User login
Pathologic superstition
When you believe in things that you don’t understand
Then you suffer
Superstition ain’t the way
– Stevie Wonder
I have always found it odd that airplanes don’t have a 13th row and hotels don’t have a 13th floor. Well, of course they do, but they are not labeled that way. Many people would hesitate to sit in the 13th row of an airplane since 13 is such an unlucky number. At least many people in the United States think the number 13 is unlucky. Thirteen is just a number in much of Asia. There, the number 4 is just as threatening as 13 is to us.
Superstitions like these are familiar to all of us.
One of my favorites is the belief that vacuum cups attached to the skin will somehow draw out toxins and generally improve health. “Cupping,” as the practice is known, is endorsed by several celebrities and famous athletes. After the treatment, a cupped patient exhibits circles of hyperemia, and no other apparent harm. I suspect that about a third of cupped patients truly think they have benefited from a good cupping, about the same number that would benefit from an orally administered placebo.
Superstitions are everywhere. Whether it is a black cat in the United States, infinite reflecting mirrors in Mexico, going back to your house after a wake in the Philippines, or whistling indoors in Lithuania, superstitions are pervasive, deeply held, and generally harmless. They are good for a good laugh as we recognize how ludicrous these unfounded fears are.
Some superstitions, though, are no laughing matter. They can be quite harmful. They are pathologic superstitions.
For example, some people believe vaccines cause autism in children. That pathologic superstition has consequences. A recent CDC report revealed that the population of unvaccinated children in the United States has quadrupled since 2001. This comes as no surprise as we hear about more measles outbreaks – and the deaths associated with them – in populations of unvaccinated children every year. A similar and pervasive pathologic superstition is the fear that an influenza vaccine will cause the flu. I wonder how many people die from this misconception.
Other people believe that their cancer can be treated, if not cured, with unproven, unconventional treatments. I cannot understand how this pathologic superstition developed. The purveyors of unconventional treatment hold much of the blame, but gullibility and ignorance may play a larger role. The consequences are tragic. A recent report demonstrated an approximately twofold increased risk of death in patients who used complementary therapies, compared with those who did not (JAMA Oncol. 2018 Oct 1;4[10]:1375-81).
These are sobering data for those of us who have in the past relented when our patients asked if they could take this or that supplement because we did not think they would cause significant harm.
Superstitions apparently are part of the human condition, evolved to attribute causation and provide order. They are a learned phenomenon. They are learned by reasonable people with normal intelligence and rational thinking. A superstition is born when someone is exposed to a false statement by someone or something they trust – a trusted other.
Trusted others exude certainty. Once established, superstitions are regrettably difficult to remove by those who are less certain, like physicians. How willing are we to say that the flu vaccine is 100% safe? Without certainty, how can a physician debunk a superstition? The techniques that we have been taught usually work, but not when faced with a pathologic superstition.
Science and experience teach us that firmly held superstitions cannot be broken with logical, stepwise reasoning. Jonathan Haidt provides a useful metaphor for this problem in his book “The Happiness Hypothesis” (Basic Books, 2006). He describes a rider on an elephant. The rider represents our rational thought and the elephant represents our emotional foundation. The rider thinks he controls the elephant, but the opposite is more likely true. In order to move the elephant in a certain direction, the rider needs to make the elephant want to turn in that direction. Otherwise, all the cajoling and arguing in the world won’t make the elephant turn. A rational argument made to someone emotionally invested in the counter argument will fail. That is why we cannot convince antivaccine parents to vaccinate their children by trying to persuade them with facts. Neither can we convince global warming skeptics to stop burning coal, gun advocates to vote for restrictions on gun ownership, or cancer patients to accept curative treatment if their values and morals are being challenged.
In a later book, “The Righteous Mind: Why Good People Are Divided by Politics and Religion” (Vintage Books, 2012), Mr. Haidt expands his hypothesis to declare that to change minds, we must appeal to underlying moral values. The challenge is to identify those moral underpinnings in our patients in order to develop an appeal likely to resonate with their emotions and values.
Superstition derives from something people learn either from trusted others or from personal experience. It does no good for physicians to deride patient beliefs and denigrate their agency in an attempt to persuade them to abandon what we consider irrational beliefs. For physicians to penetrate pathologic superstitions, they will have to become the trusted other, to understand moral foundations, to emotionally connect. That does not usually happen the first day we meet a new patient, especially a skeptical one. It takes time, and effort, to reach out and bond with the patient and their family. Only then can pathologic superstitions dissolve and a better patient-doctor relationship evolve.
During this season rife with superstition, remember that your patient’s own superstitions are part of their belief system, and your belief system may be threatening to them. Make your beliefs less threatening, become a trusted other, and appeal to their foundational values, and you can successfully break a pathologic superstition.
Dr. Kalaycio is editor in chief of Hematology News. He chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
When you believe in things that you don’t understand
Then you suffer
Superstition ain’t the way
– Stevie Wonder
I have always found it odd that airplanes don’t have a 13th row and hotels don’t have a 13th floor. Well, of course they do, but they are not labeled that way. Many people would hesitate to sit in the 13th row of an airplane since 13 is such an unlucky number. At least many people in the United States think the number 13 is unlucky. Thirteen is just a number in much of Asia. There, the number 4 is just as threatening as 13 is to us.
Superstitions like these are familiar to all of us.
One of my favorites is the belief that vacuum cups attached to the skin will somehow draw out toxins and generally improve health. “Cupping,” as the practice is known, is endorsed by several celebrities and famous athletes. After the treatment, a cupped patient exhibits circles of hyperemia, and no other apparent harm. I suspect that about a third of cupped patients truly think they have benefited from a good cupping, about the same number that would benefit from an orally administered placebo.
Superstitions are everywhere. Whether it is a black cat in the United States, infinite reflecting mirrors in Mexico, going back to your house after a wake in the Philippines, or whistling indoors in Lithuania, superstitions are pervasive, deeply held, and generally harmless. They are good for a good laugh as we recognize how ludicrous these unfounded fears are.
Some superstitions, though, are no laughing matter. They can be quite harmful. They are pathologic superstitions.
For example, some people believe vaccines cause autism in children. That pathologic superstition has consequences. A recent CDC report revealed that the population of unvaccinated children in the United States has quadrupled since 2001. This comes as no surprise as we hear about more measles outbreaks – and the deaths associated with them – in populations of unvaccinated children every year. A similar and pervasive pathologic superstition is the fear that an influenza vaccine will cause the flu. I wonder how many people die from this misconception.
Other people believe that their cancer can be treated, if not cured, with unproven, unconventional treatments. I cannot understand how this pathologic superstition developed. The purveyors of unconventional treatment hold much of the blame, but gullibility and ignorance may play a larger role. The consequences are tragic. A recent report demonstrated an approximately twofold increased risk of death in patients who used complementary therapies, compared with those who did not (JAMA Oncol. 2018 Oct 1;4[10]:1375-81).
These are sobering data for those of us who have in the past relented when our patients asked if they could take this or that supplement because we did not think they would cause significant harm.
Superstitions apparently are part of the human condition, evolved to attribute causation and provide order. They are a learned phenomenon. They are learned by reasonable people with normal intelligence and rational thinking. A superstition is born when someone is exposed to a false statement by someone or something they trust – a trusted other.
Trusted others exude certainty. Once established, superstitions are regrettably difficult to remove by those who are less certain, like physicians. How willing are we to say that the flu vaccine is 100% safe? Without certainty, how can a physician debunk a superstition? The techniques that we have been taught usually work, but not when faced with a pathologic superstition.
Science and experience teach us that firmly held superstitions cannot be broken with logical, stepwise reasoning. Jonathan Haidt provides a useful metaphor for this problem in his book “The Happiness Hypothesis” (Basic Books, 2006). He describes a rider on an elephant. The rider represents our rational thought and the elephant represents our emotional foundation. The rider thinks he controls the elephant, but the opposite is more likely true. In order to move the elephant in a certain direction, the rider needs to make the elephant want to turn in that direction. Otherwise, all the cajoling and arguing in the world won’t make the elephant turn. A rational argument made to someone emotionally invested in the counter argument will fail. That is why we cannot convince antivaccine parents to vaccinate their children by trying to persuade them with facts. Neither can we convince global warming skeptics to stop burning coal, gun advocates to vote for restrictions on gun ownership, or cancer patients to accept curative treatment if their values and morals are being challenged.
In a later book, “The Righteous Mind: Why Good People Are Divided by Politics and Religion” (Vintage Books, 2012), Mr. Haidt expands his hypothesis to declare that to change minds, we must appeal to underlying moral values. The challenge is to identify those moral underpinnings in our patients in order to develop an appeal likely to resonate with their emotions and values.
Superstition derives from something people learn either from trusted others or from personal experience. It does no good for physicians to deride patient beliefs and denigrate their agency in an attempt to persuade them to abandon what we consider irrational beliefs. For physicians to penetrate pathologic superstitions, they will have to become the trusted other, to understand moral foundations, to emotionally connect. That does not usually happen the first day we meet a new patient, especially a skeptical one. It takes time, and effort, to reach out and bond with the patient and their family. Only then can pathologic superstitions dissolve and a better patient-doctor relationship evolve.
During this season rife with superstition, remember that your patient’s own superstitions are part of their belief system, and your belief system may be threatening to them. Make your beliefs less threatening, become a trusted other, and appeal to their foundational values, and you can successfully break a pathologic superstition.
Dr. Kalaycio is editor in chief of Hematology News. He chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
When you believe in things that you don’t understand
Then you suffer
Superstition ain’t the way
– Stevie Wonder
I have always found it odd that airplanes don’t have a 13th row and hotels don’t have a 13th floor. Well, of course they do, but they are not labeled that way. Many people would hesitate to sit in the 13th row of an airplane since 13 is such an unlucky number. At least many people in the United States think the number 13 is unlucky. Thirteen is just a number in much of Asia. There, the number 4 is just as threatening as 13 is to us.
Superstitions like these are familiar to all of us.
One of my favorites is the belief that vacuum cups attached to the skin will somehow draw out toxins and generally improve health. “Cupping,” as the practice is known, is endorsed by several celebrities and famous athletes. After the treatment, a cupped patient exhibits circles of hyperemia, and no other apparent harm. I suspect that about a third of cupped patients truly think they have benefited from a good cupping, about the same number that would benefit from an orally administered placebo.
Superstitions are everywhere. Whether it is a black cat in the United States, infinite reflecting mirrors in Mexico, going back to your house after a wake in the Philippines, or whistling indoors in Lithuania, superstitions are pervasive, deeply held, and generally harmless. They are good for a good laugh as we recognize how ludicrous these unfounded fears are.
Some superstitions, though, are no laughing matter. They can be quite harmful. They are pathologic superstitions.
For example, some people believe vaccines cause autism in children. That pathologic superstition has consequences. A recent CDC report revealed that the population of unvaccinated children in the United States has quadrupled since 2001. This comes as no surprise as we hear about more measles outbreaks – and the deaths associated with them – in populations of unvaccinated children every year. A similar and pervasive pathologic superstition is the fear that an influenza vaccine will cause the flu. I wonder how many people die from this misconception.
Other people believe that their cancer can be treated, if not cured, with unproven, unconventional treatments. I cannot understand how this pathologic superstition developed. The purveyors of unconventional treatment hold much of the blame, but gullibility and ignorance may play a larger role. The consequences are tragic. A recent report demonstrated an approximately twofold increased risk of death in patients who used complementary therapies, compared with those who did not (JAMA Oncol. 2018 Oct 1;4[10]:1375-81).
These are sobering data for those of us who have in the past relented when our patients asked if they could take this or that supplement because we did not think they would cause significant harm.
Superstitions apparently are part of the human condition, evolved to attribute causation and provide order. They are a learned phenomenon. They are learned by reasonable people with normal intelligence and rational thinking. A superstition is born when someone is exposed to a false statement by someone or something they trust – a trusted other.
Trusted others exude certainty. Once established, superstitions are regrettably difficult to remove by those who are less certain, like physicians. How willing are we to say that the flu vaccine is 100% safe? Without certainty, how can a physician debunk a superstition? The techniques that we have been taught usually work, but not when faced with a pathologic superstition.
Science and experience teach us that firmly held superstitions cannot be broken with logical, stepwise reasoning. Jonathan Haidt provides a useful metaphor for this problem in his book “The Happiness Hypothesis” (Basic Books, 2006). He describes a rider on an elephant. The rider represents our rational thought and the elephant represents our emotional foundation. The rider thinks he controls the elephant, but the opposite is more likely true. In order to move the elephant in a certain direction, the rider needs to make the elephant want to turn in that direction. Otherwise, all the cajoling and arguing in the world won’t make the elephant turn. A rational argument made to someone emotionally invested in the counter argument will fail. That is why we cannot convince antivaccine parents to vaccinate their children by trying to persuade them with facts. Neither can we convince global warming skeptics to stop burning coal, gun advocates to vote for restrictions on gun ownership, or cancer patients to accept curative treatment if their values and morals are being challenged.
In a later book, “The Righteous Mind: Why Good People Are Divided by Politics and Religion” (Vintage Books, 2012), Mr. Haidt expands his hypothesis to declare that to change minds, we must appeal to underlying moral values. The challenge is to identify those moral underpinnings in our patients in order to develop an appeal likely to resonate with their emotions and values.
Superstition derives from something people learn either from trusted others or from personal experience. It does no good for physicians to deride patient beliefs and denigrate their agency in an attempt to persuade them to abandon what we consider irrational beliefs. For physicians to penetrate pathologic superstitions, they will have to become the trusted other, to understand moral foundations, to emotionally connect. That does not usually happen the first day we meet a new patient, especially a skeptical one. It takes time, and effort, to reach out and bond with the patient and their family. Only then can pathologic superstitions dissolve and a better patient-doctor relationship evolve.
During this season rife with superstition, remember that your patient’s own superstitions are part of their belief system, and your belief system may be threatening to them. Make your beliefs less threatening, become a trusted other, and appeal to their foundational values, and you can successfully break a pathologic superstition.
Dr. Kalaycio is editor in chief of Hematology News. He chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
Palliative care update highlights role of nonspecialists
The new edition of providing care for critically ill patients, not just those clinicians actively specialized in palliative care.
The Clinical Practice Guidelines for Quality Palliative Care, 4th Edition, emphasizes the importance of palliative care provided by “clinicians in primary care and specialty care practices, such as oncologists,” the guideline authors stated.
The latest revision of the guideline aims to establish a foundation for “gold-standard” palliative care for people living with serious illness, regardless of diagnosis, prognosis, setting, or age, according to the National Coalition for Hospice and Palliative Care, which published the clinical practice guidelines.
The update was developed by the National Consensus Project for Quality Palliative Care (NCP), which includes 16 national organizations with palliative care and hospice expertise, and is endorsed by more than 80 national organizations, including the American Society of Hematology and the Oncology Nurses Society.
One key reason for the update, according to the NCP, was to acknowledge that today’s health care system may not be meeting patients’ palliative care needs.
Specifically, the guidelines call on all clinicians who are not palliative specialists to integrate palliative care principles into their routine assessment of seriously ill patients with conditions such as heart failure, lung disease, and cancer.
This approach differs from the way palliative care is traditionally practiced, often by fellowship-trained physicians, trained nurses, and other specialists who provide that support.
The guidelines are organized into sections covering palliative care structure and processes, care for the patient nearing the end of life, and specific aspects of palliative care, including physical, psychological, and psychiatric; social; cultural, ethical, and legal; and spiritual, religious, and existential aspects.
“The expectation is that all clinicians caring for seriously ill patients will integrate palliative care competencies, such as safe and effective pain and symptom management and expert communication skills in their practice, and palliative care specialists will provide expertise for those with the most complex needs,” the guideline authors wrote.
Implications for treatment of oncology patients
These new guidelines represent a “blueprint for what it looks like to provide high-quality, comprehensive palliative care to people with serious illness,” said Thomas W. LeBlanc, MD, who is a medical oncologist, palliative care physician, and patient experience researcher at Duke University, Durham, N.C.
“Part of this report to is about trying to raise the game of everybody in medicine and provide a higher basic level of primary palliative care to all people with serious illness, but then also to figure out who has higher levels of needs where the specialists should be applied, since they are a scarce resource,” said Dr. LeBlanc.
An issue with that traditional model is a shortage of specialized clinicians to meet palliative care needs, said Dr. LeBlanc, whose clinical practice and research focuses on palliative care needs of patients with hematologic malignancies.
“Palliative care has matured as a field such that we are now actually facing workforce shortage issues and really fundamental questions about who needs us the most, and how we increase our reach to improve the lives of more patients and families facing serious illness,” he said in an interview.
That’s a major driver behind the emphasis in these latest guidelines on providing palliative care in the community, coordinating care, and dealing with care transitions, he added.
“I hope that this document will help to demonstrate the value and the need for palliative care specialists, and for improvements in primary care in the care of patients with hematologic diseases in general,” he said. “To me, this adds increasing legitimacy to this whole field.”
Palliative care in surgical care
These guidelines are particularly useful to surgeons in part because of their focus on what’s known as primary palliative care, said to Geoffrey P. Dunn, MD, former chair of the American College of Surgeons Committee on Surgical Palliative Care. Palliative care, the new guidelines suggest, can be implemented by nonspecialists.
Primary palliative care includes diverse skills such as breaking adverse news to patients, managing uncomplicated pain, and being able to recognize signs and symptoms of imminent demise. “These are the minimum deliverables for all people dealing with seriously ill patients,” Dr. Dunn said in an interview. “It’s palliative care that any practicing physician should be able to handle.”
Dr. Dunn concurred with Dr. LaBlanc about the workforce shortage in the palliative field. The traditional model has created a shortage of specialized clinicians to meet palliative care needs. Across the board, “staffing for palliative teams is very inconsistent,” said Dr. Dunn. “It’s a classic unfunded mandate.”
While these guidelines are a step forward in recognizing the importance of palliative care outside of the palliative care specialty, there is no reference to surgery anywhere in the text of the 141-page prepublication draft provided by the NCP, Dr. Dunn noted in the interview.
“There’s still a danger of parallel universes, where surgery is developing its own understanding of this in parallel with the more general national palliative care movement,” he said. Despite that, there is a growing connection between surgery and the broader palliative care community. That linkage is especially important given the number of seriously ill patients with high symptom burden that are seen in surgery.
“I think where surgeons are beginning to find [palliative principles] very helpful is dealing with these protracted serial discussions with families in difficult circumstances, such as how long is the life support going to be prolonged in someone with a devastating head injury, or multiple system organ failure in the elderly,” Dr. Dunn added.
The new edition of providing care for critically ill patients, not just those clinicians actively specialized in palliative care.
The Clinical Practice Guidelines for Quality Palliative Care, 4th Edition, emphasizes the importance of palliative care provided by “clinicians in primary care and specialty care practices, such as oncologists,” the guideline authors stated.
The latest revision of the guideline aims to establish a foundation for “gold-standard” palliative care for people living with serious illness, regardless of diagnosis, prognosis, setting, or age, according to the National Coalition for Hospice and Palliative Care, which published the clinical practice guidelines.
The update was developed by the National Consensus Project for Quality Palliative Care (NCP), which includes 16 national organizations with palliative care and hospice expertise, and is endorsed by more than 80 national organizations, including the American Society of Hematology and the Oncology Nurses Society.
One key reason for the update, according to the NCP, was to acknowledge that today’s health care system may not be meeting patients’ palliative care needs.
Specifically, the guidelines call on all clinicians who are not palliative specialists to integrate palliative care principles into their routine assessment of seriously ill patients with conditions such as heart failure, lung disease, and cancer.
This approach differs from the way palliative care is traditionally practiced, often by fellowship-trained physicians, trained nurses, and other specialists who provide that support.
The guidelines are organized into sections covering palliative care structure and processes, care for the patient nearing the end of life, and specific aspects of palliative care, including physical, psychological, and psychiatric; social; cultural, ethical, and legal; and spiritual, religious, and existential aspects.
“The expectation is that all clinicians caring for seriously ill patients will integrate palliative care competencies, such as safe and effective pain and symptom management and expert communication skills in their practice, and palliative care specialists will provide expertise for those with the most complex needs,” the guideline authors wrote.
Implications for treatment of oncology patients
These new guidelines represent a “blueprint for what it looks like to provide high-quality, comprehensive palliative care to people with serious illness,” said Thomas W. LeBlanc, MD, who is a medical oncologist, palliative care physician, and patient experience researcher at Duke University, Durham, N.C.
“Part of this report to is about trying to raise the game of everybody in medicine and provide a higher basic level of primary palliative care to all people with serious illness, but then also to figure out who has higher levels of needs where the specialists should be applied, since they are a scarce resource,” said Dr. LeBlanc.
An issue with that traditional model is a shortage of specialized clinicians to meet palliative care needs, said Dr. LeBlanc, whose clinical practice and research focuses on palliative care needs of patients with hematologic malignancies.
“Palliative care has matured as a field such that we are now actually facing workforce shortage issues and really fundamental questions about who needs us the most, and how we increase our reach to improve the lives of more patients and families facing serious illness,” he said in an interview.
That’s a major driver behind the emphasis in these latest guidelines on providing palliative care in the community, coordinating care, and dealing with care transitions, he added.
“I hope that this document will help to demonstrate the value and the need for palliative care specialists, and for improvements in primary care in the care of patients with hematologic diseases in general,” he said. “To me, this adds increasing legitimacy to this whole field.”
Palliative care in surgical care
These guidelines are particularly useful to surgeons in part because of their focus on what’s known as primary palliative care, said to Geoffrey P. Dunn, MD, former chair of the American College of Surgeons Committee on Surgical Palliative Care. Palliative care, the new guidelines suggest, can be implemented by nonspecialists.
Primary palliative care includes diverse skills such as breaking adverse news to patients, managing uncomplicated pain, and being able to recognize signs and symptoms of imminent demise. “These are the minimum deliverables for all people dealing with seriously ill patients,” Dr. Dunn said in an interview. “It’s palliative care that any practicing physician should be able to handle.”
Dr. Dunn concurred with Dr. LaBlanc about the workforce shortage in the palliative field. The traditional model has created a shortage of specialized clinicians to meet palliative care needs. Across the board, “staffing for palliative teams is very inconsistent,” said Dr. Dunn. “It’s a classic unfunded mandate.”
While these guidelines are a step forward in recognizing the importance of palliative care outside of the palliative care specialty, there is no reference to surgery anywhere in the text of the 141-page prepublication draft provided by the NCP, Dr. Dunn noted in the interview.
“There’s still a danger of parallel universes, where surgery is developing its own understanding of this in parallel with the more general national palliative care movement,” he said. Despite that, there is a growing connection between surgery and the broader palliative care community. That linkage is especially important given the number of seriously ill patients with high symptom burden that are seen in surgery.
“I think where surgeons are beginning to find [palliative principles] very helpful is dealing with these protracted serial discussions with families in difficult circumstances, such as how long is the life support going to be prolonged in someone with a devastating head injury, or multiple system organ failure in the elderly,” Dr. Dunn added.
The new edition of providing care for critically ill patients, not just those clinicians actively specialized in palliative care.
The Clinical Practice Guidelines for Quality Palliative Care, 4th Edition, emphasizes the importance of palliative care provided by “clinicians in primary care and specialty care practices, such as oncologists,” the guideline authors stated.
The latest revision of the guideline aims to establish a foundation for “gold-standard” palliative care for people living with serious illness, regardless of diagnosis, prognosis, setting, or age, according to the National Coalition for Hospice and Palliative Care, which published the clinical practice guidelines.
The update was developed by the National Consensus Project for Quality Palliative Care (NCP), which includes 16 national organizations with palliative care and hospice expertise, and is endorsed by more than 80 national organizations, including the American Society of Hematology and the Oncology Nurses Society.
One key reason for the update, according to the NCP, was to acknowledge that today’s health care system may not be meeting patients’ palliative care needs.
Specifically, the guidelines call on all clinicians who are not palliative specialists to integrate palliative care principles into their routine assessment of seriously ill patients with conditions such as heart failure, lung disease, and cancer.
This approach differs from the way palliative care is traditionally practiced, often by fellowship-trained physicians, trained nurses, and other specialists who provide that support.
The guidelines are organized into sections covering palliative care structure and processes, care for the patient nearing the end of life, and specific aspects of palliative care, including physical, psychological, and psychiatric; social; cultural, ethical, and legal; and spiritual, religious, and existential aspects.
“The expectation is that all clinicians caring for seriously ill patients will integrate palliative care competencies, such as safe and effective pain and symptom management and expert communication skills in their practice, and palliative care specialists will provide expertise for those with the most complex needs,” the guideline authors wrote.
Implications for treatment of oncology patients
These new guidelines represent a “blueprint for what it looks like to provide high-quality, comprehensive palliative care to people with serious illness,” said Thomas W. LeBlanc, MD, who is a medical oncologist, palliative care physician, and patient experience researcher at Duke University, Durham, N.C.
“Part of this report to is about trying to raise the game of everybody in medicine and provide a higher basic level of primary palliative care to all people with serious illness, but then also to figure out who has higher levels of needs where the specialists should be applied, since they are a scarce resource,” said Dr. LeBlanc.
An issue with that traditional model is a shortage of specialized clinicians to meet palliative care needs, said Dr. LeBlanc, whose clinical practice and research focuses on palliative care needs of patients with hematologic malignancies.
“Palliative care has matured as a field such that we are now actually facing workforce shortage issues and really fundamental questions about who needs us the most, and how we increase our reach to improve the lives of more patients and families facing serious illness,” he said in an interview.
That’s a major driver behind the emphasis in these latest guidelines on providing palliative care in the community, coordinating care, and dealing with care transitions, he added.
“I hope that this document will help to demonstrate the value and the need for palliative care specialists, and for improvements in primary care in the care of patients with hematologic diseases in general,” he said. “To me, this adds increasing legitimacy to this whole field.”
Palliative care in surgical care
These guidelines are particularly useful to surgeons in part because of their focus on what’s known as primary palliative care, said to Geoffrey P. Dunn, MD, former chair of the American College of Surgeons Committee on Surgical Palliative Care. Palliative care, the new guidelines suggest, can be implemented by nonspecialists.
Primary palliative care includes diverse skills such as breaking adverse news to patients, managing uncomplicated pain, and being able to recognize signs and symptoms of imminent demise. “These are the minimum deliverables for all people dealing with seriously ill patients,” Dr. Dunn said in an interview. “It’s palliative care that any practicing physician should be able to handle.”
Dr. Dunn concurred with Dr. LaBlanc about the workforce shortage in the palliative field. The traditional model has created a shortage of specialized clinicians to meet palliative care needs. Across the board, “staffing for palliative teams is very inconsistent,” said Dr. Dunn. “It’s a classic unfunded mandate.”
While these guidelines are a step forward in recognizing the importance of palliative care outside of the palliative care specialty, there is no reference to surgery anywhere in the text of the 141-page prepublication draft provided by the NCP, Dr. Dunn noted in the interview.
“There’s still a danger of parallel universes, where surgery is developing its own understanding of this in parallel with the more general national palliative care movement,” he said. Despite that, there is a growing connection between surgery and the broader palliative care community. That linkage is especially important given the number of seriously ill patients with high symptom burden that are seen in surgery.
“I think where surgeons are beginning to find [palliative principles] very helpful is dealing with these protracted serial discussions with families in difficult circumstances, such as how long is the life support going to be prolonged in someone with a devastating head injury, or multiple system organ failure in the elderly,” Dr. Dunn added.
Jack Rozel: Gun violence
Dr. Rozel makes his home and practices in Pittsburgh, the site of the tragic mass shooting at the Tree of Life temple.
Dr. Rozel makes his home and practices in Pittsburgh, the site of the tragic mass shooting at the Tree of Life temple.
Dr. Rozel makes his home and practices in Pittsburgh, the site of the tragic mass shooting at the Tree of Life temple.
NSAID risk score validated for CV events
Also today, the ACIP resuscitates pertussis working group, it’s important to talk to adolescents about sexual assault, and costs increase for gun injuries in children.
Amazon Alexa
Apple Podcasts
Spotify
Also today, the ACIP resuscitates pertussis working group, it’s important to talk to adolescents about sexual assault, and costs increase for gun injuries in children.
Amazon Alexa
Apple Podcasts
Spotify
Also today, the ACIP resuscitates pertussis working group, it’s important to talk to adolescents about sexual assault, and costs increase for gun injuries in children.
Amazon Alexa
Apple Podcasts
Spotify
Game changers in pediatric cancer
Although there have been significant improvements in patient outcomes for some forms of pediatric cancer, progress has been painfully slow for others. An increasing understanding of pediatric cancers is highlighting the unique molecular drivers and challenging the assumption that drugs developed in adults can be applied to children and young adults. Here, we discuss game-changing therapeutic advances and a shifting view of childhood cancers.
Unique genomic background
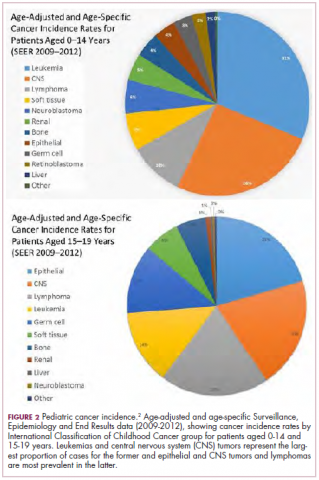
Although pediatric cancers are rare, representing just 1% of all new cancers diagnosed annually in the United States, they are the second leading cause of death in children aged 1 to 14 years. There are many different histological tumor types under the umbrella of childhood cancers, of which the most common are leukemias, central nervous system tumors, and lymphomas (Figure 1).1,2
Significant progress has been made in the treatment of certain pediatric cancers in recent decades, exemplified by pediatric acute lymphoblastic leukemia (ALL), which has been transformed from a virtually incurable cancer to one in which 5-year survival rates now reach up to 90%. In other forms of pediatric cancer, however, survival rates have stagnated and little progress has been made in the development of effective new therapies.3
Because of their rarity, pediatric cancers are difficult to study and adequate enrollment of children in clinical trials can be challenging. Pharmaceutical companies are often hesitant to test drugs in the pediatric population in patients who often cannot advocate for themselves. As a result, the activity of drugs developed in adult patients has often been inferred in pediatric patients with the same tumor type or molecular aberrations. However, as researchers have gathered more information about pediatric cancers, there has been increasing recognition of their unique attributes and the need for dedicated clinical trials in this patient population.
Pediatric cancers tend to be found in the developing mesodermic tissue, whereas adult cancers are more prevalent in the epithelial tissues. Genome sequencing studies have revealed a much lower mutational burden in pediatric cancers and the mechanisms of oncogenesis are also quite different; adult tumors can develop from a series of acquired gene mutations, but pediatric tumors tend to develop from a single catastrophic event.4,5
Even the same type of cancer in a pediatric and adult patient can be quite different, with very different underlying molecular mechanisms. In a recent genomic analysis of different types of pediatric cancer by researchers at St Jude’s Children’s Research Hospital, less than half of the identified mutated genes were found to be similar to those found in adult patients.6
A ‘magic bullet’?
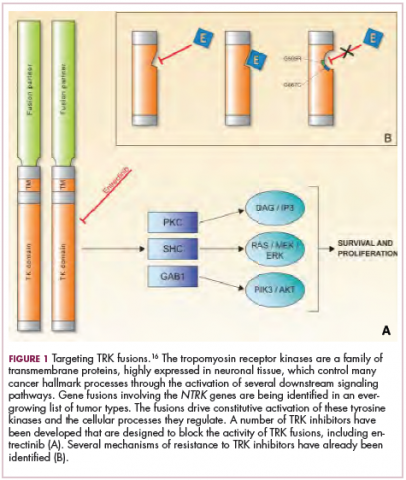
Chromosomal rearrangements are common in pediatric cancers. This type of molecular abnormality can result in a fusion of 2 different genes when the chromosome breaks apart and the pieces join back together in a muddled order. If the genetic code fuses in a manner that is “readable” by the cell, then it can drive aberrant activation of one or both genes.7 Gene fusions often involve kinase enzymes that are essential players in cell signaling pathways regulating hallmark cancer processes, such as unchecked cell proliferation. The fusion drives the constitutive activation of the kinase and, thus, these downstream signaling pathways.
One of the first chromosomal rearrangements linked to cancer, BCR-ABL1 – more commonly known as the Philadelphia chromosome – results in aberrant activation of the ABL1 kinase. It is present in nearly all cases of chronic myeloid leukemia (CML) and 3% to 5% of patients with ALL, and thus became the central focus of targeted drug development. Imatinib was initially approved by the US Food and Drug Administration (FDA) in 2001 for the treatment of adult patients with CML and had such a significant impact on the treatment landscape that it made the cover of Time magazine as a “magic bullet” in the war on cancer.8
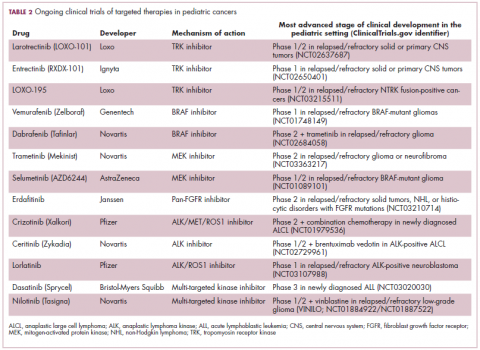
Approval was expanded into pediatric patients in 2006 and for pediatric patients with ALL in 2013. However, as with the use of most kinase inhibitors, tumors can evolve under the selective pressure of treatment, developing additional molecular abnormalities that drive resistance.9
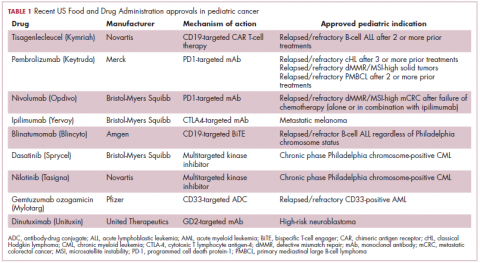
Next-generation multikinase inhibitors that more potently inhibit the BCR-ABL1 fusion protein have been developed to provide additional treatment options for patients who become resistant to imatinib. Dasatinib and nilotinib are among several drugs that have recently been approved for pediatric cancer therapy (Table 1). Both therapies were approved to treat children with Philadelphia chromosome-positive CML in the chronic phase in either the front- or second-line setting after failure of imatinib.
The approval of dasatinib was based on data from 97 patients across 2 trials, 51 of whom were newly diagnosed and 46 previously treated with imatinib. Most of the patients were treated with dasatinib 60 mg/m2 once daily. After 2 years of follow-up, more than 95% of newly diagnosed patients and 82.6% of relapsed/refractory patients had complete cytogenetic response.10
Nilotinib was approved on the basis of findings from 2 clinical trials including 69 patients – 1 trial involving patients who were refractory to or relapsed after dasatinib and imatinib treatment, and 1 that included both relapsed/refractory and newly diagnosed patients. Patients received nilotinib 230 mg/m2 twice daily, rounded to the nearest 50-mg dose, in 28-day cycles. By cycle 12, the cumulative major molecular response rate (MMR) was 47.7% in patients with relapsed/refractory disease, and 64% in newly diagnosed patients.11 Clinical trials of both drugs in the pediatric setting are ongoing.
Other prominent gene fusions
Gene fusions involving the anaplastic lymphoma kinase (ALK) occur in patients with non–small-cell lung cancer and ALK inhibitors have provided an effective new treatment option for patients whose tumors display this abnormality.
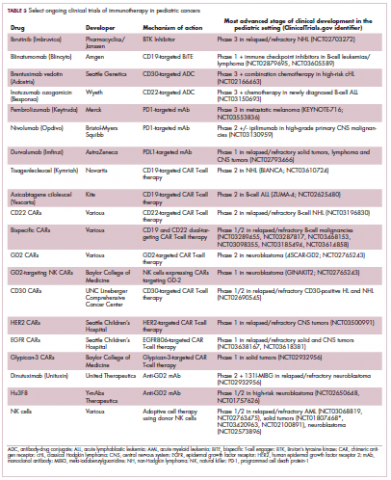
ALK fusions are also a prominent feature of several kinds of pediatric cancers and ALK inhibitors offer promise in this setting.7,12 An NPM-ALK fusion is found in 90% of pediatric anaplastic large cell lymphoma (ALCL) cases,13 whereas a variety of ALK fusions are found in up to half of patients with inflammatory myofibroblastic tumor (IMT), a rare form of soft tissue sarcoma.14 ALK inhibitors are being tested in a variety of clinical trials in pediatric patients (Table 2).
The results of a small phase 1 study of crizotinib in pediatric patients with ALK-positive ALCL (n = 26) or IMT (n = 14) were recently published. ALCL patients received crizotinib at a dose of 165 mg/m2, while IMT patients were given 100, 165, or 280 mg/m2. For the latter, the results were presented as a pooled cohort since safety and efficacy data were similar across dose levels. The overall response rate (ORR) was 83% for patients with ALCL and 86% for those with IMT. Grade 3/4 adverse events occurred in 83% and 71% of patients, respectively, and most commonly involved reduced neutrophil count.15
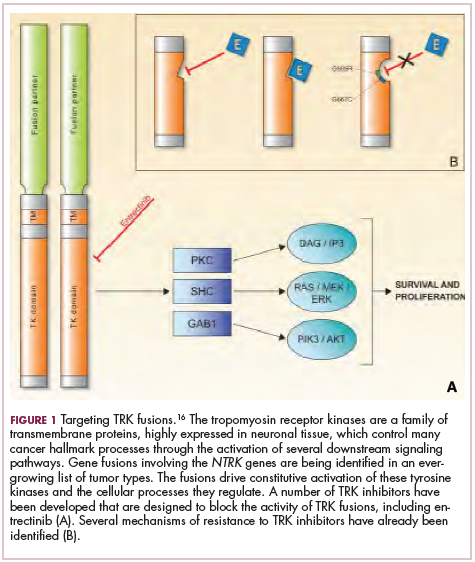
Most recently and perhaps most promisingly, fusions involving the neurotrophic tropomyosin receptor kinase (NTRK) gene have generated significant buzz. There are 3 NTRK genes, NTRK1, 2, and 3, which encode the TRKA, TRKB, and TRKC proteins, respectively.
To date, 22 different partner genes have been identified that can fuse with the NTRK genes and, as with other kinase fusions, drive constitutive activation of the receptor proteins and downstream oncogenic signaling pathways, including the mitogen-activated protein kinase (MAPK) pathway (Figure 2).
NTRK fusions are being identified in an ever-growing number of cancer types, but are typically found in a small percentage of patients. However, in certain rare pediatric tumors, including congenital infantile fibrosarcoma and papillary thyroid cancer, they are found at much higher frequencies.
TRK inhibitors have been developed to target the fusion proteins and, given the spread of NTRK fusions across different types of cancers, they offer the most substantial promise as the next tumor agnostic cancer therapy – to treat patients based on the shared presence of a molecular aberration, irrespective of the type of cancer.16
The ongoing SCOUT trial is evaluating larotrectinib (LOXO-101) in pediatric patients. Among 24 patients (17 with NTRK fusions and 7 without) with infantile fibrosarcoma (47%), soft tissue sarcoma (41%) or papillary thyroid cancer (12%), the ORR was 93%, including complete response (CR) in 13% of patients.17
Preliminary results from an ongoing phase 1/2 study of entrectinib in pediatric patients with extracranial solid tumors were also recently presented at the annual meeting of the American Society for Clinical Oncology (ASCO). Among 15 evaluable patients enrolled to date, 3 have NTRK fusions and all experienced an objective response, with 1 (a patient with IMT) ongoing at 10 months.18
CAR T cells transformative in ALL
A variety of different types of immunotherapy have been tested in patients with pediatric cancers. In general, immunotherapy has proved less effective than in adult cancers, possibly because of the lower tumor mutation burden in pediatric cancers, which means there are likely fewer cancer antigens to provoke an anti-tumor immune response.
There are notable exceptions among the disappointments, however, and most exciting is the development of chimeric antigen receptor (CAR) T cells. CAR T cells fall into a category of immunotherapy known as adoptive cell therapy (ACT), in which immune cells are harvested from a patient and grown outside the body to increase their numbers before being reinfused into the patient.
In the case of CAR T-cell therapy, the cells are genetically engineered to express a CAR that endows them with tumor-targeting capabilities. To date, the development of CAR T cells has focused on the use of the CD19 antigen as a target, which is highly expressed on a variety of B-cell malignancies, including several of the most common forms of pediatric cancer. ASCO shined the spotlight on CAR T-cell therapy this year, naming it the Advance of the Year for 2018, saying that the treatment is “poised to transform childhood ALL.”19
Two CD19-targeted CAR T-cell therapies – tisagenlecleucel and axicabtagene ciloleucel – were brought to market in 2017. Only tisagenlecleucel is approved in the pediatric ALL population, however, having been awarded approval for the treatment of patients aged up to 25 years whose disease is refractory to or relapsed after receiving at least 2 prior therapies. In the pivotal trial, complete responses were observed in more than 60% of patients.20 Clinical trials of both CAR T-cell therapies in pediatric ALL and non-Hodgkin lymphoma are ongoing (Table 3).
CD19 has also proven to be a promising target for other forms of immunotherapy, including a new type of antibody known as a bispecific T-cell engager (BiTE). In 2014, blinatumomab became the first BiTE to receive regulatory approval, for the treatment of adult patients with relapsed/refractory ALL. Blinatumomab also targets the CD3 protein on T cells and helps to bring cancer cells and cytotoxic immune cells into close enough proximity that an immunological synapse can be formed between the two, facilitating tumor cell killing.21
In 2016, the approved indication was expanded into the pediatric population based on the results of a phase 1/2 study in which the safety and efficacy of blinatumomab were evaluated in 93 pediatric patients with relapsed/refractory ALL. Among the 70 patients who received the recommended dose of 5µg/m2 a day for the first 7 days, followed by 15µg/m2 a day thereafter, 51% achieved complete remission within the first 2 cycles, 52% of whom achieved minimal residual disease (MRD).22 Most recently, the FDA expanded the indication for blinatumomab to include patients (both adults and children) who are in remission, but MRD positive.23Despite the dramatic responses, many patients relapse after treatment with CD19-targeted CAR T cells, and researchers have uncovered numerous mechanisms of resistance. Among them is the loss of the CD19 antigen on the surface of target cells, such that a CD19-positive tumor becomes CD19-negative after treatment, driving relapse.24-26Several strategies for overcoming CD19-negative relapse are already being investigated, including the development of CD22-targeted CAR T cells and bispecific CAR T cells that target both CD19 and CD22. The results of a first-in-human trial of anti-CD22 CAR T-cell therapy were recently published. Among 21 pediatric and adult patients with relapsed/refractory B-cell ALL who were treated with either 3 x 105 cells/kg, 1 x 106 cells/kg, or 3 x 106 cells/kg, complete responses were observed in 57%.27
Results from 15 pediatric patients enrolled in a trial evaluating CD22-targeted CAR T cells as salvage therapy for those who relapse after CD19-targeted CAR T cell therapy were presented at the recent Congress of the European Hematology Association in Stockholm, Sweden. Patients who had undergone a stem cell transplant received the CAR T cells at a dose of 0.9 x 105 cell/kg and those who had not undergone a transplant received a dose of 8.2 x 105 cells/kg. At 30 days after CAR T cell infusion, the CR rate was 80% and the treatment was well tolerated.28
More immunotherapy approvals
The immune checkpoint inhibitors, which work by blocking inhibitory receptors on the surface of T cells, have also had recent approvals in pediatric patient populations. Pembrolizumab and nivolumab, inhibitors of the programmed cell death receptor 1 (PD-1) protein, have both been approved for use in adult and pediatric patients (older than 12 years) with relapsed/refractory metastatic colorectal cancer (and other solid tumors in the case of pembrolizumab) that display defects in the mismatch repair pathway that fixes damaged DNA or in patients that have high levels of microsatellite instability. Both deficient mismatch repair and microsatellite instability–high can indicate a high mutation burden in a tumor, which may predict increased sensitivity to immunotherapy.29
The approval in pediatric patients in both of those instances, however, was not based on data in pediatric patient populations but extrapolated from adult patients. Pembrolizumab is also approved for the treatment of adults and pediatric patients with classical Hodgkin lymphoma (cHL) after 3 or more previous treatments, but once again efficacy in the pediatric population was inferred from clinical trials performed in adults. Most recently, pembrolizumab was approved for the treatment of adult and pediatric patients with relapsed or refractory primary mediastinal large B-cell lymphoma.30Ipilimumab, which targets a different T cell receptor – cytotoxic T lymphocyte antigen-4 (CTLA-4) – has been approved for the treatment of pediatric patients aged 12 years and older with metastatic melanoma. This expanded indication, following on from its approval in adult patients in 2011, was based on data from 2 trials in which objective responses were observed in 2 out of 17 patients, including 1 partial response that lasted 16 months.31Finally, antibody-drug conjugates (ADC), in which tumor antigen-targeting monoclonal antibodies are conjugated to cytotoxic payloads to combine the specificity of an antibody with the cell-killing potency of chemotherapy, have also generated some recent successes in pediatric cancers.
Gemtuzumab ozogamicin is an ADC that targets the CD33 protein, which is highly expressed on 85%-90% of cases of acute myeloid leukemia (AML). In 2000, it was the first ADC to be brought to market in the United States, but it was subsequently voluntarily withdrawn by the manufacturer in 2010 after confirmatory trials failed to show a survival benefit.
Recently, a meta-analysis of gemtuzumab ozogamicin trials suggested that the drug likely does improve long-term overall survival (OS) and reduce the risk of relapse and researchers developed an intermittent dosing schedule to help mitigate toxicity.32 This new dosing regimen received FDA approval in 2017 for the treatment of pediatric patients aged 2 years and older on the basis of 2 clinical trials.
In the MyloFrance-1 trial, 57 patients were administered 3 mg/m2 gemtuzumab ozogamicin on days 1, 4, and 7 followed by cytarabine consolidation therapy and demonstrated a 26% CR rate and median recurrence-free survival of 11.6 months. In the phase 3 AML-19 trial, 237 patients received gemtuzumab ozogamicin at a dose of 6 mg/m2 on day 1 and 3 mg/m2 on day 8 or best supportive care. Gemtuzumab ozogamicin improved OS from 3.6 to 4.9 months.33,34
Inotuzumab ozogamicin is a CD22-targeting ADC that has been FDA approved for the treatment of adult patients with relapsed/refractory B-cell precursor ALL since last year. The therapy has been available to pediatric patients through a compassionate access program, but it has not been extensively evaluated in this population. The results of a retrospective analysis of pediatric patients who received at least 1 dose of inotuzumab ozogamicin were presented at ASCO in 2017. Among 29 patients with heavily pretreated disease the CR rate was 62%, 72% of whom achieved MRD negativity.35
1. American Cancer Society. Key statistics for childhood cancers. https://www.cancer.org/cancer/cancer-in-children/key-statistics.html. Last revised September 10, 2018. Accessed September 16, 2018.
2. NHI/National Cancer Institute website. Unusual cancers of childhood treatment (PDQ) - Health Professional Version. https://www.cancer.gov/types/childhood-cancers/hp/unusual-cancers-childhood-pdq. Last updated August 28, 2018. Accessed September 8, 2018.
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
4. Marshall GM, Carter DR, Cheung BB, et al. The prenatal origins of cancer. Nat Rev Cancer. 2014;14(4):277-289.
5. Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339(6127):1546-1558.
6. Ma X, Liu Y, Liu Y, et al. Pan-cancer genome and transcriptome analyses of 1,699 paediatric leukaemias and solid tumours. Nature. 2018;555:371.
7. Dupain C, Harttrampf AC, Urbinati G, Geoerger B, Massaad-Massade L. Relevance of fusion genes in pediatric cancers: toward precision medicine. Molec Ther Nucleic Acids. 2017;6:315-326.
8. Lemonick MD, Park A. New hope for cancer. http://content.time.com/time/world/article/0,8599,2047900-2,00.html. Published May 28, 2001. Last accessed September 13, 2018.
9. Iqbal N, Iqbal N. Imatinib: a breakthrough of targeted therapy in cancer. https://www.hindawi.com/journals/cherp/2014/357027/. Published May 19, 2014. Accessed September 16, 2018.
10. Gore L, Kearns PR, Martino MLd, et al. Dasatinib in pediatric patients with chronic myeloid leukemia in chronic phase: results from a phase II trial. J Clin Oncol. 2018;36(13):1330-1338.
11. Novartis press release. Novartis drug Tasigna approved by FDA to treat children with rare form of leukemia. 2018; https://www.novartis.com/news/media-releases/novartis-drug-tasignar-approved-fda-treat-children-rare-form-leukemia. Released March 22, 2018. Accessed September 16, 2018.
12. Takita J. The role of anaplastic lymphoma kinase in pediatric cancers. Cancer Sci. 2017;108(10):1913-1920.
13. Turner SD, Lamant L, Kenner L, Brugieres L. Anaplastic large cell lymphoma in paediatric and young adult patients. Br J Haematol. 2016;173(4):560-572.
14. Antonescu CR, Suurmeijer AJH, Zhang L, et al. Molecular characterization of inflammatory myofibroblastic tumors with frequent ALK and ROS1 fusions and rare novel RET gene rearrangement. Am J Surg Pathol. 2015;39(7):957-967.
15. Mosse YP, Voss SD, Lim MS, et al. Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: a children's oncology group study. J Clin Oncol. 2017;35(28):3215-3221.
16. Amatu A, Sartore-Bianchi A, Siena S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5070277/. Published online March 18, 2016. Accessed September 16, 2018.
17. [Behind paywall.] Laetsch TW, DuBois SG, Mascarenhas L, et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. 2018;19(5):705-714.
18. Desai AV, Brodeur GM, Foster J, et al. Phase 1 study of entrectinib (RXDX-101), a TRK, ROS1, and ALK inhibitor, in children, adolescents, and young adults with recurrent or refractory solid tumors. J Clin Oncol. 2018;36(suppl;):abstr 10536.
19. Heymach J, Krilov L, Alberg A, et al. Clinical cancer advances 2018: annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol. 2018;36(10):1020-1044.
20. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. NEJM. 2018;378(5):439-448.
21. Wu J, Fu J, Zhang M, Liu D. Blinatumomab: a bispecific T cell engager (BiTE) antibody against CD19/CD3 for refractory acute lymphoid leukemia. J Hematol Oncol. 2015;8:104.
22. Stackelberg Av, Locatelli F, Zugmaier G, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34(36):4381-4389.
23. Gokbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131(14):1522-1531.
24. Fischer J, Paret C, El Malki K, et al. CD19 isoforms enabling resistance to CART-19 immunotherapy are expressed in B-ALL patients at initial diagnosis. J Immunother. 2017;40(5):187-195.
25. Fousek K, Watanabe J, George A, et al. Targeting CD19-negative relapsed B-acute lymphoblastic leukemia using trivalent CAR T cells. J Clin Oncol. 2018;36(5_suppl):121-121.
26. Mejstríková E, Hrusak O, Borowitz MJ, et al. CD19-negative relapse of pediatric B-cell precursor acute lymphoblastic leukemia following blinatumomab treatment. Blood Cancer J. 2017;7(12):659.
27. Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20-28.
28. Pan J, Deng B, Liu S, et al. Efficacy and safety of CD22-directed CAR T-cell therapy in 15 pediatric refractory or relapsed b acute lymphoblastic leukemia patients. Paper presented at 23rd Congress of the European Hematology Association 2018; Stockholm, Sweden.
29. Boyiadzis MM, Kirkwood JM, Marshall JL, Pritchard CC, Azad NS, Gulley JL. Significance and implications of FDA approval of pembrolizumab for biomarker-defined disease. J Immunother Cancer. 2018;6:35.
30. Drugs.com. Keytruda approval history. 2018; https://www.drugs.com/history/keytruda.html. Last update information not given. Accessed September 16, 2018.
31. Bristol Myers Squibb press release. US Food and Drug Administration expands approval of Yervoy (ipilimumab) to include pediatric patients 12 years and older with unresectable or metastatic melanoma. https://news.bms.com/press-release/corporatefinancial-news/us-food-and-drug-administration-expands-approval-yervoy-ipilim. Released July 24, 2017. Accessed September 16, 2018.
32. Hills RK, Castaigne S, Appelbaum FR, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014;15(9):986-996.
33. Amadori S, Suciu S, Selleslag D, et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Oncol. 2016;34(9):972-979.
34. Taksin AL, Legrand O, Raffoux E, et al. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia. 2007;21(1):66-71.
35. Bhojwani D, Sposto R, Shah N, et al. Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia (R/R ALL). J Clin Oncol. 2017;35(15_suppl):10512-10512.
Although there have been significant improvements in patient outcomes for some forms of pediatric cancer, progress has been painfully slow for others. An increasing understanding of pediatric cancers is highlighting the unique molecular drivers and challenging the assumption that drugs developed in adults can be applied to children and young adults. Here, we discuss game-changing therapeutic advances and a shifting view of childhood cancers.
Unique genomic background
Although pediatric cancers are rare, representing just 1% of all new cancers diagnosed annually in the United States, they are the second leading cause of death in children aged 1 to 14 years. There are many different histological tumor types under the umbrella of childhood cancers, of which the most common are leukemias, central nervous system tumors, and lymphomas (Figure 1).1,2
Significant progress has been made in the treatment of certain pediatric cancers in recent decades, exemplified by pediatric acute lymphoblastic leukemia (ALL), which has been transformed from a virtually incurable cancer to one in which 5-year survival rates now reach up to 90%. In other forms of pediatric cancer, however, survival rates have stagnated and little progress has been made in the development of effective new therapies.3
Because of their rarity, pediatric cancers are difficult to study and adequate enrollment of children in clinical trials can be challenging. Pharmaceutical companies are often hesitant to test drugs in the pediatric population in patients who often cannot advocate for themselves. As a result, the activity of drugs developed in adult patients has often been inferred in pediatric patients with the same tumor type or molecular aberrations. However, as researchers have gathered more information about pediatric cancers, there has been increasing recognition of their unique attributes and the need for dedicated clinical trials in this patient population.
Pediatric cancers tend to be found in the developing mesodermic tissue, whereas adult cancers are more prevalent in the epithelial tissues. Genome sequencing studies have revealed a much lower mutational burden in pediatric cancers and the mechanisms of oncogenesis are also quite different; adult tumors can develop from a series of acquired gene mutations, but pediatric tumors tend to develop from a single catastrophic event.4,5
Even the same type of cancer in a pediatric and adult patient can be quite different, with very different underlying molecular mechanisms. In a recent genomic analysis of different types of pediatric cancer by researchers at St Jude’s Children’s Research Hospital, less than half of the identified mutated genes were found to be similar to those found in adult patients.6
A ‘magic bullet’?
Chromosomal rearrangements are common in pediatric cancers. This type of molecular abnormality can result in a fusion of 2 different genes when the chromosome breaks apart and the pieces join back together in a muddled order. If the genetic code fuses in a manner that is “readable” by the cell, then it can drive aberrant activation of one or both genes.7 Gene fusions often involve kinase enzymes that are essential players in cell signaling pathways regulating hallmark cancer processes, such as unchecked cell proliferation. The fusion drives the constitutive activation of the kinase and, thus, these downstream signaling pathways.
One of the first chromosomal rearrangements linked to cancer, BCR-ABL1 – more commonly known as the Philadelphia chromosome – results in aberrant activation of the ABL1 kinase. It is present in nearly all cases of chronic myeloid leukemia (CML) and 3% to 5% of patients with ALL, and thus became the central focus of targeted drug development. Imatinib was initially approved by the US Food and Drug Administration (FDA) in 2001 for the treatment of adult patients with CML and had such a significant impact on the treatment landscape that it made the cover of Time magazine as a “magic bullet” in the war on cancer.8
Approval was expanded into pediatric patients in 2006 and for pediatric patients with ALL in 2013. However, as with the use of most kinase inhibitors, tumors can evolve under the selective pressure of treatment, developing additional molecular abnormalities that drive resistance.9
Next-generation multikinase inhibitors that more potently inhibit the BCR-ABL1 fusion protein have been developed to provide additional treatment options for patients who become resistant to imatinib. Dasatinib and nilotinib are among several drugs that have recently been approved for pediatric cancer therapy (Table 1). Both therapies were approved to treat children with Philadelphia chromosome-positive CML in the chronic phase in either the front- or second-line setting after failure of imatinib.
The approval of dasatinib was based on data from 97 patients across 2 trials, 51 of whom were newly diagnosed and 46 previously treated with imatinib. Most of the patients were treated with dasatinib 60 mg/m2 once daily. After 2 years of follow-up, more than 95% of newly diagnosed patients and 82.6% of relapsed/refractory patients had complete cytogenetic response.10
Nilotinib was approved on the basis of findings from 2 clinical trials including 69 patients – 1 trial involving patients who were refractory to or relapsed after dasatinib and imatinib treatment, and 1 that included both relapsed/refractory and newly diagnosed patients. Patients received nilotinib 230 mg/m2 twice daily, rounded to the nearest 50-mg dose, in 28-day cycles. By cycle 12, the cumulative major molecular response rate (MMR) was 47.7% in patients with relapsed/refractory disease, and 64% in newly diagnosed patients.11 Clinical trials of both drugs in the pediatric setting are ongoing.
Other prominent gene fusions
Gene fusions involving the anaplastic lymphoma kinase (ALK) occur in patients with non–small-cell lung cancer and ALK inhibitors have provided an effective new treatment option for patients whose tumors display this abnormality.
ALK fusions are also a prominent feature of several kinds of pediatric cancers and ALK inhibitors offer promise in this setting.7,12 An NPM-ALK fusion is found in 90% of pediatric anaplastic large cell lymphoma (ALCL) cases,13 whereas a variety of ALK fusions are found in up to half of patients with inflammatory myofibroblastic tumor (IMT), a rare form of soft tissue sarcoma.14 ALK inhibitors are being tested in a variety of clinical trials in pediatric patients (Table 2).
The results of a small phase 1 study of crizotinib in pediatric patients with ALK-positive ALCL (n = 26) or IMT (n = 14) were recently published. ALCL patients received crizotinib at a dose of 165 mg/m2, while IMT patients were given 100, 165, or 280 mg/m2. For the latter, the results were presented as a pooled cohort since safety and efficacy data were similar across dose levels. The overall response rate (ORR) was 83% for patients with ALCL and 86% for those with IMT. Grade 3/4 adverse events occurred in 83% and 71% of patients, respectively, and most commonly involved reduced neutrophil count.15
Most recently and perhaps most promisingly, fusions involving the neurotrophic tropomyosin receptor kinase (NTRK) gene have generated significant buzz. There are 3 NTRK genes, NTRK1, 2, and 3, which encode the TRKA, TRKB, and TRKC proteins, respectively.
To date, 22 different partner genes have been identified that can fuse with the NTRK genes and, as with other kinase fusions, drive constitutive activation of the receptor proteins and downstream oncogenic signaling pathways, including the mitogen-activated protein kinase (MAPK) pathway (Figure 2).
NTRK fusions are being identified in an ever-growing number of cancer types, but are typically found in a small percentage of patients. However, in certain rare pediatric tumors, including congenital infantile fibrosarcoma and papillary thyroid cancer, they are found at much higher frequencies.
TRK inhibitors have been developed to target the fusion proteins and, given the spread of NTRK fusions across different types of cancers, they offer the most substantial promise as the next tumor agnostic cancer therapy – to treat patients based on the shared presence of a molecular aberration, irrespective of the type of cancer.16
The ongoing SCOUT trial is evaluating larotrectinib (LOXO-101) in pediatric patients. Among 24 patients (17 with NTRK fusions and 7 without) with infantile fibrosarcoma (47%), soft tissue sarcoma (41%) or papillary thyroid cancer (12%), the ORR was 93%, including complete response (CR) in 13% of patients.17
Preliminary results from an ongoing phase 1/2 study of entrectinib in pediatric patients with extracranial solid tumors were also recently presented at the annual meeting of the American Society for Clinical Oncology (ASCO). Among 15 evaluable patients enrolled to date, 3 have NTRK fusions and all experienced an objective response, with 1 (a patient with IMT) ongoing at 10 months.18
CAR T cells transformative in ALL
A variety of different types of immunotherapy have been tested in patients with pediatric cancers. In general, immunotherapy has proved less effective than in adult cancers, possibly because of the lower tumor mutation burden in pediatric cancers, which means there are likely fewer cancer antigens to provoke an anti-tumor immune response.
There are notable exceptions among the disappointments, however, and most exciting is the development of chimeric antigen receptor (CAR) T cells. CAR T cells fall into a category of immunotherapy known as adoptive cell therapy (ACT), in which immune cells are harvested from a patient and grown outside the body to increase their numbers before being reinfused into the patient.
In the case of CAR T-cell therapy, the cells are genetically engineered to express a CAR that endows them with tumor-targeting capabilities. To date, the development of CAR T cells has focused on the use of the CD19 antigen as a target, which is highly expressed on a variety of B-cell malignancies, including several of the most common forms of pediatric cancer. ASCO shined the spotlight on CAR T-cell therapy this year, naming it the Advance of the Year for 2018, saying that the treatment is “poised to transform childhood ALL.”19
Two CD19-targeted CAR T-cell therapies – tisagenlecleucel and axicabtagene ciloleucel – were brought to market in 2017. Only tisagenlecleucel is approved in the pediatric ALL population, however, having been awarded approval for the treatment of patients aged up to 25 years whose disease is refractory to or relapsed after receiving at least 2 prior therapies. In the pivotal trial, complete responses were observed in more than 60% of patients.20 Clinical trials of both CAR T-cell therapies in pediatric ALL and non-Hodgkin lymphoma are ongoing (Table 3).
CD19 has also proven to be a promising target for other forms of immunotherapy, including a new type of antibody known as a bispecific T-cell engager (BiTE). In 2014, blinatumomab became the first BiTE to receive regulatory approval, for the treatment of adult patients with relapsed/refractory ALL. Blinatumomab also targets the CD3 protein on T cells and helps to bring cancer cells and cytotoxic immune cells into close enough proximity that an immunological synapse can be formed between the two, facilitating tumor cell killing.21
In 2016, the approved indication was expanded into the pediatric population based on the results of a phase 1/2 study in which the safety and efficacy of blinatumomab were evaluated in 93 pediatric patients with relapsed/refractory ALL. Among the 70 patients who received the recommended dose of 5µg/m2 a day for the first 7 days, followed by 15µg/m2 a day thereafter, 51% achieved complete remission within the first 2 cycles, 52% of whom achieved minimal residual disease (MRD).22 Most recently, the FDA expanded the indication for blinatumomab to include patients (both adults and children) who are in remission, but MRD positive.23Despite the dramatic responses, many patients relapse after treatment with CD19-targeted CAR T cells, and researchers have uncovered numerous mechanisms of resistance. Among them is the loss of the CD19 antigen on the surface of target cells, such that a CD19-positive tumor becomes CD19-negative after treatment, driving relapse.24-26Several strategies for overcoming CD19-negative relapse are already being investigated, including the development of CD22-targeted CAR T cells and bispecific CAR T cells that target both CD19 and CD22. The results of a first-in-human trial of anti-CD22 CAR T-cell therapy were recently published. Among 21 pediatric and adult patients with relapsed/refractory B-cell ALL who were treated with either 3 x 105 cells/kg, 1 x 106 cells/kg, or 3 x 106 cells/kg, complete responses were observed in 57%.27
Results from 15 pediatric patients enrolled in a trial evaluating CD22-targeted CAR T cells as salvage therapy for those who relapse after CD19-targeted CAR T cell therapy were presented at the recent Congress of the European Hematology Association in Stockholm, Sweden. Patients who had undergone a stem cell transplant received the CAR T cells at a dose of 0.9 x 105 cell/kg and those who had not undergone a transplant received a dose of 8.2 x 105 cells/kg. At 30 days after CAR T cell infusion, the CR rate was 80% and the treatment was well tolerated.28
More immunotherapy approvals
The immune checkpoint inhibitors, which work by blocking inhibitory receptors on the surface of T cells, have also had recent approvals in pediatric patient populations. Pembrolizumab and nivolumab, inhibitors of the programmed cell death receptor 1 (PD-1) protein, have both been approved for use in adult and pediatric patients (older than 12 years) with relapsed/refractory metastatic colorectal cancer (and other solid tumors in the case of pembrolizumab) that display defects in the mismatch repair pathway that fixes damaged DNA or in patients that have high levels of microsatellite instability. Both deficient mismatch repair and microsatellite instability–high can indicate a high mutation burden in a tumor, which may predict increased sensitivity to immunotherapy.29
The approval in pediatric patients in both of those instances, however, was not based on data in pediatric patient populations but extrapolated from adult patients. Pembrolizumab is also approved for the treatment of adults and pediatric patients with classical Hodgkin lymphoma (cHL) after 3 or more previous treatments, but once again efficacy in the pediatric population was inferred from clinical trials performed in adults. Most recently, pembrolizumab was approved for the treatment of adult and pediatric patients with relapsed or refractory primary mediastinal large B-cell lymphoma.30Ipilimumab, which targets a different T cell receptor – cytotoxic T lymphocyte antigen-4 (CTLA-4) – has been approved for the treatment of pediatric patients aged 12 years and older with metastatic melanoma. This expanded indication, following on from its approval in adult patients in 2011, was based on data from 2 trials in which objective responses were observed in 2 out of 17 patients, including 1 partial response that lasted 16 months.31Finally, antibody-drug conjugates (ADC), in which tumor antigen-targeting monoclonal antibodies are conjugated to cytotoxic payloads to combine the specificity of an antibody with the cell-killing potency of chemotherapy, have also generated some recent successes in pediatric cancers.
Gemtuzumab ozogamicin is an ADC that targets the CD33 protein, which is highly expressed on 85%-90% of cases of acute myeloid leukemia (AML). In 2000, it was the first ADC to be brought to market in the United States, but it was subsequently voluntarily withdrawn by the manufacturer in 2010 after confirmatory trials failed to show a survival benefit.
Recently, a meta-analysis of gemtuzumab ozogamicin trials suggested that the drug likely does improve long-term overall survival (OS) and reduce the risk of relapse and researchers developed an intermittent dosing schedule to help mitigate toxicity.32 This new dosing regimen received FDA approval in 2017 for the treatment of pediatric patients aged 2 years and older on the basis of 2 clinical trials.
In the MyloFrance-1 trial, 57 patients were administered 3 mg/m2 gemtuzumab ozogamicin on days 1, 4, and 7 followed by cytarabine consolidation therapy and demonstrated a 26% CR rate and median recurrence-free survival of 11.6 months. In the phase 3 AML-19 trial, 237 patients received gemtuzumab ozogamicin at a dose of 6 mg/m2 on day 1 and 3 mg/m2 on day 8 or best supportive care. Gemtuzumab ozogamicin improved OS from 3.6 to 4.9 months.33,34
Inotuzumab ozogamicin is a CD22-targeting ADC that has been FDA approved for the treatment of adult patients with relapsed/refractory B-cell precursor ALL since last year. The therapy has been available to pediatric patients through a compassionate access program, but it has not been extensively evaluated in this population. The results of a retrospective analysis of pediatric patients who received at least 1 dose of inotuzumab ozogamicin were presented at ASCO in 2017. Among 29 patients with heavily pretreated disease the CR rate was 62%, 72% of whom achieved MRD negativity.35
Although there have been significant improvements in patient outcomes for some forms of pediatric cancer, progress has been painfully slow for others. An increasing understanding of pediatric cancers is highlighting the unique molecular drivers and challenging the assumption that drugs developed in adults can be applied to children and young adults. Here, we discuss game-changing therapeutic advances and a shifting view of childhood cancers.
Unique genomic background
Although pediatric cancers are rare, representing just 1% of all new cancers diagnosed annually in the United States, they are the second leading cause of death in children aged 1 to 14 years. There are many different histological tumor types under the umbrella of childhood cancers, of which the most common are leukemias, central nervous system tumors, and lymphomas (Figure 1).1,2
Significant progress has been made in the treatment of certain pediatric cancers in recent decades, exemplified by pediatric acute lymphoblastic leukemia (ALL), which has been transformed from a virtually incurable cancer to one in which 5-year survival rates now reach up to 90%. In other forms of pediatric cancer, however, survival rates have stagnated and little progress has been made in the development of effective new therapies.3
Because of their rarity, pediatric cancers are difficult to study and adequate enrollment of children in clinical trials can be challenging. Pharmaceutical companies are often hesitant to test drugs in the pediatric population in patients who often cannot advocate for themselves. As a result, the activity of drugs developed in adult patients has often been inferred in pediatric patients with the same tumor type or molecular aberrations. However, as researchers have gathered more information about pediatric cancers, there has been increasing recognition of their unique attributes and the need for dedicated clinical trials in this patient population.
Pediatric cancers tend to be found in the developing mesodermic tissue, whereas adult cancers are more prevalent in the epithelial tissues. Genome sequencing studies have revealed a much lower mutational burden in pediatric cancers and the mechanisms of oncogenesis are also quite different; adult tumors can develop from a series of acquired gene mutations, but pediatric tumors tend to develop from a single catastrophic event.4,5
Even the same type of cancer in a pediatric and adult patient can be quite different, with very different underlying molecular mechanisms. In a recent genomic analysis of different types of pediatric cancer by researchers at St Jude’s Children’s Research Hospital, less than half of the identified mutated genes were found to be similar to those found in adult patients.6
A ‘magic bullet’?
Chromosomal rearrangements are common in pediatric cancers. This type of molecular abnormality can result in a fusion of 2 different genes when the chromosome breaks apart and the pieces join back together in a muddled order. If the genetic code fuses in a manner that is “readable” by the cell, then it can drive aberrant activation of one or both genes.7 Gene fusions often involve kinase enzymes that are essential players in cell signaling pathways regulating hallmark cancer processes, such as unchecked cell proliferation. The fusion drives the constitutive activation of the kinase and, thus, these downstream signaling pathways.
One of the first chromosomal rearrangements linked to cancer, BCR-ABL1 – more commonly known as the Philadelphia chromosome – results in aberrant activation of the ABL1 kinase. It is present in nearly all cases of chronic myeloid leukemia (CML) and 3% to 5% of patients with ALL, and thus became the central focus of targeted drug development. Imatinib was initially approved by the US Food and Drug Administration (FDA) in 2001 for the treatment of adult patients with CML and had such a significant impact on the treatment landscape that it made the cover of Time magazine as a “magic bullet” in the war on cancer.8
Approval was expanded into pediatric patients in 2006 and for pediatric patients with ALL in 2013. However, as with the use of most kinase inhibitors, tumors can evolve under the selective pressure of treatment, developing additional molecular abnormalities that drive resistance.9
Next-generation multikinase inhibitors that more potently inhibit the BCR-ABL1 fusion protein have been developed to provide additional treatment options for patients who become resistant to imatinib. Dasatinib and nilotinib are among several drugs that have recently been approved for pediatric cancer therapy (Table 1). Both therapies were approved to treat children with Philadelphia chromosome-positive CML in the chronic phase in either the front- or second-line setting after failure of imatinib.
The approval of dasatinib was based on data from 97 patients across 2 trials, 51 of whom were newly diagnosed and 46 previously treated with imatinib. Most of the patients were treated with dasatinib 60 mg/m2 once daily. After 2 years of follow-up, more than 95% of newly diagnosed patients and 82.6% of relapsed/refractory patients had complete cytogenetic response.10
Nilotinib was approved on the basis of findings from 2 clinical trials including 69 patients – 1 trial involving patients who were refractory to or relapsed after dasatinib and imatinib treatment, and 1 that included both relapsed/refractory and newly diagnosed patients. Patients received nilotinib 230 mg/m2 twice daily, rounded to the nearest 50-mg dose, in 28-day cycles. By cycle 12, the cumulative major molecular response rate (MMR) was 47.7% in patients with relapsed/refractory disease, and 64% in newly diagnosed patients.11 Clinical trials of both drugs in the pediatric setting are ongoing.
Other prominent gene fusions
Gene fusions involving the anaplastic lymphoma kinase (ALK) occur in patients with non–small-cell lung cancer and ALK inhibitors have provided an effective new treatment option for patients whose tumors display this abnormality.
ALK fusions are also a prominent feature of several kinds of pediatric cancers and ALK inhibitors offer promise in this setting.7,12 An NPM-ALK fusion is found in 90% of pediatric anaplastic large cell lymphoma (ALCL) cases,13 whereas a variety of ALK fusions are found in up to half of patients with inflammatory myofibroblastic tumor (IMT), a rare form of soft tissue sarcoma.14 ALK inhibitors are being tested in a variety of clinical trials in pediatric patients (Table 2).
The results of a small phase 1 study of crizotinib in pediatric patients with ALK-positive ALCL (n = 26) or IMT (n = 14) were recently published. ALCL patients received crizotinib at a dose of 165 mg/m2, while IMT patients were given 100, 165, or 280 mg/m2. For the latter, the results were presented as a pooled cohort since safety and efficacy data were similar across dose levels. The overall response rate (ORR) was 83% for patients with ALCL and 86% for those with IMT. Grade 3/4 adverse events occurred in 83% and 71% of patients, respectively, and most commonly involved reduced neutrophil count.15
Most recently and perhaps most promisingly, fusions involving the neurotrophic tropomyosin receptor kinase (NTRK) gene have generated significant buzz. There are 3 NTRK genes, NTRK1, 2, and 3, which encode the TRKA, TRKB, and TRKC proteins, respectively.
To date, 22 different partner genes have been identified that can fuse with the NTRK genes and, as with other kinase fusions, drive constitutive activation of the receptor proteins and downstream oncogenic signaling pathways, including the mitogen-activated protein kinase (MAPK) pathway (Figure 2).
NTRK fusions are being identified in an ever-growing number of cancer types, but are typically found in a small percentage of patients. However, in certain rare pediatric tumors, including congenital infantile fibrosarcoma and papillary thyroid cancer, they are found at much higher frequencies.
TRK inhibitors have been developed to target the fusion proteins and, given the spread of NTRK fusions across different types of cancers, they offer the most substantial promise as the next tumor agnostic cancer therapy – to treat patients based on the shared presence of a molecular aberration, irrespective of the type of cancer.16
The ongoing SCOUT trial is evaluating larotrectinib (LOXO-101) in pediatric patients. Among 24 patients (17 with NTRK fusions and 7 without) with infantile fibrosarcoma (47%), soft tissue sarcoma (41%) or papillary thyroid cancer (12%), the ORR was 93%, including complete response (CR) in 13% of patients.17
Preliminary results from an ongoing phase 1/2 study of entrectinib in pediatric patients with extracranial solid tumors were also recently presented at the annual meeting of the American Society for Clinical Oncology (ASCO). Among 15 evaluable patients enrolled to date, 3 have NTRK fusions and all experienced an objective response, with 1 (a patient with IMT) ongoing at 10 months.18
CAR T cells transformative in ALL
A variety of different types of immunotherapy have been tested in patients with pediatric cancers. In general, immunotherapy has proved less effective than in adult cancers, possibly because of the lower tumor mutation burden in pediatric cancers, which means there are likely fewer cancer antigens to provoke an anti-tumor immune response.
There are notable exceptions among the disappointments, however, and most exciting is the development of chimeric antigen receptor (CAR) T cells. CAR T cells fall into a category of immunotherapy known as adoptive cell therapy (ACT), in which immune cells are harvested from a patient and grown outside the body to increase their numbers before being reinfused into the patient.
In the case of CAR T-cell therapy, the cells are genetically engineered to express a CAR that endows them with tumor-targeting capabilities. To date, the development of CAR T cells has focused on the use of the CD19 antigen as a target, which is highly expressed on a variety of B-cell malignancies, including several of the most common forms of pediatric cancer. ASCO shined the spotlight on CAR T-cell therapy this year, naming it the Advance of the Year for 2018, saying that the treatment is “poised to transform childhood ALL.”19
Two CD19-targeted CAR T-cell therapies – tisagenlecleucel and axicabtagene ciloleucel – were brought to market in 2017. Only tisagenlecleucel is approved in the pediatric ALL population, however, having been awarded approval for the treatment of patients aged up to 25 years whose disease is refractory to or relapsed after receiving at least 2 prior therapies. In the pivotal trial, complete responses were observed in more than 60% of patients.20 Clinical trials of both CAR T-cell therapies in pediatric ALL and non-Hodgkin lymphoma are ongoing (Table 3).
CD19 has also proven to be a promising target for other forms of immunotherapy, including a new type of antibody known as a bispecific T-cell engager (BiTE). In 2014, blinatumomab became the first BiTE to receive regulatory approval, for the treatment of adult patients with relapsed/refractory ALL. Blinatumomab also targets the CD3 protein on T cells and helps to bring cancer cells and cytotoxic immune cells into close enough proximity that an immunological synapse can be formed between the two, facilitating tumor cell killing.21
In 2016, the approved indication was expanded into the pediatric population based on the results of a phase 1/2 study in which the safety and efficacy of blinatumomab were evaluated in 93 pediatric patients with relapsed/refractory ALL. Among the 70 patients who received the recommended dose of 5µg/m2 a day for the first 7 days, followed by 15µg/m2 a day thereafter, 51% achieved complete remission within the first 2 cycles, 52% of whom achieved minimal residual disease (MRD).22 Most recently, the FDA expanded the indication for blinatumomab to include patients (both adults and children) who are in remission, but MRD positive.23Despite the dramatic responses, many patients relapse after treatment with CD19-targeted CAR T cells, and researchers have uncovered numerous mechanisms of resistance. Among them is the loss of the CD19 antigen on the surface of target cells, such that a CD19-positive tumor becomes CD19-negative after treatment, driving relapse.24-26Several strategies for overcoming CD19-negative relapse are already being investigated, including the development of CD22-targeted CAR T cells and bispecific CAR T cells that target both CD19 and CD22. The results of a first-in-human trial of anti-CD22 CAR T-cell therapy were recently published. Among 21 pediatric and adult patients with relapsed/refractory B-cell ALL who were treated with either 3 x 105 cells/kg, 1 x 106 cells/kg, or 3 x 106 cells/kg, complete responses were observed in 57%.27
Results from 15 pediatric patients enrolled in a trial evaluating CD22-targeted CAR T cells as salvage therapy for those who relapse after CD19-targeted CAR T cell therapy were presented at the recent Congress of the European Hematology Association in Stockholm, Sweden. Patients who had undergone a stem cell transplant received the CAR T cells at a dose of 0.9 x 105 cell/kg and those who had not undergone a transplant received a dose of 8.2 x 105 cells/kg. At 30 days after CAR T cell infusion, the CR rate was 80% and the treatment was well tolerated.28
More immunotherapy approvals
The immune checkpoint inhibitors, which work by blocking inhibitory receptors on the surface of T cells, have also had recent approvals in pediatric patient populations. Pembrolizumab and nivolumab, inhibitors of the programmed cell death receptor 1 (PD-1) protein, have both been approved for use in adult and pediatric patients (older than 12 years) with relapsed/refractory metastatic colorectal cancer (and other solid tumors in the case of pembrolizumab) that display defects in the mismatch repair pathway that fixes damaged DNA or in patients that have high levels of microsatellite instability. Both deficient mismatch repair and microsatellite instability–high can indicate a high mutation burden in a tumor, which may predict increased sensitivity to immunotherapy.29
The approval in pediatric patients in both of those instances, however, was not based on data in pediatric patient populations but extrapolated from adult patients. Pembrolizumab is also approved for the treatment of adults and pediatric patients with classical Hodgkin lymphoma (cHL) after 3 or more previous treatments, but once again efficacy in the pediatric population was inferred from clinical trials performed in adults. Most recently, pembrolizumab was approved for the treatment of adult and pediatric patients with relapsed or refractory primary mediastinal large B-cell lymphoma.30Ipilimumab, which targets a different T cell receptor – cytotoxic T lymphocyte antigen-4 (CTLA-4) – has been approved for the treatment of pediatric patients aged 12 years and older with metastatic melanoma. This expanded indication, following on from its approval in adult patients in 2011, was based on data from 2 trials in which objective responses were observed in 2 out of 17 patients, including 1 partial response that lasted 16 months.31Finally, antibody-drug conjugates (ADC), in which tumor antigen-targeting monoclonal antibodies are conjugated to cytotoxic payloads to combine the specificity of an antibody with the cell-killing potency of chemotherapy, have also generated some recent successes in pediatric cancers.
Gemtuzumab ozogamicin is an ADC that targets the CD33 protein, which is highly expressed on 85%-90% of cases of acute myeloid leukemia (AML). In 2000, it was the first ADC to be brought to market in the United States, but it was subsequently voluntarily withdrawn by the manufacturer in 2010 after confirmatory trials failed to show a survival benefit.
Recently, a meta-analysis of gemtuzumab ozogamicin trials suggested that the drug likely does improve long-term overall survival (OS) and reduce the risk of relapse and researchers developed an intermittent dosing schedule to help mitigate toxicity.32 This new dosing regimen received FDA approval in 2017 for the treatment of pediatric patients aged 2 years and older on the basis of 2 clinical trials.
In the MyloFrance-1 trial, 57 patients were administered 3 mg/m2 gemtuzumab ozogamicin on days 1, 4, and 7 followed by cytarabine consolidation therapy and demonstrated a 26% CR rate and median recurrence-free survival of 11.6 months. In the phase 3 AML-19 trial, 237 patients received gemtuzumab ozogamicin at a dose of 6 mg/m2 on day 1 and 3 mg/m2 on day 8 or best supportive care. Gemtuzumab ozogamicin improved OS from 3.6 to 4.9 months.33,34
Inotuzumab ozogamicin is a CD22-targeting ADC that has been FDA approved for the treatment of adult patients with relapsed/refractory B-cell precursor ALL since last year. The therapy has been available to pediatric patients through a compassionate access program, but it has not been extensively evaluated in this population. The results of a retrospective analysis of pediatric patients who received at least 1 dose of inotuzumab ozogamicin were presented at ASCO in 2017. Among 29 patients with heavily pretreated disease the CR rate was 62%, 72% of whom achieved MRD negativity.35
1. American Cancer Society. Key statistics for childhood cancers. https://www.cancer.org/cancer/cancer-in-children/key-statistics.html. Last revised September 10, 2018. Accessed September 16, 2018.
2. NHI/National Cancer Institute website. Unusual cancers of childhood treatment (PDQ) - Health Professional Version. https://www.cancer.gov/types/childhood-cancers/hp/unusual-cancers-childhood-pdq. Last updated August 28, 2018. Accessed September 8, 2018.
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
4. Marshall GM, Carter DR, Cheung BB, et al. The prenatal origins of cancer. Nat Rev Cancer. 2014;14(4):277-289.
5. Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339(6127):1546-1558.
6. Ma X, Liu Y, Liu Y, et al. Pan-cancer genome and transcriptome analyses of 1,699 paediatric leukaemias and solid tumours. Nature. 2018;555:371.
7. Dupain C, Harttrampf AC, Urbinati G, Geoerger B, Massaad-Massade L. Relevance of fusion genes in pediatric cancers: toward precision medicine. Molec Ther Nucleic Acids. 2017;6:315-326.
8. Lemonick MD, Park A. New hope for cancer. http://content.time.com/time/world/article/0,8599,2047900-2,00.html. Published May 28, 2001. Last accessed September 13, 2018.
9. Iqbal N, Iqbal N. Imatinib: a breakthrough of targeted therapy in cancer. https://www.hindawi.com/journals/cherp/2014/357027/. Published May 19, 2014. Accessed September 16, 2018.
10. Gore L, Kearns PR, Martino MLd, et al. Dasatinib in pediatric patients with chronic myeloid leukemia in chronic phase: results from a phase II trial. J Clin Oncol. 2018;36(13):1330-1338.
11. Novartis press release. Novartis drug Tasigna approved by FDA to treat children with rare form of leukemia. 2018; https://www.novartis.com/news/media-releases/novartis-drug-tasignar-approved-fda-treat-children-rare-form-leukemia. Released March 22, 2018. Accessed September 16, 2018.
12. Takita J. The role of anaplastic lymphoma kinase in pediatric cancers. Cancer Sci. 2017;108(10):1913-1920.
13. Turner SD, Lamant L, Kenner L, Brugieres L. Anaplastic large cell lymphoma in paediatric and young adult patients. Br J Haematol. 2016;173(4):560-572.
14. Antonescu CR, Suurmeijer AJH, Zhang L, et al. Molecular characterization of inflammatory myofibroblastic tumors with frequent ALK and ROS1 fusions and rare novel RET gene rearrangement. Am J Surg Pathol. 2015;39(7):957-967.
15. Mosse YP, Voss SD, Lim MS, et al. Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: a children's oncology group study. J Clin Oncol. 2017;35(28):3215-3221.
16. Amatu A, Sartore-Bianchi A, Siena S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5070277/. Published online March 18, 2016. Accessed September 16, 2018.
17. [Behind paywall.] Laetsch TW, DuBois SG, Mascarenhas L, et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. 2018;19(5):705-714.
18. Desai AV, Brodeur GM, Foster J, et al. Phase 1 study of entrectinib (RXDX-101), a TRK, ROS1, and ALK inhibitor, in children, adolescents, and young adults with recurrent or refractory solid tumors. J Clin Oncol. 2018;36(suppl;):abstr 10536.
19. Heymach J, Krilov L, Alberg A, et al. Clinical cancer advances 2018: annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol. 2018;36(10):1020-1044.
20. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. NEJM. 2018;378(5):439-448.
21. Wu J, Fu J, Zhang M, Liu D. Blinatumomab: a bispecific T cell engager (BiTE) antibody against CD19/CD3 for refractory acute lymphoid leukemia. J Hematol Oncol. 2015;8:104.
22. Stackelberg Av, Locatelli F, Zugmaier G, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34(36):4381-4389.
23. Gokbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131(14):1522-1531.
24. Fischer J, Paret C, El Malki K, et al. CD19 isoforms enabling resistance to CART-19 immunotherapy are expressed in B-ALL patients at initial diagnosis. J Immunother. 2017;40(5):187-195.
25. Fousek K, Watanabe J, George A, et al. Targeting CD19-negative relapsed B-acute lymphoblastic leukemia using trivalent CAR T cells. J Clin Oncol. 2018;36(5_suppl):121-121.
26. Mejstríková E, Hrusak O, Borowitz MJ, et al. CD19-negative relapse of pediatric B-cell precursor acute lymphoblastic leukemia following blinatumomab treatment. Blood Cancer J. 2017;7(12):659.
27. Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20-28.
28. Pan J, Deng B, Liu S, et al. Efficacy and safety of CD22-directed CAR T-cell therapy in 15 pediatric refractory or relapsed b acute lymphoblastic leukemia patients. Paper presented at 23rd Congress of the European Hematology Association 2018; Stockholm, Sweden.
29. Boyiadzis MM, Kirkwood JM, Marshall JL, Pritchard CC, Azad NS, Gulley JL. Significance and implications of FDA approval of pembrolizumab for biomarker-defined disease. J Immunother Cancer. 2018;6:35.
30. Drugs.com. Keytruda approval history. 2018; https://www.drugs.com/history/keytruda.html. Last update information not given. Accessed September 16, 2018.
31. Bristol Myers Squibb press release. US Food and Drug Administration expands approval of Yervoy (ipilimumab) to include pediatric patients 12 years and older with unresectable or metastatic melanoma. https://news.bms.com/press-release/corporatefinancial-news/us-food-and-drug-administration-expands-approval-yervoy-ipilim. Released July 24, 2017. Accessed September 16, 2018.
32. Hills RK, Castaigne S, Appelbaum FR, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014;15(9):986-996.
33. Amadori S, Suciu S, Selleslag D, et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Oncol. 2016;34(9):972-979.
34. Taksin AL, Legrand O, Raffoux E, et al. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia. 2007;21(1):66-71.
35. Bhojwani D, Sposto R, Shah N, et al. Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia (R/R ALL). J Clin Oncol. 2017;35(15_suppl):10512-10512.
1. American Cancer Society. Key statistics for childhood cancers. https://www.cancer.org/cancer/cancer-in-children/key-statistics.html. Last revised September 10, 2018. Accessed September 16, 2018.
2. NHI/National Cancer Institute website. Unusual cancers of childhood treatment (PDQ) - Health Professional Version. https://www.cancer.gov/types/childhood-cancers/hp/unusual-cancers-childhood-pdq. Last updated August 28, 2018. Accessed September 8, 2018.
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
4. Marshall GM, Carter DR, Cheung BB, et al. The prenatal origins of cancer. Nat Rev Cancer. 2014;14(4):277-289.
5. Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339(6127):1546-1558.
6. Ma X, Liu Y, Liu Y, et al. Pan-cancer genome and transcriptome analyses of 1,699 paediatric leukaemias and solid tumours. Nature. 2018;555:371.
7. Dupain C, Harttrampf AC, Urbinati G, Geoerger B, Massaad-Massade L. Relevance of fusion genes in pediatric cancers: toward precision medicine. Molec Ther Nucleic Acids. 2017;6:315-326.
8. Lemonick MD, Park A. New hope for cancer. http://content.time.com/time/world/article/0,8599,2047900-2,00.html. Published May 28, 2001. Last accessed September 13, 2018.
9. Iqbal N, Iqbal N. Imatinib: a breakthrough of targeted therapy in cancer. https://www.hindawi.com/journals/cherp/2014/357027/. Published May 19, 2014. Accessed September 16, 2018.
10. Gore L, Kearns PR, Martino MLd, et al. Dasatinib in pediatric patients with chronic myeloid leukemia in chronic phase: results from a phase II trial. J Clin Oncol. 2018;36(13):1330-1338.
11. Novartis press release. Novartis drug Tasigna approved by FDA to treat children with rare form of leukemia. 2018; https://www.novartis.com/news/media-releases/novartis-drug-tasignar-approved-fda-treat-children-rare-form-leukemia. Released March 22, 2018. Accessed September 16, 2018.
12. Takita J. The role of anaplastic lymphoma kinase in pediatric cancers. Cancer Sci. 2017;108(10):1913-1920.
13. Turner SD, Lamant L, Kenner L, Brugieres L. Anaplastic large cell lymphoma in paediatric and young adult patients. Br J Haematol. 2016;173(4):560-572.
14. Antonescu CR, Suurmeijer AJH, Zhang L, et al. Molecular characterization of inflammatory myofibroblastic tumors with frequent ALK and ROS1 fusions and rare novel RET gene rearrangement. Am J Surg Pathol. 2015;39(7):957-967.
15. Mosse YP, Voss SD, Lim MS, et al. Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: a children's oncology group study. J Clin Oncol. 2017;35(28):3215-3221.
16. Amatu A, Sartore-Bianchi A, Siena S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5070277/. Published online March 18, 2016. Accessed September 16, 2018.
17. [Behind paywall.] Laetsch TW, DuBois SG, Mascarenhas L, et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. 2018;19(5):705-714.
18. Desai AV, Brodeur GM, Foster J, et al. Phase 1 study of entrectinib (RXDX-101), a TRK, ROS1, and ALK inhibitor, in children, adolescents, and young adults with recurrent or refractory solid tumors. J Clin Oncol. 2018;36(suppl;):abstr 10536.
19. Heymach J, Krilov L, Alberg A, et al. Clinical cancer advances 2018: annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol. 2018;36(10):1020-1044.
20. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. NEJM. 2018;378(5):439-448.
21. Wu J, Fu J, Zhang M, Liu D. Blinatumomab: a bispecific T cell engager (BiTE) antibody against CD19/CD3 for refractory acute lymphoid leukemia. J Hematol Oncol. 2015;8:104.
22. Stackelberg Av, Locatelli F, Zugmaier G, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34(36):4381-4389.
23. Gokbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131(14):1522-1531.
24. Fischer J, Paret C, El Malki K, et al. CD19 isoforms enabling resistance to CART-19 immunotherapy are expressed in B-ALL patients at initial diagnosis. J Immunother. 2017;40(5):187-195.
25. Fousek K, Watanabe J, George A, et al. Targeting CD19-negative relapsed B-acute lymphoblastic leukemia using trivalent CAR T cells. J Clin Oncol. 2018;36(5_suppl):121-121.
26. Mejstríková E, Hrusak O, Borowitz MJ, et al. CD19-negative relapse of pediatric B-cell precursor acute lymphoblastic leukemia following blinatumomab treatment. Blood Cancer J. 2017;7(12):659.
27. Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20-28.
28. Pan J, Deng B, Liu S, et al. Efficacy and safety of CD22-directed CAR T-cell therapy in 15 pediatric refractory or relapsed b acute lymphoblastic leukemia patients. Paper presented at 23rd Congress of the European Hematology Association 2018; Stockholm, Sweden.
29. Boyiadzis MM, Kirkwood JM, Marshall JL, Pritchard CC, Azad NS, Gulley JL. Significance and implications of FDA approval of pembrolizumab for biomarker-defined disease. J Immunother Cancer. 2018;6:35.
30. Drugs.com. Keytruda approval history. 2018; https://www.drugs.com/history/keytruda.html. Last update information not given. Accessed September 16, 2018.
31. Bristol Myers Squibb press release. US Food and Drug Administration expands approval of Yervoy (ipilimumab) to include pediatric patients 12 years and older with unresectable or metastatic melanoma. https://news.bms.com/press-release/corporatefinancial-news/us-food-and-drug-administration-expands-approval-yervoy-ipilim. Released July 24, 2017. Accessed September 16, 2018.
32. Hills RK, Castaigne S, Appelbaum FR, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014;15(9):986-996.
33. Amadori S, Suciu S, Selleslag D, et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Oncol. 2016;34(9):972-979.
34. Taksin AL, Legrand O, Raffoux E, et al. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia. 2007;21(1):66-71.
35. Bhojwani D, Sposto R, Shah N, et al. Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia (R/R ALL). J Clin Oncol. 2017;35(15_suppl):10512-10512.
Testing platelets reduces waste, cuts costs
BOSTON—Rapid bacterial testing of platelets in a hospital blood bank can result in both significant cost savings and reduced wastage of blood products, according to investigators.
In a single-center study, rapid bacterial testing of 6- or 7-day-old apheresis platelets resulted in projected annual cost savings of nearly $89,000 and cut the rate of platelet wastage from expiration by more than half.
Adam L. Booth, MD, of the University of Texas in Galveston, and his colleagues described this study in a poster presentation at AABB 2018 (abstract INV4).
Platelets typically have a shelf life of 5 days because longer storage increases the risk for bacterial growth and the potential for transfusion-transmitted infections, Dr. Booth and his colleagues noted.
In March 2016, the U.S. Food and Drug Administration (FDA) published a draft guidance proposing a change in regulations to allow for an extended shelf life if platelets are collected in an FDA-approved, 7-day storage container with labeling that requires testing every product with a bacterial detection device, or if the platelets are individually tested for bacterial detection using an approved device.
Dr. Booth and his colleagues wanted to see what effect the regulations, if implemented as expected, might have on acquisition costs and wastage of apheresis platelets.
The investigators reviewed their center’s platelet acquisition costs and wastage from expiration 12 months before and 6 months after implementation of a rapid bacterial testing protocol, with 6-month results projected out to 1 year for comparison purposes.
The team looked at data on bacterial testing of 6-day and 7-day-old apheresis platelets, and they excluded data on platelet units that were due to expire on day 5 because they were not stored in FDA-approved containers.
Prior to testing, the annual wastage rate was 24%, or 332 of 1,371 platelet units purchased. Using a mean per-unit cost of $516.96, the annual cost was more than $171,000.
After the start of testing, the annualized rate of wastage dropped to 10%, or 117 of 1,168 platelet units. So the annualized cost was more than $60,000.
The difference in cost—minus the cost of rapid bacterial testing (roughly $22,500)—resulted in an annual savings for the institution of nearly $89,000.
The number of units transfused and the associated costs of transfusions were similar between the time periods studied.
This study was internally funded. The authors reported having no conflicts of interest.
BOSTON—Rapid bacterial testing of platelets in a hospital blood bank can result in both significant cost savings and reduced wastage of blood products, according to investigators.
In a single-center study, rapid bacterial testing of 6- or 7-day-old apheresis platelets resulted in projected annual cost savings of nearly $89,000 and cut the rate of platelet wastage from expiration by more than half.
Adam L. Booth, MD, of the University of Texas in Galveston, and his colleagues described this study in a poster presentation at AABB 2018 (abstract INV4).
Platelets typically have a shelf life of 5 days because longer storage increases the risk for bacterial growth and the potential for transfusion-transmitted infections, Dr. Booth and his colleagues noted.
In March 2016, the U.S. Food and Drug Administration (FDA) published a draft guidance proposing a change in regulations to allow for an extended shelf life if platelets are collected in an FDA-approved, 7-day storage container with labeling that requires testing every product with a bacterial detection device, or if the platelets are individually tested for bacterial detection using an approved device.
Dr. Booth and his colleagues wanted to see what effect the regulations, if implemented as expected, might have on acquisition costs and wastage of apheresis platelets.
The investigators reviewed their center’s platelet acquisition costs and wastage from expiration 12 months before and 6 months after implementation of a rapid bacterial testing protocol, with 6-month results projected out to 1 year for comparison purposes.
The team looked at data on bacterial testing of 6-day and 7-day-old apheresis platelets, and they excluded data on platelet units that were due to expire on day 5 because they were not stored in FDA-approved containers.
Prior to testing, the annual wastage rate was 24%, or 332 of 1,371 platelet units purchased. Using a mean per-unit cost of $516.96, the annual cost was more than $171,000.
After the start of testing, the annualized rate of wastage dropped to 10%, or 117 of 1,168 platelet units. So the annualized cost was more than $60,000.
The difference in cost—minus the cost of rapid bacterial testing (roughly $22,500)—resulted in an annual savings for the institution of nearly $89,000.
The number of units transfused and the associated costs of transfusions were similar between the time periods studied.
This study was internally funded. The authors reported having no conflicts of interest.
BOSTON—Rapid bacterial testing of platelets in a hospital blood bank can result in both significant cost savings and reduced wastage of blood products, according to investigators.
In a single-center study, rapid bacterial testing of 6- or 7-day-old apheresis platelets resulted in projected annual cost savings of nearly $89,000 and cut the rate of platelet wastage from expiration by more than half.
Adam L. Booth, MD, of the University of Texas in Galveston, and his colleagues described this study in a poster presentation at AABB 2018 (abstract INV4).
Platelets typically have a shelf life of 5 days because longer storage increases the risk for bacterial growth and the potential for transfusion-transmitted infections, Dr. Booth and his colleagues noted.
In March 2016, the U.S. Food and Drug Administration (FDA) published a draft guidance proposing a change in regulations to allow for an extended shelf life if platelets are collected in an FDA-approved, 7-day storage container with labeling that requires testing every product with a bacterial detection device, or if the platelets are individually tested for bacterial detection using an approved device.
Dr. Booth and his colleagues wanted to see what effect the regulations, if implemented as expected, might have on acquisition costs and wastage of apheresis platelets.
The investigators reviewed their center’s platelet acquisition costs and wastage from expiration 12 months before and 6 months after implementation of a rapid bacterial testing protocol, with 6-month results projected out to 1 year for comparison purposes.
The team looked at data on bacterial testing of 6-day and 7-day-old apheresis platelets, and they excluded data on platelet units that were due to expire on day 5 because they were not stored in FDA-approved containers.
Prior to testing, the annual wastage rate was 24%, or 332 of 1,371 platelet units purchased. Using a mean per-unit cost of $516.96, the annual cost was more than $171,000.
After the start of testing, the annualized rate of wastage dropped to 10%, or 117 of 1,168 platelet units. So the annualized cost was more than $60,000.
The difference in cost—minus the cost of rapid bacterial testing (roughly $22,500)—resulted in an annual savings for the institution of nearly $89,000.
The number of units transfused and the associated costs of transfusions were similar between the time periods studied.
This study was internally funded. The authors reported having no conflicts of interest.
Team finds potential therapeutic target for AML
Researchers have found the cancer-associated pseudokinase Tribbles 2 (TRIB2) to be a potential therapeutic target in solid tumors and blood cancers, including acute myeloid leukemia (AML).
Previous research had described TRIB2 as a target of small-molecule protein kinase inhibitors originally designed to interfere with kinase domains of the epidermal growth factor receptor (EGFR) tyrosine kinase family.
Using a thermal shift assay, the team discovered TRIB2-binding compounds within the Published Kinase Inhibitor Set (PKIS). They then employed a biochemical drug repurposing approach to classify compounds that either stabilized or destabilized TRIB2 in vitro.
The researchers found that afatinib, which is already approved by the U.S. Food and Drug Administration to treat non-small cell lung cancer, led to rapid TRIB2 degradation in human AML cells.
Patrick A. Eyers, PhD, of the University of Liverpool in the U.K., and his colleagues published their findings in Science Signaling.
The team found afatinib to be relatively specific for EGFR and human epidermal growth factor receptor 2 (HER2) at nanomolar concentrations in cells.
The researchers confirmed that at least two TRIB2 Cys residues interact with afatinib in vitro.
The team also discovered TRIB2 could be destabilized by neratinib and osimertinib in vitro.
“Our data prove that the cellular mechanism by which TRIB2 stability is regulated by compounds is proteasome-based,” the researchers wrote, “and we speculate that an afatinib-induced conformational change might induce TRIB2 ubiquitination.”
The researchers plan to study further TRIB2 small-molecule interactions with dynamic changes in ubiquitination status.
Furthermore, they report their work demonstrates that covalent inhibitors such as afatinib have TRIB2-degrading activity in human cells at micromolar concentrations.
The researchers determined that afatinib has similar efficacy to the TRIB2-destabilizing quinazoline neratinib at similar ranges.
The team believes their data “raise the intriguing possibility that clinical inhibitors might be used as TRIB2-degrading agents in research, and possibly clinical, contexts.”
“A long-standing goal in cancer research is drug-induced degradation of oncogenic proteins,” Dr. Eyers commented. “Our study highlights how information obtained with ‘off-target’ effects of known drugs is potentially useful because it might be exploited in the future to help eliminate a protein that is involved in a completely different type of cancer.”
The TRIB proteins play many diverse roles in cell signaling, development, and cancer. According to a paper in Developmental Dynamics, they were named after the small, round, fictional organisms from the original Star Trek television series. Their major role was to eat and reproduce.
This work was funded by two U.K. Biotechnology and Biological Sciences Research Council Doctoral Training Partnership studentships, a Tools and Resources Development Fund award, Royal Society Research Grants, North West Cancer Research grants, and funding from the National Institutes of Health.
The authors disclosed no perceived conflicts of interest, although several authors are affiliated with the Structural Genomics Consortium at the University of North Carolina at Chapel Hill, which receives direct funds from various pharmaceutical companies but remains entirely independent.
Researchers have found the cancer-associated pseudokinase Tribbles 2 (TRIB2) to be a potential therapeutic target in solid tumors and blood cancers, including acute myeloid leukemia (AML).
Previous research had described TRIB2 as a target of small-molecule protein kinase inhibitors originally designed to interfere with kinase domains of the epidermal growth factor receptor (EGFR) tyrosine kinase family.
Using a thermal shift assay, the team discovered TRIB2-binding compounds within the Published Kinase Inhibitor Set (PKIS). They then employed a biochemical drug repurposing approach to classify compounds that either stabilized or destabilized TRIB2 in vitro.
The researchers found that afatinib, which is already approved by the U.S. Food and Drug Administration to treat non-small cell lung cancer, led to rapid TRIB2 degradation in human AML cells.
Patrick A. Eyers, PhD, of the University of Liverpool in the U.K., and his colleagues published their findings in Science Signaling.
The team found afatinib to be relatively specific for EGFR and human epidermal growth factor receptor 2 (HER2) at nanomolar concentrations in cells.
The researchers confirmed that at least two TRIB2 Cys residues interact with afatinib in vitro.
The team also discovered TRIB2 could be destabilized by neratinib and osimertinib in vitro.
“Our data prove that the cellular mechanism by which TRIB2 stability is regulated by compounds is proteasome-based,” the researchers wrote, “and we speculate that an afatinib-induced conformational change might induce TRIB2 ubiquitination.”
The researchers plan to study further TRIB2 small-molecule interactions with dynamic changes in ubiquitination status.
Furthermore, they report their work demonstrates that covalent inhibitors such as afatinib have TRIB2-degrading activity in human cells at micromolar concentrations.
The researchers determined that afatinib has similar efficacy to the TRIB2-destabilizing quinazoline neratinib at similar ranges.
The team believes their data “raise the intriguing possibility that clinical inhibitors might be used as TRIB2-degrading agents in research, and possibly clinical, contexts.”
“A long-standing goal in cancer research is drug-induced degradation of oncogenic proteins,” Dr. Eyers commented. “Our study highlights how information obtained with ‘off-target’ effects of known drugs is potentially useful because it might be exploited in the future to help eliminate a protein that is involved in a completely different type of cancer.”
The TRIB proteins play many diverse roles in cell signaling, development, and cancer. According to a paper in Developmental Dynamics, they were named after the small, round, fictional organisms from the original Star Trek television series. Their major role was to eat and reproduce.
This work was funded by two U.K. Biotechnology and Biological Sciences Research Council Doctoral Training Partnership studentships, a Tools and Resources Development Fund award, Royal Society Research Grants, North West Cancer Research grants, and funding from the National Institutes of Health.
The authors disclosed no perceived conflicts of interest, although several authors are affiliated with the Structural Genomics Consortium at the University of North Carolina at Chapel Hill, which receives direct funds from various pharmaceutical companies but remains entirely independent.
Researchers have found the cancer-associated pseudokinase Tribbles 2 (TRIB2) to be a potential therapeutic target in solid tumors and blood cancers, including acute myeloid leukemia (AML).
Previous research had described TRIB2 as a target of small-molecule protein kinase inhibitors originally designed to interfere with kinase domains of the epidermal growth factor receptor (EGFR) tyrosine kinase family.
Using a thermal shift assay, the team discovered TRIB2-binding compounds within the Published Kinase Inhibitor Set (PKIS). They then employed a biochemical drug repurposing approach to classify compounds that either stabilized or destabilized TRIB2 in vitro.
The researchers found that afatinib, which is already approved by the U.S. Food and Drug Administration to treat non-small cell lung cancer, led to rapid TRIB2 degradation in human AML cells.
Patrick A. Eyers, PhD, of the University of Liverpool in the U.K., and his colleagues published their findings in Science Signaling.
The team found afatinib to be relatively specific for EGFR and human epidermal growth factor receptor 2 (HER2) at nanomolar concentrations in cells.
The researchers confirmed that at least two TRIB2 Cys residues interact with afatinib in vitro.
The team also discovered TRIB2 could be destabilized by neratinib and osimertinib in vitro.
“Our data prove that the cellular mechanism by which TRIB2 stability is regulated by compounds is proteasome-based,” the researchers wrote, “and we speculate that an afatinib-induced conformational change might induce TRIB2 ubiquitination.”
The researchers plan to study further TRIB2 small-molecule interactions with dynamic changes in ubiquitination status.
Furthermore, they report their work demonstrates that covalent inhibitors such as afatinib have TRIB2-degrading activity in human cells at micromolar concentrations.
The researchers determined that afatinib has similar efficacy to the TRIB2-destabilizing quinazoline neratinib at similar ranges.
The team believes their data “raise the intriguing possibility that clinical inhibitors might be used as TRIB2-degrading agents in research, and possibly clinical, contexts.”
“A long-standing goal in cancer research is drug-induced degradation of oncogenic proteins,” Dr. Eyers commented. “Our study highlights how information obtained with ‘off-target’ effects of known drugs is potentially useful because it might be exploited in the future to help eliminate a protein that is involved in a completely different type of cancer.”
The TRIB proteins play many diverse roles in cell signaling, development, and cancer. According to a paper in Developmental Dynamics, they were named after the small, round, fictional organisms from the original Star Trek television series. Their major role was to eat and reproduce.
This work was funded by two U.K. Biotechnology and Biological Sciences Research Council Doctoral Training Partnership studentships, a Tools and Resources Development Fund award, Royal Society Research Grants, North West Cancer Research grants, and funding from the National Institutes of Health.
The authors disclosed no perceived conflicts of interest, although several authors are affiliated with the Structural Genomics Consortium at the University of North Carolina at Chapel Hill, which receives direct funds from various pharmaceutical companies but remains entirely independent.
Links between SCT and adverse outcomes
Although sickle cell trait (SCT) has been linked to adverse clinical outcomes in multiple studies, only a handful of associations have strong evidence supporting them, according to a systematic review.
Evidence was strongest for the association between SCT and venous and renal complications.
There was low-strength evidence supporting a link between SCT and exertion-related sudden death and moderate-strength evidence supporting a link between SCT and exertion-related rhabdomyolysis.
Most other associations between SCT and clinical outcomes had either low-strength evidence or insufficient data to support a link.
Rakhi P. Naik, MD, of Johns Hopkins University in Baltimore, Maryland, and her colleagues reported these findings in Annals of Internal Medicine.
The researchers’ systematic review was focused on 41 studies, most of which were population-based cohort or case-control studies.
The team rated the evidence quality of each study and grouped 24 clinical outcomes of interest into six categories: exertion-related injury, mortality, and renal, vascular, pediatric, surgery-, and trauma-related outcomes.
The researchers found low-strength evidence for a link between SCT and hematuria, end-stage renal disease, hypertension, myocardial infarction, retinopathy, diabetic vasculopathy, sudden infant death syndrome, surgery- and trauma-related injury, and overall mortality.
There was moderate-strength evidence for a link between SCT and pediatric height/weight, stroke, and heart failure/cardiomyopathy.
Exertion-related injury/death
The strength of evidence for a link between SCT and exertion-related death was low in this analysis, which included two studies of this outcome.
However, Dr. Naik and her colleagues did note that SCT may be associated with a small absolute risk of exertion-related death in extreme conditions, such as highly strenuous athletic training or the military.
There was moderate-strength evidence supporting the link between SCT and exertional rhabdomyolysis, based on two studies.
However, the researchers said the absolute risk of exertional rhabdomyolysis in SCT is small and probably occurs only in high-intensity settings, with risk modified by other genetic and environmental factors.
“We do concur with the American Society of Hematology statement recommending against routine SCT screening in athletics and supporting the consistent use of universal precautions to mitigate exertion-related risk in all persons, regardless of SCT status,” the researchers wrote.
Venous and renal outcomes
High-strength evidence linked pulmonary embolism, with or without deep-vein thrombosis, to SCT. In contrast, there was moderate-strength evidence showing no increased risk of isolated deep-vein thrombosis in individuals with SCT.
“The cause of this paradoxical observation is unknown but may be an increased risk for clot embolization in SCT,” the researchers wrote.
Renal outcomes were often attributed to SCT, and the researchers said there was high-strength evidence to support SCT as a risk factor for both proteinuria and chronic kidney disease (CKD).
Out of six studies of proteinuria, the one high-quality study showed a 1.86-fold increased risk for baseline albuminuria in African Americans with SCT versus those without. The other studies “showed a consistent direction of increased risk for proteinuria with SCT,” according to the researchers.
Out of four CKD studies, the two high-quality studies showed a 1.57- and 1.89-fold increased risk of CKD in African Americans with SCT.
Support for this review came, in part, from the National Human Genome Research Institute and the National Heart, Lung, and Blood Institute. The authors reported disclosures related to Novartis, Addmedica, and Global Blood Therapeutics, among others.
Although sickle cell trait (SCT) has been linked to adverse clinical outcomes in multiple studies, only a handful of associations have strong evidence supporting them, according to a systematic review.
Evidence was strongest for the association between SCT and venous and renal complications.
There was low-strength evidence supporting a link between SCT and exertion-related sudden death and moderate-strength evidence supporting a link between SCT and exertion-related rhabdomyolysis.
Most other associations between SCT and clinical outcomes had either low-strength evidence or insufficient data to support a link.
Rakhi P. Naik, MD, of Johns Hopkins University in Baltimore, Maryland, and her colleagues reported these findings in Annals of Internal Medicine.
The researchers’ systematic review was focused on 41 studies, most of which were population-based cohort or case-control studies.
The team rated the evidence quality of each study and grouped 24 clinical outcomes of interest into six categories: exertion-related injury, mortality, and renal, vascular, pediatric, surgery-, and trauma-related outcomes.
The researchers found low-strength evidence for a link between SCT and hematuria, end-stage renal disease, hypertension, myocardial infarction, retinopathy, diabetic vasculopathy, sudden infant death syndrome, surgery- and trauma-related injury, and overall mortality.
There was moderate-strength evidence for a link between SCT and pediatric height/weight, stroke, and heart failure/cardiomyopathy.
Exertion-related injury/death
The strength of evidence for a link between SCT and exertion-related death was low in this analysis, which included two studies of this outcome.
However, Dr. Naik and her colleagues did note that SCT may be associated with a small absolute risk of exertion-related death in extreme conditions, such as highly strenuous athletic training or the military.
There was moderate-strength evidence supporting the link between SCT and exertional rhabdomyolysis, based on two studies.
However, the researchers said the absolute risk of exertional rhabdomyolysis in SCT is small and probably occurs only in high-intensity settings, with risk modified by other genetic and environmental factors.
“We do concur with the American Society of Hematology statement recommending against routine SCT screening in athletics and supporting the consistent use of universal precautions to mitigate exertion-related risk in all persons, regardless of SCT status,” the researchers wrote.
Venous and renal outcomes
High-strength evidence linked pulmonary embolism, with or without deep-vein thrombosis, to SCT. In contrast, there was moderate-strength evidence showing no increased risk of isolated deep-vein thrombosis in individuals with SCT.
“The cause of this paradoxical observation is unknown but may be an increased risk for clot embolization in SCT,” the researchers wrote.
Renal outcomes were often attributed to SCT, and the researchers said there was high-strength evidence to support SCT as a risk factor for both proteinuria and chronic kidney disease (CKD).
Out of six studies of proteinuria, the one high-quality study showed a 1.86-fold increased risk for baseline albuminuria in African Americans with SCT versus those without. The other studies “showed a consistent direction of increased risk for proteinuria with SCT,” according to the researchers.
Out of four CKD studies, the two high-quality studies showed a 1.57- and 1.89-fold increased risk of CKD in African Americans with SCT.
Support for this review came, in part, from the National Human Genome Research Institute and the National Heart, Lung, and Blood Institute. The authors reported disclosures related to Novartis, Addmedica, and Global Blood Therapeutics, among others.
Although sickle cell trait (SCT) has been linked to adverse clinical outcomes in multiple studies, only a handful of associations have strong evidence supporting them, according to a systematic review.
Evidence was strongest for the association between SCT and venous and renal complications.
There was low-strength evidence supporting a link between SCT and exertion-related sudden death and moderate-strength evidence supporting a link between SCT and exertion-related rhabdomyolysis.
Most other associations between SCT and clinical outcomes had either low-strength evidence or insufficient data to support a link.
Rakhi P. Naik, MD, of Johns Hopkins University in Baltimore, Maryland, and her colleagues reported these findings in Annals of Internal Medicine.
The researchers’ systematic review was focused on 41 studies, most of which were population-based cohort or case-control studies.
The team rated the evidence quality of each study and grouped 24 clinical outcomes of interest into six categories: exertion-related injury, mortality, and renal, vascular, pediatric, surgery-, and trauma-related outcomes.
The researchers found low-strength evidence for a link between SCT and hematuria, end-stage renal disease, hypertension, myocardial infarction, retinopathy, diabetic vasculopathy, sudden infant death syndrome, surgery- and trauma-related injury, and overall mortality.
There was moderate-strength evidence for a link between SCT and pediatric height/weight, stroke, and heart failure/cardiomyopathy.
Exertion-related injury/death
The strength of evidence for a link between SCT and exertion-related death was low in this analysis, which included two studies of this outcome.
However, Dr. Naik and her colleagues did note that SCT may be associated with a small absolute risk of exertion-related death in extreme conditions, such as highly strenuous athletic training or the military.
There was moderate-strength evidence supporting the link between SCT and exertional rhabdomyolysis, based on two studies.
However, the researchers said the absolute risk of exertional rhabdomyolysis in SCT is small and probably occurs only in high-intensity settings, with risk modified by other genetic and environmental factors.
“We do concur with the American Society of Hematology statement recommending against routine SCT screening in athletics and supporting the consistent use of universal precautions to mitigate exertion-related risk in all persons, regardless of SCT status,” the researchers wrote.
Venous and renal outcomes
High-strength evidence linked pulmonary embolism, with or without deep-vein thrombosis, to SCT. In contrast, there was moderate-strength evidence showing no increased risk of isolated deep-vein thrombosis in individuals with SCT.
“The cause of this paradoxical observation is unknown but may be an increased risk for clot embolization in SCT,” the researchers wrote.
Renal outcomes were often attributed to SCT, and the researchers said there was high-strength evidence to support SCT as a risk factor for both proteinuria and chronic kidney disease (CKD).
Out of six studies of proteinuria, the one high-quality study showed a 1.86-fold increased risk for baseline albuminuria in African Americans with SCT versus those without. The other studies “showed a consistent direction of increased risk for proteinuria with SCT,” according to the researchers.
Out of four CKD studies, the two high-quality studies showed a 1.57- and 1.89-fold increased risk of CKD in African Americans with SCT.
Support for this review came, in part, from the National Human Genome Research Institute and the National Heart, Lung, and Blood Institute. The authors reported disclosures related to Novartis, Addmedica, and Global Blood Therapeutics, among others.
Prolonged survival in adenocarcinoma of unknown primary treated with chemoradiotherapy
Cancer of unknown primary (CUP) represents 3% to 5% of all cancer malignancies in the world.1 Since 2003, CUP has been divided into 2 subsets – favorable (20% of the cases) and unfavorable (80% of the cases) – based on histopathologic and clinical manifestations.2 The impact of locoregional therapies, such as surgery and radiation, in addition to systemic chemotherapy in adenocarcinomas of unknown primary is not well described in the literature.
Case presentation and summary
The patient was frustrated by the lack of diagnosis and extensive work-up and decided to travel to Bangladesh for several months. Upon her return in May 2015, the patient underwent dilation and curettage at an outside tertiary care center because of her persistently elevated beta-hCG levels (>500 mIU/mL; reference range for nonpregnant woman, <5 mIU/mL) that found no products of conception and excluded a malignant process. Endoscopy and colonoscopy at that time failed to reveal a primary tumor.
She was then referred to our institution. Her level of beta-hCG remained elevated, and another transvaginal ultrasound was performed but failed to reveal any masses or evidence of pregnancy. Mammogram and a breast ultrasound showed left breast lesions. Biopsy of the breast lesions was performed, and the pathology demonstrated fibrocystic changes.
The results of a PET-CT scan in August 2015 showed a lobulated abdominal mass of 5.7 x 3.7 cm, consisting of multiple periportal necrotic lymph nodes with a standardized uptake value (SUV) of 14 (Figure 1A) and a 2.0-cm hypermetabolic retroperitoneal lymph node at the aortic bifurcation level with an SUV of 8.6. The SUV is a ratio of activity per unit volume of a region of interest to the activity per unit whole body volume. An SUV of 2.5 or higher is generally considered to be indicative of malignant tissue. We conducted a detailed review of the lymph node pathologic specimen. Immunohistochemical (IHC) studies were positive for CK7, CDX2, and EMA; focally positive for PR and mammaglobin; and negative for CK20, ER, TTF-1, and WT-1. Nonspecific staining was seen with BRST2, and there was no staining with GATA3. IHC stain for HER2-NEU was equivocal. Molecular analysis did not detect BRAF, KRAS, NRAS, and PIK3CA mutations, but did find a CTNNB1 mutation. The IHC pattern suggested pancreatobiliary origin of the tumor.3
Although serum tumor marker pattern of elevated beta-hCG, AFP, and LDH can be seen in germ cell tumors, the pathology evaluation did not favor a germ cell tumor. No site of origin was evident on radiographic evaluation, and the patient was diagnosed with CUP. Based on tumor metastatic distribution and the elevated beta-hCG level,4 we suspected that an undetected pancreatic primary was possible, and we therefore chose the folinic acid, fluorouracil, irinotecan, oxaliplatin (FOLFIRINOX) chemotherapy regimen for its evidence in prolonging survival in metastatic pancreatic cancer.5 At the initiation of treatment, the patient’s elevated tumor markers were beta-hCG 953.6 mIU/mL (reference for nonpregnant woman, <5 mIU/mL) and AFP 1,800.7 ng/mL (reference range, 0.0-9.0 ng/mL). The patient began FOLFIRINOX chemotherapy in August 2015 and after 1 month of treatment, her beta-hCG and AFP levels declined notably to 1.7 mIU/mL and 11.2 ng/mL, respectively. She completed a total of 8 cycles of FOLFIRINOX in November 2015. After completion of chemotherapy, the PET-CT scan showed a decrease in fluoro-D-glucose (FDG) uptake in the porta hepatis and retroperitoneal lymph nodes (Figure 1B). SUV in the porta hepatis lymph nodes declined from 14 to 3.5. The patient’s case was presented to our institution’s multidisciplinary tumor board, and the members deemed the risk of possible lymph node dissection surgery would outweigh the benefit. It was recommended that we proceed with radiotherapy to the residual lymph node stations.
During December 2015 through February 2016, the patient underwent a course of consolidative chemoradiation therapy to the intra-abdominal lymph nodes to a dose of 5,400 cGy in 30 fractions, with concurrent capecitabine as radiosensitizer, using intensity-modulated radiation therapy. During both chemotherapy and CRT, the patient experienced nausea, vomiting, fatigue, and anorexia, which were treated with antiemetics. She completed therapy without major complications and recovered completely from the adverse effects.
Five weeks after completion of chemoradiation, a restaging PET-CT scan showed a persistent small FDG uptake in the periportal region (SUV, 4.2). After CRT, tumor markers beta-hCG and AFP declined to less than 1.2 mIU/mL and less than 2.0 ng/mL, respectively.
Discussion
CUP is divided into favorable and unfavorable subsets.1 The favorable subset includes women with adenocarcinoma involving axillary lymph nodes, women with papillary adenocarcinoma of peritoneal cavity, and adenocarcinoma with a colon profile. The unfavorable subset includes moderate to poorly differentiated adenocarcinomas (64%) and undifferentiated tumors (36%). It involves the liver in 40% to 50% of the cases, followed by lymph nodes (35%), lungs (31%), bones (28%), and the brain (15%).1,2,6 Although data suggest that CUP with lymph-node–only metastases generally fall into an unfavorable prognosis group, our patient’s survival and progression-free survival have been especially prolonged.
The combined platinum–paclitaxel-based regimens are the treatment of choice in this unfavorable subset of CUP,7,8 with patients showing 16% to 38% response rates and median overall survival times of 6.5 to 13 months.7 Platinum–gemcitabine combinations can also be used as an alternative first-line regimen, with an overall response rate of 55% and a median survival of 8 months.9 The addition of the targeted agents bevacizumab and erlotinib to the carboplatin–paclitaxel combination, followed by bevacizumab and erlotinib maintenance, has been shown to yield a median survival of 12.6 months but was not meaningfully superior to historical studies with chemotherapy alone.10
We chose the FOLFIRINOX regimen for our patient. Conroy and colleagues reported a notably improved survival of 11.1 months with that combination chemotherapy in patients with metastatic pancreatic cancer compared with 6.8 months with gemcitabine alone.5 Given the possible pancreatobiliary site of tumor origin on IHC, the lymph node pattern of spread, and the patient’s young age and robust performance status, we felt that this multiagent systemic therapy would offer the best chance of prolonged survival. FOLFIRINOX includes a platinum agent, oxaliplatin, and platinum agents are recommended to be included in chemotherapy combinations for CUP.9,10 Although there is no data to suggest the superiority of a triplet regimen over a doublet regimen in a CUP, a triplet chemotherapy regimen may be considered in select cases.
There have been only a few reports showing the effectiveness of radiotherapy in the treatment of adenocarcinomas of unknown primary outside of the head and neck. Kubisch and colleagues have reported a case of a woman with hepatic adenocarcinoma of unknown primary that was treated with chemotherapy and surgery. Upon recurrence, the patient was then treated with selective internal radiation therapy (SIRT). She was still alive 3 years after diagnosis, and there had been no tumor relapse 21 months after SIRT.11 Shiota and colleagues have reported a case of a mediastinal lymph node CUP that was treated with docetaxel and cisplatin with concurrent thoracic radiation therapy.12 The patient remained free of symptoms without regrowth of the primary site 22 months after disease onset, and exploration of the body with enhanced and PET-CT scan showed no further abnormalities.
Other reports suggest that locoregional therapy such as surgery and radiation may be of benefit to select patients with CUP. A retrospective study by Löffler and colleagues reported that patients with a limited local involvement who received radical surgery had a median overall survival of 52.7 months compared with those who received radiation (median overall survival, 19.4 months) and those who received chemotherapy alone (median overall survival, 16 months).13 A case of a metastatic undifferentiated CUP also reported a long-term (>5 years), disease-free survivor after pancreaticoduodenectomy and systemic adjuvant chemotherapy.14
Our case further demonstrates that a multidisciplinary approach to CUP may lead to excellent clinical outcomes. Chemotherapy followed by chemoradiation in our patient increased local tumor control and survival.
Adenocarcinomas of unknown primary cases should involve management by a multidisciplinary team. Clinical trials incorporating locoregional therapies for CUP in addition to systemic therapy are warranted.
1. Pavlidis N, Khaled H, Gaafar R. A mini review on cancer of unknown primary site: a clinical puzzle for the oncologists. J Adv Res. 2015;6(3):375-382.
2. Pavlidis N, Briasoulis E, Hainsworth J, Greco FA. Diagnostic and therapeutic management of cancer of an unknown primary. Eur J Cancer. 2003;39(14):1990-2005.
3. Oien KA. Pathologic evaluation of unknown primary cancer. Semin Oncol. 2009;36(1):8-37.
4. Louhimo J, Alfthan H, Stenman UH, Hagland C. Serum HCG beta and CA 72-4 are stronger prognostic factors than CEA, CA 19-9 and CA 242 in pancreatic cancer. Oncology. 2004;66(2):126-131.
5. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817-1825.
6. Pavlidis N, Pentheroudakis G. Cancer of unknown primary site. Lancet. 2012;379:1428-1435.
7. Bochtler T, Löffler H, Krämer A. Diagnosis and management of metastatic neoplasms with unknown primary. Semin Diagn Pathol. 2017;35(3):199-206.
8. Amela EY, Lauridant-Philippin G, Cousin S, Ryckewaert T, Adenis A, Penel N. Management of 'unfavourable' carcinoma of unknown primary site: synthesis of recent literature. Crit Rev Oncol Hematol. 2012;84(2):213-223.
9. Culine S, Lortholary A, Voigt J-J, et al. Cisplatin in combination with either gemcitabine or irinotecan in carcinomas of unknown primary site: results of a randomized phase II study--trial for the French study group on carcinomas of unknown primary (GEFCAPI 01). J Clin Oncol. 2003;21(18):3479-3482.
10. Hainsworth JD, Spigel DR, Thompson DS, et al. Paclitaxel/carboplatin plus bevacizumab/erlotinib in the first-line treatment of patients with carcinoma of unknown primary site. Oncologist. 2009;14(12):1189-1197.
11. Kubisch CH, Beigel F, Ihrler S, Goke B, Reiser MF, Hoffmann RT. Oesophageal ulceration after selective internal radiation therapy in a patient with carcinoma of unknown primary. Z Gastroenterol. 2010;48(5):546-550.
12. Shiota Y, Imai S, Sasaki N, et al. A case of mediastinal lymph node carcinoma of unknown primary site treated with docetaxel and cisplatin with concurrent thoracic radiation therapy. Acta Med Okayama. 2011;65(6):407-411.
13. Löffler H, Puthenparambil J, Hielscher T, Neben K, Krämer A. Patients with cancer of unknown primary: a retrospective analysis of 223 patients with adenocarcinoma or undifferentiated carcinoma. Dtsch Arztebl Int. 111(27-28):481-487.
14. Nakagawa Y, Todoroki T, Morishita Y, et al. A long-term survivor after pancreaticoduodenectomy for metastatic undifferentiated carcinoma of an unknown primary. Hepatogastroenterology. 2008;55(86-87):1557-1561.
15. Rodríguez-López JL, Toro-Bahamonde AM, Santiago-Méndez RJ, González-Cancel IF, Vélez-Cortés HA. An unusual case of colorectal adenocarcinoma presenting as an anterior mediastinal mass. Clin Colorectal Cancer. 2018;17(1):e115-e119.
Cancer of unknown primary (CUP) represents 3% to 5% of all cancer malignancies in the world.1 Since 2003, CUP has been divided into 2 subsets – favorable (20% of the cases) and unfavorable (80% of the cases) – based on histopathologic and clinical manifestations.2 The impact of locoregional therapies, such as surgery and radiation, in addition to systemic chemotherapy in adenocarcinomas of unknown primary is not well described in the literature.
Case presentation and summary
The patient was frustrated by the lack of diagnosis and extensive work-up and decided to travel to Bangladesh for several months. Upon her return in May 2015, the patient underwent dilation and curettage at an outside tertiary care center because of her persistently elevated beta-hCG levels (>500 mIU/mL; reference range for nonpregnant woman, <5 mIU/mL) that found no products of conception and excluded a malignant process. Endoscopy and colonoscopy at that time failed to reveal a primary tumor.
She was then referred to our institution. Her level of beta-hCG remained elevated, and another transvaginal ultrasound was performed but failed to reveal any masses or evidence of pregnancy. Mammogram and a breast ultrasound showed left breast lesions. Biopsy of the breast lesions was performed, and the pathology demonstrated fibrocystic changes.
The results of a PET-CT scan in August 2015 showed a lobulated abdominal mass of 5.7 x 3.7 cm, consisting of multiple periportal necrotic lymph nodes with a standardized uptake value (SUV) of 14 (Figure 1A) and a 2.0-cm hypermetabolic retroperitoneal lymph node at the aortic bifurcation level with an SUV of 8.6. The SUV is a ratio of activity per unit volume of a region of interest to the activity per unit whole body volume. An SUV of 2.5 or higher is generally considered to be indicative of malignant tissue. We conducted a detailed review of the lymph node pathologic specimen. Immunohistochemical (IHC) studies were positive for CK7, CDX2, and EMA; focally positive for PR and mammaglobin; and negative for CK20, ER, TTF-1, and WT-1. Nonspecific staining was seen with BRST2, and there was no staining with GATA3. IHC stain for HER2-NEU was equivocal. Molecular analysis did not detect BRAF, KRAS, NRAS, and PIK3CA mutations, but did find a CTNNB1 mutation. The IHC pattern suggested pancreatobiliary origin of the tumor.3
Although serum tumor marker pattern of elevated beta-hCG, AFP, and LDH can be seen in germ cell tumors, the pathology evaluation did not favor a germ cell tumor. No site of origin was evident on radiographic evaluation, and the patient was diagnosed with CUP. Based on tumor metastatic distribution and the elevated beta-hCG level,4 we suspected that an undetected pancreatic primary was possible, and we therefore chose the folinic acid, fluorouracil, irinotecan, oxaliplatin (FOLFIRINOX) chemotherapy regimen for its evidence in prolonging survival in metastatic pancreatic cancer.5 At the initiation of treatment, the patient’s elevated tumor markers were beta-hCG 953.6 mIU/mL (reference for nonpregnant woman, <5 mIU/mL) and AFP 1,800.7 ng/mL (reference range, 0.0-9.0 ng/mL). The patient began FOLFIRINOX chemotherapy in August 2015 and after 1 month of treatment, her beta-hCG and AFP levels declined notably to 1.7 mIU/mL and 11.2 ng/mL, respectively. She completed a total of 8 cycles of FOLFIRINOX in November 2015. After completion of chemotherapy, the PET-CT scan showed a decrease in fluoro-D-glucose (FDG) uptake in the porta hepatis and retroperitoneal lymph nodes (Figure 1B). SUV in the porta hepatis lymph nodes declined from 14 to 3.5. The patient’s case was presented to our institution’s multidisciplinary tumor board, and the members deemed the risk of possible lymph node dissection surgery would outweigh the benefit. It was recommended that we proceed with radiotherapy to the residual lymph node stations.
During December 2015 through February 2016, the patient underwent a course of consolidative chemoradiation therapy to the intra-abdominal lymph nodes to a dose of 5,400 cGy in 30 fractions, with concurrent capecitabine as radiosensitizer, using intensity-modulated radiation therapy. During both chemotherapy and CRT, the patient experienced nausea, vomiting, fatigue, and anorexia, which were treated with antiemetics. She completed therapy without major complications and recovered completely from the adverse effects.
Five weeks after completion of chemoradiation, a restaging PET-CT scan showed a persistent small FDG uptake in the periportal region (SUV, 4.2). After CRT, tumor markers beta-hCG and AFP declined to less than 1.2 mIU/mL and less than 2.0 ng/mL, respectively.
Discussion
CUP is divided into favorable and unfavorable subsets.1 The favorable subset includes women with adenocarcinoma involving axillary lymph nodes, women with papillary adenocarcinoma of peritoneal cavity, and adenocarcinoma with a colon profile. The unfavorable subset includes moderate to poorly differentiated adenocarcinomas (64%) and undifferentiated tumors (36%). It involves the liver in 40% to 50% of the cases, followed by lymph nodes (35%), lungs (31%), bones (28%), and the brain (15%).1,2,6 Although data suggest that CUP with lymph-node–only metastases generally fall into an unfavorable prognosis group, our patient’s survival and progression-free survival have been especially prolonged.
The combined platinum–paclitaxel-based regimens are the treatment of choice in this unfavorable subset of CUP,7,8 with patients showing 16% to 38% response rates and median overall survival times of 6.5 to 13 months.7 Platinum–gemcitabine combinations can also be used as an alternative first-line regimen, with an overall response rate of 55% and a median survival of 8 months.9 The addition of the targeted agents bevacizumab and erlotinib to the carboplatin–paclitaxel combination, followed by bevacizumab and erlotinib maintenance, has been shown to yield a median survival of 12.6 months but was not meaningfully superior to historical studies with chemotherapy alone.10
We chose the FOLFIRINOX regimen for our patient. Conroy and colleagues reported a notably improved survival of 11.1 months with that combination chemotherapy in patients with metastatic pancreatic cancer compared with 6.8 months with gemcitabine alone.5 Given the possible pancreatobiliary site of tumor origin on IHC, the lymph node pattern of spread, and the patient’s young age and robust performance status, we felt that this multiagent systemic therapy would offer the best chance of prolonged survival. FOLFIRINOX includes a platinum agent, oxaliplatin, and platinum agents are recommended to be included in chemotherapy combinations for CUP.9,10 Although there is no data to suggest the superiority of a triplet regimen over a doublet regimen in a CUP, a triplet chemotherapy regimen may be considered in select cases.
There have been only a few reports showing the effectiveness of radiotherapy in the treatment of adenocarcinomas of unknown primary outside of the head and neck. Kubisch and colleagues have reported a case of a woman with hepatic adenocarcinoma of unknown primary that was treated with chemotherapy and surgery. Upon recurrence, the patient was then treated with selective internal radiation therapy (SIRT). She was still alive 3 years after diagnosis, and there had been no tumor relapse 21 months after SIRT.11 Shiota and colleagues have reported a case of a mediastinal lymph node CUP that was treated with docetaxel and cisplatin with concurrent thoracic radiation therapy.12 The patient remained free of symptoms without regrowth of the primary site 22 months after disease onset, and exploration of the body with enhanced and PET-CT scan showed no further abnormalities.
Other reports suggest that locoregional therapy such as surgery and radiation may be of benefit to select patients with CUP. A retrospective study by Löffler and colleagues reported that patients with a limited local involvement who received radical surgery had a median overall survival of 52.7 months compared with those who received radiation (median overall survival, 19.4 months) and those who received chemotherapy alone (median overall survival, 16 months).13 A case of a metastatic undifferentiated CUP also reported a long-term (>5 years), disease-free survivor after pancreaticoduodenectomy and systemic adjuvant chemotherapy.14
Our case further demonstrates that a multidisciplinary approach to CUP may lead to excellent clinical outcomes. Chemotherapy followed by chemoradiation in our patient increased local tumor control and survival.
Adenocarcinomas of unknown primary cases should involve management by a multidisciplinary team. Clinical trials incorporating locoregional therapies for CUP in addition to systemic therapy are warranted.
Cancer of unknown primary (CUP) represents 3% to 5% of all cancer malignancies in the world.1 Since 2003, CUP has been divided into 2 subsets – favorable (20% of the cases) and unfavorable (80% of the cases) – based on histopathologic and clinical manifestations.2 The impact of locoregional therapies, such as surgery and radiation, in addition to systemic chemotherapy in adenocarcinomas of unknown primary is not well described in the literature.
Case presentation and summary
The patient was frustrated by the lack of diagnosis and extensive work-up and decided to travel to Bangladesh for several months. Upon her return in May 2015, the patient underwent dilation and curettage at an outside tertiary care center because of her persistently elevated beta-hCG levels (>500 mIU/mL; reference range for nonpregnant woman, <5 mIU/mL) that found no products of conception and excluded a malignant process. Endoscopy and colonoscopy at that time failed to reveal a primary tumor.
She was then referred to our institution. Her level of beta-hCG remained elevated, and another transvaginal ultrasound was performed but failed to reveal any masses or evidence of pregnancy. Mammogram and a breast ultrasound showed left breast lesions. Biopsy of the breast lesions was performed, and the pathology demonstrated fibrocystic changes.
The results of a PET-CT scan in August 2015 showed a lobulated abdominal mass of 5.7 x 3.7 cm, consisting of multiple periportal necrotic lymph nodes with a standardized uptake value (SUV) of 14 (Figure 1A) and a 2.0-cm hypermetabolic retroperitoneal lymph node at the aortic bifurcation level with an SUV of 8.6. The SUV is a ratio of activity per unit volume of a region of interest to the activity per unit whole body volume. An SUV of 2.5 or higher is generally considered to be indicative of malignant tissue. We conducted a detailed review of the lymph node pathologic specimen. Immunohistochemical (IHC) studies were positive for CK7, CDX2, and EMA; focally positive for PR and mammaglobin; and negative for CK20, ER, TTF-1, and WT-1. Nonspecific staining was seen with BRST2, and there was no staining with GATA3. IHC stain for HER2-NEU was equivocal. Molecular analysis did not detect BRAF, KRAS, NRAS, and PIK3CA mutations, but did find a CTNNB1 mutation. The IHC pattern suggested pancreatobiliary origin of the tumor.3
Although serum tumor marker pattern of elevated beta-hCG, AFP, and LDH can be seen in germ cell tumors, the pathology evaluation did not favor a germ cell tumor. No site of origin was evident on radiographic evaluation, and the patient was diagnosed with CUP. Based on tumor metastatic distribution and the elevated beta-hCG level,4 we suspected that an undetected pancreatic primary was possible, and we therefore chose the folinic acid, fluorouracil, irinotecan, oxaliplatin (FOLFIRINOX) chemotherapy regimen for its evidence in prolonging survival in metastatic pancreatic cancer.5 At the initiation of treatment, the patient’s elevated tumor markers were beta-hCG 953.6 mIU/mL (reference for nonpregnant woman, <5 mIU/mL) and AFP 1,800.7 ng/mL (reference range, 0.0-9.0 ng/mL). The patient began FOLFIRINOX chemotherapy in August 2015 and after 1 month of treatment, her beta-hCG and AFP levels declined notably to 1.7 mIU/mL and 11.2 ng/mL, respectively. She completed a total of 8 cycles of FOLFIRINOX in November 2015. After completion of chemotherapy, the PET-CT scan showed a decrease in fluoro-D-glucose (FDG) uptake in the porta hepatis and retroperitoneal lymph nodes (Figure 1B). SUV in the porta hepatis lymph nodes declined from 14 to 3.5. The patient’s case was presented to our institution’s multidisciplinary tumor board, and the members deemed the risk of possible lymph node dissection surgery would outweigh the benefit. It was recommended that we proceed with radiotherapy to the residual lymph node stations.
During December 2015 through February 2016, the patient underwent a course of consolidative chemoradiation therapy to the intra-abdominal lymph nodes to a dose of 5,400 cGy in 30 fractions, with concurrent capecitabine as radiosensitizer, using intensity-modulated radiation therapy. During both chemotherapy and CRT, the patient experienced nausea, vomiting, fatigue, and anorexia, which were treated with antiemetics. She completed therapy without major complications and recovered completely from the adverse effects.
Five weeks after completion of chemoradiation, a restaging PET-CT scan showed a persistent small FDG uptake in the periportal region (SUV, 4.2). After CRT, tumor markers beta-hCG and AFP declined to less than 1.2 mIU/mL and less than 2.0 ng/mL, respectively.
Discussion
CUP is divided into favorable and unfavorable subsets.1 The favorable subset includes women with adenocarcinoma involving axillary lymph nodes, women with papillary adenocarcinoma of peritoneal cavity, and adenocarcinoma with a colon profile. The unfavorable subset includes moderate to poorly differentiated adenocarcinomas (64%) and undifferentiated tumors (36%). It involves the liver in 40% to 50% of the cases, followed by lymph nodes (35%), lungs (31%), bones (28%), and the brain (15%).1,2,6 Although data suggest that CUP with lymph-node–only metastases generally fall into an unfavorable prognosis group, our patient’s survival and progression-free survival have been especially prolonged.
The combined platinum–paclitaxel-based regimens are the treatment of choice in this unfavorable subset of CUP,7,8 with patients showing 16% to 38% response rates and median overall survival times of 6.5 to 13 months.7 Platinum–gemcitabine combinations can also be used as an alternative first-line regimen, with an overall response rate of 55% and a median survival of 8 months.9 The addition of the targeted agents bevacizumab and erlotinib to the carboplatin–paclitaxel combination, followed by bevacizumab and erlotinib maintenance, has been shown to yield a median survival of 12.6 months but was not meaningfully superior to historical studies with chemotherapy alone.10
We chose the FOLFIRINOX regimen for our patient. Conroy and colleagues reported a notably improved survival of 11.1 months with that combination chemotherapy in patients with metastatic pancreatic cancer compared with 6.8 months with gemcitabine alone.5 Given the possible pancreatobiliary site of tumor origin on IHC, the lymph node pattern of spread, and the patient’s young age and robust performance status, we felt that this multiagent systemic therapy would offer the best chance of prolonged survival. FOLFIRINOX includes a platinum agent, oxaliplatin, and platinum agents are recommended to be included in chemotherapy combinations for CUP.9,10 Although there is no data to suggest the superiority of a triplet regimen over a doublet regimen in a CUP, a triplet chemotherapy regimen may be considered in select cases.
There have been only a few reports showing the effectiveness of radiotherapy in the treatment of adenocarcinomas of unknown primary outside of the head and neck. Kubisch and colleagues have reported a case of a woman with hepatic adenocarcinoma of unknown primary that was treated with chemotherapy and surgery. Upon recurrence, the patient was then treated with selective internal radiation therapy (SIRT). She was still alive 3 years after diagnosis, and there had been no tumor relapse 21 months after SIRT.11 Shiota and colleagues have reported a case of a mediastinal lymph node CUP that was treated with docetaxel and cisplatin with concurrent thoracic radiation therapy.12 The patient remained free of symptoms without regrowth of the primary site 22 months after disease onset, and exploration of the body with enhanced and PET-CT scan showed no further abnormalities.
Other reports suggest that locoregional therapy such as surgery and radiation may be of benefit to select patients with CUP. A retrospective study by Löffler and colleagues reported that patients with a limited local involvement who received radical surgery had a median overall survival of 52.7 months compared with those who received radiation (median overall survival, 19.4 months) and those who received chemotherapy alone (median overall survival, 16 months).13 A case of a metastatic undifferentiated CUP also reported a long-term (>5 years), disease-free survivor after pancreaticoduodenectomy and systemic adjuvant chemotherapy.14
Our case further demonstrates that a multidisciplinary approach to CUP may lead to excellent clinical outcomes. Chemotherapy followed by chemoradiation in our patient increased local tumor control and survival.
Adenocarcinomas of unknown primary cases should involve management by a multidisciplinary team. Clinical trials incorporating locoregional therapies for CUP in addition to systemic therapy are warranted.
1. Pavlidis N, Khaled H, Gaafar R. A mini review on cancer of unknown primary site: a clinical puzzle for the oncologists. J Adv Res. 2015;6(3):375-382.
2. Pavlidis N, Briasoulis E, Hainsworth J, Greco FA. Diagnostic and therapeutic management of cancer of an unknown primary. Eur J Cancer. 2003;39(14):1990-2005.
3. Oien KA. Pathologic evaluation of unknown primary cancer. Semin Oncol. 2009;36(1):8-37.
4. Louhimo J, Alfthan H, Stenman UH, Hagland C. Serum HCG beta and CA 72-4 are stronger prognostic factors than CEA, CA 19-9 and CA 242 in pancreatic cancer. Oncology. 2004;66(2):126-131.
5. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817-1825.
6. Pavlidis N, Pentheroudakis G. Cancer of unknown primary site. Lancet. 2012;379:1428-1435.
7. Bochtler T, Löffler H, Krämer A. Diagnosis and management of metastatic neoplasms with unknown primary. Semin Diagn Pathol. 2017;35(3):199-206.
8. Amela EY, Lauridant-Philippin G, Cousin S, Ryckewaert T, Adenis A, Penel N. Management of 'unfavourable' carcinoma of unknown primary site: synthesis of recent literature. Crit Rev Oncol Hematol. 2012;84(2):213-223.
9. Culine S, Lortholary A, Voigt J-J, et al. Cisplatin in combination with either gemcitabine or irinotecan in carcinomas of unknown primary site: results of a randomized phase II study--trial for the French study group on carcinomas of unknown primary (GEFCAPI 01). J Clin Oncol. 2003;21(18):3479-3482.
10. Hainsworth JD, Spigel DR, Thompson DS, et al. Paclitaxel/carboplatin plus bevacizumab/erlotinib in the first-line treatment of patients with carcinoma of unknown primary site. Oncologist. 2009;14(12):1189-1197.
11. Kubisch CH, Beigel F, Ihrler S, Goke B, Reiser MF, Hoffmann RT. Oesophageal ulceration after selective internal radiation therapy in a patient with carcinoma of unknown primary. Z Gastroenterol. 2010;48(5):546-550.
12. Shiota Y, Imai S, Sasaki N, et al. A case of mediastinal lymph node carcinoma of unknown primary site treated with docetaxel and cisplatin with concurrent thoracic radiation therapy. Acta Med Okayama. 2011;65(6):407-411.
13. Löffler H, Puthenparambil J, Hielscher T, Neben K, Krämer A. Patients with cancer of unknown primary: a retrospective analysis of 223 patients with adenocarcinoma or undifferentiated carcinoma. Dtsch Arztebl Int. 111(27-28):481-487.
14. Nakagawa Y, Todoroki T, Morishita Y, et al. A long-term survivor after pancreaticoduodenectomy for metastatic undifferentiated carcinoma of an unknown primary. Hepatogastroenterology. 2008;55(86-87):1557-1561.
15. Rodríguez-López JL, Toro-Bahamonde AM, Santiago-Méndez RJ, González-Cancel IF, Vélez-Cortés HA. An unusual case of colorectal adenocarcinoma presenting as an anterior mediastinal mass. Clin Colorectal Cancer. 2018;17(1):e115-e119.
1. Pavlidis N, Khaled H, Gaafar R. A mini review on cancer of unknown primary site: a clinical puzzle for the oncologists. J Adv Res. 2015;6(3):375-382.
2. Pavlidis N, Briasoulis E, Hainsworth J, Greco FA. Diagnostic and therapeutic management of cancer of an unknown primary. Eur J Cancer. 2003;39(14):1990-2005.
3. Oien KA. Pathologic evaluation of unknown primary cancer. Semin Oncol. 2009;36(1):8-37.
4. Louhimo J, Alfthan H, Stenman UH, Hagland C. Serum HCG beta and CA 72-4 are stronger prognostic factors than CEA, CA 19-9 and CA 242 in pancreatic cancer. Oncology. 2004;66(2):126-131.
5. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817-1825.
6. Pavlidis N, Pentheroudakis G. Cancer of unknown primary site. Lancet. 2012;379:1428-1435.
7. Bochtler T, Löffler H, Krämer A. Diagnosis and management of metastatic neoplasms with unknown primary. Semin Diagn Pathol. 2017;35(3):199-206.
8. Amela EY, Lauridant-Philippin G, Cousin S, Ryckewaert T, Adenis A, Penel N. Management of 'unfavourable' carcinoma of unknown primary site: synthesis of recent literature. Crit Rev Oncol Hematol. 2012;84(2):213-223.
9. Culine S, Lortholary A, Voigt J-J, et al. Cisplatin in combination with either gemcitabine or irinotecan in carcinomas of unknown primary site: results of a randomized phase II study--trial for the French study group on carcinomas of unknown primary (GEFCAPI 01). J Clin Oncol. 2003;21(18):3479-3482.
10. Hainsworth JD, Spigel DR, Thompson DS, et al. Paclitaxel/carboplatin plus bevacizumab/erlotinib in the first-line treatment of patients with carcinoma of unknown primary site. Oncologist. 2009;14(12):1189-1197.
11. Kubisch CH, Beigel F, Ihrler S, Goke B, Reiser MF, Hoffmann RT. Oesophageal ulceration after selective internal radiation therapy in a patient with carcinoma of unknown primary. Z Gastroenterol. 2010;48(5):546-550.
12. Shiota Y, Imai S, Sasaki N, et al. A case of mediastinal lymph node carcinoma of unknown primary site treated with docetaxel and cisplatin with concurrent thoracic radiation therapy. Acta Med Okayama. 2011;65(6):407-411.
13. Löffler H, Puthenparambil J, Hielscher T, Neben K, Krämer A. Patients with cancer of unknown primary: a retrospective analysis of 223 patients with adenocarcinoma or undifferentiated carcinoma. Dtsch Arztebl Int. 111(27-28):481-487.
14. Nakagawa Y, Todoroki T, Morishita Y, et al. A long-term survivor after pancreaticoduodenectomy for metastatic undifferentiated carcinoma of an unknown primary. Hepatogastroenterology. 2008;55(86-87):1557-1561.
15. Rodríguez-López JL, Toro-Bahamonde AM, Santiago-Méndez RJ, González-Cancel IF, Vélez-Cortés HA. An unusual case of colorectal adenocarcinoma presenting as an anterior mediastinal mass. Clin Colorectal Cancer. 2018;17(1):e115-e119.
The year’s top studies in child/adolescent psychiatry
BARCELONA – Prenatal exposure to selective serotonin reuptake inhibitors late in pregnancy was associated with a significantly increased risk of anxious and/or depressed behaviors at 5 years of age in the prospective Norwegian Mother and Child Cohort Study.
Other than that specific red flag, however, the outcomes of in utero exposure to maternal SSRIs were reassuringly benign. Prenatal exposure during early- or mid-pregnancy was not associated with increased risk of anxious/depressed behaviors, compared with nonexposure; that adverse effect was restricted to exposure at week 29 of pregnancy or later. Nor did in utero exposure to maternal SSRIs during any time in pregnancy pose an increased risk for pediatric externalizing, emotional, or social problems in this observational study of 8,359 Norwegian mother-child dyads, Josefina Castro-Fornieles, MD, PhD, observed at the annual congress of the European College of Neuropsychopharmacology.
The huge Norwegian study was among what she considers the four most important studies in child/adolescent psychiatry published through the first three quarters of 2018. The others she highlighted were a large longitudinal observational study that demonstrated that persistent maternal postnatal depression was strongly associated with a variety of pediatric behavioral disturbances documented during assessments at ages 3.5, 16, and 18 years; a Philadelphia study showing that multiple traumatic stressful events or any assaultive trauma experienced by children or adolescents were independently associated with significant psychopathology and neurocognitive deficits; and a Dutch brain MRI study that pinpointed a reduction in gray matter volume in the anterior cingulate cortex as a potential key mediator of the neurobiologic aftereffects of childhood sexual abuse.
She selected those studies because they shared a common theme, one that constituted her key take-home message: “When recording antecedents during a clinical assessment, both with adults and children, it is clear that we have to ask in a more detailed way – using validated scales and interviews if possible – about the mother’s prenatal problems, including psychopharmacological treatment. That is something we often don’t do in a sufficiently detailed way in our clinical practice. And it’s also important to ask about life events; abuse during childhood and adolescence can be really important. We can modulate our treatment depending upon whether there is an influence of any of these aspects,” said Dr. Castro-Fornieles, director of the Clinical Institute of Neuroscience at the Hospital Clinic of Barcelona and a recent past-president of the Spanish Society for Child and Adolescent Psychiatry.
The following are her Top 4 studies:
The Norwegian Mother and Child Cohort Study
The increased risk of anxious and/or depressed behaviors in children exposed to selective serotonin reuptake inhibitors (SSRIs) late in pregnancy did not emerge until the year-5 assessment; it wasn’t evident at the 1.5- or 3-year evaluations.
The investigators emphasized a key lesson from their study: The importance of following children with late-pregnancy exposure to maternal SSRI therapy for development of symptoms of anxiety and/or depression (J Am Acad Child Adolesc Psychiatry. 2018 Mar;57[3]:200-8). Dr. Castro-Fornieles strongly endorsed that recommendation. However, she noted what she considers an important limitation to the study: even though the University of Oslo investigators adjusted for numerous potential confounders in their risk models – including maternal body mass index, parity, education, smoking, substance use, breastfeeding, folic acid use, and other medications used during pregnancy – it’s not possible in a study such as this to control for genetic and environmental risk factors, which she suspects also were at work.
The Avon Longitudinal Study of Parents and Children in the United Kingdom
Maternal postnatal depression is common, affecting roughly 10% of mothers. But it is not invariably associated with adverse mental health outcomes in their children. This study of nearly 10,000 mothers and their children sought to identify which children were at most risk. Using the Edinburgh Postnatal Depression Scale, the international team of investigators categorized maternal postnatal depression as moderate, marked, or severe. The affective disorder was deemed persistent if scores on the Edinburgh scale were elevated at both 2 and 8 months after delivery.
Postnatal depression, whether persistent or not, was associated with roughly a 2- to 2.4-fold increase for child behavioral disturbances when assessed at age 3.5 years using the Rutter Total Problems Scale. But postnatal depression that was persistent was the real difference maker: It carried a much higher risk of adverse behavioral outcomes and cognitive deficits than did the nonpersistent version. Indeed, persistent severe postnatal depression was associated a 4.8-fold increased risk of behavioral problems at age 3.5 years, a 2.65-fold greater risk of markedly lower grades in mathematics at age 16 years, and a 7.4-fold increased prevalence of depression at 18 years of age. The investigators advised screening mothers during the first postpartum year in order to identify those with persistent postpartum depression (JAMA Psychiatry. 2018 Mar 1;75[3]:247-53).
Dr. Castro-Fornieles said an important shortcoming of the Avon study was that it did not record paternal data.
“The study didn’t consider depression or other functional measures in the father, his commitment to childrearing, and whether the family was together or divorced. I feel this is an important limitation in many studies. For me, it’s really important to consider what’s happening with the fathers,” she said.
Traumatic stress load, psychopathology, and cognition
An eye-opening report from the Philadelphia Neurodevelopmental Cohort documented a surprisingly high level of lifetime exposure to traumatic events among 9,498 youth aged 8-21 years, and the stepwise manner by which a greater traumatic stress load was associated with increasing severity of psychopathology and cognitive deficits. Notably, the study participants were recruited from general pediatric clinics in the Children’s Hospital of Philadelphia health care network; they were not patients seeking psychiatric help. And yet, extensive structured psychiatric evaluation showed that 23% of them had a history of one traumatic stressful event, 12% had two, and 1% had three or more.
In analyses adjusted for lifetime history of depression or PTSD, a higher traumatic event load was associated with increased risk of externalizing behaviors, mood/anxiety disorders, psychosis spectrum, and fear. Moreover, a high trauma stress load was associated with a 5.3-fold increased risk of suicidal thoughts and a 3.2-fold increased likelihood of cannabis use, compared with youth who had never been exposed to a traumatic event. Increased stress load also was associated with worse cognitive performance on tests of executive functioning, social cognition, and complex reasoning.
A history of assaultive trauma – being badly beaten, threatened with a weapon, or sexually abused – was associated with more severe psychopathology than in subjects with a history of nonassaultive traumatic events (Psychol Med. 2018 Apr 15:1-10).
Session moderator Carmen Moreno, MD, a child and adolescent psychiatrist at Gregorio Marañón University Hospital in Madrid, commented, “It was striking to me that the prevalence of childhood traumatic events was so high in a pediatric community sample. Is the measure the investigators chose the right measure?”
Dr. Castro-Fornieles replied that it was a very sensitive measure, in that an event many would consider part of normal life – for example, seeing a relative’s body on display in a funeral home – was scored as a traumatic exposure.
“Only one exposure is not that important,” she said. “The impact increases as you increase the number of traumatic events. And also the assaultive ones.”
Sexual abuse leaves a fingerprint
Investigators at Leiden (the Netherlands) University performed neuroimaging that looked at numerous brain regions of interest in 21 adolescents with childhood sexual abuse–related PTSD and 25 matched healthy controls. The standout finding was that the dorsal gray matter volume of the anterior cingulate cortex was significantly smaller in the teens with PTSD and a history of childhood sexual abuse (Eur Neuropsychopharmacol. 2017 Nov;27[11]:1163-71).
The investigators wanted a pure sample of patients with PTSD after childhood sexual abuse, so they excluded individuals who had experienced childhood sexual abuse and had a diagnosis of attention-deficit/hyperactivity disorder, oppositional defiant disorder, obsessive-compulsive disorder, conduct disorder, pervasive developmental disorder, bipolar disorder, or a psychotic disorder. That is both a strength and a limitation of the study, in Dr. Castro-Fornieles’ view.
“To me, that excludes too many of the children we see in our clinical settings. This work needs to be corroborated in a bigger sample, including patients with other diagnoses,” she said.
She reported having no financial conflicts regarding her presentation.
BARCELONA – Prenatal exposure to selective serotonin reuptake inhibitors late in pregnancy was associated with a significantly increased risk of anxious and/or depressed behaviors at 5 years of age in the prospective Norwegian Mother and Child Cohort Study.
Other than that specific red flag, however, the outcomes of in utero exposure to maternal SSRIs were reassuringly benign. Prenatal exposure during early- or mid-pregnancy was not associated with increased risk of anxious/depressed behaviors, compared with nonexposure; that adverse effect was restricted to exposure at week 29 of pregnancy or later. Nor did in utero exposure to maternal SSRIs during any time in pregnancy pose an increased risk for pediatric externalizing, emotional, or social problems in this observational study of 8,359 Norwegian mother-child dyads, Josefina Castro-Fornieles, MD, PhD, observed at the annual congress of the European College of Neuropsychopharmacology.
The huge Norwegian study was among what she considers the four most important studies in child/adolescent psychiatry published through the first three quarters of 2018. The others she highlighted were a large longitudinal observational study that demonstrated that persistent maternal postnatal depression was strongly associated with a variety of pediatric behavioral disturbances documented during assessments at ages 3.5, 16, and 18 years; a Philadelphia study showing that multiple traumatic stressful events or any assaultive trauma experienced by children or adolescents were independently associated with significant psychopathology and neurocognitive deficits; and a Dutch brain MRI study that pinpointed a reduction in gray matter volume in the anterior cingulate cortex as a potential key mediator of the neurobiologic aftereffects of childhood sexual abuse.
She selected those studies because they shared a common theme, one that constituted her key take-home message: “When recording antecedents during a clinical assessment, both with adults and children, it is clear that we have to ask in a more detailed way – using validated scales and interviews if possible – about the mother’s prenatal problems, including psychopharmacological treatment. That is something we often don’t do in a sufficiently detailed way in our clinical practice. And it’s also important to ask about life events; abuse during childhood and adolescence can be really important. We can modulate our treatment depending upon whether there is an influence of any of these aspects,” said Dr. Castro-Fornieles, director of the Clinical Institute of Neuroscience at the Hospital Clinic of Barcelona and a recent past-president of the Spanish Society for Child and Adolescent Psychiatry.
The following are her Top 4 studies:
The Norwegian Mother and Child Cohort Study
The increased risk of anxious and/or depressed behaviors in children exposed to selective serotonin reuptake inhibitors (SSRIs) late in pregnancy did not emerge until the year-5 assessment; it wasn’t evident at the 1.5- or 3-year evaluations.
The investigators emphasized a key lesson from their study: The importance of following children with late-pregnancy exposure to maternal SSRI therapy for development of symptoms of anxiety and/or depression (J Am Acad Child Adolesc Psychiatry. 2018 Mar;57[3]:200-8). Dr. Castro-Fornieles strongly endorsed that recommendation. However, she noted what she considers an important limitation to the study: even though the University of Oslo investigators adjusted for numerous potential confounders in their risk models – including maternal body mass index, parity, education, smoking, substance use, breastfeeding, folic acid use, and other medications used during pregnancy – it’s not possible in a study such as this to control for genetic and environmental risk factors, which she suspects also were at work.
The Avon Longitudinal Study of Parents and Children in the United Kingdom
Maternal postnatal depression is common, affecting roughly 10% of mothers. But it is not invariably associated with adverse mental health outcomes in their children. This study of nearly 10,000 mothers and their children sought to identify which children were at most risk. Using the Edinburgh Postnatal Depression Scale, the international team of investigators categorized maternal postnatal depression as moderate, marked, or severe. The affective disorder was deemed persistent if scores on the Edinburgh scale were elevated at both 2 and 8 months after delivery.
Postnatal depression, whether persistent or not, was associated with roughly a 2- to 2.4-fold increase for child behavioral disturbances when assessed at age 3.5 years using the Rutter Total Problems Scale. But postnatal depression that was persistent was the real difference maker: It carried a much higher risk of adverse behavioral outcomes and cognitive deficits than did the nonpersistent version. Indeed, persistent severe postnatal depression was associated a 4.8-fold increased risk of behavioral problems at age 3.5 years, a 2.65-fold greater risk of markedly lower grades in mathematics at age 16 years, and a 7.4-fold increased prevalence of depression at 18 years of age. The investigators advised screening mothers during the first postpartum year in order to identify those with persistent postpartum depression (JAMA Psychiatry. 2018 Mar 1;75[3]:247-53).
Dr. Castro-Fornieles said an important shortcoming of the Avon study was that it did not record paternal data.
“The study didn’t consider depression or other functional measures in the father, his commitment to childrearing, and whether the family was together or divorced. I feel this is an important limitation in many studies. For me, it’s really important to consider what’s happening with the fathers,” she said.
Traumatic stress load, psychopathology, and cognition
An eye-opening report from the Philadelphia Neurodevelopmental Cohort documented a surprisingly high level of lifetime exposure to traumatic events among 9,498 youth aged 8-21 years, and the stepwise manner by which a greater traumatic stress load was associated with increasing severity of psychopathology and cognitive deficits. Notably, the study participants were recruited from general pediatric clinics in the Children’s Hospital of Philadelphia health care network; they were not patients seeking psychiatric help. And yet, extensive structured psychiatric evaluation showed that 23% of them had a history of one traumatic stressful event, 12% had two, and 1% had three or more.
In analyses adjusted for lifetime history of depression or PTSD, a higher traumatic event load was associated with increased risk of externalizing behaviors, mood/anxiety disorders, psychosis spectrum, and fear. Moreover, a high trauma stress load was associated with a 5.3-fold increased risk of suicidal thoughts and a 3.2-fold increased likelihood of cannabis use, compared with youth who had never been exposed to a traumatic event. Increased stress load also was associated with worse cognitive performance on tests of executive functioning, social cognition, and complex reasoning.
A history of assaultive trauma – being badly beaten, threatened with a weapon, or sexually abused – was associated with more severe psychopathology than in subjects with a history of nonassaultive traumatic events (Psychol Med. 2018 Apr 15:1-10).
Session moderator Carmen Moreno, MD, a child and adolescent psychiatrist at Gregorio Marañón University Hospital in Madrid, commented, “It was striking to me that the prevalence of childhood traumatic events was so high in a pediatric community sample. Is the measure the investigators chose the right measure?”
Dr. Castro-Fornieles replied that it was a very sensitive measure, in that an event many would consider part of normal life – for example, seeing a relative’s body on display in a funeral home – was scored as a traumatic exposure.
“Only one exposure is not that important,” she said. “The impact increases as you increase the number of traumatic events. And also the assaultive ones.”
Sexual abuse leaves a fingerprint
Investigators at Leiden (the Netherlands) University performed neuroimaging that looked at numerous brain regions of interest in 21 adolescents with childhood sexual abuse–related PTSD and 25 matched healthy controls. The standout finding was that the dorsal gray matter volume of the anterior cingulate cortex was significantly smaller in the teens with PTSD and a history of childhood sexual abuse (Eur Neuropsychopharmacol. 2017 Nov;27[11]:1163-71).
The investigators wanted a pure sample of patients with PTSD after childhood sexual abuse, so they excluded individuals who had experienced childhood sexual abuse and had a diagnosis of attention-deficit/hyperactivity disorder, oppositional defiant disorder, obsessive-compulsive disorder, conduct disorder, pervasive developmental disorder, bipolar disorder, or a psychotic disorder. That is both a strength and a limitation of the study, in Dr. Castro-Fornieles’ view.
“To me, that excludes too many of the children we see in our clinical settings. This work needs to be corroborated in a bigger sample, including patients with other diagnoses,” she said.
She reported having no financial conflicts regarding her presentation.
BARCELONA – Prenatal exposure to selective serotonin reuptake inhibitors late in pregnancy was associated with a significantly increased risk of anxious and/or depressed behaviors at 5 years of age in the prospective Norwegian Mother and Child Cohort Study.
Other than that specific red flag, however, the outcomes of in utero exposure to maternal SSRIs were reassuringly benign. Prenatal exposure during early- or mid-pregnancy was not associated with increased risk of anxious/depressed behaviors, compared with nonexposure; that adverse effect was restricted to exposure at week 29 of pregnancy or later. Nor did in utero exposure to maternal SSRIs during any time in pregnancy pose an increased risk for pediatric externalizing, emotional, or social problems in this observational study of 8,359 Norwegian mother-child dyads, Josefina Castro-Fornieles, MD, PhD, observed at the annual congress of the European College of Neuropsychopharmacology.
The huge Norwegian study was among what she considers the four most important studies in child/adolescent psychiatry published through the first three quarters of 2018. The others she highlighted were a large longitudinal observational study that demonstrated that persistent maternal postnatal depression was strongly associated with a variety of pediatric behavioral disturbances documented during assessments at ages 3.5, 16, and 18 years; a Philadelphia study showing that multiple traumatic stressful events or any assaultive trauma experienced by children or adolescents were independently associated with significant psychopathology and neurocognitive deficits; and a Dutch brain MRI study that pinpointed a reduction in gray matter volume in the anterior cingulate cortex as a potential key mediator of the neurobiologic aftereffects of childhood sexual abuse.
She selected those studies because they shared a common theme, one that constituted her key take-home message: “When recording antecedents during a clinical assessment, both with adults and children, it is clear that we have to ask in a more detailed way – using validated scales and interviews if possible – about the mother’s prenatal problems, including psychopharmacological treatment. That is something we often don’t do in a sufficiently detailed way in our clinical practice. And it’s also important to ask about life events; abuse during childhood and adolescence can be really important. We can modulate our treatment depending upon whether there is an influence of any of these aspects,” said Dr. Castro-Fornieles, director of the Clinical Institute of Neuroscience at the Hospital Clinic of Barcelona and a recent past-president of the Spanish Society for Child and Adolescent Psychiatry.
The following are her Top 4 studies:
The Norwegian Mother and Child Cohort Study
The increased risk of anxious and/or depressed behaviors in children exposed to selective serotonin reuptake inhibitors (SSRIs) late in pregnancy did not emerge until the year-5 assessment; it wasn’t evident at the 1.5- or 3-year evaluations.
The investigators emphasized a key lesson from their study: The importance of following children with late-pregnancy exposure to maternal SSRI therapy for development of symptoms of anxiety and/or depression (J Am Acad Child Adolesc Psychiatry. 2018 Mar;57[3]:200-8). Dr. Castro-Fornieles strongly endorsed that recommendation. However, she noted what she considers an important limitation to the study: even though the University of Oslo investigators adjusted for numerous potential confounders in their risk models – including maternal body mass index, parity, education, smoking, substance use, breastfeeding, folic acid use, and other medications used during pregnancy – it’s not possible in a study such as this to control for genetic and environmental risk factors, which she suspects also were at work.
The Avon Longitudinal Study of Parents and Children in the United Kingdom
Maternal postnatal depression is common, affecting roughly 10% of mothers. But it is not invariably associated with adverse mental health outcomes in their children. This study of nearly 10,000 mothers and their children sought to identify which children were at most risk. Using the Edinburgh Postnatal Depression Scale, the international team of investigators categorized maternal postnatal depression as moderate, marked, or severe. The affective disorder was deemed persistent if scores on the Edinburgh scale were elevated at both 2 and 8 months after delivery.
Postnatal depression, whether persistent or not, was associated with roughly a 2- to 2.4-fold increase for child behavioral disturbances when assessed at age 3.5 years using the Rutter Total Problems Scale. But postnatal depression that was persistent was the real difference maker: It carried a much higher risk of adverse behavioral outcomes and cognitive deficits than did the nonpersistent version. Indeed, persistent severe postnatal depression was associated a 4.8-fold increased risk of behavioral problems at age 3.5 years, a 2.65-fold greater risk of markedly lower grades in mathematics at age 16 years, and a 7.4-fold increased prevalence of depression at 18 years of age. The investigators advised screening mothers during the first postpartum year in order to identify those with persistent postpartum depression (JAMA Psychiatry. 2018 Mar 1;75[3]:247-53).
Dr. Castro-Fornieles said an important shortcoming of the Avon study was that it did not record paternal data.
“The study didn’t consider depression or other functional measures in the father, his commitment to childrearing, and whether the family was together or divorced. I feel this is an important limitation in many studies. For me, it’s really important to consider what’s happening with the fathers,” she said.
Traumatic stress load, psychopathology, and cognition
An eye-opening report from the Philadelphia Neurodevelopmental Cohort documented a surprisingly high level of lifetime exposure to traumatic events among 9,498 youth aged 8-21 years, and the stepwise manner by which a greater traumatic stress load was associated with increasing severity of psychopathology and cognitive deficits. Notably, the study participants were recruited from general pediatric clinics in the Children’s Hospital of Philadelphia health care network; they were not patients seeking psychiatric help. And yet, extensive structured psychiatric evaluation showed that 23% of them had a history of one traumatic stressful event, 12% had two, and 1% had three or more.
In analyses adjusted for lifetime history of depression or PTSD, a higher traumatic event load was associated with increased risk of externalizing behaviors, mood/anxiety disorders, psychosis spectrum, and fear. Moreover, a high trauma stress load was associated with a 5.3-fold increased risk of suicidal thoughts and a 3.2-fold increased likelihood of cannabis use, compared with youth who had never been exposed to a traumatic event. Increased stress load also was associated with worse cognitive performance on tests of executive functioning, social cognition, and complex reasoning.
A history of assaultive trauma – being badly beaten, threatened with a weapon, or sexually abused – was associated with more severe psychopathology than in subjects with a history of nonassaultive traumatic events (Psychol Med. 2018 Apr 15:1-10).
Session moderator Carmen Moreno, MD, a child and adolescent psychiatrist at Gregorio Marañón University Hospital in Madrid, commented, “It was striking to me that the prevalence of childhood traumatic events was so high in a pediatric community sample. Is the measure the investigators chose the right measure?”
Dr. Castro-Fornieles replied that it was a very sensitive measure, in that an event many would consider part of normal life – for example, seeing a relative’s body on display in a funeral home – was scored as a traumatic exposure.
“Only one exposure is not that important,” she said. “The impact increases as you increase the number of traumatic events. And also the assaultive ones.”
Sexual abuse leaves a fingerprint
Investigators at Leiden (the Netherlands) University performed neuroimaging that looked at numerous brain regions of interest in 21 adolescents with childhood sexual abuse–related PTSD and 25 matched healthy controls. The standout finding was that the dorsal gray matter volume of the anterior cingulate cortex was significantly smaller in the teens with PTSD and a history of childhood sexual abuse (Eur Neuropsychopharmacol. 2017 Nov;27[11]:1163-71).
The investigators wanted a pure sample of patients with PTSD after childhood sexual abuse, so they excluded individuals who had experienced childhood sexual abuse and had a diagnosis of attention-deficit/hyperactivity disorder, oppositional defiant disorder, obsessive-compulsive disorder, conduct disorder, pervasive developmental disorder, bipolar disorder, or a psychotic disorder. That is both a strength and a limitation of the study, in Dr. Castro-Fornieles’ view.
“To me, that excludes too many of the children we see in our clinical settings. This work needs to be corroborated in a bigger sample, including patients with other diagnoses,” she said.
She reported having no financial conflicts regarding her presentation.
REPORTING FROM THE ECNP CONGRESS