User login
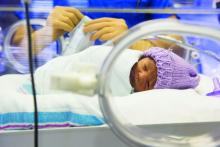
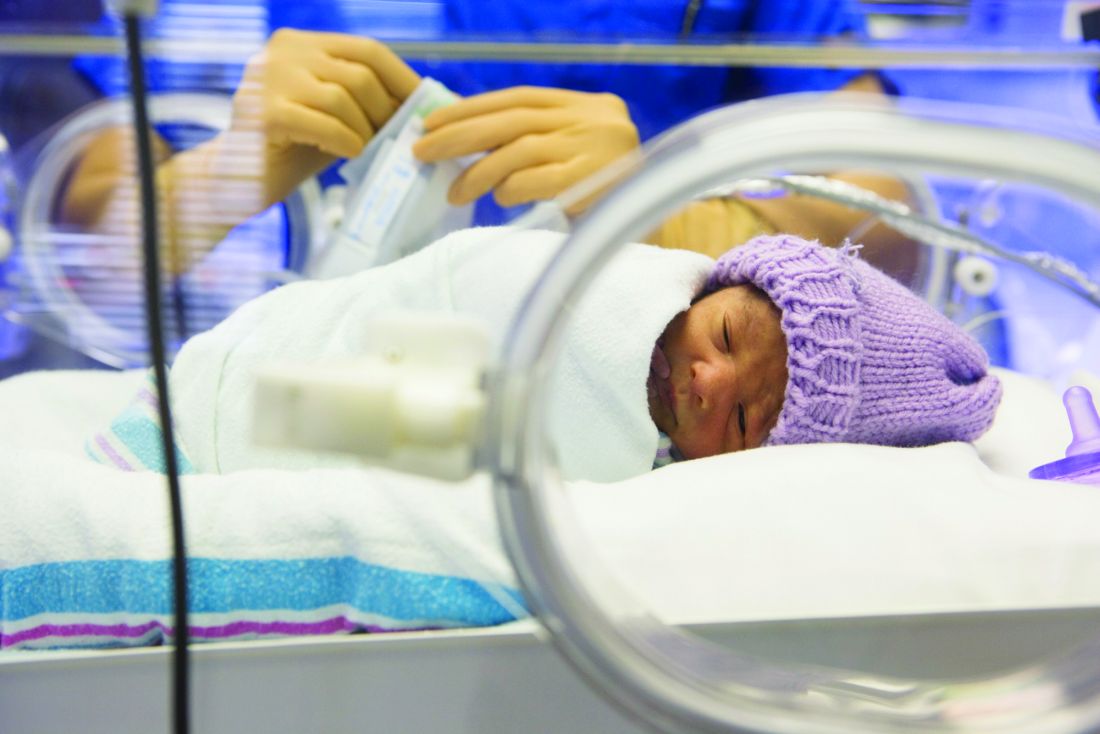
Think barrier repair for acne patients
LAS VEGAS – It’s time to start thinking about barrier repair in acne patients, Hilary E. Baldwin, MD, asserts.
“We think about barrier repair with our psoriasis patients, with our dermatitis patients, and our rosacea patients, but we don’t talk about it much with our acne patients,” Dr. Baldwin said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. “Now there is increasing evidence that acne pathophysiology may include a barrier defect.”
Both topical and systemic medications exacerbate the problem, because they are so drying, added Dr. Baldwin, medical director of the Acne Treatment & Research Center in Morristown, N.J. Benzoyl peroxide has been shown to increase transepidermal water loss (TEWL) and deplete tocopherol levels in the stratum corneum, while topical retinoids thin the stratum corneum, increase epidermal fragility, and increase TEWL.
The barrier defect may actually decrease medication use – patients are irritated and they stop taking their medication. “,” she said.
She described the results of an Internet-based survey of 200 acne patients aged 15-40 years who had been prescribed clindamycin/benzoyl peroxide 5% within the last 6 months (J Drugs Dermatol. 2011 Jun;10[6]:605-8). The researchers found that side effects lead to suboptimal use of the topical medication, as patients reported spot application, use only in flares, infrequent use, and discontinuation.
Further, 31% of patients complained to their doctors’ offices about dryness, 23% said their doctors did not understand the potential side effects, 21% lost confidence in their doctors, 11% said they were less likely to see their doctors again, and 41% reported using moisturizer to combat dryness and erythema.
“No acne visit is complete without a discussion of skin care,” Dr. Baldwin said. Quality moisturization is a must for acne patients and has been shown to improve TEWL, normalize ceramides, and repair the microbiome.
“I recommend moisturizers with ceramides, hyaluronic acid, and certainly moisturizers that have been shown to be noncomedogenic,” Dr. Baldwin said. “Most acne patients aren’t willing to use a cream moisturizer during the day – they just feel too greasy – so I have them use a cream in the evening when they are home and a lotion in the morning.”
While there has been one very specific study of using a moisturizer before topical medications, there is no good data for this practice, Dr. Baldwin said. “Clearly, though, using a moisturizer reduces irritation, so I prefer to have my patients put on the moisturizer at a different time, but if that doesn’t work, I have them put it on before the medication.”
Dr. Baldwin indicated that she is on the speakers bureau for LaRoche Posay, Galderma, and Valeant, and had received grant and/or contracted research support from Dermira and Valeant.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – It’s time to start thinking about barrier repair in acne patients, Hilary E. Baldwin, MD, asserts.
“We think about barrier repair with our psoriasis patients, with our dermatitis patients, and our rosacea patients, but we don’t talk about it much with our acne patients,” Dr. Baldwin said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. “Now there is increasing evidence that acne pathophysiology may include a barrier defect.”
Both topical and systemic medications exacerbate the problem, because they are so drying, added Dr. Baldwin, medical director of the Acne Treatment & Research Center in Morristown, N.J. Benzoyl peroxide has been shown to increase transepidermal water loss (TEWL) and deplete tocopherol levels in the stratum corneum, while topical retinoids thin the stratum corneum, increase epidermal fragility, and increase TEWL.
The barrier defect may actually decrease medication use – patients are irritated and they stop taking their medication. “,” she said.
She described the results of an Internet-based survey of 200 acne patients aged 15-40 years who had been prescribed clindamycin/benzoyl peroxide 5% within the last 6 months (J Drugs Dermatol. 2011 Jun;10[6]:605-8). The researchers found that side effects lead to suboptimal use of the topical medication, as patients reported spot application, use only in flares, infrequent use, and discontinuation.
Further, 31% of patients complained to their doctors’ offices about dryness, 23% said their doctors did not understand the potential side effects, 21% lost confidence in their doctors, 11% said they were less likely to see their doctors again, and 41% reported using moisturizer to combat dryness and erythema.
“No acne visit is complete without a discussion of skin care,” Dr. Baldwin said. Quality moisturization is a must for acne patients and has been shown to improve TEWL, normalize ceramides, and repair the microbiome.
“I recommend moisturizers with ceramides, hyaluronic acid, and certainly moisturizers that have been shown to be noncomedogenic,” Dr. Baldwin said. “Most acne patients aren’t willing to use a cream moisturizer during the day – they just feel too greasy – so I have them use a cream in the evening when they are home and a lotion in the morning.”
While there has been one very specific study of using a moisturizer before topical medications, there is no good data for this practice, Dr. Baldwin said. “Clearly, though, using a moisturizer reduces irritation, so I prefer to have my patients put on the moisturizer at a different time, but if that doesn’t work, I have them put it on before the medication.”
Dr. Baldwin indicated that she is on the speakers bureau for LaRoche Posay, Galderma, and Valeant, and had received grant and/or contracted research support from Dermira and Valeant.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – It’s time to start thinking about barrier repair in acne patients, Hilary E. Baldwin, MD, asserts.
“We think about barrier repair with our psoriasis patients, with our dermatitis patients, and our rosacea patients, but we don’t talk about it much with our acne patients,” Dr. Baldwin said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. “Now there is increasing evidence that acne pathophysiology may include a barrier defect.”
Both topical and systemic medications exacerbate the problem, because they are so drying, added Dr. Baldwin, medical director of the Acne Treatment & Research Center in Morristown, N.J. Benzoyl peroxide has been shown to increase transepidermal water loss (TEWL) and deplete tocopherol levels in the stratum corneum, while topical retinoids thin the stratum corneum, increase epidermal fragility, and increase TEWL.
The barrier defect may actually decrease medication use – patients are irritated and they stop taking their medication. “,” she said.
She described the results of an Internet-based survey of 200 acne patients aged 15-40 years who had been prescribed clindamycin/benzoyl peroxide 5% within the last 6 months (J Drugs Dermatol. 2011 Jun;10[6]:605-8). The researchers found that side effects lead to suboptimal use of the topical medication, as patients reported spot application, use only in flares, infrequent use, and discontinuation.
Further, 31% of patients complained to their doctors’ offices about dryness, 23% said their doctors did not understand the potential side effects, 21% lost confidence in their doctors, 11% said they were less likely to see their doctors again, and 41% reported using moisturizer to combat dryness and erythema.
“No acne visit is complete without a discussion of skin care,” Dr. Baldwin said. Quality moisturization is a must for acne patients and has been shown to improve TEWL, normalize ceramides, and repair the microbiome.
“I recommend moisturizers with ceramides, hyaluronic acid, and certainly moisturizers that have been shown to be noncomedogenic,” Dr. Baldwin said. “Most acne patients aren’t willing to use a cream moisturizer during the day – they just feel too greasy – so I have them use a cream in the evening when they are home and a lotion in the morning.”
While there has been one very specific study of using a moisturizer before topical medications, there is no good data for this practice, Dr. Baldwin said. “Clearly, though, using a moisturizer reduces irritation, so I prefer to have my patients put on the moisturizer at a different time, but if that doesn’t work, I have them put it on before the medication.”
Dr. Baldwin indicated that she is on the speakers bureau for LaRoche Posay, Galderma, and Valeant, and had received grant and/or contracted research support from Dermira and Valeant.
SDEF and this news organization are owned by the same parent company.
REPORTING FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
Four predictors confirmed for treatment-resistant depression
BARCELONA – Four potent clinical predictors of treatment-resistant depression have been confirmed in a validation study that showed the four factors collectively have a predictive accuracy of 87%, Alexander Kautzky, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
The four predictors – all easily assessed – are symptom severity, which was independently associated with a 3.3-fold increased likelihood of treatment-resistant depression (TRD) in a study of 916 patients with major depression at 10 referral centers in eight European countries; suicidal risk, with a 1.74-fold increased risk; comorbid anxiety disorder, with a 1.68-fold increased risk; and the lifetime number of major depressive episodes, for which the associated TRD risk climbs by 15% per prior episode, according to Dr. Kautzky of the Medical University of Vienna.
When these four predictors were put to the test in an independent validation cohort of 314 patients with major depression, the four clinical markers of TRD exhibited a sensitivity of 86%, a specificity of 88%, and an overall predictive accuracy of 87%.
In these studies, Response was defined as at least a 50% drop in MADRS score to below 22 points.
The validation of these clinical predictors of TRD is a welcome development in psychiatry. The World Health Organization ranks major depressive disorder as the No. 4 cause of disease burden worldwide, and an impressive number of antidepressant medications are available, but up to 60% of patients do not respond sufficiently to their first round of antidepressant therapy. And there is a notable absence of biological markers to aid in selecting the best initial antidepressant for a given individual, Dr. Kautzky observed.
The validation study was supported by an unrestricted research grant from Lundbeck to a European research consortium known as the Group for the Study of Resistant Depression. Dr. Kautzky reported having no financial conflicts of interest.
BARCELONA – Four potent clinical predictors of treatment-resistant depression have been confirmed in a validation study that showed the four factors collectively have a predictive accuracy of 87%, Alexander Kautzky, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
The four predictors – all easily assessed – are symptom severity, which was independently associated with a 3.3-fold increased likelihood of treatment-resistant depression (TRD) in a study of 916 patients with major depression at 10 referral centers in eight European countries; suicidal risk, with a 1.74-fold increased risk; comorbid anxiety disorder, with a 1.68-fold increased risk; and the lifetime number of major depressive episodes, for which the associated TRD risk climbs by 15% per prior episode, according to Dr. Kautzky of the Medical University of Vienna.
When these four predictors were put to the test in an independent validation cohort of 314 patients with major depression, the four clinical markers of TRD exhibited a sensitivity of 86%, a specificity of 88%, and an overall predictive accuracy of 87%.
In these studies, Response was defined as at least a 50% drop in MADRS score to below 22 points.
The validation of these clinical predictors of TRD is a welcome development in psychiatry. The World Health Organization ranks major depressive disorder as the No. 4 cause of disease burden worldwide, and an impressive number of antidepressant medications are available, but up to 60% of patients do not respond sufficiently to their first round of antidepressant therapy. And there is a notable absence of biological markers to aid in selecting the best initial antidepressant for a given individual, Dr. Kautzky observed.
The validation study was supported by an unrestricted research grant from Lundbeck to a European research consortium known as the Group for the Study of Resistant Depression. Dr. Kautzky reported having no financial conflicts of interest.
BARCELONA – Four potent clinical predictors of treatment-resistant depression have been confirmed in a validation study that showed the four factors collectively have a predictive accuracy of 87%, Alexander Kautzky, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
The four predictors – all easily assessed – are symptom severity, which was independently associated with a 3.3-fold increased likelihood of treatment-resistant depression (TRD) in a study of 916 patients with major depression at 10 referral centers in eight European countries; suicidal risk, with a 1.74-fold increased risk; comorbid anxiety disorder, with a 1.68-fold increased risk; and the lifetime number of major depressive episodes, for which the associated TRD risk climbs by 15% per prior episode, according to Dr. Kautzky of the Medical University of Vienna.
When these four predictors were put to the test in an independent validation cohort of 314 patients with major depression, the four clinical markers of TRD exhibited a sensitivity of 86%, a specificity of 88%, and an overall predictive accuracy of 87%.
In these studies, Response was defined as at least a 50% drop in MADRS score to below 22 points.
The validation of these clinical predictors of TRD is a welcome development in psychiatry. The World Health Organization ranks major depressive disorder as the No. 4 cause of disease burden worldwide, and an impressive number of antidepressant medications are available, but up to 60% of patients do not respond sufficiently to their first round of antidepressant therapy. And there is a notable absence of biological markers to aid in selecting the best initial antidepressant for a given individual, Dr. Kautzky observed.
The validation study was supported by an unrestricted research grant from Lundbeck to a European research consortium known as the Group for the Study of Resistant Depression. Dr. Kautzky reported having no financial conflicts of interest.
REPORTING FROM THE ECNP CONGRESS
Key clinical point: Patients with major depressive disorder at high risk for treatment-resistant depression are now readily identifiable.
Major finding: Four easily collected clinical variables predict treatment-resistant depression with 87% accuracy.
Study details: This project identified clinical predictors of treatment-resistant depression in a cohort of 916 patients with major depressive disorder then validated the predictors in a separate cohort of 314 patients.
Disclosures: The presenter reported having no financial conflicts regarding this study, which was supported by an unrestricted research grant from Lundbeck.
Vascular ultrasound reasonable for first-line imaging of large-vessel GCA
CHICAGO – Vascular ultrasound showed high sensitivity and specificity for diagnosing large-vessel giant-cell arteritis (LV-GCA) in a prospective study of patients with suspected new-onset disease.
The findings highlight the value of vascular ultrasound – in the hands of experienced sonographers – as a first-line imaging test in this setting, Berit Dalsgaard Nielsen, MD, reported at the annual meeting of the American College of Rheumatology.
Of 41 control subjects without LV-GCA, none had a positive ultrasound, whereas 36 of 45 LV-GCA patients had a positive ultrasound, which gives the test a specificity of 100% and sensitivity of 80%, Dr. Nielsen of Aarhus (Denmark) University Hospital said during a press briefing at the meeting.
Ultrasound was performed on the carotid artery in the neck and axillary arteries under the arm, which are easily accessible by ultrasound.
“These patients also had temporal arteries evaluated, and if we included this evaluation in the diagnostic performance, it showed a sensitivity of 91%,” she noted, explaining that temporal artery ultrasound alone conferred 71% sensitivity. “So it actually helped us identify more GCA patients.”
The study subjects were adults with suspected GCA. Inclusion criteria included age of at least 50 years, C-reactive protein of more than 15 mg/L or erythrocyte sedimentation rate of more than 40 mm, and either cranial symptoms, new-onset limb claudication, protracted constitutional symptoms, or polymyalgia rheumatica (PMR) symptoms. Patients were excluded if they had recent or ongoing glucocorticoid or disease-modifying antirheumatic drug treatment, a previous GCA or PMR diagnosis, or a large vessel inflammation that mimicked LV-GCA.
Clinical evaluations and imaging tests were performed prior to treatment initiation. The reference diagnosis was a clinical diagnosis of GCA and a positive 18F-FDG PET/CT scan, Dr Nielsen said, adding that ultrasound examinations were performed by experienced sonographers who were blinded to the PET/CT results.
Of the 86 patients included, 45 had LV-GCA with or without concomitant cranial GCA, 10 had isolated cranial GCA, 21 had PMR, and 10 were diagnosed with other diseases. The patients found to not have LV-GCA were considered control subjects.
The findings are notable because, while PET is considered the gold standard, it is very expensive and not always readily available, Dr. Nielsen said.
Additionally, while cranial-GCA patients generally present with symptoms such as headache, jaw claudication, and visual disturbances that are considered typical for GCA, LV-GCA patients rarely present with these symptoms.
Rather, these LV-GCA patients tend to present with constitutional symptoms mimicking infection or cancer, and they undergo extensive examination programs before the diagnosis is established. For this reason, diagnosis is often delayed for several months in LV-GCA patients until late in the disease course.
“During this time they often experience a decline in physical ability,” she said. “So in this disease subset of patients with GCA, there’s an unmet need for earlier recognition and earlier diagnosis.”
New recommendations from the European League Against Rheumatism call for early diagnostic imaging in all cases of suspected GCA, she added, noting that, for cranial-GCA symptoms, temporal artery ultrasound is recommended first line, but for those who present without cranial symptoms, no particular imaging modality is recommended because of a lack of comparative and diagnostic accuracy data in LV-GCA.
Biopsy has traditionally been used in these cases, but now imaging can be substituted – and vascular ultrasound is an attractive first-line option given its affordability and availability.
Indeed, the current findings support its use in this setting, she said.
“We think that these results indicate that ultrasound should not only be the first-line imaging test in patients presenting with cranial symptoms, but also in patients suspected of GCA presenting with constitutional symptoms, and if this examination is included in the standard examinations in fast-track clinics, it may overcome the delay in diagnosis and the patients can be treated earlier. It may also spare the unneeded examinations performed in these patients,” she concluded.
Dr. Nielsen disclosed a relationship with Roche.
SOURCE: Nielsen B et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 2905.
CHICAGO – Vascular ultrasound showed high sensitivity and specificity for diagnosing large-vessel giant-cell arteritis (LV-GCA) in a prospective study of patients with suspected new-onset disease.
The findings highlight the value of vascular ultrasound – in the hands of experienced sonographers – as a first-line imaging test in this setting, Berit Dalsgaard Nielsen, MD, reported at the annual meeting of the American College of Rheumatology.
Of 41 control subjects without LV-GCA, none had a positive ultrasound, whereas 36 of 45 LV-GCA patients had a positive ultrasound, which gives the test a specificity of 100% and sensitivity of 80%, Dr. Nielsen of Aarhus (Denmark) University Hospital said during a press briefing at the meeting.
Ultrasound was performed on the carotid artery in the neck and axillary arteries under the arm, which are easily accessible by ultrasound.
“These patients also had temporal arteries evaluated, and if we included this evaluation in the diagnostic performance, it showed a sensitivity of 91%,” she noted, explaining that temporal artery ultrasound alone conferred 71% sensitivity. “So it actually helped us identify more GCA patients.”
The study subjects were adults with suspected GCA. Inclusion criteria included age of at least 50 years, C-reactive protein of more than 15 mg/L or erythrocyte sedimentation rate of more than 40 mm, and either cranial symptoms, new-onset limb claudication, protracted constitutional symptoms, or polymyalgia rheumatica (PMR) symptoms. Patients were excluded if they had recent or ongoing glucocorticoid or disease-modifying antirheumatic drug treatment, a previous GCA or PMR diagnosis, or a large vessel inflammation that mimicked LV-GCA.
Clinical evaluations and imaging tests were performed prior to treatment initiation. The reference diagnosis was a clinical diagnosis of GCA and a positive 18F-FDG PET/CT scan, Dr Nielsen said, adding that ultrasound examinations were performed by experienced sonographers who were blinded to the PET/CT results.
Of the 86 patients included, 45 had LV-GCA with or without concomitant cranial GCA, 10 had isolated cranial GCA, 21 had PMR, and 10 were diagnosed with other diseases. The patients found to not have LV-GCA were considered control subjects.
The findings are notable because, while PET is considered the gold standard, it is very expensive and not always readily available, Dr. Nielsen said.
Additionally, while cranial-GCA patients generally present with symptoms such as headache, jaw claudication, and visual disturbances that are considered typical for GCA, LV-GCA patients rarely present with these symptoms.
Rather, these LV-GCA patients tend to present with constitutional symptoms mimicking infection or cancer, and they undergo extensive examination programs before the diagnosis is established. For this reason, diagnosis is often delayed for several months in LV-GCA patients until late in the disease course.
“During this time they often experience a decline in physical ability,” she said. “So in this disease subset of patients with GCA, there’s an unmet need for earlier recognition and earlier diagnosis.”
New recommendations from the European League Against Rheumatism call for early diagnostic imaging in all cases of suspected GCA, she added, noting that, for cranial-GCA symptoms, temporal artery ultrasound is recommended first line, but for those who present without cranial symptoms, no particular imaging modality is recommended because of a lack of comparative and diagnostic accuracy data in LV-GCA.
Biopsy has traditionally been used in these cases, but now imaging can be substituted – and vascular ultrasound is an attractive first-line option given its affordability and availability.
Indeed, the current findings support its use in this setting, she said.
“We think that these results indicate that ultrasound should not only be the first-line imaging test in patients presenting with cranial symptoms, but also in patients suspected of GCA presenting with constitutional symptoms, and if this examination is included in the standard examinations in fast-track clinics, it may overcome the delay in diagnosis and the patients can be treated earlier. It may also spare the unneeded examinations performed in these patients,” she concluded.
Dr. Nielsen disclosed a relationship with Roche.
SOURCE: Nielsen B et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 2905.
CHICAGO – Vascular ultrasound showed high sensitivity and specificity for diagnosing large-vessel giant-cell arteritis (LV-GCA) in a prospective study of patients with suspected new-onset disease.
The findings highlight the value of vascular ultrasound – in the hands of experienced sonographers – as a first-line imaging test in this setting, Berit Dalsgaard Nielsen, MD, reported at the annual meeting of the American College of Rheumatology.
Of 41 control subjects without LV-GCA, none had a positive ultrasound, whereas 36 of 45 LV-GCA patients had a positive ultrasound, which gives the test a specificity of 100% and sensitivity of 80%, Dr. Nielsen of Aarhus (Denmark) University Hospital said during a press briefing at the meeting.
Ultrasound was performed on the carotid artery in the neck and axillary arteries under the arm, which are easily accessible by ultrasound.
“These patients also had temporal arteries evaluated, and if we included this evaluation in the diagnostic performance, it showed a sensitivity of 91%,” she noted, explaining that temporal artery ultrasound alone conferred 71% sensitivity. “So it actually helped us identify more GCA patients.”
The study subjects were adults with suspected GCA. Inclusion criteria included age of at least 50 years, C-reactive protein of more than 15 mg/L or erythrocyte sedimentation rate of more than 40 mm, and either cranial symptoms, new-onset limb claudication, protracted constitutional symptoms, or polymyalgia rheumatica (PMR) symptoms. Patients were excluded if they had recent or ongoing glucocorticoid or disease-modifying antirheumatic drug treatment, a previous GCA or PMR diagnosis, or a large vessel inflammation that mimicked LV-GCA.
Clinical evaluations and imaging tests were performed prior to treatment initiation. The reference diagnosis was a clinical diagnosis of GCA and a positive 18F-FDG PET/CT scan, Dr Nielsen said, adding that ultrasound examinations were performed by experienced sonographers who were blinded to the PET/CT results.
Of the 86 patients included, 45 had LV-GCA with or without concomitant cranial GCA, 10 had isolated cranial GCA, 21 had PMR, and 10 were diagnosed with other diseases. The patients found to not have LV-GCA were considered control subjects.
The findings are notable because, while PET is considered the gold standard, it is very expensive and not always readily available, Dr. Nielsen said.
Additionally, while cranial-GCA patients generally present with symptoms such as headache, jaw claudication, and visual disturbances that are considered typical for GCA, LV-GCA patients rarely present with these symptoms.
Rather, these LV-GCA patients tend to present with constitutional symptoms mimicking infection or cancer, and they undergo extensive examination programs before the diagnosis is established. For this reason, diagnosis is often delayed for several months in LV-GCA patients until late in the disease course.
“During this time they often experience a decline in physical ability,” she said. “So in this disease subset of patients with GCA, there’s an unmet need for earlier recognition and earlier diagnosis.”
New recommendations from the European League Against Rheumatism call for early diagnostic imaging in all cases of suspected GCA, she added, noting that, for cranial-GCA symptoms, temporal artery ultrasound is recommended first line, but for those who present without cranial symptoms, no particular imaging modality is recommended because of a lack of comparative and diagnostic accuracy data in LV-GCA.
Biopsy has traditionally been used in these cases, but now imaging can be substituted – and vascular ultrasound is an attractive first-line option given its affordability and availability.
Indeed, the current findings support its use in this setting, she said.
“We think that these results indicate that ultrasound should not only be the first-line imaging test in patients presenting with cranial symptoms, but also in patients suspected of GCA presenting with constitutional symptoms, and if this examination is included in the standard examinations in fast-track clinics, it may overcome the delay in diagnosis and the patients can be treated earlier. It may also spare the unneeded examinations performed in these patients,” she concluded.
Dr. Nielsen disclosed a relationship with Roche.
SOURCE: Nielsen B et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 2905.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: Vascular ultrasound is reasonable for first-line maging of suspected LV-GCA.
Major finding: Vascular ultrasound had 100% specificity and 80% sensitivity.
Study details: A prospective study of 86 patients.
Disclosures: Dr. Nielsen disclosed a relationship with Roche.
Source: Nielsen BD et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 2905.
MRI Measure Distinguishes MS From Migraine
Gray matter–white matter contrast ratio may be lowest in progressive MS, compared with other MS phenotypes.
BERLIN—Gray matter–white matter (GM/WM) contrast ratio on T1 magnetization prepared rapid gradient echo (MPRAGE) is sensitive to multiple sclerosis (MS), but not to migraine pathology, according to research presented at ECTRIMS 2018. The diagnostic and prognostic value of this MRI marker could be subjects for future investigations, said the study authors.
Researchers have suggested that MRI brain T1 GM/WM contrast may be a marker of neurodegeneration in Alzheimer’s disease. Whether this contrast has diagnostic value in MS remains unknown, however. Tina Mitrovic, a research assistant at the University of Basel in Switzerland, and colleagues conducted a study to compare the T1 GM/WM contrast in patients with MS, migraineurs, and healthy controls. They also investigated whether the contrast was associated with disability outcomes in MS.
Ms. Mitrovic and colleagues analyzed precontrast 3D MPRAGE data from two independent cohorts. Cohort A was examined with 1.5-T MRI and included 124 patients with MS (65% relapsing-remitting MS; 66% female; mean disease duration, 13 years; median Expanded Disability Status Scale [EDSS] score, 2.5) and 20 healthy controls. Cohort B was examined with 3-T MRI and included 43 patients with relapsing-remitting MS (56% female; mean disease duration, three years; median EDSS score, 1.5), 19 migraineurs (47% with aura and 54% with WM abnormalities on T2-weighted scans), and 37 healthy controls.
WM lesions were segmented manually on T1- and T2-weighted images. GM and WM were segmented using Statistical Parametric Mapping 12. The researchers calculated GM and WM fractions and thresholded GM and WM masks by 95%. T1 GM/WM contrast ratio was calculated as [mean T1 intensity of WM minus mean T1 intensity of GM] divided by [mean T1 intensity of WM plus mean T1 intensity of GM]. Clinical outcomes included EDSS and Multiple Sclerosis Functional Composite (MSFC) scores. Associations between variables were investigated using univariate and multivariate general linear models. Results were bootstrapped.
Mean T1 GM/WM contrast ratio was lower in patients with MS than healthy controls in both cohorts. Furthermore, T1 GM/WM contrast ratio was lower in patients with MS than migraineurs, but the researchers observed no difference between migraine and healthy controls. Mean T1 GM/WM contrast was lowest in patients with progressive MS (11.7%), followed by those with relapsing-remitting MS or clinically isolated syndrome (12.2%) and healthy controls (12.8%). In MS, T1 GM/WM contrast was associated with WM lesion volume, disease duration, EDSS, and MSFC. A multivariable analysis with T1 GM/WM contrast as a dependent variable and age, disease duration, T1 and T2 WM lesion volume, and GM and WM fraction as independent variables indicated that GM fraction and T2 lesion volume were independently associated with T1 GM/WM contrast ratio.
Gray matter–white matter contrast ratio may be lowest in progressive MS, compared with other MS phenotypes.
Gray matter–white matter contrast ratio may be lowest in progressive MS, compared with other MS phenotypes.
BERLIN—Gray matter–white matter (GM/WM) contrast ratio on T1 magnetization prepared rapid gradient echo (MPRAGE) is sensitive to multiple sclerosis (MS), but not to migraine pathology, according to research presented at ECTRIMS 2018. The diagnostic and prognostic value of this MRI marker could be subjects for future investigations, said the study authors.
Researchers have suggested that MRI brain T1 GM/WM contrast may be a marker of neurodegeneration in Alzheimer’s disease. Whether this contrast has diagnostic value in MS remains unknown, however. Tina Mitrovic, a research assistant at the University of Basel in Switzerland, and colleagues conducted a study to compare the T1 GM/WM contrast in patients with MS, migraineurs, and healthy controls. They also investigated whether the contrast was associated with disability outcomes in MS.
Ms. Mitrovic and colleagues analyzed precontrast 3D MPRAGE data from two independent cohorts. Cohort A was examined with 1.5-T MRI and included 124 patients with MS (65% relapsing-remitting MS; 66% female; mean disease duration, 13 years; median Expanded Disability Status Scale [EDSS] score, 2.5) and 20 healthy controls. Cohort B was examined with 3-T MRI and included 43 patients with relapsing-remitting MS (56% female; mean disease duration, three years; median EDSS score, 1.5), 19 migraineurs (47% with aura and 54% with WM abnormalities on T2-weighted scans), and 37 healthy controls.
WM lesions were segmented manually on T1- and T2-weighted images. GM and WM were segmented using Statistical Parametric Mapping 12. The researchers calculated GM and WM fractions and thresholded GM and WM masks by 95%. T1 GM/WM contrast ratio was calculated as [mean T1 intensity of WM minus mean T1 intensity of GM] divided by [mean T1 intensity of WM plus mean T1 intensity of GM]. Clinical outcomes included EDSS and Multiple Sclerosis Functional Composite (MSFC) scores. Associations between variables were investigated using univariate and multivariate general linear models. Results were bootstrapped.
Mean T1 GM/WM contrast ratio was lower in patients with MS than healthy controls in both cohorts. Furthermore, T1 GM/WM contrast ratio was lower in patients with MS than migraineurs, but the researchers observed no difference between migraine and healthy controls. Mean T1 GM/WM contrast was lowest in patients with progressive MS (11.7%), followed by those with relapsing-remitting MS or clinically isolated syndrome (12.2%) and healthy controls (12.8%). In MS, T1 GM/WM contrast was associated with WM lesion volume, disease duration, EDSS, and MSFC. A multivariable analysis with T1 GM/WM contrast as a dependent variable and age, disease duration, T1 and T2 WM lesion volume, and GM and WM fraction as independent variables indicated that GM fraction and T2 lesion volume were independently associated with T1 GM/WM contrast ratio.
BERLIN—Gray matter–white matter (GM/WM) contrast ratio on T1 magnetization prepared rapid gradient echo (MPRAGE) is sensitive to multiple sclerosis (MS), but not to migraine pathology, according to research presented at ECTRIMS 2018. The diagnostic and prognostic value of this MRI marker could be subjects for future investigations, said the study authors.
Researchers have suggested that MRI brain T1 GM/WM contrast may be a marker of neurodegeneration in Alzheimer’s disease. Whether this contrast has diagnostic value in MS remains unknown, however. Tina Mitrovic, a research assistant at the University of Basel in Switzerland, and colleagues conducted a study to compare the T1 GM/WM contrast in patients with MS, migraineurs, and healthy controls. They also investigated whether the contrast was associated with disability outcomes in MS.
Ms. Mitrovic and colleagues analyzed precontrast 3D MPRAGE data from two independent cohorts. Cohort A was examined with 1.5-T MRI and included 124 patients with MS (65% relapsing-remitting MS; 66% female; mean disease duration, 13 years; median Expanded Disability Status Scale [EDSS] score, 2.5) and 20 healthy controls. Cohort B was examined with 3-T MRI and included 43 patients with relapsing-remitting MS (56% female; mean disease duration, three years; median EDSS score, 1.5), 19 migraineurs (47% with aura and 54% with WM abnormalities on T2-weighted scans), and 37 healthy controls.
WM lesions were segmented manually on T1- and T2-weighted images. GM and WM were segmented using Statistical Parametric Mapping 12. The researchers calculated GM and WM fractions and thresholded GM and WM masks by 95%. T1 GM/WM contrast ratio was calculated as [mean T1 intensity of WM minus mean T1 intensity of GM] divided by [mean T1 intensity of WM plus mean T1 intensity of GM]. Clinical outcomes included EDSS and Multiple Sclerosis Functional Composite (MSFC) scores. Associations between variables were investigated using univariate and multivariate general linear models. Results were bootstrapped.
Mean T1 GM/WM contrast ratio was lower in patients with MS than healthy controls in both cohorts. Furthermore, T1 GM/WM contrast ratio was lower in patients with MS than migraineurs, but the researchers observed no difference between migraine and healthy controls. Mean T1 GM/WM contrast was lowest in patients with progressive MS (11.7%), followed by those with relapsing-remitting MS or clinically isolated syndrome (12.2%) and healthy controls (12.8%). In MS, T1 GM/WM contrast was associated with WM lesion volume, disease duration, EDSS, and MSFC. A multivariable analysis with T1 GM/WM contrast as a dependent variable and age, disease duration, T1 and T2 WM lesion volume, and GM and WM fraction as independent variables indicated that GM fraction and T2 lesion volume were independently associated with T1 GM/WM contrast ratio.
KPNA2 is “novel prognostic factor” in RCC
High expression of karyopherin alpha 2 (KPNA2), a carrier protein that helps shuttle cancer-associated proteins from the nucleus to the cytoplasm, is an adverse prognostic factor in patients with clear cell or papillary renal cell carcinoma (RCC), according to a retrospective cohort study.
Senior author Glen Kristiansen, MD, director of the Institute of Pathology at the University Hospital Bonn (Germany), and his colleagues assessed tumor levels of KPNA2 protein by immunohistochemistry in 240 clinic patients with RCC (217 with clear cell histology, 23 with papillary histology). They also assessed tumor levels of KPNA2 mRNA in 771 patients with RCC (481 with clear cell histology, 290 with papillary cell histology) using publicly available gene expression data from the Cancer Genome Atlas (CGA).
Overall, 19% of the clinic patients’ tumors showed high expression of KPNA2 protein, according to results reported in Clinical Genitourinary Cancer. In addition, 26% of the CGA patients’ tumors showed high expression of KPNA2 mRNA.
Among patients with clear cell RCC, those with high tumor levels of KPNA2 protein survived roughly half as long as counterparts with low levels or none (74 months vs. 171 months); the difference was significant in univariate analysis (P = .012) but not in multivariate analysis that included well-known prognostic factors (HR, 1.491; P = .237). On the other hand, those with high tumor levels of KPNA2 mRNA had an elevated risk of death in both univariate analysis (HR, 2.31; P less than .001) and multivariate analysis (HR, 1.45; P = .035).
Among patients with papillary RCC, tumor levels of KPNA2 protein were not significantly associated with survival. However, those with high tumor levels of KPNA2 mRNA had a sharply elevated risk of death in both univariate analysis (HR, 9.7; P less than .001) and multivariate analysis (HR, 6.2; P = .004).
KPNA2 expression “represents a novel prognostic factor in these subtypes of RCC,” concluded Dr. Kristiansen and his coinvestigators. Therefore, this biomarker “could be used to stratify risk groups within RCC.” Collectively, the study’s findings suggest that KPNA2 is involved in both the pathogenesis and the progression of RCC. Given evidence that it helps transport p53 and fibroblast growth factor 2 at least in tumors with clear cell histology, “investigation of the nucleocytoplasmic transport through KPNA2 in [clear cell] RCC is an important task for future studies to better understand the link between elevated KPNA2 expression and altered biological processes like proliferation, cell growth, migration, invasion and tumor formation in RCC. In addition, examination of other members of [the] karyopherin-alpha family could elucidate the role of the nucleocytoplasmic transport system in pathogenesis of RCC.”
The authors reported that they had no conflict of interests.
SOURCE: Kristiansen G et al. Clin Genitourin Cancer. 2018 Oct 22. doi: 10.1016/j.clgc.2018.10.008.
High expression of karyopherin alpha 2 (KPNA2), a carrier protein that helps shuttle cancer-associated proteins from the nucleus to the cytoplasm, is an adverse prognostic factor in patients with clear cell or papillary renal cell carcinoma (RCC), according to a retrospective cohort study.
Senior author Glen Kristiansen, MD, director of the Institute of Pathology at the University Hospital Bonn (Germany), and his colleagues assessed tumor levels of KPNA2 protein by immunohistochemistry in 240 clinic patients with RCC (217 with clear cell histology, 23 with papillary histology). They also assessed tumor levels of KPNA2 mRNA in 771 patients with RCC (481 with clear cell histology, 290 with papillary cell histology) using publicly available gene expression data from the Cancer Genome Atlas (CGA).
Overall, 19% of the clinic patients’ tumors showed high expression of KPNA2 protein, according to results reported in Clinical Genitourinary Cancer. In addition, 26% of the CGA patients’ tumors showed high expression of KPNA2 mRNA.
Among patients with clear cell RCC, those with high tumor levels of KPNA2 protein survived roughly half as long as counterparts with low levels or none (74 months vs. 171 months); the difference was significant in univariate analysis (P = .012) but not in multivariate analysis that included well-known prognostic factors (HR, 1.491; P = .237). On the other hand, those with high tumor levels of KPNA2 mRNA had an elevated risk of death in both univariate analysis (HR, 2.31; P less than .001) and multivariate analysis (HR, 1.45; P = .035).
Among patients with papillary RCC, tumor levels of KPNA2 protein were not significantly associated with survival. However, those with high tumor levels of KPNA2 mRNA had a sharply elevated risk of death in both univariate analysis (HR, 9.7; P less than .001) and multivariate analysis (HR, 6.2; P = .004).
KPNA2 expression “represents a novel prognostic factor in these subtypes of RCC,” concluded Dr. Kristiansen and his coinvestigators. Therefore, this biomarker “could be used to stratify risk groups within RCC.” Collectively, the study’s findings suggest that KPNA2 is involved in both the pathogenesis and the progression of RCC. Given evidence that it helps transport p53 and fibroblast growth factor 2 at least in tumors with clear cell histology, “investigation of the nucleocytoplasmic transport through KPNA2 in [clear cell] RCC is an important task for future studies to better understand the link between elevated KPNA2 expression and altered biological processes like proliferation, cell growth, migration, invasion and tumor formation in RCC. In addition, examination of other members of [the] karyopherin-alpha family could elucidate the role of the nucleocytoplasmic transport system in pathogenesis of RCC.”
The authors reported that they had no conflict of interests.
SOURCE: Kristiansen G et al. Clin Genitourin Cancer. 2018 Oct 22. doi: 10.1016/j.clgc.2018.10.008.
High expression of karyopherin alpha 2 (KPNA2), a carrier protein that helps shuttle cancer-associated proteins from the nucleus to the cytoplasm, is an adverse prognostic factor in patients with clear cell or papillary renal cell carcinoma (RCC), according to a retrospective cohort study.
Senior author Glen Kristiansen, MD, director of the Institute of Pathology at the University Hospital Bonn (Germany), and his colleagues assessed tumor levels of KPNA2 protein by immunohistochemistry in 240 clinic patients with RCC (217 with clear cell histology, 23 with papillary histology). They also assessed tumor levels of KPNA2 mRNA in 771 patients with RCC (481 with clear cell histology, 290 with papillary cell histology) using publicly available gene expression data from the Cancer Genome Atlas (CGA).
Overall, 19% of the clinic patients’ tumors showed high expression of KPNA2 protein, according to results reported in Clinical Genitourinary Cancer. In addition, 26% of the CGA patients’ tumors showed high expression of KPNA2 mRNA.
Among patients with clear cell RCC, those with high tumor levels of KPNA2 protein survived roughly half as long as counterparts with low levels or none (74 months vs. 171 months); the difference was significant in univariate analysis (P = .012) but not in multivariate analysis that included well-known prognostic factors (HR, 1.491; P = .237). On the other hand, those with high tumor levels of KPNA2 mRNA had an elevated risk of death in both univariate analysis (HR, 2.31; P less than .001) and multivariate analysis (HR, 1.45; P = .035).
Among patients with papillary RCC, tumor levels of KPNA2 protein were not significantly associated with survival. However, those with high tumor levels of KPNA2 mRNA had a sharply elevated risk of death in both univariate analysis (HR, 9.7; P less than .001) and multivariate analysis (HR, 6.2; P = .004).
KPNA2 expression “represents a novel prognostic factor in these subtypes of RCC,” concluded Dr. Kristiansen and his coinvestigators. Therefore, this biomarker “could be used to stratify risk groups within RCC.” Collectively, the study’s findings suggest that KPNA2 is involved in both the pathogenesis and the progression of RCC. Given evidence that it helps transport p53 and fibroblast growth factor 2 at least in tumors with clear cell histology, “investigation of the nucleocytoplasmic transport through KPNA2 in [clear cell] RCC is an important task for future studies to better understand the link between elevated KPNA2 expression and altered biological processes like proliferation, cell growth, migration, invasion and tumor formation in RCC. In addition, examination of other members of [the] karyopherin-alpha family could elucidate the role of the nucleocytoplasmic transport system in pathogenesis of RCC.”
The authors reported that they had no conflict of interests.
SOURCE: Kristiansen G et al. Clin Genitourin Cancer. 2018 Oct 22. doi: 10.1016/j.clgc.2018.10.008.
FROM CLINICAL GENITOURINARY CANCER
Key clinical point:
Major finding: Survival was poorer for patients with clear cell RCC having high KPNA2 protein levels (74 vs. 171 months) or mRNA levels (hazard ratio for death, 1.45) and for patients with papillary RCC having high KPNA2 mRNA levels (HR, 6.2).
Study details: A retrospective dual cohort study of 240 clinic patients and 771 Cancer Genome Atlas patients.
Disclosures: The authors reported that they had no conflict of interests.
Source: Kristiansen G et al. Clin Genitourin Cancer. 2018 Oct 22. doi: 10.1016/j.clgc.2018.10.008.
High Systemic Inflammation at Midlife Portends Cognitive Changes in Later Life
Systemic inflammation may have an early pathogenic role in the cognitive decline that occurs before older adulthood.
ATLANTA—Individuals with higher levels of systemic inflammation during midlife experience greater rates of cognitive decline over the subsequent 20-year period, according to results from a long-term analysis presented at the 143rd Annual Meeting of the American Neurological Association.
“There is considerable evidence suggesting that abnormal immune functioning and inflammation may influence cognitive functioning and promote dementia,” said lead study author Keenan Walker, PhD, a clinical neuropsychology postdoctoral fellow at the Johns Hopkins University School of Medicine in Baltimore. “For example, several studies have found higher levels of inflammatory markers in the blood and CSF of patients with dementia, compared to nondemented individuals of a comparable age. What is less clear, however, is whether inflammation actually promotes cognitive decline or occurs simply as a result of the brain changes underlying dementia.”
To help answer this question, Dr. Walker and his colleagues evaluated blood biomarkers of inflammation in 12,727 middle-aged participants during visits one and two of the Atherosclerosis Risk in Communities (ARIC) study, a prospective epidemiologic analysis conducted in four US communities and funded by the National Heart, Lung, and Blood Institute. Visit 1 occurred between 1987 and 1989, and Visit 2 took place between 1990 and 1992. The researchers related these markers to cognitive change over the subsequent decades. Specifically, they used four biomarkers (ie, fibrinogen, white blood cell count, von Willebrand factor, and Factor VIII) to create an inflammation composite score at Visit 1 and measured C-reactive protein (CRP) at Visit 2. Next, they used measures of memory, executive function, and language to assess cognition over three visits spanning 20 years.
The average age of study participants at the first cognitive assessment was 57, 56% were women, and 21% were black. After controlling for differences in demographic variables, vascular risk factors, and comorbidities, the researchers observed that each standard deviation (SD) increase in the midlife inflammation composite score was associated with an additional 20-year global cognitive decline of –0.035 SD. They found a similar association between each SD higher midlife CRP level and additional 20-year decline in global cognition (–0.038 SD). In addition, study participants with a midlife inflammation composite score in the top quartile had a 7.6% steeper decline in global cognition, compared with participants in the lowest quartile. A similar association was observed for CRP.
“We were surprised at what we found when we looked at inflammation’s effect on individual cognitive domains,” said Dr. Walker. “Specifically, we found that inflammation was associated with declines in memory, but not declines in other domains, such as executive function or language. One possible interpretation for these findings is that inflammation may selectively influence brain regions such as the hippocampus that are necessary for memory consolidation and are also most vulnerable to Alzheimer’s disease.” He added that the current findings “provide support for the idea that systemic inflammation may have an early pathogenic role in the cognitive decline that occurs in the decades leading up to older adulthood.”
Dr. Walker acknowledged certain limitations of the study, including the attrition of participants because of deaths and dropouts over the 20-year follow-up period. “Because high levels of inflammation and comorbid disease are related to death and risk of study dropout, the sample of participants who completed the entirety of the study may represent a group that is healthier overall than the general population,” he said. “However, we took several steps to reduce any potential attrition bias using advanced statistical techniques.”
—Doug Brunk
Systemic inflammation may have an early pathogenic role in the cognitive decline that occurs before older adulthood.
Systemic inflammation may have an early pathogenic role in the cognitive decline that occurs before older adulthood.
ATLANTA—Individuals with higher levels of systemic inflammation during midlife experience greater rates of cognitive decline over the subsequent 20-year period, according to results from a long-term analysis presented at the 143rd Annual Meeting of the American Neurological Association.
“There is considerable evidence suggesting that abnormal immune functioning and inflammation may influence cognitive functioning and promote dementia,” said lead study author Keenan Walker, PhD, a clinical neuropsychology postdoctoral fellow at the Johns Hopkins University School of Medicine in Baltimore. “For example, several studies have found higher levels of inflammatory markers in the blood and CSF of patients with dementia, compared to nondemented individuals of a comparable age. What is less clear, however, is whether inflammation actually promotes cognitive decline or occurs simply as a result of the brain changes underlying dementia.”
To help answer this question, Dr. Walker and his colleagues evaluated blood biomarkers of inflammation in 12,727 middle-aged participants during visits one and two of the Atherosclerosis Risk in Communities (ARIC) study, a prospective epidemiologic analysis conducted in four US communities and funded by the National Heart, Lung, and Blood Institute. Visit 1 occurred between 1987 and 1989, and Visit 2 took place between 1990 and 1992. The researchers related these markers to cognitive change over the subsequent decades. Specifically, they used four biomarkers (ie, fibrinogen, white blood cell count, von Willebrand factor, and Factor VIII) to create an inflammation composite score at Visit 1 and measured C-reactive protein (CRP) at Visit 2. Next, they used measures of memory, executive function, and language to assess cognition over three visits spanning 20 years.
The average age of study participants at the first cognitive assessment was 57, 56% were women, and 21% were black. After controlling for differences in demographic variables, vascular risk factors, and comorbidities, the researchers observed that each standard deviation (SD) increase in the midlife inflammation composite score was associated with an additional 20-year global cognitive decline of –0.035 SD. They found a similar association between each SD higher midlife CRP level and additional 20-year decline in global cognition (–0.038 SD). In addition, study participants with a midlife inflammation composite score in the top quartile had a 7.6% steeper decline in global cognition, compared with participants in the lowest quartile. A similar association was observed for CRP.
“We were surprised at what we found when we looked at inflammation’s effect on individual cognitive domains,” said Dr. Walker. “Specifically, we found that inflammation was associated with declines in memory, but not declines in other domains, such as executive function or language. One possible interpretation for these findings is that inflammation may selectively influence brain regions such as the hippocampus that are necessary for memory consolidation and are also most vulnerable to Alzheimer’s disease.” He added that the current findings “provide support for the idea that systemic inflammation may have an early pathogenic role in the cognitive decline that occurs in the decades leading up to older adulthood.”
Dr. Walker acknowledged certain limitations of the study, including the attrition of participants because of deaths and dropouts over the 20-year follow-up period. “Because high levels of inflammation and comorbid disease are related to death and risk of study dropout, the sample of participants who completed the entirety of the study may represent a group that is healthier overall than the general population,” he said. “However, we took several steps to reduce any potential attrition bias using advanced statistical techniques.”
—Doug Brunk
ATLANTA—Individuals with higher levels of systemic inflammation during midlife experience greater rates of cognitive decline over the subsequent 20-year period, according to results from a long-term analysis presented at the 143rd Annual Meeting of the American Neurological Association.
“There is considerable evidence suggesting that abnormal immune functioning and inflammation may influence cognitive functioning and promote dementia,” said lead study author Keenan Walker, PhD, a clinical neuropsychology postdoctoral fellow at the Johns Hopkins University School of Medicine in Baltimore. “For example, several studies have found higher levels of inflammatory markers in the blood and CSF of patients with dementia, compared to nondemented individuals of a comparable age. What is less clear, however, is whether inflammation actually promotes cognitive decline or occurs simply as a result of the brain changes underlying dementia.”
To help answer this question, Dr. Walker and his colleagues evaluated blood biomarkers of inflammation in 12,727 middle-aged participants during visits one and two of the Atherosclerosis Risk in Communities (ARIC) study, a prospective epidemiologic analysis conducted in four US communities and funded by the National Heart, Lung, and Blood Institute. Visit 1 occurred between 1987 and 1989, and Visit 2 took place between 1990 and 1992. The researchers related these markers to cognitive change over the subsequent decades. Specifically, they used four biomarkers (ie, fibrinogen, white blood cell count, von Willebrand factor, and Factor VIII) to create an inflammation composite score at Visit 1 and measured C-reactive protein (CRP) at Visit 2. Next, they used measures of memory, executive function, and language to assess cognition over three visits spanning 20 years.
The average age of study participants at the first cognitive assessment was 57, 56% were women, and 21% were black. After controlling for differences in demographic variables, vascular risk factors, and comorbidities, the researchers observed that each standard deviation (SD) increase in the midlife inflammation composite score was associated with an additional 20-year global cognitive decline of –0.035 SD. They found a similar association between each SD higher midlife CRP level and additional 20-year decline in global cognition (–0.038 SD). In addition, study participants with a midlife inflammation composite score in the top quartile had a 7.6% steeper decline in global cognition, compared with participants in the lowest quartile. A similar association was observed for CRP.
“We were surprised at what we found when we looked at inflammation’s effect on individual cognitive domains,” said Dr. Walker. “Specifically, we found that inflammation was associated with declines in memory, but not declines in other domains, such as executive function or language. One possible interpretation for these findings is that inflammation may selectively influence brain regions such as the hippocampus that are necessary for memory consolidation and are also most vulnerable to Alzheimer’s disease.” He added that the current findings “provide support for the idea that systemic inflammation may have an early pathogenic role in the cognitive decline that occurs in the decades leading up to older adulthood.”
Dr. Walker acknowledged certain limitations of the study, including the attrition of participants because of deaths and dropouts over the 20-year follow-up period. “Because high levels of inflammation and comorbid disease are related to death and risk of study dropout, the sample of participants who completed the entirety of the study may represent a group that is healthier overall than the general population,” he said. “However, we took several steps to reduce any potential attrition bias using advanced statistical techniques.”
—Doug Brunk
Debunking Psoriasis Myths: Remove Psoriasis Scales Gently
Myth: Pick Psoriasis Scales to Remove Them
Patients may be inclined to pick psoriasis scales that appear in noticeable areas or on the scalp. However, they should be counseled to avoid this practice, which could cause an infection. Instead, Dr. Steven Feldman (Winston-Salem, North Carolina) suggests putting on an ointment or oil-like medication to soften the scale. “Almost any kind of moisturizer will change the reflective properties of the scale so that you don’t see the scale,” he advised. He also suggested descaling agents such as topical salicylic acid or lactic acid. His patient education video is available on the American Academy of Dermatology website should you wish to direct your patients to it.
Because salicylic acid is a keratolytic (or peeling agent), it works by causing the outer layer of skin to shed. When applied topically, it helps to soften and lift psoriasis scales. Coal tar over-the-counter products also can be used for the same purpose. The over-the-counter product guide from the National Psoriasis Foundation is a valuable resource to share with patients.
Expert Commentary
I agree that it is very important to treat scale very gently. In addition to risk for infection, picking and traumatizing scale can lead to worsening of the psoriasis. This is known as the Koebner phenomenon. The phenomenon was first described by Heinrich Koebner in 1876 as the formation of psoriatic lesions in uninvolved skin of patients with psoriasis after cutaneous trauma. This isomorphic phenomenon is now known to involve numerous diseases, among them vitiligo, lichen planus, and Darier disease.
—Jeffrey M. Weinberg, MD (New York, New York)
Feldman S. How should I remove psoriasis scale? American Academy of Dermatology website. https://www.aad.org/public/diseases/scaly-skin/psoriasis/tips-for-managing-psoriasis/how-should-i-remove-psoriasis-scale. Accessed October 31, 2018.
National Psoriasis Foundation. Over-the-counter products. https://www.aad.org/public/diseases/scaly-skin/psoriasis/tips-for-managing-psoriasis/how-should-i-remove-psoriasis-scale. Published June 2017. Accessed October 31, 2018.
Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
Myth: Pick Psoriasis Scales to Remove Them
Patients may be inclined to pick psoriasis scales that appear in noticeable areas or on the scalp. However, they should be counseled to avoid this practice, which could cause an infection. Instead, Dr. Steven Feldman (Winston-Salem, North Carolina) suggests putting on an ointment or oil-like medication to soften the scale. “Almost any kind of moisturizer will change the reflective properties of the scale so that you don’t see the scale,” he advised. He also suggested descaling agents such as topical salicylic acid or lactic acid. His patient education video is available on the American Academy of Dermatology website should you wish to direct your patients to it.
Because salicylic acid is a keratolytic (or peeling agent), it works by causing the outer layer of skin to shed. When applied topically, it helps to soften and lift psoriasis scales. Coal tar over-the-counter products also can be used for the same purpose. The over-the-counter product guide from the National Psoriasis Foundation is a valuable resource to share with patients.
Expert Commentary
I agree that it is very important to treat scale very gently. In addition to risk for infection, picking and traumatizing scale can lead to worsening of the psoriasis. This is known as the Koebner phenomenon. The phenomenon was first described by Heinrich Koebner in 1876 as the formation of psoriatic lesions in uninvolved skin of patients with psoriasis after cutaneous trauma. This isomorphic phenomenon is now known to involve numerous diseases, among them vitiligo, lichen planus, and Darier disease.
—Jeffrey M. Weinberg, MD (New York, New York)
Myth: Pick Psoriasis Scales to Remove Them
Patients may be inclined to pick psoriasis scales that appear in noticeable areas or on the scalp. However, they should be counseled to avoid this practice, which could cause an infection. Instead, Dr. Steven Feldman (Winston-Salem, North Carolina) suggests putting on an ointment or oil-like medication to soften the scale. “Almost any kind of moisturizer will change the reflective properties of the scale so that you don’t see the scale,” he advised. He also suggested descaling agents such as topical salicylic acid or lactic acid. His patient education video is available on the American Academy of Dermatology website should you wish to direct your patients to it.
Because salicylic acid is a keratolytic (or peeling agent), it works by causing the outer layer of skin to shed. When applied topically, it helps to soften and lift psoriasis scales. Coal tar over-the-counter products also can be used for the same purpose. The over-the-counter product guide from the National Psoriasis Foundation is a valuable resource to share with patients.
Expert Commentary
I agree that it is very important to treat scale very gently. In addition to risk for infection, picking and traumatizing scale can lead to worsening of the psoriasis. This is known as the Koebner phenomenon. The phenomenon was first described by Heinrich Koebner in 1876 as the formation of psoriatic lesions in uninvolved skin of patients with psoriasis after cutaneous trauma. This isomorphic phenomenon is now known to involve numerous diseases, among them vitiligo, lichen planus, and Darier disease.
—Jeffrey M. Weinberg, MD (New York, New York)
Feldman S. How should I remove psoriasis scale? American Academy of Dermatology website. https://www.aad.org/public/diseases/scaly-skin/psoriasis/tips-for-managing-psoriasis/how-should-i-remove-psoriasis-scale. Accessed October 31, 2018.
National Psoriasis Foundation. Over-the-counter products. https://www.aad.org/public/diseases/scaly-skin/psoriasis/tips-for-managing-psoriasis/how-should-i-remove-psoriasis-scale. Published June 2017. Accessed October 31, 2018.
Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
Feldman S. How should I remove psoriasis scale? American Academy of Dermatology website. https://www.aad.org/public/diseases/scaly-skin/psoriasis/tips-for-managing-psoriasis/how-should-i-remove-psoriasis-scale. Accessed October 31, 2018.
National Psoriasis Foundation. Over-the-counter products. https://www.aad.org/public/diseases/scaly-skin/psoriasis/tips-for-managing-psoriasis/how-should-i-remove-psoriasis-scale. Published June 2017. Accessed October 31, 2018.
Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
Modeling Favors Immediate AED Treatment After an Unprovoked First Seizure
Factoring in quality of life, seizure risk, and side effects, a model prefers immediate over delayed treatment.
Immediate treatment of a first unprovoked seizure may be preferable to delayed treatment over a wide range of patients, including those who are at low risk of recurrent seizures, results of a decision analysis suggest.
Taking into account quality of life, seizure risk, and antiepileptic drug (AED) side effects, a model favored treatment of a patient with a single unprovoked seizure who did not meet the International League Against Epilepsy (ILAE) definition of epilepsy, investigators reported.
The model also favored treatment of patients who did meet ILAE criteria, namely, a 10-year recurrence risk greater than 60% in a patient with a single unprovoked seizure, according to the analysis, which was published in the October 9 issue of Neurology.
Together, these findings suggest that the current ILAE epilepsy definition is “too simplistic” for deciding whether to start or withhold AED treatment after a first unprovoked seizure, said M. Brandon Westover, MD, PhD, Associate Professor of Neurology at Massachusetts General Hospital in Boston, and his coauthors.
“A more precise and patient-personalized definition of epilepsy should encompass not only seizure recurrence probability but also a multitude of other risks and benefits associated with AED treatment,” they said.
Weighing Risks and Benefits
To determine which patients with a first unprovoked seizure might benefit from immediate AED treatment, Dr. Westover and his colleagues used a decision model with measures constructed from retrospective clinical trial data.
The goal of the simulation was to determine which treatment strategy—immediate or delayed AED treatment—would maximize the patient’s expected quality-adjusted life years (QALYs). Toward that end, Dr. Westover and his coinvestigators considered three base cases, which represented various degrees of seizure-recurrence risk.
The first case was a 30-year-old man with no risk factors for recurrent seizure other than having had a first seizure. In that case, immediate and deferred AED treatment resulted in 19.04 and 18.65 QALYs, respectively.
“In dollar values, using the conservative approximation of $50,000/QALY gained, this difference in treatment outcomes would amount to $19,500 gained per individual,” Dr. Westover and his coauthors wrote in their report.
The second case was a 30-year-old woman who presented with a first unprovoked seizure and had positive MRI results that establish a high risk of recurrence. As expected, because of the high recurrence risk, this scenario also favored immediate treatment, with 15.23 and 14.75 QALYs, respectively, for the immediate and deferred strategies.
The final case was a wheelchair-bound 60-year-old woman with a first unprovoked seizure and high risk of recurrence, but also a high risk of AED adverse effects and a smaller expected quality of life reduction from further seizures. In this scenario, in which treatment might be “intuitively discouraged” because of the AED side-effect risk, the cohort simulation favored deferred AED treatment by a small margin, the investigators said.
“A high baseline risk for recurrent seizures does not by itself always favor immediate AED treatment,” they said.
Findings May Shift Discussions About Therapy
The conclusion of this decision analysis by Dr. Westover and colleagues is “likely correct” that early treatment of a first unprovoked seizure could be favorable in a wide range of clinical scenarios, according to the authors of an accompanying editorial.
The decision analysis is based on a reasonable, though not comprehensive, set of parameters to simulate base cases representative of common first-ever seizure clinical scenarios, said editorialists Claire S. Jacobs, MD, PhD, and Jong Woo Lee, MD, PhD, both with the Department of Neurology at Brigham and Women’s Hospital in Boston.
Potentially the most controversial scenario addressed in the decision model, they noted, is the patient with low seizure recurrence risk but substantial quality of life decline upon recurrence. While that patient would not meet the commonly accepted 60% recurrence risk threshold that would indicate that treatment is warranted, this model favors immediate treatment because of the potentially disruptive effect of recurrence.
This study does not address important issues such as the cost of medication and patient preferences, they pointed out, and furthermore, QALYs can be difficult to integrate into clinical decision making.
Nonetheless, the findings are worth considering in clinical practice, the authors suggested. “At the very least, this study should, however subtly, shift the starting point of discussion with the patient toward a default of immediate, rather than deferred, treatment after a first unprovoked seizure and apparent absence of disease,” said Drs. Jacob and Lee.
The study was supported by NINDS.
—Andrew D. Bowser
Suggested Reading
Bao EL, Chao LY, Ni P, et al. Antiepileptic drug treatment after an unprovoked first seizure: a decision analysis. Neurology. 2018;91(15):e1429-e1439.
Jacobs CS, Lee JW. Immediate vs delayed treatment of first unprovoked seizure: to treat, or not to treat? Neurology. 2018;91(15):684-685.
Factoring in quality of life, seizure risk, and side effects, a model prefers immediate over delayed treatment.
Factoring in quality of life, seizure risk, and side effects, a model prefers immediate over delayed treatment.
Immediate treatment of a first unprovoked seizure may be preferable to delayed treatment over a wide range of patients, including those who are at low risk of recurrent seizures, results of a decision analysis suggest.
Taking into account quality of life, seizure risk, and antiepileptic drug (AED) side effects, a model favored treatment of a patient with a single unprovoked seizure who did not meet the International League Against Epilepsy (ILAE) definition of epilepsy, investigators reported.
The model also favored treatment of patients who did meet ILAE criteria, namely, a 10-year recurrence risk greater than 60% in a patient with a single unprovoked seizure, according to the analysis, which was published in the October 9 issue of Neurology.
Together, these findings suggest that the current ILAE epilepsy definition is “too simplistic” for deciding whether to start or withhold AED treatment after a first unprovoked seizure, said M. Brandon Westover, MD, PhD, Associate Professor of Neurology at Massachusetts General Hospital in Boston, and his coauthors.
“A more precise and patient-personalized definition of epilepsy should encompass not only seizure recurrence probability but also a multitude of other risks and benefits associated with AED treatment,” they said.
Weighing Risks and Benefits
To determine which patients with a first unprovoked seizure might benefit from immediate AED treatment, Dr. Westover and his colleagues used a decision model with measures constructed from retrospective clinical trial data.
The goal of the simulation was to determine which treatment strategy—immediate or delayed AED treatment—would maximize the patient’s expected quality-adjusted life years (QALYs). Toward that end, Dr. Westover and his coinvestigators considered three base cases, which represented various degrees of seizure-recurrence risk.
The first case was a 30-year-old man with no risk factors for recurrent seizure other than having had a first seizure. In that case, immediate and deferred AED treatment resulted in 19.04 and 18.65 QALYs, respectively.
“In dollar values, using the conservative approximation of $50,000/QALY gained, this difference in treatment outcomes would amount to $19,500 gained per individual,” Dr. Westover and his coauthors wrote in their report.
The second case was a 30-year-old woman who presented with a first unprovoked seizure and had positive MRI results that establish a high risk of recurrence. As expected, because of the high recurrence risk, this scenario also favored immediate treatment, with 15.23 and 14.75 QALYs, respectively, for the immediate and deferred strategies.
The final case was a wheelchair-bound 60-year-old woman with a first unprovoked seizure and high risk of recurrence, but also a high risk of AED adverse effects and a smaller expected quality of life reduction from further seizures. In this scenario, in which treatment might be “intuitively discouraged” because of the AED side-effect risk, the cohort simulation favored deferred AED treatment by a small margin, the investigators said.
“A high baseline risk for recurrent seizures does not by itself always favor immediate AED treatment,” they said.
Findings May Shift Discussions About Therapy
The conclusion of this decision analysis by Dr. Westover and colleagues is “likely correct” that early treatment of a first unprovoked seizure could be favorable in a wide range of clinical scenarios, according to the authors of an accompanying editorial.
The decision analysis is based on a reasonable, though not comprehensive, set of parameters to simulate base cases representative of common first-ever seizure clinical scenarios, said editorialists Claire S. Jacobs, MD, PhD, and Jong Woo Lee, MD, PhD, both with the Department of Neurology at Brigham and Women’s Hospital in Boston.
Potentially the most controversial scenario addressed in the decision model, they noted, is the patient with low seizure recurrence risk but substantial quality of life decline upon recurrence. While that patient would not meet the commonly accepted 60% recurrence risk threshold that would indicate that treatment is warranted, this model favors immediate treatment because of the potentially disruptive effect of recurrence.
This study does not address important issues such as the cost of medication and patient preferences, they pointed out, and furthermore, QALYs can be difficult to integrate into clinical decision making.
Nonetheless, the findings are worth considering in clinical practice, the authors suggested. “At the very least, this study should, however subtly, shift the starting point of discussion with the patient toward a default of immediate, rather than deferred, treatment after a first unprovoked seizure and apparent absence of disease,” said Drs. Jacob and Lee.
The study was supported by NINDS.
—Andrew D. Bowser
Suggested Reading
Bao EL, Chao LY, Ni P, et al. Antiepileptic drug treatment after an unprovoked first seizure: a decision analysis. Neurology. 2018;91(15):e1429-e1439.
Jacobs CS, Lee JW. Immediate vs delayed treatment of first unprovoked seizure: to treat, or not to treat? Neurology. 2018;91(15):684-685.
Immediate treatment of a first unprovoked seizure may be preferable to delayed treatment over a wide range of patients, including those who are at low risk of recurrent seizures, results of a decision analysis suggest.
Taking into account quality of life, seizure risk, and antiepileptic drug (AED) side effects, a model favored treatment of a patient with a single unprovoked seizure who did not meet the International League Against Epilepsy (ILAE) definition of epilepsy, investigators reported.
The model also favored treatment of patients who did meet ILAE criteria, namely, a 10-year recurrence risk greater than 60% in a patient with a single unprovoked seizure, according to the analysis, which was published in the October 9 issue of Neurology.
Together, these findings suggest that the current ILAE epilepsy definition is “too simplistic” for deciding whether to start or withhold AED treatment after a first unprovoked seizure, said M. Brandon Westover, MD, PhD, Associate Professor of Neurology at Massachusetts General Hospital in Boston, and his coauthors.
“A more precise and patient-personalized definition of epilepsy should encompass not only seizure recurrence probability but also a multitude of other risks and benefits associated with AED treatment,” they said.
Weighing Risks and Benefits
To determine which patients with a first unprovoked seizure might benefit from immediate AED treatment, Dr. Westover and his colleagues used a decision model with measures constructed from retrospective clinical trial data.
The goal of the simulation was to determine which treatment strategy—immediate or delayed AED treatment—would maximize the patient’s expected quality-adjusted life years (QALYs). Toward that end, Dr. Westover and his coinvestigators considered three base cases, which represented various degrees of seizure-recurrence risk.
The first case was a 30-year-old man with no risk factors for recurrent seizure other than having had a first seizure. In that case, immediate and deferred AED treatment resulted in 19.04 and 18.65 QALYs, respectively.
“In dollar values, using the conservative approximation of $50,000/QALY gained, this difference in treatment outcomes would amount to $19,500 gained per individual,” Dr. Westover and his coauthors wrote in their report.
The second case was a 30-year-old woman who presented with a first unprovoked seizure and had positive MRI results that establish a high risk of recurrence. As expected, because of the high recurrence risk, this scenario also favored immediate treatment, with 15.23 and 14.75 QALYs, respectively, for the immediate and deferred strategies.
The final case was a wheelchair-bound 60-year-old woman with a first unprovoked seizure and high risk of recurrence, but also a high risk of AED adverse effects and a smaller expected quality of life reduction from further seizures. In this scenario, in which treatment might be “intuitively discouraged” because of the AED side-effect risk, the cohort simulation favored deferred AED treatment by a small margin, the investigators said.
“A high baseline risk for recurrent seizures does not by itself always favor immediate AED treatment,” they said.
Findings May Shift Discussions About Therapy
The conclusion of this decision analysis by Dr. Westover and colleagues is “likely correct” that early treatment of a first unprovoked seizure could be favorable in a wide range of clinical scenarios, according to the authors of an accompanying editorial.
The decision analysis is based on a reasonable, though not comprehensive, set of parameters to simulate base cases representative of common first-ever seizure clinical scenarios, said editorialists Claire S. Jacobs, MD, PhD, and Jong Woo Lee, MD, PhD, both with the Department of Neurology at Brigham and Women’s Hospital in Boston.
Potentially the most controversial scenario addressed in the decision model, they noted, is the patient with low seizure recurrence risk but substantial quality of life decline upon recurrence. While that patient would not meet the commonly accepted 60% recurrence risk threshold that would indicate that treatment is warranted, this model favors immediate treatment because of the potentially disruptive effect of recurrence.
This study does not address important issues such as the cost of medication and patient preferences, they pointed out, and furthermore, QALYs can be difficult to integrate into clinical decision making.
Nonetheless, the findings are worth considering in clinical practice, the authors suggested. “At the very least, this study should, however subtly, shift the starting point of discussion with the patient toward a default of immediate, rather than deferred, treatment after a first unprovoked seizure and apparent absence of disease,” said Drs. Jacob and Lee.
The study was supported by NINDS.
—Andrew D. Bowser
Suggested Reading
Bao EL, Chao LY, Ni P, et al. Antiepileptic drug treatment after an unprovoked first seizure: a decision analysis. Neurology. 2018;91(15):e1429-e1439.
Jacobs CS, Lee JW. Immediate vs delayed treatment of first unprovoked seizure: to treat, or not to treat? Neurology. 2018;91(15):684-685.
Platelet transfusion threshold matters for preterm infants
A lower threshold for platelet transfusions in preterm infants with severe thrombocytopenia is associated with significantly lower incidence of death and major bleeding, compared with a higher threshold, a new study suggests.
A new major bleeding episode or death occurred in 26% of infants in the high-threshold group, compared with 19% in the low-threshold group, representing a 57% higher risk of poor outcomes even after researchers adjusted for gestational age and intrauterine growth restriction (odds ratio, 1.57; P = .02).
Researchers reported the results of a trial in 660 infants with a mean gestational age of 26.6 weeks, who were randomized to a platelet infusion either at a high platelet–count threshold of 50,000/mm3 or a low threshold of 25,000/mm3.
“Although retrospective studies have suggested that platelet transfusions may cause harm in neonates independently of the disease process, data from randomized controlled trials to support this are lacking,” Anna Curley, MD, of the National Maternity Hospital in Dublin and her coauthors reported in the New England Journal of Medicine.
The rates of minor or worse bleeding were similar between the two groups, and the percentage of infants surviving with bronchopulmonary dysplasia at 36 weeks of corrected age was higher in the high-threshold group (63% vs. 54%; OR, 1.54).
The rates of serious adverse events, not including major bleeding, were similar between the high- and low-threshold groups.
The outcomes of transfusions were not influenced by other factors such as intrauterine growth restriction, gestational age, or postnatal age at randomization.
“Our trial highlights the importance of trials of platelet transfusion involving patients with conditions other than haematological malignancies,” the authors wrote.
However they acknowledged that the reasons for the differences in mortality and outcomes between the two study groups were unknown.
“Platelets have recognized immunological and inflammatory effects, outside of effects on hemostasis,” they wrote. “The effect of transfusing adult platelets to a delicately balance neonatal hemostatic system with relatively hypofunctional platelets is also poorly understood.”
The study was supported by the National Health Service Blood and Transplant Research and Development Committee and other foundations. Authors reported financial disclosures related to Sanquin and Cerus.
SOURCE: Curley A et al. N Engl J Med. 2018 Nov 2. doi: 10.1056/NEJMoa1807320.
A lower threshold for platelet transfusions in preterm infants with severe thrombocytopenia is associated with significantly lower incidence of death and major bleeding, compared with a higher threshold, a new study suggests.
A new major bleeding episode or death occurred in 26% of infants in the high-threshold group, compared with 19% in the low-threshold group, representing a 57% higher risk of poor outcomes even after researchers adjusted for gestational age and intrauterine growth restriction (odds ratio, 1.57; P = .02).
Researchers reported the results of a trial in 660 infants with a mean gestational age of 26.6 weeks, who were randomized to a platelet infusion either at a high platelet–count threshold of 50,000/mm3 or a low threshold of 25,000/mm3.
“Although retrospective studies have suggested that platelet transfusions may cause harm in neonates independently of the disease process, data from randomized controlled trials to support this are lacking,” Anna Curley, MD, of the National Maternity Hospital in Dublin and her coauthors reported in the New England Journal of Medicine.
The rates of minor or worse bleeding were similar between the two groups, and the percentage of infants surviving with bronchopulmonary dysplasia at 36 weeks of corrected age was higher in the high-threshold group (63% vs. 54%; OR, 1.54).
The rates of serious adverse events, not including major bleeding, were similar between the high- and low-threshold groups.
The outcomes of transfusions were not influenced by other factors such as intrauterine growth restriction, gestational age, or postnatal age at randomization.
“Our trial highlights the importance of trials of platelet transfusion involving patients with conditions other than haematological malignancies,” the authors wrote.
However they acknowledged that the reasons for the differences in mortality and outcomes between the two study groups were unknown.
“Platelets have recognized immunological and inflammatory effects, outside of effects on hemostasis,” they wrote. “The effect of transfusing adult platelets to a delicately balance neonatal hemostatic system with relatively hypofunctional platelets is also poorly understood.”
The study was supported by the National Health Service Blood and Transplant Research and Development Committee and other foundations. Authors reported financial disclosures related to Sanquin and Cerus.
SOURCE: Curley A et al. N Engl J Med. 2018 Nov 2. doi: 10.1056/NEJMoa1807320.
A lower threshold for platelet transfusions in preterm infants with severe thrombocytopenia is associated with significantly lower incidence of death and major bleeding, compared with a higher threshold, a new study suggests.
A new major bleeding episode or death occurred in 26% of infants in the high-threshold group, compared with 19% in the low-threshold group, representing a 57% higher risk of poor outcomes even after researchers adjusted for gestational age and intrauterine growth restriction (odds ratio, 1.57; P = .02).
Researchers reported the results of a trial in 660 infants with a mean gestational age of 26.6 weeks, who were randomized to a platelet infusion either at a high platelet–count threshold of 50,000/mm3 or a low threshold of 25,000/mm3.
“Although retrospective studies have suggested that platelet transfusions may cause harm in neonates independently of the disease process, data from randomized controlled trials to support this are lacking,” Anna Curley, MD, of the National Maternity Hospital in Dublin and her coauthors reported in the New England Journal of Medicine.
The rates of minor or worse bleeding were similar between the two groups, and the percentage of infants surviving with bronchopulmonary dysplasia at 36 weeks of corrected age was higher in the high-threshold group (63% vs. 54%; OR, 1.54).
The rates of serious adverse events, not including major bleeding, were similar between the high- and low-threshold groups.
The outcomes of transfusions were not influenced by other factors such as intrauterine growth restriction, gestational age, or postnatal age at randomization.
“Our trial highlights the importance of trials of platelet transfusion involving patients with conditions other than haematological malignancies,” the authors wrote.
However they acknowledged that the reasons for the differences in mortality and outcomes between the two study groups were unknown.
“Platelets have recognized immunological and inflammatory effects, outside of effects on hemostasis,” they wrote. “The effect of transfusing adult platelets to a delicately balance neonatal hemostatic system with relatively hypofunctional platelets is also poorly understood.”
The study was supported by the National Health Service Blood and Transplant Research and Development Committee and other foundations. Authors reported financial disclosures related to Sanquin and Cerus.
SOURCE: Curley A et al. N Engl J Med. 2018 Nov 2. doi: 10.1056/NEJMoa1807320.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: The odds of new major bleeding or death were 57% higher in preterm infants who received a platelet transfusion at a higher threshold of 50,000 per mm3 than at a lower threshold of 25,000 mm3 (P = .02).Study details: Randomized study in 660 preterm infants with severe thrombocytopenia.
Disclosures: The study was supported by the National Health Service Blood and Transplant Research and Development Committee, and other foundations. Authors reported financial disclosures related to Sanquin and Cerus.
Source: Curley A et al. N Engl J Med. 2018 Nov 2. doi: 10.1056/NEJMoa1807320.
Rate of sling removal 9 years after MUS for SUI over 3%
a British study found.
Ipek Gurol-Urganci, PhD, of the London School of Hygiene and Tropical Medicine, and her coauthors said their study comes as a result of safety concerns around the procedure, which resulted in a suspension of the operation in the United Kingdom.
“There is concern about problems that some women experience following MUS insertion, including pain, dyspareunia, persistent urinary incontinence, and exposure or erosion. However, there is little randomized, clinical trial evidence on these longer-term outcomes,” they wrote in JAMA, noting that an estimated 250,000 MUS operations were performed in 2010 in the United States.
The current study involved 95,057 women in England who underwent an MUS insertion procedure for SUI for the first time in a National Health Service hospital between 2006 and 2015. Overall, 60,194 of the women had a retropubic insertion and 34,863 had a transobturator insertion.
At 9 years after the initial insertion, the mesh was removed in 3.3% of women. The risk of removal was higher for women who had a retropubic insertion (3.6%), compared with those who had a transobturator insertion (2.7%).
“The risk of a removal was about 30% lower if the mesh sling had been inserted via the transobturator route, which may be explained by the removal of transobturator sling being a more complicated procedure,” Dr. Gurol-Urganci and her associates noted.
Mesh sling removal risk decreased with age, with the risk at 4.4% for women aged 18-39 years, compared with 2.1% in women aged 70 years and older at 9 years after insertion.
The authors wrote that the risks of removal and any reoperation (mesh removal and/or reoperation for SUI) were higher among women from a white racial/ethnic background. However, it was not possible to “disentangle explanations” for these possible differences in risk seen with patient characteristics, which ranged from higher morbidity to differences in the reasons for surgery.
Results also showed that the risk of reoperation was 4.5% at 9 years after the initial insertion, and was slightly higher for a transobturator insertion at 5.3%, compared with 4.1% for a retropubic insertion.
The risk of any reoperation, including mesh removal and/or reoperation for SUI, following the initial MUS insertion was 6.9% at 9 years (95% confidence interval, 6.7%-7.1%), but no statistically significant difference was observed between retropubic and transobturator insertion.
“The present results demonstrate that removal and reoperation risks were associated with the insertion route and patient factors,” Dr. Gurol-Urganci and her associates wrote.
“These findings may guide women and their surgeons when making decisions about surgical treatment of stress urinary incontinence,” they concluded.
The study was supported by a grant from the National Institute for Health Research Health Services and Delivery Research Programme and several of the authors reported receiving National Institute for Health Research research grants. One author reported providing consultancy services to Cambridge Medical Robotics, Femeda, and Astellas.
SOURCE: Gurol-Urganci I et al. JAMA. 2018 Oct 23. doi:10.1001/jama.2018.14997.
a British study found.
Ipek Gurol-Urganci, PhD, of the London School of Hygiene and Tropical Medicine, and her coauthors said their study comes as a result of safety concerns around the procedure, which resulted in a suspension of the operation in the United Kingdom.
“There is concern about problems that some women experience following MUS insertion, including pain, dyspareunia, persistent urinary incontinence, and exposure or erosion. However, there is little randomized, clinical trial evidence on these longer-term outcomes,” they wrote in JAMA, noting that an estimated 250,000 MUS operations were performed in 2010 in the United States.
The current study involved 95,057 women in England who underwent an MUS insertion procedure for SUI for the first time in a National Health Service hospital between 2006 and 2015. Overall, 60,194 of the women had a retropubic insertion and 34,863 had a transobturator insertion.
At 9 years after the initial insertion, the mesh was removed in 3.3% of women. The risk of removal was higher for women who had a retropubic insertion (3.6%), compared with those who had a transobturator insertion (2.7%).
“The risk of a removal was about 30% lower if the mesh sling had been inserted via the transobturator route, which may be explained by the removal of transobturator sling being a more complicated procedure,” Dr. Gurol-Urganci and her associates noted.
Mesh sling removal risk decreased with age, with the risk at 4.4% for women aged 18-39 years, compared with 2.1% in women aged 70 years and older at 9 years after insertion.
The authors wrote that the risks of removal and any reoperation (mesh removal and/or reoperation for SUI) were higher among women from a white racial/ethnic background. However, it was not possible to “disentangle explanations” for these possible differences in risk seen with patient characteristics, which ranged from higher morbidity to differences in the reasons for surgery.
Results also showed that the risk of reoperation was 4.5% at 9 years after the initial insertion, and was slightly higher for a transobturator insertion at 5.3%, compared with 4.1% for a retropubic insertion.
The risk of any reoperation, including mesh removal and/or reoperation for SUI, following the initial MUS insertion was 6.9% at 9 years (95% confidence interval, 6.7%-7.1%), but no statistically significant difference was observed between retropubic and transobturator insertion.
“The present results demonstrate that removal and reoperation risks were associated with the insertion route and patient factors,” Dr. Gurol-Urganci and her associates wrote.
“These findings may guide women and their surgeons when making decisions about surgical treatment of stress urinary incontinence,” they concluded.
The study was supported by a grant from the National Institute for Health Research Health Services and Delivery Research Programme and several of the authors reported receiving National Institute for Health Research research grants. One author reported providing consultancy services to Cambridge Medical Robotics, Femeda, and Astellas.
SOURCE: Gurol-Urganci I et al. JAMA. 2018 Oct 23. doi:10.1001/jama.2018.14997.
a British study found.
Ipek Gurol-Urganci, PhD, of the London School of Hygiene and Tropical Medicine, and her coauthors said their study comes as a result of safety concerns around the procedure, which resulted in a suspension of the operation in the United Kingdom.
“There is concern about problems that some women experience following MUS insertion, including pain, dyspareunia, persistent urinary incontinence, and exposure or erosion. However, there is little randomized, clinical trial evidence on these longer-term outcomes,” they wrote in JAMA, noting that an estimated 250,000 MUS operations were performed in 2010 in the United States.
The current study involved 95,057 women in England who underwent an MUS insertion procedure for SUI for the first time in a National Health Service hospital between 2006 and 2015. Overall, 60,194 of the women had a retropubic insertion and 34,863 had a transobturator insertion.
At 9 years after the initial insertion, the mesh was removed in 3.3% of women. The risk of removal was higher for women who had a retropubic insertion (3.6%), compared with those who had a transobturator insertion (2.7%).
“The risk of a removal was about 30% lower if the mesh sling had been inserted via the transobturator route, which may be explained by the removal of transobturator sling being a more complicated procedure,” Dr. Gurol-Urganci and her associates noted.
Mesh sling removal risk decreased with age, with the risk at 4.4% for women aged 18-39 years, compared with 2.1% in women aged 70 years and older at 9 years after insertion.
The authors wrote that the risks of removal and any reoperation (mesh removal and/or reoperation for SUI) were higher among women from a white racial/ethnic background. However, it was not possible to “disentangle explanations” for these possible differences in risk seen with patient characteristics, which ranged from higher morbidity to differences in the reasons for surgery.
Results also showed that the risk of reoperation was 4.5% at 9 years after the initial insertion, and was slightly higher for a transobturator insertion at 5.3%, compared with 4.1% for a retropubic insertion.
The risk of any reoperation, including mesh removal and/or reoperation for SUI, following the initial MUS insertion was 6.9% at 9 years (95% confidence interval, 6.7%-7.1%), but no statistically significant difference was observed between retropubic and transobturator insertion.
“The present results demonstrate that removal and reoperation risks were associated with the insertion route and patient factors,” Dr. Gurol-Urganci and her associates wrote.
“These findings may guide women and their surgeons when making decisions about surgical treatment of stress urinary incontinence,” they concluded.
The study was supported by a grant from the National Institute for Health Research Health Services and Delivery Research Programme and several of the authors reported receiving National Institute for Health Research research grants. One author reported providing consultancy services to Cambridge Medical Robotics, Femeda, and Astellas.
SOURCE: Gurol-Urganci I et al. JAMA. 2018 Oct 23. doi:10.1001/jama.2018.14997.
FROM JAMA
Key clinical point: The findings of this study may inform decision making when choosing treatment for stress urinary incontinence.
Major finding: Within 9 years of a mesh insertion for stress urinary incontinence, the rate of sling removal was 3.3% and the rate of reoperation was 4.5%.
Study details: A prospective, observational study examining long-term mesh removal and reoperations in over 95,000 women who underwent midurethral mesh operations for stress urinary incontinence between 2006 and 2015.
Disclosures: The study was supported by a grant from the National Institute for Health Research Health Services and Delivery Research Programme and several of the authors reported receiving National Institute for Health Research research grants. One author reported providing consultancy services to Cambridge Medical Robotics, Femeda, and Astellas Pharma.
Source: Gurol-Urganci I et al. JAMA. 2018 Oct 23. doi:10.1001/jama.2018.14997.