatric dermatologists could positively impact growth in this specialty. Dermatology and pediatrics training programs need to dedicate more money and developmental support for pediatric dermatologists as a way to invest in the quality of pediatric dermatology education for their trainees. By recognizing the true value of the academic contributions of pediatric dermatologists, dermatology residency programs can invest in producing trainees with greater aplomb and acumen in pediatric dermatology.
- Honig PJ, Burke L. The subspecialty of pediatric dermatology. J Am Acad Dermatol. 1986;15:123-126.
- United States Census Bureau. QuickFacts. https://www.census.gov/quickfacts/fact/table/US/PST045217#PST045217. Accessed October 19, 2018.
- An aging nation: projected number of children and older adults. United States Census Bureau website. https://www.census.gov/library/visualizations/2018/comm/historic-first.html. Published March 13, 2018. Accessed October 9, 2018.
- Hester EJ, McNealy KM, Kelloff JN, et al. Demand outstrips supply of US pediatric dermatologists: results from a national survey. J Am Acad Dermatol. 2004;50:431-434.
- Nijhawan RI, Mazza JM, Silverberg NB. Pediatric dermatology training survey of United States dermatology residency programs. Pediatr Dermatol. 2014;31:131-137.
- Admani S, Caufield M, Kim SS, et al. Understanding the pediatric dermatology workforce shortage: mentoring matters. J Pediatr. 2014;164:372-375.
- 2014 Society for Pediatric Dermatology Peds Derm Training Survey. Society for Pediatric Dermatology website. https://pedsderm.net/site/assets/files/8639/06b-peds_training_survey_responses_final.pdf. Accessed October 9, 2018.
- ABD approved pediatric dermatology fellowship programs. Society for Pediatric Dermatology website. https://pedsderm.net/training/fellowships/abd-approved-pediatric-dermatology-fellowship-programs/. Accessed October 9, 2018.
- Prindaville B, Simon SD, Horii KA. Dermatology-related outpatient visits by children: implications for workforce and pediatric education. J Am Acad Dermatol. 2016;75:228-229.
- Prindaville B, Antaya RJ, Siegfried EC. Pediatric dermatology: past, present and future [published online July 21, 2014]. Pediatr Dermatol. 2015;32:1-12.
- Fogel AL, Teng JM. The US pediatric dermatology workforce: an assessment of productivity and practice patterns. Pediatr Dermatol. 2015;32:825-829.
- Honig PJ, Burke L. The subspecialty of pediatric dermatology. J Am Acad Dermatol. 1986;15:123-126.
- United States Census Bureau. QuickFacts. https://www.census.gov/quickfacts/fact/table/US/PST045217#PST045217. Accessed October 19, 2018.
- An aging nation: projected number of children and older adults. United States Census Bureau website. https://www.census.gov/library/visualizations/2018/comm/historic-first.html. Published March 13, 2018. Accessed October 9, 2018.
- Hester EJ, McNealy KM, Kelloff JN, et al. Demand outstrips supply of US pediatric dermatologists: results from a national survey. J Am Acad Dermatol. 2004;50:431-434.
- Nijhawan RI, Mazza JM, Silverberg NB. Pediatric dermatology training survey of United States dermatology residency programs. Pediatr Dermatol. 2014;31:131-137.
- Admani S, Caufield M, Kim SS, et al. Understanding the pediatric dermatology workforce shortage: mentoring matters. J Pediatr. 2014;164:372-375.
- 2014 Society for Pediatric Dermatology Peds Derm Training Survey. Society for Pediatric Dermatology website. https://pedsderm.net/site/assets/files/8639/06b-peds_training_survey_responses_final.pdf. Accessed October 9, 2018.
- ABD approved pediatric dermatology fellowship programs. Society for Pediatric Dermatology website. https://pedsderm.net/training/fellowships/abd-approved-pediatric-dermatology-fellowship-programs/. Accessed October 9, 2018.
- Prindaville B, Simon SD, Horii KA. Dermatology-related outpatient visits by children: implications for workforce and pediatric education. J Am Acad Dermatol. 2016;75:228-229.
- Prindaville B, Antaya RJ, Siegfried EC. Pediatric dermatology: past, present and future [published online July 21, 2014]. Pediatr Dermatol. 2015;32:1-12.
- Fogel AL, Teng JM. The US pediatric dermatology workforce: an assessment of productivity and practice patterns. Pediatr Dermatol. 2015;32:825-829.
Delay in plasma exchange increases chance of poor outcomes in NMOSD
BERLIN – A delay in undertaking plasma exchange may predict a poorer outcome after a first attack of neuromyelitis optica spectrum disorder, while antibodies to myelin oligodendrocyte glycoprotein (MOG) appear to predict a more positive outcome.
“We saw that for each day of delay in plasma exchange, the Expanded Disability Status Scale [EDSS] at 6 months increased by about 0.028 points, indicating a worse prognosis,” Maxime Guillaume, MD, said at the annual congress of the European Committee for Treatment and Research in Multiples Sclerosis.
However, said Dr. Guillaume, a resident at Rouen University Hospital, France, steroids are still a reasonable first-line therapy as long as they are discontinued quickly if they don’t appear to be helping. Plasma exchange is most effective if administered less than 2 weeks after symptom onset.
His study examined 6-month outcomes among 214 attacks in 188 patients; some patients had several first attacks in different areas. Response was defined in two ways. First, patients were clinically classified as having a good response, a bad response, or no response to treatment. The second definition was based on the EDSS. Good response was an EDSS decrease of at least 2 points for an initial score of 3 or higher, or a decrease of 1 point if the initial score was less than 3. Poor response was an EDSS that decreased without reaching these thresholds.
The cohort was largely female, with a mean age of 38 years. Most (55%) were positive for antibodies against aquaporin-4. Anti-MOG antibodies were present in 30%. A total of 7.5% were negative for both antibodies, and the remainder had an undetermined serotype.
The clinical presentations varied. Most frequently, patients presented with myelitis only (44%). This was followed by optic neuritis only (34%), both myelitis and optic neuritis (8%), and myelitis plus brainstem involvement (5%). Other clinical manifestations were acute demyelinating encephalomyelitis, and encephalitis alone.
The most common treatment was methylprednisolone (73%), followed by plasma exchange (25%), which occurred at a median of 9 days after symptom onset.
Outcomes varied according to the definition of response. By clinical characteristics, there was a complete response in 41, a partial response in 122, and no response in 51. By change in EDSS, 136 had a good response and 27 a partial response; 51 were still considered nonresponders.
Dr. Guillaume conducted a multivariate analysis to determine predictive factors. In both definitions, anti-MOG antibodies nearly quadrupled the chance of a good treatment response, and delaying plasma exchange was associated with a significantly increased chance of a poor response. When judged by the clinical response definition, multiple lines of treatment also were associated with a poor response. This, he said, was another reflection of plasma exchange delay.
Dr. Guillaume had no financial disclosures.
BERLIN – A delay in undertaking plasma exchange may predict a poorer outcome after a first attack of neuromyelitis optica spectrum disorder, while antibodies to myelin oligodendrocyte glycoprotein (MOG) appear to predict a more positive outcome.
“We saw that for each day of delay in plasma exchange, the Expanded Disability Status Scale [EDSS] at 6 months increased by about 0.028 points, indicating a worse prognosis,” Maxime Guillaume, MD, said at the annual congress of the European Committee for Treatment and Research in Multiples Sclerosis.
However, said Dr. Guillaume, a resident at Rouen University Hospital, France, steroids are still a reasonable first-line therapy as long as they are discontinued quickly if they don’t appear to be helping. Plasma exchange is most effective if administered less than 2 weeks after symptom onset.
His study examined 6-month outcomes among 214 attacks in 188 patients; some patients had several first attacks in different areas. Response was defined in two ways. First, patients were clinically classified as having a good response, a bad response, or no response to treatment. The second definition was based on the EDSS. Good response was an EDSS decrease of at least 2 points for an initial score of 3 or higher, or a decrease of 1 point if the initial score was less than 3. Poor response was an EDSS that decreased without reaching these thresholds.
The cohort was largely female, with a mean age of 38 years. Most (55%) were positive for antibodies against aquaporin-4. Anti-MOG antibodies were present in 30%. A total of 7.5% were negative for both antibodies, and the remainder had an undetermined serotype.
The clinical presentations varied. Most frequently, patients presented with myelitis only (44%). This was followed by optic neuritis only (34%), both myelitis and optic neuritis (8%), and myelitis plus brainstem involvement (5%). Other clinical manifestations were acute demyelinating encephalomyelitis, and encephalitis alone.
The most common treatment was methylprednisolone (73%), followed by plasma exchange (25%), which occurred at a median of 9 days after symptom onset.
Outcomes varied according to the definition of response. By clinical characteristics, there was a complete response in 41, a partial response in 122, and no response in 51. By change in EDSS, 136 had a good response and 27 a partial response; 51 were still considered nonresponders.
Dr. Guillaume conducted a multivariate analysis to determine predictive factors. In both definitions, anti-MOG antibodies nearly quadrupled the chance of a good treatment response, and delaying plasma exchange was associated with a significantly increased chance of a poor response. When judged by the clinical response definition, multiple lines of treatment also were associated with a poor response. This, he said, was another reflection of plasma exchange delay.
Dr. Guillaume had no financial disclosures.
BERLIN – A delay in undertaking plasma exchange may predict a poorer outcome after a first attack of neuromyelitis optica spectrum disorder, while antibodies to myelin oligodendrocyte glycoprotein (MOG) appear to predict a more positive outcome.
“We saw that for each day of delay in plasma exchange, the Expanded Disability Status Scale [EDSS] at 6 months increased by about 0.028 points, indicating a worse prognosis,” Maxime Guillaume, MD, said at the annual congress of the European Committee for Treatment and Research in Multiples Sclerosis.
However, said Dr. Guillaume, a resident at Rouen University Hospital, France, steroids are still a reasonable first-line therapy as long as they are discontinued quickly if they don’t appear to be helping. Plasma exchange is most effective if administered less than 2 weeks after symptom onset.
His study examined 6-month outcomes among 214 attacks in 188 patients; some patients had several first attacks in different areas. Response was defined in two ways. First, patients were clinically classified as having a good response, a bad response, or no response to treatment. The second definition was based on the EDSS. Good response was an EDSS decrease of at least 2 points for an initial score of 3 or higher, or a decrease of 1 point if the initial score was less than 3. Poor response was an EDSS that decreased without reaching these thresholds.
The cohort was largely female, with a mean age of 38 years. Most (55%) were positive for antibodies against aquaporin-4. Anti-MOG antibodies were present in 30%. A total of 7.5% were negative for both antibodies, and the remainder had an undetermined serotype.
The clinical presentations varied. Most frequently, patients presented with myelitis only (44%). This was followed by optic neuritis only (34%), both myelitis and optic neuritis (8%), and myelitis plus brainstem involvement (5%). Other clinical manifestations were acute demyelinating encephalomyelitis, and encephalitis alone.
The most common treatment was methylprednisolone (73%), followed by plasma exchange (25%), which occurred at a median of 9 days after symptom onset.
Outcomes varied according to the definition of response. By clinical characteristics, there was a complete response in 41, a partial response in 122, and no response in 51. By change in EDSS, 136 had a good response and 27 a partial response; 51 were still considered nonresponders.
Dr. Guillaume conducted a multivariate analysis to determine predictive factors. In both definitions, anti-MOG antibodies nearly quadrupled the chance of a good treatment response, and delaying plasma exchange was associated with a significantly increased chance of a poor response. When judged by the clinical response definition, multiple lines of treatment also were associated with a poor response. This, he said, was another reflection of plasma exchange delay.
Dr. Guillaume had no financial disclosures.
REPORTING FROM ECTRIMS 2018
Key clinical point:
Major finding: Each day’s delay in receiving plasma exchange increased the mean 6-month EDSS by about 0.028 points.
Study details: The retrospective study comprised 214 attacks in 188 patients.
Disclosures: Dr. Guillaume had no financial disclosures.
Source: Guillaume M. et al. ECTRIMS 2018, Abstract 211.
Hemorrhagic Crusted Papule on the Arm
The Diagnosis: Self-healing Langerhans Cell Histiocytosis
Histopathologic examination showed an infiltrate of mononuclear cells with indented nuclei admixed with a variable dermal inflammatory infiltrate. Immunohistochemistry demonstrated cells that were strongly positive for CD1a (Figure, A) and langerin (Figure, B) antigens as well as S-100 protein (Figure, C), which was consistent with Langerhans cell histiocytosis (LCH).
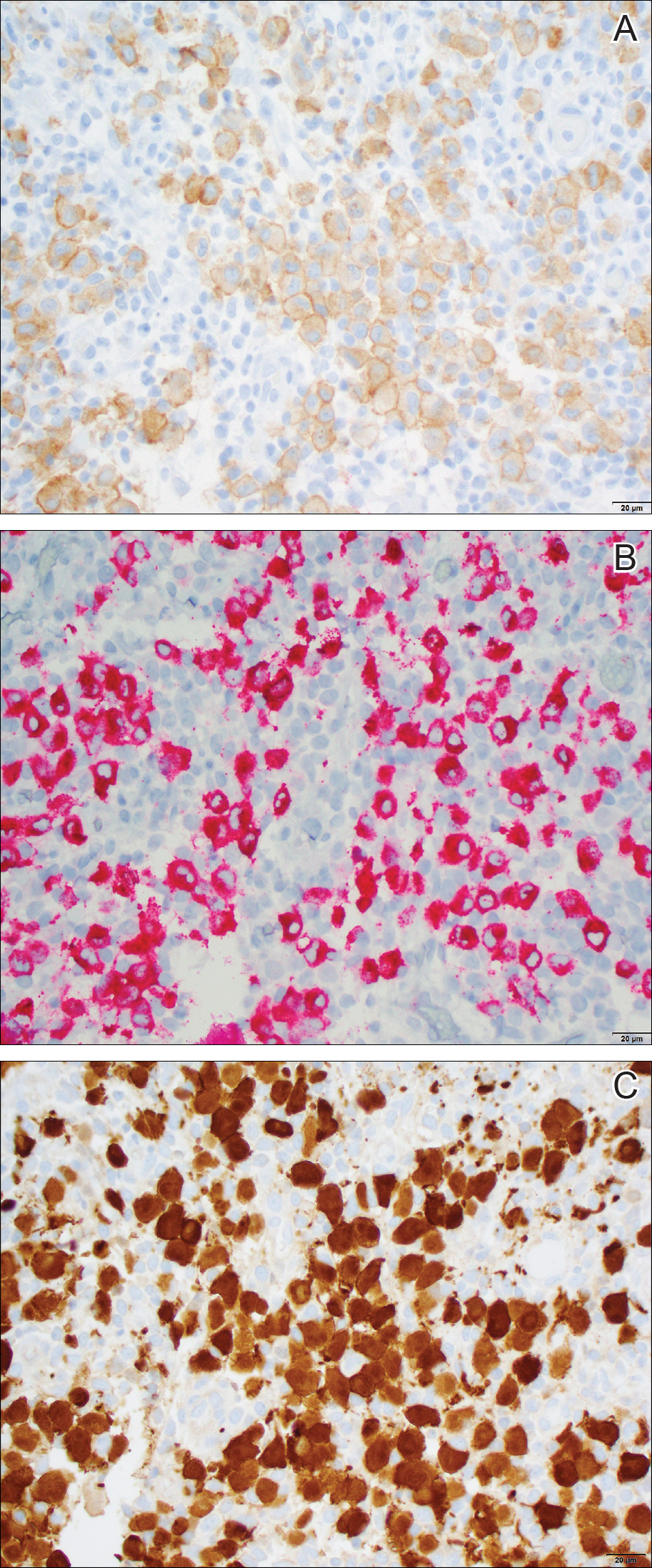
Histiocytoses are a heterogeneous group of disorders in which the infiltrating cells belong to the mononuclear phagocyte system.1,2 Langerhans cell histiocytosis is the most common dendritic cell-related histiocytosis, occurring in approximately 5 per 1 million children annually, giving it an incidence comparable to pediatric Hodgkin lymphoma and acute myeloid leukemia.1,2
Historically, there has been much debate about the pathogenesis of the disease.2 Until recently it was unknown whether LCH was primarily a neoplastic or an inflammatory disorder. Although the condition initially was thought to have a reactive etiology,1 more recent evidence suggests a clonal neoplastic process. Langerhans cell histiocytosis lesions are clonal and display malignancy-associated mechanisms such as immune evasion. Genome sequencing has revealed several mutations in precursor myeloid cells that result in the common downstream hyperactivation of the mitogen-activated protein kinase signaling pathway that regulates cell proliferation and differentiation.1
Langerhans cell histiocytosis displays a wide spectrum of clinical phenotypes, which historically were subclassified as eosinophilic granulomas (localized lesions in bone), Hand-Schüller-Christian disease (multiple organ involvement with the classic triad of skull defects, diabetes insipidus, and exophthalmos), and Letterer-Siwe disease (visceral lesions involving multiple organs).3 However, in 1997 the Reclassification Working Group of the Histiocyte Society redefined LCH as single-system single site (SS-s) LCH, single-system multisite LCH, and multisystem LCH.4
In SS-s LCH, the most common site is bone (82%), followed by the skin (12%).5 Skin SS-s LCH classically presents as multiple skin lesions at birth without systemic manifestations; the lesions spontaneously involute within a few months.6 Less commonly, skin SS-s LCH can present as a single lesion. Berger et al7 described 4 neonates with unilesional skin SS-s LCH. Since then, more than 30 cases have been reported in the literature,8 and we report herein another unilesional self-healing LCH.
The morphology of skin lesions in self-healing LCH is highly variable, with the most common being multiple erythematous crusted papules (50%), followed by eczematous scaly lesions resembling seborrheic dermatitis in intertriginous areas (37.5%).3,6 Unilesional self-healing LCH typically presents as an ulcerated or crusted nodule or papule on the trunk. This variability results in a large differential diagnosis. Self-healing LCH is easily mistaken for infectious processes including neonatal herpes simplex and varicella-zoster virus infection.9 Often, the dermatology department is consulted to rule out LCH when the asymptomatic neonate does not respond to parenteral acyclovir.
Less commonly, the magenta-colored papulonodules of self-healing LCH can mimic blueberry muffin rash and mandate a workup for intrauterine infections, especially cytomegalovirus, rubella, and blood dyscrasia.10 Other noninfectious processes in the differential of self-healing LCH include congenital infantile hemangioma, neonatal lupus erythematosus, seborrheic dermatitis (cradle cap), pyogenic granuloma, and psoriasis.3,10 Definitive diagnosis requires histopathology.
Because unilesional self-healing LCH has an excellent prognosis and usually resolves on its own, therapy is unnecessary.3,8 One large retrospective study (N=146) found that of all patients with skin lesions, 56% were managed with biopsy only.5 Other options include watchful waiting and topical corticosteroids. If the skin lesions are large, ulcerated, and/or painful, alkylating antitumor agents have been used. For extensive cutaneous disease, systemic corticosteroids combined with chemotherapy and psoralen plus UVA can be effective.6
The primary concern in the management of self-healing LCH is that the solitary skin lesion may be the harbinger of an aggressive disorder that can progress to systemic disease.5 Moreover, recurrent visceral or disseminated disease may occur months to years after resolution of solitary skin lesions.9 Studies have shown that localized and disseminated disease cannot be differentiated on the basis of clinical findings, histology, immunohistochemistry, or biomarkers.3,11 As a result, an evaluation for systemic disease should be performed at the time of diagnosis for cutaneous LCH.3,9 Minimum baseline studies recommended by the Writing Group of the Histiocyte Society include a complete blood cell count, liver function tests, coagulation studies, chest radiography, skeletal surveys, and urine osmolality testing.12 Periodic clinical follow-up is recommended for all variants of LCH.9
Our case was diagnosed as self-healing LCH based on histologic findings. No treatment was required, and at 3-month follow-up the infant was asymptomatic without recurrence and was meeting all developmental milestones.
- Berres ML, Merad M, Allen CE. Progress in understanding the pathogenesis of Langerhans cell histiocytosis: back to histiocytosis X? Br J Haematol. 2015;169:3-13.
- Jordan MB, Filipovich AH. Histiocytic disorders. In: Hoffman R, Benz EJ Jr, Silberstein LE, eds. Hematology: Basic Principles and Practice. 6th ed. Philadelphia, PA: Elsevier Saunders; 2013:686-700.
- Stein SL, Paller AS, Haut PR, et al. Langerhans cell histiocytosis presenting in the neonatal period: a retrospective case series. Arch Pediatr Adolesc Med. 2001;155:778-783.
- Favara BE, Feller AC, Pauli M, et al. Contemporary classification of histiocytic disorders. Pediatr Blood Cancer. 1997;29:157-166.
- Morimoto A, Ishida Y, Suzuki N, et al. Nationwide survey of single-system single site Langerhans cell histiocytosis in Japan. Pediatr Blood Cancer. 2010;54:98-102.
- Morren MA, Broecke KV, Vangeebergen L, et al. Diverse cutaneous presentations of Langerhans cell histiocytosis in children: a retrospective cohort study. Pediatr Blood Cancer. 2016;63:486-492.
- Berger TG, Lane AT, Headington JT, et al. A solitary variant of congenital self-healing reticulohistiocytosis: solitary Hashimoto-Pritzker disease. Pediatr Dermatol. 1986;3:230.
- Wheller L, Carman N, Butler G. Unilesional self-limited Langerhans cell histiocytosis: a case report and review of the literature. J Cutan Pathol. 2013;40:595-599.
- Battistella M, Fraitag S, Teillac DH, et al. Neonatal and early infantile cutaneous Langerhans cell histiocytosis: comparison of self-regressive and non-self-regressive forms. Arch Dermatol. 2010;146:149-156.
- Mehta V, Balachandran C, Lonikar V. Blueberry muffin baby: a pictoral differential diagnosis. Dermatol Online J. 2008;14:8.
- Kapur P, Erickson C, Rakheja D, et al. Congenital self-healing reticulohistiocytosis (Hashimoto-Pritzker disease): ten-year experience at Dallas Children's Medical Center. J Am Acad Dermatol. 2007;56:290-294.
- Writing Group of the Histiocyte Society. Histiocytosis syndromes in children. Lancet. 1987;24:208-209.
The Diagnosis: Self-healing Langerhans Cell Histiocytosis
Histopathologic examination showed an infiltrate of mononuclear cells with indented nuclei admixed with a variable dermal inflammatory infiltrate. Immunohistochemistry demonstrated cells that were strongly positive for CD1a (Figure, A) and langerin (Figure, B) antigens as well as S-100 protein (Figure, C), which was consistent with Langerhans cell histiocytosis (LCH).

Histiocytoses are a heterogeneous group of disorders in which the infiltrating cells belong to the mononuclear phagocyte system.1,2 Langerhans cell histiocytosis is the most common dendritic cell-related histiocytosis, occurring in approximately 5 per 1 million children annually, giving it an incidence comparable to pediatric Hodgkin lymphoma and acute myeloid leukemia.1,2
Historically, there has been much debate about the pathogenesis of the disease.2 Until recently it was unknown whether LCH was primarily a neoplastic or an inflammatory disorder. Although the condition initially was thought to have a reactive etiology,1 more recent evidence suggests a clonal neoplastic process. Langerhans cell histiocytosis lesions are clonal and display malignancy-associated mechanisms such as immune evasion. Genome sequencing has revealed several mutations in precursor myeloid cells that result in the common downstream hyperactivation of the mitogen-activated protein kinase signaling pathway that regulates cell proliferation and differentiation.1
Langerhans cell histiocytosis displays a wide spectrum of clinical phenotypes, which historically were subclassified as eosinophilic granulomas (localized lesions in bone), Hand-Schüller-Christian disease (multiple organ involvement with the classic triad of skull defects, diabetes insipidus, and exophthalmos), and Letterer-Siwe disease (visceral lesions involving multiple organs).3 However, in 1997 the Reclassification Working Group of the Histiocyte Society redefined LCH as single-system single site (SS-s) LCH, single-system multisite LCH, and multisystem LCH.4
In SS-s LCH, the most common site is bone (82%), followed by the skin (12%).5 Skin SS-s LCH classically presents as multiple skin lesions at birth without systemic manifestations; the lesions spontaneously involute within a few months.6 Less commonly, skin SS-s LCH can present as a single lesion. Berger et al7 described 4 neonates with unilesional skin SS-s LCH. Since then, more than 30 cases have been reported in the literature,8 and we report herein another unilesional self-healing LCH.
The morphology of skin lesions in self-healing LCH is highly variable, with the most common being multiple erythematous crusted papules (50%), followed by eczematous scaly lesions resembling seborrheic dermatitis in intertriginous areas (37.5%).3,6 Unilesional self-healing LCH typically presents as an ulcerated or crusted nodule or papule on the trunk. This variability results in a large differential diagnosis. Self-healing LCH is easily mistaken for infectious processes including neonatal herpes simplex and varicella-zoster virus infection.9 Often, the dermatology department is consulted to rule out LCH when the asymptomatic neonate does not respond to parenteral acyclovir.
Less commonly, the magenta-colored papulonodules of self-healing LCH can mimic blueberry muffin rash and mandate a workup for intrauterine infections, especially cytomegalovirus, rubella, and blood dyscrasia.10 Other noninfectious processes in the differential of self-healing LCH include congenital infantile hemangioma, neonatal lupus erythematosus, seborrheic dermatitis (cradle cap), pyogenic granuloma, and psoriasis.3,10 Definitive diagnosis requires histopathology.
Because unilesional self-healing LCH has an excellent prognosis and usually resolves on its own, therapy is unnecessary.3,8 One large retrospective study (N=146) found that of all patients with skin lesions, 56% were managed with biopsy only.5 Other options include watchful waiting and topical corticosteroids. If the skin lesions are large, ulcerated, and/or painful, alkylating antitumor agents have been used. For extensive cutaneous disease, systemic corticosteroids combined with chemotherapy and psoralen plus UVA can be effective.6
The primary concern in the management of self-healing LCH is that the solitary skin lesion may be the harbinger of an aggressive disorder that can progress to systemic disease.5 Moreover, recurrent visceral or disseminated disease may occur months to years after resolution of solitary skin lesions.9 Studies have shown that localized and disseminated disease cannot be differentiated on the basis of clinical findings, histology, immunohistochemistry, or biomarkers.3,11 As a result, an evaluation for systemic disease should be performed at the time of diagnosis for cutaneous LCH.3,9 Minimum baseline studies recommended by the Writing Group of the Histiocyte Society include a complete blood cell count, liver function tests, coagulation studies, chest radiography, skeletal surveys, and urine osmolality testing.12 Periodic clinical follow-up is recommended for all variants of LCH.9
Our case was diagnosed as self-healing LCH based on histologic findings. No treatment was required, and at 3-month follow-up the infant was asymptomatic without recurrence and was meeting all developmental milestones.
The Diagnosis: Self-healing Langerhans Cell Histiocytosis
Histopathologic examination showed an infiltrate of mononuclear cells with indented nuclei admixed with a variable dermal inflammatory infiltrate. Immunohistochemistry demonstrated cells that were strongly positive for CD1a (Figure, A) and langerin (Figure, B) antigens as well as S-100 protein (Figure, C), which was consistent with Langerhans cell histiocytosis (LCH).

Histiocytoses are a heterogeneous group of disorders in which the infiltrating cells belong to the mononuclear phagocyte system.1,2 Langerhans cell histiocytosis is the most common dendritic cell-related histiocytosis, occurring in approximately 5 per 1 million children annually, giving it an incidence comparable to pediatric Hodgkin lymphoma and acute myeloid leukemia.1,2
Historically, there has been much debate about the pathogenesis of the disease.2 Until recently it was unknown whether LCH was primarily a neoplastic or an inflammatory disorder. Although the condition initially was thought to have a reactive etiology,1 more recent evidence suggests a clonal neoplastic process. Langerhans cell histiocytosis lesions are clonal and display malignancy-associated mechanisms such as immune evasion. Genome sequencing has revealed several mutations in precursor myeloid cells that result in the common downstream hyperactivation of the mitogen-activated protein kinase signaling pathway that regulates cell proliferation and differentiation.1
Langerhans cell histiocytosis displays a wide spectrum of clinical phenotypes, which historically were subclassified as eosinophilic granulomas (localized lesions in bone), Hand-Schüller-Christian disease (multiple organ involvement with the classic triad of skull defects, diabetes insipidus, and exophthalmos), and Letterer-Siwe disease (visceral lesions involving multiple organs).3 However, in 1997 the Reclassification Working Group of the Histiocyte Society redefined LCH as single-system single site (SS-s) LCH, single-system multisite LCH, and multisystem LCH.4
In SS-s LCH, the most common site is bone (82%), followed by the skin (12%).5 Skin SS-s LCH classically presents as multiple skin lesions at birth without systemic manifestations; the lesions spontaneously involute within a few months.6 Less commonly, skin SS-s LCH can present as a single lesion. Berger et al7 described 4 neonates with unilesional skin SS-s LCH. Since then, more than 30 cases have been reported in the literature,8 and we report herein another unilesional self-healing LCH.
The morphology of skin lesions in self-healing LCH is highly variable, with the most common being multiple erythematous crusted papules (50%), followed by eczematous scaly lesions resembling seborrheic dermatitis in intertriginous areas (37.5%).3,6 Unilesional self-healing LCH typically presents as an ulcerated or crusted nodule or papule on the trunk. This variability results in a large differential diagnosis. Self-healing LCH is easily mistaken for infectious processes including neonatal herpes simplex and varicella-zoster virus infection.9 Often, the dermatology department is consulted to rule out LCH when the asymptomatic neonate does not respond to parenteral acyclovir.
Less commonly, the magenta-colored papulonodules of self-healing LCH can mimic blueberry muffin rash and mandate a workup for intrauterine infections, especially cytomegalovirus, rubella, and blood dyscrasia.10 Other noninfectious processes in the differential of self-healing LCH include congenital infantile hemangioma, neonatal lupus erythematosus, seborrheic dermatitis (cradle cap), pyogenic granuloma, and psoriasis.3,10 Definitive diagnosis requires histopathology.
Because unilesional self-healing LCH has an excellent prognosis and usually resolves on its own, therapy is unnecessary.3,8 One large retrospective study (N=146) found that of all patients with skin lesions, 56% were managed with biopsy only.5 Other options include watchful waiting and topical corticosteroids. If the skin lesions are large, ulcerated, and/or painful, alkylating antitumor agents have been used. For extensive cutaneous disease, systemic corticosteroids combined with chemotherapy and psoralen plus UVA can be effective.6
The primary concern in the management of self-healing LCH is that the solitary skin lesion may be the harbinger of an aggressive disorder that can progress to systemic disease.5 Moreover, recurrent visceral or disseminated disease may occur months to years after resolution of solitary skin lesions.9 Studies have shown that localized and disseminated disease cannot be differentiated on the basis of clinical findings, histology, immunohistochemistry, or biomarkers.3,11 As a result, an evaluation for systemic disease should be performed at the time of diagnosis for cutaneous LCH.3,9 Minimum baseline studies recommended by the Writing Group of the Histiocyte Society include a complete blood cell count, liver function tests, coagulation studies, chest radiography, skeletal surveys, and urine osmolality testing.12 Periodic clinical follow-up is recommended for all variants of LCH.9
Our case was diagnosed as self-healing LCH based on histologic findings. No treatment was required, and at 3-month follow-up the infant was asymptomatic without recurrence and was meeting all developmental milestones.
- Berres ML, Merad M, Allen CE. Progress in understanding the pathogenesis of Langerhans cell histiocytosis: back to histiocytosis X? Br J Haematol. 2015;169:3-13.
- Jordan MB, Filipovich AH. Histiocytic disorders. In: Hoffman R, Benz EJ Jr, Silberstein LE, eds. Hematology: Basic Principles and Practice. 6th ed. Philadelphia, PA: Elsevier Saunders; 2013:686-700.
- Stein SL, Paller AS, Haut PR, et al. Langerhans cell histiocytosis presenting in the neonatal period: a retrospective case series. Arch Pediatr Adolesc Med. 2001;155:778-783.
- Favara BE, Feller AC, Pauli M, et al. Contemporary classification of histiocytic disorders. Pediatr Blood Cancer. 1997;29:157-166.
- Morimoto A, Ishida Y, Suzuki N, et al. Nationwide survey of single-system single site Langerhans cell histiocytosis in Japan. Pediatr Blood Cancer. 2010;54:98-102.
- Morren MA, Broecke KV, Vangeebergen L, et al. Diverse cutaneous presentations of Langerhans cell histiocytosis in children: a retrospective cohort study. Pediatr Blood Cancer. 2016;63:486-492.
- Berger TG, Lane AT, Headington JT, et al. A solitary variant of congenital self-healing reticulohistiocytosis: solitary Hashimoto-Pritzker disease. Pediatr Dermatol. 1986;3:230.
- Wheller L, Carman N, Butler G. Unilesional self-limited Langerhans cell histiocytosis: a case report and review of the literature. J Cutan Pathol. 2013;40:595-599.
- Battistella M, Fraitag S, Teillac DH, et al. Neonatal and early infantile cutaneous Langerhans cell histiocytosis: comparison of self-regressive and non-self-regressive forms. Arch Dermatol. 2010;146:149-156.
- Mehta V, Balachandran C, Lonikar V. Blueberry muffin baby: a pictoral differential diagnosis. Dermatol Online J. 2008;14:8.
- Kapur P, Erickson C, Rakheja D, et al. Congenital self-healing reticulohistiocytosis (Hashimoto-Pritzker disease): ten-year experience at Dallas Children's Medical Center. J Am Acad Dermatol. 2007;56:290-294.
- Writing Group of the Histiocyte Society. Histiocytosis syndromes in children. Lancet. 1987;24:208-209.
- Berres ML, Merad M, Allen CE. Progress in understanding the pathogenesis of Langerhans cell histiocytosis: back to histiocytosis X? Br J Haematol. 2015;169:3-13.
- Jordan MB, Filipovich AH. Histiocytic disorders. In: Hoffman R, Benz EJ Jr, Silberstein LE, eds. Hematology: Basic Principles and Practice. 6th ed. Philadelphia, PA: Elsevier Saunders; 2013:686-700.
- Stein SL, Paller AS, Haut PR, et al. Langerhans cell histiocytosis presenting in the neonatal period: a retrospective case series. Arch Pediatr Adolesc Med. 2001;155:778-783.
- Favara BE, Feller AC, Pauli M, et al. Contemporary classification of histiocytic disorders. Pediatr Blood Cancer. 1997;29:157-166.
- Morimoto A, Ishida Y, Suzuki N, et al. Nationwide survey of single-system single site Langerhans cell histiocytosis in Japan. Pediatr Blood Cancer. 2010;54:98-102.
- Morren MA, Broecke KV, Vangeebergen L, et al. Diverse cutaneous presentations of Langerhans cell histiocytosis in children: a retrospective cohort study. Pediatr Blood Cancer. 2016;63:486-492.
- Berger TG, Lane AT, Headington JT, et al. A solitary variant of congenital self-healing reticulohistiocytosis: solitary Hashimoto-Pritzker disease. Pediatr Dermatol. 1986;3:230.
- Wheller L, Carman N, Butler G. Unilesional self-limited Langerhans cell histiocytosis: a case report and review of the literature. J Cutan Pathol. 2013;40:595-599.
- Battistella M, Fraitag S, Teillac DH, et al. Neonatal and early infantile cutaneous Langerhans cell histiocytosis: comparison of self-regressive and non-self-regressive forms. Arch Dermatol. 2010;146:149-156.
- Mehta V, Balachandran C, Lonikar V. Blueberry muffin baby: a pictoral differential diagnosis. Dermatol Online J. 2008;14:8.
- Kapur P, Erickson C, Rakheja D, et al. Congenital self-healing reticulohistiocytosis (Hashimoto-Pritzker disease): ten-year experience at Dallas Children's Medical Center. J Am Acad Dermatol. 2007;56:290-294.
- Writing Group of the Histiocyte Society. Histiocytosis syndromes in children. Lancet. 1987;24:208-209.
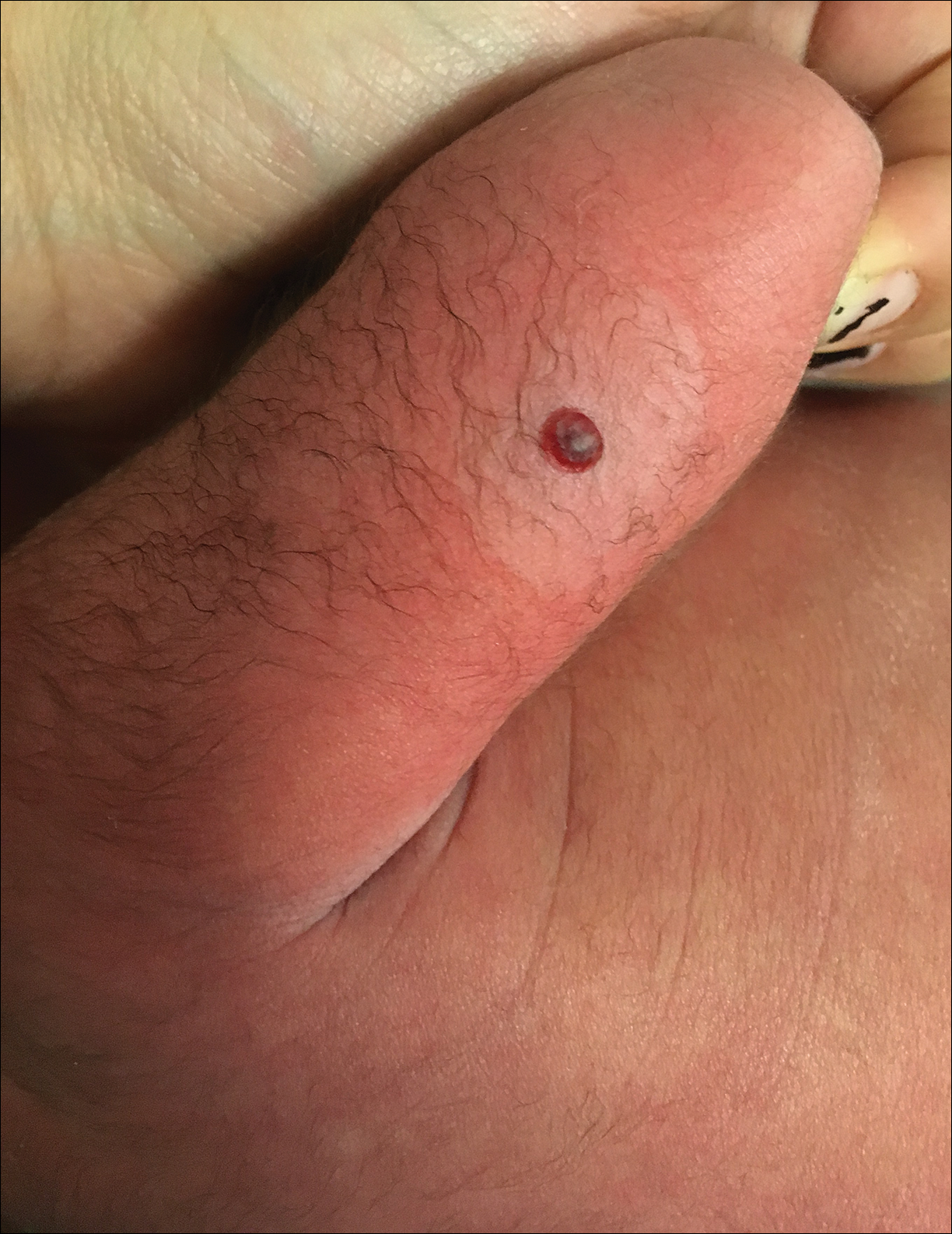
Dermatology consultation was called to the delivery room to evaluate a red, hemorrhagic, crusted, 5-mm papule on the right lateral upper arm of a preterm newborn. He appeared vigorous with an Apgar score of 7 at 1 minute and 8 at 5 minutes. Physical examination was otherwise normal. Of note, the mother presented late to prenatal care. Her herpes simplex and varicella-zoster virus status was unknown. A shave biopsy of the papule was performed at 3 days of age.
Fever, intestinal symptoms may delay diagnosis of Kawasaki disease in children
Symptoms of gastrointestinal involvement such as abdominal pain and vomiting may delay diagnosis of Kawasaki disease in pediatric patients.
“The clinical onset of Kawasaki disease with gastrointestinal involvement often leads to diagnostic and therapeutic delays – a risk factor for the development of coronary complications,” Claudia Colomba, MD, from the department of sciences for health promotion and mother and child care at the University of Palermo (Italy), and her colleagues wrote in the Journal of Pediatrics.
After caring for a boy aged 14 years at their center who presented with these symptoms, Dr. Colomba and her colleagues performed a search of the PubMed and Scopus databases and identified 33 articles with 48 total cases of Kawasaki disease with intestinal involvement between 1979 and 2017.
There were 40 cases of fever (82%), 34 cases of abdominal pain (69%), and 24 cases of vomiting (49%) at disease onset, with diarrhea occurring in 14 cases (29%) and jaundice in 1 case (2%), the researchers noted. Cardiac involvement occurred in 21 cases (43%). With regard to imaging, 38 cases of pseudo-obstruction (77%) were identified by plain radiograph, ultrasonography, and CT. Over half of the cases required surgery; of these 25 cases (51%), 8 cases involved a resection of the restricted loop and included a temporary colostomy (16%), 5 cases were exploratory laparotomy (10%), and there was 1 case with enterolysis (2%).
A total of 45 patients received medical treatment, with 12 patients (25%) receiving intravenous immunoglobulin and 18 (37%) receiving intravenous immunoglobulin plus aspirin. One patient had cyanosis and hand and foot gangrene. There were three patients who died, with massive liver necrosis identified during the autopsy of one patient. Of the other two who died, one did so 2 days after exploratory laparotomy and the other died because of Pseudomonas septic shock.
The researchers reported a good outcome in 28 patients (57%), which included 3 cases where there was no treatment.
“The diagnosis of Kawasaki disease should be considered in all children with fever, abdominal pain, and radiologic signs of pseudo-obstruction, even in the absence of typical symptoms and signs,” Dr. Colomba and her colleagues wrote. “A more comprehensive analysis including all clinical forms of Kawasaki disease would be useful to correlate intestinal involvement with worse outcomes for cardiac complications, as well as to clues to more rapid diagnosis and avoidance of unnecessary invasive procedures.”
The authors reported no relevant conflicts of interest.
SOURCE: Colomba C et al. J Pediatr. 2018 Jul 17. doi: 10.1016/j.jpeds.2018.06.034.
Adding abdominal pain–first presentation to Kawasaki disease is not unprecedented considering lymph node–first presentation was first introduced as a concept in the Journal of Pediatrics in 2013, Sarah S. Long, MD, wrote in a related editorial.
“It should not be too surprising that intestinal vasculitis could be significant in some cases,” Dr. Long said. “Might it not suggest an intestinal portal of microbe or super antigen entry, as might cervical lymphadenitis a respiratory tract portal of entry?”
Dr. Long noted diagnostic and reporting bias was most likely the cause of the 43% rate of coronary artery aneurysms reported in the study by Colomba et al, but said that “it behooves us all to consider Kawasaki disease in the differential when a child has high fever and abdominal pain.”
Dr. Long is a professor of pediatrics at Drexel University, Philadelphia. She made her comments regarding the article by Colomba et al. in the Journal of Pediatrics (2018 Jul 17. doi: 10.1016/j.jpeds.2018.09.018 ). Dr. Long is on the editorial board of the journal, served as the chief editor on and receives royalties from “Principles and Practice of Pediatric Infectious Diseases,” and serves as the associate editor of the Red Book Report of the American Academy of Pediatrics Committee on Infectious Diseases.
Adding abdominal pain–first presentation to Kawasaki disease is not unprecedented considering lymph node–first presentation was first introduced as a concept in the Journal of Pediatrics in 2013, Sarah S. Long, MD, wrote in a related editorial.
“It should not be too surprising that intestinal vasculitis could be significant in some cases,” Dr. Long said. “Might it not suggest an intestinal portal of microbe or super antigen entry, as might cervical lymphadenitis a respiratory tract portal of entry?”
Dr. Long noted diagnostic and reporting bias was most likely the cause of the 43% rate of coronary artery aneurysms reported in the study by Colomba et al, but said that “it behooves us all to consider Kawasaki disease in the differential when a child has high fever and abdominal pain.”
Dr. Long is a professor of pediatrics at Drexel University, Philadelphia. She made her comments regarding the article by Colomba et al. in the Journal of Pediatrics (2018 Jul 17. doi: 10.1016/j.jpeds.2018.09.018 ). Dr. Long is on the editorial board of the journal, served as the chief editor on and receives royalties from “Principles and Practice of Pediatric Infectious Diseases,” and serves as the associate editor of the Red Book Report of the American Academy of Pediatrics Committee on Infectious Diseases.
Adding abdominal pain–first presentation to Kawasaki disease is not unprecedented considering lymph node–first presentation was first introduced as a concept in the Journal of Pediatrics in 2013, Sarah S. Long, MD, wrote in a related editorial.
“It should not be too surprising that intestinal vasculitis could be significant in some cases,” Dr. Long said. “Might it not suggest an intestinal portal of microbe or super antigen entry, as might cervical lymphadenitis a respiratory tract portal of entry?”
Dr. Long noted diagnostic and reporting bias was most likely the cause of the 43% rate of coronary artery aneurysms reported in the study by Colomba et al, but said that “it behooves us all to consider Kawasaki disease in the differential when a child has high fever and abdominal pain.”
Dr. Long is a professor of pediatrics at Drexel University, Philadelphia. She made her comments regarding the article by Colomba et al. in the Journal of Pediatrics (2018 Jul 17. doi: 10.1016/j.jpeds.2018.09.018 ). Dr. Long is on the editorial board of the journal, served as the chief editor on and receives royalties from “Principles and Practice of Pediatric Infectious Diseases,” and serves as the associate editor of the Red Book Report of the American Academy of Pediatrics Committee on Infectious Diseases.
Symptoms of gastrointestinal involvement such as abdominal pain and vomiting may delay diagnosis of Kawasaki disease in pediatric patients.
“The clinical onset of Kawasaki disease with gastrointestinal involvement often leads to diagnostic and therapeutic delays – a risk factor for the development of coronary complications,” Claudia Colomba, MD, from the department of sciences for health promotion and mother and child care at the University of Palermo (Italy), and her colleagues wrote in the Journal of Pediatrics.
After caring for a boy aged 14 years at their center who presented with these symptoms, Dr. Colomba and her colleagues performed a search of the PubMed and Scopus databases and identified 33 articles with 48 total cases of Kawasaki disease with intestinal involvement between 1979 and 2017.
There were 40 cases of fever (82%), 34 cases of abdominal pain (69%), and 24 cases of vomiting (49%) at disease onset, with diarrhea occurring in 14 cases (29%) and jaundice in 1 case (2%), the researchers noted. Cardiac involvement occurred in 21 cases (43%). With regard to imaging, 38 cases of pseudo-obstruction (77%) were identified by plain radiograph, ultrasonography, and CT. Over half of the cases required surgery; of these 25 cases (51%), 8 cases involved a resection of the restricted loop and included a temporary colostomy (16%), 5 cases were exploratory laparotomy (10%), and there was 1 case with enterolysis (2%).
A total of 45 patients received medical treatment, with 12 patients (25%) receiving intravenous immunoglobulin and 18 (37%) receiving intravenous immunoglobulin plus aspirin. One patient had cyanosis and hand and foot gangrene. There were three patients who died, with massive liver necrosis identified during the autopsy of one patient. Of the other two who died, one did so 2 days after exploratory laparotomy and the other died because of Pseudomonas septic shock.
The researchers reported a good outcome in 28 patients (57%), which included 3 cases where there was no treatment.
“The diagnosis of Kawasaki disease should be considered in all children with fever, abdominal pain, and radiologic signs of pseudo-obstruction, even in the absence of typical symptoms and signs,” Dr. Colomba and her colleagues wrote. “A more comprehensive analysis including all clinical forms of Kawasaki disease would be useful to correlate intestinal involvement with worse outcomes for cardiac complications, as well as to clues to more rapid diagnosis and avoidance of unnecessary invasive procedures.”
The authors reported no relevant conflicts of interest.
SOURCE: Colomba C et al. J Pediatr. 2018 Jul 17. doi: 10.1016/j.jpeds.2018.06.034.
Symptoms of gastrointestinal involvement such as abdominal pain and vomiting may delay diagnosis of Kawasaki disease in pediatric patients.
“The clinical onset of Kawasaki disease with gastrointestinal involvement often leads to diagnostic and therapeutic delays – a risk factor for the development of coronary complications,” Claudia Colomba, MD, from the department of sciences for health promotion and mother and child care at the University of Palermo (Italy), and her colleagues wrote in the Journal of Pediatrics.
After caring for a boy aged 14 years at their center who presented with these symptoms, Dr. Colomba and her colleagues performed a search of the PubMed and Scopus databases and identified 33 articles with 48 total cases of Kawasaki disease with intestinal involvement between 1979 and 2017.
There were 40 cases of fever (82%), 34 cases of abdominal pain (69%), and 24 cases of vomiting (49%) at disease onset, with diarrhea occurring in 14 cases (29%) and jaundice in 1 case (2%), the researchers noted. Cardiac involvement occurred in 21 cases (43%). With regard to imaging, 38 cases of pseudo-obstruction (77%) were identified by plain radiograph, ultrasonography, and CT. Over half of the cases required surgery; of these 25 cases (51%), 8 cases involved a resection of the restricted loop and included a temporary colostomy (16%), 5 cases were exploratory laparotomy (10%), and there was 1 case with enterolysis (2%).
A total of 45 patients received medical treatment, with 12 patients (25%) receiving intravenous immunoglobulin and 18 (37%) receiving intravenous immunoglobulin plus aspirin. One patient had cyanosis and hand and foot gangrene. There were three patients who died, with massive liver necrosis identified during the autopsy of one patient. Of the other two who died, one did so 2 days after exploratory laparotomy and the other died because of Pseudomonas septic shock.
The researchers reported a good outcome in 28 patients (57%), which included 3 cases where there was no treatment.
“The diagnosis of Kawasaki disease should be considered in all children with fever, abdominal pain, and radiologic signs of pseudo-obstruction, even in the absence of typical symptoms and signs,” Dr. Colomba and her colleagues wrote. “A more comprehensive analysis including all clinical forms of Kawasaki disease would be useful to correlate intestinal involvement with worse outcomes for cardiac complications, as well as to clues to more rapid diagnosis and avoidance of unnecessary invasive procedures.”
The authors reported no relevant conflicts of interest.
SOURCE: Colomba C et al. J Pediatr. 2018 Jul 17. doi: 10.1016/j.jpeds.2018.06.034.
FROM THE JOURNAL OF PEDIATRICS
Key clinical point: Abdominal pain, fever, and radiologically identified pseudo-obstruction may delay diagnosis of Kawasaki disease in children and should be considered for these patients.
Major finding: Fever, abdominal pain, and vomiting were the most common symptoms that appeared prior to traditional Kawasaki disease symptoms.
Study details: A systematic review of 48 cases of Kawasaki disease patients with intestinal involvement.
Disclosures: The authors reported no relevant conflicts of interest.
Source: Colomba C et al. J Pediatr. 2018 Jul 17. doi: 10.1016/j.jpeds.2018.06.034.